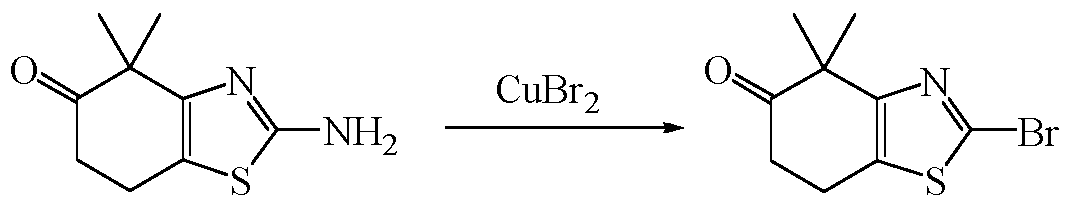WO2014190949A1 - Quadri-fused cyclic anaplastic lymphoma kinase inhibitor - Google Patents
Quadri-fused cyclic anaplastic lymphoma kinase inhibitor Download PDFInfo
- Publication number
- WO2014190949A1 WO2014190949A1 PCT/CN2014/079072 CN2014079072W WO2014190949A1 WO 2014190949 A1 WO2014190949 A1 WO 2014190949A1 CN 2014079072 W CN2014079072 W CN 2014079072W WO 2014190949 A1 WO2014190949 A1 WO 2014190949A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- alkyl
- membered
- amino
- membered heterocyclic
- Prior art date
Links
- 0 CC[C@@]1(C)*(I)=C(*C(*C(C2)=C(C)CC(*[C@]3P=C(*C)P)=C2C3P=*)=C(C)C)*1 Chemical compound CC[C@@]1(C)*(I)=C(*C(*C(C2)=C(C)CC(*[C@]3P=C(*C)P)=C2C3P=*)=C(C)C)*1 0.000 description 4
- ZBNASJDWWRBWPS-UHFFFAOYSA-N CC(C(CCC1)O)C1=O Chemical compound CC(C(CCC1)O)C1=O ZBNASJDWWRBWPS-UHFFFAOYSA-N 0.000 description 1
- AOPBDTHQAIWWMI-UHFFFAOYSA-N CC(C)(C(CCC1)=O)C1=O Chemical compound CC(C)(C(CCC1)=O)C1=O AOPBDTHQAIWWMI-UHFFFAOYSA-N 0.000 description 1
- HSYHJEQHEGMNMO-UHFFFAOYSA-N CC(C)(C)OC(N(CC1)CCN1c1nc(C(C)(C)c([nH]c2c3ccc(C#N)c2)c3C2=O)c2[s]1)=O Chemical compound CC(C)(C)OC(N(CC1)CCN1c1nc(C(C)(C)c([nH]c2c3ccc(C#N)c2)c3C2=O)c2[s]1)=O HSYHJEQHEGMNMO-UHFFFAOYSA-N 0.000 description 1
- ZPGHHWZCVJGSBS-ZIAGYGMSSA-N CC(C)(C)OC(N(C[C@]1(CN(C)C2)C=N)C[C@@]12[N]#C)=O Chemical compound CC(C)(C)OC(N(C[C@]1(CN(C)C2)C=N)C[C@@]12[N]#C)=O ZPGHHWZCVJGSBS-ZIAGYGMSSA-N 0.000 description 1
- KVOUHLVOTMOJBS-UHFFFAOYSA-N CC(C)(C)OC(N1CC2(CNC2)C1)=O Chemical compound CC(C)(C)OC(N1CC2(CNC2)C1)=O KVOUHLVOTMOJBS-UHFFFAOYSA-N 0.000 description 1
- CJHLFQNJNLWXOM-AOOOYVTPSA-N CC(C)(C)OC(N1C[C@H](CN(C)C2)[C@H]2C1)=O Chemical compound CC(C)(C)OC(N1C[C@H](CN(C)C2)[C@H]2C1)=O CJHLFQNJNLWXOM-AOOOYVTPSA-N 0.000 description 1
- MAAQYMNRQIREIS-UHFFFAOYSA-N CC(C)(c([nH]c1c2ccc(C#N)c1)c2C1=O)c2c1[s]c(N1CC(CC3)N(C)C3C1)n2 Chemical compound CC(C)(c([nH]c1c2ccc(C#N)c1)c2C1=O)c2c1[s]c(N1CC(CC3)N(C)C3C1)n2 MAAQYMNRQIREIS-UHFFFAOYSA-N 0.000 description 1
- XCNMIAILJHEWTA-UHFFFAOYSA-N CC(C)(c1c(CC2)[s]c(C)n1)C2=O Chemical compound CC(C)(c1c(CC2)[s]c(C)n1)C2=O XCNMIAILJHEWTA-UHFFFAOYSA-N 0.000 description 1
- IZOMALONLCOIJX-SNAWJCMRSA-N CC(C)C(CC(c([s]1)cnc1Br)=O)NC(/C=C/C#N)=C Chemical compound CC(C)C(CC(c([s]1)cnc1Br)=O)NC(/C=C/C#N)=C IZOMALONLCOIJX-SNAWJCMRSA-N 0.000 description 1
- ILBZSNOGPTUKHC-UHFFFAOYSA-N CC1CN(C)C1 Chemical compound CC1CN(C)C1 ILBZSNOGPTUKHC-UHFFFAOYSA-N 0.000 description 1
- KYINPWAJIVTFBW-UHFFFAOYSA-N CC1CNCC1 Chemical compound CC1CNCC1 KYINPWAJIVTFBW-UHFFFAOYSA-N 0.000 description 1
- AVFZOVWCLRSYKC-UHFFFAOYSA-N CN1CCCC1 Chemical compound CN1CCCC1 AVFZOVWCLRSYKC-UHFFFAOYSA-N 0.000 description 1
- PAMIQIKDUOTOBW-UHFFFAOYSA-N CN1CCCCC1 Chemical compound CN1CCCCC1 PAMIQIKDUOTOBW-UHFFFAOYSA-N 0.000 description 1
- PVOAHINGSUIXLS-UHFFFAOYSA-N CN1CCNCC1 Chemical compound CN1CCNCC1 PVOAHINGSUIXLS-UHFFFAOYSA-N 0.000 description 1
- FNKSTXGVEUSZJJ-UHFFFAOYSA-N CN1COCC1 Chemical compound CN1COCC1 FNKSTXGVEUSZJJ-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D519/00—Heterocyclic compounds containing more than one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system not provided for in groups C07D453/00 or C07D455/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D513/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for in groups C07D463/00, C07D477/00 or C07D499/00 - C07D507/00
- C07D513/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for in groups C07D463/00, C07D477/00 or C07D499/00 - C07D507/00 in which the condensed system contains two hetero rings
- C07D513/04—Ortho-condensed systems
Definitions
- the present invention relates to the field of medical technology, and particularly relates to a tetracyclic anaplastic lymphoma kinase inhibitor or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, and a method for preparing the same, which comprises the compound Pharmaceutical preparations and pharmaceutical compositions, and the compounds or stereoisomers thereof, or pharmaceutically acceptable salts, esters or solvates thereof, in the manufacture of a medicament for the treatment and/or prevention of ALK-mediated cancer-related diseases application.
- Background technique
- Anaplastic lymphoma kinase a member of the receptor acid kinase family, recruits downstream proteins through autophosphorylation to express specific genes and regulate cell metabolism and growth.
- ALK Anaplastic lymphoma kinase
- NSCLC non-small cell lung cancer
- ALK's small molecule inhibitors can affect the growth of tumor cells and play an anti-tumor role.
- crizotinib developed by Pfizer Inc.
- the design and screening of second-generation ALK inhibitors with good efficacy in patients with resistance to Crizotinib has significant clinical significance.
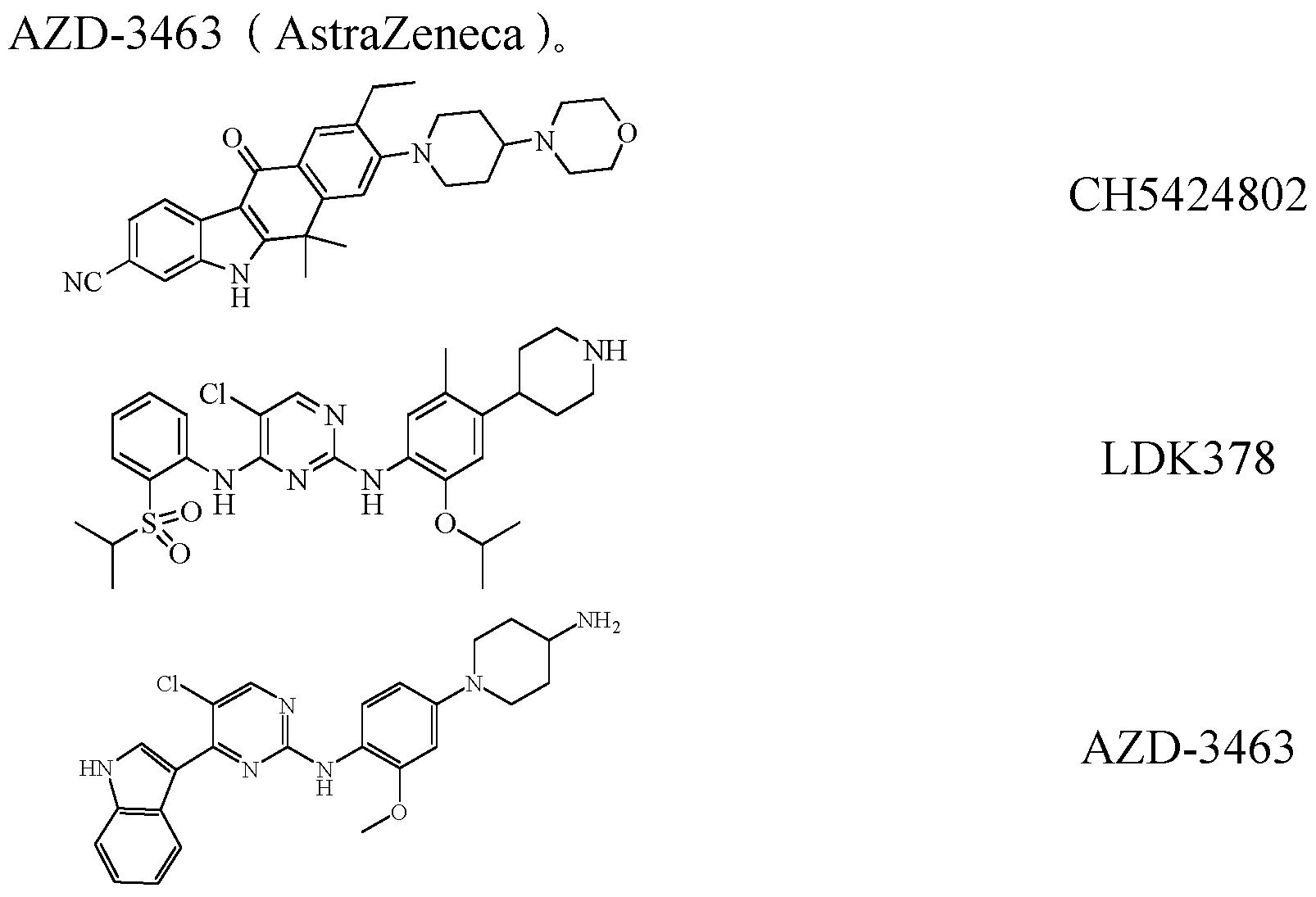
- ALK inhibitors include CH5424802 (Roche), LDK378 (Norartis), and
- the structure of the compound is modified to find a new compound structure, and efforts are made to improve the rationality of the compound.
- the nature of the drug such as increasing the exposure or bioavailability of the compound, to find small molecule inhibitors with high activity on ALK mutations, is of great significance for the clinical treatment of diseases caused by ALK mutations. Summary of the invention
- the present invention aims to develop a small molecule inhibitor against ALK, and has invented a tetracyclic anaplastic lymphoma kinase inhibitor which has a good effect on the treatment and/or prevention of ALK-mediated cancer-related diseases.
- the specific technical solutions are as follows:
- a 2 is selected from 1 2 or
- a 3 is selected from C-R 4 or N, and Ai, A 2 and A 3 are not N at the same time;
- R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxy, carboxy, nitro, halogen atom, amino, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1 -6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl;
- R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or a 14-membered cycloalkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2-6 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently Optionally substituted by one or more of the following substituents: hydroxy, carboxy, amino, cyano, halo, nitro or 3 to 14 membered heterocyclyl;
- M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
- R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
- R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
- Y is selected from N or CR 9 ;
- X is selected from 0, S or NR 9 ;
- R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3 ⁇ 8-membered cycloalkyl group;
- Q is selected from the following groups:
- R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
- R 7 is selected from a 6 to 12 membered bridged ring group, a 6 to 12 membered spirocyclic group, a 3 to 8 membered heterocyclic group or a 6 to 14 membered heterocyclic group which is optionally substituted with a substituent selected from the group consisting of Amino, hydroxy, nitro, alkane, carboxyl, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, 3 to 8 membered heterocyclic or 3 ⁇ 8-membered cycloalkyl;
- n is selected from 0, 1, 2, 3, 4, 5 or 6,
- R 7 may be the same or different
- n cannot be 0, and R 7 cannot be selected from a 3 to 8 membered heterocyclic group.
- a 2 is selected from 1 2 or
- a 3 is selected from C-R 4 or N, and Ai, A 2 and A 3 are not N at the same time;
- R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxyl, carboxyl, nitro, halogen atom, amino group, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl ;
- R 3 is selected from the group consisting of hydrogen, cyano, hydroxy, amino, halogen atom, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered ring
- the alkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently optionally one or more of the following Substituent substitution: a hydroxyl group, a carboxyl group, an amino group, a cyano group, a halogen atom, a nitro group or a 3 to 14 membered heterocyclic group;
- M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
- R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
- R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
- Y is selected from N or CR 9 ;
- X is selected from 0, S or NR 9 ;
- R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3 ⁇ 8-membered cycloalkyl group;
- Q is selected from the following groups:
- R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
- R 7 is selected from a 6 to 12 membered bridged ring group or a 6 to 12 membered spirocyclic group optionally substituted by a substituent selected from the group consisting of an amino group, a hydroxyl group, a nitro group, a halogen atom, a carboxyl group, and a C 1-6 alkane group. a group, a C 1-6 alkoxy group, a C 2-6 alkenyl group, a C 2-6 alkynyl group, a 3 to 8 membered heterocyclic group or a 3 to 8 membered cycloalkyl group;
- n 1.
- a 2 is selected from 1 2 or
- a 3 is selected from C-R 4 or N, and Ai, A 2 and A 3 are not N at the same time;
- R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxy, carboxy, nitro, halogen atom, amino, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1 -6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl;
- R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or a 14-membered cycloalkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2-6 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently Optionally substituted by one or more of the following substituents: hydroxy, carboxy, amino, cyano, halo, nitro or 3 to 14 membered heterocyclyl;
- M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
- R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
- R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
- Y is selected from N or CR 9 ;
- X is selected from 0, S or NR 9 ;
- R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3 ⁇ 8-membered cycloalkyl group;
- Q is selected from the following groups:
- R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
- R 7 is selected from a 6 to 12 membered bridged ring group optionally substituted with a substituent, a 6 to 12 membered spirocyclic group or a 3 to 8 membered hetero a cyclic group, the substituent is selected from the group consisting of an amino group, a hydroxyl group, a nitro group, a halogen atom, a carboxyl group, a ⁇ 6 alkyl group, an alkoxy group, a C 2-6 alkenyl group, a C 2-6 alkynyl group, and a 3 to 8 membered heterocyclic ring.
- n is selected from 0, 1, 2, 3, 4, 5 or 6,
- R 7 may be the same or different.
- R 1 , R 2 and R 4 are each independently selected from hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, a C 2-6 alkenyl group, a C 2-6 alkynyl group or a 3 to 8 Metacycloalkyl;
- R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or ⁇ 8-membered cycloalkyl, the C 1-6 alkyl group, C 1-6 alkoxy group, C 2-6 alkenyl group, C 2-6 alkynyl group and 3 to 8 membered cycloalkyl group may be independently Substituted by one to three substituents: a hydroxyl group, a carboxyl group, an amino group, a cyano group, a halogen atom, a nitro group or a 3 to 8 membered heterocyclic group;
- M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
- R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
- R 5 and R 6 are bonded to each other to form a 5- to 10-membered heterocyclic group or a 3- to 8-membered cycloalkyl group together with the carbon atom to which they are attached;
- Y is selected from N or CR 9 ;
- X is selected from 0, S or NR 9 ;
- R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3 ⁇ 8-membered cycloalkyl group;
- Q is selected from the following groups: (1) 4 to 7 membered heterocyclic group,
- R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 4 alkyl, C M alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
- R 7 is selected from a 6 to 10 membered bridged ring group, a 6 to 12 membered spirocyclic group, a 4 to 7 membered heterocyclic group or a 6 to 12 membered heterocyclic group which is optionally substituted with a substituent selected from the group consisting of Amino group, hydroxyl group, halogen atom, ⁇ 6 alkyl group,
- n is selected from 0, 1, 2 or 3
- R 7 may be the same or different
- n cannot be 0, and R 7 cannot be selected from a 4 to 7 membered heterocyclic group.
- ⁇ ⁇ , A 2 and A 3 are each independently selected from CH;
- R 3 is selected from hydrogen or cyano
- M is selected from Li
- R 5 and R 6 are each independently selected from hydrogen or C 1-6 alkyl
- Y is selected from N;
- X is selected from S.
- R 1 , R 2 and R 4 are each independently selected from hydrogen, a halogen atom, a C 1-6 alkyl group or a 3 to 8 membered cycloalkyl group; and R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl Acyl, alkene, CL 6 alkyl or 3 to 8 membered cycloalkyl;
- M is selected from 0, S or NR 8 , R 8 is selected from hydrogen, C 1-6 alkyl or C 1-6 alkoxy, and the C 1-6 alkyl may be optionally substituted by ⁇ 6 alkoxy ;
- R 5 and R 6 are each independently selected from hydrogen, a halogen atom, a ⁇ 6 alkyl group, a ⁇ 6 alkoxy group or a hydroxy C 1-6 alkyl group.
- R 5 and R 6 are bonded to each other to form a 5- to 6-membered heterocyclic group or a 3- to 8-membered cycloalkyl group together with the carbon atom to which they are attached;
- Y is selected from N or CR 9 ;
- X is selected from 0, S or NR 9 ;
- R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3 ⁇ 8-membered cycloalkyl group;
- Q is selected from the following groups:
- R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, C 1-6 alkylaminocarbonyl, hydroxy C 1-6 alkyl, hydroxy C 1-6 alkylamino, C M alkyl, methylsulfonyl, methylsulfonylamino, aminosulfonyl, aminosulfonylamino, C 2-6 alkenyl, C 2-6 alkynyl;
- R 7 is selected from a 7 to 10 membered bridged ring group optionally substituted with a substituent, a 7 to 11 membered spirocyclic group, a 5 to 6 membered heterocyclic group or a 6 to 10 membered heterocyclic group, and the substituent is selected from the group consisting of Amino group, hydroxyl group, halogen atom, ⁇ 6 alkyl group,
- n is selected from 0, 1, 2 or 3
- R 7 may be the same or different
- n cannot be 0, and R 7 cannot be selected from a 5- to 6-membered heterocyclic group.
- R 1 , R 2 and R 4 are each independently selected from hydrogen, methyl or ethyl;
- R 3 is selected from the group consisting of hydrogen, cyano, hydroxy, amino, fluorine, chlorine, methyl or ethyl;
- M is selected from NR 8 and R 8 is selected from hydrogen or CL 4 alkyl;
- R 5 and R 6 are each independently selected from C 1-4 alkyl
- Y is selected from N or CR 9 ;
- X is selected from S or NR 9 ;
- R 9 is selected from the group consisting of hydrogen, methyl, ethyl or n-propyl
- R 1Q is selected from amino or CL 4 alkyl
- R 7 is selected from the group consisting of 7 to 9 membered bridged ring groups, 7 to 11 membered spirocyclic groups or 5 to 6 membered heterocyclic groups.
- n is selected from 0 or 1
- n cannot be 0, and R 7 cannot be selected from a 5- to 6-membered heterocyclic group.
- Q is selected from a 4 to 7 membered heterocyclic group, preferably having 1 or 2 nitrogen atoms as a ring atom, more preferably saturated;
- R 7 is selected from 7 to 8 membered bridged ring groups, preferably containing 1 or 2 ring atoms selected from oxygen and nitrogen, more preferably saturated;
- ⁇ is selected from 1.
- Q is selected from optionally substituted with one to two identical or different substituents R 1Q of 7 ⁇ 8 membered bridged ring group, preferably containing 1 or 2 nitrogen atoms as ring atoms, more preferably a saturated,
- R 1Q is selected from amino or CL 6 alkyl
- R 7 is selected from a 4 to 7 membered heterocyclic group, preferably contains 1 or 2 ring atoms selected from oxygen and nitrogen, more preferably saturated;
- n is selected from 0 or 1, and when n is 0, R 7 is absent.
- n is selected from 0 or 1, and when n is 0, R 7 is absent.
- Q and R 7 is a bridged ring group, the bridged ring group containing at least one nitrogen atom as a ring member, and the bridge is bridged by two ring atoms (such as carbon atoms) adjacent to the nitrogen atom.
- Q is selected from, for example, "optionally substituted alkyl CL 4 - ⁇ - one,
- Q is selected from 7 to 11 membered spirocyclic groups optionally substituted by one to two identical or different R 1Q groups, preferably a spirocyclic group having 1 to 3 hetero atoms, more preferably the spiro group
- the cyclic group contains 7 to 11 ring atoms, wherein 1 or 2 ring atoms are nitrogen atoms, the remaining ring atoms are carbon atoms, and still more preferably, the spiro group is a saturated group,
- R 1Q is selected from amino or CL 4 alkyl
- R 7 does not exist.
- Q is selected from 6 to 10 membered heterocyclic groups optionally substituted by one to two identical or different R 1Q , and the heterocyclic group is preferably a heterocyclic group having 1 to 3 hetero atoms, more preferably The heterocyclic group contains 6 to 10 ring atoms, wherein 1 to 3 ring atoms are hetero atoms selected from nitrogen and oxygen, and the remaining ring atoms are carbon atoms, still more preferably, the heterocyclic group Is a saturated group,
- R 1Q is selected from amino or CL 4 alkyl
- R 7 does not exist.
- a pharmaceutical composition comprising the compound of any one of the preceding claims 1-17, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, and one or more A pharmaceutically acceptable carrier and/or diluent.
- a drug related to a disease selected from the group consisting of a brain tumor, a non-small cell lung cancer, a squamous cell carcinoma, a bladder cancer, a stomach cancer, an ovarian cancer, a peritoneal cancer, a pancreatic cancer, a breast cancer, a head and neck cancer , cervical cancer, endometrial cancer, rectal cancer, liver cancer, hepatoblastoma, papillary renal cell tumor, head and neck squamous cell tumor, nephroblastoma, renal cancer, esophageal adenocarcinoma, esophageal squamous cell Cancer, glioma, prostate cancer, thyroid cancer, female genital tract cancer, carcinoma in situ, lymphoma, neuroblastoma,
- a 2 , A 3 , M, Y, X, R 3 , R 5 , R 6 , R 7 , Q and n are as defined in the technical scheme 1-17, and Q' is as in the technical scheme 1-17
- Q' is as in the technical scheme 1-17
- the defined Q or Q, R 7 ' as defined in Technical Schemes 1-17 protected by a protecting group (PG) is protected by R 7 or protected group (PG ) as defined in Technical Schemes 1-17 R 7 as defined in Technical Schemes 1-17.
- the "prime atom” as used in the present invention means a fluorine atom, a chlorine atom, a bromine atom, an iodine atom or the like.
- the " ⁇ 6 alkyl group” of the present invention means a linear or branched alkyl group having 1 to 6 carbon atoms, and includes, for example, "C 1-4 alkyl group", “C 1-3 alkyl group” and the like. Such as methyl, ethyl, propyl, butyl, pentyl, hexyl and the like.
- the alkyl group “the present invention” refers to a specific example in which the number of carbon atoms is 1-4 in the above "C 1-6 alkyl group”.
- the "C 2-6 alkenyl group” of the present invention means a linear or branched or cyclic alkenyl group having 2 to 6 carbon atoms containing at least one of the bonds, including, for example, "C 2-4 alkenyl group”. ", "C 2-3 alkenyl”, “C 3-6 cycloalkenyl”, etc., such as ethenyl, propenyl, butenyl, pentenyl, hexenyl, butadienyl, pentadienyl And hexadienyl, cyclopentenyl, cyclopentadienyl, cyclohexenyl and cyclohexadienyl and the like.
- the joy keys can optionally be cis and trans.
- the " ⁇ 6 alkynyl group” of the present invention means a linear or branched alkynyl group having 2 to 6 carbon atoms containing at least one triple bond, and includes, for example, "C 2-6 alkynyl group”, “C” 2-4 alkynyl", “C 2-3 alkynyl” and the like, such as ethynyl, propynyl, butynyl, pentynyl, hexynyl and the like.
- the “alkylsulfonylamino group” means a group in which the above “ 6 alkyl group” is bonded to another structure through a thio group, an oxy group, an aminocarbonyl group, a sulfonyl group or a sulfonylamino group, respectively.
- C M alkylsulfonyl and “C 1-4 alkylsulfonylamino” refer to a group in which the above “ 4 alkyl group” is bonded to another structure through a sulfonyl group and a sulfonylamino group, respectively.
- hydroxy 6 alkyl group and “halogenated 6 alkyl group” of the present invention respectively mean that a hydroxyl group or a halogen atom replaces one or more hydrogen atoms on the above “d. 6 alkyl group", and passes through an alkyl group and other structures. Connected base Mission.
- hydroxyl. 14 alkyl and “halo ⁇ 14 alkyl” mean, respectively, a hydroxy group, a halogen atom substituted for one or more hydrogen atoms on the above "C M alkyl group”, and which are bonded to other structures through an alkyl group. a group, wherein "halogen atom” is as described above.
- hydroxy c 1-6 alkoxy group means a hydroxyl group, an amino group or a carbonyl group to replace the above " ⁇ 6 alkane”
- One or more hydrogen atoms on the oxy group and a group attached to the other structure through an alkoxy group.
- hydroxy C 1-6 alkylamino group as used in the present invention means a group in which an atom which can be substituted by any one of the amino groups is substituted by the above-mentioned "hydroxyl 6 alkyl group” and which is bonded to another structure through an amino group.
- ( ⁇ 6 alkyl) 2 amino and "6 alkylamino” refer to an amino group and any two atoms of a substituent can be substituted with the aforementioned ".6 alkyl group", and by A group in which an amino group is bonded to other structures.
- the "3- to 14-membered cycloalkyl group" as used in the present invention means that the alkane moiety of 3 to 14 carbon atoms is removed by a hydrogen atom-derived cyclic alkyl group, including a 3- to 8-membered cycloalkyl group (monocyclic ring), 6 -14 membered cyclocycloalkyl (bicyclic or polycyclic).
- the 3- to 8-membered cycloalkyl group means that the alkane moiety of 3 to 8 carbon atoms is removed by a hydrogen atom-derived cyclic alkyl group, and examples thereof include, but are not limited to, cyclopropyl group, cyclobutylalkyl group, cyclopentyl group. , cyclohexane, cycloheptyl, cyclooctyl, methylcyclopropane, dimethylcyclopropane, methylcyclobutane, dimethylcyclobutane, methylcyclopentanyl , dimethylcyclopentyl, methylcyclohexane, dimethylcyclohexane, and the like.
- the 6- to 14-membered cyclocycloalkyl group refers to a 6- to 14-membered cyclic group formed by two or more cyclic structures sharing two adjacent carbon atoms, and examples thereof include, but are not limited to: Bicyclo[3.1.0]hexane, bicyclo[4.1.0]heptyl, bicyclo[2.2.0]hexane, bicyclo[3.2.0]heptyl, bicyclo[4.2.0 Octyl, octahydrocyclopentadienyl, octahydro-1/7-fluorenyl, decahydronaphthyl, tetradecafluorophenanyl, re-cyclo[3.1.0]hex-2-enyl, bicyclo [4.1.0]Hept-3-enyl, bicyclo[3.2.0]hept-3-enyl, bicyclo[4.2.0]oct-3-enyl, 1,2,3,3-tetrahydrocyclo Pentadien
- hetero atom as used in the present invention means ⁇ , 0, C(0), S, SO and/or S0 2 and the like, preferably N, 0, S, more preferably N, 0.
- the "3- to 14-membered heterocyclic group" of the present invention means a 3- to 14-membered cyclic group containing one to more hetero atoms, including a 3 to 8 membered heterocyclic group (monocyclic) and 6 to 14 members.
- Heterocyclic group (bicyclic or polycyclic).
- Heterocyclyl groups can be saturated, partially unsaturated or fully unsaturated.
- a 3 to 8 membered heterocyclic group which means having 3 to 8 ring atoms (having at least one hetero atom) Monocyclic heterocyclic group.
- Specific examples include, but are not limited to, 2,5-dihydrothiophenyl, 4,5-dihydropyrazolyl, 3,4-dihydro-2/7-pyranyl, 5,6-dihydro-4/7 -1,3-oxazinyl, aziridine, azetidinyl, s-heterocyclobutane, tetrahydrofuranyl, tetrahydropyrrolyl, imidazolidinyl, pyrazolidinyl, tetrahydrofuranyl, 1,4-dioxanyl group, 1,3-dioxanyl group, 1,3-dithiacyclohexane group, morpholinyl group, piperazinyl group and the like.
- the 4- to 7-membered heterocyclic group of the present invention and the "5-6-membered heterocyclic group” refer to a specific example in which the number of ring atoms in the above "3 to 8 membered heterocyclic group” is 4 to 7 members and 5 to 6 members.
- 6-14-membered heterocyclic group means a fused ring containing 6 to 14 ring atoms (having at least one hetero atom) joined by two or more ring structures sharing two adjacent atoms with each other.
- a structure such as a benzo 3 to 8 to 8 membered heterocyclic group and a 3 to 8 membered heterocyclic group
- An isocyclic structure replaces a group formed by any substitutable hydrogen atom.
- the "6- to 12-membered heterocyclic group” and the “6- to 10-membered heterocyclic group” of the present invention mean that the above-mentioned "6- to 14-membered heterocyclic group" has a ring number of 6 to 12, 6 to 10 Specific examples of yuan.
- the "5- to 10-membered heterocyclic group" as used in the present invention refers to a specific example in which the number of ring atoms in the above “3 to 14-membered heterocyclic group” is 5 to 10 members, and for example, a 5- to 10-membered heterocyclic group includes 5 to 6 members.
- the "6- to 12-membered bridged ring group" as used in the present invention means that any two rings share two non-adjacent ring atoms and have 6 to 12 ring atoms (including carbon atoms and optional hetero atoms). structure.
- the bridged ring group can be saturated, partially unsaturated or completely unsaturated.
- the "6 12-membered spirocyclic group" as used in the present invention means a structure having at least two rings sharing a ring atom and having 6 to 12 ring atoms including a carbon atom and optionally a hetero atom.
- the spiro group can be saturated, partially unsaturated or completely unsaturated.
- the present invention provides a compound represented by the formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
- a 2 is selected from CR 2 or N;
- a 3 is selected from CR 4 or N, and Ai, A 2 and A 3 are not N at the same time;
- R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxy, carboxy, nitro, halogen atom, amino, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1 -6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl;
- R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or a 14-membered cycloalkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2-6 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently Optionally substituted by one or more of the following substituents: hydroxy, carboxy, amino, cyano, halo, nitro or 3 to 14 membered heterocyclyl;
- M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
- R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
- R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
- Y is selected from N or CR 9 ;
- X is selected from 0, S or NR 9 ;
- R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3 ⁇ 8-membered cycloalkyl group;
- Q is selected from the following groups:
- R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
- R 7 is selected from a 6 to 12 membered bridged ring group, a 6 to 12 membered spirocyclic group, a 3 to 8 membered heterocyclic group or a 6 to 14 membered heterocyclic group which is optionally substituted with a substituent selected from the group consisting of Amino, hydroxy, nitro, alkane, carboxyl, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, 3 to 8 membered heterocyclic or 3 ⁇ 8 yuan naphthenic Base
- n is selected from 0, 1, 2, 3, 4, 5 or 6,
- R 7 may be the same or different
- n cannot be 0, and R 7 cannot be selected from a 3 to 8 membered heterocyclic group.
- 1 is 1 1 .
- a 2 is CR 2 .
- a 3 is CR 4 .
- R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, halogen atom, ⁇ 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkyne Base or 3 to 8 membered cycloalkyl.
- R 1 , R 2 and R 4 are each independently selected from hydrogen, a halogen atom, a C 1-6 alkyl group or a 3 to 8 membered cycloalkyl group.
- RR 2 and R 4 are each independently selected from hydrogen, methyl or ethyl.
- R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, halogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl , C 2-6 alkynyl or 3 to 8 membered cycloalkyl, said C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl and 3 ⁇
- the 8-membered cycloalkyl group may be independently optionally substituted with one to three of the following substituents: a hydroxyl group, a carboxyl group, an amino group, a cyano group, a sulfonic acid atom, a nitro group or a 3 to 8 membered heterocyclic group.
- R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, halogen atom, ⁇ 6 alkyl or 3 to 8 membered cycloalkyl. In a preferred embodiment, R 3 is selected from the group consisting of hydrogen, cyano, hydroxy, amino, fluoro, chloro, methyl or ethyl.
- M is selected from 0, S or NR 8 and R 8 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkyne
- the C 1-6 alkyl group, the C 2-6 alkenyl group and the C 2-6 alkynyl group may be independently optionally substituted by a 6 alkoxy group.
- M is selected from 0, S or NR 8 , R 8 is selected from hydrogen, C alkyl or C alkoxy, and said C alkyl group may be optionally substituted by C alkoxy.
- M is selected from NR 8 and R 8 is selected from hydrogen or C 1-4 alkyl.
- R 5 and R 6 are each independently selected from hydrogen, a halogen atom, ⁇ 6 alkyl, C 1-6 alkoxy, hydroxy C 1-6 alkyl, C 2-6 alkenyl or The C 2-6 alkynyl group, or R 5 and R 6 are bonded to each other to form a 5- to 10-membered heterocyclic group or a 3- to 8-membered cycloalkyl group together with the carbon atom to which they are attached.
- R 5 and R 6 are each independently selected from hydrogen, a halogen atom, a ⁇ 6 alkyl group, a ⁇ 6 alkoxy group or a hydroxy group .
- R 5 and R 6 alkyl group or R 5 and R 6 are bonded to each other, Together with the carbon atom to which they are attached, a 5- to 6-membered heterocyclic group or a 3- to 8-membered cycloalkyl group is formed.
- R 5 and R 6 independently independently select a Ci_4 3 ⁇ 4* group.
- the oxime is selected from ruthenium or CR 9 , X is selected from 0, S or NR 9 ; R 9 is selected from hydrogen, C 1-6 alkyl, CM alkynyl or 3 to 8 membered cycloalkyl. In one preferred embodiment, Y is selected from N or CR 9, X is selected from S or NR 9, R 9 is selected from hydrogen, methyl, ethyl or n-propyl.
- Q is selected from the group consisting of: (1) a 4 to 7 membered heterocyclic group (or a 4 to 7 membered monocyclic heterocyclic group), (2) optionally being one to three identical or different R 1Q substituted 3 to 8 membered cycloalkyl or 6 to 12 membered heterocyclic group, and (3) optionally 7 to 10 membered bridged or substituted by 1 to 3 identical or different R 1Q A spiro ring group;
- R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, ⁇ 6 alkylamino Carbonyl, hydroxy- 6 alkyl, hydroxy- 6 alkylamino, halogenated CL 4 alkyl, CL 4 alkylsulfonyl, CL 4 alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, C 2-6 Alkeny
- Q is selected from the group consisting of: (1) a 5- to 6-membered heterocyclic group (or a 5- to 6-membered monocyclic heterocyclic group), (2) optionally being one to three identical Or a different R 1Q substituted 3 to 8 membered cycloalkyl or 6 to 10 membered heterocyclic group, and ( 3 ) a 7 to 9 membered bridged ring group optionally substituted by one to three identical or different R 1Q or 7 to 11 membered spiro group, R 1Q is selected from amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, C 1 -6 alkylaminocarbonyl group, a hydroxyl CL 6 alkyl, hydroxy ⁇ 6 alkylamino, C M substituting group, methylsulfonyl group, methylsulfonyl group, sulfamoyl group, sulfamoyl
- Q is selected from the group consisting of (1) 5 to 6 membered heterocyclic groups (or 5 to 6 membered monocyclic heterocyclic groups), (2) optionally being one to two identical or different R a 1Q- substituted 6- to 10-membered heterocyclic group, and (3) a 7- to 9-membered bridged ring group or a 7- to 11-membered spirocyclic group optionally substituted by one or two identical or different R 1Q , and R 1Q is selected from the group consisting of Amino or C M alkyl.
- Q is selected from a 4 to 7 membered heterocyclic group (or a 4 to 7 membered monocyclic heterocyclic group), preferably having 1 or 2 nitrogen atoms as a ring atom, more preferably ⁇ selected.
- the ground is saturated.
- Q is selected from one to two
- R 1 substituted 7 to 8 membered bridged ring group, preferably having 1 or 2 nitrogen atoms as a ring atom, more preferably saturated, wherein R 1Q is selected from an amino group or a 6 alkyl group .
- Q is selected from a 7 to 11-membered spiro group optionally substituted by one to two identical or different R 1Q , and the spiro group is preferably a spiro group having 1 to 3 hetero atoms, more preferably The spiro group contains 7 to 11 ring atoms, wherein 1 or 2 ring atoms are nitrogen atoms, the remaining ring atoms are carbon atoms, and still more preferably, The spiro group is a saturated group wherein R 1Q is selected from an amino group or a C M alkyl group.
- Q is selected from 6 to 10 membered heterocyclyl optionally substituted with one to two identical or different R 1Q groups, preferably having from 1 to 3 heteroatoms And a heterocyclic group, more preferably the heterocyclic group contains 6 to 10 ring atoms, wherein 1 to 3 ring atoms are hetero atoms selected from nitrogen and oxygen, and the remaining ring atoms are carbon atoms, and still more
- the heterocyclic group is a saturated group, wherein R 1Q is selected from an amino group or an alkyl group.
- Q is selected from one to two identical or different
- R 7 is selected from a 6 to 10 membered bridged ring group optionally substituted with a substituent, a 6 to 12 membered spirocyclic group, a 4 to 7 membered heterocyclic group or a 6 to 12 membered heterocyclic group.
- the substituent is selected from the group consisting of an amino group, a hydroxyl group, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, a C 2-6 alkenyl group or a C 2-6 alkynyl group.
- R 7 is selected from the group consisting of a 7 to 10 membered bridged ring group optionally substituted with a substituent, a 7 to 11 membered spirocyclic group, a 5 to 6 membered heterocyclic group or a 6 to 10 membered heterocyclic group.
- a ring group the substituent being selected from the group consisting of an amino group, a hydroxyl group, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, a C 2-6 alkenyl group or a C 2-6 alkynyl group.
- R 7 is selected from the group consisting of 7 to 9 membered bridged ring groups, 7 to 11 membered spirocyclic groups or 5 to 6 membered heterocyclic groups. In a preferred embodiment, R 7 is selected from the group consisting of 7 to 8 membered bridged ring groups, preferably containing 1 or 2 selected from the group, more preferably
- the heterocyclic group preferably contains 1 or 2 ring atoms selected from oxygen and nitrogen, more preferably saturated.
- n is selected from 0, 1, 2 or 3. In a preferred embodiment, n Selected from 0 or 1. In a preferred embodiment, n is selected from zero. In a preferred embodiment, n is selected from 1.
- ⁇ 2 and ⁇ 3 are each independently selected from CH; R 3 is selected from hydrogen or cyano; ⁇ is selected from NH; and R 5 and R 6 are each independently selected from hydrogen or C 1-6 Alkyl; Y is selected from N; X is selected from S.
- At least one of Q and R 7 is a bridged ring group containing at least one nitrogen atom as a ring member, the bridge passing through two ring atoms adjacent to the nitrogen atom (eg, carbon atom) is formed as a bridgehead atom, Q is selected from, for example, "optionally substituted C M alkyl ⁇ N ⁇ H" and N fT -, R 7 is absent or is selected from ⁇ "and a + N ⁇ yo.
- Q is a bridged ring group containing at least one nitrogen atom as a ring member, and the bridge is formed as a bridgehead atom by two rings (such as carbon atoms) adjacent to the nitrogen atom, for example Q is selected from “optionally ( ⁇ . 4 alkyl substituted) and tenth ⁇ 1-.
- R 7 is a bridged ring group containing at least one nitrogen atom as two adjacent ring atoms (eg, carbon atoms) as a bridgehead
- the present invention also includes any one of the embodiments obtained by combining the above embodiments and two or more of the above preferred embodiments.
- the pharmaceutically acceptable salt of any one of the compounds of the formula (I) of the present invention means a salt prepared from a pharmaceutically acceptable, non-toxic base or acid, including an organic acid salt, a mineral acid salt, an organic alkali salt. , inorganic alkali salt.
- Organic acid salts include formic acid, acetic acid, benzenesulfonic acid, benzoic acid, p-toluenesulfonic acid, camphorsulfonic acid, citric acid, methanesulfonic acid, ethanesulfonic acid, propanesulfonic acid, fumaric acid, gluconic acid, glutamic acid , salts of isethionate, lactic acid, maleic acid, malic acid, mandelic acid, mucic acid, pamoic acid, pantothenic acid, succinic acid, tartaric acid, and the like.
- the inorganic acid salt includes a salt of hydrobromic acid, hydrochloric acid, nitric acid, sulfuric acid, phosphoric acid or the like.
- the organic base salts include primary, secondary and tertiary amines, and the substituted amines include naturally occurring substituted amines, cyclic amines and alkali ion exchange resins selected from the group consisting of betaines, caffeine, choline, N,N,-dibenzylethylene.
- Natural amino acid salts such as glycine, alanine, valine, leucine, isoleucine, norleucine, tyrosine, cystine, cysteine, methionine, proline, hydroxyguanidine Salts of acid, histidine, ornithine, lysine, arginine, serine, and the like.
- Inorganic alkali salts include ammonium and 4 liters, sodium, potassium, Salts of calcium, magnesium, zinc, strontium, aluminum, iron, ketone, ferrous, manganese, divalent manganese, and the like.
- stereoisomer of the compound of formula (I) refers to all stereoisomers, including enantiomers, which are produced when a compound of formula (I) has a chiral center, a bond or the like. Diastereomers, cis-trans isomers, tautomers, geometric isomers, epimers, and mixtures thereof are included within the scope of the invention.
- Chiral fillers include, but are not limited to:
- the "ester" of the compound of the formula (I) of the present invention includes an ester which can be formed by esterification reaction with an alcohol when a compound of the formula (I) is present, and an organic acid or an inorganic group when the compound of the formula (I) has a hydroxyl group.
- the compound represented by the formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt or ester thereof may be in the form of a "solvate", and the solvent may be water, methanol, ethanol or the like.
- the hygroscopicity of the solvate product proceeds gradually.
- the present invention provides a pharmaceutical composition
- a pharmaceutical composition comprising a compound of the present invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, together with one or more pharmaceutically acceptable carriers and/or diluents .
- the present invention further claims any of the compounds of the above formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, and one or more pharmaceutically acceptable carriers and/or
- the pharmaceutical composition of the diluent or diluent can be formulated into any of the pharmaceutically acceptable dosage forms. It is administered to a patient in need of such treatment by oral, parenteral, rectal or pulmonary administration.
- oral administration it can be prepared into conventional solid preparations such as tablets, capsules, pills, granules, etc.; or can be prepared into oral liquid preparations, such as oral solutions, oral suspensions, syrups, etc. .
- a suitable filler, a binder, a disintegrant, a lubricant or the like may be added.
- parenteral administration it can be prepared as an injection, including injection, sterile powder for injection and concentrated solution for injection.
- the injection it can be produced by a conventional method in the prior art pharmaceutical field, and when the injection is formulated, an additional agent may be added, or a suitable additive may be added depending on the nature of the drug.
- rectal administration it can be made into a suppository or the like.
- pulmonary administration it can be made into an inhalant or a spray.
- the invention also provides a pharmaceutical composition
- a pharmaceutical composition comprising a compound of the invention or a stereoisomer thereof Or a pharmaceutically acceptable salt, ester or solvate thereof, one or more pharmaceutically acceptable carriers and/or diluents, and one or more anti-tumor agents and immunosuppressive agents.
- the antitumor agent and immunosuppressive agent are selected from the group consisting of methotrexate, capecitabine, gemcitabine, deoxyfluorouridine, pemetrexed disodium, paclitaxil, imatinib, erlotidine Nipa, lapatinib, gefitinib, vandetanib, Herceptin, bevacizumab, rituximab, trastuzumab, paclitaxel, vinorelbine, docetaxel, multiple Spirulina, hydroxycamptothecin, mitomycin, epirubicin, pirarubicin, bleomycin, letrozole, tamoxifen, fulvestrant, lysine, flutamide , leuprolide, anastrozole, ifosfamide, busulfan, cyclophosphamide, carmustine, nimustine, semustine, nitrogen mustard, melphalan, cocoaine, carboplatin
- reaction in the presence of a base (e.g., triethylamine) under heating (e.g., about 100-120 ° C) (e.g., at least 30 minutes, such as about 1-2 hours);
- a base e.g., triethylamine
- the amide) is reacted at room temperature (eg, 10-30 C) in the presence of indium trichloride (eg, at least 30 minutes, such as about 16 hours), followed by the addition of a reducing agent (eg, sodium cyanoborohydride) (eg, at least 30 Minutes, such as about 2 hours),
- a reducing agent eg, sodium cyanoborohydride
- Ai, A 2 , A 3 , M, Y, X, R 3 , R 5 , R 6 , R 7 , Q and n are as defined above, and Q' is a Q or a protected group as defined above ( PG) protected hereinbefore defined Q, R 7 'R is as hereinbefore defined hereinbefore 7 or a protected group (PG) as defined protected R 7.
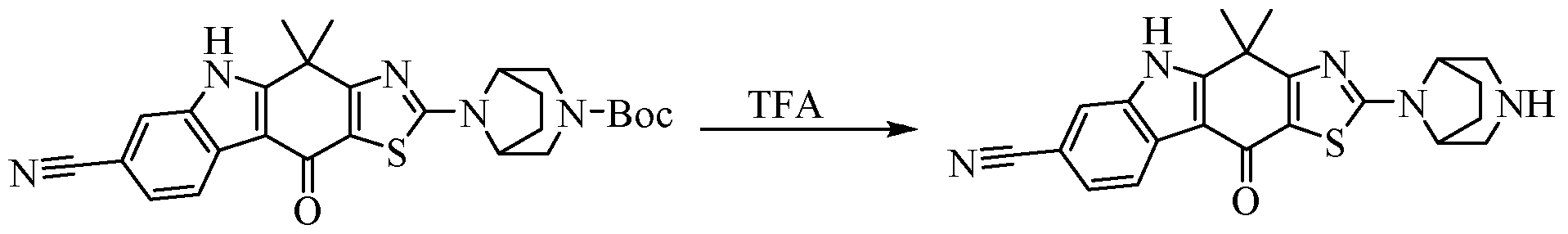
- the protecting group (PG) is a commonly used protecting group in the field of organic synthesis, including but not limited to: -Bn (benzyl), -PMB (p-methoxybenzyl), -Tos (p-toluenesulfonyl), -Fmoc (fluorenylmethoxycarbonyl), -Ac (acetyl), -SEM (2-(trimethylsilyl)ethoxymethyl), -Pht (phthaloyl) and -Alloc (allyloxy) Carbonyl).
- the preparation method of the present invention further comprises the step of deprotecting the protected Q and/or protected R 7 , and optionally alkylating (eg, methylating) the deprotected H atom. step.
- the reaction equation is as follows:
- the raw material 1 is dissolved in a solvent (for example, glacial acetic acid), and a 40% hydrobromic acid solution is added thereto. Under ice water bath, a glacial acetic acid solution in which an appropriate amount of bromine is dissolved is added dropwise, and the mixture is stirred until the raw material is consumed, and quenched by adding ice water.
- the organic solvent is extracted by an organic solvent (for example, ethyl acetate), and the combined organic phase is separated and purified by a suitable method (for example, silica gel column chromatography).
- the intermediate 1 and the starting material 2 are dissolved in a suitable solvent (e.g., tetrahydrofuran), and the mixture is heated to reflux until the reaction is completed, the solvent is removed, and the intermediate 2 is obtained by an appropriate method.
- a suitable solvent e.g., tetrahydrofuran
- Step 3 Preparation of Intermediate 3
- an organic solvent for example, acetonitrile
- the t-butyl nitrite is added dropwise in an ice water bath. After completion, the temperature is raised to room temperature, stirred (for example, 2 h), and the solvent is removed. Intermediate 3 was isolated.
- intermediate 3 the starting material 3 or its hydrochloride (e.g., its hydrochloride) is dissolved in a suitable solvent (e.g., trifluoroacetic acid, acetic acid), heated to completion, cooled, concentrated, and isolated to afford intermediate 4 as appropriate.
- a suitable solvent e.g., trifluoroacetic acid, acetic acid
- the intermediate 4 is placed in a suitable solvent, and an appropriate amount of an oxidizing agent (for example, DDQ (2,3-dichloro-5,6-dicyanio-p-benzoquinone) and methyl dichromate) is added, and the reaction is completed at room temperature, and a saturated aqueous solution of sodium hydrogencarbonate is added. It is quenched, extracted with an organic solvent (e.g., dichloromethane), and the organic phase is combined, and Intermediate 5 is isolated by an appropriate method.
- an oxidizing agent for example, DDQ (2,3-dichloro-5,6-dicyanio-p-benzoquinone) and methyl dichromate
- the intermediate 5 and the starting material 4 are dissolved in a suitable solvent (e.g., acetonitrile), and a base (e.g., methyl carbonate) is added.
- a suitable solvent e.g., acetonitrile
- a base e.g., methyl carbonate
- the intermediate 6 is dissolved in a suitable solvent (dimethyl sulfoxide and triethylamine), and a suitable oxidizing agent (for example, sulfur trioxide pyridine) is added thereto, and the mixture is stirred at room temperature until the reaction is completed, and the reaction liquid is concentrated, and the intermediate 7 is isolated by an appropriate method. .
- a suitable solvent dimethyl sulfoxide and triethylamine
- a suitable oxidizing agent for example, sulfur trioxide pyridine
- the intermediate 6 is dissolved in a suitable solvent (dichloromethane), and a suitable oxidizing agent (for example, Dess-Martin reagent) is added thereto, and the mixture is stirred at room temperature until the reaction is completed, and the reaction is quenched (for example, with water), and the intermediate 7 is isolated by an appropriate method.
- a suitable solvent for example, dichloromethane
- a suitable oxidizing agent for example, Dess-Martin reagent
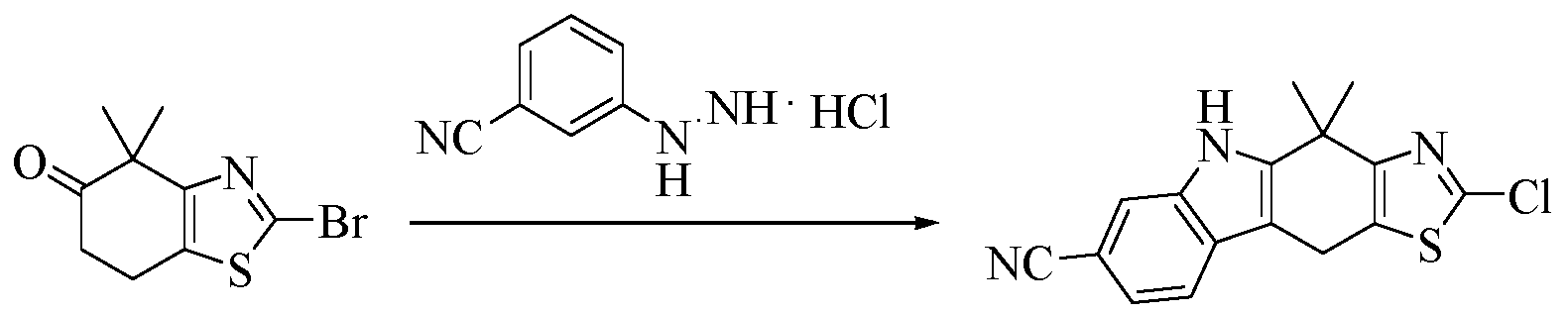
- the intermediate 5 and the starting material 4' are dissolved in a solvent (for example, N-methylpyrrolidone), a base (for example, triethylamine) is added, heated (for example, to 120 ° C), and the reaction is stirred (for example, 2 hours), and cooled ( For example, to room temperature, the reaction solution is poured into a solvent (for example, water), stirred (for example, for half an hour), filtered, and dried to give Intermediate 7.
- a solvent for example, N-methylpyrrolidone
- a base for example, triethylamine
- the raw material 1 is dissolved in a solvent (for example, glacial acetic acid), and a 40% hydrobromic acid solution is added thereto. Under ice water bath, a glacial acetic acid solution in which an appropriate amount of bromine is dissolved is added dropwise, and the mixture is stirred until the raw material is consumed, and quenched by adding ice water.
- the organic solvent is extracted by an organic solvent (for example, ethyl acetate), and the combined organic phase is separated and purified by a suitable method (for example, silica gel column chromatography).
- the intermediate 1 and the starting material 2 are dissolved in a suitable solvent (e.g., tetrahydrofuran), and the mixture is heated to reflux until the reaction is completed, the solvent is removed, and the intermediate 2 is obtained by an appropriate method.
- a suitable solvent e.g., tetrahydrofuran
- the intermediate 2 and the copper bromide are dissolved in an organic solvent (for example, acetonitrile), and the t-butyl nitrite is added dropwise in an ice water bath. After completion, the temperature is raised to room temperature, stirred (for example, 2 h), and the solvent is removed. Intermediate 3 was isolated.
- organic solvent for example, acetonitrile
- the intermediate 5 is placed in a suitable solvent, and an appropriate amount of an oxidizing agent (for example, DDQ (2,3-dichloro-5,6-dicyanio-p-benzoquinone) and potassium dichromate) is added, and the reaction is completed at room temperature, and a saturated aqueous solution of sodium hydrogencarbonate is added. It is quenched, extracted with an organic solvent (e.g., dichloromethane), and the organic phase is combined, and Intermediate 6 is isolated by an appropriate method.
- an oxidizing agent for example, DDQ (2,3-dichloro-5,6-dicyanio-p-benzoquinone) and potassium dichromate
- AA 2 , A 3 , M, Y, X, R 3 , R 5 , R 6 , Q, R 7 and n are as defined above.
- the present invention provides the use of a compound of the present invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, for the manufacture of a medicament for the treatment and/or prevention of an ALK-mediated cancer-related disease.
- the invention also provides a method of treating and/or preventing an ALK-mediated cancer-related disease, comprising administering to a subject in need thereof a therapeutically effective amount of a compound of the invention or a stereoisomer thereof, or a pharmaceutically acceptable thereof Accepted salts, esters or solvates.
- the invention also provides a compound of the invention, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, in combination with one or more antitumor agents and immunosuppressive agents, for use in therapy and / or use in drugs that prevent ALK-mediated cancer-related diseases.
- the invention also provides a method of treating and/or preventing an ALK-mediated cancer-related disease comprising administering to a subject in need thereof a therapeutically effective amount of the combination in combination with one or more anti-tumor agents and an immunosuppressant A compound of the invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof.
- the antitumor agent and immunosuppressive agent are selected from the group consisting of methotrexate, capecitabine, gemcitabine, deoxyfluorouridine, pemetrexed disodium, paclitaxil, imatinib, erlotidine Nipa, lapatinib, gefitinib, vandetanib, Herceptin, bevacizumab, rituximab, trastuzumab, paclitaxel, vinorelbine, docetaxel, multiple Spirulina, hydroxycamptothecin, mitomycin, epirubicin, pirarubicin, bleomycin, letrozole, tamoxifen, fulvestrant, lysine, flutamide , leuprolide, anastrozole, ifosfamide, busulfan, cyclophosphamide, carmustine, nimustine, Semustine, nitrogen mustard, melphalan, cyclamate, carbo
- the cancer-related diseases are selected from the group consisting of brain tumors, non-small cell lung cancer, squamous cell carcinoma, bladder cancer, gastric cancer, ovarian cancer, peritoneal cancer, pancreatic cancer, breast cancer, head and neck cancer, cervical cancer, endometrium.
- Cancer rectal cancer, liver cancer, hepatoblastoma, papillary renal cell tumor, head and neck squamous cell tumor, nephroblastoma, renal cancer, esophageal adenocarcinoma, esophageal squamous cell carcinoma, glioma, prostate Cancer, thyroid cancer, female genital tract cancer, carcinoma in situ, lymphoma, neuroblastoma, neurofibromatosis, bone cancer, skin cancer, colon cancer, testicular cancer, small cell lung cancer, gastrointestinal stromal tumor, Prostate tumors, mast cell tumors, multiple myeloma or melanoma.
- the cancer-related disease is selected from the group consisting of lymphoma (e.g., anaplastic large cell lymphoma) and lung cancer (e.g., non-small cell lung cancer).
- lymphoma e.g., anaplastic large cell lymphoma
- lung cancer e.g., non-small cell lung cancer
- the cancer-related disease is selected from the group consisting of lung cancer (e.g., non-small cell lung cancer).
- the compound of the formula (I) of the present invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, has excellent ALK inhibitory activity;
- the preparation process of the compound of the invention has a high purity and stable quality, and is easy to carry out large-scale industrial production.
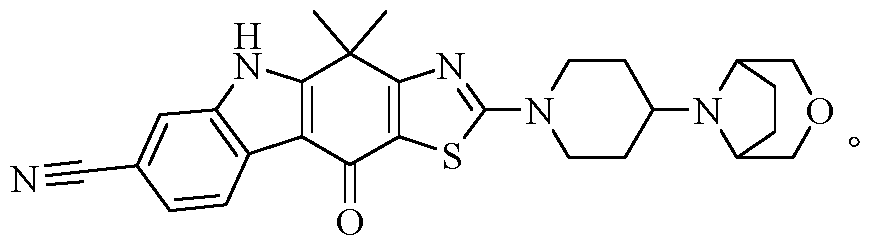
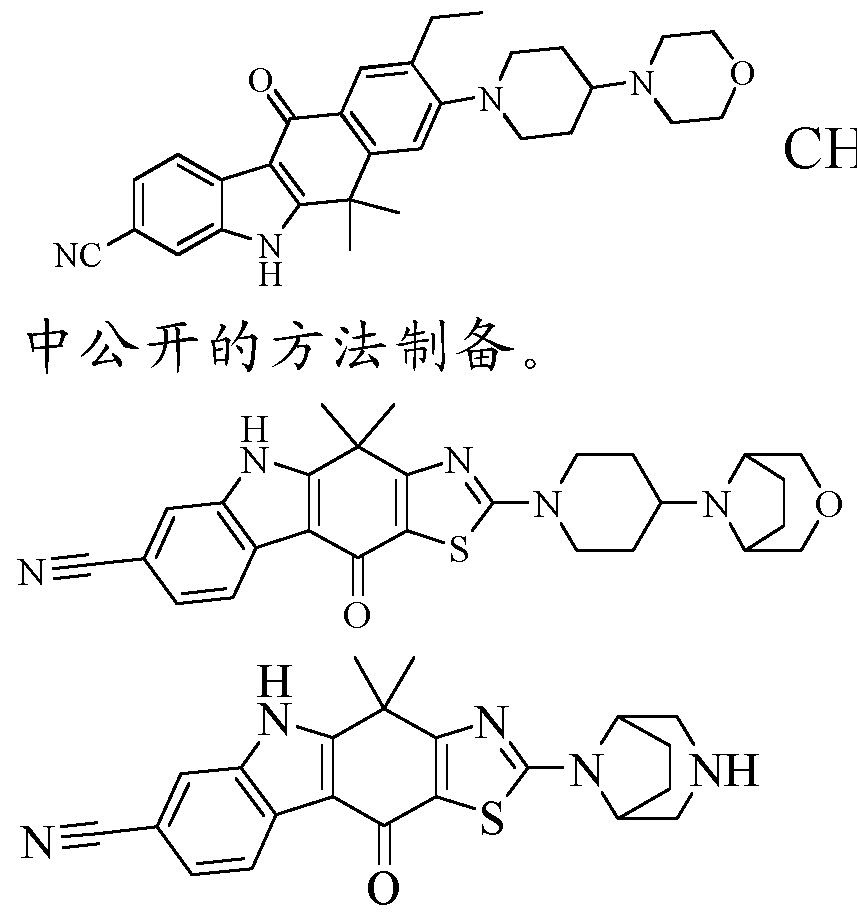
- Test Specimens Compounds 1-6, 9, 10, 13, 14, 18, 24 and 26 of the present invention, the chemical names and preparation methods thereof, can be found in the preparation examples of the respective compounds.
- Control drug CH5424802, it can be based on
- ALK Analytical '1' Lymphoma Kinase
- a 1 mM stock solution was taken and diluted with DMSO to prepare a solution having a concentration of 200 ⁇ M as a mother liquor.
- the mother liquor was diluted three times in DMSO to prepare a series of solutions, and then each concentration was diluted 80 times with ALK kinase buffer to prepare 2.5 ⁇ each test solution, the concentrations were: 2500 ⁇ , 833.33 ⁇ , 277.78 ⁇ , 92.59 ⁇ , 30.86 ⁇ , 10.29 ⁇ , 3.43 ⁇ , 1.14 ⁇ , 0.38 ⁇ , 0.13 ⁇ , 0.04 ⁇ .
- the required 5xALK kinase solution, 5 ⁇ substrate solution, and 5 ⁇ solution were separately prepared in ALK kinase buffer and used.
- Compound 26 It can be seen from Table 1 that the compound of the present invention has a good inhibitory activity against ALK kinase and is comparable to the inhibitory activity of the reference drug, and can be used for the treatment of a kinase-related disease, particularly an ALK kinase-mediated disorder or condition, which is remarkable. Clinical significance.
- Experimental Example 2 Pharmacokinetic experiments in rats of the present invention
- Test article The compounds of the present invention 1 and 7, were prepared by themselves, and their chemical names and preparation methods are shown in the preparation examples of the respective compounds.
- Control drug 5424802, which can be used as a basis
- Test animals male SD rats, 3 / route of administration / test article, weight 200-280g
- For the reference drug weigh 3.29 mg, add 0.1% Tween 80 + 2% HPC (hydroxypropyl cellulose) 6.45 mL, grind thoroughly with a tissue grinder 600 rpm, mix well, that is, as Rat PO administration drug solution.
- Preparation method of 0.1% Tween 80+2% HPC Weigh 2 g of HPC, slowly add to 80 mL of ultrapure water, stir thoroughly and dissolve thoroughly, add 0.1 mL of Tween 80, vortex and mix, continue to join The ultra-pure water is made up to a final volume of 100 mL, and vortexed and mixed.
- Preparation method of 28% HP-P-CD Weigh ⁇ - ⁇ -CD 28 g, add 80 mL of sterile water for injection, dissolve in ultrasound, vortex and mix, continue to add sterile water for injection to a final volume of 100 In mL, vortex and mix to clear the clear solution.
- test solution is administered according to the following method: experiment
- Plasma must be prepared within 30 minutes of blood collection.
- the plasma sample was taken by liquid-liquid extraction method: 20 L of plasma was added, and a solution of 600 BEBE (tert-butyl methyl ether) containing internal standard (BEZ-235) (10 ng/mL) was added, and vortex was 1500 rpm. Min, then centrifuge at 12000 rpm for 5 min, take the supernatant 300 ⁇ , blow dry under nitrogen, reconstitute with 300 acetonitrile: water (7:3, V/V), vortex and mix, LC-MS/ MS analysis.
- 600 BEBE tert-butyl methyl ether
- BEZ-235 internal standard
- plasma samples were taken by protein precipitation: 20 plasma, 200 ⁇ L of internal standard (CH5424802) in methanol (50 ng/mL), vortexed at 1500 rpm for 5 min, then centrifuged at 12,000 rpm for 5 min. Take the supernatant 100 ⁇ , add 100 ⁇ M water, vortex and mix, and analyze by LC-MS/MS.
- internal standard CH5424802
- methanol 50 ng/mL
- plasma samples were taken by protein precipitation: 30 plasma was added, 200 ⁇ L of internal standard (CH5424802) in acetonitrile was added, vortexed at 1500 rpm, and then centrifuged at 4000 rpm for 20 min. After centrifugation, take 100 L of the supernatant, add 100 more water, vortex and mix, LC-MS/MS analysis.
- internal standard CH5424802
- Test sample dose (mg/kg) AUC last (h*ng/mL) CL (L/h/kg) Control drug 1 1543 0.59 Compound 1 1 1431 0.69 Compound 7 2 7715 0.26 Table 2.2 Rat ⁇ evaluation results ( ⁇ )
- Test sample dose (mg/kg) AUC last (h*ng/mL) F (%) Control drug 2 1936 59.6 Compound 1 2 2053 72.2 Compound 7 5 9977 51.8
- AUC last represents the area under the curve of the medicine 0 ⁇ t
- F% represents absolute bioavailability
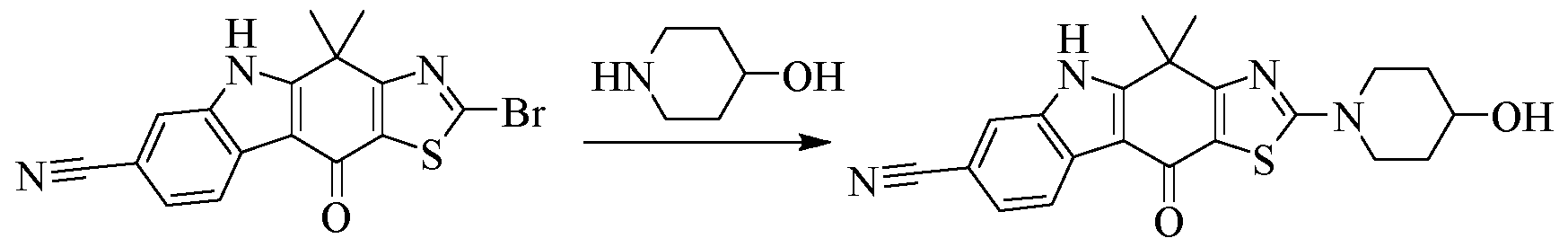
- NBS N-bromosuccinimide
- 6-Amino-3-azabicyclo[3.1.0]hexane-3-carboxylic acid tert-butyl ester 200 mg, 1.01 mmol
- triethylamine 170 mg, 1.68 mmol
- dichloromethane 20
- benzyl chloroformate 175 mg
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Provided are the compounds as shown by formula (I) or the stereoisomers thereof, or the pharmaceutically acceptable salts, esters, or solvates thereof, wherein A1, A2, A3, M, Y, X, R3, R5, R6, R7, Q and n are as defined in the description, the preparation method of these compounds, pharmaceutical preparations and pharmaceutical compositions containing these compounds, and the use of the compounds or the stereoisomers thereof, or the pharmaceutically acceptable salts, esters, or solvates thereof in the preparation of drugs for the treatment and/or prevention of diseases associated with cancers mediated by ALK.
Description
四并环类间变性淋巴瘤激酶抑制剂 Tetracyclic anaplastic lymphoma kinase inhibitor
技术领域 Technical field
本发明属于医药技术领域, 具体涉及四并环类间变性淋巴瘤激酶抑制剂 或其立体异构体、 或其药学上可接受的盐、 酯或溶剂化物, 这些化合物的制 备方法, 含有这些化合物的药物制剂和药物组合物, 以及该化合物或其立体 异构体、或其药学上可接受的盐、 酯或溶剂化物在制备治疗和 /或预防由 ALK 介导的癌症相关疾病的药物中的应用。 背景技术 The present invention relates to the field of medical technology, and particularly relates to a tetracyclic anaplastic lymphoma kinase inhibitor or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, and a method for preparing the same, which comprises the compound Pharmaceutical preparations and pharmaceutical compositions, and the compounds or stereoisomers thereof, or pharmaceutically acceptable salts, esters or solvates thereof, in the manufacture of a medicament for the treatment and/or prevention of ALK-mediated cancer-related diseases application. Background technique
间变性淋巴瘤激酶 (Anaplastic lymphoma kinase, ALK)是受体酸氨酸激酶 家族成员, 可通过自身磷酸化募集下游蛋白, 进而表达特定的基因, 调节细 胞代谢和生长。 间变性淋巴瘤激酶最早发现于间变性大细胞淋巴瘤 ( Anaplastic large cell lymphoma , ALCL ) 中, 后来发现在非小细胞肺癌 ( NSCLC ) 中亦有高表达。 Anaplastic lymphoma kinase (ALK), a member of the receptor acid kinase family, recruits downstream proteins through autophosphorylation to express specific genes and regulate cell metabolism and growth. The anaplastic lymphoma kinase was first discovered in Anaplastic large cell lymphoma (ALCL) and was later found to be highly expressed in non-small cell lung cancer (NSCLC).
ALK的小分子抑制剂可以影响肿瘤细胞的生长, 起到抗肿瘤的作用, 但 已有大量临床证明一代 ALK抑制剂克唑替尼(Crizotinib, 辉瑞公司研发), 容易产生耐药性, 因此, 设计并筛选对 Crizotinib产生耐药的患者也有良好的 疗效的二代 ALK抑制剂, 具有显著的临床意义。 ALK's small molecule inhibitors can affect the growth of tumor cells and play an anti-tumor role. However, a large number of clinically proven ALK inhibitors, crizotinib (developed by Pfizer Inc.), are prone to drug resistance. The design and screening of second-generation ALK inhibitors with good efficacy in patients with resistance to Crizotinib has significant clinical significance.
目前已知的 ALK抑制剂还包括 CH5424802( Roche )、LDK378( Novartis )、 和 Currently known ALK inhibitors include CH5424802 (Roche), LDK378 (Norartis), and
因此, 通过化合物结构修饰寻找新的化合物结构, 努力改善化合物的理
化性质, 提高成药性, 如提高化合物的暴露量或生物利用度, 来寻找对 ALK 突变有较高活性的小分子抑制剂,对于临床上因 ALK突变引起的疾病的治疗, 具有重要的意义。 发明内容 Therefore, the structure of the compound is modified to find a new compound structure, and efforts are made to improve the rationality of the compound. The nature of the drug, such as increasing the exposure or bioavailability of the compound, to find small molecule inhibitors with high activity on ALK mutations, is of great significance for the clinical treatment of diseases caused by ALK mutations. Summary of the invention
本发明以开发针对 ALK 的小分子抑制剂为目标, 发明了对治疗和 /或预 防 ALK 介导的癌症相关疾病具有良好效果的四并环类间变性淋巴瘤激酶抑 制剂。 具体的技术方案为如下: The present invention aims to develop a small molecule inhibitor against ALK, and has invented a tetracyclic anaplastic lymphoma kinase inhibitor which has a good effect on the treatment and/or prevention of ALK-mediated cancer-related diseases. The specific technical solutions are as follows:
1、 通式 ( I ) 所示的化合物或其立体异构体、 或其药学上可接受的盐、 酯或 A compound represented by the formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt or ester thereof, or
其中, among them,
选自 C- R1或 N; Selected from C-R 1 or N;
A2选自 1 2或 A 2 is selected from 1 2 or
A3选自 C- R4或 N, 且 Ai、 A2和 A3不同时为 N; A 3 is selected from C-R 4 or N, and Ai, A 2 and A 3 are not N at the same time;
R1, R2和 R4分别独立地选自氢、 羟基、 羧基、 硝基、 卤素原子、 氨基、 (C1-6烷基 )2氨基、 氰基、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14 元环烷基; R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxy, carboxy, nitro, halogen atom, amino, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1 -6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl;
R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 素原子、 CL6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14元环烷基, 所述的 C1-6烷基、 C1-6烷 氧基、 C2-6烯基、 C2-6炔基和 3〜14元环烷基可独立地任选被一至多个下列取 代基取代: 羟基、 羧基、 氨基、 氰基、 卤素原子、 硝基或 3〜14元杂环基;R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or a 14-membered cycloalkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2-6 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently Optionally substituted by one or more of the following substituents: hydroxy, carboxy, amino, cyano, halo, nitro or 3 to 14 membered heterocyclyl;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基, 所述的 C1-6烷基、 C2-6烯基和 C2-6炔基可独立地任选被 C1-6烷氧基 取代; M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
R5和 R6分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 羟基 C1-6 R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
¾^¾ 、 C2-6婦 或。2-6快 3⁄4^3⁄4, C2-6 women or. 2-6 fast
或 R5和 R6相互连接, 与它们所连接的碳原子一起形成 3〜14元杂环基或 3-14元环烷基; Or R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9;
X选自 0、 S或 N-R9; Y is selected from N or CR 9 ; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团: Q is selected from the following groups:
( 1 ) 3〜8元杂环基, (1) 3 to 8 membered heterocyclic group,
( 2 )任选被一至三个相同或不同的 R1Q取代的 3〜14元环烷基或 6〜14 元并杂环基, 和 (2) a 3 to 14 membered cycloalkyl group or a 6 to 14 membered heterocyclic group optionally substituted by one to three identical or different R 1Q groups, and
( 3 )任选被一至三个相同或不同的 R1Q取代的 6〜12元桥环基或 6〜12 元螺环基, (3) a 6 to 12 membered bridged ring group or a 6 to 12 membered spirocyclic group optionally substituted with one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 CL6烷基氨基羰基、 羟基 CL6烷基、 羟基 CL6烷基氨基、 代 CL6烷基、 d.6 烷基磺酰基、 C 烷基磺酰基氨基、氨基磺酰基、氨基磺酰基氨基、 烯基、R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
。2-6块基; . 2-6 blocks;
R7选自任选被取代基取代的 6〜12元桥环基、 6-12元螺环基、 3〜8元杂 环基或 6〜14元并杂环基, 所述取代基选自氨基、 羟基、 硝基、 素原子、 羧 基、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基、 3〜8元杂环基或 3〜8元环烷 基; R 7 is selected from a 6 to 12 membered bridged ring group, a 6 to 12 membered spirocyclic group, a 3 to 8 membered heterocyclic group or a 6 to 14 membered heterocyclic group which is optionally substituted with a substituent selected from the group consisting of Amino, hydroxy, nitro, alkane, carboxyl, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, 3 to 8 membered heterocyclic or 3 ~8-membered cycloalkyl;
n选自 0、 1、 2、 3、 4、 5或 6, n is selected from 0, 1, 2, 3, 4, 5 or 6,
条件是: requirement is:
当 n为 0时, R7不存在, When n is 0, R 7 does not exist.
当 1≥2时, R7可以相同或不同, 且 When 1 ≥ 2, R 7 may be the same or different, and
当 Q选自 3〜8元杂环基时, n不能为 0, 且 R7不能选自 3〜8元杂环基。 When Q is selected from a 3 to 8 membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 3 to 8 membered heterocyclic group.
2、 通式( I )所示的化合物或其立体异构体、 或其药学上可接受的盐、 酯或溶剂化物: 2. A compound of the formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
选自 C- R1或 N; Selected from C-R 1 or N;
A2选自 1 2或 A 2 is selected from 1 2 or
A3选自 C- R4或 N, 且 Ai、 A2和 A3不同时为 N; A 3 is selected from C-R 4 or N, and Ai, A 2 and A 3 are not N at the same time;
R1, R2和 R4分别独立地选自氢、 羟基、 羧基、 硝基、 卤素原子、 氨基、
(C1-6烷基 )2氨基、 氰基、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14 元环烷基; R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxyl, carboxyl, nitro, halogen atom, amino group, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl ;
R3选自氢、 氰基、 羟基、 氨基、 卤素原子、 C1-6烷基、 C1-6烷氧基、 C2-6 烯基、 C2-6炔基或 3〜14元环烷基, 所述的 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2 炔基和 3〜14元环烷基可独立地任选被一至多个下列取代基取代: 羟基、 羧基、 氨基、 氰基、 卤素原子、 硝基或 3〜14元杂环基; R 3 is selected from the group consisting of hydrogen, cyano, hydroxy, amino, halogen atom, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered ring The alkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently optionally one or more of the following Substituent substitution: a hydroxyl group, a carboxyl group, an amino group, a cyano group, a halogen atom, a nitro group or a 3 to 14 membered heterocyclic group;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基, 所述的 C1-6烷基、 C2-6烯基和 C2-6炔基可独立地任选被 C1-6烷氧基 取代; M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
R5和 R6分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 羟基 C1-6 R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
¾^¾ 、 C2-6婦 或。2-6快 3⁄4^3⁄4, C2-6 women or. 2-6 fast
或 R5和 R6相互连接, 与它们所连接的碳原子一起形成 3〜14元杂环基或 3-14元环烷基; Or R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 0、 S或 N-R9; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团: Q is selected from the following groups:
( 1 ) 3〜8元杂环基, (1) 3 to 8 membered heterocyclic group,
( 2 )任选被一至三个相同或不同的 R1Q取代的 6〜14元并杂环基,(2) a 6 to 14-membered heterocyclic group optionally substituted by one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 CL6烷基氨基羰基、 羟基 CL6烷基、 羟基 CL6烷基氨基、 代 CL6烷基、 d.6 烷基磺酰基、 C 烷基磺酰基氨基、氨基磺酰基、氨基磺酰基氨基、 烯基、R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
。2-6快基; . 2-6 fast base;
R7选自任选被取代基取代的 6〜12元桥环基或 6〜12元螺环基, 所述取代 基选自氨基、 羟基、 硝基、 卤素原子、 羧基、 C1-6烷基、 C1-6烷氧基、 C2-6烯 基、 C2-6炔基、 3〜8元杂环基或 3〜8元环烷基; R 7 is selected from a 6 to 12 membered bridged ring group or a 6 to 12 membered spirocyclic group optionally substituted by a substituent selected from the group consisting of an amino group, a hydroxyl group, a nitro group, a halogen atom, a carboxyl group, and a C 1-6 alkane group. a group, a C 1-6 alkoxy group, a C 2-6 alkenyl group, a C 2-6 alkynyl group, a 3 to 8 membered heterocyclic group or a 3 to 8 membered cycloalkyl group;
n为 1。 n is 1.
3、 通式( I )所示的化合物或其立体异构体、 或其药学上可接受的盐、 酯或溶剂化物:
选自 C- R1或 N; 3. A compound of the formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof: Selected from C-R 1 or N;
A2选自 1 2或 A 2 is selected from 1 2 or
A3选自 C- R4或 N, 且 Ai、 A2和 A3不同时为 N; A 3 is selected from C-R 4 or N, and Ai, A 2 and A 3 are not N at the same time;
R1, R2和 R4分别独立地选自氢、 羟基、 羧基、 硝基、 卤素原子、 氨基、 (C1-6烷基 )2氨基、 氰基、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14 元环烷基; R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxy, carboxy, nitro, halogen atom, amino, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1 -6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl;
R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 素原子、 CL6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14元环烷基, 所述的 C1-6烷基、 C1-6烷 氧基、 C2-6烯基、 C2-6炔基和 3〜14元环烷基可独立地任选被一至多个下列取 代基取代: 羟基、 羧基、 氨基、 氰基、 卤素原子、 硝基或 3〜14元杂环基;R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or a 14-membered cycloalkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2-6 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently Optionally substituted by one or more of the following substituents: hydroxy, carboxy, amino, cyano, halo, nitro or 3 to 14 membered heterocyclyl;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基, 所述的 C1-6烷基、 C2-6烯基和 C2-6炔基可独立地任选被 C1-6烷氧基 取代; M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
R5和 R6分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 羟基 C1-6 R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
¾^¾ 、 C2-6婦 或。2-6快 3⁄4^3⁄4, C2-6 women or. 2-6 fast
或 R5和 R6相互连接, 与它们所连接的碳原子一起形成 3〜14元杂环基或 3-14元环烷基; Or R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 0、 S或 N-R9; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团: Q is selected from the following groups:
( 1 )任选被一至三个相同或不同的 R1Q取代的 6〜12元桥环基或 6〜12 元螺环基, (1) a 6 to 12 membered bridged ring group or a 6 to 12 membered spirocyclic group optionally substituted with one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 CL6烷基氨基羰基、 羟基 CL6烷基、 羟基 CL6烷基氨基、 代 CL6烷基、 d.6 烷基磺酰基、 C 烷基磺酰基氨基、氨基磺酰基、氨基磺酰基氨基、 烯基、R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
。2-6快基; . 2-6 fast base;
R7选自任选被取代基取代的 6〜12元桥环基、 6〜12元螺环基或 3〜8元杂
环基, 所述取代基选自氨基、 羟基、 硝基、 卤素原子、 羧基、 ^6烷基、 烷氧基、 C2-6烯基、 C2-6炔基、 3〜8元杂环基或 3〜8元环烷基; R 7 is selected from a 6 to 12 membered bridged ring group optionally substituted with a substituent, a 6 to 12 membered spirocyclic group or a 3 to 8 membered hetero a cyclic group, the substituent is selected from the group consisting of an amino group, a hydroxyl group, a nitro group, a halogen atom, a carboxyl group, a ^ 6 alkyl group, an alkoxy group, a C 2-6 alkenyl group, a C 2-6 alkynyl group, and a 3 to 8 membered heterocyclic ring. Base or 3 to 8 membered cycloalkyl;
n选自 0、 1、 2、 3、 4、 5或 6, n is selected from 0, 1, 2, 3, 4, 5 or 6,
条件是: requirement is:
当 n为 0时, R7不存在, When n is 0, R 7 does not exist.
当 1≥2时, R7可以相同或不同。 When 1 ≥ 2, R 7 may be the same or different.
4、 如前述技术方案中任一项所述的化合物或其立体异构体、 或其药学上 可接受的盐、 酯或溶剂化物, 4. A compound according to any one of the preceding claims, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中通式( I )是通式( II ): Wherein formula (I) is of formula (II):
其中, among them,
R1, R2和 R4分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 C2-6 烯基、 C2-6炔基或 3〜8元环烷基; R 1 , R 2 and R 4 are each independently selected from hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, a C 2-6 alkenyl group, a C 2-6 alkynyl group or a 3 to 8 Metacycloalkyl;
R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 素原子、 CL6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜8元环烷基, 所述的 C1-6烷基、 C1-6烷 氧基、 C2-6烯基、 C2-6炔基和 3〜8元环烷基可独立地任选被一至三个下列取代 基取代: 羟基、 羧基, 氨基、 氰基、 卤素原子、 硝基或 3〜8元杂环基; R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or ~8-membered cycloalkyl, the C 1-6 alkyl group, C 1-6 alkoxy group, C 2-6 alkenyl group, C 2-6 alkynyl group and 3 to 8 membered cycloalkyl group may be independently Substituted by one to three substituents: a hydroxyl group, a carboxyl group, an amino group, a cyano group, a halogen atom, a nitro group or a 3 to 8 membered heterocyclic group;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基, 所述的 C1-6烷基、 C2-6烯基和 C2-6炔基可独立地任选被 C1-6烷氧基 取代; M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
R5和 R6分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 羟基 C1-6 R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
¾^¾ 、 C2-6婦 或。2-6快 3⁄4^3⁄4, C2-6 women or. 2-6 fast
或 R5和 R6相互连接, 与它们所连接的碳原子一起形成 5〜10元杂环基或 3〜8元环烷基; Or R 5 and R 6 are bonded to each other to form a 5- to 10-membered heterocyclic group or a 3- to 8-membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 0、 S或 N-R9; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团:
( 1 ) 4〜7元杂环基, Q is selected from the following groups: (1) 4 to 7 membered heterocyclic group,
( 2 )任选被一至三个相同或不同的 R1Q取代的 3〜8元环烷基或 6〜12 元并杂环基, 和 (2) a 3 to 8 membered cycloalkyl group or a 6 to 12 membered heterocyclic group optionally substituted by one to three identical or different R 1Q groups, and
( 3 )任选被一至三个相同或不同 R1Q取代的 7〜10元桥环基或 6〜12元 螺环基, (3) a 7 to 10 membered bridged ring group or a 6 to 12 membered spirocyclic group optionally substituted by one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 CL6烷基氨基羰基、 羟基 CL6烷基、 羟基 CL6烷基氨基、 代 CL4烷基、 CM 烷基磺酰基、 C 烷基磺酰基氨基、氨基磺酰基、氨基磺酰基氨基、 烯基、R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 4 alkyl, C M alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
。2-6块基; . 2-6 blocks;
R7选自任选被取代基取代的 6〜10元桥环基、 6-12元螺环基、 4〜7元杂 环基或 6〜12元并杂环基,所述取代基选自氨基、羟基、 卤素原子、 ^6烷基、R 7 is selected from a 6 to 10 membered bridged ring group, a 6 to 12 membered spirocyclic group, a 4 to 7 membered heterocyclic group or a 6 to 12 membered heterocyclic group which is optionally substituted with a substituent selected from the group consisting of Amino group, hydroxyl group, halogen atom, ^ 6 alkyl group,
C 6 ¾* 基、 。2-6婦基或。2-6快基; C 6 3⁄4* base, . 2-6 women's base or. 2-6 fast base;
n选自 0、 1、 2或 3 , n is selected from 0, 1, 2 or 3
条件是: requirement is:
当 n为 0时, R7不存在, When n is 0, R 7 does not exist.
当 1≥2时, R7可以相同或不同, 且 When 1 ≥ 2, R 7 may be the same or different, and
当 Q选自 4〜7元杂环基时, n不能为 0, 且 R7不能选自 4〜7元杂环基。 When Q is selected from a 4 to 7 membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 4 to 7 membered heterocyclic group.
5、 如前述技术方案中任一项所述的化合物或其立体异构体、 或其药学上 可接受的盐、 酯或溶剂化物, 5. A compound according to any one of the preceding claims, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, among them,
Αι , A2和 A3分别独立地选自 CH; ι ι , A 2 and A 3 are each independently selected from CH;
R3选自氢或氰基; R 3 is selected from hydrogen or cyano;
M选自丽; M is selected from Li;
R5和 R6分别独立地选自氢或 C1-6烷基; R 5 and R 6 are each independently selected from hydrogen or C 1-6 alkyl;
Y选自 N; Y is selected from N;
X选自 S。 X is selected from S.
6、 如前述技术方案中任一项所述的化合物或其立体异构体、 或其药学上 可接受的盐、 酯或溶剂化物, 6. A compound according to any one of the preceding claims, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, among them,
R1, R2和 R4分别独立地选自氢、 卤素原子、 C1-6烷基或 3〜8元环烷基; R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 素原子、 CL6烷基或
3〜8元环烷基; R 1 , R 2 and R 4 are each independently selected from hydrogen, a halogen atom, a C 1-6 alkyl group or a 3 to 8 membered cycloalkyl group; and R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl Acyl, alkene, CL 6 alkyl or 3 to 8 membered cycloalkyl;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基或 C1-6烷氧基, 所述的 C1-6 烷基可任选被 ^6烷氧基取代; M is selected from 0, S or NR 8 , R 8 is selected from hydrogen, C 1-6 alkyl or C 1-6 alkoxy, and the C 1-6 alkyl may be optionally substituted by ^ 6 alkoxy ;
R5和 R6分别独立地选自氢、 卤素原子、 ^6烷基、 ^6烷氧基或羟基 C1-6 烷基, R 5 and R 6 are each independently selected from hydrogen, a halogen atom, a ^ 6 alkyl group, a ^ 6 alkoxy group or a hydroxy C 1-6 alkyl group.
或 R5和 R6相互连接,与它们连接的碳原子一起形成 5〜6元杂环基或 3〜8 元环烷基; Or R 5 and R 6 are bonded to each other to form a 5- to 6-membered heterocyclic group or a 3- to 8-membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 0、 S或 N-R9; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团: Q is selected from the following groups:
( 1 ) 5〜6元杂环基, (1) 5 to 6 membered heterocyclic group,
( 2 )任选被一至三个相同或不同的 R1Q取代的 3〜8元环烷基或 6〜10 元并杂环基, 和 (2) a 3 to 8 membered cycloalkyl group or a 6 to 10 membered heterocyclic group optionally substituted by one to three identical or different R 1Q groups, and
( 3 )任选被一至三个相同或不同的 R1Q取代的 7〜9元桥环基或 7〜11 元螺环基, (3) a 7- to 9-membered bridged ring group or a 7 to 11-membered spiro ring group optionally substituted with one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 C1-6烷基氨基羰基、 羟基 C1-6烷基、 羟基 C1-6烷基氨基、 代 CM烷基、 甲基 磺酰基、 甲基磺酰基氨基、 氨基磺酰基、 氨基磺酰基氨基、 C2-6烯基、 C2-6炔 基; R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, C 1-6 alkylaminocarbonyl, hydroxy C 1-6 alkyl, hydroxy C 1-6 alkylamino, C M alkyl, methylsulfonyl, methylsulfonylamino, aminosulfonyl, aminosulfonylamino, C 2-6 alkenyl, C 2-6 alkynyl;
R7选自任选被取代基取代的 7〜10元桥环基、 7〜11元螺环基、 5〜6元杂环 基或 6〜10元并杂环基, 所述取代基选自氨基、 羟基、 卤素原子、 ^6烷基、R 7 is selected from a 7 to 10 membered bridged ring group optionally substituted with a substituent, a 7 to 11 membered spirocyclic group, a 5 to 6 membered heterocyclic group or a 6 to 10 membered heterocyclic group, and the substituent is selected from the group consisting of Amino group, hydroxyl group, halogen atom, ^ 6 alkyl group,
C 6 ¾* 基、 。2-6婦基或。2-6快基, C 6 3⁄4* base, . 2-6 women's base or. 2-6 fast base,
n选自 0、 1、 2或 3 , n is selected from 0, 1, 2 or 3
条件是: requirement is:
当 n为 0时, R7不存在, When n is 0, R 7 does not exist.
当 1≥2时, R7可以相同或不同, 且 When 1 ≥ 2, R 7 may be the same or different, and
当 Q选自 5〜6元杂环基时, n不能为 0, 且 R7不能选自 5〜6元杂环基。 When Q is selected from a 5- to 6-membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 5- to 6-membered heterocyclic group.
7、 如前述技术方案中任一项所述的化合物或其立体异构体、 或其药学上 可接受的盐、 酯或溶剂化物, 7. The compound of any one of the preceding claims, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, among them,
R1, R2和 R4分别独立地选自氢, 甲基或乙基;
R3选自氢、 氰基、 羟基、 氨基、 氟原子、 氯原子、 甲基或乙基; R 1 , R 2 and R 4 are each independently selected from hydrogen, methyl or ethyl; R 3 is selected from the group consisting of hydrogen, cyano, hydroxy, amino, fluorine, chlorine, methyl or ethyl;
M选自 N-R8, R8选自氢或 CL4烷基; M is selected from NR 8 and R 8 is selected from hydrogen or CL 4 alkyl;
R5和 R6分别独立地选自 C1-4烷基; R 5 and R 6 are each independently selected from C 1-4 alkyl;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 S或 N-R9; X is selected from S or NR 9 ;
R9选自氢、 甲基、 乙基或正丙基; R 9 is selected from the group consisting of hydrogen, methyl, ethyl or n-propyl;
Q选自 Q is selected from
(1) 5-6元杂环基, (1) 5-6 membered heterocyclic group,
(2)任选被一至两个相同或不同的 R1Q取代的 6〜10元并杂环基, 和(2) a 6 to 10 membered heterocyclic group optionally substituted by one to two identical or different R 1Q groups, and
(3)任选被一至两个相同或不同的 R1Q取代的 7〜9元桥环基或 7〜11元螺 环基, (3) a 7- to 9-membered bridged ring group or a 7 to 11-membered spiro ring group optionally substituted with one to two identical or different R 1Q groups,
R1Q选自氨基或 CL4烷基; R 1Q is selected from amino or CL 4 alkyl;
R7选自 7〜9元桥环基、 7〜11元螺环基或 5〜6元杂环基, R 7 is selected from the group consisting of 7 to 9 membered bridged ring groups, 7 to 11 membered spirocyclic groups or 5 to 6 membered heterocyclic groups.
n选自 0或 1 , n is selected from 0 or 1
条件是: requirement is:
当 n为 0时, R7不存在, 且 When n is 0, R 7 does not exist, and
当 Q选自 5〜6元杂环基时, n不能为 0, 且 R7不能选自 5〜6元杂环基。 When Q is selected from a 5- to 6-membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 5- to 6-membered heterocyclic group.
8、 如前述技术方案 1-7中任一项所述的化合物或其立体异构体、 或其药 学上可接受的盐、 酯或溶剂化物, 8. The compound of any one of the preceding claims 1-7, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, among them,
Q选自 4〜7元杂环基, 优选地含有 1或 2个氮原子作为环原子, 更优选 地是饱和的; Q is selected from a 4 to 7 membered heterocyclic group, preferably having 1 or 2 nitrogen atoms as a ring atom, more preferably saturated;
R7选自 7〜8元桥环基, 优选地含有 1或 2个选自氧和氮的环原子, 更优 选地是饱和的; R 7 is selected from 7 to 8 membered bridged ring groups, preferably containing 1 or 2 ring atoms selected from oxygen and nitrogen, more preferably saturated;
η选自 1。 η is selected from 1.
9、 如前述技术方案 8所述的化合物或其立体异构体、 或其药学上可接受 的盐、 酯或溶剂化物, 9. The compound of the above aspect 8, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
n选自 1 n selected from 1
10、 如前述技术方案 1-7 中任一项所述的化合物或其立体异构体、 或其 药学上可接受的盐、 酯或溶剂化物, 10. The compound of any one of the preceding claims 1-7, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, among them,
Q选自任选被一至两个相同或不同的 R1Q取代的 7〜8元桥环基,优选地含 有 1或 2个氮原子作为环原子, 更优选地是饱和的, Q is selected from optionally substituted with one to two identical or different substituents R 1Q of 7~8 membered bridged ring group, preferably containing 1 or 2 nitrogen atoms as ring atoms, more preferably a saturated,
R1Q选自氨基或 CL6烷基; R 1Q is selected from amino or CL 6 alkyl;
R7选自 4〜7元杂环基, 优选地含有 1或 2个选自氧和氮的环原子, 更优 选地是饱和的; R 7 is selected from a 4 to 7 membered heterocyclic group, preferably contains 1 or 2 ring atoms selected from oxygen and nitrogen, more preferably saturated;
n选自 0或 1 , 当 n为 0时, R7不存在。 n is selected from 0 or 1, and when n is 0, R 7 is absent.
11、 如前述技术方案 10所述的化合物或其立体异构体、 或其药学上可接 受的盐、 酯或溶剂化物, The compound according to the above aspect 10 or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, among them,
n选自 0或 1 , 当 n为 0时, R7不存在。 n is selected from 0 or 1, and when n is 0, R 7 is absent.
12、 如前述技术方案 1-11中任一项所述的化合物或其立体异构体、 或其 药学上可接受的盐、 酯或溶剂化物, 12. The compound of any one of the preceding claims 1-11, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, Q和 R7中至少一个是桥环基, 所述桥环基含有至少一个氮原子作 为环成员, 所述桥通过与该氮原子相邻的两个环原子 (如碳原子)作为桥头 原子形成,例如 Q选自 "任选被 CL4烷基取代的 -^ - 一, Wherein at least one of Q and R 7 is a bridged ring group, the bridged ring group containing at least one nitrogen atom as a ring member, and the bridge is bridged by two ring atoms (such as carbon atoms) adjacent to the nitrogen atom. forming atoms, Q is selected from, for example, "optionally substituted alkyl CL 4 - ^ - one,
5 Vy_/ i"和 5 Vy_/ i" and
s ^ \TJ
R7不存在或者选自s ^ \TJ R 7 does not exist or is selected from
13、 如前述技术方案 1-7 中任一项所述的化合物或其立体异构体、 或其 药学上可接受的盐、 酯或溶剂化物, The compound of any one of the preceding claims 1-7, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, among them,
Q选自任选被一至两个相同或不同的 R1Q取代的 7〜11元螺环基, 所述螺 环基优选是含有 1-3个杂原子的螺环基, 更优选地所述螺环基含有 7〜11个环 原子,其中 1或 2个环原子是氮原子,其余的环原子是碳原子,还更优选地, 所述螺环基是饱和基团, Q is selected from 7 to 11 membered spirocyclic groups optionally substituted by one to two identical or different R 1Q groups, preferably a spirocyclic group having 1 to 3 hetero atoms, more preferably the spiro group The cyclic group contains 7 to 11 ring atoms, wherein 1 or 2 ring atoms are nitrogen atoms, the remaining ring atoms are carbon atoms, and still more preferably, the spiro group is a saturated group,
R1Q选自氨基或 CL4烷基; R 1Q is selected from amino or CL 4 alkyl;
R7不存在。 R 7 does not exist.
14、 如前述技术方案 1-7 中任一项所述的化合物或其立体异构体、 或其 药学上可接受的盐、 酯或溶剂化物, 14. The compound of any one of the preceding claims 1-7, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
其中, among them,
Q选自任选被一至两个相同或不同的 R1Q取代的 6〜10元并杂环基, 所述 并杂环基优选是含有 1-3 个杂原子的并杂环基, 更优选地所述并杂环基含有 6〜10个环原子, 其中 1-3个环原子是选自氮和氧的杂原子, 其余的环原子是 碳原子, 还更优选地, 所述并杂环基是饱和基团, Q is selected from 6 to 10 membered heterocyclic groups optionally substituted by one to two identical or different R 1Q , and the heterocyclic group is preferably a heterocyclic group having 1 to 3 hetero atoms, more preferably The heterocyclic group contains 6 to 10 ring atoms, wherein 1 to 3 ring atoms are hetero atoms selected from nitrogen and oxygen, and the remaining ring atoms are carbon atoms, still more preferably, the heterocyclic group Is a saturated group,
R1Q选自氨基或 CL4烷基; R 1Q is selected from amino or CL 4 alkyl;
R7不存在。 R 7 does not exist.
15、 化合物或其立体异构体、 或其药学上可接受的盐、 酯或溶剂化物, 所述
15. A compound or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof,
16、 化合物或其立体异构体、 或其药学上可接受的盐、 酯或溶剂化物, 所述化合物选自:
16. A compound or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, selected from the group consisting of:
18、 一种药物组合物, 其包括前述技术方案 1-17中任一项所述的化合物 或其立体异构体、 或其药学上可接受的盐、 酯或溶剂化物与一种或多种药用 载体和 /或稀释剂。 18. A pharmaceutical composition comprising the compound of any one of the preceding claims 1-17, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, and one or more A pharmaceutically acceptable carrier and/or diluent.
19、 如技术方案 18所述的药物组合物, 其特征在于还可含有一种或多种 抗肿瘤剂和免疫抑制剂, 所述的抗肿瘤剂和免疫抑制剂选自甲氨蝶呤、 卡培
他滨、 吉西他滨、 去氧氟尿苷、 培美曲塞二钠、 帕唑帕尼、 伊马替尼、 埃罗 替尼、 拉帕替尼、 吉非替尼、 凡德他尼、 赫赛汀、 贝伐单抗、 利妥昔单抗、 曲妥珠单抗、 紫杉醇、 长春瑞滨、 多西他赛、 多柔比星、 羟基喜树碱、 丝裂 霉素、 表柔比星、 吡柔比星、 博来霉素、 来曲唑、 他莫西芬、 氟维司群、 曲 谱瑞林、 氟他胺、 亮丙瑞林、 阿那曲唑、 异环磷酰胺、 白消安、 环磷酰胺、 卡莫司汀、 尼莫司汀、 司莫司汀、 氮芥、 马法兰、 瘤可宁、 卡铂、 顺铂、 奥 沙利铂、 络铂、 拓朴特肯、 喜树碱、 拓朴替康、 依维莫司、 西罗莫斯、 特癌 适、 6-巯基嘌呤、 6-石克鸟嘌呤、 石克唑嘌呤、 菌素 D、 柔红霉素、 阿霉素、 米托 蒽醌、 争光霉素、 普卡霉素或氨鲁米特。 19. The pharmaceutical composition according to claim 18, characterized in that it further comprises one or more antitumor agents and immunosuppressive agents, said antitumor agent and immunosuppressive agent being selected from the group consisting of methotrexate and card Training Hebin, gemcitabine, deoxyfluorouridine, pemetrexed disodium, pazopanib, imatinib, erlotinib, lapatinib, gefitinib, vandetanib, hersay Tet, bevacizumab, rituximab, trastuzumab, paclitaxel, vinorelbine, docetaxel, doxorubicin, hydroxycamptothecin, mitomycin, epirubicin, Pirarubicin, bleomycin, letrozole, tamoxifen, fulvestrant, triclinin, flutamide, leuprolide, anastrozole, ifosfamide, busulfan, Cyclophosphamide, carmustine, nimustine, semustine, nitrogen mustard, melphalan, cyclamate, carboplatin, cisplatin, oxaliplatin, platinic, topotecan, camptothecin , topotecan, everolimus, sirolimus, dexamethasone, 6-mercaptopurine, 6-gram guanine, gram azole, bacteriocin D, daunorubicin, doxorubicin, Mitoxantrone, bleomycin, pucamycin or aminoglutethimide.
20、 如技术方案 1-17中任一项所述的化合物或其立体异构体、 或其药学 上可接受的盐、 酯或溶剂化物在制备用于治疗和 /或预防 ALK介导的癌症相 关疾病的药物中的应用, 所述癌症相关的疾病选自脑瘤、 非小细胞性肺癌、 鳞状上皮细胞癌、 膀胱癌、 胃癌、 卵巢癌、 腹膜癌、 胰腺癌、 乳腺癌、 头颈 癌、 子宫颈癌、 子宫内膜癌、 直肠癌、 肝癌、 肝母细胞瘤、 乳头状肾细胞瘤、 头颈部鳞状细胞瘤、 肾母细胞瘤、 肾癌、 食管腺癌、 食管鳞状细胞癌、 神经 胶质瘤、 前列腺癌、 甲状腺癌、 雌性生殖道癌、 原位癌、 淋巴瘤、 成神经细 胞瘤、 神经纤维瘤病、 骨癌、 皮肤癌、 结肠癌、 睾丸癌、 小细胞肺癌、 胃肠 道间质瘤、 前列腺肿瘤、 肥大细胞肿瘤、 多发性骨髓瘤或黑色素瘤。 一种制备下述的通式 (Γ ) 的化合物的方法, The compound according to any one of claims 1 to 17, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, for use in the treatment and/or prevention of ALK-mediated cancer The use of a drug related to a disease selected from the group consisting of a brain tumor, a non-small cell lung cancer, a squamous cell carcinoma, a bladder cancer, a stomach cancer, an ovarian cancer, a peritoneal cancer, a pancreatic cancer, a breast cancer, a head and neck cancer , cervical cancer, endometrial cancer, rectal cancer, liver cancer, hepatoblastoma, papillary renal cell tumor, head and neck squamous cell tumor, nephroblastoma, renal cancer, esophageal adenocarcinoma, esophageal squamous cell Cancer, glioma, prostate cancer, thyroid cancer, female genital tract cancer, carcinoma in situ, lymphoma, neuroblastoma, neurofibromatosis, bone cancer, skin cancer, colon cancer, testicular cancer, small cell lung cancer , gastrointestinal stromal tumors, prostate tumors, mast cell tumors, multiple myeloma or melanoma. A method of preparing a compound of the following formula (Γ),
在加热条件下反应 React under heating
或者
H-(R7)n在溶剂中在三氯化铟的存在
or H-(R 7 ) n is present in the solvent in the presence of indium trichloride
下在室温反应, 然后再加入还原剂反应, Reacting at room temperature, then adding a reducing agent,
其中, A2、 A3、 M、 Y、 X、 R3、 R5、 R6、 R7、 Q和 n如技术方案 1-17 中所定义, Q'为如技术方案 1-17中所定义的 Q或者被保护基团 (PG )保护 的如技术方案 1-17中所定义的 Q, R7'为如技术方案 1-17中所定义的 R7或者 被保护基团 (PG )保护的如技术方案 1-17中所定义的 R7。 发明详述 Wherein A 2 , A 3 , M, Y, X, R 3 , R 5 , R 6 , R 7 , Q and n are as defined in the technical scheme 1-17, and Q' is as in the technical scheme 1-17 The defined Q or Q, R 7 ' as defined in Technical Schemes 1-17 protected by a protecting group (PG) is protected by R 7 or protected group (PG ) as defined in Technical Schemes 1-17 R 7 as defined in Technical Schemes 1-17. Detailed description of the invention
I、 定义 I, definition
本发明所述的 " 素原子"是指氟原子、 氯原子、 溴原子、 碘原子等。 The "prime atom" as used in the present invention means a fluorine atom, a chlorine atom, a bromine atom, an iodine atom or the like.
本发明的所述 " ^6烷基"表示直链或支链的含有 1-6个碳原子的烷基, 包 括例如 "C1-4烷基"、 "C1-3烷基"等, 如甲基、 乙基、 丙基、 丁基、 戊基、 己 基等。 本发明所述的 烷基"指上述 "C1-6烷基"实例中碳原子数为 1-4个的 具体实例。 The "^ 6 alkyl group" of the present invention means a linear or branched alkyl group having 1 to 6 carbon atoms, and includes, for example, "C 1-4 alkyl group", "C 1-3 alkyl group" and the like. Such as methyl, ethyl, propyl, butyl, pentyl, hexyl and the like. The alkyl group "the present invention" refers to a specific example in which the number of carbon atoms is 1-4 in the above "C 1-6 alkyl group".
本发明的所述 "C2-6烯基"是指含有至少一个欢键的碳原子数为 2-6的直链 或支链或环状的烯基, 包括例如" C2-4烯基"、 "C2-3烯基"、 "C3-6环烯基 "等, 如乙烯基、 丙烯基、 丁烯基、 戊烯基、 己烯基、 丁二烯基、 戊二烯基、 己二 烯基、 环戊烯基、 环戊二烯基、 环己烯基和环己二烯基等。 欢键可任选地为 顺式和反式。 The "C 2-6 alkenyl group" of the present invention means a linear or branched or cyclic alkenyl group having 2 to 6 carbon atoms containing at least one of the bonds, including, for example, "C 2-4 alkenyl group". ", "C 2-3 alkenyl", "C 3-6 cycloalkenyl", etc., such as ethenyl, propenyl, butenyl, pentenyl, hexenyl, butadienyl, pentadienyl And hexadienyl, cyclopentenyl, cyclopentadienyl, cyclohexenyl and cyclohexadienyl and the like. The joy keys can optionally be cis and trans.
本发明的所述 " ^6炔基"是指含有至少一个三键的碳原子数为 2-6的直链 或支链的炔基, 其中包括例如 "C2-6炔基"、 "C2-4炔基"、 "C2-3炔基"等, 如 乙炔基、 丙炔基、 丁炔基、 戊炔基、 己炔基等。 The "^ 6 alkynyl group" of the present invention means a linear or branched alkynyl group having 2 to 6 carbon atoms containing at least one triple bond, and includes, for example, "C 2-6 alkynyl group", "C" 2-4 alkynyl", "C 2-3 alkynyl" and the like, such as ethynyl, propynyl, butynyl, pentynyl, hexynyl and the like.
本发明的所述 "C1-6烷基硫基"、 "C1-6烷氧基"、 "C1-6烷基氨基羰基"、 "C1-6 烷基磺酰基"、 " 6烷基磺酰基氨基"分别指上述" 6烷基"通过硫基、 氧基、 氨基羰基、磺酰基、磺酰基氨基与其他结构相连接的基团。 术语" CM烷基磺酰 基"、 "C1-4烷基磺酰基氨基"分别是指上述 " 4烷基"通过磺酰基和磺酰基氨基 与其他结构相连接的基团。 The "C 1-6 alkylthio group", "C 1-6 alkoxy group", "C 1-6 alkylaminocarbonyl group", "C 1-6 alkylsulfonyl group", " 6 " of the present invention The "alkylsulfonylamino group" means a group in which the above " 6 alkyl group" is bonded to another structure through a thio group, an oxy group, an aminocarbonyl group, a sulfonyl group or a sulfonylamino group, respectively. The term "C M alkylsulfonyl" and "C 1-4 alkylsulfonylamino" refer to a group in which the above " 4 alkyl group" is bonded to another structure through a sulfonyl group and a sulfonylamino group, respectively.
本发明的所述 "羟基 6烷基"、 "卤代 6烷基"分别指羟基、 卤素原子取 代上述 "d.6烷基"上的一个或多个氢原子, 并通过烷基与其他结构相连接的基
团。术语"羟基。14烷基"、 "卤代< 14烷基"分别指羟基、卤素原子取代上述" CM 烷基"上的一个或多个氢原子, 并通过烷基与其他结构相连接的基团, 其中"卤 素原子"如前文所述。 The "hydroxy 6 alkyl group" and "halogenated 6 alkyl group" of the present invention respectively mean that a hydroxyl group or a halogen atom replaces one or more hydrogen atoms on the above "d. 6 alkyl group", and passes through an alkyl group and other structures. Connected base Mission. The terms "hydroxyl. 14 alkyl" and "halo < 14 alkyl" mean, respectively, a hydroxy group, a halogen atom substituted for one or more hydrogen atoms on the above "C M alkyl group", and which are bonded to other structures through an alkyl group. a group, wherein "halogen atom" is as described above.
本发明的所述 "羟基 c1-6烷氧基"、 "氨基 c1-6烷氧基"、 "羰基 c1-6烷氧基" 分别指羟基、 氨基、 羰基取代上述 " ^6烷氧基 "上的一个或多个氢原子, 并通 过烷氧基与其他结构相连接的基团。 The "hydroxy c 1-6 alkoxy group", "amino c 1-6 alkoxy group", "carbonyl c 1-6 alkoxy group" of the present invention respectively mean a hydroxyl group, an amino group or a carbonyl group to replace the above "^ 6 alkane" One or more hydrogen atoms on the oxy group, and a group attached to the other structure through an alkoxy group.
本发明所述的 "羟基 C1-6烷基氨基"是指氨基中任意一个能被取代的原子 被上述 "羟基 CL6烷基"所取代, 并通过氨基与其他结构相连接的基团。 The "hydroxy C 1-6 alkylamino group" as used in the present invention means a group in which an atom which can be substituted by any one of the amino groups is substituted by the above-mentioned "hydroxyl 6 alkyl group" and which is bonded to another structure through an amino group.
本发明的所述 "( ^6烷基 )2氨基"和" 6烷基氨基"分别是指氨基中任意两 个和一个能被取代的原子被上述" .6烷基"所取代,并通过氨基与其他结构相 连接的基团。 The present invention "(^ 6 alkyl) 2 amino" and "6 alkylamino" refer to an amino group and any two atoms of a substituent can be substituted with the aforementioned ".6 alkyl group", and by A group in which an amino group is bonded to other structures.
本发明所述的 "3〜14元环烷基"是指 3〜14个碳原子的烷烃部分去除一个氢 原子衍生的环状烷基, 包括 3〜8元环烷基(单环)、 6-14元并环环烷基(双环 或多环)。 The "3- to 14-membered cycloalkyl group" as used in the present invention means that the alkane moiety of 3 to 14 carbon atoms is removed by a hydrogen atom-derived cyclic alkyl group, including a 3- to 8-membered cycloalkyl group (monocyclic ring), 6 -14 membered cyclocycloalkyl (bicyclic or polycyclic).
3〜8元环烷基, 是指 3〜8个碳原子的烷烃部分去除一个氢原子衍生的环 状烷基, 其实例包括但不限于: 环丙烷基、 环丁烷基、 环戊烷基、 环己烷基、 环庚烷基、 环辛烷基、 甲基环丙烷基、 二甲基环丙烷基、 甲基环丁烷基、 二甲 基环丁烷基、 甲基环戊烷基、 二甲基环戊烷基、 甲基环己烷基、 二甲基环己烷 基等。 The 3- to 8-membered cycloalkyl group means that the alkane moiety of 3 to 8 carbon atoms is removed by a hydrogen atom-derived cyclic alkyl group, and examples thereof include, but are not limited to, cyclopropyl group, cyclobutylalkyl group, cyclopentyl group. , cyclohexane, cycloheptyl, cyclooctyl, methylcyclopropane, dimethylcyclopropane, methylcyclobutane, dimethylcyclobutane, methylcyclopentanyl , dimethylcyclopentyl, methylcyclohexane, dimethylcyclohexane, and the like.
6〜14元并环环烷基, 是指由两个或两个以上环状结构彼此共用两个相邻 的碳原子所形成的 6〜14元环状基团, 其实例包括但不限于: 二环 [3.1.0]己烷 基、 二环 [4.1.0]庚烷基、 二环 [2.2.0]己烷基、 二环 [3.2.0]庚烷基、 二环 [4.2.0] 辛烷基、 八氢并环戊二烯基、 八氢 -1/7-茚基、 十氢化萘基、 十四氢菲基、 又 环 [3.1.0]己 -2-烯基、 双环 [4.1.0]庚 -3-烯基、 双环 [3.2.0]庚 -3-烯基、 双环 [4.2.0] 辛 -3-烯基、 1,2,3,3 -四氢并环戊二烯基、 2,3,3 ,4,7,7 -六氢 -1/7-茚基、 1,2,3,4,4 ,5,6,8 -八氢化萘基、 1,2,4 ,5,6,8 -六氢化萘基、 1,2,3,4,5,6,7,8,9,10- 十氢菲基等。 The 6- to 14-membered cyclocycloalkyl group refers to a 6- to 14-membered cyclic group formed by two or more cyclic structures sharing two adjacent carbon atoms, and examples thereof include, but are not limited to: Bicyclo[3.1.0]hexane, bicyclo[4.1.0]heptyl, bicyclo[2.2.0]hexane, bicyclo[3.2.0]heptyl, bicyclo[4.2.0 Octyl, octahydrocyclopentadienyl, octahydro-1/7-fluorenyl, decahydronaphthyl, tetradecafluorophenanyl, re-cyclo[3.1.0]hex-2-enyl, bicyclo [4.1.0]Hept-3-enyl, bicyclo[3.2.0]hept-3-enyl, bicyclo[4.2.0]oct-3-enyl, 1,2,3,3-tetrahydrocyclo Pentadienyl, 2,3,3,4,7,7-hexahydro-1/7-fluorenyl, 1,2,3,4,4,5,6,8-octahydronaphthyl, 1, 2,4,5,6,8-hexahydronaphthyl, 1,2,3,4,5,6,7,8,9,10-decahydrophenanyl and the like.
本发明所述的 "杂原子"是指 Ν、 0、 C(0)、 S、 SO和 /或 S02等, 优选 N、 0、 S, 更优选 N、 0。 The "hetero atom" as used in the present invention means Ν, 0, C(0), S, SO and/or S0 2 and the like, preferably N, 0, S, more preferably N, 0.
本发明的所述 "3〜14元杂环基"是指含有一至多个杂原子的 3〜14元环状 基团, 包括 3〜8元杂环基(单环) 和 6〜14元并杂环基(双环或多环)。 杂环 基可以是饱和的、 部分不饱和的或完全不饱和的。 The "3- to 14-membered heterocyclic group" of the present invention means a 3- to 14-membered cyclic group containing one to more hetero atoms, including a 3 to 8 membered heterocyclic group (monocyclic) and 6 to 14 members. Heterocyclic group (bicyclic or polycyclic). Heterocyclyl groups can be saturated, partially unsaturated or fully unsaturated.
3〜8元杂环基, 是指含有 3〜8个环原子 (其中至少含有一个杂原子) 的
单环杂环基。具体实例包括但不仅限于 2,5-二氢噻吩基、 4,5-二氢吡唑基、 3,4- 二氢 -2/7-吡喃基、 5,6-二氢 -4/7-1,3-噁嗪基、 氮杂环丙烷基、 氮杂环丁烷基、 石克杂环丁烷基、 四氢呋喃基、 四氢吡咯基、 咪唑烷基、 吡唑烷基、 四氢呋喃 基、 1,4-二氧杂环己烷基、 1,3-二氧杂环己烷基、 1,3-二硫杂环己烷基、 吗啉 基、 哌嗪基等。 a 3 to 8 membered heterocyclic group, which means having 3 to 8 ring atoms (having at least one hetero atom) Monocyclic heterocyclic group. Specific examples include, but are not limited to, 2,5-dihydrothiophenyl, 4,5-dihydropyrazolyl, 3,4-dihydro-2/7-pyranyl, 5,6-dihydro-4/7 -1,3-oxazinyl, aziridine, azetidinyl, s-heterocyclobutane, tetrahydrofuranyl, tetrahydropyrrolyl, imidazolidinyl, pyrazolidinyl, tetrahydrofuranyl, 1,4-dioxanyl group, 1,3-dioxanyl group, 1,3-dithiacyclohexane group, morpholinyl group, piperazinyl group and the like.
本发明所述 4〜7元杂环基"、 "5-6元杂环基"指上述 "3〜8元杂环基"中环 原子数为 4〜7元、 5〜6元的具体实例。 The 4- to 7-membered heterocyclic group of the present invention and the "5-6-membered heterocyclic group" refer to a specific example in which the number of ring atoms in the above "3 to 8 membered heterocyclic group" is 4 to 7 members and 5 to 6 members.
6-14元并杂环基,是指含有 6〜14个环原子(其中至少含有一个杂原子) 由两个或两个以上环状结构彼此共用两个相邻的原子连接起来形成的稠环结 构, 如苯并 3〜8元 〜8元杂环基并 3〜8元杂环基形成的 6-14-membered heterocyclic group means a fused ring containing 6 to 14 ring atoms (having at least one hetero atom) joined by two or more ring structures sharing two adjacent atoms with each other. a structure, such as a benzo 3 to 8 to 8 membered heterocyclic group and a 3 to 8 membered heterocyclic group
等环状结构取代任意可取代的氢原子所形成的基团。 本发明的所述 "6〜12 元 并杂环基"、 "6〜10元并杂环基"指上述 "6〜14元并杂环基"中环原子数为 6〜12 元、 6〜10元的具体实例。 An isocyclic structure replaces a group formed by any substitutable hydrogen atom. The "6- to 12-membered heterocyclic group" and the "6- to 10-membered heterocyclic group" of the present invention mean that the above-mentioned "6- to 14-membered heterocyclic group" has a ring number of 6 to 12, 6 to 10 Specific examples of yuan.
本发明所述的 "5〜10元杂环基"指上述 "3〜14元杂环基"中环原子数为 5〜10 元的具体实例, 例如 5〜10元杂环基包括 5〜6元杂环基 (单环) 和 6〜10元并 杂环基(欢环或多环)。 The "5- to 10-membered heterocyclic group" as used in the present invention refers to a specific example in which the number of ring atoms in the above "3 to 14-membered heterocyclic group" is 5 to 10 members, and for example, a 5- to 10-membered heterocyclic group includes 5 to 6 members. Heterocyclyl (monocyclic) and 6 to 10 membered heterocyclic (heterocyclic or polycyclic).
本发明所述的 "6〜12元桥环基"是指任意两个环共用两不相邻的环原子开 成的含有 6〜12个环原子(包括碳原子和任选的杂原子)的结构。 桥环基可以 是饱和的、部分不饱和的或完全不饱和的。其中包括例如" 6〜10元桥碳环基"、 "6〜8元桥碳环基"、 "7-10元桥碳环基"、 "7〜9元桥碳环基"、 "7〜8元桥碳环 基"、 "8元桥碳环基"、 "6〜12元桥杂环基"、 "6〜10元桥杂环基"、 "6〜8元桥杂 环基"、 "7〜10元桥杂环基"、 "7〜9元桥杂环基"、 "7〜8元桥杂环基"和" 8元桥 杂环基 "等。 其实例包括但不限于:
The "6- to 12-membered bridged ring group" as used in the present invention means that any two rings share two non-adjacent ring atoms and have 6 to 12 ring atoms (including carbon atoms and optional hetero atoms). structure. The bridged ring group can be saturated, partially unsaturated or completely unsaturated. These include, for example, "6 to 10 yuan bridge carbon ring group", "6 to 8 yuan bridge carbon ring group", "7-10 yuan bridge carbon ring group", "7 to 9 yuan bridge carbon ring group", "7~ 8-membered bridge carbocyclic group, "8-membered bridged carbocyclic group", "6- to 12-membered bridged heterocyclic group", "6- to 10-membered bridged heterocyclic group", "6- to 8-membered bridged heterocyclic group", "7 to 10 membered bridged heterocyclic group", "7 to 9 membered bridged heterocyclic group", "7 to 8 membered bridged heterocyclic group" and "8-membered bridged heterocyclic group" and the like. Examples include, but are not limited to:
本发明所述的 "6 12元螺环基"是指至少有两个环共享一个环原子形成的 含有 6-12个环原子(包括碳原子和任选的杂原子)的结构。 螺环基可以是饱 和的、部分不饱和的或完全不饱和的。其中包括例如 "7 12元螺碳环基"、 "7 11 元螺碳环基"、 "7-10元螺碳环基"、 "7 9元螺碳环基"、 "7 8元螺碳环基"、 "8 元螺碳环基"、 "6 12元螺杂环基"、 "7 12元螺杂环基"、 "7-11元螺杂环基"、 "7-10元螺杂环基"、 "7 9元螺杂环基"、 "7 8元螺杂环基"和" 8元螺杂环基" The "6 12-membered spirocyclic group" as used in the present invention means a structure having at least two rings sharing a ring atom and having 6 to 12 ring atoms including a carbon atom and optionally a hetero atom. The spiro group can be saturated, partially unsaturated or completely unsaturated. These include, for example, "7 12-membered spirocarbocyclyl", "7 11-membered spirocarbocyclyl", "7-10-membered spirocarbocyclyl", "79-membered spirocarbocyclyl", and "7-eight-membered spiro carbon" "Cycloalkyl", "8-membered spirocarbocyclyl", "6 12-membered spiroheterocyclyl", "7 12-membered spiroheterocyclyl", "7-11-membered spiroheterocyclyl", "7-10-membered snail" Heterocyclic group", "7 9-membered spiroheterocyclyl", "7-membered spiroheterocyclyl" and "8-membered spiroheterocyclyl"
II、 化合物 II, compound
本发明提供了通式 ( I ) 所示的化合物或其立体异构体、 或其药学上可 的盐、 酯或溶剂化物: The present invention provides a compound represented by the formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
( I )(I)
A2选自 C-R2或 N; A 2 is selected from CR 2 or N;
A3选自 C-R4或 N, 且 Ai、 A2和 A3不同时为 N; A 3 is selected from CR 4 or N, and Ai, A 2 and A 3 are not N at the same time;
R1, R2和 R4分别独立地选自氢、 羟基、 羧基、 硝基、 卤素原子、 氨基、 (C1-6烷基 )2氨基、 氰基、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14 元环烷基; R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxy, carboxy, nitro, halogen atom, amino, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1 -6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl;
R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 素原子、 CL6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14元环烷基, 所述的 C1-6烷基、 C1-6烷 氧基、 C2-6烯基、 C2-6炔基和 3〜14元环烷基可独立地任选被一至多个下列取 代基取代: 羟基、 羧基、 氨基、 氰基、 卤素原子、 硝基或 3〜14元杂环基;R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or a 14-membered cycloalkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2-6 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently Optionally substituted by one or more of the following substituents: hydroxy, carboxy, amino, cyano, halo, nitro or 3 to 14 membered heterocyclyl;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基, 所述的 C1-6烷基、 C2-6烯基和 C2-6炔基可独立地任选被 C1-6烷氧基 取代; M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
R5和 R6分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 羟基 C1-6 R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
¾^¾ 、 C2-6婦 或。2-6快 3⁄4^3⁄4, C2-6 women or. 2-6 fast
或 R5和 R6相互连接, 与它们所连接的碳原子一起形成 3〜14元杂环基或 3-14元环烷基; Or R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 0、 S或 N-R9; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团: Q is selected from the following groups:
( 1 ) 3-8元杂环基 (或, 3〜8元单环杂环基), (1) a 3-8 membered heterocyclic group (or a 3 to 8 membered monocyclic heterocyclic group),
( 2 )任选被一至三个相同或不同的 R1Q取代的 3〜14元环烷基或 6〜14元 并杂环基, 和 (2) a 3 to 14 membered cycloalkyl group or a 6 to 14 membered heterocyclic group optionally substituted by one to three identical or different R 1Q groups, and
( 3 )任选被一至三个相同或不同的 R1Q取代的 6〜12元桥环基或 6〜12元 螺环基, (3) a 6 to 12 membered bridged ring group or a 6 to 12 membered spirocyclic group optionally substituted by one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 CL6烷基氨基羰基、 羟基 CL6烷基、 羟基 CL6烷基氨基、 代 CL6烷基、 d.6 烷基磺酰基、 C 烷基磺酰基氨基、氨基磺酰基、氨基磺酰基氨基、 烯基、R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
。2-6块基; . 2-6 blocks;
R7选自任选被取代基取代的 6〜12元桥环基、 6-12元螺环基、 3〜8元杂 环基或 6〜14元并杂环基, 所述取代基选自氨基、 羟基、 硝基、 素原子、 羧 基、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基、 3〜8元杂环基或 3〜8元环烷
基; R 7 is selected from a 6 to 12 membered bridged ring group, a 6 to 12 membered spirocyclic group, a 3 to 8 membered heterocyclic group or a 6 to 14 membered heterocyclic group which is optionally substituted with a substituent selected from the group consisting of Amino, hydroxy, nitro, alkane, carboxyl, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, 3 to 8 membered heterocyclic or 3 ~8 yuan naphthenic Base
n选自 0、 1、 2、 3、 4、 5或 6, n is selected from 0, 1, 2, 3, 4, 5 or 6,
条件是: requirement is:
当 n为 0时, R7不存在, When n is 0, R 7 does not exist.
当 1≥2时, R7可以相同或不同, 且 When 1 ≥ 2, R 7 may be the same or different, and
当 Q选自 3〜8元杂环基时, n不能为 0, 且 R7不能选自 3〜8元杂环基。 在一种实施方案中, 1是 1 1。 When Q is selected from a 3 to 8 membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 3 to 8 membered heterocyclic group. In one embodiment, 1 is 1 1 .
在一种实施方案中, A2是 C-R2。 In one embodiment, A 2 is CR 2 .
在一种实施方案中, A3是 C-R4。 In one embodiment, A 3 is CR 4 .
在一种实施方案中, R1, R2和 R4分别独立地选自氢、 卤素原子、 ^6烷 基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜8元环烷基。 在一种优选的实施方 案中, R1, R2和 R4分别独立地选自氢、 素原子、 C1-6烷基或 3〜8元环烷基。 在一种优选的实施方案中, R R2和 R4分别独立地选自氢, 甲基或乙基。 In one embodiment, R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, halogen atom, ^ 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkyne Base or 3 to 8 membered cycloalkyl. In a preferred embodiment, R 1 , R 2 and R 4 are each independently selected from hydrogen, a halogen atom, a C 1-6 alkyl group or a 3 to 8 membered cycloalkyl group. In a preferred embodiment, RR 2 and R 4 are each independently selected from hydrogen, methyl or ethyl.
在一种实施方案中, R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 卤 素原子、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜8元环烷基, 所述 的 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基和 3〜8元环烷基可独立地任选 被一至三个下列取代基取代: 羟基、 羧基, 氨基、 氰基、 素原子、 硝基或 3〜8元杂环基。 在一种优选的实施方案中, R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 卤素原子、 ^6烷基或 3〜8元环烷基。 在一种优选的实施方 案中, R3选自氢、 氰基、 羟基、 氨基、 氟原子、 氯原子、 甲基或乙基。 In one embodiment, R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, halogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl , C 2-6 alkynyl or 3 to 8 membered cycloalkyl, said C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl and 3~ The 8-membered cycloalkyl group may be independently optionally substituted with one to three of the following substituents: a hydroxyl group, a carboxyl group, an amino group, a cyano group, a sulfonic acid atom, a nitro group or a 3 to 8 membered heterocyclic group. In a preferred embodiment, R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, halogen atom, ^ 6 alkyl or 3 to 8 membered cycloalkyl. In a preferred embodiment, R 3 is selected from the group consisting of hydrogen, cyano, hydroxy, amino, fluoro, chloro, methyl or ethyl.
在一种实施方案中, M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基、 C1-6 烷氧基、 C2-6烯基或 C2-6炔基, 所述的 C1-6烷基、 C2-6烯基和 C2-6炔基可独立 地任选被 ^6烷氧基取代。在一种优选的实施方案中, M选自 0、 S或 N-R8, R8选自氢、 C 烷基或 C 烷氧基,所述的 C 烷基可任选被 C 烷氧基取代。 在一种优选的实施方案中, M选自 N-R8, R8选自氢或 C1-4烷基。 In one embodiment, M is selected from 0, S or NR 8 and R 8 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkyne The C 1-6 alkyl group, the C 2-6 alkenyl group and the C 2-6 alkynyl group may be independently optionally substituted by a 6 alkoxy group. In a preferred embodiment, M is selected from 0, S or NR 8 , R 8 is selected from hydrogen, C alkyl or C alkoxy, and said C alkyl group may be optionally substituted by C alkoxy. In a preferred embodiment, M is selected from NR 8 and R 8 is selected from hydrogen or C 1-4 alkyl.
在一种实施方案中, R5和 R6分别独立地选自氢、 素原子、 ^6烷基、 C1-6烷氧基、 羟基 C1-6烷基、 C2-6烯基或 C2-6炔基, 或 R5和 R6相互连接, 与 它们所连接的碳原子一起形成 5〜10元杂环基或 3〜8元环烷基。在一种优选的 实施方案中, R5和 R6分别独立地选自氢、 卤素原子、 ^6烷基、 ^6烷氧基 或羟基 .6烷基, 或 R5和 R6相互连接, 与它们连接的碳原子一起形成 5〜6 元杂环基或 3〜8元环烷基。在一种优选的实施方案中, R5和 R6分别独立地选 白 Ci_4 ¾*基。 In one embodiment, R 5 and R 6 are each independently selected from hydrogen, a halogen atom, ^ 6 alkyl, C 1-6 alkoxy, hydroxy C 1-6 alkyl, C 2-6 alkenyl or The C 2-6 alkynyl group, or R 5 and R 6 are bonded to each other to form a 5- to 10-membered heterocyclic group or a 3- to 8-membered cycloalkyl group together with the carbon atom to which they are attached. In a preferred embodiment, R 5 and R 6 are each independently selected from hydrogen, a halogen atom, a ^ 6 alkyl group, a ^ 6 alkoxy group or a hydroxy group . 6 alkyl group, or R 5 and R 6 are bonded to each other, Together with the carbon atom to which they are attached, a 5- to 6-membered heterocyclic group or a 3- to 8-membered cycloalkyl group is formed. In a preferred embodiment, R 5 and R 6 independently independently select a Ci_4 3⁄4* group.
在一种实施方案中, Υ选自 Ν或 C-R9, X选自 0、 S或 N-R9; R9选自氢、
C1-6烷基、 CM炔基或 3〜8元环烷基。 在一种优选的实施方案中, Y选自 N或 C-R9, X选自 S或 N-R9, R9选自氢、 甲基、 乙基或正丙基。 In one embodiment, the oxime is selected from ruthenium or CR 9 , X is selected from 0, S or NR 9 ; R 9 is selected from hydrogen, C 1-6 alkyl, CM alkynyl or 3 to 8 membered cycloalkyl. In one preferred embodiment, Y is selected from N or CR 9, X is selected from S or NR 9, R 9 is selected from hydrogen, methyl, ethyl or n-propyl.
在一种实施方案中, Q选自下列基团: (1 ) 4〜7元杂环基(或, 4〜7元单 环杂环基), ( 2 )任选被一至三个相同或不同的 R1Q取代的 3〜8元环烷基或 6〜12 元并杂环基, 和(3 )任选被一至三个相同或不同 R1Q取代的 7〜10元桥环基 或 6〜12元螺环基; R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6 烷基 )2氨基、 ^6烷基氨基羰基、 羟基 ^6烷基、 羟基 ^6烷基氨基、 卤代 CL4烷基、 CL4烷基磺酰基、 CL4烷基磺酰基氨基、 氨基磺酰基、 氨基磺酰基 氨基、 C2-6烯基、 C2-6炔基。在一种优选的实施方案中, Q选自下列基团:(1 ) 5〜6元杂环基(或, 5〜6元单环杂环基), (2 )任选被一至三个相同或不同的 R1Q取代的 3〜8元环烷基或 6〜10元并杂环基, 和( 3 )任选被一至三个相同或 不同的 R1Q取代的 7〜9元桥环基或 7〜11元螺环基, R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 C1-6烷基氨基羰基、 羟基 CL6 烷基、 羟基 ^6烷基氨基、 代 CM烷基、 甲基磺酰基、 甲基磺酰基氨基、 氨基磺酰基、 氨基磺酰基氨基、 C2-6烯基、 C2-6炔基。 在一种优选的实施方案 中, Q选自 ( 1 ) 5〜6元杂环基(或, 5〜6元单环杂环基), (2 )任选被一至两 个相同或不同的 R1Q取代的 6〜10元并杂环基, 和(3 )任选被一至两个相同 或不同的 R1Q取代的 7〜9元桥环基或 7〜11元螺环基, R1Q选自氨基或 CM烷基。 在一种优选的实施方案中, Q选自 4〜7元杂环基(或, 4〜7元单环杂环基), 优选地含有 1或 2个氮原子作为环原子, 更 +优ν选。地是饱和的。 在一种优选的 In one embodiment, Q is selected from the group consisting of: (1) a 4 to 7 membered heterocyclic group (or a 4 to 7 membered monocyclic heterocyclic group), (2) optionally being one to three identical or different R 1Q substituted 3 to 8 membered cycloalkyl or 6 to 12 membered heterocyclic group, and (3) optionally 7 to 10 membered bridged or substituted by 1 to 3 identical or different R 1Q A spiro ring group; R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, ^ 6 alkylamino Carbonyl, hydroxy- 6 alkyl, hydroxy- 6 alkylamino, halogenated CL 4 alkyl, CL 4 alkylsulfonyl, CL 4 alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, C 2-6 Alkenyl, C 2-6 alkynyl. In a preferred embodiment, Q is selected from the group consisting of: (1) a 5- to 6-membered heterocyclic group (or a 5- to 6-membered monocyclic heterocyclic group), (2) optionally being one to three identical Or a different R 1Q substituted 3 to 8 membered cycloalkyl or 6 to 10 membered heterocyclic group, and ( 3 ) a 7 to 9 membered bridged ring group optionally substituted by one to three identical or different R 1Q or 7 to 11 membered spiro group, R 1Q is selected from amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, C 1 -6 alkylaminocarbonyl group, a hydroxyl CL 6 alkyl, hydroxy ^ 6 alkylamino, C M substituting group, methylsulfonyl group, methylsulfonyl group, sulfamoyl group, sulfamoyl group, C 2-6 Alkenyl, C 2-6 alkynyl. In a preferred embodiment, Q is selected from the group consisting of (1) 5 to 6 membered heterocyclic groups (or 5 to 6 membered monocyclic heterocyclic groups), (2) optionally being one to two identical or different R a 1Q- substituted 6- to 10-membered heterocyclic group, and (3) a 7- to 9-membered bridged ring group or a 7- to 11-membered spirocyclic group optionally substituted by one or two identical or different R 1Q , and R 1Q is selected from the group consisting of Amino or C M alkyl. In a preferred embodiment, Q is selected from a 4 to 7 membered heterocyclic group (or a 4 to 7 membered monocyclic heterocyclic group), preferably having 1 or 2 nitrogen atoms as a ring atom, more preferably ν selected. The ground is saturated. In a preferred
Y / ~ \ , 、 、 +N N— 施方案中, Q选自任选被一至两
Y / ~ \ , , , + NN — In the scheme, Q is selected from one to two
个相同或不同的 R1(J取代的 7〜8元桥环基, 优选地含有 1或 2个氮原子作为 环原子, 更优选地是饱和的, 其中 R1Q选自氨基或 ^6烷基。在一 The same or different R 1 (J substituted 7 to 8 membered bridged ring group, preferably having 1 or 2 nitrogen atoms as a ring atom, more preferably saturated, wherein R 1Q is selected from an amino group or a 6 alkyl group .In a
中, Q选自任选被一至两个相同或不同的 R1Q取代的 7〜11元螺环基, 所述螺 环基优选是含有 1-3个杂原子的螺环基, 更优选地所述螺环基含有 7-11个环 原子,其中 1或 2个环原子是氮原子,其余的环原子是碳原子,还更优选地,
所述螺环基是饱和基团,其中 R1Q选自氨基或 CM烷基。在一种优选的实施方 案中, Q选自任选被一至两个相同或不同的 R1Q取代的 6〜10元并杂环基, 所 述并杂环基优选是含有 1-3 个杂原子的并杂环基, 更优选地所述并杂环基含 有 6〜10个环原子, 其中 1-3个环原子是选自氮和氧的杂原子, 其余的环原子 是碳原子, 还更优选地, 所述并杂环基是饱和基团, 其中 R1Q选自氨基或 烷基。 在一种优选的实施方案中, 10 Wherein Q is selected from a 7 to 11-membered spiro group optionally substituted by one to two identical or different R 1Q , and the spiro group is preferably a spiro group having 1 to 3 hetero atoms, more preferably The spiro group contains 7 to 11 ring atoms, wherein 1 or 2 ring atoms are nitrogen atoms, the remaining ring atoms are carbon atoms, and still more preferably, The spiro group is a saturated group wherein R 1Q is selected from an amino group or a C M alkyl group. In a preferred embodiment, Q is selected from 6 to 10 membered heterocyclyl optionally substituted with one to two identical or different R 1Q groups, preferably having from 1 to 3 heteroatoms And a heterocyclic group, more preferably the heterocyclic group contains 6 to 10 ring atoms, wherein 1 to 3 ring atoms are hetero atoms selected from nitrogen and oxygen, and the remaining ring atoms are carbon atoms, and still more Preferably, the heterocyclic group is a saturated group, wherein R 1Q is selected from an amino group or an alkyl group. In a preferred embodiment, 10
Q 选自任选被一至两个相同或不同的 Q is selected from one to two identical or different
Ci_4 ¾*^- o Ci_4 3⁄4*^- o
在一种实施方案中, R7选自任选被取代基取代的 6〜10元桥环基、 6〜12 元螺环基、 4〜7元杂环基或 6〜12元并杂环基, 所述取代基选自氨基、 羟基、 卤素原子、 C1-6烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基。 在一种优选的实施 方案中, R7选自任选被取代基取代的 7〜10元桥环基、 7〜11 元螺环基、 5〜6 元杂环基或 6〜10元并杂环基, 所述取代基选自氨基、 羟基、 卤素原子、 C1-6 烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基。 在一种优选的实施方案中, R7选自 7〜9元桥环基、 7〜11元螺环基或 5〜6元杂环基。 在一种优选的实施方案中, R7选自 7〜8元桥环基, 优选地含有 1或 2个选自 原 更优选地 In one embodiment, R 7 is selected from a 6 to 10 membered bridged ring group optionally substituted with a substituent, a 6 to 12 membered spirocyclic group, a 4 to 7 membered heterocyclic group or a 6 to 12 membered heterocyclic group. The substituent is selected from the group consisting of an amino group, a hydroxyl group, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, a C 2-6 alkenyl group or a C 2-6 alkynyl group. In a preferred embodiment, R 7 is selected from the group consisting of a 7 to 10 membered bridged ring group optionally substituted with a substituent, a 7 to 11 membered spirocyclic group, a 5 to 6 membered heterocyclic group or a 6 to 10 membered heterocyclic group. a ring group, the substituent being selected from the group consisting of an amino group, a hydroxyl group, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, a C 2-6 alkenyl group or a C 2-6 alkynyl group. In a preferred embodiment, R 7 is selected from the group consisting of 7 to 9 membered bridged ring groups, 7 to 11 membered spirocyclic groups or 5 to 6 membered heterocyclic groups. In a preferred embodiment, R 7 is selected from the group consisting of 7 to 8 membered bridged ring groups, preferably containing 1 or 2 selected from the group, more preferably
元杂环基, 优选地含有 1或 2个选自氧和氮的环原子, 更优选地是饱和的。 The heterocyclic group preferably contains 1 or 2 ring atoms selected from oxygen and nitrogen, more preferably saturated.
在一种实施方案中, n选自 0、 1、 2或 3。 在一种优选的实施方案中, n
选自 0或 1。在一种优选的实施方案中, n选自 0。在一种优选的实施方案中, η选自 1。 In one embodiment, n is selected from 0, 1, 2 or 3. In a preferred embodiment, n Selected from 0 or 1. In a preferred embodiment, n is selected from zero. In a preferred embodiment, n is selected from 1.
在一种实施方案中, , Α2和 Α3分别独立地选自 CH; R3选自氢或氰基; Μ选自 NH; R5和 R6分别独立地选自氢或 C1-6烷基; Y选自 N; X选自 S。 In one embodiment, Α 2 and Α 3 are each independently selected from CH; R 3 is selected from hydrogen or cyano; Μ is selected from NH; and R 5 and R 6 are each independently selected from hydrogen or C 1-6 Alkyl; Y is selected from N; X is selected from S.
在一种实施方案中, Q和 R7中至少一个是桥环基, 所述桥环基含有至少 一个氮原子作为环成员, 所述桥通过与该氮原子相邻的两个环原子 (如碳原 子)作为桥头原子形成, 例如 Q选自 "任选被 CM烷基取代的†N^^ H " 和 NfT — , R7不存在或者选自 ^ " 和 +N^y o。 在一种实施方案中, Q是桥环基, 所述桥环基含有至少一个氮原子作为 环成员, 所述桥通过与该氮原子相邻的两个环 (如碳原子)作为桥头原 子形成, 例如 Q选自 "任选被(^.4烷基取代的 " 和十 Ν1-.In one embodiment, at least one of Q and R 7 is a bridged ring group containing at least one nitrogen atom as a ring member, the bridge passing through two ring atoms adjacent to the nitrogen atom (eg, carbon atom) is formed as a bridgehead atom, Q is selected from, for example, "optionally substituted C M alkyl † N ^^ H" and N fT -, R 7 is absent or is selected from ^ "and a + N ^ yo. In one embodiment, Q is a bridged ring group containing at least one nitrogen atom as a ring member, and the bridge is formed as a bridgehead atom by two rings (such as carbon atoms) adjacent to the nitrogen atom, for example Q is selected from "optionally (^. 4 alkyl substituted) and tenth Ν 1-.
在一种实施方案中, R7是桥环基, 所述桥环基含有至少一个氮原子作为 子相邻的两个环原子 (如碳原子)作为桥头原
In one embodiment, R 7 is a bridged ring group containing at least one nitrogen atom as two adjacent ring atoms (eg, carbon atoms) as a bridgehead
本发明还包括通过组合上述实施方案和上述优选的实施方案中的两个或 更多个所获得的任何一种实施方案。 The present invention also includes any one of the embodiments obtained by combining the above embodiments and two or more of the above preferred embodiments.
本发明的通式(I )所示的任一化合物药学上可接受的盐是指由药学上可 接受的、 非毒性碱或酸制备的盐, 包括有机酸盐、 无机酸盐、 有机碱盐、 无 机碱盐。 有机酸盐包括甲酸、 乙酸、 苯磺酸、 苯甲酸、 对甲苯磺酸、 樟脑磺 酸、 柠檬酸、 甲磺酸、 乙磺酸、 丙磺酸、 富马酸、 葡糖酸、 谷氨酸、 羟乙磺 酸、 乳酸、 马来酸、 苹果酸、 扁桃酸、 粘酸、 双羟萘酸、 泛酸、 琥珀酸、 酒 石酸等的盐。 无机酸盐包括氢溴酸、 氢氯酸、 硝酸、 硫酸、 磷酸等的盐。 The pharmaceutically acceptable salt of any one of the compounds of the formula (I) of the present invention means a salt prepared from a pharmaceutically acceptable, non-toxic base or acid, including an organic acid salt, a mineral acid salt, an organic alkali salt. , inorganic alkali salt. Organic acid salts include formic acid, acetic acid, benzenesulfonic acid, benzoic acid, p-toluenesulfonic acid, camphorsulfonic acid, citric acid, methanesulfonic acid, ethanesulfonic acid, propanesulfonic acid, fumaric acid, gluconic acid, glutamic acid , salts of isethionate, lactic acid, maleic acid, malic acid, mandelic acid, mucic acid, pamoic acid, pantothenic acid, succinic acid, tartaric acid, and the like. The inorganic acid salt includes a salt of hydrobromic acid, hydrochloric acid, nitric acid, sulfuric acid, phosphoric acid or the like.
有机碱盐包括伯、 仲和叔胺, 被取代胺包括天然存在的取代胺、 环胺和 碱离子交换树脂, 选自甜菜碱、咖啡因、胆碱、 N,N,-二苄基乙二胺、二乙胺、 2-二乙胺基乙醇、 2-二甲胺基乙醇、 乙醇胺、 乙二胺、 N-乙基-吗啉、 N-乙基 哌啶、 葡甲胺、 氨基葡萄糖、 海巴明、 异丙基胺、 甲基葡糖胺、 吗啉、 哌嗪、 哌啶、 普鲁卡因、 嘌呤、 可可碱、 三乙胺、 三甲胺、 三丙胺、 氨丁三醇等的 盐。 天然氨基酸盐如甘氨酸、 丙氨酸、 缬氨酸、 亮氨酸、 异亮氨酸、 正亮氨 酸、 酪氨酸、 胱氨酸、 半胱氨酸、 蛋氨酸、 脯氨酸、 羟基脯氨酸、 组氨酸、 鸟氨酸、 赖氨酸、 精氨酸、 丝氨酸等的盐。 无机碱盐包括铵以及 4里、 钠、 钾、
钙、 镁、 锌、 钡、 铝、 铁、 酮、 亚铁、 锰、 二价锰等的盐。 The organic base salts include primary, secondary and tertiary amines, and the substituted amines include naturally occurring substituted amines, cyclic amines and alkali ion exchange resins selected from the group consisting of betaines, caffeine, choline, N,N,-dibenzylethylene. Amine, diethylamine, 2-diethylaminoethanol, 2-dimethylaminoethanol, ethanolamine, ethylenediamine, N-ethyl-morpholine, N-ethylpiperidine, meglumine, glucosamine, Haibamin, isopropylamine, methyl glucosamine, morpholine, piperazine, piperidine, procaine, guanidine, theobromine, triethylamine, trimethylamine, tripropylamine, tromethamine, etc. salt. Natural amino acid salts such as glycine, alanine, valine, leucine, isoleucine, norleucine, tyrosine, cystine, cysteine, methionine, proline, hydroxyguanidine Salts of acid, histidine, ornithine, lysine, arginine, serine, and the like. Inorganic alkali salts include ammonium and 4 liters, sodium, potassium, Salts of calcium, magnesium, zinc, strontium, aluminum, iron, ketone, ferrous, manganese, divalent manganese, and the like.
本发明要求保护式(I )化合物的"立体异构体"是指当式(I )化合物存在 手性中心、 欢键等时, 所产生的所有立体异构体, 包括对映异构体、 非对映 异构体、 顺反异构体、 互变异构体、 几何异构体、 差向异构体及其混合物, 均包括在本发明范围中。 The present invention claims that the "stereoisomer" of the compound of formula (I) refers to all stereoisomers, including enantiomers, which are produced when a compound of formula (I) has a chiral center, a bond or the like. Diastereomers, cis-trans isomers, tautomers, geometric isomers, epimers, and mixtures thereof are included within the scope of the invention.
本发明的通式(I )所示的任一化合物合成得到的若是消旋体, 所需要的 的色谱法 (像高压制备液相、 超临界流体色谱)。 手性填料包括但不限于: The chromatographic method (such as high pressure liquid phase preparation, supercritical fluid chromatography) which is obtained by synthesizing any of the compounds represented by the formula (I) of the present invention as a racemate. Chiral fillers include, but are not limited to:
Chiralcel OJ-H, Chiralpak AD-H, Chiralpak IA, Chiralpak AS-H。 Chiralcel OJ-H, Chiralpak AD-H, Chiralpak IA, Chiralpak AS-H.
本发明通式(I )化合物的"酯"包括当式 (I ) 化合物存在羧基时, 可以 与醇发生酯化反应而形成的酯, 当式( I )化合物存在羟基时,可以有机酸、 无机酸、 有机酸盐等发生酯化反应而形成的酯。 酯在酸或者碱存在的条件 下, 可以发生水解反应生成相应的酸或醇。 The "ester" of the compound of the formula (I) of the present invention includes an ester which can be formed by esterification reaction with an alcohol when a compound of the formula (I) is present, and an organic acid or an inorganic group when the compound of the formula (I) has a hydroxyl group. An ester formed by an esterification reaction of an acid or an organic acid salt. The ester can be hydrolyzed to form the corresponding acid or alcohol in the presence of an acid or a base.
通式( I )所示的化合物或其立体异构体、或其药学上可接受的盐或酯可以 是"溶剂化物"形式, 所述的溶剂可以为水、 甲醇、 乙醇等。 例如当溶剂化物 产物的吸湿性逐渐进行。 The compound represented by the formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt or ester thereof may be in the form of a "solvate", and the solvent may be water, methanol, ethanol or the like. For example, the hygroscopicity of the solvate product proceeds gradually.
III、 组合物 III. Composition
本发明提供了一种药物组合物, 其包括本发明的化合物或其立体异构体、 或其药学上可接受的盐、 酯或溶剂化物与一种或多种药用载体和 /或稀释剂。 The present invention provides a pharmaceutical composition comprising a compound of the present invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, together with one or more pharmaceutically acceptable carriers and/or diluents .
本发明进一步要求保护包括上述的通式 (I ) 所示的任一化合物或其立体 异构体、 或者其药学上可接受的盐、 酯或溶剂化物与一种或多种药用载体和 / 或稀释剂的药物组合物,可以制成药学上可接受的任一剂型。以口服、肠胃外、 直肠或经肺给药等方式施用于需要这种治疗的患者。 用于口服给药时, 可制成 常规的固体制剂,如片剂、胶嚢剂、丸剂、颗粒剂等;也可制成口服液体制剂, 如口服溶液剂、 口服混悬剂、 糖浆剂等。 制成口服制剂时, 可以加入适宜的填 充剂、 粘合剂、 崩解剂、 润滑剂等。 用于肠胃外给药时, 可制成注射剂, 包括 注射液、 注射用无菌粉末与注射用浓溶液。 制成注射剂时, 可采用现有制药领 域中的常规方法生产, 配制注射剂时, 可以不加入附加剂, 也可才艮据药物的性 质加入适宜的附加剂。 用于直肠给药时, 可制成栓剂等。 用于经肺给药时, 可 制成吸入剂或喷雾剂等。 The present invention further claims any of the compounds of the above formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, and one or more pharmaceutically acceptable carriers and/or The pharmaceutical composition of the diluent or diluent can be formulated into any of the pharmaceutically acceptable dosage forms. It is administered to a patient in need of such treatment by oral, parenteral, rectal or pulmonary administration. For oral administration, it can be prepared into conventional solid preparations such as tablets, capsules, pills, granules, etc.; or can be prepared into oral liquid preparations, such as oral solutions, oral suspensions, syrups, etc. . When the oral preparation is prepared, a suitable filler, a binder, a disintegrant, a lubricant or the like may be added. For parenteral administration, it can be prepared as an injection, including injection, sterile powder for injection and concentrated solution for injection. When the injection is prepared, it can be produced by a conventional method in the prior art pharmaceutical field, and when the injection is formulated, an additional agent may be added, or a suitable additive may be added depending on the nature of the drug. When used for rectal administration, it can be made into a suppository or the like. For pulmonary administration, it can be made into an inhalant or a spray.
本发明还提供了一种药物组合物, 其包括本发明的化合物或其立体异构
体、 或其药学上可接受的盐、 酯或溶剂化物, 一种或多种药用载体和 /或稀释 剂, 以及一种或多种抗肿瘤剂和免疫抑制剂。 所述的抗肿瘤剂和免疫抑制剂 选自甲氨蝶呤、 卡培他滨、 吉西他滨、 去氧氟尿苷、 培美曲塞二钠、 帕唾帕 尼、 伊马替尼、 埃罗替尼、 拉帕替尼、 吉非替尼、 凡德他尼、 赫赛汀、 贝伐 单抗、 利妥昔单抗、 曲妥珠单抗、 紫杉醇、 长春瑞滨、 多西他赛、 多柔比星、 羟基喜树碱、 丝裂霉素、 表柔比星、 吡柔比星、 博来霉素、 来曲唑、 他莫西 芬、 氟维司群、 曲谱瑞林、 氟他胺、 亮丙瑞林、 阿那曲唑、 异环磷酰胺、 白 消安、 环磷酰胺、 卡莫司汀、 尼莫司汀、 司莫司汀、 氮芥、 马法兰、 瘤可宁、 卡铂、 顺铂、 奥沙利铂、 络铂、 拓朴特肯、 喜树碱、 拓朴替康、 依维莫司、 西罗莫斯、特癌适、 6-巯基嘌呤、 6-硫鸟嘌呤、硫唑嘌呤、 菌素 D、柔红霉素、 阿霉素、 米托蒽醌、 争光霉素、 普卡霉素或氨鲁米特等。 The invention also provides a pharmaceutical composition comprising a compound of the invention or a stereoisomer thereof Or a pharmaceutically acceptable salt, ester or solvate thereof, one or more pharmaceutically acceptable carriers and/or diluents, and one or more anti-tumor agents and immunosuppressive agents. The antitumor agent and immunosuppressive agent are selected from the group consisting of methotrexate, capecitabine, gemcitabine, deoxyfluorouridine, pemetrexed disodium, paclitaxil, imatinib, erlotidine Nipa, lapatinib, gefitinib, vandetanib, Herceptin, bevacizumab, rituximab, trastuzumab, paclitaxel, vinorelbine, docetaxel, multiple Spirulina, hydroxycamptothecin, mitomycin, epirubicin, pirarubicin, bleomycin, letrozole, tamoxifen, fulvestrant, lysine, flutamide , leuprolide, anastrozole, ifosfamide, busulfan, cyclophosphamide, carmustine, nimustine, semustine, nitrogen mustard, melphalan, cocoaine, carboplatin, Cisplatin, oxaliplatin, ruthenium platinum, topotecan, camptothecin, topotecan, everolimus, sirolimus, dexamethasone, 6-mercaptopurine, 6-thioguanine, Azathioprine, bacteriocin D, daunorubicin, doxorubicin, mitoxantrone, bleomycin, pucamycin or aminoglutethimide.
IV、 制备方法 IV, preparation method
) 的化合物的方法, Method of compound,
咯烷酮) 中在碱(例如三乙胺) 的存在下在加热 (例如约 100-120°C ) 条件 下反应 (例如至少 30分钟, 如约 1-2小时); The reaction in the presence of a base (e.g., triethylamine) under heating (e.g., about 100-120 ° C) (e.g., at least 30 minutes, such as about 1-2 hours);
或者 Or
酰胺)中在三氯化铟的存在下在室温(如 10-30。C )反应 (例如至少 30分钟, 如约 16小时), 然后再加入还原剂 (例如氰基硼氢化钠)反应 (例如至少 30
分钟, 如约 2小时), The amide) is reacted at room temperature (eg, 10-30 C) in the presence of indium trichloride (eg, at least 30 minutes, such as about 16 hours), followed by the addition of a reducing agent (eg, sodium cyanoborohydride) (eg, at least 30 Minutes, such as about 2 hours),
其中, Ai、 A2、 A3、 M、 Y、 X、 R3、 R5、 R6、 R7、 Q和 n如前文所定义, Q'为前文所定义的 Q或者被保护基团 (PG )保护的前文所定义的 Q, R7'为前 文所定义的 R7或者被保护基团 (PG )保护的前文所定义的 R7。 Wherein Ai, A 2 , A 3 , M, Y, X, R 3 , R 5 , R 6 , R 7 , Q and n are as defined above, and Q' is a Q or a protected group as defined above ( PG) protected hereinbefore defined Q, R 7 'R is as hereinbefore defined hereinbefore 7 or a protected group (PG) as defined protected R 7.
保护基团 (PG ) 为有机合成领域常用的保护基团, 包括但不限于: -Bn (苄基), -PMB (对甲氧基苄基), -Tos (对甲基苯磺酰基), -Fmoc (芴甲氧 羰酰基), -Ac (乙酰基), -SEM ( 2- (三甲硅基)乙氧基甲基), -Pht (邻苯二甲 酰)和 -Alloc (烯丙氧羰基)。 The protecting group (PG) is a commonly used protecting group in the field of organic synthesis, including but not limited to: -Bn (benzyl), -PMB (p-methoxybenzyl), -Tos (p-toluenesulfonyl), -Fmoc (fluorenylmethoxycarbonyl), -Ac (acetyl), -SEM (2-(trimethylsilyl)ethoxymethyl), -Pht (phthaloyl) and -Alloc (allyloxy) Carbonyl).
进一步地, 本发明的制备方法还包括将被保护的 Q和 /或被保护的 R7去 保护的步骤, 以及任选地对去保护得到的 H原子进行烷基化(如甲基化) 的 步骤。 下方法, 反应方程式如下: Further, the preparation method of the present invention further comprises the step of deprotecting the protected Q and/or protected R 7 , and optionally alkylating (eg, methylating) the deprotected H atom. step. The next method, the reaction equation is as follows:
制备方法 1 :
Preparation method 1:
中间体 7 通式(I)化合物 Intermediate 7 Compound of formula (I)
步骤 1 中间体 1的制备 Step 1 Preparation of intermediate 1
将原料 1溶于溶剂 (例如冰醋酸) 中, 加入 40%的氢溴酸溶液, 冰水浴 下, 逐滴加入溶有适量溴素的冰醋酸溶液, 搅拌至原料消耗完毕, 加入冰水 淬灭, 有机溶剂 (例如乙酸乙酯)萃取, 合并的有机相经适当方法 (例如硅 胶柱层析)分离纯化得中间体 1。 The raw material 1 is dissolved in a solvent (for example, glacial acetic acid), and a 40% hydrobromic acid solution is added thereto. Under ice water bath, a glacial acetic acid solution in which an appropriate amount of bromine is dissolved is added dropwise, and the mixture is stirred until the raw material is consumed, and quenched by adding ice water. The organic solvent is extracted by an organic solvent (for example, ethyl acetate), and the combined organic phase is separated and purified by a suitable method (for example, silica gel column chromatography).
步骤 2 中间体 2的制备 Step 2 Preparation of intermediate 2
将中间体 1和原料 2溶于适当溶剂 (例如四氢呋喃), 加热回流至反应完 毕, 除去溶剂, 经适当方法分离得中间体 2。 The intermediate 1 and the starting material 2 are dissolved in a suitable solvent (e.g., tetrahydrofuran), and the mixture is heated to reflux until the reaction is completed, the solvent is removed, and the intermediate 2 is obtained by an appropriate method.
步骤 3 中间体 3的制备
将中间体 2和溴化铜溶于有机溶剂 (例如乙腈) 中, 冰水浴下, 逐滴加 入亚硝酸叔丁酯, 完毕后升至室温, 搅拌(例如 2 h ), 除去溶剂, 经适当方 法分离得中间体 3。 Step 3 Preparation of Intermediate 3 The intermediate 2 and the copper bromide are dissolved in an organic solvent (for example, acetonitrile), and the t-butyl nitrite is added dropwise in an ice water bath. After completion, the temperature is raised to room temperature, stirred (for example, 2 h), and the solvent is removed. Intermediate 3 was isolated.
步骤 4 中间体 4的制备 Step 4 Preparation of intermediate 4
将中间体 3、 原料 3或其氢 酸盐(如其盐酸盐)溶于适当溶剂 (例如三 氟乙酸、 醋酸), 加热至反应完毕, 冷却, 浓缩, 经适当方法分离得中间体 4。 The intermediate 3, the starting material 3 or its hydrochloride (e.g., its hydrochloride) is dissolved in a suitable solvent (e.g., trifluoroacetic acid, acetic acid), heated to completion, cooled, concentrated, and isolated to afford intermediate 4 as appropriate.
步骤 5 中间体 5的制备 Step 5 Preparation of intermediate 5
将中间体 4置于适当溶剂中, 加入适量氧化剂(例如 DDQ ( 2,3-二氯 -5,6- 二氰对苯醌)和重铬酸甲), 室温反应完毕, 饱和碳酸氢钠水溶液淬灭, 有机 溶剂 (例如二氯甲烷)萃取, 合并有机相, 用适当方法分离得中间体 5。 The intermediate 4 is placed in a suitable solvent, and an appropriate amount of an oxidizing agent (for example, DDQ (2,3-dichloro-5,6-dicyanio-p-benzoquinone) and methyl dichromate) is added, and the reaction is completed at room temperature, and a saturated aqueous solution of sodium hydrogencarbonate is added. It is quenched, extracted with an organic solvent (e.g., dichloromethane), and the organic phase is combined, and Intermediate 5 is isolated by an appropriate method.
步骤 6 中间体 6的制备 Step 6 Preparation of intermediate 6
将中间体 5和原料 4溶于适当溶剂(例如乙腈),加入碱 (例如碳酸甲), 加热反应完毕, 滤除固体, 滤液浓缩, 用适当方法分离得到中间体 6。 The intermediate 5 and the starting material 4 are dissolved in a suitable solvent (e.g., acetonitrile), and a base (e.g., methyl carbonate) is added. The reaction is completed by heating, the solid is filtered, and the filtrate is concentrated, and the intermediate 6 is isolated by an appropriate method.
步骤 7 中间体 7的制备 Step 7 Preparation of intermediate 7
将中间体 6溶于适当溶剂(二甲基亚砜和三乙胺),加入合适的氧化剂(例 如三氧化硫吡啶), 室温搅拌至反应完毕, 反应液浓缩, 经适当方法分离得到 中间体 7。 The intermediate 6 is dissolved in a suitable solvent (dimethyl sulfoxide and triethylamine), and a suitable oxidizing agent (for example, sulfur trioxide pyridine) is added thereto, and the mixture is stirred at room temperature until the reaction is completed, and the reaction liquid is concentrated, and the intermediate 7 is isolated by an appropriate method. .
或者将中间体 6溶于适当溶剂 (二氯甲烷), 加入合适的氧化剂 (例如戴 斯-马丁试剂), 室温搅拌至反应完毕, 淬灭反应 (例如用水), 经适当方法分 离得到中间体 7。 Alternatively, the intermediate 6 is dissolved in a suitable solvent (dichloromethane), and a suitable oxidizing agent (for example, Dess-Martin reagent) is added thereto, and the mixture is stirred at room temperature until the reaction is completed, and the reaction is quenched (for example, with water), and the intermediate 7 is isolated by an appropriate method. .
或者将中间体 5和原料 4'溶于溶剂 (例如 N-甲基吡咯烷酮) 中, 加入碱 (例如三乙胺), 加热 (例如至 120 °C ), 搅拌反应 (例如 2小时), 冷却 (例 如至室温), 将反应液倒入溶剂 (例如水) 中, 搅拌(例如半小时), 过滤, 干燥得中间体 7。 Alternatively, the intermediate 5 and the starting material 4' are dissolved in a solvent (for example, N-methylpyrrolidone), a base (for example, triethylamine) is added, heated (for example, to 120 ° C), and the reaction is stirred (for example, 2 hours), and cooled ( For example, to room temperature, the reaction solution is poured into a solvent (for example, water), stirred (for example, for half an hour), filtered, and dried to give Intermediate 7.
步骤 8 本发明通式 (I )化合物的制备 Step 8 Preparation of the compound of the formula (I) of the present invention
将中间体 7和原料 5溶于适当溶剂 (例如 N,N-二甲基甲酰胺)加入三氯 化铟, 室温搅拌过夜, 再加入还原剂 (例如氰基硼氢化钠), 反应完毕, 加水 淬灭, 有机溶剂 (例如乙酸乙酯)萃取, 合并有机相, 用适当方法分离纯化 得本发明通式 (I )化合物。 Dissolve intermediate 7 and starting material 5 in a suitable solvent (such as N, N-dimethylformamide), add indium trichloride, stir at room temperature overnight, then add a reducing agent (such as sodium cyanoborohydride), complete the reaction, add water The mixture is quenched, extracted with an organic solvent (e.g., ethyl acetate), and the organic phase is combined and purified by an appropriate method to obtain the compound of the formula (I) of the present invention.
制备方法 2:
Preparation method 2:
通式(I)化合物 Compound of formula (I)
步骤 1 中间体 1的制备 Step 1 Preparation of intermediate 1
将原料 1溶于溶剂 (例如冰醋酸) 中, 加入 40%的氢溴酸溶液, 冰水浴 下, 逐滴加入溶有适量溴素的冰醋酸溶液, 搅拌至原料消耗完毕, 加入冰水 淬灭, 有机溶剂 (例如乙酸乙酯)萃取, 合并的有机相经适当方法 (例如硅 胶柱层析)分离纯化得中间体 1。 The raw material 1 is dissolved in a solvent (for example, glacial acetic acid), and a 40% hydrobromic acid solution is added thereto. Under ice water bath, a glacial acetic acid solution in which an appropriate amount of bromine is dissolved is added dropwise, and the mixture is stirred until the raw material is consumed, and quenched by adding ice water. The organic solvent is extracted by an organic solvent (for example, ethyl acetate), and the combined organic phase is separated and purified by a suitable method (for example, silica gel column chromatography).
步骤 2 中间体 2的制备 Step 2 Preparation of intermediate 2
将中间体 1和原料 2溶于适当溶剂 (例如四氢呋喃), 加热回流至反应完 毕, 除去溶剂, 经适当方法分离得中间体 2。 The intermediate 1 and the starting material 2 are dissolved in a suitable solvent (e.g., tetrahydrofuran), and the mixture is heated to reflux until the reaction is completed, the solvent is removed, and the intermediate 2 is obtained by an appropriate method.
步骤 3 中间体 3的制备 Step 3 Preparation of intermediate 3
将中间体 2和溴化铜溶于有机溶剂 (例如乙腈) 中, 冰水浴下, 逐滴加 入亚硝酸叔丁酯, 完毕后升至室温, 搅拌(例如 2 h ), 除去溶剂, 经适当方 法分离得中间体 3。 The intermediate 2 and the copper bromide are dissolved in an organic solvent (for example, acetonitrile), and the t-butyl nitrite is added dropwise in an ice water bath. After completion, the temperature is raised to room temperature, stirred (for example, 2 h), and the solvent is removed. Intermediate 3 was isolated.
步骤 4 中间体 5的制备 Step 4 Preparation of intermediate 5
将中间体 3、 中间体 4或其氢 酸盐(如其盐酸盐)溶于适当溶剂(例如 三氟乙酸、 醋酸), 加热至反应完毕, 冷却, 浓缩, 经适当方法分离得中间体
步骤 5 中间体 6的制备 Intermediate 3, intermediate 4 or its hydrochloride (such as its hydrochloride) is dissolved in a suitable solvent (such as trifluoroacetic acid, acetic acid), heated to completion, cooled, concentrated, and separated by an appropriate method. Step 5 Preparation of Intermediate 6
将中间体 5置于适当溶剂中, 加入适量氧化剂 (例如 DDQ(2,3-二氯 -5,6- 二氰对苯醌) 和重铬酸钾), 室温反应完毕, 饱和碳酸氢钠水溶液淬灭, 有机 溶剂 (例如二氯甲烷)萃取, 合并有机相, 用适当方法分离得中间体 6。 步骤 6 本发明通式(I )化合物的制备 The intermediate 5 is placed in a suitable solvent, and an appropriate amount of an oxidizing agent (for example, DDQ (2,3-dichloro-5,6-dicyanio-p-benzoquinone) and potassium dichromate) is added, and the reaction is completed at room temperature, and a saturated aqueous solution of sodium hydrogencarbonate is added. It is quenched, extracted with an organic solvent (e.g., dichloromethane), and the organic phase is combined, and Intermediate 6 is isolated by an appropriate method. Step 6 Preparation of the compound of the formula (I) of the present invention
将中间体 6和中间体 7溶于适当溶剂(例如 N-甲基吡咯烷酮),加入适当 碱(例如三乙胺), 加热 (例如 120°C ), 搅拌反应 (例如 2小时), 冷却至室 温, 加水淬灭, 抽滤, 滤饼经适当方法分离得到本发明通式(I )化合物。 Dissolve intermediate 6 and intermediate 7 in a suitable solvent (eg N-methylpyrrolidone), add a suitable base (eg triethylamine), heat (eg 120 ° C), stir the reaction (eg 2 hours), cool to room temperature The mixture of water is quenched, suction filtered, and the filter cake is isolated by a suitable method to obtain the compound of the formula (I) of the present invention.
反应方程式中, A A2、 A3、 M、 Y、 X、 R3、 R5、 R6、 Q、 R7和 n如前 文所定义。 In the reaction equation, AA 2 , A 3 , M, Y, X, R 3 , R 5 , R 6 , Q, R 7 and n are as defined above.
V. 治疗方法和制药用途 V. Treatment and pharmaceutical use
本发明提供了本发明的化合物或其立体异构体、或其药学上可接受的盐、 酯或溶剂化物在制备用于治疗和 /或预防 ALK介导的癌症相关疾病的药物中 的应用。 The present invention provides the use of a compound of the present invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, for the manufacture of a medicament for the treatment and/or prevention of an ALK-mediated cancer-related disease.
本发明还提供了一种治疗和 /或预防 ALK介导的癌症相关疾病的方法, 包括给予需要其的受试者治疗有效量的本发明的化合物或其立体异构体、 或 其药学上可接受的盐、 酯或溶剂化物。 The invention also provides a method of treating and/or preventing an ALK-mediated cancer-related disease, comprising administering to a subject in need thereof a therapeutically effective amount of a compound of the invention or a stereoisomer thereof, or a pharmaceutically acceptable thereof Accepted salts, esters or solvates.
本发明还提供了本发明的化合物或其立体异构体、 或其药学上可接受的 盐、 酯或溶剂化物与一种或多种抗肿瘤剂和免疫抑制剂联合地在制备用于治 疗和 /或预防 ALK介导的癌症相关疾病的药物中的应用。 The invention also provides a compound of the invention, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, in combination with one or more antitumor agents and immunosuppressive agents, for use in therapy and / or use in drugs that prevent ALK-mediated cancer-related diseases.
本发明还提供了一种治疗和 /或预防 ALK介导的癌症相关疾病的方法, 包括与一种或多种抗肿瘤剂和免疫抑制剂联合地给予需要其的受试者治疗有 效量的本发明的化合物或其立体异构体、 或其药学上可接受的盐、 酯或溶剂 化物。 The invention also provides a method of treating and/or preventing an ALK-mediated cancer-related disease comprising administering to a subject in need thereof a therapeutically effective amount of the combination in combination with one or more anti-tumor agents and an immunosuppressant A compound of the invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof.
所述的抗肿瘤剂和免疫抑制剂选自甲氨蝶呤、 卡培他滨、 吉西他滨、 去 氧氟尿苷、 培美曲塞二钠、 帕唾帕尼、 伊马替尼、 埃罗替尼、 拉帕替尼、 吉 非替尼、 凡德他尼、 赫赛汀、 贝伐单抗、 利妥昔单抗、 曲妥珠单抗、 紫杉醇、 长春瑞滨、 多西他赛、 多柔比星、 羟基喜树碱、 丝裂霉素、 表柔比星、 吡柔 比星、 博来霉素、 来曲唑、 他莫西芬、 氟维司群、 曲谱瑞林、 氟他胺、 亮丙 瑞林、 阿那曲唑、 异环磷酰胺、 白消安、 环磷酰胺、 卡莫司汀、 尼莫司汀、
司莫司汀、 氮芥、 马法兰、 瘤可宁、 卡铂、 顺铂、 奥沙利铂、 络铂、 拓朴特 肯、 喜树碱、 拓朴替康、 依维莫司、 西罗莫斯、 特癌适、 6-巯基嘌呤、 6-硫鸟 嘌呤、 硫唑嘌呤、 菌素 D、 柔红霉素、 阿霉素、 米托蒽醌、 争光霉素、 普卡 霉素或氨鲁米特。 The antitumor agent and immunosuppressive agent are selected from the group consisting of methotrexate, capecitabine, gemcitabine, deoxyfluorouridine, pemetrexed disodium, paclitaxil, imatinib, erlotidine Nipa, lapatinib, gefitinib, vandetanib, Herceptin, bevacizumab, rituximab, trastuzumab, paclitaxel, vinorelbine, docetaxel, multiple Spirulina, hydroxycamptothecin, mitomycin, epirubicin, pirarubicin, bleomycin, letrozole, tamoxifen, fulvestrant, lysine, flutamide , leuprolide, anastrozole, ifosfamide, busulfan, cyclophosphamide, carmustine, nimustine, Semustine, nitrogen mustard, melphalan, cyclamate, carboplatin, cisplatin, oxaliplatin, platinum, topotecan, camptothecin, topotecan, everolimus, sirmo , special cancer, 6-mercaptopurine, 6-thioguanine, azathioprine, bacteriocin D, daunorubicin, doxorubicin, mitoxantrone, bleomycin, pucamycin or ammonia Mitt.
所述癌症相关的疾病选自脑瘤、 非小细胞性肺癌、 鳞状上皮细胞癌、 膀 胱癌、 胃癌、 卵巢癌、 腹膜癌、 胰腺癌、 乳腺癌、 头颈癌、 子宫颈癌、 子宫 内膜癌、 直肠癌、 肝癌、 肝母细胞瘤、 乳头状肾细胞瘤、 头颈部鳞状细胞瘤、 肾母细胞瘤、 肾癌、 食管腺癌、 食管鳞状细胞癌、 神经胶质瘤、 前列腺癌、 甲状腺癌、雌性生殖道癌、 原位癌、淋巴瘤、 成神经细胞瘤、 神经纤维瘤病、 骨癌、 皮肤癌、 结肠癌、 睾丸癌、 小细胞肺癌、 胃肠道间质瘤、 前列腺肿瘤、 肥大细胞肿瘤、 多发性骨髓瘤或黑色素瘤。 The cancer-related diseases are selected from the group consisting of brain tumors, non-small cell lung cancer, squamous cell carcinoma, bladder cancer, gastric cancer, ovarian cancer, peritoneal cancer, pancreatic cancer, breast cancer, head and neck cancer, cervical cancer, endometrium. Cancer, rectal cancer, liver cancer, hepatoblastoma, papillary renal cell tumor, head and neck squamous cell tumor, nephroblastoma, renal cancer, esophageal adenocarcinoma, esophageal squamous cell carcinoma, glioma, prostate Cancer, thyroid cancer, female genital tract cancer, carcinoma in situ, lymphoma, neuroblastoma, neurofibromatosis, bone cancer, skin cancer, colon cancer, testicular cancer, small cell lung cancer, gastrointestinal stromal tumor, Prostate tumors, mast cell tumors, multiple myeloma or melanoma.
优选地, 所述癌症相关的疾病选自淋巴瘤 (如间变性大细胞淋巴瘤) 和 肺癌 (如非小细胞肺癌)。 Preferably, the cancer-related disease is selected from the group consisting of lymphoma (e.g., anaplastic large cell lymphoma) and lung cancer (e.g., non-small cell lung cancer).
更优选地, 所述癌症相关的疾病选自肺癌 (如非小细胞肺癌)。 More preferably, the cancer-related disease is selected from the group consisting of lung cancer (e.g., non-small cell lung cancer).
VI. 本发明化合物具有以下优点: VI. The compounds of the invention have the following advantages:
( 1 )本发明式(I )化合物或其立体异构体、 或者其药学上可接受的盐、 酯或溶剂化物具有优异的 ALK抑制活性; (1) The compound of the formula (I) of the present invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, has excellent ALK inhibitory activity;
( 2 )本发明式(I )化合物或其立体异构体、 或者其药学上可接受的盐、 酯或溶剂化物显示出良好的生物稳定性, 作用更持久, 生物利用度高; (2) The compound of the formula (I) of the present invention or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof exhibits good biostability, is more durable, and has high bioavailability;
( 3 )本发明化合物制备工艺筒单, 药品纯度高, 质量稳定, 易于进行大 规模工业生产。 (3) The preparation process of the compound of the invention has a high purity and stable quality, and is easy to carry out large-scale industrial production.
VII. 以下通过体外酶学抑制活性实验和大鼠体内药代动力学实验进一步 阐述本发明化合物有益效果, 但不应将此理解为本发明化合物仅具有下列有 益效果。 VII. The beneficial effects of the compounds of the present invention are further illustrated by the in vitro enzymatic inhibitory activity assay and the in vivo pharmacokinetic assay in rats, but it should not be construed that the compounds of the present invention have only the following beneficial effects.
以下活性实验、 药代动力学实验和制备实施例中所用的原料是市售可得 的。 制备实施例中化合物的命名通过 Chemical Draw 13.0软件获得。 制备实 施例 10和 12中所示的化学结构式以及在说明书和权利要求中出现的相关化 学结构式仅意图说明化合物的顺 /反构型, 而非化合物的绝对立体构型。 The following active experiments, pharmacokinetic experiments, and starting materials used in the preparation examples are commercially available. The nomenclature of the compounds in the preparation examples was obtained by Chemical Draw 13.0 software. The chemical structural formulae shown in Preparation Examples 10 and 12 and the related chemical structural formulae appearing in the specification and claims are intended only to illustrate the cis/trans configuration of the compound, rather than the absolute stereo configuration of the compound.
实验例 1 本发明化合物的体外酶学活性实验 Experimental Example 1 In vitro enzymatic activity experiment of the compound of the present invention
供试品: 本发明化合物 1-6、 9、 10、 13、 14、 18、 24和 26 , 其化学名 称和制备方法请见各化合物的制备实施例。
对照 药 : CH5424802 , 其可 以 才艮据
Test Specimens: Compounds 1-6, 9, 10, 13, 14, 18, 24 and 26 of the present invention, the chemical names and preparation methods thereof, can be found in the preparation examples of the respective compounds. Control drug: CH5424802, it can be based on
CN102459172A中公开的方法制备。 Prepared by the method disclosed in CN102459172A.
DMSO: 二甲基亚砜( Dimethyl sulfoxide ) DMSO: Dimethyl sulfoxide
DTT: 二石克苏糖醇 ( DL-Dithiothreitol ) DTT: Diphthylitol ( DL-Dithiothreitol )
SEB: 酶催化剂緩沖溶液 ( Supplement Enzymatic buffer, available from Cisbio Bioassays, http:〃 www.htrf.com/htrf-kinease, HTRF® KinEASE™-TK 62TK0PEB ) SEB: Supplement Enzymatic buffer, available from Cisbio Bioassays, http:〃 www.htrf.com/htrf-kinease, HTRF® KinEASETM-TK 62TK0PEB
ATP: 腺嘌呤核苷三磷酸 ( Adenosine Triphosphate ) ATP: Adenosine Triphosphate
ALK: 间变' 1"生淋巴瘤激酶 ( Anaplastic Lymphoma Kinase ) ALK: Analytical '1' Lymphoma Kinase
SA-XL665:链霉亲和素标记的供体( Streptavidin-XL665, available from SA-XL665: Streptavidin-XL665, available from Streptavidin-XL665
Cisbio Bioassays, http:〃 www.htrf.com/htrf-kinease, HTRF® KinEASE™-TKCisbio Bioassays, http:〃 www.htrf.com/htrf-kinease, HTRF® KinEASETM-TK
62TK0PEB ) 62TK0PEB )
2.5 χ、 5 χ、 10 x其中的 "x": 倍 实验方法: 2.5 χ, 5 χ, 10 x of "x": times Experimental method:
ALK激酶緩沖液配制: ALK Kinase Buffer Preparation:
分别取适量母液浓度为 1000 mM的 MgCl2、 2500 nM的 SEB、 100 mM 的 DTT、 5 χ酶緩沖液, 加入到超纯水中, 使得最终浓度分别为: 5 mM、 25 nM, 1 mM、 1 χ酶緩沖液, 混匀, 待用。 Appropriate amount of 1000 mM MgCl 2 , 2500 nM SEB, 100 mM DTT, 5 chymase buffer were added to the ultrapure water, so that the final concentrations were: 5 mM, 25 nM, 1 mM, 1 χEnzyme buffer, mix, stand by.
2.5 χ供试品溶液配制: 2.5 χ Preparation of test solution:
对照药的 1 mM储备液配制: 称取对照药 1.69 mg, 加入适量 DMSO 溶解, 混勾, 备用。 Prepare the 1 mM stock solution of the reference drug: Weigh 1.69 mg of the reference drug, dissolve it with appropriate amount of DMSO, mix it, and set aside.
化合物的 I mM储备液配制: 分别称取化合物适量 (具体称样量请见下 表), 加入适量 DMSO溶解, 混匀, 备用。
化合物编号 称样量 (mg) 化合物编号 称样量 (mg) Preparation of the compound's I mM stock solution: Weigh the appropriate amount of the compound (see the table below for the specific sample amount), add the appropriate amount of DMSO to dissolve, mix, and set aside. Compound No. Weighing Quantity (mg) Compound No. Weighing Quantity (mg)
1 1.71 9 1.6 1 1.71 9 1.6
2 1.69 10 1.5 2 1.69 10 1.5
3 1.46 13 1.49 3 1.46 13 1.49
4 1.56 14 1.44 4 1.56 14 1.44
5 1.46 18 1.48 5 1.46 18 1.48
6 1.75 24 1.47 6 1.75 24 1.47
26 1.59 26 1.59
分别取 1 mM储备液, 用 DMSO稀释制成浓度为 200 μΜ的溶液, 作 为母液。 用 DMSO将上述母液三倍逐级稀释制成一系列浓度的溶液, 然后 每个浓度分别用 ALK激酶緩沖液稀释 80倍, 制成各 2.5χ供试品溶液, 浓 度分别为: 2500 ηΜ、 833.33 ηΜ、 277.78 ηΜ、 92.59 ηΜ, 30.86 ηΜ、 10.29 ηΜ、 3.43 ηΜ、 1.14 ηΜ、 0.38 ηΜ、 0.13 ηΜ、 0.04 ηΜ。 A 1 mM stock solution was taken and diluted with DMSO to prepare a solution having a concentration of 200 μM as a mother liquor. The mother liquor was diluted three times in DMSO to prepare a series of solutions, and then each concentration was diluted 80 times with ALK kinase buffer to prepare 2.5 χ each test solution, the concentrations were: 2500 ηΜ, 833.33 ΜΜ, 277.78 ηΜ, 92.59 ηΜ, 30.86 ηΜ, 10.29 ηΜ, 3.43 ηΜ, 1.14 ηΜ, 0.38 ηΜ, 0.13 ηΜ, 0.04 ηΜ.
各种其他试剂配制: Various other reagents are formulated:
用 ALK激酶緩沖液分别配制所需要的 5xALK激酶溶液、 5χ底物溶液、 5χΑΤΡ溶液, 备用。 The required 5xALK kinase solution, 5 χ substrate solution, and 5 χΑΤΡ solution were separately prepared in ALK kinase buffer and used.
ALK酶学反应: ALK enzymatic reaction:
1 ) 384孔板中相对应的孔中分别加入 4 配制好的 2.5χ供试品溶液、 2 μΐ配制好的 5xALK激酶溶液, 25 °C孵育 10分钟。 1) Add 4 prepared 2.5 χ test solutions and 2 μΐ of prepared 5xALK kinase solution to the corresponding wells in a 384-well plate and incubate at 25 °C for 10 minutes.
2 )相对应的孔中再分别加入 2 μL配制好的 5x底物溶液和 2 μΐ配制好 的 5χΑΤΡ溶液, 启动酶反应, 25 °C孵育 30分钟。 2) Add 2 μL of the prepared 5x substrate solution and 2 μL of the prepared 5χΑΤΡ solution to the corresponding wells, start the enzyme reaction, and incubate at 25 °C for 30 minutes.
酶学检测: Enzymatic testing:
用检测緩沖液 (detection buffer)配制所需浓度的 SA-XL665 ,然后和等体 积的酪氨酸激酶抗体混勾, 相对应的孔中分别加入 10 配制好的此抗体 溶液, 终止反应。 25 °C孵育 lh。 Prepare the desired concentration of SA-XL665 with detection buffer, then mix it with the equivalent tyrosine kinase antibody, and add 10 prepared antibody solution to the corresponding wells to stop the reaction. Incubate at 25 °C for 1 h.
酵标仪 665nm/615nm读板。 Yeast meter 665nm/615nm reading board.
IC50: 计算抑制率(%)= (最大值-样本比值 )/(最大值-最小值) χ ΐοο, 采 用 Graph prisim软件进行曲线拟合, 得出 IC50值。
最大值: 不加化合物的阳性对照, 最小值: 不加酶的阴性对照。 实验结果和结论: IC 50 : Calculated inhibition rate (%) = (maximum - sample ratio) / (maximum - minimum value) χ ΐοο, curve fitting using Graph prisim software, resulting in IC 50 values. Maximum: Positive control without compound, Minimum: Negative control without enzyme. Experimental results and conclusions:
表 1 : 本发明化合物的体外酶学抑制活性 Table 1: In vitro enzymatic inhibitory activity of the compounds of the invention
化合物 18 Compound 18
化合物 24 Compound 24
化合物 26 由表 1可见, 本发明化合物对 ALK激酶具有良好的抑制活性, 且与对 照药抑制活性相当, 可用于治疗与激酶相关的疾病, 特别是 ALK激酶介导 的病症或病况, 具有显著的临床意义。 实验例 2 本发明化合物的大鼠体内药代动力学实验 Compound 26 It can be seen from Table 1 that the compound of the present invention has a good inhibitory activity against ALK kinase and is comparable to the inhibitory activity of the reference drug, and can be used for the treatment of a kinase-related disease, particularly an ALK kinase-mediated disorder or condition, which is remarkable. Clinical significance. Experimental Example 2 Pharmacokinetic experiments in rats of the present invention
供试品: 本发明化合物 1和 7, 自制, 其化学名称和制备方法见各化合物 的制备实施例。 对照 药 : 5424802 , 其可 以 才艮据 Test article: The compounds of the present invention 1 and 7, were prepared by themselves, and their chemical names and preparation methods are shown in the preparation examples of the respective compounds. Control drug: 5424802, which can be used as a basis
CN102459172A 化合物 1 : CN102459172A Compound 1 :
化合物 7:Compound 7:
:试动物: 雄性 SD大鼠, 3只 /给药途径 /供试品, 体重 200-280g
供试品溶液制备: : Test animals: male SD rats, 3 / route of administration / test article, weight 200-280g Preparation of test solution:
对于对照药, 称取 3.02 mg, 加入 0.592 mL DMSO (二甲基亚砜), 50 V 水浴保温使溶解, 加入 1. 184 mL PEG400 (聚乙二醇 400 ), 涡旋混匀, 50 V 水浴保温, 再加入 4.144 mL 28% captisol (磺丁基 -β-环糊精) ρΗ=3.0 盐酸溶 液, 50 °C水浴保温 20 min,过 0.22 μιη微孔滤膜即得,作为大鼠 IV给药药液; 对于对照药,称取 3.29 mg,加入 0.1%吐温 80+2%HPC (羟丙基纤维素) 6.45 mL, 用组织研磨器 600 转 /分钟研磨充分, 混合均匀, 即得, 作为大鼠 PO给 药药液。 For the control drug, weigh 3.02 mg, add 0.592 mL DMSO (dimethyl sulfoxide), dissolve in a 50 V water bath, add 1. 184 mL PEG400 (polyethylene glycol 400), vortex and mix, 50 V water bath Insulation, add 4.144 mL of 28% captisol (sulfobutyl-β-cyclodextrin) ρΗ=3.0 hydrochloric acid solution, heat in a water bath at 50 °C for 20 min, and pass through a 0.22 μηη microporous membrane to obtain IV IV as a rat. For the reference drug, weigh 3.29 mg, add 0.1% Tween 80 + 2% HPC (hydroxypropyl cellulose) 6.45 mL, grind thoroughly with a tissue grinder 600 rpm, mix well, that is, as Rat PO administration drug solution.
对于化合物 1 , 称取 3.70 mg, 加入 DMA(N,N-二甲基乙酰胺) 0.717 mL, 超声溶解, 加入 Solutol (聚乙二醇硬脂酸酯 15) 0.717 mL, 涡旋混匀, 再加入 灭菌注射用水 5.732 mL, 涡旋混匀, 即得澄清透明溶液 [作为大鼠 IV/PO给 药药液] 。 For compound 1, weigh 3.70 mg, add DMA (N,N-dimethylacetamide) 0.717 mL, dissolve in the ultrasonic solution, add Solutol (polyethylene glycol stearate 15) 0.717 mL, vortex and mix, then Add 5.732 mL of sterile water for injection and vortex to obtain a clear transparent solution [as a rat IV/PO drug solution].
对于化合物 7 , 称取 2.48 mg, 加入 DMF ( N,N-二甲基甲酰胺) 482 L, 超声溶解, 加入 PEG400 482 L涡旋混匀, 加灭菌注射用水 1.447 mL, 涡旋 混匀即得澄清透明溶液, 此溶液作为大鼠 IV给药药液; 对于化合物 7, 称取 6.29 mg, 加入 0.1% 吐温 80+2% HPC 6.116 mL 置于组织研磨器中, 1000 转 /分钟的转速, 分散均匀即得, 作为大鼠 PO给药药液。 For compound 7, weigh 2.48 mg, add DMF (N,N-dimethylformamide) 482 L, dissolve in ultrasound, add PEG400 482 L vortex to mix, add 1.474 mL of sterile water for injection, vortex and mix Clear solution is clear, this solution is used as a rat IV drug solution; for compound 7, 6.29 mg is weighed, 0.1% Tween 80+2% HPC 6.116 mL is added to the tissue grinder, 1000 rpm , Disperse evenly, as a rat PO drug solution.
28% captisol pH=3.0 盐酸溶液的配制方法: 首先配制 pH3.0盐酸溶液, 然后称取 28g captisol,緩慢加入 80 mLpH3.0盐酸溶液,超声溶解,涡旋混匀, 继续加入 pH3.0盐酸溶液定容至终体积为 100 mL, 涡旋混勾即得澄清透明溶 液。 28% captisol pH=3.0 Hydrochloric acid solution preparation method: Firstly prepare pH3.0 hydrochloric acid solution, then weigh 28g captisol, slowly add 80mL pH3.0 hydrochloric acid solution, sonicate, vortex and mix, continue to add pH3.0 hydrochloric acid solution The volume is up to 100 mL, and the vortex is clarified to clear the clear solution.
0.1% 吐温 80+2%HPC 的配制方法: 称取 HPC 2 g, 緩慢加入到 80 mL 超纯水中, 持续搅拌充分溶解, 加入 0. 1 mL吐温 80 , 涡旋混匀, 继续加入超 纯水定容至终体积为 100 mL, 涡旋混匀, 即得。 Preparation method of 0.1% Tween 80+2% HPC: Weigh 2 g of HPC, slowly add to 80 mL of ultrapure water, stir thoroughly and dissolve thoroughly, add 0.1 mL of Tween 80, vortex and mix, continue to join The ultra-pure water is made up to a final volume of 100 mL, and vortexed and mixed.
28% HP-P-CD的配制方法: 称取 ΗΡ-β-CD 28 g, 加入 80 mL灭菌注射用 水, 超声溶解, 涡旋混匀, 继续加入灭菌注射用水定容至终体积为 100 mL , 涡旋混匀即得澄清透明溶液。 Preparation method of 28% HP-P-CD: Weigh ΗΡ-β-CD 28 g, add 80 mL of sterile water for injection, dissolve in ultrasound, vortex and mix, continue to add sterile water for injection to a final volume of 100 In mL, vortex and mix to clear the clear solution.
实验方法 experimental method
给药: Dosing:
将供试品药液按照下表方法进行给药:
实验 The test solution is administered according to the following method: experiment
给药剂量 给药浓度 给药体积 供试品 动物 给药途径 Dosing dose Dosing concentration Dosing volume Test article Animal Administration route
mg/kg mg/mL mL/kg 例数 Mg/kg mg/mL mL/kg
3 尾静脉推注 ( IV ) 1 0.5 2 对照药 3 tail vein bolus ( IV ) 1 0.5 2 comparator
3 灌胃 (PO ) 2 0.5 4 3 gavage (PO ) 2 0.5 4
3 尾静脉推注 ( IV ) 1 0.5 2 化合物 1 3 tail vein bolus ( IV ) 1 0.5 2 compound 1
3 灌胃 (PO ) 2 0.5 4 3 gavage (PO ) 2 0.5 4
3 尾静脉推注 ( IV ) 2 1 2 化合物 7 3 tail vein bolus ( IV ) 2 1 2 compound 7
3 灌胃 (PO ) 5 1 5 采血: 3 gavage (PO) 5 1 5 blood collection:
对于对照药, IV给药后 0.083、 0.25、 0.5、 1、 2、 4、 6、 8、 24、 30、 48 h, PO给药后 0.167、 0.5、 1、 2、 4、 6、 8、 24、 30、 48 h进行尾静脉采血; 对于化合物 1 , IV给药后 0.083、 0.25、 0.5、 1、 2、 4、 6、 8、 24 h, PO 给药后 0.167、 0.5、 1、 2、 4、 6、 8、 24 h进行尾静脉采血; For the comparator, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 24, 30, 48 h after IV administration, 0.167, 0.5, 1, 2, 4, 6, 8, 24 after PO administration 30, 48 h for tail vein blood collection; for compound 1, IV after administration 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 24 h, PO after administration 0.167, 0.5, 1, 2, 4 Blood was collected from the tail vein at 6, 8 and 24 h;
对于化合物 7, IV给药后 0.083、 0.25、 0.5、 1、 2、 4、 6、 8、 24、 30 h, PO给药后 0.167、 0.5、 1、 2、 4、 6、 8、 24、 30 h进行尾静脉采血, For Compound 7, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 24, 30 h after IV administration, 0.167, 0.5, 1, 2, 4, 6, 8, 24, 30 after PO administration h for tail vein blood collection,
每个时间点采取 100 μΐ左右全血,高速离心机中 8000 转 /分钟离心 6 min 分离血浆,血浆于 -80°C冰箱冻存。血浆必需在血液采集后的 30分钟内制备。 At each time point, about 100 μΐ whole blood was taken, and the plasma was separated by centrifugation at 8000 rpm for 6 min in a high-speed centrifuge, and the plasma was frozen in a refrigerator at -80 °C. Plasma must be prepared within 30 minutes of blood collection.
血浆样品分析 Plasma sample analysis
对于对照药, 血浆样品采用液液萃取法: 取 20 L血浆, 加入 600 含 内标( BEZ-235 ) 的 MTBE (叔丁基甲醚)的溶液( 10 ng/mL ), 1500转 /分钟涡 旋 10 min, 然后 12000转 /分钟离心 5 min,取上清液 300 μΐ,在氮气下吹干, 用 300 乙腈:水(7:3 , V/V ) 复溶, 涡旋混匀, LC-MS/MS分析。 For the control drug, the plasma sample was taken by liquid-liquid extraction method: 20 L of plasma was added, and a solution of 600 BEBE (tert-butyl methyl ether) containing internal standard (BEZ-235) (10 ng/mL) was added, and vortex was 1500 rpm. Min, then centrifuge at 12000 rpm for 5 min, take the supernatant 300 μΐ, blow dry under nitrogen, reconstitute with 300 acetonitrile: water (7:3, V/V), vortex and mix, LC-MS/ MS analysis.
对于化合物 1 , 血浆样品采用蛋白沉淀法: 取 20 血浆, 加入 200 μL 内标( CH5424802 ) 的甲醇溶液( 50 ng/mL ), 1500转 /分钟涡旋 5 min, 然后 12000转 /分钟离心 5 min, 取上清液 100 μΐ^, 再加入 100 μΐ水, 涡旋混匀, LC-MS/MS分析。 For compound 1, plasma samples were taken by protein precipitation: 20 plasma, 200 μL of internal standard (CH5424802) in methanol (50 ng/mL), vortexed at 1500 rpm for 5 min, then centrifuged at 12,000 rpm for 5 min. Take the supernatant 100 μΐ^, add 100 μM water, vortex and mix, and analyze by LC-MS/MS.
对于化合物 7, 血浆样品采用蛋白沉淀法: 取 30 血浆, 加入 200 μL 内标( CH5424802 ) 的乙腈溶液, 1500转 /分钟涡旋, 然后 4000转 /分钟离心 20 min。 离心后, 取上清液 100 L, 再加入 100 水, 涡旋混匀, LC-MS/MS
分析。 For compound 7, plasma samples were taken by protein precipitation: 30 plasma was added, 200 μL of internal standard (CH5424802) in acetonitrile was added, vortexed at 1500 rpm, and then centrifuged at 4000 rpm for 20 min. After centrifugation, take 100 L of the supernatant, add 100 more water, vortex and mix, LC-MS/MS analysis.
表 2.1 : 大鼠 PK评价结果(IV ) Table 2.1: Rat PK evaluation results (IV)
供试品 剂量 (mg/kg) AUClast(h*ng/mL) CL (L/h/kg) 对照药 1 1543 0.59 化合物 1 1 1431 0.69 化合物 7 2 7715 0.26 表 2.2大鼠 ΡΚ评价结果( ΡΟ ) Test sample dose (mg/kg) AUC last (h*ng/mL) CL (L/h/kg) Control drug 1 1543 0.59 Compound 1 1 1431 0.69 Compound 7 2 7715 0.26 Table 2.2 Rat ΡΚ evaluation results ( ΡΟ )
供试品 剂量 (mg/kg) AUClast(h*ng/mL) F (%) 对照药 2 1936 59.6 化合物 1 2 2053 72.2 化合物 7 5 9977 51.8Test sample dose (mg/kg) AUC last (h*ng/mL) F (%) Control drug 2 1936 59.6 Compound 1 2 2053 72.2 Compound 7 5 9977 51.8
AUClast: 代表药时曲线下面积 0→t AUC last : represents the area under the curve of the medicine 0→t
CL: 代表清除率 CL: represents the clearance rate
F%: 代表绝对生物利用度 F%: represents absolute bioavailability
由表 2.1和表 2.2的实验结果可知, 与对照药相比, 本发明化合物药代动 力学性质良好。 From the experimental results of Table 2.1 and Table 2.2, it is understood that the pharmacokinetic properties of the compound of the present invention are good as compared with the control drug.
VIII.合成实施例 VIII. Synthesis Example
以下通过实施例形式的具体实施方式, 对本发明的上述内容作进一步的 详细说明。 但不应将此理解为本发明上述主题的范围仅限于以下实施例。 凡 基于本发明上述内容所实现的技术均属于本发明的范围。 The above content of the present invention will be further described in detail below by way of specific embodiments in the form of embodiments. However, the scope of the above-mentioned subject matter of the present invention should not be construed as being limited to the following examples. The techniques implemented based on the above aspects of the present invention are all within the scope of the present invention.
下述缩写所代表的定义如下: The definitions represented by the following abbreviations are as follows:
DMF: N,N-二甲基甲酰胺 DMF: N,N-dimethylformamide
NBS: N-溴代丁二酰亚胺 NBS: N-bromosuccinimide
TFA: 三氟乙酸 TFA: trifluoroacetic acid
Cbz-Cl: 氯甲酸苄酯 Cbz-Cl: benzyl chloroformate
THF: 四氢呋喃 THF: tetrahydrofuran
DDQ: 2,3-二氯 -5,6-二氰对苯醌 DDQ: 2,3-dichloro-5,6-dicyanide p-benzoquinone
Boc: 叔丁氧基羰基 Boc: tert-butoxycarbonyl
Cbz: 苄氧基羰基 实施例 1 Cbz: benzyloxycarbonyl Example 1
2-(4-(3-氧杂 -8-氮杂双环【3.2.11辛烷 -8-基)哌啶 -1-基) -4,4-二甲基 -10-氧代
-5,1 腈 (化合物 1)的制备
2-(4-(3-oxa-8-azabicyclo[3.2.11 octane-8-yl)piperidin-1-yl)-4,4-dimethyl-10-oxo -5,1 Preparation of nitrile (Compound 1)
将 2-甲基 -1,3-环己二酮(10.1 g, 80 mmol), 碘甲烷 (12.5 mL, 200 mmol )、 碳酸甲 (22.1 g, 160 mmol)溶于丙酮 (60 mL)中, 加热回流 8 小时, 冷却, 旋转 蒸发除去溶剂, 然后加入水 (200 mL), 用二氯甲烷萃取 (500 mL), 取有机层用 无水 酸钠干燥,旋转蒸发除去溶剂,冷却至 0°C ,得产物 (7.7 g, 产率 69%)。 2-Methyl-1,3-cyclohexanedione (10.1 g, 80 mmol), methyl iodide (12.5 mL, 200 mmol), and methyl carbonate (22.1 g, 160 mmol) were dissolved in acetone (60 mL). The mixture was heated to reflux for 8 hrs, then evaporated, evaporated, evaporated, evaporated, evaporated, evaporated, evaporated, evaporated. The product was obtained (7.7 g, yield 69%).
2 ) 4-溴 -2,2- 1,3-环己二酮的制备 2) Preparation of 4-bromo-2,2-1,3-cyclohexanedione
将 2,2-二甲基 -1,3-环己二酮 (6.4 g, 45.7 mmol)溶于冰醋酸 (100 mL)中, 加 入 40%的溴化氢溶液 (0.4 mL),冰水浴冷却,逐滴加入溶有溴素 (6.9 g, 43 mmol) 的冰醋酸 (25 mL)溶液, 搅拌 30分钟, 升至室温, 搅拌 2 小时, 减压除去溶 剂, 加入冰水 (100 mL), 乙酸乙酯萃取 (500 mL), 合并的有机相经饱和碳酸氢 钠溶液洗, 水洗, 减压浓缩得粗品 (6.6 g), 未经纯化直接用于下一步反应。 2,2-Dimethyl-1,3-cyclohexanedione (6.4 g, 45.7 mmol) was dissolved in glacial acetic acid (100 mL), 40% hydrogen bromide solution (0.4 mL) was added and cooled in ice water bath , a solution of bromine (6.9 g, 43 mmol) in glacial acetic acid (25 mL) was added dropwise, stirred for 30 min, warmed to room temperature, stirred for 2 h, solvent was evaporated under reduced pressure, and ice water (100 mL), acetic acid Ethyl acetate (500 mL), EtOAc (EtOAc)EtOAc.
( 3 ) 2-氨基 -4,4-二甲基 -6,7-二氢苯并 M噻唑 -5(4//)-酮的制备 (3) Preparation of 2-amino-4,4-dimethyl-6,7-dihydrobenzo Mthiazole-5(4//)-one
将 4-溴 -2,2-二甲基 -1,3-环己二酮 (4.4 g, 20 mmol), 硫脲 (1.83 g, 24 mmol) 溶于四氢呋喃 (50 mL)中, 加热回流 4 小时, 旋转蒸发除去溶剂, 剩余物用硅 胶柱层析 (二氯甲烷: 甲醇 = 30: 1)分离纯化得产物 (1.2 g)。 4-Bromo-2,2-dimethyl-1,3-cyclohexanedione (4.4 g, 20 mmol), thiourea (1.83 g, 24 mmol) in tetrahydrofuran (50 mL), heated to reflux 4 The solvent was removed by rotary evaporation, and the residue was purified mjjjjjjj
( 4 ) 2-溴 -4,4-二甲基 -6,7-二氢苯并 [ί ]噻唑 -5(4/7)-酮的制备
(4) Preparation of 2-bromo-4,4-dimethyl-6,7-dihydrobenzo[ί]thiazol-5(4/7)-one
将 2-氨基 -4,4-二甲基 -6,7-二氢苯并 W噻唑 -5(4/7)-酮(1.55 g, 7.9 mmol)、溴 化铜 (2.1 g, 9.4 mmol)溶于乙腈 (50 mL)中, 冷却至 0°C , 逐滴加入亚硝酸叔丁 酯 (1.22 g, 11.8mmol), 滴加完毕后升至室温, 搅拌 2 小时, 旋转蒸发除去溶 剂,剩余物用硅胶柱层析 (石油醚: 乙酸乙酯 = 5: 1)分离纯化得产物 (1.58 g, 产 率 77%)。 2-Amino-4,4-dimethyl-6,7-dihydrobenzothiazol-5(4/7)-one (1.55 g, 7.9 mmol), copper bromide (2.1 g, 9.4 mmol) Dissolve in acetonitrile (50 mL), cool to 0 ° C, add tert-butyl nitrite (1.22 g, 11.8 mmol) dropwise. After the addition is completed, warm to room temperature, stir for 2 hours, remove solvent by rotary evaporation, residue The product was isolated and purified (yield: EtOAc (EtOAc:EtOAc)
]咔唑 -7-甲腈的制备 Preparation of carbazole-7-carbonitrile
将 2-溴 -4,4-二甲基 -6,7-二氢苯并 W噻唑 -5(4/7)-酮(1.56 g, 6 mmol)、3-肼基 苯甲腈 (1.6 g, 12 mmol)溶于三氟乙酸 (20 mL)中,加热至 100°C ,搅拌 6 小时, 冷却, 旋转蒸发除去溶剂, 緩慢加入饱和碳酸氢钠溶液 (60 mL), 用乙酸乙酯 萃取,无水^ L酸钠干燥,旋转蒸发除去溶剂,剩余物用硅胶柱层析 (二氯甲烷: 甲醇 = 30: 1)分离纯化得产物 (0.73 g, 产率 34%)。 2-Bromo-4,4-dimethyl-6,7-dihydrobenzothiazol-5(4/7)-one (1.56 g, 6 mmol), 3-mercaptobenzonitrile (1.6 g , 12 mmol) was dissolved in trifluoroacetic acid (20 mL), heated to 100 ° C, stirred for 6 h, cooled, evaporated, evaporated, evaporated. The organic layer was dried over anhydrous EtOAc (EtOAc) (EtOAc).
( 6 ) 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈的 制备
(6) Preparation of 2-bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile
将 2-溴 -4,4-二甲基 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (537 mg, 1.5 mmol)溶于四氢呋喃 (5 mL)中,冷却至 0°C ,加入 2,3-二氯 -5,6-二氰对苯醌 (408 mg, 1.8 mmol)搅拌 1.5 小时, 然后升至 25°C , 再搅拌 2 小时, 加入 10%氢氧 化钠溶液 (25 mL), 用乙酸乙酯萃取, 取有机层用饱和氯化钠溶液洗, 无水石克 酸钠干燥, 旋转蒸发除去溶剂, 剩余物用硅胶柱层析 (二氯甲烷: 甲醇 =20: 1) 分离纯化得产物 (273 mg, 产率 49%)。 Dissolving 2-bromo-4,4-dimethyl-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (537 mg, 1.5 mmol) in tetrahydrofuran (5 mL), cool to 0 ° C, add 2,3-dichloro-5,6-dicyan-p-benzoquinone (408 mg, 1.8 mmol) for 1.5 hours, then rise to 25 ° C, then stir 2 After 10 hours, 10% sodium hydroxide solution (25 mL) was added, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. (Dichloromethane: methanol = 20: 1) The product was isolated (293 mg, yield 49%).
( 7 )2-(4-羟基哌啶 -1-基) -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6] 咔唑 -7-甲腈的制备
(7) 2-(4-Hydroxypiperidin-1-yl)-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6] Preparation of carbazole-7-carbonitrile
将 2-溴 -4,4-甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (186 mg; 0.5 mmol), 4-羟基哌啶 (152 mg, 1.5 mmol)溶于乙腈(10 mL)中, 加热至 90 °C , 反应 2 h, 旋转蒸发除去溶剂, 剩余物用硅胶柱层析 (二氯甲烷: 甲醇 =30: 1) 分离纯化得产物 (151 mg, 产率 77%)。 2-Bromo-4,4-methyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (186 mg ; 0.5 mmol The 4-hydroxypiperidine (152 mg, 1.5 mmol) was dissolved in acetonitrile (10 mL), heated to 90 ° C, EtOAc (EtOAc) Methanol = 30: 1) The product was isolated and purified (151 mg, yield 77%).
( 8 )4,4-二甲基 -10-氧代 -2-(4-氧代哌啶 -1-基) -5,10-二氢 -4/7-噻唑并 [4,5-6] 咔唑
(8) 4,4-Dimethyl-10-oxo-2-(4-oxopiperidin-1-yl)-5,10-dihydro-4/7-thiazolo[4,5-6 Carbazole
将 2-(4-羟基哌啶 -1-基) -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔 唾 -7-甲腈 (137 mg, 0.35 mmol)溶于二氯甲烷 (10 mL)中, 加入戴斯-马丁试剂 (297 mg, 0.7 mmol), 室温下反应 6 h, 加入水 (10 mL), 再用乙酸乙酯 (50 mL) 萃取, 旋转蒸发除去溶剂, 剩余物用硅胶柱层析 (二氯甲烷: 甲醇 =20: 1)分离 纯化得产物(122 mg, 产率 89%)。 2-(4-Hydroxypiperidin-1-yl)-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]pyrene -7-carbonitrile (137 mg, 0.35 mmol) was dissolved in dichloromethane (10 mL). EtOAc (EtOAc) The mixture was extracted with EtOAc (EtOAc) (EtOAc)EtOAc.
( 9 ) 2-(4-(3-氧金 -8-氮杂双环 [3.2.1]辛烷 -8-基)哌啶 -1-基) -4,4-二甲基 -10- 氧代 - -二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈的制备 (9) 2-(4-(3-Oxogold-8-azabicyclo[3.2.1]octane-8-yl)piperidin-1-yl)-4,4-dimethyl-10-oxo Preparation of di-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile
将 4,4-二甲基 -10-氧代 -2-(4-氧代哌啶 -1-基) -5,10-二氢 -4/7-噻唑并 [4,5-6]咔 唑 -7-甲腈(117 mg, 0.3 mmol), 3-氧杂 -8-氮杂双环 [3.2.1]辛烷盐酸盐 (89 mg, 0.6 mmol)溶于 N, N-二甲基甲酰胺(10 mL)中,加入三氯化铟( 66 mg, 0.3 mmol ), 室温下搅拌过夜, 加入氰基硼氢化钠 (37 mg, 0.6 mmol), 室温搅拌 2 h, 加入 水 (10 mL)淬灭, 用乙酸乙酯 (50 mL)萃取, 旋转蒸发除去溶剂, 剩余物用硅胶 柱层析 (二氯甲烷: 甲醇 =20: 1)分离纯化得终产物 (103 mg, 产率 70%)。 4,4-Dimethyl-10-oxo-2-(4-oxopiperidin-1-yl)-5,10-dihydro-4/7-thiazolo[4,5-6]indole Zylo-7-carbonitrile (117 mg, 0.3 mmol), 3-oxa-8-azabicyclo[3.2.1]octane hydrochloride (89 mg, 0.6 mmol) dissolved in N, N-dimethyl Add indium trichloride (66 mg, 0.3 mmol) to formamide (10 mL), stir at room temperature overnight, add sodium cyanoborohydride (37 mg, 0.6 mmol), stir at room temperature for 2 h, add water (10 mL) The mixture was extracted with EtOAc (EtOAc) (EtOAc) ).
分子式: C27H29N502S; 分子量: 487.62; LC-MS(m/z): 488.0 [M+H]+ ¾-NMR (400MHz, DMSO-^) δ: 12.74 (s, 1H), 8.20-8.18 (d, 1H, J = 8.4 Hz), 7.99 (s, 1 H), 7.56-7.53 (m, 1H), 4.12-4.04 (m, 2H), 3.77-3.60 (m, 2H), 3.58-3.45 (m, 4H), 3.32-3.24 (m, 3H), 2.00-1.74 (m, 6H), 1.66 (s, 6H), 1.53-1.36
(m, 2 H). Molecular formula: C 27 H 29 N 5 0 2 S; Molecular weight: 487.62; LC-MS (m/z): 488.0 [M+H] + 3⁄4-NMR (400MHz, DMSO-^) δ: 12.74 (s, 1H) , 8.20-8.18 (d, 1H, J = 8.4 Hz), 7.99 (s, 1 H), 7.56-7.53 (m, 1H), 4.12-4.04 (m, 2H), 3.77-3.60 (m, 2H), 3.58-3.45 (m, 4H), 3.32-3.24 (m, 3H), 2.00-1.74 (m, 6H), 1.66 (s, 6H), 1.53-1.36 (m, 2 H).
实施例 2 Example 2
2-(4-(8-氧杂 -3-氮杂双环【3.2.11辛烷 -3-基)哌啶 -1-基) -4,4-二甲基 -10-氧代 2-(4-(8-oxa-3-azabicyclo[3.2.11 oct-3-yl)piperidin-1-yl)-4,4-dimethyl-10-oxo
(1) 2-(4-羟基哌啶 -1-基) -4,4- 甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6] 咔唑
(1) 2-(4-Hydroxypiperidin-1-yl)-4,4-methyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6] fluorene Azole
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (260 m g, 0.70 mmol)、 4-羟基哌啶 (76 mg, 0.75 mmol)溶于乙腈 (40 mL)中,加入碳酸 钾 (138 mg, 1 mmol), 加热至 90°C搅拌反应 14小时, 冷却至室温, 旋转蒸发 除去溶剂,所得粗品用硅胶柱层析 (二氯甲烷:甲醇 =20: 1)纯化得产物 (240 mg, 产率 87%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (260 mg, 0.70) Methyl), 4-hydroxypiperidine (76 mg, 0.75 mmol) dissolved in acetonitrile (40 mL), added potassium carbonate (138 mg, 1 mmol), heated to 90 ° C, stirred for 14 hours, cooled to room temperature, rotating The solvent was evaporated, and the obtained crude material wasjjjjjjjj
(2) 4,4-二甲基 -10-氧代 -2-(4-氧代哌啶 -1-基) -5,10-二氢 -4/7-噻唑并 [4,5-6] 咔唑 (2) 4,4-Dimethyl-10-oxo-2-(4-oxopiperidin-1-yl)-5,10-dihydro-4/7-thiazolo[4,5-6 Carbazole
将 2-(4-羟基哌啶 -1-基) -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔 唑 _7-甲腈 (240 mg, 0.61 mmol)溶于二甲基亚砜 (3 mL )和三乙胺 (2 mL )中, 搅 拌下加入三氧化硫吡啶 (500 mg, 3.14 mmol ),加毕室温搅拌反应 2小时。将反 应液用硅胶柱层析纯化 (二氯甲烷:甲醇 =20: 1 )得标题化合物 (200 mg, 产率 84%)。 2-(4-Hydroxypiperidin-1-yl)-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole _7-carbonitrile (240 mg, 0.61 mmol) was dissolved in dimethyl sulfoxide (3 mL) and triethylamine (2 mL), and sulphur trioxide pyridine (500 mg, 3.14 mmol) was added with stirring. The reaction was stirred at room temperature for 2 hours. The reaction mixture was purified to mjjjjjjjjli
(3) 2-(4-(8-氧杂 -3-氮杂欢环 [3.2.1]辛烷 -3-基)哌啶 -1-基) -4,4-二甲基 -10-氧 代 -5, -7-甲腈的制备
(3) 2-(4-(8-oxa-3-azacyclo[3.2.1]octane-3-yl)piperidin-1-yl)-4,4-dimethyl-10- Preparation of oxo-5, -7-carbonitrile
将 4,4-二甲基 -10-氧代 -2-(4-氧代哌啶 -1-基) -5,10-二氢 -4/7-噻唑并 [4,5-6]咔
唑 -7_甲腈 (200 mg, 0.51 mmol)和 8-氧杂 -3-氮杂双环 [3.2.1]辛烷盐酸盐(100 mg, 0.67 mmol)溶于 N,N-二甲基甲酰胺 (5 mL)中,加入三氯化铟 (221 mg, 1 mmol), 室温搅拌 16小时, 加入氰基硼氢化钠 (30 mg, 0.48 mmol), 继续室温搅拌反应 2小时。 将反应液用水稀释, 加入乙酸乙酯 (20 mLx5)萃取, 合并有机相, 用 无水硫酸钠干燥, 过滤, 旋转蒸发除去溶剂, 所得粗品用硅胶制备板纯化 (二 氯甲烷:甲醇 =10: 1)得终产物(103 mg, 产率 41%)。 4,4-Dimethyl-10-oxo-2-(4-oxopiperidin-1-yl)-5,10-dihydro-4/7-thiazolo[4,5-6]indole Azole-7-carbonitrile (200 mg, 0.51 mmol) and 8-oxa-3-azabicyclo[3.2.1]octane hydrochloride (100 mg, 0.67 mmol) in N,N-dimethyl To the formic acid (5 mL), indium trichloride (221 mg, 1 mmol) was added, and the mixture was stirred at room temperature for 16 hours, sodium cyanoborohydride (30 mg, 0.48 mmol) was added, and the mixture was stirred at room temperature for 2 hours. The reaction mixture was diluted with water, EtOAc (EtOAc (EtOAc) 1) The final product (103 mg, yield 41%).
分子式: C27H29N502S; 分子量: 487.62; LC-MS(m/z): 488.3[M+H]+ iH-NMR (400MHz, DMSO-^) δ: 13.24 (s, 1H), 8.18 (d, 1H, J = 8.4Hz), 7.97 (s, 1H), 7.53 (d, 1H, J = 8.4 Hz), 4.09-4.21 (m, 2H), 3.91-.3.93 (m, 2H), 3.62-3.24 (m, 6H), 2.57-2.59 (m, 1H), 1.98-1.66 (m, 12H), 1.55-1.35 (2H, m). Molecular formula: C 27 H 29 N 5 0 2 S; Molecular weight: 487.62; LC-MS (m/z): 488.3 [M+H] + iH-NMR (400 MHz, DMSO-^) δ: 13.24 (s, 1H) , 8.18 (d, 1H, J = 8.4Hz), 7.97 (s, 1H), 7.53 (d, 1H, J = 8.4 Hz), 4.09-4.21 (m, 2H), 3.91-.3.93 (m, 2H) , 3.62-3.24 (m, 6H), 2.57-2.59 (m, 1H), 1.98-1.66 (m, 12H), 1.55-1.35 (2H, m).
实施例 3 Example 3
4,4-二甲基 -2-(8-吗啉代 -3-氮杂双环【3.2.11辛烷 -3-基) -10-氧代 -5,10-二氢 - g- )的制备 4,4-Dimethyl-2-(8-morpholino-3-azabicyclo[3.2.11 oct-3-yl)-10-oxo-5,10-dihydro-g-) Preparation
将 8-氧代 -3-氮杂双环 [3.2.1]辛烷 -3-甲酸叔丁基酯 (20.0 mg, 0.89 mmol)和 吗啉 (100 mg, 1.15 mmol)溶于乙腈(10 mL)中, 加入 3滴乙酸, 室温搅拌 16 小 时, 再加入三乙酰氧基硼氢化钠 (380 mg, 1.79 mmol), 继续室温搅拌反应 2小 时。 将反应液用水稀释, 加入乙酸乙酯(10 mLx3)萃取, 合并有机相, 用无水 硫酸钠干燥, 过滤, 旋转蒸发除去溶剂, 所得粗品用硅胶柱层析板纯化 (石油 醚:乙酸乙酯 =1 : 1)得产物 (210 mg, 产率 80%)。 8-Oxo-3-azabicyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester (20.0 mg, 0.89 mmol) and morpholine (100 mg, 1.15 mmol) in acetonitrile (10 mL) 3 drops of acetic acid were added, and the mixture was stirred at room temperature for 16 hours, and then sodium triacetoxyborohydride (380 mg, 1.79 mmol) was added, and the mixture was stirred at room temperature for 2 hours. The reaction mixture was diluted with EtOAc (EtOAc) (EtOAc) =1 : 1) The product was obtained (210 mg, yield 80%).
(2) 4-(3-氮杂又环 [3.2.1]辛烷 -8基)吗啉的制备 (2) Preparation of 4-(3-azacyclo-[3.2.1]octane-8-yl)morpholine
Boc-N Γ )-N O HN Γ )-N O Boc-N Γ )-N O HN Γ )-N O
将 8-吗啉代 -3-氮杂双环 [3.2.1]辛烷 -3-甲酸叔丁基酯 (210 mg, 0.71 mmol) 溶于二氯甲烷 (5 mL)中, 搅拌下加入三氟乙酸 (3 mL), 加入完毕室温搅拌反应 16小时。 将反应液旋转蒸发除去溶剂得产物(130 mg, 产率 93%)。 Dissolve 8-morpholino-3-azabicyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester (210 mg, 0.71 mmol) in dichloromethane (5 mL). Acetic acid (3 mL) was stirred at room temperature for 16 hours. The reaction solution was evaporated to dryness to give crystals (yield: 130 mg, yield 93%).
(3) 4,4-二甲基 -2-(8-吗啉代 -3-氮杂又环 [3.2.1]辛烷 -3-基) -10-氧代 _5,10-二 氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈的制备
(3) 4,4-dimethyl-2- (8-morpholino-3-aza and bicyclo [3.2.1] octan-3-yl) _ 10-oxo-5, 10-dihydro- Preparation of -4/7-thiazolo[4,5-6]carbazole-7-carbonitrile
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和 4-(3-氮杂双环 [3.2.1]辛烷 -8基)吗啉(130 mg, 0.66 mmol)溶 于 N-甲基吡咯烷酮(10 mL)中,加入三乙胺 (200 mg, 1.98 mmol),加热至 120 °C 搅拌反应 2小时, 冷却至室温, 将反应液倒入水中, 搅拌半小时, 过滤, 所 得滤饼干燥, 再用硅胶制备板纯化得终产物 (130 mg, 产率 40%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and 4-(3-azabicyclo[3.2.1]octane-8yl)morpholine (130 mg, 0.66 mmol) were dissolved in N-methylpyrrolidone (10 mL). Mg, 1.98 mmol), heated to 120 ° C. Stirring for 2 hours, cooling to room temperature, pouring the reaction into water, stirring for half an hour, filtering, drying the filter cake, and purifying with silica gel to obtain the final product (130 mg , yield 40%).
分子式: C27H29N502S; 分子量: 487.62; LC-MS(m/z): 488.3[M+H]+ Molecular formula: C 27 H 29 N 5 0 2 S; Molecular weight: 487.62; LC-MS (m/z): 488.3 [M+H] +
¾-NMR (400MHz, DMSO-^) δ: 13.12 (brs, 1H), 8.16 (d, 1H, J = 8.0Hz), 7.95 (s, 1H), 7.49 (d, 1H, J = 8.0Hz,), 3.46-3.73 (m, 10H), 2.42-2.49(m, 4H), 2.21-2.24 (m, 1H), 1.76-1.79 (m, 2H), 1.66-1.70 (m, 6 H), 1.53 -1.57 (m, 2H). 3⁄4-NMR (400MHz, DMSO-^) δ: 13.12 (brs, 1H), 8.16 (d, 1H, J = 8.0Hz), 7.95 (s, 1H), 7.49 (d, 1H, J = 8.0Hz,) , 3.46-3.73 (m, 10H), 2.42-2.49 (m, 4H), 2.21-2.24 (m, 1H), 1.76-1.79 (m, 2H), 1.66-1.70 (m, 6 H), 1.53 -1.57 (m, 2H).
实施例 4 Example 4
4,4-二甲基 -2-(3-吗啉代 -8-氮杂双环【3.2.11辛烷 -8-基) -10-氧代 -5,10-二氢 - g- 4)的制备
4,4-Dimethyl-2-(3-morpholino-8-azabicyclo[3.2.11octane-8-yl)-10-oxo-5,10-dihydro-g-4) Preparation
4,4-二甲基 -10-氧代 -2-(3-氧代 -8-氮杂又环 [3.2.1]辛烷 -8-基) -5,10-二氢 -4/7- 4,4-Dimethyl-10-oxo-2-(3-oxo-8-azabicyclo[3.2.1]octane-8-yl)-5,10-dihydro-4/7 -
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol), 8-氮杂双环 [3.2.1]辛烷 -3-酮 (150 mg, 1.2 mmol )溶于 N-甲基 吡咯烷酮(10 mL)中, 加入三乙胺 (150 mg,l .5 mmol), 加热至 120°C搅拌反应 2 小时, 冷却至室温, 将反应液倒入水中, 搅拌半小时, 过滤, 所得滤饼干 燥得产物 (210 mg, 产率 75%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl), 8-azabicyclo[3.2.1]octane-3-one (150 mg, 1.2 mmol) dissolved in N-methylpyrrolidone (10 mL), triethylamine (150 mg, 1.5) (mmol), the reaction was stirred to 120 ° C for 2 hours, cooled to room temperature, the reaction solution was poured into water, stirred for half an hour, filtered, and the obtained cake was dried to give a product (210 mg, yield 75%).
(2) 4,4-二甲基 -2-(3-吗啉代 -8-氮杂又环 [3.2.1]辛烷 -8-基) -10-氧代 -5,10-二 氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈的制备
将 4,4-二甲基 -10-氧代 -2-(3-氧代 -8-氮杂又环 [3.2.1]辛烷 -8-基) -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (210 mg, 0.5 mmol)和吗啉 (500 mg, 5.74 mmol) 溶于 N,N-二甲基甲酰胺 (10 mL)中, 加入三氯化铟 (1.3 g, 5.88 mmol), 室温搅 拌 16小时, 加入氰基硼氢化钠(100 mg, 1.59 mmol), 继续室温搅拌反应 2小 时。 将反应液用水稀释, 加入乙酸乙酯 (20 mLx 5)萃取, 有机相用无水硫酸钠 干燥, 过滤, 旋转蒸发除去溶剂, 所得粗品用硅胶制备板纯化 (二氯甲烷:甲醇 =10: 1)得终产物(110 mg, 产率 45%)。 (2) 4,4-Dimethyl-2-(3-morpholino-8-azacyclo[3.2.1]octane-8-yl)-10-oxo-5,10-dihydro Preparation of -4/7-thiazolo[4,5-6]carbazole-7-carbonitrile 4,4-Dimethyl-10-oxo-2-(3-oxo-8-azabicyclo[3.2.1]octane-8-yl)-5,10-dihydro-4/ 7-thiazolo[4,5-6]carbazole-7-carbonitrile (210 mg, 0.5 mmol) and morpholine (500 mg, 5.74 mmol) in N,N-dimethylformamide (10 mL) Indium trichloride (1.3 g, 5.88 mmol) was added, and the mixture was stirred at room temperature for 16 hours, sodium cyanoborohydride (100 mg, 1.59 mmol) was added, and the mixture was stirred at room temperature for 2 hours. The reaction mixture was diluted with water, EtOAc (EtOAc (EtOAc) (EtOAc) The end product (110 mg, yield 45%).
分子式: C27H29N502S; 分子量: 487.62; LC-MS(m/ ): 488.3 [M+H]+ iH-NMR (400MHz, DMSO- 6) δ: 13.13 (brs., 1H), 8.17 (d, 1H, J= 8.0Hz), 7.96 (s, 1H), 7.51 (d, 1H, J = 8.4Hz), 4.38 (s, 2H), 3.57 (s, 4H), 2.70-2.75 (m, 1H), 2.35 (s, 4H), 1.96-2.06 (m, 2H), 1.81-1.84 (m, 4H), 1.80 (s, 6H), 1.68-1.73 (m, 2H). Molecular formula: C 27 H 29 N 5 0 2 S; Molecular weight: 487.62; LC-MS (m/): 488.3 [M+H] + iH-NMR (400MHz, DMSO- 6 ) δ: 13.13 (brs., 1H) , 8.17 (d, 1H, J= 8.0Hz), 7.96 (s, 1H), 7.51 (d, 1H, J = 8.4Hz), 4.38 (s, 2H), 3.57 (s, 4H), 2.70-2.75 ( m, 1H), 2.35 (s, 4H), 1.96-2.06 (m, 2H), 1.81-1.84 (m, 4H), 1.80 (s, 6H), 1.68-1.73 (m, 2H).
实施例 5 Example 5
4,4-二甲基 -2-(3-甲基 -3,8-二氮杂双环【3.2.11辛烷 -8-基) -10-氧代 -5,10-二氢 - ^- 物 5)的制备
4,4-Dimethyl-2-(3-methyl-3,8-diazabicyclo[3.2.11octane-8-yl)-10-oxo-5,10-dihydro-^- Preparation of substance 5)
8-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2- 基) -3 8-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl)-3
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和 3,8-二氮杂双环 [3.2.1]辛烷 -3-甲酸叔丁基酯 (200 mg, 0.94 mmol)溶于 N-甲基吡咯烷酮(10 mL)中, 加入三乙胺(150 mg, 1.48 mmol), 加 热至 120°C搅拌反应 2小时,冷却至室温,将反应液倒入水中,搅拌半小时, 过滤, 所得滤饼干燥得产物 (290 mg, 产率 86%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and 3,8-diazabicyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester (200 mg, 0.94 mmol) dissolved in N-methylpyrrolidone (10 mL), triethylamine (150 mg, 1.48 mmol), heating to 120 ° C, stirring the reaction for 2 hours, cooling to room temperature, pouring the reaction solution into water, stirring for half an hour, filtering, the resulting filter cake was dried to give the product (290 mg, yield 86%) .
(2) 2-(3,8-二氮杂又环 [3.2.1]辛烷 -8-基) -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7- 噻唑并 [4,5-6]咔唑 -7-甲腈的制备 (2) 2-(3,8-diazacyclo[3.2.1]octane-8-yl)-4,4-dimethyl-10-oxo-5,10-dihydro-4/ Preparation of 7-thiazolo[4,5-6]carbazole-7-carbonitrile
将 8-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2- 基) -3,8-二氮杂又环 [3.2.1]辛烷 -3-甲酸叔丁基酯 (290 mg, 0.58 mmol)加到二氯 甲烷 (10 mL)中, 搅拌下加入三氟乙酸 (10 mL), 加入完毕, 室温搅拌反应 2 小时。 将反应液旋转蒸发除去溶剂, 所得粗品倒入乙醚中, 搅拌半小时, 过 滤, 所得滤饼干燥得产物 (230 mg, 产率 99%)。 8-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl) 3,8-diazacyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester (290 mg, 0.58 mmol) was added to dichloromethane (10 mL). mL), after the addition was completed, the reaction was stirred at room temperature for 2 hours. The reaction mixture was evaporated to dryness crystals crystals crystals crystals crystals
(3) 4,4-二甲基 -2-(3-甲基 -3,8-二氮杂又环 [3.2.1]辛烷 -8-基) -10-氧代 _5,10- 二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈的制备 (3) 4,4-Dimethyl-2-(3-methyl-3,8-diazacyclo[3.2.1]octane-8-yl)-10-oxo_ 5 ,10- Preparation of dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile
将 2-(3,8-二氮杂又环 [3.2.1]辛烷 -8-基) -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7- 噻唑并 [4,5-6]咔唑 -7-甲腈(180 mg, 0.45 mmol)溶于无水甲酸(10 mL)中, 加入 37%的甲醛水溶液 (8 mL), 加热至 100°C搅拌 4小时。 将反应液旋转蒸发除去 溶剂,加入饱和碳酸氢钠水溶液,搅拌半小时,过滤, 所得滤饼用甲醇洗涤, 干燥, 得产物 (60 mg, 产率 32%)。 2-(3,8-diazacyclo[3.2.1]octane-8-yl)-4,4-dimethyl-10-oxo-5,10-dihydro-4/7- Thiazolo[4,5-6]oxazol-7-carbonitrile (180 mg, 0.45 mmol) was dissolved in anhydrous formic acid (10 mL), then aq. Stir for 4 hours. The reaction mixture was evaporated to dryness crystals crystals crystals crystals crystals
分子式: C23H23N5OS; 分子量: 417.53; LC-MS(m/z): 418.2[M+H]+ iH-NMR (400MHz, -DMSO-^) δ: 12.90 (brs. , 1H), 8.19 (d, 1H, J = 8.4Hz), 7.97 (s, 1H), 7.53 (d, 1H, J = 8.4Hz), 4.35 (s, 2H), 2.68 (d, 2H, J = 10.4Hz), 2.29 (d, 2H, J = 10.8Hz), 2.15(s, 3H), 1.93 (s, 4H), 1.67 (s, 6H). Molecular formula: C 23 H 23 N 5 OS; Molecular weight: 417.53; LC-MS (m/z): 418.2 [M+H] + iH-NMR (400MHz, -DMSO-^) δ: 12.90 (brs. , 1H) , 8.19 (d, 1H, J = 8.4Hz), 7.97 (s, 1H), 7.53 (d, 1H, J = 8.4Hz), 4.35 (s, 2H), 2.68 (d, 2H, J = 10.4Hz) , 2.29 (d, 2H, J = 10.8Hz), 2.15(s, 3H), 1.93 (s, 4H), 1.67 (s, 6H).
实施例 6 Example 6
4,4-二甲基 -2-(8-甲基 -3,8-二氮杂双环【3.2.11辛烷 -3-基) -10-氧代 -5,10-二氢 - ^- 物 6)的制备
4,4-Dimethyl-2-(8-methyl-3,8-diazabicyclo[3.2.11 oct-3-yl)-10-oxo-5,10-dihydro-^- Preparation of substance 6)
(1) 8-甲基 -3,8-二氮杂又环 [3.2.1]辛烷 -3-甲酸叔丁基酯的制备 (1) Preparation of 8-methyl-3,8-diaza-cyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester
^ HCHO ^ ^ HCHO ^
ΗΝ 1 N-Boc »► -N 1 N-Boc ΗΝ 1 N-Boc »► -N 1 N-Boc
将 3,8-二氮杂双环 [3.2.1]辛烷 -3-甲酸叔丁基酯 (200 mg, 0.94 mmol)和 37% 甲醛水溶液 (2 mL)溶于乙醇 (10 mL)中, 加入 4巴炭 (50 mg), 氢气加压下室温搅 拌反应 16小时, 过滤, 旋转蒸发除去溶剂得产物(190 mg,产率 89%)。 3,8-Diazabicyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester (200 mg, 0.94 mmol) and 37% aqueous formaldehyde (2 mL) were dissolved in ethanol (10 mL) and added The mixture was stirred at room temperature for 16 hours under reduced pressure of H.sub.2, filtered, and evaporated to give the product (190 mg, yield 89%).
(2) 8_甲基 _3,8_二氮杂又环 [3.2.1]辛烷的制备 (2) Methyl 8 _ _ 3 _ 8 and diaza ring. [3 2.1] octane
TFA TFA
-Ν Ί N-Boc -Ν Ί Η
将 8-甲基 -3,8-二氮杂双环 [3.2.1]辛烷 -3-甲酸叔丁基酯(190 mg, 0.84 mmol) 溶于二氯甲烷(lO mL ) 中, 搅拌下加入三氟乙酸 (5 mL), 加入完毕, 室温搅 拌反应 16小时。 将反应液旋转蒸发除去溶剂得产物(100 mg, 产率 94%)。 -Ν Ί N-Boc -Ν Ί Η Dissolve 8-methyl-3,8-diazabicyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester (190 mg, 0.84 mmol) in dichloromethane (10 mL) and stir. Trifluoroacetic acid (5 mL) was added and the mixture was stirred at room temperature for 16 hours. The reaction solution was evaporated to dryness to give crystals (yield: 100%, yield: 94%).
(3) 4,4-二甲基 -2-(8-甲基 -3,8-二氮杂又环 [3.2.1]辛烷 -3-基) -10-氧代 _5,10- 二氢 -4/7-噻唑 [4,5-6]咔唑 -7-甲腈的制备 (3) 4,4-Dimethyl-2-(8-methyl-3,8-diazacyclo[3.2.1]octane-3-yl)-10-oxo_ 5 ,10- Preparation of dihydro-4/7-thiazole [4,5-6]carbazole-7-carbonitrile
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和 8-甲基 -3,8-二氮杂双环 [3.2.1]辛烷(100 mg, 0.79 mmol)溶于 N-甲基吡咯烷酮(10 mL)中,加入三乙胺 (200 mg, 1.98 mmol),加热至 120 °C搅 拌反应 2小时, 冷却至室温, 将反应液倒入水中, 搅拌半小时, 过滤, 所得 滤饼干燥, 再用甲醇洗涤, 干燥得终产物(130 mg, 产率 46%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and 8-methyl-3,8-diazabicyclo[3.2.1]octane (100 mg, 0.79 mmol) dissolved in N-methylpyrrolidone (10 mL) with triethylamine (200 mg) , 1.98 mmol), heat to 120 ° C, stir the reaction for 2 hours, cool to room temperature, pour the reaction solution into water, stir for half an hour, filter, and the filter cake is dried, washed with methanol, and dried to give the final product (130 mg, Yield 46%).
分子式: C23H23N5OS; 分子量: 417.53; LC-MS(m/z): 418.2[M+H]+ iH-NMR (400MHz, DMSO-^) δ: 12.72 (s, 1H), 8.19(d, 1H, J = 8.4Hz), 7.98 (s, 1H), 7.54 (d, 1H, J = 8.4Hz), 3.59 (brs., 2H), 3.15-3.29 (m, 4H), 2.24 (s, 3H), 1.97 (m, 2H), 1.65 (s, 6H), 1.54 (d, 2H, J = 7.6Hz). Molecular formula: C 23 H 23 N 5 OS; Molecular weight: 417.53; LC-MS (m/z): 418.2 [M+H] + iH-NMR (400MHz, DMSO-^) δ: 12.72 (s, 1H), 8.19 (d, 1H, J = 8.4Hz), 7.98 (s, 1H), 7.54 (d, 1H, J = 8.4Hz), 3.59 (brs., 2H), 3.15-3.29 (m, 4H), 2.24 (s , 3H), 1.97 (m, 2H), 1.65 (s, 6H), 1.54 (d, 2H, J = 7.6Hz).
实施例 7 Example 7
4,4-二甲基 -2-(7-甲基 -2,7-二氮杂螺【3.51壬烷 -2-基) -10-氧代 -5,10-二氢 - g- 9)的制备
4,4-Dimethyl-2-(7-methyl-2,7-diazaspiro[3.51 decan-2-yl)-10-oxo-5,10-dihydro-g- 9) Preparation
(1) 7-甲基 -2,7 杂螺 [3.5]壬烷 -2-甲酸叔丁基酯的制备
(1) Preparation of 7-methyl-2,7-spiro[3.5]decane-2-carboxylic acid tert-butyl ester
将 2,7-二氮杂螺 [3.5]壬烷 -2-甲酸叔丁基酯 (200 mg, 0.88 mmol)和 37%甲酪 水溶液 (2 mL)溶于乙醇 (10 mL)中, 加入钯炭 (50 mg), 氢气加压下室温搅拌反 应 16小时, 过滤, 取滤液旋转蒸发除去溶剂得产物(190 mg, 产率 90%)。 2,7-diazaspiro[3.5]decane-2-carboxylic acid tert-butyl ester (200 mg, 0.88 mmol) and 37% aqueous solution of methyl ketone (2 mL) were dissolved in ethanol (10 mL) and palladium was added. The charcoal (50 mg) was stirred under a hydrogen atmosphere at room temperature for 16 hours, filtered, and the filtrate was evaporated to give the product (190 mg, yield 90%).
(2) 7-甲基 -2,7-二氮杂螺 [3.5]壬烷的制备
将 7-甲基 -2,7-二氮杂螺 [3.5]壬烷 -2-甲酸叔丁基酯 (190 mg, 0.79 mmol)溶 于二氯甲烷 (5 mL)中, 搅拌下加入三氟乙酸 (3 mL ), 加入完毕, 室温搅拌反 应 16小时。 将反应液旋转蒸发除去溶剂得产物(110 mg, 产率 99%)。 (2) Preparation of 7-methyl-2,7-diazaspiro[3.5]decane 7-Methyl-2,7-diazaspiro[3.5]decane-2-carboxylic acid tert-butyl ester (190 mg, 0.79 mmol) was dissolved in dichloromethane (5 mL). Acetic acid (3 mL) was added and the reaction was stirred at room temperature for 16 hours. The solvent was evaporated to dryness to give the product (l.
(3) 4,4-二甲基 -2-(7-甲基 -2,7-二氮杂螺 [3.5]壬烷 -2-基) -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6] (3) 4,4-Dimethyl-2-(7-methyl-2,7-diazaspiro[3.5]decane-2-yl)-10-oxo-5,10-dihydro- 4/7-thiazolo[4,5-6]
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和 7-甲基 -2,7-二氮杂螺 [3.5]壬烷 (110 mg, 0.78 mmol )溶于 N- 甲基吡咯烷酮(10 mL)中,加入三乙胺 (200 mg, 1.98 mmol),加热至 120°C搅拌 反应 1小时, 冷却至室温, 将反应液倒入水中, 搅拌半小时, 过滤, 所得滤 饼干燥, 再用硅胶制备板纯化得终产物(130 mg, 产率 45%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and 7-methyl-2,7-diazaspiro[3.5]decane (110 mg, 0.78 mmol) were dissolved in N-methylpyrrolidone (10 mL) and triethylamine (200 mg, 1.98) Ment), heating to 120 ° C, stirring the reaction for 1 hour, cooling to room temperature, pouring the reaction solution into water, stirring for half an hour, filtering, drying the filter cake, and purifying with silica gel to obtain the final product (130 mg, yield 45%).
分子式: C24H25N5OS; 分子量: 431.56; LC-MS(m/z): 432.2[M+H]+ iH-NMR (400MHz, DMSO-^) δ: 12.85(s, IH), 8.19 (d, IH, J = 8.4Hz), 7.98 (s, IH), 7.54 (m, IH), 3.89 (s, 4H), 2.69 (m, 4H), 2.41 (s, 3H), 1.74 (m, 4H); 1.65 (s, 6H). Molecular formula: C 24 H 25 N 5 OS; Molecular weight: 431.56; LC-MS (m/z): 432.2 [M+H] + iH-NMR (400 MHz, DMSO-^) δ: 12.85 (s, IH), 8.19 (d, IH, J = 8.4 Hz), 7.98 (s, IH), 7.54 (m, IH), 3.89 (s, 4H), 2.69 (m, 4H), 2.41 (s, 3H), 1.74 (m, 4H) ; 1.65 (s, 6H).
实施例 8 Example 8
4,4-二甲基 -2-(2-甲基 -2,7-二氮杂螺【3.51壬烷 -7-基) -10-氧代 -5,10-二氢 - g- 10)的制备
4,4-Dimethyl-2-(2-methyl-2,7-diazaspiro[3.51 dec-7-yl)-10-oxo-5,10-dihydro-g- 10) Preparation
(1) 2-甲基 -2,7-二氮杂螺 [3.5]壬烷 -7-甲酸叔丁基酯的制备
(1) Preparation of 2-methyl-2,7-diazaspiro[3.5]decane-7-carboxylic acid tert-butyl ester
将 2,7-二氮杂螺 [3.5]壬烷 -7-甲酸叔丁基酯 (200 mg, 0.88 mmol)和 37%甲酪 水溶液 (2 mL)溶于乙醇 (10 mL)中, 加入钯炭 (50 mg), 氢气加压下室温搅拌反 应 16小时, 过滤, 旋转蒸发除去溶剂, 得产物(195 mg, 产率 92%)。 2,7-diazaspiro[3.5]decane-7-carboxylic acid tert-butyl ester (200 mg, 0.88 mmol) and 37% aqueous solution of methyl ketone (2 mL) were dissolved in ethanol (10 mL) and palladium was added. The charcoal (50 mg) was stirred at room temperature under reduced pressure of hydrogen for 16 hr, filtered and evaporated to give the product (195 mg, yield 92%).
(2) 2-甲基 -2,7-二氮杂螺 [3.5]壬烷的制备
将 2-甲基 -2,7-二氮杂螺 [3.5]壬烷 -7-甲酸叔丁基酯 (195 mg, 0.81 mmol )溶 于二氯甲烷 (5 mL ) 中, 搅拌下加入三氟乙酸 (3 mL ), 加毕室温搅拌反应 16 小时。 将反应液旋转蒸发除去溶剂得产物(110 mg,产率 97%)。 (2) Preparation of 2-methyl-2,7-diazaspiro[3.5]decane 2-methyl-2,7-diazaspiro[3.5]decane-7-carboxylic acid tert-butyl ester (195 mg, 0.81 mmol) was dissolved in dichloromethane (5 mL). Acetic acid (3 mL) was stirred at room temperature for 16 hours. The reaction solution was evaporated to dryness to give crystals (yield: 110 mg, yield 97%).
(3) 4,4-二甲基 -2-(2-甲基 -2,7-二氮杂螺 [3.5]壬烷 -7-基) -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6] (3) 4,4-Dimethyl-2-(2-methyl-2,7-diazaspiro[3.5]decane-7-yl)-10-oxo-5,10-dihydro- 4/7-thiazolo[4,5-6]
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和 2-甲基 -2,7-二氮杂螺 [3.5]壬烷 (110 mg, 0.78 mmol)溶于 N-甲 基吡咯烷酮(10 mL)中,加入三乙胺 (200 mg, 1.98 mmol),加热至 120°C搅拌反 应 1小时, 冷却至室温, 将反应液倒入水中, 搅拌半小时, 过滤, 所得滤饼 干燥, 再用硅胶制备板纯化得终产物 (78 mg,产率 27%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and 2-methyl-2,7-diazaspiro[3.5]decane (110 mg, 0.78 mmol) were dissolved in N-methylpyrrolidone (10 mL) and triethylamine (200 mg, 1.98) Ment), heating to 120 ° C, stirring the reaction for 1 hour, cooling to room temperature, pouring the reaction solution into water, stirring for half an hour, filtering, drying the filter cake, and purifying with silica gel to obtain the final product (78 mg, yield 27%).
分子式: C24H25N5OS; 分子量: 431.56; LC-MS(m/z): 432.2[M+H]+ iH-NMR (400MHz, DMSO-^) δ: 13.19(brs, 1H), 8.19 (d, 1H, J = 8.0Hz), 7.98 (s, 1H), 7.53 (d, 1H, J = 8.4Hz), 3.97 (s, 2H), 3.31 (s, 2H), 3,12-3.17 (m, 7H), 1.94 (m, 2H), 1.85 (s, 8H). Molecular formula: C 24 H 25 N 5 OS; Molecular weight: 431.56; LC-MS (m/z): 432.2 [M+H] + iH-NMR (400 MHz, DMSO-^) δ: 13.19 (brs, 1H), 8.19 (d, 1H, J = 8.0Hz), 7.98 (s, 1H), 7.53 (d, 1H, J = 8.4Hz), 3.97 (s, 2H), 3.31 (s, 2H), 3,12-3.17 ( m, 7H), 1.94 (m, 2H), 1.85 (s, 8H).
实施例 9 Example 9
4,4-二甲基 -2-(9-甲基 -3,9-二氮杂螺【5.51十一烷 -3-基) -10-氧代 -5,10-二氢 - g- 14)的制备
4,4-Dimethyl-2-(9-methyl-3,9-diazaspiro[5.51 undec-3-yl)-10-oxo-5,10-dihydro-g- 14 Preparation
( 1 ) 9-甲基 -3,9-二氮杂螺 [5.5]十一烷 -3-甲酸叔丁基酯的制备
(1) Preparation of 9-methyl-3,9-diazaspiro[5.5]undecane-3-carboxylic acid tert-butyl ester
将 3,9-二氮杂螺 [5.5]十一烷 -3-甲酸叔丁酯 (200 mg, 0.79 mmol)和 37%甲酪 水溶液 (2 mL)溶于乙醇 (10 mL)中, 加入钯炭 (50 mg), 氢气加压下室温搅拌反 应 16 小时, 过滤, 将滤液旋转蒸发除去溶剂得产物(192 mg,产率 91%)。 3,9-diazaspiro[5.5]undecane-3-carboxylic acid tert-butyl ester (200 mg, 0.79 mmol) and 37% aqueous solution of methyl ketone (2 mL) were dissolved in ethanol (10 mL) and palladium was added. The charcoal (50 mg) was stirred at room temperature under reduced pressure of hydrogen for 16 hr, filtered, and the solvent was evaporated to give the product (192 mg, yield 91%).
将 9-甲基 -3,9-
溶于二氯甲烷(5 mL ) 中, 搅拌下加入三氟乙酸 (3 mL), 加入完毕, 室温搅 拌反应 16小时。 将反应液旋转蒸发除去溶剂得产物(120 mg,产率 99%)。 9-methyl-3,9- It was dissolved in dichloromethane (5 mL), and trifluoroacetic acid (3 mL) was added with stirring, and the mixture was stirred at room temperature for 16 hours. The reaction solution was evaporated to dryness to give crystals (yield:
( 3 ) 4,4-二甲基 -2-(9-甲基 -3,9-二氮杂螺 [5.5]十一烷 -3-基) -10-氧代 -5,10- (3) 4,4-Dimethyl-2-(9-methyl-3,9-diazaspiro[5.5]undec-3-yl)-10-oxo-5,10-
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和 3-甲基 -3,9-二氮杂螺 [5.5]十一烷 (120 mg, 0.71 mmol)溶于 N- 甲基吡咯烷酮(10 mL)中,加入三乙胺 (200 mg, 1.98 mmol),加热至 120 °C搅拌 反应 2小时, 冷却至室温, 将反应液倒入水中, 搅拌半小时, 过滤, 所得滤 饼干燥, 再用甲醇洗涤, 干燥得产物(150 mg, 产率 49%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and 3-methyl-3,9-diazaspiro[5.5]undecane (120 mg, 0.71 mmol) were dissolved in N-methylpyrrolidone (10 mL), and triethylamine (200 mg, 1.98 mmol), heating to 120 ° C, stirring the reaction for 2 hours, cooling to room temperature, pouring the reaction solution into water, stirring for half an hour, filtering, drying the filter cake, washing with methanol, drying to obtain the product (150 mg, yield 49%).
分子式: C26H29N5OS; 分子量: 459.61 ; LC-MS(m/z): 460.3 [M+H]+ iH-NMR (400MHz, DMSO- 6) δ: 13,44 (brs., 1H), 8.18 (d,lH, J = 8Hz), 7.97(s, 1H), 7.52 (m, 1H), 3.56 (s, 4H), 2.46-2.54 (m, 4H), 2.31 (s, 3H), 1.67 (s, 6H) , 1.56(s, 8H). Molecular formula: C 26 H 29 N 5 OS; Molecular weight: 459.61 ; LC-MS (m/z): 460.3 [M+H] + iH-NMR (400MHz, DMSO- 6 ) δ: 13,44 (brs., 1H ), 8.18 (d, lH, J = 8Hz), 7.97(s, 1H), 7.52 (m, 1H), 3.56 (s, 4H), 2.46-2.54 (m, 4H), 2.31 (s, 3H), 1.67 (s, 6H), 1.56 (s, 8H).
实施例 10 Example 10
4,4-二甲基 -2- (顺式 -5-甲基六氢吡咯并【3,4-cl吡咯 -2qg -基) -10-氧代 -5,10 (化合物 18)的制备 Preparation of 4,4-dimethyl-2-(cis-5-methylhexahydropyrrolo[3,4-clpyrrole-2qg-yl)-10-oxo-5,10 (Compound 18)
-5-甲基六氢吡咯并 [3,4-c]吡咯 -2(1/7)-甲酸叔丁基酯的制备
Preparation of 5-methylhexahydropyrrolo[3,4-c]pyrrole-2(1/7)-carboxylic acid tert-butyl ester
将顺式 -六氢吡咯并 [3,4-c]吡咯 -2(1/7)-甲酸叔丁基酯 (200 mg, 0.94 mmol) 和 37%甲醛水溶液 (2 mL)溶于乙醇(10 mL)中, 加入 4巴炭 (50 mg), 氢气加压下 室温搅拌反应 16 小时,过滤,旋转蒸发除去溶剂得产物(185 mg,产率 86%)。 cis-Hexahydropyrrolo[3,4-c]pyrrole-2(1/7)-carboxylic acid tert-butyl ester (200 mg, 0.94 mmol) and 37% aqueous formaldehyde (2 mL) were dissolved in ethanol (10) In a solution of EtOAc, EtOAc (EtOAc)EtOAc.
( 2 )顺式 -2-甲基 -八氢吡咯并 [3,4-c]吡咯的制备
将顺式 -5-甲基六氢吡咯并 [3,4-c]吡咯 -2(1/7)-甲酸叔丁基酯(185 mg, 0.82 mmol)溶于二氯甲烷 (5 mL)中, 搅拌下加入三氟乙酸 (3 mL), 加入完毕, 室温 搅拌反应 16小时。 将反应液旋转蒸发除去溶剂得产物(100 mg, 产率 97%)。 (2) Preparation of cis-2-methyl-octahydropyrrolo[3,4-c]pyrrole cis-5-Methylhexahydropyrrolo[3,4-c]pyrrole-2(1/7)-carboxylic acid tert-butyl ester (185 mg, 0.82 mmol) was dissolved in dichloromethane (5 mL) Trifluoroacetic acid (3 mL) was added with stirring. After the addition was completed, the reaction was stirred at room temperature for 16 hours. The reaction solution was evaporated to dryness to give crystals (yield: 100 mg, yield 97%).
( 3 ) 4,4-二甲基 -2- (顺式 -5-甲基六氢吡咯并 [3 ,4-c]吡咯 -2(1/7)-基) -10-氧代 -5 10-二氢 -4/7 (3) 4,4-Dimethyl-2-(cis-5-methylhexahydropyrrolo[3,4-c]pyrrole-2(1/7)-yl)-10-oxo-5 10-dihydro-4/7
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和顺式 -2-甲基 -八氢吡咯并 [3 ,4-c]吡咯 (100 mg, 0.79 mmol)溶于 N-甲基吡咯烷酮(10 mL)中,加入三乙胺 (200 mg, 1.98 mmol),加热至 120°C搅 拌反应 2小时, 冷却至室温, 将反应液倒入水中, 搅拌半小时, 过滤, 所得 滤饼干燥, 再用甲醇洗涤, 干燥得终产物(150 mg, 产率 54%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and cis-2-methyl-octahydropyrrolo[3,4-c]pyrrole (100 mg, 0.79 mmol) were dissolved in N-methylpyrrolidone (10 mL), and triethylamine (200 mg, 1.98 mmol), heated to 120 ° C, stirred for 2 hours, cooled to room temperature, poured into the water, stirred for half an hour, filtered, the filter cake was dried, washed with methanol, dried to give the final product (150 mg, yield Rate 54%).
分子式: C23H23N5OS; 分子量: 417.53 ; LC-MS(m/z): 418.4[M+H]+ -NMR (400MHz, DMSO- 6) δ: 12.86 (s, 1H), 8. 19 (d, 1H, J = 8.0Hz), 7.99(s, 1H), 7.54 (d, 1H, J = 8.0 Hz), 3.70-3.74 (m, 2H), 3.43-3.46 (m, 3H), 3.08(s, 2H), 2.81 (s, 4H), 2.42 (s, 2H), 1.67 (s, 6H). Molecular formula: C 23 H 23 N 5 OS; Molecular weight: 417.53 ; LC-MS (m/z): 418.4 [M+H] + -NMR (400MHz, DMSO- 6 ) δ: 12.86 (s, 1H), 8. 19 (d, 1H, J = 8.0Hz), 7.99(s, 1H), 7.54 (d, 1H, J = 8.0 Hz), 3.70-3.74 (m, 2H), 3.43-3.46 (m, 3H), 3.08 (s, 2H), 2.81 (s, 4H), 2.42 (s, 2H), 1.67 (s, 6H).
实施例 11 Example 11
2-(6-氨基 -3-氮杂双环【3.1.01己烷 -3-基) -4,4-二甲基 -10-氧代 -5,10-二氢 - ^- 24)的制备
Preparation of 2-(6-Amino-3-azabicyclo[3.1.01hexane-3-yl)-4,4-dimethyl-10-oxo-5,10-dihydro-^- 24)
- ((; (苄氧基)羰基)氨基) -3-氮 -3-甲酸叔丁基酯的制备
- Preparation of ((; (benzyloxy)carbonyl)amino)-3-nitro-3-carboxylic acid tert-butyl ester
将 6-氨基 -3-氮杂双环 [3.1.0]己烷 -3-甲酸叔丁基酯 (200 mg, 1.01 mmol)和 三乙胺 (170 mg, 1.68 mmol)溶于二氯甲烷 (20 mL)中, 滴加氯甲酸苄酯 (175 mg, 6-Amino-3-azabicyclo[3.1.0]hexane-3-carboxylic acid tert-butyl ester (200 mg, 1.01 mmol) and triethylamine (170 mg, 1.68 mmol) were dissolved in dichloromethane (20) In mL), add benzyl chloroformate (175 mg,
1.03 mmol) , 室温搅拌反应 16小时, 旋转蒸发除去溶剂, 所得粗品用硅胶柱 层析 (石油醚:乙酸乙酯 =5 : 1 )纯化得产物 (270 mg, 产率 81%)。 The reaction was stirred at room temperature for 16 hr. EtOAc (EtOAc:EtOAc)
(2) 3-氮杂欢环 [3.1.0]己烷 -6-基氨基甲酸苄基酯的制备
(2) Preparation of 3-azabicyclo[3.1.0]hexane-6-ylcarbamic acid benzyl ester
将 6- ((; (苄氧基)羰基)氨基) -3-氮杂又环 [3.1.0]己烷 -3-甲酸叔丁基酯 (270 mg, 0.81 mmol)溶于二氯甲烷 (10 mL)中, 搅拌下加入三氟乙酸( 5 mL ), 加入 完毕, 室温搅拌反应 16小时。将反应液旋转蒸发除去溶剂得产物(180 mg, 产 率 95%)。 6-((; (Benzyloxy)carbonyl)amino)-3-azabicyclo[3.1.0]hexane-3-carboxylic acid tert-butyl ester (270 mg, 0.81 mmol) was dissolved in dichloromethane. In 10 mL), trifluoroacetic acid (5 mL) was added with stirring. After the addition was completed, the reaction was stirred at room temperature for 16 hours. The reaction solution was rotary evaporated to remove the solvent to give product (180 mg, yield 95%).
(3) 3-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2- 基) -3 (3) 3-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl ) -3
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和 3-氮杂双环 [3.1.0]己烷 -6-基氨基甲酸苄基酯 (180 mg, 0.77 mmol)溶于 N-甲基吡咯烷酮(10 mL)中, 加入三乙胺(150 mg, 1.5 mmol), 加 热至 120°C搅拌反应 1小时,冷却至室温,将反应液倒入水中,搅拌半小时, 过滤, 所得滤饼干燥, 再用甲醇洗涤, 干燥得产物 (250 mg, 产率 71%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and 3-azabicyclo[3.1.0]hexane-6-ylcarbamic acid benzyl ester (180 mg, 0.77 mmol) dissolved in N-methylpyrrolidone (10 mL). Mg, 1.5 mmol), heated to 120 ° C, stirred for 1 hour, cooled to room temperature, poured into the water, stirred for half an hour, filtered, the filter cake was dried, washed with methanol, dried to give the product (250 mg, Yield 71%).
(4) 2-(6-氨基 -3-氮杂又环 [3.1.0]己烷-3-基)-4,4-二甲基-10-氧代_5,10-二氢 -4/7-
(4) 2- (6-amino-3-aza and bicyclo [3.1.0] hexane-3-yl) -4,4-dimethyl-10-oxo _ 5, 10-dihydro-4 /7-
将 3-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2- 基) -3-氮杂又环 [3.1.0]己烷 -6-基氨基甲酸苄基酯 (250 mg, 0.48 mmol)溶于溴化 氢乙酸溶液 (10 mL)中, 室温搅拌反应 5小时, 将反应液倒入水中, 用氨水调 节 pH值至 8 , 过滤, 所得滤饼干燥后用硅胶柱层析 (二氯甲烷:甲醇 =10: 1)纯 化得产物 (96 mg,产率 52%)。 3-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl) 3-Azacyclo[3.1.0]hexane-6-ylcarbamic acid benzyl ester (250 mg, 0.48 mmol) was dissolved in hydrogen bromide acetic acid solution (10 mL), and the reaction was stirred at room temperature for 5 hours. The liquid was poured into water, the pH was adjusted to 8 with aqueous ammonia, and filtered, and the obtained filter cake was dried and purified by silica gel column chromatography (dichloromethane:methanol = 10:1) (96 mg, yield 52%).
分子式: C21H19N5OS; 分子量: 389.48; LC-MS(m/z): 390.2[M+H]+ -NMR (400MHz, DMSO-^) δ: 8.19 (d, 1H, J = 8.0Hz), 7.97 (s, 1H), 7.53 (d, 1H, J= 8.4Hz), 3.51 (s, 4H), 2.03 (s, 1H), 1.65 (s, 8H). Molecular formula: C 21 H 19 N 5 OS; Molecular weight: 389.48; LC-MS (m/z): 390.2 [M+H] + -NMR (400MHz, DMSO-^) δ: 8.19 (d, 1H, J = 8.0 Hz), 7.97 (s, 1H), 7.53 (d, 1H, J= 8.4Hz), 3.51 (s, 4H), 2.03 (s, 1H), 1.65 (s, 8H).
实施例 12 Example 12
4,4-二甲基 -2- (反式 -八氢 吡啶并【3,4- >lil,41噁嗪 -6-基) -10-氧代 -5.10- 二氢 - g-噻唑并【4,5- >1咔唑 -7-甲腈 (化合物 26)的制备
4,4-dimethyl-2-(trans-octahydropyridyl[3,4->lil,41oxazin-6-yl)-10-oxo-5.10-dihydro-g-thiazole Preparation of 4,5->1 carbazole-7-carbonitrile (Compound 26)
( 1 )反式 -: 氢 -1/7-吡啶并 [3,4- >][1 ,4]噁嗪 -1,6(5/7)-二甲酸 1-苄基 6-叔丁 基酯的制备
(1) trans-: hydrogen-1/7-pyrido[3,4->][1 ,4]oxazine-1,6(5/7)-dicarboxylic acid 1-benzyl 6-tert-butyl Preparation of ester
将反式 -八氢 -6/7-吡啶并 [3,4-6][l ,4]噁嗪 -6-甲酸叔丁基酯 (200 mg, 0.83 mmol)和三乙胺 (170 mg, 1.68 mmol)溶于二氯甲烷 (20 mL)中,滴加氯甲酸苄酯 (170 mg, 1 mmol), 室温搅拌反应 16 小时, 旋转蒸发除去溶剂, 所得粗品用 硅胶柱层析 (石油醚: 乙酸乙酯 =5 : 1 )纯化得产物 (250 mg, 产率 80%)。 Trans-octahydro-6/7-pyrido[3,4-6][l,4]oxazine-6-carboxylic acid tert-butyl ester (200 mg, 0.83 mmol) and triethylamine (170 mg, 1.68 mmol) was dissolved in dichloromethane (20 mL), benzyl chloroformate (170 mg, 1 mmol) was added dropwise, and the mixture was stirred at room temperature for 16 hours. The solvent was removed by rotary evaporation. Ethyl acetate = 5 : 1 ) purified product (250 mg, yield 80%).
( 2 )反式 -八氢 -1/7-吡啶并 [3,4-b][l ,4]噁嗪 -1-甲酸苄基酯的制备
(2) Preparation of trans-octahydro-1/7-pyrido[3,4-b][l,4]oxazine-1-carboxylic acid benzyl ester
将反式 -六氢 吡啶并 [3,4-/)][l,4]噁嗪 -1,6(5//)-二曱酸 1-苄基 6-叔丁基 酯 (250 mg, 0.66 mmol)溶于二氯曱烷 ( 10 mL)中, 搅拌下加入三氟乙酸 (5 mL), 加入完毕,室温搅拌反应 16小时。将反应液旋转蒸发除去溶剂得产物(180 mg, 产率 98%)。 Trans-hexahydropyrido[3,4-/)][l,4]oxazine-1,6(5//)-didecanoic acid 1-benzyl 6-tert-butyl ester (250 mg, 0.66 mmol) was dissolved in dichloromethane (10 mL), trifluoroacetic acid (5 mL) was added with stirring, and the mixture was stirred at room temperature for 16 hours. The reaction solution was evaporated to dryness to give crystals (yield: 180 mg, yield 98%).
( 3 ) 6-(7-氰基 -4,4-二甲基 -10-氧代 -5, 10-二氢 -4//-噻唑并 [4,5-6]咔唑 -2- 基) -反式 -八氢 吡啶并 [3,4-Ζ)][1,4]噁嗪 -1 -甲酸苄基酯的制备
(3) 6-(7-Cyano-4,4-dimethyl-10-oxo-5, 10-dihydro-4//-thiazolo[4,5-6]oxazol-2-yl - Preparation of trans-octahydropyrido[3,4-indole][1,4]oxazine-1 -formic acid benzyl ester
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-b]咔唑 -7-甲腈 (200 mg, 0.54 mmol)和反式 -八氢 -1/7-吡啶并 [3,4-/>][1,4]噁嗪 -1 -曱酸苄基酯 (180 mg, 0.65 mmol )溶于 N-甲基吡咯烷酮(10 mL)中,加入三乙胺 (150 mg, 1.5 mmol) , 加热至 120°C搅拌反应 2小时, 冷却至室温, 将反应液倒入水中, 搅拌半小 时,过滤,滤饼干燥,所得粗品用甲醇洗涤,干燥得产物 (260 mg, 产率 85%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-b]carbazole-7-carbonitrile (200 mg, 0.54 Methyl) and trans-octahydro-1/7-pyrido[3,4-][1,4]oxazine-1 -decanoic acid benzyl ester (180 mg, 0.65 mmol) dissolved in N-A To the pyrrolidone (10 mL), add triethylamine (150 mg, 1.5 mmol), heat to 120 ° C, stir the reaction for 2 hours, cool to room temperature, pour the reaction into water, stir for half an hour, filter, filter cake dry The obtained crude product was washed with methanol and dried to give product (260 mg, yield: 85%).
( 4 ) 4,4-二曱基 -2- (反式 -八氢 -6 -吡啶并 [3,4-6][1 ,4]噁嗪 -6-基) -10-氧代 -5. 10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-曱腈的制备
(4) 4,4-Dimercapto-2-(trans-octahydro-6-pyrido[3,4-6][1 ,4]oxazin-6-yl)-10-oxo-5 Preparation of 10-Dihydro-4/7-thiazolo[4,5-6]carbazole-7-indolecarbonitrile
将 6-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2-基) - 反式 -八氢 吡啶并 [3,4-6][1 ,4]噁嗪 -1-甲酸苄基酯 (190 mg, 0.33 mmol)溶于 溴化氢乙酸溶液 (10 mL)中, 室温搅拌反应 5小时, 将反应液倒入水中, 用氨 水调节 pH值至 8 ,过滤,所得滤饼干燥后,用硅胶柱层析 (二氯甲烷:甲醇 =10: 1) 纯化得终产物 (76 mg, 产率 53%)。 6-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl) Trans-octahydropyrido[3,4-6][1,4]oxazine-1-carboxylic acid benzyl ester (190 mg, 0.33 mmol) dissolved in hydrogen bromide acetic acid solution (10 mL), stirred at room temperature After reacting for 5 hours, the reaction solution was poured into water, and the pH was adjusted to 8 with aqueous ammonia. After filtration, the obtained cake was dried and purified by silica gel column chromatography (dichloromethane:methanol = 10:1) to give the final product (76 mg , yield 53%).
分子式: C23H23N502S; 分子量: 433.53 ; LC-MS(m/z): 434.2 [M+H]+ iH-NMR (400MHz, DMSO- 6-) δ: 13. 15 (brs. , IH), 8. 18 (d, IH, J = 8.0Hz), 7.97 (s, IH), 7.53 (d, IH, J = 8.0Hz), 3.96-4.08 (m, 2H), 3.75-3.77 (m, IH), 3.48-3.51 (m, IH), 3.21-3.27 (m, IH), 3.12-3.16 (m, IH), 3.07-3.11 (m, IH), 2.96-3.02 (m, IH), 2.73-2.84 (m, 2H), 1.74-1.77 (m, IH), 1.67 (s, 6H), 1.32-1.48 (m, IH). Molecular formula: C 23 H 23 N 5 0 2 S; Molecular weight: 433.53 ; LC-MS (m/z): 434.2 [M+H] + iH-NMR (400MHz, DMSO- 6 -) δ: 13. 15 (brs . , IH), 8. 18 (d, IH, J = 8.0Hz), 7.97 (s, IH), 7.53 (d, IH, J = 8.0Hz), 3.96-4.08 (m, 2H), 3.75-3.77 (m, IH), 3.48-3.51 (m, IH), 3.21-3.27 (m, IH), 3.12-3.16 (m, IH), 3.07-3.11 (m, IH), 2.96-3.02 (m, IH) , 2.73-2.84 (m, 2H), 1.74-1.77 (m, IH), 1.67 (s, 6H), 1.32-1.48 (m, IH).
实施例 13 Example 13
2-(3,8-二氮杂双环【3.2.11辛烷 -8-基) -4,4-二甲基 -10-氧代 -5,10-二氢 - g-噻 唑并 的制备
Preparation of 2-(3,8-diazabicyclo[3.2.11 Octane-8-yl)-4,4-dimethyl-10-oxo-5,10-dihydro-g-thiazole
在 10 L的三口瓶中, 加入丙酮 (4.4 L), 碳酸钾 (1.14 kg, 8.25 mol), 2國甲基 环己烷 -1,3-二酮 (700 g, 5.55 mol),在室温下将碘甲烷 (1.17 kg, 8.24 mol)滴加到 体系中, 四小时加完, 加完后将体系升温至 55 °C , 保温过夜。 反应完毕后, 将体系过滤, 滤饼用乙酸乙酯 (1 L)洗涤三次; 合并有机相。 旋转蒸发除去溶 剂, 加入乙酸乙酯 (3 L), 用饱和氯化钠水溶液洗涤三次, 无水石克酸钠干燥, 将有机相旋转蒸发除去溶剂, 得油状物, 加入石油醚, -25 °C下, 析晶, 抽滤 得产物 (330 g, 产率 42%)。 In a 10 L three-necked flask, add acetone (4.4 L), potassium carbonate (1.14 kg, 8.25 mol), and 2-methylcyclohexane-1,3-dione (700 g, 5.55 mol) at room temperature. Methyl iodide (1.17 kg, 8.24 mol) was added dropwise to the system, and the addition was completed in four hours. After the addition, the system was heated to 55 ° C and kept overnight. After completion of the reaction, the system was filtered, and the filter cake was washed three times with ethyl acetate (1 L); The solvent was removed by rotary evaporation, ethyl acetate (3 L) was evaporated,EtOAcEtOAcjjjjjjjjjjjjjjj The product was isolated (330 g, yield 42%) by crystallization.
在 10 L的三口瓶中, 加入醋酸 (2 L)、 40%氢溴酸 (20.6 mL)、 2,2-二甲基 环己烷 -1,3-二酮 (330 g, 2.35 mol);将溴素 (376g, 2.35mol)溶解于醋酸 (2.5 L)中, 在 0°C下, 緩慢滴加到体系中, 升至室温过夜。 将醋酸蒸干, 加入乙酸乙酯 (3 L)溶解, 用饱和氯化钠水溶液萃取三次, 用饱和碳酸钠水溶液调至弱碱性。 干燥有机相, 旋转蒸发除去溶剂得到粗品 (200 g)。 In a 10 L three-necked flask, acetic acid (2 L), 40% hydrobromic acid (20.6 mL), 2,2-dimethylcyclohexane-1,3-dione (330 g, 2.35 mol); Bromide (376 g, 2.35 mol) was dissolved in acetic acid (2.5 L), slowly added dropwise to the system at 0 ° C, and allowed to warm to room temperature overnight. The acetic acid was evaporated to dryness, taken-up ethyl acetate (3 L), and then extracted three times with a saturated aqueous sodium chloride aqueous solution and diluted with saturated aqueous sodium carbonate. The organic phase was dried and evaporated <RTI ID=0.0>
(3) 2-氨基 -4,4-二甲基 -6,7-二氢苯并 W噻唑 -5(4/7)-酮的制备 (3) Preparation of 2-amino-4,4-dimethyl-6,7-dihydrobenzothiazol-5(4/7)-one
将 4-溴 -2,2-二甲基环己基 -1,3-二酮的粗品 (200 g)溶解于 THF(1 L)中, 加 入石克脲 (77.4 g, 1 mol), 加热回流过夜, 蒸干 THF, 经柱层析纯化得到产物 (50 g, 0.255 mol)。 The crude product (200 g) of 4-bromo-2,2-dimethylcyclohexyl-1,3-dione was dissolved in THF (1 L), and sulphonic acid (77.4 g, 1 mol) was added and heated to reflux. After overnight, the THF was evaporated to dryness and purified by column chromatography to afford product (50 g, 0.255 mol).
2-氨基 -4,4-二甲基 -6,7-二氢苯并 W噻唑 -5(4/7)-酮 (50 g, 0.255 mol)溶解于 乙腈 (1 L)中, 加入 CuBr2(68.3 g, 0.306 mol), 将稀释的亚硝酸叔丁酯 (36.4 g, 0.36 mol), 在 -10°C下滴入反应体系, 加完后 TLC监测, 反应完成, 旋转蒸发 除去溶剂, 加入乙酸乙酯, 乙酸乙酯相用饱和氯化钠水溶液萃取三次, 合并 有机相, 无水^ L酸钠干燥, 旋转蒸发除去溶剂, 经柱层析纯化得产物 (35 g,产 率 53%)。 2-Amino-4,4-dimethyl-6,7-dihydrobenzothiazol-5(4/7)-one (50 g, 0.255 mol) dissolved in acetonitrile (1 L), added CuBr 2 (68.3 g, 0.306 mol), diluted tert-butyl nitrite (36.4 g, 0.36 mol) was added dropwise to the reaction system at -10 ° C. After the addition, TLC was monitored, the reaction was completed, and the solvent was removed by rotary evaporation. The ethyl acetate and ethyl acetate phases were extracted three times with a saturated aqueous solution of sodium chloride, and the organic phase was combined, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation and purified by column chromatography (35 g, yield 53%) .
唑并 [4,5-6]咔唑 -7-甲腈的制备 Preparation of oxazo[4,5-6]carbazole-7-carbonitrile
2-溴 -4,4-二甲基 -6,7-二氢苯并 W噻唑 -5(4/7)-酮 (35 g, 0.135 mol)和 3-肼基 苯甲腈盐酸盐 (45.4 g,0.269 mol)溶解于醋酸 (1 L)中。 100°C加热回流 2h后反应 完毕, 加入水、 乙酸乙酯萃取, 合并乙酸乙酯相, 无水石克酸钠干燥, 旋转蒸
发除去溶剂, 经柱层析纯化得产物 (17.6 g,产率 42%)。 2-Bromo-4,4-dimethyl-6,7-dihydrobenzothiazol-5(4/7)-one (35 g, 0.135 mol) and 3-mercaptobenzonitrile hydrochloride ( 45.4 g, 0.269 mol) was dissolved in acetic acid (1 L). After heating at 100 ° C for 2 h, the reaction was completed, and the mixture was extracted with water and ethyl acetate. The ethyl acetate phase was combined, dried over anhydrous sodium sulfate, and evaporated. The solvent was removed and purified by column chromatography to yield (17.6 g, yield 42%).
(6-1) 2-氯 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈的 制备
(6-1) 2-Chloro-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile preparation
将 2-氯 -4,4-二甲基 -5,10-二氢 -4/7-噻唑并 [4,5-b]咔唑 -7-甲腈 (3 g, 9.6 mmol) 溶解于 THF/水 (V/V=9: l)的混合溶液 (50 mL)中, 将 DDQ(3.81 g, 16.8 mmol)加 入到体系中, 氧化过夜。加入乙酸乙酯水萃取, 水洗三次,合并乙酸乙酯相, 旋转蒸发除去溶剂, 剩余物经柱层析纯化得产物(127 mg,产率 4%)。 Dissolving 2-chloro-4,4-dimethyl-5,10-dihydro-4/7-thiazolo[4,5-b]carbazole-7-carbonitrile (3 g, 9.6 mmol) in THF In a mixed solution of water/V (V/V = 9:1) (50 mL), DDQ (3.81 g, 16.8 mmol) was added to the system and oxidized overnight. Extraction with ethyl acetate water, washing with water three times, ethyl acetate phase was combined, solvent was evaporated, and the residue was purified by column chromatography (127 mg, yield 4%).
或者 Or
(6-2) 2-氯 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈的 制备
(6-2) 2-Chloro-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile preparation
将 2-氯 -4,4-二甲基 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (7.6 g, 24.2 mmol)溶解于丙酮 /醋酸 (V/V=2: l)的混合溶剂(220 mL)中, 将重铬酸甲 (15.7 g, 53.4 mmol)加入到体系中,氧化到反应完成,向体系中加入亚石克酸钠,加入水、 乙酸乙酯萃取, 将乙酸乙酯相用饱和氯化钠水溶液洗涤三次, 合并乙酸乙酯 相, 旋转蒸发除去溶剂, 剩余物经柱层析, 得到产物 (5.25 g, 产率 66%)。 Dissolving 2-chloro-4,4-dimethyl-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (7.6 g, 24.2 mmol) in acetone In a mixed solvent of acetic acid (V/V = 2: l) (220 mL), a solution of dichromate (15.7 g, 53.4 mmol) was added to the system, and oxidation was completed until the reaction was completed, and succinic acid was added to the system. Sodium, extracted with water and ethyl acetate. The ethyl acetate phase was washed three times with saturated aqueous sodium chloride, and the ethyl acetate phase was combined, and the solvent was removed by rotary evaporation. 66%).
(7) 8-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2- 基) - (7) 8-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl ) -
将 2-氯 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (300 mg, 0.92 mmol)和 3,8-二氮杂双环 [3.2.1]辛烷 -3-甲酸叔丁基酯 (200 mg, 0.94 mmol)溶于 N-甲基吡咯烷酮(10 mL)中, 加入三乙胺(150 mg, 1.48 mmol), 加 热至 100°C搅拌反应 1小时,冷却至室温,将反应液倒入水中,搅拌半小时, 过滤,所得滤饼干燥后用硅胶柱层析 (二氯甲烷:甲醇 =20: 1)纯化即得产物 (360 mg, 产率 78%)。 2-Chloro-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (300 mg, 0.92 Methyl) and 3,8-diazabicyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester (200 mg, 0.94 mmol) dissolved in N-methylpyrrolidone (10 mL), triethylamine (150 mg, 1.48 mmol), heated to 100 ° C, stirred for 1 hour, cooled to room temperature, poured into water, stirred for half an hour, filtered, and the obtained filter cake was dried and purified by silica gel column chromatography (dichloromethane: Methanol = 20: 1) Purification afforded the product (360 mg, yield 78%).
(8) 2-(3,8-二氮杂又环 [3.2.1]辛烷 -8-基) -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-
[4,5-6]咔唑 -7-甲腈的制备 (8) 2-(3,8-diazacyclo[3.2.1]octane-8-yl)-4,4-dimethyl-10-oxo-5,10-dihydro-4/ 7- Preparation of [4,5-6]carbazole-7-carbonitrile
将 8-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2- 基) -3,8-二氮杂双环 [3.2.1]辛烷 -3-甲酸叔丁基酯 (360 mg, 0.71 mmol)加至二氯 甲烷 (10 mL)中,搅拌下加入三氟乙酸 (5 mL),加入完毕室温搅拌反应 2小时。 将反应液旋转蒸发除去溶剂, 所得粗品倒入饱和碳酸氢钠水溶液中, 搅拌半 小时, 过滤, 所得滤饼用水洗涤, 干燥即得终产物 (250 mg, 产率 87%)。 8-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl) 3,8-Diazabicyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester (360 mg, 0.71 mmol) was added to dichloromethane (10 mL). After the addition, the reaction was stirred at room temperature for 2 hours. The reaction mixture was evaporated to dryness crystals crystals crystals crystals crystals crystals
分子式: C22H21N5OS; 分子量: 403.50; LC-MS(m/z): 404.2[M+H]+ Molecular formula: C 22 H 21 N 5 OS; Molecular weight: 403.50; LC-MS (m/z): 404.2 [M+H] +
-NMR (400MHz, DMSO- 6) δ: 12.78 (s, 1H), 8.19 (d, 1H, J = 8.0 Hz), 7.99 (s, 1H), 7.55 (dd, 1H, J7=1.2Hz, J2 = 8.0Hz), 4.38 (s, 2H), 3.11(d, 2H, J = 12 Hz), 2.91 (d, 2H, J = 12.0 Hz), 2.00-2.07 (m, 4H), 1.67 (s, 6H). -NMR (400MHz, DMSO- 6 ) δ: 12.78 (s, 1H), 8.19 (d, 1H, J = 8.0 Hz), 7.99 (s, 1H), 7.55 (dd, 1H, J7=1.2Hz, J2 = 8.0 Hz), 4.38 (s, 2H), 3.11 (d, 2H, J = 12 Hz), 2.91 (d, 2H, J = 12.0 Hz), 2.00-2.07 (m, 4H), 1.67 (s, 6H) .
实施例 14 Example 14
4,4-二甲基 -2-(6-甲基 -2,6-二氮杂螺【3.31庚烷 -2-基) -10-氧代 -5,10-二氢 - g- 13)的制备
4,4-Dimethyl-2-(6-methyl-2,6-diazaspiro[3.31heptan-2-yl)-10-oxo-5,10-dihydro-g- 13) Preparation
(1) 6-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2- 基) -
(1) 6-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl ) -
将 2-溴 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -7-甲腈 (250 mg, 0.67 mmol)和 2,6-二氮杂螺 [3.3]庚烷 -2-甲酸叔丁基酯 (200 mg, 1.01 mmol) 溶于 N-甲基吡咯烷酮(10 mL)中,加入三乙胺(150 mg, 1.48 mmol),加热至 100°C 搅拌反应 1小时, 冷却至室温, 将反应液倒入水中, 搅拌半小时, 过滤, 所 得滤饼干燥得产物 (280 mg,产率 85%)。 2-Bromo-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]carbazole-7-carbonitrile (250 mg, 0.67 Methyl) and 2,6-diazaspiro[3.3]heptane-2-carboxylic acid tert-butyl ester (200 mg, 1.01 mmol) dissolved in N-methylpyrrolidone (10 mL) with triethylamine (150) Mg, 1.48 mmol), heated to 100 ° C, stirred for 1 hour, cooled to room temperature, poured into water, stirred for half an hour, filtered, and the obtained filter cake was dried to give product (280 mg, yield 85%).
(2) 4,4-二甲基 -10-氧代 -2-(2,6-二氮杂螺 [3.3]庚烷 -2-基) -5,10-二氢 -4/7-噻 唑并 [4,5-6]咔唑 -7-甲腈的制备
(2) 4,4-Dimethyl-10-oxo-2-(2,6-diazaspiro[3.3]heptan-2-yl)-5,10-dihydro-4/7-thiazole And preparation of [4,5-6]carbazole-7-carbonitrile
将 6-(7-氰基 -4,4-二甲基 -10-氧代 -5,10-二氢 -4/7-噻唑并 [4,5-6]咔唑 -2- 基) -2,6-二氮杂螺 [3.3]庚烷 -2-甲酸叔丁基酯 (280 mg, 0.57 mmol)加至二氯甲烷 (10 mL)中, 搅拌下加入三氟乙酸 (10 mL), 加入完毕, 室温搅拌反应 2小时。 将反应液旋转蒸发除去溶剂, 所得粗品倒入乙醚中, 搅拌半小时, 过滤, 所 得滤饼干燥得产物 (220 mg, 产率 99%)。 6-(7-Cyano-4,4-dimethyl-10-oxo-5,10-dihydro-4/7-thiazolo[4,5-6]oxazol-2-yl) Add 2,6-diazaspiro[3.3]heptane-2-carboxylic acid tert-butyl ester (280 mg, 0.57 mmol) to dichloromethane (10 mL). After the addition was completed, the reaction was stirred at room temperature for 2 hours. The reaction mixture was evaporated to dryness crystals crystals crystals crystals crystals crystals
(3) 4,4-二甲基 -2-(6-甲基 -2,6-二氮杂螺 [3.3]庚烷 -2-基) -10-氧代 -5,10-二氢 -4/7-
(3) 4,4-Dimethyl-2-(6-methyl-2,6-diazaspiro[3.3]heptan-2-yl)-10-oxo-5,10-dihydro- 4/7-
将 4,4-二甲基 -10-氧代 -2-(2,6-二氮杂螺 [3.3]庚烷 -2-基) -5,10-二氢 -4/7-噻唑 并 [4,5-6]咔唑 -7-甲腈 (220 mg, 0.56 mmol)溶于甲醇(10 mL)和四氢呋喃的(10 mL)混合溶剂中,加入冰乙酸 (1 mL)、 37%的甲醛水溶液 (2 mL)和钯炭 (50 mg), 氢气加压下室温搅拌 24小时。 过滤, 将滤液旋转蒸发除去溶剂, 加入浓氨水 4,4-Dimethyl-10-oxo-2-(2,6-diazaspiro[3.3]heptan-2-yl)-5,10-dihydro-4/7-thiazolo[ 4,5-6]carbazole-7-carbonitrile (220 mg, 0.56 mmol) was dissolved in a mixture of methanol (10 mL) and tetrahydrofuran (10 mL), glacial acetic acid (1 mL), 37% formaldehyde Aqueous solution (2 mL) and palladium on carbon (50 mg) were stirred at room temperature under hydrogen pressure for 24 hours. Filtration, rotary evaporation of the filtrate to remove the solvent, add concentrated ammonia
(2 mL),搅拌 5分钟,旋转蒸发除去溶剂,加入二氯甲烷 (20 mL)和甲醇 (2 mL) 的混合溶剂,搅拌并过滤,所得滤液浓缩,用硅胶制备板纯化得终产物(182 mg; 产率 80%)。 (2 mL), stirring for 5 minutes, the solvent was removed by rotary evaporation, and a solvent mixture of dichloromethane (20 mL) and methanol (2 mL) was added, and the mixture was stirred and filtered. Mg ; yield 80%).
分子式: C22H21N5OS; 分子量: 403.50; LC-MS(m/z): 404.2[M+H]+ iH-NMR (400MHz, DMSO- 6) δ: 13.09(s, 1H), 8.18 (d, 1H, J = 8.0Hz),Molecular formula: C 22 H 21 N 5 OS; Molecular weight: 403.50; LC-MS (m/z): 404.2 [M+H] + iH-NMR (400MHz, DMSO- 6 ) δ: 13.09 (s, 1H), 8.18 (d, 1H, J = 8.0Hz),
7.98 (s, 1H), 7.54 (dd, 1H, J = 8.0Hz), 4.31 (s, 4H), 4.15 (brs., 4H), 2.69 (s, 3H),7.98 (s, 1H), 7.54 (dd, 1H, J = 8.0Hz), 4.31 (s, 4H), 4.15 (brs., 4H), 2.69 (s, 3H),
1.66 (s, 6H). 1.66 (s, 6H).
根据制备方法部分中举例说明的的制备方法, 以类似于上述实施例的方 式, 制备了下述化合物: According to the preparation method exemplified in the preparation method section, the following compounds were prepared in a manner similar to the above examples:
Claims
1、 通式( I )所示的化合物或其立体异构体、 或其药学上可接受的盐 酯或 A compound represented by the formula (I) or a stereoisomer thereof, or a pharmaceutically acceptable salt thereof or
其中, among them,
选自 C- R1或 N; Selected from C-R 1 or N;
A2选自 1 2或 A 2 is selected from 1 2 or
A3选自 C- R4或 N, 且 Ai、 A2和 A3不同时为 N; A 3 is selected from C-R 4 or N, and Ai, A 2 and A 3 are not N at the same time;
R1 , R2和 R4分别独立地选自氢、 羟基、 羧基、 硝基、 卤素原子、 氨基、 (C1-6烷基 )2氨基、 氰基、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14 元环烷基; R 1 , R 2 and R 4 are each independently selected from the group consisting of hydrogen, hydroxy, carboxy, nitro, halogen atom, amino, (C 1-6 alkyl) 2 amino, cyano, C 1-6 alkyl, C 1 -6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or 3 to 14 membered cycloalkyl;
R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 素原子、 CL6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜14元环烷基, 所述的 C1-6烷基、 C1-6烷 氧基、 C2-6烯基、 C2-6炔基和 3〜14元环烷基可独立地任选被一至多个下列取 代基取代: 羟基、 羧基、 氨基、 氰基、 卤素原子、 硝基或 3〜14元杂环基;R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or a 14-membered cycloalkyl group, the C 1-6 alkyl group, the C 1-6 alkoxy group, the C 2-6 alkenyl group, the C 2-6 alkynyl group and the 3 to 14 membered cycloalkyl group may be independently Optionally substituted by one or more of the following substituents: hydroxy, carboxy, amino, cyano, halo, nitro or 3 to 14 membered heterocyclyl;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基, 所述的 C1-6烷基、 C2-6烯基和 C2-6炔基可独立地任选被 C1-6烷氧基 取代; M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy;
R5和 R6分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 羟基 C1-6 R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
¾^¾ 、 C2-6婦 或。2-6快 3⁄4^3⁄4, C2-6 women or. 2-6 fast
或 R5和 R6相互连接, 与它们所连接的碳原子一起形成 3〜14元杂环基或 3-14元环烷基; Or R 5 and R 6 are bonded to each other to form a 3 to 14 membered heterocyclic group or a 3 to 14 membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 0、 S或 N-R9; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团: Q is selected from the following groups:
( 1 ) 3〜8元杂环基, (1) 3 to 8 membered heterocyclic group,
( 2 )任选被一至三个相同或不同的 R1Q取代的 3〜14元环烷基或 6〜14 元并杂环基, 和
( 3 )任选被一至三个相同或不同的 R1Q取代的 6〜12元桥环基或 6〜12 元螺环基, (2) a 3 to 14 membered cycloalkyl group or a 6 to 14 membered heterocyclic group optionally substituted by one to three identical or different R 1Q groups, and (3) a 6 to 12 membered bridged ring group or a 6 to 12 membered spirocyclic group optionally substituted with one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 CL6烷基氨基羰基、 羟基 CL6烷基、 羟基 CL6烷基氨基、 代 CL6烷基、 d.6 烷基磺酰基、 C 烷基磺酰基氨基、氨基磺酰基、氨基磺酰基氨基、 烯基、R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 6 alkyl, d. 6 alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
。2-6块基; . 2-6 blocks;
R7选自任选被取代基取代的 6〜12元桥环基、 6-12元螺环基、 3〜8元杂 环基或 6〜14元并杂环基, 所述取代基选自氨基、 羟基、 硝基、 素原子、 羧 基、 C1-6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基、 3〜8元杂环基或 3〜8元环烷 基; R 7 is selected from a 6 to 12 membered bridged ring group, a 6 to 12 membered spirocyclic group, a 3 to 8 membered heterocyclic group or a 6 to 14 membered heterocyclic group which is optionally substituted with a substituent selected from the group consisting of Amino, hydroxy, nitro, alkane, carboxyl, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, 3 to 8 membered heterocyclic or 3 ~8-membered cycloalkyl;
n选自 0、 1、 2、 3、 4、 5或 6, n is selected from 0, 1, 2, 3, 4, 5 or 6,
条件是: requirement is:
当 n为 0时, R7不存在, When n is 0, R 7 does not exist.
当 1≥2时, R7可以相同或不同, 且 When 1 ≥ 2, R 7 may be the same or different, and
当 Q选自 3〜8元杂环基时, n不能为 0, 且 R7不能选自 3〜8元杂环基。 When Q is selected from a 3 to 8 membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 3 to 8 membered heterocyclic group.
2、如权利要求 1所述的化合物或其立体异构体、或其药学上可接受的盐 酯或溶剂化物, 其中通式( I )是通式( II ): The compound according to claim 1 or a stereoisomer thereof, or a pharmaceutically acceptable salt or solvate thereof, wherein the formula (I) is a formula (II):
其中, among them,
R1, R2和 R4分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 C2-6 烯基、 C2-6炔基或 3〜8元环烷基; R 1 , R 2 and R 4 are each independently selected from hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, a C 2-6 alkenyl group, a C 2-6 alkynyl group or a 3 to 8 Metacycloalkyl;
R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 素原子、 CL6烷基、 C1-6烷氧基、 C2-6烯基、 C2-6炔基或 3〜8元环烷基, 所述的 C1-6烷基、 C1-6烷 氧基、 C2-6烯基、 C2-6炔基和 3〜8元环烷基可独立地任选被一至三个下列取代 基取代: 羟基、 羧基, 氨基、 氰基、 卤素原子、 硝基或 3〜8元杂环基; R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl, alicyclic, CL 6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl or ~8-membered cycloalkyl, the C 1-6 alkyl group, C 1-6 alkoxy group, C 2-6 alkenyl group, C 2-6 alkynyl group and 3 to 8 membered cycloalkyl group may be independently Substituted by one to three substituents: a hydroxyl group, a carboxyl group, an amino group, a cyano group, a halogen atom, a nitro group or a 3 to 8 membered heterocyclic group;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基、 C1-6烷氧基、 C2-6烯基或 C2-6炔基, 所述的 C1-6烷基、 C2-6烯基和 C2-6炔基可独立地任选被 C1-6烷氧基 取代;
R5和 R6分别独立地选自氢、 卤素原子、 C1-6烷基、 C1-6烷氧基、 羟基 C1-6 M is selected from 0, S or NR 8 and R 8 is selected from hydrogen, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl or C 2-6 alkynyl, said C 1 -6 alkyl, C 2-6 alkenyl and C 2-6 alkynyl may be independently optionally substituted by C 1-6 alkoxy; R 5 and R 6 are each independently selected from the group consisting of hydrogen, a halogen atom, a C 1-6 alkyl group, a C 1-6 alkoxy group, and a hydroxyl group C 1-6
¾^¾ 、 C2-6婦 或。2-6快 3⁄4^3⁄4, C2-6 women or. 2-6 fast
或 R5和 R6相互连接, 与它们所连接的碳原子一起形成 5〜10元杂环基或 3〜8元环烷基; Or R 5 and R 6 are bonded to each other to form a 5- to 10-membered heterocyclic group or a 3- to 8-membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 0、 S或 N-R9; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团: Q is selected from the following groups:
( 1 ) 4〜7元杂环基, (1) 4 to 7 membered heterocyclic group,
( 2 )任选被一至三个相同或不同的 R1Q取代的 3〜8元环烷基或 6〜12 元并杂环基, 和 (2) a 3 to 8 membered cycloalkyl group or a 6 to 12 membered heterocyclic group optionally substituted by one to three identical or different R 1Q groups, and
( 3 )任选被一至三个相同或不同 R1Q取代的 7〜10元桥环基或 6〜12元 螺环基, (3) a 7 to 10 membered bridged ring group or a 6 to 12 membered spirocyclic group optionally substituted by one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 CL6烷基氨基羰基、 羟基 CL6烷基、 羟基 CL6烷基氨基、 代 CL4烷基、 CM 烷基磺酰基、 C 烷基磺酰基氨基、氨基磺酰基、氨基磺酰基氨基、 烯基、R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, CL 6 alkylaminocarbonyl, hydroxy CL 6 Alkyl, hydroxy CL 6 alkylamino, substituted CL 4 alkyl, C M alkylsulfonyl, C alkylsulfonylamino, aminosulfonyl, aminosulfonylamino, alkenyl,
。2-6块基; . 2-6 blocks;
R7选自任选被取代基取代的 6〜10元桥环基、 6-12元螺环基、 4〜7元杂 环基或 6〜12元并杂环基,所述取代基选自氨基、羟基、 卤素原子、 ^6烷基、R 7 is selected from a 6 to 10 membered bridged ring group, a 6 to 12 membered spirocyclic group, a 4 to 7 membered heterocyclic group or a 6 to 12 membered heterocyclic group which is optionally substituted with a substituent selected from the group consisting of Amino group, hydroxyl group, halogen atom, ^ 6 alkyl group,
C 6 ¾* 基、 。2-6婦基或。2-6快基; C 6 3⁄4* base, . 2-6 women's base or. 2-6 fast base;
n选自 0、 1、 2或 3 , n is selected from 0, 1, 2 or 3
条件是: requirement is:
当 n为 0时, R7不存在, When n is 0, R 7 does not exist.
当 1≥2时, R7可以相同或不同, 且 When 1 ≥ 2, R 7 may be the same or different, and
当 Q选自 4〜7元杂环基时, n不能为 0, 且 R7不能选自 4〜7元杂环基。 When Q is selected from a 4 to 7 membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 4 to 7 membered heterocyclic group.
3、 如权利要求 1或 2所述的化合物或其立体异构体、 或其药学上可接受 的盐、 酯或溶剂化物: 3. A compound according to claim 1 or 2, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
其中, among them,
Αι , A2和 A3分别独立地选自 CH; ι ι , A 2 and A 3 are each independently selected from CH;
R3选自氢或氰基; R 3 is selected from hydrogen or cyano;
M选自丽; M is selected from Li;
R5和 R6分别独立地选自氢或 C1-6烷基;
Y选自 N; R 5 and R 6 are each independently selected from hydrogen or C 1-6 alkyl; Y is selected from N;
X选自 S。 X is selected from S.
4、如权利要求 2所述的化合物或其立体异构体、或其药学上可接受的盐、 酯或溶剂化物: 4. A compound according to claim 2, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
其中, among them,
R1 , R2和 R4分别独立地选自氢、 卤素原子、 C1-6烷基或 3〜8元环烷基; R3选自氢、 氰基、 硝基、 羟基、 氨基、 磺酰基、 素原子、 CL6烷基或 3〜8元环烷基; R 1 , R 2 and R 4 are each independently selected from hydrogen, a halogen atom, a C 1-6 alkyl group or a 3 to 8 membered cycloalkyl group; and R 3 is selected from the group consisting of hydrogen, cyano, nitro, hydroxy, amino, sulfonyl An acyl group, a phenol atom, a CL 6 alkyl group or a 3 to 8 membered cycloalkyl group;
M选自 0、 S或 N-R8, R8选自氢、 C1-6烷基或 C1-6烷氧基, 所述的 C1-6 烷基可任选被 ^6烷氧基取代; M is selected from 0, S or NR 8 , R 8 is selected from hydrogen, C 1-6 alkyl or C 1-6 alkoxy, and the C 1-6 alkyl may be optionally substituted by ^ 6 alkoxy ;
R5和 R6分别独立地选自氢、 卤素原子、 ^6烷基、 ^6烷氧基或羟基 C1-6 烷基, R 5 and R 6 are each independently selected from hydrogen, a halogen atom, a ^ 6 alkyl group, a ^ 6 alkoxy group or a hydroxy C 1-6 alkyl group.
或 R5和 R6相互连接,与它们连接的碳原子一起形成 5〜6元杂环基或 3〜8 元环烷基; Or R 5 and R 6 are bonded to each other to form a 5- to 6-membered heterocyclic group or a 3- to 8-membered cycloalkyl group together with the carbon atom to which they are attached;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 0、 S或 N-R9; X is selected from 0, S or NR 9 ;
R9选自氢、 CL6烷基、 C2.6炔基或 3〜8元环烷基; . R 9 is selected from hydrogen, CL 6 alkyl, C 2 6 alkynyl, or 3~8-membered cycloalkyl group;
Q选自下列基团: Q is selected from the following groups:
( 1 ) 5〜6元杂环基, (1) 5 to 6 membered heterocyclic group,
( 2 )任选被一至三个相同或不同的 R1Q取代的 3〜8元环烷基或 6〜10 元并杂环基, 和 (2) a 3 to 8 membered cycloalkyl group or a 6 to 10 membered heterocyclic group optionally substituted by one to three identical or different R 1Q groups, and
( 3 )任选被一至三个相同或不同的 R1Q取代的 7〜9元桥环基或 7〜11 元螺环基, (3) a 7- to 9-membered bridged ring group or a 7 to 11-membered spiro ring group optionally substituted with one to three identical or different R 1Q groups,
R1Q选自氨基、 C1-6烷基、 C1-6烷氧基、 C1-6烷基氨基、 (C1-6烷基 )2氨基、 C1-6烷基氨基羰基、 羟基 C1-6烷基、 羟基 C1-6烷基氨基、 代 CM烷基、 甲基 磺酰基、 甲基磺酰基氨基、 氨基磺酰基、 氨基磺酰基氨基、 C2-6烯基、 C2-6炔 基; R 1Q is selected from the group consisting of amino, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkylamino, (C 1-6 alkyl) 2 amino, C 1-6 alkylaminocarbonyl, hydroxy C 1-6 alkyl, hydroxy C 1-6 alkylamino, C M alkyl, methylsulfonyl, methylsulfonylamino, aminosulfonyl, aminosulfonylamino, C 2-6 alkenyl, C 2-6 alkynyl;
R7选自任选被取代基取代的 7〜10元桥环基、 7〜11元螺环基、 5〜6元杂环 基或 6〜10元并杂环基, 所述取代基选自氨基、 羟基、 卤素原子、 ^6烷基、R 7 is selected from a 7 to 10 membered bridged ring group optionally substituted with a substituent, a 7 to 11 membered spirocyclic group, a 5 to 6 membered heterocyclic group or a 6 to 10 membered heterocyclic group, and the substituent is selected from the group consisting of Amino group, hydroxyl group, halogen atom, ^ 6 alkyl group,
C 6 ¾* 基、 。2-6婦基或。2-6快基, C 6 3⁄4* base, . 2-6 women's base or. 2-6 fast base,
n选自 0、 1、 2或 3 , n is selected from 0, 1, 2 or 3
条件是:
当 n为 0时, R7不存在, requirement is: When n is 0, R 7 does not exist.
当 1≥2时, R7可以相同或不同, 且 When 1 ≥ 2, R 7 may be the same or different, and
当 Q选自 5〜6元杂环基时, n不能为 0, 且 R7不能选自 5〜6元杂环基。 When Q is selected from a 5- to 6-membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 5- to 6-membered heterocyclic group.
5、如权利要求 4所述的化合物或其立体异构体、或其药学上可接受的盐、 酯或溶剂化物: 5. A compound according to claim 4, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
其中, among them,
R1, R2和 R4分别独立地选自氢, 甲基或乙基; R 1 , R 2 and R 4 are each independently selected from hydrogen, methyl or ethyl;
R3选自氢、 氰基、 羟基、 氨基、 氟原子、 氯原子、 甲基或乙基; R 3 is selected from the group consisting of hydrogen, cyano, hydroxy, amino, fluorine, chlorine, methyl or ethyl;
M选自 N-R8, R8选自氢或 CL4烷基; M is selected from NR 8 and R 8 is selected from hydrogen or CL 4 alkyl;
R5和 R6分别独立地选自 C1-4烷基; R 5 and R 6 are each independently selected from C 1-4 alkyl;
Y选自 N或 C-R9; Y is selected from N or CR 9 ;
X选自 S或 N-R9; X is selected from S or NR 9 ;
R9选自氢、 甲基、 乙基或正丙基; R 9 is selected from the group consisting of hydrogen, methyl, ethyl or n-propyl;
Q选自 Q is selected from
(1) 5-6元杂环基, (1) 5-6 membered heterocyclic group,
(2)任选被一至两个相同或不同的 R1Q取代的 6〜10元并杂环基, 和(2) a 6 to 10 membered heterocyclic group optionally substituted by one to two identical or different R 1Q groups, and
(3)任选被一至两个相同或不同的 R1Q取代的 7〜9元桥环基或 7〜11元螺 环基, (3) a 7- to 9-membered bridged ring group or a 7 to 11-membered spiro ring group optionally substituted with one to two identical or different R 1Q groups,
R1Q选自氨基或 CL4烷基; R 1Q is selected from amino or CL 4 alkyl;
R7选自 7〜9元桥环基、 7〜11元螺环基或 5〜6元杂环基, R 7 is selected from the group consisting of 7 to 9 membered bridged ring groups, 7 to 11 membered spirocyclic groups or 5 to 6 membered heterocyclic groups.
n选自 0或 1 , n is selected from 0 or 1
条件是: requirement is:
当 n为 0时, R7不存在, 且 When n is 0, R 7 does not exist, and
当 Q选自 5〜6元杂环基时, n不能为 0, 且 R7不能选自 5〜6元杂环基。 When Q is selected from a 5- to 6-membered heterocyclic group, n cannot be 0, and R 7 cannot be selected from a 5- to 6-membered heterocyclic group.
6、如权利要求 1所述的化合物或其立体异构体、或其药学上可接受的盐、 酯或溶剂化物: 6. A compound according to claim 1 or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
其中, among them,
Q选自 4〜7元杂环基, 优选地含有 1或 2个氮原子作为环原子, 更优选 地是饱和的; Q is selected from a 4 to 7 membered heterocyclic group, preferably having 1 or 2 nitrogen atoms as a ring atom, more preferably saturated;
R7选自 7〜8元桥环基, 优选地含有 1或 2个选自氧和氮的环原子, 更优 选地是饱和的;
n选自 1。 R 7 is selected from 7 to 8 membered bridged ring groups, preferably containing 1 or 2 ring atoms selected from oxygen and nitrogen, more preferably saturated; n is selected from 1.
7、如权利要求 6所述的化合物或其立体异构体、或其药学上可接受的盐 酯或溶剂化物: 7. A compound according to claim 6 or a stereoisomer thereof, or a pharmaceutically acceptable salt or solvate thereof:
其中, among them,
η选自 1。 η is selected from 1.
8、如权利要求 1所述的化合物或其立体异构体、或其药学上可接受的盐、 酯或溶剂化物: The compound of claim 1 or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
其中, among them,
Q选自任选被一至两个相同或不同的 R1Q取代的 7〜8元桥环基,优选地含 有 1或 2个氮原子作为环原子, 更优选地是饱和的, Q is selected from optionally substituted with one to two identical or different substituents R 1Q of 7~8 membered bridged ring group, preferably containing 1 or 2 nitrogen atoms as ring atoms, more preferably a saturated,
R1Q选自氨基或 CL6烷基; R 1Q is selected from amino or CL 6 alkyl;
R7选自 4〜7元杂环基, 优选地含有 1或 2个选自氧和氮的环原子, 更优 选地是饱和的; R 7 is selected from a 4 to 7 membered heterocyclic group, preferably contains 1 or 2 ring atoms selected from oxygen and nitrogen, more preferably saturated;
n选自 0或 1 , 当 n为 0时, R7不存在。 n is selected from 0 or 1, and when n is 0, R 7 is absent.
9、如权利要求 8所述的化合物或其立体异构体、或其药学上可接受的盐 酯或溶剂化物: 9. A compound according to claim 8 or a stereoisomer thereof, or a pharmaceutically acceptable salt or solvate thereof:
其中, among them,
Q选自任选被一至两个相同或不同的 R1Q取代的Q is selected from the group consisting of one or two identical or different R 1Q
或- - N、 N- - R1Q选自氨基或 CL4烷基; Or - N, N- - R 1Q is selected from amino or CL 4 alkyl;
R选自 、 R is selected from ,
+Ν 0 、+Ν 0,
10、 如权利要求 1所述的化合物或其立体异构体、 或其药学上可接受的 盐、 酯或溶剂化物: 10. A compound according to claim 1 or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
其中, among them,
Q选自任选被一至两个相同或不同的 R1Q取代的 7〜11元螺环基, 所述螺 环基优选是含有 1-3个杂原子的螺环基, 更优选地所述螺环基含有 7-11个环 原子,其中 1或 2个环原子是氮原子,其余的环原子是碳原子,还更优选地, 所述螺环基是饱和基团, Q is selected from 7 to 11 membered spirocyclic groups optionally substituted by one to two identical or different R 1Q groups, preferably a spirocyclic group having 1 to 3 hetero atoms, more preferably the spiro group The cyclic group contains 7 to 11 ring atoms, wherein 1 or 2 ring atoms are nitrogen atoms, the remaining ring atoms are carbon atoms, and still more preferably, the spiro group is a saturated group,
R1Q选自氨基或 CL4烷基; R 1Q is selected from amino or CL 4 alkyl;
R7不存在。 R 7 does not exist.
11、 如权利要求 1 所述的化合物或其立体异构体、 或其药学上可接受的 盐、 酯或溶剂化物: 11. A compound according to claim 1 or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof:
其中, among them,
Q选自任选被一至两个相同或不同的 R1Q取代的 6〜10元并杂环基, 所述 并杂环基优选是含有 1-3 个杂原子的并杂环基, 更优选地所述并杂环基含有 6〜10个环原子, 其中 1-3个环原子是选自氮和氧的杂原子, 其余的环原子是 碳原子, 还更优选地, 所述并杂环基是饱和基团, Q is selected from 6 to 10 membered heterocyclic groups optionally substituted by one to two identical or different R 1Q , and the heterocyclic group is preferably a heterocyclic group having 1 to 3 hetero atoms, more preferably The heterocyclic group contains 6 to 10 ring atoms, wherein 1 to 3 ring atoms are hetero atoms selected from nitrogen and oxygen, and the remaining ring atoms are carbon atoms, still more preferably, the heterocyclic group Is a saturated group,
R1Q选自氨基或 CL4烷基; R 1Q is selected from amino or CL 4 alkyl;
R7不存在。 R 7 does not exist.
12、 如权利要求 1所述的化合物或其立体异构体、 或其药学上可接受的 盐、 酯或溶剂化物, 所述化合物选自: 12. The compound of claim 1 or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, selected from the group consisting of:
L9 L9
13、 一种药物组合物, 其包括权利要求 1-12任一权利要求所述的化合物 或其立体异构体、 或其药学上可接受的盐、 酯或溶剂化物与一种或多种药用 载体和 /或稀释剂。 A pharmaceutical composition comprising a compound according to any one of claims 1 to 12, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, and one or more drugs Use a carrier and / or a diluent.
14、 如权利要求 13所述的药物组合物, 其特征在于还可含有一种或多种 抗肿瘤剂和免疫抑制剂, 所述的抗肿瘤剂和免疫抑制剂选自甲氨蝶呤、 卡培 他滨、 吉西他滨、 去氧氟尿苷、 培美曲塞二钠、 帕唑帕尼、 伊马替尼、 埃罗 替尼、 拉帕替尼、 吉非替尼、 凡德他尼、 赫赛汀、 贝伐单抗、 利妥昔单抗、 曲妥珠单抗、 紫杉醇、 长春瑞滨、 多西他赛、 多柔比星、 羟基喜树碱、 丝裂 霉素、 表柔比星、 吡柔比星、 博来霉素、 来曲唑、 他莫西芬、 氟维司群、 曲 谱瑞林、 氟他胺、 亮丙瑞林、 阿那曲唑、 异环磷酰胺、 白消安、 环磷酰胺、 卡莫司汀、 尼莫司汀、 司莫司汀、 氮芥、 马法兰、 瘤可宁、 卡铂、 顺铂、 奥 沙利铂、 络铂、 拓朴特肯、 喜树碱、 拓朴替康、 依维莫司、 西罗莫斯、 特癌 适、 6-巯基嘌呤、 6-石克鸟嘌呤、 石克唑嘌呤、 菌素 D、 柔红霉素、 阿霉素、 米托 蒽醌、 争光霉素、 普卡霉素或氨鲁米特。 The pharmaceutical composition according to claim 13, which may further comprise one or more antitumor agents and immunosuppressive agents, wherein said antitumor agent and immunosuppressive agent are selected from the group consisting of methotrexate and card Peitabin, gemcitabine, deoxyfluorouridine, pemetrexed disodium, pazopanib, imatinib, erlotinib, lapatinib, gefitinib, vandetanib, hep Statin, bevacizumab, rituximab, trastuzumab, paclitaxel, vinorelbine, docetaxel, doxorubicin, hydroxycamptothecin, mitomycin, epirubicin , pirarubicin, bleomycin, letrozole, tamoxifen, fulvestrant, triclinin, flutamide, leuprolide, anastrozole, ifosfamide, busulfan , cyclophosphamide, carmustine, nimustine, semustine, nitrogen mustard, melphalan, cyclamate, carboplatin, cisplatin, oxaliplatin, platinum, topotecan, hi-tree Alkali, Topotecan, Everolimus, Sirolimus, Tetracarcinoma, 6-mercaptopurine, 6-stone guanine, gramazole Methotrexate, streptozotocin D, daunorubicin, doxorubicin, mitoxantrone, bleomycin, mithramycin or aminoglutethimide.
15、 如权利要求 1-12任一项所述的化合物或其立体异构体、 或其药学上 可接受的盐、 酯或溶剂化物在制备用于治疗和 /或预防 ALK介导的癌症相关 疾病的药物中的应用, 所述癌症相关的疾病选自脑瘤、 非小细胞性肺癌、 鳞 状上皮细胞癌、 膀胱癌、 胃癌、 卵巢癌、 腹膜癌、 胰腺癌、 乳腺癌、 头颈癌、 子宫颈癌、 子宫内膜癌、 直肠癌、 肝癌、 肝母细胞瘤、 乳头状肾细胞瘤、 头 颈部鳞状细胞瘤、 肾母细胞瘤、 肾癌、 食管腺癌、 食管鳞状细胞癌、 神经胶 质瘤、 前列腺癌、 甲状腺癌、 雌性生殖道癌、 原位癌、 淋巴瘤、 成神经细胞 瘤、 神经纤维瘤病、 骨癌、 皮肤癌、 结肠癌、 睾丸癌、 小细胞肺癌、 胃肠道 间质瘤、 前列腺肿瘤、 肥大细胞肿瘤、 多发性骨髓瘤或黑色素瘤。 15. A compound according to any one of claims 1 to 12, or a stereoisomer thereof, or a pharmaceutically acceptable salt, ester or solvate thereof, for use in the manufacture and/or prevention of ALK-mediated cancer. The use of a disease-related disease selected from the group consisting of a brain tumor, a non-small cell lung cancer, a squamous cell carcinoma, a bladder cancer, a stomach cancer, an ovarian cancer, a peritoneal cancer, a pancreatic cancer, a breast cancer, a head and neck cancer, Cervical cancer, endometrial cancer, rectal cancer, liver cancer, hepatoblastoma, papillary renal cell tumor, head and neck squamous cell tumor, nephroblastoma, renal cancer, esophageal adenocarcinoma, esophageal squamous cell carcinoma , glioma, prostate cancer, thyroid cancer, female genital tract cancer, carcinoma in situ, lymphoma, neuroblastoma, neurofibromatosis, bone cancer, skin cancer, colon cancer, testicular cancer, small cell lung cancer, Gastrointestinal stromal tumors, prostate tumors, mast cell tumors, multiple myeloma or melanoma.
16、 一种制备下述的通式 (Γ ) 的化合物的方法,
16. A method of preparing a compound of the formula (Γ) described below,
其包括以下步骤: It includes the following steps:
在加热条件下反应 React under heating
或者 Or
下在室温反应, 然后再加入还原剂反应, Reacting at room temperature, then adding a reducing agent,
其中, A2、 A3、 M、 Y、 X、 R3、 R5、 R6、 R7、 Q和 n如权利要求 1 中所定义, Q'为如权利要求 1中所定义的 Q或者被保护基团 (PG )保护的如 权利要求 1中所定义的 Q , R7'为如权利要求 1中所定义的 R7或者被保护基团 ( PG )保护的如权利要求 1中所定义的 R7。
Wherein A 2 , A 3 , M, Y, X, R 3 , R 5 , R 6 , R 7 , Q and n are as defined in claim 1, Q' is Q as defined in claim 1 or Q, R 7 ' as defined in claim 1 protected by a protecting group (PG) is R 7 as defined in claim 1 or protected by a protecting group (PG) as defined in claim 1. R 7 .
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201480031459.6A CN105579459B (en) | 2013-06-01 | 2014-06-03 | Four and ring class anaplastic lymphoma kinase inhibitor |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201310214667.7 | 2013-06-01 | ||
| CN201310214667 | 2013-06-01 | ||
| CN201410111912 | 2014-03-24 | ||
| CN201410111912.6 | 2014-03-24 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014190949A1 true WO2014190949A1 (en) | 2014-12-04 |
Family
ID=51988037
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2014/079072 WO2014190949A1 (en) | 2013-06-01 | 2014-06-03 | Quadri-fused cyclic anaplastic lymphoma kinase inhibitor |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN105579459B (en) |
| WO (1) | WO2014190949A1 (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102459172A (en) * | 2009-06-10 | 2012-05-16 | 中外制药株式会社 | Tetracyclic compounds |
| CN103052386A (en) * | 2010-08-20 | 2013-04-17 | 中外制药株式会社 | Composition containing tetracyclic compound |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102443009B (en) * | 2010-09-30 | 2014-04-16 | 山东轩竹医药科技有限公司 | Fused ring kinase inhibitor |
-
2014
- 2014-06-03 CN CN201480031459.6A patent/CN105579459B/en active Active
- 2014-06-03 WO PCT/CN2014/079072 patent/WO2014190949A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102459172A (en) * | 2009-06-10 | 2012-05-16 | 中外制药株式会社 | Tetracyclic compounds |
| CN103052386A (en) * | 2010-08-20 | 2013-04-17 | 中外制药株式会社 | Composition containing tetracyclic compound |
Also Published As
| Publication number | Publication date |
|---|---|
| CN105579459A (en) | 2016-05-11 |
| CN105579459B (en) | 2018-03-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10919913B2 (en) | Spiro[3H-indole-3,2′-pyrrolidin]-2(1H)-one compounds and derivatives as MDM2-p53 inhibitors | |
| US10882866B1 (en) | Spiro[3H-indole-3,2′-pyrrolidin]-2(1H)-one compounds and derivatives as MDM2-P53 inhibitors | |
| US10138251B2 (en) | Spiro[3H-indole-3,2′-pyrrolidin]-2(1H)-one compounds and derivatives as MDM2-P53 inhibitors | |
| EP3164401A1 (en) | New spiro[3h-indole-3,2´-pyrrolidin]-2(1h)-one compounds and derivatives as mdm2-p53 inhibitors | |
| TW202340209A (en) | Annulated 2-amino-3-cyano thiophenes and derivatives for the treatment of cancer | |
| JP2021503479A (en) | Substituted heteroaryl compound and usage | |
| WO2014190949A1 (en) | Quadri-fused cyclic anaplastic lymphoma kinase inhibitor | |
| CN104230960B (en) | Four-ring anaplastic lymphoma kinase inhibitor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201480031459.6 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14804314 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14804314 Country of ref document: EP Kind code of ref document: A1 |