WO2014183169A1 - Method for producing hollow structures - Google Patents
Method for producing hollow structures Download PDFInfo
- Publication number
- WO2014183169A1 WO2014183169A1 PCT/AU2014/050038 AU2014050038W WO2014183169A1 WO 2014183169 A1 WO2014183169 A1 WO 2014183169A1 AU 2014050038 W AU2014050038 W AU 2014050038W WO 2014183169 A1 WO2014183169 A1 WO 2014183169A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- metal
- hollow structures
- shelled
- shelled hollow
- particles
- Prior art date
Links
- 238000004519 manufacturing process Methods 0.000 title description 9
- 238000000034 method Methods 0.000 claims abstract description 66
- 229910052751 metal Inorganic materials 0.000 claims abstract description 35
- 239000002184 metal Substances 0.000 claims abstract description 35
- 239000011246 composite particle Substances 0.000 claims abstract description 29
- 150000002736 metal compounds Chemical class 0.000 claims abstract description 26
- 229910044991 metal oxide Inorganic materials 0.000 claims abstract description 25
- 150000004706 metal oxides Chemical class 0.000 claims abstract description 25
- 238000001694 spray drying Methods 0.000 claims abstract description 18
- 238000010438 heat treatment Methods 0.000 claims abstract description 16
- 150000002894 organic compounds Chemical class 0.000 claims abstract description 16
- 239000011368 organic material Substances 0.000 claims abstract description 16
- 239000002245 particle Substances 0.000 claims description 66
- 239000005720 sucrose Substances 0.000 claims description 42
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 37
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 34
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 31
- 229930006000 Sucrose Natural products 0.000 claims description 31
- 229910052595 hematite Inorganic materials 0.000 claims description 18
- LIKBJVNGSGBSGK-UHFFFAOYSA-N iron(3+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Fe+3].[Fe+3] LIKBJVNGSGBSGK-UHFFFAOYSA-N 0.000 claims description 18
- 239000011019 hematite Substances 0.000 claims description 17
- 229910000000 metal hydroxide Inorganic materials 0.000 claims description 17
- 150000004692 metal hydroxides Chemical class 0.000 claims description 17
- 239000000203 mixture Substances 0.000 claims description 16
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 claims description 12
- 229910001416 lithium ion Inorganic materials 0.000 claims description 12
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 claims description 11
- 238000001354 calcination Methods 0.000 claims description 11
- 239000000356 contaminant Substances 0.000 claims description 11
- 229910001385 heavy metal Inorganic materials 0.000 claims description 10
- 238000002156 mixing Methods 0.000 claims description 9
- 239000002243 precursor Substances 0.000 claims description 9
- 239000007787 solid Substances 0.000 claims description 8
- 150000001336 alkenes Chemical class 0.000 claims description 7
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 6
- 150000001335 aliphatic alkanes Chemical class 0.000 claims description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 6
- 150000001875 compounds Chemical class 0.000 claims description 6
- 239000007772 electrode material Substances 0.000 claims description 6
- 239000008103 glucose Substances 0.000 claims description 6
- 150000002500 ions Chemical class 0.000 claims description 6
- 230000001590 oxidative effect Effects 0.000 claims description 6
- 239000004372 Polyvinyl alcohol Substances 0.000 claims description 5
- 229910052799 carbon Inorganic materials 0.000 claims description 5
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 5
- 239000002904 solvent Substances 0.000 claims description 5
- 238000006356 dehydrogenation reaction Methods 0.000 claims description 4
- 239000000843 powder Substances 0.000 claims description 4
- 238000012545 processing Methods 0.000 claims description 4
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 claims description 3
- 230000003247 decreasing effect Effects 0.000 claims description 3
- 235000006408 oxalic acid Nutrition 0.000 claims description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 claims description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 claims description 2
- 239000002202 Polyethylene glycol Substances 0.000 claims description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 2
- 150000004703 alkoxides Chemical class 0.000 claims description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 claims description 2
- 239000012876 carrier material Substances 0.000 claims description 2
- 229940050410 gluconate Drugs 0.000 claims description 2
- 235000001727 glucose Nutrition 0.000 claims description 2
- 150000002506 iron compounds Chemical class 0.000 claims description 2
- 229910001960 metal nitrate Inorganic materials 0.000 claims description 2
- 229920001223 polyethylene glycol Polymers 0.000 claims description 2
- 235000019422 polyvinyl alcohol Nutrition 0.000 claims description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 2
- 239000000758 substrate Substances 0.000 claims description 2
- 229910021653 sulphate ion Inorganic materials 0.000 claims description 2
- 229910001510 metal chloride Inorganic materials 0.000 claims 1
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 51
- 229910052785 arsenic Inorganic materials 0.000 description 44
- 239000000243 solution Substances 0.000 description 40
- RQNWIZPPADIBDY-UHFFFAOYSA-N arsenic atom Chemical compound [As] RQNWIZPPADIBDY-UHFFFAOYSA-N 0.000 description 37
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 29
- 239000000463 material Substances 0.000 description 29
- 230000015572 biosynthetic process Effects 0.000 description 24
- MVFCKEFYUDZOCX-UHFFFAOYSA-N iron(2+);dinitrate Chemical compound [Fe+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O MVFCKEFYUDZOCX-UHFFFAOYSA-N 0.000 description 19
- 238000001179 sorption measurement Methods 0.000 description 18
- 229910052742 iron Inorganic materials 0.000 description 15
- 235000013980 iron oxide Nutrition 0.000 description 15
- 239000004005 microsphere Substances 0.000 description 13
- 239000002131 composite material Substances 0.000 description 12
- 238000003860 storage Methods 0.000 description 12
- 238000003786 synthesis reaction Methods 0.000 description 12
- 238000003917 TEM image Methods 0.000 description 11
- -1 phosphate anions Chemical class 0.000 description 11
- 239000007921 spray Substances 0.000 description 11
- 230000008602 contraction Effects 0.000 description 10
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 9
- 239000000047 product Substances 0.000 description 9
- 239000003463 adsorbent Substances 0.000 description 8
- 150000001450 anions Chemical class 0.000 description 7
- 238000000137 annealing Methods 0.000 description 7
- 239000010405 anode material Substances 0.000 description 7
- 229920000447 polyanionic polymer Polymers 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 6
- 239000011651 chromium Substances 0.000 description 6
- 230000001351 cycling effect Effects 0.000 description 6
- 229910052744 lithium Inorganic materials 0.000 description 6
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 5
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 5
- 238000002441 X-ray diffraction Methods 0.000 description 5
- 239000011149 active material Substances 0.000 description 5
- 239000011230 binding agent Substances 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 229910052804 chromium Inorganic materials 0.000 description 5
- 239000007789 gas Substances 0.000 description 5
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 5
- 239000010452 phosphate Substances 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- 229910019142 PO4 Inorganic materials 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 239000010941 cobalt Substances 0.000 description 4
- 229910017052 cobalt Inorganic materials 0.000 description 4
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 238000000445 field-emission scanning electron microscopy Methods 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- NPFOYSMITVOQOS-UHFFFAOYSA-K iron(III) citrate Chemical compound [Fe+3].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NPFOYSMITVOQOS-UHFFFAOYSA-K 0.000 description 4
- 239000011159 matrix material Substances 0.000 description 4
- 229910052759 nickel Inorganic materials 0.000 description 4
- 238000005839 oxidative dehydrogenation reaction Methods 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 239000007858 starting material Substances 0.000 description 4
- 239000011800 void material Substances 0.000 description 4
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 3
- 239000005977 Ethylene Substances 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 239000004411 aluminium Substances 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 3
- SMYKVLBUSSNXMV-UHFFFAOYSA-K aluminum;trihydroxide;hydrate Chemical compound O.[OH-].[OH-].[OH-].[Al+3] SMYKVLBUSSNXMV-UHFFFAOYSA-K 0.000 description 3
- 229910052787 antimony Inorganic materials 0.000 description 3
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 3
- 229910052793 cadmium Inorganic materials 0.000 description 3
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000012612 commercial material Substances 0.000 description 3
- 238000000354 decomposition reaction Methods 0.000 description 3
- 238000009792 diffusion process Methods 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 239000003792 electrolyte Substances 0.000 description 3
- 238000000605 extraction Methods 0.000 description 3
- 238000000349 field-emission scanning electron micrograph Methods 0.000 description 3
- 238000002173 high-resolution transmission electron microscopy Methods 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- VBMVTYDPPZVILR-UHFFFAOYSA-N iron(2+);oxygen(2-) Chemical class [O-2].[Fe+2] VBMVTYDPPZVILR-UHFFFAOYSA-N 0.000 description 3
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 239000002114 nanocomposite Substances 0.000 description 3
- 239000002086 nanomaterial Substances 0.000 description 3
- 238000004098 selected area electron diffraction Methods 0.000 description 3
- 239000002002 slurry Substances 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- LLZRNZOLAXHGLL-UHFFFAOYSA-J titanic acid Chemical compound O[Ti](O)(O)O LLZRNZOLAXHGLL-UHFFFAOYSA-J 0.000 description 3
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- XHCLAFWTIXFWPH-UHFFFAOYSA-N [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] XHCLAFWTIXFWPH-UHFFFAOYSA-N 0.000 description 2
- 239000006230 acetylene black Substances 0.000 description 2
- 238000001994 activation Methods 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 239000001569 carbon dioxide Substances 0.000 description 2
- 229910002092 carbon dioxide Inorganic materials 0.000 description 2
- 230000003197 catalytic effect Effects 0.000 description 2
- 239000013043 chemical agent Substances 0.000 description 2
- 239000000571 coke Substances 0.000 description 2
- 239000007771 core particle Substances 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 239000003651 drinking water Substances 0.000 description 2
- 235000020188 drinking water Nutrition 0.000 description 2
- 210000002969 egg yolk Anatomy 0.000 description 2
- 238000002848 electrochemical method Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000001125 extrusion Methods 0.000 description 2
- 239000010419 fine particle Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 229910052750 molybdenum Inorganic materials 0.000 description 2
- 239000011733 molybdenum Substances 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000011164 primary particle Substances 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000004227 thermal cracking Methods 0.000 description 2
- 229910052718 tin Inorganic materials 0.000 description 2
- 239000011135 tin Substances 0.000 description 2
- VXUYXOFXAQZZMF-UHFFFAOYSA-N titanium(IV) isopropoxide Chemical compound CC(C)O[Ti](OC(C)C)(OC(C)C)OC(C)C VXUYXOFXAQZZMF-UHFFFAOYSA-N 0.000 description 2
- 229910052720 vanadium Inorganic materials 0.000 description 2
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 2
- 229910001935 vanadium oxide Inorganic materials 0.000 description 2
- ONDPHDOFVYQSGI-UHFFFAOYSA-N zinc nitrate Chemical compound [Zn+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O ONDPHDOFVYQSGI-UHFFFAOYSA-N 0.000 description 2
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- DJHGAFSJWGLOIV-UHFFFAOYSA-K Arsenate3- Chemical compound [O-][As]([O-])([O-])=O DJHGAFSJWGLOIV-UHFFFAOYSA-K 0.000 description 1
- 101100223811 Caenorhabditis elegans dsc-1 gene Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- JJLJMEJHUUYSSY-UHFFFAOYSA-L Copper hydroxide Chemical compound [OH-].[OH-].[Cu+2] JJLJMEJHUUYSSY-UHFFFAOYSA-L 0.000 description 1
- 239000005750 Copper hydroxide Substances 0.000 description 1
- 229910002483 Cu Ka Inorganic materials 0.000 description 1
- OIFBSDVPJOWBCH-UHFFFAOYSA-N Diethyl carbonate Chemical compound CCOC(=O)OCC OIFBSDVPJOWBCH-UHFFFAOYSA-N 0.000 description 1
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 description 1
- 229910017356 Fe2C Inorganic materials 0.000 description 1
- 229910015136 FeMn Inorganic materials 0.000 description 1
- 229910013872 LiPF Inorganic materials 0.000 description 1
- 101150058243 Lipf gene Proteins 0.000 description 1
- 229910018654 Mn Ox Inorganic materials 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- WGLPBDUCMAPZCE-UHFFFAOYSA-N Trioxochromium Chemical compound O=[Cr](=O)=O WGLPBDUCMAPZCE-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- WQHONKDTTOGZPR-UHFFFAOYSA-N [O-2].[O-2].[Mn+2].[Fe+2] Chemical class [O-2].[O-2].[Mn+2].[Fe+2] WQHONKDTTOGZPR-UHFFFAOYSA-N 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 229910021502 aluminium hydroxide Inorganic materials 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000006183 anode active material Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 229940000489 arsenate Drugs 0.000 description 1
- HAYXDMNJJFVXCI-UHFFFAOYSA-N arsenic(5+) Chemical compound [As+5] HAYXDMNJJFVXCI-UHFFFAOYSA-N 0.000 description 1
- AQLMHYSWFMLWBS-UHFFFAOYSA-N arsenite(1-) Chemical compound O[As](O)[O-] AQLMHYSWFMLWBS-UHFFFAOYSA-N 0.000 description 1
- UNTBPXHCXVWYOI-UHFFFAOYSA-O azanium;oxido(dioxo)vanadium Chemical compound [NH4+].[O-][V](=O)=O UNTBPXHCXVWYOI-UHFFFAOYSA-O 0.000 description 1
- 238000000498 ball milling Methods 0.000 description 1
- 230000003796 beauty Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 239000006229 carbon black Substances 0.000 description 1
- 239000002041 carbon nanotube Substances 0.000 description 1
- 229910021393 carbon nanotube Inorganic materials 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 239000004568 cement Substances 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000013626 chemical specie Substances 0.000 description 1
- 229910000423 chromium oxide Inorganic materials 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 229910001956 copper hydroxide Inorganic materials 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- IEJIGPNLZYLLBP-UHFFFAOYSA-N dimethyl carbonate Chemical compound COC(=O)OC IEJIGPNLZYLLBP-UHFFFAOYSA-N 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 238000004146 energy storage Methods 0.000 description 1
- 238000005562 fading Methods 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 229910021389 graphene Inorganic materials 0.000 description 1
- 229910002804 graphite Inorganic materials 0.000 description 1
- 239000010439 graphite Substances 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 238000000024 high-resolution transmission electron micrograph Methods 0.000 description 1
- 239000011796 hollow space material Substances 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 238000001027 hydrothermal synthesis Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000010842 industrial wastewater Substances 0.000 description 1
- 238000009830 intercalation Methods 0.000 description 1
- 230000002687 intercalation Effects 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 235000014413 iron hydroxide Nutrition 0.000 description 1
- DALUDRGQOYMVLD-UHFFFAOYSA-N iron manganese Chemical compound [Mn].[Fe] DALUDRGQOYMVLD-UHFFFAOYSA-N 0.000 description 1
- KFZAUHNPPZCSCR-UHFFFAOYSA-N iron zinc Chemical compound [Fe].[Zn] KFZAUHNPPZCSCR-UHFFFAOYSA-N 0.000 description 1
- NCNCGGDMXMBVIA-UHFFFAOYSA-L iron(ii) hydroxide Chemical compound [OH-].[OH-].[Fe+2] NCNCGGDMXMBVIA-UHFFFAOYSA-L 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910001463 metal phosphate Inorganic materials 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 229910003455 mixed metal oxide Inorganic materials 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 239000002073 nanorod Substances 0.000 description 1
- 239000002135 nanosheet Substances 0.000 description 1
- 239000003345 natural gas Substances 0.000 description 1
- BFDHFSHZJLFAMC-UHFFFAOYSA-L nickel(ii) hydroxide Chemical compound [OH-].[OH-].[Ni+2] BFDHFSHZJLFAMC-UHFFFAOYSA-L 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 238000010951 particle size reduction Methods 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 238000003672 processing method Methods 0.000 description 1
- 238000010298 pulverizing process Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000006479 redox reaction Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 230000009919 sequestration Effects 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 238000004729 solvothermal method Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000004230 steam cracking Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000011232 storage material Substances 0.000 description 1
- 238000002411 thermogravimetry Methods 0.000 description 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- YONPGGFAJWQGJC-UHFFFAOYSA-K titanium(iii) chloride Chemical compound Cl[Ti](Cl)Cl YONPGGFAJWQGJC-UHFFFAOYSA-K 0.000 description 1
- 238000004627 transmission electron microscopy Methods 0.000 description 1
- 238000001106 transmission high energy electron diffraction data Methods 0.000 description 1
- ZSDSQXJSNMTJDA-UHFFFAOYSA-N trifluralin Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O ZSDSQXJSNMTJDA-UHFFFAOYSA-N 0.000 description 1
- 229910000406 trisodium phosphate Inorganic materials 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
- 230000037303 wrinkles Effects 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/52—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron
- H01M4/525—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron of mixed oxides or hydroxides containing iron, cobalt or nickel for inserting or intercalating light metals, e.g. LiNiO2, LiCoO2 or LiCoOxFy
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J13/00—Colloid chemistry, e.g. the production of colloidal materials or their solutions, not otherwise provided for; Making microcapsules or microballoons
- B01J13/02—Making microcapsules or microballoons
- B01J13/20—After-treatment of capsule walls, e.g. hardening
- B01J13/22—Coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/16—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of arsenic, antimony, bismuth, vanadium, niobium, tantalum, polonium, chromium, molybdenum, tungsten, manganese, technetium or rhenium
- B01J23/20—Vanadium, niobium or tantalum
- B01J23/22—Vanadium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/16—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of arsenic, antimony, bismuth, vanadium, niobium, tantalum, polonium, chromium, molybdenum, tungsten, manganese, technetium or rhenium
- B01J23/24—Chromium, molybdenum or tungsten
- B01J23/26—Chromium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/70—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper
- B01J23/74—Iron group metals
- B01J23/745—Iron
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/40—Catalysts, in general, characterised by their form or physical properties characterised by dimensions, e.g. grain size
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/50—Catalysts, in general, characterised by their form or physical properties characterised by their shape or configuration
- B01J35/51—Spheres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/0009—Use of binding agents; Moulding; Pressing; Powdering; Granulating; Addition of materials ameliorating the mechanical properties of the product catalyst
- B01J37/0018—Addition of a binding agent or of material, later completely removed among others as result of heat treatment, leaching or washing,(e.g. forming of pores; protective layer, desintegrating by heat)
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/0009—Use of binding agents; Moulding; Pressing; Powdering; Granulating; Addition of materials ameliorating the mechanical properties of the product catalyst
- B01J37/0027—Powdering
- B01J37/0045—Drying a slurry, e.g. spray drying
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/08—Heat treatment
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/288—Treatment of water, waste water, or sewage by sorption using composite sorbents, e.g. coated, impregnated, multi-layered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C5/00—Preparation of hydrocarbons from hydrocarbons containing the same number of carbon atoms
- C07C5/32—Preparation of hydrocarbons from hydrocarbons containing the same number of carbon atoms by dehydrogenation with formation of free hydrogen
- C07C5/327—Formation of non-aromatic carbon-to-carbon double bonds only
- C07C5/333—Catalytic processes
- C07C5/3332—Catalytic processes with metal oxides or metal sulfides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C5/00—Preparation of hydrocarbons from hydrocarbons containing the same number of carbon atoms
- C07C5/42—Preparation of hydrocarbons from hydrocarbons containing the same number of carbon atoms by dehydrogenation with a hydrogen acceptor
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/485—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of mixed oxides or hydroxides for inserting or intercalating light metals, e.g. LiTi2O4 or LiTi2OxFy
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/396—Distribution of the active metal ingredient
- B01J35/397—Egg shell like
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/396—Distribution of the active metal ingredient
- B01J35/398—Egg yolk like
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/60—Catalysts, in general, characterised by their form or physical properties characterised by their surface properties or porosity
- B01J35/61—Surface area
- B01J35/613—10-100 m2/g
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/60—Catalysts, in general, characterised by their form or physical properties characterised by their surface properties or porosity
- B01J35/63—Pore volume
- B01J35/633—Pore volume less than 0.5 ml/g
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/0009—Use of binding agents; Moulding; Pressing; Powdering; Granulating; Addition of materials ameliorating the mechanical properties of the product catalyst
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/281—Treatment of water, waste water, or sewage by sorption using inorganic sorbents
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/283—Treatment of water, waste water, or sewage by sorption using coal, charred products, or inorganic mixtures containing them
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to a method for making hollow structures, such as hollow particles or spheres. More specifically, the present invention relates to a method for forming hollow structures having a plurality of shells.
- the hollow structures may be used in a lithium ion battery anode or to remove contaminants, such as heavy metals and polyanions, from solution.
- Hematite (a-FeiOs), the most stable form of iron oxide, has been extensively studied as water splitting photoanodes, media for the absorptive removal of arsenic and phosphate and heavy metal species from water, gas sensors, and active anode materials for lithium ion batteries (LJBs).
- Lithium ion battery technology is facing ever increasing challenges in its traditional applications in consumer electronics where the power demands of portable electronic equipment are increasing at a greater rate than improvements in charge storage capacity of the batteries powering them, leading to shorter run times between charges. It is widely recognised that there is no feasible alternative to lithium ion batteries for powering portable electronic equipment in the short to medium term.
- iron oxides are well known as materials that adsorb polyanion contaminants from water including arsenic (1IT) and arsenic (V) species, phosphate anions and some heavy metals including lead, cadmium and chromium.
- arsenic (1IT) and arsenic (V) species phosphate anions and some heavy metals including lead, cadmium and chromium.
- arsenic removal hematite works by adsorbing multilayers of arsenic species on the surface of the material.
- strategies to improve the performance of adsorbent materials have focussed on increasing surface area and leading commercial materials typically have surface areas in the range 200-300 m 2 /g.
- Chromium based oxides and vanadium based oxides have proven to be very effective catalysts for oxidative dehydrogenation of ethane to ethylene.
- the catalytic conversion of alkanes into their corresponding alkenes by the oxidative or nonoxidative dehydrogenation are of increasing importance because of growing demand for alkenes.
- Ethane is the second major component of natural gas.
- Thermal cracking of hydrocarbons, such as ethane in the presence of steam is currently the main source of ethylene.
- steam cracking of ethane to ethylene is a highly endotbermic process, which must be performed at high temperatures and consumes a great deal of energy.
- Oxidative dehydrogenation of ethane by oxygen or carbon dioxide has been proposed as an alternative route to the process of thermal cracking of ethane. Jt is an exothermic process and can be performed at lower temperatures. The low temperature operation and exothermic reactions can significantly reduce the external heat input and lessen the coke formation.
- Spray drying is a widely applied technology in chemical, pharmaceutical, and food industry. Jt enables simple, continuous, and scalable production of fine particles. Spray drying involves forming a solution or slurry and spraying the solution or slurry through a hot gas to dry the solution or slurry and to form fine particles of material.
- the present invention is directed to a method for forming hollow structures, which may at least partially overcome at least one of the abovementioned disadvantages or provide the consumer with a useful or commercial choice.
- the present invention provides a method for forming multi-shelled or yolk-shell hollow structures comprising metal oxide, the method comprising the steps of:
- the solution is formed by dissolving a metal compound and at least one organic compound in water.
- the at least one dissolved metal compound is formed by dissolving a metal-containing compound in a solvent.
- the solvent is preferably water.
- the at least one dissolved metal compound may comprise a metal nitrate, a metai chloride, or a metal sulphate.
- Other metal compounds that are soluble may also be used.
- inorganic metal compounds are preferred, in some instances, organic metal compounds may be used, such as a metal acetate, a metal alkoxide, a metal citrate, a metal lactate, a metal gluconate (such as a metal D-gluconate), or a metal oxalate. It will be appreciated that this list is not exhaustive and the other metal compounds may be used.
- An ammonium-metal compound may be used.
- the at least one dissolved metal compound suitably includes iron.
- the at least one dissolved metal compound suitably includes zinc, tin, vanadium, manganese, titanium, chromium, nickel, cobalt, aluminium or mixtures of two or more thereof, such as mixtures of nickel/manganese, or nickel/cobalt/manganese, or nickel/cobalt/aluminium.
- the method of the present invention may be used to form hollow structures of FejO ? , T1O2, SnCh, ⁇ , FeMnO*, Cr ⁇ Os, vanadium oxide, etc.
- the at least one dissolved metal compound may comprise two or more dissolved metal compounds.
- the two or more dissolved metal compounds may comprise an iron compound and another metal compound.
- the at least one organic compound comprises an organic compound that dissolves in water.
- the at least one organic compound may comprise an organic compound that can be removed by calcination.
- the organic compound acts as an organic matrix in the composite particles formed by the spraydrying step.
- the organic matrix assists in confining the salt and binding the composite particle, forming a homogenous mixture after spray drying and allowing for fine control over the nanostructures during calcination. It also acts to lower the hygroscopicity of the spray dried composite particles.
- the organic material may comprise sucrose, glucose, oxalic acid, polyvinyl alcohol, polyvinyl pyrrolidone, polyethylene glycol and citric acid. Other organic materials may be used.
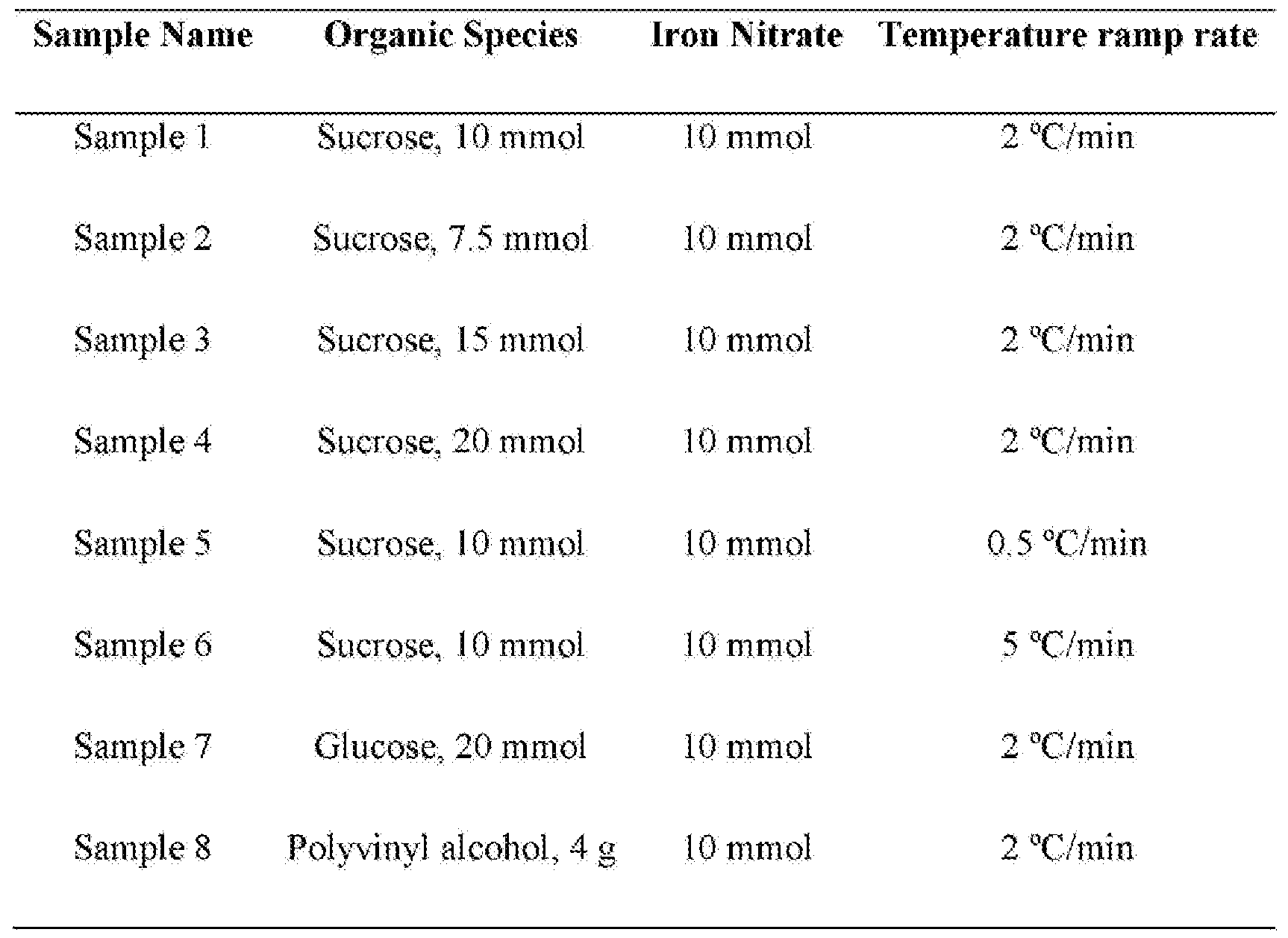
- the molar ratio of organic compound to dissolved metal in the solution may vary depending upon the metal compound(s) and organic material being used. For example, if iron nitrate and sucrose are being used as starting materials, the molar ratio of organic compound to dissolved metal in the solution may vary from 5/10 to 15/10, more preferably from 7.5/10 to 10/10. However, using other metal compounds, or using other organic materials may result in quite different molar ratios of organic compound to dissolved metal being used to form the multi-shelled hollow structures.
- a glucose/iron nitrate ratio of 20/10 can lead to multilayered FejOi
- an oxalic acid/ammonium metavanadate ratio of 30/10 can lead to yolk-shell structured V2O .
- the skill person would really appreciate that quite simple experimental work will be able to ascertain a range of suitable ratios of organic compound to dissolved metal in the solution for the particular starting materials being used.
- the as-synthesized composite particles will typically comprise solid particles.
- the particle size of the composite particles is largely determined by the size of the nozzle in the spray drier, the flow rate of solution to the nozzle and the dry conditions in the spray drier. Persons skilled in the art will appreciate that particles of a desired size may be formed across a relatively wide range of particle sizes using spray drying techniques.
- the as-synthesized composite particles may comprise hollow particles or collapsed hollow particles.
- the present inventors believe that the form of the as- synthesised composite particles is somewhat dependent upon the organic material that is being used.
- the temperature of the composite particles is increased at a predetermined rate by increasing the temperature at a constant rate, such as by increasing the temperature at a constant rate of between 0.5 e C per minute and 10°C per minute (for example, 0.5 °C/min, 2 T/min, 5 °C7min and 10 °C/min).
- the temperature is increased at a predetermined rate by increasing the temperature at a non-constant rate or a non-linear rate.
- the composite particles are removed from the spray drier or are recovered from the spray drier prior to the heating step.
- the composite particles are heated by placing the composite particles in a furnace and increasing the temperature of the furnace at a predetermined rate
- the furnace is at room temperature ( ⁇ 25° €) at the commencement of the heating step.
- the heating step is desirably conducted until the temperature is increased to or above a minimum temperature at which the organic material is removed from the particles and the desired metal oxide phase or phases have been formed.
- TGA thermogravimetric analyser
- the multi-shelled hollow structures formed in the present invention may be maintained at a high temperature for a period of time in order to improve the crystallinity of those structures.
- the multi-shelled hollow structures may comprise multi-shelled hollow particles or multi-shelled hollow spheres.
- the multi-shelled hollow structures comprise three or more shells wherein shells of decreasing size are located within larger shells.
- the multi- shelled hollow structures comprise at least four shells, or suitably comprise four shells.
- all of the shells comprise hollow shells.
- the multi-shelled hollow structures suitably do not have a solid core.
- the yolk-shell hollow structures may comprise yolk-shell hollow spheres or yolk-shell hollow particles.
- the core particles i.e., the yolk
- the yolk may be solid.
- the metal oxide that forms the multi-shelled hollow structures may comprise hematite (Fe O. .
- the metal oxide that forms the multi-shelled hollow structures may comprise a complex metal oxide or a mixed metal oxide containing two or more metals in the oxide structure.
- the two or more metals may comprise iron and one or more other metals.
- the multi-shelled hollow structures may comprise particles having a size falling within the range of 200 to 4000nm, or within the range of 400 to 2000 nm.
- the multi-shelled hollow structures may comprise a plurality of particles joined to other particles to form an aggregate.
- the plurality of particles may be formed into an aggregate by necking at regions where one particle comes into contact with an adjacent particle. At these regions, "bridges" may be formed between the particles to thereby hold the particles together in a fused aggregate of particles such that the size of the aggregates is larger than that of the primary particle building blocks.
- the invention resides broadly in a method for forming aggregate particles ("fused aggregates") from multi-shelled hollow structures, the method comprising the steps of mixing the multi-shelled hollow structures with a chemical species that is a metal hydroxide or is capable of forming a metal hydroxide to form a mixture, and processing the mixture to produce the aggregate particles.
- any suitable metal hydroxide may be used, such as, but not limited to, copper hydroxide, titanium hydroxide, aluminium hydroxide, iron hydroxide, nickel hydroxide or the like, or a combination thereof.
- the metal hydroxide may be provided in any suitable form, such as, but not limited to a solid, solution or gel. More preferably, however, the metal hydroxide is in the form of a gel.
- the metal hydroxide gel comprises aluminium hydroxide gel.
- the metal hydroxide gel is a wet metal hydroxide gel.
- fused aggregates may be formed by mixing the multi-shelled hollow structures with a chemical agent that is a precursor to metal hydroxides.
- the chemical agent precursor precipitates to form a metal hydroxide that cements the multi-shelled hollow structures together.
- metal hydroxides from metal -based precursors via reactions such as hydrolysis and precipitation are well known to those in the art.
- a titanium hydroxide species could be formed using a precursor such as titanium isopropoxide or titanium trichloride which undergo hydrolysis to form the titanium hydroxide product.
- the multi-shelled hollow structures and the metal hydroxide gel may be mixed using any suitable technique. Preferably, however, the mixing of the multi-shelled hollow structures and the metal hydroxide gel is achieved using mechanical mixing. ⁇ skilled addressee will understand that the exact nature of the mechanical mixer used to achieve the mixing is not critical.
- the mixing of the multi-shelled hollow structures and the metal hydroxide gel will result in the formation of a mixture
- the mixture may be processed to form the aggregate particles.
- the mixture may be processed using any suitable technique, such as extruding the mixture through a screen, sieve, mould or the like, or any suitable combination thereof.
- the choice of processing method used will depend on the desired particle size of the final fused aggregates.
- the mixture may be extruded through a screen or sieve.
- the size of the apertures in the screen or the sieve is not critical, and a skilled addressee wilt understand that the size of the apertures in the screen or sieve through which the mixture is extruded will be dependent on the desired size of the aggregate particles.
- the aggregate particles may be dried and or calcined. Following drying and/or calcination, the fused aggregates may be further processed to refine the particle size. By providing control of the particle size in this step, less precise processing of the mixture prior to dryingcalcination to control particle size is required. Methods for achieving particle size control in this manner include, but are not limited to, milling techniques such as ball milling, crushing, particle size reduction using high shear forces, high pressure homogenisation and other techniques known to those skilled in the art.
- electrodes typically contain a number of components including the active, charge storage material, a particle additive to promote electronic conductivity such as carbon black or acetylene black and a polymeric binder that acts to hold together all of these particulate components into a self-supporting structure. These mixed components are typically coated onto a metal foil which acts as a current collector in a battery.
- binder materials and conductivity enhancers perform an important function, they do not contribute to the charge storage capacity of the electrode and therefore essentially dilute the charge storage capacity of the active material.
- the multi-shelled hollow structures comprise microspheres.
- the present invention provides a method for forming multi-shelled hollow structures comprising metal oxide, the method comprising the steps of
- the composite particles are formed by spray drying.
- the composite particles are then subjected to a separate heating or an annealing step in which the composite particles are subjected to increasing temperature at a predetermined rate. This converts the metal species or metal compound to a metal oxide whilst also removing the organic material from the particles.
- the present inventors have postulated that during the heating step in the non-equilibrium heat treatment, induced heterogeneous contraction dominates and is responsible for the formation of the metal oxide multi-shelled hollow structures. More specifically, the composite particles are not homogeneously heated during the heating annealing; instead, there exists a temperature gradient ( ⁇ ) along the radial direction of the particles. The ⁇ leads to the first formation of a metal oxide shell at the surface of the composite particles. This shell is relatively rigid, and it can prevent the further contraction of the particles. Two forces of opposite directions exert at the interface of the composite core and the metal oxide shell.
- the contraction force (Fc) promotes the inward shrinkage of the core, while the adhesion force (Fa) induces the outward contraction.
- Fc The contraction force
- Fa adhesion force
- the Fc exceeds Fa, leading to the detachment of the core from the preformed shell.
- the abovementioned process can be repeated several times until the ⁇ become negligible.
- the Fa surpasses Fc, and the mass diffusion trend is reversed. That is, the inner core shrinks outwardly, leaving a cavity at the centre.
- multi-shelled hollow structures having a number of hollow shells.
- the present invention provides multi-shelled hollow structures comprising a metal oxide, the multi-shelled hollow structures comprising at least three hollow shells.
- the multi-shelled hollow structures comprise at least four hollow shells. In another embodiment, the multi-shelled hollow structures comprise four hollow shells. In some embodiments, the multi-shelled hollow structures suitably do not have a solid core. In other embodiments, the multi-shelled hollow structures have multiple hollow shells and a solid core within the inner shell.
- the multi-shelled hollow structures of the present invention may be suitable for use as an active material on or in the anode of lithium ion batteries. Therefore, in a further aspect, the present invention provides an electrode material for a lithium ion battery anode, the electrode material including multi-shelled hollow structures in accordance with the present invention, as described herein.
- a lithium ion battery using the anode of the present invention is provided.
- the multi-shelled hollow structures and the anode electrodes of the present invention can function as drop-in replacements for conventional anode active materials and anode electrodes.
- no special processing of the active material or of the assembled anode is required and these components are compatible with the use of other conventional components used to make batteries such as electrolytes, separators, current collectors, cathodes and cell packing materials.
- batteries such as electrolytes, separators, current collectors, cathodes and cell packing materials.
- the multi-shelled hollow structures of the present invention may also be suitable for removing contaminants, such as heavy metal ions, from solutions. Accordingly, in another aspect, the present invention provides a method for removing heavy metal ions dissolved in a solution, the method comprising contacting the solution with multi-shelled hollow structures in accordance with the present invention, as described herein.
- the heavy metal ions that may be removed from the solution include arsenic, lead, cadmium, chromium, antimony, cobalt, copper, selenium and molybdenum.
- the present inventors have also found that the multi-shelled hollow structures in (lie present invention may be used to remove polyanions, such as phosphate, from solution. Accordingly, in yet a further aspect, the present invention provides a method for removing polyanions from solution, the method comprising contacting the solution with multi-shelled hollow structures in accordance with the present invention, as described herein.
- the polyanions to be removed may be selected from arsenite, arsenate, phosphate. Other polyanions may also be removed.
- the multi-shelled hollow structures used to remove heavy metal ions or phosphate from solution preferably comprise hematite (Fe Oj) chromium oxide, vanadium oxide, zinc iron ternary oxide and manganese-iron ternary oxide (Fe-Mn-O x , such as ternary iron-manganese- oxides such as MnFe ⁇ 0 and FeMn>0,i).
- the surface area of the material is typically lower that 100 m 2 /g and more typically lower than 50 m 2 /g. These values of surface area are significantly lower than those of commercial iron oxide materials used in the removal of arsenic species where surface areas of commercial materials can be in the range of 200-300 m 2 /g. Such high surface areas are desired for maximising capacity to adsorb arsenic species as it is understood that arsenic adsorption is a surface phenomenon, and as such, larger surface areas impart a larger capacity for arsenic species removal.
- the present inventors postulate that the higher arsenic adsorption capacities seen in the material of the present invention relative to commercial materials, despite the much lower surface areas measured, may result from the presence of the internal voids within each multi-shelled hollow structure particle which acts as a reservoir for sequestration of contaminant species.
- the concave shape of the inside walls of the multi-shelled hollow structures together with the internal void space may be acting to stabilise captured contaminant.
- adsorption to iron oxide is understood to occur by the build-up of multi-layers of arsenic species on the surface of the iron oxide according to the Freundlich isotherm model and it may be that the structure of the multi-shelled hollow materials of the present invention act to stabilise these multilayers of contaminants.
- Adsorbent materials are often used in a particulate or granular form held within a cartridge or other container through which contaminated water requiring treatment is passed. To ensure the adsorbent material does not exit the container with the treated water, its exit is impeded by a mesh-like structure. In order for this to be effective, the particle size of the adsorbent material must be at least of a certain size. This is also important in ensuring reasonable flow rates through a treatment system since smaller particles tend to pack more tightly than larger particles, leaving lower amounts of interparticle void space for water to pass through thus requiring large back pressures to move water through the material.
- Adsorbent material may be used as a pure powder, it may be attached to the surface of a carrier material or to a substrate or may be incorporated within an extruded or pressed porous filtration block such as those made from activated carbon particles.
- the choice of product format depends on the adsorbent particle size requirements of the application, the amount of arsenic to be removed and other factors.
- the adsorbent material will be present as a collection of pure iron oxide particulates contained within a metal or plastic filtration tank through which contaminated water passes, as is commonly used in the art.
- pure iron oxide will be used to maximise absorption capacity.
- the iron oxide absorption media of the present invention may be used in conjunction with water filter pitchers that are based on activated carbon. These devices typically only contain activated carbon and thus are not capable of adsorbing arsenic species from contaminated water. Particles of iron oxide could be included into the filtration media in order to provide an arsenic removal capability for these products.
- the iron oxide could be included as a discrete powder mixed in with particles of activated carbon, as a discrete powder contained separately from the activated carbon, as particles fixed to the surface of die activated carbon particles, incorporated into a carbon block fabricated by extrusion or pressing or incorporated using other methods known to those skilled in the art.
- the multi-shelled hollow structures or yolk shell structures could be packed in reactor beds for the treatment of industrial waste water.
- the multi-shelled hollow structures of the present invention may also be suitable for oxidative or non-oxidative dehydrogenation of alkanes to alkenes. Accordingly, in a further aspect the present invention provides a method for the oxidative or non-oxidative dehydrogenation of alkanes to alkenes, characterised in that multi -shelled hollow structures of the present invention are present during the oxidative or non-oxidalive dehydrogenation of alkanes to alkenes.
- Figure 1 shows a schematic illustration for the formation of a-FeiOj MSHSs in accordance with the postulated synthesis pathway
- Figure 2 shows an FESEM image of iron nitrate-sucrose composite microspheres formed alter spray drying (sucrose iron nitrate ratio: 10/10), but before annealing;
- Figure 3 shows a TGA (thermogravimetric analyser) curve of the iron nitrate-sucrose composites (sucrose/iron nitrate ratio: 10/10) during heating thereof to elevated temperature.
- Figure 4a shows a digital photo of the a-Fe 2 0 3 MSHSs
- Figure 4b shows an XRD pattern of the a-Fe 2 0 3 MSHSs
- Figures 5(a) to 5(f) show FESEM images (a, b) at an accelerating voltage of 5 and 15 kV, respectively.
- Figures 6(a) to 6(f) show TEM images of Sample 2 (a and b, sucrose iron nitrate ratio: 7.5/10), Sample 1 (c, sucrose iron nitrate ratio: 10/10), Sample 3 (d and e, sucrose iron nitrate ratio: 15/10), and Sample 4 (f, sucrose/iron nitrate: 20/10);
- Figures 7(a) to 7(e) show FESEM (a - c) and TEM (d, e) images of a-F ⁇ O* hollow structures (Sample 5, temperate ramp rate: 0.5 °C/min).
- the accelerating voltage is 5 kV; for c, the accelerating voltage is 15 kV;
- Figures 8(a) to 8(e) show FESEM (a - c) and TEM (d, e) images of a-F ⁇ hollow structures (Sample 6, temperature ramp rate: 5 °C/min). For (a) and (b), the accelerating voltage is 5 kV; for (c), the accelerating voltage is 15 kV; [0069] Figures 9(a) to 9(d) show TEM images of a-FejO* hollow structures using glucose (a and b. Sample 7) and PVA (c and d, Sample 8) as the organic species;
- Figures 1 (a) to 10(e) show FESEM (a, b) t TEM (c, d), SAED (e) and HRTEM (f) images of ZnFe 2 0 yolk-shell structures;
- Figure 11 shows XRD pattern of the ZnFe 2 >4 yolk-shell structures
- Figure 12 shows CVs (cyclic voltrammetry) of a-FeiC ⁇ MSHSs for the initial three cycles at a rate of 0.1 mA s "1 ;
- Figures 13(a) to 13(d) show (a) Representative charge-discharge profiles of a-FeaOs MSHSs at a current density of 400 mA g "1 in the voltage window of 0.05 - 3.0 V; (b) cycling performance of a-FejOj MSHSs at a current density of 400 mA g "1 ; (c) representative charge- discharge profiles of a-FejO MSHSs at different current densities (100 - 3200 mA h g *1 ); (d) rate performance of -FejQ ⁇ MSHSs.
- current density of the first two cycles are 50 mA g *1 ;
- Figure 14 shows Coulombic efficiency vs. cycle number
- Figure 15 shows TEM images of ⁇ 2 ⁇ 3 multi-shelled hollow spheres formed in accordance with an embodiment of the present invention
- Figure 16 shows TEM images of V2O5 yolk-shell structures formed in accordance with an embodiment of the present invention
- Figure 17 shows XRD pattern of V2O5 yolk-shell structures
- Figure 18 shows a TEM image of a-Fe 2 03 MSHSs prepared by calcining an iron nitrate-sucrose composite at 600 °C for 5 hours in air.
- Figure 19 shows a drinking water treatment device.
- Figure 20 illustrates the arsenic removal capabilities of a-Fe 2 C>3 multi-shelled hollow structures when used in the drinking water treatment device of Figure 19.
- Figures 21 (a) to 21 (g) show TEM images of a-Fe2O.ii multi-shelled hollow structures with an iron citrate precursor using sucrose as the organic species.
- Figure 22 illustrates the adsorption of anions other than arsenic anions using a-Fe 2 0* multi-shelled hollow structures.
- Figure 23 illustrates granular particles produced using a-Fe 0 3 multi-shelled hollow structure material.
- the examples mainly relate to the preparation of a-Fe ⁇ O? multi-shelled hollow structures.
- the a-FejOs MSHSs hollow microspheres were prepared by a spray drying method followed by annealing in air.
- a typical synthesis (Sample I, see Table 1), Fe(N03>*9H 0 (10 mmol) and sucrose (10 mmol) were dissolved in H 2 0 (100 mL) to form a clear solution.
- the resulting solution was then spray dried using a Buchi mini spray drier B-290 at an inlet temperature of 220 °C, an aspirator rate of 100 %, a rotameter setting of 60 mm, and a pump rate of 5 % (1.5 mL/min). Nitrogen was used as the drying gas.
- X-ray diffraction (X D) patterns were collected on a German Bruker D8 Advanced X-Ray DifFractometer with Ni filtered Cu Ka radiation (40 kV, 30 mA).
- Field emission scanning electron microscopy (FESE ) images were obtained on a JEOL JSM 7800 microscope with an accelerating voltage of 5.0 or 15.0 kV.
- Transmission electron microscopy (TE ) images were taken on Tecnai F20 (200 kV) and JEOL 1010 (100 kV) microscopes.
- Thermo gravimetric analysis ⁇ TGA was carried out on a TGA/DSC 1 STAR* System under air flow (25 - 1000 °C, 5 °C/min).
- Nitrogen adsorption isotherms were measured at 77 K using a TriStar II Surface Area and Porosity analyser (Micromeritics). The samples were degassed under vacuum for 6 hours at 200 °C before analysis. BET surface area was calculated from the adsorption branch in relative pressure (p/po) range of 0.05 - 0.30.
- the electrochemical measurements were carried out in homemade two-electrode Swagelok type cells.
- the working electrode is consisted of active material, conductive acetylene black, and polyvinylidene fluoride binder in a weight ratio of 70:20:10.
- Lithium chips were used as both the counter electrode and reference electrode.
- 1 M LiPF « in a mixture of ethylene carbonate, dimethyl carbonate, and diethyl carbonate (1: 1 :1 in volume) was used as the electrolyte.
- Cell assembly was carried out in an Ar-ftlled glovebox with moisture and oxygen concentrations below 1.0 ppm.
- Cyclic voltaminetry (CV) measurements were performed on a Solartron 1480 Multistat instrument. The galvanostatic charge-discharge tests were performed on a MTI 8 Channels Battery Analyzer.
- the synthesis of a-Fe ⁇ O* MS Ss is based on a simple spray drying method followed by annealing in air.
- the first step involves the preparation of iron nitrate-sucrose composite microspheres by spray drying; while the second step is annealing the composite spheres in air at 400 °C.
- the non-equilibrium heat treatment induced heterogeneous contraction dominates the second step and is responsible for the formation of the ⁇ -FejOj MSHSs. More specifically, the iron nitrate-sucrose microspheres are not homogeneously heated during the annealing; instead, there exists a temperature gradient ( ⁇ ) along the radical direction.
- the ⁇ leads to the first formation of a a-F ⁇ Oi shell at the surface of the composite spheres.
- This shell is relatively rigid, and it can prevent the further contraction of the microspheres.
- Two forces of opposite directions exert at the interface of the composite core and a-Fe 2 0 5 shell.
- the contraction force (Fc) promotes the inward shrinkage of the core, while the adhesion force (Fa) induces the outward contraction.
- the Fc exceeds Fa, leading to the detachment of the core from the preformed shell.
- the abovementioned process (shell formation and core detachment) can be repeated for several times until the ⁇ become negligible.
- the Fa surpasses Fc, and the mass diffusion trend is reversed. That is, the inner core shrinks outward, leaving a cavity at the centre.
- FIG. 1 A schematic diagram showing the postulated synthesis pathway is show in Figure 1.
- Figure 2 shows an FESEM image of the iron nitrate-sucrose composite particles formed after spray drying of the iron nitrate-sucrose solution in the spray drier.
- FIG. 3 shows a TGA (thermogravimetric analyser) curve of the iron nitrate-sucrose composites (sucrose/iron nitrate mole ratio: 10/10).
- TGA thermogravimetric analyser
- the morphology and structure of the products were examined by SEM and TEM.
- the products consists of microspheres with a size in the range of 400 - 2000 nm.
- a relatively low accelerating voltage of 5 kV Figure 5(a)
- an inner core particle is exposed, indicating the microspheres actually have a yolk-shell or multi-shell structure.
- a high accelerating voltage of 15 kV the penetration capability of the electrons in SEM is greatly enhanced.
- This unique multi-shelled hollow structure gives rise to a relatively high surface area of 17.2 m 2 g " ' and pore volume of 0.1 cm 3 g " ⁇
- the temperature ramp rate may also ptay an important role on the morphology and structure of the products: a low ramp rate will lead to a small ⁇ , and thus fewer shells; while a high ramp rate will result in a high ⁇ , and thus more shells.
- a low ramp rate of 0.5 °C/min single-shelled hollow spheres and yolk-shell structures are formed ( Figure 7).
- a high ramp rate of 2 and 5 Vmin multi- shelled hollow spheres are formed ( Figure 8).
- sample 1 Figure 5
- sample 6 with a ramp rate of 5 °C Figure 8 shows more broken spheres.
- Figures 21a to 2 lg illustrate the use of different quantities of sucrose as the organic species utilized as the matrix to synthesize a-Fe 2 0 3 hollow spheres having an iron citrate precursor.
- 10 mmol of iron citrate was dissolved in 100ml of water to form a clear solution.
- the resulting solution was then spray dried using a Buchi mini spray drier B-290 using air as the drying gas.
- the as-made samples were then annealed in air at 400 3 C for 5 hours with a temperature ramp rate of 2'C/hour, with the resulting iron oxide structures illustrated in Figures 21 a to 2lg. High arsenic absorption capacity was confirmed for these a-Fe 2 0 3 structures produced from the iron citrate precursor.
- the cycling performance of the a-FejOs electrode was evaluated by activating (lie half cells at 50 mA g *1 for two cycles and then cycling at 400 or 1600 mA g " ⁇
- the ⁇ - ⁇ 2 ⁇ 1 ⁇ 4 MSHSs delivers an initial discharge capacity of 1443 mA h g "1 and a charge capacity of and 1067 mA h g ' ⁇ respectively ( Figure 13a).
- a high and stable capacity of ⁇ 1000 and 900 mA h g * ' can be achieved at a current of 400 and 1600 mA g * ' ( Figure 13b), respectively.
- the coulombic efficiency for the first cycle is around 75%, while it stabilizes at ⁇ 98% after the activation process ( Figure 14).
- the electrochemical performances of the a-Fe20j MSHSs are superior or at least comparable to those of high-performance FeaC -based anode materials reported recently, such as a-FeiOs nanorods, a-FeaO? hollow spheres, Fe20j decorated carbon nanotubes, and Fe2 ⁇ ) reduced graphene oxide nanocomposites.
- the enhanced electrochemical performances can be attributed to the unique structural characteristics of the a-Fe 2 03 ⁇ 4 MSHSs.
- the permeable and thin shells greatly shorten the distances for Li * diffusion; the void space effectively accommodates the drastic volume change and alleviates the strain during Li * insertion/extraction. All these features contribute to the stable high-rate performance of the ⁇ -Fe 2 0 3 MSHSs.
- nanoporous hematite were also investigated for arsenic removal. Without wishing to be bound by theory, the present inventors have hypothesized that the existence of voids within layers might accommodate arsenic species for adsorption purpose.
- Na 2 HA 0 4 .7H 2 0 Sigma Aldrich
- NaHAsC Adjax Finekem
- 0.01M HCI and M IO ⁇ NaOH were used for pH adjustment. Distilled water was used throughout the experiment.
- a solution containing 400 ppm As(IH) was prepared by dissolving 693. S mg of NaHAsCh in 1 L H 2 0. Consequently, 1.65 g of Na 2 HAs04.7H 2 0 was dissolved in 1 L H 2 0 to make 400 ppm of As(V) solution. Serial dilutions were performed to obtain arsenic concentrations of 200ppm, 1 ppm, and 50ppb for both As(V) and As(IIJ) solutions. A dosage 20 mg of nanoporous hematite was added into each 50 mL solution. All solutions were shaken for 24 h at room temperature at a speed of 200 rpm.
- WAGTECH arsenic kit rM was used to quantify the amount of arsenic adsorbed. The filter paper was calibrated prior to each arsenic quantification.
- nanoporous hematite is beneficial for arsenic removal, as it can lower down the As(V) concentration from lppm to 8ppb (table 3), which is below the stringent discharge limit.
- a-FejCh multi-shelled hollow structures made in accordance with the present invention will also be able to remove other heavy metal ions, such as lead, cadmium, chromium, antimony and molybdenum, as well as removing phosphate ions from solution.
- heavy metal ions such as lead, cadmium, chromium, antimony and molybdenum
- nanoporous hematite was used as an additive to the filter media of a standard activated carbon-based water purification system.
- the addition of the nanoporous hematite conveys an arsenic removal capability on the system enabled by the hematite.
- FIG. 1 The test is illustrated in Figure 1 .
- a commercial water filter pitcher 10 (Brita® Maxtra) was used as the standard water filter device.
- the standard media 1 1 in this device is composed of activated carbon and ion exchange resin with a total mass of 30g.
- O.lg of a-Fe Oj multi-shelled hollow structures 12 made in accordance with the present invention were added to the 30g of standard media 1 by mixing the a-Fe20i multi-shelled hollow structures 12 into the standard media 1 1.
- the modified filter 1 containing 0. lg of a-Fe 2 0? multi-shelled hollow structures 12 reduced the As(V) concentration in the effluent 15 from I OOppb to around Oppb
- FIG 22 there is illustrated the adsorption of oxyanions other than arsenic oxyanions using a-Fe 2 0:t multi-shelled hollow structures synthesised in accordance with the present invention.
- This Figure demonstrates the ability of the non-arsenic anions to complete for adsorption sites on the iron oxide structures and, in doing so, reduce the capacity of the iron oxide to adsorb arsenic species.
- Figure 16 shows TEM images of V2O5 yolk-shell structures formed in accordance with an embodiment of the present invention.
- Figure 17 shows XRD pattern of V2O5 yolk-shell structures. Again, multiple shells can be seen.
- Figure 18 shows a TEM image of a-FezOj MSHSs prepared by calcining an iron nitrate-sucrose composite at 600 °C for 5 hours in air.
- a multi shelled material has been obtained, showing that higher calcination temperatures are not detrimental to formation of the multi-shelled hollow structures.
- calcination to temperatures above the minimum temperature at which the organic material has been removed and the metal oxide phase(s) has been fully formed may not be required unless the higher temperature results in improved crystallinity in the material.
- Embodiments of die present invention provide a facile method for the high-yield and large-scale synthesis of a-Fe20* multi-shelled hollow spheres (MSHSs) by spray drying.
- MSHSs multi-shelled hollow spheres
- the resultant o-Fe 2 0j hollow spheres exhibit high specific capacity, excellent rate capability and cycling stability.
- the method can also be used to make hollow spheres of other metal oxides. They can also be used to remove contaminants, such as heavy metals and polyanions, from solutions, such as water streams.
- the final a-FejO* weight percentage in the granules was between 60% and 95% and this value was controlled by the proportion of aluminium hydroxide gel added to the a-Fe 2 0 3 multi-shelled hollow spheres.
- the arsenic absorption capacity of the granulated a-Fe ⁇ Oi multi-shelled hollow spheres was not reduced compared to the a-Fe ⁇ Ch multi-shelled hollow spheres primary particles.
- the particle size of the granulated material 20 was in the order of millimetres in length.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Inorganic Chemistry (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Hydrology & Water Resources (AREA)
- Environmental & Geological Engineering (AREA)
- Water Supply & Treatment (AREA)
- Compounds Of Iron (AREA)
Abstract
A method for forming multi-shelled hollow structures comprising metal oxide, the method comprising the steps of: forming a solution containing at least one dissolved metal compound and at least one organic compound, spray drying the solution to form composite particles containing at least one metal species and organic material, and heating the composite particles by increasing temperature at a predetermined rate to form the multi-shelled hollow structures comprising metal oxide.
Description
TITLE
Method for producing hollow structures
TECHNICAL FIELD
[0001] The present invention relates to a method for making hollow structures, such as hollow particles or spheres. More specifically, the present invention relates to a method for forming hollow structures having a plurality of shells. The hollow structures may be used in a lithium ion battery anode or to remove contaminants, such as heavy metals and polyanions, from solution.
BACKGROUND ART
[0002] Hollow structures, a unique family of materials with low density and high surface area versus conventional materials, and shell permeability, have attracted tremendous attentions in drug delivery, gas sensors, adsorption, as well as energy storage and conversion. Recently, efforts have been dedicated to the rational design of complex multi-shelled hollow structures due to bodi their aesthetic beauty and the opportunity for further physical chemical properties tailoring by manipulating the shell structure. The most popular approach for the fabrication of multi-shelled hollow structures involves the shell-by-shell deposition of desired materials onto sacrificial templates followed by selective template removal. Hard templates, such as monodispersed polymer, silica, carbon, and metal/metal oxide nanoparticles, or soft ones, such as micelles, have been widely utilized as the sacrificial templates. However, the templating approach is usually tedious and costly; and the more complex the target structure, the more complicated the synthesis procedure. Therefore, it is highly desirable to develop a simple and scalable method for the fabrication of MSHSs.
[0003] Hematite (a-FeiOs), the most stable form of iron oxide, has been extensively studied as water splitting photoanodes, media for the absorptive removal of arsenic and phosphate and heavy metal species from water, gas sensors, and active anode materials for lithium ion batteries (LJBs). Lithium ion battery technology is facing ever increasing challenges in its traditional applications in consumer electronics where the power demands of portable electronic equipment are increasing at a greater rate than improvements in charge storage capacity of the batteries powering them, leading to shorter run times between charges. It is widely recognised that there is no feasible alternative to lithium ion batteries for powering portable electronic equipment in the short to medium term. Efforts to increase the charge storage capacity of lithium ion batteries have largely focussed on the development of anode materials with inherently large lithium
storage capabilities, in particular, silicon, tin, aluminium, antimony, tin oxide and lithium metal. In the case of silicon for example, practical charge storage capacity is approximately 3600 mAh/g compared with 372 mAh/g for conventional graphite anode material. Despite the large charge storage capacities of these materials, their practical implementation has been hampered by poor cycle life that results from the significant expansion and subsequent contraction in the volume of the active material that occurs on lithium insertion and extraction processes respectively as the battery is cycled. Hematite offers a charge storage capacity of 1007 mAh/g, can be fabricated from abundant raw materials, is low cost and is environmentally benign. Despite these advantages, the application of ct-FejO. in LIBs is still hampered by the same large volume variations during Li* insertion/extraction (~ % %) that afflict other high capacity anode materials, which causes the notorious problem of pulverization and the subsequent capacity fading. To alleviate the volume change issue in anode materials, several strategies have been proposed. One effective strategy is designing unique nanostructures, such as hollow structures and porous materials. The idea is to utilize the free space in the nanostructured electrode material to partially accommodate the drastic volume change. However, the creation of free space in a material also reduces the tap density of the material which in a lithium ion battery translates into a lower volumetric charge storage capacity. As such, the introduction of free space to improve cycle life must be balanced against the original desire to maintain charge storage capacity. Another commonly used approach is fabricating nanocomposites of carbon and a-FejO?, where the carbon acts as a buffering layer. However, the synthesis of state-of-the-art a-FejO-t based nanostructures and nanocomposites usually involves expensive sacrificial templates, cumbersome muitisteps, or time-consuming hydrothermal/solvothermal reactions in autoclaves. This situation inspires us to search for simple, economic, and scalable ways to produce a-FeiOj anode materials with satisfactory specific capacity and rate capability.
[0004] In the field of water treatment, iron oxides are well known as materials that adsorb polyanion contaminants from water including arsenic (1IT) and arsenic (V) species, phosphate anions and some heavy metals including lead, cadmium and chromium. In the case of arsenic removal, hematite works by adsorbing multilayers of arsenic species on the surface of the material. As such, strategies to improve the performance of adsorbent materials have focussed on increasing surface area and leading commercial materials typically have surface areas in the range 200-300 m2/g.
[0005] Chromium based oxides and vanadium based oxides, either binary or ternary oxides, have proven to be very effective catalysts for oxidative dehydrogenation of ethane to ethylene. The catalytic conversion of alkanes into their corresponding alkenes by the oxidative or
nonoxidative dehydrogenation are of increasing importance because of growing demand for alkenes. Ethane is the second major component of natural gas. Thermal cracking of hydrocarbons, such as ethane, in the presence of steam is currently the main source of ethylene. However, steam cracking of ethane to ethylene is a highly endotbermic process, which must be performed at high temperatures and consumes a great deal of energy. Moreover, high temperature may result in other undesirable reactions producing coke, the existence of which deteriorates the catalytic activity and reactor performance. Oxidative dehydrogenation of ethane by oxygen or carbon dioxide has been proposed as an alternative route to the process of thermal cracking of ethane. Jt is an exothermic process and can be performed at lower temperatures. The low temperature operation and exothermic reactions can significantly reduce the external heat input and lessen the coke formation.
[0006] Spray drying is a widely applied technology in chemical, pharmaceutical, and food industry. Jt enables simple, continuous, and scalable production of fine particles. Spray drying involves forming a solution or slurry and spraying the solution or slurry through a hot gas to dry the solution or slurry and to form fine particles of material.
[0007] It will be clearly understood that, if a prior art publication is referred to herein, this reference does not constitute an admission that the publication forms part of the common general knowledge in the art in Australia or in any other country.
SUMMARY OF INVENTION
[0008] The present invention is directed to a method for forming hollow structures, which may at least partially overcome at least one of the abovementioned disadvantages or provide the consumer with a useful or commercial choice.
[0009] In a first aspect, the present invention provides a method for forming multi-shelled or yolk-shell hollow structures comprising metal oxide, the method comprising the steps of:
- forming a solution containing at least one dissolved metal compound and at least one organic compound,
- spray drying the solution to form composite particles containing at least one metal species and organic material, and
- heating the composite particles by increasing temperature at a predetermined rate to form the multi-shelled or yolk-shell hollow structures comprising metal oxide.
[0010] In one embodiment, the solution is formed by dissolving a metal compound and at
least one organic compound in water.
[001 1] In some embodiments, the at least one dissolved metal compound is formed by dissolving a metal-containing compound in a solvent. The solvent is preferably water.
[0012] The at least one dissolved metal compound may comprise a metal nitrate, a metai chloride, or a metal sulphate. Other metal compounds that are soluble may also be used. Although inorganic metal compounds are preferred, in some instances, organic metal compounds may be used, such as a metal acetate, a metal alkoxide, a metal citrate, a metal lactate, a metal gluconate (such as a metal D-gluconate), or a metal oxalate. It will be appreciated that this list is not exhaustive and the other metal compounds may be used. An ammonium-metal compound may be used.
[0013] The at least one dissolved metal compound suitably includes iron. In another embodiment, the at least one dissolved metal compound suitably includes zinc, tin, vanadium, manganese, titanium, chromium, nickel, cobalt, aluminium or mixtures of two or more thereof, such as mixtures of nickel/manganese, or nickel/cobalt/manganese, or nickel/cobalt/aluminium..
[0014] The method of the present invention may be used to form hollow structures of FejO?, T1O2, SnCh, Μηθχ, FeMnO*, Cr^Os, vanadium oxide, etc.
[0015] The at least one dissolved metal compound may comprise two or more dissolved metal compounds. The two or more dissolved metal compounds may comprise an iron compound and another metal compound.
[0016] In some embodiments, the at least one organic compound comprises an organic compound that dissolves in water. The at least one organic compound may comprise an organic compound that can be removed by calcination.
[0017] Without wishing to be bound by theory, the present inventors have postulated that the organic compound acts as an organic matrix in the composite particles formed by the spraydrying step. The organic matrix assists in confining the salt and binding the composite particle, forming a homogenous mixture after spray drying and allowing for fine control over the nanostructures during calcination. It also acts to lower the hygroscopicity of the spray dried composite particles.
[0018] The organic material may comprise sucrose, glucose, oxalic acid, polyvinyl alcohol, polyvinyl pyrrolidone, polyethylene glycol and citric acid. Other organic materials may be used.
[0019] The molar ratio of organic compound to dissolved metal in the solution may vary depending upon the metal compound(s) and organic material being used. For example, if iron nitrate and sucrose are being used as starting materials, the molar ratio of organic compound to dissolved metal in the solution may vary from 5/10 to 15/10, more preferably from 7.5/10 to 10/10. However, using other metal compounds, or using other organic materials may result in quite different molar ratios of organic compound to dissolved metal being used to form the multi-shelled hollow structures. For example, a glucose/iron nitrate ratio of 20/10 can lead to multilayered FejOi, an oxalic acid/ammonium metavanadate ratio of 30/10 can lead to yolk-shell structured V2O . The skill person would really appreciate that quite simple experimental work will be able to ascertain a range of suitable ratios of organic compound to dissolved metal in the solution for the particular starting materials being used.
[0020] In some embodiments, the as-synthesized composite particles will typically comprise solid particles. The particle size of the composite particles is largely determined by the size of the nozzle in the spray drier, the flow rate of solution to the nozzle and the dry conditions in the spray drier. Persons skilled in the art will appreciate that particles of a desired size may be formed across a relatively wide range of particle sizes using spray drying techniques.
[0021] In other embodiments, the as-synthesized composite particles may comprise hollow particles or collapsed hollow particles. The present inventors believe that the form of the as- synthesised composite particles is somewhat dependent upon the organic material that is being used.
[0022] In some embodiments, the temperature of the composite particles is increased at a predetermined rate by increasing the temperature at a constant rate, such as by increasing the temperature at a constant rate of between 0.5eC per minute and 10°C per minute (for example, 0.5 °C/min, 2 T/min, 5 °C7min and 10 °C/min).
[0023] in other embodiments, the temperature is increased at a predetermined rate by increasing the temperature at a non-constant rate or a non-linear rate.
[0024] In some embodiments, the composite particles are removed from the spray drier or are recovered from the spray drier prior to the heating step.
[0025] In some embodiments, the composite particles are heated by placing the composite particles in a furnace and increasing the temperature of the furnace at a predetermined rate
[0026] In some embodiments, the furnace is at room temperature (~25°€) at the
commencement of the heating step. The heating step is desirably conducted until the temperature is increased to or above a minimum temperature at which the organic material is removed from the particles and the desired metal oxide phase or phases have been formed. For example, in preparing haematite hollow structures from an initial composition containing sucrose and iron nitrate, the thermogravimetric analyser (TGA) curve shows that the burn off of sucrose and decomposition of iron nitrate is complete at 400°C. Therefore, in using this combination of starting materials, there is little benefit in extending the heating step above a temperature of 400°C. However, higher calcination temperatures (for example up to 600°C) were tried and the desired hollow microstructures were obtained. The skilled person will appreciate that the minimum temperature to which the composite particles must be heated will be very in accordance with the starting materials utilised.
[0027] In some embodiments, the multi-shelled hollow structures formed in the present invention may be maintained at a high temperature for a period of time in order to improve the crystallinity of those structures.
[0028] The multi-shelled hollow structures may comprise multi-shelled hollow particles or multi-shelled hollow spheres.
[0029] In some embodiments, the multi-shelled hollow structures comprise three or more shells wherein shells of decreasing size are located within larger shells. Suitably, the multi- shelled hollow structures comprise at least four shells, or suitably comprise four shells.
[0030] In desirable embodiments of the present invention, all of the shells comprise hollow shells. The multi-shelled hollow structures suitably do not have a solid core.
[0031] In some embodiments, the yolk-shell hollow structures may comprise yolk-shell hollow spheres or yolk-shell hollow particles. In other embodiments, the core particles (i.e., the yolk) may be solid.
[0032] The metal oxide that forms the multi-shelled hollow structures may comprise hematite (Fe O. . The metal oxide that forms the multi-shelled hollow structures may comprise a complex metal oxide or a mixed metal oxide containing two or more metals in the oxide structure. The two or more metals may comprise iron and one or more other metals.
[0033] The multi-shelled hollow structures may comprise particles having a size falling within the range of 200 to 4000nm, or within the range of 400 to 2000 nm.
[0034] In some embodiments, the multi-shelled hollow structures may comprise a plurality of particles joined to other particles to form an aggregate. The plurality of particles may be formed into an aggregate by necking at regions where one particle comes into contact with an adjacent particle. At these regions, "bridges" may be formed between the particles to thereby hold the particles together in a fused aggregate of particles such that the size of the aggregates is larger than that of the primary particle building blocks. In water treatment applications, small particles often wash through the filter housing containing the particles, causing discolouration of the filtered water, loss of contaminant adsorption capacity in the filter and the loss of contaminant-containing particles into the treated water stream. The use of larger particles can prevent this toss of material. Small particles also pack more densely than larger particles providing smaller interpartictes dimensions for water to flow through. This has the effect of decreasing the flow rate of water through media compared to media composed of larger particles where interparticle void spaces are larger. Lower flow rates in turn limit the productivity of water treatment systems and makes blockage of filter systems more likely to occur, in contrast, fused aggregates with their larger particle size have the advantages of allowing higher flow rates to be achieved with a lower probability of blocking.
[0035] In another aspect, the invention resides broadly in a method for forming aggregate particles ("fused aggregates") from multi-shelled hollow structures, the method comprising the steps of mixing the multi-shelled hollow structures with a chemical species that is a metal hydroxide or is capable of forming a metal hydroxide to form a mixture, and processing the mixture to produce the aggregate particles.
[0036] Any suitable metal hydroxide may be used, such as, but not limited to, copper hydroxide, titanium hydroxide, aluminium hydroxide, iron hydroxide, nickel hydroxide or the like, or a combination thereof. The metal hydroxide may be provided in any suitable form, such as, but not limited to a solid, solution or gel. More preferably, however, the metal hydroxide is in the form of a gel. Preferably, the metal hydroxide gel comprises aluminium hydroxide gel. Preferably, the metal hydroxide gel is a wet metal hydroxide gel.
[0037] In place of the metal hydroxide, fused aggregates may be formed by mixing the multi-shelled hollow structures with a chemical agent that is a precursor to metal hydroxides. In this case, the chemical agent precursor precipitates to form a metal hydroxide that cements the multi-shelled hollow structures together. The formation of metal hydroxides from metal -based precursors via reactions such as hydrolysis and precipitation are well known to those in the art. For example, a titanium hydroxide species could be formed using a precursor such as titanium
isopropoxide or titanium trichloride which undergo hydrolysis to form the titanium hydroxide product.
[0038] The multi-shelled hollow structures and the metal hydroxide gel may be mixed using any suitable technique. Preferably, however, the mixing of the multi-shelled hollow structures and the metal hydroxide gel is achieved using mechanical mixing. Λ skilled addressee will understand that the exact nature of the mechanical mixer used to achieve the mixing is not critical.
[0039] It is envisaged that the mixing of the multi-shelled hollow structures and the metal hydroxide gel will result in the formation of a mixture Once mixing is complete, the mixture may be processed to form the aggregate particles. The mixture may be processed using any suitable technique, such as extruding the mixture through a screen, sieve, mould or the like, or any suitable combination thereof. The choice of processing method used will depend on the desired particle size of the final fused aggregates. Most preferably, the mixture may be extruded through a screen or sieve. The size of the apertures in the screen or the sieve is not critical, and a skilled addressee wilt understand that the size of the apertures in the screen or sieve through which the mixture is extruded will be dependent on the desired size of the aggregate particles.
[0040] Once extruded, the aggregate particles may be dried and or calcined. Following drying and/or calcination, the fused aggregates may be further processed to refine the particle size. By providing control of the particle size in this step, less precise processing of the mixture prior to dryingcalcination to control particle size is required. Methods for achieving particle size control in this manner include, but are not limited to, milling techniques such as ball milling, crushing, particle size reduction using high shear forces, high pressure homogenisation and other techniques known to those skilled in the art.
[0041] In lithium ion battery applications electrodes typically contain a number of components including the active, charge storage material, a particle additive to promote electronic conductivity such as carbon black or acetylene black and a polymeric binder that acts to hold together all of these particulate components into a self-supporting structure. These mixed components are typically coated onto a metal foil which acts as a current collector in a battery. In addition, while binder materials and conductivity enhancers perform an important function, they do not contribute to the charge storage capacity of the electrode and therefore essentially dilute the charge storage capacity of the active material. Consequently, it is generally the objective of those skilled in the art to minimise the use of binders, conductivity enhancers and other non- active additives while still maintaining an electrode with suitable mechanical strength and
electronic conductivity. Relative to larger particles, it is generally observed that smaller particles, due to their higher surface area compared to larger particles, require larger amounts of binder in the electrode to achieve a suitable level of mechanical robustness. Therefore, the use of larger particles has the advantage of requiring less binder, allowing higher charge storage capacities to be achieved.
[0042] In other embodiments, the multi-shelled hollow structures comprise microspheres.
[0043] In a second aspect, the present invention provides a method for forming multi-shelled hollow structures comprising metal oxide, the method comprising the steps of
- forming a solution by dissolving at least one metal-containing compound and organic material in a solvent,
- spray drying the solution to form composite particles comprising the at least one metal - containing compound and the organic material, and
- heating the composite particles by increasing temperature at a predetermined rate to form the multi-shelled hollow structures comprising metal oxide.
[0044] In the method of the present invention, the composite particles are formed by spray drying. The composite particles are then subjected to a separate heating or an annealing step in which the composite particles are subjected to increasing temperature at a predetermined rate. This converts the metal species or metal compound to a metal oxide whilst also removing the organic material from the particles.
[0045] Without wishing to be bound by theory, the present inventors have postulated that during the heating step in the non-equilibrium heat treatment, induced heterogeneous contraction dominates and is responsible for the formation of the metal oxide multi-shelled hollow structures. More specifically, the composite particles are not homogeneously heated during the heating annealing; instead, there exists a temperature gradient (ΔΤ) along the radial direction of the particles. The ΔΤ leads to the first formation of a metal oxide shell at the surface of the composite particles. This shell is relatively rigid, and it can prevent the further contraction of the particles. Two forces of opposite directions exert at the interface of the composite core and the metal oxide shell. The contraction force (Fc) promotes the inward shrinkage of the core, while the adhesion force (Fa) induces the outward contraction. With a large ΔΤ, the Fc exceeds Fa, leading to the detachment of the core from the preformed shell. The abovementioned process (shell formation and core detachment) can be repeated several times until the ΔΤ become negligible. With a small ΔΤ, the Fa surpasses Fc, and the mass diffusion trend is reversed. That
is, the inner core shrinks outwardly, leaving a cavity at the centre. As a result, multi-shelled hollow structures having a number of hollow shells.
[0046] In a further aspect, the present invention provides multi-shelled hollow structures comprising a metal oxide, the multi-shelled hollow structures comprising at least three hollow shells.
[0047] In some embodiments, the multi-shelled hollow structures comprise at least four hollow shells. In another embodiment, the multi-shelled hollow structures comprise four hollow shells. In some embodiments, the multi-shelled hollow structures suitably do not have a solid core. In other embodiments, the multi-shelled hollow structures have multiple hollow shells and a solid core within the inner shell.
[0048] The multi-shelled hollow structures of the present invention may be suitable for use as an active material on or in the anode of lithium ion batteries. Therefore, in a further aspect, the present invention provides an electrode material for a lithium ion battery anode, the electrode material including multi-shelled hollow structures in accordance with the present invention, as described herein.
[0049] In a further embodiment, a lithium ion battery using the anode of the present invention is provided. The multi-shelled hollow structures and the anode electrodes of the present invention can function as drop-in replacements for conventional anode active materials and anode electrodes. As such, no special processing of the active material or of the assembled anode is required and these components are compatible with the use of other conventional components used to make batteries such as electrolytes, separators, current collectors, cathodes and cell packing materials. Those skilled in the art will be well placed to select appropriate components in the construction of anode electrodes and battery devices using the multi-shelled hollow structures of the present invention.
[0050] The multi-shelled hollow structures of the present invention may also be suitable for removing contaminants, such as heavy metal ions, from solutions. Accordingly, in another aspect, the present invention provides a method for removing heavy metal ions dissolved in a solution, the method comprising contacting the solution with multi-shelled hollow structures in accordance with the present invention, as described herein.
[00S1] The heavy metal ions that may be removed from the solution include arsenic, lead, cadmium, chromium, antimony, cobalt, copper, selenium and molybdenum.
[0052] The present inventors have also found that the multi-shelled hollow structures in (lie present invention may be used to remove polyanions, such as phosphate, from solution. Accordingly, in yet a further aspect, the present invention provides a method for removing polyanions from solution, the method comprising contacting the solution with multi-shelled hollow structures in accordance with the present invention, as described herein. The polyanions to be removed may be selected from arsenite, arsenate, phosphate. Other polyanions may also be removed.
[0053] The multi-shelled hollow structures used to remove heavy metal ions or phosphate from solution preferably comprise hematite (Fe Oj) chromium oxide, vanadium oxide, zinc iron ternary oxide and manganese-iron ternary oxide (Fe-Mn-Ox, such as ternary iron-manganese- oxides such as MnFe∑0 and FeMn>0,i).
[0054] Where the multi-shelled hollow structures of the present invention are composed of iron oxide and have been designed for use in the removal of arsenic species from solution, the surface area of the material is typically lower that 100 m2/g and more typically lower than 50 m2/g. These values of surface area are significantly lower than those of commercial iron oxide materials used in the removal of arsenic species where surface areas of commercial materials can be in the range of 200-300 m2/g. Such high surface areas are desired for maximising capacity to adsorb arsenic species as it is understood that arsenic adsorption is a surface phenomenon, and as such, larger surface areas impart a larger capacity for arsenic species removal. Without wishing to be bound by theory, the present inventors postulate that the higher arsenic adsorption capacities seen in the material of the present invention relative to commercial materials, despite the much lower surface areas measured, may result from the presence of the internal voids within each multi-shelled hollow structure particle which acts as a reservoir for sequestration of contaminant species. The concave shape of the inside walls of the multi-shelled hollow structures together with the internal void space may be acting to stabilise captured contaminant. In the case of arsenic species, adsorption to iron oxide is understood to occur by the build-up of multi-layers of arsenic species on the surface of the iron oxide according to the Freundlich isotherm model and it may be that the structure of the multi-shelled hollow materials of the present invention act to stabilise these multilayers of contaminants.
[0055] Adsorbent materials are often used in a particulate or granular form held within a cartridge or other container through which contaminated water requiring treatment is passed. To ensure the adsorbent material does not exit the container with the treated water, its exit is impeded by a mesh-like structure. In order for this to be effective, the particle size of the
adsorbent material must be at least of a certain size. This is also important in ensuring reasonable flow rates through a treatment system since smaller particles tend to pack more tightly than larger particles, leaving lower amounts of interparticle void space for water to pass through thus requiring large back pressures to move water through the material.
[0056] The use of the adsorbent materials of the present invention is compatible with existing engineering solutions used in the water treatment industry. As such, the materials of the present invention have been designed to be a drop-in solution. Adsorbent material may be used as a pure powder, it may be attached to the surface of a carrier material or to a substrate or may be incorporated within an extruded or pressed porous filtration block such as those made from activated carbon particles. The choice of product format depends on the adsorbent particle size requirements of the application, the amount of arsenic to be removed and other factors. In large scale water utility or industrial arsenic removal processes it is common that the adsorbent material will be present as a collection of pure iron oxide particulates contained within a metal or plastic filtration tank through which contaminated water passes, as is commonly used in the art. Here, in order to minimise the frequency of adsorption media changeovers (replacing spent iron oxide media) pure iron oxide will be used to maximise absorption capacity. In residential applications the iron oxide absorption media of the present invention may be used in conjunction with water filter pitchers that are based on activated carbon. These devices typically only contain activated carbon and thus are not capable of adsorbing arsenic species from contaminated water. Particles of iron oxide could be included into the filtration media in order to provide an arsenic removal capability for these products. This would not lead to a significant decrease in the volume of activated carbon present in the filter (and hence a reduction in filtration capacity for other contaminants) as long as the arsenic adsorption capacity of the iron oxide was sufficiently high that only a small volume of material was required to remove arsenic to suitable levels. Here, the iron oxide could be included as a discrete powder mixed in with particles of activated carbon, as a discrete powder contained separately from the activated carbon, as particles fixed to the surface of die activated carbon particles, incorporated into a carbon block fabricated by extrusion or pressing or incorporated using other methods known to those skilled in the art. Optionally, the multi-shelled hollow structures or yolk shell structures could be packed in reactor beds for the treatment of industrial waste water.
[0057] The multi-shelled hollow structures of the present invention may also be suitable for oxidative or non-oxidative dehydrogenation of alkanes to alkenes. Accordingly, in a further aspect the present invention provides a method for the oxidative or non-oxidative dehydrogenation of alkanes to alkenes, characterised in that multi -shelled hollow structures of
the present invention are present during the oxidative or non-oxidalive dehydrogenation of alkanes to alkenes.
[0058] Any of the features described herein can be combined in any combination with any one or more of the other features described herein within the scope of the invention
[0059] The reference to any prior art in this specification is not, and should not be taken as an acknowledgement or any form of suggestion that the prior art forms part of the common general knowledge.
BRIEF DESCRIPTION OF DRAWINGS
[0060] Figure 1 shows a schematic illustration for the formation of a-FeiOj MSHSs in accordance with the postulated synthesis pathway;
[0061] Figure 2 shows an FESEM image of iron nitrate-sucrose composite microspheres formed alter spray drying (sucrose iron nitrate ratio: 10/10), but before annealing;
[0062] Figure 3 shows a TGA (thermogravimetric analyser) curve of the iron nitrate-sucrose composites (sucrose/iron nitrate ratio: 10/10) during heating thereof to elevated temperature.
[0063] Figure 4a shows a digital photo of the a-Fe203 MSHSs;
[0064] Figure 4b shows an XRD pattern of the a-Fe203 MSHSs;
[0065] Figures 5(a) to 5(f) show FESEM images (a, b) at an accelerating voltage of 5 and 15 kV, respectively. TEM images (c, d), SAED pattern (e), and HRTEM image (f) of a-FejOj MSHSs (Sample 1);
[0066] Figures 6(a) to 6(f) show TEM images of Sample 2 (a and b, sucrose iron nitrate ratio: 7.5/10), Sample 1 (c, sucrose iron nitrate ratio: 10/10), Sample 3 (d and e, sucrose iron nitrate ratio: 15/10), and Sample 4 (f, sucrose/iron nitrate: 20/10);
[0067] Figures 7(a) to 7(e) show FESEM (a - c) and TEM (d, e) images of a-F^O* hollow structures (Sample 5, temperate ramp rate: 0.5 °C/min). For a and b, the accelerating voltage is 5 kV; for c, the accelerating voltage is 15 kV;
[0068] Figures 8(a) to 8(e) show FESEM (a - c) and TEM (d, e) images of a-F^ hollow structures (Sample 6, temperature ramp rate: 5 °C/min). For (a) and (b), the accelerating voltage is 5 kV; for (c), the accelerating voltage is 15 kV;
[0069] Figures 9(a) to 9(d) show TEM images of a-FejO* hollow structures using glucose (a and b. Sample 7) and PVA (c and d, Sample 8) as the organic species;
[0070] Figures 1 (a) to 10(e) show FESEM (a, b)t TEM (c, d), SAED (e) and HRTEM (f) images of ZnFe20 yolk-shell structures;
[0071] Figure 11 shows XRD pattern of the ZnFe2 >4 yolk-shell structures;
[0072] Figure 12 shows CVs (cyclic voltrammetry) of a-FeiC^ MSHSs for the initial three cycles at a rate of 0.1 mA s"1;
[0073] Figures 13(a) to 13(d) show (a) Representative charge-discharge profiles of a-FeaOs MSHSs at a current density of 400 mA g"1 in the voltage window of 0.05 - 3.0 V; (b) cycling performance of a-FejOj MSHSs at a current density of 400 mA g"1; (c) representative charge- discharge profiles of a-FejO MSHSs at different current densities (100 - 3200 mA h g*1); (d) rate performance of -FejQ^ MSHSs. For a and b, current density of the first two cycles are 50 mA g*1;
[0074] Figure 14 shows Coulombic efficiency vs. cycle number;
[0075] Figure 15 shows TEM images of Ο2Ο3 multi-shelled hollow spheres formed in accordance with an embodiment of the present invention;
[0076] Figure 16 shows TEM images of V2O5 yolk-shell structures formed in accordance with an embodiment of the present invention;
[0077] Figure 17 shows XRD pattern of V2O5 yolk-shell structures; and
[0078] Figure 18 shows a TEM image of a-Fe203 MSHSs prepared by calcining an iron nitrate-sucrose composite at 600 °C for 5 hours in air.
[0079] Figure 19 shows a drinking water treatment device.
[0080] Figure 20 illustrates the arsenic removal capabilities of a-Fe2C>3 multi-shelled hollow structures when used in the drinking water treatment device of Figure 19.
[0081 ] Figures 21 (a) to 21 (g) show TEM images of a-Fe2O.ii multi-shelled hollow structures with an iron citrate precursor using sucrose as the organic species.
[0082] Figure 22 illustrates the adsorption of anions other than arsenic anions using a-Fe20*
multi-shelled hollow structures.
[0083] Figure 23 illustrates granular particles produced using a-Fe 03 multi-shelled hollow structure material.
EXAMPLES
[0084] The examples mainly relate to the preparation of a-Fe^O? multi-shelled hollow structures.
[0085] The a-FejOs MSHSs hollow microspheres were prepared by a spray drying method followed by annealing in air. In a typical synthesis (Sample I, see Table 1), Fe(N03>*9H 0 (10 mmol) and sucrose (10 mmol) were dissolved in H20 (100 mL) to form a clear solution. The resulting solution was then spray dried using a Buchi mini spray drier B-290 at an inlet temperature of 220 °C, an aspirator rate of 100 %, a rotameter setting of 60 mm, and a pump rate of 5 % (1.5 mL/min). Nitrogen was used as the drying gas. These settings resulted in an outlet temperature of - 130 °C. The spray dried samples (iron nitrate-sucrose composite) were then annealed in air at 400 °C for 5 hours with a temperature ramp rate of 2 °C/min. To study the influence of synthesis parameters on the structures, a series of samples were prepared by adjusting the feeding ratio of reagents, the temperature ramp rate, and the choices of organic matrices. The detailed synthesis conditions for all samples are listed in Table 1.
Table 1. Synthesis conditions for a-FejO? hollow structures/nanostruclures.
Sample Name Organic Species Iron Nitrate Temperature ramp rate
Sample I Sucrose, 10 mmol 10 mmol 2 °C7min
Sample 2 Sucrose, 7.5 tnmol 10 mmol 2 °C/min
Sample 3 Sucrose, 15 mmol 10 mmol 2 "C/min
Sample 4 Sucrose, 20 mmol 10 mmol 2 /min
Sample 5 Sucrose, 10 mmol 10 mmol 0.5 °C/min
Sample 6 Sucrose, 10 mmol 10 mmol 5 °C/min
Sample 7 Glucose, 20 mmol 10 mmol 2 °C/min
Sample 8 Polyvinyl alcohol, 4 g 10 mmol 2 "C/min
[0086] Characterization
[0087] X-ray diffraction (X D) patterns were collected on a German Bruker D8 Advanced X-Ray DifFractometer with Ni filtered Cu Ka radiation (40 kV, 30 mA). Field emission scanning electron microscopy (FESE ) images were obtained on a JEOL JSM 7800 microscope with an accelerating voltage of 5.0 or 15.0 kV. Transmission electron microscopy (TE ) images were taken on Tecnai F20 (200 kV) and JEOL 1010 (100 kV) microscopes. Thermo gravimetric analysis <TGA) was carried out on a TGA/DSC 1 STAR* System under air flow (25 - 1000 °C, 5 °C/min). Nitrogen adsorption isotherms were measured at 77 K using a TriStar II Surface Area and Porosity analyser (Micromeritics). The samples were degassed under vacuum for 6 hours at 200 °C before analysis. BET surface area was calculated from the adsorption branch in relative pressure (p/po) range of 0.05 - 0.30.
[0088] Electrochemical Measurement
[0089] The electrochemical measurements were carried out in homemade two-electrode Swagelok type cells. The working electrode is consisted of active material, conductive acetylene black, and polyvinylidene fluoride binder in a weight ratio of 70:20:10. Lithium chips were used as both the counter electrode and reference electrode. 1 M LiPF« in a mixture of ethylene
carbonate, dimethyl carbonate, and diethyl carbonate (1: 1 :1 in volume) was used as the electrolyte. Cell assembly was carried out in an Ar-ftlled glovebox with moisture and oxygen concentrations below 1.0 ppm. Cyclic voltaminetry (CV) measurements were performed on a Solartron 1480 Multistat instrument. The galvanostatic charge-discharge tests were performed on a MTI 8 Channels Battery Analyzer.
[0090] The synthesis of a-Fe^O* MS Ss is based on a simple spray drying method followed by annealing in air. The first step involves the preparation of iron nitrate-sucrose composite microspheres by spray drying; while the second step is annealing the composite spheres in air at 400 °C. The non-equilibrium heat treatment induced heterogeneous contraction dominates the second step and is responsible for the formation of the α-FejOj MSHSs. More specifically, the iron nitrate-sucrose microspheres are not homogeneously heated during the annealing; instead, there exists a temperature gradient (ΔΤ) along the radical direction. The ΔΤ leads to the first formation of a a-F^Oi shell at the surface of the composite spheres. This shell is relatively rigid, and it can prevent the further contraction of the microspheres. Two forces of opposite directions exert at the interface of the composite core and a-Fe205 shell. The contraction force (Fc) promotes the inward shrinkage of the core, while the adhesion force (Fa) induces the outward contraction. With a large ΔΤ, the Fc exceeds Fa, leading to the detachment of the core from the preformed shell. The abovementioned process (shell formation and core detachment) can be repeated for several times until the ΔΤ become negligible. With a small ΔΤ, the Fa surpasses Fc, and the mass diffusion trend is reversed. That is, the inner core shrinks outward, leaving a cavity at the centre.
[0091] A schematic diagram showing the postulated synthesis pathway is show in Figure 1. Figure 2 shows an FESEM image of the iron nitrate-sucrose composite particles formed after spray drying of the iron nitrate-sucrose solution in the spray drier.
[0092] The composite iron nitrate-sucrose particles were then heated. Figure 3 shows a TGA (thermogravimetric analyser) curve of the iron nitrate-sucrose composites (sucrose/iron nitrate mole ratio: 10/10). As can be seen from figure 3, the weight of the panicles decreases as temperature increases, due to decomposition of the iron nitrate to iron oxide and due to decomposition and calcination of the sucrose. It is believed that the sucrose is eventually converted to carbon dioxide, which is removed from the particles. This can also be seen from figure 3, no further weight loss is identified after the temperature reaches 400°C, indicating that further heating above 400°C should not be required to fully form the α-FeiO? multi-shelled hollow particles.
[0093] Well-developed a-Fe^Oj MSHSs can be synthesized in a large scale using inexpensive iron nitrate and sucrose as the precursors (Figure 4a). The as-prepared product is first characterized by XRD to gain insight into the crystallographic structure. As shown in Figure 4b, all the diffraction peaks can be well indexed to rhombohedra phase a-FejOj (JCPDS Card
No. 33-0664), also known as hematite, with a R3 space group. No other peaks can be detected, indicating the high purity of the products. The relatively broad diffraction peaks suggesting the nanocrystalline characteristic of the sample. By applying Scherrer equation on the strongest (104) diffraction peak, die average crystallite size is determined to be 24 nm.
[0094] The morphology and structure of the products were examined by SEM and TEM. As can be seen from Figure 5(a) and 5(b), the products consists of microspheres with a size in the range of 400 - 2000 nm. With a relatively low accelerating voltage of 5 kV (Figure 5(a)), one can find that the microspheres are not perfectly smooth; instead, wrinkles can be observed on the surface. From the broken part of a microsphere, an inner core particle is exposed, indicating the microspheres actually have a yolk-shell or multi-shell structure. With a high accelerating voltage of 15 kV, the penetration capability of the electrons in SEM is greatly enhanced. Thus, the inner cores can be clearly discerned even though the microspheres are intact (Figure 5(b)). Low magnification TEM images (Figure 5(c) and 5(d)) undisputedly demonstrate the microspheres have a multi-shelled structure and most of the spheres have four shells. The thickness of the shell is around 20 nm, which is generally agrees with the crystallite size determined from XRD. The selected area electron diffraction (SAED) pattern (Figure 5(e)) of the microspheres shows a series of concentric rings, which can be assigned to the (012), (104), (1 10), (113), (024) and (1 6) diffractions of a-FejOj, respectively. A typical high resolution TEM (HRTEM) image is shown in Figure 5(f), where the (012) lattice fringes of a-FejO^ can be clearly observed.
[0095] This unique multi-shelled hollow structure gives rise to a relatively high surface area of 17.2 m2 g"' and pore volume of 0.1 cm3 g"\
[0096] The effect of iron nitrate sucrose on the structure of the products has been studied (Figure 6(a) to 6(f)). The optimised sucrose/iron nitrate ratio (mole ratio) for the fabrication of a- Fe203 MSHSs is found to be in the range of 7.5/10 - 10/10 (Figure 6(a) - 6(c)). With a sucrose iron nitrate of less than 5/10, no iron nitrate-sucrose composite spheres can be formed by spray drying (data not shown). With a sucrose iron nitrate of more than 15/10, a-FejOj nanosheets rather than hollow spheres are formed (Figure 6(d) - 6(f)).
[0097] According to the heterogeneous contraction mechanism, the temperature ramp rate may also ptay an important role on the morphology and structure of the products: a low ramp
rate will lead to a small ΔΤ, and thus fewer shells; while a high ramp rate will result in a high ΔΤ, and thus more shells. With a low ramp rate of 0.5 °C/min, single-shelled hollow spheres and yolk-shell structures are formed (Figure 7). With a high ramp rate of 2 and 5 Vmin, multi- shelled hollow spheres are formed (Figure 8). Compared to sample 1 (Figure 5) with a ramp rate of 2 °C/min, sample 6 with a ramp rate of 5 °C (Figure 8) shows more broken spheres.
[0098] Besides sucrose, other organic species, such as glucose (Figure 9(a) and 9(b)) and polyvinyl alcohol (Figure 9(c) and 9(d)) can also be utilized as the matrix to synthesize a-FejO? hollow spheres. This strategy is not limited to the fabrication of a-Fe203 hollow spheres. As another example, ZnFe>0.t yolk-shell structures (Figure 10 and 1 1) can also be fabricated by this approach by simply replacing one third of the iron nitrate with zinc nitrate.
[0099] Figures 21a to 2 lg illustrate the use of different quantities of sucrose as the organic species utilized as the matrix to synthesize a-Fe203 hollow spheres having an iron citrate precursor. In the synthesis of the a-FejOs hollow spheres in Figures 21a to 21g, 10 mmol of iron citrate was dissolved in 100ml of water to form a clear solution. In Figure 21a no sucrose was added to the solution, while in Figure 21b 2.5 mmol of sucrose was added, in Figure 21c 5 mmol was added, in Figure 2 Id 7.5 mmol was added, in Figure 21e 10 mmol was added, in Figure 21f 12.5 mmol was added and in Figure 2 lg 15 mmol was added.
[00100] The resulting solution was then spray dried using a Buchi mini spray drier B-290 using air as the drying gas. The as-made samples were then annealed in air at 4003C for 5 hours with a temperature ramp rate of 2'C/hour, with the resulting iron oxide structures illustrated in Figures 21 a to 2lg. High arsenic absorption capacity was confirmed for these a-Fe203 structures produced from the iron citrate precursor.
[00101] To study the lithium storage capabilities of the a-Fe203 MSHSs, CV and galvanostatic charge/discharge cycling were carried out based on the half-cell configuration. The CVs for the initial three cycles at a rate of 0,1 mV s*1 are shown in Figure 12. Three cathodic peaks can be observed in the first cycle. The first peak centred at 1.58 eV can be attributed to the intercalation of lithium into the crystal structure of a-FejOj the second peak centred at 0.93 eV can be attributed to the reduction of Fe * to Fe*~; and the third peak centred at 0.58 eV can be attributed to the reduction of Fe2^ to Fe and the irreversible reduction of the electrolyte. Tn the first anodic process, only one broad peak located between I 5 and 2.0 V can be observed, corresponding to the oxidation of Fe to Fe3 \ From the second cycle onward, a pair of broad cathodic/anodic peaks can be observed and the CV curves generally overlaps, suggesting a good reversibility the redox reaction (Fe3* + 3e" «→ Fe).
[00102] The cycling performance of the a-FejOs electrode was evaluated by activating (lie half cells at 50 mA g*1 for two cycles and then cycling at 400 or 1600 mA g"\ At a current of SOmAg"1, the α-Ρβ2<¼ MSHSs delivers an initial discharge capacity of 1443 mA h g"1 and a charge capacity of and 1067 mA h g'\ respectively (Figure 13a). After the activation process, a high and stable capacity of ~ 1000 and 900 mA h g*' can be achieved at a current of 400 and 1600 mA g*' (Figure 13b), respectively. The coulombic efficiency for the first cycle is around 75%, while it stabilizes at ~ 98% after the activation process (Figure 14).
[00103] The rate performance of the a-Fe20.? was investigated by cycling the material at various currents ranging from 100 to 3200 in A g*1 for 5 cycles. Representative charge-discharge profiles (the third cycle at each rate) are shown in Figure 13c. As the current density increases from 100 to 200, 400, 800, 1600 and 3200 mA g"\ the capacity only decreases slightly from 1228 to 1 193, 1 102, 1013, 913 and 784 mA h g 'iFigure 13c and 13d), indicating the excellent rate capability of the a-Fe203 MSHSs. After reaching its highest value, that is 3200 mA g"1, the current density is reduced to 200 mA g"1 gradually. A capacity of 1 176 mA h g*1 can be recovered at 200 mA g*\ which is 98.6 % of the capacity at the same current density before the high rate measurement.
[00104] Notably, the electrochemical performances of the a-Fe20j MSHSs are superior or at least comparable to those of high-performance FeaC -based anode materials reported recently, such as a-FeiOs nanorods, a-FeaO? hollow spheres, Fe20j decorated carbon nanotubes, and Fe2<) reduced graphene oxide nanocomposites. The enhanced electrochemical performances can be attributed to the unique structural characteristics of the a-Fe20¾ MSHSs. The permeable and thin shells greatly shorten the distances for Li* diffusion; the void space effectively accommodates the drastic volume change and alleviates the strain during Li* insertion/extraction. All these features contribute to the stable high-rate performance of the < -Fe203 MSHSs.
EXAMPLE - Nanoporous hematite for As removal
[00105] The properties of nanoporous hematite were also investigated for arsenic removal. Without wishing to be bound by theory, the present inventors have hypothesized that the existence of voids within layers might accommodate arsenic species for adsorption purpose.
[00106] Na2HA 04.7H20 (Sigma Aldrich) and NaHAsC (Ajax Finekem) were used as As(V) and As(Ill) sources, respectively. 0.01M HCI and M IO^ NaOH were used for pH adjustment. Distilled water was used throughout the experiment.
Batch experiments;
[00107] A solution containing 400 ppm As(IH) was prepared by dissolving 693. S mg of NaHAsCh in 1 L H20. Consequently, 1.65 g of Na2HAs04.7H20 was dissolved in 1 L H20 to make 400 ppm of As(V) solution. Serial dilutions were performed to obtain arsenic concentrations of 200ppm, 1 ppm, and 50ppb for both As(V) and As(IIJ) solutions. A dosage 20 mg of nanoporous hematite was added into each 50 mL solution. All solutions were shaken for 24 h at room temperature at a speed of 200 rpm.
[00108] WAGTECH arsenic kitrM was used to quantify the amount of arsenic adsorbed. The filter paper was calibrated prior to each arsenic quantification.
[00109] The removal at both high and low As concentrations are presented below.
Table 2. Summarized results of As removal at high concentrations using nanoporous Fe205
All pH values were recorded under stirring. All measurements were in duplicate
[00110] From the above tabulated results, it can be concluded that nanoporous hematite is beneficial for arsenic removal, as it can lower down the As(V) concentration from lppm to 8ppb (table 3), which is below the stringent discharge limit.
[001 11] It is expected that the a-FejCh multi-shelled hollow structures made in accordance with the present invention will also be able to remove other heavy metal ions, such as lead, cadmium, chromium, antimony and molybdenum, as well as removing phosphate ions from solution.
[001 12] To demonstrate the effectiveness of nanoporous hematite in arsenic removal in the case that the hematite is used in combination with an activated carbon-based water treatment system, nanoporous hematite was used as an additive to the filter media of a standard activated
carbon-based water purification system. The addition of the nanoporous hematite conveys an arsenic removal capability on the system enabled by the hematite.
[001 13] The test is illustrated in Figure 1 . In this test, a commercial water filter pitcher 10 (Brita® Maxtra) was used as the standard water filter device. The standard media 1 1 in this device is composed of activated carbon and ion exchange resin with a total mass of 30g. For the device modified for arsenic removal, O.lg of a-Fe Oj multi-shelled hollow structures 12 made in accordance with the present invention were added to the 30g of standard media 1 by mixing the a-Fe20i multi-shelled hollow structures 12 into the standard media 1 1.
[001 14] An aqueous solution 13 containing l Oppb As(V) was fed through both standard filter (not shown) and a modified filter 14 under gravity, and the concentration of As(V) was measured in the effluent 15. As illustrated in Figure 20, it was found that the standard filter (not shown) reduced As(V) concentration in the water from lOOppb to 40ppb with .1 litre of water poured through the filter.
[00115] By contrast, the modified filter 1 containing 0. lg of a-Fe20? multi-shelled hollow structures 12 reduced the As(V) concentration in the effluent 15 from I OOppb to around Oppb
Example - Adsorption of anions other than arsenic anions
[00116] In Figure 22 there is illustrated the adsorption of oxyanions other than arsenic oxyanions using a-Fe20:t multi-shelled hollow structures synthesised in accordance with the present invention. This Figure demonstrates the ability of the non-arsenic anions to complete for adsorption sites on the iron oxide structures and, in doing so, reduce the capacity of the iron oxide to adsorb arsenic species.
[00117] In this Example, the As(IU) and As(V) adsorption capacity of the a-Fe2<->3 multi- shelled hollow structures was studied using 400ppm arsenic contaminated waters that also contained additional non-arsenic anions. The non-arsenic anions were present at a concentration of lg L and were provided by the addition of Na2S04, Na3P04, Na2C03, and NaSiOv a-Fe20? multi-shelled hollow structures were present in the solutions at a dosage of 20mg per 50mL of solution
[00118] The effect of the addition of competing anions on As(IlI) and As(V) adsorption performance is illustrated in Figure 22. In all cases, the addition of the competing anions reduces the ability of the a-Fe20¾ multi -shelled hollow structures to adsorb arsenic anions, thereby demonstrating that the <x-Fe203 multi-shelled hollow structures also adsorb non-arsenic
anions. The decrease in the adsorption of arsenic anions due to the presence of competing anions is summarised in Table 5 below.
Table 5 Summarized results of reduced arsenic anion adsorption due to competing anions
Example - Production of CrjOj multi-shelled hollow spheres.
[00119] The method of the present invention has been used to form Cr205 multi-shelled hollow spheres. Figure 15 shows TEM images of the (¾<¼ multi-shelled hollow spheres that were formed. Multiple shells can be clearly seen.
Example - Production of V2O5 yolk-shell structures.
[00120] Figure 16 shows TEM images of V2O5 yolk-shell structures formed in accordance with an embodiment of the present invention. Figure 17 shows XRD pattern of V2O5 yolk-shell structures. Again, multiple shells can be seen.
Example - Formation of a-F^Oa MSHSs prepared by calcining an iron nitrate-sucrose composite.
[00121] Figure 18 shows a TEM image of a-FezOj MSHSs prepared by calcining an iron nitrate-sucrose composite at 600 °C for 5 hours in air. As can be seen, a multi shelled material has been obtained, showing that higher calcination temperatures are not detrimental to formation of the multi-shelled hollow structures. However, it will be appreciated that calcination to temperatures above the minimum temperature at which the organic material has been removed and the metal oxide phase(s) has been fully formed may not be required unless the higher temperature results in improved crystallinity in the material.
[00122] Embodiments of die present invention provide a facile method for the high-yield and large-scale synthesis of a-Fe20* multi-shelled hollow spheres (MSHSs) by spray drying. The resultant o-Fe20j hollow spheres exhibit high specific capacity, excellent rate capability and cycling stability. The method can also be used to make hollow spheres of other metal oxides. They can also be used to remove contaminants, such as heavy metals and polyanions, from solutions, such as water streams.
[00123] Example - Synthesis of granular particles based on a FeaOj particles
[00124] In this Example, granules were produced using the extrusion granulation method. 0.5g of a*Fe2Ch multi-shelled hollow spheres were mechanically mixed with wet aluminium hydroxide gel. The resulting uniform mixture was extruded through a 20 mesh sieve to produce granules that were dried at 50*C - 100*.
[00125] The final a-FejO* weight percentage in the granules was between 60% and 95% and this value was controlled by the proportion of aluminium hydroxide gel added to the a-Fe203 multi-shelled hollow spheres.
[00126] The arsenic absorption capacity of the granulated a-Fe∑Oi multi-shelled hollow spheres was not reduced compared to the a-Fe^Ch multi-shelled hollow spheres primary particles. As illustrated in Figure 23, the particle size of the granulated material 20 was in the order of millimetres in length.
[00127] In the present specification and claims (if any), the word 'comprising' and its derivatives including 'comprises' and 'comprise' include each of the stated integers but does not exclude the inclusion of one or more further integers.
[00128] Reference throughout this specification to One embodiment' or 'an embodiment' means that a particular feature, structure, or characteristic described in connection with the embodiment is included in at least one embodiment of the present invention. Thus, the appearance of the phrases 'in one embodiment' or in an embodiment' in various places throughout this specification are not necessarily all referring to the same embodiment. Furthermore, the particular features, structures, or characteristics may be combined in any suitable manner in one or more combinations.
[00129] En compliance with the statute, the invention has been described in language more or less specific to structural or methodical features. It is to be understood that the invention is not limited to specific features shown or described since the means herein described comprises
preferred forms of putting the invention into effect. The invention is, therefore, claimed in any of its forms or modifications within the proper scope of the appended claims (if any) appropriately interpreted by those skilled in the art.
Claims
1. A method for forming multi-shelled hollow structures comprising metal oxide, the method comprising the steps of:
- forming a solution containing at least one dissolved metal compound and at least one organic compound,
- spray drying the solution to form composite particles containing at least one metal species and organic material, and
- heating the composite particles by increasing temperature at a predetermined rate to form the multi-shelled hollow structures comprising metal oxide.
2. A method according to claim 1 wherein the solution is formed by dissolving a metal compound and at least one organic compound in water.
3. A method according to claim 1 or claim 2 wherein the at least one dissolved metal compound is formed by dissolving a metal-containing compound in a solvent.
4. A method according to any one of claims 1 to 3 wherein the at least one dissolved metal compound comprises a metal nitrate, a metal chloride, a metal sulphate, a metal acetate, a metal alkoxide, a metal citrate, a metal lactate, a metal gluconate, a metal oxalate or an ammonium- metal compound.
5. A method according to any one of the preceding claims wherein the at least one dissolved metal compound comprises two or more dissolved metal compounds.
6. A method according to claim 5 wherein the two or more dissolved metal compounds comprise an iron compound and another metal compound.
7. A method according to any one of the preceding claims wherein the organic compound comprises an organic compound that can be removed by calcination.
8. A method according to any one of the preceding claims wherein the organic material comprises sucrose, glucose, oxalic acid, polyvinyl alcohol, polyvinyl pyrrolidone, polyethylene glycol or citric acid
9. A method according to any one of the preceding claims wherein the temperature of the composite particles is increased at a predetermined rate by increasing the temperature at a constant rate.
10. A method according to any one of the preceding claims wherein the multi-shelled hollow structures are maintained at a high temperature for a period of time in order to improve the crystallinity of the structures.
11 A method according to any one of the preceding claims wherein the metal oxide formed in the multi-shelled hollow structures is hematite.
12. A method for forming multi-shelled hollow structures comprising metal oxide, the method comprising the steps of
- forming a solution by dissolving at least one metal-containing compound and organic material in a solvent,
- spray drying the solution to form composite particles comprising the at least one metal- containing compound and the organic material, and
- heating the composite particles by increasing temperature at a predetermined rate to fonn the multi-shelled hollow structures comprising metal oxide.
13. A method according to claim 12 wherein the multi-shelled hollow structures comprise at least three hollow shells.
14. A method according to claim 12 or claim 13 wherein the multi-shelled hollow structures do not have a solid core.
15. Multi-shelled hollow structures comprising a metal oxide, the multi-shelled hollow structures comprising at least three hollow shells.
16. Multi-shelled hollow structures according to claim 15 wherein the at least three hollow shells comprise shells of decreasing size located within larger shells.
17 Multi-shelled hollow structures according to claim 15 or claim 16 wherein all of the at least three shells are hollow.
18. Multi-shelled hollow structures according to claim 15 or claim 16 wherein the structures further compri e a solid core.
19. Multi-shelled hollow structures according to any one of claims 15 to IS wherein the structure comprise particles having a size falling within the range of 200nm to 4000nm.
20. Multi-shelled hollow structures according to any one of claims 15 to 19 wherein the metal oxide comprises hematite.
21. A fused aggregate of multi-shelled hollow structures comprising a plurality of particles as formed by the method claimed in any one of claims 1 to 14 or as claimed in any one of claims 15 to 20 joined to other particles to form an aggregate.
22. A method for forming the aggregate of claim 21 comprising the steps of mixing the multi-shelled hollow structures with a metal hydroxide or a precursor to a metal hydroxide to form a mixture, and processing the mixture to form aggregate particles.
23. An electrode material for a lithium ion battery anode, the electrode material including multi-shelled hollow structures as claimed in claims 15 to 20, or as formed by the method of any one of claims 1 to 14.
24. A method for removing heavy metal ions dissolved in a solution, the method comprising contacting the solution with multi-shelled hollow structures as claimed in claims IS to 20, or as formed by the method of any one of claims 1 to 14.
25. A lithium ion battery comprising the electrode material as claimed in claim 23.
26. A water filter system for the removal of contaminants, the water Miter system comprising multi-shelled hollow structures as claimed in claims 15 to 20 or as formed by the method of any one of claims 1 to 14, or the fused aggregates of claim 2 or as formed by the method of claim 22, wherein the multi-shelled hollow structures or the fused aggregates are optionally present with activated carbon.
27. A water filter system according to claim 26 wherein the multi-shelled hollow structures or the fused aggregates and the activated carbon, if present, are in the form of a powder.
28. A water filter system according to claim 26 wherein the multi-shelled hollow structures or the fused aggregates are attached to the surface of a carrier material or to a substrate
29. A water filter system according to claim 26 wherein the multi-shelled hollow structures or the fused aggregates are incorporated within an extruded or pressed carbon filter block.
30. A method for the oxidative or nonoxidative dehydrogenation of alkanes to alkenes, characterised in that multi-shelled hollow structures as claimed in claims 15 to20, or as formed by the method of any one of claims I to 14, are present during the oxidative or nonoxidative dehydrogenation of alkanes to alkenes.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2013901725A AU2013901725A0 (en) | 2013-05-15 | Method for Producing Hollow Structures | |
| AU2013901725 | 2013-05-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014183169A1 true WO2014183169A1 (en) | 2014-11-20 |
Family
ID=51897521
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/AU2014/050038 WO2014183169A1 (en) | 2013-05-15 | 2014-05-15 | Method for producing hollow structures |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2014183169A1 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20180145554A1 (en) * | 2015-05-26 | 2018-05-24 | Siemens Aktiengesellschaft | Resistance Covering For A Corona Shield Of An Electric Machine |
| CN110404540A (en) * | 2019-07-30 | 2019-11-05 | 华中科技大学 | The preparation method and its product of a kind of Openworks shape iron selenium derivative catalyst and application |
| CN114142026A (en) * | 2021-12-02 | 2022-03-04 | 河南师范大学 | Manganese-based polyanion positive electrode material, preparation method thereof and sodium-ion battery |
| CN114471535A (en) * | 2022-01-24 | 2022-05-13 | 桂林理工大学 | Preparation method and application of manganese-titanium composite oxide catalytic material with hollow sphere structure assembled by rods |
| CN116216763A (en) * | 2021-12-02 | 2023-06-06 | 中国科学院过程工程研究所 | Multi-valence metal oxide hollow multi-shell composite material and preparation method and application thereof |
| WO2024098957A1 (en) * | 2022-11-10 | 2024-05-16 | 东江环保股份有限公司 | Preparation method and use of nano copper phosphide |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040038832A1 (en) * | 2002-08-23 | 2004-02-26 | Osram Sylvania Inc. | Spherical tungsten disulfide powder |
-
2014
- 2014-05-15 WO PCT/AU2014/050038 patent/WO2014183169A1/en active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040038832A1 (en) * | 2002-08-23 | 2004-02-26 | Osram Sylvania Inc. | Spherical tungsten disulfide powder |
Non-Patent Citations (2)
| Title |
|---|
| HONG, Y. J.; ET AL.: "One-pot facile synthesis of double-shelled Sn02 yolk-shell-structured powders by continuous process as anode materials for Li-ion batteries'';", ADV. MATER., vol. 25, 1 February 2013 (2013-02-01), pages 2279 - 2283 * |
| JING HU ET AL.: "Fabrication and application of inorganic hollow spheres", CHEM. SOC. REV., vol. 40, 2011, pages 5472 - 5491 * |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20180145554A1 (en) * | 2015-05-26 | 2018-05-24 | Siemens Aktiengesellschaft | Resistance Covering For A Corona Shield Of An Electric Machine |
| CN110404540A (en) * | 2019-07-30 | 2019-11-05 | 华中科技大学 | The preparation method and its product of a kind of Openworks shape iron selenium derivative catalyst and application |
| CN110404540B (en) * | 2019-07-30 | 2020-06-02 | 华中科技大学 | Preparation method of hollow-out iron-selenium derivative catalyst, product and application thereof |
| CN114142026A (en) * | 2021-12-02 | 2022-03-04 | 河南师范大学 | Manganese-based polyanion positive electrode material, preparation method thereof and sodium-ion battery |
| CN116216763A (en) * | 2021-12-02 | 2023-06-06 | 中国科学院过程工程研究所 | Multi-valence metal oxide hollow multi-shell composite material and preparation method and application thereof |
| CN114142026B (en) * | 2021-12-02 | 2023-10-20 | 河南师范大学 | Manganese-based polyanion positive electrode material, preparation method thereof and sodium ion battery |
| CN114471535A (en) * | 2022-01-24 | 2022-05-13 | 桂林理工大学 | Preparation method and application of manganese-titanium composite oxide catalytic material with hollow sphere structure assembled by rods |
| WO2024098957A1 (en) * | 2022-11-10 | 2024-05-16 | 东江环保股份有限公司 | Preparation method and use of nano copper phosphide |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Orooji et al. | Recent advances in nanomaterial development for lithium ion-sieving technologies | |
| Xu et al. | Extraction of lithium with functionalized lithium ion-sieves | |
| Zhu et al. | Recent progress in the syntheses and applications of multishelled hollow nanostructures | |
| Zhang et al. | Rice husks as a sustainable silica source for hierarchical flower-like metal silicate architectures assembled into ultrathin nanosheets for adsorption and catalysis | |
| US11440811B2 (en) | Ternary precursor particles and method for manufacturing the same | |
| Li et al. | Battery performance and photocatalytic activity of mesoporous anatase TiO2 nanospheres/graphene composites by template‐free self‐assembly | |
| Wu et al. | Smart construction of 3D N-doped graphene honeycombs with (NH 4) 2 SO 4 as a multifunctional template for Li-ion battery anode:“A choice that serves three purposes” | |
| Sun et al. | Co 3 O 4 nanocrystals with predominantly exposed facets: synthesis, environmental and energy applications | |
| KR101182271B1 (en) | Porous manganese oxide absorbent for Lithium having spinel type structure and a method of manufacturing the same | |
| Wan et al. | Facile synthesis of mesoporous NiCo2O4 fibers with enhanced photocatalytic performance for the degradation of methyl red under visible light irradiation | |
| Liu et al. | Anisotropic Co 3 O 4 porous nanocapsules toward high-capacity Li-ion batteries | |
| WO2014183169A1 (en) | Method for producing hollow structures | |
| Li et al. | Highly selective separation of lithium with hierarchical porous lithium-ion sieve microsphere derived from MXene | |
| Li et al. | Fabrication of 2D/2D nanosheet heterostructures of ZIF-derived Co 3 S 4 and gC 3 N 4 for asymmetric supercapacitors with superior cycling stability | |
| Hu et al. | Facile synthesis of porous Mn2O3 hierarchical microspheres for lithium battery anode with improved lithium storage properties | |
| Zhang et al. | A scalable three-dimensional porous λ-MnO2/rGO/Ca-alginate composite electroactive film with potential-responsive ion-pumping effect for selective recovery of lithium ions | |
| Feng et al. | Tailoring CoO–ZnO nanorod and nanotube arrays for Li-ion battery anode materials | |
| Liang et al. | Hydrothermal synthesis, self-assembly and electrochemical performance of α-Fe2O3 microspheres for lithium ion batteries | |
| JP5700338B2 (en) | Lithium adsorbent production method, lithium concentration method, and lithium concentration apparatus | |
| Meng et al. | Recent advances of hierarchically porous bifunctional oxygen electrocatalysts derived from metal–organic frameworks for Zn–air batteries | |
| Park et al. | Prussian blue analogue nanocubes with hollow interior and porous walls encapsulated within reduced graphene oxide nanosheets and their sodium-ion storage performances | |
| Kim et al. | Formation of ordered macroporous ZnFe2O4 anode materials for highly reversible lithium storage | |
| Zhang et al. | Cu3V2O8 hollow spheres in photocatalysis and primary lithium batteries | |
| Pal et al. | Morphology-mediated tailoring of the performance of porous nanostructured Mn 2 O 3 as an anode material | |
| Assefi et al. | Recycling of Ni-Cd batteries by selective isolation and hydrothermal synthesis of porous NiO nanocuboid |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14797919 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14797919 Country of ref document: EP Kind code of ref document: A1 |