WO2014019442A1 - Benzofuran derivatives, preparation method and medical use thereof - Google Patents
Benzofuran derivatives, preparation method and medical use thereof Download PDFInfo
- Publication number
- WO2014019442A1 WO2014019442A1 PCT/CN2013/079031 CN2013079031W WO2014019442A1 WO 2014019442 A1 WO2014019442 A1 WO 2014019442A1 CN 2013079031 W CN2013079031 W CN 2013079031W WO 2014019442 A1 WO2014019442 A1 WO 2014019442A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- aryl
- heteroaryl
- alkyl
- cycloalkyl
- Prior art date
Links
- 0 **(C=C1)C=Cc([o]2)c1c(*)c2S([n]1c(*)cc(CN(*)*)c1)(=O)=O Chemical compound **(C=C1)C=Cc([o]2)c1c(*)c2S([n]1c(*)cc(CN(*)*)c1)(=O)=O 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
Definitions
- the present invention relates to a novel class of benzofuran derivatives, a process for the preparation thereof, and a pharmaceutical composition containing the same, and as a therapeutic agent, particularly as a gastric acid secretion inhibitor and a potassium ion competitive acid blocker (P- Medium use of CABs).
- a novel class of benzofuran derivatives a process for the preparation thereof, and a pharmaceutical composition containing the same, and as a therapeutic agent, particularly as a gastric acid secretion inhibitor and a potassium ion competitive acid blocker (P- Medium use of CABs).
- Peptic ulcer is a common disease, and the incidence rate varies from time to time and from region to region. The usual incidence rate is about 10 to 20% of the total population.
- the usual incidence rate is about 10 to 20% of the total population.
- Proton Pump also known as gastric acid pump
- H + /K + -adenosine triphosphatase H + /K + -ATPase
- H + and K + exchange are performed by ATP degradation, and the ⁇ + is specifically pumped into the gastric cavity to form a strong acid state in the stomach.
- a proton pump is a heterodimer consisting of two subunits of alpha and beta across the membrane.
- the a subunit has 10 helical transmembrane segments ( ⁇ 1 ⁇ 10), which are mainly responsible for the catalytic activity of the enzyme and provide a ruthenium binding site, and also a cation binding site, also known as a catalytic subunit; functional expression of the enzyme
- a single transmembrane beta subunit is required to participate.
- PPIs are weak base and lipophilic compounds that can rapidly pass through the cell membrane of the stomach wall, accumulate in the strong acid secretion tubules, and convert to sulfenamide compounds under H + catalysis, and H + /K + -ATPase transmembrane region
- the thiol group on the cysteine residue is covalently bound to form a disulfide bond, which inactivates the proton pump, thereby inhibiting central or peripherally mediated gastric acid secretion.
- the first generation of PPIs significantly inhibited gastric acid secretion stimulated by basal, nocturnal gastric acid, pentagastrin, and test meals.
- the effect of time of administration on the efficacy of the drug slow acid onset at night, and instability under acidic conditions (often formulated into enteric preparations, this In a case where it takes several hours to show the effect), dependence on CYP450 enzyme (a large difference in blood concentration of PPIs between different patients, which may lead to a huge difference in acid suppression between different patients), etc. Effects and clinical applications.
- the new generation of PPIs has obvious advantages in the treatment of Gastroesophageal Reflux Disease (GERD) and other acid-related diseases.
- GSD Gastroesophageal Reflux Disease
- P-CABs potassium-competitive acid blockers
- H + /K + -ATPase activity by competitively binding H + , and its mechanism of action is significantly different.
- the above PPIs can therefore be referred to as acid pump blockers.
- P-CABs are lipophilic, weakly basic, have a high dissociation constant and are stable at low pH.
- P-CABs are immediately ionized, ionized form Inhibition of H + /K + -ATPase by ionic binding, preventing H + transport and acid secretion into the gastric cavity, without the activation of microcapsules and microtubules and acid concentrated in the cells of the stomach wall, can rapidly increase the pH value in the stomach , the enzyme activity is restored after dissociation. Humans and animals can absorb quickly after oral administration and reach the peak plasma concentration. Clinical and animal studies have also shown that P-CABs are more effective than PPIs or H2 blockers and have a higher pH-raising effect. Some of the P-CABs have entered the sputum and phase III clinical studies. P-CABs have the following potential advantages: rapid onset, maximum effect in 1 hour; blood concentration is linearly related to oral dose, suggesting that the drug can easily achieve the best acid suppression state.
- P-CABs potassium competing acid blockers
- the object of the present invention is to provide a compound represented by the formula (I), and tautomers, mesomers, racemates, enantiomers, diastereomers thereof, Mixture forms and pharmaceutically acceptable salts, as well as metabolites and metabolic precursors or prodrugs. as follows:
- R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R Substituted by a substituent of 6 or -C(0)OR 6 ;
- R 2 is selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or heteroaryl group is optionally further one Or a plurality selected from the group consisting of halogen, cyano, hydroxy, amino, alkyl, alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 Substituted by a substituent of -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R 6 or -C(0)OR 6 ;
- R 3 is selected from a hydrogen atom or an alkyl group
- R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane Base, alkoxy group, cycloalkyl group, heterocyclic group,
- the aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, alkyl, hydroxyalkyl, alkoxy, cycloalkyl ,heterocyclyl, aryl,heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R 6 or Substituted by a substituent of -C(0)OR 6 ;
- R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring
- the radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
- R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group
- the aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted with a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
- n 0, 1, or 2.
- a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer Or a mixture thereof, or a pharmaceutically acceptable salt thereof wherein R 2 is an alkyl group, preferably an alkyl group.
- a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 3 is a hydrogen atom.
- a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 5 is a hydrogen atom.
- a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof wherein R 1 is selected from aryl or heteroaryl, wherein the aryl or heteroaryl is optionally further further selected from one or more selected from halogen or - Substituted by a substituent of OR 6 , R 6 is an alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group.
- a compound of formula (I) or tautomerized thereof a form, a meso form, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is an aryl group, wherein the aryl group Optionally further substituted by one or more substituents selected from halogen or -OR 6 , R 6 is alkyl, preferably alkyl, said alkyl optionally further further selected from one or more selected from cycloalkyl Substituted by a substituent, the cycloalkyl group is preferably a C 3 -C 6 cycloalkyl group, more preferably a cyclopropyl group; the aryl group means a 6 to 14 membered all carbon monocyclic or thick having a conjugated ⁇ -electron system
- the polycyclic group is preferably a 6 to 10 membered aryl group, more preferably a
- a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof wherein R 1 is a heteroaryl group, said heteroaryl group means a heteroaromatic system comprising 1 to 4 hetero atoms, 5 to 14 ring atoms Wherein the hetero atom comprises oxygen, sulfur and nitrogen; preferably a heteroaromatic ring containing from 5 to 10 ring atoms, wherein from 1 to 4 heteroatoms selected from oxygen, sulfur or nitrogen; more preferably from 5 to 6 A ring atom, wherein the heteroaromatic ring contains from 1 to 4 heteroatoms selected from oxygen, sulfur or nitrogen, most preferably a pyridyl group.
- the present invention also relates to a compound of the formula ( ⁇ - ⁇ ) or a tautomer, enantiomer, diastereomer, mesogen, racemate, formula thereof, Or a pharmaceutically acceptable salt thereof:
- R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R Substituted by a substituent of 6 or -C(0)OR 6 ;
- R 2 is selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or heteroaryl group is optionally further one Or a plurality selected from the group consisting of halogen, cyano, hydroxy, amino, alkyl, alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 Substituted by a substituent of -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R 6 or -C(0)OR 6 ;
- R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane
- the radical, alkoxy, cycloalkyl, heterocyclyl, aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, Alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0) NR 7 R 8 , -S(0) m R 6 Substituted with a substituent of -C(0)R 6 -OC(0)R 6 or -C(0)OR 6 ;
- R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring
- the radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
- R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group
- the aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted by a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
- PG is an amino protecting group, preferably a tert-butoxycarbonyl group
- n 0, 1, or 2.
- a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein PG is t-butoxycarbonyl.
- a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof wherein R 1 is selected from aryl or heteroaryl, wherein the aryl or heteroaryl is optionally further further selected from one or more selected from halogen or - Substituted by a substituent of OR 6 , R 6 is an alkyl group, preferably a C alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group, preferably a cycloalkyl group C 3 to C 6 cycloalkyl, more preferably cyclopropyl.
- aryl group means a 6 to 14 membered all-carbon monocyclic or fused polycyclic group having a conjugated ⁇ -electron system
- R 1 is preferably a 6- to 10-membered aryl group, more preferably a phenyl group or a benzo group.
- Tetrahydrofuranyl most preferably phenyl; said heteroaryl refers to a heteroaromatic system containing from 1 to 4 heteroatoms, from 5 to 14 ring atoms, wherein the heteroatoms include oxygen, sulfur and nitrogen; R 1 is preferably a heteroaromatic ring comprising 5 to 10 ring atoms, wherein 1 to 4 hetero atoms selected from oxygen, sulfur or nitrogen; more preferably 5 to 6 ring atoms, wherein 1 to 4 are selected from oxygen
- the heteroaromatic ring of a hetero atom of sulfur or nitrogen is most preferably a pyridyl group.
- a compound of the formula ( ⁇ - ⁇ ) or a tautomer, a mesogen, a racemate, an enantiomer, a non-pair a conjugate, or a mixture thereof, or a pharmaceutically acceptable salt thereof wherein R 1 is selected from aryl, wherein the aryl group is optionally further substituted with one or more substituents selected from halogen or -OR 6 Substituting, R 6 is an alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group.
- a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof wherein R 1 is selected from heteroaryl.
- Another aspect of the invention relates to the preparation of a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer thereof, Or a mixture thereof, or a pharmaceutically acceptable salt thereof, the method comprising
- the compound of the formula (IA) is deprotected in a solvent under acidic conditions to give a compound of the formula (I);
- PG is an amino protecting group
- 1 ⁇ 1 5 is as defined in the formula (I), wherein R 3 is preferably a hydrogen atom ⁇
- R 3 is preferably a hydrogen atom ⁇
- Preparation of a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer, a mixture thereof or a mixture thereof A method of using a salt wherein PG is a tert-butoxycarbonyl group.
- the present invention also relates to a compound of the formula (IB) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer, or a mixture thereof , or a pharmaceutically acceptable salt thereof:
- R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R Substituted by a substituent of 6 or -C(0)OR 6 ;
- R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane
- the radical, alkoxy, cycloalkyl, heterocyclyl, aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, Alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 - Substituted by a substituent of C(0)R 6 -OC(0)R 6 or -C(0)OR 6 ;
- R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring
- the radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
- R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group
- the aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted with a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
- n 0, 1, or 2.
- a compound of the formula (IB) or a mutual mutation thereof a form, a meso form, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 4 is a hydrogen atom.
- a compound of the formula (IB) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof wherein R 1 is selected from aryl or heteroaryl, wherein the aryl or heteroaryl is optionally further further selected from one or more selected from halogen or - Substituted by a substituent of OR 6 , R 6 is an alkyl group, preferably a C alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group, preferably a cycloalkyl group C 3 to C 6 cycloalkyl, more preferably cyclopropyl.
- aryl group means a 6 to 14 membered all-carbon monocyclic or fused polycyclic group having a conjugated ⁇ -electron system
- R 1 is preferably a 6- to 10-membered aryl group, more preferably a phenyl group or a benzo group.
- Tetrahydrofuranyl most preferably phenyl; said heteroaryl refers to a heteroaromatic system containing from 1 to 4 heteroatoms, from 5 to 14 ring atoms, wherein the heteroatoms include oxygen, sulfur and nitrogen; R 1 is preferably a heteroaromatic ring comprising 5 to 10 ring atoms, wherein 1 to 4 hetero atoms selected from oxygen, sulfur or nitrogen; more preferably 5 to 6 ring atoms, wherein 1 to 4 are selected from oxygen
- the heteroaromatic ring of a hetero atom of sulfur or nitrogen is most preferably a pyridyl group.
- a compound of the formula ( ⁇ - ⁇ ) or a tautomer, a mesogen, a racemate, an enantiomer, a non-pair a conjugate, or a mixture thereof, or a pharmaceutically acceptable salt thereof wherein R 1 is selected from aryl, wherein the aryl group is optionally further substituted with one or more substituents selected from halogen or -OR 6 Substituting, R 6 is an alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group.
- Another aspect of the invention relates to the preparation of a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer thereof, Or a method of the mixture thereof, or a pharmaceutically acceptable salt thereof, the method comprising the steps of:
- ⁇ 11 5 is as defined in the compound of formula (I).
- a pharmaceutical composition comprising a therapeutically effective amount of a compound represented by the formula (I) or a tautomer thereof, a mesogen, a foreign body A rot, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, diluent or excipient.
- Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the preparation of a gastric acid secretion inhibitor.
- Another aspect of the invention relates to a method of inhibiting gastric acid secretion comprising administering to a patient in need of treatment a therapeutically effective amount of a compound of formula (I) or a tautomer, racemate, pair thereof a conjugate, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same.
- Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, as a gastric acid secretion inhibitor.
- Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for the preparation of an H + /K + -adenosine triphosphatase (H + /K + -ATPase) inhibitor.
- Another aspect of the invention relates to a method of inhibiting H + /K + -adenosine triphosphatase (H + /K + -ATPase), the method comprising administering to a patient in need of treatment a therapeutically effective amount of the formula (I) a compound, or a tautomer, a racemate, an enantiomer, a diastereomer thereof, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same, or a pharmaceutical composition thereof .
- H + /K + -adenosine triphosphatase H + /K + -ATPase
- Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, which is an H + /K + - adenosine triphosphatase (H + /K + -ATPase) inhibitor.
- Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the preparation of potassium ion competitive acid blockers (P-CABs).
- Another aspect of the invention relates to a method for competing acid to block potassium ions, which comprises administering to a patient in need of treatment a therapeutically effective amount of a compound of the formula (I) or a tautomer thereof, a foreign body A rot, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same.
- Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, as a potassium ion competitive acid blocker (P-CABs).
- P-CABs potassium ion competitive acid blocker
- the present invention also relates to a compound of the formula (I) or a tautomer, a racemate, an enantiomer, a diastereomer thereof, or a mixture thereof, or a pharmaceutically acceptable compound thereof A salt, or a pharmaceutical composition comprising the same, for the treatment or prevention of peptic ulcer, Zollinger-Ellison syndrome, gastritis, erosive esophagitis, reflux esophagitis, symptomatic gastroesophageal reflux disease ( Symptomatic GERD), Barrett's esophagitis, functional dyspepsia, Helicobacter pylori infection, gastric cancer, gastric MALT lymphoma, non-steroidal anti-inflammatory drugs (NSAIDs) caused by ulcers or post-operative stress Use in a drug having hyperacidity or ulceration; or in the preparation of a medicament for inhibiting upper gastrointestinal bleeding caused by peptic ulcer, acute stress ulcer, hemorrhagic gastritis or invasive stress.
- Peptic ulcers include, but are not limited to, gastric ulcers, duodenal ulcers or anastomotic ulcers; symptomatic gastroesophageal reflux disease (symptomatic GERD) includes, but is not limited to, non-erosive reflux disease or no esophagitis Gastroesophageal reflux disease.
- symptomatic GERD symptomatic gastroesophageal reflux disease
- Another aspect of the invention relates to the treatment or prevention of peptic ulcer, Zollinger-Ellison syndrome, gastritis, erosive esophagitis, reflux esophagitis, symptomatic gastroesophageal reflux disease (symptomatic GERD), Barrett's esophagitis, functional dyspepsia, Helicobacter pylori infection, gastric cancer, gastric MALT lymphoma, non-steroidal anti-inflammatory drugs (NSAIDs) caused by ulcers or post-operative stress-induced gastric acid a method of multiple or ulceration; or a method of inhibiting upper gastrointestinal bleeding caused by peptic ulcer, acute stress ulcer, hemorrhagic gastritis or invasive stress, the method comprising administering a therapeutically effective amount to a patient in need of treatment a compound represented by (I) or a tautomer, a racemate, an enantiomer, a diastereomer, a mixture thereof, a pharmaceutically acceptable salt thereof, or
- Peptic ulcers include, but are not limited to, gastric ulcer, duodenal ulcer or anastomotic ulcer; symptomatic gastroesophageal reflux disease (symptomatic GERD) These include, but are not limited to, non-erosive reflux disease or gastroesophageal reflux disease without esophagitis.
- symptomatic GERD symptomatic gastroesophageal reflux disease
- Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or A medicinal salt, or a pharmaceutical composition comprising the same, for treating or preventing peptic ulcer, Zollinger-Ellison syndrome, gastritis, erosive esophagitis, reflux esophagitis, symptomatic gastroesophageal Reflux disease (symptomatic GERD), Barrett esophagitis, functional dyspepsia, Helicobacter pylori infection, gastric cancer, gastric MALT lymphoma, non-steroidal anti-inflammatory drugs (NSAIDs) caused by ulcers or after surgery A drug that causes hyperacidity or ulceration caused by stress; or a drug that inhibits upper gastrointestinal bleeding caused by peptic ulcer, acute stress ulcer, hemorrhagic gastritis, or invasive stress.
- NSAIDs non-steroidal anti-inflammatory drugs
- Peptic ulcers include, but are not limited to, gastric ulcers, duodenal ulcers or anastomotic ulcers; symptomatic gastroesophageal reflux disease (symptomatic GERD) includes, but is not limited to, non-erosive reflux disease or no esophagitis Gastroesophageal reflux disease.
- symptomatic GERD symptomatic gastroesophageal reflux disease
- Alkyl means a saturated aliphatic hydrocarbon group including straight chain and branched chain groups of 1 to 20 carbon atoms. An alkyl group having 1 to 10 carbon atoms is preferred, and an alkyl group having 1 to 6 carbon atoms is more preferred.
- Non-limiting examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, sec-butyl, n-pentyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2 -methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1, 3-dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 2,3-dimethylbutyl, n-heptyl, 2-methylhexyl, 3-methylhexyl, 4-methylhexyl, 5-methylhexyl,
- lower alkyl groups having 1 to 6 carbon atoms More preferred are lower alkyl groups having 1 to 6 carbon atoms, and non-limiting examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t-butyl, sec-butyl Base, n-pentyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2-methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethyl Butyl, 2,2-dimethylbutyl, 1,3-dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl Base, 2,3-dimethylbutyl and the like.
- the alkyl group may be substituted or unsubstituted, and when substituted, the substituent may be substituted at any available point of attachment, preferably one or more of the following groups, independently selected from alkyl, alkenyl, Block, alkoxy, alkylthio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, Cycloalkoxy, heterocycloalkoxy, cycloalkylthio, heterocycloalkylthio, oxo, -OR-NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 , -OC(0)R 6 , -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
- Cycloalkyl means a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent comprising from 3 to 20 carbon atoms, preferably from 3 to 12 carbon atoms, more preferably the cycloalkyl ring comprises from 3 to 10 The carbon atom, most preferably the cycloalkyl ring contains from 3 to 6 carbon atoms.
- Non-limiting examples of monocyclic cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cyclohexadienyl, cycloheptyl, cycloheptatriene Alkenyl, cyclooctyl and the like.
- Polycyclic cycloalkyl groups include spiro, fused, and bridged cycloalkyl groups.
- Spirocycloalkyl means a polycyclic group of 5 to 20 members which shares a carbon atom (referred to as a spiro atom) between the monocyclic rings. These may contain one or more double bonds, but none of the rings are fully conjugated. ⁇ electronic system. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- the spirocycloalkyl group is classified into a monospirocycloalkyl group, a bispirocycloalkyl group or a polyspirocycloalkyl group, preferably a monospirocycloalkyl group and a bispirocycloalkyl group, depending on the number of common spiro atoms between the ring and the ring.
- fused cycloalkyl means 5 to 20 members, each ring of the system sharing an adjacent carbon atom of an all-carbon polycyclic group with other rings in the system, wherein one or more rings may contain one or more Two double bonds, but none of the rings have a fully conjugated ⁇ -electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- the bicyclic ring, the tricyclic ring, the tetracyclic ring or the polycyclic fused ring alkyl group may be classified according to the number of the constituent rings, and preferably a bicyclic ring or a tricyclic ring, more preferably.
- Non-limiting examples of fused cycloalkyl groups include
- Bridge cycloalkyl means 5 to 20 members, any two rings sharing two carbon-free all-carbon polycyclic groups, which may contain one or more double bonds, but none of the rings have a total The ⁇ electronic system of the yoke. It is preferably 6 to 14 members, more preferably 7 to 10 members. Depending on the number of constituent rings, it may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic bridged cycloalkyl group, preferably a bicyclic ring, a tricyclic ring or a tetracyclic ring, and more preferably a bicyclic ring or a tricyclic ring.
- bridged cycloalkyl groups include
- the cycloalkyl ring may be fused to an aryl, heteroaryl or heterocycloalkyl ring, wherein the ring to which the parent structure is attached is a cycloalkyl group, non-limiting examples include indanyl, tetrahydrogen Naphthyl, benzocycloheptyl and the like.
- the cycloalkyl group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, block, alkoxy, alkylthio Base, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, Heterocycloalkylthio, oxo, -OR 6 , -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC(0 R 6 , -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
- Heterocyclyl means a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent comprising from 3 to 20 ring atoms wherein one or more of the ring atoms are selected from nitrogen, oxygen or S(0) m ( Wherein m is a hetero atom of the integer 0 to 2), but does not include a ring moiety of -0-0-, -0-S- or -SS-, and the remaining ring atoms are carbon.
- It preferably comprises from 3 to 12 ring atoms, wherein from 1 to 4 are heteroatoms, more preferably the heterocycloalkyl ring contains from 3 to 10 ring atoms, more preferably the heterocycloalkyl ring contains from 5 to 6 ring atoms.
- monocyclic heterocycloalkyl groups include pyrrolidinyl, piperidinyl, piperazinyl, morpholinyl, thiomorpholinyl, homopiperazinyl, pyranyl, tetrahydrofuranyl and the like.
- Polycyclic heterocycloalkyl groups include spiro, fused, and bridged heterocyclic groups.
- spiroheterocyclyl means a polycyclic heterocyclic group of 5 to 20 members in which one atom (called a spiro atom) is shared between the monocyclic rings, wherein one or more ring atoms are selected from nitrogen, oxygen or S(0) m A hetero atom (where m is an integer from 0 to 2), and the remaining ring atoms are carbon. These may contain one or more double bonds, but none of the rings have a fully conjugated pi-electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- the spirocycloalkyl group is classified into a monospiroheterocyclic group, a dispiroheterocyclic group or a polyspirocyclic group according to the number of common spiro atoms between the rings, preferably a monospirocycloalkyl group and a bispirocycloalkyl group. More preferably, it is 4 yuan / 4 yuan, 4 yuan / 5 yuan, 4 yuan / 6 yuan, 5 yuan / 5 yuan or 5 yuan / 6 yuan monospirocycloalkyl.
- “Fused heterocyclic group” means 5 to 20 members, each ring in the system shares an adjacent pair of atomic polycyclic heterocyclic groups with other rings in the system, and one or more rings may contain one or more a bond, but none of the rings have a fully conjugated ⁇ -electron system in which one or more ring atoms are selected from nitrogen, oxygen or S(0) m (where m is an integer from 0 to 2), and the remaining ring atoms are carbon. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- fused heterocyclic groups include
- “Bridge heterocyclyl” refers to a polycyclic heterocyclic group of 5 to 14 members in which two rings share two atoms which are not directly bonded, and these may contain one or more double bonds, but none of the rings have a complete conjugation
- a ⁇ -electron system in which one or more ring atoms are selected from nitrogen, oxygen or S(0) m (where m is an integer from 0 to 2), and the remaining ring atoms are carbon. It is preferably 6 to 14 members, more preferably 7 to 10 members. 7 to 10 yuan.
- a bicyclic, tricyclic, tetracyclic or polycyclic bridged cycloalkyl group preferably a bicyclic ring, a tricyclic ring or a tetracyclic ring, and more preferably a bicyclic ring or a tricyclic ring.
- the heterocyclyl ring may be fused to an aryl, heteroaryl or cycloalkyl ring, wherein the ring to which the parent structure is attached is a heterocyclic group,
- the heterocyclic group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups, independently selected from the group consisting of alkyl, alkenyl, block, alkoxy, alkylthio Base, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, Heterocycloalkylthio, oxo, -OR 6 , -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 , -OC(0)R 6 , -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
- Aryl means a 6 to 14 membered all-carbon monocyclic or fused polycyclic ring (ie, a ring that shares a pair of adjacent carbon atoms) having a conjugated ⁇ -electron system, preferably 6 to 10 members, such as phenyl. And naphthyl.
- the aryl ring may be fused to a heteroaryl, heterocyclic or cycloalkyl ring, wherein the ring to which the parent structure is attached is an aryl ring,
- the aryl group may be substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups, independently selected from the group consisting of alkyl, alkenyl, block, alkoxy, alkylthio, alkane.
- Heteroaryl means a heteroaromatic system containing from 1 to 4 heteroatoms, 5 to 14 ring atoms, wherein the heteroatoms include oxygen, sulfur and nitrogen. It is preferably 5 to 10 yuan.
- the heteroaryl group is preferably a 5- or 6-membered compound such as a furyl group, a thienyl group, a pyridyl group, a pyrrolyl group, an N-alkylpyrrolyl group, a pyrimidinyl group, a pyrazinyl group, an imidazolyl group, a tetrazolyl group or the like.
- the heteroaryl ring may be fused to an aryl, heterocyclic or cycloalkyl ring, wherein the parent structure is attached
- the heteroaryl group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, block, alkoxy, alkylthio Base, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, Heterocycloalkylthio, -OR 6 , -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC(0)R 6 -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
- Alkoxy means -o-(indenyl) and -o-(unsubstituted cycloalkyl), wherein alkyl is as defined above. Non-limiting examples include methoxy, ethoxy, propoxy, butoxy, cyclopropoxy, cyclobutoxy, cyclopentyloxy, cyclohexyloxy and the like.
- the alkoxy group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups, independently selected from the group consisting of an alkyl group, an alkenyl group, a block group, an alkoxy group, and an alkane group.
- Haloalkyl means an alkyl group substituted by one or more halogens, wherein alkyl is as defined above.
- Haldroxy means an -OH group.
- Hydroalkyl means an alkyl group substituted by a hydroxy group, wherein the alkyl group is as defined above.
- Halogen means fluoro, chloro, bromo or iodo.
- Amino means -NH 2 .
- Neitro means -N0 2 .
- Benzyl refers to -CH 2 - phenyl.
- Carboxy means -C(0)OH.
- the "carboxylate group” means -C(0)0(alkyl) or (cycloalkyl), wherein the alkyl group and the cycloalkyl group are as defined above.
- amino protecting group is used to keep the amino group unchanged when the other parts of the molecule are reacted.
- the group to be removed protects the amino group.
- Non-limiting examples include formyl, alkylcarbonyl, alkoxycarbonyl, benzoyl, aralkylcarbonyl, aralkoxycarbonyl, trityl, phthaloyl, N,N-dimethyl Aminoaminomethylene, substituted silyl, and the like. These groups may be optionally substituted with from 1 to 3 substituents selected from halogen, alkoxy or nitro.
- the amino protecting group is preferably a tert-butoxycarbonyl group.
- heterocyclic group optionally substituted by an alkyl group means that an alkyl group may be, but not necessarily, present, including the case where the heterocyclic group is substituted by an alkyl group and the case where the heterocyclic group is not substituted by an alkyl group.
- Substituted means that one or more hydrogen atoms in the group, preferably up to 5, more preferably 1 to 3, hydrogen atoms are independently substituted with each other by a corresponding number of substituents. It goes without saying that the substituents are only in their possible chemical positions, and those skilled in the art will be able to determine (by experiment or theory) substitutions that may or may not be possible without undue effort. For example, an amino group or a hydroxyl group having a free hydrogen may be unstable when combined with a carbon atom having an unsaturated (e.g., olefinic) bond.
- “Pharmaceutical composition” means a mixture containing one or more of the compounds described herein, or a physiologically/pharmaceutically acceptable salt or prodrug thereof, and other chemical components, as well as other components such as physiological/pharmaceutically acceptable carriers. And excipients.
- the purpose of the pharmaceutical composition is to promote administration to an organism, to facilitate absorption of the active ingredient and to exert biological activity.
- the preparation method of the medicinal salt comprises the following steps:
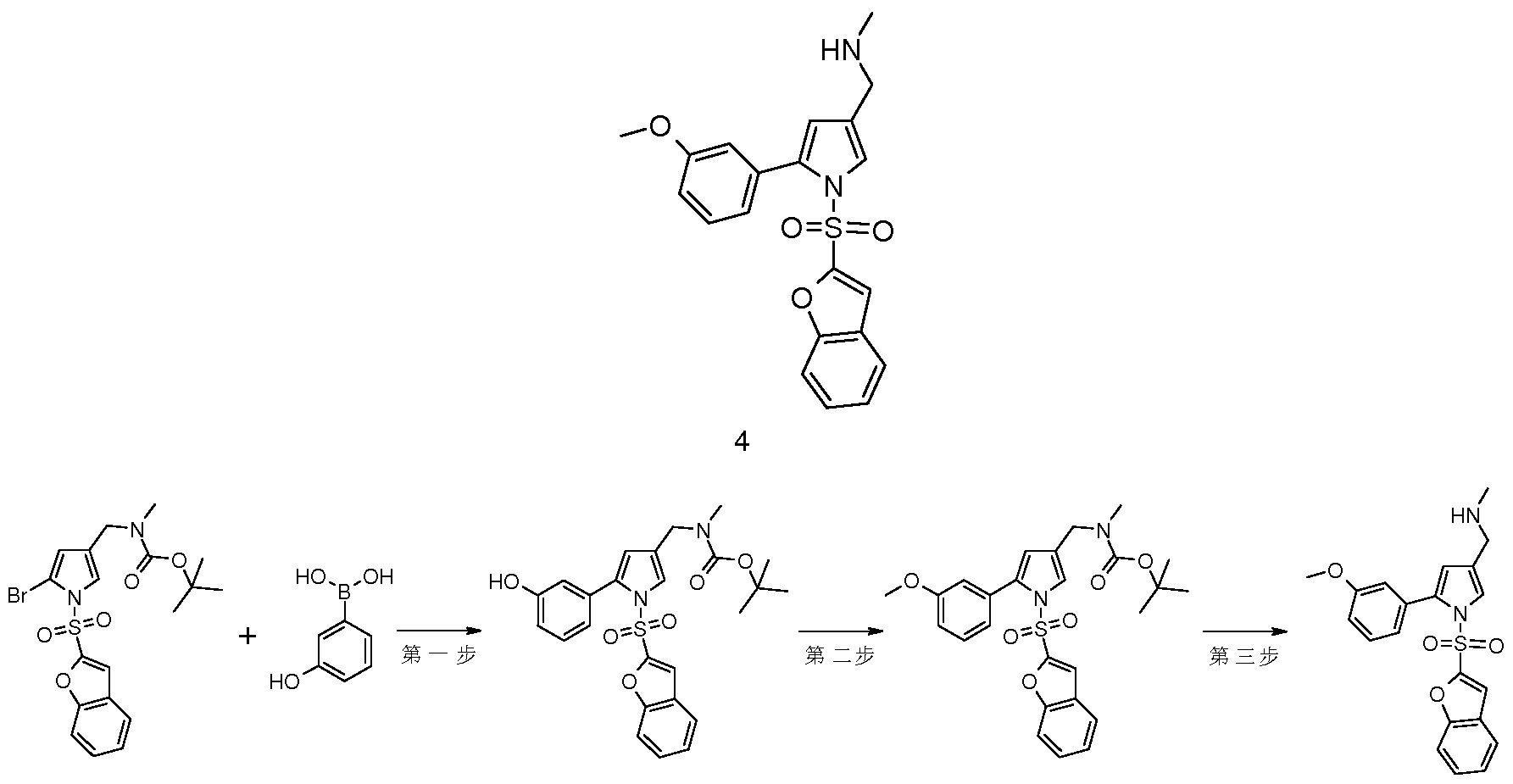
- the azole compound (a) and the benzofuransulfonyl chloride compound (b) are reacted in a solvent under basic conditions to obtain a benzofuransulfonyl-substituted pyrrole compound (C), a benzofuransulfonyl-substituted pyrrole compound ( C) and
- R 1 -substituted boronic acid ester or boric acid is subjected to catalytic catalysis in a solvent under basic conditions to obtain R benzofuransulfonyl-substituted pyrrole compound (IA), R benzofuransulfonyl-substituted pyrrole
- the compound (IA) is deprotected in a solvent under acidic conditions to give a compound of the formula (I).
- X is a halogen
- R 5 is as defined in the compound of formula (I) wherein R 3 is preferably a hydrogen atom
- PG is an amino protecting group, preferably a tert-butoxycarbonyl group.
- Agents that provide acidic conditions include, but are not limited to, trifluoroacetic acid, formic acid, acetic acid, hydrochloric acid, sulfuric acid, methanesulfonic acid.
- the alkaline condition reagent includes an organic base and an inorganic base
- the organic base includes, but not limited to, triethylamine, N,N-diisopropylethylamine, n-butyllithium, potassium t-butoxide
- Inorganic bases include, but are not limited to, sodium hydride, sodium carbonate, sodium bicarbonate, potassium carbonate, potassium bicarbonate or cesium carbonate.
- Catalysts include, but are not limited to, [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride, tetrakistriphenylphosphine palladium, palladium dichloride, palladium acetate or tris(dibenzylidene) Acetone) dipalladium.
- Solvents used include, but are not limited to, tetrahydrofuran, dichloromethane, 1,4-dioxane, water, methanol, ethanol, dimethyl sulfoxide or N,N-dimethylformamide.
- Option II tetrahydrofuran, dichloromethane, 1,4-dioxane, water, methanol, ethanol, dimethyl sulfoxide or N,N-dimethylformamide.
- the pyrrole formaldehyde compound (d) is reacted with a benzofuransulfonyl chloride compound (b) in a solvent under basic conditions to obtain a benzofuransulfonyl-substituted pyrrole formaldehyde compound (IB), a benzofuransulfonyl-substituted pyrrole formaldehyde.
- a reducing agent such as sodium borohydride
- the reagents providing basic conditions include organic bases including, but not limited to, triethylamine, N,N-diisopropylethylamine, n-butyllithium, potassium t-butoxide, and the like.
- organic bases including, but not limited to, triethylamine, N,N-diisopropylethylamine, n-butyllithium, potassium t-butoxide, and the like.
- the inorganic bases mentioned include, but are not limited to, sodium hydride, sodium carbonate, sodium hydrogencarbonate, potassium carbonate, potassium hydrogencarbonate or cesium carbonate.
- Reducing agents include, but are not limited to, sodium borohydride, sodium triacetoxyborohydride, sodium nitrile borohydride or lithium aluminum hydride.
- Solvents used include, but are not limited to, tetrahydrofuran, dichloromethane, 1,4-dioxane, water, methanol, ethanol, dimethyl sulfoxide or N,N-dimethylformamide. Detailed ways
- the structure of the compound is determined by nuclear magnetic resonance (NMR) or mass spectrometry (MS).
- NMR nuclear magnetic resonance
- MS mass spectrometry
- the NMR was measured by a Bruker AVANCE-400 nuclear magnetic apparatus, and the solvent was deuterated dimethyl sulfoxide ( ) ⁇ - ⁇ 3 ⁇ 4), deuterated chloroform (CDC1 3 ) deuterated methanol (CD 3 OD), and the internal standard was tetramethyl.
- silane CTMS chemical shifts are given 10- 6 Cppm) as a unit.
- the MS was measured using a FINMGAN LCQAd (ESI) mass spectrometer (manufacturer: Thermo, model: Finnigan LCQ advantage MAX).
- the HPLC was measured using an Agilent 1200 DAD high pressure liquid chromatograph (Sunfire C18 150 x 4.6 mm column) and a Waters 2695-2996 high pressure liquid chromatograph (Gimini C18 150 x 4.6 mm column).
- the average inhibition rate of the kinase and the IC 5Q value were determined using a NovoStar plate reader (BMG, Germany).
- the thin layer chromatography silica gel plate uses Yantai Yellow Sea HSGF254 or Qingdao GF254 silica gel plate.
- the silica gel plate used for thin layer chromatography (TLC) has a specification of 0.15 mm ⁇ 0.2 mm, and the thin layer chromatography separation and purification product adopts the specification of 0.4 mm. ⁇ 0.5 mm silica gel plate.
- the known starting materials of the present invention may be synthesized by or according to methods known in the art, or may be purchased from ABCR GmbH & Co. KG, Acros Organnics, Aldrich Chemical Company, Accela ChemBio Inc. Companies such as Dare Chemicals.
- An argon atmosphere or a nitrogen atmosphere means that the reaction flask is connected to an argon or nitrogen balloon having a volume of about 1 L.
- the solution in the reaction means an aqueous solution unless otherwise specified.
- the temperature of the reaction was room temperature unless otherwise specified.
- Room temperature is the optimum reaction temperature, and the temperature range is from 20 ° C to 30 ° C.
- the progress of the reaction in the examples was monitored by thin layer chromatography (TLC).
- TLC thin layer chromatography
- the system used for the reaction was: A: dichloromethane and methanol system, B: n-hexane and ethyl acetate system, C: petroleum ether And the ethyl acetate system, D: acetone, the volume ratio of the solvent is adjusted depending on the polarity of the compound.
- the system of the eluent for column chromatography and the system for developing the thin layer chromatography of the purified compound include: A: dichloromethane and methanol system, B: n-hexane and ethyl acetate system, C: n-hexane and acetone System, D: hexamethylene, E: ethyl acetate, the volume ratio of the solvent is adjusted depending on the polarity of the compound, and may be adjusted by adding a small amount of triethylamine and an acidic or alkaline reagent.
- A dichloromethane and methanol system
- B n-hexane and ethyl acetate system
- C n-hexane and acetone System
- D hexamethylene
- E ethyl acetate
- the volume ratio of the solvent is adjusted depending on the polarity of the compound, and may be adjusted by adding a small amount of triethylamine and an acidic
- reaction mixture was quenched by the addition of 2 mL of a saturated aqueous solution of ammonium chloride, and 50 mL of ethyl acetate was added, and then washed with saturated sodium chloride solution (10 mL ⁇ 2), dried over anhydrous sodium sulfate, filtered, and concentrated.
- the resulting residue was purified by chromatography eluting to afford titled product ((1-(benzofuran-2-ylsulfonyl)-5-(3-methoxyphenyl)-1H-pyrrole-3- Tert-butyl)methyl (meth)carbamate 4c (37 mg, pale yellow solid), yield: 44.0%.
- 1-(benzofuran-2-yl) Sulfonyl)-5-(pyridin-3-yl)-1H-pyrrol-3-yl)methylXmethyl)carbamic acid tert-butyl ester 9b 43 mg, 0.09 mmol
- V/V 4:1
- the compound is formulated in 100% DMSO to a suitable concentration: 10000, 1000, 100, 10, 1, O.lnM;
- buffer 1 50mmol / L HEPEs-Tris, 5mmol / L magnesium chloride, pH 6.5;
- ATP Dilute ATP to 2 mM with buffer 1;
- malachite green solution 0.12% malachite green dissolved in 2.5 moles of sulfuric acid, 7.5% ammonium molybdate and 11% Tween 20 when used in a ratio of 100:25:2;
- pig gastric mucosa microsomes (rich in H + /K + -ATPase), the extraction method is sucrose gradient centrifugation: the pig stomach is washed with tap water, immersed in 3mol / L concentrated brine for 1-2 minutes, and then wiped dry. The gastric mucosa was separated, mashed, and then suspended in 0.25 mol/L sucrose, 1 mmol/LEDTA, 10 mmol/L tris-HCl solution; homogenized, (100 g: 330 ml, fully uniform and then added 300 ml) The slurry is centrifuged at 20000G for 30 minutes.
- the IC 5Q value of the compound can be calculated from the inhibition rates at different concentrations.
- the compounds of the present invention have significant inhibitory activity against H + /K + -ATPase.
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The present invention relates to benzofuran derivatives, the preparation method and medical use thereof. Specifically, the present invention relates to novel benzofuran derivatives represented by formula (I), the preparation method thereof, the pharmaceutical composition containing said derivative, the use of said derivative as a therapeutic agent especially as gastric acid secretion inhibitors and potassium-competitive acid blockers (P-CABs), wherein the respective substituent of formula (I) is defined as those in the specification.
Description
苯并呋喃类衍生物、 其制备方法及其在医药上的应用 技术领域 Benzofuran derivative, preparation method thereof and application thereof in medicine
本发明涉及一类新的苯并呋喃类衍生物、 其制备方法及含有该衍生物的药物 组合物以及其作为治疗剂特别是作为胃酸分泌抑制剂和钾离子竞争性酸阻滞剂 (P-CABs)的中用途。 背景技术 The present invention relates to a novel class of benzofuran derivatives, a process for the preparation thereof, and a pharmaceutical composition containing the same, and as a therapeutic agent, particularly as a gastric acid secretion inhibitor and a potassium ion competitive acid blocker (P- Medium use of CABs). Background technique
消化性溃疡是一种常见病, 不同时期、 不同地区的发病率会有所不同, 通常发 病率约占人口总数的 10〜20%。 随着社会发展, 人们生活方式的变化, 因吸烟、 饮 酒、 情绪紧张及药物剌激等引起的消化性溃疡发病率正逐渐增高, 正在严重影响 人们的工作和生活。 现在医学界对其确切的发病机制还不清楚, 但是抑制胃酸分 泌己成为治疗此类疾病公认的首选方法。 Peptic ulcer is a common disease, and the incidence rate varies from time to time and from region to region. The usual incidence rate is about 10 to 20% of the total population. With the development of society, changes in people's lifestyles, the incidence of peptic ulcers caused by smoking, drinking, emotional stress and drug stimulation are gradually increasing, which is seriously affecting people's work and life. It is still unclear about the exact pathogenesis of the medical community, but inhibition of gastric acid secretion has become the accepted method of choice for the treatment of such diseases.
自 1988年第一个质子泵抑制剂 (Proton Pump Inhibitors, PPIs)上市以来, 至今 全球已有数个 PPIs产品上市。 经过多年的临床应用, PPIs已经成为治疗胃酸相关 性疾病的首选药物。 质子泵 (Proton Pump)又称胃酸泵, 其实质为 H+/K+-腺苷三磷 酸酶(H+/K+-ATPase), 是胃分泌 H+的最终共同途径, 它存在于胃壁细胞分泌小管 的细胞膜上, 借助 ATP降解供能进行 H+、 K+交换, 特异性地将 Η+泵入胃腔, 形 成胃内强酸状态。 质子泵是一种异质二聚体, 由跨膜的 α和 β两个亚单位组成。 a 亚基有 10个螺旋跨膜片段 (Μ1〜Μ10), 主要负责酶的催化活性及提供 ΑΤΡ结合位 点, 同时也是阳离子的结合位点, 亦称为催化亚基; 酶的功能性表达则需要单次 跨膜的 β亚基参与。 PPIs均为弱碱、 亲脂性化合物, 能迅速穿过胃壁细胞膜, 聚 集在强酸性分泌小管中, 在 H+催化作用下转化为次磺酰胺类化合物, 与 H+/K+-ATPase跨膜区半胱氨酸残基上的巯基共价结合形成二硫键, 使质子泵失活, 从而抑制中枢或外周介导的胃酸分泌。 Since the first Proton Pump Inhibitors (PPIs) were launched in 1988, several PPIs have been on the market worldwide. After years of clinical application, PPIs have become the drug of choice for the treatment of gastric acid-related diseases. Proton Pump, also known as gastric acid pump, is essentially H + /K + -adenosine triphosphatase (H + /K + -ATPase), which is the ultimate common pathway for gastric secretion of H + , which is present in gastric parietal cells. On the cell membrane of the secretory tubule, H + and K + exchange are performed by ATP degradation, and the Η + is specifically pumped into the gastric cavity to form a strong acid state in the stomach. A proton pump is a heterodimer consisting of two subunits of alpha and beta across the membrane. The a subunit has 10 helical transmembrane segments (Μ1~Μ10), which are mainly responsible for the catalytic activity of the enzyme and provide a ruthenium binding site, and also a cation binding site, also known as a catalytic subunit; functional expression of the enzyme A single transmembrane beta subunit is required to participate. PPIs are weak base and lipophilic compounds that can rapidly pass through the cell membrane of the stomach wall, accumulate in the strong acid secretion tubules, and convert to sulfenamide compounds under H + catalysis, and H + /K + -ATPase transmembrane region The thiol group on the cysteine residue is covalently bound to form a disulfide bond, which inactivates the proton pump, thereby inhibiting central or peripherally mediated gastric acid secretion.
第一代 PPIs对基础、 夜间胃酸和五肽胃泌素、 试餐等刺激的胃酸分泌有明显 的抑制作用。 但因在药动学及药效学方面的局限性, 包括生物利用度、 给药时间 对药效的影响、 夜间酸突破起效慢、 酸性条件下不稳定 (经常需配制成肠制剂, 这 种情况下需要数小时才能表现出效果)、 对 CYP450 酶的依赖性 (不同患者之间的 PPIs血药浓度存在巨大差异, 可能导致不同患者间抑酸效果的巨大差异)等因素, 影响了治疗效果与临床应用。 与第一代 PPIs相比, 新一代 PPIs在治疗胃食管返流 病 (Gastroesophageal Reflux Disease, GERD)及其他酸相关性疾病时具有明显优势。 The first generation of PPIs significantly inhibited gastric acid secretion stimulated by basal, nocturnal gastric acid, pentagastrin, and test meals. However, due to limitations in pharmacokinetics and pharmacodynamics, including bioavailability, the effect of time of administration on the efficacy of the drug, slow acid onset at night, and instability under acidic conditions (often formulated into enteric preparations, this In a case where it takes several hours to show the effect), dependence on CYP450 enzyme (a large difference in blood concentration of PPIs between different patients, which may lead to a huge difference in acid suppression between different patients), etc. Effects and clinical applications. Compared with the first generation of PPIs, the new generation of PPIs has obvious advantages in the treatment of Gastroesophageal Reflux Disease (GERD) and other acid-related diseases.
钾竞争性酸阻滞齐 UCPotassium-Competitive Acid Blockers, P-CABs) 作为一类新 型抑酸剂, 通过竞争性地结合 H+而抑制 H+/K+-ATPase的活性, 其作用机制明显不 同于上述 PPIs, 因此可称为酸泵阻滞剂。 P-CABs具有亲脂性、 弱碱性、 解离常数 高和在低 pH值时稳定的特点。 在酸性环境下, P-CABs立刻离子化, 离子化形式
通过离子型结合抑制 H+/K+-ATPase, 阻止 H+运送以及酸分泌到胃腔中, 不需要集 中于胃壁细胞的微囊和微管及酸的激活, 能迅速升高胃内 pH值, 离解后酶活性恢 复。 人和动物口服后能吸收迅速, 达到血浆浓度的峰值。 临床和动物实验也表明, P-CABs比 PPIs或 H2受体阻滞剂起效更快,升高 pH的作用更强,其中部分 P-CABs 制剂已进入 Π期和 III期临床研究。 P-CABs具备以下潜在优势: 起效迅速, 在 1 小时内就能达到最大效果; 血药浓度与口服给药剂量线性相关, 提示该类药物可 以比较容易地达到最佳抑酸状态。 As a new class of antacids, potassium-competitive acid blockers, P-CABs, inhibit H + /K + -ATPase activity by competitively binding H + , and its mechanism of action is significantly different. The above PPIs can therefore be referred to as acid pump blockers. P-CABs are lipophilic, weakly basic, have a high dissociation constant and are stable at low pH. In an acidic environment, P-CABs are immediately ionized, ionized form Inhibition of H + /K + -ATPase by ionic binding, preventing H + transport and acid secretion into the gastric cavity, without the activation of microcapsules and microtubules and acid concentrated in the cells of the stomach wall, can rapidly increase the pH value in the stomach , the enzyme activity is restored after dissociation. Humans and animals can absorb quickly after oral administration and reach the peak plasma concentration. Clinical and animal studies have also shown that P-CABs are more effective than PPIs or H2 blockers and have a higher pH-raising effect. Some of the P-CABs have entered the sputum and phase III clinical studies. P-CABs have the following potential advantages: rapid onset, maximum effect in 1 hour; blood concentration is linearly related to oral dose, suggesting that the drug can easily achieve the best acid suppression state.
目前公开了一系列的钾竞争性酸阻滞剂 (P-CABs)的专利申请, 其中包括 WO200504196K WO2006134460 WO2009041447或 WO2010021149等。 A series of patent applications for potassium competing acid blockers (P-CABs) are disclosed, including WO200504196K WO2006134460 WO2009041447 or WO2010021149.
尽管目前已公开了一系列的钾竞争性酸阻滞剂 (P-CABs)抑制剂, 但仍需要开 发新的具有更好的药效的化合物, 经过不断努力, 本发明设计具有通式(I )所示的 结构的化合物, 并发现具有此类结构的化合物表现出优异的效果和作用。 发明内容 Although a series of potassium competitive acid blocker (P-CABs) inhibitors have been disclosed, there is still a need to develop new compounds with better pharmacodynamics, and the design of the present invention has a general formula (I) The compound of the structure shown, and the compound having such a structure was found to exhibit excellent effects and effects. Summary of the invention
本发明的目的在于提供一种通式( I )所示的化合物, 以及它们的互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 混合物形式和可药用的盐, 以及代谢产物和代谢前体或前药。 如下: The object of the present invention is to provide a compound represented by the formula (I), and tautomers, mesomers, racemates, enantiomers, diastereomers thereof, Mixture forms and pharmaceutically acceptable salts, as well as metabolites and metabolic precursors or prodrugs. as follows:
其巾: Its towel:
R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被一个或多个选自 卤素、 氰基、 烷基、 卤代烷基、 羟烷基、 环烷基、 -OR6、 杂环基、 芳基、 杂芳基、 -NR7R8、 -C(0)NR7R8 -S(0)mR6 -C(0)R6 -OC(0)R6或 -C(0)OR6的取代基所取代;R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R Substituted by a substituent of 6 or -C(0)OR 6 ;
R2选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述烷基、 环 烷基、 杂环基、 芳基或杂芳基任选进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0)NR7R8 -S(0)mR6 -C(0)R6 -OC(0)R6或 -C(0)OR6的取代基所取代; R 2 is selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or heteroaryl group is optionally further one Or a plurality selected from the group consisting of halogen, cyano, hydroxy, amino, alkyl, alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 Substituted by a substituent of -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R 6 or -C(0)OR 6 ;
R3选自氢原子或烷基; R 3 is selected from a hydrogen atom or an alkyl group;
R4或 R5各自独立地选自氢原子、 卤素、 氰基、 硝基、 羟基、 烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、
芳基或杂芳基各自独立地任选进一步被一个或多个选自卤素、 硝基、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0)NR7R8 -S(0)mR6 -C(0)R6 -OC(0)R6或 -C(0)OR6的取代基所取代; R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane Base, alkoxy group, cycloalkyl group, heterocyclic group, The aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, alkyl, hydroxyalkyl, alkoxy, cycloalkyl ,heterocyclyl, aryl,heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R 6 or Substituted by a substituent of -C(0)OR 6 ;
R6选自氢原子、 烷基、 羟基、 卤素、 烷氧基、 环烷基、 杂环基、 芳基或杂芳 基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选 进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所 取代; R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring The radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
R7或 R8各自独立地选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其 中所述的烷基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或 多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所取代; 且 R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group The aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted with a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
m为 0、 1或 2。 在本发明的一个具体实施方案中, 一种通式( I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 其中 R2为烷基, 优选为 烷基。 在本发明的另一个具体实施方案中, 一种通式(I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 其中 R3为氢原子。 在本发明的另一个具体实施方案中,一种通式(1 ))所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 其中 R4为氢原子。 在本发明的另一个具体实施方案中, 一种通式(I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 其中 R5为氢原子。 在本发明的另一个具体实施方案中, 一种通式(I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 其中 R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被一 个或多个选自卤素或 -OR6的取代基所取代, R6为烷基, 所述的烷基任选进一步被 一个或多个选自环烷基的取代基所取代。 在本发明的另一个具体实施方案中, 一种通式( I )所示的化合物或其互变异构
体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 其中 R1为芳基, 其中所述芳基任选进一步被一个或多个选自卤素或 -OR6的取代基所取代, R6为烷基, 优选 烷基, 所述的烷基任选进一步被一 个或多个选自环烷基的取代基所取代, 所述环烷基优选 C3〜C6环烷基, 更优选环 丙基;所述的芳基指具有共轭的 π电子体系的 6至 14元全碳单环或稠合多环基团, 优选为 6至 10元芳基, 更优选苯基或苯并四氢呋喃基, 最优选为苯基。 在本发明的另一个具体实施方案中, 一种通式( I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 其中 R1为杂芳基, 所述的杂芳基指包含 1至 4个杂原子, 5至 14个 环原子的杂芳族体系, 其中杂原子包括氧、硫和氮; 优选为包含 5至 10个环原子, 其中含 1至 4个选自氧、 硫或氮的杂原子的杂芳族环; 更优选为包含 5至 6个环 原子, 其中含 1至 4个选自氧、 硫或氮的杂原子的杂芳族环, 最优选为吡啶基。 m is 0, 1, or 2. In a particular embodiment of the invention, a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer Or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 2 is an alkyl group, preferably an alkyl group. In another embodiment of the present invention, a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 3 is a hydrogen atom. In another embodiment of the present invention, a compound represented by the formula (1)) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer Isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 4 is a hydrogen atom. In another embodiment of the present invention, a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 5 is a hydrogen atom. In another embodiment of the present invention, a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is selected from aryl or heteroaryl, wherein the aryl or heteroaryl is optionally further further selected from one or more selected from halogen or - Substituted by a substituent of OR 6 , R 6 is an alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group. In another embodiment of the invention, a compound of formula (I) or tautomerized thereof a form, a meso form, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is an aryl group, wherein the aryl group Optionally further substituted by one or more substituents selected from halogen or -OR 6 , R 6 is alkyl, preferably alkyl, said alkyl optionally further further selected from one or more selected from cycloalkyl Substituted by a substituent, the cycloalkyl group is preferably a C 3 -C 6 cycloalkyl group, more preferably a cyclopropyl group; the aryl group means a 6 to 14 membered all carbon monocyclic or thick having a conjugated π-electron system The polycyclic group is preferably a 6 to 10 membered aryl group, more preferably a phenyl group or a benzotetrahydrofuranyl group, and most preferably a phenyl group. In another embodiment of the present invention, a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is a heteroaryl group, said heteroaryl group means a heteroaromatic system comprising 1 to 4 hetero atoms, 5 to 14 ring atoms Wherein the hetero atom comprises oxygen, sulfur and nitrogen; preferably a heteroaromatic ring containing from 5 to 10 ring atoms, wherein from 1 to 4 heteroatoms selected from oxygen, sulfur or nitrogen; more preferably from 5 to 6 A ring atom, wherein the heteroaromatic ring contains from 1 to 4 heteroatoms selected from oxygen, sulfur or nitrogen, most preferably a pyridyl group.
物形式、 或其可药用的盐。 本发明还涉及一种通式(Ι-Α)所示的化合物或其互变异构体、 对映异构体、 非 对映异构体、 内消旋体、 外消旋体、 式, 或其可药用的盐: Form, or a pharmaceutically acceptable salt thereof. The present invention also relates to a compound of the formula (Ι-Α) or a tautomer, enantiomer, diastereomer, mesogen, racemate, formula thereof, Or a pharmaceutically acceptable salt thereof:
( Ι-Α
可作为合成通式(I )所示的化合物的中间体, 其中: ( Ι-Α It can be used as an intermediate for synthesizing a compound represented by the general formula (I), wherein:
R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被一个或多个选自 卤素、 氰基、 烷基、 卤代烷基、 羟烷基、 环烷基、 -OR6、 杂环基、 芳基、 杂芳基、 -NR7R8、 -C(0)NR7R8 -S(0)mR6 -C(0)R6 -OC(0)R6或 -C(0)OR6的取代基所取代;R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R Substituted by a substituent of 6 or -C(0)OR 6 ;
R2选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述烷基、 环 烷基、 杂环基、 芳基或杂芳基任选进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0)NR7R8 -S(0)mR6 -C(0)R6 -OC(0)R6或 -C(0)OR6的取代基所取代; R 2 is selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or heteroaryl group is optionally further one Or a plurality selected from the group consisting of halogen, cyano, hydroxy, amino, alkyl, alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 Substituted by a substituent of -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R 6 or -C(0)OR 6 ;
R4或 R5各自独立地选自氢原子、 卤素、 氰基、 硝基、 羟基、 烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或多个选自卤素、 硝基、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0) NR7R8、 -S(0)mR6 -C(0)R6 -OC(0)R6或 -C(0)OR6的取代基所取代; R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane The radical, alkoxy, cycloalkyl, heterocyclyl, aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, Alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0) NR 7 R 8 , -S(0) m R 6 Substituted with a substituent of -C(0)R 6 -OC(0)R 6 or -C(0)OR 6 ;
R6选自氢原子、 烷基、 羟基、 卤素、 烷氧基、 环烷基、 杂环基、 芳基或杂芳 基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选 进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所 取代; R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring The radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
R7或 R8各自独立地选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其 中所述的烷基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或 多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所取代; R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group The aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted by a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
PG为氨基保护基, 优选叔丁氧羰基; 且 PG is an amino protecting group, preferably a tert-butoxycarbonyl group;
m为 0、 1或 2。 在本发明的另一个具体实施方案中, 一种通式(I-A )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 PG为叔丁氧羰基。 在本发明的一个具体实施方案中, 一种通式(I-A )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 其中 R2为烷基, 优选 烷基。 在本发明的另一个具体实施方案中, 一种通式(I-A )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R4为氢原子。
在本发明的另一个具体实施方案中, 一种通式(I-A )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R5为氢原子。 在本发明的另一个具体实施方案中, 一种通式(I-A )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被 一个或多个选自卤素或 -OR6的取代基所取代, R6为烷基, 优选 C^ 烷基, 所述 的烷基任选进一步被一个或多个选自环烷基的取代基所取代, 所述环烷基优选 C3〜C6环烷基, 更优选环丙基。 进一步, 所述的芳基指具有共轭的 π电子体系的 6 至 14元全碳单环或稠合多环基团, R1优选为 6至 10元芳基, 更优选苯基或苯并 四氢呋喃基, 最优选为苯基; 所述的杂芳基指包含 1至 4个杂原子, 5至 14个环 原子的杂芳族体系,其中杂原子包括氧、硫和氮; R1优选为包含 5至 10个环原子, 其中含 1至 4个选自氧、 硫或氮的杂原子的杂芳族环; 更优选为包含 5至 6个环 原子, 其中含 1至 4个选自氧、 硫或氮的杂原子的杂芳族环, 最优选为吡啶基。 在本发明的另一个具体实施方案中, 一种通式(Ι-Α )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R1选自芳基, 其中所述芳基任选进一步被一个或多个选自卤 素或 -OR6的取代基所取代, R6为烷基, 所述的烷基任选进一步被一个或多个选自 环烷基的取代基所取代。 在本发明的另一个具体实施方案中, 一种通式(I-A )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R1选自杂芳基。 本发明的另一方面涉及一种制备通式(I )所示的化合物或其互变异构体、 内消 旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药用的 盐的方法, 该方法包括以 m is 0, 1, or 2. In another embodiment of the present invention, a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein PG is t-butoxycarbonyl. In a particular embodiment of the invention, a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer Or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 2 is an alkyl group, preferably an alkyl group. In another embodiment of the present invention, a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 4 is a hydrogen atom. In another embodiment of the present invention, a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 5 is a hydrogen atom. In another embodiment of the present invention, a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is selected from aryl or heteroaryl, wherein the aryl or heteroaryl is optionally further further selected from one or more selected from halogen or - Substituted by a substituent of OR 6 , R 6 is an alkyl group, preferably a C alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group, preferably a cycloalkyl group C 3 to C 6 cycloalkyl, more preferably cyclopropyl. Further, the aryl group means a 6 to 14 membered all-carbon monocyclic or fused polycyclic group having a conjugated π-electron system, and R 1 is preferably a 6- to 10-membered aryl group, more preferably a phenyl group or a benzo group. Tetrahydrofuranyl, most preferably phenyl; said heteroaryl refers to a heteroaromatic system containing from 1 to 4 heteroatoms, from 5 to 14 ring atoms, wherein the heteroatoms include oxygen, sulfur and nitrogen; R 1 is preferably a heteroaromatic ring comprising 5 to 10 ring atoms, wherein 1 to 4 hetero atoms selected from oxygen, sulfur or nitrogen; more preferably 5 to 6 ring atoms, wherein 1 to 4 are selected from oxygen The heteroaromatic ring of a hetero atom of sulfur or nitrogen is most preferably a pyridyl group. In another embodiment of the present invention, a compound of the formula (Ι-Α) or a tautomer, a mesogen, a racemate, an enantiomer, a non-pair a conjugate, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is selected from aryl, wherein the aryl group is optionally further substituted with one or more substituents selected from halogen or -OR 6 Substituting, R 6 is an alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group. In another embodiment of the present invention, a compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is selected from heteroaryl. Another aspect of the invention relates to the preparation of a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer thereof, Or a mixture thereof, or a pharmaceutically acceptable salt thereof, the method comprising
( I-A ) ( ! ) ( I-A ) ( ! )
通式 (I-A)化合物在溶剂中, 酸性条件下脱保护得到通式( I )化合物;
其中: PG为氨基保护基; 1^〜1 5的定义如通式(I )中所述, 其中 R3优选为氢 原子 < 在本发明的另一个具体实施方案中, 一种如上所述的制备通式(I )所示的化合 物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混 合物形式、 或其可药用的盐的方法, 其中 PG为叔丁氧羰基。 本发明还涉及一种通式(I-B )所示的化合物或其互变异构体、 内消旋体、 外消 旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐: The compound of the formula (IA) is deprotected in a solvent under acidic conditions to give a compound of the formula (I); Wherein: PG is an amino protecting group; 1^~1 5 is as defined in the formula (I), wherein R 3 is preferably a hydrogen atom < In another embodiment of the invention, one is as described above Preparation of a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer, a mixture thereof or a mixture thereof A method of using a salt wherein PG is a tert-butoxycarbonyl group. The present invention also relates to a compound of the formula (IB) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer, or a mixture thereof , or a pharmaceutically acceptable salt thereof:
可作为合成通式(I )所示的化合物的中间体, 其中: It can be used as an intermediate for synthesizing a compound represented by the general formula (I), wherein:
R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被一个或多个选自 卤素、 氰基、 烷基、 卤代烷基、 羟烷基、 环烷基、 -OR6、 杂环基、 芳基、 杂芳基、 -NR7R8、 -C(0)NR7R8 -S(0)mR6 -C(0)R6 -OC(0)R6或 -C(0)OR6的取代基所取代;R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 -OC(0)R Substituted by a substituent of 6 or -C(0)OR 6 ;
R4或 R5各自独立地选自氢原子、 卤素、 氰基、 硝基、 羟基、 烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或多个选自卤素、 硝基、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0)NR7R8 -S(0)mR6 -C(0)R6 -OC(0)R6或 -C(0)OR6的取代基所取代; R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane The radical, alkoxy, cycloalkyl, heterocyclyl, aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, Alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 - Substituted by a substituent of C(0)R 6 -OC(0)R 6 or -C(0)OR 6 ;
R6选自氢原子、 烷基、 羟基、 卤素、 烷氧基、 环烷基、 杂环基、 芳基或杂芳 基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选 进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所 取代; R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring The radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
R7或 R8各自独立地选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其 中所述的烷基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或 多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所取代; 且 R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group The aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted with a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
m为 0、 1或 2。 在本发明的另一个具体实施方案中, 一种通式(I-B )所示的化合物或其互变异
构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R4为氢原子。 在本发明的另一个具体实施方案中, 一种通式(I-B )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R5为氢原子。 在本发明的另一个具体实施方案中, 一种通式(I-B )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被 一个或多个选自卤素或 -OR6的取代基所取代, R6为烷基, 优选 C^ 烷基, 所述 的烷基任选进一步被一个或多个选自环烷基的取代基所取代, 所述环烷基优选 C3〜C6环烷基, 更优选环丙基。 进一步, 所述的芳基指具有共轭的 π电子体系的 6 至 14元全碳单环或稠合多环基团, R1优选为 6至 10元芳基, 更优选苯基或苯并 四氢呋喃基, 最优选为苯基; 所述的杂芳基指包含 1至 4个杂原子, 5至 14个环 原子的杂芳族体系,其中杂原子包括氧、硫和氮; R1优选为包含 5至 10个环原子, 其中含 1至 4个选自氧、 硫或氮的杂原子的杂芳族环; 更优选为包含 5至 6个环 原子, 其中含 1至 4个选自氧、 硫或氮的杂原子的杂芳族环, 最优选为吡啶基。 在本发明的另一个具体实施方案中, 一种通式(Ι-Β )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R1选自芳基, 其中所述芳基任选进一步被一个或多个选自卤 素或 -OR6的取代基所取代, R6为烷基, 所述的烷基任选进一步被一个或多个选自 环烷基的取代基所取代。 在本发明的另一个具体实施方案中, 一种通式(I-B )所示的化合物或其互变异 构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或 其可药用的盐, 其中 R1选自杂芳基。 本发明的另一方面涉及一种制备通式(I )所示的化合物或其互变异构体、 内消 旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药用的 盐的方法, 该方法包括以下步骤:
m is 0, 1, or 2. In another embodiment of the present invention, a compound of the formula (IB) or a mutual mutation thereof a form, a meso form, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 4 is a hydrogen atom. In another embodiment of the present invention, a compound of the formula (IB) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer A form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 5 is a hydrogen atom. In another embodiment of the present invention, a compound of the formula (IB) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is selected from aryl or heteroaryl, wherein the aryl or heteroaryl is optionally further further selected from one or more selected from halogen or - Substituted by a substituent of OR 6 , R 6 is an alkyl group, preferably a C alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group, preferably a cycloalkyl group C 3 to C 6 cycloalkyl, more preferably cyclopropyl. Further, the aryl group means a 6 to 14 membered all-carbon monocyclic or fused polycyclic group having a conjugated π-electron system, and R 1 is preferably a 6- to 10-membered aryl group, more preferably a phenyl group or a benzo group. Tetrahydrofuranyl, most preferably phenyl; said heteroaryl refers to a heteroaromatic system containing from 1 to 4 heteroatoms, from 5 to 14 ring atoms, wherein the heteroatoms include oxygen, sulfur and nitrogen; R 1 is preferably a heteroaromatic ring comprising 5 to 10 ring atoms, wherein 1 to 4 hetero atoms selected from oxygen, sulfur or nitrogen; more preferably 5 to 6 ring atoms, wherein 1 to 4 are selected from oxygen The heteroaromatic ring of a hetero atom of sulfur or nitrogen is most preferably a pyridyl group. In another embodiment of the present invention, a compound of the formula (Ι-Β) or a tautomer, a mesogen, a racemate, an enantiomer, a non-pair a conjugate, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is selected from aryl, wherein the aryl group is optionally further substituted with one or more substituents selected from halogen or -OR 6 Substituting, R 6 is an alkyl group, and the alkyl group is optionally further substituted with one or more substituents selected from a cycloalkyl group. In another embodiment of the present invention, a compound of the formula (IB) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer a form, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is selected from heteroaryl. Another aspect of the invention relates to the preparation of a compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer thereof, Or a method of the mixture thereof, or a pharmaceutically acceptable salt thereof, the method comprising the steps of:
( I-B ) ( I-B )
通式 (I-B)化合物与胺基化合物反应得到通式( I )化合物; The compound of the formula (I-B) is reacted with an amine compound to give a compound of the formula (I);
其中: ^〜115的定义如通式(I )化合物中所述。 进一步, 本发明的另一方面涉及一种药物组合物, 所述药物组合物含有治疗 有效量的如通式(I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异 构体、 非对映异构体、 或其混合物形式、 或其可药用的盐和药学上可接受的载体、 稀释剂或赋形剂。 本发明的另一方面涉及通式(I )所示的化合物或其互变异构体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物 组合物在制备胃酸分泌抑制剂中的用途。 本发明的另一方面涉及一种抑制胃酸分泌的方法, 该方法包括给予需要治疗 的患者有效治疗量的通式(I )所示的化合物或其互变异构体、 外消旋体、 对映异构 体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物组合物。 本发明的另一方面涉及通式(I )所示的化合物或其互变异构体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物 组合物, 其作为胃酸分泌抑制剂。 本发明的另一方面涉及通式(I )所示的化合物或其互变异构体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物 组合物在制备 H+/K+-腺苷三磷酸酶 (H+/K+-ATPase)抑制剂中的用途。 本发明的另一方面涉及一种抑制 H+/K+-腺苷三磷酸酶 (H+/K+-ATPase)的方法, 该方法包括给予需要治疗的患者有效治疗量的通式( I )所示的化合物或其互变异 构体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药用的 盐, 或包含其的药物组合物。
本发明的另一方面涉及通式(I )所示的化合物或其互变异构体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物 组合物, 其作为 H+/K+-腺苷三磷酸酶 (H+/K+-ATPase)抑制剂。 本发明的另一方面涉及通式(I )所示的化合物或其互变异构体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物 组合物在制备钾离子竞争性酸阻滞剂 (P-CABs)中的用途。 本发明的另一方面涉及一种竞争性酸阻滞钾离子的方法, 该方法包括给予需 要治疗的患者有效治疗量的通式(I )所示的化合物或其互变异构体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物 组合物。 本发明的另一方面涉及通式(I )所示的化合物或其互变异构体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物 组合物, 其作为钾离子竞争性酸阻滞剂 (P-CABs)。 本发明还涉及通式(I )所示的化合物或其互变异构体、外消旋体、对映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物组合物在制 备治疗或预防消化性溃疡、 卓-艾 (Zollinger-Ellison)综合征、 胃炎、 糜烂性食管炎、 反流性食管炎、 症状性胃食管反流疾病 (症状性 GERD)、 巴雷特 (Barrett)食管炎、 功能性消化不良、 幽门螺旋杆菌感染、 胃癌、 胃 MALT 淋巴瘤、 非甾体抗炎药 (NSAIDs)引起的溃疡或手术后应激导致的胃酸过多或溃疡的药物中的用途; 或者 在制备抑制由于消化性溃疡、 急性应激性溃疡、 出血性胃炎或侵入性应激造成的 上消化道出血的药物中的用途。 其中消化性溃疡包括但不限于胃溃疡、 十二指肠 溃疡或吻合口溃疡; 症状性胃食管反流疾病 (症状性 GERD)包括但不限于非糜烂性 的反流性疾病或无食管炎的胃食管反流疾病。 本发明的另一方面涉及一种治疗或预防消化性溃疡、 卓-艾 (Zollinger-Ellison) 综合征、 胃炎、 糜烂性食管炎、 反流性食管炎、 症状性胃食管反流疾病 (症状性 GERD), 巴雷特 (Barrett)食管炎、 功能性消化不良、 幽门螺旋杆菌感染、 胃癌、 胃 MALT 淋巴瘤、 非甾体抗炎药 (NSAIDs)引起的溃疡或手术后应激导致的胃酸过多 或溃疡的方法; 或者抑制由于消化性溃疡、 急性应激性溃疡、 出血性胃炎或侵入 性应激造成的上消化道出血的方法, 该方法包括给予需要治疗的患者有效治疗量 的通式(I )所示的化合物或其互变异构体、外消旋体、对映异构体、非对映异构体、 混合物形式、 及其可药用的盐, 或包含其的药物组合物。 其中消化性溃疡包括但 不限于胃溃疡、十二指肠溃疡或吻合口溃疡;症状性胃食管反流疾病 (症状性 GERD)
包括但不限于非糜烂性的反流性疾病或无食管炎的胃食管反流疾病。 本发明的另一方面涉及通式(I )所示的化合物或其互变异构体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 或包含其的药物 组合物, 其作为治疗或预防消化性溃疡、卓-艾 (Zollinger-Ellison)综合征、 胃炎、 糜 烂性食管炎、反流性食管炎、症状性胃食管反流疾病 (症状性 GERD)、巴雷特 (Barrett) 食管炎、 功能性消化不良、 幽门螺旋杆菌感染、 胃癌、 胃 MALT淋巴瘤、 非甾体 抗炎药 (NSAIDs)引起的溃疡或手术后应激导致的胃酸过多或溃疡的药物; 或者作 为抑制由于消化性溃疡、 急性应激性溃疡、 出血性胃炎或侵入性应激造成的上消 化道出血的药物。 其中消化性溃疡包括但不限于胃溃疡、 十二指肠溃疡或吻合口 溃疡; 症状性胃食管反流疾病 (症状性 GERD)包括但不限于非糜烂性的反流性疾病 或无食管炎的胃食管反流疾病。 发明的详细说明 Wherein: ^~11 5 is as defined in the compound of formula (I). Further, another aspect of the present invention relates to a pharmaceutical composition comprising a therapeutically effective amount of a compound represented by the formula (I) or a tautomer thereof, a mesogen, a foreign body A rot, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, diluent or excipient. Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the preparation of a gastric acid secretion inhibitor. Another aspect of the invention relates to a method of inhibiting gastric acid secretion comprising administering to a patient in need of treatment a therapeutically effective amount of a compound of formula (I) or a tautomer, racemate, pair thereof a conjugate, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same. Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, as a gastric acid secretion inhibitor. Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for the preparation of an H + /K + -adenosine triphosphatase (H + /K + -ATPase) inhibitor. Another aspect of the invention relates to a method of inhibiting H + /K + -adenosine triphosphatase (H + /K + -ATPase), the method comprising administering to a patient in need of treatment a therapeutically effective amount of the formula (I) a compound, or a tautomer, a racemate, an enantiomer, a diastereomer thereof, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same, or a pharmaceutical composition thereof . Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, which is an H + /K + - adenosine triphosphatase (H + /K + -ATPase) inhibitor. Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the preparation of potassium ion competitive acid blockers (P-CABs). Another aspect of the invention relates to a method for competing acid to block potassium ions, which comprises administering to a patient in need of treatment a therapeutically effective amount of a compound of the formula (I) or a tautomer thereof, a foreign body A rot, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same. Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, as a potassium ion competitive acid blocker (P-CABs). The present invention also relates to a compound of the formula (I) or a tautomer, a racemate, an enantiomer, a diastereomer thereof, or a mixture thereof, or a pharmaceutically acceptable compound thereof A salt, or a pharmaceutical composition comprising the same, for the treatment or prevention of peptic ulcer, Zollinger-Ellison syndrome, gastritis, erosive esophagitis, reflux esophagitis, symptomatic gastroesophageal reflux disease ( Symptomatic GERD), Barrett's esophagitis, functional dyspepsia, Helicobacter pylori infection, gastric cancer, gastric MALT lymphoma, non-steroidal anti-inflammatory drugs (NSAIDs) caused by ulcers or post-operative stress Use in a drug having hyperacidity or ulceration; or in the preparation of a medicament for inhibiting upper gastrointestinal bleeding caused by peptic ulcer, acute stress ulcer, hemorrhagic gastritis or invasive stress. Peptic ulcers include, but are not limited to, gastric ulcers, duodenal ulcers or anastomotic ulcers; symptomatic gastroesophageal reflux disease (symptomatic GERD) includes, but is not limited to, non-erosive reflux disease or no esophagitis Gastroesophageal reflux disease. Another aspect of the invention relates to the treatment or prevention of peptic ulcer, Zollinger-Ellison syndrome, gastritis, erosive esophagitis, reflux esophagitis, symptomatic gastroesophageal reflux disease (symptomatic GERD), Barrett's esophagitis, functional dyspepsia, Helicobacter pylori infection, gastric cancer, gastric MALT lymphoma, non-steroidal anti-inflammatory drugs (NSAIDs) caused by ulcers or post-operative stress-induced gastric acid a method of multiple or ulceration; or a method of inhibiting upper gastrointestinal bleeding caused by peptic ulcer, acute stress ulcer, hemorrhagic gastritis or invasive stress, the method comprising administering a therapeutically effective amount to a patient in need of treatment a compound represented by (I) or a tautomer, a racemate, an enantiomer, a diastereomer, a mixture thereof, a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same Things. Peptic ulcers include, but are not limited to, gastric ulcer, duodenal ulcer or anastomotic ulcer; symptomatic gastroesophageal reflux disease (symptomatic GERD) These include, but are not limited to, non-erosive reflux disease or gastroesophageal reflux disease without esophagitis. Another aspect of the invention relates to a compound of the formula (I) or a tautomer, racemate, enantiomer, diastereomer thereof, or a mixture thereof, or A medicinal salt, or a pharmaceutical composition comprising the same, for treating or preventing peptic ulcer, Zollinger-Ellison syndrome, gastritis, erosive esophagitis, reflux esophagitis, symptomatic gastroesophageal Reflux disease (symptomatic GERD), Barrett esophagitis, functional dyspepsia, Helicobacter pylori infection, gastric cancer, gastric MALT lymphoma, non-steroidal anti-inflammatory drugs (NSAIDs) caused by ulcers or after surgery A drug that causes hyperacidity or ulceration caused by stress; or a drug that inhibits upper gastrointestinal bleeding caused by peptic ulcer, acute stress ulcer, hemorrhagic gastritis, or invasive stress. Peptic ulcers include, but are not limited to, gastric ulcers, duodenal ulcers or anastomotic ulcers; symptomatic gastroesophageal reflux disease (symptomatic GERD) includes, but is not limited to, non-erosive reflux disease or no esophagitis Gastroesophageal reflux disease. Detailed description of the invention
除非有相反陈述, 否则下列用在说明书和权利要求书中的术语具有下述含义。 "烷基 "指饱和的脂族烃基团, 包括 1至 20个碳原子的直链和支链基团。 优 选含有 1至 10个碳原子的烷基, 更优选含有 1至 6个碳原子的烷基。 非限制性实 施例包括甲基、 乙基、 正丙基、 异丙基、 正丁基、 异丁基、 叔丁基、 仲丁基、 正 戊基、 1,1-二甲基丙基、 1,2-二甲基丙基、 2,2-二甲基丙基、 1-乙基丙基、 2-甲基丁 基、 3-甲基丁基、正己基、 1-乙基 -2-甲基丙基、 1,1,2-三甲基丙基、 1,1-二甲基丁基、 1,2-二甲基丁基、 2,2-二甲基丁基、 1,3-二甲基丁基、 2-乙基丁基、 2-甲基戊基、 3- 甲基戊基、 4-甲基戊基、 2,3-二甲基丁基、 正庚基、 2-甲基己基、 3-甲基己基、 4- 甲基己基、 5-甲基己基、 2,3-二甲基戊基、 2,4-二甲基戊基、 2,2-二甲基戊基、 3,3- 二甲基戊基、 2-乙基戊基、 3-乙基戊基、正辛基、 2,3-二甲基己基、 2,4-二甲基己基、 2,5-二甲基己基、 2,2-二甲基己基、 3,3-二甲基己基、 4,4-二甲基己基、 2-乙基己基、 3-乙基己基、 4-乙基己基、 2-甲基 -2-乙基戊基、 2-甲基 -3-乙基戊基、 正壬基、 2-甲 基 -2-乙基己基、 2-甲基 -3-乙基己基、 2,2-二乙基戊基、 正癸基、 3,3-二乙基己基、 2,2-二乙基己基, 及其各种支链异构体等。 更优选的是含有 1至 6个碳原子的低级 烷基, 非限制性实施例包括甲基、 乙基、 正丙基、 异丙基、 正丁基、 异丁基、 叔 丁基、 仲丁基、 正戊基、 1,1-二甲基丙基、 1,2-二甲基丙基、 2,2-二甲基丙基、 1-乙 基丙基、 2-甲基丁基、 3-甲基丁基、 正己基、 1-乙基 -2-甲基丙基、 1,1,2-三甲基丙 基、 1,1-二甲基丁基、 1,2-二甲基丁基、 2,2-二甲基丁基、 1,3-二甲基丁基、 2-乙基 丁基、 2-甲基戊基、 3-甲基戊基、 4-甲基戊基、 2,3-二甲基丁基等。 烷基可以是取 代的或未取代的, 当被取代时, 取代基可以在任何可使用的连接点上被取代, 优 选为一个或多个以下基团, 独立地选自烷基、 烯基、 块基、 烷氧基、 烷硫基、 烷 基氨基、 卤素、 巯基、 羟基、 硝基、 氰基、 环烷基、 杂环烷基、 芳基、 杂芳基、
环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基、氧代、 -OR -NR7R8、 -C(0)NR7R8 -S(0)mR6、 -C(0)R6、 -OC(0)R6、 -NR7C(0)R8、 -NR7C(0)OR8或 -C(0)OR6。 Unless otherwise stated, the following terms used in the specification and claims have the following meanings. "Alkyl" means a saturated aliphatic hydrocarbon group including straight chain and branched chain groups of 1 to 20 carbon atoms. An alkyl group having 1 to 10 carbon atoms is preferred, and an alkyl group having 1 to 6 carbon atoms is more preferred. Non-limiting examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, sec-butyl, n-pentyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2 -methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1, 3-dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 2,3-dimethylbutyl, n-heptyl, 2-methylhexyl, 3-methylhexyl, 4-methylhexyl, 5-methylhexyl, 2,3-dimethylpentyl, 2,4-dimethylpentyl, 2,2-dimethyl Pentyl, 3,3-dimethylpentyl, 2-ethylpentyl, 3-ethylpentyl, n-octyl, 2,3-dimethylhexyl, 2,4-dimethylhexyl, 2,5-Dimethylhexyl, 2,2-dimethylhexyl, 3,3-dimethylhexyl, 4,4-dimethylhexyl, 2-ethylhexyl, 3-ethylhexyl, 4- Ethylhexyl, 2-methyl-2-ethylpentyl, 2 -methyl-3-ethylpentyl, n-decyl, 2-methyl-2-ethylhexyl, 2-methyl-3-ethylhexyl, 2,2-diethylpentyl, n-decyl , 3,3-diethylhexyl, 2,2-diethylhexyl, and various branched isomers thereof. More preferred are lower alkyl groups having 1 to 6 carbon atoms, and non-limiting examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t-butyl, sec-butyl Base, n-pentyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2-methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethyl Butyl, 2,2-dimethylbutyl, 1,3-dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl Base, 2,3-dimethylbutyl and the like. The alkyl group may be substituted or unsubstituted, and when substituted, the substituent may be substituted at any available point of attachment, preferably one or more of the following groups, independently selected from alkyl, alkenyl, Block, alkoxy, alkylthio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, Cycloalkoxy, heterocycloalkoxy, cycloalkylthio, heterocycloalkylthio, oxo, -OR-NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 , -OC(0)R 6 , -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
"环烷基"指饱和或部分不饱和单环或多环环状烃取代基, 其包括 3至 20个 碳原子, 优选包括 3至 12个碳原子, 更优选环烷基环包含 3至 10个碳原子, 最 优选环烷基环包含 3至 6个碳原子。 单环环烷基的非限制性实施例包含环丙基、 环丁基、 环戊基、 环戊烯基、 环己基、 环己烯基、 环己二烯基、 环庚基、 环庚三 烯基、 环辛基等。 多环环烷基包括螺环、 稠环和桥环的环烷基。 "Cycloalkyl" means a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent comprising from 3 to 20 carbon atoms, preferably from 3 to 12 carbon atoms, more preferably the cycloalkyl ring comprises from 3 to 10 The carbon atom, most preferably the cycloalkyl ring contains from 3 to 6 carbon atoms. Non-limiting examples of monocyclic cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cyclohexadienyl, cycloheptyl, cycloheptatriene Alkenyl, cyclooctyl and the like. Polycyclic cycloalkyl groups include spiro, fused, and bridged cycloalkyl groups.
"螺环烷基"指 5至 20元, 单环之间共用一个碳原子 (称螺原子)的多环基团, 这些可以含有一个或多个双键, 但没有一个环具有完全共轭的 π 电子系统。 优选 为 6至 14元, 更优选为 7至 10元。 根据环与环之间共用螺原子的数目将螺环烷 基分为单螺环烷基、 双螺环烷基基或多螺环烷基, 优选为单螺环烷基和双螺环烷 基。 更优选为 4元 /4元、 4元 /5元、 4元 /6元、 5元 /5元或 5元 /6元单螺环烷基。 螺环烷基的非限制性实
"Spirocycloalkyl" means a polycyclic group of 5 to 20 members which shares a carbon atom (referred to as a spiro atom) between the monocyclic rings. These may contain one or more double bonds, but none of the rings are fully conjugated. π electronic system. It is preferably 6 to 14 members, more preferably 7 to 10 members. The spirocycloalkyl group is classified into a monospirocycloalkyl group, a bispirocycloalkyl group or a polyspirocycloalkyl group, preferably a monospirocycloalkyl group and a bispirocycloalkyl group, depending on the number of common spiro atoms between the ring and the ring. . More preferably, it is 4 yuan / 4 yuan, 4 yuan / 5 yuan, 4 yuan / 6 yuan, 5 yuan / 5 yuan or 5 yuan / 6 yuan monospirocycloalkyl. Non-limiting real
"稠环烷基"指 5至 20元, 系统中的每个环与体系中的其他环共享毗邻的一 对碳原子的全碳多环基团, 其中一个或多个环可以含有一个或多个双键, 但没有 一个环具有完全共轭的 π电子系统。 优选为 6至 14元, 更优选为 7至 10元。 根 据组成环的数目可以分为双环、 三环、 四环或多环稠环烷基, 优选为双环或三环, 更优 。 稠环烷基的非限制性实施例包含 "Fused cycloalkyl" means 5 to 20 members, each ring of the system sharing an adjacent carbon atom of an all-carbon polycyclic group with other rings in the system, wherein one or more rings may contain one or more Two double bonds, but none of the rings have a fully conjugated π-electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members. The bicyclic ring, the tricyclic ring, the tetracyclic ring or the polycyclic fused ring alkyl group may be classified according to the number of the constituent rings, and preferably a bicyclic ring or a tricyclic ring, more preferably. Non-limiting examples of fused cycloalkyl groups include
"桥环烷基"指 5至 20元, 任意两个环共用两个不直接连接的碳原子的全碳 多环基团, 这些可以含有一个或多个双键, 但没有一个环具有完全共轭的 π 电子 系统。 优选为 6至 14元, 更优选为 7至 10元。 根据组成环的数目可以分为双环、 三环、 四环或多环桥环烷基, 优选为双环、 三环或四环, 更有选为双环或三环。 桥环烷基的非限制性实施例包含 "Bridge cycloalkyl" means 5 to 20 members, any two rings sharing two carbon-free all-carbon polycyclic groups, which may contain one or more double bonds, but none of the rings have a total The π electronic system of the yoke. It is preferably 6 to 14 members, more preferably 7 to 10 members. Depending on the number of constituent rings, it may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic bridged cycloalkyl group, preferably a bicyclic ring, a tricyclic ring or a tetracyclic ring, and more preferably a bicyclic ring or a tricyclic ring. Non-limiting examples of bridged cycloalkyl groups include
所述环烷基环可以稠合于芳基、 杂芳基或杂环烷基环上, 其中与母体结构连接在 一起的环为环烷基, 非限制性实施例包括茚满基、 四氢萘基、 苯并环庚烷基等。 环烷基可以是任选取代的或未取代的, 当被取代时, 取代基优选为一个或多个以 下基团, 独立地选自烷基、 烯基、 块基、 烷氧基、 烷硫基、 烷基氨基、 卤素、 巯 基、 羟基、 硝基、 氰基、 环烷基、 杂环烷基、 芳基、 杂芳基、 环烷氧基、 杂环烷 氧基、环烷硫基、杂环烷硫基、氧代、 -OR6、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6、 -NR7C(0)R8、 -NR7C(0)OR8或 -C(0)OR6。 The cycloalkyl ring may be fused to an aryl, heteroaryl or heterocycloalkyl ring, wherein the ring to which the parent structure is attached is a cycloalkyl group, non-limiting examples include indanyl, tetrahydrogen Naphthyl, benzocycloheptyl and the like. The cycloalkyl group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, block, alkoxy, alkylthio Base, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, Heterocycloalkylthio, oxo, -OR 6 , -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC(0 R 6 , -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
"杂环基"指饱和或部分不饱和单环或多环环状烃取代基, 其包括 3至 20个 环原子, 其中一个或多个环原子选自氮、氧或 S(0)m (其中 m是整数 0至 2)的杂原 子, 但不包括 -0-0-、 -0-S-或 -S-S-的环部分, 其余环原子为碳。 优选包括 3至 12 个环原子, 其中 1〜4个是杂原子, 更优选杂环烷基环包含 3至 10个环原子, 更 优选杂环烷基环包含 5至 6个环原子。 单环杂环烷基的非限制性实施例包含吡咯 烷基、 哌啶基、 哌嗪基、 吗啉基、 硫代吗啉基、 高哌嗪基、 吡喃基、 四氢呋喃基 等。 多环杂环烷基包括螺环、 稠环和桥环的杂环基。 "Heterocyclyl" means a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent comprising from 3 to 20 ring atoms wherein one or more of the ring atoms are selected from nitrogen, oxygen or S(0) m ( Wherein m is a hetero atom of the integer 0 to 2), but does not include a ring moiety of -0-0-, -0-S- or -SS-, and the remaining ring atoms are carbon. It preferably comprises from 3 to 12 ring atoms, wherein from 1 to 4 are heteroatoms, more preferably the heterocycloalkyl ring contains from 3 to 10 ring atoms, more preferably the heterocycloalkyl ring contains from 5 to 6 ring atoms. Non-limiting examples of monocyclic heterocycloalkyl groups include pyrrolidinyl, piperidinyl, piperazinyl, morpholinyl, thiomorpholinyl, homopiperazinyl, pyranyl, tetrahydrofuranyl and the like. Polycyclic heterocycloalkyl groups include spiro, fused, and bridged heterocyclic groups.
"螺杂环基"指 5至 20元, 单环之间共用一个原子 (称螺原子)的多环杂环基 团, 其中一个或多个环原子选自氮、 氧或 S(0)m (其中 m是整数 0至 2)的杂原子, 其余环原子为碳。这些可以含有一个或多个双键, 但没有一个环具有完全共轭的 π 电子系统。 优选为 6至 14元, 更优选为 7至 10元。 根据环与环之间共用螺原子 的数目将螺环烷基分为单螺杂环基、 双螺杂环基或多螺杂环基, 优选为单螺环烷 基和双螺环烷基。 更优选为 4元 /4元、 4元 /5元、 4元 /6元、 5元 /5元或 5元 /6元 单螺环烷基。 螺环烷 "spiroheterocyclyl" means a polycyclic heterocyclic group of 5 to 20 members in which one atom (called a spiro atom) is shared between the monocyclic rings, wherein one or more ring atoms are selected from nitrogen, oxygen or S(0) m A hetero atom (where m is an integer from 0 to 2), and the remaining ring atoms are carbon. These may contain one or more double bonds, but none of the rings have a fully conjugated pi-electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members. The spirocycloalkyl group is classified into a monospiroheterocyclic group, a dispiroheterocyclic group or a polyspirocyclic group according to the number of common spiro atoms between the rings, preferably a monospirocycloalkyl group and a bispirocycloalkyl group. More preferably, it is 4 yuan / 4 yuan, 4 yuan / 5 yuan, 4 yuan / 6 yuan, 5 yuan / 5 yuan or 5 yuan / 6 yuan monospirocycloalkyl. Spirocycloalkane
"稠杂环基"指 5至 20元, 系统中的每个环与体系中的其他环共享毗邻的一 对原子的多环杂环基团, 一个或多个环可以含有一个或多个双键, 但没有一个环 具有完全共轭的 π电子系统, 其中一个或多个环原子选自氮、 氧或 S(0)m (其中 m 是整数 0至 2)的杂原子, 其余环原子为碳。 优选为 6至 14元, 更优选为 7至 10 元。 根据组成环的数目可以分为双环、 三环、 四环或多环稠杂环烷基, 优选为双 环或三环, 更优选为 5元 /5元或 5元 /6元双环稠杂环基。 稠杂环基的非限制性实 施例包含 "Fused heterocyclic group" means 5 to 20 members, each ring in the system shares an adjacent pair of atomic polycyclic heterocyclic groups with other rings in the system, and one or more rings may contain one or more a bond, but none of the rings have a fully conjugated π-electron system in which one or more ring atoms are selected from nitrogen, oxygen or S(0) m (where m is an integer from 0 to 2), and the remaining ring atoms are carbon. It is preferably 6 to 14 members, more preferably 7 to 10 members. It may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic fused heterocycloalkyl group according to the number of constituent rings, preferably a bicyclic or tricyclic ring, more preferably a 5-member/5-membered or 5-membered/6-membered bicyclic fused heterocyclic group. . Non-limiting examples of fused heterocyclic groups include
"桥杂环基"指 5至 14元, 任意两个环共用两个不直接连接的原子的多环杂 环基团, 这些可以含有一个或多个双键, 但没有一个环具有完全共轭的 π 电子系 统, 其中一个或多个环原子选自氮、 氧或 S(0)m (其中 m是整数 0至 2)的杂原子, 其余环原子为碳。 优选为 6至 14元, 更优选为 7至 10元。 7至 10元。 根据组成 环的数目可以分为双环、 三环、 四环或多环桥环烷基, 优选为双环、 三环或四环, 更有选为双环或三环。 桥环烷基的非 施 :
"Bridge heterocyclyl" refers to a polycyclic heterocyclic group of 5 to 14 members in which two rings share two atoms which are not directly bonded, and these may contain one or more double bonds, but none of the rings have a complete conjugation A π-electron system in which one or more ring atoms are selected from nitrogen, oxygen or S(0) m (where m is an integer from 0 to 2), and the remaining ring atoms are carbon. It is preferably 6 to 14 members, more preferably 7 to 10 members. 7 to 10 yuan. Depending on the number of constituent rings, it may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic bridged cycloalkyl group, preferably a bicyclic ring, a tricyclic ring or a tetracyclic ring, and more preferably a bicyclic ring or a tricyclic ring. Non-application of bridged cycloalkyl groups:
所述杂环基环可以稠合于芳基、 杂芳基或环烷基环上, 其中与母体结构连接在 起的环为杂环基,
The heterocyclyl ring may be fused to an aryl, heteroaryl or cycloalkyl ring, wherein the ring to which the parent structure is attached is a heterocyclic group,
等。 杂环基可以是任选取代的或未取代的, 当被取代时, 取代基优选为一个或多 个以下基团, 独立地选自烷基、 烯基、 块基、 烷氧基、 烷硫基、 烷基氨基、 卤素、 巯基、 羟基、 硝基、 氰基、 环烷基、 杂环烷基、 芳基、 杂芳基、 环烷氧基、 杂环 烷氧基、 环烷硫基、 杂环烷硫基、 氧代、 -OR6、 -NR7R8、 -C(0)NR7R8 -S(0)mR6 -C(0)R6、 -OC(0)R6、 -NR7C(0)R8、 -NR7C(0)OR8或 -C(0)OR6。 Wait. The heterocyclic group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups, independently selected from the group consisting of alkyl, alkenyl, block, alkoxy, alkylthio Base, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, Heterocycloalkylthio, oxo, -OR 6 , -NR 7 R 8 , -C(0)NR 7 R 8 -S(0) m R 6 -C(0)R 6 , -OC(0)R 6 , -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
"芳基"指具有共轭的 π电子体系的 6至 14元全碳单环或稠合多环 (也就是共 享毗邻碳原子对的环)基团, 优选为 6至 10元, 例如苯基和萘基。所述芳基环可以 稠合于杂芳基、 杂环基或环烷基环上, 其中与母体结构连接在一起的环为芳基环, "Aryl" means a 6 to 14 membered all-carbon monocyclic or fused polycyclic ring (ie, a ring that shares a pair of adjacent carbon atoms) having a conjugated π-electron system, preferably 6 to 10 members, such as phenyl. And naphthyl. The aryl ring may be fused to a heteroaryl, heterocyclic or cycloalkyl ring, wherein the ring to which the parent structure is attached is an aryl ring,
芳基可以是取代的或未取代的, 当被取代时, 取代基优选为一个或多个以下基团, 独立地选自烷基、 烯基、 块基、 烷氧基、 烷硫基、 烷基氨基、 ^素、 巯基、 羟基、 硝基、 氰基、 环烷基、 杂环烷基、 芳基、 杂芳基、 环烷氧基、 杂环烷氧基、 环浣 硫基、 杂环烷硫基、 -OR6、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6、
-NR7C(0)R8、 -NR7C(0)OR8或 -C(0)OR6。 The aryl group may be substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups, independently selected from the group consisting of alkyl, alkenyl, block, alkoxy, alkylthio, alkane. Amino, arginyl, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cyclodecylthio, heterocycle Alkylthio group, -OR 6 , -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC(0)R 6 , -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
"杂芳基"指包含 1至 4个杂原子, 5至 14个环原子的杂芳族体系, 其中杂 原子包括氧、 硫和氮。 优选为 5至 10元。 杂芳基优选为是 5元或 6元, 例如呋喃 基、 噻吩基、 吡啶基、 吡咯基、 N-烷基吡咯基、 嘧啶基、 吡嗪基、 咪唑基、 四唑 基等。 所述杂芳基环可以稠合于芳基、 杂环基或环烷基环上, 其中与母体结构连 接在一 "Heteroaryl" means a heteroaromatic system containing from 1 to 4 heteroatoms, 5 to 14 ring atoms, wherein the heteroatoms include oxygen, sulfur and nitrogen. It is preferably 5 to 10 yuan. The heteroaryl group is preferably a 5- or 6-membered compound such as a furyl group, a thienyl group, a pyridyl group, a pyrrolyl group, an N-alkylpyrrolyl group, a pyrimidinyl group, a pyrazinyl group, an imidazolyl group, a tetrazolyl group or the like. The heteroaryl ring may be fused to an aryl, heterocyclic or cycloalkyl ring, wherein the parent structure is attached
杂芳基可以是任选取代的或未取代的, 当被取代时, 取代基优选为一个或多个以 下基团, 独立地选自烷基、 烯基、 块基、 烷氧基、 烷硫基、 烷基氨基、 卤素、 巯 基、 羟基、 硝基、 氰基、 环烷基、 杂环烷基、 芳基、 杂芳基、 环烷氧基、 杂环烷 氧基、 环烷硫基、 杂环烷硫基、 -OR6、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6、 -NR7C(0)R8、 -NR7C(0)OR8或 -C(0)OR6。 The heteroaryl group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, block, alkoxy, alkylthio Base, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, Heterocycloalkylthio, -OR 6 , -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC(0)R 6 -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
"烷氧基"指 -o- (浣基)和 -o- (未取代的环烷基), 其中烷基的定义如上所述。 非限制性实施例包含甲氧基、 乙氧基、 丙氧基、 丁氧基、 环丙氧基、 环丁氧基、 环戊氧基、 环己氧基等。 烷氧基可以是任选取代的或未取代的, 当被取代时, 取 代基优选为一个或多个以下基团, 独立地选自为烷基、 烯基、 块基、 烷氧基、 烷 硫基、 烷基氨基、 卤素、 巯基、 羟基、 硝基、 氰基、 环烷基、 杂环烷基、 芳基、 杂芳基、环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基、 -OR6、-NR7R8、-C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6、 -NR7C(0)R8、 -NR7C(0)OR8或 -C(0)OR6。 "Alkoxy" means -o-(indenyl) and -o-(unsubstituted cycloalkyl), wherein alkyl is as defined above. Non-limiting examples include methoxy, ethoxy, propoxy, butoxy, cyclopropoxy, cyclobutoxy, cyclopentyloxy, cyclohexyloxy and the like. The alkoxy group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups, independently selected from the group consisting of an alkyl group, an alkenyl group, a block group, an alkoxy group, and an alkane group. Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio , heterocycloalkylthio, -OR 6 , -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC(0)R 6 , -NR 7 C(0)R 8 , -NR 7 C(0)OR 8 or -C(0)OR 6 .
"卤代烷基"指烷基被一个或多个卤素取代, 其中烷基的定义如上所述。 "Haloalkyl" means an alkyl group substituted by one or more halogens, wherein alkyl is as defined above.
"羟基"指 -OH基团。 "Hydroxy" means an -OH group.
"羟烷基"指被羟基取代的烷基, 其中烷基的定义如上所述。 "Hydroxyalkyl" means an alkyl group substituted by a hydroxy group, wherein the alkyl group is as defined above.
"卤素"指氟、 氯、 溴或碘。 "Halogen" means fluoro, chloro, bromo or iodo.
"氨基"指 -NH2。 "Amino" means -NH 2 .
"氰基"指 -CN。 "Cyano" means -CN.
"硝基"指 -N02。 "Nitro" means -N0 2 .
"苄基"指 -CH2-苯基。 "Benzyl" refers to -CH 2 - phenyl.
"氧代基"指 =0。 "Oxo group" means =0.
"羧基"指 -C(0)OH。 "Carboxy" means -C(0)OH.
"羧酸酯基"指 -C(0)0(烷基)或 (环烷基), 其中烷基、环烷基的定义如上所述。 The "carboxylate group" means -C(0)0(alkyl) or (cycloalkyl), wherein the alkyl group and the cycloalkyl group are as defined above.
"氨基保护基"是为了使分子其它部位进行反应时氨基保持不变, 用易于脱
去的基团对氨基进行保护。 非限制性实施例包含甲酰基、 烷基羰基、 烷氧基羰基、 苯甲酰基、 芳烷基羰基、 芳烷氧基羰基、 三苯甲基、 邻苯二甲酰基、 N,N-二甲基 氨基亚甲基、 取代的甲硅烷基等。 这些基团可任选地被选自卤素、 烷氧基或硝基 中的 1-3个取代基所取代。 氨基保护基优选为叔丁氧羰基。 "Amino protecting group" is used to keep the amino group unchanged when the other parts of the molecule are reacted. The group to be removed protects the amino group. Non-limiting examples include formyl, alkylcarbonyl, alkoxycarbonyl, benzoyl, aralkylcarbonyl, aralkoxycarbonyl, trityl, phthaloyl, N,N-dimethyl Aminoaminomethylene, substituted silyl, and the like. These groups may be optionally substituted with from 1 to 3 substituents selected from halogen, alkoxy or nitro. The amino protecting group is preferably a tert-butoxycarbonyl group.
"任选"或 "任选地"意味着随后所描述地事件或环境可以但不必发生, 该 说明包括该事件或环境发生或不发生地场合。例如, "任选被烷基取代的杂环基团" 意味着烷基可以但不必须存在, 该说明包括杂环基团被烷基取代的情形和杂环基 团不被烷基取代的情形。 "Optional" or "optionally" means that the subsequently described event or environment may, but need not, occur, including where the event or environment occurs or does not occur. For example, "heterocyclic group optionally substituted by an alkyl group" means that an alkyl group may be, but not necessarily, present, including the case where the heterocyclic group is substituted by an alkyl group and the case where the heterocyclic group is not substituted by an alkyl group. .
"取代的"指基团中的一个或多个氢原子, 优选为最多 5个, 更优选为 1〜3 个氢原子彼此独立地被相应数目的取代基取代。 不言而喻, 取代基仅处在它们的 可能的化学位置, 本领域技术人员能够在不付出过多努力的情况下确定 (通过实验 或理论)可能或不可能的取代。例如, 具有游离氢的氨基或羟基与具有不饱和 (如烯 属)键的碳原子结合时可能是不稳定的。 "Substituted" means that one or more hydrogen atoms in the group, preferably up to 5, more preferably 1 to 3, hydrogen atoms are independently substituted with each other by a corresponding number of substituents. It goes without saying that the substituents are only in their possible chemical positions, and those skilled in the art will be able to determine (by experiment or theory) substitutions that may or may not be possible without undue effort. For example, an amino group or a hydroxyl group having a free hydrogen may be unstable when combined with a carbon atom having an unsaturated (e.g., olefinic) bond.
"药物组合物"表示含有一种或多种本文所述化合物或其生理学上 /可药用的 盐或前体药物与其他化学组分的混合物, 以及其他组分例如生理学 /可药用的载体 和赋形剂。 药物组合物的目的是促进对生物体的给药, 利于活性成分的吸收进而 发挥生物活性。 "Pharmaceutical composition" means a mixture containing one or more of the compounds described herein, or a physiologically/pharmaceutically acceptable salt or prodrug thereof, and other chemical components, as well as other components such as physiological/pharmaceutically acceptable carriers. And excipients. The purpose of the pharmaceutical composition is to promote administration to an organism, to facilitate absorption of the active ingredient and to exert biological activity.
m和 R6〜R8的定义如通式( I )化合物中所述。 本发明化合物的合成方法 m and R 6 to R 8 are as defined in the compound of the formula (I). Method for synthesizing the compound of the present invention
为了完成本发明的合成目的, 本发明采用如下合成技术方案: In order to accomplish the synthetic purposes of the present invention, the present invention employs the following synthetic technical solutions:
本发明通式(I )所述的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异 构体、 非对映异构体、 或其混合物形式、 或其可药用的盐的制备方法包括以下步 驟: a compound of the formula (I), or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or a compound thereof The preparation method of the medicinal salt comprises the following steps:
吡咯类化合物 (a)与苯并呋喃磺酰氯化合物 (b)在碱性条件下溶剂中反应得到苯 并呋喃磺酰基取代的吡咯类化合物 (C), 苯并呋喃磺酰基取代的吡咯类化合物 (C)与 The azole compound (a) and the benzofuransulfonyl chloride compound (b) are reacted in a solvent under basic conditions to obtain a benzofuransulfonyl-substituted pyrrole compound (C), a benzofuransulfonyl-substituted pyrrole compound ( C) and
R1取代的硼酸酯或硼酸在碱性条件下, 于溶剂中经催化剂催化进行反应得到 R\ 苯并呋喃磺酰基取代的吡咯类化合物 (I-A), R 苯并呋喃磺酰基取代的吡咯类化 合物 (I-A)在酸性条件下溶剂中脱保护得到通式( I )化合物。 其中 X为卤素;
R5的定义如通式(I )化合物中所述, 其中 R3优选为氢原子; PG为氨基保护基, 优 选叔丁氧羰基。 The R 1 -substituted boronic acid ester or boric acid is subjected to catalytic catalysis in a solvent under basic conditions to obtain R benzofuransulfonyl-substituted pyrrole compound (IA), R benzofuransulfonyl-substituted pyrrole The compound (IA) is deprotected in a solvent under acidic conditions to give a compound of the formula (I). Wherein X is a halogen; R 5 is as defined in the compound of formula (I) wherein R 3 is preferably a hydrogen atom; PG is an amino protecting group, preferably a tert-butoxycarbonyl group.
提供酸性条件的试剂包括但不限于三氟醋酸、 甲酸、 乙酸、 盐酸、 硫酸、 甲 磺酸。 Agents that provide acidic conditions include, but are not limited to, trifluoroacetic acid, formic acid, acetic acid, hydrochloric acid, sulfuric acid, methanesulfonic acid.
碱性条件的试剂包括有机碱和无机碱类, 所述的有机碱类包括但不限于三乙 胺、 N,N-二异丙基乙胺、 正丁基锂、 叔丁醇钾, 所述的无机碱类包括但不限于氢 化钠、 碳酸钠、 碳酸氢钠、 碳酸钾、 碳酸氢钾或碳酸铯。 The alkaline condition reagent includes an organic base and an inorganic base, and the organic base includes, but not limited to, triethylamine, N,N-diisopropylethylamine, n-butyllithium, potassium t-butoxide, Inorganic bases include, but are not limited to, sodium hydride, sodium carbonate, sodium bicarbonate, potassium carbonate, potassium bicarbonate or cesium carbonate.
催化剂包括但不限于 [1,1'-双 (二苯基膦基)二茂铁]二氯化钯、 四-三苯基膦钯、 二氯化钯、 醋酸钯或三 (二亚苄基丙酮)二钯。 Catalysts include, but are not limited to, [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride, tetrakistriphenylphosphine palladium, palladium dichloride, palladium acetate or tris(dibenzylidene) Acetone) dipalladium.
所用溶剂包括但不限于: 四氢呋喃、 二氯甲烷、 1,4-二氧六环、 水、 甲醇、 乙 醇、 二甲基亚砜或 N,N-二甲基甲酰胺。 方案二: Solvents used include, but are not limited to, tetrahydrofuran, dichloromethane, 1,4-dioxane, water, methanol, ethanol, dimethyl sulfoxide or N,N-dimethylformamide. Option II:
吡咯甲醛类化合物 (d)与苯并呋喃磺酰氯化合物 (b)在碱性条件下溶剂中反应得 到苯并呋喃磺酰基取代的吡咯甲醛类化合物 (I-B), 苯并呋喃磺酰基取代的吡咯甲 醛类化合物 (I-B)与胺基化合物 (e)在还原剂如硼氢化钠作用下还原氨化得到通式( I ) 化合物。 其中 Ri〜R5的定义如通式(I )中所述。 The pyrrole formaldehyde compound (d) is reacted with a benzofuransulfonyl chloride compound (b) in a solvent under basic conditions to obtain a benzofuransulfonyl-substituted pyrrole formaldehyde compound (IB), a benzofuransulfonyl-substituted pyrrole formaldehyde. The reductive amination of the compound (IB) with the amine compound (e) under the action of a reducing agent such as sodium borohydride gives the compound of the formula (I). Wherein R to R 5 are as defined in the formula (I).
提供碱性条件的试剂包括有机碱和无机碱类, 所述的有机碱类包括但不限于 三乙胺、 N,N-二异丙基乙胺、 正丁基锂、 叔丁醇钾, 所述的无机碱类包括但不限 于氢化钠、 碳酸钠、 碳酸氢钠、 碳酸钾、 碳酸氢钾或碳酸铯。 The reagents providing basic conditions include organic bases including, but not limited to, triethylamine, N,N-diisopropylethylamine, n-butyllithium, potassium t-butoxide, and the like. The inorganic bases mentioned include, but are not limited to, sodium hydride, sodium carbonate, sodium hydrogencarbonate, potassium carbonate, potassium hydrogencarbonate or cesium carbonate.
还原剂包括但不限于硼氢化钠、 三乙酰氧基硼氢化钠、 腈基硼氢化钠或氢化 铝锂。 Reducing agents include, but are not limited to, sodium borohydride, sodium triacetoxyborohydride, sodium nitrile borohydride or lithium aluminum hydride.
所用溶剂包括但不限于: 四氢呋喃、 二氯甲烷、 1,4-二氧六环、 水、 甲醇、 乙 醇、 二甲基亚砜或 N,N-二甲基甲酰胺。 具体实施方式 Solvents used include, but are not limited to, tetrahydrofuran, dichloromethane, 1,4-dioxane, water, methanol, ethanol, dimethyl sulfoxide or N,N-dimethylformamide. Detailed ways
以下结合实施例进一步描述本发明, 但这些实施例并非限制着本发明的范围。 本发明实施例中未注明具体条件的实验方法, 通常按照常规条件, 或按照原 料或商品制造厂商所建议的条件。 未注明具体来源的试剂, 为市场购买的常规试 剂。
实施例 The invention is further described in the following examples, but these examples are not intended to limit the scope of the invention. Experimental methods in which no specific conditions are specified in the examples of the present invention are generally carried out according to conventional conditions or according to the conditions recommended by the raw material or commodity manufacturer. Reagents not specified for specific sources, are routine reagents purchased by the market. Example
化合物的结构是通过核磁共振 (NMR)或质谱 (MS)来确定的。 NMR的测定是用 BrukerAVANCE-400核磁仪, 测定溶剂为氘代二甲基亚砜 ( )ΜΛ -ί¾)、 氘代氯仿 (CDC13) 氘代甲醇 (; CD3OD), 内标为四甲基硅烷 CTMS), 化学位移是以 10— 6Cppm) 作为单位给出。 The structure of the compound is determined by nuclear magnetic resonance (NMR) or mass spectrometry (MS). The NMR was measured by a Bruker AVANCE-400 nuclear magnetic apparatus, and the solvent was deuterated dimethyl sulfoxide ( ) ΜΛ - ί 3⁄4), deuterated chloroform (CDC1 3 ) deuterated methanol (CD 3 OD), and the internal standard was tetramethyl. silane CTMS), chemical shifts are given 10- 6 Cppm) as a unit.
MS的测定用 FINMGAN LCQAd (ESI)质谱仪 (生产商: Thermo,型号: Finnigan LCQ advantage MAX)。 The MS was measured using a FINMGAN LCQAd (ESI) mass spectrometer (manufacturer: Thermo, model: Finnigan LCQ advantage MAX).
HPLC的测定使用安捷伦 1200DAD高压液相色谱仪 (Sunfire C18 150x4.6mm 色谱柱)和 Waters 2695-2996高压液相色谱仪 (Gimini C18 150x4.6mm色谱柱)。 The HPLC was measured using an Agilent 1200 DAD high pressure liquid chromatograph (Sunfire C18 150 x 4.6 mm column) and a Waters 2695-2996 high pressure liquid chromatograph (Gimini C18 150 x 4.6 mm column).
激酶平均抑制率及 IC5Q值的测定用 NovoStar酶标仪 (德国 BMG公司)。 The average inhibition rate of the kinase and the IC 5Q value were determined using a NovoStar plate reader (BMG, Germany).
薄层层析硅胶板使用烟台黄海 HSGF254或青岛 GF254硅胶板, 薄层色谱法 (TLC)使用的硅胶板采用的规格是 0.15 mm〜0.2 mm, 薄层层析分离纯化产品采用 的规格是 0.4 mm〜0.5 mm硅胶板。 The thin layer chromatography silica gel plate uses Yantai Yellow Sea HSGF254 or Qingdao GF254 silica gel plate. The silica gel plate used for thin layer chromatography (TLC) has a specification of 0.15 mm~0.2 mm, and the thin layer chromatography separation and purification product adopts the specification of 0.4 mm. ~0.5 mm silica gel plate.
柱层析一般使用烟台黄海 200〜300目硅胶为载体。 Column chromatography generally uses Yantai Yellow Sea 200~300 mesh silica gel as a carrier.
本发明的已知的起始原料可以采用或按照本领域已知的方法来合成, 或可购 买自 ABCR GmbH & Co.KG, Acros Organnics, Aldrich Chemical Company, 韶远 化学科技 (; Accela ChemBio Inc) 达瑞化学品等公司。 The known starting materials of the present invention may be synthesized by or according to methods known in the art, or may be purchased from ABCR GmbH & Co. KG, Acros Organnics, Aldrich Chemical Company, Accela ChemBio Inc. Companies such as Dare Chemicals.
实施例中如无特殊说明, 反应均在氩气氛或氮气氛下进行。 In the examples, the reactions were all carried out under an argon atmosphere or a nitrogen atmosphere unless otherwise specified.
氩气氛或氮气氛是指反应瓶连接一个约 1L容积的氩气或氮气气球。 An argon atmosphere or a nitrogen atmosphere means that the reaction flask is connected to an argon or nitrogen balloon having a volume of about 1 L.
实施例中如无特殊说明, 反应中的溶液是指水溶液。 In the examples, the solution in the reaction means an aqueous solution unless otherwise specified.
实施例中如无特殊说明, 反应的温度为室温。 In the examples, the temperature of the reaction was room temperature unless otherwise specified.
室温为最适宜的反应温度, 温度范围是 20°C〜30°C。 Room temperature is the optimum reaction temperature, and the temperature range is from 20 ° C to 30 ° C.
实施例中的反应进程的监测采用薄层色谱法 (TLC), 反应所使用的展开剂的体 系有: A: 二氯甲烷和甲醇体系, B: 正己烷和乙酸乙酯体系, C: 石油醚和乙酸 乙酯体系, D: 丙酮, 溶剂的体积比根据化合物的极性不同而进行调节。 The progress of the reaction in the examples was monitored by thin layer chromatography (TLC). The system used for the reaction was: A: dichloromethane and methanol system, B: n-hexane and ethyl acetate system, C: petroleum ether And the ethyl acetate system, D: acetone, the volume ratio of the solvent is adjusted depending on the polarity of the compound.
纯化化合物采用的柱层析的洗脱剂的体系和薄层色谱法的展开剂的体系包括: A: 二氯甲烷和甲醇体系, B: 正己烷和乙酸乙酯体系, C: 正己烷和丙酮体系, D: 正己浣, E: 乙酸乙酯, 溶剂的体积比根据化合物的极性不同而进行调节, 也可以 加入少量的三乙胺和酸性或碱性试剂等进行调节。 实施例 1 The system of the eluent for column chromatography and the system for developing the thin layer chromatography of the purified compound include: A: dichloromethane and methanol system, B: n-hexane and ethyl acetate system, C: n-hexane and acetone System, D: hexamethylene, E: ethyl acetate, the volume ratio of the solvent is adjusted depending on the polarity of the compound, and may be adjusted by adding a small amount of triethylamine and an acidic or alkaline reagent. Example 1
l-Π-ί苯并呋喃 -2-基磺酰基) -5-(2-氟苯基) -1H-吡咯 -3-基) 甲基甲胺
L-Π-ίbenzofuran-2-ylsulfonyl)-5-(2-fluorophenyl)-1H-pyrrol-3-yl)methylmethylamine
1 a 1 b 1 c 1 1 a 1 b 1 c 1
第一步 First step
l-(苯并呋喃 -2-基磺酰基) -5-P-氟苯基) -1H-吡咯 -3-甲醛 冰浴下, 将 5-(2-氟苯基) -1H-吡咯 -3-甲醛 la (100 mg, 0.53 mmol, 根据现有文 献 WO2007026916制备而得)溶解于 4 mL四氢呋喃中, 向反应液中加入氢化钠 (106 mg, 60%),加毕,搅拌反应液 30分钟。再向反应液中加入苯并呋喃 -2-磺酰氯 lb (172 mg, 0.79 mmol, 根据现有文献 WO2006047302制备而得), 加毕, 室温搅拌 18小时。 加水淬灭反应, 反应液用乙酸乙酯萃取 (5 mLx3), 合并有机相, 水相用 6 M盐酸调 节 ρΗ<1, 用二氯甲烷萃取 (100 mLx2), 合并有机相, 无水硫酸钠干燥, 过滤, 滤 液减压浓縮,用硅胶柱色谱法以洗脱剂体系 B纯化所得残余物,得到标题产物 1- (苯 并呋喃 -2-基磺酰基) -5-(2-氟苯基) -1H-吡咯 -3-甲醛 lc (131 mg, 白色固体), 产率: 67.2%。 1-(2-Fluorophenyl)-1H-pyrrole-3 under ice bath with l-(benzofuran-2-ylsulfonyl)-5-P-fluorophenyl)-1H-pyrrole-3-carbaldehyde - Formaldehyde la (100 mg, 0.53 mmol, prepared according to the prior art WO2007026916) was dissolved in 4 mL of tetrahydrofuran, sodium hydride (106 mg, 60%) was added to the reaction mixture, and the reaction mixture was stirred for 30 minutes. Further, benzofuran-2-sulfonyl chloride lb (172 mg, 0.79 mmol, prepared according to the prior art WO2006047302) was added to the reaction mixture, and the mixture was stirred at room temperature for 18 hours. The reaction mixture was quenched with water and EtOAc (EtOAc (EtOAc)EtOAc. The mixture was dried, filtered, and evaporated tolululululululululululululululululululululu -1H-pyrrole-3-carbaldehyde lc (131 mg, white solid), Yield: 67.2%.
MS m/z (ESI): 370.1 [M+l] MS m/z (ESI): 370.1 [M+l]
第二步 Second step
l-G-(苯并呋喃 -2-基磺酰基) 氟苯基) -1H-吡咯 -3-基) -N-甲基甲胺 将 1- (;苯并呋喃 -2-基磺酰基) -5-0氟苯基) -1H-吡咯 -3-甲醛 lc (130 mg, 0.35 mmol)溶解于 1.5 mL 甲胺醇溶液 (25%)中,搅拌 2小时。加入硼氢化钠 (40 mg, 1.05 mmol), 加毕, 搅拌反应 2小时。 反应液减压浓縮, 用硅胶柱色谱法以洗脱剂体系 A 纯化所得残余物, 得到标题产物 1-(1-(苯并呋喃 -2-基磺酰基) -5-(2-氟苯基) -1H- 吡咯 -3-基) 甲基甲胺 l C57 mg, 黄色油状物), 产率: 42.5%。 lG-(benzofuran-2-ylsulfonyl)fluorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine 1-(;benzofuran-2-ylsulfonyl)-5 -0 fluorophenyl) -1H-pyrrole-3-carbaldehyde lc (130 mg, 0.35 mmol) was dissolved in 1.5 mL of methylamine (25%) and stirred for 2 hr. Sodium borohydride (40 mg, 1.05 mmol) was added, and the reaction was stirred for 2 hours. The reaction mixture was concentrated under reduced pressure. mjjjjjjjjjjjj -1H-pyrrol-3-yl)methylmethylamine 1 C57 mg, yellow oil), Yield: 42.5%.
MS m/z (ESI): 385.2 [M+l] MS m/z (ESI): 385.2 [M+l]
1H NMR (400 MHz, COCl3-d6): δ 7.64-7.66 (m, 1H), 7.50-7.53 (m,3H), 7.35-7.46 (m, 2H) 7.27-7.27 (m, 1H), 7.13-7.17 (m, 1Η),7.04-7.09 (m, 3H), 6.40 (m, 2H), 3.75 (s, 2H), 2.53 (s, 3H)
实施例 21H NMR (400 MHz, COCl 3 -d 6 ): δ 7.64-7.66 (m, 1H), 7.50-7.53 (m, 3H), 7.35-7.46 (m, 2H) 7.27-7.27 (m, 1H), 7.13 -7.17 (m, 1Η), 7.04-7.09 (m, 3H), 6.40 (m, 2H), 3.75 (s, 2H), 2.53 (s, 3H) Example 2
-Π-ί苯并呋喃 -2-基磺酰基) -5-(2-氯苯基) -1H-吡咯 -3-基) -N-甲基甲胺 -Π-ίbenzofuran-2-ylsulfonyl)-5-(2-chlorophenyl)-1H-pyrrole-3-yl)-N-methylmethylamine
第一步 First step
((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔丁酯 冰浴下, 将氢化纳 (0.5 g, 60%)加入到 20 mL四氢呋喃溶液中, 分批加入 ((5-溴 -1H-吡咯 -3-基)甲基 X甲基)氨基甲酸叔丁酯 2a (1.80 g, 6.22 mmol, 根据现有文献 WO2008108380制备而得), 加毕, 搅拌反应 30分钟。 向反应液中分批加入苯并呋 喃 -2-磺酰氯 lb (1.34 g, 6.22 mmol), 加毕, 撤去冰浴, 反应液室温搅拌 2小时。 用 10 mL饱和氯化铵溶液淬灭反应, 加入 20 mL水, 用乙酸乙酯萃取 (30 mLx4), 合 并有机相, 用饱和氯化钠溶液洗涤 (lO rnLx l), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用硅胶柱色谱法以洗脱剂体系 C纯化所得残余物, 得到标题产物 ((1- (苯并呋喃 -2- 基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (1.45 g, 浅黄色固 体), 产率: 49.7%。 ((1-(benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl) (methyl)carbamic acid tert-butyl ester, under ice bath, sodium hydride (0.5 g , 60%) was added to 20 mL of tetrahydrofuran solution, and ((5-bromo-1H-pyrrol-3-yl)methylxmethyl)carbamic acid tert-butyl ester 2a (1.80 g, 6.22 mmol, according to the present Prepared by the literature WO2008108380), after the addition, the reaction was stirred for 30 minutes. To the reaction liquid, benzofuran-2-sulfonyl chloride lb (1.34 g, 6.22 mmol) was added portionwise, and the mixture was stirred and evaporated, and the mixture was stirred at room temperature for 2 hours. The reaction was quenched with 10 mL of aq. EtOAc. EtOAc (EtOAc m. Filtration, concentrating the filtrate, and the residue obtained from silica gel column chromatography elute -Methyl)methyl (meth)carbamate tert-butyl ester 2b (1.45 g, pale yellow solid), Yield: 49.7%.
MS m/z (ESI): 413.1 [M-55] MS m/z (ESI): 413.1 [M-55]
第二步 Second step
((1- (苯并呋喃 -2-基磺酰基) -5-(2-氯苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔丁酯 将 ((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (100 mg, 0.21 mmol)、2-氯-苯硼酸 (34 mg, 0.23 mmol)和碳酸钠 (34 mg, 0.32 mmol) 依次加入到 6 mL的 1,4-二氧六环和水 (V/V=5: l)混合溶剂中, 加毕, 搅拌均匀, 再 加入 [1,1'-双 (;二苯基膦基)二茂铁]二氯化钯 (15 mg, 0.02 mmol), 加热至 100°C, 搅 拌反应 2小时。 向反应液中加入 50 mL乙酸乙酯, 再用饱和氯化钠溶液洗涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C纯化所得 残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5-(2-氯苯基) -1H-吡咯 -3-基)甲 基 X甲基)氨基甲酸叔丁酯 2c (47 mg, 浅黄色油状物), 产率: 44.8%。 ((1-(benzofuran-2-ylsulfonyl)-5-(2-chlorophenyl)-1H-pyrrol-3-yl)methyl) (methyl)carbamic acid tert-butyl ester ((1) - (benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 2b (100 mg, 0.21 mmol), 2-chloro- Phenylboronic acid (34 mg, 0.23 mmol) and sodium carbonate (34 mg, 0.32 mmol) were added to 6 mL of a mixed solvent of 1,4-dioxane and water (V/V=5:1). After stirring well, [1,1'-bis(;diphenylphosphino)ferrocene]palladium dichloride (15 mg, 0.02 mmol) was added, and the mixture was heated to 100 ° C, and the reaction was stirred for 2 hours. 50 mL of ethyl acetate was added to the reaction mixture, and the mixture was washed with a saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated, and the residue was purified by thin layer chromatography To give the title product ((1-(benzofuran-2-ylsulfonyl)-5-(2-chlorophenyl)-1H-pyrrol-3-yl)methylxmethyl)carbamic acid tert-butyl ester 2c (47 mg, light yellow oil), Yield: 44.8%.
MS m/z (ESI): 445.2 [M-55]
第三步 MS m/z (ESI): 445.2 [M-55] third step
l-G-(苯并呋喃 -2-基磺酰基) 氯苯基) -1H-吡咯 -3-基) -N-甲基甲胺 将 1-(苯并呋喃 -2-基磺酰基) 氯苯基) -1H-吡咯 -3-基)甲基 X甲基) 氨基甲 酸叔丁酯 2c (47 mg, 0.09 mmol)加入到5 mL二氯甲烷和三氟醋酸(V/V=4: l)混合溶 剂中, 搅拌反应 1小时。 反应液减压浓縮后加入 5 mL氨水和 40 mL二氯甲烷, 搅拌 均匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展 开剂体系 A纯化所得残余物, 得到标题产物 1-(1- (苯并呋喃 -2-基磺酰基) -5-(2-氯苯 基) -1H-吡咯 -3-基) 甲基甲胺 2 C23 mg, 浅黄色油状物), 产率: 49.0%。 lG-(benzofuran-2-ylsulfonyl)chlorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine 1-(benzofuran-2-ylsulfonyl)chlorophenyl -1H-pyrrol-3-yl)methyl X methyl) tert-butyl carbamate 2c (47 mg, 0.09 mmol) was added to 5 mL of dichloromethane and trifluoroacetic acid (V/V = 4: l). The solvent was stirred for 1 hour in a solvent. The reaction solution was concentrated under reduced pressure, and then 5 mL aqueous ammonia and 40 mL of dichloromethane were added, and the mixture was stirred and then allowed to stand. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. The residue obtained was purified to give the title product 1-(1-(benzofuran-2-ylsulfonyl)-5-(2-chlorophenyl)-1H-pyrrol-3-yl)methylmethylamine 2 C23 Mg, light yellow oil), Yield: 49.0%.
MS m/z (ESI): 401.2 [M+l] MS m/z (ESI): 401.2 [M+l]
1H NMR (400 MHz, CDC13) δ 7.60-7.62 (m, 1H), 7.45-7.50 (m, 3H), 7.33-7.35 (m, 3H) 7.27-7.32 (m, 2H), 7.01-7.02 (m, 1H), 6.31-6.32 (m, 1H), 3.71 (s, 2H), 2.50 (s, 3H) 实施例 3 1H NMR (400 MHz, CDC1 3 ) δ 7.60-7.62 (m, 1H), 7.45-7.50 (m, 3H), 7.33-7.35 (m, 3H) 7.27-7.32 (m, 2H), 7.01-7.02 (m , 1H), 6.31-6.32 (m, 1H), 3.71 (s, 2H), 2.50 (s, 3H) Example 3
l-Π-ί苯并呋喃 -2-基磺酰基) - -(4-氟苯基) -1H-吡咯 -3-基) 甲基甲胺 L-Π-ίbenzofuran-2-ylsulfonyl)--(4-fluorophenyl)-1H-pyrrole-3-yl)methylmethylamine
第一步 First step
((1- (苯并呋喃 -2-基磺酰基) -5-(4-氟苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔丁 将 ((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (150 mg, 0.32 mmol) 4-氟苯硼酸 3a (49 mg, 0.35 mmol)和碳酸钠 (51 mg, 0.48 mmol)依次加入到 6 mL的 1, 4-二氧六环和水 (V/V=5: l)混合溶剂中, 加毕, 搅拌均 匀,再加入 [1,1'-双 (二苯基膦基)二茂铁]二氯化钯 (23 mg, 0.03 mmol),加热至 100°C, 搅拌反应 2小时。 向反应液中加入 50 mL乙酸乙酯, 再用饱和氯化钠溶液洗涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C纯化所得 残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5-(4-氟苯基) -1H-吡咯 -3-基)甲
基) (甲基)氨基甲酸叔丁酯 3b (72 mg, 浅黄色油状物), 产率: 46.5%。 ((1-(benzofuran-2-ylsulfonyl)-5-(4-fluorophenyl)-1H-pyrrol-3-yl)methyl) (methyl)carbamic acid tert-butyl ((1- (benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 2b (150 mg, 0.32 mmol) 4-fluorophenylboronic acid 3a (49 mg, 0.35 mmol) and sodium carbonate (51 mg, 0.48 mmol) were added to 6 mL of a mixture solvent of 1,4-dioxane and water (V/V=5:1), added, stirred. After uniform addition, [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (23 mg, 0.03 mmol) was added, and the mixture was heated to 100 ° C, and the reaction was stirred for 2 hours. 50 mL of ethyl acetate was added to the reaction mixture, and the mixture was washed with a saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated, and the residue was purified by thin layer chromatography , the title product ((1-(benzofuran-2-ylsulfonyl)-5-(4-fluorophenyl)-1H-pyrrol-3-yl)) Tert-butyl (methyl)carbamate 3b (72 mg, pale yellow oil), yield: 46.5%.
MS m/z (ESI): 429.2 [M-55] MS m/z (ESI): 429.2 [M-55]
第二步 Second step
l-G-(苯并呋喃 -2-基磺酰基) -5-(4-氟苯基) -1H-吡咯 -3-基) -N-甲基甲胺 将 1-(苯并呋喃 -2-基磺酰基) -5-(4-氟苯基) -1H-吡咯 -3-基)甲基 X甲基) 氨基甲 酸叔丁酯 3b (72 mg, 0.15 mmol)加入到5 mL二氯甲烷和三氟醋酸(V/V=4:l)混合溶 剂中, 搅拌反应 1小时。 反应液减压浓縮后加入 5 mL氨水和 40 mL二氯甲烷, 搅拌 均匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展 开剂体系 A纯化所得残余物, 得到标题产物 1-(1- (苯并呋喃 -2-基磺酰基) -5-(4-氟苯 基) -1H-吡咯 -3-基) 甲基甲胺 3 C27 mg, 浅黄色固体), 产率: 47.4%。 L-(benzofuran-2-ylsulfonyl)-5-(4-fluorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine 1-(benzofuran-2-yl) Sulfonyl)-5-(4-fluorophenyl)-1H-pyrrol-3-yl)methylXmethyl)-tert-butyl carbamate 3b (72 mg, 0.15 mmol) was added to 5 mL of dichloromethane and In a mixed solvent of fluoroacetic acid (V/V = 4:1), the reaction was stirred for 1 hour. The reaction solution was concentrated under reduced pressure, and then 5 mL aqueous ammonia and 40 mL of dichloromethane were added, and the mixture was stirred and then allowed to stand. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. The residue obtained is purified to give the titled product 1-(1-(benzofuran-2-ylsulfonyl)-5-(4-fluorophenyl)-1H-pyrrol-3-yl)methylmethylamine 3 C27 Mg, pale yellow solid), Yield: 47.4%.
MS m/z (ESI): 385.2 [M+l] MS m/z (ESI): 385.2 [M+l]
1H NMR (400 MHz, CDC13) δ 7.58-7.60 (m, 1H), 7.47-7.48 (m, 2H), 7.40-7.42 (m, 1H) 7.28-7.31 (m, 1H), 7.25-7.27 (m, 2H), 6.96-6.99 (m, 3H), 6.24-6.25 (m, 1H), 3.66 (s, 2H), 2.49 (s, 3H) 实施例 4 1H NMR (400 MHz, CDC1 3 ) δ 7.58-7.60 (m, 1H), 7.47-7.48 (m, 2H), 7.40-7.42 (m, 1H) 7.28-7.31 (m, 1H), 7.25-7.27 (m , 2H), 6.96-6.99 (m, 3H), 6.24-6.25 (m, 1H), 3.66 (s, 2H), 2.49 (s, 3H) Example 4
l-Π-ί苯并呋喃 -2-基磺酰基) -5-(3-甲氧基苯基) -1H-吡咯 -3-基) -N-甲基甲胺 L-Π-ίbenzofuran-2-ylsulfonyl)-5-(3-methoxyphenyl)-1H-pyrrole-3-yl)-N-methylmethylamine
第一步 first step
1- (苯并呋喃 -2-基磺酰基) -5- 3-羟基苯基 )-lH-吡咯 -3-基)甲基 X甲基)氨基甲酸叔丁 酯 1-(benzofuran-2-ylsulfonyl)-5-hydroxyphenyl)-lH-pyrrole-3-yl)methyl X-methyl)carbamic acid tert-butyl ester
将 ((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (300 mg, 0.64 mmol) 3-羟基苯硼酸 4a (97 mg, 0.71 mmol)和碳酸钠(188 mg, 0.96 mmol)依次加入到 12 mL的 1,4-二氧六环和水 (V/V=5:l)混合溶剂中, 加毕, 搅 拌均匀, 再加入 [1,1'-双 (;二苯基膦基)二茂铁]二氯化钯 C44 mg, 0.06 mmol), 加热至 100°C, 搅拌反应 2小时。 向反应液中加入 60 mL乙酸乙酯, 再用饱和氯化钠溶液洗
涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C纯 化所得残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5-(3-羟基苯基) -1H-吡咯 -3-基)甲基 X甲基)氨基甲酸叔丁酯 4b (160 mg, 浅黄色油状物), 产率: 51.8%。 MS m/z (ESI): 427.2 [M-55] ((1-(Benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 2b (300 mg, 0.64 mmol) 3 -Hydroxybenzeneboronic acid 4a (97 mg, 0.71 mmol) and sodium carbonate (188 mg, 0.96 mmol) were added to 12 mL of a mixture of 1,4-dioxane and water (V/V = 5:1). After the addition, the mixture was stirred well, and then [1,1'-bis(;diphenylphosphino)ferrocene]palladium dichloride (C44 mg, 0.06 mmol) was added thereto, and the mixture was heated to 100 ° C, and the reaction was stirred for 2 hours. Add 60 mL of ethyl acetate to the reaction solution and wash with saturated sodium chloride solution. The title compound ((1-(benzofuran-2-ylsulfonyl)) was obtained from EtOAc (EtOAc) -5-(3-Hydroxyphenyl)-1H-pyrrol-3-yl)methylXmethyl)carbamic acid tert-butyl ester 4b (160 mg,yield of pale yellow oil), yield: 51.8%. MS m/z (ESI): 427.2 [M-55]
第二步 Second step
((1- (苯并呋喃 -2-基磺酰基) -5-(3-甲氧基苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔 丁酯 ((1-(Benzofuran-2-ylsulfonyl)-5-(3-methoxyphenyl)-1H-pyrrole-3-yl)methyl) tert-butyl (methyl)carbamate
将 ((1- (苯并呋喃 -2-基磺酰基) -5-(3-羟基苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲 酸叔丁酯 4b C80 mg, 0.17 mmol)加入到 5 mL的 N,N-二甲基甲酰胺中, 冰浴冷却, 分批加入氢化钠 (12 mg, 0.25 mmol), 加毕, 搅拌反应 30分钟。 向反应液中加入碘 甲浣 (36 mg, 0.25 mmol), 加毕, 撤去冰浴, 室温搅拌反应 1小时。 向反应液中加 入 2 mL饱和氯化铵溶液淬灭反应, 加入 50 mL乙酸乙酯, 再用饱和氯化钠溶液洗涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C纯化 所得残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5-(3-甲氧基苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔丁酯 4c (37 mg, 浅黄色固体), 产率: 44.0%。 ((1-(Benzofuran-2-ylsulfonyl)-5-(3-hydroxyphenyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 4b C80 mg , 0.17 mmol) was added to 5 mL of N,N-dimethylformamide, cooled in an ice bath, sodium hydride (12 mg, 0.25 mmol) was added portionwise, and the reaction was stirred for 30 minutes. Iodoformamidine (36 mg, 0.25 mmol) was added to the reaction mixture, and the mixture was added, the ice bath was removed, and the mixture was stirred at room temperature for 1 hour. The reaction mixture was quenched by the addition of 2 mL of a saturated aqueous solution of ammonium chloride, and 50 mL of ethyl acetate was added, and then washed with saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and concentrated. The resulting residue was purified by chromatography eluting to afford titled product ((1-(benzofuran-2-ylsulfonyl)-5-(3-methoxyphenyl)-1H-pyrrole-3- Tert-butyl)methyl (meth)carbamate 4c (37 mg, pale yellow solid), yield: 44.0%.
MS m/z (ESI): 441.3 [M-55] MS m/z (ESI): 441.3 [M-55]
第三步 third step
1-(1- (苯并呋喃 -2-基磺酰基) -5-(3-甲氧基苯基) -1H-吡咯 -3-基) -N-甲基甲胺 将 ((1- (苯并呋喃 -2-基磺酰基) -5-(3-甲氧基苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基 甲酸叔丁酯 4c (37 mg, 0.07 1^^1)加入到5 二氯甲烷和三氟醋酸(^/¥=4:1)混合 溶剂中, 搅拌反应 1小时。 反应液减压浓縮后加入 3 mL氨水和 50 mL二氯甲烷, 搅 拌均匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以 展开剂体系 A纯化所得残余物,得到标题产物 1-(1- (苯并呋喃 -2-基磺酰基) -5-(3-甲氧 基苯基) -1H-吡咯 -3-基) 甲基甲胺 4 C12 mg, 浅黄色油状物), 产率: 40.0%。 MS m/z (ESI): 397.2 [M+l] 1-(1-(benzofuran-2-ylsulfonyl)-5-(3-methoxyphenyl)-1H-pyrrol-3-yl)-N-methylmethylamine ((1-( Benzofuran-2-ylsulfonyl)-5-(3-methoxyphenyl)-1H-pyrrol-3-yl)methyl) tert-butyl (methyl)carbamate 4c (37 mg, 0.07 1 ^^1) was added to a mixed solvent of 5 dichloromethane and trifluoroacetic acid (^/¥=4:1), and the reaction was stirred for 1 hour. The reaction solution was concentrated under reduced pressure, and then 3 mL aqueous ammonia and 50 mL of dichloromethane were added, and the mixture was stirred and then allowed to stand. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. The residue obtained is purified to give the title product 1-(1-(benzofuran-2-ylsulfonyl)-5-(3-methoxyphenyl)-1H-pyrrol-3-yl)methylmethylamine 4 C12 mg, pale yellow oil), Yield: 40.0%. MS m/z (ESI): 397.2 [M+l]
1H NMR (400 MHz, CDC13) δ 7.56-7.58 (m, 1H), 7.46-7.47 (m, 2H), 7.40-7.42 (m, 1H) 7.30-7.32 (m, 1H), 7.17-7.19 (m, 1H), 6.91-6.92 (m, 1H), 6.78-6.87 (m, 2H), 6.77-6.78 (m, 1H), 6.24-6.25 (m, 1H), 3.67 (s, 3H), 3.66 (s, 2H), 2.48 (s, 3H) 实施例 5 1H NMR (400 MHz, CDC1 3 ) δ 7.56-7.58 (m, 1H), 7.46-7.47 (m, 2H), 7.40-7.42 (m, 1H) 7.30-7.32 (m, 1H), 7.17-7.19 (m , 1H), 6.91-6.92 (m, 1H), 6.78-6.87 (m, 2H), 6.77-6.78 (m, 1H), 6.24-6.25 (m, 1H), 3.67 (s, 3H), 3.66 (s , 2H), 2.48 (s, 3H) Example 5
苯并呋喃 -2-基磺酰基) -5-(3- (环丙基甲氧基)苯基) -1H-吡咯 -3-基) -N-甲基甲胺
Benzofuran-2-ylsulfonyl)-5-(3-(cyclopropylmethoxy)phenyl)-1H-pyrrol-3-yl)-N-methylmethylamine
((1- (苯并呋喃 -2-基磺酰基) -5-(3- (环丙基甲氧基)苯基) -1H-吡咯 -3-基)甲基) (甲基)氨 基甲酸叔丁酯 ((1-(benzofuran-2-ylsulfonyl)-5-(3-(cyclopropylmethoxy)phenyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid Tert-butyl ester
氩气氛保护下, 依次将 ((1- (苯并呋喃 -2-基磺酰基) -5-(3-羟基苯基) -1H-吡咯 -3- 基)甲基) (甲基)氨基甲酸叔丁酯 4b (80 mg, 0.17 mmol)、溴甲基环丙烷 5a (45 mg, 0.33 mmol)和碳酸钾 (35 mg, 0.26 mmol)加入到 5 mL的 N,N-二甲基甲酰胺中, 加热 至 60°C, 搅拌反应 5小时。 向反应液中加入 50 mL乙酸乙酯, 再用饱和氯化钠溶液 洗涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C 纯化所得残余物, 得到标题产物 (G- (苯并呋喃 -2-基磺酰基) -5- H环丙基甲氧基)苯 基) -1H-吡咯 -3-基)甲基 X甲基)氨基甲酸叔丁酯 5b (45 mg, 浅黄色固体), 产率: 50.6%。 Under the protection of an argon atmosphere, ((1-(benzofuran-2-ylsulfonyl)-5-(3-hydroxyphenyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-Butyl ester 4b (80 mg, 0.17 mmol), bromomethylcyclopropane 5a (45 mg, 0.33 mmol) and potassium carbonate (35 mg, 0.26 mmol) were added to 5 mL of N,N-dimethylformamide Heat to 60 ° C and stir the reaction for 5 hours. 50 mL of ethyl acetate was added to the reaction mixture, and the mixture was washed with a saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. , the title product (G-(benzofuran-2-ylsulfonyl)-5-H cyclopropylmethoxy)phenyl)-1H-pyrrol-3-yl)methylxmethyl)carbamic acid Butyrate 5b (45 mg, pale yellow solid), Yield: 50.6%.
MS m/z (ESI): 481.3 [M-55] MS m/z (ESI): 481.3 [M-55]
第二步 Second step
1-G- (苯并呋喃 -2-基磺酰基) -5-(3- (环丙基甲氧基)苯基) -1H-吡咯 -3-基) -N-甲基 甲胺 1-G-(benzofuran-2-ylsulfonyl)-5-(3-(cyclopropylmethoxy)phenyl)-1H-pyrrole-3-yl)-N-methylmethylamine
将 ((1- (苯并呋喃 -2-基磺酰基) -5-(3- (环丙基甲氧基)苯基) -1H-吡咯 -3-基)甲 基 X甲基)氨基甲酸叔丁酯 5b (45 mg, 0.08 mmol)加入到 5 mL二氯甲烷和三氟醋酸 (V/V=4: l)混合溶剂中, 搅拌反应 1小时。 反应液减压浓縮后加入 3 mL氨水和 40 mL 二氯甲烷, 搅拌均匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 A纯化所得残余物, 得到标题产物 1-(1- (苯并呋喃 -2-基磺 酰基 )-5-(3- (环丙基甲氧基)苯基) -1H-吡咯 -3-基) 甲基甲胺 5 (14 mg, 浅黄色固 体), 产率: 37.8%。 ((1-(Benzofuran-2-ylsulfonyl)-5-(3-(cyclopropylmethoxy)phenyl)-1H-pyrrol-3-yl)methylXmethyl)carbamic acid tert-Butyl ester 5b (45 mg, 0.08 mmol) was added to a mixed solvent of 5 mL of dichloromethane and trifluoroacetic acid (V/V = 4:1), and the reaction was stirred for 1 hour. The reaction solution was concentrated under reduced pressure, and then, 3 mL aqueous ammonia and 40 mL of dichloromethane were added, and the mixture was stirred and then allowed to stand. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. A resulting residue was purified to give the title product l- (l- (benzofuran-2-yl-sulfonyl) - 5 - (3- (cyclopropylmethoxy) phenyl) lH-pyrrol-3-yl Methylmethylamine 5 (14 mg, pale yellow solid), Yield: 37.8%.
MS m/z (ESI): 437.5 [M+l] MS m/z (ESI): 437.5 [M+l]
1H NMR (400 MHz, CDC13) δ 7.56-7.58 (m, 1H), 7.46-7.47 (m, 2H), 7.40-7.42 (m, 1H) 7.30-7.32 (m, 1H), 7.16-7.18 (m, 1H), 6.93-6.95 (m, 2H), 6.84-6.86 (m, 1H), 6.72-6.73
(m, 1H), 6.24-6.25 (m, 1H), 3.67 (s, 2H), 3.60-3.61 (m, 2H), 2.49 (s, 3H), 1.20-1.22 (m: 1H), 0.60-0.63 (m, 2H), 0.28-030 (m, 2H) 实施例 6 1H NMR (400 MHz, CDC1 3 ) δ 7.56-7.58 (m, 1H), 7.46-7.47 (m, 2H), 7.40-7.42 (m, 1H) 7.30-7.32 (m, 1H), 7.16-7.18 (m , 1H), 6.93-6.95 (m, 2H), 6.84-6.86 (m, 1H), 6.72-6.73 (m, 1H), 6.24-6.25 (m, 1H), 3.67 (s, 2H), 3.60-3.61 (m, 2H), 2.49 (s, 3H), 1.20-1.22 (m : 1H), 0.60-0.63 (m, 2H), 0.28-030 (m, 2H) Example 6
l-Π-ί苯并呋喃 -2-基磺酰基) -5 H-吡咯 -3-基) 甲基甲胺 L-Π-ίbenzofuran-2-ylsulfonyl)-5H-pyrrole-3-yl)methylmethylamine
第一步 First step
((1- (苯并呋喃 -2-基磺酰基) -5-(4-氯苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔丁 酯 ((1-(Benzofuran-2-ylsulfonyl)-5-(4-chlorophenyl)-1H-pyrrole-3-yl)methyl) tert-butyl (methyl)carbamate
将 ((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (100 mg, 0.21 mmol) 4-氯苯硼酸 6a (34 mg, 0.23 mmol)和碳酸钠 (34 mg, 0.32 mmol)依次加入到 6 mL的 1, 4-二氧六环和水 (V/V=5: l)混合溶剂中, 加毕, 搅拌均 匀,再加入 [1,1'-双 (二苯基膦基)二茂铁]二氯化钯 (15 mg, 0.02 mmol),加热至 100°C, 搅拌反应 2小时。 向反应液中加入 50 mL乙酸乙酯, 再用饱和氯化钠溶液洗涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C纯化所得 残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5-(4-氯苯基) -1H-吡咯 -3-基)甲 基 X甲基)氨基甲酸叔丁酯 6b (43 mg, 浅黄色固体), 产率: 41.0%。 ((1-(Benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 2b (100 mg, 0.21 mmol) 4 -Chlorophenylboronic acid 6a (34 mg, 0.23 mmol) and sodium carbonate (34 mg, 0.32 mmol) were added to 6 mL of a mixture solvent of 1,4-dioxane and water (V/V = 5:1). After the addition, the mixture was stirred well, and then [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (15 mg, 0.02 mmol) was added thereto, and the mixture was heated to 100 ° C, and the reaction was stirred for 2 hours. 50 mL of ethyl acetate was added to the reaction mixture, and the mixture was washed with a saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated, and the residue was purified by thin layer chromatography To give the title product ((1-(benzofuran-2-ylsulfonyl)-5-(4-chlorophenyl)-1H-pyrrol-3-yl)methylxmethyl)carbamic acid tert-butyl ester 6b (43 mg, pale yellow solid), Yield: 41.0%.
MS m/z (ESI): 413.1 [M-55] MS m/z (ESI): 413.1 [M-55]
第二步 Second step
l-G-(苯并呋喃 -2-基磺酰基) -5-(4-氯苯基) -1H-吡咯 -3-基) -N-甲基甲胺 将 ((1- (苯并呋喃 -2-基磺酰基) -5-(4-氯苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸 叔丁酯 6b (43 mg, 0.08 mmol)加入到 5 mL二氯甲烷和三氟醋酸 (V/V=4: l)混合溶剂 中, 搅拌反应 1小时。 反应液减压浓縮后加入 3 mL氨水和 50 mL二氯甲烷, 搅拌均 匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开 剂体系 A纯化所得残余物, 得到标题产物 1-(1- (苯并呋喃 -2-基磺酰基) -5-(4-氯苯 基) -1H-吡咯 -3-基) 甲基甲胺 6 C13 mg, 浅黄色油状物), 产率: 38.2%。
MS m/z (ESI): 401.2 [M+l] lG-(benzofuran-2-ylsulfonyl)-5-(4-chlorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine ((1-(benzofuran-2) -ylsulfonyl)-5-(4-chlorophenyl)-1H-pyrrol-3-yl)methyl) tert-butyl (methyl)carbamate 6b (43 mg, 0.08 mmol) added to 5 mL of dichloro In a mixed solvent of methane and trifluoroacetic acid (V/V = 4:1), the reaction was stirred for 1 hour. The reaction solution was concentrated under reduced pressure, and then 3 mL aqueous ammonia and 50 mL of dichloromethane were added, and the mixture was stirred and then allowed to stand. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. The residue obtained is purified to give the titled product 1-(1-(benzofuran-2-ylsulfonyl)-5-(4-chlorophenyl)-1H-pyrrol-3-yl)methylmethylamine 6 C13 Mg, light yellow oil), Yield: 38.2%. MS m/z (ESI): 401.2 [M+l]
1H NMR (400 MHz, CDC13) δ 7.58-7.60 (m, 1H), 7.47-7.48 (m, 2H), 7.41-7.42 (m, 1H) 7.32-7.34 (m, 1H), 7.29-7.32 (m, 1H), 7.22-7.24 (m, 2H), 7.21-7.22 (m, 1H), 7.01-7.02 (m, 1H), 6.26-6.27 (m, 1H), 3.68 (s, 2H), 2.48 (s, 3H) 实施例 7 1H NMR (400 MHz, CDC1 3 ) δ 7.58-7.60 (m, 1H), 7.47-7.48 (m, 2H), 7.41-7.42 (m, 1H) 7.32-7.34 (m, 1H), 7.29-7.32 (m , 1H), 7.22-7.24 (m, 2H), 7.21-7.22 (m, 1H), 7.01-7.02 (m, 1H), 6.26-6.27 (m, 1H), 3.68 (s, 2H), 2.48 (s , 3H) Example 7
1-Π- -2-基磺酰基) -5-(2,3- -5-基) -1H-吡咯 -3-基) -N-甲基甲胺 1-Π--2-ylsulfonyl)-5-(2,3- -5-yl)-1H-pyrrole-3-yl)-N-methylmethylamine
第一步 First step
((1- (苯并呋喃 -2-基磺酰基) -5-(2,3-二氢苯并呋喃 -5-基) -1H-吡咯 -3-基)甲基 )(甲基)氨 基甲酸叔丁酯 ((L- (benzofuran - 2 - yl) -5- (2, 3-dihydro-benzofuran-5-yl) lH-pyrrol-3-yl) methyl) (methyl) amino Tert-butyl formate
将 ((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (150 mg, 0.32 mmol) 2,3-二氢 -1-苯并呋喃 -5-基硼酸 7a (58 mg, 0.35 mmol)和 碳酸钠 (51 mg, 0.48 mmol)依次加入到 6 mL的 1, 4-二氧六环和水 (V/V=5:l)混合溶 剂中, 加毕, 搅拌均匀, 再加入 [1,1'-双 (二苯基膦基)二茂铁]二氯化钯 (23 mg, 0.03 mmol), 加热至 100°C, 搅拌反应 2小时。 向反应液中加入 50 mL乙酸乙酯, 再用饱 和氯化钠溶液洗涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以 展开剂体系 C纯化所得残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5-(2,3-二 氢苯并呋喃 -5-基) -1H-吡咯 -3-基)甲基 X甲基)氨基甲酸叔丁酯 7b (83 mg, 浅黄色油 状物), 产率: 51.2%。 ((1-(Benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 2b (150 mg, 0.32 mmol) 2 , 3-dihydro-1-benzofuran-5-ylboronic acid 7a (58 mg, 0.35 mmol) and sodium carbonate (51 mg, 0.48 mmol) were added sequentially to 6 mL of 1,4-dioxane and water (V/V=5:1) in a mixed solvent, add and stir well, then add [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (23 mg, 0.03 mmol) Heat to 100 ° C and stir the reaction for 2 hours. 50 mL of ethyl acetate was added to the reaction mixture, and the mixture was washed with a saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated, and the residue was purified by thin layer chromatography , the title product is obtained ((1-(benzofuran-2-ylsulfonyl)-5-(2,3-dihydrobenzofuran-5-yl)-1H-pyrrol-3-yl)methyl-X- Tert-butyl carbamate 7b (83 mg, light yellow oil), yield: 51.2%.
MS m/z (ESI): 453.3 [M-55] MS m/z (ESI): 453.3 [M-55]
第二步 Second step
l-G-(苯并呋喃 -2-基磺酰基) -5-(2,3-二氢苯并呋喃 -5-基) -1H-吡咯 -3-基) -N-甲基甲胺 将 1 苯并呋喃 -2-基磺酰基) -5-(2,3-二氢苯并呋喃 -5-基) -1H-吡咯 -3-基)甲 基 X甲基)氨基甲酸叔丁酯 7b (83 mg, 0.16 mmol)加入到 8 mL二氯甲烷和三氟醋酸 (V/V=3:l)混合溶剂中, 搅拌反应 1小时。 反应液减压浓縮后加入 2 mL氨水和 50 mL
二氯甲烷, 搅拌均匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 A纯化所得残余物, 得到标题产物 1-(1- (苯并呋喃 -2-基磺 酰基) -5-(2,3-二氢苯并呋喃 -5-基) -1H-吡咯 -3-基) 甲基甲胺 7 36 mg,浅黄色油状 物), 产率: 53.7%。 lG-(benzofuran-2-ylsulfonyl)-5-(2,3-dihydrobenzofuran-5-yl)-1H-pyrrol-3-yl)-N-methylmethylamine 1 benzene And furan-2-ylsulfonyl)-5-(2,3-dihydrobenzofuran-5-yl)-1H-pyrrol-3-yl)methylXmethyl)carbamic acid tert-butyl ester 7b (83 Mg, 0.16 mmol) was added to 8 mL of a mixed solvent of dichloromethane and trifluoroacetic acid (V/V = 3:1), and the reaction was stirred for 1 hour. The reaction solution was concentrated under reduced pressure, then 2 mL aqueous ammonia and 50 mL Dichloromethane, the mixture was stirred, and the mixture was partitioned. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. (benzofuran-2-ylsulfonyl)-5-(2,3-dihydrobenzofuran-5-yl)-1H-pyrrol-3-yl)methylmethylamine 7 36 mg, light yellow oil ), Yield: 53.7%.
MS m/z (ESI): 409.3 [M+l] MS m/z (ESI): 409.3 [M+l]
1H NMR (400 MHz, CDC13) δ 7.58-7.60 (m, 1H), 7.47-7.49 (m, 2H), 7.37-7.38 (m, 1H) 7.33-7.35 (m, 1H), 7.04-7.05 (m, 1H), 6.96-6.98 (m, 2H), 6.67-6.69 (m, 1H), 6.17-6.18 (m, 1H), 4.58 (t, / = 8.8 Hz, 2H), 3.65 (s, 2H), 3.07 (t, / = 8.8 Hz, 2H), 2.48 (s, 3H) 实施例 8 1H NMR (400 MHz, CDC1 3 ) δ 7.58-7.60 (m, 1H), 7.47-7.49 (m, 2H), 7.37-7.38 (m, 1H) 7.33-7.35 (m, 1H), 7.04-7.05 (m , (1,1H) 3.07 (t, / = 8.8 Hz, 2H), 2.48 (s, 3H) Example 8
l-Π-ί苯并呋喃 -2-基磺酰基) ) -1H-吡咯 -3-基) 甲基甲胺 L-Π-ίbenzofuran-2-ylsulfonyl) ) -1H-pyrrole-3-yl)methylmethylamine
第一步 First step
((1- (苯并呋喃 -2-基磺酰基) -5-(3-氯苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔丁酯 将 ((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (100 mg, 0.21 mmol) 3-氯苯硼酸 8a (34 mg, 0.23 mmol)和碳酸钠 (34 mg, 0.32 mmol)依次加入到 6 mL的 1, 4-二氧六环和水 (V/V=5:l)混合溶剂中, 加毕, 搅拌均 匀,再加入 [1,1'-双 (二苯基膦基)二茂铁]二氯化钯 (15 mg, 0.02 mmol),加热至 100°C, 搅拌反应 3小时。 向反应液中加入 40 mL乙酸乙酯, 再用饱和氯化钠溶液洗涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C纯化所得 残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5-(3-氯苯基) -1H-吡咯 -3-基)甲 基 X甲基)氨基甲酸叔丁酯 8b (51 mg, 浅黄色固体), 产率: 48.6%。 ((1-(benzofuran-2-ylsulfonyl)-5-(3-chlorophenyl)-1H-pyrrol-3-yl)methyl) (methyl)carbamic acid tert-butyl ester ((1) - (benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 2b (100 mg, 0.21 mmol) 3-chlorobenzeneboronic acid 8a (34 mg, 0.23 mmol) and sodium carbonate (34 mg, 0.32 mmol) were added to 6 mL of a mixed solvent of 1,4-dioxane and water (V/V=5:1), and the mixture was added. After stirring well, [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (15 mg, 0.02 mmol) was added, and the mixture was heated to 100 ° C, and the reaction was stirred for 3 hours. 40 mL of ethyl acetate was added to the reaction solution, and the mixture was washed with a saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated, and the residue was purified by a thin layer chromatography , the title product ((1-(benzofuran-2-ylsulfonyl)-5-(3-chlorophenyl)-1H-pyrrol-3-yl)methylxmethyl)carbamic acid tert-butyl ester 8b (51 mg, pale yellow solid), Yield: 48.6%.
MS m/z (ESI): 413.1 [M-55] MS m/z (ESI): 413.1 [M-55]
第二步 Second step
l-G-(苯并呋喃 -2-基磺酰基) -5- 3-氯苯基) -1H-吡咯 -3-基) -N-甲基甲胺 将 ((1- (苯并呋喃 -2-基磺酰基) -5-(3-氯苯基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸
叔丁酯 8b (51 mg, 0.10 1^^)1)加入到5 11 二氯甲烷和三氟醋酸(^/¥=4:1)混合溶剂 中, 搅拌反应 1小时。 反应液减压浓縮后加入 3 mL氨水和 40 mL二氯甲烷, 搅拌均 匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开 剂体系 A纯化所得残余物, 得到标题产物 1-(1- (苯并呋喃 -2-基磺酰基) -5-(3-氯苯 基) -1H-吡咯 -3-基) 甲基甲胺 8 C15 mg, 浅黄色油状物), 产率: 36.6%。 lG-(benzofuran-2-ylsulfonyl)-5-chlorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine ((1-(benzofuran-2-) Sulfamoyl)-5-(3-chlorophenyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-Butyl ester 8b (51 mg, 0.10 1 ^^) 1) was added to a mixed solvent of 5 11 dichloromethane and trifluoroacetic acid (^/¥ = 4:1), and the mixture was stirred for 1 hour. After the reaction mixture was concentrated under reduced pressure, 3 mL aqueous ammonia and 40 mL of dichloromethane were added, and the mixture was stirred and then allowed to stand. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. The residue obtained was purified to give the title product 1-(1-(benzofuran-2-ylsulfonyl)-5-(3-chlorophenyl)-1H-pyrrol-3-yl)methylmethylamine 8 C15 Mg, light yellow oil), Yield: 36.6%.
MS m/z (ESI): 401.2 [M+l] MS m/z (ESI): 401.2 [M+l]
1H NMR (400 MHz, CDC13) δ 7.59-7.61 (m, 1H), 7.48-7.50 (m, 2H), 7.41-7.42 (m, 1H) 7.34-7.35 (m, 2H), 7.24-7.27 (m, 3H), 7.06-7.07 (m, 1H), 6.28-6.29 (m, 1H), 3.67 (s, 2H), 2.48 (s, 3H) 实施例 9 1H NMR (400 MHz, CDC1 3 ) δ 7.59-7.61 (m, 1H), 7.48-7.50 (m, 2H), 7.41-7.42 (m, 1H) 7.34-7.35 (m, 2H), 7.24-7.27 (m , 3H), 7.06-7.07 (m, 1H), 6.28-6.29 (m, 1H), 3.67 (s, 2H), 2.48 (s, 3H) Example 9
1-Π- (苯并呋喃 -2-基磺酰基) - - (吡啶 -3-基) -1H-吡咯 -3-基) -N-甲基甲胺 1-Π-(benzofuran-2-ylsulfonyl)--(pyridin-3-yl)-1H-pyrrole-3-yl)-N-methylmethylamine
第一步 First step
((1- (苯并呋喃 -2-基磺酰基) -5- (吡啶 -3-基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔丁 酯 ((1-(Benzofuran-2-ylsulfonyl)-5-(pyridin-3-yl)-1H-pyrrole-3-yl)methyl) tert-butyl (methyl)carbamate
将 ((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (100 mg, 0.21 mmol) 吡啶 -3-硼酸 9a (29 mg, 0.23 mmol)和碳酸钠 (34 mg, 0.32 mmol)依次加入到 6 mL的 1, 4-二氧六环和水 (V/V=5:l)混合溶剂中, 加毕, 搅拌均 匀,再加入 [1,1'-双 (二苯基膦基)二茂铁]二氯化钯 (15 mg, 0.02 mmol),加热至 100°C, 搅拌反应 3小时。 向反应液中加入 50 mL乙酸乙酯, 再用饱和氯化钠溶液洗涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C纯化所得 残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5- (吡啶 -3-基) -1H-吡咯 -3-基)甲 基 X甲基)氨基甲酸叔丁酯 9b (43 mg, 浅黄色固体), 产率: 43.0%。 ((1-(Benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 2b (100 mg, 0.21 mmol) pyridine -3-Boronic acid 9a (29 mg, 0.23 mmol) and sodium carbonate (34 mg, 0.32 mmol) were added to 6 mL of 1,4-dioxane and water (V/V = 5:1) solvent. After the addition, the mixture was stirred well, and then [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (15 mg, 0.02 mmol) was added thereto, and the mixture was heated to 100 ° C, and the reaction was stirred for 3 hours. 50 mL of ethyl acetate was added to the reaction mixture, and the mixture was washed with a saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated, and the residue was purified by thin layer chromatography To give the title product ((1-(benzofuran-2-ylsulfonyl)-5-(pyridin-3-yl)-1H-pyrrol-3-yl)methylxmethyl)carbamic acid tert-butyl ester 9b (43 mg, pale yellow solid), Yield: 43.0%.
MS m/z (ESI): 468.2 [M+l] MS m/z (ESI): 468.2 [M+l]
第二步
l-G-(苯并呋喃 -2-基磺酰基) -5- (吡啶 -3-基) -1H-吡咯 -3-基) -N-甲基甲胺 将 1- (苯并呋喃 -2-基磺酰基) -5- (吡啶 -3-基) -1H-吡咯 -3-基)甲基 X甲基)氨基甲 酸叔丁酯 9b (43 mg, 0.09 mmol)加入到 2.5 mL二氯甲烷和三氟醋酸 (V/V=4: l)混合 溶剂中, 搅拌反应 1小时。 反应液减压浓縮后加入 2 mL氨水和 30 mL二氯甲烷, 搅 拌均匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以 展开剂体系 A纯化所得残余物, 得到标题产物 1-(1-(苯并呋喃 -2-基磺酰基) -5- (吡啶 -3-基) -1H-吡咯 -3-基) 甲基甲胺 9 (12 mg, 浅黄色油状物 产率: 35.3%。 Second step lG-(benzofuran-2-ylsulfonyl)-5-(pyridin-3-yl)-1H-pyrrol-3-yl)-N-methylmethylamine 1-(benzofuran-2-yl) Sulfonyl)-5-(pyridin-3-yl)-1H-pyrrol-3-yl)methylXmethyl)carbamic acid tert-butyl ester 9b (43 mg, 0.09 mmol) was added to 2.5 mL dichloromethane and In a mixed solvent of fluoroacetic acid (V/V = 4:1), the reaction was stirred for 1 hour. The reaction solution was concentrated under reduced pressure, and then 2 mL aqueous ammonia and 30 mL of dichloromethane were added, and the mixture was stirred and then allowed to stand. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. The residue obtained is purified to give the titled product 1-(1-(benzofuran-2-ylsulfonyl)-5-(pyridin-3-yl)-1H-pyrrol-3-yl)methylmethylamine 9 ( 12 mg, pale yellow oil Yield: 35.3%.
MS m/z (ESI): 368.2 [M+l] MS m/z (ESI): 368.2 [M+l]
1H NMR (400 MHz, CDC13) δ 8.61-8.63 (m, 1H), 8.46-8.47 (m, 1H), 7.76-7.78 (m, 1H) 7.60-7.62 (m, 1H), 7.46-7.49 (m, 3H), 7.31-7.34 (m, 2H), 7.06-7.07 (m, 1H), 6.35-6.36 (m, 1H), 3.69 (s, 2H), 2.49 (s, 3H) 实施例 10 1H NMR (400 MHz, CDC1 3 ) δ 8.61-8.63 (m, 1H), 8.46-8.47 (m, 1H), 7.76-7.78 (m, 1H) 7.60-7.62 (m, 1H), 7.46-7.49 (m , 3H), 7.31-7.34 (m, 2H), 7.06-7.07 (m, 1H), 6.35-6.36 (m, 1H), 3.69 (s, 2H), 2.49 (s, 3H) Example 10
1-Π- (苯并呋喃 -2-基磺酰基) - ) -1H-吡咯 -3-基) -N-甲基甲胺 1-Π-(benzofuran-2-ylsulfonyl)-)-1H-pyrrole-3-yl)-N-methylmethylamine
第一步 First step
((1- (苯并呋喃 -2-基磺酰基) -5- (吡啶 -4-基) -1H-吡咯 -3-基)甲基) (甲基)氨基甲酸叔丁 将 ((1- (苯并呋喃 -2-基磺酰基) -5-溴 -1H-吡咯 -3-基)甲基 )(甲基)氨基甲酸叔丁酯 2b (100 mg, 0.21 mmol)、 吡啶 -4-硼酸 10a (29 mg, 0.23 mmol)和碳酸钠 (34 mg, 0.32 mmol)依次加入到 6 mL的 1, 4-二氧六环和水 (V/V=5: l)混合溶剂中, 加毕, 搅 拌均匀, 再加入 [1,1'-双 (二苯基膦基)二茂铁]二氯化钯 (15 mg, 0.02 mmol), 加热至 100°C, 搅拌反应 3小时。 向反应液中加入 50 mL乙酸乙酯, 再用饱和氯化钠溶液洗 涤 (10 mLx2), 无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以展开剂体系 C纯 化所得残余物, 得到标题产物 ((1- (苯并呋喃 -2-基磺酰基) -5- (吡啶 -4-基) -1H-吡咯 -3- 基)甲基) (甲基)氨基甲酸叔丁酯 10b (51 mg, 浅黄色固体), 产率: 51.0%。
MS m/z (ESI): 468.3 [M+l] ((1-(benzofuran-2-ylsulfonyl)-5-(pyridin-4-yl)-1H-pyrrol-3-yl)methyl) (methyl)carbamic acid tert-butyl ((1- (benzofuran-2-ylsulfonyl)-5-bromo-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 2b (100 mg, 0.21 mmol), pyridine-4-boronic acid 10a (29 mg, 0.23 mmol) and sodium carbonate (34 mg, 0.32 mmol) were added to 6 mL of a mixed solvent of 1,4-dioxane and water (V/V=5:1), and added. After stirring well, [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (15 mg, 0.02 mmol) was added, and the mixture was heated to 100 ° C, and the reaction was stirred for 3 hours. 50 mL of ethyl acetate was added to the reaction mixture, and the mixture was washed with a saturated sodium chloride solution (10 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated, and the residue was purified by thin layer chromatography , the title product ((1-(benzofuran-2-ylsulfonyl)-5-(pyridin-4-yl)-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester 10b (51 mg, pale yellow solid), Yield: 51.0%. MS m/z (ESI): 468.3 [M+l]
第二步 Second step
l-G-(苯并呋喃 -2-基磺酰基) -5- (吡啶 -4-基) -1H-吡咯 -3-基) -N-甲基甲胺 将 1- (苯并呋喃 -2-基磺酰基) -5- (吡啶 -4-基) -1H-吡咯 -3-基)甲基 X甲基)氨基甲 酸叔丁酯 10b (51 mg, 0.11 mmol)加入到 5 mL二氯甲烷和三氟醋酸 (V/V=4:l)混合 溶剂中, 搅拌反应 1小时。 反应液减压浓縮后加入 5 mL氨水和 40 mL二氯甲烷, 搅 拌均匀后静置分层, 有机层用无水硫酸钠干燥, 过滤, 浓縮滤液, 用薄层层析以 展开剂体系 A纯化所得残余物, 得到标题产物 1-(1-(苯并呋喃 -2-基磺酰基) -5- (吡啶 -4-基) -1H-吡咯 -3-基) 甲基甲胺 10 (17 mg, 浅黄色油状物 产率: 42.5%。 MS m/z (ESI): 368.3 [M+l] lG-(benzofuran-2-ylsulfonyl)-5-(pyridin-4-yl)-1H-pyrrol-3-yl)-N-methylmethylamine 1-(benzofuran-2-yl) Sulfonyl)-5-(pyridin-4-yl)-1H-pyrrol-3-yl)methylXmethyl)carbamic acid tert-butyl ester 10b (51 mg, 0.11 mmol) was added to 5 mL dichloromethane and In a mixed solvent of fluoroacetic acid (V/V = 4:1), the reaction was stirred for 1 hour. The reaction solution was concentrated under reduced pressure, and then 5 mL aqueous ammonia and 40 mL of dichloromethane were added, and the mixture was stirred and then allowed to stand. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. The residue obtained is purified to give the titled product 1-(1-(benzofuran-2-ylsulfonyl)-5-(pyridin-4-yl)-1H-pyrrol-3-yl)methylmethylamine 10 ( Yield: 42.5%. MS m/z (ESI): 368.3 [M+l]
1H NMR (400 MHz, CDC13) δ 8.57-8.58 (m, 2H), 7.60-7.62 (m, 1H), 7.48-7.51 (m, 3H) 7.32-7.34 (m, 2H), 7.27-7.28 (m, 1H), 7.09-7.10 (m, 1H), 6.44-6.45 (m, 1H), 3.74 (s, 2H), 2.50 (s, 3H) 测试例: 1H NMR (400 MHz, CDC1 3 ) δ 8.57-8.58 (m, 2H), 7.60-7.62 (m, 1H), 7.48-7.51 (m, 3H) 7.32-7.34 (m, 2H), 7.27-7.28 (m , 1H), 7.09-7.10 (m, 1H), 6.44-6.45 (m, 1H), 3.74 (s, 2H), 2.50 (s, 3H) Test example:
H+/K+-ATPase生物学评价 Biological evaluation of H + /K + -ATPase
下面的体外筛选试验是用来测定本发明化合物对于 H+/K+-ATPase 酶活性的 抑制作用。 The following in vitro screening assays were used to determine the inhibition of H + /K + -ATPase enzymatic activity by the compounds of the invention.
实验材料及仪器: Experimental materials and instruments:
1、 猪胃粘膜微粒体 (富含 H+/K+-ATPaSe ) (自提) 1, pig gastric mucosa microsomes (rich in H + /K + -ATPa Se ) (self-lifting)
2、 ATP (sigma-aldrich, A1852-1VL) 2. ATP (sigma-aldrich, A1852-1VL)
3、 孔雀石绿 (Malachite green) (sigma-aldrich, 213020-25G) 3. Malachite green (sigma-aldrich, 213020-25G)
4、 钼酸铵 (Ammonium molybdate) (sigma-aldrich, 277908-5G)。 实验步骤简述如下: 4. Ammonium molybdate (sigma-aldrich, 277908-5G). The experimental steps are briefly described as follows:
一、 试剂准备 First, reagent preparation
1、化合物用 100%DMSO配制成合适的浓度: 10000, 1000, 100, 10, 1, O.lnM; 1. The compound is formulated in 100% DMSO to a suitable concentration: 10000, 1000, 100, 10, 1, O.lnM;
2、 缓冲液 1 : 50mmol/L HEPEs-Tris, 5mmol/L氯化镁, pH6.5; 2, buffer 1 : 50mmol / L HEPEs-Tris, 5mmol / L magnesium chloride, pH 6.5;
3、 缓冲液 2: 50mmol/L HEPEs-Tris, 5mmol/L氯化镁, pH6.5, 10mmol/L氯 化钾, pH=6.5; 3. Buffer 2: 50 mmol/L HEPEs-Tris, 5 mmol/L magnesium chloride, pH 6.5, 10 mmol/L potassium chloride, pH=6.5;
4、 ATP: 用缓冲液 1稀释 ATP至 2mM; 4. ATP: Dilute ATP to 2 mM with buffer 1;
5、 孔雀石绿溶液: 0.12% 孔雀石绿溶于 2.5摩尔硫酸, 7.5%钼酸铵和 11%的 Tween 20 使用时按 100:25:2 比例混合; 5, malachite green solution: 0.12% malachite green dissolved in 2.5 moles of sulfuric acid, 7.5% ammonium molybdate and 11% Tween 20 when used in a ratio of 100:25:2;
6、 猪胃粘膜微粒体 (富含 H+/K+-ATPase), 提取方法为蔗糖梯度离心: 把猪 胃用自来水清洗, 浸入 3mol/L浓盐水 1-2分钟, 然后擦干。 将胃粘膜分离, 剁碎, 然后悬于 0.25mol/L蔗糖, lmmol/LEDTA, 10mmol/Ltris-HCl溶液; 匀浆处理, (比 例 100g: 330ml, 充分均匀完成后再加 300ml)将获得的匀浆在 20000G离心 30分
钟, 去除沉淀; 取上清液在 100000G离心 90分钟,取沉淀; 把沉淀悬于 0.25mol/L 蔗糖溶液并在底部加入 0.25mol/L蔗糖加入 7.5%聚蔗糖, 100000G离心 5小时。 收集处于两液面层之间的物质, 用 0.25mol/L蔗糖溶液边摇晃边清洗, 获得的微粒 体酶放置于 -80°C保存备用。 二、 实验过程: 6, pig gastric mucosa microsomes (rich in H + /K + -ATPase), the extraction method is sucrose gradient centrifugation: the pig stomach is washed with tap water, immersed in 3mol / L concentrated brine for 1-2 minutes, and then wiped dry. The gastric mucosa was separated, mashed, and then suspended in 0.25 mol/L sucrose, 1 mmol/LEDTA, 10 mmol/L tris-HCl solution; homogenized, (100 g: 330 ml, fully uniform and then added 300 ml) The slurry is centrifuged at 20000G for 30 minutes. Clock, the precipitate was removed; the supernatant was centrifuged at 100,000 G for 90 minutes, and a precipitate was taken; the precipitate was suspended in a 0.25 mol/L sucrose solution and 0.25 mol/L sucrose was added to the bottom to add 7.5% polysucrose, and centrifuged at 100,000 G for 5 hours. The material between the two liquid layers was collected, washed with a 0.25 mol/L sucrose solution while shaking, and the obtained microsomal enzyme was stored at -80 ° C for storage. Second, the experimental process:
向 79ul 缓冲液 2中加入 lOul的胃粘膜微粒体 (H+/K+-ATPase ), 再加入 lul 的化合物溶液, 然后加入 lOul 2mM的 ATP启动反应。 在 37°C 反应 30分钟。 加 入 30ul孔雀石绿溶液终止反应, 室温平衡 20分钟, 在 620nm处读吸收光。 10 μl of gastric mucosal microsomes (H + /K + -ATPase ) were added to 79 ul of buffer 2, and then 1 ul of the compound solution was added, followed by the addition of 10 ul of 2 mM ATP to initiate the reaction. The reaction was carried out at 37 ° C for 30 minutes. The reaction was terminated by the addition of 30 ul of malachite green solution, equilibrated at room temperature for 20 minutes, and the absorbed light was read at 620 nm.
同时, 进行相同体积, 不加氯化钾的反应作为背景, 在计算酶活性时减去。 化合物的 IC5Q值可通过不同浓度下的抑制率计算得出。 At the same time, the same volume, without the addition of potassium chloride as a background, was subtracted from the calculation of the enzyme activity. The IC 5Q value of the compound can be calculated from the inhibition rates at different concentrations.
、 实验结果: 化合物的 IC, experimental results: IC of the compound
结论: 本发明化合物对 H+/K+-ATPase 具有明显的抑制活性。
Conclusion: The compounds of the present invention have significant inhibitory activity against H + /K + -ATPase.
Claims
1、 一种通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映 异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐: A compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or Its pharmaceutically acceptable salt:
其巾: Its towel:
R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被一个或多个选自 卤素、 氰基、 烷基、 卤代烷基、 羟烷基、 环烷基、 -OR6、 杂环基、 芳基、 杂芳基、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6或 -C(0)OR6的取代基所取代;R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC( 0) substituted with a substituent of R 6 or -C(0)OR 6 ;
R2选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述烷基、 环 烷基、 杂环基、 芳基或杂芳基任选进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0) NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6或 -C(0)OR6的取代基所取代; R 2 is selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or heteroaryl group is optionally further one Or a plurality selected from the group consisting of halogen, cyano, hydroxy, amino, alkyl, alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 Substituting a substituent of -C(0) NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC(0)R 6 or -C(0)OR 6 ;
R3选自氢原子或烷基; R 3 is selected from a hydrogen atom or an alkyl group;
R4或 R5各自独立地选自氢原子、 卤素、 氰基、 硝基、 羟基、 烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或多个选自卤素、 硝基、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6或 -C(0)OR6的取代基所取代; R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane The radical, alkoxy, cycloalkyl, heterocyclyl, aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, Alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 Substituted with a substituent of -C(0)R 6 , -OC(0)R 6 or -C(0)OR 6 ;
R6选自氢原子、 烷基、 羟基、 卤素、 烷氧基、 环烷基、 杂环基、 芳基或杂芳 基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选 进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所 取代; R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring The radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
R7或 R8各自独立地选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其 中所述的烷基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或 多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所取代; 且 R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group The aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted with a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
m为 0、 1或 2。
m is 0, 1, or 2.
2、 根据权利要求 1所述的通式(I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐, 其 中 R2为烷基。 2. A compound of the formula (I) according to claim 1 or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer thereof, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 2 is an alkyl group.
3、 根据权利要求 1或 2所述的通式( I )所示的化合物或其互变异构体、 内消 旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药用的 盐, 其中 R3为氢原子。 3. A compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to claim 1 or 2. Or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 3 is a hydrogen atom.
4、根据权利要求 1〜3任意一项所述的通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药 用的盐, 其中 R4为氢原子。 The compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 3; Isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 4 is a hydrogen atom.
5、根据权利要求 1〜4任意一项所述的通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药 用的盐, 其中 R5为氢原子。 The compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 4; Isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 5 is a hydrogen atom.
6、根据权利要求 1〜5任意一项所述的通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药 用的盐, 其中 R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被一个或 多个选自卤素或 -OR6的取代基所取代, 其中 R6为烷基, 所述的烷基任选进一步被 一个或多个选自环烷基的取代基所取代。 The compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 5; Isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is selected from aryl or heteroaryl, wherein the aryl or heteroaryl is optionally further selected from one or more selected from halogen or Substituted by a substituent of -OR 6 wherein R 6 is alkyl, said alkyl optionally being further substituted with one or more substituents selected from cycloalkyl.
7、 根据权利要求 1~6任意一项所述的通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药 用的盐, 其中 R1为芳基, 所述芳基任选进一步被一个或多个选自卤素或 -OR6的取 代基所取代, R6为烷基, 所述的烷基任选进一步被一个或多个选自环烷基的取代 基所取代。 The compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 6. Isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is an aryl group, which is optionally further substituted with one or more substituents selected from halogen or -OR 6 , R 6 is an alkyl group, which is optionally further substituted with one or more substituents selected from cycloalkyl groups.
8、 根据权利要求 1~6任意一项所述的通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药 用的盐, 其中 R1为杂芳基。 The compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 6. Isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein R 1 is heteroaryl.
9、 根据权利要求 1~8任意一项所述的通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药 用的盐, 其中该化合物为:
The compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 8. Isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, wherein the compound is:
10、 一种通式( I-A )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对 映异构体、 非对映异构体、 或其混 可药用的盐: 10. A compound of the formula (IA) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer, or a mixture thereof Salt:
( I-A ) ( I-A )
其中: among them:
R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被一个或多个选自 卤素、 氰基、 烷基、 卤代烷基、 羟烷基、 环烷基、 -OR6、 杂环基、 芳基、 杂芳基、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6或 -C(0)OR6的取代基所取代; R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC( 0) substituted with a substituent of R 6 or -C(0)OR 6 ;
R2选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述烷基、 环 浣基、 杂环基、 芳基或杂芳基任选进一步被一个或多个选自卤素、 氰基、 羟基、
氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6或 -C(0)OR6的取代基所取代; R 2 is selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cyclodecyl group, heterocyclic group, aryl group or heteroaryl group is optionally further one Or a plurality selected from the group consisting of halogen, cyano, hydroxyl, Amino, alkyl, alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 , Substituted by a substituent of S(0) m R 6 , -C(0)R 6 , -OC(0)R 6 or -C(0)OR 6 ;
R4或 R5各自独立地选自氢原子、 卤素、 氰基、 硝基、 羟基、 烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或多个选自卤素、 硝基、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6或 -C(0)OR6的取代基所取代; R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane The radical, alkoxy, cycloalkyl, heterocyclyl, aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, Alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 Substituted with a substituent of -C(0)R 6 , -OC(0)R 6 or -C(0)OR 6 ;
R6选自氢原子、 烷基、 羟基、 卤素、 烷氧基、 环烷基、 杂环基、 芳基或杂芳 基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选 进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所 取代; R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring The radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
R7或 R8各自独立地选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其 中所述的烷基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或 多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所取代; R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group The aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted by a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
PG为氨基保护基; 且 PG is an amino protecting group;
m为 0、 1或 2。 m is 0, 1, or 2.
11、 一种制备根据权利要求 1所述的通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药 用的盐的方法, 该方 11. A compound of the formula (I) according to claim 1 or a tautomer, a mesogen, a racemate, an enantiomer thereof, a diastereomer Method of the body, or a mixture thereof, or a pharmaceutically acceptable salt thereof, the method
通式 (I-A)化合物在溶剂中, 酸性条件下脱保护得到通式( I )化合物; 其中: PG为氨基保护基; 1^〜1 5的定义如权利要求 1中所述, 其中 R3优选 为氢原子。 Deprotection of a compound of the formula (IA) in a solvent under acidic conditions affords a compound of the formula (I); wherein: PG is an amino protecting group; 1^~1 5 is as defined in claim 1, wherein R 3 is preferred It is a hydrogen atom.
12、 一种通式( Ι-Β )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐:
其巾: 12. A compound of the formula (Ι-Β) or a tautomer, a mesogen, a racemate, an enantiomer, a diastereomer, or a mixture thereof , or a pharmaceutically acceptable salt thereof: Its towel:
R1选自芳基或杂芳基, 其中所述芳基或杂芳基任选进一步被一个或多个选自 卤素、 氰基、 烷基、 卤代烷基、 羟烷基、 环烷基、 -OR6、 杂环基、 芳基、 杂芳基、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6或 -C(0)OR6的取代基所取代;R 1 is selected from an aryl or heteroaryl group, wherein the aryl or heteroaryl group is further further selected from one or more selected from the group consisting of halogen, cyano, alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, OR 6 , heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 , -C(0)R 6 , -OC( 0) substituted with a substituent of R 6 or -C(0)OR 6 ;
R4或 R5各自独立地选自氢原子、 卤素、 氰基、 硝基、 羟基、 烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或多个选自卤素、 硝基、 氰基、 羟基、 氨基、烷基、 ^代烷基、羟烷基、烷氧基、环烷基、杂环基、芳基、杂芳基、 -NR7R8、 -C(0)NR7R8、 -S(0)mR6、 -C(0)R6、 -OC(0)R6或 -C(0)OR6的取代基所取代; R 4 or R 5 are each independently selected from a hydrogen atom, a halogen, a cyano group, a nitro group, a hydroxyl group, an alkyl group, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein said alkane The radical, alkoxy, cycloalkyl, heterocyclyl, aryl or heteroaryl are each independently optionally further selected from one or more selected from the group consisting of halogen, nitro, cyano, hydroxy, amino, alkyl, Alkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, aryl, heteroaryl, -NR 7 R 8 , -C(0)NR 7 R 8 , -S(0) m R 6 Substituted with a substituent of -C(0)R 6 , -OC(0)R 6 or -C(0)OR 6 ;
R6选自氢原子、 烷基、 羟基、 卤素、 烷氧基、 环烷基、 杂环基、 芳基或杂芳 基, 其中所述的烷基、 烷氧基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选 进一步被一个或多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所 取代; R 6 is selected from a hydrogen atom, an alkyl group, a hydroxyl group, a halogen, an alkoxy group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, alkoxy group, cycloalkyl group, heterocyclic ring The radical, aryl or heteroaryl are each, independently, optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkane. Substituted by a substituent of a heterocyclic group, a heterocyclic group, an aryl group, a heteroaryl group, a carboxyl group or a carboxylate group;
R7或 R8各自独立地选自氢原子、 烷基、 环烷基、 杂环基、 芳基或杂芳基, 其 中所述的烷基、 环烷基、 杂环基、 芳基或杂芳基各自独立地任选进一步被一个或 多个选自卤素、 氰基、 羟基、 氨基、 氧代基、 烷基、 卤代烷基、 羟烷基、 烷氧基、 环烷基、 杂环基、 芳基、 杂芳基、 羧基或羧酸酯基的取代基所取代; 且 R 7 or R 8 are each independently selected from a hydrogen atom, an alkyl group, a cycloalkyl group, a heterocyclic group, an aryl group or a heteroaryl group, wherein the alkyl group, cycloalkyl group, heterocyclic group, aryl group or hetero group The aryl groups are each independently optionally further selected from one or more selected from the group consisting of halogen, cyano, hydroxy, amino, oxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, cycloalkyl, heterocyclyl, Substituted with a substituent of an aryl, heteroaryl, carboxy or carboxylate group;
m为 0、 1或 2。 m is 0, 1, or 2.
13、 一种制备根据权利要求 1所述的通式( I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其可药 用的盐的方法, 该方法包括以下步骤:
通式 (I-B)化合物与胺基化合物反应得到通式( I )化合物; 13. A compound of the formula (I) according to claim 1 or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof. A method of the body, or a mixture thereof, or a pharmaceutically acceptable salt thereof, the method comprising the steps of: A compound of the formula (IB) is reacted with an amine compound to give a compound of the formula (I);
其中: 1^〜1 5的定义如权利要求 1中所述。 Wherein: 1^~1 5 is as defined in claim 1.
14、 一种药物组合物, 所述药物组合物含有治疗有效量的根据权利要求 1〜9 任意一项所述的通式(I )所示的化合物或其互变异构体、 内消旋体、 外消旋体、 对 映异构体、 非对映异构体、 或其混合物形式、 或其可药用的盐和药学上可接受的 载体、 稀释剂或赋形剂。 A pharmaceutical composition comprising a therapeutically effective amount of the compound of the formula (I) according to any one of claims 1 to 9 or a tautomer thereof, mesogenic a form, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, diluent or excipient.
15、 根据权利要求 1〜9任意一项所述的通式( I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 或根据权利要求 14所述的药物组合物在制备胃酸分泌抑制剂中的用 途。 The compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 9. Use of the isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or the pharmaceutical composition according to claim 14 for the preparation of a gastric acid secretion inhibitor.
16、 根据权利要求 1〜9任意一项所述的通式( I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 或根据权利要求 14所述的药物组合物在制备 H+/K+-ATPaSe抑制剂 中的用途。 The compound of the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 9. Use of the isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or the pharmaceutical composition according to claim 14 for the preparation of an H + /K+-ATP aSe inhibitor.
17、 根据权利要求 1〜9任意一项所述的通式( I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐, 或根据权利要求 14所述的药物组合物在制备钾离子竞争性酸阻滞剂 中的用途。 The compound represented by the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 9. Use of the isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or the pharmaceutical composition according to claim 14 for the preparation of a potassium ion competitive acid retarder.
18、 根据权利要求 1〜9任意一项所述的通式( I )所示的化合物或其互变异构 体、 内消旋体、 外消旋体、 对映异构体、 非对映异构体、 或其混合物形式、 或其 可药用的盐,或根据权利要求 14所述的药物组合物在制备治疗或预防消化性溃疡、 卓 -艾综合征、 胃炎、 糜烂性食管炎、 反流性食管炎、 症状性胃食管反流疾病、 巴 雷特食管炎、 功能性消化不良、 幽门螺旋杆菌感染、 胃癌、 胃 MALT淋巴瘤、 非 甾体抗炎药引起的溃疡或手术后应激导致的胃酸过多或溃疡的药物中的用途; 或 者在制备抑制由于消化性溃疡、 急性应激性溃疡、 出血性胃炎或侵入性应激造成
的上消化道出血的药物中的用途。 The compound represented by the formula (I) or a tautomer, a mesogen, a racemate, an enantiomer or a diastereomer thereof according to any one of claims 1 to 9. The isomer, or a mixture thereof, or a pharmaceutically acceptable salt thereof, or the pharmaceutical composition according to claim 14 for the treatment or prevention of peptic ulcer, Zhuo-Eye syndrome, gastritis, erosive esophagitis, Reflux esophagitis, symptomatic gastroesophageal reflux disease, Barrett's esophagitis, functional dyspepsia, Helicobacter pylori infection, gastric cancer, gastric MALT lymphoma, ulcers caused by non-steroidal anti-inflammatory drugs or after surgery Use in drugs that cause hyperacidity or ulceration; or in the preparation of inhibition due to peptic ulcer, acute stress ulcer, hemorrhagic gastritis or invasive stress The use of drugs for upper gastrointestinal bleeding.
19、 根据权利要求 18所述的用途, 其中所述的消化性溃疡选自胃溃疡、 十二 指肠溃疡或吻合口溃疡; 所述的症状性胃食管反流疾病选自非糜烂性的反流性疾 病或无食管炎的胃食管反流疾病。
The use according to claim 18, wherein the peptic ulcer is selected from the group consisting of a gastric ulcer, a duodenal ulcer or an anastomotic ulcer; and the symptomatic gastroesophageal reflux disease is selected from a non-erosive reflex Fluidic disease or gastroesophageal reflux disease without esophagitis.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201380004866.3A CN104039776B (en) | 2012-08-03 | 2013-07-09 | Benzofuran derivative, its preparation method and in application pharmaceutically |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210274018 | 2012-08-03 | ||
| CN201210274018.1 | 2012-08-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014019442A1 true WO2014019442A1 (en) | 2014-02-06 |
Family
ID=50027220
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2013/079031 WO2014019442A1 (en) | 2012-08-03 | 2013-07-09 | Benzofuran derivatives, preparation method and medical use thereof |
Country Status (3)
| Country | Link |
|---|---|
| CN (1) | CN104039776B (en) |
| TW (1) | TW201406753A (en) |
| WO (1) | WO2014019442A1 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113620930A (en) * | 2021-07-12 | 2021-11-09 | 南京烁慧医药科技有限公司 | Compound containing sulfonamide structure, preparation method and application thereof, and pharmaceutical composition and application thereof |
| CN114105962A (en) * | 2021-10-26 | 2022-03-01 | 南京烁慧医药科技有限公司 | Compound containing sulfonamide structure, preparation method and application thereof, and pharmaceutical composition and application thereof |
| CN114349737A (en) * | 2020-04-26 | 2022-04-15 | 南京烁慧医药科技有限公司 | Compound containing sulfonamide structure and application thereof, and pharmaceutical composition and application thereof |
| CN115557876A (en) * | 2022-10-26 | 2023-01-03 | 四川国康药业有限公司 | 3-arylcyclylsulfonyl-1-N-heteropyrrole derivative for treating peptic ulcer, and its preparing process and application |
| WO2023280290A1 (en) * | 2021-07-09 | 2023-01-12 | 天地恒一制药股份有限公司 | Pyrrole sulfonyl derivative, and preparation method therefor and use thereof |
| WO2024217519A1 (en) * | 2023-04-21 | 2024-10-24 | 天地恒一制药股份有限公司 | Pyrrole sulfonyl derivative, and preparation method therefor and use thereof |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007026916A1 (en) * | 2005-08-30 | 2007-03-08 | Takeda Pharmaceutical Company Limited | 1-heterocyclylsulfonyl, 2-aminomethyl, 5- (hetero-) aryl substituted 1-h-pyrrole derivatives as acid secretion inhibitors |
| US20080139639A1 (en) * | 2004-09-30 | 2008-06-12 | Takeda Pharmaceutical Company Limited | Proton Pump Inhibitors |
| US20100210696A1 (en) * | 2007-09-28 | 2010-08-19 | Takeda Pharmaceutical Company Limited | 5-membered heterocyclic compound |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101418000B (en) * | 2007-10-24 | 2010-12-22 | 山东轩竹医药科技有限公司 | DPP-IV inhibitor derivates containing benzofuran sulfonyl ureas |
| US20100056491A1 (en) * | 2008-08-29 | 2010-03-04 | Memory Pharmaceuticals Corporation | 4'-amino cyclic compounds having 5-ht6 receptor affinity |
-
2013
- 2013-07-09 WO PCT/CN2013/079031 patent/WO2014019442A1/en active Application Filing
- 2013-07-09 CN CN201380004866.3A patent/CN104039776B/en active Active
- 2013-07-26 TW TW102126839A patent/TW201406753A/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080139639A1 (en) * | 2004-09-30 | 2008-06-12 | Takeda Pharmaceutical Company Limited | Proton Pump Inhibitors |
| WO2007026916A1 (en) * | 2005-08-30 | 2007-03-08 | Takeda Pharmaceutical Company Limited | 1-heterocyclylsulfonyl, 2-aminomethyl, 5- (hetero-) aryl substituted 1-h-pyrrole derivatives as acid secretion inhibitors |
| US20100210696A1 (en) * | 2007-09-28 | 2010-08-19 | Takeda Pharmaceutical Company Limited | 5-membered heterocyclic compound |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114349737A (en) * | 2020-04-26 | 2022-04-15 | 南京烁慧医药科技有限公司 | Compound containing sulfonamide structure and application thereof, and pharmaceutical composition and application thereof |
| WO2023280290A1 (en) * | 2021-07-09 | 2023-01-12 | 天地恒一制药股份有限公司 | Pyrrole sulfonyl derivative, and preparation method therefor and use thereof |
| CN113620930A (en) * | 2021-07-12 | 2021-11-09 | 南京烁慧医药科技有限公司 | Compound containing sulfonamide structure, preparation method and application thereof, and pharmaceutical composition and application thereof |
| CN113620930B (en) * | 2021-07-12 | 2022-08-16 | 南京烁慧医药科技有限公司 | Compound containing sulfonamide structure, preparation method and application thereof, and pharmaceutical composition and application thereof |
| WO2023284159A1 (en) * | 2021-07-12 | 2023-01-19 | 南京烁慧医药科技有限公司 | Compound containing sulfamide structure, and preparation method therefor and application thereof, and pharmaceutical composition and application |
| CN114105962A (en) * | 2021-10-26 | 2022-03-01 | 南京烁慧医药科技有限公司 | Compound containing sulfonamide structure, preparation method and application thereof, and pharmaceutical composition and application thereof |
| CN115557876A (en) * | 2022-10-26 | 2023-01-03 | 四川国康药业有限公司 | 3-arylcyclylsulfonyl-1-N-heteropyrrole derivative for treating peptic ulcer, and its preparing process and application |
| WO2024217519A1 (en) * | 2023-04-21 | 2024-10-24 | 天地恒一制药股份有限公司 | Pyrrole sulfonyl derivative, and preparation method therefor and use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201406753A (en) | 2014-02-16 |
| CN104039776A (en) | 2014-09-10 |
| CN104039776B (en) | 2016-06-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2013347383B2 (en) | Pyrrole sulfonamide derivative, preparation method for same, and medical application thereof | |
| CA2629527C (en) | 2-(phenyl or heterocyclic)-1h-phenantrho[9,10-d]imidazoles as mpges-1 inhibitors | |
| JP7575952B2 (en) | 15-PGDH inhibitors | |
| WO2014019442A1 (en) | Benzofuran derivatives, preparation method and medical use thereof | |
| WO2014036897A1 (en) | Imidazoline derivatives, preparation methods thereof, and their applications in medicine | |
| JP2012512888A (en) | Carbazole carboxamide compounds useful as kinase inhibitors | |
| PT2089367E (en) | Pyrazoline compounds as mineralocorticoid receptor antagonists | |
| WO2017152857A1 (en) | Indoleamine-2,3-dioxygenase inhibitor containing nitrogen alkylated and arylated sulphoxide imines | |
| TW201702226A (en) | Urea derivative or pharmacologically acceptable salt thereof | |
| TW201206906A (en) | Dihydropyrimidinone derivative and pharmaceutical use thereof | |
| CN114409656B (en) | PIM kinase inhibitors | |
| WO2019062328A1 (en) | Aniline-substituted 1,2-dihydropyrrol[3,4-c]pyridin/pyrimidin-3-one derivative and use | |
| TW200901980A (en) | Benzimidazole cannabinoid agonists | |
| JP7451765B2 (en) | Pyridine acetamide derivatives as CDK inhibitors, their preparation methods and uses | |
| CA2730071A1 (en) | Antineoplastic derivatives of 4-oxo-l, 4-dihydro-quinolin?, preparation thereof, and therapeutic use thereof | |
| JP2022537415A (en) | Compounds for inhibiting FGFR4 | |
| JP7406008B2 (en) | Polycyclic amide derivatives as CDK9 inhibitors, their preparation methods and uses | |
| CN107428682B (en) | Amide derivatives, preparation method and medical application thereof | |
| CN116891464A (en) | AT2R agonist | |
| CN117209501A (en) | Sulfonamide derivatives and uses thereof | |
| WO2021091593A1 (en) | Rock kinase inhibitors | |
| CN110105275B (en) | Amide derivatives, preparation method and medical application thereof | |
| US11912719B2 (en) | Substituted isoquinolines as rock kinase inhibitors | |
| WO2023078392A1 (en) | 2-(aryl-2-yl) morpholine and deuterated derivative thereof, preparation method therefor and application thereof | |
| WO2019141095A1 (en) | Amidine and guanidine derivative, preparation method therefor and medical use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13825388 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13825388 Country of ref document: EP Kind code of ref document: A1 |