WO2013047889A1 - Pharmaceutical composition for treating autoimmune diseases - Google Patents
Pharmaceutical composition for treating autoimmune diseases Download PDFInfo
- Publication number
- WO2013047889A1 WO2013047889A1 PCT/JP2012/075587 JP2012075587W WO2013047889A1 WO 2013047889 A1 WO2013047889 A1 WO 2013047889A1 JP 2012075587 W JP2012075587 W JP 2012075587W WO 2013047889 A1 WO2013047889 A1 WO 2013047889A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sirna
- seq
- expression
- pharmaceutical composition
- heavy chain
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1138—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against receptors or cell surface proteins
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering N.A.
Definitions
- the present invention relates to a novel use of a functional nucleic acid related to CD98. More specifically, the present invention relates to a pharmaceutical composition for treating autoimmune disease using CD98 siRNA. More specifically, the present invention relates to a therapeutic agent for diabetes (particularly type 1 diabetes) and a pharmaceutical composition for treating rheumatoid arthritis.
- CD98 is a heterodimeric glycoprotein of approximately 120 kDa that is widely expressed on the cell membrane and is thought to be associated with amino acid transport, increased cell membrane expression and cell fusion associated with integrin-mediated adhesion .
- the structure of CD98 is heterozygous by disulfide bond with one of approximately 80 kDa type II transmembrane protein CD98hc (heavy chain: 4F2hc, SLC3A2) and one of six types of approximately 40 kDa light chain (LAT1, LAT2, y + LAT1, y + LAT2, xCT, asc). It constitutes a dimer and functions as an amino acid transporter.
- CD98hc The heavy chain of CD98 (CD98hc) has a role of transferring a dimer to the cell membrane, and the light chain (lc) is considered to have a transporter function.
- CD98hc was first discovered as a T cell activation antigen and is a glycoprotein having various functions. Its function is highly expressed in proliferating lymphocytes, tumor cells, and other highly proliferative cells, and plays an important role in integrin-dependent signals that promote tumorigenesis.
- Amino acid transport system that CD98 is involved is composed from one to reflect the diversity of the amino acid molecules as substrates six transporters (the light chain of about 40 kDa), the transport substrate selectivity and Na + It is classified into various transport systems by dependence.
- CD98 heavy chain is present in the cell membrane on the blood vessel side of the epithelial cells and functions to transport the transporter to the cell membrane on the blood vessel side of the epithelial cells.
- CD98 is known to be highly expressed in various cancer cells. The reason for this is that cancer cells need to actively take in essential amino acids necessary for growth in order to promote cell growth, and it is considered that neutral amino acid transporters are overexpressed there.
- L-type amino acid transporter 1 has been cloned as an amino acid transporter that is highly expressed specifically in cancer cells (Kanai et al., J. Biol. Chem. (1998), 273). , 23629-23632).
- LAT1 forms a complex with CD98 heavy chain and has a function of transporting neutral amino acids having large side chains such as leucine, valine, phenylalanine, tyrosine, tryptophan, methionine, and histidine independent of sodium ions. ing.
- This LAT1 is low in expression or not observed in most normal tissues except brain, placenta, bone marrow, and testis, but colon cancer, stomach cancer, breast cancer, pancreatic cancer, renal cancer, laryngeal cancer, esophagus
- CD98 CD98 heavy chain and light chain
- LAT1 suppressing amino acid uptake
- Patent Documents 2 and 3 In addition, in order to suppress amino acid transport of CD98 (a complex of heavy chain and light chain), an inhibitory effect on cancer cells using an anti-CD98 antibody has been reported in a cancer transplanted mouse model (Patent Documents 2 and 3). As described above, various attempts have been made to use anti-CD98 antibodies as anticancer agents. However, there are almost no efforts for applications other than anticancer drugs. Moreover, no attempt has been made to try to evaluate the therapeutic effect of the anticancer agent and the like by suppressing the expression of CD98 using siRNA.
- the object of the present invention is to provide a pharmaceutical composition for autoimmune diseases using a functional nucleic acid of CD98.
- an object of the present invention is to provide a pharmaceutical composition containing siRNA that suppresses CD98 heavy chain expression as an active ingredient.
- the present inventor has identified a monoclonal antibody that recognizes the heavy chain of CD98, and found that this monoclonal antibody suppresses activation of T cells.
- the present inventors have found that this antibody does not affect amino acid transport and suppresses cell adhesion via fibronectin. Then, when this antibody was used to treat diabetic NOD mice, it was possible to suppress the activation of CD4 + T cells and return the diabetic state to the normal level (PCT / JP2011 / 057432). Therefore, the present inventor treated diabetic NOD mice using siRNA that suppresses the expression of CD98 heavy chain.
- the siRNA against CD98 heavy chain not only suppressed the progression of type 1 diabetes, but also the treatment. I found out that I can do it.
- siRNA capable of suppressing the expression of CD98 heavy chain was administered to rheumatoid arthritis model mice, it was confirmed that the onset of arthritis was suppressed.
- a pharmaceutical composition containing siRNA of CD98 as an active ingredient is effective for treatment / prevention of autoimmune diseases (type 1 diabetes, rheumatoid arthritis, etc.). From these findings, it was shown that the siRNA of the present invention is useful as a therapeutic agent for autoimmune diseases.
- a pharmaceutical composition for preventing or treating an autoimmune disease comprising a functional nucleic acid that suppresses the expression of CD98 heavy chain as an active ingredient.
- the functional nucleic acid is siRNA.
- the autoimmune disease is type 1 diabetes or rheumatoid arthritis.
- the siRNA is a double-stranded RNA.
- the above (4) description, wherein the double-stranded RNA is an siRNA containing one or more of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4, or SEQ ID NO: 5 and SEQ ID NO: 6.
- the double-stranded RNA is an siRNA containing one or more of SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and SEQ ID NO: 10, or SEQ ID NO: 11 and SEQ ID NO: 12.
- Pharmaceutical composition (7) Double-stranded siRNA having any one of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4, or SEQ ID NO: 5 and SEQ ID NO: 6.
- Double-stranded siRNA in which the double-stranded RNA portion has any one of SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and SEQ ID NO: 10, or SEQ ID NO: 11 and SEQ ID NO: 12.
- a method for treating an autoimmune disease comprising administering to a mammal an effective amount of a functional nucleic acid that suppresses the expression of CD98 heavy chain.
- the functional nucleic acid is siRNA.
- the siRNA is the double-stranded siRNA of (7) or (8).
- the autoimmune disease is type 1 diabetes or rheumatoid arthritis.
- the pharmaceutical composition and the therapeutic method of the present invention are useful for the treatment of autoimmune diseases. Very useful. It is particularly effective for type 1 diabetes and rheumatoid arthritis, for which there has been no effective treatment.
- FIG. 1 T cells of NOD mice that have developed type 1 diabetes are administered to NOD model mice (NOD-SCID mice) with severe combined immunodeficiency. After administration, mice are administered mouse siRNA against CD98 heavy chain (4 nmol / mouse) or control siRNA on days 2, 5, and 8. The day on which T cells are administered is taken as the starting date.
- FIG. 1 is a diagram in which the expression of CD98 heavy chain in peripheral blood mononuclear cells after 3 days was evaluated by flow cytometry.
- the gray (GRAY) part is the measurement result when the CD98 antibody was administered.
- the dotted line portion shows the measurement results when the control siRNA is administered, and the solid line portion shows the measurement results when the siRNA against CD98 heavy chain is administered.
- FIG. 1 is a diagram in which the expression of CD98 heavy chain in peripheral blood mononuclear cells after 3 days was evaluated by flow cytometry.
- the gray (GRAY) part is the measurement result when the CD98 antibody was administered.
- the dotted line portion shows the measurement
- FIG. 2 is a diagram showing blood glucose levels measured and evaluated in order to see the therapeutic effect of type 1 diabetes. The experiment was conducted with 5 mice as a group, and the symbol * indicates a statistically significant difference (p ⁇ 0.05).
- a dotted line part is a result at the time of administering control siRNA, and a continuous line part is a result at the time of administering siRNA with respect to CD98 heavy chain.
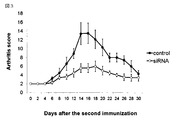
- FIG. 3 shows the results of intraperitoneal administration of siRNA on day 0, day 1, day 2, day 3, day 4 using rheumatoid model mice (collagen-induced arthritis model). . The result shows that the onset of rheumatism is greatly reduced by administration of mouse siRNA.
- FIG. 1 shows that the onset of rheumatism is greatly reduced by administration of mouse siRNA.
- FIG. 4 is a diagram in which human siRNA was administered to Jurkat cells derived from human T lymphoma, and changes in the expression of CD98 protein on the cell surface were measured and evaluated using a flow cytometer. Compared with the untreated group and the control siRNA administration group, the suppression of the expression of CD98 protein was greatly suppressed by administering human siRNA.
- a pharmaceutical composition for preventing or treating an autoimmune disease comprising a functional nucleic acid that suppresses the expression of CD98 heavy chain as an active ingredient
- CD98 heavy chain a method for treating an autoimmune disease, which comprises administering an effective amount of a functional nucleic acid that suppresses the expression of an ".
- CD98 is a known protein, and the DNA sequence and amino acid sequence are described in Patent Document 2 and the like.
- CD98 in the present invention is not limited to these sequences, and the number of amino acid and DNA mutations and mutation sites are not limited as long as the function of CD98 is maintained.
- “functional nucleic acid” refers to siRNA, miRNA, aptamer, ribozyme, and antisense nucleic acid having a function capable of suppressing protein expression.
- Preferred examples include siRNA. That is, the siRNA of the present invention refers to an siRNA that suppresses gene expression of CD98 heavy chain. Specifically, it refers to siRNA that can suppress the gene expression of CD98 by 50% or more, and preferably siRNA that can suppress 80% or more compared to the control group (when control siRNA is used). siRNA is known to induce sequence-specific suppression of gene expression called RNA interference.
- siRNA is generally composed of a double-stranded RNA portion and an overhang portion at the 3 ′ end of the sense strand and the antisense strand.
- siRNAs can be designed by methods known to those skilled in the art. For example, a selected DNA sequence (preferably 19 to 21 bases) is directly converted into an RNA sequence (sense strand) and its antisense strand as a double-stranded RNA portion, and an overhang portion is added.
- the overhang portion is an arbitrary nucleic acid (ribonucleic acid or deoxynucleic acid) having 1 or 2 bases, and uridine (U) or thymidine (dT) is preferable.
- the siRNA of the present invention is not particularly limited as long as it is designed based on the DNA sequence of CD98, preferably human CD98 heavy chain, and can suppress the expression of CD98 heavy chain.
- the suppression of expression is desirably specific for the CD98 heavy chain. Whether it is specific or not can be confirmed by conducting a publicly available BLAST search.
- the overhang part is not essential.
- the siRNA of the present invention also includes any molecule having the same effect as the siRNA of the present invention within the administration subject.
- An example of such a molecule is shRNA.
- shRNA is RNA having a short hairpin structure, and is an RNA molecule having a stem-loop structure so that a part of a single strand forms a complementary strand with another region.
- ShRNA in which the double-stranded RNA portion has the same structure as the siRNA of the present invention is also included in the siRNA of the present invention.
- a DNA that can express the siRNA of the present invention by being administered to an administration subject is also included in the siRNA of the present invention.
- Such a DNA is used by constructing a DNA encoding siRNA into an expression vector (for example, a vector such as adenovirus, adeno-associated virus, herpes virus, lentivirus).
- an expression vector for example, a vector such as adenovirus, adeno-associated virus, herpes virus, lentivirus.
- a functional nucleic acid when used as a pharmaceutical composition, it is desirable to include a modification for improving the properties as a therapeutic agent or a transport carrier such as a liposome.
- nucleotide modifications or analogs can be introduced. For example, improved nuclease resistance and / or improved cell permeability. Nuclease resistance is brought about by any method known in the art that does not interfere with the biological activity of the antisense, siRNA, shRNA and / or ribozyme.
- An example of a modification that can be added to an oligonucleotide for the purpose of improving nuclease resistance is modification of a heteroatom's phosphorus or oxygen in the phosphate backbone. For example, methyl phosphate, phosphorothioate, phosphorodithioate, and morpholino oligomers.
- the functional nucleic acid when used as a pharmaceutical composition or a treatment method in the present invention, there are a method of using the functional nucleic acid itself for treatment and a method of expressing the functional nucleic acid using a vector and using it for the treatment. To do.
- the functional nucleic acid itself is used for treatment, it is desirable to prepare an aqueous solution to which a stabilizer such as atelocollagen, a pH regulator and the like are added and administered parenterally as it is.
- an expression vector particularly a mammalian expression vector, particularly for expression in a patient requiring the treatment of the present invention.
- Expression vectors are well known in the art and preferably include plasmids, cosmids, and viral expression systems. Examples of preferred virus expression systems are adenovirus, retrovirus, lentivirus and the like.
- methods for introducing vectors into cells and tissues are well known in the art. Preferred examples include transfection, lipofection, electroporation, and infection with recombinant viral vectors.
- the “mammal” of the present invention include rodents such as rats, mice, and guinea pigs, such as dogs and cats, and humans and monkeys.
- the “autoimmune disease” of the present invention is a disease caused by the immune system raising autoantibodies against endogenous antigens.
- type 1 diabetes rheumatoid arthritis, ulcerative colitis, psoriasis, Crohn's disease, systemic lupus erythematosus And Graves' disease.
- Previous studies have revealed that any autoimmune disease is caused by abnormal overactivation of T cells.
- CD4 positive or CD8 positive T lymphocytes (T cells) are excessively activated to promote the production of autoantibodies or cause tissue destruction, which has a great influence on the progress of disease states. It is a fact that.
- the siRNA of the present invention suppresses the activation of T lymphocytes (T cells) similarly to the anti-CD98 antibody, the above-described autoimmune diseases can be treated using the siRNA of the present invention.
- the siRNA of the present invention is effective for the treatment of type 1 diabetes, and as shown in FIG. 3, it is effective for the treatment of rheumatoid arthritis.
- the siRNA of the present invention can be used for the treatment of autoimmune diseases such as type 1 diabetes, rheumatoid arthritis, ulcerative colitis, psoriasis, Crohn's disease, systemic lupus erythematosus, and Graves' disease.
- preferred uses of the siRNA of the present invention are type 1 diabetes, rheumatoid arthritis, ulcerative colitis, psoriasis, Crohn's disease, systemic lupus erythematosus, and Graves' disease, and particularly preferred uses include type 1 diabetes or Rheumatoid arthritis can be mentioned.
- “Rheumatoid arthritis” of the present invention refers to an autoimmune disease in which joints, bones, muscles, ligaments, tendons, etc. hurt, and the most frequent autoimmune disease, which is often difficult to treat following a very chronic course It is.
- the siRNA of the present invention having an action of suppressing activation of T cells (T lymphocytes) is suitable for the treatment of rheumatoid arthritis.
- the “type 1 diabetes” of the present invention refers to a disease in which pancreatic ⁇ -cells are destroyed mainly by autoimmunity and insulin production is depleted, resulting in insulin deficiency.
- the siRNA of the present invention having an activity of suppressing activation of T cells (T lymphocytes) is suitable for the prevention and treatment of type 1 diabetes.
- the pharmaceutical composition is used together with the above-described functional nucleic acid and one or more kinds of conventional pharmaceutically acceptable additives. Is preferably prepared and administered.
- the pharmaceutical composition of the present invention is preferably administered parenterally, and can be administered intravenously, intramuscularly, intraperitoneally, or subcutaneously.
- the dose of the functional nucleic acid of the present invention varies depending on the administration subject, administration method, and the like. For example, when administered parenterally, for example, about 0.01 to about 1 day per day for an autoimmune disease patient (60 kg). 30 mg, preferably about 0.1 to 20 mg, more preferably about 0.1 to 10 mg can be intravenously injected.
- Experimental Materials a) Animals: Type 1 diabetes model mouse (NOD mouse): NOD-SCID mice were purchased from Japan SLC (Hamamatsu, Japan). All mice were kept under aseptic conditions in the Tokushima University Animal Center, and all experiments were conducted according to animal management and utilization guidelines. The development of diabetes in NOD mice was monitored by measuring the urine sugar concentration and fasting blood glucose level of each mouse every week.
- a mouse having a blood concentration of 250 mg / dl or more was considered diabetic. When female mice with NOD reach 20-25 weeks of age, they have type 1 diabetes at a rate of 80% or more.
- a fluorescent dye-conjugated antibody eBioscience, UAS
- TCRV ⁇ 2 TCRV ⁇ 5
- Mouse siRNA A mixture of the three mouse siRNAs shown in Table 1 below for suppressing the expression of CD98 heavy chain, or a control siRNA having no target gene (B-Bridge) was used. 2) Method: A mixture of the above three siRNAs for suppressing the expression of CD98 heavy chain, or a control siRNA having no target gene and atelocollagen (Koken, Japan) were mixed according to the experimental procedure to prepare a siRNA solution.
- the siRNA solution was intraperitoneally administered at 4 nmol / mouse to a severe combined immunodeficiency model mouse (NOD-SCID mouse) to which T cells of diabetic NOD mice were administered.
- the administration schedule of siRNA is the 2nd day, the 5th day, and the 8th day. The day on which the T cells are administered is taken as the starting date.
- Three days after administration of the siRNA solution blood was collected, white blood cells were isolated, stained with anti-mouse CD98 antibody, and evaluated by flow cytometry. 3) Results: The evaluation results by flow cytometry are shown in FIG.
- the gray (GRAY) part is the measurement result when the CD98 antibody was administered.
- FIG. 2 shows the results of administering CD98 heavy chain siRNA to the type 1 diabetes model mouse (NOD-SCID mouse). As shown in FIG. 2, it was found that the progression of type 1 diabetes in this mouse can be suppressed by administration of siRNA. This result indicates that CD98 plays a crucial role in the development of type 1 diabetes and that treatment with siRNA targeting the CD98 heavy chain is self-induced due to type 1 diabetes and other T cells.
- Example 2 Rheumatoid arthritis progression inhibition test with siRNA for suppressing expression of CD98 heavy chain
- Experimental materials Production of rheumatoid model mice (collagen-induced arthritis model mice): DBA / 1J mice (7-week-old female, 10 mice) were divided into two groups, respectively, and administered with an emulsion in which bovine collagen II and complete adjuvant were mixed and emulsified. Furthermore, the second bovine collagen II was administered on the 26th day, and the onset of arthritis was observed and evaluated. In FIG. 3, the clinical score is expressed as the mean ⁇ standard deviation. The horizontal axis represents the number of days after the second collagen immunization.
- CD98 plays a very important role in the development of type 1 diabetes, and treatment using siRNA targeting the CD98 heavy chain is very useful for the prevention and treatment of autoimmune diseases (rheumatic). Is effective.
- Example 3 CD98 expression progression suppression test of human leukemia T cells (Jurkat cells) by siRNA for suppressing expression of CD98 heavy chain 1)
- Experimental materials Jurkat cells: Jurkat cells that highly express human CD98 protein were purchased from ATCC.
- a siRNA was prepared from a mixture of the three human siRNAs shown in Table 2 below for suppression of CD98 heavy chain expression (requested from B-Bridge) and used.
- 3) Results The ratio of CD98 expression on the Jurkat cell surface was measured and evaluated with a flow cytometer, and the results are shown in FIG. As shown in FIG. 4, in the control siRNA administration group, the protein expression of CD98 was the same as that in the non-treatment group. However, in the group administered with human siRNA, protein expression of CD98 was suppressed by about 75%. This indicates that these three types of human siRNA can be used to prevent and treat type 1 diabetes and other autoimmune diseases caused by T cells.
- the pharmaceutical composition for prevention and treatment of autoimmune diseases of the present invention prevents and treats autoimmune diseases (for example, in type 1 diabetes and rheumatoid arthritis) by a new mechanism of action that suppresses the expression of CD98 by siRNA. To get.
- autoimmune diseases for example, in type 1 diabetes and rheumatoid arthritis
- siRNA siRNA
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Diabetes (AREA)
- Molecular Biology (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- Rheumatology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Plant Pathology (AREA)
- Endocrinology (AREA)
- Biophysics (AREA)
- Emergency Medicine (AREA)
- Transplantation (AREA)
- Obesity (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Hematology (AREA)
- Pain & Pain Management (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The purpose of the present invention is to provide a pharmaceutical composition and a treatment method both for treating/preventing autoimmune diseases.
It is found that a pharmaceutical composition containing siRNA that can inhibit the expression of a CD98 heavy chain is useful for the prevention/treatment of autoimmune diseases. As a result, it becomes possible to provide a novel treatment method using the siRNA for autoimmune diseases such as type-1 diabetes and rheumatoid arthritis.
Description
本発明は、CD98に関する機能性核酸の新規用途に関する。より詳細には、本発明は、CD98のsiRNAを用いた自己免疫疾患治療用医薬組成物に関するものである。更に詳しくは、糖尿病治療剤(特に1型糖尿病)や関節リウマチ治療用医薬組成物に関するものである。
The present invention relates to a novel use of a functional nucleic acid related to CD98. More specifically, the present invention relates to a pharmaceutical composition for treating autoimmune disease using CD98 siRNA. More specifically, the present invention relates to a therapeutic agent for diabetes (particularly type 1 diabetes) and a pharmaceutical composition for treating rheumatoid arthritis.
CD98とは、細胞膜上に幅広く発現している約120kDaのヘテロ2量体の糖タンパク質であり、アミノ酸輸送、増加した細胞膜発現とインテグリンが仲介する接着に付随する細胞融合に関連すると考えられている。CD98の構造は、約80kDaII型膜貫通型タンパク質CD98hc(重鎖:4F2hc、SLC3A2)と6種類ある約40kDaの軽鎖(LAT1,LAT2,y+LAT1,y+LAT2,xCT,asc)の1つとジスルフィド結合によるヘテロ2量体を構成し、アミノ酸トランスポーターとして機能している。CD98の重鎖(CD98hc)は細胞膜に2量体を移動させる役割を持ち、軽鎖(lc)がトランスポーターの機能を持つと考えられている。
CD98hcは、最初、T細胞活性化抗原として発見されたものであり、様々な機能を持った糖タンパク質である。その機能としては、増殖中リンパ球、腫瘍細胞、その他の増殖能の高い細胞で高発現し、腫瘍形成を促進するインテグリン依存性シグナルに重要な役割を果たしている。
CD98が関与するアミノ酸輸送システムは、基質となるアミノ酸分子の多様性を反映して6種類のトランスポーター(約40kDaの軽鎖)の一つから構成されており、その輸送基質選択性とNa+依存性により種々の輸送系に分類されている。例えば、中性アミノ酸輸送系L、小型中性アミノ酸輸送系asc、中性および塩基性アミノ酸輸送系y+L、シスチン、塩基性および中性アミノ酸輸送系x−cに相当する特定のトランスポーター(LAT1,LAT2,y+LAT1,y+LAT2,xCT,asc)とジスルフィド結合している。そして、CD98重鎖は、上皮細胞の血管側の細胞膜に存在し、上記のトランスポーターを上皮細胞の血管側の細胞膜へ移送する働きをしている。
また、CD98は、多種の癌細胞で高発現していることが知られている。その理由として、癌細胞は細胞増殖を促進させるために、増殖に必要な必須アミノ酸を積極的に取り込む必要があり、そこで中性アミノ酸トランスポーターが過剰発現していると考えられる。例えば、癌細胞に特異的に高く発現しているアミノ酸トランスポーターとして、L−type amino acid transporter 1(LAT1)がクローニングされている(Kanai et al.,J.Biol.Chem.(1998),273,23629−23632)。LAT1は、CD98重鎖と複合体を形成し、ロイシン、バリン、フェニルアラニン、チロシン、トリプトファン、メチオニン、ヒスチジン等の大型の側鎖を持つ中性アミノ酸をナトリウムイオン非依存的に輸送する機能を有している。このLAT1は、脳、胎盤、骨髄、精巣を除く殆どの正常組織において、その発現が低いか、あるいは認められないものであるが、大腸癌、胃癌、乳癌、膵癌、腎癌、喉頭癌、食道癌、肺癌等のヒト悪性腫瘍組織では、CD98と共に発現が亢進していることが知られている(非特許文献1)。
そこで、CD98重鎖や軽鎖(LAT1)の発現を低下させ、アミノ酸取り込みを抑制すると、腫瘍の増殖が抑えられることが考えられた。即ち、軽鎖(LAT1)の発現を低下させると、腫瘍の増殖抑制が起きることが癌移植マウスモデルで報告されている(特許文献1)。そこで、LAT1の活性を抑えることは癌の治療法に有望であると考えられた。
また、CD98(重鎖と軽鎖の複合体)のアミノ酸輸送を抑制するため、抗CD98抗体を用いた癌細胞の抑制効果が癌移植マウスモデルで報告されている(特許文献2、3)。このように、抗CD98抗体を使用して、抗癌剤として使用する試みについては、色々取り組みが行われている。しかし、抗癌剤以外の用途に関する取り組みは、ほとんど行われていない状況である。
しかも、siRNAを用いてCD98の発現を抑制し、上記抗癌剤等に関する治療効果を評価する試みについては、全く行なわれていない状況である。 CD98 is a heterodimeric glycoprotein of approximately 120 kDa that is widely expressed on the cell membrane and is thought to be associated with amino acid transport, increased cell membrane expression and cell fusion associated with integrin-mediated adhesion . The structure of CD98 is heterozygous by disulfide bond with one of approximately 80 kDa type II transmembrane protein CD98hc (heavy chain: 4F2hc, SLC3A2) and one of six types of approximately 40 kDa light chain (LAT1, LAT2, y + LAT1, y + LAT2, xCT, asc). It constitutes a dimer and functions as an amino acid transporter. The heavy chain of CD98 (CD98hc) has a role of transferring a dimer to the cell membrane, and the light chain (lc) is considered to have a transporter function.
CD98hc was first discovered as a T cell activation antigen and is a glycoprotein having various functions. Its function is highly expressed in proliferating lymphocytes, tumor cells, and other highly proliferative cells, and plays an important role in integrin-dependent signals that promote tumorigenesis.
Amino acid transport system that CD98 is involved, is composed from one to reflect the diversity of the amino acid molecules as substrates six transporters (the light chain of about 40 kDa), the transport substrate selectivity and Na + It is classified into various transport systems by dependence. For example, specific transporters corresponding to the neutral amino acid transport system L, small neutral amino acid transport system asc, neutral and basic amino acid transport system y + L, cystine, basic and neutral amino acid transport system xc (LAT1, LAT2, y + LAT1, y + LAT2, xCT, asc) and disulfide bonds. The CD98 heavy chain is present in the cell membrane on the blood vessel side of the epithelial cells and functions to transport the transporter to the cell membrane on the blood vessel side of the epithelial cells.
Further, CD98 is known to be highly expressed in various cancer cells. The reason for this is that cancer cells need to actively take in essential amino acids necessary for growth in order to promote cell growth, and it is considered that neutral amino acid transporters are overexpressed there. For example, L-type amino acid transporter 1 (LAT1) has been cloned as an amino acid transporter that is highly expressed specifically in cancer cells (Kanai et al., J. Biol. Chem. (1998), 273). , 23629-23632). LAT1 forms a complex with CD98 heavy chain and has a function of transporting neutral amino acids having large side chains such as leucine, valine, phenylalanine, tyrosine, tryptophan, methionine, and histidine independent of sodium ions. ing. This LAT1 is low in expression or not observed in most normal tissues except brain, placenta, bone marrow, and testis, but colon cancer, stomach cancer, breast cancer, pancreatic cancer, renal cancer, laryngeal cancer, esophagus In human malignant tumor tissues such as cancer and lung cancer, it is known that expression is enhanced together with CD98 (Non-patent Document 1).
Therefore, it was considered that tumor growth can be suppressed by reducing the expression of CD98 heavy chain and light chain (LAT1) and suppressing amino acid uptake. That is, it has been reported in a mouse model transplanted with cancer that suppression of tumor growth occurs when the expression of the light chain (LAT1) is decreased (Patent Document 1). Therefore, suppressing LAT1 activity was considered promising for cancer therapy.
In addition, in order to suppress amino acid transport of CD98 (a complex of heavy chain and light chain), an inhibitory effect on cancer cells using an anti-CD98 antibody has been reported in a cancer transplanted mouse model (Patent Documents 2 and 3). As described above, various attempts have been made to use anti-CD98 antibodies as anticancer agents. However, there are almost no efforts for applications other than anticancer drugs.
Moreover, no attempt has been made to try to evaluate the therapeutic effect of the anticancer agent and the like by suppressing the expression of CD98 using siRNA.
CD98hcは、最初、T細胞活性化抗原として発見されたものであり、様々な機能を持った糖タンパク質である。その機能としては、増殖中リンパ球、腫瘍細胞、その他の増殖能の高い細胞で高発現し、腫瘍形成を促進するインテグリン依存性シグナルに重要な役割を果たしている。
CD98が関与するアミノ酸輸送システムは、基質となるアミノ酸分子の多様性を反映して6種類のトランスポーター(約40kDaの軽鎖)の一つから構成されており、その輸送基質選択性とNa+依存性により種々の輸送系に分類されている。例えば、中性アミノ酸輸送系L、小型中性アミノ酸輸送系asc、中性および塩基性アミノ酸輸送系y+L、シスチン、塩基性および中性アミノ酸輸送系x−cに相当する特定のトランスポーター(LAT1,LAT2,y+LAT1,y+LAT2,xCT,asc)とジスルフィド結合している。そして、CD98重鎖は、上皮細胞の血管側の細胞膜に存在し、上記のトランスポーターを上皮細胞の血管側の細胞膜へ移送する働きをしている。
また、CD98は、多種の癌細胞で高発現していることが知られている。その理由として、癌細胞は細胞増殖を促進させるために、増殖に必要な必須アミノ酸を積極的に取り込む必要があり、そこで中性アミノ酸トランスポーターが過剰発現していると考えられる。例えば、癌細胞に特異的に高く発現しているアミノ酸トランスポーターとして、L−type amino acid transporter 1(LAT1)がクローニングされている(Kanai et al.,J.Biol.Chem.(1998),273,23629−23632)。LAT1は、CD98重鎖と複合体を形成し、ロイシン、バリン、フェニルアラニン、チロシン、トリプトファン、メチオニン、ヒスチジン等の大型の側鎖を持つ中性アミノ酸をナトリウムイオン非依存的に輸送する機能を有している。このLAT1は、脳、胎盤、骨髄、精巣を除く殆どの正常組織において、その発現が低いか、あるいは認められないものであるが、大腸癌、胃癌、乳癌、膵癌、腎癌、喉頭癌、食道癌、肺癌等のヒト悪性腫瘍組織では、CD98と共に発現が亢進していることが知られている(非特許文献1)。
そこで、CD98重鎖や軽鎖(LAT1)の発現を低下させ、アミノ酸取り込みを抑制すると、腫瘍の増殖が抑えられることが考えられた。即ち、軽鎖(LAT1)の発現を低下させると、腫瘍の増殖抑制が起きることが癌移植マウスモデルで報告されている(特許文献1)。そこで、LAT1の活性を抑えることは癌の治療法に有望であると考えられた。
また、CD98(重鎖と軽鎖の複合体)のアミノ酸輸送を抑制するため、抗CD98抗体を用いた癌細胞の抑制効果が癌移植マウスモデルで報告されている(特許文献2、3)。このように、抗CD98抗体を使用して、抗癌剤として使用する試みについては、色々取り組みが行われている。しかし、抗癌剤以外の用途に関する取り組みは、ほとんど行われていない状況である。
しかも、siRNAを用いてCD98の発現を抑制し、上記抗癌剤等に関する治療効果を評価する試みについては、全く行なわれていない状況である。 CD98 is a heterodimeric glycoprotein of approximately 120 kDa that is widely expressed on the cell membrane and is thought to be associated with amino acid transport, increased cell membrane expression and cell fusion associated with integrin-mediated adhesion . The structure of CD98 is heterozygous by disulfide bond with one of approximately 80 kDa type II transmembrane protein CD98hc (heavy chain: 4F2hc, SLC3A2) and one of six types of approximately 40 kDa light chain (LAT1, LAT2, y + LAT1, y + LAT2, xCT, asc). It constitutes a dimer and functions as an amino acid transporter. The heavy chain of CD98 (CD98hc) has a role of transferring a dimer to the cell membrane, and the light chain (lc) is considered to have a transporter function.
CD98hc was first discovered as a T cell activation antigen and is a glycoprotein having various functions. Its function is highly expressed in proliferating lymphocytes, tumor cells, and other highly proliferative cells, and plays an important role in integrin-dependent signals that promote tumorigenesis.
Amino acid transport system that CD98 is involved, is composed from one to reflect the diversity of the amino acid molecules as substrates six transporters (the light chain of about 40 kDa), the transport substrate selectivity and Na + It is classified into various transport systems by dependence. For example, specific transporters corresponding to the neutral amino acid transport system L, small neutral amino acid transport system asc, neutral and basic amino acid transport system y + L, cystine, basic and neutral amino acid transport system xc (LAT1, LAT2, y + LAT1, y + LAT2, xCT, asc) and disulfide bonds. The CD98 heavy chain is present in the cell membrane on the blood vessel side of the epithelial cells and functions to transport the transporter to the cell membrane on the blood vessel side of the epithelial cells.
Further, CD98 is known to be highly expressed in various cancer cells. The reason for this is that cancer cells need to actively take in essential amino acids necessary for growth in order to promote cell growth, and it is considered that neutral amino acid transporters are overexpressed there. For example, L-type amino acid transporter 1 (LAT1) has been cloned as an amino acid transporter that is highly expressed specifically in cancer cells (Kanai et al., J. Biol. Chem. (1998), 273). , 23629-23632). LAT1 forms a complex with CD98 heavy chain and has a function of transporting neutral amino acids having large side chains such as leucine, valine, phenylalanine, tyrosine, tryptophan, methionine, and histidine independent of sodium ions. ing. This LAT1 is low in expression or not observed in most normal tissues except brain, placenta, bone marrow, and testis, but colon cancer, stomach cancer, breast cancer, pancreatic cancer, renal cancer, laryngeal cancer, esophagus In human malignant tumor tissues such as cancer and lung cancer, it is known that expression is enhanced together with CD98 (Non-patent Document 1).
Therefore, it was considered that tumor growth can be suppressed by reducing the expression of CD98 heavy chain and light chain (LAT1) and suppressing amino acid uptake. That is, it has been reported in a mouse model transplanted with cancer that suppression of tumor growth occurs when the expression of the light chain (LAT1) is decreased (Patent Document 1). Therefore, suppressing LAT1 activity was considered promising for cancer therapy.
In addition, in order to suppress amino acid transport of CD98 (a complex of heavy chain and light chain), an inhibitory effect on cancer cells using an anti-CD98 antibody has been reported in a cancer transplanted mouse model (
Moreover, no attempt has been made to try to evaluate the therapeutic effect of the anticancer agent and the like by suppressing the expression of CD98 using siRNA.
本発明は、CD98の機能性核酸による自己免疫疾患の医薬組成物の提供を目的とする。特に、CD98重鎖の発現抑制型のsiRNAを有効成分とする医薬組成物の提供を目的とする。
The object of the present invention is to provide a pharmaceutical composition for autoimmune diseases using a functional nucleic acid of CD98. In particular, an object of the present invention is to provide a pharmaceutical composition containing siRNA that suppresses CD98 heavy chain expression as an active ingredient.
本発明者は、CD98の重鎖を認識するモノクロナール抗体を特定し、このモノクロナール抗体がT細胞の活性化を抑制することを見出した。そして、この抗体は、アミノ酸輸送に影響を与えず、フィブロネクチンを介した細胞接着を抑制することを見出した。そして、この抗体を用いて糖尿病NODマウスを治療したところ、CD4+T細胞の活性化を抑制し、糖尿病の状態を通常のレベルに戻すことが出来た(PCT/JP2011/057432)。
そこで、本発明者は、CD98重鎖の発現を抑制するsiRNAを用いて、糖尿病NODマウスの治療を行なったところ、CD98の重鎖に対するsiRNAが1型糖尿病の進行を抑制するだけでなく、治療できることを見出した。即ち、これまでインスリンの補充療法以外に適切な治療方法のなかった膵臓β細胞のアポトーシスに基づく1型糖尿病に対して、本抗体が非常に有効であることが示された。更に関節リウマチモデルマウスにCD98重鎖の発現を抑制できるsiRNAを投与したところ、関節炎の発症が抑制されることを確認した。このような知見は、CD98のsiRNAを有効成分として含む医薬組成物が、自己免疫疾患(1型糖尿病や関節リュウマチ等)の治療・予防に有効であることを示すものである。
これらの知見から、本発明のsiRNAは、自己免疫疾患の治療薬として有用であることが示された。本発明者は、以上の知見に基づいて本発明を完成した。
すなわち本発明は以下のとおりである。
(1)CD98重鎖の発現を抑制する機能性核酸を有効成分として含むことを特徴とする自己免疫疾患を予防又は治療するための医薬組成物。
(2)上記機能性核酸がsiRNAである、上記(1)記載の医薬組成物。
(3)上記自己免疫疾患が1型糖尿病又は関節リウマチである、上記(1)又は(2)の医薬組成物。
(4)上記siRNAが二本鎖RNAである、上記(2)記載の医薬組成物。
(5)上記二本鎖RNAが、配列番号1と配列番号2、配列番号3と配列番号4、あるいは配列番号5と配列番号6の一つ以上を含有するsiRNAである、上記(4)記載の医薬組成物。
(6)上記二本鎖RNAが、配列番号7と配列番号8、配列番号9と配列番号10、あるいは配列番号11と配列番号12の一つ以上を含有するsiRNAである、上記(4)記載の医薬組成物。
(7)配列番号1と配列番号2、配列番号3と配列番号4、あるいは配列番号5と配列番号6のいずれかの配列を有する二本鎖siRNA。
(8)二本鎖RNA部分が、配列番号7と配列番号8、配列番号9と配列番号10、あるいは配列番号11と配列番号12のいずれかの配列を有する二本鎖siRNA。
(9)哺乳類に、CD98重鎖の発現を抑制する機能性核酸を有効量投与することを特徴とする自己免疫疾患の治療方法。
(10)上記機能性核酸が、siRNAである、上記(9)記載の治療方法。
(11)上記siRNAが、上記(7)または(8)の2本鎖siRNAである、上記(10)記載の治療方法。
(12)上記自己免疫疾患が、1型糖尿病又は関節リウマチである上記(9)~(11)のいずれかに記載の治療方法。 The present inventor has identified a monoclonal antibody that recognizes the heavy chain of CD98, and found that this monoclonal antibody suppresses activation of T cells. The present inventors have found that this antibody does not affect amino acid transport and suppresses cell adhesion via fibronectin. Then, when this antibody was used to treat diabetic NOD mice, it was possible to suppress the activation of CD4 + T cells and return the diabetic state to the normal level (PCT / JP2011 / 057432).
Therefore, the present inventor treated diabetic NOD mice using siRNA that suppresses the expression of CD98 heavy chain. The siRNA against CD98 heavy chain not only suppressed the progression oftype 1 diabetes, but also the treatment. I found out that I can do it. That is, it was shown that this antibody is very effective against type 1 diabetes based on apoptosis of pancreatic β cells, for which there has been no appropriate therapeutic method other than insulin replacement therapy. Further, when siRNA capable of suppressing the expression of CD98 heavy chain was administered to rheumatoid arthritis model mice, it was confirmed that the onset of arthritis was suppressed. Such findings indicate that a pharmaceutical composition containing siRNA of CD98 as an active ingredient is effective for treatment / prevention of autoimmune diseases (type 1 diabetes, rheumatoid arthritis, etc.).
From these findings, it was shown that the siRNA of the present invention is useful as a therapeutic agent for autoimmune diseases. The present inventor completed the present invention based on the above findings.
That is, the present invention is as follows.
(1) A pharmaceutical composition for preventing or treating an autoimmune disease comprising a functional nucleic acid that suppresses the expression of CD98 heavy chain as an active ingredient.
(2) The pharmaceutical composition according to (1) above, wherein the functional nucleic acid is siRNA.
(3) The pharmaceutical composition according to (1) or (2), wherein the autoimmune disease istype 1 diabetes or rheumatoid arthritis.
(4) The pharmaceutical composition according to (2) above, wherein the siRNA is a double-stranded RNA.
(5) The above (4) description, wherein the double-stranded RNA is an siRNA containing one or more of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4, or SEQ ID NO: 5 and SEQ ID NO: 6. Pharmaceutical composition.
(6) The above (4) description, wherein the double-stranded RNA is an siRNA containing one or more of SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and SEQ ID NO: 10, or SEQ ID NO: 11 and SEQ ID NO: 12. Pharmaceutical composition.
(7) Double-stranded siRNA having any one of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4, or SEQ ID NO: 5 and SEQ ID NO: 6.
(8) Double-stranded siRNA in which the double-stranded RNA portion has any one of SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and SEQ ID NO: 10, or SEQ ID NO: 11 and SEQ ID NO: 12.
(9) A method for treating an autoimmune disease, comprising administering to a mammal an effective amount of a functional nucleic acid that suppresses the expression of CD98 heavy chain.
(10) The method according to (9) above, wherein the functional nucleic acid is siRNA.
(11) The method according to (10) above, wherein the siRNA is the double-stranded siRNA of (7) or (8).
(12) The method according to any one of (9) to (11), wherein the autoimmune disease istype 1 diabetes or rheumatoid arthritis.
そこで、本発明者は、CD98重鎖の発現を抑制するsiRNAを用いて、糖尿病NODマウスの治療を行なったところ、CD98の重鎖に対するsiRNAが1型糖尿病の進行を抑制するだけでなく、治療できることを見出した。即ち、これまでインスリンの補充療法以外に適切な治療方法のなかった膵臓β細胞のアポトーシスに基づく1型糖尿病に対して、本抗体が非常に有効であることが示された。更に関節リウマチモデルマウスにCD98重鎖の発現を抑制できるsiRNAを投与したところ、関節炎の発症が抑制されることを確認した。このような知見は、CD98のsiRNAを有効成分として含む医薬組成物が、自己免疫疾患(1型糖尿病や関節リュウマチ等)の治療・予防に有効であることを示すものである。
これらの知見から、本発明のsiRNAは、自己免疫疾患の治療薬として有用であることが示された。本発明者は、以上の知見に基づいて本発明を完成した。
すなわち本発明は以下のとおりである。
(1)CD98重鎖の発現を抑制する機能性核酸を有効成分として含むことを特徴とする自己免疫疾患を予防又は治療するための医薬組成物。
(2)上記機能性核酸がsiRNAである、上記(1)記載の医薬組成物。
(3)上記自己免疫疾患が1型糖尿病又は関節リウマチである、上記(1)又は(2)の医薬組成物。
(4)上記siRNAが二本鎖RNAである、上記(2)記載の医薬組成物。
(5)上記二本鎖RNAが、配列番号1と配列番号2、配列番号3と配列番号4、あるいは配列番号5と配列番号6の一つ以上を含有するsiRNAである、上記(4)記載の医薬組成物。
(6)上記二本鎖RNAが、配列番号7と配列番号8、配列番号9と配列番号10、あるいは配列番号11と配列番号12の一つ以上を含有するsiRNAである、上記(4)記載の医薬組成物。
(7)配列番号1と配列番号2、配列番号3と配列番号4、あるいは配列番号5と配列番号6のいずれかの配列を有する二本鎖siRNA。
(8)二本鎖RNA部分が、配列番号7と配列番号8、配列番号9と配列番号10、あるいは配列番号11と配列番号12のいずれかの配列を有する二本鎖siRNA。
(9)哺乳類に、CD98重鎖の発現を抑制する機能性核酸を有効量投与することを特徴とする自己免疫疾患の治療方法。
(10)上記機能性核酸が、siRNAである、上記(9)記載の治療方法。
(11)上記siRNAが、上記(7)または(8)の2本鎖siRNAである、上記(10)記載の治療方法。
(12)上記自己免疫疾患が、1型糖尿病又は関節リウマチである上記(9)~(11)のいずれかに記載の治療方法。 The present inventor has identified a monoclonal antibody that recognizes the heavy chain of CD98, and found that this monoclonal antibody suppresses activation of T cells. The present inventors have found that this antibody does not affect amino acid transport and suppresses cell adhesion via fibronectin. Then, when this antibody was used to treat diabetic NOD mice, it was possible to suppress the activation of CD4 + T cells and return the diabetic state to the normal level (PCT / JP2011 / 057432).
Therefore, the present inventor treated diabetic NOD mice using siRNA that suppresses the expression of CD98 heavy chain. The siRNA against CD98 heavy chain not only suppressed the progression of
From these findings, it was shown that the siRNA of the present invention is useful as a therapeutic agent for autoimmune diseases. The present inventor completed the present invention based on the above findings.
That is, the present invention is as follows.
(1) A pharmaceutical composition for preventing or treating an autoimmune disease comprising a functional nucleic acid that suppresses the expression of CD98 heavy chain as an active ingredient.
(2) The pharmaceutical composition according to (1) above, wherein the functional nucleic acid is siRNA.
(3) The pharmaceutical composition according to (1) or (2), wherein the autoimmune disease is
(4) The pharmaceutical composition according to (2) above, wherein the siRNA is a double-stranded RNA.
(5) The above (4) description, wherein the double-stranded RNA is an siRNA containing one or more of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4, or SEQ ID NO: 5 and SEQ ID NO: 6. Pharmaceutical composition.
(6) The above (4) description, wherein the double-stranded RNA is an siRNA containing one or more of SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and SEQ ID NO: 10, or SEQ ID NO: 11 and SEQ ID NO: 12. Pharmaceutical composition.
(7) Double-stranded siRNA having any one of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4, or SEQ ID NO: 5 and SEQ ID NO: 6.
(8) Double-stranded siRNA in which the double-stranded RNA portion has any one of SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and SEQ ID NO: 10, or SEQ ID NO: 11 and SEQ ID NO: 12.
(9) A method for treating an autoimmune disease, comprising administering to a mammal an effective amount of a functional nucleic acid that suppresses the expression of CD98 heavy chain.
(10) The method according to (9) above, wherein the functional nucleic acid is siRNA.
(11) The method according to (10) above, wherein the siRNA is the double-stranded siRNA of (7) or (8).
(12) The method according to any one of (9) to (11), wherein the autoimmune disease is
本発明によれば、CD98重鎖の発現を抑制する機能性核酸を投与することにより、高い治療効果を得ることができるため、本発明の医薬組成物および治療法は、自己免疫疾患の治療に極めて有用である。特に、これまで効果的な治療方法のなかった、1型糖尿病と関節リウマチに有効である。
According to the present invention, since a high therapeutic effect can be obtained by administering a functional nucleic acid that suppresses the expression of CD98 heavy chain, the pharmaceutical composition and the therapeutic method of the present invention are useful for the treatment of autoimmune diseases. Very useful. It is particularly effective for type 1 diabetes and rheumatoid arthritis, for which there has been no effective treatment.
図1は1型糖尿病を発症したNODマウスのT細胞を重症複合型免疫不全症のNODモデルマウス(NOD−SCIDマウス)に投与する。投与後のマウスに、CD98重鎖に対するマウスsiRNA(4nmol/mouse)、あるいはコントロールのsiRNAを、2日目、5日目、8日目に投与する。なお、T細胞を投与した日を起算日とする。
図1は、3日後の末梢血単核球細胞のCD98重鎖の発現をフローサイトメトリーで評価した図である。灰色(GRAY)の部分は、CD98抗体を投与した場合の測定結果である。点線部分は、コントロールのsiRNAを投与した場合の測定結果であり、実線部分は、CD98重鎖に対するsiRNAを投与した場合の測定結果を示している。
図2は1型糖尿病の治療効果を見るために、血糖値を測り、評価した図である。マウス5匹を一群として実験を行い、*印は統計的な有意差を示す(p<0.05)。点線部分は、コントロールのsiRNAを投与した場合の結果であり、実線部分は、CD98重鎖に対するsiRNAを投与した場合の結果である。
図3はリウマチモデルマウス(コラーゲン誘導性関節炎モデル)を使用し、siRNAを0日目、1日目、2日目、3日目、4日目に腹腔内投与した結果を表わした図である。マウスsiRNAの投与により、リウマチの発症が大きく軽減された結果が示されている。
図4はヒトTリンパ腫由来のJurkat細胞に対してヒトsiRNAを投与し、細胞表面におけるCD98タンパクの発現の変化を、フローサイトメーターで測定、評価した図である。無処置群、コントロールsiRNA投与群と比較し、ヒトsiRNAを投与することにより、CD98タンパクの発現の抑制が大きく抑制されたことを示している。 In FIG. 1, T cells of NOD mice that have developedtype 1 diabetes are administered to NOD model mice (NOD-SCID mice) with severe combined immunodeficiency. After administration, mice are administered mouse siRNA against CD98 heavy chain (4 nmol / mouse) or control siRNA on days 2, 5, and 8. The day on which T cells are administered is taken as the starting date.
FIG. 1 is a diagram in which the expression of CD98 heavy chain in peripheral blood mononuclear cells after 3 days was evaluated by flow cytometry. The gray (GRAY) part is the measurement result when the CD98 antibody was administered. The dotted line portion shows the measurement results when the control siRNA is administered, and the solid line portion shows the measurement results when the siRNA against CD98 heavy chain is administered.
FIG. 2 is a diagram showing blood glucose levels measured and evaluated in order to see the therapeutic effect oftype 1 diabetes. The experiment was conducted with 5 mice as a group, and the symbol * indicates a statistically significant difference (p <0.05). A dotted line part is a result at the time of administering control siRNA, and a continuous line part is a result at the time of administering siRNA with respect to CD98 heavy chain.
FIG. 3 shows the results of intraperitoneal administration of siRNA onday 0, day 1, day 2, day 3, day 4 using rheumatoid model mice (collagen-induced arthritis model). . The result shows that the onset of rheumatism is greatly reduced by administration of mouse siRNA.
FIG. 4 is a diagram in which human siRNA was administered to Jurkat cells derived from human T lymphoma, and changes in the expression of CD98 protein on the cell surface were measured and evaluated using a flow cytometer. Compared with the untreated group and the control siRNA administration group, the suppression of the expression of CD98 protein was greatly suppressed by administering human siRNA.
図1は、3日後の末梢血単核球細胞のCD98重鎖の発現をフローサイトメトリーで評価した図である。灰色(GRAY)の部分は、CD98抗体を投与した場合の測定結果である。点線部分は、コントロールのsiRNAを投与した場合の測定結果であり、実線部分は、CD98重鎖に対するsiRNAを投与した場合の測定結果を示している。
図2は1型糖尿病の治療効果を見るために、血糖値を測り、評価した図である。マウス5匹を一群として実験を行い、*印は統計的な有意差を示す(p<0.05)。点線部分は、コントロールのsiRNAを投与した場合の結果であり、実線部分は、CD98重鎖に対するsiRNAを投与した場合の結果である。
図3はリウマチモデルマウス(コラーゲン誘導性関節炎モデル)を使用し、siRNAを0日目、1日目、2日目、3日目、4日目に腹腔内投与した結果を表わした図である。マウスsiRNAの投与により、リウマチの発症が大きく軽減された結果が示されている。
図4はヒトTリンパ腫由来のJurkat細胞に対してヒトsiRNAを投与し、細胞表面におけるCD98タンパクの発現の変化を、フローサイトメーターで測定、評価した図である。無処置群、コントロールsiRNA投与群と比較し、ヒトsiRNAを投与することにより、CD98タンパクの発現の抑制が大きく抑制されたことを示している。 In FIG. 1, T cells of NOD mice that have developed
FIG. 1 is a diagram in which the expression of CD98 heavy chain in peripheral blood mononuclear cells after 3 days was evaluated by flow cytometry. The gray (GRAY) part is the measurement result when the CD98 antibody was administered. The dotted line portion shows the measurement results when the control siRNA is administered, and the solid line portion shows the measurement results when the siRNA against CD98 heavy chain is administered.
FIG. 2 is a diagram showing blood glucose levels measured and evaluated in order to see the therapeutic effect of
FIG. 3 shows the results of intraperitoneal administration of siRNA on
FIG. 4 is a diagram in which human siRNA was administered to Jurkat cells derived from human T lymphoma, and changes in the expression of CD98 protein on the cell surface were measured and evaluated using a flow cytometer. Compared with the untreated group and the control siRNA administration group, the suppression of the expression of CD98 protein was greatly suppressed by administering human siRNA.
−本発明の第一の態様−
本発明の一つの態様としては、「CD98重鎖の発現を抑制する機能性核酸を有効成分として含むことを特徴とする自己免疫疾患を予防又は治療するための医薬組成物」および「CD98重鎖の発現を抑制する機能性核酸を有効量投与することを特徴とする自己免疫疾患の治療方法」が挙げられる。ここで「CD98」は公知のタンパク質であり、DNA配列とアミノ酸配列は、特許文献2等に記載されている。本発明における「CD98」は、これらの配列に限定されるものではなく、CD98の機能が保持される限り、アミノ酸やDNAの変異数や変異部位には制限はないものとする。
本発明において「機能性核酸」とは、タンパク質の発現を抑制できる機能を持ったsiRNA、miRNA、アプタマー、リボザイム、アンチセンス核酸のことを言う。好ましいものとしては、siRNAを挙げることができる。即ち、本発明のsiRNAとは、CD98重鎖の遺伝子発現を抑制するsiRNAをさす。具体的には、対照群(コントロールsiRNAを使用した場合)と比較してCD98の遺伝子発現を50%以上抑制できるsiRNAを、好ましくは80%以上抑制できるsiRNAをいう。siRNAは、RNA干渉と呼ばれる配列特異的な遺伝子発現の抑制を誘導することが知られている。siRNAは、一般的に二本鎖のRNA部分と、センス鎖およびアンチセンス鎖の3’末端のオーバーハング部分から構成される。siRNAは、当業者にとって公知の方法によって設計することができる。例えば、選択したDNA配列(19~21塩基が望ましい)をそのままRNA配列に変換したもの(センス鎖)とそのアンチセンス鎖を二本鎖RNA部分とし、オーバーハング部を付加する。オーバーハング部は、1又は2塩基の任意の核酸(リボ核酸またはデオキシ核酸)であるが、ウリジン(U)もしくはチミジン(dT)が好ましい。本発明のsiRNAは、CD98、好ましくはヒトのCD98重鎖のDNA配列に基づいて設計され、CD98重鎖の発現を抑制できるsiRNAであれば、特に限定されるものではない。発現の抑制は、CD98重鎖に特異的であることが望ましい。特異的であるかどうかは、一般公開されているBLAST検索を実施することにより確認できる。またオーバーハング部分は必須ではない。
また本発明のsiRNAには、投与対象内で本発明のsiRNAと同じ効果を有する任意の分子も含まれる。このような分子としては、例えば、shRNAが挙げられる。shRNAとはショートヘアピン構造型のRNAであり、一本鎖の一部の領域が他の領域と相補鎖を形成するためにステムループ構造を有するRNA分子である。二本鎖RNA部分が、本発明のsiRNAと同一構造を有するshRNAも本発明のsiRNAに含まれる。他には、投与対象に投与することによって本発明のsiRNAを発現することができるようなDNAも本発明のsiRNAに含まれる。このようなDNAは、siRNAをコードするDNAを発現ベクター(例えばアデノウイルス、アデノ随伴ウイルス、ヘルペスウイルス、レンチウイルスなどのベクター)に組み込んで構築して使用される。
本発明において、機能性核酸を医薬組成物として使用する場合には、治療薬としての特性を改善するための修飾、あるいはリポソームなどの輸送担体に含包することが望ましい。ポリヌクレオチドの治療薬としての特性を改善するには、ヌクレオチドの修飾または類似体を導入することができる。例えば、ヌクレアーゼ耐性の向上および/または細胞透過性の向上などである。ヌクレアーゼ耐性は、アンチセンス、siRNA、shRNAおよび/またはリボザイムの生理活性を妨げないような技術上周知の任意の方法によりもたらされる。ヌクレアーゼ耐性の向上を目的にオリゴヌクレオチドに加えることができる修飾の例としては、リン酸骨格中のヘテロ原子のリンまたは酸素の修飾である。例えば、メチルリン酸、ホスホロチオエート、ホスホロジチオエート、およびモルホリノオリゴマーなどである。また、技術上周知の他の修飾により、生理活性は維持したまま、ヌクレアーゼに対する安定性を大幅に高めるようにしてもよい。
更に、本発明において機能性核酸を医薬組成物や治療方法として使用する場合には、機能性核酸そのものを治療に用いる方法と、ベクターを用いて機能性核酸を発現させて治療に用いる方法が存在する。
機能性核酸そのものを治療に用いる場合には、アテロコラーゲン等の安定化剤、pH調節剤などを添加した水溶液を作製し、そのまま非経口で投与することが望ましい。
ベクターを用いてin vivo発現を行なう場合には、特に本発明の治療を必要とする患者体内での発現に際しては、発現ベクター、特に哺乳動物発現ベクターを使用するのが望ましい。発現ベクターは技術上周知であり、好ましくはプラスミド、コスミド、ウイルス発現系を含む。好ましいウイルス発現系の例としてはアデノウイルス、レトロウイルス、レンチウイルスなどである。また細胞や組織にベクターを導入する方法は技術上周知である。好ましい例としては、トランスフェクション、リポフェクション、エレクトロポレーション、および組み換えウイルスベクターによる感染などである。
本発明の「哺乳動物」とは、例えばラット、マウス、モルモット等のげっ歯類、例えばイヌやネコ、更には例えばヒトやサルの霊長類を挙げることができる。
本発明の「自己免疫疾患」とは、免疫系が内因性抗原に対する自己抗体を起こすことで起こる疾患であり、例えば1型糖尿病、関節リウマチ、潰瘍性大腸炎、乾癬、クローン病、全身性エリテマトーデス、バセドウ病等を挙げることができる。いずれの自己免疫疾患も、T細胞の異常な過剰活性化がその原因となっていることがこれまでの研究から明らかになっている。特に、CD4陽性あるいはCD8陽性のTリンパ球(T細胞)が過剰に活性化し、自己抗体の産生を促したり、組織破壊を引き起こしたりすることが病状の進展に大きな影響を与えることは広く知られている事実である。従って、本発明のsiRNAが抗CD98抗体と同様にTリンパ球(T細胞)の活性化を抑制することから、本発明のsiRNAを用いて、上記の自己免疫疾患の治療を行なうことができる。例えば、図2に示されるように、本発明のsiRNAは、1型糖尿病の治療に有効であり、また、図3に示されるように、関節リウマチの治療に有効である。このように、本発明のsiRNAは、例えば1型糖尿病、関節リウマチ、潰瘍性大腸炎、乾癬、クローン病、全身性エリテマトーデス、バセドウ病等の自己免疫疾患の治療に使用することができる。以上のように、本発明のsiRNAの好ましい用途としては、1型糖尿病、関節リウマチ、潰瘍性大腸炎、乾癬、クローン病、全身性エリテマトーデス、バセドウ病であり、特に好ましい用途として、1型糖尿病あるいは関節リウマチを挙げることができる。
本発明の「関節リウマチ」とは、関節や骨、筋肉、じん帯、腱などが痛む自己免疫疾患のことを言い、きわめて慢性の経過をたどり治療に難渋することが多いもっとも頻度の高い自己免疫疾患である。関節リウマチは、CD4陽性T細胞が過剰に活性化し、様々なサイトカインを産生することに加えて、CD8陽性T細胞が関節組織を破壊することによって病状が進展することが知られている。そのため、T細胞(Tリンパ球)の活性化抑制作用を持つ、本発明のsiRNAは、関節リウマチの治療に好適なものである。
本発明の「1型糖尿病」とは、主に自己免疫により膵β細胞が破壊され、インスリンの産生が枯渇して、インスリン不足に陥る疾患を言う。また、糖尿病が重症化し、インスリン抵抗性が亢進し、膵臓β細胞が疲弊し、アポトーシスが起こり始めると、2型糖尿病から結果として、1型糖尿病に推移して来ることになる。1型糖尿病の発症にはCD4陽性およびCD8陽性T細胞のいずれもが寄与し、最終的にCD8陽性T細胞が膵臓β細胞を破壊することによりインスリンの欠乏が惹起される。従って、T細胞(Tリンパ球)の活性化抑制作用を持つ、本発明のsiRNAは、1型糖尿病の予防と治療に好適なものである。
本発明の医薬組成物により、自己免疫疾患を治療する際には、上記の機能性核酸と共に、通常の製剤学的に許容しうる1又は2種以上の製剤用添加物を用いて医薬組成物を製造して投与することが好ましい。本発明の医薬組成物の投与方法は非経口投与が望ましく、静脈投与、筋肉内投与、腹腔内投与、または皮下投与などを行うことができる。
本発明の機能性核酸の投与量は、投与対象、投与方法等により異なるが、例えば非経口投与する場合は、自己免疫疾患の患者(60kg)に対して、例えば、一日約0.01~30mg、好ましくは約0.1~20mg、より好ましくは約0.1~10mgを静脈注射することができる。 -First embodiment of the present invention-
As one aspect of the present invention, “a pharmaceutical composition for preventing or treating an autoimmune disease comprising a functional nucleic acid that suppresses the expression of CD98 heavy chain as an active ingredient” and “CD98 heavy chain” And a method for treating an autoimmune disease, which comprises administering an effective amount of a functional nucleic acid that suppresses the expression of an ". Here, “CD98” is a known protein, and the DNA sequence and amino acid sequence are described inPatent Document 2 and the like. “CD98” in the present invention is not limited to these sequences, and the number of amino acid and DNA mutations and mutation sites are not limited as long as the function of CD98 is maintained.
In the present invention, “functional nucleic acid” refers to siRNA, miRNA, aptamer, ribozyme, and antisense nucleic acid having a function capable of suppressing protein expression. Preferred examples include siRNA. That is, the siRNA of the present invention refers to an siRNA that suppresses gene expression of CD98 heavy chain. Specifically, it refers to siRNA that can suppress the gene expression of CD98 by 50% or more, and preferably siRNA that can suppress 80% or more compared to the control group (when control siRNA is used). siRNA is known to induce sequence-specific suppression of gene expression called RNA interference. siRNA is generally composed of a double-stranded RNA portion and an overhang portion at the 3 ′ end of the sense strand and the antisense strand. siRNAs can be designed by methods known to those skilled in the art. For example, a selected DNA sequence (preferably 19 to 21 bases) is directly converted into an RNA sequence (sense strand) and its antisense strand as a double-stranded RNA portion, and an overhang portion is added. The overhang portion is an arbitrary nucleic acid (ribonucleic acid or deoxynucleic acid) having 1 or 2 bases, and uridine (U) or thymidine (dT) is preferable. The siRNA of the present invention is not particularly limited as long as it is designed based on the DNA sequence of CD98, preferably human CD98 heavy chain, and can suppress the expression of CD98 heavy chain. The suppression of expression is desirably specific for the CD98 heavy chain. Whether it is specific or not can be confirmed by conducting a publicly available BLAST search. The overhang part is not essential.
The siRNA of the present invention also includes any molecule having the same effect as the siRNA of the present invention within the administration subject. An example of such a molecule is shRNA. shRNA is RNA having a short hairpin structure, and is an RNA molecule having a stem-loop structure so that a part of a single strand forms a complementary strand with another region. ShRNA in which the double-stranded RNA portion has the same structure as the siRNA of the present invention is also included in the siRNA of the present invention. In addition, a DNA that can express the siRNA of the present invention by being administered to an administration subject is also included in the siRNA of the present invention. Such a DNA is used by constructing a DNA encoding siRNA into an expression vector (for example, a vector such as adenovirus, adeno-associated virus, herpes virus, lentivirus).
In the present invention, when a functional nucleic acid is used as a pharmaceutical composition, it is desirable to include a modification for improving the properties as a therapeutic agent or a transport carrier such as a liposome. To improve the therapeutic properties of the polynucleotide, nucleotide modifications or analogs can be introduced. For example, improved nuclease resistance and / or improved cell permeability. Nuclease resistance is brought about by any method known in the art that does not interfere with the biological activity of the antisense, siRNA, shRNA and / or ribozyme. An example of a modification that can be added to an oligonucleotide for the purpose of improving nuclease resistance is modification of a heteroatom's phosphorus or oxygen in the phosphate backbone. For example, methyl phosphate, phosphorothioate, phosphorodithioate, and morpholino oligomers. In addition, other modifications known in the art may greatly increase the stability against nucleases while maintaining physiological activity.
Furthermore, when the functional nucleic acid is used as a pharmaceutical composition or a treatment method in the present invention, there are a method of using the functional nucleic acid itself for treatment and a method of expressing the functional nucleic acid using a vector and using it for the treatment. To do.
When the functional nucleic acid itself is used for treatment, it is desirable to prepare an aqueous solution to which a stabilizer such as atelocollagen, a pH regulator and the like are added and administered parenterally as it is.
When performing in vivo expression using a vector, it is desirable to use an expression vector, particularly a mammalian expression vector, particularly for expression in a patient requiring the treatment of the present invention. Expression vectors are well known in the art and preferably include plasmids, cosmids, and viral expression systems. Examples of preferred virus expression systems are adenovirus, retrovirus, lentivirus and the like. In addition, methods for introducing vectors into cells and tissues are well known in the art. Preferred examples include transfection, lipofection, electroporation, and infection with recombinant viral vectors.
Examples of the “mammal” of the present invention include rodents such as rats, mice, and guinea pigs, such as dogs and cats, and humans and monkeys.
The “autoimmune disease” of the present invention is a disease caused by the immune system raising autoantibodies against endogenous antigens. For example,type 1 diabetes, rheumatoid arthritis, ulcerative colitis, psoriasis, Crohn's disease, systemic lupus erythematosus And Graves' disease. Previous studies have revealed that any autoimmune disease is caused by abnormal overactivation of T cells. In particular, it is widely known that CD4 positive or CD8 positive T lymphocytes (T cells) are excessively activated to promote the production of autoantibodies or cause tissue destruction, which has a great influence on the progress of disease states. It is a fact that. Therefore, since the siRNA of the present invention suppresses the activation of T lymphocytes (T cells) similarly to the anti-CD98 antibody, the above-described autoimmune diseases can be treated using the siRNA of the present invention. For example, as shown in FIG. 2, the siRNA of the present invention is effective for the treatment of type 1 diabetes, and as shown in FIG. 3, it is effective for the treatment of rheumatoid arthritis. Thus, the siRNA of the present invention can be used for the treatment of autoimmune diseases such as type 1 diabetes, rheumatoid arthritis, ulcerative colitis, psoriasis, Crohn's disease, systemic lupus erythematosus, and Graves' disease. As described above, preferred uses of the siRNA of the present invention are type 1 diabetes, rheumatoid arthritis, ulcerative colitis, psoriasis, Crohn's disease, systemic lupus erythematosus, and Graves' disease, and particularly preferred uses include type 1 diabetes or Rheumatoid arthritis can be mentioned.
“Rheumatoid arthritis” of the present invention refers to an autoimmune disease in which joints, bones, muscles, ligaments, tendons, etc. hurt, and the most frequent autoimmune disease, which is often difficult to treat following a very chronic course It is. In rheumatoid arthritis, it is known that CD4-positive T cells are excessively activated and various cytokines are produced, and in addition, CD8-positive T cells destroy joint tissues and progress in disease states. Therefore, the siRNA of the present invention having an action of suppressing activation of T cells (T lymphocytes) is suitable for the treatment of rheumatoid arthritis.
The “type 1 diabetes” of the present invention refers to a disease in which pancreatic β-cells are destroyed mainly by autoimmunity and insulin production is depleted, resulting in insulin deficiency. In addition, when diabetes becomes severe, insulin resistance increases, pancreatic β-cells become exhausted, and apoptosis begins to occur, type 2 diabetes results in transition to type 1 diabetes. Both CD4 positive and CD8 positive T cells contribute to the onset of type 1 diabetes, and finally, CD8 positive T cells destroy pancreatic β cells, leading to insulin deficiency. Therefore, the siRNA of the present invention having an activity of suppressing activation of T cells (T lymphocytes) is suitable for the prevention and treatment of type 1 diabetes.
When treating an autoimmune disease with the pharmaceutical composition of the present invention, the pharmaceutical composition is used together with the above-described functional nucleic acid and one or more kinds of conventional pharmaceutically acceptable additives. Is preferably prepared and administered. The pharmaceutical composition of the present invention is preferably administered parenterally, and can be administered intravenously, intramuscularly, intraperitoneally, or subcutaneously.
The dose of the functional nucleic acid of the present invention varies depending on the administration subject, administration method, and the like. For example, when administered parenterally, for example, about 0.01 to about 1 day per day for an autoimmune disease patient (60 kg). 30 mg, preferably about 0.1 to 20 mg, more preferably about 0.1 to 10 mg can be intravenously injected.
本発明の一つの態様としては、「CD98重鎖の発現を抑制する機能性核酸を有効成分として含むことを特徴とする自己免疫疾患を予防又は治療するための医薬組成物」および「CD98重鎖の発現を抑制する機能性核酸を有効量投与することを特徴とする自己免疫疾患の治療方法」が挙げられる。ここで「CD98」は公知のタンパク質であり、DNA配列とアミノ酸配列は、特許文献2等に記載されている。本発明における「CD98」は、これらの配列に限定されるものではなく、CD98の機能が保持される限り、アミノ酸やDNAの変異数や変異部位には制限はないものとする。
本発明において「機能性核酸」とは、タンパク質の発現を抑制できる機能を持ったsiRNA、miRNA、アプタマー、リボザイム、アンチセンス核酸のことを言う。好ましいものとしては、siRNAを挙げることができる。即ち、本発明のsiRNAとは、CD98重鎖の遺伝子発現を抑制するsiRNAをさす。具体的には、対照群(コントロールsiRNAを使用した場合)と比較してCD98の遺伝子発現を50%以上抑制できるsiRNAを、好ましくは80%以上抑制できるsiRNAをいう。siRNAは、RNA干渉と呼ばれる配列特異的な遺伝子発現の抑制を誘導することが知られている。siRNAは、一般的に二本鎖のRNA部分と、センス鎖およびアンチセンス鎖の3’末端のオーバーハング部分から構成される。siRNAは、当業者にとって公知の方法によって設計することができる。例えば、選択したDNA配列(19~21塩基が望ましい)をそのままRNA配列に変換したもの(センス鎖)とそのアンチセンス鎖を二本鎖RNA部分とし、オーバーハング部を付加する。オーバーハング部は、1又は2塩基の任意の核酸(リボ核酸またはデオキシ核酸)であるが、ウリジン(U)もしくはチミジン(dT)が好ましい。本発明のsiRNAは、CD98、好ましくはヒトのCD98重鎖のDNA配列に基づいて設計され、CD98重鎖の発現を抑制できるsiRNAであれば、特に限定されるものではない。発現の抑制は、CD98重鎖に特異的であることが望ましい。特異的であるかどうかは、一般公開されているBLAST検索を実施することにより確認できる。またオーバーハング部分は必須ではない。
また本発明のsiRNAには、投与対象内で本発明のsiRNAと同じ効果を有する任意の分子も含まれる。このような分子としては、例えば、shRNAが挙げられる。shRNAとはショートヘアピン構造型のRNAであり、一本鎖の一部の領域が他の領域と相補鎖を形成するためにステムループ構造を有するRNA分子である。二本鎖RNA部分が、本発明のsiRNAと同一構造を有するshRNAも本発明のsiRNAに含まれる。他には、投与対象に投与することによって本発明のsiRNAを発現することができるようなDNAも本発明のsiRNAに含まれる。このようなDNAは、siRNAをコードするDNAを発現ベクター(例えばアデノウイルス、アデノ随伴ウイルス、ヘルペスウイルス、レンチウイルスなどのベクター)に組み込んで構築して使用される。
本発明において、機能性核酸を医薬組成物として使用する場合には、治療薬としての特性を改善するための修飾、あるいはリポソームなどの輸送担体に含包することが望ましい。ポリヌクレオチドの治療薬としての特性を改善するには、ヌクレオチドの修飾または類似体を導入することができる。例えば、ヌクレアーゼ耐性の向上および/または細胞透過性の向上などである。ヌクレアーゼ耐性は、アンチセンス、siRNA、shRNAおよび/またはリボザイムの生理活性を妨げないような技術上周知の任意の方法によりもたらされる。ヌクレアーゼ耐性の向上を目的にオリゴヌクレオチドに加えることができる修飾の例としては、リン酸骨格中のヘテロ原子のリンまたは酸素の修飾である。例えば、メチルリン酸、ホスホロチオエート、ホスホロジチオエート、およびモルホリノオリゴマーなどである。また、技術上周知の他の修飾により、生理活性は維持したまま、ヌクレアーゼに対する安定性を大幅に高めるようにしてもよい。
更に、本発明において機能性核酸を医薬組成物や治療方法として使用する場合には、機能性核酸そのものを治療に用いる方法と、ベクターを用いて機能性核酸を発現させて治療に用いる方法が存在する。
機能性核酸そのものを治療に用いる場合には、アテロコラーゲン等の安定化剤、pH調節剤などを添加した水溶液を作製し、そのまま非経口で投与することが望ましい。
ベクターを用いてin vivo発現を行なう場合には、特に本発明の治療を必要とする患者体内での発現に際しては、発現ベクター、特に哺乳動物発現ベクターを使用するのが望ましい。発現ベクターは技術上周知であり、好ましくはプラスミド、コスミド、ウイルス発現系を含む。好ましいウイルス発現系の例としてはアデノウイルス、レトロウイルス、レンチウイルスなどである。また細胞や組織にベクターを導入する方法は技術上周知である。好ましい例としては、トランスフェクション、リポフェクション、エレクトロポレーション、および組み換えウイルスベクターによる感染などである。
本発明の「哺乳動物」とは、例えばラット、マウス、モルモット等のげっ歯類、例えばイヌやネコ、更には例えばヒトやサルの霊長類を挙げることができる。
本発明の「自己免疫疾患」とは、免疫系が内因性抗原に対する自己抗体を起こすことで起こる疾患であり、例えば1型糖尿病、関節リウマチ、潰瘍性大腸炎、乾癬、クローン病、全身性エリテマトーデス、バセドウ病等を挙げることができる。いずれの自己免疫疾患も、T細胞の異常な過剰活性化がその原因となっていることがこれまでの研究から明らかになっている。特に、CD4陽性あるいはCD8陽性のTリンパ球(T細胞)が過剰に活性化し、自己抗体の産生を促したり、組織破壊を引き起こしたりすることが病状の進展に大きな影響を与えることは広く知られている事実である。従って、本発明のsiRNAが抗CD98抗体と同様にTリンパ球(T細胞)の活性化を抑制することから、本発明のsiRNAを用いて、上記の自己免疫疾患の治療を行なうことができる。例えば、図2に示されるように、本発明のsiRNAは、1型糖尿病の治療に有効であり、また、図3に示されるように、関節リウマチの治療に有効である。このように、本発明のsiRNAは、例えば1型糖尿病、関節リウマチ、潰瘍性大腸炎、乾癬、クローン病、全身性エリテマトーデス、バセドウ病等の自己免疫疾患の治療に使用することができる。以上のように、本発明のsiRNAの好ましい用途としては、1型糖尿病、関節リウマチ、潰瘍性大腸炎、乾癬、クローン病、全身性エリテマトーデス、バセドウ病であり、特に好ましい用途として、1型糖尿病あるいは関節リウマチを挙げることができる。
本発明の「関節リウマチ」とは、関節や骨、筋肉、じん帯、腱などが痛む自己免疫疾患のことを言い、きわめて慢性の経過をたどり治療に難渋することが多いもっとも頻度の高い自己免疫疾患である。関節リウマチは、CD4陽性T細胞が過剰に活性化し、様々なサイトカインを産生することに加えて、CD8陽性T細胞が関節組織を破壊することによって病状が進展することが知られている。そのため、T細胞(Tリンパ球)の活性化抑制作用を持つ、本発明のsiRNAは、関節リウマチの治療に好適なものである。
本発明の「1型糖尿病」とは、主に自己免疫により膵β細胞が破壊され、インスリンの産生が枯渇して、インスリン不足に陥る疾患を言う。また、糖尿病が重症化し、インスリン抵抗性が亢進し、膵臓β細胞が疲弊し、アポトーシスが起こり始めると、2型糖尿病から結果として、1型糖尿病に推移して来ることになる。1型糖尿病の発症にはCD4陽性およびCD8陽性T細胞のいずれもが寄与し、最終的にCD8陽性T細胞が膵臓β細胞を破壊することによりインスリンの欠乏が惹起される。従って、T細胞(Tリンパ球)の活性化抑制作用を持つ、本発明のsiRNAは、1型糖尿病の予防と治療に好適なものである。
本発明の医薬組成物により、自己免疫疾患を治療する際には、上記の機能性核酸と共に、通常の製剤学的に許容しうる1又は2種以上の製剤用添加物を用いて医薬組成物を製造して投与することが好ましい。本発明の医薬組成物の投与方法は非経口投与が望ましく、静脈投与、筋肉内投与、腹腔内投与、または皮下投与などを行うことができる。
本発明の機能性核酸の投与量は、投与対象、投与方法等により異なるが、例えば非経口投与する場合は、自己免疫疾患の患者(60kg)に対して、例えば、一日約0.01~30mg、好ましくは約0.1~20mg、より好ましくは約0.1~10mgを静脈注射することができる。 -First embodiment of the present invention-
As one aspect of the present invention, “a pharmaceutical composition for preventing or treating an autoimmune disease comprising a functional nucleic acid that suppresses the expression of CD98 heavy chain as an active ingredient” and “CD98 heavy chain” And a method for treating an autoimmune disease, which comprises administering an effective amount of a functional nucleic acid that suppresses the expression of an ". Here, “CD98” is a known protein, and the DNA sequence and amino acid sequence are described in
In the present invention, “functional nucleic acid” refers to siRNA, miRNA, aptamer, ribozyme, and antisense nucleic acid having a function capable of suppressing protein expression. Preferred examples include siRNA. That is, the siRNA of the present invention refers to an siRNA that suppresses gene expression of CD98 heavy chain. Specifically, it refers to siRNA that can suppress the gene expression of CD98 by 50% or more, and preferably siRNA that can suppress 80% or more compared to the control group (when control siRNA is used). siRNA is known to induce sequence-specific suppression of gene expression called RNA interference. siRNA is generally composed of a double-stranded RNA portion and an overhang portion at the 3 ′ end of the sense strand and the antisense strand. siRNAs can be designed by methods known to those skilled in the art. For example, a selected DNA sequence (preferably 19 to 21 bases) is directly converted into an RNA sequence (sense strand) and its antisense strand as a double-stranded RNA portion, and an overhang portion is added. The overhang portion is an arbitrary nucleic acid (ribonucleic acid or deoxynucleic acid) having 1 or 2 bases, and uridine (U) or thymidine (dT) is preferable. The siRNA of the present invention is not particularly limited as long as it is designed based on the DNA sequence of CD98, preferably human CD98 heavy chain, and can suppress the expression of CD98 heavy chain. The suppression of expression is desirably specific for the CD98 heavy chain. Whether it is specific or not can be confirmed by conducting a publicly available BLAST search. The overhang part is not essential.
The siRNA of the present invention also includes any molecule having the same effect as the siRNA of the present invention within the administration subject. An example of such a molecule is shRNA. shRNA is RNA having a short hairpin structure, and is an RNA molecule having a stem-loop structure so that a part of a single strand forms a complementary strand with another region. ShRNA in which the double-stranded RNA portion has the same structure as the siRNA of the present invention is also included in the siRNA of the present invention. In addition, a DNA that can express the siRNA of the present invention by being administered to an administration subject is also included in the siRNA of the present invention. Such a DNA is used by constructing a DNA encoding siRNA into an expression vector (for example, a vector such as adenovirus, adeno-associated virus, herpes virus, lentivirus).
In the present invention, when a functional nucleic acid is used as a pharmaceutical composition, it is desirable to include a modification for improving the properties as a therapeutic agent or a transport carrier such as a liposome. To improve the therapeutic properties of the polynucleotide, nucleotide modifications or analogs can be introduced. For example, improved nuclease resistance and / or improved cell permeability. Nuclease resistance is brought about by any method known in the art that does not interfere with the biological activity of the antisense, siRNA, shRNA and / or ribozyme. An example of a modification that can be added to an oligonucleotide for the purpose of improving nuclease resistance is modification of a heteroatom's phosphorus or oxygen in the phosphate backbone. For example, methyl phosphate, phosphorothioate, phosphorodithioate, and morpholino oligomers. In addition, other modifications known in the art may greatly increase the stability against nucleases while maintaining physiological activity.
Furthermore, when the functional nucleic acid is used as a pharmaceutical composition or a treatment method in the present invention, there are a method of using the functional nucleic acid itself for treatment and a method of expressing the functional nucleic acid using a vector and using it for the treatment. To do.
When the functional nucleic acid itself is used for treatment, it is desirable to prepare an aqueous solution to which a stabilizer such as atelocollagen, a pH regulator and the like are added and administered parenterally as it is.
When performing in vivo expression using a vector, it is desirable to use an expression vector, particularly a mammalian expression vector, particularly for expression in a patient requiring the treatment of the present invention. Expression vectors are well known in the art and preferably include plasmids, cosmids, and viral expression systems. Examples of preferred virus expression systems are adenovirus, retrovirus, lentivirus and the like. In addition, methods for introducing vectors into cells and tissues are well known in the art. Preferred examples include transfection, lipofection, electroporation, and infection with recombinant viral vectors.
Examples of the “mammal” of the present invention include rodents such as rats, mice, and guinea pigs, such as dogs and cats, and humans and monkeys.
The “autoimmune disease” of the present invention is a disease caused by the immune system raising autoantibodies against endogenous antigens. For example,
“Rheumatoid arthritis” of the present invention refers to an autoimmune disease in which joints, bones, muscles, ligaments, tendons, etc. hurt, and the most frequent autoimmune disease, which is often difficult to treat following a very chronic course It is. In rheumatoid arthritis, it is known that CD4-positive T cells are excessively activated and various cytokines are produced, and in addition, CD8-positive T cells destroy joint tissues and progress in disease states. Therefore, the siRNA of the present invention having an action of suppressing activation of T cells (T lymphocytes) is suitable for the treatment of rheumatoid arthritis.
The “
When treating an autoimmune disease with the pharmaceutical composition of the present invention, the pharmaceutical composition is used together with the above-described functional nucleic acid and one or more kinds of conventional pharmaceutically acceptable additives. Is preferably prepared and administered. The pharmaceutical composition of the present invention is preferably administered parenterally, and can be administered intravenously, intramuscularly, intraperitoneally, or subcutaneously.
The dose of the functional nucleic acid of the present invention varies depending on the administration subject, administration method, and the like. For example, when administered parenterally, for example, about 0.01 to about 1 day per day for an autoimmune disease patient (60 kg). 30 mg, preferably about 0.1 to 20 mg, more preferably about 0.1 to 10 mg can be intravenously injected.
以下に実施例を挙げて本発明を説明するが、実施例は本発明をより良く理解するために例示するものであって、本発明の範囲がこれらの実施例に限定されることを意図するものではない。
(実施例1)CD98重鎖の発現抑制用のsiRNAによる1型糖尿病の進行抑制試験
1)実験材料:
a)動物:
1型糖尿病モデルマウス(NODマウス):NOD−SCIDマウスは、日本SLC社(浜松、日本)から購入した。すべてのマウスは徳島大学の動物センター内で無菌条件下で飼育され、すべての実験は動物の管理と利用のガイドラインに則って実施された。
NODマウスの糖尿病発症状況は、このマウスの尿中の糖濃度と空腹時の血糖値を一週間毎に測定してモニターした。250mg/dl以上の血中濃度を持つマウスが糖尿病であるとした。NODの雌性マウスが20~25週令になると80%以上の割合で、1型糖尿病になる。
b)フローサイトメトリー:
脾臓、膵臓リンパ節、膵臓から得られるリンパ球は、CD4、CD8、TCRVα2とTCRVβ5に対する蛍光色素の結合抗体(eBioscience社、UAS)で染色された。幾つかの実験では、細胞をメモンシンの存在下、250ng/mlのPMA(シグマ社)と1μg/mlのイオノマイシン(シグマ社)で5時間刺激し、CD4又はCD8用の表面染色をした。更に、グランザイムB、IFN−γ又はIL−17用の細胞内染色をした。7−アミノアクチノマイシンD(シグマ社)は、死細胞を除くために使用された。
実験操作は、マニュアルに則って実施した。データは、FACSのコントロール下で集計され、FlowJoソフト(スリースター、米国)を使用して行った。
c)マウスsiRNA:
CD98重鎖の発現抑制用の下記表1の3種のマウスsiRNAの混合物、又は標的遺伝子を持たないコントロールのsiRNA(B−Bridge社)を作製し、使用した。
2)方法:
上記CD98重鎖の発現抑制用の3種のsiRNAの混合物、又は標的遺伝子を持たないコントロールのsiRNAとアテロコラーゲン(高研、日本)とを実験手順書に従い混合し、siRNA溶液を調製した。
糖尿病NODマウスのT細胞が投与された重症複合型免疫不全症モデルマウス(NOD−SCIDマウス)に、上記siRNA溶液を4nmol/マウスになるように腹腔内投与した。siRNAの投与スケジュールは、2日目、5日目と8日目である。なお、T細胞が投与された日を起算日とする。上記siRNA溶液の投与後3日目に、血液を採取し、白血球を単離して、抗マウスCD98抗体で染色して、フローサイトメトリーで評価した。
3)結果:
フローサイトメトリーでの評価結果を図1に示す。灰色(GRAY)の部分は、CD98抗体を投与した場合の測定結果である。点線部分は、コントロールのsiRNAを投与した場合の測定結果であり、実線部分は、CD98重鎖に対するsiRNAを投与した場合の測定結果を示している。
統計解析として、すべての実験において群間の有意差は、マン−ホワイトニイ Uテストを用いて計算された。差がp<0.05の場合に有意差があると考えられる。
また、上記の1型糖尿病モデルマウス(NOD−SCIDマウス)にCD98重鎖のsiRNAを投与した結果を図2に示す。図2に示されるように、このマウスの1型糖尿病の進行をsiRNAの投与で抑制できることが分かった。この結果は、CD98が1型糖尿病の進展に極めて重大な役割を果していることを示すと共に、CD98重鎖をターゲットとするsiRNAを用いた治療が、1型糖尿病や他のT細胞に起因する自己免疫疾患の予防と治療に非常に有効であることを示している。
(実施例2)CD98重鎖の発現抑制用のsiRNAによる関節リウマチの進行抑制試験
1)実験材料:
リウマチ・モデルマウス(コラーゲン誘導性関節炎モデルマウス)の作製:
DBA/1Jマウス(7週齢の雌、10匹)のそれぞれ2群に分け、牛コラーゲンIIと完全アジュバントを混合乳化させたエマルジョン投与を投与した。更に、26日目に2回目の牛コラーゲンIIを投与し、関節炎の発症を観察評価した。
図3では、その臨床スコアの平均±標準偏差で表している。横軸は二回目のコラーゲン免疫後からの日数を表している。
2)方法:
DBA/1Jマウス(雌性)にII型コラーゲンを投与した日を起算日として、実施例1に記載の3種のsiRNA混合溶液を4nmol/マウスになるように腹腔内投与した。siRNAの投与スケジュールは、0日目、1日目、2日目、3日目と4日目である。
なお、siRNA混合溶液は、実施例1に記載のようにsiRNAをアテロコラーゲンと用事調整で混合し、作製後直ちにマウスに投与した。
3)結果:
上記のリウマチモデルマウス(DBA/1Jマウス)にCD98重鎖のsiRNAを投与したところ、図3に示すように、リウマチの進行を抑制できることが分かった。このように、CD98が1型糖尿病の進展に極めて重大な役割を果していることを示すと共に、CD98重鎖をターゲットとするsiRNAを用いた治療が、自己免疫疾患(リウマチ)の予防と治療に非常に有効であることを示している。
(実施例3)CD98重鎖の発現抑制用のsiRNAによるヒト白血病T細胞(Jurkat細胞)のCD98発現進行抑制試験
1)実験材料:
・Jurkat細胞: ヒトCD98タンパクを高発現するJurkat細胞をATCCから購入した。
・トランスフェクション試薬(FuGENE8):非リポソーム系トランスフェクション試薬であるFuGENE8をロシュ・アプライド・サイエンス社から購入した。
・ヒトsiRNA:
CD98重鎖の発現抑制用の下記表2の3種のヒトsiRNAの混合物のsiRNAを作製(B−Bridge社に依頼)し、使用した。
2)方法:
Jurkat細胞にCD98に対して上記ヒトsiRNAを使用し、Fugene8をつかって遺伝子導入し、2日後に細胞表面上のCD98発現をFITC標識したヒトCD98抗体を用いて、フローサイトメーターで測定し、非処理を100としたときの発現量を測定した。
3)結果:
Jurkat細胞表面におけるCD98発現の比率をフローサイトメーターにより測定、評価し、その結果を図4に示す。図4に示されるように、コントロールsiRNA投与群では、CD98のタンパク発現は非処理群と同じであった。しかし、ヒトsiRNAの投与群では、CD98のタンパク発現が約75%抑制された。このことから、これらの3種のヒトsiRNAを使用して、1型糖尿病や他のT細胞に起因する自己免疫疾患の予防と治療ができることを示している。 EXAMPLES The present invention will be described below with reference to examples. However, the examples are provided for better understanding of the present invention, and the scope of the present invention is intended to be limited to these examples. It is not a thing.
Example 1Type 1 Diabetes Progression Inhibition Test Using siRNA for Suppression of CD98 Heavy Chain Expression 1) Experimental Materials:
a) Animals:
Type 1 diabetes model mouse (NOD mouse): NOD-SCID mice were purchased from Japan SLC (Hamamatsu, Japan). All mice were kept under aseptic conditions in the Tokushima University Animal Center, and all experiments were conducted according to animal management and utilization guidelines.
The development of diabetes in NOD mice was monitored by measuring the urine sugar concentration and fasting blood glucose level of each mouse every week. A mouse having a blood concentration of 250 mg / dl or more was considered diabetic. When female mice with NOD reach 20-25 weeks of age, they havetype 1 diabetes at a rate of 80% or more.
b) Flow cytometry:
Lymphocytes obtained from the spleen, pancreatic lymph node, and pancreas were stained with a fluorescent dye-conjugated antibody (eBioscience, UAS) against CD4, CD8, TCRVα2 and TCRVβ5. In some experiments, cells were stimulated with 250 ng / ml PMA (Sigma) and 1 μg / ml ionomycin (Sigma) for 5 hours in the presence of memonsin and surface stained for CD4 or CD8. Furthermore, intracellular staining for granzyme B, IFN-γ or IL-17 was performed. 7-aminoactinomycin D (Sigma) was used to remove dead cells.
The experimental operation was performed according to the manual. Data were collected under FACS control and performed using FlowJo software (Three Star, USA).
c) Mouse siRNA:
A mixture of the three mouse siRNAs shown in Table 1 below for suppressing the expression of CD98 heavy chain, or a control siRNA having no target gene (B-Bridge) was used.
2) Method:
A mixture of the above three siRNAs for suppressing the expression of CD98 heavy chain, or a control siRNA having no target gene and atelocollagen (Koken, Japan) were mixed according to the experimental procedure to prepare a siRNA solution.
The siRNA solution was intraperitoneally administered at 4 nmol / mouse to a severe combined immunodeficiency model mouse (NOD-SCID mouse) to which T cells of diabetic NOD mice were administered. The administration schedule of siRNA is the 2nd day, the 5th day, and the 8th day. The day on which the T cells are administered is taken as the starting date. Three days after administration of the siRNA solution, blood was collected, white blood cells were isolated, stained with anti-mouse CD98 antibody, and evaluated by flow cytometry.
3) Results:
The evaluation results by flow cytometry are shown in FIG. The gray (GRAY) part is the measurement result when the CD98 antibody was administered. The dotted line portion shows the measurement results when the control siRNA is administered, and the solid line portion shows the measurement results when the siRNA against CD98 heavy chain is administered.
As a statistical analysis, significant differences between groups in all experiments were calculated using the Mann-Whiteny U test. A significant difference is considered when the difference is p <0.05.
FIG. 2 shows the results of administering CD98 heavy chain siRNA to thetype 1 diabetes model mouse (NOD-SCID mouse). As shown in FIG. 2, it was found that the progression of type 1 diabetes in this mouse can be suppressed by administration of siRNA. This result indicates that CD98 plays a crucial role in the development of type 1 diabetes and that treatment with siRNA targeting the CD98 heavy chain is self-induced due to type 1 diabetes and other T cells. It is very effective for prevention and treatment of immune diseases.
(Example 2) Rheumatoid arthritis progression inhibition test with siRNA for suppressing expression of CD98 heavy chain 1) Experimental materials:
Production of rheumatoid model mice (collagen-induced arthritis model mice):
DBA / 1J mice (7-week-old female, 10 mice) were divided into two groups, respectively, and administered with an emulsion in which bovine collagen II and complete adjuvant were mixed and emulsified. Furthermore, the second bovine collagen II was administered on the 26th day, and the onset of arthritis was observed and evaluated.
In FIG. 3, the clinical score is expressed as the mean ± standard deviation. The horizontal axis represents the number of days after the second collagen immunization.
2) Method:
Starting from the day when type II collagen was administered to DBA / 1J mice (female), the three siRNA mixed solutions described in Example 1 were intraperitoneally administered to 4 nmol / mouse. The administration schedule of siRNA is the 0th day, the 1st day, the 2nd day, the 3rd day, and the 4th day.
As described in Example 1, the siRNA mixed solution was mixed with siRNA and atelocollagen by errand adjustment and administered to mice immediately after preparation.
3) Results:
When siRNA of CD98 heavy chain was administered to the above rheumatic model mice (DBA / 1J mice), it was found that the progression of rheumatism can be suppressed as shown in FIG. Thus, CD98 plays a very important role in the development oftype 1 diabetes, and treatment using siRNA targeting the CD98 heavy chain is very useful for the prevention and treatment of autoimmune diseases (rheumatic). Is effective.
(Example 3) CD98 expression progression suppression test of human leukemia T cells (Jurkat cells) by siRNA for suppressing expression of CD98 heavy chain 1) Experimental materials:
Jurkat cells: Jurkat cells that highly express human CD98 protein were purchased from ATCC.
-Transfection reagent (FuGENE8): FuGENE8, a non-liposome transfection reagent, was purchased from Roche Applied Science.
-Human siRNA:
A siRNA was prepared from a mixture of the three human siRNAs shown in Table 2 below for suppression of CD98 heavy chain expression (requested from B-Bridge) and used.
2) Method:
Jurkat cells were transfected with the above human siRNA againstCD98 using Fugene 8, and after 2 days, CD98 expression on the cell surface was measured with a flow cytometer using a human CD98 antibody labeled with FITC. The expression level when the treatment was set to 100 was measured.
3) Results:
The ratio of CD98 expression on the Jurkat cell surface was measured and evaluated with a flow cytometer, and the results are shown in FIG. As shown in FIG. 4, in the control siRNA administration group, the protein expression of CD98 was the same as that in the non-treatment group. However, in the group administered with human siRNA, protein expression of CD98 was suppressed by about 75%. This indicates that these three types of human siRNA can be used to prevent and treattype 1 diabetes and other autoimmune diseases caused by T cells.
(実施例1)CD98重鎖の発現抑制用のsiRNAによる1型糖尿病の進行抑制試験
1)実験材料:
a)動物:
1型糖尿病モデルマウス(NODマウス):NOD−SCIDマウスは、日本SLC社(浜松、日本)から購入した。すべてのマウスは徳島大学の動物センター内で無菌条件下で飼育され、すべての実験は動物の管理と利用のガイドラインに則って実施された。
NODマウスの糖尿病発症状況は、このマウスの尿中の糖濃度と空腹時の血糖値を一週間毎に測定してモニターした。250mg/dl以上の血中濃度を持つマウスが糖尿病であるとした。NODの雌性マウスが20~25週令になると80%以上の割合で、1型糖尿病になる。
b)フローサイトメトリー:
脾臓、膵臓リンパ節、膵臓から得られるリンパ球は、CD4、CD8、TCRVα2とTCRVβ5に対する蛍光色素の結合抗体(eBioscience社、UAS)で染色された。幾つかの実験では、細胞をメモンシンの存在下、250ng/mlのPMA(シグマ社)と1μg/mlのイオノマイシン(シグマ社)で5時間刺激し、CD4又はCD8用の表面染色をした。更に、グランザイムB、IFN−γ又はIL−17用の細胞内染色をした。7−アミノアクチノマイシンD(シグマ社)は、死細胞を除くために使用された。
実験操作は、マニュアルに則って実施した。データは、FACSのコントロール下で集計され、FlowJoソフト(スリースター、米国)を使用して行った。
c)マウスsiRNA:
CD98重鎖の発現抑制用の下記表1の3種のマウスsiRNAの混合物、又は標的遺伝子を持たないコントロールのsiRNA(B−Bridge社)を作製し、使用した。
上記CD98重鎖の発現抑制用の3種のsiRNAの混合物、又は標的遺伝子を持たないコントロールのsiRNAとアテロコラーゲン(高研、日本)とを実験手順書に従い混合し、siRNA溶液を調製した。
糖尿病NODマウスのT細胞が投与された重症複合型免疫不全症モデルマウス(NOD−SCIDマウス)に、上記siRNA溶液を4nmol/マウスになるように腹腔内投与した。siRNAの投与スケジュールは、2日目、5日目と8日目である。なお、T細胞が投与された日を起算日とする。上記siRNA溶液の投与後3日目に、血液を採取し、白血球を単離して、抗マウスCD98抗体で染色して、フローサイトメトリーで評価した。
3)結果:
フローサイトメトリーでの評価結果を図1に示す。灰色(GRAY)の部分は、CD98抗体を投与した場合の測定結果である。点線部分は、コントロールのsiRNAを投与した場合の測定結果であり、実線部分は、CD98重鎖に対するsiRNAを投与した場合の測定結果を示している。
統計解析として、すべての実験において群間の有意差は、マン−ホワイトニイ Uテストを用いて計算された。差がp<0.05の場合に有意差があると考えられる。
また、上記の1型糖尿病モデルマウス(NOD−SCIDマウス)にCD98重鎖のsiRNAを投与した結果を図2に示す。図2に示されるように、このマウスの1型糖尿病の進行をsiRNAの投与で抑制できることが分かった。この結果は、CD98が1型糖尿病の進展に極めて重大な役割を果していることを示すと共に、CD98重鎖をターゲットとするsiRNAを用いた治療が、1型糖尿病や他のT細胞に起因する自己免疫疾患の予防と治療に非常に有効であることを示している。
(実施例2)CD98重鎖の発現抑制用のsiRNAによる関節リウマチの進行抑制試験
1)実験材料:
リウマチ・モデルマウス(コラーゲン誘導性関節炎モデルマウス)の作製:
DBA/1Jマウス(7週齢の雌、10匹)のそれぞれ2群に分け、牛コラーゲンIIと完全アジュバントを混合乳化させたエマルジョン投与を投与した。更に、26日目に2回目の牛コラーゲンIIを投与し、関節炎の発症を観察評価した。
図3では、その臨床スコアの平均±標準偏差で表している。横軸は二回目のコラーゲン免疫後からの日数を表している。
2)方法:
DBA/1Jマウス(雌性)にII型コラーゲンを投与した日を起算日として、実施例1に記載の3種のsiRNA混合溶液を4nmol/マウスになるように腹腔内投与した。siRNAの投与スケジュールは、0日目、1日目、2日目、3日目と4日目である。
なお、siRNA混合溶液は、実施例1に記載のようにsiRNAをアテロコラーゲンと用事調整で混合し、作製後直ちにマウスに投与した。
3)結果:
上記のリウマチモデルマウス(DBA/1Jマウス)にCD98重鎖のsiRNAを投与したところ、図3に示すように、リウマチの進行を抑制できることが分かった。このように、CD98が1型糖尿病の進展に極めて重大な役割を果していることを示すと共に、CD98重鎖をターゲットとするsiRNAを用いた治療が、自己免疫疾患(リウマチ)の予防と治療に非常に有効であることを示している。
(実施例3)CD98重鎖の発現抑制用のsiRNAによるヒト白血病T細胞(Jurkat細胞)のCD98発現進行抑制試験
1)実験材料:
・Jurkat細胞: ヒトCD98タンパクを高発現するJurkat細胞をATCCから購入した。
・トランスフェクション試薬(FuGENE8):非リポソーム系トランスフェクション試薬であるFuGENE8をロシュ・アプライド・サイエンス社から購入した。
・ヒトsiRNA:
CD98重鎖の発現抑制用の下記表2の3種のヒトsiRNAの混合物のsiRNAを作製(B−Bridge社に依頼)し、使用した。
Jurkat細胞にCD98に対して上記ヒトsiRNAを使用し、Fugene8をつかって遺伝子導入し、2日後に細胞表面上のCD98発現をFITC標識したヒトCD98抗体を用いて、フローサイトメーターで測定し、非処理を100としたときの発現量を測定した。
3)結果:
Jurkat細胞表面におけるCD98発現の比率をフローサイトメーターにより測定、評価し、その結果を図4に示す。図4に示されるように、コントロールsiRNA投与群では、CD98のタンパク発現は非処理群と同じであった。しかし、ヒトsiRNAの投与群では、CD98のタンパク発現が約75%抑制された。このことから、これらの3種のヒトsiRNAを使用して、1型糖尿病や他のT細胞に起因する自己免疫疾患の予防と治療ができることを示している。 EXAMPLES The present invention will be described below with reference to examples. However, the examples are provided for better understanding of the present invention, and the scope of the present invention is intended to be limited to these examples. It is not a thing.
Example 1
a) Animals:
The development of diabetes in NOD mice was monitored by measuring the urine sugar concentration and fasting blood glucose level of each mouse every week. A mouse having a blood concentration of 250 mg / dl or more was considered diabetic. When female mice with NOD reach 20-25 weeks of age, they have
b) Flow cytometry:
Lymphocytes obtained from the spleen, pancreatic lymph node, and pancreas were stained with a fluorescent dye-conjugated antibody (eBioscience, UAS) against CD4, CD8, TCRVα2 and TCRVβ5. In some experiments, cells were stimulated with 250 ng / ml PMA (Sigma) and 1 μg / ml ionomycin (Sigma) for 5 hours in the presence of memonsin and surface stained for CD4 or CD8. Furthermore, intracellular staining for granzyme B, IFN-γ or IL-17 was performed. 7-aminoactinomycin D (Sigma) was used to remove dead cells.
The experimental operation was performed according to the manual. Data were collected under FACS control and performed using FlowJo software (Three Star, USA).
c) Mouse siRNA:
A mixture of the three mouse siRNAs shown in Table 1 below for suppressing the expression of CD98 heavy chain, or a control siRNA having no target gene (B-Bridge) was used.
A mixture of the above three siRNAs for suppressing the expression of CD98 heavy chain, or a control siRNA having no target gene and atelocollagen (Koken, Japan) were mixed according to the experimental procedure to prepare a siRNA solution.
The siRNA solution was intraperitoneally administered at 4 nmol / mouse to a severe combined immunodeficiency model mouse (NOD-SCID mouse) to which T cells of diabetic NOD mice were administered. The administration schedule of siRNA is the 2nd day, the 5th day, and the 8th day. The day on which the T cells are administered is taken as the starting date. Three days after administration of the siRNA solution, blood was collected, white blood cells were isolated, stained with anti-mouse CD98 antibody, and evaluated by flow cytometry.
3) Results:
The evaluation results by flow cytometry are shown in FIG. The gray (GRAY) part is the measurement result when the CD98 antibody was administered. The dotted line portion shows the measurement results when the control siRNA is administered, and the solid line portion shows the measurement results when the siRNA against CD98 heavy chain is administered.
As a statistical analysis, significant differences between groups in all experiments were calculated using the Mann-Whiteny U test. A significant difference is considered when the difference is p <0.05.
FIG. 2 shows the results of administering CD98 heavy chain siRNA to the
(Example 2) Rheumatoid arthritis progression inhibition test with siRNA for suppressing expression of CD98 heavy chain 1) Experimental materials:
Production of rheumatoid model mice (collagen-induced arthritis model mice):
DBA / 1J mice (7-week-old female, 10 mice) were divided into two groups, respectively, and administered with an emulsion in which bovine collagen II and complete adjuvant were mixed and emulsified. Furthermore, the second bovine collagen II was administered on the 26th day, and the onset of arthritis was observed and evaluated.
In FIG. 3, the clinical score is expressed as the mean ± standard deviation. The horizontal axis represents the number of days after the second collagen immunization.
2) Method:
Starting from the day when type II collagen was administered to DBA / 1J mice (female), the three siRNA mixed solutions described in Example 1 were intraperitoneally administered to 4 nmol / mouse. The administration schedule of siRNA is the 0th day, the 1st day, the 2nd day, the 3rd day, and the 4th day.
As described in Example 1, the siRNA mixed solution was mixed with siRNA and atelocollagen by errand adjustment and administered to mice immediately after preparation.
3) Results:
When siRNA of CD98 heavy chain was administered to the above rheumatic model mice (DBA / 1J mice), it was found that the progression of rheumatism can be suppressed as shown in FIG. Thus, CD98 plays a very important role in the development of
(Example 3) CD98 expression progression suppression test of human leukemia T cells (Jurkat cells) by siRNA for suppressing expression of CD98 heavy chain 1) Experimental materials:
Jurkat cells: Jurkat cells that highly express human CD98 protein were purchased from ATCC.
-Transfection reagent (FuGENE8): FuGENE8, a non-liposome transfection reagent, was purchased from Roche Applied Science.
-Human siRNA:
A siRNA was prepared from a mixture of the three human siRNAs shown in Table 2 below for suppression of CD98 heavy chain expression (requested from B-Bridge) and used.
Jurkat cells were transfected with the above human siRNA against
3) Results:
The ratio of CD98 expression on the Jurkat cell surface was measured and evaluated with a flow cytometer, and the results are shown in FIG. As shown in FIG. 4, in the control siRNA administration group, the protein expression of CD98 was the same as that in the non-treatment group. However, in the group administered with human siRNA, protein expression of CD98 was suppressed by about 75%. This indicates that these three types of human siRNA can be used to prevent and treat
本発明の自己免疫疾患の予防・治療用医薬組成物は、siRNAによるCD98の発現抑制という新たな作用機序により、自己免疫疾患(例えば、1型糖尿病や関節リウマチでなど)を予防・治療し得るものである。本発明の医薬組成物を用いることにより、これまで適切な治療方法がなかった、自己免疫疾患の治療方法に新たな道を開くものとなっている。
The pharmaceutical composition for prevention and treatment of autoimmune diseases of the present invention prevents and treats autoimmune diseases (for example, intype 1 diabetes and rheumatoid arthritis) by a new mechanism of action that suppresses the expression of CD98 by siRNA. To get. The use of the pharmaceutical composition of the present invention opens up a new way to a method for treating an autoimmune disease, which has not had a suitable method for treatment so far.
The pharmaceutical composition for prevention and treatment of autoimmune diseases of the present invention prevents and treats autoimmune diseases (for example, in
Claims (12)
- CD98重鎖の発現を抑制する機能性核酸を有効成分として含むことを特徴とする自己免疫疾患を予防又は治療するための医薬組成物。 A pharmaceutical composition for preventing or treating an autoimmune disease comprising a functional nucleic acid that suppresses the expression of CD98 heavy chain as an active ingredient.
- 上記機能性核酸がsiRNAである、請求項1に記載の組成物。 The composition according to claim 1, wherein the functional nucleic acid is siRNA.
- 上記自己免疫疾患が1型糖尿病又は関節リウマチである、請求項1又は2に記載の組成物。 The composition according to claim 1 or 2, wherein the autoimmune disease is type 1 diabetes or rheumatoid arthritis.
- 上記siRNAが二本鎖RNAである、請求項2に記載の医薬組成物。 The pharmaceutical composition according to claim 2, wherein the siRNA is a double-stranded RNA.
- 上記二本鎖RNAが、配列番号1と配列番号2、配列番号3と配列番号4、あるいは配列番号5と配列番号6の一つ以上を含有するsiRNAである、請求項4に記載の医薬組成物。 The pharmaceutical composition according to claim 4, wherein the double-stranded RNA is an siRNA containing one or more of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4, or SEQ ID NO: 5 and SEQ ID NO: 6. object.
- 上記二本鎖RNAが、配列番号7と配列番号8、配列番号9と配列番号10、あるいは配列番号11と配列番号12の一つ以上を含有するsiRNAである、請求項4記載の医薬組成物。 The pharmaceutical composition according to claim 4, wherein the double-stranded RNA is an siRNA containing one or more of SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and SEQ ID NO: 10, or SEQ ID NO: 11 and SEQ ID NO: 12. .
- 配列番号1と配列番号2、配列番号3と配列番号4、あるいは配列番号5と配列番号6のいずれかの配列を有する二本鎖siRNA。 Double-stranded siRNA having any one of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4, or SEQ ID NO: 5 and SEQ ID NO: 6.
- 二本鎖RNA部分が、配列番号7と配列番号8、配列番号9と配列番号10、あるいは配列番号11と配列番号12のいずれかの配列を有する二本鎖siRNA。 A double-stranded siRNA in which the double-stranded RNA portion has any one of SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and SEQ ID NO: 10, or SEQ ID NO: 11 and SEQ ID NO: 12.
- 哺乳類に、CD98重鎖の発現を抑制する機能性核酸を有効量投与することを特徴とする自己免疫疾患の治療方法。 A method for treating an autoimmune disease, comprising administering to a mammal an effective amount of a functional nucleic acid that suppresses the expression of CD98 heavy chain.
- 上記機能性核酸が、siRNAである、請求項9記載の治療方法。 The treatment method according to claim 9, wherein the functional nucleic acid is siRNA.
- 上記siRNAが、請求項7または8の2本鎖siRNAである、上記(10)記載の治療方法。 The treatment method according to (10) above, wherein the siRNA is the double-stranded siRNA according to claim 7 or 8.
- 上記自己免疫疾患が、1型糖尿病又は関節リウマチである、請求項9~11のいずれかに記載の治療方法。 The method according to any one of claims 9 to 11, wherein the autoimmune disease is type 1 diabetes or rheumatoid arthritis.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011210392 | 2011-09-27 | ||
| JP2011-210392 | 2011-09-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013047889A1 true WO2013047889A1 (en) | 2013-04-04 |
Family
ID=47995911
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/075587 WO2013047889A1 (en) | 2011-09-27 | 2012-09-26 | Pharmaceutical composition for treating autoimmune diseases |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JPWO2013047889A1 (en) |
| WO (1) | WO2013047889A1 (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010540595A (en) * | 2007-10-04 | 2010-12-24 | フエー・イー・ベー・フエー・ゼツト・ウエー | Extracellular targets for Alzheimer's disease |
| WO2012026526A1 (en) * | 2010-08-27 | 2012-03-01 | 国立大学法人宮崎大学 | Hemokinin-1 receptor and hemokinin-1-derived peptide |
-
2012
- 2012-09-26 WO PCT/JP2012/075587 patent/WO2013047889A1/en active Application Filing
- 2012-09-26 JP JP2013536480A patent/JPWO2013047889A1/en active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010540595A (en) * | 2007-10-04 | 2010-12-24 | フエー・イー・ベー・フエー・ゼツト・ウエー | Extracellular targets for Alzheimer's disease |
| WO2012026526A1 (en) * | 2010-08-27 | 2012-03-01 | 国立大学法人宮崎大学 | Hemokinin-1 receptor and hemokinin-1-derived peptide |
Non-Patent Citations (6)
| Title |
|---|
| CANTOR J ET AL.: "Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity", THE JOURNAL OF IMMUNOLOGY, vol. 187, no. 2, 15 July 2011 (2011-07-15), pages 851 - 860 * |
| DIAZ LA ET AL.: "A role for CD98 in rheumatoid arthritis and in the regulation of superantigenic responses", ARTHRITIS & RHEUMATISM, vol. 37, no. 9, September 1994 (1994-09-01), pages S313, XP008163310 * |
| KUDO Y ET AL.: "RNA interference-induced reduction in CD98 expression suppresses cell fusion during syncytialization of human placental BeWo cells", FEBS LETTERS, vol. 577, no. 3, 19 November 2004 (2004-11-19), pages 473 - 477, XP004647184, DOI: doi:10.1016/j.febslet.2004.10.047 * |
| LAROUI H ET AL.: "Silencing of CD98 Expression Using a Nanoparticle-Based Delivery System Ameliorates Induced Colitis in Mice", GASTROENTEROLOGY, vol. 140, no. 5, May 2011 (2011-05-01), pages S10 - S11, XP008163309, DOI: doi:10.1016/S0016-5085(11)60044-9 * |
| LIAN G ET AL.: "Manipulation of CD98 resolves type 1 diabetes in nonobese diabetic mice", THE JOURNAL OF IMMUNOLOGY, vol. 188, no. 5, 1 March 2012 (2012-03-01), pages 2227 - 2234 * |
| PINHO MJ ET AL.: "Overexpression of non-functional LAT1/4F2hc in renal proximal tubular epithelial cells from the spontaneous hypertensive rat", CELLULAR PHYSIOLOGY AND BIOCHEMISTRY, vol. 20, no. 5, 2007, pages 535 - 548 * |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2013047889A1 (en) | 2015-03-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11071716B2 (en) | Combinations of mRNAs encoding immune modulating polypeptides and uses thereof | |
| US11660341B2 (en) | mRNA combination therapy for the treatment of cancer | |
| US11447773B2 (en) | Stabilized HNF4A saRNA compositions and methods of use | |
| US11311543B2 (en) | Compositions and methods for inhibiting PTPN22 | |
| KR20150038531A (en) | Methods and compositions for in vivo induction of pancreatic beta cell formation | |
| ES2645366T3 (en) | Treatment of angiogenesis disorders | |
| WO2013047889A1 (en) | Pharmaceutical composition for treating autoimmune diseases | |
| KR20180068524A (en) | SS18-SSX fusion gene specific siRNA and pharmaceutical composition for preventing or treating of cancer containing the same | |
| WO2014148216A1 (en) | Pharmaceutical composition for treatment of inflammatory bowel disease | |
| CN116234908A (en) | Morpholino to promote FOXP3S |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12836573 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2013536480 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12836573 Country of ref document: EP Kind code of ref document: A1 |