WO2013000924A1 - 1-ARYL-4-METHYL-[1,2,4]TRIAZOLO[4,3-a]QUINOXALINE DERIVATIVES - Google Patents
1-ARYL-4-METHYL-[1,2,4]TRIAZOLO[4,3-a]QUINOXALINE DERIVATIVES Download PDFInfo
- Publication number
- WO2013000924A1 WO2013000924A1 PCT/EP2012/062381 EP2012062381W WO2013000924A1 WO 2013000924 A1 WO2013000924 A1 WO 2013000924A1 EP 2012062381 W EP2012062381 W EP 2012062381W WO 2013000924 A1 WO2013000924 A1 WO 2013000924A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- triazolo
- quinoxaline
- chlorophenyl
- quinoxalin
- Prior art date
Links
- RKBZHWQDHMRQNE-UHFFFAOYSA-N CCCCOc(cc1C(NN)=O)ccc1Cl Chemical compound CCCCOc(cc1C(NN)=O)ccc1Cl RKBZHWQDHMRQNE-UHFFFAOYSA-N 0.000 description 1
- ZVAHQRLUAXRLAF-UHFFFAOYSA-N Cc(nc(ccc(OC(F)(F)F)c1)c1n1)c1Cl Chemical compound Cc(nc(ccc(OC(F)(F)F)c1)c1n1)c1Cl ZVAHQRLUAXRLAF-UHFFFAOYSA-N 0.000 description 1
- UQHNHWHCASOISS-UHFFFAOYSA-N Cc1nc(cc(cc2)Br)c2nc1Cl Chemical compound Cc1nc(cc(cc2)Br)c2nc1Cl UQHNHWHCASOISS-UHFFFAOYSA-N 0.000 description 1
- JZYZNOOUMHUMNP-UHFFFAOYSA-N Cc1nc(cc(cc2)OC(F)(F)F)c2nc1Cl Chemical compound Cc1nc(cc(cc2)OC(F)(F)F)c2nc1Cl JZYZNOOUMHUMNP-UHFFFAOYSA-N 0.000 description 1
- GCQVXKHFLSRFSR-UHFFFAOYSA-N Cc1nc(ccc(Br)c2)c2nc1Cl Chemical compound Cc1nc(ccc(Br)c2)c2nc1Cl GCQVXKHFLSRFSR-UHFFFAOYSA-N 0.000 description 1
- JVGYNXBNLFSTNR-UHFFFAOYSA-N Cc1nc(ccc(C=C)c2)c2[n]2c(-c(c([N+]([O-])=O)ccc3)c3Cl)nnc12 Chemical compound Cc1nc(ccc(C=C)c2)c2[n]2c(-c(c([N+]([O-])=O)ccc3)c3Cl)nnc12 JVGYNXBNLFSTNR-UHFFFAOYSA-N 0.000 description 1
- DXCDCZVIUFFBMN-UHFFFAOYSA-N Cc1nc(ccc(C=O)c2)c2[n]2c(-c3ccccc3Cl)nnc12 Chemical compound Cc1nc(ccc(C=O)c2)c2[n]2c(-c3ccccc3Cl)nnc12 DXCDCZVIUFFBMN-UHFFFAOYSA-N 0.000 description 1
- IQFAUAIVCNIZMA-UHFFFAOYSA-N Cc1nc(ccc(OC)c2)c2[n]2c(-c3ccccc3Cl)nnc12 Chemical compound Cc1nc(ccc(OC)c2)c2[n]2c(-c3ccccc3Cl)nnc12 IQFAUAIVCNIZMA-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4985—Pyrazines or piperazines ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/041—Heterocyclic compounds
- A61K51/044—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins
- A61K51/0463—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/32—Alcohol-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/36—Opioid-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/58—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances
- G01N33/60—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances involving radioactive labelled substances
Definitions
- the present invention relates to novel l-aryl-4-methyl-[l,2,4]triazolo[4,3-a]- quinoxaline derivatives as inhibitors of phosphodiesterase 2 (PDE2) and to a lesser extent of phosphodiesterase 10 (PDE10) or as inhibitors of both, phosphodiesterases 2 and 10.
- PDE2 phosphodiesterase 2
- PDE10 phosphodiesterase 10
- the invention is also directed to pharmaceutical compositions comprising such compounds, to processes for preparing such compounds and compositions, and to the use of such compounds and compositions for the prevention and treatment of disorders in which PDE2 is involved, or disorders in which both PDE2 and PDE10 are involved, such as neurological and psychiatric disorders, and endocrinological or metabolic diseases.
- the present invention also relates to radiolabeled compounds which may be useful for imaging and quantifying the PDE2 enzyme in tissues, for example, using positron-emission tomography (PET).
- PET positron-emission tomography
- the invention is also directed to compositions comprising such compounds, to processes for preparing such compounds and compositions, to the use of such compounds and compositions for imaging a tissue, cells or a host, in vitro or in vivo and to precursors of said compounds.
- WO-2010/101230 discloses [l,2,4]triazolo[4,3-a]quinoxalin-4(5H)-ones as PDE9 inhibitors useful for treating urination disorders.
- Phosphodiesterases are a family of enzymes encoded by 21 genes and subdivided into 11 distinct families according to structural and functional properties. These enzymes metabolically inactivate widely occurring intracellular second messengers, 3 ',5 '-cyclic adenosine monophosphate (cAMP) and 3 ',5 '-cyclic guanosine monophosphate (cGMP). These two messengers regulate a wide variety of biological processes, including pro -inflammatory mediator production and action, ion channel function, muscle contraction, learning, differentiation, apoptosis, lipogenesis, glycogeno lysis, and gluconeogenesis.
- cAMP 3 ',5 '-cyclic adenosine monophosphate
- cGMP 3 ',5 '-cyclic guanosine monophosphate
- PKA protein kinase A
- PKG protein kinase G
- Intracellular concentrations of cAMP and cGMP are strictly regulated by the rate of biosynthesis by cyclases and by the rate of degradation by PDEs.
- PDEs are hydrolases that inactivate cAMP and cGMP by catalytic hydrolysis of the 3 '-ester bond, forming the inactive 5 '-monophosphate (Scheme A).
- the PDE families can be divided into three groups: i) the cAMP-specific PDEs, which include PDE4, 7 and 8; ii) the cGMP-selective enzymes PDE5, 6 and 9; and iii) the dual- substrate PDEs, PDE1, 2 and 3, as well as PDE10 and 11.
- PDEs are expressed differentially throughout the organism, including the central nervous system. Different PDE isozymes therefore may have different physiological functions. Compounds that inhibit selectively PDE families or isozymes may display particular therapeutic activity, fewer side effects, or both.
- Phosphodiesterase 2A inactivates intracellular signalling mechanisms reliant on cyclic nucleotide signalling mediated by cAMP and cGMP via their degradation. Such signalling pathways are known to play a role in the regulation of genes involved in the induction of synaptic plasticity.
- PDE2A modulation may be a target for alleviating cognitive deficits seen in people suffering from disorders such as for example, schizophrenia, Alzheimer's disease, Parkinson's disease and other CNS disorders associated with cognitive dysfunction.
- Phosphodiesterase 2A is more abundantly expressed in the brain relative to peripheral tissues.
- the high expression of PDE2 in the limbic system suggests that PDE2 may modulate neuronal signalling involved in emotion, perception, concentration, learning and memory. Additionally, PDE2 is expressed in the nucleus accumbens, the olfactory bulb, the olfactory tubercle and the amygdala, supporting the suggestion that PDE2 may also be involved in anxiety and depression.
- PDE2 inhibitors have been shown to be beneficial in the reduction of oxidative stress-induced anxiety, supporting their use in the treatment of anxiety in neuropsychiatric and neurodegenerative disorders that involve oxidative stress, such as Alzheimer's disease, Parkinson's disease and multiple sclerosis.
- PDE2 inhibitors have been shown to enhance long term potentiation of synaptic transmission and to improve memory acquisition and consolidation in the object recognition and in the social recognition tests in rats. Furthermore, PDE2 inhibitors have been shown to reverse the MK-801 induced working memory deficit in the
- PDE2 inhibitors have also been shown to display activity in forced swim test and light/dark box models; and to show anxiolytic- like effects in elevated plus-maze, hole-board and open-field tests and to prevent stress-induced changes in apoptosis and behaviour.
- PDE2 inhibitors may be useful in the treatment of memory deficiency, cognitive disorders, anxiety, bipolar disorder and depression.
- PDE10 has the most restricted distribution with high expression only in the brain and testes. In the brain, PDE10A mR A and protein are highly expressed in a majority of striatal Medium Spiny Neurons (MSNs). This unique distribution of PDE10A in the brain, together with its increased pharmacological characterization, indicates a potential use of PDE10A inhibitors for treating
- PDE10 inhibitors may possess a pharmacological profile similar to that of the current antipsychotics which mainly treat positive symptoms of schizophrenia, but also having the potential to improve the negative and cognitive symptoms of schizophrenia, while lacking the non-target related side effects such as EPS or prolactin release, that are often observed with the currently available antipsychotics.
- PDE10 inhibitors can be used to raise levels of cAMP and /or cGMP within cells that express the PDE10 enzyme, for example neurons that comprise the basal ganglia
- PDE10 inhibitors may be useful in treating schizophrenia and additionally, a variety of conditions as described herein, for example, Parkinson's Disease, Huntington's Disease, addiction and depression.
- PDE10 inhibitors may be also useful in other conditions such as obesity, non- insulin dependent diabetes, bipolar disorder, obsessive compulsive disorder and pain.
- PDE2 inhibitors may provide an advantageous balance of properties in the treatment of disorders selected from, but not limited to, cognitive disorders, anxiety, depression, and movement disorders; compounds that are PDE2 and PDE10 inhibitors may show utility in schizophrenia, Parkinson's disease, Huntington's disease, addiction, depression, and anxiety, with an additional beneficial effect in cognitive deficits and/or drug-induced extrapyramidal symptoms observed in these patient populations.
- the present compounds are compounds which, due to their novel mode of action are potentially useful in the treatment of diseases related to PDE2 enzyme activity or diseases related to the activity of the PDE2 and 10 enzymes.
- the present invention is directed to a l-aryl-4-methyl-[l,2,4]triazolo[4,3-a]- quinoxalines of formula (I)
- R 1 is phenyl or pyridinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, Ci_ 6 alkyl, Ci_ 6 alkyl substituted with 1, 2 or 3 halo substituents, Ci_ 6 alkyloxy, (C3- 6 cycloalkyl)Ci_ 3 alkyloxy, Ci_ 6 alkyloxy substituted with 1, 2 or 3 halo substituents, (Ci_ 6 alkyloxy)Ci_ 3 alkyl and (C i _ 6 alkyloxy)C i _ 3 alkyloxy;
- R 2 is selected from the group consisting of hydrogen, halo, trifluoromethyl,
- R 3 is hydrogen or methyl
- R 4 is selected from the group consisting of hydrogen; Ci_ 3 alkyl optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, hydroxy, Ci_ 3 alkoxy, mono- and di(Ci_ 3 alkyl)amino, C 3 _ 6 cycloalkyl, phenyl, 3,4,5- trimethoxyphenyl, pyridinyl, pyridinyl substituted with halo, morpholinyl, pyrrolidinyl, piperidinyl, and piperidinyl substituted with methyl; C 3 - 6 cycloalkyl; tetrahydropyranyl; l-methylpiperidin-4-yl; 4-hydroxycyclohexan-l-yl; 3,4,5-trimethoxyphenyl; Ci_ 3 alkyl- carbonyl; and pyridinyl; or
- NR 3 R 4 is pyrrolidinyl, piperidinyl or morpholinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo,
- R 5 is selected from the group consisting of hydrogen; Ci_ 3 alkyl; Ci_ 3 alkyl substituted with pyridinyl, phenyl, pyrrolidinyl or morpholinyl; phenyl; and pyridinyl; or a pharmaceutically acceptable salt or a solvate thereof, provided that R 2 is other than hydrogen when R 1 is phenyl, 4-methylphenyl,
- Illustrative of the invention is a pharmaceutical composition comprising a pharmaceutically acceptable carrier and any of the compounds described above.
- An illustration of the invention is a pharmaceutical composition made by mixing any of the compounds described above and a pharmaceutically acceptable carrier.
- Illustrating the invention is a process for making a pharmaceutical composition comprising mixing any of the compounds described above and a pharmaceutically acceptable carrier.
- Exemplifying the invention are methods of treating a disorder mediated by the PDE2 enzyme or by the PDE2 and PDE10 enzymes, comprising administering to a subject in need thereof a therapeutically effective amount of any of the compounds or pharmaceutical compositions described above.
- An example of the invention is a method of treating a disorder selected from the group consisting of neurological and psychiatric disorders, and endocrinological or metabolic diseases comprising administering to a subject in need thereof, a
- An example of the invention is a method of treating a disorder selected from the group of neurological and psychiatric disorders selected from psychotic disorders and conditions; anxiety disorders; movement disorders; drug abuse; mood disorders;
- neurodegenerative disorders disorders or conditions comprising as a symptom a deficiency in attention and/or cognition; pain; autistic disorder; and metabolic disorders, comprising administering to a subject in need thereof, a therapeutically effective amount of any of the compounds or pharmaceutical compositions described above.
- An example of the invention is a method of treating a disorder selected from the group consisting of neurological and psychiatric disorders, and endocrinological or metabolic diseases comprising administering to a subject in need thereof, a
- An example of the invention is a method of treating a disorder selected from the group of neurological and psychiatric disorders selected from psychotic disorders and conditions; anxiety disorders; movement disorders; drug abuse; mood disorders;
- neurodegenerative disorders disorders or conditions comprising as a symptom a deficiency in attention and/or cognition; pain; autistic disorder; and metabolic disorders, comprising administering to a subject in need thereof, a therapeutically effective amount of any of the compounds or pharmaceutical compositions described above.
- Also exemplifying the invention is a compound or a pharmaceutical composition described above for use as a medicament.
- a compound according to the present invention or a pharmaceutical composition according to the invention for use in the treatment, prevention, amelioration, control or reduction of the risk of various neurological and psychiatric disorders associated with phosphodiesterase 2 or associated with phosphodiesterases 2 and 10 dysfunction in a mammal, including a human, the treatment or prevention of which is affected or facilitated by the inhibition of phosphodiesterase 2 or by the inhibition of phosphodiesterases 2 and 10.
- An example of the invention is a compound according to the present invention or a pharmaceutical composition according to the invention for use in the treatment, prevention, amelioration, control or reduction of the risk of various disorders selected from psychotic disorders and conditions; anxiety disorders; movement disorders; drug abuse; mood disorders; neurodegenerative disorders; disorders or conditions comprising as a symptom a deficiency in attention and/or cognition; pain; autistic disorder; and metabolic disorders.
- An example of the invention is a method of treating a disorder selected from the group consisting of Alzheimer's disease, mild cognitive impairment, senility, dementia, dementia with Lewy bodies, Down's syndrome, dementia associated with stroke, dementia associated with Parkinson's disease and dementia associated with beta- amyloid, preferably Alzheimer's disease, comprising administering to a subject in need thereof, a therapeutically effective amount of any of the compounds or pharmaceutical compositions described above.
- Another example of the invention is any of the compounds described above for use in treating: (a) Alzheimer's Disease, (b) mild cognitive impairment, (c) senility, (d) dementia, (e) dementia with Lewy bodies, (f) Down's syndrome, (g) dementia associated with stroke, (h) dementia associated with Parkinson's disease, (i) dementia associated with beta-amyloid, (j) depressive disorders and (k) anxiety disorders, in a subject in need thereof.
- Another aspect of the invention relates to precursor compounds for the synthesis of radio labelled compounds of formula (I).
- Illustrative of the invention is a sterile solution comprising a radio labelled compound of Formula (I).
- Exemplifying the invention is a use of a radiolabeled compound of formula (I) as described herein, for, or a method of, imaging a tissue, cells or a host, in vitro or in vivo.
- a method of imaging a tissue, cells or a host comprising contacting with or administering to a tissue, cells or a host a compound of formula (I) as described herein, and imaging the tissue, cells or host with a positron-emission tomography imaging system.
- the present invention is directed to compounds of formula (I) as defined hereinbefore, and pharmaceutically acceptable salts thereof.
- the compounds of formula (I) are inhibitors of the phosphodiesterase 2 enzyme (PDE2) and to a lesser extent of phosphodiesterase 10 (PDE10), or are inhibitors of the phosphodiesterase 2 and phosphodiesterase 10 enzymes (PDE2 and PDE10) and may be useful in the treatment of neurological and psychiatric disorders, and endocrinological or metabolic diseases.
- the present invention relates to a compound of formula (I), or a stereochemically isomeric form thereof, as defined herein, wherein
- R 1 is phenyl or pyridinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, Ci_ 6 alkyl, trifluoromethyl, Ci_ 6 alkyloxy, (C3- 6 cycloalkyl)Ci_ 3 alkyloxy and trifluoromethoxy;
- R 2 is selected from the group consisting of hydrogen, halo, trifluoromethyl,
- R 3 is hydrogen or methyl
- R 4 is selected from the group consisting of hydrogen; Ci_ 3 alkyl optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, hydroxy, Ci_ 3 alkoxy, mono- and di(Ci_ 3 alkyl)amino, C 3 _ 6 cycloalkyl, phenyl, 3,4,5- trimethoxyphenyl, pyridinyl, pyridinyl substituted with halo, morpholinyl, pyrrolidinyl, piperidinyl, and piperidinyl substituted with methyl; C 3 _ 6 Cycloalkyl; tetrahydropyranyl;
- NR 3 R 4 is pyrrolidinyl, piperidinyl or morpholinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo,
- R 5 is selected from the group consisting of hydrogen; Ci_ 3 alkyl; Ci_ 3 alkyl substituted with pyridinyl, phenyl, pyrrolidinyl or morpholinyl; phenyl; and pyridinyl; or a pharmaceutically acceptable salt or a solvate thereof, provided that R 2 is other than hydrogen when R 1 is phenyl, 4-methylphenyl,
- the present invention relates to a compound of formula (I), wherein
- R 1 is phenyl or pyridinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, Ci_ 6 alkyl, and Ci_ 6 alkyloxy;
- R 2 is selected from the group consisting of hydrogen, halo, trifluoromethoxy, 1,1-difluoroethoxy, cyano, (C 3 _ 6 Cycloalkyl)carbonyl, C 2 _ 6 alkenyl, a radical of formula -L'-NR , or a radical of formula -L 2 -0-R 5 ;
- R 3 is hydrogen or methyl
- R 4 is selected from the group consisting of hydrogen; Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of halo, hydroxy, Ci_ 3 alkoxy, mono- and di(Ci_ 3 alkyl)amino, phenyl, 3,4,5-trimethoxyphenyl, pyridinyl, pyridinyl substituted with halo, morpholinyl, pyrrolidinyl, and piperidinyl; tetrahydropyranyl;
- NR 3 R 4 is pyrrolidinyl, piperidinyl or morpholinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo,
- R 5 is selected from the group consisting of hydrogen; Ci_ 3 alkyl; Ci_ 3 alkyl substituted with pyridinyl, phenyl, or morpholinyl; phenyl; and pyridinyl; or a pharmaceutically acceptable salt or a solvate thereof, provided that R 2 is other than hydrogen when R 1 is phenyl, 4-methylphenyl,
- R 2 is not hydrogen, and the rest of variables as previously defined in any of the above embodiments.
- the present invention relates to a compound of formula (I), wherein
- R 1 is phenyl or pyridinyl each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, and Ci_ 6 alkyloxy;
- R 2 is selected from the group consisting of halo, cyano, a radical of formula -L 1 -NR 3 R 4 ; or a radical of formula -L 2 -0-R 5 ;
- R 3 is hydrogen or methyl
- R 4 is selected from the group consisting of Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of halo, Ci_ 3 alkoxy, mono- and di(Ci_ 3 alkyl)amino, phenyl, pyridinyl, pyridinyl substituted with halo, morpholinyl, and piperidinyl; l-methylpiperidin-4-yl; 3,4,5-trimethoxyphenyl; pyridinyl; or
- NR 3 R 4 is pyrrolidinyl, piperidinyl or morpholinyl each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo and hydroxyl; or 4-methylpiperazin-l-yl;
- R 5 is Ci_ 3 alkyl substituted with pyridinyl; or a pharmaceutically acceptable salt or a solvate thereof.
- the present invention relates to a compound of formula (I), wherein R 2 is bound to the scaffold at position 8 and R 1 and R 2 are as previously defined.
- the present invention is directed to a compound of formula ( ⁇ )
- R 1 and R 2 are as previously defined in any of the above embodiments, or a pharmaceutically acceptable salt or solvate thereof.
- the present invention relates to a compound of formula (I), wherein R 1 is pyridinyl substituted with Ci_ 6 alkyloxy and R 2 is as previously defined in any of the above embodiments, or a pharmaceutically acceptable salt or a solvate thereof.
- the present invention relates to a compound of formula (I), wherein R 1 and R 2 are as previously defined in any of the above embodiments, and wherein
- R 3a is hydrogen or methyl
- R 4a is selected from the group consisting of Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of C 3 - 6 cycloalkyl and phenyl; C 3 - 6 cyclo- alkyl; tetrahydropyranyl; 4-hydroxycyclohexan-l-yl; and pyridinyl; or
- NR 3a R 4a is pyrrolidinyl, piperidinyl or morpholinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, trifluoromethyl, hydroxyl, Ci_ 3 alkyloxy, mono- and di(Ci_ 3 alkyl)amino, hydroxyl- Ci_ 3 alkyl, haloCi_ 3 alkyl, and methoxyCi_ 3 alkyl; or 4-methylpiperazin-l-yl; or
- R 3b is hydrogen and R 4b is Ci_ 3 alkyl
- N R 3b R 4b is morpholinyl .
- R 3c is hydrogen or methyl
- R 4c is selected from the group consisting of hydrogen; Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of halo, hydroxy, Ci_ 3 alkoxy, mono- and di(Ci_ 3 alkyl)amino, phenyl, pyridinyl, pyridinyl substituted with halo, morpholinyl, pyrrolidinyl, and piperidinyl; l-methylpiperidin-4-yl; and 3,4,5- trimethoxyphenyl; or
- R 3d is hydrogen or methyl
- R 4d is selected from the group consisting of hydrogen; Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of Ci_ 3 alkoxy, and morpholinyl; C3- 6 cycloalkyl; l-methylpiperidin-4-yl; and Ci_ 3 alkylcarbonyl; or
- NR 3d R 4d is 4-methylpiperazin-l-yl
- -L 2 -0-R 5 is selected from -covalent bond-0-R 5a wherein R 5a is selected from the group consisting of hydrogen; Ci_ 3 alkyl; Ci_ 3 alkyl substituted with pyridinyl, pyrrolidinyl or morpholinyl; and pyridinyl; or
- R 5b is selected from the group consisting of hydrogen; Ci_ 3 alkyl; and phenyl; or
- R 5c is selected from the group consisting of hydrogen
- Ci_ 3 alkyl and Ci_ 3 alkyl substituted with pyridinyl or phenyl; or -CH(CF 3 )-0-H;
- the present invention relates to a compound of formula (I), wherein R 1 and R 2 are as previously defined in any of the above embodiments, and wherein
- R 3a is hydrogen or methyl
- R 4a is selected from the group consisting of Ci_ 3 alkyl optionally substituted with phenyl; tetrahydropyranyl; 4-hydroxycyclohexan-l-yl; and pyridinyl; or
- NR 3a R 4a is pyrrolidinyl, piperidinyl or morpholinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, trifluoromethyl, hydroxyl, Ci_ 3 alkyloxy, mono- and di(Ci_3 alky 1) amino, hydroxyl- Ci_ 3 alkyl, haloCi_ 3 alkyl, and methoxyCi_ 3 alkyl; or 4-methylpiperazin-l-yl; or
- R 3b is hydrogen and R 4b is Ci_ 3 alkyl
- NR 3b R 4b is morpholinyl
- R 4c is selected from the group consisting of hydrogen; Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of halo, hydroxy, Ci_3alkoxy, mono- and di(Ci_3alkyl)amino, phenyl, pyridinyl, pyridinyl substituted with halo, morpholinyl, pyrrolidinyl, and piperidinyl; l-methylpiperidin-4-yl; and 3,4,5- trimethoxyphenyl; or
- R 3d is hydrogen or methyl
- R 4d is selected from the group consisting of hydrogen; Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of Ci_ 3 alkoxy, and morpholinyl; l-methylpiperidin-4-yl; and Ci_ 3 alkylcarbonyl; or
- NR 3d R 4d is 4-methylpiperazin-l-yl
- R 5a is selected from the group consisting of hydrogen; Ci_ 3 alkyl; Ci_ 3 alkyl substituted with pyridinyl or morpholinyl; and pyridinyl; or
- Ci_ 3 alkyl and Ci_ 3 alkyl substituted with pyridinyl or phenyl; or
- the present invention relates to a compound of formula (I), wherein R 1 and R 2 are as previously defined in any of the above embodiments, and wherein
- -L 1 -NR 3 R 4 is selected from -CH 2 -NR 3a R 4a wherein
- R 3a is hydrogen or methyl
- R 4a is selected from the group consisting of Ci_ 3 alkyl optionally substituted with phenyl; and pyridinyl; or
- NR 3a R 4a is pyrrolidinyl, piperidinyl or morpholinyl, each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, and hydro xyl; or 4-methylpiperazin-l-yl; or
- R 3b is hydrogen and R 4b is Ci_ 3 alkyl
- NR 3b R 4b is morpholinyl
- R 3c is hydrogen or methyl
- R 4c is selected from the group consisting of Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of halo, Ci_3alkoxy, mono- and di(Ci_ 3 alkyl)amino, phenyl, pyridinyl, pyridinyl substituted with halo, morpholinyl, and piperidinyl; l-methylpiperidin-4-yl; and 3,4,5-trimethoxyphenyl; or -covalent bond-NR 3d R 4d wherein
- R 3d is hydrogen or methyl
- R 4d is selected from the group consisting of hydrogen; Ci_ 3 alkyl optionally substituted with a substituent selected from the group consisting of Ci_ 3 alkoxy, and morpholinyl; and l-methylpiperidin-4-yl; or
- NR 3d R 4d is 4-methylpiperazin- 1 -yl
- the present invention relates to a compound of formula (I) as defined herein, wherein R 1 is 5-butoxypyridin-3-yl or 5-butoxy-2-chlorophenyl and R 2 is In a further embodiment, the present invention relates to a compound of formula (I) as defined herein, wherein R 1 is 5-butoxypyridin-3-yl or 5-butoxy-2-chlorophenyl and R 2 is In a further embodiment, the present invention relates to a compound of formula (I) as defined herein, wherein R 1 is 5-butoxypyridin-3-yl or 5-butoxy-2-chlorophenyl and R 2 is In a further embodiment, the present invention relates to a compound of formula (I) as defined herein, wherein R 1 is 5-butoxypyridin-3-yl or 5-butoxy-2-chlorophenyl and R 2 is In a further embodiment, the present invention relates to a compound of formula (I) as defined herein, wherein R 1 is 5-butoxypyridin
- R 1 is 2-chloro-4-fluorophenyl or 2-chloro-6-fluorophenyl and R 2 is
- the present invention relates to a compound of formula ( ⁇ ) as defined herein, wherein
- R 1 is phenyl or pyridinyl each optionally substituted with 1 or 2 substituents independently selected from the group consisting of halo, (C3_ 6 cycloalkyl)Ci_ 3 alkyloxy and Ci_ 6 alkyloxy; and
- R 2 is -CH 2 -NR 3a R 4a ;
- R 3a is hydrogen or methyl
- R 4a is selected from the group consisting of Ci_ 3 alkyl; or
- the invention relates to a compound of Formula
- R 1 is phenyl substituted with halo and Ci_ 6 alkyloxy, or pyridinyl substituted with
- the invention relates to a compound of Formula ( ⁇ ), as described herein, wherein
- R 1 is phenyl substituted with chloro and Ci_ 6 alkyloxy, in particular ethoxy, isopropoxy or butoxy; or pyridinyl substituted with Ci_ 6 alkyloxy or (C 3 - 6 cycloalkyl)Ci_ 3 alkyloxy, in particular butoxy or cyclopropylmethoxy; and
- R 2 is -CH 2 -NHCH 3 , -CH 2 -N(CH 3 ) 2 or -CH 2 -(4-morpholinyl); or a pharmaceutically acceptable salt or a solvate thereof.
- the invention relates to a compound of Formula ( ⁇ ), as described herein, wherein
- R 1 is phenyl substituted with halo and Ci_ 6 alkyloxy, or pyridinyl substituted with
- Ci_ 6 alkyloxy; and R 2 is as previously defined;
- the invention relates to a compound of Formula ( ⁇ ), as described herein, wherein
- R 1 is phenyl substituted with chloro and Ci_ 6 alkyloxy, in particular ethoxy, isopropoxy or butoxy; or pyridinyl substituted with Ci_ 6 alkyloxy, in particular butoxy; and
- R 2 is as previously defined
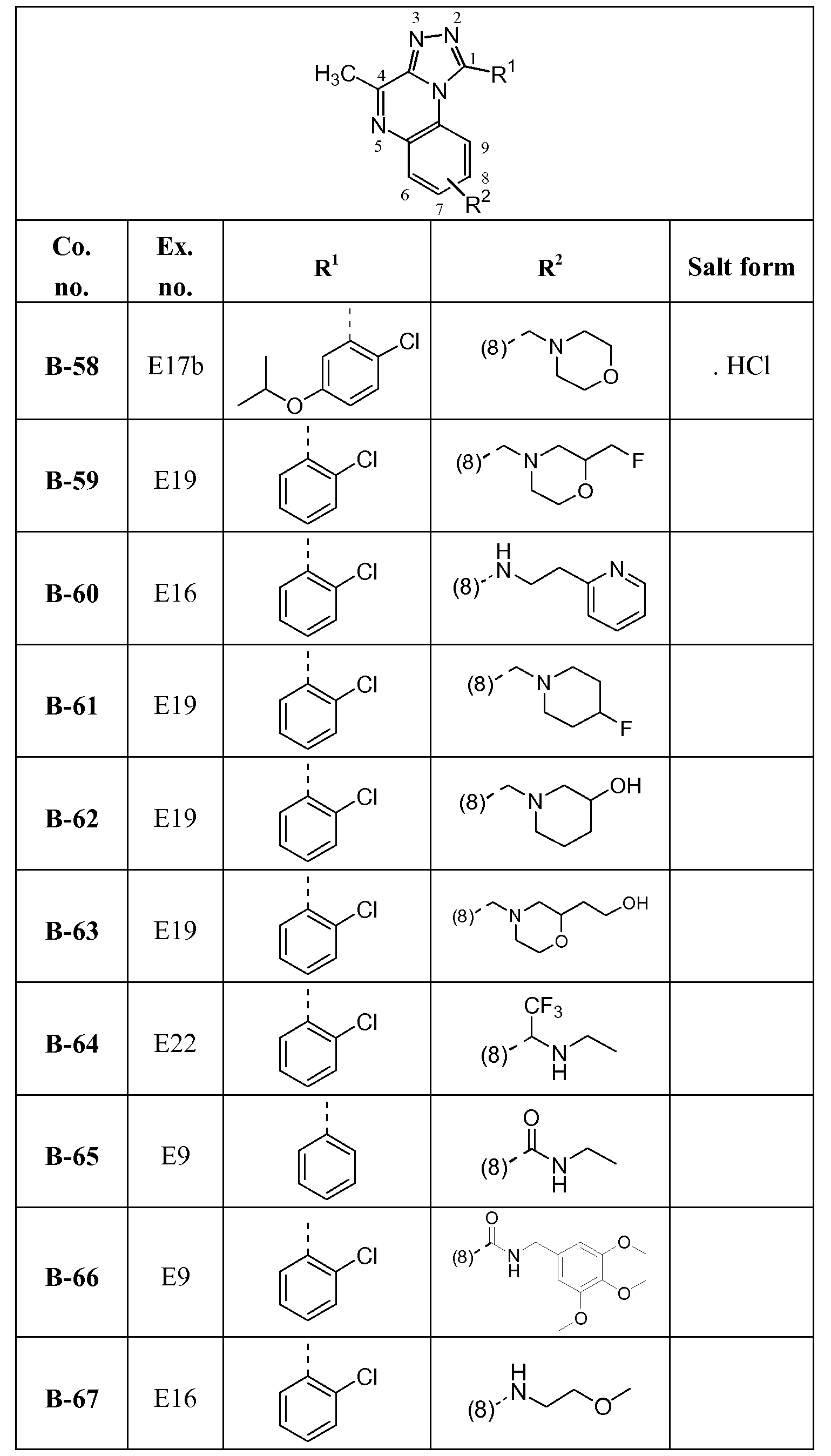
- the compound is selected from Ethyl l-(2-chlorophenyl)-4-methyl[l ,2,4]triazolo[4,3-a]quinoxaline-8-carboxylate; Ethyl l-(2-chlorophenyl)-4-methyl[l ,2,4]triazolo[4,3-a]quinoxaline-7-carboxylate; Ethyl 4-methyl- 1 -phenyl[ 1 ,2,4]triazolo[4,3-a]quinoxaline-8-carboxylate;
- the compound is selected from l-(5-Butoxypyridin-3-yl)-4-methyl-8-(morpholin-4-ylmethyl)[l,2,4]triazolo[4,3- ajquinoxaline, or a hydrochloride salt thereof, or an oxalate salt thereof; 1 - [2-Chloro-5 -( 1 -methylethoxy)phenyl] -4-methyl- 8-(morpho lin-4- ylmethyl)[l,2,4]triazolo[4,3-a]quinoxaline or a hydrochloride salt thereof;
- the compound is selected from l-(2-Chlorophenyl)-4-methyl-8-(2-pyridin-2-ylethoxy)[l,2,4]triazolo[4,3- ajquinoxaline;
- the invention also relates to radiolabelled compounds of Formula (I).
- the invention relates to a compound of Formula (I-u)
- ring A is phenyl or pyridinyl, R is halo or trifluoromethyl, n is 0 or 1 and R 2 is as defined herein in the compounds of Formula (I); or of Formula [ 3 H]-(I-p) wherein R 1 , R 3 and R 4 , are as defined herein in the compounds of
- the radio labelled compound of Formula (I) is l-(5-Butoxypyridin-3-yl)-4-methyl-8-[morpholin-4-yl( 3 Hi)methyl][l ,2,4]triazolo[4,3- a]quinoxaline; or
- the invention relates to an intermediate compound having the Formula (XVI)
- ring A is phenyl or pyridinyl, R is halo or trifluoromethyl, n is 0 or 1 and R 2 is as defined herein in the compounds of Formula (I); or having the Formula (XIII)
- R 1 is as defined herein in the compounds of Formula (I);
- the compounds of Formula [ 3 H]-(I-p) or (I-u) and compositions comprising the compounds of Formula [ 3 H]-(I-p) or (I-u) can be used for imaging a tissue, cells or a host, in vitro or in vivo.
- the invention relates to a method of imaging or quantifying the PDE2 enzyme in a tissue, cells or a host in vitro or in vivo.
- the cells and tissues are preferably central nervous system cells and tissues in which the PDE2 enzyme is abundant.
- the PDE2 enzyme is abundant in central nervous system tissue, more in particular, in central nervous system tissue forming the brain; more in particular, PDE2 is expressed in olfactory bulb, olfactory tubercle, cortex, striatum, hippocampus, habenula, amygdala, thalamus, hypothalamus and substantia nigra.
- the host is a mammal.
- the compound of Formula (I) is administered intravenously, for example, by injection with a syringe or by means of a peripheral intravenous line, such as a short catheter.
- the compound of Formula (I-u) or a sterile solution comprising a compound of Formula (I-u) may in particular be administered by intravenous administration in the arm, into any identifiable vein, in particular in the back of the hand, or in the median cubital vein at the elbow.
- the invention relates to a method of imaging a tissue or cells in a mammal, comprising the intravenous administration of a compound of Formula (I-u), as defined herein, or a composition comprising a compound of Formula (I-u) to the mammal, and imaging the tissue or cells with a positron-emission tomography imaging system.
- the invention relates to a method of imaging a tissue or cells in a human, comprising the intravenous administration of a compound of Formula (I-u), as defined herein, or a sterile formulation comprising a compound of Formula (I-u) to the human, and imaging the tissue or cells with a positron-emission tomography imaging system.
- the invention relates to a method of imaging or quantifying the PDE2 enzyme in a mammal, comprising the intravenous administration of a compound of Formula (I-u), or a composition comprising a compound of Formula (I-u) to the mammal, and imaging with a positron-emission tomography imaging system.
- the invention relates to the use of a compound of Formula (I-u) for imaging a tissue, cells or a host, in vitro or in vivo, or the invention relates to a compound of Formula (I-u), for use in imaging a tissue, cells or a host in vitro or in vivo, using positron-emission tomography.
- Halo shall denote fluoro, chloro and bromo;
- Ci_6alkyl and “Ci_3alkyl” as used herein as a group or part of a group shall denote a straight or branched saturated alkyl group having 1, 2, 3, 4, 5, or 6 carbon atoms or 1, 2 or 3 carbon atoms, respectively e.g.
- C2- 6 alkenyl as used herein as a group or part of a group refers to a linear or branched hydrocarbon group containing from 2 to 6 carbon atoms and containing a carbon carbon double bond
- Ci_6alkyloxy and "Ci_3alkyloxy” shall denote an ether radical wherein Ci_6alkyl and Ci_ 3 alkyl are as defined before
- haloCi_ 3 alkyl shall denote Ci_ 3 alkyl as defined before, substituted with 1 halo atom as defined before
- C 3 _6cycloalkyl shall denote cyclopropyl, cyclobutyl,
- subject refers to an animal, preferably a mammal, most preferably a human, who is or has been the object of treatment, observation or experiment.
- terapéuticaally effective amount means that amount of active compound or pharmaceutical agent that elicits the biological or medicinal response in a tissue system, animal or human that is being sought by a researcher, veterinarian, medical doctor or other clinician, which includes alleviation of the symptoms of the disease or disorder being treated.
- composition is intended to encompass a product comprising the specified ingredients in the specified amounts, as well as any product which results, directly or indirectly, from combinations of the specified ingredients in the specified amounts.
- the term "host” refers to a mammal, in particular to humans, mice, dogs and rats.
- cell refers to a cell expressing or incorporating the PDE2 enzyme. It will be appreciated that some of the compounds of Formula (I) and their pharmaceutically acceptable addition salts and solvates thereof may contain one or more centres of chirality and exist as stereoisomeric forms.
- the invention includes all stereoisomers of the compounds of the invention either as a pure stereoisomer or as a mixture of two or more stereoisomers.
- Enantiomers are stereoisomers that are non-superimposable mirror images of each other.
- a 1 : 1 mixture of a pair of enantiomers is a racemate or racemic mixture.
- Diastereomers are stereoisomers that are not enantiomers, i.e. they are not related as mirror images. If a compound contains a double bond, the substituents may be in the E or the Z configuration. If a compound contains an at least disubstituted non-aromatic cyclic group, the substituents may be in the cis or trans configuration.
- the invention includes enantiomers, diastereomers, racemates, E isomers, Z isomers, cis isomers, trans isomers and mixtures thereof, whenever chemically possible.
- the absolute configuration is specified according to the Cahn-Ingold-Prelog system.
- the configuration at an asymmetric atom is specified by either R or S.
- Resolved stereoisomers whose absolute configuration is not known can be designated by (+) or (-) depending on the direction in which they rotate plane polarized light.
- resolved enantiomers whose absolute configuration is not known can be designated by (+) or (-) depending on the direction in which they rotate plane polarized light.
- stereoisomer is substantially free, i.e. associated with less than 50%, preferably less than 20%, more preferably less than 10%, even more preferably less than 5%, in particular less than 2% and most preferably less than 1%, of the other stereoisomers.
- a compound of Formula (I) is for instance specified as (R)
- a compound of Formula (I) is for instance specified as E
- Z Z isomer
- a compound of Formula (I) is for instance specified as cis, this means that the compound is substantially free of the trans isomer.
- some of the compounds of the present invention may form solvates with water (i.e., hydrates) or common organic solvents, and such solvates are also intended to be encompassed within the scope of this invention.
- Radiolabelled compounds of Formula (I) may comprise a radioactive isotope selected from the group of 3 H, n C, 18 F, 122 1, 123 I, 125 I, 131 I, 75 Br, 76 Br, 77 Br and 82 Br.
- the radioactive isotope is selected from the group of 3 H, n C and 18 F.
- the salts of the compounds of this invention refer to non- toxic "pharmaceutically acceptable salts".
- Other salts may, however, be useful in the preparation of compounds according to this invention or of their pharmaceutically acceptable salts.
- Suitable pharmaceutically acceptable salts of the compounds include acid addition salts which may, for example, be formed by mixing a solution of the compound with a solution of a pharmaceutically acceptable acid such as hydrochloric acid, sulfuric acid, fumaric acid, maleic acid, succinic acid, acetic acid, benzoic acid, citric acid, tartaric acid, carbonic acid or phosphoric acid.
- suitable pharmaceutically acceptable salts thereof may include alkali metal salts, e.g., sodium or potassium salts; alkaline earth metal salts, e.g., calcium or magnesium salts; and salts formed with suitable organic ligands, e.g., quaternary ammonium salts.
- Representative acids which may be used in the preparation of pharmaceutically acceptable salts include, but are not limited to, the following: acetic acid, 2,2- dichloroactic acid, acylated amino acids, adipic acid, alginic acid, ascorbic acid, L-aspartic acid, benzenesulfonic acid, benzoic acid, 4-acetamidobenzoic acid,
- Representative bases which may be used in the preparation of pharmaceutically acceptable salts include, but are not limited to, the following: ammonia, L-arginine, benethamine, benzathine, calcium hydroxide, choline, dimethylethanolamine, diethanolamine, diethylamine, 2-(diethylamino)-ethanol, ethanolamine, ethylene-diamine, N-methyl-glucamine, hydrabamine, lH-imidazole, L-lysine, magnesium hydroxide, 4-(2-hydroxyethyl)- morpholine, piperazine, potassium hydroxide, l-(2-hydroxyethyl)-pyrrolidine, secondary amine, sodium hydroxide, triethanolamine, tromethamine and zinc hydroxide.
- the compounds according to the invention can generally be prepared by a succession of steps, each of which is known to the skilled person.
- the transformations of different functional groups present in the final compounds into other functional groups according to Formula (I) can be performed as well by synthesis methods well known to the person skilled in the art.
- the compounds can be prepared according to the following synthesis methods.
- R 2 H (l-a), halo (l-b), CF 3 (l-c), OCF 3 (l-d), CN (l-e),
- Step 1 An intermediate compound of Formula (II) can be reacted with a commercially available compound of Formula (III), wherein R 6 is Ci_3-alkyl such as for example methyl or ethyl in an inert solvent such as, for example, toluene stirring the reaction mixture at a suitable temperature, typically at 100-130 °C, using conventional heating or under microwave irradiation, for the required time to achieve completion of the reaction, typically 3 hours for conventional heating.
- R 6 is hydrogen the reaction is performed in a mixture of acetic acid and water and the stirring is performed at room temperature overnight.
- This reaction usually affords a mixture of the two possible regio isomers of Formula (IV), which can be separated at this step or in one of the following steps by chromatographic methods, either by column chromatography or HPLC.
- Compounds of Formula (II) are either commercially available or described in chemical literature and can be prepared by simple standard synthetic procedures well known to the skilled person.
- Step 2 Intermediate compounds of Formula (IV) can react, in presence or absence of a solvent such as for example 1,2-dichloroethane, with phosphorous oxychloride, stirring the reaction mixture at a suitable temperature, typically at 100-120 °C, using conventional heating or under microwave irradiation, for the required time to achieve completion of the reaction, typically 2-4 hours for conventional heating.
- a solvent such as for example 1,2-dichloroethane
- Step 3 An intermediate compound of Formula (V) can react with an intermediate compound of Formula (VI) in a solvent, such as, for example, ethanol, n-butanol or tetrahydrofuran stirring the reaction mixture at a suitable temperature, typically at 100-160 °C, using conventional heating or under microwave irradiation, for the required time to achieve completion of the reaction, typically 15-20 minutes at 160° C for microwave heating, affording final compounds of Formula (I).
- a solvent such as, for example, ethanol, n-butanol or tetrahydrofuran stirring the reaction mixture at a suitable temperature, typically at 100-160 °C, using conventional heating or under microwave irradiation, for the required time to achieve completion of the reaction, typically 15-20 minutes at 160° C for microwave heating, affording final compounds of Formula (I).
- a solvent such as, for example, ethanol, n-butanol or tetrahydrofuran stirring the reaction mixture at a suitable temperature, typically at 100-160 °C,
- Step 1 Intermediate compounds of Formula (V) can be treated with hydrazine hydrate in an inert solvent, such as methanol or ethanol, following simple standard synthetic procedures well known to the skilled person yielding intermediate compounds of
- Step 2 Intermediate compounds of Formula (VII) can react with intermediate compounds of Formula (VIII) following simple standard synthetic procedures well known to the skilled person to give intermediate compounds of Formula (IX).
- Step 3 Intermediate compounds of Formula (IX) can react, in presence or absence of a solvent such as for example 1 ,2-dichloroethane, with phosphorous oxychloride, stirring the reaction mixture at a suitable temperature, typically at 80-100 °C, using a solvent such as for example 1 ,2-dichloroethane, with phosphorous oxychloride, stirring the reaction mixture at a suitable temperature, typically at 80-100 °C, using
- Step 1 Final compounds of Formula (I-g) may be used as starting materials for a conventional hydrolysis reaction very well known to the person skilled in the art.
- compounds of Formula (I-g) can react in presence of a base, such as for example sodium or potassium hydroxide, in a mixture of solvents such as, for example, tetrahydrofuran and water stirring the reaction mixture at a suitable temperature, typically room temperature, for the required time to achieve completion of the reaction, typically 18 hours.
- a base such as for example sodium or potassium hydroxide
- solvents such as, for example, tetrahydrofuran and water stirring the reaction mixture at a suitable temperature, typically room temperature, for the required time to achieve completion of the reaction, typically 18 hours.
- This reaction step affords intermediate compounds of Formula (X).
- Step 2 Intermediate compounds of Formula (X) can react with an alkylating agent, of formula R 5 -Z, wherein R 5 is selected from the group consisting of Ci_ 3 alkyl; Ci_ 3 alkyl substituted with pyridinyl, phenyl or morpholinyl; and pyridinyl and Z is a suitable leaving group such as halo, for example bromo or iodo, in the presence of a suitable base such as l ,8-diazabicyclo[5.4.0]undec-7-ene (DBU), in an inert solvent such as, for example, dimethylformamide, stirring the reaction mixture at a suitable temperature, typically room temperature, for the required time to achieve completion of the reaction, typically 2-3 hours.
- This reaction step affords final compounds of Formula (I-i).
- Intermediate compounds of formula (X) can react with an amine of formula NHR 3 R 4 , wherein R 3 and R 4 are as previously defined, in the presence of a coupling reagent, such as for example 2-(7-aza-lH-benzotriazole-l-yl)-l,l,3,3-tetramethyluronium hexafluorophosphate (HATU) and a base such as ⁇ , ⁇ -diisopropyl ethylamine, in a mixture of inert solvents such as, for example, ⁇ , ⁇ -dimethylformamide and dichloromethane, stirring the reaction mixture at a suitable temperature, typically room temperature, for the required time to achieve completion of the reaction, typically 2-3 hours.
- a coupling reagent such as for example 2-(7-aza-lH-benzotriazole-l-yl)-l,l,3,3-tetramethyluronium hexafluorophosphate (HATU) and a base such as ⁇
- Final compounds of Formula (I-b) may react with an amine of formula NHR 3 R 4 wherein R 3 and R 4 are as previously defined, in an inert solvent, such as, for example, toluene in presence of a complexing agent, such as for example XantPhos, a palladium catalyst, such as Palladium(II) acetate, a base such as for example triethylamine, and carbon monoxide.
- a complexing agent such as for example XantPhos
- a palladium catalyst such as Palladium(II) acetate
- a base such as for example triethylamine
- carbon monoxide carbon monoxide
- Step 1 Final compounds of Formula (I-g) or (I-i) may react with the Lawesson's reagent (2,4-bis-(4-methoxyphenyl)-l,3-dithia-2,4-diphosphetane 2,4-disulfide), in an inert solvent such as, for example, toluene and stirring the reaction mixture at a suitable temperature, typically 150° C, for the required time to achieve completion of the reaction, typically 24 hours.
- This reaction step affords intermediate compounds of Formula (XI).
- Step 2 Intermediate compounds of Formula (XI) can react in an inert solvent such as, for example, tetrahydrofuran in presence of Raney®-Nickel, stirring the reaction mixture at a suitable temperature, such as room temperature, for the required time to achieve completion of the reaction, typically 1 hour.
- This reaction step affords final compounds of Formula (I-j).
- Step 1 Final compounds of Formula (I-b) may be also used as precursors for a hydro xylation reaction.
- a compound of Formula (I-b) can react with bis- (pinacolato) diboron in an inert solvent such as, for example, 1,4-dioxane in presence of a palladium catalyst, such as [l,l'-Bis(diphenylphosphino)ferrocene]dichloro- palladium (II), a base such as for example potassium acetate, stirring the reaction mixture at a suitable temperature, such as 110-130° C, for the required time to consume all starting material, typically 1 hour.
- a palladium catalyst such as [l,l'-Bis(diphenylphosphino)ferrocene]dichloro- palladium (II)
- a base such as for example potassium acetate
- Step 2 Compounds of Formula (I-k) may be used as intermediate reagents for a conventional Mitsunobu reaction, which is well known to the person skilled in the art.
- a compound of Formula (I-k) can react with alcohols of formula R 5 -OH, wherein R 5 is selected from the group consisting of Ci_ 3 alkyl; Ci_ 3 alkyl substituted with pyridinyl, phenyl or morpholinyl; and pyridinyl and in the presence of diethyl-, ⁇ -tert- butyl- or diisopropyl azodicarboxylate and triphenylphosphine, in an inert solvent such as for example tetrahydrofuran, stirring the reaction mixture at a suitable temperature, typically at 120 °C under microwave irradiation, for a suitable period of time to allow completion of the reaction, typically 15-20 minutes.
- This reaction step affords final compound of Formula (1-1).
- a compound of Formula (I-b) can react with an amine of formula NHR 3 R 4 , wherein R 3 and R 4 are as previously defined, in an inert solvent, such as, for example, toluene or a mixture of 1 ,4-dioxane/water, in presence of a complexing agent, such as 4,5-bis- (diphenylphosphino)-9,9-dimethylxanthene (XantPhos) or 2-dichlorohexylphosphino- 2',4',6'-triisopropylbiphenyl (XPhos), a palladium catalyst, such as Palladium(II) acetate or tris(dibenzylideneacetone)dipalladium(0), and a base such as for example caesium carbonate, stirring the reaction mixture at a suitable temperature, such as 1 10-130° C, using conventional heating or microwave irradiation, for the required time to achieve completion of the reaction, typically 10-15 minutes for
- a compound of Formula (I-b) can also react with an intermediate compound of Formula (XII) in an inert solvent or mixture of solvents, such as, for example, a mixture of tetrahydrofuran and water in presence of a complexing agent such as 2-dichlorohexylphosphino-2',4',6'-triisopropylbiphenyl (XPhos), a palladium catalyst, such as Palladium (II) acetate, and a base such as for example caesium carbonate stirring the reaction mixture at a suitable temperature, such as 110-120° C, using conventional heating or microwave irradiation, for the required time to achieve completion of the reaction, typically 45 minutes for conventional heating.
- Intermediate compounds of Formula (XII) can be either commercially available or can be prepared by methods described in chemical literature well known to the skilled person.
- Step 1 Final compounds of Formula (I-b) may also be used as precursors for the synthesis of final compounds of Formula (I-o), Formula (I-p), Formula (I-q) and Formula (I-r).
- a compound of Formula (I-b) can react with tributylvinyl tin, in an inert solvent such as, for example, toluene in presence of a palladium catalyst, such as (triphenylphosphine)tetrakis Palladium(0), and a salt such as, for example, lithium chloride stirring the reaction mixture at a suitable temperature, such as 120-130° C, using conventional heating or microwave irradiation, for the required time to achieve completion of the reaction, typically 1 hour for conventional heating.
- This reaction step affords a final compound of Formula (I-o).
- Step 2 A compound of Formula (I-o) can be oxidized by standard procedures well known to the person skilled in the art, such as, for example, by ozonolysis or by reaction with a mixture of osmium tetroxide and sodium periodate yielding an intermediate compound of Formula (XIII).
- Step 3a An intermediate compound of Formula (XIII) can react with an amine of formula NHR 3 R 4 , wherein R 3 and R 4 are as previously defined, in a conventional reductive amination reaction, which is well known to the skilled person.
- a compound of Formula (XIII) can react with an amine of formula NHR 3 R 4 as previously defined in an inert solvent, such as for example, 1,2-dichloroetane, stirring the reaction mixture at a suitable temperature, typically at 80-120 °C for 10-20 minutes under microwave irradiation, in the presence of a reducing agent, such as tributoxy cyanoborohydride or sodium borohydride.
- a reducing agent such as tributoxy cyanoborohydride or sodium borohydride.
- the reaction can be stirred either at room temperature or by microwave heating for the required time to achieve completion of the reaction, typically 20 min at 80° C for microwave heating. This reaction step yields a final compound of Formula (I-p).
- Step 3b An intermediate compound of Formula (XIII) can also react with
- trimethyl(trifluoromethyl) silane in a inert solvent such as, for example,
- Step 4 A final compound of formula (I-q) can react with methanesulfonyl chloride in an inert solvent, such as, for example, dichlorometane in the presence of a base, such as pyridine, stirring the reaction at a suitable temperature, typically room temperature for the required time to consume all starting material, typically overnight. Then, the mixture can be reacted with a primary or secondary amine stirring the reaction at a suitable temperature, typically room temperature for the required time to achieve completion of the reaction, typically 4 hours. This reaction step affords a final compound of Formula (I-r).
- Step 1 Intermediate compounds of Formula (XIII) can react with a Grignard reagent following standard synthetic procedures well known to the skilled person.
- a compound of Formula (XIII) can react with an appropriate Grignard Reagent in an inert solvent, such as, for example, tetrahydrofuran stirring the reaction mixture at a suitable temperature, typically at 45 °C, using conventional heating, for the required time to achieve completion of the reaction, typically 30 minutes.
- an inert solvent such as, for example, tetrahydrofuran stirring the reaction mixture at a suitable temperature, typically at 45 °C, using conventional heating, for the required time to achieve completion of the reaction, typically 30 minutes.
- Step 2 Intermediate compounds of Formula (XIV) can be oxidized following reaction procedures well known to the people skilled in the art.
- a compound of Formula (XIV) can react with an appropriate oxidizing agent, such as, for example, Manganese dioxide in the presence of an inert solvent, such as, for example, dichloromethane stirring the reaction mixture at suitable temperature, typically room temperature for the required time to achieve completion of the reaction, usually 4 hours.
- an oxidizing agent such as, for example, Manganese dioxide
- an inert solvent such as, for example, dichloromethane stirring the reaction mixture at suitable temperature, typically room temperature for the required time to achieve completion of the reaction, usually 4 hours.
- Step 1 Final compounds of Formula (I-b) may also be used as precursors for the synthesis of Final compounds of Formula (I-t).
- a compound of Formula (I-b) can react with tributyl(l-ethoxyvinyl) tin in an inert solvent, such as, for example, toluene in the presence of a palladium catalyst, such as (triphenylphosphine)tetrakis
- Step 2 Intermediate compounds of Formula (XV) can react with Xenon difluoride and hydrogen fluoride-pyridine complex, in an inert solvent, such as dichloromethane, stirring the reaction at a suitable temperature, such as room temperature, for the required time to achieve completion of the reaction, typically overnight. This reaction step yields final compounds of Formula (I-t).
- an inert solvent such as dichloromethane
- Step 1 (a) A compound of Formula (V) can be reacted with a compound of Formula (Via) wherein ring A is phenyl or pyridinyl, R 8 is halo or trifluoromethyl, n is 0 or 1 and R 2 is as previously defined for compounds of Formula (I), according to the conditions described under Scheme 1, Method A, Step 3.
- Step 1 A compound of Formula (VII) can be reacted with a compound of formula (Villa) wherein ring A is phenyl or pyridinyl, R 8 is halo or trifluoromethyl, n is 0 or 1 and R 2 is as previously defined for compounds of Formula (I), according to the conditions described under Scheme 1, Method B, Step 2.
- Step 2 Intermediate compound of Formula (IXa) can react, in presence or absence of a solvent such as for example 1,2-dichloroethane, with phosphorous oxychloride, stirring the reaction mixture at a suitable temperature, typically at 80-100 °C, using a solvent such as for example 1,2-dichloroethane, with phosphorous oxychloride, stirring the reaction mixture at a suitable temperature, typically at 80-100 °C, using
- Step 3 Intermediate compound of Formula (XVI) can undergo a nucleophilic aromatic substitution reaction with a source of [ 18 F]fluoride ([ 18 F]F " ) such as for example [ 18 F]F ⁇ / 2 C0 3 /Kryptofix® 222 complex, or [ 18 F]KF-K 222 (wherein Kryptofix® 222 and K 222 mean 4,7,13,16,21,24-hexaoxa-l,10-diazabicyclo[8.8.8]hexacosane; also known as 2.2.2) in an inert solvent such as for example anhydrous DMF under appropriate reaction conditions, such as heating in a microwave, for example at 140 0 or conditions known to the skilled person (for a review, see for example P. W. Miller et al. Angew. Chem. Int. Ed. 2008, 47, 8998-9033).
- a source of [ 18 F]fluoride such as for example [ 18 F]F ⁇
- Tritiated compounds of Formula (I-p), referred to herein as [ 3 H]-(I-p) may be prepared from compounds of formula (XIII) by reaction with an amine of formula NHR 3 R 4 , wherein R 3 and R 4 are as previously defined, in a reductive amination reaction using tritium in the presence of a catalyst, under conditions known to the skilled person, in two steps.
- a compound of formula (XIII) can react in a first step with an amine of formula NHR 3 R 4 as previously defined in an inert solvent, such as for example, dichloromethane, optionally in the presence of a dehydrating agent such as titanium tetra(isopropoxide) stirring the reaction mixture at a suitable temperature, typically at room temperature under an inert atmosphere.
- a dehydrating agent such as titanium tetra(isopropoxide) stirring the reaction mixture at a suitable temperature, typically at room temperature under an inert atmosphere.
- the second step involves the addition of another inert aprotic solvent, such as for example, tetrahydrofuran, and reacting the intermediate imine in the presence of a reducing agent, such as tritium, and in the presence of a catalyst, such as Pt on carbon.
- the reaction can be stirred at room temperature for the required time to achieve completion of the reaction, typically 60 min at room temperature.
- This reaction step yields a final compound of Formula [ 3
- Some compounds according to the invention were isolated as acid addition salt forms or isolated as free base and then converted to the acid addition salt forms.
- the acid addition salt forms of the compounds according to the invention for example the HCl salt forms unless otherwise described, several procedures known to those skilled in the art can be used.
- the free base can be dissolved in isopropanol, diisopropylether, diethyl ether and/or dichloromethane and subsequently, 1 to 2 equivalents of the appropriate acid, for example a 6N HCl solution in 2-propanol or a 2N HCl solution in diethyl ether, can be added dropwise.
- the mixture typically is stirred for 10 min or longer after which the product can be filtered off.
- the HCl salt is usually dried in vacuo.
- the values of salt stoichiometry as provided hereinbefore and hereinafter, are those obtained
- the compounds according to the invention inhibit PDE2 enzyme activity, in particular PDE2A, and to a lesser extent they inhibit PDE10 enzyme activity, in particular PDE10A, or inhibit both, PDE2 and PDE10 enzyme activity, in particular PDE2A and PDE10A enzyme activity and hence raise the levels of cAMP or cGMP within cells that express PDE2, or PDE2 and PDE10. Accordingly, inhibition of PDE2 or of PDE2 and PDE10 enzyme activity may be useful in the treatment of diseases caused by deficient amounts of cAMP or cGMP in cells. PDE2 or PDE2 and PDE10 inhibitors may also be of benefit in cases in which raising the amount of cAMP or cGMP above normal levels results in a therapeutic effect. Inhibitors of PDE2 or inhibitors of PDE2 and PDE10 may be used to treat neurological and psychiatric disorders, and endocrinological or metabolic diseases.
- the present invention relates to a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the present invention, for use as a medicine, as well as to the use of a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention or a pharmaceutical composition according to the invention for the manufacture of a medicament.
- the present invention also relates to a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the present invention or a pharmaceutical composition according to the invention for use in the treatment or prevention of, in particular treatment of, a condition in a mammal, including a human, the treatment or prevention of which is affected or facilitated by the inhibition of phosphodiesterase 2 enzyme or phosphodiesterase 2 and 10 enzymes.
- the present invention also relates to the use of a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the present invention or a
- composition according to the invention for the manufacture of a medicament for the treatment or prevention of, in particular treatment of, a condition in a mammal, including a human, the treatment or prevention of which is affected or facilitated by the inhibition of phosphodiesterase 2 enzyme or of phosphodiesterase 2 and 10 enzymes.
- the present invention also relates to a compound of formula (I) or a
- the present invention relates to the use of a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention or a pharmaceutical composition according to the invention for the manufacture of a medicament for treating, preventing, ameliorating, controlling or reducing the risk of various neurological and psychiatric disorders associated with phosphodiesterase 2 or associated with phosphodiesterases 2 and 10 dysfunction in a mammal, including a human, the treatment or prevention of which is affected or facilitated by the inhibition of phosphodiesterase 2 or by the inhibition of phosphodiesterases 2 and 10.
- a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof or composition according to the invention for the manufacture of a medicament for e.g. the treatment of a subject, e.g. a mammal
- a method of e.g. treatment of a subject comprising administering to a subject in need of such e.g. treatment, an effective amount of a compound of formula (I) or a
- the indications that may be treated with PDE2 inhibitors, or with PDE2 and PDE10 inhibitors, either alone or in combination with other drugs include, but are not limited to, those diseases thought to be mediated in part by the basal ganglia, prefrontal cortex and hippocampus.
- neurological and psychiatric disorders selected from psychotic disorders and conditions; anxiety disorders; movement disorders; drug abuse; mood disorders; neurodegenerative disorders; disorders or conditions comprising as a symptom a deficiency in attention and/or cognition; pain; autistic disorder or autism; and metabolic disorders.
- the psychotic disorders and conditions associated with PDE2 or with PDE2 and PDE10 dysfunction include one or more of the following conditions or diseases: schizophrenia, for example of the paranoid, disorganized, catatonic, undifferentiated or residual type; schizophreniform disorder; schizoaffective disorder, such as delusional or depressive type; delusional disorder; substance-induced psychotic disorder such as psychosis induced by alcohol, amphetamine, cannabis, cocaine, hallucinogens, inhalants, opioids, or phencyclidine; personality disorders of the paranoid type; and personality disorder of the schizoid type.
- the anxiety disorders include panic disorder; agoraphobia; specific phobia; social phobia; obsessive-compulsive disorder; post-traumatic stress disorder; acute stress disorder; and generalized anxiety disorder.
- movement disorders include Huntington's disease and dyskinesia; Parkinson's disease; restless leg syndrome and essential tremor.
- Tourette's syndrome and other tic disorders can be included.
- the central nervous system disorder is a substance-related disorder selected from the group of alcohol abuse; alcohol dependence; alcohol withdrawal; alcohol withdrawal delirium; alcohol- induced psychotic disorder; amphetamine dependence; amphetamine withdrawal; cocaine dependence; cocaine withdrawal;
- mood disorders and mood episodes include depression, mania and bipolar disorders.
- the mood disorder is selected from the group of bipolar disorders (I and II); cyclothymic disorder; depression; dysthymic disorder; major depressive disorder; treatment-resistant depression; and substance-induced mood disorder.
- neurodegenerative disorders include Parkinson's disease
- Huntington's disease dementia such as for example Alzheimer's disease; multi-infarct dementia; AIDS-related dementia or fronto temperal dementia.
- the neurodegenerative disorder or condition comprises dysfunction of striatal medium spiny neurons responses.
- disorders or conditions comprising as a symptom a deficiency in attention and/or cognition include dementia, such as Alzheimer's disease; multi-infarct dementia; dementia due to Lewy body disease; alcoholic dementia or substance- induced persisting dementia; dementia associated with intracranial tumours or cerebral trauma; dementia associated with Huntington's disease; dementia associated with
- Parkinson's disease AIDS-related dementia; dementia due to Pick's disease; dementia due to Creutzfeldt- Jakob disease; other diseases include delirium; amnestic disorder; post-traumatic stress disorder; stroke; progressive supranuclear palsy; mental retardation; a learning disorder; attention-deficit/hyperactivity disorder (ADHD); mild cognitive disorder; Asperger's syndrome; and age-related cognitive impairment.
- ADHD attention-deficit/hyperactivity disorder
- pain includes acute and chronic states, severe pain, intractable pain, neuropathic pain and post-traumatic pain, cancer pain, non-cancer pain, pain disorder associated with psychological factors, pain disorder associated with a general medical condition or pain disorder associated with both psychological factors and a general medical condition.
- metabolic disorders include diabetes, in particular type 1 or type 2 diabetes, and related disorders such as obesity.
- Additional related disorders include syndrome X, impaired glucose tolerance, impaired fasting glucose, gestational diabetes, maturity-onset diabetes of the young (MODY), latent autoimmune diabetes adult (LAD A), associated diabetic dyslipidemia, hyperglycemia, hyperinsulinemia, dyslipidemia, hypertriglyceridemia, and insulin resistance.
- the psychotic disorder is selected from the group of schizophrenia, delusional disorder, schizoaffective disorder, schizophreniform disorder and
- the central nervous system disorder is a personality disorder selected from the group of obsessive-compulsive personality disorder and schizoid, schizotypal disorder.
- the central nervous system disorder is a mood disorder selected from the group of bipolar disorders (I & II), cyclothymic disorder, depression, dysthymic disorder, major depressive disorder; treatment-resistant depression; and
- the central nervous system disorder is attention-deficit/hyperactivity disorder.
- the central nervous system disorder is a cognitive disorder selected from the group of delirium, substance-induced persisting delirium, dementia, dementia due to HIV disease, dementia due to Huntington's disease, dementia due to Parkinson's disease, dementia of the Alzheimer's type, substance-induced persisting dementia and mild cognitive impairment.
- disorders treated by the compounds of formula (I) or a pharmaceutically acceptable salt or a solvate thereof of the present invention are selected from schizophrenia; obsessive-compulsive disorder; generalized anxiety disorder; Huntington's disease; dyskinesia; Parkinson's disease; depression; bipolar disorders; dementia such as Alzheimer's disease; attention-deficit/hyperactivity disorder; drug abuse; pain; autism; diabetes and obesity.
- the disorders treated by the compounds of formula (I) or a pharmaceutically acceptable salt or a solvate thereof of the present invention are schizophrenia, including positive and negative symptoms thereof, and cognitive deficits, such as impaired attention or memory.
- anxiety obsessive- compulsive disorder
- post-traumatic stress disorder generalized anxiety disorder
- schizophrenia depression
- attention-deficit/hyperactivity disorder Alzheimer's disease
- dementia due to Huntington's disease dementia due to Parkinson's disease
- dementia of the Alzheimer's type dementia of the Alzheimer's type
- substance-induced persisting dementia and mild cognitive impairment are of particular importance.
- central nervous system disorders include schizoanxiety disorder, and comorbid depression and anxiety, in particular major depressive disorder with comorbid generalized anxiety disorder, social anxiety disorder, or panic disorder; it is understood that comorbid depression and anxiety may also be referred to by the terms anxious depression, mixed anxiety depression, mixed anxiety-depressive disorder, or major depressive disorder with anxiety symptoms, which are used indistinctively herein.
- DSM-IV Diagnostic & Statistical Manual of Mental Disorders
- the invention also relates to a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention, for use in the treatment of any one of the diseases mentioned hereinbefore.
- the invention also relates to a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention for use in treating any one of the diseases mentioned hereinbefore.
- the invention also relates to a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention, for the treatment or prevention, in particular treatment, of any one of the diseases mentioned hereinbefore.
- the invention also relates to the use of a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention, for the manufacture of a medicament for the treatment or prevention of any one of the disease conditions mentioned hereinbefore.
- the invention also relates to the use of a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention for the manufacture of a medicament for the treatment of any one of the disease conditions mentioned hereinbefore.
- the compounds of formula (I) or a pharmaceutically acceptable salt or a solvate thereof of the present invention can be administered to mammals, preferably humans, for the treatment or prevention of any one of the diseases mentioned hereinbefore.
- a method of treating a disorder or disease mentioned hereinbefore comprising administering to a subject in need thereof, a therapeutically effective amount of any of the compounds of formula (I) or a pharmaceutically acceptable salt or a solvate thereof or pharmaceutical compositions described herein.
- Said methods comprise the administration, i.e. the systemic or topical administration, preferably oral administration, of a therapeutically effective amount of a compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention to warm-blooded animals, including humans.
- the invention also relates to a method for the prevention and/or treatment of any one of the diseases mentioned hereinbefore comprising administering a therapeutically effective amount of compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof according to the invention to a patient in need thereof.
- PDE2 inhibitors or PDE2 and 10 inhibitors described herein can be used alone, in combination or in combination with other pharmaceutical agents such as other agents used in the treatment of psychoses, such as schizophrenia and bipolar disorder, obsessive-compulsive disorder, Parkinson's disease, cognitive impairment and/or memory loss, e.g.
- nicotinic a-7 agonists PDE4 inhibitors, other PDE2 inhibitors, other PDE10 inhibitors, other PDE2 and 10 inhibitors, calcium channel blockers, muscarinic ml and m2 modulators, adenosine receptor modulators, ampakines, NMDA-R modulators, mGluR modulators, dopamine modulators, serotonin modulators, cannabinoid modulators, and cholinesterase inhibitors (e.g. donepezil, rivastigmine, and galantamine).
- pharmaceutically acceptable salt or a solvate thereof of the present invention may be utilized in combination with one or more other drugs in the treatment, prevention, control, amelioration, or reduction of risk of diseases or conditions for which compounds of Formula (I) or the other drugs may have utility, where the combination of the drugs together are safer or more effective than either drug alone.
- a therapeutically effective amount of the PDE2 inhibitors or PDE2 and 10 inhibitors of the present invention is the amount sufficient to inhibit the PDE2 enzyme or both PDE2 and PDE10 enzymes and that this amount varies inter alia, depending on the type of disease, the concentration of the compound in the therapeutic formulation, and the condition of the patient.
- an amount of PDE2 inhibitor or PDE2 and 10 inhibitor to be administered as a therapeutic agent for treating diseases in which inhibition of the PDE2 enzyme is beneficial or in which inhibition of both PDE2 and PDE10 enzymes is beneficial, such as the disorders described herein, will be determined on a case by case by an attending physician.
- a suitable dose is one that results in a concentration of the PDE2 inhibitor or PDE2 and 10 inhibitor at the treatment site in the range of 0.5 nM to 200 ⁇ , and more usually 5 nM to 50 ⁇ .
- a patient in need of treatment likely will be administered between 0.001 mg/kg to 15 mg/kg body weight, in particular from 0.01 mg/kg to 2.50 mg/kg body weight, in particular, from 0.01 to 1.5 mg/kg body weight, in particular from 0.1 mg/kg to 0.50 mg/kg body weight.
- the amount of a compound according to the present invention, also referred to here as the active ingredient, which is required to achieve a therapeutical effect will, of course vary on case-by-case basis, vary with the particular compound, the route of administration, the age and condition of the recipient, and the particular disorder or disease being treated.
- a method of treatment may also include administering the active ingredient on a regimen of between one and four intakes per day.
- the compounds according to the invention are preferably formulated prior to admission.
- suitable pharmaceutical formulations are prepared by known procedures using well known and readily available ingredients.
- the radio labelled compounds according to the present invention find various applications for imaging tissues, cells or a host, both in vitro and in vivo. Thus, for instance, they can be used to map the differential distribution of PDE2 enzyme in subjects of different age and sex. Further, they allow one to explore for differential distribution of PDE2 enzyme in subjects afflicted by different diseases or disorders. Thus, abnormal distribution may be helpful in diagnosis, case finding, stratification of subject populations, and in monitoring disease progression in individual subjects.
- the radioligands (for example, compounds of Formula [ 3 H]-(I-p) or (I-u)) may further find utility in determining PDE2 enzyme occupancy by other ligands. Since the radioligand is administered in trace amounts, no therapeutic effect may be attributed to the administration of the radioligands according to the invention.
- the present invention also provides compositions for preventing or treating diseases in which inhibition of PDE2 is beneficial or inhibition of both PDE2 and 10 is beneficial, such as neurological and psychiatric disorders, and endocrinological or metabolic diseases.
- Said compositions comprising a therapeutically effective amount of a compound according to formula (I) and a pharmaceutically acceptable carrier or diluent.
- the present invention further provides a pharmaceutical composition comprising a compound according to the present invention, together with a pharmaceutically acceptable carrier or diluent.
- a pharmaceutically acceptable carrier or diluent must be "acceptable" in the sense of being compatible with the other ingredients of the composition and not deleterious to the recipients thereof.
- compositions of this invention may be prepared by any methods well known in the art of pharmacy.
- a therapeutically effective amount of the particular compound, in base form or addition salt form, as the active ingredient is combined in intimate admixture with a pharmaceutically acceptable carrier, which may take a wide variety of forms depending on the form of preparation desired for administration.
- a pharmaceutically acceptable carrier which may take a wide variety of forms depending on the form of preparation desired for administration.
- These pharmaceutical compositions are desirably in unitary dosage form suitable, preferably, for systemic administration such as oral, percutaneous or parenteral administration; or topical administration such as via inhalation, a nose spray, eye drops or via a cream, gel, shampoo or the like.
- any of the usual pharmaceutical media may be employed, such as, for example, water, glycols, oils, alcohols and the like in the case of oral liquid preparations such as suspensions, syrups, elixirs and solutions: or solid carriers such as starches, sugars, kaolin, lubricants, binders, disintegrating agents and the like in the case of powders, pills, capsules and tablets. Because of their ease in administration, tablets and capsules represent the most advantageous oral dosage unit form, in which case solid pharmaceutical carriers are obviously employed.
- the carrier will usually comprise sterile water, at least in large part, though other ingredients, for example, to aid solubility, may be included.
- Injectable solutions may be prepared in which the carrier comprises saline solution, glucose solution or a mixture of saline and glucose solution. Injectable suspensions may also be prepared in which case appropriate liquid carriers, suspending agents and the like may be employed.
- the carrier optionally comprises a penetration enhancing agent and/or a suitable wettable agent, optionally combined with suitable additives of any nature in minor proportions, which additives do not cause any significant deleterious effects on the skin. Said additives may facilitate the administration to the skin and/or may be helpful for preparing the desired compositions.
- transdermal patch e.g., as a transdermal patch, as a spot-on or as an ointment.
- Dosage unit form as used in the specification and claims herein refers to physically discrete units suitable as unitary dosages, each unit containing a predetermined quantity of active ingredient calculated to produce the desired therapeutic effect in association with the required pharmaceutical carrier.
- dosage unit forms are tablets (including scored or coated tablets), capsules, pills, powder packets, wafers, injectable solutions or suspensions, teaspoonfuls, tablespoonfuls and the like, and segregated multiples thereof.
- the pharmaceutical composition will comprise from 0.05 to 99 % by weight, preferably from 0.1 to 70 % by weight, more preferably from 0.1 to 50 % by weight of the active ingredient, and, from 1 to 99.95 % by weight, preferably from 30 to 99.9 % by weight, more preferably from 50 to 99.9 % by weight of a pharmaceutically acceptable carrier, all percentages being based on the total weight of the composition.
- the present compounds can be used for systemic administration such as oral, percutaneous or parenteral administration; or topical administration such as via inhalation, a nose spray, eye drops or via a cream, gel, shampoo or the like.
- systemic administration such as oral, percutaneous or parenteral administration; or topical administration such as via inhalation, a nose spray, eye drops or via a cream, gel, shampoo or the like.
- topical administration such as via inhalation, a nose spray, eye drops or via a cream, gel, shampoo or the like.
- the compounds are preferably orally administered.
- the exact dosage and frequency of administration depends on the particular compound according to formula (I) used, the particular condition being treated, the severity of the condition being treated, the age, weight, sex, extent of disorder and general physical condition of the particular patient as well as other medication the individual may be taking, as is well known to those skilled in the art. Furthermore, it is evident that said effective daily amount may be lowered or increased depending on the response of the treated subject and/or depending on the evaluation of the physician prescribing the compounds of the instant invention.
- suitable unit doses for the compounds of the present invention can, for example, preferably contain between 0.1 mg to about 1000 mg of the active compound.
- a preferred unit dose is between 1 mg to about 500 mg.
- a more preferred unit dose is between 1 mg to about 300mg.
- Even more preferred unit dose is between 1 mg to about 100 mg.
- Such unit doses can be administered more than once a day, for example, 2, 3, 4, 5 or 6 times a day, but preferably 1 or 2 times per day, so that the total dosage for a 70 kg adult is in the range of 0.001 to about 15 mg per kg weight of subject per administration.
- a preferred dosage is 0.01 to about 1.5 mg per kg weight of subject per administration, and such therapy can extend for a number of weeks or months, and in some cases, years.
- the specific dose level for any particular patient will depend on a variety of factors including the activity of the specific compound employed; the age, body weight, general health, sex and diet of the individual being treated; the time and route of administration; the rate of excretion; other drugs that have previously been administered; and the severity of the particular disease undergoing therapy, as is well understood by those of skill in the area.
- a typical dosage can be one 1 mg to about 100 mg tablet or 1 mg to about 300 mg taken once a day, or, multiple times per day, or one time-release capsule or tablet taken once a day and containing a proportionally higher content of active ingredient.
- the time-release effect can be obtained by capsule materials that dissolve at different pH values, by capsules that release slowly by osmotic pressure, or by any other known means of controlled release.
- compositions, methods and kits provided above, one of skill in the art will understand that preferred compounds for use in each are those compounds that are noted as preferred above. Still further preferred compounds for the compositions, methods and kits are those compounds provided in the non-limiting Examples below.
- LCMS liquid chromatography/mass spectrometry
- GCMS gas chromatography/mass spectrometry
- HPLC high- performance liquid chromatography
- RP HPLC reverse phase high- performance liquid chromatography
- aq means aqueous
- Boc means tert- butoxycarbonyl
- nBuLi means n-butyllithium
- BuOH means 1-butanol
- DBU means 2,3,4,6, 7, 8,9, 10-octahydropyrimidol[l,2-a]azepine
- DCE means 1,2-dichloro- ethane
- DCM means dichloromethane
- DIPE means diisopropyl ether
- DIPEA means diisopropylethyl amine
- DF means N,N-dimethylformamide
- EtOH means ethanol
- EtOAc means ethyl
- Microwave assisted reactions were performed in a single-mode reactor: Biotage InitiatorTM Sixty microwave reactor (Biotage) or in a multimode reactor: MicroSYNTH Labstation (Milestone, Inc.).
- Hydrogenation reactions were performed in a continuous flow hydrogenator H-CUBE ® from ThalesNano Nanotechnology Inc.
- TLC Thin layer chromatography
- silica gel 60 F254 plates Merck
- Open column chromatography was performed on silica gel, mesh 230-400 particle size and 60 A pore size (Merck) under standard techniques.
- Automated flash column chromatography was performed using ready-to-connect cartridges from Merck, on irregular silica gel, particle size 15-40 ⁇ (normal phase disposable flash columns) on an SPOT or LAFLASH system from Armen Instrument.
- a batch of the regioisomeric mixture was separated by suspending the mixture in methanol and ammonium hydroxide (q.s.), warming up to reflux and cooling down to room temperature.
- the precipitate that formed was filtered, water was added to the filtrate and the precipitate that formed was also recovered by filtration. Two additional cycles were repeated to obtain a precipitate containing a 94:6 mixture of I-3a:I-3b.
- Intermediates (I6-a) and (I-6b) were synthesized following the same approach described for intermediate 4. Starting from a mixture of intermediates (I-5a) and (I-5b) (l . lg, 4.51 mmol), intermediates (I-6a) and (I-6b) (0.9 g, 76%) were obtained.
- Tributylvinyl tin (0.18 mL, 0.61 mmol) was added to a stirred solution of intermediate (I-20a) (0.2 g, 0.511 mmol), LiCl (0.065 g, 1.53 mmol) and (tetrakis)triphenyl- phosphine palladium(O) (0.023 g, 0.02 mmol) in toluene (7 mL).
- the mixture was heated at 120° C for 1.5 h. After cooling to r.t. the r.m. was partitioned between EtOAc and H 2 0. The organic layer was washed with brine, separated, dried (Na 2 S0 4 ) and concentrated in vacuo.
- Tributyl-(l-ethoxyvinyl) tin (0.217 mL, 0.64 mmol) was added to a stirred solution of compound B-3a (0.2 g, 0.53 mmol), palladium(O) (tetrakis)triphenylphosphine (0.025 g, 0.02 mmol) and LiCl (0.068 g, 1.61 mmol) in toluene (2 ml) at r.t.
- the mixture was then heated at 120° C for 20 min under microwave irradiation. After that, HCl (aq. 2M, 1.5 mL) was added and the reaction was heated again at 80° C for 10 min under microwave irradiation.
- intermediate 1-26 (also referred to as compound B-190) was obtained (0.114 g, 89%).
- Intermediate 1-34 was synthesized following a similar approach described for intermediate 1-16. Starting from 1-33 (0.046 g, 0.127 mmol) intermediate 1-34 obtained as pale yellow solid (0.031 g, 66.5%).
- intermediate I-4a To a solution of intermediate I-4a (5 g, 19.4 mmol) in BuOH (40 ml) intermediate 1-12 (4.06 g, 19.4 mmol) was added. The r.m. was heated in a sealed reactor at 160° C for 30 min. The mixture was then evaporated till dryness and the residue taken up in EtOAc. The organic layer was washed with NaHC0 3 (sat. sol), then separated, dried (MgS0 4 ), filtered and the solvent evaporated in vacuo.
- triphenylphosphine (1.52 g, 5.79 mmol), in THF (36 mL) was heated in a microwave oven for 20 min at 120° C (the reaction mixture was divided in three batches). Then 1 equiv. more of di-tert-butylazadicarboxylate and triphenylphosphine were added and the r.m. was heated again at the same conditions as before. Then the solvent was evaporated, the crude compound taken up in aq. sat. NaHC0 3 and then extracted with DCM. The organic layer was separated, dried (Na 2 S0 4 ), filtered and the solvent concentrated in vacuo.
- Trimethyl(trifluoromethyl) silane (0.105 g, 0.74 mmol) was added to a stirred suspension of intermediate 1-16 (0.2 g, 0.62 mmol) containing a catalytic amount of CsF (0.003 g, 0.025 mmol) in 1 ,2-dimethoxyethane (4 mL) at r.t. and under argon atmosphere. After being stirred for 30 min at r.t., the mixture was treated with HC1 (1M in H 2 0, 1.24 mL) and stirred for further 15 min. Then the r.m. was diluted with EtOAc, the organic layer was separated, dried (Na 2 S0 4 ), filtered and the solvent concentrated in vacuo.
- Methanesulphonyl chloride (0.079 mL, 1.02 mmol) was added to a stirred solution of final product B-21 (0.08 g, 0.2 mmol) and pyridine (0.161 mL, 2.04 mmol) dissolved in DCM (1 mL). The mixture was stirred at r.t. overnight, then it was basified with NaHC0 3 (sat. sol) and extracted with DCM. The organic layer was separated, dried (Na 2 S0 4 ), filtered and the solvent concentrated in vacuo. Then, morpholine (0.528 mL, 6.11 mmol) was added to the crude residue and the r.m. was stirred at r.t. 4 h.
- KHMDS Potassium hexamethyldisilazide
- the reaction mixture was degassed (3x) and placed under tritium atmosphere (750 mbar at room temperature) for 60 minutes at room temperature. The tritium atmosphere was removed and the volatile components lyophilized to a waste ampoule.
- the crude mixture was rinsed and lyophilized with MeOH (3 x 0.15 mL), filtered over an acrodisk® and dissolved in ethanol (10 mL). This stock solution was purified over prep-HPLC and resulted in 230 MBq with a radiochemical purity of >98% and specific activity of 726 GBq/mmol.
- [ ie F]fluoride [ ie F]F was produced by an [ 0(p,n) F] reaction by irradiation of 2 mL of 97% enriched [ 18 0]H 2 0 (Rotem HYOX18, Rotem Industries, Beer Sheva, Israel) in a niobium target using 18-MeV protons from a Cyclone 18/9 cyclotron (Ion Beam Applications, Louvain-la-Neuve, Belgium). After irradiation, the resultant [ 18 F]F ⁇ was separated from [ 18 0]H 2 0 using a SepPakTM Light Accell plus QMA anion exchange cartridge (Waters, C0 3 2 form).
- [ 18 F]F ⁇ was eluted from the cartridge using a mixture of 0.38 mL of a solution containing K 2 C0 3 (0.00247 g) and Kryptofix 222 (0.00279 g) dissolved in H 2 0/MeCN (0.75 mL; 5:95 v/v) and 0.38 mL MeCN.
- the solution was evaporated under a stream of helium at 80 °C and 35 watt by applying microwave heating and further dried by azeo tropic distillation using MeCN (1 mL) at a
- the precursor for the radiolabeling, 1-35 (0.0013 g, 0.0029 mmol) was dissolved in anhydrous DMF (0.35 mL), this solution was added to the dried [ 18 F]F7K 2 C0 3 /Kryptofix ® 222 complex, and the nucleophilic substitution reaction was carried out using microwave heating at 140 °C and 50 watt for 6 min.
- the crude mixture was diluted with 0.05 M NaOAc buffer pH 5.5 (0.6 mL) and injected onto the HPLC system consisting of a semi- preparative XBridgeTM column (C 18 , 5 ⁇ , 4.6 mm x 150 mm; Waters) that was eluted with a mixture of 0.05 M NaOAc buffer pH 5.5 and EtOH (73:27 v/v) at a flow rate of 1 mL/min.
- UV detection of the HPLC eluate was performed at 254 nm.
- radiolabeled product [ 18 F]B-23 was collected after about 25 min. The collected peak corresponding to [ 18 F]B-23 was then diluted with saline (Mini Plasco ® , Braun,
- the HPLC measurement was performed using an HP 1100 (Agilent Technologies) system comprising a pump (quaternary or binary) with degasser, an autosampler, a column oven, a diode-array detector (DAD) and a column as specified in the respective methods below.
- Flow from the column was split to the MS spectrometer.
- the MS detector was configured with either an electrospray ionization source or an ESCI dual ionization source (electrospray combined with atmospheric pressure chemical ionization). Nitrogen was used as the nebulizer gas.
- the source temperature was maintained at 140 °C. Data acquisition was performed with MassLynx-Openlynx software.
- the UPLC (Ultra Performance Liquid Chromatography) measurement was performed using an Acquity UPLC (Waters) system comprising a sampler organizer, a binary pump with degasser, a four column's oven, a diode-array detector (DAD) and a column as specified in the respective methods below. Column flow was used without split to the MS detector.
- the MS detector was configured with an ESCI dual ionization source (electrospray combined with atmospheric pressure chemical ionization). Nitrogen was used as the nebulizer gas. The source temperature was maintained at 140 °C. Data acquisition was performed with MassLynx-Openlynx software.
- the LC measurement was performed using an Acquity UPLC (Waters) system comprising a binary pump, a sample organizer, a column heater (set at 55 °C), a diode- array detector (DAD) and a column as specified in the respective methods below.
- Flow from the column was split to a MS spectrometer.
- the MS detector was configured with an electrospray ionization source. Mass spectra were acquired by scanning from 100 to 1000 in 0.18 seconds using a dwell time of 0.02 seconds.
- the capillary needle voltage was 3.5 kV and the source temperature was maintained at 140 °C. Nitrogen was used as the nebulizer gas.
- Data acquisition was performed with a Waters-Micromass
- the HPLC measurement was performed using an Agilent 1100 module comprising a pump, a diode-array detector (DAD) (Agilent 1200) (wavelength used 254 nm), a column heater and a column as specified in the respective methods below.
- Flow from the column was split to a Agilent MSD Serie G1956A.
- MS detector was configured with API-ES (atmospheric pressure electrospray ionization). Mass spectra were acquired by scanning from 105 to 1400.
- the capillary needle voltage was 3000 V for positive ionization mode. Fragmentation voltage was 70 V. Drying gas temperature was maintained at 350 °C at a flow of 12 1/min.
- Reversed phase HPLC was carried out on a Sunfire-C18 column (2.5 ⁇ , 2.1 x 30 mm) from Waters, with a flow rate of 1.0 ml/min, at 60°C.
- the gradient conditions used are: 95 % A (0.5 g/1 ammonium acetate solution + 5 % of acetonitrile), 2.5 % B (acetonitrile), 2.5 % C (methanol) to 50 % B, 50 % C in 6.5 minutes, kept till 7.0 minutes and equilibrated to initial conditions at 7.3 minutes until 9.0 minutes.
- High-resolution mass spectra (Time of Flight, TOF detector) were acquired by scanning from 100 to 750 in 0.5 seconds using a dwell time of 0.3 seconds.
- the capillary needle voltage was 2.5 kV for positive ionization mode and 2.9 kV for negative ionization mode.
- the cone voltage was 20 V for both positive and negative ionization modes.
- Leucine-Enkephaline was the standard substance used for the lock mass calibration.
- Reversed phase UPLC was carried out on a BEH-C18 column (1.7 ⁇ , 2.1 x 50 mm) from Waters, with a flow rate of 1.0 ml/min, at 50°C without split to the MS detector.
- the gradient conditions used are: 95 % A (0.5 g/1 ammonium acetate solution + 5 % acetonitrile), 5 % B (acetonitrile), to 40 % A, 60 % B in 4.4 minutes, to 5 % A, 95 % B in 5.6 minutes, kept till 5.8 minutes and equilibrated to initial conditions at 6.0 minutes until 7.0 minutes.
- Injection volume 0.5 ⁇ .
- Low-resolution mass spectra (single quadrupole, SQD detector) were acquired by scanning from 100 to 1000 in 0.1 seconds using an inter-channel delay of 0.08 second.
- the capillary needle voltage was 3 kV.
- the cone voltage was 25 V for positive ionization mode and 30 V for negative ionization mode.
- BEH-C18 column (1.7 ⁇ , 2.1 x 50 mm) from Waters, with a flow rate of 1.0 ml/min, at 50°C without split to the MS detector.
- the gradient conditions used are: 95 % A (0.5 g/1 ammonium acetate solution + 5 % acetonitrile), 5 % B (acetonitrile), to 40 % A, 60 % B in 2.8 minutes, to 5 % A, 95 % B in 3.6 minutes, kept till 3.8 minutes and equilibrated to initial conditions at 4.0 minutes until 5.0 minutes.
- Injection volume 0.5 ⁇ .
- Low-resolution mass spectra (single quadrupole, SQD detector) were acquired by scanning from 100 to 1000 in 0.1 seconds using an inter-channel delay of 0.08 second.
- the capillary needle voltage was 3 kV.
- the cone voltage was 25 V for positive ionization mode and 30 V for negative ionization mode.
- Reversed phase UPLC was carried out on a BEH-C18 column (1.7 ⁇ , 2.1 x 50 mm) from Waters, with a flow rate of 1.0 ml/min, at 50°C without split to the MS detector.
- the gradient conditions used are: 95 % A (0.5 g/1 ammonium acetate solution + 5 % acetonitrile), 5 % B (acetonitrile), to 40 % A, 60 % B in 3.8 minutes, to 5 % A, 95 % B in 4.6 minutes, kept till 5.0 minutes.
- Low-resolution mass spectra (single quadrupole, SQD detector) were acquired by scanning from 100 to 1000 in 0.1 seconds using an inter-channel delay of 0.08 second.
- the capillary needle voltage was 3 kV.
- the cone voltage was 25 V for positive ionization mode and 30 V for negative ionization mode.
- Reversed phase HPLC was carried out on a YMC pack ODS-AQ C 18 column (3 ⁇ , 50 mm x 4.6 mm) with a flow rate of 2.6 mL/min, at 35°C.
- a gradient elution was performed from 95% (H 2 0 + 0.1% HCOOH)/5% CH 3 CN to 5% (H 2 0 + 0.1% HCOOH)/95% CH 3 CN in 4.8 min and held for 1.0 min; then to 95% (H 2 0 + 0.1% HCOOH)/5% CH 3 CN in 0.2 min.
- the injection volume was 2 ⁇ ⁇ . Acquisition ranges were set to 190-400 nm for the UV-PDA detector and 100-1400 m/z for the MS detector.
- Reversed phase HPLC was carried out on an Xterra MS C18 column (3.5 ⁇ , 4.6 x 100 mm) with a flow rate of 1.6 ml/min.
- Three mobile phases (mobile phase A: 95% 25 mM ammoniumacetate + 5 % acetonitrile; mobile phase B: acetonitrile; mobile phase C: methanol) were employed to run a gradient condition from 100 % A to 50 % B and 50 % C in 6.5 minutes, to 100 % B in 0.5 minute, 100 % B for 1 minute and reequilibrate with 100 % A for 1.5 minutes.
- An injection volume of 10 ⁇ was used.
- Cone voltage was 10 V for positive ionization mode and 20 V for negative ionization mode.
- Reversed phase HPLC was carried out on an Eclipse Plus-C18 column (3.5 ⁇ , 2.1 x 30 mm) from Agilent, with a flow rate of 1.0 ml/min, at 60°C without split to the MS detector.
- the gradient conditions used are: 95 % A (0.5 g/1 ammonium acetate solution + 5 % acetonitrile), 5 % B (mixture of acetonitrile / methanol, 1/1), kept 0.2 minutes, to 100 % B in 3.0 minutes, kept till 3.15 minutes and equilibrated to initial conditions at 3.30 minutes until 5.0 minutes.
- Reversed phase UPLC was carried out on a RRHD Eclipse Plus-C18 (1.8 ⁇ , 2.1 x 50 mm) from Agilent, with a flow rate of 1.0 ml/min, at 50°C without split to the MS detector.
- the gradient conditions used are: 95 % A (0.5 g/1 ammonium acetate solution + 5 % acetonitrile), 5 % B (acetonitrile), to 40 % A, 60 % B in 1.2 minutes, to 5 % A, 95 % B in 1.8 minutes, kept till 2.0 minutes. Injection volume 2.0 ⁇ .
- Low-resolution mass spectra (single quadrupole, SQD detector) were acquired by scanning from 100 to 1000 in 0.1 seconds using an inter- channel delay of 0.08 second.
- the capillary needle voltage was 3 kV.
- the cone voltage was 25 V for positive ionization mode and 30 V for negative ionization mode.
- Reversed phase UPLC Ultra Performance Liquid Chromatography
- BEH bridged ethylsiloxane/silica hybrid
- Waters Acquity a flow rate of 0.8 ml/min.
- Two mobile phases 10 mM NH 4 AcO in H 2 0/CH 3 CN 95/5 ; mobile phase B : CH 3 CN
- Cone voltage was 10 V for positive ionization mode and 20 V for negative ionization mode.
- Reversed phase HPLC was carried out on a SB-C18 lpk column (4.6 x 30 mm, 1.8 ⁇ ) with a flow rate of 4.0 ml/min, at 65°C.
- a gradient elution was performed from 88 % H 2 0 and 12 % CH 3 CN to 88 % CH 3 CN / 12 % H 2 0 in 1.10 minutes and and held for 0.50 minutes, then to 88% H 2 0 / 12% CH 3 CN in 0.2 min and held for 0.40 minutes.
- the injection volume was 1 MS acquisition range and UV detector were set to 150-1200 m/z and 254 nm respectively
- the GC measurement was performed using a 6890 Series Gas Chromatograph (Agilent Technologies) system comprising a 7683 Series injector and autosampler, a column oven and a column as specified in the respective methods below, coupled to a 5973N MSD Mass Selective Detector (single quadrupole, Agilent Technologies).
- the MS detector was configured with an electronic impact ionization source / chemical ionization source (EI/CI). EI low-resolution mass spectra were acquired by scanning from 50 to 550 at a rate of 14.29 scans/s.
- the source temperature was maintained at 230°C. Helium was used as the nebulizer gas.
- Data acquisition was performed with Chemstation-Open Action software.
- GC was carried out on a J&W HP-5MS column (20 m x 0.18 mm, 0.18 ⁇ ) from Agilent Technologies, with a flow rate of 0.7 ml/min.
- the temperature gradient applied was: initial temperature 50°C, hold for 2.0 min, then a 50°C/min ramp applied for 5.0 min until 300°C and hold for 3.0 min in a 10 min run.
- Front inlet temperature was 250°C.
- Split injection mode was used, 0.2 ⁇ injection volume, with a 50/1 ratio into the GC/MS system.
- Values are either peak values or melt ranges, and are obtained with experimental uncertainties that are commonly associated with this analytical method.
- melting points were determined in open capillary tubes on a Mettler FP62 apparatus. Melting points were measured with a temperature gradient of 1, 3, 5 or 10 °C/minute. Maximum temperature was 300 °C. The melting point was read from a digital display.
- melting points were determined with a DSC823e Mettler- Toledo (indicated with DSC in table 2). Melting points were measured with a temperature gradient of 30 °C/minute. Maximum temperature was 400 °C.
- the compounds provided in the present invention are inhibitors of PDE2, particularly of PDE2A, and to a lesser extent of PDE10, particularly of PDE10A, or of PDE2 and PDE10, particularly, of PDE2A and PDE10A.
- the behaviour of representative PDE2 inhibitors or PDE2 and PDE10 inhibitors according to Formula (I) is shown in Tables 3-5 below.
- hPDE2A Human recombinant PDE2A was expressed in Sf9 cells using a recombinant rPDElOA baculo virus construct. Cells were harvested after 48 h of infection and the hPDE2A protein was purified by metal chelate chromatography on Ni-sepharose 6FF . Tested compounds were dissolved and diluted in 100% DMSO to a concentration 100 fold of the final concentration in the assay. Compound dilutions (0.4 ⁇ ) were added in 384 well plates to 20 ⁇ of incubation buffer (50 mM Tris pH 7.8, 8.3 mM MgCl 2 , 1.7 mM EGTA).
- hPDE2A enzyme in incubation buffer was added and the reaction was started by addition of 10 ⁇ substrate to a final concentration of 10 ⁇ cGMP and 0.01 ⁇ 3 H-cGMP.
- the reaction was incubated for 45 minutes at room temperature. After incubation, the reaction was stopped with 20 ⁇ of of stop solution consisting of 17.8 mg/ml PDE SPA scintillation proximity assay) beads supplemented with 200 mM ZnCl 2 . After sedimentation of the beads during 30 minutes the radioactivity was measured in a Perkin Elmer Topcount scintillation counter and results were expressed as cpm. For blanc values the enzyme was omitted from the reaction and replaced by incubation buffer.
- Control values were obtained by addition of a final concentration of 1% DMSO instead of compound.
- a best fit curve is fitted by a minimum sum of squares method to the plot of % of control value substracted with blanc value versus compound concentration and the half maximal inhibitory concentration (IC 50 ) value is derived from this curve.
- Rat recombinant PDEIOA (rPDE10A2) was expressed in Sf9 cells using a recombinant rPDElOA baculo virus construct.
- Cells were harvested after 48 h of infection and the rPDElOA protein was purified by metal chelate chromatography on Ni-sepharose 6FF.
- Tested compounds were dissolved and diluted in 100% DMSO to a concentration 100 fold of the final concentration in the assay.
- Compound dilutions (0.4 ⁇ ) were added in 384 well plates to 20 ⁇ of incubation buffer (50 mM Tris pH 7.8, 8.3 mM MgCl 2 , 1.7 mM EGTA).
- pIC 5 o corresponds to the -log IC50 expressed in mol/L.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Hematology (AREA)
- Addiction (AREA)
- Diabetes (AREA)
- Psychiatry (AREA)
- Epidemiology (AREA)
- Obesity (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Physics & Mathematics (AREA)
- Immunology (AREA)
- Pain & Pain Management (AREA)
- Psychology (AREA)
- Optics & Photonics (AREA)
- Analytical Chemistry (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Pathology (AREA)
- Microbiology (AREA)
- General Physics & Mathematics (AREA)
- Biochemistry (AREA)
- Food Science & Technology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
Abstract
Description
Claims
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| MX2013015201A MX344600B (en) | 2011-06-27 | 2012-06-26 | 1-ARYL-4-METHYL-[1,2,4]TRIAZOLO[4,3-a]QUINOXALINE DERIVATIVES. |
| EP12729637.4A EP2723744B1 (en) | 2011-06-27 | 2012-06-26 | 1-ARYL-4-METHYL-[1,2,4]TRIAZOLO[4,3-a]QUINOXALINE DERIVATIVES |
| CA2838645A CA2838645C (en) | 2011-06-27 | 2012-06-26 | 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]quinoxaline derivatives |
| US14/128,835 US10604523B2 (en) | 2011-06-27 | 2012-06-26 | 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]quinoxaline derivatives |
| CN201280031255.3A CN103619846B (en) | 2011-06-27 | 2012-06-26 | 1-aryl-4-methyl-[1,2,4] triazole [4,3-a] quinoxaline derivant |
| AU2012277912A AU2012277912B2 (en) | 2011-06-27 | 2012-06-26 | 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]quinoxaline derivatives |
| ES12729637.4T ES2575092T3 (en) | 2011-06-27 | 2012-06-26 | 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline derivatives |
| BR112013033375-8A BR112013033375B1 (en) | 2011-06-27 | 2012-06-26 | Derivatives of 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]quinoxaline, their use, pharmaceutical composition that comprises them, process of preparation thereof, sterile solution and intermediate compound |
| JP2014517667A JP6115962B2 (en) | 2011-06-27 | 2012-06-26 | 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline derivatives |
| EA201490165A EA027418B1 (en) | 2011-06-27 | 2012-06-26 | 1-ARYL-4-METHYL-[1,2,4]TRIAZOLO[4,3-a]QUINOXALINE DERIVATIVES, PHARMACEUTICAL COMPOSITION BASED THEREON, PROCESSES FOR PREPARING AND USING THE SAME, INTERMEDIATES |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11171519 | 2011-06-27 | ||
| EP11171519.9 | 2011-06-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013000924A1 true WO2013000924A1 (en) | 2013-01-03 |
Family
ID=44370665
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2012/062381 WO2013000924A1 (en) | 2011-06-27 | 2012-06-26 | 1-ARYL-4-METHYL-[1,2,4]TRIAZOLO[4,3-a]QUINOXALINE DERIVATIVES |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US10604523B2 (en) |
| EP (1) | EP2723744B1 (en) |
| JP (1) | JP6115962B2 (en) |
| CN (1) | CN103619846B (en) |
| AU (1) | AU2012277912B2 (en) |
| BR (1) | BR112013033375B1 (en) |
| CA (1) | CA2838645C (en) |
| EA (1) | EA027418B1 (en) |
| ES (1) | ES2575092T3 (en) |
| MX (1) | MX344600B (en) |
| WO (1) | WO2013000924A1 (en) |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014001314A1 (en) * | 2012-06-26 | 2014-01-03 | Janssen Pharmaceutica Nv | Combinations comprising pde 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and pde 10 inhibitors for use in the treatment of neurological or metabolic disorders |
| WO2014019979A1 (en) | 2012-07-31 | 2014-02-06 | Boehringer Ingelheim International Gmbh | 4-methyl-2,3,5,9,9b-pentaaza-cyclopenta[a]naphthalenes |
| WO2014118039A1 (en) * | 2013-01-31 | 2014-08-07 | F. Hoffmann-La Roche Ag | Radiolabeled compounds |
| WO2014139983A1 (en) | 2013-03-13 | 2014-09-18 | H. Lundbeck A/S | [1,2,4]triazolo[4,3-a]quinoxalines as dual pde2/pde10 inhibitors |
| JP2014526453A (en) * | 2011-09-09 | 2014-10-06 | ハー・ルンドベック・アクチエゼルスカベット | Pyridine compounds and their use |
| WO2015106032A1 (en) | 2014-01-08 | 2015-07-16 | Intra-Cellular Therapies, Inc. | Products and pharmaceutical compositions |
| WO2015164508A1 (en) | 2014-04-23 | 2015-10-29 | Dart Neuroscience, Llc | Substituted [1,2,4] triazolo [1,5-a] pyrimidin-7-yl compounds as pde2 inhibitors |
| WO2016149058A1 (en) | 2015-03-17 | 2016-09-22 | Merck Sharp & Dohme Corp. | Triazolyl pyrimidinone compounds as pde2 inhibitors |
| WO2016154081A1 (en) | 2015-03-26 | 2016-09-29 | Merck Sharp & Dohme Corp. | Pyrazolyl pyrimidinone compounds as pde2 inhibitors |
| WO2016209749A1 (en) | 2015-06-25 | 2016-12-29 | Merck Sharp & Dohme Corp. | Substituted pyrazolo/imidazolo bicyclic compounds as pde2 inhibitors |
| US9540379B2 (en) | 2011-01-31 | 2017-01-10 | Boehringer Ingelheim International Gmbh | (1,2,4)triazolo[4,3-A]quinoxaline derivatives as inhibitors of phosphodiesterases |
| EP3156405A1 (en) | 2015-10-13 | 2017-04-19 | Boehringer Ingelheim International GmbH | Spirocyclic ether derivatives of pyrazolo[1,5-a]pyrimidine-3-carboxamide |
| WO2018083098A1 (en) | 2016-11-02 | 2018-05-11 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidine derivatives as pde2 inhibitors |
| WO2018083103A1 (en) | 2016-11-02 | 2018-05-11 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidine compounds as pde2 inhibitors |
| WO2018083101A1 (en) | 2016-11-02 | 2018-05-11 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidine compounds as pde2 inhibitors |
| CN108349979A (en) * | 2015-11-02 | 2018-07-31 | 詹森药业有限公司 | [1,2,4] triazol [1,5-a] pyrimidin-7-yl compound |
| US10105349B2 (en) | 2014-12-06 | 2018-10-23 | Intra-Cellular Therapies, Inc. | Organic compounds |
| EP3302486A4 (en) * | 2015-06-04 | 2018-11-07 | Merck Sharp & Dohme Corp. | Dihydropyrazolopyrimidinone compounds as pde2 inhibitors |
| US10160762B2 (en) | 2015-05-29 | 2018-12-25 | Merck Sharp & Dohme Corp. | 6-alkyl dihydropyrazolopyrimidinone compounds as PDE2 inhibitors |
| US10195201B2 (en) | 2015-05-05 | 2019-02-05 | Merck Sharp & Dohme Corp. | Heteroaryl-pyrimidinone compounds as PDE2 inhibitors |
| US10239882B2 (en) | 2014-11-05 | 2019-03-26 | Dart Neuroscience (Cayman) Ltd. | Substituted 5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-amine compounds as PDE2 inhibitors |
| US10287293B2 (en) | 2015-07-01 | 2019-05-14 | Merck Sharp & Dohme Corp. | Bicyclic heterocyclic compounds as PDE2 inhibitors |
| US10285989B2 (en) | 2015-05-15 | 2019-05-14 | Merck Sharp & Dohme Corp. | Pyrimidinone amide compounds as PDE2 inhibitors |
| US10300064B2 (en) | 2014-12-06 | 2019-05-28 | Intra-Cellular Therapies, Inc. | Organic compounds |
| WO2019101970A1 (en) | 2017-11-23 | 2019-05-31 | Oslo University Hospital Hf | Treatment of tachycardia |
| US10357481B2 (en) | 2015-07-01 | 2019-07-23 | Merck Sharp & Dohme Corp. | Substituted triazolo bicyclic compounds as PDE2 inhibitors |
| US10604523B2 (en) | 2011-06-27 | 2020-03-31 | Janssen Pharmaceutica Nv | 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]quinoxaline derivatives |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017165311A1 (en) * | 2016-03-21 | 2017-09-28 | Perlstein Lab Pbc | Fused heterocyclic organic compounds and uses thereof |
| CN107827828B (en) * | 2017-11-21 | 2021-03-30 | 南京华漫新材料科技有限公司 | Quinoxaline derivative containing phenylhydrazide skeleton, preparation method thereof and application thereof in preparation of antitumor drugs |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010101230A1 (en) | 2009-03-05 | 2010-09-10 | アステラス製薬株式会社 | Quinoxaline compounds |
| WO2010138833A1 (en) * | 2009-05-29 | 2010-12-02 | Wyeth | SUBSTITUTED IMIDAZO[1,5-a]QUINOXALINES AS INHIBITORS OF PHOSPHODIESTERASE 10 |
Family Cites Families (213)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5584421A (en) | 1978-12-21 | 1980-06-25 | Mitsui Cokes Kogyo Kk | Method of making fibrous carbon |
| US4242513A (en) | 1979-03-05 | 1980-12-30 | Appleton Papers Inc. | Lactone compounds containing a heterocyclic radical |
| EP0226641B1 (en) | 1985-05-25 | 1989-10-18 | Yoshitomi Pharmaceutical Industries, Ltd. | Oxodiazine compounds |
| AU5921890A (en) | 1989-06-13 | 1991-01-08 | Smithkline Beecham Corporation | Monokine activity interference |
| AU6355190A (en) | 1989-06-13 | 1991-01-17 | Smithkline Beecham Corporation | Inhibition of interleukin-1 and tumor necrosis factor production by monocytes and/or macrophages |
| AU622330B2 (en) | 1989-06-23 | 1992-04-02 | Takeda Chemical Industries Ltd. | Condensed heterocyclic compounds having a nitrogen atom in the bridgehead for use as fungicides |
| KR910011852A (en) | 1989-12-04 | 1991-08-07 | 폴 디. 매튜카이티스 | Imidazo [1,2-a] pyridinylalkyl compounds for the treatment of neurotoxin disorders |
| CA2084290A1 (en) | 1990-06-12 | 1991-12-13 | Jerry L. Adams | Inhibition of 5-lipoxygenase and cyclooxygenase pathway mediated diseases |
| US5137876A (en) | 1990-10-12 | 1992-08-11 | Merck & Co., Inc. | Nucleoside antiviral and anti-inflammatory compounds and compositions and methods for using same |
| JPH06504779A (en) | 1990-12-13 | 1994-06-02 | スミスクライン・ビーチャム・コーポレイション | New CSAIDS |
| ZA919797B (en) | 1990-12-13 | 1992-10-28 | Smithkline Beecham Corp | Bicyclo-5,6-dihydro-7h-pyrrolo-1,20-imidazol-7-ols and 7-ones |
| US5683998A (en) | 1991-04-23 | 1997-11-04 | Toray Industries, Inc. | Tricyclic triazolo derivatives, processes for producing the same and the uses of the same |
| CN1033327C (en) * | 1991-04-23 | 1996-11-20 | 东丽株式会社 | Process for preparing tricyclotriazolo derivatives and their use |
| DK31093D0 (en) | 1993-03-19 | 1993-03-19 | Novo Nordisk As | |
| US5486525A (en) | 1993-12-16 | 1996-01-23 | Abbott Laboratories | Platelet activating factor antagonists: imidazopyridine indoles |
| US5869486A (en) | 1995-02-24 | 1999-02-09 | Ono Pharmaceutical Co., Ltd. | Fused pyrimidines and pyriazines as pharmaceutical compounds |
| AU5348396A (en) | 1995-05-01 | 1996-11-21 | Fujisawa Pharmaceutical Co., Ltd. | Imidazo 1,2-a pyridine and imidazo 1,2-a pyridezine derivati ves and their use as bone resorption inhibitors |
| US6992188B1 (en) | 1995-12-08 | 2006-01-31 | Pfizer, Inc. | Substituted heterocyclic derivatives |
| US20010007867A1 (en) | 1999-12-13 | 2001-07-12 | Yuhpyng L. Chen | Substituted 6,5-hetero-bicyclic derivatives |
| CA2281525A1 (en) | 1997-02-18 | 1998-08-20 | Neurocrine Biosciences, Inc. | Biazacyclic crf antagonists |
| AU6691798A (en) | 1997-03-07 | 1998-09-22 | Metabasis Therapeutics, Inc. | Novel indole and azaindole inhibitors of fructose-1,6-bisphosphatase |
| EP1012151B1 (en) | 1997-09-02 | 2002-08-07 | Bristol-Myers Squibb Pharma Company | Heterocyclyl-substituted ring-fused pyridines and pyrimidines as corticotropin releasing hormone (crh) antagonists, useful for treating cns and stress-related disorders |
| JP2002519373A (en) | 1998-07-02 | 2002-07-02 | エーザイ株式会社 | Pharmaceutical compositions and their use |
| US6248755B1 (en) | 1999-04-06 | 2001-06-19 | Merck & Co., Inc. | Pyrrolidine modulators of chemokine receptor activity |
| JP4032566B2 (en) | 1999-06-21 | 2008-01-16 | 東レ株式会社 | Light emitting element |
| JP2001057292A (en) | 1999-08-20 | 2001-02-27 | Toray Ind Inc | Luminescent element |
| GB9919778D0 (en) | 1999-08-21 | 1999-10-27 | Zeneca Ltd | Chemical compounds |
| CO5271670A1 (en) | 1999-10-29 | 2003-04-30 | Pfizer Prod Inc | ANTIGONISTS OF THE CORTICITROPINE RELEASE FACTOR AND RELATED COMPOSITIONS |
| EP1293213A1 (en) | 2000-02-14 | 2003-03-19 | Japan Tobacco Inc. | Preventives/remedies for postoperative stress |
| EP1149583A3 (en) | 2000-04-13 | 2001-11-14 | Pfizer Products Inc. | Combinations of corticotropin releasing factor antagonists and growth hormone secretagogues |
| US6403588B1 (en) | 2000-04-27 | 2002-06-11 | Yamanouchi Pharmaceutical Co., Ltd. | Imidazopyridine derivatives |
| JP2004532792A (en) | 2000-07-14 | 2004-10-28 | ブリストル−マイヤーズ・スクイブ・ファーマ・カンパニー | Imidazo [1,2-a] pyrazine for treating neuropathy |
| AU2001295992A1 (en) | 2000-10-24 | 2002-05-06 | Sankyo Company Limited | Imidazopyridine derivatives |
| AU2002224927A1 (en) | 2000-12-13 | 2002-06-24 | Basf Aktiengesellschaft | Use of substituted imidazoazines, novel imidazoazines, methods for the production thereof, and agents containing these compounds |
| EA007491B1 (en) | 2001-01-22 | 2006-10-27 | Мерк Энд Ко., Инк. | Nucleoside derivatives as inhibitors of rna-dependent rna viral polymerase |
| TWI312347B (en) | 2001-02-08 | 2009-07-21 | Eisai R&D Man Co Ltd | Bicyclic nitrogen-containing condensed ring compounds |
| GB0103926D0 (en) | 2001-02-17 | 2001-04-04 | Astrazeneca Ab | Chemical compounds |
| SE0100568D0 (en) | 2001-02-20 | 2001-02-20 | Astrazeneca Ab | Compounds |
| ES2316546T3 (en) | 2001-02-20 | 2009-04-16 | Astrazeneca Ab | 2-ARYLAMINE-PYRIMIDINS FOR THE TREATMENT OF ASSOCIATED DISORDERS TO GSK3. |
| MXPA03007779A (en) | 2001-03-14 | 2004-11-12 | Lilly Co Eli | Retinoid x receptor modulators. |
| ES2310202T3 (en) | 2001-04-26 | 2009-01-01 | EISAI R&D MANAGEMENT CO., LTD. | CONDENSED CYCLING COMPOUND CONTAINING NITROGEN THAT HAS A PIRAZOLIL GROUP AS A SUBSTITUTING GROUP AND PHARMACEUTICAL COMPOSITION OF THE SAME. |
| US20030027820A1 (en) | 2001-05-30 | 2003-02-06 | Alteon, Inc. | Method for treating fibrotic diseases or other indications V |
| JP2004536807A (en) | 2001-05-30 | 2004-12-09 | アルテオン インコーポレイテッド | How to treat glaucoma V |
| ATE337316T1 (en) | 2001-06-21 | 2006-09-15 | Smithkline Beecham Corp | IMIDAZO 1,2-AÖPYRIDINE DERIVATIVES FOR THE PROPHYLAXIS AND TREATMENT OF HERPES INFECTIONS |
| CA2450555A1 (en) | 2001-06-25 | 2003-01-03 | Merck & Co., Inc. | (pyrimidyl)(phenyl)substituted fused heteroaryl p38 inhibiting and pkg kinase inhibiting compounds |
| IL159811A0 (en) | 2001-07-13 | 2004-06-20 | Neurogen Corp | Heteroaryl substituted fused bicyclic heteroaryl compounds as gabaa receptor ligands |
| EP1432712B1 (en) | 2001-10-05 | 2006-05-17 | SmithKline Beecham Corporation | Imidazo-pyridine derivatives for use in the treatment of herpes viral infection |
| US20050079176A1 (en) | 2002-01-22 | 2005-04-14 | Pierson Iii Richard N. | Treating stress response with chemokine receptor ccr5 modulators |
| CN1312154C (en) | 2002-03-05 | 2007-04-25 | 伊莱利利公司 | Purine derivatives as kinase inhibitors |
| JP2003313126A (en) | 2002-04-23 | 2003-11-06 | Sankyo Co Ltd | Medicine comprising imidazopyridine derivative as active ingredient |
| JP2004002826A (en) | 2002-04-24 | 2004-01-08 | Sankyo Co Ltd | High molecular imidazopyridine derivative |
| US20050165232A1 (en) | 2002-05-13 | 2005-07-28 | Richard Beresis | Phenyl substituted imidaopyridines and phenyl substituted benzimidazoles |
| WO2003099840A1 (en) | 2002-05-24 | 2003-12-04 | Isis Pharmaceuticals, Inc. | Oligonucleotides having modified nucleoside units |
| EP1539748A1 (en) | 2002-07-31 | 2005-06-15 | Smithkline Beecham Corporation | 2-phenylpyridin-4-yl derivatives as alk5 inhibitors |
| GB0217783D0 (en) | 2002-07-31 | 2002-09-11 | Glaxo Group Ltd | Compounds |
| DE60309342T2 (en) | 2002-08-09 | 2007-05-16 | Eli Lilly And Co., Indianapolis | BENZIMIDAZOLE AND BENZOTHIAZOLE AS INHIBITORS OF MAP KINASE |
| AU2003255845A1 (en) | 2002-08-22 | 2004-03-11 | Piramed Limited | Phosphadidylinositol 3,5-biphosphate inhibitors as anti-viral agents |
| UA80296C2 (en) | 2002-09-06 | 2007-09-10 | Biogen Inc | Imidazolopyridines and methods of making and using the same |
| AU2003270489A1 (en) | 2002-09-09 | 2004-03-29 | Cellular Genomics, Inc. | 6-ARYL-IMIDAZO(1,2-a)PYRAZIN-8-YLAMINES, METHOD OF MAKING, AND METHOD OF USE THEREOF |
| WO2004024074A2 (en) | 2002-09-13 | 2004-03-25 | Merck & Co., Inc. | Fused heterobicyclo substituted phenyl metabotropic glutamate-5 modulators |
| NZ538566A (en) | 2002-09-19 | 2008-02-29 | Schering Corp | Imidazopyridines as cyclin dependent kinase inhibitors |
| US7576085B2 (en) | 2002-09-23 | 2009-08-18 | Schering Corporation | Imidazopyrazines as cyclin dependent kinase inhibitors |
| AU2003275031B2 (en) | 2002-09-23 | 2006-08-17 | Schering Corporation | Novel imidazopyrazines as cyclin dependent kinase inhibitors |
| MXPA05003120A (en) | 2002-09-23 | 2005-06-22 | Schering Corp | Imidazopyrazines as cyclin dependent kinase inhibitors. |
| AU2003271185A1 (en) | 2002-10-15 | 2004-05-04 | Takeda Pharmaceutical Company Limited | Imidazopyridine derivative, process for producing the same, and use |
| LT1576138T (en) | 2002-11-15 | 2017-06-26 | Idenix Pharmaceuticals Llc | 2`-methyl nucleosides in combination with interferon and flaviviridae mutation |
| CA2507026A1 (en) | 2002-11-22 | 2004-06-10 | Takeda Pharmaceutical Company Limited | Imidazole derivatives, their production and use |
| EP1582516B1 (en) | 2003-01-10 | 2013-07-17 | Idemitsu Kosan Co., Ltd. | Nitrogenous heterocyclic derivative and organic electroluminescent element employing the same |
| WO2004072081A1 (en) | 2003-02-10 | 2004-08-26 | Cellular Genomics, Inc. | Certain 8-heteroaryl-6-phenyl-imidazo[1,2-a]pyrazines as modulators of kinase activity |
| US7186832B2 (en) | 2003-02-20 | 2007-03-06 | Sugen Inc. | Use of 8-amino-aryl-substituted imidazopyrazines as kinase inhibitors |
| US7329668B2 (en) | 2003-02-25 | 2008-02-12 | Bristol-Myers Squibb Company | Pyrazolopurine-based tricyclic compounds and pharmaceutical compositions comprising same |
| PA8595001A1 (en) | 2003-03-04 | 2004-09-28 | Pfizer Prod Inc | NEW CONDENSED HETEROAROMATIC COMPOUNDS THAT ARE INHIBITORS OF THE TRANSFORMING GROWTH FACTOR (TGF) |
| US7041671B2 (en) | 2003-04-02 | 2006-05-09 | Pfizer Inc | Pyrrolo[1,2-b]pyridazine compounds and their uses |
| US20060100235A1 (en) | 2003-04-11 | 2006-05-11 | Novo Nordisk A/S | Pharmaceutical use of substituted 1,2,4-triazoles |
| WO2004089416A2 (en) | 2003-04-11 | 2004-10-21 | Novo Nordisk A/S | Combination of an 11beta-hydroxysteroid dehydrogenase type 1 inhibitor and an antihypertensive agent |
| US20060094699A1 (en) | 2003-04-11 | 2006-05-04 | Kampen Gita Camilla T | Combination therapy using an 11beta-hydroxysteroid dehydrogenase type 1 inhibitor and a glucocorticoid receptor agonist to minimize the side effects associated with glucocorticoid receptor agonist therapy |
| WO2004103991A1 (en) | 2003-05-20 | 2004-12-02 | 'chemical Diversity Research Institute', Ltd. | 2-substituted piperidines, focused library and a pharmaceutical compound |
| WO2005020885A2 (en) | 2003-05-21 | 2005-03-10 | Isis Pharmaceuticals, Inc. | Compositions and methods for the treatment of severe acute respiratory syndrome (sars) |
| EP1630152A4 (en) | 2003-05-30 | 2009-09-23 | Takeda Pharmaceutical | Condensed ring compound |
| WO2005014599A1 (en) | 2003-06-04 | 2005-02-17 | Cellular Genomics, Inc. | Imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton’s tyrosine kinase by such compounds |
| WO2005005429A1 (en) | 2003-06-30 | 2005-01-20 | Cellular Genomics, Inc. | Certain heterocyclic substituted imidazo[1,2-a]pyrazin-8-ylamines and methods of inhibition of bruton’s tyrosine kinase by such compounds |
| MXPA06000554A (en) | 2003-07-14 | 2006-07-03 | Arena Pharm Inc | Fused-aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto. |
| US7259164B2 (en) | 2003-08-11 | 2007-08-21 | Cgi Pharmaceuticals, Inc. | Certain substituted imidazo[1,2-a]pyrazines, as modulators of kinase activity |
| KR100553752B1 (en) | 2003-10-13 | 2006-02-20 | 삼성에스디아이 주식회사 | Imidazole ring containing compound and organic electroluminescence display device |
| AU2004287847A1 (en) | 2003-10-28 | 2005-05-19 | Sepracor Inc. | Imidazo(1,2-a)pyridine anxiolytics |
| US20050288295A1 (en) | 2003-11-11 | 2005-12-29 | Currie Kevin S | Certain imidazo[1,2-a]pyrazin-8-ylamines, method of making, and method of use thereof |
| WO2005080355A1 (en) | 2004-02-12 | 2005-09-01 | Neurogen Corporation | Imidazo-pyridazines, triazolo-pyridazines and related benzodiazepine receptor ligands |
| US7306631B2 (en) | 2004-03-30 | 2007-12-11 | The Procter & Gamble Company | Keratin dyeing compounds, keratin dyeing compositions containing them, and use thereof |
| JP2005343889A (en) | 2004-05-06 | 2005-12-15 | Taisho Pharmaceut Co Ltd | Imidazopyridine derivative |
| AU2005251408A1 (en) | 2004-06-09 | 2005-12-22 | Oncalis Ag | Protein kinase inhibitors |
| GB0413605D0 (en) | 2004-06-17 | 2004-07-21 | Addex Pharmaceuticals Sa | Novel compounds |
| CA2577947A1 (en) | 2004-08-31 | 2006-03-09 | Banyu Pharmaceutical Co., Ltd. | Novel substituted imidazole derivative |
| CA2584248A1 (en) | 2004-10-15 | 2006-04-27 | Biogen Idec Ma Inc. | Methods of treating vascular injuries |
| MX2007005125A (en) | 2004-10-28 | 2007-07-04 | Irm Llc | Compounds and compositions as hedgehog pathway modulators. |
| CA2586259A1 (en) | 2004-11-08 | 2006-05-11 | Banyu Pharmaceutical Co., Ltd. | Novel fused imidazole derivative |
| JP2008519843A (en) | 2004-11-10 | 2008-06-12 | シージーアイ ファーマスーティカル インコーポレーテッド | Specific imidazo [1,2-a] virazine-8-iramines, method for producing the same, and method of use relating thereto |
| EP1838312A4 (en) | 2004-12-17 | 2010-01-20 | Smithkline Beecham Corp | Chemical compounds |
| JPWO2006070943A1 (en) | 2004-12-28 | 2008-06-12 | 武田薬品工業株式会社 | Condensed imidazole compounds and uses thereof |
| US7998974B2 (en) | 2005-03-03 | 2011-08-16 | Sirtris Pharmaceuticals, Inc. | Fused heterocyclic compounds and their use as sirtuin modulators |
| US7666880B2 (en) | 2005-03-21 | 2010-02-23 | S*Bio Pte Ltd. | Imidazo[1,2-A]pyridine derivatives: preparation and pharmaceutical applications |
| MX2007011600A (en) | 2005-03-21 | 2007-12-07 | Lilly Co Eli | Imidazopyridazine compounds. |
| TW200700064A (en) | 2005-03-24 | 2007-01-01 | Glaxo Group Ltd | Novel compounds |
| EP1879896A1 (en) | 2005-04-05 | 2008-01-23 | Eli Lilly And Company | Imidazopyridazine compounds |
| US7572807B2 (en) | 2005-06-09 | 2009-08-11 | Bristol-Myers Squibb Company | Heteroaryl 11-beta-hydroxysteroid dehydrogenase type I inhibitors |
| GB0513423D0 (en) | 2005-06-30 | 2005-08-03 | Glaxo Group Ltd | Novel compounds |
| MX2008000463A (en) | 2005-07-11 | 2008-03-11 | Novartis Ag | Indolylmaleimide derivatives. |
| JP2009502734A (en) | 2005-07-29 | 2009-01-29 | アステラス製薬株式会社 | Fused heterocycles as Lck inhibitors |
| WO2007025043A2 (en) | 2005-08-23 | 2007-03-01 | Idenix Pharmaceuticals, Inc. | Seven-membered ring nucleosides |
| WO2007025090A2 (en) | 2005-08-25 | 2007-03-01 | Kalypsys, Inc. | Heterobicyclic and - tricyclic inhibitors of mapk/erk kinase |
| CA2620223A1 (en) | 2005-09-02 | 2007-03-08 | Abbott Laboratories | Novel imidazo based heterocycles |
| US20070078136A1 (en) | 2005-09-22 | 2007-04-05 | Bristol-Myers Squibb Company | Fused heterocyclic compounds useful as kinase modulators |
| US7723336B2 (en) | 2005-09-22 | 2010-05-25 | Bristol-Myers Squibb Company | Fused heterocyclic compounds useful as kinase modulators |
| EP1934214B1 (en) | 2005-09-27 | 2010-04-07 | F.Hoffmann-La Roche Ag | Oxadiazolyl pyrazolo-pyrimidines as mglur2 antagonists |
| WO2007036732A1 (en) | 2005-09-30 | 2007-04-05 | Astrazeneca Ab | Imidazo [1,2-a] pyridine having anti-cell-proliferation activity |
| JO2769B1 (en) | 2005-10-26 | 2014-03-15 | جانسين فارماسوتيكا ان. في | Fast Dissociting Dopamine 2 Receptor Antagonists |
| WO2007056468A1 (en) | 2005-11-10 | 2007-05-18 | Schering Corporation | Methods for inhibiting protein kinases |
| RU2008122967A (en) | 2005-11-10 | 2009-12-20 | Шеринг Корпорейшн (US) | IMIDAZOPYRAZINES AS PROTEINKINASE INHIBITORS |
| WO2007078815A2 (en) | 2005-12-16 | 2007-07-12 | Cytokinetics, Inc. | Certain chemical entities, compositions, and methods |
| EP2474546A1 (en) | 2006-01-23 | 2012-07-11 | Vertex Pharmaceuticals Inc. | Thiophene-carboxamides useful as inhibitors of protein kinases |
| JP2009524689A (en) | 2006-01-25 | 2009-07-02 | スミスクライン ビーチャム コーポレーション | Compound |
| US20090203732A1 (en) | 2006-03-02 | 2009-08-13 | Dashyant Dhanak | Thiazolones for Use as P13 Kinase Inhibitors |
| US20090175852A1 (en) | 2006-06-06 | 2009-07-09 | Schering Corporation | Imidazopyrazines as protein kinase inhibitors |
| AR061229A1 (en) | 2006-06-06 | 2008-08-13 | Schering Corp | IMIDAZOPIRAZINS AS INHIBITORS OF PROTEIN QUINASA |
| ITVA20060041A1 (en) | 2006-07-05 | 2008-01-06 | Dialectica Srl | USE OF COMPOUNDS ADMINOTIAZOLIC DERIVATIVES, OF THEIR PHARMACEUTICAL COMPOSITIONS, IN THE TREATMENT OF DISEASES CHARACTERIZED BY THE ABNORMAL REPRESSION OF GENE TRANSCRIPTION, PARTICULARLY THE HUNTINGTON'S DISEASE |
| WO2008011557A2 (en) | 2006-07-20 | 2008-01-24 | Borchardt Allen J | Heteroaryl inhibitors of rho kinase |
| WO2008014219A2 (en) | 2006-07-24 | 2008-01-31 | Smithkline Beecham Corporation | Thiozolidinedione derivatives as p13 kinase inhibitors |
| WO2008027812A2 (en) | 2006-08-28 | 2008-03-06 | Forest Laboratories Holdings Limited | Imidazopyridine and imidazopyrimidine derivatives |
| US20100144743A1 (en) | 2006-09-05 | 2010-06-10 | Board Of Regents, The University Of Texas System | Compositions and methods for inhibition of tyrosine kinases |
| EP2471529A3 (en) | 2006-09-05 | 2012-10-10 | Emory University | Kinase Inhibitors for Preventing or Treating Pathogen Infection and Method of Use Thereof |
| JP2010502716A (en) | 2006-09-07 | 2010-01-28 | バイオジェン・アイデック・エムエイ・インコーポレイテッド | Modulator of interleukin 1 receptor related kinase |
| EP2068869A4 (en) | 2006-10-06 | 2011-05-25 | Abbott Lab | Novel imidazothiazoles and imidazoxazoles |
| US7563920B2 (en) | 2006-10-30 | 2009-07-21 | Draximage Limited | Methods for preparing 2-methoxyisobutylisonitrile and tetrakis(2-methoxyisobutylisonitrile)copper(I) tetrafluoroborate |
| WO2008057402A2 (en) | 2006-11-02 | 2008-05-15 | Cytovia, Inc. | N-aryl-isoxazolopyrimidin-4-amines and related compounds as activators of caspases and inducers of apoptosis and the use thereof |
| EP2079746A2 (en) | 2006-11-08 | 2009-07-22 | Schering Corporation | Imidazopyrazines as protein kinase inhibitors |
| CA2670042A1 (en) | 2006-11-20 | 2008-05-29 | Alantos Pharmaceuticals Holding, Inc. | Heterobicyclic matrix metalloprotease inhibitors |
| US7622584B2 (en) | 2006-11-24 | 2009-11-24 | Samsung Mobile Display Co., Ltd. | Imidazopyridine-based compound and organic light emitting diode including organic layer comprising the imidazopyridine-based compound |
| CA2670870A1 (en) | 2006-12-04 | 2008-06-12 | Astrazeneca Ab | Antibacterial polycyclic urea compounds |
| US7977336B2 (en) | 2006-12-28 | 2011-07-12 | Banyu Pharmaceutical Co. Ltd | Aminopyrimidine derivatives as PLK1 inhibitors |
| AU2008205642B2 (en) | 2007-01-12 | 2013-06-06 | Msd K.K. | Spirochromanon derivatives |
| US20080242862A1 (en) | 2007-03-27 | 2008-10-02 | Calderwood David J | Novel imidazo based heterocycles |
| CA2682231A1 (en) | 2007-03-28 | 2008-10-09 | Array Biopharma Inc. | Imidazo[1,2-a]pyridine compounds as receptor tyrosine kinase inhibitors |
| CN103214482B (en) | 2007-04-03 | 2016-06-29 | 阵列生物制药公司 | Imidazo [1,2-a] pyridine compounds as receptor tyrosine kinase inhibitors |
| KR20090130109A (en) | 2007-04-10 | 2009-12-17 | 하. 룬트벡 아크티에 셀스카브 | Heteroaryl amide analogues as p2x7 antagonists |
| WO2008133192A1 (en) | 2007-04-19 | 2008-11-06 | Takeda Pharmaceutical Company Limited | Fused imidazole compound and use thereof |
| WO2008134553A1 (en) | 2007-04-26 | 2008-11-06 | Xenon Pharmaceuticals Inc. | Methods of using bicyclic compounds in treating sodium channel-mediated diseases |
| US20100305113A1 (en) | 2007-05-09 | 2010-12-02 | Hans-Georg Capraro | Substituted Imidazopyridazines as Lipid Kinase Inhibitors |
| PL2155758T3 (en) | 2007-05-10 | 2013-06-28 | Biocryst Pharm Inc | Tetrahydrofuro[3,4-d]dioxolane compounds for use in the treatment of viral infections and cancer |
| CN101932580B (en) | 2007-06-01 | 2013-05-22 | 葛兰素史密丝克莱恩有限责任公司 | Imidazopyridine kinase inhibitors |
| PE20090365A1 (en) | 2007-06-14 | 2009-04-04 | Schering Corp | IMIDAZOPYRAZINES AS PROTEIN KINASE INHIBITORS |
| KR101030007B1 (en) | 2007-06-15 | 2011-04-20 | 삼성모바일디스플레이주식회사 | Heteroaromatic cycle containing compound method of preparing the same and an organic light emitting device comprising the same |
| EP2170882A1 (en) | 2007-06-26 | 2010-04-07 | Gilead Colorado, Inc. | Imidazopyridinyl thiazolyl histone deacetylase inhibitors |
| US8188083B2 (en) | 2007-06-28 | 2012-05-29 | Abbott Laboratories | Triazolopyridazines |
| FR2918061B1 (en) | 2007-06-28 | 2010-10-22 | Sanofi Aventis | 6-CYCLOAMINO-3- (PYRIDIN-4-YL) IMIDAZO-1,2-B1-PYRIDAZINE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC USE. |
| FR2918986B1 (en) | 2007-07-19 | 2009-09-04 | Sanofi Aventis Sa | 6-CYCLOAMINO-3- (PYRIDAZIN-4-YL) IMIDAZO [1,2-B] -PYRIDAZINE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| CN101790515A (en) | 2007-07-27 | 2010-07-28 | 埃科特莱茵药品有限公司 | trans-3-aza-bicyclo[3.1.0]hexane derivatives |
| MX2010001340A (en) | 2007-07-31 | 2010-06-02 | Schering Corp | Anti-mitotic agent and aurora kinase inhibitor combination as anti-cancer treatment. |
| CA2695989A1 (en) | 2007-08-10 | 2009-02-19 | Glaxosmithkline Llc | Certain nitrogen containing bicyclic chemical entities for treating viral infections |
| JP5327652B2 (en) | 2007-08-14 | 2013-10-30 | バイエル・インテレクチュアル・プロパティ・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング | Fused imidazole for cancer treatment |
| EP2190844B3 (en) | 2007-08-15 | 2013-07-17 | Arena Pharmaceuticals, Inc. | Imidazo[1,2-a]pyridine derivatives as modulators of the 5-ht2a serotonin receptor useful for the treatment of disorders related thereto |
| GB0716292D0 (en) | 2007-08-21 | 2007-09-26 | Biofocus Dpi Ltd | Imidazopyrazine compounds |
| US20110046127A1 (en) | 2007-11-08 | 2011-02-24 | Paolo Pevarello | Imidazopyridazines for Use as Protein Kinase Inhibitors |
| EP2217069A4 (en) | 2007-11-09 | 2012-03-14 | Salk Inst For Biological Studi | Non-nucleoside reverse transcriptase inhibitors |
| WO2009070584A1 (en) * | 2007-11-30 | 2009-06-04 | Wyeth | Aryl and heteroaryl fused imidazo[1,5-a]pyrazines as inhibitors of phosphodiesterase 10 |
| JP5643105B2 (en) | 2007-12-14 | 2014-12-17 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | Novel imidazo [1,2-a] pyridine and imidazo [1,2-b] pyridazine derivatives |
| WO2009086123A1 (en) | 2007-12-21 | 2009-07-09 | Wyeth | Imidazo [1,2-a] pyridine compounds |
| JP5355421B2 (en) | 2007-12-21 | 2013-11-27 | 出光興産株式会社 | Organic electroluminescence device |
| AU2008345688A1 (en) | 2007-12-21 | 2009-07-09 | Wyeth Llc | Imidazo [1,2-b] pyridazine compounds as modulators of liver X receptors |
| FR2926556B1 (en) | 2008-01-22 | 2010-02-19 | Sanofi Aventis | N-AZABICYCLIC CARBOXAMIDE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC APPLICATION |
| WO2009097233A1 (en) | 2008-01-28 | 2009-08-06 | Schering Corporation | Imidazopyrazines as protein kinase inhibitors |
| ATE555116T1 (en) | 2008-02-26 | 2012-05-15 | Merck Sharp & Dohme | AHCY HYDROLASE INHIBITORS FOR THE TREATMENT OF HYPERHOMOCYSTEINEMIA |
| JP2011515401A (en) | 2008-03-20 | 2011-05-19 | アムジエン・インコーポレーテツド | Aurora kinase modulator and methods of use |
| JP5637982B2 (en) | 2008-04-09 | 2014-12-10 | インフィニティー ファーマシューティカルズ, インコーポレイテッド | Inhibitors of fatty acid amide hydrolase |
| DE102008017853A1 (en) | 2008-04-09 | 2009-10-15 | Merck Patent Gmbh | thienopyrimidines |
| US8012941B2 (en) | 2008-04-23 | 2011-09-06 | Gilead Sciences, Inc. | Carba-nucleoside analogs for antiviral treatment |
| WO2010036407A2 (en) | 2008-05-15 | 2010-04-01 | Biocryst Pharmaceuticals, Inc. | Antiviral nucleoside analogs |
| US8497278B2 (en) | 2008-05-19 | 2013-07-30 | Sunovion Pharmaceuticals Inc. | Imidazo[1,2-a]pyridine compounds |
| CN102112475A (en) | 2008-05-29 | 2011-06-29 | 西特里斯药业公司 | Imidazopyridine and related analogs as sirtuin modulators |
| AR072008A1 (en) | 2008-06-13 | 2010-07-28 | Merck & Co Inc | HETEROBICICLIC COMPOUNDS AS QUINASA P38 INHIBITION AGENTS |
| ES2605815T3 (en) | 2008-07-01 | 2017-03-16 | Ptc Therapeutics, Inc. | Bmi-1 protein expression modulators |
| NZ590320A (en) | 2008-07-14 | 2012-12-21 | Gilead Sciences Inc | Fused heterocyclyc inhibitors of histone deacetylase and/or cyclin-dependent kinases |
| WO2010011837A1 (en) | 2008-07-24 | 2010-01-28 | Bristol-Myers Squibb Company | Fused heterocyclic compounds useful as kinase modulators |
| US8518952B2 (en) | 2008-08-06 | 2013-08-27 | Pfizer Inc. | 6 substituted 2-heterocyclylamino pyrazine compounds as CHK-1 inhibitors |
| FR2934994B1 (en) | 2008-08-12 | 2010-09-17 | Sanofi Aventis | DERIVATIVES OF 2-ALKYL-6CYCLOAMINO-3- (PYRIDIN-4-YL) IMIDAZ-1,2-B! PYRIDAZINE, THEIR PREPARATION AND THEIR THERAPEUTIC APPLICATION |
| US8198449B2 (en) | 2008-09-11 | 2012-06-12 | Bristol-Myers Squibb Company | Compounds for the treatment of hepatitis C |
| WO2010033906A2 (en) | 2008-09-19 | 2010-03-25 | President And Fellows Of Harvard College | Efficient induction of pluripotent stem cells using small molecule compounds |
| WO2010048149A2 (en) | 2008-10-20 | 2010-04-29 | Kalypsys, Inc. | Heterocyclic modulators of gpr119 for treatment of disease |
| JP5416944B2 (en) | 2008-10-23 | 2014-02-12 | ユー・ディー・シー アイルランド リミテッド | Organic electroluminescence device |
| WO2010054253A1 (en) | 2008-11-07 | 2010-05-14 | Biotie Therapies Gmbh | Triazine derivatives as inhibitors of phosphodiesterases |
| WO2010059836A1 (en) | 2008-11-20 | 2010-05-27 | Decode Genetics Ehf | Substituted aza-bridged bicyclics for cardiovascular and cns disease |
| WO2010059838A2 (en) | 2008-11-20 | 2010-05-27 | Decode Genetics Ehf | Pde4 inhibitors selective for the long form of pde4 for treating inflammation and avoiding side effects |
| GB0822981D0 (en) | 2008-12-17 | 2009-01-21 | Summit Corp Plc | Compounds for treatment of duchenne muscular dystrophy |
| CA2746307C (en) * | 2008-12-17 | 2013-11-19 | Amgen Inc. | Aminopyridine and carboxypyridine compounds as phosphodiesterase 10 inhibitors |
| JP5210187B2 (en) | 2009-01-22 | 2013-06-12 | ユー・ディー・シー アイルランド リミテッド | Organic electroluminescence device |
| EP2210891A1 (en) | 2009-01-26 | 2010-07-28 | Domain Therapeutics | New adenosine receptor ligands and uses thereof |
| WO2010088518A2 (en) | 2009-01-31 | 2010-08-05 | Kalypsys, Inc. | Heterocyclic modulators of gpr119 for treatment of disease |
| US20110305637A1 (en) | 2009-02-24 | 2011-12-15 | Janssen Pharmaceutica N.V. | Radiolabelled pde10 ligands |
| US8367222B2 (en) | 2009-02-27 | 2013-02-05 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device |
| AU2010226490A1 (en) | 2009-03-20 | 2011-10-06 | Amgen Inc. | Inhibitors of PI3 kinase |
| JP5528427B2 (en) | 2009-03-24 | 2014-06-25 | 出光興産株式会社 | Organic electroluminescence device |
| KR101792837B1 (en) | 2009-04-16 | 2017-11-02 | 푼다시온 센트로 나시오날 드 인베스티가시오네스 온콜로기카스 카를로스Ⅲ | Imidazopyrazines for use as kinase inhibitors |
| TW201107329A (en) | 2009-07-30 | 2011-03-01 | Oncotherapy Science Inc | Fused imidazole derivative having ttk inhibitory action |
| JP5709752B2 (en) | 2009-08-19 | 2015-04-30 | 出光興産株式会社 | Aromatic amine derivative and organic electroluminescence device using the same |
| CA2778949C (en) | 2009-10-30 | 2018-02-27 | Janssen Pharmaceutica Nv | Imidazo[1,2-b]pyridazine derivatives and their use as pde10 inhibitors |
| CN102596907B (en) | 2009-11-16 | 2014-12-17 | 出光兴产株式会社 | aromatic amine derivative and organic electroluminescent element using same |
| US9073927B2 (en) | 2010-01-22 | 2015-07-07 | Fundacion Centro Nacional De Investigaciones Oncologicas Carlos Iii | Inhibitors of PI3 kinase |
| AR080754A1 (en) | 2010-03-09 | 2012-05-09 | Janssen Pharmaceutica Nv | IMIDAZO DERIVATIVES (1,2-A) PIRAZINA AND ITS USE AS PDE10 INHIBITORS |
| US9540379B2 (en) * | 2011-01-31 | 2017-01-10 | Boehringer Ingelheim International Gmbh | (1,2,4)triazolo[4,3-A]quinoxaline derivatives as inhibitors of phosphodiesterases |
| AU2012247564B2 (en) | 2011-04-28 | 2016-05-26 | Janssen Pharmaceutica Nv | Salt of an inhibitor of phosphodiesterase 10 enzyme |
| JP6115962B2 (en) | 2011-06-27 | 2017-04-19 | ヤンセン ファーマシューティカ エヌ.ベー. | 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline derivatives |
| EP2763989A1 (en) | 2011-09-09 | 2014-08-13 | H. Lundbeck A/S | Pyridine compounds and uses thereof |
| WO2013034755A1 (en) | 2011-09-09 | 2013-03-14 | H. Lundbeck A/S | Triazolopyrazine derivatives and their use for treating neurological and psychiatric disorders |
| EP2869822B1 (en) | 2012-07-09 | 2016-09-14 | Janssen Pharmaceutica, N.V. | Inhibitors of phosphodiesterase 10 enzyme |
| JP6247969B2 (en) | 2014-03-17 | 2017-12-13 | 本田技研工業株式会社 | Motorcycle exhaust system |
-
2012
- 2012-06-26 JP JP2014517667A patent/JP6115962B2/en not_active Expired - Fee Related
- 2012-06-26 US US14/128,835 patent/US10604523B2/en not_active Expired - Fee Related
- 2012-06-26 AU AU2012277912A patent/AU2012277912B2/en not_active Ceased
- 2012-06-26 BR BR112013033375-8A patent/BR112013033375B1/en not_active IP Right Cessation
- 2012-06-26 CA CA2838645A patent/CA2838645C/en active Active
- 2012-06-26 EP EP12729637.4A patent/EP2723744B1/en active Active
- 2012-06-26 CN CN201280031255.3A patent/CN103619846B/en not_active Expired - Fee Related
- 2012-06-26 EA EA201490165A patent/EA027418B1/en not_active IP Right Cessation
- 2012-06-26 MX MX2013015201A patent/MX344600B/en active IP Right Grant
- 2012-06-26 WO PCT/EP2012/062381 patent/WO2013000924A1/en active Application Filing
- 2012-06-26 ES ES12729637.4T patent/ES2575092T3/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010101230A1 (en) | 2009-03-05 | 2010-09-10 | アステラス製薬株式会社 | Quinoxaline compounds |
| WO2010138833A1 (en) * | 2009-05-29 | 2010-12-02 | Wyeth | SUBSTITUTED IMIDAZO[1,5-a]QUINOXALINES AS INHIBITORS OF PHOSPHODIESTERASE 10 |
Non-Patent Citations (13)
| Title |
|---|
| AGGARWAL, RANJANA ET AL: "Hypervalent iodine-mediated synthesis of 1-aryl-4-methyl-1,2,4-triazolo[4,3-a]quinoxalines by oxidative cyclization of arene carbaldehyde-3-methylquinoxalin-2-yl hydrazones", SYNTHETIC COMMUNICATIONS , 36(13), 1873-1878 CODEN: SYNCAV; ISSN: 0039-7911, vol. 36, no. 13, 2006, pages 1873 - 1878, XP002657377 * |
| BARATTI, C.M.; BOCCIA, M.M., BEHAV. PHARMACOL., vol. 10, 1999, pages 731 - 737 |
| DOMEK-LOPACIFISKA KU; STROSZNAJDER JB, MOL NEUROBIOL., vol. 41, no. 2-3, 2010, pages 129 - 37 |
| GREEN CHEMISTRY, vol. 6, 2004, pages 156 - 157 |
| JOURNAL OF FLUORINE CHEMISTRY, vol. 130, no. 10, 2009, pages 886 - 893 |
| KUMAR P ET AL., BEHAV PHARMACOL., vol. 21, no. 3, May 2010 (2010-05-01), pages 217 - 30 |
| KUMAR, DALIP ET AL: "An expeditious synthesis of 1-aryl-4-methyl-1,2,4-triazolo[4,3- a]quinoxalines under solvent-free conditions using iodobenzene diacetate", GREEN CHEMISTRY , 6(3), 156-157 CODEN: GRCHFJ; ISSN: 1463-9262, vol. 6, no. 3, 27 January 2004 (2004-01-27), pages 156 - 157, XP002657376 * |
| MENNITTI, F. S. ET AL., NATURE REV. DRUG DISCOVERY, vol. 5, 2006, pages 660 - 669 |
| P. W. MILLER ET AL., ANGEW. CHEM. INT. ED., vol. 47, 2008, pages 8998 - 9033 |
| PRICKAERTS, J. ET AL., NEUROSCIENCE, vol. 113, 2002, pages 349 - 359 |
| REIERSON, G.W. ET AL., CURRENT NEUROPHARMACOLOGY, vol. 9, 2011, pages 715 - 727 |
| SYNTHETIC COMMUNICATIONS, vol. 36, 2006, pages 1873 - 1878 |
| WEST, A.R.; TSENG K.Y., NEUROSCIENCE, vol. 5, 2011, pages 55 - 64 |
Cited By (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9540379B2 (en) | 2011-01-31 | 2017-01-10 | Boehringer Ingelheim International Gmbh | (1,2,4)triazolo[4,3-A]quinoxaline derivatives as inhibitors of phosphodiesterases |
| US10604523B2 (en) | 2011-06-27 | 2020-03-31 | Janssen Pharmaceutica Nv | 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]quinoxaline derivatives |
| JP2014526453A (en) * | 2011-09-09 | 2014-10-06 | ハー・ルンドベック・アクチエゼルスカベット | Pyridine compounds and their use |
| CN104411312B (en) * | 2012-06-26 | 2018-03-06 | 詹森药业有限公司 | The combination for being used in treatment neurological or dysbolism use including PDE2 the inhibitor such as methyl of 1 aryl 4 [1,2,4] triazole [4,3 A] quinoxaline compounds and PDE10 inhibitor |
| WO2014001314A1 (en) * | 2012-06-26 | 2014-01-03 | Janssen Pharmaceutica Nv | Combinations comprising pde 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and pde 10 inhibitors for use in the treatment of neurological or metabolic disorders |
| CN104411312A (en) * | 2012-06-26 | 2015-03-11 | 詹森药业有限公司 | Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders |
| AU2013283426B2 (en) * | 2012-06-26 | 2018-02-22 | Janssen Pharmaceutica Nv | Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders |
| JP2015522575A (en) * | 2012-06-26 | 2015-08-06 | ヤンセン ファーマシューティカ エヌ.ベー. | PDE2 inhibitors, such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] -quinoxaline compounds, and PDE10 inhibitors for use in the treatment of neurological or metabolic disorders combination |
| US9669035B2 (en) | 2012-06-26 | 2017-06-06 | Janssen Pharmaceutica Nv | Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl-[1,2,4]triazolo-[4,3-A]]quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological of metabolic disorders |
| US9085584B2 (en) | 2012-07-31 | 2015-07-21 | Boehringer Ingelheim International Gmbh | Substituted pyrido[3,2-E][1,2,4]-triazolo[4,3-A]pyrazines for the treatment of central nervous system disorders |
| WO2014019979A1 (en) | 2012-07-31 | 2014-02-06 | Boehringer Ingelheim International Gmbh | 4-methyl-2,3,5,9,9b-pentaaza-cyclopenta[a]naphthalenes |
| WO2014118039A1 (en) * | 2013-01-31 | 2014-08-07 | F. Hoffmann-La Roche Ag | Radiolabeled compounds |
| CN104955825A (en) * | 2013-01-31 | 2015-09-30 | 霍夫曼-拉罗奇有限公司 | Radiolabeled compounds |
| WO2014139983A1 (en) | 2013-03-13 | 2014-09-18 | H. Lundbeck A/S | [1,2,4]triazolo[4,3-a]quinoxalines as dual pde2/pde10 inhibitors |
| WO2015106032A1 (en) | 2014-01-08 | 2015-07-16 | Intra-Cellular Therapies, Inc. | Products and pharmaceutical compositions |
| EP3597649A1 (en) | 2014-04-23 | 2020-01-22 | Dart NeuroScience (Cayman) Ltd | Substituted [1,2,4]triazolo[1,5-a]pyrimidin-7-yl compounds as pde2 inhibitors |
| WO2015164508A1 (en) | 2014-04-23 | 2015-10-29 | Dart Neuroscience, Llc | Substituted [1,2,4] triazolo [1,5-a] pyrimidin-7-yl compounds as pde2 inhibitors |
| US11186582B2 (en) | 2014-04-23 | 2021-11-30 | Dart Neuroscience, (Cayman) LTD. | Substituted [1,2,4]triazolo[1,5-a]pyrimidin-7-yl compounds as PDE2 inhibitors |
| US9932345B2 (en) | 2014-04-23 | 2018-04-03 | Dart Neuroscience (Cayman) Ltd. | Substituted [1,2,4]triazolo[1,5-A]pyrimidin-7-yl compounds as PDE2 inhibitors |
| US10501465B2 (en) | 2014-04-23 | 2019-12-10 | Dart Neuroscience (Cayman) Ltd. | Substituted [1,2,4]triazolo[1,5-A]pyrimidin-7-yl compounds as PDE2 inhibitors |
| US10239882B2 (en) | 2014-11-05 | 2019-03-26 | Dart Neuroscience (Cayman) Ltd. | Substituted 5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-amine compounds as PDE2 inhibitors |
| US10543194B2 (en) | 2014-12-06 | 2020-01-28 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US10300064B2 (en) | 2014-12-06 | 2019-05-28 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US10105349B2 (en) | 2014-12-06 | 2018-10-23 | Intra-Cellular Therapies, Inc. | Organic compounds |
| WO2016149058A1 (en) | 2015-03-17 | 2016-09-22 | Merck Sharp & Dohme Corp. | Triazolyl pyrimidinone compounds as pde2 inhibitors |
| US10358435B2 (en) | 2015-03-17 | 2019-07-23 | Merck Sharp & Dohme Corp. | Triazolyl pyrimidinone compounds as PDE2 inhibitors |
| WO2016154081A1 (en) | 2015-03-26 | 2016-09-29 | Merck Sharp & Dohme Corp. | Pyrazolyl pyrimidinone compounds as pde2 inhibitors |
| US10287269B2 (en) | 2015-03-26 | 2019-05-14 | Merck Sharp & Dohme Corp. | Pyrazolyl pyrimidinone compounds as PDE2 inhibitors |
| US10195201B2 (en) | 2015-05-05 | 2019-02-05 | Merck Sharp & Dohme Corp. | Heteroaryl-pyrimidinone compounds as PDE2 inhibitors |
| US10285989B2 (en) | 2015-05-15 | 2019-05-14 | Merck Sharp & Dohme Corp. | Pyrimidinone amide compounds as PDE2 inhibitors |
| US10160762B2 (en) | 2015-05-29 | 2018-12-25 | Merck Sharp & Dohme Corp. | 6-alkyl dihydropyrazolopyrimidinone compounds as PDE2 inhibitors |
| US10174037B2 (en) | 2015-06-04 | 2019-01-08 | Merck Sharp & Dohme Corp. | Dihydropyrazolopyrimidinone compounds as PDE2 inhibitors |
| EP3302486A4 (en) * | 2015-06-04 | 2018-11-07 | Merck Sharp & Dohme Corp. | Dihydropyrazolopyrimidinone compounds as pde2 inhibitors |
| WO2016209749A1 (en) | 2015-06-25 | 2016-12-29 | Merck Sharp & Dohme Corp. | Substituted pyrazolo/imidazolo bicyclic compounds as pde2 inhibitors |
| US10647727B2 (en) | 2015-06-25 | 2020-05-12 | Merck Sharp & Dohme Corp. | Substituted pyrazolo/imidazolo bicyclic compounds as PDE2 inhibitors |
| US10287293B2 (en) | 2015-07-01 | 2019-05-14 | Merck Sharp & Dohme Corp. | Bicyclic heterocyclic compounds as PDE2 inhibitors |
| US10357481B2 (en) | 2015-07-01 | 2019-07-23 | Merck Sharp & Dohme Corp. | Substituted triazolo bicyclic compounds as PDE2 inhibitors |
| US10023575B2 (en) | 2015-10-13 | 2018-07-17 | Boehringer Ingelheim International Gmbh | Cyclic ether derivatives of pyrazolo[1,5-a]pyrimidine-3-carboxyamide |
| US10479794B2 (en) | 2015-10-13 | 2019-11-19 | Boehringer Ingelheim International Gmbh | Cyclic ether derivatives of pyrazolo[1,5-a]pyrimidine-3-carboxyamide |
| US11691977B2 (en) | 2015-10-13 | 2023-07-04 | Boehringer Ingelheim International Gmbh | Cyclic ether derivatives of pyrazolo[1,5-A]pyrimidine-3-carboxyamide |
| EP3156405A1 (en) | 2015-10-13 | 2017-04-19 | Boehringer Ingelheim International GmbH | Spirocyclic ether derivatives of pyrazolo[1,5-a]pyrimidine-3-carboxamide |
| US10875867B2 (en) | 2015-10-13 | 2020-12-29 | Boehringer Ingelheim International Gmbh | Cyclic ether derivatives of pyrazolo[1,5-a]pyrimidine-3-carboxyamide |
| CN108349979B (en) * | 2015-11-02 | 2021-04-09 | 詹森药业有限公司 | [1,2,4] triazolo [1,5-a ] pyrimidin-7-yl compounds |
| CN108349979A (en) * | 2015-11-02 | 2018-07-31 | 詹森药业有限公司 | [1,2,4] triazol [1,5-a] pyrimidin-7-yl compound |
| US10947239B2 (en) | 2015-11-02 | 2021-03-16 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidin-7-yl compound |
| WO2018083098A1 (en) | 2016-11-02 | 2018-05-11 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidine derivatives as pde2 inhibitors |
| US10947242B2 (en) | 2016-11-02 | 2021-03-16 | Janssen Pharmaceutica, Nv | [1,2,4]triazolo[1,5and#8208;A]pyrimidine compounds as PDE2 inhibitors |
| WO2018083103A1 (en) | 2016-11-02 | 2018-05-11 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidine compounds as pde2 inhibitors |
| US11053248B2 (en) | 2016-11-02 | 2021-07-06 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidine compounds as PDE2 inhibitors |
| AU2017353315B2 (en) * | 2016-11-02 | 2021-09-16 | Janssen Pharmaceutica Nv | (1,2,4)triazolo(1,5-a)pyrimidine compounds as PDE2 inhibitors |
| WO2018083101A1 (en) | 2016-11-02 | 2018-05-11 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidine compounds as pde2 inhibitors |
| EA039102B1 (en) * | 2016-11-02 | 2021-12-03 | Янссен Фармацевтика Нв | [1,2,4]TRIAZOLO[1,5-a]PYRIMIDINE COMPOUNDS AS PDE2 INHIBITORS |
| US11319321B2 (en) | 2016-11-02 | 2022-05-03 | Janssen Pharmaceutica Nv | [1,2,4]triazolo[1,5-a]pyrimidine compounds as PDE2 inhibitors |
| US11419874B2 (en) | 2017-11-23 | 2022-08-23 | Oslo University Hospital Hf | Treatment of tachycardia |
| WO2019101970A1 (en) | 2017-11-23 | 2019-05-31 | Oslo University Hospital Hf | Treatment of tachycardia |
Also Published As
| Publication number | Publication date |
|---|---|
| US20140147386A1 (en) | 2014-05-29 |
| AU2012277912B2 (en) | 2017-03-23 |
| US10604523B2 (en) | 2020-03-31 |
| BR112013033375B1 (en) | 2022-05-10 |
| EA201490165A1 (en) | 2014-04-30 |
| ES2575092T3 (en) | 2016-06-24 |
| CN103619846A (en) | 2014-03-05 |
| AU2012277912A1 (en) | 2013-12-19 |
| CA2838645C (en) | 2020-03-10 |
| MX2013015201A (en) | 2014-02-17 |
| BR112013033375A2 (en) | 2016-09-06 |
| CA2838645A1 (en) | 2013-01-03 |
| EA027418B1 (en) | 2017-07-31 |
| EP2723744A1 (en) | 2014-04-30 |
| JP6115962B2 (en) | 2017-04-19 |
| EP2723744B1 (en) | 2016-03-23 |
| JP2014518234A (en) | 2014-07-28 |
| MX344600B (en) | 2016-12-20 |
| CN103619846B (en) | 2016-08-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2838645C (en) | 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]quinoxaline derivatives | |
| JP7030827B2 (en) | Aminopyrimidine compounds useful as SSAO inhibitors | |
| KR101753826B1 (en) | 1,2,4-triazolo [4,3-a] pyridine derivatives and their use for the treatment or prevention of neurological and psychiatric disorders | |
| US20120053168A1 (en) | Fused benzoazepines as neuronal nicotinic acetylcholine receptor ligands | |
| EP3038466B1 (en) | 2,2-difluorodioxolo a2a receptor antagonists | |
| JP2017527588A (en) | P2X7 modulated N-acyl-triazolopyrazine | |
| KR102171706B1 (en) | Inhibitors of phosphodiesterase 10 enzyme | |
| CA3129516A1 (en) | Low affinity poly(ad-ribose) polymerase 1 dependent cytotoxic agents | |
| CA2876969A1 (en) | Substituted bicyclic alkoxy pyrazole analogs as allosteric modulators of mglur5 receptors | |
| WO2012092530A1 (en) | Naphthyridinone analogs as mglur5 positive allosteric modulators | |
| JP2023534803A (en) | 7-(piperidin-1-yl)-4H-pyrimido[1,2-B]pyridazin-4-one derivatives as positive allosteric modulators of the muscarinic acetylcholine receptor M4 | |
| TW202330533A (en) | 7h-pyrrolo[2,3-d]pyrimidines and preparation and uses thereof | |
| EP2863909B1 (en) | Combinations comprising 4-methyl-[1,2,4]triazolo[4,3-a]quinoxaline compounds as pde 2 inhibitors and pde 10 inhibitors for use in the treatment of neurological or metabolic disorders | |
| CA3103047A1 (en) | Oga inhibitor compounds | |
| TW201311698A (en) | 1,4-diazabicyclo[3.2.2]nonanes as neuronal nicotinic acetylcholine receptor ligands |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12729637 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2838645 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2014517667 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2013/015201 Country of ref document: MX |
|
| ENP | Entry into the national phase |
Ref document number: 2012277912 Country of ref document: AU Date of ref document: 20120626 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012729637 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14128835 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 201490165 Country of ref document: EA |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112013033375 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112013033375 Country of ref document: BR Kind code of ref document: A2 Effective date: 20131224 |