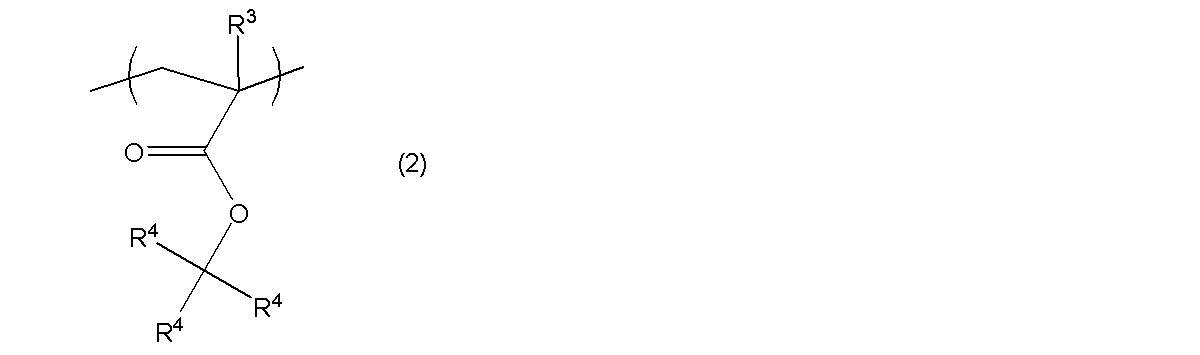WO2011108665A1 - Radiation-sensitive resin composition and resist pattern formation method - Google Patents
Radiation-sensitive resin composition and resist pattern formation method Download PDFInfo
- Publication number
- WO2011108665A1 WO2011108665A1 PCT/JP2011/054967 JP2011054967W WO2011108665A1 WO 2011108665 A1 WO2011108665 A1 WO 2011108665A1 JP 2011054967 W JP2011054967 W JP 2011054967W WO 2011108665 A1 WO2011108665 A1 WO 2011108665A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- carbon atoms
- polymer
- repeating unit
- hydrogen atom
- Prior art date
Links
- 0 CC(C)(C)CC(*)(C(C)(C)C)C(O*NS(*)(=O)=O)=O Chemical compound CC(C)(C)CC(*)(C(C)(C)C)C(O*NS(*)(=O)=O)=O 0.000 description 3
- OHWURTQGCZEEHA-UHFFFAOYSA-N CC(C)(C)C(OC(C1CC2C3C1)C3OC2=O)=O Chemical compound CC(C)(C)C(OC(C1CC2C3C1)C3OC2=O)=O OHWURTQGCZEEHA-UHFFFAOYSA-N 0.000 description 1
- VSYDNHCEDWYFBX-UHFFFAOYSA-N CC1(CCCC1)OC(C(C)=C)=O Chemical compound CC1(CCCC1)OC(C(C)=C)=O VSYDNHCEDWYFBX-UHFFFAOYSA-N 0.000 description 1
- DCTVCFJTKSQXED-UHFFFAOYSA-N CCC1(C2CC(C3)CC1CC3C2)OC(C(C)=C)=O Chemical compound CCC1(C2CC(C3)CC1CC3C2)OC(C(C)=C)=O DCTVCFJTKSQXED-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/039—Macromolecular compounds which are photodegradable, e.g. positive electron resists
- G03F7/0392—Macromolecular compounds which are photodegradable, e.g. positive electron resists the macromolecular compound being present in a chemically amplified positive photoresist composition
- G03F7/0397—Macromolecular compounds which are photodegradable, e.g. positive electron resists the macromolecular compound being present in a chemically amplified positive photoresist composition the macromolecular compound having an alicyclic moiety in a side chain
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/38—Esters containing sulfur
- C08F220/387—Esters containing sulfur and containing nitrogen and oxygen
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/0046—Photosensitive materials with perfluoro compounds, e.g. for dry lithography
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/20—Exposure; Apparatus therefor
- G03F7/2041—Exposure; Apparatus therefor in the presence of a fluid, e.g. immersion; using fluid cooling means
Definitions
- the present invention relates to a radiation-sensitive resin composition that provides a chemically amplified resist capable of accurately forming a pattern having a desired shape while suppressing the occurrence of variations in line width, and a resist pattern forming method using the same.
- lithography technology capable of microfabrication at a level of 0.10 ⁇ m or less is eagerly desired in order to obtain a higher degree of integration.
- near ultraviolet rays such as i-line are used as exposure light, and it is extremely difficult to perform fine processing at a level of 0.10 ⁇ m or less (sub quarter micron level) with this near ultraviolet rays. Therefore, in order to enable microfabrication at a level of 0.10 ⁇ m or less, development of a lithography technique using radiation having a shorter wavelength is being performed.
- Examples of radiation having a shorter wavelength include an emission line spectrum of a mercury lamp, far ultraviolet rays such as an excimer laser, an X-ray, and an electron beam.
- far ultraviolet rays such as an excimer laser, an X-ray, and an electron beam.
- KrF excimer laser wavelength 248 nm
- ArF excimer laser wavelength 193 nm
- a component having an acid-dissociable functional group and a component that generates an acid upon irradiation with radiation hereinafter referred to as “exposure”) (hereinafter referred to as “acid generator”), and these chemical amplification effects And the like (hereinafter referred to as “chemically amplified resist”).
- exposure a component having an acid-dissociable functional group and a component that generates an acid upon irradiation with radiation
- exposure hereinafter referred to as “acid generator”
- chemically amplified resist As a chemically amplified resist, specifically, a composition containing a resin having a tert-butyl ester group of carboxylic acid or a tert-butyl carbonate group of phenol and an acid generator has been reported.
- the tert-butyl ester group or tert-butyl carbonate group present in the resin is dissociated by the action of an acid generated by exposure, and the resin has an acidic group composed of a carboxyl group or a phenolic hydroxyl group.
- the exposed portion of the photoresist film becomes readily soluble in an alkali developer, so that a desired resist pattern can be formed.
- a finer resist pattern for example, a fine resist pattern having a line width of about 45 nm.
- the light source wavelength of the exposure apparatus can be shortened, the numerical aperture (NA) of the lens can be increased, and the like.
- NA numerical aperture
- shortening the light source wavelength requires a new exposure apparatus, but such an apparatus is expensive.
- the numerical aperture of the lens is increased, the resolution and the depth of focus are in a trade-off relationship. Therefore, there is a problem that the depth of focus is lowered even if the resolution can be improved.
- a liquid immersion lithography (liquid immersion lithography) method has been reported as a lithography technique for solving such problems.
- This method is a method in which a liquid for immersion exposure (for example, pure water, fluorine-based inert liquid, or the like) is interposed between the lens and the photoresist film (on the photoresist film) at the time of exposure.
- the exposure optical path space that has been conventionally filled with an inert gas such as air or nitrogen is filled with an immersion exposure liquid having a refractive index (n) larger than that of air or the like.
- the present invention provides a radiation-sensitive resin composition that provides a chemically amplified resist capable of accurately forming a pattern having a desired shape while suppressing the occurrence of variations in line width, and a resist pattern forming method using the same.
- the purpose is to do.
- the present invention is as follows. 1.
- [A] A polymer containing a repeating unit represented by the following general formula (1) and having an acid-dissociable group, [B] a radiation-sensitive acid generator, and [C] a polymer containing a fluorine atom And the content rate of the fluorine atom contained in the said polymer [A] is less than the content rate of the fluorine atom contained in the said polymer [C],
- the radiation sensitive resin composition characterized by the above-mentioned.
- R 1 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated carbon atom having 1 to 20 carbon atoms
- a hydrogen group, and Q 1 is a divalent linking group.
- the content of the repeating unit represented by the general formula (1) is 5 to 50 mol% when the total amount of the repeating units constituting the polymer [A] is 100 mol%.
- Radiation sensitive resin composition 3. 3.
- R 3 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 4 is independently a linear or branched alkyl group having 1 to 4 carbon atoms.
- the polymer [A] further contains at least one repeating unit selected from the group consisting of repeating units represented by the following general formulas (3-1) to (3-6):
- R 5 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 6 is a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms
- R 7 is a single bond or a methylene group
- R 8 is a hydrogen atom or a methoxy group
- R 9 is an oxygen atom or a methylene group
- p is 1, 2 or 3
- m is 0 or 1
- R 21 represents a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 22 represents a fluorinated hydrocarbon group having 1 to 30 carbon atoms
- R 23 represents Independently a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms
- R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group
- Q 3 is (g + 1)
- Q 4 is a divalent linking group and g is 1, 2 or 3. provided that in formulas (c1-2) and (c1-3), all R 23 Is not a hydrogen atom.) 6).
- R 1 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated carbon atom having 1 to 20 carbon atoms
- a hydrogen group, and Q 1 is a divalent linking group.
- a step of forming a photoresist film on a substrate Placing a liquid for immersion exposure on the photoresist film, and subjecting the photoresist film to immersion exposure via the liquid for immersion exposure; Developing the photoresist film exposed to immersion and forming a resist pattern; and
- a resist pattern forming method comprising:
- the radiation-sensitive resin composition and the radiation-sensitive resin composition for immersion exposure of the present invention it is possible to provide a chemically amplified resist capable of maintaining a good LWR while maintaining a wide exposure margin. it can. Further, according to the pattern forming method of the present invention, the chemically amplified resist to be used is excellent in developability, and it is possible to accurately form a pattern having a desired shape while suppressing the occurrence of variations in line width.
- (meth) acryl means acryl and methacryl.
- the radiation-sensitive resin composition of the present invention includes [A] a repeating unit represented by the following general formula (1) (hereinafter referred to as “repeating unit (I-1)”).
- a polymer having an acid dissociable group hereinafter referred to as “polymer [A]”
- [B] a radiation-sensitive acid generator hereinafter referred to as “acid generator [B]”
- [C ] A polymer containing a fluorine atom (hereinafter referred to as “polymer [C]”), and the content of fluorine atom contained in the polymer [A] is the same as that of the fluorine atom contained in the polymer [C]. Less than the content rate.
- the fluorine atom content in the polymers [A] and [C] can be measured by 13 C-NMR.
- the “acid-dissociable group” is a group in which an alkali-soluble site is protected with a protecting group, and means a group that is not “alkali-soluble” until the protecting group is removed with an acid.
- R 1 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated carbon atom having 1 to 20 carbon atoms
- a hydrogen group, and Q 1 is a divalent linking group.
- Another radiation-sensitive resin composition for immersion exposure according to the present invention is a composition containing the polymer [A] and the acid generator [B].
- Polymer [A] This polymer [A] is a polymer containing the repeating unit (I-1) represented by the above general formula (1) and having an acid dissociable group, and preferably becomes alkali-soluble by the action of an acid. It is an alkali-insoluble or hardly alkali-soluble polymer.
- alkali insoluble or hardly soluble in alkali means that the photoresist film under the alkali development conditions employed when forming a resist pattern from a photoresist film formed using the composition of the present invention.
- a film having a thickness of 100 nm formed using only the polymer [A] instead of it means that 50% or more of the initial film thickness of this film remains after development.
- the acid dissociable group contained in the polymer [A] may be contained in the repeating unit (I-1), and may be contained in other repeating units contained in the polymer, if necessary.
- the polymer [A] is preferably a copolymer further containing other repeating units as described later.
- R 1 is a hydrogen atom, a trifluoromethyl group, or an alkyl group having 1 to 4 carbon atoms.
- alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like.
- R 1 is preferably a hydrogen atom or a methyl group.
- R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated hydrocarbon group having 1 to 20 carbon atoms, an alkyl group having 1 to 20 carbon atoms, a carbon number of 1 -20 fluorinated alkyl group, cycloalkyl group having 3-20 carbon atoms, fluorinated cycloalkyl group having 3-20 carbon atoms, and the like.
- alkyl group having 1 to 20 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, sec-butyl group, tert-butyl group, n-pentyl group, n- Examples include hexyl group, n-heptyl group, octyl group and the like.
- fluorinated alkyl group having 1 to 20 carbon atoms include a difluoromethyl group, a perfluoromethyl group, a 2,2-difluoroethyl group, a 2,2,2-trifluoroethyl group, a perfluoroethyl group, 2 , 2,3,3-tetrafluoropropyl group, perfluoroethylmethyl group, perfluoropropyl group, 2,2,3,3,4,4-hexafluorobutyl group, perfluorobutyl group, 1,1-dimethyl -2,2,3,3-tetrafluoropropyl group, 1,1-dimethyl-2,2,3,3,3-pentafluoropropyl group, 2- (perfluoropropyl) ethyl group, 2,2,3 , 3,4,4,5,5-octafluoropentyl group, perfluoropentyl group, 1,1-dimethyl-2,2,3,3,4,
- a perfluoromethyl group, a perfluoroethyl group, a perfluoropropyl group, a perfluorobutyl group, and a perfluorooctyl group are preferable.
- the cycloalkyl group having 3 to 20 carbon atoms include a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, and the like.
- a fluorinated cycloalkyl group having 3 to 20 carbon atoms at least one hydrogen atom of the cycloalkyl group may be substituted with a fluorine atom.
- Q 1 is a divalent linking group, preferably a divalent hydrocarbon group.
- the divalent hydrocarbon group is preferably a chain hydrocarbon group or a cyclic hydrocarbon group, and specific examples thereof include methylene group, ethylene group, 1,3-propylene group and 1,2-propylene group.
- Hydrocarbon ring group 4 carbon atoms such as norbornylene group such as 1,4-norbornylene group, 2,5-norbornylene group, adamantylene group such as 1,5-adamantylene group, 2,6-adamantylene group, etc.
- a crosslinked cyclic hydrocarbon group such as a bicyclic to tetracyclic hydrocarbon group which is 30.
- Q 1 contains a divalent aliphatic cyclic hydrocarbon group, it has an alkylene group having 1 to 4 carbon atoms as a spacer between the —NH— group and the aliphatic cyclic hydrocarbon group. Is preferred. That is, Q 1 can be a divalent hydrocarbon group in which an aliphatic cyclic hydrocarbon group and an alkylene group having 1 to 4 carbon atoms are linked.
- Q 1 in the general formula (1) is preferably a 2,5-norbornylene group, a hydrocarbon group containing a 2,6-norbornylene group, an ethylene group, or a 1,2-propylene group.
- the polymer [A] may contain only one type of repeating unit (I-1), or may contain two or more types.
- Examples of the monomer that gives the repeating unit (I-1) represented by the general formula (1) include compounds represented by the following formulas (1-1) and (1-2).
- the content of the repeating unit (I-1) constituting the polymer [A] is preferably 5 to 50 mol% with respect to 100 mol% in total of all the repeating units constituting the polymer [A]. More preferably, it is 5 to 30 mol%. If the content of the repeating unit (I-1) is too small, the LWR improving effect may not be observed. On the other hand, if the content of the repeating unit (I-1) is too large, the contrast after development is impaired, and a good pattern shape may not be obtained.
- the polymer [A] contains, in addition to the repeating unit (I-1), a repeating unit having an acid dissociable group represented by the following general formula (2) (hereinafter referred to as “repeating unit (I)”. -2) ”) and repeating units having a lactone skeleton represented by the following general formulas (3-1) to (3-6) (hereinafter also referred to as” repeating units (I-3) "). ) May be included.
- R 3 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 4 is independently a linear or branched alkyl group having 1 to 4 carbon atoms.
- It may be an alicyclic hydrocarbon group or a derivative group thereof.
- R 5 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 6 is a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms
- R 7 is a single bond or a methylene group
- R 8 is a hydrogen atom or a methoxy group
- R 9 is an oxygen atom or a methylene group
- p is 1, 2 or 3 and m is 0 or 1
- Repeating unit (I-2) This repeating unit (I-2) is a unit having an acid dissociable group.
- R 3 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like.
- R 3 is preferably a hydrogen atom or a methyl group.
- R 4 s are independently of each other a linear or branched alkyl group having 1 to 4 carbon atoms, or a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms. It is.
- Examples of linear and branched alkyl groups having 1 to 4 carbon atoms represented by R 4 include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -Methylpropyl group, tert-butyl group and the like.
- Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms represented by R 4 include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, and 1- (2-cyclopentylethyl).
- dodecanyl group alicyclic alkyl group such as adamantyl group, and the like.
- a cyclopentyl group a cyclohexyl group, a bicyclo [2.2.1] heptyl group and an adamantyl group are preferred.
- a bridged skeleton such as an adamantane skeleton, a norbornane skeleton, a tricyclodecane skeleton, and a tetracyclododecane skeleton, cyclobutane, cyclopentane, cyclohexane, and cycloheptane.
- Groups having a cycloalkane skeleton such as cyclooctane; the hydrogen atoms contained in these groups are methyl, ethyl, n-propyl, isopropyl, n-butyl, 2-methylpropyl, 1-methyl Groups substituted with one or more linear, branched or cyclic alkyl groups having 1 to 10 carbon atoms such as propyl group and tert-butyl group And a group having an alicyclic skeleton such as.
- ester bond portion —C (R 4 ) 3 in the general formula (2) include tert-butyl group, 1-n- (1-ethyl-1-methyl) propyl group, 1-n- (1 , 1-dimethyl) propyl group, 1-n- (1,1-dimethyl) butyl group, 1-n- (1,1-dimethyl) pentyl group, 1- (1,1-diethyl) propyl group, 1- n- (1,1-diethyl) butyl group, 1-n- (1,1-diethyl) pentyl group, 1- (1-methyl) cyclopentyl group, 1- (1-ethyl) cyclopentyl group, 1- (1 -N-propyl) cyclopentyl group, 1- (1-isopropyl) cyclopentyl group, 1- (1-methyl) cyclohexyl group, 1- (1-ethyl) cyclohexyl group, 1- (1-n-propyl
- a group substituted with one or more of 4 linear, branched or cyclic alkyl groups for example, a methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group, etc.
- a group substituted with one or more of 4 linear, branched or cyclic alkyl groups are examples of 4 linear, branched or cyclic alkyl groups.
- the polymer [A] may contain only one type of repeating unit (I-2), or may contain two or more types.
- Examples of the monomer that gives the repeating unit (I-2) include (meth) acrylic acid 2-methyladamantyl-2-yl ester, (meth) acrylic acid 2-methyl-3-hydroxyadamantyl-2-yl ester, (Meth) acrylic acid 2-ethyladamantyl-2-yl ester, (meth) acrylic acid 2-ethyl-3-hydroxyadamantyl-2-yl ester, (meth) acrylic acid 2-n-propyladamantyl-2-yl ester (Meth) acrylic acid 2-isopropyladamantyl-2-yl ester, (meth) acrylic acid-2-methylbicyclo [2.2.1] hept-2-yl ester, (meth) acrylic acid-2-ethylbicyclo ester [2.2.1] Hept-2-yl ester, (meth) acrylic acid-8-methyltricyclo [5.2.1.0] , 6] decan-8-yl ester, (meth) ethyl-8-acrylic acid tricycl
- the content of the repeating unit (I-2) is 100 mol% in total of all the repeating units constituting the polymer [A]. On the other hand, it is preferably 15 to 85 mol%, more preferably 25 to 75 mol%, still more preferably 30 to 60 mol%. If the content of the repeating unit (I-2) is too small, the contrast after development is impaired, and a good pattern shape may not be obtained. On the other hand, when the content of the repeating unit (I-2) is too large, the adhesion with the base substrate becomes insufficient, and the pattern film may be peeled off.
- Repeating unit (I-3) This repeating unit (I-3) is a unit having a lactone skeleton. When the polymer [A] contains this repeating unit (I-3), the adhesion of the resist pattern to the substrate can be improved.
- R 5 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms.
- alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like.
- R 5 is preferably a hydrogen atom or a methyl group.
- R 6 represents a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms.
- the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like.
- a hydrogen atom contained in the alkyl group is substituted with at least one of a hydroxyl group, a cyano group, a carboxyl group, a halogen atom, and the like. Can be.
- the polymer [A] may contain only one type of repeating unit (I-3), or may contain two or more types.
- Examples of the monomer giving the repeating unit (I-3) include compounds represented by the following general formulas (3-1m) to (3-6m).
- the following general formula (3-1m) is a monomer that gives the repeating unit represented by the above general formula (3-1), and is represented by the following general formulas (3-2m) to (3-6m).
- these compounds are monomers that give the repeating units represented by the general formulas (3-2) to (3-6), respectively.
- R 5 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 6 is a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms
- R 7 is a single bond or a methylene group
- R 8 is a hydrogen atom or a methoxy group
- R 9 is an oxygen atom or a methylene group
- p is 1, 2 or 3 and m is 0 or 1
- Examples of the monomer giving the repeating unit (I-3) include (meth) acrylic acid-5-oxo-4-oxa-tricyclo [4.2.1.0 3,7 ] non-2-yl ester, (Meth) acrylic acid-9-methoxycarbonyl-5-oxo-4-oxa-tricyclo [4.2.1.0 3,7 ] non-2-yl ester, (meth) acrylic acid-5-oxo-4 -Oxa-tricyclo [5.2.1.0 3,8 ] dec-2-yl ester, (meth) acrylic acid-10-methoxycarbonyl-5-oxo-4-oxa-tricyclo [5.2.1.
- the content of the repeating unit (I-3) is 100 mol% in total of all the repeating units constituting the polymer [A]. On the other hand, it is preferably 5 to 80 mol%, more preferably 10 to 70 mol%, and still more preferably 10 to 60 mol%. If the content of the repeating unit (I-3) is too small, the adhesion to the substrate may be insufficient and the pattern may be peeled off. On the other hand, if the amount is excessive, the solubility in an alkali developer may be insufficient and development defects may increase.
- the polymer [A] includes, as other repeating units, in addition to the repeating units (I-2) and (I-3), a repeating unit having an alicyclic structure other than those described above (hereinafter referred to as “repeating unit ( I-4) ”), and a repeating unit derived from an aromatic unsaturated compound (hereinafter also referred to as“ repeating unit (I-5) ”).
- Repeating unit (I-4) is another repeating unit having an alicyclic structure excluding the above repeating units (I-2) and (I-3).
- etching resistance can be improved.
- Examples of the repeating unit (I-4) include repeating units represented by the following general formula (4). (Wherein R 11 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 12 is a monovalent group containing an alicyclic hydrocarbon group having 4 to 20 carbon atoms. is there.)
- the alkyl group having 1 to 4 carbon atoms represented by R 11 includes a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, Examples thereof include a 1-methylpropyl group and a tert-butyl group.
- R 11 is preferably a hydrogen atom or a methyl group.
- R 12 is a monovalent group containing an alicyclic hydrocarbon group having 4 to 20 carbon atoms, and may be a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms. It may be a derivative group of a hydrocarbon group.
- Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, a 1- (2-cyclopentylethyl) group, a cyclohexyl group, and a cyclohexylmethyl group.
- Group 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1-cycloheptylethyl) group, 1- (2-cycloheptylethyl) ) Group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2.1.0 2,6 ] decanyl group, tricyclo [3. 3.1.1 3,7 ] decanyl group, tetracyclo [6.2.1.1 3,6 . 0 2,7 ] dodecanyl group, alicyclic alkyl group such as adamantyl group, and the like.
- R 12 represents a hydrogen atom contained in the alicyclic hydrocarbon group as a methyl group, an ethyl group, an n-propyl group, an isopropyl group.
- a linear, branched or cyclic alkyl group having 1 to 4 carbon atoms such as n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group, hydroxyl group, cyano group, carbon
- a hydroxyalkyl group, a carboxyl group, a group substituted with one or more oxygen atoms or one or more of oxygen atoms can be used.
- the polymer [A] may contain only one type of repeating unit (I-4), or may contain two or more types.
- Examples of the monomer that gives the repeating unit (I-4) include (meth) acrylic acid-bicyclo [2.2.1] hept-2-yl ester, (meth) acrylic acid-bicyclo [2.2.2]. ] Oct-2-yl ester, (meth) acrylic acid-tricyclo [5.2.1.0 2,6 ] dec-7-yl ester, (meth) acrylic acid-tetracyclo [6.2.1.1 3 , 6 . 0 2,7 ] dodec-9-yl ester, (meth) acrylic acid-tricyclo [3.3.1.1 3,7 ] dec-1-yl ester, (meth) acrylic acid-tricyclo [3.3. 1.1,7 ] dec-2-yl ester and the like.
- the upper limit of the content of the repeating unit (I-4) is 100 mol in total of all the repeating units constituting the polymer [A].
- Repeating unit (I-5) This repeating unit (I-5) is a repeating unit derived from an aromatic unsaturated compound.
- the aromatic structure part is preferably contained in the side chain of the polymer.
- the polymer [A] may contain only one type of repeating unit (I-5), or may contain two or more types.
- aromatic unsaturated compounds examples include styrene, ⁇ -methylstyrene, 2-methylstyrene, 3-methylstyrene, 4-methylstyrene, 2-methoxystyrene, 3-methoxystyrene, 4-methoxystyrene, 4- (2-tert-butoxycarbonylethyloxy) styrene, 2-hydroxystyrene, 3-hydroxystyrene, 4-hydroxystyrene, 2-hydroxy- ⁇ -methylstyrene, 3-hydroxy- ⁇ -methylstyrene, 4-hydroxy- ⁇ -Methylstyrene, 2-methyl-3-hydroxystyrene, 4-methyl-3-hydroxystyrene, 5-methyl-3-hydroxystyrene, 2-methyl-4-hydroxystyrene, 3-methyl-4-hydroxystyrene, 3 , 4-Dihydroxystyrene, 2,4,6-trihydro Styrene, 4-tert-butoxysty
- the upper limit of the content of the repeating unit (I-5) is 100 mol in total of all the repeating units constituting the polymer [A].
- T1 It consists of the repeating unit (I-1), the repeating unit (I-2) and the repeating unit (I-3), and the content of all of these repeating units constituting the polymer [A] Preferably, 5 to 50 mol%, 15 to 85 mol% and 5 to 80 mol%, more preferably 5 to 30 mol%, 30 to 70 mol% and 10 to 70 mol%, respectively, with respect to the total of 100 mol% Is a polymer.
- the repeating unit (I-1), the repeating unit (I-2), the repeating unit (I-3) and the repeating unit (I-4) are contained in the polymer [A].
- the content of the fluorine atom contained in the polymer [A] is less than the content of the fluorine atom contained in the polymer [C]. It is a feature. Thereby, the (C) polymer is likely to be unevenly distributed on the surface layer of the photoresist film. Accordingly, the water repellency of the surface layer portion of the photoresist film to be formed can be increased, and when applying immersion exposure, good water repellency can be obtained without separately forming an upper film (immersion upper film) on the surface. A photoresist film can be formed.
- the fluorine atom content in the polymer [A] is smaller than that of the polymer [C] described later.
- the content of fluorine atoms is usually less than 10% by mass, preferably 0.1 to 9% by mass, more preferably 1 to 6% by mass, when the total amount of the polymer [A] is 100% by mass. is there.
- the fluorine atom content in the polymer [A] is smaller than that of the polymer [C]
- the polymer [C] is likely to be unevenly distributed on the surface layer of the photoresist film. Therefore, when immersion exposure is applied, it is not necessary to separately form an immersion upper layer film, so that the immersion exposure can be carried out smoothly, the occurrence of variations in line width is suppressed, and a pattern with a desired shape is accurately obtained.
- a chemically amplified resist that can be well formed can be obtained.
- the weight average molecular weight (hereinafter referred to as “Mw”) of the polymer [A] is preferably 1,000 to 100,000, more preferably 1 in terms of polystyrene by gel permeation chromatography (GPC). 3,000 to 30,000, more preferably 1,000 to 20,000. If the Mw is less than 1,000, a photoresist film having excellent heat resistance may not be obtained. On the other hand, if Mw exceeds 100,000, the developability of the photoresist film may deteriorate.
- the polymer [A] is a monomer that forms the repeating unit (I-1) in the presence of a radical polymerization initiator such as hydroperoxide, dialkyl peroxide, diacyl peroxide, and azo compound. It can manufacture by polymerizing the monomer raw material containing this in a suitable solvent.
- the polymerization temperature is usually 40 ° C. to 150 ° C., preferably 50 ° C. to 120 ° C.
- the polymerization time is usually 1 to 48 hours, preferably 1 to 24 hours.
- the solvent examples include alkanes such as n-pentane, n-hexane, n-heptane, n-octane, n-nonane and n-decane; cycloalkanes such as cyclohexane, cycloheptane and cyclooctane; decalin, norbornane and the like Alicyclic hydrocarbons; aromatic hydrocarbons such as benzene, toluene, xylene, ethylbenzene, cumene; halogenated hydrocarbons such as chlorobutane, bromohexane, dichloroethane, hexamethylene dibromide, chlorobenzene; ethyl acetate, acetic acid saturated carboxylic acid esters such as n-butyl, isobutyl acetate and methyl propionate; ketones such as acetone, 2-butanone, 4-methyl-2-pent
- polymer [A] used for manufacture of the radiation sensitive resin composition of this invention or the radiation sensitive resin composition for immersion exposure it is so preferable that there is little content of impurities, such as a halogen and a metal.
- impurities such as a halogen and a metal.
- the purification method include chemical purification methods such as washing with water and liquid-liquid extraction, and methods combining these chemical purification methods with physical purification methods such as ultrafiltration and centrifugation.
- the content is preferably 0.1% by mass or less, more preferably 0.07% by mass or less, and still more preferably 0.05% by mass or less with respect to the polymer [A].
- this content is 0.1% by mass or less, it is possible to suppress the amount of the eluate in the immersion exposure liquid such as water that is in contact with the immersion exposure.
- the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure is stored, no foreign matter is generated, and the radiation-sensitive resin composition of the present invention or radiation exposure for immersion exposure is performed.
- the low molecular weight component derived from the said monomer raw material means a monomer, a dimer, a trimer, an oligomer, etc., and can be normally made into a component with a molecular weight of 500 or less.
- Such components having a molecular weight of 500 or less are removed by, for example, chemical purification methods such as washing with water and liquid-liquid extraction, or a combination of these chemical purification methods and physical purification methods such as ultrafiltration and centrifugation. can do.
- the low molecular weight component derived from the monomer raw material can be analyzed by subjecting the crude product containing the polymer [A] to high performance liquid chromatography (HPLC).
- Acid generator [B] This acid generator [B] is used when the photoresist film formed by the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure is exposed through the immersion exposure liquid. , A component that generates an acid derived from the acid generator [B] from the exposed portion. By the action of the acid, the acid dissociable group contained in the polymer [A] is dissociated from the polymer in the exposed portion. The polymer from which the acid dissociable group is dissociated becomes readily soluble in an alkali developer. Then, a positive resist pattern having a desired shape can be obtained by removing an unnecessary portion of the photoresist film using an alkali developer.
- the acid generator [B] contained in the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure conventionally known radiation-sensitive acid generators can be applied.
- compounds described in paragraphs [0080] to [0113] of JP-A-2009-134088 can be used.
- Examples of the acid generator [B] include triphenylsulfonium salt compounds, 4-cyclohexylphenyldiphenylsulfonium salt compounds, 4-tert-butylphenyldiphenylsulfonium salt compounds, tri (4-tert-butylphenyl) sulfonium salt compounds, diphenyl Iodonium salt compound, bis (4-tert-butylphenyl) iodonium salt compound, 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium salt compound, 1- (3,5-dimethyl-4-hydroxy Phenyl) tetrahydrothiophenium salt compounds, succinimide compounds, bicyclo [2.2.1] hept-5-ene-2,3-dicarboximide compounds, and the like. These acid generators may be used alone or in combination of two or more.
- triphenylsulfonium salt compound examples include triphenylsulfonium trifluoromethanesulfonate, triphenylsulfonium nonafluoro-n-butanesulfonate, triphenylsulfonium perfluoro-n-octanesulfonate, triphenylsulfonium 2-bicyclo [2.2.1].
- triphenylsulfonium tricyclo [3.3.1.1 3,7 ] decanyl difluoromethanesulfonate, triphenylsulfonium 2- ( 3- tetracyclo [4.4.0.1 2,5 .1 7,10] dodecanyl) -1,1-difluoroethanesulfonate, triphenylsulfonium N, N'-bis (nonafluoro -n- butanesulfonyl) imidate And triphenylsulfonium camphorsulfonate.
- 4-cyclohexylphenyldiphenylsulfonium salt compound examples include 4-cyclohexylphenyldiphenylsulfonium trifluoromethanesulfonate, 4-cyclohexylphenyldiphenylsulfonium nonafluoro-n-butanesulfonate, 4-cyclohexylphenyldiphenylsulfonium perfluoro-n-octanesulfonate, 4-cyclohexylphenyldiphenylsulfonium 2-bicyclo [2.2.1] hept-2-yl-1,1,2,2-tetrafluoroethanesulfonate, 4-cyclohexylphenyldiphenylsulfonium 2- (3-tetracyclo [4.
- dodecanyl) -1,1-difluoroethanesulfonate, 4-cyclohexylphenyldiphenylsulfate Examples include onium N, N′-bis (nonafluoro-n-butanesulfonyl) imidate, 4-cyclohexylphenyldiphenylsulfonium camphorsulfonate, and the like.
- 4-tert-butylphenyldiphenylsulfonium salt compound examples include 4-tert-butylphenyldiphenylsulfonium trifluoromethanesulfonate, 4-tert-butylphenyldiphenylsulfonium nonafluoro-n-butanesulfonate, 4-tert-butylphenyldiphenylsulfonium. And perfluoro-n-octane sulfonate.
- tri (4-tert-butylphenyl) sulfonium salt compound examples include tri (4-tert-butylphenyl) sulfonium trifluoromethanesulfonate, tri (4-tert-butylphenyl) sulfonium nonafluoro-n-butanesulfonate, and the like. It is done.
- Examples of the 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium salt compound include 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium trifluoromethanesulfonate, 1- (4 -N-butoxynaphthalen-1-yl) tetrahydrothiophenium nonafluoro-n-butanesulfonate, 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium perfluoro-n-octanesulfonate, 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium 2-bicyclo [2.2.1] hept-2-yl-1,1,2,2-tetrafluoroethanesulfonate, 1- (4- n-Butoxynaphthalen-1-yl) tetrahydrothiophenium 2- (3-tetracycline [4.4
- Examples of the 1- (3,5-dimethyl-4-hydroxyphenyl) tetrahydrothiophenium salt compound include 1- (3,5-dimethyl-4-hydroxyphenyl) tetrahydrothiophenium trifluoromethanesulfonate, 1- (3 , 5-dimethyl-4-hydroxyphenyl) tetrahydrothiophenium nonafluoro-n-butanesulfonate.
- the content of the acid generator [B] in the radiation-sensitive resin composition or the radiation-sensitive resin composition for immersion exposure of the present invention is preferably 0.1 with respect to 100 parts by mass of the polymer [A]. -30 parts by mass, more preferably 2-27 parts by mass, still more preferably 5-25 parts by mass.
- the content of the acid generator [B] is within this range, the sensitivity and developability of the formed photoresist film can be maintained high.
- a sensitivity and resolution may not be enough.
- paintability of a composition is not enough and a favorable pattern shape may not be obtained.
- the polymer [C] contained in the radiation-sensitive resin composition of the present invention is a polymer containing a fluorine atom and has a higher fluorine atom content than the polymer [A].
- the fluorine atom content is preferably 5% by mass or more, more preferably 5 to 40% by mass, still more preferably 8 to 30% by mass, particularly preferably 100% by mass of the entire polymer [C]. 10 to 20% by mass.
- the distribution of the polymer [C] is high on the surface of the photoresist film due to the oil repellency of the polymer [C]. Tend to be.
- the polymer [C] tends to be unevenly distributed on the outermost surface of the photoresist film. Therefore, for the purpose of blocking the photoresist film and the immersion medium, it is not necessary to form an immersion upper layer film on the photoresist film, and it can be suitably used for the immersion exposure method as it is.
- the difference between the fluorine atom content in the polymer [A] and the fluorine atom content in the polymer [C] is preferably 1 mass. % Or more, more preferably 3% by mass or more, and particularly preferably 5% by mass or more.
- the polymer [C] is a polymer containing at least a repeating unit containing a fluorine atom, and may have a repeating unit containing no fluorine atom.
- the repeating unit containing a fluorine atom is preferably at least one repeating unit selected from the group consisting of repeating units represented by the following general formulas (c1-1) to (c1-3) (hereinafter these are combined) "Repeating unit (III-1)").
- R 21 represents a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 22 represents a fluorinated hydrocarbon group having 1 to 30 carbon atoms
- R 23 represents Independently a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms
- R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group
- Q 3 is (g + 1)
- Q 4 is a divalent linking group and g is 1, 2 or 3. provided that in formulas (c1-2) and (c1-3), all R 23 Is not a hydrogen atom.
- repeating unit (III-1) The polymer [C] is a repeating unit represented by the general formula (c1-1) (hereinafter referred to as “repeating unit (III-1-1)”), represented by the general formula (c1-2). Repeating units (hereinafter referred to as “repeating units (III-1-2)”) and repeating units represented by the above general formula (c1-3) (hereinafter referred to as “repeating units (III-1-3)”.
- an acid generator [B] in a photoresist film, and an acid diffusion inhibitor contained as necessary And the like can be prevented from eluting into the immersion exposure liquid.
- the receding contact angle of the immersion exposure liquid on the surface of the photoresist film can be increased, so that droplets derived from the immersion exposure liquid are less likely to remain on the photoresist film. Generation of defects due to the liquid can be suppressed.
- This repeating unit (III-1-1) is a repeating unit represented by the above general formula (c1-1).
- R 21 represents a hydrogen atom, a trifluoromethyl group, or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like.
- R 1 is preferably a hydrogen atom or a methyl group.
- R 22 is a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and at least one hydrogen atom in the hydrocarbon group having 1 to 30 carbon atoms is a fluorine atom. It is a group formed by substitution.
- R 22 is preferably a linear or branched alkyl group having 1 to 6 carbon atoms in which at least one hydrogen atom is substituted with a fluorine atom; at least one hydrogen atom is substituted with a fluorine atom And a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms or a derivative group thereof.
- Examples of the linear or branched alkyl group having 1 to 6 carbon atoms in which at least one hydrogen atom is substituted with a fluorine atom include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, and 1-butyl.
- Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms in which at least one hydrogen atom is substituted with a fluorine atom include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, 1- (2-cyclopentylethyl) group, cyclohexyl group, cyclohexylmethyl group, 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1 -Cycloheptylethyl) group, 1- (2-cycloheptylethyl) group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2 1.0 2,6 ] decanyl group, tricyclo [3.3.1.1 3,7
- dodecanyl group 0 2,7 ] dodecanyl group, adamantyl group and the like, a partially fluorinated alkyl group and a perfluoroalkyl group in which at least one hydrogen atom is substituted with a fluorine atom.
- a hydrogen atom contained in these groups is substituted with a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -A group substituted with one or more linear, branched or cyclic alkyl groups having 1 to 10 carbon atoms such as methylpropyl group and tert-butyl group.
- Monomers that give the repeating unit (III-1-1) represented by the general formula (c1-1) include trifluoromethyl (meth) acrylate, 2,2,2-trifluoroethyl (meth) ) Acrylic acid ester, Perfluoroethyl (meth) acrylic acid ester, Perfluoro n-propyl (meth) acrylic acid ester, Perfluoroisopropyl (meth) acrylic acid ester, Perfluoro n-butyl (meth) acrylic acid ester, Per Fluoroisobutyl (meth) acrylic acid ester, perfluoro tert-butyl (meth) acrylic acid ester, 2- (1,1,1,3,3,3-hexafluoropropyl) (meth) acrylic acid ester, 1- ( 2,2,3,3,4,4,5,5-octafluoropentyl) (meth) acrylic acid ester Perfluorocyclohexylmethyl (meth) acrylate, 1- (2,2,3,3,3-p
- the polymer [C] may contain only one type of repeating unit (III-1-1), or may contain two or more types.
- repeating units (III-1-2) and (III-1-3) are repeating units represented by the above general formulas (c1-2) and (c1-3), respectively.
- R 21 can be directly applied to the description of R 21 in formula (c1-1).
- R 23 is a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and all R 23 are hydrogen atoms There is no.
- the plurality of R 23 may be the same as or different from each other.
- R 23 is a fluorinated hydrocarbon group having 1 to 30 carbon atoms, the description of R 22 can be applied as it is.
- the partial structure containing R 23 represented by the following general formula includes the structures represented by the following formulas (1) to (5): can do.
- R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group.
- alkali dissociable group refers to a group that substitutes a hydrogen atom in a polar functional group such as a hydroxyl group or a carboxyl group and dissociates in the presence of an alkali.
- the polymer [C] is a polymer containing the repeating unit (c1-2) and / or the repeating unit (c1-3), and these repeating units contain an acid dissociable group
- the solubility of the polymer [C] contained in the exposed part of the photoresist film can be improved, which is preferable. This is because in the exposure step in the resist pattern forming method described later, the acid generated in the exposed portion of the photoresist film reacts with the acid dissociable group contained in the polymer [C] to generate a polar group. it is conceivable that.
- the polymer [C] is a polymer containing the repeating unit (c1-2) and / or the repeating unit (c1-3), and these repeating units contain an alkali dissociable group
- the affinity of the polymer [C] for the developer can be improved, which is preferable. This is considered to be because the polymer [C] reacts with the developer to generate a polar group in the development step in the pattern forming method described later.
- Examples of the acid dissociable group include a tert-butoxycarbonyl group, a tetrahydropyranyl group, a tetrahydrofuranyl group, a (thiotetrahydropyranylsulfanyl) methyl group, a (thiotetrahydrofuranylsulfanyl) methyl group, an alkoxy-substituted methyl group, and an alkylsulfanyl group.
- Substituted methyl group general formula [—C (R 29 ) 3 ] (wherein R 29 are each independently a linear or branched alkyl group having 1 to 4 carbon atoms, and having 4 to 20 carbon atoms.
- alkoxyl group (substituent) in the alkoxy-substituted methyl group examples include an alkoxyl group having 1 to 4 carbon atoms, that is, a methoxy group, an ethoxy group, an n-propoxy group, and an n-butoxy group.
- alkyl group (substituent) in the alkylsulfanyl-substituted methyl group examples include an alkyl group having 1 to 4 carbon atoms, that is, a methyl group, an ethyl group, an n-propyl group, an n-butyl group, and the like.
- R 29 is a linear or branched alkyl group having 1 to 4 carbon atoms, or 1 having 4 to 20 carbon atoms.
- Examples of the linear and branched alkyl group having 1 to 4 carbon atoms represented by R 29 include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -Methylpropyl group, tert-butyl group and the like.
- Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms represented by R 29 include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, and 1- (2-cyclopentylethyl).
- dodecanyl group alicyclic alkyl group such as adamantyl group, and the like.
- alicyclic hydrocarbon groups composed of alicyclic rings derived from norbornane, tricyclodecane, tetracyclododecane, adamantane, cyclopentane or cyclohexane are preferred.
- R 29 is a derivative group of the alicyclic hydrocarbon group
- R 29 represents a hydrogen atom contained in the alicyclic hydrocarbon group as a methyl group, an ethyl group, an n-propyl group, an isopropyl group. 1 or more or 1 or more of linear, branched or cyclic alkyl groups having 1 to 4 carbon atoms such as a group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group and tert-butyl group Or a group substituted with or the like.
- the general formula [—C (R 29 ) 3 ] is a divalent alicyclic carbon atom having 4 to 20 carbon atoms formed by bonding any two of three R 29 to each other. It may have a hydrogen group and a derivative group thereof, and examples thereof include a bridged skeleton such as an adamantane skeleton, a norbornane skeleton, a tricyclodecane skeleton, a tetracyclododecane skeleton, a cyclobutylene group, a cyclopentylene group, Divalent monocyclic hydrocarbon groups such as cyclohexylene group, cycloheptylene group, cyclooctylene group; the hydrogen atoms contained in these groups are methyl, ethyl, n-propyl, isopropyl, n- Substituted with one or more linear, branched or cyclic alkyl groups having 1 to 4 carbon atoms such as butyl
- Groups having an alicyclic skeleton such groups.
- a divalent monocyclic hydrocarbon group such as a cyclopentylene group or a cyclohexylene group, or a hydrogen atom contained in the alicyclic hydrocarbon group (monocyclic hydrocarbon group) is represented by the number of carbon atoms.
- a group substituted with one or more of 1 to 4 linear, branched or cyclic alkyl groups is preferred.
- Preferred examples of the general formula [—C (R 29 ) 3 ] include a tert-butyl group, a 1-n- (1-ethyl-1-methyl) propyl group, and a 1-n- (1,1-dimethyl) group.
- the acid dissociable group is preferably a group represented by the above general formula [—C (R 29 ) 3 ], a tert-butoxycarbonyl group, an alkoxy-substituted methyl group, or the like.
- a tert-butoxycarbonyl group and an alkoxy-substituted methyl group are preferable.
- an alkoxy-substituted methyl group and a group represented by the above general formula [—C (R 29 ) 3 ] are preferable.
- the alkali-dissociable group is not particularly limited as long as it exhibits the above properties.
- Examples of the alkali dissociable group in the general formula (c1-2) include groups represented by the following general formula (R1-1). (Wherein R 30 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms.)
- R 30 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms, and at least one hydrogen atom in the hydrocarbon group having 1 to 10 carbon atoms is substituted with a fluorine atom. It is a group consisting of R 30 is preferably a linear or branched perfluoroalkyl group having 1 to 10 carbon atoms, and particularly preferably a trifluoromethyl group.
- examples of the alkali dissociable group in the general formula (c1-3) include groups represented by the following general formulas (R1-2) to (R1-4). (Wherein R 31 s are independently of each other a halogen atom, an alkyl group having 1 to 10 carbon atoms, an alkoxyl group having 1 to 10 carbon atoms, an acyl group having 1 to 10 carbon atoms, or an acyloxy group having 1 to 10 carbon atoms.
- R 33 is, independently of each other, a hydrogen atom or an alkyl group having 1 to 10 carbon atoms, and is a divalent carbon atom formed by bonding two R 33 together, It may be an alicyclic hydrocarbon having 4 to 20 or a derivative group thereof, m1 is 0, 1, 2, 3, 4 or 5, and m2 is 0, 1, 2, 3 or 4. is there.)
- R 31 when R 31 is a halogen atom, it can be a fluorine atom, a chlorine atom, a bromine atom or an iodine atom. Of these, fluorine atoms are preferred.
- the alkyl group having 1 to 10 carbon atoms represented by R 31 includes a methyl group, an ethyl group, an n-propyl group, an isopropyl group, and 1-butyl.
- Examples of the alkoxyl group having 1 to 10 carbon atoms represented by R 31 include methoxy group, ethoxy group, n-propoxy group, n-butoxy group, sec-butoxy group, tert-butoxy group, n-pentyloxy group, n- Examples thereof include linear and branched alkyl groups such as a hexyloxy group.
- Examples of the acyl group having 1 to 10 carbon atoms represented by R 31 include acetyl group, propionyl group, methoxycarbonyl group, ethoxycarbonyl group, n-propoxycarbonyl group, isopropoxycarbonyl group, n-butoxycarbonyl group, isobutoxy group.
- Examples include carbonyl group, sec-butoxycarbonyl group, tert-butoxycarbonyl group, n-pentoxycarbonyl group, n-hexyloxycarbonyl group, n-heptyloxycarbonyl group, n-octyloxycarbonyl group and the like.
- Examples of the acyloxy group having 1 to 10 carbon atoms represented by R 31 include an acetoxy group, an ethylyloxy group, a butyryloxy group, a tert-butyryloxy group, a tert-amylyloxy group, an n-hexanecarbonyloxy group, and an n-octanecarboxyl group.
- Examples include a nitroxy group.
- R 31 may or be identical to each other or may be different.
- R 33 is a hydrogen atom or an alkyl group having 1 to 10 carbon atoms.
- R 33 is an alkyl group having 1 to 10 carbon atoms, methyl group, ethyl group, n-propyl group, isopropyl group, 1-butyl group, 2-butyl group, 2- (2-methylpropyl) group, 1-pentyl group, 2-pentyl group, 3-pentyl group, 1- (2-methylbutyl) group, 1- (3-methylbutyl) group, 2- (2-methylbutyl) group, 2- (3-methylbutyl) group , Neopentyl group, 1-hexyl group, 2-hexyl group, 3-hexyl group, 1- (2-methylpentyl) group, 1- (3-methylpentyl) group, 1- (4-methylpentyl) group, 2 -(2-methylpentyl) group, 2- (3-methylpentyl) group, 2- (4-methylpentyl) group,
- examples of the divalent alicyclic hydrocarbon having 4 to 20 carbon atoms formed by bonding two R 33 to each other in the general formula (R1-4) include an adamantane skeleton and a norbornane skeleton. And a bridged skeleton such as a tricyclodecane skeleton and a tetracyclododecane skeleton, and a group having a cycloalkane skeleton such as cyclobutane, cyclopentane, cyclohexane, cycloheptane, and cyclooctane.
- the hydrogen atom contained in the alicyclic hydrocarbon group is a methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2 -A group substituted with one or more linear, branched or cyclic alkyl groups having 1 to 4 carbon atoms such as methylpropyl group, 1-methylpropyl group, tert-butyl group, etc. Can do.
- Examples of the group represented by the general formula (R1-4) include a methyl group, an ethyl group, a 1-propyl group, a 2-propyl group, a 1-butyl group, a 2-butyl group, a 1-pentyl group, and a 2-pentyl group.
- the repeating unit (III-1-2) and the repeating unit (III-1-3) in which R 24 is a hydrogen atom are polar groups. It will have some hydroxyl or carboxyl group.
- the affinity of the polymer [C] for the developer can be improved in the development step of the pattern forming method described later.
- Q 3 is a (g + 1) -valent linking group.
- examples of such a group include a single bond, a (g + 1) valent hydrocarbon group having 1 to 30 carbon atoms and a derivative group thereof.
- the (g + 1) -valent hydrocarbon group can be a group including a chain structure or a cyclic structure.
- Chain structure hydrocarbon groups include methane, ethane, propane, butane, 2-methylpropane, pentane, 2-methylbutane, 2,2-dimethylpropane, hexane, heptane, octane, nonane, decane, etc.
- a hydrocarbon group having a structure in which (g + 1) hydrogen atoms are removed from 1 to 10 chain hydrocarbons can be obtained.
- Examples of the cyclic hydrocarbon group include cyclobutane, cyclopentane, cyclohexane, bicyclo [2.2.1] heptane, bicyclo [2.2.2] octane, and tricyclo [5.2.1.0 2,6. ]
- Q 3 in the general formula (c1-2) may be a derivative group of the hydrocarbon group, and an oxygen atom, a sulfur atom, an imino group, a carbonyl group, —CO—O— or —CO—NH—. It can be set as the group provided with.
- Examples of Q 3 having an oxygen atom, a sulfur atom, an imino group, a carbonyl group, —CO—O— or —CO—NH— include groups represented by the following general formula. (Wherein, R 61 s are independently of each other a single bond, divalent, an aliphatic hydrocarbon group having 1 to 10 carbon atoms or a derivative group thereof, divalent, and having 4 to 20 carbon atoms. An alicyclic hydrocarbon group or a derivative group thereof, or a divalent aromatic hydrocarbon group having 6 to 30 carbon atoms or a derivative group thereof, and g is 1, 2, or 3.
- Examples of the divalent aliphatic hydrocarbon group having 1 to 10 carbon atoms represented by R 61 in the above general formula include a methylene group, an ethylene group, a 1,3-propylene group, and a 1,2-propylene group.
- the divalent and alicyclic hydrocarbon group having 4 to 20 carbon atoms represented by R 61 includes a cyclobutylene group such as 1,3-cyclobutylene group, 1,3- 3 to 10 carbon atoms, such as a cyclopentylene group such as a cyclopentylene group, a cyclohexylene group such as a 1,4-cyclohexylene group, and a cyclooctylene group such as a 1,5-cyclooctylene group Monocyclic hydrocarbon ring groups such as cycloalkylene groups; norbornylene groups such as 1,4-norbornylene groups and 2,5-norbornylene groups, adamantylene groups such as 1,5-adamantylene groups and 2,6-adamantylene groups And a bridged cyclic hydrocarbon group such as a 2 to 4 cyclic hydrocarbon ring group having 4 to 20 carbon atoms, such as a group.
- the divalent aromatic hydrocarbon group represented by R 61 having 6 to 30 carbon atoms is obtained by removing two hydrogen atoms from an aromatic hydrocarbon such as benzene or naphthalene. It can be an aromatic hydrocarbon group having a different structure.
- any of the chain hydrocarbon group, the cyclic hydrocarbon group, and the aromatic hydrocarbon group can be a derivative group, and at least one hydrogen atom contained in the hydrocarbon group is methylated.
- Linear, branched or cyclic having 1 to 10 carbon atoms such as a group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group Groups having an alicyclic skeleton such as one or more alkyl groups substituted with one or more alkyl groups.
- g is 1, 2 or 3, and when g is 2 or 3, a plurality of structural parts represented by the following general formula in the general formula (c1-2) May be the same as or different from each other.
- R 23 is independently of each other a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms
- R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group. is there.
- Q 4 in the general formula (c1-3) can be the same as Q 3 when g in the general formula (c1-3) is 1.
- the repeating unit (III-1-2) represented by the general formula (c1-2) is exemplified by the following general formula. (Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group, and Q 3 is (G + 1) is a valent linking group, and g is 1, 2 or 3.)
- Examples of the monomer that gives the repeating unit (III-1-2) represented by the general formula (c1-2) are exemplified by the following general formula. (Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group.)
- the compound in which R 24 is an acid dissociable group or an alkali dissociable group uses, for example, a compound in which R 24 in the above general formula is a hydrogen atom as a raw material. Can be synthesized.
- R 24 is an alkali dissociative group, for example, as a synthesis method of a monomer having the structure represented by the general formula (R1-1), the compound R 24 is a hydrogen atom in the above general formula And a method of fluoroacylation.
- Specific methods are exemplified below. (1) A method in which an alcohol and a fluorocarboxylic acid are condensed and esterified in the presence of an acid. (2) A method in which an alcohol and a fluorocarboxylic acid halide are condensed and esterified in the presence of a base.
- the repeating unit (III-1-3) represented by the general formula (c1-3) is exemplified by the following general formula. (Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 24 is a hydrogen atom, an acid-dissociable group or an alkali-dissociable group, and Q 4 is It is a divalent linking group.)
- Examples of the monomer that gives the repeating unit (III-1-3) represented by the general formula (c1-3) are exemplified by the following general formula. (Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group.)
- the compound in which R 24 is an acid dissociable group or an alkali dissociable group is, for example, a compound in which R 24 in the above general formula is a hydrogen atom, or It can be synthesized by using a derivative compound as a raw material.
- a monomer having a structure represented by the general formulas (R1-2) to (R1-4) wherein R 24 is an alkali-dissociable group is represented by the following general formula (m-2-3): It can be synthesized by reacting the compound represented by the following general formulas (m-2-4-1) to (m-2--4-3).
- R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 23 is independently a hydrogen atom, a fluorine atom or a fluorinated group having 1 to 30 carbon atoms.
- R 51 is a halogen atom or a hydroxyl group
- Q 4 is a divalent linking group
- two R 23 are not hydrogen atoms.
- R 31 s are independently of each other a halogen atom, an alkyl group having 1 to 10 carbon atoms, an alkoxyl group having 1 to 10 carbon atoms, an acyl group having 1 to 10 carbon atoms, or an acyloxy group having 1 to 10 carbon atoms.
- R 55 is a halogen atom
- R 56 is a halogen atom
- m1 is 0, 1, 2, 3, 4 or 5
- m2 is 0, 1, 2, 3 or 4
- R 55 is preferably a chlorine atom.
- R 56 is preferably a bromine atom.
- R 24 is an alkali dissociable group and, for example, a monomer having a structure represented by the above general formulas (R1-2) to (R1-4) is represented by the following general formula (m-2-5) ) And a compound represented by the following general formula (m-2-6) can be synthesized.
- R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms
- R 23 is independently a hydrogen atom, a fluorine atom or a fluorinated group having 1 to 30 carbon atoms.
- R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group
- R 59 is a halogen atom or a hydroxyl group
- Q 4 is a divalent linking group.
- two R 23 are not hydrogen atoms.
- the polymer [C] contains at least one repeating unit (III) selected from repeating units (III-1-1), (III-1-2) and (III-1-3).
- a polymer containing -1) is preferred.
- Each of the repeating units (III-1) contained may be only one type, or two or more types.
- the polymer [C] contains at least two kinds of repeating units selected from the repeating units (III-1-1), (III-1-2) and (III-1-3).
- a polymer is more preferable, and a polymer including repeating units (III-1-2) and (III-1-3) is particularly preferable.
- the content of the repeating unit (III-1) constituting the polymer [C] is preferably 20 to 90 mol% with respect to 100 mol% in total of all the repeating units constituting the polymer [C]. More preferably, it is 30 to 90 mol%, still more preferably 30 to 85 mol%. When the content of the repeating unit (III-1) is within this range, it is particularly effective from the viewpoint of ensuring water repellency after coating and compatibility of contact angle with the developer after PEB.
- the polymer [C] includes, in addition to the repeating unit (III-1), a repeating unit having an acid dissociable group (hereinafter referred to as “repeating unit (III-2)”), an alkali-soluble group. And a repeating unit having a lactone skeleton or a cyclic carbonate skeleton (hereinafter referred to as “repeating unit (III-4)”) and the like. But you can.
- Repeating unit (III-2) By including this repeating unit (III-2) in the polymer [C], the difference between the advancing contact angle and the receding contact angle in the photoresist film can be reduced, and the scanning speed during exposure can be improved. Can respond more.
- the repeating unit (III-2) is preferably a repeating unit represented by the following general formula (c2-1). (Wherein R 25 is a hydrogen atom, a methyl group, a trifluoromethyl group or a hydroxymethyl group, R 26 is a linear or branched alkyl group having 1 to 4 carbon atoms, and k is 1, 2, 3 or 4.)
- the linear or branched alkyl group having 1 to 4 carbon atoms represented by R 26 includes a methyl group, ethyl group, n-propyl group, isopropyl group, n- Examples thereof include a butyl group, a 2-methylpropyl group, a 1-methylpropyl group, and a tert-butyl group.
- the polymer [C] may contain the repeating unit (III-2) alone or in combination of two or more.
- the content thereof is preferably 80 mol with respect to 100 mol% in total of all the repeating units constituting the polymer [C]. % Or less, more preferably 10 to 80 mol%, still more preferably 20 to 80 mol%, particularly preferably 30 to 70 mol%.
- the content of the repeating unit (III-2) is in the above range, the difference between the advancing contact angle and the receding contact angle in the photoresist film can be reduced.
- Repeating unit (III-3) By including this repeating unit (III-3) in the polymer [C], the solubility of the photoresist film can be improved in the development step of the pattern forming method described later.
- the alkali-soluble group in the repeating unit (III-3) is preferably a functional group having a hydrogen atom having a pKa of 4 to 11.
- alkali-soluble group examples include the following general formula (c3-a) and formula (c3-b). (Wherein R 36 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms.)
- R 36 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms, and at least one hydrogen atom in the hydrocarbon group having 1 to 10 carbon atoms is substituted with a fluorine atom. It is a group consisting of In the present invention, R 36 is preferably a trifluoromethyl group.
- the structure of the main chain in the repeating unit (III-3) is not particularly limited, but is preferably the structure of the main chain in a polymer such as (meth) acrylic acid ester or ⁇ -trifluoroacrylic acid ester.
- the repeating unit (III-3) is exemplified by the following general formulas (c3-a-1) and (c3-b-1).
- R 27 is a hydrogen atom, a methyl group or a trifluoromethyl group
- R 28 is a single bond, or a divalent, linear, branched or cyclic group having 1 to 20 carbon atoms.
- S 36 is a saturated or unsaturated hydrocarbon group
- R 36 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms.
- R 28 in the above general formulas (c3-a-1) and (c3-b-1) is divalent and has 1 to 20 carbon atoms, linear, branched or cyclic, saturated or unsaturated.
- it is a hydrocarbon group
- specific examples thereof include a methylene group, an ethylene group, a 1,3-propylene group and a 1,2-propylene group, a propylene group, a tetramethylene group, a pentamethylene group, a hexamethylene group.
- a saturated chain hydrocarbon group a cyclobutylene group such as 1,3-cyclobutylene group, a cyclopentylene group such as 1,3-cyclopentylene group, and a cyclohexylene group such as 1,4-cyclohexylene group
- a monocyclic hydrocarbon ring group such as a cycloalkylene group having 3 to 10 carbon atoms such as a cyclooctylene group such as 1,5-cyclooctylene group; 1,4-norbornylene group, 2,5-norbornylene
- a bridged cyclic group such as a 2-4 cyclic hydrocarbon ring group having 4 to 30 carbon atoms, such as a norbornylene group such as a group, adamantylene group such as 1,5-adamantylene group, 2,6-adamantylene group, etc. Hydrocarbon group, etc. And the like.
- the polymer [C] may contain the repeating unit (III-3) alone or in combination of two or more.
- the content thereof is preferably 50 mol with respect to 100 mol% in total of all the repeating units constituting the polymer [C]. % Or less, more preferably 5 to 30 mol%, still more preferably 5 to 20 mol%.
- the content of the repeating unit (III-3) is in the above range, the solubility of the photoresist film can be improved in the development step of the pattern forming method described later.
- Repeating unit (III-4) By including this repeating unit (III-4) in the polymer [C], it is possible to improve the hydrophilicity during alkali development while ensuring the hydrophobicity of the photoresist film during immersion exposure. Repeating unit that can be.
- the content thereof is preferably 50 mol with respect to 100 mol% in total of all the repeating units constituting the polymer [C]. % Or less, more preferably 5 to 30 mol%, still more preferably 5 to 20 mol%.
- the content of the repeating unit (III-4) is in the above range, the hydrophilicity during alkali development can be improved while ensuring the hydrophobicity of the photoresist film during immersion exposure.
- the weight average molecular weight (Mw) of the polymer [C] is preferably 1,000 to 50,000, more preferably 1,000 to 40,000, and still more preferably 1,000 in terms of polystyrene by GPC. ⁇ 30,000. If Mw is less than 1,000, a photoresist film having a sufficient receding contact angle may not be obtained. On the other hand, if Mw exceeds 50,000, the developability of the photoresist film may deteriorate. Further, the ratio (Mw / Mn) obtained using the number average molecular weight (Mn) in terms of polystyrene by GPC and Mw is preferably 1 to 5, more preferably 1 to 4.
- the above polymer [C] is a single monomer that will form the repeating unit (III-1) in the presence of a radical polymerization initiator such as hydroperoxide, dialkyl peroxide, diacyl peroxide, and azo compound. It can manufacture by polymerizing the monomer raw material containing a body in a suitable solvent. In the polymerization system, a chain transfer agent may coexist if necessary.
- the polymerization temperature is usually 40 ° C. to 150 ° C., preferably 50 ° C. to 120 ° C.
- the polymerization time is usually 1 to 48 hours, preferably 1 to 24 hours.
- the solvent examples include alkanes such as n-pentane, n-hexane, n-heptane, n-octane, n-nonane and n-decane; cycloalkanes such as cyclohexane, cycloheptane and cyclooctane; decalin, norbornane and the like Alicyclic hydrocarbons; aromatic hydrocarbons such as benzene, toluene, xylene, ethylbenzene, cumene; halogenated hydrocarbons such as chlorobutane, bromohexane, dichloroethane, hexamethylene dibromide, chlorobenzene; ethyl acetate, acetic acid saturated carboxylic acid esters such as n-butyl, isobutyl acetate and methyl propionate; ketones such as acetone, 2-butanone, 4-methyl-2-pent
- polymer [C] used for manufacture of the radiation sensitive resin composition of this invention it is so preferable that there is little content of impurities, such as a halogen and a metal.
- impurities such as a halogen and a metal.
- the purification method include chemical purification methods such as washing with water and liquid-liquid extraction, and methods combining these chemical purification methods with physical purification methods such as ultrafiltration and centrifugation.
- the content of the polymer [C] in the radiation-sensitive resin composition of the present invention is preferably 0.1 to 20 parts by mass, more preferably 1 to 100 parts by mass with respect to 100 parts by mass of the polymer [A].
- the amount is 10 parts by mass, more preferably 1 to 7.5 parts by mass. If the content of the polymer [C] is too small, the effect of containing the polymer [C] may not be sufficient. On the other hand, if the content of the polymer [C] is too large, the water repellency on the surface of the photoresist film becomes too high, and development failure may occur.
- the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure includes, in addition to the above essential components, an acid diffusion inhibitor, a lactone compound, a solvent, a surfactant, a sensitizer, and a halation.
- An inhibitor, an adhesion assistant, a storage stabilizer, an antifoaming agent, an alicyclic additive, and the like may be included.
- Acid Diffusion Inhibitor controls the diffusion phenomenon of the acid generated from the acid generator [B] in the photoresist film by immersion exposure, and suppresses an undesirable chemical reaction in the unexposed area. It is a component having By blending this acid diffusion inhibitor, the shape of the patterned resist film portion to be obtained and its dimensional fidelity can be improved.
- Examples of the acid diffusion inhibitor include a compound represented by the following general formula (E) (hereinafter referred to as “nitrogen-containing compound (I)”), a compound having two nitrogen atoms (hereinafter referred to as “nitrogen-containing compound (II)”. ) “), Compounds having 3 or more nitrogen atoms (hereinafter referred to as” nitrogen-containing compound (III) "), amide group-containing compounds, urea compounds, nitrogen-containing heterocyclic compounds, and the like. These acid diffusion inhibitors may be used alone or in combination of two or more.
- R 61 are each independently a hydrogen atom, or a substituted or unsubstituted, linear, branched or cyclic alkyl group, aryl group or aralkyl group.
- Nitrogen-containing compounds (I) include monoalkylamines such as n-hexylamine, n-heptylamine, n-octylamine, n-nonylamine, n-decylamine; di-n-butylamine, di-n-pentylamine Dialkylamines such as di-n-hexylamine, di-n-heptylamine, di-n-octylamine, di-n-nonylamine, di-n-decylamine; triethylamine, tri-n-propylamine, tri- trialkylamines such as n-butylamine, tri-n-pentylamine, tri-n-hexylamine, tri-n-heptylamine, tri-n-octylamine, tri-n-nonylamine, tri-n-decylamine; Aniline, N-methylaniline, N, N-dimethylaniline,
- nitrogen-containing compound (II) examples include ethylenediamine, N, N, N ′, N′-tetramethylethylenediamine, N, N, N ′, N′-tetrakis (2-hydroxypropyl) ethylenediamine, tetramethylenediamine, hexa Methylenediamine, 4,4′-diaminodiphenylmethane, 4,4′-diaminodiphenyl ether, 4,4′-diaminobenzophenone, 4,4′-diaminodiphenylamine, 2,2′-bis (4-aminophenyl) propane, 2 -(3-aminophenyl) -2- (4-aminophenyl) propane, 1,4-bis [1- (4-aminophenyl) -1-methylethyl] benzene, 1,3-bis [1- (4 -Aminophenyl) -1-methylethyl] benzene and the like.
- nitrogen-containing compound (III) examples include polymers of polyethyleneimine, polyallylamine, dimethylaminoethylacrylamide, and the like.
- amide group-containing compound examples include formamide, N-methylformamide, N, N-dimethylformamide, acetamide, N-methylacetamide, N, N-dimethylacetamide, propionamide, benzamide, pyrrolidone, N-methylpyrrolidone and the like. It is done.
- urea compound examples include urea, methylurea, 1,1-dimethylurea, 1,3-dimethylurea, 1,1,3,3-tetramethylurea, 1,3-diphenylurea, tributylthiourea and the like.
- nitrogen-containing heterocyclic compound examples include pyridine, 2-methylpyridine, 4-methylpyridine, 2-ethylpyridine, 4-ethylpyridine, 2-phenylpyridine, 4-phenylpyridine, N-methyl-4-phenylpyridine, Pyridines such as nicotine, nicotinic acid, nicotinamide, quinoline, 8-oxyquinoline, acridine; piperidines such as piperidine, tert-butyl-4-hydroxy-1-piperidinecarboxylate; pyrazine, pyrazole, pyridazine, quinosaline, Examples include purine, pyrrolidine, morpholine, 4-methylmorpholine, piperazine, 1,4-dimethylpiperazine, 1,4-diazabicyclo [2.2.2] octane.
- a compound represented by the following general formula (D1-0) can also be used.
- X + is a cation represented by the following general formula (D1-1) or (D1-2), Z ⁇ is OH ⁇
- R D11 is , An optionally substituted fluorinated alkyl group, an alicyclic fluorinated hydrocarbon group, or a fluorinated aryl group.
- R D2 , R D3, and R D4 are each independently a hydrogen atom, an alkyl group, an alkoxyl group, a hydroxyl group, or a halogen atom.
- R D5 and R D6 are each independently a hydrogen atom, an alkyl group, an alkoxyl group, a hydroxyl group, or a halogen atom.
- the compound represented by the general formula (D1-0) is used as an acid diffusion control agent that is decomposed by exposure and loses acid diffusion controllability (hereinafter also referred to as “photodegradable acid diffusion control agent”). It is.
- the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure contains the compound represented by the general formula (D1-0)
- an acid diffuses in the exposed portion, Since the acid diffusion is controlled in the unexposed area, the contrast between the exposed area and the unexposed area is excellent (that is, the boundary between the exposed area and the unexposed area becomes clear). Therefore, such a composition is effective in improving LWR and MEEF (Mask Error Enhancement Factor (amplification factor of line width deviation due to mask width deviation)).
- X + is a cation represented by the general formula (D1-1) or (D1-2) as described above.
- R D2 , R D3 and R D4 are each independently a hydrogen atom, an alkyl group, an alkoxyl group, a hydroxyl group or a halogen atom.
- a hydrogen atom, an alkyl group, an alkoxy group, and a halogen atom are preferable because the compound represented by the above formula (D1-0) has an effect of reducing solubility in a developer.
- R D5 and R D6 are each independently a hydrogen atom, an alkyl group, an alkoxyl group, a hydroxyl group, or a halogen atom. Of these, a hydrogen atom, an alkyl group, and a halogen atom are preferable.
- Z ⁇ represents, as described above, OH ⁇ , an anion represented by the general formula (D1-3): R D1 —COO — , and the general formula (D1-4): R An anion represented by D1— SO 3 — , or an anion represented by formula (D1-5): R D1 —N —— SO 2 —R D11 (provided that the formula (D1-3) to In (D1-5), R D1 represents an optionally substituted alkyl group, an alicyclic hydrocarbon group, or an aryl group, and R D11 represents an optionally substituted fluorinated alkyl group, an alicyclic group. A fluorinated hydrocarbon group or a fluorinated aryl group).
- Z ⁇ in the general formula (D1-0) is preferably an anion represented by the following formula (1a) (that is, in the anion represented by the general formula (D1-3), R D1 is a phenyl group.
- Anion) an anion represented by the following formula (1b) (ie, an anion represented by the general formula (D1-4), wherein R D1 is 1,7,7-trimethylbicyclo [2.2.1] heptane-
- an anion represented by the following formula (1c) that is, an anion represented by the general formula (D1-5)
- R D1 is a butyl group
- the photodegradable acid diffusion controller is represented by the above general formula (D1-0), and specifically, is a sulfonium salt compound or an iodonium salt compound that satisfies the above conditions.
- sulfonium salt compounds include triphenylsulfonium hydroxide, triphenylsulfonium acetate, triphenylsulfonium salicylate, diphenyl-4-hydroxyphenylsulfonium hydroxide, diphenyl-4-hydroxyphenylsulfonium acetate, diphenyl-4-hydroxyphenyl.
- sulfonium salicylate examples include sulfonium 10-camphor sulfonate, 4-tert-butoxyphenyl diphenylsulfonium 10-camphor sulfonate, and the like.
- these compounds may be used independently and may be used in combination of 2 or more.
- iodonium salt compound examples include bis (4-tert-butylphenyl) iodonium hydroxide, bis (4-tert-butylphenyl) iodonium acetate, bis (4-tert-butylphenyl) iodonium hydroxide, bis (4 -Tert-butylphenyl) iodonium acetate, bis (4-tert-butylphenyl) iodonium salicylate, 4-tert-butylphenyl-4-hydroxyphenyliodonium hydroxide, 4-tert-butylphenyl-4-hydroxyphenyliodonium Acetate, 4-tert-butylphenyl-4-hydroxyphenyliodonium salicylate, bis (4-tert-butylphenyl) iodonium 10-camphorsulfo Over DOO, such as diphenyl iodonium 10-camphorsulfonate, and the like.
- these compounds may be used
- a nitrogen-containing compound (I), a nitrogen-containing compound (II), a nitrogen-containing heterocyclic compound, and a photodegradable acid diffusion controller are preferable.
- the content thereof is preferably based on 100 parts by mass of the polymer [A]. Is 10 parts by mass or less, more preferably 5 parts by mass or less. However, the lower limit of the content is usually 0.01 parts by mass. When there is too much content of the said acid diffusion inhibitor, the sensitivity of a photoresist film may fall.
- Solvent is preferably a solvent that can dissolve at least the polymers [A] and [C] and the acid generator [B] to bring the composition of the present invention into a solution state.
- the solvent include linear or branched ketones, cyclic ketones, alkylene glycol monoalkyl ethers, alkylene glycol dialkyl ethers, alkylene glycol monoalkyl ether acetates, carboxylic acid esters, and hydroxyl groups. Examples thereof include carboxylic acid esters, carboxylic acid esters having an alkoxy group, alcohols, and aromatic hydrocarbons.
- these solvents may be used independently and may be used in combination of 2 or more type.
- the amount of the solvent used is such that the total solid concentration of the radiation-sensitive resin composition or the radiation-sensitive resin composition for immersion exposure of the present invention is preferably 1 to 50% by mass, more preferably 1 to 25% by mass.
- the amount is such that
- the composition of the present invention can be used as a composition solution by, for example, filtration using a filter having a pore size of about 0.2 ⁇ m.
- Lactone compound This lactone compound is a component that imparts the effect of segregating the contained polymer [C] to the surface layer of the photoresist film in order to efficiently develop water repellency on the surface of the photoresist film in immersion exposure. It is.
- the composition containing the lactone compound By using the composition containing the lactone compound, the elution of components from the photoresist film to the immersion exposure liquid is suppressed without impairing basic resist characteristics such as LWR, development defects, and pattern collapse resistance. be able to.
- basic resist characteristics such as LWR, development defects, and pattern collapse resistance.
- lactone compound imparting the above action examples include ⁇ -butyrolactone, valerolactone, mevalonic lactone, norbornane lactone and the like. These compounds may be used alone or in combination of two or more.
- the content thereof is usually 100 parts by mass of the polymer [A]. 30 to 200 parts by mass, preferably 50 to 150 parts by mass. If the content of the lactone compound is too large, the basic performance of the resist is lowered, and a pattern having a good shape may not be obtained.
- the radiation-sensitive resin composition for immersion exposure according to the present invention may contain another polymer such as the polymer [C] in addition to the polymer [A].
- the content of the other polymer is usually 0.1 to 20 parts by mass, preferably 1 to 10 parts by mass, more preferably 1 to 7.5 parts by mass with respect to 100 parts by mass of the polymer [A]. It is.
- the other polymer is the polymer [C]
- the content is too small, the effect of containing the polymer [C] may not be sufficient.
- the content of the polymer [C] is too large, the water repellency on the surface of the photoresist film becomes too high, and development failure may occur.
- the radiation-sensitive resin composition and the radiation-sensitive resin composition for immersion exposure of the present invention are particularly useful as a chemically amplified resist.
- the acid-dissociable group in the polymer component is dissociated by the action of the acid generated from the acid generator [B] by exposure to generate a carboxyl group.
- the exposed portion of the resist The solubility in the alkaline developer becomes higher, and the exposed portion is dissolved and removed by the alkaline developer to obtain a positive resist pattern.
- the resist pattern forming method of the present invention is a step of forming a photoresist film on a substrate using the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure (hereinafter referred to as “resist pattern forming method”). , “First step”), and a step of placing the immersion exposure liquid on the photoresist film and subjecting the photoresist film to immersion exposure via the immersion exposure liquid (hereinafter referred to as “second step”). And a step of developing a photoresist film subjected to immersion exposure to form a resist pattern (hereinafter referred to as “third step”).
- the first step is a step of forming a photoresist film on the substrate using the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure.
- the method for applying the composition include spin coating, cast coating, roll coating, and the like.
- the substrate include a silicon wafer and a wafer coated with aluminum.
- a specific example of the first step is a method of volatilizing the solvent in the coating film by applying the composition so that the obtained photoresist film has a predetermined thickness and then pre-baking (PB). . Thereby, a uniform photoresist film is formed.
- the thickness of the photoresist film is not particularly limited, but is usually 0.05 to 0.40 ⁇ m.
- the prebaking conditions are appropriately selected depending on the composition of the composition, but are preferably 30 ° C to 200 ° C, more preferably 50 ° C to 170 ° C. It is.
- an organic or inorganic antireflection film can also be formed on the substrate.
- a protective film can be provided on the photoresist film as disclosed in, for example, Japanese Patent Laid-Open No. 5-188598.
- an immersion protective film may be provided on the photoresist film as disclosed in, for example, JP-A-2005-352384. it can.
- the second step is a step in which an immersion exposure liquid is disposed on the photoresist film, and the photoresist film is subjected to immersion exposure via the immersion exposure liquid.
- the radiation illustrated below is usually used as exposure light through a photomask having a mask pattern for forming a desired pattern, and the surface of the photoresist film is irradiated with this radiation. .
- the radiation passes through the opening of the photomask, further passes through the exposure lens, and reaches the photoresist film.
- the exposed portion in the photoresist film is removed by the third step.
- the immersion exposure liquid pure water, a long chain or cyclic aliphatic compound, or the like can be used.
- radiation is used as the exposure light for immersion exposure.
- the radiation can be appropriately selected according to the type of the acid generator in the composition.
- visible light, ultraviolet light, far ultraviolet light, X-rays, charged particle beams, and the like can be used.
- far ultraviolet rays represented by ArF excimer laser (wavelength 193 nm) or KrF excimer laser (wavelength 248 nm) are preferable, and ArF excimer laser (wavelength 193 nm) is particularly preferable.
- exposure conditions such as an exposure amount, can be suitably selected according to the composition of the composition, the type when an additive is contained, and the like.
- a step of baking the exposed film (hereinafter referred to as “PEB”) may be provided as necessary.
- PEB heating conditions are appropriately selected depending on the composition of the composition, but are preferably 30 ° C. to 200 ° C., more preferably 50 ° C. to 170 ° C., from the viewpoint of facilitating the crosslinking reaction.
- the third step is a step of developing a photoresist film subjected to immersion exposure to form a resist pattern.
- this step by using the developer, the unexposed portion in the second step is removed, and a pattern reflecting the pattern of the exposed portion, that is, the opening of the photomask, is left and formed.
- an alkaline aqueous solution obtained by dissolving an alkaline compound in water is usually used.
- the alkaline compound include sodium hydroxide, potassium hydroxide, sodium carbonate, sodium silicate, sodium metasilicate, ammonia, ethylamine, n-propylamine, diethylamine, di-n-propylamine, triethylamine, methyldiethylamine, ethyl Dimethylamine, triethanolamine, tetramethylammonium hydroxide, pyrrole, piperidine, choline, 1,8-diazabicyclo- [5.4.0] -7-undecene, 1,5-diazabicyclo- [4.3.0] -5-nonene and the like.
- tetramethylammonium hydroxide is preferred.
- concentration of the alkaline compound is usually 10% by mass or less. If this concentration is too high, the exposed area may also be dissolved in the developer.
- the developer may be a solution containing only the alkaline compound or a composition containing an organic solvent, a surfactant, and the like.
- organic solvent include ketones such as acetone, methyl ethyl ketone, methyl isobutyl ketone, cyclopentanone, cyclohexanone, 3-methylcyclopentanone, and 2,6-dimethylcyclohexanone; methanol, ethanol, n-propyl alcohol, isopropyl alcohol, alcohols such as n-butyl alcohol, tert-butyl alcohol, cyclopentanol, cyclohexanol, 1,4-hexanediol, 1,4-hexanedimethylol; ethers such as tetrahydrofuran and dioxane; ethyl acetate, n-acetate Examples include esters such as butyl and isoamyl acetate; aromatic hydrocarbons such as tolu
- the content thereof is preferably 100 parts by volume or less with respect to 100 parts by volume of the alkaline aqueous solution.
- developability may fall and the image development residue of an unexposed part may increase.
- the pattern remaining on the substrate is usually washed with water and dried.
- the GPC measurement method of the polymer obtained by the following synthesis example is as follows.
- the weight average molecular weight (Mw) of the polymer is a GPC column (hereinafter referred to as model name) manufactured by Tosoh Corporation.
- Two "G2000HXL", one "G3000HXL” and one "G4000HXL” are connected to elute tetrahydrofuran.
- the measurement was performed by gel permeation chromatography (GPC) using monodisperse polystyrene as a standard under conditions of a flow rate of 1.0 mL / min and a column temperature of 40 ° C. as a solvent.
- the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours. After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Next, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 80.1 g, yield 80%).
- Synthesis Example 2 (Synthesis of polymer (A-2)) Compound (S1-1) 37.39 g (45 mol%), compound (S1-2) 12.26 g (10 mol%), compound (S1-4) 6.45 g (5 mol%) and compound (S1- 3) 43.90 g (40 mol%) was dissolved in 100 g of 2-butanone, and then 4.05 g of 2,2′-azobisisobutyronitrile was added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes. After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C.
- the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours. After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Then, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 71 g, yield 71%).
- the copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 45: 9: 5: 41, respectively. This is referred to as a polymer (A-2). Mw of this polymer (A-2) was 7,300, and the fluorine atom content was 1.4%.
- Synthesis Example 3 (Synthesis of polymer (A-3)) Compound (S1-1) 32.49 g (40 mol%), Compound (S1-2) 11.99 g (10 mol%), Compound (S1-4) 12.61 g (10 mol%) and Compound (S1- 3) 42.91 g (40 mol%) was dissolved in 100 g of 2-butanone, and further 3.96 g of 2,2′-azobisisobutyronitrile was added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
- the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 70 g, yield 70%).
- the copolymer was analyzed by 13 C-NMR.
- the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 39: 11: 10: 40, respectively.
- Mw of this polymer (A-3) was 7,100, and the fluorine atom content was 2.8%.
- Synthesis Example 4 (Synthesis of polymer (A-4)) Compound (S1-1) 22.90 g (30 mol%), Compound (S1-2) 11.27 g (10 mol%), Compound (S1-4) 35.57 g (30 mol%) and Compound (S1- 3) 30.26 g (30 mol%) was dissolved in 100 g of 2-butanone, and 3.73 g of 2,2′-azobisisobutyronitrile was further added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
- the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 69 g, yield 69%).
- the copolymer was analyzed by 13 C-NMR.
- the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 30: 10: 29: 31, respectively.
- Mw of this polymer (A-4) was 6,800, and the fluorine atom content was 7.8%.
- Synthesis Example 5 (Synthesis of polymer (A-5)) Compound (S1-1) 31.27 g (40 mol%), compound (S1-2) 11.54 g (10 mol%), compound (S1-5) 15.87 g (10 mol%) and compound (S1- 3) 41.31 g (40 mol%) was dissolved in 100 g of 2-butanone, and 3.82 g of 2,2′-azobisisobutyronitrile was further added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
- Synthesis Example 6 (Synthesis of polymer (C-1)) Compound (S3-1) 14.5 g (20 mol%) and compound (S3-2) 84.6 g (80 mol%) shown below are dissolved in 2-butanone 150 g, and 2,2′-azobis is further dissolved. A monomer solution was prepared by adding 3.47 g of isobutyronitrile. Meanwhile, the inside of the 500 mL three-necked flask was purged with nitrogen gas for 30 minutes. After purging with nitrogen, the inside of the three-necked flask was heated to 80 ° C. while stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel.
- the mixture was further stirred at 80 ° C. for 3 hours.
- the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 600 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice with 300 g of methanol as a slurry, and then filtered. Next, the white powder was dried at 50 ° C. for 17 hours to obtain a colorless solid copolymer (yield 72 g, yield 72%).
- the copolymer was analyzed by 13 C-NMR, whereby the content (mol%) of repeating units derived from the compound (S3-1) and the compound (S3-2) was 21:79, respectively. This is referred to as a polymer (C-1).
- This polymer (C-1) had an Mw of 6,400 and a fluorine atom content of 12.8%.
- Example 1 100 parts of polymer (A-2), 12 parts of acid generator (B-1), 5 parts of polymer (C-1), 0.8 part of acid diffusion inhibitor, solvent (F-1) 1,980 parts and 848 parts of solvent (F-2) were mixed to obtain a uniform solution. Then, the radiation sensitive resin composition (composition solution) was manufactured by filtering using a membrane filter with a pore diameter of 200 nm (see Table 1).
- Examples 2 to 6 and Comparative Example 1 A radiation-sensitive resin composition (composition solution) was produced in the same manner as in Example 1 except that the raw material components were used according to the formulation described in Table 1 (see Table 1).
- the radiation sensitive resin composition was spin-coated using a semiconductor manufacturing apparatus (model name “CLEAN TRACK ACT12”, manufactured by Tokyo Electron Ltd.). Then, PB (110 ° C., 60 seconds) and cooling (23 ° C., 30 seconds) were performed to form a 100 nm-thick photoresist layer.
- the silicon wafer for evaluation on which a resist pattern (line and space pattern) was formed was obtained by spin-drying by shaking off at 2000 rpm for 15 seconds.
- the exposure amount for forming a pattern of 48 nm line / 96 nm pitch in the mask size with a target size of 48 nm line / 96 nm pitch was determined as the optimum exposure amount.
- Comparative Example 1 is an example using a radiation-sensitive resin composition containing a polymer that does not contain a repeating unit represented by the general formula (1) according to the present invention. Is as high as 4.7, which is not preferable. On the other hand, the radiation sensitive resin compositions of Examples 1 to 6 were excellent with an LWR of less than 4.
- a chemically amplified resist capable of maintaining a good line width roughness (LWR) while maintaining a wide exposure margin can be obtained.
- LWR line width roughness
- X-rays such as far-ultraviolet rays, synchrotron radiation, and other charged particle beams such as electron beams, such as KrF excimer laser and ArF excimer laser. Is preferred.
- electron beams such as KrF excimer laser and ArF excimer laser.
- it is very useful for the manufacture of a semiconductor device which is expected to be further miniaturized in the future.
Landscapes
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- General Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Materials For Photolithography (AREA)
Abstract
Provided are a radiation-sensitive resin composition, from which a chemically amplified resist showing reduced line width roughness and ensuring the formation of a desired pattern shape at a high accuracy can be obtained, and a method for forming a resist pattern using the same. The radiation-sensitive resin composition comprises a polymer [A] containing a repeating unit represented by general formula (1) and having an acid-dissociating group, a radiation-sensitive acid generator [B], and a fluorine-containing polymer [C], wherein the ratio of fluorine atoms contained in polymer [A] is smaller than the ratio of fluorine atoms contained in polymer [C].
In general formula (1), R1 represents a hydrogen atom, a trifluoromethyl group or a C1-4 alkyl group; R2 represents a C1-20 hydrocarbon group or a C1-20 fluorohydrocarbon group; and Q1 represents a divalent linking group.
Description
本発明は、ライン幅のばらつきの発生を抑制して、所望形状のパターンを精度良く形成することのできる化学増幅型レジストを与える感放射線性樹脂組成物及びそれを用いたレジストパターン形成方法に関する。
The present invention relates to a radiation-sensitive resin composition that provides a chemically amplified resist capable of accurately forming a pattern having a desired shape while suppressing the occurrence of variations in line width, and a resist pattern forming method using the same.
集積回路素子を製造する微細加工の分野においては、より高い集積度を得るために、0.10μm以下のレベルでの微細加工が可能なリソグラフィ技術が切望されている。しかし、従来のリソグラフィ技術では、露光光としてi線等の近紫外線を用いており、この近紫外線では、0.10μm以下のレベル(サブクオーターミクロンレベル)の微細加工は極めて困難である。そこで、0.10μm以下のレベルでの微細加工を可能にするために、より波長の短い放射線を使用したリソグラフィ技術の開発が行われている。より波長の短い放射線としては、例えば、水銀灯の輝線スペクトル、エキシマレーザー等の遠紫外線、X線、電子線等を挙げることができる。これらの中でも、KrFエキシマレーザー(波長248nm)やArFエキシマレーザー(波長193nm)が注目されている。
In the field of microfabrication for manufacturing integrated circuit elements, lithography technology capable of microfabrication at a level of 0.10 μm or less is eagerly desired in order to obtain a higher degree of integration. However, in the conventional lithography technique, near ultraviolet rays such as i-line are used as exposure light, and it is extremely difficult to perform fine processing at a level of 0.10 μm or less (sub quarter micron level) with this near ultraviolet rays. Therefore, in order to enable microfabrication at a level of 0.10 μm or less, development of a lithography technique using radiation having a shorter wavelength is being performed. Examples of radiation having a shorter wavelength include an emission line spectrum of a mercury lamp, far ultraviolet rays such as an excimer laser, an X-ray, and an electron beam. Among these, KrF excimer laser (wavelength 248 nm) and ArF excimer laser (wavelength 193 nm) are attracting attention.
エキシマレーザーが注目されたことに伴い、エキシマレーザー用のフォトレジスト膜の材料が数多く提案されている。例えば、酸解離性官能基を有する成分と、放射線の照射(以下、「露光」という)により酸を発生する成分(以下、「酸発生剤」という)と、を含有し、これらの化学増幅効果を利用した組成物(以下、「化学増幅型レジスト」という)等を挙げることができる。化学増幅型レジストとして、具体的には、カルボン酸のtert-ブチルエステル基あるいはフェノールのtert-ブチルカーボナート基を有する樹脂と酸発生剤とを含有する組成物が報告されている。この組成物は、露光により発生する酸の作用により、樹脂中に存在するtert-ブチルエステル基あるいはtert-ブチルカーボナート基が解離して、樹脂が、カルボキシル基あるいはフェノール性水酸基からなる酸性基を有するようになる。その結果、フォトレジスト膜の露光部がアルカリ現像液に易溶性となるため、所望のレジストパターンを形成することができる。
Along with the attention of excimer lasers, many materials for photoresist films for excimer lasers have been proposed. For example, it contains a component having an acid-dissociable functional group and a component that generates an acid upon irradiation with radiation (hereinafter referred to as “exposure”) (hereinafter referred to as “acid generator”), and these chemical amplification effects And the like (hereinafter referred to as “chemically amplified resist”). As a chemically amplified resist, specifically, a composition containing a resin having a tert-butyl ester group of carboxylic acid or a tert-butyl carbonate group of phenol and an acid generator has been reported. In this composition, the tert-butyl ester group or tert-butyl carbonate group present in the resin is dissociated by the action of an acid generated by exposure, and the resin has an acidic group composed of a carboxyl group or a phenolic hydroxyl group. To have. As a result, the exposed portion of the photoresist film becomes readily soluble in an alkali developer, so that a desired resist pattern can be formed.
しかしながら、微細加工の分野においては、更に微細なレジストパターン(例えば、線幅が45nm程度の微細なレジストパターン)を形成することが切望されている。更に微細なレジストパターンを形成可能にするためには、例えば、露光装置の光源波長の短波長化や、レンズの開口数(NA)を増大させること等を挙げることができる。しかし、光源波長の短波長化には、新たな露光装置が必要になるが、このような装置は高額なものである。また、レンズの開口数を増大させる場合、解像度と焦点深度がトレードオフの関係にあるため、解像度を向上させることができても、焦点深度が低下するという問題がある。
However, in the field of microfabrication, it is desired to form a finer resist pattern (for example, a fine resist pattern having a line width of about 45 nm). In order to make it possible to form a finer resist pattern, for example, the light source wavelength of the exposure apparatus can be shortened, the numerical aperture (NA) of the lens can be increased, and the like. However, shortening the light source wavelength requires a new exposure apparatus, but such an apparatus is expensive. Further, when the numerical aperture of the lens is increased, the resolution and the depth of focus are in a trade-off relationship. Therefore, there is a problem that the depth of focus is lowered even if the resolution can be improved.
そこで、近年、このような問題を解決するリソグラフィ技術として、液浸露光(リキッドイマージョンリソグラフィ)法という方法が報告されている。この方法は、露光時に、レンズとフォトレジスト膜との間(フォトレジスト膜上)に液浸露光用液体(例えば、純水、フッ素系不活性液体等)を介在させるという方法である。この方法によれば、従来、空気や窒素等の不活性ガスで満たされていた露光光路空間を、空気等よりも屈折率(n)の大きい液浸露光用液体で満たすことになるため、従来と同様の露光光源を用いた場合であっても、露光装置の光源波長を短波長化等した場合と同様の効果、即ち、高い解像性が得られる。また、焦点深度の低下がない。
Therefore, in recent years, a liquid immersion lithography (liquid immersion lithography) method has been reported as a lithography technique for solving such problems. This method is a method in which a liquid for immersion exposure (for example, pure water, fluorine-based inert liquid, or the like) is interposed between the lens and the photoresist film (on the photoresist film) at the time of exposure. According to this method, the exposure optical path space that has been conventionally filled with an inert gas such as air or nitrogen is filled with an immersion exposure liquid having a refractive index (n) larger than that of air or the like. Even when the same exposure light source is used, the same effect as when the light source wavelength of the exposure apparatus is shortened, that is, high resolution can be obtained. Moreover, there is no reduction in the depth of focus.
従って、このような液浸露光法によれば、既存の装置に実装されているレンズを用いて、低コストで、解像性に優れ、更には焦点深度にも優れるレジストパターンを形成することができる。そのため、液浸露光法に用いられる感放射線性樹脂組成物としてフッ素系ポリマーを添加したものが開示されている(例えば、特許文献1~3参照)。
Therefore, according to such an immersion exposure method, it is possible to form a resist pattern that is low in cost, excellent in resolution, and also excellent in depth of focus, using a lens mounted on an existing apparatus. it can. Therefore, a radiation-sensitive resin composition used in the immersion exposure method is disclosed in which a fluorine-based polymer is added (see, for example, Patent Documents 1 to 3).
半導体分野において、より高い集積度が求められるなか、より優れた感度や広い露光マージンを有するだけでなく、ライン幅の粗さ(LWR:Line Width Roughness)を小さく形成することができる感放射線性樹脂組成物が必要となってきている。
In the semiconductor field, a higher degree of integration is required. In addition to superior sensitivity and a wide exposure margin, a radiation-sensitive resin that can be formed with a small line width roughness (LWR: Line Width Roughness). Compositions are becoming necessary.
本発明は、ライン幅のばらつきの発生を抑制して、所望形状のパターンを精度良く形成することのできる化学増幅型レジストを与える感放射線性樹脂組成物及びそれを用いたレジストパターン形成方法を提供することを目的とする。
The present invention provides a radiation-sensitive resin composition that provides a chemically amplified resist capable of accurately forming a pattern having a desired shape while suppressing the occurrence of variations in line width, and a resist pattern forming method using the same. The purpose is to do.
本発明は、以下のとおりである。
1.[A]下記一般式(1)で表される繰り返し単位を含み、酸解離性基を有する重合体、[B]感放射線性酸発生剤、及び、[C]フッ素原子を含む重合体を含有し、上記重合体[A]に含まれるフッ素原子の含有率が、上記重合体[C]に含まれるフッ素原子の含有率よりも少ないことを特徴とする感放射線性樹脂組成物。
(式中、R1は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R2は、炭素数1~20の炭化水素基又は炭素数1~20のフッ素化炭化水素基であり、Q1は、2価の連結基である。)
2.上記重合体[A]を構成する繰り返し単位の全量を100モル%としたときに、上記一般式(1)で表される繰り返し単位の含有率が5~50モル%である上記1に記載の感放射線性樹脂組成物。
3.上記重合体[A]が、更に、下記一般式(2)で表される繰り返し単位を含む上記1又は2に記載の感放射線性樹脂組成物。
(式中、R3は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R4は、相互に独立に、炭素数1~4の直鎖状若しくは分岐状のアルキル基、又は、炭素数4~20の1価の脂環式炭化水素基である。また、2つのR4が相互に結合して形成された、2価であって、炭素数4~20の脂環式炭化水素基又はその誘導体基であってもよい。)
4.上記重合体[A]が、更に、下記一般式(3-1)~(3-6)で表される繰り返し単位からなる群より選択された少なくとも1つの繰り返し単位を含む上記1乃至3のいずれかに記載の感放射線性樹脂組成物。
(式中、R5は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R6は、水素原子又は炭素数1~4の置換若しくは非置換のアルキル基であり、R7は、単結合又はメチレン基であり、R8は、水素原子又はメトキシ基であり、R9は、酸素原子又はメチレン基であり、pは1、2又は3であり、mは0又は1である。)
5.上記重合体[C]が、下記一般式(c1-1)~(c1-3)で表される繰り返し単位からなる群より選択された少なくとも1つの繰り返し単位を含む上記1乃至4のいずれかに記載の感放射線性樹脂組成物。
(式中、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R22は、炭素数1~30のフッ素化炭化水素基であり、R23は、相互に独立に、水素原子、フッ素原子又は炭素数1~30のフッ素化炭化水素基であり、R24は、水素原子、酸解離性基又はアルカリ解離性基であり、Q3は、(g+1)価の連結基であり、Q4は、2価の連結基であり、gは、1、2又は3である。但し、式(c1-2)及び(c1-3)において、全てのR23が水素原子である場合はない。)
6.[A]下記一般式(1)で表される繰り返し単位を含み、酸解離性基を有する重合体、及び、[B]感放射線性酸発生剤を含有することを特徴とする液浸露光用感放射線性樹脂組成物。
(式中、R1は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R2は、炭素数1~20の炭化水素基又は炭素数1~20のフッ素化炭化水素基であり、Q1は、2価の連結基である。)
7.上記1乃至6のいずれかに記載の感放射線性樹脂組成物を用いて、基板上にフォトレジスト膜を形成する工程と、
上記フォトレジスト膜上に液浸露光用液体を配置し、上記液浸露光用液体を介して上記フォトレジスト膜を液浸露光する工程と、
液浸露光された上記フォトレジスト膜を現像してレジストパターンを形成する工程と、
を備えることを特徴とするレジストパターン形成方法。 The present invention is as follows.
1. [A] A polymer containing a repeating unit represented by the following general formula (1) and having an acid-dissociable group, [B] a radiation-sensitive acid generator, and [C] a polymer containing a fluorine atom And the content rate of the fluorine atom contained in the said polymer [A] is less than the content rate of the fluorine atom contained in the said polymer [C], The radiation sensitive resin composition characterized by the above-mentioned.
(Wherein R 1 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated carbon atom having 1 to 20 carbon atoms) A hydrogen group, and Q 1 is a divalent linking group.)
2. 2. The content of the repeating unit represented by the general formula (1) is 5 to 50 mol% when the total amount of the repeating units constituting the polymer [A] is 100 mol%. Radiation sensitive resin composition.
3. 3. The radiation sensitive resin composition according to 1 or 2 above, wherein the polymer [A] further contains a repeating unit represented by the following general formula (2).
(Wherein R 3 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 4 is independently a linear or branched alkyl group having 1 to 4 carbon atoms. Or a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms, or a divalent formed by bonding two R 4 to each other and having 4 to 20 carbon atoms. (It may be an alicyclic hydrocarbon group or a derivative group thereof.)
4). Any of 1 to 3 above, wherein the polymer [A] further contains at least one repeating unit selected from the group consisting of repeating units represented by the following general formulas (3-1) to (3-6): A radiation-sensitive resin composition according to claim 1.
(Wherein R 5 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 6 is a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms, R 7 is a single bond or a methylene group, R 8 is a hydrogen atom or a methoxy group, R 9 is an oxygen atom or a methylene group, p is 1, 2 or 3, and m is 0 or 1)
5. Any one of the above 1 to 4 wherein the polymer [C] contains at least one repeating unit selected from the group consisting of repeating units represented by the following general formulas (c1-1) to (c1-3) The radiation sensitive resin composition as described.
(Wherein R 21 represents a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 22 represents a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and R 23 represents Independently a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms, R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group, and Q 3 is (g + 1) Q 4 is a divalent linking group and g is 1, 2 or 3. provided that in formulas (c1-2) and (c1-3), all R 23 Is not a hydrogen atom.)
6). [A] For immersion exposure, comprising a polymer having a repeating unit represented by the following general formula (1) and having an acid-dissociable group, and [B] a radiation-sensitive acid generator Radiation sensitive resin composition.
(Wherein R 1 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated carbon atom having 1 to 20 carbon atoms) A hydrogen group, and Q 1 is a divalent linking group.)
7). Using the radiation-sensitive resin composition according to any one of 1 to 6 above, a step of forming a photoresist film on a substrate;
Placing a liquid for immersion exposure on the photoresist film, and subjecting the photoresist film to immersion exposure via the liquid for immersion exposure;
Developing the photoresist film exposed to immersion and forming a resist pattern; and
A resist pattern forming method comprising:
1.[A]下記一般式(1)で表される繰り返し単位を含み、酸解離性基を有する重合体、[B]感放射線性酸発生剤、及び、[C]フッ素原子を含む重合体を含有し、上記重合体[A]に含まれるフッ素原子の含有率が、上記重合体[C]に含まれるフッ素原子の含有率よりも少ないことを特徴とする感放射線性樹脂組成物。
2.上記重合体[A]を構成する繰り返し単位の全量を100モル%としたときに、上記一般式(1)で表される繰り返し単位の含有率が5~50モル%である上記1に記載の感放射線性樹脂組成物。
3.上記重合体[A]が、更に、下記一般式(2)で表される繰り返し単位を含む上記1又は2に記載の感放射線性樹脂組成物。
4.上記重合体[A]が、更に、下記一般式(3-1)~(3-6)で表される繰り返し単位からなる群より選択された少なくとも1つの繰り返し単位を含む上記1乃至3のいずれかに記載の感放射線性樹脂組成物。
5.上記重合体[C]が、下記一般式(c1-1)~(c1-3)で表される繰り返し単位からなる群より選択された少なくとも1つの繰り返し単位を含む上記1乃至4のいずれかに記載の感放射線性樹脂組成物。
6.[A]下記一般式(1)で表される繰り返し単位を含み、酸解離性基を有する重合体、及び、[B]感放射線性酸発生剤を含有することを特徴とする液浸露光用感放射線性樹脂組成物。
7.上記1乃至6のいずれかに記載の感放射線性樹脂組成物を用いて、基板上にフォトレジスト膜を形成する工程と、
上記フォトレジスト膜上に液浸露光用液体を配置し、上記液浸露光用液体を介して上記フォトレジスト膜を液浸露光する工程と、
液浸露光された上記フォトレジスト膜を現像してレジストパターンを形成する工程と、
を備えることを特徴とするレジストパターン形成方法。 The present invention is as follows.
1. [A] A polymer containing a repeating unit represented by the following general formula (1) and having an acid-dissociable group, [B] a radiation-sensitive acid generator, and [C] a polymer containing a fluorine atom And the content rate of the fluorine atom contained in the said polymer [A] is less than the content rate of the fluorine atom contained in the said polymer [C], The radiation sensitive resin composition characterized by the above-mentioned.
2. 2. The content of the repeating unit represented by the general formula (1) is 5 to 50 mol% when the total amount of the repeating units constituting the polymer [A] is 100 mol%. Radiation sensitive resin composition.
3. 3. The radiation sensitive resin composition according to 1 or 2 above, wherein the polymer [A] further contains a repeating unit represented by the following general formula (2).
4). Any of 1 to 3 above, wherein the polymer [A] further contains at least one repeating unit selected from the group consisting of repeating units represented by the following general formulas (3-1) to (3-6): A radiation-sensitive resin composition according to claim 1.
5. Any one of the above 1 to 4 wherein the polymer [C] contains at least one repeating unit selected from the group consisting of repeating units represented by the following general formulas (c1-1) to (c1-3) The radiation sensitive resin composition as described.
6). [A] For immersion exposure, comprising a polymer having a repeating unit represented by the following general formula (1) and having an acid-dissociable group, and [B] a radiation-sensitive acid generator Radiation sensitive resin composition.
7). Using the radiation-sensitive resin composition according to any one of 1 to 6 above, a step of forming a photoresist film on a substrate;
Placing a liquid for immersion exposure on the photoresist film, and subjecting the photoresist film to immersion exposure via the liquid for immersion exposure;
Developing the photoresist film exposed to immersion and forming a resist pattern; and
A resist pattern forming method comprising:
本発明の感放射線性樹脂組成物及び液浸露光用感放射線性樹脂組成物によれば、広い露光マージンを維持したまま、LWRを良好に維持することが可能な化学増幅型レジストを与えることができる。
また、本発明のパターン形成方法によれば、用いる化学増幅型レジストの現像性に優れ、ライン幅のばらつきの発生を抑制して、所望形状のパターンを精度良く形成することができる。 According to the radiation-sensitive resin composition and the radiation-sensitive resin composition for immersion exposure of the present invention, it is possible to provide a chemically amplified resist capable of maintaining a good LWR while maintaining a wide exposure margin. it can.
Further, according to the pattern forming method of the present invention, the chemically amplified resist to be used is excellent in developability, and it is possible to accurately form a pattern having a desired shape while suppressing the occurrence of variations in line width.
また、本発明のパターン形成方法によれば、用いる化学増幅型レジストの現像性に優れ、ライン幅のばらつきの発生を抑制して、所望形状のパターンを精度良く形成することができる。 According to the radiation-sensitive resin composition and the radiation-sensitive resin composition for immersion exposure of the present invention, it is possible to provide a chemically amplified resist capable of maintaining a good LWR while maintaining a wide exposure margin. it can.
Further, according to the pattern forming method of the present invention, the chemically amplified resist to be used is excellent in developability, and it is possible to accurately form a pattern having a desired shape while suppressing the occurrence of variations in line width.
以下、本発明について、詳細に説明する。尚、下記記載において、「(メタ)アクリル」は、アクリル及びメタクリルを意味する。
Hereinafter, the present invention will be described in detail. In the following description, “(meth) acryl” means acryl and methacryl.
1.感放射線性樹脂組成物
本発明の感放射線性樹脂組成物は、[A]下記一般式(1)で表される繰り返し単位(以下、「繰り返し単位(I-1)」という。)を含み、酸解離性基を有する重合体(以下、「重合体[A]」という。)、[B]感放射線性酸発生剤(以下、「酸発生剤[B]」という。)、及び、[C]フッ素原子を含む重合体(以下、「重合体[C]」という。)を含有し、重合体[A]に含まれるフッ素原子の含有率が、重合体[C]に含まれるフッ素原子の含有率よりも少ない。尚、上記重合体[A]及び[C]におけるフッ素原子の含有率は、13C-NMRにより測定することができる。
ここで、「酸解離性基」は、アルカリ可溶性部位が保護基で保護された状態になっている基であり、酸で保護基が脱離されるまでは「アルカリ可溶性」ではない基を意味する。
(式中、R1は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R2は、炭素数1~20の炭化水素基又は炭素数1~20のフッ素化炭化水素基であり、Q1は、2価の連結基である。)
1. Radiation-sensitive resin composition The radiation-sensitive resin composition of the present invention includes [A] a repeating unit represented by the following general formula (1) (hereinafter referred to as “repeating unit (I-1)”). A polymer having an acid dissociable group (hereinafter referred to as “polymer [A]”), [B] a radiation-sensitive acid generator (hereinafter referred to as “acid generator [B]”), and [C ] A polymer containing a fluorine atom (hereinafter referred to as “polymer [C]”), and the content of fluorine atom contained in the polymer [A] is the same as that of the fluorine atom contained in the polymer [C]. Less than the content rate. The fluorine atom content in the polymers [A] and [C] can be measured by 13 C-NMR.
Here, the “acid-dissociable group” is a group in which an alkali-soluble site is protected with a protecting group, and means a group that is not “alkali-soluble” until the protecting group is removed with an acid. .
(Wherein R 1 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated carbon atom having 1 to 20 carbon atoms) A hydrogen group, and Q 1 is a divalent linking group.)
本発明の感放射線性樹脂組成物は、[A]下記一般式(1)で表される繰り返し単位(以下、「繰り返し単位(I-1)」という。)を含み、酸解離性基を有する重合体(以下、「重合体[A]」という。)、[B]感放射線性酸発生剤(以下、「酸発生剤[B]」という。)、及び、[C]フッ素原子を含む重合体(以下、「重合体[C]」という。)を含有し、重合体[A]に含まれるフッ素原子の含有率が、重合体[C]に含まれるフッ素原子の含有率よりも少ない。尚、上記重合体[A]及び[C]におけるフッ素原子の含有率は、13C-NMRにより測定することができる。
ここで、「酸解離性基」は、アルカリ可溶性部位が保護基で保護された状態になっている基であり、酸で保護基が脱離されるまでは「アルカリ可溶性」ではない基を意味する。
Here, the “acid-dissociable group” is a group in which an alkali-soluble site is protected with a protecting group, and means a group that is not “alkali-soluble” until the protecting group is removed with an acid. .
また、他の本発明の液浸露光用感放射線性樹脂組成物は、上記の重合体[A]及び酸発生剤[B]を含有する組成物である。
Further, another radiation-sensitive resin composition for immersion exposure according to the present invention is a composition containing the polymer [A] and the acid generator [B].
1-1.重合体[A]
この重合体[A]は、上記一般式(1)で表される繰り返し単位(I-1)を含み、酸解離性基を有する重合体であり、好ましくは、酸の作用によりアルカリ可溶性となるアルカリ不溶性又はアルカリ難溶性の重合体である。ここで、「アルカリ不溶性又はアルカリ難溶性」とは、本発明の組成物を用いて形成されたフォトレジスト膜から、レジストパターンを形成する際に採用されるアルカリ現像条件下で、このフォトレジスト膜の代わりに重合体[A]のみを用いて形成した、厚さ100nmの被膜を現像した場合に、この被膜の初期膜厚の50%以上が現像後に残存する性質を意味する。
上記重合体[A]に含まれる酸解離性基は、上記繰り返し単位(I-1)に含まれてよいし、必要により、この重合体に含まれる他の繰り返し単位に含まれてもよい。本発明においては、この重合体[A]は、後述するように、他の繰り返し単位を更に含む共重合体であることが好ましい。 1-1. Polymer [A]
This polymer [A] is a polymer containing the repeating unit (I-1) represented by the above general formula (1) and having an acid dissociable group, and preferably becomes alkali-soluble by the action of an acid. It is an alkali-insoluble or hardly alkali-soluble polymer. Here, “alkali insoluble or hardly soluble in alkali” means that the photoresist film under the alkali development conditions employed when forming a resist pattern from a photoresist film formed using the composition of the present invention. When a film having a thickness of 100 nm formed using only the polymer [A] instead of is developed, it means that 50% or more of the initial film thickness of this film remains after development.
The acid dissociable group contained in the polymer [A] may be contained in the repeating unit (I-1), and may be contained in other repeating units contained in the polymer, if necessary. In the present invention, the polymer [A] is preferably a copolymer further containing other repeating units as described later.
この重合体[A]は、上記一般式(1)で表される繰り返し単位(I-1)を含み、酸解離性基を有する重合体であり、好ましくは、酸の作用によりアルカリ可溶性となるアルカリ不溶性又はアルカリ難溶性の重合体である。ここで、「アルカリ不溶性又はアルカリ難溶性」とは、本発明の組成物を用いて形成されたフォトレジスト膜から、レジストパターンを形成する際に採用されるアルカリ現像条件下で、このフォトレジスト膜の代わりに重合体[A]のみを用いて形成した、厚さ100nmの被膜を現像した場合に、この被膜の初期膜厚の50%以上が現像後に残存する性質を意味する。
上記重合体[A]に含まれる酸解離性基は、上記繰り返し単位(I-1)に含まれてよいし、必要により、この重合体に含まれる他の繰り返し単位に含まれてもよい。本発明においては、この重合体[A]は、後述するように、他の繰り返し単位を更に含む共重合体であることが好ましい。 1-1. Polymer [A]
This polymer [A] is a polymer containing the repeating unit (I-1) represented by the above general formula (1) and having an acid dissociable group, and preferably becomes alkali-soluble by the action of an acid. It is an alkali-insoluble or hardly alkali-soluble polymer. Here, “alkali insoluble or hardly soluble in alkali” means that the photoresist film under the alkali development conditions employed when forming a resist pattern from a photoresist film formed using the composition of the present invention. When a film having a thickness of 100 nm formed using only the polymer [A] instead of is developed, it means that 50% or more of the initial film thickness of this film remains after development.
The acid dissociable group contained in the polymer [A] may be contained in the repeating unit (I-1), and may be contained in other repeating units contained in the polymer, if necessary. In the present invention, the polymer [A] is preferably a copolymer further containing other repeating units as described later.
1-1-1.繰り返し単位(I-1)
上記一般式(1)において、R1は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基である。炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。R1としては、水素原子及びメチル基が好ましい。 1-1-1. Repeating unit (I-1)
In the general formula (1), R 1 is a hydrogen atom, a trifluoromethyl group, or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. . R 1 is preferably a hydrogen atom or a methyl group.
上記一般式(1)において、R1は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基である。炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。R1としては、水素原子及びメチル基が好ましい。 1-1-1. Repeating unit (I-1)
In the general formula (1), R 1 is a hydrogen atom, a trifluoromethyl group, or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. . R 1 is preferably a hydrogen atom or a methyl group.
また、上記一般式(1)において、R2は、炭素数1~20の炭化水素基又は炭素数1~20のフッ素化炭化水素基であり、炭素数1~20のアルキル基、炭素数1~20のフッ素化アルキル基、炭素数3~20のシクロアルキル基、炭素数3~20のフッ素化シクロアルキル基等が挙げられる。
炭素数1~20のアルキル基の具体例としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、sec-ブチル基、tert-ブチル基、n-ペンチル基、n-ヘキシル基、n-ヘプチル基、オクチル基等が挙げられる。
炭素数1~20のフッ素化アルキル基の具体例としては、ジフルオロメチル基、パーフルオロメチル基、2,2-ジフルオロエチル基、2,2,2-トリフルオロエチル基、パーフルオロエチル基、2,2,3,3-テトラフルオロプロピル基、パーフルオロエチルメチル基、パーフルオロプロピル基、2,2,3,3,4,4―ヘキサフルオロブチル基、パーフルオロブチル基、1,1-ジメチル-2,2,3,3―テトラフルオロプロピル基、1,1-ジメチル-2,2,3,3,3-ペンタフルオロプロピル基、2-(パーフルオロプロピル)エチル基、2,2,3,3,4,4,5,5-オクタフルオロペンチル基、パーフルオロペンチル基、1,1-ジメチル-2,2,3,3,4,4-ヘキサフルオロブチル基、2-(パーフルオロブチル)エチル基、2,2,3,3,4,4,5,5,6,6-デカフルオロヘキシル基、パーフルオロペンチルメチル基、パーフルオロヘキシル基、1,1-ジメチル-2,2,3,3,4,4,5,5-オクタフルオロペンチル基、1,1-ジメチル-2,2,3,3,4,4,5,5,5-ノナフルオロペンチル基、2-(パーフルオロペンチル)エチル基、2,2,3,3,4,4,5,5,6,6,7,7-ドデカフルオロヘプチル基、パーフルオロヘキシルメチル基、パーフルオロヘプチル基、2-(パーフルオロヘキシル)エチル基、2,2,3,3,4,4,5,5,6,6,7,7,8,8-テトラデカフルオロオクチル基、パーフルオロヘプチルメチル基、パーフルオロオクチル基、2-(パーフルオロヘプチル)エチル基、2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-ヘキサデカフルオロノニル基、パーフルオロオクチルメチル基、パーフルオロノニル基、2-(パーフルオロオクチル)エチル基、2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10-オクタデカフルオロデシル基、パーフルオロノニルメチル基、パーフルオロデシル基、2,2,3,4,4,4-ヘキサフルオロブチル基、2,2,3,3,4,4,4-ヘプタフルオロブチル基、3,3,4,4,5,5,6,6,6-ノナフルオロヘキシル基、3,3,4,4,5,5,6,6,7,7,8,8,8-トリデカフルオロオクチル基等が挙げられる。これらのうち、パーフルオロメチル基、パーフルオロエチル基、パーフルオロプロピル基、パーフルオロブチル基及びパーフルオロオクチル基が好ましい。
炭素数3~20のシクロアルキル基の具体例としては、シクロプロピル基、シクロブチル基、シクロペンチル基、シクロへキシル基等が挙げられる。
また、炭素数3~20のフッ素化シクロアルキル基の具体例としては、上記シクロアルキル基の水素原子の少なくとも1つがフッ素原子に置換されてなるものとすることができる。 In the general formula (1), R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated hydrocarbon group having 1 to 20 carbon atoms, an alkyl group having 1 to 20 carbon atoms, a carbon number of 1 -20 fluorinated alkyl group, cycloalkyl group having 3-20 carbon atoms, fluorinated cycloalkyl group having 3-20 carbon atoms, and the like.
Specific examples of the alkyl group having 1 to 20 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, sec-butyl group, tert-butyl group, n-pentyl group, n- Examples include hexyl group, n-heptyl group, octyl group and the like.
Specific examples of the fluorinated alkyl group having 1 to 20 carbon atoms include a difluoromethyl group, a perfluoromethyl group, a 2,2-difluoroethyl group, a 2,2,2-trifluoroethyl group, a perfluoroethyl group, 2 , 2,3,3-tetrafluoropropyl group, perfluoroethylmethyl group, perfluoropropyl group, 2,2,3,3,4,4-hexafluorobutyl group, perfluorobutyl group, 1,1-dimethyl -2,2,3,3-tetrafluoropropyl group, 1,1-dimethyl-2,2,3,3,3-pentafluoropropyl group, 2- (perfluoropropyl) ethyl group, 2,2,3 , 3,4,4,5,5-octafluoropentyl group, perfluoropentyl group, 1,1-dimethyl-2,2,3,3,4,4-hexafluorobutyl group, 2- (perfluoro (Robutyl) ethyl group, 2,2,3,3,4,4,5,5,6,6-decafluorohexyl group, perfluoropentylmethyl group, perfluorohexyl group, 1,1-dimethyl-2,2 , 3,3,4,4,5,5-octafluoropentyl group, 1,1-dimethyl-2,2,3,3,4,4,5,5,5-nonafluoropentyl group, 2- ( Perfluoropentyl) ethyl group, 2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoroheptyl group, perfluorohexylmethyl group, perfluoroheptyl group, 2- ( Perfluorohexyl) ethyl group, 2,2,3,3,4,4,5,5,6,6,7,7,8,8-tetradecafluorooctyl group, perfluoroheptylmethyl group, perfluorooctyl Group, 2- (perfluoroheptyl) Til group, 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-hexadecafluorononyl group, perfluorooctylmethyl group, perfluorononyl Group, 2- (perfluorooctyl) ethyl group, 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10-octadeca Fluorodecyl group, perfluorononylmethyl group, perfluorodecyl group, 2,2,3,4,4,4-hexafluorobutyl group, 2,2,3,3,4,4,4-heptafluorobutyl group 3,3,4,4,5,5,6,6,6-nonafluorohexyl group, 3,3,4,4,5,5,6,6,7,7,8,8,8- Examples include a tridecafluorooctyl group. Of these, a perfluoromethyl group, a perfluoroethyl group, a perfluoropropyl group, a perfluorobutyl group, and a perfluorooctyl group are preferable.
Specific examples of the cycloalkyl group having 3 to 20 carbon atoms include a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, and the like.
As a specific example of a fluorinated cycloalkyl group having 3 to 20 carbon atoms, at least one hydrogen atom of the cycloalkyl group may be substituted with a fluorine atom.
炭素数1~20のアルキル基の具体例としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、sec-ブチル基、tert-ブチル基、n-ペンチル基、n-ヘキシル基、n-ヘプチル基、オクチル基等が挙げられる。
炭素数1~20のフッ素化アルキル基の具体例としては、ジフルオロメチル基、パーフルオロメチル基、2,2-ジフルオロエチル基、2,2,2-トリフルオロエチル基、パーフルオロエチル基、2,2,3,3-テトラフルオロプロピル基、パーフルオロエチルメチル基、パーフルオロプロピル基、2,2,3,3,4,4―ヘキサフルオロブチル基、パーフルオロブチル基、1,1-ジメチル-2,2,3,3―テトラフルオロプロピル基、1,1-ジメチル-2,2,3,3,3-ペンタフルオロプロピル基、2-(パーフルオロプロピル)エチル基、2,2,3,3,4,4,5,5-オクタフルオロペンチル基、パーフルオロペンチル基、1,1-ジメチル-2,2,3,3,4,4-ヘキサフルオロブチル基、2-(パーフルオロブチル)エチル基、2,2,3,3,4,4,5,5,6,6-デカフルオロヘキシル基、パーフルオロペンチルメチル基、パーフルオロヘキシル基、1,1-ジメチル-2,2,3,3,4,4,5,5-オクタフルオロペンチル基、1,1-ジメチル-2,2,3,3,4,4,5,5,5-ノナフルオロペンチル基、2-(パーフルオロペンチル)エチル基、2,2,3,3,4,4,5,5,6,6,7,7-ドデカフルオロヘプチル基、パーフルオロヘキシルメチル基、パーフルオロヘプチル基、2-(パーフルオロヘキシル)エチル基、2,2,3,3,4,4,5,5,6,6,7,7,8,8-テトラデカフルオロオクチル基、パーフルオロヘプチルメチル基、パーフルオロオクチル基、2-(パーフルオロヘプチル)エチル基、2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-ヘキサデカフルオロノニル基、パーフルオロオクチルメチル基、パーフルオロノニル基、2-(パーフルオロオクチル)エチル基、2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10-オクタデカフルオロデシル基、パーフルオロノニルメチル基、パーフルオロデシル基、2,2,3,4,4,4-ヘキサフルオロブチル基、2,2,3,3,4,4,4-ヘプタフルオロブチル基、3,3,4,4,5,5,6,6,6-ノナフルオロヘキシル基、3,3,4,4,5,5,6,6,7,7,8,8,8-トリデカフルオロオクチル基等が挙げられる。これらのうち、パーフルオロメチル基、パーフルオロエチル基、パーフルオロプロピル基、パーフルオロブチル基及びパーフルオロオクチル基が好ましい。
炭素数3~20のシクロアルキル基の具体例としては、シクロプロピル基、シクロブチル基、シクロペンチル基、シクロへキシル基等が挙げられる。
また、炭素数3~20のフッ素化シクロアルキル基の具体例としては、上記シクロアルキル基の水素原子の少なくとも1つがフッ素原子に置換されてなるものとすることができる。 In the general formula (1), R 2 is a hydrocarbon group having 1 to 20 carbon atoms or a fluorinated hydrocarbon group having 1 to 20 carbon atoms, an alkyl group having 1 to 20 carbon atoms, a carbon number of 1 -20 fluorinated alkyl group, cycloalkyl group having 3-20 carbon atoms, fluorinated cycloalkyl group having 3-20 carbon atoms, and the like.
Specific examples of the alkyl group having 1 to 20 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, sec-butyl group, tert-butyl group, n-pentyl group, n- Examples include hexyl group, n-heptyl group, octyl group and the like.
Specific examples of the fluorinated alkyl group having 1 to 20 carbon atoms include a difluoromethyl group, a perfluoromethyl group, a 2,2-difluoroethyl group, a 2,2,2-trifluoroethyl group, a perfluoroethyl group, 2 , 2,3,3-tetrafluoropropyl group, perfluoroethylmethyl group, perfluoropropyl group, 2,2,3,3,4,4-hexafluorobutyl group, perfluorobutyl group, 1,1-dimethyl -2,2,3,3-tetrafluoropropyl group, 1,1-dimethyl-2,2,3,3,3-pentafluoropropyl group, 2- (perfluoropropyl) ethyl group, 2,2,3 , 3,4,4,5,5-octafluoropentyl group, perfluoropentyl group, 1,1-dimethyl-2,2,3,3,4,4-hexafluorobutyl group, 2- (perfluoro (Robutyl) ethyl group, 2,2,3,3,4,4,5,5,6,6-decafluorohexyl group, perfluoropentylmethyl group, perfluorohexyl group, 1,1-dimethyl-2,2 , 3,3,4,4,5,5-octafluoropentyl group, 1,1-dimethyl-2,2,3,3,4,4,5,5,5-nonafluoropentyl group, 2- ( Perfluoropentyl) ethyl group, 2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoroheptyl group, perfluorohexylmethyl group, perfluoroheptyl group, 2- ( Perfluorohexyl) ethyl group, 2,2,3,3,4,4,5,5,6,6,7,7,8,8-tetradecafluorooctyl group, perfluoroheptylmethyl group, perfluorooctyl Group, 2- (perfluoroheptyl) Til group, 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-hexadecafluorononyl group, perfluorooctylmethyl group, perfluorononyl Group, 2- (perfluorooctyl) ethyl group, 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10-octadeca Fluorodecyl group, perfluorononylmethyl group, perfluorodecyl group, 2,2,3,4,4,4-hexafluorobutyl group, 2,2,3,3,4,4,4-heptafluorobutyl group 3,3,4,4,5,5,6,6,6-nonafluorohexyl group, 3,3,4,4,5,5,6,6,7,7,8,8,8- Examples include a tridecafluorooctyl group. Of these, a perfluoromethyl group, a perfluoroethyl group, a perfluoropropyl group, a perfluorobutyl group, and a perfluorooctyl group are preferable.
Specific examples of the cycloalkyl group having 3 to 20 carbon atoms include a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, and the like.
As a specific example of a fluorinated cycloalkyl group having 3 to 20 carbon atoms, at least one hydrogen atom of the cycloalkyl group may be substituted with a fluorine atom.
上記一般式(1)において、Q1は、2価の連結基であり、好ましくは、2価の炭化水素基である。2価の炭化水素基は、鎖状炭化水素基及び環状炭化水素基のいずれも好ましく、その具体例としては、メチレン基、エチレン基、1,3-プロピレン基及び1,2-プロピレン基に例示されるプロピレン基、テトラメチレン基、ペンタメチレン基、ヘキサメチレン基、ヘプタメチレン基、オクタメチレン基、ノナメチレン基、デカメチレン基、ウンデカメチレン基、ドデカメチレン基、トリデカメチレン基、テトラデカメチレン基、ペンタデカメチレン基、ヘキサデカメチレン基、ヘプタデカメチレン基、オクタデカメチレン基、ノナデカメチレン基、インサレン基、1-メチル-1,3-プロピレン基、2-メチル-1,3-プロピレン基、2-メチル-1,2-プロピレン基、1-メチル-1,4-ブチレン基、2-メチル-1,4-ブチレン基、メチリデン基、エチリデン基、プロピリデン基、2-プロピリデン基等の飽和鎖状炭化水素基;1,3-シクロブチレン基等のシクロブチレン基、1,3-シクロペンチレン基等のシクロペンチレン基、1,4-シクロへキシレン基等のシクロへキシレン基、1,5-シクロオクチレン基等のシクロオクチレン基等の、炭素数3~10であるシクロアルキレン基等の単環式炭化水素環基;1,4-ノルボルニレン基、2,5-ノルボルニレン基等のノルボルニレン基、1,5-アダマンチレン基、2,6-アダマンチレン基等のアダマンチレン基等の、炭素数4~30である2~4環式炭化水素環基等の架橋環式炭化水素基等が挙げられる。
In the general formula (1), Q 1 is a divalent linking group, preferably a divalent hydrocarbon group. The divalent hydrocarbon group is preferably a chain hydrocarbon group or a cyclic hydrocarbon group, and specific examples thereof include methylene group, ethylene group, 1,3-propylene group and 1,2-propylene group. Propylene group, tetramethylene group, pentamethylene group, hexamethylene group, heptamethylene group, octamethylene group, nonamethylene group, decamethylene group, undecamethylene group, dodecamethylene group, tridecamethylene group, tetradecamethylene group, Pentadecamethylene group, hexadecamethylene group, heptacamethylene group, octadecamethylene group, nonadecamethylene group, insalen group, 1-methyl-1,3-propylene group, 2-methyl-1,3-propylene group, 2-methyl-1,2-propylene group, 1-methyl-1,4-butylene group, 2-methyl-1,4-butyl group Saturated chain hydrocarbon groups such as a len group, a methylidene group, an ethylidene group, a propylidene group and a 2-propylidene group; a cyclobutylene group such as a 1,3-cyclobutylene group and a cyclopentylene such as a 1,3-cyclopentylene group Monocyclic such as cycloalkylene group having 3 to 10 carbon atoms such as ylene group, cyclohexylene group such as 1,4-cyclohexylene group, cyclooctylene group such as 1,5-cyclooctylene group, etc. Hydrocarbon ring group; 4 carbon atoms such as norbornylene group such as 1,4-norbornylene group, 2,5-norbornylene group, adamantylene group such as 1,5-adamantylene group, 2,6-adamantylene group, etc. And a crosslinked cyclic hydrocarbon group such as a bicyclic to tetracyclic hydrocarbon group which is 30.
Q1が、2価の脂肪族環状炭化水素基を含む場合には、-NH-基とこの脂肪族環状炭化水素基との間に、スペーサーとして、炭素数1~4のアルキレン基を有することが好ましい。即ち、Q1は、脂肪族環状炭化水素基及び炭素数1~4のアルキレン基が連結した2価の炭化水素基とすることができる。
When Q 1 contains a divalent aliphatic cyclic hydrocarbon group, it has an alkylene group having 1 to 4 carbon atoms as a spacer between the —NH— group and the aliphatic cyclic hydrocarbon group. Is preferred. That is, Q 1 can be a divalent hydrocarbon group in which an aliphatic cyclic hydrocarbon group and an alkylene group having 1 to 4 carbon atoms are linked.
上記一般式(1)におけるQ1としては、2,5-ノルボルニレン基、2,6-ノルボルニレン基を含む炭化水素基、エチレン基、及び、1,2-プロピレン基が好ましい。
Q 1 in the general formula (1) is preferably a 2,5-norbornylene group, a hydrocarbon group containing a 2,6-norbornylene group, an ethylene group, or a 1,2-propylene group.
上記重合体[A]は、繰り返し単位(I-1)を1種のみ含んでよいし、2種以上を含んでもよい。
The polymer [A] may contain only one type of repeating unit (I-1), or may contain two or more types.
上記一般式(1)で表される繰り返し単位(I-1)を与える単量体としては、下記式(1-1)及び(1-2)で表される化合物が挙げられる。
Examples of the monomer that gives the repeating unit (I-1) represented by the general formula (1) include compounds represented by the following formulas (1-1) and (1-2).
上記重合体[A]を構成する繰り返し単位(I-1)の含有量は、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは5~50モル%、より好ましくは5~30モル%である。繰り返し単位(I-1)の含有量が少なすぎると、LWRの改善効果が見られない場合がある。一方、繰り返し単位(I-1)の含有量が多すぎると、現像後のコントラストが損なわれ、良好なパターン形状が得られない場合がある。
The content of the repeating unit (I-1) constituting the polymer [A] is preferably 5 to 50 mol% with respect to 100 mol% in total of all the repeating units constituting the polymer [A]. More preferably, it is 5 to 30 mol%. If the content of the repeating unit (I-1) is too small, the LWR improving effect may not be observed. On the other hand, if the content of the repeating unit (I-1) is too large, the contrast after development is impaired, and a good pattern shape may not be obtained.
1-1-2.他の繰り返し単位
上記重合体[A]は、上記繰り返し単位(I-1)以外に、下記一般式(2)で表される、酸解離性基を有する繰り返し単位(以下、「繰り返し単位(I-2)」という。)、及び、下記一般式(3-1)~(3-6)で表される、ラクトン骨格を有する繰り返し単位(以下、「繰り返し単位(I-3)」ともいう。)を含んでもよい。
(式中、R3は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R4は、相互に独立に、炭素数1~4の直鎖状若しくは分岐状のアルキル基、又は、炭素数4~20の1価の脂環式炭化水素基である。また、2つのR4が相互に結合して形成された、2価であって、炭素数4~20の脂環式炭化水素基又はその誘導体基であってもよい。)
(式中、R5は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R6は、水素原子又は炭素数1~4の置換若しくは非置換のアルキル基であり、R7は、単結合又はメチレン基であり、R8は、水素原子又はメトキシ基であり、R9は、酸素原子又はメチレン基であり、pは1、2又は3であり、mは0又は1である。)
1-1-2. Other Repeating Units The polymer [A] contains, in addition to the repeating unit (I-1), a repeating unit having an acid dissociable group represented by the following general formula (2) (hereinafter referred to as “repeating unit (I)”. -2) ") and repeating units having a lactone skeleton represented by the following general formulas (3-1) to (3-6) (hereinafter also referred to as" repeating units (I-3) "). ) May be included.
(Wherein R 3 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 4 is independently a linear or branched alkyl group having 1 to 4 carbon atoms. Or a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms, or a divalent formed by bonding two R 4 to each other and having 4 to 20 carbon atoms. (It may be an alicyclic hydrocarbon group or a derivative group thereof.)
(Wherein R 5 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 6 is a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms, R 7 is a single bond or a methylene group, R 8 is a hydrogen atom or a methoxy group, R 9 is an oxygen atom or a methylene group, p is 1, 2 or 3, and m is 0 or 1)
上記重合体[A]は、上記繰り返し単位(I-1)以外に、下記一般式(2)で表される、酸解離性基を有する繰り返し単位(以下、「繰り返し単位(I-2)」という。)、及び、下記一般式(3-1)~(3-6)で表される、ラクトン骨格を有する繰り返し単位(以下、「繰り返し単位(I-3)」ともいう。)を含んでもよい。
1-1-2-1.繰り返し単位(I-2)
この繰り返し単位(I-2)は、酸解離性基を有する単位である。
上記一般式(2)において、R3は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基である。炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。R3としては、水素原子及びメチル基が好ましい。 1-1-2-1. Repeating unit (I-2)
This repeating unit (I-2) is a unit having an acid dissociable group.
In the above general formula (2), R 3 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. . R 3 is preferably a hydrogen atom or a methyl group.
この繰り返し単位(I-2)は、酸解離性基を有する単位である。
上記一般式(2)において、R3は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基である。炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。R3としては、水素原子及びメチル基が好ましい。 1-1-2-1. Repeating unit (I-2)
This repeating unit (I-2) is a unit having an acid dissociable group.
In the above general formula (2), R 3 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. . R 3 is preferably a hydrogen atom or a methyl group.
上記一般式(2)において、R4は、相互に独立に、炭素数1~4の直鎖状若しくは分岐状のアルキル基、又は、炭素数4~20の1価の脂環式炭化水素基である。
In the above general formula (2), R 4 s are independently of each other a linear or branched alkyl group having 1 to 4 carbon atoms, or a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms. It is.
R4により表した、炭素数1~4の直鎖状及び分岐状のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。
また、R4により表した、炭素数4~20の1価の脂環式炭化水素基としては、シクロペンチル基、シクロペンチルメチル基、1-(1-シクロペンチルエチル)基、1-(2-シクロペンチルエチル)基、シクロヘキシル基、シクロヘキシルメチル基、1-(1-シクロヘキシルエチル)基、1-(2-シクロヘキシルエチル基)、シクロヘプチル基、シクロヘプチルメチル基、1-(1-シクロヘプチルエチル)基、1-(2-シクロヘプチルエチル)基、2-ノルボルニル基、ビシクロ[2.2.1]ヘプチル基、ビシクロ[2.2.1]オクチル基、トリシクロ[5.2.1.02,6]デカニル基、トリシクロ[3.3.1.13,7]デカニル基、テトラシクロ[6.2.1.13,6.02,7]ドデカニル基、アダマンチル基等の脂環式アルキル基等が挙げられる。これらのうち、シクロペンチル基、シクロヘキシル基、ビシクロ[2.2.1]ヘプチル基及びアダマンチル基が好ましい。 Examples of linear and branched alkyl groups having 1 to 4 carbon atoms represented by R 4 include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -Methylpropyl group, tert-butyl group and the like.
Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms represented by R 4 include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, and 1- (2-cyclopentylethyl). ) Group, cyclohexyl group, cyclohexylmethyl group, 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1-cycloheptylethyl) group, 1- (2-cycloheptylethyl) group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2.1.0 2,6 ] Decanyl group, tricyclo [3.3.1.1 3,7 ] decanyl group, tetracyclo [6.2.1.1 3,6 . 0 2,7 ] dodecanyl group, alicyclic alkyl group such as adamantyl group, and the like. Of these, a cyclopentyl group, a cyclohexyl group, a bicyclo [2.2.1] heptyl group and an adamantyl group are preferred.
また、R4により表した、炭素数4~20の1価の脂環式炭化水素基としては、シクロペンチル基、シクロペンチルメチル基、1-(1-シクロペンチルエチル)基、1-(2-シクロペンチルエチル)基、シクロヘキシル基、シクロヘキシルメチル基、1-(1-シクロヘキシルエチル)基、1-(2-シクロヘキシルエチル基)、シクロヘプチル基、シクロヘプチルメチル基、1-(1-シクロヘプチルエチル)基、1-(2-シクロヘプチルエチル)基、2-ノルボルニル基、ビシクロ[2.2.1]ヘプチル基、ビシクロ[2.2.1]オクチル基、トリシクロ[5.2.1.02,6]デカニル基、トリシクロ[3.3.1.13,7]デカニル基、テトラシクロ[6.2.1.13,6.02,7]ドデカニル基、アダマンチル基等の脂環式アルキル基等が挙げられる。これらのうち、シクロペンチル基、シクロヘキシル基、ビシクロ[2.2.1]ヘプチル基及びアダマンチル基が好ましい。 Examples of linear and branched alkyl groups having 1 to 4 carbon atoms represented by R 4 include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -Methylpropyl group, tert-butyl group and the like.
Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms represented by R 4 include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, and 1- (2-cyclopentylethyl). ) Group, cyclohexyl group, cyclohexylmethyl group, 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1-cycloheptylethyl) group, 1- (2-cycloheptylethyl) group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2.1.0 2,6 ] Decanyl group, tricyclo [3.3.1.1 3,7 ] decanyl group, tetracyclo [6.2.1.1 3,6 . 0 2,7 ] dodecanyl group, alicyclic alkyl group such as adamantyl group, and the like. Of these, a cyclopentyl group, a cyclohexyl group, a bicyclo [2.2.1] heptyl group and an adamantyl group are preferred.
更に、上記一般式(2)で表される繰り返し単位において、3つのR4のうちいずれか2つが互いに結合して形成された、2価であって、炭素数4~20の脂環式炭化水素基及びその誘導体基を有してもよく、その例としては、アダマンタン骨格、ノルボルナン骨格、トリシクロデカン骨格、テトラシクロドデカン骨格等の有橋式骨格や、シクロブタン、シクロペンタン、シクロヘキサン、シクロヘプタン、シクロオクタン等のシクロアルカン骨格を有する基;これらの基に含まれる水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~10の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等の脂環式骨格を有する基が挙げられる。
Further, in the repeating unit represented by the above general formula (2), a divalent alicyclic carbon atom having 4 to 20 carbon atoms formed by bonding any two of the three R 4 s to each other. It may have a hydrogen group and a derivative group thereof. Examples thereof include a bridged skeleton such as an adamantane skeleton, a norbornane skeleton, a tricyclodecane skeleton, and a tetracyclododecane skeleton, cyclobutane, cyclopentane, cyclohexane, and cycloheptane. Groups having a cycloalkane skeleton such as cyclooctane; the hydrogen atoms contained in these groups are methyl, ethyl, n-propyl, isopropyl, n-butyl, 2-methylpropyl, 1-methyl Groups substituted with one or more linear, branched or cyclic alkyl groups having 1 to 10 carbon atoms such as propyl group and tert-butyl group And a group having an alicyclic skeleton such as.
上記一般式(2)におけるエステル結合部-C(R4)3の好ましい例としては、tert-ブチル基、1-n-(1-エチル-1-メチル)プロピル基、1-n-(1,1-ジメチル)プロピル基、1-n-(1,1-ジメチル)ブチル基、1-n-(1,1-ジメチル)ペンチル基、1-(1,1-ジエチル)プロピル基、1-n-(1,1-ジエチル)ブチル基、1-n-(1,1-ジエチル)ペンチル基、1-(1-メチル)シクロペンチル基、1-(1-エチル)シクロペンチル基、1-(1-n-プロピル)シクロペンチル基、1-(1-イソプロピル)シクロペンチル基、1-(1-メチル)シクロヘキシル基、1-(1-エチル)シクロヘキシル基、1-(1-n-プロピル)シクロヘキシル基、1-(1-イソプロピル)シクロヘキシル基、1-{1-メチル-1-(2-ノルボニル)}エチル基、1-{1-メチル-1-(2-テトラシクロデカニル)}エチル基、1-{1-メチル-1-(1-アダマンチル)}エチル基、2-(2-メチル)ノルボニル基、2-(2-エチル)ノルボニル基、2-(2-n-プロピル)ノルボニル基、2-(2-イソプロピル)ノルボニル基、2-(2-メチル)テトラシクロデカニル基、2-(2-エチル)テトラシクロデカニル基、2-(2-n-プロピル)テトラシクロデカニル基、2-(2-イソプロピル)テトラシクロデカニル基、1-(1-メチル)アダマンチル基、1-(1-エチル)アダマンチル基、1-(1-n-プロピル)アダマンチル基、1-(1-イソプロピル)アダマンチル基や、これらの基に含まれる水素原子を、例えば、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~4の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等が挙げられる。
Preferable examples of the ester bond portion —C (R 4 ) 3 in the general formula (2) include tert-butyl group, 1-n- (1-ethyl-1-methyl) propyl group, 1-n- (1 , 1-dimethyl) propyl group, 1-n- (1,1-dimethyl) butyl group, 1-n- (1,1-dimethyl) pentyl group, 1- (1,1-diethyl) propyl group, 1- n- (1,1-diethyl) butyl group, 1-n- (1,1-diethyl) pentyl group, 1- (1-methyl) cyclopentyl group, 1- (1-ethyl) cyclopentyl group, 1- (1 -N-propyl) cyclopentyl group, 1- (1-isopropyl) cyclopentyl group, 1- (1-methyl) cyclohexyl group, 1- (1-ethyl) cyclohexyl group, 1- (1-n-propyl) cyclohexyl group, 1- (1-Isopropyl Cyclohexyl, 1- {1-methyl-1- (2-norbornyl)} ethyl, 1- {1-methyl-1- (2-tetracyclodecanyl)} ethyl, 1- {1-methyl-1 -(1-adamantyl)} ethyl group, 2- (2-methyl) norbornyl group, 2- (2-ethyl) norbornyl group, 2- (2-n-propyl) norbornyl group, 2- (2-isopropyl) norbornyl Group, 2- (2-methyl) tetracyclodecanyl group, 2- (2-ethyl) tetracyclodecanyl group, 2- (2-n-propyl) tetracyclodecanyl group, 2- (2-isopropyl) Tetracyclodecanyl group, 1- (1-methyl) adamantyl group, 1- (1-ethyl) adamantyl group, 1- (1-n-propyl) adamantyl group, 1- (1-isopropyl) adamantyl group, etc. of For example, a methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group, etc. And a group substituted with one or more of 4 linear, branched or cyclic alkyl groups.
上記重合体[A]は、繰り返し単位(I-2)を1種のみ含んでよいし、2種以上を含んでもよい。
The polymer [A] may contain only one type of repeating unit (I-2), or may contain two or more types.
上記繰り返し単位(I-2)を与える単量体としては、(メタ)アクリル酸2-メチルアダマンチル-2-イルエステル、(メタ)アクリル酸2-メチル-3-ヒドロキシアダマンチル-2-イルエステル、(メタ)アクリル酸2-エチルアダマンチル-2-イルエステル、(メタ)アクリル酸2-エチル-3-ヒドロキシアダマンチル-2-イルエステル、(メタ)アクリル酸2-n-プロピルアダマンチル-2-イルエステル、(メタ)アクリル酸2-イソプロピルアダマンチル-2-イルエステル、(メタ)アクリル酸-2-メチルビシクロ[2.2.1]ヘプト-2-イルエステル、(メタ)アクリル酸-2-エチルビシクロ[2.2.1]ヘプト-2-イルエステル、(メタ)アクリル酸-8-メチルトリシクロ[5.2.1.02,6]デカン-8-イルエステル、(メタ)アクリル酸-8-エチルトリシクロ[5.2.1.02,6]デカン-8-イルエステル、(メタ)アクリル酸-4-メチルテトラシクロ[6.2.13,6.02,7]ドデカン-4-イルエステル、(メタ)アクリル酸-4-エチルテトラシクロ[6.2.13,6.02,7]ドデカン-4-イルエステル、(メタ)アクリル酸1-(ビシクロ[2.2.1]ヘプト-2-イル)-1-メチルエチルエステル、(メタ)アクリル酸1-(トリシクロ[5.2.1.02,6]デカン-8-イル)-1-メチルエチルエステル、(メタ)アクリル酸1-(テトラシクロ[6.2.13,6.02,7]ドデカン-4-イル)-1-メチルエチルエステル、(メタ)アクリル酸1-(アダマンタン-1-イル)-1-メチルエチルエステル、(メタ)アクリル酸1-(3-ヒドロキシアダマンタン-1-イル)-1-メチルエチルエステル、(メタ)アクリル酸1,1-ジシクロヘキシルエチルエステイル、(メタ)アクリル酸1,1-ジ(ビシクロ[2.2.1]ヘプト-2-イル)エチルエステル、(メタ)アクリル酸1,1-ジ(トリシクロ[5.2.1.02,6]デカン-8-イル)エチルエステル、(メタ)アクリル酸1,1-ジ(テトラシクロ[6.2.13,6.02,7]ドデカン-4-イル)エチルエステル、(メタ)アクリル酸1,1-ジ(アダマンタン-1-イル)エチルエステル、(メタ)アクリル酸1-メチル-1-シクロペンチルエステル、(メタ)アクリル酸1-エチル-1-シクロペンチルエステル、(メタ)アクリル酸1-メチル-1-シクロヘキシルエステル、(メタ)アクリル酸1-エチル-1-シクロヘキシルエステル等が挙げられる。
Examples of the monomer that gives the repeating unit (I-2) include (meth) acrylic acid 2-methyladamantyl-2-yl ester, (meth) acrylic acid 2-methyl-3-hydroxyadamantyl-2-yl ester, (Meth) acrylic acid 2-ethyladamantyl-2-yl ester, (meth) acrylic acid 2-ethyl-3-hydroxyadamantyl-2-yl ester, (meth) acrylic acid 2-n-propyladamantyl-2-yl ester (Meth) acrylic acid 2-isopropyladamantyl-2-yl ester, (meth) acrylic acid-2-methylbicyclo [2.2.1] hept-2-yl ester, (meth) acrylic acid-2-ethylbicyclo ester [2.2.1] Hept-2-yl ester, (meth) acrylic acid-8-methyltricyclo [5.2.1.0] , 6] decan-8-yl ester, (meth) ethyl-8-acrylic acid tricyclo [5.2.1.0 2,6] decan-8-yl ester, (meth) methyl-4-acrylic acid tetra Cyclo [6.2.1 3,6 . 0 2,7 ] dodecan-4-yl ester, (meth) acrylic acid-4-ethyltetracyclo [6.2.1 3,6 . 0 2,7 ] dodecan-4-yl ester, (meth) acrylic acid 1- (bicyclo [2.2.1] hept-2-yl) -1-methylethyl ester, (meth) acrylic acid 1- (tricyclo [5.2.1.0 2,6 ] decan-8-yl) -1-methylethyl ester, (meth) acrylic acid 1- (tetracyclo [6.2.1 3,6 0.0 2,7 ] dodecane -4-yl) -1-methylethyl ester, (meth) acrylic acid 1- (adamantan-1-yl) -1-methylethyl ester, (meth) acrylic acid 1- (3-hydroxyadamantan-1-yl) -1-methyl ethyl ester, (meth) acrylic acid 1,1-dicyclohexylethyl ester, (meth) acrylic acid 1,1-di (bicyclo [2.2.1] hept-2-yl) ethyl ester, Meth) acrylic acid 1,1-di (tricyclo [5.2.1.0 2,6] decan-8-yl) ethyl ester, (meth) acrylic acid 1,1-di (tetracyclo [6.2.1 3,6 .0 2,7 ] dodecan-4-yl) ethyl ester, (meth) acrylic acid 1,1-di (adamantan-1-yl) ethyl ester, (meth) acrylic acid 1-methyl-1-cyclopentyl Examples include esters, (meth) acrylic acid 1-ethyl-1-cyclopentyl ester, (meth) acrylic acid 1-methyl-1-cyclohexyl ester, (meth) acrylic acid 1-ethyl-1-cyclohexyl ester, and the like.
上記単量体のうち、(メタ)アクリル酸2-メチルアダマンチル-2-イルエステル、(メタ)アクリル酸2-エチルアダマンチル-2-イルエステル、(メタ)アクリル酸-2-メチルビシクロ[2.2.1]ヘプト-2-イルエステル、(メタ)アクリル酸-2-エチルビシクロ[2.2.1]ヘプト-2-イルエステル、(メタ)アクリル酸1-(ビシクロ[2.2.1]ヘプト-2-イル)-1-メチルエチルエステル、(メタ)アクリル酸1-(アダマンタン-1-イル)-1-メチルエチルエステル、(メタ)アクリル酸1-メチル-1-シクロペンチルエステル、(メタ)アクリル酸1-エチル-1-シクロペンチルエステル、(メタ)アクリル酸1-メチル-1-シクロヘキシルエステル及び(メタ)アクリル酸1-エチル-1-シクロヘキシルエステルが特に好ましい。
Among the above monomers, (meth) acrylic acid 2-methyladamantyl-2-yl ester, (meth) acrylic acid 2-ethyladamantyl-2-yl ester, (meth) acrylic acid-2-methylbicyclo [2. 2.1] hept-2-yl ester, (meth) acrylic acid-2-ethylbicyclo [2.2.1] hept-2-yl ester, (meth) acrylic acid 1- (bicyclo [2.2.1] ] Hept-2-yl) -1-methylethyl ester, (meth) acrylic acid 1- (adamantan-1-yl) -1-methylethyl ester, (meth) acrylic acid 1-methyl-1-cyclopentyl ester, ( (Meth) acrylic acid 1-ethyl-1-cyclopentyl ester, (meth) acrylic acid 1-methyl-1-cyclohexyl ester and (meth) acrylic acid 1-ethyl 1-cyclohexyl ester is particularly preferred.
上記重合体[A]が繰り返し単位(I-2)を含む場合、この繰り返し単位(I-2)の含有量は、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは15~85モル%、より好ましくは25~75モル%、更に好ましくは30~60モル%である。繰り返し単位(I-2)の含有量が少なすぎると、現像後のコントラストが損なわれ、良好なパターン形状が得られない場合がある。一方、繰り返し単位(I-2)の含有量が多すぎると、下地基板との密着性が不十分となり、パターン皮膜が剥がれてしまう場合がある。
When the polymer [A] contains the repeating unit (I-2), the content of the repeating unit (I-2) is 100 mol% in total of all the repeating units constituting the polymer [A]. On the other hand, it is preferably 15 to 85 mol%, more preferably 25 to 75 mol%, still more preferably 30 to 60 mol%. If the content of the repeating unit (I-2) is too small, the contrast after development is impaired, and a good pattern shape may not be obtained. On the other hand, when the content of the repeating unit (I-2) is too large, the adhesion with the base substrate becomes insufficient, and the pattern film may be peeled off.
1-1-2-2.繰り返し単位(I-3)
この繰り返し単位(I-3)は、ラクトン骨格を有する単位である。上記重合体[A]が、この繰り返し単位(I-3)を含む場合、レジストパターンの基板への密着性を向上させることができる。 1-1-2-2. Repeating unit (I-3)
This repeating unit (I-3) is a unit having a lactone skeleton. When the polymer [A] contains this repeating unit (I-3), the adhesion of the resist pattern to the substrate can be improved.
この繰り返し単位(I-3)は、ラクトン骨格を有する単位である。上記重合体[A]が、この繰り返し単位(I-3)を含む場合、レジストパターンの基板への密着性を向上させることができる。 1-1-2-2. Repeating unit (I-3)
This repeating unit (I-3) is a unit having a lactone skeleton. When the polymer [A] contains this repeating unit (I-3), the adhesion of the resist pattern to the substrate can be improved.
上記一般式(3-1)~(3-6)において、R5は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基である。炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。R5としては、水素原子及びメチル基が好ましい。
In the above general formulas (3-1) to (3-6), R 5 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. . R 5 is preferably a hydrogen atom or a methyl group.
上記一般式(3-1)において、R6は、水素原子又は炭素数1~4の置換若しくは非置換のアルキル基である。
炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。
また、置換基を有する炭素数1~4のアルキル基においては、このアルキル基に含まれる水素原子が、例えば、ヒドロキシル基、シアノ基、カルボキシル基、ハロゲン原子等の少なくとも1種に置換されてなるものとすることができる。 In the general formula (3-1), R 6 represents a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms.
Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. .
In the alkyl group having 1 to 4 carbon atoms having a substituent, a hydrogen atom contained in the alkyl group is substituted with at least one of a hydroxyl group, a cyano group, a carboxyl group, a halogen atom, and the like. Can be.
炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。
また、置換基を有する炭素数1~4のアルキル基においては、このアルキル基に含まれる水素原子が、例えば、ヒドロキシル基、シアノ基、カルボキシル基、ハロゲン原子等の少なくとも1種に置換されてなるものとすることができる。 In the general formula (3-1), R 6 represents a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms.
Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. .
In the alkyl group having 1 to 4 carbon atoms having a substituent, a hydrogen atom contained in the alkyl group is substituted with at least one of a hydroxyl group, a cyano group, a carboxyl group, a halogen atom, and the like. Can be.
上記重合体[A]は、繰り返し単位(I-3)を1種のみ含んでよいし、2種以上を含んでもよい。
The polymer [A] may contain only one type of repeating unit (I-3), or may contain two or more types.
上記繰り返し単位(I-3)を与える単量体としては、下記一般式(3-1m)~(3-6m)で表される化合物等が挙げられる。
尚、下記一般式(3-1m)は、上記一般式(3-1)で表した繰り返し単位を与える単量体であり、下記一般式(3-2m)~(3-6m)で表される化合物についても、同様に、上記一般式(3-2)~(3-6)で表した繰り返し単位を、それぞれ、与える単量体である。
(式中、R5は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R6は、水素原子又は炭素数1~4の置換若しくは非置換のアルキル基であり、R7は、単結合又はメチレン基であり、R8は、水素原子又はメトキシ基であり、R9は、酸素原子又はメチレン基であり、pは1、2又は3であり、mは0又は1である。)
Examples of the monomer giving the repeating unit (I-3) include compounds represented by the following general formulas (3-1m) to (3-6m).
The following general formula (3-1m) is a monomer that gives the repeating unit represented by the above general formula (3-1), and is represented by the following general formulas (3-2m) to (3-6m). Similarly, these compounds are monomers that give the repeating units represented by the general formulas (3-2) to (3-6), respectively.
(Wherein R 5 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 6 is a hydrogen atom or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms, R 7 is a single bond or a methylene group, R 8 is a hydrogen atom or a methoxy group, R 9 is an oxygen atom or a methylene group, p is 1, 2 or 3, and m is 0 or 1)
尚、下記一般式(3-1m)は、上記一般式(3-1)で表した繰り返し単位を与える単量体であり、下記一般式(3-2m)~(3-6m)で表される化合物についても、同様に、上記一般式(3-2)~(3-6)で表した繰り返し単位を、それぞれ、与える単量体である。
The following general formula (3-1m) is a monomer that gives the repeating unit represented by the above general formula (3-1), and is represented by the following general formulas (3-2m) to (3-6m). Similarly, these compounds are monomers that give the repeating units represented by the general formulas (3-2) to (3-6), respectively.
上記繰り返し単位(I-3)を与える単量体としては、(メタ)アクリル酸-5-オキソ-4-オキサ-トリシクロ[4.2.1.03,7]ノナ-2-イルエステル、(メタ)アクリル酸-9-メトキシカルボニル-5-オキソ-4-オキサ-トリシクロ[4.2.1.03,7]ノナ-2-イルエステル、(メタ)アクリル酸-5-オキソ-4-オキサ-トリシクロ[5.2.1.03,8]デカ-2-イルエステル、(メタ)アクリル酸-10-メトキシカルボニル-5-オキソ-4-オキサ-トリシクロ[5.2.1.03,8]ノナ-2-イルエステル、(メタ)アクリル酸-6-オキソ-7-オキサ-ビシクロ[3.2.1]オクタ-2-イルエステル、(メタ)アクリル酸-4-メトキシカルボニル-6-オキソ-7-オキサ-ビシクロ[3.2.1]オクタ-2-イルエステル、(メタ)アクリル酸-7-オキソ-8-オキサ-ビシクロ[3.3.1]オクタ-2-イルエステル、(メタ)アクリル酸-4-メトキシカルボニル-7-オキソ-8-オキサ-ビシクロ[3.3.1]オクタ-2-イルエステル、(メタ)アクリル酸-2-オキソテトラヒドロピラン-4-イルエステル、(メタ)アクリル酸-4-メチル-2-オキソテトラヒドロピラン-4-イルエステル、(メタ)アクリル酸-4-エチル-2-オキソテトラヒドロピラン-4-イルエステル、(メタ)アクリル酸-4-プロピル-2-オキソテトラヒドロピラン-4-イルエステル、(メタ)アクリル酸-5-オキソテトラヒドロフラン-3-イルエステル、(メタ)アクリル酸-2,2-ジメチル-5-オキソテトラヒドロフラン-3-イルエステル、(メタ)アクリル酸-4,4-ジメチル-5-オキソテトラヒドロフラン-3-イルエステル、(メタ)アクリル酸-2-オキソテトラヒドロフラン-3-イルエステル、(メタ)アクリル酸-4,4-ジメチル-2-オキソテトラヒドロフラン-3-イルエステル、(メタ)アクリル酸-5,5-ジメチル-2-オキソテトラヒドロフラン-3-イルエステル、(メタ)アクリル酸-2-オキソテトラヒドロフラン-3-イルエステル、(メタ)アクリル酸-5-オキソテトラヒドロフラン-2-イルメチルエステル、(メタ)アクリル酸-3,3-ジメチル-5-オキソテトラヒドロフラン-2-イルメチルエステル、(メタ)アクリル酸-4,4-ジメチル-5-オキソテトラヒドロフラン-2-イルメチルエステル等が挙げられる。
Examples of the monomer giving the repeating unit (I-3) include (meth) acrylic acid-5-oxo-4-oxa-tricyclo [4.2.1.0 3,7 ] non-2-yl ester, (Meth) acrylic acid-9-methoxycarbonyl-5-oxo-4-oxa-tricyclo [4.2.1.0 3,7 ] non-2-yl ester, (meth) acrylic acid-5-oxo-4 -Oxa-tricyclo [5.2.1.0 3,8 ] dec-2-yl ester, (meth) acrylic acid-10-methoxycarbonyl-5-oxo-4-oxa-tricyclo [5.2.1. 0 3,8 ] Nona-2-yl ester, (meth) acrylic acid-6-oxo-7-oxa-bicyclo [3.2.1] oct-2-yl ester, (meth) acrylic acid-4-methoxy Carbonyl-6-oxo-7- Xa-bicyclo [3.2.1] oct-2-yl ester, (meth) acrylic acid-7-oxo-8-oxa-bicyclo [3.3.1] oct-2-yl ester, (meth) acrylic Acid-4-methoxycarbonyl-7-oxo-8-oxa-bicyclo [3.3.1] oct-2-yl ester, (meth) acrylic acid-2-oxotetrahydropyran-4-yl ester, (meth) Acrylic acid-4-methyl-2-oxotetrahydropyran-4-yl ester, (meth) acrylic acid-4-ethyl-2-oxotetrahydropyran-4-yl ester, (meth) acrylic acid-4-propyl-2 -Oxotetrahydropyran-4-yl ester, (meth) acrylic acid-5-oxotetrahydrofuran-3-yl ester, (meth) acrylic acid-2,2 -Dimethyl-5-oxotetrahydrofuran-3-yl ester, (meth) acrylic acid-4,4-dimethyl-5-oxotetrahydrofuran-3-yl ester, (meth) acrylic acid-2-oxotetrahydrofuran-3-yl ester (Meth) acrylic acid-4,4-dimethyl-2-oxotetrahydrofuran-3-yl ester, (meth) acrylic acid-5,5-dimethyl-2-oxotetrahydrofuran-3-yl ester, (meth) acrylic acid -2-Oxotetrahydrofuran-3-yl ester, (meth) acrylic acid-5-oxotetrahydrofuran-2-ylmethyl ester, (meth) acrylic acid-3,3-dimethyl-5-oxotetrahydrofuran-2-ylmethyl ester , (Meth) acrylic acid-4,4-dimethyl-5-oxy Tetrahydrofuran-2-yl methyl ester.
上記重合体[A]が繰り返し単位(I-3)を含む場合、この繰り返し単位(I-3)の含有量は、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは5~80モル%、より好ましくは10~70モル%、更に好ましくは10~60モル%である。繰り返し単位(I-3)の含有量が過小であると、基板との密着性が不十分となりパターンが剥がれてしまう場合がある。一方、過剰であると、アルカリ現像液への溶解性が不十分となり現像欠陥が増加してしまう場合がある。
When the polymer [A] contains the repeating unit (I-3), the content of the repeating unit (I-3) is 100 mol% in total of all the repeating units constituting the polymer [A]. On the other hand, it is preferably 5 to 80 mol%, more preferably 10 to 70 mol%, and still more preferably 10 to 60 mol%. If the content of the repeating unit (I-3) is too small, the adhesion to the substrate may be insufficient and the pattern may be peeled off. On the other hand, if the amount is excessive, the solubility in an alkali developer may be insufficient and development defects may increase.
上記重合体[A]は、他の繰り返し単位として、上記繰り返し単位(I-2)及び(I-3)以外に、更に、上記以外の脂環構造を有する繰り返し単位(以下、「繰り返し単位(I-4)」ともいう。)、芳香族系の不飽和化合物に由来する繰り返し単位(以下、「繰り返し単位(I-5)」ともいう。)等を含んでもよい。
The polymer [A] includes, as other repeating units, in addition to the repeating units (I-2) and (I-3), a repeating unit having an alicyclic structure other than those described above (hereinafter referred to as “repeating unit ( I-4) ”), and a repeating unit derived from an aromatic unsaturated compound (hereinafter also referred to as“ repeating unit (I-5) ”).
1-1-2-3.繰り返し単位(I-4)
この繰り返し単位(I-4)は、上記繰り返し単位(I-2)及び(I-3)を除く、脂環構造を有する他の繰り返し単位である。上記重合体[A]が、この繰り返し単位(I-4)を含む場合、エッチング耐性を向上させることができる。
上記繰り返し単位(I-4)としては、下記一般式(4)で表される繰り返し単位が挙げられる。
(式中、R11は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R12は、炭素数4~20の脂環式炭化水素基を含む1価の基である。)
1-1-2-3. Repeating unit (I-4)
This repeating unit (I-4) is another repeating unit having an alicyclic structure excluding the above repeating units (I-2) and (I-3). When the polymer [A] contains the repeating unit (I-4), etching resistance can be improved.
Examples of the repeating unit (I-4) include repeating units represented by the following general formula (4).
(Wherein R 11 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 12 is a monovalent group containing an alicyclic hydrocarbon group having 4 to 20 carbon atoms. is there.)
この繰り返し単位(I-4)は、上記繰り返し単位(I-2)及び(I-3)を除く、脂環構造を有する他の繰り返し単位である。上記重合体[A]が、この繰り返し単位(I-4)を含む場合、エッチング耐性を向上させることができる。
上記繰り返し単位(I-4)としては、下記一般式(4)で表される繰り返し単位が挙げられる。
This repeating unit (I-4) is another repeating unit having an alicyclic structure excluding the above repeating units (I-2) and (I-3). When the polymer [A] contains the repeating unit (I-4), etching resistance can be improved.
Examples of the repeating unit (I-4) include repeating units represented by the following general formula (4).
上記一般式(4)において、R11により表した、炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。R11としては、水素原子及びメチル基が好ましい。
In the general formula (4), the alkyl group having 1 to 4 carbon atoms represented by R 11 includes a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, Examples thereof include a 1-methylpropyl group and a tert-butyl group. R 11 is preferably a hydrogen atom or a methyl group.
R12は、炭素数4~20の脂環式炭化水素基を含む1価の基であり、炭素数4~20の1価の脂環式炭化水素基であってよいし、この脂環式炭化水素基の誘導体基であってもよい。炭素数4~20の1価の脂環式炭化水素基としては、シクロペンチル基、シクロペンチルメチル基、1-(1-シクロペンチルエチル)基、1-(2-シクロペンチルエチル)基、シクロヘキシル基、シクロヘキシルメチル基、1-(1-シクロヘキシルエチル)基、1-(2-シクロヘキシルエチル基)、シクロヘプチル基、シクロヘプチルメチル基、1-(1-シクロヘプチルエチル)基、1-(2-シクロヘプチルエチル)基、2-ノルボルニル基、ビシクロ[2.2.1]ヘプチル基、ビシクロ[2.2.1]オクチル基、トリシクロ[5.2.1.02,6]デカニル基、トリシクロ[3.3.1.13,7]デカニル基、テトラシクロ[6.2.1.13,6.02,7]ドデカニル基、アダマンチル基等の脂環式アルキル基等が挙げられる。
R 12 is a monovalent group containing an alicyclic hydrocarbon group having 4 to 20 carbon atoms, and may be a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms. It may be a derivative group of a hydrocarbon group. Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, a 1- (2-cyclopentylethyl) group, a cyclohexyl group, and a cyclohexylmethyl group. Group, 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1-cycloheptylethyl) group, 1- (2-cycloheptylethyl) ) Group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2.1.0 2,6 ] decanyl group, tricyclo [3. 3.1.1 3,7 ] decanyl group, tetracyclo [6.2.1.1 3,6 . 0 2,7 ] dodecanyl group, alicyclic alkyl group such as adamantyl group, and the like.
また、R12が、上記脂環式炭化水素基の誘導体基である場合、R12は、この脂環式炭化水素基に含まれる水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~4の直鎖状、分岐状又は環状のアルキル基、ヒドロキシル基、シアノ基、炭素数1~10のヒドロキシアルキル基、カルボキシル基、酸素原子の1種以上あるいは1個以上で置換した基等とすることができる。
When R 12 is a derivative group of the above alicyclic hydrocarbon group, R 12 represents a hydrogen atom contained in the alicyclic hydrocarbon group as a methyl group, an ethyl group, an n-propyl group, an isopropyl group. A linear, branched or cyclic alkyl group having 1 to 4 carbon atoms such as n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group, hydroxyl group, cyano group, carbon A hydroxyalkyl group, a carboxyl group, a group substituted with one or more oxygen atoms or one or more of oxygen atoms can be used.
上記重合体[A]は、繰り返し単位(I-4)を1種のみ含んでよいし、2種以上を含んでもよい。
The polymer [A] may contain only one type of repeating unit (I-4), or may contain two or more types.
上記繰り返し単位(I-4)を与える単量体としては、(メタ)アクリル酸-ビシクロ[2.2.1]ヘプト-2-イルエステル、(メタ)アクリル酸-ビシクロ[2.2.2]オクタ-2-イルエステル、(メタ)アクリル酸-トリシクロ[5.2.1.02,6]デカ-7-イルエステル、(メタ)アクリル酸-テトラシクロ[6.2.1.13,6.02,7]ドデカ-9-イルエステル、(メタ)アクリル酸-トリシクロ[3.3.1.13,7]デカ-1-イルエステル、(メタ)アクリル酸-トリシクロ[3.3.1.13,7]デカ-2-イルエステル等が挙げられる。
Examples of the monomer that gives the repeating unit (I-4) include (meth) acrylic acid-bicyclo [2.2.1] hept-2-yl ester, (meth) acrylic acid-bicyclo [2.2.2]. ] Oct-2-yl ester, (meth) acrylic acid-tricyclo [5.2.1.0 2,6 ] dec-7-yl ester, (meth) acrylic acid-tetracyclo [6.2.1.1 3 , 6 . 0 2,7 ] dodec-9-yl ester, (meth) acrylic acid-tricyclo [3.3.1.1 3,7 ] dec-1-yl ester, (meth) acrylic acid-tricyclo [3.3. 1.1,7 ] dec-2-yl ester and the like.
上記重合体[A]が繰り返し単位(I-4)を含む場合、この繰り返し単位(I-4)の含有量の上限は、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは70モル%、より好ましくは60モル%である。この繰り返し単位(I-4)の含有量が多すぎると、解像性が低下し、良好なパターン形状が得られない場合がある。
When the polymer [A] contains the repeating unit (I-4), the upper limit of the content of the repeating unit (I-4) is 100 mol in total of all the repeating units constituting the polymer [A]. % Is preferably 70 mol%, more preferably 60 mol%. If the content of the repeating unit (I-4) is too large, resolution may be lowered and a good pattern shape may not be obtained.
1-1-2-4.繰り返し単位(I-5)
この繰り返し単位(I-5)は、芳香族系の不飽和化合物に由来する繰り返し単位である。 1-1-2-4. Repeating unit (I-5)
This repeating unit (I-5) is a repeating unit derived from an aromatic unsaturated compound.
この繰り返し単位(I-5)は、芳香族系の不飽和化合物に由来する繰り返し単位である。 1-1-2-4. Repeating unit (I-5)
This repeating unit (I-5) is a repeating unit derived from an aromatic unsaturated compound.
上記繰り返し単位(I-5)において、芳香族構造部は、重合体の側鎖に含まれることが好ましい。
上記重合体[A]は、繰り返し単位(I-5)を1種のみ含んでよいし、2種以上を含んでもよい。 In the above repeating unit (I-5), the aromatic structure part is preferably contained in the side chain of the polymer.
The polymer [A] may contain only one type of repeating unit (I-5), or may contain two or more types.
上記重合体[A]は、繰り返し単位(I-5)を1種のみ含んでよいし、2種以上を含んでもよい。 In the above repeating unit (I-5), the aromatic structure part is preferably contained in the side chain of the polymer.
The polymer [A] may contain only one type of repeating unit (I-5), or may contain two or more types.
上記芳香族系の不飽和化合物としては、スチレン、α-メチルスチレン、2-メチルスチレン、3-メチルスチレン、4-メチルスチレン、2-メトキシスチレン、3-メトキシスチレン、4-メトキシスチレン、4-(2-tert-ブトキシカルボニルエチルオキシ)スチレン、2-ヒドロキシスチレン、3-ヒドロキシスチレン、4-ヒドロキシスチレン、2-ヒドロキシ-α-メチルスチレン、3-ヒドロキシ-α-メチルスチレン、4-ヒドロキシ-α-メチルスチレン、2-メチル-3-ヒドロキシスチレン、4-メチル-3-ヒドロキシスチレン、5-メチル-3-ヒドロキシスチレン、2-メチル-4-ヒドロキシスチレン、3-メチル-4-ヒドロキシスチレン、3,4-ジヒドロキシスチレン、2,4,6-トリヒドロキシスチレン、4-tert-ブトキシスチレン、4-tert-ブトキシ-α-メチルスチレン、4-(2-エチル-2-プロポキシ)スチレン、4-(2-エチル-2-プロポキシ)-α-メチルスチレン、4-(1-エトキシエトキシ)スチレン、4-(1-エトキシエトキシ)-α-メチルスチレン、(メタ)アクリル酸フェニル、(メタ)アクリル酸ベンジル、アセナフチレン、5-ヒドロキシアセナフチレン、1-ビニルナフタレン、2-ビニルナフタレン、2-ヒドロキシ-6-ビニルナフタレン、1-ナフチル(メタ)アクリレート、2-ナフチル(メタ)アクリレート、1-ナフチルメチル(メタ)アクリレート、1-アントリル(メタ)アクリレート、2-アントリル(メタ)アクリレート、9-アントリル(メタ)アクリレート、9-アントリルメチル(メタ)アクリレート、1-ビニルピレン等が挙げられる。
Examples of the aromatic unsaturated compounds include styrene, α-methylstyrene, 2-methylstyrene, 3-methylstyrene, 4-methylstyrene, 2-methoxystyrene, 3-methoxystyrene, 4-methoxystyrene, 4- (2-tert-butoxycarbonylethyloxy) styrene, 2-hydroxystyrene, 3-hydroxystyrene, 4-hydroxystyrene, 2-hydroxy-α-methylstyrene, 3-hydroxy-α-methylstyrene, 4-hydroxy-α -Methylstyrene, 2-methyl-3-hydroxystyrene, 4-methyl-3-hydroxystyrene, 5-methyl-3-hydroxystyrene, 2-methyl-4-hydroxystyrene, 3-methyl-4-hydroxystyrene, 3 , 4-Dihydroxystyrene, 2,4,6-trihydro Styrene, 4-tert-butoxystyrene, 4-tert-butoxy-α-methylstyrene, 4- (2-ethyl-2-propoxy) styrene, 4- (2-ethyl-2-propoxy) -α-methylstyrene, 4- (1-ethoxyethoxy) styrene, 4- (1-ethoxyethoxy) -α-methylstyrene, phenyl (meth) acrylate, benzyl (meth) acrylate, acenaphthylene, 5-hydroxyacenaphthylene, 1-vinyl Naphthalene, 2-vinylnaphthalene, 2-hydroxy-6-vinylnaphthalene, 1-naphthyl (meth) acrylate, 2-naphthyl (meth) acrylate, 1-naphthylmethyl (meth) acrylate, 1-anthryl (meth) acrylate, 2 -Anthryl (meth) acrylate, 9-anthryl (meth) acryl Rate, 9-anthrylmethyl (meth) acrylate, 1-vinylpyrene and the like.
上記重合体[A]が繰り返し単位(I-5)を含む場合、この繰り返し単位(I-5)の含有量の上限は、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは40モル%、より好ましくは30モル%である。この繰り返し単位(I-5)の含有量が多すぎると、露光時に、露光光の透過率が低下し、良好なパターンプロファイルが得られない場合がある。
When the polymer [A] contains the repeating unit (I-5), the upper limit of the content of the repeating unit (I-5) is 100 mol in total of all the repeating units constituting the polymer [A]. % Is preferably 40 mol%, more preferably 30 mol%. If the content of the repeating unit (I-5) is too large, the exposure light transmittance may be reduced during exposure, and a good pattern profile may not be obtained.
1-1-2-5.繰り返し単位の好ましい組み合わせ
上記重合体[A]を構成する繰り返し単位の組み合わせ及びその含有量について、以下に示す。
(T1)繰り返し単位(I-1)、繰り返し単位(I-2)及び繰り返し単位(I-3)とからなり、これらの含有量が、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、それぞれ、好ましくは5~50モル%、15~85モル%及び5~80モル%、より好ましくは5~30モル%、30~70モル%及び10~70モル%である重合体。
(T2)繰り返し単位(I-1)、繰り返し単位(I-2)、繰り返し単位(I-3)及び繰り返し単位(I-4)とからなり、これらの含有量が、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、それぞれ、好ましくは5~50モル%、15~85モル%、5~80モル%及び5~80モル%、より好ましくは5~30モル%、30~70モル%、10~50モル%及び5~50モル%である重合体。 1-1-2-5. Preferred combinations of repeating units The combinations of repeating units constituting the polymer [A] and their contents are shown below.
(T1) It consists of the repeating unit (I-1), the repeating unit (I-2) and the repeating unit (I-3), and the content of all of these repeating units constituting the polymer [A] Preferably, 5 to 50 mol%, 15 to 85 mol% and 5 to 80 mol%, more preferably 5 to 30 mol%, 30 to 70 mol% and 10 to 70 mol%, respectively, with respect to the total of 100 mol% Is a polymer.
(T2) The repeating unit (I-1), the repeating unit (I-2), the repeating unit (I-3) and the repeating unit (I-4) are contained in the polymer [A]. Are preferably 5 to 50 mol%, 15 to 85 mol%, 5 to 80 mol%, and 5 to 80 mol%, more preferably 5 to 30 mol, respectively, with respect to a total of 100 mol% of all repeating units constituting Polymers that are mol%, 30-70 mol%, 10-50 mol%, and 5-50 mol%.
上記重合体[A]を構成する繰り返し単位の組み合わせ及びその含有量について、以下に示す。
(T1)繰り返し単位(I-1)、繰り返し単位(I-2)及び繰り返し単位(I-3)とからなり、これらの含有量が、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、それぞれ、好ましくは5~50モル%、15~85モル%及び5~80モル%、より好ましくは5~30モル%、30~70モル%及び10~70モル%である重合体。
(T2)繰り返し単位(I-1)、繰り返し単位(I-2)、繰り返し単位(I-3)及び繰り返し単位(I-4)とからなり、これらの含有量が、上記重合体[A]を構成する全ての繰り返し単位の合計100モル%に対して、それぞれ、好ましくは5~50モル%、15~85モル%、5~80モル%及び5~80モル%、より好ましくは5~30モル%、30~70モル%、10~50モル%及び5~50モル%である重合体。 1-1-2-5. Preferred combinations of repeating units The combinations of repeating units constituting the polymer [A] and their contents are shown below.
(T1) It consists of the repeating unit (I-1), the repeating unit (I-2) and the repeating unit (I-3), and the content of all of these repeating units constituting the polymer [A] Preferably, 5 to 50 mol%, 15 to 85 mol% and 5 to 80 mol%, more preferably 5 to 30 mol%, 30 to 70 mol% and 10 to 70 mol%, respectively, with respect to the total of 100 mol% Is a polymer.
(T2) The repeating unit (I-1), the repeating unit (I-2), the repeating unit (I-3) and the repeating unit (I-4) are contained in the polymer [A]. Are preferably 5 to 50 mol%, 15 to 85 mol%, 5 to 80 mol%, and 5 to 80 mol%, more preferably 5 to 30 mol, respectively, with respect to a total of 100 mol% of all repeating units constituting Polymers that are mol%, 30-70 mol%, 10-50 mol%, and 5-50 mol%.
1-1-3.フッ素原子の含有率
本発明の感放射線性樹脂組成物において、上記重合体[A]に含まれるフッ素原子の含有率が、重合体[C]に含まれるフッ素原子の含有率よりも少ないことが特徴である。これにより、(C)重合体がフォトレジスト膜の表層に偏在し易くなる。従って、形成するフォトレジスト膜の表層部分の撥水性を高めることができ、液浸露光を適用する場合にその表面に上層膜(液浸上層膜)を別途形成しなくても良好な撥水性を有するフォトレジスト膜を形成することができる。
上記重合体[A]におけるフッ素原子の含有率は、後述する重合体[C]よりも小さいものである。フッ素原子の含有率は、重合体[A]全体を100質量%とした場合に、通常、10質量%未満であり、好ましくは0.1~9質量%、より好ましくは1~6質量%である。上記重合体[A]におけるフッ素原子の含有率が重合体[C]よりも小さいことにより、重合体[C]がフォトレジスト膜の表層に偏在し易くなる。従って、液浸露光を適用する場合に液浸上層膜を別途形成する必要がなく、液浸露光を円滑に進めることができ、ライン幅のばらつきの発生を抑制して、所望形状のパターンを精度良く形成することのできる化学増幅型レジストを得ることができる。 1-1-3. Content of Fluorine Atom In the radiation sensitive resin composition of the present invention, the content of the fluorine atom contained in the polymer [A] is less than the content of the fluorine atom contained in the polymer [C]. It is a feature. Thereby, the (C) polymer is likely to be unevenly distributed on the surface layer of the photoresist film. Accordingly, the water repellency of the surface layer portion of the photoresist film to be formed can be increased, and when applying immersion exposure, good water repellency can be obtained without separately forming an upper film (immersion upper film) on the surface. A photoresist film can be formed.
The fluorine atom content in the polymer [A] is smaller than that of the polymer [C] described later. The content of fluorine atoms is usually less than 10% by mass, preferably 0.1 to 9% by mass, more preferably 1 to 6% by mass, when the total amount of the polymer [A] is 100% by mass. is there. When the fluorine atom content in the polymer [A] is smaller than that of the polymer [C], the polymer [C] is likely to be unevenly distributed on the surface layer of the photoresist film. Therefore, when immersion exposure is applied, it is not necessary to separately form an immersion upper layer film, so that the immersion exposure can be carried out smoothly, the occurrence of variations in line width is suppressed, and a pattern with a desired shape is accurately obtained. A chemically amplified resist that can be well formed can be obtained.
本発明の感放射線性樹脂組成物において、上記重合体[A]に含まれるフッ素原子の含有率が、重合体[C]に含まれるフッ素原子の含有率よりも少ないことが特徴である。これにより、(C)重合体がフォトレジスト膜の表層に偏在し易くなる。従って、形成するフォトレジスト膜の表層部分の撥水性を高めることができ、液浸露光を適用する場合にその表面に上層膜(液浸上層膜)を別途形成しなくても良好な撥水性を有するフォトレジスト膜を形成することができる。
上記重合体[A]におけるフッ素原子の含有率は、後述する重合体[C]よりも小さいものである。フッ素原子の含有率は、重合体[A]全体を100質量%とした場合に、通常、10質量%未満であり、好ましくは0.1~9質量%、より好ましくは1~6質量%である。上記重合体[A]におけるフッ素原子の含有率が重合体[C]よりも小さいことにより、重合体[C]がフォトレジスト膜の表層に偏在し易くなる。従って、液浸露光を適用する場合に液浸上層膜を別途形成する必要がなく、液浸露光を円滑に進めることができ、ライン幅のばらつきの発生を抑制して、所望形状のパターンを精度良く形成することのできる化学増幅型レジストを得ることができる。 1-1-3. Content of Fluorine Atom In the radiation sensitive resin composition of the present invention, the content of the fluorine atom contained in the polymer [A] is less than the content of the fluorine atom contained in the polymer [C]. It is a feature. Thereby, the (C) polymer is likely to be unevenly distributed on the surface layer of the photoresist film. Accordingly, the water repellency of the surface layer portion of the photoresist film to be formed can be increased, and when applying immersion exposure, good water repellency can be obtained without separately forming an upper film (immersion upper film) on the surface. A photoresist film can be formed.
The fluorine atom content in the polymer [A] is smaller than that of the polymer [C] described later. The content of fluorine atoms is usually less than 10% by mass, preferably 0.1 to 9% by mass, more preferably 1 to 6% by mass, when the total amount of the polymer [A] is 100% by mass. is there. When the fluorine atom content in the polymer [A] is smaller than that of the polymer [C], the polymer [C] is likely to be unevenly distributed on the surface layer of the photoresist film. Therefore, when immersion exposure is applied, it is not necessary to separately form an immersion upper layer film, so that the immersion exposure can be carried out smoothly, the occurrence of variations in line width is suppressed, and a pattern with a desired shape is accurately obtained. A chemically amplified resist that can be well formed can be obtained.
1-1-4.平均分子量
上記重合体[A]の重量平均分子量(以下、「Mw」という。)は、ゲルパーミエーションクロマトグラフィ(GPC)法によるポリスチレン換算で、好ましくは1,000~100,000、より好ましくは1,000~30,000、更に好ましくは1,000~20,000である。Mwが1,000未満であると、耐熱性に優れたフォトレジスト膜が得られない場合がある。一方、Mwが100,000を超えると、フォトレジスト膜の現像性が低下する場合がある。
また、GPCによるポリスチレン換算の数平均分子量(以下、「Mn」という。)と、Mwとを用いて得られた比(Mw/Mn)は、好ましくは1~5、より好ましくは1~3である。 1-1-4. Average Molecular Weight The weight average molecular weight (hereinafter referred to as “Mw”) of the polymer [A] is preferably 1,000 to 100,000, more preferably 1 in terms of polystyrene by gel permeation chromatography (GPC). 3,000 to 30,000, more preferably 1,000 to 20,000. If the Mw is less than 1,000, a photoresist film having excellent heat resistance may not be obtained. On the other hand, if Mw exceeds 100,000, the developability of the photoresist film may deteriorate.
The ratio (Mw / Mn) obtained by using GPC in terms of polystyrene-equivalent number average molecular weight (hereinafter referred to as “Mn”) and Mw is preferably 1 to 5, more preferably 1 to 3. is there.
上記重合体[A]の重量平均分子量(以下、「Mw」という。)は、ゲルパーミエーションクロマトグラフィ(GPC)法によるポリスチレン換算で、好ましくは1,000~100,000、より好ましくは1,000~30,000、更に好ましくは1,000~20,000である。Mwが1,000未満であると、耐熱性に優れたフォトレジスト膜が得られない場合がある。一方、Mwが100,000を超えると、フォトレジスト膜の現像性が低下する場合がある。
また、GPCによるポリスチレン換算の数平均分子量(以下、「Mn」という。)と、Mwとを用いて得られた比(Mw/Mn)は、好ましくは1~5、より好ましくは1~3である。 1-1-4. Average Molecular Weight The weight average molecular weight (hereinafter referred to as “Mw”) of the polymer [A] is preferably 1,000 to 100,000, more preferably 1 in terms of polystyrene by gel permeation chromatography (GPC). 3,000 to 30,000, more preferably 1,000 to 20,000. If the Mw is less than 1,000, a photoresist film having excellent heat resistance may not be obtained. On the other hand, if Mw exceeds 100,000, the developability of the photoresist film may deteriorate.
The ratio (Mw / Mn) obtained by using GPC in terms of polystyrene-equivalent number average molecular weight (hereinafter referred to as “Mn”) and Mw is preferably 1 to 5, more preferably 1 to 3. is there.
1-1-5.製造方法
上記重合体[A]は、ハイドロパーオキシド、ジアルキルパーオキシド、ジアシルパーオキシド、アゾ化合物等のラジカル重合開始剤の存在下、繰り返し単位(I-1)を形成することとなる単量体を含む単量体原料を、適当な溶媒中において重合することにより、製造することができる。
重合温度は、通常、40℃~150℃、好ましくは50℃~120℃である。また、重合時間は、通常、1~48時間、好ましくは1~24時間である。 1-1-5. Production Method The polymer [A] is a monomer that forms the repeating unit (I-1) in the presence of a radical polymerization initiator such as hydroperoxide, dialkyl peroxide, diacyl peroxide, and azo compound. It can manufacture by polymerizing the monomer raw material containing this in a suitable solvent.
The polymerization temperature is usually 40 ° C. to 150 ° C., preferably 50 ° C. to 120 ° C. The polymerization time is usually 1 to 48 hours, preferably 1 to 24 hours.
上記重合体[A]は、ハイドロパーオキシド、ジアルキルパーオキシド、ジアシルパーオキシド、アゾ化合物等のラジカル重合開始剤の存在下、繰り返し単位(I-1)を形成することとなる単量体を含む単量体原料を、適当な溶媒中において重合することにより、製造することができる。
重合温度は、通常、40℃~150℃、好ましくは50℃~120℃である。また、重合時間は、通常、1~48時間、好ましくは1~24時間である。 1-1-5. Production Method The polymer [A] is a monomer that forms the repeating unit (I-1) in the presence of a radical polymerization initiator such as hydroperoxide, dialkyl peroxide, diacyl peroxide, and azo compound. It can manufacture by polymerizing the monomer raw material containing this in a suitable solvent.
The polymerization temperature is usually 40 ° C. to 150 ° C., preferably 50 ° C. to 120 ° C. The polymerization time is usually 1 to 48 hours, preferably 1 to 24 hours.
上記溶媒としては、n-ペンタン、n-ヘキサン、n-ヘプタン、n-オクタン、n-ノナン、n-デカン等のアルカン類;シクロヘキサン、シクロヘプタン、シクロオクタン等のシクロアルカン類;デカリン、ノルボルナン等の脂環式炭化水素類;ベンゼン、トルエン、キシレン、エチルベンゼン、クメン等の芳香族炭化水素類;クロロブタン、ブロモヘキサン、ジクロロエタン、ヘキサメチレンジブロミド、クロロベンゼン等のハロゲン化炭化水素類;酢酸エチル、酢酸n-ブチル、酢酸イソブチル、プロピオン酸メチル等の飽和カルボン酸エステル類;アセトン、2-ブタノン、4-メチル-2-ペンタノン、2-ヘプタノン等のケトン類;テトラヒドロフラン、ジメトキシエタン、ジエトキシエタン等のエーテル類;メタノール、エタノール、1-プロパノール、2-プロパノール、4-メチル-2-ペンタノール等のアルコール類等が挙げられる。尚、これらの溶媒は、単独で用いてよいし、2種以上を組み合わせて用いてもよい。
Examples of the solvent include alkanes such as n-pentane, n-hexane, n-heptane, n-octane, n-nonane and n-decane; cycloalkanes such as cyclohexane, cycloheptane and cyclooctane; decalin, norbornane and the like Alicyclic hydrocarbons; aromatic hydrocarbons such as benzene, toluene, xylene, ethylbenzene, cumene; halogenated hydrocarbons such as chlorobutane, bromohexane, dichloroethane, hexamethylene dibromide, chlorobenzene; ethyl acetate, acetic acid saturated carboxylic acid esters such as n-butyl, isobutyl acetate and methyl propionate; ketones such as acetone, 2-butanone, 4-methyl-2-pentanone and 2-heptanone; tetrahydrofuran, dimethoxyethane, diethoxyethane and the like Ethers; methanol, Ethanol, 1-propanol, 2-propanol, alcohols such as 4-methyl-2-pentanol. These solvents may be used alone or in combination of two or more.
尚、本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物の製造に用いられる重合体[A]においては、ハロゲン、金属等の不純物の含有量が少ないほど好ましい。このような不純物の含有量が少ない場合、フォトレジスト膜の感度、解像度、プロセス安定性、パターン形状等を更に向上させることができる。従って、上記方法により合成された重合体[A]を含む粗生成物を精製に供することが好ましい。精製法としては、水洗、液々抽出等の化学的精製法や、これらの化学的精製法と限外ろ過、遠心分離等の物理的精製法とを組み合わせた方法等が挙げられる。
In addition, in polymer [A] used for manufacture of the radiation sensitive resin composition of this invention or the radiation sensitive resin composition for immersion exposure, it is so preferable that there is little content of impurities, such as a halogen and a metal. When the content of such impurities is small, the sensitivity, resolution, process stability, pattern shape and the like of the photoresist film can be further improved. Therefore, it is preferable to subject the crude product containing the polymer [A] synthesized by the above method to purification. Examples of the purification method include chemical purification methods such as washing with water and liquid-liquid extraction, and methods combining these chemical purification methods with physical purification methods such as ultrafiltration and centrifugation.
また、上記重合体[A]においては、単量体原料に由来する低分子量成分の含有量が少ないほど好ましい。具体的には、この含有量は、重合体[A]に対して、好ましくは0.1質量%以下、より好ましくは0.07質量%以下、更に好ましくは0.05質量%以下である。この含有量が0.1質量%以下である場合には、液浸露光時に接触した、水等の液浸露光用液体への溶出物の量を抑制することができる。更に、本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物の保管時に、異物を生成することがなく、本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物の塗布時においても、塗布ムラを抑制することができ、レジストパターン形成時における欠陥の発生をも、十分に抑制することができる。
尚、上記単量体原料に由来する低分子量成分は、モノマー、ダイマー、トリマー、オリゴマー等を意味し、通常、分子量500以下の成分とすることができる。このような分子量500以下の成分は、例えば、水洗、液々抽出等の化学的精製法や、これらの化学的精製法と限外ろ過、遠心分離等の物理的精製法との組み合わせ等により除去することができる。また、上記単量体原料に由来する低分子量成分は、重合体[A]を含む粗生成物を高速液体クロマトグラフィ(HPLC)に供することより分析することができる。 Moreover, in the said polymer [A], it is so preferable that there is little content of the low molecular weight component originating in a monomer raw material. Specifically, the content is preferably 0.1% by mass or less, more preferably 0.07% by mass or less, and still more preferably 0.05% by mass or less with respect to the polymer [A]. When this content is 0.1% by mass or less, it is possible to suppress the amount of the eluate in the immersion exposure liquid such as water that is in contact with the immersion exposure. Furthermore, when the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure is stored, no foreign matter is generated, and the radiation-sensitive resin composition of the present invention or radiation exposure for immersion exposure is performed. Even when the conductive resin composition is applied, uneven application can be suppressed, and the occurrence of defects when forming the resist pattern can be sufficiently suppressed.
In addition, the low molecular weight component derived from the said monomer raw material means a monomer, a dimer, a trimer, an oligomer, etc., and can be normally made into a component with a molecular weight of 500 or less. Such components having a molecular weight of 500 or less are removed by, for example, chemical purification methods such as washing with water and liquid-liquid extraction, or a combination of these chemical purification methods and physical purification methods such as ultrafiltration and centrifugation. can do. The low molecular weight component derived from the monomer raw material can be analyzed by subjecting the crude product containing the polymer [A] to high performance liquid chromatography (HPLC).
尚、上記単量体原料に由来する低分子量成分は、モノマー、ダイマー、トリマー、オリゴマー等を意味し、通常、分子量500以下の成分とすることができる。このような分子量500以下の成分は、例えば、水洗、液々抽出等の化学的精製法や、これらの化学的精製法と限外ろ過、遠心分離等の物理的精製法との組み合わせ等により除去することができる。また、上記単量体原料に由来する低分子量成分は、重合体[A]を含む粗生成物を高速液体クロマトグラフィ(HPLC)に供することより分析することができる。 Moreover, in the said polymer [A], it is so preferable that there is little content of the low molecular weight component originating in a monomer raw material. Specifically, the content is preferably 0.1% by mass or less, more preferably 0.07% by mass or less, and still more preferably 0.05% by mass or less with respect to the polymer [A]. When this content is 0.1% by mass or less, it is possible to suppress the amount of the eluate in the immersion exposure liquid such as water that is in contact with the immersion exposure. Furthermore, when the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure is stored, no foreign matter is generated, and the radiation-sensitive resin composition of the present invention or radiation exposure for immersion exposure is performed. Even when the conductive resin composition is applied, uneven application can be suppressed, and the occurrence of defects when forming the resist pattern can be sufficiently suppressed.
In addition, the low molecular weight component derived from the said monomer raw material means a monomer, a dimer, a trimer, an oligomer, etc., and can be normally made into a component with a molecular weight of 500 or less. Such components having a molecular weight of 500 or less are removed by, for example, chemical purification methods such as washing with water and liquid-liquid extraction, or a combination of these chemical purification methods and physical purification methods such as ultrafiltration and centrifugation. can do. The low molecular weight component derived from the monomer raw material can be analyzed by subjecting the crude product containing the polymer [A] to high performance liquid chromatography (HPLC).
1-2.酸発生剤[B]
この酸発生剤[B]は、本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物によって形成されたフォトレジスト膜が液浸露光用液体を介して露光された際に、露光部からこの酸発生剤[B]に由来する酸を発生させる成分である。この酸の作用によって、露光部において、重合体[A]に含まれる酸解離性基が、この重合体から解離する。そして、酸解離性基が解離した重合体は、アルカリ現像液に易溶性となる。その後、アルカリ現像液を用いてフォトレジスト膜の不要部分を除去することにより、所望の形状を有するポジ型のレジストパターンを得ることができる。 1-2. Acid generator [B]
This acid generator [B] is used when the photoresist film formed by the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure is exposed through the immersion exposure liquid. , A component that generates an acid derived from the acid generator [B] from the exposed portion. By the action of the acid, the acid dissociable group contained in the polymer [A] is dissociated from the polymer in the exposed portion. The polymer from which the acid dissociable group is dissociated becomes readily soluble in an alkali developer. Then, a positive resist pattern having a desired shape can be obtained by removing an unnecessary portion of the photoresist film using an alkali developer.
この酸発生剤[B]は、本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物によって形成されたフォトレジスト膜が液浸露光用液体を介して露光された際に、露光部からこの酸発生剤[B]に由来する酸を発生させる成分である。この酸の作用によって、露光部において、重合体[A]に含まれる酸解離性基が、この重合体から解離する。そして、酸解離性基が解離した重合体は、アルカリ現像液に易溶性となる。その後、アルカリ現像液を用いてフォトレジスト膜の不要部分を除去することにより、所望の形状を有するポジ型のレジストパターンを得ることができる。 1-2. Acid generator [B]
This acid generator [B] is used when the photoresist film formed by the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure is exposed through the immersion exposure liquid. , A component that generates an acid derived from the acid generator [B] from the exposed portion. By the action of the acid, the acid dissociable group contained in the polymer [A] is dissociated from the polymer in the exposed portion. The polymer from which the acid dissociable group is dissociated becomes readily soluble in an alkali developer. Then, a positive resist pattern having a desired shape can be obtained by removing an unnecessary portion of the photoresist film using an alkali developer.
本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物に含有される酸発生剤[B]としては、従来、公知の感放射線性酸発生剤を適用することができる。例えば、特開2009-134088号公報の段落[0080]~[0113]等に記載されている化合物等を用いることができる。
As the acid generator [B] contained in the radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure, conventionally known radiation-sensitive acid generators can be applied. For example, compounds described in paragraphs [0080] to [0113] of JP-A-2009-134088 can be used.
上記酸発生剤[B]としては、トリフェニルスルホニウム塩化合物、4-シクロヘキシルフェニルジフェニルスルホニウム塩化合物、4-tert-ブチルフェニルジフェニルスルホニウム塩化合物、トリ(4-tert-ブチルフェニル)スルホニウム塩化合物、ジフェニルヨードニウム塩化合物、ビス(4-tert-ブチルフェニル)ヨードニウム塩化合物、1-(4-n-ブトキシナフタレン-1-イル)テトラヒドロチオフェニウム塩化合物、1-(3,5-ジメチル-4-ヒドロキシフェニル)テトラヒドロチオフェニウム塩化合物、スクシンイミド類化合物、ビシクロ[2.2.1]ヘプト-5-エン-2,3-ジカルボキシイミド類化合物等が挙げられる。これらの酸発生剤は、単独で用いてよいし、2種以上を組み合わせて用いてもよい。
Examples of the acid generator [B] include triphenylsulfonium salt compounds, 4-cyclohexylphenyldiphenylsulfonium salt compounds, 4-tert-butylphenyldiphenylsulfonium salt compounds, tri (4-tert-butylphenyl) sulfonium salt compounds, diphenyl Iodonium salt compound, bis (4-tert-butylphenyl) iodonium salt compound, 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium salt compound, 1- (3,5-dimethyl-4-hydroxy Phenyl) tetrahydrothiophenium salt compounds, succinimide compounds, bicyclo [2.2.1] hept-5-ene-2,3-dicarboximide compounds, and the like. These acid generators may be used alone or in combination of two or more.
上記トリフェニルスルホニウム塩化合物としては、トリフェニルスルホニウムトリフルオロメタンスルホネート、トリフェニルスルホニウムノナフルオロ-n-ブタンスルホネート、トリフェニルスルホニウムパーフルオロ-n-オクタンスルホネート、トリフェニルスルホニウム2-ビシクロ[2.2.1]ヘプト-2-イル-1,1,2,2-テトラフルオロエタンスルホネート、トリフェニルスルホニウム=トリシクロ[3.3.1.13,7]デカニルジフルオロメタンスルホナート、トリフェニルスルホニウム2-(3-テトラシクロ[4.4.0.12,5.17,10]ドデカニル)-1,1-ジフルオロエタンスルホネート、トリフェニルスルホニウムN,N’-ビス(ノナフルオロ-n-ブタンスルホニル)イミデート、トリフェニルスルホニウムカンファースルホネート等が挙げられる。
Examples of the triphenylsulfonium salt compound include triphenylsulfonium trifluoromethanesulfonate, triphenylsulfonium nonafluoro-n-butanesulfonate, triphenylsulfonium perfluoro-n-octanesulfonate, triphenylsulfonium 2-bicyclo [2.2.1]. ] Hept-2-yl-1,1,2,2-tetrafluoroethanesulfonate, triphenylsulfonium = tricyclo [3.3.1.1 3,7 ] decanyl difluoromethanesulfonate, triphenylsulfonium 2- ( 3- tetracyclo [4.4.0.1 2,5 .1 7,10] dodecanyl) -1,1-difluoroethanesulfonate, triphenylsulfonium N, N'-bis (nonafluoro -n- butanesulfonyl) imidate And triphenylsulfonium camphorsulfonate.
上記4-シクロヘキシルフェニルジフェニルスルホニウム塩化合物としては、4-シクロヘキシルフェニルジフェニルスルホニウムトリフルオロメタンスルホネート、4-シクロヘキシルフェニルジフェニルスルホニウムノナフルオロ-n-ブタンスルホネート、4-シクロヘキシルフェニルジフェニルスルホニウムパーフルオロ-n-オクタンスルホネート、4-シクロヘキシルフェニルジフェニルスルホニウム2-ビシクロ[2.2.1]ヘプト-2-イル-1,1,2,2-テトラフルオロエタンスルホネート、4-シクロヘキシルフェニルジフェニルスルホニウム2-(3-テトラシクロ[4.4.0.12,5.17,10]ドデカニル)-1,1-ジフルオロエタンスルホネート、4-シクロヘキシルフェニルジフェニルスルホニウムN,N’-ビス(ノナフルオロ-n-ブタンスルホニル)イミデート、4-シクロヘキシルフェニルジフェニルスルホニウムカンファースルホネート等が挙げられる。
Examples of the 4-cyclohexylphenyldiphenylsulfonium salt compound include 4-cyclohexylphenyldiphenylsulfonium trifluoromethanesulfonate, 4-cyclohexylphenyldiphenylsulfonium nonafluoro-n-butanesulfonate, 4-cyclohexylphenyldiphenylsulfonium perfluoro-n-octanesulfonate, 4-cyclohexylphenyldiphenylsulfonium 2-bicyclo [2.2.1] hept-2-yl-1,1,2,2-tetrafluoroethanesulfonate, 4-cyclohexylphenyldiphenylsulfonium 2- (3-tetracyclo [4. 4.0.1 2,5 .1 7,10 ] dodecanyl) -1,1-difluoroethanesulfonate, 4-cyclohexylphenyldiphenylsulfate Examples include onium N, N′-bis (nonafluoro-n-butanesulfonyl) imidate, 4-cyclohexylphenyldiphenylsulfonium camphorsulfonate, and the like.
上記4-tert-ブチルフェニルジフェニルスルホニウム塩化合物としては、4-tert-ブチルフェニルジフェニルスルホニウムトリフルオロメタンスルホネート、4-tert-ブチルフェニルジフェニルスルホニウムノナフルオロ-n-ブタンスルホネート、4-tert-ブチルフェニルジフェニルスルホニウムパーフルオロ-n-オクタンスルホネート等が挙げられる。
上記トリ(4-tert-ブチルフェニル)スルホニウム塩化合物としては、トリ(4-tert-ブチルフェニル)スルホニウムトリフルオロメタンスルホネート、トリ(4-tert-ブチルフェニル)スルホニウムノナフルオロ-n-ブタンスルホネート等が挙げられる。 Examples of the 4-tert-butylphenyldiphenylsulfonium salt compound include 4-tert-butylphenyldiphenylsulfonium trifluoromethanesulfonate, 4-tert-butylphenyldiphenylsulfonium nonafluoro-n-butanesulfonate, 4-tert-butylphenyldiphenylsulfonium. And perfluoro-n-octane sulfonate.
Examples of the tri (4-tert-butylphenyl) sulfonium salt compound include tri (4-tert-butylphenyl) sulfonium trifluoromethanesulfonate, tri (4-tert-butylphenyl) sulfonium nonafluoro-n-butanesulfonate, and the like. It is done.
上記トリ(4-tert-ブチルフェニル)スルホニウム塩化合物としては、トリ(4-tert-ブチルフェニル)スルホニウムトリフルオロメタンスルホネート、トリ(4-tert-ブチルフェニル)スルホニウムノナフルオロ-n-ブタンスルホネート等が挙げられる。 Examples of the 4-tert-butylphenyldiphenylsulfonium salt compound include 4-tert-butylphenyldiphenylsulfonium trifluoromethanesulfonate, 4-tert-butylphenyldiphenylsulfonium nonafluoro-n-butanesulfonate, 4-tert-butylphenyldiphenylsulfonium. And perfluoro-n-octane sulfonate.
Examples of the tri (4-tert-butylphenyl) sulfonium salt compound include tri (4-tert-butylphenyl) sulfonium trifluoromethanesulfonate, tri (4-tert-butylphenyl) sulfonium nonafluoro-n-butanesulfonate, and the like. It is done.
上記1-(4-n-ブトキシナフタレン-1-イル)テトラヒドロチオフェニウム塩化合物としては、1-(4-n-ブトキシナフタレン-1-イル)テトラヒドロチオフェニウムトリフルオロメタンスルホネート、1-(4-n-ブトキシナフタレン-1-イル)テトラヒドロチオフェニウムノナフルオロ-n-ブタンスルホネート、1-(4-n-ブトキシナフタレン-1-イル)テトラヒドロチオフェニウムパーフルオロ-n-オクタンスルホネート、1-(4-n-ブトキシナフタレン-1-イル)テトラヒドロチオフェニウム2-ビシクロ[2.2.1]ヘプト-2-イル-1,1,2,2-テトラフルオロエタンスルホネート、1-(4-n-ブトキシナフタレン-1-イル)テトラヒドロチオフェニウム2-(3-テトラシクロ[4.4.0.12,5.17,10]ドデカニル)-1,1-ジフルオロエタンスルホネート等が挙げられる。
Examples of the 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium salt compound include 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium trifluoromethanesulfonate, 1- (4 -N-butoxynaphthalen-1-yl) tetrahydrothiophenium nonafluoro-n-butanesulfonate, 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium perfluoro-n-octanesulfonate, 1- (4-n-butoxynaphthalen-1-yl) tetrahydrothiophenium 2-bicyclo [2.2.1] hept-2-yl-1,1,2,2-tetrafluoroethanesulfonate, 1- (4- n-Butoxynaphthalen-1-yl) tetrahydrothiophenium 2- (3-tetracycline [4.4.0.1 2,5 .1 7,10] dodecanyl) -1,1-difluoroethanesulfonate, and the like.
上記1-(3,5-ジメチル-4-ヒドロキシフェニル)テトラヒドロチオフェニウム塩化合物としては、1-(3,5-ジメチル-4-ヒドロキシフェニル)テトラヒドロチオフェニウムトリフルオロメタンスルホネート、1-(3,5-ジメチル-4-ヒドロキシフェニル)テトラヒドロチオフェニウムノナフルオロ-n-ブタンスルホネート等が挙げられる。
Examples of the 1- (3,5-dimethyl-4-hydroxyphenyl) tetrahydrothiophenium salt compound include 1- (3,5-dimethyl-4-hydroxyphenyl) tetrahydrothiophenium trifluoromethanesulfonate, 1- (3 , 5-dimethyl-4-hydroxyphenyl) tetrahydrothiophenium nonafluoro-n-butanesulfonate.
本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物における酸発生剤[B]の含有量は、上記重合体[A]100質量部に対して、好ましくは0.1~30質量部、より好ましくは2~27質量部、更に好ましくは5~25質量部である。上記酸発生剤[B]の含有量が、この範囲にあると、形成したフォトレジスト膜における感度及び現像性を高く維持することができる。尚、酸発生剤[B]の含有量が少なすぎると、感度及び解像度が十分でない場合がある。一方、酸発生剤[B]の含有量が多すぎると、組成物の塗布性が十分でなく、良好なパターン形状が得られない場合がある。
The content of the acid generator [B] in the radiation-sensitive resin composition or the radiation-sensitive resin composition for immersion exposure of the present invention is preferably 0.1 with respect to 100 parts by mass of the polymer [A]. -30 parts by mass, more preferably 2-27 parts by mass, still more preferably 5-25 parts by mass. When the content of the acid generator [B] is within this range, the sensitivity and developability of the formed photoresist film can be maintained high. In addition, when there is too little content of acid generator [B], a sensitivity and resolution may not be enough. On the other hand, when there is too much content of acid generator [B], the applicability | paintability of a composition is not enough and a favorable pattern shape may not be obtained.
1-3.重合体[C]
本発明の感放射線性樹脂組成物に含有される重合体[C]は、フッ素原子を含む重合体であり、上記重合体[A]よりもフッ素原子の含有率が高いものである。フッ素原子の含有率は、重合体[C]全体を100質量%とした場合に、好ましくは5質量%以上、より好ましくは5~40質量%、更に好ましくは8~30質量%、特に好ましくは10~20質量%である。本発明の感放射線性樹脂組成物を用いて、フォトレジスト膜を形成した場合、この重合体[C]の撥油性に起因して、フォトレジスト膜の表面において重合体[C]の分布が高くなる傾向がある。即ち、重合体[C]が、フォトレジスト膜の最表面に偏在する傾向がある。従って、フォトレジスト膜と液浸媒体とを遮断することを目的として、フォトレジスト膜に、液浸上層膜を形成する必要がなく、そのまま、液浸露光法に好適に用いることができる。
尚、本発明の効果を十分に発揮するためには、上記重合体[A]におけるフッ素原子の含有率と、上記重合体[C]におけるフッ素原子の含有率との差が、好ましくは1質量%以上、より好ましくは3質量%以上、特に好ましくは5質量%以上である。 1-3. Polymer [C]
The polymer [C] contained in the radiation-sensitive resin composition of the present invention is a polymer containing a fluorine atom and has a higher fluorine atom content than the polymer [A]. The fluorine atom content is preferably 5% by mass or more, more preferably 5 to 40% by mass, still more preferably 8 to 30% by mass, particularly preferably 100% by mass of the entire polymer [C]. 10 to 20% by mass. When a photoresist film is formed using the radiation sensitive resin composition of the present invention, the distribution of the polymer [C] is high on the surface of the photoresist film due to the oil repellency of the polymer [C]. Tend to be. That is, the polymer [C] tends to be unevenly distributed on the outermost surface of the photoresist film. Therefore, for the purpose of blocking the photoresist film and the immersion medium, it is not necessary to form an immersion upper layer film on the photoresist film, and it can be suitably used for the immersion exposure method as it is.
In order to sufficiently exert the effect of the present invention, the difference between the fluorine atom content in the polymer [A] and the fluorine atom content in the polymer [C] is preferably 1 mass. % Or more, more preferably 3% by mass or more, and particularly preferably 5% by mass or more.
本発明の感放射線性樹脂組成物に含有される重合体[C]は、フッ素原子を含む重合体であり、上記重合体[A]よりもフッ素原子の含有率が高いものである。フッ素原子の含有率は、重合体[C]全体を100質量%とした場合に、好ましくは5質量%以上、より好ましくは5~40質量%、更に好ましくは8~30質量%、特に好ましくは10~20質量%である。本発明の感放射線性樹脂組成物を用いて、フォトレジスト膜を形成した場合、この重合体[C]の撥油性に起因して、フォトレジスト膜の表面において重合体[C]の分布が高くなる傾向がある。即ち、重合体[C]が、フォトレジスト膜の最表面に偏在する傾向がある。従って、フォトレジスト膜と液浸媒体とを遮断することを目的として、フォトレジスト膜に、液浸上層膜を形成する必要がなく、そのまま、液浸露光法に好適に用いることができる。
尚、本発明の効果を十分に発揮するためには、上記重合体[A]におけるフッ素原子の含有率と、上記重合体[C]におけるフッ素原子の含有率との差が、好ましくは1質量%以上、より好ましくは3質量%以上、特に好ましくは5質量%以上である。 1-3. Polymer [C]
The polymer [C] contained in the radiation-sensitive resin composition of the present invention is a polymer containing a fluorine atom and has a higher fluorine atom content than the polymer [A]. The fluorine atom content is preferably 5% by mass or more, more preferably 5 to 40% by mass, still more preferably 8 to 30% by mass, particularly preferably 100% by mass of the entire polymer [C]. 10 to 20% by mass. When a photoresist film is formed using the radiation sensitive resin composition of the present invention, the distribution of the polymer [C] is high on the surface of the photoresist film due to the oil repellency of the polymer [C]. Tend to be. That is, the polymer [C] tends to be unevenly distributed on the outermost surface of the photoresist film. Therefore, for the purpose of blocking the photoresist film and the immersion medium, it is not necessary to form an immersion upper layer film on the photoresist film, and it can be suitably used for the immersion exposure method as it is.
In order to sufficiently exert the effect of the present invention, the difference between the fluorine atom content in the polymer [A] and the fluorine atom content in the polymer [C] is preferably 1 mass. % Or more, more preferably 3% by mass or more, and particularly preferably 5% by mass or more.
上記重合体[C]は、フッ素原子を含む繰り返し単位を少なくとも含む重合体であり、フッ素原子を含まない繰り返し単位を有してもよい。
フッ素原子を含む繰り返し単位は、好ましくは下記一般式(c1-1)~(c1-3)で表される繰り返し単位からなる群より選択された少なくとも1つの繰り返し単位(以下、これらを併せて、「繰り返し単位(III-1)」という。)である。
(式中、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R22は、炭素数1~30のフッ素化炭化水素基であり、R23は、相互に独立に、水素原子、フッ素原子又は炭素数1~30のフッ素化炭化水素基であり、R24は、水素原子、酸解離性基又はアルカリ解離性基であり、Q3は、(g+1)価の連結基であり、Q4は、2価の連結基であり、gは、1、2又は3である。但し、式(c1-2)及び(c1-3)において、全てのR23が水素原子である場合はない。)
The polymer [C] is a polymer containing at least a repeating unit containing a fluorine atom, and may have a repeating unit containing no fluorine atom.
The repeating unit containing a fluorine atom is preferably at least one repeating unit selected from the group consisting of repeating units represented by the following general formulas (c1-1) to (c1-3) (hereinafter these are combined) "Repeating unit (III-1)").
(Wherein R 21 represents a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 22 represents a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and R 23 represents Independently a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms, R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group, and Q 3 is (g + 1) Q 4 is a divalent linking group and g is 1, 2 or 3. provided that in formulas (c1-2) and (c1-3), all R 23 Is not a hydrogen atom.)
フッ素原子を含む繰り返し単位は、好ましくは下記一般式(c1-1)~(c1-3)で表される繰り返し単位からなる群より選択された少なくとも1つの繰り返し単位(以下、これらを併せて、「繰り返し単位(III-1)」という。)である。
The repeating unit containing a fluorine atom is preferably at least one repeating unit selected from the group consisting of repeating units represented by the following general formulas (c1-1) to (c1-3) (hereinafter these are combined) "Repeating unit (III-1)").
1-3-1.繰り返し単位(III-1)
上記重合体[C]が、上記一般式(c1-1)で表される繰り返し単位(以下、「繰り返し単位(III-1-1)」という。)、上記一般式(c1-2)で表される繰り返し単位(以下、「繰り返し単位(III-1-2)」という。)、及び、上記一般式(c1-3)で表される繰り返し単位(以下、「繰り返し単位(III-1-3)」という。)、からなる群より選択された少なくとも1つの繰り返し単位(III-1)を含む場合、フォトレジスト膜中の酸発生剤[B]や、必要に応じて含まれる酸拡散抑制剤等の添加剤が、液浸露光用液体に溶出するのを抑制することができる。また、フォトレジスト膜の表面における、液浸露光用液体の後退接触角を大きくすることができ、液浸露光用液体に由来する液滴が、フォトレジスト膜上に残り難くなり、液浸露光用液体に起因する欠陥の発生を抑制することができる。 1-3-1. Repeating unit (III-1)
The polymer [C] is a repeating unit represented by the general formula (c1-1) (hereinafter referred to as “repeating unit (III-1-1)”), represented by the general formula (c1-2). Repeating units (hereinafter referred to as “repeating units (III-1-2)”) and repeating units represented by the above general formula (c1-3) (hereinafter referred to as “repeating units (III-1-3)”. In the case of containing at least one repeating unit (III-1) selected from the group consisting of :) an acid generator [B] in a photoresist film, and an acid diffusion inhibitor contained as necessary And the like can be prevented from eluting into the immersion exposure liquid. In addition, the receding contact angle of the immersion exposure liquid on the surface of the photoresist film can be increased, so that droplets derived from the immersion exposure liquid are less likely to remain on the photoresist film. Generation of defects due to the liquid can be suppressed.
上記重合体[C]が、上記一般式(c1-1)で表される繰り返し単位(以下、「繰り返し単位(III-1-1)」という。)、上記一般式(c1-2)で表される繰り返し単位(以下、「繰り返し単位(III-1-2)」という。)、及び、上記一般式(c1-3)で表される繰り返し単位(以下、「繰り返し単位(III-1-3)」という。)、からなる群より選択された少なくとも1つの繰り返し単位(III-1)を含む場合、フォトレジスト膜中の酸発生剤[B]や、必要に応じて含まれる酸拡散抑制剤等の添加剤が、液浸露光用液体に溶出するのを抑制することができる。また、フォトレジスト膜の表面における、液浸露光用液体の後退接触角を大きくすることができ、液浸露光用液体に由来する液滴が、フォトレジスト膜上に残り難くなり、液浸露光用液体に起因する欠陥の発生を抑制することができる。 1-3-1. Repeating unit (III-1)
The polymer [C] is a repeating unit represented by the general formula (c1-1) (hereinafter referred to as “repeating unit (III-1-1)”), represented by the general formula (c1-2). Repeating units (hereinafter referred to as “repeating units (III-1-2)”) and repeating units represented by the above general formula (c1-3) (hereinafter referred to as “repeating units (III-1-3)”. In the case of containing at least one repeating unit (III-1) selected from the group consisting of :) an acid generator [B] in a photoresist film, and an acid diffusion inhibitor contained as necessary And the like can be prevented from eluting into the immersion exposure liquid. In addition, the receding contact angle of the immersion exposure liquid on the surface of the photoresist film can be increased, so that droplets derived from the immersion exposure liquid are less likely to remain on the photoresist film. Generation of defects due to the liquid can be suppressed.
1-3-1-1.繰り返し単位(III-1-1)
この繰り返し単位(III-1-1)は、上記一般式(c1-1)で表される繰り返し単位である。
上記一般式(c1-1)において、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基である。炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。R1としては、水素原子及びメチル基が好ましい。 1-3-1. Repeating unit (III-1-1)
This repeating unit (III-1-1) is a repeating unit represented by the above general formula (c1-1).
In the general formula (c1-1), R 21 represents a hydrogen atom, a trifluoromethyl group, or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. . R 1 is preferably a hydrogen atom or a methyl group.
この繰り返し単位(III-1-1)は、上記一般式(c1-1)で表される繰り返し単位である。
上記一般式(c1-1)において、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基である。炭素数1~4のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。R1としては、水素原子及びメチル基が好ましい。 1-3-1. Repeating unit (III-1-1)
This repeating unit (III-1-1) is a repeating unit represented by the above general formula (c1-1).
In the general formula (c1-1), R 21 represents a hydrogen atom, a trifluoromethyl group, or an alkyl group having 1 to 4 carbon atoms. Examples of the alkyl group having 1 to 4 carbon atoms include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group and the like. . R 1 is preferably a hydrogen atom or a methyl group.
また、上記一般式(c1-1)において、R22は、炭素数1~30のフッ素化炭化水素基であり、炭素数1~30の炭化水素基における水素原子の少なくとも1つが、フッ素原子に置換されてなる基である。
R22は、好ましくは、少なくとも1つの水素原子が、フッ素原子で置換された、炭素数1~6の直鎖状又は分岐状のアルキル基;少なくとも1つの水素原子が、フッ素原子で置換された、炭素数4~20の1価の脂環式炭化水素基又はその誘導体基等である。 In the general formula (c1-1), R 22 is a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and at least one hydrogen atom in the hydrocarbon group having 1 to 30 carbon atoms is a fluorine atom. It is a group formed by substitution.
R 22 is preferably a linear or branched alkyl group having 1 to 6 carbon atoms in which at least one hydrogen atom is substituted with a fluorine atom; at least one hydrogen atom is substituted with a fluorine atom And a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms or a derivative group thereof.
R22は、好ましくは、少なくとも1つの水素原子が、フッ素原子で置換された、炭素数1~6の直鎖状又は分岐状のアルキル基;少なくとも1つの水素原子が、フッ素原子で置換された、炭素数4~20の1価の脂環式炭化水素基又はその誘導体基等である。 In the general formula (c1-1), R 22 is a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and at least one hydrogen atom in the hydrocarbon group having 1 to 30 carbon atoms is a fluorine atom. It is a group formed by substitution.
R 22 is preferably a linear or branched alkyl group having 1 to 6 carbon atoms in which at least one hydrogen atom is substituted with a fluorine atom; at least one hydrogen atom is substituted with a fluorine atom And a monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms or a derivative group thereof.
上記少なくとも1つの水素原子が、フッ素原子で置換された、炭素数1~6の直鎖状又は分岐状のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、1-ブチル基、2-ブチル基、2-(2-メチルプロピル)基、1-ペンチル基、2-ペンチル基、3-ペンチル基、1-(2-メチルブチル)基、1-(3-メチルブチル)基、2-(2-メチルブチル)基、2-(3-メチルブチル)基、ネオペンチル基、1-ヘキシル基、2-ヘキシル基、3-ヘキシル基、1-(2-メチルペンチル)基、1-(3-メチルペンチル)基、1-(4-メチルペンチル)基、2-(2-メチルペンチル)基、2-(3-メチルペンチル)基、2-(4-メチルペンチル)基、3-(2-メチルペンチル)基、3-(3-メチルペンチル)基等の直鎖状若しくは分岐状のアルキル基における水素原子の少なくとも1つがフッ素原子に置換された、部分フッ素化アルキル基及びパーフルオロアルキル基が挙げられる。
Examples of the linear or branched alkyl group having 1 to 6 carbon atoms in which at least one hydrogen atom is substituted with a fluorine atom include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, and 1-butyl. Group, 2-butyl group, 2- (2-methylpropyl) group, 1-pentyl group, 2-pentyl group, 3-pentyl group, 1- (2-methylbutyl) group, 1- (3-methylbutyl) group, 2- (2-methylbutyl) group, 2- (3-methylbutyl) group, neopentyl group, 1-hexyl group, 2-hexyl group, 3-hexyl group, 1- (2-methylpentyl) group, 1- (3 -Methylpentyl) group, 1- (4-methylpentyl) group, 2- (2-methylpentyl) group, 2- (3-methylpentyl) group, 2- (4-methylpentyl) group, 3- (2 -Methylpentyl) group, 3- (3-methyl Rupenchiru) at least one of the hydrogen atoms in a linear or branched alkyl group such as a group has been substituted with fluorine atom, a partially fluorinated alkyl group and perfluoroalkyl group.
上記少なくとも1つの水素原子が、フッ素原子で置換された、炭素数4~20の1価の脂環式炭化水素基としては、シクロペンチル基、シクロペンチルメチル基、1-(1-シクロペンチルエチル)基、1-(2-シクロペンチルエチル)基、シクロヘキシル基、シクロヘキシルメチル基、1-(1-シクロヘキシルエチル)基、1-(2-シクロヘキシルエチル基)、シクロヘプチル基、シクロヘプチルメチル基、1-(1-シクロヘプチルエチル)基、1-(2-シクロヘプチルエチル)基、2-ノルボルニル基、ビシクロ[2.2.1]ヘプチル基、ビシクロ[2.2.1]オクチル基、トリシクロ[5.2.1.02,6]デカニル基、トリシクロ[3.3.1.13,7]デカニル基、テトラシクロ[6.2.1.13,6.02,7]ドデカニル基、アダマンチル基等における水素原子の少なくとも1つがフッ素原子に置換された、部分フッ素化アルキル基及びパーフルオロアルキル基が挙げられる。
また、この部分フッ素化アルキル基の誘導体基としては、これらの基に含まれる水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~10の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等とすることができる。 Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms in which at least one hydrogen atom is substituted with a fluorine atom include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, 1- (2-cyclopentylethyl) group, cyclohexyl group, cyclohexylmethyl group, 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1 -Cycloheptylethyl) group, 1- (2-cycloheptylethyl) group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2 1.0 2,6 ] decanyl group, tricyclo [3.3.1.1 3,7 ] decanyl group, tetracyclo [6.2.1.1 3,6 . 0 2,7 ] dodecanyl group, adamantyl group and the like, a partially fluorinated alkyl group and a perfluoroalkyl group in which at least one hydrogen atom is substituted with a fluorine atom.
In addition, as a derivative group of the partially fluorinated alkyl group, a hydrogen atom contained in these groups is substituted with a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -A group substituted with one or more linear, branched or cyclic alkyl groups having 1 to 10 carbon atoms such as methylpropyl group and tert-butyl group.
また、この部分フッ素化アルキル基の誘導体基としては、これらの基に含まれる水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~10の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等とすることができる。 Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms in which at least one hydrogen atom is substituted with a fluorine atom include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, 1- (2-cyclopentylethyl) group, cyclohexyl group, cyclohexylmethyl group, 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1 -Cycloheptylethyl) group, 1- (2-cycloheptylethyl) group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2 1.0 2,6 ] decanyl group, tricyclo [3.3.1.1 3,7 ] decanyl group, tetracyclo [6.2.1.1 3,6 . 0 2,7 ] dodecanyl group, adamantyl group and the like, a partially fluorinated alkyl group and a perfluoroalkyl group in which at least one hydrogen atom is substituted with a fluorine atom.
In addition, as a derivative group of the partially fluorinated alkyl group, a hydrogen atom contained in these groups is substituted with a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -A group substituted with one or more linear, branched or cyclic alkyl groups having 1 to 10 carbon atoms such as methylpropyl group and tert-butyl group.
上記一般式(c1-1)で表される繰り返し単位(III-1-1)を与える単量体としては、トリフルオロメチル(メタ)アクリル酸エステル、2,2,2-トリフルオロエチル(メタ)アクリル酸エステル、パーフルオロエチル(メタ)アクリル酸エステル、パーフルオロn-プロピル(メタ)アクリル酸エステル、パーフルオロイソプロピル(メタ)アクリル酸エステル、パーフルオロn-ブチル(メタ)アクリル酸エステル、パーフルオロイソブチル(メタ)アクリル酸エステル、パーフルオロtert-ブチル(メタ)アクリル酸エステル、2-(1,1,1,3,3,3-ヘキサフルオロプロピル)(メタ)アクリル酸エステル、1-(2,2,3,3,4,4,5,5-オクタフルオロペンチル)(メタ)アクリル酸エステル、パーフルオロシクロヘキシルメチル(メタ)アクリル酸エステル、1-(2,2,3,3,3-ペンタフルオロプロピル)(メタ)アクリル酸エステル、1-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-ヘプタデカフルオロデシル)(メタ)アクリル酸エステル、1-(5-トリフルオロメチル-3,3,4,4,5,6,6,6-オクタフルオロヘキシル)(メタ)アクリル酸エステル等が挙げられる。
Monomers that give the repeating unit (III-1-1) represented by the general formula (c1-1) include trifluoromethyl (meth) acrylate, 2,2,2-trifluoroethyl (meth) ) Acrylic acid ester, Perfluoroethyl (meth) acrylic acid ester, Perfluoro n-propyl (meth) acrylic acid ester, Perfluoroisopropyl (meth) acrylic acid ester, Perfluoro n-butyl (meth) acrylic acid ester, Per Fluoroisobutyl (meth) acrylic acid ester, perfluoro tert-butyl (meth) acrylic acid ester, 2- (1,1,1,3,3,3-hexafluoropropyl) (meth) acrylic acid ester, 1- ( 2,2,3,3,4,4,5,5-octafluoropentyl) (meth) acrylic acid ester Perfluorocyclohexylmethyl (meth) acrylate, 1- (2,2,3,3,3-pentafluoropropyl) (meth) acrylate, 1- (3,3,4,4,5,5 , 6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecyl) (meth) acrylic acid ester, 1- (5-trifluoromethyl-3,3,4, 4,5,6,6,6-octafluorohexyl) (meth) acrylic acid ester and the like.
上記重合体[C]は、繰り返し単位(III-1-1)を1種のみ含んでよいし、2種以上を含んでもよい。
The polymer [C] may contain only one type of repeating unit (III-1-1), or may contain two or more types.
1-3-1-2.繰り返し単位(III-1-2)及び(III-1-3)
この繰り返し単位(III-1-2)及び(III-1-3)は、それぞれ、上記一般式(c1-2)及び(c1-3)で表される繰り返し単位である。
上記一般式(c1-2)及び(c1-3)において、R21は、上記一般式(c1-1)におけるR21の説明をそのまま適用することができる。 1-3-1-2. Repeating units (III-1-2) and (III-1-3)
These repeating units (III-1-2) and (III-1-3) are repeating units represented by the above general formulas (c1-2) and (c1-3), respectively.
In the general formula (c1-2) and (c1-3), R 21 can be directly applied to the description of R 21 in formula (c1-1).
この繰り返し単位(III-1-2)及び(III-1-3)は、それぞれ、上記一般式(c1-2)及び(c1-3)で表される繰り返し単位である。
上記一般式(c1-2)及び(c1-3)において、R21は、上記一般式(c1-1)におけるR21の説明をそのまま適用することができる。 1-3-1-2. Repeating units (III-1-2) and (III-1-3)
These repeating units (III-1-2) and (III-1-3) are repeating units represented by the above general formulas (c1-2) and (c1-3), respectively.
In the general formula (c1-2) and (c1-3), R 21 can be directly applied to the description of R 21 in formula (c1-1).
上記一般式(c1-2)及び(c1-3)において、R23は、水素原子、フッ素原子又は炭素数1~30のフッ素化炭化水素基であり、全てのR23が水素原子である場合はない。尚、複数のR23は、互いに同一であってよいし、異なってもよい。
R23が、炭素数1~30のフッ素化炭化水素基である場合、R22の説明をそのまま適用することができる。 In the above general formulas (c1-2) and (c1-3), when R 23 is a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and all R 23 are hydrogen atoms There is no. The plurality of R 23 may be the same as or different from each other.
When R 23 is a fluorinated hydrocarbon group having 1 to 30 carbon atoms, the description of R 22 can be applied as it is.
R23が、炭素数1~30のフッ素化炭化水素基である場合、R22の説明をそのまま適用することができる。 In the above general formulas (c1-2) and (c1-3), when R 23 is a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and all R 23 are hydrogen atoms There is no. The plurality of R 23 may be the same as or different from each other.
When R 23 is a fluorinated hydrocarbon group having 1 to 30 carbon atoms, the description of R 22 can be applied as it is.
また、上記一般式(c1-2)及び(c1-3)において、下記一般式で表される、R23を含む部分構造としては、下記式(1)~(5)で表される構造とすることができる。
In the general formulas (c1-2) and (c1-3), the partial structure containing R 23 represented by the following general formula includes the structures represented by the following formulas (1) to (5): can do.
上記式で表される構造のうち、上記一般式(c1-2)においては、上記式(5)で表される構造が好ましく、上記一般式(c1-3)においては、上記式(3)で表される構造が好ましい。
Of the structures represented by the above formula, in the general formula (c1-2), the structure represented by the above formula (5) is preferable, and in the above general formula (c1-3), the above formula (3) The structure represented by these is preferable.
上記一般式(c1-2)及び(c1-3)において、R24は、水素原子、酸解離性基又はアルカリ解離性基である。
ここで、「アルカリ解離性基」とは、例えば、ヒドロキシル基、カルボキシル基等の極性官能基中の水素原子を置換する基であって、アルカリの存在下で解離する基をいう。 In the above general formulas (c1-2) and (c1-3), R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group.
Here, the “alkali dissociable group” refers to a group that substitutes a hydrogen atom in a polar functional group such as a hydroxyl group or a carboxyl group and dissociates in the presence of an alkali.
ここで、「アルカリ解離性基」とは、例えば、ヒドロキシル基、カルボキシル基等の極性官能基中の水素原子を置換する基であって、アルカリの存在下で解離する基をいう。 In the above general formulas (c1-2) and (c1-3), R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group.
Here, the “alkali dissociable group” refers to a group that substitutes a hydrogen atom in a polar functional group such as a hydroxyl group or a carboxyl group and dissociates in the presence of an alkali.
上記重合体[C]が、繰り返し単位(c1-2)及び/又は繰り返し単位(c1-3)を含む重合体であって、且つ、これらの繰り返し単位が、酸解離性基を含む場合には、フォトレジスト膜における露光部に含まれる重合体[C]の溶解性を向上させることができ、好ましい。これは、後述のレジストパターン形成方法における露光工程において、フォトレジスト膜における露光部で発生した酸と、重合体[C]に含まれる酸解離性基とが反応して極性基を生じるためであると考えられる。
When the polymer [C] is a polymer containing the repeating unit (c1-2) and / or the repeating unit (c1-3), and these repeating units contain an acid dissociable group The solubility of the polymer [C] contained in the exposed part of the photoresist film can be improved, which is preferable. This is because in the exposure step in the resist pattern forming method described later, the acid generated in the exposed portion of the photoresist film reacts with the acid dissociable group contained in the polymer [C] to generate a polar group. it is conceivable that.
また、上記重合体[C]が、繰り返し単位(c1-2)及び/又は繰り返し単位(c1-3)を含む重合体であって、且つ、これらの繰り返し単位が、アルカリ解離性基を含む場合には、この重合体[C]の現像液に対する親和性を向上させることができ、好ましい。これは、後述のパターン形成方法における現像工程において、重合体[C]が、現像液と反応し、極性基を生じるためであると考えられる。
Further, when the polymer [C] is a polymer containing the repeating unit (c1-2) and / or the repeating unit (c1-3), and these repeating units contain an alkali dissociable group In this case, the affinity of the polymer [C] for the developer can be improved, which is preferable. This is considered to be because the polymer [C] reacts with the developer to generate a polar group in the development step in the pattern forming method described later.
上記酸解離性基としては、tert-ブトキシカルボニル基、テトラヒドロピラニル基、テトラヒドロフラニル基、(チオテトラヒドロピラニルスルファニル)メチル基、(チオテトラヒドロフラニルスルファニル)メチル基や、アルコキシ置換メチル基、アルキルスルファニル置換メチル基、一般式[-C(R29)3](式中、R29は、相互に独立に、炭素数1~4の直鎖状若しくは分岐状のアルキル基、炭素数4~20の1価の脂環式炭化水素基若しくはその誘導体基である。また、2つのR29が相互に結合して形成された、2価であって、炭素数4~20の脂環式炭化水素基若しくはその誘導体基であってもよい。)で表される基等が挙げられる。
Examples of the acid dissociable group include a tert-butoxycarbonyl group, a tetrahydropyranyl group, a tetrahydrofuranyl group, a (thiotetrahydropyranylsulfanyl) methyl group, a (thiotetrahydrofuranylsulfanyl) methyl group, an alkoxy-substituted methyl group, and an alkylsulfanyl group. Substituted methyl group, general formula [—C (R 29 ) 3 ] (wherein R 29 are each independently a linear or branched alkyl group having 1 to 4 carbon atoms, and having 4 to 20 carbon atoms. A monovalent alicyclic hydrocarbon group or a derivative group thereof, and a divalent alicyclic hydrocarbon group having 4 to 20 carbon atoms formed by bonding two R 29 to each other Or a derivative group thereof).
上記アルコキシ置換メチル基におけるアルコキシル基(置換基)としては、炭素数1~4のアルコキシル基、即ち、メトキシ基、エトキシ基、n-プロポキシ基、n-ブトキシ基等が挙げられる。
上記アルキルスルファニル置換メチル基におけるアルキル基(置換基)としては、炭素数1~4のアルキル基、即ち、メチル基、エチル基、n-プロピル基、n-ブチル基等が挙げられる。 Examples of the alkoxyl group (substituent) in the alkoxy-substituted methyl group include an alkoxyl group having 1 to 4 carbon atoms, that is, a methoxy group, an ethoxy group, an n-propoxy group, and an n-butoxy group.
Examples of the alkyl group (substituent) in the alkylsulfanyl-substituted methyl group include an alkyl group having 1 to 4 carbon atoms, that is, a methyl group, an ethyl group, an n-propyl group, an n-butyl group, and the like.
上記アルキルスルファニル置換メチル基におけるアルキル基(置換基)としては、炭素数1~4のアルキル基、即ち、メチル基、エチル基、n-プロピル基、n-ブチル基等が挙げられる。 Examples of the alkoxyl group (substituent) in the alkoxy-substituted methyl group include an alkoxyl group having 1 to 4 carbon atoms, that is, a methoxy group, an ethoxy group, an n-propoxy group, and an n-butoxy group.
Examples of the alkyl group (substituent) in the alkylsulfanyl-substituted methyl group include an alkyl group having 1 to 4 carbon atoms, that is, a methyl group, an ethyl group, an n-propyl group, an n-butyl group, and the like.
また、上記酸解離性基である上記一般式[-C(R29)3]において、R29は、炭素数1~4の直鎖状若しくは分岐状のアルキル基、炭素数4~20の1価の脂環式炭化水素基若しくはその誘導体基である。
In the general formula [—C (R 29 ) 3 ], which is the acid dissociable group, R 29 is a linear or branched alkyl group having 1 to 4 carbon atoms, or 1 having 4 to 20 carbon atoms. A valent alicyclic hydrocarbon group or a derivative group thereof.
R29により表した、炭素数1~4の直鎖状及び分岐状のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。
また、R29により表した、炭素数4~20の1価の脂環式炭化水素基としては、シクロペンチル基、シクロペンチルメチル基、1-(1-シクロペンチルエチル)基、1-(2-シクロペンチルエチル)基、シクロヘキシル基、シクロヘキシルメチル基、1-(1-シクロヘキシルエチル)基、1-(2-シクロヘキシルエチル基)、シクロヘプチル基、シクロヘプチルメチル基、1-(1-シクロヘプチルエチル)基、1-(2-シクロヘプチルエチル)基、2-ノルボルニル基、ビシクロ[2.2.1]ヘプチル基、ビシクロ[2.2.1]オクチル基、トリシクロ[5.2.1.02,6]デカニル基、トリシクロ[3.3.1.13,7]デカニル基、テトラシクロ[6.2.1.13,6.02,7]ドデカニル基、アダマンチル基等の脂環式アルキル基等が挙げられる。これらのうち、ノルボルナン、トリシクロデカン、テトラシクロドデカン、アダマンタン、シクロペンタン又はシクロヘキサンに由来する脂環族環からなる脂環式炭化水素基が好ましい。 Examples of the linear and branched alkyl group having 1 to 4 carbon atoms represented by R 29 include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -Methylpropyl group, tert-butyl group and the like.
Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms represented by R 29 include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, and 1- (2-cyclopentylethyl). ) Group, cyclohexyl group, cyclohexylmethyl group, 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1-cycloheptylethyl) group, 1- (2-cycloheptylethyl) group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2.1.0 2,6 ] Decanyl group, tricyclo [3.3.1.1 3,7 ] decanyl group, tetracyclo [6.2.1.1 3,6 . 0 2,7 ] dodecanyl group, alicyclic alkyl group such as adamantyl group, and the like. Of these, alicyclic hydrocarbon groups composed of alicyclic rings derived from norbornane, tricyclodecane, tetracyclododecane, adamantane, cyclopentane or cyclohexane are preferred.
また、R29により表した、炭素数4~20の1価の脂環式炭化水素基としては、シクロペンチル基、シクロペンチルメチル基、1-(1-シクロペンチルエチル)基、1-(2-シクロペンチルエチル)基、シクロヘキシル基、シクロヘキシルメチル基、1-(1-シクロヘキシルエチル)基、1-(2-シクロヘキシルエチル基)、シクロヘプチル基、シクロヘプチルメチル基、1-(1-シクロヘプチルエチル)基、1-(2-シクロヘプチルエチル)基、2-ノルボルニル基、ビシクロ[2.2.1]ヘプチル基、ビシクロ[2.2.1]オクチル基、トリシクロ[5.2.1.02,6]デカニル基、トリシクロ[3.3.1.13,7]デカニル基、テトラシクロ[6.2.1.13,6.02,7]ドデカニル基、アダマンチル基等の脂環式アルキル基等が挙げられる。これらのうち、ノルボルナン、トリシクロデカン、テトラシクロドデカン、アダマンタン、シクロペンタン又はシクロヘキサンに由来する脂環族環からなる脂環式炭化水素基が好ましい。 Examples of the linear and branched alkyl group having 1 to 4 carbon atoms represented by R 29 include a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, a 2-methylpropyl group, 1 -Methylpropyl group, tert-butyl group and the like.
Examples of the monovalent alicyclic hydrocarbon group having 4 to 20 carbon atoms represented by R 29 include a cyclopentyl group, a cyclopentylmethyl group, a 1- (1-cyclopentylethyl) group, and 1- (2-cyclopentylethyl). ) Group, cyclohexyl group, cyclohexylmethyl group, 1- (1-cyclohexylethyl) group, 1- (2-cyclohexylethyl group), cycloheptyl group, cycloheptylmethyl group, 1- (1-cycloheptylethyl) group, 1- (2-cycloheptylethyl) group, 2-norbornyl group, bicyclo [2.2.1] heptyl group, bicyclo [2.2.1] octyl group, tricyclo [5.2.1.0 2,6 ] Decanyl group, tricyclo [3.3.1.1 3,7 ] decanyl group, tetracyclo [6.2.1.1 3,6 . 0 2,7 ] dodecanyl group, alicyclic alkyl group such as adamantyl group, and the like. Of these, alicyclic hydrocarbon groups composed of alicyclic rings derived from norbornane, tricyclodecane, tetracyclododecane, adamantane, cyclopentane or cyclohexane are preferred.
また、R29が、上記脂環式炭化水素基の誘導体基である場合、R29は、この脂環式炭化水素基に含まれる水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~4の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等とすることができる。
When R 29 is a derivative group of the alicyclic hydrocarbon group, R 29 represents a hydrogen atom contained in the alicyclic hydrocarbon group as a methyl group, an ethyl group, an n-propyl group, an isopropyl group. 1 or more or 1 or more of linear, branched or cyclic alkyl groups having 1 to 4 carbon atoms such as a group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group and tert-butyl group Or a group substituted with or the like.
更に、上記一般式[-C(R29)3]は、3つのR29のうちいずれか2つが互いに結合して形成された、2価であって、炭素数4~20の脂環式炭化水素基及びその誘導体基を有してもよく、その例としては、アダマンタン骨格、ノルボルナン骨格、トリシクロデカン骨格、テトラシクロドデカン骨格等の有橋式骨格や、シクロブチレン基、シクロペンチレン基、シクロヘキシレン基、シクロヘプチレン基、シクロオクチレン基等の、2価の単環式炭化水素基;これらの基に含まれる水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~4の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等の脂環式骨格を有する基が挙げられる。これらのうち、シクロペンチレン基、シクロヘキシレン基等の、2価の単環式炭化水素基や、この脂環式炭化水素基(単環式炭化水素基)に含まれる水素原子を、炭素数1~4の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基が好ましい。
Further, the general formula [—C (R 29 ) 3 ] is a divalent alicyclic carbon atom having 4 to 20 carbon atoms formed by bonding any two of three R 29 to each other. It may have a hydrogen group and a derivative group thereof, and examples thereof include a bridged skeleton such as an adamantane skeleton, a norbornane skeleton, a tricyclodecane skeleton, a tetracyclododecane skeleton, a cyclobutylene group, a cyclopentylene group, Divalent monocyclic hydrocarbon groups such as cyclohexylene group, cycloheptylene group, cyclooctylene group; the hydrogen atoms contained in these groups are methyl, ethyl, n-propyl, isopropyl, n- Substituted with one or more linear, branched or cyclic alkyl groups having 1 to 4 carbon atoms such as butyl, 2-methylpropyl, 1-methylpropyl and tert-butyl. Groups having an alicyclic skeleton such groups. Among these, a divalent monocyclic hydrocarbon group such as a cyclopentylene group or a cyclohexylene group, or a hydrogen atom contained in the alicyclic hydrocarbon group (monocyclic hydrocarbon group) is represented by the number of carbon atoms. A group substituted with one or more of 1 to 4 linear, branched or cyclic alkyl groups is preferred.
上記一般式[-C(R29)3]の好ましい例としては、tert-ブチル基、1-n-(1-エチル-1-メチル)プロピル基、1-n-(1,1-ジメチル)プロピル基、1-n-(1,1-ジメチル)ブチル基、1-n-(1,1-ジメチル)ペンチル基、1-(1,1-ジエチル)プロピル基、1-n-(1,1-ジエチル)ブチル基、1-n-(1,1-ジエチル)ペンチル基、1-(1-メチル)シクロペンチル基、1-(1-エチル)シクロペンチル基、1-(1-n-プロピル)シクロペンチル基、1-(1-イソプロピル)シクロペンチル基、1-(1-メチル)シクロヘキシル基、1-(1-エチル)シクロヘキシル基、1-(1-n-プロピル)シクロヘキシル基、1-(1-イソプロピル)シクロヘキシル基、1-{1-メチル-1-(2-ノルボニル)}エチル基、1-{1-メチル-1-(2-テトラシクロデカニル)}エチル基、1-{1-メチル-1-(1-アダマンチル)}エチル基、2-(2-メチル)ノルボニル基、2-(2-エチル)ノルボニル基、2-(2-n-プロピル)ノルボニル基、2-(2-イソプロピル)ノルボニル基、2-(2-メチル)テトラシクロデカニル基、2-(2-エチル)テトラシクロデカニル基、2-(2-n-プロピル)テトラシクロデカニル基、2-(2-イソプロピル)テトラシクロデカニル基、1-(1-メチル)アダマンチル基、1-(1-エチル)アダマンチル基、1-(1-n-プロピル)アダマンチル基、1-(1-イソプロピル)アダマンチル基や、これらの基に含まれる水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~4の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等が挙げられる。
Preferred examples of the general formula [—C (R 29 ) 3 ] include a tert-butyl group, a 1-n- (1-ethyl-1-methyl) propyl group, and a 1-n- (1,1-dimethyl) group. Propyl group, 1-n- (1,1-dimethyl) butyl group, 1-n- (1,1-dimethyl) pentyl group, 1- (1,1-diethyl) propyl group, 1-n- (1, 1-diethyl) butyl group, 1-n- (1,1-diethyl) pentyl group, 1- (1-methyl) cyclopentyl group, 1- (1-ethyl) cyclopentyl group, 1- (1-n-propyl) Cyclopentyl group, 1- (1-isopropyl) cyclopentyl group, 1- (1-methyl) cyclohexyl group, 1- (1-ethyl) cyclohexyl group, 1- (1-n-propyl) cyclohexyl group, 1- (1- Isopropyl) cyclohexyl group, 1 -{1-methyl-1- (2-norbornyl)} ethyl group, 1- {1-methyl-1- (2-tetracyclodecanyl)} ethyl group, 1- {1-methyl-1- (1- Adamantyl)} ethyl group, 2- (2-methyl) norbornyl group, 2- (2-ethyl) norbornyl group, 2- (2-n-propyl) norbornyl group, 2- (2-isopropyl) norbornyl group, 2- (2-methyl) tetracyclodecanyl group, 2- (2-ethyl) tetracyclodecanyl group, 2- (2-n-propyl) tetracyclodecanyl group, 2- (2-isopropyl) tetracyclodecanyl group Groups, 1- (1-methyl) adamantyl group, 1- (1-ethyl) adamantyl group, 1- (1-n-propyl) adamantyl group, 1- (1-isopropyl) adamantyl group, and these groups Hydrogen atom Straight chain, branched or straight chain having 1 to 4 carbon atoms such as methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group, etc. And a group substituted with one or more cyclic alkyl groups.
上記酸解離性基としては、上記一般式[-C(R29)3]で表される基、tert-ブトキシカルボニル基、アルコキシ置換メチル基等が好ましい。
上記繰り返し単位(c1-2)においては、tert-ブトキシカルボニル基、及び、アルコキシ置換メチル基が好ましい。
また、上記繰り返し単位(c1-3)においては、アルコキシ置換メチル基、及び、上記一般式[-C(R29)3]で表される基が好ましい。 The acid dissociable group is preferably a group represented by the above general formula [—C (R 29 ) 3 ], a tert-butoxycarbonyl group, an alkoxy-substituted methyl group, or the like.
In the repeating unit (c1-2), a tert-butoxycarbonyl group and an alkoxy-substituted methyl group are preferable.
In the repeating unit (c1-3), an alkoxy-substituted methyl group and a group represented by the above general formula [—C (R 29 ) 3 ] are preferable.
上記繰り返し単位(c1-2)においては、tert-ブトキシカルボニル基、及び、アルコキシ置換メチル基が好ましい。
また、上記繰り返し単位(c1-3)においては、アルコキシ置換メチル基、及び、上記一般式[-C(R29)3]で表される基が好ましい。 The acid dissociable group is preferably a group represented by the above general formula [—C (R 29 ) 3 ], a tert-butoxycarbonyl group, an alkoxy-substituted methyl group, or the like.
In the repeating unit (c1-2), a tert-butoxycarbonyl group and an alkoxy-substituted methyl group are preferable.
In the repeating unit (c1-3), an alkoxy-substituted methyl group and a group represented by the above general formula [—C (R 29 ) 3 ] are preferable.
また、上記アルカリ解離性基としては、上記の性質を示すものであれば、特に限定されない。上記一般式(c1-2)におけるアルカリ解離性基としては、下記一般式(R1-1)で表される基が挙げられる。
(式中、R30は、炭素数1~10のフッ素化炭化水素基である。)
The alkali-dissociable group is not particularly limited as long as it exhibits the above properties. Examples of the alkali dissociable group in the general formula (c1-2) include groups represented by the following general formula (R1-1).
(Wherein R 30 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms.)
上記一般式(R1-1)において、R30は、炭素数1~10のフッ素化炭化水素基であり、炭素数1~10の炭化水素基における水素原子の少なくとも1つが、フッ素原子に置換されてなる基である。このR30は、好ましくは、直鎖状又は分岐状の炭素数1~10のパーフルオロアルキル基であり、特に好ましくはトリフルオロメチル基である。
In the general formula (R1-1), R 30 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms, and at least one hydrogen atom in the hydrocarbon group having 1 to 10 carbon atoms is substituted with a fluorine atom. It is a group consisting of R 30 is preferably a linear or branched perfluoroalkyl group having 1 to 10 carbon atoms, and particularly preferably a trifluoromethyl group.
また、上記一般式(c1-3)におけるアルカリ解離性基としては、下記一般式(R1-2)~(R1-4)で表される基が挙げられる。
(式中、R31は、相互に独立に、ハロゲン原子、炭素数1~10のアルキル基、炭素数1~10のアルコキシル基、炭素数1~10のアシル基又は炭素数1~10のアシロキシ基であり、R33は、相互に独立に、水素原子又は炭素数1~10のアルキル基である。また、2つのR33が相互に結合して形成された、2価であって、炭素数4~20の脂環式炭化水素又はその誘導体基であってもよい。m1は、0、1、2、3、4又は5であり、m2は、0、1、2、3又は4である。)
In addition, examples of the alkali dissociable group in the general formula (c1-3) include groups represented by the following general formulas (R1-2) to (R1-4).
(Wherein R 31 s are independently of each other a halogen atom, an alkyl group having 1 to 10 carbon atoms, an alkoxyl group having 1 to 10 carbon atoms, an acyl group having 1 to 10 carbon atoms, or an acyloxy group having 1 to 10 carbon atoms. R 33 is, independently of each other, a hydrogen atom or an alkyl group having 1 to 10 carbon atoms, and is a divalent carbon atom formed by bonding two R 33 together, It may be an alicyclic hydrocarbon having 4 to 20 or a derivative group thereof, m1 is 0, 1, 2, 3, 4 or 5, and m2 is 0, 1, 2, 3 or 4. is there.)
上記一般式(R1-2)及び(R1-3)において、R31がハロゲン原子である場合、フッ素原子、塩素原子、臭素原子又はヨウ素原子とすることができる。これらのうち、フッ素原子が好ましい。
In the above general formulas (R1-2) and (R1-3), when R 31 is a halogen atom, it can be a fluorine atom, a chlorine atom, a bromine atom or an iodine atom. Of these, fluorine atoms are preferred.
上記一般式(R1-2)及び(R1-3)において、R31により表した、炭素数1~10のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、1-ブチル基、2-ブチル基、2-(2-メチルプロピル)基、1-ペンチル基、2-ペンチル基、3-ペンチル基、1-(2-メチルブチル)基、1-(3-メチルブチル)基、2-(2-メチルブチル)基、2-(3-メチルブチル)基、ネオペンチル基、1-ヘキシル基、2-ヘキシル基、3-ヘキシル基、1-(2-メチルペンチル)基、1-(3-メチルペンチル)基、1-(4-メチルペンチル)基、2-(2-メチルペンチル)基、2-(3-メチルペンチル)基、2-(4-メチルペンチル)基、3-(2-メチルペンチル)基、3-(3-メチルペンチル)基、1-ヘプチル基、1-オクチル基、1-ノニル基、1-デシル基等の直鎖状及び分岐状のアルキル基が挙げられる。
In the above general formulas (R1-2) and (R1-3), the alkyl group having 1 to 10 carbon atoms represented by R 31 includes a methyl group, an ethyl group, an n-propyl group, an isopropyl group, and 1-butyl. Group, 2-butyl group, 2- (2-methylpropyl) group, 1-pentyl group, 2-pentyl group, 3-pentyl group, 1- (2-methylbutyl) group, 1- (3-methylbutyl) group, 2- (2-methylbutyl) group, 2- (3-methylbutyl) group, neopentyl group, 1-hexyl group, 2-hexyl group, 3-hexyl group, 1- (2-methylpentyl) group, 1- (3 -Methylpentyl) group, 1- (4-methylpentyl) group, 2- (2-methylpentyl) group, 2- (3-methylpentyl) group, 2- (4-methylpentyl) group, 3- (2 -Methylpentyl) group, 3- (3-methylpe Straight chain and branched alkyl groups such as 1-heptyl group, 1-heptyl group, 1-octyl group, 1-nonyl group and 1-decyl group.
R31により表した、炭素数1~10のアルコキシル基としては、メトキシ基、エトキシ基、n-プロポキシ基、n-ブトキシ基、sec-ブトキシ基、tert-ブトキシ基、n-ペンチロキシ基、n-ヘキシロキシ基等の直鎖状及び分岐状のアルキル基が挙げられる。
Examples of the alkoxyl group having 1 to 10 carbon atoms represented by R 31 include methoxy group, ethoxy group, n-propoxy group, n-butoxy group, sec-butoxy group, tert-butoxy group, n-pentyloxy group, n- Examples thereof include linear and branched alkyl groups such as a hexyloxy group.
R31により表した、炭素数1~10のアシル基としては、アセチル基、プロピオニル基、メトキシカルボニル基、エトキシカルボニル基、n-プロポキシカルボニル基、イソプロポキシカルボニル基、n-ブトキシカルボニル基、イソブトキシカルボニル基、sec-ブトキシカルボニル基、tert-ブトキシカルボニル基、n-ペントキシカルボニル基、n-ヘキシルオキシカルボニル基、n-ヘプチルオキシカルボニル基、n-オクチルオキシカルボニル基等が挙げられる。
Examples of the acyl group having 1 to 10 carbon atoms represented by R 31 include acetyl group, propionyl group, methoxycarbonyl group, ethoxycarbonyl group, n-propoxycarbonyl group, isopropoxycarbonyl group, n-butoxycarbonyl group, isobutoxy group. Examples include carbonyl group, sec-butoxycarbonyl group, tert-butoxycarbonyl group, n-pentoxycarbonyl group, n-hexyloxycarbonyl group, n-heptyloxycarbonyl group, n-octyloxycarbonyl group and the like.
また、R31により表した、炭素数1~10のアシロキシ基としては、アセトキシ基、エチリルオキシ基、ブチリルオキシ基、tert-ブチリルオキシ基、tert-アミリルオキシ基、n-ヘキサンカルボニロキシ基、n-オクタンカルボニロキシ基等が挙げられる。
Examples of the acyloxy group having 1 to 10 carbon atoms represented by R 31 include an acetoxy group, an ethylyloxy group, a butyryloxy group, a tert-butyryloxy group, a tert-amylyloxy group, an n-hexanecarbonyloxy group, and an n-octanecarboxyl group. Examples include a nitroxy group.
上記一般式(R1-2)及び(R1-3)において、R31が2つ以上ある場合、R31は、互いに同一であってよいし、異なってもよい。
In the general formula (R1-2) and (R1-3), if R 31 is two or more, R 31 may or be identical to each other or may be different.
上記一般式(R1-4)において、R33は、水素原子又は炭素数1~10のアルキル基である。
R33が、炭素数1~10のアルキル基である場合、メチル基、エチル基、n-プロピル基、イソプロピル基、1-ブチル基、2-ブチル基、2-(2-メチルプロピル)基、1-ペンチル基、2-ペンチル基、3-ペンチル基、1-(2-メチルブチル)基、1-(3-メチルブチル)基、2-(2-メチルブチル)基、2-(3-メチルブチル)基、ネオペンチル基、1-ヘキシル基、2-ヘキシル基、3-ヘキシル基、1-(2-メチルペンチル)基、1-(3-メチルペンチル)基、1-(4-メチルペンチル)基、2-(2-メチルペンチル)基、2-(3-メチルペンチル)基、2-(4-メチルペンチル)基、3-(2-メチルペンチル)基、3-(3-メチルペンチル)基、1-ヘプチル基、1-オクチル基、1-ノニル基、1-デシル基等の直鎖状及び分岐状のアルキル基が挙げられる。 In the above general formula (R1-4), R 33 is a hydrogen atom or an alkyl group having 1 to 10 carbon atoms.
When R 33 is an alkyl group having 1 to 10 carbon atoms, methyl group, ethyl group, n-propyl group, isopropyl group, 1-butyl group, 2-butyl group, 2- (2-methylpropyl) group, 1-pentyl group, 2-pentyl group, 3-pentyl group, 1- (2-methylbutyl) group, 1- (3-methylbutyl) group, 2- (2-methylbutyl) group, 2- (3-methylbutyl) group , Neopentyl group, 1-hexyl group, 2-hexyl group, 3-hexyl group, 1- (2-methylpentyl) group, 1- (3-methylpentyl) group, 1- (4-methylpentyl) group, 2 -(2-methylpentyl) group, 2- (3-methylpentyl) group, 2- (4-methylpentyl) group, 3- (2-methylpentyl) group, 3- (3-methylpentyl) group, 1 -Heptyl group, 1-octyl group, 1-nonyl group And linear and branched alkyl groups such as 1-decyl group.
R33が、炭素数1~10のアルキル基である場合、メチル基、エチル基、n-プロピル基、イソプロピル基、1-ブチル基、2-ブチル基、2-(2-メチルプロピル)基、1-ペンチル基、2-ペンチル基、3-ペンチル基、1-(2-メチルブチル)基、1-(3-メチルブチル)基、2-(2-メチルブチル)基、2-(3-メチルブチル)基、ネオペンチル基、1-ヘキシル基、2-ヘキシル基、3-ヘキシル基、1-(2-メチルペンチル)基、1-(3-メチルペンチル)基、1-(4-メチルペンチル)基、2-(2-メチルペンチル)基、2-(3-メチルペンチル)基、2-(4-メチルペンチル)基、3-(2-メチルペンチル)基、3-(3-メチルペンチル)基、1-ヘプチル基、1-オクチル基、1-ノニル基、1-デシル基等の直鎖状及び分岐状のアルキル基が挙げられる。 In the above general formula (R1-4), R 33 is a hydrogen atom or an alkyl group having 1 to 10 carbon atoms.
When R 33 is an alkyl group having 1 to 10 carbon atoms, methyl group, ethyl group, n-propyl group, isopropyl group, 1-butyl group, 2-butyl group, 2- (2-methylpropyl) group, 1-pentyl group, 2-pentyl group, 3-pentyl group, 1- (2-methylbutyl) group, 1- (3-methylbutyl) group, 2- (2-methylbutyl) group, 2- (3-methylbutyl) group , Neopentyl group, 1-hexyl group, 2-hexyl group, 3-hexyl group, 1- (2-methylpentyl) group, 1- (3-methylpentyl) group, 1- (4-methylpentyl) group, 2 -(2-methylpentyl) group, 2- (3-methylpentyl) group, 2- (4-methylpentyl) group, 3- (2-methylpentyl) group, 3- (3-methylpentyl) group, 1 -Heptyl group, 1-octyl group, 1-nonyl group And linear and branched alkyl groups such as 1-decyl group.
また、上記一般式(R1-4)において、2つのR33が相互に結合して形成された、2価である、炭素数4~20の脂環式炭化水素としては、アダマンタン骨格、ノルボルナン骨格、トリシクロデカン骨格、テトラシクロドデカン骨格等の有橋式骨格や、シクロブタン、シクロペンタン、シクロヘキサン、シクロヘプタン、シクロオクタン等のシクロアルカン骨格を有する基等が挙げられる。更に、上記脂環式炭化水素基の誘導体基である場合、この脂環式炭化水素基に含まれる水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~4の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等とすることができる。
In addition, examples of the divalent alicyclic hydrocarbon having 4 to 20 carbon atoms formed by bonding two R 33 to each other in the general formula (R1-4) include an adamantane skeleton and a norbornane skeleton. And a bridged skeleton such as a tricyclodecane skeleton and a tetracyclododecane skeleton, and a group having a cycloalkane skeleton such as cyclobutane, cyclopentane, cyclohexane, cycloheptane, and cyclooctane. Further, in the case of the derivative group of the alicyclic hydrocarbon group, the hydrogen atom contained in the alicyclic hydrocarbon group is a methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2 -A group substituted with one or more linear, branched or cyclic alkyl groups having 1 to 4 carbon atoms such as methylpropyl group, 1-methylpropyl group, tert-butyl group, etc. Can do.
上記一般式(R1-4)で表される基としては、メチル基、エチル基、1-プロピル基、2-プロピル基、1-ブチル基、2-ブチル基、1-ペンチル基、2-ペンチル基、3-ペンチル基、1-(2-メチルブチル)基、1-(3-メチルブチル)基、2-(3-メチルブチル)基、ネオペンチル基、1-ヘキシル基、2-ヘキシル基、3-ヘキシル基、1-(2-メチルペンチル)基、1-(3-メチルペンチル)基、1-(4-メチルペンチル)基、2-(3-メチルペンチル)基、2-(4-メチルペンチル)基、3-(2-メチルペンチル)基等が挙げられる。これらのうち、メチル基、エチル基、1-プロピル基、2-プロピル基、1-ブチル基及び2-ブチル基が好ましい。
Examples of the group represented by the general formula (R1-4) include a methyl group, an ethyl group, a 1-propyl group, a 2-propyl group, a 1-butyl group, a 2-butyl group, a 1-pentyl group, and a 2-pentyl group. Group, 3-pentyl group, 1- (2-methylbutyl) group, 1- (3-methylbutyl) group, 2- (3-methylbutyl) group, neopentyl group, 1-hexyl group, 2-hexyl group, 3-hexyl Group, 1- (2-methylpentyl) group, 1- (3-methylpentyl) group, 1- (4-methylpentyl) group, 2- (3-methylpentyl) group, 2- (4-methylpentyl) group Group, 3- (2-methylpentyl) group and the like. Of these, methyl, ethyl, 1-propyl, 2-propyl, 1-butyl and 2-butyl are preferred.
上記一般式(c1-2)及び(c1-3)において、R24が水素原子である、繰り返し単位(III-1-2)、及び、繰り返し単位(III-1-3)は、極性基であるヒドロキシル基又はカルボキシル基を有することになる。重合体[C]が、このような繰り返し単位を含む場合、後述するパターン形成方法の現像工程において、重合体[C]の現像液に対する親和性を向上させることができる。
In the general formulas (c1-2) and (c1-3), the repeating unit (III-1-2) and the repeating unit (III-1-3) in which R 24 is a hydrogen atom are polar groups. It will have some hydroxyl or carboxyl group. When the polymer [C] contains such a repeating unit, the affinity of the polymer [C] for the developer can be improved in the development step of the pattern forming method described later.
上記一般式(c1-2)において、Q3は、(g+1)価の連結基である。このような基としては、単結合、(g+1)価であり、且つ、炭素数1~30の炭化水素基及びその誘導体基等が挙げられる。
In the above general formula (c1-2), Q 3 is a (g + 1) -valent linking group. Examples of such a group include a single bond, a (g + 1) valent hydrocarbon group having 1 to 30 carbon atoms and a derivative group thereof.
上記(g+1)価の炭化水素基は、鎖状構造又は環状構造を含む基とすることができる。
鎖状構造の炭化水素基としては、メタン、エタン、プロパン、ブタン、2-メチルプロパン、ペンタン、2-メチルブタン、2,2-ジメチルプロパン、ヘキサン、ヘプタン、オクタン、ノナン、デカン等の、炭素数1~10の鎖状炭化水素から、水素原子を(g+1)個取り除いた構造の炭化水素基等とすることができる。
また、環状構造の炭化水素基としては、シクロブタン、シクロペンタン、シクロヘキサン、ビシクロ[2.2.1]ヘプタン、ビシクロ[2.2.2]オクタン、トリシクロ[5.2.1.02,6]デカン、トリシクロ[3.3.1.13,7]デカン等の、炭素数4~20の脂環式炭化水素から、水素原子を(g+1)個取り除いた構造の脂環族炭化水素基;ベンゼン、ナフタレン等の、炭素数6~30の芳香族炭化水素から、水素原子を(g+1)個取り除いた構造の芳香族炭化水素基等とすることができる。 The (g + 1) -valent hydrocarbon group can be a group including a chain structure or a cyclic structure.
Chain structure hydrocarbon groups include methane, ethane, propane, butane, 2-methylpropane, pentane, 2-methylbutane, 2,2-dimethylpropane, hexane, heptane, octane, nonane, decane, etc. A hydrocarbon group having a structure in which (g + 1) hydrogen atoms are removed from 1 to 10 chain hydrocarbons can be obtained.
Examples of the cyclic hydrocarbon group include cyclobutane, cyclopentane, cyclohexane, bicyclo [2.2.1] heptane, bicyclo [2.2.2] octane, and tricyclo [5.2.1.0 2,6. ] An alicyclic hydrocarbon group having a structure in which (g + 1) hydrogen atoms are removed from an alicyclic hydrocarbon having 4 to 20 carbon atoms such as decane, tricyclo [3.3.1.1 3,7 ] decane, etc. An aromatic hydrocarbon group having a structure in which (g + 1) hydrogen atoms are removed from an aromatic hydrocarbon having 6 to 30 carbon atoms, such as benzene and naphthalene.
鎖状構造の炭化水素基としては、メタン、エタン、プロパン、ブタン、2-メチルプロパン、ペンタン、2-メチルブタン、2,2-ジメチルプロパン、ヘキサン、ヘプタン、オクタン、ノナン、デカン等の、炭素数1~10の鎖状炭化水素から、水素原子を(g+1)個取り除いた構造の炭化水素基等とすることができる。
また、環状構造の炭化水素基としては、シクロブタン、シクロペンタン、シクロヘキサン、ビシクロ[2.2.1]ヘプタン、ビシクロ[2.2.2]オクタン、トリシクロ[5.2.1.02,6]デカン、トリシクロ[3.3.1.13,7]デカン等の、炭素数4~20の脂環式炭化水素から、水素原子を(g+1)個取り除いた構造の脂環族炭化水素基;ベンゼン、ナフタレン等の、炭素数6~30の芳香族炭化水素から、水素原子を(g+1)個取り除いた構造の芳香族炭化水素基等とすることができる。 The (g + 1) -valent hydrocarbon group can be a group including a chain structure or a cyclic structure.
Chain structure hydrocarbon groups include methane, ethane, propane, butane, 2-methylpropane, pentane, 2-methylbutane, 2,2-dimethylpropane, hexane, heptane, octane, nonane, decane, etc. A hydrocarbon group having a structure in which (g + 1) hydrogen atoms are removed from 1 to 10 chain hydrocarbons can be obtained.
Examples of the cyclic hydrocarbon group include cyclobutane, cyclopentane, cyclohexane, bicyclo [2.2.1] heptane, bicyclo [2.2.2] octane, and tricyclo [5.2.1.0 2,6. ] An alicyclic hydrocarbon group having a structure in which (g + 1) hydrogen atoms are removed from an alicyclic hydrocarbon having 4 to 20 carbon atoms such as decane, tricyclo [3.3.1.1 3,7 ] decane, etc. An aromatic hydrocarbon group having a structure in which (g + 1) hydrogen atoms are removed from an aromatic hydrocarbon having 6 to 30 carbon atoms, such as benzene and naphthalene.
更に、上記一般式(c1-2)におけるQ3は、上記炭化水素基の誘導体基であってよく、酸素原子、硫黄原子、イミノ基、カルボニル基、-CO-O-又は-CO-NH-を備える基とすることができる。
酸素原子、硫黄原子、イミノ基、カルボニル基、-CO-O-又は-CO-NH-を有するQ3としては、下記一般式で表される基が挙げられる。
(式中、R61は、相互に独立に、単結合、2価であって、炭素数1~10の脂肪族炭化水素基若しくはその誘導体基、2価であって、炭素数4~20の脂環族炭化水素基若しくはその誘導体基、又は、2価であって、炭素数6~30の芳香族炭化水素基若しくはその誘導体基であり、gは、1、2又は3である。)
Further, Q 3 in the general formula (c1-2) may be a derivative group of the hydrocarbon group, and an oxygen atom, a sulfur atom, an imino group, a carbonyl group, —CO—O— or —CO—NH—. It can be set as the group provided with.
Examples of Q 3 having an oxygen atom, a sulfur atom, an imino group, a carbonyl group, —CO—O— or —CO—NH— include groups represented by the following general formula.
(Wherein, R 61 s are independently of each other a single bond, divalent, an aliphatic hydrocarbon group having 1 to 10 carbon atoms or a derivative group thereof, divalent, and having 4 to 20 carbon atoms. An alicyclic hydrocarbon group or a derivative group thereof, or a divalent aromatic hydrocarbon group having 6 to 30 carbon atoms or a derivative group thereof, and g is 1, 2, or 3.
酸素原子、硫黄原子、イミノ基、カルボニル基、-CO-O-又は-CO-NH-を有するQ3としては、下記一般式で表される基が挙げられる。
Examples of Q 3 having an oxygen atom, a sulfur atom, an imino group, a carbonyl group, —CO—O— or —CO—NH— include groups represented by the following general formula.
上記一般式において、R61により表した、2価であり、且つ、炭素数1~10の脂肪族炭化水素基としては、メチレン基、エチレン基、1,3-プロピレン基及び1,2-プロピレン基に例示されるプロピレン基、テトラメチレン基、ペンタメチレン基、ヘキサメチレン基、ヘプタメチレン基、オクタメチレン基、ノナメチレン基、デカメチレン基、1-メチル-1,3-プロピレン基、2-メチル-1,3-プロピレン基、2-メチル-1,2-プロピレン基、1-メチル-1,4-ブチレン基、2-メチル-1,4-ブチレン基等が挙げられる。
Examples of the divalent aliphatic hydrocarbon group having 1 to 10 carbon atoms represented by R 61 in the above general formula include a methylene group, an ethylene group, a 1,3-propylene group, and a 1,2-propylene group. Propylene group, tetramethylene group, pentamethylene group, hexamethylene group, heptamethylene group, octamethylene group, nonamethylene group, decamethylene group, 1-methyl-1,3-propylene group, 2-methyl-1 , 3-propylene group, 2-methyl-1,2-propylene group, 1-methyl-1,4-butylene group, 2-methyl-1,4-butylene group and the like.
上記一般式において、R61により表した、2価であり、且つ、炭素数4~20の脂環族炭化水素基としては、1,3-シクロブチレン基等のシクロブチレン基、1,3-シクロペンチレン基等のシクロペンチレン基、1,4-シクロへキシレン基等のシクロへキシレン基、1,5-シクロオクチレン基等のシクロオクチレン基等の、炭素数3~10であるシクロアルキレン基等の単環式炭化水素環基;1,4-ノルボルニレン基、2,5-ノルボルニレン基等のノルボルニレン基、1,5-アダマンチレン基、2,6-アダマンチレン基等のアダマンチレン基等の、炭素数4~20である2~4環式炭化水素環基等の架橋環式炭化水素基等が挙げられる。
In the above general formula, the divalent and alicyclic hydrocarbon group having 4 to 20 carbon atoms represented by R 61 includes a cyclobutylene group such as 1,3-cyclobutylene group, 1,3- 3 to 10 carbon atoms, such as a cyclopentylene group such as a cyclopentylene group, a cyclohexylene group such as a 1,4-cyclohexylene group, and a cyclooctylene group such as a 1,5-cyclooctylene group Monocyclic hydrocarbon ring groups such as cycloalkylene groups; norbornylene groups such as 1,4-norbornylene groups and 2,5-norbornylene groups, adamantylene groups such as 1,5-adamantylene groups and 2,6-adamantylene groups And a bridged cyclic hydrocarbon group such as a 2 to 4 cyclic hydrocarbon ring group having 4 to 20 carbon atoms, such as a group.
上記一般式において、R61により表した、2価であり、且つ、炭素数6~30の芳香族炭化水素基としては、ベンゼン、ナフタレン等の芳香族炭化水素から、水素原子を、2個取り除いた構造の芳香族炭化水素基とすることができる。
In the above general formula, the divalent aromatic hydrocarbon group represented by R 61 having 6 to 30 carbon atoms is obtained by removing two hydrogen atoms from an aromatic hydrocarbon such as benzene or naphthalene. It can be an aromatic hydrocarbon group having a different structure.
また、上記のように、鎖状炭化水素基、環状炭化水素基及び芳香族炭化水素基は、いずれも、誘導体基とすることができ、炭化水素基に含まれる少なくとも1つの水素原子を、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等の炭素数1~10の直鎖状、分岐状又は環状のアルキル基の1種以上あるいは1個以上で置換した基等の脂環式骨格を有する基が挙げられる。
In addition, as described above, any of the chain hydrocarbon group, the cyclic hydrocarbon group, and the aromatic hydrocarbon group can be a derivative group, and at least one hydrogen atom contained in the hydrocarbon group is methylated. Linear, branched or cyclic having 1 to 10 carbon atoms such as a group, ethyl group, n-propyl group, isopropyl group, n-butyl group, 2-methylpropyl group, 1-methylpropyl group, tert-butyl group Groups having an alicyclic skeleton such as one or more alkyl groups substituted with one or more alkyl groups.
上記一般式(c1-2)において、gは、1、2又は3であり、gが2又は3の場合、上記一般式(c1-2)において複数の、下記一般式で表される構造部は、互いに同一であってよいし、異なってもよい。
(式中、R23は、相互に独立に、水素原子、フッ素原子又は炭素数1~30のフッ素化炭化水素基であり、R24は、水素原子、酸解離性基又はアルカリ解離性基である。)
In the general formula (c1-2), g is 1, 2 or 3, and when g is 2 or 3, a plurality of structural parts represented by the following general formula in the general formula (c1-2) May be the same as or different from each other.
(Wherein R 23 is independently of each other a hydrogen atom, a fluorine atom or a fluorinated hydrocarbon group having 1 to 30 carbon atoms, and R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group. is there.)
上記一般式(c1-3)におけるQ4は、上記一般式(c1-3)におけるgが1であるときのQ3と同様とすることができる。
Q 4 in the general formula (c1-3) can be the same as Q 3 when g in the general formula (c1-3) is 1.
上記一般式(c1-2)で表される繰り返し単位(III-1-2)としては、下記一般式に例示される。
(式中、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R24は、水素原子、酸解離性基又はアルカリ解離性基であり、Q3は、(g+1)価の連結基であり、gは、1、2又は3である。)
The repeating unit (III-1-2) represented by the general formula (c1-2) is exemplified by the following general formula.
(Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group, and Q 3 is (G + 1) is a valent linking group, and g is 1, 2 or 3.)
上記一般式(c1-2)で表される繰り返し単位(III-1-2)を与える単量体としては、下記一般式に例示される。
(式中、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R24は、水素原子、酸解離性基又はアルカリ解離性基である。)
Examples of the monomer that gives the repeating unit (III-1-2) represented by the general formula (c1-2) are exemplified by the following general formula.
(Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group.)
上記一般式で表された5種の単量体において、R24が酸解離性基又はアルカリ解離性基である化合物は、例えば、上記一般式におけるR24が水素原子である化合物を原料として用いることにより合成することができる。
R24がアルカリ解離性基であって、例えば、上記一般式(R1-1)で表される構造を含む単量体の合成方法としては、上記一般式におけるR24が水素原子である化合物を、フルオロアシル化する方法等が挙げられる。具体的な方法としては、以下に例示される。
(1)酸の存在下、アルコールとフルオロカルボン酸を縮合させてエステル化する方法。(2)塩基の存在下、アルコールとフルオロカルボン酸ハロゲン化物を縮合させてエステル化する方法。 In the five types of monomers represented by the above general formula, the compound in which R 24 is an acid dissociable group or an alkali dissociable group uses, for example, a compound in which R 24 in the above general formula is a hydrogen atom as a raw material. Can be synthesized.
And R 24 is an alkali dissociative group, for example, as a synthesis method of a monomer having the structure represented by the general formula (R1-1), the compound R 24 is a hydrogen atom in the above general formula And a method of fluoroacylation. Specific methods are exemplified below.
(1) A method in which an alcohol and a fluorocarboxylic acid are condensed and esterified in the presence of an acid. (2) A method in which an alcohol and a fluorocarboxylic acid halide are condensed and esterified in the presence of a base.
R24がアルカリ解離性基であって、例えば、上記一般式(R1-1)で表される構造を含む単量体の合成方法としては、上記一般式におけるR24が水素原子である化合物を、フルオロアシル化する方法等が挙げられる。具体的な方法としては、以下に例示される。
(1)酸の存在下、アルコールとフルオロカルボン酸を縮合させてエステル化する方法。(2)塩基の存在下、アルコールとフルオロカルボン酸ハロゲン化物を縮合させてエステル化する方法。 In the five types of monomers represented by the above general formula, the compound in which R 24 is an acid dissociable group or an alkali dissociable group uses, for example, a compound in which R 24 in the above general formula is a hydrogen atom as a raw material. Can be synthesized.
And R 24 is an alkali dissociative group, for example, as a synthesis method of a monomer having the structure represented by the general formula (R1-1), the compound R 24 is a hydrogen atom in the above general formula And a method of fluoroacylation. Specific methods are exemplified below.
(1) A method in which an alcohol and a fluorocarboxylic acid are condensed and esterified in the presence of an acid. (2) A method in which an alcohol and a fluorocarboxylic acid halide are condensed and esterified in the presence of a base.
上記一般式(c1-3)で表される繰り返し単位(III-1-3)としては、下記一般式に例示される。
(式中、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R24は、水素原子、酸解離性基又はアルカリ解離性基であり、Q4は、2価の連結基である。)
The repeating unit (III-1-3) represented by the general formula (c1-3) is exemplified by the following general formula.
(Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, R 24 is a hydrogen atom, an acid-dissociable group or an alkali-dissociable group, and Q 4 is It is a divalent linking group.)
上記一般式(c1-3)で表される繰り返し単位(III-1-3)を与える単量体としては、下記一般式に例示される。
(式中、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R24は、水素原子、酸解離性基又はアルカリ解離性基である。)
Examples of the monomer that gives the repeating unit (III-1-3) represented by the general formula (c1-3) are exemplified by the following general formula.
(Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group.)
上記一般式で表された4種の単量体において、R24が酸解離性基又はアルカリ解離性基である化合物は、例えば、上記一般式におけるR24が水素原子である化合物、又は、その誘導体化合物を原料として用いることにより合成することができる。
R24がアルカリ解離性基であって、例えば、上記一般式(R1-2)~(R1-4)で表される構造を含む単量体は、下記一般式(m-2-3)で表される化合物と、下記一般式(m-2-4-1)~(m-2-4-3)で表される化合物とを反応させることにより合成することができる。
(式中、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R23は、相互に独立に、水素原子、フッ素原子又は炭素数1~30のフッ素化炭化水素基であり、R51は、ハロゲン原子又はヒドロキシル基であり、Q4は、2価の連結基であり、2つのR23が水素原子である場合はない。)
(式中、R31は、相互に独立に、ハロゲン原子、炭素数1~10のアルキル基、炭素数1~10のアルコキシル基、炭素数1~10のアシル基又は炭素数1~10のアシロキシ基であり、R55は、ハロゲン原子であり、R56は、ハロゲン原子であり、m1は、0、1、2、3、4又は5であり、m2は、0、1、2、3又は4である。)
In the four types of monomers represented by the above general formula, the compound in which R 24 is an acid dissociable group or an alkali dissociable group is, for example, a compound in which R 24 in the above general formula is a hydrogen atom, or It can be synthesized by using a derivative compound as a raw material.
A monomer having a structure represented by the general formulas (R1-2) to (R1-4) wherein R 24 is an alkali-dissociable group is represented by the following general formula (m-2-3): It can be synthesized by reacting the compound represented by the following general formulas (m-2-4-1) to (m-2--4-3).
(Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 23 is independently a hydrogen atom, a fluorine atom or a fluorinated group having 1 to 30 carbon atoms. (It is a hydrocarbon group, R 51 is a halogen atom or a hydroxyl group, Q 4 is a divalent linking group, and two R 23 are not hydrogen atoms.)
(Wherein R 31 s are independently of each other a halogen atom, an alkyl group having 1 to 10 carbon atoms, an alkoxyl group having 1 to 10 carbon atoms, an acyl group having 1 to 10 carbon atoms, or an acyloxy group having 1 to 10 carbon atoms. R 55 is a halogen atom, R 56 is a halogen atom, m1 is 0, 1, 2, 3, 4 or 5, and m2 is 0, 1, 2, 3 or 4)
R24がアルカリ解離性基であって、例えば、上記一般式(R1-2)~(R1-4)で表される構造を含む単量体は、下記一般式(m-2-3)で表される化合物と、下記一般式(m-2-4-1)~(m-2-4-3)で表される化合物とを反応させることにより合成することができる。
A monomer having a structure represented by the general formulas (R1-2) to (R1-4) wherein R 24 is an alkali-dissociable group is represented by the following general formula (m-2-3): It can be synthesized by reacting the compound represented by the following general formulas (m-2-4-1) to (m-2--4-3).
上記一般式(m-2-4-1)において、R55は、好ましくは塩素原子である。また、上記一般式(m-2-4-2)において、R56は、好ましくは臭素原子である。
In the above general formula (m-2-4-1), R 55 is preferably a chlorine atom. In the general formula (m-2-4-2), R 56 is preferably a bromine atom.
また、R24がアルカリ解離性基であって、例えば、上記一般式(R1-2)~(R1-4)で表される構造を含む単量体は、下記一般式(m-2-5)で表される化合物と、下記一般式(m-2-6)で表される化合物とを反応させることにより合成することができる。
(式中、R21は、水素原子、トリフルオロメチル基又は炭素数1~4のアルキル基であり、R23は、相互に独立に、水素原子、フッ素原子又は炭素数1~30のフッ素化炭化水素基であり、R24は、水素原子、酸解離性基又はアルカリ解離性基であり、R59は、ハロゲン原子又はヒドロキシル基であり、Q4は、2価の連結基である。但し、式(m-2-5)において、2つのR23が水素原子である場合はない。)
In addition, R 24 is an alkali dissociable group and, for example, a monomer having a structure represented by the above general formulas (R1-2) to (R1-4) is represented by the following general formula (m-2-5) ) And a compound represented by the following general formula (m-2-6) can be synthesized.
(Wherein R 21 is a hydrogen atom, a trifluoromethyl group or an alkyl group having 1 to 4 carbon atoms, and R 23 is independently a hydrogen atom, a fluorine atom or a fluorinated group having 1 to 30 carbon atoms. A hydrocarbon group, R 24 is a hydrogen atom, an acid dissociable group or an alkali dissociable group, R 59 is a halogen atom or a hydroxyl group, and Q 4 is a divalent linking group. In the formula (m-2-5), two R 23 are not hydrogen atoms.)
上記重合体[C]は、上記のように、繰り返し単位(III-1-1)、(III-1-2)及び(III-1-3)から選ばれた少なくとも1種の繰り返し単位(III-1)を含む重合体であることが好ましい。そして、含まれる繰り返し単位(III-1)は、それぞれ、1種のみであってよいし、2種以上であってもよい。
本発明においては、上記重合体[C]は、繰り返し単位(III-1-1)、(III-1-2)及び(III-1-3)から選ばれた少なくとも2種の繰り返し単位を含む重合体であることがより好ましく、繰り返し単位(III-1-2)及び(III-1-3)を含む重合体であることが特に好ましい。 As described above, the polymer [C] contains at least one repeating unit (III) selected from repeating units (III-1-1), (III-1-2) and (III-1-3). A polymer containing -1) is preferred. Each of the repeating units (III-1) contained may be only one type, or two or more types.
In the present invention, the polymer [C] contains at least two kinds of repeating units selected from the repeating units (III-1-1), (III-1-2) and (III-1-3). A polymer is more preferable, and a polymer including repeating units (III-1-2) and (III-1-3) is particularly preferable.
本発明においては、上記重合体[C]は、繰り返し単位(III-1-1)、(III-1-2)及び(III-1-3)から選ばれた少なくとも2種の繰り返し単位を含む重合体であることがより好ましく、繰り返し単位(III-1-2)及び(III-1-3)を含む重合体であることが特に好ましい。 As described above, the polymer [C] contains at least one repeating unit (III) selected from repeating units (III-1-1), (III-1-2) and (III-1-3). A polymer containing -1) is preferred. Each of the repeating units (III-1) contained may be only one type, or two or more types.
In the present invention, the polymer [C] contains at least two kinds of repeating units selected from the repeating units (III-1-1), (III-1-2) and (III-1-3). A polymer is more preferable, and a polymer including repeating units (III-1-2) and (III-1-3) is particularly preferable.
上記重合体[C]を構成する繰り返し単位(III-1)の含有量は、上記重合体[C]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは20~90モル%、より好ましくは30~90モル%、更に好ましくは30~85モル%である。繰り返し単位(III-1)の含有割合がこの範囲内である場合には、塗布後の撥水性確保と、PEB後の現像液に対する接触角の親和性の両立という観点から特に有効である。
The content of the repeating unit (III-1) constituting the polymer [C] is preferably 20 to 90 mol% with respect to 100 mol% in total of all the repeating units constituting the polymer [C]. More preferably, it is 30 to 90 mol%, still more preferably 30 to 85 mol%. When the content of the repeating unit (III-1) is within this range, it is particularly effective from the viewpoint of ensuring water repellency after coating and compatibility of contact angle with the developer after PEB.
1-3-2.他の繰り返し単位
上記重合体[C]は、上記繰り返し単位(III-1)以外に、酸解離性基を有する繰り返し単位(以下、「繰り返し単位(III-2)」という。)、アルカリ可溶性基を有する繰り返し単位(以下、「繰り返し単位(III-3)」という。)、ラクトン骨格又は環状カーボネート骨格を有する繰り返し単位(以下、「繰り返し単位(III-4)」という。)等を、更に含んでもよい。 1-3-2. Other Repeating Units The polymer [C] includes, in addition to the repeating unit (III-1), a repeating unit having an acid dissociable group (hereinafter referred to as “repeating unit (III-2)”), an alkali-soluble group. And a repeating unit having a lactone skeleton or a cyclic carbonate skeleton (hereinafter referred to as “repeating unit (III-4)”) and the like. But you can.
上記重合体[C]は、上記繰り返し単位(III-1)以外に、酸解離性基を有する繰り返し単位(以下、「繰り返し単位(III-2)」という。)、アルカリ可溶性基を有する繰り返し単位(以下、「繰り返し単位(III-3)」という。)、ラクトン骨格又は環状カーボネート骨格を有する繰り返し単位(以下、「繰り返し単位(III-4)」という。)等を、更に含んでもよい。 1-3-2. Other Repeating Units The polymer [C] includes, in addition to the repeating unit (III-1), a repeating unit having an acid dissociable group (hereinafter referred to as “repeating unit (III-2)”), an alkali-soluble group. And a repeating unit having a lactone skeleton or a cyclic carbonate skeleton (hereinafter referred to as “repeating unit (III-4)”) and the like. But you can.
1-3-2-1.繰り返し単位(III-2)
この繰り返し単位(III-2)は、上記重合体[C]に含まれることにより、フォトレジスト膜における前進接触角と後退接触角との差を小さくすることができ、露光時のスキャン速度向上に、より対応することができる。 1-3-2-1. Repeating unit (III-2)
By including this repeating unit (III-2) in the polymer [C], the difference between the advancing contact angle and the receding contact angle in the photoresist film can be reduced, and the scanning speed during exposure can be improved. Can respond more.
この繰り返し単位(III-2)は、上記重合体[C]に含まれることにより、フォトレジスト膜における前進接触角と後退接触角との差を小さくすることができ、露光時のスキャン速度向上に、より対応することができる。 1-3-2-1. Repeating unit (III-2)
By including this repeating unit (III-2) in the polymer [C], the difference between the advancing contact angle and the receding contact angle in the photoresist film can be reduced, and the scanning speed during exposure can be improved. Can respond more.
上記繰り返し単位(III-2)は、下記一般式(c2-1)で表される繰り返し単位が好ましい。
(式中、R25は、水素原子、メチル基、トリフルオロメチル基又はヒドロキシメチル基であり、R26は、炭素数1~4の直鎖状又は分岐状のアルキル基であり、kは、1、2、3又は4である。)
The repeating unit (III-2) is preferably a repeating unit represented by the following general formula (c2-1).
(Wherein R 25 is a hydrogen atom, a methyl group, a trifluoromethyl group or a hydroxymethyl group, R 26 is a linear or branched alkyl group having 1 to 4 carbon atoms, and k is 1, 2, 3 or 4.)
上記一般式(c2-1)において、R26により表した、炭素数1~4の直鎖状又は分岐状のアルキル基としては、メチル基、エチル基、n-プロピル基、イソプロピル基、n-ブチル基、2-メチルプロピル基、1-メチルプロピル基、tert-ブチル基等が挙げられる。
In the general formula (c2-1), the linear or branched alkyl group having 1 to 4 carbon atoms represented by R 26 includes a methyl group, ethyl group, n-propyl group, isopropyl group, n- Examples thereof include a butyl group, a 2-methylpropyl group, a 1-methylpropyl group, and a tert-butyl group.
上記重合体[C]は、上記繰り返し単位(III-2)を単独で含んでよいし、2種以上の組み合わせで含んでもよい。
The polymer [C] may contain the repeating unit (III-2) alone or in combination of two or more.
上記重合体[C]が、繰り返し単位(III-2)を含む場合、その含有量は、上記重合体[C]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは80モル%以下、より好ましくは10~80モル%、更に好ましくは20~80モル%、特に好ましくは30~70モル%である。繰り返し単位(III-2)の含有量が上記範囲にあると、フォトレジスト膜における前進接触角と後退接触角との差を小さくすることができる。
When the polymer [C] contains the repeating unit (III-2), the content thereof is preferably 80 mol with respect to 100 mol% in total of all the repeating units constituting the polymer [C]. % Or less, more preferably 10 to 80 mol%, still more preferably 20 to 80 mol%, particularly preferably 30 to 70 mol%. When the content of the repeating unit (III-2) is in the above range, the difference between the advancing contact angle and the receding contact angle in the photoresist film can be reduced.
1-3-2-2.繰り返し単位(III-3)
この繰り返し単位(III-3)は、上記重合体[C]に含まれることにより、後述するパターン形成方法の現像工程において、フォトレジスト膜の溶解性を向上させることができる。 1-3-2-2. Repeating unit (III-3)
By including this repeating unit (III-3) in the polymer [C], the solubility of the photoresist film can be improved in the development step of the pattern forming method described later.
この繰り返し単位(III-3)は、上記重合体[C]に含まれることにより、後述するパターン形成方法の現像工程において、フォトレジスト膜の溶解性を向上させることができる。 1-3-2-2. Repeating unit (III-3)
By including this repeating unit (III-3) in the polymer [C], the solubility of the photoresist film can be improved in the development step of the pattern forming method described later.
上記繰り返し単位(III-3)におけるアルカリ可溶性基は、好ましくは、pKaが4~11の水素原子を有する官能基である。
The alkali-soluble group in the repeating unit (III-3) is preferably a functional group having a hydrogen atom having a pKa of 4 to 11.
上記アルカリ可溶性基としては、下記一般式(c3-a)及び式(c3-b)に例示される。
(式中、R36は、炭素数1~10のフッ素化炭化水素基である。)
Examples of the alkali-soluble group include the following general formula (c3-a) and formula (c3-b).
(Wherein R 36 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms.)
上記一般式(c3-a)において、R36は、炭素数1~10のフッ素化炭化水素基であり、炭素数1~10の炭化水素基における水素原子の少なくとも1つが、フッ素原子に置換されてなる基である。本発明において、R36は、トリフルオロメチル基が好ましい。
In the general formula (c3-a), R 36 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms, and at least one hydrogen atom in the hydrocarbon group having 1 to 10 carbon atoms is substituted with a fluorine atom. It is a group consisting of In the present invention, R 36 is preferably a trifluoromethyl group.
上記繰り返し単位(III-3)における主鎖の構造は、特に限定されないが、(メタ)アクリル酸エステル、α-トリフルオロアクリル酸エステル等の重合体における主鎖の構造であることが好ましい。
The structure of the main chain in the repeating unit (III-3) is not particularly limited, but is preferably the structure of the main chain in a polymer such as (meth) acrylic acid ester or α-trifluoroacrylic acid ester.
上記繰り返し単位(III-3)としては、下記一般式(c3-a-1)及び(c3-b-1)に例示される。
(式中、R27は、水素原子、メチル基又はトリフルオロメチル基であり、R28は、単結合、又は、2価であって、炭素数1~20の直鎖状、分岐状若しくは環状の、飽和あるいは不飽和の炭化水素基であり、R36は、炭素数1~10のフッ素化炭化水素基である。)
The repeating unit (III-3) is exemplified by the following general formulas (c3-a-1) and (c3-b-1).
(Wherein R 27 is a hydrogen atom, a methyl group or a trifluoromethyl group, and R 28 is a single bond, or a divalent, linear, branched or cyclic group having 1 to 20 carbon atoms. S 36 is a saturated or unsaturated hydrocarbon group, and R 36 is a fluorinated hydrocarbon group having 1 to 10 carbon atoms.)
上記一般式(c3-a-1)及び(c3-b-1)におけるR28が、2価であって、炭素数1~20の直鎖状、分岐状若しくは環状の、飽和あるいは不飽和の炭化水素基である場合、その具体例としては、メチレン基、エチレン基、1,3-プロピレン基及び1,2-プロピレン基に例示されるプロピレン基、テトラメチレン基、ペンタメチレン基、ヘキサメチレン基、ヘプタメチレン基、オクタメチレン基、ノナメチレン基、デカメチレン基、ウンデカメチレン基、ドデカメチレン基、トリデカメチレン基、テトラデカメチレン基、ペンタデカメチレン基、ヘキサデカメチレン基、ヘプタデカメチレン基、オクタデカメチレン基、ノナデカメチレン基、インサレン基、1-メチル-1,3-プロピレン基、2-メチル-1,3-プロピレン基、2-メチル-1,2-プロピレン基、1-メチル-1,4-ブチレン基、2-メチル-1,4-ブチレン基、メチリデン基、エチリデン基、プロピリデン基、2-プロピリデン基等の飽和鎖状炭化水素基;1,3-シクロブチレン基等のシクロブチレン基、1,3-シクロペンチレン基等のシクロペンチレン基、1,4-シクロへキシレン基等のシクロへキシレン基、1,5-シクロオクチレン基等のシクロオクチレン基等の、炭素数3~10であるシクロアルキレン基等の単環式炭化水素環基;1,4-ノルボルニレン基、2,5-ノルボルニレン基等のノルボルニレン基、1,5-アダマンチレン基、2,6-アダマンチレン基等のアダマンチレン基等の、炭素数4~30である2~4環式炭化水素環基等の架橋環式炭化水素基等が挙げられる。
R 28 in the above general formulas (c3-a-1) and (c3-b-1) is divalent and has 1 to 20 carbon atoms, linear, branched or cyclic, saturated or unsaturated. When it is a hydrocarbon group, specific examples thereof include a methylene group, an ethylene group, a 1,3-propylene group and a 1,2-propylene group, a propylene group, a tetramethylene group, a pentamethylene group, a hexamethylene group. , Heptamethylene group, octamethylene group, nonamethylene group, decamethylene group, undecamethylene group, dodecamethylene group, tridecamethylene group, tetradecamethylene group, pentadecamethylene group, hexadecamethylene group, heptacamethylene group, octa Decamethylene group, nonacamethylene group, insalen group, 1-methyl-1,3-propylene group, 2-methyl-1,3-propylene Group, 2-methyl-1,2-propylene group, 1-methyl-1,4-butylene group, 2-methyl-1,4-butylene group, methylidene group, ethylidene group, propylidene group, 2-propylidene group, etc. A saturated chain hydrocarbon group; a cyclobutylene group such as 1,3-cyclobutylene group, a cyclopentylene group such as 1,3-cyclopentylene group, and a cyclohexylene group such as 1,4-cyclohexylene group A monocyclic hydrocarbon ring group such as a cycloalkylene group having 3 to 10 carbon atoms such as a cyclooctylene group such as 1,5-cyclooctylene group; 1,4-norbornylene group, 2,5-norbornylene A bridged cyclic group such as a 2-4 cyclic hydrocarbon ring group having 4 to 30 carbon atoms, such as a norbornylene group such as a group, adamantylene group such as 1,5-adamantylene group, 2,6-adamantylene group, etc. Hydrocarbon group, etc. And the like.
上記重合体[C]は、上記繰り返し単位(III-3)を単独で含んでよいし、2種以上の組み合わせで含んでもよい。
The polymer [C] may contain the repeating unit (III-3) alone or in combination of two or more.
上記重合体[C]が、繰り返し単位(III-3)を含む場合、その含有量は、上記重合体[C]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは50モル%以下、より好ましくは5~30モル%、更に好ましくは5~20モル%である。繰り返し単位(III-3)の含有量が上記範囲にあると、後述するパターン形成方法の現像工程において、フォトレジスト膜の溶解性を向上させることができる。
When the polymer [C] contains the repeating unit (III-3), the content thereof is preferably 50 mol with respect to 100 mol% in total of all the repeating units constituting the polymer [C]. % Or less, more preferably 5 to 30 mol%, still more preferably 5 to 20 mol%. When the content of the repeating unit (III-3) is in the above range, the solubility of the photoresist film can be improved in the development step of the pattern forming method described later.
1-3-2-3.繰り返し単位(III-4)
この繰り返し単位(III-4)は、上記重合体[C]に含まれることにより、液浸露光時において、フォトレジスト膜の疎水性を確保しつつ、アルカリ現像時における親水性を向上させることができる繰り返し単位である。 1-3-2-3. Repeating unit (III-4)
By including this repeating unit (III-4) in the polymer [C], it is possible to improve the hydrophilicity during alkali development while ensuring the hydrophobicity of the photoresist film during immersion exposure. Repeating unit that can be.
この繰り返し単位(III-4)は、上記重合体[C]に含まれることにより、液浸露光時において、フォトレジスト膜の疎水性を確保しつつ、アルカリ現像時における親水性を向上させることができる繰り返し単位である。 1-3-2-3. Repeating unit (III-4)
By including this repeating unit (III-4) in the polymer [C], it is possible to improve the hydrophilicity during alkali development while ensuring the hydrophobicity of the photoresist film during immersion exposure. Repeating unit that can be.
上記重合体[C]が、繰り返し単位(III-4)を含む場合、その含有量は、上記重合体[C]を構成する全ての繰り返し単位の合計100モル%に対して、好ましくは50モル%以下、より好ましくは5~30モル%、更に好ましくは5~20モル%である。繰り返し単位(III-4)の含有量が上記範囲にあると、液浸露光時において、フォトレジスト膜の疎水性を確保しつつ、アルカリ現像時における親水性を向上させることができる。
When the polymer [C] contains the repeating unit (III-4), the content thereof is preferably 50 mol with respect to 100 mol% in total of all the repeating units constituting the polymer [C]. % Or less, more preferably 5 to 30 mol%, still more preferably 5 to 20 mol%. When the content of the repeating unit (III-4) is in the above range, the hydrophilicity during alkali development can be improved while ensuring the hydrophobicity of the photoresist film during immersion exposure.
1-3-2-4.繰り返し単位の好ましい組み合わせ
上記重合体[C]を構成する繰り返し単位の組み合わせ及びその含有量について、以下に示す。
繰り返し単位(III-1)及び繰り返し単位(III-2)とからなり、これらの含有量が、上記重合体[C]を構成する全ての繰り返し単位の合計100モル%に対して、それぞれ、好ましくは20~90モル%及び10~80モル%、より好ましくは30~90モル%及び10~70モル%である重合体。 1-3-2-4. Preferred combinations of repeating units The combinations of repeating units constituting the polymer [C] and their contents are shown below.
It consists of the repeating unit (III-1) and the repeating unit (III-2), and the content thereof is preferably respectively based on 100 mol% in total of all the repeating units constituting the polymer [C]. Are polymers of 20 to 90 mol% and 10 to 80 mol%, more preferably 30 to 90 mol% and 10 to 70 mol%.
上記重合体[C]を構成する繰り返し単位の組み合わせ及びその含有量について、以下に示す。
繰り返し単位(III-1)及び繰り返し単位(III-2)とからなり、これらの含有量が、上記重合体[C]を構成する全ての繰り返し単位の合計100モル%に対して、それぞれ、好ましくは20~90モル%及び10~80モル%、より好ましくは30~90モル%及び10~70モル%である重合体。 1-3-2-4. Preferred combinations of repeating units The combinations of repeating units constituting the polymer [C] and their contents are shown below.
It consists of the repeating unit (III-1) and the repeating unit (III-2), and the content thereof is preferably respectively based on 100 mol% in total of all the repeating units constituting the polymer [C]. Are polymers of 20 to 90 mol% and 10 to 80 mol%, more preferably 30 to 90 mol% and 10 to 70 mol%.
1-3-3.平均分子量
上記重合体[C]の重量平均分子量(Mw)は、GPCによるポリスチレン換算で、好ましくは1,000~50,000、より好ましくは1,000~40,000、更に好ましくは1,000~30,000である。Mwが1,000未満であると、十分な後退接触角を有するフォトレジスト膜が得られない場合がある。一方、Mwが50,000を超えると、フォトレジスト膜の現像性が低下する場合がある。
また、GPCによるポリスチレン換算の数平均分子量(Mn)と、Mwとを用いて得られた比(Mw/Mn)は、好ましくは1~5、より好ましくは1~4である。 1-3-3. Average Molecular Weight The weight average molecular weight (Mw) of the polymer [C] is preferably 1,000 to 50,000, more preferably 1,000 to 40,000, and still more preferably 1,000 in terms of polystyrene by GPC. ~ 30,000. If Mw is less than 1,000, a photoresist film having a sufficient receding contact angle may not be obtained. On the other hand, if Mw exceeds 50,000, the developability of the photoresist film may deteriorate.
Further, the ratio (Mw / Mn) obtained using the number average molecular weight (Mn) in terms of polystyrene by GPC and Mw is preferably 1 to 5, more preferably 1 to 4.
上記重合体[C]の重量平均分子量(Mw)は、GPCによるポリスチレン換算で、好ましくは1,000~50,000、より好ましくは1,000~40,000、更に好ましくは1,000~30,000である。Mwが1,000未満であると、十分な後退接触角を有するフォトレジスト膜が得られない場合がある。一方、Mwが50,000を超えると、フォトレジスト膜の現像性が低下する場合がある。
また、GPCによるポリスチレン換算の数平均分子量(Mn)と、Mwとを用いて得られた比(Mw/Mn)は、好ましくは1~5、より好ましくは1~4である。 1-3-3. Average Molecular Weight The weight average molecular weight (Mw) of the polymer [C] is preferably 1,000 to 50,000, more preferably 1,000 to 40,000, and still more preferably 1,000 in terms of polystyrene by GPC. ~ 30,000. If Mw is less than 1,000, a photoresist film having a sufficient receding contact angle may not be obtained. On the other hand, if Mw exceeds 50,000, the developability of the photoresist film may deteriorate.
Further, the ratio (Mw / Mn) obtained using the number average molecular weight (Mn) in terms of polystyrene by GPC and Mw is preferably 1 to 5, more preferably 1 to 4.
1-3-4.製造方法
上記重合体[C]は、ハイドロパーオキシド、ジアルキルパーオキシド、ジアシルパーオキシド、アゾ化合物等のラジカル重合開始剤の存在下、繰り返し単位(III-1)等を形成することとなる単量体を含む単量体原料を、適当な溶媒中において重合することにより、製造することができる。重合系においては、必要に応じて、連鎖移動剤を併存させてもよい。
重合温度は、通常、40℃~150℃、好ましくは50℃~120℃である。また、重合時間は、通常、1~48時間、好ましくは1~24時間である。 1-3-4. Production Method The above polymer [C] is a single monomer that will form the repeating unit (III-1) in the presence of a radical polymerization initiator such as hydroperoxide, dialkyl peroxide, diacyl peroxide, and azo compound. It can manufacture by polymerizing the monomer raw material containing a body in a suitable solvent. In the polymerization system, a chain transfer agent may coexist if necessary.
The polymerization temperature is usually 40 ° C. to 150 ° C., preferably 50 ° C. to 120 ° C. The polymerization time is usually 1 to 48 hours, preferably 1 to 24 hours.
上記重合体[C]は、ハイドロパーオキシド、ジアルキルパーオキシド、ジアシルパーオキシド、アゾ化合物等のラジカル重合開始剤の存在下、繰り返し単位(III-1)等を形成することとなる単量体を含む単量体原料を、適当な溶媒中において重合することにより、製造することができる。重合系においては、必要に応じて、連鎖移動剤を併存させてもよい。
重合温度は、通常、40℃~150℃、好ましくは50℃~120℃である。また、重合時間は、通常、1~48時間、好ましくは1~24時間である。 1-3-4. Production Method The above polymer [C] is a single monomer that will form the repeating unit (III-1) in the presence of a radical polymerization initiator such as hydroperoxide, dialkyl peroxide, diacyl peroxide, and azo compound. It can manufacture by polymerizing the monomer raw material containing a body in a suitable solvent. In the polymerization system, a chain transfer agent may coexist if necessary.
The polymerization temperature is usually 40 ° C. to 150 ° C., preferably 50 ° C. to 120 ° C. The polymerization time is usually 1 to 48 hours, preferably 1 to 24 hours.
上記溶媒としては、n-ペンタン、n-ヘキサン、n-ヘプタン、n-オクタン、n-ノナン、n-デカン等のアルカン類;シクロヘキサン、シクロヘプタン、シクロオクタン等のシクロアルカン類;デカリン、ノルボルナン等の脂環式炭化水素類;ベンゼン、トルエン、キシレン、エチルベンゼン、クメン等の芳香族炭化水素類;クロロブタン、ブロモヘキサン、ジクロロエタン、ヘキサメチレンジブロミド、クロロベンゼン等のハロゲン化炭化水素類;酢酸エチル、酢酸n-ブチル、酢酸イソブチル、プロピオン酸メチル等の飽和カルボン酸エステル類;アセトン、2-ブタノン、4-メチル-2-ペンタノン、2-ヘプタノン等のケトン類;テトラヒドロフラン、ジメトキシエタン、ジエトキシエタン等のエーテル類;メタノール、エタノール、1-プロパノール、2-プロパノール、4-メチル-2-ペンタノール等のアルコール類等が挙げられる。尚、これらの溶媒は、単独で用いてよいし、2種以上を組み合わせて用いてもよい。
Examples of the solvent include alkanes such as n-pentane, n-hexane, n-heptane, n-octane, n-nonane and n-decane; cycloalkanes such as cyclohexane, cycloheptane and cyclooctane; decalin, norbornane and the like Alicyclic hydrocarbons; aromatic hydrocarbons such as benzene, toluene, xylene, ethylbenzene, cumene; halogenated hydrocarbons such as chlorobutane, bromohexane, dichloroethane, hexamethylene dibromide, chlorobenzene; ethyl acetate, acetic acid saturated carboxylic acid esters such as n-butyl, isobutyl acetate and methyl propionate; ketones such as acetone, 2-butanone, 4-methyl-2-pentanone and 2-heptanone; tetrahydrofuran, dimethoxyethane, diethoxyethane and the like Ethers; methanol, Ethanol, 1-propanol, 2-propanol, alcohols such as 4-methyl-2-pentanol. These solvents may be used alone or in combination of two or more.
尚、本発明の感放射線性樹脂組成物の製造に用いられる重合体[C]においては、ハロゲン、金属等の不純物の含有量が少ないほど好ましい。このような不純物の含有量が少ない場合、フォトレジスト膜の感度、解像度、プロセス安定性、パターン形状等を更に向上させることができる。従って、上記方法により合成された重合体[C]を含む粗生成物を精製に供することが好ましい。精製法としては、水洗、液々抽出等の化学的精製法や、これらの化学的精製法と限外ろ過、遠心分離等の物理的精製法とを組み合わせた方法等が挙げられる。
In addition, in polymer [C] used for manufacture of the radiation sensitive resin composition of this invention, it is so preferable that there is little content of impurities, such as a halogen and a metal. When the content of such impurities is small, the sensitivity, resolution, process stability, pattern shape and the like of the photoresist film can be further improved. Therefore, it is preferable to subject the crude product containing the polymer [C] synthesized by the above method to purification. Examples of the purification method include chemical purification methods such as washing with water and liquid-liquid extraction, and methods combining these chemical purification methods with physical purification methods such as ultrafiltration and centrifugation.
1-3-5.含有量
本発明の感放射線性樹脂組成物における重合体[C]の含有量は、上記重合体[A]100質量部に対して、好ましくは0.1~20質量部、より好ましくは1~10質量部、更に好ましくは1~7.5質量部である。上記重合体[C]の含有量が少なすぎると、重合体[C]を含有させる効果が十分ではない場合がある。一方、上記重合体[C]の含有量が多すぎると、フォトレジスト膜の表面における撥水性が高くなりすぎて、現像不良が起こる場合がある。 1-3-5. Content The content of the polymer [C] in the radiation-sensitive resin composition of the present invention is preferably 0.1 to 20 parts by mass, more preferably 1 to 100 parts by mass with respect to 100 parts by mass of the polymer [A]. The amount is 10 parts by mass, more preferably 1 to 7.5 parts by mass. If the content of the polymer [C] is too small, the effect of containing the polymer [C] may not be sufficient. On the other hand, if the content of the polymer [C] is too large, the water repellency on the surface of the photoresist film becomes too high, and development failure may occur.
本発明の感放射線性樹脂組成物における重合体[C]の含有量は、上記重合体[A]100質量部に対して、好ましくは0.1~20質量部、より好ましくは1~10質量部、更に好ましくは1~7.5質量部である。上記重合体[C]の含有量が少なすぎると、重合体[C]を含有させる効果が十分ではない場合がある。一方、上記重合体[C]の含有量が多すぎると、フォトレジスト膜の表面における撥水性が高くなりすぎて、現像不良が起こる場合がある。 1-3-5. Content The content of the polymer [C] in the radiation-sensitive resin composition of the present invention is preferably 0.1 to 20 parts by mass, more preferably 1 to 100 parts by mass with respect to 100 parts by mass of the polymer [A]. The amount is 10 parts by mass, more preferably 1 to 7.5 parts by mass. If the content of the polymer [C] is too small, the effect of containing the polymer [C] may not be sufficient. On the other hand, if the content of the polymer [C] is too large, the water repellency on the surface of the photoresist film becomes too high, and development failure may occur.
1-4.添加剤
本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物は、上記必須成分に加えて、酸拡散抑制剤、ラクトン化合物、溶剤、界面活性剤、増感剤、ハレーション防止剤、接着助剤、保存安定化剤、消泡剤、脂環族添加剤等を含んでもよい。 1-4. Additives The radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure includes, in addition to the above essential components, an acid diffusion inhibitor, a lactone compound, a solvent, a surfactant, a sensitizer, and a halation. An inhibitor, an adhesion assistant, a storage stabilizer, an antifoaming agent, an alicyclic additive, and the like may be included.
本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物は、上記必須成分に加えて、酸拡散抑制剤、ラクトン化合物、溶剤、界面活性剤、増感剤、ハレーション防止剤、接着助剤、保存安定化剤、消泡剤、脂環族添加剤等を含んでもよい。 1-4. Additives The radiation-sensitive resin composition of the present invention or the radiation-sensitive resin composition for immersion exposure includes, in addition to the above essential components, an acid diffusion inhibitor, a lactone compound, a solvent, a surfactant, a sensitizer, and a halation. An inhibitor, an adhesion assistant, a storage stabilizer, an antifoaming agent, an alicyclic additive, and the like may be included.
1-4-1.酸拡散抑制剤
この酸拡散抑制剤は、液浸露光により、上記酸発生剤[B]から生じる酸のフォトレジスト膜中における拡散現象を制御し、未露光部における好ましくない化学反応を抑制する作用を有する成分である。この酸拡散抑制剤を配合することにより、得られるパターン化されたレジスト膜部の形状及びその寸法忠実性を向上させることができる。 1-4-1. Acid Diffusion Inhibitor This acid diffusion inhibitor controls the diffusion phenomenon of the acid generated from the acid generator [B] in the photoresist film by immersion exposure, and suppresses an undesirable chemical reaction in the unexposed area. It is a component having By blending this acid diffusion inhibitor, the shape of the patterned resist film portion to be obtained and its dimensional fidelity can be improved.
この酸拡散抑制剤は、液浸露光により、上記酸発生剤[B]から生じる酸のフォトレジスト膜中における拡散現象を制御し、未露光部における好ましくない化学反応を抑制する作用を有する成分である。この酸拡散抑制剤を配合することにより、得られるパターン化されたレジスト膜部の形状及びその寸法忠実性を向上させることができる。 1-4-1. Acid Diffusion Inhibitor This acid diffusion inhibitor controls the diffusion phenomenon of the acid generated from the acid generator [B] in the photoresist film by immersion exposure, and suppresses an undesirable chemical reaction in the unexposed area. It is a component having By blending this acid diffusion inhibitor, the shape of the patterned resist film portion to be obtained and its dimensional fidelity can be improved.
上記酸拡散抑制剤としては、下記一般式(E)で表される化合物(以下、「含窒素化合物(I)」という。)、窒素原子を2個有する化合物(以下、「含窒素化合物(II)」という。)、窒素原子を3個以上有する化合物(以下、「含窒素化合物(III)」という。)、アミド基含有化合物、ウレア化合物、含窒素複素環化合物等が挙げられる。これらの酸拡散抑制剤は、単独で用いてよいし、2種以上を組み合わせて用いてもよい。
(式中、R61は、相互に独立に、水素原子、あるいは、置換又は非置換の、直鎖状、分岐状若しくは環状のアルキル基、アリール基又はアラルキル基である。)
Examples of the acid diffusion inhibitor include a compound represented by the following general formula (E) (hereinafter referred to as “nitrogen-containing compound (I)”), a compound having two nitrogen atoms (hereinafter referred to as “nitrogen-containing compound (II)”. ) "), Compounds having 3 or more nitrogen atoms (hereinafter referred to as" nitrogen-containing compound (III) "), amide group-containing compounds, urea compounds, nitrogen-containing heterocyclic compounds, and the like. These acid diffusion inhibitors may be used alone or in combination of two or more.
(In the formula, R 61 are each independently a hydrogen atom, or a substituted or unsubstituted, linear, branched or cyclic alkyl group, aryl group or aralkyl group.)
含窒素化合物(I)としては、n-ヘキシルアミン、n-ヘプチルアミン、n-オクチルアミン、n-ノニルアミン、n-デシルアミン等のモノアルキルアミン類;ジ-n-ブチルアミン、ジ-n-ペンチルアミン、ジ-n-ヘキシルアミン、ジ-n-ヘプチルアミン、ジ-n-オクチルアミン、ジ-n-ノニルアミン、ジ-n-デシルアミン等のジアルキルアミン類;トリエチルアミン、トリ-n-プロピルアミン、トリ-n-ブチルアミン、トリ-n-ペンチルアミン、トリ-n-ヘキシルアミン、トリ-n-ヘプチルアミン、トリ-n-オクチルアミン、トリ-n-ノニルアミン、トリ-n-デシルアミン等のトリアルキルアミン類;アニリン、N-メチルアニリン、N,N-ジメチルアニリン、2-メチルアニリン、3-メチルアニリン、4-メチルアニリン、4-ニトロアニリン、2, 6-ジイソプロピルアニリン、ジフェニルアミン、トリフェニルアミン、1-ナフチルアミン、2-(4-アミノフェニル)-2-(3-ヒドロキシフェニル)プロパン、2-(4-アミノフェニル)-2-(4-ヒドロキシフェニル)プロパン等の芳香族アミン類等が挙げられる。
Nitrogen-containing compounds (I) include monoalkylamines such as n-hexylamine, n-heptylamine, n-octylamine, n-nonylamine, n-decylamine; di-n-butylamine, di-n-pentylamine Dialkylamines such as di-n-hexylamine, di-n-heptylamine, di-n-octylamine, di-n-nonylamine, di-n-decylamine; triethylamine, tri-n-propylamine, tri- trialkylamines such as n-butylamine, tri-n-pentylamine, tri-n-hexylamine, tri-n-heptylamine, tri-n-octylamine, tri-n-nonylamine, tri-n-decylamine; Aniline, N-methylaniline, N, N-dimethylaniline, 2-methylaniline, 3-methylaline Phosphorus, 4-methylaniline, 4-nitroaniline, 2, 6-diisopropylaniline, diphenylamine, triphenylamine, 1-naphthylamine, 2- (4-aminophenyl) -2- (3-hydroxyphenyl) propane, 2- And aromatic amines such as (4-aminophenyl) -2- (4-hydroxyphenyl) propane.
上記含窒素化合物(II)としては、エチレンジアミン、N,N,N’,N’-テトラメチルエチレンジアミン、N,N,N’,N’-テトラキス(2-ヒドロキシプロピル)エチレンジアミン、テトラメチレンジアミン、ヘキサメチレンジアミン、4,4’-ジアミノジフェニルメタン、4,4’-ジアミノジフェニルエーテル、4,4’-ジアミノベンゾフェノン、4,4’-ジアミノジフェニルアミン、2,2’-ビス(4-アミノフェニル)プロパン、2-(3-アミノフェニル)-2-(4-アミノフェニル)プロパン、1,4-ビス[1-(4-アミノフェニル)-1-メチルエチル]ベンゼン、1,3-ビス[1-(4-アミノフェニル)-1-メチルエチル]ベンゼン等が挙げられる。
Examples of the nitrogen-containing compound (II) include ethylenediamine, N, N, N ′, N′-tetramethylethylenediamine, N, N, N ′, N′-tetrakis (2-hydroxypropyl) ethylenediamine, tetramethylenediamine, hexa Methylenediamine, 4,4′-diaminodiphenylmethane, 4,4′-diaminodiphenyl ether, 4,4′-diaminobenzophenone, 4,4′-diaminodiphenylamine, 2,2′-bis (4-aminophenyl) propane, 2 -(3-aminophenyl) -2- (4-aminophenyl) propane, 1,4-bis [1- (4-aminophenyl) -1-methylethyl] benzene, 1,3-bis [1- (4 -Aminophenyl) -1-methylethyl] benzene and the like.
上記含窒素化合物(III)としては、ポリエチレンイミン、ポリアリルアミン、ジメチルアミノエチルアクリルアミドの重合体等が挙げられる。
Examples of the nitrogen-containing compound (III) include polymers of polyethyleneimine, polyallylamine, dimethylaminoethylacrylamide, and the like.
上記アミド基含有化合物としては、ホルムアミド、N-メチルホルムアミド、N,N-ジメチルホルムアミド、アセトアミド、N-メチルアセトアミド、N,N-ジメチルアセトアミド、プロピオンアミド、ベンズアミド、ピロリドン、N-メチルピロリドン等が挙げられる。
Examples of the amide group-containing compound include formamide, N-methylformamide, N, N-dimethylformamide, acetamide, N-methylacetamide, N, N-dimethylacetamide, propionamide, benzamide, pyrrolidone, N-methylpyrrolidone and the like. It is done.
上記ウレア化合物としては、尿素、メチルウレア、1,1-ジメチルウレア、1,3-ジメチルウレア、1,1,3,3-テトラメチルウレア、1,3-ジフェニルウレア、トリブチルチオウレア等が挙げられる。
Examples of the urea compound include urea, methylurea, 1,1-dimethylurea, 1,3-dimethylurea, 1,1,3,3-tetramethylurea, 1,3-diphenylurea, tributylthiourea and the like.
上記含窒素複素環化合物としては、ピリジン、2-メチルピリジン、4-メチルピリジン、2-エチルピリジン、4-エチルピリジン、2-フェニルピリジン、4-フェニルピリジン、N-メチル-4-フェニルピリジン、ニコチン、ニコチン酸、ニコチン酸アミド、キノリン、8-オキシキノリン、アクリジン等のピリジン類;ピペリジン、tert-ブチル-4-ヒドロキシ-1-ピペリジンカルボキシレート等のピペリジン類;ピラジン、ピラゾール、ピリダジン、キノザリン、プリン、ピロリジン、モルホリン、4-メチルモルホリン、ピペラジン、1,4-ジメチルピペラジン、1,4-ジアザビシクロ[2.2.2]オクタン等が挙げられる。
Examples of the nitrogen-containing heterocyclic compound include pyridine, 2-methylpyridine, 4-methylpyridine, 2-ethylpyridine, 4-ethylpyridine, 2-phenylpyridine, 4-phenylpyridine, N-methyl-4-phenylpyridine, Pyridines such as nicotine, nicotinic acid, nicotinamide, quinoline, 8-oxyquinoline, acridine; piperidines such as piperidine, tert-butyl-4-hydroxy-1-piperidinecarboxylate; pyrazine, pyrazole, pyridazine, quinosaline, Examples include purine, pyrrolidine, morpholine, 4-methylmorpholine, piperazine, 1,4-dimethylpiperazine, 1,4-diazabicyclo [2.2.2] octane.
また、酸拡散抑制剤としては、下記一般式(D1-0)で表される化合物を用いることもできる。
X+Z- (D1-0)
(式中、X+は、下記一般式(D1-1)又は(D1-2)で表されるカチオンであり、Z-は、OH-、一般式(D1-3):RD1-COO-で表されるアニオン、一般式(D1-4):RD1-SO3 -で表されるアニオン、又は、一般式(D1-5):RD1-N--SO2-RD11で表されるアニオンである(但し、一般式(D1-3)~(D1-5)において、RD1は、置換されていてもよいアルキル基、脂環式炭化水素基又はアリール基である。RD11は、置換されていてもよいフッ素化アルキル基、脂環式フッ素化炭化水素基、又は、フッ素化アリール基である。)。) As the acid diffusion inhibitor, a compound represented by the following general formula (D1-0) can also be used.
X + Z - (D1-0)
Wherein X + is a cation represented by the following general formula (D1-1) or (D1-2), Z − is OH − , general formula (D1-3): R D1 —COO − An anion represented by general formula (D1-4): R D1 —SO 3 — , or an anion represented by general formula (D1-5): R D1 —N − —SO 2 —R D11 that is an anion (provided that in the general formula (D1-3) ~ (D1-5), R D1 is an optionally substituted alkyl group, an alicyclic hydrocarbon group or an aryl group .R D11 is , An optionally substituted fluorinated alkyl group, an alicyclic fluorinated hydrocarbon group, or a fluorinated aryl group.)
X+Z- (D1-0)
(式中、X+は、下記一般式(D1-1)又は(D1-2)で表されるカチオンであり、Z-は、OH-、一般式(D1-3):RD1-COO-で表されるアニオン、一般式(D1-4):RD1-SO3 -で表されるアニオン、又は、一般式(D1-5):RD1-N--SO2-RD11で表されるアニオンである(但し、一般式(D1-3)~(D1-5)において、RD1は、置換されていてもよいアルキル基、脂環式炭化水素基又はアリール基である。RD11は、置換されていてもよいフッ素化アルキル基、脂環式フッ素化炭化水素基、又は、フッ素化アリール基である。)。) As the acid diffusion inhibitor, a compound represented by the following general formula (D1-0) can also be used.
X + Z - (D1-0)
Wherein X + is a cation represented by the following general formula (D1-1) or (D1-2), Z − is OH − , general formula (D1-3): R D1 —COO − An anion represented by general formula (D1-4): R D1 —SO 3 — , or an anion represented by general formula (D1-5): R D1 —N − —SO 2 —R D11 that is an anion (provided that in the general formula (D1-3) ~ (D1-5), R D1 is an optionally substituted alkyl group, an alicyclic hydrocarbon group or an aryl group .R D11 is , An optionally substituted fluorinated alkyl group, an alicyclic fluorinated hydrocarbon group, or a fluorinated aryl group.)
上記一般式(D1-0)で表される化合物は、露光により分解して酸拡散制御性を失う酸拡散制御剤(以下、「光分解性酸拡散制御剤」ともいう。)として用いられるものである。そして、本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物が、上記一般式(D1-0)で表される化合物を含有する場合、露光部では酸が拡散し、未露光部では酸の拡散が制御されるため、露光部及び未露光部のコントラストに優れる(即ち、露光部と未露光部の境界部分が明確になる)。従って、そのような組成物は、LWR、MEEF(Mask Error Enhancement Factor(マスク幅のずれによるライン幅のずれの増幅因子))の改善に有効である。
The compound represented by the general formula (D1-0) is used as an acid diffusion control agent that is decomposed by exposure and loses acid diffusion controllability (hereinafter also referred to as “photodegradable acid diffusion control agent”). It is. When the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure contains the compound represented by the general formula (D1-0), an acid diffuses in the exposed portion, Since the acid diffusion is controlled in the unexposed area, the contrast between the exposed area and the unexposed area is excellent (that is, the boundary between the exposed area and the unexposed area becomes clear). Therefore, such a composition is effective in improving LWR and MEEF (Mask Error Enhancement Factor (amplification factor of line width deviation due to mask width deviation)).
上記一般式(D1-0)において、X+は、上述したように、上記一般式(D1-1)又は(D1-2)で表されるカチオンである。そして、一般式(D1-1)中のRD2、RD3及びRD4は、相互に独立に、水素原子、アルキル基、アルコキシル基、ヒドロキシル基又はハロゲン原子である。これらのうち、上記式(D1-0)で表される化合物において、現像液に対する溶解性を低下させる効果を有することから、水素原子、アルキル基、アルコキシ基及びハロゲン原子が好ましい。また、一般式(D1-2)におけるRD5及びRD6は、相互に独立に、水素原子、アルキル基、アルコキシル基、ヒドロキシル基又はハロゲン原子である。これらのうち、水素原子、アルキル基及びハロゲン原子が好ましい。
In the general formula (D1-0), X + is a cation represented by the general formula (D1-1) or (D1-2) as described above. In the general formula (D1-1), R D2 , R D3 and R D4 are each independently a hydrogen atom, an alkyl group, an alkoxyl group, a hydroxyl group or a halogen atom. Among these, a hydrogen atom, an alkyl group, an alkoxy group, and a halogen atom are preferable because the compound represented by the above formula (D1-0) has an effect of reducing solubility in a developer. In the general formula (D1-2), R D5 and R D6 are each independently a hydrogen atom, an alkyl group, an alkoxyl group, a hydroxyl group, or a halogen atom. Of these, a hydrogen atom, an alkyl group, and a halogen atom are preferable.
上記一般式(D1-0)において、Z-は、上述したように、OH-、一般式(D1-3):RD1-COO-で表されるアニオン、一般式(D1-4):RD1-SO3
-で表されるアニオン、又は、一般式(D1-5):RD1-N--SO2-RD11で表されるアニオンである(但し、一般式(D1-3)~(D1-5)において、RD1は、置換されていてもよいアルキル基、脂環式炭化水素基又はアリール基である。RD11は、置換されていてもよいフッ素化アルキル基、脂環式フッ素化炭化水素基、又は、フッ素化アリール基である。)。
In the general formula (D1-0), Z − represents, as described above, OH − , an anion represented by the general formula (D1-3): R D1 —COO — , and the general formula (D1-4): R An anion represented by D1— SO 3 — , or an anion represented by formula (D1-5): R D1 —N —— SO 2 —R D11 (provided that the formula (D1-3) to In (D1-5), R D1 represents an optionally substituted alkyl group, an alicyclic hydrocarbon group, or an aryl group, and R D11 represents an optionally substituted fluorinated alkyl group, an alicyclic group. A fluorinated hydrocarbon group or a fluorinated aryl group).
上記一般式(D1-0)におけるZ-は、好ましくは、下記式(1a)で表されるアニオン(即ち、一般式(D1-3)で表されるアニオンにおいて、RD1がフェニル基であるアニオン)、下記式(1b)で表されるアニオン(即ち、一般式(D1-4)で表されるアニオンにおいて、RD1が1,7,7-トリメチルビシクロ[2.2.1]ヘプタン-2-オン由来の基であるアニオン)、及び、下記式(1c)で表されるアニオン(即ち、一般式(D1-5)で表されるアニオンにおいて、RD1がブチル基であり、RD11がトリフルオロメチル基であるアニオン)である。
Z − in the general formula (D1-0) is preferably an anion represented by the following formula (1a) (that is, in the anion represented by the general formula (D1-3), R D1 is a phenyl group. Anion), an anion represented by the following formula (1b) (ie, an anion represented by the general formula (D1-4), wherein R D1 is 1,7,7-trimethylbicyclo [2.2.1] heptane- An anion which is a group derived from 2-one) and an anion represented by the following formula (1c) (that is, an anion represented by the general formula (D1-5)), wherein R D1 is a butyl group, and R D11 Is an anion) which is a trifluoromethyl group.
上記光分解性酸拡散制御剤は、上記一般式(D1-0)で表されるものであり、具体的には、上記条件を満たすスルホニウム塩化合物又はヨードニウム塩化合物である。
The photodegradable acid diffusion controller is represented by the above general formula (D1-0), and specifically, is a sulfonium salt compound or an iodonium salt compound that satisfies the above conditions.
上記スルホニウム塩化合物としては、トリフェニルスルホニウムハイドロオキサイド、トリフェニルスルホニウムアセテート、トリフェニルスルホニウムサリチレート、ジフェニル-4-ヒドロキシフェニルスルホニウムハイドロオキサイド、ジフェニル-4-ヒドロキシフェニルスルホニウムアセテート、ジフェニル-4-ヒドロキシフェニルスルホニウムサリチレート、トリフェニルスルホニウム10-カンファースルホネート、4-tert-ブトキシフェニル・ジフェニルスルホニウム10-カンファースルホネート等が挙げられる。尚、これらの化合物は、単独で用いてよいし、2つ以上を組み合わせて用いてもよい。
Examples of the sulfonium salt compounds include triphenylsulfonium hydroxide, triphenylsulfonium acetate, triphenylsulfonium salicylate, diphenyl-4-hydroxyphenylsulfonium hydroxide, diphenyl-4-hydroxyphenylsulfonium acetate, diphenyl-4-hydroxyphenyl. Examples thereof include sulfonium salicylate, triphenylsulfonium 10-camphor sulfonate, 4-tert-butoxyphenyl diphenylsulfonium 10-camphor sulfonate, and the like. In addition, these compounds may be used independently and may be used in combination of 2 or more.
また、上記ヨードニウム塩化合物としては、ビス(4-tert-ブチルフェニル)ヨードニウムハイドロオキサイド、ビス(4-tert-ブチルフェニル)ヨードニウムアセテート、ビス(4-tert-ブチルフェニル)ヨードニウムハイドロオキサイド、ビス(4-tert-ブチルフェニル)ヨードニウムアセテート、ビス(4-tert-ブチルフェニル)ヨードニウムサリチレート、4-tert-ブチルフェニル-4-ヒドロキシフェニルヨードニウムハイドロオキサイド、4-tert-ブチルフェニル-4-ヒドロキシフェニルヨードニウムアセテート、4-tert-ブチルフェニル-4-ヒドロキシフェニルヨードニウムサリチレート、ビス(4-tert-ブチルフェニル)ヨードニウム10-カンファースルホネート、ジフェニルヨードニウム10-カンファースルホネート等が挙げられる。尚、これらの化合物は、単独で用いてよいし、2つ以上を組み合わせて用いてもよい。
上記光分解性酸拡散制御剤は、1種単独でまたは2種以上を組み合わせて使用することができる。 Examples of the iodonium salt compound include bis (4-tert-butylphenyl) iodonium hydroxide, bis (4-tert-butylphenyl) iodonium acetate, bis (4-tert-butylphenyl) iodonium hydroxide, bis (4 -Tert-butylphenyl) iodonium acetate, bis (4-tert-butylphenyl) iodonium salicylate, 4-tert-butylphenyl-4-hydroxyphenyliodonium hydroxide, 4-tert-butylphenyl-4-hydroxyphenyliodonium Acetate, 4-tert-butylphenyl-4-hydroxyphenyliodonium salicylate, bis (4-tert-butylphenyl) iodonium 10-camphorsulfo Over DOO, such as diphenyl iodonium 10-camphorsulfonate, and the like. In addition, these compounds may be used independently and may be used in combination of 2 or more.
The said photodegradable acid diffusion control agent can be used individually by 1 type or in combination of 2 or more types.
上記光分解性酸拡散制御剤は、1種単独でまたは2種以上を組み合わせて使用することができる。 Examples of the iodonium salt compound include bis (4-tert-butylphenyl) iodonium hydroxide, bis (4-tert-butylphenyl) iodonium acetate, bis (4-tert-butylphenyl) iodonium hydroxide, bis (4 -Tert-butylphenyl) iodonium acetate, bis (4-tert-butylphenyl) iodonium salicylate, 4-tert-butylphenyl-4-hydroxyphenyliodonium hydroxide, 4-tert-butylphenyl-4-hydroxyphenyliodonium Acetate, 4-tert-butylphenyl-4-hydroxyphenyliodonium salicylate, bis (4-tert-butylphenyl) iodonium 10-camphorsulfo Over DOO, such as diphenyl iodonium 10-camphorsulfonate, and the like. In addition, these compounds may be used independently and may be used in combination of 2 or more.
The said photodegradable acid diffusion control agent can be used individually by 1 type or in combination of 2 or more types.
上記酸拡散抑制剤としては、含窒素化合物(I)、含窒素化合物(II)、含窒素複素環化合物及び光分解性酸拡散制御剤が好ましい。
As the acid diffusion inhibitor, a nitrogen-containing compound (I), a nitrogen-containing compound (II), a nitrogen-containing heterocyclic compound, and a photodegradable acid diffusion controller are preferable.
本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物が、酸拡散抑制剤を含有する場合、その含有量は、上記重合体[A]100質量部に対して、好ましくは10質量部以下、より好ましくは5質量部以下である。但し、含有量の下限値は、通常、0.01質量部である。上記酸拡散抑制剤の含有量が多すぎると、フォトレジスト膜の感度が低下する場合がある。
When the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure contains an acid diffusion inhibitor, the content thereof is preferably based on 100 parts by mass of the polymer [A]. Is 10 parts by mass or less, more preferably 5 parts by mass or less. However, the lower limit of the content is usually 0.01 parts by mass. When there is too much content of the said acid diffusion inhibitor, the sensitivity of a photoresist film may fall.
1-4-2.溶剤
この溶剤は、少なくとも、重合体[A]及び[C]並びに酸発生剤[B]を溶解して、本発明の組成物を溶液状態にすることができるものが好ましい。
上記溶剤としては、直鎖状又は分岐状のケトン類、環状のケトン類、アルキレングリコールモノアルキルエーテル類、アルキレングリコールジアルキルエーテル類、アルキレングリコールモノアルキルエーテルアセテート類、カルボン酸エステル類、ヒドロキシル基を有するカルボン酸エステル類、アルコキシ基を有するカルボン酸エステル類、アルコール類、芳香族炭化水素類等が挙げられる。尚、これらの溶剤は、単独で用いてよいし、2種以上を組み合わせて用いてもよい。 1-4-2. Solvent This solvent is preferably a solvent that can dissolve at least the polymers [A] and [C] and the acid generator [B] to bring the composition of the present invention into a solution state.
Examples of the solvent include linear or branched ketones, cyclic ketones, alkylene glycol monoalkyl ethers, alkylene glycol dialkyl ethers, alkylene glycol monoalkyl ether acetates, carboxylic acid esters, and hydroxyl groups. Examples thereof include carboxylic acid esters, carboxylic acid esters having an alkoxy group, alcohols, and aromatic hydrocarbons. In addition, these solvents may be used independently and may be used in combination of 2 or more type.
この溶剤は、少なくとも、重合体[A]及び[C]並びに酸発生剤[B]を溶解して、本発明の組成物を溶液状態にすることができるものが好ましい。
上記溶剤としては、直鎖状又は分岐状のケトン類、環状のケトン類、アルキレングリコールモノアルキルエーテル類、アルキレングリコールジアルキルエーテル類、アルキレングリコールモノアルキルエーテルアセテート類、カルボン酸エステル類、ヒドロキシル基を有するカルボン酸エステル類、アルコキシ基を有するカルボン酸エステル類、アルコール類、芳香族炭化水素類等が挙げられる。尚、これらの溶剤は、単独で用いてよいし、2種以上を組み合わせて用いてもよい。 1-4-2. Solvent This solvent is preferably a solvent that can dissolve at least the polymers [A] and [C] and the acid generator [B] to bring the composition of the present invention into a solution state.
Examples of the solvent include linear or branched ketones, cyclic ketones, alkylene glycol monoalkyl ethers, alkylene glycol dialkyl ethers, alkylene glycol monoalkyl ether acetates, carboxylic acid esters, and hydroxyl groups. Examples thereof include carboxylic acid esters, carboxylic acid esters having an alkoxy group, alcohols, and aromatic hydrocarbons. In addition, these solvents may be used independently and may be used in combination of 2 or more type.
上記溶剤の使用量は、本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物の全固形分濃度が、好ましくは1~50質量%、より好ましくは1~25質量%となるような量である。
本発明の組成物は、例えば、孔径0.2μm程度のフィルターを用いたろ過によって、組成物溶液として用いることができる。 The amount of the solvent used is such that the total solid concentration of the radiation-sensitive resin composition or the radiation-sensitive resin composition for immersion exposure of the present invention is preferably 1 to 50% by mass, more preferably 1 to 25% by mass. The amount is such that
The composition of the present invention can be used as a composition solution by, for example, filtration using a filter having a pore size of about 0.2 μm.
本発明の組成物は、例えば、孔径0.2μm程度のフィルターを用いたろ過によって、組成物溶液として用いることができる。 The amount of the solvent used is such that the total solid concentration of the radiation-sensitive resin composition or the radiation-sensitive resin composition for immersion exposure of the present invention is preferably 1 to 50% by mass, more preferably 1 to 25% by mass. The amount is such that
The composition of the present invention can be used as a composition solution by, for example, filtration using a filter having a pore size of about 0.2 μm.
1-4-3.ラクトン化合物
このラクトン化合物は、液浸露光において、フォトレジスト膜の表面における撥水性を効率よく発現させるために、含まれる重合体[C]を、フォトレジスト膜の表層に偏析させる効果を付与する成分である。そして、このラクトン化合物を含有する組成物を用いることにより、LWR、現像欠陥、パターン倒れ耐性等のレジスト基本特性を損なうことなく、フォトレジスト膜から液浸露光用液体への成分の溶出を抑制することができる。また、高速スキャンにより液浸露光を行った場合にも、液滴を残すことなく、フォトレジスト膜の表面における撥水性を維持することができ、ウォーターマーク欠陥等の不良現象を抑制することができる。 1-4-3. Lactone compound This lactone compound is a component that imparts the effect of segregating the contained polymer [C] to the surface layer of the photoresist film in order to efficiently develop water repellency on the surface of the photoresist film in immersion exposure. It is. By using the composition containing the lactone compound, the elution of components from the photoresist film to the immersion exposure liquid is suppressed without impairing basic resist characteristics such as LWR, development defects, and pattern collapse resistance. be able to. In addition, even when immersion exposure is performed by high-speed scanning, water repellency on the surface of the photoresist film can be maintained without leaving droplets, and defective phenomena such as watermark defects can be suppressed. .
このラクトン化合物は、液浸露光において、フォトレジスト膜の表面における撥水性を効率よく発現させるために、含まれる重合体[C]を、フォトレジスト膜の表層に偏析させる効果を付与する成分である。そして、このラクトン化合物を含有する組成物を用いることにより、LWR、現像欠陥、パターン倒れ耐性等のレジスト基本特性を損なうことなく、フォトレジスト膜から液浸露光用液体への成分の溶出を抑制することができる。また、高速スキャンにより液浸露光を行った場合にも、液滴を残すことなく、フォトレジスト膜の表面における撥水性を維持することができ、ウォーターマーク欠陥等の不良現象を抑制することができる。 1-4-3. Lactone compound This lactone compound is a component that imparts the effect of segregating the contained polymer [C] to the surface layer of the photoresist film in order to efficiently develop water repellency on the surface of the photoresist film in immersion exposure. It is. By using the composition containing the lactone compound, the elution of components from the photoresist film to the immersion exposure liquid is suppressed without impairing basic resist characteristics such as LWR, development defects, and pattern collapse resistance. be able to. In addition, even when immersion exposure is performed by high-speed scanning, water repellency on the surface of the photoresist film can be maintained without leaving droplets, and defective phenomena such as watermark defects can be suppressed. .
上記作用を付与するラクトン化合物としては、γ-ブチロラクトン、バレロラクトン、メバロニックラクトン、ノルボルナンラクトン等が挙げられる。これらの化合物は、単独で用いてよいし、2つ以上を組み合わせて用いてもよい。
Examples of the lactone compound imparting the above action include γ-butyrolactone, valerolactone, mevalonic lactone, norbornane lactone and the like. These compounds may be used alone or in combination of two or more.
本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物が、上記ラクトン化合物を含有する場合、その含有量は、上記重合体[A]100質量部に対して、通常、30~200質量部、好ましくは50~150質量部である。このラクトン化合物の含有量が多すぎると、レジストの基本性能が低下し、良好な形状を有するパターンが得られない場合がある。
When the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure contains the lactone compound, the content thereof is usually 100 parts by mass of the polymer [A]. 30 to 200 parts by mass, preferably 50 to 150 parts by mass. If the content of the lactone compound is too large, the basic performance of the resist is lowered, and a pattern having a good shape may not be obtained.
本発明の液浸露光用感放射線性樹脂組成物は、上記重合体[A]以外に、上記重合体[C]等の他の重合体を含有してもよい。
他の重合体の含有量は、上記重合体[A]100質量部に対して、通常、0.1~20質量部、好ましくは1~10質量部、更に好ましくは1~7.5質量部である。他の重合体が上記重合体[C]である場合、その含有量が少なすぎると、重合体[C]を含有させる効果が十分ではない場合がある。一方、上記重合体[C]の含有量が多すぎると、フォトレジスト膜の表面における撥水性が高くなりすぎて、現像不良が起こる場合がある。 The radiation-sensitive resin composition for immersion exposure according to the present invention may contain another polymer such as the polymer [C] in addition to the polymer [A].
The content of the other polymer is usually 0.1 to 20 parts by mass, preferably 1 to 10 parts by mass, more preferably 1 to 7.5 parts by mass with respect to 100 parts by mass of the polymer [A]. It is. When the other polymer is the polymer [C], if the content is too small, the effect of containing the polymer [C] may not be sufficient. On the other hand, if the content of the polymer [C] is too large, the water repellency on the surface of the photoresist film becomes too high, and development failure may occur.
他の重合体の含有量は、上記重合体[A]100質量部に対して、通常、0.1~20質量部、好ましくは1~10質量部、更に好ましくは1~7.5質量部である。他の重合体が上記重合体[C]である場合、その含有量が少なすぎると、重合体[C]を含有させる効果が十分ではない場合がある。一方、上記重合体[C]の含有量が多すぎると、フォトレジスト膜の表面における撥水性が高くなりすぎて、現像不良が起こる場合がある。 The radiation-sensitive resin composition for immersion exposure according to the present invention may contain another polymer such as the polymer [C] in addition to the polymer [A].
The content of the other polymer is usually 0.1 to 20 parts by mass, preferably 1 to 10 parts by mass, more preferably 1 to 7.5 parts by mass with respect to 100 parts by mass of the polymer [A]. It is. When the other polymer is the polymer [C], if the content is too small, the effect of containing the polymer [C] may not be sufficient. On the other hand, if the content of the polymer [C] is too large, the water repellency on the surface of the photoresist film becomes too high, and development failure may occur.
本発明の感放射線性樹脂組成物及び液浸露光用感放射線性樹脂組成物は、特に、化学増幅型レジストとして有用である。上記化学増幅型レジストにおいては、露光により酸発生剤[B]から発生した酸の作用によって、重合体成分中の酸解離性基が解離して、カルボキシル基を生じ、その結果、レジストの露光部のアルカリ現像液に対する溶解性が高くなり、該露光部がアルカリ現像液によって溶解、除去され、ポジ型のレジストパターンが得られる。
The radiation-sensitive resin composition and the radiation-sensitive resin composition for immersion exposure of the present invention are particularly useful as a chemically amplified resist. In the chemically amplified resist, the acid-dissociable group in the polymer component is dissociated by the action of the acid generated from the acid generator [B] by exposure to generate a carboxyl group. As a result, the exposed portion of the resist The solubility in the alkaline developer becomes higher, and the exposed portion is dissolved and removed by the alkaline developer to obtain a positive resist pattern.
2.レジストパターンの形成方法
本発明のレジストパターン形成方法は、上記本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物を用いて基板上にフォトレジスト膜を形成する工程(以下、「第1工程」という。)と、このフォトレジスト膜上に液浸露光用液体を配置し、液浸露光用液体を介してフォトレジスト膜を液浸露光する工程(以下、「第2工程」という。)と、液浸露光されたフォトレジスト膜を現像してレジストパターンを形成する工程(以下、「第3工程」という。)と、を備えることを特徴とする。 2. Method for Forming Resist Pattern The resist pattern forming method of the present invention is a step of forming a photoresist film on a substrate using the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure (hereinafter referred to as “resist pattern forming method”). , “First step”), and a step of placing the immersion exposure liquid on the photoresist film and subjecting the photoresist film to immersion exposure via the immersion exposure liquid (hereinafter referred to as “second step”). And a step of developing a photoresist film subjected to immersion exposure to form a resist pattern (hereinafter referred to as “third step”).
本発明のレジストパターン形成方法は、上記本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物を用いて基板上にフォトレジスト膜を形成する工程(以下、「第1工程」という。)と、このフォトレジスト膜上に液浸露光用液体を配置し、液浸露光用液体を介してフォトレジスト膜を液浸露光する工程(以下、「第2工程」という。)と、液浸露光されたフォトレジスト膜を現像してレジストパターンを形成する工程(以下、「第3工程」という。)と、を備えることを特徴とする。 2. Method for Forming Resist Pattern The resist pattern forming method of the present invention is a step of forming a photoresist film on a substrate using the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure (hereinafter referred to as “resist pattern forming method”). , “First step”), and a step of placing the immersion exposure liquid on the photoresist film and subjecting the photoresist film to immersion exposure via the immersion exposure liquid (hereinafter referred to as “second step”). And a step of developing a photoresist film subjected to immersion exposure to form a resist pattern (hereinafter referred to as “third step”).
第1工程は、上記本発明の感放射線性樹脂組成物又は液浸露光用感放射線性樹脂組成物を用いて基板上にフォトレジスト膜を形成する工程である。組成物の塗布方法としては、回転塗布、流延塗布、ロール塗布等の方法が挙げられる。基板としては、シリコンウエハ、アルミニウムで被覆されたウエハ等が挙げられる。
上記第1工程の具体例としては、得られるフォトレジスト膜が所定の膜厚となるように組成物を塗布し、その後、プレベーク(PB)することによって塗膜中の溶剤を揮発させる方法である。これにより、均一なフォトレジスト膜が形成される。
フォトレジスト膜の厚さは、特に限定されないが、通常、0.05~0.40μmである。
また、プレベークの条件は、組成物の構成によって、適宜、選択されるが、好ましくは30℃~200℃であり、より好ましくは50℃~170℃である。である。 The first step is a step of forming a photoresist film on the substrate using the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure. Examples of the method for applying the composition include spin coating, cast coating, roll coating, and the like. Examples of the substrate include a silicon wafer and a wafer coated with aluminum.
A specific example of the first step is a method of volatilizing the solvent in the coating film by applying the composition so that the obtained photoresist film has a predetermined thickness and then pre-baking (PB). . Thereby, a uniform photoresist film is formed.
The thickness of the photoresist film is not particularly limited, but is usually 0.05 to 0.40 μm.
The prebaking conditions are appropriately selected depending on the composition of the composition, but are preferably 30 ° C to 200 ° C, more preferably 50 ° C to 170 ° C. It is.
上記第1工程の具体例としては、得られるフォトレジスト膜が所定の膜厚となるように組成物を塗布し、その後、プレベーク(PB)することによって塗膜中の溶剤を揮発させる方法である。これにより、均一なフォトレジスト膜が形成される。
フォトレジスト膜の厚さは、特に限定されないが、通常、0.05~0.40μmである。
また、プレベークの条件は、組成物の構成によって、適宜、選択されるが、好ましくは30℃~200℃であり、より好ましくは50℃~170℃である。である。 The first step is a step of forming a photoresist film on the substrate using the radiation sensitive resin composition of the present invention or the radiation sensitive resin composition for immersion exposure. Examples of the method for applying the composition include spin coating, cast coating, roll coating, and the like. Examples of the substrate include a silicon wafer and a wafer coated with aluminum.
A specific example of the first step is a method of volatilizing the solvent in the coating film by applying the composition so that the obtained photoresist film has a predetermined thickness and then pre-baking (PB). . Thereby, a uniform photoresist film is formed.
The thickness of the photoresist film is not particularly limited, but is usually 0.05 to 0.40 μm.
The prebaking conditions are appropriately selected depending on the composition of the composition, but are preferably 30 ° C to 200 ° C, more preferably 50 ° C to 170 ° C. It is.
尚、本発明においては、組成物の潜在能力を最大限に引き出すため、例えば、特公平6-12452号公報(特開昭59-93448号公報)等に開示されているように、使用される基板上に有機系あるいは無機系の反射防止膜を形成しておくこともできる。また、環境雰囲気中に含まれる塩基性不純物等の影響を防止するため、例えば、特開平5-188598号公報等に開示されているように、フォトレジスト膜上に保護膜を設けることもできる。更に、フォトレジスト膜からの酸発生剤等の流出を防止するため、例えば、特開2005-352384号公報等に開示されているように、フォトレジスト膜上に液浸用保護膜を設けることもできる。尚、これらの技術は併用することができる。
In the present invention, in order to maximize the potential of the composition, it is used, for example, as disclosed in Japanese Patent Publication No. 6-12452 (Japanese Patent Laid-Open No. 59-93448). An organic or inorganic antireflection film can also be formed on the substrate. In order to prevent the influence of basic impurities contained in the environmental atmosphere, a protective film can be provided on the photoresist film as disclosed in, for example, Japanese Patent Laid-Open No. 5-188598. Further, in order to prevent the acid generator and the like from flowing out of the photoresist film, an immersion protective film may be provided on the photoresist film as disclosed in, for example, JP-A-2005-352384. it can. These techniques can be used in combination.
第2工程は、フォトレジスト膜上に液浸露光用液体を配置し、液浸露光用液体を介してフォトレジスト膜を液浸露光する工程である。この工程においては、通常、所望のパターンを形成するためのマスクパターンを有するフォトマスクを介して、下記に例示する放射線を、露光光として用い、この放射線を、上記フォトレジスト膜の表面に照射する。これにより、放射線は、フォトマスクの開口部を通過し、更に露光用のレンズを通過して、フォトレジスト膜に達する。フォトレジスト膜における露光部は、第3工程により除去される。
The second step is a step in which an immersion exposure liquid is disposed on the photoresist film, and the photoresist film is subjected to immersion exposure via the immersion exposure liquid. In this step, the radiation illustrated below is usually used as exposure light through a photomask having a mask pattern for forming a desired pattern, and the surface of the photoresist film is irradiated with this radiation. . Thereby, the radiation passes through the opening of the photomask, further passes through the exposure lens, and reaches the photoresist film. The exposed portion in the photoresist film is removed by the third step.
上記液浸露光用液体としては、純水、長鎖又は環状の脂肪族化合物等を用いることができる。また、液浸露光用の露光光は、放射線が使用される。この放射線は、組成物中の酸発生剤の種類に応じて、適宜、選択することができるが、例えば、可視光線、紫外線、遠紫外線、X線、荷電粒子線等を用いることができる。これらのうち、ArFエキシマレーザー(波長193nm)あるいはKrFエキシマレーザー(波長248nm)で代表される遠紫外線が好ましく、特にArFエキシマレーザー(波長193nm)が好ましい。なお、露光量等の露光条件は、組成物の構成、添加剤が含有された場合のその種類等に応じて、適宜、選択することができる。
As the immersion exposure liquid, pure water, a long chain or cyclic aliphatic compound, or the like can be used. Further, radiation is used as the exposure light for immersion exposure. The radiation can be appropriately selected according to the type of the acid generator in the composition. For example, visible light, ultraviolet light, far ultraviolet light, X-rays, charged particle beams, and the like can be used. Among these, far ultraviolet rays represented by ArF excimer laser (wavelength 193 nm) or KrF excimer laser (wavelength 248 nm) are preferable, and ArF excimer laser (wavelength 193 nm) is particularly preferable. In addition, exposure conditions, such as an exposure amount, can be suitably selected according to the composition of the composition, the type when an additive is contained, and the like.
上記第2工程の後、必要に応じて、露光された膜をベーク(以下、「PEB」という。)する工程を備えてもよい。この工程により、露光部に含まれる重合体の架橋反応が円滑に進めることができる。
このPEBの加熱条件は、組成物の組成によって、適宜、選択されるが、架橋反応の円滑化の観点から、好ましくは30℃~200℃であり、より好ましくは50℃~170℃である。 After the second step, a step of baking the exposed film (hereinafter referred to as “PEB”) may be provided as necessary. By this step, the cross-linking reaction of the polymer contained in the exposed part can proceed smoothly.
The PEB heating conditions are appropriately selected depending on the composition of the composition, but are preferably 30 ° C. to 200 ° C., more preferably 50 ° C. to 170 ° C., from the viewpoint of facilitating the crosslinking reaction.
このPEBの加熱条件は、組成物の組成によって、適宜、選択されるが、架橋反応の円滑化の観点から、好ましくは30℃~200℃であり、より好ましくは50℃~170℃である。 After the second step, a step of baking the exposed film (hereinafter referred to as “PEB”) may be provided as necessary. By this step, the cross-linking reaction of the polymer contained in the exposed part can proceed smoothly.
The PEB heating conditions are appropriately selected depending on the composition of the composition, but are preferably 30 ° C. to 200 ° C., more preferably 50 ° C. to 170 ° C., from the viewpoint of facilitating the crosslinking reaction.
第3工程は、液浸露光されたフォトレジスト膜を現像してレジストパターンを形成する工程である。この工程において、現像液を用いることにより、上記第2工程における未露光部が除去され、露光部、即ち、上記フォトマスクの開口部のパターンを反映したパターンが、残存、形成される。
The third step is a step of developing a photoresist film subjected to immersion exposure to form a resist pattern. In this step, by using the developer, the unexposed portion in the second step is removed, and a pattern reflecting the pattern of the exposed portion, that is, the opening of the photomask, is left and formed.
上記現像液としては、通常、アルカリ性化合物を水に溶解させてなるアルカリ性水溶液が用いられる。このアルカリ性化合物としては、水酸化ナトリウム、水酸化カリウム、炭酸ナトリウム、けい酸ナトリウム、メタけい酸ナトリウム、アンモニア、エチルアミン、n-プロピルアミン、ジエチルアミン、ジ-n-プロピルアミン、トリエチルアミン、メチルジエチルアミン、エチルジメチルアミン、トリエタノールアミン、テトラメチルアンモニウムヒドロキシド、ピロール、ピペリジン、コリン、1,8-ジアザビシクロ-[5.4.0]-7-ウンデセン、1,5-ジアザビシクロ-[4.3.0]-5-ノネン等が挙げられる。これらは、単独で用いてよいし、2つ以上を組み合わせて用いてもよい。また、上記化合物のうち、テトラメチルアンモニウムヒドロキシドが好ましい。上記アルカリ性化合物の濃度は、通常、10質量%以下である。この濃度が高すぎると、露光部も現像液に溶解する場合がある。
As the developer, an alkaline aqueous solution obtained by dissolving an alkaline compound in water is usually used. Examples of the alkaline compound include sodium hydroxide, potassium hydroxide, sodium carbonate, sodium silicate, sodium metasilicate, ammonia, ethylamine, n-propylamine, diethylamine, di-n-propylamine, triethylamine, methyldiethylamine, ethyl Dimethylamine, triethanolamine, tetramethylammonium hydroxide, pyrrole, piperidine, choline, 1,8-diazabicyclo- [5.4.0] -7-undecene, 1,5-diazabicyclo- [4.3.0] -5-nonene and the like. These may be used alone or in combination of two or more. Of the above compounds, tetramethylammonium hydroxide is preferred. The concentration of the alkaline compound is usually 10% by mass or less. If this concentration is too high, the exposed area may also be dissolved in the developer.
上記現像液は、上記アルカリ性化合物のみを含む溶液であってよいし、有機溶剤、界面活性剤等を含む組成物であってもよい。
上記有機溶剤としては、アセトン、メチルエチルケトン、メチルイソブチルケトン、シクロペンタノン、シクロヘキサノン、3-メチルシクロペンタノン、2,6-ジメチルシクロヘキサノン等のケトン類;メタノール、エタノール、n-プロピルアルコール、イソプロピルアルコール、n-ブチルアルコール、tert-ブチルアルコール、シクロペンタノール、シクロヘキサノール、1,4-ヘキサンジオール、1,4-ヘキサンジメチロール等のアルコール類;テトラヒドロフラン、ジオキサン等のエーテル類;酢酸エチル、酢酸n-ブチル、酢酸イソアミル等のエステル類;トルエン、キシレン等の芳香族炭化水素類や、フェノール、アセトニルアセトン、ジメチルホルムアミド等が挙げられる。これらの有機溶媒は、単独で用いてよいし、2種以上を組み合わせて用いてもよい。 The developer may be a solution containing only the alkaline compound or a composition containing an organic solvent, a surfactant, and the like.
Examples of the organic solvent include ketones such as acetone, methyl ethyl ketone, methyl isobutyl ketone, cyclopentanone, cyclohexanone, 3-methylcyclopentanone, and 2,6-dimethylcyclohexanone; methanol, ethanol, n-propyl alcohol, isopropyl alcohol, alcohols such as n-butyl alcohol, tert-butyl alcohol, cyclopentanol, cyclohexanol, 1,4-hexanediol, 1,4-hexanedimethylol; ethers such as tetrahydrofuran and dioxane; ethyl acetate, n-acetate Examples include esters such as butyl and isoamyl acetate; aromatic hydrocarbons such as toluene and xylene; phenol, acetonylacetone, and dimethylformamide. These organic solvents may be used alone or in combination of two or more.
上記有機溶剤としては、アセトン、メチルエチルケトン、メチルイソブチルケトン、シクロペンタノン、シクロヘキサノン、3-メチルシクロペンタノン、2,6-ジメチルシクロヘキサノン等のケトン類;メタノール、エタノール、n-プロピルアルコール、イソプロピルアルコール、n-ブチルアルコール、tert-ブチルアルコール、シクロペンタノール、シクロヘキサノール、1,4-ヘキサンジオール、1,4-ヘキサンジメチロール等のアルコール類;テトラヒドロフラン、ジオキサン等のエーテル類;酢酸エチル、酢酸n-ブチル、酢酸イソアミル等のエステル類;トルエン、キシレン等の芳香族炭化水素類や、フェノール、アセトニルアセトン、ジメチルホルムアミド等が挙げられる。これらの有機溶媒は、単独で用いてよいし、2種以上を組み合わせて用いてもよい。 The developer may be a solution containing only the alkaline compound or a composition containing an organic solvent, a surfactant, and the like.
Examples of the organic solvent include ketones such as acetone, methyl ethyl ketone, methyl isobutyl ketone, cyclopentanone, cyclohexanone, 3-methylcyclopentanone, and 2,6-dimethylcyclohexanone; methanol, ethanol, n-propyl alcohol, isopropyl alcohol, alcohols such as n-butyl alcohol, tert-butyl alcohol, cyclopentanol, cyclohexanol, 1,4-hexanediol, 1,4-hexanedimethylol; ethers such as tetrahydrofuran and dioxane; ethyl acetate, n-acetate Examples include esters such as butyl and isoamyl acetate; aromatic hydrocarbons such as toluene and xylene; phenol, acetonylacetone, and dimethylformamide. These organic solvents may be used alone or in combination of two or more.
上記現像液が有機溶剤を含む場合、その含有量は、アルカリ性水溶液100体積部に対して、好ましくは100体積部以下である。この有機溶剤の含有量が多すぎる場合、現像性が低下して、未露光部の現像残りが多くなる場合がある。
上記第3工程の後、基板上に残存したパターンは、通常、水で洗浄して乾燥される。 When the developer contains an organic solvent, the content thereof is preferably 100 parts by volume or less with respect to 100 parts by volume of the alkaline aqueous solution. When there is too much content of this organic solvent, developability may fall and the image development residue of an unexposed part may increase.
After the third step, the pattern remaining on the substrate is usually washed with water and dried.
上記第3工程の後、基板上に残存したパターンは、通常、水で洗浄して乾燥される。 When the developer contains an organic solvent, the content thereof is preferably 100 parts by volume or less with respect to 100 parts by volume of the alkaline aqueous solution. When there is too much content of this organic solvent, developability may fall and the image development residue of an unexposed part may increase.
After the third step, the pattern remaining on the substrate is usually washed with water and dried.
以上の工程を備えることにより、広い露光マージンを維持しつつ、LWRの小さいライン・アンド・スペースパターンを形成することができる。
By providing the above steps, a line and space pattern with a small LWR can be formed while maintaining a wide exposure margin.
以下、本発明を実施例に基づいて具体的に説明するが、本発明はこれらの実施例に限定されるものではない。尚、合成例、実施例及び比較例における、「部」及び「%」は、特に断らない限り、質量基準である。
Hereinafter, the present invention will be specifically described based on examples, but the present invention is not limited to these examples. In the synthesis examples, examples and comparative examples, “parts” and “%” are based on mass unless otherwise specified.
下記の合成例により得られた重合体のGPC測定方法は、以下の通りである。
重合体の重量平均分子量(Mw)は、東ソー社製GPCカラム(以下、型式名)である、「G2000HXL」2本、「G3000HXL」1本及び「G4000HXL」1本を連結して、テトラヒドロフランを溶出溶媒とし、流量1.0mL/分及びカラム温度40℃の条件で、単分散ポリスチレンを標準とするゲルパーミエーションクロマトグラフィ(GPC)により測定した。 The GPC measurement method of the polymer obtained by the following synthesis example is as follows.
The weight average molecular weight (Mw) of the polymer is a GPC column (hereinafter referred to as model name) manufactured by Tosoh Corporation. Two "G2000HXL", one "G3000HXL" and one "G4000HXL" are connected to elute tetrahydrofuran. The measurement was performed by gel permeation chromatography (GPC) using monodisperse polystyrene as a standard under conditions of a flow rate of 1.0 mL / min and a column temperature of 40 ° C. as a solvent.
重合体の重量平均分子量(Mw)は、東ソー社製GPCカラム(以下、型式名)である、「G2000HXL」2本、「G3000HXL」1本及び「G4000HXL」1本を連結して、テトラヒドロフランを溶出溶媒とし、流量1.0mL/分及びカラム温度40℃の条件で、単分散ポリスチレンを標準とするゲルパーミエーションクロマトグラフィ(GPC)により測定した。 The GPC measurement method of the polymer obtained by the following synthesis example is as follows.
The weight average molecular weight (Mw) of the polymer is a GPC column (hereinafter referred to as model name) manufactured by Tosoh Corporation. Two "G2000HXL", one "G3000HXL" and one "G4000HXL" are connected to elute tetrahydrofuran. The measurement was performed by gel permeation chromatography (GPC) using monodisperse polystyrene as a standard under conditions of a flow rate of 1.0 mL / min and a column temperature of 40 ° C. as a solvent.
1.重合体[A]及び[C]
合成例1(重合体(A-1)の合成)
下記に示す、化合物(S1-1)33.11g(40mol%)、化合物(S1-2)12.22g(10mol%)及び化合物(S1-3)54.67g(50mol%)を、2-ブタノン200gに溶解し、更に、2,2'-アゾビスイソブチロニトリル8.08gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量80.1g、収率80%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、41:10:49であった。これを、重合体(A-1)とする。この重合体(A-1)のMwは7,300であり、フッ素原子の含有率は0%であった。
1. Polymers [A] and [C]
Synthesis Example 1 (Synthesis of polymer (A-1))
Compound (S1-1) 33.11 g (40 mol%), compound (S1-2) 12.22 g (10 mol%) and compound (S1-3) 54.67 g (50 mol%) shown below were converted into 2-butanone. A monomer solution was prepared by dissolving 8.02 g of 2,2′-azobisisobutyronitrile. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Next, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 80.1 g, yield 80%). When this copolymer was analyzed by 13 C-NMR, the content (mol%) of repeating units derived from the compound (S1-1), the compound (S1-2), and the compound (S1-3) was 41:10:49. This is designated as polymer (A-1). Mw of this polymer (A-1) was 7,300, and the fluorine atom content was 0%.
合成例1(重合体(A-1)の合成)
下記に示す、化合物(S1-1)33.11g(40mol%)、化合物(S1-2)12.22g(10mol%)及び化合物(S1-3)54.67g(50mol%)を、2-ブタノン200gに溶解し、更に、2,2'-アゾビスイソブチロニトリル8.08gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量80.1g、収率80%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、41:10:49であった。これを、重合体(A-1)とする。この重合体(A-1)のMwは7,300であり、フッ素原子の含有率は0%であった。
Synthesis Example 1 (Synthesis of polymer (A-1))
Compound (S1-1) 33.11 g (40 mol%), compound (S1-2) 12.22 g (10 mol%) and compound (S1-3) 54.67 g (50 mol%) shown below were converted into 2-butanone. A monomer solution was prepared by dissolving 8.02 g of 2,2′-azobisisobutyronitrile. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Next, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 80.1 g, yield 80%). When this copolymer was analyzed by 13 C-NMR, the content (mol%) of repeating units derived from the compound (S1-1), the compound (S1-2), and the compound (S1-3) was 41:10:49. This is designated as polymer (A-1). Mw of this polymer (A-1) was 7,300, and the fluorine atom content was 0%.
合成例2(重合体(A-2)の合成)
下記に示す、化合物(S1-1)37.39g(45mol%)、化合物(S1-2)12.26g(10mol%)、化合物(S1-4)6.45g(5mol%)及び化合物(S1-3)43.90g(40mol%)を、2-ブタノン100gに溶解し、更に、2,2'-アゾビスイソブチロニトリル4.05gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量71g、収率71%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)、化合物(S1-4)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、45:9:5:41であった。これを、重合体(A-2)とする。この重合体(A-2)のMwは7,300であり、フッ素原子の含有率は1.4%であった。
Synthesis Example 2 (Synthesis of polymer (A-2))
Compound (S1-1) 37.39 g (45 mol%), compound (S1-2) 12.26 g (10 mol%), compound (S1-4) 6.45 g (5 mol%) and compound (S1- 3) 43.90 g (40 mol%) was dissolved in 100 g of 2-butanone, and then 4.05 g of 2,2′-azobisisobutyronitrile was added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Then, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 71 g, yield 71%). The copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 45: 9: 5: 41, respectively. This is referred to as a polymer (A-2). Mw of this polymer (A-2) was 7,300, and the fluorine atom content was 1.4%.
下記に示す、化合物(S1-1)37.39g(45mol%)、化合物(S1-2)12.26g(10mol%)、化合物(S1-4)6.45g(5mol%)及び化合物(S1-3)43.90g(40mol%)を、2-ブタノン100gに溶解し、更に、2,2'-アゾビスイソブチロニトリル4.05gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量71g、収率71%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)、化合物(S1-4)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、45:9:5:41であった。これを、重合体(A-2)とする。この重合体(A-2)のMwは7,300であり、フッ素原子の含有率は1.4%であった。
Compound (S1-1) 37.39 g (45 mol%), compound (S1-2) 12.26 g (10 mol%), compound (S1-4) 6.45 g (5 mol%) and compound (S1- 3) 43.90 g (40 mol%) was dissolved in 100 g of 2-butanone, and then 4.05 g of 2,2′-azobisisobutyronitrile was added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Then, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 71 g, yield 71%). The copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 45: 9: 5: 41, respectively. This is referred to as a polymer (A-2). Mw of this polymer (A-2) was 7,300, and the fluorine atom content was 1.4%.
合成例3(重合体(A-3)の合成)
下記に示す、化合物(S1-1)32.49g(40mol%)、化合物(S1-2)11.99g(10mol%)、化合物(S1-4)12.61g(10mol%)及び化合物(S1-3)42.91g(40mol%)を、2-ブタノン100gに溶解し、更に、2,2'-アゾビスイソブチロニトリル3.96gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量70g、収率70%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)、化合物(S1-4)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、39:11:10:40であった。これを、重合体(A-3)とする。この重合体(A-3)のMwは7,100であり、フッ素原子の含有率は2.8%であった。
Synthesis Example 3 (Synthesis of polymer (A-3))
Compound (S1-1) 32.49 g (40 mol%), Compound (S1-2) 11.99 g (10 mol%), Compound (S1-4) 12.61 g (10 mol%) and Compound (S1- 3) 42.91 g (40 mol%) was dissolved in 100 g of 2-butanone, and further 3.96 g of 2,2′-azobisisobutyronitrile was added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Subsequently, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 70 g, yield 70%). The copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 39: 11: 10: 40, respectively. This is designated as polymer (A-3). Mw of this polymer (A-3) was 7,100, and the fluorine atom content was 2.8%.
下記に示す、化合物(S1-1)32.49g(40mol%)、化合物(S1-2)11.99g(10mol%)、化合物(S1-4)12.61g(10mol%)及び化合物(S1-3)42.91g(40mol%)を、2-ブタノン100gに溶解し、更に、2,2'-アゾビスイソブチロニトリル3.96gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量70g、収率70%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)、化合物(S1-4)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、39:11:10:40であった。これを、重合体(A-3)とする。この重合体(A-3)のMwは7,100であり、フッ素原子の含有率は2.8%であった。
Compound (S1-1) 32.49 g (40 mol%), Compound (S1-2) 11.99 g (10 mol%), Compound (S1-4) 12.61 g (10 mol%) and Compound (S1- 3) 42.91 g (40 mol%) was dissolved in 100 g of 2-butanone, and further 3.96 g of 2,2′-azobisisobutyronitrile was added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Subsequently, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 70 g, yield 70%). The copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 39: 11: 10: 40, respectively. This is designated as polymer (A-3). Mw of this polymer (A-3) was 7,100, and the fluorine atom content was 2.8%.
合成例4(重合体(A-4)の合成)
下記に示す、化合物(S1-1)22.90g(30mol%)、化合物(S1-2)11.27g(10mol%)、化合物(S1-4)35.57g(30mol%)及び化合物(S1-3)30.26g(30mol%)を、2-ブタノン100gに溶解し、更に、2,2'-アゾビスイソブチロニトリル3.73gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量69g、収率69%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)、化合物(S1-4)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、30:10:29:31であった。これを、重合体(A-4)とする。この重合体(A-4)のMwは6,800であり、フッ素原子の含有率は7.8%であった。
Synthesis Example 4 (Synthesis of polymer (A-4))
Compound (S1-1) 22.90 g (30 mol%), Compound (S1-2) 11.27 g (10 mol%), Compound (S1-4) 35.57 g (30 mol%) and Compound (S1- 3) 30.26 g (30 mol%) was dissolved in 100 g of 2-butanone, and 3.73 g of 2,2′-azobisisobutyronitrile was further added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Subsequently, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 69 g, yield 69%). The copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 30: 10: 29: 31, respectively. This is designated as polymer (A-4). Mw of this polymer (A-4) was 6,800, and the fluorine atom content was 7.8%.
下記に示す、化合物(S1-1)22.90g(30mol%)、化合物(S1-2)11.27g(10mol%)、化合物(S1-4)35.57g(30mol%)及び化合物(S1-3)30.26g(30mol%)を、2-ブタノン100gに溶解し、更に、2,2'-アゾビスイソブチロニトリル3.73gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量69g、収率69%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)、化合物(S1-4)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、30:10:29:31であった。これを、重合体(A-4)とする。この重合体(A-4)のMwは6,800であり、フッ素原子の含有率は7.8%であった。
Compound (S1-1) 22.90 g (30 mol%), Compound (S1-2) 11.27 g (10 mol%), Compound (S1-4) 35.57 g (30 mol%) and Compound (S1- 3) 30.26 g (30 mol%) was dissolved in 100 g of 2-butanone, and 3.73 g of 2,2′-azobisisobutyronitrile was further added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Subsequently, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 69 g, yield 69%). The copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-4), and the compound (S1-3) ( mol%) was 30: 10: 29: 31, respectively. This is designated as polymer (A-4). Mw of this polymer (A-4) was 6,800, and the fluorine atom content was 7.8%.
合成例5(重合体(A-5)の合成)
下記に示す、化合物(S1-1)31.27g(40mol%)、化合物(S1-2)11.54g(10mol%)、化合物(S1-5)15.87g(10mol%)及び化合物(S1-3)41.31g(40mol%)を、2-ブタノン100gに溶解し、更に、2,2'-アゾビスイソブチロニトリル3.82gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量81g、収率81%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)、化合物(S1-5)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、39:10:10:41であった。これを、重合体(A-5)とする。この重合体(A-5)のMwは7,800であり、フッ素原子の含有率は2.6%であった。
Synthesis Example 5 (Synthesis of polymer (A-5))
Compound (S1-1) 31.27 g (40 mol%), compound (S1-2) 11.54 g (10 mol%), compound (S1-5) 15.87 g (10 mol%) and compound (S1- 3) 41.31 g (40 mol%) was dissolved in 100 g of 2-butanone, and 3.82 g of 2,2′-azobisisobutyronitrile was further added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Subsequently, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 81 g, yield 81%). The copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-5), and the compound (S1-3) ( mol%) was 39: 10: 10: 41, respectively. This is designated as polymer (A-5). This polymer (A-5) had an Mw of 7,800 and a fluorine atom content of 2.6%.
下記に示す、化合物(S1-1)31.27g(40mol%)、化合物(S1-2)11.54g(10mol%)、化合物(S1-5)15.87g(10mol%)及び化合物(S1-3)41.31g(40mol%)を、2-ブタノン100gに溶解し、更に、2,2'-アゾビスイソブチロニトリル3.82gを投入して単量体溶液を準備した。一方、100gの2-ブタノンを1,000mLの三口フラスコに投入し、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内の2-ブタノンを攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を2,000gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、400gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末(共重合体)を50℃で17時間乾燥した(収量81g、収率81%)。この共重合体を、13C-NMR分析したところ、化合物(S1-1)、化合物(S1-2)、化合物(S1-5)及び化合物(S1-3)に由来する繰り返し単位の含有率(mol%)は、それぞれ、39:10:10:41であった。これを、重合体(A-5)とする。この重合体(A-5)のMwは7,800であり、フッ素原子の含有率は2.6%であった。
Compound (S1-1) 31.27 g (40 mol%), compound (S1-2) 11.54 g (10 mol%), compound (S1-5) 15.87 g (10 mol%) and compound (S1- 3) 41.31 g (40 mol%) was dissolved in 100 g of 2-butanone, and 3.82 g of 2,2′-azobisisobutyronitrile was further added to prepare a monomer solution. On the other hand, 100 g of 2-butanone was put into a 1,000 mL three-necked flask and purged with nitrogen gas for 30 minutes.
After purging with nitrogen, 2-butanone in the three-necked flask was heated to 80 ° C. with stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 2,000 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice as a slurry with 400 g of methanol and then filtered. Subsequently, the white powder (copolymer) was dried at 50 ° C. for 17 hours (yield 81 g, yield 81%). The copolymer was analyzed by 13 C-NMR. As a result, the content of repeating units derived from the compound (S1-1), the compound (S1-2), the compound (S1-5), and the compound (S1-3) ( mol%) was 39: 10: 10: 41, respectively. This is designated as polymer (A-5). This polymer (A-5) had an Mw of 7,800 and a fluorine atom content of 2.6%.
合成例6(重合体(C-1)の合成)
下記に示す、化合物(S3-1)14.5g(20mol%)及び化合物(S3-2)84.6g(80mol%)を、2-ブタノン150gに溶解し、更に、2,2'-アゾビスイソブチロニトリル3.47gを投入して単量体溶液を準備した。一方、500mLの三口フラスコ内を、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内を攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を600gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、300gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末を50℃で17時間乾燥し、無色固体の共重合体を得た(収量72g、収率72%)。この共重合体を、13C-NMR分析したところ、化合物(S3-1)及び化合物(S3-2)に由来する繰り返し単位の含有率(mol%)は、それぞれ、21:79であった。これを、重合体(C-1)とする。この重合体(C-1)のMwは6,400であり、フッ素原子の含有率は12.8%であった。
Synthesis Example 6 (Synthesis of polymer (C-1))
Compound (S3-1) 14.5 g (20 mol%) and compound (S3-2) 84.6 g (80 mol%) shown below are dissolved in 2-butanone 150 g, and 2,2′-azobis is further dissolved. A monomer solution was prepared by adding 3.47 g of isobutyronitrile. Meanwhile, the inside of the 500 mL three-necked flask was purged with nitrogen gas for 30 minutes.
After purging with nitrogen, the inside of the three-necked flask was heated to 80 ° C. while stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 600 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice with 300 g of methanol as a slurry, and then filtered. Next, the white powder was dried at 50 ° C. for 17 hours to obtain a colorless solid copolymer (yield 72 g, yield 72%). The copolymer was analyzed by 13 C-NMR, whereby the content (mol%) of repeating units derived from the compound (S3-1) and the compound (S3-2) was 21:79, respectively. This is referred to as a polymer (C-1). This polymer (C-1) had an Mw of 6,400 and a fluorine atom content of 12.8%.
下記に示す、化合物(S3-1)14.5g(20mol%)及び化合物(S3-2)84.6g(80mol%)を、2-ブタノン150gに溶解し、更に、2,2'-アゾビスイソブチロニトリル3.47gを投入して単量体溶液を準備した。一方、500mLの三口フラスコ内を、30分間窒素ガスによりパージした。
窒素パージの後、三口フラスコ内を攪拌しながら、80℃に加熱した。次いで、事前に準備した上記単量体溶液を、滴下漏斗を用いて、3時間かけて滴下した。滴下終了後、更に、80℃で3時間撹拌した。
重合終了後、重合溶液を水冷により、30℃以下に冷却した。そして、この重合溶液を600gのメタノールへ投入し、白色粉末を析出させ、その後、これを濾別した。濾別された白色粉末を、2度、300gのメタノールにてスラリー状で洗浄した後、濾別した。次いで、白色粉末を50℃で17時間乾燥し、無色固体の共重合体を得た(収量72g、収率72%)。この共重合体を、13C-NMR分析したところ、化合物(S3-1)及び化合物(S3-2)に由来する繰り返し単位の含有率(mol%)は、それぞれ、21:79であった。これを、重合体(C-1)とする。この重合体(C-1)のMwは6,400であり、フッ素原子の含有率は12.8%であった。
Compound (S3-1) 14.5 g (20 mol%) and compound (S3-2) 84.6 g (80 mol%) shown below are dissolved in 2-butanone 150 g, and 2,2′-azobis is further dissolved. A monomer solution was prepared by adding 3.47 g of isobutyronitrile. Meanwhile, the inside of the 500 mL three-necked flask was purged with nitrogen gas for 30 minutes.
After purging with nitrogen, the inside of the three-necked flask was heated to 80 ° C. while stirring. Subsequently, the monomer solution prepared in advance was dropped over 3 hours using a dropping funnel. After completion of dropping, the mixture was further stirred at 80 ° C. for 3 hours.
After completion of the polymerization, the polymerization solution was cooled to 30 ° C. or less by water cooling. And this polymerization solution was thrown into 600 g of methanol, white powder was deposited, and this was separated by filtration after that. The white powder separated by filtration was washed twice with 300 g of methanol as a slurry, and then filtered. Next, the white powder was dried at 50 ° C. for 17 hours to obtain a colorless solid copolymer (yield 72 g, yield 72%). The copolymer was analyzed by 13 C-NMR, whereby the content (mol%) of repeating units derived from the compound (S3-1) and the compound (S3-2) was 21:79, respectively. This is referred to as a polymer (C-1). This polymer (C-1) had an Mw of 6,400 and a fluorine atom content of 12.8%.
2.感放射線性樹脂組成物の製造及び評価
組成物を調製するための他の原料成分を以下に示す。
2-1.酸発生剤[B]
(1)B-1
4-シクロヘキシルフェニルジフェニルスルホニウムノナフルオロ-n-ブタンスルホネート
(2)B-2
トリフェニルスルホニウム=トリシクロ[3.3.1.13,7]デカニルジフルオロメタンスルホナート 2. Production and Evaluation of Radiation Sensitive Resin Composition Other raw material components for preparing the composition are shown below.
2-1. Acid generator [B]
(1) B-1
4-cyclohexylphenyldiphenylsulfonium nonafluoro-n-butanesulfonate (2) B-2
Triphenylsulfonium = tricyclo [3.3.1.1 3,7 ] decanyl difluoromethanesulfonate
組成物を調製するための他の原料成分を以下に示す。
2-1.酸発生剤[B]
(1)B-1
4-シクロヘキシルフェニルジフェニルスルホニウムノナフルオロ-n-ブタンスルホネート
(2)B-2
トリフェニルスルホニウム=トリシクロ[3.3.1.13,7]デカニルジフルオロメタンスルホナート 2. Production and Evaluation of Radiation Sensitive Resin Composition Other raw material components for preparing the composition are shown below.
2-1. Acid generator [B]
(1) B-1
4-cyclohexylphenyldiphenylsulfonium nonafluoro-n-butanesulfonate (2) B-2
Triphenylsulfonium = tricyclo [3.3.1.1 3,7 ] decanyl difluoromethanesulfonate
2-2.酸拡散抑制剤[D]
(1)D-1
tert-ブチル-4-ヒドロキシ-1-ピペリジンカルボキシレート
(2)D-2
2,6-ジイソプロピルアニリン 2-2. Acid diffusion inhibitor [D]
(1) D-1
tert-Butyl-4-hydroxy-1-piperidinecarboxylate (2) D-2
2,6-Diisopropylaniline
(1)D-1
tert-ブチル-4-ヒドロキシ-1-ピペリジンカルボキシレート
(2)D-2
2,6-ジイソプロピルアニリン 2-2. Acid diffusion inhibitor [D]
(1) D-1
tert-Butyl-4-hydroxy-1-piperidinecarboxylate (2) D-2
2,6-Diisopropylaniline
2-3.溶剤[F]
(1)F-1
プロピレングリコールモノメチルエーテルアセテート
(2)F-2
シクロヘキサノン 2-3. Solvent [F]
(1) F-1
Propylene glycol monomethyl ether acetate (2) F-2
Cyclohexanone
(1)F-1
プロピレングリコールモノメチルエーテルアセテート
(2)F-2
シクロヘキサノン 2-3. Solvent [F]
(1) F-1
Propylene glycol monomethyl ether acetate (2) F-2
Cyclohexanone
実施例1
重合体(A-2)100部と、酸発生剤(B-1)12部と、重合体(C-1)5部と、酸拡散抑制剤0.8部と、溶剤(F-1)1,980部及び溶剤(F-2)848部とを混合して均一溶液とした。その後、孔径200nmのメンブランフィルターを用いてろ過することにより、感放射線性樹脂組成物(組成物溶液)を製造した(表1参照)。 Example 1
100 parts of polymer (A-2), 12 parts of acid generator (B-1), 5 parts of polymer (C-1), 0.8 part of acid diffusion inhibitor, solvent (F-1) 1,980 parts and 848 parts of solvent (F-2) were mixed to obtain a uniform solution. Then, the radiation sensitive resin composition (composition solution) was manufactured by filtering using a membrane filter with a pore diameter of 200 nm (see Table 1).
重合体(A-2)100部と、酸発生剤(B-1)12部と、重合体(C-1)5部と、酸拡散抑制剤0.8部と、溶剤(F-1)1,980部及び溶剤(F-2)848部とを混合して均一溶液とした。その後、孔径200nmのメンブランフィルターを用いてろ過することにより、感放射線性樹脂組成物(組成物溶液)を製造した(表1参照)。 Example 1
100 parts of polymer (A-2), 12 parts of acid generator (B-1), 5 parts of polymer (C-1), 0.8 part of acid diffusion inhibitor, solvent (F-1) 1,980 parts and 848 parts of solvent (F-2) were mixed to obtain a uniform solution. Then, the radiation sensitive resin composition (composition solution) was manufactured by filtering using a membrane filter with a pore diameter of 200 nm (see Table 1).
実施例2~6及び比較例1
原料成分を、表1に記載の配合処方に従って用いたこと以外は、実施例1と同様にして、感放射線性樹脂組成物(組成物溶液)を製造した(表1参照)。 Examples 2 to 6 and Comparative Example 1
A radiation-sensitive resin composition (composition solution) was produced in the same manner as in Example 1 except that the raw material components were used according to the formulation described in Table 1 (see Table 1).
原料成分を、表1に記載の配合処方に従って用いたこと以外は、実施例1と同様にして、感放射線性樹脂組成物(組成物溶液)を製造した(表1参照)。 Examples 2 to 6 and Comparative Example 1
A radiation-sensitive resin composition (composition solution) was produced in the same manner as in Example 1 except that the raw material components were used according to the formulation described in Table 1 (see Table 1).
各感放射線性樹脂組成物を用いて、以下の要領で、シリコンウエハに配された反射防止膜上に、ライン・アンド・スペースパターンを形成し、LWRを評価した。その結果を表1に併記した。
(1)レジストパターンの形成
12インチシリコンウエハ表面に、下層反射防止膜形成用組成物(商品名「ARC66」、日産化学社製)を、半導体製造装置(型式名「Lithius Pro-i」、東京エレクトロン社製)を使用して、スピンコートした。次いで、PB(205℃、60秒間)を行うことにより、膜厚105nmの反射防止層を形成した。 Using each of the radiation sensitive resin compositions, a line and space pattern was formed on the antireflection film disposed on the silicon wafer in the following manner, and LWR was evaluated. The results are also shown in Table 1.
(1) Formation of resist pattern On the surface of a 12-inch silicon wafer, a composition for forming a lower antireflection film (trade name “ARC66”, manufactured by Nissan Chemical Co., Ltd.) Spin-coated using Electron). Next, PB (205 ° C., 60 seconds) was performed to form an antireflection layer having a thickness of 105 nm.
(1)レジストパターンの形成
12インチシリコンウエハ表面に、下層反射防止膜形成用組成物(商品名「ARC66」、日産化学社製)を、半導体製造装置(型式名「Lithius Pro-i」、東京エレクトロン社製)を使用して、スピンコートした。次いで、PB(205℃、60秒間)を行うことにより、膜厚105nmの反射防止層を形成した。 Using each of the radiation sensitive resin compositions, a line and space pattern was formed on the antireflection film disposed on the silicon wafer in the following manner, and LWR was evaluated. The results are also shown in Table 1.
(1) Formation of resist pattern On the surface of a 12-inch silicon wafer, a composition for forming a lower antireflection film (trade name “ARC66”, manufactured by Nissan Chemical Co., Ltd.) Spin-coated using Electron). Next, PB (205 ° C., 60 seconds) was performed to form an antireflection layer having a thickness of 105 nm.
その後、半導体製造装置(型式名「CLEAN TRACK ACT12」、東京エレクトロン社製)を使用して、感放射線性樹脂組成物をスピンコートした。そして、PB(110℃、60秒間)し、冷却(23℃、30秒間)することにより、膜厚100nmのフォトレジスト層を形成した。
Then, the radiation sensitive resin composition was spin-coated using a semiconductor manufacturing apparatus (model name “CLEAN TRACK ACT12”, manufactured by Tokyo Electron Ltd.). Then, PB (110 ° C., 60 seconds) and cooling (23 ° C., 30 seconds) were performed to form a 100 nm-thick photoresist layer.
次いで、ArF液浸露光装置(商品名「S610C」、NIKON社製)を使用して、NA:1.30、Crosspoleの光学条件にて、ターゲットサイズが48nmライン/96nmピッチのマスクを介して露光した。その後、半導体製造装置(型式名「Lithius Pro-i」、東京エレクトロン社製)のホットプレート上で、PEB(95℃、60秒間)し、冷却(23℃、30秒間)した。次に、現像カップのGPノズルにて、2.38%テトラメチルアンモニウムヒドロキシド水溶液を現像液としてパドル現像(10秒間)し、超純水でリンスした。その後、2000rpm、15秒間振り切りで、スピンドライすることにより、レジストパターン(ライン・アンド・スペースパターン)が形成された評価用シリコンウエハを得た。このとき、ターゲットサイズが48nmライン/96nmピッチのマスク寸法において、48nmライン/96nmピッチのパターンを形成する露光量を最適露光量とした。
Next, using an ArF immersion exposure apparatus (trade name “S610C”, manufactured by NIKON Corporation), exposure is performed through a mask having a target size of 48 nm line / 96 nm pitch under optical conditions of NA: 1.30 and Crosspore. did. Thereafter, PEB (95 ° C., 60 seconds) was performed and cooled (23 ° C., 30 seconds) on a hot plate of a semiconductor manufacturing apparatus (model name “Lithius Pro-i”, manufactured by Tokyo Electron Ltd.). Next, paddle development (10 seconds) was performed using a 2.38% tetramethylammonium hydroxide aqueous solution as a developer with a GP nozzle of the developing cup, and rinsed with ultrapure water. Thereafter, the silicon wafer for evaluation on which a resist pattern (line and space pattern) was formed was obtained by spin-drying by shaking off at 2000 rpm for 15 seconds. At this time, the exposure amount for forming a pattern of 48 nm line / 96 nm pitch in the mask size with a target size of 48 nm line / 96 nm pitch was determined as the optimum exposure amount.
(2)LWRの測定
走査型電子顕微鏡を用いて、最適露光量にて解像した48nmライン/96nmピッチのパターンを、その上方から観察し、線幅を、任意のポイントで10点測定し、その測定値の3σ(ばらつき)を、LWR(単位:nm)とした。 (2) Measurement of LWR Using a scanning electron microscope, a 48 nm line / 96 nm pitch pattern resolved at the optimum exposure dose was observed from above, and the line width was measured at 10 arbitrary points. The 3σ (variation) of the measured value was defined as LWR (unit: nm).
走査型電子顕微鏡を用いて、最適露光量にて解像した48nmライン/96nmピッチのパターンを、その上方から観察し、線幅を、任意のポイントで10点測定し、その測定値の3σ(ばらつき)を、LWR(単位:nm)とした。 (2) Measurement of LWR Using a scanning electron microscope, a 48 nm line / 96 nm pitch pattern resolved at the optimum exposure dose was observed from above, and the line width was measured at 10 arbitrary points. The 3σ (variation) of the measured value was defined as LWR (unit: nm).
表1から明らかなように、比較例1は、本発明に係る一般式(1)で表される繰り返し単位を含まない重合体を含有する感放射線性樹脂組成物を用いた例であり、LWRが4.7と高く、好ましくない。一方、実施例1~6の感放射線性樹脂組成物は、LWRが4未満であり、優れていた。
As is clear from Table 1, Comparative Example 1 is an example using a radiation-sensitive resin composition containing a polymer that does not contain a repeating unit represented by the general formula (1) according to the present invention. Is as high as 4.7, which is not preferable. On the other hand, the radiation sensitive resin compositions of Examples 1 to 6 were excellent with an LWR of less than 4.
本発明の感放射線性樹脂組成物を用いると、広い露光マージンを維持しつつ、ライン幅粗さ(LWR)を良好に維持することが可能な化学増幅型レジストを得ることができる。特に、KrFエキシマレーザー及びArFエキシマレーザーに代表される遠紫外線、シンクロトロン放射線等のX線、電子線等の荷電粒子線、等の放射線を使用する微細加工に有用な化学増幅型レジストの形成に好適である。そして、今後、更に微細化が進むと予想される半導体デバイスの製造に極めて有用である。
When the radiation-sensitive resin composition of the present invention is used, a chemically amplified resist capable of maintaining a good line width roughness (LWR) while maintaining a wide exposure margin can be obtained. In particular, for the formation of chemically amplified resists useful for microfabrication using X-rays such as far-ultraviolet rays, synchrotron radiation, and other charged particle beams such as electron beams, such as KrF excimer laser and ArF excimer laser. Is preferred. And it is very useful for the manufacture of a semiconductor device which is expected to be further miniaturized in the future.
Claims (7)
- [A]下記一般式(1)で表される繰り返し単位を含み、酸解離性基を有する重合体、[B]感放射線性酸発生剤、及び、[C]フッ素原子を含む重合体を含有し、前記重合体[A]に含まれるフッ素原子の含有率が、前記重合体[C]に含まれるフッ素原子の含有率よりも少ないことを特徴とする感放射線性樹脂組成物。
- 前記重合体[A]を構成する繰り返し単位の全量を100モル%としたときに、前記一般式(1)で表される繰り返し単位の含有率が5~50モル%である請求項1に記載の感放射線性樹脂組成物。 The content of the repeating unit represented by the general formula (1) is 5 to 50 mol%, when the total amount of the repeating units constituting the polymer [A] is 100 mol%. Radiation sensitive resin composition.
- 前記重合体[A]が、更に、下記一般式(2)で表される繰り返し単位を含む請求項1又は2に記載の感放射線性樹脂組成物。
- 前記重合体[A]が、更に、下記一般式(3-1)~(3-6)で表される繰り返し単位からなる群より選択された少なくとも1つの繰り返し単位を含む請求項1乃至3のいずれかに記載の感放射線性樹脂組成物。
- 前記重合体[C]が、下記一般式(c1-1)~(c1-3)で表される繰り返し単位からなる群より選択された少なくとも1つの繰り返し単位を含む請求項1乃至4のいずれかに記載の感放射線性樹脂組成物。
- [A]下記一般式(1)で表される繰り返し単位を含み、酸解離性基を有する重合体、及び、[B]感放射線性酸発生剤を含有することを特徴とする液浸露光用感放射線性樹脂組成物。
- 請求項1乃至6のいずれかに記載の感放射線性樹脂組成物を用いて、基板上にフォトレジスト膜を形成する工程と、
前記フォトレジスト膜上に液浸露光用液体を配置し、前記液浸露光用液体を介して前記フォトレジスト膜を液浸露光する工程と、
液浸露光された前記フォトレジスト膜を現像してレジストパターンを形成する工程と、
を備えることを特徴とするレジストパターン形成方法。 A step of forming a photoresist film on a substrate using the radiation-sensitive resin composition according to claim 1;
Placing an immersion exposure liquid on the photoresist film, and immersion exposing the photoresist film through the immersion exposure liquid;
Developing the photoresist film that has been subjected to immersion exposure to form a resist pattern; and
A resist pattern forming method comprising:
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012503263A JPWO2011108665A1 (en) | 2010-03-04 | 2011-03-03 | Radiation-sensitive resin composition and resist pattern forming method |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010-048389 | 2010-03-04 | ||
| JP2010048389 | 2010-03-04 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011108665A1 true WO2011108665A1 (en) | 2011-09-09 |
Family
ID=44542312
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/054967 WO2011108665A1 (en) | 2010-03-04 | 2011-03-03 | Radiation-sensitive resin composition and resist pattern formation method |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JPWO2011108665A1 (en) |
| TW (1) | TW201144932A (en) |
| WO (1) | WO2011108665A1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014209203A (en) * | 2013-03-27 | 2014-11-06 | 東京応化工業株式会社 | Resist composition, method of forming resist pattern, and compound |
| WO2016039327A1 (en) * | 2014-09-12 | 2016-03-17 | Jsr株式会社 | Method for manufacturing structure having recessed pattern, resin composition, method for forming electroconductive film, electronic circuit, and electronic device |
| WO2019131953A1 (en) * | 2017-12-27 | 2019-07-04 | Jsr株式会社 | Pattern-forming method and radiation-sensitive composition |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007304545A (en) * | 2005-09-13 | 2007-11-22 | Fujifilm Corp | Positive resist composition and pattern-forming method using same |
| WO2009044666A1 (en) * | 2007-10-01 | 2009-04-09 | Jsr Corporation | Radiation-sensitive composition |
| JP2009237379A (en) * | 2008-03-27 | 2009-10-15 | Fujifilm Corp | Positive photosensitive composition and pattern forming method using the same |
-
2011
- 2011-03-03 WO PCT/JP2011/054967 patent/WO2011108665A1/en active Application Filing
- 2011-03-03 JP JP2012503263A patent/JPWO2011108665A1/en active Pending
- 2011-03-04 TW TW100107321A patent/TW201144932A/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007304545A (en) * | 2005-09-13 | 2007-11-22 | Fujifilm Corp | Positive resist composition and pattern-forming method using same |
| WO2009044666A1 (en) * | 2007-10-01 | 2009-04-09 | Jsr Corporation | Radiation-sensitive composition |
| JP2009237379A (en) * | 2008-03-27 | 2009-10-15 | Fujifilm Corp | Positive photosensitive composition and pattern forming method using the same |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014209203A (en) * | 2013-03-27 | 2014-11-06 | 東京応化工業株式会社 | Resist composition, method of forming resist pattern, and compound |
| JP2018076323A (en) * | 2013-03-27 | 2018-05-17 | 東京応化工業株式会社 | Compound |
| WO2016039327A1 (en) * | 2014-09-12 | 2016-03-17 | Jsr株式会社 | Method for manufacturing structure having recessed pattern, resin composition, method for forming electroconductive film, electronic circuit, and electronic device |
| JPWO2016039327A1 (en) * | 2014-09-12 | 2017-06-22 | Jsr株式会社 | Manufacturing method of structure having concave pattern, resin composition, method of forming conductive film, electronic circuit and electronic device |
| US10392699B2 (en) | 2014-09-12 | 2019-08-27 | Jsr Corporation | Method for manufacturing structure having recessed pattern, resin composition, method for forming electroconductive film, electronic circuit, and electronic device |
| WO2019131953A1 (en) * | 2017-12-27 | 2019-07-04 | Jsr株式会社 | Pattern-forming method and radiation-sensitive composition |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2011108665A1 (en) | 2013-06-27 |
| TW201144932A (en) | 2011-12-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5445454B2 (en) | Positive radiation sensitive composition and resist pattern forming method | |
| EP2003148B1 (en) | Radiation-sensitive resin composition comprising a fluorine-containing polymer | |
| KR101701523B1 (en) | Radiation-sensitive resin composition, and resist pattern formation method | |
| JP5146606B2 (en) | Radiation sensitive resin composition, resist pattern forming method, and polymer | |
| US20100285405A1 (en) | Radiation-sensitive resin composition | |
| KR20140050053A (en) | Photoresist composition | |
| JP5835319B2 (en) | Resist pattern forming method, radiation-sensitive resin composition, and resist film | |
| JP6048532B2 (en) | Radiation-sensitive resin composition and resist pattern forming method | |
| JP5581726B2 (en) | Radiation-sensitive resin composition and resist pattern forming method | |
| JP5487921B2 (en) | Photoresist composition, photoresist composition and polymer for immersion exposure, and method for forming resist pattern | |
| JP5765340B2 (en) | Radiation-sensitive resin composition and resist pattern forming method | |
| JP5515449B2 (en) | Radiation-sensitive resin composition and resist pattern forming method | |
| JP5343535B2 (en) | Radiation-sensitive resin composition, resist film forming method and resist pattern forming method using the same | |
| WO2011108665A1 (en) | Radiation-sensitive resin composition and resist pattern formation method | |
| JP5783168B2 (en) | Photoresist composition and resist pattern forming method | |
| JP5434709B2 (en) | Radiation-sensitive resin composition and polymer | |
| JP5157932B2 (en) | Radiation sensitive resin composition | |
| JP2011075750A (en) | Radiation sensitive resin composition for chemically amplified resist and polymer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11750774 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012503263 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11750774 Country of ref document: EP Kind code of ref document: A1 |