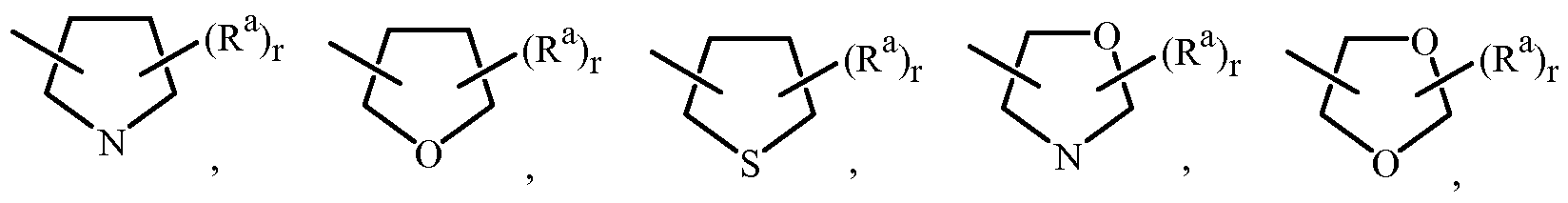WO2010101973A1 - Fungicidal pyrazoles - Google Patents
Fungicidal pyrazoles Download PDFInfo
- Publication number
- WO2010101973A1 WO2010101973A1 PCT/US2010/026003 US2010026003W WO2010101973A1 WO 2010101973 A1 WO2010101973 A1 WO 2010101973A1 US 2010026003 W US2010026003 W US 2010026003W WO 2010101973 A1 WO2010101973 A1 WO 2010101973A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- dimethyl
- independently selected
- alkyl
- pyrazol
- ring
- Prior art date
Links
- 230000000855 fungicidal effect Effects 0.000 title claims description 41
- 150000003217 pyrazoles Chemical class 0.000 title description 9
- 150000001875 compounds Chemical class 0.000 claims abstract description 480
- 125000001424 substituent group Chemical group 0.000 claims abstract description 178
- 239000000203 mixture Substances 0.000 claims abstract description 136
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract description 105
- 238000000034 method Methods 0.000 claims abstract description 75
- 125000000623 heterocyclic group Chemical group 0.000 claims abstract description 51
- 150000003839 salts Chemical class 0.000 claims abstract description 42
- 229910052717 sulfur Inorganic materials 0.000 claims abstract description 34
- 229910052760 oxygen Inorganic materials 0.000 claims abstract description 33
- 201000010099 disease Diseases 0.000 claims abstract description 31
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 31
- 229920006395 saturated elastomer Polymers 0.000 claims abstract description 16
- -1 cyano, cyanomethyl Chemical group 0.000 claims description 211
- 239000000417 fungicide Substances 0.000 claims description 199
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 89
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 84
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 78
- 229910052736 halogen Inorganic materials 0.000 claims description 72
- 150000002367 halogens Chemical class 0.000 claims description 66
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 60
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 claims description 55
- 125000000217 alkyl group Chemical group 0.000 claims description 45
- 229910052794 bromium Inorganic materials 0.000 claims description 42
- 229910052801 chlorine Inorganic materials 0.000 claims description 42
- 239000007787 solid Substances 0.000 claims description 42
- 229910052757 nitrogen Inorganic materials 0.000 claims description 41
- 125000004076 pyridyl group Chemical group 0.000 claims description 40
- 125000004122 cyclic group Chemical group 0.000 claims description 39
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 39
- 125000003545 alkoxy group Chemical group 0.000 claims description 37
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 32
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 claims description 31
- OKKJLVBELUTLKV-UHFFFAOYSA-N methanol Substances OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 31
- JFDZBHWFFUWGJE-UHFFFAOYSA-N benzonitrile Chemical compound N#CC1=CC=CC=C1 JFDZBHWFFUWGJE-UHFFFAOYSA-N 0.000 claims description 30
- 229910052731 fluorine Inorganic materials 0.000 claims description 30
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 29
- 239000003085 diluting agent Substances 0.000 claims description 29
- 125000004183 alkoxy alkyl group Chemical group 0.000 claims description 28
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 28
- 125000001188 haloalkyl group Chemical group 0.000 claims description 27
- 239000007788 liquid Substances 0.000 claims description 27
- 229910052799 carbon Inorganic materials 0.000 claims description 26
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 24
- 125000004432 carbon atom Chemical group C* 0.000 claims description 23
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims description 23
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 23
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 23
- 125000003373 pyrazinyl group Chemical group 0.000 claims description 23
- 125000002098 pyridazinyl group Chemical group 0.000 claims description 23
- 239000004094 surface-active agent Substances 0.000 claims description 23
- 150000001721 carbon Chemical group 0.000 claims description 22
- 125000004448 alkyl carbonyl group Chemical group 0.000 claims description 21
- 125000002813 thiocarbonyl group Chemical group *C(*)=S 0.000 claims description 20
- 125000004414 alkyl thio group Chemical group 0.000 claims description 19
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 18
- 125000004453 alkoxycarbonyl group Chemical group 0.000 claims description 18
- 125000006274 (C1-C3)alkoxy group Chemical group 0.000 claims description 17
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 claims description 17
- 125000004737 (C1-C6) haloalkoxy group Chemical group 0.000 claims description 15
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 claims description 15
- 125000000000 cycloalkoxy group Chemical group 0.000 claims description 15
- 125000006677 (C1-C3) haloalkoxy group Chemical group 0.000 claims description 14
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 claims description 14
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 14
- 125000000278 alkyl amino alkyl group Chemical group 0.000 claims description 14
- 125000004985 dialkyl amino alkyl group Chemical group 0.000 claims description 14
- 125000004438 haloalkoxy group Chemical group 0.000 claims description 13
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 13
- 125000005119 alkyl cycloalkyl group Chemical group 0.000 claims description 12
- 125000004434 sulfur atom Chemical group 0.000 claims description 12
- 239000003795 chemical substances by application Substances 0.000 claims description 11
- 125000004995 haloalkylthio group Chemical group 0.000 claims description 11
- 125000005347 halocycloalkyl group Chemical group 0.000 claims description 11
- 125000004390 alkyl sulfonyl group Chemical group 0.000 claims description 10
- 125000006254 cycloalkyl carbonyl group Chemical group 0.000 claims description 10
- 125000004441 haloalkylsulfonyl group Chemical group 0.000 claims description 10
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 claims description 9
- 125000006645 (C3-C4) cycloalkyl group Chemical group 0.000 claims description 9
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 claims description 9
- 125000000304 alkynyl group Chemical group 0.000 claims description 9
- 125000001316 cycloalkyl alkyl group Chemical group 0.000 claims description 9
- 125000005366 cycloalkylthio group Chemical group 0.000 claims description 9
- 125000004785 fluoromethoxy group Chemical group [H]C([H])(F)O* 0.000 claims description 9
- 244000000004 fungal plant pathogen Species 0.000 claims description 9
- 125000005842 heteroatom Chemical group 0.000 claims description 9
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 claims description 8
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 claims description 8
- 125000002816 methylsulfanyl group Chemical group [H]C([H])([H])S[*] 0.000 claims description 8
- 229910006067 SO3−M Inorganic materials 0.000 claims description 7
- 125000004455 (C1-C3) alkylthio group Chemical group 0.000 claims description 6
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 claims description 6
- 150000001768 cations Chemical class 0.000 claims description 6
- 125000004450 alkenylene group Chemical group 0.000 claims description 5
- 125000003806 alkyl carbonyl amino group Chemical group 0.000 claims description 5
- 125000006350 alkyl thio alkyl group Chemical group 0.000 claims description 5
- 125000004419 alkynylene group Chemical group 0.000 claims description 5
- 125000005724 cycloalkenylene group Chemical group 0.000 claims description 5
- 125000002993 cycloalkylene group Chemical group 0.000 claims description 5
- 125000004970 halomethyl group Chemical group 0.000 claims description 5
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 claims description 5
- 125000004769 (C1-C4) alkylsulfonyl group Chemical group 0.000 claims description 4
- 125000006700 (C1-C6) alkylthio group Chemical group 0.000 claims description 4
- 241000607479 Yersinia pestis Species 0.000 claims description 4
- 125000002947 alkylene group Chemical group 0.000 claims description 4
- 125000004400 (C1-C12) alkyl group Chemical group 0.000 claims description 3
- 125000004767 (C1-C4) haloalkoxy group Chemical group 0.000 claims description 3
- 125000006710 (C2-C12) alkenyl group Chemical group 0.000 claims description 3
- 125000006711 (C2-C12) alkynyl group Chemical group 0.000 claims description 3
- 125000004070 6 membered heterocyclic group Chemical group 0.000 claims description 3
- GNBGNFVTOFWLSA-UHFFFAOYSA-N [4-(2-chloro-4-fluorophenyl)-2,5-dimethylpyrazol-3-yl]-(2,4-difluorophenyl)methanol Chemical compound C=1C=C(F)C=C(Cl)C=1C=1C(C)=NN(C)C=1C(O)C1=CC=C(F)C=C1F GNBGNFVTOFWLSA-UHFFFAOYSA-N 0.000 claims description 3
- 125000005196 alkyl carbonyloxy group Chemical group 0.000 claims description 3
- 125000005278 alkyl sulfonyloxy group Chemical group 0.000 claims description 3
- 125000005280 halo alkyl sulfonyloxy group Chemical group 0.000 claims description 3
- 125000004440 haloalkylsulfinyl group Chemical group 0.000 claims description 3
- 125000003161 (C1-C6) alkylene group Chemical group 0.000 claims description 2
- 125000006729 (C2-C5) alkenyl group Chemical group 0.000 claims description 2
- VXXSVCZAHLUQEJ-UHFFFAOYSA-N (2-bromo-4-fluorophenyl)-[4-(2,4-difluorophenyl)-2,5-dimethylpyrazol-3-yl]methanol Chemical compound C=1C=C(F)C=C(F)C=1C=1C(C)=NN(C)C=1C(O)C1=CC=C(F)C=C1Br VXXSVCZAHLUQEJ-UHFFFAOYSA-N 0.000 claims 1
- 125000004768 (C1-C4) alkylsulfinyl group Chemical group 0.000 claims 1
- WLLBTGPVVUDNIM-UHFFFAOYSA-N 4-[4-(2,6-difluorophenyl)-2,5-dimethylpyrazol-3-yl]oxy-3-fluorobenzonitrile Chemical compound FC=1C=CC=C(F)C=1C=1C(C)=NN(C)C=1OC1=CC=C(C#N)C=C1F WLLBTGPVVUDNIM-UHFFFAOYSA-N 0.000 claims 1
- VDBLUBUZHAZTHA-UHFFFAOYSA-N 4-[4-(2-bromo-4-fluorophenyl)-2,5-dimethylpyrazol-3-yl]oxy-3-fluorobenzonitrile Chemical compound C=1C=C(F)C=C(Br)C=1C=1C(C)=NN(C)C=1OC1=CC=C(C#N)C=C1F VDBLUBUZHAZTHA-UHFFFAOYSA-N 0.000 claims 1
- GQMGMVRAMWNLHW-UHFFFAOYSA-N 4-[5-(2-chloro-4,6-difluoroanilino)-1,3-dimethylpyrazol-4-yl]-3-fluorobenzonitrile Chemical compound C=1C=C(C#N)C=C(F)C=1C=1C(C)=NN(C)C=1NC1=C(F)C=C(F)C=C1Cl GQMGMVRAMWNLHW-UHFFFAOYSA-N 0.000 claims 1
- GQUSTSWMNBDBJG-UHFFFAOYSA-N 4-[5-(4-chloro-2,6-difluoroanilino)-1,3-dimethylpyrazol-4-yl]-3-fluorobenzonitrile Chemical compound C=1C=C(C#N)C=C(F)C=1C=1C(C)=NN(C)C=1NC1=C(F)C=C(Cl)C=C1F GQUSTSWMNBDBJG-UHFFFAOYSA-N 0.000 claims 1
- 239000000543 intermediate Substances 0.000 abstract description 30
- 244000053095 fungal pathogen Species 0.000 abstract description 2
- 239000000460 chlorine Substances 0.000 description 243
- 229940126208 compound 22 Drugs 0.000 description 141
- WWTBZEKOSBFBEM-SPWPXUSOSA-N (2s)-2-[[2-benzyl-3-[hydroxy-[(1r)-2-phenyl-1-(phenylmethoxycarbonylamino)ethyl]phosphoryl]propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)O)C(=O)C(CP(O)(=O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1C=CC=CC=1)CC1=CC=CC=C1 WWTBZEKOSBFBEM-SPWPXUSOSA-N 0.000 description 139
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 126
- 241000196324 Embryophyta Species 0.000 description 49
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 49
- 239000000243 solution Substances 0.000 description 47
- 230000015572 biosynthetic process Effects 0.000 description 41
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 39
- 238000002360 preparation method Methods 0.000 description 39
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 36
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 34
- 239000011541 reaction mixture Substances 0.000 description 34
- 241000233866 Fungi Species 0.000 description 31
- 238000006243 chemical reaction Methods 0.000 description 31
- 238000009472 formulation Methods 0.000 description 28
- 239000000047 product Substances 0.000 description 28
- 238000003786 synthesis reaction Methods 0.000 description 28
- 125000004182 2-chlorophenyl group Chemical group [H]C1=C([H])C(Cl)=C(*)C([H])=C1[H] 0.000 description 27
- 239000002904 solvent Substances 0.000 description 27
- 235000019441 ethanol Nutrition 0.000 description 26
- XERJKGMBORTKEO-VZUCSPMQSA-N (1e)-2-(ethylcarbamoylamino)-n-methoxy-2-oxoethanimidoyl cyanide Chemical compound CCNC(=O)NC(=O)C(\C#N)=N\OC XERJKGMBORTKEO-VZUCSPMQSA-N 0.000 description 24
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 24
- 238000005160 1H NMR spectroscopy Methods 0.000 description 22
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 22
- AZQWKYJCGOJGHM-UHFFFAOYSA-N 1,4-benzoquinone Chemical compound O=C1C=CC(=O)C=C1 AZQWKYJCGOJGHM-UHFFFAOYSA-N 0.000 description 20
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 20
- 230000012010 growth Effects 0.000 description 20
- 239000003112 inhibitor Substances 0.000 description 20
- 239000004480 active ingredient Substances 0.000 description 19
- 239000012267 brine Substances 0.000 description 19
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 19
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 18
- 229930182558 Sterol Natural products 0.000 description 17
- 235000003702 sterols Nutrition 0.000 description 17
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 16
- 125000001309 chloro group Chemical group Cl* 0.000 description 16
- 150000003432 sterols Chemical class 0.000 description 16
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 16
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 15
- 239000012074 organic phase Substances 0.000 description 15
- KXDAEFPNCMNJSK-UHFFFAOYSA-N Benzamide Chemical compound NC(=O)C1=CC=CC=C1 KXDAEFPNCMNJSK-UHFFFAOYSA-N 0.000 description 14
- 230000009471 action Effects 0.000 description 14
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 14
- 125000003118 aryl group Chemical group 0.000 description 13
- 229910052739 hydrogen Inorganic materials 0.000 description 13
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 12
- 229910052740 iodine Inorganic materials 0.000 description 12
- 239000003921 oil Substances 0.000 description 12
- 235000019198 oils Nutrition 0.000 description 12
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical group C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 11
- 150000001412 amines Chemical class 0.000 description 11
- ZMXDDKWLCZADIW-UHFFFAOYSA-N dimethylformamide Substances CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 11
- 125000005843 halogen group Chemical group 0.000 description 11
- 238000010992 reflux Methods 0.000 description 11
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 10
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 10
- 239000003153 chemical reaction reagent Substances 0.000 description 10
- ACMXQHFNODYQAT-UHFFFAOYSA-N cyflufenamid Chemical compound FC1=CC=C(C(F)(F)F)C(C(NOCC2CC2)=NC(=O)CC=2C=CC=CC=2)=C1F ACMXQHFNODYQAT-UHFFFAOYSA-N 0.000 description 10
- 235000014113 dietary fatty acids Nutrition 0.000 description 10
- 239000000194 fatty acid Substances 0.000 description 10
- 229930195729 fatty acid Natural products 0.000 description 10
- 230000002538 fungal effect Effects 0.000 description 10
- 239000000741 silica gel Substances 0.000 description 10
- 229910002027 silica gel Inorganic materials 0.000 description 10
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 9
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 150000001298 alcohols Chemical class 0.000 description 9
- 238000004440 column chromatography Methods 0.000 description 9
- 230000008099 melanin synthesis Effects 0.000 description 9
- 239000000126 substance Substances 0.000 description 9
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 8
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 8
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 8
- 230000003115 biocidal effect Effects 0.000 description 8
- CRQQGFGUEAVUIL-UHFFFAOYSA-N chlorothalonil Chemical compound ClC1=C(Cl)C(C#N)=C(Cl)C(C#N)=C1Cl CRQQGFGUEAVUIL-UHFFFAOYSA-N 0.000 description 8
- HAORKNGNJCEJBX-UHFFFAOYSA-N cyprodinil Chemical compound N=1C(C)=CC(C2CC2)=NC=1NC1=CC=CC=C1 HAORKNGNJCEJBX-UHFFFAOYSA-N 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
- FBOUIAKEJMZPQG-BLXFFLACSA-N diniconazole-M Chemical compound C1=NC=NN1/C([C@H](O)C(C)(C)C)=C/C1=CC=C(Cl)C=C1Cl FBOUIAKEJMZPQG-BLXFFLACSA-N 0.000 description 8
- 239000012039 electrophile Substances 0.000 description 8
- 230000003647 oxidation Effects 0.000 description 8
- 238000007254 oxidation reaction Methods 0.000 description 8
- 150000003871 sulfonates Chemical class 0.000 description 8
- 235000001508 sulfur Nutrition 0.000 description 8
- 238000011282 treatment Methods 0.000 description 8
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 7
- LSBDFXRDZJMBSC-UHFFFAOYSA-N 2-phenylacetamide Chemical compound NC(=O)CC1=CC=CC=C1 LSBDFXRDZJMBSC-UHFFFAOYSA-N 0.000 description 7
- 239000002253 acid Substances 0.000 description 7
- 125000004429 atom Chemical class 0.000 description 7
- 125000002837 carbocyclic group Chemical group 0.000 description 7
- 150000003857 carboxamides Chemical class 0.000 description 7
- 210000004027 cell Anatomy 0.000 description 7
- 239000004495 emulsifiable concentrate Substances 0.000 description 7
- 239000008187 granular material Substances 0.000 description 7
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 7
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 7
- 239000012299 nitrogen atmosphere Substances 0.000 description 7
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N phenylbenzene Natural products C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 7
- 150000003904 phospholipids Chemical class 0.000 description 7
- 229910000027 potassium carbonate Inorganic materials 0.000 description 7
- NMVCBWZLCXANER-UHFFFAOYSA-N pyriofenone Chemical compound COC1=C(OC)C(OC)=CC(C)=C1C(=O)C1=C(C)C(Cl)=CN=C1OC NMVCBWZLCXANER-UHFFFAOYSA-N 0.000 description 7
- WRPIRSINYZBGPK-UHFFFAOYSA-N quinoxyfen Chemical compound C1=CC(F)=CC=C1OC1=CC=NC2=CC(Cl)=CC(Cl)=C12 WRPIRSINYZBGPK-UHFFFAOYSA-N 0.000 description 7
- 229910000104 sodium hydride Inorganic materials 0.000 description 7
- 238000001228 spectrum Methods 0.000 description 7
- 238000006467 substitution reaction Methods 0.000 description 7
- 239000011593 sulfur Substances 0.000 description 7
- 239000000725 suspension Substances 0.000 description 7
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 7
- 239000008096 xylene Substances 0.000 description 7
- ZMYFCFLJBGAQRS-IRXDYDNUSA-N (2R,3S)-epoxiconazole Chemical compound C1=CC(F)=CC=C1[C@@]1(CN2N=CN=C2)[C@H](C=2C(=CC=CC=2)Cl)O1 ZMYFCFLJBGAQRS-IRXDYDNUSA-N 0.000 description 6
- RYAUSSKQMZRMAI-ALOPSCKCSA-N (2S,6R)-4-[3-(4-tert-butylphenyl)-2-methylpropyl]-2,6-dimethylmorpholine Chemical compound C=1C=C(C(C)(C)C)C=CC=1CC(C)CN1C[C@H](C)O[C@H](C)C1 RYAUSSKQMZRMAI-ALOPSCKCSA-N 0.000 description 6
- MGNFYQILYYYUBS-UHFFFAOYSA-N 1-[3-(4-tert-butylphenyl)-2-methylpropyl]piperidine Chemical compound C=1C=C(C(C)(C)C)C=CC=1CC(C)CN1CCCCC1 MGNFYQILYYYUBS-UHFFFAOYSA-N 0.000 description 6
- DNCYBUMDUBHIJZ-UHFFFAOYSA-N 1h-pyrimidin-6-one Chemical compound O=C1C=CN=CN1 DNCYBUMDUBHIJZ-UHFFFAOYSA-N 0.000 description 6
- UFNOUKDBUJZYDE-UHFFFAOYSA-N 2-(4-chlorophenyl)-3-cyclopropyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol Chemical compound C1=NC=NN1CC(O)(C=1C=CC(Cl)=CC=1)C(C)C1CC1 UFNOUKDBUJZYDE-UHFFFAOYSA-N 0.000 description 6
- PCCSBWNGDMYFCW-UHFFFAOYSA-N 5-methyl-5-(4-phenoxyphenyl)-3-(phenylamino)-1,3-oxazolidine-2,4-dione Chemical compound O=C1C(C)(C=2C=CC(OC=3C=CC=CC=3)=CC=2)OC(=O)N1NC1=CC=CC=C1 PCCSBWNGDMYFCW-UHFFFAOYSA-N 0.000 description 6
- 239000005730 Azoxystrobin Substances 0.000 description 6
- 239000005740 Boscalid Substances 0.000 description 6
- 239000005755 Cyflufenamid Substances 0.000 description 6
- 239000005757 Cyproconazole Substances 0.000 description 6
- 239000005762 Dimoxystrobin Substances 0.000 description 6
- 102000004190 Enzymes Human genes 0.000 description 6
- 108090000790 Enzymes Proteins 0.000 description 6
- 239000005767 Epoxiconazole Substances 0.000 description 6
- 239000005772 Famoxadone Substances 0.000 description 6
- 239000005778 Fenpropimorph Substances 0.000 description 6
- 239000005800 Kresoxim-methyl Substances 0.000 description 6
- 239000005868 Metconazole Substances 0.000 description 6
- 239000005810 Metrafenone Substances 0.000 description 6
- 102000029749 Microtubule Human genes 0.000 description 6
- 108091022875 Microtubule Proteins 0.000 description 6
- 229910004749 OS(O)2 Inorganic materials 0.000 description 6
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 6
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 6
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 6
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 6
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 6
- WFDXOXNFNRHQEC-GHRIWEEISA-N azoxystrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1OC1=CC(OC=2C(=CC=CC=2)C#N)=NC=N1 WFDXOXNFNRHQEC-GHRIWEEISA-N 0.000 description 6
- WYEMLYFITZORAB-UHFFFAOYSA-N boscalid Chemical compound C1=CC(Cl)=CC=C1C1=CC=CC=C1NC(=O)C1=CC=CN=C1Cl WYEMLYFITZORAB-UHFFFAOYSA-N 0.000 description 6
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 6
- WXUZAHCNPWONDH-DYTRJAOYSA-N dimoxystrobin Chemical compound CNC(=O)C(=N\OC)\C1=CC=CC=C1COC1=CC(C)=CC=C1C WXUZAHCNPWONDH-DYTRJAOYSA-N 0.000 description 6
- VMNULHCTRPXWFJ-UJSVPXBISA-N enoxastrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1CO\N=C(/C)\C=C\C1=CC=C(Cl)C=C1 VMNULHCTRPXWFJ-UJSVPXBISA-N 0.000 description 6
- 229940088598 enzyme Drugs 0.000 description 6
- FQKUGOMFVDPBIZ-UHFFFAOYSA-N flusilazole Chemical compound C=1C=C(F)C=CC=1[Si](C=1C=CC(F)=CC=1)(C)CN1C=NC=N1 FQKUGOMFVDPBIZ-UHFFFAOYSA-N 0.000 description 6
- 125000001072 heteroaryl group Chemical group 0.000 description 6
- 239000001257 hydrogen Substances 0.000 description 6
- RONFGUROBZGJKP-UHFFFAOYSA-N iminoctadine Chemical compound NC(N)=NCCCCCCCCNCCCCCCCCN=C(N)N RONFGUROBZGJKP-UHFFFAOYSA-N 0.000 description 6
- 230000002401 inhibitory effect Effects 0.000 description 6
- 230000005764 inhibitory process Effects 0.000 description 6
- WPOOICLZIIBUBM-UHFFFAOYSA-H iron;iron(3+);methyl-dioxido-oxo-$l^{5}-arsane Chemical compound [Fe].[Fe+3].[Fe+3].C[As]([O-])([O-])=O.C[As]([O-])([O-])=O.C[As]([O-])([O-])=O WPOOICLZIIBUBM-UHFFFAOYSA-H 0.000 description 6
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 6
- XWPZUHJBOLQNMN-UHFFFAOYSA-N metconazole Chemical compound C1=NC=NN1CC1(O)C(C)(C)CCC1CC1=CC=C(Cl)C=C1 XWPZUHJBOLQNMN-UHFFFAOYSA-N 0.000 description 6
- HIIRDDUVRXCDBN-OBGWFSINSA-N metominostrobin Chemical compound CNC(=O)C(=N\OC)\C1=CC=CC=C1OC1=CC=CC=C1 HIIRDDUVRXCDBN-OBGWFSINSA-N 0.000 description 6
- AMSPWOYQQAWRRM-UHFFFAOYSA-N metrafenone Chemical compound COC1=CC=C(Br)C(C)=C1C(=O)C1=C(C)C=C(OC)C(OC)=C1OC AMSPWOYQQAWRRM-UHFFFAOYSA-N 0.000 description 6
- 210000004688 microtubule Anatomy 0.000 description 6
- 230000006540 mitochondrial respiration Effects 0.000 description 6
- FLVBXVXXXMLMOX-UHFFFAOYSA-N proquinazid Chemical compound C1=C(I)C=C2C(=O)N(CCC)C(OCCC)=NC2=C1 FLVBXVXXXMLMOX-UHFFFAOYSA-N 0.000 description 6
- 239000012312 sodium hydride Substances 0.000 description 6
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 6
- CXNIUSPIQKWYAI-UHFFFAOYSA-N xantphos Chemical compound C=12OC3=C(P(C=4C=CC=CC=4)C=4C=CC=CC=4)C=CC=C3C(C)(C)C2=CC=CC=1P(C=1C=CC=CC=1)C1=CC=CC=C1 CXNIUSPIQKWYAI-UHFFFAOYSA-N 0.000 description 6
- YLZGKZDEFJIHIJ-UHFFFAOYSA-N (1-methylbenzimidazol-2-yl) carbamate Chemical compound C1=CC=C2N(C)C(OC(N)=O)=NC2=C1 YLZGKZDEFJIHIJ-UHFFFAOYSA-N 0.000 description 5
- PPDBOQMNKNNODG-NTEUORMPSA-N (5E)-5-(4-chlorobenzylidene)-2,2-dimethyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentanol Chemical compound C1=NC=NN1CC1(O)C(C)(C)CC\C1=C/C1=CC=C(Cl)C=C1 PPDBOQMNKNNODG-NTEUORMPSA-N 0.000 description 5
- XBYRMPXUBGMOJC-UHFFFAOYSA-N 1,2-dihydropyrazol-3-one Chemical class OC=1C=CNN=1 XBYRMPXUBGMOJC-UHFFFAOYSA-N 0.000 description 5
- PXMNMQRDXWABCY-UHFFFAOYSA-N 1-(4-chlorophenyl)-4,4-dimethyl-3-(1H-1,2,4-triazol-1-ylmethyl)pentan-3-ol Chemical compound C1=NC=NN1CC(O)(C(C)(C)C)CCC1=CC=C(Cl)C=C1 PXMNMQRDXWABCY-UHFFFAOYSA-N 0.000 description 5
- JVVRJMXHNUAPHW-UHFFFAOYSA-N 1h-pyrazol-5-amine Chemical class NC=1C=CNN=1 JVVRJMXHNUAPHW-UHFFFAOYSA-N 0.000 description 5
- STMIIPIFODONDC-UHFFFAOYSA-N 2-(2,4-dichlorophenyl)-1-(1H-1,2,4-triazol-1-yl)hexan-2-ol Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(O)(CCCC)CN1C=NC=N1 STMIIPIFODONDC-UHFFFAOYSA-N 0.000 description 5
- MNHVNIJQQRJYDH-UHFFFAOYSA-N 2-[2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl]-1,2-dihydro-1,2,4-triazole-3-thione Chemical compound N1=CNC(=S)N1CC(C1(Cl)CC1)(O)CC1=CC=CC=C1Cl MNHVNIJQQRJYDH-UHFFFAOYSA-N 0.000 description 5
- WNZQDUSMALZDQF-UHFFFAOYSA-N 2-benzofuran-1(3H)-one Chemical compound C1=CC=C2C(=O)OCC2=C1 WNZQDUSMALZDQF-UHFFFAOYSA-N 0.000 description 5
- SOUGWDPPRBKJEX-UHFFFAOYSA-N 3,5-dichloro-N-(1-chloro-3-methyl-2-oxopentan-3-yl)-4-methylbenzamide Chemical compound ClCC(=O)C(C)(CC)NC(=O)C1=CC(Cl)=C(C)C(Cl)=C1 SOUGWDPPRBKJEX-UHFFFAOYSA-N 0.000 description 5
- RQDJADAKIFFEKQ-UHFFFAOYSA-N 4-(4-chlorophenyl)-2-phenyl-2-(1,2,4-triazol-1-ylmethyl)butanenitrile Chemical compound C1=CC(Cl)=CC=C1CCC(C=1C=CC=CC=1)(C#N)CN1N=CN=C1 RQDJADAKIFFEKQ-UHFFFAOYSA-N 0.000 description 5
- YEJRWHAVMIAJKC-UHFFFAOYSA-N 4-Butyrolactone Chemical compound O=C1CCCO1 YEJRWHAVMIAJKC-UHFFFAOYSA-N 0.000 description 5
- 239000005964 Acibenzolar-S-methyl Substances 0.000 description 5
- 239000005741 Bromuconazole Substances 0.000 description 5
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 5
- TWFZGCMQGLPBSX-UHFFFAOYSA-N Carbendazim Natural products C1=CC=C2NC(NC(=O)OC)=NC2=C1 TWFZGCMQGLPBSX-UHFFFAOYSA-N 0.000 description 5
- 239000005747 Chlorothalonil Substances 0.000 description 5
- 239000005760 Difenoconazole Substances 0.000 description 5
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 5
- 239000005775 Fenbuconazole Substances 0.000 description 5
- 239000005788 Fluxapyroxad Substances 0.000 description 5
- 101000615488 Homo sapiens Methyl-CpG-binding domain protein 2 Proteins 0.000 description 5
- 239000005796 Ipconazole Substances 0.000 description 5
- 102100021299 Methyl-CpG-binding domain protein 2 Human genes 0.000 description 5
- 239000005818 Picoxystrobin Substances 0.000 description 5
- 239000005822 Propiconazole Substances 0.000 description 5
- 239000005824 Proquinazid Substances 0.000 description 5
- 239000005825 Prothioconazole Substances 0.000 description 5
- 239000005869 Pyraclostrobin Substances 0.000 description 5
- 239000005829 Pyriofenone Substances 0.000 description 5
- 239000005831 Quinoxyfen Substances 0.000 description 5
- 239000005839 Tebuconazole Substances 0.000 description 5
- 239000005857 Trifloxystrobin Substances 0.000 description 5
- 239000005859 Triticonazole Substances 0.000 description 5
- 239000008346 aqueous phase Substances 0.000 description 5
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 5
- 239000004305 biphenyl Substances 0.000 description 5
- 229910052796 boron Inorganic materials 0.000 description 5
- 229940118790 boscalid Drugs 0.000 description 5
- HJJVPARKXDDIQD-UHFFFAOYSA-N bromuconazole Chemical compound ClC1=CC(Cl)=CC=C1C1(CN2N=CN=C2)OCC(Br)C1 HJJVPARKXDDIQD-UHFFFAOYSA-N 0.000 description 5
- DKVNPHBNOWQYFE-UHFFFAOYSA-N carbamodithioic acid Chemical compound NC(S)=S DKVNPHBNOWQYFE-UHFFFAOYSA-N 0.000 description 5
- JNPZQRQPIHJYNM-UHFFFAOYSA-N carbendazim Chemical compound C1=C[CH]C2=NC(NC(=O)OC)=NC2=C1 JNPZQRQPIHJYNM-UHFFFAOYSA-N 0.000 description 5
- 239000006013 carbendazim Substances 0.000 description 5
- 239000003054 catalyst Substances 0.000 description 5
- 210000002421 cell wall Anatomy 0.000 description 5
- 230000001276 controlling effect Effects 0.000 description 5
- BQYJATMQXGBDHF-UHFFFAOYSA-N difenoconazole Chemical compound O1C(C)COC1(C=1C(=CC(OC=2C=CC(Cl)=CC=2)=CC=1)Cl)CN1N=CN=C1 BQYJATMQXGBDHF-UHFFFAOYSA-N 0.000 description 5
- 239000012990 dithiocarbamate Substances 0.000 description 5
- 235000013399 edible fruits Nutrition 0.000 description 5
- 150000002148 esters Chemical class 0.000 description 5
- 239000000284 extract Substances 0.000 description 5
- 150000004665 fatty acids Chemical class 0.000 description 5
- SXSGXWCSHSVPGB-UHFFFAOYSA-N fluxapyroxad Chemical compound FC(F)C1=NN(C)C=C1C(=O)NC1=CC=CC=C1C1=CC(F)=C(F)C(F)=C1 SXSGXWCSHSVPGB-UHFFFAOYSA-N 0.000 description 5
- 239000002917 insecticide Substances 0.000 description 5
- QTYCMDBMOLSEAM-UHFFFAOYSA-N ipconazole Chemical compound C1=NC=NN1CC1(O)C(C(C)C)CCC1CC1=CC=C(Cl)C=C1 QTYCMDBMOLSEAM-UHFFFAOYSA-N 0.000 description 5
- ZOTBXTZVPHCKPN-HTXNQAPBSA-N kresoxim-methyl Chemical compound CO\N=C(\C(=O)OC)C1=CC=CC=C1COC1=CC=CC=C1C ZOTBXTZVPHCKPN-HTXNQAPBSA-N 0.000 description 5
- CFHGBZLNZZVTAY-UHFFFAOYSA-N lawesson's reagent Chemical compound C1=CC(OC)=CC=C1P1(=S)SP(=S)(C=2C=CC(OC)=CC=2)S1 CFHGBZLNZZVTAY-UHFFFAOYSA-N 0.000 description 5
- 239000008188 pellet Substances 0.000 description 5
- 229910052698 phosphorus Inorganic materials 0.000 description 5
- IBSNKSODLGJUMQ-SDNWHVSQSA-N picoxystrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1COC1=CC=CC(C(F)(F)F)=N1 IBSNKSODLGJUMQ-SDNWHVSQSA-N 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- STJLVHWMYQXCPB-UHFFFAOYSA-N propiconazole Chemical compound O1C(CCC)COC1(C=1C(=CC(Cl)=CC=1)Cl)CN1N=CN=C1 STJLVHWMYQXCPB-UHFFFAOYSA-N 0.000 description 5
- 125000006239 protecting group Chemical group 0.000 description 5
- HZRSNVGNWUDEFX-UHFFFAOYSA-N pyraclostrobin Chemical compound COC(=O)N(OC)C1=CC=CC=C1COC1=NN(C=2C=CC(Cl)=CC=2)C=C1 HZRSNVGNWUDEFX-UHFFFAOYSA-N 0.000 description 5
- 150000003254 radicals Chemical class 0.000 description 5
- 238000006722 reduction reaction Methods 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- 230000029058 respiratory gaseous exchange Effects 0.000 description 5
- AKEJUJNQAAGONA-UHFFFAOYSA-N sulfur trioxide Chemical group O=S(=O)=O AKEJUJNQAAGONA-UHFFFAOYSA-N 0.000 description 5
- 125000001544 thienyl group Chemical group 0.000 description 5
- 230000014616 translation Effects 0.000 description 5
- ONCZDRURRATYFI-TVJDWZFNSA-N trifloxystrobin Chemical compound CO\N=C(\C(=O)OC)C1=CC=CC=C1CO\N=C(/C)C1=CC=CC(C(F)(F)F)=C1 ONCZDRURRATYFI-TVJDWZFNSA-N 0.000 description 5
- NHOWDZOIZKMVAI-UHFFFAOYSA-N (2-chlorophenyl)(4-chlorophenyl)pyrimidin-5-ylmethanol Chemical compound C=1N=CN=CC=1C(C=1C(=CC=CC=1)Cl)(O)C1=CC=C(Cl)C=C1 NHOWDZOIZKMVAI-UHFFFAOYSA-N 0.000 description 4
- SAPGTCDSBGMXCD-UHFFFAOYSA-N (2-chlorophenyl)-(4-fluorophenyl)-pyrimidin-5-ylmethanol Chemical compound C=1N=CN=CC=1C(C=1C(=CC=CC=1)Cl)(O)C1=CC=C(F)C=C1 SAPGTCDSBGMXCD-UHFFFAOYSA-N 0.000 description 4
- LDVVMCZRFWMZSG-OLQVQODUSA-N (3ar,7as)-2-(trichloromethylsulfanyl)-3a,4,7,7a-tetrahydroisoindole-1,3-dione Chemical compound C1C=CC[C@H]2C(=O)N(SC(Cl)(Cl)Cl)C(=O)[C@H]21 LDVVMCZRFWMZSG-OLQVQODUSA-N 0.000 description 4
- QNBTYORWCCMPQP-JXAWBTAJSA-N (Z)-dimethomorph Chemical compound C1=C(OC)C(OC)=CC=C1C(\C=1C=CC(Cl)=CC=1)=C/C(=O)N1CCOCC1 QNBTYORWCCMPQP-JXAWBTAJSA-N 0.000 description 4
- QWEWLLNSJDTOKH-UHFFFAOYSA-N 1,3-thiazole-2-carboxamide Chemical compound NC(=O)C1=NC=CS1 QWEWLLNSJDTOKH-UHFFFAOYSA-N 0.000 description 4
- JWUCHKBSVLQQCO-UHFFFAOYSA-N 1-(2-fluorophenyl)-1-(4-fluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanol Chemical compound C=1C=C(F)C=CC=1C(C=1C(=CC=CC=1)F)(O)CN1C=NC=N1 JWUCHKBSVLQQCO-UHFFFAOYSA-N 0.000 description 4
- LUBJCRLGQSPQNN-UHFFFAOYSA-N 1-Phenylurea Chemical compound NC(=O)NC1=CC=CC=C1 LUBJCRLGQSPQNN-UHFFFAOYSA-N 0.000 description 4
- WKBPZYKAUNRMKP-UHFFFAOYSA-N 1-[2-(2,4-dichlorophenyl)pentyl]1,2,4-triazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(CCC)CN1C=NC=N1 WKBPZYKAUNRMKP-UHFFFAOYSA-N 0.000 description 4
- PZBPKYOVPCNPJY-UHFFFAOYSA-N 1-[2-(allyloxy)-2-(2,4-dichlorophenyl)ethyl]imidazole Chemical compound ClC1=CC(Cl)=CC=C1C(OCC=C)CN1C=NC=C1 PZBPKYOVPCNPJY-UHFFFAOYSA-N 0.000 description 4
- PFFIDZXUXFLSSR-UHFFFAOYSA-N 1-methyl-N-[2-(4-methylpentan-2-yl)-3-thienyl]-3-(trifluoromethyl)pyrazole-4-carboxamide Chemical compound S1C=CC(NC(=O)C=2C(=NN(C)C=2)C(F)(F)F)=C1C(C)CC(C)C PFFIDZXUXFLSSR-UHFFFAOYSA-N 0.000 description 4
- HZJKXKUJVSEEFU-UHFFFAOYSA-N 2-(4-chlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)hexanenitrile Chemical compound C=1C=C(Cl)C=CC=1C(CCCC)(C#N)CN1C=NC=N1 HZJKXKUJVSEEFU-UHFFFAOYSA-N 0.000 description 4
- KWLVWJPJKJMCSH-UHFFFAOYSA-N 2-(4-chlorophenyl)-N-{2-[3-methoxy-4-(prop-2-yn-1-yloxy)phenyl]ethyl}-2-(prop-2-yn-1-yloxy)acetamide Chemical compound C1=C(OCC#C)C(OC)=CC(CCNC(=O)C(OCC#C)C=2C=CC(Cl)=CC=2)=C1 KWLVWJPJKJMCSH-UHFFFAOYSA-N 0.000 description 4
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 4
- SBUKOHLFHYSZNG-UHFFFAOYSA-N 4-dodecyl-2,6-dimethylmorpholine Chemical compound CCCCCCCCCCCCN1CC(C)OC(C)C1 SBUKOHLFHYSZNG-UHFFFAOYSA-N 0.000 description 4
- NRTLIYOWLVMQBO-UHFFFAOYSA-N 5-chloro-1,3-dimethyl-N-(1,1,3-trimethyl-1,3-dihydro-2-benzofuran-4-yl)pyrazole-4-carboxamide Chemical compound C=12C(C)OC(C)(C)C2=CC=CC=1NC(=O)C=1C(C)=NN(C)C=1Cl NRTLIYOWLVMQBO-UHFFFAOYSA-N 0.000 description 4
- GOFJDXZZHFNFLV-UHFFFAOYSA-N 5-fluoro-1,3-dimethyl-N-[2-(4-methylpentan-2-yl)phenyl]pyrazole-4-carboxamide Chemical compound CC(C)CC(C)C1=CC=CC=C1NC(=O)C1=C(F)N(C)N=C1C GOFJDXZZHFNFLV-UHFFFAOYSA-N 0.000 description 4
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 4
- 239000005734 Benalaxyl Substances 0.000 description 4
- 239000005739 Bordeaux mixture Substances 0.000 description 4
- 239000005745 Captan Substances 0.000 description 4
- 239000005746 Carboxin Substances 0.000 description 4
- JJLJMEJHUUYSSY-UHFFFAOYSA-L Copper hydroxide Chemical compound [OH-].[OH-].[Cu+2] JJLJMEJHUUYSSY-UHFFFAOYSA-L 0.000 description 4
- 239000005750 Copper hydroxide Substances 0.000 description 4
- 239000005752 Copper oxychloride Substances 0.000 description 4
- 239000005754 Cyazofamid Substances 0.000 description 4
- 239000005756 Cymoxanil Substances 0.000 description 4
- 239000005758 Cyprodinil Substances 0.000 description 4
- 239000005761 Dimethomorph Substances 0.000 description 4
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 4
- 239000005765 Dodemorph Substances 0.000 description 4
- 239000005769 Etridiazole Substances 0.000 description 4
- 239000005774 Fenamidone Substances 0.000 description 4
- 239000005777 Fenpropidin Substances 0.000 description 4
- 239000005780 Fluazinam Substances 0.000 description 4
- 239000005782 Fluopicolide Substances 0.000 description 4
- 239000005784 Fluoxastrobin Substances 0.000 description 4
- 239000005785 Fluquinconazole Substances 0.000 description 4
- 239000005786 Flutolanil Substances 0.000 description 4
- 239000005787 Flutriafol Substances 0.000 description 4
- 239000005789 Folpet Substances 0.000 description 4
- ZRALSGWEFCBTJO-UHFFFAOYSA-N Guanidine Chemical compound NC(N)=N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- 239000005795 Imazalil Substances 0.000 description 4
- 239000005867 Iprodione Substances 0.000 description 4
- 239000005802 Mancozeb Substances 0.000 description 4
- 239000005804 Mandipropamid Substances 0.000 description 4
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 4
- 239000005807 Metalaxyl Substances 0.000 description 4
- 239000005811 Myclobutanil Substances 0.000 description 4
- MZRVEZGGRBJDDB-UHFFFAOYSA-N N-Butyllithium Chemical compound [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 4
- 102000004316 Oxidoreductases Human genes 0.000 description 4
- 108090000854 Oxidoreductases Proteins 0.000 description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 239000005813 Penconazole Substances 0.000 description 4
- 239000005815 Penflufen Substances 0.000 description 4
- 239000005816 Penthiopyrad Substances 0.000 description 4
- 229930182764 Polyoxin Natural products 0.000 description 4
- 239000005820 Prochloraz Substances 0.000 description 4
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 4
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 4
- 239000005848 Tribasic copper sulfate Substances 0.000 description 4
- 239000005860 Valifenalate Substances 0.000 description 4
- UELITFHSCLAHKR-UHFFFAOYSA-N acibenzolar-S-methyl Chemical group CSC(=O)C1=CC=CC2=C1SN=N2 UELITFHSCLAHKR-UHFFFAOYSA-N 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- AKNQMEBLVAMSNZ-UHFFFAOYSA-N azaconazole Chemical compound ClC1=CC(Cl)=CC=C1C1(CN2N=CN=C2)OCCO1 AKNQMEBLVAMSNZ-UHFFFAOYSA-N 0.000 description 4
- 229950000294 azaconazole Drugs 0.000 description 4
- RIOXQFHNBCKOKP-UHFFFAOYSA-N benomyl Chemical compound C1=CC=C2N(C(=O)NCCCC)C(NC(=O)OC)=NC2=C1 RIOXQFHNBCKOKP-UHFFFAOYSA-N 0.000 description 4
- MITFXPHMIHQXPI-UHFFFAOYSA-N benzoxaprofen Natural products N=1C2=CC(C(C(O)=O)C)=CC=C2OC=1C1=CC=C(Cl)C=C1 MITFXPHMIHQXPI-UHFFFAOYSA-N 0.000 description 4
- 235000010290 biphenyl Nutrition 0.000 description 4
- YNHIGQDRGKUECZ-UHFFFAOYSA-L bis(triphenylphosphine)palladium(ii) dichloride Chemical compound [Cl-].[Cl-].[Pd+2].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-L 0.000 description 4
- OIPMQULDKWSNGX-UHFFFAOYSA-N bis[[ethoxy(oxo)phosphaniumyl]oxy]alumanyloxy-ethoxy-oxophosphanium Chemical compound [Al+3].CCO[P+]([O-])=O.CCO[P+]([O-])=O.CCO[P+]([O-])=O OIPMQULDKWSNGX-UHFFFAOYSA-N 0.000 description 4
- 238000009835 boiling Methods 0.000 description 4
- 229940117949 captan Drugs 0.000 description 4
- GYSSRZJIHXQEHQ-UHFFFAOYSA-N carboxin Chemical compound S1CCOC(C)=C1C(=O)NC1=CC=CC=C1 GYSSRZJIHXQEHQ-UHFFFAOYSA-N 0.000 description 4
- 230000032823 cell division Effects 0.000 description 4
- HKMOPYJWSFRURD-UHFFFAOYSA-N chloro hypochlorite;copper Chemical compound [Cu].ClOCl HKMOPYJWSFRURD-UHFFFAOYSA-N 0.000 description 4
- VNFPBHJOKIVQEB-UHFFFAOYSA-N clotrimazole Chemical compound ClC1=CC=CC=C1C(N1C=NC=C1)(C=1C=CC=CC=1)C1=CC=CC=C1 VNFPBHJOKIVQEB-UHFFFAOYSA-N 0.000 description 4
- 229960004022 clotrimazole Drugs 0.000 description 4
- 239000013256 coordination polymer Substances 0.000 description 4
- 150000001879 copper Chemical class 0.000 description 4
- 229910001956 copper hydroxide Inorganic materials 0.000 description 4
- QTMDXZNDVAMKGV-UHFFFAOYSA-L copper(ii) bromide Chemical compound [Cu+2].[Br-].[Br-] QTMDXZNDVAMKGV-UHFFFAOYSA-L 0.000 description 4
- 238000006880 cross-coupling reaction Methods 0.000 description 4
- YXKMMRDKEKCERS-UHFFFAOYSA-N cyazofamid Chemical compound CN(C)S(=O)(=O)N1C(C#N)=NC(Cl)=C1C1=CC=C(C)C=C1 YXKMMRDKEKCERS-UHFFFAOYSA-N 0.000 description 4
- 230000034994 death Effects 0.000 description 4
- SWXVUIWOUIDPGS-UHFFFAOYSA-N diacetone alcohol Chemical compound CC(=O)CC(C)(C)O SWXVUIWOUIDPGS-UHFFFAOYSA-N 0.000 description 4
- JMXKCYUTURMERF-UHFFFAOYSA-N dodemorph Chemical compound C1C(C)OC(C)CN1C1CCCCCCCCCCC1 JMXKCYUTURMERF-UHFFFAOYSA-N 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 229960002125 enilconazole Drugs 0.000 description 4
- 230000000967 entomopathogenic effect Effects 0.000 description 4
- IGUYEXXAGBDLLX-UHFFFAOYSA-N ethyl 3-(3,5-dichlorophenyl)-5-methyl-2,4-dioxo-1,3-oxazolidine-5-carboxylate Chemical compound O=C1C(C(=O)OCC)(C)OC(=O)N1C1=CC(Cl)=CC(Cl)=C1 IGUYEXXAGBDLLX-UHFFFAOYSA-N 0.000 description 4
- KQTVWCSONPJJPE-UHFFFAOYSA-N etridiazole Chemical compound CCOC1=NC(C(Cl)(Cl)Cl)=NS1 KQTVWCSONPJJPE-UHFFFAOYSA-N 0.000 description 4
- LMVPQMGRYSRMIW-KRWDZBQOSA-N fenamidone Chemical compound O=C([C@@](C)(N=C1SC)C=2C=CC=CC=2)N1NC1=CC=CC=C1 LMVPQMGRYSRMIW-KRWDZBQOSA-N 0.000 description 4
- JFSPBVWPKOEZCB-UHFFFAOYSA-N fenfuram Chemical compound O1C=CC(C(=O)NC=2C=CC=CC=2)=C1C JFSPBVWPKOEZCB-UHFFFAOYSA-N 0.000 description 4
- UTOHZQYBSYOOGC-UHFFFAOYSA-N fenpyrazamine Chemical compound O=C1N(C(C)C)N(C(=O)SCC=C)C(N)=C1C1=CC=CC=C1C UTOHZQYBSYOOGC-UHFFFAOYSA-N 0.000 description 4
- UZCGKGPEKUCDTF-UHFFFAOYSA-N fluazinam Chemical compound [O-][N+](=O)C1=CC(C(F)(F)F)=C(Cl)C([N+]([O-])=O)=C1NC1=NC=C(C(F)(F)F)C=C1Cl UZCGKGPEKUCDTF-UHFFFAOYSA-N 0.000 description 4
- GBOYJIHYACSLGN-UHFFFAOYSA-N fluopicolide Chemical compound ClC1=CC(C(F)(F)F)=CN=C1CNC(=O)C1=C(Cl)C=CC=C1Cl GBOYJIHYACSLGN-UHFFFAOYSA-N 0.000 description 4
- UFEODZBUAFNAEU-NLRVBDNBSA-N fluoxastrobin Chemical compound C=1C=CC=C(OC=2C(=C(OC=3C(=CC=CC=3)Cl)N=CN=2)F)C=1C(=N/OC)\C1=NOCCO1 UFEODZBUAFNAEU-NLRVBDNBSA-N 0.000 description 4
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 description 4
- PTCGDEVVHUXTMP-UHFFFAOYSA-N flutolanil Chemical compound CC(C)OC1=CC=CC(NC(=O)C=2C(=CC=CC=2)C(F)(F)F)=C1 PTCGDEVVHUXTMP-UHFFFAOYSA-N 0.000 description 4
- HKIOYBQGHSTUDB-UHFFFAOYSA-N folpet Chemical compound C1=CC=C2C(=O)N(SC(Cl)(Cl)Cl)C(=O)C2=C1 HKIOYBQGHSTUDB-UHFFFAOYSA-N 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 125000005640 glucopyranosyl group Chemical group 0.000 description 4
- 150000002314 glycerols Chemical class 0.000 description 4
- 239000004009 herbicide Substances 0.000 description 4
- AGKSTYPVMZODRV-UHFFFAOYSA-N imibenconazole Chemical compound C1=CC(Cl)=CC=C1CSC(CN1N=CN=C1)=NC1=CC=C(Cl)C=C1Cl AGKSTYPVMZODRV-UHFFFAOYSA-N 0.000 description 4
- 150000002460 imidazoles Chemical class 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- ONUFESLQCSAYKA-UHFFFAOYSA-N iprodione Chemical compound O=C1N(C(=O)NC(C)C)CC(=O)N1C1=CC(Cl)=CC(Cl)=C1 ONUFESLQCSAYKA-UHFFFAOYSA-N 0.000 description 4
- 239000003446 ligand Substances 0.000 description 4
- YKSNLCVSTHTHJA-UHFFFAOYSA-L maneb Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S YKSNLCVSTHTHJA-UHFFFAOYSA-L 0.000 description 4
- 229920000940 maneb Polymers 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- BCTQJXQXJVLSIG-UHFFFAOYSA-N mepronil Chemical compound CC(C)OC1=CC=CC(NC(=O)C=2C(=CC=CC=2)C)=C1 BCTQJXQXJVLSIG-UHFFFAOYSA-N 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- ZQEIXNIJLIKNTD-GFCCVEGCSA-N metalaxyl-M Chemical compound COCC(=O)N([C@H](C)C(=O)OC)C1=C(C)C=CC=C1C ZQEIXNIJLIKNTD-GFCCVEGCSA-N 0.000 description 4
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Natural products C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 4
- ZQEIXNIJLIKNTD-UHFFFAOYSA-N methyl N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alaninate Chemical compound COCC(=O)N(C(C)C(=O)OC)C1=C(C)C=CC=C1C ZQEIXNIJLIKNTD-UHFFFAOYSA-N 0.000 description 4
- CJPQIRJHIZUAQP-UHFFFAOYSA-N methyl N-(2,6-dimethylphenyl)-N-(phenylacetyl)alaninate Chemical compound CC=1C=CC=C(C)C=1N(C(C)C(=O)OC)C(=O)CC1=CC=CC=C1 CJPQIRJHIZUAQP-UHFFFAOYSA-N 0.000 description 4
- CIEXPHRYOLIQQD-UHFFFAOYSA-N methyl N-(2,6-dimethylphenyl)-N-2-furoylalaninate Chemical compound CC=1C=CC=C(C)C=1N(C(C)C(=O)OC)C(=O)C1=CC=CO1 CIEXPHRYOLIQQD-UHFFFAOYSA-N 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- HDZGCSFEDULWCS-UHFFFAOYSA-N monomethylhydrazine Chemical compound CNN HDZGCSFEDULWCS-UHFFFAOYSA-N 0.000 description 4
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 4
- 239000002736 nonionic surfactant Substances 0.000 description 4
- JHIPUJPTQJYEQK-ZLHHXESBSA-N orysastrobin Chemical compound CNC(=O)C(=N\OC)\C1=CC=CC=C1CO\N=C(/C)\C(=N\OC)\C(\C)=N\OC JHIPUJPTQJYEQK-ZLHHXESBSA-N 0.000 description 4
- UWVQIROCRJWDKL-UHFFFAOYSA-N oxadixyl Chemical compound CC=1C=CC=C(C)C=1N(C(=O)COC)N1CCOC1=O UWVQIROCRJWDKL-UHFFFAOYSA-N 0.000 description 4
- 239000007800 oxidant agent Substances 0.000 description 4
- 230000010627 oxidative phosphorylation Effects 0.000 description 4
- AMEKQAFGQBKLKX-UHFFFAOYSA-N oxycarboxin Chemical compound O=S1(=O)CCOC(C)=C1C(=O)NC1=CC=CC=C1 AMEKQAFGQBKLKX-UHFFFAOYSA-N 0.000 description 4
- 239000000575 pesticide Substances 0.000 description 4
- 150000003014 phosphoric acid esters Chemical class 0.000 description 4
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical compound C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 description 4
- 150000004885 piperazines Chemical class 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- YEBIHIICWDDQOL-YBHNRIQQSA-N polyoxin Polymers O[C@@H]1[C@H](O)[C@@H](C(C=O)N)O[C@H]1N1C(=O)NC(=O)C(C(O)=O)=C1 YEBIHIICWDDQOL-YBHNRIQQSA-N 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- TVLSRXXIMLFWEO-UHFFFAOYSA-N prochloraz Chemical compound C1=CN=CN1C(=O)N(CCC)CCOC1=C(Cl)C=C(Cl)C=C1Cl TVLSRXXIMLFWEO-UHFFFAOYSA-N 0.000 description 4
- DWTVBEZBWMDXIY-UHFFFAOYSA-N pyrametostrobin Chemical compound COC(=O)N(OC)C1=CC=CC=C1COC1=C(C)C(C=2C=CC=CC=2)=NN1C DWTVBEZBWMDXIY-UHFFFAOYSA-N 0.000 description 4
- URXNNPCNKVAQRA-XMHGGMMESA-N pyraoxystrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1COC1=CC(C=2C=CC(Cl)=CC=2)=NN1C URXNNPCNKVAQRA-XMHGGMMESA-N 0.000 description 4
- 150000003222 pyridines Chemical class 0.000 description 4
- 150000003230 pyrimidines Chemical class 0.000 description 4
- QJBZDBLBQWFTPZ-UHFFFAOYSA-N pyrrolnitrin Chemical compound [O-][N+](=O)C1=C(Cl)C=CC=C1C1=CNC=C1Cl QJBZDBLBQWFTPZ-UHFFFAOYSA-N 0.000 description 4
- FBQQHUGEACOBDN-UHFFFAOYSA-N quinomethionate Chemical compound N1=C2SC(=O)SC2=NC2=CC(C)=CC=C21 FBQQHUGEACOBDN-UHFFFAOYSA-N 0.000 description 4
- 239000007921 spray Substances 0.000 description 4
- 150000003457 sulfones Chemical class 0.000 description 4
- 150000003462 sulfoxides Chemical class 0.000 description 4
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 4
- LITQZINTSYBKIU-UHFFFAOYSA-F tetracopper;hexahydroxide;sulfate Chemical compound [OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[Cu+2].[Cu+2].[Cu+2].[Cu+2].[O-]S([O-])(=O)=O LITQZINTSYBKIU-UHFFFAOYSA-F 0.000 description 4
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 4
- 150000003852 triazoles Chemical class 0.000 description 4
- DBXFMOWZRXXBRN-LWKPJOBUSA-N valifenalate Chemical compound CC(C)OC(=O)N[C@@H](C(C)C)C(=O)NC(CC(=O)OC)C1=CC=C(Cl)C=C1 DBXFMOWZRXXBRN-LWKPJOBUSA-N 0.000 description 4
- 150000003738 xylenes Chemical class 0.000 description 4
- FJBGIXKIXPUXBY-UHFFFAOYSA-N {2-[3-(4-chlorophenyl)propyl]-2,4,4-trimethyl-1,3-oxazolidin-3-yl}(imidazol-1-yl)methanone Chemical compound C1=CN=CN1C(=O)N1C(C)(C)COC1(C)CCCC1=CC=C(Cl)C=C1 FJBGIXKIXPUXBY-UHFFFAOYSA-N 0.000 description 4
- YNWVFADWVLCOPU-MDWZMJQESA-N (1E)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pent-1-en-3-ol Chemical compound C1=NC=NN1/C(C(O)C(C)(C)C)=C/C1=CC=C(Cl)C=C1 YNWVFADWVLCOPU-MDWZMJQESA-N 0.000 description 3
- OILXMJHPFNGGTO-UHFFFAOYSA-N (22E)-(24xi)-24-methylcholesta-5,22-dien-3beta-ol Natural products C1C=C2CC(O)CCC2(C)C2C1C1CCC(C(C)C=CC(C)C(C)C)C1(C)CC2 OILXMJHPFNGGTO-UHFFFAOYSA-N 0.000 description 3
- RQOCXCFLRBRBCS-UHFFFAOYSA-N (22E)-cholesta-5,7,22-trien-3beta-ol Natural products C1C(O)CCC2(C)C(CCC3(C(C(C)C=CCC(C)C)CCC33)C)C3=CC=C21 RQOCXCFLRBRBCS-UHFFFAOYSA-N 0.000 description 3
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Polymers OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 3
- CXNPLSGKWMLZPZ-GIFSMMMISA-N (2r,3r,6s)-3-[[(3s)-3-amino-5-[carbamimidoyl(methyl)amino]pentanoyl]amino]-6-(4-amino-2-oxopyrimidin-1-yl)-3,6-dihydro-2h-pyran-2-carboxylic acid Chemical compound O1[C@@H](C(O)=O)[C@H](NC(=O)C[C@@H](N)CCN(C)C(N)=N)C=C[C@H]1N1C(=O)N=C(N)C=C1 CXNPLSGKWMLZPZ-GIFSMMMISA-N 0.000 description 3
- CKPCAYZTYMHQEX-NBVRZTHBSA-N (e)-1-(2,4-dichlorophenyl)-n-methoxy-2-pyridin-3-ylethanimine Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(=N/OC)/CC1=CC=CN=C1 CKPCAYZTYMHQEX-NBVRZTHBSA-N 0.000 description 3
- WURBVZBTWMNKQT-UHFFFAOYSA-N 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-one Chemical compound C1=NC=NN1C(C(=O)C(C)(C)C)OC1=CC=C(Cl)C=C1 WURBVZBTWMNKQT-UHFFFAOYSA-N 0.000 description 3
- VGPIBGGRCVEHQZ-UHFFFAOYSA-N 1-(biphenyl-4-yloxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-ol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)OC(C=C1)=CC=C1C1=CC=CC=C1 VGPIBGGRCVEHQZ-UHFFFAOYSA-N 0.000 description 3
- LQDARGUHUSPFNL-UHFFFAOYSA-N 1-[2-(2,4-dichlorophenyl)-3-(1,1,2,2-tetrafluoroethoxy)propyl]1,2,4-triazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(COC(F)(F)C(F)F)CN1C=NC=N1 LQDARGUHUSPFNL-UHFFFAOYSA-N 0.000 description 3
- YIKWKLYQRFRGPM-UHFFFAOYSA-N 1-dodecylguanidine acetate Chemical compound CC(O)=O.CCCCCCCCCCCCN=C(N)N YIKWKLYQRFRGPM-UHFFFAOYSA-N 0.000 description 3
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 3
- NIOPZPCMRQGZCE-WEVVVXLNSA-N 2,4-dinitro-6-(octan-2-yl)phenyl (E)-but-2-enoate Chemical compound CCCCCCC(C)C1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1OC(=O)\C=C\C NIOPZPCMRQGZCE-WEVVVXLNSA-N 0.000 description 3
- YTOPFCCWCSOHFV-UHFFFAOYSA-N 2,6-dimethyl-4-tridecylmorpholine Chemical compound CCCCCCCCCCCCCN1CC(C)OC(C)C1 YTOPFCCWCSOHFV-UHFFFAOYSA-N 0.000 description 3
- YABFPHSQTSFWQB-UHFFFAOYSA-N 2-(4-fluorophenyl)-1-(1,2,4-triazol-1-yl)-3-(trimethylsilyl)propan-2-ol Chemical compound C=1C=C(F)C=CC=1C(O)(C[Si](C)(C)C)CN1C=NC=N1 YABFPHSQTSFWQB-UHFFFAOYSA-N 0.000 description 3
- OWDLFBLNMPCXSD-UHFFFAOYSA-N 2-chloro-N-(2,6-dimethylphenyl)-N-(2-oxotetrahydrofuran-3-yl)acetamide Chemical compound CC1=CC=CC(C)=C1N(C(=O)CCl)C1C(=O)OCC1 OWDLFBLNMPCXSD-UHFFFAOYSA-N 0.000 description 3
- 229940044120 2-n-octyl-4-isothiazolin-3-one Drugs 0.000 description 3
- AVGVFDSUDIUXEU-UHFFFAOYSA-N 2-octyl-1,2-thiazolidin-3-one Chemical compound CCCCCCCCN1SCCC1=O AVGVFDSUDIUXEU-UHFFFAOYSA-N 0.000 description 3
- ZRDUSMYWDRPZRM-UHFFFAOYSA-N 2-sec-butyl-4,6-dinitrophenyl 3-methylbut-2-enoate Chemical compound CCC(C)C1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1OC(=O)C=C(C)C ZRDUSMYWDRPZRM-UHFFFAOYSA-N 0.000 description 3
- NRAYWXLNSHEHQO-UHFFFAOYSA-N 3-(1-benzothiophen-2-yl)-5,6-dihydro-1,4,2-oxathiazine 4-oxide Chemical compound O=S1CCON=C1C1=CC2=CC=CC=C2S1 NRAYWXLNSHEHQO-UHFFFAOYSA-N 0.000 description 3
- FSCWZHGZWWDELK-UHFFFAOYSA-N 3-(3,5-dichlorophenyl)-5-ethenyl-5-methyl-2,4-oxazolidinedione Chemical compound O=C1C(C)(C=C)OC(=O)N1C1=CC(Cl)=CC(Cl)=C1 FSCWZHGZWWDELK-UHFFFAOYSA-N 0.000 description 3
- XTDZGXBTXBEZDN-UHFFFAOYSA-N 3-(difluoromethyl)-N-(9-isopropyl-1,2,3,4-tetrahydro-1,4-methanonaphthalen-5-yl)-1-methylpyrazole-4-carboxamide Chemical compound CC(C)C1C2CCC1C1=C2C=CC=C1NC(=O)C1=CN(C)N=C1C(F)F XTDZGXBTXBEZDN-UHFFFAOYSA-N 0.000 description 3
- WYVVKGNFXHOCQV-UHFFFAOYSA-N 3-iodoprop-2-yn-1-yl butylcarbamate Chemical compound CCCCNC(=O)OCC#CI WYVVKGNFXHOCQV-UHFFFAOYSA-N 0.000 description 3
- XHZWFUVEKDDQPF-UHFFFAOYSA-N 5-bromo-1h-pyrazole Chemical class BrC1=CC=NN1 XHZWFUVEKDDQPF-UHFFFAOYSA-N 0.000 description 3
- NEKULYKCZPJMMJ-UHFFFAOYSA-N 5-chloro-N-{1-[4-(difluoromethoxy)phenyl]propyl}-6-methylpyrimidin-4-amine Chemical compound C=1C=C(OC(F)F)C=CC=1C(CC)NC1=NC=NC(C)=C1Cl NEKULYKCZPJMMJ-UHFFFAOYSA-N 0.000 description 3
- OQMZNAMGEHIHNN-UHFFFAOYSA-N 7-Dehydrostigmasterol Natural products C1C(O)CCC2(C)C(CCC3(C(C(C)C=CC(CC)C(C)C)CCC33)C)C3=CC=C21 OQMZNAMGEHIHNN-UHFFFAOYSA-N 0.000 description 3
- ZKHQWZAMYRWXGA-KQYNXXCUSA-J ATP(4-) Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)[C@H]1O ZKHQWZAMYRWXGA-KQYNXXCUSA-J 0.000 description 3
- ZKHQWZAMYRWXGA-UHFFFAOYSA-N Adenosine triphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O ZKHQWZAMYRWXGA-UHFFFAOYSA-N 0.000 description 3
- YCIPQJTZJGUXND-UHFFFAOYSA-N Aglaia odorata Alkaloid Natural products C1=CC(OC)=CC=C1C1(C(C=2C(=O)N3CCCC3=NC=22)C=3C=CC=CC=3)C2(O)C2=C(OC)C=C(OC)C=C2O1 YCIPQJTZJGUXND-UHFFFAOYSA-N 0.000 description 3
- 239000005727 Amisulbrom Substances 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- 239000005735 Benalaxyl-M Substances 0.000 description 3
- 239000005736 Benthiavalicarb Substances 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 239000005738 Bixafen Substances 0.000 description 3
- 239000005742 Bupirimate Substances 0.000 description 3
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 3
- XFXPMWWXUTWYJX-UHFFFAOYSA-N Cyanide Chemical compound N#[C-] XFXPMWWXUTWYJX-UHFFFAOYSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- 102000053602 DNA Human genes 0.000 description 3
- 108020004414 DNA Proteins 0.000 description 3
- 239000005759 Diethofencarb Substances 0.000 description 3
- 239000005764 Dithianon Substances 0.000 description 3
- 239000005766 Dodine Substances 0.000 description 3
- 102000015782 Electron Transport Complex III Human genes 0.000 description 3
- 108010024882 Electron Transport Complex III Proteins 0.000 description 3
- DNVPQKQSNYMLRS-NXVQYWJNSA-N Ergosterol Natural products CC(C)[C@@H](C)C=C[C@H](C)[C@H]1CC[C@H]2C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@H]3CC[C@]12C DNVPQKQSNYMLRS-NXVQYWJNSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 239000005776 Fenhexamid Substances 0.000 description 3
- 239000005779 Fenpyrazamine Substances 0.000 description 3
- 239000005781 Fludioxonil Substances 0.000 description 3
- 239000005783 Fluopyram Substances 0.000 description 3
- 239000005791 Fuberidazole Substances 0.000 description 3
- SQUHHTBVTRBESD-UHFFFAOYSA-N Hexa-Ac-myo-Inositol Natural products CC(=O)OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O SQUHHTBVTRBESD-UHFFFAOYSA-N 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- 239000005797 Iprovalicarb Substances 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 239000005799 Isopyrazam Substances 0.000 description 3
- NWUWYYSKZYIQAE-ZBFHGGJFSA-N L-(R)-iprovalicarb Chemical compound CC(C)OC(=O)N[C@@H](C(C)C)C(=O)N[C@H](C)C1=CC=C(C)C=C1 NWUWYYSKZYIQAE-ZBFHGGJFSA-N 0.000 description 3
- 239000005805 Mepanipyrim Substances 0.000 description 3
- 239000005806 Meptyldinocap Substances 0.000 description 3
- 239000005808 Metalaxyl-M Substances 0.000 description 3
- 239000005809 Metiram Substances 0.000 description 3
- IUOKJNROJISWRO-UHFFFAOYSA-N N-(2-cyano-3-methylbutan-2-yl)-2-(2,4-dichlorophenoxy)propanamide Chemical compound CC(C)C(C)(C#N)NC(=O)C(C)OC1=CC=C(Cl)C=C1Cl IUOKJNROJISWRO-UHFFFAOYSA-N 0.000 description 3
- NQRFDNJEBWAUBL-UHFFFAOYSA-N N-[cyano(2-thienyl)methyl]-4-ethyl-2-(ethylamino)-1,3-thiazole-5-carboxamide Chemical compound S1C(NCC)=NC(CC)=C1C(=O)NC(C#N)C1=CC=CS1 NQRFDNJEBWAUBL-UHFFFAOYSA-N 0.000 description 3
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 3
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 3
- 241000233654 Oomycetes Species 0.000 description 3
- KYGZCKSPAKDVKC-UHFFFAOYSA-N Oxolinic acid Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC2=C1OCO2 KYGZCKSPAKDVKC-UHFFFAOYSA-N 0.000 description 3
- 239000004100 Oxytetracycline Substances 0.000 description 3
- KFSLWBXXFJQRDL-UHFFFAOYSA-N Peracetic acid Chemical compound CC(=O)OO KFSLWBXXFJQRDL-UHFFFAOYSA-N 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- 239000004349 Polyvinylpyrrolidone-vinyl acetate copolymer Substances 0.000 description 3
- 239000005823 Propineb Substances 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 229940123185 Squalene epoxidase inhibitor Drugs 0.000 description 3
- 102000019259 Succinate Dehydrogenase Human genes 0.000 description 3
- 108010012901 Succinate Dehydrogenase Proteins 0.000 description 3
- 239000005840 Tetraconazole Substances 0.000 description 3
- GNVMUORYQLCPJZ-UHFFFAOYSA-M Thiocarbamate Chemical compound NC([S-])=O GNVMUORYQLCPJZ-UHFFFAOYSA-M 0.000 description 3
- 102100029677 Trehalase Human genes 0.000 description 3
- 108010087472 Trehalase Proteins 0.000 description 3
- 239000005846 Triadimenol Substances 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 239000005858 Triflumizole Substances 0.000 description 3
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 3
- 102000004243 Tubulin Human genes 0.000 description 3
- 108090000704 Tubulin Proteins 0.000 description 3
- 239000005863 Zoxamide Substances 0.000 description 3
- PSLUFJFHTBIXMW-WYEYVKMPSA-N [(3r,4ar,5s,6s,6as,10s,10ar,10bs)-3-ethenyl-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-6-(2-pyridin-2-ylethylcarbamoyloxy)-5,6,6a,8,9,10-hexahydro-2h-benzo[f]chromen-5-yl] acetate Chemical compound O([C@@H]1[C@@H]([C@]2(O[C@](C)(CC(=O)[C@]2(O)[C@@]2(C)[C@@H](O)CCC(C)(C)[C@@H]21)C=C)C)OC(=O)C)C(=O)NCCC1=CC=CC=N1 PSLUFJFHTBIXMW-WYEYVKMPSA-N 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 230000000895 acaricidal effect Effects 0.000 description 3
- 239000000642 acaricide Substances 0.000 description 3
- 239000002168 alkylating agent Substances 0.000 description 3
- 229940100198 alkylating agent Drugs 0.000 description 3
- 239000011717 all-trans-retinol Substances 0.000 description 3
- 235000019169 all-trans-retinol Nutrition 0.000 description 3
- 150000001408 amides Chemical class 0.000 description 3
- BREATYVWRHIPIY-UHFFFAOYSA-N amisulbrom Chemical compound CN(C)S(=O)(=O)N1C=NC(S(=O)(=O)N2C3=CC(F)=CC=C3C(Br)=C2C)=N1 BREATYVWRHIPIY-UHFFFAOYSA-N 0.000 description 3
- IMHBYKMAHXWHRP-UHFFFAOYSA-N anilazine Chemical compound ClC1=CC=CC=C1NC1=NC(Cl)=NC(Cl)=N1 IMHBYKMAHXWHRP-UHFFFAOYSA-N 0.000 description 3
- 239000003945 anionic surfactant Substances 0.000 description 3
- XRWSZZJLZRKHHD-WVWIJVSJSA-N asunaprevir Chemical compound O=C([C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)OC1=NC=C(C2=CC=C(Cl)C=C21)OC)N[C@]1(C(=O)NS(=O)(=O)C2CC2)C[C@H]1C=C XRWSZZJLZRKHHD-WVWIJVSJSA-N 0.000 description 3
- CJPQIRJHIZUAQP-MRXNPFEDSA-N benalaxyl-M Chemical compound CC=1C=CC=C(C)C=1N([C@H](C)C(=O)OC)C(=O)CC1=CC=CC=C1 CJPQIRJHIZUAQP-MRXNPFEDSA-N 0.000 description 3
- LJOZMWRYMKECFF-UHFFFAOYSA-N benodanil Chemical compound IC1=CC=CC=C1C(=O)NC1=CC=CC=C1 LJOZMWRYMKECFF-UHFFFAOYSA-N 0.000 description 3
- VVSLYIKSEBPRSN-PELKAZGASA-N benthiavalicarb Chemical compound C1=C(F)C=C2SC([C@@H](C)NC(=O)[C@@H](NC(O)=O)C(C)C)=NC2=C1 VVSLYIKSEBPRSN-PELKAZGASA-N 0.000 description 3
- USRKFGIXLGKMKU-ABAIWWIYSA-N benthiavalicarb-isopropyl Chemical compound C1=C(F)C=C2SC([C@@H](C)NC(=O)[C@H](C(C)C)NC(=O)OC(C)C)=NC2=C1 USRKFGIXLGKMKU-ABAIWWIYSA-N 0.000 description 3
- 208000036815 beta tubulin Diseases 0.000 description 3
- LDLMOOXUCMHBMZ-UHFFFAOYSA-N bixafen Chemical compound FC(F)C1=NN(C)C=C1C(=O)NC1=CC=C(F)C=C1C1=CC=C(Cl)C(Cl)=C1 LDLMOOXUCMHBMZ-UHFFFAOYSA-N 0.000 description 3
- CXNPLSGKWMLZPZ-UHFFFAOYSA-N blasticidin-S Natural products O1C(C(O)=O)C(NC(=O)CC(N)CCN(C)C(N)=N)C=CC1N1C(=O)N=C(N)C=C1 CXNPLSGKWMLZPZ-UHFFFAOYSA-N 0.000 description 3
- DSKJPMWIHSOYEA-UHFFFAOYSA-N bupirimate Chemical compound CCCCC1=C(C)N=C(NCC)N=C1OS(=O)(=O)N(C)C DSKJPMWIHSOYEA-UHFFFAOYSA-N 0.000 description 3
- JHRWWRDRBPCWTF-OLQVQODUSA-N captafol Chemical compound C1C=CC[C@H]2C(=O)N(SC(Cl)(Cl)C(Cl)Cl)C(=O)[C@H]21 JHRWWRDRBPCWTF-OLQVQODUSA-N 0.000 description 3
- RXDMAYSSBPYBFW-UHFFFAOYSA-N carpropamid Chemical compound C=1C=C(Cl)C=CC=1C(C)NC(=O)C1(CC)C(C)C1(Cl)Cl RXDMAYSSBPYBFW-UHFFFAOYSA-N 0.000 description 3
- 239000003093 cationic surfactant Substances 0.000 description 3
- PFIADAMVCJPXSF-UHFFFAOYSA-N chloroneb Chemical compound COC1=CC(Cl)=C(OC)C=C1Cl PFIADAMVCJPXSF-UHFFFAOYSA-N 0.000 description 3
- APEJMQOBVMLION-UHFFFAOYSA-N cinnamamide Chemical compound NC(=O)C=CC1=CC=CC=C1 APEJMQOBVMLION-UHFFFAOYSA-N 0.000 description 3
- 229940125961 compound 24 Drugs 0.000 description 3
- 239000010949 copper Substances 0.000 description 3
- 229910052802 copper Inorganic materials 0.000 description 3
- 239000013078 crystal Substances 0.000 description 3
- 230000007123 defense Effects 0.000 description 3
- 230000017858 demethylation Effects 0.000 description 3
- 238000010520 demethylation reaction Methods 0.000 description 3
- WURGXGVFSMYFCG-UHFFFAOYSA-N dichlofluanid Chemical compound CN(C)S(=O)(=O)N(SC(F)(Cl)Cl)C1=CC=CC=C1 WURGXGVFSMYFCG-UHFFFAOYSA-N 0.000 description 3
- BIXZHMJUSMUDOQ-UHFFFAOYSA-N dichloran Chemical compound NC1=C(Cl)C=C([N+]([O-])=O)C=C1Cl BIXZHMJUSMUDOQ-UHFFFAOYSA-N 0.000 description 3
- YEJGPFZQLRMXOI-PKEIRNPWSA-N diclocymet Chemical compound N#CC(C(C)(C)C)C(=O)N[C@H](C)C1=CC=C(Cl)C=C1Cl YEJGPFZQLRMXOI-PKEIRNPWSA-N 0.000 description 3
- UWQMKVBQKFHLCE-UHFFFAOYSA-N diclomezine Chemical compound C1=C(Cl)C(C)=C(Cl)C=C1C1=NNC(=O)C=C1 UWQMKVBQKFHLCE-UHFFFAOYSA-N 0.000 description 3
- 229940004812 dicloran Drugs 0.000 description 3
- LNJNFVJKDJYTEU-UHFFFAOYSA-N diethofencarb Chemical compound CCOC1=CC=C(NC(=O)OC(C)C)C=C1OCC LNJNFVJKDJYTEU-UHFFFAOYSA-N 0.000 description 3
- CJHXCRMKMMBYJQ-UHFFFAOYSA-N dimethirimol Chemical compound CCCCC1=C(C)NC(N(C)C)=NC1=O CJHXCRMKMMBYJQ-UHFFFAOYSA-N 0.000 description 3
- PYZSVQVRHDXQSL-UHFFFAOYSA-N dithianon Chemical compound S1C(C#N)=C(C#N)SC2=C1C(=O)C1=CC=CC=C1C2=O PYZSVQVRHDXQSL-UHFFFAOYSA-N 0.000 description 3
- AWZOLILCOUMRDG-UHFFFAOYSA-N edifenphos Chemical compound C=1C=CC=CC=1SP(=O)(OCC)SC1=CC=CC=C1 AWZOLILCOUMRDG-UHFFFAOYSA-N 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- DNVPQKQSNYMLRS-SOWFXMKYSA-N ergosterol Chemical compound C1[C@@H](O)CC[C@]2(C)[C@H](CC[C@]3([C@H]([C@H](C)/C=C/[C@@H](C)C(C)C)CC[C@H]33)C)C3=CC=C21 DNVPQKQSNYMLRS-SOWFXMKYSA-N 0.000 description 3
- BBXXLROWFHWFQY-UHFFFAOYSA-N ethirimol Chemical compound CCCCC1=C(C)NC(NCC)=NC1=O BBXXLROWFHWFQY-UHFFFAOYSA-N 0.000 description 3
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 3
- 238000001704 evaporation Methods 0.000 description 3
- 230000008020 evaporation Effects 0.000 description 3
- VDLGAVXLJYLFDH-UHFFFAOYSA-N fenhexamid Chemical compound C=1C=C(O)C(Cl)=C(Cl)C=1NC(=O)C1(C)CCCCC1 VDLGAVXLJYLFDH-UHFFFAOYSA-N 0.000 description 3
- FKLFBQCQQYDUAM-UHFFFAOYSA-N fenpiclonil Chemical compound ClC1=CC=CC(C=2C(=CNC=2)C#N)=C1Cl FKLFBQCQQYDUAM-UHFFFAOYSA-N 0.000 description 3
- WDQNIWFZKXZFAY-UHFFFAOYSA-M fentin acetate Chemical compound CC([O-])=O.C1=CC=CC=C1[Sn+](C=1C=CC=CC=1)C1=CC=CC=C1 WDQNIWFZKXZFAY-UHFFFAOYSA-M 0.000 description 3
- NJVOZLGKTAPUTQ-UHFFFAOYSA-M fentin chloride Chemical compound C=1C=CC=CC=1[Sn](C=1C=CC=CC=1)(Cl)C1=CC=CC=C1 NJVOZLGKTAPUTQ-UHFFFAOYSA-M 0.000 description 3
- BFWMWWXRWVJXSE-UHFFFAOYSA-M fentin hydroxide Chemical compound C=1C=CC=CC=1[Sn](C=1C=CC=CC=1)(O)C1=CC=CC=C1 BFWMWWXRWVJXSE-UHFFFAOYSA-M 0.000 description 3
- NYPJDWWKZLNGGM-UHFFFAOYSA-N fenvalerate Aalpha Natural products C=1C=C(Cl)C=CC=1C(C(C)C)C(=O)OC(C#N)C(C=1)=CC=CC=1OC1=CC=CC=C1 NYPJDWWKZLNGGM-UHFFFAOYSA-N 0.000 description 3
- WHDGWKAJBYRJJL-UHFFFAOYSA-K ferbam Chemical compound [Fe+3].CN(C)C([S-])=S.CN(C)C([S-])=S.CN(C)C([S-])=S WHDGWKAJBYRJJL-UHFFFAOYSA-K 0.000 description 3
- GOWLARCWZRESHU-AQTBWJFISA-N ferimzone Chemical compound C=1C=CC=C(C)C=1C(/C)=N\NC1=NC(C)=CC(C)=N1 GOWLARCWZRESHU-AQTBWJFISA-N 0.000 description 3
- 239000000706 filtrate Substances 0.000 description 3
- MUJOIMFVNIBMKC-UHFFFAOYSA-N fludioxonil Chemical compound C=12OC(F)(F)OC2=CC=CC=1C1=CNC=C1C#N MUJOIMFVNIBMKC-UHFFFAOYSA-N 0.000 description 3
- KVDJTXBXMWJJEF-UHFFFAOYSA-N fluopyram Chemical compound ClC1=CC(C(F)(F)F)=CN=C1CCNC(=O)C1=CC=CC=C1C(F)(F)F KVDJTXBXMWJJEF-UHFFFAOYSA-N 0.000 description 3
- GNVDAZSPJWCIQZ-UHFFFAOYSA-N flusulfamide Chemical compound ClC1=CC([N+](=O)[O-])=CC=C1NS(=O)(=O)C1=CC=C(Cl)C(C(F)(F)F)=C1 GNVDAZSPJWCIQZ-UHFFFAOYSA-N 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- UYJUZNLFJAWNEZ-UHFFFAOYSA-N fuberidazole Chemical compound C1=COC(C=2NC3=CC=CC=C3N=2)=C1 UYJUZNLFJAWNEZ-UHFFFAOYSA-N 0.000 description 3
- 125000000524 functional group Chemical group 0.000 description 3
- 238000007429 general method Methods 0.000 description 3
- 150000002429 hydrazines Chemical class 0.000 description 3
- KGVPNLBXJKTABS-UHFFFAOYSA-N hymexazol Chemical compound CC1=CC(O)=NO1 KGVPNLBXJKTABS-UHFFFAOYSA-N 0.000 description 3
- 239000005457 ice water Substances 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 229960000367 inositol Drugs 0.000 description 3
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 3
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 3
- FCOAHACKGGIURQ-UHFFFAOYSA-N iprobenfos Chemical compound CC(C)OP(=O)(OC(C)C)SCC1=CC=CC=C1 FCOAHACKGGIURQ-UHFFFAOYSA-N 0.000 description 3
- UFHLMYOGRXOCSL-UHFFFAOYSA-N isoprothiolane Chemical compound CC(C)OC(=O)C(C(=O)OC(C)C)=C1SCCS1 UFHLMYOGRXOCSL-UHFFFAOYSA-N 0.000 description 3
- WLPCAERCXQSYLQ-UHFFFAOYSA-N isotianil Chemical compound ClC1=NSC(C(=O)NC=2C(=CC=CC=2)C#N)=C1Cl WLPCAERCXQSYLQ-UHFFFAOYSA-N 0.000 description 3
- PVTHJAPFENJVNC-MHRBZPPQSA-N kasugamycin Chemical compound N[C@H]1C[C@H](NC(=N)C(O)=O)[C@@H](C)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1O PVTHJAPFENJVNC-MHRBZPPQSA-N 0.000 description 3
- 150000002576 ketones Chemical class 0.000 description 3
- 230000003859 lipid peroxidation Effects 0.000 description 3
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Inorganic materials [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- CIFWZNRJIBNXRE-UHFFFAOYSA-N mepanipyrim Chemical compound CC#CC1=CC(C)=NC(NC=2C=CC=CC=2)=N1 CIFWZNRJIBNXRE-UHFFFAOYSA-N 0.000 description 3
- IXJOSTZEBSTPAG-UHFFFAOYSA-N methasulfocarb Chemical compound CNC(=O)SC1=CC=C(OS(C)(=O)=O)C=C1 IXJOSTZEBSTPAG-UHFFFAOYSA-N 0.000 description 3
- 229920000257 metiram Polymers 0.000 description 3
- 239000004530 micro-emulsion Substances 0.000 description 3
- 239000002480 mineral oil Substances 0.000 description 3
- 230000011278 mitosis Effects 0.000 description 3
- 150000002780 morpholines Chemical class 0.000 description 3
- IWWKIOTVAJOMJT-UHFFFAOYSA-N n-(2,2,2-trichloro-1-morpholin-4-ylethyl)formamide Chemical compound O=CNC(C(Cl)(Cl)Cl)N1CCOCC1 IWWKIOTVAJOMJT-UHFFFAOYSA-N 0.000 description 3
- 230000001069 nematicidal effect Effects 0.000 description 3
- 239000005645 nematicide Substances 0.000 description 3
- 125000002524 organometallic group Chemical group 0.000 description 3
- 229960000321 oxolinic acid Drugs 0.000 description 3
- IWVCMVBTMGNXQD-PXOLEDIWSA-N oxytetracycline Chemical compound C1=CC=C2[C@](O)(C)[C@H]3[C@H](O)[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-PXOLEDIWSA-N 0.000 description 3
- 229960000625 oxytetracycline Drugs 0.000 description 3
- 235000019366 oxytetracycline Nutrition 0.000 description 3
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 3
- 239000012071 phase Substances 0.000 description 3
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 3
- 150000003053 piperidines Chemical class 0.000 description 3
- 235000019448 polyvinylpyrrolidone-vinyl acetate copolymer Nutrition 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- QXJKBPAVAHBARF-BETUJISGSA-N procymidone Chemical compound O=C([C@]1(C)C[C@@]1(C1=O)C)N1C1=CC(Cl)=CC(Cl)=C1 QXJKBPAVAHBARF-BETUJISGSA-N 0.000 description 3
- KKMLIVYBGSAJPM-UHFFFAOYSA-L propineb Chemical compound [Zn+2].[S-]C(=S)NC(C)CNC([S-])=S KKMLIVYBGSAJPM-UHFFFAOYSA-L 0.000 description 3
- VTGOHKSTWXHQJK-UHFFFAOYSA-N pyrimidin-2-ol Chemical compound OC1=NC=CC=N1 VTGOHKSTWXHQJK-UHFFFAOYSA-N 0.000 description 3
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 3
- NPCOQXAVBJJZBQ-UHFFFAOYSA-N reduced coenzyme Q9 Natural products COC1=C(O)C(C)=C(CC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)C)C(O)=C1OC NPCOQXAVBJJZBQ-UHFFFAOYSA-N 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000027756 respiratory electron transport chain Effects 0.000 description 3
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229910001415 sodium ion Inorganic materials 0.000 description 3
- 229910052938 sodium sulfate Inorganic materials 0.000 description 3
- 239000002689 soil Substances 0.000 description 3
- PUYXTUJWRLOUCW-UHFFFAOYSA-N spiroxamine Chemical compound O1C(CN(CC)CCC)COC11CCC(C(C)(C)C)CC1 PUYXTUJWRLOUCW-UHFFFAOYSA-N 0.000 description 3
- 239000007858 starting material Substances 0.000 description 3
- 229960005322 streptomycin Drugs 0.000 description 3
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- IWVCMVBTMGNXQD-UHFFFAOYSA-N terramycin dehydrate Natural products C1=CC=C2C(O)(C)C3C(O)C4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-UHFFFAOYSA-N 0.000 description 3
- 150000003512 tertiary amines Chemical class 0.000 description 3
- WOSNCVAPUOFXEH-UHFFFAOYSA-N thifluzamide Chemical compound S1C(C)=NC(C(F)(F)F)=C1C(=O)NC1=C(Br)C=C(OC(F)(F)F)C=C1Br WOSNCVAPUOFXEH-UHFFFAOYSA-N 0.000 description 3
- YFNCATAIYKQPOO-UHFFFAOYSA-N thiophanate Chemical compound CCOC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OCC YFNCATAIYKQPOO-UHFFFAOYSA-N 0.000 description 3
- 230000032258 transport Effects 0.000 description 3
- BAZVSMNPJJMILC-UHFFFAOYSA-N triadimenol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)OC1=CC=C(Cl)C=C1 BAZVSMNPJJMILC-UHFFFAOYSA-N 0.000 description 3
- DQJCHOQLCLEDLL-UHFFFAOYSA-N tricyclazole Chemical compound CC1=CC=CC2=C1N1C=NN=C1S2 DQJCHOQLCLEDLL-UHFFFAOYSA-N 0.000 description 3
- HSMVPDGQOIQYSR-KGENOOAVSA-N triflumizole Chemical compound C1=CN=CN1C(/COCCC)=N/C1=CC=C(Cl)C=C1C(F)(F)F HSMVPDGQOIQYSR-KGENOOAVSA-N 0.000 description 3
- RROQIUMZODEXOR-UHFFFAOYSA-N triforine Chemical compound O=CNC(C(Cl)(Cl)Cl)N1CCN(C(NC=O)C(Cl)(Cl)Cl)CC1 RROQIUMZODEXOR-UHFFFAOYSA-N 0.000 description 3
- 229940040064 ubiquinol Drugs 0.000 description 3
- QNTNKSLOFHEFPK-UPTCCGCDSA-N ubiquinol-10 Chemical compound COC1=C(O)C(C)=C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)C(O)=C1OC QNTNKSLOFHEFPK-UPTCCGCDSA-N 0.000 description 3
- 239000001993 wax Substances 0.000 description 3
- WYRSGXAIHNMKOL-UHFFFAOYSA-N $l^{1}-sulfanylethane Chemical compound CC[S] WYRSGXAIHNMKOL-UHFFFAOYSA-N 0.000 description 2
- QSLPNSWXUQHVLP-UHFFFAOYSA-N $l^{1}-sulfanylmethane Chemical compound [S]C QSLPNSWXUQHVLP-UHFFFAOYSA-N 0.000 description 2
- MYUPFXPCYUISAG-UHFFFAOYSA-N (2,4-dichlorophenyl)(phenyl)pyrimidin-5-ylmethanol Chemical compound C=1N=CN=CC=1C(C=1C(=CC(Cl)=CC=1)Cl)(O)C1=CC=CC=C1 MYUPFXPCYUISAG-UHFFFAOYSA-N 0.000 description 2
- STBLNCCBQMHSRC-BATDWUPUSA-N (2s)-n-[(3s,4s)-5-acetyl-7-cyano-4-methyl-1-[(2-methylnaphthalen-1-yl)methyl]-2-oxo-3,4-dihydro-1,5-benzodiazepin-3-yl]-2-(methylamino)propanamide Chemical compound O=C1[C@@H](NC(=O)[C@H](C)NC)[C@H](C)N(C(C)=O)C2=CC(C#N)=CC=C2N1CC1=C(C)C=CC2=CC=CC=C12 STBLNCCBQMHSRC-BATDWUPUSA-N 0.000 description 2
- GIWOBQLAIGEECV-UHFFFAOYSA-N (4-fluorophenyl) n-[1-[1-(4-cyanophenyl)ethylsulfonyl]butan-2-yl]carbamate Chemical compound C=1C=C(F)C=CC=1OC(=O)NC(CC)CS(=O)(=O)C(C)C1=CC=C(C#N)C=C1 GIWOBQLAIGEECV-UHFFFAOYSA-N 0.000 description 2
- BKBSMMUEEAWFRX-NBVRZTHBSA-N (E)-flumorph Chemical compound C1=C(OC)C(OC)=CC=C1C(\C=1C=CC(F)=CC=1)=C\C(=O)N1CCOCC1 BKBSMMUEEAWFRX-NBVRZTHBSA-N 0.000 description 2
- MPIPASJGOJYODL-SFHVURJKSA-N (R)-isoconazole Chemical compound ClC1=CC(Cl)=CC=C1[C@@H](OCC=1C(=CC=CC=1Cl)Cl)CN1C=NC=C1 MPIPASJGOJYODL-SFHVURJKSA-N 0.000 description 2
- XGWIJUOSCAQSSV-XHDPSFHLSA-N (S,S)-hexythiazox Chemical compound S([C@H]([C@@H]1C)C=2C=CC(Cl)=CC=2)C(=O)N1C(=O)NC1CCCCC1 XGWIJUOSCAQSSV-XHDPSFHLSA-N 0.000 description 2
- GVTLFGJNTIRUEG-ZHACJKMWSA-N (e)-n-(3-methoxyphenyl)-3-phenylprop-2-enamide Chemical compound COC1=CC=CC(NC(=O)\C=C\C=2C=CC=CC=2)=C1 GVTLFGJNTIRUEG-ZHACJKMWSA-N 0.000 description 2
- FNQJDLTXOVEEFB-UHFFFAOYSA-N 1,2,3-benzothiadiazole Chemical compound C1=CC=C2SN=NC2=C1 FNQJDLTXOVEEFB-UHFFFAOYSA-N 0.000 description 2
- OWQPOVKKUWUEKE-UHFFFAOYSA-N 1,2,3-benzotriazine Chemical compound N1=NN=CC2=CC=CC=C21 OWQPOVKKUWUEKE-UHFFFAOYSA-N 0.000 description 2
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 2
- YGTAZGSLCXNBQL-UHFFFAOYSA-N 1,2,4-thiadiazole Chemical compound C=1N=CSN=1 YGTAZGSLCXNBQL-UHFFFAOYSA-N 0.000 description 2
- CSNIZNHTOVFARY-UHFFFAOYSA-N 1,2-benzothiazole Chemical compound C1=CC=C2C=NSC2=C1 CSNIZNHTOVFARY-UHFFFAOYSA-N 0.000 description 2
- JLHMJWHSBYZWJJ-UHFFFAOYSA-N 1,2-thiazole 1-oxide Chemical compound O=S1C=CC=N1 JLHMJWHSBYZWJJ-UHFFFAOYSA-N 0.000 description 2
- UUFQTNFCRMXOAE-UHFFFAOYSA-N 1-methylmethylene Chemical compound C[CH] UUFQTNFCRMXOAE-UHFFFAOYSA-N 0.000 description 2
- LEZWWPYKPKIXLL-UHFFFAOYSA-N 1-{2-(4-chlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl}imidazole Chemical compound C1=CC(Cl)=CC=C1COC(C=1C(=CC(Cl)=CC=1)Cl)CN1C=NC=C1 LEZWWPYKPKIXLL-UHFFFAOYSA-N 0.000 description 2
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical compound CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 2
- QMQZIXCNLUPEIN-UHFFFAOYSA-N 1h-imidazole-2-carbonitrile Chemical compound N#CC1=NC=CN1 QMQZIXCNLUPEIN-UHFFFAOYSA-N 0.000 description 2
- BNYCHCAYYYRJSH-UHFFFAOYSA-N 1h-pyrazole-5-carboxamide Chemical compound NC(=O)C1=CC=NN1 BNYCHCAYYYRJSH-UHFFFAOYSA-N 0.000 description 2
- AAILEWXSEQLMNI-UHFFFAOYSA-N 1h-pyridazin-6-one Chemical compound OC1=CC=CN=N1 AAILEWXSEQLMNI-UHFFFAOYSA-N 0.000 description 2
- VCKTYXPOCYGHLN-UHFFFAOYSA-N 2-(2,6-difluoro-4-methoxyphenyl)-3-oxobutanenitrile Chemical compound COC1=CC(F)=C(C(C#N)C(C)=O)C(F)=C1 VCKTYXPOCYGHLN-UHFFFAOYSA-N 0.000 description 2
- QIAUDGXIPNBTDC-UHFFFAOYSA-N 2-(2,6-difluoro-4-methoxyphenyl)acetonitrile Chemical compound COC1=CC(F)=C(CC#N)C(F)=C1 QIAUDGXIPNBTDC-UHFFFAOYSA-N 0.000 description 2
- IZXIZTKNFFYFOF-UHFFFAOYSA-N 2-Oxazolidone Chemical compound O=C1NCCO1 IZXIZTKNFFYFOF-UHFFFAOYSA-N 0.000 description 2
- BOTNFCTYKJBUMU-UHFFFAOYSA-N 2-[4-(2-methylpropyl)piperazin-4-ium-1-yl]-2-oxoacetate Chemical compound CC(C)C[NH+]1CCN(C(=O)C([O-])=O)CC1 BOTNFCTYKJBUMU-UHFFFAOYSA-N 0.000 description 2
- IQHSSYROJYPFDV-UHFFFAOYSA-N 2-bromo-1,3-dichloro-5-(trifluoromethyl)benzene Chemical group FC(F)(F)C1=CC(Cl)=C(Br)C(Cl)=C1 IQHSSYROJYPFDV-UHFFFAOYSA-N 0.000 description 2
- ZQMRDENWZKMOTM-UHFFFAOYSA-N 2-butoxy-6-iodo-3-propylchromen-4-one Chemical compound C1=C(I)C=C2C(=O)C(CCC)=C(OCCCC)OC2=C1 ZQMRDENWZKMOTM-UHFFFAOYSA-N 0.000 description 2
- FEFZGUWAYDEBHK-UHFFFAOYSA-N 2-cyano-n'-hydroxyethanimidamide Chemical compound ON=C(N)CC#N FEFZGUWAYDEBHK-UHFFFAOYSA-N 0.000 description 2
- CYEJMVLDXAUOPN-UHFFFAOYSA-N 2-dodecylphenol Chemical compound CCCCCCCCCCCCC1=CC=CC=C1O CYEJMVLDXAUOPN-UHFFFAOYSA-N 0.000 description 2
- YIWUKEYIRIRTPP-UHFFFAOYSA-N 2-ethylhexan-1-ol Chemical compound CCCCC(CC)CO YIWUKEYIRIRTPP-UHFFFAOYSA-N 0.000 description 2
- IRTLROCMFSDSNF-UHFFFAOYSA-N 2-phenyl-1h-pyrrole Chemical compound C1=CNC(C=2C=CC=CC=2)=C1 IRTLROCMFSDSNF-UHFFFAOYSA-N 0.000 description 2
- CJXBHFANXQMZBF-UHFFFAOYSA-N 2-phenylethanethioamide Chemical compound NC(=S)CC1=CC=CC=C1 CJXBHFANXQMZBF-UHFFFAOYSA-N 0.000 description 2
- WAIIVJKIXMLKTR-UHFFFAOYSA-N 2h-triazole-4-sulfonamide Chemical compound NS(=O)(=O)C1=CNN=N1 WAIIVJKIXMLKTR-UHFFFAOYSA-N 0.000 description 2
- CAAMSDWKXXPUJR-UHFFFAOYSA-N 3,5-dihydro-4H-imidazol-4-one Chemical compound O=C1CNC=N1 CAAMSDWKXXPUJR-UHFFFAOYSA-N 0.000 description 2
- OVFHHJZHXHZIHT-UHFFFAOYSA-N 3-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-yl)quinazolin-4-one Chemical compound ClC1=CC(Cl)=CC=C1N1C(=O)C2=CC=CC=C2N=C1N1N=CN=C1 OVFHHJZHXHZIHT-UHFFFAOYSA-N 0.000 description 2
- FOGYNLXERPKEGN-UHFFFAOYSA-N 3-(2-hydroxy-3-methoxyphenyl)-2-[2-methoxy-4-(3-sulfopropyl)phenoxy]propane-1-sulfonic acid Chemical compound COC1=CC=CC(CC(CS(O)(=O)=O)OC=2C(=CC(CCCS(O)(=O)=O)=CC=2)OC)=C1O FOGYNLXERPKEGN-UHFFFAOYSA-N 0.000 description 2
- BZGLBXYQOMFXAU-UHFFFAOYSA-N 3-(2-methylpiperidin-1-yl)propyl 3,4-dichlorobenzoate Chemical compound CC1CCCCN1CCCOC(=O)C1=CC=C(Cl)C(Cl)=C1 BZGLBXYQOMFXAU-UHFFFAOYSA-N 0.000 description 2
- DHTJFQWHCVTNRY-UHFFFAOYSA-N 3-[5-(4-chlorophenyl)-2,3-dimethyl-1,2-oxazolidin-3-yl]pyridine Chemical compound CN1OC(C=2C=CC(Cl)=CC=2)CC1(C)C1=CC=CN=C1 DHTJFQWHCVTNRY-UHFFFAOYSA-N 0.000 description 2
- YNSCKPCDFIDINW-UHFFFAOYSA-N 3-[[2-[[1-[2-(dimethylamino)acetyl]-6-methoxy-4,4-dimethyl-2,3-dihydroquinolin-7-yl]amino]-7h-pyrrolo[2,3-d]pyrimidin-4-yl]amino]thiophene-2-carboxamide Chemical compound COC1=CC(C(CCN2C(=O)CN(C)C)(C)C)=C2C=C1NC(N=C1NC=CC1=1)=NC=1NC=1C=CSC=1C(N)=O YNSCKPCDFIDINW-UHFFFAOYSA-N 0.000 description 2
- JZTPKAROPNTQQV-UHFFFAOYSA-N 3-fluorobenzonitrile Chemical compound FC1=CC=CC(C#N)=C1 JZTPKAROPNTQQV-UHFFFAOYSA-N 0.000 description 2
- RUKDVLFJSMVBLV-UHFFFAOYSA-N 5-iodo-1h-pyrazole Chemical class IC1=CC=NN1 RUKDVLFJSMVBLV-UHFFFAOYSA-N 0.000 description 2
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 2
- 239000005660 Abamectin Substances 0.000 description 2
- VVJKKWFAADXIJK-UHFFFAOYSA-N Allylamine Chemical compound NCC=C VVJKKWFAADXIJK-UHFFFAOYSA-N 0.000 description 2
- 239000005726 Ametoctradin Substances 0.000 description 2
- 241000235349 Ascomycota Species 0.000 description 2
- 108700003918 Bacillus Thuringiensis insecticidal crystal Proteins 0.000 description 2
- 241000193388 Bacillus thuringiensis Species 0.000 description 2
- 241000221198 Basidiomycota Species 0.000 description 2
- KHBQMWCZKVMBLN-UHFFFAOYSA-N Benzenesulfonamide Chemical compound NS(=O)(=O)C1=CC=CC=C1 KHBQMWCZKVMBLN-UHFFFAOYSA-N 0.000 description 2
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 2
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 2
- ZZVVDIVWGXTDRQ-BSYVCWPDSA-N Buthiobate Chemical compound C=1C=CN=CC=1\N=C(/SCCCC)SCC1=CC=C(C(C)(C)C)C=C1 ZZVVDIVWGXTDRQ-BSYVCWPDSA-N 0.000 description 2
- KCBAMQOKOLXLOX-BSZYMOERSA-N CC1=C(SC=N1)C2=CC=C(C=C2)[C@H](C)NC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNCCCONC(=O)C4=C(C(=C(C=C4)F)F)NC5=C(C=C(C=C5)I)F)O Chemical compound CC1=C(SC=N1)C2=CC=C(C=C2)[C@H](C)NC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNCCCONC(=O)C4=C(C(=C(C=C4)F)F)NC5=C(C=C(C=C5)I)F)O KCBAMQOKOLXLOX-BSZYMOERSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- RAPBNVDSDCTNRC-UHFFFAOYSA-N Chlorobenzilate Chemical compound C=1C=C(Cl)C=CC=1C(O)(C(=O)OCC)C1=CC=C(Cl)C=C1 RAPBNVDSDCTNRC-UHFFFAOYSA-N 0.000 description 2
- 239000005749 Copper compound Substances 0.000 description 2
- 229910021595 Copper(I) iodide Inorganic materials 0.000 description 2
- 229910021590 Copper(II) bromide Inorganic materials 0.000 description 2
- VMQMZMRVKUZKQL-UHFFFAOYSA-N Cu+ Chemical compound [Cu+] VMQMZMRVKUZKQL-UHFFFAOYSA-N 0.000 description 2
- JPVYNHNXODAKFH-UHFFFAOYSA-N Cu2+ Chemical class [Cu+2] JPVYNHNXODAKFH-UHFFFAOYSA-N 0.000 description 2
- 108010052832 Cytochromes Proteins 0.000 description 2
- 102000018832 Cytochromes Human genes 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- 241000537219 Deltabaculovirus Species 0.000 description 2
- YXHKONLOYHBTNS-UHFFFAOYSA-N Diazomethane Chemical compound C=[N+]=[N-] YXHKONLOYHBTNS-UHFFFAOYSA-N 0.000 description 2
- LWLJUMBEZJHXHV-UHFFFAOYSA-N Dienochlor Chemical compound ClC1=C(Cl)C(Cl)=C(Cl)C1(Cl)C1(Cl)C(Cl)=C(Cl)C(Cl)=C1Cl LWLJUMBEZJHXHV-UHFFFAOYSA-N 0.000 description 2
- 239000005897 Etoxazole Substances 0.000 description 2
- 239000005958 Fenamiphos (aka phenamiphos) Substances 0.000 description 2
- 239000005656 Fenazaquin Substances 0.000 description 2
- 239000005657 Fenpyroximate Substances 0.000 description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 244000068988 Glycine max Species 0.000 description 2
- 235000010469 Glycine max Nutrition 0.000 description 2
- 241000555709 Guignardia Species 0.000 description 2
- 239000005661 Hexythiazox Substances 0.000 description 2
- 239000005794 Hymexazol Substances 0.000 description 2
- PPCUNNLZTNMXFO-ACCUITESSA-N Imicyafos Chemical compound CCCSP(=O)(OCC)N1CCN(CC)\C1=N/C#N PPCUNNLZTNMXFO-ACCUITESSA-N 0.000 description 2
- WLLGXSLBOPFWQV-UHFFFAOYSA-N MGK 264 Chemical compound C1=CC2CC1C1C2C(=O)N(CC(CC)CCCC)C1=O WLLGXSLBOPFWQV-UHFFFAOYSA-N 0.000 description 2
- BYBLEWFAAKGYCD-UHFFFAOYSA-N Miconazole Chemical compound ClC1=CC(Cl)=CC=C1COC(C=1C(=CC(Cl)=CC=1)Cl)CN1C=NC=C1 BYBLEWFAAKGYCD-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- XFOXDUJCOHBXRC-UHFFFAOYSA-N N-Ethyl-N-methyl-4-(trifluoromethyl)-2-(3,4-dimethoxyphenyl)benzamide Chemical compound CCN(C)C(=O)C1=CC=C(C(F)(F)F)C=C1C1=CC=C(OC)C(OC)=C1 XFOXDUJCOHBXRC-UHFFFAOYSA-N 0.000 description 2
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 2
- CHJJGSNFBQVOTG-UHFFFAOYSA-N N-methyl-guanidine Natural products CNC(N)=N CHJJGSNFBQVOTG-UHFFFAOYSA-N 0.000 description 2
- XQJQCBDIXRIYRP-UHFFFAOYSA-N N-{2-[1,1'-bi(cyclopropyl)-2-yl]phenyl}-3-(difluoromethyl)-1-methyl-1pyrazole-4-carboxamide Chemical compound FC(F)C1=NN(C)C=C1C(=O)NC1=CC=CC=C1C1C(C2CC2)C1 XQJQCBDIXRIYRP-UHFFFAOYSA-N 0.000 description 2
- 239000007832 Na2SO4 Substances 0.000 description 2
- IOVCWXUNBOPUCH-UHFFFAOYSA-M Nitrite anion Chemical compound [O-]N=O IOVCWXUNBOPUCH-UHFFFAOYSA-M 0.000 description 2
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 2
- 239000012425 OXONE® Substances 0.000 description 2
- 239000005950 Oxamyl Substances 0.000 description 2
- 239000005814 Pencycuron Substances 0.000 description 2
- XYFCBTPGUUZFHI-UHFFFAOYSA-N Phosphine Chemical compound P XYFCBTPGUUZFHI-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 239000005821 Propamocarb Substances 0.000 description 2
- 239000005663 Pyridaben Substances 0.000 description 2
- 239000005828 Pyrimethanil Substances 0.000 description 2
- 235000004443 Ricinus communis Nutrition 0.000 description 2
- 238000000297 Sandmeyer reaction Methods 0.000 description 2
- 239000005834 Sedaxane Substances 0.000 description 2
- 239000005835 Silthiofam Substances 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 239000005837 Spiroxamine Substances 0.000 description 2
- 229930182692 Strobilurin Natural products 0.000 description 2
- ULUAUXLGCMPNKK-UHFFFAOYSA-N Sulfobutanedioic acid Chemical class OC(=O)CC(C(O)=O)S(O)(=O)=O ULUAUXLGCMPNKK-UHFFFAOYSA-N 0.000 description 2
- 239000005658 Tebufenpyrad Substances 0.000 description 2
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 2
- 239000005843 Thiram Substances 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- 239000005845 Tolclofos-methyl Substances 0.000 description 2
- 239000005847 Triazoxide Substances 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 229910052770 Uranium Inorganic materials 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- 229930195482 Validamycin Natural products 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 240000008042 Zea mays Species 0.000 description 2
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 239000005870 Ziram Substances 0.000 description 2
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 2
- 238000005054 agglomeration Methods 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- QGLZXHRNAYXIBU-WEVVVXLNSA-N aldicarb Chemical compound CNC(=O)O\N=C\C(C)(C)SC QGLZXHRNAYXIBU-WEVVVXLNSA-N 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 150000004996 alkyl benzenes Chemical class 0.000 description 2
- 150000008052 alkyl sulfonates Chemical class 0.000 description 2
- GGKQIOFASHYUJZ-UHFFFAOYSA-N ametoctradin Chemical compound NC1=C(CCCCCCCC)C(CC)=NC2=NC=NN21 GGKQIOFASHYUJZ-UHFFFAOYSA-N 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 229960002587 amitraz Drugs 0.000 description 2
- QXAITBQSYVNQDR-ZIOPAAQOSA-N amitraz Chemical compound C=1C=C(C)C=C(C)C=1/N=C/N(C)\C=N\C1=CC=C(C)C=C1C QXAITBQSYVNQDR-ZIOPAAQOSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 230000000844 anti-bacterial effect Effects 0.000 description 2
- 125000006615 aromatic heterocyclic group Chemical group 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 229960000892 attapulgite Drugs 0.000 description 2
- 150000003851 azoles Chemical class 0.000 description 2
- 229940097012 bacillus thuringiensis Drugs 0.000 description 2
- 239000003899 bactericide agent Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- 239000012965 benzophenone Substances 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- 239000003124 biologic agent Substances 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 229920001400 block copolymer Polymers 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 229930188620 butyrolactone Natural products 0.000 description 2
- KBPLFHHGFOOTCA-UHFFFAOYSA-N caprylic alcohol Natural products CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- 235000013339 cereals Nutrition 0.000 description 2
- PSOVNZZNOMJUBI-UHFFFAOYSA-N chlorantraniliprole Chemical compound CNC(=O)C1=CC(Cl)=CC(C)=C1NC(=O)C1=CC(Br)=NN1C1=NC=CC=C1Cl PSOVNZZNOMJUBI-UHFFFAOYSA-N 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 229940125833 compound 23 Drugs 0.000 description 2
- 229940125878 compound 36 Drugs 0.000 description 2
- 229940126540 compound 41 Drugs 0.000 description 2
- 239000012141 concentrate Substances 0.000 description 2
- 150000001880 copper compounds Chemical class 0.000 description 2
- 229910000365 copper sulfate Inorganic materials 0.000 description 2
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 2
- RWGFKTVRMDUZSP-UHFFFAOYSA-N cumene Chemical compound CC(C)C1=CC=CC=C1 RWGFKTVRMDUZSP-UHFFFAOYSA-N 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- AIMMVWOEOZMVMS-UHFFFAOYSA-N cyclopropanecarboxamide Chemical compound NC(=O)C1CC1 AIMMVWOEOZMVMS-UHFFFAOYSA-N 0.000 description 2
- WCMMILVIRZAPLE-UHFFFAOYSA-M cyhexatin Chemical compound C1CCCCC1[Sn](C1CCCCC1)(O)C1CCCCC1 WCMMILVIRZAPLE-UHFFFAOYSA-M 0.000 description 2
- MWKFXSUHUHTGQN-UHFFFAOYSA-N decan-1-ol Chemical compound CCCCCCCCCCO MWKFXSUHUHTGQN-UHFFFAOYSA-N 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 2
- 239000012954 diazonium Substances 0.000 description 2
- 150000001989 diazonium salts Chemical class 0.000 description 2
- 238000006193 diazotization reaction Methods 0.000 description 2
- UOAMTSKGCBMZTC-UHFFFAOYSA-N dicofol Chemical compound C=1C=C(Cl)C=CC=1C(C(Cl)(Cl)Cl)(O)C1=CC=C(Cl)C=C1 UOAMTSKGCBMZTC-UHFFFAOYSA-N 0.000 description 2
- 125000004786 difluoromethoxy group Chemical group [H]C(F)(F)O* 0.000 description 2
- NKDDWNXOKDWJAK-UHFFFAOYSA-N dimethoxymethane Chemical compound COCOC NKDDWNXOKDWJAK-UHFFFAOYSA-N 0.000 description 2
- SWSQBOPZIKWTGO-UHFFFAOYSA-N dimethylaminoamidine Natural products CN(C)C(N)=N SWSQBOPZIKWTGO-UHFFFAOYSA-N 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 229960003913 econazole Drugs 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- DWRKFAJEBUWTQM-UHFFFAOYSA-N etaconazole Chemical compound O1C(CC)COC1(C=1C(=CC(Cl)=CC=1)Cl)CN1N=CN=C1 DWRKFAJEBUWTQM-UHFFFAOYSA-N 0.000 description 2
- IXSZQYVWNJNRAL-UHFFFAOYSA-N etoxazole Chemical compound CCOC1=CC(C(C)(C)C)=CC=C1C1N=C(C=2C(=CC=CC=2F)F)OC1 IXSZQYVWNJNRAL-UHFFFAOYSA-N 0.000 description 2
- ZCJPOPBZHLUFHF-UHFFFAOYSA-N fenamiphos Chemical compound CCOP(=O)(NC(C)C)OC1=CC=C(SC)C(C)=C1 ZCJPOPBZHLUFHF-UHFFFAOYSA-N 0.000 description 2
- DMYHGDXADUDKCQ-UHFFFAOYSA-N fenazaquin Chemical compound C1=CC(C(C)(C)C)=CC=C1CCOC1=NC=NC2=CC=CC=C12 DMYHGDXADUDKCQ-UHFFFAOYSA-N 0.000 description 2
- XQUXKZZNEFRCAW-UHFFFAOYSA-N fenpropathrin Chemical compound CC1(C)C(C)(C)C1C(=O)OC(C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 XQUXKZZNEFRCAW-UHFFFAOYSA-N 0.000 description 2
- YYJNOYZRYGDPNH-MFKUBSTISA-N fenpyroximate Chemical compound C=1C=C(C(=O)OC(C)(C)C)C=CC=1CO/N=C/C=1C(C)=NN(C)C=1OC1=CC=CC=C1 YYJNOYZRYGDPNH-MFKUBSTISA-N 0.000 description 2
- 238000007667 floating Methods 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- IPENDKRRWFURRE-UHFFFAOYSA-N fluoroimide Chemical compound C1=CC(F)=CC=C1N1C(=O)C(Cl)=C(Cl)C1=O IPENDKRRWFURRE-UHFFFAOYSA-N 0.000 description 2
- KGXUEPOHGFWQKF-ZCXUNETKSA-N flutianil Chemical compound COC1=CC=CC=C1N(CCS\1)C/1=C(C#N)/SC1=CC(C(F)(F)F)=CC=C1F KGXUEPOHGFWQKF-ZCXUNETKSA-N 0.000 description 2
- TVFIYRKPCACCNL-UHFFFAOYSA-N furan-2-carboxamide Chemical compound NC(=O)C1=CC=CO1 TVFIYRKPCACCNL-UHFFFAOYSA-N 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 235000013773 glyceryl triacetate Nutrition 0.000 description 2
- 238000000227 grinding Methods 0.000 description 2
- 150000004820 halides Chemical class 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- CATSNJVOTSVZJV-UHFFFAOYSA-N heptan-2-one Chemical compound CCCCCC(C)=O CATSNJVOTSVZJV-UHFFFAOYSA-N 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- ZSIAUFGUXNUGDI-UHFFFAOYSA-N hexan-1-ol Chemical compound CCCCCCO ZSIAUFGUXNUGDI-UHFFFAOYSA-N 0.000 description 2
- AOGQPLXWSUTHQB-UHFFFAOYSA-N hexyl acetate Chemical compound CCCCCCOC(C)=O AOGQPLXWSUTHQB-UHFFFAOYSA-N 0.000 description 2
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 2
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 2
- 125000001841 imino group Chemical group [H]N=* 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 238000011835 investigation Methods 0.000 description 2
- MLFHJEHSLIIPHL-UHFFFAOYSA-N isoamyl acetate Chemical compound CC(C)CCOC(C)=O MLFHJEHSLIIPHL-UHFFFAOYSA-N 0.000 description 2
- ZXEKIIBDNHEJCQ-UHFFFAOYSA-N isobutanol Chemical compound CC(C)CO ZXEKIIBDNHEJCQ-UHFFFAOYSA-N 0.000 description 2
- 229960004849 isoconazole Drugs 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- HJOVHMDZYOCNQW-UHFFFAOYSA-N isophorone Chemical compound CC1=CC(=O)CC(C)(C)C1 HJOVHMDZYOCNQW-UHFFFAOYSA-N 0.000 description 2
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 2
- 150000002540 isothiocyanates Chemical class 0.000 description 2
- 229920005610 lignin Chemical class 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 2
- 229940126707 lipid peroxidation inhibitor Drugs 0.000 description 2
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 2
- YNESATAKKCNGOF-UHFFFAOYSA-N lithium bis(trimethylsilyl)amide Chemical compound [Li+].C[Si](C)(C)[N-][Si](C)(C)C YNESATAKKCNGOF-UHFFFAOYSA-N 0.000 description 2
- RENRQMCACQEWFC-UGKGYDQZSA-N lnp023 Chemical compound C1([C@H]2N(CC=3C=4C=CNC=4C(C)=CC=3OC)CC[C@@H](C2)OCC)=CC=C(C(O)=O)C=C1 RENRQMCACQEWFC-UGKGYDQZSA-N 0.000 description 2
- IUYHWZFSGMZEOG-UHFFFAOYSA-M magnesium;propane;chloride Chemical compound [Mg+2].[Cl-].C[CH-]C IUYHWZFSGMZEOG-UHFFFAOYSA-M 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- CKFGINPQOCXMAZ-UHFFFAOYSA-N methanediol Chemical compound OCO CKFGINPQOCXMAZ-UHFFFAOYSA-N 0.000 description 2
- BYFVQGSSOPBYMR-UHFFFAOYSA-N methoxycarbamic acid Chemical compound CONC(O)=O BYFVQGSSOPBYMR-UHFFFAOYSA-N 0.000 description 2
- KJIXWGGDFINGJL-UHFFFAOYSA-N methyl 2-(2,4,6-trifluorophenyl)acetate Chemical compound COC(=O)CC1=C(F)C=C(F)C=C1F KJIXWGGDFINGJL-UHFFFAOYSA-N 0.000 description 2
- NJTBUDSSZFYJAO-UHFFFAOYSA-N methyl 3-oxo-2-(2,4,6-trifluorophenyl)butanoate Chemical compound COC(=O)C(C(C)=O)C1=C(F)C=C(F)C=C1F NJTBUDSSZFYJAO-UHFFFAOYSA-N 0.000 description 2
- 239000012022 methylating agents Substances 0.000 description 2
- 229960002509 miconazole Drugs 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 210000001700 mitochondrial membrane Anatomy 0.000 description 2
- 229910052901 montmorillonite Inorganic materials 0.000 description 2
- XGXNTJHZPBRBHJ-UHFFFAOYSA-N n-phenylpyrimidin-2-amine Chemical compound N=1C=CC=NC=1NC1=CC=CC=C1 XGXNTJHZPBRBHJ-UHFFFAOYSA-N 0.000 description 2
- OZGNYLLQHRPOBR-DHZHZOJOSA-N naftifine Chemical compound C=1C=CC2=CC=CC=C2C=1CN(C)C\C=C\C1=CC=CC=C1 OZGNYLLQHRPOBR-DHZHZOJOSA-N 0.000 description 2
- 229960004313 naftifine Drugs 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- BOPGDPNILDQYTO-NNYOXOHSSA-N nicotinamide-adenine dinucleotide Chemical compound C1=CCC(C(=O)N)=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OC[C@@H]2[C@H]([C@@H](O)[C@@H](O2)N2C3=NC=NC(N)=C3N=C2)O)O1 BOPGDPNILDQYTO-NNYOXOHSSA-N 0.000 description 2
- 229930027945 nicotinamide-adenine dinucleotide Natural products 0.000 description 2
- GJQIMXVRFNLMTB-UHFFFAOYSA-N nonyl acetate Chemical compound CCCCCCCCCOC(C)=O GJQIMXVRFNLMTB-UHFFFAOYSA-N 0.000 description 2
- YTYGAJLZOJPJGH-UHFFFAOYSA-N noviflumuron Chemical compound FC1=C(Cl)C(OC(F)(F)C(C(F)(F)F)F)=C(Cl)C=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F YTYGAJLZOJPJGH-UHFFFAOYSA-N 0.000 description 2
- 239000012038 nucleophile Substances 0.000 description 2
- 230000000269 nucleophilic effect Effects 0.000 description 2
- 238000007344 nucleophilic reaction Methods 0.000 description 2
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 2
- YLYBTZIQSIBWLI-UHFFFAOYSA-N octyl acetate Chemical compound CCCCCCCCOC(C)=O YLYBTZIQSIBWLI-UHFFFAOYSA-N 0.000 description 2
- KZAUOCCYDRDERY-UHFFFAOYSA-N oxamyl Chemical compound CNC(=O)ON=C(SC)C(=O)N(C)C KZAUOCCYDRDERY-UHFFFAOYSA-N 0.000 description 2
- IMTNSEPDLICZMZ-UHFFFAOYSA-N oxathiine-3-carboxamide Chemical compound NC(=O)C1=CC=COS1 IMTNSEPDLICZMZ-UHFFFAOYSA-N 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 150000002940 palladium Chemical class 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- LXNAVEXFUKBNMK-UHFFFAOYSA-N palladium(II) acetate Substances [Pd].CC(O)=O.CC(O)=O LXNAVEXFUKBNMK-UHFFFAOYSA-N 0.000 description 2
- 229910052625 palygorskite Inorganic materials 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- OGYFATSSENRIKG-UHFFFAOYSA-N pencycuron Chemical compound C1=CC(Cl)=CC=C1CN(C(=O)NC=1C=CC=CC=1)C1CCCC1 OGYFATSSENRIKG-UHFFFAOYSA-N 0.000 description 2
- WBTYBAGIHOISOQ-UHFFFAOYSA-N pent-4-en-1-yl 2-[(2-furylmethyl)(imidazol-1-ylcarbonyl)amino]butanoate Chemical compound C1=CN=CN1C(=O)N(C(CC)C(=O)OCCCC=C)CC1=CC=CO1 WBTYBAGIHOISOQ-UHFFFAOYSA-N 0.000 description 2
- LKPLKUMXSAEKID-UHFFFAOYSA-N pentachloronitrobenzene Chemical compound [O-][N+](=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1Cl LKPLKUMXSAEKID-UHFFFAOYSA-N 0.000 description 2
- 125000006340 pentafluoro ethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 2
- 150000002989 phenols Chemical class 0.000 description 2
- 125000001639 phenylmethylene group Chemical group [H]C(=*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 2
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- IBBMAWULFFBRKK-UHFFFAOYSA-N picolinamide Chemical compound NC(=O)C1=CC=CC=N1 IBBMAWULFFBRKK-UHFFFAOYSA-N 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 229920002689 polyvinyl acetate Polymers 0.000 description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 description 2
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- NROKBHXJSPEDAR-UHFFFAOYSA-M potassium fluoride Chemical compound [F-].[K+] NROKBHXJSPEDAR-UHFFFAOYSA-M 0.000 description 2
- 229910001414 potassium ion Inorganic materials 0.000 description 2
- OKBMCNHOEMXPTM-UHFFFAOYSA-M potassium peroxymonosulfate Chemical compound [K+].OOS([O-])(=O)=O OKBMCNHOEMXPTM-UHFFFAOYSA-M 0.000 description 2
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical group [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- WHHIPMZEDGBUCC-UHFFFAOYSA-N probenazole Chemical compound C1=CC=C2C(OCC=C)=NS(=O)(=O)C2=C1 WHHIPMZEDGBUCC-UHFFFAOYSA-N 0.000 description 2
- WZZLDXDUQPOXNW-UHFFFAOYSA-N propamocarb Chemical compound CCCOC(=O)NCCCN(C)C WZZLDXDUQPOXNW-UHFFFAOYSA-N 0.000 description 2
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 2
- ZYHMJXZULPZUED-UHFFFAOYSA-N propargite Chemical compound C1=CC(C(C)(C)C)=CC=C1OC1C(OS(=O)OCC#C)CCCC1 ZYHMJXZULPZUED-UHFFFAOYSA-N 0.000 description 2
- QLNJFJADRCOGBJ-UHFFFAOYSA-N propionamide Chemical compound CCC(N)=O QLNJFJADRCOGBJ-UHFFFAOYSA-N 0.000 description 2
- 238000001243 protein synthesis Methods 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- YRRBXJLFCBCKNW-UHFFFAOYSA-N prothiocarb Chemical compound CCSC(=O)NCCCN(C)C YRRBXJLFCBCKNW-UHFFFAOYSA-N 0.000 description 2
- JOOMJVFZQRQWKR-UHFFFAOYSA-N pyrazophos Chemical compound N1=C(C)C(C(=O)OCC)=CN2N=C(OP(=S)(OCC)OCC)C=C21 JOOMJVFZQRQWKR-UHFFFAOYSA-N 0.000 description 2
- CRFYLQMIDWBKRT-LPYMAVHISA-N pyribencarb Chemical compound C1=C(Cl)C(CNC(=O)OC)=CC(C(\C)=N\OCC=2N=C(C)C=CC=2)=C1 CRFYLQMIDWBKRT-LPYMAVHISA-N 0.000 description 2
- VTRWMTJQBQJKQH-UHFFFAOYSA-N pyributicarb Chemical compound COC1=CC=CC(N(C)C(=S)OC=2C=C(C=CC=2)C(C)(C)C)=N1 VTRWMTJQBQJKQH-UHFFFAOYSA-N 0.000 description 2
- DWFZBUWUXWZWKD-UHFFFAOYSA-N pyridaben Chemical compound C1=CC(C(C)(C)C)=CC=C1CSC1=C(Cl)C(=O)N(C(C)(C)C)N=C1 DWFZBUWUXWZWKD-UHFFFAOYSA-N 0.000 description 2
- ZLIBICFPKPWGIZ-UHFFFAOYSA-N pyrimethanil Chemical compound CC1=CC(C)=NC(NC=2C=CC=CC=2)=N1 ZLIBICFPKPWGIZ-UHFFFAOYSA-N 0.000 description 2
- XRJLAOUDSILTFT-UHFFFAOYSA-N pyroquilon Chemical compound O=C1CCC2=CC=CC3=C2N1CC3 XRJLAOUDSILTFT-UHFFFAOYSA-N 0.000 description 2
- 229960002132 pyrrolnitrin Drugs 0.000 description 2
- WUKKREVJKMPFTB-UHFFFAOYSA-N pyrrolo[2,3-h]quinolin-2-one Chemical compound C1=C2N=CC=C2C2=NC(=O)C=CC2=C1 WUKKREVJKMPFTB-UHFFFAOYSA-N 0.000 description 2
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 2
- 229920002477 rna polymer Polymers 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- MXMXHPPIGKYTAR-UHFFFAOYSA-N silthiofam Chemical compound CC=1SC([Si](C)(C)C)=C(C(=O)NCC=C)C=1C MXMXHPPIGKYTAR-UHFFFAOYSA-N 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 235000017550 sodium carbonate Nutrition 0.000 description 2
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 2
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 2
- JQWHASGSAFIOCM-UHFFFAOYSA-M sodium periodate Chemical compound [Na+].[O-]I(=O)(=O)=O JQWHASGSAFIOCM-UHFFFAOYSA-M 0.000 description 2
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 239000011877 solvent mixture Substances 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 241000894007 species Species 0.000 description 2
- GOLXNESZZPUPJE-UHFFFAOYSA-N spiromesifen Chemical compound CC1=CC(C)=CC(C)=C1C(C(O1)=O)=C(OC(=O)CC(C)(C)C)C11CCCC1 GOLXNESZZPUPJE-UHFFFAOYSA-N 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- 239000000021 stimulant Substances 0.000 description 2
- NVBFHJWHLNUMCV-UHFFFAOYSA-N sulfamide Chemical compound NS(N)(=O)=O NVBFHJWHLNUMCV-UHFFFAOYSA-N 0.000 description 2
- 239000004546 suspension concentrate Substances 0.000 description 2
- 230000002195 synergetic effect Effects 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- 239000003760 tallow Substances 0.000 description 2
- ZZYSLNWGKKDOML-UHFFFAOYSA-N tebufenpyrad Chemical compound CCC1=NN(C)C(C(=O)NCC=2C=CC(=CC=2)C(C)(C)C)=C1Cl ZZYSLNWGKKDOML-UHFFFAOYSA-N 0.000 description 2
- LWLJEQHTPVPKSJ-UHFFFAOYSA-N tebufloquin Chemical compound C1=C(C(C)(C)C)C=C2C(OC(=O)C)=C(C)C(C)=NC2=C1F LWLJEQHTPVPKSJ-UHFFFAOYSA-N 0.000 description 2
- XQTLDIFVVHJORV-UHFFFAOYSA-N tecnazene Chemical compound [O-][N+](=O)C1=C(Cl)C(Cl)=CC(Cl)=C1Cl XQTLDIFVVHJORV-UHFFFAOYSA-N 0.000 description 2
- XLNZEKHULJKQBA-UHFFFAOYSA-N terbufos Chemical compound CCOP(=S)(OCC)SCSC(C)(C)C XLNZEKHULJKQBA-UHFFFAOYSA-N 0.000 description 2
- ISIJQEHRDSCQIU-UHFFFAOYSA-N tert-butyl 2,7-diazaspiro[4.5]decane-7-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCCC11CNCC1 ISIJQEHRDSCQIU-UHFFFAOYSA-N 0.000 description 2
- 229940072172 tetracycline antibiotic Drugs 0.000 description 2
- WJCNZQLZVWNLKY-UHFFFAOYSA-N thiabendazole Chemical compound S1C=NC(C=2NC3=CC=CC=C3N=2)=C1 WJCNZQLZVWNLKY-UHFFFAOYSA-N 0.000 description 2
- 239000004308 thiabendazole Substances 0.000 description 2
- 229960004546 thiabendazole Drugs 0.000 description 2
- 235000010296 thiabendazole Nutrition 0.000 description 2
- INWVNNCOIIHEPX-UHFFFAOYSA-N thiadiazole-4-carboxamide Chemical compound NC(=O)C1=CSN=N1 INWVNNCOIIHEPX-UHFFFAOYSA-N 0.000 description 2
- 150000003568 thioethers Chemical class 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- KUAZQDVKQLNFPE-UHFFFAOYSA-N thiram Chemical compound CN(C)C(=S)SSC(=S)N(C)C KUAZQDVKQLNFPE-UHFFFAOYSA-N 0.000 description 2
- 229960002447 thiram Drugs 0.000 description 2
- VJQYLJSMBWXGDV-UHFFFAOYSA-N tiadinil Chemical compound N1=NSC(C(=O)NC=2C=C(Cl)C(C)=CC=2)=C1C VJQYLJSMBWXGDV-UHFFFAOYSA-N 0.000 description 2
- OBZIQQJJIKNWNO-UHFFFAOYSA-N tolclofos-methyl Chemical compound COP(=S)(OC)OC1=C(Cl)C=C(C)C=C1Cl OBZIQQJJIKNWNO-UHFFFAOYSA-N 0.000 description 2
- HYVWIQDYBVKITD-UHFFFAOYSA-N tolylfluanid Chemical compound CN(C)S(=O)(=O)N(SC(F)(Cl)Cl)C1=CC=C(C)C=C1 HYVWIQDYBVKITD-UHFFFAOYSA-N 0.000 description 2
- 229960002622 triacetin Drugs 0.000 description 2
- IQGKIPDJXCAMSM-UHFFFAOYSA-N triazoxide Chemical compound N=1C2=CC=C(Cl)C=C2[N+]([O-])=NC=1N1C=CN=C1 IQGKIPDJXCAMSM-UHFFFAOYSA-N 0.000 description 2
- 230000004102 tricarboxylic acid cycle Effects 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- 241000701451 unidentified granulovirus Species 0.000 description 2
- JARYYMUOCXVXNK-IMTORBKUSA-N validamycin Chemical compound N([C@H]1C[C@@H]([C@H]([C@H](O)[C@H]1O)O[C@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)CO)[C@H]1C=C(CO)[C@H](O)[C@H](O)[C@H]1O JARYYMUOCXVXNK-IMTORBKUSA-N 0.000 description 2
- 235000013311 vegetables Nutrition 0.000 description 2
- UGOMMVLRQDMAQQ-UHFFFAOYSA-N xphos Chemical group CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 UGOMMVLRQDMAQQ-UHFFFAOYSA-N 0.000 description 2
- VNDYJBBGRKZCSX-UHFFFAOYSA-L zinc bromide Chemical compound Br[Zn]Br VNDYJBBGRKZCSX-UHFFFAOYSA-L 0.000 description 2
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 description 2
- DUBNHZYBDBBJHD-UHFFFAOYSA-L ziram Chemical compound [Zn+2].CN(C)C([S-])=S.CN(C)C([S-])=S DUBNHZYBDBBJHD-UHFFFAOYSA-L 0.000 description 2
- BQPPJGMMIYJVBR-UHFFFAOYSA-N (10S)-3c-Acetoxy-4.4.10r.13c.14t-pentamethyl-17c-((R)-1.5-dimethyl-hexen-(4)-yl)-(5tH)-Delta8-tetradecahydro-1H-cyclopenta[a]phenanthren Natural products CC12CCC(OC(C)=O)C(C)(C)C1CCC1=C2CCC2(C)C(C(CCC=C(C)C)C)CCC21C BQPPJGMMIYJVBR-UHFFFAOYSA-N 0.000 description 1
- ZXQYGBMAQZUVMI-RDDWSQKMSA-N (1S)-cis-(alphaR)-cyhalothrin Chemical compound CC1(C)[C@H](\C=C(/Cl)C(F)(F)F)[C@@H]1C(=O)O[C@@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 ZXQYGBMAQZUVMI-RDDWSQKMSA-N 0.000 description 1
- AOSZTAHDEDLTLQ-AZKQZHLXSA-N (1S,2S,4R,8S,9S,11S,12R,13S,19S)-6-[(3-chlorophenyl)methyl]-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-9,13-dimethyl-6-azapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one Chemical compound C([C@@H]1C[C@H]2[C@H]3[C@]([C@]4(C=CC(=O)C=C4[C@@H](F)C3)C)(F)[C@@H](O)C[C@@]2([C@@]1(C1)C(=O)CO)C)N1CC1=CC=CC(Cl)=C1 AOSZTAHDEDLTLQ-AZKQZHLXSA-N 0.000 description 1
- UKSZBOKPHAQOMP-SVLSSHOZSA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 UKSZBOKPHAQOMP-SVLSSHOZSA-N 0.000 description 1
- AGMMRUPNXPWLGF-AATRIKPKSA-N (2,3,5,6-tetrafluoro-4-methylphenyl)methyl 2,2-dimethyl-3-[(e)-prop-1-enyl]cyclopropane-1-carboxylate Chemical compound CC1(C)C(/C=C/C)C1C(=O)OCC1=C(F)C(F)=C(C)C(F)=C1F AGMMRUPNXPWLGF-AATRIKPKSA-N 0.000 description 1
- XOFNMNLYGPKKOV-UHFFFAOYSA-N (2-chloro-4-fluorophenyl)boronic acid Chemical compound OB(O)C1=CC=C(F)C=C1Cl XOFNMNLYGPKKOV-UHFFFAOYSA-N 0.000 description 1
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- LZTIMERBDGGAJD-SNAWJCMRSA-N (2e)-2-(nitromethylidene)-1,3-thiazinane Chemical compound [O-][N+](=O)\C=C1/NCCCS1 LZTIMERBDGGAJD-SNAWJCMRSA-N 0.000 description 1
- XDEHMKQLKPZERH-BYPYZUCNSA-N (2s)-2-amino-3-methylbutanamide Chemical compound CC(C)[C@H](N)C(N)=O XDEHMKQLKPZERH-BYPYZUCNSA-N 0.000 description 1
- CHGIKSSZNBCNDW-UHFFFAOYSA-N (3beta,5alpha)-4,4-Dimethylcholesta-8,24-dien-3-ol Natural products CC12CCC(O)C(C)(C)C1CCC1=C2CCC2(C)C(C(CCC=C(C)C)C)CCC21 CHGIKSSZNBCNDW-UHFFFAOYSA-N 0.000 description 1
- OOKAZRDERJMRCJ-KOUAFAAESA-N (3r)-7-[(1s,2s,4ar,6s,8s)-2,6-dimethyl-8-[(2s)-2-methylbutanoyl]oxy-1,2,4a,5,6,7,8,8a-octahydronaphthalen-1-yl]-3-hydroxy-5-oxoheptanoic acid Chemical compound C1=C[C@H](C)[C@H](CCC(=O)C[C@@H](O)CC(O)=O)C2[C@@H](OC(=O)[C@@H](C)CC)C[C@@H](C)C[C@@H]21 OOKAZRDERJMRCJ-KOUAFAAESA-N 0.000 description 1
- VYRMNTABYYNYHW-UHFFFAOYSA-N (4-chloro-2,6-difluorophenyl)-[4-(2-chloro-4-fluorophenyl)-2,5-dimethylpyrazol-3-yl]methanol Chemical compound C=1C=C(F)C=C(Cl)C=1C=1C(C)=NN(C)C=1C(O)C1=C(F)C=C(Cl)C=C1F VYRMNTABYYNYHW-UHFFFAOYSA-N 0.000 description 1
- XUNYDVLIZWUPAW-UHFFFAOYSA-N (4-chlorophenyl) n-(4-methylphenyl)sulfonylcarbamate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)NC(=O)OC1=CC=C(Cl)C=C1 XUNYDVLIZWUPAW-UHFFFAOYSA-N 0.000 description 1
- MGAXYKDBRBNWKT-UHFFFAOYSA-N (5-oxooxolan-2-yl)methyl 4-methylbenzenesulfonate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)OCC1OC(=O)CC1 MGAXYKDBRBNWKT-UHFFFAOYSA-N 0.000 description 1
- OIIOPWHTJZYKIL-PMACEKPBSA-N (5S)-5-[[[5-[2-chloro-3-[2-chloro-3-[6-methoxy-5-[[[(2S)-5-oxopyrrolidin-2-yl]methylamino]methyl]pyrazin-2-yl]phenyl]phenyl]-3-methoxypyrazin-2-yl]methylamino]methyl]pyrrolidin-2-one Chemical compound C1(=C(N=C(C2=C(C(C3=CC=CC(=C3Cl)C3=NC(OC)=C(N=C3)CNC[C@H]3NC(=O)CC3)=CC=C2)Cl)C=N1)OC)CNC[C@H]1NC(=O)CC1 OIIOPWHTJZYKIL-PMACEKPBSA-N 0.000 description 1
- ALSTYHKOOCGGFT-KTKRTIGZSA-N (9Z)-octadecen-1-ol Chemical compound CCCCCCCC\C=C/CCCCCCCCO ALSTYHKOOCGGFT-KTKRTIGZSA-N 0.000 description 1
- WCXDHFDTOYPNIE-RIYZIHGNSA-N (E)-acetamiprid Chemical compound N#C/N=C(\C)N(C)CC1=CC=C(Cl)N=C1 WCXDHFDTOYPNIE-RIYZIHGNSA-N 0.000 description 1
- PGOOBECODWQEAB-UHFFFAOYSA-N (E)-clothianidin Chemical compound [O-][N+](=O)\N=C(/NC)NCC1=CN=C(Cl)S1 PGOOBECODWQEAB-UHFFFAOYSA-N 0.000 description 1
- CFRPSFYHXJZSBI-DHZHZOJOSA-N (E)-nitenpyram Chemical compound [O-][N+](=O)/C=C(\NC)N(CC)CC1=CC=C(Cl)N=C1 CFRPSFYHXJZSBI-DHZHZOJOSA-N 0.000 description 1
- IQVNEKKDSLOHHK-FNCQTZNRSA-N (E,E)-hydramethylnon Chemical compound N1CC(C)(C)CNC1=NN=C(/C=C/C=1C=CC(=CC=1)C(F)(F)F)\C=C\C1=CC=C(C(F)(F)F)C=C1 IQVNEKKDSLOHHK-FNCQTZNRSA-N 0.000 description 1
- ZFHGXWPMULPQSE-SZGBIDFHSA-N (Z)-(1S)-cis-tefluthrin Chemical compound FC1=C(F)C(C)=C(F)C(F)=C1COC(=O)[C@@H]1C(C)(C)[C@@H]1\C=C(/Cl)C(F)(F)F ZFHGXWPMULPQSE-SZGBIDFHSA-N 0.000 description 1
- HOKKPVIRMVDYPB-UVTDQMKNSA-N (Z)-thiacloprid Chemical compound C1=NC(Cl)=CC=C1CN1C(=N/C#N)/SCC1 HOKKPVIRMVDYPB-UVTDQMKNSA-N 0.000 description 1
- IAKOZHOLGAGEJT-UHFFFAOYSA-N 1,1,1-trichloro-2,2-bis(p-methoxyphenyl)-Ethane Chemical compound C1=CC(OC)=CC=C1C(C(Cl)(Cl)Cl)C1=CC=C(OC)C=C1 IAKOZHOLGAGEJT-UHFFFAOYSA-N 0.000 description 1
- PTTUMBGORBMNBN-UHFFFAOYSA-N 1,2,3-trifluoro-5-nitrobenzene Chemical compound [O-][N+](=O)C1=CC(F)=C(F)C(F)=C1 PTTUMBGORBMNBN-UHFFFAOYSA-N 0.000 description 1
- ZZXUZKXVROWEIF-UHFFFAOYSA-N 1,2-butylene carbonate Chemical compound CCC1COC(=O)O1 ZZXUZKXVROWEIF-UHFFFAOYSA-N 0.000 description 1
- FVRXOULDGSWPPO-UHFFFAOYSA-N 1,2-dihydropyrazole-3-thione Chemical class SC1=CC=NN1 FVRXOULDGSWPPO-UHFFFAOYSA-N 0.000 description 1
- UPNNXUSUOSTIIM-UHFFFAOYSA-N 1,2-dithietane Chemical group C1CSS1 UPNNXUSUOSTIIM-UHFFFAOYSA-N 0.000 description 1
- NODLZCJDRXTSJO-UHFFFAOYSA-N 1,3-dimethylpyrazole Chemical compound CC=1C=CN(C)N=1 NODLZCJDRXTSJO-UHFFFAOYSA-N 0.000 description 1
- IMLSAISZLJGWPP-UHFFFAOYSA-N 1,3-dithiolane Chemical compound C1CSCS1 IMLSAISZLJGWPP-UHFFFAOYSA-N 0.000 description 1
- ZRHUHDUEXWHZMA-UHFFFAOYSA-N 1,4-dihydropyrazol-5-one Chemical class O=C1CC=NN1 ZRHUHDUEXWHZMA-UHFFFAOYSA-N 0.000 description 1
- ITVVHXZQWSCZBC-UHFFFAOYSA-N 1-(2,4-dichlorophenyl)propan-2-one Chemical compound CC(=O)CC1=CC=C(Cl)C=C1Cl ITVVHXZQWSCZBC-UHFFFAOYSA-N 0.000 description 1
- XFRVVPUIAFSTFO-UHFFFAOYSA-N 1-Tridecanol Chemical compound CCCCCCCCCCCCCO XFRVVPUIAFSTFO-UHFFFAOYSA-N 0.000 description 1
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 1
- SQAINHDHICKHLX-UHFFFAOYSA-N 1-naphthaldehyde Chemical compound C1=CC=C2C(C=O)=CC=CC2=C1 SQAINHDHICKHLX-UHFFFAOYSA-N 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- XYTLYKGXLMKYMV-UHFFFAOYSA-N 14alpha-methylzymosterol Natural products CC12CCC(O)CC1CCC1=C2CCC2(C)C(C(CCC=C(C)C)C)CCC21C XYTLYKGXLMKYMV-UHFFFAOYSA-N 0.000 description 1
- AVRPFRMDMNDIDH-UHFFFAOYSA-N 1h-quinazolin-2-one Chemical class C1=CC=CC2=NC(O)=NC=C21 AVRPFRMDMNDIDH-UHFFFAOYSA-N 0.000 description 1
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 1
- TVFWYUWNQVRQRG-UHFFFAOYSA-N 2,3,4-tris(2-phenylethenyl)phenol Chemical class C=1C=CC=CC=1C=CC1=C(C=CC=2C=CC=CC=2)C(O)=CC=C1C=CC1=CC=CC=C1 TVFWYUWNQVRQRG-UHFFFAOYSA-N 0.000 description 1
- JKTAIYGNOFSMCE-UHFFFAOYSA-N 2,3-di(nonyl)phenol Chemical compound CCCCCCCCCC1=CC=CC(O)=C1CCCCCCCCC JKTAIYGNOFSMCE-UHFFFAOYSA-N 0.000 description 1
- 239000000263 2,3-dihydroxypropyl (Z)-octadec-9-enoate Substances 0.000 description 1
- BJSVKBGQDHUBHZ-UHFFFAOYSA-N 2,4,6-trifluoroaniline Chemical compound NC1=C(F)C=C(F)C=C1F BJSVKBGQDHUBHZ-UHFFFAOYSA-N 0.000 description 1
- ABGGPKIFVAIRGU-UHFFFAOYSA-N 2,4-difluoro-1-isothiocyanatobenzene Chemical compound FC1=CC=C(N=C=S)C(F)=C1 ABGGPKIFVAIRGU-UHFFFAOYSA-N 0.000 description 1
- WCGPCBACLBHDCI-UHFFFAOYSA-N 2,4-difluorobenzaldehyde Chemical compound FC1=CC=C(C=O)C(F)=C1 WCGPCBACLBHDCI-UHFFFAOYSA-N 0.000 description 1
- 125000004215 2,4-difluorophenyl group Chemical group [H]C1=C([H])C(*)=C(F)C([H])=C1F 0.000 description 1
- JXPVQFCUIAKFLT-UHFFFAOYSA-N 2,5-dimethyl-1h-pyrazol-3-one Chemical compound CC1=CC(=O)N(C)N1 JXPVQFCUIAKFLT-UHFFFAOYSA-N 0.000 description 1
- QFUSCYRJMXLNRB-UHFFFAOYSA-N 2,6-dinitroaniline Chemical class NC1=C([N+]([O-])=O)C=CC=C1[N+]([O-])=O QFUSCYRJMXLNRB-UHFFFAOYSA-N 0.000 description 1
- JNYAEWCLZODPBN-UHFFFAOYSA-N 2-(1,2-dihydroxyethyl)oxolane-3,4-diol Polymers OCC(O)C1OCC(O)C1O JNYAEWCLZODPBN-UHFFFAOYSA-N 0.000 description 1
- NGEKZFHYZPHNKQ-UHFFFAOYSA-N 2-(2,4,6-trifluorophenyl)acetic acid Chemical compound OC(=O)CC1=C(F)C=C(F)C=C1F NGEKZFHYZPHNKQ-UHFFFAOYSA-N 0.000 description 1
- PBZTWPWMQQLXME-UHFFFAOYSA-N 2-(2,4-difluorophenyl)-3-oxobutanenitrile Chemical compound CC(=O)C(C#N)C1=CC=C(F)C=C1F PBZTWPWMQQLXME-UHFFFAOYSA-N 0.000 description 1
- AGAOESUOSOGZOD-UHFFFAOYSA-N 2-(2,4-difluorophenyl)acetonitrile Chemical compound FC1=CC=C(CC#N)C(F)=C1 AGAOESUOSOGZOD-UHFFFAOYSA-N 0.000 description 1
- QEBYEVQKHRUYPE-UHFFFAOYSA-N 2-(2-chlorophenyl)-5-[(1-methylpyrazol-3-yl)methyl]-4-[[methyl(pyridin-3-ylmethyl)amino]methyl]-1h-pyrazolo[4,3-c]pyridine-3,6-dione Chemical compound C1=CN(C)N=C1CN1C(=O)C=C2NN(C=3C(=CC=CC=3)Cl)C(=O)C2=C1CN(C)CC1=CC=CN=C1 QEBYEVQKHRUYPE-UHFFFAOYSA-N 0.000 description 1
- JCRQCVDDBZVTMB-UHFFFAOYSA-N 2-(bromomethyl)-1,3-difluoro-5-methoxybenzene Chemical compound COC1=CC(F)=C(CBr)C(F)=C1 JCRQCVDDBZVTMB-UHFFFAOYSA-N 0.000 description 1
- MDFXJBQEWLCGHP-MFOYZWKCSA-N 2-[2-[(z)-(pyridine-4-carbonylhydrazinylidene)methyl]phenoxy]acetic acid Chemical compound OC(=O)COC1=CC=CC=C1\C=N/NC(=O)C1=CC=NC=C1 MDFXJBQEWLCGHP-MFOYZWKCSA-N 0.000 description 1
- KGXUEPOHGFWQKF-UHFFFAOYSA-N 2-[2-fluoro-5-(trifluoromethyl)phenyl]sulfanyl-2-[3-(2-methoxyphenyl)-1,3-thiazolidin-2-ylidene]acetonitrile Chemical compound COC1=CC=CC=C1N(CCS1)C1=C(C#N)SC1=CC(C(F)(F)F)=CC=C1F KGXUEPOHGFWQKF-UHFFFAOYSA-N 0.000 description 1
- ZDOOQPFIGYHZFV-UHFFFAOYSA-N 2-ethyl-4-[(4-phenoxyphenoxy)methyl]-1,3-dioxolane Chemical compound O1C(CC)OCC1COC(C=C1)=CC=C1OC1=CC=CC=C1 ZDOOQPFIGYHZFV-UHFFFAOYSA-N 0.000 description 1
- LFOIDLOIBZFWDO-UHFFFAOYSA-N 2-methoxy-6-[6-methoxy-4-[(3-phenylmethoxyphenyl)methoxy]-1-benzofuran-2-yl]imidazo[2,1-b][1,3,4]thiadiazole Chemical compound N1=C2SC(OC)=NN2C=C1C(OC1=CC(OC)=C2)=CC1=C2OCC(C=1)=CC=CC=1OCC1=CC=CC=C1 LFOIDLOIBZFWDO-UHFFFAOYSA-N 0.000 description 1
- AWSZRJQNBMEZOI-UHFFFAOYSA-N 2-methoxyethyl 2-(4-tert-butylphenyl)-2-cyano-3-oxo-3-[2-(trifluoromethyl)phenyl]propanoate Chemical compound C=1C=C(C(C)(C)C)C=CC=1C(C#N)(C(=O)OCCOC)C(=O)C1=CC=CC=C1C(F)(F)F AWSZRJQNBMEZOI-UHFFFAOYSA-N 0.000 description 1
- LQAQMOIBXDELJX-UHFFFAOYSA-N 2-methoxyprop-2-enoic acid Chemical class COC(=C)C(O)=O LQAQMOIBXDELJX-UHFFFAOYSA-N 0.000 description 1
- SDTMFDGELKWGFT-UHFFFAOYSA-N 2-methylpropan-2-olate Chemical compound CC(C)(C)[O-] SDTMFDGELKWGFT-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- XFKYJMGXZXJYBS-UHFFFAOYSA-N 3,4,5-trifluorobenzonitrile Chemical compound FC1=CC(C#N)=CC(F)=C1F XFKYJMGXZXJYBS-UHFFFAOYSA-N 0.000 description 1
- MBMDRPGKRRJGSQ-UHFFFAOYSA-N 3,4-dihydrodioxazine Chemical compound C1NOOC=C1 MBMDRPGKRRJGSQ-UHFFFAOYSA-N 0.000 description 1
- CQXZSEXZQVKCHW-UHFFFAOYSA-N 3,5-difluorobenzonitrile Chemical compound FC1=CC(F)=CC(C#N)=C1 CQXZSEXZQVKCHW-UHFFFAOYSA-N 0.000 description 1
- KROYDXBSPISGNO-UHFFFAOYSA-N 3-(2,6-difluorophenyl)-4,4-bis(ethylsulfanyl)but-3-en-2-one Chemical compound CCSC(SCC)=C(C(C)=O)C1=C(F)C=CC=C1F KROYDXBSPISGNO-UHFFFAOYSA-N 0.000 description 1
- GPUHJQHXIFJPGN-UHFFFAOYSA-N 3-(3,5-dichlorophenyl)-1-(3-methylbutanoyl)imidazolidine-2,4-dione Chemical compound O=C1N(C(=O)CC(C)C)CC(=O)N1C1=CC(Cl)=CC(Cl)=C1 GPUHJQHXIFJPGN-UHFFFAOYSA-N 0.000 description 1
- XIUXHKYVNPGCEP-UHFFFAOYSA-N 3-bromo-4-[4-(2,4-difluorophenyl)-2,5-dimethylpyrazol-3-yl]oxybenzonitrile Chemical compound C=1C=C(F)C=C(F)C=1C=1C(C)=NN(C)C=1OC1=CC=C(C#N)C=C1Br XIUXHKYVNPGCEP-UHFFFAOYSA-N 0.000 description 1
- GNNSNXJRJGUOFW-UHFFFAOYSA-N 3-chloro-4-[5-(2-chloro-4,6-difluoroanilino)-1,3-dimethylpyrazol-4-yl]benzonitrile Chemical compound C=1C=C(C#N)C=C(Cl)C=1C=1C(C)=NN(C)C=1NC1=C(F)C=C(F)C=C1Cl GNNSNXJRJGUOFW-UHFFFAOYSA-N 0.000 description 1
- DKTYKODJDGLPEN-UHFFFAOYSA-N 3-chloro-4-[5-(4-chloro-2,6-difluoroanilino)-1,3-dimethylpyrazol-4-yl]benzonitrile Chemical compound C=1C=C(C#N)C=C(Cl)C=1C=1C(C)=NN(C)C=1NC1=C(F)C=C(Cl)C=C1F DKTYKODJDGLPEN-UHFFFAOYSA-N 0.000 description 1
- RZRNAYUHWVFMIP-GDCKJWNLSA-N 3-oleoyl-sn-glycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)CO RZRNAYUHWVFMIP-GDCKJWNLSA-N 0.000 description 1
- FPTJELQXIUUCEY-UHFFFAOYSA-N 3beta-Hydroxy-lanostan Natural products C1CC2C(C)(C)C(O)CCC2(C)C2C1C1(C)CCC(C(C)CCCC(C)C)C1(C)CC2 FPTJELQXIUUCEY-UHFFFAOYSA-N 0.000 description 1
- NMWKWBPNKPGATC-UHFFFAOYSA-N 4,5,6,7-tetrachloro-2-benzofuran-1(3H)-one Chemical compound ClC1=C(Cl)C(Cl)=C2COC(=O)C2=C1Cl NMWKWBPNKPGATC-UHFFFAOYSA-N 0.000 description 1
- IGYJYMBGLMYKSZ-UHFFFAOYSA-N 4-(2,4-difluoroanilino)-3-(2,6-difluorophenyl)-4-methylsulfanylbut-3-en-2-one Chemical compound FC=1C=CC=C(F)C=1C(C(C)=O)=C(SC)NC1=CC=C(F)C=C1F IGYJYMBGLMYKSZ-UHFFFAOYSA-N 0.000 description 1
- DDNSAKDLVNQOHK-UHFFFAOYSA-N 4-(2,5-dimethylpyrazol-3-yl)oxy-3,5-difluorobenzonitrile Chemical compound CN1N=C(C)C=C1OC1=C(F)C=C(C#N)C=C1F DDNSAKDLVNQOHK-UHFFFAOYSA-N 0.000 description 1
- RAKKOIQLYSDCJT-UHFFFAOYSA-N 4-[4-(2-chloro-4-fluorophenyl)-2,5-dimethylpyrazol-3-yl]oxy-3-fluorobenzonitrile Chemical compound C=1C=C(F)C=C(Cl)C=1C=1C(C)=NN(C)C=1OC1=CC=C(C#N)C=C1F RAKKOIQLYSDCJT-UHFFFAOYSA-N 0.000 description 1
- QDFVXXBCJYNKKC-UHFFFAOYSA-N 4-[4-(4-chlorophenyl)-4-cyclopropylbutyl]-1-fluoro-2-phenoxybenzene Chemical compound C1=C(OC=2C=CC=CC=2)C(F)=CC=C1CCCC(C=1C=CC(Cl)=CC=1)C1CC1 QDFVXXBCJYNKKC-UHFFFAOYSA-N 0.000 description 1
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 1
- BCFOOQRXUXKJCL-UHFFFAOYSA-N 4-amino-4-oxo-2-sulfobutanoic acid Chemical class NC(=O)CC(C(O)=O)S(O)(=O)=O BCFOOQRXUXKJCL-UHFFFAOYSA-N 0.000 description 1
- WVGCPEDBFHEHEZ-UHFFFAOYSA-N 4-bromo-1h-pyrazole Chemical compound BrC=1C=NNC=1 WVGCPEDBFHEHEZ-UHFFFAOYSA-N 0.000 description 1
- LLNQWPTUJJYTTE-UHFFFAOYSA-N 4-iodopyrazole Chemical compound IC=1C=NNC=1 LLNQWPTUJJYTTE-UHFFFAOYSA-N 0.000 description 1
- 125000004172 4-methoxyphenyl group Chemical group [H]C1=C([H])C(OC([H])([H])[H])=C([H])C([H])=C1* 0.000 description 1
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 1
- UHPMCKVQTMMPCG-UHFFFAOYSA-N 5,8-dihydroxy-2-methoxy-6-methyl-7-(2-oxopropyl)naphthalene-1,4-dione Chemical compound CC1=C(CC(C)=O)C(O)=C2C(=O)C(OC)=CC(=O)C2=C1O UHPMCKVQTMMPCG-UHFFFAOYSA-N 0.000 description 1
- JFCBXEAFNPFTTC-UHFFFAOYSA-N 5-bromo-4-(2-chloro-4-fluorophenyl)-1,3-dimethylpyrazole Chemical compound CC1=NN(C)C(Br)=C1C1=CC=C(F)C=C1Cl JFCBXEAFNPFTTC-UHFFFAOYSA-N 0.000 description 1
- XJFIKRXIJXAJGH-UHFFFAOYSA-N 5-chloro-1,3-dihydroimidazo[4,5-b]pyridin-2-one Chemical group ClC1=CC=C2NC(=O)NC2=N1 XJFIKRXIJXAJGH-UHFFFAOYSA-N 0.000 description 1
- WWJLCYHYLZZXBE-UHFFFAOYSA-N 5-chloro-1,3-dihydroindol-2-one Chemical compound ClC1=CC=C2NC(=O)CC2=C1 WWJLCYHYLZZXBE-UHFFFAOYSA-N 0.000 description 1
- XFJBGINZIMNZBW-CRAIPNDOSA-N 5-chloro-2-[4-[(1r,2s)-2-[2-(5-methylsulfonylpyridin-2-yl)oxyethyl]cyclopropyl]piperidin-1-yl]pyrimidine Chemical compound N1=CC(S(=O)(=O)C)=CC=C1OCC[C@H]1[C@@H](C2CCN(CC2)C=2N=CC(Cl)=CN=2)C1 XFJBGINZIMNZBW-CRAIPNDOSA-N 0.000 description 1
- LLPOBYGXVLYIJR-UHFFFAOYSA-N 5-methylidene-1,4,2,3-dioxadithiolane Chemical class C=C1OSSO1 LLPOBYGXVLYIJR-UHFFFAOYSA-N 0.000 description 1
- IBSREHMXUMOFBB-JFUDTMANSA-N 5u8924t11h Chemical compound O1[C@@H](C)[C@H](O)[C@@H](OC)C[C@@H]1O[C@@H]1[C@@H](OC)C[C@H](O[C@@H]2C(=C/C[C@@H]3C[C@@H](C[C@@]4(O3)C=C[C@H](C)[C@@H](C(C)C)O4)OC(=O)[C@@H]3C=C(C)[C@@H](O)[C@H]4OC\C([C@@]34O)=C/C=C/[C@@H]2C)/C)O[C@H]1C.C1=C[C@H](C)[C@@H]([C@@H](C)CC)O[C@]11O[C@H](C\C=C(C)\[C@@H](O[C@@H]2O[C@@H](C)[C@H](O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](OC)C3)[C@@H](OC)C2)[C@@H](C)\C=C\C=C/2[C@]3([C@H](C(=O)O4)C=C(C)[C@@H](O)[C@H]3OC\2)O)C[C@H]4C1 IBSREHMXUMOFBB-JFUDTMANSA-N 0.000 description 1
- QQLGQHGKSPJXTO-UHFFFAOYSA-N 6,8-diiodo-2-propoxy-3-propylquinazolin-4-one Chemical compound C1=C(I)C=C2C(=O)N(CCC)C(OCCC)=NC2=C1I QQLGQHGKSPJXTO-UHFFFAOYSA-N 0.000 description 1
- GFALABICUSQHNW-UHFFFAOYSA-N 6-bromo-2-propoxy-3-propylpyrido[2,3-d]pyrimidin-4-one Chemical compound C1=C(Br)C=C2C(=O)N(CCC)C(OCCC)=NC2=N1 GFALABICUSQHNW-UHFFFAOYSA-N 0.000 description 1
- FVQAEZOUDXAZGN-UHFFFAOYSA-N 6-bromo-2-propoxy-3-propylquinazolin-4-one Chemical compound C1=C(Br)C=C2C(=O)N(CCC)C(OCCC)=NC2=C1 FVQAEZOUDXAZGN-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- UHYISDCXHNDRHZ-UHFFFAOYSA-N 7h-[1,3]thiazolo[5,4-e]benzotriazole Chemical compound C1=CC2=NCSC2=C2N=NN=C21 UHYISDCXHNDRHZ-UHFFFAOYSA-N 0.000 description 1
- PLLBRTOLHQQAQQ-UHFFFAOYSA-N 8-methylnonan-1-ol Chemical compound CC(C)CCCCCCCO PLLBRTOLHQQAQQ-UHFFFAOYSA-N 0.000 description 1
- MTVNAPYHLASOSX-UHFFFAOYSA-N 9,9-dimethylxanthene Chemical compound C1=CC=C2C(C)(C)C3=CC=CC=C3OC2=C1 MTVNAPYHLASOSX-UHFFFAOYSA-N 0.000 description 1
- 230000002407 ATP formation Effects 0.000 description 1
- 239000005875 Acetamiprid Substances 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 241001133760 Acoelorraphe Species 0.000 description 1
- 102000055025 Adenosine deaminases Human genes 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 241000223600 Alternaria Species 0.000 description 1
- 241001149961 Alternaria brassicae Species 0.000 description 1
- 241000213004 Alternaria solani Species 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical class NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- 241001530056 Athelia rolfsii Species 0.000 description 1
- 239000005878 Azadirachtin Substances 0.000 description 1
- 241000193363 Bacillus thuringiensis serovar aizawai Species 0.000 description 1
- 241001147758 Bacillus thuringiensis serovar kurstaki Species 0.000 description 1
- 239000005884 Beta-Cyfluthrin Substances 0.000 description 1
- 239000005653 Bifenazate Substances 0.000 description 1
- 239000005874 Bifenthrin Substances 0.000 description 1
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical group N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 description 1
- 241001480061 Blumeria graminis Species 0.000 description 1
- 241001465180 Botrytis Species 0.000 description 1
- 241000123650 Botrytis cinerea Species 0.000 description 1
- 240000002791 Brassica napus Species 0.000 description 1
- 235000004977 Brassica sinapistrum Nutrition 0.000 description 1
- 241000233685 Bremia lactucae Species 0.000 description 1
- 239000005885 Buprofezin Substances 0.000 description 1
- JFLRKDZMHNBDQS-UCQUSYKYSA-N CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C(=C[C@H]3[C@@H]2CC(=O)O1)C)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C.CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C=C[C@H]3C2CC(=O)O1)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C Chemical compound CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C(=C[C@H]3[C@@H]2CC(=O)O1)C)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C.CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C=C[C@H]3C2CC(=O)O1)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C JFLRKDZMHNBDQS-UCQUSYKYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 244000020518 Carthamus tinctorius Species 0.000 description 1
- 235000003255 Carthamus tinctorius Nutrition 0.000 description 1
- 241000530549 Cercospora beticola Species 0.000 description 1
- 241000819038 Chichester Species 0.000 description 1
- 102000005469 Chitin Synthase Human genes 0.000 description 1
- 108700040089 Chitin synthases Proteins 0.000 description 1
- 239000005886 Chlorantraniliprole Substances 0.000 description 1
- 239000005944 Chlorpyrifos Substances 0.000 description 1
- 239000005945 Chlorpyrifos-methyl Substances 0.000 description 1
- 239000005887 Chromafenozide Substances 0.000 description 1
- 239000005888 Clothianidin Substances 0.000 description 1
- 244000060011 Cocos nucifera Species 0.000 description 1
- 235000013162 Cocos nucifera Nutrition 0.000 description 1
- 241000222199 Colletotrichum Species 0.000 description 1
- 241001429695 Colletotrichum graminicola Species 0.000 description 1
- 241000222235 Colletotrichum orbiculare Species 0.000 description 1
- 229940126657 Compound 17 Drugs 0.000 description 1
- 229910021591 Copper(I) chloride Inorganic materials 0.000 description 1
- 241000371644 Curvularia ravenelii Species 0.000 description 1
- 239000005889 Cyantraniliprole Substances 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 241001135545 Cydia pomonella granulovirus Species 0.000 description 1
- 239000005655 Cyflumetofen Substances 0.000 description 1
- 239000005946 Cypermethrin Substances 0.000 description 1
- 239000005891 Cyromazine Substances 0.000 description 1
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 description 1
- 102000003849 Cytochrome P450 Human genes 0.000 description 1
- 102100030497 Cytochrome c Human genes 0.000 description 1
- 108010075031 Cytochromes c Proteins 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- 239000005892 Deltamethrin Substances 0.000 description 1
- 206010012422 Derealisation Diseases 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 241001306390 Diaporthe ampelina Species 0.000 description 1
- JDZSMXLTQNHBRF-UHFFFAOYSA-N Dichlozoline Chemical compound O=C1C(C)(C)OC(=O)N1C1=CC(Cl)=CC(Cl)=C1 JDZSMXLTQNHBRF-UHFFFAOYSA-N 0.000 description 1
- 239000005893 Diflubenzuron Substances 0.000 description 1
- 239000005947 Dimethoate Substances 0.000 description 1
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 1
- YFPJFKYCVYXDJK-UHFFFAOYSA-N Diphenylphosphine oxide Chemical compound C=1C=CC=CC=1[P+](=O)C1=CC=CC=C1 YFPJFKYCVYXDJK-UHFFFAOYSA-N 0.000 description 1
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 1
- 239000005894 Emamectin Substances 0.000 description 1
- 241000588694 Erwinia amylovora Species 0.000 description 1
- 241000221785 Erysiphales Species 0.000 description 1
- 241000221787 Erysiphe Species 0.000 description 1
- 241000896222 Erysiphe polygoni Species 0.000 description 1
- 239000005895 Esfenvalerate Substances 0.000 description 1
- FNELVJVBIYMIMC-UHFFFAOYSA-N Ethiprole Chemical compound N1=C(C#N)C(S(=O)CC)=C(N)N1C1=C(Cl)C=C(C(F)(F)F)C=C1Cl FNELVJVBIYMIMC-UHFFFAOYSA-N 0.000 description 1
- HMIBKHHNXANVHR-UHFFFAOYSA-N Fenothiocarb Chemical compound CN(C)C(=O)SCCCCOC1=CC=CC=C1 HMIBKHHNXANVHR-UHFFFAOYSA-N 0.000 description 1
- 239000005898 Fenoxycarb Substances 0.000 description 1
- 239000005900 Flonicamid Substances 0.000 description 1
- 239000005901 Flubendiamide Substances 0.000 description 1
- 241000223218 Fusarium Species 0.000 description 1
- 241000223195 Fusarium graminearum Species 0.000 description 1
- 241000223221 Fusarium oxysporum Species 0.000 description 1
- 241000221779 Fusarium sambucinum Species 0.000 description 1
- 108091006027 G proteins Proteins 0.000 description 1
- 102000030782 GTP binding Human genes 0.000 description 1
- 108091000058 GTP-Binding Proteins 0.000 description 1
- 241001149475 Gaeumannomyces graminis Species 0.000 description 1
- 239000005903 Gamma-cyhalothrin Substances 0.000 description 1
- 241001620302 Glomerella <beetle> Species 0.000 description 1
- BKLIAINBCQPSOV-UHFFFAOYSA-N Gluanol Natural products CC(C)CC=CC(C)C1CCC2(C)C3=C(CCC12C)C4(C)CCC(O)C(C)(C)C4CC3 BKLIAINBCQPSOV-UHFFFAOYSA-N 0.000 description 1
- 239000007818 Grignard reagent Substances 0.000 description 1
- 244000020551 Helianthus annuus Species 0.000 description 1
- 235000003222 Helianthus annuus Nutrition 0.000 description 1
- 241001181532 Hemileia vastatrix Species 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 241000549404 Hyaloperonospora parasitica Species 0.000 description 1
- 239000005906 Imidacloprid Substances 0.000 description 1
- FKWDSATZSMJRLC-UHFFFAOYSA-N Iminoctadine acetate Chemical compound CC([O-])=O.CC([O-])=O.CC([O-])=O.NC([NH3+])=NCCCCCCCC[NH2+]CCCCCCCCN=C(N)[NH3+] FKWDSATZSMJRLC-UHFFFAOYSA-N 0.000 description 1
- 239000005907 Indoxacarb Substances 0.000 description 1
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 1
- KGEKLUUHTZCSIP-UHFFFAOYSA-N Isobornyl acetate Natural products C1CC2(C)C(OC(=O)C)CC1C2(C)C KGEKLUUHTZCSIP-UHFFFAOYSA-N 0.000 description 1
- 239000004440 Isodecyl alcohol Substances 0.000 description 1
- 102000004195 Isomerases Human genes 0.000 description 1
- 108090000769 Isomerases Proteins 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 239000004166 Lanolin Substances 0.000 description 1
- LOPKHWOTGJIQLC-UHFFFAOYSA-N Lanosterol Natural products CC(CCC=C(C)C)C1CCC2(C)C3=C(CCC12C)C4(C)CCC(C)(O)C(C)(C)C4CC3 LOPKHWOTGJIQLC-UHFFFAOYSA-N 0.000 description 1
- 238000005684 Liebig rearrangement reaction Methods 0.000 description 1
- 229920001732 Lignosulfonate Polymers 0.000 description 1
- 240000006240 Linum usitatissimum Species 0.000 description 1
- 235000004431 Linum usitatissimum Nutrition 0.000 description 1
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 description 1
- 239000005912 Lufenuron Substances 0.000 description 1
- 241001344131 Magnaporthe grisea Species 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 239000005949 Malathion Substances 0.000 description 1
- 239000005914 Metaflumizone Substances 0.000 description 1
- MIFOMMKAVSCNKQ-HWIUFGAZSA-N Metaflumizone Chemical compound C1=CC(OC(F)(F)F)=CC=C1NC(=O)N\N=C(C=1C=C(C=CC=1)C(F)(F)F)\CC1=CC=C(C#N)C=C1 MIFOMMKAVSCNKQ-HWIUFGAZSA-N 0.000 description 1
- 239000005956 Metaldehyde Substances 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 239000005916 Methomyl Substances 0.000 description 1
- 239000005917 Methoxyfenozide Substances 0.000 description 1
- 241001518731 Monilinia fructicola Species 0.000 description 1
- FTCOKXNKPOUEFH-UHFFFAOYSA-N Myclozolin Chemical compound O=C1C(COC)(C)OC(=O)N1C1=CC(Cl)=CC(Cl)=C1 FTCOKXNKPOUEFH-UHFFFAOYSA-N 0.000 description 1
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-diisopropylethylamine Substances CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 1
- PHSPJQZRQAJPPF-UHFFFAOYSA-N N-alpha-Methylhistamine Chemical compound CNCCC1=CN=CN1 PHSPJQZRQAJPPF-UHFFFAOYSA-N 0.000 description 1
- 150000001204 N-oxides Chemical class 0.000 description 1
- 102000006746 NADH Dehydrogenase Human genes 0.000 description 1
- 108010086428 NADH Dehydrogenase Proteins 0.000 description 1
- CAHGCLMLTWQZNJ-UHFFFAOYSA-N Nerifoliol Natural products CC12CCC(O)C(C)(C)C1CCC1=C2CCC2(C)C(C(CCC=C(C)C)C)CCC21C CAHGCLMLTWQZNJ-UHFFFAOYSA-N 0.000 description 1
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 1
- 241001329956 Nothopassalora personata Species 0.000 description 1
- 241001668536 Oculimacula yallundae Species 0.000 description 1
- 240000007817 Olea europaea Species 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 229910019201 POBr3 Inorganic materials 0.000 description 1
- 241000736122 Parastagonospora nodorum Species 0.000 description 1
- 241000315044 Passalora arachidicola Species 0.000 description 1
- 241001223281 Peronospora Species 0.000 description 1
- 241000582441 Peronospora tabacina Species 0.000 description 1
- 241000233679 Peronosporaceae Species 0.000 description 1
- 241000682645 Phakopsora pachyrhizi Species 0.000 description 1
- 239000005921 Phosmet Substances 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 241000233614 Phytophthora Species 0.000 description 1
- 241000233616 Phytophthora capsici Species 0.000 description 1
- 241000233618 Phytophthora cinnamomi Species 0.000 description 1
- 241000233622 Phytophthora infestans Species 0.000 description 1
- 241000233624 Phytophthora megasperma Species 0.000 description 1
- 241000233629 Phytophthora parasitica Species 0.000 description 1
- 239000005923 Pirimicarb Substances 0.000 description 1
- 241001281803 Plasmopara viticola Species 0.000 description 1
- 241001337928 Podosphaera leucotricha Species 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- MKIMSXGUTQTKJU-UHFFFAOYSA-N Propamocarb hydrochloride Chemical compound [Cl-].CCCOC(=O)NCCC[NH+](C)C MKIMSXGUTQTKJU-UHFFFAOYSA-N 0.000 description 1
- 102000001253 Protein Kinase Human genes 0.000 description 1
- 241000589615 Pseudomonas syringae Species 0.000 description 1
- 241001281802 Pseudoperonospora Species 0.000 description 1
- 241001281805 Pseudoperonospora cubensis Species 0.000 description 1
- 241000221300 Puccinia Species 0.000 description 1
- 241000343500 Puccinia arachidis Species 0.000 description 1
- 241000221301 Puccinia graminis Species 0.000 description 1
- 241001123559 Puccinia hordei Species 0.000 description 1
- 241001123569 Puccinia recondita Species 0.000 description 1
- 241001123583 Puccinia striiformis Species 0.000 description 1
- 239000005925 Pymetrozine Substances 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- 241000520648 Pyrenophora teres Species 0.000 description 1
- 241000190117 Pyrenophora tritici-repentis Species 0.000 description 1
- VQXSOUPNOZTNAI-UHFFFAOYSA-N Pyrethrin I Natural products CC(=CC1CC1C(=O)OC2CC(=O)C(=C2C)CC=C/C=C)C VQXSOUPNOZTNAI-UHFFFAOYSA-N 0.000 description 1
- 239000005926 Pyridalyl Substances 0.000 description 1
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 1
- MWMQNVGAHVXSPE-UHFFFAOYSA-N Pyriprole Chemical compound ClC=1C=C(C(F)(F)F)C=C(Cl)C=1N1N=C(C#N)C(SC(F)F)=C1NCC1=CC=CC=N1 MWMQNVGAHVXSPE-UHFFFAOYSA-N 0.000 description 1
- 239000005927 Pyriproxyfen Substances 0.000 description 1
- 241000233639 Pythium Species 0.000 description 1
- 241000918585 Pythium aphanidermatum Species 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 235000019484 Rapeseed oil Nutrition 0.000 description 1
- 241001361634 Rhizoctonia Species 0.000 description 1
- 241000813090 Rhizoctonia solani Species 0.000 description 1
- 241001518834 Rutstroemia Species 0.000 description 1
- 229930001406 Ryanodine Natural products 0.000 description 1
- 241000221662 Sclerotinia Species 0.000 description 1
- 241000221696 Sclerotinia sclerotiorum Species 0.000 description 1
- 241001533598 Septoria Species 0.000 description 1
- 244000000231 Sesamum indicum Species 0.000 description 1
- 235000003434 Sesamum indicum Nutrition 0.000 description 1
- 239000004965 Silica aerogel Substances 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-NQAPHZHOSA-N Sorbitol Polymers OCC(O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-NQAPHZHOSA-N 0.000 description 1
- 241000592344 Spermatophyta Species 0.000 description 1
- 241000579741 Sphaerotheca <fungi> Species 0.000 description 1
- 239000005929 Spinetoram Substances 0.000 description 1
- GOENIMGKWNZVDA-OAMCMWGQSA-N Spinetoram Chemical compound CO[C@@H]1[C@H](OCC)[C@@H](OC)[C@H](C)O[C@H]1OC1C[C@H]2[C@@H]3C=C4C(=O)[C@H](C)[C@@H](O[C@@H]5O[C@H](C)[C@H](CC5)N(C)C)CCC[C@H](CC)OC(=O)CC4[C@@H]3CC[C@@H]2C1 GOENIMGKWNZVDA-OAMCMWGQSA-N 0.000 description 1
- 239000005930 Spinosad Substances 0.000 description 1
- 239000005665 Spiromesifen Substances 0.000 description 1
- 239000005931 Spirotetramat Substances 0.000 description 1
- 102000005782 Squalene Monooxygenase Human genes 0.000 description 1
- 108020003891 Squalene monooxygenase Proteins 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- 239000005937 Tebufenozide Substances 0.000 description 1
- 239000005938 Teflubenzuron Substances 0.000 description 1
- 239000005939 Tefluthrin Substances 0.000 description 1
- 239000005940 Thiacloprid Substances 0.000 description 1
- 239000005941 Thiamethoxam Substances 0.000 description 1
- 239000005842 Thiophanate-methyl Substances 0.000 description 1
- 101710183280 Topoisomerase Proteins 0.000 description 1
- 239000005942 Triflumuron Substances 0.000 description 1
- 238000006887 Ullmann reaction Methods 0.000 description 1
- 241000510929 Uncinula Species 0.000 description 1
- 241000317942 Venturia <ichneumonid wasp> Species 0.000 description 1
- 241000228452 Venturia inaequalis Species 0.000 description 1
- 241001123668 Verticillium dahliae Species 0.000 description 1
- 241000589636 Xanthomonas campestris Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000016383 Zea mays subsp huehuetenangensis Nutrition 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 241001360088 Zymoseptoria tritici Species 0.000 description 1
- 239000001940 [(1R,4S,6R)-1,7,7-trimethyl-6-bicyclo[2.2.1]heptanyl] acetate Substances 0.000 description 1
- 239000001089 [(2R)-oxolan-2-yl]methanol Substances 0.000 description 1
- QQODLKZGRKWIFG-RUTXASTPSA-N [(R)-cyano-(4-fluoro-3-phenoxyphenyl)methyl] (1S)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate Chemical compound CC1(C)C(C=C(Cl)Cl)[C@@H]1C(=O)O[C@@H](C#N)C1=CC=C(F)C(OC=2C=CC=CC=2)=C1 QQODLKZGRKWIFG-RUTXASTPSA-N 0.000 description 1
- KVIZNNVXXNFLMU-AIIUZBJTSA-N [2,3,5,6-tetrafluoro-4-(methoxymethyl)phenyl]methyl (1r,3r)-2,2-dimethyl-3-[(e)-prop-1-enyl]cyclopropane-1-carboxylate Chemical compound FC1=C(F)C(COC)=C(F)C(F)=C1COC(=O)[C@H]1C(C)(C)[C@@H]1\C=C\C KVIZNNVXXNFLMU-AIIUZBJTSA-N 0.000 description 1
- OOWCJRMYMAMSOH-UHFFFAOYSA-N [2,3,5,6-tetrafluoro-4-(methoxymethyl)phenyl]methyl 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate Chemical compound FC1=C(F)C(COC)=C(F)C(F)=C1COC(=O)C1C(C)(C)C1C=C(C)C OOWCJRMYMAMSOH-UHFFFAOYSA-N 0.000 description 1
- XYTNGEADGUXZCN-UHFFFAOYSA-N [5-chloro-4-(2,6-difluorophenyl)-2-methylpyrazol-3-yl]-(4-fluorophenyl)methanol Chemical compound CN1N=C(Cl)C(C=2C(=CC=CC=2F)F)=C1C(O)C1=CC=C(F)C=C1 XYTNGEADGUXZCN-UHFFFAOYSA-N 0.000 description 1
- 229950008167 abamectin Drugs 0.000 description 1
- YASYVMFAVPKPKE-UHFFFAOYSA-N acephate Chemical compound COP(=O)(SC)NC(C)=O YASYVMFAVPKPKE-UHFFFAOYSA-N 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- ZCZSIDMEHXZRLG-UHFFFAOYSA-N acetic acid heptyl ester Natural products CCCCCCCOC(C)=O ZCZSIDMEHXZRLG-UHFFFAOYSA-N 0.000 description 1
- CSCPPACGZOOCGX-WFGJKAKNSA-N acetone d6 Chemical compound [2H]C([2H])([2H])C(=O)C([2H])([2H])[2H] CSCPPACGZOOCGX-WFGJKAKNSA-N 0.000 description 1
- GDZNYEZGJAFIKA-UHFFFAOYSA-N acetoprole Chemical compound NC1=C(S(C)=O)C(C(=O)C)=NN1C1=C(Cl)C=C(C(F)(F)F)C=C1Cl GDZNYEZGJAFIKA-UHFFFAOYSA-N 0.000 description 1
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 1
- 239000012346 acetyl chloride Substances 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- QBYJBZPUGVGKQQ-SJJAEHHWSA-N aldrin Chemical compound C1[C@H]2C=C[C@@H]1[C@H]1[C@@](C3(Cl)Cl)(Cl)C(Cl)=C(Cl)[C@@]3(Cl)[C@H]12 QBYJBZPUGVGKQQ-SJJAEHHWSA-N 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 150000001345 alkine derivatives Chemical class 0.000 description 1
- 150000004703 alkoxides Chemical class 0.000 description 1
- 229920000180 alkyd Polymers 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 230000002152 alkylating effect Effects 0.000 description 1
- RMRFFCXPLWYOOY-UHFFFAOYSA-N allyl radical Chemical group [CH2]C=C RMRFFCXPLWYOOY-UHFFFAOYSA-N 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 150000001448 anilines Chemical class 0.000 description 1
- 230000002528 anti-freeze Effects 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 150000004982 aromatic amines Chemical class 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 238000000065 atmospheric pressure chemical ionisation Methods 0.000 description 1
- 239000005667 attractant Substances 0.000 description 1
- RRZXIRBKKLTSOM-XPNPUAGNSA-N avermectin B1a Chemical compound C1=C[C@H](C)[C@@H]([C@@H](C)CC)O[C@]11O[C@H](C\C=C(C)\[C@@H](O[C@@H]2O[C@@H](C)[C@H](O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](OC)C3)[C@@H](OC)C2)[C@@H](C)\C=C\C=C/2[C@]3([C@H](C(=O)O4)C=C(C)[C@@H](O)[C@H]3OC\2)O)C[C@H]4C1 RRZXIRBKKLTSOM-XPNPUAGNSA-N 0.000 description 1
- VEHPJKVTJQSSKL-UHFFFAOYSA-N azadirachtin Natural products O1C2(C)C(C3(C=COC3O3)O)CC3C21C1(C)C(O)C(OCC2(OC(C)=O)C(CC3OC(=O)C(C)=CC)OC(C)=O)C2C32COC(C(=O)OC)(O)C12 VEHPJKVTJQSSKL-UHFFFAOYSA-N 0.000 description 1
- FTNJWQUOZFUQQJ-NDAWSKJSSA-N azadirachtin A Chemical compound C([C@@H]([C@]1(C=CO[C@H]1O1)O)[C@]2(C)O3)[C@H]1[C@]23[C@]1(C)[C@H](O)[C@H](OC[C@@]2([C@@H](C[C@@H]3OC(=O)C(\C)=C\C)OC(C)=O)C(=O)OC)[C@@H]2[C@]32CO[C@@](C(=O)OC)(O)[C@@H]12 FTNJWQUOZFUQQJ-NDAWSKJSSA-N 0.000 description 1
- FTNJWQUOZFUQQJ-IRYYUVNJSA-N azadirachtin A Natural products C([C@@H]([C@]1(C=CO[C@H]1O1)O)[C@]2(C)O3)[C@H]1[C@]23[C@]1(C)[C@H](O)[C@H](OC[C@@]2([C@@H](C[C@@H]3OC(=O)C(\C)=C/C)OC(C)=O)C(=O)OC)[C@@H]2[C@]32CO[C@@](C(=O)OC)(O)[C@@H]12 FTNJWQUOZFUQQJ-IRYYUVNJSA-N 0.000 description 1
- CJJOSEISRRTUQB-UHFFFAOYSA-N azinphos-methyl Chemical group C1=CC=C2C(=O)N(CSP(=S)(OC)OC)N=NC2=C1 CJJOSEISRRTUQB-UHFFFAOYSA-N 0.000 description 1
- 235000015278 beef Nutrition 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229940054066 benzamide antipsychotics Drugs 0.000 description 1
- 150000003936 benzamides Chemical class 0.000 description 1
- 150000001556 benzimidazoles Chemical class 0.000 description 1
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 1
- 150000008366 benzophenones Chemical class 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- PUJDIJCNWFYVJX-UHFFFAOYSA-N benzyl carbamate Chemical compound NC(=O)OCC1=CC=CC=C1 PUJDIJCNWFYVJX-UHFFFAOYSA-N 0.000 description 1
- RRIWSQXXBIFKQM-UHFFFAOYSA-N benzylcarbamic acid Chemical class OC(=O)NCC1=CC=CC=C1 RRIWSQXXBIFKQM-UHFFFAOYSA-N 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- VHLKTXFWDRXILV-UHFFFAOYSA-N bifenazate Chemical compound C1=C(NNC(=O)OC(C)C)C(OC)=CC=C1C1=CC=CC=C1 VHLKTXFWDRXILV-UHFFFAOYSA-N 0.000 description 1
- OMFRMAHOUUJSGP-IRHGGOMRSA-N bifenthrin Chemical compound C1=CC=C(C=2C=CC=CC=2)C(C)=C1COC(=O)[C@@H]1[C@H](\C=C(/Cl)C(F)(F)F)C1(C)C OMFRMAHOUUJSGP-IRHGGOMRSA-N 0.000 description 1
- MUALRAIOVNYAIW-UHFFFAOYSA-N binap Chemical compound C1=CC=CC=C1P(C=1C(=C2C=CC=CC2=CC=1)C=1C2=CC=CC=C2C=CC=1P(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 MUALRAIOVNYAIW-UHFFFAOYSA-N 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000000853 biopesticidal effect Effects 0.000 description 1
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical compound OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 description 1
- 125000001626 borono group Chemical group [H]OB([*])O[H] 0.000 description 1
- 150000001649 bromium compounds Chemical class 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- PRLVTUNWOQKEAI-VKAVYKQESA-N buprofezin Chemical compound O=C1N(C(C)C)\C(=N\C(C)(C)C)SCN1C1=CC=CC=C1 PRLVTUNWOQKEAI-VKAVYKQESA-N 0.000 description 1
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000000480 butynyl group Chemical group [*]C#CC([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005622 butynylene group Chemical class 0.000 description 1
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 1
- 229910000024 caesium carbonate Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000004490 capsule suspension Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- DUEPRVBVGDRKAG-UHFFFAOYSA-N carbofuran Chemical compound CNC(=O)OC1=CC=CC2=C1OC(C)(C)C2 DUEPRVBVGDRKAG-UHFFFAOYSA-N 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 150000001728 carbonyl compounds Chemical class 0.000 description 1
- IRUJZVNXZWPBMU-UHFFFAOYSA-N cartap Chemical compound NC(=O)SCC(N(C)C)CSC(N)=O IRUJZVNXZWPBMU-UHFFFAOYSA-N 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 238000003889 chemical engineering Methods 0.000 description 1
- 239000007810 chemical reaction solvent Substances 0.000 description 1
- 230000003559 chemosterilizing effect Effects 0.000 description 1
- CWFOCCVIPCEQCK-UHFFFAOYSA-N chlorfenapyr Chemical compound BrC1=C(C(F)(F)F)N(COCC)C(C=2C=CC(Cl)=CC=2)=C1C#N CWFOCCVIPCEQCK-UHFFFAOYSA-N 0.000 description 1
- UISUNVFOGSJSKD-UHFFFAOYSA-N chlorfluazuron Chemical compound FC1=CC=CC(F)=C1C(=O)NC(=O)NC(C=C1Cl)=CC(Cl)=C1OC1=NC=C(C(F)(F)F)C=C1Cl UISUNVFOGSJSKD-UHFFFAOYSA-N 0.000 description 1
- 125000004218 chloromethyl group Chemical group [H]C([H])(Cl)* 0.000 description 1
- SBPBAQFWLVIOKP-UHFFFAOYSA-N chlorpyrifos Chemical compound CCOP(=S)(OCC)OC1=NC(Cl)=C(Cl)C=C1Cl SBPBAQFWLVIOKP-UHFFFAOYSA-N 0.000 description 1
- HPNSNYBUADCFDR-UHFFFAOYSA-N chromafenozide Chemical compound CC1=CC(C)=CC(C(=O)N(NC(=O)C=2C(=C3CCCOC3=CC=2)C)C(C)(C)C)=C1 HPNSNYBUADCFDR-UHFFFAOYSA-N 0.000 description 1
- 235000012716 cod liver oil Nutrition 0.000 description 1
- 239000003026 cod liver oil Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- OXBLHERUFWYNTN-UHFFFAOYSA-M copper(I) chloride Chemical compound [Cu]Cl OXBLHERUFWYNTN-UHFFFAOYSA-M 0.000 description 1
- DOBRDRYODQBAMW-UHFFFAOYSA-N copper(i) cyanide Chemical compound [Cu+].N#[C-] DOBRDRYODQBAMW-UHFFFAOYSA-N 0.000 description 1
- LSXDOTMGLUJQCM-UHFFFAOYSA-M copper(i) iodide Chemical compound I[Cu] LSXDOTMGLUJQCM-UHFFFAOYSA-M 0.000 description 1
- OPQARKPSCNTWTJ-UHFFFAOYSA-L copper(ii) acetate Chemical compound [Cu+2].CC([O-])=O.CC([O-])=O OPQARKPSCNTWTJ-UHFFFAOYSA-L 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 238000012272 crop production Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 230000001955 cumulated effect Effects 0.000 description 1
- DVBUIBGJRQBEDP-UHFFFAOYSA-N cyantraniliprole Chemical compound CNC(=O)C1=CC(C#N)=CC(C)=C1NC(=O)C1=CC(Br)=NN1C1=NC=CC=C1Cl DVBUIBGJRQBEDP-UHFFFAOYSA-N 0.000 description 1
- 125000000392 cycloalkenyl group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000004976 cyclobutylene group Chemical group 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000004956 cyclohexylene group Chemical group 0.000 description 1
- 125000002933 cyclohexyloxy group Chemical group C1(CCCCC1)O* 0.000 description 1
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 1
- 125000006638 cyclopentyl carbonyl group Chemical group 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000004979 cyclopentylene group Chemical group 0.000 description 1
- 125000001887 cyclopentyloxy group Chemical group C1(CCCC1)O* 0.000 description 1
- 125000006255 cyclopropyl carbonyl group Chemical group [H]C1([H])C([H])([H])C1([H])C(*)=O 0.000 description 1
- 125000004980 cyclopropylene group Chemical group 0.000 description 1
- 125000004186 cyclopropylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C1([H])[H] 0.000 description 1
- APJLTUBHYCOZJI-VZCXRCSSSA-N cyenopyrafen Chemical compound CC1=NN(C)C(\C(OC(=O)C(C)(C)C)=C(/C#N)C=2C=CC(=CC=2)C(C)(C)C)=C1C APJLTUBHYCOZJI-VZCXRCSSSA-N 0.000 description 1
- 229960001591 cyfluthrin Drugs 0.000 description 1
- QQODLKZGRKWIFG-QSFXBCCZSA-N cyfluthrin Chemical compound CC1(C)[C@@H](C=C(Cl)Cl)[C@H]1C(=O)O[C@@H](C#N)C1=CC=C(F)C(OC=2C=CC=CC=2)=C1 QQODLKZGRKWIFG-QSFXBCCZSA-N 0.000 description 1
- ZXQYGBMAQZUVMI-UNOMPAQXSA-N cyhalothrin Chemical compound CC1(C)C(\C=C(/Cl)C(F)(F)F)C1C(=O)OC(C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 ZXQYGBMAQZUVMI-UNOMPAQXSA-N 0.000 description 1
- 229960005424 cypermethrin Drugs 0.000 description 1
- KAATUXNTWXVJKI-UHFFFAOYSA-N cypermethrin Chemical compound CC1(C)C(C=C(Cl)Cl)C1C(=O)OC(C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 KAATUXNTWXVJKI-UHFFFAOYSA-N 0.000 description 1
- LVQDKIWDGQRHTE-UHFFFAOYSA-N cyromazine Chemical compound NC1=NC(N)=NC(NC2CC2)=N1 LVQDKIWDGQRHTE-UHFFFAOYSA-N 0.000 description 1
- 229950000775 cyromazine Drugs 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 229960002483 decamethrin Drugs 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000008260 defense mechanism Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- OWZREIFADZCYQD-NSHGMRRFSA-N deltamethrin Chemical compound CC1(C)[C@@H](C=C(Br)Br)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 OWZREIFADZCYQD-NSHGMRRFSA-N 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 230000005595 deprotonation Effects 0.000 description 1
- 238000010537 deprotonation reaction Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- WOWBFOBYOAGEEA-UHFFFAOYSA-N diafenthiuron Chemical compound CC(C)C1=C(NC(=S)NC(C)(C)C)C(C(C)C)=CC(OC=2C=CC=CC=2)=C1 WOWBFOBYOAGEEA-UHFFFAOYSA-N 0.000 description 1
- JYIMWRSJCRRYNK-UHFFFAOYSA-N dialuminum;disodium;oxygen(2-);silicon(4+);hydrate Chemical compound O.[O-2].[O-2].[O-2].[O-2].[O-2].[O-2].[Na+].[Na+].[Al+3].[Al+3].[Si+4] JYIMWRSJCRRYNK-UHFFFAOYSA-N 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- FHIVAFMUCKRCQO-UHFFFAOYSA-N diazinon Chemical compound CCOP(=S)(OCC)OC1=CC(C)=NC(C(C)C)=N1 FHIVAFMUCKRCQO-UHFFFAOYSA-N 0.000 description 1
- WMKGGPCROCCUDY-PHEQNACWSA-N dibenzylideneacetone Chemical compound C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 WMKGGPCROCCUDY-PHEQNACWSA-N 0.000 description 1
- 125000003963 dichloro group Chemical group Cl* 0.000 description 1
- DFBKLUNHFCTMDC-PICURKEMSA-N dieldrin Chemical compound C([C@H]1[C@H]2[C@@]3(Cl)C(Cl)=C([C@]([C@H]22)(Cl)C3(Cl)Cl)Cl)[C@H]2[C@@H]2[C@H]1O2 DFBKLUNHFCTMDC-PICURKEMSA-N 0.000 description 1
- 229950006824 dieldrin Drugs 0.000 description 1
- NGPMUTDCEIKKFM-UHFFFAOYSA-N dieldrin Natural products CC1=C(Cl)C2(Cl)C3C4CC(C5OC45)C3C1(Cl)C2(Cl)Cl NGPMUTDCEIKKFM-UHFFFAOYSA-N 0.000 description 1
- JXSJBGJIGXNWCI-UHFFFAOYSA-N diethyl 2-[(dimethoxyphosphorothioyl)thio]succinate Chemical compound CCOC(=O)CC(SP(=S)(OC)OC)C(=O)OCC JXSJBGJIGXNWCI-UHFFFAOYSA-N 0.000 description 1
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 1
- 229940019503 diflubenzuron Drugs 0.000 description 1
- QBSJHOGDIUQWTH-UHFFFAOYSA-N dihydrolanosterol Natural products CC(C)CCCC(C)C1CCC2(C)C3=C(CCC12C)C4(C)CCC(C)(O)C(C)(C)C4CC3 QBSJHOGDIUQWTH-UHFFFAOYSA-N 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- MCWXGJITAZMZEV-UHFFFAOYSA-N dimethoate Chemical compound CNC(=O)CSP(=S)(OC)OC MCWXGJITAZMZEV-UHFFFAOYSA-N 0.000 description 1
- VAYGXNSJCAHWJZ-UHFFFAOYSA-N dimethyl sulfate Chemical compound COS(=O)(=O)OC VAYGXNSJCAHWJZ-UHFFFAOYSA-N 0.000 description 1
- FFHWGQQFANVOHV-UHFFFAOYSA-N dimethyldioxirane Chemical compound CC1(C)OO1 FFHWGQQFANVOHV-UHFFFAOYSA-N 0.000 description 1
- YKBZOVFACRVRJN-UHFFFAOYSA-N dinotefuran Chemical compound [O-][N+](=O)\N=C(/NC)NCC1CCOC1 YKBZOVFACRVRJN-UHFFFAOYSA-N 0.000 description 1
- 150000004844 dioxiranes Chemical class 0.000 description 1
- SZXQTJUDPRGNJN-UHFFFAOYSA-N dipropylene glycol Chemical compound OCCCOCCCO SZXQTJUDPRGNJN-UHFFFAOYSA-N 0.000 description 1
- YDEXUEFDPVHGHE-GGMCWBHBSA-L disodium;(2r)-3-(2-hydroxy-3-methoxyphenyl)-2-[2-methoxy-4-(3-sulfonatopropyl)phenoxy]propane-1-sulfonate Chemical compound [Na+].[Na+].COC1=CC=CC(C[C@H](CS([O-])(=O)=O)OC=2C(=CC(CCCS([O-])(=O)=O)=CC=2)OC)=C1O YDEXUEFDPVHGHE-GGMCWBHBSA-L 0.000 description 1
- 239000004491 dispersible concentrate Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 150000004863 dithiolanes Chemical class 0.000 description 1
- LQZZUXJYWNFBMV-UHFFFAOYSA-N dodecan-1-ol Chemical compound CCCCCCCCCCCCO LQZZUXJYWNFBMV-UHFFFAOYSA-N 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000009837 dry grinding Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- XJLZCPIILZRCPS-ANMPWZFDSA-N eburicol Chemical compound C([C@@]12C)C[C@H](O)C(C)(C)[C@@H]1CCC1=C2CC[C@]2(C)[C@@H]([C@H](C)CCC(=C)C(C)C)CC[C@]21C XJLZCPIILZRCPS-ANMPWZFDSA-N 0.000 description 1
- 238000007350 electrophilic reaction Methods 0.000 description 1
- CXEGAUYXQAKHKJ-NSBHKLITSA-N emamectin B1a Chemical compound C1=C[C@H](C)[C@@H]([C@@H](C)CC)O[C@]11O[C@H](C\C=C(C)\[C@@H](O[C@@H]2O[C@@H](C)[C@H](O[C@@H]3O[C@@H](C)[C@H](NC)[C@@H](OC)C3)[C@@H](OC)C2)[C@@H](C)\C=C\C=C/2[C@]3([C@H](C(=O)O4)C=C(C)[C@@H](O)[C@H]3OC\2)O)C[C@H]4C1 CXEGAUYXQAKHKJ-NSBHKLITSA-N 0.000 description 1
- 239000004497 emulsifiable granule Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- RDYMFSUJUZBWLH-SVWSLYAFSA-N endosulfan Chemical compound C([C@@H]12)OS(=O)OC[C@@H]1[C@]1(Cl)C(Cl)=C(Cl)[C@@]2(Cl)C1(Cl)Cl RDYMFSUJUZBWLH-SVWSLYAFSA-N 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000008686 ergosterol biosynthesis Effects 0.000 description 1
- NYPJDWWKZLNGGM-RPWUZVMVSA-N esfenvalerate Chemical compound C=1C([C@@H](C#N)OC(=O)[C@@H](C(C)C)C=2C=CC(Cl)=CC=2)=CC=CC=1OC1=CC=CC=C1 NYPJDWWKZLNGGM-RPWUZVMVSA-N 0.000 description 1
- LRMHFDNWKCSEQU-UHFFFAOYSA-N ethoxyethane;phenol Chemical compound CCOCC.OC1=CC=CC=C1 LRMHFDNWKCSEQU-UHFFFAOYSA-N 0.000 description 1
- 125000006437 ethyl cyclopropyl group Chemical group 0.000 description 1
- 125000004705 ethylthio group Chemical group C(C)S* 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 235000019387 fatty acid methyl ester Nutrition 0.000 description 1
- 150000002194 fatty esters Chemical class 0.000 description 1
- HJUFTIJOISQSKQ-UHFFFAOYSA-N fenoxycarb Chemical compound C1=CC(OCCNC(=O)OCC)=CC=C1OC1=CC=CC=C1 HJUFTIJOISQSKQ-UHFFFAOYSA-N 0.000 description 1
- 235000021323 fish oil Nutrition 0.000 description 1
- 235000004426 flaxseed Nutrition 0.000 description 1
- RLQJEEJISHYWON-UHFFFAOYSA-N flonicamid Chemical compound FC(F)(F)C1=CC=NC=C1C(=O)NCC#N RLQJEEJISHYWON-UHFFFAOYSA-N 0.000 description 1
- 230000009969 flowable effect Effects 0.000 description 1
- ZGNITFSDLCMLGI-UHFFFAOYSA-N flubendiamide Chemical compound CC1=CC(C(F)(C(F)(F)F)C(F)(F)F)=CC=C1NC(=O)C1=CC=CC(I)=C1C(=O)NC(C)(C)CS(C)(=O)=O ZGNITFSDLCMLGI-UHFFFAOYSA-N 0.000 description 1
- GBIHOLCMZGAKNG-CGAIIQECSA-N flucythrinate Chemical compound O=C([C@@H](C(C)C)C=1C=CC(OC(F)F)=CC=1)OC(C#N)C(C=1)=CC=CC=1OC1=CC=CC=C1 GBIHOLCMZGAKNG-CGAIIQECSA-N 0.000 description 1
- GJEREQYJIQASAW-UHFFFAOYSA-N flufenerim Chemical compound CC(F)C1=NC=NC(NCCC=2C=CC(OC(F)(F)F)=CC=2)=C1Cl GJEREQYJIQASAW-UHFFFAOYSA-N 0.000 description 1
- RYLHNOVXKPXDIP-UHFFFAOYSA-N flufenoxuron Chemical compound C=1C=C(NC(=O)NC(=O)C=2C(=CC=CC=2F)F)C(F)=CC=1OC1=CC=C(C(F)(F)F)C=C1Cl RYLHNOVXKPXDIP-UHFFFAOYSA-N 0.000 description 1
- 125000004428 fluoroalkoxy group Chemical group 0.000 description 1
- 125000003709 fluoroalkyl group Chemical group 0.000 description 1
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 description 1
- 238000005187 foaming Methods 0.000 description 1
- 244000000049 foliar pathogen Species 0.000 description 1
- KVGLBTYUCJYMND-UHFFFAOYSA-N fonofos Chemical compound CCOP(=S)(CC)SC1=CC=CC=C1 KVGLBTYUCJYMND-UHFFFAOYSA-N 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 235000019256 formaldehyde Nutrition 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- ZXQYGBMAQZUVMI-GCMPRSNUSA-N gamma-cyhalothrin Chemical compound CC1(C)[C@@H](\C=C(/Cl)C(F)(F)F)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 ZXQYGBMAQZUVMI-GCMPRSNUSA-N 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000035784 germination Effects 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 229960005150 glycerol Drugs 0.000 description 1
- 239000001087 glyceryl triacetate Substances 0.000 description 1
- 229940087559 grape seed Drugs 0.000 description 1
- 239000003630 growth substance Substances 0.000 description 1
- 239000010440 gypsum Substances 0.000 description 1
- 229910052602 gypsum Inorganic materials 0.000 description 1
- 125000004969 haloethyl group Chemical group 0.000 description 1
- CNKHSLKYRMDDNQ-UHFFFAOYSA-N halofenozide Chemical compound C=1C=CC=CC=1C(=O)N(C(C)(C)C)NC(=O)C1=CC=C(Cl)C=C1 CNKHSLKYRMDDNQ-UHFFFAOYSA-N 0.000 description 1
- 230000002140 halogenating effect Effects 0.000 description 1
- 230000026030 halogenation Effects 0.000 description 1
- 238000005658 halogenation reaction Methods 0.000 description 1
- JPXGPRBLTIYFQG-UHFFFAOYSA-N heptan-4-yl acetate Chemical compound CCCC(CCC)OC(C)=O JPXGPRBLTIYFQG-UHFFFAOYSA-N 0.000 description 1
- 230000002363 herbicidal effect Effects 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- RGNPBRKPHBKNKX-UHFFFAOYSA-N hexaflumuron Chemical compound C1=C(Cl)C(OC(F)(F)C(F)F)=C(Cl)C=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F RGNPBRKPHBKNKX-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003707 hexyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- RCBVKBFIWMOMHF-UHFFFAOYSA-L hydroxy-(hydroxy(dioxo)chromio)oxy-dioxochromium;pyridine Chemical compound C1=CC=NC=C1.C1=CC=NC=C1.O[Cr](=O)(=O)O[Cr](O)(=O)=O RCBVKBFIWMOMHF-UHFFFAOYSA-L 0.000 description 1
- 229940056881 imidacloprid Drugs 0.000 description 1
- YWTYJOPNNQFBPC-UHFFFAOYSA-N imidacloprid Chemical compound [O-][N+](=O)\N=C1/NCCN1CC1=CC=C(Cl)N=C1 YWTYJOPNNQFBPC-UHFFFAOYSA-N 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- VBCVPMMZEGZULK-NRFANRHFSA-N indoxacarb Chemical compound C([C@@]1(OC2)C(=O)OC)C3=CC(Cl)=CC=C3C1=NN2C(=O)N(C(=O)OC)C1=CC=C(OC(F)(F)F)C=C1 VBCVPMMZEGZULK-NRFANRHFSA-N 0.000 description 1
- 239000012442 inert solvent Substances 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 150000004694 iodide salts Chemical class 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 230000002262 irrigation Effects 0.000 description 1
- 238000003973 irrigation Methods 0.000 description 1
- 239000003621 irrigation water Substances 0.000 description 1
- 229940117955 isoamyl acetate Drugs 0.000 description 1
- 229940035429 isobutyl alcohol Drugs 0.000 description 1
- HOQADATXFBOEGG-UHFFFAOYSA-N isofenphos Chemical compound CCOP(=S)(NC(C)C)OC1=CC=CC=C1C(=O)OC(C)C HOQADATXFBOEGG-UHFFFAOYSA-N 0.000 description 1
- JMMWKPVZQRWMSS-UHFFFAOYSA-N isopropanol acetate Natural products CC(C)OC(C)=O JMMWKPVZQRWMSS-UHFFFAOYSA-N 0.000 description 1
- NWUWYYSKZYIQAE-UHFFFAOYSA-N isopropyl (3-methyl-1-{[1-(4-methylphenyl)ethyl]amino}-1-oxobutan-2-yl)carbamate Chemical compound CC(C)OC(=O)NC(C(C)C)C(=O)NC(C)C1=CC=C(C)C=C1 NWUWYYSKZYIQAE-UHFFFAOYSA-N 0.000 description 1
- 229940011051 isopropyl acetate Drugs 0.000 description 1
- 125000001810 isothiocyanato group Chemical group *N=C=S 0.000 description 1
- 230000000155 isotopic effect Effects 0.000 description 1
- GWYFCOCPABKNJV-UHFFFAOYSA-M isovalerate Chemical compound CC(C)CC([O-])=O GWYFCOCPABKNJV-UHFFFAOYSA-M 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- 150000002545 isoxazoles Chemical class 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 150000004797 ketoamides Chemical class 0.000 description 1
- 150000003893 lactate salts Chemical class 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 239000005910 lambda-Cyhalothrin Substances 0.000 description 1
- 235000019388 lanolin Nutrition 0.000 description 1
- 229940039717 lanolin Drugs 0.000 description 1
- CAHGCLMLTWQZNJ-RGEKOYMOSA-N lanosterol Chemical compound C([C@]12C)C[C@@H](O)C(C)(C)[C@H]1CCC1=C2CC[C@]2(C)[C@H]([C@H](CCC=C(C)C)C)CC[C@@]21C CAHGCLMLTWQZNJ-RGEKOYMOSA-N 0.000 description 1
- 229940058690 lanosterol Drugs 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 231100001231 less toxic Toxicity 0.000 description 1
- 229940087305 limonene Drugs 0.000 description 1
- 235000001510 limonene Nutrition 0.000 description 1
- 229910001416 lithium ion Inorganic materials 0.000 description 1
- PWPJGUXAGUPAHP-UHFFFAOYSA-N lufenuron Chemical compound C1=C(Cl)C(OC(F)(F)C(C(F)(F)F)F)=CC(Cl)=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F PWPJGUXAGUPAHP-UHFFFAOYSA-N 0.000 description 1
- 229960000521 lufenuron Drugs 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- YCCXQARVHOPWFJ-UHFFFAOYSA-M magnesium;ethane;chloride Chemical compound [Mg+2].[Cl-].[CH2-]C YCCXQARVHOPWFJ-UHFFFAOYSA-M 0.000 description 1
- 235000009973 maize Nutrition 0.000 description 1
- 229960000453 malathion Drugs 0.000 description 1
- 150000002689 maleic acids Chemical class 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- MAGPZHKLEZXLNU-UHFFFAOYSA-N mandelamide Chemical compound NC(=O)C(O)C1=CC=CC=C1 MAGPZHKLEZXLNU-UHFFFAOYSA-N 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 239000008204 material by function Substances 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- GKKDCARASOJPNG-UHFFFAOYSA-N metaldehyde Chemical compound CC1OC(C)OC(C)OC(C)O1 GKKDCARASOJPNG-UHFFFAOYSA-N 0.000 description 1
- NNKVPIKMPCQWCG-UHFFFAOYSA-N methamidophos Chemical compound COP(N)(=O)SC NNKVPIKMPCQWCG-UHFFFAOYSA-N 0.000 description 1
- CKJNUZNMWOVDFN-UHFFFAOYSA-N methanone Chemical compound O=[CH-] CKJNUZNMWOVDFN-UHFFFAOYSA-N 0.000 description 1
- MEBQXILRKZHVCX-UHFFFAOYSA-N methidathion Chemical compound COC1=NN(CSP(=S)(OC)OC)C(=O)S1 MEBQXILRKZHVCX-UHFFFAOYSA-N 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- UHXUZOCRWCRNSJ-QPJJXVBHSA-N methomyl Chemical compound CNC(=O)O\N=C(/C)SC UHXUZOCRWCRNSJ-QPJJXVBHSA-N 0.000 description 1
- 229930002897 methoprene Natural products 0.000 description 1
- 229950003442 methoprene Drugs 0.000 description 1
- QCAWEPFNJXQPAN-UHFFFAOYSA-N methoxyfenozide Chemical compound COC1=CC=CC(C(=O)NN(C(=O)C=2C=C(C)C=C(C)C=2)C(C)(C)C)=C1C QCAWEPFNJXQPAN-UHFFFAOYSA-N 0.000 description 1
- KBHDSWIXRODKSZ-UHFFFAOYSA-N methyl 5-chloro-2-(trifluoromethylsulfonylamino)benzoate Chemical compound COC(=O)C1=CC(Cl)=CC=C1NS(=O)(=O)C(F)(F)F KBHDSWIXRODKSZ-UHFFFAOYSA-N 0.000 description 1
- 150000004702 methyl esters Chemical class 0.000 description 1
- BSDQITJYKQHXQR-UHFFFAOYSA-N methyl prop-2-eneperoxoate Chemical compound COOC(=O)C=C BSDQITJYKQHXQR-UHFFFAOYSA-N 0.000 description 1
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 1
- 229960001952 metrifonate Drugs 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 229910052618 mica group Inorganic materials 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000002438 mitochondrial effect Effects 0.000 description 1
- 230000004898 mitochondrial function Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- KRTSDMXIXPKRQR-AATRIKPKSA-N monocrotophos Chemical compound CNC(=O)\C=C(/C)OP(=O)(OC)OC KRTSDMXIXPKRQR-AATRIKPKSA-N 0.000 description 1
- RZRNAYUHWVFMIP-UHFFFAOYSA-N monoelaidin Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-UHFFFAOYSA-N 0.000 description 1
- 125000006372 monohalo methyl group Chemical group 0.000 description 1
- 239000012452 mother liquor Substances 0.000 description 1
- AEXITZJSLGALNH-UHFFFAOYSA-N n'-hydroxyethanimidamide Chemical compound CC(N)=NO AEXITZJSLGALNH-UHFFFAOYSA-N 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N n-Octanol Natural products CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- JCPCLLBVKYTARN-UHFFFAOYSA-N n-[2-[4-[3-(4-chlorophenyl)prop-2-ynoxy]-3-methoxyphenyl]ethyl]-2-(ethylsulfonylamino)-3-methylbutanamide Chemical compound COC1=CC(CCNC(=O)C(C(C)C)NS(=O)(=O)CC)=CC=C1OCC#CC1=CC=C(Cl)C=C1 JCPCLLBVKYTARN-UHFFFAOYSA-N 0.000 description 1
- BOBIZDGUDNVINH-UHFFFAOYSA-N n-[2-[4-[3-(4-chlorophenyl)prop-2-ynoxy]-3-methoxyphenyl]ethyl]-2-(methanesulfonamido)-3-methylbutanamide Chemical compound COC1=CC(CCNC(=O)C(NS(C)(=O)=O)C(C)C)=CC=C1OCC#CC1=CC=C(Cl)C=C1 BOBIZDGUDNVINH-UHFFFAOYSA-N 0.000 description 1
- YNKFZRGTXAPYFD-UHFFFAOYSA-N n-[[2-chloro-3,5-bis(trifluoromethyl)phenyl]carbamoyl]-2,6-difluorobenzamide Chemical compound FC1=CC=CC(F)=C1C(=O)NC(=O)NC1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1Cl YNKFZRGTXAPYFD-UHFFFAOYSA-N 0.000 description 1
- LPOIGVZLNWEGJG-UHFFFAOYSA-N n-benzyl-5-(4-methylpiperazin-1-yl)-2-nitroaniline Chemical compound C1CN(C)CCN1C1=CC=C([N+]([O-])=O)C(NCC=2C=CC=CC=2)=C1 LPOIGVZLNWEGJG-UHFFFAOYSA-N 0.000 description 1
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229950006238 nadide Drugs 0.000 description 1
- 150000002790 naphthalenes Chemical class 0.000 description 1
- 229940079888 nitenpyram Drugs 0.000 description 1
- 125000006574 non-aromatic ring group Chemical group 0.000 description 1
- 229920000847 nonoxynol Polymers 0.000 description 1
- NJPPVKZQTLUDBO-UHFFFAOYSA-N novaluron Chemical compound C1=C(Cl)C(OC(F)(F)C(OC(F)(F)F)F)=CC=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F NJPPVKZQTLUDBO-UHFFFAOYSA-N 0.000 description 1
- 238000001668 nucleic acid synthesis Methods 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 239000004533 oil dispersion Substances 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 229940055577 oleyl alcohol Drugs 0.000 description 1
- XMLQWXUVTXCDDL-UHFFFAOYSA-N oleyl alcohol Natural products CCCCCCC=CCCCCCCCCCCO XMLQWXUVTXCDDL-UHFFFAOYSA-N 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 239000012044 organic layer Substances 0.000 description 1
- 125000002734 organomagnesium group Chemical group 0.000 description 1
- 150000002902 organometallic compounds Chemical class 0.000 description 1
- 238000010653 organometallic reaction Methods 0.000 description 1
- XSXHWVKGUXMUQE-UHFFFAOYSA-N osmium dioxide Inorganic materials O=[Os]=O XSXHWVKGUXMUQE-UHFFFAOYSA-N 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- COWNFYYYZFRNOY-UHFFFAOYSA-N oxazolidinedione Chemical compound O=C1COC(=O)N1 COWNFYYYZFRNOY-UHFFFAOYSA-N 0.000 description 1
- 150000001475 oxazolidinediones Chemical class 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- AUONHKJOIZSQGR-UHFFFAOYSA-N oxophosphane Chemical compound P=O AUONHKJOIZSQGR-UHFFFAOYSA-N 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- BHAAPTBBJKJZER-UHFFFAOYSA-N p-anisidine Chemical compound COC1=CC=C(N)C=C1 BHAAPTBBJKJZER-UHFFFAOYSA-N 0.000 description 1
- UQPUONNXJVWHRM-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 UQPUONNXJVWHRM-UHFFFAOYSA-N 0.000 description 1
- LCCNCVORNKJIRZ-UHFFFAOYSA-N parathion Chemical compound CCOP(=S)(OCC)OC1=CC=C([N+]([O-])=O)C=C1 LCCNCVORNKJIRZ-UHFFFAOYSA-N 0.000 description 1
- RLBIQVVOMOPOHC-UHFFFAOYSA-N parathion-methyl Chemical group COP(=S)(OC)OC1=CC=C([N+]([O-])=O)C=C1 RLBIQVVOMOPOHC-UHFFFAOYSA-N 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N pentanoic acid group Chemical class C(CCCC)(=O)O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 229960000490 permethrin Drugs 0.000 description 1
- RLLPVAHGXHCWKJ-UHFFFAOYSA-N permethrin Chemical compound CC1(C)C(C=C(Cl)Cl)C1C(=O)OCC1=CC=CC(OC=2C=CC=CC=2)=C1 RLLPVAHGXHCWKJ-UHFFFAOYSA-N 0.000 description 1
- 150000004965 peroxy acids Chemical class 0.000 description 1
- 235000020030 perry Nutrition 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 239000003016 pheromone Substances 0.000 description 1
- BULVZWIRKLYCBC-UHFFFAOYSA-N phorate Chemical compound CCOP(=S)(OCC)SCSCC BULVZWIRKLYCBC-UHFFFAOYSA-N 0.000 description 1
- IOUNQDKNJZEDEP-UHFFFAOYSA-N phosalone Chemical compound C1=C(Cl)C=C2OC(=O)N(CSP(=S)(OCC)OCC)C2=C1 IOUNQDKNJZEDEP-UHFFFAOYSA-N 0.000 description 1
- LMNZTLDVJIUSHT-UHFFFAOYSA-N phosmet Chemical compound C1=CC=C2C(=O)N(CSP(=S)(OC)OC)C(=O)C2=C1 LMNZTLDVJIUSHT-UHFFFAOYSA-N 0.000 description 1
- RGCLLPNLLBQHPF-HJWRWDBZSA-N phosphamidon Chemical compound CCN(CC)C(=O)C(\Cl)=C(/C)OP(=O)(OC)OC RGCLLPNLLBQHPF-HJWRWDBZSA-N 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- UXCDUFKZSUBXGM-UHFFFAOYSA-N phosphoric tribromide Chemical compound BrP(Br)(Br)=O UXCDUFKZSUBXGM-UHFFFAOYSA-N 0.000 description 1
- 229910000073 phosphorus hydride Inorganic materials 0.000 description 1
- 125000005543 phthalimide group Chemical class 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- YFGYUFNIOHWBOB-UHFFFAOYSA-N pirimicarb Chemical compound CN(C)C(=O)OC1=NC(N(C)C)=NC(C)=C1C YFGYUFNIOHWBOB-UHFFFAOYSA-N 0.000 description 1
- 244000000003 plant pathogen Species 0.000 description 1
- 239000003880 polar aprotic solvent Substances 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 150000004291 polyenes Chemical class 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 235000015277 pork Nutrition 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011698 potassium fluoride Substances 0.000 description 1
- 235000003270 potassium fluoride Nutrition 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- QYMMJNLHFKGANY-UHFFFAOYSA-N profenofos Chemical compound CCCSP(=O)(OCC)OC1=CC=C(Br)C=C1Cl QYMMJNLHFKGANY-UHFFFAOYSA-N 0.000 description 1
- 125000006412 propinylene group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- 229940080818 propionamide Drugs 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- RUOJZAUFBMNUDX-UHFFFAOYSA-N propylene carbonate Chemical compound CC1COC(=O)O1 RUOJZAUFBMNUDX-UHFFFAOYSA-N 0.000 description 1
- 108060006633 protein kinase Proteins 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- QHMTXANCGGJZRX-WUXMJOGZSA-N pymetrozine Chemical compound C1C(C)=NNC(=O)N1\N=C\C1=CC=CN=C1 QHMTXANCGGJZRX-WUXMJOGZSA-N 0.000 description 1
- DDIQWGKUSJOETH-UHFFFAOYSA-N pyrafluprole Chemical compound ClC=1C=C(C(F)(F)F)C=C(Cl)C=1N1N=C(C#N)C(SCF)=C1NCC1=CN=CC=N1 DDIQWGKUSJOETH-UHFFFAOYSA-N 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- HYJYGLGUBUDSLJ-UHFFFAOYSA-N pyrethrin Natural products CCC(=O)OC1CC(=C)C2CC3OC3(C)C2C2OC(=O)C(=C)C12 HYJYGLGUBUDSLJ-UHFFFAOYSA-N 0.000 description 1
- VJFUPGQZSXIULQ-XIGJTORUSA-N pyrethrin II Chemical compound CC1(C)[C@H](/C=C(\C)C(=O)OC)[C@H]1C(=O)O[C@@H]1C(C)=C(C\C=C/C=C)C(=O)C1 VJFUPGQZSXIULQ-XIGJTORUSA-N 0.000 description 1
- AEHJMNVBLRLZKK-UHFFFAOYSA-N pyridalyl Chemical group N1=CC(C(F)(F)F)=CC=C1OCCCOC1=C(Cl)C=C(OCC=C(Cl)Cl)C=C1Cl AEHJMNVBLRLZKK-UHFFFAOYSA-N 0.000 description 1
- UDJFFSGCRRMVFH-UHFFFAOYSA-N pyrido[2,3-d]pyrimidine Chemical compound N1=CN=CC2=CC=CN=C21 UDJFFSGCRRMVFH-UHFFFAOYSA-N 0.000 description 1
- MIOBBYRMXGNORL-UHFFFAOYSA-N pyrifluquinazon Chemical compound C1C2=CC(C(F)(C(F)(F)F)C(F)(F)F)=CC=C2N(C(=O)C)C(=O)N1NCC1=CC=CN=C1 MIOBBYRMXGNORL-UHFFFAOYSA-N 0.000 description 1
- QDGHXQFTWKRQTG-UHFFFAOYSA-N pyrimidin-2-ylhydrazine Chemical class NNC1=NC=CC=N1 QDGHXQFTWKRQTG-UHFFFAOYSA-N 0.000 description 1
- FUXJMHXHGDAHPD-UHFFFAOYSA-N pyrimidine-2-carboxamide Chemical compound NC(=O)C1=NC=CC=N1 FUXJMHXHGDAHPD-UHFFFAOYSA-N 0.000 description 1
- NHDHVHZZCFYRSB-UHFFFAOYSA-N pyriproxyfen Chemical compound C=1C=CC=NC=1OC(C)COC(C=C1)=CC=C1OC1=CC=CC=C1 NHDHVHZZCFYRSB-UHFFFAOYSA-N 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- 238000007348 radical reaction Methods 0.000 description 1
- 229920005604 random copolymer Polymers 0.000 description 1
- 239000001044 red dye Substances 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 230000002940 repellent Effects 0.000 description 1
- 230000001850 reproductive effect Effects 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 239000011369 resultant mixture Substances 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 125000006413 ring segment Chemical group 0.000 description 1
- 229940080817 rotenone Drugs 0.000 description 1
- JUVIOZPCNVVQFO-UHFFFAOYSA-N rotenone Natural products O1C2=C3CC(C(C)=C)OC3=CC=C2C(=O)C2C1COC1=C2C=C(OC)C(OC)=C1 JUVIOZPCNVVQFO-UHFFFAOYSA-N 0.000 description 1
- JJSYXNQGLHBRRK-SFEDZAPPSA-N ryanodine Chemical compound O([C@@H]1[C@]([C@@]2([C@]3(O)[C@]45O[C@@]2(O)C[C@]([C@]4(CC[C@H](C)[C@H]5O)O)(C)[C@@]31O)C)(O)C(C)C)C(=O)C1=CC=CN1 JJSYXNQGLHBRRK-SFEDZAPPSA-N 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical class C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 description 1
- 150000004671 saturated fatty acids Chemical class 0.000 description 1
- 235000003441 saturated fatty acids Nutrition 0.000 description 1
- 108010010116 scytalone dehydratase Proteins 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 239000003620 semiochemical Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 235000010288 sodium nitrite Nutrition 0.000 description 1
- 229960001922 sodium perborate Drugs 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- NYCVSSWORUBFET-UHFFFAOYSA-M sodium;bromite Chemical compound [Na+].[O-]Br=O NYCVSSWORUBFET-UHFFFAOYSA-M 0.000 description 1
- YKLJGMBLPUQQOI-UHFFFAOYSA-M sodium;oxidooxy(oxo)borane Chemical compound [Na+].[O-]OB=O YKLJGMBLPUQQOI-UHFFFAOYSA-M 0.000 description 1
- 239000004550 soluble concentrate Substances 0.000 description 1
- JNYAEWCLZODPBN-CTQIIAAMSA-N sorbitan Polymers OCC(O)C1OCC(O)[C@@H]1O JNYAEWCLZODPBN-CTQIIAAMSA-N 0.000 description 1
- 229960002920 sorbitol Drugs 0.000 description 1
- 108010025009 spectrin-like proteins Proteins 0.000 description 1
- VNFWTIYUKDMAOP-UHFFFAOYSA-N sphos Chemical compound COC1=CC=CC(OC)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 VNFWTIYUKDMAOP-UHFFFAOYSA-N 0.000 description 1
- 229940014213 spinosad Drugs 0.000 description 1
- CLSVJBIHYWPGQY-GGYDESQDSA-N spirotetramat Chemical compound CCOC(=O)OC1=C(C=2C(=CC=C(C)C=2)C)C(=O)N[C@@]11CC[C@H](OC)CC1 CLSVJBIHYWPGQY-GGYDESQDSA-N 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000003206 sterilizing agent Substances 0.000 description 1
- 239000012258 stirred mixture Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 125000005504 styryl group Chemical group 0.000 description 1
- 125000003107 substituted aryl group Chemical group 0.000 description 1
- 235000011044 succinic acid Nutrition 0.000 description 1
- 150000003444 succinic acids Chemical class 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 150000003445 sucroses Chemical class 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 1
- JXHJNEJVUNHLKO-UHFFFAOYSA-N sulprofos Chemical compound CCCSP(=S)(OCC)OC1=CC=C(SC)C=C1 JXHJNEJVUNHLKO-UHFFFAOYSA-N 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000004548 suspo-emulsion Substances 0.000 description 1
- 239000000271 synthetic detergent Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 239000005936 tau-Fluvalinate Substances 0.000 description 1
- INISTDXBRIBGOC-XMMISQBUSA-N tau-fluvalinate Chemical compound N([C@H](C(C)C)C(=O)OC(C#N)C=1C=C(OC=2C=CC=CC=2)C=CC=1)C1=CC=C(C(F)(F)F)C=C1Cl INISTDXBRIBGOC-XMMISQBUSA-N 0.000 description 1
- QYPNKSZPJQQLRK-UHFFFAOYSA-N tebufenozide Chemical compound C1=CC(CC)=CC=C1C(=O)NN(C(C)(C)C)C(=O)C1=CC(C)=CC(C)=C1 QYPNKSZPJQQLRK-UHFFFAOYSA-N 0.000 description 1
- ROZUQUDEWZIBHV-UHFFFAOYSA-N tecloftalam Chemical compound OC(=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1C(=O)NC1=CC=CC(Cl)=C1Cl ROZUQUDEWZIBHV-UHFFFAOYSA-N 0.000 description 1
- CJDWRQLODFKPEL-UHFFFAOYSA-N teflubenzuron Chemical compound FC1=CC=CC(F)=C1C(=O)NC(=O)NC1=CC(Cl)=C(F)C(Cl)=C1F CJDWRQLODFKPEL-UHFFFAOYSA-N 0.000 description 1
- IOGXOCVLYRDXLW-UHFFFAOYSA-N tert-butyl nitrite Chemical compound CC(C)(C)ON=O IOGXOCVLYRDXLW-UHFFFAOYSA-N 0.000 description 1
- 239000012414 tert-butyl nitrite Substances 0.000 description 1
- CIHOLLKRGTVIJN-UHFFFAOYSA-N tert‐butyl hydroperoxide Chemical compound CC(C)(C)OO CIHOLLKRGTVIJN-UHFFFAOYSA-N 0.000 description 1
- UBCKGWBNUIFUST-YHYXMXQVSA-N tetrachlorvinphos Chemical compound COP(=O)(OC)O\C(=C/Cl)C1=CC(Cl)=C(Cl)C=C1Cl UBCKGWBNUIFUST-YHYXMXQVSA-N 0.000 description 1
- BSYVTEYKTMYBMK-UHFFFAOYSA-N tetrahydrofurfuryl alcohol Chemical compound OCC1CCCO1 BSYVTEYKTMYBMK-UHFFFAOYSA-N 0.000 description 1
- CZDYPVPMEAXLPK-UHFFFAOYSA-N tetramethylsilane Chemical compound C[Si](C)(C)C CZDYPVPMEAXLPK-UHFFFAOYSA-N 0.000 description 1
- NWWZPOKUUAIXIW-FLIBITNWSA-N thiamethoxam Chemical compound [O-][N+](=O)\N=C/1N(C)COCN\1CC1=CN=C(Cl)S1 NWWZPOKUUAIXIW-FLIBITNWSA-N 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 150000003558 thiocarbamic acid derivatives Chemical class 0.000 description 1
- 125000000858 thiocyanato group Chemical group *SC#N 0.000 description 1
- BAKXBZPQTXCKRR-UHFFFAOYSA-N thiodicarb Chemical compound CSC(C)=NOC(=O)NSNC(=O)ON=C(C)SC BAKXBZPQTXCKRR-UHFFFAOYSA-N 0.000 description 1
- 125000000101 thioether group Chemical group 0.000 description 1
- QGHREAKMXXNCOA-UHFFFAOYSA-N thiophanate-methyl Chemical compound COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC QGHREAKMXXNCOA-UHFFFAOYSA-N 0.000 description 1
- 229930192474 thiophene Natural products 0.000 description 1
- QSOHVSNIQHGFJU-UHFFFAOYSA-L thiosultap disodium Chemical compound [Na+].[Na+].[O-]S(=O)(=O)SCC(N(C)C)CSS([O-])(=O)=O QSOHVSNIQHGFJU-UHFFFAOYSA-L 0.000 description 1
- 230000009974 thixotropic effect Effects 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 235000010215 titanium dioxide Nutrition 0.000 description 1
- WPALTCMYPARVNV-UHFFFAOYSA-N tolfenpyrad Chemical compound CCC1=NN(C)C(C(=O)NCC=2C=CC(OC=3C=CC(C)=CC=3)=CC=2)=C1Cl WPALTCMYPARVNV-UHFFFAOYSA-N 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-M toluene-4-sulfonate Chemical compound CC1=CC=C(S([O-])(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-M 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- YWSCPYYRJXKUDB-KAKFPZCNSA-N tralomethrin Chemical compound CC1(C)[C@@H](C(Br)C(Br)(Br)Br)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 YWSCPYYRJXKUDB-KAKFPZCNSA-N 0.000 description 1
- XQJQCBDIXRIYRP-STQMWFEESA-N trans-(1S,2R)-sedaxane Chemical compound FC(F)C1=NN(C)C=C1C(=O)NC1=CC=CC=C1[C@H]1[C@H](C2CC2)C1 XQJQCBDIXRIYRP-STQMWFEESA-N 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- NKNFWVNSBIXGLL-UHFFFAOYSA-N triazamate Chemical compound CCOC(=O)CSC1=NC(C(C)(C)C)=NN1C(=O)N(C)C NKNFWVNSBIXGLL-UHFFFAOYSA-N 0.000 description 1
- NFACJZMKEDPNKN-UHFFFAOYSA-N trichlorfon Chemical compound COP(=O)(OC)C(O)C(Cl)(Cl)Cl NFACJZMKEDPNKN-UHFFFAOYSA-N 0.000 description 1
- BDCUDAOHXKZZAJ-UHFFFAOYSA-N tricyclopropylbismuthane Chemical compound C1CC1[Bi](C1CC1)C1CC1 BDCUDAOHXKZZAJ-UHFFFAOYSA-N 0.000 description 1
- ZDRNMODJXFOYMN-UHFFFAOYSA-N tridecyl acetate Chemical compound CCCCCCCCCCCCCOC(C)=O ZDRNMODJXFOYMN-UHFFFAOYSA-N 0.000 description 1
- 229940087291 tridecyl alcohol Drugs 0.000 description 1
- MCVUKOYZUCWLQQ-UHFFFAOYSA-N tridecylbenzene Chemical class CCCCCCCCCCCCCC1=CC=CC=C1 MCVUKOYZUCWLQQ-UHFFFAOYSA-N 0.000 description 1
- 125000002827 triflate group Chemical group FC(S(=O)(=O)O*)(F)F 0.000 description 1
- XAIPTRIXGHTTNT-UHFFFAOYSA-N triflumuron Chemical compound C1=CC(OC(F)(F)F)=CC=C1NC(=O)NC(=O)C1=CC=CC=C1Cl XAIPTRIXGHTTNT-UHFFFAOYSA-N 0.000 description 1
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 1
- 108010013280 ubiquinol oxidase Proteins 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 150000004670 unsaturated fatty acids Chemical class 0.000 description 1
- 235000021122 unsaturated fatty acids Nutrition 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 239000004562 water dispersible granule Substances 0.000 description 1
- 239000004563 wettable powder Substances 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 229940102001 zinc bromide Drugs 0.000 description 1
- 239000011592 zinc chloride Substances 0.000 description 1
- 235000005074 zinc chloride Nutrition 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
- 235000014692 zinc oxide Nutrition 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C323/00—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups
- C07C323/22—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and doubly-bound oxygen atoms bound to the same carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/12—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/18—One oxygen or sulfur atom
- C07D231/20—One oxygen atom attached in position 3 or 5
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/38—Nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D407/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00
- C07D407/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing two hetero rings
- C07D407/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
Definitions
- This invention relates to certain pyrazoles, their //-oxides, salts and compositions, and methods of their use as fungicides.
- JP09208620 discloses JV-phenylpyrazolylamine and JV-pyridylpyrazolylamine derivatives as insecticides, herbicides and fungicides; however the fungicides of the present invention are not disclosed in this publication.
- This invention is directed to compounds of Formula 1 (including all geometric and stereoisomers), iV-oxides, and salts thereof, agricultural compositions containing them and their use as fungicides:
- R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 2 -C 4 alkenyl, C 2 -C 4 alkynyl, C 3 -
- C 7 cycloalkyl CO 2 R 5 , C(O)NR 6 R 7 , cyano, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy or C 2 -C 5 alkoxyalkyl; or
- R 1 is phenyl optionally substituted with up to 3 R 8 ; or a five- or six-membered nitrogen-containing aromatic heterocycle optionally substituted with up to 3 substituents independently selected from R 9a on carbon atom ring members and
- R la is H
- R la and R 1 are taken together with the carbon atom to which they are attached to form a cyclopropyl ring optionally substituted with up to 2 substituents independently selected from halogen and methyl;
- R 2 is CH 3 , CH 2 CH 3 , halogen, cyano, cyanomethyl, halomethyl, hydroxymethyl, methoxy or methylthio; or cyclopropyl optionally substituted with up to 2 substituents independently selected from halogen and methyl; each R 3 is independently selected from halogen, cyano, nitro, amino, methylamino, dimethylamino, formylamino, C 2 -C 3 alkylcarbonylamino, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 1 -C 3 alkylthio, C 1 -C 3 haloalkylthio, C 1 -C 3 alkylsulf ⁇ nyl, C 1 -C 3 haloalkylsulfmyl, C 1 -C 3 alkylsulfonyl, C 1 -C
- R 4 is H, formyl, C 2 -C 5 alkenyl, C 3 -C 5 alkynyl, C 3 -C 7 cycloalkyl, -SO 3 -M + ,
- R 10 is H, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl;
- R 6 and R 7 are independently selected from H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 7 cycloalkyl, C 4 -Cg cycloalkylalkyl and C 4 -Cg alkylcycloalkyl; or
- R 6 and R 7 are taken together with the nitrogen atom to which they are connected to form a four- to seven-membered nonaromatic heterocyclic ring containing ring members, in addition to the connecting ring nitrogen atom, selected from carbon atoms and optionally up to one ring member selected from O, S(O) n and NR 13 ; each R 8 , R 9a and R 9b is independently selected from halogen, C ⁇ C 2 alkyl, C ⁇ C 2 haloalkyl, C 1 -C 2 alkoxy, C 1 -C 2 haloalkoxy, cyano, nitro, SCH 3 , S(O)CH 3 and
- R 10 is C 1 -C 6 alkyl or C 1 -C 6 haloalkyl; each R 11 is independently C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 2 -C 7 alkoxyalkyl, C 2 -C 7 alkylaminoalkyl, C 2 -Cg dialkylaminoalkyl, C 1 -C 6 alkylthio or C 2 -C 7 alkylthioalkyl; each R 12 is independently C 3 -C 7 cycloalkyl, C 1 -C 4 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfmyl, C 1 -C 4 alkylsulfonyl or cyano; R 13 is H, C 1 -C 3 alkyl or C 2 -C 3 haloalkyl; each R 14 is independently H, cyano, C 1 -C 3
- R 15 is H, C 1 -C 4 alkyl or OR 18 ;
- R 16 is C 1 -C 4 alkyl or OR 18 ; or R 15 and R 16 are taken together as -OCH 2 CH 2 O-;
- each R 19 and R 20 is independently H or CH 3 ;
- each R 22 is independently H, C 1 -C 6 alkyl, C 1 -C 6
- each R 23a and R 23b is independently H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 2 -C 6 alkenyl, C 3 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 3 -C 6 halocycloalkyl, C 2 -C 6 alkylcarbonyl, C 2 -C 6 alkoxycarbonyl, C 2 -C 6 (alkylthio)carbonyl, C 2 -C 6 alkoxy(thiocarbonyl),
- each R 24 and R 25 is independently H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 2 -C 6 alkenyl, C 3 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 3 -C 6 halocycloalkyl, C 2 -C 6 alkylcarbonyl, C 2 -C 6 alkoxycarbonyl, C 2 -C 6 (alkylthi
- this invention pertains to a compound of Formula 1 (including all geometric and stereoisomers), an JV-oxide or a salt thereof.
- This invention also relates to a fungicidal composition comprising a compound of Formula 1, an JV-oxide, or a salt thereof, and at least one additional component selected from the group consisting of surfactants, solid diluents and liquid diluents.
- This invention also relates to a fungicidal composition
- a fungicidal composition comprising: (a) a compound of Formula 1, an JV-oxide, or a salt thereof, and (b) at least one other fungicide (e.g., at least one other fungicide having a different site of action).
- This invention further relates to a method for controlling plant diseases caused by fungal plant pathogens comprising applying to the plant or portion thereof, or to the plant seed, a fungicidally effective amount of a compound of the invention (e.g., as a composition described herein).
- This invention also relates to a composition
- a composition comprising a compound of Formula 1, an N-oxide, or a salt thereof, and at least one invertebrate pest control compound or agent.
- the invention also relates to compounds of Formula 2 (including all geometric and stereoisomers) and salts thereof
- X is NH
- the terms “comprises,” “comprising,” “includes,” “including,” “has,” “having,” “contains”, “containing,” “characterized by” or any other variation thereof, are intended to cover a non-exclusive inclusion, subject to any limitation explicitly indicated.
- a composition, mixture, process or method that comprises a list of elements is not necessarily limited to only those elements but may include other elements not expressly listed or inherent to such composition, mixture, process or method.
- the phrase “consisting of appears in a clause of the body of a claim, rather than immediately following the preamble it limits only the element set forth in that clause; other elements are not excluded from the claim as a whole.
- transitional phrase consisting essentially of is used to define a composition or method that includes materials, steps, features, components, or elements, in addition to those literally disclosed, provided that these additional materials, steps, features, components, or elements do not materially affect the basic and novel characteristic(s) of the claimed invention.
- plant includes members of Kingdom Plantae, particularly seed plants (Spermatopsida), at all life stages, including young plants (e.g., germinating seeds developing into seedlings) and mature, reproductive stages (e.g., plants producing flowers and seeds).
- Portions of plants include geotropic members typically growing beneath the surface of the growing medium (e.g., soil), such as roots, tubers, bulbs and corms, and also members growing above the growing medium, such as foliage (including stems and leaves), flowers, fruits and seeds.
- the term “seedling”, used either alone or in a combination of words means a young plant developing from the embryo of a seed.
- the term “alkylating agent” refers to a chemical compound in which a carbon-containing radical is bound through a carbon atom to leaving group such as halide or sulfonate, which is displaceable by bonding of a nucleophile to said carbon atom. Unless otherwise indicated, the term “alkylating” does not limit the carbon-containing radical to alkyl; the carbon-containing radicals in alkylating agents include the variety of carbon-bound substituent radicals specified for R 1 .
- a molecular fragment i.e. radical
- a series of atom symbols e.g., C, H, N, O, S
- the point or points of attachment may be explicitly indicated by a hyphen ("-").
- -SCN indicates that the point of attachment is the sulfur atom (i.e. thiocyanato, not isothiocyanato).
- alkyl used either alone or in compound words such as “alkylthio” or “haloalkyl” includes straight-chain or branched alkyl, such as, methyl, ethyl, n-propyl, /-propyl, or the different butyl, pentyl or hexyl isomers.
- alkenyl includes straight-chain or branched alkenes such as ethenyl, 1-propenyl, 2-propenyl, and the different butenyl isomers.
- Alkenyl also includes polyenes such as 1,2-propadienyl.
- Alkynyl includes straight-chain or branched alkynes such as ethynyl, 1-propynyl, 2-propynyl and the different butynyl isomers.
- Alkynylene denotes a straight-chain or branched alkynediyl containing one triple bond.
- alkynylene examples include CH 2 C ⁇ C, C ⁇ CCH 2 and the different butynylene, pentynylene and hexynylene isomers.
- Alkoxy includes, for example, methoxy, ethoxy, n-propyloxy, isopropyloxy and the different butoxy, pentoxy and hexyloxy isomers.
- Alkoxyalkyl denotes alkoxy substitution on alkyl. Examples of “alkoxyalkyl” include CH 3 OCH 2 , CH 3 OCH 2 CH 2 , CH 3 CH 2 OCH 2 , CH 3 CH 2 CH 2 CH 2 OCH 2 and CH 3 CH 2 OCH 2 CH 2 .
- Alkylthio includes branched or straight-chain alkylthio moieties such as methylthio, ethylthio, and the different propylthio, butylthio, pentylthio and hexylthio isomers.
- Alkylsulfmyl includes both enantiomers of an alkylsulf ⁇ nyl group. Examples of “alkylsulfmyl” include CH 3 S(O)-, CH 3 CH 2 S(O)-, CH 3 CH 2 CH 2 S(O)-, (CH 3 ) 2 CHS(O)- and the different butylsulfinyl isomers.
- alkylsulfonyl examples include CH 3 S(O) 2 -, CH 3 CH 2 S(O) 2 -, CH 3 CH 2 CH 2 S(O) 2 -, (CH 3 ) 2 CHS(O) 2 -, and the different butylsulfonyl isomers.
- Alkylthioalkyl denotes alkylthio substitution on alkyl.
- alkylthioalkyl include CH 3 SCH 2 , CH 3 SCH 2 CH 2 , CH 3 CH 2 SCH 2 , CH 3 CH 2 CH 2 CH 2 SCH 2 and CH 3 CH 2 SCH 2 CH 2 .
- Alkylaminoalkyl denotes a straight-chain or branched alkyl moieties bonded to a nitrogen atom of an amino(straight-chain or branched)alkyl moiety.
- alkylaminoalkyl examples include CH 3 NHCH 2 -, (CH 3 ) 2 CHNHCH 2 - and CH 3 NHCH(CH 3 )-.
- Dialkylaminoalkyl denotes two independent straight-chain or branched alkyl moieties bonded to a nitrogen atom of an amino (straight-chain or branched)alkyl moiety.
- dialkylaminoalkyl examples include (CH 3 ) 2 NCH r , (CH 3 ) 2 CH(CH 3 )NCH 2 - and (CH 3 ) 2 NCH(CH 3 )-.
- Cycloalkyl includes, for example, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl.
- alkylcycloalkyl denotes alkyl substitution on a cycloalkyl moiety and includes, for example, ethylcyclopropyl, z-propylcyclobutyl, 3-methylcyclopentyl and 4-methylcyclohexyl.
- cycloalkylalkyl denotes cycloalkyl substitution on an alkyl moiety.
- cycloalkylalkyl examples include cyclopropylmethyl, cyclopentylethyl, and other cycloalkyl moieties bonded to straight-chain or branched alkyl groups.
- cycloalkoxy denotes cycloalkyl linked through an oxygen atom such as cyclopentyloxy and cyclohexyloxy.
- Cycloalkenyl includes carbocyclic rings that contain only one double bond such as cyclopentenyl and cyclohexenyl, as well as carbocyclic rings with more than one double bond such as 1,3- and 1 ,4-cyclohexadienyl, but are not aromatic.
- cycloalkylene denotes a cycloalkanediyl ring.
- cycloalkylene examples include cyclopropylene, cyclobutylene, cyclopentylene and cyclohexylene.
- cycloalkenylene denotes a cycloalkenediyl ring containing one olefmic bond.
- Examples of “cycloalkenylene” include cylopropenediyl and cyclpentenediyl.
- halogen either alone or in compound words such as “haloalkyl”, or when used in descriptions such as “alkyl substituted with halogen” includes fluorine, chlorine, bromine or iodine. Further, when used in compound words such as “haloalkyl”, or when used in descriptions such as “alkyl substituted with halogen” said alkyl may be partially or fully substituted with halogen atoms which may be the same or different. Examples of “haloalkyl” or “alkyl substituted with halogen” include F 3 C-, ClCH 2 -, CF 3 CH 2 - and CF 3 CCl 2 -.
- halocycloalkyl examples include CH 2 FO-, CHF 2 O-, CF 3 O-, CCl 3 CH 2 O-, HCF 2 CH 2 CH 2 O- and CF 3 CH 2 O-.
- fluoroalkoxy examples include CH 2 FO-, CHF 2 O-, CF 3 O- HCF 2 CH 2 CH 2 O- and CF 3 CH 2 O-.
- fluoromethoxy examples include CH 2 FO-, CHF 2 O- and CF 3 O-.
- haloalkylthio examples include CCl 3 S-, CF 3 S-, CCl 3 CH 2 S- and ClCH 2 CH 2 CH 2 S-.
- haloalkylsulfmyl examples include CF 3 S(O)-, CCl 3 S(O)-, CF 3 CH 2 S(O)- and CF 3 CF 2 S(O)-.
- haloalkylsulfonyl include CF 3 S(O) 2 -, CCl 3 S(O) 2 -, CF 3 CH 2 S(O) 2 - and CF 3 CF 2 S(O) 2 -.
- Cj-C The total number of carbon atoms in a substituent group is indicated by the "Cj-C;" prefix where i and j are numbers from 1 to 12.
- C 1 -C 4 alkylsulfonyl designates methylsulfonyl through butylsulfonyl
- C 2 alkoxyalkyl designates CH 3 OCH 2 -
- C 3 alkoxyalkyl designates, for example, CH 3 CH(OCH 3 )-, CH 3 OCH 2 CH 2 - or CH 3 CH 2 OCH 2 -
- C 4 alkoxyalkyl designates the various isomers of an alkyl group substituted with an alkoxy group containing a total of four carbon atoms, examples including CH 3 CH 2 CH 2 OCH 2 - and CH 3 CH 2 OCH 2 CH 2 -.
- an optionally substituted group may have a substituent at each substitutable position of the group, and each substitution is independent of the other.
- unsubstituted in connection with a group such as a ring or ring system means the group does not have any substituents other than its one or more attachments to the remainder of Formula 1.
- optionally substituted means that the number of substituents can be zero. Unless otherwise indicated, optionally substituted groups may be substituted with as many optional substituents as can be accommodated by replacing a hydrogen atom with a non-hydrogen substituent on any available carbon or nitrogen atom. The number of optional substituents may be restricted by an expressed limitation. For example, the phrase “optionally substituted with up to 3 substituents selected from R 9a on carbon ring members" means that 0, 1, 2 or 3 substituents can be present (if the number of potential connection points allows).
- the phrase "optionally substituted with up to 5 substituents selected from R 3 on carbon ring members” means that 0, 1, 2, 3, 4 or 5 substituents can be present if the number of available connection points allows.
- a range specified for the number of substituents e.g., r being an integer from 0 to 4 or from 0 to 3 for 5- and 6-membered nitrogen-containing heterocycles in Exhibit A
- the number of positions available for substituents on a ring e.g., 2 positions available for (R a ) r on U-27 in Exhibit A
- the actual higher end of the range is recognized to be the number of available positions.
- said substituents are independently selected from the group of defined substituents, e.g., (R 3 ) p in Table 1 where p is 0, 1, 2, 3, 4 or 5.
- substituents which can be hydrogen, for example R 1 , R 4 , R 5 , R 6 , R 7 or R 13 , then when this substituent is taken as hydrogen, it is recognized that this is equivalent to said group being unsubstituted.
- variable group When a variable group is shown to be optionally attached to a position, for example (R a ) r in H-23 of Exhibit 1 , wherein r may be 0, then hydrogen may be at the position even if not recited in the variable group definition.
- hydrogen atoms When one or more positions on a group are said to be "not substituted” or “unsubstituted”, then hydrogen atoms are attached to take up any free valency.
- a "ring” as a component of Formula 1 is carbocyclic or heterocyclic.
- the term “ring system” as a component of Formula 1 denotes two fused rings (e.g., a phenyl ring fused to a pyridinyl ring to form quinolinyl).
- carbocyclic ring denotes a ring wherein the atoms forming the ring backbone are selected only from carbon. Unless otherwise indicated, a carbocyclic ring can be a saturated, partially unsaturated, or fully unsaturated ring. "Saturated carbocyclic” refers to a ring having a backbone consisting of carbon atoms linked to one another by single bonds; unless otherwise specified, the remaining carbon valences are occupied by hydrogen atoms.
- heterocyclic ring or “heterocycle” denote a ring or ring system in which at least one atom forming the ring backbone is not carbon, e.g., nitrogen, oxygen or sulfur. Typically a heterocyclic ring contains no more than 4 nitrogens, no more than 2 oxygens and no more than 2 sulfurs. Unless otherwise indicated, a heterocyclic ring can be a saturated, partially unsaturated, or fully unsaturated ring.
- saturated heterocyclic ring refers to a heterocyclic ring containing only single bonds between ring members.
- a partially unsaturated heterocyclic ring is intermediate between a saturated heterocyclic ring and a fully unsaturated heterocyclic ring (which may be aromatic). Therefore, as referred to in the present disclosure and claims, the term “partially unsaturated heterocyclic ring” denotes a heterocyclic ring comprising at least one ring member bonded to an adjacent ring member through a double bond and which conceptually potentially accommodates a number of non-cumulated double bonds between adjacent ring members (i.e. in its fully unsaturated counterpart form) greater than the number of double bonds present (i.e. in its partially unsaturated form).
- heterocyclic ring When a fully unsaturated heterocyclic ring satisfies H ⁇ ckel's rule, then said ring is also called a "heteroaromatic ring” or “aromatic heterocyclic ring".
- the terms “heteroaromatic ring system” and “heteroaromatic bicyclic ring system” denote a ring system in which at least one atom forming the ring backbone is not carbon, e.g., nitrogen, oxygen or sulfur, and at least one ring is aromatic. Unless otherwise indicated, heterocyclic rings and ring systems can be attached through any available carbon or nitrogen by replacement of a hydrogen on said carbon or nitrogen.
- Aromatic indicates that each of the ring atoms is essentially in the same plane and has a /?-orbital perpendicular to the ring plane, and that (4n + T) ⁇ electrons, where n is a positive integer, are associated with the ring to comply with H ⁇ ckel's rule.
- aromatic heterocyclic ring system denotes a heterocyclic ring system in which at least one ring of the ring system is aromatic.
- nonaromatic ring system denotes a carbocyclic or heterocyclic ring system that may be fully saturated, as well as partially or fully unsaturated, provided that none of the rings in the ring system are aromatic.
- four- to seven-membered nonaromatic heterocyclic ring refers to rings containing four to seven ring members and which do not satisfy H ⁇ ckel's rule. This term (as used where R 6 and R 7 are taken together) is not limited by carbon atoms only and can include ring members selected from O, S(O) n and NR 13 .
- Q 1 , Q 2 or R 1 comprises a phenyl or a 6-membered fully unsaturated heterocyclic ring
- the ortho, meta and para positions of each ring is relative to the connection of the ring to the remainder of Formula 1.
- Q 1 , Q 2 and R 1 can be (among others) phenyl optionally substituted with one or more substituents selected from a group of substituents as defined in the Summary of the Invention.
- An example of phenyl optionally substituted with one to five substituents is the ring illustrated as U-57 in Exhibit A, wherein R 8 is as defined in the Summary of the Invention for R 8 and q is an integer from 0 to 5.
- substituents on the ring or ring system of Q 1 or Q 2 are optional, 0 to 5 substituents may be present, limited only by the number of available points of attachment.
- the ring members selected from up to 2 O, up to 2 S and up to 4 N atoms are optional, provided at least one ring member is not carbon (e.g., N, O or S).
- the nitrogen atom ring members may be oxidized as //-oxides, because compounds relating to Formula 1 also include iV-oxide derivatives.
- R 1 can be (among others) 5- or 6-membered nitrogen-containing aromatic heterocycle, which may be optionally substituted with one or more substituents selected from a group of substituents as defined in the Summary of Invention.
- R 1 is phenyl or a 5- or 6- membered nitrogen-containing aromatic heterocycle, it may be attached to the remainder of Formula 1 through any available carbon or nitrogen ring atom, unless otherwise described.
- the ring or ring system of Q 1 or Q 2 may be attached to the remainder of Formula 1 through any available carbon or nitrogen ring atom, unless otherwise described.
- Examples of a 5- to 6-membered fully unsaturated heterocyclic ring include the rings H-I through H-39 illustrated in Exhibit 1, and examples of an 8- to 10-membered heteroaromatic bicyclic ring system include the ring systems B-I through B-39 illustrated in Exhibit 2.
- variable R a is any substituent as defined in the Summary of the Invention for Q 1 , Q 2 or R 1 (e.g., a Q 1 ring or ring system is optionally substituted with R 3 on carbon ring members and cyano, C j -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 ⁇ C 6 cycloalkyl, C 1 -C 6 alkoxy, C 2 -C 6 alkoxyalkyl, C 2 -C 6 alkylcarbonyl, C 2 -C 6 alkoxycarbonyl, C 2 -C 6 alkylaminoalkyl and C 3 ⁇ C 6 dialkylaminoalkyl on nitrogen atom ring members) and r is an integer from 0 to 5 for Q 1 and Q 2 or from 0 to 3 for R 1 , limited by the number of available positions on each depicted ring or ring system.
- R a is any substituent as defined in the Summary of the Invention for Q 2 (e.g., a Q 2 ring is optionally substituted with R 3 on carbon ring members and cyano, C ⁇ -Cg alkyl, C 2 - C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 1 -C 6 alkoxy, C 2 -C 6 alkoxyalkyl, C 2 -C 6 alkylcarbonyl, C 2 -C 6 alkoxycarbonyl, C 2 -C 6 alkylaminoalkyl and C 3 -C 6 dialkylaminoalkyl on nitrogen atom ring members) and r is an integer from 0 to 5, limited by the number of available positions on each depicted ring or ring
- Examples of a 5- or 6-membered nitrogen-containing heterocycle optionally substituted with from one or more substituents of particular note for Q 1 , Q 2 and R 1 include the rings U-I through U-56 illustrated in Exhibit A wherein R a is any substituent as defined in the Summary of the Invention for Q 1 Q 2 and R 1 , respectively (i.e.
- R 3 on carbon atom ring members, and the recited list of possible substituents on nitrogen atom ring members; and for R 1 , R 9a on carbon ring members and R 9 ⁇ on nitrogen ring members) and r is an integer ranging from 0 to 4 for Q 1 and Q 2 and from 0 to 3 for R 1 , limited by the number of available positions on each U group. Note that some U groups can only be substituted with less than 4 R a groups (e.g., U-4 through U-43 and U-47 through U-56).
- r is limited to the integers 0 or 1 , and r being 0 means that the U group is unsubstituted and a hydrogen is present at the position indicated by (R a ) r .
- R a groups are shown in the structures H-I through H-39, B-I through B-39, P-I through P-40, and U-I through U-57 in Exhibits 1 through 3 and Exhibit A, it is noted that they do not need to be present since they are optional substituents.
- the nitrogen atoms that require substitution to fill their valence are substituted with H or R a . Note that when the attachment point between (R a ) r and the H, B, P or U group in Exhibits 1 through 3 and Exhibit A is illustrated as floating, (R a ) r can be attached to any available carbon atom or nitrogen atom of the H, B, P or U group.
- R 6 and R 7 are taken together to form a four- to seven-membered nonaromatic heterocyclic ring
- examples of where R 6 and R 7 are taken together to form a four- to seven-membered nonaromatic heterocyclic ring include the rings G-I through G-28 as illustrated in Exhibit 4. Note that when R 6 and R 7 are taken together to form a ring comprising a ring selected from G-25 through G-28, G 2 is selected from O, S(O) n or NR 13 . Note that when G 2 is N, the nitrogen atom can complete its valence by substitution with either H or the substituents corresponding to R 13 as defined in the Summary of Invention.
- Compounds of this invention can exist as one or more stereoisomers.
- the various stereoisomers include enantiomers, diastereomers, atropisomers and geometric isomers.
- one stereoisomer may be more active and/or may exhibit beneficial effects when enriched relative to the other stereoisomer(s) or when separated from the other stereoisomer(s). Additionally, the skilled artisan knows how to separate, enrich, and/or to selectively prepare said stereoisomers.
- the compounds of the invention may be present as a mixture of stereoisomers, individual stereoisomers or as an optically active form.
- nitrogen-containing heterocycles can form JV-oxides since the nitrogen requires an available lone pair for oxidation to the oxide; one skilled in the art will recognize those nitrogen-containing heterocycles which can form TV-oxides.
- tertiary amines can form iV-oxides.
- Synthetic methods for the preparation of iV-oxides of heterocycles and tertiary amines are very well known by one skilled in the art including the oxidation of heterocycles and tertiary amines with peroxy acids such as peracetic and m-chloroperbenzoic acid (MCPBA), hydrogen peroxide, alkyl hydroperoxides such as t-butyl hydroperoxide, sodium perborate, and dioxiranes such as dimethyldioxirane.
- MCPBA peroxy acids
- alkyl hydroperoxides such as t-butyl hydroperoxide
- sodium perborate sodium perborate
- dioxiranes such as dimethyldioxirane
- salts of chemical compounds are in equilibrium with their corresponding nonsalt forms, salts share the biological utility of the nonsalt forms.
- the salts of the compounds of Formula 1 include acid-addition salts with inorganic or organic acids such as hydrobromic, hydrochloric, nitric, phosphoric, sulfuric, acetic, butyric, fumaric, lactic, maleic, malonic, oxalic, propionic, salicylic, tartaric, 4-toluenesulfonic or valeric acids.
- Non-crystalline forms include embodiments which are solids such as waxes and gums as well as embodiments which are liquids such as solutions and melts.
- Crystalline forms include embodiments which represent essentially a single crystal type and embodiments which represent a mixture of polymorphs (i.e. different crystalline types).
- polymorph refers to a particular crystalline form of a chemical compound that can crystallize in different crystalline forms, these forms having different arrangements and/or conformations of the molecules in the crystal lattice.
- polymorphs can have the same chemical composition, they can also differ in composition due the presence or absence of co-crystallized water or other molecules, which can be weakly or strongly bound in the lattice. Polymorphs can differ in such chemical, physical and biological properties as crystal shape, density, hardness, color, chemical stability, melting point, hygroscopicity, suspensibility, dissolution rate and biological availability.
- a polymorph of a compound represented by Formula 1 can exhibit beneficial effects (e.g., suitability for preparation of useful formulations, improved biological performance) relative to another polymorph or a mixture of polymorphs of the same compound represented by Formula 1.
- Preparation and isolation of a particular polymorph of a compound represented by Formula 1 can be achieved by methods known to those skilled in the art including, for example, crystallization using selected solvents and temperatures.
- Embodiments of the present invention as described in the Summary of the Invention include (where Formula 1 as used in the following Embodiments includes //-oxides and salts, geometric isomers, stereoisomers and atropisomers thereof ):
- Embodiment 1 A compound of Formula 1 wherein X is O, S(O) m , NR 4 , CR 15 R 16 or
- Embodiment 2 A compound of Formula 1 wherein X is O, S(0) m , NR 4 or CR 15 R 16 .
- Embodiment 4 A compound of Formula 1 wherein X is O, NR 4 or CR 15 R 16 .
- Embodiment 5. A compound of Formula 1 wherein X is O, S(0) m or NR 4 .
- Embodiment 6. A compound of Formula 1 wherein X is O or S(0) m .
- Embodiment 7. A compound of Formula 1 wherein X is O.
- Embodiment 8. A compound of Formula 1 wherein X is NR 4 .
- Embodiment 9 A compound of Formula 1 wherein X is O or NR 4 .
- Embodiment 11. A compound of Formula 1 or any one of Embodiments 1 through 10 wherein when Q 1 is a six-membered ring (e.g., phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl) and an R 3 substituent is located at a meta position
- R 3 substituent is selected from F, Cl, Br and cyano (-CN).
- Embodiment 11a A compound of Formula 1 or any one of Embodiments 1 through 11 wherein when Q 1 is a six-membered ring and an R 3 substituent is located at a meta position (relative to the connection of the Q 1 ring to the remainder of
- R 3 substituent is F.
- R 3 substituent is F.
- Q 1 is a six-membered ring (e.g., phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl) substituted with only one R 3 substituent, then said R 3 substituent is attached at an ortho position (relative to the connection of the Q 1 ring to the remainder of Formula 1).
- Embodiment 14 A compound of Embodiment 13 wherein Q 1 is phenyl, thienyl, pyridinyl, pyridazinyl, pyrazinyl or pyrimidinyl, each optionally substituted with up to 5 substituents independently selected from R 3 .
- Embodiment 15 A compound of Embodiment 14 wherein Q 1 is phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, each substituted with from 1 to 4 substituents independently selected from R 3 .
- Embodiment 16 A compound of Embodiment 15 wherein Q 1 is phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, each substituted with 1 , 2 or 3 substituents independently selected from R 3 .
- a compound of Embodiment 16 wherein the substituents are located at the ortho and/or para positions (relative to the connection of the Q 1 ring to the remainder of Formula 1) of the phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl of Q * .
- Embodiment 18 A compound of Embodiment 16 or 17 wherein Q 1 is phenyl or pyridinyl, each substituted with 1 , 2 or 3 substituents independently selected from R 3 .
- Embodiment 19 A compound of Embodiment 18 wherein Q 1 is phenyl or pyridinyl, each substituted with 2 or 3 substituents independently selected from R 3 .
- Embodiment 20 A compound of Embodiment 18 wherein Q 1 is phenyl or pyridinyl, each substituted with 2 or 3 substituents independently selected from R 3 .
- Embodiment 21 wherein Q 1 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 .
- Embodiment 23 A compound of Embodiment 21 wherein Q 1 is phenyl substituted at the 2- and 4-positions with substituents independently selected from R 3 .
- Embodiment 24 A compound of Embodiment 21 wherein Q 1 is phenyl substituted at the 2- and 6-positions with substituents independently selected from R 3 .
- Embodiment 25 A compound of Embodiment 18 wherein Q 1 is pyridinyl substituted with 1, 2 or 3 substituents independently selected from R 3 .
- Embodiment 26 A compound of Embodiment 25 wherein Q 1 is pyridinyl substituted with 1 or 2 substituents independently selected from R 3 .
- Embodiment 27 A compound of Embodiment 26 wherein Q 1 is pyridinyl substituted with 1 substituent independently selected from R 3 .
- Embodiment 28 A compound of Formula 1 or any one of Embodiments 1 through 27 wherein when Q 2 is a six-membered ring (e.g., phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl) and an R 3 substituent is located at a meta position
- Q 2 is a six-membered ring (e.g., phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl) and an R 3 substituent is located at a meta position
- R 3 substituent is selected from F, Cl, Br and cyano (-CN).
- Embodiment 29 A compound of Formula 1 or any one of Embodiments 1 through 28 wherein when Q 2 is a six-membered ring and an R 3 substituent is located at a meta position (relative to the connection of the Q 2 ring to the remainder of
- Embodiment 30 A compound of Formula 1 or any one of Embodiments 1 through 29 wherein when Q 2 is a six-membered ring (e.g., phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl) substituted with only one R 3 substituent, then said R 3 substituent is attached at an ortho position (relative to the connection of the Q 2 ring to the remainder of Formula 1).
- Q 2 is a six-membered ring (e.g., phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl) substituted with only one R 3 substituent, then said R 3 substituent is attached at an ortho position (relative to the connection of the Q 2 ring to the remainder of Formula 1).
- Embodiment 31 A compound of Formula 1 or any one of Embodiments 1 through 30 wherein Q 2 is phenyl, thienyl, pyridinyl, pyridazinyl, pyrazinyl, pyrimidinyl, naphthalenyl, quinolinyl, isoquinolinyl or quinoxalinyl, each optionally substituted with up to 5 substituents independently selected from R 3 .
- Embodiment 32 is phenyl, thienyl, pyridinyl, pyridazinyl, pyrazinyl, pyrimidinyl, naphthalenyl, quinolinyl, isoquinolinyl or quinoxalinyl, each optionally substituted with up to 5 substituents independently selected from R 3 .
- Embodiment 32 Embodiment 32.
- Embodiment 33 A compound of Embodiment 32 wherein Q 2 is phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, each substituted with from 1 to 4 substituents independently selected from R 3 .
- Embodiment 34 A compound of any one of Embodiments 31 through 33 wherein Q 2 is phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, each substituted with 1 ,
- Embodiment 35 A compound of Embodiment 34 wherein the substituents are located at the ortho and/or para positions (relative to the connection of the Q 2 ring to the remainder of Formula 1) of the phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl of Q 2 .
- Embodiment 36 A compound of any one of Embodiments 34 or 35 wherein Q 2 is phenyl or pyridinyl, each substituted with 1, 2 or 3 substituents independently selected from R 3 .
- Embodiment 37 A compound of Embodiment 36 wherein Q 2 is phenyl substituted with
- Embodiment 38 A compound of Embodiment 37 wherein Q 2 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 ; or phenyl substituted at the 2- and 4-positions with substituents independently selected from R 3 ; or phenyl substituted at the 2- and 6-positions with substituents independently selected from R 3 .
- Embodiment 39 A compound of Embodiment 38 wherein Q 2 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 .
- Embodiment 40 A compound of Embodiment 40 wherein Q 2 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 .
- Embodiment 42. A compound of Embodiment 36 wherein Q 2 is pyridinyl substituted with 1, 2 or 3 substituents independently selected from R 3 .
- Embodiment 43 A compound of Embodiment 42 wherein Q 2 is pyridinyl substituted with 1 or 2 subsituents independently selected from R 3 .
- Embodiment 44 A compound of Embodiment 43 wherein Q 2 is pyridinyl substituted with 1 substituent selected from R 3 .
- Embodiment 45 A compound of Formula 1 or any one of Embodiments 1 through 44 wherein at least one of Q 1 and Q 2 is phenyl optionally substituted with R 3 (e.g., optionally substituted with up to 5 substituents independently selected from R 3 ).
- Embodiment 46 Embodiment 46.
- Embodiment 45 wherein at least one of Q 1 and Q 2 is phenyl substituted with 2, 3 or 4 substituents independently selected from R 3 .
- Embodiment 47 A compound of Embodiment 46 wherein at least one Q 1 and Q 2 is phenyl substituted with 2 or 3 substituents independently selected from R 3 .
- Embodiment 48 A compound of Embodiment 47 wherein each of Q 1 and Q 2 is phenyl substituted with 2 or 3 substituents independently selected from R 3 .
- Embodiment 49 Embodiment 49.
- R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, CO 2 R 5 , C(O)NR 6 R 7 , cyano, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy or C 2 -C 5 alkoxyalkyl; or
- R 1 is a five- or six-membered nitrogen-containing aromatic heterocycle optionally substituted with up to 3 substituents independently selected from R 9a on carbon atom ring members and R 9 ⁇ on nitrogen atom ring members.
- Embodiment 50 A compound of Embodiment 49 wherein R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, cyano, C 1 -C 6 alkoxy or C 1 -C 6 haloalkoxy; or
- R 1 is pyridinyl, pyrimidinyl, pyrazolyl or oxazolyl, each optionally substituted with up to 3 substituents independently selected from R 9a on carbon atom ring members and R 9 ⁇ on nitrogen atom ring members.
- Embodiment 51 A compound of Embodiment 50 wherein R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, CO 2 R 5 , C(O)NR 6 R 7 , cyano, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy or C 2 -C 5 alkoxyalkyl.
- Embodiment 52 Embodiment 52.
- Embodiment 53 A compound of Embodiment 52 wherein R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, cyano, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy or C 2 -C 5 alkoxyalkyl.
- Embodiment 54 A compound of Embodiment 53 wherein R 1 is H, halogen or C 1 -C 6 alkyl.
- Embodiment 55 A compound of Embodiment 54 wherein R 1 is H or CH 3 .
- Embodiment 56 A compound of Embodiment 55 wherein R 1 is H.
- Embodiment 57 A compound of Formula 1 or any one of Embodiments 1 through 56 wherein R 1 is other than an optionally substituted phenyl or an optionally substituted five- or six-membered nitrogen-containing aromatic heterocycle.
- R la is H.
- Embodiment 60 A compound of Formula 1 or any one of Embodiments 1 through 59 wherein R 2 is CH 3 , CH 2 CH 3 , halogen, cyano, cyanomethyl, monohalomethyl, hydroxymethyl, methoxy or methylthio; or cyclopropyl optionally substituted with up to 2 substituents independently selected from halogen and methyl.
- Embodiment 61 A compound of Embodiment 60 wherein R 2 is CH 3 , CH 2 CH 3 , Cl, Br or I.
- Embodiment 62. A compound of Embodiment 61 wherein R 2 is CH 3 , CH 2 CH 3 , Cl or
- Embodiment 63 A compound of Embodiment 62 wherein R 2 is CH 3 , Cl or Br.
- Embodiment 64 A compound of Embodiment 63 wherein R 2 is CH 3 or Cl.
- Embodiment 65 A compound of Embodiment 64 wherein R 2 is CH 3 .
- Embodiment 66 A compound of Embodiment 62 wherein R 2 is Cl or Br.
- Embodiment 67 A compound of Embodiment 66 wherein R 2 is Cl.
- Embodiment 68 A compound of Formula 1 or any one of Embodiments 1 through 67 wherein R 5 is H or C 1 -C 6 alkyl.
- Embodiment 69 A compound of Embodiment 68 wherein R 5 is H, CH 3 or CH 2 CH 3 .
- Embodiment 70 A compound of Embodiment 68 wherein R 5 is C 1 -C 6 alkyl.
- Embodiment 71 A compound of Embodiment 69 or 70 wherein R 5 is CH 3 or CH 2 CH 3 .
- Embodiment 72 A compound of Formula 1 or any one of Embodiments 1 through 71 wherein when R 6 is separate (i.e. not taken together with R 7 to form a ring), then
- R 6 is H or C 1 -C 6 alkyl.
- Embodiment 73 A compound of Embodiment 72 wherein R 6 is H.
- Embodiment 74. A compound of Formula 1 or any one of Embodiments 1 through 73 wherein when R 7 is separate (i.e. not taken together with R 6 to form a ring), then
- R 7 is H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl or C 4 -C 8 alkylcycloalkyl.
- Embodiment 75 A compound of Embodiment 74 wherein R 7 is H or C 1 -C 6 alkyl.
- Embodiment 76 A compound of Embodiment 75 wherein R 7 is H.
- Embodiment 77 A compound of Embodiment 75 wherein R 7 is H.
- Embodiment 78 A compound of Embodiment 77 wherein when R 6 and R 7 are taken together with the nitrogen atom to which they are connected to form a nonaromatic heterocyclic ring, the ring is six-membered and contains one ring member selected from O and NR 13 in addition to the connecting nitrogen atom and ring members selected from carbon atoms.
- Embodiment 79 A compound of Embodiment 77 wherein R 6 and R 7 are taken together with the nitrogen atom to which they are connected to form a piperidine ring.
- Embodiment 80 A compound of Embodiment 78 wherein R 6 and R 7 are taken together with the nitrogen atom to which they are connected to form a piperazine or morpholine ring.
- Embodiment 81. A compound of Formula 1 or any one of Embodiments 1 through 80 wherein each R 8 is independently selected from halogen, C 1 -C 2 alkyl, C 1 -C 2 haloalkyl, C 1 -C 2 alkoxy, C 1 -C 2 haloalkoxy, cyano and nitro.
- Embodiment 82 A compound of Embodiment 81 wherein each R 8 is independently selected from halogen, C 1 -C 2 alkyl, C 1 -C 2 alkoxy, cyano and nitro.
- Embodiment 83 A compound of Embodiment 82 wherein each R 8 is independently Cl or F.
- Embodiment 84. A compound of Formula 1 or any one of Embodiments 1 through 83 wherein each R 9a is independently selected from halogen, C 1 -C 2 alkyl, C 1 -C 2 haloalkyl, C 1 -C 2 alkoxy, C 1 -C 2 haloalkoxy, cyano and nitro.
- Embodiment 85 A compound of Embodiment 84 wherein each R 9a is independently selected from halogen, C 1 -C 2 alkyl, C 1 -C 2 alkoxy, cyano and nitro.
- Embodiment 86. A compound of Embodiment 85 wherein each R 9a is independently selected from Cl, F, CH 3 , -OCH 3 and cyano.
- Embodiment 87. A compound of Embodiment 86 wherein each R 9a is independently Cl or F.
- Embodiment 88. A compound of Formula 1 or any one of Embodiments 1 through 87 wherein each R 9 ⁇ is independently C 1 -C 2 alkyl.
- Embodiment 89 A compound of Formula 1 or any one of Embodiments 1 through 88 wherein each R 3 is independently selected from halogen, cyano, nitro, amino, methylamino, dimethylamino, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy,
- Embodiment 90 A compound of Embodiment 89 wherein each R 3 is independently selected from halogen, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy and -U-V-T.
- Embodiment 91 A compound of Embodiment 89 wherein each R 3 is independently selected from halogen, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy and -U-V-T.
- each R 3 is independently selected from F, Cl, Br, cyano, nitro, CH 3 , CF 3 , -OCH 3 , -OCHF 2 and -U-V-T.
- Embodiment 92 A compound of Formula 1 or any one of Embodiments 1 through 91 wherein at least one R 3 substituent on the ring or ring system of Q 1 or Q 2 is
- Embodiment 93 A compound of Formula 1 or any one of Embodiments 1 through 91 wherein each R 3 is other than -U-V-T.
- Embodiment 94 A compound of Embodiment 89 wherein each R 3 is independently selected from halogen, cyano, nitro, amino, methylamino, dimethylamino, C 1 -
- Embodiment 95 Embodiment 95.
- each R 3 is independently selected from halogen, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy and C 1 -C 3 haloalkoxy.
- Embodiment 96 A compound of Embodiment 95 wherein each R 3 is independently selected from halogen, cyano, C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkoxy and
- Embodiment 97 A compound of Embodiment 96 wherein each R 3 is independently selected from F, Cl, Br, cyano, C 1 -C 2 alkyl, C 1 -C 2 haloalkyl, C 1 -C 2 alkoxy and
- Embodiment 98 A compound of Embodiment 97 wherein each R 3 is independently selected from F, Cl, Br, cyano, methyl, C 1 -C 2 alkoxy and fluoromethoxy.
- Embodiment 99 A compound of Embodiment 98 wherein each R 3 is independently selected from F, Cl, cyano, methyl, C 1 -C 2 alkoxy and fluoromethoxy.
- Embodiment 100 A compound of Embodiment 95 wherein each R 3 is independently selected from F, Cl, Br, cyano, nitro, CH 3 , CF 3 , -OCH 3 and -OCHF 2 .
- Embodiment 101 A compound of any one of Embodiments 89 through 98 or 100 wherein each R 3 is independently selected from F, Cl, Br, cyano and methoxy.
- Embodiment 102 A compound of Embodiment 101 wherein each R 3 is independently selected from F, Cl, Br and cyano.
- Embodiment 103 A compound of Embodiment 101 wherein each R 3 is independently selected from F, Cl, cyano and -OCH 3 .
- Embodiment 104 A compound of Formula 1 or any one of Embodiments 1 through 92 wherein each U is independently O or NR 22 .
- Embodiment 105 A compound of Embodiment 104 wherein each U is independently O or NH.
- Embodiment 106. A compound of Formula 1 or any one of Embodiments 1 through 92 and 104 through 105 wherein each V is C 2 -C 4 alkylene.
- Embodiment 107. A compound of Formula 1 or any one of Embodiments 1 through 92 and 104 through 106 wherein each T is independently NR 23a R 23b or OR 24 .
- Embodiment 108 A compound of Formula 1 or any one of Embodiments 1 through 92 and 104 through 107 wherein each R 23a and R 23b is independently H, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl.
- Embodiment 109. A compound of Formula 1 or any one of Embodiments 1 through 92 and 104 through 108 wherein each R 24 is independently H, C 1 -C 6 alkyl or C 1 -
- Embodiment 110 A compound of Formula 1 or any one of Embodiments 1 through 109 wherein when an R 3 substituent attached to phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl Of Q 1 Or Q 2 is other than F, Cl, Br, cyano, methyl, C 1 -
- R 3 substituent is attached at the para position (of the phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl ring).
- Embodiment 111 A compound of Formula 1 or any one of Embodiments 1 through 110 wherein R 4 is H, formyl, C 3 -C 7 cycloalkyl or -SR 10 ; or C 1 -C 6 alkyl or C 1 -C 6 haloalkyl, each optionally substituted with up to 2 R 12 .
- Embodiment 112 A compound of Embodiment 111 wherein R 4 is H, formyl, C 3 -C 7 cycloalkyl or -SR 10 ; or C 1 -C 6 alkyl substituted with one R 12 .
- Embodiment 113 A compound of Embodiment 112 wherein R 4 is H, formyl,
- Embodiment 114 A compound of Embodiment 113 wherein R 4 is H, formyl, cyclopropyl or -CH 2 CN.
- Embodiment 115 A compound of Embodiment 113 wherein R 4 is H, formyl,
- Embodiment 116 A compound of Embodiment 115 wherein R 4 is H, formyl or cyclopropyl.
- Embodiment 117 A compound of Embodiment 114 or 116 wherein R 4 is H.
- Embodiment 118 A compound of Formula 1 or any one of Embodiments 1 through 117 wherein R 13 is H or CH 3 .
- Embodiment 119 A compound of Embodiment 118 wherein R 13 is CH 3 .
- Embodiment 120 A compound of Formula 1 or any one of Embodiments 1 through 119 wherein each R 12 is independently C 3 -C 7 cycloalkyl, Q-C 4 alkoxy or cyano.
- Embodiment 121 A compound of Embodiment 120 wherein each R 12 is independently cyclopropyl, -OCH 3 or cyano.
- Embodiment 122 A compound of Embodiment 120 wherein each R 12 is independently
- Embodiment 123 A compound of Embodiment 122 wherein each R 12 is independently cyclopropyl or -OCH 3 .
- Embodiment 124 A compound of Formula 1 or any one of Embodiments 1 through 123 wherein R 10 is CH 3 , CH 2 CH 3 , CF 3 or CF 2 CF 3 .
- Embodiment 125 A compound of Embodiment 124 wherein R 10 is CH 3 .
- Embodiment 126. A compound of Formula 1 or any one of Embodiments 1 through 125 wherein R 11 is C 1 -C 6 alkyl, C 1 -C 6 alkoxy or C 1 -C 6 alkylthio.
- Embodiment 127 A compound of Embodiment 126 wherein R 11 is CH 3 , CH 2 CH 3 ,
- Embodiment 128 A compound of Embodiment 127 wherein R 11 is CH 3 , -OCH 3 or
- Embodiment 129 A compound of Formula 1 or any one of Embodiments 1 through 128 wherein R 15 is H or CH 3 .
- Embodiment 130 A compound of Embodiment 129 wherein R 15 is H.
- Embodiment 131 A compound of Formula 1 or any one of Embodiments 1 through 130 wherein R 16 is CH 3 or OR 18 .
- Embodiment 132 A compound of Embodiment 131 wherein R 16 is OR 18 .
- Embodiment 133 A compound of Formula 1 or any one of Embodiments 1 through 132 wherein R 18 is H.
- Embodiment 134 A compound of Formula 1 or any one of Embodiments 1 through 133 wherein W is O.
- Embodiment 135. A compound of Formula 1 or any one of Embodiments 1 through 134 wherein M + is a cation selected from sodium, potassium and lithium ions.
- Embodiment 136. A compound of Embodiment 135 wherein M + is a cation selected from sodium and potassium ions.
- Embodiment 137 A compound of Embodiment 136 wherein M + is a sodium ion.
- Embodiment 138. A compound of Formula 1 or any one of Embodiments 1 through 137 wherein m is 0.
- Embodiment 139. A compound of Formula 1 or any one of Embodiments 1 through 138 wherein n is 0.
- Embodiments of this invention can be combined in any manner, and the descriptions of variables in the embodiments pertain not only to the compounds of Formula 1 but also to the starting compounds and intermediate compounds (e.g. compounds of Formula 2) useful for preparing the compounds of Formula 1.
- embodiments of this invention including Embodiments 1-139 above as well as any other embodiments described herein, and any combination thereof, pertain to the compositions and methods of the present invention. Combinations of Embodiments 1-139 are illustrated by:
- Embodiment A A compound of Formula 1 wherein
- Q 1 is phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, each substituted with from 1 to 4 substituents independently selected from R 3 ; provided that when an R 3 substituent is located at a meta position, then said R 3 substituent is selected from F, Cl, Br and cyano;
- Q 2 is phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, each substituted with 1 , 2 or 3 substituents independently selected from R 3 , provided that when an R 3 substituent is located at a meta position, then said R 3 substituent is selected from F, Cl, Br and cyano;
- X is O, NR 4 , C(O) or CR 15 R 16 ;
- R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, CO 2 R 5 , C(O)NR 6 R 7 , cyano, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy or C 2 -C 5 alkoxyalkyl;
- R la is H
- R 6 is H or C 1 -C 6 alkyl
- R 7 is H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl or C 4 -C 8 alkylcycloalkyl; or
- R 6 and R 7 are taken together with the nitrogen atom to which they are connected to form a four- to seven-membered nonaromatic heterocyclic ring containing ring members, in addition to the connecting nitrogen atom, selected from carbon atoms and up to one ring member selected from O and NR 13 ; each R 12 is independently C 3 -C 7 cycloalkyl, C 1 -C 4 alkoxy or cyano; R 13 is H or CH 3 ; R 15 is H or CH 3 ; and R 16 is OR 18 .
- Embodiment B A compound of Embodiment A wherein
- Q 1 is phenyl or pyridinyl, each substituted with 1, 2 or 3 substituents independently selected from R 3 ;
- Q 2 is phenyl or pyridinyl, each substituted with 1 , 2 or 3 substituents independently selected from R 3 ;
- R 1 is H, halogen or C 1 -C 6 alkyl
- R 2 is CH 3 , Cl or Br; each R 3 is independently selected from halogen, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy and -U-V-T; R 4 is H, formyl, C 3 -C 7 cycloalkyl or -SR 10 ; or C 1 -C 6 alkyl substituted with one R 12 ; each R 12 is independently cyclopropyl, -OCH 3 or cyano; R 15 is H; each U is independently O or NH; each V is C 2 -C 4 alkylene; each T is independently NR 23a R 23b or OR 24 ; each R 23a and R 23b is independently H, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl; and each R 24 is independently H, C 1 -C 6 alkyl or C 1 -C 6
- Embodiment C A compound of Embodiment B wherein at least one of Q 1 and Q 2 is phenyl substituted with 2 or 3 substituents independently selected from R 3 ;
- R 1 is H or CH 3 ;
- R 2 is CH 3 ;
- R 4 is H;
- each R 3 is independently selected from halogen, cyano, C 1 -C 3 alkyl, C 1 -C 3 haloalkyl,
- Embodiment D A compound of Embodiment C wherein Q 1 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 ; or phenyl substituted at the 2- and 4-positions with substituents independently selected from R 3 ; or phenyl substituted at the 2- and 6-positions with substituents independently selected from R 3 ;
- Q 2 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 ; or phenyl substituted at the 2- and 4-positions with substituents independently selected from R 3 ; or phenyl substituted at the 2- and 6-positions with substituents independently selected from R 3 ;
- X is O, NR 4 or CR 15 R 16 ;
- R 1 is H; each R 3 is independently selected from F, Cl, Br, cyano, C 1 -C 2 alkyl, C 1 -C 2 haloalkyl,
- Embodiment E A compound of Embodiment D wherein each R 3 is independently selected from F, Cl, Br, cyano, methyl, C 1 -C 2 alkoxy and fluoromethoxy.
- Embodiment F A compound of Embodiment E wherein X is O or NH; and each R 3 is independently selected from F, Cl, Br, cyano and methoxy.
- Specific embodiments include compounds of Formula 1 selected from the group consisting of:
- This invention provides a fungicidal composition comprising a compound of Formula 1 (including all geometric and stereoisomers, iV-oxides, and salts thereof), and at least one other fungicide.
- a fungicidal composition comprising a compound corresponding to any of the compound embodiments described above.
- This invention provides a fungicidal composition comprising a fungicidally effective amount of a compound of Formula 1 (including all geometric and stereoisomers, iV-oxides, and salts thereof), and at least one additional component selected from the group consisting of surfactants, solid diluents and liquid diluents.
- compositions comprising a compound corresponding to any of the compound embodiments described above.
- This invention provides a method for controlling plant diseases caused by fungal plant pathogens comprising applying to the plant or portion thereof, or to the plant seed, a fungicidally effective amount of a compound of Formula 1 (including all geometric and stereoisomers, iV-oxides, and salts thereof).
- a compound of Formula 1 including all geometric and stereoisomers, iV-oxides, and salts thereof.
- methods comprising applying a fungicidally effective amount of a compound corresponding to any of the compound embodiments describe above.
- the compounds are applied as compositions of this invention.
- compounds of Formula 1 that are compounds of Formula IP (including all geometric and stereoisomers), iV-oxides, and salts thereof, and also agricultural compositions containing them and their use as fungicides:
- Q 1 and Q 2 are independently phenyl, thienyl, pyridinyl, pyridazinyl, pyrazinyl or pyrimidinyl, each optionally substituted with up to 5 substituents independently selected from R 3 ;
- X is O, S(O) m or NR 4 ;
- R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 2 -C 4 alkenyl, C 2 -C 4 alkynyl, C 3 - C 7 cycloalkyl, CO 2 R 5 , C(O)NR 6 R 7 , cyano, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy or
- R 1 is phenyl optionally substituted with up to 3 R 8 ; or a five- or six-membered nitrogen-containing aromatic heterocycle optionally substituted with up to 3 substituents independently selected from R 9a on carbon atom ring members and R 9 ⁇ on nitrogen atom ring members;
- R 2 is CH 3 , CH 2 CH 3 , cyclopropyl or halogen; each R 3 is independently selected from halogen, cyano, nitro, amino, methylamino, dimethylamino, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 1 -C 3 alkylthio, C 1 -C 3 haloalkylthio, C 1 -C 3 alkylsulfmyl, C 1 -C 3 haloalkylsulfinyl, C 1 -C 3 alkylsulfonyl, C 1 -C 3 haloalkylsulfonyl, C 3 -C 4 cycloalkyl, C 3 -C 7 cycloalkoxy, C 4 -C 6 alkylcycloalkyl, C 4 -C 6 cycloalkylalkyl
- R 5 is H, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl;
- R 6 and R 7 are independently selected from H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 7 cycloalkyl, C 4 -C 8 cycloalkylalkyl and C 4 -C 8 alkylcycloalkyl; or
- R 6 and R 7 are taken together with the nitrogen atom to which they are connected to form a four- to seven-membered nonaromatic heterocyclic ring containing ring members, in addition to the connecting ring nitrogen atom, selected from carbon atoms and optionally up to one ring member selected from O, S(O) n and NR 13 ; each R 8 , R 9a and R 915 is independently selected from halogen, C 1 -C 2 alkyl, C 1 -C 2 haloalkyl, C 1 -C 2 alkoxy, C 1 -C 2 haloalkoxy, cyano, nitro, SCH 3 , S(O)CH 3 and S(O) 2 CH 3 ;
- R 10 is C 1 -C 6 alkyl or C 1 -C 6 haloalkyl
- R 11 is C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 2 -C 7 alkoxyalkyl, C 2 -C 7 alkylaminoalkyl, C 2 -C 8 dialkylaminoalkyl, C 1 -C 6 alkylthio or C 2 -C 7 alkylthioalkyl; each R 12 is independently C 3 -C 7 cycloalkyl, C 1 -C 4 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulf ⁇ nyl or C 1 -C 4 alkylsulfonyl; R 13 is H, C 1 -C 3 alkyl or C 2 -C 3 haloalkyl; W is O or S; M + is a cation; m is 0, 1 or 2; and n is 0, 1 or 2.
- Q 1 is phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, each substituted with from 1 to 4 substituents independently selected from R 3 ;
- Q 2 is phenyl, pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, each substituted with 1 , 2 or 3 substituents independently selected from R 3
- X is O or NR 4 ;
- R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, CO 2 R 5 , C(O)NR 6 R 7 , cyano, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy or C 2 -C 5 alkoxyalkyl; or R 1 is a five- or six-membered nitrogen-containing aromatic heterocycle optionally substituted with up to 3 substituents independently selected from R 9a on carbon atom ring members and R 9 ⁇ on nitrogen atom ring members; R 2 is CH 3 , CH 2 CH 3 , Cl or Br; each R 3 is independently selected from halogen, cyano, nitro, amino, methylamino, dimethylamino, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy C 1 -C 3 alkylthio, C 1
- R 7 is H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl or C 4 -C 8 alkylcycloalkyl; or R 6 and R 7 are taken together with the nitrogen atom to which they are connected to form a four- to seven-membered nonaromatic heterocyclic ring containing ring members, in addition to the connecting nitrogen atom, selected from carbon atoms and up to one ring member selected from O and NR 13 ; each R 8 is independently selected from halogen, C 1 -C 2 alkyl, C 1 -C 2 haloalkyl, C 1 -C 2 alkoxy, C 1 -C 2 haloalkoxy, cyano and nitro; each R 9a is independently selected from halogen, C 1 -C 2 alkyl, C 1 -C 2 haloalkyl, C 1 -C 2 alkoxy, C 1 -C 2 haloalkoxy, cyano and nitro; each R9b
- R 13 is H or CH 3 .
- Embodiment BP A compound of Embodiment AP wherein
- Q 1 is phenyl or pyridinyl, each substituted with 1, 2 or 3 substituents independently selected from R 3 ;
- Q 2 is phenyl or pyridinyl, each substituted with 1, 2 or 3 substituents independently selected from R 3 ;
- X is NR 4 ;
- R 1 is H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, cyano, C 1 -C 6 alkoxy or C 1 -C 6 haloalkoxy;
- R 2 is CH 3 ; each R 3 is independently selected from halogen, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 3 alkoxy and C 1 -C 3 haloalkoxy;
- R 4 is H, formyl, C 3 -C 7 cycloalkyl or -SR 10 ; or C 1 -C 6 alkyl substituted
- Q 1 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 ; or
- Q 1 is phenyl substituted at the 2- and 4-positions with substituents independently selected from R 3 ;
- Q 2 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 ;
- X is O;
- R 2 is CH 3 ; each R 3 is independently selected from F, Cl, Br, cyano, nitro, CH 3 , CF 3 , -OCH 3 , and
- R 4 is H, formyl or cyclopropyl.
- Embodiment DP A compound of Embodiment CP wherein
- Q 1 is phenyl substituted at the 2- and 4-positions with substituents independently selected from R 3 ;
- Q 2 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 ; each R 3 is independently selected from F, Cl, cyano and -OCH 3 ;
- R 4 is H.
- Embodiment EP A compound of Embodiment BP wherein
- Q 1 is phenyl substituted at the 2- and 4-positions with substituents independently selected from R 3 ;
- Q 2 is phenyl substituted at the 2-, 4- and 6-positions with substituents independently selected from R 3 ; each R 3 is independently selected from F, Cl, CN and -OCH 3 ; and
- R 4 is H.
- fungicidal composition comprising a fungicidally effective amount of a compound of Formula IP (including all geometric and stereoisomers, iV-oxides, and salts thereof) or any one of counterpart embodiments that are embodiment counterparts to
- Embodiments 1 through 139 and Embodiments A through F e.g., Embodiment AP, BP, CP,
- DP or EP DP or EP
- additional component selected from the group consisting of surfactants, solid diluents and liquid diluents.
- a method for controlling plant diseases caused by fungal plant pathogens comprising applying to the plant or portion thereof, or to the plant seed, a fungicidally effective amount of a compound of Formula IP
- sulfoxides and sulfones of Formula Ib can be made via oxidation of the linking sulfur atom on sulfides of Formula Ia (i.e. Formula 1 wherein X is S(O) m and m is 0).
- a compound of Formula Ib wherein m is 1 (i.e. sulfoxides) or m is 2 (i.e. sulfones) is prepared by oxidizing a corresponding sulfide of Formula Ia with a suitable oxidizing agent.
- an oxidizing agent in an amount from 1 to 4 equivalents depending on the oxidation state of the product desired is added to a solution of the compound of Formula Ia in a solvent.
- Useful oxidizing agents include Oxone® (potassium peroxymonosulfate), hydrogen peroxide, sodium periodate, peracetic acid and 3-chloroperbenzoic acid.
- the solvent is selected with regard to the oxidizing agent employed.
- Aqueous ethanol or aqueous acetone is preferably used with potassium peroxymonosulfate, and dichloromethane is generally preferable with 3-chloroperbenzoic acid.
- Useful reaction temperatures typically range from -78 to 90 0 C. Particular procedures useful for oxidizing sulfides to sulfoxides and sulfones are described by Brand et al., J. Agric. Food Chem. 1984, 32, 221-226 and references cited therein.
- compounds of Formula 1 in which X is NH and R la is H can be prepared by the reaction of lH-pyrazole compounds of Formula 2 with various alkylating agents (e.g., Formula 3), such as iodoalkanes, alkylsulfonates (e.g., mesylate (OMs) or tosylate (OTs)) or trialkyl phosphates, preferably in the presence of an organic or inorganic base such as l,8-diazabicyclo[5.4.0]undec-7-ene, potassium carbonate or potassium hydroxide, and in a solvent such as N, ⁇ /-dimethylformamide, tetrahydrofuran, toluene or water.
- alkylating agents e.g., Formula 3
- alkylating agents e.g., Formula 3
- alkylating agents e.g., Formula 3
- alkylsulfonates e.g., mesylate (OMs)
- Compounds of Formula 1 wherein CHR 1 R ⁇ forms an optionally substituted cyclopropyl ring can likewise be prepared by reaction of a pyrazole of Formula 2 with an organometallic reagent, such as tricyclopropylbismuth, in the presence of a catalyst, such as copper acetate, under conditions known in the art. See, for example, J. Am. Chem. Soc. 2007, 129(1), 44-45. Of note as starting materials in the method of Scheme 2 are compounds of Formula 2 specifically disclosed in Tables 588 through 671 below.
- compounds of Formula 1 can be prepared by the reaction of compounds of Formula 4 (i.e. 5-aminopyrazoles for X being NR 4 , 5-hydroxypyrazoles (5 -pyrazolones) for X being O, or 5-mercaptopyrazoles for X being S) with aromatic compounds of Formula 5 containing a leaving group G (i.e. halogen or (halo)alkylsulfonate), optionally in the presence of a metal catalyst, and generally in the presence of a base and a polar aprotic solvent such as ⁇ /, ⁇ /-dimethylformamide or dimethyl sulfoxide.
- a leaving group G i.e. halogen or (halo)alkylsulfonate
- compounds of Formula 5 in which Q 2 is an electron-deficient heteroaromatic ring, or a benzene ring with electron-withdrawing substituents react by direct displacement of the leaving group G from the ring to provide compounds of Formula 1.
- G is typically Cl, Br, I or a sulfonate (e.g., OS(O) 2 CH 3 ).
- Compounds of Formula 5 are commercially available or their preparation is known in the art. Of note are embodiments of the method of Scheme 3 wherein a compound of Formula 4 is used to prepare a corresponding compound of Formula 1 specifically disclosed in Tables 85 through 252 below.
- a metal catalyst e.g., metal or metal salt
- G is Br or I or a sulfonate such as OS(O) 2 CF 3 or OS(O) 2 (CF 2 ) 3 CF 3 .
- copper salt complexes e.g., CuI with JV. ⁇ /'-dimethyl- ethylenediamine, proline or bipyridyl
- palladium complexes e.g., tris(dibenzylidene- acetone)dipalladium(O)
- palladium salts e.g., palladium acetate
- ligands such as 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (i.e. "Xantphos"), 2-dicyclohexyl- phosphino-2',4',6'-triisopropylbiphenyl (i.e.
- Xphos 2,2'-bis(diphenylphosphino)- l,l'-binaphthalene
- BINAP 2,2'-bis(diphenylphosphino)- l,l'-binaphthalene
- a base such as potassium carbonate, cesium carbonate, sodium phenoxide or sodium tert-butoxide
- solvent such as ⁇ /, ⁇ /-dimethylformamide, 1 ,2-dimethoxy ethane, dimethyl sulfoxide, 1,4-dioxane or toluene, optionally mixed with alcohols such as ethanol, can be used.
- compounds of Formula Ic i.e.
- Formula 1 in which X is NR 4 and R 4 is H can be prepared by reaction of compounds of Formula 6 (i.e. 5-bromopyrazoles or other pyrazoles substituted at the 5 -position with a leaving group) with compounds of Formula 7 under metal-catalyzed conditions similar to those described above for Scheme 3.
- compounds of Formula 7 are commercially available or their preparation is known in the art.
- compounds of Formula 6 wherein G is Br or I can be prepared by reaction of 5-aminopyrazoles of Formula 4a (i.e. Formula 4 wherein X is NH) under diazotization conditions either in the presence of, or followed by combination with, copper salts containing bromide or iodide.
- 5-aminopyrazoles of Formula 4a i.e. Formula 4 wherein X is NH
- diazotization conditions either in the presence of, or followed by combination with, copper salts containing bromide or iodide.
- tert-butyl nitrite to a solution of a 5-aminopyrazole of Formula 4a in the presence of CuBr 2 in a solvent such as acetonitrile provides the corresponding 5-bromopyrazole of Formula 6.
- a 5-aminopyrazole of Formula 4a can be converted to a diazonium salt and then to a corresponding 5-halopyrazole of Formula 6 by treatment with sodium nitrite in solvents such as water, acetic acid or trifluoroacetic acid, in the presence of a mineral acid typically containing the same halide atom (such as aqueous HI solution for G being I), followed by treatment with the corresponding copper(I) or copper(II) salt according to general procedures well known to those skilled in the art.
- solvents such as water, acetic acid or trifluoroacetic acid
- G is Br or I.
- 5-bromopyrazoles of Formula 6a i.e. Formula 6 wherein G is Br
- 5-hydroxypyrazoles of Formula 4b i.e. Formula 4 wherein X is O
- phosphorus tribromide as described in Tetrahedron Lett. 2000, 47(24), 4713.
- 5-hydroxypyrazoles of Formula 4b can also be used to prepare 5-fluoroalkylsulfonyl (e.g, 5-trifluoromethanesulfonyl, 5-nonafluorobutylsulfonyl) pyrazoles of Formula 6b (i.e. Formula 6 wherein G is fluoroalkylsulfonyl) as described in Synlett 2004, 5, 795.
- 5-fluoroalkylsulfonyl e.g, 5-trifluoromethanesulfonyl, 5-nonafluorobutylsulfonyl
- Formula 6b i.e. Formula 6 wherein G is fluoroalkylsulfonyl
- Rf is fluoroalkyl such as CF3 or (CF 2 ) 2 CF3
- a 4-bromo or iodo pyrazole of Formula 10 Reaction of a 4-bromo or iodo pyrazole of Formula 10 with a boronic acid, trialkyltin, zinc or organomagnesium reagent of Formula 11 in the presence of a palladium or nickel catalyst having appropriate ligands (e.g., triphenylphosphine (PPI1 3 ), dibenzylideneacetone (dba), dicyclohexyl(2',6'-dimethoxy- [l,l'-biphenyl]-2-yl)phosphine (SPhos)) and a base, if needed, affords the corresponding compound of Formula 1.
- PPI1 3 triphenylphosphine
- dba dibenzylideneacetone
- SPhos dicyclohexyl(2',6'-dimethoxy- [l,l'-biphenyl]-2-yl)phosphin
- a substituted aryl boronic acid or derivative e.g., Formula 11 wherein Q 1 is optionally substituted phenyl or heterocyclyl and M is B(OH) 2 , B(OC(CH 3 ) 2 C(CH 3 ) 2 ⁇ )) or B(O-Z-Pr) 3 / Li - reacts with a 4-bromo- or 4-iodopyrazole of Formula 10 in the presence of dichlorobis(triphenylphosphine) palladium(II) and aqueous base such as sodium carbonate or potassium hydroxide, in solvents such as 1,4-dioxane, 1 ,2-dimethoxyethane, toluene or ethyl alcohol, or under anhydrous conditions with a ligand such as phosphine oxide or phosphite ligand (e.g., diphenylphosphine oxide) and potassium fluoride in a solvent such as 1,4-dio
- Sn(Me) 3 , Sn(Bu) 3 , ZnCl, MgBr, 10 G is Br, I MgCl or MgCl-LiCl.
- compounds of Formula 4a i.e. Formula 4 wherein X is NH
- compounds of Formula 11 such as Q i -B(OH) 2 (Formula Ha)
- transition-metal-catalyzed cross-coupling reaction conditions as described for the method of Scheme 8.
- halogenating reagents such as bromine, sodium bromite, ⁇ /-bromosuccinimide (NBS) or JV-iodosuccinimide (NIS)
- solvents such as acetic
- compounds of Formula 13 wherein A is H or a protecting group can be converted into intermediates corresponding to Formula 10 wherein Q 2 is replaced by A or a protecting group, respectively, which are useful for preparing compounds of Formula 1.
- Compounds of Formula 13 wherein A is H can be be prepared by methods known in the art; see, for example, Synlett 2004, 5, 795-798, US 4256902 and references cited therein.
- some compounds of Formula 13 wherein A is H, particularly those in which R 2 is methyl, ethyl or halogen, are commercially available.
- compounds of Formula 13 wherein X is O, S(O ) m or NR 4 , m is 0, and A is Q 2 can be prepared from corresponding compounds of Formula 13a (i.e. Formula 13 wherein A is H) by procedures analogous those used for the method of Scheme 3.
- Compounds of Formula 13 wherein X is S i.e. S(O ) m wherein m is 0
- Compounds of Formula 13a are commercially available or can be prepared by methods known in the art.
- CHR 1 R ⁇ G is F, Cl, Br, I, NO 2 , OS(O) 2 CF 3 , etc.
- Formula Id i.e. Formula 1 wherein X is CR 15 R 16 , R 15 is H, R 16 is OR 18 and R 18 is H
- an organometallic reagent i.e. Formula 26
- an alkyllithium preferably n-butyllithium, or an alkylmagnesium reagent, preferably isopropylmagnesium chloride (optionally complexed with lithium chloride
- a sulfur electrophile i.e. Formula 27
- carbonyl electrophile i.e. Formula 28, 29 or 30
- Reaction temperatures can range from -90 0 C to the boiling point of the reaction solvent; temperatures of -78 0 C to ambient temperature are generally preferred, with temperatures of -78 to -10 0 C preferred when an alkyllithium reagent is used, and -20 0 C to ambient temperature preferred with use of alkylmagnesium reagents.
- solvents are useful, such as toluene, ethyl ether, tetrahydrofuran or dimethoxymethane; anhydrous tetrahydrofuran is preferred.
- a second metallic component such as zinc chloride, zinc bromide or a monovalent copper salt, such as copper(I) iodide or copper(I) cyanide, can advantageously be added before the electrophile in cases in which the electrophile is Q 2 C(O)Cl (i.e. Formula 30).
- the Q 2 -containing sulfur and carbonyl intermediates of Formulae 27, 28, 29 and 30 are commercially available or can be prepared by methods known in the art.
- 5-thiopyrazole compounds of Formula 4c can be prepared by reaction of corresponding 5-hydroxypyrazole compounds of Formula 4b with P 2 S 5 (see, for example, Justus Liebigs Annalen der Chemie 1908, 361, 251) or with Lawesson's Reagent (2,4-bis-(4-methoxyphenyl)-l,3-dithia-2,4-diphosphetane 2,4-disulfide; see, for example, International Patent Publication WO 2005/118575) in solvents such as toluene, xylene or tetrahydrofuran.
- P 2 S 5 see, for example, Justus Liebigs Annalen der Chemie 1908, 361, 251
- Lawesson's Reagent 2,4-bis-(4-methoxyphenyl)-l,3-dithia-2,4-diphosphetane 2,4-disulfide
- solvents such as toluene, x
- compounds of Formula Ic i.e. Formula 1 wherein X is NR 4 and R 4 is H
- a solvent such as ethanol or methanol
- an acid or base catalyst such as acetic acid, piperidine or sodium methoxide
- R 32 is H or lower alkyl (e.g., CH 3 , CH 2 CH 3 or (CH 2 ) 2 CH 3 )
- compounds of Formula 2 wherein X is NH can be similarly prepared by condensing compounds of Formula 17 with hydrazine. This method is described in Chemistry of Heterocyclic Compounds 2005, 41(1), 105-110.
- compounds of Formula 17 (wherein, e.g., R 2 is methyl, ethyl or optionally substituted cyclopropyl and R 33 is H or lower alkyl such as CH 3 , CH 2 CH 3 or (CH 2 ) 2 CH 3 ) can be prepared by reaction of corresponding ketene dithioacetal compounds of Formula 18 with compounds of formula Q 2 -NH 2 (i.e.
- Formula 7) optionally in the presence of a base, such as sodium hydride or ethylmagnesium chloride, in solvents such as toluene, tetrahydrofuran or dimethoxymethane, at temperatures ranging from -10 0 C to the boiling point of the solvent.
- a base such as sodium hydride or ethylmagnesium chloride
- solvents such as toluene, tetrahydrofuran or dimethoxymethane, at temperatures ranging from -10 0 C to the boiling point of the solvent.
- R 33 is H or lower alkyl (e.g., CH 3 , CH 2 CH 3 or (CH 2 ) 2 CH 3 )
- compounds of Formula 17a i.e. tautomer of Formula 17 wherein R 33 is H
- compounds of Formula 19a can be prepared by reaction of corresponding isothiocyanate compounds of Formula 19 with arylacetone compounds of Formula 20 wherein R 2 is methyl, ethyl or optionally substituted cyclopropyl; see, for example, Zhurnal Organicheskoi Khimii 1982, 0 18(12), 2501.
- Bases useful for this reaction include sodium hydride, alkoxide bases (e.g., potassium te/t-butoxide or sodium ethoxide), potassium hydroxide, sodium hydroxide, potassium carbonate, or amine bases (e.g., triethylamine or JV,jV-diisopropylethylamine).
- alkoxide bases e.g., potassium te/t-butoxide or sodium ethoxide
- potassium hydroxide sodium hydroxide
- potassium carbonate e.g., triethylamine or JV,jV-diisopropylethylamine
- amine bases e.g., triethylamine or JV,jV-diisopropylethylamine.
- solvents such as tetrahydrofuran, ether, toluene, ⁇ f,iV-dimethyl- formamide, alcohols (e.g., ethanol), esters (e
- Solvents are chosen for compatibility with the base selected, as is well- known in the art. Reaction temperatures can range from -78 0 C to the boiling point of the solvent.
- One useful mixture of base and solvent is potassium tert-butoxide in tetrahydrofuran, to which at -70 to 0 0 C is added a combined solution of an isothiocyanate of Formula 19 and a carbonyl compound of Formula 20.
- Ketothioamides of Formula 17 can be also be prepared by allowing the corresponding ketoamides to react with sulfurizing agents such as Lawesson's reagent or P 2 S 5 ; see, for example, HeIv. Chim. Act. 1998, 57(7), 1207.
- sulfurizing agents such as Lawesson's reagent or P 2 S 5 ; see, for example, HeIv. Chim. Act. 1998, 57(7), 1207.
- R la are H and R 2 is OCH 3 ) can be prepared by reacting corresponding compounds of
- compounds of Formula 31 wherein X is NH can be prepared by condensation of corresponding isothiocyanates of Formula 19 with esters of Formula 33 wherein R 33 is lower alkyl (e.g., methyl, ethyl, propyl) in the presence of a strong, non- nucleophilic base such as sodium hydride or lithium hexamethyldisilazide, in an inert solvent such as tetrahydrofuran (analogous to the method of Scheme 18), followed by reaction of the intermediate with hydrazine or an acid salt of hydrazine, such as, for example, an acetate or hydrochloride salt (analogous to the method of Scheme 16).
- a strong, non- nucleophilic base such as sodium hydride or lithium hexamethyldisilazide
- an inert solvent such as tetrahydrofuran
- R 33 is lower alkyl
- the methylating agent can be included in the reaction mixture with the compounds of Formulae 21 and 22 without isolation of the cyano ketoamide intermediate.
- compounds of Formula 23 can also be prepared by a method analogous to Scheme 17 wherein the C(O)R 2 of the compound of Formula 18 is replaced by cyano.
- the resultant compound of Formula 23 is then reacted with an alkylhydrazine of Formula 15 to form the corresponding 3-aminopyrazole compound of Formula 24 using general procedures known in the art; see, for example, J. Chem. Soc. Perkin 1 1988, 2, 169-173 and J. Med. Chem. 2003, 46(7), 1229-1241.
- the amino group of the compound of Formula 24 can then be converted to R 2 being halogen in Formula Ic by a diazotization reaction using conditions known in the art, such as those previously described for Scheme 5.
- R is halogen
- compounds of Formula 2 wherein X is NH and R 2 is halogen can be similarly prepared by condensing compounds of Formula 23 with hydrazine instead of an alkylhydrazine of Formula 15.
- compounds of Formula Ih i.e. Formula 1 wherein X in NR 4
- compounds of Formula Ic i.e. Formula 1 wherein X is NH
- an electrophile comprising R 4 (i.e. Formula 25) typically in the presence of a base such as NaH and a polar solvent such as ⁇ f, ⁇ /-dimethylformamide.
- electrophile comprising R 4 means a chemical compound capable of transferring an R 4 moiety to a nucleophile (such as the nitrogen atom attached to Q 2 in Formula Ic).
- electrophiles comprising R 4 have the formula R 4 Lg wherein Lg is a nucleofuge (i.e. leaving group in nucleophilic reactions).
- Typical nucleofuges include halogens (e.g., Cl, Br, I) and sulfonates (e.g., OS(O) 2 CH 3 , OS(O) 2 CF 3 , OS(O) 2 -(4-CH 3 -Ph)).
- electrophiles comprising R 4 do not comprise a nucleofuge; an example is sulfur trioxide (SO 3 ), which after deprotonation (such as by a base of the formulae M + H ⁇ wherein M + is a cation) of the nitrogen atom attached to Q 2 in Formula Ic, can bond to the nitrogen atom as a -SO 3 ⁇ M + substituent.
- SO 3 sulfur trioxide
- aromatic amines anilines
- aromatic halides such as bromides or iodides prepared via the Sandmeyer reaction can react with alcohols under copper-catalyzed conditions, such as the Ullmann reaction or known modifications thereof, to provide compounds of Formula 1 that contain alkoxy substituents.
- some halogen groups such as fluorine or chlorine, can be displaced with alcohols under basic conditions to provide compounds of Formula 1 containing the corresponding alkoxy substituents.
- the resultant alkoxy compounds can themselves be used in further reactions to prepare compounds of Formula 1 wherein R 3 is -U-V-T (see, for example, PCT Publication WO 2007/149448 A2).
- Compounds of Formula 1 or precursors thereof in which R 2 or R 3 is halide, preferably bromide or iodide, are particularly useful intermediates for transition metal-catalyzed cross-coupling reactions to prepare compounds of Formula 1.
- sulfide groups can be oxidized to the corresponding sulfoxides or sulfones by conditions well-known in the art.
- ketones can be readily converted to ketals using general methods known in the art, thus providing compounds of Formula 1 wherein X is CR 15 R 16 and R 15 and R 16 are taken together as -OCH 2 CH 2 O-.
- compounds of Formula 1 wherein X is CR 15 R 16 , R 15 is C 1 -C 4 alkyl, R 16 is OR 18 , and R 18 is H can be prepared by adding an alkyl Grignard reagent to the corresponding compounds of Formula 1 wherein X is C(O).
- the reaction mixture was heated to 70 0 C, and a solution of 2,4-difluorophenylacetonitrile (3.9 g, 25 mmol), ethyl acetate (3.8 mL, 38 mmol) and xylenes (1 mL) was added dropwise over 15 min. Additional xylenes (5 mL) was added to aid stirring.
- the reaction mixture was heated for 2 h, then allowed to cool. Water (50 mL) was added, and the mixture was extracted with hexanes (50 mL). The aqueous phase was then acidified to p ⁇ 3-4 with 1 N aqueous HCl solution.
- Step B Preparation of 4-(2,4-Difluorophenyl)-l,3-dimethyl-lH-pyrazol-5-amine
- 9,9-dimethylxanthene (460 mg, 0.80 mmol) and powdered potassium carbonate (5.5 g, 40 mmol) were combined in anhydrous 1,4-dioxane (20 mL), and the mixture was sparged with a subsurface stream of N 2 gas for 10 min.
- 4-(2,4-Difluorophenyl)-l,3-dimethyl- lH-pyrazol-5-amine i.e. the product of Step B) (0.89 g, 4.0 mmol) was added in one portion, and l-bromo-3-chlorobenzene (0.47 mL, 4.0 mmol) was added via a syringe.
- the reaction mixture was heated at reflux under a nitrogen atmosphere for 3 h. Additional l-bromo-3-chlorobenzene (0.09 mL, 0.8 mmol) was added, and heating was continued for
- Example 1 (2.4 g, 10 mmol) in acetonitrile (50 mL), and the mixture was stirred and cooled in an ice-water bath while tert-butyi nitrite (90% technical grade, 2.33 mL, 17.7 mmol) was added dropwise over 5 min. The reaction mixture was allowed to warm slowly to ambient temperature. Aqueous HCl solution (20 mL) was added, and then ethyl acetate was added
- the filter pad was washed with ethyl acetate (20 mL), and the phases were separated.
- the organic phase was washed with 1.0 N aqueous hydrochloric acid solution and brine, dried over MgSO ⁇ and concentrated to leave the title compound as an orange-brown semisolid (2.8 g).
- Step A) (0.20 g, 0.66 mmol), palladium(II) acetate (15 mg, 0.066 mmol), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (76 mg, 0.13 mmol) and powdered potassium carbonate (1.8 g, 13 mmol) were combined in anhydrous 1,4-dioxane (3 mL), and the mixture was sparged with a subsurface stream of N 2 gas for 10 min. 2,6-Difluoro-
- Step B Preparation of ⁇ -Acetyl-2,6-difluoro-4-methoxybenzeneacetonitrile Solid sodium ethoxide (4.7 g, 66 mmol) was stirred in a mixture of xylene (20 mL) and ethanol (10 mL) and heated to 50 0 C.
- Step C Preparation of 4-(2,6-Difluoro-4-methoxyphenyl)-l,3-dimethyl-lH-pyrazole- 5 -amine ⁇ -Acetyl-2,6-difluoro-4-methoxybenzeneacetonitrile (i.e. the product of Step B) (8.03 g, 35.7 mmol) and acetic acid (5 mL) were stirred in ethanol (35 mL), and methylhydrazine (1.91 mL, 35.7 mmol) was added. The reaction mixture was heated at reflux for 16 h, cooled, and then poured into water (100 mL). The resulting mixture was extracted with ethyl acetate (100 mL).
- Step D Preparation of 5-Bromo-4-(2,6-difluoro-4-methoxyphenyl)-l,3-dimethyl- lH-pyrazole
- Aqueous hydrochloric acid solution (25 mL) was added, then ethyl acetate (25 mL) was added, and the resulting mixture was filtered through a 2-cm pad of Celite® diatomaceous filter aid.
- the filter pad was washed with ethyl acetate (50 mL), and the phases were separated.
- the organic phase was washed with 1 N aqueous HCl solution (25 mL) and brine (25 mL), dried over MgSO 4 , and concentrated.
- 2,4,6-Trifluoroaniline (0.28 g, 1.9 mmol) was added in one portion, and the reaction mixture was heated at reflux under nitrogen for 22 h. The reaction mixture was cooled, then filtered through Celite® diatomaceous filter aid. The filter pad was washed with ethyl acetate (2O mL), and the filtrate was washed with water (10 mL) and brine (10 mL), dried over MgSO ⁇ and concentrated to leave a semisolid residue.
- Step B Preparation of 3,5-Difluoro-4-[(4-iodo-l,3-dimethyl-lH-pyrazol-
- Step A Preparation of ⁇ -Acetyl-2,4-dichloro-iV-(2,4-difiuorophenyl)benzene- ethanethioamide
- 2,4-Difluorophenyl isothiocyanate (0.27 mL, 2.0 mmol) was added to a stirred suspension of sodium hydride (60% in mineral oil) (112 mg, 2.8 mmol) in anhydrous tetrahydrofuran (4 mL) cooled in an ice-water bath under a nitrogen atmosphere.
- a solution of (2,4-dichlorophenyl)-2-propanone (570 mg, 2.8 mmol) in tetrahydrofuran (4 mL) was added dropwise over 5 min. The resultant yellow solution was stirred at 5-10 0 C for 1 h. Water (10 mL) was carefully added, and the reaction mixture was extracted with ethyl acetate (10 mL).
- reaction mixture was quenched by the addition of saturated aqueous
- Step B Preparation of Methyl ⁇ -acetyl-2,4,6-trifluorobenzeneacetate
- reaction mixture was stirred for an additional 30 minutes, and then while maintaining the -65 0 C temperature, a solution of freshly distilled acetyl chloride (0.80 mL, 11 mmol) in dry tetrahydrofuran (3 mL) was added dropwise.
- the reaction mixture was allowed to warm slowly to ambient temperature, and then water (30 mL) was added.
- the resultant mixture was extracted with ethyl acetate (60 mL).
- the aqueous phase was acidified with 1 N hydrochloric acid and extracted with ethyl acetate (60 mL). Only the first extract was retained, because thin layer chromatographic analysis showed the second extract to contain apparent polar impurities besides additional desired product.
- This solid was suspended in a small volume of ethyl acetate (about 5 mL), an equal volume of hexanes was gradually added, and the suspension was stirred for 30 minutes.
- the solid component was collected on a glass frit, washed with small portions of ethyl acetate/ hexanes (1 :1 and 1 :2 v:v), and allowed to dry in air to provide a white solid (1.02 g). Evaporation of the mother liquor and treatment of the resultant residue with small volumes of ethyl acetate and hexanes as already described provided an additional 0.13 g of solid containing the title product (1.15 g total).
- Step D Preparation of 5-(2,6-Difluoro-4-nitrophenoxy)-l,3-dimethyl-4-(2,4,6- trifluorophenyl)-lH-pyrazole l,3-Dimethyl-4-(2,4,6-trifluorophenyl)-lH-pyrazol-5-ol (i.e. the product of Step C) (0.310 g, 1.28 mmol), was combined with 3,4,5-trifluoronitrobenzene (157 ⁇ L, 1.35 mmol) and potassium carbonate powder (0.27 g, 2 mmol) in dry ⁇ f, ⁇ /-dimethylformamide (4 mL). This mixture was stirred and heated at 80 0 C for 45 minutes and then allowed to cool.
- reaction mixture was diluted with water (10 mL) and extracted with ethyl acetate (2 x 10 mL). The organic phase was washed with water and with brine, dried over MgSO ⁇ and concentrated to leave a viscous residue. This residue was purified by column chromatography through silica gel eluted with a gradient of ethyl acetate (30% to 100%) in hexane to give the title product, a compound of the present invention, as an off- white solid (209 mg).
- Q 1 is 2,6-di-F-Ph, and R 2 is Me.
- 2-C1-4-F 2-F-4-C1 2,4-di-Cl 2,6-di-Cl 2,4,6-tri-Cl
- the present disclosure also includes Tables 2 through 84, each of which is constructed the same as Table 1 above, except that the row heading in Table 1 (i.e. "Q 1 is 2,6-di-F-Ph, and R 2 is Me.") is replaced with the respective row heading shown below.
- Table 2 the row heading is "Q 1 is 2,6-di-F-Ph, and R 2 is CL", and (R 3 ) p is as defined in Table 1 above.
- the first entry in Table 2 specifically discloses 2-chloro-4-(2,6- difluorophenyl)- ⁇ /-(2-fluorophenyl)-l -methyl- lH-pyrazol-5-amine.
- Tables 3 through 84 are constructed similarly.
- Q 1 is 2,6-di-F -Ph, and R 2 is Cl.
- 47 Q 1 is 2-Br-Ph, and R 2 is Cl.
- J Q 1 is 2,6-di-F -Ph, and R 2 is Br.
- Q 1 is 2-Br-Ph, and R 2 is Br.
- Q 1 is 2,4,6-tri -F-Ph, and R 2 is Me.
- Q 1 is 2,4-di-Cl-Ph, and R 2 is Me.
- Q 1 is 2,4,6-tri -F-Ph, and R 2 is Cl.
- Q 1 is 2,4,6-tri -F-Ph, and R 2 is Br.
- Q 1 is 2,4-di-Cl-Ph, and R 2 is Br.
- Q 1 is 2,6-di-F -4-OMe-Ph, and R 2 is Me.
- Q 1 is 2,6-di-Cl-Ph, and R 2 is Me.
- Q 1 is 2,6-di-F 4-OMe-Ph, and R 2 is Cl.
- 56 Q 1 is 2,6-di-Cl-Ph, and R 2 is Cl.
- 12 Q 1 is 2,6-di-F -4-OMe-Ph, and R 2 is Br.
- 57 Q 1 is 2,6-di-Cl-Ph, and R 2 is Br.
- 12A Q 1 is 2,6-di-F-4-OEt-Ph, and R 2 is Me.
- 58 Q 1 is 2-F-4-MeO-Ph, and R 2 is Me.
- 12B Q 1 is 2,6-di-F-4-OEt-Ph, and R 2 is Cl.
- 59 Q 1 is 2-F-4-MeO-Ph, and R 2 is Cl.
- 12C Q 1 is 2,6-di-F-4-OEt-Ph, and R 2 is Br.
- 60 Q 1 is 2-F-4-MeO-Ph, and R 2 is Br.
- Q 1 is 2,6-di-F-4-CN-Ph, and R 2 is Me.
- 6OA Q 1 is 2-F-4-EtO-Ph, and R 2 is Me.
- Q 1 is 2,6-di-F-4-CN-Ph, and R 2 is Cl.
- 6OB Q 1 is 2-F-4-EtO-Ph, and R 2 is Cl.
- Q 1 is 2,6-di-F-4-CN-Ph, and R 2 is Br.
- 6OC Q 1 is 2-F-4-EtO-Ph, and R 2 is Br.
- Q 1 is 2-Cl-4-F-Ph, and R 2 is Me.
- 61 Q 1 is 2-Cl-4-MeO-Ph, and R 2 is Me.
- Q 1 is 2-Cl-4-F-Ph, and R 2 is Cl.
- 62 Q 1 is 2-Cl-4-MeO-Ph, and R 2 is Cl.
- Q 1 is 2-Cl-4-F-Ph, and R 2 is Br.
- Q 1 is 2-Cl-4-MeO-Ph, and R 2 is Br.
- Q 1 is 2-Cl-6-F-Ph, and R 2 is Me.
- 63A Q 1 is 2-Cl-4-EtO-Ph, and R 2 is Me.
- Q 1 is 2-Cl-6-F-Ph, and R 2 is Cl.
- 63B Q 1 is 2-Cl-4-EtO-Ph, and R 2 is Cl.
- Q 1 is 4-Cl-2,6-di-F-Ph, and R 2 is Cl.
- 66B Q 1 is 2-Br-4-EtO-Ph, and R 2 is Cl.
- 27 Q 1 is 4-Cl-2,6-di-F-Ph, and R 2 is Br.
- 66C Q 1 is 2-Br-4-EtO-Ph, and R 2 is Br.
- 28 Q 1 is 2-Br-4-F-Ph, and R 2 is Me.
- 67 Q 1 is 2-F-4-CN-Ph, and R 2 is Me.
- 29 Q 1 is 2-Br-4-F-Ph, and R 2 is Cl.
- 68 Q 1 is 2-F-4-CN-Ph, and R 2 is Cl.
- Q 1 is 2-Cl-Ph, and R 2 is Me.
- 82 Q 1 is 2,5-di-Cl-3-thienyl, and R 2 is Me.
- 44 Q 1 is 2-Cl-Ph, and R 2 is Cl.
- 83 Q 1 is 2,5-di-Cl-3-thienyl, and R 2 is Cl.
- 45 Q 1 is 2-Cl-Ph, and R 2 is Br.
- 84 Q 1 is 2,5-di-Cl-3-thienyl, and R 2 is Br.
- 46 is 2-Br-Ph, and R 2 is Me.
- Q 1 is 2,6-di-F-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Me.
- 2-F-4-Br 2-Br-6-F 2-Cl-4-Br 2-Br-4-Cl 2-I-4-F
- the present disclosure also includes Tables 86 through 280, each of which is constructed the same as Table 85 above, except that the row heading in Table 85 (i.e. "pi is 2,6-di-F-Ph, X is O, RI and R ⁇ a are both H, and R ⁇ is Me.") is replaced with the respective row heading shown below.
- Table 86 the row heading is "Q 1 is 2,6-di-F-Ph, X is O, R 1 and R la are both H, and R 2 is Cl.” and (R 3 ) p is as defined in Table 85 above.
- Table 87 through 280 are constructed similarly.
- Q 1 is 2,6-di-F-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2,6-di-F-4-CN-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2,6-di-F-4-CN-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Br.
- 100 Q 1 is 2-Cl-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 101 Q 1 is 2-Cl-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 102 Q 1 is 2-Cl-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 103 Q 1 is 2-Cl-6-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 104 Q 1 is 2-Cl-6-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 105 Q 1 is 2-Cl-6-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 106 Q 1 is 2-Cl-4,6-di-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 107 Q 1 is 2-Cl-4,6-di-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 108 Q 1 is 2-Cl-4,6-di-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 109 Q 1 is 4-Cl-2,6-di-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 110 Q 1 is 4-Cl-2,6-di-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 111 Q 1 is 4-Cl-2,6-di-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 112 Q 1 is 2-Br-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 113 Q 1 is 2-Br-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 114 Q 1 is 2-Br-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 115 Q 1 is 2-Br-6-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 116 Q 1 is 2-Br-6-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 117 Q 1 is 2-Br-6-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 118 Q 1 is 2-Me-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 119 Q 1 is 2-Me-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 120 Q 1 is 2-Me-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 121 Q 1 is 2-1-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 122 QI is 2-1-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 123 Q 1 is 2-1-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 124 Q 1 is 2-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 126 Q 1 is 2-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 127 Q 1 is 2-Cl-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 128 Q 1 is 2-Cl-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 129 Q 1 is 2-Cl-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 130 Q 1 is 2-Br-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 131 Q 1 is 2-Br-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 132 Q 1 is 2-Br-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 133 Q 1 is 2-F-4-Cl-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 134 Q 1 is 2-F-4-Cl-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 135 Q 1 is 2-F-4-Cl-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- 136 Q 1 is 2,4-di-Cl-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- Q 1 is 2,6-di-Cl-Ph, X is O, R 1 and R la are both H, and R 2 is Br.
- 142 Q 1 is 2-F-4-MeO-Ph, X is O, R 1 and R l a are both H, and R 2 is Me.
- 143 Q 1 is 2-F-4-MeO-Ph, X is O, R 1 and R l a are both H, and R 2 is Cl.
- 144 Q 1 is 2-F-4-MeO-Ph, X is O, R 1 and R l a are both H, and R 2 is Br.
- Q 1 is 2-F-4-EtO-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-Cl-4-EtO-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-Cl-4-EtO-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-Cl-4-EtO-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-Br-4-MeO-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-Br-4-EtO-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Me.
- 150B Q 1 is 2-Br-4-EtO-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-Br-4-EtO-Ph
- X is O
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2,5-di-Cl-3-pyridinyl
- X is O
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2,5-di-Cl-3-pyridinyl
- X is O
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2,5-di-Cl-3-pyridinyl
- X is O
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-Cl-3-thienyl
- X is O
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-Cl-3-thienyl
- X is O
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2,5-di-Cl-3-thienyl
- X is O
- R 1 and R la are both H
- R 2 is Cl.
- Q 1 is 2,6-di-F-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2,6-di-F-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- 180A Q 1 is 2,6-di-F-4-OEt-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- 180B Q 1 is 2,6-di-F-4-OEt-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- 180C Q 1 is 2,6-di-F-4-OEt-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- 181 Q 1 is 2,6-di-F-4-CN-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- 182 Q 1 is 2,6-di-F-4-CN-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- 183 Q 1 is 2,6-di-F-4-CN-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- 184 Q 1 is 2-Cl-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- 185 Q 1 is 2-Cl-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- Q 1 is 2-Cl-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- Q 1 is 2-Cl-6-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- 188 is 2-Cl-6-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- Q 1 is 2-Cl-6-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- Q 1 is 2-Cl-4,6-di-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- Q 1 is 2-Cl-4,6-di-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- Q 1 is 2-Cl-4,6-di-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- Q 1 is 4-Cl-2,6-di-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- Q 1 is 4-Cl-2,6-di-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- Q 1 is 4-Cl-2,6-di-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- 196 Q 1 is 2-Br-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- Q 1 is 2-Br-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- 198 Q 1 is 2-Br-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- 199 Q 1 is 2-Br-6-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- 200 Q 1 is 2-Br-6-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- 201 Q 1 is 2-Br-6-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- 202 Q 1 is 2-Me-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- 203 Q 1 is 2-Me-4-F-Ph, X is S, R 1 and R la are both H, and R 2 is Cl.
- 204 Q 1 is 2-Me-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- 205 Q 1 is 2-1-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- 206 QI is 2-1-4-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- 207 Q 1 is 2-1-4-F-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-F-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-Cl-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-Br-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-F-4-Cl-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-F-4-Cl-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-F-4-Cl-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2,4-di-Cl-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2,4-di-Cl-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2,4-di-Cl-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2,6-di-Cl-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-F-4-MeO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-F-4-MeO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-F-4-MeO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-F-4-EtO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-F-4-EtO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-F-4-EtO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-Cl-4-MeO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Me.
- 230 Q 1 is 2-Cl-4-MeO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-Cl-4-MeO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-Cl-4-EtO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-Cl-4-EtO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Cl.
- Q 1 is 2-Cl-4-EtO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-Br-4-MeO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- Q 1 is 2-Br-4-EtO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Me.
- Q 1 is 2-Br-4-EtO-Ph
- X is S
- R 1 and R l a are both H
- R 2 is Br.
- 240 Q 1 is 2-Cl-4-CN-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- 241 Q 1 is 2-Br-4-CN-Ph, X is S, R 1 and R l a are both H, and R 2 is Me.
- 242 Q 1 is 2-Br-4-CN-Ph, X is S, R 1 and R l a are both H, and R 2 is Cl.
- 243 Q 1 is 2-Br-4-CN-Ph, X is S, R 1 and R l a are both H, and R 2 is Br.
- 252 Q 1 is 2,5-di-Cl-3-thienyl, X is S, R 1 and R l a are both H, and R 2 is Br.
- 253 Q 1 is 2,6-di-F-Ph, X is NH, R 1 and R l a form c-Pr, and R 2 is Me.
- 254 Q 1 is 2,4-di-F-Ph, X is O, R 1 is Et, R l a is H, and R 2 is Cl.
- 255 Q 1 is 2,4,6-tri-F-Ph, X is S, R 1 and R l a are both H, and R 2 is Et.
- 256 Q 1 is 2,6-di-F-4-OMe-Ph, X is CHOH, R 1 and R l a are both H, and R 2 is CF 3 .
- 258 Q 1 is 2-Cl-4-F-Ph, X is CHOH, X is O, R 1 is c-Pr, R l a is H, and R 2 is Br.
- 259 Q 1 is 2-Cl-6-F-Ph, X is S, R 1 and R l a form c-Pr, and R 2 is Me.
- 260 Q 1 is 2-Cl-4,6-di-F-Ph, X is CHOH, R 1 is c-Pr, R l a is H, and R 2 is Cl.
- 261 Q 1 is 4-Cl-2,6-di-F-Ph, X is NH, X is NH, R 1 is Et, R l a is H, and R 2 is Br.
- 262 Q 1 is 2-Br-4-F-Ph, X is O, R 1 and R l a are both H, and R 2 is Et.
- 264 Q 1 is 2-Me-4-F-Ph, X is CHOH, R 1 and R l a are both H, and R 2 is c-Pr.
- 265 Q 1 is 2-1-4-F-Ph, X is NH, R 1 is CH 2 CF 3 , R l a is H, and R 2 is Me.
- 266 Q 1 is 2-F-Ph, X is O, R 1 is CH 2 F, R l a is H, and R 2 is Cl.
- 267 Q 1 is 2-Cl-Ph, X is S, R 1 and R l a are both H, and R 2 is CH 2 Cl.
- Q 1 is 2-F-4-MeO-Ph
- X is CHOCH 3
- R 1 and R l a are both H
- R 2 is Cl
- Q 1 is 2-Cl-4-MeO-Ph
- X is NH
- R 1 and R l a form c-Pr
- R 2 is Br
- Q 1 is 2-Br-4-MeO-Ph
- X is O
- R 1 is «-Pr
- R l a is H
- R 2 is Me.
- Q 1 is 2-F-4-CN-Ph
- X is NH
- R 1 is CH 2 C ⁇ CH
- R l a is H
- R 2 is Cl.
- Q 1 is 2,6-di-F-Ph
- X is CHOH
- R 2 is Me
- the present disclosure also includes Tables 282 through 448, each of which is constructed the same as Table 281 above, except that the row heading in Table 281 (i.e. "Q 1 is 2,6-di-F-Ph, X is CHOH, and R ⁇ is Me.") is replaced with the respective row heading shown below.
- Table 282 the row heading is "Q 1 is 2,6-di-F-Ph, X is CHOH, and R 2 is Cl.” and (R 3 ) p is as defined in Table 281 above.
- Table 282 specifically discloses 3-chloro-4-(2,6-difluorophenyl)- ⁇ -(4-fluorophenyl)- 1 -methyl- lH-pyrazole-5-methanol.
- Tables 283 through 448 are constructed similarly.
- Q 1 is 2,4-di-F-Ph
- X is CHOH
- R 2 is Cl
- Q 1 is 2,6-di-F-4-OEt-Ph
- X is CHOH
- R 2 is Me.
- Q 1 is 2,6-di-F-4-OEt-Ph
- X is CHOH
- R 2 is Cl
- Q 1 is 2,6-di-F-4-OEt-Ph
- X is CHOH
- R 2 is Br.
- Q 1 is 2,6-di-F-4-CN-Ph
- X is CHOH
- R 2 is Me.
- Q 1 is 2,6-di-F-4-CN-Ph
- X is CHOH
- R 2 is Br
- Q 1 is 2-Cl-4-F-Ph, X is CHOH, and R 2 is Cl.
- Q 1 is 2-Cl-6-F-Ph
- X is CHOH
- R 2 is Me
- 301 Q 1 is 2-Cl-6-F-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-Cl-4,6-di-F-Ph
- X is CHOH
- R 2 is Me.
- 304 Q 1 is 2-Cl-4,6-di-F-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 4-Cl-2,6-di-F-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-Br-4-F-Ph
- X is CHOH
- R 2 is Me
- Q 1 is 2-Br-4-F-Ph
- X is CHOH
- R 2 is Cl
- Q 1 is 2-Br-6-F-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-Me-4-F-Ph, X is CHOH, and R 2 is Me.
- Q 1 is 2-Me-4-F-Ph, X is CHOH, and R 2 is Cl.
- Q 1 is 2-1-4-F-Ph, X is CHOH, and R 2 is Me.
- Q 1 is 2-1-4-F-Ph
- X is CHOH
- R 2 is Cl.
- Q 1 is 2-1-4-F-Ph, X is CHOH, and R 2 is Br.
- 320 Q 1 is 2-F-Ph, X is CHOH, and R 2 is Me.
- Q 1 is 2-F-Ph, X is CHOH, and R 2 is Cl.
- Q 1 is 2-F-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-Cl-Ph, X is CHOH, and R 2 is Me.
- Q 1 is 2-Cl-Ph, X is CHOH, and R 2 is Cl.
- Q 1 is 2-Cl-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-Br-Ph, X is CHOH, and R 2 is Me.
- Q 1 is 2-Br-Ph, X is CHOH, and R 2 is Cl.
- 328 Q 1 is 2-Br-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-F-4-Cl-Ph, X is CHOH, and R 2 is Me.
- Q 1 is 2-F-4-Cl-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2,4-di-Cl-Ph
- X is CHOH
- R 2 is Me
- 333 Q 1 is 2,4-di-Cl-Ph, X is CHOH, and R 2 is Cl.
- Q 1 is 2,4-di-Cl-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2,6-di-Cl-Ph
- X is CHOH
- R 2 is Me
- Q 1 is 2,6-di-Cl-Ph
- X is CHOH
- R 2 is Cl
- Q 1 is 2,6-di-Cl-Ph
- X is CHOH
- R 2 is Br
- Q 1 is 2-F-4-MeO-Ph
- X is CHOH
- R 2 is Me
- Q 1 is 2-F-4-MeO-Ph
- X is CHOH
- R 2 is Cl
- 340 Q 1 is 2-F-4-MeO-Ph, X is CHOH, and R 2 is Br.
- 340A Q 1 is 2-F-4-EtO-Ph, X is CHOH, and R 2 is Me.
- 340B Q 1 is 2-F-4-EtO-Ph, X is CHOH, and R 2 is Cl.
- 340C Q 1 is 2-F-4-EtO-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-Cl-4-MeO-Ph
- X is CHOH
- R 2 is Me
- Q 1 is 2-Cl-4-MeO-Ph
- X is CHOH
- R 2 is Cl
- Q 1 is 2-Cl-4-MeO-Ph
- X is CHOH
- R 2 is Br.
- Q 1 is 2-Cl-4-EtO-Ph
- X is CHOH
- R 2 is Me.
- Q 1 is 2-Cl-4-EtO-Ph
- X is CHOH
- R 2 is Cl
- Q 1 is 2-Cl-4-EtO-Ph
- X is CHOH
- R 2 is Br.
- Q 1 is 2-Br-4-MeO-Ph
- X is CHOH
- R 2 is Me
- Q 1 is 2-Br-4-MeO-Ph
- X is CHOH
- R 2 is Cl
- Q 1 is 2-Br-4-MeO-Ph
- X is CHOH
- R 2 is Br.
- Q 1 is 2-Br-4-EtO-Ph
- X is CHOH
- R 2 is Me.
- Q 1 is 2-F-4-CN-Ph, X is CHOH, and R 2 is Me.
- Q 1 is 2-F-4-CN-Ph, X is CHOH, and R 2 is Cl.
- Q 1 is 2-F-4-CN-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-Cl-4-CN-Ph
- X is CHOH
- R 2 is Me
- Q 1 is 2-Cl-4-CN-Ph, X is CHOH, and R 2 is Cl.
- Q 1 is 2-Cl-4-CN-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2-Br-4-CN-Ph, X is CHOH, and R 2 is Me.
- Q 1 is 2-Br-4-CN-Ph, X is CHOH, and R 2 is Cl.
- Q 1 is 2-Br-4-CN-Ph, X is CHOH, and R 2 is Br.
- Q 1 is 2,5-di-Cl-3-pyridinyl
- X is CHOH
- R 2 is Me.
- Q 1 is 2,5-di-Cl-3-pyridinyl
- X is CHOH
- R 2 is Cl
- Q 1 is 2,5-di-Cl-3-pyridinyl
- X is CHOH
- R 2 is Br.
- Q 1 is 2-Cl-3-thienyl
- X is CHOH
- R 2 is Me.
- Q 1 is 2-Cl-3-thienyl
- X is CHOH
- R 2 is Br.
- Q 1 is 2,5-di-Cl-3-thienyl
- X is CHOH
- R 2 is Me.
- Q 1 is 2,5-di-Cl-3-thienyl
- X is CHOH
- R 2 is Cl
- Q 1 is 2,5-di-Cl-3-thienyl
- X is CHOH
- R 2 is Br.
- Q 1 is 2,6-di-F-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2,6-di-F-Ph
- X is C(O)
- R 2 is Br.
- Q 1 is 2,4-di-F-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2,4-di-F-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2,4,6-tri-F-Ph
- X is C(O)
- R 2 is Me.
- Q 1 is 2,4,6-tri-F-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2,6-di-F-4-OMe-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2,6-di-F-4-OMe-Ph, X is C(O), and R 2 is Br.
- a Q 1 is 2,6-di-F-4-OEt-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2,6-di-F-4-OEt-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2,6-di-F-4-OEt-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2,6-di-F-4-CN-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2,6-di-F-4-CN-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2,6-di-F-4-CN-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Cl-4-F-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Cl-6-F-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-Cl-6-F-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Cl-6-F-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Cl-4,6-di-F-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-Cl-4,6-di-F-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Cl-4,6-di-F-Ph, X is C(O), and R 2 is Br.
- Q 1 is 4-Cl-2,6-di-F-Ph, X is C(O), and R 2 is Me.
- Q 1 is 4-Cl-2,6-di-F-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 4-Cl-2,6-di-F-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Br-4-F-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Br-4-F-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Br-6-F-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-Br-6-F-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Me-4-F-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Cl-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-F-4-Cl-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-F-4-Cl-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-F-4-Cl-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2,4-di-Cl-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2,4-di-Cl-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2,6-di-Cl-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2,6-di-Cl-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-F-4-MeO-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-F-4-MeO-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-F-4-EtO-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-F-4-EtO-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-F-4-EtO-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Cl-4-MeO-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-Cl-4-MeO-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Cl-4-MeO-Ph
- X is C(O)
- R 2 is Br.
- Q 1 is 2-Cl-4-EtO-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-Cl-4-EtO-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Cl-4-EtO-Ph
- X is C(O)
- R 2 is Br.
- Q 1 is 2-Br-4-MeO-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Br-4-EtO-Ph, X is C(O), and R 2 is Me.
- 430B Q 1 is 2-Br-4-EtO-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-F-4-CN-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-F-4-CN-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Cl-4-CN-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-Cl-4-CN-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Cl-4-CN-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2-Br-4-CN-Ph, X is C(O), and R 2 is Me.
- Q 1 is 2-Br-4-CN-Ph, X is C(O), and R 2 is Cl.
- Q 1 is 2-Br-4-CN-Ph, X is C(O), and R 2 is Br.
- Q 1 is 2,5-di-Cl-3-pyridinyl
- X is C(O)
- R 2 is Cl.
- Q 1 is 2,5-di-Cl-3-pyridinyl
- X is C(O)
- R 2 is Br.
- Q 1 is 2-Cl-3-thienyl
- X is C(O)
- R 2 is Me.
- Q 1 is 2-Cl-3-thienyl
- X is C(O)
- R 2 is Cl
- Q 1 is 2-Cl-3-thienyl
- X is C(O)
- R 2 is Br.
- Q 1 is 2,6-di-F-Ph, and X is NH.
- the present disclosure also includes Tables 450 through 587, each of which is constructed the same as Table 449 above, except that the row heading in Table 449 (i.e. "Q is 2,6-di-F-Ph, and X is NH.") is replaced with the respective row heading shown below.
- Table 450 the row heading is "Q 1 is 2,4-di-F-Ph, and X is NH.”
- Q 2 is as defined in Table 449 above.
- the first entry in Table 450 specifically discloses 2-chloro- ⁇ /-[4-(2,4-difluorophenyl)-l,3-dimethyl-lH-pyrazol-5-yl]-3-pyridinamine.
- Tables 451 through 587 are constructed similarly.
- 450 Q 1 is 2,4-di-F-Ph, and X is NH.
- 519 Q 1 is 2-Cl-Ph, and X is S.
- 451 Q 1 is 2,4,6-tri-F-Ph, and X is NH.
- 520 Q 1 is 2-Br-Ph, and X is S.
- Q 1 is 2,6-di-F-4-OMe-Ph, and X is NH.
- Q 1 is 2-F-4-Cl-Ph, and X is S.
- Q 1 is 2,6-di-F-4-CN-Ph, and X is NH.
- Q 1 is 2,4-di-Cl-Ph, and X is S.
- Q 1 is 2-Cl-4-F-Ph, and X is NH. 523 Q 1 is 2,6-di-Cl-Ph, and X is S.
- Q 1 is 2-Cl-6-F-Ph, and X is NH.
- Q 1 is 2-F-4-MeO-Ph, and X is S.
- Q 1 is 2-Cl-4,6-di-F-Ph, and X is NH.
- Q 1 is 2-Cl-4-MeO-Ph, and X is S.
- Q 1 is 4-Cl-2,6-di-F-Ph, and X is NH.
- 526 Q 1 is 2-Br-4-MeO-Ph, and X is S.
- Q 1 is 2-Br-4-F-Ph, and X is NH.
- 527 Q 1 is 2-F-4-CN-Ph, and X is S.
- Q 1 is 2-Br-6-F-Ph, and X is NH.
- 528 Q 1 is 2-Cl-4-CN-Ph, and X is S.
- 461 Q 1 is 2-1-4-F-Ph, and X is NH.
- 530 Q 1 is 2,5-di-Cl-3-pyridinyl, and X is S.
- Table Row Heading Table Row Heading
- 462 Q 1 is 2-F-Ph, and X is NH.
- 531 Q 1 is 2-Cl-3-thienyl, and X is S.
- Q 1 is 2-Cl-Ph, and X is NH.
- 532 Q 1 is 2,5-di-Cl-3-thienyl, and X is S.
- Q 1 is 2-Br-Ph, and X is NH.
- Q 1 is 2,6-di-F-Ph, and X is CHOH.
- Q 1 is 2-F-4-Cl-Ph, and X is NH.
- 534 Q 1 is 2,4-di-F-Ph, and X is CHOH.
- Q 1 is 2,4-di-Cl-Ph, and X is NH.
- Q 1 is 2,4,6-tri-F-Ph, and X is CHOH.
- Q 1 is 2,6-di-Cl-Ph, and X is NH.
- Q 1 is 2,6-di-F-4-OMe-Ph, and X is CHOH.
- Q 1 is 2-F-4-MeO-Ph, and X is NH.
- Q 1 is 2,6-di-F-4-CN-Ph, and X is CHOH.
- Q 1 is 2-Cl-4-MeO-Ph, and X is NH.
- Q 1 is 2-Cl-4-F-Ph, and X is CHOH.
- Q 1 is 2-Br-4-MeO-Ph, and X is NH.
- Q 1 is 2-Cl-6-F-Ph, and X is CHOH.
- Q 1 is 2-F-4-CN-Ph, and X is NH.
- 540 Q 1 is 2-Cl-4,6-di-F-Ph, and X is CHOH.
- Q 1 is 2-Cl-4-CN-Ph, and X is NH.
- Q 1 is 4-Cl-2,6-di-F-Ph, and X is CHOH.
- Q 1 is 2-Br-4-CN-Ph, and X is NH.
- Q 1 is 2-Br-4-F-Ph, and X is CHOH.
- Q 1 is 2,5-di-Cl-3-pyridinyl, and X is NH.
- 543 Q 1 is 2-Br-6-F-Ph, and X is CHOH.
- Q 1 is 2-Cl-3-thienyl, and X is NH.
- Q 1 is 2-Me-4-F-Ph, and X is CHOH.
- Q 1 is 2,5-di-Cl-3-thienyl, and X is NH.
- Q 1 is 2-1-4-F-Ph, and X is CHOH.
- Q 1 is 2,6-di-F-Ph, and X is O.
- Q 1 is 2-F-Ph, and X is CHOH.
- Q 1 is 2,4-di-F-Ph, and X is O.
- Q 1 is 2-Cl-Ph, and X is CHOH.
- Q 1 is 2,4,6-tri-F-Ph, and X is O.
- Q 1 is 2-Br-Ph, and X is CHOH.
- Q 1 is 2,6-di-F-4-OMe-Ph, and X is O.
- Q 1 is 2-F-4-Cl-Ph, and X is CHOH.
- 481 Q 1 is 2,6-di-F-4-CN-Ph, and X is O.
- 550 Q 1 is 2,4-di-Cl-Ph, and X is CHOH.
- Q 1 is 2-Cl-4-F-Ph, and X is O.
- Q 1 is 2,6-di-Cl-Ph, and X is CHOH.
- Q 1 is 2-Cl-6-F-Ph, and X is O.
- Q 1 is 2-F-4-MeO-Ph, and X is CHOH.
- Q 1 is 2-Cl-4,6-di-F-Ph, and X is O.
- Q 1 is 2-Cl-4-MeO-Ph, and X is CHOH.
- Q 1 is 4-Cl-2,6-di-F-Ph, and X is O.
- Q 1 is 2-Br-4-MeO-Ph, and X is CHOH.
- Q 1 is 2-Br-6-F-Ph, and X is O.
- Q 1 is 2-Cl-4-CN-Ph, and X is CHOH.
- Q 1 is 2-Me-4-F-Ph, and X is O.
- Q 1 is 2-Br-4-CN-Ph, and X is CHOH.
- 489 Q 1 is 2-1-4-F-Ph, and X is O.
- 558 Q 1 is 2,5-di-Cl-pyridin-3-yl, and X is CHOH.
- Q 1 is 2-F-Ph, and X is O.
- Q 1 is 2,5-di-Cl-thien-3-yl, and X is CHOH.
- 492 Q 1 is 2-Br-Ph, and X is O.
- 561 Q 1 is 2,4-di-F-Ph, and X is C(O).
- Q 1 is 2-F-4-Cl-Ph, and X is O.
- 562 Q 1 is 2,4,6-tri-F-Ph, and X is C(O).
- Q 1 is 2,6-di-Cl-Ph, and X is O.
- Q 1 is 2,6-di-F-4-CN-Ph, and X is C(O).
- 496 Q 1 is 2-F-4-MeO-Ph, and X is O.
- 565 Q 1 is 2-Cl-4-F-Ph, and X is C(O).
- Q 1 is 2-Cl-4-MeO-Ph, and X is O.
- 566 Q 1 is 2-Cl-6-F-Ph, and X is C(O).
- 570 Q 1 is 2-Br-6-F-Ph, and X is C(O).
- 502 Q 1 is 2,5-di-Cl-pyridin-3-yl, and X is O.
- 571 Q 1 is 2-Me-4-F-Ph, and X is C(O).
- 503 Q 1 is 2-Cl-thien-3-yl, and X is O.
- 572 Q 1 is 2-1-4-F-Ph, and X is C(O).
- 504 Q 1 is 2,5-di-Cl-thien-3-yl, and X is O.
- 573 Q 1 is 2-F-Ph, and X is C(O).
- 505 Q 1 is 2,6-di-F-Ph, and X is S.
- 578 Q 1 is 2,6-di-Cl-Ph, and X is C(O).
- 510 Q 1 is 2-Cl-4-F-Ph, and X is S.
- 579 Q 1 is 2-F-4-MeO-Ph, and X is C(O).
- 511 Q 1 is 2-Cl-6-F-Ph, and X is S.
- 580 Q 1 is 2-Cl-4-MeO-Ph, and X is C(O).
- 512 Q 1 is 2-Cl-4,6-di-F-Ph, and X is S.
- 581 Q 1 is 2-Br-4-MeO-Ph, and X is C(O).
- Q 1 is 4-Cl-2,6-di-F-Ph, and X is S.
- Q 1 is 2-F-4-CN-Ph, and X is C(O).
- 514 Q 1 is 2-Br-4-F-Ph, and X is S.
- 583 Q 1 is 2-Cl-4-CN-Ph, and X is C(O).
- 515 Q 1 is 2-Br-6-F-Ph, and X is S.
- Q 1 is 2-Br-4-CN-Ph, and X is C(O).
- 516 Q 1 is 2-Me-4-F-Ph, and X is S. 585 Q 1 is 2,5-di-Cl-3-pyridinyl, and X is C(O).
- 517 Q 1 is 2-1-4-F-Ph, and X is S. 586 Q 1 is 2-Cl-3-thienyl, and X is C(O). 518 Q 1 is 2-F-Ph, and X is S. 587 Q 1 is 2,5-di-Cl-3-thienyl, and X is C(O).
- Q 1 is 2,6-di-F-Ph, and R 2 is Me.
- the present disclosure also includes Tables 589 through 671, each of which is constructed the same as Table 588 above, except that the row heading in Table 588 (i.e. "Q 1 is 2,6-di-F-Ph, and R 2 is Me.") is replaced with the respective row heading shown below.
- Table 589 the row heading is "Q 1 is 2,6-di-F-Ph, and R 2 is CL", and (R 3 ) p is as defined in Table 588 above.
- the first entry in Table 589 specifically discloses 5-chloro-4-(2,6-difluorophenyl)- ⁇ /-(2-fluorophenyl)-lH-pyrazol-3-amine.
- Tables 589 through 671 are constructed similarly.
- Q 1 is 2,6-di-F-Ph, and R 2 is Br.
- 635 Q 1 is 2-Br-Ph, and R 2 is Br.
- Q 1 is 2,4-di-F-Ph, and R 2 is Me.
- Q 1 is 2-F-4-Cl-Ph, and R 2 is Me.
- Q 1 is 2,4-di-F-Ph, and R 2 is Cl.
- Q 1 is 2-F-4-Cl-Ph, and R 2 is Cl.
- Q 1 is 2,4-di-F-Ph, and R 2 is Br.
- Q 1 is 2-F-4-Cl-Ph, and R 2 is Br.
- Q 1 is 2,4,6-tri-F-Ph, and R 2 is Me.
- Q 1 is 2,4-di-Cl-Ph, and R 2 is Me.
- Q 1 is 2,4,6-tri-F-Ph, and R 2 is Cl.
- 640 Q 1 is 2,4-di-Cl-Ph, and R 2 is Cl.
- Q 1 is 2,6-di-F-4-OMe-Ph, and R 2 is Me.
- Q 1 is 2,6-di-Cl-Ph, and R 2 is Me.
- Q 1 is 2,6-di-F-4-OMe-Ph, and R 2 is Br.
- Q 1 is 2,6-di-Cl-Ph, and R 2 is Br.
- Q 1 is 2,6-di-F-4-OEt-Ph, and R 2 is Me.
- Q 1 is 2-F-4-MeO-Ph, and R 2 is Me.
- Q 1 is 2,6-di-F-4-OEt-Ph, and R 2 is Cl.
- 646 Q 1 is 2-F-4-MeO-Ph, and R 2 is Cl.
- Q 1 is 2,6-di-F-4-OEt-Ph, and R 2 is Br. 647 Q 1 is 2-F-4-MeO-Ph, and R 2 is Br.
- 600 Q 1 is 2,6-di-F-4-CN-Ph, and R 2 is Me.
- 647A Q 1 is 2-F-4-EtO-Ph, and R 2 is Me.
- 601 Q 1 is 2,6-di-F-4-CN-Ph, and R 2 is Cl.
- 647B Q 1 is 2-F-4-EtO-Ph, and R 2 is Cl.
- 602 Q 1 is 2,6-di-F-4-CN-Ph, and R 2 is Br.
- 647C Q 1 is 2-F-4-EtO-Ph, and R 2 is Br.
- Q 1 is 2-Cl-4-F-Ph, and R 2 is Me.
- 648 Q 1 is 2-Cl-4-MeO-Ph, and R 2 is Me.
- 604 Q 1 is 2-Cl-4-F-Ph, and R 2 is Cl.
- 649 Q 1 is 2-Cl-4-MeO-Ph, and R 2 is Cl.
- Q 1 is 2-Cl-4-F-Ph, and R 2 is Br.
- 650 Q 1 is 2-Cl-4-MeO-Ph, and R 2 is Br.
- 606 Q 1 is 2-Cl-6-F-Ph, and R 2 is Me.
- 650A Q 1 is 2-Cl-4-EtO-Ph, and R 2 is Me.
- 607 Q 1 is 2-Cl-6-F-Ph, and R 2 is Cl.
- 650B Q 1 is 2-Cl-4-EtO-Ph, and R 2 is Cl.
- 608 Q 1 is 2-Cl-6-F-Ph, and R 2 is Br.
- 650C Q 1 is 2-Cl-4-EtO-Ph, and R 2 is Br.
- 609 Q 1 is 2-Cl-4,6-di-F-Ph, and R 2 is Me.
- 651 Q 1 is 2-Br-4-MeO-Ph, and R 2 is Me.
- 610 Q 1 is 2-Cl-4,6-di-F-Ph, and R 2 is Cl.
- 652 Q 1 is 2-Br-4-MeO-Ph, and R 2 is Cl.
- 611 Q 1 is 2-Cl-4,6-di-F-Ph, and R 2 is Br.
- 653 Q 1 is 2-Br-4-MeO-Ph, and R 2 is Br.
- 612 Q 1 is 4-Cl-2,6-di-F-Ph, and R 2 is Me.
- 653A Q 1 is 2-Br-4-EtO-Ph, and R 2 is Me.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Dentistry (AREA)
- Wood Science & Technology (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Agronomy & Crop Science (AREA)
- General Health & Medical Sciences (AREA)
- Pest Control & Pesticides (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Plural Heterocyclic Compounds (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Abstract
Description
Claims
Priority Applications (25)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA2750862A CA2750862C (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| BRPI1005812A BRPI1005812B8 (en) | 2009-03-03 | 2010-03-03 | COMPOUND, FUNGICIDAL COMPOSITIONS AND METHOD TO CONTROL DISEASES IN PLANTS |
| ES10707187.0T ES2562178T3 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| AU2010221440A AU2010221440B2 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| KR1020117023011A KR101752925B1 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| RS20160051A RS54531B1 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| PL10707187T PL2403834T3 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| US13/254,205 US9062005B2 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| RU2011139893/04A RU2577247C2 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| UAA201109619A UA124518C2 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| DK10707187.0T DK2403834T3 (en) | 2009-03-03 | 2010-03-03 | fungicidal pyrazoles |
| SI201031129T SI2403834T1 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| CN2010800198065A CN102421756A (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| MEP-2016-26A ME02349B (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| MX2011008900A MX2011008900A (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles. |
| JP2011553063A JP6285090B2 (en) | 2009-03-03 | 2010-03-03 | Bactericidal and fungicidal pyrazole |
| NZ594181A NZ594181A (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| EP10707187.0A EP2403834B1 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| IL214184A IL214184A (en) | 2009-03-03 | 2011-07-19 | Fungicidal pyrazoles |
| ZA2011/05391A ZA201105391B (en) | 2009-03-03 | 2011-07-21 | Fungicidal pyrazoles |
| US14/695,689 US9655361B2 (en) | 2009-03-03 | 2015-04-24 | Fungicidal pyrazoles |
| HRP20160111T HRP20160111T1 (en) | 2009-03-03 | 2016-02-01 | Fungicidal pyrazoles |
| US15/496,048 US20170223959A1 (en) | 2009-03-03 | 2017-04-25 | Fungicidal pyrazoles |
| US16/173,167 US10448639B2 (en) | 2009-03-03 | 2018-10-29 | Fungicidal pyrazoles |
| US16/572,759 US20200008425A1 (en) | 2009-03-03 | 2019-09-17 | Fungicidal pyrazoles |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15704609P | 2009-03-03 | 2009-03-03 | |
| US61/157,046 | 2009-03-03 | ||
| US30405310P | 2010-02-12 | 2010-02-12 | |
| US61/304,053 | 2010-02-12 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/254,205 A-371-Of-International US9062005B2 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
| US14/695,689 Division US9655361B2 (en) | 2009-03-03 | 2015-04-24 | Fungicidal pyrazoles |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010101973A1 true WO2010101973A1 (en) | 2010-09-10 |
Family
ID=42139982
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/026003 WO2010101973A1 (en) | 2009-03-03 | 2010-03-03 | Fungicidal pyrazoles |
Country Status (30)
| Country | Link |
|---|---|
| US (5) | US9062005B2 (en) |
| EP (2) | EP2403834B1 (en) |
| JP (4) | JP6285090B2 (en) |
| KR (1) | KR101752925B1 (en) |
| CN (2) | CN107011326B (en) |
| AR (1) | AR075713A1 (en) |
| AU (1) | AU2010221440B2 (en) |
| BR (1) | BRPI1005812B8 (en) |
| CA (1) | CA2750862C (en) |
| CO (1) | CO6361933A2 (en) |
| DK (1) | DK2403834T3 (en) |
| ES (1) | ES2562178T3 (en) |
| GE (1) | GEP20156211B (en) |
| HR (1) | HRP20160111T1 (en) |
| HU (1) | HUE026926T2 (en) |
| IL (1) | IL214184A (en) |
| ME (1) | ME02349B (en) |
| MX (1) | MX2011008900A (en) |
| MY (1) | MY159364A (en) |
| NZ (1) | NZ594181A (en) |
| PE (2) | PE20130039A1 (en) |
| PL (1) | PL2403834T3 (en) |
| PT (1) | PT2403834E (en) |
| RS (1) | RS54531B1 (en) |
| RU (1) | RU2577247C2 (en) |
| SI (1) | SI2403834T1 (en) |
| TW (1) | TWI548344B (en) |
| UA (1) | UA124518C2 (en) |
| WO (1) | WO2010101973A1 (en) |
| ZA (1) | ZA201105391B (en) |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012031061A3 (en) * | 2010-09-01 | 2013-04-25 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles and their mixtures |
| WO2013126283A1 (en) | 2012-02-20 | 2013-08-29 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2013192126A1 (en) | 2012-06-22 | 2013-12-27 | E. I. Du Pont De Nemours And Company | Fungicidal 4-methylanilino pyrazoles |
| WO2013116251A3 (en) * | 2012-02-01 | 2014-02-20 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazole mixtures |
| US8754115B2 (en) | 2010-09-01 | 2014-06-17 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2014130241A1 (en) | 2013-02-20 | 2014-08-28 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2015026646A1 (en) | 2013-08-20 | 2015-02-26 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2015171392A1 (en) | 2014-05-06 | 2015-11-12 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2016012424A1 (en) | 2014-07-24 | 2016-01-28 | Bayer Cropscience Aktiengesellschaft | Fungicidal pyrazole derivatives |
| WO2016026830A1 (en) * | 2014-08-21 | 2016-02-25 | Bayer Cropscience Aktiengesellschaft | Novel fungicidal pyrazole derivatives |
| WO2016149311A1 (en) | 2015-03-19 | 2016-09-22 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2018052838A1 (en) | 2016-09-16 | 2018-03-22 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| US9932325B2 (en) | 2016-06-16 | 2018-04-03 | Denali Therapeutics Inc. | Compounds, compositions, and methods |
| WO2020051402A1 (en) | 2018-09-06 | 2020-03-12 | Fmc Corporation | Fungicidal nitroanilino substituted pyrazoles |
| US11028080B2 (en) | 2016-03-11 | 2021-06-08 | Denali Therapeutics Inc. | Substituted pyrimidines as LRKK2 inhibitors |
| WO2021183721A1 (en) | 2020-03-11 | 2021-09-16 | Fmc Corporation | Fungicidal mixtures containing pyrazole derivatives. |
| US11214565B2 (en) | 2015-11-20 | 2022-01-04 | Denali Therapeutics Inc. | Compound, compositions, and methods |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AR075713A1 (en) * | 2009-03-03 | 2011-04-20 | Du Pont | FUNGICIDE PIRAZOLS |
| US20140235689A1 (en) * | 2013-02-20 | 2014-08-21 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles |
| US20140288074A1 (en) * | 2013-03-22 | 2014-09-25 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles |
| TWI652012B (en) * | 2013-05-20 | 2019-03-01 | 杜邦股份有限公司 | Solid form of fungicidal pyrazole |
| TWI652014B (en) * | 2013-09-13 | 2019-03-01 | 美商艾佛艾姆希公司 | Heterocyclic substituted bicycloazole insecticide |
| CA2984202A1 (en) * | 2015-04-29 | 2016-11-03 | The State Of Israel, Ministry Of Agriculture & Rural Development, Agricultural Research Organization (Aro) (Volcani Center) | Anti-phytopathogenic compositions |
| AU2016272624A1 (en) * | 2015-06-01 | 2017-12-21 | Ishihara Sangyo Kaisha, Ltd. | Antibacterial composition and method for control of plant disease |
| CN107286097B (en) | 2017-07-13 | 2019-06-25 | 青岛清原化合物有限公司 | Polybenzobisoxazole humulone C crystal form and its preparation method and application |
| CN113015433A (en) * | 2018-09-14 | 2021-06-22 | Fmc公司 | Fungicidal halomethyl ketones and hydrates |
| CN113549053B (en) * | 2020-04-23 | 2022-09-13 | 沈阳中化农药化工研发有限公司 | Pyrazoloquine (azolyl) ether compound and application thereof |
Citations (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2891855A (en) | 1954-08-16 | 1959-06-23 | Geigy Ag J R | Compositions and methods for influencing the growth of plants |
| US3060084A (en) | 1961-06-09 | 1962-10-23 | Du Pont | Improved homogeneous, readily dispersed, pesticidal concentrate |
| US3235361A (en) | 1962-10-29 | 1966-02-15 | Du Pont | Method for the control of undesirable vegetation |
| US3299566A (en) | 1964-06-01 | 1967-01-24 | Olin Mathieson | Water soluble film containing agricultural chemicals |
| US3309192A (en) | 1964-12-02 | 1967-03-14 | Du Pont | Method of controlling seedling weed grasses |
| US3920442A (en) | 1972-09-18 | 1975-11-18 | Du Pont | Water-dispersible pesticide aggregates |
| US4144050A (en) | 1969-02-05 | 1979-03-13 | Hoechst Aktiengesellschaft | Micro granules for pesticides and process for their manufacture |
| US4172714A (en) | 1976-12-20 | 1979-10-30 | E. I. Du Pont De Nemours And Company | Dry compactible, swellable herbicidal compositions and pellets produced therefrom |
| GB2054555A (en) * | 1979-07-27 | 1981-02-18 | Abbott Lab | Pyrazolyl amino imidazolines |
| US4256902A (en) | 1977-12-23 | 1981-03-17 | Montedison S.P.A. | Process for the preparation of 3-chloro-5-hydroxypyrazoles |
| GB2095558A (en) | 1981-03-30 | 1982-10-06 | Avon Packers Ltd | Formulation of agricultural chemicals |
| DE3246493A1 (en) | 1982-12-16 | 1984-06-20 | Bayer Ag, 5090 Leverkusen | METHOD FOR PRODUCING WATER-DISPERSIBLE GRANULES |
| WO1991013546A1 (en) | 1990-03-12 | 1991-09-19 | E.I. Du Pont De Nemours And Company | Water-dispersible or water-soluble pesticide granules from heat-activated binders |
| US5180587A (en) | 1988-06-28 | 1993-01-19 | E. I. Du Pont De Nemours And Company | Tablet formulations of pesticides |
| US5208030A (en) | 1989-08-30 | 1993-05-04 | Imperial Chemical Industries Plc | Active ingredient dosage device |
| US5232701A (en) | 1990-10-11 | 1993-08-03 | Sumitomo Chemical Company, Limited | Boron carbonate and solid acid pesticidal composition |
| WO1994026722A1 (en) | 1993-05-12 | 1994-11-24 | E.I. Du Pont De Nemours And Company | Fungicidal fused bicyclic pyrimidinones |
| JPH08208620A (en) * | 1995-02-03 | 1996-08-13 | Takeda Chem Ind Ltd | Aminopyrazole derivative, its production and use |
| JPH09208620A (en) | 1996-02-02 | 1997-08-12 | Sumitomo Bakelite Co Ltd | Curable elastomer and its production |
| US6066638A (en) | 1995-07-05 | 2000-05-23 | E. I. Du Pont De Nemours And Company | Fungicidal pyrimidinones |
| US6087381A (en) * | 1997-05-22 | 2000-07-11 | G. D. Searle & Company | Pyrazole derivatives as p38 kinase inhibitors |
| US6262058B1 (en) | 1996-03-11 | 2001-07-17 | Syngenta Crop Protection, Inc. | Pyrimidin-4-one derivatives as pesticide |
| US6277858B1 (en) | 1997-09-12 | 2001-08-21 | Syngenta Crop Protection, Inc. | Pyrimidin-4-one and pyrimidin-4-thione as fungicide |
| WO2003010149A1 (en) | 2001-07-25 | 2003-02-06 | Bayer Cropscience Ag | Pyrazolylcarboxanilides as fungicides |
| WO2003024222A1 (en) | 2001-09-21 | 2003-03-27 | E. I. Du Pont De Nemours And Company | Anthranilamide arthropodicide treatment |
| WO2005118575A1 (en) | 2004-06-01 | 2005-12-15 | Boehringer Ingelheim International Gmbh | Non nucleoside reverse transcriptase inhibitors |
| WO2007149448A2 (en) | 2006-06-21 | 2007-12-27 | E. I. Du Pont De Nemours And Company | Pyrazinones as cellular proliferation inhibitors |
| WO2008093639A1 (en) * | 2007-01-29 | 2008-08-07 | Takeda Pharmaceutical Company Limited | Pyrazole compound |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2076801A (en) * | 1980-04-14 | 1981-12-09 | Erba Farmitalia | alpha , beta -Disubstituted Acrylamido Cephalosporins |
| JPH01319478A (en) * | 1988-06-20 | 1989-12-25 | Mitsui Toatsu Chem Inc | Immunomodulator |
| US5189040A (en) * | 1990-05-28 | 1993-02-23 | Sumitomo Chemical Company, Limited | Pyrazole derivatives, method for producing the same and agricultural and/or horticultural fungicides containing the same as active ingredient |
| FR2676057B1 (en) * | 1991-05-02 | 1995-03-03 | Therapeutique Moder Lab | DERIVATIVES OF 1,2-DITHIOLE-3-THIONE, PROCESS FOR THE PREPARATION OF THESE DERIVATIVES, AS WELL AS THE USE OF THESE DERIVATIVES AS SYNTHESIS INTERMEDIATES OR AS MEDICAMENTS. |
| FR2682379B1 (en) | 1991-10-09 | 1994-02-11 | Rhone Poulenc Agrochimie | NEW FUNGICIDAL PHENYLPYRAZOLES. |
| WO1993019054A1 (en) | 1992-03-26 | 1993-09-30 | Dowelanco | N-heterocyclic nitro anilines as fungicides |
| JPH0665237A (en) * | 1992-05-07 | 1994-03-08 | Nissan Chem Ind Ltd | Substituted pyrazole derivative and germicide for agriculture and horticulture |
| JPH0816059B2 (en) * | 1993-05-21 | 1996-02-21 | 三井東圧化学株式会社 | Immunomodulator |
| DE4405207A1 (en) * | 1994-02-18 | 1995-08-24 | Bayer Ag | N-pyrazolylanilines and N-pyrazolylaminopyridines |
| JP2000095778A (en) * | 1998-09-28 | 2000-04-04 | Ube Ind Ltd | Pyrazole derivative, production thereof and horticultural bactericide |
| AR042067A1 (en) | 2002-11-27 | 2005-06-08 | Bayer Pharmaceuticals Corp | USEFUL ANILINOPIRAZOL DERIVATIVES IN THE TREATMENT OF DIABETES |
| GB0315657D0 (en) | 2003-07-03 | 2003-08-13 | Astex Technology Ltd | Pharmaceutical compounds |
| WO2007027842A1 (en) | 2005-08-31 | 2007-03-08 | Bayer Healthcare Llc | Anilinopyrazole derivatives useful for the treatment of diabetes |
| PE20091953A1 (en) | 2008-05-08 | 2010-01-09 | Du Pont | SUBSTITUTED AZOLS AS FUNGICIDES |
| AR075713A1 (en) * | 2009-03-03 | 2011-04-20 | Du Pont | FUNGICIDE PIRAZOLS |
| WO2011051958A1 (en) | 2009-10-30 | 2011-05-05 | E.I. Du Pont De Nemours And Company | Fungicidal pyrazolones |
| TW201117722A (en) | 2009-11-04 | 2011-06-01 | Du Pont | Fungicidal mixtures |
| WO2012023143A1 (en) | 2010-08-19 | 2012-02-23 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| TWI504350B (en) | 2010-09-01 | 2015-10-21 | Du Pont | Fungicidal pyrazoles and their mixtures |
| TW201245155A (en) | 2010-09-01 | 2012-11-16 | Du Pont | Fungicidal pyrazoles |
| MX2013003390A (en) | 2010-09-29 | 2013-05-31 | Du Pont | Fungicidal imidazoles. |
-
2010
- 2010-03-02 AR ARP100100624A patent/AR075713A1/en active IP Right Grant
- 2010-03-03 EP EP10707187.0A patent/EP2403834B1/en active Active
- 2010-03-03 RU RU2011139893/04A patent/RU2577247C2/en active
- 2010-03-03 BR BRPI1005812A patent/BRPI1005812B8/en active IP Right Grant
- 2010-03-03 CA CA2750862A patent/CA2750862C/en active Active
- 2010-03-03 SI SI201031129T patent/SI2403834T1/en unknown
- 2010-03-03 TW TW099106178A patent/TWI548344B/en active
- 2010-03-03 AU AU2010221440A patent/AU2010221440B2/en active Active
- 2010-03-03 MY MYPI2011003394A patent/MY159364A/en unknown
- 2010-03-03 JP JP2011553063A patent/JP6285090B2/en active Active
- 2010-03-03 ME MEP-2016-26A patent/ME02349B/en unknown
- 2010-03-03 GE GEAP201012393A patent/GEP20156211B/en unknown
- 2010-03-03 RS RS20160051A patent/RS54531B1/en unknown
- 2010-03-03 DK DK10707187.0T patent/DK2403834T3/en active
- 2010-03-03 EP EP15178327.1A patent/EP2966063A1/en not_active Withdrawn
- 2010-03-03 PE PE2012001543A patent/PE20130039A1/en active IP Right Grant
- 2010-03-03 CN CN201710017930.1A patent/CN107011326B/en active Active
- 2010-03-03 ES ES10707187.0T patent/ES2562178T3/en active Active
- 2010-03-03 PT PT107071870T patent/PT2403834E/en unknown
- 2010-03-03 NZ NZ594181A patent/NZ594181A/en unknown
- 2010-03-03 CN CN2010800198065A patent/CN102421756A/en active Pending
- 2010-03-03 WO PCT/US2010/026003 patent/WO2010101973A1/en active Application Filing
- 2010-03-03 KR KR1020117023011A patent/KR101752925B1/en active IP Right Review Request
- 2010-03-03 PL PL10707187T patent/PL2403834T3/en unknown
- 2010-03-03 US US13/254,205 patent/US9062005B2/en active Active
- 2010-03-03 HU HUE10707187A patent/HUE026926T2/en unknown
- 2010-03-03 PE PE2011001568A patent/PE20120101A1/en not_active Application Discontinuation
- 2010-03-03 UA UAA201109619A patent/UA124518C2/en unknown
- 2010-03-03 MX MX2011008900A patent/MX2011008900A/en active IP Right Grant
-
2011
- 2011-07-19 IL IL214184A patent/IL214184A/en active IP Right Grant
- 2011-07-21 ZA ZA2011/05391A patent/ZA201105391B/en unknown
- 2011-09-01 CO CO11112622A patent/CO6361933A2/en active IP Right Grant
-
2015
- 2015-04-24 US US14/695,689 patent/US9655361B2/en active Active
- 2015-05-20 JP JP2015102333A patent/JP2015172062A/en not_active Withdrawn
-
2016
- 2016-02-01 HR HRP20160111T patent/HRP20160111T1/en unknown
- 2016-12-27 JP JP2016252244A patent/JP2017081965A/en not_active Withdrawn
-
2017
- 2017-04-25 US US15/496,048 patent/US20170223959A1/en not_active Abandoned
- 2017-07-24 JP JP2017142601A patent/JP6641326B2/en active Active
-
2018
- 2018-10-29 US US16/173,167 patent/US10448639B2/en active Active
-
2019
- 2019-09-17 US US16/572,759 patent/US20200008425A1/en not_active Abandoned
Patent Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2891855A (en) | 1954-08-16 | 1959-06-23 | Geigy Ag J R | Compositions and methods for influencing the growth of plants |
| US3060084A (en) | 1961-06-09 | 1962-10-23 | Du Pont | Improved homogeneous, readily dispersed, pesticidal concentrate |
| US3235361A (en) | 1962-10-29 | 1966-02-15 | Du Pont | Method for the control of undesirable vegetation |
| US3299566A (en) | 1964-06-01 | 1967-01-24 | Olin Mathieson | Water soluble film containing agricultural chemicals |
| US3309192A (en) | 1964-12-02 | 1967-03-14 | Du Pont | Method of controlling seedling weed grasses |
| US4144050A (en) | 1969-02-05 | 1979-03-13 | Hoechst Aktiengesellschaft | Micro granules for pesticides and process for their manufacture |
| US3920442A (en) | 1972-09-18 | 1975-11-18 | Du Pont | Water-dispersible pesticide aggregates |
| US4172714A (en) | 1976-12-20 | 1979-10-30 | E. I. Du Pont De Nemours And Company | Dry compactible, swellable herbicidal compositions and pellets produced therefrom |
| US4256902A (en) | 1977-12-23 | 1981-03-17 | Montedison S.P.A. | Process for the preparation of 3-chloro-5-hydroxypyrazoles |
| GB2054555A (en) * | 1979-07-27 | 1981-02-18 | Abbott Lab | Pyrazolyl amino imidazolines |
| GB2095558A (en) | 1981-03-30 | 1982-10-06 | Avon Packers Ltd | Formulation of agricultural chemicals |
| DE3246493A1 (en) | 1982-12-16 | 1984-06-20 | Bayer Ag, 5090 Leverkusen | METHOD FOR PRODUCING WATER-DISPERSIBLE GRANULES |
| US5180587A (en) | 1988-06-28 | 1993-01-19 | E. I. Du Pont De Nemours And Company | Tablet formulations of pesticides |
| US5208030A (en) | 1989-08-30 | 1993-05-04 | Imperial Chemical Industries Plc | Active ingredient dosage device |
| WO1991013546A1 (en) | 1990-03-12 | 1991-09-19 | E.I. Du Pont De Nemours And Company | Water-dispersible or water-soluble pesticide granules from heat-activated binders |
| US5232701A (en) | 1990-10-11 | 1993-08-03 | Sumitomo Chemical Company, Limited | Boron carbonate and solid acid pesticidal composition |
| WO1994026722A1 (en) | 1993-05-12 | 1994-11-24 | E.I. Du Pont De Nemours And Company | Fungicidal fused bicyclic pyrimidinones |
| JPH08208620A (en) * | 1995-02-03 | 1996-08-13 | Takeda Chem Ind Ltd | Aminopyrazole derivative, its production and use |
| US6245770B1 (en) | 1995-07-05 | 2001-06-12 | E. I. Du Pont De Nemours And Company | Fungicidal pyrimidinones |
| US6066638A (en) | 1995-07-05 | 2000-05-23 | E. I. Du Pont De Nemours And Company | Fungicidal pyrimidinones |
| JPH09208620A (en) | 1996-02-02 | 1997-08-12 | Sumitomo Bakelite Co Ltd | Curable elastomer and its production |
| US6262058B1 (en) | 1996-03-11 | 2001-07-17 | Syngenta Crop Protection, Inc. | Pyrimidin-4-one derivatives as pesticide |
| US6087381A (en) * | 1997-05-22 | 2000-07-11 | G. D. Searle & Company | Pyrazole derivatives as p38 kinase inhibitors |
| US6277858B1 (en) | 1997-09-12 | 2001-08-21 | Syngenta Crop Protection, Inc. | Pyrimidin-4-one and pyrimidin-4-thione as fungicide |
| WO2003010149A1 (en) | 2001-07-25 | 2003-02-06 | Bayer Cropscience Ag | Pyrazolylcarboxanilides as fungicides |
| WO2003024222A1 (en) | 2001-09-21 | 2003-03-27 | E. I. Du Pont De Nemours And Company | Anthranilamide arthropodicide treatment |
| WO2005118575A1 (en) | 2004-06-01 | 2005-12-15 | Boehringer Ingelheim International Gmbh | Non nucleoside reverse transcriptase inhibitors |
| WO2007149448A2 (en) | 2006-06-21 | 2007-12-27 | E. I. Du Pont De Nemours And Company | Pyrazinones as cellular proliferation inhibitors |
| WO2008093639A1 (en) * | 2007-01-29 | 2008-08-07 | Takeda Pharmaceutical Company Limited | Pyrazole compound |
| EP2128138A1 (en) * | 2007-01-29 | 2009-12-02 | Takeda Pharmaceutical Company Limited | Pyrazole compound |
Non-Patent Citations (46)
| Title |
|---|
| "Comprehensive Heterocyclic Chemistry II", 1996, PERGAMON PRESS |
| "Comprehensive Heterocyclic Chemistry", 1984, PERGAMON PRESS |
| "Developments in formulation technology", 2000, PJB PUBLICATIONS |
| "McCutcheon's Emulsifiers and Detergents", MCCUTCHEON'S DIVISION, THE MANUFACTURING CONFECTIONER PUBLISHING CO. |
| "McCutcheon's Volume 2: Functional Materials", vol. 2, MCCUTCHEON'S DIVISION, THE MANUFACTURING CONFECTIONER PUBLISHING CO. |
| "Perry's Chemical Engineer's Handbook", 1963, MCGRAW-HILL, pages: 8 - 57 |
| "The BioPesticide Manual", 2001, BRITISH CROP PROTECTION COUNCIL |
| "The Pesticide Manual", 2003, BRITISH CROP PROTECTION COUNCIL |
| A. S. DAVIDSON; B. MILWIDSKY: "Synthetic Detergents", 1987, JOHN WILEY AND SONS |
| ANGEWANDTE CHEMIE, INTERNATIONAL EDITION, vol. 47, no. 25, 2008, pages 4695 - 4698 |
| ANNALEN DER CHEMIE, vol. 436, 1924, pages 88 |
| BRAND ET AL., J. AGRIC. FOOD CHEM., vol. 32, 1984, pages 221 - 226 |
| BROWNING: "Agglomeration", CHEMICAL ENGINEERING, 4 December 1967 (1967-12-04), pages 147 - 48 |
| CHEMISTRY OFHETEROCYCLIC COMPOUNDS, vol. 41, no. 1, 2005, pages 105 - 110 |
| G. W. H. CHEESEMAN; E. S. G. WERSTIUK: "Advances in Heterocyclic Chemistry", vol. 22, ACADEMIC PRESS, pages: 390 - 392 |
| GREENE, T. W.; WUTS, P. G. M.: "Protective Groups in Organic Synthesis", 1991, WILEY |
| H. SAUTER ET AL., ANGEW. CHEM. INT. ED., vol. 38, 1999, pages 1328 - 1349 |
| HANCE ET AL.: "Weed Control Handbook", 1989, BLACKWELL SCIENTIFIC PUBLICATIONS |
| HELV. CHIM. ACT., vol. 81, no. 7, 1998, pages 1207 |
| J. AM. CHEM. SOC., vol. 76, 1954, pages 501 |
| J. AM. CHERN. SOC., vol. 129, no. 1, 2007, pages 44 - 45 |
| J. BIOL. CHEM., vol. 264, 1989, pages 14543 - 48 |
| J. BIOL. CHEM., vol. 267, 1992, pages 13175 - 79 |
| J. CHEM. SOC. PERKIN 1, vol. 2, 1988, pages 169 - 173 |
| J. HETEROCYCL. CHEM., vol. 12, no. 1, 1975, pages 139 |
| J. MED. CHEM., vol. 46, no. 7, 2003, pages 1229 - 1241 |
| JOURNAL FOR PRAKTISCHE CHEMIE (LEIPZIG), vol. 83, 1911, pages 171 |
| K. H. KUCK ET AL.: "Modern Selective Fungicides - Properties, Applications and Mechanisms of Action", 1995, GUSTAV FISCHER VERLAG, pages: 205 - 258 |
| K. H. KUCK ET AL.: "Modern Selective Fungicides - Properties. Applications and Mechanisms ofAction", 1995, GUSTAV FISCHER VERLAG, pages: 205 - 258 |
| KLINGMAN: "Weed Control as a Science", 1961, JOHN WILEY AND SONS, INC., pages: 81 - 96 |
| M. R. GRIMMETT; B. R. T. KEENE: "Advances in Heterocyclic Chemistry", vol. 43, ACADEMIC PRESS, pages: 149 - 161 |
| M. TISLER; B. STANOVNIK: "Advances in Heterocyclic Chemistry", vol. 9, ACADEMIC PRESS, pages: 285 - 291 |
| M. TISLER; B. STANOVNIK: "Comprehensive Heterocyclic Chemistry", vol. 3, PERGAMON PRESS, pages: 18 - 20 |
| MARSDEN: "Solvents Guide", 1950, INTERSCIENCE |
| METHODS ENZYMOL., vol. 126, 1986, pages 253 - 71 |
| MIYAURA; BUCHWALD, CROSS COUPLING REACTIONS: A PRACTICAL GUIDE, 2002 |
| PFEIFFER W D ET AL: "SYNTHESE UND REAKTIVITAET VON 6H-1,3,4-SELENADIAZINEN//SYNTHESIS AND REACTIVITY OF 6H-1,3,4-SELENADIAZINES", DIE PHARMAZIE, GOVI VERLAG PHARMAZEUTISCHER VERLAG GMBH, ESCHBORN, DE, vol. 10, no. 48, 1 January 1993 (1993-01-01), pages 732 - 735, XP001093841, ISSN: 0031-7144 * |
| SISELY; WOOD: "Encyclopedia of Surface Active Agents", 1964, CHEMICAL PUBL. CO., INC. |
| SYNLETT, vol. 5, 2004, pages 795 |
| SYNLETT, vol. 5, 2004, pages 795 - 798 |
| T. L. GILCHRIST: "Comprehensive Organic Synthesis", vol. 7, PERGAMON PRESS, pages: 748 - 750 |
| T. S. WOODS: "Pesticide Chemistry and Bioscience, The Food-Environnient Challenge", 1999, THE ROYAL SOCIETY OF CHEMISTRY, article "Thc Formulator's Toolbox - Product Forms for Modern Agriculture", pages: 120 - 133 |
| TETRAHEDRON LETT., vol. 41, no. 24, 2000, pages 4713 |
| TSUJI: "Palladium in Organic Synthesis", 2005, SPRINGER |
| TSUJI: "Transition Metal Reagents and Catalysts: Innovations in Organic Synthesis", 2002, JOHN WILEY AND SONS |
| WATKINS ET AL.: "Handbook of Insecticide Dust Diluents and Carriers", DORLAND BOOKS |
Cited By (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9107412B2 (en) | 2010-09-01 | 2015-08-18 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles and their mixtures |
| US20130129839A1 (en) * | 2010-09-01 | 2013-05-23 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles and their mixtures |
| EP3590340A1 (en) * | 2010-09-01 | 2020-01-08 | FMC Corporation | Intermediates for the synthesis of fungicidal pyrazoles |
| JP2013536866A (en) * | 2010-09-01 | 2013-09-26 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Bactericidal and fungicidal pyrazole and mixtures thereof |
| US9596853B2 (en) | 2010-09-01 | 2017-03-21 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles and their mixtures |
| WO2012031061A3 (en) * | 2010-09-01 | 2013-04-25 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles and their mixtures |
| US8754115B2 (en) | 2010-09-01 | 2014-06-17 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles |
| AU2011295864B2 (en) * | 2010-09-01 | 2016-02-11 | Fmc Corporation | Fungicidal pyrazoles and their mixtures |
| US20150305339A1 (en) * | 2010-09-01 | 2015-10-29 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles and their mixtures |
| US9131693B2 (en) | 2010-09-01 | 2015-09-15 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles |
| TWI568721B (en) * | 2012-02-01 | 2017-02-01 | 杜邦股份有限公司 | Fungicidal pyrazole mixtures |
| CN104394693B (en) * | 2012-02-01 | 2016-10-05 | 纳幕尔杜邦公司 | Antifungal pyrazoles mixture |
| CN104394693A (en) * | 2012-02-01 | 2015-03-04 | 纳幕尔杜邦公司 | Fungicidal pyrazole mixtures |
| RU2664576C1 (en) * | 2012-02-01 | 2018-08-21 | Е.И.Дюпон Де Немур Энд Компани | Mixtures of fungicidal pyrazole |
| JP2015505561A (en) * | 2012-02-01 | 2015-02-23 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニーE.I.Du Pont De Nemours And Company | Bactericidal and fungicidal pyrazole mixture |
| US10015966B2 (en) | 2012-02-01 | 2018-07-10 | E I Du Pont De Nemours And Company | Fungicidal pyrazole mixtures |
| EP3254563A1 (en) * | 2012-02-01 | 2017-12-13 | E. I. du Pont de Nemours and Company | Intermediates in the preparation of fungicidal pyrazoles |
| RU2632981C2 (en) * | 2012-02-01 | 2017-10-11 | Е.И.Дюпон Де Немур Энд Компани | Mixtures of fungucid pyrazoles |
| WO2013116251A3 (en) * | 2012-02-01 | 2014-02-20 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazole mixtures |
| AU2013215296B2 (en) * | 2012-02-01 | 2016-07-07 | Fmc Corporation | Fungicidal pyrazole mixtures |
| CN106342817A (en) * | 2012-02-01 | 2017-01-25 | 纳幕尔杜邦公司 | Fungicidal pyrazole mixtures |
| WO2013126283A1 (en) | 2012-02-20 | 2013-08-29 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| CN104540808A (en) * | 2012-06-22 | 2015-04-22 | 杜邦公司 | Fungicidal 4-methylanilino pyrazoles |
| WO2013192126A1 (en) | 2012-06-22 | 2013-12-27 | E. I. Du Pont De Nemours And Company | Fungicidal 4-methylanilino pyrazoles |
| JP2015525242A (en) * | 2012-06-22 | 2015-09-03 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニーE.I.Du Pont De Nemours And Company | Bactericidal and fungicidal 4-methylanilinopyrazole |
| WO2014130241A1 (en) | 2013-02-20 | 2014-08-28 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2015026646A1 (en) | 2013-08-20 | 2015-02-26 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2015171392A1 (en) | 2014-05-06 | 2015-11-12 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| WO2016012424A1 (en) | 2014-07-24 | 2016-01-28 | Bayer Cropscience Aktiengesellschaft | Fungicidal pyrazole derivatives |
| US10246420B2 (en) | 2014-07-24 | 2019-04-02 | Bayer Cropscience Aktiengesellschaft | Pyrazole derivatives |
| WO2016026830A1 (en) * | 2014-08-21 | 2016-02-25 | Bayer Cropscience Aktiengesellschaft | Novel fungicidal pyrazole derivatives |
| WO2016149311A1 (en) | 2015-03-19 | 2016-09-22 | E I Du Pont De Nemours And Company | Fungicidal pyrazoles |
| US11214565B2 (en) | 2015-11-20 | 2022-01-04 | Denali Therapeutics Inc. | Compound, compositions, and methods |
| US11840529B2 (en) | 2016-03-11 | 2023-12-12 | Denali Therapeutics Inc. | Substituted pyrimidines as LRKK2 inhibitors |
| US11028080B2 (en) | 2016-03-11 | 2021-06-08 | Denali Therapeutics Inc. | Substituted pyrimidines as LRKK2 inhibitors |
| US9932325B2 (en) | 2016-06-16 | 2018-04-03 | Denali Therapeutics Inc. | Compounds, compositions, and methods |
| US10590114B2 (en) | 2016-06-16 | 2020-03-17 | Denali Therapautics Inc. | Compounds, compositions, and methods |
| US11111235B2 (en) | 2016-06-16 | 2021-09-07 | Denali Therapeutics Inc. | Compounds, compositions, and methods |
| US11591316B2 (en) | 2016-06-16 | 2023-02-28 | Denali Therapeutics Inc. | Compounds, compositions, and methods |
| US11834439B2 (en) | 2016-06-16 | 2023-12-05 | Denali Therapeutics Inc. | Compounds, compositions, and methods |
| WO2018052838A1 (en) | 2016-09-16 | 2018-03-22 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazoles |
| CN112969688A (en) * | 2018-09-06 | 2021-06-15 | Fmc公司 | Fungicidal nitroaniline substituted pyrazoles |
| WO2020051402A1 (en) | 2018-09-06 | 2020-03-12 | Fmc Corporation | Fungicidal nitroanilino substituted pyrazoles |
| TWI819078B (en) * | 2018-09-06 | 2023-10-21 | 美商富曼西公司 | Fungicidal nitroanilino substituted pyrazoles |
| EP3847160B1 (en) * | 2018-09-06 | 2024-05-08 | FMC Corporation | Fungicidal nitroanilino substituted pyrazoles |
| CN112969688B (en) * | 2018-09-06 | 2024-06-04 | Fmc公司 | Fungicidal nitroaniline-substituted pyrazoles |
| EP4388876A2 (en) | 2018-09-06 | 2024-06-26 | FMC Corporation | Fungicidal nitroanilino substituted pyrazoles |
| WO2021183721A1 (en) | 2020-03-11 | 2021-09-16 | Fmc Corporation | Fungicidal mixtures containing pyrazole derivatives. |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10448639B2 (en) | Fungicidal pyrazoles | |
| EP2611297B1 (en) | Fungicidal pyrazoles | |
| WO2013126283A1 (en) | Fungicidal pyrazoles | |
| WO2012024586A1 (en) | Fungicidal pyrazoles | |
| EP2864293B1 (en) | Fungicidal 4-methylanilino pyrazoles | |
| EP2330891A1 (en) | Fungicidal pyridazines | |
| US20120135995A1 (en) | Fungicidal diphenyl-substituted pyridazines | |
| US20130143929A1 (en) | Fungicidal imidazoles | |
| WO2011051958A1 (en) | Fungicidal pyrazolones |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201080019806.5 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10707187 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 594181 Country of ref document: NZ Ref document number: 2010221440 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 5680/DELNP/2011 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2750862 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2010221440 Country of ref document: AU Date of ref document: 20100303 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010707187 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2011/008900 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13254205 Country of ref document: US Ref document number: 11112622 Country of ref document: CO Ref document number: 001568-2011 Country of ref document: PE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011002134 Country of ref document: CL Ref document number: 2011553063 Country of ref document: JP Ref document number: 12011501753 Country of ref document: PH |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12393 Country of ref document: GE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: a201109619 Country of ref document: UA |
|
| ENP | Entry into the national phase |
Ref document number: 20117023011 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2011139893 Country of ref document: RU Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 001543-2012 Country of ref document: PE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: P-2016/0051 Country of ref document: RS |
|
| ENP | Entry into the national phase |
Ref document number: PI1005812 Country of ref document: BR Kind code of ref document: A2 Effective date: 20110830 |