WO2010089764A2 - Improved process for the preparation of nebivolol hydrochloride - Google Patents
Improved process for the preparation of nebivolol hydrochloride Download PDFInfo
- Publication number
- WO2010089764A2 WO2010089764A2 PCT/IN2010/000004 IN2010000004W WO2010089764A2 WO 2010089764 A2 WO2010089764 A2 WO 2010089764A2 IN 2010000004 W IN2010000004 W IN 2010000004W WO 2010089764 A2 WO2010089764 A2 WO 2010089764A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- compound
- dihydro
- benzopyran
- acid
- Prior art date
Links
- OQJLGKBTBSSWAV-UHFFFAOYSA-N O=CC(CCc1c2)Oc1ccc2F Chemical compound O=CC(CCc1c2)Oc1ccc2F OQJLGKBTBSSWAV-UHFFFAOYSA-N 0.000 description 3
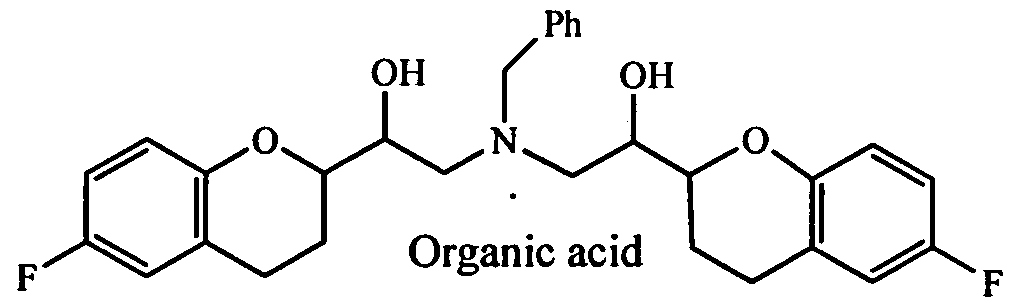
- QQLSKGJUQXHWHG-UHFFFAOYSA-N C[N](CC(C(CC1)Oc(cc2)c1cc2F)O)(CC(C(CCc1c2)Oc1ccc2F)O)Cc1ccccc1 Chemical compound C[N](CC(C(CC1)Oc(cc2)c1cc2F)O)(CC(C(CCc1c2)Oc1ccc2F)O)Cc1ccccc1 QQLSKGJUQXHWHG-UHFFFAOYSA-N 0.000 description 1
- STEPXTPIBUXRLE-UHFFFAOYSA-N OC(CN(CC(C(CC1)Oc(cc2)c1cc2F)O)Cc1ccccc1)C(CC1)Oc(cc2)c1cc2F Chemical compound OC(CN(CC(C(CC1)Oc(cc2)c1cc2F)O)Cc1ccccc1)C(CC1)Oc(cc2)c1cc2F STEPXTPIBUXRLE-UHFFFAOYSA-N 0.000 description 1
- HAIDNNYCHKHYHX-UHFFFAOYSA-N OCC(CCc1c2)Oc1ccc2F Chemical compound OCC(CCc1c2)Oc1ccc2F HAIDNNYCHKHYHX-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D311/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings
- C07D311/02—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D311/04—Benzo[b]pyrans, not hydrogenated in the carbocyclic ring
- C07D311/58—Benzo[b]pyrans, not hydrogenated in the carbocyclic ring other than with oxygen or sulphur atoms in position 2 or 4
Definitions
- the present invention relates to an improved process for the preparation of nebivolol and its pharmaceutically acceptable salts, especially the hydrochloride salt.
- the present invention also relates to organic acid salts of benzyl protected nebivolol and their polymorphic forms.
- Nebivolol hydrochloride is chemically known as ( ⁇ R, ⁇ 'R,2R,2'S)- re/- ⁇ , ⁇ '-[iminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanol] hydrochloride represented by the following structural formula- 1.
- Nebivolol is useful in the treatment and prevention of coronary vascular disorders Beta blockers are used in the treatment of high blood pressure, control of angina, arrhythmia, post myocardial infection, heart failure and migraine or essential tremor. Nebivolol is a highly selective beta blocker and has been found to be useful for the management of hyper tension. Nebivolol is a ⁇ l -adrenoceptor blocking drug, or ⁇ -blocker, distinguished from other members of its drug class by its additional nitric oxide (NO)-mediated vasodilatory effects.
- NO nitric oxide
- nebivolol may also slow or prevent some of the vascular complications associated with hypertension, by improving arterial compliance and reducing peripheral vascular resistance.
- Nebivolol, its pharmaceutically acceptable salts and process for their preparation was first disclosed in US 4654362.
- the disclosed process involves the esterification of 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid provides ethyl 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-carboxylate, which on reduction with bis(2- methylethoxy)aluminate in methyl benzene provides 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-methanol.
- Nebivolol is prepared by treating the 6-fluoro-3,4-dihydro-2-[[(phenylmethyl)amino]methyl]-2H-l- benzopyran-2-methanol with 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran and subsequent debenzylation, which is time consuming and lengthy process leading to increase in the cost of production.
- the present invention provides an improved and economical process for the preparation of nebivolol and its pharmaceutically acceptable salts without isolating 6-fluoro-3,4-dihydro-2-[[(phenylmethyl)amino]methyl]-2H-l-benzopyran-2-methanol and proceeds through crystalline oxalic acid salt of benzyl protected nebivolol, which improves the yield and purity and overcomes the all the prior art problems.
- the first aspect of the present invention is to provide an improved process for the preparation of nebivolol hydrochloride, which comprise of the following steps; a) Reacting 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2 with alcohol in presence of a suitable catalyst to provide alkyl 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with a suitable reducing agent in a suitable solvent to provide 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde compound of formula-5, which is treated in-situ with trimethylsulfoxonium iodide in the presence of a suitable base in a suitable solvent to provide 6-fluoro-3,4
- the second aspect of the present invention is to provide an improved process for the preparation of nebivolol hydrochloride, which comprise of the following steps; a) Reacting the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2 with alcohol in presence of a suitable catalyst to provide alkyl 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with a suitable reducing agent in a suitable solvent to provide 6-fluoro-3 ,4-dihydro-2H- 1 -benzopyran-2-methanol compound of formula-4, c) oxidizing the compound of formula-4 with sodium hypochlorite in presence of a suitable catalyst in a suitable solvent to provide 6-fluoro-3,4-dihydro-2
- the third aspect of the present invention is to provide novel organic acid salts of benzyl protected nebivolol compound of general formula-7.
- the fourth aspect of the present invention is to provide a crystalline form of oxalic acid salt of benzyl protected nebivolol.
- the crystalline oxalic acid salt of benzyl protected nebivolol of the present invention is characterized by its PXRD diffractogram.
- Figure-1 Illustrates the powder X-ray diffraction pattern of nebivolol hydrochloride compound of formula- 1.
- Figure-2 Illustrates the powder X-ray diffraction pattern of crystalline oxalic acid salt of benzyl protected nebivolol compound of formula- 7a.
- Figure-3 Illustrates the photograph of nebivolol hydrochloride obtained as per the process of the present invention as seen through the microscope.
- alkyl refers to Ci to C 4 alkyl, including methyl, ethyl, n-propyl, isopropyl, n-butyl and isobutyl.
- benzyl protected nebivolol refers to the compound which is chemically known as ( ⁇ R, ⁇ 'R,2R,2'S)-re/- ⁇ , ⁇ '-[benzyliminobis (methylene)]bis[6-fluoro-3 ,4-dihydro-2H- 1 -benzopyran-2-methanol] .
- the present invention relates to an improved process for the preparation of nebivolol and its pharmaceutically acceptable salts, especially hydrochloride salt compound of formula- 1.
- the first aspect of the present invention provides an improved process for the preparation of nebivolol hydrochloride compound of formula- 1,
- Formula-2 with suitable alcohol selected from methanol, ethanol, isopropanol and butanol, preferably methanol in presence of a suitable catalyst selected from sulfuric acid, hydrochloric acid, paratoluene sulfonic acid, preferably sulfuric acid to provide the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3,
- Formula-3 where in R is alkyl b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with suitable reducing agent like DIBAL-H, vitride, preferably DIBAL-H in a suitable hydrocarbon solvent selected from toluene, heptane and hexane, preferably toluene to provide the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- carboxaldehyde compound of formula-5,
- suitable reducing agent like DIBAL-H, vitride, preferably DIBAL-H in a suitable hydrocarbon solvent selected from toluene, heptane and hexane, preferably toluene
- Formula-5 which on in-situ treatment with trimethylsulfoxonium iodide in presence of a suitable base selected from sodium tertiary butoxide and potassium tertiary butoxide, preferably sodium tertiary butoxide in a suitable polar aprotic solvent like dimethyl sulfoxide, dimethyl acetamide and dimethylformamide, preferably dimethylsulfoxide to provide the 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6,
- a suitable catalyst like palladium-carbon in a suitable solvent like methanol, ethanol, isopropanol, preferably methanol followed by treating with hydrochloric acid in a suitable alcoholic solvent like methanol, ethanol and isopropan
- the second aspect of the present invention is to provide an improved process for the preparation of nebivolol hydrochloride compound of formula- 1, which comprises of the following steps; a) Reacting 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2
- Formula-2 with suitable alcohol like methanol, ethanol, isopropanol and butanol, preferably methanol in presence of a suitable catalyst selected from sulfuric acid, hydrochloric acid, paratoluene sulfonic acid, preferably sulfuric acid to provide the alkyl 6-fluoro- 3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3,
- a suitable catalyst selected from sulfuric acid, hydrochloric acid, paratoluene sulfonic acid, preferably sulfuric acid to provide the alkyl 6-fluoro- 3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3,
- a suitable solvent selected from chloro solvents like methylene chloride and chloroform; hydrocarbon solvents like toluene, heptane, hexane and cyclohexane, preferably chloro solvent like methylene chloride to provide the 6-fiuoro-3,4-dihydro- 2H-l-benzopyran-2-carbox
- 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanol compound of - * formula-4 also directly obtained from 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- ⁇ . carboxylic acid compound of formula-2, by reacting it with sodium borohydride and BF 3 - v etherate complex.
- the third aspect of the present invention provides novel organic acid salts of ⁇ * benzyl protected nebivolol compound of formula-7, with the proviso that the organic acid is not an oxalic acid.
- the organic acid is selected from tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid.
- the novel organic acid salt of benzyl protected nebivolol compound of the present invention is prepared by treating the benzyl protected nebivolol with a suitable acid like tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid in a suitable solvent selected from alcohol solvents like methanol, ethanol, 1-propanol, isopronaol; ester solvents like ethyl acetate, methyl acetate, isopropyl acetate; ether solvents like diisopropyl ether, diethyl ether, dimethyl ether and tetrahydrofuran; hydrocarbon solvents like toluene, heptane, hexane and cyclohexane or mixtures thereof.
- organic acid salts of benzyl protected nebivolol compound of general formula-7 of the present invention used as an intermediate or processing aid for the preparation of highly pure nebivolol or its pharmaceutically acceptable salts, especially hydrochloride compound of formula- 1.
- the fourth aspect of the present invention provides a crystalline form of oxalic acid salt of benzyl protected nebivolol compound of formula-7a having the following structure.
- the crystalline form of the present invention is characterized by its strong powder X-ray diffraction peaks (expressed in degrees 2 ⁇ ) at 6.9, 14.9, 15.2, 18.7, 20.7, 25.9, 28.9, 30.3, 37.0, 39.9 and 45.8 ⁇ 0.2 degrees 2 ⁇ .
- novel crystalline form of oxalic acid salt of benzylated nebivolol compound of formula-7a of the present invention useful in the preparation of highly pure nebivolol and its pharmaceutically acceptable salts.
- Highly pure nebivolol hydrochloride of the present invention refers to the compound with purity greater than 99.00%, preferably 99.50% by High performance Liquid Chromatography. Nebivolol hydrochloride of the present invention can be further micronized or milled to get the desired particle size.
- impurity A The following are structural formulae of the process related impurities (herein designated as impurity A, B, C, D and E) which are formed during the preparation of nebivolol hydrochloride.
- impurity-A The following are structural formulae of the process related impurities (herein designated as impurity A, B, C, D and E) which are formed during the preparation of nebivolol hydrochloride.
- nebivolol hydrochloride was analyzed by HPLC using the following conditions: Column: Hypersil BDS Cl 8, 250X 4.6 mm, 5 ⁇ m or equivalent; Flow rate: 1.0 ml/min; wavelength: 220 nm; Temperature: 25°C; Load: 20 ⁇ l; Run time: 50 min; and using acetonitrile: water (1 :1) as a diluent.
- the details of impurities and their RRT are as follows:
- XRD analysis of crystalline oxalic acid salt of benzyl protected nebivolol and nebivolol hydrochloride were carried out using SIEMENS/D-5000 X-Ray diffractometer using Cu, Ka radiation of wavelength 1.54 A° and continuous scan speed of 0.045°/min.
- Morphology of nebivolol hydrochloride was recorded in the following method: The samples are molded on alumina stubs using double adhesive tape, coated with gold using HUS-5GB vacuum evaporator and observed in Hitachi S-520 Scanning Electron Microscope at an acculation voltage of 10 KV.
- Example-1 Preparation of methyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- carboxylate:
- DIBAL 300 ml was added to a solution of methyl 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-carboxylate (50 gram) in toluene (250 ml) at -75 to -70 0 C and stirred for 3 hours.
- the reaction mixture was quenched with methanol at -75 to -7O 0 C and then acidified with aqueous hydrochloric acid.
- the organic and aqueous layers were separated at 25-35°C, aqueous layer extracted with toluene.
- the combined organic layer washed with aqueous acetic acid followed by sodium chloride solution and then dried with sodium sulphate.
- Example-4 Preparation of 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran: DIBAL (300 ml) was added to a solution of methyl 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-carboxylate (50 gram) in toluene (250 ml) at -75 to -70 0 C and stirred for 3 hours. The reaction mixture was quenched with methanol at -75 to -70 0 C and then acidified with aqueous hydrochloric acid. The organic and aqueous layers were separated at 25-35°C, aqueous layer extracted with toluene.
- Example-6 Preparation of oxalate salt of benzyl protected nebivolol: A mixture of 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran (50 g), benzyl amine (14 g) and methanol (300 ml) was heated to 65-70 0 C and stirred for 6 hours. The solvent from the reaction mixture was distilled off completely under reduced pressure at below 60 0 C. The obtained residue was cooled and dissolved in ethyl acetate. The reaction mixture was acidified with hydrochloric acid, stirred for 10 minutes then the organic and aqueous layers were separated.
- Benzyl protected nebivolol oxalate 50 g was dissolved in methanol (2.5 1) by heating to 50-55 0 C.
- Palladium carbon (5 g) in water was added to the above solution taken in hydrogenator.
- the hydrogen pressure 4.0 kg/cm 2 was applied and maintained for 3 hours at 25-30 0 C.
- the reaction mixture was filtered through hyflow and washed the bed with methanol.
- the methanol from the filtrate was distilled off at 65 -75 0 C under reduced pressure and then IPA hydrochloric acid (45 ml) was added to it and stirred for 1.5 hours at 65-70 0 C.
- the reaction mixture was cooled to 35-40 0 C and methanol was added to it.
- the reaction mixture was subjected to carbon treatment and filtered through hyflow.
- the isoproanol hydrochloric acid (5 ml) was added to the filtrate and stirred for 30 minutes at 60-65 0 C.
- the methanol was distilled off from the reaction mixture up to 70% under reduced pressure at 65-75°C.
- the reaction mixture was slowly cooled to 33 -35 0 C and stirred for 4 hours. The obtained solid was filtered, washed with methanol and dried to provide the title compound.
- Example-10 Preparation of 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanoI: Methanol (100 ml) was added to a mixture of methyl 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-carboxylate (100 g), sodium borohydride (17.6 g) in tetrahydrofuran (250 ml) and stirred for 3.5 hours. The reaction mixture was quenched with chilled water and the reaction mixture was extracted with ethyl acetate. The ethyl acetate layer was washed with sodium bicarbonate solution followed by sodium chloride solution. The solvent from the ethyl acetate layer was distilled off completely under reduced pressure at below 60 0 C to get the title compound. Yield: 85 grams
- Example-11 Preparation of 6-fluoro-3,4-dihydro-2-oxiranyI-2H-l-benzopyran: Sodium hypochlorite (220 ml) was slowly added to a mixture of 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-methanol (50 g), TEMPO (0.1 gram), potassium bromide (3.3 g) and methylene chloride (600 ml) at -10 to 0 0 C and stirred for 20 minutes. The reaction mixture was quenched with sodium thiosulphate solution. The layers were separated and the organic layer was washed with sodium bicarbonate, water and saturated sodium chloride solution respectively. The combined organic layer dried with sodium sulphate.
- Example-12 Preparation of maleic acid salt of benzyl protected nebivolol.
- Example-13 Preparation of salicylic acid salt of benzyl protected nebivolol:
- the salicylic acid salt of benzyl protected nebivolol has been prepared in an analogous manner to example- 12 using the salicylic acid (1.67 gram) in place of maleic acid.
- Example-14 Preparation of fumaric acid salt of benzyl protected nebivolol: . . . :.
- the fumaric acid salt of benzyl protected nebivolol has been prepared in an .*,- analogous manner to example- 12 using the fumaric acid (1.4 gram) in place of maleic - ⁇ ; acid. Yield: 1.9 grams; M.R: 123-126°C
- Example-15 Purification of nebivolol hydrochloride compound of formula-1: Nebivolol hydrochloride (10 grams) was dissolved in methanol (150 ml) by heating to 65-70 0 C and treated with carbon and stirred for 45 minutes at 65-70 0 C. The reaction mixture was filtered through the hyflow and washed the bed with methanol. The 70% of the solvent from the filtrate was distilled off and the reaction mixture was cooled to 30-35 0 C then stirred for 45 minutes. The solid obtained was filtered, washed with methanol and then dried to get high pure nebivolol hydrochloride.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
An improved process for the preparation of nebivolol and its pharmaceutically acceptable salts is disclosed Concretely, the process involves a) esterifying 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid to provide alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate to provide 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde, which is subsequently converted to 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran, c) reacting 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran with benzyl amine in a suitable solvent followed by treatment with suitable organic acid, and recrystalhzing the obtained compound to provide the corresponding organic acid salt of benzyl protected nebivolol, d) debenzylating the benzyl protected nebivolol salt, followed by treatment with hydrochloric acid to provide nebivolol hydrochloride
Description
Improved Process for the Preparation of Nebivolol Hydrochloride
Related Application:
This application claims the benefit of priority of our Indian patent application number 19/CHE/2009 filed on 5th January 2009, which is incorporated herein by reference.
Field of the Invention: The present invention relates to an improved process for the preparation of nebivolol and its pharmaceutically acceptable salts, especially the hydrochloride salt. The present invention also relates to organic acid salts of benzyl protected nebivolol and their polymorphic forms. Nebivolol hydrochloride is chemically known as (αR,α'R,2R,2'S)- re/-α,α'-[iminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanol] hydrochloride represented by the following structural formula- 1.
Formula- 1
Nebivolol is useful in the treatment and prevention of coronary vascular disorders Beta blockers are used in the treatment of high blood pressure, control of angina, arrhythmia, post myocardial infection, heart failure and migraine or essential tremor. Nebivolol is a highly selective beta blocker and has been found to be useful for the management of hyper tension. Nebivolol is a βl -adrenoceptor blocking drug, or β-blocker, distinguished from other members of its drug class by its additional nitric oxide (NO)-mediated vasodilatory effects. Consequently, it effectively lowers blood pressure by blocking βl -adrenoceptors in the heart and vasculature, nebivolol may also slow or prevent some of the vascular complications associated with hypertension, by improving arterial compliance and reducing peripheral vascular resistance.
Background of the Invention: Nebivolol, its pharmaceutically acceptable salts and process for their preparation was first disclosed in US 4654362. The disclosed process involves the esterification of
6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid provides ethyl 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-carboxylate, which on reduction with bis(2- methylethoxy)aluminate in methyl benzene provides 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-methanol. Thus obtained alcohol is reacted with oxalyl chloride and then with triethyl amine to provide the corresponding 6-fluoro-3,4-dihydro-2H-l-benzopyran- 2-carboxaldehyde. The 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde when treated with sodium hydride and then with trimethyl sulfoxonium iodide in dimethyl sulfoxide provides 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran. This on treatment with benzyl amine provides 3,4-dihydro-2-[[(phenylmethyl)amino]methyl]-2H-l- benzopyran-2-methanol, which on subsequent reaction with 6-fluoro-3,4-dihydro-2- oxiranyl-2H-l-benzopyran provides benzyl protected nebivolol. The benzyl protected nebivolol on deprotection with palladium carbon in methanol, followed by treatment with hydrochloric acid provides nebivolol hydrochloride. The disclosed process involves the usage of sodium hydride, which is commercially not recommendable.
The different processes for the preparation of nebivolol hydrochloride have been reported in EP 1803715, EP 1803716, WO 2006/025070, WO 2006/016376, WO 2007/009143, WO 2007/083318, WO 2008/040528 and WO 2008/064826 patent publications.
Ih general, all the reported processes involve the isolation of 6-fluoro-3,4- dihydro-2-[[(phenylmethyl)amino]methyl]-2H-l -benzopyran-2-methanol, by reacting the 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran with benzyl amine. Nebivolol is prepared by treating the 6-fluoro-3,4-dihydro-2-[[(phenylmethyl)amino]methyl]-2H-l- benzopyran-2-methanol with 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran and subsequent debenzylation, which is time consuming and lengthy process leading to increase in the cost of production.
The present invention provides an improved and economical process for the preparation of nebivolol and its pharmaceutically acceptable salts without isolating 6-fluoro-3,4-dihydro-2-[[(phenylmethyl)amino]methyl]-2H-l-benzopyran-2-methanol and proceeds through crystalline oxalic acid salt of benzyl protected nebivolol, which improves the yield and purity and overcomes the all the prior art problems.
Brief Description of the Invention:
The first aspect of the present invention is to provide an improved process for the preparation of nebivolol hydrochloride, which comprise of the following steps; a) Reacting 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2 with alcohol in presence of a suitable catalyst to provide alkyl 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with a suitable reducing agent in a suitable solvent to provide 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde compound of formula-5, which is treated in-situ with trimethylsulfoxonium iodide in the presence of a suitable base in a suitable solvent to provide 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l- benzopyran compound of formula-6, c) reacting the 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6 with benzyl amine in a suitable solvent followed by treatment with suitable organic acid, and recrystallization of the obtained compound in a suitable solvent to provide the corresponding organic acid salt of benzyl protected nebivolol compound of general formula-7, d) debenzylating the benzyl protected nebivolol salt compound of general formula-7 using hydrogen in presence of a suitable catalyst in a suitable solvent, followed by treatment with hydrochloric acid in a suitable solvent to provide nebivolol hydrochloride compound of formula- 1.
The second aspect of the present invention is to provide an improved process for the preparation of nebivolol hydrochloride, which comprise of the following steps; a) Reacting the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2 with alcohol in presence of a suitable catalyst to provide alkyl 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with a suitable reducing agent in a suitable solvent to provide 6-fluoro-3 ,4-dihydro-2H- 1 -benzopyran-2-methanol compound of formula-4,
c) oxidizing the compound of formula-4 with sodium hypochlorite in presence of a suitable catalyst in a suitable solvent to provide 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-carboxaldehyde compound of formula-5, d) converting the obtained 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde compound of formula-5 into nebivolol hydrochloride.
The third aspect of the present invention is to provide novel organic acid salts of benzyl protected nebivolol compound of general formula-7.
The fourth aspect of the present invention is to provide a crystalline form of oxalic acid salt of benzyl protected nebivolol. The crystalline oxalic acid salt of benzyl protected nebivolol of the present invention is characterized by its PXRD diffractogram.
Brief Description of the Drawings: Figure-1: Illustrates the powder X-ray diffraction pattern of nebivolol hydrochloride compound of formula- 1.
Figure-2: Illustrates the powder X-ray diffraction pattern of crystalline oxalic acid salt of benzyl protected nebivolol compound of formula- 7a.
Figure-3: Illustrates the photograph of nebivolol hydrochloride obtained as per the process of the present invention as seen through the microscope.
Detailed Description of the Invention:
As used herein, the term "alkyl" refers to Ci to C4 alkyl, including methyl, ethyl, n-propyl, isopropyl, n-butyl and isobutyl.
As used herein, the term "benzyl protected nebivolol" refers to the compound which is chemically known as (αR,α'R,2R,2'S)-re/-α,α'-[benzyliminobis (methylene)]bis[6-fluoro-3 ,4-dihydro-2H- 1 -benzopyran-2-methanol] .
The present invention relates to an improved process for the preparation of nebivolol and its pharmaceutically acceptable salts, especially hydrochloride salt compound of formula- 1.
The first aspect of the present invention provides an improved process for the preparation of nebivolol hydrochloride compound of formula- 1,
Formula- 1
Which comprise of the following steps, a) Reacting the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2
Formula-2 with suitable alcohol selected from methanol, ethanol, isopropanol and butanol, preferably methanol in presence of a suitable catalyst selected from sulfuric acid, hydrochloric acid, paratoluene sulfonic acid, preferably sulfuric acid to provide the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3,
Formula-3 where in R is alkyl, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with suitable reducing agent like DIBAL-H, vitride, preferably DIBAL-H in a suitable hydrocarbon solvent selected from toluene, heptane and hexane, preferably toluene to provide the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- carboxaldehyde compound of formula-5,
Formula-5
which on in-situ treatment with trimethylsulfoxonium iodide in presence of a suitable base selected from sodium tertiary butoxide and potassium tertiary butoxide, preferably sodium tertiary butoxide in a suitable polar aprotic solvent like dimethyl sulfoxide, dimethyl acetamide and dimethylformamide, preferably dimethylsulfoxide to provide the 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6,
Formula-6 c) reacting the 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6 with benzyl amine in a suitable solvent selected from alcohol solvents methanol, ethanol and isopropanol and ester solvents like ethyl acetate, isopropyl acetate solvent, preferably alcohol solvents like methanol followed by treatment with suitable organic acids like oxalic acid, tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid, and recrystallization of the obtained compound in a suitable solvent selected from ether solvents like diisopropyl ether and diethyl; nitrile solvents like acetonitrile and hydrocarbon solvents like toluene, heptane, hexane and cyclohexane to provide the corresponding organic acid salt of benzyl protected nebivolol compound of general formula-7,
Formula-7 d) debenzylating the benzyl protected nebivolol salt compound of general formula-7 using hydrogen in the presence of a suitable catalyst like palladium-carbon in a suitable solvent like methanol, ethanol, isopropanol, preferably methanol followed by treating with hydrochloric acid in a suitable alcoholic solvent like methanol, ethanol and isopropanol or in a ester solvent like ethyl acetate; preferably isopropanol, and the recrystallization of the obtained compound in a suitable alcohol solvents selected
from methanol, ethanol, isopropanol or mixtures thereof, preferably methanol to provide the pure nebivolol hydrochloride compound of formula- 1.
The second aspect of the present invention is to provide an improved process for the preparation of nebivolol hydrochloride compound of formula- 1, which comprises of the following steps; a) Reacting 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2
Formula-2 with suitable alcohol like methanol, ethanol, isopropanol and butanol, preferably methanol in presence of a suitable catalyst selected from sulfuric acid, hydrochloric acid, paratoluene sulfonic acid, preferably sulfuric acid to provide the alkyl 6-fluoro- 3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3,
Formula-3 wherein R is alkyl, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with a suitable reducing agent selected from DIBAL-H, sodium borohydride and the like, preferably sodium borohydride in a suitable solvent selected from alcohol solvents like methanol, ethanol, isopropanol or ether solvents like tetrahydrofuran or mixtures thereof to provide the 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-methanol compound of formula-4,
Formula-4 c) oxidizing the compound of formula-4 with sodium hypochlorite in the presence of a suitable catalyst like 2,2,6,6-tetramethyl piperidinyl oxy free radical (TEMPO)/KBr
in a suitable solvent selected from chloro solvents like methylene chloride and chloroform; hydrocarbon solvents like toluene, heptane, hexane and cyclohexane, preferably chloro solvent like methylene chloride to provide the 6-fiuoro-3,4-dihydro- 2H-l-benzopyran-2-carboxaldehyde compound of formula-5,
Formula-5 d) converting the obtained 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde compound of formula-5 into nebivolol hydrochloride.
The 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde compound of formula-5 obtained by the above process converted into the nebivolol hydrochloride by the process described in the first aspect of the invention or by the process known in the art.
Further, the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanol compound of -* formula-4 also directly obtained from 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- ^. carboxylic acid compound of formula-2, by reacting it with sodium borohydride and BF3- v etherate complex.
The third aspect of the present invention provides novel organic acid salts of ~* benzyl protected nebivolol compound of formula-7, with the proviso that the organic acid is not an oxalic acid.
Formula-7 The organic acid is selected from tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid.
The novel organic acid salt of benzyl protected nebivolol compound of the present invention is prepared by treating the benzyl protected nebivolol with a suitable acid like tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid in a suitable solvent selected from alcohol solvents like methanol, ethanol, 1-propanol, isopronaol; ester solvents like ethyl acetate, methyl acetate, isopropyl acetate; ether solvents like diisopropyl ether, diethyl ether, dimethyl ether and tetrahydrofuran; hydrocarbon solvents like toluene, heptane, hexane and cyclohexane or mixtures thereof.
The organic acid salts of benzyl protected nebivolol compound of general formula-7 of the present invention used as an intermediate or processing aid for the preparation of highly pure nebivolol or its pharmaceutically acceptable salts, especially hydrochloride compound of formula- 1.
The fourth aspect of the present invention provides a crystalline form of oxalic acid salt of benzyl protected nebivolol compound of formula-7a having the following structure.
Formula-7a
The crystalline form of the present invention is characterized by its strong powder X-ray diffraction peaks (expressed in degrees 2Θ) at 6.9, 14.9, 15.2, 18.7, 20.7, 25.9, 28.9, 30.3, 37.0, 39.9 and 45.8 ±0.2 degrees 2Θ.
The novel crystalline form of oxalic acid salt of benzylated nebivolol compound of formula-7a of the present invention useful in the preparation of highly pure nebivolol and its pharmaceutically acceptable salts.
Highly pure nebivolol hydrochloride of the present invention refers to the compound with purity greater than 99.00%, preferably 99.50% by High performance
Liquid Chromatography. Nebivolol hydrochloride of the present invention can be further micronized or milled to get the desired particle size.
The following are structural formulae of the process related impurities (herein designated as impurity A, B, C, D and E) which are formed during the preparation of nebivolol hydrochloride. Impurity-A:
Impurity-B:
Impurity-D:
Impurity-E:
The related substance of nebivolol hydrochloride was analyzed by HPLC using the following conditions: Column: Hypersil BDS Cl 8, 250X 4.6 mm, 5 μm or equivalent; Flow rate: 1.0 ml/min; wavelength: 220 nm; Temperature: 25°C; Load: 20 μl; Run time: 50 min; and using acetonitrile: water (1 :1) as a diluent.
The details of impurities and their RRT are as follows:
XRD analysis of crystalline oxalic acid salt of benzyl protected nebivolol and nebivolol hydrochloride were carried out using SIEMENS/D-5000 X-Ray diffractometer using Cu, Ka radiation of wavelength 1.54 A° and continuous scan speed of 0.045°/min.
Morphology of nebivolol hydrochloride was recorded in the following method: The samples are molded on alumina stubs using double adhesive tape, coated with gold using HUS-5GB vacuum evaporator and observed in Hitachi S-520 Scanning Electron Microscope at an acculation voltage of 10 KV.
The present invention is schematically represented as below
Formula-3 Formula-4
DIBAL-H NaOCl
Formula-5
Formula-6
Benzyl amine Methanol Organic acid
Pd/C Methanol IPA HCl
The process described in the present invention was demonstrated in examples illustrated below. These examples are provided as illustration only and therefore should not be construed as limitation of the scope of the invention. Examples: Example-1: Preparation of methyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- carboxylate:
Mixture of 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid (100 g), methanol (1000 ml) and sulfuric acid (1.0 ml) was heated to reflux for 3 hours, then distilled off the solvent completely under reduced pressure. Water (400 ml) was added to the obtained residue and basified with sodiumbicaronate solution. The reaction mixture was extracted into methylene chloride. The methylene chloride was distilled off from the reaction mixture under reduced pressure at below 450C to get the title compound as a semi solid. Example-2: Preparation of methyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- carboxylate:
Mixture of 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid (50 g), *'•■! methanol (500 ml) and sulfuric acid (0.5 ml) was heated to refluxed for 3 hours, then *ϋ distilled off the solvent completely under reduced pressure. Water (200 ml) was added to <*'.;; the obtained residue and basified with sodiumbicaronate solution and the reaction -ha mixture was stirred for 30 minutes at 25-3O0C. The obtained solid was filtered off, ■&■? washed with water and then dried to get the title compound as a solid. Yield: 42 grams; M.R: 39-43°C Example-3: Preparation of 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran:
DIBAL (300 ml) was added to a solution of methyl 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-carboxylate (50 gram) in toluene (250 ml) at -75 to -700C and stirred for 3 hours. The reaction mixture was quenched with methanol at -75 to -7O0C and then acidified with aqueous hydrochloric acid. The organic and aqueous layers were separated at 25-35°C, aqueous layer extracted with toluene. The combined organic layer washed with aqueous acetic acid followed by sodium chloride solution and then dried with sodium sulphate. A suspension of trimethylsulfoxonium iodide (63 g) and sodium tertiary
butoxide (26 g) in dimethyl sulfoxide (250 ml) was stirred for 1.5 hours at 7-15°C and to this mixture, added the combined organic layer obtained above. The reaction mixture was stirred for 3 hours at 25-300C and then quenched with ice water. The layers were separated and aqueous layer was extracted with ethyl acetate. The combined organic layer washed with water followed by sodium chloride solution, then dried with sodium sulphate. Distilled off the solvent from the organic layer to get the title compound. Yield: 35 grams
Example-4: Preparation of 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran: DIBAL (300 ml) was added to a solution of methyl 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-carboxylate (50 gram) in toluene (250 ml) at -75 to -700C and stirred for 3 hours. The reaction mixture was quenched with methanol at -75 to -700C and then acidified with aqueous hydrochloric acid. The organic and aqueous layers were separated at 25-35°C, aqueous layer extracted with toluene. The combined organic layer washed with aqueous acetic acid followed by sodium chloride solution and then dried with sodium sulphate. A suspension of trimethylsulfoxonium iodide (63 g) and sodium tertiary butoxide (26 g) in dimethyl sulfoxide (250 ml) was stirred for 1.5 hours at 7-15°C and to this mixture, added the combined organic layer obtained above. The reaction mixture was stirred for 3 hours at 25-300C and then quenched with ice water. The layers were separated and aqueous layer was extracted with ethyl acetate. The combined organic layer washed with water followed by sodium chloride solution, then dried with sodium sulphate. Distilled off the solvent completely from the organic layer under reduced pressure and the obtained residue was subjected to high vacuum distillation to collect the title compound. Yield: 29 grams
Example-5: Preparation of oxalate salt of benzyl protected nebivolol:
A mixture of 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran (50 g), benzyl amine (14 g) and methanol (300 ml) was heated to 65-700C and stirred for 6 hours. The solvent from the reaction mixture was distilled off completely under reduced pressure at below 600C. The obtained residue was cooled and dissolved in ethyl acetate. The reaction mixture was acidified with hydrochloric acid, stirred for 10 minutes then the organic and
aqueous layers were separated. The organic layer washed with water and then oxalic acid (65 g) was added to it, stirred for 60 minutes at 25-30°C. The solvent was distilled off from the reaction mixture under reduced pressure at below 600C and the obtained residue dissolved in acetonitrile at 30-35°C and stirred. Diisopropyl ether (100 ml) was added to the reaction mixture and stirred for 3 hours. The solid obtained was filtered, washed with acetonitrile and finally recrystallised from acetonitrile to get the title compound. Yield: 30 grams
Example-6: Preparation of oxalate salt of benzyl protected nebivolol: A mixture of 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran (50 g), benzyl amine (14 g) and methanol (300 ml) was heated to 65-700C and stirred for 6 hours. The solvent from the reaction mixture was distilled off completely under reduced pressure at below 600C. The obtained residue was cooled and dissolved in ethyl acetate. The reaction mixture was acidified with hydrochloric acid, stirred for 10 minutes then the organic and aqueous layers were separated. The organic layer washed with water and then oxalic acid (65 g) was added to it, stirred for 60 minutes at 25-300C. The solvent was distilled off from the reaction mixture under reduced pressure at below 600C, the obtained residue was dissolved in acetonitrile at 30-350C and stirred for 3 hours. The solid obtained was filtered, washed with acetonitrile and finally recrystallised from acetonitrile to get the title compound. Yield: 29 grams
Example-7: Preparation of oxalate salt of benzyl protected nebivolol:
A mixture of 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran (50 g), benzyl amine (14 g) and methanol (300 ml) was heated to 65-700C and stirred for 6 hours. The solvent from the reaction mixture was distilled off completely under reduced pressure at below 600C. The obtained residue was cooled and dissolved in ethyl acetate. The reaction mixture was acidified with hydrochloric acid, stirred for 10 minutes then the organic and aqueous layers were separated. The organic layer washed with water and then oxalic acid (65 g) was added to it, stirred for 60 minutes at 25-300C. The solvent was distilled off from the reaction mixture under reduced pressure at below 600C and the obtained residue dissolved in acetonitrile at 30-350C and stirred. Cyclohexane (100 ml) was added to the
reaction mixture and stirred for 3 hours. The solid obtained was filtered, washed with acetonitrile and finally recrystallised from acetonitrile to get the title compound. Yield: 28.6 grams Exmaple-8: Preparation of nebivolol hydrochloride:
Benzyl protected nebivolol oxalate (50 g) was dissolved in methanol (2.5 1) by heating to 50-550C. Palladium carbon (5 g) in water was added to the above solution taken in hydrogenator. The hydrogen pressure 4.0 kg/cm2 was applied and maintained for 3 hours at 25-300C. After the completion of the reaction, the reaction mixture was filtered through hyflow and washed the bed with methanol. The methanol from the filtrate was distilled off at 65 -750C under reduced pressure and then IPA hydrochloric acid (45 ml) was added to it and stirred for 1.5 hours at 65-700C. The reaction mixture was cooled to 35-400C and methanol was added to it. The reaction mixture was subjected to carbon treatment and filtered through hyflow. The isoproanol hydrochloric acid (5 ml) was added to the filtrate and stirred for 30 minutes at 60-650C. The methanol was distilled off from the reaction mixture up to 70% under reduced pressure at 65-75°C. The reaction mixture was slowly cooled to 33 -350C and stirred for 4 hours. The obtained solid was filtered, washed with methanol and dried to provide the title compound.
Yield: 20 grams
Example-9: Purification of nebivolol hydrochloride:
A mixture of nebivolol hydrochloride (11 g) and methanol (44 ml) was heated to 65-7O0C and stirred for 45 minutes. The reaction mixture was cooled to 30-350C and stirred for 45 minutes. The solid was filtered and washed with methanol. The obtained solid was dissolved in methanol (150 ml) by heating to 65-700C and treated with carbon and stirred for 45 minutes at 65-700C. The reaction mixture was filtered through the hyflow and washed the bed with methanol. The 70% of the solvent from the filtrate was distilled off and the reaction mixture was cooled to 30-350C then stirred for 45 minutes. The solid obtained was filtered, washed with methanol and then dried to get high pure nebivolol hydrochloride.
Yield: 5.5 grams; Particle size Distribution: D (0.1): 16 μm ; D (0.5): 87 μm; D (0.9): 210 μm; D[4,3]: 375 μm
Purity by HPLC: 99.90 %; Impurity A: Not detected; Impurity B: Not detected; Impurity C: 0.04 %; Impurity D: 0.03%; Impurity E: Not detected
Example-10: Preparation of 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanoI: Methanol (100 ml) was added to a mixture of methyl 6-fluoro-3,4-dihydro-2H-l- benzopyran-2-carboxylate (100 g), sodium borohydride (17.6 g) in tetrahydrofuran (250 ml) and stirred for 3.5 hours. The reaction mixture was quenched with chilled water and the reaction mixture was extracted with ethyl acetate. The ethyl acetate layer was washed with sodium bicarbonate solution followed by sodium chloride solution. The solvent from the ethyl acetate layer was distilled off completely under reduced pressure at below 600C to get the title compound. Yield: 85 grams
Example-11: Preparation of 6-fluoro-3,4-dihydro-2-oxiranyI-2H-l-benzopyran: Sodium hypochlorite (220 ml) was slowly added to a mixture of 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-methanol (50 g), TEMPO (0.1 gram), potassium bromide (3.3 g) and methylene chloride (600 ml) at -10 to 00C and stirred for 20 minutes. The reaction mixture was quenched with sodium thiosulphate solution. The layers were separated and the organic layer was washed with sodium bicarbonate, water and saturated sodium chloride solution respectively. The combined organic layer dried with sodium sulphate. A suspension of trimethyl sulfoxonium iodide (72.5 g) and sodium tertiary butoxide (30.5 g) in dimethyl sulfoxide (250 ml) was stirred for 1.5 hours at 7-15°C and to this mixture, added the combined organic layer obtained above. The reaction mixture was stirred for 3 hours at 25-3O0C and then quenched with ice water. The layers were separated and aqueous layer was extracted with ethyl acetate. The combined organic layers washed with water followed by sodium chloride solution, then dried with sodium sulphate. Distilled off the solvent completely from the organic layer to get the title compound
Yield: 31 grams
Example-12: Preparation of maleic acid salt of benzyl protected nebivolol.
A mixture of 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran (4 g), benzyl amine (1.12 g) and methanol (24 ml) was heated to 65-700C and stirred for 6 hours. The
solvent from the reaction mixture was distilled off completely under reduced pressure at below 600C. The obtained residue was cooled and dissolved in ethyl acetate. The reaction mixture was acidified with hydrochloric acid, stirred for 10 minutes then the organic and aqueous layers were separated. The organic layer washed with water and then maleic acid (1.4 g) was added to it and stirred for 12 hours at 25-300C. The solvent was distilled off from the reaction mixture, acetonitrile was added to the residue and stirred for 3 hours at 25-300C. Diisopropylether (25 ml) was added and stirred for 2 hours at 25-300C. The obtained solid filtered and washed with diisopropyl ether and dried to get the title compound. Yield: 2 grams; M.R: 157-163°C
Example-13: Preparation of salicylic acid salt of benzyl protected nebivolol:
The salicylic acid salt of benzyl protected nebivolol has been prepared in an analogous manner to example- 12 using the salicylic acid (1.67 gram) in place of maleic acid.
Yield: 3 grams; M.R: 105-1090C
Example-14: Preparation of fumaric acid salt of benzyl protected nebivolol: ... :.
The fumaric acid salt of benzyl protected nebivolol has been prepared in an .*,- analogous manner to example- 12 using the fumaric acid (1.4 gram) in place of maleic - ■; acid. Yield: 1.9 grams; M.R: 123-126°C
Example-15: Purification of nebivolol hydrochloride compound of formula-1: Nebivolol hydrochloride (10 grams) was dissolved in methanol (150 ml) by heating to 65-700C and treated with carbon and stirred for 45 minutes at 65-700C. The reaction mixture was filtered through the hyflow and washed the bed with methanol. The 70% of the solvent from the filtrate was distilled off and the reaction mixture was cooled to 30-350C then stirred for 45 minutes. The solid obtained was filtered, washed with methanol and then dried to get high pure nebivolol hydrochloride. Yield: 5.2 grams; Purity by HPLC: 99.91% Particle Size Distribution: D (0.1): 0.156 μm ; D (0.5): 20.05 μm; D (0.9): 93.82 μm; D[4,3]: 36.91 μm
Claims
1. An improved process for the preparation of nebivolol hydrochloride compound of formula- 1,
Formula- 1
Which comprise of the following steps, a) Reacting the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound offormula-2
Formula-2 with suitable alcohol in presence of a suitable catalyst to provide the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3,
Formula-3 wherein R is C1-C4 alkyl, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with suitable reducing agent in a suitable solvent to provide the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde compound of formula-5,
Formula-5 which on in-situ treatment with trimethylsulfoxonium iodide in presence of a suitable base in a suitable polar aprotic solvent to provide the 6-fIuoro-3,4- dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6,
Formula-6 c) reacting the 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6 with benzyl amine in a suitable solvent followed by subsequent treatment with suitable organic acid, and recrystallisation of the obtained compound in a suitable solvent to provide the corresponding organic acid salt compound of general formula-7,
Formula-7 d) debenzylating the benzyl protected nebivolol salt compound of general formula-7 using hydrogen in the presence of a suitable catalyst in a suitable solvent followed by treating with hydrochloric acid in a suitable solvent, and recrystallisation of the obtained compound in a suitable solvent to provide nebivolol hydrochloride compound of formula- 1.
2. A process according to claim 1, wherein i) in step a) the alcohol is selected from methanol, ethanol, isopropanol and butanol; catalyst is selected from sulfuric acid, hydrochloric acid and paratoluene sulfonic acid; ii) in step b) reducing agent is selected from DIBAL-H and vitride; solvent is selected from hydrocarbon solvents like toluene, heptane and hexane; polar aprotic solvents like dimethylsulfoxide, dimethylformamide and dimethyl acetamide; and base is selected from sodium tertiary butoxide and potassium tertiary butoxide; iii) in step c) solvent is selected from alcoholic solvents like methanol, ethanol and isopropanol, ester solvent like ethyl acetate, isopropyl acetate; and organic acid is selected from oxalic acid, tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid; and the solvent for recrystallisation is selected from ether solvent like diisopropyl ether; nitrile solvent like acetonitrile; hydrocarbon solvents like toluene, heptane, hexane and cyclohexane or mixtures thereof; iv) in step d) hydrogenation catalyst is selected from palladium-carbon and solvent is selected from alcohol solvents like methanol, ethanol, isopropanol and butanol; ester solvents like ethyl acetate and isopropyl acetate or mixtures thereof.
3. An improved process for the preparation of nebivolol hydrochloride compound of formula- 1, which comprise of the following steps, a) reacting the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2
Formula-2 with methanol in presence of sulfuric acid to provide methyl 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-carboxylate compound of formula-3a,
Formula-3a b) reducing the methyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of formula-3a with DIBAL-H in toluene to provide the 6-fluoro-3,4- dihydro-2H-l-benzopyran-2-carboxaldehyde compound of formula-5,
Formula-5 which on in-situ treatment with trimethylsulfoxonium iodide in presence of a sodium tertiary butoxide in dimethylsulfoxide to provide the 6-fluoro-3,4- dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6,
Formula-6 c) reacting the 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6 with benzyl amine in methanol followed by subsequent treatment with oxalic acid in ethylacetate, and recrystallisation of the obtained compound in a acetonitrile or diisopropyl ether or mixtures thereof to provide the oxalic acid salt of benzyl protected nebivolol compound of formula-7a,
Formula-7a d) debenzylating the benzyl protected nebivolol oxalate compound of formula-7a using hydrogen in the presence of palladium/carbon in methanol followed by treating with hydrochloric acid in isopropyl alcohol, and recrystallisation of the obtained compound from methanol provides the pure nebivolol hydrochloride compound of formula- 1.
4. An improved process for the preparation of nebivolol hydrochloride compound of formula- 1, which comprise of the following steps; a) reacting the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound offormula-2
Formula-2 with suitable alcohol in presence of a suitable catalyst to provide the alkyl 6- fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3,
Formula-3 wherein R is C1-C4 alkyl, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with a suitable reducing agent in a suitable solvent to provide the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanol compound of formula-4,
Formula-4 c) oxidizing the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanol compound of formula-4 with sodium hypochlorite in the presence of a suitable catalyst in a suitable solvent to provide the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- carboxaldehyde compound of formula-5,
Formula-5 which on in-situ treatment with trimethylsulfoxonium iodide in presence of a suitable base and in a suitable polar aprotic solvent to provide the 6-fluoro-3,4- dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6,
Formula-6 d) reacting the 6-fluoro-3,4-dihydro-2-oxiranyl-2H-l-benzopyran compound of formula-6 with benzyl amine in a suitable solvent followed by subsequent treatment with suitable organic acid, and recrystallisation of the obtained compound in a suitable solvent to provide the corresponding organic acid salt of benzyl protected nebivolol compound of general formula-7,
Formula-7 e) debenzylating the benzyl protected nebivolol salt compound of general formula-7 using hydrogen in the presence of a suitable catalyst in a suitable solvent followed by treating with hydrochloric acid in a suitable solvent, recrystallisation of the obtained compound in a suitable solvent to provide nebivolol hydrochloride compound of formula- 1.
5. A process according to claim 4, wherein i) in step a) the alcohol is selected from methanol, ethanol, isopropanol and butanol; catalyst is selected from sulfuric acid, hydrochloric acid, paratoluene sulfonic acid; ii) in step b) reducing agent is selected from DIBAL-H and sodium borohydride; solvent is selected from alcohols solvents like methanol, ethanol, isopropanol or tetrahydrofuran; iii) in step c) the catalyst is 2,2,6,6-tetramethyl piperidinyl oxy free radical
(TEMPO)/KBr; suitable solvent is selected from chloro solvent like methylene chloride and chloro form; hydrocarbon solvents like toluene, heptane, hexane and cyclohexane; and the base used is selected from sodium tertiary butoxide, potassium tertiary butoxide; and polar aprotic solvent is selected from dimethyl sulfoxide, dimethylformamide and dimethyl acetamide; iv) in step d) solvent is selected from alcohol solvents like methanol, ethanol and isopropanol, ester solvents like ethyl acetate, isopropyl acetate solvent; and organic acid is selected from oxalic acid, tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid; v) in step e) hydrogenation catalyst is selected from palladium-carbon and solvent is selected from alcohol solvents like methanol, ethanol, isopropanol and butanol; ester solvents like ethyl acetate and isopropyl acetate or mixtures thereof.
6. A process for the preparation of 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- carboxaldehyde compound of formula-5, which comprise of the following steps; a) reacting the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylic acid compound of formula-2 with suitable alcohol in presence of a suitable catalyst to provide the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3, b) reducing the alkyl 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxylate compound of general formula-3 with a suitable reducing agent in a suitable solvent to provide the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanol compound of formula-4, c) oxidizing the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2 -methanol compound-^of formula-4 with sodium hypochlorite in the presence of a suitable catalyst like 2,2,6,6-tetramethyl piperidinyloxy free radical (TEMPO)/KBr in a suitable solvent to provide the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-carboxaldehyde compound of formula-5.
7. Organic acid salts of benzyl protected nebivolol compound of general formula-7 having the following structure
Formula-7 with the proviso that the organic acid is not an oxalic acid.
8. The organic acid salts according to claim 7, is selected from tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid.
9. Use of organic acid salts of benzyl protected nebivolol compound of general formula- 7 as claimed in claim 7, as an intermediate or processing aid to prepare the highly pure nebivolol or its pharmaceutically acceptable salts.
10. A process for the preparation of novel organic acid salt of benzyl protected nebivolol compound of general formula-7,
Formula-7 which comprise of treating the benzyl protected nebivolol with a suitable acid in suitable solvent selected from alcohol solvents like methanol, ethanol, 1-propanol, isopronaol; ester solvents like ethyl acetate, methyl acetate, isopropyl acetate; ether solvents like diisopropyl ether, diethyl ether, dimethyl ether and tetrahydrofuran; hydrocarbon solvents like toluene, heptane, hexane and cyclohexane or mixtures thereof to provide the corresponding organic acid salt of benzyl protected nebivolol compound of formula-7.
11. A process according to claim 10, wherein the acid is selected from tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid
12. A process for the preparation of highly pure nebivolol hydrochloride compound of formula- 1, which comprise of preparing organic acid salt of benzyl protected nebivolol as per the process of claim 10 and converting it into nebivolol hydrochloride.
13. Nebivolol hydrochloride containing any of the impurities A, B, C, D and E, less than 0.1 area-% by HPLC; Wherein the said nebivolol hydrochloride obtained by the process, which comprises of debenzylating the benzyl protected nebivolol salt compound of general formula-7
Formula-7 wherein the organic acid is selected from tartaric acid, maleic acid, fumaric acid, salicylic acid and malic acid, using hydrogen in the presence of palladium-carbon in a suitable solvent selected from alcohol solvents like methanol, ethanol, isopropanol followed by treating with hydrochloric acid in a suitable alcoholic solvent like methanol, ethanol, n-propanol and isopropanol or ester solvent like ethyl acetate to provide nebivolol hydrochloride.
14. Crystalline form of oxalic acid salt of benzyl protected nebivolol having the following structural formula
15. Use of crystalline oxalic acid salt of benzyl protected nebivolol as claimed in claim 14, as an intermediate or processing aid in the preparation of highly pure nebivolol or its pharmaceutically acceptable salts.
16. A process for the preparation of 6-fluoro-3,4-dihydro-2H-l-benzopyran-2- carboxaldehyde compound of formula-5
Formula-5 which comprises of oxidizing the 6-fluoro-3,4-dihydro-2H-l-benzopyran-2-methanol compound of formula-4
Formula-4 with sodium hypochlorite in the presence of 2,2,6,6-tetramethyl piperidinyloxy free radical (TEMPO)/KBr in a suitable solvent selected from chloro solvents like methylene chloride or hydrocarbon solvent like toluene.
********
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN19/CHE/2009 | 2009-01-05 | ||
| IN19CH2009 | 2009-01-05 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2010089764A2 true WO2010089764A2 (en) | 2010-08-12 |
| WO2010089764A3 WO2010089764A3 (en) | 2010-11-04 |
Family
ID=42542465
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2010/000004 WO2010089764A2 (en) | 2009-01-05 | 2010-01-05 | Improved process for the preparation of nebivolol hydrochloride |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2010089764A2 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102329291A (en) * | 2011-07-27 | 2012-01-25 | 上海现代制药股份有限公司 | Method for preparing 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-formaldehyde |
| WO2013018053A1 (en) | 2011-08-02 | 2013-02-07 | Menarini International Operations Luxembourg S.A. | Process for the preparation of epoxides as intermediates for the synthesis of nebivolol |
| EP2907809A1 (en) | 2014-02-14 | 2015-08-19 | Corden Pharma International GmbH | Base-free process for the preparation of ketone intermediates usable for manufacture of nebivolol |
| EP2907810A1 (en) | 2014-02-14 | 2015-08-19 | Corden Pharma International GmbH | A new method for producing nebivolol hydrochloride of high purity |
| DE102014107132A1 (en) | 2014-05-20 | 2015-11-26 | Corden Pharma International Gmbh | Process for the preparation of epoxides which can be used in the preparation of nebivolol and its derivatives |
| WO2016185492A1 (en) * | 2015-05-19 | 2016-11-24 | Ipca Laboratories Limited | Process for preparation of nebivolol and it's salts |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4654362A (en) * | 1983-12-05 | 1987-03-31 | Janssen Pharmaceutica, N.V. | Derivatives of 2,2'-iminobisethanol |
| WO2004041805A1 (en) * | 2002-11-06 | 2004-05-21 | EGIS Gyógyszergyár Rt. | NEW PROCESS FOR THE PREPARATION OF RACEMIC ([2S[2R*[R[R*]]]] and ([2R[2S*[S[S*]]]]-(±)- α,α' -[imino-bis(methylene)]bis[6-fluorochroman-2-methanol] AND ITS PURE [2S[2R*[R[R*]& |
| WO2006016376A1 (en) * | 2004-08-11 | 2006-02-16 | Hetero Drugs Limited | A novel process for preparation of nebivolol intermediates |
| WO2006025070A2 (en) * | 2004-07-30 | 2006-03-09 | Torrent Pharmaceuticals Limited | Nebivolol and its pharmaceutically acceptable salts, process for preparation and pharmaceutical compositions of nebivolol |
-
2010
- 2010-01-05 WO PCT/IN2010/000004 patent/WO2010089764A2/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4654362A (en) * | 1983-12-05 | 1987-03-31 | Janssen Pharmaceutica, N.V. | Derivatives of 2,2'-iminobisethanol |
| WO2004041805A1 (en) * | 2002-11-06 | 2004-05-21 | EGIS Gyógyszergyár Rt. | NEW PROCESS FOR THE PREPARATION OF RACEMIC ([2S[2R*[R[R*]]]] and ([2R[2S*[S[S*]]]]-(±)- α,α' -[imino-bis(methylene)]bis[6-fluorochroman-2-methanol] AND ITS PURE [2S[2R*[R[R*]& |
| WO2006025070A2 (en) * | 2004-07-30 | 2006-03-09 | Torrent Pharmaceuticals Limited | Nebivolol and its pharmaceutically acceptable salts, process for preparation and pharmaceutical compositions of nebivolol |
| WO2006016376A1 (en) * | 2004-08-11 | 2006-02-16 | Hetero Drugs Limited | A novel process for preparation of nebivolol intermediates |
Non-Patent Citations (1)
| Title |
|---|
| DATABASE CASREACT 06 November 2009 Database accession no. 152:525671 * |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102329291A (en) * | 2011-07-27 | 2012-01-25 | 上海现代制药股份有限公司 | Method for preparing 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-formaldehyde |
| WO2013018053A1 (en) | 2011-08-02 | 2013-02-07 | Menarini International Operations Luxembourg S.A. | Process for the preparation of epoxides as intermediates for the synthesis of nebivolol |
| EP2907809A1 (en) | 2014-02-14 | 2015-08-19 | Corden Pharma International GmbH | Base-free process for the preparation of ketone intermediates usable for manufacture of nebivolol |
| EP2907810A1 (en) | 2014-02-14 | 2015-08-19 | Corden Pharma International GmbH | A new method for producing nebivolol hydrochloride of high purity |
| DE102014107132A1 (en) | 2014-05-20 | 2015-11-26 | Corden Pharma International Gmbh | Process for the preparation of epoxides which can be used in the preparation of nebivolol and its derivatives |
| WO2016185492A1 (en) * | 2015-05-19 | 2016-11-24 | Ipca Laboratories Limited | Process for preparation of nebivolol and it's salts |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010089764A3 (en) | 2010-11-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2010089764A2 (en) | Improved process for the preparation of nebivolol hydrochloride | |
| CN112047888B (en) | Method for synthesizing enzalutamide | |
| JP2003513974A (en) | Method for producing imidazolidinone αv-integrin antagonist and intermediate for production | |
| WO2011021223A2 (en) | Novel salts of ethyl (3r, 4s, 5r)-4,5-imino-3-(l-ethylpropoxy)-1- cvclohexene-1-carboxylate and its use | |
| MXPA03003459A (en) | Crystalline venlafaxine base and novel polymorphs of venlafaxine hydrochloride, processes for preparing thereof. | |
| WO2010113183A2 (en) | Process for the preparation of 1-[[[3-[2-(dimethylamino)ethyl]-1h-indol-5-yl]methyl]sulfonyl] pyrrolidine and its pharmaceutically acceptable salts | |
| US8378106B2 (en) | Method for preparing argatroban monohydrate and a process for its synthesis | |
| CA2629720A1 (en) | Synthesis and preparations of intermediates and polymorphs thereof useful for the preparation of donepezil hydrochloride | |
| WO2019008520A1 (en) | A process for preparing alectinib or a pharmaceutically acceptable salt thereof | |
| CN114933580A (en) | Process for the preparation of caronic anhydride | |
| CA2351528C (en) | Process for the preparation of a piperazine derivative | |
| EP2094693B1 (en) | A method for the preparation of solifenacin | |
| US20100305328A1 (en) | Process for preparation of piperidine carboxylic acid | |
| CN114149360B (en) | Preparation method of high-purity nitrendipine bulk drug | |
| AU2001278094A1 (en) | Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1-piperindinyl]-1-hydroxybutyl]-alpha, alpha-dimethylbenzene acetic acid and its hydrochloride | |
| WO2002102777A2 (en) | NOVEL CRYSTALLINE FORMS OF 4-[4-[4-(HYDROXYDIPHENYLMETHYL)-1-PIPERINDINYL]-1-HYDROXYBUTYL]-α, α-DIMETHYLBENZENE ACETIC ACID AND ITS HYDROCHLORIDE | |
| BE897000A (en) | NOVEL 1,4-DIHYDROPYRIDINE DERIVATIVES, THEIR PREPARATION AND THEIR USE AS MEDICAMENTS | |
| US20070254959A1 (en) | Process for Obtaining Tolterodine | |
| EP2053043A1 (en) | Crystalline salt of montelukast | |
| WO2005023769A1 (en) | Process for the preparation of amlodipine salts | |
| EP1907377A1 (en) | A process for the preparation of almotriptan | |
| EP2072510A1 (en) | Crystalline form of azelastine | |
| NO330042B1 (en) | Process for the preparation of acid salts of gemifloxacin and intermediate for the preparation of the same. | |
| KR100469030B1 (en) | Synthesis of cisapride | |
| CN115785067A (en) | Preparation method of impurity compound of esomeprazole thioether |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10738282 Country of ref document: EP Kind code of ref document: A2 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10738282 Country of ref document: EP Kind code of ref document: A2 |