WO2010029134A1 - Toughened cyanoacrylate compositions - Google Patents
Toughened cyanoacrylate compositions Download PDFInfo
- Publication number
- WO2010029134A1 WO2010029134A1 PCT/EP2009/061770 EP2009061770W WO2010029134A1 WO 2010029134 A1 WO2010029134 A1 WO 2010029134A1 EP 2009061770 W EP2009061770 W EP 2009061770W WO 2010029134 A1 WO2010029134 A1 WO 2010029134A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cyanoacrylate
- composition
- polyethylene
- cyanoacrylates
- polyvinyl acetate
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J4/00—Adhesives based on organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond ; adhesives, based on monomers of macromolecular compounds of groups C09J183/00 - C09J183/16
- C09J4/06—Organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond in combination with a macromolecular compound other than an unsaturated polymer of groups C09J159/00 - C09J187/00
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/30—Nitriles
- C08F222/32—Alpha-cyano-acrylic acid; Esters thereof
- C08F222/322—Alpha-cyano-acrylic acid ethyl ester, e.g. ethyl-2-cyanoacrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/30—Nitriles
- C08F222/32—Alpha-cyano-acrylic acid; Esters thereof
- C08F222/327—Alpha-cyano-acrylic acid alkoxy ester
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F210/00—Copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond
- C08F210/02—Ethene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/04—Anhydrides, e.g. cyclic anhydrides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
- C08L23/08—Copolymers of ethene
- C08L23/0846—Copolymers of ethene with unsaturated hydrocarbons containing other atoms than carbon or hydrogen atoms
- C08L23/0853—Vinylacetate
Definitions
- This invention relates to toughened cyanoacrylate compositions which exhibit improved peel strengths and fixture speeds.
- the compositions according to the invention are suitable for use as adhesives for quick bonding of a variety of substrates.
- Cyanoacrylate compositions are well known in the art as excellent adhesives. In particular they are well known as one-component reactive adhesives, quick bonding and suitable for a wide variety of substrates. However, one of the main drawbacks of these compositions is that they tend to be brittle following cure and have low peel strengths. A variety of additives and fillers have been proposed for addition to cyanoacrylate adhesive compositions to improve toughness and peel strengths.
- U.S. Patent No. 4,102,945 (Gleave) describes a cyanoacrylate adhesive having enhanced peel strengths in which a cyanoacrylate is thickened by a copolymer or terpolymer including vinylidene chloride-acrylonitrile copolymers.
- U. S . Patent No . 4,440,910 (O ' Connor) is directed to cyanoacrylate compositions having improved toughness, achieved through the addition of elastomers, i.e., acrylic rubbers.
- elastomers i.e., acrylic rubbers.
- These rubbers are either (i) homopolymers of alkyl esters of acrylic acid; (ii) copolymers of another polymerizable monomer, such as lower alkenes, with an alkyl ester of acrylic acid or with an alkoxy ester of acrylic acid; (iii) copolymers of alkyl esters of acrylic acid; (iv) copolymers of alkoxy esters of acrylic acid; and (v) mixtures thereof.
- U.S. Patent 4,444,933 suggests the addition of a vinyl chloride/vinyl acetate copolymer to a cyanoacrylate adhesive to reduce adhesion to human skin.
- U.S. Patent No. 4,560,723 discloses a cyanoacrylate adhesive composition containing a toughening agent comprising a core-shell polymer and a sustainer comprising an organic compound containing one or more unsubstituted or substituted aryl groups. The sustainer is reported to improve retention of toughness after heat aging of cured bonds of the adhesive.
- U.S. Patent No. 4,713,405 discloses an ⁇ -cyanoacrylate adhesive composition of matter consisting essentially of ⁇ -cyanoacrylate, fumed silica having a surface treated with a dimethyldichlorosilane, and trialkyl borate.
- U.S. Patent No. 5,340,873 discloses a cyanoacrylate adhesive composition having improved toughness by including an effective toughening amount of a polyester polymer derived from a dibasic aliphatic or aromatic carboxylic acid and a glycol.
- U.S. Patent No. 5,739,205 discloses an ⁇ -cyanoacrylate adhesive composition which comprises (a) 100 parts by weight of an ⁇ -cyanoacrylate compound, (b) 10 through 20 parts by weight of (I) polyalkyl methacrylates having a weight average molecular weight of 100,000 through 300,000, or (II) copolymers of alkyl methacrylates and other methacrylates or acrylates, said copolymers having the same weight average molecular weight as that of the polyalkyl methacrylates (I), (c) 2 through 20 parts by weight of ultrafme anhydrous silicas, and (d) 0.001 through 20 parts by weight of certain quick curing additives, (b)-(d) being on the basis of (a) 100 parts by weight of ⁇ -cyanoacrylate compounds.
- U.S. Patent No. 5,994,464 discloses a cyanoacrylate adhesive composition containing a cyanoacrylate monomer, an elastomer miscible or compatible with the cyanoacrylate monomer, and a core-shell polymer being compatible, but not miscible, with the cyanoacrylate monomer.
- U.S. Patent No. 6,475,331 discloses a cyanoacrylate adhesive composition
- a cyanoacrylate adhesive composition comprising: (a) a cyanoacrylate component; and (b) an accelerator component consisting essentially of (i) calixarenes, oxacalixarenes, or a combination thereof, and (ii) at least one crown ether, wherein said composition exhibits a fixturing speed of less than 20 seconds for bonding two substrates, at least one of which is constructed of a material selected from steel, epoxy glass, and balsawood.
- elastomeric polymers as toughening agents in cyanoacrylate adhesive compositions is known.
- One group of particularly suitable elastomeric polymers is the group comprising copolymers of methyl aery late and ethylene, manufactured by DuPont, under the name of VAMAC, such as VAMAC N123 AND VAMAC B-124.
- VAMAC N123 and VAMAC B- 124 are reported by DuPont to be a master batch of ethylene/acrylic elastomer.
- Henkel Corporation (as successor to Loctite Corporation) has sold for a number of years rubber toughened cyanoacrylate adhesive products under the trade name BLACK MAX, which employ as the rubber toughening component the DuPont materials called VAMAC B-124 and N 123.
- Henkel has sold in the past clear and substantially colorless rubber toughened cyanoacrylate adhesive products, namely LOCTITE 4203, 4204, and 4205, which employ as the rubber toughening component the DuPont material, VAMAC G. While VAMAC G contains no fillers to provide color or stabilizers, it does contain processing aids.
- processing aids, or release systems are reported to be ARMEEN 18D and stearic acid in combination with GAFAC RL-210 (or with VANFRE UN, or SERVOXYL VPAZ-100).
- polyethylene glycol ether wax is also used as a processing aid. Waxes such as this interfere with the physical properties of cyanoacrylate compositions.
- processing aids used in the manufacture of the VAMAC type elastomers are detrimental to adhesion and therefore give poor performance.
- VAMAC VCS rubber appears to be the base rubber, from which the remaining members of the VAMAC product line are compounded.
- VAMAC VCS is a reaction product of the combination of ethylene, methyl acrylate and monomers having carboxylic acid cure sites, which once formed is then substantially free of processing aids such as the release agents octadecyl amine, complex organic phosphate esters and/or stearic acid, and anti-oxidants, such as substituted diphenyl amine.
- processing aids such as the release agents octadecyl amine, complex organic phosphate esters and/or stearic acid, and anti-oxidants, such as substituted diphenyl amine.
- VAMAC VMX 1012 and VCD 6200 rubbers which are made from ethylene and methyl acrylate. It is believed that the VAMAC VMX 1012 rubber possesses little or no carboxylic acid in the polymer backbone. Like the VAMAC VCS rubber, the VAMAC VMX 1012 and VCD 6200 rubbers are substantially free of processing aids such as the release agents octadecyl amine, complex organic phosphate esters and/or stearic acid, and anti-oxidants, such as substituted diphenyl amine, noted above.
- processing aids such as the release agents octadecyl amine, complex organic phosphate esters and/or stearic acid, and anti-oxidants, such as substituted diphenyl amine, noted above.
- the present invention is thus directed to an alternative cyanoacrylate adhesive composition which provides improved performance and toughness compared to known toughened cyanoacrylate compositions.
- the invention provides a cyanoacrylate composition comprising:
- a toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate.
- the composition according to the invention has been found to demonstrate increased performance over known cyanoacrylate compositions.
- the present invention is directed to a cyanoacrylate composition which demonstrates substantially enhanced toughness and has an improved peel strength and fixturing speed compared to known toughened cyanoacrylate compositions.
- the toughening agent comprises a co-polymer of polyethylene and polyvinyl acetate.
- Agents which are particularly suitable for use in accordance with the present invention are those agents comprising co-polymers of polyethylene and polyvinyl acetate which are sold under the trade name LEVAMELT by Lanxess
- a range of LEVAMELT agents is available and includes for example,
- LEVAMELT 400 represents an ethylene- vinyl acetate copolymer comprising 40 wt% vinyl acetate.
- the LEVAMELT products are supplied in granular form. The granules are almost colourless and dusted with silica and talc. The product may also be supplied in bales of 25 kg under the trade name
- toughening agents comprising a copolymer of polyethylene and polyvinyl acetate permits the use of other monomers such as methyl cyanoacrylate, ethyl-2-cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates (such as n-butyl-2- cyanoacrylate), octyl cyanoacrylates, allyl cyanoacrylate, ⁇ -methoxy ethyl cyanoacrylate, propargyl cyanoacrylates and combinations thereof.
- monomers such as methyl cyanoacrylate, ethyl-2-cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates (such as n-butyl-2- cyanoacrylate), octyl cyanoacrylates, allyl cyanoacrylate, ⁇ -methoxy ethyl cyanoacrylate, propargyl cyanoacrylates and combinations thereof.
- the monomers can be toughened by varying the ratios of polyethylene and polyvinyl acetate in the elastomer. Suitable elastomers can be selected for use in toughening a particular monomer. Elastomers comprising copolymers comprising different ratios of polyethylene and polyvinyl acetate may be used to toughen different monomers.
- a particularly preferred toughening agent for use in accordance with the present invention comprises a copolymer of polyethylene and polyvinyl acetate wherein the vinyl acetate is present in an amount of 90 wt%.
- compositions according to the invention provide increased performance compared to known toughened cyanoacrylate compositions.
- cyanoacrylate compositions according to the present invention show increased toughness, measured for instance as an increased peel strength.
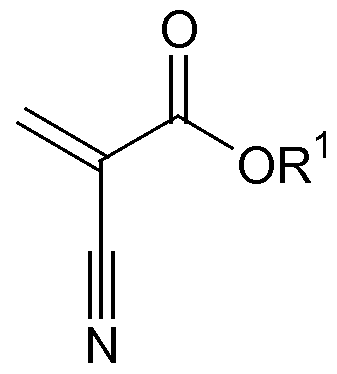
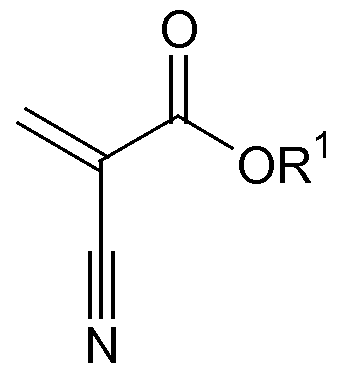
- the cyanoacrylate component suitably comprises a monomeric structure represented by:
- R 1 is C 1-15 alkyl, C 2-15 alkoxyalkyl, C 3-15 cycloalkyl, C 2-15 alkenyl, C 6-15 aryl, C 7-15 aralkyl, C 3 - I s allyl, C 1-15 alkylhalide, or C 1-15 haloalkyl and mixtures thereof.
- the cyanoacrylate component may be selected from the group consisting of methyl cyanoacrylate, ethyl-2-cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates, octyl cyanoacrylates, allyl cyanoacrylates, alkoxyalkyl cyanoacryalates
- the cyanoacrylate component comprises ethyl-2-cyanoacrylate.
- composition values are given in weight percent unless otherwise noted.
- the co-polymer of polyethylene and polyvinyl acetate is present in an amount of 1 to about 20% by weight.
- the co-polymer of polyethylene and polyvinyl acetate is present in an amount of 5 to about 10% by weight.
- the present invention is directed to a cyanoacrylate composition which demonstrates enhanced toughness including a cyanoacrylate material; a toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate and one or more additives selected from plasticizers, accelerators, fillers, opacif ⁇ ers, inhibitors, thixotrophy conferring agents, stabilizers, dyes, thermal degradation reducers, adhesion promoters, shock resistance conferring agents and combinations thereof, where upon cure, the cyanoacrylate composition has an average T peel strength on mild steel of more than about 3 N/mm after curing at room temperature for about 72 hours and a fixture speed on mild steel of less than 360 seconds.
- the present invention is directed to a method of bonding two or more substrates including the steps of providing at least two substrates; dispensing, on at least a portion of a surface of one or both of the at least two substrates, a cyanoacrylate composition including about 1 to about 20% by weight of the toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate; contacting the surfaces of the at least two substrates having the cyanoacrylate composition therebetween; and curing the cyanoacrylate composition.
- the present invention is directed to a bonded assembly including: a first substrate having a first surface; another substrate having a second surface; and a cured cyanoacrylate composition disposed between the first and second surfaces, the composition having included prior to cure a cyanoacrylate component; and a toughening agent comprising about 1 to about 20% by weight of the toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate.
- the T peel strength on mild steel is greater than about 3 N/mm after room temperature cure for about 72 hours, and a fixture speed on mild steel of less than 360 seconds.
- the invention is directed to reaction products of the inventive compositions.
- the invention is directed to a method of preparing the inventive compositions.
- the invention is directed to a method of conferring one or more of the following properties to rubber toughened cyanoacrylate compositions, improved shelf life, fixture speed, improved shear strength development over time and improved side impact strength and fracture toughness, which includes the method of providing a cyanoacrylate component and a toughening agent component comprising a co-polymer of polyethylene and polyvinyl acetate and mixing together said components.
- Fig. 1 depicts a plot of the effect of LEVAMELT 900 (copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate) on T peel strength for each of the monomers ⁇ -methoxyethyl cyanoacrylate and ethyl cyanoacrylate.
- LEVAMELT 900 copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate
- Fig. 2 depicts a plot of the effect of LEVAMELT 900 (copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate) on T peel strength for the monomer ⁇ -methoxyethyl cyanoacrylate over a period of 3 days at room temperature and a period of 3 days at room temperature and 1 day at 80 °C.
- LEVAMELT 900 copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate
- Fig. 3 depicts a plot of the effect of LEVAMELT 900 (copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate) on T peel strength for the monomer ethyl cyanoacrylate over a period of 3 days at room temperature and a period of 3 days at room temperature and 1 day at 80 °C.
- LEVAMELT 900 copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate
- the cyanoacrylate compositions of the present invention comprise toughening agents which provide enhanced toughness, such as improved peel strengths in the cured compositions.
- the preferred toughening agent comprises an elastomeric co-polymer of polyethylene and polyvinyl acetate.
- the toughening agents which are preferably used in the compositions according to the invention comprise LEVAMELT elastomers which are available from Lanxess. It will be appreciated that other suitable copolymers of polyethylene and polyvinyl acetate could also be used.
- LEVAMELT elastomers are high performance elastomers.
- Various grades of LEVAMELT elastomers are available from Lanxess. They dissolve more readily than other tougheners currently used in the art, for example, VAMAC. They are readily available in both monomers and perform better than VAMAC in ethyl cyanoacrylate.
- LEVAMELT consists of methylene units forming a saturated main chain with pendant acetate groups. The presence of a fully saturated main chain is an indication that LEVAMELT is a particularly stable polymer. It does not contain any reactive double bonds which make conventional rubbers prone to aging reactions, ozone and UV light. The saturated backbone makes it robust.
- the LEVAMELT elastomers are available without any of the processing aids that are used for the most of the VAMAC elastomers.
- these elastomers facilitate the use of other monomers such as methyl cyanoacrylate, ethyl-2- cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates, octyl cyanoacrylates, allyl cyanoacrylates, ⁇ -methoxyethyl cyanoacrylate, propargyl cyanoacrylate and mixtures thereof.
- the solubilities of these materials change in different monomers and also the ability to toughen changes as a result of the solubility.
- the LEVAMELT elastomers are available in pellet form and are easier to formulate than other known elastomeric toughening agents. Furthermore, these elastomers are less expensive than other known elastomers. Thus these elastomers allow for the formulation of a more cost-effective toughened cyanoacrylate composition which provides better performance compared to known toughened cyanoacrylate compositions.
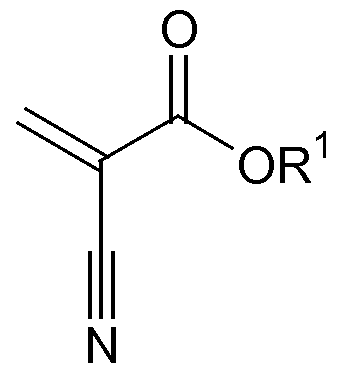
- the cyanoacrylate compositions of the present invention include a cyanoacrylate component which includes cyanoacrylate monomers, such as those represented by the structure:
- R 1 is selected from R 1 is C 1-15 alkyl, C 2-15 alkoxyalkyl, C 3-15 cycloalkyl, C 2-15 alkenyl, C 6-15 aryl, C 7 -I 5 aralkyl, C 3 -I 5 allyl, C 1-15 alkylhalide, or C 1-15 haloalkyl and mixtures thereof.
- the cyanoacrylate monomer is selected from methyl cyanoacrylate, ethyl-2-cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates (such as n-butyl-2-cyanoacrylate), octyl cyanoacrylates, allyl cyanoacrylate, ⁇ -methoxyethyl cyanoacrylate, propargyl cyanoacrylate and combinations thereof.
- a particularly desirable cyanoacrylate monomer is ethyl-2-cyanoacrylate.
- the cyanoacrylate component should be included in the compositions in an amount within the range of about 70% by weight to about 95% by weight, preferably about 75% by weight to about 90% by weight.
- compositions of the present invention may also contain additives such as stabilizers, accelerators, plasticizers, fillers, opacif ⁇ ers, inhibitors, thixotrophy conferring agents, dyes, fluorescence markers, thermal degradation reducers, adhesion promoters, thermal resistance conferring agents and combinations thereof, and the like.
- Cured cyanoacrylate compositions are typically rigid, brittle materials, having low crack resistance and therefore low T peel strength. Additives may be included in order to modify these properties. These additives are known to those of skill in the art.
- the compositions according to the invention may further comprise free radical inhibitors, such as hydroquinones or MMBP (2,2'-methylenebis(6-tert-butyl-4- methylphenol).
- the inhibitor when present, is preferably present in an amount of about 0.001% by weight to about 2.0% by weight, preferably 0.02% to about 0.5% by weight.
- Accelerators that may be useful in the cyanoacrylate compositions include for example calixarenes, oxacalixarenes, and combinations thereof. Of the calixarenes and oxacalixarenes, many are known, and are reported in the patent literature. See, e.g. U.S. Patent Nos. 4,556,700, 4,622,414, 4,636,539, 4,695,615, 4,718,966, and 4,855,461, the disclosures of each of which are hereby expressly incorporated herein by reference.
- crown ether Another potentially useful accelerator component is a crown ether.
- a host of crown ethers are known.
- examples which may be used herein either individually or in combination, or in combination with the calixarenes and oxacalixarenes described above include 15-crown-5, 18-crown-6, dibenzo-18-crown-6, benzo-15-crown-5, dibenzo-24-crown-8, dibenzo-30-crown-lO, tribenzo-18-crown-6, asym-dibenzo-22-crown-6, dibenzo- 14-crown-4, dicyclohexyl- 18-crown-6, dicyclohexyl-24-crown-8, cyclohexyl-12-crown-4, l,2-decalyl-15-crown-5, 1,2- naphtho-15-crown-5, 3,4,5-naphthyl-16-crown-5, l,2-methyl-benzo-18-crown
- R 3 and R 4 are organo groups which do not themselves cause polymerization of the cyanoacrylate monomer, R 5 is H or CH 3 and n is an integer of between 1 and 4.
- suitable R 3 and R 4 groups are C 1-15 alkyl groups, C 1-15 alkoxy groups such as methoxy, and C 1-15 aryloxy groups such as phenoxy.
- the R 3 and R 4 groups may contain halogen or other substituents, an example being trifluoropropyl.
- groups not suitable as R 4 and R 3 groups are basic groups such as amino, substituted amino and alkylamino.
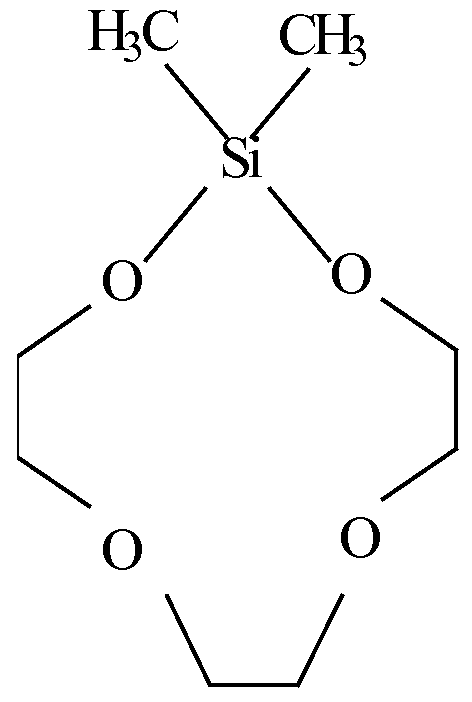
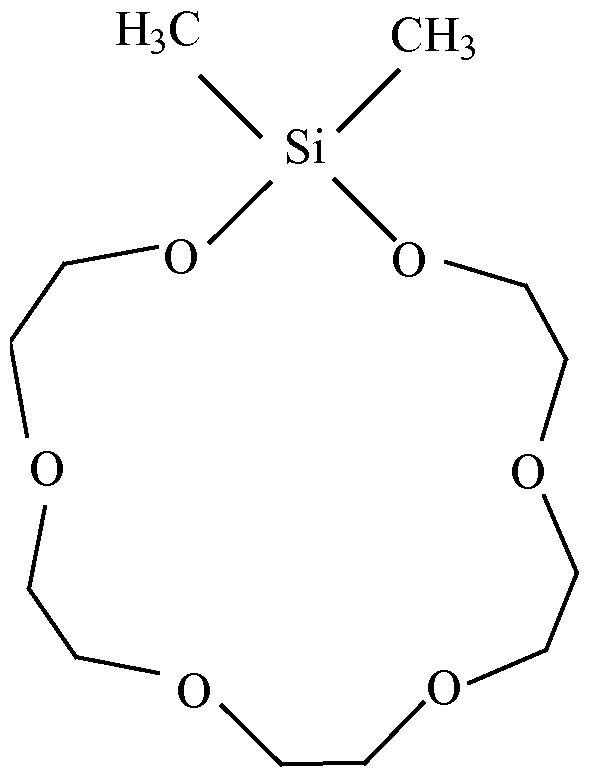
- silacrown compounds useful in the inventive compositions include:
- accelerators and accelerator packages (i.e., the combination of two or more accelerators) may be found in U.S. Patent Nos. 6,294,629 — an accelerator component including (i) calixarenes, oxacalixarenes, silacrowns, cyclodextrins or a combination thereof, and (ii) poly(ethyleneglycol) di(meth)acrylates, ethoxylated hydric compounds, and combinations thereof --, 6,475,331— an accelerator component including (i) calixarenes, oxacalixarenes, or a combination thereof, and (ii) at least one crown ether — , and 6,835,789 — chemical class embraced by
- R is a member selected from the group consisting of hydrogen, C 1-15 alkyl, C 1-15 alkyloxy, C 2-15 alkyl thioethers, C 1-15 haloalkyl, C 1-15 carboxylic acid and esters thereof, Co-i 5 sulfmic, C 0-15 sulfonic and C 0-15 sulfurous acids and esters thereof, C 0-15 phosphinic, Co-i 5 phosphonic and C 0-15 phosphorous acids and esters thereof, Z is a single or double bond, n is 1-12, p is 1-3, R' is the same as R, and g is the same as n; such as
- the accelerator component used in accordance with the present invention comprises 18- crown-6.
- the accelerator component should be included in the compositions in an amount within the range of from about 0.01% to about 10% by weight, with the range of about 0.05% to about 2% by weight being desirable, and about 0.1% to about 1% by weight of the total composition being particularly desirable.
- Itaconic anhydride may also be added to the cyanoacrylate component to further aid in durability and impact, heat, and moisture resistance. Itaconic anhydride when present in the composition increases adhesion to mild steel.
- Plasticizers may also be added to the cyanoacrylate component, and when so added are preferably present in an amount of about 10% by weight to about 50% by weight, more preferably about 10% by weight to about 25% by weight of the total composition.
- inventive compositions may also be rendered thixotropic by the addition of thixotrophy conferring agents, such as fumed silica. See U.S. Patent Nos.
- 4,533,422 (Litke) and 4,477,607 (Litke). These agents, when used, should be used in an amount less than about 15% by weight, such as within the range of about 0.5% by weight to about 10% by weight of the total composition.
- cyanoacrylate component The combination of the cyanoacrylate monomer and the additives will be referred to herein as the cyanoacrylate component.
- Thermal resistance conferring agents such as citric acid, are also desirable for use herein.
- compositions according to the invention may be readily prepared by adding predetermined amounts of the toughening agent components to the cyanoacrylate component and stirring or agitating for a sufficient time at an appropriate temperature to achieve a homogenous solution or suspension.
- temperatures much above room temperature are not necessary, though in formulations containing high levels of fillers, thickeners, and the like, moderate heating may be desirable to speed dissolution of the various additives.
- the improved toughness of the cured compositions of the invention is manifested through various physical properties, e.g. 180° peel strength, impact strength and tensile shear strength. These strengths are useful properties of an adhesive bond, being parameters of what is loosely referred to as the bond strength. Referring for simplicity to the procedures of American Standard Test Methods, peel strength is determined in accordance with ASTM No. D 903-49; impact strength is determined in accordance with ASTM No. D-950; and tensile shear strength is determined in accordance with ASTM No. D- 1002. The reader is referred to these standards for a full description of the tests.
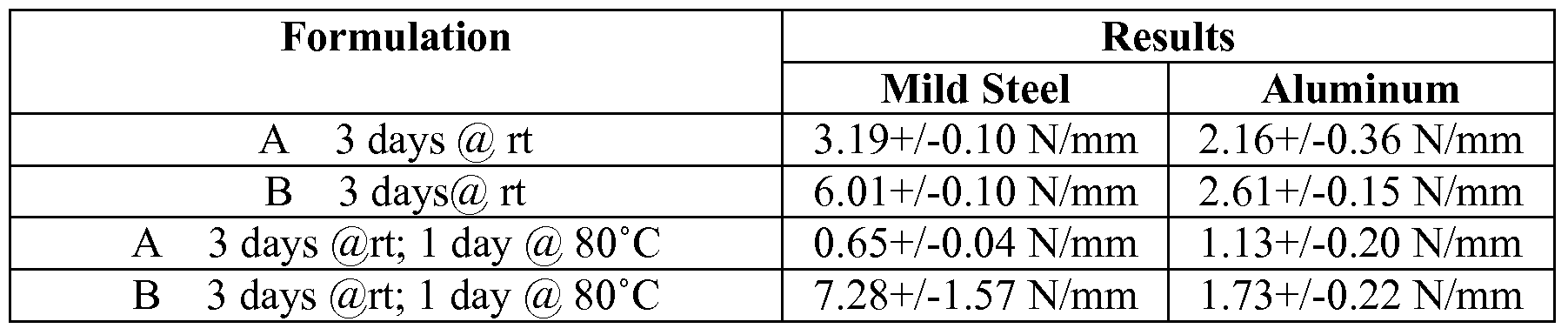
- Formulations A and B were prepared with methane sulfonic acid and
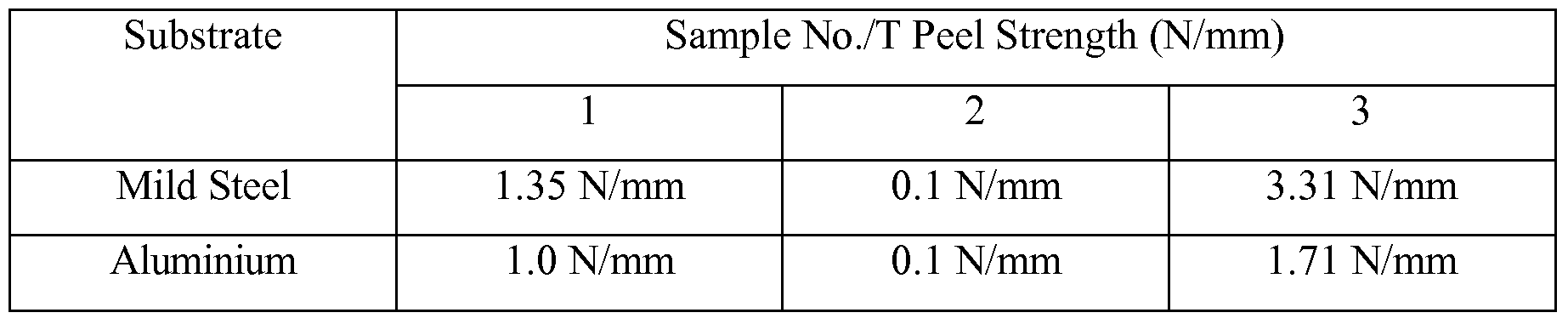
- the substrates used in determining T peel strength in Table 4 are mild steel bonded to mild steel and aluminium bonded to aluminum.
- Non-toughened cyanoacrylate compositions that are evaluated in adhesive applications have low T peel strengths, typically from about 0.2 -0.4 N/mm. It has been found that the addition of a copolymer of polyethylene and polyvinyl acetate to a cyanoacrylate monomer increases the T peel strength of the cured cyanoacrylate composition.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Adhesives Or Adhesive Processes (AREA)
Abstract
This invention relates to toughened cyanoacrylate compositions which exhibit improved peel strengths and fixture speeds. The toughened cyanoacrylate compositions described include a toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate.
Description
TOUGHENED CYANOACRYLATE COMPOSITIONS
BACKGROUND OF THE INVENTION Field of the Invention
[0001] This invention relates to toughened cyanoacrylate compositions which exhibit improved peel strengths and fixture speeds. The compositions according to the invention are suitable for use as adhesives for quick bonding of a variety of substrates.
Brief Description Of Related Technology
[0002] Cyanoacrylate compositions are well known in the art as excellent adhesives. In particular they are well known as one-component reactive adhesives, quick bonding and suitable for a wide variety of substrates. However, one of the main drawbacks of these compositions is that they tend to be brittle following cure and have low peel strengths. A variety of additives and fillers have been proposed for addition to cyanoacrylate adhesive compositions to improve toughness and peel strengths. [0003] U.S. Patent No. 4,102,945 (Gleave) describes a cyanoacrylate adhesive having enhanced peel strengths in which a cyanoacrylate is thickened by a copolymer or terpolymer including vinylidene chloride-acrylonitrile copolymers. [0004] U. S . Patent No . 4,440,910 (O ' Connor) is directed to cyanoacrylate compositions having improved toughness, achieved through the addition of elastomers, i.e., acrylic rubbers. These rubbers are either (i) homopolymers of alkyl esters of acrylic acid; (ii) copolymers of another polymerizable monomer, such as lower alkenes, with an alkyl ester of acrylic acid or with an alkoxy ester of acrylic acid; (iii) copolymers of alkyl esters of acrylic acid; (iv) copolymers of alkoxy esters of acrylic acid; and (v) mixtures thereof.
[0005] U.S. Patent 4,444,933 (Columbus) suggests the addition of a vinyl chloride/vinyl acetate copolymer to a cyanoacrylate adhesive to reduce adhesion to human skin.
[0006] U.S. Patent No. 4,560,723 (Millet) discloses a cyanoacrylate adhesive composition containing a toughening agent comprising a core-shell polymer and a sustainer comprising an organic compound containing one or more unsubstituted or substituted aryl groups. The sustainer is reported to improve retention of toughness after heat aging of cured bonds of the adhesive.
[0007] U.S. Patent No. 4,713,405 (Koga) discloses an α-cyanoacrylate adhesive composition of matter consisting essentially of α-cyanoacrylate, fumed silica having a surface treated with a dimethyldichlorosilane, and trialkyl borate. [0008] U.S. Patent No. 5,340,873 (Mitry) discloses a cyanoacrylate adhesive composition having improved toughness by including an effective toughening amount of a polyester polymer derived from a dibasic aliphatic or aromatic carboxylic acid and a glycol.
[0009] U.S. Patent No. 5,739,205 (Nishino) discloses an α-cyanoacrylate adhesive composition which comprises (a) 100 parts by weight of an α-cyanoacrylate compound, (b) 10 through 20 parts by weight of (I) polyalkyl methacrylates having a weight average molecular weight of 100,000 through 300,000, or (II) copolymers of alkyl methacrylates and other methacrylates or acrylates, said copolymers having the same weight average molecular weight as that of the polyalkyl methacrylates (I), (c) 2 through 20 parts by weight of ultrafme anhydrous silicas, and (d) 0.001 through 20 parts by weight of certain quick curing additives, (b)-(d) being on the basis of (a) 100 parts by weight of α-cyanoacrylate compounds.
[0010] U.S. Patent No. 5,994,464 (Ohsawa) discloses a cyanoacrylate adhesive composition containing a cyanoacrylate monomer, an elastomer miscible or compatible with the cyanoacrylate monomer, and a core-shell polymer being compatible, but not miscible, with the cyanoacrylate monomer.
[0011] U.S. Patent No. 6,475,331 (O'Connor) discloses a cyanoacrylate adhesive composition comprising: (a) a cyanoacrylate component; and (b) an accelerator component consisting essentially of (i) calixarenes, oxacalixarenes, or a combination thereof, and (ii) at least one crown ether, wherein said composition exhibits a fixturing speed of less than 20 seconds for bonding two substrates, at least one of which is constructed of a material selected from steel, epoxy glass, and balsawood.
[0012] The use of elastomeric polymers as toughening agents in cyanoacrylate adhesive compositions is known. One group of particularly suitable elastomeric polymers is the group comprising copolymers of methyl aery late and ethylene, manufactured by DuPont, under the name of VAMAC, such as VAMAC N123 AND VAMAC B-124. VAMAC N123 and VAMAC B- 124 are reported by DuPont to be a master batch of ethylene/acrylic elastomer.
[0013] Henkel Corporation (as successor to Loctite Corporation) has sold for a number of years rubber toughened cyanoacrylate adhesive products under the trade name BLACK MAX, which employ as the rubber toughening component the DuPont materials called VAMAC B-124 and N 123. In addition Henkel has sold in the past clear and substantially colorless rubber toughened cyanoacrylate adhesive products, namely LOCTITE 4203, 4204, and 4205, which employ as the rubber toughening component the DuPont material, VAMAC G. While VAMAC G contains no fillers to provide color or stabilizers, it does contain processing aids. These processing aids, or release systems are reported to be ARMEEN 18D and stearic acid in combination with GAFAC RL-210 (or with VANFRE UN, or SERVOXYL VPAZ-100). In addition it is believed that polyethylene glycol ether wax is also used as a processing aid. Waxes such as this interfere with the physical properties of cyanoacrylate compositions. In particular, it is believed that the processing aids used in the manufacture of the VAMAC type elastomers are detrimental to adhesion and therefore give poor performance. [0014] VAMAC VCS rubber appears to be the base rubber, from which the remaining members of the VAMAC product line are compounded. VAMAC VCS is a reaction product of the combination of ethylene, methyl acrylate and monomers having carboxylic acid cure sites, which once formed is then substantially free of processing aids such as the release agents octadecyl amine, complex organic phosphate esters and/or stearic acid, and anti-oxidants, such as substituted diphenyl amine. [0015] Recently DuPont has provided to the market under the trade designation
VAMAC VMX 1012 and VCD 6200, rubbers which are made from ethylene and methyl acrylate. It is believed that the VAMAC VMX 1012 rubber possesses little or no carboxylic acid in the polymer backbone. Like the VAMAC VCS rubber, the VAMAC VMX 1012 and VCD 6200 rubbers are substantially free of processing aids such as the
release agents octadecyl amine, complex organic phosphate esters and/or stearic acid, and anti-oxidants, such as substituted diphenyl amine, noted above. [0016] Notwithstanding the state of the art and the commercial success experienced by Henkel Corporation with its line of rubber toughened cyanoacrylate adhesive products, it would be desirable to provide an alternative, more cost-effective cyanoacrylate adhesive composition with improved toughness and peel strengths, while maintaining a high fixture speed.
SUMMARY OF THE INVENTION
[0017] The present invention is thus directed to an alternative cyanoacrylate adhesive composition which provides improved performance and toughness compared to known toughened cyanoacrylate compositions.
[0018] The invention provides a cyanoacrylate composition comprising:
(i) a cyanoacrylate component; and
(ii) a toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate.
[0019] The composition according to the invention has been found to demonstrate increased performance over known cyanoacrylate compositions. In particular, the present invention is directed to a cyanoacrylate composition which demonstrates substantially enhanced toughness and has an improved peel strength and fixturing speed compared to known toughened cyanoacrylate compositions.
[0020] The toughening agent comprises a co-polymer of polyethylene and polyvinyl acetate. Agents which are particularly suitable for use in accordance with the present invention are those agents comprising co-polymers of polyethylene and polyvinyl acetate which are sold under the trade name LEVAMELT by Lanxess
Limited.
[0021] A range of LEVAMELT agents is available and includes for example,
LEVAMELT 400, LEVAMELT 600 and LEVAMELT 900. These agents differ in the amount of vinyl acetate present. For example, LEVAMELT 400 represents an ethylene- vinyl acetate copolymer comprising 40 wt% vinyl acetate. The LEVAMELT products are supplied in granular form. The granules are almost colourless and dusted with silica and talc. The product may also be supplied in bales of 25 kg under the trade name
LEVAPREN.
[0022] The use of a toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate allows different cyanoacrylate esters to be formed as tough adhesives.
[0023] The use of toughening agents comprising a copolymer of polyethylene and polyvinyl acetate permits the use of other monomers such as methyl cyanoacrylate, ethyl-2-cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates (such as n-butyl-2- cyanoacrylate), octyl cyanoacrylates, allyl cyanoacrylate, β-methoxy ethyl cyanoacrylate, propargyl cyanoacrylates and combinations thereof.
[0024] The monomers can be toughened by varying the ratios of polyethylene and polyvinyl acetate in the elastomer. Suitable elastomers can be selected for use in toughening a particular monomer. Elastomers comprising copolymers comprising different ratios of polyethylene and polyvinyl acetate may be used to toughen different monomers. A particularly preferred toughening agent for use in accordance with the present invention comprises a copolymer of polyethylene and polyvinyl acetate wherein the vinyl acetate is present in an amount of 90 wt%.
[0025] The compositions according to the invention provide increased performance compared to known toughened cyanoacrylate compositions. In particular, cyanoacrylate compositions according to the present invention show increased toughness, measured for instance as an increased peel strength.
[0026] The cyanoacrylate component suitably comprises a monomeric structure represented by:
where R1 is C1-15 alkyl, C2-15 alkoxyalkyl, C3-15 cycloalkyl, C2-15 alkenyl, C6-15 aryl, C7-15 aralkyl, C3-Is allyl, C1-15 alkylhalide, or C1-15 haloalkyl and mixtures thereof.
[0027] The cyanoacrylate component may be selected from the group consisting of methyl cyanoacrylate, ethyl-2-cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates, octyl cyanoacrylates, allyl cyanoacrylates, alkoxyalkyl cyanoacryalates
(such as β-methoxyethyl cyanoacrylate), propargyl cyanoacrylate and mixtures thereof.
[0028] In one aspect of the invention, the cyanoacrylate component comprises ethyl-2-cyanoacrylate.
[0029] As used herein all composition values are given in weight percent unless otherwise noted.
[0030] Desirably, the co-polymer of polyethylene and polyvinyl acetate is present in an amount of 1 to about 20% by weight.
[0031] Desirably, the co-polymer of polyethylene and polyvinyl acetate is present in an amount of 5 to about 10% by weight.
[0032] In another aspect the present invention is directed to a cyanoacrylate composition which demonstrates enhanced toughness including a cyanoacrylate material; a toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate and one or more additives selected from plasticizers, accelerators, fillers, opacifϊers, inhibitors, thixotrophy conferring agents, stabilizers, dyes, thermal degradation reducers, adhesion promoters, shock resistance conferring agents and combinations thereof, where upon cure, the cyanoacrylate composition has an average T peel strength on mild steel of more than about 3 N/mm after curing at room temperature for about 72 hours and a fixture speed on mild steel of less than 360 seconds.
[0033] In yet another aspect, the present invention is directed to a method of bonding two or more substrates including the steps of providing at least two substrates; dispensing, on at least a portion of a surface of one or both of the at least two substrates, a cyanoacrylate composition including about 1 to about 20% by weight of the toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate; contacting the surfaces of the at least two substrates having the cyanoacrylate composition therebetween; and curing the cyanoacrylate composition.
[0034] In still another aspect, the present invention is directed to a bonded assembly including: a first substrate having a first surface; another substrate having a second surface; and a cured cyanoacrylate composition disposed between the first and
second surfaces, the composition having included prior to cure a cyanoacrylate component; and a toughening agent comprising about 1 to about 20% by weight of the toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate.
Preferably, the T peel strength on mild steel is greater than about 3 N/mm after room temperature cure for about 72 hours, and a fixture speed on mild steel of less than 360 seconds.
[0035] In addition the invention is directed to reaction products of the inventive compositions.
[0036] Also, the invention is directed to a method of preparing the inventive compositions.
[0037] And the invention is directed to a method of conferring one or more of the following properties to rubber toughened cyanoacrylate compositions, improved shelf life, fixture speed, improved shear strength development over time and improved side impact strength and fracture toughness, which includes the method of providing a cyanoacrylate component and a toughening agent component comprising a co-polymer of polyethylene and polyvinyl acetate and mixing together said components.
[0038] The invention will be more fully understood by a reading of the section entitled "Detailed Description of the Invention" which follows.
BRIEF DESCRIPTION OF THE FIGURES
[0039] The invention will be described in more detail with reference to the accompanying drawing in which:
Fig. 1 depicts a plot of the effect of LEVAMELT 900 (copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate) on T peel strength for each of the monomers β-methoxyethyl cyanoacrylate and ethyl cyanoacrylate.
Fig. 2 depicts a plot of the effect of LEVAMELT 900 (copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate) on T peel strength for the monomer β-methoxyethyl cyanoacrylate over a period of 3 days at room temperature and a period of 3 days at room temperature and 1 day at 80 °C.
Fig. 3 depicts a plot of the effect of LEVAMELT 900 (copolymer of polyethylene and polyvinyl acetate comprising 90 wt% vinyl acetate) on T peel strength for the monomer
ethyl cyanoacrylate over a period of 3 days at room temperature and a period of 3 days at room temperature and 1 day at 80 °C.
DETAILED DESCRIPTION OF THE INVENTION
[0040] The cyanoacrylate compositions of the present invention comprise toughening agents which provide enhanced toughness, such as improved peel strengths in the cured compositions.
Toughening Agent
[0041] The preferred toughening agent comprises an elastomeric co-polymer of polyethylene and polyvinyl acetate. The toughening agents which are preferably used in the compositions according to the invention comprise LEVAMELT elastomers which are available from Lanxess. It will be appreciated that other suitable copolymers of polyethylene and polyvinyl acetate could also be used.
Vinyl Acetate Ethylene Vinyl Acetate Copolymer
[0042] The LEVAMELT elastomers are high performance elastomers. Various grades of LEVAMELT elastomers are available from Lanxess. They dissolve more readily than other tougheners currently used in the art, for example, VAMAC. They are readily available in both monomers and perform better than VAMAC in ethyl cyanoacrylate. LEVAMELT consists of methylene units forming a saturated main chain with pendant acetate groups. The presence of a fully saturated main chain is an indication that LEVAMELT is a particularly stable polymer. It does not contain any reactive double bonds which make conventional rubbers prone to aging reactions, ozone and UV light. The saturated backbone makes it robust.
[0043] Further, the LEVAMELT elastomers are available without any of the processing aids that are used for the most of the VAMAC elastomers. As a result, these elastomers facilitate the use of other monomers such as methyl cyanoacrylate, ethyl-2-
cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates, octyl cyanoacrylates, allyl cyanoacrylates, β-methoxyethyl cyanoacrylate, propargyl cyanoacrylate and mixtures thereof. This allows different cyanoacrylate esters to be formulated as tough adhesives. Interestingly, depending on the ratio of polyethylene/polyvinylacetate, the solubilities of these materials change in different monomers and also the ability to toughen changes as a result of the solubility.
[0044] The LEVAMELT elastomers are available in pellet form and are easier to formulate than other known elastomeric toughening agents. Furthermore, these elastomers are less expensive than other known elastomers. Thus these elastomers allow for the formulation of a more cost-effective toughened cyanoacrylate composition which provides better performance compared to known toughened cyanoacrylate compositions.
Cyanoacrylate Component
[0045] The cyanoacrylate compositions of the present invention include a cyanoacrylate component which includes cyanoacrylate monomers, such as those represented by the structure:
where R1 is selected from R1 is C1-15 alkyl, C2-15 alkoxyalkyl, C3-15 cycloalkyl, C2-15 alkenyl, C6-15 aryl, C7-I5 aralkyl, C3-I5 allyl, C1-15 alkylhalide, or C1-15 haloalkyl and mixtures thereof. Preferably, the cyanoacrylate monomer is selected from methyl cyanoacrylate, ethyl-2-cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates (such as n-butyl-2-cyanoacrylate), octyl cyanoacrylates, allyl cyanoacrylate, β-methoxyethyl cyanoacrylate, propargyl cyanoacrylate and combinations thereof. A particularly desirable cyanoacrylate monomer is ethyl-2-cyanoacrylate. The cyanoacrylate component should be included in the compositions in an amount within the range of about 70% by weight to about 95% by weight, preferably about 75% by weight to about 90% by weight.
[0046] The compositions of the present invention may also contain additives such as stabilizers, accelerators, plasticizers, fillers, opacifϊers, inhibitors, thixotrophy conferring agents, dyes, fluorescence markers, thermal degradation reducers, adhesion promoters, thermal resistance conferring agents and combinations thereof, and the like. Cured cyanoacrylate compositions are typically rigid, brittle materials, having low crack resistance and therefore low T peel strength. Additives may be included in order to modify these properties. These additives are known to those of skill in the art. [0047] The compositions according to the invention may further comprise free radical inhibitors, such as hydroquinones or MMBP (2,2'-methylenebis(6-tert-butyl-4- methylphenol). The inhibitor, when present, is preferably present in an amount of about 0.001% by weight to about 2.0% by weight, preferably 0.02% to about 0.5% by weight. [0048] Accelerators that may be useful in the cyanoacrylate compositions include for example calixarenes, oxacalixarenes, and combinations thereof. Of the calixarenes and oxacalixarenes, many are known, and are reported in the patent literature. See, e.g. U.S. Patent Nos. 4,556,700, 4,622,414, 4,636,539, 4,695,615, 4,718,966, and 4,855,461, the disclosures of each of which are hereby expressly incorporated herein by reference.
[0049] Another potentially useful accelerator component is a crown ether. A host of crown ethers are known. For instance, examples which may be used herein either individually or in combination, or in combination with the calixarenes and oxacalixarenes described above include 15-crown-5, 18-crown-6, dibenzo-18-crown-6, benzo-15-crown-5, dibenzo-24-crown-8, dibenzo-30-crown-lO, tribenzo-18-crown-6, asym-dibenzo-22-crown-6, dibenzo- 14-crown-4, dicyclohexyl- 18-crown-6, dicyclohexyl-24-crown-8, cyclohexyl-12-crown-4, l,2-decalyl-15-crown-5, 1,2- naphtho-15-crown-5, 3,4,5-naphthyl-16-crown-5, l,2-methyl-benzo-18-crown-6, 1,2- methylbenzo-5, 6-methylbenzo-18-crown-6, l,2-t-butyl-18-crown-6, l,2-vinylbenzo-15- crown-5, l,2-vinylbenzo-18-crown-6, l,2-t-butyl-cyclohexyl-18-crown-6, asym- dibenzo-22-crown-6 and l,2-benzo-l,4-benzo-5-oxygen-20-crown-7. See U.S. Patent No. 4,837,260 (Sato), the disclosure of which is hereby expressly incorporated here by reference.
[0050] Other suitable accelerators include those described in U.S. Patent No.
5,312,864 (Wenz), which are hydroxyl group derivatives of an CC-, β- or γ-cyclodextrin
which is at least partly soluble in the cyanoacrylate; in U.S. Patent No. 4,906,317 (Liu), which are silacrown compounds to accelerate fϊxturing and cure on de-activating substrates such as wood, examples of which are within the following structure:
( OCH2CH ) k= where R3 and R4 are organo groups which do not themselves cause polymerization of the cyanoacrylate monomer, R5 is H or CH3 and n is an integer of between 1 and 4. Examples of suitable R3 and R4 groups are C1-15 alkyl groups, C1-15 alkoxy groups such as methoxy, and C1-15 aryloxy groups such as phenoxy. The R3 and R4 groups may contain halogen or other substituents, an example being trifluoropropyl. However, groups not suitable as R4 and R3 groups are basic groups such as amino, substituted amino and alkylamino.
[0051] Specific examples of silacrown compounds useful in the inventive compositions include:
dimethylsila- 11 -crown-4;
dimethylsila- 14-cro wn-5 ;
and dimethylsila-17-crown-6.
[0052] Other accelerators, and accelerator packages (i.e., the combination of two or more accelerators) may be found in U.S. Patent Nos. 6,294,629 — an accelerator component including (i) calixarenes, oxacalixarenes, silacrowns, cyclodextrins or a combination thereof, and (ii) poly(ethyleneglycol) di(meth)acrylates, ethoxylated hydric compounds, and combinations thereof --, 6,475,331— an accelerator component including (i) calixarenes, oxacalixarenes, or a combination thereof, and (ii) at least one
crown ether — , and 6,835,789 — chemical class embraced by
where R is a member selected from the group consisting of hydrogen, C1-15 alkyl, C1-15 alkyloxy, C2-15 alkyl thioethers, C1-15 haloalkyl, C1-15 carboxylic acid and esters thereof, Co-i 5 sulfmic, C0-15 sulfonic and C0-15 sulfurous acids and esters thereof, C0-15 phosphinic, Co-i 5 phosphonic and C0-15 phosphorous acids and esters thereof, Z is a single or double bond, n is 1-12, p is 1-3, R' is the same as R, and g is the same as n; such as
19 where n and m combined is greater than or equal to 12, the disclosures of each of which are hereby expressly incorporated herein by reference. Desirably, however, the accelerator component used in accordance with the present invention comprises 18- crown-6.
[0053] The accelerator component should be included in the compositions in an amount within the range of from about 0.01% to about 10% by weight, with the range of about 0.05% to about 2% by weight being desirable, and about 0.1% to about 1% by weight of the total composition being particularly desirable.
[0054] Itaconic anhydride may also be added to the cyanoacrylate component to further aid in durability and impact, heat, and moisture resistance. Itaconic anhydride when present in the composition increases adhesion to mild steel.
[0055] Plasticizers may also be added to the cyanoacrylate component, and when so added are preferably present in an amount of about 10% by weight to about 50% by weight, more preferably about 10% by weight to about 25% by weight of the total composition.
[0056] The inventive compositions may also be rendered thixotropic by the addition of thixotrophy conferring agents, such as fumed silica. See U.S. Patent Nos.
4,533,422 (Litke) and 4,477,607 (Litke). These agents, when used, should be used in an
amount less than about 15% by weight, such as within the range of about 0.5% by weight to about 10% by weight of the total composition.
[0057] The combination of the cyanoacrylate monomer and the additives will be referred to herein as the cyanoacrylate component.
[0058] Thermal resistance conferring agents, such as citric acid, are also desirable for use herein.
[0059] The compositions according to the invention may be readily prepared by adding predetermined amounts of the toughening agent components to the cyanoacrylate component and stirring or agitating for a sufficient time at an appropriate temperature to achieve a homogenous solution or suspension. Typically, temperatures much above room temperature are not necessary, though in formulations containing high levels of fillers, thickeners, and the like, moderate heating may be desirable to speed dissolution of the various additives.
[0060] The improved toughness of the cured compositions of the invention is manifested through various physical properties, e.g. 180° peel strength, impact strength and tensile shear strength. These strengths are useful properties of an adhesive bond, being parameters of what is loosely referred to as the bond strength. Referring for simplicity to the procedures of American Standard Test Methods, peel strength is determined in accordance with ASTM No. D 903-49; impact strength is determined in accordance with ASTM No. D-950; and tensile shear strength is determined in accordance with ASTM No. D- 1002. The reader is referred to these standards for a full description of the tests.
EXAMPLES
[0061] The following examples are illustrative of the invention and not intended to limit the scope of the invention in any way. The examples describe the preparation and use of the cyanoacrylate compositions of the present invention.
Example 1
[0062] The following formulations were prepared from the constituents listed in
Table 1 in the respective amounts. The effect of LEVAMELT 900 on T peel strength for each of the monomers, β-methoxy ethyl cyanoacrylate and ethyl cyanoacrylate, is
demonstrated contrasted to a control without LEVAMELT 900. The results are shown in Table 2 and Figure 1.
Table 1
Table 2
Example 2
[0063] Formulations A and B were prepared with methane sulfonic acid and
SO2 as stabilisers according to the following process. Itaconic anhydride and phosphoric acid were added to the stabilised cyanoacrylate. Crown ether (18-crown-6) was then added and dissolved microparticles (55μm polyethylene microparticles available from Inhance, which are constructed of polyethylene in the form of a particle having an average particle size of less than 500 μm and whose surface has been modified such that the outermost surface thereof has been activated through exposure to an oxidation process) were subsequently added and dispersed. LEVAMELT 900 was added portion wise to this solution and mixed thoroughly over 15 minutes. [0064] Details of the compositions are set out in Table 3 below:
Table 3
Formulation A B β-methoxyethylcyanoacrylate 86.35%
Ethylcyano acrylate — 86.35%
Itaconic anhydride 1.25% 1.25%
Phosphoric acid 0.25% 0.25%
Crown Ether 0.25% 0.25%
LEVAMELT 900 10.0% 10.0%
UH-1250
(55μm polyethylene microparticles available from
Inhance) 0.5% 0.5%
Silica 1.0% 1.0%
[0065] The test results for each of Formulations A and B are presented in Table
4 and Figures 2 and 3. The substrates used in determining T peel strength in Table 4 are mild steel bonded to mild steel and aluminium bonded to aluminum.
Table 4
Performance
[0066] Non-toughened cyanoacrylate compositions that are evaluated in adhesive applications have low T peel strengths, typically from about 0.2 -0.4 N/mm. It has been found that the addition of a copolymer of polyethylene and polyvinyl acetate to a cyanoacrylate monomer increases the T peel strength of the cured cyanoacrylate composition.
[0067] It is evident from the information provided in the table above that the elastomer LEVAMELT 900 works well with ethyl cyanoacrylate monomer. [0068] The toughened cyanoacrylate compositions according to the invention appear to provide excellent adhesion on both mild steel and aluminium. The performance of the inventive compositions can be improved by the addition of fibres if necessary.
Claims
1. A cyanoacrylate composition comprising: (i) a cyanoacrylate component; and
(ii) a toughening agent comprising a co-polymer of polyethylene and polyvinyl acetate.
2. The composition of claim 1 wherein said cyanoacrylate component comprises a monomeric structure represented by:
3. The composition of claim 2 wherein said cyanoacrylate component comprises a member selected from the group consisting of methyl cyanoacrylate, ethyl-2- cyanoacrylate, propyl cyanoacrylates, butyl cyanoacrylates, octyl cyanoacrylates, allyl cyanoacrylates, β-methoxyethyl cyanoacrylate, propargyl cyanoacrylates and combinations thereof.
4. The composition of claim 1 wherein said cyanoacrylate component comprises ethyl-2-cyanoacrylate.
5. The composition of claim 1 wherein said co-polymer of polyethylene and polyvinyl acetate is present in an amount of 1 to about 20% by weight.
6. The composition of claim 5 wherein said co-polymer of polyethylene and polyvinyl acetate is present in an amount of 5 to about 10% by weight.
7. The composition of claim 1 further comprising fumed silica.
8. The composition of claim 6, wherein said fumed silica is present in an amount (by weight) of from about 0.5 to about 10%.
9. The composition of claim 1 further comprising at least one additive selected from the group consisting of stabilizers, accelerators, plasticizers, fillers, opacifϊers, thickeners, viscosity modifiers, inhibitors, thixotrophy conferring agents, dyes, thermal degradation inhibitors, adhesion promoters, shock resistance-conferring agents and combinations thereof.
10. A method of bonding two or more substrates comprising the steps of: providing at least two substrates; dispensing, on a surface of one or both of the at least two substrates, a cyanoacrylate composition of claim 1 ; contacting the surfaces of the at least two substrates having the cyanoacrylate composition thereon; and exposing the cyanoacrylate composition to cure conditions.
11. A bonded assembly comprising: a first substrate having a first surface; another substrate having a second surface; and a cured cyanoacrylate composition disposed between said first and second surfaces, said composition, prior to cure, comprising: a cyanoacrylate component; a toughening agent comprising a co -polymer of polyethylene and polyvinyl acetate.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US9571508P | 2008-09-10 | 2008-09-10 | |
| US61/095,715 | 2008-09-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010029134A1 true WO2010029134A1 (en) | 2010-03-18 |
Family
ID=41404238
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2009/061770 WO2010029134A1 (en) | 2008-09-10 | 2009-09-10 | Toughened cyanoacrylate compositions |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2010029134A1 (en) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018202384A1 (en) * | 2017-05-05 | 2018-11-08 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| WO2019068690A1 (en) * | 2017-10-06 | 2019-04-11 | Henkel IP & Holding GmbH | Toughened cyanoacrylate compositions |
| GB2567867A (en) * | 2017-10-27 | 2019-05-01 | Henkel IP & Holding GmbH | Toughened, low odor/low bloom cyanoacrylate compositions |
| GB2567868A (en) * | 2017-10-27 | 2019-05-01 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| GB2567869A (en) * | 2017-10-27 | 2019-05-01 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| WO2020035579A1 (en) | 2018-08-16 | 2020-02-20 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| WO2021203234A1 (en) * | 2020-04-07 | 2021-10-14 | Henkel Ag & Co. Kgaa | Low odour cyanoacrylate composition |
| WO2023023082A1 (en) * | 2021-08-16 | 2023-02-23 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS59145271A (en) * | 1983-02-05 | 1984-08-20 | Taoka Chem Co Ltd | Adhesive composition |
| US4533422A (en) * | 1983-08-31 | 1985-08-06 | Loctite Corporation | Thixotropic cyanoacrylate compositions |
| JPS6225185A (en) * | 1985-07-25 | 1987-02-03 | Alpha Giken:Kk | Alpha-cyanoacrylate adhesive composition |
| US6475331B1 (en) * | 2001-06-26 | 2002-11-05 | Henkel Loctite Corporation | Cyanoacrylate compositions |
| US20040131827A1 (en) * | 2003-01-06 | 2004-07-08 | Loctite (R&D) Limited | Toughened cyanoacrylate compositions |
| US7390851B1 (en) * | 2003-01-06 | 2008-06-24 | Loctite (R&D) Limited | Toughened cyanoacrylate compositions |
-
2009
- 2009-09-10 WO PCT/EP2009/061770 patent/WO2010029134A1/en active Application Filing
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS59145271A (en) * | 1983-02-05 | 1984-08-20 | Taoka Chem Co Ltd | Adhesive composition |
| US4533422A (en) * | 1983-08-31 | 1985-08-06 | Loctite Corporation | Thixotropic cyanoacrylate compositions |
| JPS6225185A (en) * | 1985-07-25 | 1987-02-03 | Alpha Giken:Kk | Alpha-cyanoacrylate adhesive composition |
| US6475331B1 (en) * | 2001-06-26 | 2002-11-05 | Henkel Loctite Corporation | Cyanoacrylate compositions |
| US20040131827A1 (en) * | 2003-01-06 | 2004-07-08 | Loctite (R&D) Limited | Toughened cyanoacrylate compositions |
| US7390851B1 (en) * | 2003-01-06 | 2008-06-24 | Loctite (R&D) Limited | Toughened cyanoacrylate compositions |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110741055A (en) * | 2017-05-05 | 2020-01-31 | 汉高知识产权控股有限责任公司 | Cyanoacrylate compositions |
| US11555135B2 (en) | 2017-05-05 | 2023-01-17 | Henkel Ag & Co. Kgaa | Cyanoacrylate compositions |
| WO2018202384A1 (en) * | 2017-05-05 | 2018-11-08 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| WO2019068690A1 (en) * | 2017-10-06 | 2019-04-11 | Henkel IP & Holding GmbH | Toughened cyanoacrylate compositions |
| US11299651B2 (en) | 2017-10-06 | 2022-04-12 | Henkel Ag & Co. Kgaa | Toughened cyanoacrylate compositions |
| CN111433244B (en) * | 2017-10-06 | 2022-04-05 | 汉高知识产权控股有限责任公司 | Toughened cyanoacrylate compositions |
| CN111433244A (en) * | 2017-10-06 | 2020-07-17 | 汉高知识产权控股有限责任公司 | Toughened cyanoacrylate compositions |
| WO2019081759A1 (en) * | 2017-10-27 | 2019-05-02 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| GB2567869B (en) * | 2017-10-27 | 2021-08-11 | Henkel IP & Holding GmbH | Toughened humidity/thermal resistant cyanoacrylate compositions |
| JP7394755B2 (en) | 2017-10-27 | 2023-12-08 | ヘンケル・アクチェンゲゼルシャフト・ウント・コムパニー・コマンディットゲゼルシャフト・アウフ・アクチェン | cyanoacrylate composition |
| GB2567868B (en) * | 2017-10-27 | 2020-05-06 | Henkel IP & Holding GmbH | Toughened low odour cyanoacrylate compositions |
| CN111263778A (en) * | 2017-10-27 | 2020-06-09 | 汉高知识产权控股有限责任公司 | Cyanoacrylate compositions |
| CN111278938A (en) * | 2017-10-27 | 2020-06-12 | 汉高知识产权控股有限责任公司 | Toughened low odor/low blooming cyanoacrylate compositions |
| WO2019081762A1 (en) * | 2017-10-27 | 2019-05-02 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| GB2567867B (en) * | 2017-10-27 | 2020-09-16 | Henkel IP & Holding GmbH | Toughened, low odor/low bloom cyanoacrylate compositions |
| JP2021500453A (en) * | 2017-10-27 | 2021-01-07 | ヘンケル アイピー アンド ホールディング ゲゼルシャフト ミット ベシュレンクテル ハフツング | Cyanoacrylate composition |
| WO2019081753A1 (en) * | 2017-10-27 | 2019-05-02 | Henkel IP & Holding GmbH | Toughened, low odor/low bloom cyanoacrylate compositions |
| GB2567867A (en) * | 2017-10-27 | 2019-05-01 | Henkel IP & Holding GmbH | Toughened, low odor/low bloom cyanoacrylate compositions |
| GB2567869A (en) * | 2017-10-27 | 2019-05-01 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| US11299652B2 (en) | 2017-10-27 | 2022-04-12 | Henkel Ag & Co. Kgaa | Cyanoacrylate compositions |
| GB2567868A (en) * | 2017-10-27 | 2019-05-01 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| CN111263778B (en) * | 2017-10-27 | 2022-08-30 | 汉高股份有限及两合公司 | Cyanoacrylate compositions |
| WO2020035579A1 (en) | 2018-08-16 | 2020-02-20 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
| US12065554B2 (en) | 2018-08-16 | 2024-08-20 | Henkel Ag & Co. Kgaa | Cyanoacrylate compositions |
| WO2021203234A1 (en) * | 2020-04-07 | 2021-10-14 | Henkel Ag & Co. Kgaa | Low odour cyanoacrylate composition |
| WO2023023082A1 (en) * | 2021-08-16 | 2023-02-23 | Henkel IP & Holding GmbH | Cyanoacrylate compositions |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2010029134A1 (en) | Toughened cyanoacrylate compositions | |
| EP2121777B1 (en) | Cyanoacrylate compositions incorporating graphite platelets | |
| JP6794460B2 (en) | Cyanoacrylate composition | |
| US10947418B2 (en) | Cyanoacrylate compositions | |
| MX2008000578A (en) | Toughened cyanoacrylate compositions. | |
| WO2004111147A2 (en) | Cyanoacrylate compositions | |
| KR20160009024A (en) | Cyanoacrylate compositions | |
| US6833196B1 (en) | Acrylic-toughened cyanoacrylate compositions | |
| JP2004522827A (en) | Cyanoacrylate compositions curable into flexible polymeric materials | |
| EP3700950A1 (en) | Cyanoacrylate compositions | |
| US20040131827A1 (en) | Toughened cyanoacrylate compositions | |
| US7390851B1 (en) | Toughened cyanoacrylate compositions | |
| US7687561B1 (en) | Toughened cyanoacrylate compositions | |
| TWI825036B (en) | Toughened, low odor/low bloom cyanoacrylate compositions | |
| JP2844946B2 (en) | Adhesive composition | |
| KR20200076680A (en) | Cyanoacrylate composition | |
| TWI806962B (en) | Cyanoacrylate compositions | |
| EP2424933A1 (en) | Cyanoacrylate compositions | |
| CA3121794A1 (en) | Adhesive composition with retarding additive | |
| JPH0125352B2 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09782885 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 09782885 Country of ref document: EP Kind code of ref document: A1 |