WO2007107878A2 - Solid dosage forms of hypnotic agent - Google Patents
Solid dosage forms of hypnotic agent Download PDFInfo
- Publication number
- WO2007107878A2 WO2007107878A2 PCT/IB2007/000809 IB2007000809W WO2007107878A2 WO 2007107878 A2 WO2007107878 A2 WO 2007107878A2 IB 2007000809 W IB2007000809 W IB 2007000809W WO 2007107878 A2 WO2007107878 A2 WO 2007107878A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- solid dosage
- dosage form
- starch
- zaleplon
- group
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4858—Organic compounds
Definitions
- the present invention relates to solid dosage forms of nonbenzodiazepine hypnotic agent. More particularly, the present invention relates to solid dosage forms of Zaleplon.
- the present invention also relates to process for preparing solid dosage forms of Zalepion. Background of the invention
- Zaleplon is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class. Chemically Zaleplon is N-[3-(3-cyanopyrazolo[l,5-a]pyrimidin-7- yl)phenyl]-N-ethylacetamide. Zaleplon is disclosed and claimed in US patent No 4,626,538. It is commercially available in capsule dosage form under the trademark of SONATA ® for the treatment of Insomnia. The inactive ingredients of the commercial capsule dosage form of Zaleplon are: microcrystalline cellulose, pregelatinized starch, silicon dioxide, sodium lauryl sulfate, magnesium stearate, and lactose.
- Magnesium stearate is the most commonly used lubricant in pharmaceutical tablets.
- Pregelatinized starch is incompatible with magnesium stearate and effects the disintegration and dissolution rate of the formulation.
- Incompatibility between magnesium stearate and pregelatinised starch is well known in the prior art as described in technical data sheet published by Colorcon for Hydrochlorothiazide formulations.
- formulations containing magnesium stearate become over lubricated and show increased disintegration time and thereby reduce the dissolution rate of capsules containing insoluble actives like hydrochlorothiazide, zaleplon etc.
- These formulations, which contain magnesium stearate show slow dissolution profile.
- the main objective of the present invention is to provide solid dosage forms of Zaleplon.
- Another objective of the present invention is to provide simple, cost effective and efficient process for preparing solid dosage forms of Zaleplon.
- Another objective of the present invention is to provide solid dosage forms of Zalelpon in such a way that it will comply with the reference product in terms of in vitro parameters like dissolution, disintegration, etc and in vivo parameters for bioequivalence such as C max , AUC, T max etc.
- the present invention provides solid dosage forms comprising Zaleplon and stearic acid as lubricant.
- the present invention also provides process for preparing solid dosage forms of Zaleplon wherein the composition is prepared by blending Zaleplon with one or more pharmaceutically acceptable excipients lubricating the blend with stearic acid and finally filling into capsules or compressing into tablets.
- the solid dosage form of the present invention is in the form of tablet or capsule.
- solid dosage forms of Zaleplon further comprise one or more pharmaceutical acceptable excipients such as diluents, binders, disintegrants, surfactants and glidants.
- Suitable diluents used according to the present invention are selected from calcium phosphate-dibasic, calcium carbonate, sucrose, lactose, cellulose- microcrystalline, cellulose powdered, calcium silicate, kaolin, starch, starch pregelatinized, polyols such as mannitol, sorbitol, lactitol, xylitol, maltitol or combinations thereof.
- Suitable binders used according to the present invention are selected from hydroxy propyl cellulose, hydroxypropyl methylcellulose, gelatin, hydroxy ethyl cellulose, povidone, polyvinyl alcohol, copovidone, ethylcellulose, starch and methylcellulose or a combination thereof.
- Suitable disintegrants used according to the present invention are selected from croscarmellose sodium, crospovidone, sodium starch glycolate, starch, sodium carboxymethylcellulose, pregelatinised starch, hydroxypropylcellulose or combination thereof.
- Suitable surfactants used according to present invention are selected from sodium lauryl sulfate, polysorbates, sorbitan esters or combination thereof.
- Suitable glidants used according to the present invention are selected from magnesium trisilicate, talc, tribasic calcium phosphate, glyceryl monostearate, glyceryl stearate and colloidal silica or a combination thereof.
- the solid dosage forms of Zaleplon when the solid dosage forms of Zaleplon is tablet, may be prepared by dry granulation (slugging or compaction), wet granulation, and direct compression.
- the pharmaceutical composition of the present invention is prepared by direct compression or direct filling into capsules.
- Suitable solvents used for preparing solid dosage forms by wet granulation are selected from water, ethanol, methanol, and isopropanol or a combination thereof.
- process for the preparation of solid dosage forms comprising Zaleplon and stearic acid as lubricant as follows: i) blending Zaleplon with one or more diluents, binder, disintegrants, surfactants, glidants, ii) lubricating the blend of step (i) with stearic acid and iii) filling the blend into capsules or compressing into tablets.
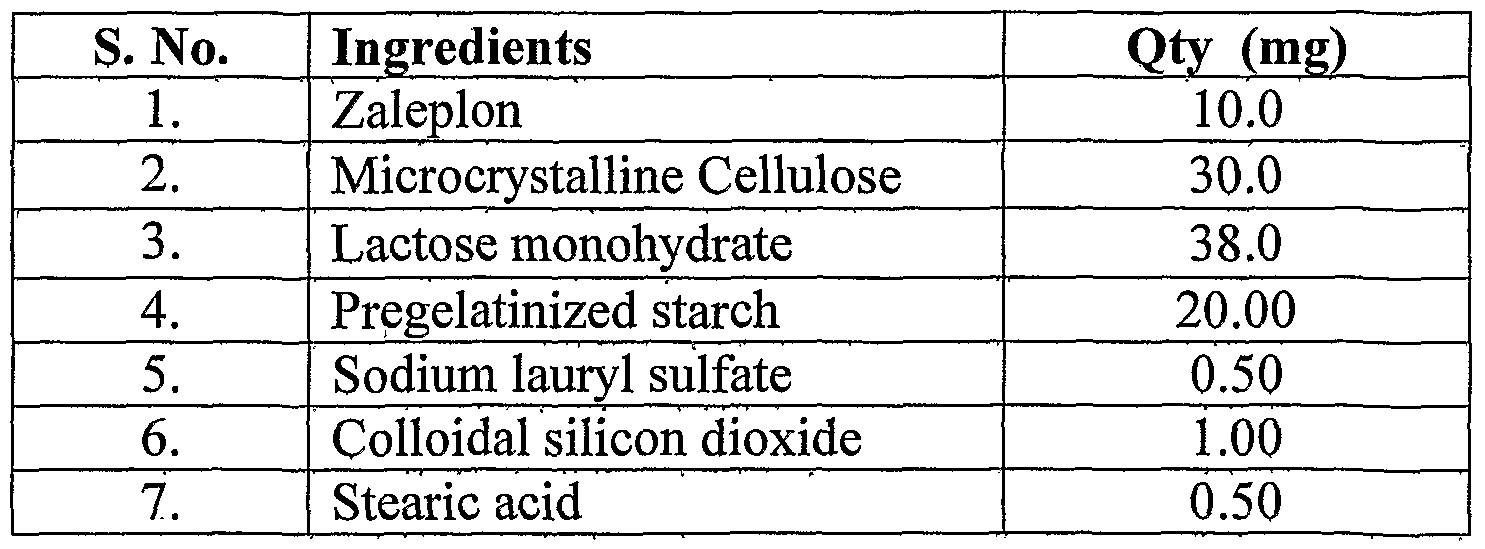
- the processing steps involved are: , j i) sifted and blended Zaleplon, microcrystalline cellulose, lactose monohydrate, pregelatinized starch, sodium lauryl sulfate and colloidal sijico ⁇ s dioxide, : ii) lubricated the blend of step (i) with stearic acid and finally ; iii) the lubricated blend was filled into capsules or compressed into tajblets.
- the solid dosage forms of Zaleplon disclosed in example 2-6 were prepared by a similar procedure described in example 1. '
- the Zaleplon capsules prepared according to the present invention were tested for drug release in water using USP apparatus 2 with paddle speed at 75 rpm.
- the samples of the media were periodically withdrawn and spectrophotometrically analyzed for Zaleplon content.
- the dissolution profile data is given in Table 1.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The present invention relates to solid dosage forms of nonbenzodiazepine hypnotic agent. More particularly, the present invention relates to solid dosage forms of Zaleplon. The present invention also relates to a process for preparing solid dosage forms of Zaleplon.
Description
SOLID DOSAGE FORMS OF HYPNOTIC AGENT
Filed of the invention
The present invention relates to solid dosage forms of nonbenzodiazepine hypnotic agent. More particularly, the present invention relates to solid dosage forms of Zaleplon.
The present invention also relates to process for preparing solid dosage forms of Zalepion. Background of the invention
Zaleplon is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class. Chemically Zaleplon is N-[3-(3-cyanopyrazolo[l,5-a]pyrimidin-7- yl)phenyl]-N-ethylacetamide. Zaleplon is disclosed and claimed in US patent No 4,626,538. It is commercially available in capsule dosage form under the trademark of SONATA® for the treatment of Insomnia. The inactive ingredients of the commercial capsule dosage form of Zaleplon are: microcrystalline cellulose, pregelatinized starch, silicon dioxide, sodium lauryl sulfate, magnesium stearate, and lactose.
Magnesium stearate is the most commonly used lubricant in pharmaceutical tablets. Pregelatinized starch is incompatible with magnesium stearate and effects the disintegration and dissolution rate of the formulation. Incompatibility between magnesium stearate and pregelatinised starch is well known in the prior art as described in technical data sheet published by Colorcon for Hydrochlorothiazide formulations. During long-term capsule filling operation, formulations containing magnesium stearate become over lubricated and show increased disintegration time and thereby reduce the dissolution rate of capsules containing insoluble actives like hydrochlorothiazide, zaleplon etc. These formulations, which contain magnesium stearate, show slow dissolution profile. Hence, there is a need for selection of proper lubricant in the dosage form, which doesn't impair dissolution rate and is devoid of compatibility
problems. Hence, the focus of present invention was to evaluate and select compatible lubricant for formulating Zaleplon dosage forms.
Objective of the invention
Accordingly, the main objective of the present invention is to provide solid dosage forms of Zaleplon.
Yet, another objective of the present invention is to provide simple, cost effective and efficient process for preparing solid dosage forms of Zaleplon.
Yet, another objective of the present invention is to provide solid dosage forms of Zalelpon in such a way that it will comply with the reference product in terms of in vitro parameters like dissolution, disintegration, etc and in vivo parameters for bioequivalence such as Cmax, AUC, Tmax etc.
Summary of the invention
Accordingly, the present invention provides solid dosage forms comprising Zaleplon and stearic acid as lubricant. The present invention also provides process for preparing solid dosage forms of Zaleplon wherein the composition is prepared by blending Zaleplon with one or more pharmaceutically acceptable excipients lubricating the blend with stearic acid and finally filling into capsules or compressing into tablets.
Detailed description of the invention In an embodiment the solid dosage form of the present invention, is in the form of tablet or capsule.
In yet another embodiment of the present invention, solid dosage forms of Zaleplon further comprise one or more pharmaceutical acceptable excipients such as diluents, binders, disintegrants, surfactants and glidants. Suitable diluents used according to the present invention are selected from calcium phosphate-dibasic, calcium carbonate, sucrose, lactose, cellulose- microcrystalline, cellulose powdered, calcium silicate, kaolin, starch, starch pregelatinized, polyols such as mannitol, sorbitol, lactitol, xylitol, maltitol or combinations thereof.
Suitable binders used according to the present invention are selected from hydroxy propyl cellulose, hydroxypropyl methylcellulose, gelatin, hydroxy ethyl cellulose, povidone, polyvinyl alcohol, copovidone, ethylcellulose, starch and methylcellulose or a combination thereof. Suitable disintegrants used according to the present invention are selected from croscarmellose sodium, crospovidone, sodium starch glycolate, starch, sodium carboxymethylcellulose, pregelatinised starch, hydroxypropylcellulose or combination thereof.
Suitable surfactants used according to present invention are selected from sodium lauryl sulfate, polysorbates, sorbitan esters or combination thereof.
Suitable glidants used according to the present invention are selected from magnesium trisilicate, talc, tribasic calcium phosphate, glyceryl monostearate, glyceryl stearate and colloidal silica or a combination thereof.
In yet another embodiment of the present invention, when the solid dosage forms of Zaleplon is tablet, may be prepared by dry granulation (slugging or compaction), wet granulation, and direct compression. Preferably, the pharmaceutical composition of the present invention is prepared by direct compression or direct filling into capsules.
Suitable solvents used for preparing solid dosage forms by wet granulation are selected from water, ethanol, methanol, and isopropanol or a combination thereof.
In yet another embodiment of the present invention, there is provided process for the preparation of solid dosage forms comprising Zaleplon and stearic acid as lubricant as follows: i) blending Zaleplon with one or more diluents, binder, disintegrants, surfactants, glidants, ii) lubricating the blend of step (i) with stearic acid and iii) filling the blend into capsules or compressing into tablets.
The following examples further exemplify the invention and are not intended to limit the scope of the invention. It is obvious to those skilled in the art to find out the composition for other dosage forms and substitute the equivalent excipients as described in this specification or with the one known to the industry.
Example 1
The processing steps involved are: , j i) sifted and blended Zaleplon, microcrystalline cellulose, lactose monohydrate, pregelatinized starch, sodium lauryl sulfate and colloidal sijicoή s dioxide, : ii) lubricated the blend of step (i) with stearic acid and finally ; iii) the lubricated blend was filled into capsules or compressed into tajblets. The solid dosage forms of Zaleplon disclosed in example 2-6 were prepared by a similar procedure described in example 1. '
Example 2
Example 4
Example 5
Dissolution profile:
The Zaleplon capsules prepared according to the present invention were tested for drug release in water using USP apparatus 2 with paddle speed at 75 rpm. The samples of the media were periodically withdrawn and spectrophotometrically analyzed for Zaleplon content. The dissolution profile data is given in Table 1.
Table 1
Claims
1. A solid dosage form comprising Zaleplon and stearic acid as lubricant.
2. The solid dosage form as claimed in claim 1, further comprises one or more pharmaceutically acceptable excipients comprising diluents, binders, disintegrants, surfactants and glidants.
3. The solid dosage form as claimed in claim 2, wherein the diluent is selected from the group consisting of calcium phosphate-dibasic, calcium carbonate, lactose, sucrose, cellulose-microcrystalline, cellulose powdered, calcium silicate, kaolin, starch, starch pregelatinized, polyols such as mannitol, sorbitol, lactitol, xylitol, maltitol or combinations thereof.
4. The solid dosage form as claimed in claim 2, wherein the binder is selected from the group consisting of hydroxy propyl cellulose, hydroxypropyl methylcellulose, gelatin, hydroxy ethyl cellulose, povidone, polyvinyl alcohol, copovidone, ethylcellulose, starch and methylcellulose or a combination thereof.
5. The solid dosage form as claimed in claim 2, wherein the disintegrant is selected from the group consisting of croscarmellose sodium, crospovidone, sodium starch glycolate, starch, sodium carboxymethylcellulose, pregelatinised starch, hydroxypropylcellulose or combination thereof.
6. The solid dosage form as claimed in claim 2, wherein the surfactant is selected from the group consisting of sodium lauryl sulfate, polysorbates, sorbitan esters or combination thereof.
7. The solid dosage form as claimed in claim 2, wherein the glidant is selected from the group consisting of magnesium trisilicate, talc, tribasic calcium phosphate, glyceryl monostearate, glyceryl stearate and colloidal silica or a combination thereof.
8. A process for the preparation of solid dosage form comprising Zaleplon and stearic acid as lubricant, which comprises the steps of i) blending Zaleplon with one or more diluents, binder, disintegrants, surfactants, glidants, ii) lubricating the blend of step (i) with stearic acid and iii) filling the blend into capsules or compressing into tablets.
9. The solid dosage form as claimed in claim 1, in the form of tablets or capsules.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN488/CHE/2006 | 2006-03-17 | ||
| IN488CH2006 | 2006-03-17 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007107878A2 true WO2007107878A2 (en) | 2007-09-27 |
| WO2007107878A3 WO2007107878A3 (en) | 2008-04-03 |
Family
ID=38522808
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2007/000809 WO2007107878A2 (en) | 2006-03-17 | 2007-03-19 | Solid dosage forms of hypnotic agent |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2007107878A2 (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4626538A (en) * | 1983-06-23 | 1986-12-02 | American Cyanamid Company | [7-(3-disubstituted amino)phenyl]pyrazolo[1,5-a]pyrimidines |
| WO2004045589A1 (en) * | 2002-11-15 | 2004-06-03 | Elan Pharmaceuticals, Inc. | Modified release composition comprising a short-acting hypnotic for treatment of sleep disorders |
| WO2005023813A1 (en) * | 2003-09-04 | 2005-03-17 | Cipla Limited | Zaleplon synthesis |
| WO2006045618A1 (en) * | 2004-10-28 | 2006-05-04 | Jagotec Ag | Dosage form time-lagged of drugs for the therapy of insomnia |
-
2007
- 2007-03-19 WO PCT/IB2007/000809 patent/WO2007107878A2/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4626538A (en) * | 1983-06-23 | 1986-12-02 | American Cyanamid Company | [7-(3-disubstituted amino)phenyl]pyrazolo[1,5-a]pyrimidines |
| WO2004045589A1 (en) * | 2002-11-15 | 2004-06-03 | Elan Pharmaceuticals, Inc. | Modified release composition comprising a short-acting hypnotic for treatment of sleep disorders |
| WO2005023813A1 (en) * | 2003-09-04 | 2005-03-17 | Cipla Limited | Zaleplon synthesis |
| WO2006045618A1 (en) * | 2004-10-28 | 2006-05-04 | Jagotec Ag | Dosage form time-lagged of drugs for the therapy of insomnia |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2007107878A3 (en) | 2008-04-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107530348B (en) | Pharmaceutical composition containing JAK kinase inhibitor or pharmaceutically acceptable salt thereof | |
| US20080008752A1 (en) | Pharmaceutical compositions of memantine | |
| CZ16814U1 (en) | Pharmaceutical tablet comprising tablet matrix and tamsulosin | |
| AU746889B2 (en) | Pharmaceutical composition comprising entacapone or nitecapone as well as a cross-linked cellulose derivative | |
| EP2029134A1 (en) | Stabilized pharmaceutical compositions comprising fesoterodine | |
| WO2008027600A2 (en) | Imatinib compositions | |
| JP4773456B2 (en) | Oral preparation with improved bioavailability | |
| EP3013327A1 (en) | Pharmaceutical capsule composite formulation comprising tadalafil and tamsulosin | |
| TW201442712A (en) | Formulations of organic compounds | |
| US10668073B2 (en) | Pharmaceutical composition containing 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[4-methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione or a pharmaceutically acceptable salt thereof | |
| WO2007049868A1 (en) | Stabilized pharmaceutical oral preparation containing clopidogrel bisulfate | |
| WO2012136839A1 (en) | Dry formulation and pharmaceutical composition comprising fesoterodine or a salt or a solvate thereof | |
| US20130085145A1 (en) | Imatinib mesilate pharmaceutical tablet | |
| JP6679578B2 (en) | Ceritinib formulation | |
| WO2016012898A1 (en) | Oral pharmaceutical composition of lurasidone | |
| WO2007107878A2 (en) | Solid dosage forms of hypnotic agent | |
| WO2007090595A1 (en) | Solid formulations of valacyclovir hydrochloride | |
| WO2005089720A1 (en) | Valsartan tablets and the process for the preparation thereof | |
| WO2005065662A1 (en) | Solid dosage formulations of galantamine | |
| JP2005187464A (en) | Solid preparation and method for producing the same | |
| US20120329831A1 (en) | Pharmaceutical composition of donepezil | |
| KR101809886B1 (en) | Minimized Oral Dosage Formulation of Clarithromycin | |
| EP4260848A1 (en) | Pharmaceutical composition for solid dosage form containing nilotinib and process for its preparation | |
| JP7585043B2 (en) | Pharmaceutical compositions containing lenalidomide | |
| CN108721239A (en) | A kind of sustained release preparation and preparation method thereof for treating Alzheimer disease |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07734132 Country of ref document: EP Kind code of ref document: A2 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07734132 Country of ref document: EP Kind code of ref document: A2 |