COMPOSITION FOR FORMING A LASER-MARKABLE COATING AND PROCESS FOR FORMING A MARKING BY LASER EXPOSURE
CROSS-REFERENCE TO RELATED APPLICATION
The present application claims the benefit of U.S. Provisional Application No. 60/634.099 filed December 8, 2004.
BACKGROUND
Product and package labeling is becoming increasingly important in various industries, and it is generally beneficial to provide clearly visible, sharp, high contrast marks. In some applications, it can be beneficial to provide color images rather than black and white images.
Among conventional processes, printing, embossing, stamping and label application are predominant means for product marking. However, it can be desirable in a particular application to allow for frequent information changes, such as individualized product identification, coding, production date, lot number, or expiration date marking. Accordingly, there is a need for marking means which enables the rapid change of content.
Laser beam marking is a growing area of interest and offers several advantages over conventional marking technologies in terms of cost, marking speed, environmental concerns, safety and/or equipment maintenance. For example, markings can be formed by use of laser ablation. One disadvantage of employing conventional laser ablation means is that it can require strong interaction of the marking substrate with the laser beam to yield significant color or density changes in comparison with unmarked areas. Packaging materials such as plastic films, containers and glass bottles, can lack sufficient interaction with laser beam energy, the interaction can fail to yield sufficient contrast changes on the material, and/or the interaction can cause undesirable damage to the substrate surface.
A coating can be formed on the substrate that is capable of absorbing energy of a laser beam to yield visible marks on the coated substrate. This type of laser- markable coating can contain pigments, dyes, binders, as well as other coating additives. The coating composition can contain a binder which functions substantially
as a film forming agent. Besides being utilized for its film-forming function, binders can be used in various applications to obtain special effects in laser-markable coating compositions.
Use of conventional binders can lead to various adverse effects which can be described as interference mark effects. The formation of such interference marks can contribute to low mark quality of the marked material. Interference mark effects can be manifested in several different ways. For example, a whiteness, opacity, or haziness can occur in the area near laser exposure, which can be visible with the naked eye. A conventional binder can undergo a physical change when exposed to a laser beam to produce microvoids, bubbles, crosslinks, fine particulates and/or inclusions, which can result in opacity or otherwise degradation of the mark. The interference marks can lead to low mark density, poor color purity, and/or visually unsharp/distorted images in the marked region of the material. Machine and/or human readability can be reduced when the intended marks to be formed by laser exposure are lower in quality than required.
In view of the above, a need exists for providing means for improving laser mark quality for a laser-markable material, for example, by reducing or substantially eliminating the formation of interference marks in the material as a result of the laser exposure.
SUMMARY
In accordance with one aspect, a composition for forming a laser-markable coating is provided, comprising: (a) a first component of a color-forming agent, wherein upon exposure to a laser the first component is capable of reacting with a second component of the color-forming agent to generate a color; and (b) a binder comprising a substituted or unsubstituted polyurethane.
In accordance with another aspect, a laser-markable material is provided, comprising: (a) a coating comprising a substituted or unsubstituted polyurethane compound; and (b) a laser-markable layer, wherein the coating is in contact with the laser-markable layer.
In accordance with another aspect, a process of forming a marking by laser exposure is provided, comprising applying a composition comprising the coating
composition to a substrate to form a coating, and exposing at least a part of the coating to a laser.
In accordance with a further aspect, a process of forming a marking by laser exposure is provided, comprising combining the coating composition with a second composition comprising the second component, applying the resulting composition to a substrate to form a coating, and exposing at least a part of the coating to a laser.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows an image of two exemplary coatings exposed to a CO2 laser.
Figures 2A to 2H are images of various exemplary coatings exposed to a CO2 laser.
Figures 3 A to 3E are images of various exemplary coatings exposed to a CO2 laser.
Figures 4A to 4H are images of various exemplary coatings exposed to a CO2 laser.
DETAILED DESCRIPTION
The coating composition is useful for forming a coating such as a laser- recordable layer on a substrate. The coating can constitute a part of a multi-layered laser-markable material. By employing the coating composition, a laser mark of relatively high quality can be obtained.
The coating composition includes at least one component of a color-forming agent. The color-forming agent can contribute to the generation of a color upon exposure to a laser. For example, the color-forming agent can include at least one component which reacts with at least another component upon exposure to a laser, wherein such reaction results in the generation of a color. The color-forming agent can include an electron donor dye precursor, an electron acceptor developer, or both
such components, wherein the reaction between such compounds upon exposure to a laser results in a generation of a color.
In a preferred embodiment, multiple coating compositions can be formed wherein a first coating composition includes the electron donor dye precursor and the second coating composition includes the electron acceptor developer. Such first and second compositions can be maintained separately to improve stability of the compositions, and can be combined and/or mixed together prior to use.
The electron donor dye precursor can include, for example, a triphenylmethane phthalide series compound, a fluorane series compound, a phenothiazine series compound, an indolyl phthalide series compound, a leucoauramine series compound, a rhodamine lactam series compound, a triphenylmethane series compound, a triazene series compound, a spiropyran series compound, a fluorene series compound, a pyridine series compound, a pyradine series compound and a combination thereof. The electron acceptor developer, for reacting with the electron donor dye precursor, can include an acidic substance such as activated bentonite, a metal salt of salicylate, a phenol compound, an organic acid or a metallic salt thereof, an oxybenzoate and a combination thereof.
The composition can include at least one auxiliary additive such as, for example, a surfactant, an anti-foam agent, a plasticizer, a rheological agent, a biocide, an antistatic agent, a solvent, a photoinitiator for radiation curing or combinations thereof. The auxiliary additive can also include an additive for improving laser- marking performance such as a heat transfer agent, a melting agent, an ultraviolet ray absorbing agent, an antioxidant or combinations thereof.
The heat transfer agent can include a compound which is capable of absorbing CO2 laser emission energy at 943 cm"1, and converting same to heat. The heat transfer agent can include, for example, mica, fumed silica, fumed alumina, and various inorganic and organic compounds having strong absorption in the wavelength range of 900 cm"1 to 1000 cm"1. The melting agent can function to improve laser responsiveness. Examples can include an aromatic ether, a thioether, an ester aliphatic amide, a ureide or combinations thereof. The ultraviolet ray absorbing agent can include, for example, a benzophenone series ultraviolet ray absorbing agent, a benzotriazole series ultraviolet ray absorbing agent, a salicylic acid series ultraviolet
ray absorbing agent, a cyanoacrylate series ultraviolet ray absorbing agent, an oxalic acid anilide series ultraviolet ray absorbing agent or combinations thereof. The antioxidant can include, for example, a hindered amine series antioxidant, a hindered phenol series antioxidant, an aniline series antioxidant, a quinoline series antioxidant or combinations thereof.
The coating composition also includes a binder which can function as a medium for the color-forming agent. Preferably, the binder is capable of being processed into a coating or film. The binder includes a substituted or unsubstituted polyurethane compound. The substituted or unsubstituted polyurethane compound can include a polyurethane formed from the reaction of an isocyanate with, for example, various organic compounds as discussed in "Polyurethane Handbook," 2nd Ed., edited by Dr. Gunter Oertel, Hanser Publishers, Munich, pp. 17-25 (1994), the contents of which are herein incorporated by reference. Any substituted or unsubstituted polyurethane compound suitable for forming a coating can be used such as, for example, a polyester-derived polyurethane, a polyether-derived polyurethane, a polycarbonate-derived polyurethane, a castor oil-derived polyurethane, or combinations thereof. The substituted or unsubstituted polyurethane compound can be present in an amount of at least about 50% by weight of the total binder content. Preferably, the substituted or unsubstituted polyurethane compound can be present in an amount effective to reduce or substantially eliminate the formation of interference marks. For example, the substituted or unsubstituted polyurethane can yield substantially no interference marks after exposure to laser energy, for example, a CO2 laser beam. Preferably, the binder is substantially chemically inert with respect to the color-forming agent, and therefore preferably does not interference with the color- forming reaction. The binder can be a water-soluble resin.
In an exemplary embodiment, the polyurethane compound can constitute substantially all of the binder present in the coating composition. Alternatively, additional binder materials can be used in combination with the polyurethane compound. Examples of such additional binder materials include starch and modified derivatives, cellulose and modified derivatives, gelatin, casein, gum arabic, pectin, sodium alginate, silicate resin, polyvinyl alcohol, polyacrylic resin, epoxy, polystyrene, polyester, polyacrylic amide, styrene-acrylic acid copolymer, styrene-
butadiene copolymer, ethylene-vinyl acetate copolymer, styrene-maleic anhydride copolymer, ethylene-maleic anhydride copolymer, isobutylene-maleic anhydride copolymer, polyvinyl pyrrolidone, acrylic, ethylene-acrylic acid copolymer, vinyl acetate-acrylic acid copolymer and combinations thereof. An additional binder can be employed, for example, when a special technical property which is imparted by such additional binder, is desired.
The coating composition can contain any suitable amount of binder. In an exemplary embodiment, the binder can be present in an amount of at least about 50% of the total solids weight of the coating composition, hi an exemplary embodiment, the binder can be present in an amount from about 5% to about 40%, more preferably from about 10% to about 20%, and most preferably about 15% of the total solid weight in the coating composition.
The coating composition can be a single-part coating composition which contains substantially all of the various components of the coating composition. Alternatively, multiple coating compositions can be used to provide storage stability prior to use of the compositions, and the binder can be incorporated into any of the multiple coating compositions.
The coating composition can be used to form a coating or film using any suitable technique. For example, the coating or film can be aqueous-based, solvent- based such as an organic-solvent-based, radiation-curable such by as UV radiation, and/or an electron beam-curable. The binder containing the polyurethane compound can be employed as the binder material to reduce or substantially eliminate interference mark effects independent of the specific coating formation method of the coating composition.
Any suitable electron donor dye precursor that is compatible with an electron acceptor developer can be used in the color-forming agent. Compounds represented by general structural Formula 1 can be employed which are capable of being incorporated into the microcapsules in very high concentration and can providing high mark densities: Formula 1
wherein, R
1 and R
2 represent a alkyl group, such as a butyl group, a sec-butyl group, a tert. -butyl group, a propyl group, an ethyl group, a methyl group, etc.; R
3 represents a hydrogen, or a alkyl group, such as a butyl group, a sec-butyl group, a tert. -butyl group, a propyl group, a ethyl group, a methyl group, etc.; and R
4 represents an imino-benzene group or a hydrogen. An exemplary compound is shown below as Formula 2: Formula 2
In a preferred embodiment, the solubility of the electron donor dye precursor can be greater than about 10g/100g of ethyl acetate, more preferably greater than about 15g/100g of ethyl acetate, and most preferably greater than about 18g/100g of ethyl acetate.
In an exemplary embodiment, the electron donor dye precursor contains greater than about 80% by weight, more preferably greater than about 90%, and most preferably about 100% by weight, of compound(s) represented by structural the above Formula 1.
The color-forming agent can be incorporated in the coating composition using any suitable technique. For example, the color-forming agent can be incorporated by a) dispersing the color-forming agent in solid powder form into the binder medium, b) dissolving the color-forming agent in a solvent and adding the solution of color forming agents to the binder medium, and c) micro-encapsulating the color forming agents and dispersing the encapsulated color forming agents into the binder medium. In an exemplary embodiment, the color forming agents are microencapsulated and dispersed in the binder medium.
At least one of the components of the color-forming agent can be present in the coating composition in the form of a microcapsule. For example, the electron donor dye precursor and/or the electron acceptor developer can be microencapsulated. This can depend on, for example, whether it is advisable to protect either or both of such components from being contacted by any other components of the coating composition. In an exemplary embodiment, the dye precursor can be microencapsulated and separated from the developer.
An exemplary process for micro-encapsulating a component of the color- forming agent such as an electron donor dye precursor will now be described. For encapsulation, a surface polymerization process can be employed, such that the electron donor dye precursor that becomes a core of the microcapsules is dissolved or dispersed in a hydrophobic organic solvent to prepare an oily phase, which is then mixed with an aqueous phase obtained by dissolving a water-soluble polymer in water. The resulting material is then subjected to emulsification and dispersion by using, for example, an homogenizer, followed by heating, so as to conduct a polymer- forming reaction at the interface of the oily droplets, whereby a microcapsule wall of a polymer substance is formed. Reactants for forming the polymer substance can be added to the interior of the oily droplets and/or the exterior of the oily droplets. Specific examples of the polymer substance include polyurethane, polyurea, polyamide, polyester, polycarbonate, a urea-formaldehyde resin, a melamine resin. Among these, polyurethane, polyurea, polyamide, polyester and polycarbonate are preferred, and polyurethane and polyurea are particularly preferred. For example, in the case where polyurea is used as the microcapsule wall material, the microcapsule wall can be easily formed by reacting a polyisocyanate, such as diisocyanate,
triisocyanate, tetraisocyanate or a polyisocyanate prepolymer, with a polyamine, such as diamine, triamine or tetramine, a prepolymer having two or more amino groups, piperazine or a derivative thereof, or a polyol, in the aqueous phase by the interface polymerization process.
A composite wall formed with polyurea and polyamide or a composite wall formed with polyurethane and polyamide can be prepared in such a manner that, for example, a polyisocyanate and a secondary substance for forming the capsule wall through reaction therewith (for example, an acid chloride, a polyamine or a polyol) are mixed with an aqueous solution of a water-soluble polymer (aqueous phase) or an oily medium to be encapsulated (oily phase), and subjected to emulsifϊcation and dispersion, followed by heating. The production process of the composite wall formed with polyurea and polyamide is described in detail in JP- A-58-66948.
As the polyisocyanate compound, a compound having an isocyanate group of three or more functional groups is preferred, and a difunctional isocyanate compound may be used in combination therewith. Specific examples thereof include a diisocyanate, such as xylene diisocyanate or a hydrogenated product thereof, hexamethylene diisocyanate or a hydrogenated product thereof, tolylene diisocyanate or a hydrogenated product thereof and isophorone diisocyanate, as the main component; a dimer or a trimer thereof (burette or isocyanaurate); a compound having polyfunctionality as an adduct product of a polyol, such as trimethylolpropane, and a difunctional isocyanate, such as xylylene diisocyanate; a compound of an adduct product of a polyol, such as trimethylolpropane, and a difunctional isocyanate, such as xylylene diisocyanate, having a polymer compound, such as polyether having an active hydrogen, such as polyoxyethylene oxide, introduced therein; and a formalin condensation product of benzeneisocyanate.
The compounds described in JP-A-62-212190, JP-A-4-26189, JP-A-5-317694 and Japanese Patent Application No. 8-268721 can be preferably used. Specific examples of the polyol and/or the polyamine added to the aqueous phase and/or the oily phase as one constitutional component of the microcapsule wall through the reaction with the polyisocyanate include propylene glycol, glycerin, trimethylolpropane, triethanolamine, sorbitol and hexamethylenediamine. In the case where a polyol is added, a polyurethane wall is formed. An exemplary
polyisocyanate, polyol, reaction catalyst and polyamine for forming a the microcapsules are described in "Polyurethane Handbook" written by Keiji Iwata, and published by Nikkan Kogyo Shimbun, Ltd. (1987) and "Polyurethane Handbook," 2nd Ed., edited by Dr. Gϋnter Oertel, Hanser Publishers, Munich (1994).
In an exemplary embodiment, at least about 90% of the total volume of the dye precursor particles is present in microcapsules having an average particle diameter of from about 0.3 μm to about 12 μm, preferably from about 0.2 μm and about 5 μm, and most preferably from about 0.2 μm and about 2 μm. Preferably, the microcapsules have an average particle diameter of from about 0.3 to about 12 μm, preferably from about 0.2 μm and about 5 μm, and most preferably from about 0.2 μm and about 2 μm. The thickness of the microcapsule wall can be from about about O.Olμm and about 0.3μm. Particle size of the microcapsules in the suspension can be measured by diluting the suspension into aqueous solution and using laser scattering method based on Mie-scattering theory to measure the particle size and distribution. Typical equipment used for such measurement are Horiba's LA series, Beckman Coulter's LS series or Malvern Instruments' Mastersizer series.
The microencapsulation reaction can also be controlled so that the microcapsule wall has a glass transition temperature, Tg, of from about 150°C to about 1900C, preferably from about 160°C to about 1800C, and most preferably from about 165°C to about 175°C. The Tg of the microcapsule wall can be measured by using conventional differential thermal analysis methods, such as DSC (Differential Scanning Calorimeters) or DDSC (Dynamic DSC), which measures specific heat (Cp) change over different temperature ranges. Both a microcapsule-containing suspension and a blank suspension are placed in the sample trays before measurement. Typical equipment used for such measurements are Perkin Elmer Diamond DSC, Sapphire DSC, HyperDSC™, or TA Instruments Q-series.
Various reaction conditions of the microcapsule preparation process can be controlled and adjusted in order to obtain microcapsules having the preferred characteristics. These conditions cam include, for example, emulsification process of the electron donor dye precursor, addition rates and amounts of the polyisocyanate and polyamine to form the microcapsule wall, as well as mixing and reaction temperature, time, and agitation, hi the reaction, the reaction rate can be increased,
for example, by either maintaining a high reaction temperature or by adding an appropriate polymerization catalyst.
The microcapsule wall may further contain, depending on the specific application, a metal-containing dye, a charge adjusting agent, such as nigrosin, and/or other additive substances. These additives may be contained in the capsule wall during wall formation or at other times during the microencapsulation process. In order to adjust the charging property of the surface of the capsule wall, a monomer, such as a vinyl monomer, can be graft-polymerized depending on necessity.
Furthermore, in order to make a microcapsule wall having excellent substance permeability at low temperature and having the quality of high coloring properties, a plasticizer can be used that is suitable for the polymer that is used as the wall material. The plasticizer can have a melting point of about 50 degrees C or more, and more preferably about 120 degrees C or more. Among plasticizers, those in a solid state at ordinary temperature can be preferably employed. For example, in the case where the wall material comprises polyurea or polyurethane, as a plasticizer a hydroxyl compound, a carbamate compound, an aromatic alkoxy compound, an organic sulfoneamide compound, an aliphatic amide compound, an arylamide compound or combinations thereof can be used.
As a hydrophobic organic solvent used for forming the core of the microcapsule by dissolving the electron donor dye precursor compound upon preparing the oily phase, an organic solvent having a boiling point of from about 100 to about 300 degrees C can be used. Specific examples thereof include an ester compound, dimethylnaphthalene, diethylnaphthalene, diisopropylnaphthalene, dimethylbiphenyl, diisopropyldiphenyl, diisobutylbiphenyl, 1 -methyl- 1- dimethylphenyl-2-phenylmethane, 1 -ethyl- 1 -dimethylphenyl- 1 -phenylmethane, 1 - propyl- 1-dimethylphenyl-l-phenylmethane, triarylmethane (such as tritoluylmethane or toluyldiphenylmethane), a terphenyl compound (such as terphenyl), an alkyl compound, an alkylated diphenyl ether (such as propyldiphenyl ether), hydrogenated terphenyl (such as hexahydroterphenyl) and diphenylterphenyl. Among these, an ester compound can be preferably used from the standpoint of emulsification stability of the emulsion dispersion. Examples of the ester compound include a phosphate, such as triphenyl phosphate, tricresyl phosphate, butyl phosphate, octyl phosphate or
cresylphenyl phosphate; a phthalate, such as dibutyl phthalate, 2-ethylhexyl phthalate, ethyl phthalate, octyl phthalate or butylbenzyl phthalate; dioctyl tetrahydrophthalate; a benzoate, such as ethyl benzoate, propyl benzoate, butyl benzoate, isopentyl benzoate or benzyl benzoate; an abietate, such as ethyl abietate or benzyl abietate; dioctyl adipate; isodecyl succinate; dioctyl azelate; an oxalate, such as dibutyl oxalate or dipentyl oxalate; diethyl malonate; amaleate, such as dimethylmaleate, diethyl maleate ordibutyl maleate; tributyl citrate; a sorbate, such as methyl sorbate, ethyl sorbate or butyl sorbate; a sebacate, such as dibutyl sebacate or dioctyl sebacate; an ethylene glycol ester, such as a formic acid monoester or diester, a butyric acid monoester or diester, a lauric acid monoester or diester, a palmitic acid monoester or diester, a stearic acid monoester or diester, or an oleic acid monoester or diester; triacetin; diethyl carbonate; diphenyl carbonate; ethylene carbonate; propylene carbonate; and a borate, such as tributyl borate or tripentyl borate.
The hydrophobic organic solvent can be used alone or in combinations of two or more. Among these, tricresyl phosphate can be preferably used, either singly or as a mixture with other solvents since it provides high emulsion stability. In the case where the electron donor dye precursor to be encapsulated has poor solubility in the hydrophobic organic solvent, a low boiling point solvent having high solubility can additionally be used in combination. Examples of the low boiling point solvent include ethyl acetate, isopropyl acetate, butyl acetate and methylene chloride.
In an exemplary embodiment where the electron donor dye precursor compound is used in the laser-sensitive recording layer of the laser-sensitive recording material, the content of the electron donor dye precursor is preferably from about 0.1 to about 5.0 g/m , and more preferably from about 1.0 to about 4.0 g/m . While not wishing to be bound by any particular theory, it is believed that when the content of the electron donor dye precursor is in the range of from about 0.1 to 5.0 g/m2, a sufficient coloring density can be obtained, and when the content is 5.0 g/m2or less, a sufficient coloring density can be achieved while the transparency of the laser-sensitive recording layer can also be maintained.
During microcapsule formation, water-soluble resins can be added to the aqueous phase of the reaction mixture as a binder in order to stabilize the emulsified dispersion and formed microcapsules. The type and addition amount of the water-
soluble resins can be selected so that the viscosity of the coating composition has a viscosity of from about 5 centipoise (cP) to about 30 cP, preferably from about 10 cP to about 25 cP, and most preferably from about 10 cP to about 20 cP. Viscosity can be measured using Brookfield Programmable DV-II+ viscometer with small sample adapter plus a S21 spindle at 100-200 RPM. Regular RV series spindle can also be used depending on sample quantity.
In the preparation of the coating composition, the mixing ratio of the oily phase to the aqueous phase (oily phase weight/aqueous phase weight) is preferably from about 0.02 to about 0.6, and more preferably from about 0.1 to about 0.4. For example, when the mixing ratio is in the range of from 0.02 to 0.6, a suitable viscosity can be maintained. This can provide both an improved productivity of use for coating the composition as well as optimized stability of the coating composition.
In order to further uniformly emulsify and disperse the oily phase and the aqueous phase, a surfactant can be added to at least one of the oily phase and the aqueous phase. Any suitable surfactant for emulsification can be used. The addition amount of the surfactant can be from about 0.1% to about 5%, more preferably from about 0.5 to about 2%, based on the weight of the oily phase. As the surfactant contained in the aqueous phase, one that does not cause precipitation or aggregation through an action with the binder can be used by appropriately selecting from anionic and nonionic surfactants. Preferred examples of the surface-active agent include sodium alkylbenzenesulfonate, sodium alkylsulfate, sodium dioctyl sulfosuccinate and a polyalkylene glycol (such as poly oxy ethylene nonylphenyl ether).
The emulsification can be conducted by subjecting the oily phase containing the foregoing components and the aqueous phase containing the binder and the surfactant to a device generally used for fine particle emulsification, such as high speed agitation or ultrasonic wave dispersion by using a known emulsifying apparatus, such as a homogenizer, Manton Gaulin, an ultrasonic wave disperser, a dissolver or a KADY mill. After emulsification, the emulsion can be heated to a temperature of from 30 to 70° C for accelerating the capsule wall-forming reaction. During the reaction, water can be added to the emulsion to decrease the probability of collision of the capsules or that sufficient agitation is conducted to prevent aggregation of the capsules.
A dispersion containing the polyurethane compound may further be added during the reaction for reducing or substantially preventing aggregation. Formation of a carbon dioxide gas can be observed with progress of the reaction, and termination of the formation can be determined as completion of the capsule wall-forming reaction. In general, the reaction can be conducted for several hours to obtain the objective microcapsules.
Examples of the electron acceptor compound, which is capable of reacting with the electron donor dye precursor, include an acidic substance, such as activated bentonite, metal salt of salicylate, phenol compound, organic acid or its metallic salt, oxybenzoate or combinations thereof.
Specific examples thereof include a bisphenol compound, such as 2,2-bis(4'- hydroxyphenyl)propane (generic name: bisphenol A), 2,2-bis(4- hydroxyphenyl)pentane, 2,2-bis(4'-hydroxy-3',5'-dichlorophenyl)propane, l,l-bis(4'- hydroxyphenyl)cyclohexane, 2,2-bis(4'-hydroxyphenyl) hexane, l,l-bis(4'- hydroxyphenyl)propane, l,l-bis(4'-hydroxyphenyl)butane, l,l-bis(4'- hydroxyphenyl)pentane, l,l-bis(4'-hydroxyphenyl)hexane, 1,1 -bis (41- hydroxyphenyl)heptane, l,l-bis(4'-hydroxyphenyl) octane, l,l-bis(4'- hydroxyphenyl)-2-methylpentane, 1 , 1 -bis(4'-hydroxypenyl)-2-ethylhexane, 1 , 1 -bis(4'- hydroxyphenyl)dodecane, 1 ,4-bis(p-hydroxyphenylcumyl)benzene, 1 ,3-bis(p- hydroxyphenylcymyl)benzene, bis(p-hydroxyphenyl) sulfone, bis(3-allyl-4- hydroxyphenyl)sulfone and bis(p-hydroxyphenyl)acetic acid benzyl ester; a salicylic acid derivative, such as 3,5-di-.alpha.-methylbenzylsalicylic acid, 3,5-di-tert- butylsalicylic acid, 3-.alpha.-.alpha.-dimethylbenzylsalicylic acid and 4-(.beta.-p- methoxyphenoxyethoxy)salicylic acid; a polyvalent metallic salt thereof (in particular, a zinc salt and an aluminum salt are preferred); an oxybenzoate, such as p- hydroxybenzoic acid benzyl ester, p-hydroxybenzoic acid 2-ethylhexyl ester and .beta.-resorcinic acid 2-phenxyethyl ester; and a phenol compound, such as p- phenylphenol, 3,5-diphenylphenol, cumylphenol, 4-hydroxy-4'- phenoxydiphenylsulfone. Among these, the metal salts of salicylate can be preferred employed, for example, zinc salicylate. For example, it is possible to achieve good coloring characteristics by using such developer. Additional electron acceptor developers that can be used are disclosed in U.S. Patent Nos. 6,797,318, 5,409,797
and US 5,691,757, the contents of which are incorporated by reference herein. The electron acceptor compounds may be used singly or in a combination of two or more.
The electron acceptor compound may be used, for example, as a solid dispersion prepared in a sand mill with water-soluble polymers, organic bases, and other color formation aids or may be used as an emulsion dispersion by dissolution in a high boiling point organic solvent that is only slightly water-soluble or is water- insoluble, mixing with waterborne polyurethane and its modified derivatives as the binder (aqueous phase), followed by emulsification, for example, by a homogenizer. In this case, a low boiling point solvent can be used as a dissolving assistant depending on necessity.
Furthermore, the electron acceptor compound and the organic base may be separately subjected to emulsion dispersion, and also may be dissolved in a high boiling point solvent after mixing, followed by subjecting to emulsion dispersion. The emulsion dispersion particle diameter can be about 1 μm or less. In this case, the high boiling point organic solvent used can be appropriately selected, for example, from the high boiling point oils described in JP-A-2-141279. Among these, the use of an ester compound is preferred from the standpoint of emulsion stability of the emulsion dispersion, and tricresyl phosphate is particularly preferred. The oils can be used as a mixture thereof and as a mixture with other oils.
In an exemplary coating composition, the binder can be present from an amount of about 5% to about 50%, preferably from about 10 % to about 30%, more preferably about 15% of total solid weight of the coating composition containing the electron acceptor developer.
A coating composition containing the electron acceptor developer and a second coating composition containing the electron donor dye precursor can be mixed together to prepare a mixed coating dispersion which is subsequently coated on a substrate for use as a laser-sensitive recording layer for laser marking. In this process, the two coating compositions can be mixed in any suitable ratio, for example, such that the ratio of total weight of electron donor dye precursor(s) and the total weight of the developer(s) is from about 1:0.5 to about 1:30, preferably from about 1:1 to about 1:0.
In order to safely and uniformly coat the laser-sensitive recording layer coating composition and to maintain the strength of the coated film, besides the 2 coating compositions described above, extra amount of binder resins and auxiliary additives can be used. In addition, to coat a substrate with the mixed costing dispersion to prepare a laser-sensitive recording layer, a known coating method applied to an aqueous or organic solvent series coating composition can be used for coating the laser-sensitive recording layer coating composition on a substrate.
In an exemplary embodiment, a laser-markable material is provided which includes a coating comprising a substituted or unsubstituted polyurethane compound; and a laser-markable layer. The coating can be in contact with the laser-markable layer.
The laser-markable material can include additional layers such as a protective layer, an intermediate layer, an undercoating layer (a primer layer), a light reflection preventing layer, and the like. The protective layer can be the uppermost layer of the material, and can be arranged above and/or in contact with the laser-sensitive recording layer. The function of the protective layer is to provide protection for the laser-sensitive recording layer against physical damage such as rubbing, moisture attack, to strengthen the resistance against instant heat impact, etc. The intermediate layer can be applied on the laser-sensitive recording layer. The function of this layer is to reduce or prevent intermixing of the layers and also for blocking a gas (such as oxygen) that may be harmful in order to preserve an image after formation. The undercoating layer, light reflection preventing layer and other functional layers such as an adhesion layer can be applied onto the substrate before coating the laser- sensitive recording layer.
Since the protective layer is of interest to provide protection for the color forming layer in a laser markable material, a protective coating composition can also be provided according to an exemplary embodiment. For example, the protective coating composition not only can provide the demanded protection as described above, but also be effective to reduce or eliminate the formation of interference marks that affect the mark quality of a laser marked material. The binder quantity for the protective layers can be, for example, about 50% of total solid weight in the coating composition. The percentage of binder quantity can vary in from about 10% to about
80% according to different application, more preferably from about 30% to about 60% by weight.
Using substantially only a polyurethane compound as the binder for the additional layers is preferred for a good mark quality. A combination between polyurethane and other type of resins, such as acrylic, epoxy, cellulose, etc., can be a selected when a special technical property is demanded for a laser markable material. For example, the amount of polyurethane and its modified derivatives is preferably not less than 50% of the total binder quantity in a coating composition to reduce or avoid intensifying the interference mark effect.
The additional layer(s) can include auxiliary additives such as regular coating additives, such as surfactants, anti-foam agents, plasticizers, rheological agents, biocides, antistatic agents, solvents, water, photoinitiator for radiation curing, hardening agents, etc. For example, the additional layer(s) can include a fine particle substance having a refractive index of from about 1.45 to about 1.75 from the standpoint that the transparency of the laser markable material is maintained.
Aspects of the present invention will now be illustrated and exemplified in greater detail with reference to the following examples, but it will be understood that the invention is not necessarily limited thereto.
EXAMPLES
Example 1: Laser Exposure of Various Binder Materials
Several different types of binder materials were exposed to a CO2 laser to determine the effects thereof. The experimental procedure included the following: a) coating the sample resin solution on a 1" x 4" glass slide using a K Control Coater (RK Print Coat Instruments, Ltd.), wherein No. 8 coating bar is used to product a film thickness of 100 micrometers when wet; b) drying the coated resin solution overnight under ambient condition; c) scanning the coated resins with a Domino SlOO laser maker (Domino Amjet, Inc.) under equal laser intensity; d) observing interference mark (such as micro bubble, foaming effect) formation under Leica GZ6 microscope, and ranking the amount of interference markings (foaming effect) formed from 1 to 10; e) rescanning the sample having the maximum foaming effect and the sample having the minimum foaming effect with varying laser dosages by adjusting the scan
speed, and observing the differences in foaming effect in relation to the rate of laser irradiation. The experimental results are shown in the following Table 1-1 :
Table 1-1
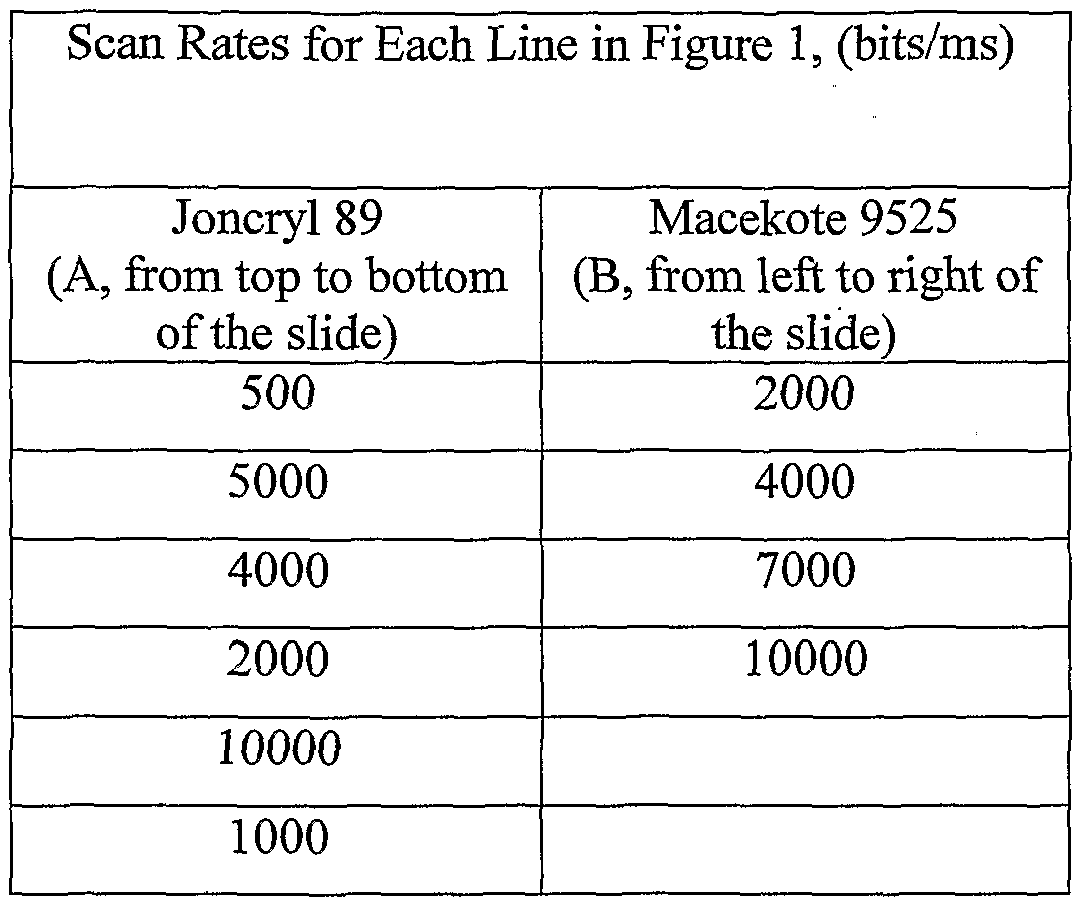
As can be seen from Table 1-1, Joncryl 89 yielded the highest degree of foaming effect, and Macekote 9525 yielded the least degree of foaming effect at the scan rate of 2000 bits/ms (bits per millisecond). The two were rescanned (according step(e) discussed above) with varying laser scanning rates, and the results are shown in Figure 1, wherein A corresponds to Joncryl 89 and B corresponds to Macekote 9525.
The various scan rates employed to generate the marks shown in Figure 1 are summarized in Table 1-2:
Table 1-2
As clearly shown in Figure 1, Joncryl 89 (A) and Macekote 9525 (B) produced very different responses when scanned at comparable rates. Joncryl 89 foamed conspicuously while Macekote 9525 had only minimal foaming effect. Especially at the scan rate of 10,000 bits/ms, Macekote 9525 had comparatively little response to the laser beam. The experimental results show that Macekote 9525 (a polyether- based polyurethane) provided superior results in comparison with the other tested resins in terms of generating less interference marks under CO2 laser exposure.
Example 2: Laser Exposure of Various Inventive and Comparative Binders
The effects of laser exposure of five inventive polyurethane dispersions (Sample Nos. 3 to 7) were compared to those of polyvinyl alcohol and styrened acrylate (comparative Sample Nos. 1 and 2, respectively). A blank glass slide was used as a reference.
Table 2-1
The experimental procedure included the following: a) coating the tested sample solution on a 1" x 4" glass slide using a K Control Coater (RK Print Coat Instruments, Ltd.), wherein No. 7 coating bar was used to produce a film thickness of 80 micrometers when wet; b) drying the coated sample solution overnight under ambient condition; c) exposing the coated samples with a Domino SlOO laser maker (Domino Amjet, Inc.) under a matrix exposure. The matrix exposure consisted of 70 of the same mark, the letter "M", and was applied onto each of the coated samples, using a Domino S-100 CO2 laser marker with a f=80mm lens, which provides 35mm x 35mm marking field and a spot size of from about 250 to about 280 μm. The design of the test marking matrix was such that each row consisted of 7 characters, with increasing laser power output from 26.5% to 100% (5.2W→19.6W from left to right), and 20% power increment between neighboring characters, and each column consisted of 10 characters, with increasing marking speed from 1300 bits/msec to 9500 bits/msec (from bottom to top), and 20% speed increment between neighboring characters.
A picture was taken for the CO2 laser matrix-exposed samples on a black background. The number of white "M" letters and degree of whiteness of the letter were compared to determine the sample that had minimum response to CO2 laser energy. The photographs are shown in Figures 2A to 2H, which correspond to Polyvinyl Alcohol, Joncryl 89, MaceKote 9525, Alberdingk U400N, Alberdingk U
2101 VP, Alberdingk U 9152VP, Alberdingk CUR 21 and a blank glass slide, respectively. The experimental results show that polyurethane and its derivatives including polyether-based polyurethane, polyester-based polyurethane, polycarbonate-based polyurethane and castor oil-based polyurethane can provide improved performance in comparison with polyvinyl alcohol and styrened acrylate, in reducing interference marking caused by CO2 laser exposure.
Example 3: Preparation and Laser Exposure of a Coating Composition formed from Two Parts
In this experiment, various polyurethane compounds were used as a binder in making two parts of a coating composition, in which Part A was a coating composition containing the micro-encapsulated dye precursor, and Part B was a coating composition containing the electron acceptor-type developer. Polyvinvl alcohol was used in this experiment as a reference binder to compare the experimental results.
1) Preparation of Part A - coating composition containing micro-encapsulated dye precursor
13.3 g of electron donor-type dye precursor (PSD-184, Nippon Soda) and 0.47 g of a UV light absorbing agent (Tinuvin P, Ciba Geigy Corp.) were added in 20 g of ethyl acetate and dissolved by heating up to 7O0C, and then cooled down to 45°C. 12.6 g of capsule wall material (D-140N, Mitsui Takeda Chemical Co., Ltd.) was added into the ethyl acetate solution. The above ethyl acetate solution was added in 53 g of 6%w/w polyvinyl alcohol aqueous solution (Kurary Poval MP- 103, Kuraray Co., Ltd.) and emulsified with a homogenizer at 15,000 rpm for 5 minutes. 8Og of water and 0.75 g of tetraethylenepentamine were added and mixed with a stirrer at 400 rpm for 4 hours for encapsulation reaction. Part A was completed, and the coating composition is referred to as Aref.
The particle size distribution of the encapsulated electron donor-type dye precursor particles and the viscosity of the liquid coating composition were measured with Beckman Coulter's LS-100Q particle size analyzer and Brookfield
Programmable DV-II+ viscometer with S21 small size spindle at 100-200 RPM. The Tg of the microcapsule wall was measured by using Perkin Elmer's Diamond DSC, Sapphire DSC, HyperDSC™, or TA Instruments' Q-series. A blank suspension without microcapsule was prepared under the same conditions as a reference sample. Both the microcapsule-containing suspension and the blank suspension were placed in the sample trays before measurement.
Other test solutions (A1, A2; A3, and A4) were made in the same manner as Aref, wherein the quantity of binder sample was adjusted if necessary to equal that of Aref based on its solid content. Table 3-1 lists the various Part A coating compositions which were formed:
Table 3-1
2) Preparation of Part B - coating composition containing the electron acceptor- type developer
4.2g of a UV light absorbing agent (Tinuvin 328, Ciba Geigy), 1.0 g of tricresylphosphate, and 36.4g of developer (RO54, Sanko Chemicals) were added in 16.Og of ethyl acetate, and dissolved by heating up to 700C. The ethyl acetate solution was added to the aqueous solution described in Table 3-2 and dispersed with a homogenizer at 12,000 rpm for 5 minutes.
Table 3-2
Aqueous solution for emulsified developer dispersion
Water, 68.4g
15%w/w Poly-vinylalcohol (Poval PVA205, Kurary Co.,Ltd.), 19.8g 8%w/w Poly-vinylalcohol (Poval PVA217, Kurary Co.,Ltd.), 55.7g Surfactant A (W-502, Waco Pure Chemical Industries), 11.2g
Surfactant B (NEOPELEX G- 15, Kao), 11.2g
Part B was completed at this step, and the coating composition is hereinafter referred to as Bref.
Other sample Part B solutions (B1, B2, B3, and B4) were made in the same manner as Bref, wherein the quantity used for each sample was adjusted if necessary to equal that of Bref, based on its solid content. Table 3-3 lists the sample Part B coating compositions which were formed:
Table 3-3
3) Preparation of coating pot solutions by mixing Part A and Part B
Each of the Part A samples was mixed with its corresponding Part B sample (Aj+Bi). The mixing ratio was as set forth below:
Part A 5.04 g
Part B 19.13 g
Deionized Water 6.40 g
To make coating pot solution 30.57 g
The coating pot solutions formed from from A;+Bj are referred to hereafter as Ti.
4) Coat the coating pot solution on PET film
Each of the above mixtures was coated in an amount of 15ml/m2 on a film of A4 size and 75μm thickness PET, which was preliminarily coated with SBR lutex and gelatin, and the following laser marking was conducted after drying. Coating was conducted using a K Control Coater (RK Print Coat Instruments, Ltd.), and a No. 3 coating bar was used to form a film thickness of 24 micrometers when wet.
5) Laser Exposure
The coated samples were exposed by a Domino SlOO laser maker (Domino Amjet, Inc.) under a matrix exposure as described in Example 2. The mark density of a specific letter "M" that best represents the marking results after receiving a fixed quantity of laser energy in the matrix was observed, and the experimental results are shown in Figures 3A to 3E. A letter "M" in the matrix, representing a specific laser exposure condition (Laser on time=53μs, and Mark speed^OSO bits/ms) was selected to compare the mark density of each tested sample. The density in the same position of the letter was measured. As can be seen from the Figures, employing a substituted or unsubstituted polyurethane compound as a binder in the coating composition was effective to improve the mark density of a laser markable material.
Example 4: Laser Exposure of a Binder-containing Protective Layer
Various polyurethane compounds were used as a binder to form sample protective layer coating compositions. Polyvinyl alcohol was used as a reference binder to compare the experimental results.
1) Preparation of the protective coat composition
The compounds listed in Table 4-1 were added one by one, wherein each successive ingredient was added after the previous one fully dissolved or dispersed.
Table 4-1
The protective coating composition was completed at this step. The coating composition is referred to hereinafter as PCref-
Other sample solutions (PC1, PC2, PC3, and PC4) were prepared in the same manner as for PCref, wherein the amount of binder used was adjusted if necessary to equal that of PCref based on its solid content. Table 4-2 lists the various sample protective layer coating compositions which were formed:
Table 4-2
2) Coating the protective layer coating composition on a color forming layer
Each of the coated films (T1, T2, T3 and T4) in Example 3 was coated with the protective layer coating composition prepared above. T; was coated with PQ and PCref to observe any differences in laser mark quality. For instance, T1 was coated with PC1 and PCref, and so on. Coating was conducted using a K Control Coater (RK Print Coat Instruments, Ltd.), wherein a No. 3 coating bar was used to give the film thickness of 24 micrometer when wet.
3) Laser Exposure
The coated samples were exposed by a Domino SlOO laser maker (Domino Amjet, Inc.) under a matrix exposure described in Example 2. The mark density of a specific letter "M" that best represents the marking result after receiving a fixed quantity of laser energy in the matrix was observed, and the experimental results are shown in Figures 4A to 4H. A letter "M" in the matrix, representing a specific laser exposure condition (Laser on time=53μs, and Mark speed=2030 bits/ms), was selected to compare the mark density of each tested sample. The density in the same position of the letter was measured. As can be seen from the figures, replacing polyvinyl alcohol with the polyurethane compounds as a binder in a protective layer showed improvements in retaining the mark density of markings formed in the recording layer of a laser-markable material.