PHARMACEUTICAL INTERMEDIATES AND A PROCESS FOR THE PREPARATION THEREOF
FIELD OF THE INVENTION
The present invention relates to new pharmaceutical intermediates and a process for the preparation thereof.
STATE OF THE ART
Optically active aryl-isopropanols are important pharmaceutical intermediates. According to DE 2832604 steroid compounds are prepared in a stereoselective synthesis by using the corresponding enantiomeric aryl-isopropanol derivatives. In /. Am. Chem. Soc. (1995), 777, 12358-12359 the synthesis of talampanel is described; the key intermediate is also an enantiomeric aryl-isopropanol derivative. According to Bull. Soc. Chim. 130, 450-458 (1993) for the formation of the amino group of suitable stereo chemistry of (S)- phenfluramine as starting material an optically active aryl- isopropanol derivative is used.
Racemic aryl-isopropanols of the general Formula I
of the present invention are known compounds. The new racemic aryl-isopropanols of the general Formula
of the present invention can be prepared by methods analogous to those described in prior art. [HU 194550; J. Med. Chem., (1978), 21, 448-56].
According to prior art optically active aryl-isopropanols can be prepared by chemical or biochemical or enzyme catalyzed reactions.
InJ. Chem. Soc. 2987 (1956) and/. Am. Chem. Soc. 91, 996 (1969) chemical methods are described for the preparation of optically active l-phenyl-propane-2-ol. According to this process the racemic alcohol is converted into the semiester by reacting with phthalic anhydride in pyridine, whereupon the semiester obtained is reacted with brαcine in acetone, the precipitated diastereomic salt is recrystallized in 4-6 times and finally only one of the enantiomers is obtained in a yield of 19 %. This process is accompanied by several disadvantages. On the one hand the diastereomic salt is recrystallized several times which is disadvantageous economically and the yield obtained is very low. On the other hand even this low yield
necessities the use of a large quantity of resoluting agent detrimental to the health.
According to Tetrahedron Letters 34, 4145 (1993) l-(4-fluσro- phenyl)-propane-2-one is synthetized by using an enantioselective reduction; the reaction is carried out with borane in the presence of chiral oxazaborolidine catalyst. However, the optical purity of the alcohol obtained is not satisfactory; the enantiomer access is only 50 %. The product obtained can be used for stereoselective synthesis only after further purification and resolution. The authors have not even tried to isolate a pure enantiomeric product.
An enantioselective process is described in Bull. Soc. Chini. Fr. 130., 450 (1993) for the synthesis of optically active l-(3- trifluoromethyl-phenyl)-2-propanol. The optically active alcohol is prepared by reacting the lithium salt obtained by the reaction of optically active propylene oxide and 3-trifluoromethyl-bromo-benzene by using butyl-lithium at -780C. For economical and technological reasons, however, this process is not quite suitable for industrial scale manufacture because of the reactant and the extreme reaction conditions are used.
In J. Am. Chem. Soc. (1995), UT3 12358-12359 the preparation of ($-l-benzo[l,3]dioxol-5-yl-propane-2-ol by- enzymatic reduction is described. The benzo[l,3]dioxol-5-yl- propane-2-one is converted into the dextrorotatory optically active alcohol by using a reducing enzyme occurring in yeast. However, the reaction requires a very large volume and there this process is uneconomical.
An other process also requiring large volumes is described in DE 2832602. The (S>3-methoxy-phenyl-2-ρroρanol and (S)- 3,5-dimethoxy-phenyl-2-propanol are prepared by subjecting the corresponding ketones to enzymatic reduction.
In J. Chem. Soc. Chem. Commun. 1277-1278 (1985) the preparation of (^-3-methoxy-phenyl-2-propanol is disclosed. However, the authors do not describe the details of the process.
According to Biosci. Biotechnol. Biochem. 58 (7), 1330 (1994) racemic alcohols are separated by means of enzyme catalyzed reaction of racemic O-acylated l-phenyl-2-propanol derivatives. Said enzymatic hydrolysis is carried out for a weak in a very diluted solution and the mixture of ester-alcohols obtained is separated by preparative thin-layer chromatography. The optically active l-phenyl-2-propanol
obtained is converted into the corresponding optically active semiester without isolation.
The separation of racemic l-benzo[l,3]dioxol-5-yl-propane-2- ol into the enantiomers is described in Tetrahedron Asymmetry 14 333-337 (2003). The essence of said process is that racemic aryl-isopropanol is acylated in the presence of a suitable lipase enzyme. As a result of the enantioselective acylation a (+)-rotating alcohol and a (-)-rotating acylated product are obtained. As acylating agent vinyl acetate is used in a hexane solution. However, during working up of the reaction mixture the acylated product and the alcohol used can be separated only by means of chromatography. This is not surprising because the optically active acylated aryl- isopropanol derivative obtained in the reaction and the optically active aryl-isopropanol being still present in the reaction mixture have very similar solubility properties. Additionally, compounds of this type are generally oily substances having low melting point. This obvious separation method is, however, unsuitable for industrial scale manufacture.
Summarized, it can be stated that the known procedures have serious drawbacks. The materials and reactants used in the chemical synthesis are detrimental to the environment and their
enantioselectivity is in many cases unsatisfactory. The biologically based methods can be only carried out in very diluted solutions requiring large reaction volume. Prior art fails to describe industrial scale methods for the separation of end products having similar physical properties formed in the synthesis.
The object of the present invention is the development of new enantiomeric aryl-isopropanol derivatives which enable the enantioselective synthesis of numerous new pharmaceutical active ingredients. It is a further object of the present invention to elaborate a process which eliminate the drawbacks of the methods described in prior art, can be performed in a reaction mixture of reasonable concentration, requires a relatively short reaction time, can be performed on industrial scale and provides an easy separation of the antipode forms of the process.
The above objects are solved by the present invention.
SUMMARY OF THE INVENTION
According to the present invention there are provided compounds of the general Formula I in which the chiral carbon atom is of (^-configuration
[wherein
R3 is hydrogen;
R1 and R2 independently from each other stand for hydrogen, Ci-4-alkyl, C1-4-alkoxy, aryloxy, aralkoxy, trifluoromethyl, cyano or halogen; or R and R together form methylenedioxy; with the proviso that at least one of symbols R1 and R2 is other than hydrogen; with the proviso that if R1 and R2 stand for alkoxy, one of said substituents is attached to position 2 of the phenyl ring; with the further proviso that if R1 and R2 together form methylenedioxy, said group together with the phenyl ring form l-benzo[l,3]dioxol-4-yl; or if one of symbols R1 and R is cyano is attached to position 2, the other group is other than hydrogen or methoxy attached to position 3; or if one of symbols of R1 and R2 is hydrogen, the other is different from methoxy attached to position 2 or 3; or the chiral carbon atom is of (Rj-confϊguration and
R is hydrogen;
R and R independently from each other stand for hydrogen, Ci
-4-alkyl, Q^-alkoxy, aryloxy, aralkoxy, trifluoromethyl, cyano or halogen; or R
1 and R
2 together form methylenedioxy; with the proviso that at least one of symbols R
1 and R
2 is other than hydrogen; with the further proviso that if R
1 and R
2 are Ci
-4-alkoxy, one of said alkoxy groups is attached to position 2 of the phenyl
ring; with the further proviso that if R1 and R2 together form methylenedioxy, said group together with the phenyl ring form l-benzo[l,3]dioxol-4-yl; or if one of symbols R1 and R2 stands for trifluoromethyl attached to position 3 or fluorine attached to position 4, the other symbol is other than hydrogen; or the chiral carbon atom is of (^-configuration and
R3 is Ci_4-acyl;
R1 and R2 independently from each other represent hydrogen, C1-4-alkyl, C1-4-alkoxy, aryloxy, aralkoxy, trifluoromethyl, cyano or halogen; or R1 and R2 together form methylenedioxy; with the proviso that at least one of symbols R1 and R2 is other than hydrogen; with the further proviso that if R3 is acetyl and at least one of symbols of R and R is hydrogen, the substituent attached to position 4 of the phenyl ring is other than methoxy; or the chiral carbon atom is of ^-configuration and
R3 is Ci.4-acyl;
R1 and R2 independently from each other stand for hydrogen, Ci_4-alkyl, Ci_4-alkoxy, aryloxy, aralkoxy, trifluoromethyl, cyano or halogen; or R1 and R2 together form methylenedioxy; with the proviso that at least one of symbols R1 and R2 is other than hydrogen; with the further proviso that if R3 is acetyl and R1 and R2 together
form methylenedioxy, said group together with the phenyl ring form l-benzo[l,3]dioxol-4-yl; or R3 is acyl in which the group R5 binds said acyl group with a further carboxy group; wherein
R5 stands for substituted or unsubstituted alkenyl or aryl; with the proviso that if R3 stands for phthalyl, one of symbols R1 and R2 represents methoxy attached to position 3 of the phenyl ring and the other is different from hydrogen or methoxy attached to position 4 of the phenyl ring; and salts of said racemic or optically active enantiomers formed with optically active bases with the exception of salts formed with optically active brucine as base and compounds of the general Formula I in which R3 is phthalyl, one of symbols R1 and R2 is methoxy attached to position to 3 of the phenyl ring and the other is hydrogen or methoxy attached to position 4 of the phenyl ring.
According to a further aspect of the present invention there is provided a process for the preparation of enantiomers of aryl- isopropanol derivatives of the general Formula I (wherein R
3 is hydrogen; R
1 and R
2 independently from each other stand for hydrogen, C^-alkyl, Ci
-4-alkoxy, aryloxy, aralkoxy, trifluoromethyl, cyano or halogen; or R
1 and R
2 together form
methylenedioxy) by subjecting the corresponding racemic alcohol of the general Formula
(wherein R1 and R2 independently from each other stands for hydrogen, C^-alkyl, C1_4-alkoxy, aryloxy, aralkoxy, trifluoromethyl, cyano or halogen; or R1 and R2 together form methylenedioxy) to enantioselective acylation in an enzyme catalyzed reaction; thereafter acylating the enantiomeric aryl- isopropanol of the general Formula
which remained unchanged under the conditions of the enzyme-catalyzed reaction (wherein R
1 and R
2 independently from each other stands for hydrogen, C
1-4-alkyl, C
1-4-alkoxy, aryloxy, aralkoxy, trifluoromethyl, cyano or halogen; or R
1 and R together form methylenedioxy) with a dicarboxyiic acid derivative in the presence of an acylated enantiomer of the general Formula
formed in the enzyme catalyzed reaction (wherein R4 is C1-4- alkyl; R1 and R2 independently from each other stands for hydrogen, C1-4-alkyl, C1-4-alkoxy, aryloxy, aralkoxy, trifluoromethyl, cyano or halogen; or R1 and R2 together form methylenedioxy) converting the dicarboxylic acid derivative of the general Formula
(wherein R stands for alkyl, branched chain alkyl, alkenyl or aryl; R1 and R2 independently from each other stands for hydrogen, C1-4-alkyl, C1-4-alkoxy,. aralkoxy, trifluoromethyl, cyano or halogen; or R and R together form methylenedioxy) into a salt by using an aqueous base in the presence of a water- insoluble solvent; thereafter separating the aqueous phase which contains the dicarboxylic acid derivative of the general Formula I/D from the organic phase which contains the acylated enantiomeric derivative of the general Formula I/C; thereafter working up both phases by methods known per se to obtain the individual enantiomeric derivatives; and thereafter optionally converting said enantiomeric derivatives into other derivatives and/or purifying the same.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is based on the recognition that the separation of two enantiomers of aryl-isopropanols is high enantioselectivity can be carried out by using simple conventional extraction method of pharmaceutical industry by forming derivatives of the two enantiomers, one of said enantiomers being water-soluble and the other being soluble in water-immiscible solvents.
On subjecting acylation a racemic compound of the general Formula I/A by methods known from prior art [Poppe-Novak: Selectiv Biocatalysis A Synthetic Approach, VCH Weinheim New York (1992)] in the presence of a lipase enzyme due to the kinetic control only the (-)-enantiomer is acylated to yield a derivative of the general Formula I/C, whereby the other (+)- enantiomer of the general Formula I/B remains in the reaction mixture in unchanged form. On acylating the (+)-enantiomer aryl-isopropanol of the general Formula I/B with a dicarboxylic acid anhydride a semiester-semiacidic form beside the other enantiomer, namely the acylated derivative of the general Formula I/C.
The semiester-semiacid formed from the dextrorotatory enantiomer can be separated from the acylated derivative of the laevorotatory enantiomer by means of an alkaline aqueous and organic solvent extraction. The salt formed from the semiester-semiacid of the general Formula I/D goes into the aqueous phase, whereby the acylated derivative of the general Formula I/C remains in the organic phase.
The phases are than worked up in a manner known per se. The enantiomer-pure semiester-semiacid of the general Formula I/D is recovered from the aqueous phase. On hydrolysing said compound one of the enantiomeric alcohols of the general Formula I/B is obtained, while the other enantiomeric alcohol is formed by subjecting the enantiomer of the general Formula I/C obtained from the organic phase to hydrolysis.
If the purity of the separated enantiomers is not satisfactory, purification can be readily carried out by subjecting the salts of said dicarboxylic semiesters formed with a chiral base to resolution. Accordingly the aryl-isopropanol of the general Formula I/B having non-satisfactory optical purity is acylated with a dicarboxylic acid dihydrate in a manner known per se to yield the semiester-semiacid of the general Formula I/D; from said compound a salt is formed with an optically active base, the diastereomic salt is subjected to recrystallization and
thereby a salt having the desired optical purity is obtained. The semiester-semiacid of the general Formula I/D of satisfactory optical purity is set free from said salt and hydrolysis of the latter compound gives an aryl-isopropanol of the general Formula IfB having high optical purity.
The enzyme catalyzed reaction according to the present invention is carried out by using a lipase enzyme in an acylating solvent or a mixture of said acylating solvent and a further solvent. As acylating solvent preferably vinyl acetate or vinyl butylate, particularly vinyl acetate can be used. One may also proceed by performing the reaction in a solvent of said acylating solvent formed with another solvent, or in the presence of a suitable solvent solution. As further solvent preferably ether-type solvents (particularly diethyl ether) or saturated hydrocarbons (preferably hexane or heptane) can be used. Acylation can also be performed in the presence of a phosphate buffer (pH 7.5). As lipase enzyme preferably Pseudomonas fhiorescens, Pseudomonas cepacia, Candida cylindracea, Candida antarctica or Mucor miehei lipase, particularly Pseudomonas cepacia lipase, particularly advantageously Amano® PS-C can be used. The enzymatic reaction can be carried out at a temperature between 10-60
0C, particularly 20-40
0C, most advantageously at room
temperature. The reaction time is generally 4-72 hours, preferably 6-48 hours, the most advantageously 6-30 hours.
The mixture of the acylated compound of the general Formula I/C and the derivative of the general Formula I/B can be separated by formation of chemical derivatives. On acylating with a dicarboxylic acid derivative from the compound of the general Formula I/B a semiester-semiacid of the general Formula I/D is formed, whereby the acylated enantiomer of the general Formula I/C remains unchanged. As dicarboxylic acid derivative one can use derivatives of dicarboxylic acid containing alkyl, branched chain alkyl, substituted alkyl, alkenyl, substituted alkenyl or aryl groups, preferably derivatives of maleic acid, succinic acid or phthalic acid. It is preferably to use dicarboxylic acid anhydrates. Acylation of the compounds of the general Formula I/B can be carried out preferably in an inert solvent. As reaction medium preferably chlorinated hydrocarbon, acetonitrile, tetrahydrofurane, particularly dichloromethane can be used. One can work in the presence of a catalyst, preferably an organic base, particularly triethyl amine or diethyl amino-pyridine. The semiester- semiacid derivative of the general Formula I/D is converted into a salt by using an aqueous base. In the reaction an alkali metal or an alkaline earth metal hydroxide, hydrogen carbonate or carbonate solution or suspension, particularly an alkali
carbonate solution, most advantageously sodium carbonate solution can be used. A two-phase reaction mixture is formed after the salt formation, whereby the organic phase contains the compound of the general Formula I/C and the aqueous phase contains the salt of the semiester-semiacid derivative of the general Formula I/D. The two phases are separated and worked up by methods known per se. One may proceed by drying and evaporating the organic phase and, if necessary, purifying the compound of the general Formula I/C by distillation in vacuo. The aqueous phase can be acidified and on adding a water non- miscible solvent, the organic acid solution of the semiester- semiacid of the general Formula I/D is obtained.
On subjecting the compounds of the general Formulae I/C and I/D to hydrolysis the two enantiomeric alcohols of the general Formula I/B are obtained.
If the purity of the separated enantiomers is not satisfactory, further purification can be readily carried out by subjecting the salts of the dicarboxylic acid-semiesters formed with chiral bases to resolution.
As chiral base preferably optically active organic amines can be used, e.g. (2^-(+)-l-phenyl-ethyl-amine, ($-(-)- 1-phenyl- ethyl-amine, (+)-dehydroabiethyl-amine, quinine, (-)-l-(4-
nitrophenyl)-2-amine- 1,3 -propanediol or ($-(+)-2-benzyl- amino-1-butanol. Resolution is carried out in an organic solvent. At reaction medium a dipolar aprotic solvent, ether- type solvent, preferably ethyl acetate, acetone, diisopropyl ether, diethyl ether or a mixture of said solvents can be used.
It is known that on crystallization from an inert solvent as a result of thermodynamical control one of the diastereomeric salts precipitates in crystalline form. The precipitated salt is separated by filtration. The filtered diastereomeric salt obtained in said stereoselective process contains the semiester of one of the optically active aryl isopropanol enantiomers, while the semiester of the other optically active aryl isopropanol enantiomer remains in the mother lye. If the optical purity of the aryl isopropanol enantiomer semiester component of the filtered diastereomeric salt is not satisfactory, it can be further purified by recrystallization.
The corresponding semiester-semiacid of the general Formula I/D is set free from the diastereomeric salt by using an aqueous acid which is then recovered from the reaction mixture by extraction. On evaporating the extract the semiester-semiacid of the general Formula I/D is obtained which is subjected to hydrolysis. The reaction mixture of said hydrolysis step results
in the optically pure aryl isopropanol of the general Formula IfB which can be purified by distillation, if necessary.
According to a further feature of the present invention there are provided the following intermediates:
(±)_? (_)_, and (+)-2-(l-benzo[l,3]dioxol-5-yl)-l-methyl-ethyl- hydrogen-maleate,
(±)-, (-)-, and (+)-2-(l-benzo[l,3]dioxol-5-yl)-l-methyl-ethyl- hydrogen-succinate,
(±)-, (-)-, and (+)-2-(l-benzo[l,3]dioxol-5-yl)-l-methyl-ethyl- hydrogen-phthalate,
(S^-(9-phenyl-ethyl-ammonium-{(+)-2-(l-benzo[l,3]dioxol-5- yl)- 1 -methyl-ethyl-maleate} , fRj-f'+j-phenyl-ethyl-ammonium- {(+)-2-(l -benzo[ 1 ,3]dioxol-
5-yl)- 1 -methyl-ethyl-maleate} ,
CSJ-(9-phenyl-ethyl-ammonium- {(-)-2-(l -benzo[ 1 ,3]dioxol-5- yl)- 1 -methyl-ethyl-maleate} ,
^-f+j-phenyl-ethyl-ammonium- {(-)-2-(l-benzo[ 1 ,3]dioxol-5- yl)- 1 -methyl-ethyl-maleate} ,
(+)-dehydroabiethyl-ammonium- {(-)-2-(l -benzo[l ,3]dioxol-5- yl)- 1 -methyl-ethyl-maleate} ,
(±)-, (-)-, and (+)-2-(4-chloro-ρhenyl)-l-methyl-ethyl- hydrogen-maleate,
(±)-, (-)-, and (+)-2-(4-chloro-ρhenyl)-l-methyl-ethyl- hydrogen-succinate,
(±)-, (-)-, and (+)-2-(4-chloro-phenyl)-l-methyl-ethyl- hydrogen-phthalate,
(-)-2-(4-chloro-phenyl)- 1 -methyl-ethyl-acetate,
(+)- and (-)-l-(4-chloro-phenyl)-2-propanol,
(ϊ?j-f"+j-phenyl-ethyl-arnmonmm-[(-)-2-(4-chloro-phenyl)-l- methyl-ethyl-maleate],
(2?y)-^+J-phenyl-ethyl-anraionium-[(-)-2-(4-chloro-phenyl)-l- methyl-ethyl-succinate] , fSJ-(^-phenyl-ethyl-ammonium-[(-)-2-(4-chloro-phenyl)-l- methyl-ethyl-succinate] ,
(R)-f+j-phenyl-ethyl-ammonium-[(+)-2-(4-chloro-phenyl)- 1 - methyl-ethyl-maleate] ,
(^-f-y)-phenyl-ethyl-ammonium-[(+)-2-(4-chloro-phenyl)-l- methyl-ethyl-maleate],
(+)-dehydroabiethyl-ammonmm- [(+)-2-(4-chloro-phenyl)- 1 - methyl-ethyl-maleate] ,
(+)-dehydroabiethyl-ammonium- [(-)-2-(4-chloro-phenyl)- 1 - methyl-ethyl-maleate] ,
(7?j-^+j-phenyl-ethyl-ammonium-[(-)-2-(4-chloro-phenyl)-l- methyl-ethyl-phthalate] ,
(^)-(9-phenyl-ethyl-ammonium-[(-)-2-(4-chloro-phenyl)- 1 - methyl-ethyl-phthalate] ,
(+)-2-(4-chloro-phenyl)- 1 -methyl-ethyl-hydrogen-succinate quinine salt,
(-)-2-hydroxy- 1 -(hydroxy-methyl)-2-(4-nitro-phenyl)-ethyl- ammonium- [(+)-2-(4-chloro-phenyl)- 1 -methyl-ethyl- phthalate],
(±)-, (-)-, and (+)-2-(3-chloro-ρhenyl)-l-methyl-ethyl- hydrogen-maleate,
(±)-, (-)-, and (+)-2-(3-chloro-ρhenyl)-l-methyl-ethyl- hydrogen-succinate,
(±)-, (-)-, and (+)-2-(3-chloro-phenyl)-l-methyl-ethyl- hydrogen-phthalate,
(-)-2-(3 -chloro-phenyl)- 1 -methyl-ethyl-acetate,
(+)- and (-)-l-(3-chloro-phenyl)-2-propanol,
(+)-dehydroabiethyl-amnionium-[(+)-2-(3-chloro-phenyl)-l- methyl-ethyl— maleate] ,
(+)-dehydroabiethyl-ammonium-[(-)-2-(3-chloro-phenyl)-l- methyl-ethyl— maleate] ,
(Rj-f+/)-phenyl-ethyl-amtnonium-[(-)-2-(3-chloro-phenyl)-l- methyl-ethyl-maleate] ,
(^-(-)-phenyl-ethyl-ammonium-[(-)-2-(3 ,4-dichloro-phenyl)- 1 - methyl-ethyl-phtalate] ,
(±)-, (-)-, and (+)-2-(3 ,4-dichloro-phenyl)- 1-methyl-ethyl- hydrogen-maleate,
(±)-, (-)-, and (+)-2-(3,4-dichloro-phenyl)-l-methyl-ethyl- hydrogen-succinate,
(±)-, (-)-, and (+)-2-(3,4-dichloro-phenyl)-l-methyl-ethyl- hydrogen-phthalate,
(-)-2-(3,4-dichloro-phenyl)- 1 -methyl-ethyl-acetate,
(+)- and (-)-l-(3,4-dichloro-phenyl)-2-propanol,
(RJ-^+j-phenyl-ethyl-ammonium-[(-)-2-(3,4-dichloro-phenyl)-
1 -methyl-ethyl-maleate] ,
(^?y)-(/+j-phenyl-ethyl-amnionium-[(+)-2-(3,4-dichloro-phenyl)-
1 -methyl-ethyl-phthalate],
(^-(9-phenyl-ethyl-animonium-[(-)-2-(3,4-dichloro-phenyl)-l- methyl-ethyl-succinate] ,
(^)-(9-phenyl-ethyl-amnionium- [(-)-2-(3 ,4-dichloro-phenyl)- 1 - methyl-ethyl-phthalate] ,
(+)-dehydroabiethyl-ammonium-[(+)-2-(3,4-dichloro-phenyl)-
1 -methyl-ethyl-maleate] ,
(+)-dehydroabiethyl-ammonium- [(+)-2-(3 ,4-dichloro-phenyl)-
1 -methyl-ethyl-succinate] ,
(±)-, (-)-, and (+)-2-(3-bromo-4-methoxy-phenyl)-l-methyl- ethyl-hydrogen-maleate,
(±)-5 (-)-, and (+)-2-(3-bromo-4-methoxy-phenyl)-l-methyl- ethyl-hydrogen-succinate,
(±)-, (-)-, and (+)-2-(3-bromo-4-methoxy-phenyl)-l-methyl- ethyl-hydrogen-phthalate,
(-)-2-(3-bromo-4-methoxy-phenyl)- 1 -methyl-ethyl-acetate,
(+)- and (-)-l-(3-bromo-4-methoxy-phenyl)-2-propanol,
(+)-dehydroabiethyl- ammonium- [(-)-2-(3 -bromo-4-methoxy- phenyl)- 1 -methyl-ethyl-maleate] ,
(^Rj-f+j-phenyl-ethyl-ammonram-[(+)-2-(3-bromo-4-methoxy- phenyl)- 1 -methyl-ethyl-succinate] ,
(Ry)-f+/)-phenyl-ethyl-ammonium-[(-)-2-(3-bromo-4-methoxy- phenyl)- 1 -methyl-ethyl-phthalate],
(5^-(+)-benzyl-[l-(hydroxy-methyl)-propyl]-ammonium-[(-)-2-
(3-bromo-4-methoxy-phenyl)-l-methyl-ethyl-phthalate],
(±)-, (-)-, and (+)-2-(4-fluoro-ρhenyl)-l-methyl-ethyl- hydrogen-maleate,
(±)-, (-)-, and (+)-2-(4-fluoro-phenyl)-l-methyl-ethyl- hydrogen-succinate,
(±)-, (-)-, and (+)-2-(4-fluoro-ρhenyl)-l-methyl-ethyl- hydrogen-phthalate,
(-)-2-(4-fluoro-phenyl)- 1 -methyl-ethyl-acetate,
(+)- 1 -(4-fluoro-phenyl)-2-propanol.
The essence of the present invention is the combination of an enzymatic route on the one hand and a chemical derivative- formation on the other. Thus the present invention provides a unique, completely unknown route for the separation of two enantiomers which is suitable for an industrial scale resolution.
The present invention provides the following significant advantages:
- the enzymatic reaction is carried out under usual conditions of chemical industry in a relatively concentrated reaction mixture;
- the optically pure derivatives can be separated by a simple method by using a conventional liquid-liquid extraction method;
- neither extreme temperature conditions, nor special equipment is required;
- the enantioselective activity of the reaction can be influenced by choosing the reaction conditions, reaction time, temperature and the type of the lipase enzyme;
- the enantiomer purity of the product obtained can be further improved with a significantly smaller reactant and apparatus need than the resolution of the complete amount of the racemic aryl-isopropanol;
- the non-desired enantiomer pure antipode can be converted into the desired enantiomer by methods known from prior art in a simple way.
Summarized, the present invention provides a new industrial scale process for the preparation of partially known and partially new optically active aryl-isopropanols.
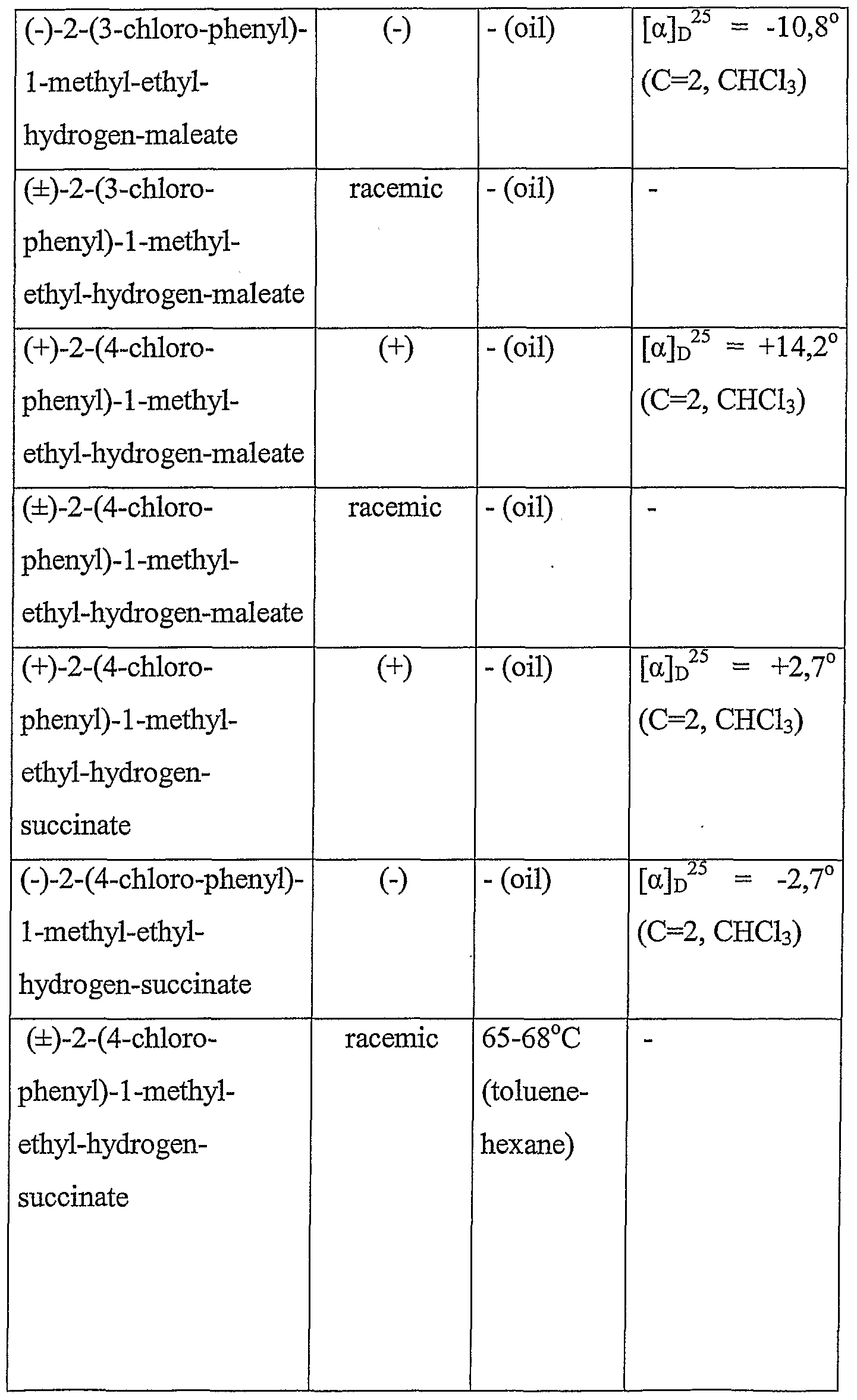
For example physical properties of several new optically active aryl-isopropanols according to our invention, some new salts of known optically active intermediers with chiral bases and some racemic compounds prepared as reference compounds are as follows:
Further details of the present invention are to be found in the following Examples without limiting the scope of protection to said Examples.
Example 1
Resolution of l-benzo[1.3]dioxol-5-yl-2-propanol by using
Pseudomonas cepacia lipase
To a solution of 35.00 g (194.23 mmoles) of 1- benzo[1.3]dioxol-5-yl-2-propanol in 175 ml of vinyl-acetate 10.50 g of Ps eudomonas cepacia lipase (Amano® PS-C) bond to a carrier are added. The mixture is stirred at room temperature for 6 hours, filtered, the enzyme is washed 4 times in 10 ml of vinyl-acetate each and the filtrate is evaporated in vacuo. Thus 39.1 g of a mixture of (+)-l-benzo[1.3]dioxol-5- yl-2-propanol and (-)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl- ethyl-acetate are obtained in the form of a yellow oil.
The yellow oil obtained is dissolved in 100 ml of dichloro- methane, 17.50 ml (125.73 mmoles, d=0.727) of triethyl- amine, and in one portion 11.20 g (114.22 mmoles) of maleic anhydride are added. The reaction mixture gradually warms up, while its colour turns into dark brown. The reaction mixture is heated to boiling for 5 minutes, cooled and washed once with 100 ml, once with 15 ml of 1.5 molar hydrochloric acid and twice with 15 ml of water each. The dichloro-methane phase is extracted once with 125 ml and twice with 15 ml of a 1 molar sodium carbonate solution. The aqueous layers are united and washed 3 times with 15 ml of dichloro-methane each. [The aqueous phase contains the sodium salt of (+)-2-(l- benzo[l .3]dioxol-5-yl)-l-methyl-ethyl-hydrogen-maleate.] The dichloro-methane phases are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 21.60 g (97.19
inmoles, 100.1 %) of (-)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl- ethyl-acetate are obtained in the form of a yellow oil. The optical rotation is [α]D 25= -4.5° (c=2, chloroform).
21.40 g of the (-)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl-ethyl- acetate thus obtained, 30 ml of water, 15 ml of methanol and 6 g of sodium hydroxide are heated to boiling for 5 minutes, whereupon the methanol is distilled off in vacuo. The mixture is cooled and extracted once with 50 ml and 3 times with 15 ml of diethyl-ether. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (16.67 g) is subjected to distillation in vacuo. Thus 16.08 g (89.23 mmoles, 91.9 %) of Q-l-benzo[1.3]dioxol-5- yl-2-propanol are obtained in the form of an oil. The optical rotation is [α]D 25= -26.4° (c=2, chloroform), [α]365 25= -103,4° (c=2, chloroform).
To the aqueous phase containing the sodium salt of (+)-2-(l- benzo[l .3]dioxol-5-yl)-l-methyl-ethyl-hydrogen-maleate 30 ml of 37 % hydrochloric acid are added and it is extracted once in 50 ml and 3 times in 15 ml of dichloro-methane each. The dichloro-methane layers are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus in the form of a brown oil 24.38 g (87.61 mmoles, yield 90.2 %) of (+)-2-(l-
benzo[l .3]dioxol-5-yl)-l-methyl-ethyl-hydrogen-maleate are obtained. Optical purity: [α]D 25= +17.3° (c=2, chloroform).
23.98 g of (+)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl-ethyl- hydrogen-maleate are heated to boiling in a mixture of 36 ml of water and 12 g of sodium hydroxide for 1 minute. The mixture is cooled and extracted 4 times with 50 ml of diethyl- ether each. The ethereal phases are united, washed and dried over sodium sulfate and the solvent is distilled off in vacuo. The residual crude oil (14.55 g) is distilled off in vacuo. Thus 14.16 g (78.58 mmoles, 80.9 %) of oily (+)-l- benzo[1.3]dioxol-5-yl-2-propanol are obtained. Optical purity: [α]D 25= +32.2° (c=2, chloroform), [α]365 25= +124.1° (c=2, chloroform).
Example 2
Resolution of l-benzo[1.3]dioxol-5-yl-2-propanoI with
Candida antarctica lipase
To a solution of 3.50 g (19.42 mmoles) of l-benzo[1.3]dioxol- 5-yl-2-propanol in 10.5 ml of vinyl-acetate 300 mg of Candida antarctica lipase (Novozym® 435) bond to a carrier are added. The mixture is stirred at room temperature for 24 hours, filtered, the enzyme is washed 4 times in 1 ml of vinyl-acetate each and the filtrate is evaporated in vacuo. Thus 3.76 g of a
mixture of (+)-l-benzo[1.3]dioxol-5-yl-2-propanol and (-)-2- (l-benzo[1.3]dioxol-5~yl)-l-methyl-ethyl-acetate are obtained in the form of a yellow oil.
The yellow oil thus obtained is dissolved in 10 ml of dichloro- methane, whereupon 1.75 ml (12.57 mmoles, d=0.727) of triethyl-amine and in one portion 1.12 g (11.42 mmoles) of maleic anhydride are added. The reaction mixture gradually warms up, whereby its colour turns dark brown. The reaction mixture is heated to boiling for 5 minutes, cooled, washed once with 10 ml, once with 3 ml of 1.5 molar hydrochloric acid and twice with 3 ml of water each. The dichloro-methane layer is extracted once with 12.5 ml and twice with 3 ml of a 1 molar sodium-carbonate solution each. The aqueous layers are united and washed 3 times with 3 ml of dichloro-methane each. [The aqueous phase contains the sodium salt of a (+)-2-(l- benzo[1.3]dioxol-5-yl)-l-methyl-ethyl-hydrogen-maleate.] The dichloro-methane are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 2.62 g (11.79 mmoles, yield 121.4 %) of (-)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl- ethyl-acetate are obtained. Optical rotation: [α]D 25= -2.9° (c=2, chloroform).
A mixture of the (-)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl- ethyl-acetate thus obtained, 5 ml of water, 3 ml of methanol
and 0.6 g of sodium hydroxide is heated to boiling for 5 minutes, whereupon the methanol is distilled off in vacuo. The mixture is cooled and extracted 4 times with 10 ml of diethyl- ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 2.03 g (11.26 mmoles, yield 116.0 %) of (-)-l-benzo[1.3]dioxol-5-yl- 2-propanol are obtained in the form of an oil. Optical rotation: [α]D 25= -16.6° (c=2, chloroform).
To the aqueous phase containing the sodium salt of the maleic acid semiester of (+)-l~benzo[1.3]dioxol-5-yl-2-propanol 3.5 ml of 37 % hydrochloric acid are added. The mixture is extracted 4 times with 10 ml of dichloro-methane each. The dichloro-methane phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 1.86 g (6.68 mmoles, yield 68.9 %) of (+)-2-(l-benzo[1.3]dioxol-5-yl)-l- methyl-ethyl-hydrogen-maleate are obtained in the form of a brown oil. Optical rotation: [α]D 25= +16.9° (c=2, chloroform).
A mixture of (+)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl-ethyl- hydrogen-maleate, 4 ml of water and 1.2 g of sodium hydroxide is heated to boiling for 1 minute. The reaction mixture is cooled and extracted 4 times with 10 ml of diethyl- ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 1.09 g (6.05
mmoles, yield 62.3 %) of (+)-l-benzo[1.3]dioxol-5-yl-2- propanol are obtained in the form of an oil. Optical rotation: [α]D 25= +31.4° (c=2, chloroform).
Example 3
Enantiomer purification of (-)-l-benzo[1.3]dioxol-5-yl-2- propanol via the (2?j-(+)-l-phenyl-ethyl-ammoiiium salt of a maleic acid semiester
a) Preparation of(-)-2-(l-benzo[1.3]dioxol-5-yl)-l- methyl-ethyl-hydrogen-maleate
To a solution of 15.00 g (83.24 mmoles) of (-)-l- benzo[1.3]dioxol-5-yl-2-propanol {[α]
D 25- -15.3° (c=2, chloroform)} in 60 ml of dichloro-methane 13.4 ml (96.26 mmoles, d=0.727) of triethyl-amine and in one portion 8.80 g (89.74 mmoles) of maleic anhydride are added. The reaction mixture warms gradually up, while its colour turns dark brown. The reaction mixture is stirred for 10 minutes, cooled, washed once with 80 ml, once with 20 ml of 1.5 molar hydrochloric acid and once with 20 ml of water. The dichloro-methane phase is dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 24.14 g (86.76 mmoles, yield 97.7 %) of
(-)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl-ethyl-hydrogen- maleate are obtained in the form of an oily crystalline product. Optical rotation: [α]D 25= -11.0° (c=2, chloroform).
b) Enantiomer purification of (-)-2-(l-benzo[1.3]dioxol- 5-yl)-l-methyl-ethyl-hydrogen-maleate and the decomposition of the semiester
10.00 g (35.94 mmoles) of (-)-2-(l-benzo[1.3]dioxol-5-yl)-l- methyl-ethyl-hydrogen-maleate {[α]D 25= -11.0° (c=2, chloroform)} are dissolved in 10 ml in ethyl-acetate under heating. The dark brown solution is cooled to room temperature and 2.5 g (20.63 mmoles) of fR)-(+)-l-phenyl~ ethyl-amine and 30 ml diethyl-ether are added. The mixture is seeded with a small amount of diastereomic salt and allowed to stand at room temperature for 2 hours. The mixture is filtered, washed 4 times with 4 ml of diethyl-ether each and dried. Thus 6.78 g (16.97 mmoles, yield 47.2%) of almost white crystalline f'Ry)-f+j-l-phenyl-ethyl-ammonium-{(-)-2-(l-benzo[1.3]dioxol- 5-yl)-l-methyl-ethyl-maleate} are obtained.
The crystals are dissolved in 20 ml of hot ethyl-acetate and allowed to crystallize at room temperature for an hour. The
mixture is filtered, the crystals are washed 3 times with 2.5 ml of ethyl-acetate each and dried. Thus 6.08 g (15.22 mmoles, yield 42.3%) of almost white crystalline (Rj-f+j-1-phenyl- ethyl-ammonium-{(-)-2-(l-benzo[1.3]dioxol-5-yl)-l-methyl- ethyl-maleate} are obtained.
The crystals are dissolved in 18 ml of hot ethyl-acetate and allowed to crystallize at room temperature for an hour. The mixture is filtered, the crystals are washed 3 times with 2.5 ml of ethyl- acetate each and dried. Thus 5.27 g (13.19 mmoles, yield 36.7%) of almost white crystalline (R)-(+)-l-pheny[- ethyl-ammonium- {(-)-2-(l~benzo[l .3]dioxol-5-yl)-l-methyl- ethyl-maleate} . Optical rotation: [a]O 25= -6.1° (c=2, glacial acetic acid).
A mixture of 4.87g (12.19 mmoles) of the above salt, 40 ml of ethyl-acetate and 5 ml of 20% hydrochloric acid is stirred for 15 minutes. The layers of the clear, two-phase solution are separated, whereupon the ethyl-acetate phase is washed 3 times with 5 ml of 1.5 molar hydrochloric acid each, dried over sodium sulfate and the solvent is evaporated in vacuo. Thus 3.38 g (12.14 mmoles, yield 33.8%) of (-)-2-(l- benzo[l .3]dioxol-5-yl)-l-methyl-ethyl-hydrogen-maleate are obtained. Optical rotation: [α]
D 25= -15.3° (c=2, chloroform).
A mixture of 2.98 g (7.46 mmoles) of the above semiester, 4.5 ml of water and 1.5 g of sodium hydroxide is heated to boiling for 1 minute. The reaction mixture is cooled and extracted 4 times with 10 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 1.68 g of an oil is obtained, which is subjected to distillation in vacuo. Thus 1.52 g (8.43 mmoles, yield 23.5 %) of oily (-)-l-benzo[1.3]dioxol-5-yl-2-propanol are obtained. Optical rotation: [a]O 25= -31.3° (c=2, chloroform).
Example 4
Resolution of l-(3-chloro-phenyl)-2-propanoI with
Pseudomonas cepacia lipase
To a solution of 35.00 g (205.11 mmoles) of l-(3-chloro- phenyl)-2-propanol in 105 ml of vinyl-acetate 3.00 g of Pseudomonas cepacia lipase (Amano® PS-C) bond to a carrier are added. The mixture is stirred at room temperature for 36 hours, filtered, the enzyme is washed 4 times with 10 ml of vinyl-acetate each and the filtrate is evaporated in vacuo. Thus 41.48 g of a mixture of (+)-l-(3-chloro-phenyl)-2-propanol and a (-)-2-(3-chloro-phenyl)-l -methyl-ethyl-acetate are obtained in the form of a yellow oil.
The yellow oil thus obtained is dissolved in 100 ml dichloro- methane, whereupon 17.5 ml (125.73 mmoles, $=0,727) of triethyl-amine and in one portion 10.00 g (101.98 mmoles) of maleic anhydride are added. The reaction mixture gradually warms up, while its colour turns dark brown. The reaction mixture is heated to boiling for 5 minutes, then cooled, washed once in 100 ml and once with 10 ml of 1.5 molar hydrochloric acid and twice with 15 ml of water each. The dichloro-methane phase is extracted once with 100 ml and twice with 5 ml of a 1 molar sodium-carbonate solution each. The aqueous layers are united and washed 3 times with 15 ml of dichloro-methane each. [The aqueous layer contains the sodium salt of (+)-2-(3- chloro-phenyl)- 1 -methyl-ethyl-hydrogen-maleate.] The dichloro-methane phases are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 26.1 g (122.72 mmoles, yield 119.7 %) of (-)-2-(3-chloro-phenyl)-l -methyl- ethyl-acetate are obtained in the form of a yellow oil. Optical rotation: [α]D 25= -3.0° (c=2, chloroform).
A mixture of 25.80 g of (-)-2-(3-chloro-phenyl)-l -methyl- ethyl-acetate thus obtained, 15 ml of water, 25 ml of methanol and 7 g of sodium hydroxide is heated to boiling for 5 minutes, whereupon the methanol is removed in vacuo. The mixture is cooled and extracted once with 50 ml and 3 times with 15 ml of diethyl-ether each. The ethereal phases are united, dried
over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (21.25 g) is subjected to distillation in vacuo. Thus 20.33 g (l 19.14 mmoles, 116.2 %) of oily (-)-l-(3- chloro-phenyl)-2-propanol are obtained. Optical rotation: [α]D 25= -19.8° (c=2, chloroform).
To the aqueous phase containing the sodium salt of (+)-2-(3- chloro-phenyl)-l-methyl-ethyl-hydrogen-maleate 50 ml of 20 % hydrochloric acid are added. The mixture is extracted, once with 50 ml and 3 times with 15 ml of dichloro-methane each. The dichloro-methane phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 20.82 g (77.49 mmoles, 75.6 %) of (+)-2-(3-chloro-phenyl)-l-methyl- ethyl-hydrogen-maleate are obtained in the form of a brown oil. Optical rotation: [α]D 25= +14.2° (c=2, chloroform).
A mixture of 20.52 g of (+)-2-(3-chloro-phenyl)-l-methyl- ethyl-hydrogen-maleate, 60 ml of water and 8 g of sodium hydroxide is heated to boiling for 1 minute. The reaction mixture is cooled and extracted 4 times with 30 ml of diethyl- ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 12.42 g of an oil are obtained which is subjected to distillation in vacuo. Thus 11.73 g (68.74 mmoles, 67.0 %) of oily (+)-l-(3-chlσro-
phenyl)-2-propanol are obtained. Optical rotation: [α]D = +34.4° (c=2, chloroform).
Example 5
Enantiomer purification of (-)-l-(3-chIoro-phenyl)-2- propanol via the (+)-dehydroabiethyI-ammonium salt of the maleic acid semiester
a) Preparation of(-)-2-(3-chloro-phenyl)-l-methyl-ethyl- hydrogen-maleate
A mixture of 20.33 g (119.14 mmoles, [α]D 25= -19.8° (c=2, chloroform)) of (-)-l-(3-chloro-phenyl)-2-propanol, 80 ml of dichloro-methane, 18.50 ml of triethyl-amine (132.91 mmoles) and 12.50 g (127.47 mmoles) of maleic acid anhydride is heated to boiling for 10 minutes. The reaction mixture is cooled, washed once with 100 ml and once with 10 ml of 1.5 molar hydrochloric acid and once with 20 ml of water, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 32.01 g (119.13 mmoles, 100.0%) of (-)-2-(3-chloro- phenyl)-l-methyl-ethyl-hydrogen-maleate are obtained in the form of a dark brown oil. Optical rotation: [α]D 25= -8.3° (c=2, chloroform).
b) Enantiomer purification of (-)-2-(3-chloro-phenyl)-l- methyl-ethyl-hydrogen-maleate and decomposition of the semiester
To a solution of 16.00 g (59.54 mmoles) of (-)-2-(3-chloro- ρhenyl)-l-methyl-ethyl-hydrogen-maleate {[α]D 25= -8.3° (c=2, chloroform)} and 80 ml of ethyl-acetate 28.0 g (technical grade, 60 %, 59.5 mmoles) of (+)-dehydroabiethyl-amine are added. The clear solution is seeded, allowed to crystallize for 10 hours, filtered, washed 5 times with 5 ml of ethyl-acetate each and dried. Thus 13.13 g (23.69 mmoles, 39.8%) of (+)- dehydroabiethyl-ammonium-[(-)-2-(3-chloro-phenyl)- 1 - methyl-ethyl-maleate are obtained.
The salt thus obtained is dissolved in 350 ml of hot acetone and the solution to evaporated to 86 g. The mixture is allowed crystallize at room temperature for 1 hour. After filtration the crystals are washed 5 times with 5 ml of ethyl-acetate each and dried. Thus 10.20 g (18.40 mmoles, 30.9%) of (+)- dehydroabiethyl-ammonium- [(-)-2-(3 -chloro-phenyl)- 1 - methyl-ethyl-maleate] are obtained.
10.20 g of (+)-dehydroabiethyl-ammonium-[(-)-2-(3-chloro- phenyl)-l -methyl-ethyl-maleate] are dissolved in 300 ml of hot acetone. The solution is evaporated to 63 g. The mixture is
allowed to crystallize at room temperature for 1 hour, filtered, the crystals are washed 5 times with 5 ml of ethyl-acetate each and dried. Thus 7.97 g (14.38 mmoles, 24.2%) of (+)- dehydroabiethyl-ammonium-[(-)-2-(3-chloro-phenyl)- 1 - methyl-ethyl-maleate] are obtained. Optical rotation: [ccfo — +10.9° (c=2, acetic acid). Mp.: 140-1430C.
7.97 g of the salt thus obtained are dissolved in 25 ml of methanol, whereupon a solution of 0.80 g of sodium hydroxide in 5 ml of water and thereafter 100 ml of water are added. The reaction mixture is extracted 3 times with 30 ml of dichloro- methane each, acidified with 3 ml of 37 % hydrochloric acid and extracted 4 times with 20 ml of dichloro-methane each. The dichloro-methane phases are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 3.34 g (12.43 mmoles, 20.9 %) of (-)-2-(3-chloro-phenyl)-l-methyl- ethyl-hydrogen-maleate are obtained in the form of an oil.
3.34 g of (-)-2-(3-chloro-phenyl)-l-memyl-ethyl-hydrogen- maleate are dissolved in a mixture of 2.5 g sodium hydroxide and 6 ml of water and heated to boiling for 1 minute. The reaction mixture is cooled, extracted 4 times with 20 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual crude oil (2.04 g) is distilled off in vacuo. Thus 1.82 g
(10.67 mmoles, 17.9 %) of (-)-l-(3-chloro-ρhenyl)-2-ρropanol a arree o obbttaaiinneedd.. Optical rotation: [α]D 25=: -33.8° (c=2, chloroform).
Example 6
Resolution of l-(4-chloro-phenyI)-2-propanol with
Pseudomonas cepacia lipase
To a solution of 30.00 g (175.81 mmoles) of l-(4-chloro- phenyl)-2-propanol in 150 ml of vinyl-acetate 9.00 g Pseudomonas cepacia lipase (Amano® PS-C) bond to a carrier are added. The mixture is stirred at room temperature for 48 hours, filtered, the enzyme is washed 4 times with 10 ml of vinyl-acetate each and the filtrate is evaporated in vacuo. Thus 37.71 g of a mixture of (+)-l-(4-chloro-phenyl)-2-propanol and (-)-2-(4-chloro-phenyl)-l -methyl-ethyl-acetate are obtained in the form of a yellow oil.
The yellow oil thus obtained is dissolved in 100 ml of dichloro-methane, whereupon 15.9 ml (114.23 mmoles, d=0.727) of triethyl-amine and in one portion 10.20 g (104.02 mmoles) maleic anhydride are added. The reaction mixture warms up gradually and its colour turns dark brown. The reaction mixture is heated to boiling for 5 minutes, cooled, washed once with 100 ml and once with 15 ml of 1.5 molar
hydrochloric acid twice with 15 ml of water each. The dichloro-methane phase is extracted once with 115 ml and twice with 15 ml of a 1 molar sodium carbonate solution. The aqueous layers are united and washed 3 times with 15 ml of dichloro-methane each. [The aqueous phase contains the sodium salt of (+)-2-(4-chloro-phenyl)-l-methyl-ethyl- hydrogen-maleate.] The dichloro-methane phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 29.08 g (136.74 mmoles, 155.6 %) of (-)-l-(3- chloro-phenyl)-2-propil-acetate are obtained in the form of a yellow oil. Optical rotation : [α]D 25= -2.6° (c=2, chloroform).
A mixture of the (-)-2-(4-chloro-phenyl)-l -methyl-ethyl- acetate thus obtained, 45 ml of water, 20 ml of methanol and 10 g of sodium hydroxide is heated to boiling for 5 minutes. The methanol is distilled off in vacuo. The residue is cooled and extracted once with 50 ml and 3 times with 15 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (23.25 g) is subjected to distillation in vacuo. Thus 21.97 g (128.75 mmoles, 146.5 %) of (-)-l-(4-chloro-phenyl> 2-propanol are obtained. Optical rotation: [α]D 25= -8.6° {c-2, chloroform).
To the aqueous phase containing the sodium salt of (+)-2-(4- chloro-phenyl)-l-methyl-ethyl-hydrogen-maleate 60 ml of 20 % hydrochloric acid are added and the mixture is extracted once with 50 ml and 3 times with 15 ml of dichloro-methane each. The dichloro-methane phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 10.16 g (37.81 mmoles, 43.0 %) of (-)-2-(4-chloro-phenyl)-l- methyl-ethyl-hydrogen-maleate are obtained in the form of a brown oil. Optical rotation [α]D 25= +14.2° (c=2, chloroform).
A mixture of 9.76 g (+)-2-(4-chloro-phenyl)-l-methyl-ethyl- hydrogen-maleate thus obtained, 25 ml of water and 5 g of sodium hydroxide is heated to boiling for 1 minutes. The reaction mixture is cooled and extracted 4 times with 10 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (5.92 g) is distilled off in vacuo. Thus 5.73 g (33.58 mmoles, 38.2 %) of (+)-l-(4-chloro-phenyl)-2-ρroρanol are obtained in the form of an oil. Optical rotation: [α]D 25= +31.2° (c=2, chloroform).
Example 7
Enantiomer purification of (-)-l-(4-chloro-phenyl)-2- propanol via the fφ-(-)-l-phenyI-ethyl-ammonium salt of the succinic acid semiester
a) Preparation of(-)-2-(4-chloro-phenyl)-l-methyl-ethyl- hydrogen-succinate
A mixture of 6.25 g (36.63 mmoles) of (-)-l-(4-chloro- ρhenyl)-2-proρanol {[α]D 25= -16.2° (c=2, chloroform)} 30 ml of dichloro-methane, 5.7 ml of triethyl-amine (40.96 mmoles), a catalytic amount of N,N-dimethylamino-pyridine and 4.00 g (39.97 mmoles) of succinic acid anhydride is heated to boiling for 1 hour. The reaction mixture is cooled, washed once with 35 ml and once with 10 ml of 1.5 molar hydrochloric acid and once with 20 ml of water, dried over sodium sulfate and the solvent is removed in vacuo. Thus 9.83 g (36.31 mmoles, 99.1 %) of (-)-2-(4-chloro-ρhenyl)- 1 -methyl-ethyl-hydrogen- succinate are obtained in the form of a yellow oil. Optical rotation: [α]D 25= -1.4° (c=2, chloroform).
b) Enantiomer purification of (-)-2-(4-chloro-phenyl)-l- methyl-ethyl-hydrogen-sucdnate and decomposition of the semiester
9.83 g (36.31 mmoles) of (-)-2-(4-chloro-phenyl)-l-methyl- ethyl-hydrogen-succinate {[α]D 25= -1.4° (c=2, chloroform)} are dissolved in 10 ml of acetone. The solution 4.40 g (36.31 mmoles) of (^-('-j-phenyl-ethylamine are added, the solution is seeded and allowed to crystallize at 10 0C for 2 hours. The crystals are filtered, washed twice with 5 ml of ethyl-acetate and dried. Thus 4.67 g (11.92 mmoles, 32.8%) of white crystalline (S)-(-)- l-phenyl-ethyl-ammonium-[(-)-2-(4-chloro- phenyl)-l-methyl-ethyl-succinate] are obtained.
The crystals are dissolved in 10 ml of hot acetone. The solution is allowed to crystallize at 10 0C for 10 hours. The crystals are filtered, washed twice with 5 ml of ethyl-acetate each and dried. Thus 3.85 g (9.82 mmoles, 27.1%) of white crystalline (^)-('-y )-l-phenyl-ethyl-ammonium-[(-)-2-(4-chloro-phenyl)-l- methyl-ethyl-succinate] are obtained.
The crystals are dissolved in 10 ml of hot acetone and the solution is allowed to crystallize -10 0C for 2 hours. The crystals are filtered, washed twice with 5 ml of ethyl-acetate each and dried. Thus 3,48 g (8.88 mmoles, 24.5%) of white
crystalline (^)-(9-l-phenyl-ethyl-ammonium-[(-)-2-(4-chloro- phenyl)-l-methyl-ethyl-succinate] are obtained. Optical rotation: [α]D 25= -4.3° (c=2, methanol). Mp.: 113-115 0C.
The crystals are suspended in 30 ml of ethyl-acetate, washed 3 times with 10 ml of 1.5 molar hydrochloric acid each and once with 20 ml of water, dried over sodium sulfate and the solvent is removed in vacuo. Thus 2.25 g (8.31 mmoles, 22.9 %) of (-)-2-(4-chloro-phenyl)- 1 -methyl-ethyl-hydrogen-succinate are obtained in the form of a yellow oil. Optical rotation: [α]o = -2.7° (c=2, chloroform).
A mixture of (-)-2-(4-chloro-phenyl)-l-methyl-ethyl-hydrogen- succinate thus obtained, 4.5 ml of water and 1.5 g of sodium hydroxide is heated to boiling for 1 minute. The reaction mixture is cooled and extracted 4 times with 10 ml of diethyl- ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is removed in vacuo. The residual oil (1.44 g) is subjected to distillation in vacuo. Thus 1.27 g (7.44 mmoles, 20.5 %) of (-)-l-(4-chloro-phenyl)-2-propanol are obtained in the form of a crystalline oil. Optical rotation: [α]
D 25= -31.2° (c=2, chloroform).
Example 8
Resolution of l-(3,4-dichloro-phenyI)-2-propanol with
Pseudomonas cepacia lipase
To a solution of 35.00 g (170.67 mmoles) of l-(3,4-dichloro- phenyl)-2-propanol and 105 ml of vinyl-acetate 3.00 g of Pseudomonas cepacia lipase (Amano® PS-C) bond to a carrier are added. The reaction mixture is stirred at room temperature for 30 hours, filtered, the enzyme is washed 4 times with 10 ml of vinyl-acetate each and the filtrate is evaporated in vacuo. Thus 39.82 g of a mixture of (+)-l-(3,4-dichloro-ρhenyl)-2- propanol and (-)-2-(3,4-dichloro-phenyl)-l -methyl-ethyl- acetate are obtained in the form of a yellow oil.
The oil is dissolved in 100 ml of dichloro-methane, whereupon 14.5 ml (104.42 mmoles, d=0.727) of triethyl-amine and in one portion 8.50 g (86.73 mmoles) of maleic anhydride are added. The reaction mixture gradually warms up, while its colour turns dark brown. The reaction mixture is heated to boiling for 5 minutes, cooled, washed once with 100 ml and once with 10 ml of 1.5 molar hydrochloric acid and twice with 15 ml of water each. A dichloro-methane layer is extracted once with 100 ml and twice with 5 ml of 1 molar sodium carbonate solution each. The aqueous layers are united and washed 3 times with 15 ml of dichloro-methane each. [The aqueous
phase contains the sodium salt of the maleic acid semiester of (+)-l-(3,4-dichloro-phenyl)-2-propanol.] The dichloro- methane layers are united, dried over sodium sulfate and the solvent is removed in vacuo. Thus 23.24 g (94.04 mmoles, 110.2 %) of (-)-2-(3,4-dichloro-phenyl)-l-methyl-ethyl-acetate are obtained in the form of a yellow oil. Optical rotation: [α]D 25= -2.9° (c=2, chloroform).
A mixture of 23.04 g of (-)-2-(3,4-dichloro-ρhenyl)-l -methyl- ethyl-acetate thus obtained, 15 ml of water, 25 ml of methanol and 5.5 g of sodium hydroxide is heated to boiling for 5 minutes whereupon the methanol is distilled off in vacuo. The residue is cooled and extracted once with 50 ml and 3 times with 15 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (19.06 g) is subjected to distillation in vacuo. Thus 18.70 g (91.18 mmoles, 106.8 %) of (-)-l-(3,4- dichloro-phenyl)-2-propanol are obtained in the form of an oil. Optical rotation: [α]D 25= -18.8° (c=2, chloroform).
To the aqueous phase containing the sodium salt of (+)-2-(3,4- dichloro-phenyl)-l-methyl-ethyl-hydrogen-maleate 50 ml of 20 % hydrochloric acid are added. The reaction mixture is extracted once with 50 ml and 3 times with 15 ml of dichloro- methane each. The dichloro-methane phases are united, dried
over sodium sulfate and the solvent is distilled off in vacuo. Thus 20.24 g (66.77 mmoles, 78.2 %) of (+)-2-(3,4-dichloro- phenyl)-l-methyl-ethyl-hydrogen-maleate are obtained in the form of a brown oil. Optical rotation: [OL]Ό 25= +8.5° (c=2, chloroform).
A mixture of 20.4 g of the (+)-2-(3,4-dichloro-ρhenyl)-l- methyl-ethyl-hydrogen-maleate thus obtained, 60 ml of water and 7 g of sodium hydroxide is heated to boiling for 1 minute. The reaction mixture is cooled and extracted 4 times with 30 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (13.07 g) is subjected to distillation in vacuo. Thus 12.13 g (59.16 mmoles, 69.2 %) of (+)-l-(3,4-dichloro- phenyl)-2-propanol are obtained in the form of an oil. Optical rotation : [α]D 25= +28.8° (c=2, chloroform). On standing the oil is solidified, mp.: 48-520C.
Examp ■Λlae. 9
Enantiomer purification of (-)-l-(3,4-dichloro-phenyl)-2- propanol via the $H-)-l-phenyl-ethyl-ammonium saIt of the phthalic acid semiester
a) Preparation of(-)-2-(3,4-dichloro-phenyl)-l-methyl- ethyl-hydrogen-phthalate
A mixture of 7.10 g (34.62 mmoles) of (-)-l-(3,4-dichloro- ρhenyl)-2-propanol {[α]D 25= -18.8° (c=2, chloroform)}, 20 ml of dichloro-methane 5.50 ml of triethyl- amine (39.54 mmoles), a catalytic amount of N,N-dimethyl-amino-pyridine and 5.3O g (35.78 mmoles) of phthalic anhydride is heated to boiling for an hour. The reaction mixture is cooled, washed once with 30 ml and once with 10 ml of 1.5 molar hydrochloric acid each and once with 20 ml of water, dried over sodium sulfate and the solvent is removed in vacuo. Thus 12.19 g (34.51 mmoles, 99.7%) of (-)-2-(3,4-dichloro-phenyl)-l-methyl-ethyl- hydrogen-phthalate are obtained in the form of a brown oil. Optical rotation: [α]D 25= -28.3° (c=2, chloroform).
b) Enantiomer purification of(-)-2-(3,4-dichloro- phenyl)-l-methyl-ethyl-hydrogen-phthalate and decomposition of the semiester
To a solution of 12.19 g (34.51 mmoles) of (-)-2-(3,4-dichloro- phenyl)-l-methyl-ethyl-hydrogen-phthalate {[α]D 25= -28.3° (c=2, chloroform)} and 50 ml of diisopropyl-ether 4.1 g (33.83 mmoles) of (S)-(-)- 1-phenyl-ethyl-amine are added. The two- phase solution is stirred at room temperature overnight. After crystallization the mixture is filtered, washed 3 times with 5 ml of diisopropyl-ether and dried. Thus 8.26 g (17.41 mmoles,
50.5%) of (^>(9-l-ρhenyl-ethyl-ammonium-[(-)-2-(3,4- dichloro-phenyl)-l-memyl-ethyl-phthalate] are obtained.
The crystals obtained are dissolved in 8 ml of hot ethyl-acetate. The solution is allowed to crystallize at -10 0C for 6 hours. The crystals are filtered, washed 5 times with 2.5 ml of diisopropyl- ether each and dried. Thus 6.15 g of (^-^-l-phenyl-ethyl- ammonium-[(-)-2-(3,4-dichloro-phenyl)- 1 -methyl-ethyl- phthalate] are obtained.
The salt is dissolved in 6 ml of hot ethyl-acetate. The solution is allowed to crystallize at -10 0C for 6 hours, filtered, washed 5 times with 2.5 ml of diisopropyl-ether each and dried. Thus 5.56 g (11.72 mmoles, 34.0%) of white crystalline (S)-(-)-l- phenyl-ethyl-ammonium- [(-)-2-(3 ,4-dichloro-phenyl)- 1 - methyl-ethyl-phthalate] are obtained. Optical rotation: [α]D 25= -36.6° (c=l. methanol). [α]D 25= -36.5° (c=2, acetic acid). Mp.: 102-104 0C
To 5.46 g of the salt thus obtained 30 ml of ethyl-acetate are added. The mixture is washed 3 times with 10 ml of 1.5 molar hydrochloric acid each and once with 10 ml of water, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 3.97 g (11.24 mmoles, 32.6 %) of (-)-2-(3,4-dichloro- phenyl)- 1-methyl-ethyl-hydrogen-phthalate are obtained in the
form of an oil. Optical rotation: [α]D 25= -38.6° (c=2, chloroform).
Mp.: 82-86 0C (after recrystallization from toluol/hexane
90-920C)
A mixture of the (-)-2-(3,4-dichloro-phenyl)-l-methyl-ethyl- hydrogen-phthalate thus obtained, 10 ml of water and 3.0 g of sodium hydroxide is heated to boiling for 1 minute. The reaction mixture is cooled and extracted 4 times with 20 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (2.04 g) is subjected to distillation in vacuo. Thus 1.85 g (9.02 mmoles, 26.1 %) of (-)-l-(3,4-dichloro-phenyl)-2- propanol are obtained. Optical rotation: [α]D 25= -25.3° (c=2, chloroform). Mp.: 42-46 0C.
Example 10
Resolution of l-(3-bromo-4-methoxy-phenyl)-2-propanol with Pseudomonas cepacia lipase
To a solution of 35.00 g (142.79 mmoles) of l-(3-bromo-4- methoxy-phenyl)-2-propanol and 105 ml of vinyl-acetate 3.00 g of Pseudomonas cepacia lipase (Amano® PS-C) bond to a carrier are added. The reaction mixture is stirred at room temperature for 30 hours, filtered, the enzyme is washed 4
times with 10 ml of vinyl-acetate each and the filtrate is evaporated in vacuo. Thus 39.26 g of a mixture of (+)-l-(3- bromo-4-methoxy-phenyl)-2-propanol and (-)-2-(3-bromo-4- methoxy-phenyl)-l -methyl-ethyl-acetate are obtained in the form of a yellow oil.
The yellow oil thus obtained is dissolved in 100 ml of dichloro-methane, whereupon 12.2 ml (87.65 mmoles, d=0.727) triethyl-amine and in one portion 7.20 g (73,42 mmoles) of maleic anhydride are added. The reaction mixture gradually warms up and its colour turns dark brown. The reaction mixture is heated to boiling for 5 minutes, cooled, washed once with 85 ml and once with 10 ml of a 1.5 molar hydrochloric acid and twice with 15 ml of water. The dichloro- methane phases are extracted once with 100 ml and twice with 5 ml of a 1 molar sodium carbonate solution. The aqueous layers are united and washed 3 times with 15 ml of dichloro- methane each. [The aqueous phase contains the sodium salt of (+)-2-(3~bromo-4-methoxy-phenyl)- 1 -methyl-ethyl-hydrogen- maleate.] The dichloro-methane phases are united, washed over sodium sulfate and the solvent is distilled off in vacuo. Thus 23.54 g (81.98 mmoles, 114.8 %) of (-)-2-(3-bromo-4- methoxy-phenyl)-l -methyl-ethyl-acetate are obtained in the form of a yellow oil. Optical rotation: [α]
D 25= -2.9° (c=2, chloroform).
A mixture of 23.34 g of (-)-2-(3-bromo-4-methoxy-phenyl)-l- methyl-ethyl-acetate thus obtained, 15 ml of water, 25 ml of methanol and 5.5 g of sodium hydroxide is heated to boiling for 5 minutes, whereupon the methanol is distilled off in vacuo. The mixture is cooled and extracted 3 times with 25 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (20.14 g) is subjected to distillation in vacuo. Thus 19.77 g (80.66 mmoles, 113.0 %) of (-)-l-(3-bromo-4- methoxy-phenyl)-2-propanol are obtained in the form of an oil. Optical rotation: [α]D 25= -16.2° (c=2, chloroform).
To the aqueous phase containing the sodium salt of (+)-2-(3- bromo-4-methoxy-phenyl)- 1 -methyl-ethyl-hydrogen-maleate 50 ml of 20 % hydrochloric acid are added and the mixture is extracted once with 50 ml and 3 times with 15 ml of dichloro- methane each. The dichloro-methane phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 19.25 g (55.09 mmoles, 78.6 %) of (+)-2-(3-bromo-4- methoxy-phenyl)- 1 -methyl-ethyl-hydrogen-maleate are obtained in the form of yellow oil. Optical rotation: [α]
D 25= +9.3° (c=2, chloroform).
A mixture of 19.04 g of (+)-2-(3-bromo-4-methoxy-phenyi)-l- methyl-ethyl-hydrogen-maleate, 60 ml of water and 7 g of sodium hydroxide is heated to boiling for 1 minutes. After cooling the mixture is extracted 4 times with 30 ml of diethyl- ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual oil (12.56 g) is subjected to distillation in vacuo. Thus 11.86 g (48.39 mmoles, 67.8 %) of (+)-l-(3-bromo-4-methoxy- phenyl)-2-propanol are obtained in the form of an oil. Optical rotation: [α]D 25= +25.4° (c=2, chloroform). On standing the oil solidifies, mp.: 43-46°C.
Example 11
Enantiomer purification of (-)-l-(3-bromo-4-methoxy- phenyl)-2-propanol with the (+)-dehydroabiethyI- ammonium salt of the maleic acid semiester
a) Preparation of(-)-2-(3-bromo-4-methoxy-phenyl)-l- methyl-ethyl-hydrGgen-maleate
A mixture of 10.00 g (40.80 mmoles) of (-)-l-(3-bromo-4- methoxy-phenyl)-2-propanol [α]
D 25=: -16.2° (c=2, chloroform), 50 ml of dichloro-methane, 6.30 ml of triethyl-amine (45.26 mmoles) and 4.20 g (42.83 mmoles) of maleic anhydride is heated to boiling for 10 minutes. The reaction mixture is
cooled, washed once with 40 ml, once with 10 ml of 1.5 molar hydrochloric acid and once with 20 ml of water, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 13.95 g (40.65 mmoles, 99.6 %) of (-)-2-(3-bromo-4-methoxy- phenyl)-l-methyl-ethyl-hydrogen-maleate are obtained in the form of a dark brown oil. Optical rotation: [α]o25=r -6.3° (0=2, chloroform).
b) Enantiomer purification of (-)-2-(3-bromo-4-methoxy- phenyl)-l~methyl-ethyl-hydrogen~maleate and decomposition of the semiester
To a solution of 13.95 g (40.65 mmoles) of (-)-2-(3-bromo-4- methoxy-phenyl)- 1 -methyl-ethyl-hydrogen-maleate { [α]D 25= -6.3° (c=2, chloroform)} in 14 ml of ethyl-acetate 12.0 g (92 %, 38.7 mmoles) of (+)-dehydroabiethyl-amine are added. The clear solution is seeded. The solution is allowed to crystallize for 10 hours. The crystals are filtered, washed 6 times with 5 ml of ethyl-acetate each and dried. Thus 16.02 g (25.77 mmoles, 63,4%) of (+)-dehydroabiethyl-ammonium-[(-)-2-(3- bromo-4-methoxy-phenyl)- 1 -methyl-ethyl-maleate] are obtained.
The salt thus obtained is dissolved in 70 ml of hot acetone. The solution is allowed to crystallize at -10 0C for 2 hours. The
crystals are filtered, washed 4 times with 5 ml of ethyl-acetate each and dried. Thus 11.2O g (17.82 mmoles, 43.8%) of (+)- dehydroabiethyl-ammonium-[(-)-2-(3-bromo-4-methoxy- phenyl)-l-methyl-ethyl-maleate] are obtained.
The salt thus obtained is dissolved in 50 ml of hot acetone. The solution is allowed to crystallize at -10 0C for 2 hours. The crystals are filtered, washed 5 times with 5 ml of ethyl-acetate each and dried. Thus 8.36 g (13.30 mmoles, 32.7%) of (+)- dehydroabiethyl-ammonium-[(-)-2-(3-bromo-4-methoxy- phenyl)- 1 -methyl-ethyl— maleate] are obtained. Optical rotation: [α]D 25= +22.0° (c=2, methanol), [α]D 25= +10.9° (c=2, acetic acid). Mp.: 139-141°C.
8.36 g of the salt thus obtained are suspended in 25 ml of methanol, whereupon a solution of 0.70 g of sodium hydroxide in 5 ml of water and 100 ml of water are added. The reaction mixture is extracted 3 times with 30 ml of dichloro-methane each, acidified with 3 ml of 37 % hydrochloric acid and extracted 4 times with 20 ml of dichloro-methane each. The dichloro-methane phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 4.33 g (12.62 mmoles, 31.0%) of (-)-2-(3-bromo-4-methoxy-phenyl)-l- methyl-ethyl-hydrogen-maleate are obtained in the form of an oil. Optical rotation: [α]
D 25= -8.5° (c=2, chloroform).
A mixture of the (-)-2-(3-bromo-4-methoxy-phenyl)-l-methyl- ethyl-hydrogen-maleate thus obtained, 6 ml of water and 2.O g of sodium hydroxide is heated to boiling for 1 minute. After cooling the mixture is extracted 4 times with 20 ml of diethyl- ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual crude oil (2.81 g) is subjected to distillation in vacuo. Thus 2.52 g (10.28 mmoles, 25.3 %) of (->l-(3-bromo-4-methoxy- phenyl)-2-propanol are obtained. Optical rotation: [α]D 25= -24.6° (c=2, chloroform). Mp.: 43-46 0C.
Example 12
Resolution of l-(4-fluor-phenyl)-2-propanoI with
Pseudomonas cepacia lipase
To a solution of 5.00 g (32.43 mmoles) of l-(4-fluor-phenyl)- 2-propanol in 25 ml of vinyl-acetate 1.5 g of Pseudomonas cepacia lipase (Amano® PS-C) bond to a carrier are added. The reaction mixture is stirred at room temperature for 15 hours, filtered, the enzyme is washed 4 times with 5 ml of vinyl-acetate each and the filtrate is evaporated in vacuo. Thus 6.26 g of a mixture of a (+)-l-(4-fluor-phenyl)-2-propanol and (-)-2-(4-fluor-phenyl)-l -methyl-ethyl-acetate are obtained in the form of a yellow oil.
The oil thus obtained is dissolved in 15 ml of dichloro- methane, whereupon 2.8 ml (20.12 mmoles, d=0.727) of triethyl-amine and in one portion 1.80 g (18.36 mmoles) of maleic anhydride are added. The reaction mixture warms up gradually, while its colour turns dark brown. The reaction mixture is heated to boiling for 5 minutes, cooled, washed once with 45 ml and once with 10 ml of 1.5 molar hydrochloric acid and twice with 10 ml of water each. The dichloro-methane layer is extracted once with 20 ml and twice with 5 ml of 1 molar sodium carbonate solution each. The aqueous layers are united and washed 3 times with 10 ml of dichloro-methane each. [The aqueous phase contains the sodium salt of (+)-2-(4- fluor-phenyl)-l-methyl-ethyl-hydrogen-maleate.] The dichloro- methane phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 3.80 g (19.37 mmoles, 119.4 %) of (-)-2-(4-fluor-ρhenyl)-l -methyl-ethyl-acetate are obtained in the form of a yellow oil. Optical rotation: [α]D 25= -4.2° (c=2, chloroform).
A mixture of 3,40 g of (-)-2-(4-fluor-phenyl)-l -methyl-ethyl- acetate, 4 ml of water, 6 ml of methanol and 1.2 g of sodium hydroxide are heated to boiling for 5 minutes, whereupon the methanol is distilled off in vacuo. The reaction mixture is cooled and extracted and 4 times with 10 ml of diethyl-ether each. The ethereal phases are united, dried over sodium sulfate and the
solvent is distilled off in vacuo. The residual oil (2.46 g) is distilled off in vacuo. Thus 2.39 g (15.50 mmoles, 95.6 %) of (-)-l-(4-fluor-phenyl)-2-propanol are obtained in the form of an oil . Optical rotation: [α]D 25= -20.5° (c=2, chloroform).
To the aqueous phase containing the sodium salt of (+)-2-(4- fluor-phenyl)-l-methyl-ethyl-hydrogen-maleate 6 ml of 37 % hydrochloric acid are added and the mixture is extracted 4 times with 15 ml of dichloro-methane each. The dichloro- methane phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. Thus 3.07 g (12.17 mmoles, 75.06 %) of (+)-2-(4-fluor-phenyl)-l -methyl-ethyl -hydrogen- maleate are obtained in the form of a yellow oil. Optical rotation: [α]D 25= +18.5° (c=2, chloroform).
A mixture of '2.67 g of (+)-2-(4-fluor-phenyl)-l-methyl~ethyl- hydrogen-maleate, 4.5 ml of water and 1.5 g of sodium hydroxide is heated to boiling for 1 minute. After cooling the reaction mixture is extracted 4 times with 10 ml of diethyl- ether each. The ethereal phases are united, dried over sodium sulfate and the solvent is distilled off in vacuo. The residual crude oil (1.58 g) is subjected to distillation in vacuo. Thus 1.47 g (9.53 mmoles, 58.8 %) of (+)-l-(4-fluor-phenyl)-2- propanol are obtained. Optical rotation: [α]D 25= +32.2° (c=2, chloroform).