WO2005075193A1 - 積層体及びその製造方法 - Google Patents
積層体及びその製造方法 Download PDFInfo
- Publication number
- WO2005075193A1 WO2005075193A1 PCT/JP2005/001873 JP2005001873W WO2005075193A1 WO 2005075193 A1 WO2005075193 A1 WO 2005075193A1 JP 2005001873 W JP2005001873 W JP 2005001873W WO 2005075193 A1 WO2005075193 A1 WO 2005075193A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- photochromic
- ultraviolet
- surface layer
- compound
- optical substrate
- Prior art date
Links
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B27/08—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
Definitions
- the present invention relates to a laminate suitable as an optical article having photochromic properties, such as a photochromic spectacle lens.
- Photochromism is a reversible effect in which a compound rapidly changes its color when irradiated with light containing ultraviolet rays, such as sunlight or the light of a mercury lamp, and returns to its original color when the light is stopped and left in a dark place. It is applied to various uses.
- photochromism is also applied in the field of spectacle lenses, and plastic lenses having added retortochromic properties by adding various photochromic compounds having the above properties have been obtained.
- Konimide compounds, spiroxazine compounds, chromene compounds and the like have been found as photomix compounds.
- the photochromic properties of the photochromic plastic lenses produced by these methods are closely related to the properties of the resin (or raw material monomer composition) that forms the matrix of the photochromic compound. Many studies have been made to improve the characteristics. For example, lowering the glass transition temperature (T g) of the lens substrate, Or use a combination of a specific long-chain alkylene glycol dimethacrylate and a polyfunctional methacrylate having three or more radically polymerizable groups as the raw material monomer for the matrix resin. By adopting measures such as expanding the free space of the photomask to facilitate the movement of the photo molecules, we succeeded in obtaining a photochromic lens with relatively good photochromic properties such as color density and fading speed. Prior art 1).
- the coating film containing the photochromic compound has a thickness of only several tens of microns, so that it can be obtained by impregnation or kneading. Durability tends to be lower than that of photochromic lenses.
- the UV absorber When a UV-absorbing i
- the problem of precipitation of the ultraviolet absorber at the time of curing can be improved by reducing the amount of the ultraviolet absorber to be added, but there is a practical problem in terms of optical characteristics.
- an object of the present invention is to provide a photochromic product having a high photochromic durability, in which the oxidative deterioration of the photochromic compound is effectively prevented without lowering the coloring density, and a method for producing the same. That
- Another object of the present invention is to prevent the precipitation of an ultraviolet absorber, effectively prevent white turbidity and reduce the optical properties of an optical substrate such as a lens.
- An object of the present invention is to provide a photochromic product and a method for producing the same, which effectively prevent oxidation deterioration of the product.
- Still another object of the present invention is to effectively prevent bleed-out of an ultraviolet absorber even when used for a long period of time, and to effectively prevent the ultraviolet absorber from oxidatively deteriorating a photomix compound.
- the lipstick is durable photo A chromic product and a method for producing the same.
- an optical substrate having a photochromic surface layer portion composed of a resin in which a photochromic compound is dispersed on at least one surface, and a thickness formed on the photochromic surface layer portion of the optical substrate
- a UV-absorbing film having a wavelength of 0.1 to 100 m.
- the UV-absorbing film has a light transmittance at 360 nm of 50 ⁇ 1 ⁇ 2 or more and a light transmittance of 320 nm. (Hereinafter, may be referred to as a photochromic laminate).
- a light transmittance of 560 nm of the ultraviolet absorbing film is 85% or more;
- the ultraviolet absorbing film is a coating layer containing an inorganic oxide containing titanium as an ultraviolet absorbing agent
- an optical article comprising the above photochromic laminate.
- the photochromic laminate described above includes a silicone coating agent containing colloidal particles of an inorganic compound that selectively absorbs ultraviolet light having a wavelength of 320 nm as an ultraviolet absorber, on the photochromic surface layer of the optical substrate. It can be manufactured by applying a UV curable film and curing it to form an ultraviolet absorbing film having a thickness of 0.1 to 100 m.
- the photochromic laminate is formed by applying an organic coating agent containing an ultraviolet absorber that selectively absorbs ultraviolet light having a wavelength of 320 nm on the photochromic surface layer of the optical substrate. It can also be manufactured by curing an ultraviolet ray absorbing film having a thickness of 0.1 to 100 m.
- the photochromic laminate described above is further provided with an ultraviolet absorber that selectively absorbs ultraviolet light having a wavelength of 320 nm on the surface of the photochromic layer to form a film having a film thickness of 0. It can also be manufactured by forming an ultraviolet absorbing film.
- the ultraviolet absorbing film can be formed directly on the photochromic surface layer, or the primer layer or the effect of the present invention can be added. In other embodiments, it can be formed on the photochromic surface layer via another functional layer.
- the light transmission characteristics of the UV absorbing film formed on the photochromic laminate are as follows.
- the UV absorbing film of the same composition and thickness is formed on quartz glass, and the wavelength at each wavelength is calculated by subtracting the reflection of quartz glass. It can be easily confirmed by measuring the light transmittance.
- IA Just measure the light absorption. For example, a spectrophotometer "! Is equipped with a 5 ° specular reflection measuring device, the relative reflectance with aluminum is measured, and the absorption of the ultraviolet absorbing film is calculated from the relative reflectance to obtain the light of each wavelength. The transmittance can be determined.
- the term “selectively absorb ultraviolet light having a length of 320 nm” means that, when the absorption intensity of ultraviolet light having a wavelength of 320 nm is set to 100%, the wavelength of 360 nm Means that the ultraviolet light absorption intensity is 80% or less, preferably 60% or less.
- Such UV absorbers having a selective absorption ability at a wavelength of 320 nm are often achieved in those having an absorption maximum wavelength of 300 to 330 nm.
- the photochromic laminate of the present invention when the ultraviolet absorbing film formed on the photochromic surface layer has a thickness of 0.1 to 100 m, a predetermined light beam Due to the transmission characteristics, the photochromic durability is improved without lowering the basic optical characteristics of the optical substrate to "F". Since the rate is 10% or less, the photochromic compound existing in the photochromic rhombic layer can be effectively prevented from being oxidized and degraded by violet rays, and the photochromic durability can be improved. However, at the same time, such an ultraviolet absorbing film has a light transmittance at 360 nm of 50% or more, so that the photochromic reaction is not hindered by the ultraviolet absorbing film, and Concentration (a reduction has been effectively avoided.
- the photochromic laminate ⁇ of the present invention may include a silicone-based coating agent containing an ultraviolet absorber that selectively absorbs 32 Onm ultraviolet rays. Alternatively, it is produced by applying and curing an organic coating agent on the photochromic surface layer portion, or by vapor-depositing the ultraviolet absorber on the photochromic surface layer portion.
- the light transmittance at 560 nm is 85 ⁇ 1 ⁇ 2 or more), and the bleeding of the ultraviolet absorber is effectively suppressed, so that the performance of the ultraviolet absorbing film can be prevented from deteriorating.
- the deposition and bleed-out of the ultraviolet absorbing agent can be similarly prevented.
- the ultraviolet absorbing film formed by using the silicone-based coating agent is also excellent in oxygen barrier properties, so that the photochromic compound is remarkably highly oxidatively degraded by ultraviolet rays and is most suitable. .
- the photochromic laminate of the present invention can be suitably used as a photochromic optical article such as a photochromic plastic lens.
- the photochromic laminate of the present invention is obtained by forming an ultraviolet absorber having a specific thickness and a specific light transmittance on a photochromic surface layer formed on an optical substrate.
- the optical substrate means a transparent plate having a pair of front and back main surfaces, and the four dogs may be curved, and their thickness does not necessarily have to be constant.
- At least one surface of the optical substrate is made of a resin in which a photochromic compound is dispersed.
- the photochromic surface layer to be formed has a shape, and the presence of the photochromic surface layer has a reversible color change due to a predetermined photomix reaction.
- Such a photochromic layer portion may be formed on the entire surface of the transparent plate-like body having a predetermined shape, may be formed on the entire surface of one surface, or may be formed on the other side, depending on the application. May be partially formed on the surface of the substrate.
- the entire optical substrate may be formed of a resin in which photochromic conversion is dispersed, and may be formed so that a photochromic reaction occurs not only on the surface but also inside.
- optical substrate examples include a photochromic plastic lens manufactured by an impregnation method, a kneading method, or an Ifc coating method; glass, etc.
- a photochromic optical component provided with photochromic properties in the same manner as the coating method is exemplified.
- photochromic plastic lens manufactured by the impregnation method for example, those disclosed in US Pat. No. 5,739,243 (the prior art 1) can be suitably used.
- This hard fc body includes (A) a polymerizable monomer having an L scale Rockwell hardness of 40 or less, and (a trifunctional or higher functional polymerizable monomer having a BOL scale Rockwell hardness of 60 or more). Is obtained by curing a curable composition comprising (C) a bifunctional polymerizable monomer having an L-scale Rockwell hardness of 60 or more, and a photochromic compound l.
- the L-slur rock hardness of the above-mentioned polymerizable monomer means the hardness of a polymer obtained by homopolymerizing this monomer.
- the photokeromic plastic lens or photomic optical component manufactured by the coating method is a coating agent composed of a curable composition disclosed in International Publication No. 03Z0119667 Pamphlet. It is preferable that a photochromic coat layer (photochromic surface layer portion) is formed using the above.
- the curable composition comprises a radical polymerizable monomer having a silanol group or a radical polymerizable monomer having a group capable of forming a silanol group by hydrolyzing water, And a photochromic compound each containing a specific amount, and can be suitably used as a curable composition containing no amine compound as a coating agent.
- a known photochromic compound such as a fulgimide compound, a spiroxazine compound, or a chromene compound, which can be used in the stiffening composition used in the production of the above-mentioned optical substrate, may be used without limitation. it can.
- Examples of the above-mentioned fulgimide compound, spiroxane compound and chromene compound include, for example, JP-A-2-28154, JP-A-62-288830, WO94Z228. Compounds described in the specification of Japanese Patent No. 50, WO96 / 145,966 and the like can be used.
- chromene compounds are preferred because they have high durability of photochromic properties and good color development density and fading speed. It is. These chromene compounds, from the fact that the effect of maintaining the selected [Koyoru color density of the ultraviolet absorbing film in the present invention are remarkably exhibited, is preferably used Q
- chromene compounds include compounds described in International Publication No. 01 Z60811, U.S. Pat.No. 6,340,765, and: Japanese Patent No. 6,252,194.
- the most preferred cupene compound is a chromene compound represented by the following general formula (1).
- R 3 , R 4 and R 5 each independently represent a hydrogen atom, an alkyl group, an alkoxyl group, an aralkoxy group, an amino group, a substituted amino group, a cyano group, a substituted or unsubstituted aryl group, a halogen atom
- a condensed heterocyclic group in which an aromatic hydrocarbon ring or an aromatic heterocyclic ring is fused to the heterocyclic group
- o is an integer from 0 to 6
- R 1 and R 2 each independently represent a group represented by the following formula (3) or (4), a substituted or unsubstituted aryl group, a substituted or unsubstituted heteroaryl group, or an alkyl group; Group. Further, R 1 and R 2 may together form an aliphatic hydrocarbon ring or an aromatic hydrocarbon ring.
- R 6 is a substituted or unsubstituted aryl group or a substituted or unsubstituted heteroaryl group
- R 7 is a hydrogen atom, an alkyl group, or a halogen atom
- p is “! It is an integer of 3.
- R 8 is a substituted or unsubstituted aryl group or a substituted or unsubstituted heteroaryl group, and p ′ is an integer of 1 to 3.
- the compounds represented by the following (5) to (10) are particularly preferable from the viewpoint of photochromic properties such as coloring density and fading speed and durability. .
- R 9 and R 1 ° have the same meanings as R 1 and R 2 in the formula (1), respectively, and R 11 and R 12 have the same meanings as R 5 in the formula (1),
- q and q ' are each an integer of 1-2.
- R 13 and R 14 have the same meanings as R 11 and R 12 in the formula (1).
- R 15 and R 16 have the same meaning as R 5 in the formula (1),
- L is the following formula, , ⁇ F "CH '+ P +, individual Rip—,
- P is an oxygen atom or a sulfur atom
- R 17 is an alkylene group having 1 to 6 carbon atoms
- s, s ′ and s ′′ are each an integer of 1 to 4.
- r and are each independently 1 or 2.
- R 19 has the same meaning as R 1 and R 2 in the formula (1), and R 21 and R 22 have the same meaning as R 5 in the formula (1), V is 1 or 2.
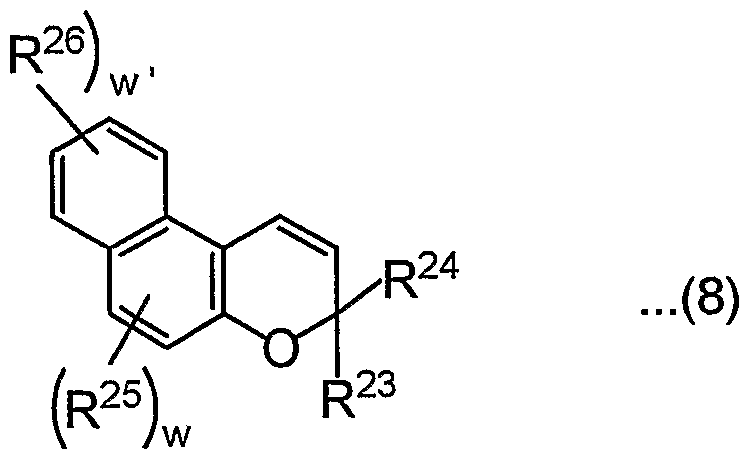
- R 23 and R 24 have the same meanings as R 1 and R 2 in the formula (1),
- R 25 and R 26 have the same meaning as R 5 in the formula (1),
- w and w ' are each independently 1 or 2.
- R 27 and R 28 have the same meanings as R 1 and t ⁇ R 2 in the formula (1),
- R 29 , R 3 °, R 31 and R 32 have the same meaning as R 5 in the formula (1), and X and x ′ are each independently 1 or 2.
- R 33 and R 34 have the same meaning as R 1 and R 2 in the formula (1), R 35 , R 36 and R 37 have the same meanings as R 5 in the formula (1), and ring Q is an aliphatic hydrocarbon ring;
- a chromene compound having the following structure is also preferred. Particularly preferably used.
- the photochromic compound described above can be used by appropriately mixing a plurality of types in order to develop an appropriate color tone.
- the surface layer of the photochromic compound is formed by the resin composition in which the above-described photochromic compound is dispersed, and the concentration of the photochromic compound in such a resin composition is: From the viewpoint that good color density can be obtained, the content is preferably in the range of 0.002 to 20% by weight.
- the entire optical substrate is formed of the above resin composition (that is, the inside has the same composition as the photochromic surface layer portion).
- the initial coloring referred to coloring in a non-irradiated state
- the content of the photochromic compound in the resin composition is particularly preferably in the range of 0.01 to 1% by weight.
- the same viewpoint as described above is applied.
- the content of the photochromic compound in the photochromic surface layer is preferably in the range of 0.1 to 15% by weight, and the thickness of the photochromic surface layer is 10 to 40; Um.
- the content of the photochromic compound in the surface layer is particularly preferably 0.1 to 5% by weight.
- the rephotochromic surface layer is formed by a coating method or an impregnation method.
- Oxygen and harmful ultraviolet rays cause oxidative degradation of photomix compounds.
- the thinner the part where the mic compound is dispersed the more susceptible to oxygen degradation, which is the diffusion of oxygen. That is, an optical substrate having a photochromic surface layer formed by a coating method or the like has a property that oxidative deterioration of a photochromic compound is more likely to occur than an optical substrate formed by a kneading method.
- the present invention applied the present invention to an optical substrate by a coating method or the like in order to prevent the oxidative deterioration of such a photochromic compound by providing an ultraviolet absorbing film having a specific light transmission characteristic described later.
- the advantage of the present invention that the photochromic compound is prevented from oxidizing and improving the photochromic durability is extremely:
- a resin in which a photochromic compound is dispersed (hereinafter referred to as a photochromic resin composition) is used to prevent yellowing and improve moldability.
- Various additives known per se may be included for the purpose of improving the durability, improving the color development of 31 degrees, improving the fading speed, and the like.
- Surfactants can be exemplified as such additives.
- a nonionic surfactant, an anionic surfactant, or a cationic surfactant can be used, and a nonionic surfactant is used because of its solubility in a radical polymerizable monomer that forms a resin component that becomes a matrix. Preferably, it is used.
- nonionic surfactants that can be preferably used include sorbitan fatty acid ester, glycerin fatty acid ester, decaglycerin fatty acid ester, propylene glycol 'pentaerythryl I ⁇ fatty acid ester, polyoxyethylene sorbitan fatty acid ester, Polyxylene sorbit fatty acid ester, polyoxyethylene glycerin fatty acid ester, polyethylene glycol fatty acid ester, polyoxyethylene alkyl ether, polyoxyethylene phytosterol phytostanol, polyoxyethylene polyoxypropylene alkyl ether, polyoxy Ethylene J-Rekylphenyl ether, Polyoxyethylene castor oil 'Hardened castor oil, Polyoxyethylene lanolin' Lanolin alcohol 'Mitsuro C derivatives, polyoxyethylene alkylamines ⁇ fatty acid amides, polyoxyethylene alkylphenylformaldehyde condensates, surfactants having a single polyoxyethylene alkyl,
- additives examples include various antioxidants such as hindered phenolic antioxidants and zeolite antioxidants, various radical scavengers such as phenolic radical scavengers, and various additives such as hindered amine light stabilizers.
- Light stabilizers, ultraviolet absorbers such as benzotriazole-based compounds and benzophenone-based compounds, and the like can be used alone or in combination of two or more.
- Such an additive may be generally contained in the photochromic resin composition in an amount of 0.001 to 20% by weight, respectively.
- hindered amine-based light stabilizers are particularly preferred because they have a high effect of preventing deterioration of the photochromic compound.
- bis (1,2,2,6,6-pentamethyl-4-piperidyl) Hindamines such as Sebaket, ADK STAB LA-52, LA-57, A-62, LA-63, LA-67, LA-77, LA-82, manufactured by Asahi Denka Kogyo Co., Ltd. are preferably used. You.
- the addition amount of such a hindered amine light stabilizer is most preferably in the range of 0.1 to 10% by weight, particularly in the range of 1 to 10% by weight.
- release agents In addition to the various additives described above, release agents, anti-coloring agents, anti-static agents, fluorescent dyes, dyes, pigments, fragrances, plasticizers, etc., as long as the photochromic properties are not impaired. It may be added to the resin composition.
- the ultraviolet absorbing film formed on the photochromic surface layer portion of the optical substrate described above must have a thickness of 0.1 to 100 m. If the thickness of the film is less than 0.1 m, sufficient durability-improving curing cannot be obtained, and if it exceeds 1 O OjUm, it becomes difficult to obtain a film having a uniform thickness, and the thickness varies. As a result, the optical characteristics of the laminate deteriorate.
- this ultraviolet absorbing film is formed by a coating method or a vapor deposition method as described later. Particularly, in the case of an ultraviolet absorbing film formed by a coating method, its durability, optical characteristics, and the like are low.
- the thickness is in the range of 0.5-30 im
- the thickness of the ultraviolet absorbing film formed by the vapor deposition method is preferably in the range of 0.1 to 1 jum.
- such an ultraviolet absorbing film has a light transmittance at 360 nm of 50% or more, preferably 55% or more, and most preferably 60% or more. It is also important that the light transmittance in nm be 10 ⁇ 1 ⁇ 2 or less, preferably 5% or less, and most preferably 30/0 or less. That is, the oxidative deterioration of the photochromic compound described above is strongly caused by light having a short wavelength of around 320 nm, while the excitation of the i-to-mouth compound is favorably performed by light having a wavelength of around 360 nm.
- an ultraviolet absorbing film having the above-mentioned light transmission characteristics it is possible to effectively prevent the oxidative deterioration of the photochromic compound and improve the durability of the photochromic without lowering the coloring density. Can be done. For example, if the light transmittance at 360, which is 8 millimeters of ultraviolet absorption, is lower than the above range, the color density of the photomixer compound will be reduced, and the light transmittance at 320 nm will be lower than the above IE. If it is higher than the range, the oxidative deterioration of the photochromic compound cannot be effectively suppressed, and a sufficient improvement in the photochromic durability cannot be obtained. That is, in the present invention, since the ultraviolet absorbing film has the above-mentioned light transmission characteristics, it is possible to achieve both the improvement in the durability of the photomask S-ock compound and the color density.
- the ultraviolet absorbing film has excellent transparency without opaque foreign matter (for example, a precipitate of the ultraviolet absorbing agent) that can be visually confirmed. Therefore, the preferable range varies depending on the refractive index of the film due to the reflection at the film interface.
- the light transmittance at 560 nm of this ultraviolet absorbing film is preferably 85 o / o or more. Preferably, it is 90% or more.
- the photochromic laminate described above uses an ultraviolet absorber that selectively absorbs CO ultraviolet light having a wavelength of 320 nm, and a predetermined light beam within the above-described thickness range according to the amount of the ultraviolet absorber used.
- the film is manufactured by selecting a film thickness having characteristics I and forming an ultraviolet absorbing film on the photochromic surface layer of the optical substrate.
- a method of forming an ultraviolet absorbing film used in such a production method a film having no ultraviolet absorbing agent deposited and having a light transmittance of 580 nm or more at 560 nm can be obtained.
- a method (a) of a coating method using a silicone-based coating containing an ultraviolet absorber (a); A method (b) by a coating method using an organic coating agent and a method (c) by a UV absorber 0> deposition are suitably employed.
- a UV-absorbing film is formed by applying a silicone-based coating agent containing a UV-absorbing agent to the surface layer of the photomix and curing the applied film.
- colloidal particles of an inorganic compound that selectively absorbs ultraviolet light having a wavelength of 320 nm are used.
- inorganic conjugates include metal oxides such as zinc oxide, cerium oxide, zirconium oxide, iron oxide, and titanium oxide, and composite oxides containing these metal oxides.
- metal oxides such as zinc oxide, cerium oxide, zirconium oxide, iron oxide, and titanium oxide
- composite oxides containing these metal oxides zinc oxide, titanium oxide, cerium oxide, and composite oxides containing these metal oxides are preferable because they have no coloring, and composite metal oxides containing titanium oxide are most preferable.
- a titanium oxide / zirconium oxide / tin oxide composite metal oxide and a titanium oxide / zirconium oxide / silicon oxide composite metal oxide are most preferred.
- the content of the metal oxide that selectively absorbs a wavelength of 320 rpm as described above is 30% by weight or more, more preferably 50% by weight. It is preferable that the content of titanium oxide is not less than these amounts.
- Such colloidal particles of a metal oxide or a composite oxide can be prepared by a so-called sol-gel method, and a sol containing such a colloidal element is suitably used industrially or as a reagent. used
- the colloidal particles of the above inorganic compound may contain a small amount of other inorganic compound components in # particles as long as they can selectively absorb ultraviolet light having a wavelength of 320 m.
- the content of such an ultraviolet absorber in the silicone-based coating agent is adjusted so that the light transmittance of 360 nm and the light transmittance of 32 O nm of the obtained ultraviolet absorbing film are in the above-mentioned ranges.
- the UV-absorbing film contains 15 to 90% by weight, more preferably 15 to 70% by weight. More When the ultraviolet absorber is used as a metal oxide, the content is most preferably 15 to 50% by weight. When the ultraviolet absorber is used as a composite oxide containing these metal oxides, Most preferably, the content is between 25 and 70% by weight.
- the thickness of the ultraviolet absorbing film is set in the range described above (0.1 to 100 / m). In such a range, it is preferable to adjust the film thickness in accordance with the amount of the ultraviolet absorbing agent. For example, it is preferable to increase the film thickness when the amount of the ultraviolet absorber is small, and to reduce the film thickness when the amount is large. If the film thickness is more than necessary than the amount of the ultraviolet absorber in the film, cracks and the like may occur in the ultraviolet absorber film. For example, when the amount (concentration) of the ultraviolet absorber in the ultraviolet absorbing film is 15 to 70% by weight, the film thickness is in the range of 0.5 to 30 m, more preferably 1 to 1 Ojtm. Preferably, it is in the range.
- the silicone coating agent used in the method (a) contains a hydrolyzable organic silicon compound or a hydrolyzate thereof as a curing component.
- a hydrolyzable organic gayne compound or its hydrolyzate those generally used as a silane coupling agent are preferably used.
- organic gayne compounds include r-methacryloyloxypropyltrimethoxysilane, r-methacryloyloxypropyltriethoxysilane, vinyltrialkoxysilane, aryltrialkoxysilane, and r-glycido.
- the curing component comprising such an organic gayne compound or a hydrolyzate thereof is used in such an amount that the content in the ultraviolet absorbing film (cured film) becomes 10 to 25% by weight.
- the silicone-based coating agent may contain other additives (for example, acid, leveling agent, curing catalyst, etc.) and an organic solvent in addition to the above-mentioned ultraviolet absorber.
- additives for example, acid, leveling agent, curing catalyst, etc.
- organic solvent in addition to the above-mentioned ultraviolet absorber.
- the acid is used for accelerating the hydrolysis and condensation of the organic gay compound as a curing component, and a mineral acid such as hydrochloric acid is preferably used. Such acids are generally used in an amount of from 1 to 10 mmol per mol of organic gay compound.
- the organic solvent is used to adjust the viscosity of the coating agent to enhance the coating property, or is used as a dispersion medium (sol) of the above-mentioned colloidal particles. Methanol, isopropanol, t-butyl alcohol, Diacetone alcohol, ethylene glycol monoisopropyl ether, and dioxane can be suitably used.
- the content of such an organic solvent in the coating agent is generally 40 to 9% by weight &).
- Leveling agents include sorbitan fatty acid ester, glycerin fatty acid ft3 ⁇ 4 ester, decaglycerin fatty acid ester, propylene glycol 'penta; I: ristolyl fatty acid ester, polyoxyethylene sorbitan fatty acid ester, polyoxyethylene sorbit fatty acid ester And polyoxyethylene glycerin fatty acid ester, polyethylene glycol fatty acid ester, polyoxyethylene alkyl ether and the like.
- the content of such a leveling agent is about 0.01 to 3% by mass per coating agent.
- perchloric acids such as perchloric acid, ammonium perchlorate, magnesium perchlorate, Cu (II), Zn (II), Co (II), Ni (II), B e (ll), Ce (lll), Ta (111), Ti (lll), Mn (lll), Lho (111), Cr (III), V (III), Co (III), F acetyl acetonate having e (III), AI (III), Ce (IV), Zr (IV), V (IV), etc. as a central atom; amino acids such as amines and glycine; Lewis acids; Metal salts; and the like are preferably used.
- the above-mentioned silicone-based coating agent may contain colloidal particles of an inorganic oxide such as colloidal silica as a reinforcing agent or the like as long as the light transmission characteristics of the obtained ultraviolet absorbing film are not impaired. .
- the above-described ultraviolet absorbing film is formed by coating using an organic coating agent containing an ultraviolet absorbing agent.
- the UV absorber incorporated in the organic coating agent also selectively absorbs UV light having a wavelength of 320 n.
- the method (a) Inorganic UV absorbers such as inorganic compounds (especially inorganic oxides) used in the field can be used, and organic UV absorbers lj can be used. You can also. It is preferable to use an organic UV absorber from the viewpoint of preventing clouding due to precipitation of the UV absorber during film formation.
- organic UV absorbers among the compounds belonging to benzophenone type, benzotriazole type, salicylate type, cyanoacrylate type, hydroxybenzoate type, benzoxazidinone type, and triazine type, there are 32%.
- a material that satisfies the condition of selectively absorbing ultraviolet light having a wavelength of 0 nm and is capable of forming a film having the above-described light transmission characteristics is used alone or in combination of two or more.
- Preferred are cyanoacrylates, salicylates, and hydroxybenzoates because they have a large maximum absorption in the short-wavelength ultraviolet region of 320 nm and it is difficult to lower the color density of photochromic compounds. It is.
- Many benzotriazole and benzophenone organic ultraviolet absorbers do not satisfy the above requirements, and cannot be used alone.
- organic UV absorbers that can be particularly preferably used include ethyl 2-cyano-1,3,3-diphenyl acrylate, octyl 2-cyano-3,3-diphenyl acrylate, 2 '-Ethylhexyl-ul-cyanoacrylate UV absorbers such as 2-cyano 3,3-diphenyl acrylate; salicylate UV absorbers such as phenyl sulfite and p-t-butylphenyl salicylate: 2, 4 —Di-tert-butyl 2 '3 ′, 5′-Di-tert-butyl —4′-Hydroxybenzoate ultraviolet absorbers such as hydroxybenzoate.
- the content of these ultraviolet absorbers is also adjusted so that the light transmission characteristics (ultraviolet absorption characteristics) of the cured film satisfies the above-mentioned conditions in the same manner as in the above method (a).
- the amount is set in the range of 0.1 to 10% by weight, preferably 0.1 to 5% by weight, based on the solid content of the system coating agent. It is preferable to adjust the film thickness in accordance with the amount used.
- the concentration of the ultraviolet absorbing agent is 0.1 to 5% by weight
- the thickness of the formed ultraviolet absorbing film is preferably in the range of 0.5 to 30 m.
- the organic coating agent containing the above-mentioned ultraviolet absorber is a hydrocarbon-based polymerizable monomer (a polymerizable monomer having a main skeleton formed by a hydrocarbon) that gives a transparent cured product. And may contain an oxygen atom, a nitrogen atom, a sulfur atom, etc. if partially contained) as a curing component.
- a hydrocarbon-based polymerizable monomer component includes a monofunctional or polyfunctional (meth) acrylate compound, a monofunctional or polyfunctional vinyl compound, a monofunctional or polyfunctional epoxy compound, a polyfunctional urethane (polyisourethane).
- Known polymerizable monomers that are known to give transparent cured products, such as chemical compounds, monofunctional or polyfunctional hydroxy compounds, and mixtures of these monomers are particularly useful (without any two restrictions).
- Specific examples of monomers that can be suitably used include the following monomers.
- 2,2-bis (4-acryloyloxypoly X ethylene glycol phenyl) propane with an average molecular weight of 7776 propane, methyl ether polyethylene glycol methacrylate with an average molecular weight of 475, methylstyrene, vinylnaphthalene, monomethyl Styrene dimer, diaryl phthalate, diethylene glycol;
- 1,6-Hexanediol diglycidyl ether 1,6-Hexanediol diglycidyl ether, ethylene glycol diglycidyl ether, propylene glycol diglycidyl ether, trimethylolpropane diglycidyl ether, glycerol polyglycidyl ether, diglycerol polyglycidyl ether, pentaerythryl Tall triglycidyl ether, bisphenol diglycidyl ether, bis-1,2,2-hydro Xycyclohexylpropane diglycidyl ether and the like.
- Aromatic isocyanate compounds such as thiophosphate and tetramethyl xylene diisocyanate; trimethylhexamethylene diisocyanate, hexamethylene gesocyanate, isophoron diisocyanate, hydrogenated 4, 4-diphenylmethane diisocyanate, hydrogenated xylene diisocyanate, lysine diisocyanate, lysine ester triisocyanate, 1,6,11 1- decanetriisocyanate, 1, 8-diisocyanate- 1-isocyanate methyloctane, 1, 3, 6 —Polysodium compounds obtained by combining an aliphatic iso
- Alkylene glycols such as ethylene glycol, 1,2-propylene glycol, 1,3-butanediol, 1,4-butanediol, 1,6-hexanediol, neopentyl glycol, dipropylene glycol, diethylene glycol; polypropylene glycol, Polyalkylene glycols such as polyethylene glycol and polytetramethylene glycol; poly (alkylene adipates) such as poly (methylene adipate), poly (tetramethylene adipate), poly (hexamethylene adipate), and poly (neopenthylene adipate) ) Classes: Poly (1,4-butane) glycol, poly (1,4-butane) glycol, poly (1,4-butane) glycol, etc.
- Polybutadiene glycols such as poly (1,2-butanediene) glycol: Poly (alkylene carbonate) s such as poly (hexamethylene carbonate); Polyester polyols; "!, 2,4-butanetriol, 1,2 Two or more hydroxy groups such as 2,6-hexanetriol Polyols containing: silicone polyols and the like.
- the above-mentioned organic coating agent preferably contains a curing catalyst.
- a radical polymerization initiator, a photopolymerization initiator and the like are appropriately used depending on the reactivity of the polymerizable monomer used.
- radical polymerization initiator examples include benzoyl peroxide, p-chlorobenzoic peroxide, decanoyl peroxide, lauroyl peroxide, acetyl peroxide and the like. 1-butyl veroxy
- Peroxyesters such as 2-ethylhexanoate, t-butylperoxydicarbonate, cumylperoxyneodecanate, t-butylperoxybenzoate and the like: diisopropylperoxydicarbonate, di-2-ethylethyl Peroxypolycarbonates such as silver oxydicarbonate and G-sec monobutyloxy carbonate; 2,2, -azobisisobutyronitrile, 2,2'-azobis (4-dimethylvaleronitrile), 2 And azo compounds such as 1,2, -azobis (2-methylbutyronitrile) and 1,1'-azobis (cyclohexane-11-carbonitrile).
- the amount of such radical polymerization initiator used depends on the type, polymerization conditions, type and composition of the polymerizable monomer component used, and cannot be unconditionally limited. It is preferably used in the range of 0.01 to 10 parts by weight per 100 parts by weight.
- photopolymerization initiators examples include benzoin, benzoin methyl ether, benzoin butyl ether, benzophenol, aetophenone 4,4 'dichlorobenzophenone, ethoxyacetophenone, 2-hydroxy-2-methyl-11-phenylpropane, one-one, Benzylmethyl ketal, 1- (4-hydroxypropyl) -2-hydroxy-2-methylpropane 1-one, 1-hydroxycyclohexylphenyl ketone, 2-isopropylthiooxanthone, acylphosphine Side, diphosphine phosphide and the like.
- Such a photopolymerization initiator is generally used in an amount of 0.001 to 5 parts by weight per 100 parts by weight of the total radical polymerizable monomer.
- various epoxy resin curing agents various organic gay resin curing agents, and the like can be used as the curing catalyst other than the above.
- specific examples of such curing agents include: Kinds of organic acids and their acid anhydrides; Nitrogen-containing organic compounds such as tertiary amine compounds: various metal complex compounds such as organotin compounds and organozinc compounds or metal alkoxides; organic carboxylic acids of metal salts And various salts such as salts and carbonates.
- the amount added at this time is preferably from 0.1 to 5 parts by weight, particularly preferably from 0.5 to 2 parts by weight, per 100 parts by weight of the total weight of the polymerizable monomer.
- the organic coating agent may contain an organic solvent for dilution, if necessary, or may contain a stabilizer other than the ultraviolet absorber.
- the organic solvent is not particularly limited as long as it dissolves the monomer component and various additives, and examples thereof include toluene, xylene, and ethyl acetate.
- additives other than the ultraviolet absorber hindered amine light stabilizers, hindered phenol-based antioxidants, sulfur-containing secondary antioxidants, phosphorus-containing secondary antioxidants, nickel-based singlet Oxygen scavengers and the like can be mentioned.
- the method of applying the coating agent to a predetermined portion of the optical substrate is not particularly limited, and may be spin coating, diving, spin diving, or the like. Can be applied.
- the thickness of the finally obtained ultraviolet absorbing film can be controlled by adjusting the number of revolutions in spin coating, the viscosity of the coating agent, and the like.
- the pretreatment Prior to application of these coating agents, it is preferable to pretreat the surface of the optical substrate in order to improve the adhesion between the ultraviolet absorbing film and the optical substrate.
- the pretreatment include chemical treatment with a basic aqueous solution or an acidic aqueous solution, polishing using an abrasive, plasma treatment using atmospheric pressure plasma and low pressure plasma, corona discharge treatment, UV ozone treatment, and the like. be able to. It is also preferable from the viewpoint of adhesion to form a single layer of the primer by pretreatment and form an ultraviolet absorbing film on the single layer of the primer.
- the primer layer is formed by subjecting the surface treatment as described above as necessary to various primers typified by a urethane-based primer, preferably a moisture-hardened urethane-based primer to a photomask of an optical substrate. What is necessary is just to apply
- the thickness of such a primer layer is usually about 2 to 10 ⁇ m.o
- the coating layer applied to a predetermined portion of the optical substrate surface is cured according to the type of the coating agent. Is formed. For example, when a silicone-based coating agent is used (the above method (a)), heat condensation The recoating layer is cured to form an ultraviolet absorbing film.
- the coating layer is cured by thermal polymerization and photopolymerization to form an ultraviolet absorbing film.
- thermal polymerization it is generally sufficient to heat at a temperature of 40 to 200 ° C. for 5 minutes to 30 hours.
- the polymerization is carried out in an inert atmosphere such as nitrogen.
- a metal halide lamp, high-pressure mercury lamp, xenon lamp, electrodeless discharge light source D or V bulb, etc. is used as the light source, light is irradiated for 1 second to 30 minutes at a light intensity of 10 to 200 mW / cm2. Good.
- an ultraviolet absorbing film is separately prepared using these coating agents, and the obtained film is used with an adhesive or the like. It can be adhered to a predetermined portion of the optical substrate surface.
- the above-described ultraviolet absorbing film is formed by depositing an ultraviolet absorbing agent.
- the ultraviolet absorbent used in this method is an inorganic oxide that selectively absorbs ultraviolet light having a wavelength of 320 nm as exemplified in the above method (a), such as zinc oxide, cerium oxide, zirconium oxide, iron oxide, and oxide.
- Metal oxides such as titanium, and composite oxides containing these metal oxides.
- An ultraviolet absorbing film is formed by forming such an inorganic oxide as an ultraviolet absorber into a film by vapor deposition using a vacuum vapor deposition technique such as CVD, PVD or sputtering.
- the thickness of the ultraviolet absorbing film (deposited film) formed by vapor deposition is in the range of 0.1 to 1 im, the above-mentioned light transmission characteristics can be satisfied.
- an ultraviolet absorbing film is formed by vapor deposition, it is necessary to previously form a silicone-based coating film as a primer layer on the substrate surface in order to improve the adhesion of the ultraviolet absorbing film (deposited film).
- the thickness of such a primer layer is also about 2 to 10 Um.
- the method (a) uses oxygen due to the fact that the obtained ultraviolet absorbing film (cured film) is hard and dense.
- the photochromic compound has an extremely high effect of preventing oxidative deterioration of the photochromic compound, and has an extremely high effect of improving the durability of the photochromic compound.
- the ultraviolet absorbing film is usually provided as a single layer structure, but is preferably 0.1 to 1 / m, preferably 0.5 to 30 im (in the case of an ultraviolet absorbing film formed by a vapor deposition method, If the thickness is within the range of "! ⁇ 1 im), it may be provided as a multi-layer structure with a small number of layers. It can be formed easily, does not require much labor and costs, and has poor appearance such as cracks. 4 From the viewpoint that it is unlikely to occur, a single-layer structure is most preferable, and a multi-layer structure is also preferably within 3 ;!
- the photochromic laminate of the present invention can be used as it is as a photochromic optical article.
- the ultraviolet absorbing film is formed by the method of the above (a) and the ultraviolet absorbing film is not a film, it is preferable to coat the ultraviolet absorbing film with a hard coat material. Any known hard coat film can be used without any limitation, such as a silane coupling agent, gay silicon, zirconium, antimony, aluminum, etc.
- the photochromic laminate of the present invention can be formed by using a hard coat liquid mainly composed of a sol of an oxide of the present invention or a hard coat liquid mainly composed of an organic polymer.
- anti-reflection treatment by coating or the like of a thin film deposition and organic polymer material of the film to the coated surface with the coating material made of a metal oxide such as S i 0 2, the processing of the antistatic treatment or the like and 2 It is also possible to perform the processing.
- photochromic laminate of the present invention it can be used without limitation for the purpose of irradiating light including ultraviolet rays to exhibit its photochromic properties, but, for example, contains a large amount of harmful light of 320 nm. It is suitable for $ f in applications that irradiate sunlight or mercury lamps. Examples>
- photochromic compounds were used for producing optical substrates in Examples and Comparative Examples.
- a plastic lens obtained by polymerizing diethylene glycol bisaryl carbonate was prepared.
- a moisture-curable primer (Primer PFR4, manufactured by Takebayashi Chemical Industry Co., Ltd.) and ethyl acetate are mixed in a weight ratio of 9: 1, and the mixture is thoroughly stirred under a nitrogen atmosphere until uniform to obtain a primer coating solution.
- the above primer coating solution is spin-coated on the surface of the lens substrate using a spin coater (a spin coater 1H-DX 2 manufactured by MI KAS II), and the mixture is allowed to stand at room temperature.
- the composition was cured in 20 minutes to produce a lens substrate having a single primer layer on the surface of the lens substrate.
- a mixture of radically polymerizable monomers was prepared with 10 parts by weight of glycidyl methacrylate (EB-1830, Daicel UCB). Next, 2.35 parts by weight of the photochromic compound A was added to 100 parts by weight of the radical polymerizable monomer mixture. 0.2 parts by weight of photomix compound B and photomix compound fee
- Silane coupling agent 7 parts by weight were added and mixed well to prepare a photochromic polymerizable composition (photochromic coating solution).
- a photochromic polymerizable composition photochromic coating solution.
- a mixture of 1-hydroxycyclohexyl phenyl ketone and bis (2,6-dimethoxybenzo ⁇ T-l, 4,4-trimethyl-pentylphosphine oxide (weight ratio: 3 Vs. 1) was used.
- An ultraviolet absorbing film was formed on the photochromic coating layer of the optical substrate prepared as described above as in O) to obtain a photochromic laminate.
- GTS r-glycidoxypropyl trialkoxysilane
- TAA t-Butyl alcohol
- Leveling agent (“L 7001” manufactured by Nippon Tunicer Co., Ltd.) According to 0.025 g, after mixing each component, 2.09 g of a 0.05 N aqueous hydrochloric acid solution was added dropwise with stirring, followed by stirring for 4 hours. . In the above mixture,
- photochromic laminate having an ultraviolet absorbing film having a thickness of 2 m.
- the ultraviolet absorption characteristics and the photochromic properties of this photochromic laminate were evaluated by the following methods.
- the silicone-based coating agent containing the ultraviolet absorber used above was spin-coated and cured on quartz glass under exactly the same conditions as above to produce a cured film (ultraviolet absorbing film) having the same film thickness.
- a cured film ultraviolet absorbing film
- the light transmittance at 320 nm, 360 nm and 560 nm of the cured film the ultraviolet absorption characteristics of the ultraviolet absorbing film in the above photochromic laminate were evaluated.
- the light transmittance at 560 nm was 90 ⁇ 1 ⁇ 2, and it was transparent without visual turbidity.
- the light transmittance at 360 nm was 74%, and the light transmittance at 320 was 1 ⁇ 1 ⁇ 2. Table 2 shows the results.
- the photochromic properties of the obtained photochromic laminate were evaluated by measuring the color density, durability and yellowness by the following methods. The results are shown in Table 3.
- Color density A (A b s.); Using the xenon lamp L-2480 (30 OW) SHL-100 manufactured by Hamamatsu Tonics, the obtained photochromic laminate was passed through an air port mass filter (manufactured by Koingen Co., Ltd.) at 20 ° C ⁇ 1 °. The light was irradiated for 120 seconds to develop the color.
- the absorbance at the maximum absorption wavelength was determined by a spectrophotometer (Instant Multichannel Photodetector MCPD1000) manufactured by Otsuka Electronics Co., Ltd., and the color density was calculated by the following method.
- the obtained photochromic laminate was accelerated and degraded at 50 o'clock by a xenon weather meter X25 manufactured by Suga Test Instruments Co., Ltd.
- the color density before and after the accelerated aging was determined (Alpha:.! Test It color density, A 5:. Color density after test) was measured, the value of ⁇ (A 5 ZA..) > 1 00 ⁇ was defined as the residual ratio (%), and was used as an index of color development durability. The higher the residual ratio, the higher the durability of coloring.
- a photochromic laminate was produced in the same manner as in Example 1, except that the ultraviolet absorbing film was changed as follows.
- a hard coating solution (product name: 160-74 NT) manufactured by SC International was prepared.
- This coating solution is a hard coating solution containing titanium oxide, the solid content concentration is about 33% by weight, and the refractive index of the hard coating film is 1.60 in catalog value.
- 4 g of this coating solution was applied on the photochromic coating layer of the optical substrate prepared previously using the same spin coater as described above.
- the spin coating conditions were 600 rpm and 10 seconds. After spin coating, pre-drying was performed at 70 ° C for 15 minutes, and then main curing was performed at 120 ° C for 1 hour to obtain a photomix laminate.
- the thickness of the ultraviolet absorbing film obtained at this time was 4.8 ⁇ m.
- a photochromic laminate was produced in the same manner as in Example 1, except that the ultraviolet absorbing film was formed using an organic coating agent as follows.
- the respective components were sufficiently mixed to prepare an organic coating agent.
- the photopolymerization initiator 1-hydroxycyclohexylphenyl ketone and bis (2,6 A mixture of dimethoxybenzoyl 2,4,4-trimethyl-pentylphosphinoxide (weight ratio: 3 to 1) was used.
- a photochromic laminate was produced in the same manner as in Example 1, except that the ultraviolet absorbing film was formed by vapor deposition of a metal oxide as described below.
- a general-purpose silicone-based coating agent (alkoxysilane Z-silicone sol system; TS-56H, manufactured by Tokuyama Corporation) is applied to the surface of the optical substrate prepared above by diving. For 2 hours to form a primer layer.
- the thickness of this primer layer was 1.6 ⁇ m.
- the coating composition II does not contain an ultraviolet absorber, and no ultraviolet absorber is present in one layer of this primer.
- a thin layer of titanium oxide (UV absorbing film) of titanium oxide was formed on the primer layer by vapor deposition to obtain a photochromic laminate having a UV absorbing film (vapor deposited film) on the surface of the photochromic coat layer. .
- the thickness of this deposited film was 0.2.
- the ultraviolet absorption properties and the photochromic properties of this photochromic laminate were evaluated in the same manner as in Example 1, and the results are shown in Tables 2 and 3.
- the sol of the ultraviolet absorber to be added to the silicone coating agent is converted into methanol-dispersed titanium oxide zirconium oxide Z silicon oxide composite metal oxide fine particle sol (weight ratio: 205575, solid content concentration: 30% by weight).
- Change the silicone coating A photochromic laminate was manufactured in the same manner as in Example 1 except that the composition of the coating agent was changed as shown in Table 1.
- the UV absorption characteristics and photochromic characteristics of the optical substrate (substrate before the vapor-deposited film was formed) formed on the surface of the primer layer prepared in Example 6 were evaluated in the same manner as in Example 1, and the results were evaluated.
- the results are shown in Tables 2 and 3.
- the ultraviolet absorption characteristic is a characteristic of the primer layer.
- oxide microparticles (UV (Not an absorbent).
- a photochromic laminate having an ultraviolet absorbing film having a thickness of 2 m was produced in exactly the same manner as in Example 1, and the ultraviolet absorption and photochromic properties of the photochromic laminate were measured. Evaluation was performed in the same manner as in Example 1, and the results are shown in Tables 2 and 3.
Landscapes
- Optical Filters (AREA)
- Laminated Bodies (AREA)
Abstract
Description
Claims
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05709925.1A EP1714778B1 (en) | 2004-02-03 | 2005-02-02 | Laminate and process for producing the same |
| AU2005210427A AU2005210427B9 (en) | 2004-02-03 | 2005-02-02 | Layered product and method of producing the same |
| US10/588,378 US7441893B2 (en) | 2004-02-03 | 2005-02-02 | Layered product and method of producing the same |
| ES05709925T ES2707808T3 (es) | 2004-02-03 | 2005-02-02 | Laminado y proceso para la producción del mismo |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2004-027157 | 2004-02-03 | ||
| JP2004027157 | 2004-02-03 | ||
| JP2004-265424 | 2004-09-13 | ||
| JP2004265424 | 2004-09-13 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2005075193A1 true WO2005075193A1 (ja) | 2005-08-18 |
Family
ID=34840127
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2005/001873 WO2005075193A1 (ja) | 2004-02-03 | 2005-02-02 | 積層体及びその製造方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US7441893B2 (ja) |
| EP (1) | EP1714778B1 (ja) |
| AU (1) | AU2005210427B9 (ja) |
| ES (1) | ES2707808T3 (ja) |
| HU (1) | HUE042515T2 (ja) |
| WO (1) | WO2005075193A1 (ja) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011071183A1 (ja) * | 2009-12-10 | 2011-06-16 | 株式会社トクヤマ | フォトクロミック組成物 |
| US9119710B2 (en) * | 2005-09-08 | 2015-09-01 | Calhoun Vision, Inc. | Adjustable optical elements with enhanced ultraviolet protection |
| WO2018230513A1 (ja) | 2017-06-14 | 2018-12-20 | 株式会社トクヤマ | 光学物品用プライマー組成物および積層体 |
| KR20210104707A (ko) | 2018-12-17 | 2021-08-25 | 가부시끼가이샤 도꾸야마 | 광학 재료용 경화성 조성물 및 광학 재료 |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100118376A1 (en) * | 2007-04-09 | 2010-05-13 | Dong-Joo Kwon | Multi-layered photochromic sheet and photochromic glass prepared therefrom |
| JP2008279425A (ja) * | 2007-04-12 | 2008-11-20 | Nikon Corp | フォトクロミック装飾されたボディ部材 |
| GB0708692D0 (en) | 2007-05-04 | 2007-06-13 | Innovia Films Ltd | Seelable, pealable film |
| SI2147044T1 (sl) | 2007-05-24 | 2013-12-31 | Innovia Films Limited | Film z nizko emisivnostjo |
| GB0714418D0 (en) | 2007-07-24 | 2007-09-05 | Innovia Films Ltd | UV barrier film |
| US8547635B2 (en) | 2010-01-22 | 2013-10-01 | Oakley, Inc. | Lenses for 3D eyewear |
| MX2012011406A (es) * | 2010-04-01 | 2012-11-29 | Tokuyama Corp | Composicion curable fotocromica. |
| US9158207B2 (en) | 2011-08-09 | 2015-10-13 | Carl Zeiss Smt Gmbh | Optical component comprising radiation protective layer |
| DE102011080639A1 (de) * | 2011-08-09 | 2012-10-11 | Carl Zeiss Smt Gmbh | Optische Komponente mit Strahlungs-Schutzschicht |
| RU2505845C2 (ru) * | 2012-01-11 | 2014-01-27 | Федеральное Государственное Бюджетное Образовательное Учреждение Высшего Профессионального Образования "Дагестанский Государственный Технический Университет" (Дгту) | Способ изготовления линз для стоматологических очков |
| CA2946610A1 (en) * | 2014-05-30 | 2015-12-03 | Cabela's Incorporated | System and method for application of chromic compositions |
| TWI585196B (zh) * | 2014-12-31 | 2017-06-01 | 聖高拜塑膠製品公司 | 陽光控制膜、包含彼等之總成及製造彼等之方法 |
| CN107654006B (zh) * | 2017-09-21 | 2020-08-25 | 广东兴发精密制造有限公司 | 可控玻璃幕墙 |
| CN113448005B (zh) * | 2021-06-29 | 2022-12-02 | 江西沃格光电股份有限公司 | 光学复合膜及其制备方法、应用和显示装置 |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1419985A (en) | 1973-01-22 | 1976-01-07 | American Cyanamid Co | Photochromic filter for human spectacles |
| JPH05297510A (ja) | 1992-04-21 | 1993-11-12 | Nissan Motor Co Ltd | フォトクロミック積層体 |
| JPH0848940A (ja) * | 1994-04-21 | 1996-02-20 | Seiko Epson Corp | 被膜形成用塗布液および合成樹脂製レンズ |
| JPH0876150A (ja) * | 1994-09-01 | 1996-03-22 | Affinity Kk | 棒状体およびそれを使用したブラインド |
| JPH09241263A (ja) * | 1996-03-05 | 1997-09-16 | Fuji Photo Film Co Ltd | 感光性組成物及びそれを用いた要素及びフォトクロミック化合物 |
| US5694240A (en) | 1994-06-24 | 1997-12-02 | Bausch & Lomb Incorporated | Multilayer anti-reflective and ultraviolet blocking coating for sunglasses |
| JPH10265241A (ja) * | 1996-06-28 | 1998-10-06 | Nippon Oil Co Ltd | 紫外線吸収透明板 |
| JPH10316453A (ja) * | 1997-05-14 | 1998-12-02 | Affinity Kk | 積層体およびそれを使用した窓 |
| JPH1135326A (ja) * | 1997-07-18 | 1999-02-09 | Noevir Co Ltd | モリブデン又はタングステン含有微粒子酸化チタン、及びこれを含有する皮膚外用剤 |
| JPH11133204A (ja) * | 1997-09-01 | 1999-05-21 | Seiko Epson Corp | 硬化膜付きプラスチックレンズ |
| JP2002523267A (ja) * | 1998-08-31 | 2002-07-30 | コーニング インコーポレイテッド | 被覆された紫外線吸収ガラス |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8610709D0 (en) | 1986-05-01 | 1986-06-04 | Plinkington Bros Plc | Photochromic lenses |
| JPH0745502B2 (ja) | 1987-11-10 | 1995-05-17 | 株式会社トクヤマ | 新規化合物及びそれを含む組成物 |
| JPH01135326A (ja) | 1987-11-18 | 1989-05-29 | Matsushita Electric Ind Co Ltd | 電気掃除機 |
| JPH06192651A (ja) * | 1992-12-24 | 1994-07-12 | Toray Ind Inc | フォトクロミック複合体 |
| GB9306587D0 (en) | 1993-03-30 | 1993-05-26 | Pilkington Plc | Photochromic compounds |
| IL115803A (en) | 1994-11-03 | 2000-02-17 | Ppg Industries Inc | Indeno-naphthopyran derivatives useful for photochromic articles |
| US5739243A (en) * | 1996-11-27 | 1998-04-14 | Ppg Industries, Inc. | Polymerizable composition |
| US6113813A (en) * | 1999-03-11 | 2000-09-05 | Goudjil; Kamal | Photochromic ultraviolet protective shield |
| US6340765B1 (en) * | 1999-05-20 | 2002-01-22 | Tokuyama Corporation | Chromene compound |
| EP1122251B1 (en) * | 1999-06-03 | 2005-07-20 | Tokuyama Corporation | Chromene compounds |
| JP4016119B2 (ja) | 1999-07-19 | 2007-12-05 | 株式会社トクヤマ | 硬化性組成物 |
| JP4157245B2 (ja) | 2000-02-21 | 2008-10-01 | 株式会社トクヤマ | クロメン化合物 |
| US6547390B1 (en) * | 2000-09-11 | 2003-04-15 | Exxene Corporation | Top stabilized photochromic lens system |
| EP1433814B1 (en) | 2001-07-27 | 2007-12-19 | Tokuyama Corporation | Curable composition, cured article obtained therefrom, and photochromic optical material and process for producing the same |
| KR100399250B1 (ko) * | 2001-08-30 | 2003-09-26 | 김수진 | 광 가역성 변색 도수 편광 선글라스 렌즈 및 제조방법 |
| US7189456B2 (en) * | 2004-03-04 | 2007-03-13 | Transitions Optical, Inc. | Photochromic optical article |
-
2005
- 2005-02-02 US US10/588,378 patent/US7441893B2/en active Active
- 2005-02-02 EP EP05709925.1A patent/EP1714778B1/en active Active
- 2005-02-02 ES ES05709925T patent/ES2707808T3/es active Active
- 2005-02-02 HU HUE05709925A patent/HUE042515T2/hu unknown
- 2005-02-02 AU AU2005210427A patent/AU2005210427B9/en not_active Ceased
- 2005-02-02 WO PCT/JP2005/001873 patent/WO2005075193A1/ja active Application Filing
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1419985A (en) | 1973-01-22 | 1976-01-07 | American Cyanamid Co | Photochromic filter for human spectacles |
| JPH05297510A (ja) | 1992-04-21 | 1993-11-12 | Nissan Motor Co Ltd | フォトクロミック積層体 |
| JPH0848940A (ja) * | 1994-04-21 | 1996-02-20 | Seiko Epson Corp | 被膜形成用塗布液および合成樹脂製レンズ |
| US5694240A (en) | 1994-06-24 | 1997-12-02 | Bausch & Lomb Incorporated | Multilayer anti-reflective and ultraviolet blocking coating for sunglasses |
| JPH0876150A (ja) * | 1994-09-01 | 1996-03-22 | Affinity Kk | 棒状体およびそれを使用したブラインド |
| JPH09241263A (ja) * | 1996-03-05 | 1997-09-16 | Fuji Photo Film Co Ltd | 感光性組成物及びそれを用いた要素及びフォトクロミック化合物 |
| JPH10265241A (ja) * | 1996-06-28 | 1998-10-06 | Nippon Oil Co Ltd | 紫外線吸収透明板 |
| JPH10316453A (ja) * | 1997-05-14 | 1998-12-02 | Affinity Kk | 積層体およびそれを使用した窓 |
| JPH1135326A (ja) * | 1997-07-18 | 1999-02-09 | Noevir Co Ltd | モリブデン又はタングステン含有微粒子酸化チタン、及びこれを含有する皮膚外用剤 |
| JPH11133204A (ja) * | 1997-09-01 | 1999-05-21 | Seiko Epson Corp | 硬化膜付きプラスチックレンズ |
| JP2002523267A (ja) * | 1998-08-31 | 2002-07-30 | コーニング インコーポレイテッド | 被覆された紫外線吸収ガラス |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9119710B2 (en) * | 2005-09-08 | 2015-09-01 | Calhoun Vision, Inc. | Adjustable optical elements with enhanced ultraviolet protection |
| WO2011071183A1 (ja) * | 2009-12-10 | 2011-06-16 | 株式会社トクヤマ | フォトクロミック組成物 |
| WO2018230513A1 (ja) | 2017-06-14 | 2018-12-20 | 株式会社トクヤマ | 光学物品用プライマー組成物および積層体 |
| KR20200018444A (ko) | 2017-06-14 | 2020-02-19 | 가부시키가이샤 도쿠야마 | 광학 물품용 프라이머 조성물 및 적층체 |
| US11807769B2 (en) | 2017-06-14 | 2023-11-07 | Tokuyama Corporation | Primer composition for optical articles, and laminate |
| KR20210104707A (ko) | 2018-12-17 | 2021-08-25 | 가부시끼가이샤 도꾸야마 | 광학 재료용 경화성 조성물 및 광학 재료 |
| US12077623B2 (en) | 2018-12-17 | 2024-09-03 | Tokuyama Corporation | Curable composition for optical materials, and optical material |
Also Published As
| Publication number | Publication date |
|---|---|
| US7441893B2 (en) | 2008-10-28 |
| HUE042515T2 (hu) | 2019-06-28 |
| AU2005210427B9 (en) | 2010-03-04 |
| AU2005210427A1 (en) | 2005-08-18 |
| EP1714778A4 (en) | 2008-10-08 |
| AU2005210427B2 (en) | 2010-02-11 |
| ES2707808T3 (es) | 2019-04-05 |
| EP1714778A1 (en) | 2006-10-25 |
| EP1714778B1 (en) | 2018-11-21 |
| US20070127133A1 (en) | 2007-06-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2005075193A1 (ja) | 積層体及びその製造方法 | |
| JP5404551B2 (ja) | フォトクロミック性光学物品の製造方法 | |
| EP2733155B1 (en) | Photochromic curable composition | |
| JP5738280B2 (ja) | フォトクロミック硬化性組成物 | |
| JP6230165B2 (ja) | フォトクロミック硬化性組成物 | |
| WO2012133749A1 (ja) | フォトクロミックレンズ | |
| JP4606215B2 (ja) | フォトクロミック性光学物品及びその製造方法 | |
| JP4500696B2 (ja) | 積層体及びその製造方法 | |
| KR20200135353A (ko) | 포토크로믹 경화성 조성물 | |
| US9873819B2 (en) | Curable composition and photochromic composition | |
| KR20220150275A (ko) | 습기 경화형 폴리우레탄 조성물 및 적층체 | |
| JP2017068059A (ja) | 透光性ハードコート積層体の製造方法 | |
| JP6016397B2 (ja) | フォトクロミックレンズの製造方法 | |
| JP2013109254A (ja) | フォトクロミックレンズ | |
| WO2022191161A1 (ja) | フォトクロミック硬化性組成物 | |
| JP2011215240A (ja) | フォトクロミックレンズ | |
| JP2017042981A (ja) | フォトクロミック積層体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2005210427 Country of ref document: AU |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWW | Wipo information: withdrawn in national office |
Country of ref document: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2005210427 Country of ref document: AU Date of ref document: 20050202 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005709925 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005210427 Country of ref document: AU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005709925 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007127133 Country of ref document: US Ref document number: 10588378 Country of ref document: US |
|
| WWP | Wipo information: published in national office |
Ref document number: 10588378 Country of ref document: US |