WO2005033367A1 - Electrode - Google Patents
Electrode Download PDFInfo
- Publication number
- WO2005033367A1 WO2005033367A1 PCT/SE2004/001428 SE2004001428W WO2005033367A1 WO 2005033367 A1 WO2005033367 A1 WO 2005033367A1 SE 2004001428 W SE2004001428 W SE 2004001428W WO 2005033367 A1 WO2005033367 A1 WO 2005033367A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- electrode
- metal oxide
- coating layer
- precursors
- platinum
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/052—Electrodes comprising one or more electrocatalytic coatings on a substrate
- C25B11/053—Electrodes comprising one or more electrocatalytic coatings on a substrate characterised by multilayer electrocatalytic coatings
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C18/00—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating
- C23C18/02—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating by thermal decomposition
- C23C18/12—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating by thermal decomposition characterised by the deposition of inorganic material other than metallic material
- C23C18/1204—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating by thermal decomposition characterised by the deposition of inorganic material other than metallic material inorganic material, e.g. non-oxide and non-metallic such as sulfides, nitrides based compounds
- C23C18/1208—Oxides, e.g. ceramics
- C23C18/1216—Metal oxides
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C18/00—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating
- C23C18/02—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating by thermal decomposition
- C23C18/12—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating by thermal decomposition characterised by the deposition of inorganic material other than metallic material
- C23C18/1225—Deposition of multilayers of inorganic material
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/055—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the substrate or carrier material
- C25B11/057—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the substrate or carrier material consisting of a single element or compound
- C25B11/061—Metal or alloy
- C25B11/063—Valve metal, e.g. titanium
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/073—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material
- C25B11/091—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material consisting of at least one catalytic element and at least one catalytic compound; consisting of two or more catalytic elements or catalytic compounds
- C25B11/093—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material consisting of at least one catalytic element and at least one catalytic compound; consisting of two or more catalytic elements or catalytic compounds at least one noble metal or noble metal oxide and at least one non-noble metal oxide
Definitions
- Electrode The present invention relates to an electrode, a method of preparing the electrode, and the use thereof.
- Background of the invention Electrodes coated with titanium oxide, iridium oxide, and ruthenium oxide are commercially used today in electrolytic cells. Such electrodes can be prepared in accordance with EP 715 002 B1 disclosing a method wherein an anhydrous solvent comprising precursors of mixed metal oxides are deposited on a substrate to form an electrocatalytic oxide coating.
- electrodes produced by this method have a fairly low activity resulting in ohmic losses and high cell voltages in electrolytic cells, which leads to increased electric energy consumption.
- a further problem of conventional electrodes are their relatively short service life. The present invention intends to solve these problems.
- the present invention relates, to a method of preparing an electrode comprising providing an. electrode substrate, depositing on said electrode substrate a first substantially aqueous coating solution comprising precursors of a valve metal oxide and of at least two platinum group metal oxides, treating the first coating solution to provide a first metal oxide coating layer on the electrode substrate, depositing on said first coating layer a second substantially organic coating solution comprising precursors of a valve metal oxide and of at least one platinum group metal oxide, wherein at least one of the precursors is in organic form, treating said second coating solution to provide a second metal oxide coating layer on the first coating layer.
- the electrode substrate can be any valve metal or valve metal surfaced substrate such as titanium, tantalum, zirconium, niobium, tungsten, silicon or alloys thereof, preferably titanium.
- Valve metals are known as film-forming metals having the property, when connected as an electrode in the electrolyte in which the coated electrode is expected to operate, of rapidly forming a passivating oxide film which protects the underlying metal from corrosion by the electrolyte.
- the substrate can have any suitable shape such as a rod, tube, woven or knitted wire, perforated or non-perforated plate, louver, or mesh, e.g. an expanded mesh. Titanium or other valve metal clad on a conducting metal core or substrate can also be used.
- the electrode substrate is perforated or has the shape of a mesh having openings with a diameter from about 1 to about 10, more preferably from about 2 to about 5 mm.
- the electrode substrate is roughened using chemicals means such as etching, e.g. acid etching, or mechanical such as blasting, e.g. sand blasting, grit blasting by means of e.g. aluminium oxide grits.
- etching e.g. acid etching
- blasting e.g. sand blasting, grit blasting by means of e.g. aluminium oxide grits.
- the substrate surface has a roughness R a from about 2 to about 12, more preferably from about 3 to about 6, and most preferably from about 4 to about 5 ⁇ m as measured using the SURFTEST 212 surface roughness tester (Mitutoyo,
- the precursors of the platinum group metal oxides dissolved in the first coating solution preferably comprise at least two water-soluble compounds of platinum, iridium, palladium, rhodium, osmium, and ruthenium, more preferably ruthenium and at least one of iridium, palladium, platinum, rhodium, and osmium, and most preferably ruthenium and iridium.
- Suitable precursors include e.g. RuCI 3 , H 2 RuCI 6 , lrCI 3 , and mixtures thereof.
- the precursors are soluble also in acidified aqueous solutions.
- Suitable valve metal oxide precursors include water-soluble compounds of aluminium, zirconium, bismuth, tungsten, niobium, titanium, silicon and tantalum, preferably titanium, e.g. TiCI .
- the first coating solution is acidified, suitably by hydrochloric acid and/or other mineral acids to a pH of from about 0 to about 5, more preferably from about 0 to about 2.
- at least about 70, preferably at least about 90, and most preferably at least about 95 volume percent of the solvent in the substantially aqueous coating solution is comprised of water.
- the first coating solution is suitably deposited on the substrate by applying the solution on the electrode substrate, preferably until the total loading of the first layer is from about 0.5 to about 10, more preferably from about 1 to about 6, and most preferably from about 1.5 to about 3 g metal /m 2 .
- the process of depositing the coating solution can be repeated in order to obtain a thicker layer having the desired metal oxide content. It is desirable to let the coating air dry after each repetition at a temperature from about 20 to about 70, preferably from about 20 to about 50 °C. The drying can take from about 10 to about 20 minutes.
- the coating solution can then be heat treated at a temperature from about 300 to about 600, preferably from about 450 to about 550 °C for suitably about 10 to about 30.
- Suitable platinum group oxide precursors of the second coating solution include organic compounds, such as organic salts and acids of ruthenium, osmium, rhodium, iridium, palladium, and platinum, and mixtures thereof, preferably ruthenium and optionally at least one of iridium, palladium, rhodium, and osmium, and most preferably ruthenium and iridium.
- Suitable valve metal oxide precursors can include e.g. organic compounds such as organic salts and acids thereof include water-soluble compounds of aluminium, zirconium, bismuth, tungsten, niobium, titanium, silicon and tantalum, preferably titanium.
- At least one of the precursor compounds is present in its organic form, i.e. includes organic compounds such as organic metal salts or acids such as e.g. titanium alcoxide, tetrabuthyl titanate, and/or tetrapentyl titanate.
- organic compounds such as organic metal salts or acids such as e.g. titanium alcoxide, tetrabuthyl titanate, and/or tetrapentyl titanate.
- coating solutions for providing the second or outermost coating layer containing at least one precursor in organic form in a substantially organic coating solution results in an electrode having increased activity when deposited on the first coating layer.
- at least about 70, preferably at least about 90, and most preferably at least about 95 volume percent of the solvent in the substantially organic coating solution is comprised of organic solvent.
- Preferred organic solvents of the second coating are examples of the second coating .
- the second coating solutions include alcohols, preferably lower alcohols, more preferably acidified anhydrous, lower alkyl alcohols having from about 3 to about 5 carbons atoms, such as 1-buthanol, 1-propanol, 2- propanol, 1-pentanol and 2-pentanol and 3-methyl-2-butanol.
- the second coating solutions preferably include a concentrated acid, such as a mineral acid, e.g. hydrochloric acid adjusting the pH to from about -1 to about 5, preferably from about -1 to about 2.
- the second coating solution is suitably applied to the obtained first coating layer until the total metal loading of the second layer is from about 1 to about 10, preferably from about 1.5 to about 3.5 g metal/m 2 !
- the deposition process can be repeated in order to obtain a thicker second coating layer or a further coating layer on the second coating layer.
- the loading of the second coating solution is preferably from about 1 to about 10, most preferably from about 1.5 to about 3.5 g metal/m 2 .
- the second coating solution is air dried and heat treated in the same way as the first coating solution so as to form the second coating layer.
- precursors of the two platinum metal oxides are dissolved in the first coating, solution in a mole ratio of about 1:2 to about 2:1 , preferably from about 2:3 to about 3:2.
- at least two precursors of platinum metal oxides are dissolved in the second coating solution in the same mole ratio as in the first coating solution.
- precursors of the platinum and valve metal oxides are dissolved in the coating solutions in a mole ratio of valve metal to platinum metal(s) of about 1:2 to about 2: 1 , preferably from about 4:5 to about 1:1.
- precursors of iridium and ruthenium oxides are dissolved in at least one of the first and/or the second coating solutions in a mole ratio of about 1:2 to about 2:1 , preferably from about 2:3 to about 3:2.
- precursors of titanium, iridium and/or ruthenium are dissolved in the coating solutions in a mole ratio of titanium to iridium and ruthenium of about 1 :2 to about 2: 1 , preferably from about 4:5 to about 1 :1.
- Each coating solution is suitably deposited by immersion of the electrode substrate in the coating solution or by means of other suitable methods such as spraying, e.g. electrostatic spraying, rolling or brush painting. Even though a process providing two layers (with the defined coatings) is preferred, further layers may also be adhered.
- the invention also relates to an electrode obtainable by the method as disclosed herein.
- the invention also relates to an electrode comprising an electrode substrate, a .
- first coating layer having a charge/projected area from about 10 to about 200, preferably from about 25 to about 200, and most preferably from about 25 to about 190 mC/cm 2 (mCo.ulomb/cm 2 ), said first coating layer comprising a valve metal oxide and at least two platinum group metal oxides deposited on said electrode substrate, and a second coating layer having a charge/projected area from about 210 to about 1000, more preferably from about 250 to about 1000, and most preferably from about 300 to about 800 mC/cm 2 , said second layer comprising a valve metal oxide and at least one platinum group metal oxide deposited on the first coating layer.
- the charge/projected area was measured by an electro-double layer measurement with cyclic voltammograms in sulphuric acid.
- the measuring condition of the cyclic voltammograms was 50mV/second at a sweep rate in the range of 0.3 to 1.1V (vs. RHE (Reversible Hydrogen Electrode)) in 0.5 sulphuric acid.
- the measured values in mC/cm 2 are proportional to the active specific surface area of the electrodes. More information about this method can be found in L.D. Burke et al, Electroanal. Chem. 96(1976) 19-27 and R.F. Savinell et al, J. Electrochem. Soc. 137(1990) 489-494.
- an electrode according to the invention shows a superior activity while providing higher stability and longer service life in view of existing electrodes.
- the electrode substrate is as described herein.
- the electrode substrate is suitably perforated or has the shape of a mesh having openings with a diameter from about 1 to about 10, more preferably from about 2 to about 5 mm. It has been found that the electrodes with openings within the defined ranges when immersed in an operated cell produce small bubbles of evolved gas, which in turn results in an increased homogeneous current distribution and lower ohmic loss, particularly in a membrane cell.
- the coating layers of the electrode may comprise platinum group metal oxides, such as oxides of iridium, palladium, rhodium, osmium, and ruthenium, preferably oxides of ruthenium and at least one of iridium, rhodium, osmium, more preferably oxides of ruthenium and iridium.
- the coating layers also comprise at least one valve metal oxide such as an oxide of titanium, tantalum, zirconium, niobium, tungsten, and silicon, preferably titanium.
- the roughness R a of the electrode is from about 2 to about 12, more preferably from about 3 to about 6, and most preferably from about 4 to about 5 ⁇ m.
- the metal oxide layers preferably contain from about 40 to about 70 mole percent counted as valve metal, preferably as tantalum and/or titanium, from about 20 to about 30 mole percent of ruthenium oxide counted as ruthenium, and from about 10 to about 30 mole percent of another platinum group metal oxide counted as metal.
- the oxide coating on the electrode substrate is also effective in increasing the service life of the electrode by retarding the corrosion of the platinum group metals. Even though a process providing two layers (with the defined coatings) is preferred, further layers optionally with same or similar chemical composition may also be adhered.
- the invention also relates to the use of the electrode in an electrolytic cell.
- the electrode is used as an anode, preferably as a dimensionally stable anode, particularly in an ion membrane cell for the production of e.g. alkali metal hydroxide, particularly sodium hydroxide.
- alkali metal hydroxide particularly sodium hydroxide.
- Example 1 A titanium expanded mesh having a thickness of 1 mm and length and width of 80 and 24 mm respectively was used as electrode substrate after having been degreased and pickled in boiling hydrochloric acid.
- a first coating solution was deposited on the substrate having a molar ratio of Ti:Ru:lr of 2:1:1 , in which the total Ir+Ru concentration was 50 g/l.
- the solution was prepared by dissolving ruthenium trichloride, iridium trichloride, and titanium tetrachloride in a hydrochloric acid based solution. The solution was then dried at 60 °C followed by thermal decomposition at 460 °C for 10 minutes. This deposition step was repeated three times.
- a second coating solution was then prepared by mixing hexachloro ruthenic acid and hexachloro iridic acid into a titanium solution comprising tetrabuthyl ortho titanate in n-propyl alcohol. 10 volume percent of HCI was added to the alcohol solution. The molar ratio of Ti:Ru:lr was 2:1:1. The total Ir+Ru concentration was 30 g/l. The deposition and thermal decomposition of the second coating solution on the substrate was made in the same way as the first coating solution. The obtained electrode sample was then stabilised at 520 °C for 60 minutes.
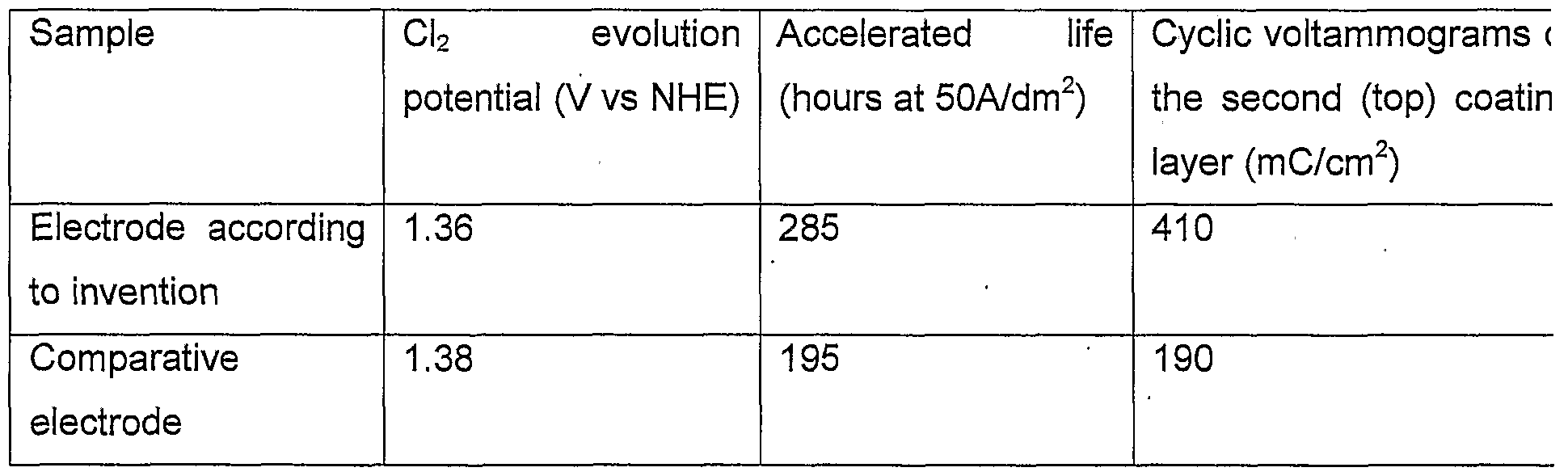
- the chlorine evolution potential at 90 °C in a 300 g/l NaCI solution was tested at pH 2 for the electrode (used as anode) and for a comparative electrode produced in the same way as the first coating layer but with six repetitions instead of three.
- the current density was 40 A/dm 2 .
- Table 1 below shows the difference between the two electrodes.
- An accelerated life test was also performed in a Na 2 SO 4 *10H 2 O 250 g/l electrolyte at 60 °C at a pH of 2.
- the current density was 50A/dm 2 .
- the electrodouble layer measurement by cyclic voltammograms was performed in 0.5M sulphuric acid. Measuring conditions were 0.3 to 1.1 V vs. RHE at a sweep rate of 50mV/second.
- a second coating solution was prepared by mixing ruthenium chloride into a titanium solution comprising tetrabuthyl ortho titanate in n-butyl alcohol. 10 volume percent of HCI was added to the alcohol solution. The molar ratio of Ti:Ru was 2:1. The total Ru concentration was 40 g/l.
- An electrode with a first oxide layer prepared according to Example 1 was then coated with this second coating solution. The deposition and thermal decomposition was made in the same way as in Example 1. Chlorine potential and electrodouble layer measurements, according to Example 1 , were then performed on the obtained electrode. Table 2 below shows the results of these measurements.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Metallurgy (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Mechanical Engineering (AREA)
- General Chemical & Material Sciences (AREA)
- Thermal Sciences (AREA)
- Physics & Mathematics (AREA)
- Inorganic Chemistry (AREA)
- Electrochemistry (AREA)
- Ceramic Engineering (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
- Hybrid Cells (AREA)
- Electroluminescent Light Sources (AREA)
- Chemically Coating (AREA)
Abstract
Description
Claims
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2004800264108A CN1849414B (en) | 2003-10-08 | 2004-10-06 | Electrode |
| AU2004277578A AU2004277578B2 (en) | 2003-10-08 | 2004-10-06 | Electrode |
| PL04775517T PL1670973T3 (en) | 2003-10-08 | 2004-10-06 | Electrode |
| EP04775517.8A EP1670973B1 (en) | 2003-10-08 | 2004-10-06 | Electrode |
| JP2006532238A JP5037133B2 (en) | 2003-10-08 | 2004-10-06 | Electrode preparation method and electrode |
| CA2541311A CA2541311C (en) | 2003-10-08 | 2004-10-06 | Dual-layered electrode for electrolysis |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP03445107.0 | 2003-10-08 | ||
| EP03445107 | 2003-10-08 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2005033367A1 true WO2005033367A1 (en) | 2005-04-14 |
| WO2005033367A8 WO2005033367A8 (en) | 2006-04-06 |
Family
ID=34400645
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/SE2004/001428 WO2005033367A1 (en) | 2003-10-08 | 2004-10-06 | Electrode |
Country Status (9)
| Country | Link |
|---|---|
| EP (1) | EP1670973B1 (en) |
| JP (1) | JP5037133B2 (en) |
| KR (1) | KR100787276B1 (en) |
| CN (2) | CN101942673A (en) |
| AU (1) | AU2004277578B2 (en) |
| CA (1) | CA2541311C (en) |
| PL (1) | PL1670973T3 (en) |
| WO (1) | WO2005033367A1 (en) |
| ZA (1) | ZA200601219B (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ITMI20102193A1 (en) * | 2010-11-26 | 2012-05-27 | Industrie De Nora Spa | ANODE FOR CHLORINE ELECTROLYTIC EVOLUTION |
| WO2019162518A1 (en) * | 2018-02-26 | 2019-08-29 | T&W Engineering A/S | Electrode for detecting bioelectrical signals |
| KR20190140755A (en) * | 2018-06-12 | 2019-12-20 | 주식회사 엘지화학 | Anode for electrolysis and preparation method thereof |
| EP3808449A4 (en) * | 2018-06-12 | 2022-03-30 | Japan Science and Technology Agency | Catalyst and method of use thereof |
| US11396709B2 (en) | 2017-08-11 | 2022-07-26 | Lg Chem, Ltd. | Electrode for electrolysis and preparation method thereof |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100812990B1 (en) * | 2006-11-08 | 2008-03-13 | 고등기술연구원연구조합 | Manufacturing method of mono-polar electrode |
| EP2390385B1 (en) * | 2010-05-25 | 2015-05-06 | Permelec Electrode Ltd. | Anode for electrolysis and manufacturing method thereof |
| JP5456744B2 (en) * | 2010-11-04 | 2014-04-02 | ペルメレック電極株式会社 | Electrolytic sampling method |
| CN102400203B (en) * | 2011-11-09 | 2014-06-18 | 广东达志环保科技股份有限公司 | Chromium plating anode of trivalent chromium chloride system |
| WO2016066544A1 (en) * | 2014-10-27 | 2016-05-06 | Industrie De Nora S.P.A. | Electrode for electrochlorination processes and method of manufacturing thereof |
| AR106068A1 (en) * | 2015-09-25 | 2017-12-06 | Akzo Nobel Chemicals Int Bv | ELECTRODE AND PROCESS FOR ITS MANUFACTURE |
| AR106069A1 (en) * | 2015-09-25 | 2017-12-06 | Akzo Nobel Chemicals Int Bv | ELECTRODE AND PROCESS FOR ITS MANUFACTURE |
| CN108299868A (en) * | 2016-08-25 | 2018-07-20 | 先丰通讯股份有限公司 | Catalyst coating and use its anode |
| CN106367779A (en) * | 2016-11-07 | 2017-02-01 | 南昌专腾科技有限公司 | Titanium-based porous electrode material and preparation method thereof |
| CN107142496A (en) * | 2017-04-10 | 2017-09-08 | 广东卓信环境科技股份有限公司 | Active masking liquid of a kind of internal layer and preparation method thereof |
| EP3492631B1 (en) * | 2017-08-11 | 2021-03-03 | LG Chem, Ltd. | Electrolytic electrode and manufacturing method therefor |
| CN108070877B (en) * | 2017-11-09 | 2020-07-07 | 江苏安凯特科技股份有限公司 | Cathode for electrolytic production and preparation method thereof |
| CN108048862B (en) * | 2017-11-16 | 2020-04-28 | 江苏安凯特科技股份有限公司 | Anode for chlorine evolution and preparation method thereof |
| CN108048865B (en) * | 2017-11-17 | 2020-04-28 | 江苏安凯特科技股份有限公司 | Electrode and preparation method and application thereof |
| KR102503040B1 (en) * | 2018-12-21 | 2023-02-23 | 주식회사 엘지화학 | Anode Comprising Metal Phosphide Complex and Preparation Method thereof |
| EP3748042A1 (en) | 2019-06-03 | 2020-12-09 | Permascand Ab | Electrode assembly for electrochemical processes and method of restoring the same |
| CN113151885B (en) * | 2021-03-15 | 2023-03-21 | 广州鸿葳科技股份有限公司 | Titanium anode for electroplating and preparation method thereof |
| WO2024127921A1 (en) * | 2022-12-14 | 2024-06-20 | デノラ・ペルメレック株式会社 | Positive electrode for chlorine generation electrolysis |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3773554A (en) * | 1970-03-18 | 1973-11-20 | Ici Ltd | Electrodes for electrochemical processes |
| US4070504A (en) * | 1968-10-29 | 1978-01-24 | Diamond Shamrock Technologies, S.A. | Method of producing a valve metal electrode with valve metal oxide semi-conductor face and methods of manufacture and use |
| US4443317A (en) * | 1981-10-08 | 1984-04-17 | Tdk Electronics Co., Ltd. | Electrode for electrolysis and process for its production |
| EP0715002A1 (en) * | 1994-11-30 | 1996-06-05 | The Dow Chemical Company | Stable coating solutions for preparing electrocatalytic mixed oxide coatings on metal substrates or metal-coated conductive substrates, and dimensionally stable anodes produced from such solutions |
| EP0867527A1 (en) * | 1997-02-27 | 1998-09-30 | Aragonesas Industrias Y Energia, S.A. | Electrode with catalytic coating for electrochemical processes and manufacture thereof |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BR8006373A (en) * | 1979-10-08 | 1981-04-14 | Diamond Shamrock Corp | ELECTRODE FOR USE IN ELECTRIC PROCESSES, PROCESS FOR ITS MANUFACTURING, AND USE OF THE ELECTRODE |
| GB2083837B (en) * | 1980-08-18 | 1984-06-27 | Diamond Shamrock Corp | Manufacture of electrode with manganese dioxide coating valve metal base intermediate semiconducting layer |
| JPH0660427B2 (en) * | 1988-05-31 | 1994-08-10 | ティーディーケイ株式会社 | Oxygen generating electrode and method for manufacturing the same |
| JPH05209299A (en) * | 1992-01-28 | 1993-08-20 | Nippon Steel Corp | Insoluble electrode and its production |
| US5587058A (en) * | 1995-09-21 | 1996-12-24 | Karpov Institute Of Physical Chemicstry | Electrode and method of preparation thereof |
| JP3725685B2 (en) * | 1997-11-21 | 2005-12-14 | ペルメレック電極株式会社 | Hydrogen peroxide production equipment |
| US6217729B1 (en) * | 1999-04-08 | 2001-04-17 | United States Filter Corporation | Anode formulation and methods of manufacture |
| CN1179068C (en) * | 2000-08-22 | 2004-12-08 | 黄永昌 | Titanium base iridium dioxide electrode with tin-antiomony intermediate layer |
-
2004
- 2004-10-06 CA CA2541311A patent/CA2541311C/en not_active Expired - Fee Related
- 2004-10-06 PL PL04775517T patent/PL1670973T3/en unknown
- 2004-10-06 ZA ZA200601219A patent/ZA200601219B/en unknown
- 2004-10-06 JP JP2006532238A patent/JP5037133B2/en not_active Expired - Fee Related
- 2004-10-06 WO PCT/SE2004/001428 patent/WO2005033367A1/en active Application Filing
- 2004-10-06 CN CN2010102936821A patent/CN101942673A/en active Pending
- 2004-10-06 AU AU2004277578A patent/AU2004277578B2/en not_active Ceased
- 2004-10-06 KR KR1020067006852A patent/KR100787276B1/en not_active IP Right Cessation
- 2004-10-06 EP EP04775517.8A patent/EP1670973B1/en not_active Expired - Lifetime
- 2004-10-06 CN CN2004800264108A patent/CN1849414B/en not_active Expired - Fee Related
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4070504A (en) * | 1968-10-29 | 1978-01-24 | Diamond Shamrock Technologies, S.A. | Method of producing a valve metal electrode with valve metal oxide semi-conductor face and methods of manufacture and use |
| US3773554A (en) * | 1970-03-18 | 1973-11-20 | Ici Ltd | Electrodes for electrochemical processes |
| US4443317A (en) * | 1981-10-08 | 1984-04-17 | Tdk Electronics Co., Ltd. | Electrode for electrolysis and process for its production |
| EP0715002A1 (en) * | 1994-11-30 | 1996-06-05 | The Dow Chemical Company | Stable coating solutions for preparing electrocatalytic mixed oxide coatings on metal substrates or metal-coated conductive substrates, and dimensionally stable anodes produced from such solutions |
| EP0867527A1 (en) * | 1997-02-27 | 1998-09-30 | Aragonesas Industrias Y Energia, S.A. | Electrode with catalytic coating for electrochemical processes and manufacture thereof |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ITMI20102193A1 (en) * | 2010-11-26 | 2012-05-27 | Industrie De Nora Spa | ANODE FOR CHLORINE ELECTROLYTIC EVOLUTION |
| WO2012069653A1 (en) * | 2010-11-26 | 2012-05-31 | Industrie De Nora S.P.A. | Anode for electrolytic evolution of chlorine |
| EA023645B1 (en) * | 2010-11-26 | 2016-06-30 | Индустрие Де Нора С.П.А. | Anode for electrolytic evolution of chlorine |
| US11634827B2 (en) | 2010-11-26 | 2023-04-25 | Industrie De Nora S.P.A. | Anode for electrolytic evolution of chlorine |
| US11396709B2 (en) | 2017-08-11 | 2022-07-26 | Lg Chem, Ltd. | Electrode for electrolysis and preparation method thereof |
| WO2019162518A1 (en) * | 2018-02-26 | 2019-08-29 | T&W Engineering A/S | Electrode for detecting bioelectrical signals |
| KR20190140755A (en) * | 2018-06-12 | 2019-12-20 | 주식회사 엘지화학 | Anode for electrolysis and preparation method thereof |
| EP3808449A4 (en) * | 2018-06-12 | 2022-03-30 | Japan Science and Technology Agency | Catalyst and method of use thereof |
| US11499239B2 (en) | 2018-06-12 | 2022-11-15 | Lg Chem, Ltd. | Anode for electrolysis and preparation method thereof |
| US11965255B2 (en) | 2018-06-12 | 2024-04-23 | Japan Science And Technology Agency | Catalyst and method of use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2541311C (en) | 2010-06-01 |
| CA2541311A1 (en) | 2005-04-14 |
| WO2005033367A8 (en) | 2006-04-06 |
| AU2004277578A1 (en) | 2005-04-14 |
| EP1670973A1 (en) | 2006-06-21 |
| CN1849414A (en) | 2006-10-18 |
| KR100787276B1 (en) | 2007-12-20 |
| KR20060085676A (en) | 2006-07-27 |
| EP1670973B1 (en) | 2018-04-11 |
| ZA200601219B (en) | 2007-05-30 |
| PL1670973T3 (en) | 2018-09-28 |
| AU2004277578B2 (en) | 2008-07-17 |
| JP2007507612A (en) | 2007-03-29 |
| CN101942673A (en) | 2011-01-12 |
| CN1849414B (en) | 2011-01-26 |
| JP5037133B2 (en) | 2012-09-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2004277578B2 (en) | Electrode | |
| Chen et al. | Corrosion resistance mechanism of a novel porous Ti/Sn-Sb-RuOx/β-PbO2 anode for zinc electrowinning | |
| US8580091B2 (en) | Multi-layer mixed metal oxide electrode and method for making same | |
| Zhao et al. | Study on the performance of an improved Ti/SnO 2–Sb 2 O 3/PbO 2 based on porous titanium substrate compared with planar titanium substrate | |
| US7332065B2 (en) | Electrode | |
| Moradi et al. | Addition of IrO2 to RuO2+ TiO2 coated anodes and its effect on electrochemical performance of anodes in acid media | |
| Huang et al. | Electrochemical behavior of IrO2-Ta2O5/Ti anodes prepared with different surface pretreatments of Ti substrate | |
| JPS592753B2 (en) | How to get the job done | |
| JP2012092449A (en) | Electrode for manufacturing chlorine by electrolysis | |
| MXPA05003643A (en) | Coatings for the inhibition of undesirable oxidation in an electrochemical cell. | |
| Chen et al. | Controllable preparation of Ti/TiO2-NTs/PbO2–CNTs–MnO2 layered composite materials with excellent electrocatalytic activity for the OER in acidic media | |
| JP2003507580A (en) | Cathode usable for electrolysis of aqueous solution | |
| JP3883597B2 (en) | Novel stable coating solutions for producing improved electrocatalytic mixed oxide coatings on metal substrates or metal-coated conductive substrates, and dimensionally stable anodes produced from such solutions | |
| US7566389B2 (en) | Electrode | |
| US3986942A (en) | Electrolytic process and apparatus | |
| CA2529190C (en) | Electrode with electroconductive titanium oxide and process for manufacturing same | |
| CN107075702A (en) | Electrode with duplex coating, its use and preparation method | |
| CN101338437A (en) | Method for preparing graded multicomponent metal mixing oxide anode | |
| KR20110139126A (en) | Electrode for electrolytic production of chlorine | |
| JPS6160147B2 (en) | ||
| JP2836840B2 (en) | Electrode for chlorine generation and method for producing the same | |
| CA2030092C (en) | Electrocatalytic coating | |
| TW202225486A (en) | Electrolyser for electrochlorination processes and a self-cleaning electrochlorination system | |
| JP3868513B2 (en) | Electrode for seawater electrolysis and method for producing the same | |
| JPS6147231B2 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200480026410.8 Country of ref document: CN |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DPEN | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2004277578 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2004775517 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006/01219 Country of ref document: ZA Ref document number: 200601219 Country of ref document: ZA |
|
| ENP | Entry into the national phase |
Ref document number: 2004277578 Country of ref document: AU Date of ref document: 20041006 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004277578 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2541311 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1186/CHENP/2006 Country of ref document: IN |
|
| CFP | Corrected version of a pamphlet front page | ||
| CR1 | Correction of entry in section i |
Free format text: IN PCT GAZETTE 15/2005 REPLACE "(71) APPLICANT (FOR ALL DESIGNATED STATES EXCEPT US): EKA CHEMICALS AB ¢SE/SE!; S-445 80 BOHUS (SE)." BY "(71) APPLICANT (FOR SE ONLY): EKA CHEMICALS AB ¢SE/SE!; S-445 80 BOHUS (SE)." |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006532238 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020067006852 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004775517 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020067006852 Country of ref document: KR |
|
| ENP | Entry into the national phase |
Ref document number: 2004277578 Country of ref document: AU Date of ref document: 20041006 Kind code of ref document: B |