WO2005000339A2 - Melanocortin receptor 4(mc4) agonists and their uses - Google Patents
Melanocortin receptor 4(mc4) agonists and their uses Download PDFInfo
- Publication number
- WO2005000339A2 WO2005000339A2 PCT/US2004/016625 US2004016625W WO2005000339A2 WO 2005000339 A2 WO2005000339 A2 WO 2005000339A2 US 2004016625 W US2004016625 W US 2004016625W WO 2005000339 A2 WO2005000339 A2 WO 2005000339A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- arg
- fmoc
- compound
- tyr
- phe
- Prior art date
Links
- 0 C*C(CCNC(NC)=N)=O Chemical compound C*C(CCNC(NC)=N)=O 0.000 description 4
- QFYVYBYJZNOOHB-NSCUHMNNSA-N C/C=C/C(CN(CCN1)C1=N)=O Chemical compound C/C=C/C(CN(CCN1)C1=N)=O QFYVYBYJZNOOHB-NSCUHMNNSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/665—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans derived from pro-opiomelanocortin, pro-enkephalin or pro-dynorphin
- C07K14/68—Melanocyte-stimulating hormone [MSH]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/12—Cyclic peptides, e.g. bacitracins; Polymyxins; Gramicidins S, C; Tyrocidins A, B or C
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/10—Drugs for genital or sexual disorders; Contraceptives for impotence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/48—Drugs for disorders of the endocrine system of the pancreatic hormones
Definitions
- the present invention relates to peptide agonists of the MC4 receptor and as such are useful in the treatment of disorders responsive to the activation of this receptor, such as obesity, diabetes mellitus, and male and/or female sexual dysfunction.
- the proopiomelanocortin (POMC) gene encodes a 31-36 kDa pre-proliormone, from which seven mature peptide hormones are derived. POMC processing occurs in a tissue specific manner yielding four distinct melanocortin peptides: adrenocorticotropic hormone (ACTH), ⁇ -melanocyte stimulating hormone ( ⁇ -MSH), ⁇ -MSH, and ⁇ -MSH. Five melanocortin receptors have thus far been identified and are referred to herein as MCI, MC2, MC3, MC4, and MC5. MCI, whose primary endogenous ligand is ⁇ -MSH, is associated with pigmentation.
- MC2 whose primary endogenous ligand is ACTH, is associated with steroidogenesis.
- MC2 is distinctly different from the other melanocortin receptors and is not expected to interact with endogenous or synthetic MSHs other than ACTH or analogues thereof (Schi ⁇ th et al., Life Sciences 59(10):797-801, 1996).
- MC5 is believed to have two primary ligands, ⁇ -MSEC and ACTH, and is associated with exocrine Amenand sebaceous gland lipid secretion.
- Diverse lines of evidence including genetic and pharmacological data obtained in rodents and humans, support a role for the MC4 receptor in the regulation of energy homeostasis, specifically regulating food intake and metabolism.
- MC4 receptors The distribution of MC4 receptors in the brain correlates well with the areas in the brain which show high sensitivity to melanocortin-mediated feeding behavior (MacNeil et al, Eur. J. JPharm. 440(2-3): 141 -57, 2002).
- the MC4 receptor is believed to be significantly involved in regulating body weight as evidenced by the fact that Mc4r -I- mice are obese, and humans with mutations in the melanocortin MC4 receptor gene are obese.
- MC4 receptor agonists may be beneficial for the treatment of obesity.
- the development of selective peptide agonists for melanocortin receptors has closely followed the identification of the various melanocortin receptor subtypes and their perceived primary ligands.
- ⁇ -MSH a 13-amino acid peptide
- MCI melanocortin receptors
- MC3-MC5 melanocortin receptors
- NDP ⁇ -MSH is a more potent, protease resistant, but still non-selective analogue of ⁇ -MSH.
- the lactam derived from the 4-10 fragment of ⁇ -NDP-MSH, known as MTII, is even more potent in vivo than NDP- ⁇ -MSH but is non-selective.
- WO 00/35952 discloses certain peptides cyclized via disulfide bridges having utility as MC4 agonists.
- MC4 agonists with pharmaceutically desirable selectivity, potency and efficacy, for use as a pharmaceutical, in particular, for the treatment of obesity.
- MC4 agonists with a clinically desirable pharmacology and safety profile.
- Obesity is a common and very serious public health problem in the United States and throughout the world. According to recent statistics, more than 25% of the United States population and 27% of the Canadian population are overweight. Kuczmarski, Amer. J. of Clin. Nutr. 55:495S-502S, 1992; Reeder et al, Can. Med. Assn. J, 23:226-33, 1992. Upper body obesity is the strongest risk factor known for type II diabetes mellitus, and is a strong risk factor for cardiovascular disease and cancer as well. Recent estimates for the medical cost of obesity are $150,000,000,000 worldwide.
- male and/or Female sexual Dysfunction The MC4 receptor appears to play role in other physiological functions as well, namely controlling grooming behavior, erection, and blood pressure.
- Female sexual dysfunction encompasses, without limitation, conditions such as a lack of sexual desire and related arousal disorders, inhibited orgasm, lubrication difficulties, and vaginismus.
- Erectile dysfunction is a disorder involving the failure of a male mammal to achieve erection, ejaculation, or both.
- Symptoms of erectile dysfunction include an inability to achieve or maintain an erection, ejaculatory failure, premature ejaculation, and inability to achieve an orgasm.
- An increase in erectile dysfunction is often associated with age and is generally caused by a physical disease or as a side effect of drug treatment.
- the term "impotence" is often times employed to describe this prevalent condition. Synthetic melanocortin receptor agonists have been found to initiate erections in men with psychogenic erectile dysfunction (Wessells et al, "Synthetic Melanotropic Peptide Initiates Erections in Men With Psychogenic Erectile Dysfunction: Double-Blind, Placebo Controlled Crossover Study," J.
- Diabetes Diabetes is a disease in which a mammal's ability to regulate glucose levels in the blood is impaired because the mammal has a reduced ability to convert glucose to glycogen for storage in muscle and liver cells. In Type I diabetes, this reduced ability to store glucose is caused by reduced insulin production.
- Type II Diabetes or “non-insulin dependent diabetes mellitus” (NIDDM) is a form of diabetes which is due to a profound resistance to insulin stimulating or regulatory effect on glucose and lipid metabolism in the main insulin-sensitive tissues, muscle, liver, and adipose tissue.
- the constellation of symptoms which includes hyperinsulinemia, combined with hypertension, elevated body weight, elevated triglycerides and elevated LDL, is known as Syndrome X.
- Applicants have discovered compounds that have an unexpectedly high affinity for the MC4 receptor and are selective for the MC4 receptor over other melanocortin receptor subtypes.
- the present invention is directed to compounds represented by the following Structural Formula I:
- W is Glu, Gin, Asp, Asn, Ala, Gly, Thr, Ser, Pro, Met, He, Nal, Arg, His, Tyr, Trp, Phe, Lys, Leu, Cya, or is absent;
- R 1 is -H, -C(O)CH 3 , -C(O)(CH 2 ) ⁇ -4 CH 3; -C(O)(CH 2 ) M HC( ⁇ H) ⁇ H 2 , Tyr- ⁇ Arg-, Ac-Tyr- ⁇ -hArg-, gluconoyl-Tyr-Arg-, Ac-diaminobutyryl-, Ac-diaminopropionyl-, N-propionyl-, N-butyryl-, N- ⁇ valeryl-, N-methyl-Tyr-Arg-, N-glutaryl-Tyr-Arg-, N-succinyl-Tyr-Arg-, R 6 -SO 2 NHC(
- R 2 is -H, -NH 2 , -NHC(O)CH 3 , -NHC(O)(CH 2 ) 1 . 4 CH 3 , -NH-TyrC(O)CH 3 , R 6 SO 2 NH-, Ac-Cya-NH-, Tyr-N-H-, HO-(C 6 H 5 )-CH 2 CH 2 C(O)NH-, or CH 3 -(C 6 H 5 )-C(0)CH 2 CH 2 C(O)NH-;
- R 3 is -C 4 straight or branched alkyl, NH 2 -CH 2 -(CH 2 ) q -, HO-CH 2 -, (CH 3 ) 2 CHNH(CH 2 ) 4 -, R 6 (CH 2 ) q -, R 6 SO 2 NH-, Ser, He,
- R 6 is a phenyl or C 8 -C ⁇ 4 bicyclic aryl; mis 1 or 2; nis 1,2, 3, or 4;
- R 9 is(CH 2 ) p or(CH 3 ) 2 C-; p is 1 or 2;
- R 10 is NH- or is absent;
- R 7 is a 5- or 6-membered heteroaryl or a 5- or 6-rnembered heteroaryl ring optionally substituted with R 4 ;
- R 4 is H, -C 4 straight or branched alkyl, phenyl, benzyl, or (C 6 H 5 )-CH 2 -O-CH 2 -;
- R 8 is phenyl, a phenyl ring optionally substituted with X, or cyclohexyl;
- X is H, Cl, F, Br, methyl, or methoxy;
- R n is -C(O) or -CH 2 ;
- ⁇ R 5 is -NH remember -OH, glycinol, NH 2 -Pro-Ser-, NH 2 -Pro-Lys-, HO-Ser-, HO-Pro-Ser-, HO-Lys-, -Ser alcohol, -Ser
- W is a single bond, Glu, Gin, Asp, Asn, Ala, Gly, Thr, Ser, Pro, Met, He, Val, Arg, His, Tyr, Trp, or Phe;
- R 1 is -H, -C(O)CH 3 , -C(O)(CH 2 ) 1 - 4 CH 3) -C(O)(CH 2 ) 1 - 4 -NHC(NH)NH 2 , Tyr- ⁇ Arg, gluconoyl-Tyr-Arg, Ac-Dab, Ac-D>ap, N-succinyl-Tyr-Arg, N-propionyl, N-valeryl, N-glutaryl-Tyr-Arg, N-butyryl, , wherein R .2 z i.
- R 3 is C 1 -C 4 straight or branched alkyl, Ser, He,
- Another preferred embodiment of the present invention includes compounds of
- W is Glu, Gin, Asp, Asn, Ala, Gly, Thr, Ser, Pro, Met, He, Val, Arg, His, Tyr, Trp, Phe, Lys, Leu, Cya, or is absent;
- R 1 is -H, -C(O)CH 3 , -C(O)(CH 2 ) 1 - 4 CH 3 ⁇ -C(O)(CH 2 ) 1-4 NHC(NH)NH 2 , Tyr- ⁇ Arg-, Ac-Tyr- ⁇ -hArg-, gluconoyl-Tyr-Arg-, Ac-diaminobutyryl-, Ac-diaminopropionyl-, N-propionyl-, N-butyryl-, N-valeryl-, N-methyl-Tyr-Arg-, N-glutaryl-Tyr-Arg-, N-succinyl-Tyr-Arg-, R 6 -SO 2 NHC(O)CH
- R 2 is -H, -NH 2 , -NHC(O)CH 3 , -NHC(O)(C-H 2 ) 1 - 4 CH 3 , -NH-TyrC(O)CH 3 , R 6 SO 2 NH-, Ac-Cya-NH-, Tyr-NH-, HO-(C 6 H 5 )-CH 2 CH 2 C(O)NH-, or CH 3 - ⁇ C 6 H 5 )-C(O)CH 2 CH 2 C(O)NH-;
- R 3 is C 1 -C straight or branched alkyl, NH 2 -CH 2 -(CH 2 ) q -, HO-CH 2 -, (CH 3 ) 2 CHNH(CH 2 ) 4 -, R 6 (CH 2 ) q -, R 6 S0 2 NH-, Ser, He,
- R 6 is a phenyl or C 8 -C 14 bicyclic aryl; m is 1 or 2; p is 1 or 2; R 4 is H, -C 4 straight or branched alkyl, phenyl, benzyl, or (C 6 H 5 )-CH 2 -O-CH 2 -; X is H, Cl, F, Br, methyl, or methoxy; and R 5 is -NH 2 , -OH, glycinol, NH 2 -Pro-Ser-, H 2 -Pro-Lys-, HO-Ser-, HO-Pro-Ser-, HO-Lys-, -Ser alcohol, -Ser-Pro alcohol, -Lys-Pro alcohol, HOCH 2 CH 2 -O-CH 2 CH 2 NH-, NH 2 -Phe-Arg-, NH 2 -Glu-, NH 2 CH 2 RCH 2 NH-, R
- Structural Formula HI wherein W is Glu or a single bond (viz., is absent); R 4 is H or CH 3 ; X is H, Cl, F, or Br; and R 5 is NH 2 or OH.
- a preferred embodiment includes compounds of Structural Formula m wherein W is Glu or is absent; R 1 is H-, Ac-, Arg-, Ac-Arg-, or Ac -D-Arg-; m is 1 or 2; p is 1; and R 5 is H 2 or OH.
- Another preferred embodiment of the invention includes a compound of Structural Formula III wherein W is absent; R 1 is Ac-; m is 2; p is 1; and R 5 is NH 2 .
- Another preferred embodiment of the invention includes a compound of Structural Formula HI wherein W is Glu; R 1 is Ac-Arg-; m is 1; p is 1; and R 5 is NH 2 .
- Another preferred embodiment of the invention includes a compound of Structural Formula HI wherein W is absent; R 1 is H; m is 2; p is 1; and R 5 is NH 2 .
- Another preferred embodiment of the invention includes a compound of Structural Formula HI wherein W is absent; R 1 is Arg-; m is 2; p is 1; and R 5 is OH.
- a most preferred embodiment of the; present invention includes a compound of Structural Formula HI wherein W is Glu; R 1 is Ac-D-Arg-; m is 1; p is 1; and R 5 is NH 2 .
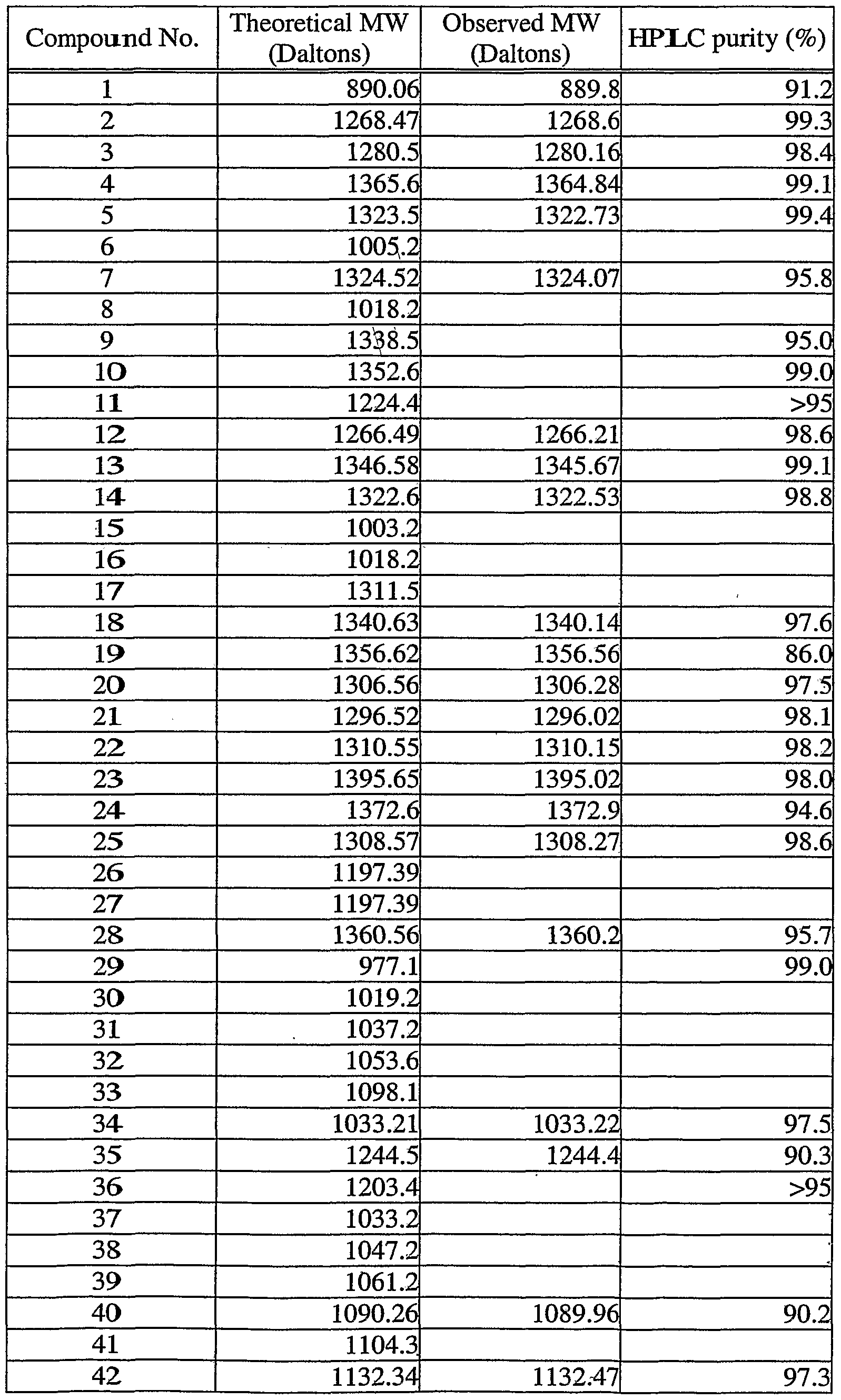
- the present invention includes, but is not limited to, those compounds listed in the following table:
- a preferred embodiment of the invention includes Compound Nos.48, 52, 132, 137, and 155. More preferred is a group consisting of Compound Numbers 52 and 137. Another more preferred embodiment includes Compound Number 137, denoted by the name Ac-cyclo[hCys-His-D-Phe-Arg-Trp-Cys]-N ⁇ 2 . A most preferred embodiment of the present invention includes Compound Number 52, denoted by the name Ac-D-Arg- cyclo[Cys-Glu-His-D-Phe-Arg-Tr ⁇ -Cys]-NH 2 .
- the present invention relates to pharmaceutical compositions comprising at least one compound of the present invention, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier.
- the present invention relates to a method for agonizing the MC4 receptor, which comprises administering to a patient in need thereof an effective amount of a compound represented by Structural Formula I, Structural Formula H, or Structural Formula HI, or a pharmaceutical salt thereof.
- the present invention relates to a method of treating obesity in a mammal, comprising the step of administering to the mammal in need thereof a pharmaceutically effective amount of at least one compound of Structural Formula I, Structural Formula H, or Structural Formula HI, or a pharmaceutical salt thereof.
- the present invention relates to a method of treating diabetes mellitus in a mammal, comprising the step of administering to the mammal in heed thereof a pharmaceutically effective amount of at least one compound of Structural Formula I, Structural Formula H, or Structural Formula HI, or a pharmaceutical salt thereof.
- the present invention relates to a method of treating male and/or female sexual dysfunction in a mammal, comprising the step of administering to the mammal in need thereof a pharmaceutically effective amount of at least one compound of Structural Formula I, Structural Formula H, or Structural Formula HI, or a pharmaceutical salt thereof.
- the present invention is further related to the use of the compound of Structural Formula I, Structural Formula H, or Structural Formula HI, or a pharmaceutical salt thereof, as a medicament.
- the present invention is further related to the use of the compound of Structural Formula I, Structural Formula H, or Structural Formula HI, or a pharmaceutical salt thereof, in the manufacture of a medicament for treating obesity.
- the present invention is further related to the use of the compound of Structural Formula I, Structural Formula II, or Structural Formula HI, or a pharmaceutical salt thereof, in the manufacture of a medicament for treating diabetes mellitus.
- the present invention is further related to the use of the compound of Structural Formula I, Structural Formula H, or Structural Formula IH, or a pharmaceutical salt thereof, in the manufacture of a medicament for treating sexual dysfunction.
- the compounds of the present invention also can be effective in treating and preventing diabetes mellitus, and male and female sexual dysfunction.
- the compounds can be associated with a more favorable safety profile than compounds currently used to treat these conditions.
- the terms used to describe the instant invention have the following meanings herein.
- a compound represented by Structural Formula I, Structural Formula H, or Structural Formula HI has more than one chiral substituent, it may exist in diastereoisomeric forms.
- the diastereoisomeric pairs may be separated by methods known to those skilled in the art (for example, chromatography or crystallization), and the individual enantiomers within each pair may be separated using methods familiar to the skilled artisan.
- the present invention includes each diastereoisomer of compounds of Structural Formula I, Structural Formula H, and Structural Formula IH, and mixtures thereof.
- Certain compounds of Structural Formula I, Structural Formula H, and Structural Formula IH may exist in different stable conformational forms, which may be separable. Torsional asymmetry due to restricted rotation about an asymmetric single bond, for example because of steric hindrance or ring strain, may permit separation of different conformers.
- the present invention includes each conformational isomer of compounds of Structural Formula I, Structural Formula H, and Structural Formula HI, and mixtures thereof.
- Certain compounds of Structural Formula I, Structural Formula II, and Structural Formula HI may exist in zwitterionic form, and the present invention includes each zwitterionic form of compounds of Structural Formula I, Structural Formula H, or Structural Formula HI, and mixtures thereof.
- C 1 -C 4 straight or branched alkyl means a straight chained or branched hydrocarbon having 1 to 4 carbon atoms, which is completely saturated and unsubstituted.
- C 3 -C 7 cycloalkyl refers to a saturated, unsubstituted hydrocarbon ring having 3 to 7 carbon atoms.
- C 1 -C 4 straight or branched heteroalkyl refers to a straight chained or branched hydrocarbon having 1 to 4 carbon atoms, which is completely saturated and unsubstituted, that also contains at least one "heteroatom.”
- a “heteroatom” is nitrogen, oxygen, or sulfur.
- C 3 -C 7 heterocycloalkyl refers to a saturated, unsubstituted hydrocarbon ring having 3 to 7 carbon atoms, which also contains at least one "heteroatom.”
- -C 4 straight or branched alkyl, C -C 7 cycloalkyl, -C 4 straight or branched heteroalkyl, and C 3 -C- 7 heterocycloalkyl may be used as generic modifiers to describe a genus of substituents on another functional group such as a carbonyl, sulfonyl, or sulfonamide.
- a "C 3 -C 7 cycloalkylcarbonyl” refers to a genus of saturated, unsubstituted hydrocarbon rings having 3 to 7 carbon atoms that are bonded to a carbonyl group.
- a "C 8 -C 14 bicyclic aryl” refers to two or three hydrocarbon rings fused together, having 8 to 14 carbon atoms, such as naphthalene.
- a C S -C M bicyclic aryl ring system has at least one aromatic ring.
- a "5- or 6-membered heteroaryl” refers to a monocyclic aromatic ring having 5 or 6 atoms, of which 1-4 atoms are heteroatoms.
- An "8- to 14-membered bicyclic heteroaryl" ring refers to two or three hydrocarbon rings fused together, having 8 to 14 atoms, at least one aromatic ring, and 1-4 heteroatoms.
- a phenyl, benzyl, benzoyl, -Cu bicyclic aryl, 5- or 6-membered heteroaryl, or 8- to 14-membered bicyclic heteroaryl may be unsubstituted or substituted with -C 4 straight or branched alkyl, F, Cl, Br, -OH, methoxy, phenyl, benzyl, benzoyl, or benzyloxymethyl.
- phenyl, benzyl, benzoyl, C S -CH bicyclic aryl, 5- or 6-membered heteroaryl, and 8- to 14-membered bicyclic heteroaryl may be used as generic modifiers to describe a genus of substituents on another functional group such as a carbonyl, sulfonyl, or sulfonamide.
- a "C%-C bicyclic arylsulfonyl” refers to a genus of bicyclic aryl rings having 8 to 14 carbon atoms that are bonded to a sulfonyl group.
- Modified amino acids are indicated by parentheses around the amino acid and the modification thereto (e.g., (4-Cl-D-Phe) is a 4-chloro modification on the D-isomer of phenylalanine).
- (4-Cl-D-Phe) is a 4-chloro modification on the D-isomer of phenylalanine.
- the single letter designations are as defined and do not refer to single letter amino acids corresponding to those letters.
- ⁇ -Phe means the D-form of the amino acid.
- An “amino alcohol” is an amino acid that has been modified by reducing the carbonyl group of the C-terminus to a methyl group. Amino alcohols are denoted h>y the general nomenclature “Xaa alcohol,” wherein Xaa is the specific amino acid from which the carbonyl group has been removed.
- Ser alcohol has the structure H 2 N-CH(CH 2 OH)-CH 2 OH as opposed to the Ser amino acid structure of H 2 N-CH(CH 2 OH)-COOH.
- Single bond refers to a structure that does not contain an amino acid at the specified position. It is used to signify that an amino acid is absent from that position such that the carbonyl adjacent to that position on one side and the amine adjacent to that position on the other side form a peptide bond with each other.
- “*” means that both the D- and L- isomers are possible.
- Ac refers to acetyl (i.e., -C(O)CH 3 ).
- Orn refers to ornithine.
- hCys refers to homocysteine.
- hArg refers to homoarginine.
- Lys(ipr) refers to lysine(N-isopropyl).
- Cit refers to citrulline.
- nLeu refers to norleucine.
- Me refers to methyl.
- OMe refers to methoxy.
- Cya refers to cysteic acid.
- Dap refers to diaminopropionyl.
- Dab refers to diaminobutyryl.
- MC4 agonist refers to a compound that has affinity for the MC4 receptor and results in measurable biological activity in cells, tissues, and organisms containing the MC4 receptor.
- “selective” means having an activation preference for a certain receptor over other receptors which can be quantified based on whole cell, tissue, or organism assays which demonstrate receptor activity. Selectivity is ascertained by comparison of EC 50 values at the relevant receptors referenced.
- “Pharmaceutically-acceptable salt” refers to salts of the compounds of the Structural Formula I, Structural Formula H, or Structural Formula IH that are substantially non-toxic to mammals. Typical pharmaceutically acceptable salts include those salts prepared by reaction of the compounds of the present invention with a mineral or organic acid or an organic or inorganic base. Such salts are known as acid addition and base addition salts, respectively.
- a pharmaceutical "acid addition salt” is a salt formed by reaction of the free base form of a compound of formula I with a pharmaceutical acid, such as described in the Encyclopedia of Pharmaceutical Technology, editors lames Swarbrick and James C. Boylan, Nol.
- salt forms include, but are not limited to the: acetate, benzoate, benzenesulfonate, 4-chlorobenzenesulfo ⁇ iate; citrate; ethanesulfonate; fumarate; d-gluconate; d-glucuronate; glutarate; glycolate; hippurate; hydrochloride; 2- hydroxyethanesulfonate; dl-lactate; maleate; d-malate; 1-malate; malonate; d-mandelate; 1-mandelate; methanesulfonate; 1,5-napthalenedisulfonate; 2-naphthalenesulfonate; phosphate; salicylate; succinate; sulfate; d-tartrate; 1-tartrate; and p-toluenesulfonate.
- a pharmaceutical "base addition” salt is a salt formed by reaction of the free acid form of a compound of formula I with a pharmaceutical base, such as described in the Encyclopedia of Pharmaceutical Technology, supra. Specific salt forms include, “ but are not limited to the: calcium, diethanolamine, diethylamine, ethylenediamine, lysine, magnesium, piperazine, potassium, sodium, and tromethamine (Tris, Trizma) salts.
- active ingredient means the compounds generically described " by Structural Formula I, Structural Formula H, or Structural Formula HI, as well as tine salts of such compounds.
- compositions of the present invention are prepared by procedures known in the art using well-known and readily available ingredients.
- treating and “treat”, as used herein, include their generally accepted meanings, i.e., alleviating, ameliorating, managing, preventing, prohibiting, restraining, slowing, stopping, or reversing the progression or severity of a pathological condition, or sequela thereof, described herein.
- the diseases, disorders or conditions for which compounds of the present invention are useful in treating include (1) obesity, (2) diabetes mellitus, and (3) male and/or female sexual dysfunction.
- Preventing refers to reducing the likelihood that the recipient will incur or develop any of the pathological conditions described herein.
- the term “preventing” is particularly applicable to a patient that is susceptible to the particular pathological condition as determined by medical diagnosis.
- “Pharmaceutically effective amount” means that amount of a compound, or salt thereof, that will elicit the biological or medical response of a tissue, system, or mammal and/or is capable of treating the conditions described herein, or that is capable of agonizing the MC3 and/or M C4 receptors.
- An “effective amount” of the peptide administered to a subject will also depend on the type and severity of the disease or condition and on the characteristics of the subject, such as general health, age, sex, body weight and tolerance to drugs.
- the recipient patient's physician should determine the therapeutic dose administered in light of the relevant circumstances.
- a pharmaceutically effective amount can be administered prophylactically to a patient thought to be susceptible to development of a disease or condition. Such amount, when administered prophylactically to a patient, can also be effective to prevent or lessen the severity of the mediated condition.
- the dosage regimen utilizing the compounds of the present invention is selected by one of ordinary skill in the medical or veterinary arts, in view of a variety of factors, including, without limitation, the route of administration, the prior medical history of the recipient, the pathological condition or symptom being treated, the severity of the condition/symptom being treated, and the age and sex- of the recipient patient. However, it will be understood that the therapeutic dose administered will be determined by the attending physician in the light of the relevant circumstances.
- an effecti e minimum daily dose of a compound of the present invention will exceed about O.01 mg.
- an effective maximum daily dose will not exceed about 1000 mg.
- an effective minimum daily dose will be between about 0.05 mg and 50 mg, more preferably between 0.1 mg and 10 mg.
- an effective minimum daily dose of an MC4R agonist peptide in the present invention will exceed about 2 ⁇ g/kg and will not exceed about 20 ⁇ g/kg.
- the exact dose may be determined, in accordance with the standard practice in the medical arts of "dose titrating" the recipient; that is, initially administering a low dose of the compound, and gradually increasing the does until the desired therapeutic effect is observed.
- the desired dose may be presented in a single dose or as divided doses administered at appropriate intervals.
- a "mammal" is an individual animal that is a member of the taxonomic class
- the class Mammalia includes humans, monkeys, chimpanzees, gorillas, cattle, swine, horses, sheep, dogs, cats, mi ⁇ e, and rats.
- the attending physician of ordinary skill can identify humans who will benefit from administration of the compounds and compositions of the preterit invention.
- the term "patient” includes human and non-human animals such as companion animals (dogs and cats and the like), farm animals, and laboratory animals.
- the term "pharmaceutical” when used herein as an adjective means substantially non-deleterious to the recipient patient.
- a pharmaceutically effective amount of a compound of Structural Formula I, Structural Formula ⁇ , or Structural Formula HI can be used for the preparation of a medicament useful for treating weight loss, obesity, diabetes and male and female sexual dysfunction.
- compositions are prepared by known procedures using well-known and readily available ingredients. Such procedures may include, e.g., conventional mixing, dissolving, granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping or lyophilizing processes. Because compounds of the invention contain an acidic moiety (i.e., carboxy), the compounds of the invention may be formulated as a pharmaceutical base addition salt thereof, e.g., as the sodium salt. Similarly, because compounds of the invention contain a basic moiety (i.e., amino), the compounds can be formulated as a pharmaceutical acid addition salt, e.g., as the acetate salt.
- an acidic moiety i.e., carboxy
- the compounds of the invention may be formulated as a pharmaceutical base addition salt thereof, e.g., as the sodium salt.
- compounds of the invention contain a basic moiety (i.e., amino)
- the compounds can be formulated as a pharmaceutical acid addition salt, e.
- the active ingredient (a compound of the present invention) will usually be mixed with a carrier, or diluted by a carrier, or enclosed within a carrier.
- the carrier serves as a diluent, it may be a solid, semisolid, or liquid material that acts as a vehicle, excipient, or medium for the active ingredient.
- the compositions can be in the form of, e.g., a suspension, solution, or sterile injectable solution.
- An injectable formulation for example, a sterile injectable aqueous or oleaginous suspension, can be prepared using suitable dispersing or wetting agents and suspending agents.
- the sterile injectable formulation may be a solution or suspension in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3-butanediol.
- acceptable vehicles and solvents that may be employed are water, sterile water for injection (WFT), bacteriostatic water for injection (BWFI), Ringer's solution, and isotonic sodium chloride solution.
- WFT sterile water for injection
- BWFI bacteriostatic water for injection
- Ringer's solution bacteriostatic water for injection
- isotonic sodium chloride solution sterile fixed oils are conventionally employed as a solvent or suspending medium. Fixed oils and fatty acids, such as oleic acid, may be employed in the preparation of an injectable formulation.
- the compounds of the present invention, and the pharmaceutically acceptable salts have valuable pharmacological properties and can be used in pharmaceutical compositions containing a pharmaceutically effective amount of a compound of the present invention, or pharmaceutically acceptable salts thereof, in combination with one or more pharmaceutically acceptable excipients.
- Excipients may include substances such as carriers, diluents, fillers, flavoring agents, sweeteners, lubricants, solubilizers, suspending agents, wetting agents, binders, disintegrating agents, encapsulating material, antimicrobial agents, and other conventional adjuvants. Proper formulation is dependent upon the route of administration chosen as well as any interactions between excipients.
- compositions typically contain from about 1 to about 99 weight percent of the active ingredient, which is a compound of the present invention.
- Solid form formulations may include powders, tablets, and capsules.
- a solid carrier can be one or more substance that may also act as flavoring agents, lubricants, solubilizers, suspending agents, binders, tablet disintegrating agents, and encapsulating material.
- Sterile liquid formulations may include suspensions, emulsions, syrups, and elixirs.
- the active ingredient may be dissolved or suspended in a pharmaceutically acceptable carrier, such as sterile water, sterile organic solvent, or a mixture of both sterile water and sterile organic solvent.
- the injectable formulation may be sterilized, for example, by filtration through a bacteria- or virus-retaining filter, by radi tion, or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable media just prior to use.
- the compounds of the present invention may be formulated in a unit dosage form prior to administration to the recipient patient.
- a "unit dosage form" is a physically discrete unit containing a unit dose, suitable for administration in human subjects or other mammals.
- a unit dosage form can be a capsule or tablet, or a number of capsules or tablets.
- a "unit dose” is a predetermined quantity of the active compound of the present invention, calculated to produce the desired therapeutic effect, generally in association with one or more pharmaceutically acceptable excipients.
- the quantity of active ingredient in a unit dose may be varied or adjusted from about O.Ol to about 1000 milligrams according to the particular treatment involved.
- the compounds of the present invention can be administered in a single daily dose, or the total daily dose may be administered in divided doses, two, three, or more times per day, or by continuous infusion. Where delivery is via transdermal forms, of course, administration is continuous.
- the compounds of the present invention can be administered by a variety of routes, including the oral, subcutaneous, topical, parenteral (e.g., intravenous and intramuscular), bronchial, or intranasal routes.
- parenteral e.g., intravenous and intramuscular
- bronchial e.g., bronchial
- intranasal routes e.g., intranasal routes.
- Continuous infusion of a compound of the present invention refers to controlled parenteral delivery of the peptide to a patient for an extended period of time.
- Administration via continuous infusion may be accomplished by, but is not limited to, delivery via pump, depot, suppository, pessary, transdermal patch or other topical administration (such as buccal, sublingual, spray, ointment, creme, or gel) using, for example, subcutaneous, intramuscular, intraperitoneal, intravenous, intracerebral, or intraarterial administration.
- a pump delivering a compound of the present invention into the body may be implanted in the patient's body.
- the patient may wear a pump externally, being attached to the patient's body via catheter, needle, or some other connective means. Any pump that is suitable for the delivery of pharmaceuticals to a patient may be used. Examples include pumps such as those disclosed in US Pat. No.
- a depot is a biocompatible polymer system containing a compound of the present invention and delivering the peptide over time.
- examples include microspheres, microcapsules, nanoparticles, liposomes, a hydrogel, or other polymeric implants.
- Preferred periods for delivery of agonist by depot include one week, two weeks, and one month periods. If needed, another depot will be delivered to the patient for continued delivery of peptide.
- Engineering a compound of the present invention to have a prolonged half-life will also result in continuous delivery of the MC4 receptor agonist to the receptor.
- modifications include conjugations with larger proteins such as albumin, antibody and antigen or chemical modifications that may increase half-life by linking fatty acids, polyethylene glycol (PEG) polymers, and other agents.
- Combination therapy includes administration of a single pharmaceutical dosage composition which contains a compound of Structural Formula I, Structural Formula H, or Structural Formula IH, and one or more additional active agents, as well as administration of a compound of Structural Formula I, Structural Formula ⁇ , or Structural Formula IH, and each active agent in its own separate pharmaceutical dosage formulation.
- a compound of Structural Formula I, Structural Formula ⁇ , or Structural Formula IH, and one or more additional active agents can be administered at essentially the same time, i.e., concurrently, or at separately staggered times, i.e., sequentially; combination therapy is understood to include all of these regimens.
- a preferred combination therapy for the treatment of obesity is the use of a compound of the present invention in combination with sibutramine (or active metabolites of sibutramine, e.g., desmethyl sibutramine and di-desmethyl sibutramine), preferably with sibutramine hydrochloride monohydrate.
- Another preferred combination is the use of a compound of the present invention in combination with orlistat.
- a preferred combination therapy for the treatment of sexual dysfunction is the use of a compound of the present invention in combination with sildenafil citrate.
- Another preferred combination is the use of a compound of the present invention in combination with tadalafil.
- Yet another preferred combination is the use of a compound of the present invention in combination with vardenafil, preferably vardenafil hydrochloride.
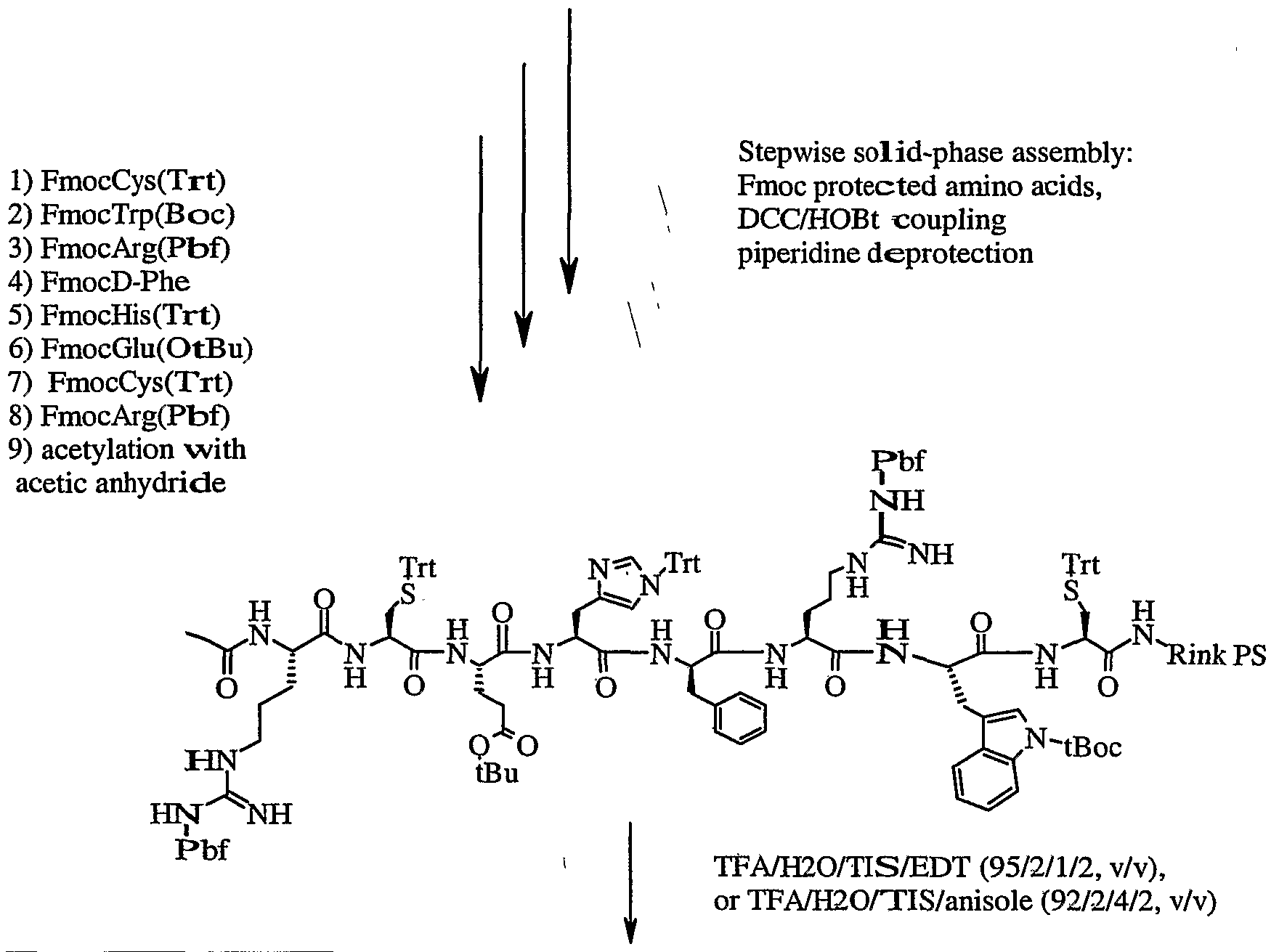
- All peptides of the present invention can be synthesized by solid-phase synthesis methods
- the protected amino acids and Rink resin can be purchased from Nova Biochem or Midwest Biotech.
- Acetylation of the -amino group, after the chain assembly, is carried out off-line with 5 equivalents acetic anhydride, 10 equivalents DIEA in dry DMF or NMP, 1 h at room temperature.
- the finished peptide is simultaneously deprotected and cleaved from the resin using a scavenger cocktail of TFA/H 2 O/TIS/EDT (95/2/1/2, v/v), or TFA/H 2 O/TIS/anisole (92/2/4-/2, v/v) 2 hours at room temperature.
- the solvents are then evaporated under vacuum, and the peptide is precipitated and washed three times with cold diethyl ether to remove the scavengers.
- the crude product is used directly in the cyclization reaction.
- Cyclization protocol The oxidation of the free cysteine sulfhydryl groups is acco plished by either air oxidation in 0.2 M ammonium acetate buffer containing 20% dimethyl sulfoxide (DMSO) at pH 7.0, or by treatment with 2,2'-pyridyldisulfide in 2.7 M guanidine buffer -containing 30% DMSO.
- DMSO dimethyl sulfoxide
- 2,2'-pyridyldisulfide in 2.7 M guanidine buffer -containing 30% DMSO.
- the final product is isolated by high performance liquid chromatography. Purification Purification is accomplished using standard preparative HPLC techniques. Immediately following the cyclization, the peptide is diluted and loaded onto an HPLC column and eluted with an aqueous 0.1% trifluoroacetic acid/acetonitrile gradient while monitoring at 214 nm. The appropriate fractions are pooled and lyophilized. Further characterization of the final product is performed
- the peptide is adsorbed onto a 2.1 x 25 cm Zorbax C18 preparative column, which is equilibrated with 0.1%TFA/H 2 O. The column is then washed with 2 volumes of 0.1 ammonium acetate/5% acetonitrile followed by 2 column volumes of water. The peptide is eluted using 2% acetic acid and lyophilized.
- Example 2 Synthesis of Compound No. 1: Ac-cyclorCys-His-D-Phe-Arg-Trp-Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc-
- Example 3 Synthesis of Compound No. 2: Ac-C y a-Ar g -c y clorCvs-Ala-His-D-Phe-Ar g -Trp-Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc- Glu(OtBu) in step 6 is replaced with Fmoc-Ala. Between steps 8 and 9, one extra step of Fmoc-Cya (Fmoc-cysteic acid) is added.
- Example 4 Synthesis of Compound No. 3: Ac-Tyr- Arg-cyclo FCvs- Ala-His-p-Phe-Arg-Trp-Cysl -NH? Can be prepared according to Example 1, with the exception that Fmoc-Ala is used instead of Fmoc-Glu(OtBu) in step 6. Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 5 Synthesis of Compound No.4: Ac-Tyr-Arg-cvclorCys-Arg-His-D-Phe-Arg-Trp-Cys1--SlH2 Can be prepared according to Exarnple 1, with the exception that Fmoc-Arg(Pbf) is used instead of Fmoc-Glu(OtBu) in step 6. Fmoc-Tyr(tBu) is used between steps 8 and 9.
- Example 6 Synthesis of Compound No. 5: Ac-Tyr-Arg-cvclorCvs-Asn-His-D-Phe-Arg-Trp-Cvsl-NH ?
- Example 7 S y nthesis of Compound No. 6: Ac-c y clorCvs-Asp-His- p -Phe-Ar g -Trp-C y sl-NH? Can be prepared according to Example 1, with the exception that Fmoc-Arg(Pbf) is not used in step 8. Fmoc- Asp is used instead of Fmoc-Glu(OtBu) in step 6.
- Example 8 Synthesis of Compound No.
- Fmoc-Gln is used instead of Fmoc-Glu(OtBu) in step 6.
- Example 10 Synthesis of Compound No. 9: Ac-Tyr-Arg-cvclorCys-Gln-ffis-p-Phe-Arg-Trp-Cysl-OH Can be prepared according to Example 1, with the exception that: Step 1 Fmoc- Cys(Trt) is not used; Fmoc-Gln(Trt) is used instead of Fmoc-Glu(OtBu) in step 6.
- preloaded Fmoc-Cys(Trt)-Wang resin Wang, J. Am. Chem. Soc. 95:1328-33,
- Example 11 Synthesis of Compound No. 10: Ac-Tyr-Arg-cvclofCys-Gln-His-p-Phe-Arg-T -Cysl-OMe Can be prepared according to Example 10. After the cleavage, cyclization, and purification, the peptide (Compound No. 9) is dissolved in dry methanol. Then, hydrochloride gas is bubbled into the methanol solution for about half minute. The reaction is allowed to proceed at room temperature for ten minutes. The solvents are removed under vacuum, and the final product is purified as specified in Example 1.
- Example 12 Synthesis of Compound No.
- Example 13 Synthesis of Compound No. 12: Ac-Tyr-Arg-cvclofCvs-Glv-His- p -Phe-Ar- g -Trp-C y sl-NH? Can be prepared according to Example 1, with the exception that Fmoc-Gly is used instead of Fmoc-Glu(OtBu) in step 6. Fmoc-Tyr(tBu) is used between steps 8 and 9.
- Example 14 Synthesis of Compound No. 13: Ac-Tyr-Arg-cvclorCys-His-His- p -Phe-Arg-Trp-Cvsl-NH?
- Example 18 Synthesis of Compound No. 17: N-methyl-Tyr-Arg-cyclofCys-Met-His-p-Phe-Arg-Trp-Cysl-NH? Can be prepared according to Example 1, with the exception that acetylation with acetic anhydride in step 9 is not used. Fmoc-N-methyl-Tyr is used after step 8. In addition, Fmoc-Met is used instead of Fmoc-Glu(OtBu) in step 6.
- Example 19 Synthesis of Compound No. 18: Ac-T y r-Arg-c y clorCys-Met-His- p -Phe-Arg-Trp-C y sl-NH?
- Example 20 Synthesis of Compound No. 19: Ac-Tyr-Arg-cyclorCys-Phe-His-p-Phe-Arg-Trp-Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc-Phe is used instead of Fmoc-Glu(OtBu) in step 6. Fmoc-Tyr(tBu) is used between steps 8 and 9.
- Example 21 Synthesis of Compound No.
- Fmoc-Tyr(tBu) is used between steps 8 and 9.
- Example 23 Synthesis of Compound No. 22: Ac-Tyr-Arg-cyclorCvs-Thr-His-p-Phe--Arg-Trp-Cys1-NH? Can be prepared according to Example 1, with the exception that Fmoc-Thr is used instead of Fmoc-Glu(OfBu) in step 6. Fmoc-Tyr(tBu) is used between steps 8 and 9.
- Example 24 Synthesis of Compound No. 23: Ac-Tyr-Arg-cvclorCvs-Trp-His-p-Phe--Arg-Tr ⁇ -Cvs1-NH?
- Example 25 Synthesis of Compound NTo. 24: Ac-Tyr-Arg-cvclorCys-Tyr-His-p-Phe-Arg-Trp-Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc-Tyr(tBu) is used instead of Fmoc-Glu(OtBu) in step 6. Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 26 Synthesis of Compound No.
- Glu(OtBu) in step 6 is replaced with Fmoc-Cya.
- Fmoc-Tyr (tBu) is added between steps 8 and 9.
- peptide cyclization is carried out on resin using 10 equivalents of iodine in DMF for 2 h at room temperature.
- Example 30 Synthesis of Compound No. 29: cyclorCys-Glu-His-p-Phe-Arg-Trp-Cysl-NH? Can be prepared according to Example 1, with the exception that steps 8 and 9 are omitted.
- Example 31 Synthesis of Compound No. 30: Ac-cvclofCvs-Glu-His-p-Phe-Arg-T ⁇ -Cysl-NH?
- Example 32 Synthesis of Compound No. 31: Ac-cvclorC ⁇ s-Glu-His-(4-F-p-PheVArg-T ⁇ -Cys1-NH? Can be prepared according to Example 1, with the exception that Fmoc-Arg(Pbf) in step 8 is not used. In addition, Fmoc-4-F-r>-Phe is used in step 4 instead of Fmoc-o- Phe.
- Example 33 Synthesis of Compound No. 32: Ac-cvclorCvs-Glu-His-.4-Cl-p-Phe)-Arg-T ⁇ -Cvs1-NH?
- Example 34 Synthesis of Compound No. 33: Ac-cyclorCvs-Glu-His-(4-Br-p-Phe)-Arg-T ⁇ -Cvs1-NH? Can be prepared according to Example 1, with the exception that Fmoc-Arg(Pbf) in step 8 is not used. In addition, Fmoc-4-Br-D-Phe is used instead of Fmoc-p-Phe.
- Example 35 Synthesis of Compound No.
- Fmoc--Arg Pbf is not used in step 8.
- Example 37 Synthesis of Compound No. 36: Ac-cvclorCvs-Glu-His-p-Phe--Arg-T ⁇ -Cvs1-Ser-Pro-NH? Can be prepared according to Example 1, with the exception that Fmoc-Ser and Fmoc-Pro are used prior to step 1. Fmoc-ArgfPbf) is not used in step 8.
- step 9 is carried out with propionic acid DCC/HOBt instead of acetic anhydride.
- Example 39 Synthesis of Compound No. 38: N-butyryl-cyclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cysl-NH? Can be prepared according to Example 1, with the exception that step 8 is not carried out.
- step 9 is carried out with butyric acid/DCC/HOBt instead of acetic anhydride.
- Example 40 Synthesis of Compound No. 39: N-valeryl-cyclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cvsl-NH? Can be prepared according to Example 1, with the exception that step 8 is not carried out.
- step 9 is carried out with valerianic acid/DCC/HOBt instead of acetic anhydride.
- Example 41 Synthesis of Compound No. 40: 3-guanidinopropionyl-cvclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cvsl-NH The peptide resin Cys(Trt)Glu(OtBu)His(Trt)-p-Phe-Arg(Pbf)T ⁇ (Boc)Cys(Trt)-
- Rink-PS is assembled by standard Fmoc chemistry as previously described.
- the resin is then treated with a threefold excess of commercially obtained FmocHNCH 2 CH 2 COOH activated with DCC/HOBt in DMF for 1.5 hrs.
- the Fmoc group is removed with 30% piperidine in DMF, and the resin washed with additional DMF and DCM.
- the resin is then suspended in NMP and treated with 2.0 equivalents of N,N-di(Boc)-l- guanylpyrazole and 2.0 equivalents of DIEA in NMP and shaken overnight at room temperature. (Bernatowicz, Wu, and Matsueda, J. Org. Chem. 57(8):2497-2502, 1992).
- Example 42 Synthesis of Compound No. 41: 4-guanidinobutyryl-cvclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cysl-NH2
- the peptide is prepared as in Example 40 above with the exception that FmocHNCH 2 CH 2 CH 2 COOH is utilized in place of Fmoc-HNCH 2 CH 2 COOH.
- Example 43 Synthesis of Compound No.
- N-terminal Fmoc group is removed by treatment with 30% piperidine in DMF.
- the free N-terminus is treated with 5 equivalents of acetic anhydride and
- Example 45 Synthesis of Compound No. 44: Ac-Dab-cvclo.Cvs-Gl ⁇ -ffis-p-Phe-Arg-T ⁇ -Cvsl-NH? Can be prepared according to Example 1, with the exception that the Arg-Cys- Glu-His-o-Phe-Arg-T ⁇ -Cys resin is not treated with acetic anhydride, but instead with
- N-terminal Fmoc group is removed hy treatment with 30% piperidine in DMF.
- the free N-terminus is treated with 5 equivalents of acetic anhydride and 10 equivalents
- Example 46 Synthesis of Compound No. 45: Arg-cyclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cysl-OH Can be prepared according to Example 1, with the exception that acetylation with acetic anhydride in step 9 is not used. In addition, Wang resin is used instead of Rink resin.
- Example 47 Synthesis of Compound No. 46: p-Arg-cyclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cysl-NH?
- Example 48 Synthesis of Compound No. 47: Ac-p-Arg-cyclorCvs-Glu-His-Phe-Arg-T ⁇ -Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc-p-Phe in step 4 and Fmoc-Arg(pbf) in step 8 are replaced with Fmoc -Phe and Fmoc-o-Arg(pbf), respectively.
- Example 49 Synthesis of Compound No.
- Example 53 Synthesis of Compound No.52: Ac-D-Arg-cyclofCvs-Glu-His- p -Phe-Arg-T ⁇ -Cysl-NH Can be prepared according to Example 1 , with the exception that Fmoc-o-
- Arg(Pbf) is used in step 8 instead of Fmoc-Arg(Pbf).
- Example 54 Synthesis of Compound No. 53: Ac-p-Arg-cvclofCvs-Glu-His-p-Phe-Arg-Trp-Cysl-OH Can be prepared according to Example 1, with the exception that Fmoc-o- Arg(pbf) is used instead of Fmoc-Arg(pbf) in step 8. In addition, Wang resin is used instead of
- Example 55 Synthesis of Compound No. 54: Ac-hArg-cvclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cvsl-NH? Can be prepared according to Example 1, with the exception that Fmoc-hArg(Pb»f) is used in step 8 instead of Fmoc-Arg(Pbf).
- Example 56 Synthesis of Compound No. 55: Ac-Cit-cvclofCvs-Glu-His-p-Phe-Arg-Trp-Cvsl-NH? Can be prepared according to Example 1, with the exception that Fmoc-Cit is us «d in step 8 instead of Fmoc-Arg(Pbf).
- Example 57 Synthesis of Compound No.
- Example 58 Synthesis of Compound No. 57: Ac-Leu-cvclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cvsl-NH? Can be prepared according to Example 1, with the exception that Fmoc-Leu is used instead of Fmoc-Arg(Pbf) in step 8.
- Example 59 Synthesis of Compound No. 58: Ac-Lvs-cyclo.rCys-Glu-His-p-Phe-Arg-T ⁇ -Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc-Lys Boc) is used in step 8 instead of Fmoc-Arg(Pbf).
- Example 60 Synthesis of Compound No. 59: Ac-Lvs ipr)-cyclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc-
- Example 61 Synthesis of Compound No. 60: Ac-nLeu-cvclorCys-Glu-HLis-p-Phe-Arg-T ⁇ -Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc-nLeu is used instead of Fmoc-Arg(Pbf) in step 8.
- Example 62 Synthesis of Compound No. 61: Ac-nLeu-cvclo[Cys-Glu-His-p-Phe-Arg-T ⁇ -Cvsl-Ser-Pro-NH? Can be prepared according to Example 1, with the exception that Fmoc-Ser and Fmoc-Pro are used prior to step 1. Li addition, Fmoc-nLeu is used instead of Fmoc-
- Example 63 S y nthesis of Compound No.62: Ac-Orn-c y clorCvs-Glu-His- p -Phe-Arg-T ⁇ -C y sl-NH? Can be prepared according to Example 1, with the exception that Fmoc-Orn is used in step 8 instead of Fmoc-Arg(Pbf).
- Example 64 Synthesis of Compound No. 63: Ac-Nal-cvclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cysl-N ⁇ ? Can be prepared according to Example 1, with the exception that Fmoc- Val is used instead of Fmoc-Arg(Pbf) in step 8.
- Example 65 Synthesis of Compound No. 64: N-(2-naphthalenesulfonyl)- p -Arg-cvclorCys-Glu-His- p -Phe-Arg-T ⁇ -Cysl--NH 2 Can be prepared according to Example 1, with the exception that Fmoc-A-rg(pbf) in step 8 and acetic anhydride in step 9 are replaced with Fmoc-p-Arg(pbf) and
- Example 66 Synthesis of Compound No. 65: N-(4-(2-naphthalenesulfonamido)-4-oxo-but ⁇ rylV p-Arg-cyclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cysl-NH? Can be prepared according to Example 1, with the exception that Fmoc-A-rg(pbf) in step 8 and acetic anhydride in step 9 are replaced with Fmoc-p-Arg(pbf) and succinic anhydride, respectively.
- naphthalene 2'-sulfonamide is carried out as follows: after step 9, the resin is swollen in DCM and washed several times with dry DMF. Then, 5 equivalents of naphthalene 2' -sulfonamide, 10 equivalents of PyBOP, and 10 equivalents of DIEA in dry DMF are added to the resin with a catalytic amount of DMAP (4-(N,N'-dimethylamino)pyridine). The coupling reaction is allowed to proceed at room temperature for 3 h, and the resin is washed and dried.
- DMAP 4-(N,N'-dimethylamino)pyridine
- Glu-His-p-Phe-Arg-T ⁇ -Cys resin is not treated with acetic anhydride, but instead with an excess of 3-(4-methylbenzoyl)propionic acid activated with DCC/HOBt.
- the cyclization and purification are carried out as in Example 1.
- Example 69 Synthesis of Compound No. 68: Tyr-Arg-cvclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cysl-NH? Can be prepared according to Example 1, with the exception that acetylation with acetic anhydride in step 9 is not used. Fmoc-Tyr(tBu) is added after step 8.
- Example 70 Synthesis of Compound No.
- Example 72 Synthesis of Compound No. 71: Tyr-Arg-cvclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cvsl-Glu-N ⁇ 2 Can be prepared according to Example 1, with the exception that Fmoc-Glu is used prior to step 1. Fmoc-Tyr(tBu) is added after step 8. Acetylation with acetic anhydride in step 9 is omitted.
- Example 73 Synthesis of Compound No.
- N-succinyl-Tyr-Ajrg-cyclofCys-Glu-His-p-Phe-Arg-T ⁇ -Cys1-NH 2 Can be prepared according to Example 1, with the exception that step 9 is carried out with succinyl anhydride instead of acetic anhydride. Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 76 Synthesis of Compound No.
- N-glutaryl-Tyr-Arg-cvclorCys-Glu-His-p-Phe-Arg-Trp-Cysl-OH Can be prepared according to Example 1, with the exception that step 9 is carried out with glutaryl anhydride instead of acetic anhydride.
- Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Wang resin is used instead of Rink resin.
- Example 78 Synthesis of Compound No. 77: N-gluconoyl-Tyr-Arg-cyclofCys-Glu-His-p-Phe-Arg-T ⁇ -Cys1-NH 2
- step 9 is not carried out.
- Fmoc-Tyr(tBu) is added between steps 8 and 9. The peptide is dissolved in
- Example 80 Synthesis of Compound No. 79: Ac-Tyr-D-Ar -cyclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cvs1-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-r>-
- Example 83 Synthesis of Compound No. 84: Ac-Tyr-Arg-cvclorCvs-Glu-(l-Me-His -(4-F-p-PheVArg-Trp-Cvsl--NH? and Synthesis of Compound No. 85: Ac-Tyr-Arg-cvclorCys-Glu-( ' l-Me-p-His")-(4-F-p-Phe')-Arg-Trp-Cvs1-NH? Can be prepared according to Example 1, with the exception that Frnoc-1-Me-His is used in step 5 instead of Fmoc-His(Trt). Fmoc-4-F-o-Phe is used instead of Fmoc-o-
- F noc-Tyr(tBu) is added between steps 8 and 9. Due to the unprotected side chain of Fmoc-( 1-Me-His), this residue is racemerized during the coupling, which affords two peptides: Ac-Tyr-Arg-cyclo[Cys-Glu-(l-Me-His)-(4-F-p-Phe)-Arg-T ⁇ -Cys]--NH 2 and Ac-Tyr-A-rg-cyclo[Cys-Glu-(l-Me-o-His)-(4-F-p-Phe)-Arg-T ⁇ -Cys]-NH 2 .
- the two peptide-isomers are easily separated on HPLC.
- the absolute configurations of the 1-Me-His residue in each peptide are defined by two-dimensional
- Example 84 Synthesis of Compound No. 86: Ac-Tyr-Arg-cyclorCvs-Glu-His-(4-Cl-p-Phe)-Arg-Trp-Cys]-N ⁇ ? Can be prepared according to Example 1, with the exception that Fmoc-4-Cl-p-
- step 4 Phe is used in step 4 instead of Fmoc-p-Phe and Fmoc-(l-Me-His) is used in step 5 instead of Fmoc-His(Trt), respectively.
- Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 86 Synthesis of Compound No. 89: Ac-Tyr-Arg-cvclorCvs-Glu-His-r4-Br-p-Phe -Arg-T ⁇ -Cysl-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-4-Br-o- Phe is used instead of Fmoc-p-Phe in step 4. Fmoc-Tyr(fBu) is added between steps 8 and 9.
- Example 87 Synthesis of Compound No.
- step 4 Phe is used in step 4 instead of Fmoc-p-Phe and Fmoc-( 1-Me-His) is used in step 5 instead of Fmoc-His(Trt), respectively.
- Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 88 Synthesis of Compound No. 92: Ac-Tyr-Arg-cvclorCvs-Glu-His-(4-Me-p-Phe -Arg-T ⁇ -Cys1-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-4-Me-o-
- Example 91 Synthesis of Compound No. 96: Ac-Tyr-A-rg-cvclorCys-Glu-(3-Me-HisVp-Phe-Arg-T ⁇ -Cysl-N ⁇ 7 Can be prepared according to Example 1, with the exception that Fmoc-3-Me-His is used in step 5 instead of Fmoc-His(Trt). Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 92 Synthesis of Compound No.
- Example 93 Synthesis of Compound No. 101: Ac-Tyr-Arg-c vcloFCvs-Glu-( 1 -Bom-His)-p-Phe- Arg-T ⁇ -Cvsl-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-1-Bom- His is used in step 5 instead of Fmoc-His(Trt). Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 94 Synthesis of Compound No.
- Example 99 Synthesis of Compound No. 115: Ac-Tyr-Arg-cvclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cys1-2-(2-arninoetho ⁇ y)ethanol Can be prepared according to Example 1, with the exception that
- Tyr(tBu) is used between steps 8 and 9.
- Example 101 Synthesis of Compound No. 117: Ac-Tyr-Arg-cyclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cvsl-N ⁇ -(CH?) fi -NH? Can be prepared according to Example 1, with the exception that
- Glu(OtBu) is used prior to step 1.
- Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 103 Synthesis of Compound No. 119: Ac-Tyr-Arg-cvclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cvs1-Ser-Pro-NH 2 Can he prepared according to Example 1, with the exception that Fmoc-Ser and
- Fmoc-Pro are used prior to step 1.
- Fmoc-Tyr is used between steps 8 and 9.
- Example 104 Synthesis of Compound No. 120: Ac-Tyr- Arg-cvclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cvs]-S er-Pro alcohol
- Wang resin was preloaded with Fmoc-prolinol according to a published method (Yan and Mayer, J. Org. Chem. 68:1161-62, 2003), and then Fmoc-
- Fmoc-Tyr(tBu) is added between steps 8 and 9. Due to the unprotected side chain of Fmoc-( 1-Me-His), this residue is racemerized during the coupling, which affords two peptides: Ac-Tyr-Cit-cyclo[Cys-Glu-(l-Me-His)-p-Phe-Arg-T ⁇ -Cys]-NH 2 and Ac-Tyr-Cit-cyclo[Cys- jlu-(l-Me-p-His)-p-Phe-Arg-T ⁇ p-Cys3-NH 2 .
- the two peptide-isomers are easily separated on HPLC
- the absolute configurations of the 1-Me-His residue in each peptide are defined by two-dimensional
- Example 110 Synthesis of Compound No. 126: Ac-Tyr-hArg-cvclorCys-Glu-His-p-Phe-Arg-T ⁇ -Cvsl-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-hArg(Pbf) is used in step 8 instead of Fmoc-Arg(Pbf). Fmoc-Tyr (OtBu) is added between steps 8 and 9.
- Example 111 Synthesis of Compound No. 127 : Ac-Tyr-(l- ⁇ -hArg)-cvclofCys-Glu-His-p-Phe-Arg-T ⁇ -Cys1-NH?
- Example 112 Synthesis of Compound No. 128 : Ac-Tyr-Lys-cyclofCys-Glu-His-p-Phe-Arg-T ⁇ p-Cvs1-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-Lys(Boc) is used in step 8 instead of Fmoc-Arg(Pbf). Fmoc-Tyr(tBu) is used between steps 8 and 9.
- Example 113 Synthesis of Compound No.
- Fmoc-Tyr(tBu) is used between steps 8 and 9.
- Example 115 Synthesis of Compound No. 131 : N-succinyl-Tyr-Arg-cvclorCvs-Glu-His-p-Phe-Arg-T ⁇ -Cysl-OH Can be prepared according to Example 1, with the exception that step 9 is carried out with succinyl anhydride instead of acetic anhydride. Fmoc-Tyr(tBu) is added between steps 8 and 9. Wang resin is used instead of Rink resin.
- Example 116 Synthesis of Compound No.
- Example 120 S y nthesis of Compound No. 136: Ac-cvclorhC y s--His-Phe-Ar g -T ⁇ -C y sl-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-
- Example 122 Synthesis of Compound No. 138: Ac-cyclorhCvs--His-p-Phe-Arg-T ⁇ -Cys]-OH Can be prepared according to Example 1, with the exception that homocysteine is used instead of cysteine in step 7, and Fmoc-Arg(Pbf) is omitted from step 8. Wang resin is used instead of Rink resin.
- Example 123 Synthesis of Compound No. 139: Ac-cyclorhCvs-His-(4-F-p-PheVArg-T ⁇ -Cvs1-N ⁇ 2 Can be prepared according to Example 1, with the exception that Fmoc-
- Fmoc-Cys(Trt) in step 7 is replaced with Fmoc-hCys(Trt).
- acetic acid anhydride is replaced with cyclopropane carboxylic acid, which is pre-activated with DIG (1,3-diisopropyl- carbodiimide)/HOBt ( 1 -hydroxylbenzotriazole) .
- DIG 1,3-diisopropyl- carbodiimide
- HBt 1 -hydroxylbenzotriazole
- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- Fmoc-hCys(Trt) is used in step 7 instead of Fmoc-Cys(Trt).
- acetic anhydride is replaced with cyclobutane carboxylic acid, which is pre-activated with DIC (1,3-diisopropyl- carbodiimideVHOBt ( 1 -hydroxylbenzotriazole) .
- Example 127 Synthesis of Compound No. 143: N-cyclopentanecarbonyl-cvclofhCys-His-p-Phe-Arg-T ⁇ -Cvs1-NH 2 Can be prepared according to Example 1, with t ie exception that Fmoc-
- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- Fmoc-hCys(Trt) is used in step 7 instead of Fmoc-Cys(Trt).
- acetic anhydride is replaced with cyclohexane carboxylic acid, which is pre-activated with DIC (1,3-diisopropyl- carbodiimide)/HOBt (l-hydroxylbenzotriazole).
- N-hexanoyl-cvclorhCys-His-p-Phe-Ajg-T ⁇ -Cvs1-NH 2 Can be prepared according to Example 1, with the exception that Fmoc- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- Fmoc-hCys(Trt) is used in step 7 instead of Fmoc-Cys(Trt).
- acetic anhydride is replaced with n-hexanoic acid, which is pre-activated with DIC (1,3-diisopropylcarbodiimide)/
- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- Fmoc-hCys(Trt) is used in step 7 instead of Fmoc-Cys(Trt).
- acetic anhydride is replaced with benzoic acid, which is pre-activated with DIC (l,3-diisopropylcarbodiimide)/HOBt
- Example 131 Synthesis of Compound No. 147: 4-phenylbutyryl-c y clorhCys-B-is-D-Phe-Arg-T ⁇ -C y s1-NH2 Can be prepared according to Example 1, with the exception that Fmoc-
- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- Fmoc-hCys(Trt) is used in step 7 instead of Fmoc-Cys(Trt).
- acetic anhydride is replaced with 4-phenylbutyric acid, which is pre-activated with DIC (1,3-diisopropylcarbodiimide) /HOBt (1-hydroxylbenzotriazole).
- Example 132 Synthesis of Compound NO. 148: 3-guanidinopropionyl-cyclorhCvs-His-p-Phe-Arg-T ⁇ -Cys1-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-
- Glu(OtBu) in step 6 is not used.
- Fmoc-Cys(Trt) in step 7 and Fmoc-Arg(pbf) in step 8 are replaced with Fmoc-hCys(Trt) and Fmoc- ⁇ -Ala (Frnoc-3-amino propionic acid), respectively.
- step 9 acetylation is replaced the following treatment
- Fmoc-Cys(Trt) in step 7 and Fmoc-Arg(pbf) in step 8 are replaced with Fmoc-hCys(Trt) and Fmoc-5 -amino- valeric acid, respectively.
- step 9 acetylation is replaced the, following treatment (guanidylation): After Fmoc deprotection, the resin is incubated with 10 equivalents of N,N'-bis(tert- butoxycarbonyl)-lH-pyrazole-l-carboxamidine and 10 equivalents of DIEA in NMP (N-methylpyrrolidone) overnight at room temperature.
- NMP N-methylpyrrolidone
- step 6 Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- Fmoc-hCys(Trt) is used in step 7 instead of Fmoc-Cys(Trt).
- acetic anhydride is replaced with succinic acid anhydride.
- one more step is added after step 9. Attaching the phenylsulfonamide is as follows: after the step 9, the resin is swollen in DCM and washed several times with dry DMF.
- Example 138 Synthesis of Compound No. 154: p-A ⁇ g-cvclofhCvs-ffis-p-Phe-Arg-T ⁇ -Cvs]-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-Arg(pbf) in step 8 is replaced with Fmoc-o-A ⁇ g(pbf) and Fmoc-Glu(OtBu), and acetylation with acetic anhydride in steps 6 and 9, respectively, are not used.
- Example 139 Synthesis of Compound No. 155: Arg-cyclorhCys-His-p-Phe-Arg-T ⁇ -Cvsl-OH Can be prepared according to Example 1, with the exception that Fmoc- Glu(OtBu) acetylation with acetic anhydride in steps 6 and 9, respectively, are not used.
- Fmoc-hCys(Trt) is used instead of Fmoc-
- Example 141 Synthesis of Compound No. 158: Ac-Arg-cyclorhCys-H ⁇ s-p-Phe-Arg-T ⁇ -Cvs1-NH 2 Can be prepared according to Ex-ample 1, with the exception that Fmoc- Glu(OtBu) in step 6 is not used. In addition, homocysteine is used instead of cysteine in step 7.
- Example 142 Synthesis of Compound No.
- Glu(OtBu) in step 6 is not used.
- Fmoc-hCys(Trt) and Fmoc-Gly are used in steps 7 and 8 instead of Fmoc-Cys(Trt) and Fmoc-Arg(pbf), respectively.
- Acetic anhydride in step 9 is replaced with phenylsulfonylchloride.
- Example 145 Synthesis of Compound No. 162: Tyr-Arg-cvclorhCys-His-p-Phe-Arg-T ⁇ -Cys1-NH 2 Can be prepared according to E ample 1, with the exception that Fmoc-
- Example 147 Synthesis of Compound No. 164: Ac-Tyr-Arg-cvclorhCvs ⁇ His-p-Phe-Arg-T ⁇ -Cvsl-NH? Can be prepared according to Example 1, with the exception that homocysteine is used instead of cysteine in step 7. Fmoc-Glu(OfBu) is not used in step 6. Fmoc-TyrttBn) is added between steps 8 and 9.
- Example 148 Synthesis of Compound No. 165: Ac-Tyr- Arg-cyclorhCys-His-p-Phe-Arg-Trp-Cysl-OH Can be prepared according to Example 1, with the exception that Fmoc-
- Glu(OtBu) is not used.
- homocysteine is used instead of cysteine in step 7.
- Example 152 Synthesis of Compound No. 169: Ac-cvclorhCvs--as-(4-Cl-p-Phe)-Arg-T ⁇ -penicillamine1-NH 2 Can be prepared according to Example 1, with the exception that Fmoc-
- N-hexanoyl-cvclorhCvs-His-p-Phe-Arg-T ⁇ -penicillaminel-NH 2 Can be prepared according to Example 1, with the exception that Fmoc- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used. Fmoc-hCys(Trt) and Fmoc-penicillamine(Trt) are used in steps 7 and 1, respectively, instead of Fmoc-
- Fmoc-Cys(Trt) in steps 1 and 7 are replaced with Fmoc-penicillamine(Trt) and Fmoc-hCys(Trt), respectively.
- acetic acid anhydride is replaced with cyclopentane carboxylic acid, which is pre-activated with DIC (l,3-diisopropylcarbodiimide)/HOBt (1-hydroxylbenzotriazole).
- DIC l,3-diisopropylcarbodiimide
- HBt 1-hydroxylbenzotriazole
- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- Fmoc-hCys(Trt) and Fmoc-penicillamine(Trt) are used in steps 7 and 1, respectively, instead of Fmoc-
- Fmoc-hCys(Trt) and Fmoc-penicillamine(Trt) are used in steps 7 and 1, respectively, instead of Fmoc- Cys(Trt).
- acetic anhydride is replaced with 4-phenylbutyric acid, which is pre-activated with DIC (l,3-diisopropylcarbodiimide) HOBt (1-hydroxylbenzotriazole).
- DIC l,3-diisopropylcarbodiimide
- HOBt 1-hydroxylbenzotriazole
- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- Fmoc- hCys(Trt) and Fmoc-penicillamine(Trt) are used in steps 7 and 1, respectively, instead of
- Glu(OtBu) in step 6 is not used.
- Fmoc-Cys(Trt) in steps 1 and 7, and Fmoc-Arg(pbf) in step 8, are replaced with Fmoc-penicillamine(Trt), Fmoc-hCys(Trt) and Fmoc-nLeu, respectively.
- Example 161 Synthesis of Compound No. 178: N-phenylsulfonyl-Glv-cvclorhCys--ffis- p -Phe-Arg-T ⁇ -penicillamine1-NH 2 Can be prepared according to Example 1, with the exception that Fmoc- Glu(OtBu) in step 6 is not used.
- Fmoc-penicillamine(Trt), Fmoc-hCys(Trt) and Fmoc-Gly are used in steps 1 , 7, and 8 instead of Fmoc-Cys(Trt), Fmoc-Cys(Trt), and Fmoc-Arg(pbf), respectively.
- Acetic anhydride in step 9 is replaced with phenylsulfonylchloiide.
- Arg-cvclorCvs-His-(4-Cl-p-Phe)-Arg-T ⁇ -hCvsl-N ⁇ 2 Can be prepared according to Example 1, with the exception that Fmoc- Glu(OtBu) in step 6 and acetylation with acetic anhydride in step 9 are not used. In addition, Fmoc-hCys(Trt) and Fmoc-4-Cl-o-Phe are used instead of Fmoc-Cys(Trt) and
- Glu(OtBu) in step 6 is not used.
- Fmoc-hCys(Trt) is used instead of Fmoc-
- Glu(OtBu) in step 6 is not used.
- Fmoc-hCys(Trt) and Fmoc-4-F-p-Phe are used instead of Fmoc-Cys ⁇ rt) in step 1 and Fmoc-p-Phe in step 4, respectively.
- Example 174 Synthesis of Compound No. 191: Ac-Arg-cvclorCys-His-(4-Cl-p-Phe)-Arg-T ⁇ -hCvsl-NH? Can be prepared according to Example 1, with the exception that Fmoc- Glu(OtBu) in step 6 is not used.
- Fmoc-hCys(Trt) and Fmoc-4-Cl-D-Phe are used instead of Fmoc-Cys(Trt) in step 1 and Fmoc-p-Phe in step 4, respectively.
- Example 176 Synthesis of Compound No. 193: Ac-cvclorhCvs-ffis- p -Phe-Ar g -T ⁇ -hC y sl-NH? Can be prepared according to Example 1, with the exception that Fmoc-hCys(Trt) is used instead of Fmoc-Cys(Trt) in steps 1 and 7. Fmoc-Glu(OtBu) is not used in step 6. Fmoc-Arg(Pbf) is not used in step 8.
- Example 177 Synthesis of Compound No.
- Fmoc-Tyr(tBu) is added between steps 8 and 9.
- Example 180 Synthesis of Compound No. 197: Ac-Tyr-Arg-cvclorhCys-Glu-His-p-Phe-Arg-T ⁇ -hCvsl-NH? Can be prepared according to Example 1, with the exception that Fnxoc-hCys(Trt) is used instead of Fmoc-Cys(Trt) in steps 1 and 7. Fmoc-Tyr(tBu) is added fcetween steps 8 and 9.
- Example 181 Synthesis of Compound No.
- Glu(OtBu) in step 6 and Fmoc-Arg(pbf) in step 8 are not used.
- the cyclization to form the disulfide bond is not carried out. Instead, the crude peptide (200 mg) is suspended in 200 mL of dichloromethane/acetonitrile (1:1 v/v) containing 3 mL of 1.0 M TBAF (tetrabutyl ammonium fluoride in THF) and stirring at room temperature for 30 min.
- MBHA resin (Midwest Biotech) is utilized as the solid support.
- the couplings are carried out either manually by single coupling each residue with a three-fold excess of amino acid activated with DCC/HOBt or by automated methods using an ABI 431 A or ABI 433A synthesizer programmed with the manufacturer's standard t-Boc protocol.
- N-terminal acetylation is accomplished with 5 equivalents acetic anhydride, 10 equivalents DIEA in dry DMF, 1 hour at room temperature.
- the tryptophan formyl group is deprotected by treatment of the resin-bound peptide with 20% piperidine in DMF, followed by washing with DMF and dichloromethane.
- the peptides are simultaneously cleaved from the resin and deprotected by treatment with liquid hydrogen fluoride at 0°C for 1 hour in the presence m-cresol and thiocresol scavengers.
- the peptides are recovered by ether precipitation, washed with ether, extracted into aqueous acetic acid, and lyophilized.
- Cyclization protocol The oxidation of the free cysteine sulfhydryl groups is accomplished either by air oxidation in 0.2 M ammonium acetate buffer containing 20% dimethyl sulfoxide (DMSO) at pH 7.0, or by treatment with 2,2'-pyridyldisulfide in 2.7 M guanidine buffer containing 30% DMSO. In each case, the final product is isolated by high performance liquid chromatography.
- DMSO dimethyl sulfoxide
- the peptide is diluted and loaded onto an HPLC column and eluted with an aqueous 0.1% trifluoroacetic acid/acetonitrile gradient while monitoring at 214nm. The appropriate fractions are pooled and lyophilized. Further characterization of the final product is performed using analytical HPLC and mass spectral analysis.
- Example 183 Synthesis of Compound No. 97: Ac-Tyr-Arg-cvclorCys-Glu- ⁇ 5-Me-HisVp-Phe-Arg-T ⁇ -Cys1-NH2 and Synthesis of Compound No. 98: Ac-Tyr-Ar g -cvclorC y s-Glu- ( 5-Me- p -His ') - p -Phe-Ar g -T ⁇ -C y sl- l2 Can be prepared according to Example 182, with the exception that Boc-5-Me- (D/L)-His(3-Boc) is used in step 5 instead of Boc-His(3-Bom).
- Pyrazolyl-(D/L)Ala is used in step 5 instead of Boc-His(3-Bom).
- the two peptide-isomers are easily separated on HPLC, which affords: Ac-Tyr-Arg-cyclo[Cys-Glu-(l-pyrazVlyl-p-Ala)-p-Phe-Arg-T ⁇ -Cys]-NH 2 and Ac-Tyr- Arg-c yclo[Cys-Glu-( 1 -pyrazolyl- Ala)-o-Phe- Arg-T ⁇ -Cys] -NH 2
- the absolute configurations of this His residue replacement in each peptide are defined by two-dimensional NMR techniques with proper standard peptides and controls.
- Example 188 Construction of MC receptor expression plasmids Construction of human MCI expression plasmid: Human MCI cDNA is cloned by PCR using human genomic DNA (Clontech Cat. # 6550-1) as a template. A forward hMCl gene-specific primer containing initiation codon (ATG and EcoRI site and a reverse hMCl gene specific primer containing a stop codon and Xbal site are used in the PCR. The full-length hMCl cDNA generated by PCR is cloned into pUClS/Smal . plasmid (Pharmacia Cat. # 27-5266-01), and the correct hMCl cDNA is confirmed by DNA sequencing.
- Human MC3 cDNA is cloned by PCR using human genomic DNA (Clontech Cat. # 6550-1) as a template.
- a forward hMC3 gene-specific primer containing initiation codon (ATG) and EcoRI site and a reverse hMC3 gene specific primer containing a stop codon and Xbal site are used in the PCR.
- hMC3 cDNA generated by PCR is cloned into pUC 18/SmaI plasmid (Pharmacia Cat# 27-5266-01), and the correct hMC3 cDNA is confirmed by DNA sequencing.
- the sequenced pUC18hMC3 is digested with EcoRI and Xbal, and the hMC3 cDNA fragment is then subcloned into pcDNA3.1 (Invitrogen Cat. # N790-20) to generate expression plasmid pCD ⁇ A3-hMC3.
- Human MC4 (hMC4) cDNA is cloned in a similar way as hMC3 cDNA by PCR using human fetal brain cDNA (Clontech Cat. # 7402-1) as a template.
- the hMC4 cDNA PCR product is digested with EcoRI/Xbal, and then subcloned into pCIneo (Promega Cat. # El 841) and sequenced.
- the resulting hMC4R plasmid has two mutations, which are then corrected to create the hMC4 cDNA encoding the correct hMC4 protein.
- Human MC5 cDNA is cloned by PCR using human genomic DNA (Clontech Cat. # 6550-1) as a template.
- a forward hMC5 gene-specific primer containing initiation codon (ATG) and ffindlH site and a reverse hMC5 gene specific primer containing a stop codon and Xbal site are used in the PCR.
- the full-length hMC5 cDNA generated by PCR is cloned into ⁇ UC18/SmaI plasmid (Pharmacia Cat.
- hMC5 cDNA is-confirmed by DNA sequencing.
- the sequenced pUC18hMC5 is digested with EcoRI and Xbal, and the hMC5 cDNA fragment is then subcloned into pcDNA3.1 (Invitrogen Cat. # N790-20) to generate expression plasmid pCD ⁇ A3-hMC5.
- Stable HEK-293 cells expressing human MCRs Stable 293 cells expressing all hMCRs are generated by co-transfecting HEK-293 cells with pCDNA3-hMC4R and a CRE-luciferase reporter plasmid following the protocol of Lipofectamine Plus Reagent (Invitrogen, Cat.
- Genticin G4128
- IO Luciferase Reporter Gene Assay kit
- Example 189 Melanocortin Receptor Whole Cell cAMP Accumulation Assay Hank's Balanced Salt Solution without phenol red (HBSS-092), 1 M HEPES, Dulbecco's Modified Eagle Media (DMEM), Fetal Bovine Serum (FBS), Antibiotic/ Antimycotic Solution, and sodium acetate are obtained from GibcoBRL. Triton X-100, ascorbic acid, cAMP, and 3-isobutyl-l -methyl-xanthine (IBMX) are purchased from Sigma. Bovine Serum Albumin (BSA) is obtained from Roche. SPA PVT antibody-binding beads type II anti-sheep beads and 125 I cAMP are obtained from Amersham.
- BSA Bovine Serum Albumin
- Anti-goat cAMP antibody is obtained from ICN. Enzyme Free Cell Dissociation Solution Hank's based is obtained from Specialty Media. NDP-o-MSH is obtained from Calbiochem. Dimethylsulfoxide (DMSO) is obtained from Aldrich.
- compounds are prepared as 10 mM and NDP- ⁇ MSH (control) as 33.3 ⁇ M stock solutions in 100% DMSO. These solutions are serially diluted in 100% DMSO. The compound plate is further diluted in compound dilution buffer (HBSS-092, 1 mM Ascorbic Acid, 1 mM IBMX, 0.6% DMSO, 0.1% BSA) to yield a final concentration range in the assay between 600 nM - 6 pM for compound and 100 nM - 1 pM for NDP- ⁇ MSH control in 0.5% DMSO. Twenty ⁇ L of compound solution are transferred from this plate into four PET 96-well plates (all assays are performed in duplicate for each receptor).
- compound dilution buffer HBSS-092, 1 mM Ascorbic Acid, 1 mM IBMX, 0.6% DMSO, 0.1% BSA
- HEK 293 cells stably transfected with the human MC3R or MC4R are grown in DMEM containing 10 % FBS and 1% Antibiotic/Antimycotic Solution.
- the cells are dislodged with enzyme free cell dissociation solution and re-suspended in cell buffer (HBSS-092, 0.1 % BSA, 10 mM HEPES) at 1 x 10° cells/mL.
- cell buffer HBSS-092, 0.1 % BSA, 10 mM HEPES
- Forty ⁇ L of cell suspension are added per well to PET 96-well plates containing 20 ⁇ L of diluted compound or control. Plates are incubated at 37°C in a waterbath for 20 minutes.
- the assay is stopped by adding 50 ⁇ L Quench Buffer (50 mM sodium acetate, 0.25% Triton X-100).
- Radioligand binding assays are run in SPA buffer (50 mM sodium acetate, 0.1% BSA). The beads, antibody, and radioligand are diluted in SPA buffer to provide sufficient volume for each 96-well plate. To each quenched assay well is added 100 ⁇ L cocktail containing 33.33 ⁇ L of beads, 33.33 ⁇ L antibody, and 33.33 ⁇ L 125 I-cAMP. This is based on a final concentration of 6.3 mg/mL beads, 0.65% anti-goat antibody, and
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Endocrinology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Diabetes (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Reproductive Health (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Hematology (AREA)
- Toxicology (AREA)
- Obesity (AREA)
- Biochemistry (AREA)
- Emergency Medicine (AREA)
- Gynecology & Obstetrics (AREA)
- Child & Adolescent Psychology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
Claims
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| BRPI0410731-4A BRPI0410731A (en) | 2003-06-19 | 2004-06-17 | compound, pharmaceutical composition, methods for agonizing the mc4 receptor, treating obesity, treating diabetes mellitus and treating male and / or female sexual dysfunction in a mammal, and, using a compound |
| MXPA05013951A MXPA05013951A (en) | 2003-06-19 | 2004-06-17 | Melanocortin recptor 4(mc4) agonists and their uses. |
| AU2004251616A AU2004251616A1 (en) | 2003-06-19 | 2004-06-17 | Melanocortin receptor 4(MC4) agonists and their uses |
| CA002530024A CA2530024A1 (en) | 2003-06-19 | 2004-06-17 | Melanocortin receptor 4(mc4) agonists and their uses |
| EA200600055A EA200600055A1 (en) | 2003-06-19 | 2004-06-17 | AGONISTS OF MELANOCORTIN 4 (MK4) RECEPTOR AND THEIR APPLICATION |
| EP04753454A EP1644023A2 (en) | 2003-06-19 | 2004-06-17 | Melanocortin recptor 4(mc4) agonists and their uses |
| JP2006517152A JP2006527773A (en) | 2003-06-19 | 2004-06-17 | Melanocortin receptor 4 (MC4) agonist and its use |
| US10/556,689 US20070105759A1 (en) | 2003-06-19 | 2004-06-17 | Melanocortin receptor 4 (mc4) agonists and their uses |
| IL171931A IL171931A0 (en) | 2003-06-19 | 2005-11-13 | Melanocortin receptor 4(mc4) agonists and their uses |
| NO20060259A NO20060259L (en) | 2003-06-19 | 2006-01-18 | Melanocortin receptor 4 (MC4) agonist and uses thereof |
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US47974003P | 2003-06-19 | 2003-06-19 | |
| US60/479,740 | 2003-06-19 | ||
| US55734704P | 2004-03-29 | 2004-03-29 | |
| US60/557,347 | 2004-03-29 | ||
| US57073704P | 2004-05-13 | 2004-05-13 | |
| US57067604P | 2004-05-13 | 2004-05-13 | |
| US60/570,676 | 2004-05-13 | ||
| US60/570,737 | 2004-05-13 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| WO2005000339A2 true WO2005000339A2 (en) | 2005-01-06 |
| WO2005000339A3 WO2005000339A3 (en) | 2005-02-03 |
| WO2005000339A8 WO2005000339A8 (en) | 2005-04-21 |
Family
ID=33556652
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2004/016625 WO2005000339A2 (en) | 2003-06-19 | 2004-06-17 | Melanocortin receptor 4(mc4) agonists and their uses |
Country Status (17)
| Country | Link |
|---|---|
| US (1) | US20070105759A1 (en) |
| EP (1) | EP1644023A2 (en) |
| JP (1) | JP2006527773A (en) |
| KR (1) | KR20060014444A (en) |
| AR (1) | AR044824A1 (en) |
| AU (1) | AU2004251616A1 (en) |
| BR (1) | BRPI0410731A (en) |
| CA (1) | CA2530024A1 (en) |
| CR (1) | CR8159A (en) |
| EA (1) | EA200600055A1 (en) |
| EC (1) | ECSP056236A (en) |
| IL (1) | IL171931A0 (en) |
| MX (1) | MXPA05013951A (en) |
| NO (1) | NO20060259L (en) |
| PE (1) | PE20050284A1 (en) |
| TW (1) | TW200514791A (en) |
| WO (1) | WO2005000339A2 (en) |
Cited By (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006073772A1 (en) * | 2005-01-05 | 2006-07-13 | Eli Lilly And Company | Polyethylene glycol linked mc4r or mc3r agonist peptides |
| WO2007008684A2 (en) | 2005-07-08 | 2007-01-18 | Societe De Conseils De Recherches Et D'applications Scientifiques S.A.S. | Ligands of melanocortin receptors |
| WO2008017381A1 (en) | 2006-08-08 | 2008-02-14 | Sanofi-Aventis | Arylaminoaryl-alkyl-substituted imidazolidine-2,4-diones, processes for preparing them, medicaments comprising these compounds, and their use |
| EP1915167A2 (en) * | 2005-07-08 | 2008-04-30 | Societe De Conseils De Recherches Et D'applications Scientifiques S.A.S. | Melanocortin receptor ligands |
| WO2009021740A2 (en) | 2007-08-15 | 2009-02-19 | Sanofis-Aventis | Substituted tetrahydronaphthalenes, process for the preparation thereof and the use thereof as medicaments |
| WO2010003624A2 (en) | 2008-07-09 | 2010-01-14 | Sanofi-Aventis | Heterocyclic compounds, processes for their preparation, medicaments comprising these compounds, and the use thereof |
| US7662771B2 (en) | 2003-08-20 | 2010-02-16 | Emisphere Technologies, Inc. | Compounds, methods and formulations for the oral delivery of a glucagon-like peptide (GLP)-1 compound or a melanocortin-4 receptor (MC4) agonist peptide |
| EP2167112A2 (en) * | 2007-06-15 | 2010-03-31 | Ipsen Pharma S.A.S. | Cyclic peptide melanocortin receptor ligands |
| WO2010047982A1 (en) | 2008-10-22 | 2010-04-29 | Merck Sharp & Dohme Corp. | Novel cyclic benzimidazole derivatives useful anti-diabetic agents |
| WO2010051206A1 (en) | 2008-10-31 | 2010-05-06 | Merck Sharp & Dohme Corp. | Novel cyclic benzimidazole derivatives useful anti-diabetic agents |
| WO2010068601A1 (en) | 2008-12-08 | 2010-06-17 | Sanofi-Aventis | A crystalline heteroaromatic fluoroglycoside hydrate, processes for making, methods of use and pharmaceutical compositions thereof |
| US20100311647A1 (en) * | 2007-11-05 | 2010-12-09 | Halem Heather A | Use of melanocortins to treat insulin sensitivity |
| WO2011023754A1 (en) | 2009-08-26 | 2011-03-03 | Sanofi-Aventis | Novel crystalline heteroaromatic fluoroglycoside hydrates, pharmaceuticals comprising these compounds and their use |
| EP2300036A1 (en) * | 2008-06-09 | 2011-03-30 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptides for treatment of sexual dysfunction |
| US7947841B2 (en) | 2003-08-20 | 2011-05-24 | Emisphere Technologies, Inc. | Compounds, methods and formulations for the oral delivery of a glucagon-like peptide (GLP)-1 compound or a melanocortin-4 receptor (MC4) agonist peptide |
| WO2011106273A1 (en) | 2010-02-25 | 2011-09-01 | Merck Sharp & Dohme Corp. | Novel cyclic benzimidazole derivatives useful anti-diabetic agents |
| CN102458436A (en) * | 2009-06-08 | 2012-05-16 | 帕拉丁科技公司 | Melanocortin receptor-specific peptides |
| WO2012116145A1 (en) | 2011-02-25 | 2012-08-30 | Merck Sharp & Dohme Corp. | Novel cyclic azabenzimidazole derivatives useful as anti-diabetic agents |
| WO2012120056A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Tetrasubstituted oxathiazine derivatives, method for producing them, their use as medicine and drug containing said derivatives and the use thereof |
| WO2012120053A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Branched oxathiazine derivatives, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120057A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Novel substituted phenyl-oxathiazine derivatives, method for producing them, drugs containing said compounds and the use thereof |
| WO2012120051A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Benzyl-oxathiazine derivates substituted with adamantane or noradamantane, medicaments containing said compounds and use thereof |
| WO2012120054A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120050A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Novel substituted phenyl-oxathiazine derivatives, method for producing them, drugs containing said compounds and the use thereof |
| WO2012120058A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Oxathiazine derivatives which are substituted with benzyl or heteromethylene groups, method for producing them, their use as medicine and drug containing said derivatives and the use thereof |
| WO2012120055A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120052A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Oxathiazine derivatives substituted with carbocycles or heterocycles, method for producing same, drugs containing said compounds, and use thereof |
| US8318451B2 (en) | 2008-01-02 | 2012-11-27 | Danisco Us Inc. | Process of obtaining ethanol without glucoamylase using Pseudomonas saccharophila G4-amylase variants thereof |
| US8455618B2 (en) | 2009-06-08 | 2013-06-04 | Astrazeneca Ab | Melanocortin receptor-specific peptides |
| WO2013102047A1 (en) | 2011-12-29 | 2013-07-04 | Rhythm Pharmaceuticals, Inc. | Method of treating melanocortin-4 receptor-associated disorders in heterozygous carriers |
| US8492517B2 (en) | 2009-11-23 | 2013-07-23 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific cyclic peptides |
| US8563000B2 (en) | 2007-05-25 | 2013-10-22 | Ipsen Pharma S.A.S. | Melanocortin receptor ligands modified with hydantoin |
| WO2014022528A1 (en) | 2012-08-02 | 2014-02-06 | Merck Sharp & Dohme Corp. | Antidiabetic tricyclic compounds |
| WO2014130608A1 (en) | 2013-02-22 | 2014-08-28 | Merck Sharp & Dohme Corp. | Antidiabetic bicyclic compounds |
| WO2014144260A1 (en) * | 2013-03-15 | 2014-09-18 | Rhythm Metabolic, Inc. | Peptide compositions |
| WO2014139388A1 (en) | 2013-03-14 | 2014-09-18 | Merck Sharp & Dohme Corp. | Novel indole derivatives useful as anti-diabetic agents |
| US8933194B2 (en) | 2009-11-23 | 2015-01-13 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific linear peptides |
| WO2014144842A3 (en) * | 2013-03-15 | 2015-04-09 | Rhythm Metabolic, Inc. | Pharmaceutical compositions |
| WO2015051725A1 (en) | 2013-10-08 | 2015-04-16 | Merck Sharp & Dohme Corp. | Antidiabetic tricyclic compounds |
| US9273098B2 (en) | 2009-06-08 | 2016-03-01 | Palatin Technologies, Inc. | Lactam-bridged melanocortin receptor-specific peptides |
| WO2018106518A1 (en) | 2016-12-06 | 2018-06-14 | Merck Sharp & Dohme Corp. | Antidiabetic heterocyclic compounds |
| WO2018118670A1 (en) | 2016-12-20 | 2018-06-28 | Merck Sharp & Dohme Corp. | Antidiabetic spirochroman compounds |
| EP4029513A1 (en) | 2015-09-30 | 2022-07-20 | Rhythm Pharmaceuticals, Inc. | Melanocortin-4 receptor agonists for the treatment of disorders characterised by pomc gene hypermethylation |
| CN115010793A (en) * | 2022-06-17 | 2022-09-06 | 中国农业大学 | Preparation method and application of rose-leaf malformation virus coat protein polyclonal antibody |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090305960A1 (en) * | 2008-06-09 | 2009-12-10 | Palatin Technologies, Inc | Melanocortin Receptor-Specific Peptides for Treatment of Obesity / 669 |
| KR101917854B1 (en) * | 2017-08-24 | 2018-11-12 | 한국콜마주식회사 | Peptides having capacity of binding to cell receptor and cosmetic composition comprising the same |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999054358A1 (en) * | 1998-04-17 | 1999-10-28 | Quadrant Holdings Cambridge Limited | Melanocortin receptor ligands |
| WO2000033658A1 (en) * | 1998-12-09 | 2000-06-15 | Eleanor Roosevelt Institute | Composition and method for regulation of body weight and associated conditions |
| WO2000035952A2 (en) * | 1998-12-14 | 2000-06-22 | Melacure Therapeutics Ab | Compounds for control of eating, growth and body weight |
| WO2000058361A1 (en) * | 1999-03-29 | 2000-10-05 | The Procter & Gamble Company | Melanocortin receptor ligands |
| US20020143141A1 (en) * | 2000-08-30 | 2002-10-03 | Li Chen | Selective cyclic peptides with melanocortin-4 receptor (MC4-R) agonist activity |
| WO2003006604A2 (en) * | 2001-07-12 | 2003-01-23 | Merck & Co., Inc. | Cyclic peptides as potent and selective melanocortin-4 receptor agonists |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4485039A (en) * | 1982-06-11 | 1984-11-27 | University Patents, Inc. | Synthetic analogues of α-melanotropin |
| US5674839A (en) * | 1987-05-22 | 1997-10-07 | Competitive Technologies, Inc. | Cyclic analogs of alpha-MSH fragments |
| US6716810B1 (en) * | 1998-12-09 | 2004-04-06 | Eleanor Roosevelt Institute | Composition and method for regulation of body weight and associated conditions |
| US6579968B1 (en) * | 1999-06-29 | 2003-06-17 | Palatin Technologies, Inc. | Compositions and methods for treatment of sexual dysfunction |
| US6659982B2 (en) * | 2000-05-08 | 2003-12-09 | Sterling Medivations, Inc. | Micro infusion drug delivery device |
-
2004
- 2004-06-17 KR KR1020057024261A patent/KR20060014444A/en not_active Application Discontinuation
- 2004-06-17 AU AU2004251616A patent/AU2004251616A1/en not_active Abandoned
- 2004-06-17 EA EA200600055A patent/EA200600055A1/en unknown
- 2004-06-17 BR BRPI0410731-4A patent/BRPI0410731A/en not_active Application Discontinuation
- 2004-06-17 US US10/556,689 patent/US20070105759A1/en not_active Abandoned
- 2004-06-17 JP JP2006517152A patent/JP2006527773A/en not_active Withdrawn
- 2004-06-17 WO PCT/US2004/016625 patent/WO2005000339A2/en active Search and Examination
- 2004-06-17 MX MXPA05013951A patent/MXPA05013951A/en unknown
- 2004-06-17 EP EP04753454A patent/EP1644023A2/en not_active Withdrawn
- 2004-06-17 CA CA002530024A patent/CA2530024A1/en not_active Abandoned
- 2004-06-18 PE PE2004000599A patent/PE20050284A1/en not_active Application Discontinuation
- 2004-06-18 AR ARP040102134A patent/AR044824A1/en unknown
- 2004-06-18 TW TW093117823A patent/TW200514791A/en unknown
-
2005
- 2005-11-13 IL IL171931A patent/IL171931A0/en unknown
- 2005-12-16 EC EC2005006236A patent/ECSP056236A/en unknown
- 2005-12-16 CR CR8159A patent/CR8159A/en unknown
-
2006
- 2006-01-18 NO NO20060259A patent/NO20060259L/en not_active Application Discontinuation
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999054358A1 (en) * | 1998-04-17 | 1999-10-28 | Quadrant Holdings Cambridge Limited | Melanocortin receptor ligands |
| WO2000033658A1 (en) * | 1998-12-09 | 2000-06-15 | Eleanor Roosevelt Institute | Composition and method for regulation of body weight and associated conditions |
| WO2000035952A2 (en) * | 1998-12-14 | 2000-06-22 | Melacure Therapeutics Ab | Compounds for control of eating, growth and body weight |
| WO2000058361A1 (en) * | 1999-03-29 | 2000-10-05 | The Procter & Gamble Company | Melanocortin receptor ligands |
| US20020143141A1 (en) * | 2000-08-30 | 2002-10-03 | Li Chen | Selective cyclic peptides with melanocortin-4 receptor (MC4-R) agonist activity |
| WO2003006604A2 (en) * | 2001-07-12 | 2003-01-23 | Merck & Co., Inc. | Cyclic peptides as potent and selective melanocortin-4 receptor agonists |
Non-Patent Citations (3)
| Title |
|---|
| CHEUNG ADRIAN WAI-HING ET AL: "Structure-activity relationship of linear peptide Bu-His-DPhe-Arg-Trp-Gly-NH2 at the human melanocortin-1 and -4 receptors: Histidine substitution." BIOORGANIC AND MEDICINAL CHEMISTRY LETTERS, vol. 13, no. 1, 6 January 2003 (2003-01-06), pages 133-137, XP001183563 ISSN: 0960-894X * |
| HASKELL-LUEVANO C ET AL: "Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors" JOURNAL OF MEDICINAL CHEMISTRY, AMERICAN CHEMICAL SOCIETY. WASHINGTON, US, vol. 44, no. 13, 2001, pages 2247-2252, XP002970859 ISSN: 0022-2623 * |
| PROIETTO J ET AL: "NOVEL ANTI-OBESITY DRUGS" EXPERT OPINION ON INVESTIGATIONAL DRUGS, ASHLEY PUBLICATIONS LTD., LONDON, GB, vol. 9, no. 6, June 2000 (2000-06), pages 1317-1326, XP001004696 ISSN: 1354-3784 * |
Cited By (107)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7662771B2 (en) | 2003-08-20 | 2010-02-16 | Emisphere Technologies, Inc. | Compounds, methods and formulations for the oral delivery of a glucagon-like peptide (GLP)-1 compound or a melanocortin-4 receptor (MC4) agonist peptide |
| US8552039B2 (en) | 2003-08-20 | 2013-10-08 | Emisphere Technologies, Inc. | Compounds, methods and formulations for the oral delivery of a glucagon-like peptide (GLP-1) compound or a melanocortin-4 receptor (MC4) agonist peptide |
| US8765796B2 (en) | 2003-08-20 | 2014-07-01 | Emisphere Technologies, Inc. | Compounds, methods and formulations for the oral delivery of a glucagon-like peptide (GLP-1) compound or a melanocortin-4 receptor (MC4) agonist peptide |
| US7947841B2 (en) | 2003-08-20 | 2011-05-24 | Emisphere Technologies, Inc. | Compounds, methods and formulations for the oral delivery of a glucagon-like peptide (GLP)-1 compound or a melanocortin-4 receptor (MC4) agonist peptide |
| WO2006073772A1 (en) * | 2005-01-05 | 2006-07-13 | Eli Lilly And Company | Polyethylene glycol linked mc4r or mc3r agonist peptides |
| JP2015232003A (en) * | 2005-07-08 | 2015-12-24 | イプセン ファルマ ソシエテ パール アクシオン サンプリフィエIpsen Pharma S.A.S. | Melanocortin receptor ligand |
| US9458195B2 (en) | 2005-07-08 | 2016-10-04 | Ipsen Pharma S.A.S. | Melanocortin receptor ligands |
| WO2007008684A3 (en) * | 2005-07-08 | 2009-05-07 | Sod Conseils Rech Applic | Ligands of melanocortin receptors |
| EP3925614A3 (en) * | 2005-07-08 | 2022-03-23 | Ipsen Pharma | Melanocortin receptor ligands |
| JP2009500427A (en) * | 2005-07-08 | 2009-01-08 | ソシエテ・ドゥ・コンセイユ・ドゥ・ルシェルシュ・エ・ダプリカーション・シャンティフィック・エス・ア・エス | Melanocortin receptor ligand |
| CN103755786A (en) * | 2005-07-08 | 2014-04-30 | 益普生制药股份有限公司 | Melanocortin receptor ligands |
| EP1915167A4 (en) * | 2005-07-08 | 2010-04-07 | Ipsen Pharma | Melanocortin receptor ligands |
| JP2010090130A (en) * | 2005-07-08 | 2010-04-22 | Ipsen Pharma Sas | Melanocortin receptor ligand |
| EP3354273A1 (en) * | 2005-07-08 | 2018-08-01 | Ipsen Pharma | Melanocortin receptor ligands |
| US9850280B2 (en) | 2005-07-08 | 2017-12-26 | Ipsen Pharma S.A.S. | Melanocortin receptor ligands |
| US8349797B2 (en) | 2005-07-08 | 2013-01-08 | Ipsen Pharma S.A.S. | Ligands of melanocortin receptors |
| EP1915167A2 (en) * | 2005-07-08 | 2008-04-30 | Societe De Conseils De Recherches Et D'applications Scientifiques S.A.S. | Melanocortin receptor ligands |
| EP2236151A1 (en) * | 2005-07-08 | 2010-10-06 | Ipsen Pharma | Melanocortin receptor ligands |
| EP2548568A3 (en) * | 2005-07-08 | 2013-07-17 | Ipsen Pharma | Melanocortin receptor ligands |
| WO2007008684A2 (en) | 2005-07-08 | 2007-01-18 | Societe De Conseils De Recherches Et D'applications Scientifiques S.A.S. | Ligands of melanocortin receptors |
| JP2013253090A (en) * | 2005-07-08 | 2013-12-19 | Ipsen Pharma Sas | Melanocortin receptor ligands |
| JP2009500426A (en) * | 2005-07-08 | 2009-01-08 | ソシエテ・ドゥ・コンセイユ・ドゥ・ルシェルシュ・エ・ダプリカーション・シャンティフィック・エス・ア・エス | Melanocortin receptor ligand |
| US8039435B2 (en) | 2005-07-08 | 2011-10-18 | Ipsen Pharma S.A.S. | Melanocortin receptor ligands |
| EP2286825A3 (en) * | 2005-07-08 | 2011-07-27 | Ipsen Pharma | Melanocortin receptor ligands |
| US20110183886A1 (en) * | 2005-07-08 | 2011-07-28 | Zheng Xin Dong | Melanocortin receptor ligands |
| CN105837661A (en) * | 2005-07-08 | 2016-08-10 | 益普生制药股份有限公司 | Melanocortin receptor ligands |
| WO2008017381A1 (en) | 2006-08-08 | 2008-02-14 | Sanofi-Aventis | Arylaminoaryl-alkyl-substituted imidazolidine-2,4-diones, processes for preparing them, medicaments comprising these compounds, and their use |
| US20160176925A1 (en) * | 2007-05-25 | 2016-06-23 | Ipsen Pharma S.A.S. | Melanocortin receptor ligands modified with hydantoin |
| US9296783B2 (en) | 2007-05-25 | 2016-03-29 | Ipsen Pharma S.A.S. | Melanocortin receptor ligands modified with hydantoin |
| US9758549B2 (en) | 2007-05-25 | 2017-09-12 | Ipsen Pharma S.A.S. | Melanocortin receptor ligands modified with hydantoin |
| US8563000B2 (en) | 2007-05-25 | 2013-10-22 | Ipsen Pharma S.A.S. | Melanocortin receptor ligands modified with hydantoin |
| EP2167112A4 (en) * | 2007-06-15 | 2012-01-25 | Ipsen Pharma Sas | Cyclic peptide melanocortin receptor ligands |
| US20100173834A1 (en) * | 2007-06-15 | 2010-07-08 | Zheng Xin Dong | Cyclic peptide melanocortin receptor ligands |
| EP2167112A2 (en) * | 2007-06-15 | 2010-03-31 | Ipsen Pharma S.A.S. | Cyclic peptide melanocortin receptor ligands |
| WO2009021740A2 (en) | 2007-08-15 | 2009-02-19 | Sanofis-Aventis | Substituted tetrahydronaphthalenes, process for the preparation thereof and the use thereof as medicaments |
| US9439943B2 (en) | 2007-11-05 | 2016-09-13 | Ipsen Pharma S.A.S. | Use of melanocortins to treat insulin sensitivity |
| US9155777B2 (en) * | 2007-11-05 | 2015-10-13 | Ipsen Pharma S.A.S. | Use of melanocortins to treat insulin sensitivity |
| US20100311647A1 (en) * | 2007-11-05 | 2010-12-09 | Halem Heather A | Use of melanocortins to treat insulin sensitivity |
| US9827286B2 (en) | 2007-11-05 | 2017-11-28 | Ipsen Pharma S.A.S. | Use of melanocortins to treat insulin sensitivity |
| US8318451B2 (en) | 2008-01-02 | 2012-11-27 | Danisco Us Inc. | Process of obtaining ethanol without glucoamylase using Pseudomonas saccharophila G4-amylase variants thereof |
| US8729224B2 (en) | 2008-06-09 | 2014-05-20 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptides for treatment of female sexual dysfunction |
| EP2300036A1 (en) * | 2008-06-09 | 2011-03-30 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptides for treatment of sexual dysfunction |
| CN102131514B (en) * | 2008-06-09 | 2014-08-20 | 帕拉丁科技公司 | Melanocortin receptor-specific peptides for treatment of sexual dysfunction |
| EP2300036A4 (en) * | 2008-06-09 | 2012-01-18 | Palatin Technologies Inc | Melanocortin receptor-specific peptides for treatment of sexual dysfunction |
| US8487073B2 (en) | 2008-06-09 | 2013-07-16 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptides for treatment of sexual dysfunction |
| CN102131514A (en) * | 2008-06-09 | 2011-07-20 | 帕拉丁科技公司 | Melanocortin receptor-specific peptides for treatment of sexual dysfunction |
| WO2010003624A2 (en) | 2008-07-09 | 2010-01-14 | Sanofi-Aventis | Heterocyclic compounds, processes for their preparation, medicaments comprising these compounds, and the use thereof |
| WO2010047982A1 (en) | 2008-10-22 | 2010-04-29 | Merck Sharp & Dohme Corp. | Novel cyclic benzimidazole derivatives useful anti-diabetic agents |
| WO2010051206A1 (en) | 2008-10-31 | 2010-05-06 | Merck Sharp & Dohme Corp. | Novel cyclic benzimidazole derivatives useful anti-diabetic agents |
| WO2010068601A1 (en) | 2008-12-08 | 2010-06-17 | Sanofi-Aventis | A crystalline heteroaromatic fluoroglycoside hydrate, processes for making, methods of use and pharmaceutical compositions thereof |
| US10632171B2 (en) | 2009-06-08 | 2020-04-28 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptides |
| EP2440227B1 (en) * | 2009-06-08 | 2017-10-18 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptides |
| US8455617B2 (en) | 2009-06-08 | 2013-06-04 | Astrazeneca Ab | Melanocortin receptor-specific peptides |
| CN102458436A (en) * | 2009-06-08 | 2012-05-16 | 帕拉丁科技公司 | Melanocortin receptor-specific peptides |
| US8455618B2 (en) | 2009-06-08 | 2013-06-04 | Astrazeneca Ab | Melanocortin receptor-specific peptides |
| US9458201B2 (en) | 2009-06-08 | 2016-10-04 | Palatin Technologies, Inc. | Melanocortin receptor-specific heptapeptides |
| US10179804B2 (en) | 2009-06-08 | 2019-01-15 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptides |
| US9273098B2 (en) | 2009-06-08 | 2016-03-01 | Palatin Technologies, Inc. | Lactam-bridged melanocortin receptor-specific peptides |
| US8846601B2 (en) | 2009-06-08 | 2014-09-30 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptides |
| CN105037502A (en) * | 2009-06-08 | 2015-11-11 | 帕拉丁科技公司 | Melanocortin receptor-specific peptides |
| CN102458436B (en) * | 2009-06-08 | 2015-06-03 | 帕拉丁科技公司 | Melanocortin receptor-specific peptides |
| US9040663B2 (en) | 2009-06-08 | 2015-05-26 | Astrazeneca Ab | Melanocortin receptor-specific peptides |
| WO2011023754A1 (en) | 2009-08-26 | 2011-03-03 | Sanofi-Aventis | Novel crystalline heteroaromatic fluoroglycoside hydrates, pharmaceuticals comprising these compounds and their use |
| US10711039B2 (en) | 2009-11-23 | 2020-07-14 | Palatin Technologies, Inc. | Melanocortin receptor-specific peptide with C-terminal naphthylalanine |
| US8933194B2 (en) | 2009-11-23 | 2015-01-13 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific linear peptides |
| US10017539B2 (en) | 2009-11-23 | 2018-07-10 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific cyclic hexapeptides |
| US8877890B2 (en) | 2009-11-23 | 2014-11-04 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific cyclic peptides |
| US10106578B2 (en) | 2009-11-23 | 2018-10-23 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific linear peptides |
| US8492517B2 (en) | 2009-11-23 | 2013-07-23 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific cyclic peptides |
| US9580466B2 (en) | 2009-11-23 | 2017-02-28 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific linear peptides |
| US9447148B2 (en) | 2009-11-23 | 2016-09-20 | Palatin Technologies, Inc. | Melanocortin-1 receptor-specific cyclic peptides |
| WO2011106273A1 (en) | 2010-02-25 | 2011-09-01 | Merck Sharp & Dohme Corp. | Novel cyclic benzimidazole derivatives useful anti-diabetic agents |
| WO2012116145A1 (en) | 2011-02-25 | 2012-08-30 | Merck Sharp & Dohme Corp. | Novel cyclic azabenzimidazole derivatives useful as anti-diabetic agents |
| EP3243385A1 (en) | 2011-02-25 | 2017-11-15 | Merck Sharp & Dohme Corp. | Novel cyclic azabenzimidazole derivatives useful as anti-diabetic agents |
| WO2012120051A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Benzyl-oxathiazine derivates substituted with adamantane or noradamantane, medicaments containing said compounds and use thereof |
| WO2012120057A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Novel substituted phenyl-oxathiazine derivatives, method for producing them, drugs containing said compounds and the use thereof |
| WO2012120054A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120050A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Novel substituted phenyl-oxathiazine derivatives, method for producing them, drugs containing said compounds and the use thereof |
| WO2012120053A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Branched oxathiazine derivatives, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120058A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Oxathiazine derivatives which are substituted with benzyl or heteromethylene groups, method for producing them, their use as medicine and drug containing said derivatives and the use thereof |
| WO2012120052A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Oxathiazine derivatives substituted with carbocycles or heterocycles, method for producing same, drugs containing said compounds, and use thereof |
| WO2012120055A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120056A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Tetrasubstituted oxathiazine derivatives, method for producing them, their use as medicine and drug containing said derivatives and the use thereof |
| US10167312B2 (en) | 2011-12-29 | 2019-01-01 | Rhythm Pharmaceuticals, Inc. | Method of treating melanocortin-4 receptor-associated disorders in heterozygous carriers |
| US10954268B2 (en) | 2011-12-29 | 2021-03-23 | Rhythm Pharmaceuticals, Inc. | Method of treating melanocortin-4 receptor-associated disorders in heterozygous carriers |
| US11702448B2 (en) | 2011-12-29 | 2023-07-18 | Rhythm Pharmaceuticals, Inc. | Method of treating melanocortin-4 receptor-associated disorders in heterozygous carriers |
| US9845339B2 (en) | 2011-12-29 | 2017-12-19 | Rhythm Pharmaceuticals, Inc. | Method of treating melanocortin-4 receptor-associated disorders in heterozygous carriers |
| EP3988108A1 (en) | 2011-12-29 | 2022-04-27 | Rhythm Pharmaceuticals, Inc. | Method of treating melanocortin-4 receptor-associated disorders in heterozygous carriers |
| WO2013102047A1 (en) | 2011-12-29 | 2013-07-04 | Rhythm Pharmaceuticals, Inc. | Method of treating melanocortin-4 receptor-associated disorders in heterozygous carriers |
| EP3539551A1 (en) | 2011-12-29 | 2019-09-18 | Rhythm Pharmaceuticals, Inc. | Method of treating melanocortin-4 receptor-associated disorders in heterozygous carriers |
| WO2014022528A1 (en) | 2012-08-02 | 2014-02-06 | Merck Sharp & Dohme Corp. | Antidiabetic tricyclic compounds |
| WO2014130608A1 (en) | 2013-02-22 | 2014-08-28 | Merck Sharp & Dohme Corp. | Antidiabetic bicyclic compounds |
| WO2014139388A1 (en) | 2013-03-14 | 2014-09-18 | Merck Sharp & Dohme Corp. | Novel indole derivatives useful as anti-diabetic agents |
| EP3778623A1 (en) * | 2013-03-15 | 2021-02-17 | Rhythm Pharmaceuticals, Inc. | Pharmaceutical compositions |
| US11129869B2 (en) | 2013-03-15 | 2021-09-28 | Rhythm Pharmaceuticals, Inc. | Pharmaceutical compositions |
| RU2690377C2 (en) * | 2013-03-15 | 2019-06-03 | Ритм Фармасьютикалз, Инк. (Usa) | Pharmaceutical compositions |
| US10196425B2 (en) | 2013-03-15 | 2019-02-05 | Rhythm Pharmaceuticals, Inc. | Peptide compositions |
| US10858399B2 (en) | 2013-03-15 | 2020-12-08 | Rhythm Pharmaceuticals, Inc. | Peptide compositions |
| WO2014144260A1 (en) * | 2013-03-15 | 2014-09-18 | Rhythm Metabolic, Inc. | Peptide compositions |
| US12077612B2 (en) | 2013-03-15 | 2024-09-03 | Rhythm Pharmaceuticals, Inc. | Peptide compositions |
| WO2014144842A3 (en) * | 2013-03-15 | 2015-04-09 | Rhythm Metabolic, Inc. | Pharmaceutical compositions |
| AU2014227712B2 (en) * | 2013-03-15 | 2018-08-02 | Rhythm Pharmaceuticals, Inc. | Peptide compositions |
| WO2015051725A1 (en) | 2013-10-08 | 2015-04-16 | Merck Sharp & Dohme Corp. | Antidiabetic tricyclic compounds |
| EP4029513A1 (en) | 2015-09-30 | 2022-07-20 | Rhythm Pharmaceuticals, Inc. | Melanocortin-4 receptor agonists for the treatment of disorders characterised by pomc gene hypermethylation |
| WO2018106518A1 (en) | 2016-12-06 | 2018-06-14 | Merck Sharp & Dohme Corp. | Antidiabetic heterocyclic compounds |
| WO2018118670A1 (en) | 2016-12-20 | 2018-06-28 | Merck Sharp & Dohme Corp. | Antidiabetic spirochroman compounds |
| CN115010793A (en) * | 2022-06-17 | 2022-09-06 | 中国农业大学 | Preparation method and application of rose-leaf malformation virus coat protein polyclonal antibody |
Also Published As
| Publication number | Publication date |
|---|---|
| TW200514791A (en) | 2005-05-01 |
| WO2005000339A8 (en) | 2005-04-21 |
| WO2005000339A3 (en) | 2005-02-03 |
| EP1644023A2 (en) | 2006-04-12 |
| KR20060014444A (en) | 2006-02-15 |
| IL171931A0 (en) | 2006-04-10 |
| CA2530024A1 (en) | 2005-01-06 |
| EA200600055A1 (en) | 2006-08-25 |
| NO20060259L (en) | 2006-03-14 |
| MXPA05013951A (en) | 2006-02-24 |
| AU2004251616A1 (en) | 2005-01-06 |
| US20070105759A1 (en) | 2007-05-10 |
| CR8159A (en) | 2006-02-09 |
| JP2006527773A (en) | 2006-12-07 |
| ECSP056236A (en) | 2006-04-19 |
| AR044824A1 (en) | 2005-10-05 |
| BRPI0410731A (en) | 2006-06-20 |
| PE20050284A1 (en) | 2005-05-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20070105759A1 (en) | Melanocortin receptor 4 (mc4) agonists and their uses | |
| DE60038734T2 (en) | Melanocortin receptor Ligands | |
| JP5615353B2 (en) | Melanocortin receptor specific peptide | |
| US6239107B1 (en) | Conjugates of lipophilic moieties and fragments of vasoactive intestinal peptide (VIP) | |
| JP2009508885A (en) | Y4 selective receptor agonists for therapeutic intervention | |
| US20060293223A1 (en) | Uses of melanocortin-3 receptor (mc3r) agonist peptides | |
| JPH08511541A (en) | Anti-inflammatory compositions and methods for DES-TYR dynorphin and analogs | |
| US20040122013A1 (en) | Analogs of nocicettin | |
| US5869450A (en) | Anti-inflammatory compositions and method with corticotropin-releasing factor analogs | |
| JP2949129B2 (en) | Motilin-like polypeptide with gastrointestinal motility-stimulating activity | |
| WO1997032898A9 (en) | Anti-inflammatory crf analogs, their compositions and use | |
| US20220143196A1 (en) | Conjugation of mcr1 ligand with cytotoxic drugs for treating skin cancer | |
| WO2006073772A1 (en) | Polyethylene glycol linked mc4r or mc3r agonist peptides | |
| WO2006073771A2 (en) | Polyethylene glycol linked mc4r or mc3r agonist peptides | |
| IL295151A (en) | Diamine-linked receptor-specific cyclic peptides | |
| US20240270821A1 (en) | Gip/glp1/gcg tri-receptor agonists and uses thereof | |
| WO2014075137A1 (en) | Peptides incorporating amino-substituted lactams for treatment of retinopathy | |
| AU2005202153A1 (en) | Urotensin-II agonists | |
| KR20080063342A (en) | Y4 selective receptor agonists for therapeutic interventions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| CFP | Corrected version of a pamphlet front page |
Free format text: UNDER (54) PUBLISHED TITLE REPLACED BY CORRECT TITLE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 543218 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2004251616 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 171931 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007105759 Country of ref document: US Ref document number: 10556689 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 2004251616 Country of ref document: AU Date of ref document: 20040617 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004251616 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2530024 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 05124743 Country of ref document: CO |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005/10189 Country of ref document: ZA Ref document number: 200510189 Country of ref document: ZA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2004753454 Country of ref document: EP Ref document number: 1020057024261 Country of ref document: KR Ref document number: CR2005-008159 Country of ref document: CR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/2005/013951 Country of ref document: MX Ref document number: 20048171781 Country of ref document: CN Ref document number: 2006517152 Country of ref document: JP Ref document number: 12005502300 Country of ref document: PH |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2745/KOLNP/2005 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200600055 Country of ref document: EA |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020057024261 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004753454 Country of ref document: EP |
|
| DPEN | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: PI0410731 Country of ref document: BR |
|
| WWP | Wipo information: published in national office |
Ref document number: 10556689 Country of ref document: US |