DESCRIPTION
STEEL SHEET FOR CONTAINER AND METHOD OF PRODUCING THE SAME
Technical Field
The present invention relates to a steel sheet, used as a material for a can produced by welding, and a method of producing the steel sheet, as represented by the production of a three-piece can. That is, the present invention provides an ultra-thin material, for a container, which can be produced with a high productivity and is excellent in formability at a weld, in the fields of the production of steel sheets and the production of cans.
Background Art A container, as a can drum formed by welding and called a three-piece can, is used in the field of the production of beverage cans, food cans and the like. Flange forming is applied to a can drum for expanding the openings thereof so that a can bottom and a can top may be attached to the can drum and, in this case, it is required for the weld to have an excellent formability. In addition, in case of a large container or the like, welding is often adopted when a metal handle is attached to the container and sometimes the strength, particularly the fatigue strength, at a weld is a problem.
In the meantime, the thickness of a steel sheet for a container has become thinner, from the viewpoint of cost reduction, and this leads to the deterioration of the ductility and fatigue property of the steel sheet. Therefore, a good formability and a high strength at a weld are also required of an ultra-thin steel sheet. Further, an ultra-thin steel sheet that has been cold-
rolled to the thickness of the end product is apt to generate buckling, called a heat-buckle, during continuous annealing, and that causes a very poor strip threading performance and a marked deterioration of productivity.
To solve the above problems, Japanese Unexamined Patent Publication Nos. H3-257123 and H2-118026 and other publications disclose a DR material produced by the so- called DR method (double reduced method), wherein a steel sheet having a thickness thicker than the final product is processed during annealing and, after the annealing, the thickness of the final product is obtained by secondary cold-rolling. However, in the steel sheet hardened by the secondary cold-rolling, the softening of the material at a weld is caused by the recovery and recrystallization of the material due to the heat generated during welding and, thus, the stress concentration in the vicinity of the weld is increased • and the formability and fatigue property are deteriorated.
Further, Japanese Unexamined Patent Publication No. H10-72640 discloses a method of securing the strength of a steel sheet by increasing solute N, but, by this method, the stress concentration at a weld is excessive and thus the formability and fatigue strength of the weld are not improved sufficiently.
Yet further, Japanese Unexamined Patent Publication No. H2-118028 discloses a method of improving flange formability by decreasing solute C and N or by improving the Lankford value. Further, Japanese Unexamined Patent Publication No. S63-89625 discloses a technology of improving flange formability by fining grain sizes by the addition of Nb and B. Further, Japanese Unexamined Patent Publication No. S61-34159 discloses a technology of improving flange formability by fining cementite, and Japanese Unexamined Patent Publication No. S63-310922 discloses a technology of improving flange formability by
specifying the conditions of overaging heat treatment.
In the meantime, Japanese Unexamined Patent Publication No. S63-317625 discloses a technology of avoiding the cracking of a weld by adding Ti, Nb and B and thus increasing the strength of the weld, in regard to the softening of a heat affected zone by welding as the cause of the cracking when a steel sheet for automobile use is subjected to spot welding. However, in case of an ultra-thin material, cracking of a base material becomes conspicuous when the strength of a weld is excessive, and the flange formability is rather deteriorated.
As mentioned above, the mechanism of the improvement of flange formability has not been clarified yet, and thus various measures for the improvement have been taken, but measures do not take the threading performance of an ultra-thin material in an annealing furnace into consideration and a mere addition of Ti, Nb and B makes the recrystallization temperature of a steel sheet high and makes it necessary to raise the annealing temperature and, thus, the strip threading performance in an annealing furnace is markedly deteriorated.
Disclosure of the Invention The present invention, which has been established in view of the above-mentioned situation, provides a steel sheet for a container. The present invention makes it possible to produce an ultra-thin material used for a container having a weld with a high productivity but without the deterioration of the strip threading performance during annealing, to improve the formability of the weld during can manufacturing, and to reduce cracking at the weld which is a problem when it is used. The present invention is a method of improving flange formability at a weld during can forming and fatigue strength at the weld during use by appropriately preparing a base material so that the material quality of
the weld, which is apt to generate a stress concentration during forming or use, may be suitable for the object. That is, the present invention is a method, for a B added ultra-low carbon steel, of further improving the properties by regulating the form of nitrides within an appropriate range and adding elements in very small amounts .
More specifically, the present invention is a steel sheet for a container, excellent in formability and fatigue property at a weld, and a method of producing the steel sheet. The present invention is composed of the following items:
(1) A steel sheet for a container, excellent in formability and fatigue property at a weld, characterized by: containing, in mass,
C: 0.0050% or less,
N: 0.0060% or less, and
B so that the ratio B/N may be within the range from 0.40 to 2.00; and having AlN and BN in the steel satisfy the expression, (N existing as ALN)/(N existing as BN) ≤ 0.40.
(2) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to the item (1), characterized by: further containing, in mass,
Al: 0.040% or less; and the ratio Al/B being 30 or less.
(3) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to the item (1) or (2), characterized by further containing, in mass, 0: 0.0010 to 0.0070%.
(4) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to
any one of the items (1) to (3), characterized by further containing, in mass, Si: 0.015 to 2.00%, Mn: 0.05 to 2.00%, and P: 0.005 to 0.080%.
(5) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to any one of the items (1) to (4), characterized by further containing, in mass,
Ti: 0.010% or less and Nb: 0.010% or less.
(6) A steel sheet for a container according to any one of the items (1) to (5), characterized in that the sulfides contained in the steel satisfy the expression, (S existing as Cu sulfides )/(S existing as Mn sulfides) ≤ 0.10.
(7) A method of producing a steel sheet for a container, excellent in formability and fatigue property at a weld, characterized by controlling the reheating temperature of a slab to 1,100°C or higher in hot-rolling when the steel sheet for a container is produced in the ordinary production processes using a steel containing the components according to any one of the items (1) to (6).
(8) A method of producing a steel sheet for a container, excellent in formability and fatigue property at a weld, characterized by controlling the coiling temperature to 730 °C or lower in hot-rolling when the steel sheet for a container is produced in the ordinary production processes using a steel containing the components according to any one of the items (1) to (6).
(9) A method of producing a steel sheet for a
container, excellent in formability and fatigue property at a weld, characterized by controlling the annealing temperature after cold-rolling to 700°C or lower when the steel sheet for a container is produced in the ordinary production processes using a steel containing the components according to any one of the items (1) to (6).
(10) A steel sheet for a container, excellent in formability and fatigue property at a weld, characterized by: containing, in mass,
C: 0.0005 to 0.040%,
Si: 0.002 to 0.50%,
Mn: 0.03 to 2.00%,
P: 0.002 to 0.080%, S: 0.0100 to 0.0600%,
Al: 0.0010 to 0.0700%, and
N: 0.0020 to 0.0300%; the content of N dissolved in the steel sheet being 20 to
300 ppm; and the balance consisting of Fe and unavoidable impurities.
(11) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to the item (10), characterized by further containing, in mass, one or more of, Nb: 0.0005 to 0.0050%, Ti: 0.0005 to 0.0050%, and B: 0.0010% or less.
(12) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to the item (10) or (11), characterized by further containing, in mass, 0: 0.0015 to 0.0090%.
(13) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to
any one of the items (10) to (12), characterized by further containing, in mass, one or more of, Cu: 0.0005 to 0.050%, Ni: 0.0005 to 0.100%, and Cr: 0.0005 to 0.100%.
(14) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to any one of the items (10) to (13), characterized by further containing, in mass, Sn: 0.0002 to 0.0050%.
(15) A steel sheet for a container, excellent in formability and fatigue property at a weld, according to any one of the items (1) to (14), characterized in that the sulfides contained in the steel satisfy the expression,
(S existing as Cu sulfides )/(S existing as Mn sulfides) <
0.30.
(16) A method of producing a steel sheet for a container, excellent in formability and fatigue property at a weld, according to any one of the items (10) to (15), characterized in that the steel sheet is subjected to a secondary cold-rolling under the reduction ratio of 20% or less after the processes of continuous casting of molten steel, hot-rolling, pickling, cold-rolling and annealing.
(17) A method of producing a steel sheet for a container, excellent in formability and fatigue property at a weld, according to any one of the items (1) to (16), characterized by commencing hot-rolling under the condition that the heat history in the temperature range from 1,000 to 1,300°C after producing a slab by continuously casting molten steel and before commencing the hot-rolling satisfies the expression,
temperature (°C) x time (min.) ≤ 200,000.
(18) A method of producing a steel sheet for a container, excellent in formability and fatigue property at a weld, according to any one of the items (1) to (17), characterized by hot-rolling the steel sheet by controlling the average cooling rate, in the period of time from the commencement of the finish hot-rolling to the coiling after the completion of the finish hot- rolling, to 30°C/sec. or less.
Brief Description of the Drawings
Figure 1 is a view showing the method of evaluating the workability of a weld. Figure 2 is a view showing the method of evaluating a weld by a tensile test.
Figure 3 is a view showing the method of evaluating the fatigue strength of a weld.
Figure 4 is a graph showing the relation between (N existing as ALN)/(N existing as BN) and workability.
Figure 5 is a graph showing the relation between (N existing as ALN)/(N existing as BN) and fatigue strength.
Best Mode for Carrying out the Invention Embodiment 1
The present invention according to claims 1 to 9 will hereunder be explained in detail.
Firstly, chemical components will be explained hereunder. In the explanation, the amount of each chemical component is expressed in terms of mass %.
C content is generally preferred to be as low as possible from the viewpoint of workability, and therefore the upper limit thereof is set at 0.0050%. In particular, when a good ductility with a low aging property is required, the property can be improved remarkably by reducing C content down to 0.0015% or less. However, because an excessive reduction of C content
causes not only a cost to increase but also a steel sheet to soften and hence the strength of a can to deteriorate, the lower limit is preferred to be 0.0030%.
N is an important element to control the formation of nitrides, which is an important requirement of the present invention. Because an excessive content of N causes excessive formation of nitrides and hence the object of the present invention cannot be achieved, the upper limit thereof is set at 0.0060%. When the addition amount of B is comparatively small, as will be explained later, a problem with an aging property caused by the residue of solute N may arise, and therefore it is preferable to control N content to 0.0030% or less in order to reduce the aging effect. Further, if the N content is controlled to 0.0020% or less by applying a vacuum degassing treatment sufficiently, the formation of nitrides is suppressed and, in particular, formability is improved.
B is added as an indispensable element in the preset invention, because B affects the form of nitrides, changes material properties at the heat affected zone of a weld, lowers the recrystallization temperature of a steel sheet' hen it is added properly, hence makes it possible to anneal a steel sheet at a lower temperature, and, as a result, improves the strip threading performance during annealing. However, an excessive addition of B causes a weld to harden excessively, thus workability to deteriorate, a recrystallization temperature to rise necessitating a rise in an annealing temperature, and, as a result, heat-buckling to occur easily. An important point is the ratio of B to N, and the ratio B/N is set at 0.40 to 2.00, preferably 0.60 to 1.40.
An important requirement in the present invention is to control the kind and amount of nitrides, and the ratio of the N amount existing as AlN to the N amount existing as BN in a boron added ultra-low carbon steel must be
0.40 or less, preferably 0.20 or less.
Here, the N amount existing as A1N is a value obtained by analyzing Al amount in a residue when a steel sheet is dissolved in an iodine alcohol solution and then calculating the N amount regarding the whole Al amount as a constituent of AlN. Likewise, the N amount existing as BN is a value obtained by analyzing the B amount in a residue when a steel sheet is dissolved in an iodine alcohol solution and then calculating N amount regarding the whole B amount as a constituent of BN.
As mentioned above, in order to control nitrides, the addition amounts of Al and B, the ratio between them, the oxides which act as the precipitation nuclei of nitrides, namely the 0 content in a steel, and heat history throughout the all production processes are important factors. By controlling Al/B to 30 or less, preferably 20 or less, and Al to 0.040% or less, preferably 0.020% or less, solute N existing excessively in a steel combines with B more preferentially than Al when nitrides precipitate, and, by so doing, the kind and amount of nitrides can be controlled desirably.
0 is effective for controlling nitrides when the content is within the range from 0.0010 to 0.0070%. The reason is thought to be that 0 in a steel exists as oxides containing Si, Al, Mn, and Fe and further microelements such as Ca, Mg, etc., acts effectively as the precipitation nuclei of nitrides when the existing 0 amount is appropriate, and thus makes a desirable control of nitrides possible. However, an excessive amount of 0 in a steel coarsens oxides, acts as the origin of cracks during working, and hence markedly deteriorates the product quality. Therefore, the upper limit of 0 content is set at 0.0070%.
In order to desirably control the form of oxides as mentioned above or to improve workability and fatigue strength by adjusting the strength of a base steel sheet and thus mitigating stress concentration in the vicinity
of a weld, Si, Mn, P, etc. can be added. The addition amounts are set at Si: 0.015 to 2.00%, Mn: 0.05 to 2.00% and P: 0.005 to 0.080%, respectively. When the addition amounts miss the ranges, the form of oxides changes, or a weld softens or hardens unusually and, therefore, desired properties cannot be obtained.
The provisions of the upper limits of Ti and Nb, which are added for improving drawability when draw- forming or the like is involved or unavoidably contained in a steel by coming from scraps, etc. during a steelmaking process, are also important requirements in the present invention. Therefore, the content of each element is set at 0.010% or less. When each content exceeds the upper limit, the recrystallization temperature of a steel sheet rises, the strip threading performance in an annealing process markedly deteriorates, a crystal structure coarsens and softens extraordinarily by the influence of heat in the vicinity of a weld, the stress concentration is accelerated at the portion, and, as a result, formability and fatigue strength sometimes deteriorate.
Further, with regard to sulfides in a steel, suppressing the formation of Cu sulfides is also important. In general, S in a steel must be fixed as sulfides in relation to the hot-rolling performance and therefore it is preferable to fix S as MnS in the present invention.
In the present invention, the ratio of (S existing as Cu sulfides) to (S existing as MnS) is set at 0.10 or less. The reason is that the fine precipitation of Cu sulfides causes not only a recrystallization temperature of a steel sheet to rise but also complex-precipitates with B and Al nitrides to form and thus the form of nitrides to be undesirable. Here, (S existing as Cu sulfides) is a value obtained by quantitatively measuring the Cu amount in a residue obtained by the electrolytic extraction of a
steel sheet and then converting the Cu amount into S amount using the expression, Cu/S = 2/1, and (S existing as Mn sulfides) a value obtained by quantitatively measuring Mn amount in a residue obtained by the electrolytic extraction of a steel sheet and then converting the Mn amount into S amount using the expression, Mn/S = 1/1.
The production processes in the present invention include hot-rolling, coiling, pickling, cold-rolling, annealing and skin-pass, etc. which are generally employed.
With regard to a heat history in the production processes, the influences of a reheating temperature of a slab, a coiling temperature during hot-rolling and an annealing temperature after cold-rolling are predominant, and, by restricting a reheating temperature of a slab during hot-rolling to 1,100°C or higher, a coiling temperature during hot-rolling to 730 °C or lower and an annealing temperature after cold-rolling to 700 °C or lower, the workability and fatigue strength of a weld can be further improved. Though the reasons are not clear, it is thought that the forms of nitrides or of precipitates other than nitrides influence the improvement . By restricting an annealing temperature after cold- rolling to 700°C or lower, the occurrence of heat- buckling is suppressed and thus the strip threading performance in an annealing process is improved, and therefore the industrial significance is considerable. As mentioned above, though the mechanism of improving workability and fatigue strength at a weld by controlling the form of nitrides has not been clarified, as a phenomenon, the hardness of a material at a weld and at a heat affected zone in the vicinity thereof is properly adjusted and, by so doing, the stress concentration to the portions is mitigated and a desirable hardness can be obtained. At a weld and in the
vicinity thereof, nitrides are dissolved by the temperature rise during welding and the hardness is determined by fine nitrides wherein both solute N and solute B remain without fully dissolved and fine nitrides precipitating again during cooling. Therefore, in order to obtain the desirable and preferable forms of solute N, solute B and nitrides, it is necessary to control in advance the form of nitrides in a steel before welding as specified in the present invention. In the production of a thin steel sheet for a container, there are some cases where a steel sheet which is subjected to 2CR rolling after annealing for securing the strength of a container and hardened by the work- hardening is used. In such a steel sheet too, the effects of improving workability and fatigue strength at a weld can be obtained by the present invention. Further, in case of adding elements for improving corrosion resistance and other properties too, the effects of the present invention are not lost. Even when Sn, W, Mo, Ca, Cr, Ni, V, Sb, etc. are contained for improving the properties which are not referred to in the description of the present invention, such as the workability including drawability and secondary workability and the corrosion resistance of a steel sheet, a strip threading performance in each process, and the like, the effects of the present invention are not lost at all. However, when these elements are added excessively, a strip threading performance during annealing worsens because of the rise of a recrystallization temperature, and therefore it is preferable to control each amount of the elements to 0.10% or less and the total amount thereof to 0.50% or less.
A steel sheet according to the present invention is generally used as the substrate of a surface treated steel sheet and, in that case too, the effects of the present invention are not spoiled at all by the surface
treatment. As a surface treatment for a can, a treatment by tin, chromium (tin-free), nickel, zinc, aluminum or the like is adopted. Further, as the substrate of a laminated steel sheet covered by an organic film, which has come to be used recently, a steel sheet according to the present invention can be adopted without spoiling the effects of the present invention.
Embodiment 2 The present invention according to claims 10 to 16 will hereunder be explained in detail.
Firstly, chemical components will be explained hereunder. In the explanation, the amount of each chemical component is expressed in terms of mass %. C, when the amount exceeds 0.040%, coarsens carbides and acts as the origin of fracture at a site where stress is concentrated in the vicinity of a weld. On the other hand, an excessive reduction of C content causes the cost to increase and hence the lower limit is set at 0.0005%. Si is generally preferred to be as low as possible from the viewpoint of corrosion resistance. However, when a steel sheet is applied to a so-called laminated steel sheet, the surface of which is coated with a resin film, and the demand for which is increasing recently, the Si content is preferred to be high from the viewpoint of suppressing the deterioration of corrosion resistance and suppressing stress concentration at a weld. On the other hand, an excessive reduction of Si makes it difficult to control the form of oxides desirably as it will be mentioned later, and therefore the Si content is set at 0.002 to 0.5%.
Mn has the same effect as Si and the most proper range is determined to be 0.03 to 2.00%, preferably 0.05 to 1.00%. P is preferred to be as low as possible from the viewpoint of corrosion resistance and stress concentration at a weld. However, P is a useful element
for adjusting the strength of a steel sheet at a low cost. The regulation range of P content is set at 0.002 to 0.080%, preferably 0.002 to 0.030%.
S forms sulfides by combining with Mn, Cu, Ti, etc. in a steel. S is an indispensable and important element in the present invention for mitigating stress concentration at a weld by having an appropriate amount of sulfides exist in a steel. S content of at least 0.0100% is required for obtaining the effects of the present invention. However, an excessive amount of S content sometimes coarsens sulfides and acts as the origin of fracture, and therefore the upper limit is set at 0.0600%.
Al, when the amount is too low in relation to the amount of oxygen, which will be explained later, makes deoxidization at a steelmaking process insufficient. On the other hand, when the amount is excessive, not only is solute N not secured but also fine AlN is formed abundantly and the recrystallization temperature of a steel sheet rises, and this results in remarkable deterioration of a strip threading performance in an annealing process. Therefore, Al content is determined to be in the range from 0.0010 to 0.0700%.
N is an important element for controlling solute N which is one of the important requirements in the present invention. N is added by 0.0020% or more because the effects of the present invention become insufficient when the addition amount is low. On the other hand, when N is contained abundantly, Fe nitrides are generated in a great amount even when Al content is low and they act as the origin of fracture at a weld. Therefore, the upper limit is set at 0.0300%. Here, with regard to a method of adding N, N can be added either by adding N at the stage of molten steel as it is applied to a regular steel sheet or by adding N at a heat treatment of' a steel sheet in an atmosphere containing ammonia, that is, by nitriding.
The amount of solute N is calculated by subtracting the amount of precipitated N, which can be measured by a method of dissolving a steel in a bromine ester solution, from the total N amount in a steel. When a solute N amount is small, the softening in the vicinity of a weld cannot be suppressed, but in tern, when it is excessive, aging becomes large and ductility deteriorates. Therefore, the amount of solute N is restricted in the range from 20 to 300 ppm. Since Nb, Ti and B form precipitates by combining with N and moreover Ti forms precipitates by combining with S, a trace addition amount of the elements act effectively on controlling the solute N and the form of sulfides and make the effects of the present invention more conspicuous. On the other hand, when the addition amount is excessive, the solute N and the form of sulfides become undesirable, and therefore, undesirably, not only the effects of the present invention tend to be spoiled but also the recrystallization temperature of a steel sheet tends to rise and a strip threading performance during annealing tends to deteriorate. The desirable ranges of the elements are as follows; Nb: 0.0005 to 0.0050%, Ti: 0.0005 to 0.0050% and B: 0.0010% or less. 0 is an important element for properly controlling the form of oxides, which is one of the important factors of the present invention. When the 0 content is too small, the amount of oxides for suppressing the softening of a material caused by the heat generation of welding is insufficient and thus a sufficient effect cannot be obtained. On the other hand, when the 0 content is excessive, the oxides become the origin of fracture during forming. Therefore, when 0 is added, the range of the content is determined to be from 0.0015 to 0.0090%, preferably from 0.0030 to 0.0090%.
Though the reason why an oxygen amount produces a remarkable effect in the present invention has not been
determined yet, it is thought that fine oxides suppress grain growth in a high temperature range during welding, the fine oxides themselves do not change their forms with such a temperature rise as is obtained in the vicinity of a weld, and thus the effect of suppressing softening is maintained. Actually, the prescription on the sizes, numbers and densities of the oxides of Fe, Al, Si and Mn becomes important, and as far as the contents of Al, Si, Mn and 0 are controlled within the ranges specified by the present invention, the forms of the oxides can be controlled desirably and preferable effects can be obtained as long as usual production conditions are adopted.
Cu, Ni and Cr have the functions of improving the corrosion resistance of a steel sheet and also of suppressing the softening of a material during welding, and therefore the elements are added as occasion demands. Since an excessive addition of the elements causes the deterioration of the ductility of a material, it is preferable, when the elements are added, to control them within the following ranges; Cu: 0.0005 to 0.050%, Ni: 0.0005 to 0.10% and Cr: 0.0005 to 0.100%.
Sn is an element which generally segregates at grain boundaries. As Sn has the effects of suppressing the abnormal grain growth caused by heat generation during welding and hence suppressing the softening of a material, the element can be contained in a steel. As an excessive addition of Sn causes ductility to deteriorate, it is preferable, when it is added, to control the element within the range from 0.0002 to 0.0050%.
In a steel sheet according to the present invention, by properly controlling the form of sulfides, the softening of a weld can be suppressed and hence the desirable properties can be obtained. Though the size, number and density, etc. of sulfides can be specified, in the present invention, the sulfides in a steel are regulated by the expression, (S existing as Cu
sulfides )/(S existing as Mn sulfides) < 0.30. Though the reason of the above-mentioned effect is not clear, it is estimated that, as CuS is inferior to MnS in the stability at a high temperature, CuS dissolves or coarsens with a temperature rise during welding and the effect of suppressing the softening of a material is apt to be lost.
Here, (S existing as Cu sulfides) is a value obtained by quantitatively measuring Cu amount in a residue obtained by the electrolytic extraction of a steel sheet and then converting the Cu amount into S amount using the atom ratio, Cu/S = 2/1, and (S existing as Mn sulfides) a value obtained by quantitatively measuring Mn amount in a residue obtained by the electrolytic extraction of a steel sheet and then converting the Mn amount into S amount using the atomic ratio, Mn/S=l/1.
A method of satisfying the expression, (S existing as Cu sulfides )/(S existing as Mn sulfides) < 0.30, is not particularly restricted and, it can be attained, for instance, by specifying the chemical components, notably the ratio between Mn and Cu. Also, it can be attained by controlling hot-rolling conditions, in particular an average cooling rate between the entry of hot-rolling and the start of coiling (for instance, controlling the cooling rate to 10 to 50°C/sec. or the like), or by a combination thereof.
A steel according to the present invention is produced through the processes of; continuous casting of molten steel, hot-rolling, pickling, cold-rolling, annealing and, after that, secondary cold-rolling for controlling the shape or the material properties of a steel sheet. Here, in case of a steel sheet which is subjected to a high reduction ratio in the secondary cold-rolling, namely which is hardened by a work hardening effect, the material properties are easily recovered in the vicinity of a weld by the temperature
rise during welding and the steel sheet tends to soften. Therefore, the reduction ratio is preferred to be 20% or less.
Even when W, Mo, Ca, V, Sb, etc. are contained for improving the properties which are not referred to in the description of the present invention, such as the workability including drawability and secondary workability and the corrosion resistance of a steel sheet, a strip threading performance in each process, and .the like, the effects of the present invention are not lost at all.
A steel sheet according to the present invention is generally used as the substrate of a surface treated steel sheet and, in that case too, the effects of the present invention are not spoiled at all by the surface treatment. As a surface treatment for a can, a treatment by tin, chromium (tin-free), nickel, zinc, aluminum or the like is adopted. Further, as the substrate of a laminated steel sheet covered by an organic film, which has been used recently, a steel sheet according to the present invention can be adopted without spoiling the effects of the present invention.
The present invention according to claims 17 and 18 will hereunder be explained in detail. A steel according to the present invention is characterized by dispersing the second phase composed of oxides, nitrides, sulfides and the like in a base phase mainly composed of Fe and, in order to obtain the effects of the present invention, their forms must be controlled properly. For the purpose, it is particularly effective to control the heat history before a hot-rolling process. As an example, it is preferable to carry out a hot- rolling by commencing the hot-rolling under the condition that the heat history in the temperature range from 1,000 to 1,300°C after producing a slab by continuously casting molten steel and before commencing the hot-rolling satisfies the expression, temperature (°C) x time (min.)
≤ 200,000, and by controlling the average cooling rate in the period of time from the commencement of the finish hot-rolling to the coiling after the completion of the finish hot-rolling to 30°C/sec. or less. Though the reason is not clear, it is thought that, when a steel sheet is retained at a high temperature for a long time, sulfides and nitrides coarsen and disperse as the second phase in extremely large size, in particular, making oxides act as their precipitation nuclei, and, by so doing, the effect of suppressing the softening of a steel sheet caused by the influence of the heat during welding decreases. In general, it is not preferable that the cooling rate is excessively high in the temperature range of about 1,000°C or lower after finish hot-rolling. It is thought that this is because, when the cooling rate in the temperature range is high, the sizes of nitrides and sulfides become very fine when formerly dissolved N and S precipitate as nitrides and sulfides, they dissolve even by the heat during the welding of a product sheet, and the effect of suppressing the softening of a material tends to disappear. In particular, when a cooling rate at this stage is high, S is apt to form Cu sulfides, and therefore the stability against the heat during welding is further decreased. Considering the above situations, as a method of controlling sulfides and nitrides into appropriate shapes, it is recommendable that the method of carrying out a hot-rolling by commencing the hot- rolling under the condition that the heat history in the temperature range from 1,000 to 1,300°C after producing a slab by continuously casting molten steel and before commencing the hot-rolling satisfies the expression, temperature (°C) x time (min.) ≤ 200,000, and controls the average cooling rate in the period of time from the commencement of the finish hot-rolling to the coiling after the completion of the finish hot-rolling to
30°C/sec. or less. Here, the method of controlling the
heat history in the temperature range from 1,000 to 1,300°C after producing a slab by continuously casting molten steel and before commencing the hot-rolling so as to satisfy the expression, temperature (°C) x time (min.) ≤ 200,000, includes so-called direct rolling (CC-DR), wherein hot-rolling is commenced without soaking a slab in a reheating furnace or the like after casting, and so- called thin slab continuous casting, wherein a hot- rolling process is simplified or eliminated by making the thickness of a cast slab thin.
Example 1
The workability of a weld was evaluated, as shown in Figure 1, by welding a quadrangular steel sheet with seam welding and forming it into a cylindrical shape by the bond along a weld seam, as in the case of manufacturing the can drum of a regular three-piece beverage can, expanding an opening by thrusting a conical die into the opening, and calculating the amount of deformation until cracking occurred at the opening end using the following expression;
{(Diameter at crack generation) - (Initial diameter )}/( Initial diameter) (1).
The strength of a weld was evaluated, as shown in Figure 2, by welding two quadrangular steel sheets with spot welding at a welding current just lower than the current at which welding burrs occurred, and measuring the maximum load at a tensile test.
The fatigue strength of a weld was evaluated, as shown in Figure 3, by cutting out a strap 20 mm in width having a weld in the center from a welded cylindrical can drum formed as shown in Figure 1, subjecting the strap to a fatigue tensile test by one side oscillation, and measuring the maximum load at which it can withstand 10 million cycles.
Heat buckling was judged by whether or not heat buckling occurred when cold-rolled coils having identical
thickness and width were passed through an identical annealing line at the temperature of the recrystallization temperature + 40 °C, and the results were expressed by the marks, O: no occurrence, Δ: little occurrence and x: frequent occurrence.
The effects of the present invention were evaluated by synthetically judging the above-mentioned four evaluation items and expressed by the marks, © : excellent (invented steels), O: good (invented steels), Δ: good in some of the evaluation items (invented steels) and x: ordinary. level (comparative steels).
Example 1-1
The steels having the chemical components shown in Table 1 were cast into slabs 250 mm in thickness, then hot-rolled sheets 2.0 mm in thickness were produced at the slab reheating temperature of 1,150°C and the coiling temperature of 650°C, and then steel sheets 0.16 mm in thickness were produced through the processes of pickling, cold-rolling at the reduction ratio of 92%, annealing at 680°C for 1 min., and then skin pass rolling at the reduction ratio of 3%, and the produced steel sheets were evaluated.
As it is clear from Table 2, the steels produced within the ranges specified in the present invention show excellent properties in all the evaluation items such as the workability, the strength and the fatigue strength of the welds and the heat-buckling resistance.
Example 1-2
The steels having various amounts of Ti and Nb as shown in Table 3 were evaluated. The production conditions were the same as those of Example 1.
As it is clear from Table 4, the steels produced within the preferable ranges show excellent properties in all the evaluation items such as the workability, the
strength and the fatigue strength of the welds and the heat-buckling resistance.
Example 1-3 The steels having various ratios of CuS to MnS as shown in Table 5 were evaluated. The production conditions were the same as those of Example 1.
As it is clear from Table 6, the steels produced within the preferable ranges show excellent properties in all the evaluation items such as the workability, the strength and the fatigue strength of the welds and the heat-buckling resistance.
Example 1-4 The steels produced under the different conditions in and after hot-rolling were evaluated. The production conditions other than the slab reheating temperature and the coiling temperature in hot-rolling and the annealing temperature after cold-rolling were the same as those of Example 1. The results are shown in Figures 4 and 5.
Figure 4 shows the relation between (N existing as AlN)/(N existing as BN) and the workability and Figure 5 the relation between (N existing as AlN)/(N existing as BN) and the fatigue strength. The production conditions 1 and 2 in these figures are as follows;
Production condition 1: slab reheating temperature >
1,100°C, or coiling temperature < 730°C, or annealing temperature <
700°C, Production condition 2: slab reheating temperature <
1,100°C, and coiling temperature >
730°C, and annealing temperature >
700°C. As it is clear from Figures 4 and 5, the steels produced within the preferable ranges show excellent properties in the workability and the fatigue strength of the welds.
As explained above, by the present invention, it is possible to reduce the bad formability caused by welding and the fracture during the usage of a can having a weld. Further, as a steel according to the present invention has excellent properties even at an annealing temperature lower than an ordinary annealing temperature, the occurrence of heat buckling can be avoided and an ultra- thin material for a container can be produced with a high efficiency.
*1: Heat history in the temperature range from 1,000 to 1,300°C until hot-rolling commences: temperature (°C) x time (min. ) /10,000 *2: Average cooling rate in the period of time from the commencement of the finish hot- rolling to the coiling after the completion of the finish hot-rolling: °C/sec.
-J
Example 2
The workability of a weld was evaluated, as shown in Figure 1, by forming a quadrangular steel sheet into a cylindrical shape with seam welding, as in the case of manufacturing the can drum of a regular three-piece beverage can, expanding an opening by thrusting a conical die into the opening, and calculating the amount of deformation until cracking occurred at the opening end using the following expression; {(Diameter at crack generation) - (Initial diameter) }/ (Initial diameter) (1).
The strength of a weld was evaluated, as shown in Figure 2, by welding two quadrangular steel sheets with spot welding at a welding current just lower than the current at which welding burrs occurred, and measuring the maximum load at a tensile test.
The fatigue strength of a weld was evaluated, as shown in Figure 3, by cutting out a strap 20 mm in width having a weld in the center from a welded cylindrical can drum formed as shown in Figure 6, subjecting the strap to a fatigue tensile test at one side oscillation, and measuring the maximum load at which it can withstand 10 million cycles.
Heat buckling was judged by whether or not heat buckling occurred when cold-rolled coils having identical thickness and width were passed through an identical annealing line at the temperature of the recrystallization temperature + 40 °C, and the results were expressed by the marks, O: no occurrence, Δ: little occurrence and x: frequent occurrence.
The effects of the present invention were evaluated by synthetically judging the above-mentioned four evaluation items and expressed by the marks, © : excellent (invented steels), O: good (invented steels), Δ: good in some of the evaluation items (invented steels) and x: ordinary level (comparative steels).
Concrete example 5
The steels having the chemical components shown in Table 7 were cast into slabs 250 mm in thickness, then hot-rolled sheets 2.2 mm in thickness were produced at the slab reheating temperature of 1,150°C and the coiling temperatures of 520 to 730°C, and then steel sheets 0.16 mm in thickness were produced through the processes of pickling, cold-rolling at the reduction ratio of 92%, annealing at 660 to 720°C for 1 min., and rolling at the reduction ratio of 10%, and the produced steel sheets were evaluated. The results are shown in Table 8.
As it is clear from Table 8, the steels produced within the ranges specified in the present invention show excellent properties in all the evaluation items such as the workability, the strength and the fatigue strength of the welds and the heat-buckling resistance.
Table 7
Table 8
*1: Heat history in the temperature range from 1,000 to 1,300°C until hot-rolling commences: temperature (°C) x time (min. ) /10 , 000 *2: Average cooling rate in the period of time from the commencement of the finish hot- rolling to the coiling after the completion of the finish hot-rolling: °C/sec.
Concrete example 6
The steels having various amounts of 0 as shown in Table 9 were evaluated. The production conditions were the same as those of Example 1. The results are shown in Table 10.
As it is clear from Table 10, the steels produced within the preferable ranges show excellent properties in all the evaluation items such as the workability, the strength and the fatigue strength of the welds and the heat-buckling resistance.
Table 10
*1: Heat history in the temperature range from 1,000 to 1,300°C until hot-rolling commences: temperature (°C) x time (min. )/10, 000 *2: Average cooling rate in the period of time from the commencement of the finish hot- rolling to the coiling after the completion of the finish hot-rolling: °C/sec.
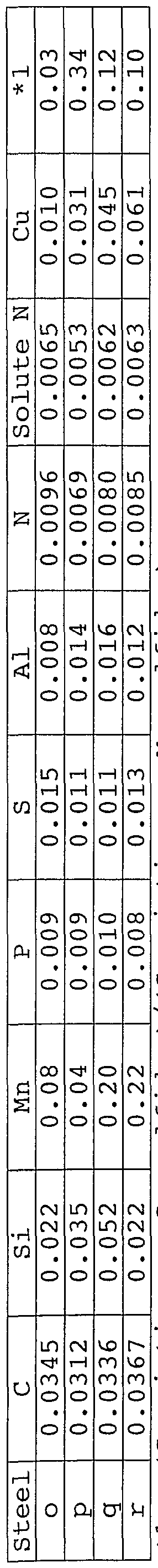
Concrete example 7
The steels having various amounts of Cu as shown in Table 11 were evaluated. The production conditions were the same as those of Example 1. The results are shown in Table 12.
As it is clear from Table 12, the steels produced within the preferable ranges show excellent properties in all the evaluation items such as the workability, the strength and the fatigue strength of the welds and the heat-buckling resistance.
*1: (S existing as Cu sulfides )/(S existing as Mn sulfides)
Table 12
*1: Heat history in the temperature range from 1,000 to 1,300°C until hot-rolling commences: temperature (°C) x time (min. ) /10 , 000 *2: Average cooling rate in the period of time from the commencement of the finish hot- rolling to the coiling after the completion of the finish hot-rolling: °C/sec.
As explained above, by the present invention, it is possible to reduce the bad formability caused by welding and the fracture during the usage of a can having a weld. Further, since a steel according to the present invention has excellent properties even at an annealing temperature lower than an ordinary annealing temperature, the occurrence of heat buckling can be avoided and an ultra- thin material for a container can be produced with a high efficiency.