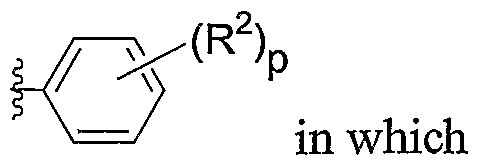APPLICATION FOR PATENT
Substituted 3-Pyridyl Pyrroles and 3-
Pyridyl Pyrazoles as CI 7,20 Lyase
Inhibitors
Background of the Invention Steroid biosynthesis begins in cells of the adrenal gland where the initial product in sterol biosynthesis, cholesterol, is converted into the adrenal steroid hormones aldosterone, hydro cortisone, and corticosterone by a series of P 50 -mediated hydroxylation steps. The cholesterol side-chain cleavage activity that represents the first step in steroid hormone biosynthesis is a P450 -mediated oxidation and cleavage of a pair of adjacent methylene groups to two carbonyl fragments, pregnenolone and isocaprylaldehyde (see Walsh (1979) Enzymatic Reaction Mechanisms; W.H. Freeman and Company, pp. 474-77). Another critical set of enzymatic conversions in steroid metabolism is facilitated by 17-alpha- hydroxylase-17,20-lyase (CYP 17, P450 17). CYP 17 is a bifunctional enzyme which possesses both a C17,20-lyase activity and a C17-hydroxylase activity. Significantly, these two alternative enzymatic activities of CYP 17 result in the formation of critically different intermediates in steroid biosynthesis and each activity appear to be differentially and developmentally regulated (see e.g. PAllemand et al. (2000) Eur. J. Clin. Invest. 30: 28-33).
The C17,20-lyase activity of CYP 17 catalyzes the conversion of 17α-hydroxy- pregnenolone and 17α-hydroxy-progesterone to dehydroepiandrosterone (DHEA) and delta4-androstenedione (androstenedione) respectively. Both DHEA and androstenedione lyase products are key intermediates in the synthesis of not only the androgens testosterone and dihydrotestosterone (DHT), but also the estrogens 17-beta-estradiol and estrone. Indeed, adrenal and ovarian estrogens are the main sources of estrogens in postmenopausal women (see e.g. Harris et al. (1988) Br. J. Cancer 58: 493-6). In contrast, the C17-hydroxylase activity of CYP 17 catalyzes the conversion of the common intermediate progesterone to 17- hydroxyprogesterone, a precursor of cortisol. Therefore the first activity of CYP 17, the C17-hydroxylase activity, promotes the formation of glucocorticoids while the second
activity of CYP 17, the C17,20-lyase activity, promotes the formation of sex hormones - particularly androgens including testosterone as well as estrogens.
Prostate cancer is currently one of the most frequently diagnosed forms of cancer in men in the U.S. and Europe. Prostate cancer is typically androgen-dependent and, accordingly, the reduction in androgen production via surgical or pharmacological castration remains the major treatment option for this indication. However, complete rather than partial withdrawal of androgens may be more effective in treating prostate cancer (Labrie, F. et al, Prostate, 1983, 4, 579 and Crawford, E.D. et al, N. Engl J. Med., 1989, 321, 419). Phannacological inhibition of CYP 17 may be a promising alternative treatment to antiandrogens and LHRH agonists in that testicular, adrenal, and peripheral androgen biosynthesis would be reduced rather than only testicular androgen production (Njar V, et al, J. Med. Chem., 1998, 41, 902). One such CYP 17 inhibitor, the fungicide ketoconazole, has been used previously for prostate cancer treatment (Trachtenberg, J., J. Urol, 1984, 132, 61 and Williams, G. et al, Br. J. Urol, 1986, 58, 45). However, this drug is a relatively non-selective inhibitor of cytochrome P450 (CYP) enzymes, has weak CYP 17 activity, and has a number of notable side effects associated with it including liver damage (De Coster, R. et al, J. Steroid Biochem. Mol. Biol, 1996, 56, 133 and Lake-Bakaar, G. et al, Br. Med. J., 1987, 294, 419).
The importance of potent and selective inhibitors of CYP 17 as potential prostate cancer treatments has been the subject of numerous studies and reviews (Njar, V. et al, Curr. Pharm. Design, 1999, 5, 163; Barrie, S.E. et al, Endocr. Relat. Cancer, 1996, 3, 25 and Jarman, M. et al, Nat. Prod. Rep., 1998, 495). Finasteride, a 5α-reductase inhibitor, is an approved treatment for benign prostatic hyperplasia (BPH), although it is only effective with patients exhibiting minimal disease. While finasteride reduces serum DHT levels, it increases testosterone levels, and may therefore be insufficient for prostate cancer treatment (Peters, D. H. et al, Drugs, 1993, 46, 177). Certain anti-androgenic steroids, for example, cyproterone acetate (17α-acetoxy-6-chloro-lα, 2α-methylene-4,6-pregnadiene-3,20-dione), have been tested as adjuvant treatments for prostate cancer. Many other steroids have been tested as hydroxylase/lyase inhibitors. See, for example, PCT Specification WO 92/00992 (Schering AG) which describes anti-androgenic steroids having a pyrazole or triazole ring fused to the A ring at the 2,3 -position, or European specifications EP-A288053 and EP-
A413270 (Menell Dow) wliich propose 17β-cyclopropylamino-androst-5-en-3β-ol or -4-en- 3 -one and their derivatives.
In addition to the use of CYP 17 inhibitors in the treatment of prostate cancer, a second potential indication would be for estrogen-dependent breast cancer. In postmenopausal patients with advanced breast cancer, treatment with high doses of ketoconazole resulted in suppression of both testosterone and estradiol levels, implicating CYP17 as a potential target for hormone therapy (Harris, A. L. et al, Br. J. Cancer, 1988, 58, 493).
Chemotherapy is usually not highly effective, and is not a practical option for most patients with prostate cancer because of the adverse side effects which are particularly detrimental in older patients. However, the majority of patients initially respond to hormone ablative therapy although they eventually relapse, as is typical with all cancer treatments (McGuire, in: Hormones and Cancer,. Iacobelli et al. Eds.; Raven Press, New York, 1980, Vol. 15, 337-344). Current treatment by orchidectomy or administration of gonadotropin- releasing hormone (GnRH) agonists results in reduced androgen production by the testis, but does not interfere with androgen synthesis by the adrenals. Following three months of treatment with a GnRH agonist, testosterone and DHT concentrations in the prostate remained at 25% and 10%, respectively, of pretreatment levels (Forti et al, J. Clin. Endocrinol Metab., 1989, 68, 461). Similarly, about 20% of castrated patients in relapse had significant levels of DHT in their prostatic tissue (Geller et al, J. Urol, 1984, 132, 693). These findings suggest that the adrenals contribute precursor androgens to the prostate. This is supported by clinical studies of patients receiving combined treatment with either GnRH or orchidectomy and an anti-androgen, such as flutamide, to block the actions of androgens, including adrenal androgens. Such patients have increased progression-free survival time compared to patients treated with GnRH agonist or orchidectomy alone (Crawford et al. , N Engl J. Med., 1989, 321, 419 and Labrie et al, Cancer Suppl, 1993, 71, 1059).
Although patients initially respond to endocrine therapy, they frequently relapse. It was reported recently that in 30% of recurring tumors of patients treated with endocrine therapy, high-level androgen receptor (AR) amplification was found (Visakorpi, et al, Nature Genetics, 1995, 9, 401). Also, flutamide tends to interact with mutant ARs, and stimulate prostatic cell growth. This suggests that AR amplification may facilitate tumor cell growth in low androgen concentrations. Thus, total androgen blockade as first line
therapy may be more effective than conventional androgen deprivation by achieving maximum suppression of androgen concentrations which may also prevent AR amplification. It is presently unclear whether sequential treatment with different agents can prolong the benefits of the initial therapy. This strategy has been found effective in breast cancer treatment. New agents which act by different mechanisms could produce second responses in a portion of relapsed patients. Although the percentage of patients who respond to second-line hormonal therapy may be relatively low, a substantial number of patients may benefit because of the high incidence of prostate cancer. Furthermore, there is the potential for developing more potent agents than current therapies, none of which are completely effective in blocking androgen effects.
The need exists for C 17,20 lyase inhibitors that overcome the above-mentioned deficiencies.
Summary of the Invention The invention provides substituted 3-pyridyl heterocyclic compounds which inhibit the lyase activity of enzymes, e.g., 17α-hydroxylase-C 17,20 lyase. Compounds of the invention have the formula I
(I) in which X represents CH or N.
When X is CH,
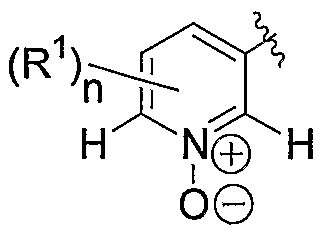
L represents a bond or a linker of formula -(CH2)m- in which m is 1 or 2 ; and A represents
H N H in which R1 is C M alkyl or C -5 cycloalkyl; and n is
0, l, or 2; or
, provided that G is other than a pyridyl or an N- oxide-containing group; and
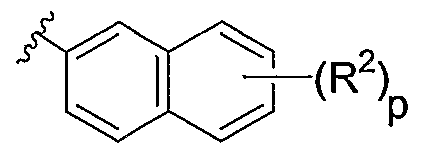
G represents
-(R2)r
ATA / in which R2 is C^ alkyl, -4. alkoxy, halogen, NO2, or
CN; and p is O, 1, or 2;
in which R
3 is H, , or C
M alkyl;
-e alkyl; C
3.
6 cycloalkyl; Q2.
4 alkenyl; or
When X is N,
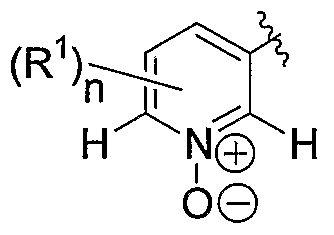
L represents abond; A represents
<R,, HS NΪ H in which R1 is C 1- alkyl or C3-5 cycloalkyl; and n is
0, 1, or 2; or
_ provided that G is other than a pyridyl or an N- oxide-containing group; and G represents
in which R
2 is Ci-
4 alkyl, Ci-
4 alkoxy, halogen, NO
2, or CN; and p is O, l, or 2;
Pharmaceutically acceptable salts of these materials are also within the scope of the invention.
The invention also provides pharmaceutical compositions for inhibiting lyase activity, comprising a compound of the invention and a pharmaceutically acceptable carrier.
The invention also provides methods for inhibiting lyases, comprising contacting the lyase with a compound of the invention. More particularly, the invention provides a method of inhibiting a 17α-hydroxylase-C 17,20 lyase, comprising contacting a 17α-hydroxylase- C 17,20 lyase with a compound of the invention.
The invention further provides methods for treating diseases which can benefit from an inhibition of a lyase enzyme. Exemplary diseases are lyase-associated diseases, e.g., diseases resulting from an excess of androgens or estrogens. For example, the invention provides a method for treating cancer in a subject, comprising administering to the subject a pharmaceutically effective amount of a compound of the invention, such that the cancer is treated.
The method of treatment may be applied where the subject is equine, canine, feline, or a primate, in particular, a human. The cancer may, for example, be prostate or breast cancer. Accordingly, a method for treating prostate cancer in a subject, comprises administering to the subject a therapeutically effective amount of a compound of the invention, such that the prostate cancer in the subject is treated. Similarly, a method for treating breast cancer in a subject comprises administering to the subject a therapeutically effective amount of a compound of the invention, such that the breast cancer in the subject is treated.
Detailed Description of the Invention
The invention is based at least in part on the discovery that substituted 3-pyridyl heterocyclic compounds inhibit the enzyme 17α-hydroxylase-C 17,20 lyase.
In a preferred embodiment, compounds of the invention have the formula I
A^X" L_G
(I) in which X represents CH or N. When X is CH,
L represents a bond or a linker of formula -(CH2)m- in which m is 1 or 2 ; and A represents
in which R
1 is C
1.
4 alkyl or C
3-
5 cycloalkyl; and n is 0, 1, or 2; or
, provided that G is other than a pyridyl or an N- oxide-containing group; and G represents
in which R is Cμ alkyl, Cμ alkoxy, halogen, NO
2, or
CN; and p is 0, 1, or 2;
When X is N, L represents a bond;
A represents
H N H i
n hich R
1 is C
1-4 alkyl; and n is 0, 1, or 2; or
and G represents
in which R
2 is halogen, and p is 0, 1, or 2. Pharmaceutically acceptable salts of these materials are also within the scope of the invention.
In a more preferred embodiment, compounds of the invention have the formula I
(I) in which X represents CH, and
L represents a bond or a linker of formula -(CH2)m- in which m is 1 or 2 ; and A represents
H N H in which R1 is C alkyl or C3.5 cycloalkyl; and n is l; or
, provided that G is other than an N-oxide- containing group; and G represents
R
2 is Cμ alkyl, C
M alkoxy, halogen, NO
2, or
CN; and p is O, 1, or 2;
is H,
, or CM alkyl;
Pharmaceutically acceptable salts of these materials are also within the scope of the invention.
In a most preferred embodiment, compounds of the invention have the formula
in which X represents CH and
L represents a bond or a linker of formula -(CH2)m- in which m is 1 or 2 ; and A represents
(R,) H rt N H in which R1 is C M alkyl or C3.5 cycloalkyl; and n is 1; or
is C
M alkyl, C alkoxy, halogen, NO
2, or
CN; and p is 0, 1, or 2. Pharmaceutically acceptable salts of these materials are also within the scope of the invention.
Definitions
The invention is based at least in part on the discovery that substituted 3-pyridyl pyrroles and pyrrazoles compounds inhibit the enzyme 17α-hydroxylase-C 17,20 lyase.
For convenience, certain terms employed in the specification, examples, and appended claims are collected here.
The term "agonist" of an enzyme refers to a compound that binds to the enzyme and stimulates the action of the naturally occurring enzyme, or a compound which mimics the activity of the naturally occurring enzyme.
The term "antagonist" of an enzyme refers to a compound that binds to the enzyme and inhibits the action of the naturally occurring enzyme.
The term "analog" of a compound refers to a compound having a some structural similarity to a particular compound and having essentially the same type of biological activity as the compound.
The term "CYP 17 substrate" includes any of the various steroid hormones acted upon by a CYP 17 or a CYP17-like P45o enzyme. Examples include pregnenolone, progesterone and their 17α-hydroxylated forms. Pregnenolone is converted to DHEA via a CYP17 C17,20-lyase reaction, but is also subject to C17α-hydroxylation via the C17,20- lyase activity. Progesterone is converted to delta 4- androstenedione via a CYP17 C17,20- lyase reaction, but is also subject to C17 alpha-hydroxylation via the C17-hydroxylase activity to form 17-hydroxyl-progesterone, a precursor to hydrocortisone (i.e. cortisol).
The term "CYP 17 metabolite" refers to any of the steroid hormones that are synthesized from a cholesterol precursor via a CYP17-mediated reaction, such as a C17- hydroxylase reaction or a C 17,20-lyase reaction. Examples of CYP 17 metabolites include the androgens, such as testosterone, which are synthesized via a CYP 17 CI 7,20-lyase reaction from CYP 17 substrate precursors such as pregnenolone (converted to DHEA by the CYP 17 CI 7,20-lyase activity), and progesterone (converted to delta 4- androstenedione by the CYP 17 CI 7,20-lyase activity). Progestagens such as progesterone are primarily synthesized in the corpus luteum. The androgens are responsible for, among other things, development of male secondary sex characteristics and are primarily synthesized in the testis. Other examples include the estrogens, which are also synthesized from a cholesterol precursor via a CYP17-mediated reaction. The estrogens are responsible for, among other things, the development of female secondary sex characteristics and they also participate in the ovarian cycle and are primarily synthesized in the ovary. Another group of CYP 17 metabolites are the glucocorticoids, such as hydrocortisone (i.e. cortisol), which is
synthesized from progesterone via a CYP17-mediated reaction. The glucocorticoids, among other functions, promote gluconeogenesis and the formation of glycogen and also enhance the degradation of fat. The glucocorticoids are primarily .synthesized in the adrenal cortex.
The term "CYP 17 metabolite" is further meant to include other steroid hormones which, although not necessarily synthesized by a CYP17-mediated reaction, may nonetheless be understood by the skilled artisan to be readily affected by an alteration in a CYP17-mediated activity. For example, the mineralocorticoids, such as aldosterone, are derived from cholesterol via a progesterone intermediate. Since progesterone is also converted to the glucocorticoids and sex steroids via CYP17-mediated reactions, an alteration of a CYP 17 activity can alter the amount of progesterone available for conversion to aldosterone. For example, inhibition of CYP 17 activity can increase the amount of progesterone available for conversion into aldosterone. Therefore, inhibition of CYP 17 can lead to an increase in the level of aldosterone. The mineralocorticoids function, among other things, to increase reabsorption of sodium ions, chloride ions, and bicarbonate ions by the kidney, which leads to an increase in blood volume and blood pressure. The mineralocorticoids are primarily synthesized in the adrenal cortex.
The term "CYP 17 metabolite-associated disease or disorder" refers to a disease or disorder wliich maybe treated by alteration of the level of one or more CYP17 metabolites. Examples include a hormone dependent cancer, such as an androgen-dependent prostate cancer, which may be treated by inhibiting CYP 17-mediated androgen synthesis, and an estrogen-dependent breast cancer or ovarian cancer, which may be treated by inhibiting CYP 17-mediated estrogen synthesis. Other examples of "CYP 17 metabolite-associated diseases or disorders" are Cushing's disease, hypertension, prostatic hyperplasia, and glucocorticoid deficiency. Patients with Cushing's syndrome are relatively insensitive to glucocorticoid feedback and exhibit an oversecretion of cortisol devoid of a circadian cycle (see e.g. Newell-Price & Grossman (2001) Ann. Endocrinol. 62: 173-9). Another CYP17 metabolite-associated disease or disorder is hypertension. Mineralocorticoid excess causes hypertension by facilitating the sodium retention at renal tubules.
The term "derivative" of a compound refers to another compound which can be derived, e.g., by chemical synthesis, from the original compound. Thus a derivative of a compound has certain structural similarities with the original compound.
"Disease associated with an abnormal activity or level of a lyase" refers to diseases in which an abnormal activity or protein level of a lyase is present in certain cells, and in which the abnormal activity or protein level of the lyase is at least partly responsible for the disease. A "disease associated with a lyase" refers to a disease that can be treated with a lyase inhibitor, such as the compounds disclosed herein.
A "lyase" refers to an enzyme having a lyase activity.
"Lyase activity" refers to the activity of an enzyme to catalyze the cleavage of the bond C17-C20 in 17α-hydroxy-pregnenolone and 17α-hydroxy-progesterone to form dehydroepiandrosterone (DHEA) and delta4-androstenedione, respectively. Lyase activity also refers to the cleavage of a similar bond in related compounds.
A "lyase inhibitor" is a compound which inhibits at least part of the activity of a lyase in a cell. The inhibition can be at least about 20%, preferably at least about 40%, even more preferably at least about 50%, 70%, 80%, 90%, 95%, and most preferably at least about 98% of the activity of the lyase.
A "patient" or "subject" to be treated by the subject method can mean either a human or non-human animal.
"Treating" a disease refers to preventing, curing or improving at least one symptom of a disease. The following definitions pertain to the chemical structure of compounds:
The term "heteroatom" as used herein means an atom of nitrogen, oxygen, or sulfur.
The term "alkyl" refers to the radicals of saturated aliphatic groups, including straight-chain alkyl groups and branched-chain alkyl groups.
The term "cycloalkyl" (alicyclic) refers to radicals of cycloalkyl compounds, examples being cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, etc.
The term "aralkyl", as used herein, refers to an alkyl group substituted with an aryl group (e.g., an aromatic or heteroaromatic group).
The terms "alkenyl" and "alkynyl" refer to unsaturated aliphatic groups that contain at least one double or triple bond respectively.
Unless the number of carbons is otherwise specified, "lower alkyl" as used herein means an alkyl group but having from one to six carbons, preferably from one to four carbon atoms in its backbone structure. Likewise, "lower alkenyl" and "lower alkynyl" have similar chain lengths. Preferred alkyl groups are lower alkyls. The term "aryl" as used herein means an aromatic group of 6 to 14 carbon atoms in the ring(s), for example, phenyl and naphthyl. As indicated, the term "aryl" includes polycyclic ring systems having two or more rings in which two or more carbons are common to two adjoining rings (the rings are "fused rings") wherein at least one of the rings is aromatic. The term "heteroaryl" as used herein means an aromatic group which contains at least one heteroatom in at least one ring. Typical examples include 5-, 6- and 7-membered single-ring aromatic groups that may include from one to four heteroatoms. Examples include pyrrole, furan, thiophene, imidazole, oxazole, thiazole, triazole, tetrazole, pyrazole, pyridine, pyrazine, pyridazine and pyrimidine, and the like. These aryl groups may also be referred to as "aryl heterocycles" or "heteroaromatics."
The terms ortho, meta and para apply to 1,2-, 1,3- and 1,4-disubstituted benzenes, respectively. For example, the names 1,2-dimethylbenzene and ort/zo-dimethylbenzene are synonymous.
The terms "alkoxyl" or "alkoxy" as used herein refer to moiety in which an alkyl group is bonded to an oxygen atom, which is in turn bonded to the rest of the molecule. Examples are methoxy, ethoxy, propyloxy, tert-butoxy, etc.
As used herein, the term "nitro" means -NO2; the term "halogen" designates -F, -CI, - Br or -I; the term "sulfhydryl" means -SH; the term "hydroxyl" means -OH; and the term "sulfonyl" means -SO2-.
The terms triflyl, tosyl, mesyl, and nonaflyl are art-recognized and refer to trifluoromethanesulfonyl, j?-toluenesulfonyl, methanesulfonyl, and nonafluorobutanesulfonyl groups, respectively. The terms triflate, tosylate, mesylate, and nonaflate are art-recognized and refer to trifluoromethanesulfonate ester, >-toluenesulfonate ester, methanesulfonate ester, and nonafluorobutanesulfonate ester functional groups and molecules that contain said groups, respectively.
The abbreviations Me, Et, Ph, Tf, Nf, Ts, Ms represent methyl, ethyl, phenyl, trifluoromethanesulfonyl, nonafluorobutanesulfonyl, j»-toluenesulfonyl and methanesulfonyl, respectively. A more comprehensive list of the abbreviations utilized by organic chemists of ordinary skill in the art appears in the first issue of each volume of the Journal of Organic Chemistτγ;(i.e., J. Org. Chem. 2002, 67(1), 24A. The abbreviations contained in said list, and all abbreviations utilized by organic chemists of ordinary skill in the art are hereby incorporated by reference.
As used herein, the definition of each expression, e.g. alkyl, m, n, etc., when it occurs more than once in any structure, is intended to be independent of its definition elsewhere in the same structure.
It will be understood that "substitution" or "substituted with" includes the implicit proviso that such substitution is in accordance with pennitted valence of the substituted atom and the substituent, and that the substitution results in a stable compound, e.g., which does not spontaneously undergo transformation such as by reanangement, cyclization, elimination, etc.
As used herein, the term "substituted" is contemplated to include all permissible substituents of organic compounds. In a broad aspect, the permissible substituents include acyclic and cyclic, branched and unbranched, carbocyclic and heterocyclic, aromatic and nonaromatic substituents of organic compounds. Illustrative substituents include, for example, those described herein above. The permissible substituents can be one or more and the same or different for appropriate organic compounds. For purposes of this invention, the heteroatoms such as nitrogen may have hydrogen substituents and/or any permissible substituents of organic compounds described herein which satisfy the valences of the heteroatoms. The phrase "protecting group" as used herein means temporary substituents which protect a potentially reactive functional group from undesired chemical transformations. Examples of such protecting groups include esters of carboxylic acids, silyl ethers of alcohols, and acetals and ketals of aldehydes and ketones, respectively. The field of protecting group chemistry has been reviewed (Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 3rd ed.; Wiley: New York, 1999).
Abbreviations and Acronyms
When the following abbreviations are used throughout the disclosure, they have the followi meaning:
A angstrom amu atomic mass units
Ar argon
CD2CI2 methylene chloride-d
CDCI3 chloroform-^-'
CH2C12 methylene chloride
CI chemical ionization (for mass spectrometry)
CPM counts per minute
DMSO dimethylsulfoxide
DMSO- tf dimethylsulfoxide-G-
El electron impact (for mass spectrometry)
EPA Environmental Protection Agency (as in EPA vial) eq equivalent(s)
ES electrospray (for mass spectrometry)
EtOAc ethyl acetate g gram
GCMS gas chromatography/mass spectrometry h hours
HCl hydrochloric acid
1H NMR proton nuclear magnetic resonance
HEPES 4-(2-Hydroxyethyl peperazine-1 -ethane sulfonic acid)
HPLC high performance liquid chromatography
LC/MS liquid chromatography / mass spectroscopy
M molar
MeOH methanol mg milligram min minute(s) mL milliliter mm millimeter
mmol millimol
MS mass spectrometry
MTBE methyl tert-butyl ether m/z mass to charge ratio (for mass spectrometry)
NaHCO3 sodium bicarbonate
NaOH sodium hydroxide
Na2SO4 sodium sulfate
NH4C1 ammonium chloride
NMR Nuclear magnetic
OTf trifluoroacetate (triflate)
OTs ^-toluenesulfonate (tosylate) psi pounds per square inch
Rf TLC retention factor rt room temperature
SPA Scintillation Proximity Assay
TFA trifluoroacetic acid
THF tetrahydrofuran
TMS tetramethylsilane
TLC thin layer chromatography tR retention time μL microliter
Compoun ds of the Invention
Exemplary compounds of the invention are set forth in Table 1 below. The exemplary compounds of Tables 1 are producible from known compounds (or from starting materials which, in turn, are producible from known compounds), through the general preparative methods described in the General Methods or Examples.
Table 1. Exemplary Compounds of the Invention
Certain compounds of the present invention may exist in particular geometric or stereoisomeric fonns. The present invention contemplates all such compounds, including cis- and trαrø-isomers, R- and S-enantiomers, diastereomers, (D)-isomers, (L)-isomers, the racemic mixtures thereof, and other mixtures thereof, as falling within the scope of the invention. Additional asymmetric carbon atoms may be present in a substituent such as an alkyl group. All such isomers, as well as mixtures thereof, are intended to be included in this invention.
If, for instance, a particular enantiomer of a compound of the present invention is desired, it may be prepared by asymmetric synthesis, or by derivation with a chiral auxiliary, where the resulting diastereomeric mixture is separated and the auxiliary group cleaved to provide the pure desired enantiomers. Alternatively, where the molecule contains a basic functional group, such as amino, or an acidic functional group, such as carboxyl, diastereomeric salts are formed with an appropriate optically-active acid or base, followed by resolution of the diastereomers thus formed by fractional crystallization or
chromatographic means well known in the art, and subsequent recovery of the pure enantiomers.
Compounds may contain a basic functional group, such as amino or alkylamino, and are, thus, capable of forming pharmaceutically-acceptable salts with pharmaceutically- acceptable acids. The term "pharmaceutically-acceptable salts" in this respect, refers to the relatively non-toxic, inorganic and organic acid addition salts of compounds of the present invention. These salts can be prepared in situ during the final isolation and purification of the compounds of the invention, or by separately reacting a purified compound of the invention in its free base form with a suitable organic or inorganic acid, and isolating the salt thus formed. Representative salts include the hydrobromide, hydrochloride, sulfate, bisulfate, phosphate, nitrate, acetate, valerate, oleate, palmitate, stearate, laurate, benzoate, lactate, phosphate, tosylate, citrate, maleate, fumarate, succinate, tartrate, napthylate, mesylate, glucoheptonate, lactobionate, and laurylsulphonate salts and the like. (See, for example, Berge et al. (1977) "Pharmaceutical Salts", J. Pharm. Sci. 66:1-19). Pharmaceutically acceptable salts of the subject compounds include the conventional nontoxic salts or quaternary ammonium salts of the compounds, e.g., from non-toxic organic or inorganic acids. For example, such conventional nontoxic salts include those derived from inorganic acids such as hydrochloric, hydrobromic, sulfuric, sulfamic, phosphoric, nitric, and the like; and the salts prepared from organic acids such as acetic, propionic, succinic, glycolic, stearic, lactic, malic, tartaric, citric, ascorbic, palmitic, maleic, hydroxymaleic, phenylacetic, glutamic, benzoic, salicyclic, sulfanilic, 2-acetoxybenzoic, fumaric, toluenesulfonic, methanesulfonic, ethane disulfonic, oxalic, isothionic, and the like.
In other cases, the compounds of the present invention may contain one or more acidic functional groups and, thus, are capable of forming pharmaceutically-acceptable salts with pharmaceutically-acceptable bases. These salts can be prepared in situ during the final isolation and purification of the compounds, or by separately reacting the purified compound in its free acid form with a suitable base, such as the hydroxide, carbonate or bicarbonate of a pharmaceutically-acceptable metal cation, with ammonia, or with a pharmaceutically- acceptable organic primary, secondary or tertiary amine. Representative alkali or alkaline earth salts include the lithium, sodium, potassium, calcium, magnesium, and aluminum salts and the like. Representative organic amines useful for the formation of base addition salts include ethylamine, diethylamine, ethylenediamine, ethanolamine, diethanolamine, piperazine and the like. (See, for example, Berge et al., supra).
Contemplated equivalents of the compounds described above include compounds wliich otherwise conespond thereto, and which have the same general properties thereof (e.g., functioning as 17α-hydroxylase-Cl 7,20-lyase inhibitors), wherein one or more simple
variations of substituents are made which do not adversely affect the efficacy of the compound in binding to 17α-hydroxylase-Cl 7,20-lyase receptors. In general, the compounds of the present invention may be prepared by the methods illustrated in the general reaction schemes as, for example, described below, or by modifications thereof, using readily available starting materials, reagents and conventional synthesis procedures. In these reactions, it is also possible to make use of variants which are in themselves known, but are not mentioned here.
Diseases that can be treated with the compounds of the invention The present invention also provides a method of inhibiting a lyase, e.g., 17α- hydroxylase-C 17,20 lyase, comprising contacting a lyase with a compound of the invention. The activity can be inhibited by at least 20%, preferably at least about 50%, more preferably at least about 60%, 70%, 80%, 90%, 95%, and most preferably at least about 98%. In one embodiment, the invention provides a method for inhibiting a lyase in vitro. In a preferred embodiment, the lyase is in vivo or ex vivo. For example, the invention provides methods for inhibiting a lyase in a cell, comprising contacting the cell with a compound of the invention, such that the activity of the lyase is inhibited. The cell may further be contacted with a composition stimulating the uptake of the compound into the cell. In one embodiment, the invention provides a method for inhibiting a lyase in a cell of a subject, comprising administering to the subject a therapuetically effective amount of a compound of the present invention, or a formulation comprising a compound of the present invention, such that the lyase is inhibited in a cell of the subject. The subject can be a subject having a disease associated with a lyase, e.g., cancer. Preferred types of cancer that can be treated according to the invention include prostate cancer and breast cancer. Other diseases that can be treated include diseases in which it is desired to prevent or inhibit the formation of a hormone selected from the group consisting of the androgens testosterone and dihydrotestosterone (DHT) and the estrogens 17β-estradiol and estrone. Generally, any disease that be treated by inhibiting the activity of a lyase, e.g., 17α-Hydroxylase-Cl 7,20-lyase, can be treated with the compounds of the invention. In general, the invention provides methods and compositions for the treatment of
CYP 17 metabolite-associated diseases and disorders. Examples include particularly sex steroid honnone dependent cancers, such as androgen-dependent prostate cancer, which may be treated by inhibiting CYP 17-mediated androgen synthesis, and estrogen-dependent breast
cancer or ovarian cancer, which may be treated by inhibiting CYP 17-mediated estrogen synthesis.
For example, adeno carcinoma of the prostate is a common disease that causes significant morbidity and mortality in the adult male population (see Han and Nelson (2000) Expert Opin Pharmacother. 1 : 443-9). Hormonal therapy for prostate cancer is considered when a patient fails with initial curative therapy, such as radical prostatectomy or definitive radiation therapy, or if he is found with an advanced disease. Hormonal agents have been developed to exploit the fact that prostate cancer growth is dependent on androgen. Non- steroidal anti-androgens (NSAAs) block androgen at the cellular level. Castration is another, albeit drastic means of decreasing androgens levels in order to treat or prevent prostate cancer. The methods and compositions of the invention are useful in inhibiting the C 17,20 lyase activity of CYP 17 and thereby decreasing levels of androgen production and the associated growth of androgen-dependent cancers such as prostate cancer.
In another example, breast cancer, particularly breast cancer in postmenopausal women, can be treated by administration of a C 17,20-lyase inhibitor of the invention because adrenal and ovarian androgens are the main precursors of the estrogens which stimulate the growth of hormone dependent breast cancer. In addition, breast cancer can be treated with inhibitors of aromatase that prevent interconversion of estrogens and adrenal and ovarian androgens (see Harris et al. (1983) Eur. J. Cancer Clin. Oncol. 19: 11). Patients failing to respond to aromatase inhibitors show elevated levels of androgens in response to aromatase inhibitor treatment (see Harris et al. (1988) Br. J. Cancer 58: 493-6). Accordingly sequential blockade to inhibit androgen production as well as inhibit aromatase may produce greater estrogen suppression and enhanced therapeutic effects in treating breast and other estrogen hormone-dependent forms of cancer. Therefore the inhibitors of the invention may be used alone or in combination with other drugs to treat or prevent hormone-dependent cancers such as breast and prostate cancer.
Furthermore, susceptibility to prostate cancer and breast cancer has been associated with particular polymorphic alleles of the CYP 17 gene (see e.g. McKean-Cowdin (2001) Cancer Res 61: 848-9; Haiman et al. (2001) Cancer Epidmeiol Biomarkers 10: 743-8; Huang et al. (2001) Cancer Res 59: 4870-5). Accordingly, the compositions of the invention are particularly suited to treating or preventing hormone-dependent cancers in individuals
genetically predisposed to such cancers, particularly those predisposed due to an alteration in the CYP 17 gene.
Another group of CYP 17 metabolite-associated diseases or disorders amenable to treatment with the compositions and methods of the invention include those associated with mineralocorticoid excess such as hypertension caused by sodium retension at renal tubules. Such a mechanism operates in hypertension such as primary hyperaldosteronism and some forms of congenital adrenal hyperplasia. Recently, deficient cortisol metabolism in the aldosterone target organ has been recognized as a novel form of hypertension known as apparent mineralocorticoid excess. Disorders associated with mineralocorticoid synthesis include abnormalities of mineralocorticoid synthesis and/or metabolism which profoundly affect the regulation of electrolyte and water balance and of blood pressure (see e.g. Connell et al. (2001) Baillieres Best Pract Res Clin Endocrinol Metab 15:43-60). Characteristic changes in extracellular potassium, sodium and hydrogen ion concentrations are usually diagnostic of such disorders. Serious deficiency may be acquired, for example in Addison's disease, or inherited. In most of the inherited syndromes, the precise molecular changes in specific steroidogenic enzymes have been identified. Mineralocorticoid excess may be caused by aldosterone or 11-deoxycorticosterone by inadequate conversion of cortisol to cortisone by 1 lbeta-hydroxysteroid dehydrogenase type 2 in target tissues, by glucocorticoid receptor deficiency or by constitutive activation of renal sodium channels. Changes in electrolyte balance and renin as well as the abnormal pattern of corticosteroid metabolism are usually diagnostic. Where these abnormalities are inherited (e.g. 1 lbeta- or 17alpha- hydroxylase deficiencies, glucocorticoid remediable hyperaldosteronism (GRA), receptor defects, Liddle's syndrome), the molecular basis is again usually known and, in some cases, may provide the simplest diagnostic tests. Primary aldosteronism, although readily identifiable, presents problems of differential diagnosis, important because optimal treatment is different for each variant. Finally, a significant proportion of patients with essential hypertension show characteristics of mild mineralocorticoid excess, for example low renin levels. As described above, a decrease in CYP 17 activity can result in an alteration in mineralorticoid (e.g. aldosterone) biosynthesis. Accordingly, the "CYP 17 metabolite- associated diseases or disorders" of the invention would include those associated with altered levels of aldosterone production (e.g. hypertension, primary adrenal hyperplasia).
Still other examples of CYP 17 metabolite-associated diseases or disorders" are Cushing's disease, prostatic hyperplasia, glucocorticoid deficiency, and endometrial cancer.
The subject that can be treated according to the invention can be a mammal, e.g., a primate, equine, canine, bovine, ovine, porcine, or feline. In preferred embodiments of this method, the mammal is a human. In other embodiments, the invention provides methods for inhibiting the lyase activity of enzymes that are present in organisms other than mammals, e.g., yeast and fungus, e.g., mildew. Certain compounds of the invention may function as antifungal compounds.
Methods of administering the compounds of the invention
The therapeutic methods of the invention generally comprise administering to a subject in need thereof, a pharmaceutically effective amount of a compound. The compounds of the invention can be administered in an amount effective to inhibit the activity of a 17α-Hydroxylase-Cl 7,20-lyase. The compounds of this invention may be administered to mammals, preferably humans, either alone or, preferably, in combination with pharmaceutically acceptable carriers, excipients or diluents, in a pharmaceutical composition, according to standard pharmaceutical practice. The compounds can be administered orally or parenterally, including the intravenous, intramuscular, intraperitoneal, subcutaneous, rectal and topical routes of administration. Toxicity and therapeutic efficacy of the compounds can be determined by standard pharmaceutical procedures in cell cultures or experimental animals, e.g., for determining the LD5o (the dose lethal to 50% of the population) and the EDs0 (the dose therapeutically effective in 50% of the population). The dose ratio between toxic and therapeutic effects is the therapeutic index and it can be expressed as the ratio LD50/ED50. Compounds which exhibit large therapeutic indices are preferred. While compounds that exhibit toxic side effects may be used, care should be taken to design a delivery system that targets such reagents to the site of affected tissue in order to minimize potential damage to normal cells and, thereby, reduce side effects.
Data obtained from cell culture assays and animal studies can be used in formulating a range of dosage for use in humans. The dosage of such reagents lies preferably within a range of circulating concentrations that include the ED50 with little or no toxicity. The
dosage may vary within this range depending upon the dosage form employed and the route of administration utilized. For any reagent used in the method of the invention, the therapeutically effective dose can be estimated initially from cell culture assays. A dose may be formulated in animal models to achieve a circulating plasma concentration range that includes the ICso (i.e., the concentration of the test compound which achieves a half- maximal inhibition of symptoms) as determined in cell culture. Such information can be used to more accurately determine useful doses in humans. The compounds of the invention have an IC50 less than lOμM as determined by the biochemical or cellular assay described herein. Some compounds of the invention are effective at concentrations of lOnM, lOOnM, or 1 μM. Based on these numbers, it is possible to derive an appropriate dosage for administration to subjects.
Pharmaceutical compositions containing a compound of the invention may be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsions, hard or soft capsules, or syrups or elixirs. Compositions intended for oral use may be prepared according to any method known to the art for the manufacture of pharmaceutical compositions and such compositions may contain one or more agents selected from the group consisting of sweetening agents, flavoring agents, coloring agents and preserving agents in order to provide pharmaceutically elegant and palatable preparations. Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients which are suitable for the manufacture of tablets. These excipients may be for example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, microcrystalline cellulose, sodium crosscarmellose, corn starch, or alginic acid; binding agents, for example starch, gelatin, polyvinyl-pyrrolidone or acacia, and lubricating agents, for example, magnesium stearate, stearic acid or talc. The tablets may be uncoated or they may be coated by known techniques to mask the unpleasant taste of the drug or delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period. For example, a water soluble taste masking material such as hydroxypropylmethyl-cellulose or hydroxypropylcellulose, or a time delay material such as ethyl cellulose, cellulose acetate buryrate may be employed.
Formulations for oral use may also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate,
calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water soluble carrier such as polyethyleneglycol or an, oil medium, for example peanut oil, liquid paraffin, or olive oil.
Aqueous suspensions contain the active material in admixture with excipients suitable for the manufacture of aqueous suspensions. Such excipients are suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydroxypropylmethylcellulose, sodium alginate, polyvinyl-pynolidone, gum tragacanth and gum acacia; dispersing or wetting agents may be a naturally-occurring phosphatide, for example lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethylene-oxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example polyethylene sorbitan monooleate. The aqueous suspensions may also contain one or more preservatives, for example ethyl, or n-propyl p- hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose, saccharin or aspartame.
Oily suspensions may be formulated by suspending the active ingredient in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in mineral oil such as liquid paraffin. The oily suspensions may contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol. Sweetening agents such as those set forth above, and flavoring agents may be added to provide a palatable oral preparation. These compositions may be preserved by the addition of an anti-oxidant such as butylated hydroxyanisol or alpha-tocopherol. Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the compound of the invention in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives. Suitable dispersing or wetting agents and suspending agents are exemplified by those already mentioned above. Additional excipients, for example sweetening, flavoring and coloring agents, may also be present. These compositions may be preserved by the addition of an anti-oxidant such as ascorbic acid.
Pharmaceutical compositions of the invention may also be in the form of an oil-in- water emulsions. The oily phase may be a vegetable oil, for example olive oil or arachis oil, or a mineral oil, for example liquid paraffin or mixtures of these. Suitable emulsifying agents may be naturally-occurring phosphatides, for example soy bean lecithin, and esters or partial esters derived from fatty acids and hexitol anhydrides, for example sorbitan monooleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monooleate. The emulsions may also contain sweetening, flavouring agents, preservatives and antioxidants.
Syrups and elixirs may be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also contain a demulcent, a preservative, flavoring and coloring agents and antioxidant.
Pharmaceutical compositions may be in the form of a sterile injectable aqueous solutions. Among the acceptable vehicles and solvents that may be employed are water, Ringer's solution and isotonic sodium chloride solution. Sterile injectable preparation may also be a sterile injectable oil-in-water microemulsion where the compound of the invention is dissolved in the oily phase. For example, the active ingredient may be first dissolved in a mixture of soybean oil and lecithin. The oil solution then introduced into a water and glycerol mixture and processed to fonn a microemulation. The injectable solutions or microemulsions may be introduced into a patient's bloodstream by local bolus injection. Alternatively, it may be advantageous to administer the solution or microemulsion in such a way as to maintain a constant circulating concentration of the instant compound. In order to maintain such a constant concentration, a continuous intravenous delivery device may be utilized. An example of such a device is the Deltec CADD-PLUS™ model 5400 intravenous pump.
The pharmaceutical compositions may be in the form of a sterile injectable aqueous or oleagenous suspension for intramuscular and subcutaneous administration. This suspension may be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents which have been mentioned above. The sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic parenterally-acceptable diluent or solvent, for example as a solution in 1,3-butane diol. In
addition, sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose any bland fixed oil may be employed including synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid find use in the preparation of injectables. Compounds of the invention may also be administered in the form of a suppositories for rectal administration of the drug. These compositions can be prepared by mixing the drug with a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug. Such materials include cocoa butter, glycerinated gelatin, hydrogenated vegetable oils, mixtures of polyethylene glycols of various molecular weights and fatty acid esters of polyethylene glycol.
For topical use, creams, ointments, jellies, solutions or suspensions, etc., containing the compound of the invention can be employed. For purposes of this application, topical application shall include mouth washes and gargles. The compounds for the present invention can be administered in intranasal form via topical use of suitable intranasal vehicles and delivery devices, or via transdermal routes, using those forms of transdermal skin patches well known to those of ordinary skill in the art. To be administered in the form of a transdermal delivery system, the dosage administration will preferably be continuous rather than intermittent throughout the dosage regimen.
The compounds of the invention may also be co-administered with other well known therapeutic agents that are selected for their particular usefulness against the condition that is being treated. The compounds may be administered simultaneously or sequentially. For example, the instant compounds may be useful in combination with known anti-cancer and cytotoxic agents. Similarly, the instant compounds may be useful in combination with agents that are effective in the treatment and prevention of osteoporosis, inflammation, neurofibromatosis, restinosis, and viral infections. The instant compounds may also be useful in combination with inhibitors of other components of signaling pathways of cell surface growth factor receptors. Drugs can be co-administered to a subject being treated with a compound of the invention include antineoplastic agents selected from vinca alkaloids, epipodophyllotoxins,
anthracycline antibiotics, actinomycin D, plicamycin, puromycin, gramicidin D, taxol, colchicine, cytochalasin B, emetine, maytansine, or amsacrine. Methods for the safe and effective administration of most of these chemotherapeutic agents are known to those skilled in the art. In addition, their administration is described in the standard literature. For example, the administration of many of the chemotherapeutic agents is described in the "Physicians' Desk Reference" (PDR), e.g., 1996 edition (Medical Economics Company, Montvale, NJ. 07645-1742, USA).
Radiation therapy, including x-rays or gamma rays which are delivered from either an externally applied beam or by implantation of tiny radioactive sources, may also be used in combination with a compound of the invention to treat a disease, e.g., cancer.
When a composition according to this invention is administered into a human subject, the daily dosage will normally be determined by the prescribing physician with the dosage generally varying according to the age, weight, and response of the individual patient, as well as the severity of the patient's symptoms.
Kits of the invention
In one embodiment, materials and reagents required for administering the compounds of the invention may be assembled together in a kit. When the components of the kit are provided in one or more liquid solutions, the liquid solution preferably is an aqueous solution, with a sterile aqueous solution being particularly preferred.
The kit may further comprise one or more other drugs, e.g., a chemo- or radiotherapeutic agent. These normally will be a separate formulation, but may be formulated into a single pharmaceutically acceptable composition. The container means may itself be geared for administration, such as an inhalant, syringe, pipette, eye dropper, or other such like apparatus, from which the formulation may be applied to an infected area of the body, such as the lungs, or injected into an animal, or even applied to and mixed with the other components of the kit.
The compositions of these kits also may be provided in dried or lyophilized forms. When reagents or components are provided as a dried form, reconstitution generally is by the addition of a suitable solvent. It is envisioned that the solvent also may be provided in
another container means. The kits of the invention may also include an instruction sheet defining administration of the agent.
The kits of the present invention also will typically include a means for containing the vials in close confinement for commercial sale such as, e.g., injection or blow-molded plastic containers into wliich the desired vials are retained. Irrespective of the number or type of containers, the kits of the invention also may comprise, or be packaged with a separate instrument for assisting with the injection/administration or placement of the ultimate complex composition within the body of an animal. Such an instrument maybe an inhalant, syringe, pipette, forceps, measured spoon, eye dropper or any such medically approved delivery vehicle. Other instrumentation includes devices that permit the reading or monitoring of reactions or amounts of compounds or polypeptides.
General Method for the Preparation of Compounds of Formula I
3-(3-Pyridyl)pyrcoles la (Formula I, where X represents CH) and the 3-(3- pyridyl)pyrazoles lb (Formula I, where X represents N), wherein A, L, and G are as defined in hereinabove are prepared by the general method described below, according to methods described below, or according to methods commonly employed in the art.
(la) (lb) (I, X = CH (I. X = N)
The 3-bromopyridines IN used to prepare compounds of Formula la are prepared according to method described by Comins (Comins, D. L., Smith, R., Stroud, E., Heterocycles, Nol. 22, No. 2, 1984, 339) or by other methods commonly employed in the art to prepare 4-alkyl-3- bromopyridines. The requisite 3-bromopyridines III, where q = 0 or 1, are commercially available, can be prepared by Suzuki coupling with alkyl or cycloalkylboronic acids with 3,5-dibromopyridine or with 3-(trifluoromethanesulfonyloxy)-5-bromopyridine, or by other methods commonly employed in the art. Treatment of 3-bromopyridines IN with an alkyl acrylate N, preferably methyl acrylate, in the presence of a palladium (II) or a palladium (0) catalyst, preferably a palladium (II) catalyst, and in the presence of a phosphine, preferably (o-tolyl)3P, provides vinyl pyridines NL Alternatively, vinyl pyridines Nl can be prepared
according to the methods used in the preparation of Intermediates A, B, and I-N below, or by other methods commonly employed in the art. Treatment of vinyl pyridines VI with an aryl methyl isocyanide and an alkali bis(trimethylsilyl)amide, preferably tosyl methyl isocyanide and sodium bis(trimethylsilyl)amide respectively, affords intermediates NIL Hydrolysis and decarboxylation affords pyridyl pyrroles VIII. Alternatively, the methods used in the preparation of Intermediates A, B, and I-Ν below, or other methods commonly employed in the art, may be used to prepare intermediates NIII. Alkylation with a G-L-X alkylating agent IX (where L = CH2 or (CH2)2, and X = chloride, bromide, iodide, tosylate, triflate, or other leaving group commonly employed in the art) then provides compounds of Formula la. General methods A and B detail experimental procedures that may be employed to prepare compounds of Formula la, where L = CH2 or (CH2)2). The requisite alkylating agents IX are commercially available or can be prepared from commercially available compounds employing methods commonly found in the art.
Scheme 1. General Preparation of Compounds of Formula la (L is CH2 or (CH2)2)
VIII
G-L— X IX
Ν^L/°
la
Compounds of Formula la, where L is a bond, are prepared from intermediate NIII according to Method C described below, by the following literature methods (Tetrahedron 1999, 55, 12757-12770, Tetrahedron Lett. 1998, 39, 2941-2944, and Tetrahedron Lett. 1990, 31,
12,1665-1668. or by other Suzuki conditions commonly employed in the art (Scheme 2). The texts of the above references are hereby incorporated by reference.
Scheme 2. General Preparation of Compounds of Formula la (L is a Bond)

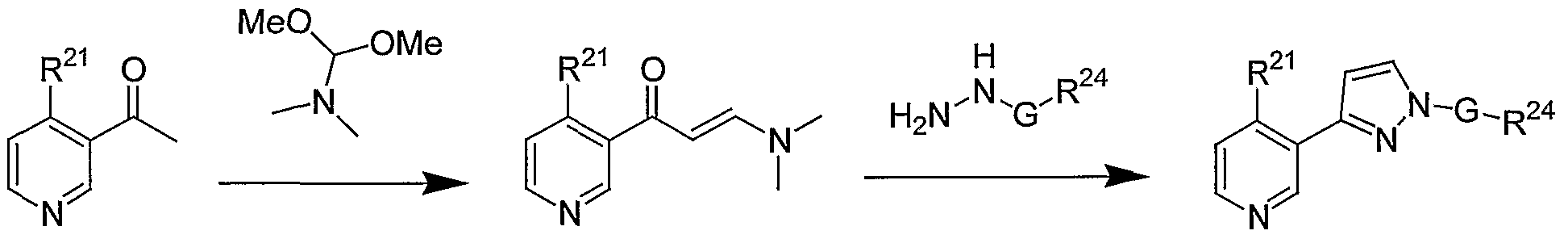
3-(3-Pyridyl)pyrazoles of Formula lb are prepared by the route shown in Scheme 3 or according to methods commonly employed in the art. The 3-acetypyridines XIII used to prepare compounds of Formula lb are prepared according to method described by Comins (Comins, D. L., Smith, R., Stroud, E., Heterocycles, Nol. 22, No. 2, 1984, 339) or by other methods commonly employed in the art to prepare 4-alkyl-3-acetylpyridines. The requisite 3-acetylpyridines XII, where q = 0 or 1, are commercially available, can be prepared by Suzuki coupling with alkyl or cycloalkylboronic acids with 3-acetyl-5-bromopyridine or by other methods commonly employed in the art. The requisite 3-acetyl-5-bromopyridine can be prepared from 5-bromonicotinic acid, methyl 5-bromonicotinate, or from methyl 5- bromonicotinylacetate using methods commonly employed in the art. Alternatively, the synthesis of XIII can begin with either 5-bromonicotinic acid, methyl 5-bromonicotinate, or from methyl 5-bromonicotinylacetate, rearranging the steps and employing methods commonly employed in the art to convert the acid, ester or acetate functionalities to the acetyl moiety of XIII. Treatment of XIII with an alkoxybis(dialkylamino)methane, preferably methoxybis(dimethylamino)methane at a temperature between 80 - 180 °C the intermediate enamine XIV. Preferably the reaction is run neat and the temperature is between 100-120 °C. Other methods commonly employed in the art to prepare aryl enamines from aryl acetyl may be employed. Enamines XIV are then treated with arylhydrazines XV to provide compounds of Formula lb. Hydrazines XV are commercially available or are prepared using literature methods employed in the art. Most preferably, the Method D described below is used to convert acetyl pyridines XIII to compounds of Formula lb.
Scheme 3. General Preparation of Compounds of Formula lb
H2N.N, G H
XV N-|
A N lb
The present invention is further illustrated by the following examples which should not be construed as limiting in any way. The contents of all cited references (including literature references, issued patents, published patent applications as cited throughout this application) are hereby expressly incorporated by reference.
Examples
Preparation of the compounds of the invention
General. All reagents are commercially available unless otherwise specified. Reagents were used as received unless otherwise specified. Proton NMR data is reported downfield from TMS; coupling constants are in hertz. LC/MS mass spectral data were obtained using a Hewlett-Packard 1100 HPLC equipped with a quaternary pump, a variable wavelength detector set at 254 nm, a YMC pro C-18 column (2 x 23 mm, 120A), and a Finnigan LCQ ion trap mass spectrometer with electrospray ionization. Spectra were scanned from 120-1200 amu using a variable ion time according to the number of ions in the source. The eluents were A: 2% acetonitrile in water with 0.02% TFA and B: 2% water in acetonitrile with 0.018% TFA. Gradient elution from 10% B to 95% B over 3.5 minutes at a flowrate of 1.0 mL/min was used with an initial hold of 0.5 minutes and a final hold at 95% B of 0.5 minutes. Total run time was 6.5 minutes. Purification by HPLC was performed using a Gilson HPLC system (UV/VIS-155 detector, 215 liquid handler, 306 pumps, 819 injection valve and an 81 IC mixer, the column was a YMC Pro C18 (75 x 30, 5μm, 120A); the eluents were A: water with 0.1% TFA, and B: acetonitrile with 0.1% TFA; gradient
elution from 10% B to 90% B over 12 minutes with a final hold at 90% B for 2 minutes; flowrate was 25 mL per minute. NMR data are in agreement with the structure of all prepared compounds. Elemental analyses were obtained at Robertson Microlit Laboratories, Madison NJ. Melting points are uncoreected.
Preparation of Intermediate A: 3-(lH-Pyrrol-3-yl)pyridine
Step 1. Trans-3-(3-pyridyl)acrylic acid (7.0 g, 47 mmol), Cs2CO3 (18.35 g, 56 mmol), and methyl iodide (3.50 mL, 56 mmol) were refluxed together in acetonitrile (100 mL) for 2 h. The dark brown reaction was cooled, filtered, and the solid washed with acetonitrile (50 mL). The solvent of the combined filtrates was evaporated in vacuo, and the residue partitioned between EtOAc (150 mL) and H2O (100 mL). The separated organic layer was washed with H2O (3 x 100 mL), then with brine (50 mL) and dried over Na2SO . After filtration, the solvent was evaporated in vacuo, and the residue purified via flash chromatography (15-30% EtOAc/hexane), affording 4.92 g (64%) of the ester as a yellow solid. Step 2. A solution of the ester (2.5 g, 15 mmol) and tosylmethyl isocyanide (TosMIC, 3.15 g, 16 mmol) in THF (100 mL) at rt was treated dropwise with 1.0 M sodium bis(trimethylsilyl)amide in THF (16 mL, 16 mmol). The reaction became cloudy with a precipitate. The reaction was stirred overnight and then the solvent evaporated in vacuo. The residue was partitioned between EtOAc (200 mL) and H2O (200 mL). The separated organic layer was washed with H2O (2 x 125 mL), then brine (200 mL) and dried over Na2SO4. After filtration, the solvent was evaporated in vacuo, and the residue purified via flash chromatography (40-70% EtOAc/hexane), giving 2.05 g (66%) of the pyrrole ester as a yellow-orange solid.
Step 3. A mixture of the pyrrole ester (3.0 g, 15 mmol) and sodium hydroxide (5.93 g, 150 mmol) in MeOH (40 mL) was heated at reflux for 3 h. The MeOH was evaporated in vacuo, the residue dissolved in H2O (100 mL), and acidified to ~ pH 1 with concentrated aqueous HCl. The reaction mixture was heated to reflux overnight. After cooling, the reaction mixture was neutralized with solid NaHCO , giving a pinkish precipitate. The mixture was extracted with EtOAc (4 x 75 mL). The combined extracts were washed with H2O (50 mL), brine (50 mL), and then dried over Na2SO4. After filtration, the solvent was evaporated in vacuo and the residue purified via flash chromatography (30-40% EtOAc/hexane), giving 2.03 g (95%) of the pyridyl pyrrole as a light-yellow solid.
Preparation of Intermediate B: 4-Methyl-3-(lH-pyrrol-3-yI) pyridine
Step 1. A suspension of 3-bromo-4-picoline (30.86 g, 0.174 mol), palladium acetate (1.95 g, 0.0087 mol), tri-o-tolyl phosphine (4.08 g, 0.013 mol), methyl acrylate (23.80 mL, 0.264 mol) and triethylamine (87.8 mL, 0.63 mol) in DMF (297 mL) was degassed with argon, then heated to 70-75 °C for 16 h. The reaction mixture was poured into water (1500 mL), then extracted with EtOAc (2 x 1.2 L, 1 x 0.6 L). Combined organic layer washed with water (2x 0.6L), dried over anhydrous sodium sulfate and concentrated to dryness to give 34.12g of crude methyl-3-(4-methyl-3-pyridinyl)-2-propenoate: GCMS (El) tR = 7.2 min, 177 (M ); 1H NMR (CDC13) δ 8.70 (1H, s), 8.45 (1H, d), 7.90 (1H, d), 7.12 (1H, d), 6.44 (1H, d), 3.82 (3H, s), 2.43 (3H, s).
Step 2. To a solution of the crude methyl-3-(4-methyl-3-pyridinyl)-2-propenoate (34.0 g, 0.192 mol), tosyl methyl isocyanide (40.0 g, 0.205 mol) in THF (1.7 L) was added at room temperature sodium bis(trimethylsilyl)amide (288 mL, 0.288 mol, 1.0M solution in THF) over a period of 30-40 min. The resulting brown suspension was stirred at rt for 12-14 h. As
the reaction was incomplete, an additional amount of sodium bis(trimethylsilyl)amide (100 mL, 0.10 mol, 1.0M solution in THF) was added to the reaction mixture at rt. After stirring for and additonal 7 h, the reaction mixture was concentrated to dryness and the residue partitioned between EtOAc (3 x 200 mL) and water (225 mL). The combined organic layers was washed with water (2 x 150 mL) and dried over anhydrous sodium sulfate.
Concentration to dryness gave a 34.20 g of methyl-4-(4-methyl-3-pyridinyl)-lH-pyrrole-3- carboxylate as a brown oil: LCMS tR 0.84 min, 217 (M+H+); 1H NMR (CDC13) δ 8.30 (IH, d), 8.28 (IH, s), 7.45 (IH, t), 7.10 (IH, d), 6.60 (IH, t), 3.59 (3H, s), 2.15 (3H, s). Step 3. A suspension of methyl-4-(4-methyl-3-pyridinyl)-lH-pyrrole-3-carboxylate (34.0 g, 0.16 mol), sodium hydroxide (64.0 g, 1.6 mol) in methanol (451 mL) was heated to reflux for 16-18 h. The reaction mixture was concentrated to dryness. Water (1.1 L) was added and the pH adjusted to ~1.0 using concentrated hydrochloric acid (250 mL). This suspension was then heated to reflux for 16-18 h. The reaction mixture was cooled to room temperature and neutralized with solid sodium bicarbonate. The aqueous mixture was extracted with EtOAc (4 x 500 mL). The combined organic layer was washed with water (500 mL), washed with brine (400 mL), and then dried over anhydrous sodium sulfate. The filtrate was concentrated to dryness giving a brown liquid which on silica gel chromatography, using 20-45% ethyl aceate-hexanes as eluent, gave 12.05 g of 4-methyl-3-(lH-pyrrol-3-yl) pyridine as a brownish white product: LC/MS tR 0.63 min, 159 (M+H+); 1H NMR (CDC13) δ 8.58 (IH, s), 8.32 (IH, d), 7.14 (IH, d), 6.95 (IH, m), 6.90 (IH, m), 6.43 (IH, m), 2.44 (3H, s).
Preparation of Intermediates C - H
Using the procedure described by Comins (Comins, D. L.; Smith, R. K.; Stroud, E. D. Heterocycles (1984) 22, 339-44) the following bromopyridines are prepared:
4-Ethyl-3-bromopyridine (Intermediate C) 4-Butyl-3-bromopyridine (Intermediate D) 4-(2-Propyl)-3-bromopyridine (Intermediate E) 4-(ter^-Butyl)-3-bromopyridine (Intermediate F) 4-(Cyclohexyl)-3-bromopyridine (Intermediate G) 4-Phenyl-3-bromopyridine (Intermediate H)
Preparation of Intermediates I - N:
Using the procedure described above for 4-methyl-3-(lH-pyrrol-3-yl) pyridine, the following pyrrole intermediates are prepared:
4-Ethyl-3-(lH-pyrrol-3-yl) pyridine (Intermediate I) 4-Butyl-3-(lH-pyrrol-3-yl) pyridine (Intermediate J) 4-(2-Propyl)-3-(lH-pyrrol-3-yl) pyridine (Intermediate K) 4-(tert-Butyl)-3-(lH-pyrrol-3-yl) pyridine (Intermediate L) 4-Cyclohexyl-3-(lH-pyrrol-3-yl) pyridine (Intermediate M) 4-Phenyl-3-(lH-pyrrol-3-yl) pyridine (Intermediate N)
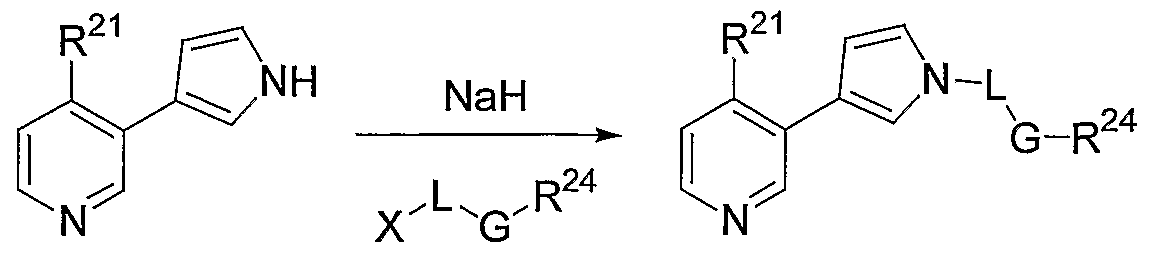
General Method A.
(X = CI, Br)
To a suspension of 95% sodium hydride (0.76 mmol) in DMF (5 mL) is added a solution of the 4-(R21)-3-(lH-pyrrol-3-yl) pyridine (0.7 mmol) dissolved in anhydrous DMF (5 mL) drop wise at rt. The reaction is stirred 20 min. The alkyl or aryl halide (0.7 mmol) is added dropwise and the reaction mixture stirred overnight. The reaction mixture is diluted with H2O (60 mL), then extracted with EtOAc (4 x 15 mL). The combined organic extract is washed with H2O (2 x 25 mL), then with brine (25 mL) and dried over Na2SO . After filtration, the solvent is evaporated in vacuo, and the residue purified via column chromatography (15-20% EtOAc/hexane), affording the target pyrrole derivatives in 80- 100% yields. The target pyrrole derivatives are characterized by NMR and MS.
General Method B.
(X = CI, Br)
The 4-(R1)-3-(lH-pynol-3-yl) pyridine (100 mg, 1 eq) and anhydrous DMF (10 mL) are place into a 40 mL EPA vial with NaH (1 eq) and the mixture is shaken at room temperature for 45 min. The halide is added into the vial and the resulting suspension is stirred at room temperature for 3 days. After the reaction is completed, the mixture is concentrated. The resulting residue is taken up into dichloromethane, washed with water, dried and concentrated. The crude product is purified using a Gilson HPLC and the products characterized by NMR and MS.
General Method C.
X = CH, CR25 , N
The 4-(R21)-3-(lH-pyrrol-3-yl)pyridine (0.63 mmol) and an aryl boronic acid (1.26 mmol) are dissolved in dichloromethane (7 mL, reagent grade). To this is added cupric acetate (1.26 mmol), pyridine (1.26 mmol) and triethylamine (1.26 mmol). Also, to this reaction is added 4A activated molecular sieves (0.25 g). The reaction is stirred at rt for 48 h with a septum and needle exposing the reaction to air. The reaction is filtered and washed with methanol. The filtrate is concentrated under reduced pressure to give a green residue. The compound is purified by a Gilson HPLC using a YMC-Pack Pro C18 Prep Column (150 mm x 20 mm I.D. S-5 μm, 12 nm). The mobile phase starts with 10 % CH3CN/water (0.1 % TFA) for 3 min and then goes from 10 % to 90 % CH3CN/water (0.1 % TFA) over 7 min. It is optionally furthered purified by preparative TLC, mobile phase 20 - 50 % EtOAc/Hexane. Product yields are generally in the range of 10 - 50%.
Table 2. Examples 1 - 25: Pyridyl Pyrrole Derivatives Prepared from Intermediates A and B using General Methods A and B.
Table 1 Note a: HPLC - electrospray mass spectra (HPLC ES-MS) were obtained using a Hewlett-Packard 1100 HPLC equipped with a quaternary pump, a variable wavelength detector set at 254 nm, a YMC pro C-18 column (2 x 23 mm, 120A), and a Finnigan LCQ ion trap mass spectrometer with electrospray ionization. Spectra were scanned from 120- 1200 amu using a variable ion time according to the number of ions in the source. The eluents were A: 2% acetonitrile in water with 0.02% TFA and B: 2% water in acetonirile with 0.018% TFA. Gradient elution from 10% B to 95% over 3.5 minutes at a flowrate of 1.0 mL/min was used with an initial hold of 0.5 minutes and a final hold at 95% B of 0.5 minutes. Total run time was 6.5 minutes. Table Note b: Molecular ion data obtained via electrospray ionization.
The following Examples of 4-(substituted)-3-(3-pyrrolyl)pyridine derivatives are prepared from Intermediates I-N according to General Methods A and B:
Table 3. Examples 26 - 145 : Pyridyl Pyrrole Derivatives which may be Prepared from General Intermediates I-N by General Methods A and B.
The following Examples of 4-(substituted)-3-(3-pynolyl)pyridine derivatives are prepared from Intermediates I-N according to General Methods A and B:
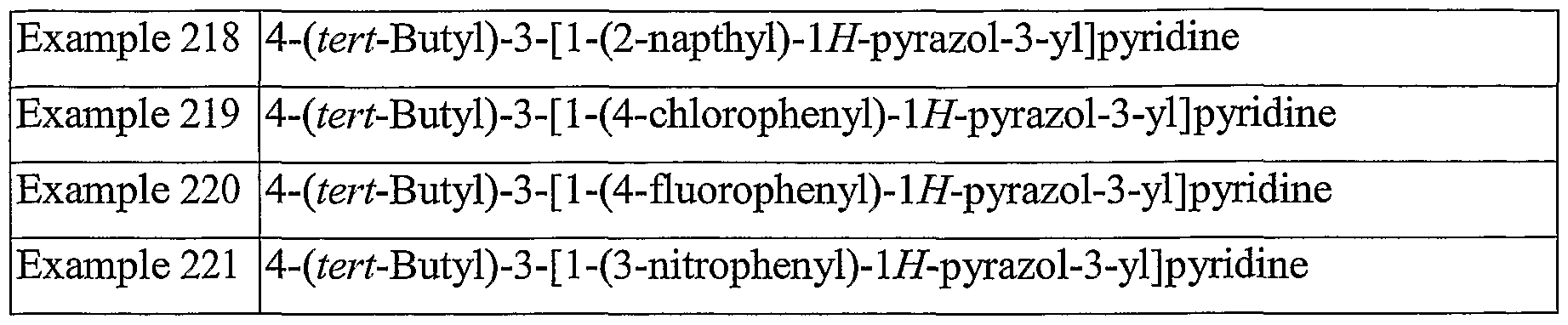
Table 4. Examples 146 - 155: Pyridyl Pyrrole Derivatives which may be Prepared from Intermediate B by General Method C.
Example No. Compound Name
Example 146 4-methyl-3 -(1 -(4-methylphenyl)- 1 H-pyrrol-3 -yl)pyridine
Example 147 4-methyl-3 -( 1 -(3 -methylphenyl)- 1 H-pyrrol-3 -yl)pyridine
Example 148 4-methyl-3-(l-(2-methylphenyl)-lH-pyrrol-3-yl)pyridine
Example 149 4-methyl-3 -(1 -(4-methoxyphenyl)- 1 H-pyrrol-3 -yl)pyridine
Example 150 4-methyl-3 -(1 -(4-fluorophenyl)- 1 H-pyrrol-3 -yl)pyridine
Example 151 4-methyl-3-(l-(4-cyanophenyl)-lH-pyrrol-3-yl)pyridine
Example 152 4-methyl-3 -( 1 -(3 -(trifluoromethyl)phenyl)- 1 H-pyrrol-3 -yl)pyridine
Example 153 4-methyl-3 -( 1 -(3 -pyridyl)- 1 H-pyrrol-3 -yl)pyridine
Example 154 4-methyl-3 -(1 -(4-chlorophenyl)- 1 H-pyrrol-3 -yl)pyridine
Example 155 4-methyl-3 -(1 -(2-thiophenyl)- 1 H-pyrrol-3 -yl)pyridine
Preparation of Intermediate O: 4-Methyl-3-acetylpyridine
Step 1. A solution of 3-acetylpyridine (100 g, 0.82 mol), dimethyl sulfide (400 mL, 5.4 mol) and copper (I) iodide (7.94 g, 0.041 mol) in anhydrous THF (2 L) was stiff ed at rt under an Ar. Phenyl chloroformate (0.4 mL, 0.82 mol) was then added, producing a dark brown precipitate. After 30 minutes, the mixture was cooled below -21 °C and methyl
magnesium bromide (1.4 M in 3: 1 toluene-THF, 586 mL, 0.82 mol) was added over 50 min, keeping the reaction temperature below -15 °C. The color lightened as the mixture became a solution; a lime green precipitate formed near the end of the addition, but redissolved upon completion. The mixture was stirred and allowed to warm slowly; after 2 h it had warmed to 8.8 °C. Saturated aqueous ammonium chloride solution (500 mL) was added; after stirring 10 min, the mixture was poured into a separatory funnel with water (500 mL). The organic phase was separated, washed with brine (500 mL), dried (Na2SO ), filtered and then concentrated in vacuo. The residue was purified by silica gel chromatography using a hexane-EtOAc gradient to afford 134.3 g (63.7) of the intermediate dihydropyridine. Step 2. A solution of the intermediate dihydropyridine (134.3 g, 0.52 mol) in dichloromethane (100 mL) was added to a stirred suspension of sulfur (16.67 g, 0.52 mol) in decalin and slowly heated to reflux under an Ar sweep. After refluxing 1 h, the mixture was allowed to cool to rt, then filtered through a pad of silica gel. After eluting the decalin with hexane, elution with a hexane-diethyl ether gradient afforded 49.4 g (70.3%) of 4-methyl-3- acetylpyridine as a reddish-brown oil: TLC R/0.19 (diethyl ether); TLC R 0.14 (1:1 hexane-EtOAc); 1H NMR (CD2C12) δ 8.9 (s, IH), 8.5 (d, IH), 7.2 (dd, IH), 2.6 (s, 3H); GCMS 135 (M+).
Preparation of Intermediate P: 4-Ethyl-3-acetylpyridine
Stepl. 3-Acetylρyridine (5.0 g, 0.0413 mol), copper iodide (7.86 g, 0.0413 mol) and dimethyl sulfide (20.0 mL, 0.272 mol) were dissolved in THF (100 mL, anhydrous). This was stirred at rt for 15 min. To the reaction was added dropwise phenyl chloroformate (5.5 mL, 0.0441 mol) over 10 min. This reaction was then stirred under Ar for 1 h. The reaction was cooled to -25 °C and ethylmagnesium bromide (1M in THF, 44.1 mL, 0.0441 mol) was added dropwise over 40 min. The reaction was stirred at -25 °C for 30 min, then was wanned to rt and quenched with 20% NH4C1 (35 mL). The mixture was extracted with EtOAc, washed with 20% NH4C1, brine, and then dried over sodium sulfate. Regioisomers were produced in a 2:1 ratio (desired: undesired). The organic was concentrated to dryness
and the crude oil was purified by column chromatography (mobile phase 5% EtOAc/Hexane). Phenyl 3-acetyl-4-ethyl-l(4H)-pyridine carboxylate was obtained as an orange oil in 40.6 % yield, (4.55 g). Step 2. Phenyl 3-acetyl-4-ethyl-l(4H)-pyridinecarboxylate (3.26 g, 0.0120 mol) and sulfur (0.385 g, 0.0120 mol) were dissolved into decalin (15 mL). The reaction was heated to reflux for 17 h under Ar. The reaction mixture was then poured onto a silica gel column and washed with copious amounts of hexane. The product was then eluted from the column with a gradient mobile phase (5% EtOAc/hexane to 30% EtOAc/hexane). The product-containing fractions were concentrated to dryness to give an orange oil, 1.16 g (64.8%): R/0.12 (20% EtOAc/hexane).
Preparation of Intermediate Q: 4-(2~Propyl)-3-acetylpyridine
Step 1. To a mixture of Cul (78.5g, 0.412 mol), dimethyl sulphide (203 mL, 2.76 mol) and 3-acetyl pyridine (50.0g, 0.412 mol) in anhydrous THF (1100 mL) at rt was added phenyl chloroformate (55.2 mL, 0.44 mol) and the mixture was stirred for 40-50 min. To this suspension at -25 to -20°C was added isopropyl magnesium chloride (220 mL, 0.44 mol, 2.0 M solution in THF) over 30-40 min. The mixture was stirred at this temperature for 30 min, then warmed slowly to rt over 1.0-1.5 h. The reaction mixture was quenched with 20% NH C1 (350 mL), followed by extraction of the aqueous layer with EtOAc (700 mL). The organic layer was washed with 20% NH4C1 (350 mL), then brine (250 mL) and dried over anhydrous Na2SO4. Silica gel chromatography using a 3-10% EtOAc-hexane gradiant yielded 43.5 g of crude 3-acetyl-4-isopropyl-l-phenoxycarbonyl-l,4-dihydropyridine. Step 2. A mixture of the crude dihydropyridine (43.5 g, 0.153 mol) and sulphur (4.9 g, 0.153 mol) were heated at reflux in decalin (175 mL) for 3 h, then cooled to rt. Purificationby silica gel chromatography, eluting first with hexanes, then with a 5-30% EtOAc-hexane gradiant, gave 19.3 g (78%) of the title compound: TLC R 0.19 (25% EtOAc-hexane); GCMS (El) tR = 6.2 min, 163 (M+); 1H NMR (CDC13) δ 8.76 (s, IH), 8.57 (d, IH), 7.30 (d, IH), 3.55 (m, IH), 2.60 (s, 3H), 1.22 (d, 6H).
Preparation of Intermediate R: 4-Cyclopropyl-3-acetylpyridine
Step 1. Cyclopropyl bromide (50.0 g, 413 mmol) was dissolved in 500 mL of anhydrous THF. Dry magnesium (lO.O g, 411 mmol) was charged to a round-bottomed flask containing a catalytic amount of iodine. 20% of the solution of the cyclopropyl bromide solution was then charged into the flask. After observing bubble formation, the remaining cyclopropyl bromide solution was added over 15 min, thereby causing the reaction mixture to reflux. After 30 min, a 5.0 mL aliquot of the reaction mixture was removed to determine the concentration of the Grignard reagent. This analysis was performed according to the following procedure: 2 mg of 1,10 phenanthroline was added to a 50 mL flask with 10 mL of benzene; the 5.0 mL aliquot was then added; and the resulting mixture was titrated to the reddish-purple endpoint with 2.4 mL of 1.0 M butan-2-ol in p-xylene. Concentration was thus 0.48 M, which implied a 58% conversion to the desired Grignard reagent. Step 2. 780 mg of Cul (4.10 mmol) was added to a round-bottomed flask under inert (Ar) conditions. A suspension was then formed by the addition of 100 mL of THF. 40 mL of dimethyl sulfide was added, yielding a clear yellow solution. 3-Acetylpyridine (10.0 g, 82.7 mmol) was then dissolved in 70 mL of THF and added to the yellow solution. Finally, 13.6 g (86.8 mmol) of phenyl chloroformate was dissolved in 50 mL of THF and the resulting solution was added slowly, resulting in the formation of a precipitate. The mixture was then cooled to -20 °C by packing the flask in dry ice. 172 mL (82.6 mmol) of the Grignard solution from above was then added dropwise over 20 minutes while maintaining the temperature below -5 °C. The reaction mixture was allowed to warm to rt and then quenched with 400 mL of 20% aqueous ammonium chloride. Ethyl acetate (200 mL) was • added. The organic layer was collected and the aqeuous layer was washed with ethyl acetate (400 mL). The organic layers were combined, washed with brine, and then concentrated in vacuo. The residue was dissolved in dichloromethane and chromatographed on silica gel using a Biotage Flash 75 L column, first eluting with 2 L of 10% EtOAc-hexane, and then eluting with 4 L of 15% EtOAc-hexane. The fractions containing the desired compound
were combined and concentrated in vacuo, providing 12.2 g of an oil: 1H NMR (CDC13) δ 7.98 (s, IH, broad) 7.44 (t, 2H), 7.31 (t, IH), 7.21 (d, 2H), 6.99 (s, IH, broad), 5.20 (s, IH, broad), 3.23 (t, IH, broad), 2.40 (s, 3H), 0.91 (m, IH), 0.53-0.33 (m, 3H), 0.20 (sx, IH); LCMS (ES) m/z 284.0 (M+H+). Step 3. 12.2 g (43.0 mmol) of the dihydropyridine was transfened into a round-bottomed flask containing 143 mL of decahydronaphthalene. Sulfur (1.38 g, 43.0 mmol) was added and the flask was heated in an oil bath at 180 °C. Over 4 h an additional 1.38 g of sulfur was added. The heat was then turned off and the reaction was diluted with 500 mL of MTBE. The organic layer was extracted twice with 250 mL portions of 1.0 N HCl. 500 mL of dichloromethane was added to the aqueous layer, which was then made basic with 1.0 N
NaOH. The organic layer was then washed with 250 mL of brine, dried with sodium sulfate, filtered, and concentrated to obtain 2.13 g of an oil. The acidic aqueous layers were extracted again with 500 mL of dichloromethane. The organic layer was dried with sodium sulfate, filtered into the oil obtained from above, and concentrated in vacuo to obtain a total of 3.63 g (27% from 3-acetylpyridine): 1H NMR (CDC13) δ 8.83 (s, IH), 8.54 (d, IH), 6.93 (d, IH), 2.71 (m, IH), 2.71 (s, 3H), 1.28 (d, 2H), 0.92 (d, 2H); LCMS (ES) m/z 162.1 (MH+); GCMS (CI) m/z 162 (M+H+).
Preparation of Intermediate S: 4-(l-Propyl)-3-acetylpyridine
4-(l-Propyl)-3-acetylpyridine was prepared according to the method used to prepare 4-ethyl- 3-acetylpyridine: LCMS tR = 0.82 min; 164 (M+H+); 1H NMR (CDC13) δ 8.86 (s, IH), 8.56 (d, J= 5 Hz, IH), 7.20 (d, J= 5 Hz, IH), 2.85 (t, J= 8 Hz, 2H), 2.63 (s, 3H), 1.61 (m, 2H), 0.97 1, J= 7 Hz, 3H).
Preparation of Intermediate T: 4-(fert-Butyl)-3-acetylpyridine
4-(tert-Butyl)-3 -acetylpyridine was prepared according to the method used to prepare 4- ethyl-3 -acetylpyridine to first give the intermediate phenyl 3-acetyl-4-tert-butyl-l(4H)- pyridinecarboxylate [HPLC tR = 3.32 min; TLC R = 0.51 (5% EtOAc/hexane); 1H NMR (CD2C12) δ 0.82 (s, 9H), 2.38 (s, 3H), 3.44 (d, IH), 5.36-5.32 (m, IH), 6.82 (d, IH) 7.48- 7.19 (m, 5H), 8.02 (s, IH); LCMS (ES) m/z 300.3 (M+H+)], which was then aromatized with sulfur to give the desired product 4-(tert-butyl)-3-acetylpyridine: HPLC: tR = 0.28; TLC Rf 0.31 (EtOAc); LCMS (ES) m/z 177.9 (M+H+).
General Method D.
Step 1. A solution of the acetylpyridine (1 eq) and N, N-dimethylformamide dimethyl acetal (3 eq) is heated at 105 °C for 6.5 h. The volatile materials are evaporated and the crude enamine is used for the next reaction without fhrther purification.
Step 2. A solution of the crude enamine (100 mg, 1 eq), and the hydrazine (1.5 eq) in EtOH is refluxed for 2 h. The reaction mixture is concentrated and the residue is purified by HPLC or column chromatography to give the desired pyridyl pyrrole.
Table 5. Examples 156-166: Pyridyl Pyrrole Derivatives Prepared from 3-Acetyl Pyridine and from Intermediate O by General Method D.
Table 5 Note a: HPLC - electrospray mass spectra (HPLC ES-MS) were obtained using a Hewlett-Packard 1100 HPLC equipped with a quaternary pump, a variable wavelength detector set at 254 nm, a YMC pro C-18 column (2 x 23 mm, 120A), and a Finnigan LCQ ion trap mass spectrometer with electrospray ionization. Spectra were scanned from 120- 1200 amu using a variable ion time according to the number of ions in the source. The eluents were A: 2% acetonitrile in water with 0.02% TFA and B: 2% water in acetonitrile with 0.018% TFA. Gradient elution from 10% B to 95% over 3.5 minutes at a flowrate of
1.0 mL/min was used with an initial hold of 0.5 minutes and a final hold at 95% B of 0.5 minutes. Total run time was 6.5 minutes.
Table Note b: Molecular ion data obtained via electrospray ionization.
The following Examples of 4-(substituted)-3-(3-pyrazolyl)pyridine derivatives are prepared from Intermediates P-R according to General Method D:
Table 6. Examples 167-221: Pyridyl Pyrazole Derivatives which may be Prepared from Intermediates P-T by General Method D.
Determination of the activity of the compounds of the invention
C 17,20 Lyase inhibitory activity of compounds can be determined using, e.g., the biochemical or the cellular assays set forth in the Examples. A person of skill in the art will recognize that variants of these assays can also be used.
The compounds of the invention can also be tested in animal models, e.g., animal models of prostate or breast cancer.
Each of the compounds of the invention was subjected to a biochemical assay and a cellular assay for determining its C 17,20 lyase inhibitory activity.
Human and murine C17,20 lyase biochemical assays:
Recombinant human C 17,20 lyase (hLyase) was expressed in (Sf9) cells, and hLyase enriched microsomes were prepared from cultures as described in the following reference: Baculovirus Expression of Bovine P450 in Sf9 Cells and Comparison with Expression in Yeast, Mammalian Cells, and E. Coli. Barnes H. J.; Jenkins, C. M.; Waterman, M. R, Archives of Biochemistry and Biophysics (1994) 315(2) 489-494. Recombinant murine C 17,20 lyase (mLyase) was prepared in a similar manner. hLyase and mLyase preparations were titrated using assay conditions to determine protein concentrations to be used for assays. Both mLyase and hLyase assays were run in an identical manner except that cytochrome b5 was omitted in the murine assays.
Test compounds were diluted 1:4, serially in six steps, with 100% DMSO starting from 800 μM going to 51.2 nM reserving the first 2 columns for the generation of a standard curve. Each of these compound solutions in 100% DMSO was further diluted twenty fold in H2O to obtain compound concentrations ranging from 40 μM to 2.56 nM in 5% DMSO. Dehydroepiandrosterone (DHEA) standards were serially diluted in 100% DMSO from 400 μM down to 120 nM in half-log dilutions. Each dilution was further diluted twenty fold in H2O to obtain 20 μM to 6 nM solutions in 5% DMSO using the first 2 columns. Five μl of these 5% DMSO dilutions were used in the assay.
Clear-bottomed opaque 96 well assay plates were loaded with 50 μL of assay buffer (50 mM Na3PO , pH 7.5) and 5 μL of the diluted compounds were added to the wells. Thirty μL of substrate solution (7 mM NADPH (Sigma N1630), 3.35 μM 17-OH- pregnenolone (Steraloids Q4710), 3.35 μg/mL human cytochrome b5 (Panvera P2252) in 50 mM sodium phosphate pH 7.5 buffer) was added to all wells. Reactions were initiated with the addition of 10 μL hLyase or mLyase in assay buffer.
Enzymatic reactions were allowed to run for 2 hours at room temperature with gentle agitation. Reactions were terminated with the addition of 50 μM (final concentration) YM116, a potent C 17,20 lyase inhibitor. The concentration of DHEA generated by hLyase was detennined by radioimmunoassay (RIA) as described below.
0.08 μCi 3H-DHEA (1.6 μCi/mL) (NEN (NET814)) in scintillation proximity assay (SPA) buffer (100 mM Tris-HCl, pH 7.5, 50 mM NaCl, 0.5% BSA (Sigma A9647), 0.2% Tween 20) was added to each well. Fifty μL DHEA rabbit antiserum with anti-rabbit SPA beads in SPA buffer was added to all wells. Anti DHEA rabbit antiserum was obtained from Endocrine Sciences (D7-421) (1 mL H2O to the vial) and anti-Rabbit SPA Beads were obtained from Amersham (RPNQ 0016) (6mL SPA buffer to the bottle). Mixtures were allowed to equilibrate with gentle agitation for 1 hour followed by an overnight equilibration with no agitation. 3H-DHEA bound to the SPA beads was determined by scintillation counting. The concentration of DHEA generated in each reaction was calculated from raw data
(CPM) and the standard curve. The lyase inhibitory activity of each compound was determined as the concentration of DHEA generated in the presence of test compounds, expressed as a percent inhibition compared to the DHEA concentration generated in the absence of test compounds (l-(nM DHEA formed in the presence of test compound/nM DHEA formed in the absence of test compounds) x 100).
Human C17,20 cellular assay:
Human 293 lyase cells were prepared as described above for the Sf9 cells
[Baculovirus Expression of Bovine Cytochrome P 50 in Sf9 Cells and Comparison with Expression in Yeast, Mammalian Cells, and E.Coli. Barnes, H. J.; Jenkins, C. M.;
Waterman, M. R. Archives of Biochemistry and Biophysics (1994) 315 (2) 489-494]. The
cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) /10% FBS/ l%S/P/l%L-Glu/0.8mg/mLG418/HEPES.
On day one, human 293 lyase cells were plated at 10,000 cells/well/lOOμL in columns 2-12 of a 96-well tissue culture plate (Falcon 3075), and allowed to attach overnight (each mother plate needs two cell plates).
On day two, 100 μL H2O was added to all the wells of a daughter plate (one mother plate one daughter plate Costar 3365). DHEA standard was diluted with RPMI (4.5 μL of 500 μM into 3 mL RPMI, then 1:3 serial dilutions). The media from columns 2-12 of the cell plate was removed and replaced with 100 μL RPMI without phenol red. Diluted DHEA standards (100 μL) at a concentration of 750, 250, 83.3, 27.7, 9.2, 3, 1 and 0.3 nM were added to column 1 of the cell plate. 50 μL of 100% DMSO was added to columns 1 and 2 of the mother plate. 5 μL of compound was transfened from mother plate to daughter plate, then from the daughter plate to a cell plate using a robot. The cell plate was incubated for 10 minutes at room temperature. 15 μL of 10 mM 17-OH-pregnenolone (Steraloids (Q4710) (10 mM stock in 100% DMSO)) was diluted in 30 mL RPMI to obtain a solution of 5 μM 17-OH-pregnenolone. 10 μL of this solution was added to all the wells of the cell plate, except that column received only DMSO. The plate was then incubated for one hour at 37°C.
The amount of DHEA produced was determined as follows. 90 μL media was removed from each well of the cell plate and placed into an SPA assay plate (Wallac Isoplate #1450). 50 μL of 3H-DHEA (1.6 μCi/mL, New England Nuclear (Catalog # NET814)) was added to each well of the SPA assay plate. 50 μL of anti-DHEA anti-rabbit SPA beads (20 μL/mL AB with 10 mg/mL SPA beads) were then added to each well of the plate. The plate was incubated overnight, and the radioactivity counted as described above. The first two columns of the plate were reserved for a standard curve of DHEA and the no compound controls.
The raw data (CPM) was converted to a concentration of DHEA formed (nM) by use of the standard curve. The lyase inhibitory activity of the compounds was determined as the amount of DHEA formed in the presence of compound compared to the amount fonned in the absence of compound in the form of a percent inhibition (1- (nM DHEA formed with compound/nM DHEA formed without compound) x 100).
A test compound was considered to be active if the IC50 in the human C 17,20 biochemical assay or in the human C 17,20 cellular assay was less than 10 μM. All the
compounds tested have IC50 in the human C17,20 biochemical assay or the human C17,20 cellular assay of less than 10 μM.
Equivalents
Those skilled in the art will recognize, or be able to ascertain using no more than routine experimentation, many equivalents to the specific embodiments of the invention described herein. Such equivalents are intended to be encompassed by the following claims.