WO2001046315A2 - Vinylcyclohexan basierende einschichtfolien - Google Patents
Vinylcyclohexan basierende einschichtfolien Download PDFInfo
- Publication number
- WO2001046315A2 WO2001046315A2 PCT/EP2000/012483 EP0012483W WO0146315A2 WO 2001046315 A2 WO2001046315 A2 WO 2001046315A2 EP 0012483 W EP0012483 W EP 0012483W WO 0146315 A2 WO0146315 A2 WO 0146315A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- vinylcyclohexane
- polymer
- block
- weight
- Prior art date
Links
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D65/00—Wrappers or flexible covers; Packaging materials of special type or form
- B65D65/38—Packaging materials of special type or form
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/18—Manufacture of films or sheets
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2325/00—Characterised by the use of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring; Derivatives of such polymers
- C08J2325/02—Homopolymers or copolymers of hydrocarbons
Definitions
- the invention relates to a monofilm made of a homo- or copolymer based on vinylcyclohexane, which is characterized by low density, high transparency, an excellent water vapor barrier effect and good processability.
- the type and extent of defects are important for the barrier effect of a film material, since these can make a decisive contribution to the undesired diffusion of gases or aroma substances. This is particularly important because when thermoforming a sheet material, e.g. When deep-drawing for the production of blister packs, the influence of such defects on parts of the blister with thin walls becomes particularly great.
- PVC films are often used as packaging materials for moisture-sensitive goods. Since the water vapor barrier effect of pure PVC is often not sufficient, these films are additionally coated with polyvinylidene chloride.
- Halogen-containing polymers generally have densities greater than 2 g / cm 3 and thus a high specific basis weight. To avoid environmental pollution caused by the burning of halogen-containing polymers, efforts have been made for years to develop halogen-free polymers with a water vapor barrier effect.
- Polypropylene films are more advantageous from an ecological point of view and have good mechanical properties, but due to the partially crystalline nature of this thermoplastic, they have to be processed in narrow processing windows during thermoforming. The water vapor barrier effect of these materials can also be insufficient for many applications. Since the appearance of the packaging or the visual presentation of the packaged goods can be of great importance, a high level of transparency is often required. Polypropylene materials have disadvantages here.
- Polycycloolefins are made from comparatively expensive (co) monomers such as tetracyclododecene, ethylene tetracyclododecene, norbornene and others, which has to be assessed critically before the cost pressure mentioned above with polymeric packaging materials with barrier effect.
- Films made from polystyrene or polystyrene copolymers are known in the art. There are countless materials with a wide range of properties. Due to the wide range of applications of styrene-containing polymers (injection molded articles, polymer foams, etc.) in addition to the use of foils, the starting monomers used are very inexpensive.
- the present invention therefore relates to monofilms containing a vinylcyclohexane-based polymer or a mixture of vinylcyclohexane-based polymer with at least one polar polymer.
- the thickness of the film can vary widely depending on the application. It is generally 0.001 to 2 mm, preferably 0.005 to 1.5 mm, in particular 0.01 to 1 mm and very particularly preferably 0.01 to 0.7 mm. In the case of use as a flexible container, the thickness can also be up to 7 mm, preferably up to 5 mm.
- the Fc lie according to the invention can furthermore be provided with one or more layers, for example a paper or metal layer.
- Polymers based on vinylcyclohexane are understood to mean homopolymers of vinylcyclohexane and copolymers or block copolymers of vinylcyclohexane and other copolymers.
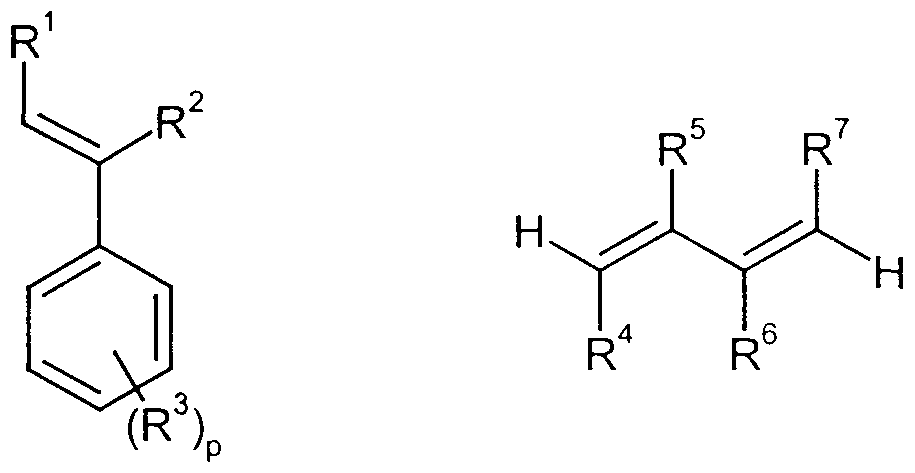
- Preferred as a homo- or copolymer is a vinylcyclohexane-based polymer with the recurring structural unit of the formula (I)
- Rl and R2 are independently hydrogen or are preferably C j -C 4 alkyl and
- R ⁇ and R ⁇ independently of one another for hydrogen or for C j -C 6 alkyl, preferably C ] -C 4 alkyl, in particular methyl and / or ethyl, or R ⁇ and R ⁇ together for alkylene, preferably C3 or C 4 -alkylene (fused-on 5- or 6-membered cycloaliphatic ring),
- R5 is hydrogen or C j -CG alkyl, preferably C j -C 4 - alkyl,
- Rl, R ⁇ and R ⁇ independently of one another in particular represent hydrogen or methyl.
- (optionally substituted polystyrene) can preferably be used and incorporated into the polymer: olefins with generally 2 to 10 carbon atoms, such as, for example and preferably, ethylene, propylene, isoprene, isobutylene, butadiene, particularly preferably Isoprene and / or butadiene, Cj-Cg- preferably C j -C 4 alkyl esters
- Acrylic or methacrylic acid unsaturated cycloaliphatic hydrocarbons, e.g. Cyclopentadiene, cyclohexene, cyclohexadiene, optionally substituted norbornene, dicyclopentadiene, dihydrocyclopentadiene, optionally substituted tetracyclododecenes, nuclear alkylated styrenes, ⁇ -methylstyrene, divinylbenzene, vinyl esters, vinyl acids, vinyl ethers, vinyl acetate, for example acrylic cyanide, such as vinyl cyanide,
- the polymers can generally contain up to 60% by weight, preferably up to 50% by weight, particularly preferably up to 40% by weight, of comonomers (based on the polymer).
- the polymers can very particularly preferably contain 1 to 30% by weight of comonomers.
- the vinylcyclohexane (co) polymers generally have absolute molecular weights Mw weight average of 1,000-10,000,000, preferably 60,000-1,000,000, very particularly preferably 70,000-600,000, determined by light scattering.
- the vinylcyclohexane-based polymers can be both iso- and syndiotactic.
- a group of particularly suitable polymers are the vinylcyclohexane-based polymers described in syndiotactic form, preferably with a syndiotactic diad fraction of from 50.1 to 74%, in particular from 52 to
- the polymers can have a linear chain structure as well as
- Units have branching points (eg graft copolymers).
- the branching centers contain, for example, star-shaped or branched polymers.
- the polymers according to the invention can have other geometric shapes of the primary, secondary, Tertiary, possibly quaternary, polymer structures have what is called their helix, double helix, leaflet, etc. or mixtures of these structures.
- Both random and block-like polymers can be used as copolymers.
- Block copolymers include di-blocks, tri-blocks, multi-blocks and star-shaped block copolymers.
- the vinylcyclohexane based (co) polymers are made by
- styrene with the corresponding monomers are polymerized radically, anionically, cationically, or by metal complex initiators or catalysts and the unsaturated aromatic bonds are then completely or partially hydrogenated (cf. e.g. WO 94/21694, EP-A 322 731).
- a block copolymer with at least three blocks is preferably used, which contains at least one hard block and at least one soft block, the hard block having at least 50, preferably 60, particularly preferably 65% by weight repeating units of the general formula (II)
- R and R ⁇ independently of one another denote hydrogen or Cj-Cö-alkyl, preferably C j -C ⁇ alkyl, R3 for hydrogen or for C 1 -C 6 -alkyl, preferably C 1 -C 4 -alkyl, in particular methyl and / or ethyl, or for fused alkylene, preferably C3 or C4-alkylene (fused-on 5- or 6-membered cycloaliphatic ring),
- p represents an integer from 0.1 to 5, preferably 0.1 to 3,
- the repetition units in the soft block can be distributed statistically, alternating or gradient.
- the repeating units according to formula (II) in the hard and soft block can be either the same or different.
- a hard block and a soft block can themselves in turn contain different repeating units according to formula (II).
- the hard blocks of the block copolymers which can be used according to the invention as polymer component A) may contain at most 35% by weight of further repeating units which are based on customary, optionally substituted olefinic comonomers, preferably cyclohexadiene, norbornene, dicyclopentadiene substituted by C 1 -C 4 -alkyl. Dihydrocyclopentadiene, tetracyclododecene, vinyl esters, vinyl ethers,
- the suitable block copolymer can optionally contain further soft blocks of repeating units based on saturated, optionally substituted by C ] -C4-alkyl, aliphatic hydrocarbon chains with 2 to 10, preferably 2 to 5 carbon atoms and their isomer form.
- the proportion of hard blocks (based on the total polymer) is generally 65 to 97% by weight, preferably 75 to 95% by weight, and the proportion of soft blocks 3 to 35% by weight, preferably 5 to 25% by weight. -%.
- the block copolymer that can be used generally has molecular weights (number average) of 5,000-1,000,000, preferably 50,000-500,000, particularly preferably 80,000-200,000, determined according to gel permeation chromatography, calibrated with the polystyrene standard.
- the molecular weight (number average) of the hard blocks is generally 650-970,000, preferably 6,500-480,000, particularly preferred
- the molecular weight of the soft blocks is generally 150-350,000, preferably 1,500-170,000, particularly preferably 2,400-70,000.
- the block copolymer can contain hard or soft blocks with different molecular weights.
- the linkage of the chain components can have a small proportion of head-to-head linkage.
- the copolymers can be linear or branched through centers. They can also have a star-shaped structure. Linear block copolymers are preferred in the context of this invention.
- the block copolymer can have different block structures, and the end blocks can be a hard or soft block independently of one another. For example, they can be structured as follows: A 1 - (B , - A) n ; B 1 - (A , - B ') n ; (A '- BX;
- the hard and soft blocks in the block copolymer are generally incompatible with each other. This incompatibility leads to phase separation on a microscopic scale.
- the polymer component which can be used as component A) is preferably prepared in such a way that in a living polymerization process vinyl aromatic monomers of the general formula (III) for the hard blocks and conjugated dienes of the general formula (IV) and optionally vinyl aromatic monomers of the general formula ( III) for the soft blocks
- R, R ⁇ , R3 and p have the meaning given above and R4 to R ⁇ are independently hydrogen, C] -C4 alkyl, preferably methyl,
- the monomers of the formula (III) can be either the same or different for the hard and soft block of the prepolymer.
- a hard block and a soft block can have different repeating units based on monomers of
- the following can preferably be used as comonomers in the polymerization and incorporated into the hard blocks: in each case cyclohexadiene, vinylcyclohexane, vinylcyclohexene, norbornene, dicyclopentadiene, dihydrocyclopentadiene, tetracyclododecene, nucleus-alkylated styrenes optionally substituted by C] -C4-alkyl. Methylstyrene, divinylbenzene, vinyl esters, vinyl ethers, vinyl acetate, maleic acid derivatives and (meth) acrylic acid derivatives, etc. or a mixture thereof.
- the prepolymer can be prepared by a living polymerization process, e.g. a living anionic polymerization or a living radical polymerization can be prepared.
- a living polymerization process which can be initiated by alkali metals or by alkali metal alkyl compounds such as methyl lithium and butylhthium is particularly suitable.
- Suitable solvents for such polymerization are hydrocarbons such as e.g. Cyclohexane, hexane, pentane, benzene, toluene, etc. and ethers such as e.g. Diethyl ether, methyl tert-butyl ether, tetrahydrofuran.
- Block copolymers with specific block segments can be produced by sequential addition of monomer or monomer mixture.
- a styrene-isoprene or butadiene diblock copolymer can be produced by adding the styrene monomer after the diene has fully polymerized.
- the chain structure is denoted by the symbol (I) m - (S) n or (B) m - (S) n or, in simplified terms, by IS or BS, m, n mean degree of polymerization in the respective blocks.
- block copolymers with a mixing block can be produced by using the favorable cross-polymerization parameters and starting the polymerization in a monomer mixture.
- styrene / butadiene diblock copolymer with a diene-rich mixing block can be used as Soft block by initiation in a mixture of styrene and
- the polymer chain contains a diene-rich soft block, a transition phase with increasing styrene incorporation rate and a styrene block that ends the chain.
- the chain structure is denoted by the symbol (I , / s ) m - (S) n or (B B / s ) m - (S) n or simplified by I IS S or B BS S, where I 1S and B BS each for the isoprene-rich and butadiene-rich
- HI IS S Stand soft block.
- HB BS S The corresponding hydrogenated products are referred to as HI IS S or HB BS S.
- multi-block copolymers can be produced with both mixed and certain soft blocks.
- Examples are Triblock SI 1S S, I IS SI and Pentablock S (I IS S) 2 , (I 1S S) 2 I.
- the symbols are self-explanatory.
- the corresponding hydrogenated products are referred to as H-SI 1S S, HI IS SI or HS (I IS S) 2 , H- (I IS S) 2 I.
- Control of the molecular weight in anionic polymerization is possible by varying the monomer / initiator ratio. The theoretical molecular weight can be calculated using the following equation:
- solvents such as solvents, co-solvents and cocatalysts can also have a sensitive effect on the chain structure.
- Hydrocarbons like. e.g. Cyclohexane, toluene or benzene are preferred as solvents for the polymerization in the present invention, since block copolymers with a mixing block can be formed in such solvents and the diene monomer preferably polymerizes to the highly elastic 1,4-polydiene.
- An oxygen or nitrogen containing cosolvent such as e.g.
- Tetrahydrofuran, dimethoxyethane or N, N, N ', N'-tetramethylethylenediamine effects a statistical polymerization and at the same time a preferred 1,2-polymerization of conjugated dienes. In contrast, it does
- Alkali metal alcoholate such as e.g. Lithium tert-butoxide also has a statistical polymerization, but has little influence on the 1,2- / 1,4-ratio of diene polymerization.
- the microstructure of the soft blocks in prepolymer is decisive for the microstructure of the soft blocks in the corresponding hydrogenated block copolymer.
- a poly-l, 4-butadiene block when hydrogenated to give a polyethylene
- the hydrogenation product of poly-1,2-butadiene has too high a glass transition temperature and is therefore not elastic.
- the hydrogenation of poly-butadiene block with suitable 1.2 / 1.4 ratios can provide an elastic poly (ethylene-co-butylene) segment.
- 1,4-polymerization is preferred, since an alternating poly (ethylene-propylene) elastomer block results after hydrogenation.
- Temperature, pressure and monomer concentration are largely uncritical for the polymerization.
- the preferred temperature, pressure and monomer concentration for the polymerization are in the range from -60 ° C. to 130 ° C., particularly preferably from 20 ° C. to 100 ° C, 0.8 to 6 bar and 5 to 30 wt .-% (based on the sum of the amount of monomer and solvent).
- the process for producing the block copolymers is carried out either with or without, but preferably without, a work-up between the polymerization and hydrogenation stages in order to isolate the prepolymer.
- Any workup can be carried out by known methods such as precipitation in a non-solvent such as Cj-C4 alcohol and C3-C5 ketone, evaporation extrusion or stripping, etc. are done.
- the prepolymer is redissolved in a solvent for hydrogenation.
- the prepolymer solution can be hydrogenated directly - if appropriate after chain termination and if appropriate diluted with the same inert solvent as in the polymerization or with another inert solvent.
- a saturated hydrocarbon such as e.g. Cyclohexane, hexane or mixtures thereof are particularly preferred as solvents for the process.
- the vinylcyclohexane-based copolymers or homopolymers are prepared by polymerizing derivatives of styrene with the corresponding monomers by radical, anionic, cationic or metal complex initiators or catalysts and then hydrogenating the unsaturated aromatic bonds completely or partially (cf. e.g. WO 94/21694, EP-A 322 731).
- WO 94/21694 describes a process for the complete hydrogenation of alkenyl aromatic polymers and poly (alkenyl aromatic) / polydiene block copolymers by heterogeneous catalysis.
- a large number of known hydrogenation catalysts can be used as catalysts.
- Preferred metal catalysts are mentioned, for example, in WO 94/21 694 or WO 96/34 896.
- Any catalyst known for the hydrogenation reaction can be used as the catalyst.
- Large catalysts are suitable Surface (e.g. 100 - 600 m 2 / g) and small average pore diameter (e.g. 20 - 500 ⁇ ).
- catalysts with a small surface area (for example> 10 m 2 / g) and large average pore diameters are also suitable, which are characterized by the fact that 98% of the pore volume has pores with pore diameters greater than 600 ⁇ (for example approx. 1,000 - 4,000 ⁇ ) (see e.g. US-A 5,654,253, US-A 5,612,422,
- JP-A 03076706 In particular, Raney nickel, nickel on silicon dioxide or silicon dioxide / aluminum oxide, nickel on carbon as a support and / or noble metal catalysts, for. B. Pt, Ru, Rh, Pd used.
- Polymer is generally 80 to 1, preferably 50 to 10, in particular 40 to 15% by weight.
- the hydrogenation is generally carried out at temperatures between 0 and 500 ° C, preferably between 20 and 250 ° C, in particular between 60 and 200 ° C.
- the reaction is generally carried out at pressures from 1 bar to 1000 bar, preferably 20 to 300 bar, in particular 40 to 200 bar.
- the process generally leads to a virtually complete hydrogenation of the aromatic units and, if appropriate, of double bonds in the main chain.
- the degree of hydrogenation is higher than 97%, particularly preferably higher than 99%.
- the degree of hydrogenation can be determined, for example, by NMR or UV spectroscopy.
- the amount of catalyst used depends on the procedure.
- the process can be carried out continuously, semi-continuously or batchwise.
- the ratio of catalyst to prepolymer is generally 0.3-0.001, preferably 0.2-0.005, particularly preferably 0.15-0.01.
- the invention further relates to a process for producing the film according to the invention by the extrusion process known per se.
- the procedure is such that the polymer is metered into a conveyor screw via a metering device, heated and melted and extruded as a melt through a flat die.
- the melt film is drawn off via a roller system, the film is oriented one after the other or simultaneously in the longitudinal direction and / or in the transverse direction with or without tempering (in the longitudinal direction by means of snow-running rollers, in the transverse direction, for example with the aid of a tenter frame), heat-set, corona or flame treated and finally wound. It has proven to be advantageous to temper the draw-off roller or rollers in accordance with the glass transition temperature of the polymer.
- the film can also be stretched.
- the layer containing the polyvinylcyclohexane-based polymer can contain a further polar polymer in addition to the vinylcyclohexane-based polymer. Based on the lower transparency of single-layer films
- polar polymers are preferably selected from the group consisting of polycarbonate, polyamide, polyester and polyurethane or mixtures thereof, particularly preferably
- the layer preferably contains up to 8% by weight of a polar polymer or a mixture thereof based on the total amount of polymer in this layer; 0.1 to 6% by weight, in particular 0.5 to 5% by weight, are particularly preferred. % of a polar polymer or a mixture thereof, very particularly preferably the layer contains up to 3% by weight of polar monomer.
- Suitable polyamides are known homopolyamides, copolyamides and mixtures of these polyamides. These can be partially crystalline and or amorphous polyamides.
- Polyamide-6, polyamide-6,6, mixtures and corresponding copolymers of these components are suitable as partially crystalline polyamides.
- partially crystalline polyamides the acid component of which is wholly or partly selected from at least one acid consisting of the group consisting of terephthalic acid, isophthalic acid, suberic acid, sebacic acid, azelaic acid, adipic acid, cyclohexanedicarboxylic acid, the diamine component of which is wholly or partly selected from m - and / or p-xylylene diamine, hexamethylene diamine, 2,2,4-trimethylhexamethylene diamine, 2,4,4-trimethylhexamethylene diamine and isophorone diamine and the composition of which is known in principle.
- polyamides which are made wholly or partly from lactams with 7 to 12 carbon atoms in the ring, optionally with the use of one or more of the starting components mentioned above.
- Particularly preferred partially crystalline polyamides are polyamide 6 and polyamide 6,6 and their mixtures.
- Known products can be used as amorphous polyamides. They are obtained by polycondensation of diamines, preferably selected from ethylene diamine, hexamethylene diamine, decamethylene diamine, 2,2,4- and / or 2,4,4-trimethylhexamethylene diamine, m- and / or p-xylylene diamine, bis- (4- aminocyclohexyl) methane, bis (4-aminocyclohexyl) propane, 3,3'-dimethyl
- 4,4'-diamino-dicyclohexylmethane 3-aminomethyl-3,5,5-trimethylcyclohexylamine, 2,5- and / or 2,6-bis- (aminomethyl) norbornane and or 1,4-diaminomethylcyclohexane or mixtures thereof with dicarboxylic acids, preferably selected from oxalic acid, adipic acid, azelaic acid, decanedicarboxylic acid, heptadecanedicarboxylic acid, 2, 2,4- and / or 2,4,4-trimethyladipic acid, isophthalic acid and terephthalic acid or mixtures thereof.
- dicarboxylic acids preferably selected from oxalic acid, adipic acid, azelaic acid, decanedicarboxylic acid, heptadecanedicarboxylic acid, 2, 2,4- and / or 2,4,4-trimethyladipic acid, isophthalic
- Copolymers which are obtained by polycondensation of several monomers are also suitable, and copolymers which are prepared with the addition of aminocarboxylic acids such as aminocaproic acid, aminoundecanoic acid or aminolauric acid or their lacquer.
- aminocarboxylic acids such as aminocaproic acid, aminoundecanoic acid or aminolauric acid or their lacquer.
- Particularly suitable amorphous polyamides are the polyamides made from isophthalic acid, hexamethylene diamine and other diamines such as 4,4'-diaminodicyclohexyl methane, isophorone diamine, 2,2,4- and or 2,4,4-trimethylhexamethylene diamine, 2,5- and / or 2,6-bis (aminomethyl) norbornene; or from isophthalic acid,
- 4,4'-diamino-dicyclohexylmethane and caprolactam or from isophthalic acid, 3,3'-dimethyl-4,4'-diamino-dicyclohexylmethane and laurolactam; or from terephthalic acid and the isomer mixture of 2,24- and / or 2,4,4-trimethylhexamethylene diamine.
- polyamide-6, polyamide-6,6, polyamide-8, -10, -1 1 and -12 are preferred.
- Polyamide-6 and polyamide-6,6 are particularly preferred.
- the polyamides preferably have a relative viscosity (measured on a 1% strength by weight solution in m-cresol at 25 ° C.) from 2 to 5, particularly preferably from 2.5 to 4.
- Preferred polyalkylene terephthalates can be prepared from terephthalic acid (or its reactive derivatives) and aliphatic or cycloaliphatic diols with 2 to 10 carbon atoms according to known methods (Kunststoff-Handbuch, Vol. VIII, p. 695 ff, Karl-HanserNerlag, Kunststoff 1973) ,
- Preferred polyalkylene terephthalates contain at least 80, preferably 90 mol%, based on the dicarboxylic acid component, terephthalic acid residues and at least 80, preferably at least 90 mol%, based on the diol component, diols selected from the group consisting of ethylene glycol, propylene glycol, 1,4- Butanediol, cyclohexane 1, 4-dimethanol or mixtures thereof.
- the preferred polyalkylene terephthalates can contain up to
- other aromatic dicarboxylic acids with 8 to 14 carbon atoms or ahphatic dicarboxylic acids with 4 to 12 carbon atoms such as residues of phthalic acid, isophthalic acid, ⁇ aphthalene-2,6-dicarboxylic acid, 4 , 4'-diphenyldicarboxylic acid, succinic, adipic, sebacic acid, azelaic acid, cyclohexanediacetic acid
- the preferred polyalkylene terephthalates can in addition to the above. Contain diols up to 20 mol%, preferably up to 10 mol%, other aliphatic diols with 3 to 12 carbon atoms or cycloaliphatic diols with 6 to 21 carbon atoms, for example and preferably residues of 1,3-propanediol -Ethylpropandiol-1, 3, neopentylglycol,
- the polyalkylene terephthalates can be prepared by incorporating relatively small amounts of trihydric or tetravalent alcohols or 3- or 4-basic carboxylic acid, e.g. are described in DE-A 19 00 270 and US-A 3 692 744.
- preferred branching agents are trimesic acid, trimellitic acid, trimethylolethane and propane and pentaerythritol.
- polyalkylene terephthalates which are composed solely of terephthalic acid and ethylene glycol, propylene glycol or 1,4-butanediol or their copolymers with 1,4-dicyclohexanedimethanol and mixtures of these polyalkylene terephthalates.
- the polyalkylene terephthalates generally have an intrinsic viscosity of about 0.4 to 1.5 dl / g, preferably 0.5 to 1.3 dl / g, each measured in phenol / o-dichlorobenzene (1: 1 wt . Parts) at 25 ° C.
- Polyesters are described for example in EP-A774490.
- Polyolefins in the sense of the invention are polyethylene, polypropylene, poly-1-butene and polymethylpentene, which may contain small amounts of non-conjugated dienes in copolymerized form. Polyolefins are known and are described in Roempp's chemistry dictionary and in the literature cited therein. Polypropylene is preferred. Polycarbonates in the sense of the invention are those as described, for example, in EP-A 640 655.
- Preferred aromatic polycarbonates are polycarbonates based on 2,2-bis (4-hydroxyphenyl) propane or one of the other diphenols mentioned as preferred in EP-A 640 655. Those based on 2,2-bis (4-hydroxyphenyl) propane, 2,2-bis (3,5-dimethyl-4-hydroxyphenyl) propane or 1,1-bis- are very particularly preferred. (4-hydroxyphenyl) -3,3,5-trimethylcyclohexane or mixtures of 2,2-bis (4-hydroxyphenyl) propane and 1,1-bis (4-hydroxyphenyl-3,3,5-trimethylcyclohexane ,
- Polycarbonates based on bisphenol A are very particularly preferred.
- the aromatic polycarbonates generally have average molecular weights M w of approximately 10,000 to 200,000, preferably 20,000 to 80,000 (determined by gel chromatography after prior calibration).
- Additives may be added to the film according to the invention, which are added to the polymer or the polymer mixture used for film production before or during the
- Processing can be added.
- the aim of such an additive is to facilitate processing or to improve the properties of the end product.
- Important groups of additives are antiblocking agents, thermal or oxidation stabilizers, antistatic agents, bio-stabilizers, coloring agents, lubricants, light protection agents, optical brighteners, additives to improve printability, etc.
- Suitable antiblocking agents are inorganic additives such as silicon dioxide, calcium carbonate, magnesium silicate, aluminum silicate, calcium phosphate and the like and / or incompatible organic polymers such as polyamides, polyesters, polyurethanes, polycarbonates etc.
- the effective amount of antiblocking agent lies in
- the particle size is between 1 and 6 ⁇ m, in particular 2 and 5 ⁇ m, particles with a spherical shape being particularly suitable.
- thermal stabilizers can be used as stabilizers.
- sterically hindered phenols, phosphorus compounds and lactone derivatives can be used either alone or as binary or ternary mixtures.
- Ternary mixtures in particular those made from Igranox 1010 (phenol component), Irgafos P-EPQ (phosphorus compound) and HP 136 (lactone derivative) from CIBA Specialty Chemicals, Basel, Switzerland, are particularly preferred.
- Preferred antistatic agents are alkali alkane sulfonates, polyether-modified, ie ethoxylated and / or propoxylated polydiorganosiloxanes (polydialkylsiloxanes, polyalkylphenylsiloxanes etc.) and / or the essentially straight-chain and saturated aliphatic, tertiary amines with an aliphatic radical having 10 to 20 carbon atoms -Hydroxy- (-C-C 4 ) alkyl groups are substituted, N, N-bis (2-hydroxyethyl) alkylamines having 10 to 20 carbon atoms, preferably 12 to 18 carbon atoms, being particularly suitable in the alkyl radical.
- the effective amount of antistatic is in the range of 0.05 to 0.3% by weight.
- Suitable dyes are dyes and pigments, which can be of an inorganic or organic nature. Examples are titanium dioxide, carbon black, oxides and / or mixed oxides of chromium, nickel, iron, azo pigments and phthalocyanines.
- Lubricants are hydrocarbons such as paraffin oils, waxes (e.g. polyethylene and
- Polypropylene waxes higher alcohols and carboxylic acids, carboxylic acid esters and amides, glycerides, higher ahphatic acid amides, higher ahphatic acid esters and polydimethylsiloxanes.
- the effective amount of lubricant is in the range of 0.01 to 4% by weight, particularly preferably 0.25 to 1% by weight.
- Light stabilizers are additives that protect films from exposure to light. Different classes are distinguished depending on the mechanism of action. Most films contain hydroperoxide decomposers (metal complexes, sulfur-containing organic compounds), quenchers (e.g. nickel complexes of phenols containing sulfide groups), radical scavengers (e.g. sterically hindered amines) and less often UV-
- Absorbers e.g. hydroxybenzophenones, hydroxyphenybenzotriazoles
- the effective amount of light stabilizers is between 0.01 and 3% by weight, particularly preferably 0.5 and 2% by weight.
- Optical brighteners are additives that compensate for the yellowish appearance of some plastic films (blue effect) caused by the absorption of blue wavelength ranges. Examples are triazine-phenycoumarins, benzotriazole-phenyl-coumarins and benzooxazoles.
- the effective amount is between 0.001 and 0.1% by weight, particularly preferably 0.01 and 0.05% by weight.
- Low molecular weight resins can be added to further improve the desired physical properties (eg film stiffness, shrinkage behavior, optics, water vapor permeability).
- Compatible hydrocarbon resins are low molecular weight polymers whose molecular weight M w is generally in a range from 300 to 8,000, preferably 400 to 5,000, particularly preferably 500 to
- the proportion of the resin is in a range of 1 to 30% by weight, preferably 2 to 30% by weight. Hydrocarbon resins are preferred.
- the printability of the film can be improved without a significant loss of the water vapor barrier effect by appropriately increasing the polarity of the surface. This can be done by corona treatment or by adding organic or inorganic additives.
- Polymers such as polypropylene or polyethylene as homopolymers or copolymers, polyesters, polyamides, polycarbonate, polyacetals, polyurethanes, acrylate or methacrylate polymers or mixtures thereof are suitable as organic additives.
- the layer preferably contains 0.05 up to 8% by weight. Titanium dioxide, barium sulfate, calcium sulfate or calcium carbonate are suitable as inorganic pigments.
- Sealing layers can be applied to the films according to the invention in order to improve the adhesion to films or containers made of thermoplastic polymers.
- the sealing layers must be adapted.
- materials for sealing layers e.g. Ethylene and propylene polymers with different proportions of polar groups as they result from the copolymerization e.g. with vinyl acetate, acrylate monomers are available or polymers based on copolymers of ethylene or propylene with alpha-olefins and polar monomers are used.
- grafted (e.g. maleic anhydride) ethylene and propylene copolymers can be used as sealing layers.
- the sealing temperatures can be set by selecting the correct composition.
- the films according to the invention can be used in a variety of ways, e.g. as food packaging, blister packaging, packaging of electronic items and electrical capacitor foils ..
- Example 1 non-oriented film made of PVCH homopolymer
- the autoclave is flushed with inert gas (nitrogen).
- the polymer solution used is filtered through a filter SBF-101-S16 (Loeffler, Filter-Technik, Nettersheim, Germany) and added to the autoclave together with the catalyst (Table 1).
- inert gas and then hydrogen are applied several times.
- the respective hydrogen pressure is set and heated to the corresponding reaction temperature with stirring.
- the reaction pressure is kept constant after the onset of hydrogen uptake.
- the reaction time is defined from the heating up of the batch to the time when the hydrogen uptake approaches its saturation value.
- the polymer solution is filtered through a pressure filter which is covered with a Teflon fabric (B43-MU10, company Dr. M, Gurnnendorf, Switzerland).
- the product solution is then filtered through a 0.2 ⁇ m Teflon filter (Pall Filtertechnik, Dreieich, Germany).
- the polymer solution is stabilized with Irganox XP 420 FF (CIBA Specialty Chemicals, Basel, Switzerland) and filtered again with the 0.2 ⁇ m filter mentioned.
- the polymer solution is then freed from the solvent at 240 ° C., the melt is filtered through a 20 ⁇ m filter and the product is processed into granules. Neither aromatic nor olefinic carbon-carbon double bonds can be detected using 1 H NMR spectroscopy. Table 1
- the PVCH homopolymer from Example 1 is at a melt temperature of 310 ° C with a single screw extruder (screw diameter 60 mm, screw length
- This triblock copolymer made up of PVCH and alternating ethylene-propylene (E / P) middle block soft segments with a PVCH gradient (E / P content
- polymer synthesis The polymer is prepared analogously to Example 3 from 153 g of styrene and 27 g
- the temperature of the casting roll is 120 ° C.
- a 50 ⁇ m thick, clearly transparent film was obtained which had a take-off speed of 6 m / min. was wound up.
- Extruder (30 mm screw diameter, screw length 25D) via a flat extruded film nozzle (220 mm lip width, gap width adjustable between 0.01 - 0.6 mm).
- the temperature of the casting roll is 90 ° C.
- a 50 ⁇ m thick, clearly transparent film is obtained, which has a peeling speed of 7.3 m / min. was wound up.
- the melt flow index is measured according to DIN ISO 1133 with a Meltflixer LT from SWO Polymertechnik GmbH at 280 ° C under a weight of 2.16 kg.
- the weight average molecular weight M w which is given in the description of the examples, indicates the weight average molecular weight.
- a two-detector gel permeation chromatography is used for its determination (0.5% by weight solution, solvent tetrahydrofuran, 25 ° C., polystyrene standard).
- the detection is carried out by means of UV absorption spectroscopy at 254 nm and by means of Differential refractometry of the fractions.
- the calibration is carried out using several polystyrene standards of known molecular weights.
- Injection molded standard bodies (flat bars 80x10x4 mm) of the samples are examined by means of DMA (dynamic mechanical analysis).
- the complex thrust module is determined depending on the temperature at a measuring frequency of 1 Hz.
- the test is based on DIN EN ISO 527 on 15 mm wide test strips.
- the tensile modulus of elasticity results from the quotient of the stress difference at 0.05 and 0.25% elongation in relation to the clamping cross-section.
- the tensile strength is the maximum stress that the sample carries during the tensile test.
- the elongation at break is the elongation when the sample breaks.
- the water vapor permeability WDD of the samples is determined by two different methods depending on the water vapor permeability.
- the determination according to DIN 53 122, Part 1 is based on the gravimetric method, with water vapor permeability ⁇ 1 gd " - ⁇ becomes the determination based on DIN 53 122 Part 2 made after the electrolysis process.
- the regularly reflected light component is referred to as gloss, based on a light beam incident at 20 ° to the plumb line.
- the gloss is specified in gloss units GE, which are based on a black glass standard.
- Table 2 contains the water vapor permeability measured in Examples 1-4 as well as optical and mechanical measurement data. It can be seen that polyvinylcyclohexane-based monofilms have a specific water vapor barrier effect greater than polystyrene films by at least a decimal potency. It is clear from example 3 that a comonomer incorporation results in a further improvement in the water vapor barrier effect. The films are highly transparent, as evidenced by the low levels of haze. Water vapor barrier films based on vinylcyclohexane-containing polymers expand the technically available spectrum of commercial water vapor barrier films by highly transparent polyolefin films based on inexpensive raw materials.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Health & Medical Sciences (AREA)
- Mechanical Engineering (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Laminated Bodies (AREA)
Abstract
Description
Claims
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU30090/01A AU3009001A (en) | 1999-12-22 | 2000-12-11 | Vinylcyclohexane-based single layer films |
| JP2001547219A JP2003518186A (ja) | 1999-12-22 | 2000-12-11 | ビニルシクロヘキサン系単層フィルム |
| EP00990699A EP1244745A2 (de) | 1999-12-22 | 2000-12-11 | Vinylcyclohexan basierende einschichtfolien |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19962018A DE19962018A1 (de) | 1999-12-22 | 1999-12-22 | Vinylcyclohexan basierende Einschichtfolien |
| DE19962018.0 | 1999-12-22 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2001046315A2 true WO2001046315A2 (de) | 2001-06-28 |
| WO2001046315A3 WO2001046315A3 (de) | 2001-12-06 |
Family
ID=7933815
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2000/012483 WO2001046315A2 (de) | 1999-12-22 | 2000-12-11 | Vinylcyclohexan basierende einschichtfolien |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20030027937A1 (de) |
| EP (1) | EP1244745A2 (de) |
| JP (1) | JP2003518186A (de) |
| AU (1) | AU3009001A (de) |
| DE (1) | DE19962018A1 (de) |
| TW (1) | TW546341B (de) |
| WO (1) | WO2001046315A2 (de) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10007672A1 (de) * | 2000-02-19 | 2001-08-23 | Mitsubishi Polyester Film Gmbh | Weiß-opake, schwer entflammbare, UV-stabilisierte Folie mit niedriger Transparenz aus einem kristallisierbaren Thermoplasten |
| JP5027718B2 (ja) * | 2008-04-08 | 2012-09-19 | 株式会社日本触媒 | 熱可塑性樹脂体とその製造方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1054303A (de) * | 1962-07-20 | |||
| DE1131885B (de) * | 1961-01-28 | 1962-06-20 | Basf Ag | Verfahren zur Herstellung von Polymerisaten des Vinylcyclohexans |
| US3051670A (en) * | 1959-11-13 | 1962-08-28 | American Viscose Corp | Coating composition comprising cellulose derivative and a crystalline polymer |
| WO1999032528A1 (de) * | 1997-12-18 | 1999-07-01 | Bayer Aktiengesellschaft | Vinylcyclohexan basierende polymere |
-
1999
- 1999-12-22 DE DE19962018A patent/DE19962018A1/de not_active Withdrawn
-
2000
- 2000-12-08 TW TW089126134A patent/TW546341B/zh not_active IP Right Cessation
- 2000-12-11 JP JP2001547219A patent/JP2003518186A/ja active Pending
- 2000-12-11 US US10/168,641 patent/US20030027937A1/en not_active Abandoned
- 2000-12-11 EP EP00990699A patent/EP1244745A2/de not_active Withdrawn
- 2000-12-11 WO PCT/EP2000/012483 patent/WO2001046315A2/de active Application Filing
- 2000-12-11 AU AU30090/01A patent/AU3009001A/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3051670A (en) * | 1959-11-13 | 1962-08-28 | American Viscose Corp | Coating composition comprising cellulose derivative and a crystalline polymer |
| DE1131885B (de) * | 1961-01-28 | 1962-06-20 | Basf Ag | Verfahren zur Herstellung von Polymerisaten des Vinylcyclohexans |
| GB1054303A (de) * | 1962-07-20 | |||
| WO1999032528A1 (de) * | 1997-12-18 | 1999-07-01 | Bayer Aktiengesellschaft | Vinylcyclohexan basierende polymere |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2001046315A3 (de) | 2001-12-06 |
| DE19962018A1 (de) | 2001-06-28 |
| TW546341B (en) | 2003-08-11 |
| EP1244745A2 (de) | 2002-10-02 |
| JP2003518186A (ja) | 2003-06-03 |
| AU3009001A (en) | 2001-07-03 |
| US20030027937A1 (en) | 2003-02-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE112006000818B4 (de) | Hydrierungsprodukt eines Blockcopolymers, Zusammensetzung und Verwendung | |
| EP0765909B1 (de) | Polyolefinfolie mit Cycloolefinpolymer, Verfahren zu ihrer Herstellung und ihre Verwendung | |
| EP1498438B1 (de) | Blockcopolymer und zusammensetzung davon | |
| DE112006002443T5 (de) | Blockcopolymeres und Verfahren zur Herstellung einer Schrumpffolie | |
| DE4313430A1 (de) | Matte, biaxial orientierte Polypropylen-Mehrschichtfolie, Verfahren zu ihrer Herstellung und ihre Verwendung | |
| DE60020710T2 (de) | Hydrierte blockcopolymerzusammensetzungen und verwendungen davon | |
| WO2000032646A1 (de) | Blockcopolymere auf vinylcyclohexanbasis | |
| DE19815895C2 (de) | Hydrierte Blockcopolymere, diese enthaltende Zusammensetzungen und Formkörper | |
| DE2443873A1 (de) | Weichgemachte, thermoplastische copolymere | |
| DE10392130T5 (de) | Polymerharz auf Styrol-Basis und Zusammensetzung desselben | |
| EP0195505B1 (de) | Polypropylenzusammensetzung und daraus zweiaxial verstreckter Film | |
| EP1266935B1 (de) | Styrol-copolymerzusammensetzung | |
| DE60131904T2 (de) | Blockcopolymerzusammensetzung | |
| DE69107238T2 (de) | Polyolefinharzzusammensetzungen und Verfahren zu ihrer Herstellung. | |
| DE2156681C3 (de) | Polymere Massen auf der Basis von Polypropylen | |
| DE3938927C2 (de) | ||
| DE69811704T2 (de) | Kautschukmodifizierte monovinyliden-aromatische polymermischungen | |
| DE4318031A1 (de) | Harzhaltige, biaxial orientierte Polypropylen-Mehrschichtfolie, Verfahren zu ihrer Herstellung und ihre Verwendung | |
| EP0651011B1 (de) | Harzhaltige, biaxial orientierte Polypropylenfolie, Verfahren zu ihrer Herstellung und ihre Verwendung | |
| DE60300724T2 (de) | Thermoplastische Polymersetzungen mit Barriereigenschaften, Folie oder Schicht, Schichtstruktur, Verpackungsmaterial, Behälter und Verpackungskissen | |
| WO2001046315A2 (de) | Vinylcyclohexan basierende einschichtfolien | |
| EP1252016A1 (de) | Thermoformbare mehrschichtfolie mit einer schicht aus vinylcyclohexan basierendem polymer | |
| DE102004059783A1 (de) | Transparente Mischungen linearer Styrol-Butadien-Blockcopolymerer | |
| JP4791021B2 (ja) | 水添共重合体及びその組成物 | |
| JP5221318B2 (ja) | 熱収縮性フィルム |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CR CU CZ DE DK DM DZ EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE TR BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| AK | Designated states |
Kind code of ref document: A3 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CR CU CZ DE DK DM DZ EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A3 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE TR BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2000990699 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 10168641 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref country code: JP Ref document number: 2001 547219 Kind code of ref document: A Format of ref document f/p: F |
|
| WWP | Wipo information: published in national office |
Ref document number: 2000990699 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |