明 細 書 Specification
安息香酸エステル化合物及び除草剤並びにその使用方法 Benzoic acid ester compound, herbicide and method of using the same
技術分野 Technical field
本発明は一般式(I) で表される安息香酸ェステル化合物及び該化合物を有効成 分として含有する除草剤並びにその使用方法に関するものである。 The present invention relates to a benzoate ester compound represented by the general formula (I), a herbicide containing the compound as an effective component, and a method for using the same.
背景技術 Background art
特開平 3— 1 6 3 0 6 3号公報には本発明化合物を一般式中に含む 3 —置換フ ヱ二ルビラゾール誘導体が除草剤として有用であることが記載されている。 しか し、 本願発明の一般式(I) で表される安息香酸エステル化合物について物性、 使 用方法等の具体的記載は全く記載されていない。 Japanese Patent Application Laid-Open No. 3-163603 describes that a 3-substituted pentavirazole derivative containing the compound of the present invention in the general formula is useful as a herbicide. However, there is no description at all of the benzoic acid ester compound represented by the general formula (I) of the present invention, such as physical properties and methods of use.
現在、 低薬量で活性を示し、 作物と雑草との選択が高い除草剤の創出が強く求 められており、 特に雑草防除を効率良く行うためには、 茎葉処理除草剤ばかりで なく、 土壌処理除草剤も必要である。 農作業には各種の作業が必要であるが、 除 草作業は中でも重要かつ労力のかかる作業である。 除草作業が他の作業と同時に できるか又は他の作業とぶっからない時期にできれば農業生産の効率化につなが る。 近年、 農業用機械の発展とともに多種の作業を併行して実施できるものが現 れてきており、 田植えと同時に肥料や農薬を散布できる機械が知られている。 耕 運とともに除草剤を散布すればその後の除草作業を省くことができるので効率的 である。 At present, there is a strong demand for the creation of herbicides that are active at low doses and have high selection between crops and weeds.Efficient weed control requires not only foliar treatment herbicides but also soil Processed herbicides are also needed. Agricultural work requires various types of work, but weeding is an important and labor-intensive task. If weeding work can be done at the same time as other work or at a time when it does not conflict with other work, it will lead to more efficient agricultural production. In recent years, with the development of agricultural machinery, machines that can carry out various types of work in parallel have emerged. Machines that can spray fertilizers and pesticides simultaneously with rice planting are known. Spraying the herbicide along with cultivation is efficient because subsequent weeding work can be omitted.
本発明者等は低薬量で活性が高く、 土壌処理における選択性が高い除草剤を開 発すべく鋭意研究を重ねた結果、 本願発明の一般式(I) で表される安息香酸エス テル化合物が文献未記載の新規化合物であり、 特開平 3— 1 6 3 0 6 3号公報に 開示の化合物に比して優れた除草活性を示し、 土壌処理において高い選択性を有 することを見出し、 本発明を完成させたものである。 The present inventors have conducted intensive studies to develop a herbicide having a high activity at a low dose and a high selectivity in soil treatment. As a result, the benzoate ester compound represented by the general formula (I) of the present invention was obtained. Is a novel compound not described in the literature, exhibits excellent herbicidal activity as compared with the compound disclosed in JP-A-3-16363, and has high selectivity in soil treatment. The present invention has been completed.
発明の開示 Disclosure of the invention
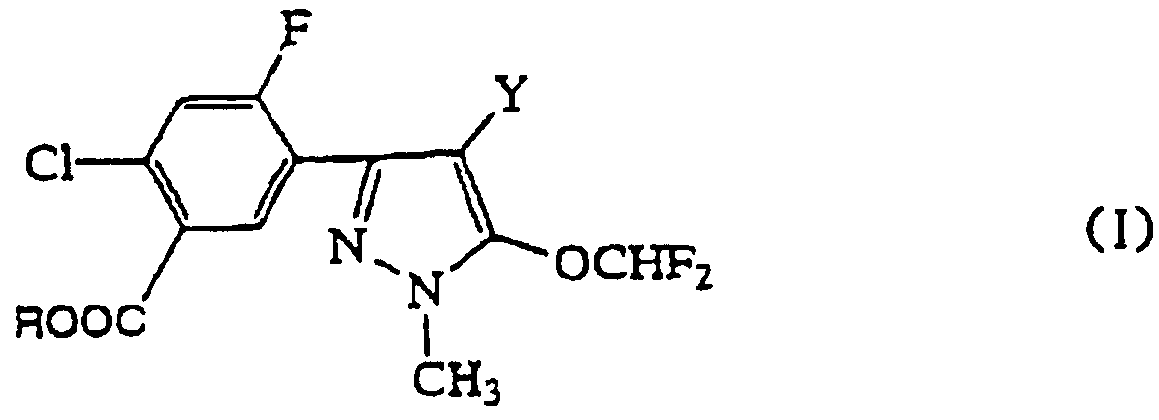
本発明は一般式(I) :
The present invention provides a compound of the general formula (I):
(式中、 Yが臭素原子の場合、 Rはイソプロピル基、 一 CHsCOOR1 (式 中、 !^はじ —じ ァルキル基を示す。 ) 又は— CH (CH3) COOR 1 (式 中、 R1は前記に同じ。 ) を示し、 Yが塩素原子の場合、 Rはイソプロピル基を 示す。 ) (In the formula, when Y is a bromine atom, R is an isopropyl group, and CHsCOOR 1 (in the formula, denotes a ^ alkyl group.) Or — CH (CH 3 ) COOR 1 (where R 1 is Same as above.)), And when Y is a chlorine atom, R represents an isopropyl group.)
で表される安息香酸エステル化合物及び該化合物を有効成分とする除草剤並びに その使用方法に関するものである。 ' And a herbicide containing the compound as an active ingredient and a method of using the same. '
本発明の一般式(I) の定義中、 「C i一 C3アルキル基」 とは、 炭素原子数 1 〜 3の直鎖状又は分枝状のアルキル基を示し、 例えばメチル基、 ェチル基、 ノル マルプロピル基、 イソプロピル基を示す。 In the definition of the general formula (I) of the present invention, “C i -C 3 alkyl group” means a linear or branched alkyl group having 1 to 3 carbon atoms, for example, a methyl group, an ethyl group , A normal propyl group and an isopropyl group.
本発明の好ましい化合物としては、 例えば 2—クロ口一 5— (4—クロ口一 5 ージフルォロメ トキシ一 1—メチルー 1 H—ピラゾーノレ一 3—ィル) ― 4ーフノレ ォロ安息香酸 イソプロピノレ、 2—クロ口一 5— (4—ブロモ一 5—ジフルォロ メ トキシ— 1—メチルー 1 H—ピラゾール一 3—ィル) —4一フルォロ安息香酸 イソプロピルを例示することができる。 The preferred compounds of the present invention include, for example, 2-chloro-5- (4-chloro-5-difluoromethoxy-1--1-methyl-1H-pyrazolone-3-yl) -4-isopropynole 4-benzoylbenzoate, —Chloro-1- (4-bromo-5-difluoromethoxy) -1-methyl-1H-pyrazole-13-yl) —Isopropyl-4-fluorobenzoate.
発明を実施するための形態 BEST MODE FOR CARRYING OUT THE INVENTION
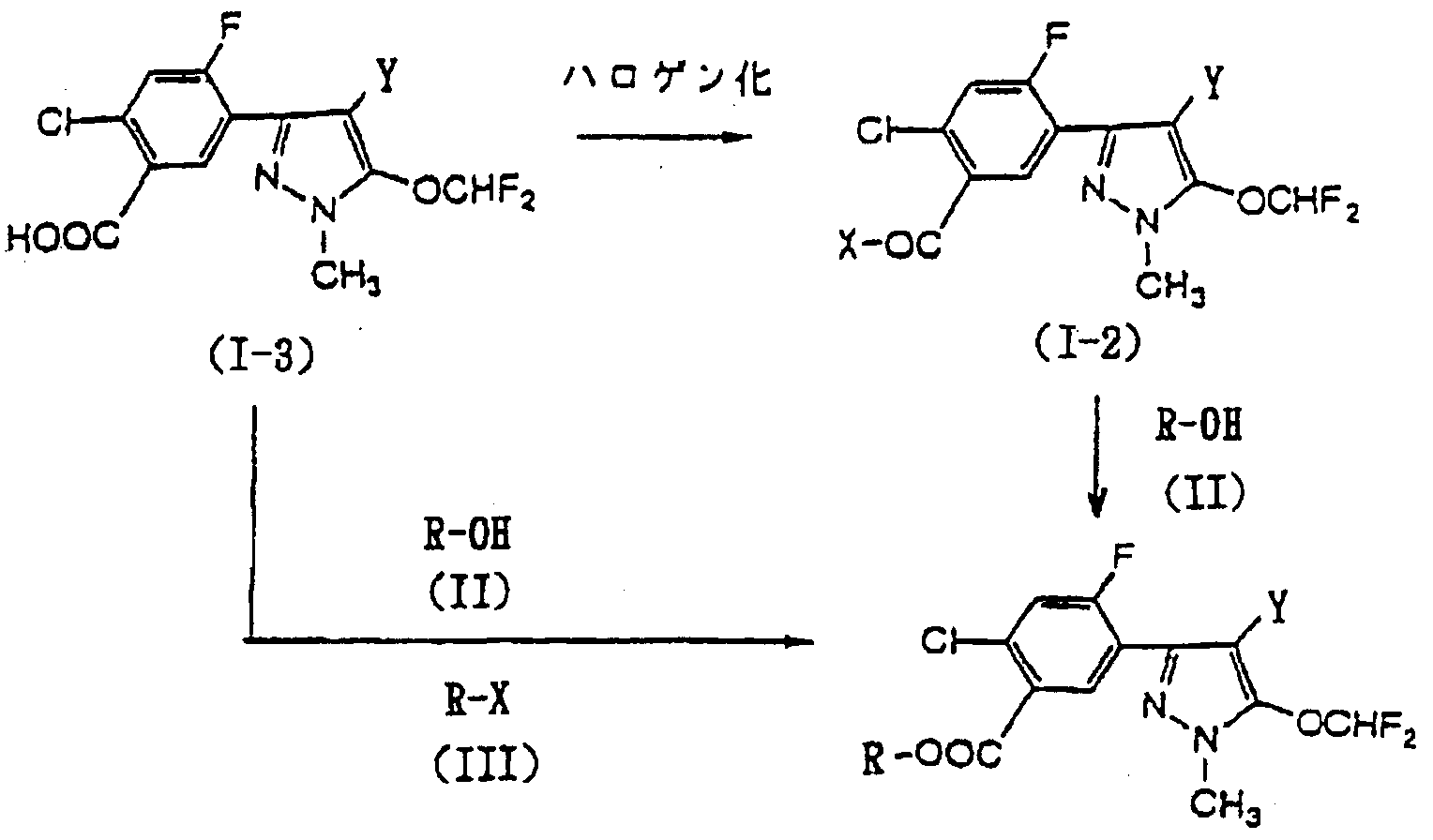
本発明化合物は特開平 3— 1 63063号公報記載の方法に従って製造するこ とができるが、 例えば以下に図示する製造方法により製造することもできる。
The compound of the present invention can be produced according to the method described in JP-A-3-163063. For example, it can also be produced by the production method shown below.
(式中、 R及び Yは前記に同じく し、 Ζはハロゲン原子を示す。 ) (In the formula, R and Y are as defined above, and Ζ represents a halogen atom.)
一般式(1-3) で表される化合物を不活性溶媒の存在下又は不存在下のハロゲン 化剤によりハロゲン化反応を行い、 一般式(1-2) で表される酸ハライ ド類とし、 該酸ハライ ド類を単離し又は単離せずして一般式(II)で表されるアルコール類と 反応させるか、 一般式(1-3) で表される化合物を不活性溶媒の存在下又は不存在 下、 鉱酸類の存在下に一般式(Π)で表されるアルコール類と反応させるか、 又は 一般式(1-3) で表される化合物を不活性溶媒の存在下又は不存在下、 脱ハロゲン 化剤の存在下の一般式(III) で表されるハライ ド類と反応させることにより、 目 的とする一般式(I) で表される安息香酸エステル化合物を製造することができる。 The compound represented by the general formula (1-3) is subjected to a halogenation reaction with a halogenating agent in the presence or absence of an inert solvent to obtain an acid halide represented by the general formula (1-2). The acid halide is isolated or not isolated and reacted with an alcohol represented by the general formula (II), or the compound represented by the general formula (1-3) is reacted in the presence of an inert solvent. Or reacting with an alcohol represented by the general formula (III) in the presence or absence of a mineral acid, or a compound represented by the general formula (1-3) in the presence or absence of an inert solvent By reacting with a halide represented by the general formula (III) in the presence of a dehalogenating agent, it is possible to produce the desired benzoate compound represented by the general formula (I). it can.
( 1 ) . 一般式(1-3) →—般式(1-2) . (1). General formula (1-3) → General formula (1-2).
本反応は酸ハライド化の反応であり、 本反応で使用できる不活性溶媒としては、 例えば塩化メチレン、 クロ口ホルム、 四塩化炭素等のハロゲン化炭化水素類、 ベ ンゼン、 トルエン、 キシレン等の芳香族炭化水素類、 メチルセ口ソルブ、 ジェチ ルエーテル、 ジイソプロピルエーテル、 ジォキサン、 テトラヒ ドロフラン等の鎖 状又は環状エーテル類等を使用することができ、 これらの不活性溶媒は単独で又 は混合して使用することもできる。 This reaction is an acid halide reaction. Examples of inert solvents that can be used in this reaction include halogenated hydrocarbons such as methylene chloride, chloroform, and carbon tetrachloride, and aromatic solvents such as benzene, toluene, and xylene. Acyclic or cyclic ethers such as aromatic hydrocarbons, methyl sorb, methyl ether, diisopropyl ether, dioxane, and tetrahydrofuran can be used, and these inert solvents are used alone or in combination. You can also.
ハロゲン化剤としては、 例えば塩化チォニル、 五塩化リン、 三塩化リン等を使 用することができ、 ハロゲン化剤の使用量は一般式(I - 3) で表される化合物に対
して等モル乃至過剰モルの範囲から適宜選択して使用すれば良く、 好ましくは過 剰量使用するのが良い。 又、 本反応を促進させる目的で触媒量のトリェチルアミ ン、 ピリジン、 ジメチルホルムアミ ド、 テトラメチルチオ尿素等を添加しても反 応することができる。 As the halogenating agent, for example, thionyl chloride, phosphorus pentachloride, phosphorus trichloride and the like can be used. The amount of the halogenating agent used is based on the compound represented by the general formula (I-3). The amount may be appropriately selected from the range of equimolar to excess mole, and preferably used in excess. The reaction can also be performed by adding a catalytic amount of triethylamine, pyridine, dimethylformamide, tetramethylthiourea or the like for the purpose of accelerating the reaction.
反応温度は室温乃至使用する不活性溶媒の沸点域から選択すれば良く、 反応時 間は反応量、 反応温度によって一定しないが、 数分乃至 4 8時間の範囲から適宜 選択すれば良い。 The reaction temperature may be selected from the range of room temperature to the boiling point of the inert solvent to be used. The reaction time is not fixed depending on the reaction amount and the reaction temperature, but may be appropriately selected from the range of several minutes to 48 hours.
反応終了後、 目的物を含む反応液から過剰のハロゲン化剤及び溶媒を留去等し て除き、 目的物を単離すれば良く、 必要に応じて再結晶法、 蒸留法等の方法によ り精製を行い、 次の反応に供することもでき、 単離精製せずして次の反応に供す ることもできる。 After completion of the reaction, the target compound may be isolated by removing the excess halogenating agent and solvent from the reaction solution containing the target compound by distillation or the like, and the target compound may be isolated by a method such as a recrystallization method or a distillation method, if necessary. It can be used for the next reaction after purification, or can be used for the next reaction without isolation and purification.
( 2 ) . 一般式(1-2) 又は一般式(1-3) ——般式(I) (2). General formula (1-2) or General formula (1-3) — General formula (I)
本反応は、 エステル化反応では、 一般式(Π)で表されるアルコール類を鉱酸類、 例えば濃硫酸等の存在下に過剰に使用し、 反応剤及び溶剤として使用することが できる。 又、 一般式(1-3) 化合物はアルカリ金属原子等の塩の形で使用すること もできる。 更に、 不活性溶媒及び脱ハ πゲン化剤の存在下にエステル化反応を行 うことができ、 この場合使用できる不活性溶媒としては、 例えば酸ハライ ド化反 応で使用する不活性溶媒の他に、 アセトン等のケトン類、 ジメチルホルムアミ ド、 ジメチルスルホキシド、 ジメチルイミダゾール、 ジメチルァセトアミ ド等の非プ ロトン性溶媒を使用することができる。 In this reaction, in the esterification reaction, the alcohol represented by the general formula (II) is used in excess in the presence of a mineral acid, for example, concentrated sulfuric acid, and can be used as a reactant and a solvent. Further, the compound of the general formula (1-3) can be used in the form of a salt such as an alkali metal atom. Further, the esterification reaction can be carried out in the presence of an inert solvent and a depilation agent. In this case, examples of the inert solvent that can be used include those of the inert solvent used in the acid halide reaction. In addition, non-protonic solvents such as ketones such as acetone, dimethylformamide, dimethylsulfoxide, dimethylimidazole, and dimethylacetamide can be used.
一般式(II)で表されるアルコール類又は一般式(ΙΠ) で表されるハライ ド類の 使用量は等モル乃至過剰モルの範囲から適宜選択して使用すれば良く、 好ましく は過剰モル使用するのが良い。 The amount of the alcohol represented by the general formula (II) or the halide represented by the general formula (II) may be appropriately selected and used from the range of equimolar to excess molar, and is preferably used in excess molar. Good to do.
本反応で使用できる脱ハロゲン化剤としては、 無機塩基又は有機塩基を使用す ることができ、 例えば水酸化ナトリウム、 水酸化カリウム等のアルカリ金属原子 の水酸化物、 有機塩基としては、 例えばトリェチルァミン等の三級ァミン類、 4 ージメチルァミノピリジン、 1, 8—ジァザビシクロ [ 5, 4, 0 ] — 7—ゥン デセン等の等の有機塩基等を使用することができ、 その使用量は一般式(1-2) で 表される酸ハライ ド類に対して等モル乃至過剰モルの範囲から適宜選択して使用
すれば良い。 As the dehalogenating agent that can be used in this reaction, an inorganic base or an organic base can be used. For example, a hydroxide of an alkali metal atom such as sodium hydroxide or potassium hydroxide, and an organic base such as triethylamine And organic bases such as 4-dimethylaminopyridine, 1,8-diazabicyclo [5,4,0] —7-pinedecene, etc., and the amount used is The acid halide represented by the general formula (1-2) is appropriately selected and used in the range of equimolar to excess molar. Just do it.
反応温度は o°c乃至使用する溶媒の沸点域から選択すれば良く、 好ましくは 0 °C乃至 1 50°Cの範囲から選択すれば良い。 The reaction temperature may be selected from the range of o ° C to the boiling point of the solvent used, preferably from 0 ° C to 150 ° C.
反応時間は、 反応量、 反応温度等によって一定しないが、 数分乃至 48時間の 範囲から選択すれば良い。 The reaction time is not fixed depending on the reaction amount, the reaction temperature and the like, but may be selected from a range of several minutes to 48 hours.
反応終了後は、 反応液から常法により、 例えば溶媒抽出等の操作を行い、 必要 に応じて再結晶法、 蒸留法、 カラムクロマトグラフィー法等の方法により精製す ることにより、 一般式(I) で表される安息香酸エステル化合物を製造することが できる。 After completion of the reaction, the reaction solution is subjected to an operation such as solvent extraction by a conventional method and, if necessary, purified by a method such as a recrystallization method, a distillation method, or a column chromatography method to obtain a compound represented by the general formula (I) ) Can be produced.
第 1表に一般式(I) で表される安息香酸エステル化合物の代表的な化合物を例 示する。 Table 1 shows typical benzoate compounds represented by the general formula (I).
一般式 (I) : General formula (I):
No Y R 物性 (屈折率) No Y R Physical properties (refractive index)
1 CI - i- C3H7 nD 1.5273(24.3 。C) 1 CI-i-C 3 H 7 nD 1.5273 (24.3.C)
2 Br - i- C3H7 nD 1.5403(28.7 。C) 2 Br - i- C 3 H 7 nD 1.5403 (28.7 .C)
3 Br - CH2C00CH3 nD 1.5465(26.2 。C) 3 Br - CH 2 C00CH 3 nD 1.5465 (26.2 .C)
4 Br -CH2C00C2H5 nD 1.5420(26.3 。C) 4 Br -CH 2 C00C 2 H 5 nD 1.5420 (26.3.C)
5 Br -CH(CH3)C00CH3 nD 1.5398(26.9 。C) 5 Br -CH (CH 3) C00CH 3 nD 1.5398 (26.9 .C)
6 Br -CH(CH3)C00C2H5 nD 1.5343(28.7 。C)
本発明の一般式(I) で表される安息香酸ェステル化合物を有効成分とする除草 剤は、 例えばィヌビエ (イネ科 1年生、 水田の害草) 、 タマガヤッリ (力ャッリ ダサ科丄年生草、 水田の害草) 、 マツバイ (力ャッリグサ科多年生草、 湿地、 水 路、 水田に発生、 水田の多年生害草) 、 ゥリカヮ (ォモダカ科、 水田、 湿地、 溝 に発生する多年生害草) 、 ホタルイ (力ャッリグサ科多年生草、 水田、 湿地、 溝 に発生) 、 スズメノテツボウ (イネ科雑草、 水田、 低湿地に発生) 、 ェンパク (イネ科越年草、 平地、 荒地、 畑地に発生) 、 ョモギ (キク科多年生草、 山野、 畑地に発生) 、 メヒシバ (イネ科 1年生草、 畑、 樹園地の強害草) 、 ギシギシ (タデ科多年生草、 畑地、 道端に発生) 、 コゴメガャッリ (力ャッリグサ科 1年 生草、 ァオビュ (ヒュ科 1年生草、 空き地、 道端、 畑地に発生) 、 ォナモミ (キ ク科 1年生草、 畑地の害草) 、 ィチビ (ァオイ科 1年生草、 畑地の害草) 、 ヨウ シュチョウセンアサガオ (ヒルガオ科 1年生草、 畑地の害草) 、 ォオイヌノフグ リ (ゴマノハグサ科 1〜 2年生草、 畑地の害草) 、 ヤエムダラ (ァカネ科 1年生 草、 畑地、 樹園地の害草) 等の水田、 畑、 樹園地、 湿地等に発生する 1年生及び 多年生雑草等を除草するのに有用である。 6 Br -CH (CH 3) C00C 2 H 5 nD 1.5343 (28.7 .C) Herbicides containing the benzoate ester compound represented by the general formula (I) of the present invention as an active ingredient include, for example, barnyardgrass (Poaceae, first grade, paddy field weeds), tamagayari (Ricaridasa family, annual grass, paddy field) Pests), pine trees (perennial grasses of the scrophulariaceae, wetlands, waterways, and paddy fields, perennial grasses of paddy fields), ゥ rika ヮ (perennial grasses of the ophidaceae, paddy fields, wetlands, ditches), fireflies Perennial grasses, perennial grasses, rice fields, wetlands, ditches, sparrows (grass weeds, rice fields, lowlands), Empak (grass annuals, flatlands, wastelands, fields), sagebrush (kiku) Perennial grass, occurring in the mountains and fields, grasshoppers (first-year grasses, fields, and orchards), rubbing (perennials, perennials, fields, and roadsides), kogomajarari Grass family 1st year grass, Aobu (Hygaceae 1st year grass, vacant lot, roadside, upland), bonafami (Asteraceae 1st year grass, upland grass), Ichibi (1st year grass, upland grass) Grass), Ipomoea japonicus (Goldenaceae, 1st year grass, field grass), Ooinoufuguri (1st to 2th year grass, field grass), Yaemdara (1st year grass, grassland, orchard) It is useful for weeding annual and perennial weeds that occur in paddy fields, fields, orchards, wetlands, etc.
本発明の一般式(I) で表される安息香酸ェステル化合物を有効成分とする除草 剤は出芽前及び出芽後にある雑草に対して優れた除草効果、 特に出芽前の処理に より優れた除草効果を示す。 その態様は有用植物の植え付け予定地に予め処理す ると力 \ 有用植物の播種、 植え付け等の後 (有用植物が樹園のごとく既に定植さ れている場合を含む) 雑草の発生前から発生始期に処理することにより本発明除 草剤の有する特徴ある生理活性を効果的に発現させることができる。 The herbicide containing the benzoic acid ester compound represented by the general formula (I) of the present invention as an active ingredient has an excellent herbicidal effect on weeds before and after budding, particularly, an excellent herbicidal effect by treatment before budding. Is shown. The mode of application is that if pre-treated at the site where the useful plants are to be planted, power is applied \ after sowing and planting of the useful plants (including when the useful plants have already been planted like an orchard) Before the emergence of weeds By treating at an early stage, the characteristic physiological activity of the herbicide of the present invention can be effectively expressed.
しかし、 本発明の除草剤はこのような態様おいてのみ使用されねばならないと レ、うものではなく、 例えば本発明除草剤は水田用除草剤として使用することがで き、 一般雑草の除草剤としても使用することができ、 例えば刈り取り跡、 休耕田 畑、 畦畔、 農道、 水路、 牧草造成地、 墓地、 公園、 道路、 運動場、 建物の周辺の 空き地、 開墾地、 線路端、 森林等の一般雑草の防除するために使用することもで きる。 この場合、 雑草の発生始期までに処理するのが経済的にも最も効果的であ るが、 必ずしもこれに限定されず、 生育期にある雑草をも防除することが可能で ある。 好ましくは雑草発生前に使用すると良い。
本発明の一般式(I) で表される安息香酸エステル化合物を有効成分を除草剤と して使用する場合、 農薬製剤上の常法に従い、 使用上都合の良い形状に製剤して 使用するのが一般的である。 However, the herbicide of the present invention must be used only in such an embodiment. However, for example, the herbicide of the present invention can be used as a herbicide for paddy fields, and a herbicide for general weeds. For example, mowing marks, fallow fields, levees, agricultural roads, waterways, pastures, cemeteries, parks, roads, playgrounds, vacant lots around buildings, reclaimed land, track ends, forests, etc. It can also be used to control weeds. In this case, it is most economically effective to treat weeds until the beginning of their emergence, but it is not necessarily limited to this, and weeds in the growing season can be controlled. Preferably, it is used before the occurrence of weeds. When the benzoate compound represented by the general formula (I) of the present invention is used as an active ingredient as a herbicide, the benzoate compound is formulated into a convenient form in accordance with a conventional method for agricultural chemicals. Is common.
即ち、 一般式(I) で表される安息香酸エステル化合物は、 これらを適当な不活 性担体に又は必要に応じて補助剤と一緒に、 適当な割合に配合して溶解、 分離、 懸濁、 混合、 含浸、 吸着若しくは付着させ、 適宜の剤形、 例えば懸濁剤、 乳剤、 液剤、 水和剤、 粒剤、 粉剤、 錠剤等に製剤して使用すれば良い。 That is, the benzoate compound represented by the general formula (I) is dissolved, separated, suspended by mixing these in an appropriate inactive carrier or, if necessary, together with an auxiliary in an appropriate ratio. It may be mixed, impregnated, adsorbed or adhered and formulated into an appropriate dosage form, such as a suspension, emulsion, liquid, wettable powder, granule, powder, tablet or the like.
本発明で使用できる不活性担体としては固体又は液体の何れであっても良く、 固体の担体になりうる材料としては、 例えばダイズ粉、 穀物粉、 木粉、 樹皮粉、 鋸粉、 タバコ茎粉、 クルミ殻粉、 ふすま、 繊維素粉末、 植物エキス抽出後の残渣、 粉砕合成樹脂等の合成重合体、 粘土類 (例えばカオリン、 ベントナイ ト、 酸性白 土等) 、 タノレク類 (例えばタルク、 ピロフィライ ド等) 、 シリカ類 (例えば珪藻 土、 珪砂、 雲母、 ホワイ トカーボン 〔含水微粉珪素、 含水珪酸ともいわれる合成 高分散珪酸で、 製品により珪酸カルシウムを主成分として含むものもある。 〕 ) 、 活性炭、 ィォゥ粉末、 軽石、 焼成珪藻土、 レンガ粉砕物、 フライアッシュ、 砂、 炭酸カルシウム、 燐酸カルシウム等の無機鉱物性粉末、 硫安、 燐安、 硝安、 尿素、 塩安等の化学肥料、 堆肥等を挙げることができ、 これらは単独で又は二種以上の 混合物として使用することができる。 The inert carrier that can be used in the present invention may be either solid or liquid. Examples of the material that can be a solid carrier include soybean flour, cereal flour, wood flour, bark flour, saw flour, and tobacco stem flour. , Walnut shell powder, bran, cellulose powder, residue after extracting plant extracts, synthetic polymers such as pulverized synthetic resin, clays (for example, kaolin, bentonite, acid clay), tanoleks (for example, talc, pyrophyllide) ), Silica (for example, diatomaceous earth, silica sand, mica, and white carbon [Synthetic high-dispersion silicic acid also known as hydrous finely divided silicon and hydrous silicic acid, some of which contain calcium silicate as a main component depending on the product.)], Activated carbon, zeolite Powder, pumice, calcined diatomaceous earth, crushed brick, fly ash, sand, inorganic mineral powder such as calcium carbonate, calcium phosphate, and ammonium sulfate Phosphate weaker ammonium nitrate, can be mentioned urea, fertilizer salts depreciation etc., compost and the like, which may be used alone or as a mixture of two or more.
液体の担体になりうる材料としては、 それ自体溶媒能を有するものの他、 溶媒 能を有さずとも補助剤の助けにより本発明化合物を分散させうることとなるもの であれば良く、 例えば水、 アルコール類 (例えばメタノール、 エタノール、 イソ プロパノール、 ブタノール、 エチレングリコール等) 、 ケトン類 (例えばァセト ン、 メチルェチルケトン、 メチルイソブチルケトン、 ジィソブチルケトン、 シク 口へキサノン等) 、 エーテル類 (例えばェチルエーテル、 ジォキサン、 セロソル ブ、 ジプロピルエーテル、 テトラヒドロフラン等) 、 脂肪族炭化水素類 (例えば ケロシン、 鉱油等) 、 芳香族炭化水素類 (例えばベンゼン、 トルエン、 キシレン、 ソルベントナフサ、 アルキルナフタレン等) 、 ハロゲン化炭化水素類 (例えばジ クロロェタン、 クロ口ホルム、 四塩化炭素等) 、 エステル類 (例えば酢酸ェチル、 ジイソプロピルフタレート、 ジブチルフタレ一ト、 ジォクチルフタレート等) 、
アミ ド類 (例えばジメチルホルムアミ ド、 ジェチルホルムアミ ド、 ジメチルァセ トアミ ド等) 、 二トリル類 (例えばァセトニトリル等) 、 ジメチルスルホキシド 類等を挙げることができ、 これらは単独で又は二種以上の混合物して使用するこ とができる。 As a material that can be a liquid carrier, other than a material having a solvent function itself, any material that does not have a solvent function and can disperse the compound of the present invention with the aid of an auxiliary agent may be used. Alcohols (eg, methanol, ethanol, isopropanol, butanol, ethylene glycol, etc.), ketones (eg, acetone, methylethyl ketone, methyl isobutyl ketone, diisobutyl ketone, cyclohexanone, etc.), ethers (eg, Ethyl ether, dioxane, cellosolve, dipropyl ether, tetrahydrofuran, etc.), aliphatic hydrocarbons (eg, kerosene, mineral oil, etc.), aromatic hydrocarbons (eg, benzene, toluene, xylene, solvent naphtha, alkyl naphthalene, etc.), halogen Hydrocarbons (Eg, dichloroethane, chloroform, carbon tetrachloride, etc.), esters (eg, ethyl acetate, diisopropyl phthalate, dibutyl phthalate, dioctyl phthalate, etc.), Examples include amides (eg, dimethylformamide, getylformamide, dimethylacetamide, etc.), nitriles (eg, acetonitrile, etc.), dimethylsulfoxides, and the like. These may be used alone or in combination of two or more. It can be used as a mixture.
他の補助剤としては、 次に例示する代表的な補助剤をあげることができ、 これ らの補助剤は目的に応じて使用され、 単独で、 ある場合は二種以上の補助剤を併 用し、 又ある場合には全く補助剤を使用しないことも可能である。 Examples of other adjuvants include the following representative adjuvants.These adjuvants are used according to the purpose, and may be used alone or, in some cases, in combination of two or more adjuvants. However, in some cases, it is possible to use no adjuvant at all.
本発明化合物の乳化、 分散、 可溶化及び Z又は湿潤の目的のために界面活性剤 が使用され、 例えばポリオキシエチレンアルキルエーテル、 ポリオキシエチレン アルキルァリールエーテル、 ポリオキシエチレン高級脂肪酸エステル、 ポリオキ シエチレン樹脂酸エステル、 ポリオキシエチレンソルビタンモノラウレート、 ポ リオキシエチレンソルビタンモノォレエート、 アルキルァリールスルホン酸塩、 ナフタレンスルホン酸縮合物、 リグニンスルホン酸塩、 高級アルコール硫酸エス テル等の界面活性剤を例示することができる。 A surfactant is used for the purpose of emulsifying, dispersing, solubilizing and Z or wetting the compound of the present invention, for example, polyoxyethylene alkyl ether, polyoxyethylene alkyl aryl ether, polyoxyethylene higher fatty acid ester, polyoxyethylene. Surfactants such as resin acid ester, polyoxyethylene sorbitan monolaurate, polyoxyethylene sorbitan monooleate, alkylaryl sulfonate, naphthalene sulfonic acid condensate, lignin sulfonate, and higher alcohol sulfate ester Can be exemplified.
又、 本発明化合物の分散安定化、 粘着及び/又は結合の目的のために、 例えば カゼイン、 ゼラチン、 澱粉、 メチルセノレロース、 カルボキシメチノレセルロース、 アラビアゴム、 ポリビニルアルコール、 松根油、 糠油、 ベントナイ ト、 リグニン スルホン酸塩等の補助剤を使用することもできる。 Further, for the purpose of dispersion stabilization, adhesion and / or binding of the compound of the present invention, for example, casein, gelatin, starch, methylsenorellose, carboxymethinolecellulose, gum arabic, polyvinyl alcohol, pine oil, bran oil, bentonite Auxiliaries such as lignin sulfonate can also be used.
固体製品の流動性改良のために、 例えばワックス、 ステアリン酸塩、 燐酸アル キルェステル等の補助剤を使用でき、 懸濁性製品の解こう剤として、 例えばナフ タレンスルホン酸縮合物、 縮合燐酸塩等の補助剤を使用することもできる。 For improving the fluidity of solid products, auxiliary agents such as wax, stearate and alkylester phosphate can be used.As peptizers for suspending products, for example, naphthalenesulfonic acid condensate, condensed phosphate, etc. Auxiliaries can also be used.
消泡剤としては、 例えばシリコーン油等の補助剤を使用することもできる。 本発明化合物の配合割合は必要に応じて加減することができ、 例えば粉剤或い は粒剤とする場合は 0 . 0 1〜 5 0重量%、 又乳剤或レヽは水和剤とする場合も同 様 0 . 0:!〜 5 0重量%が適当である。 As an antifoaming agent, for example, an auxiliary agent such as silicone oil can be used. The compounding ratio of the compound of the present invention can be adjusted as required. For example, when it is used as a powder or granules, it is 0.01 to 50% by weight, and when it is used as an emulsion or a wettable powder. Same 0 :! ~ 50% by weight is suitable.
本発明化合物を有効成分とする除草剤は各種雑草を枯殺し若しくは生育を抑制 するために、 そのまま又は水等で適宜希釈し、 若しくは懸濁させた形で殺草若し くは生育抑制に有効な量を当該雑草に、 又は当該雑草の発生若しくは生育が好ま しくない場所において茎葉又は土壌に適用して使用すればよレ、。
本発明化合物を含有する除草剤の使用量は種々の因子、 例えば目的、 対象雑草、 作物の生育状況、 雑草の発生傾向、 天候、 環境条件、 剤型、 施用方法、 施用場所、 施用時期等により変動するが、 有効成分化合物として 1ヘクタール当たり 0. 1 g〜 10 k gの範囲から目的に応じて適宜選択すれば良レ、。 The herbicide containing the compound of the present invention as an active ingredient is effective for killing or suppressing growth of various kinds of weeds, either as it is, or appropriately diluted with water or the like, or suspended to form a suspension. A large amount can be applied to the weed or to the foliage or soil in places where the occurrence or growth of the weed is not preferred. The amount of the herbicide containing the compound of the present invention may vary depending on various factors, for example, the purpose, the target weed, the growth status of the crop, the tendency of weed occurrence, the weather, the environmental conditions, the dosage form, the method of application, the place of application, and the time of application. Although it fluctuates, it is advisable to appropriately select the active ingredient compound from the range of 0.1 g to 10 kg per hectare according to the purpose.
本発明化合物を含有する除草剤を更に防除対象雑草、 防除適期の拡大のため、 或いは薬量の低減をはかる目的で他の除草剤と混合して使用することも可能であ る。 The herbicide containing the compound of the present invention can be used in admixture with other herbicides for controlling weeds to be controlled, extending the suitable period of control, or reducing the dose.
本発明の一般式(I) で表される安息香酸ェステル化合物を含有する除草剤は土 壤処理において低薬量で優れた除草活性を有し、 且つ作物と.雑草間の選択性が高 く、 農業生産性の向上に有用なものである。 The herbicide containing the benzoate ester compound represented by the general formula (I) of the present invention has excellent herbicidal activity at a low dose in soil treatment, and has high selectivity between crops and weeds. It is useful for improving agricultural productivity.
実施例 Example
以下に本発明の代表的な実施例、 製剤例及び試験例を示すが、 本発明はこれら に限定されるものではない。 尚、 製剤例中、 部とあるのは重量部を示す。 Hereinafter, typical examples, preparation examples, and test examples of the present invention are shown, but the present invention is not limited thereto. In the preparation examples, “parts” means “parts by weight”.
実施例 1. 2_クロ口一 5— (4—クロロー 5—ジフノレオロメ トキシ一 1—メチ ル一 1 H—ピラゾールー 3—ィル) 一4—フルォロ安息香酸 イソプロピル (ィ匕 合物 No. 1 ) の製造 Example 1. 2-chloro-1-5- (4-chloro-5-diphnoleolomethoxy-1-methyl-1H-pyrazol-3-yl) isopropyl-14-fluorobenzoate (diamide compound No. 1) Manufacturing of
2—クロロー 5— (4一クロロー 5—ジフルォロメ トキシ一 1—メチルー 1 H ーピラゾールー 3—ィル) —4—フルォロ安息香酸 1. 705 g (0. 0047 7モル) 、 ジメチルスルホキシド 40mし 無水炭酸カリウム 0. 989 g (0. 00716モル) 及び沃化イソプロピル 1. 218 g (0. 0071 6モル) の 混合液を室温下に 4時間反応させた。 反応終了後、 反応混合液を氷水 200m l 中へ注ぎ、 目的物を酢酸ェチルで抽出し、 有機層を水洗後、 飽和食塩水で洗浄し、 無水硫酸マグネシウムで乾燥させた後、 溶媒を減圧下に留去し、 得られた残渣を " 〃 レ々ロマトグラフィ一にて精製することにより目的物を油状物として 1.
858 g得た。 2-chloro-5- (4-chloro-5-difluoromethoxy-1-methyl-1H-pyrazole-3-yl) -4-fluorobenzoic acid 1.705 g (0.0047 7 mol), dimethylsulfoxide 40m and anhydrous potassium carbonate A mixture of 0.989 g (0.00716 mol) and 1.218 g (0.0071 6 mol) of isopropyl iodide was reacted at room temperature for 4 hours. After completion of the reaction, the reaction mixture was poured into 200 ml of ice water, the desired product was extracted with ethyl acetate, the organic layer was washed with water, washed with saturated saline, dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The target product was converted to an oily substance by purifying the obtained residue by HPLC. 858 g were obtained.
物性: nD l. 5273 (24. 3°C) Physical properties: nD l. 5273 (24.3 ° C)
収率: 97. 8% Yield: 97.8%
実施例 2. 2_クロ口一 5— (4—ブロモー 5—ジフルォロメ トキシ一 1—メチ ル— 1 H—ピラゾール— 3 _ィル) 一 4—フルォロ安息香酸 イソプロピル (ィ匕 合物 No. 2) の製造 Example 2. 2-chloro-1-5- (4-bromo-5-difluoromethoxy-1-methyl-1H-pyrazole-3-yl) -14-isopropyl-4-benzoate isopropyl ) Manufacturing of
2—クロロー 5— (4—ブロモー 5—ジフノレオ口メ トキシ一 1ーメチノレ一 1 H —ビラゾ―ル _ 3一ィル) 一 4 _フルォロ安息香酸 0. i 5 g (0. 00037 モル) 、 ジメチルスルホキシド 20m 1、 無水炭酸カリウム 0. 077 g (0. 00056モル) 及び沃化イソプロピノレ 0. 095 g (0. 00056モル) の 混合液を室温下に 4時間反応させた。 反応終了後、 反応混合液を氷水 8 Om 1中 へ注ぎ、 目的物を酢酸ェチルで抽出し、 有機層を水洗後、 飽和食塩水で洗浄し、 無水硫酸マグネシウムで乾燥させた後、 溶媒を減圧下に留去し、 得られた残渣を シリカゲルク口マトグラフィ一にて精製することにより目的物を油状物として 0. 1 2 g得た。 2- chloro 5- (4- bromo-5-Jifunoreo port main butoxy one 1 Mechinore one 1 H - Birazo - Le _ three to I le) one 4 _ Furuoro benzoate 0. i 5 g (0. 00037 mol), dimethyl A mixed solution of 20 ml of sulfoxide, 0.077 g (0.00056 mol) of anhydrous potassium carbonate and 0.095 g (0.00056 mol) of isopropynole iodide was reacted at room temperature for 4 hours. After completion of the reaction, the reaction mixture was poured into ice water (8 Om1), the desired product was extracted with ethyl acetate, the organic layer was washed with water, washed with saturated saline, dried over anhydrous magnesium sulfate, and then the solvent was depressurized. The residue was purified by silica gel gel chromatography to obtain 0.12 g of the desired product as an oil.
物性: nD l. 5403 (28. 7°C) Physical properties: nD l. 5403 (28.7 ° C)
収率: 72. 4% Yield: 72.4%
製剤例 1. Formulation example 1.
第 1表記載の化合物 50部 Compounds listed in Table 1 50 parts
キシレン 40部 Xylene 40 parts
ポリォキシエチレンノニルフエニルエーテルと Polyoxyethylene nonylphenyl ether
アルキルベンゼンスルホン酸カルシウムとの混合物 1 0部 Mixture with calcium alkylbenzenesulfonate 10 parts
以上を均一に混合溶解して乳剤とする。 The above are uniformly mixed and dissolved to form an emulsion.
製剤例 2.
第 1表記載の化合物 3部 Formulation example 2. Compounds listed in Table 1 3 parts
クレ一粉末 8 2部 Cray powder 8 2 parts
珪藻土粉末 1 5部 Diatomaceous earth powder 1 5 parts
以上を均一に混合粉砕して粉剤とする ( The above is uniformly mixed and pulverized into a powder (
製剤例 3. Formulation example 3.
第 1表記載の化合物 5部 Compounds listed in Table 1 5 parts
ベントナイ トとクレーの混合粉末 90部 90 parts mixed powder of bentonite and clay
リグニンスルホン酸カノレシゥム 5部 Lignin sulfonic acid canolesum 5 parts
以上を均一に混合し、 適量の水を加えて混練し、 造粒、 乾燥して粒剤とする。 製剤例 4. The above components are mixed uniformly, kneaded by adding an appropriate amount of water, granulated and dried to obtain granules. Formulation example 4.
第 1表記載の化合物 20部 Compounds listed in Table 1 20 parts
カオリンと合成高分散珪酸 7 5部 Kaolin and synthetic high dispersion silicic acid 75 parts
ポリオキシエチレンノニノレフエニノレエーテ とァノレ Polyoxyethylene Noninolefenenolate and Anole
キルベンゼンスノレホン酸カノレシゥムとの混合物 5部 5 parts of a mixture with kilbenzenesnolephonate canolesum
以上を均一に混合粉碎して水和剤とする。 The above is uniformly mixed and ground to form a wettable powder.
試験例 1. 出芽前の畑地雑草に対する除草効果 Test Example 1. Herbicidal effect on field weeds before emergence
縦 1 0 cmX横 20 cmX高さ 5 cmのプラスチック製バットに土壌を詰め、 これにィヌカミツレ、 ハコべ、 スミ レ及びコムギ (品種: アンザ、 An z a) の 種子を播種覆土した。 これに第 1表記載の化合物又は比較化合物を有効成分とす る薬剤を所定濃度の散布液として処理した。 処理後 28日後に除草効果を調査し た。 肉眼判定による判定値 〔0 (活性なし又は薬害なし) 〜 1 0 0 (完全枯 死) 〕 を記した。 コムギに対する薬害を処理後 7日後と処理後 2 8日後に調査し、 肉眼判定値による判定値を記した。 The soil was packed in a plastic vat measuring 10 cm in length, 20 cm in width, and 5 cm in height, and seeds were covered with soybean chamomile, Hakobe, violet and wheat (variety: Anza, Anza). To this, a drug having a compound shown in Table 1 or a comparative compound as an active ingredient was applied as a spray solution having a predetermined concentration. The herbicidal effect was investigated 28 days after the treatment. The judgment value by the naked eye judgment [0 (no activity or no phytotoxicity) to 100 (complete withering)] was recorded. The phytotoxicity to wheat was investigated 7 days after treatment and 28 days after treatment, and the judgment value by the visual judgment value was recorded.
尚、 肉眼判定 20以上の薬害の場合、 収量に影響があり、 農業生産上許容でき ないものである。 結果を第 1表に示す。
第 2表 In addition, if the damage is more than 20 with the naked eye judgment, it will affect the yield and is unacceptable for agricultural production. The results are shown in Table 1. Table 2
比較化合物:特開平 3— 1 6 3 0 6 3号公報記載の化合物 N o . 4 9 8を使用 した。 Comparative compound: Compound No. 498 described in JP-A-3-16363 was used.
〔2—クロロー 5— (4 _クロ口一 5—ジフノレオロメ トキシ一 1 —メチル— 1 H—ピラゾールー 3—ィル) —4—フルォロ安息 香酸 メチル〕
[2-Chloro-5- (4-chloro-5-difnoleolomethoxy-1 1-methyl-1H-pyrazole-3-yl) -methyl 4-fluorobenzoate]