WO2000052124A1 - Stabilized bleach compositions - Google Patents
Stabilized bleach compositions Download PDFInfo
- Publication number
- WO2000052124A1 WO2000052124A1 PCT/US2000/005291 US0005291W WO0052124A1 WO 2000052124 A1 WO2000052124 A1 WO 2000052124A1 US 0005291 W US0005291 W US 0005291W WO 0052124 A1 WO0052124 A1 WO 0052124A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- hgand
- transition
- organic
- macropolycychc
- donor atoms
- Prior art date
Links
- DAVLXWLUGZJMOK-DTORHVGOSA-N C(C1)CN2[C@H]3N1CCN[C@H]3NCC2 Chemical compound C(C1)CN2[C@H]3N1CCN[C@H]3NCC2 DAVLXWLUGZJMOK-DTORHVGOSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/0005—Other compounding ingredients characterised by their effect
- C11D3/0084—Antioxidants; Free-radical scavengers
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/0005—Other compounding ingredients characterised by their effect
- C11D3/0042—Reducing agents
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/168—Organometallic compounds or orgometallic complexes
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/39—Organic or inorganic per-compounds
- C11D3/3902—Organic or inorganic per-compounds combined with specific additives
- C11D3/3905—Bleach activators or bleach catalysts
- C11D3/3932—Inorganic compounds or complexes
Definitions
- the present invention relates to stabilized bleaching and detergent compositions which comp ⁇ se a catalytically effective amount of a transition-metal bleach catalyst which is a complex of a transition-metal and a cross-bridged macropolycychc hgand and an anti-oxidant or reducing agent which serves to stabilize said catalyst.
- the present invention further relates to a method for bleaching/cleaning fab ⁇ c with a catalytically effective amount of said stabilized transition-metal bleach catalyst wherein the method is performed substantially free of any organic or inorganic peroxygen compound or precursors to any organic or inorganic peroxygen compound
- Bleaching of fab ⁇ c is essentially exposing soiled or stained fabnc to a chemical reaction the purpose of which is to eliminate the soil or stam.
- bleaching involved exposure of fab ⁇ c to a solution of hypochlonte. Therefore, fab ⁇ c which was colored or dyed via sensitive pigments were excluded from treatment with bleach.
- formulators developed va ⁇ ous forms of bleach inter alia peroxygen bleaching systems which typically comp ⁇ se a source of hydrogen peroxide and a bleach activator. This combination of source of hydrogen peroxide and activator plays a dominating role in effective, safe bleaching compositions.
- An effective example of this peroxygen bleaching system employs perborate (peroxygen source) and nonanoyloxybenzene sulfonate (activator).
- Said stable bleaching system will comprise a transition-metal catalyst which does not loose its effectiveness due to the instability of the catalyst to oxidation and/or reduction post formulation.
- the present invention meets the aforementioned needs in that it has been surprisingly discovered that bleaching systems comp ⁇ sing a transition-metal catalyst which are capable of bleaching soils and stains in the absence of a source of hydrogen peroxide or other peroxygen bleaching agent can be successfully stabilized against the unwanted degradation of the transition- metal catalyst.
- the addition of an effective amount of an anti-oxidant and/or free radical scavenger or an reducing agent can stabilize a nil hydrogen peroxide bleaching system.
- a first aspect of the present invention relates to stabilized bleaching compositions comprising: A) a catalytically effective amount of a transition-metal bleach catalyst which is a complex of a transition-metal and a cross-bridged macropolycychc gand;
- a stabilizing agent selected from l) one or more anti-oxidants; n) one or more reducing agents; in) and mixtures thereof;
- the present invention further relates to a method for cleaning and/or bleaching soils and stains on fab ⁇ cs, said method comp ⁇ sing the step of contacting the fab ⁇ c in need of cleaning and/or bleaching with an aqueous solution containing a composition which is substantially free of a peroxygen source and which compnses.
- compositions and methods of the present invention are suitable for cleaning/bleachmg any surface in need of stam removal.
- hard surface cleaners and automatic dishwashing compositions can employ the bleach catalysts of the present invention m applications which are substantially free of any organic or inorganic peroxygen compounds.
- the present invention relates to the surprising discovery that transition-metal catalysts which are capable of bleaching of soils and stains in the absence of an added peroxygen bleach can be suitably stabilized by the addition of one or more stabilizing agents.
- the stabilizing agents suitable for use in the present invention are present at varying level depending upon the formulation inter aha liquid, solid, gel, and the concentration of the transition-metal present. Absence of Peroxygen Sources
- compositions of the present invention as well as the methods for cleaning and/or bleaching of fab ⁇ c which utilize the compositions of the present invention are substantially free of any peroxygen source such as hydrogen peroxide, peroxyacid etc.
- the compositions of the present invention need only have an effective amount of the herein below descnbed catalyst present for effective bleaching.
- the term "substantially free” is defined as "the formulator does not include in the composition any peroxygen compound or source of peroxygen at a level required for either effective bleaching without a transition metal catalyst, or which would provide an increase in effectiveness of bleaching in the presence of a transition metal catalyst as defined herein.” Therefore, as will be further descnbed herein below, effective bleaching of stains can be accomplished by simply adding an aqueous or non-aqueous solution of a catalyst as descnbed herein to fabric which is stained, preferably the fabnc is in an aqueous solution when contacted with the catalyst.
- compositions of the present invention do not require any peroxygen source, but the presence of any minor amounts will not effect the performance of the bleaching compositions described herein.
- Formulators may typically include a small amount of a source of hydrogen peroxide into compositions for the purposes of stabilizing enzymes, for example, a minor amount of perborate may be added. However, this amount of perborate is typically so minor that it has no effect on the bleaching capacity of the compositions of the present invention.
- compositions are still defined as "substantially free” of a source of peroxygen as defined herein above if they do not provide additional bleaching activity on stains under typical use conditions.
- a “substantially free” composition can include an amount of peroxygen source provided the degree to which the catalyst is effective is substantially the same as if the source of peroxygen were absent.
- any composition which comprises less than 0.1%), preferably less than 0.01% of a primary oxidant, such as a pre-formed peracid or a source of hydrogen peroxide is considered “substantially free” as further defined herein above.
- a primary oxidant such as a pre-formed peracid or a source of hydrogen peroxide
- any laundry liquor, laundry wash water, pre-soak bath, or other fab ⁇ c or surface cleaning solution, wherein the present catalysts are used and which comprises less than 0.001% by weight of a source of peroxygen, pre-formed or otherwise formed in situ is defined herein as "substantially free” as defined herein above.
- the catalysts of the present invention are used to bleach stains on fab ⁇ c, or otherwise clean/bleach a hard surface or dishware, and the solution containing the catalyst has a concentration of a source of peroxygen less than 0.001% > , that solution is defined herein as "substantially free" of a source of peroxygen.
- compositions of the present invention comp ⁇ se an effective amount of a bleach catalyst.
- an effective amount is defined as "an amount of the transition-metal bleach catalyst present in the present invention compositions, or during use according to the present invention methods, that is sufficient, under whatever comparative or use conditions are employed, to result m at least partial oxidation of the matenal sought to be oxidized by the composition or method.”
- the material to be oxidized is an unwanted substance inter aha food and beverage stains, greasy/oily stains, body soils on fabric, however, this is not the limitation to which the invention is applicable Oxidation in the absence of a source of peroxygen has wide applicability and the present invention is not limited solely to bleaching and/or cleaning of fabric.
- automatic dishwashing compositions are an embodiment of the present invention wherein bleaching of a stam w ith a composition and/or with a solution which is "substantially free" of a source of peroxygen is a part of the present invention
- a solution which is "substantially free” of a source of peroxygen is a part of the present invention
- hard surface cleaning compositions and solutions which comprise hard surface cleaning compositions which are "substantially free” of a source of peroxygen are "substantially free" of a source of peroxygen.
- compositions of the present invention comprise from about 1 ppb (0.0000001%), more preferably from about 100 ppb (0.00001%), yet more preferably from about 500 ppb (0.00005%), still more preferably from about 1 ppm (0.0001%) to about 99.9%, more preferably to about 50%, yet more preferably to about 5%, still more preferably to about 500 ppm (0.05%o) by weight of the composition, of a transition-metal bleach catalyst as described herein below.
- the transition-metal bleach catalyst of the present invention comprises: i) a transition metal selected from the group consisting of Mn(LI), Mn(UI), Mn(IV),
- a cross-bridged macropolycychc hgand being coordinated by four or five donor atoms to the same transition metal, said gand comprising: a) an organic macrocycle ring containing four or more donor atoms (preferably at least 3, more preferably at least 4, of these donor atoms are
- N separated from each other by covalent linkages of 2 or 3 non-donor atoms, two to five (preferably three to four, more preferably four) of these donor atoms being coordinated to the same transition metal atom in the complex; b) a cross-bridged chain which covalently connects at least 2 non-adjacent donor atoms of the organic macrocycle ring, said covalently connected non-adjacent donor atoms being bridgehead donor atoms which are coordinated to the same transition metal in the complex, and wherein said cross-bridged chain compnses from 2 to about 10 atoms (preferably the cross-bridged chain is selected from 2, 3 or 4 non-donor atoms, and
- non-donor atoms with a further donor atom b) optionally, one or more non-macropolycyclic ligands, preferably selected from the group consisting of H2 ⁇ .
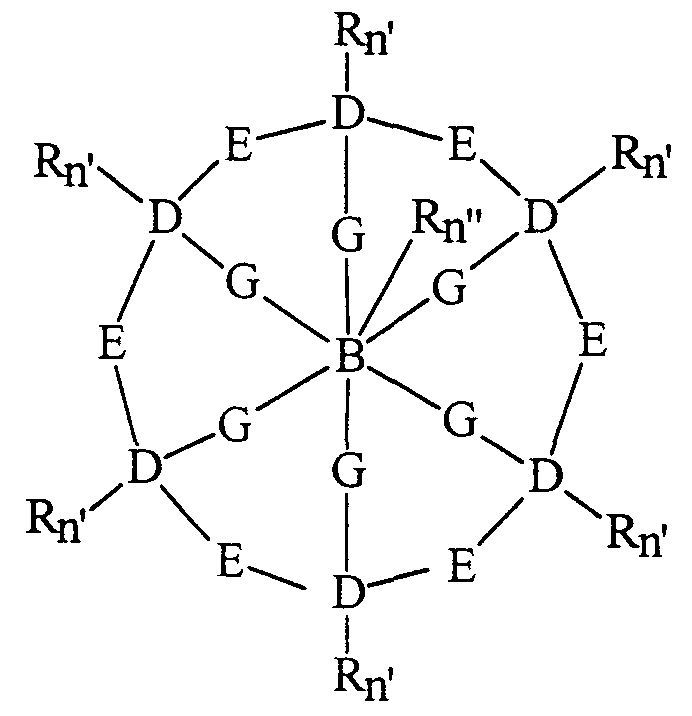
- the preferred cross-b ⁇ dged macropolycychc ligands are is selected from the group consisting of a) a cross-bridged macropolycychc hgand of formula (I) having denticity of 4 or 5-
- each E unit represents the moiety having the formula:
- each E units the sum of a + a' is independently selected from 1 to 5; each G unit is a moiety (CR n )b; each R unit is independently selected from H, alkyl, alkenyl, alkynyl, aryl, alkylaryl, and heteroaryl, or two or more R units are covalently bonded to form an aromatic, heteroaromatic, cycloalkyl, or heterocycloalkyl ⁇ ng; each D unit is a donor atom independently selected from the group consisting of nitrogen, oxygen, sulfur, and phosphorous, and at least two atoms which comp ⁇ se D units are bridgehead donor atoms coordinated to the transition metal; B units are a carbon atom, a D unit, or a cycloalkyl or heterocychc ring; each n is an integer independently selected from 1 and 2, completing the valence of the carbon atoms to which the R units are covalently
- Transition-metal bleach catalysts useful in the invention compositions can in general include known compounds where they conform with the invention definition, as well as, more preferably, any of a large number of novel compounds expressly designed for the present laundry or cleaning uses.
- suitable catalysts according to the present invention include:
- Manganese(II) D ⁇ chloro-4J 0-d ⁇ methyl-3 -phenyl- 1 ,4,7,10-tetraazab ⁇ cyclo[5.5 J]tetradecane
- Manganese(Ll) D ⁇ chloro-5J2-d ⁇ methyl-4,9-d ⁇ phenyl-l,5,8J2-tetraazab ⁇ cyclo[6.6J]hexadecane
- Manganese(LI) D ⁇ chloro-4, 10-d ⁇ methyl-3,8-d ⁇ phenyl- 1,4,7,10-tetraazab ⁇ cyclo[5.5 J]tetradecane
- Manganese(EI) D ⁇ chloro-5J2-d ⁇ methyl-2,H-d ⁇ phenyl-l,5,8J2-tetraazab ⁇ cyclo[6.6J]hexadecane
- Manganese(II) D ⁇ chloro-4J0-d ⁇ methyl-4,9-d ⁇ phenyl-l,4JJ0-tetraazab ⁇ cyclo[5JJ]tetradecane
- Manganese(II) D ⁇ chloro-l,5,8J2-tetraazab ⁇ cyclo[6 6J]hexadecane
- Manganese(II) D ⁇ chloro-l,4JJ0-tetraazab ⁇ cyclo[5JJ]tetradecane
- Manganese(II) D ⁇ chloro-l,5,8J2-tetraazab ⁇ cyclo[6.6J]hexadecane
- Iron(II) D ⁇ chloro-l,4JJ0-tetraazab ⁇ cyclo[5.5J]tetradecane Iron(II)
- Manganese(LII) Chloride D ⁇ chloro-5J2-d ⁇ methyl-l,4JJ0J3-pentaazab ⁇ cyclo[8 5 2]heptadecane
- Manganese(II) D ⁇ chloro-14,20-d ⁇ methyl-lJ0J4,20-tetraazat ⁇ yclo[8 6 6]docosa-3(8),4,6-tnene
- Manganese(LI) D ⁇ chloro-4,H-d ⁇ methyl-l,4J,H-tetraazab ⁇ cyclo[6 5 2]pentadecane
- Manganese(LI) D ⁇ chloro-5J2-d ⁇ methyl-l,5,8J2-tetraazab ⁇ cyclo(7 6 2]heptadecane
- Manganese( ⁇ ) D ⁇ chloro-5J3-d ⁇ methyl-l,5,9J3-tetraazab ⁇ cyclo[7 7 2]heptadecane
- Manganese(LI) D ⁇ aquo-3J0-d ⁇ carboxy-5J2-d ⁇ methyl-l,5,8J2-tetraazab ⁇ cyclo[6.6J]hexadecane
- Manganese(II) Chloro-20-methyl-l,9,20,24,25-pentaaza-tetracyclo[7.7 7J 3 J.l 1 U5.jpentacosa-
- Monometallic, mononuclear complexes are preferred.
- a monometallic transition-metal bleach catalyst contains only one transition metal atom per mole of complex.
- a monometallic, mononuclear complex is one in which any donor atoms of the essential macrocychc hgand are bonded to the same transition metal atom, that is, the essential hgand does not "b ⁇ dge" across two or more transition-metal atoms.
- compositions of the present invention will comp ⁇ se an effective amount of the anti-oxidant, preferably from about 0.01%, more preferably from about 0.1%, most preferably from about 0.2% to about 10%, preferably to about 5%, more preferably to about 1% by weight of an anti-oxidant.
- the anti-oxidant is a free radical scavenger.
- One class of anti-oxidants suitable for use in the present invention are the alkylated phenols having the general formula: wherein R is C ⁇ -C 22 linear or branched alkyl, preferably methyl or branched C 3 -C 6 alkyl; C 3 -C 6 alkoxy, preferably methoxy; R 1 is a C 3 -C 6 branched alkyl, preferably tert-butyl; x is 1 or 2.
- Another class of anti-oxidants suitable for use in the present invention are benzofuran denvatives having the formula:
- R 1 and R 2 are each independently C ⁇ -C 4 alkyl or R 1 and R 2 can be taken together to form a C 5 -C 6 cyclic hydrocarbyl moiety;
- R 4 is C ⁇ -C 6 alkyl;
- R 5 is hydrogen or -C(0)R 3 wherein R 3 is hydrogen or C r C 19 alkyl;
- R 6 is C C 6 alkyl;
- R 7 is hydrogen or C,-C 6 alkyl;
- X is -CH 2 OH, or - CH 2 A wherein A is a nitrogen comp ⁇ smg unit, phenyl, or substituted phenyl.
- Preferred nitrogen compnsmg A units include ammo, pyrro dmo, pipe ⁇ dmo, morpholmo, piperazmo, and mixtures thereof.
- Non-limiting examples of anti-oxidants suitable for use in the present invention include phenols inter alia 2,6-di-tert-butylphenol. 2,6-d ⁇ -/ert-butyl-4-methylphenol. mixtures of 2 and 3- terf-butyl-4-methoxyphenol, and other ingredients including include propyl gallate, ter- butylhydroqumone, benzoic acid denvatives such as methoxy benzoic acid, methylbenzoic acid, dichloro benzoic acid, dimethyl benzoic acid, 5-hydroxy-2J.4,6J-pentamethyl-2J-d ⁇ hydro-l- benzofuran-3-one, 5-hydroxy-3-methylene-2J,4.6J-pentamethyl-2J-d ⁇ hydro-benzofuran, 5- benzyloxy-3-hydroxymethyl-2J,4.6J-pentamethyl-2J-d ⁇ hydro-l -benzofuran, 3-hydroxymethyl- 5-methoxy-2J,4.6J-pentamethyl-2J
- compositions of the present invention will compnse an effective amount of one or more reducing agents, preferably from about 0.001%, more preferably from about 0.01%, most preferably from about 0.02% to about 1%, preferably to about 0.5%, more preferably to about 0.1%) by weight of a reducing agent.
- reducing agent is an inorganic compound
- Non-limiting examples of reducing agents include compounds capable of yielding sulfite ions such as sodium sulfite, potassium sulfite, ammonium sulfite, sodium hydrogen sulfite, sodium metabisulfite, potassium metabisulfite, potassium hydrogen sulfite, pyrosulphite salts, sodium borohyd ⁇ de, lithium borohyd ⁇ de, lithium aluminum isopropoxide, and mixtures thereof.
- sulfite ions such as sodium sulfite, potassium sulfite, ammonium sulfite, sodium hydrogen sulfite, sodium metabisulfite, potassium metabisulfite, potassium hydrogen sulfite, pyrosulphite salts, sodium borohyd ⁇ de, lithium borohyd ⁇ de, lithium aluminum isopropoxide, and mixtures thereof.
- the present invention further relates to a method for using stabilized bleach catalysts without the requirement of a peroxygen source of peroxygen to clean soil from and stains from fab ⁇ c.
- the present invention therefore, relates to a method for bleaching soils and stains on fabric m the absence of a bleaching agent, said method comp ⁇ smg the step of contacting fab ⁇ c m need of cleaning with an aqueous or non-aqueous solution containing a composition which is substantially free of a peroxygen source, comp ⁇ sing: a) a catalytically effective amount of a transition-metal bleach catalyst which is a complex of a transition-metal and a cross-b ⁇ dged macropolycychc hgand; b) an effective amount of a stabilizing agent, said agent selected from l) one or more anti-oxidants; n) one or more reducing agents; in) and mixtures thereof; and c) the balance earners and other adjunct ingredients; provided the concentration of said transition metal bleach catalyst m the solution is at least about 0.01 ppb and said composition is substantially free of any organic or inorganic peroxygen compounds.
- the solution which compnses the transition-metal bleach catalyst has a solution concentration of catalyst of from about 1 ppb, more preferably from about 10 ppb, yet more preferably from about 100 ppb.
- 100 ppb parts per billion is a solution which compnses 0.00001% by weight, of a catalyst.
- solutions which compnses less than 0.001% of a source of peroxygen are solutions which are "substantially free" of any organic or inorganic peroxygen compounds.
- Methods directed entirely to large scale bleaching per se may utilized a higher concentration of catalyst, for example, 1 ppm or higher m order to reduce the contact time of the fabric with the catalyst containing solution.
- a higher concentration of catalyst for example, 1 ppm or higher m order to reduce the contact time of the fabric with the catalyst containing solution.
- the bleaching, pre-soak, pre-treatment, laundry or automatic diswashmg, or hard surface cleaning compositions of the present invention may further comp ⁇ se one or more earners and adjunct ingredients.
- compositions according to the present invention may compnse- a) a catalytically effective amount of a transition-metal bleach catalyst which is a complex of a transition-metal and a cross-bridged macropolycychc hgand; and b) optionally from about 0.001% to about 90% by weight, of one or more dye fixing agents; c) optionally from about 0.01% to about 50% by weight, of one or more cellulose reactive dye fixing agents; d) optionally from about 0.01% to about 15% by weight, of a chlo ⁇ ne scavenger; e) optionally about 0.005% to about 1% by weight, of one or more crystal growth inhibitors; f) optionally from about 0.01% to about 20% by weight, of a fabnc abrasion reducing polymer; g) optionally from about 1% to about 12% by weight, of one or more liquid earners; h) optionally from about 0.001%> to about 1% by weight, of an enzyme; l) optional
- surfactants selected from the group consisting of aniomc, cationic, nomonic, ampholytic, zwitte ⁇ omc surfactants, and mixtures thereof.
- the bleaching, pre-soak, pre-treatment, and laundry detergent compositions of the present invention may compnse at least about 0.01% by weight, preferably from about 0.1% to about 60%, preferably to about 30% by weight, of a detersive surfactant system, said system is compnsed of one or more category of surfactants depending upon the embodiment, said categories of surfactants are selected from the group consisting of aniomc, cationic, nomonic, zwitte ⁇ onic, ampholytic surfactants, and mixtures thereof. Within each category of surfactant, more than one type of surfactant of surfactant can be selected. For example, preferably the solid (i.e. granular) and viscous semi-solid (i.e. gelatinous, pastes, etc.) systems of the present invention, surfactant is preferably present to the extent of from about 0.1% to 60 %, preferably to about 30% by weight of the composition.
- Nonhmiting examples of surfactants useful herein include- a) C ⁇ -Ci 8 alkyl benzene sulfonates (LAS); b) C 10 -C 2 o primary, branched-chain and random alkyl sulfates (AS); c) C 10 -C 18 secondary (2,3) alkyl sulfates having the formula.
- LAS alkyl benzene sulfonates
- AS branched-chain and random alkyl sulfates
- C 10 -C 18 secondary (2,3) alkyl sulfates having the formula.
- x and (y + 1) are integers of at least about 7, preferably at least about 9; said surfactants disclosed U.S. 3J34J58 Morris, issued February 8, 1966; U S. 5,075,041 Lutz, issued December 24, 1991; U.S. 5,349,101 Lutz et al., issued September 20, 1994; and U.S.
- R 7 is C5-C31 alkyl
- R ⁇ is selected from the group consisting of hydrogen, C1 -C4 alkyl, C1-C4 hydroxyalkyl
- Q is a polyhydroxyalkyl moiety having a linear alkyl chain with at least 3 hydroxyls directly connected to the chain, or an alkoxy lated derivative thereof; preferred alkoxy is ethoxy or propoxy, and mixtures thereof; preferred Q is derived from a reducing sugar m a reductive animation reaction, more preferably Q is a glycityl moiety;
- Q is more preferably selected from the group consisting of -CH 2 (CHOH) n CH 2 OH, -CH(CH 2 OH)(CHOH) n _ jCH ⁇ OH, -CH 2 (CHOH) 2 -(CHOR')(CHOH)CH 2 OH, and alkoxylated denvatives thereof, wherein n is an integer from 3 to 5, inclusive
- the bleaching, pre-soak, pre-treatment, and laundry detergent compositions of the present invention can also compnse from about 0.001% to about 100% of one or more (preferably a mixture of two or more) mid-cham branched surfactants, preferably mid-chain branched alkyl alkoxy alcohols having the formula:
- M is a water soluble cation and may compnses more than one type of cation, for example, a mixture of sodium and potassium
- the index w is an integer from 0 to 13.
- x is an integer from 0 to 13;
- y is an integer from 0 to 13;
- z is an integer of at least 1; provided w - x + y + z is from 8 to 14.
- EO and PO represent ethyleneoxy units and propyleneoxy units having the formula:
- alkoxy units inter alia 1J -propyleneoxy, butoxy, and mixtures thereof are suitable as alkoxy units appended to the mid-chain branched alkyl moieties.
- the mid-cham branched surfactants are preferably mixtures which comp ⁇ se a surfactant system. Therefore, when the surfactant system comprises an alkoxylated surfactant, the index m indicates the average degree of alkoxylation withm the mixture of surfactants. As such, the index m is at least about 0.01, preferably withm the range of from about 0J, more preferably from about 0.5, most preferably from about 1 to about 30, preferably to about 10, more preferably to about 5.
- the value of the index m represents a distribution of the average degree of alkoxylation corresponding to m, or it may be a single specific chain with alkoxylation (e.g., ethoxylation and/or propoxylation) of exactly the number of units corresponding to m.
- the preferred mid-cham branched surfactants of the present invention which are suitable for use in the surfactant systems of the present invention have the formula:
- a, b, d, and e are integers such that a + b is from 10 to 16 and d + e is from 8 to 14, M is selected from sodium, potassium, magnesium, ammonium and substituted ammonium, and mixtures thereof.
- the surfactant systems of the present invention which comprise mid-cham branched surfactants are preferably formulated in two embodiments.
- a first preferred embodiment comprises mid-cham branched surfactants which are formed from a feedstock which compnses 25% or less of mid-cham branched alkyl units. Therefore, prior to admixture with any other conventional surfactants, the mid-chain branched surfactant component will compnse 25% or less of surfactant molecules which are non-linear surfactants.
- a second preferred embodiment comprises mid-cham branched surfactants which are formed from a feedstock which comprises from about 25% to about 70% of mid-chain branched alkyl units. Therefore, prior to admixture with any other conventional surfactants, the mid-cham branched surfactant component will comprise from about 25% to about 70% surfactant molecules which are non-linear surfactants.
- the surfactant systems of the laundry detergent compositions of the present invention can also comprise from about 0.001%>, preferably from about 1%, more preferably from about 5%, most preferably from about 10% to about 100%, preferably to about 60%, more preferably to about 30% by weight, of the surfactant system, of one or more (preferably a mixture of two or more) mid-chain branched alkyl arylsulfonate surfactants, preferably surfactants wherein the aryl unit is a benzene ⁇ ng having the formula:
- L is an acyclic hydrocarbyl moiety compnsing from 6 to 18 carbon atoms; R , R , and R are each independently hydrogen or C C 3 alkyl, provided R 1 and R 2 are not attached at the terminus of the L unit; M is a water soluble cation having charge q wherein a and b are taken together to satisfy charge neutrality.
- compositions of the present invention especially when comp ⁇ sing surfactants, preferably comp ⁇ se one or more detergent builders or builder systems.
- the compositions will typically comprise at least about 1% builder, preferably from about 5%, more preferably from about 10% to about 80%, preferably to about 50%, more preferably to about 30% by weight, of detergent builder.
- the level of builder can vary widely depending upon the end use of the composition and its desired physical form. When present, the compositions will typically compnse at least about 1% builder. Formulations typically comprise from about 5% to about 50%, more typically about 5% to about 30%, by weight, of detergent builder. Granular formulations typically compnse from about 10% to about 80%, more typically from about 15% to about 50% > by weight, of the detergent builder. Lower or higher levels of builder, however, are not meant to be excluded.
- Inorganic or P-containmg detergent builders include, but are not limited to, the alkali metal, ammonium and alkanolammonium salts of polyphosphates (exemplified by the t ⁇ polyphosphates, pyrophosphates, and glassy polymeric meta-phosphates), phosphonates, phytic acid, silicates, carbonates (including bicarbonates and sesquicarbonates), sulphates, and aluminosihcates.
- non-phosphate builders are required in some locales.
- compositions herein function surprisingly well even in the presence of the so-called “weak” builders (as compared with phosphates) such as citrate, or in the so-called “underbuilt” situation that may occur with zeolite or layered silicate builders.
- silicate builders are the alkali metal silicates described m U.S. 4,664,839 Rieck, issued May 12, 1987.
- NaSK.S-6 is the trademark for a crystalline layered silicate marketed by Hoechst (commonly abbreviated herein as "SKS-6").
- Examples of carbonate builders are the alkaline earth and alkali metal carbonates as disclosed in German Patent Application No. 2,321,001 published on November 15, 1973.
- Alummosilicate builders are useful in the present invention. Examples of suitable alummosilicate builders are descnbed in U.S. 4,274,975 Corkhill et al. included herein by reference.
- Alummosilicate builders are of great importance in most currently marketed heavy duty granular detergent compositions, and can also be a significant builder ingredient in liquid detergent formulations.
- Alummosilicate builders include those having the empincal formula:
- Organic detergent builders suitable for the purposes of the present invention include, but are not restncted to, a wide va ⁇ ety of polycarboxylate compounds.
- polycarboxylate refers to compounds having a plurality of carboxylate groups, preferably at least 3 carboxylates.
- Polycarboxylate builder can generally be added to the composition in acid form, but can also be added m the form of a neutralized salt. When utilized in salt form, alkali metals, such as sodium, potassium, and lithium, or alkanolammonium salts are preferred.
- Citrate builders e.g., citric acid and soluble salts thereof (particularly sodium salt), are polycarboxylate builders of particular importance for heavy duty liquid detergent formulations due to their availability from renewable resources and their biodegradabihty. Citrates can also be used m granular compositions, especially in combination with zeolite and/or layered silicate builders. Oxydisuccmates are also especially useful in such compositions and combinations. Dispersants
- polymenc dispersing agents which include polyme ⁇ c polycarboxylates and polyethylene glycols, are suitable for use m the present invention.
- Polymenc polycarboxylate matenals can be prepared by polymenzmg or copolyme ⁇ zing suitable unsaturated monomers, preferably in their acid form.
- Unsaturated monomenc acids that can be polymenzed to form suitable polymenc polycarboxylates include acrylic acid, maleic acid (or maleic anhydnde), fumanc acid, itacomc acid, acomtic acid, mesaconic acid, citraconic acid and methylenemalonic acid.
- polymenc polycarboxylates herein or monomenc segments containing no carboxylate radicals such as vmylmethyl ether, styrene, ethylene, etc. is suitable provided that such segments do not constitute more than about 40% by weight.
- Particularly suitable polymenc polycarbox lates can be denved from acrylic acid.
- acrylic acid-based polymers which are useful herein are the water-soluble salts of polymenzed acrylic acid.
- the average molecular weight of such polymers in the acid form preferably ranges from about 2,000 to 10,000, more preferably from about 4,000 to 7,000 and most preferably from about 4,000 to 5,000.
- Water-soluble salts of such acrylic acid polymers can include, for example, the alkali metal, ammonium and substituted ammonium salts Soluble polymers of this type are known materials. Use of polyacrylates of this type in detergent compositions has been disclosed, for example, in Diehl, U.S. Patent 3,308,067, issued march 7, 1967.
- Acryhc/maleic-based copolymers may also be used as a preferred component of the dispersing/anti-redeposition agent.
- Such matenals include the water-soluble salts of copolymers of acrylic acid and maleic acid.
- the average molecular weight of such copolymers m the acid form preferably ranges from about 2,000, preferably from about 5,000, more preferably from about 7,000 to 100,000, more preferably to 75,000, most preferably to 65,000.
- the ratio of acrylate to maleate segments in such copolymers will generally range from about 30:1 to about 1 : 1, more preferably from about 10:1 to 2: 1.
- Water-soluble salts of such acrylic acid/maleic acid copolymers can include, for example, the alkali metal, ammonium and substituted ammonium salts.
- Soluble acrylate/maleate copolymers of this type are known matenals which are described in European Patent Application No. 66915, published December 15, 1982, as well as m EP 193J60, published September 3, 1986, which also descnbes such polymers comp ⁇ sing hydroxypropylacrylate.
- Still other useful dispersing agents include the maleic/acryhc/vinyl alcohol terpolymers.
- Such matenals are also disclosed m EP 193,360, including, for example, the 45/45/10 terpolymer of acry c/maleic/vmyl alcohol.
- PEG polyethylene glycol
- PEG can exhibit dispersing agent performance as well as act as a clay soil removal-antiredeposition agent.
- Typical molecular weight ranges for these purposes range from about 500 to about 100,000, preferably from about 1 ,000 to about 50,000, more preferably from about 1 ,500 to about 10,000.
- Polyaspartate and polyglutamate dispersing agents may also be used, especially in conjunction with zeolite builders.
- Dispersing agents such as polyaspartate preferably have a molecular weight (avg.) of about 10,000.
- Soil Release Agents such as polyaspartate preferably have a molecular weight (avg.) of about 10,000.
- compositions according to the present invention may optionally comprise one or more soil release agents.
- soil release agents will generally compnse from about 0.01%), preferably from about 0J%, more preferably from about 0J%> to about 10%, preferably to about 5%, more preferably to about 3% by weight, of the composition.
- Polyme ⁇ c soil release agents are characte ⁇ zed by having both hydrophihc segments, to hydrophihze the surface of hydrophobic fibers, such as polyester and nylon, and hydrophobic segments, to deposit upon hydrophobic fibers and remain adhered thereto through completion of the laundry cycle and, thus, serve as an anchor for the hydrophihc segments.
- the detergent and cleaning compositions herein may also optionally contain one or more types of detergent enzymes.
- Such enzymes can include other proteases, amylases, cellulases and hpases.
- Such matenals are known in the art and are commercially available under such trademarks as . They may be incorporated into the non-aqueous liquid detergent compositions herein in the form of suspensions, "marumes" or "pnlls".
- Another suitable type of enzyme comprises those in the form of slurnes of enzymes in nomonic surfactants, e.g., the enzymes marketed by Novo Nordisk under the tradename "SL” or the microencapsulated enzymes marketed by Novo Nordisk under the tradename "LDP.” Suitable enzymes and levels of use are described in U.S. Pat. No. 5,576,282, 5,705,464 and 5,710,115.
- Enzymes added to the compositions herein in the form of conventional enzyme pnlls are especially preferred for use herein.
- Such pnlls will generally range in size from about 100 to 1 ,000 microns, more preferably from about 200 to 800 microns and will be suspended throughout the non-aqueous liquid phase of the composition.
- Prills m the compositions of the present invention have been found, in companson with other enzyme forms, to exhibit especially desirable enzyme stability in terms of retention of enzymatic activity over time.
- compositions which utilize enzyme prills need not contain conventional enzyme stabilizing such as must frequently be used when enzymes are incorporated into aqueous liquid detergents.
- enzymes added to the compositions herein may be in the form of granulates, preferably T-granulates.
- "Detersive enzyme" means any enzyme having a cleaning, stam removing or otherwise beneficial effect in a laundry, hard surface cleaning or personal care detergent composition.
- Preferred detersive enzymes are hydrolases such as proteases, amylases and hpases
- Preferred enzymes for laundry purposes include, but are not limited to, proteases, cellulases, hpases and peroxidases.
- Highly preferred for automatic dishwashing are amylases and/or proteases, including both current commercially available types and improved types which, though more and more bleach compatible though successive improvements, have a remaining degree of bleach deactivation susceptibility.
- suitable enzymes include, but are not limited to, hemicellulases, peroxidases, proteases, cellulases, xylanases, hpases, phosphohpases, esterases, cutmases, pectmases, keratanases, reductases, oxidases, phenoloxidases, hpoxygenases, hgmnases, pullulanases, tannases, pentosanases, malanases, ⁇ -glucanases, arabmosidases, hyaluromdase, chondroitmase, laccase, and known amylases, or mixtures thereof.
- cellulases useful in the present invention include both bactenal or fungal cellulases
- Suitable cellulases are disclosed in U.S. Patent 4,435,307, J61078384 and WO96/02653 which discloses fungal cellulase produced respectively from Humicola msolens, Tnchoderma, Thielavia and Sporot ⁇ chum.
- EP 739 982 describes cellulases isolated from novel Bacillus species. Suitable cellulases are also disclosed in GB-A-2 075.028, GB-A-2.095.275; DE-OS-2.247.832 and W095/26398
- cellulases examples include cellulases produced by a strain of Humicola msolens (Humicola g ⁇ sea var. thermoidea), particularly the Humicola strain DSM 1800.
- suitable cellulases are cellulases o ⁇ gmated from Humicola msolens having a molecular weight of about 50KDa, an isoelectnc point of 5.5 and containing 415 ammo acids; and a ⁇ 43kD endoglucanase derived from Humicola msolens, DSM 1800, exhibiting cellulase activity; a preferred endoglucanase component has the ammo acid sequence disclosed in WO 91/17243.
- suitable cellulases are the EGHI cellulases from Tnchoderma longibrachiatum described in WO94/21801 to Genencor. Especially suitable cellulases are the cellulases having color care benefits. Examples of such cellulases are cellulases descnbed in European patent application No 91202879J, filed November 6, 1991 (Novo). Carezyme and Celluzyme (Novo Nordisk A/S) are especially useful.
- Suitable hpase enzymes for detergent usage include those produced by microorganisms of the Pseudomonas group, such as Pseudomonas stutze ⁇ ATCC 19.154, as disclosed in British Patent 1,372,034.
- Suitable hpases include those which show a positive immunological cross-reaction with the antibody of the hpase, produced by the microorganism Pseudomonas fluorescent LAM 1057. This hpase is available from Amano Pharmaceutical Co Ltd., Nagoya, Japan, under the trade name Lipase P "Amano,” hereinafter referred to as "Amano- P".
- hpases include Amano-CES, hpases ex Chromobacter viscosum, e.g. Chromobacter viscosum var lipolyticum NRRLB 3673 from Toyo Jozo Co., Tagata, Japan, Chromobacter viscosum hpases from U.S. Biochemical Corp., U.S.A. and Disoynth Co., The Netherlands, and hpases ex Pseudomonas gladioli.
- Chromobacter viscosum e.g. Chromobacter viscosum var lipolyticum NRRLB 3673 from Toyo Jozo Co., Tagata, Japan
- Chromobacter viscosum hpases from U.S. Biochemical Corp., U.S.A. and Disoynth Co., The Netherlands
- hpases ex Pseudomonas gladioli.
- hpases such as Ml Lipase* ⁇ an( L ⁇ poma ⁇ R (Gist-Brocades) and LipolaseR and Lipolase Ultra ⁇ (Novo) which have found to be very effective when used in combination with the compositions of the present invention.
- lipolytic enzymes described m EP 258 068, WO 92/05249 and WO 95/22615 by Novo Nordisk and in WO 94/03578, WO 95/35381 and WO 96/00292 by Unilever
- cutmases [EC 3.1.1.50] which can be considered as a special kind of lipase, namely hpases which do not require mterfacial activation. Addition of cutmases to cleaning compositions have been described in e.g. WO-A-88/09367 (Genencor); WO 90/09446 (Plant Genetic System) and WO 94/14963 and WO 94/14964 (Unilever).
- Lipases and or cutmases when present, are normally incorporated in the cleaning composition at levels from 0.0001% to 2% of pure enzyme by weight of the cleaning composition.
- phosphohpases may be incorporated into the cleaning compositions of the present invention.
- suitable phosphohpases included: EC 3.U.32 Phosphohpase Al; EC 3 1 1 4 Phosphohpase A2; EC 3.U.5 Lysophohpase; EC 3.1.4.3 Phosphohpase C, EC 3 1.4 4. Phospohpase D.
- phosphohpases include LECITASE® from Novo Nordisk A/S of Denmark and Phosphohpase A2 from Sigma.
- amylases are also included.
- the combined action of the phosphohpase and amylase provide substantive stain removal, especially on greasy/oily, starchy and highly colored stains and soils.
- the phosphohpase and amylase when present, are incorporated into the compositions of the present invention at a pure enzyme weight ratio between 4500J and 1 :5, more preferably between 50: 1 and 1: 1.
- Suitable proteases are the subtihsins which are obtained from particular strains of B subtihs and B. hcheniformis (subtihsm BPN and BPN')
- One suitable protease is obtained from a strain of Bacillus, having maximum activity throughout the pH range of 8-12, developed and sold as ESPERASE® by Novo Industries A S of Denmark, hereinafter "Novo". The preparation of this enzyme and analogous enzymes is described in GB 1.243,784 to Novo.
- Proteolytic enzymes also encompass modified bactenal se ⁇ ne proteases, such as those described in European Patent Application Se ⁇ al Number 87 303761.8, filed April 28, 1987 (particularly pages 17, 24 and 98), and which is called herein "Protease B", and in European Patent Application 199,404, Venegas, published October 29, 1986, which refers to a modified bactenal senne protealytic enzyme which is called "Protease A" herein.
- Protease C is a vanant of an alkaline senne protease from Bacillus in which Lysine replaced argmine at position 27, tyrosme replaced vahne at position 104, se ⁇ ne replaced asparagme at position 123, and alanine replaced threonme at position 274
- Protease C is descnbed in EP 90915958:4, corresponding to WO 91/06637, Published May 16. 1991 Genetically modified va ⁇ ants, particularly of Protease C, are also included herein
- a preferred protease referred to as "Protease D” is a carbonyl hydrolase as descnbed in U.S. Patent No. 5,677,272, and WO95/10591 Also suitable is a carbonyl hydrolase vanant of the protease descnbed in WO95/10591, having an amino acid sequence denved by replacement of a plurality of ammo acid residues replaced in the precursor enzyme corresponding to position +210 in combination with one or more of the follow ing residues ⁇ +33, +62, +67, +76, +100, +101, +103, +104, +107, +128, +129, +130, +132. - 135.
- protease from Bacillus sp. NCLMB 40338 described in WO 93/18140 A to Novo.
- Enzymatic detergents comprising protease, one or more other enzymes, and a reversible protease inhibitor are described in WO 92/03529 A to Novo.
- a protease having decreased adsorption and increased hydrolysis is available as described m WO 95/07791 to Procter & Gamble.
- a recombinant trypsin-hke protease for detergents suitable herein is described in WO 94/25583 to Novo Other suitable proteases are descnbed in EP 516 200 by Unilever.
- proteases are described m PCT publications: WO 95/30010; WO 95/30011 ; and WO 95/29979 Suitable proteases are commercially available as ESPERASE® , ALCALASE®, DURAZYM®, SAVLNASE®, EVERLASE® and KAN ASE® all from Novo Nordisk A S of Denmark, and as MAXATASE®, MAXACAL®, PROPERASE® and MAXAPEM® all from Genencor International (formerly Gist-Brocades of The Netherlands).
- proteolytic enzymes when present, are incorporated in the cleaning compositions of the present invention a level of from 0.0001% to 2%, preferably from 0.001% to 0.2%, more preferably from 0.005% to 0.1% pure enzyme by weight of the composition.
- Amylases ( ⁇ and/or ⁇ ) can be included for removal of carbohydrate-based stains.
- WO94/02597 descnbes cleaning compositions which incorporate mutant amylases. See also W095/ 10603.
- Other amylases known for use in cleaning compositions include both ⁇ - and ⁇ - amylases.
- ⁇ -Amylases are known m the art and include those disclosed in US Pat. no. 5,003,257, EP 252,666; WO/91/00353; FR 2,676,456; EP 285,123; EP 525,610; EP 368,341; and B ⁇ tish Patent specification no. 1,296,839 (Novo).
- amylases are stability-enhanced amylases described m W094/18314 and WO96/05295, Genencor, and amylase vanants having additional modification in the immediate parent available from Novo Nordisk A S, disclosed in WO 95/10603. Also suitable are amylases described in EP 277 216.
- Examples of commercial ⁇ -amylases products are Purafect Ox Am® from Genencor and
- Termamyl®, Ban® ,Fungamyl® and Duramyl® all available from Novo Nordisk A S Denmark W095/26397 descnbes other suitable amylases : ⁇ -amylases charactensed by having a specific activity at least 25% higher than the specific activity of Termamyl® at a temperature range of 25°
- amylolytic enzymes when present, are incorporated in the cleaning compositions of the present invention a level of from 0.0001% to 2%, preferably from 0.00018% to 0.06%, more preferably from 0.00024% to 0.048% pure enzyme by weight of the composition
- the above-mentioned enzymes may be of any suitable ongin, such as vegetable, animal, bacterial, fungal and yeast origin Ongin can further be mesophihc or extremophihc (psychrophihc, psychrotrophic, thermophihc, barophi c, alkalophihc, acidophihc, halophihc, etc.). Purified or non-purified forms of these enzymes may be used.
- the variants may be designed such that the compatibility of the enzyme to commonly encountered ingredients of such compositions is increased.
- the variant may be designed such that the optimal pH, bleach or chelant stability, catalytic activity and the like, of the enzyme variant is tailored to suit the particular cleaning application
- attention should be focused on amino acids sensitive to oxidation m the case of bleach stability and on surface charges for the surfactant compatibility.
- the lsoelectnc point of such enzymes may be modified by the substitution of some charged amino acids, e.g. an increase in isoelect ⁇ c point may help to improve compatibility with aniomc surfactants.
- the stability of the enzymes may be further enhanced by the creation of e g. additional salt bndges and enforcing calcium binding sites to increase chelant stability.
- detersive enzymes when present, are normally incorporated in the cleaning composition at levels from 0.0001% to 2% of pure enzyme by weight of the cleaning composition.
- the enzymes can be added as separate single ingredients (pnlls, granulates, stabilized liquids, etc... containing one enzyme ) or as mixtures of two or more enzymes ( e.g. cogranulates ).
- enzyme oxidation scavengers examples include ethoxylated tetraethylene polyammes.
- a range of enzyme matenals and means for their incorporation into synthetic detergent compositions is also disclosed in WO 9307263 and WO 9307260 to Genencor International, WO 8908694, and U.S. 3,553,139, January 5, 1971 to McCarty et al. Enzymes are further disclosed in U.S. 4,101,457, and in U.S. 4,507,219. Enzyme materials useful for liquid detergent formulations, and their incorporation into such formulations, are disclosed in U.S. 4,261,868. Amylase enzymes are suitable for use in the compositions of the present invention.
- Amylase enzymes and variants used in the present invention include, but are not limited to, the amylase enzymes described m WO 95/26397 and in WO 96/23873 (Novo). These enzymes are incorporated into cleaning compositions at a level of from about 0.0001%, preferably from about 0.00018%>, more preferably from about 0.00024%, most preferably from about 0.05% to about 0.1%, preferably to about 0.060%, more preferably to about 0.048%> by weight of the cleaning compositions of pure enzyme.
- amylase vanants are preferably selected from the group consisting of ⁇ -amylase variants.
- Suitable ⁇ -amylase variants for use in the present invention include, but are not limited to the following ⁇ -amylases:
- SEQ LD No. 1 or an ⁇ -amylase being at least 80% homologous with the ammo acid sequence shown in SEQ LD No. 1 and/or;
- ⁇ -amylase according to (l) comprising the amino acid sequence shown m SEQ LD No. 2 or an ⁇ -amylase being at least 80% homologous with the ammo acid sequence shown in SEQ LD No. 2 and/or;
- (vn) ⁇ -amylase showing positive immunological cross-reactivity with antibodies raised against an ⁇ -amylase having an amino acid sequence corresponding respectively to SEQ LD No. 1 , LD No. 2, or LD No. 3 and/or;
- (vni) vanant of a parent ⁇ -amylase wherein the parent ⁇ -amylase (1) has one of the ammo acid sequences shown in SEQ LD No. 1, LD No. 2, or LD No. 4, respectively, or (2) displays at least 80% homology with one or more of said ammo acid sequences, and/or displays lmmunological cross-reactivity with an antibody raised against an ⁇ -amylase having one of said ammo acid sequences, and or is encoded by a DNA sequence which hybridizes with the same probe as a DNA sequence encoding an ⁇ -amylase having one of said ammo acid sequences, in which variants: (A) at least one amino acid residue of said parent ⁇ -amylase has been deleted, and or (B) at least one ammo acid residue of said parent ⁇ -amylase has been replaced by a different amino acid residue; and or (C) at least one amino acid residue has been inserted relative to said parent ⁇ -amylase; said variant having an ⁇
- a polypeptide is considered to be X% homologous to the parent amylase if a comparison of the respective amino acid sequences, performed via algonthms, such as the one descnbed by Lipman and Pearson m Science 227, 1985, p. 1435, reveals an identity of X%.
- the term "obtainable from” is intended not only to indicate an amylase produced by a Bacillus strain but also an amylase encoded by a DNA sequence isolated from such a Bacillus strain and produced in a host organism transformed with the DNA sequence.
- Enzyme Stabilizers are intended not only to indicate an amylase produced by a Bacillus strain but also an amylase encoded by a DNA sequence isolated from such a Bacillus strain and produced in a host organism transformed with the DNA sequence.
- Enzymes for use in detergents can be stabilized by vanous techniques. Enzyme stabilization techniques are disclosed and exemplified in U.S. 3,600,319, EP 199,405 and EP 200,586. Enzyme stabilization systems are also described, for example, in U.S. 3,519,570. A useful Bacillus, sp. AC 13 giving proteases, xylanases and cellulases, is descnbed in WO
- the enzymes employed herein can be stabilized by the presence of water-soluble sources of calcium and/or magnesium ions in the finished compositions which provide such ions to the enzymes. Suitable enzyme stabilizers and levels of use are descnbed m U.S. Pat. Nos. 5,705,464, 5,710,115 and 5,576,282. The following is a non-hmitmg example of the preparation of a bleach catalyst which effectively bleaches stains in the absence of a source of peroxygen.
- N,N'-b ⁇ s(2-am ⁇ noethyl)-l J-propaned ⁇ am ⁇ ne (5.00g, 31J mmol) and absolute ethanol (100 mL).
- the solution is stirred under argon and cooled to 15°C using an ice bath.
- Aqueous glyoxal (4.78 g., 33 mmol, 40% in water) is added dropwise with stirnng.
- the solution is concentrated under reduced pressure to yield a clear, colorless oil.
- the isolated oil has the formula 1
- Cyclic amine 1 (6.0 g) is suspended in acetonitnle ( 100 mL). Potassium carbonate (25 g) and 1,3-propanediol ditosylate (12.61 g, 32.8 mmol) is added. The solution is stirred vigorously at RT overnight. The reaction is then warmed to 70°C and filtered hot with glass fiber filter paper and vacuum filtration. The resulting solid is washed with acetonitnle (100 mL). The acetonitnle filtrate is concentrated under reduced pressure to yield a light green oil having the formula 2:
- HDL Heavy Duty Liquid
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Inorganic Chemistry (AREA)
- Biochemistry (AREA)
- Detergent Compositions (AREA)
- Catalysts (AREA)
Abstract
Description
Claims
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU36127/00A AU772358B2 (en) | 1999-03-02 | 2000-02-29 | Stabilized bleach compositions |
| JP2000602736A JP2002538268A (en) | 1999-03-02 | 2000-02-29 | Stabilized bleach composition |
| BR0008715-7A BR0008715A (en) | 1999-03-02 | 2000-02-29 | Bleach stabilized compositions |
| DE60025472T DE60025472T2 (en) | 1999-03-02 | 2000-02-29 | STABILIZED BLEACHING COMPOSITIONS |
| EP00914782A EP1157088B1 (en) | 1999-03-02 | 2000-02-29 | Stabilized bleach compositions |
| CA002364538A CA2364538A1 (en) | 1999-03-02 | 2000-02-29 | Bleach compositions comprising cross-bridged macropolycyclic transition metal bleach catalysts and a stabilizing agent |
| MXPA01008890A MXPA01008890A (en) | 1999-03-02 | 2000-02-29 | Stabilized bleach compositions. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12249299P | 1999-03-02 | 1999-03-02 | |
| US60/122,492 | 1999-03-02 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09914528 A-371-Of-International | 2001-08-29 | ||
| US10/142,070 Continuation US6653270B2 (en) | 1999-03-02 | 2002-05-09 | Stabilized bleach compositions |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2000052124A1 true WO2000052124A1 (en) | 2000-09-08 |
Family
ID=22403015
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2000/005291 WO2000052124A1 (en) | 1999-03-02 | 2000-02-29 | Stabilized bleach compositions |
Country Status (14)
| Country | Link |
|---|---|
| EP (1) | EP1157088B1 (en) |
| JP (1) | JP2002538268A (en) |
| KR (1) | KR100453614B1 (en) |
| CN (1) | CN1342194A (en) |
| AR (1) | AR022803A1 (en) |
| AT (1) | ATE315626T1 (en) |
| AU (1) | AU772358B2 (en) |
| BR (1) | BR0008715A (en) |
| CA (1) | CA2364538A1 (en) |
| CZ (1) | CZ20013157A3 (en) |
| DE (1) | DE60025472T2 (en) |
| ES (1) | ES2255992T3 (en) |
| MX (1) | MXPA01008890A (en) |
| WO (1) | WO2000052124A1 (en) |
Cited By (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001048299A1 (en) * | 1999-12-24 | 2001-07-05 | Unilever Plc | Composition and method for bleaching a substrate |
| WO2001048298A1 (en) * | 1999-12-24 | 2001-07-05 | Unilever Plc | Method of treating a textile |
| WO2001064994A1 (en) * | 2000-03-01 | 2001-09-07 | Unilever Plc | Method for reducing dye fading of fabrics in laundry bleaching compositions |
| WO2002026267A2 (en) * | 2000-09-25 | 2002-04-04 | The Procter & Gamble Company | Manganes complexes for magnetic resonance imaging |
| WO2002068574A1 (en) * | 2001-02-28 | 2002-09-06 | Unilever N.V. | Liquid cleaning compositions and their use |
| US6518231B2 (en) | 2000-12-18 | 2003-02-11 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Enhancement of air bleaching catalysts |
| US6551977B2 (en) | 2001-03-14 | 2003-04-22 | Unilever Home & Personal Care Usa, A Division Of Conopco, Inc. | Air bleaching catalysts with enhancer and moderating agent |
| US6586383B2 (en) | 2001-03-14 | 2003-07-01 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Air bleaching catalysts with moderating agent |
| EP1369472A1 (en) * | 2002-06-06 | 2003-12-10 | Unilever N.V. | Preserved enhancement of bleaching catalyst |
| WO2003104377A1 (en) * | 2002-06-06 | 2003-12-18 | Unilever N.V. | Enhancement of bleaching catalysts |
| US6720299B2 (en) | 2001-02-16 | 2004-04-13 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Bleaching composition of enhanced stability and a process for making such a composition |
| EP1700904A1 (en) | 2005-03-11 | 2006-09-13 | Unilever N.V. | Liquid detergent composition |
| EP1700907A1 (en) | 2005-03-11 | 2006-09-13 | Unilever N.V. | Liquid bleaching composition |
| US7572761B2 (en) | 2005-11-14 | 2009-08-11 | Evonik Degussa Gmbh | Process for cleaning and softening fabrics |
| EP2135934A1 (en) | 2008-06-16 | 2009-12-23 | Unilever PLC | Use of a laundry detergent composition |
| US7956027B2 (en) | 2006-07-27 | 2011-06-07 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| US8153576B2 (en) | 2006-07-27 | 2012-04-10 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| EP2476743A1 (en) | 2011-04-04 | 2012-07-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Method of laundering fabric |
| WO2012104159A1 (en) | 2011-01-31 | 2012-08-09 | Unilever Plc | Alkaline liquid detergent compositions |
| EP2522715A1 (en) | 2011-05-13 | 2012-11-14 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Aqueous concentrated laundry detergent compositions |
| EP2522714A1 (en) | 2011-05-13 | 2012-11-14 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Aqueous concentrated laundry detergent compositions |
| WO2012156250A1 (en) | 2011-05-13 | 2012-11-22 | Unilever Plc | Aqueous concentrated laundry detergent compositions |
| WO2013092052A1 (en) | 2011-12-20 | 2013-06-27 | Unilever Plc | Isotropic liquid detergents comprising soil release polymer |
| WO2013171210A1 (en) | 2012-05-16 | 2013-11-21 | Unilever Plc | Laundry detergent compositions comprising polyalkoxylated polyethyleneimine |
| US8658590B2 (en) | 2006-07-27 | 2014-02-25 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| WO2014114570A1 (en) | 2013-01-23 | 2014-07-31 | Unilever Plc | An uncoloured laundry additive material for promotion of anti redeposition of particulate soil |
| EP2770044A1 (en) | 2013-02-20 | 2014-08-27 | Unilever PLC | Lamellar gel with amine oxide |
| US8945671B2 (en) | 2007-12-19 | 2015-02-03 | Evonik Treibacher Gmbh | Method for producing encapsulated sodium percarbonate particles |
| WO2016155993A1 (en) | 2015-04-02 | 2016-10-06 | Unilever Plc | Composition |
| WO2017198574A1 (en) | 2016-05-17 | 2017-11-23 | Unilever Plc | Liquid laundry detergent compositions |
| WO2017198438A1 (en) | 2016-05-17 | 2017-11-23 | Unilever Plc | Liquid laundry detergent compositions |
| WO2017202923A1 (en) | 2016-05-27 | 2017-11-30 | Unilever Plc | Laundry composition |
| WO2018127390A1 (en) | 2017-01-06 | 2018-07-12 | Unilever N.V. | Stain removing composition |
| WO2019038186A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
| WO2019038187A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103174008A (en) * | 2011-11-24 | 2013-06-26 | 东华大学 | Application of bipyridyl tetranitrogen metal complex to low-temperature scouring and bleaching auxiliary for textiles |
| TWI819375B (en) * | 2021-09-13 | 2023-10-21 | 南亞塑膠工業股份有限公司 | Method of decolorizing polyester fabric |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5366510A (en) * | 1992-06-09 | 1994-11-22 | Eric Wasinger | Process for desizing and color fading garments |
| EP0743356A1 (en) * | 1995-05-16 | 1996-11-20 | Bayer Corporation | Universal rinse reagent composition for use in hematological analyses of whole blood samples |
| WO1998039098A1 (en) * | 1997-03-07 | 1998-09-11 | The University Of Kansas | Catalysts and methods for catalytic oxidation |
| WO1998039405A1 (en) * | 1997-03-07 | 1998-09-11 | The Procter & Gamble Company | Bleach compositions containing metal bleach catalyst, and bleach activators and/or organic percarboxylic acids |
| WO1999000473A1 (en) * | 1997-06-27 | 1999-01-07 | The Procter & Gamble Company | Non-aqueous detergent compositions containing bleach |
-
2000
- 2000-02-29 CZ CZ20013157A patent/CZ20013157A3/en unknown
- 2000-02-29 ES ES00914782T patent/ES2255992T3/en not_active Expired - Lifetime
- 2000-02-29 BR BR0008715-7A patent/BR0008715A/en not_active IP Right Cessation
- 2000-02-29 CA CA002364538A patent/CA2364538A1/en not_active Abandoned
- 2000-02-29 DE DE60025472T patent/DE60025472T2/en not_active Expired - Fee Related
- 2000-02-29 AU AU36127/00A patent/AU772358B2/en not_active Ceased
- 2000-02-29 KR KR10-2001-7011137A patent/KR100453614B1/en not_active IP Right Cessation
- 2000-02-29 WO PCT/US2000/005291 patent/WO2000052124A1/en active IP Right Grant
- 2000-02-29 JP JP2000602736A patent/JP2002538268A/en not_active Withdrawn
- 2000-02-29 EP EP00914782A patent/EP1157088B1/en not_active Expired - Lifetime
- 2000-02-29 AT AT00914782T patent/ATE315626T1/en not_active IP Right Cessation
- 2000-02-29 CN CN00804473A patent/CN1342194A/en active Pending
- 2000-02-29 MX MXPA01008890A patent/MXPA01008890A/en active IP Right Grant
- 2000-03-01 AR ARP000100905A patent/AR022803A1/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5366510A (en) * | 1992-06-09 | 1994-11-22 | Eric Wasinger | Process for desizing and color fading garments |
| EP0743356A1 (en) * | 1995-05-16 | 1996-11-20 | Bayer Corporation | Universal rinse reagent composition for use in hematological analyses of whole blood samples |
| WO1998039098A1 (en) * | 1997-03-07 | 1998-09-11 | The University Of Kansas | Catalysts and methods for catalytic oxidation |
| WO1998039405A1 (en) * | 1997-03-07 | 1998-09-11 | The Procter & Gamble Company | Bleach compositions containing metal bleach catalyst, and bleach activators and/or organic percarboxylic acids |
| WO1999000473A1 (en) * | 1997-06-27 | 1999-01-07 | The Procter & Gamble Company | Non-aqueous detergent compositions containing bleach |
Cited By (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6642195B2 (en) | 1999-12-24 | 2003-11-04 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Method of treating a textile |
| WO2001048298A1 (en) * | 1999-12-24 | 2001-07-05 | Unilever Plc | Method of treating a textile |
| BE1013476A5 (en) * | 1999-12-24 | 2002-02-05 | Unilever Nv | PROCESS FOR TREATING A TEXTILE. |
| BE1013475A5 (en) * | 1999-12-24 | 2002-02-05 | Unilever Nv | Composition and method of laundering substrate. |
| WO2001048299A1 (en) * | 1999-12-24 | 2001-07-05 | Unilever Plc | Composition and method for bleaching a substrate |
| US6569354B2 (en) | 1999-12-24 | 2003-05-27 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Composition and method for bleaching a substrate |
| WO2001064994A1 (en) * | 2000-03-01 | 2001-09-07 | Unilever Plc | Method for reducing dye fading of fabrics in laundry bleaching compositions |
| WO2002026267A2 (en) * | 2000-09-25 | 2002-04-04 | The Procter & Gamble Company | Manganes complexes for magnetic resonance imaging |
| WO2002026267A3 (en) * | 2000-09-25 | 2003-04-03 | Procter & Gamble | Manganes complexes for magnetic resonance imaging |
| US6518231B2 (en) | 2000-12-18 | 2003-02-11 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Enhancement of air bleaching catalysts |
| US6720299B2 (en) | 2001-02-16 | 2004-04-13 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Bleaching composition of enhanced stability and a process for making such a composition |
| WO2002068574A1 (en) * | 2001-02-28 | 2002-09-06 | Unilever N.V. | Liquid cleaning compositions and their use |
| US6586383B2 (en) | 2001-03-14 | 2003-07-01 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Air bleaching catalysts with moderating agent |
| US6551977B2 (en) | 2001-03-14 | 2003-04-22 | Unilever Home & Personal Care Usa, A Division Of Conopco, Inc. | Air bleaching catalysts with enhancer and moderating agent |
| AU2002304851B2 (en) * | 2001-03-14 | 2005-06-09 | Unilever Plc | Bleaching catalysts with unsaturated surfactant and antioxidant |
| EP1369472A1 (en) * | 2002-06-06 | 2003-12-10 | Unilever N.V. | Preserved enhancement of bleaching catalyst |
| WO2003104377A1 (en) * | 2002-06-06 | 2003-12-18 | Unilever N.V. | Enhancement of bleaching catalysts |
| EP1700904A1 (en) | 2005-03-11 | 2006-09-13 | Unilever N.V. | Liquid detergent composition |
| EP1700907A1 (en) | 2005-03-11 | 2006-09-13 | Unilever N.V. | Liquid bleaching composition |
| US7572761B2 (en) | 2005-11-14 | 2009-08-11 | Evonik Degussa Gmbh | Process for cleaning and softening fabrics |
| US8658590B2 (en) | 2006-07-27 | 2014-02-25 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| US7956027B2 (en) | 2006-07-27 | 2011-06-07 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| US8153576B2 (en) | 2006-07-27 | 2012-04-10 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| US8945671B2 (en) | 2007-12-19 | 2015-02-03 | Evonik Treibacher Gmbh | Method for producing encapsulated sodium percarbonate particles |
| EP2135934A1 (en) | 2008-06-16 | 2009-12-23 | Unilever PLC | Use of a laundry detergent composition |
| WO2012104159A1 (en) | 2011-01-31 | 2012-08-09 | Unilever Plc | Alkaline liquid detergent compositions |
| EP2476743A1 (en) | 2011-04-04 | 2012-07-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Method of laundering fabric |
| WO2012136427A1 (en) | 2011-04-04 | 2012-10-11 | Unilever Plc | Method of laundering fabric |
| EP2522715A1 (en) | 2011-05-13 | 2012-11-14 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Aqueous concentrated laundry detergent compositions |
| EP2522714A1 (en) | 2011-05-13 | 2012-11-14 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Aqueous concentrated laundry detergent compositions |
| WO2012156250A1 (en) | 2011-05-13 | 2012-11-22 | Unilever Plc | Aqueous concentrated laundry detergent compositions |
| WO2013092052A1 (en) | 2011-12-20 | 2013-06-27 | Unilever Plc | Isotropic liquid detergents comprising soil release polymer |
| WO2013171210A1 (en) | 2012-05-16 | 2013-11-21 | Unilever Plc | Laundry detergent compositions comprising polyalkoxylated polyethyleneimine |
| WO2014114570A1 (en) | 2013-01-23 | 2014-07-31 | Unilever Plc | An uncoloured laundry additive material for promotion of anti redeposition of particulate soil |
| EP2770044A1 (en) | 2013-02-20 | 2014-08-27 | Unilever PLC | Lamellar gel with amine oxide |
| WO2016155993A1 (en) | 2015-04-02 | 2016-10-06 | Unilever Plc | Composition |
| WO2017198574A1 (en) | 2016-05-17 | 2017-11-23 | Unilever Plc | Liquid laundry detergent compositions |
| WO2017198438A1 (en) | 2016-05-17 | 2017-11-23 | Unilever Plc | Liquid laundry detergent compositions |
| WO2017202923A1 (en) | 2016-05-27 | 2017-11-30 | Unilever Plc | Laundry composition |
| WO2018127390A1 (en) | 2017-01-06 | 2018-07-12 | Unilever N.V. | Stain removing composition |
| WO2019038186A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
| WO2019038187A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
Also Published As
| Publication number | Publication date |
|---|---|
| AU772358B2 (en) | 2004-04-22 |
| MXPA01008890A (en) | 2002-04-24 |
| ATE315626T1 (en) | 2006-02-15 |
| KR100453614B1 (en) | 2004-10-20 |
| ES2255992T3 (en) | 2006-07-16 |
| AU3612700A (en) | 2000-09-21 |
| CN1342194A (en) | 2002-03-27 |
| DE60025472T2 (en) | 2006-09-28 |
| EP1157088A1 (en) | 2001-11-28 |
| DE60025472D1 (en) | 2006-04-06 |
| BR0008715A (en) | 2002-01-02 |
| CA2364538A1 (en) | 2000-09-08 |
| KR20010103787A (en) | 2001-11-23 |
| AR022803A1 (en) | 2002-09-04 |
| JP2002538268A (en) | 2002-11-12 |
| CZ20013157A3 (en) | 2002-07-17 |
| EP1157088B1 (en) | 2006-01-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1157088A1 (en) | Stabilized bleach compositions | |
| EP1129164A1 (en) | Bleach compositions | |
| EP0549271B1 (en) | Bleach activation using a manganese compound and an organic ligand | |
| AU622362B2 (en) | Bleach activation | |
| CA1231653A (en) | Bleaching and cleaning composition | |
| CA2212115C (en) | Automatic dishwashing compositions comprising cobalt catalysts | |
| US5616546A (en) | Automatic dishwashing compositions comprising multiperacid-forming bleach activators | |
| WO1996023860A1 (en) | Automatic dishwashing compositions comprising cobalt chelated catalysts | |
| WO1996023861A1 (en) | Automatic dishwashing compositions comprising cobalt (iii) catalysts | |
| JPS62292898A (en) | Use to enzymatic per-hydrolytic system and bleaching | |
| US6653270B2 (en) | Stabilized bleach compositions | |
| US5041142A (en) | Peroxymetallates and their use as bleach activating catalysts | |
| US6667288B2 (en) | Bleach compositions | |
| CN100457718C (en) | Crystalline modification of a manganese complex | |
| MXPA97005918A (en) | Detergent compositions that include whitening activators that form multiperac |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 00804473.2 Country of ref document: CN |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AL AM AT AT AU AZ BA BB BG BR BY CA CH CN CR CU CZ CZ DE DE DK DK DM EE EE ES FI FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 36127/00 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: IN/PCT/2001/00743/DE Country of ref document: IN |
|
| ENP | Entry into the national phase |
Ref document number: 2364538 Country of ref document: CA Ref document number: 2364538 Country of ref document: CA Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2000 602736 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 09914528 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PV2001-3157 Country of ref document: CZ Ref document number: 1020017011137 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/2001/008890 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2000914782 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020017011137 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 2000914782 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWP | Wipo information: published in national office |
Ref document number: PV2001-3157 Country of ref document: CZ |
|
| WWG | Wipo information: grant in national office |
Ref document number: 1020017011137 Country of ref document: KR |
|
| WWG | Wipo information: grant in national office |
Ref document number: 36127/00 Country of ref document: AU |
|
| WWG | Wipo information: grant in national office |
Ref document number: 2000914782 Country of ref document: EP |