WO1998051673A1 - Novel imidazole and pyrrole derivatives useful as fungicides - Google Patents
Novel imidazole and pyrrole derivatives useful as fungicides Download PDFInfo
- Publication number
- WO1998051673A1 WO1998051673A1 PCT/GB1998/001208 GB9801208W WO9851673A1 WO 1998051673 A1 WO1998051673 A1 WO 1998051673A1 GB 9801208 W GB9801208 W GB 9801208W WO 9851673 A1 WO9851673 A1 WO 9851673A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- optionally substituted
- compounds
- alkyl
- compound
- phenyl
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/30—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members
- C07D207/32—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
- C07D207/325—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms with substituted hydrocarbon radicals directly attached to the ring nitrogen atom
- C07D207/327—Radicals substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D233/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings
- C07D233/54—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
- C07D233/64—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with substituted hydrocarbon radicals attached to ring carbon atoms, e.g. histidine
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/04—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/10—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a carbon chain containing aromatic rings
Definitions
- This invention relates to new derivatives of imidazole or pyrrole useful as fungicides.
- W is N or CH
- A is a phenyl or a 5 or 6 membered heterocyclyl group, each of which is optionally substituted one or more times by the same or different group 0.1 ;
- Q and ⁇ which may be the same or different, are Y-(X) n -, optionally substituted amino, halogen, cyano, nitro, or two adjacent groups together with the carbon atoms to which they are attached can form an optionally substituted benzo or heterocyclic ring;
- R 1 is C ⁇
- Y and R 2 which may be the same or different, are alkyl, cycloalkyl, cycloalkenyl, alkenyl, alkynyl, phenyl or heterocyclyl, each of which is optionally substituted, hydrogen or acyl;
- X is oxygen or sulfur;
- n is 0 or 1;
- s is 0 to 2, together with complexes with metal salts, as well as salts with bases of compounds which are acids and salts with acids of compounds which are bases.
- Alkyl groups are preferably of 1 to 20, e.g. 1 to 6, carbon atoms.
- Alkenyl and alkynyl groups are generally of 2 to 6 carbon atoms.
- Cycloalkyl or cycloalkenyl groups are preferably of 3 to 8 carbon atoms.
- Substituents when present on any alkyl, cycloalkyl, cycloalkenyl, alkenyl or alkynyl moiety include halogen, azido, cyano, optionally substituted alkoxy, optionally substituted alkylthio, hydroxy, nitro, optionally substituted amino, acyl, acyloxy, optionally substituted phenyl, optionally substituted heterocyclyl, optionally substituted phenoxy and optionally substituted heterocyclyloxy.
- Cycloalkyl or cycloalkenyl groups may also be substituted by alkyl, alkenyl or alkynyl
- Substituents when present on any phenyl or heterocyclyl group are usually one or more of the same groups as defined for Q.
- heterocyclyl includes both aromatic and non-aromatic heterocyclyl groups.
- Heterocyclyl groups are generally 5, 6 or 7-membered rings containing up to 4 hetero atoms selected from nitrogen, oxygen and sulfur.
- Examples of heterocyclyl groups are furyl, thienyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolyl, dioxolanyl, oxazolyl, thiazolyl, imidazolyl, imidazolinyl, imidazolidinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, isoxazolyl, isothiazolyl, oxadiazolyl, triazolyl, thiadiazolyl, pyranyl, pyridyl, piperidinyl, dioxanyl, morphoiino, dithianyl, thiomorpholino, pyridazinyl,
- Amino groups may be substituted for example by one or two optionally substituted alkyl or acyl, or two substituents can form a ring, preferably a 5 to 7-membered ring, which may be substituted and may contain other heteroatoms, for example morpholine, thiomorpholine, or piperidine. This ring can be substituted as for heterocyclyl.
- acyl includes the residue of sulfur and phosphorus-containing acids as well as carboxylic acids.
- Examples of acyl groups are thus -COR 5 , -COOR 5 , -CXNR 5 R 6 , -CON(R 5 )OR 6 , -COONR 5 R 6 , -CON(R 5 )NR 6 R 7 , -COSR 5 , -CSSR 5 , -S(O) p R 5 ,
- R 5 , R 6 and R 7 which may be the same or different, are hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted cycloalkenyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted phenyl or optionally substituted heterocyclyl, or R 5 and R®, or R ⁇ and R 7 , together with the atom(s) to which they are attached can form a ring, q is 1 or 2 and X is O or S.
- Complexes are usually formed from a salt of formula MAn2, in which M is a divalent metal cation, e.g. copper, manganese, cobalt, nickel, iron or zinc and An is one equivalent of an anion, e.g. chloride, nitrate or sulfate.
- M is a divalent metal cation, e.g. copper, manganese, cobalt, nickel, iron or zinc
- An is one equivalent of an anion, e.g. chloride, nitrate or sulfate.
- W is preferably N.
- Rl is preferably methyl or ethyl.
- R 2 is preferably hydrogen or C--
- Z is a heterocyclic group it is preferably carbon linked to the phenyl. Especially preferred is 1 ,2,4-oxadiazol-3-yl, substituted in the 5-position by methyl or ethyl.
- A is preferably phenyl, which is generally substituted by one or two Ql groups.
- Q.1 is preferably chloro or methoxy or two adjacent Q 1 groups form a methylenedioxy ring.
- s is preferably 0.
- W is CH
- s is preferably 1
- Q is generally in the 2-position and is preferably alkyl or haloalkyl, especially methyl.
- the compounds of the invention have particularly advantageous activity against fungal pathogens of plants and usually those pathogens of Ascomycete origin, especially against mildew diseases of plants, particularly cereal powdery mildew (Erysiphe graminis), cucumber powdery mildew (Erysiphe cichoracearum), vine downy mildew ⁇ Uncinula necato ⁇ , and apple powdery mildew ⁇ Podosphaera leuchotricha).
- mildew diseases of plants particularly cereal powdery mildew (Erysiphe graminis), cucumber powdery mildew (Erysiphe cichoracearum), vine downy mildew ⁇ Uncinula necato ⁇ , and apple powdery mildew ⁇ Podosphaera leuchotricha).
- mildew diseases of plants particularly cereal powdery mildew (Erysiphe graminis), cucumber powdery mildew (Erysiphe cichoracearum), vine downy mildew
- rice blast (Pyricularia oryzae), cereal eyespot (Pseudocercosporella herpotrichoides), rice sheath blight (Pellicularia sasakii), grey mould (Botrytis cinerea), wheat brown rust (Puccinia recondita), late tomato or potato blight (Phytophthora infestans), apple scab (Venturia inaequalis) and glume blotch (Leptosphaeria nodorum).
- Some compounds may be active against only a few pathogens whereas others may have a broader spectrum of activity.
- the compounds of the invention are generally formulated in conventional compositions used for fungicides. These compositions can contain one or more additional pesticides, for example compounds known to possess herbicidal, fungicidal, insecticidal, acaricidal or nematicidal properties. In addition the compounds may be merely mixed with the additional pesticide.
- the diluent or carrier in the composition of the invention can be a solid or a liquid optionally in association with a surface-active agent, for example a dispersing agent, emulsifying agent or wetting agent.
- Suitable surface-active agents include anionic compounds such as a carboxylate, for example a metal carboxylate of a long chain fatty acid; an N-acylsarcosinate; mono- or di-esters of phosphoric acid with fatty alcohol ethoxylates or salts of such esters; fatty alcohol sulfates such as sodium dodecyl sulfate, sodium octadecyl sulfate or sodium cetyl sulfate; ethoxylated fatty alcohol sulfates; ethoxylated alkylphenol sulfates; lignin sulfonates; petroleum sulfonates; alkyl-aryl sulfonates such as alkyl-benzene s
- butyl-naphthalene sulfonate salts of sulfonated naphthalene-formaldehyde condensates; salts of sulfonated phenol-formaldehyde condensates; or more complex sulfonates such as the amide sulfonates, e.g. the sulfonated condensation product of oleic acid and N-methyl taurine or the dialkyl sulfosuccinates, e.g. the sodium sulfonate of dioctyl succinate.
- amide sulfonates e.g. the sulfonated condensation product of oleic acid and N-methyl taurine or the dialkyl sulfosuccinates, e.g. the sodium sulfonate of dioctyl succinate.
- Nonionic agents include condensation products of fatty acid esters, fatty alcohols, fatty acid amides or fatty-alkyl- or alkenyl-substituted phenols with ethylene oxide, fatty esters of polyhydric alcohol ethers, e.g. sorbitan fatty acid esters, condensation products of such esters with ethylene oxide, e.g. polyoxyethylene sorbitan fatty acid esters, block copolymers of ethylene oxide and propylene oxide, acetylenic glycols such as 2,4,7,9-tetramethyl-5-decyne-4,7-diol, or ethoxylated acetylenic glycols.
- a cationic surface-active agent examples include, for instance, an aliphatic mono-, di-, or polyamine as an acetate, naphthenate or oleate; an oxygen-containing amine such as an amine oxide or polyoxyethylene alkylamine; an amide-linked amine prepared by the condensation of a carboxylic acid with a di- or polyamine; or a quaternary ammonium salt.
- compositions of the invention can take any form known in the art for the formulation of agrochemicals, for example, a solution, a dispersion, an aqueous emulsion, a dusting powder, a seed dressing, a fumigant, a smoke, a dispersible powder, an emulsifiable concentrate or granules. Moreover it can be in a suitable form for direct application or as a concentrate or primary composition which requires dilution with a suitable quantity of water or other diluent before application.
- the composition comprises a compound of the invention dispersed in a liquid medium, preferably water. It is often convenient to supply the consumer with a primary composition which can be diluted with water to form a dispersion having the desired concentration.
- the primary composition can be provided in any one of the following forms. It can be a dispersible solution which comprises a compound of the invention dissolved in a water-miscible solvent with the addition of a dispersing agent.
- a further alternative comprises a compound of the invention in the form of a finely ground powder in association with a dispersing agent and intimately mixed with water to give a paste or cream which can if desired be added to an emulsion of oil in water to give a dispersion of active ingredient in an aqueous oil emulsion.
- An emulsifiable concentrate comprises a compound of the invention dissolved in a water-immiscible solvent together with an emulsifying agent and which is formed into an emulsion on mixing with water.
- a dusting powder comprises a compound of the invention intimately mixed with a solid pulverulent diluent, for example, kaolin.
- a granular solid comprises a compound of the invention associated with similar diluents to those which may be employed in dusting powders, but the mixture is granulated by known methods. Alternatively it comprises the active ingredient adsorbed or absorbed on a pre-granular diluent, for example, Fuller's earth, attapulgite or limestone grit.
- a wettable powder usually comprises the active ingredient in admixture with a suitable surfactant and an inert powder diluent such as china clay.
- a suitable concentrate particularly when the product is a solid, is a flowable suspension concentrate which is formed by grinding the compound with water, a wetting agent and a suspending agent.
- the concentration of the active ingredient in the composition of the present invention is preferably within the range of 1 to 30 per cent by weight, especially 5 to 30 per cent by weight.
- the amount of active ingredient can vary widely and can be, for example, from 5 to 95 per cent by weight of the composition.
- the concentration of the active ingredient in the composition of the present invention, as applied to plants is preferably within the range of 0.0001 to 1.0 per cent by weight, especially 0.0001 to 0.01 per cent by weight.
- the amount of active ingredient can vary widely and can be, for example, from 5 to 95 per cent by weight of the composition.
- the compound is generally applied to seeds, plants or their habitat.
- the compound can be applied directly to the soil before, at or after drilling so that the presence of active compound in the soil can control the growth of fungi which may attack seeds.
- the active compound can be applied in any manner which allows it to be intimately mixed with the soil such as by spraying, by broadcasting a solid form of granules, or by applying the active ingredient at the same time as drilling by inserting it in the same drill as the seeds.
- a suitable application rate is within the range of from 5 to 1000 g per hectare, more preferably from 10 to 500 g per hectare.
- the active compound can be applied directly to the plant by, for example, spraying or dusting either at the time when the fungus has begun to appear on the plant or before the appearance of fungus as a protective measure.
- spraying or dusting either at the time when the fungus has begun to appear on the plant or before the appearance of fungus as a protective measure.
- the preferred mode of application is by foliar spraying. It is generally important to obtain good control of fungi in the early stages of plant growth as this is the time when the plant can be most severely damaged.
- the spray or dust can conveniently contain a pre- or post-emergence herbicide if this is thought necessary.
- it is practicable to treat the roots of a plant before or during planting for example, by dipping the roots in a suitable liquid or solid composition.
- a suitable rate of application is from 0.025 to 5 kg per hectare, preferably from 0.05 to 1 kg per hectare.
- the compounds of the invention can be applied to plants, or parts thereof, which have been genetically modified to exhibit a trait such as fungal and/or herbicidal resistance.
- the starting compounds are either known or can be prepared in known manner.
- Example 2 2-[2-(4-Methoxyphenyl)imidazol-1-yl]benzonitrile, from Example 1 (4.6 g), hydroxylamine hydrochloride (2.3 g) and potassium carbonate (2.3 g) were heated under reflux in aqueous ethanol overnight. The mixture was cooled and the solvent removed under reduced pressure. The residue was washed with water and the solid collected by filtration. The solid was washed with dichloromethane to give 2-[2-(4- methoxyphenyl)imidazol-1-yl]benzamide oxime, m.p. 187-190°C. (Compound 2)
- Example 3 The product of Example 2 (1.2 g) and acetic anhydride (0.8 ml) were heated under reflux for 2 hours in acetic acid (50 ml). The mixture was cooled and the solvent removed under reduced pressure. The residue was treated with water and extracted with dichloromethane. The organic layer was dried and evaporated. The residue was purified by silica gel column chromatography to give 5- ⁇ 2-[2-(4-methoxyphenyl)- imidazol-1-yl]phenyl ⁇ -3-methyl-1 ,2,4-oxadiazole, as an orange semi-solid. (Compound 3)
- N,N-dimethyl-1 ,1-dimethoxyethylamine (5.7 g) was heated under reflux for 1 1 /2 hours. The mixture was evaporated to dryness to give crude Nl-[1-(dimethylamino)- ethylidene]-2-[2-(4-methoxyphenyl)1 H-1-imidazolyl]benzamide.
- a solution of this compound (2.14 g) in acetic acid (10 ml) was treated with hydroxylamine hydrochloride (0.6 g), dioxane (10 ml) and aqueous sodium hydroxide (4.4.ml of 2M) and the mixture heated at 90°C for 2 hours.
- Example 7 A solution of the product of Example 6 (0.71 g) in dioxane (25 ml) was heated under reflux for 3 days. The solvent was evaporated under reduced pressure and the residue purified by silica gel column chromatography to give 3- ⁇ 2-[2-(1 ,3-benzodioxol- 5-yl)-1/-/-1-imidazolyl]phenyl ⁇ -5-methyl-1,2,4-oxadiazole, as a yellow gum. (Compound 7) The following compounds of the invention were prepared in an analogous manner to one of the previous examples
- Compounds are assessed for activity against one or more of the following:
- Erysiphe graminis f. sp. tritici wheat powdery mildew Pyricularia oryzae: rice blast
- Leptosphaeria nodorum glume blotch
- Plasmopara viticola downy mildew of vines Aqueous solutions or dispersions of the compounds at the desired concentration, including a wetting agent, were applied by spray or by drenching the stem base of the test plants, as appropriate. After a given time, plants or plant parts were inoculated with appropriate test pathogens and kept under controlled environmental conditions suitable for maintaining plant growth and development of the disease. After an appropriate time, the degree of infection of the affected part of the plant was visually estimated. Compounds are assessed on a score of 1 to 3 where 1 is little or no control, 2 is moderate control and 3 is good to total control. At a concentration of 500 ppm (w/v) or less, the following compounds scored 2 or more against the fungi specified.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Compounds of formula (I) where: W is N or CH; A is a phenyl or a 5 or 6 membered heterocyclyl group, each of which is optionally substituted one or more times by the same or different group Q1; Z is COOR1, -C(NH¿2)=NOR?2, or an oxadiazolyl or thiazolyl group, substituted by alkyl, haloalkyl or cycloalkyl; Q and Q1, which may be the same or different, are Y-(X)¿n?-, optionally substituted amino, halogen, cyano, nitro, or two adjacent groups together with the carbon atoms to which they are attached can form an optionally substituted benzo or heterocyclic ring; R?1¿ is C-¿1-6?-alkyl; Y and R?2¿, which may be the same or different, are alkyl, cycloalkyl, cycloalkenyl, alkenyl, alkynyl, phenyl or heterocyclyl, each of which is optionally substituted, hydrogen or acyl; X is oxygen or sulfur; n is 0 or 1; and s is 0 to 2, together with complexes with metal salts, as well as salts with bases of compounds which are acids and salts with acids of compounds which are bases, are useful as fungicides especially phytopathogenic fungicides.
Description
NOVEL IMIDAZOLE AND PYRROLE DERIVATIVES USEFUL AS FUNGICIDES
This invention relates to new derivatives of imidazole or pyrrole useful as fungicides.
In one aspect the invention provides compounds of formula I
W is N or CH;
A is a phenyl or a 5 or 6 membered heterocyclyl group, each of which is optionally substituted one or more times by the same or different group 0.1 ; Z is COOR"1 , -C(NH2)=NOR2, or an oxadiazolyl or thiazolyl group, substituted by alkyl, haloalkyl or cycloalkyl, e.g. cyclopropyl;
Q and θΛ which may be the same or different, are Y-(X)n-, optionally substituted amino, halogen, cyano, nitro, or two adjacent groups together with the carbon atoms to which they are attached can form an optionally substituted benzo or heterocyclic ring;
R1 is C~|_6-alkyl;
Y and R2, which may be the same or different, are alkyl, cycloalkyl, cycloalkenyl, alkenyl, alkynyl, phenyl or heterocyclyl, each of which is optionally substituted, hydrogen or acyl; X is oxygen or sulfur; n is 0 or 1; s is 0 to 2, together with complexes with metal salts, as well as salts with bases of compounds which are acids and salts with acids of compounds which are bases.
Alkyl groups are preferably of 1 to 20, e.g. 1 to 6, carbon atoms. Alkenyl and alkynyl groups are generally of 2 to 6 carbon atoms. Cycloalkyl or cycloalkenyl groups are preferably of 3 to 8 carbon atoms.
Substituents, when present on any alkyl, cycloalkyl, cycloalkenyl, alkenyl or alkynyl moiety include halogen, azido, cyano, optionally substituted alkoxy, optionally substituted alkylthio, hydroxy, nitro, optionally substituted amino, acyl, acyloxy, optionally substituted phenyl, optionally substituted heterocyclyl, optionally substituted phenoxy and optionally substituted heterocyclyloxy.
Cycloalkyl or cycloalkenyl groups may also be substituted by alkyl, alkenyl or alkynyl
Substituents when present on any phenyl or heterocyclyl group are usually one or more of the same groups as defined for Q.
The term heterocyclyl includes both aromatic and non-aromatic heterocyclyl groups. Heterocyclyl groups are generally 5, 6 or 7-membered rings containing up to 4 hetero atoms selected from nitrogen, oxygen and sulfur. Examples of heterocyclyl groups are furyl, thienyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolyl, dioxolanyl, oxazolyl, thiazolyl, imidazolyl, imidazolinyl, imidazolidinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, isoxazolyl, isothiazolyl, oxadiazolyl, triazolyl, thiadiazolyl, pyranyl, pyridyl, piperidinyl, dioxanyl, morphoiino, dithianyl, thiomorpholino, pyridazinyl, pyrimidinyl, pyrazinyl, piperazinyl, triazinyl, thiazolinyl, benzimidazolyl, tetrazolyl, benzoxazolyl, imidazopyridinyl, 1,3-benzoxazinyl, 1 ,3-benzothiazinyl, oxazolopyridinyl, benzofuranyl, quinolinyl, quinazolinyl, quinoxalinyl, sulfolanyl, dihydroquinazolinyl, benzothiazolyl, phthalimido, benzofuranyl, azepinyl, oxazepinyl, thiazepinyl, diazepinyl and benzodiazepinyl.
Amino groups may be substituted for example by one or two optionally substituted alkyl or acyl, or two substituents can form a ring, preferably a 5 to 7-membered ring, which may be substituted and may contain other heteroatoms, for example morpholine, thiomorpholine, or piperidine. This ring can be substituted as for heterocyclyl.
The term acyl includes the residue of sulfur and phosphorus-containing acids as well as carboxylic acids. Examples of acyl groups are thus -COR5, -COOR5, -CXNR5R6, -CON(R5)OR6, -COONR5R6, -CON(R5)NR6R7, -COSR5, -CSSR5, -S(O)pR5,
-S(O)2OR5, -S(O)pNR5R6, -P(=X)(OR5)(OR6), -CO-COOR5, where where R5, R6 and R7, which may be the same or different, are hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted cycloalkenyl, optionally
substituted alkenyl, optionally substituted alkynyl, optionally substituted phenyl or optionally substituted heterocyclyl, or R5 and R®, or R^ and R 7, together with the atom(s) to which they are attached can form a ring, q is 1 or 2 and X is O or S.
Complexes are usually formed from a salt of formula MAn2, in which M is a divalent metal cation, e.g. copper, manganese, cobalt, nickel, iron or zinc and An is one equivalent of an anion, e.g. chloride, nitrate or sulfate.
W is preferably N.
Rl is preferably methyl or ethyl.
R2 is preferably hydrogen or C--|_4-alkanoyl
When Z is a heterocyclic group it is preferably carbon linked to the phenyl. Especially preferred is 1 ,2,4-oxadiazol-3-yl, substituted in the 5-position by methyl or ethyl.
A is preferably phenyl, which is generally substituted by one or two Ql groups. Q.1 is preferably chloro or methoxy or two adjacent Q1 groups form a methylenedioxy ring.
When W is N, s is preferably 0.
When W is CH, s is preferably 1 , in which case Q is generally in the 2-position and is preferably alkyl or haloalkyl, especially methyl.
The compounds of the invention have particularly advantageous activity against fungal pathogens of plants and usually those pathogens of Ascomycete origin, especially against mildew diseases of plants, particularly cereal powdery mildew (Erysiphe graminis), cucumber powdery mildew (Erysiphe cichoracearum), vine downy mildew {Uncinula necatoή, and apple powdery mildew {Podosphaera leuchotricha). However some compounds may be active against other pathogens of Deuteromycete, Ascomycete, Phycomycete and Basidiomycete origin, e.g. rice blast (Pyricularia oryzae), cereal eyespot (Pseudocercosporella herpotrichoides), rice sheath blight (Pellicularia sasakii), grey mould (Botrytis cinerea), wheat brown rust (Puccinia recondita), late tomato or potato blight (Phytophthora infestans), apple scab (Venturia inaequalis) and
glume blotch (Leptosphaeria nodorum). Some compounds may be active against only a few pathogens whereas others may have a broader spectrum of activity.
The compounds of the invention are generally formulated in conventional compositions used for fungicides. These compositions can contain one or more additional pesticides, for example compounds known to possess herbicidal, fungicidal, insecticidal, acaricidal or nematicidal properties. In addition the compounds may be merely mixed with the additional pesticide.
The diluent or carrier in the composition of the invention can be a solid or a liquid optionally in association with a surface-active agent, for example a dispersing agent, emulsifying agent or wetting agent. Suitable surface-active agents include anionic compounds such as a carboxylate, for example a metal carboxylate of a long chain fatty acid; an N-acylsarcosinate; mono- or di-esters of phosphoric acid with fatty alcohol ethoxylates or salts of such esters; fatty alcohol sulfates such as sodium dodecyl sulfate, sodium octadecyl sulfate or sodium cetyl sulfate; ethoxylated fatty alcohol sulfates; ethoxylated alkylphenol sulfates; lignin sulfonates; petroleum sulfonates; alkyl-aryl sulfonates such as alkyl-benzene sulfonates or lower alkylnaphthalene sulfonates, e.g. butyl-naphthalene sulfonate; salts of sulfonated naphthalene-formaldehyde condensates; salts of sulfonated phenol-formaldehyde condensates; or more complex sulfonates such as the amide sulfonates, e.g. the sulfonated condensation product of oleic acid and N-methyl taurine or the dialkyl sulfosuccinates, e.g. the sodium sulfonate of dioctyl succinate. Nonionic agents include condensation products of fatty acid esters, fatty alcohols, fatty acid amides or fatty-alkyl- or alkenyl-substituted phenols with ethylene oxide, fatty esters of polyhydric alcohol ethers, e.g. sorbitan fatty acid esters, condensation products of such esters with ethylene oxide, e.g. polyoxyethylene sorbitan fatty acid esters, block copolymers of ethylene oxide and propylene oxide, acetylenic glycols such as 2,4,7,9-tetramethyl-5-decyne-4,7-diol, or ethoxylated acetylenic glycols. Examples of a cationic surface-active agent include, for instance, an aliphatic mono-, di-, or polyamine as an acetate, naphthenate or oleate; an oxygen-containing amine such as an amine oxide or polyoxyethylene alkylamine; an amide-linked amine prepared by the condensation of a carboxylic acid with a di- or polyamine; or a quaternary ammonium salt.
The compositions of the invention can take any form known in the art for the formulation of agrochemicals, for example, a solution, a dispersion, an aqueous emulsion, a dusting powder, a seed dressing, a fumigant, a smoke, a dispersible powder, an emulsifiable concentrate or granules. Moreover it can be in a suitable form for direct application or as a concentrate or primary composition which requires dilution with a suitable quantity of water or other diluent before application.
As a dispersion, the composition comprises a compound of the invention dispersed in a liquid medium, preferably water. It is often convenient to supply the consumer with a primary composition which can be diluted with water to form a dispersion having the desired concentration. The primary composition can be provided in any one of the following forms. It can be a dispersible solution which comprises a compound of the invention dissolved in a water-miscible solvent with the addition of a dispersing agent. A further alternative comprises a compound of the invention in the form of a finely ground powder in association with a dispersing agent and intimately mixed with water to give a paste or cream which can if desired be added to an emulsion of oil in water to give a dispersion of active ingredient in an aqueous oil emulsion.
An emulsifiable concentrate comprises a compound of the invention dissolved in a water-immiscible solvent together with an emulsifying agent and which is formed into an emulsion on mixing with water.
A dusting powder comprises a compound of the invention intimately mixed with a solid pulverulent diluent, for example, kaolin.
A granular solid comprises a compound of the invention associated with similar diluents to those which may be employed in dusting powders, but the mixture is granulated by known methods. Alternatively it comprises the active ingredient adsorbed or absorbed on a pre-granular diluent, for example, Fuller's earth, attapulgite or limestone grit.
A wettable powder usually comprises the active ingredient in admixture with a suitable surfactant and an inert powder diluent such as china clay.
Another suitable concentrate, particularly when the product is a solid, is a flowable suspension concentrate which is formed by grinding the compound with water, a wetting agent and a suspending agent.
The concentration of the active ingredient in the composition of the present invention is preferably within the range of 1 to 30 per cent by weight, especially 5 to 30 per cent by weight. In a primary composition the amount of active ingredient can vary widely and can be, for example, from 5 to 95 per cent by weight of the composition.
The concentration of the active ingredient in the composition of the present invention, as applied to plants is preferably within the range of 0.0001 to 1.0 per cent by weight, especially 0.0001 to 0.01 per cent by weight. In a primary composition, the amount of active ingredient can vary widely and can be, for example, from 5 to 95 per cent by weight of the composition.
In the method of the invention the compound is generally applied to seeds, plants or their habitat. Thus, the compound can be applied directly to the soil before, at or after drilling so that the presence of active compound in the soil can control the growth of fungi which may attack seeds. When the soil is treated directly the active compound can be applied in any manner which allows it to be intimately mixed with the soil such as by spraying, by broadcasting a solid form of granules, or by applying the active ingredient at the same time as drilling by inserting it in the same drill as the seeds. A suitable application rate is within the range of from 5 to 1000 g per hectare, more preferably from 10 to 500 g per hectare.
Alternatively the active compound can be applied directly to the plant by, for example, spraying or dusting either at the time when the fungus has begun to appear on the plant or before the appearance of fungus as a protective measure. In both such cases the preferred mode of application is by foliar spraying. It is generally important to obtain good control of fungi in the early stages of plant growth as this is the time when the plant can be most severely damaged. The spray or dust can conveniently contain a pre- or post-emergence herbicide if this is thought necessary. Sometimes, it is practicable to treat the roots of a plant before or during planting, for example, by dipping the roots in a suitable liquid or solid composition. When the active compound is applied directly to the plant a suitable rate of application is from 0.025 to 5 kg per hectare, preferably from 0.05 to 1 kg per hectare.
In addition, the compounds of the invention can be applied to plants, or parts thereof, which have been genetically modified to exhibit a trait such as fungal and/or herbicidal resistance.
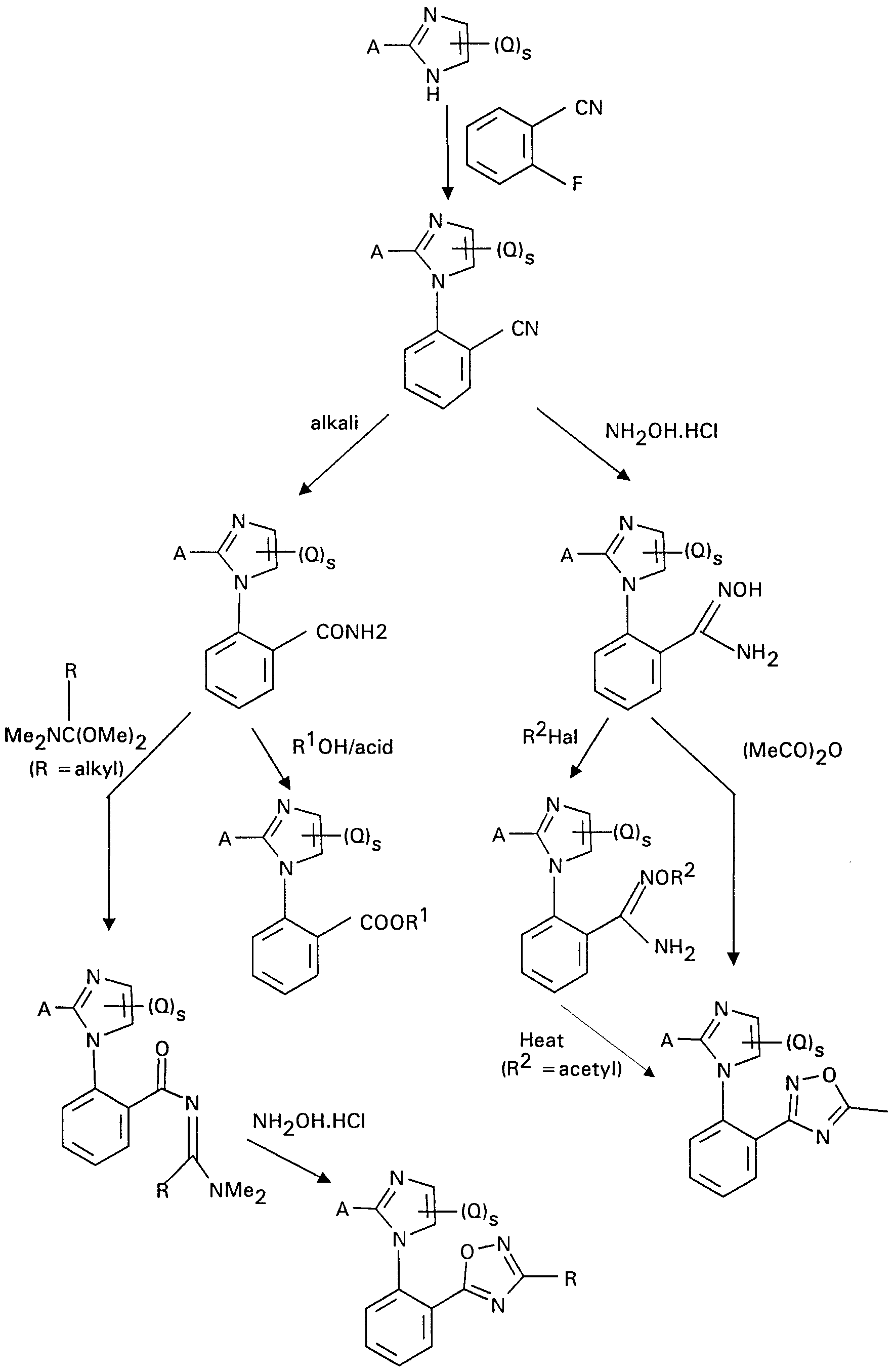
Typical methods of preparing compounds of the invention are shown in the following reaction schemes.
a)W=N
The starting compounds are either known or can be prepared in known manner.
The invention is illustrated in the following Examples. Structures of isolated novel compounds were confirmed by nmr and/or other appropriate analyses.
Example 1
4-Methoxybenzonitrile (20 g) was dissolved in dry methanol and cooled in an ice bath. Hydrogen chloride gas was bubbled through the solution for 14 hour. The resulting solution was allowed to stand in a refrigerator overnight. The deposited solid was collected by filtration and washed several times with diethyl ether to give methyl 4-methoxybenzimidate hydrochloride.
This compound (28.97 g) and 2,2-dimethoxyethylamine (16.6 ml) were stirred together in dry tetrahydrofuran at room temperature for 4 hour. The reaction mixture was then heated under reflux for 2 hours, treated with 2M hydrochloric acid (87 ml) and then heated under reflux overnight. The mixture was cooled, the solvent removed under reduced pressure and the residue treated with aqueous sodium hydrogen carbonate. The solid was collected by filtration, washed several times with water and air dried to give 2-(4-methoxyphenyl)imidazole, m.p. 169-177°C
This compound (7 g), 2-fluorobenzonitrile (4.5 ml) and potassium carbonate (14.4 g) were stirred together in dimethyl sulfoxide (130 ml). The reaction mixture was heated to 100°C and stirred at this temperature overnight. The mixture was cooled, poured into ice and water and the solid collected by filtration. The solid was washed several times with water and light petroleum (b.p. 40-60 °C). The solid was dissolved in dichloromethane, washed with water, dried and evaporated to give 2-[2-(4-methoxyphenyl)imidazol-1-yl]benzonitrile, m.p. 155-157°C.
This compound (7.4 g) and potassium hydroxide (4.53 g) were heated under reflux in water until all the solid had dissolved. The mixture was filtered hot and the filtrate
acidified with acetic acid. The aqueous solution was evaporated and the residue treated with methanol. The methanol solution was evaporated to give 2-[2-(4-methoxyphenyl)imidazol-1-yl]benzamide, m.p. 197-199°C
This compound (1 g) was dissolved in dry methanol and heated to reflux. Hydrogen chloride gas was bubbled through the reaction mixture for 3 hours, whilst maintaining the reflux. Gas addition was stopped and the reaction was heated under reflux for a further 24 hours. The mixture was cooled, evaporated and the residue partitioned between dichloromethane and aqueous sodium hydrogen carbonate. The organic layer was dried and evaporated under reduced pressure. The product was purified by silica gel column chromatography to give methyl 2-[2-(4-methoxyphenyl)imidazol- 1-yl]benzoate, m.p. 109-111 °C. (Compound 1)
Example 2 2-[2-(4-Methoxyphenyl)imidazol-1-yl]benzonitrile, from Example 1 (4.6 g), hydroxylamine hydrochloride (2.3 g) and potassium carbonate (2.3 g) were heated under reflux in aqueous ethanol overnight. The mixture was cooled and the solvent removed under reduced pressure. The residue was washed with water and the solid collected by filtration. The solid was washed with dichloromethane to give 2-[2-(4- methoxyphenyl)imidazol-1-yl]benzamide oxime, m.p. 187-190°C. (Compound 2)
Example 3
The product of Example 2 (1.2 g) and acetic anhydride (0.8 ml) were heated under reflux for 2 hours in acetic acid (50 ml). The mixture was cooled and the solvent removed under reduced pressure. The residue was treated with water and extracted with dichloromethane. The organic layer was dried and evaporated. The residue was purified by silica gel column chromatography to give 5-{2-[2-(4-methoxyphenyl)- imidazol-1-yl]phenyl}-3-methyl-1 ,2,4-oxadiazole, as an orange semi-solid. (Compound 3)
Example 4
2-[2-(4-methoxyphenyl)imidazol-1-yl]benzamide (3.47 g), from Example 1, and
N,N-dimethyl-1 ,1-dimethoxyethylamine (5.7 g) was heated under reflux for 11/2 hours. The mixture was evaporated to dryness to give crude Nl-[1-(dimethylamino)- ethylidene]-2-[2-(4-methoxyphenyl)1 H-1-imidazolyl]benzamide.
A solution of this compound (2.14 g) in acetic acid (10 ml) was treated with hydroxylamine hydrochloride (0.6 g), dioxane (10 ml) and aqueous sodium hydroxide (4.4.ml of 2M) and the mixture heated at 90°C for 2 hours. The mixture was cooled, poured into water and extracted with ethyl acetate. The organic layer was dried and evaporated. The residue was purified by silica gel column chromatography to give 5-{2-[2-(4-methoxyphenyl)-1 HA -imidazolyl]phenyl}-3-methyl-1 ,2,4-oxadiazole, m.p. 1 51 -3 °C (Compound 4)
Example 5 p-Toluenesulfonic acid (0.2 g) and a solution of methyl anthranilate (6 g) and
1-phenyl-1 ,4-pentanedione (7.04 g) in toluene (25 ml) was heated under reflux under Dean and Stark conditions for 13 hours. The mixture was allowed to cool and solvent removed under reduced pressure. The residue was dissolved in ether, washed with water and then hydrochloric acid and again water and dried. The solvent was removed under reduced pressure and the residue purified by silica column chromatography to give methyl 2-(2-methyl-5-phenyl-1/-/-1-pyrrolyl)benzoate, as a dark orange oil. (Compound 5)
Example 6
Compound 9 (see in table) (0.86 g) was dissolved in dry tetrahydrofuran (20 ml) and acetyl chloride (0.19 ml) was added. The mixture was heated for 10 minutes, cooled and the solvent evaporated under reduced pressure. The residue was dissolved in water and added to saturated aqueous sodium hydrogen carbonate and extracted with dichloromethane. The extract was dried and evaporated under reduced pressure to give 2-[2-(1 ,3-benzodioxol-5-yl)-1/-/-1-imidazolyl]-1-benzamide O-acetyloxime, as an orange gum. (Compound 6)
Example 7 A solution of the product of Example 6 (0.71 g) in dioxane (25 ml) was heated under reflux for 3 days. The solvent was evaporated under reduced pressure and the residue purified by silica gel column chromatography to give 3-{2-[2-(1 ,3-benzodioxol- 5-yl)-1/-/-1-imidazolyl]phenyl}-5-methyl-1,2,4-oxadiazole, as a yellow gum. (Compound 7)
The following compounds of the invention were prepared in an analogous manner to one of the previous examples
Compounds are assessed for activity against one or more of the following:
Erysiphe graminis f. sp. hordei: barley powdery mildew
Erysiphe graminis f. sp. tritici: wheat powdery mildew Pyricularia oryzae: rice blast
Leptosphaeria nodorum: glume blotch
Plasmopara viticola: downy mildew of vines Aqueous solutions or dispersions of the compounds at the desired concentration, including a wetting agent, were applied by spray or by drenching the stem base of the test plants, as appropriate. After a given time, plants or plant parts were inoculated with appropriate test pathogens and kept under controlled environmental conditions suitable for maintaining plant growth and development of the disease. After an appropriate time, the degree of infection of the affected part of the plant was visually estimated. Compounds are assessed on a score of 1 to 3 where 1 is little or no control, 2 is moderate control and 3 is good to total control. At a concentration of 500 ppm (w/v) or less, the following compounds scored 2 or more against the fungi specified.
Erysiphe graminis f sp. tritici 2-4, 6-1 5, 1 7
Erysiphe graminis f sp. hordeii
1
Pyricularia oryzae
14 Leptosphaeria nodorum
9, 14
Plasmopara viticola
5
Claims
1. Compounds of formula I
W is N or CH;
A is a phenyl or a 5 or 6 membered heterocyclyl group, each of which is optionally substituted one or more times by the same or different group Q1 ;
Z is COOR"1 , -C(NH2)=NOR2, or an oxadiazolyl or thiazolyl group, substituted by alkyl, haloalkyl or cycloalkyl;
Q and ╬╕ which may be the same or different, are Y-(X)n-, optionally substituted amino, halogen, cyano, nitro, or two adjacent groups together with the carbon atoms to which they are attached can form an optionally substituted benzo or heterocyclic ring; R1 is C~|_6-alkyl;
Y and R2, which may be the same or different, are alkyl, cycloalkyl, cycloalkenyl, alkenyl, alkynyl, phenyl or heterocyclyl, each of which is optionally substituted, hydrogen or acyl;
X is oxygen or sulfur; n is O or l; s is 0 to 2, together with complexes with metal salts, as well as salts with bases of compounds which are acids and salts with acids of compounds which are bases.
2. Fungicidal compositions which comprise a compound as defined in claim 1 in admixture with an agriculturally acceptable diluent or carrier.
3. A method of combating phytopathogenic fungi at a locus infested or liable to be infested therewith, which comprises applying to the locus a compound as defined in claim 1.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU72187/98A AU7218798A (en) | 1997-05-09 | 1998-05-08 | Novel imidazole and pyrrole derivatives useful as fungicides |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB9709317.3 | 1997-05-09 | ||
| GBGB9709317.3A GB9709317D0 (en) | 1997-05-09 | 1997-05-09 | Fungicides |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1998051673A1 true WO1998051673A1 (en) | 1998-11-19 |
Family
ID=10811976
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB1998/001208 WO1998051673A1 (en) | 1997-05-09 | 1998-05-08 | Novel imidazole and pyrrole derivatives useful as fungicides |
Country Status (3)
| Country | Link |
|---|---|
| AU (1) | AU7218798A (en) |
| GB (1) | GB9709317D0 (en) |
| WO (1) | WO1998051673A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009137651A2 (en) * | 2008-05-08 | 2009-11-12 | E. I. Du Pont De Nemours And Company | Fungicidal substituted azoles |
| JP2013509408A (en) * | 2009-10-29 | 2013-03-14 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Heterobicyclic substituted azolylbenzene fungicides and fungicides |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1563664A (en) * | 1977-01-06 | 1980-03-26 | Sumitomo Chemical Co | N-benzoylanthranilates processes for producing them and compositions containing them |
| US4339448A (en) * | 1978-11-02 | 1982-07-13 | Basf Aktiengesellschaft | Imidazole-copper complex compounds and fungicides containing them |
| WO1997010228A1 (en) * | 1995-09-16 | 1997-03-20 | Agrevo Uk Limited | Substituted benzoic or thiobenzoic acid anilides as fungicides |
-
1997
- 1997-05-09 GB GBGB9709317.3A patent/GB9709317D0/en active Pending
-
1998
- 1998-05-08 WO PCT/GB1998/001208 patent/WO1998051673A1/en active Application Filing
- 1998-05-08 AU AU72187/98A patent/AU7218798A/en not_active Abandoned
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1563664A (en) * | 1977-01-06 | 1980-03-26 | Sumitomo Chemical Co | N-benzoylanthranilates processes for producing them and compositions containing them |
| US4339448A (en) * | 1978-11-02 | 1982-07-13 | Basf Aktiengesellschaft | Imidazole-copper complex compounds and fungicides containing them |
| WO1997010228A1 (en) * | 1995-09-16 | 1997-03-20 | Agrevo Uk Limited | Substituted benzoic or thiobenzoic acid anilides as fungicides |
Non-Patent Citations (1)
| Title |
|---|
| STETTER H ET AL: "Preparation of bi- and tricyclic pyrrole systems", LIEBIGS ANN. CHEM., no. 5, 1980, pages 703 - 714, XP002073707 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009137651A2 (en) * | 2008-05-08 | 2009-11-12 | E. I. Du Pont De Nemours And Company | Fungicidal substituted azoles |
| WO2009137538A2 (en) * | 2008-05-08 | 2009-11-12 | E. I. Du Pont De Nemours And Company | Fungicidal substituted azoles |
| WO2009137538A3 (en) * | 2008-05-08 | 2012-04-19 | E. I. Du Pont De Nemours And Company | Fungicidal substituted azoles |
| WO2009137651A3 (en) * | 2008-05-08 | 2012-04-26 | E. I. Du Pont De Nemours And Company | Fungicidal substituted azoles |
| JP2013509408A (en) * | 2009-10-29 | 2013-03-14 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Heterobicyclic substituted azolylbenzene fungicides and fungicides |
Also Published As
| Publication number | Publication date |
|---|---|
| AU7218798A (en) | 1998-12-08 |
| GB9709317D0 (en) | 1997-06-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1150944B1 (en) | N2-phenylamidine derivatives | |
| WO1997030047A1 (en) | Fungicidal 1,2,4-oxadiazoles and analogues | |
| US5304530A (en) | Acrylate fungicides | |
| EP1204322B1 (en) | Fungicides | |
| US5756524A (en) | Anilide derivatives as fungicides | |
| EP1179528B1 (en) | Fungicidal phenylamidine derivatives | |
| EP0794950A1 (en) | Derivatives of anthranilic acid useful as fungicides | |
| EP0378308B1 (en) | Acrylate fungicides | |
| WO1996017840A1 (en) | Heterocyclyl substituted hydroxyacetamide derivatives as fongicides | |
| WO1997007099A1 (en) | Alpha-methoxymethylene or alpha-methoxyimino naphthylacetic acid derivatives and their use as fungicides | |
| EP1178037B1 (en) | Fungicidal phenylimidate derivatives | |
| WO1997010228A1 (en) | Substituted benzoic or thiobenzoic acid anilides as fungicides | |
| EP1178035B1 (en) | Fungicidal phenylimine derivatives | |
| WO1998051673A1 (en) | Novel imidazole and pyrrole derivatives useful as fungicides | |
| US6933307B2 (en) | Fungicidal phenylimine derivatives | |
| EP0819115A1 (en) | Fungicidal compounds | |
| WO1998004525A1 (en) | Fungicidal n-aryl five-membered cyclic imides | |
| US5268383A (en) | Heterocyclic derivatives of alkoxyacrylates with a fungicidal activity | |
| GB2225011A (en) | Fungicidal acrylates | |
| WO1998050352A1 (en) | Dithiocarbazonic acid derivatives as pesticides | |
| EP0416746A2 (en) | Fungicides containing an alkoxycarbonylvinyl group or an alkyloxyimino group |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AU BR CA CN CZ HU ID IL JP KR MX PL RO RU TR UA US VN |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| NENP | Non-entry into the national phase |
Ref country code: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: JP Ref document number: 1998548884 Format of ref document f/p: F |
|
| 122 | Ep: pct application non-entry in european phase |