USRE37105E1 - Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit - Google Patents
Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit Download PDFInfo
- Publication number
- USRE37105E1 USRE37105E1 US09/453,582 US45358299A USRE37105E US RE37105 E1 USRE37105 E1 US RE37105E1 US 45358299 A US45358299 A US 45358299A US RE37105 E USRE37105 E US RE37105E
- Authority
- US
- United States
- Prior art keywords
- methyl
- norbornene
- lubricant
- additive
- graft copolymer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- DXMMVTGPVOZXHG-UHFFFAOYSA-N C.CSC1=NN=C(S)S1 Chemical compound C.CSC1=NN=C(S)S1 DXMMVTGPVOZXHG-UHFFFAOYSA-N 0.000 description 3
- 0 *.*.*.*.*.*C.*SC1=NN=C(SC)S1 Chemical compound *.*.*.*.*.*C.*SC1=NN=C(SC)S1 0.000 description 2
- VJGRGPDJKBSGDE-UHFFFAOYSA-N CCCC(C)CC(C(C)CCCCC(C)C)C1CCCC1C(C)SC1=NN=C(S)S1 Chemical compound CCCC(C)CC(C(C)CCCCC(C)C)C1CCCC1C(C)SC1=NN=C(S)S1 VJGRGPDJKBSGDE-UHFFFAOYSA-N 0.000 description 2
- MEBOMLJIEXWJIE-UHFFFAOYSA-N CCCC(C)CC1C(CCCCC(C)C)C2CCC(C(C)SC3=NN=C(S)S3)C1C2 Chemical compound CCCC(C)CC1C(CCCCC(C)C)C2CCC(C(C)SC3=NN=C(S)S3)C1C2 MEBOMLJIEXWJIE-UHFFFAOYSA-N 0.000 description 2
- QCQMPTDWOWMRCS-UHFFFAOYSA-N CCSC1=NN=C(SC)S1 Chemical compound CCSC1=NN=C(SC)S1 QCQMPTDWOWMRCS-UHFFFAOYSA-N 0.000 description 2
- CFWGYKRJMYXYND-UHFFFAOYSA-N CSC1=NN=C(S)S1 Chemical compound CSC1=NN=C(S)S1 CFWGYKRJMYXYND-UHFFFAOYSA-N 0.000 description 2
- BIGYLAKFCGVRAN-UHFFFAOYSA-N SC1=NN=C(S)S1 Chemical compound SC1=NN=C(S)S1 BIGYLAKFCGVRAN-UHFFFAOYSA-N 0.000 description 1
- DJCISYVIXPWVHY-UHFFFAOYSA-N SC1=NN=C(SSC2=NN=C(S)S2)S1 Chemical compound SC1=NN=C(SSC2=NN=C(S)S2)S1 DJCISYVIXPWVHY-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M151/00—Lubricating compositions characterised by the additive being a macromolecular compound containing sulfur, selenium or tellurium
- C10M151/02—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F8/00—Chemical modification by after-treatment
- C08F8/34—Introducing sulfur atoms or sulfur-containing groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M143/00—Lubricating compositions characterised by the additive being a macromolecular hydrocarbon or such hydrocarbon modified by oxidation
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M143/00—Lubricating compositions characterised by the additive being a macromolecular hydrocarbon or such hydrocarbon modified by oxidation
- C10M143/12—Lubricating compositions characterised by the additive being a macromolecular hydrocarbon or such hydrocarbon modified by oxidation containing conjugated diene
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M143/00—Lubricating compositions characterised by the additive being a macromolecular hydrocarbon or such hydrocarbon modified by oxidation
- C10M143/14—Lubricating compositions characterised by the additive being a macromolecular hydrocarbon or such hydrocarbon modified by oxidation containing non-conjugated diene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2800/00—Copolymer characterised by the proportions of the comonomers expressed
- C08F2800/20—Copolymer characterised by the proportions of the comonomers expressed as weight or mass percentages
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/02—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/06—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing conjugated dienes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/08—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing non-conjugated dienes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2221/00—Organic macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2221/02—Macromolecular compounds obtained by reactions of monomers involving only carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2221/00—Organic macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2221/04—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
Definitions
- the present invention is directed to lubricants, especially lubricating oils. More particularly, the invention relates to a class of ashless and phosphorus-free antiwear, anti-fatigue, dispersant additives having viscosity index improving credit that are preferably derived from an ethylene-propylene diene modified copolymer (EPDM) and a 2-mercapto-1,3,4-thiadiazole derivative.
- EPDM ethylene-propylene diene modified copolymer
- 2-mercapto-1,3,4-thiadiazole derivative 2-mercapto-1,3,4-thiadiazole derivative
- Zinc dialkyldithiophosphates have been used in formulated oils as antiwear additives.
- zinc dialkyldithiophosphates give rise to ash, which contributes to particular matter in automotive exhaust emissions. Regulatory agencies are seeking to reduce emissions of zinc into the environment.
- the phosphorus of these compounds is also suspected of limiting the service life of the catalytic converters that are used on cars to reduce pollution. It is important to limit the particulate matter and pollution formed during engine use for toxicological and environmental reasons, but it is also important to maintain the antiwear properties of the lubricating oil.
- Additives that protect engines against sludge formation generally contain nitrogen. These additives are also known as dispersants and/or detergents in the formulation of crankcase lubricating oil compositions.
- the preparation of many of the known dispersant/detergent compounds is based on the reaction of an alkenylsuccinic acid or anhydride with an amine or polyamine to produce an alkenyl succinimide or an alkenylsuccinamic acid or anhydride as an intermediate. This is advantageous since these products, if not completely reacted with amine or polyamine, can cause rust in an engine. In most cases, to produce an alkenyl succinimide, an intermediate must first be manufactured and then further reacted. Thus, two steps are required in the manufacturing process.
- Ethylene-propylene copolymers and ethylene-alpha olefin non-conjugated diene copolymers that have been grafted and derivatized to provide valuable properties in lubricating oil compositions are well known.
- U.S. Pat. No. 3,522,180 discloses a method for the preparation of an ethylene-propylene copolymer substrate effective as a viscosity index improver for lubricating oils.
- U.S. Pat. No. 4,026,809 discloses graft copolymers of a methacrylate ester and an ethylene-propylene-alkylidene norbornene terpolymer as a viscosity index improver for lubricating oils.
- U.S. Pat. No. 4,089,794 discloses ethylene copolymers derived from ethylene and one or more C 3 to C 28 alpha olefins solution-grafted with an ethylenically-unsaturated carboxylic acid material followed by a reaction with a polyfunctional material, such as a polyamine, a polyol, or a hydroxylamine that is reactive with the carboxyl groups of the acid.
- a polyfunctional material such as a polyamine, a polyol, or a hydroxylamine that is reactive with the carboxyl groups of the acid.
- U.S. Pat. Nos. 4,137,185 and 4,144,181 disclose an oil-soluble, derivatized ethylene copolymer derived from about 2 to 98 wt. percent ethylene, and one or more C 3 to C 28 alpha-olefins, e.g., propylene. These compounds are preferably solution-grafted under an inert atmosphere and at elevated temperatures in the presence of a high-temperature, decomposable free-radical initiator with an ethylenically-unsaturated dicarboxylic acid material.
- the graft copolymer is reacted with a polyamine having at least two primary amine groups, e.g., an alkylene polyamine such as diethylene triamine, to form carboxyl-grafted polymeric imide, usually maleimide, derivatives.
- the derivatives are reacted with an anhydride of a C 1 to C 30 hydrocarbyl substituted acid, preferably acetic anhydride, to yield an oil-soluble, stable amide derivative of the polyamine that exhibits minimal viscosity change over an extended period of time.
- Useful number average molecular weights (M n ) of the copolymers range from about 700 to 500,000. If the molecular weight is in the range of 10,000 to 500,000, then these copolymers are also useful as multifunctional viscosity index improvers.
- U.S. Pat. No. 4,146,489 discloses graft copolymers wherein the backbone polymer is a rubbery, oil-soluble ethylene-propylene copolymer or ethylene-propylene diene modified terpolymer and the graft monomer is a C-vinylpyridine or N-vinylpyrrolidone that imparts dispersant properties to hydrocarbon fuels and combined viscosity index improvement and dispersant properties to lubricating oils for internal combustion engines.
- the graft copolymers are prepared by intimate admixture of the backbone polymer, monomer to be grafted, and a free radical initiator at a temperature below initiation temperature, followed by a temperature increase to or above the initiation temperature, thus providing a product containing little or no by-product.
- U.S. Pat. No. 4,234,435 discloses carboxylic acid acylating agents derived from polyalkenes and a carboxylic reactant having a molecular weight from about 1,300 to 5,000 and having at least 1.3 carboxylic groups per equivalent of polyalkene.
- U.S. Pat. No. 4,320,019 discloses a multipurpose lubricating additive prepared by the reaction of an interpolymer of ethylene and a C 1 to C 8 alpha-monoolefin with an olefinic carboxylic acid acylating agent to form an acylating reaction intermediate that is then reacted with an amine.
- U.S. Pat. No. 4,340,689 discloses a process for grafting a functional organic group onto an ethylene copolymer or an ethylene-propylene-diene terpolymer.
- U.S. Pat. No. 4,357,250 discloses a reaction product of a copolymer and an olefin carboxylic acid via the “ene” reaction followed by a reaction with monoamine-polyamine mixture.
- U.S. Pat. No. 4,382,007 discloses a dispersant-viscosity index improver prepared by reacting a polyamine-derived dispersant with an oxidized ethylene-propylene polymer or an ethylene-propylene-diene terpolymer.
- U.S. Pat. No. 4,668,834 discloses low molecular weight copolymers comprised of ethylene, an alphaolefin and, optionally, a nonconjugated polyene, which copolymers have a viscosity index of at least about 75 and vinylidene-type unsaturation.
- the copolymers are said to possess unexpected advantages as intermediates in epoxy-grafted electrical encapsulation compositions.
- U.S. Pat. No. 4,863,623 discloses multifunctional grafted and derivatized copolymers that provide viscosity index improvement, dispersancy, and antioxidant properties in a multigrade lubricating oil composition.
- the additive composition comprises a graft and amine-derivatized copolymer prepared from ethylene and at least one C 3 to C 10 alpha-monoolefin and, optionally, a polyene selected from non-conjugated dienes and trienes comprising from about 15 to 80 mole percent of ethylene, from about 20 to 85 mole percent of the C 3 to C 10 alpha-monoolefin, and from about 0 to 15 mole percent of the polyene having an average molecular weight ranging from about 5,000 to 500,000, which has been reacted with at least one olefinic carboxylic acid acylating agent to one or more acylating reaction intermediates characterized by having a carboxylic acid acylating function within their structure and reacting
- the amino-aromatic polyamine compound is a member selected from the group consisting of an N-arylphenylenediamine, an aminothiazole, an amino carbazole, an aminoindole, an aminopyrrole, an amino indazolinone, an aminomercaptotriazole, and an aminoperimidine to form the graft and amine-derivatized copolymer.
- a lubricating oil composition containing the additive is also disclosed.
- U.S. Pat. No. 4,904,403 discloses compounds derived from 2,5-dimercapto-1,3,4-thiadiazole and one or two moles of polyolefin having 5 to 400 carbon atoms.
- the 5-position of the 2-mercapto-1,3,4-thiadiazole can be substituted by an alkylthio, a 2-hydroxyalkylthio, an amino, or a hydroxy group.
- the compounds are said to be effective dispersants, antiwear agents, and antioxidants when incorporated into lubricating compositions.
- U.S. Pat. No. 5,075,383 discloses an additive composition comprising a graft and amine-derivatized copolymer prepared from ethylene and at least one C 3 to C 10 alpha-monoolefin and, optionally, a polyene selected from non-conjugated dienes and trienes comprising from about 15 to 80 mole percent of ethylene, from about 20 to 85 mole percent of the C 3 to C 10 alpha-monoolefin and from about 0 to 15 mole percent of the polyene.
- the copolymer has an average molecular weight ranging from about 5,500 to 50,000 and has grafted thereon at least 1.8 molecules of a carboxylic acid acylating function per molecule of the copolymer.
- the grafted copolymer is reacted with an amino-aromatic polyamine compound from the group consisting of an N-arylphenylenediamine, an aminocarbazole, and an aminoperimidine to form the graft and amine-derivatized copolymer.
- a lubricating oil composition containing the additive is also disclosed.
- U.S. Pat. No. 5,162,086 discloses an additive composition comprising a graft and amine-derivatized copolymer prepared from ethylene and at least one C 3 to C 10 alpha-monoolefin and, optionally, a polyene selected from non-conjugated dienes and trienes comprising from about 15 to 80 mole percent of ethylene, from about 20 to 85 mole percent of the C 3 to C 10 alpha-monoolefin and from about 0 to 15 mole percent of the polyene.
- the copolymer has a number average molecular weight ranging from about 5,500 to 50,000 and has grafted thereon at least 1.8 molecules of a carboxylic acid acylating function per molecule of the copolymer.
- the grafted copolymer is reacted with an amine substituted phenothiazine to form the graft and amine-derivatized copolymer.
- a lubricating oil composition containing the additive is also disclosed.
- U.S. Pat. No. 5,188,745 discloses an additive composition comprising a graft and amine-derivatized copolymer prepared from ethylene and at least one C 3 to C 10 alpha-monoolefin and, optionally, a polyene selected from non-conjugated dienes and trienes comprising from about 15 to 80 mole percent of ethylene, from about 20 to 85 mole percent of the C 3 to C 10 alpha-monoolefin, and from about 0 to 15 mole percent of the polyene.
- the copolymer has an average molecular weight ranging from about 5,500 to 500,000 and has been reacted with at least one olefinic carboxylic acid acylating agent to form one or more acylating reaction intermediates characterized by having a carboxylic acid acylating function within their structure.
- This reaction intermediate is then reacted with an N-(2-aminoalkyl)imidazolidone to form the grafted derivatized copolymer.
- a lubricating oil composition containing the additive is also disclosed.
- U.S. Pat. No. 5,200,102 discloses an additive composition comprising a graft and derivatized copolymer prepared from ethylene and at least one C 3 to C 10 alpha-monoolefin and, optionally, a polyene selected from non-conjugated dienes and trienes comprising from about 15 to 80 mole percent of ethylene, from about 20 to 85 mole percent of the C 3 to C 10 alpha-monoolefin and from about 0 to 15 mole percent of the polyene having an average molecular weight ranging from about 5,000 to 500,000, which has been reacted with at least one olefinic carboxylic acid acylating agent to form one or more acylating reaction intermediates characterized by having a carboxylic acid acylating function within their structure.
- the reaction intermediate is reacted with an amino alkylthio thiadiazole to form the graft derivatized copolymer.
- U.S. Pat. No. 5,474,694 discloses an additive composition comprising a graft and amine-derivatized copolymer prepared from ethylene and at least one C 3 to C 10 alpha-monoolefin and, optionally, a polyene selected from non-conjugated dienes and trienes comprising from about 15 to 80 mole percent of ethylene, from about 20 to 85 mole percent of the C 3 to C 10 alpha-monoolefin, and from about 0 to 15 mole percent of the polyene.
- the copolymer has a number average molecular weight ranging from about 5,500 to 50,000 and has grafted thereon at least 1.8 molecules of a carboxylic acid acylating function per molecule of the copolymer.
- the grafted copolymer is reacted with an amino alcohol compound selected from the group consisting of a 2-anilinoalcohol, a (2-hydroxyalkyl)pyridine, a 4-(2-hydroxyalkyl)morpholine, a 1-(2-hydroxyalkyl)piperazine, and a 1-(2-hydroxyalkyl)2-pyrrolidine.
- An object of this invention is to provide a novel graft copolymer composition.
- Another object of this invention is to provide a lubricant additive and lubricant composition containing such an additive, which has improved dispersancy and antiwear properties and which can withstand the stresses imposed by modern internal combustion engines.
- the present invention is directed to lubricants, especially lubricating oils, and more particularly to a class of ashless and phosphorus-free antiwear, anti-fatigue, dispersant additives having viscosity index improving credit that are preferably derived from an EPDM copolymer and a 2-mercapto-1,3,4-thiadiazole.
- EPDM is intended to be interpreted in a broad sense, such that “P” stands for any C 3 -C 10 alpha monoolefin and “D” stands for both dienes and trienes.
- polymer and copolymer are used generically herein to encompass ethylene copolymers, terpolymers, or interpolymers. These materials can contain minor amounts of other olefinic monomers so long as their basic characteristics are not materially changed.
- the present invention relates to a graft copolymer comprising:
- A a copolymer prepared by the interpolymerization of a mixture of monomers comprising:
- a polyene being a member selected from the group consisting of non-conjugated dienes and trienes
- the present invention relates to a lubricant additive comprising a graft copolymer comprising:
- A a copolymer prepared by the interpolymerization of a mixture of monomers comprising:
- a polyene being a member selected from the group consisting of non-conjugated dienes and trienes
- the present invention relates to a lubricant comprising a lubricant additive comprising a graft copolymer comprising:
- A a copolymer prepared by the interpolymerization of a mixture of monomers comprising:
- a polyene being a member selected from the group consisting of non-conjugated dienes and trienes
- the lubricant be a lubricating oil.
- the additives of this invention can be used as either partial or complete replacements for the zinc dialkyldithiophosphates and/or alkenyl succinimides or other dispersants currently used. They can also be used in combination with other additives typically found in motor oils, as well as with other ashless antiwear additives. These additives, due to their polymeric nature, can also be used to provide viscosity index improver credit and, thus, can be used as partial or complete replacements for viscosity index improvers currently known in the art.
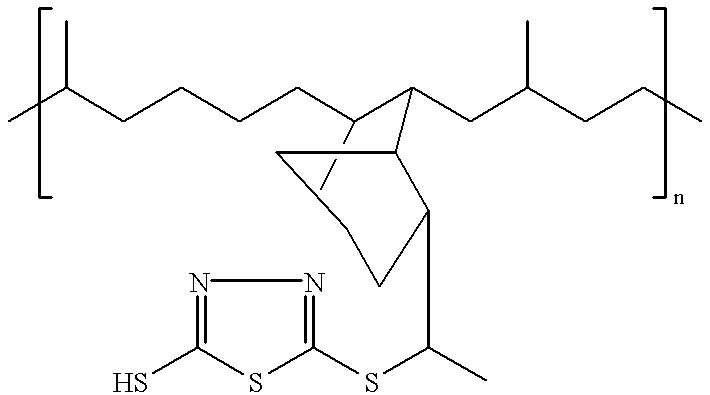
- the ashless and phosphorus-free, anti-fatigue, antiwear, viscosity index improving lubricating oil dispersants of the present invention are derived from an EPDM copolymer and a 2-mercapto-1,3,4-thiadiazole, preferably 2,5-dimercapto-1,3,4 thiadiazole, and can preferably have the following structure:
- n is an integer in the range of about 1 to about 10.
- the graft copolymer lubricant additives of the present invention can be prepared by means of a solution polymerization process.
- the graft copolymer of the present invention when used as a lubricant additive, comprises an ethylene copolymer of a C 3 -C 12 alpha-monoolefin and a non-conjugated diene or triene having an average molecular weight ranging from about 500 to 40,000, preferably 2,000 to 10,000, on which has been grafted from about 0.1 to about 20 percent by weight, preferably from about 0.5 to about 10 percent by weight, of a mercapto-1,3,4-thiadiazole.
- copolymers employed in the practice of this invention are useful intermediates in the production of grafted compositions.
- copolymers employed in the practice of this invention are copolymers of ethylene, an alphaolefin having the formula H 2 C ⁇ CHR wherein R is an alkyl radical comprising 1 to 10 carbon atoms and a nonconjugated polyene.
- alphaolefins employed in the practice of this invention are compounds of the formula CH 2 ⁇ CHR wherein R is an alkyl radical containing from one to ten carbon atoms. When R contains more than two carbon atoms, such a radical can be a straight chain or branched.
- Preferred alphaolefins include propylene, 1-butene, 1-pentene, 1-hexene, 3-methyl-1-pentene, 1-heptene, 1-octene, and 1-decene.
- polyenes employed in the practice of this invention are nonconjugated.
- nonconjugated polyenes are aliphatic dienes such as 1,4-hexadiene, 1,5-hexadiene, 1,4-pentadiene, 2-methyl-1,4-pentadiene, 3-methyl-1,4 hexadiene, 4-methyl-1,3-hexadiene, 1,7-octadiene, 1,9-decadiene, exo- and endodicyclopentadiene and the like; exo- and endo-alkenylnorbornenes, such as 5-propenyl-, 5-(buten-2-yl)-, and 5-(2-methylbuten-[2′]-yl) norbornene and the like; alkylalkenylnorbornenes, such as 5-methyl-6-propenylnorbornene and the like; alkylidenenorbornenes, such as 5-methyl-, 5-ethylidene-
- Any triene component has at least two non-conjugated double bonds and up to about 30 carbon atoms in the chain.
- Typical trienes useful in preparing the copolymer of the present invention are 1-isopropylidene-3a,4,7,7a-tetrahydroindene, 1-isopropylidenedicyclopentadiene, dehydroisodicyclopentadiene, and 2-(2-methylene-4-methyl-3-pentenyl) ⁇ 2.2.1 ⁇ bicyclo-5-heptene.
- a mixture of more than one triene can be used in the preparation of the copolymer. Mixtures of one or more trienes with one or more dienes can also be used.
- the molar ethylene content of the copolymers of this invention is preferably in the range of between about 20 and about 80 percent, is more preferably between about 30 and about 70 percent, and is most preferably between about 35 and about 65 percent, although higher or lower ethylene contents can be present.
- the nonconjugated polyene molar percent generally ranges between about 0.1 and about 25 percent.
- the remaining mole percent of such copolymers, up to 100 percent, is comprised of alphaolefin.
- the copolymer employed in the practice of this invention may possess vinylidene-type unsaturation.
- one end of such polymer has the formula P—CR ⁇ CH 2 wherein R is as defined above, for the alphaolefins which can be employed, and P represents the polymer chain.
- composition of this invention comprises copolymer chains with vinylidene-type unsaturization
- at least about 30 percent of the chains will possess such unsaturation.
- at least about 50 percent, more preferably at least about 60 percent, and most preferably at least about 75 percent, of such polymer chains exhibit vinylidene-type unsaturation.
- the percentage of polymer chains exhibiting vinylidene-type unsaturation can be determined by FTIR spectroscopic analysis or titration.
- copolymer and the composition of this invention can be prepared employing a Group 4 element of the Periodic Table as defined in the Handbook of Chemistry and Physics, (CRC Press, 66th Ed., 1985-1986) catalyst and an aluminoxane cocatalyst.
- the catalysts that can be employed are generally of the Formula Q n MJ 4-n wherein Q is cyclopentadiene, cyclopentadiene substituted with up to five C 1 to C 6 alkyl groups, or indene; M is zirconium, titanium, or hafnium; J is C 1 to C 4 alkyl, halogen, CH 2 AlR′′ 2 , CH 2 CH 2 AlR′′ 2 or CH 2 CH (AlR′′ 2 ) 2 wherein R′′ is C 1 to C 6 alkyl or OAl(C 1 -C 6 alkyl) 2 ; and n is 1, 2, or 3.
- Q is cyclopentadiene, methylcyclopentadiene or indene; M is zirconium or titanium; J is methyl, ethyl, Cl or Br; and n is 2 or 3. Most preferably Q is cyclopentadiene; M is zirconium; J is methyl or chlorine; and n is 2.
- Representative catalysts include: (C 5 H 5 ) 3 TiC 2 H 5 ; (C 5 H 5 ) 2 ; (CH 3 C 5 H 4 ) 2 HaCl 2 ; (C 5 H 5 ) 2 ZrCH 3 Cl; (C 5 H 5 ) 3 ZrC 2 H 5 ; (C 5 H 5 ) 2 Zr(CH 3 ) 2 ; (C 5 H 5 ) 2 ZrCl 2 ; and (C 5 H 5 ) 2 ZrBr 2 .
- the cocatalysts that are typically employed to produce the polymer intermediates of this invention are aluminoxanes either having the linear formula (a) R′ 2 AlO—(AlR′O) n —AlR′ 2 or the cyclic formula (b) (—AlR′O—) n+2 wherein R′ is linear or branched C 1 to C 6 alkyl and n is an integer of 2 to 40; preferably R′ is methyl or ethyl and n is 6 to 20. Most preferably cocatalysts have the formula (b) wherein R′ is methyl and n is 10 to 20.
- Preferred cocatalysts include linear or cyclic methaluminoxane, ethylaluminoxane, and butylaluminoxane.
- the catalysts system is employed so that the Al/M molar ratio, wherein M is as defined above, is between about 10 and 10,000, is preferably between about 20 and about 5,000, and most preferably between about 40 and about 2,000.
- Polymerization is generally conducted at temperatures ranging between about 20° and about 100° C., preferably between about 30° and about 80° C.
- Reaction time is not critical and can vary from several hours or more to several minutes or less, depending upon factors such as reaction temperature, the monomers to be copolymerized, and the like.
- One of ordinary skill in the art can readily obtain the optimum reaction time for a given set of reaction parameters by routine experimentation.
- the polymerization can be conducted employing liquid monomer, such as liquid propylene, as the reaction medium.
- polymerization can be accomplished in the presence of a hydrocarbon inert to the polymerization such as butane, pentane, isopentane, isopentane, hexane, isooctane, decane, toluene, xylene, and the like.
- the polymerization can be performed in the presence of hydrogen to further lower polymer molecular weight.
- the reaction must be monitored to ensure that the vinylidene-type unsaturization is not reduced to less than about 30 percent of the polymer chains.
- alphaolefin, ethylene, polyene, and the reaction medium, if any is present are charged at appropriate ratios to a suitable reactor.
- the reaction must be monitored to ensure that all ingredients are dry with the reactants typically being passed through molecular sieves or other drying means prior to their introduction into the reactor. Subsequently, either the catalyst and then the cocatalyst, or first the cocatalyst and then the catalyst are introduced while agitating the reaction mixture, thereby causing polymerization to commence.
- the catalyst and cocatalyst can be premixed in a solvent and then charged to the reactor. As polymer is being formed, additional monomers can be added to the reactor. Upon completion of the reaction, unreacted monomer and solvent are either “flashed off” or distilled off, if necessary by vacuum, and the low molecular weight copolymer is withdrawn from the reactor.
- the polymerization can be conducted in a continuous manner by simultaneously feeding the reaction medium, if employed, monomers, catalyst, and cocatalyst to a reactor and withdrawing solvent, unreacted monomer and polymer from the reactor so as to allow a residence time of ingredients long enough for forming copolymer of the desired molecular weight and separating the copolymer from the reaction mixture.
- the polymerization reaction used to form the polymer substrate can be carried out in the presence of a Ziegler-Natta catalyst in a solvent medium.
- the polymerization can be conducted employing liquid monomer, such as liquid propylene, as the reaction medium, or in the presence of a hydrocarbon inert to the polymerization such as butane, pentane, isopentane, hexane, isooctane, decane, toluene, xylene, and the like.
- hexane is initially introduced into the reactor and the temperature raised to about 30° C.
- the appropriate quantities of dry propylene, ethylene, termonomer (e.g., triene), and hydrogen are fed into the reactor.
- the appropriate reactor pressure is a function of the desired product.
- the catalyst system consisting of vanadium oxytrichloride, aluminum sesquichloride, and a halogenated ester modifier are added to initiate polymerization.
- a thiadiazole is grafted onto the prescribed copolymer backbone.
- the thiadiazole is a 2-mercapto-1,3,4-thiazole characterized by the structural formula
- R 1 is hydrogen, alkyl, alkenyl, aryl, alkaryl, aralkyl, or 2-hydroxyalkyl;
- X is sulfur
- Y is 0 or 1
- R 2 is hydrogen or
- R 1 is alkyl, it is preferably an alkyl of from 1 to 40 carbon atoms and can have either a straight chain or a branched chain, for example, methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl, eicosyl, heneicosyl, docosyl, tricosyl, tetracosyl, pentacosyl, triacontyl, pentatriacontyl, tetracontyl, and the like, and isomers and mixtures thereof.
- R 1 is alkylene, it is preferably an alkylene of from 2 to 40 carbon atoms and can have either a straight chain or a branched chain, e.g., ethylene, propylene, butylene, pentylene, hexylene, heptylene, octylene, nonylene, decylene, undecylene, dodecylene, tridecylene, tetradecylene, pentadecylene, hexadecylene, heptadecylene, octadecylene, nonadecylene, eicosylene, heneicosylene, dococylene, tricosylene, tetracosylene, pentacosylene, triacontylene, pentatriacontylene, tetracontylene, and the like, and isomers and mixtures thereof.
- ethylene propylene, butylene, pentylene, hexylene
- R 1 is 2-hydroxyalkyl, it is preferably a 2-hydroxyalkyl of from 1 to 40 carbon atoms and can be either a straight chain or a branched chain, e.g. 2-hydroxymethyl, 2-hydroxyethyl, 2-hydroxypropyl, 2-hydroxybutyl, 2-hydroxypentyl, 2-hydroxyhexyl, 2-hydroxyheptyl, 2-hydroxyoctyl, 2-hydroxynonyl, 2-hydroxydecyl, 2-hydroxyundecyl, 2-hydroxydodecyl, 2-hydroxytridecyl, 2-hydroxytetradeoyl, 2-hydroxypentadecyl, 2-hydroxyhexadecyl, 2-hydroxyheptadecyl, 2-hydroxyoctadecyl, 2-hydroxynonadecyl, 2-hydroxyeicosyl, 2-hydroxyheneicosyl, 2-hydroxydocosyl, 2-hydroxytrioosyl, 2-hydroxytetracosyl, 2-hydroxypentacosyl, 2-hydroxytriacont
- R 1 is aryl, aralkyl, or alkaryl, it preferably comprises from 6 to 12 carbon atoms. Additionally, one or more of the ring carbon atoms can be replaced by an atom of a suitable alternative element, for example, nitrogen, oxygen, or sulfur.
- R 1 can be a residue or benzene, toluene, xylene, indene, naphthalene, alpha-methylnaphthalene, beta-methylnaphthalene, diphenyl, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, chrysene, naphthacene, pyridine, picoline, quinoline, isoquinoline, quinaldine, indole, acridine, carbazole, hemimellitene, pseudocumene, mesitylene, prehnitene, isodurene, durene, pentamethylbenzene, hexamethylbenzene, ethylbenzene, propylbenzene, cumene, butylbenzene, cymene, triethylbenzene, hexaethylbenzene,
- R 1 be the reacted moiety after the reaction of the diene or triene in a fatty acid or fatty acid ester, for example, oleic acid, linoleic acid, methyl oleate, butyl oleate, butyl tallate, methyl tallate, octyl tallate, glyceryl monooleate, glyceryl monotallate, or pentaerythritol trioleate.
- a fatty acid or fatty acid ester for example, oleic acid, linoleic acid, methyl oleate, butyl oleate, butyl tallate, methyl tallate, octyl tallate, glyceryl monooleate, glyceryl monotallate, or pentaerythritol trioleate.
- VANCHEM® DMTD Thiadiazoles useful in the practice of the present invention can be obtained commercially, for example, as the products sold under the trademarks VANCHEM® DMTD and VANLUBE® 829, both available from R. T. Vanderbilt Company, Inc.
- VANCHEM® DMTD The chemical structure of the VANCHEM® DMTD brand product is understood to be:
- the grafting of the thiadiazole onto the copolymer can be done in the presence of heat, in the neat polymer, or the polymer dissolved in oil, or a hydrocarbon solvent, such as hexanes or xylenes. Alternatively, the grafting can be conducted in the presence of a free radical initiator.
- Free radical initiators that can be used are preferably peroxides, hydroperoxides, or azo compounds and, most preferably, are those that have a boiling point higher than about 100° C. and decompose thermally within the grafting temperature range to provide free radicals.
- Representative of these free radical initiators are azobutyronitrile and 2,5-dimethyl-hex-3-yne-2,5 bis-tertiary-butyl peroxide.
- the initiator is used in an amount of between about 0.005 percent and about 1.0 percent by weight based on the weight of the reaction mixture solution.
- the grafting is preferably performed in an inert atmosphere, such as under nitrogen blanketing.
- the additives of the present invention are useful as components in lubricant compositions. They can be employed in a variety of oils of lubricating viscosity including natural and synthetic lubricating oils and mixtures thereof.
- the additives can be employed in crankcase lubricating oils for spark-ignited and compression-ignited internal combustion engines.
- the compositions can also be used in gas engines, or turbines, automatic transmission fluids, gear lubricants, metal-working lubricants, hydraulic fluids and other lubricating oil and grease compositions. Their use in motor fuel compositions is also contemplated.
- the lubricant compositions of the invention contain the graft copolymer additives in a concentration ranging from about 0.1 to 30 weight percent.
- a concentration range for the additives ranging from about 0.5 to 15 weight percent based on the total weight of the lubricant composition is preferred.
- a still more preferred concentration range is from about 1.0 to about 7.5 weight percent.
- Oil concentrates of the additives can contain from about 1.0 to 50 weight percent of the additive reaction product in a carrier or dilutive oil of lubricating oil viscosity.
- the additives of the present invention can be employed in lubricant compositions together with conventional lubricant additives.
- the typical additives found in lubricating oil compositions are dispersants, detergents, rust inhibitors, antioxidants, antifoamants, friction modifiers, viscosity index improvers, and pour point depressants.
- a quantity of 83.4 grams of a 9 percent 5-ethylidene-2-norbornene and 52/48 ethylene/propylene terpolymer sold under the trademark Trilene®-47 was combined with 2.1 grams (0.056 mol) of 2,5-dimercapto-1,3,4-thiadiazole and 300 milliliters reagent xylenes in a one-liter resin kettle equipped with a mechanical stirrer, a reflux condenser, a thermocouple, and a gas inlet port. The system was heated to reflux (139° C.), and the conditions were maintained for 15 hours.
- the reaction was cooled to 50° C., and 214 grams of 100 P mineral oil were added.
- the xylenes were removed by vacuum distillation (130° C. at seven millibars vacuum for one hour).
- the product was then pressure filtered using a five-micron filter, under 100 psig N 2 at 100° C. A quantity of 273 grams of a hazy, olive-green, very thick liquid product was recovered.
- the product was pressure filtered using a one-micron filter under 100 psig N 2 at 100° C. whereupon a clear, medium-yellow, very thick liquid product was obtained.
- Trilene®-47 brand terpolymer A quantity of 70.1 grams of the Trilene®-47 brand terpolymer was combined with 3.5 grams (0.046 mol) 2,5-dimercapto-1,3,4-thiadiazole and 321 grams of 100 P mineral oil in a one-liter resin kettle reactor equipped with a mechanical stirrer, a thermocouple, and gas inlet and outlet ports. The system was heated to 170° C., and the conditions were maintained for nine hours.
- the product was pressure filtered using a one-micron filter, under 100 psig N 2 at 75° C., which yielded a final product that was a clear, dark brown liquid of moderate viscosity.
- a quantity of 106.2 grams of a Trilene®-45 brand terpolymer (a 9.5 percent dicyclopentadiene, 50/50 ethylene/propylene terpolymer) was combined with 3.5 grams (0.07 mol) 2,5-dimercapto-1,3,4-thiadiazole, 300 milliliters of reagent xylenes, and 0.83 gram (0.0031 mol) dicumyl peroxide in a one-liter resin kettle equipped with a mechanical stirrer, a reflux condenser, a thermocouple, and a gas inlet port. The system was heated to reflux (139° C.), and the conditions were maintained for 16 hours.
- the reaction was cooled to 100° C., and a quantity of 326 grams of 100 P mineral oil was added. The reaction was then cooled further to 60° C., whereupon 300 milliliters of reagent hexanes were added. The reaction was cooled still further to 5° C. to 10° C. and held at that temperature for one hour. The product was then pressure filtered using a five-micron filter under 100 psig N 2 . The filtrate was hazy yellow. The product was pressure filtered two more times using a one-micron filter. The solvent was then removed by vacuum distillation (130° C. at seven millibars vacuum for one hour). The product was a hazy reddish-brown. This final product was then vacuum filtered through a Celite 545 brand filter material, whereupon a clear, thick reddish-brown liquid product was obtained.
- Trilene®-47 brand terpolymer A quantity of 106.7 grams of the Trilene®-47 brand terpolymer was combined with 11.3 grams (0.07 mol) 2,5-dimercapto-1,3,4-thiadiazole, 300 milliliters of reagent xylenes, and 1.0 gram (0.0037 mol) dicumyl peroxide in a one-liter kettle reactor equipped with a mechanical stirrer, a reflux condenser, a thermocouple, and a gas inlet port. The system was heated to reflux (139° C.), and the conditions were maintained for eight hours.
- the reaction was cooled to 100° C., and a quantity of 298 grams of 100 P mineral oil was added. Vacuum distillation was used to remove the xylenes (100° C. at seven millibars vacuum for one-half hour).
- the product was a milky, light yellow liquid. It was cooled to 30° C., and 400 milliters of reagent hexanes were added. This mixture was agitated for one-half hour.
- the product was vacuum filtered through Celite 545 brand filter material. The product was slightly turbid. The solution was then passed through the Celite brand filter material a second time, yielding a clear, light-yellow solution. The solvent was removed by vacuum distillation (90° C. at seven millibars vacuum for one hour) whereupon a clear, amber liquid of moderate viscosity was obtained.
- Trilene®-47 brand terpolymer A quantity of 83.4 grams of the Trilene®-47 brand terpolymer was combined with 2.1 grams (0.056 mol) of 2,5-dimercapto-1,3,4-thiadiazole and 300 milliliters of reagent xylenes in a one-liter resin kettle equipped with a mechanical stirrer, a reflux condenser, a thermocouple, and a gas inlet port. The system was heated to reflux (139° C.), and the conditions were maintained for 15 hours.
- the reaction was cooled to 50° C., and 214 grams of 100 P mineral oil were added.
- the xylenes were removed by vacuum distillation (130° C. at seven millibars vacuum for one hour).
- the product was then pressure filtered using a five-micron filter, under 100 psig N 2 at 100° C. A quantity of 273 grams of a hazy, olive-green, very thick liquid product was recovered.
- the product was pressure filtered using a one-micron filter under 100 psig N 2 at 100° C. whereupon a clear, medium-yellow, very thick liquid product was obtained.
- the antiwear properties of the reaction product of this invention in a fully formulated lubricating oil were determined in the “Four-Ball Wear Test,” described below, under the ASTM D 4172 test conditions.
- the fully formulated lubricating oils tested in this example also contained 1.0 weight percent cumene hydroperoxide.
- the additives were tested for effectiveness in two motor oil formulations, as described in Table 2, and compared to identical formulations with an without any zinc dialkyldithiophosphate. In Table 1 the numerical value of the test results (Average Wear Scar Diameter millimeters (mm)) decreases with an increase in effectiveness. In many instances, antiwear additives are effective in lubricating oil containing no other additives.
- the “Four-Ball Wear Test” evaluates the antiwear performance of oil and grease formulations and transportation fuels, such as diesel.
- a Four-Ball Wear Test machine is used to perform this evaluation.
- Four balls are arranged in an equilateral tetrahedron.
- the lower three balls are clamped securely in a test cup filled with lubricant and the upper ball is held by a chuck that is motor-driven.
- the upper ball rotates against the fixed lower balls.
- Load is applied in an upward direction through a weight/lever arm system. Loading is through a continuously variable pneumatic loading system. Heaters allow operation at elevated oil temperatures.
- the three stationary steel balls are immersed in 10 milliliters of formulated oil to be tested, and the fourth steel ball is rotated on top of the three stationary balls in “point-to-point contact.”
- the machine is operated for one hour at 75° C. with a load of 40 kilograms and a rotational speed of 1,200 revolutions per minute.
- the oils for dispersancy were fully formulated SAE 80W-90 API GL-5 quality gear oils.
- the dispersant test was run with 50 milliliters of sample. The oil was placed into a four-ounce jar and then put into an air circulating oven for 168 hours at 150° C. The samples were analyzed by two methods: a blotter test and a percent hexanes insolubles test.
- the blotter test uses one drop of oil placed on a piece of filter paper. The ring formed is evaluated visually for how the sludge is dispersed across the paper. A uniform ring is rated excellent, while separation with sludge staying toward the center is rated very poor.
- the percent hexane insolubles test was done by diluting the oil with hexanes, centrifuging, collecting the solid mass, and repeating the process two more times. Then the hexanes were removed, and the solids were oven-dried and weighed. The results of the blotter test and the percent hexane insolubles test are in Table 3.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Lubricants (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Liquid Carbonaceous Fuels (AREA)
- Graft Or Block Polymers (AREA)
Abstract
Description
| TABLE 1 |
| Four-Ball Wear Results |
| Wear Scar | Weight Percent | ||
| Compound | Formulation | Diameter, mm | Active Additive |
| Example 1 | A | 0.64 | 1.1 |
| Example 1 | A | 0.84 | 1.0 |
| Example 3 | A | 0.77 | 1.0 |
| Example 4 | A | 0.84 | 1.0 |
| Example 5 | A | 0.73 | 1.0 |
| No Antiwear | A | 0.93 | 0.0 |
| Additive | |||
| ZDDP | A | 0.46 | 1.01 |
| Trilene ® 45 | A | 1.00 | 1.0 |
| Trilene ® 47 | A | 0.96 | 1.0 |
| Example 1 | B | 0.82 | 1.1 |
| Example 2 | B | 0.83 | 1.0 |
| No Antiwear | B | 0.98 | 0.0 |
| Additive | |||
| ZDDP | B | 0.53 | 1.01 |
| Trilene ® 47 | B | 0.93 | 1.0 |
| 11.0 weight percent ZDDP is equal to 0.1 weight percent phosphorus in the fully formulated oil. | |||
| TABLE 2 |
| SA 10W-30 Motor Oil Formulations |
| Formulation A | Wt. % | Formulation B | Wt. % |
| Solvent | 22.8 | Solvent | 22.8 |
| Neutral 100 | Neutral 100 | ||
| Solvent | 60.0 | Solvent | 60.0 |
| Neutral 150 | Neutral 150 | ||
| Succinimide | 7.5 | Succinimeide | 7.5 |
| Dispersant | Dispersant | ||
| Overbased Calcium | 2.0 | Overbased Calcium | 2.0 |
| Phenate Detergent | Sulfonate Detergent | ||
| Neutral Calcium | 0.5 | Neutral Calcium | 0.5 |
| Sulfonate Detergent | Sulfonate Detergent | ||
| Antioxidant | 0.5 | Antioxidant | 0.5 |
| Rust Inhibitor | 0.1 | Rust Inhibitor | 0.1 |
| Pour Point | 0.1 | Pour Point | 0.1 |
| Depressant | Depressant | ||
| OCP VI Improver | 5.5 | OCP VI Improver | 5.5 |
| Antiwear Additive2 | 1.0 | Antiwear Additive | 1.0 |
| 2In the case of No Antiwear Additive in Table 1, solvent neutral 150 is put in its place at 1.0 weight percent. | |||
| TABLE 3 |
| Dispersant Test Results |
| % Hexane | Wt. % Active | ||||
| Compound | Blotter | Insolubles | Additive | ||
| Example 1 | Excellent | 1.2 | 3 | ||
| Succinimide | |||||
| Dispersant | Excellent | 1.0 | 3 | ||
| No Dispersant | Very Poor | 6.3 | 0 | ||
Claims (29)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/453,582 USRE37105E1 (en) | 1997-02-03 | 1999-12-02 | Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/794,111 US5698500A (en) | 1997-02-03 | 1997-02-03 | Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit |
| US09/453,582 USRE37105E1 (en) | 1997-02-03 | 1999-12-02 | Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/794,111 Reissue US5698500A (en) | 1997-02-03 | 1997-02-03 | Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| USRE37105E1 true USRE37105E1 (en) | 2001-03-20 |
Family
ID=25161744
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/794,111 Ceased US5698500A (en) | 1997-02-03 | 1997-02-03 | Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit |
| US09/453,582 Expired - Lifetime USRE37105E1 (en) | 1997-02-03 | 1999-12-02 | Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/794,111 Ceased US5698500A (en) | 1997-02-03 | 1997-02-03 | Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US5698500A (en) |
| EP (1) | EP0961789B1 (en) |
| JP (1) | JP3332935B2 (en) |
| AT (1) | ATE244732T1 (en) |
| CA (1) | CA2278430C (en) |
| DE (1) | DE69816275T2 (en) |
| WO (1) | WO1998033828A1 (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6288144B1 (en) | 1999-04-20 | 2001-09-11 | Uniroyal Chemical Company, Inc. | Processing of coupled, filled polyolefins |
| ATE281481T1 (en) * | 1999-12-30 | 2004-11-15 | Uniroyal Chem Co Inc | COMPOSITIONS CONTAINING N-(4-ANILINOPHENYL)AMIDE-BASED AMINIC ANTIOXIDANTS |
| US7338348B2 (en) | 2003-08-29 | 2008-03-04 | Black & Decker Inc. | Dust collection system for a belt sander |
| WO2011119229A1 (en) * | 2010-03-24 | 2011-09-29 | C.W. Machine Worx, Ltd. | Dust suppression apparatus |
| WO2018047589A1 (en) * | 2016-09-06 | 2018-03-15 | 株式会社大阪ソーダ | Polymer modified with thiol compound, photo-curable composition including said polymer, and use therefor |
| JP7354293B2 (en) * | 2019-12-26 | 2023-10-02 | 株式会社クラレ | Viscosity index improver comprising conjugated diene graft polymer and oil composition |
Citations (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3522180A (en) | 1967-09-28 | 1970-07-28 | Texaco Inc | Lubricating oil compositions containing amorphous ethylene-propylene copolymers |
| US4026809A (en) | 1974-12-19 | 1977-05-31 | Texaco Inc. | Lubricating compositions containing methacrylate ester graft copolymers as useful viscosity index improvers |
| US4089794A (en) | 1975-06-25 | 1978-05-16 | Exxon Research & Engineering Co. | Polymeric additives for fuels and lubricants |
| US4107059A (en) * | 1977-06-27 | 1978-08-15 | Pennwalt Corporation | Polymer of 1,2,4-thiadiazole and lubricants containing it as an additive |
| US4137185A (en) | 1977-07-28 | 1979-01-30 | Exxon Research & Engineering Co. | Stabilized imide graft of ethylene copolymeric additives for lubricants |
| US4144181A (en) | 1977-04-29 | 1979-03-13 | Exxon Research & Engineering Co. | Polymeric additives for fuels and lubricants |
| US4146489A (en) | 1975-07-31 | 1979-03-27 | Rohm And Haas Company | Polyolefin graft copolymers |
| US4234435A (en) | 1979-02-23 | 1980-11-18 | The Lubrizol Corporation | Novel carboxylic acid acylating agents, derivatives thereof, concentrate and lubricant compositions containing the same, and processes for their preparation |
| US4320019A (en) | 1978-04-17 | 1982-03-16 | The Lubrizol Corporation | Multi-purpose additive compositions and concentrates containing same |
| US4340689A (en) | 1979-09-17 | 1982-07-20 | Copolymer Rubber & Chemical Corporation | Method of grafting EPM and EPDM polymers |
| US4357250A (en) | 1978-04-17 | 1982-11-02 | The Lubrizol Corporation | Nitrogen-containing terpolymer-based compositions useful as multi-purpose lubricant additives |
| US4382007A (en) | 1981-02-02 | 1983-05-03 | Texaco Inc. | Novel dispersant-VI improvers and lubricating oil containing same |
| US4668834A (en) | 1985-10-16 | 1987-05-26 | Uniroyal Chemical Company, Inc. | Low molecular weight ethylene-alphaolefin copolymer intermediates |
| US4761482A (en) * | 1987-04-23 | 1988-08-02 | R. T. Vanderbilt Company, Inc. | Terpene derivatives of 2,5-dimercapto-1,3,4-thiadiazoles and lubricating compositions containing same |
| US4863623A (en) | 1988-03-24 | 1989-09-05 | Texaco Inc. | Novel VI improver, dispersant, and anti-oxidant additive and lubricating oil composition containing same |
| US4904403A (en) | 1989-06-12 | 1990-02-27 | R. T. Vanderbilt Company, Inc. | Polyalkylated 1,3,4-thiadiazoles and lubricating compositions containing same |
| US5075383A (en) | 1990-04-11 | 1991-12-24 | Texaco Inc. | Dispersant and antioxidant additive and lubricating oil composition containing same |
| US5162086A (en) | 1991-05-22 | 1992-11-10 | Texaco Inc. | Dispersant additive and lubricating oil composition containing same |
| US5188745A (en) | 1991-12-23 | 1993-02-23 | Texaco Inc. | Viton seal compatible dispersant and lubricating oil composition containing same |
| US5200102A (en) | 1991-12-02 | 1993-04-06 | Texaco Inc. | Multifunctional olefin copolymer and lubricating oil composition |
| US5460740A (en) * | 1990-12-31 | 1995-10-24 | Texaco Inc. | Acylated mono and/or bis-succinimides lubricating oil additives |
| US5474694A (en) | 1992-09-21 | 1995-12-12 | Texaco Inc. | Lubricating oil composition |
| US5490864A (en) * | 1991-08-02 | 1996-02-13 | Texaco Inc. | Anti-wear lubricity additive for low-sulfur content diesel fuels |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0199453A3 (en) * | 1985-04-24 | 1988-04-13 | Texaco Development Corporation | Hydrocarbon compositions containing polyolefin graft polymers |
| US4699723A (en) * | 1986-08-20 | 1987-10-13 | Texaco Inc. | Dispersant-antioxidant multifunction viscosity index improver |
| US4964880A (en) * | 1989-06-09 | 1990-10-23 | Exxon Research & Engineering Company | Distillate fuels containing mono alkyl substituted derivatives of thiadiazoles |
-
1997
- 1997-02-03 US US08/794,111 patent/US5698500A/en not_active Ceased
-
1998
- 1998-01-23 AT AT98904954T patent/ATE244732T1/en not_active IP Right Cessation
- 1998-01-23 WO PCT/US1998/002241 patent/WO1998033828A1/en active IP Right Grant
- 1998-01-23 CA CA002278430A patent/CA2278430C/en not_active Expired - Fee Related
- 1998-01-23 DE DE69816275T patent/DE69816275T2/en not_active Expired - Lifetime
- 1998-01-23 JP JP53323998A patent/JP3332935B2/en not_active Expired - Fee Related
- 1998-01-23 EP EP98904954A patent/EP0961789B1/en not_active Expired - Lifetime
-
1999
- 1999-12-02 US US09/453,582 patent/USRE37105E1/en not_active Expired - Lifetime
Patent Citations (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3522180A (en) | 1967-09-28 | 1970-07-28 | Texaco Inc | Lubricating oil compositions containing amorphous ethylene-propylene copolymers |
| US4026809A (en) | 1974-12-19 | 1977-05-31 | Texaco Inc. | Lubricating compositions containing methacrylate ester graft copolymers as useful viscosity index improvers |
| US4089794A (en) | 1975-06-25 | 1978-05-16 | Exxon Research & Engineering Co. | Polymeric additives for fuels and lubricants |
| US4146489B1 (en) | 1975-07-31 | 1983-11-08 | ||
| US4146489A (en) | 1975-07-31 | 1979-03-27 | Rohm And Haas Company | Polyolefin graft copolymers |
| US4144181A (en) | 1977-04-29 | 1979-03-13 | Exxon Research & Engineering Co. | Polymeric additives for fuels and lubricants |
| US4107059A (en) * | 1977-06-27 | 1978-08-15 | Pennwalt Corporation | Polymer of 1,2,4-thiadiazole and lubricants containing it as an additive |
| US4137185A (en) | 1977-07-28 | 1979-01-30 | Exxon Research & Engineering Co. | Stabilized imide graft of ethylene copolymeric additives for lubricants |
| US4320019A (en) | 1978-04-17 | 1982-03-16 | The Lubrizol Corporation | Multi-purpose additive compositions and concentrates containing same |
| US4357250A (en) | 1978-04-17 | 1982-11-02 | The Lubrizol Corporation | Nitrogen-containing terpolymer-based compositions useful as multi-purpose lubricant additives |
| US4234435A (en) | 1979-02-23 | 1980-11-18 | The Lubrizol Corporation | Novel carboxylic acid acylating agents, derivatives thereof, concentrate and lubricant compositions containing the same, and processes for their preparation |
| US4340689A (en) | 1979-09-17 | 1982-07-20 | Copolymer Rubber & Chemical Corporation | Method of grafting EPM and EPDM polymers |
| US4382007A (en) | 1981-02-02 | 1983-05-03 | Texaco Inc. | Novel dispersant-VI improvers and lubricating oil containing same |
| US4668834B1 (en) | 1985-10-16 | 1996-05-07 | Uniroyal Chem Co Inc | Low molecular weight ethylene-alphaolefin copolymer intermediates |
| US4668834A (en) | 1985-10-16 | 1987-05-26 | Uniroyal Chemical Company, Inc. | Low molecular weight ethylene-alphaolefin copolymer intermediates |
| US4761482A (en) * | 1987-04-23 | 1988-08-02 | R. T. Vanderbilt Company, Inc. | Terpene derivatives of 2,5-dimercapto-1,3,4-thiadiazoles and lubricating compositions containing same |
| US4863623A (en) | 1988-03-24 | 1989-09-05 | Texaco Inc. | Novel VI improver, dispersant, and anti-oxidant additive and lubricating oil composition containing same |
| US4904403A (en) | 1989-06-12 | 1990-02-27 | R. T. Vanderbilt Company, Inc. | Polyalkylated 1,3,4-thiadiazoles and lubricating compositions containing same |
| US5075383A (en) | 1990-04-11 | 1991-12-24 | Texaco Inc. | Dispersant and antioxidant additive and lubricating oil composition containing same |
| US5460740A (en) * | 1990-12-31 | 1995-10-24 | Texaco Inc. | Acylated mono and/or bis-succinimides lubricating oil additives |
| US5162086A (en) | 1991-05-22 | 1992-11-10 | Texaco Inc. | Dispersant additive and lubricating oil composition containing same |
| US5490864A (en) * | 1991-08-02 | 1996-02-13 | Texaco Inc. | Anti-wear lubricity additive for low-sulfur content diesel fuels |
| US5200102A (en) | 1991-12-02 | 1993-04-06 | Texaco Inc. | Multifunctional olefin copolymer and lubricating oil composition |
| US5188745A (en) | 1991-12-23 | 1993-02-23 | Texaco Inc. | Viton seal compatible dispersant and lubricating oil composition containing same |
| US5474694A (en) | 1992-09-21 | 1995-12-12 | Texaco Inc. | Lubricating oil composition |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2278430A1 (en) | 1998-08-06 |
| DE69816275D1 (en) | 2003-08-14 |
| DE69816275T2 (en) | 2004-05-27 |
| EP0961789B1 (en) | 2003-07-09 |
| WO1998033828A1 (en) | 1998-08-06 |
| EP0961789A1 (en) | 1999-12-08 |
| CA2278430C (en) | 2008-04-01 |
| ATE244732T1 (en) | 2003-07-15 |
| JP3332935B2 (en) | 2002-10-07 |
| US5698500A (en) | 1997-12-16 |
| JP2000508375A (en) | 2000-07-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5075383A (en) | Dispersant and antioxidant additive and lubricating oil composition containing same | |
| US4863623A (en) | Novel VI improver, dispersant, and anti-oxidant additive and lubricating oil composition containing same | |
| US6916767B2 (en) | Antioxidant amines based on n-(4aniliophenyl) amides antioxidant amines based on n-(4-anilinophenyl) amides | |
| EP0922752B1 (en) | Highly grafted, multi-functional olefin copolymer VI modifiers | |
| US5409623A (en) | Functionalized graft co-polymer as a viscosity and index improver, dispersant, and anti-oxidant additive and lubricating oil composition containing same | |
| US5188745A (en) | Viton seal compatible dispersant and lubricating oil composition containing same | |
| US5112508A (en) | VI improver, dispersant, and antioxidant additive and lubricating oil composition | |
| US5429757A (en) | Multifunctional copolymer and lubricating oil composition | |
| US5182041A (en) | Dispersant - anti-oxidant additive and lubricating oil composition containing same | |
| US5474694A (en) | Lubricating oil composition | |
| US6586646B1 (en) | Vinylidene-containing polymers and uses thereof | |
| US5162086A (en) | Dispersant additive and lubricating oil composition containing same | |
| EP0417904B1 (en) | Method for producing dispersant, vi improver, additive for lubricating oil | |
| USRE37105E1 (en) | Lubricants containing ashless antiwear-dispersant additive having viscosity index improver credit | |
| US5094765A (en) | Lubricating oil composition | |
| CA2021959C (en) | Dispersant and anti-oxidant additive and lubricating oil composition containing same | |
| US5167845A (en) | Polymeric additives to enhance anti-wear, anti-oxidancy, and dispersancy in lubricants | |
| US5147569A (en) | Lubricant additive to enhance anti-wear, anti-oxidancy, and dispersancy thereof | |
| EP0396297B1 (en) | Dispersant - anti-oxidant additive and lubricating oil composition containing same | |
| US5200102A (en) | Multifunctional olefin copolymer and lubricating oil composition | |
| EP0461774B1 (en) | Dispersant, antioxidant and VI improver and lubricating oil composition containing same | |
| WO1998039399A1 (en) | Ashless friction modifier with viscosity index improving credit and lubricating oil composition containing same | |
| AU9704398A (en) | Highly grafted, multi-functional olefin copolymer VI modifiers |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| FEPP | Fee payment procedure |
Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| AS | Assignment |
Owner name: CITIBANK, N.A., DELAWARE Free format text: SECURITY AGREEMENT;ASSIGNORS:CHEMTURA CORPORATION;A & M CLEANING PRODUCTS, LLC;AQUA CLEAR INDUSTRIES, LLC;AND OTHERS;REEL/FRAME:022668/0658 Effective date: 20090318 |
|
| FPAY | Fee payment |
Year of fee payment: 12 |
|
| AS | Assignment |
Owner name: CITIBANK, N.A., DELAWARE Free format text: AMENDED AND RESTATED INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNORS:CHEMTURA CORPORATION;A & M CLEANING PRODUCTS, LLC;AQUA CLEAR INDUSTRIES, LLC;AND OTHERS;REEL/FRAME:023998/0001 Effective date: 20100212 Owner name: CITIBANK, N.A.,DELAWARE Free format text: AMENDED AND RESTATED INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNORS:CHEMTURA CORPORATION;A & M CLEANING PRODUCTS, LLC;AQUA CLEAR INDUSTRIES, LLC;AND OTHERS;REEL/FRAME:023998/0001 Effective date: 20100212 |
|
| AS | Assignment |
Owner name: BANK OF AMERICA, N. A., CONNECTICUT Free format text: SECDOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNORS:CHEMTURA CORPORATION;BIOLAB FRANCHISE COMPANY, LLC;BIO-LAB, INC.;AND OTHERS;REEL/FRAME:027881/0347 Effective date: 20101110 Owner name: BANK OF AMERICA, N.A., CONNECTICUT Free format text: FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNORS:CHEMTURA CORPORATION;BIOLAB FRANCHISE COMPANY, LLC;BIO-LAB, INC.;AND OTHERS;REEL/FRAME:026028/0622 Effective date: 20101110 Owner name: BIOLAB FRANCHISE COMPANY, LLC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: WRL OF INDIANA, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: WEBER CITY ROAD LLC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: RECREATIONAL WATER PRODUCTS, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: MONOCHEM, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: UNIROYAL CHEMICAL COMPANY LIMITED (DELAWARE), CONN Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: NAUGATUCK TREATMENT COMPANY, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: LAUREL INDUSTRIES HOLDINGS, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: KEM MANUFACTURING CORPORATION, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: HOMECARE LABS, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: GREAT LAKES CHEMICAL GLOBAL, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: ISCI, INC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: GT SEED TREATMENT, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: GREAT LAKES CHEMICAL CORPORATION, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: GLCC LAUREL, LLC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: CROMPTON HOLDING CORPORATION, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: CNK CHEMICAL REALTY CORPORATION, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: CROMPTON MONOCHEM, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: CROMPTON COLORS INCORPORATED, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: BIOLAB, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: BIOLAB TEXTILES ADDITIVES, LLC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: ASEPSIS, INC., CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: AQUA CLEAR INDUSTRIES, LLC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: BIOLAB COMPANY STORE, LLC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: ASCK, INC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: A & M CLEANING PRODUCTS, LLC, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 Owner name: CHEMTURA CORPORATION, CONNECTICUT Free format text: INTELLECTUAL PROPERTY SECURITY RELEASE AGREEMENT;ASSIGNOR:CITIBANK, N.A.;REEL/FRAME:026039/0142 Effective date: 20101110 |
|
| AS | Assignment |
Owner name: GREAT LAKES CHEMICAL GLOBAL, INC., CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: CROMPTON HOLDING CORPORATION, CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: BIOLAB FRANCHISE COMPANY, LLC, GEORGIA Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: BIO-LAB, INC., CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: WEBER CITY ROAD LLC, CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: RECREATIONAL WATER PRODUCTS, INC., GEORGIA Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: GT SEED TREATMENT, INC., CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: LAUREL INDUSTRIES HOLDINGS, INC., CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: CROMPTON COLORS INCORPORATED, CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: HOMECARE LABS, INC., CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: GREAT LAKES CHEMICAL CORPORATION, CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: CHEMTURA CORPORATION, CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: GLCC LAUREL, LLC, CONNECTICUT Free format text: RELEASE OF SECOND LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042449/0001 Effective date: 20170421 Owner name: WEBER CITY ROAD LLC, CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: CROMPTON HOLDING CORPORATION, CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: HOMECARE LABS, INC., CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: GREAT LAKES CHEMICAL CORPORATION, CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: GT SEED TREATMENT, INC., CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: RECREATIONAL WATER PRODUCTS, INC., GEORGIA Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: LAUREL INDUSTRIES HOLDINGS, INC., CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: BIO-LAB, INC., CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: CROMPTON COLORS INCORPORATED, CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: CHEMTURA CORPORATION, CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: GLCC LAUREL, LLC, CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: BIOLAB FRANCHISE COMPANY, LLC, GEORGIA Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 Owner name: GREAT LAKES CHEMICAL GLOBAL, INC., CONNECTICUT Free format text: RELEASE OF FIRST LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:042447/0508 Effective date: 20170421 |