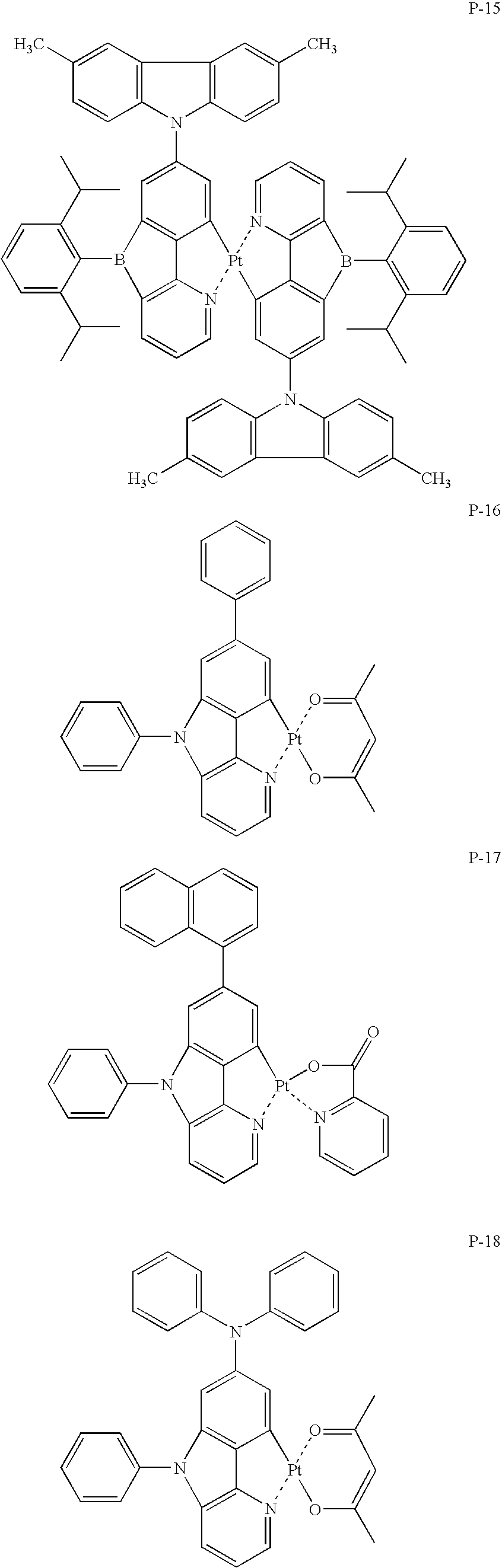
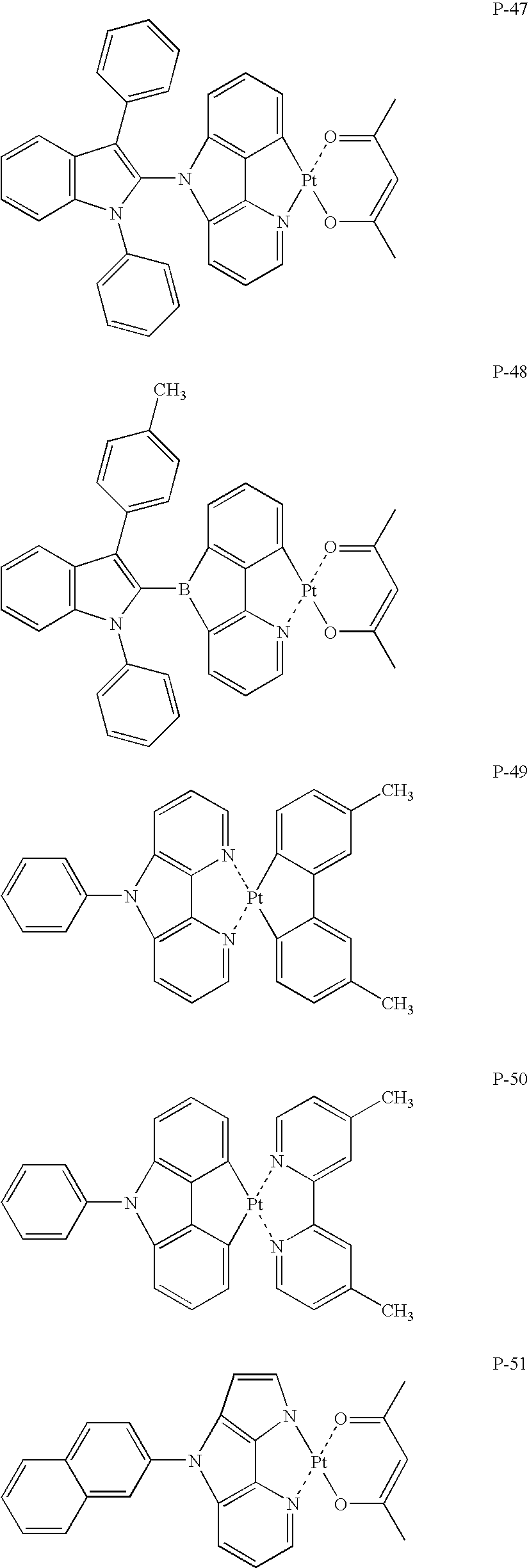
US7790890B2 - Organic electroluminescence element material, organic electroluminescence element, display device and illumination device - Google Patents
Organic electroluminescence element material, organic electroluminescence element, display device and illumination device Download PDFInfo
- Publication number
- US7790890B2 US7790890B2 US10/598,971 US59897104A US7790890B2 US 7790890 B2 US7790890 B2 US 7790890B2 US 59897104 A US59897104 A US 59897104A US 7790890 B2 US7790890 B2 US 7790890B2
- Authority
- US
- United States
- Prior art keywords
- emission
- layer
- organic
- organic electroluminescence
- electroluminescence element
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related, expires
Links
- 239000000463 material Substances 0.000 title claims abstract description 102
- 238000005401 electroluminescence Methods 0.000 title claims abstract description 53
- 238000005286 illumination Methods 0.000 title claims abstract description 25
- 150000004696 coordination complex Chemical class 0.000 claims abstract description 39
- 230000005764 inhibitory process Effects 0.000 claims description 57
- 125000001424 substituent group Chemical group 0.000 claims description 18
- 125000006615 aromatic heterocyclic group Chemical group 0.000 claims description 16
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 16
- 229910052757 nitrogen Inorganic materials 0.000 claims description 14
- 125000002029 aromatic hydrocarbon group Chemical group 0.000 claims description 12
- 239000000470 constituent Substances 0.000 claims description 12
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 12
- 229910052741 iridium Inorganic materials 0.000 claims description 10
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 claims description 10
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical group [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims description 7
- 229910052796 boron Inorganic materials 0.000 claims description 7
- 230000000737 periodic effect Effects 0.000 claims description 7
- 229910052697 platinum Inorganic materials 0.000 claims description 7
- 229910052799 carbon Inorganic materials 0.000 claims description 5
- 125000004432 carbon atom Chemical group C* 0.000 claims description 2
- 239000003446 ligand Substances 0.000 abstract description 25
- 239000010410 layer Substances 0.000 description 257
- 238000000034 method Methods 0.000 description 44
- 150000001875 compounds Chemical class 0.000 description 42
- 239000002019 doping agent Substances 0.000 description 40
- 238000002347 injection Methods 0.000 description 32
- 239000007924 injection Substances 0.000 description 32
- 229910052751 metal Inorganic materials 0.000 description 26
- 239000002184 metal Substances 0.000 description 26
- 230000005525 hole transport Effects 0.000 description 25
- 238000002360 preparation method Methods 0.000 description 23
- 239000000872 buffer Substances 0.000 description 22
- 239000000126 substance Substances 0.000 description 19
- 239000000758 substrate Substances 0.000 description 18
- 230000027756 respiratory electron transport chain Effects 0.000 description 17
- 229910052782 aluminium Inorganic materials 0.000 description 16
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 16
- 238000001704 evaporation Methods 0.000 description 14
- 239000011159 matrix material Substances 0.000 description 14
- 239000000203 mixture Substances 0.000 description 14
- -1 hydroxyethyl group Chemical group 0.000 description 13
- 239000003086 colorant Substances 0.000 description 12
- 230000006870 function Effects 0.000 description 10
- 238000010438 heat treatment Methods 0.000 description 9
- 229910052749 magnesium Inorganic materials 0.000 description 9
- 239000011777 magnesium Substances 0.000 description 9
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 8
- 239000007983 Tris buffer Substances 0.000 description 8
- 230000008020 evaporation Effects 0.000 description 8
- PQXKHYXIUOZZFA-UHFFFAOYSA-M lithium fluoride Chemical compound [Li+].[F-] PQXKHYXIUOZZFA-UHFFFAOYSA-M 0.000 description 8
- 0 [1*]C1cc(c)-c(c)c1C Chemical compound [1*]C1cc(c)-c(c)c1C 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 238000000151 deposition Methods 0.000 description 7
- 230000008021 deposition Effects 0.000 description 7
- 230000003287 optical effect Effects 0.000 description 7
- 229920000642 polymer Polymers 0.000 description 6
- 239000002356 single layer Substances 0.000 description 6
- 230000000694 effects Effects 0.000 description 5
- 239000011521 glass Substances 0.000 description 5
- IBHBKWKFFTZAHE-UHFFFAOYSA-N n-[4-[4-(n-naphthalen-1-ylanilino)phenyl]phenyl]-n-phenylnaphthalen-1-amine Chemical group C1=CC=CC=C1N(C=1C2=CC=CC=C2C=CC=1)C1=CC=C(C=2C=CC(=CC=2)N(C=2C=CC=CC=2)C=2C3=CC=CC=C3C=CC=2)C=C1 IBHBKWKFFTZAHE-UHFFFAOYSA-N 0.000 description 5
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 5
- 238000000059 patterning Methods 0.000 description 5
- 239000011347 resin Substances 0.000 description 5
- 229920005989 resin Polymers 0.000 description 5
- TVIVIEFSHFOWTE-UHFFFAOYSA-K tri(quinolin-8-yloxy)alumane Chemical compound [Al+3].C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1 TVIVIEFSHFOWTE-UHFFFAOYSA-K 0.000 description 5
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical group C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 4
- 229940126062 Compound A Drugs 0.000 description 4
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 4
- QVQLCTNNEUAWMS-UHFFFAOYSA-N barium oxide Chemical compound [Ba]=O QVQLCTNNEUAWMS-UHFFFAOYSA-N 0.000 description 4
- 150000001721 carbon Chemical group 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 229910052738 indium Inorganic materials 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 4
- 238000001771 vacuum deposition Methods 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 3
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 3
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical group C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 3
- 125000000609 carbazolyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 description 3
- 230000001747 exhibiting effect Effects 0.000 description 3
- 229910052731 fluorine Inorganic materials 0.000 description 3
- 125000002883 imidazolyl group Chemical group 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 150000002503 iridium Chemical class 0.000 description 3
- 239000004973 liquid crystal related substance Substances 0.000 description 3
- 229910052744 lithium Inorganic materials 0.000 description 3
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical group C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 3
- 150000004866 oxadiazoles Chemical class 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 125000003226 pyrazolyl group Chemical group 0.000 description 3
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 3
- MCJGNVYPOGVAJF-UHFFFAOYSA-N quinolin-8-ol Chemical class C1=CN=C2C(O)=CC=CC2=C1 MCJGNVYPOGVAJF-UHFFFAOYSA-N 0.000 description 3
- 238000007789 sealing Methods 0.000 description 3
- 238000004528 spin coating Methods 0.000 description 3
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical class C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 238000013519 translation Methods 0.000 description 3
- 150000003852 triazoles Chemical group 0.000 description 3
- 238000007738 vacuum evaporation Methods 0.000 description 3
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical group C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 2
- BCMCBBGGLRIHSE-UHFFFAOYSA-N 1,3-benzoxazole Chemical group C1=CC=C2OC=NC2=C1 BCMCBBGGLRIHSE-UHFFFAOYSA-N 0.000 description 2
- VQGHOUODWALEFC-UHFFFAOYSA-N 2-phenylpyridine Chemical compound C1=CC=CC=C1C1=CC=CC=N1 VQGHOUODWALEFC-UHFFFAOYSA-N 0.000 description 2
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 2
- 229920008347 Cellulose acetate propionate Polymers 0.000 description 2
- DUMSKQUKLVSSII-UHFFFAOYSA-N ICC1CCCC1 Chemical compound ICC1CCCC1 DUMSKQUKLVSSII-UHFFFAOYSA-N 0.000 description 2
- KWYHDKDOAIKMQN-UHFFFAOYSA-N N,N,N',N'-tetramethylethylenediamine Chemical compound CN(C)CCN(C)C KWYHDKDOAIKMQN-UHFFFAOYSA-N 0.000 description 2
- PJANXHGTPQOBST-VAWYXSNFSA-N Stilbene Natural products C=1C=CC=CC=1/C=C/C1=CC=CC=C1 PJANXHGTPQOBST-VAWYXSNFSA-N 0.000 description 2
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical group C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- WZJYKHNJTSNBHV-UHFFFAOYSA-N benzo[h]quinoline Chemical compound C1=CN=C2C3=CC=CC=C3C=CC2=C1 WZJYKHNJTSNBHV-UHFFFAOYSA-N 0.000 description 2
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical group C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 2
- 229920001940 conductive polymer Polymers 0.000 description 2
- 239000004020 conductor Substances 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 229910001873 dinitrogen Inorganic materials 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical group C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 125000001153 fluoro group Chemical group F* 0.000 description 2
- 125000001041 indolyl group Chemical group 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 150000002504 iridium compounds Chemical class 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 229960003540 oxyquinoline Drugs 0.000 description 2
- XNGKCOFXDHYSGR-UHFFFAOYSA-N perillene Chemical compound CC(C)=CCCC=1C=COC=1 XNGKCOFXDHYSGR-UHFFFAOYSA-N 0.000 description 2
- LFSXCDWNBUNEEM-UHFFFAOYSA-N phthalazine Chemical group C1=NN=CC2=CC=CC=C21 LFSXCDWNBUNEEM-UHFFFAOYSA-N 0.000 description 2
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical compound N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 2
- SIOXPEMLGUPBBT-UHFFFAOYSA-N picolinic acid Chemical compound OC(=O)C1=CC=CC=N1 SIOXPEMLGUPBBT-UHFFFAOYSA-N 0.000 description 2
- 229920000139 polyethylene terephthalate Polymers 0.000 description 2
- 239000005020 polyethylene terephthalate Substances 0.000 description 2
- 239000002861 polymer material Substances 0.000 description 2
- 230000000379 polymerizing effect Effects 0.000 description 2
- 229920000123 polythiophene Polymers 0.000 description 2
- LVTJOONKWUXEFR-FZRMHRINSA-N protoneodioscin Natural products O(C[C@@H](CC[C@]1(O)[C@H](C)[C@@H]2[C@]3(C)[C@H]([C@H]4[C@@H]([C@]5(C)C(=CC4)C[C@@H](O[C@@H]4[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@@H](O)[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@H](CO)O4)CC5)CC3)C[C@@H]2O1)C)[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](CO)O1 LVTJOONKWUXEFR-FZRMHRINSA-N 0.000 description 2
- 125000003373 pyrazinyl group Chemical group 0.000 description 2
- 125000005581 pyrene group Chemical group 0.000 description 2
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical group C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 2
- 125000000714 pyrimidinyl group Chemical group 0.000 description 2
- 229910052761 rare earth metal Inorganic materials 0.000 description 2
- 150000002910 rare earth metals Chemical class 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 238000005215 recombination Methods 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- 239000004332 silver Substances 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 235000021286 stilbenes Nutrition 0.000 description 2
- 125000005504 styryl group Chemical group 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 229910052715 tantalum Inorganic materials 0.000 description 2
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 2
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 2
- 238000002834 transmittance Methods 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- OIAQMFOKAXHPNH-UHFFFAOYSA-N 1,2-diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC=C1C1=CC=CC=C1 OIAQMFOKAXHPNH-UHFFFAOYSA-N 0.000 description 1
- YJTKZCDBKVTVBY-UHFFFAOYSA-N 1,3-Diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC(C=2C=CC=CC=2)=C1 YJTKZCDBKVTVBY-UHFFFAOYSA-N 0.000 description 1
- XJKSTNDFUHDPQJ-UHFFFAOYSA-N 1,4-diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=C(C=2C=CC=CC=2)C=C1 XJKSTNDFUHDPQJ-UHFFFAOYSA-N 0.000 description 1
- MVWPVABZQQJTPL-UHFFFAOYSA-N 2,3-diphenylcyclohexa-2,5-diene-1,4-dione Chemical class O=C1C=CC(=O)C(C=2C=CC=CC=2)=C1C1=CC=CC=C1 MVWPVABZQQJTPL-UHFFFAOYSA-N 0.000 description 1
- KJNZQKYSNAQLEO-UHFFFAOYSA-N 2-(4-methylphenyl)pyridine Chemical compound C1=CC(C)=CC=C1C1=CC=CC=N1 KJNZQKYSNAQLEO-UHFFFAOYSA-N 0.000 description 1
- NBYLBWHHTUWMER-UHFFFAOYSA-N 2-Methylquinolin-8-ol Chemical compound C1=CC=C(O)C2=NC(C)=CC=C21 NBYLBWHHTUWMER-UHFFFAOYSA-N 0.000 description 1
- UUNIOFWUJYBVGQ-UHFFFAOYSA-N 2-amino-4-(3,4-dimethoxyphenyl)-10-fluoro-4,5,6,7-tetrahydrobenzo[1,2]cyclohepta[6,7-d]pyran-3-carbonitrile Chemical compound C1=C(OC)C(OC)=CC=C1C1C(C#N)=C(N)OC2=C1CCCC1=CC=C(F)C=C12 UUNIOFWUJYBVGQ-UHFFFAOYSA-N 0.000 description 1
- OGGKVJMNFFSDEV-UHFFFAOYSA-N 3-methyl-n-[4-[4-(n-(3-methylphenyl)anilino)phenyl]phenyl]-n-phenylaniline Chemical compound CC1=CC=CC(N(C=2C=CC=CC=2)C=2C=CC(=CC=2)C=2C=CC(=CC=2)N(C=2C=CC=CC=2)C=2C=C(C)C=CC=2)=C1 OGGKVJMNFFSDEV-UHFFFAOYSA-N 0.000 description 1
- YXYUIABODWXVIK-UHFFFAOYSA-N 4-methyl-n,n-bis(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 YXYUIABODWXVIK-UHFFFAOYSA-N 0.000 description 1
- MEIBOBDKQKIBJH-UHFFFAOYSA-N 4-methyl-n-[4-[1-[4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]-4-phenylcyclohexyl]phenyl]-n-(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)C1(CCC(CC1)C=1C=CC=CC=1)C=1C=CC(=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 MEIBOBDKQKIBJH-UHFFFAOYSA-N 0.000 description 1
- ZOKIJILZFXPFTO-UHFFFAOYSA-N 4-methyl-n-[4-[1-[4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]cyclohexyl]phenyl]-n-(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)C1(CCCCC1)C=1C=CC(=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 ZOKIJILZFXPFTO-UHFFFAOYSA-N 0.000 description 1
- DUSWRTUHJVJVRY-UHFFFAOYSA-N 4-methyl-n-[4-[2-[4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]propan-2-yl]phenyl]-n-(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)C(C)(C)C=1C=CC(=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 DUSWRTUHJVJVRY-UHFFFAOYSA-N 0.000 description 1
- MVIXNQZIMMIGEL-UHFFFAOYSA-N 4-methyl-n-[4-[4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]phenyl]-n-(4-methylphenyl)aniline Chemical group C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)C=1C=CC(=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 MVIXNQZIMMIGEL-UHFFFAOYSA-N 0.000 description 1
- XIQGFRHAIQHZBD-UHFFFAOYSA-N 4-methyl-n-[4-[[4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]-phenylmethyl]phenyl]-n-(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)C(C=1C=CC=CC=1)C=1C=CC(=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 XIQGFRHAIQHZBD-UHFFFAOYSA-N 0.000 description 1
- ZDASUJMDVPTNTF-UHFFFAOYSA-N 5,7-dibromo-8-quinolinol Chemical compound C1=CN=C2C(O)=C(Br)C=C(Br)C2=C1 ZDASUJMDVPTNTF-UHFFFAOYSA-N 0.000 description 1
- ZYASLTYCYTYKFC-UHFFFAOYSA-N 9-methylidenefluorene Chemical class C1=CC=C2C(=C)C3=CC=CC=C3C2=C1 ZYASLTYCYTYKFC-UHFFFAOYSA-N 0.000 description 1
- VIJYEGDOKCKUOL-UHFFFAOYSA-N 9-phenylcarbazole Chemical compound C1=CC=CC=C1N1C2=CC=CC=C2C2=CC=CC=C21 VIJYEGDOKCKUOL-UHFFFAOYSA-N 0.000 description 1
- VVJKKWFAADXIJK-UHFFFAOYSA-N Allylamine Chemical class NCC=C VVJKKWFAADXIJK-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- XNDNELPGIFCKHY-MGCZUKKZSA-M C1=C2CCCN3C4=C5C(=C23)N(=C1)[Ir]/C5=C/C=C\4.CC1=CC(C)=O[Ir]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CCN1C2=C3C4=C1/C=C(C)\C=C/4[Ir]N3=CC=C2.CCN1C2=C3C4=C1/C=C(C1=CC=CC=C1)\C=C/4[Ir]N3=CC=C2.CCN1C2=C3C4=C1/C=N\C=C/4[Ir]N3=CC=C2 Chemical compound C1=C2CCCN3C4=C5C(=C23)N(=C1)[Ir]/C5=C/C=C\4.CC1=CC(C)=O[Ir]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CCN1C2=C3C4=C1/C=C(C)\C=C/4[Ir]N3=CC=C2.CCN1C2=C3C4=C1/C=C(C1=CC=CC=C1)\C=C/4[Ir]N3=CC=C2.CCN1C2=C3C4=C1/C=N\C=C/4[Ir]N3=CC=C2 XNDNELPGIFCKHY-MGCZUKKZSA-M 0.000 description 1
- SFNWVXBROWNOHM-UHFFFAOYSA-N C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=N3)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=N3)=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CCC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.CC(C)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C1=CC=C(C(C)(C)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.CC(C)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.CC1=C(C2=C(C)C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.FC(F)(F)C(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C(F)(F)F Chemical compound C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=N3)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=N3)=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CCC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.CC(C)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C1=CC=C(C(C)(C)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.CC(C)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.CC1=C(C2=C(C)C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.FC(F)(F)C(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C(F)(F)F SFNWVXBROWNOHM-UHFFFAOYSA-N 0.000 description 1
- MEQNJBNULKXGHG-PSBMRQRRSA-J C1=CC(N2C3=C4C(=CC=C3)[Ir]N3=CC=CC2=C43)=CN=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=O[Ir]3(O2)C2=NC=CC4=C2C2=C(C=CC=N23)N4C2=CC=CC=C2)C=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)B3C1=C2C=CC=CC2=CC2=C1C=CC=C2.CC1=CC(C)=O[Ir]2(O1)C1=NC=CC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CCN1C2=C3C(=CC=C2)[Ir]N2=CC=CC1=C32.O=C1O[Ir@@]2(C3=CN=CC4=C3C3=C(C=CC=N32)B4C2=C(C3=CC=CC=C3)C=CC=C2C2=CC=CC=C2)N2=C1C=CC=C2 Chemical compound C1=CC(N2C3=C4C(=CC=C3)[Ir]N3=CC=CC2=C43)=CN=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=O[Ir]3(O2)C2=NC=CC4=C2C2=C(C=CC=N23)N4C2=CC=CC=C2)C=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)B3C1=C2C=CC=CC2=CC2=C1C=CC=C2.CC1=CC(C)=O[Ir]2(O1)C1=NC=CC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CCN1C2=C3C(=CC=C2)[Ir]N2=CC=CC1=C32.O=C1O[Ir@@]2(C3=CN=CC4=C3C3=C(C=CC=N32)B4C2=C(C3=CC=CC=C3)C=CC=C2C2=CC=CC=C2)N2=C1C=CC=C2 MEQNJBNULKXGHG-PSBMRQRRSA-J 0.000 description 1
- CVKYNQAZUUMWTA-RZKGLWFWSA-K C1=CC(N2C3=C4C(=CC=C3)[Pt]3(C5=C\C=C/C6=C\5C5=C(C=CC=N53)N6C3=CN=CC=C3)N3=CC=CC2=C43)=CN=C1.C1=CC2=CC=CC(N3C4=C5C6=C3/C=C\C=C\6[Pt]3(C6=CC=CC7=C6C6=C(C=CC=N63)N7C3=CC=CC6=C3C=CC=C6)N5=CC=C4)=C2C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=O[Pt]3(O2)C2=CC=CC4=C2C2=C(C=CC=N23)N4C2=CC=CC=C2)C=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=NC=CC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CCN1C2=C3C(=CC=C2)[Pt]2(C4=C\C=C/C5=C\4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32 Chemical compound C1=CC(N2C3=C4C(=CC=C3)[Pt]3(C5=C\C=C/C6=C\5C5=C(C=CC=N53)N6C3=CN=CC=C3)N3=CC=CC2=C43)=CN=C1.C1=CC2=CC=CC(N3C4=C5C6=C3/C=C\C=C\6[Pt]3(C6=CC=CC7=C6C6=C(C=CC=N63)N7C3=CC=CC6=C3C=CC=C6)N5=CC=C4)=C2C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=O[Pt]3(O2)C2=CC=CC4=C2C2=C(C=CC=N23)N4C2=CC=CC=C2)C=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=NC=CC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CCN1C2=C3C(=CC=C2)[Pt]2(C4=C\C=C/C5=C\4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32 CVKYNQAZUUMWTA-RZKGLWFWSA-K 0.000 description 1
- RUVUSGZNSDBCFA-SOMAOQDOSA-I C1=CC2=C(C=C1)C(N1C3=C4C(=CC=C3)[Ir]N3=CC=CC1=C43)=CC=C2.CC1=CC(C)=O[Ir]2(O1)C1=CC(N3C4=CC=CC=C4C4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=NC=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.O=C1O[Ir@@]2(C3=CN=CC4=C3C3=C(C=CC=N32)N4C2=CC=CC=C2)N2=C1C=CC=C2 Chemical compound C1=CC2=C(C=C1)C(N1C3=C4C(=CC=C3)[Ir]N3=CC=CC1=C43)=CC=C2.CC1=CC(C)=O[Ir]2(O1)C1=CC(N3C4=CC=CC=C4C4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=NC=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.O=C1O[Ir@@]2(C3=CN=CC4=C3C3=C(C=CC=N32)N4C2=CC=CC=C2)N2=C1C=CC=C2 RUVUSGZNSDBCFA-SOMAOQDOSA-I 0.000 description 1
- COHCFZROOYKROU-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)CCCCC3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(OC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(OC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CN=C5)C=C3)C=C1)C1=C2C=NC=C1.C1=CC2=C(C=C1)N(C1=CC=C(SC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(SC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CN=C5)C=C3)C=C1)C1=C2C=NC=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(CC4=CC=C(N5C6=C(C=C(C7=CC=CC=C7)C=C6)C6=C5C=CC(C5=CC=CC=C5)=C6)C=C4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.CC1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(N4C5=C(C=C(C)C=C5)C5=C4C=CC(C)=C5)C=C3)C=C1)C1=C2C=C(C)C=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)CCCCC3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(OC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(OC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CN=C5)C=C3)C=C1)C1=C2C=NC=C1.C1=CC2=C(C=C1)N(C1=CC=C(SC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(SC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CN=C5)C=C3)C=C1)C1=C2C=NC=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(CC4=CC=C(N5C6=C(C=C(C7=CC=CC=C7)C=C6)C6=C5C=CC(C5=CC=CC=C5)=C6)C=C4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.CC1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(N4C5=C(C=C(C)C=C5)C5=C4C=CC(C)=C5)C=C3)C=C1)C1=C2C=C(C)C=C1 COHCFZROOYKROU-UHFFFAOYSA-N 0.000 description 1
- AEDKTULHFIKTSW-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=N5)C=C3)C=C1)C1=C2N=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=NC=C5)C=C3)C=C1)C1=C2C=CN=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4N=CC=C5)C=C3)C=C1)C1=C2C=CC=N1.C1=CC2=C(C=N1)C1=C(C=CN=C1)N2C1=CC=C(C2=CC=C(CC3=CC=C(C4=CC=C(N5C6=C(C=NC=C6)C6=C5C=CN=C6)C=C4)C=C3)C=C2)C=C1.C1=CC2=C(C=N1)C1=C(C=CN=C1)N2C1=CC=C(C2=CC=C(N3C4=C(C=NC=C4)C4=C3C=CN=C4)C=C2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC=C(C2(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)CCC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)CC2)C=C1.CC(C)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CN=C3)C=C1)C1=CC=C(C(C)(C)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CN=C4)C=C2)C=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=N5)C=C3)C=C1)C1=C2N=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=NC=C5)C=C3)C=C1)C1=C2C=CN=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4N=CC=C5)C=C3)C=C1)C1=C2C=CC=N1.C1=CC2=C(C=N1)C1=C(C=CN=C1)N2C1=CC=C(C2=CC=C(CC3=CC=C(C4=CC=C(N5C6=C(C=NC=C6)C6=C5C=CN=C6)C=C4)C=C3)C=C2)C=C1.C1=CC2=C(C=N1)C1=C(C=CN=C1)N2C1=CC=C(C2=CC=C(N3C4=C(C=NC=C4)C4=C3C=CN=C4)C=C2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC=C(C2(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)CCC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)CC2)C=C1.CC(C)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CN=C3)C=C1)C1=CC=C(C(C)(C)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CN=C4)C=C2)C=C1 AEDKTULHFIKTSW-UHFFFAOYSA-N 0.000 description 1
- NGPJUNHOPKMYEB-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CN=C5)C=C3)C=C1)C1=C2C=NC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=N5)C=C3)C=C1)C1=C2N=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=NC=C5)C=C3)C=C1)C1=C2C=CN=C1.C1=CC2=C(C=N1)C1=C(C=CN=C1)N2C1=CC=C(C2=CC=C(N3C4=C(C=NC=C4)C4=C3C=CN=C4)C=C2)C=C1.FC(F)(F)C(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CN=C3)C=C1)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CN=C3)C=C1)C(F)(F)F Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CN=C5)C=C3)C=C1)C1=C2C=NC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=N5)C=C3)C=C1)C1=C2N=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(CC3=CC=C(N4C5=C(C=CC=C5)C5=C4C=NC=C5)C=C3)C=C1)C1=C2C=CN=C1.C1=CC2=C(C=N1)C1=C(C=CN=C1)N2C1=CC=C(C2=CC=C(N3C4=C(C=NC=C4)C4=C3C=CN=C4)C=C2)C=C1.FC(F)(F)C(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CN=C3)C=C1)(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CN=C3)C=C1)C(F)(F)F NGPJUNHOPKMYEB-UHFFFAOYSA-N 0.000 description 1
- XRYLQJNUTPHIGW-DPDCUOCVSA-L C1=CC2=C3C(=C1)[Pt]1(C4=C\C=C/C5=C\4C4=C6C(=CC=N41)CCCN65)N1=CC=C4CCCN2C4=C31.CC1=CC(C)=O[Pt]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CC1=CC=C(N2C3=C4C5=C2/C=C\C=C/5[Pt]2(OC(C)=CC(C)=O2)N4=CC=C3)C=C1.CCN1C2=C3C(=CC(C)=C2)[Pt]2(/C4=C/C(C)=C\C5=C4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32.CCN1C2=C3C(=CC(C4=CC=CC=C4)=C2)[Pt]2(/C4=C/C(C5=CC=CC=C5)=C\C5=C4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32 Chemical compound C1=CC2=C3C(=C1)[Pt]1(C4=C\C=C/C5=C\4C4=C6C(=CC=N41)CCCN65)N1=CC=C4CCCN2C4=C31.CC1=CC(C)=O[Pt]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CC1=CC=C(N2C3=C4C5=C2/C=C\C=C/5[Pt]2(OC(C)=CC(C)=O2)N4=CC=C3)C=C1.CCN1C2=C3C(=CC(C)=C2)[Pt]2(/C4=C/C(C)=C\C5=C4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32.CCN1C2=C3C(=CC(C4=CC=CC=C4)=C2)[Pt]2(/C4=C/C(C5=CC=CC=C5)=C\C5=C4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32 XRYLQJNUTPHIGW-DPDCUOCVSA-L 0.000 description 1
- GHWCGFWHQOEOSG-UHFFFAOYSA-N C1=CC2=C3C4=C(C=CC=C4C=C2)[Ir]24(C5=C6C(=CC=C5)C=CC5=C6N2=CC=C5)(/C2=C/C=C\C5=C2C2=C(C=C5)/C=C\C=N/24)N3=C1.C1=CC=N2C(=C1)C1=C(C=CC=C1)[Ir]213(C2=C(C=CC=C2)C2=CC=CC=N21)C1=C(C=CC=C1)C1=C/C=C/C=N\13.CC1=CC2=C(C=C1)C1=CC=CC=N1[Ir]213(C2=C(C=CC(C)=C2)C2=CC=CC=N21)C1=C(C=CC(C)=C1)C1=C/C=C/C=N\13.CC1CC(C)O[Ir]2(O1)C1=C(SC=C1)C1=CC=CC=N12.CCCC[PH](CCCC)(CCCC)[Ir]12(Cl)(C3=C4C(=CC=C3)C=CC3=C4N1=CC=C3)C1=C3\C4=C(C=CC=N42)C=C\C3=C\C=C\1 Chemical compound C1=CC2=C3C4=C(C=CC=C4C=C2)[Ir]24(C5=C6C(=CC=C5)C=CC5=C6N2=CC=C5)(/C2=C/C=C\C5=C2C2=C(C=C5)/C=C\C=N/24)N3=C1.C1=CC=N2C(=C1)C1=C(C=CC=C1)[Ir]213(C2=C(C=CC=C2)C2=CC=CC=N21)C1=C(C=CC=C1)C1=C/C=C/C=N\13.CC1=CC2=C(C=C1)C1=CC=CC=N1[Ir]213(C2=C(C=CC(C)=C2)C2=CC=CC=N21)C1=C(C=CC(C)=C1)C1=C/C=C/C=N\13.CC1CC(C)O[Ir]2(O1)C1=C(SC=C1)C1=CC=CC=N12.CCCC[PH](CCCC)(CCCC)[Ir]12(Cl)(C3=C4C(=CC=C3)C=CC3=C4N1=CC=C3)C1=C3\C4=C(C=CC=N42)C=C\C3=C\C=C\1 GHWCGFWHQOEOSG-UHFFFAOYSA-N 0.000 description 1
- SDQFBOIZTPGIKS-UHFFFAOYSA-K C1=CC2=CC=CC3=C2N(=C1)[Al]12(O3)(OC3=C4C(=CC=C3)/C=C\C=N/41)OC1=C3C(=CC=C1)/C=C\C=N/32.C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=C5C=CC=CC5=CC=C4)C=C3)C=C2)C2=CC=CC3=C2C=CC=C3)C=C1.C1=CC=C(N2C3=CC=CC=C3[Ir]N3=CC=CC=C23)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC=C(C2=CC=C(N3C4=CC=CC=C4C4=C3C=CC=C4)C=C2)C=C1.CC1(C)C2=CC=CC=C2[Ir]N2=CC=CC=C21.CC1=NC2=C(C=CC3=C2N=C(C)C=C3C2=CC=CC=C2)C(C2=CC=CC=C2)=C1 Chemical compound C1=CC2=CC=CC3=C2N(=C1)[Al]12(O3)(OC3=C4C(=CC=C3)/C=C\C=N/41)OC1=C3C(=CC=C1)/C=C\C=N/32.C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=C5C=CC=CC5=CC=C4)C=C3)C=C2)C2=CC=CC3=C2C=CC=C3)C=C1.C1=CC=C(N2C3=CC=CC=C3[Ir]N3=CC=CC=C23)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC=C(C2=CC=C(N3C4=CC=CC=C4C4=C3C=CC=C4)C=C2)C=C1.CC1(C)C2=CC=CC=C2[Ir]N2=CC=CC=C21.CC1=NC2=C(C=CC3=C2N=C(C)C=C3C2=CC=CC=C2)C(C2=CC=CC=C2)=C1 SDQFBOIZTPGIKS-UHFFFAOYSA-K 0.000 description 1
- CCBYMVUEBYHLQH-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC4=C5C(=C3)[Ir]N3=CC=CC(=C53)N4C3=CC=CC=C3)=C2)C=C1.CCN1C2=C3C(=CC(N4C5=CC=CC=C5C5=C4C=CC=C5)=C2)[Pt]2(/C4=C/C(N5C6=CC=CC=C6C6=C5C=CC=C6)=C\C5=C4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32.CCN1C2=C3C(=CN=C2)[Pt]2(/C4=C/N=C\C5=C4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32.CCN1C2=C3C4=C1/C=C(C1=CC=CC=C1)\C=C/4[Pt]1(N3=CC=C2)N2=CC=CN2B(N2C=CC=N2)(N2C=CC=N2)N2C=CC=N21 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC4=C5C(=C3)[Ir]N3=CC=CC(=C53)N4C3=CC=CC=C3)=C2)C=C1.CCN1C2=C3C(=CC(N4C5=CC=CC=C5C5=C4C=CC=C5)=C2)[Pt]2(/C4=C/C(N5C6=CC=CC=C6C6=C5C=CC=C6)=C\C5=C4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32.CCN1C2=C3C(=CN=C2)[Pt]2(/C4=C/N=C\C5=C4C4=C(C=CC=N42)N5CC)N2=CC=CC1=C32.CCN1C2=C3C4=C1/C=C(C1=CC=CC=C1)\C=C/4[Pt]1(N3=CC=C2)N2=CC=CN2B(N2C=CC=N2)(N2C=CC=N2)N2C=CC=N21 CCBYMVUEBYHLQH-UHFFFAOYSA-N 0.000 description 1
- GHAPMCNNFHCELJ-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC4=C5C(=C3)[Pt]3(C6=CC(C7=CC(C8=CC=CC=C8)=CC(C8=CC=CC=C8)=C7)=CC7=C6C6=C(C=CC=N63)N7C3=CC=CC=C3)N3=CC=CC(=C53)N4C3=CC=CC=C3)=C2)C=C1.C1=CC=C(N2C3=C4C(=CC=C3)[Pt]3(C5=CC=CC6=C5C5=C(C=CC=N53)N6C3=CC=CC=C3)N3=CC=CC2=C43)C=C1.CC1=CC2=C3C(=C1)[Pt]1(C4=CC(C)=CC5=C4C4=C(C=CC=N41)N5C1=CC=CC=C1)N1=CC=CC(=C31)N2C1=CC=CC=C1.FC(F)(F)C1=CC(C(F)(F)F)=C2C3=C1N(C1=CC=CC=C1)C1=C3N(=CC=C1)[Pt]21C2=C(C(F)(F)F)C=C(C(F)(F)F)C3=C2C2=C(C=CC=N21)N3C1=CC=CC=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC4=C5C(=C3)[Pt]3(C6=CC(C7=CC(C8=CC=CC=C8)=CC(C8=CC=CC=C8)=C7)=CC7=C6C6=C(C=CC=N63)N7C3=CC=CC=C3)N3=CC=CC(=C53)N4C3=CC=CC=C3)=C2)C=C1.C1=CC=C(N2C3=C4C(=CC=C3)[Pt]3(C5=CC=CC6=C5C5=C(C=CC=N53)N6C3=CC=CC=C3)N3=CC=CC2=C43)C=C1.CC1=CC2=C3C(=C1)[Pt]1(C4=CC(C)=CC5=C4C4=C(C=CC=N41)N5C1=CC=CC=C1)N1=CC=CC(=C31)N2C1=CC=CC=C1.FC(F)(F)C1=CC(C(F)(F)F)=C2C3=C1N(C1=CC=CC=C1)C1=C3N(=CC=C1)[Pt]21C2=C(C(F)(F)F)C=C(C(F)(F)F)C3=C2C2=C(C=CC=N21)N3C1=CC=CC=C1 GHAPMCNNFHCELJ-UHFFFAOYSA-N 0.000 description 1
- VTKZUCAQMQSZTB-UHFFFAOYSA-N C1=CC=C(C2=CC3=C4C(=C2)[Ir]N2=CC=CC(=C42)B3C2=C(C3=CC=CC=C3)C=CC=C2C2=CC=CC=C2)C=C1.CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(C4=CC=CC5=C4C=CC=C5)=C2)[Ir]N2=CC=CC1=C32.CC1=CC(C)=C(C2=CC3=C4C(=C2)[Ir]N2=CC=CC(=C42)B3C2=C(C(C)C)C=CC=C2C(C)C)C(C)=C1.CC1=CC2=C3C(=C1)[Ir]N1=CC=CC(=C31)B2C1=C(C)C=CC=C1C Chemical compound C1=CC=C(C2=CC3=C4C(=C2)[Ir]N2=CC=CC(=C42)B3C2=C(C3=CC=CC=C3)C=CC=C2C2=CC=CC=C2)C=C1.CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(C4=CC=CC5=C4C=CC=C5)=C2)[Ir]N2=CC=CC1=C32.CC1=CC(C)=C(C2=CC3=C4C(=C2)[Ir]N2=CC=CC(=C42)B3C2=C(C(C)C)C=CC=C2C(C)C)C(C)=C1.CC1=CC2=C3C(=C1)[Ir]N1=CC=CC(=C31)B2C1=C(C)C=CC=C1C VTKZUCAQMQSZTB-UHFFFAOYSA-N 0.000 description 1
- MJFTWPOJMUSUNA-UHFFFAOYSA-N C1=CC=C(C2=CC3=C4C(=C2)[Pt]2(C5=CC(C6=CC=CC=C6)=CC6=C5C5=C(C=CC=N52)B6C2=C(C5=CC=CC=C5)C=CC=C2C2=CC=CC=C2)N2=CC=CC(=C42)B3C2=C(C3=CC=CC=C3)C=CC=C2C2=CC=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C4C(=C2)[Pt]2(C5=CC(N(C6=CC=CC=C6)C6=CC=CC=C6)=CC6=C5C5=C(C=CC=N52)N6C2=CC=CC=C2)N2=CC=CC(=C42)N3C2=CC=CC=C2)C=C1.C1=CC=C(N2C3=C4C(=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=C3)[Pt]3(C5=CC(N6C7=CC=CC=C7C7=C6C=CC=C7)=CC6=C5C5=C(C=CC=N53)N6C3=CC=CC=C3)N3=CC=CC2=C43)C=C1 Chemical compound C1=CC=C(C2=CC3=C4C(=C2)[Pt]2(C5=CC(C6=CC=CC=C6)=CC6=C5C5=C(C=CC=N52)B6C2=C(C5=CC=CC=C5)C=CC=C2C2=CC=CC=C2)N2=CC=CC(=C42)B3C2=C(C3=CC=CC=C3)C=CC=C2C2=CC=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C4C(=C2)[Pt]2(C5=CC(N(C6=CC=CC=C6)C6=CC=CC=C6)=CC6=C5C5=C(C=CC=N52)N6C2=CC=CC=C2)N2=CC=CC(=C42)N3C2=CC=CC=C2)C=C1.C1=CC=C(N2C3=C4C(=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=C3)[Pt]3(C5=CC(N6C7=CC=CC=C7C7=C6C=CC=C7)=CC6=C5C5=C(C=CC=N53)N6C3=CC=CC=C3)N3=CC=CC2=C43)C=C1 MJFTWPOJMUSUNA-UHFFFAOYSA-N 0.000 description 1
- RSWAKLUEAJDNSY-UHFFFAOYSA-N C1=CC=C(C2=CC=CC(C3=CC=CC=C3)=C2B2C3=C4C5=C2/C=C2/SC6=C(C=CC=C6)/C2=C/5[Ir]N4=CC=C3)C=C1.CCN1C2=C3C4=C1/C=C1/SC5=C(C=CC=C5)/C1=C/4[Ir]N3=CC=C2 Chemical compound C1=CC=C(C2=CC=CC(C3=CC=CC=C3)=C2B2C3=C4C5=C2/C=C2/SC6=C(C=CC=C6)/C2=C/5[Ir]N4=CC=C3)C=C1.CCN1C2=C3C4=C1/C=C1/SC5=C(C=CC=C5)/C1=C/4[Ir]N3=CC=C2 RSWAKLUEAJDNSY-UHFFFAOYSA-N 0.000 description 1
- ZXAKKKUCVLJPLI-RKFQPGFBSA-P C1=CC=C(C2=CC=N3C(=C2)C2=N(C=CC(C4=CC=CC=C4)=C2)[Os]32[PH](C3=CC=CC=C3)(C3=CC=CC=C3)C=C[PH]2(C2=CC=CC=C2)C2=CC=CC=C2)C=C1 Chemical compound C1=CC=C(C2=CC=N3C(=C2)C2=N(C=CC(C4=CC=CC=C4)=C2)[Os]32[PH](C3=CC=CC=C3)(C3=CC=CC=C3)C=C[PH]2(C2=CC=CC=C2)C2=CC=CC=C2)C=C1 ZXAKKKUCVLJPLI-RKFQPGFBSA-P 0.000 description 1
- QLLAEZLQZRJNIJ-UHFFFAOYSA-N C1=CC=C(C2CC(C3=CC=CC=C3)O[Ir]3(O2)C2=C(SC4=C2C=CC=C4)C2=CC=CC=N23)C=C1.CC1CC(C)O[Ir]2(O1)C1=C(/C=C/C=C/1)C1=CC=CC=N12.CC1CC(C)O[Ir]2(O1)C1=C(C3=CC=CC=N32)\C(F)=C/C(F)=C\1.CC1CC(C)O[Ir]2(O1)C1=C(SC3=C1C=CC=C3)C1=CC=CC=N12.CC1CC(C)O[Ir]2(O1)C1=C(SC3=C1C=CC=C3)C1=CC=CC=N12 Chemical compound C1=CC=C(C2CC(C3=CC=CC=C3)O[Ir]3(O2)C2=C(SC4=C2C=CC=C4)C2=CC=CC=N23)C=C1.CC1CC(C)O[Ir]2(O1)C1=C(/C=C/C=C/1)C1=CC=CC=N12.CC1CC(C)O[Ir]2(O1)C1=C(C3=CC=CC=N32)\C(F)=C/C(F)=C\1.CC1CC(C)O[Ir]2(O1)C1=C(SC3=C1C=CC=C3)C1=CC=CC=N12.CC1CC(C)O[Ir]2(O1)C1=C(SC3=C1C=CC=C3)C1=CC=CC=N12 QLLAEZLQZRJNIJ-UHFFFAOYSA-N 0.000 description 1
- QRLZUZRZBSQPMH-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C4C(=C2)[Ir]N2=CC=CC(=C42)N3C2=CC=CC=C2)C=C1.C1=CC=C(N2C3=C4C(=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=C3)[Ir]N3=CC=CC2=C43)C=C1.C1=CC=C(N2C3=C4C(=CC=C3)[Ir]N3=CC=CC2=C43)C=C1.CC1=CC2=C3C(=C1)[Ir]N1=CC=CC(=C31)N2C1=CC=CC=C1.FC(F)(F)C1=CC(C(F)(F)F)=C2[Ir]N3=CC=CC4=C3C2=C1N4C1=CC=CC=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C4C(=C2)[Ir]N2=CC=CC(=C42)N3C2=CC=CC=C2)C=C1.C1=CC=C(N2C3=C4C(=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=C3)[Ir]N3=CC=CC2=C43)C=C1.C1=CC=C(N2C3=C4C(=CC=C3)[Ir]N3=CC=CC2=C43)C=C1.CC1=CC2=C3C(=C1)[Ir]N1=CC=CC(=C31)N2C1=CC=CC=C1.FC(F)(F)C1=CC(C(F)(F)F)=C2[Ir]N3=CC=CC4=C3C2=C1N4C1=CC=CC=C1 QRLZUZRZBSQPMH-UHFFFAOYSA-N 0.000 description 1
- FGEPMVCWQPFXGW-YYEDWNSSSA-J C1=CC=C(N2C3=C4C(=CC=C3)[Ir]3(C5=CC=CC2=C54)N2=CC=CC=C2C2=CC=CC=N23)C=C1.C1=CC=C([P+](C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.C1=CC=C([P+](C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC1=CC(C)=O[Ir]2(O1)N1/C=C\C3=C1C1=C(/C=C\C=N/12)N3C1=CC=C2C=CC=CC2=C1.CC1=CC(C)=O[Ir]2(O1)N1C3=C(/C=C\1C)N(C1=CC=CC=C1)C1=C3/N2=C\C=C/1.CC1=CC=C(C2=C(B3C4=C5C(=CC=C4)[Ir]4(OC(C)=CC(C)=O4)N4=CC=CC3=C54)N(C3=CC=CC=C3)C3=C2C=CC=C3)C=C1.CC1=CC=C2C(=C1)C1=CC(C)=CC=C1[Ir]21N2=CC=CC3=C2C2=C(C=CC=N21)N3C1=CC=CC=C1.CC1=CC=N2C(=C1)C1=CC(C)=CC=N1[Ir]21C2=CC=CC3=C2C2=C(C=CC=C21)N3C1=CC=CC=C1.[Cl-] Chemical compound C1=CC=C(N2C3=C4C(=CC=C3)[Ir]3(C5=CC=CC2=C54)N2=CC=CC=C2C2=CC=CC=N23)C=C1.C1=CC=C([P+](C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.C1=CC=C([P+](C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC1=CC(C)=O[Ir]2(O1)N1/C=C\C3=C1C1=C(/C=C\C=N/12)N3C1=CC=C2C=CC=CC2=C1.CC1=CC(C)=O[Ir]2(O1)N1C3=C(/C=C\1C)N(C1=CC=CC=C1)C1=C3/N2=C\C=C/1.CC1=CC=C(C2=C(B3C4=C5C(=CC=C4)[Ir]4(OC(C)=CC(C)=O4)N4=CC=CC3=C54)N(C3=CC=CC=C3)C3=C2C=CC=C3)C=C1.CC1=CC=C2C(=C1)C1=CC(C)=CC=C1[Ir]21N2=CC=CC3=C2C2=C(C=CC=N21)N3C1=CC=CC=C1.CC1=CC=N2C(=C1)C1=CC(C)=CC=N1[Ir]21C2=CC=CC3=C2C2=C(C=CC=C21)N3C1=CC=CC=C1.[Cl-] FGEPMVCWQPFXGW-YYEDWNSSSA-J 0.000 description 1
- JGGFEPTZOYRAHP-RIRCKPMCSA-J C1=CC=C(N2C3=C4C(=CC=C3)[Ir]3(C5=CC=CC2=C54)N2=CC=CC=C2C2=CC=CC=N23)C=C1.C1=CC=C([P+](C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C4=C1/C=C\C=N/4[Ir]1(C4=CC=CC=C4C4=CC=CC=C41)N3=CC=C2.CC1=CC(C)=O[Ir]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)B3C1=CC(C2=CC=CC=C2)=CC(C2=CC=CC=C2)=C1.CC1=CC(C)=O[Ir]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)N1/C=C\C3=C1C1=C(/C=C\C=N/12)N3C1=CC=CC=C1.CC1=CC=C(N2C3=C4C5=C2/C=C\C=C/5[Ir]2(OC(C)=CC(C)=O2)N4=CC=C3)C=C1 Chemical compound C1=CC=C(N2C3=C4C(=CC=C3)[Ir]3(C5=CC=CC2=C54)N2=CC=CC=C2C2=CC=CC=N23)C=C1.C1=CC=C([P+](C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C4=C1/C=C\C=N/4[Ir]1(C4=CC=CC=C4C4=CC=CC=C41)N3=CC=C2.CC1=CC(C)=O[Ir]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)B3C1=CC(C2=CC=CC=C2)=CC(C2=CC=CC=C2)=C1.CC1=CC(C)=O[Ir]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)N1/C=C\C3=C1C1=C(/C=C\C=N/12)N3C1=CC=CC=C1.CC1=CC=C(N2C3=C4C5=C2/C=C\C=C/5[Ir]2(OC(C)=CC(C)=O2)N4=CC=C3)C=C1 JGGFEPTZOYRAHP-RIRCKPMCSA-J 0.000 description 1
- VJHVCRYNCFHKAZ-YQRUSNOGSA-J C1=CC=C(N2C3=C4C(=CC=C3)[Pt]3(C5=CC=CC2=C54)N2=CC=CC=C2C2=CC=CC=N23)C=C1.C1=CC=C(N2C3=C4C(=CC=C3)[Pt]3(C5=CC=CC2=C54)N2=CC=CC=C2C2=CC=CC=N23)C=C1.CC(C)C(C)(B1C2=C3C4=C1/C=C\C=N/4[Pt]1(C4=CC=CC=C4C4=CC=CC=C41)N3=CC=C2)C(C)C.CC1=CC(C)=O[Pt]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C(C2=CC=CC=C2)=CC(C2=CC=CC=C2)=C1C(C)C.CC1=CC(C)=O[Pt]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)N1C=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)N1C=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1 Chemical compound C1=CC=C(N2C3=C4C(=CC=C3)[Pt]3(C5=CC=CC2=C54)N2=CC=CC=C2C2=CC=CC=N23)C=C1.C1=CC=C(N2C3=C4C(=CC=C3)[Pt]3(C5=CC=CC2=C54)N2=CC=CC=C2C2=CC=CC=N23)C=C1.CC(C)C(C)(B1C2=C3C4=C1/C=C\C=N/4[Pt]1(C4=CC=CC=C4C4=CC=CC=C41)N3=CC=C2)C(C)C.CC1=CC(C)=O[Pt]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C(C2=CC=CC=C2)=CC(C2=CC=CC=C2)=C1C(C)C.CC1=CC(C)=O[Pt]2(O1)/C1=C/C=C\C3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)N1C=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)N1C=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1 VJHVCRYNCFHKAZ-YQRUSNOGSA-J 0.000 description 1
- SURLRVSKGXJWDY-TVAVUMNESA-K C1=CC=N2[Pt]C3=C(/C=C/C=C/3)C2=C1.CC1=CC(C)=O[Pt]2(O1)C1=C(C3=CC=CC=N32)\C(F)=C/C(F)=C\1.CCC1=C(CC)/C2=C/C3=N4/C(=C\C5=C(CC)C(CC)=C6/C=C7/C(CC)=C(CC)C8=N7[Pt@@]4(N65)N2C1=C8)C(CC)=C3CC.FC1=C\C2=C(C3=CC=CC=N3[Ir]2)/C(F)=C\1.O=C1O[Ir]2(C3=C(C4=CC=CC=N42)/C(C2=CC=C(F)C=C2)=C\C(F)=C\3)N2=CC=CC=C12.O=C1O[Ir]2(C3=C(C4=CC=CC=N42)/C(F)=C\C(F)=C\3)N2=CC=CC=C12 Chemical compound C1=CC=N2[Pt]C3=C(/C=C/C=C/3)C2=C1.CC1=CC(C)=O[Pt]2(O1)C1=C(C3=CC=CC=N32)\C(F)=C/C(F)=C\1.CCC1=C(CC)/C2=C/C3=N4/C(=C\C5=C(CC)C(CC)=C6/C=C7/C(CC)=C(CC)C8=N7[Pt@@]4(N65)N2C1=C8)C(CC)=C3CC.FC1=C\C2=C(C3=CC=CC=N3[Ir]2)/C(F)=C\1.O=C1O[Ir]2(C3=C(C4=CC=CC=N42)/C(C2=CC=C(F)C=C2)=C\C(F)=C\3)N2=CC=CC=C12.O=C1O[Ir]2(C3=C(C4=CC=CC=N42)/C(F)=C\C(F)=C\3)N2=CC=CC=C12 SURLRVSKGXJWDY-TVAVUMNESA-K 0.000 description 1
- MSDMPJCOOXURQD-UHFFFAOYSA-N C545T Chemical compound C1=CC=C2SC(C3=CC=4C=C5C6=C(C=4OC3=O)C(C)(C)CCN6CCC5(C)C)=NC2=C1 MSDMPJCOOXURQD-UHFFFAOYSA-N 0.000 description 1
- DSHQYPWMOOPUJS-XCXAHRAVSA-K CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(C4=CC=CC5=C4C=CC=C5)=C2)[Ir]N2=CC=CC1=C32.CC1=CC(C)=C(B2C3=C4C(=CC(C5=CC=CC=C5)=C3)[Ir]N3=CC=CC2=C43)C(C)=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C(F)(F)F)C=C(C(F)(F)F)C3=C1C1=C(C=CC=N12)B3C(C(C)C)(C(C)C)C(C)C.CC1=CC(C)=O[Ir]2(O1)C1=CC(C3=CC=CC4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CC1=CC(C)=O[Ir]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C Chemical compound CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(C4=CC=CC5=C4C=CC=C5)=C2)[Ir]N2=CC=CC1=C32.CC1=CC(C)=C(B2C3=C4C(=CC(C5=CC=CC=C5)=C3)[Ir]N3=CC=CC2=C43)C(C)=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C(F)(F)F)C=C(C(F)(F)F)C3=C1C1=C(C=CC=N12)B3C(C(C)C)(C(C)C)C(C)C.CC1=CC(C)=O[Ir]2(O1)C1=CC(C3=CC=CC4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CC1=CC(C)=O[Ir]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C DSHQYPWMOOPUJS-XCXAHRAVSA-K 0.000 description 1
- DQKKDYJXIJGLFX-UHFFFAOYSA-N CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(C4=CC=CC5=C4C=CC=C5)=C2)[Pt]2(C4=CC(C5=CC=CC6=C5C=CC=C6)=CC5=C4C4=C(C=CC=N42)B5C2=C(C(C)C)C=CC=C2C(C)C)N2=CC=CC1=C32.CC1=CC(C)=C(C2=CC3=C4C(=C2)[Pt]2(C5=CC(C6=C(C)C=C(C)C=C6C)=CC6=C5C5=C(C=CC=N52)B6C2=C(C(C)C)C=CC=C2C(C)C)N2=CC=CC(=C42)B3C2=C(C(C)C)C=CC=C2C(C)C)C(C)=C1.CC1=CC2=C3C(=C1)[Pt]1(C4=CC(C)=CC5=C4C4=C(C=CC=N41)B5C1=C(C)C=CC=C1C)N1=CC=CC(=C31)B2C1=C(C)C=CC=C1C Chemical compound CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(C4=CC=CC5=C4C=CC=C5)=C2)[Pt]2(C4=CC(C5=CC=CC6=C5C=CC=C6)=CC5=C4C4=C(C=CC=N42)B5C2=C(C(C)C)C=CC=C2C(C)C)N2=CC=CC1=C32.CC1=CC(C)=C(C2=CC3=C4C(=C2)[Pt]2(C5=CC(C6=C(C)C=C(C)C=C6C)=CC6=C5C5=C(C=CC=N52)B6C2=C(C(C)C)C=CC=C2C(C)C)N2=CC=CC(=C42)B3C2=C(C(C)C)C=CC=C2C(C)C)C(C)=C1.CC1=CC2=C3C(=C1)[Pt]1(C4=CC(C)=CC5=C4C4=C(C=CC=N41)B5C1=C(C)C=CC=C1C)N1=CC=CC(=C31)B2C1=C(C)C=CC=C1C DQKKDYJXIJGLFX-UHFFFAOYSA-N 0.000 description 1
- XXOBIDZONPBEEC-DPDCUOCVSA-K CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(C4=CC=CC5=C4C=CC=C5)=C2)[Pt]2(C4=C\C(C5=CC=CC6=C5C=CC=C6)=C/C5=C\4C4=C(C=CC=N42)B5C2=CC=CC=C2)N2=CC=CC1=C32.CC1=CC(C)=C(B2C3=C4C(=CC(C5=CC=CC=C5)=C3)[Pt]3(C5=C\C(C6=CC=CC=C6)=C/C6=C\5C5=C(C=CC=N53)B6C3=CC=CC=C3)N3=CC=CC2=C43)C(C)=C1.CC1=CC(C)=O[Pt@@]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CC1=CC(C)=O[Pt@@]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)B3C1=C2C=CC=CC2=CC2=C1C=CC=C2.O=C1O[Pt@@]2(C3=CN=CC4=C3C3=C(C=CC=N32)B4C2=C(C3=CC=CC=C3)C=CC=C2C2=CC=CC=C2)N2=C1C=CC=C2 Chemical compound CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(C4=CC=CC5=C4C=CC=C5)=C2)[Pt]2(C4=C\C(C5=CC=CC6=C5C=CC=C6)=C/C5=C\4C4=C(C=CC=N42)B5C2=CC=CC=C2)N2=CC=CC1=C32.CC1=CC(C)=C(B2C3=C4C(=CC(C5=CC=CC=C5)=C3)[Pt]3(C5=C\C(C6=CC=CC=C6)=C/C6=C\5C5=C(C=CC=N53)B6C3=CC=CC=C3)N3=CC=CC2=C43)C(C)=C1.CC1=CC(C)=O[Pt@@]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)B3C1=C(C(C)C)C=CC=C1C(C)C.CC1=CC(C)=O[Pt@@]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)B3C1=C2C=CC=CC2=CC2=C1C=CC=C2.O=C1O[Pt@@]2(C3=CN=CC4=C3C3=C(C=CC=N32)B4C2=C(C3=CC=CC=C3)C=CC=C2C2=CC=CC=C2)N2=C1C=CC=C2 XXOBIDZONPBEEC-DPDCUOCVSA-K 0.000 description 1
- ORTJJAJNUNZUBI-UHFFFAOYSA-N CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(N(C4=CC=CC=C4)C4=CC=CC=C4)=C2)[Ir]N2=CC=CC1=C32.CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(N4C5=CC=CC=C5C5=C4C=CC=C5)=C2)[Ir]N2=CC=CC1=C32.CC1=CC2=C(C=C1)N(C1=CC3=C4C(=C1)[Ir]N1=CC=CC(=C41)B3C1=C(C(C)C)C=CC=C1C(C)C)C1=C2C=C(C)C=C1.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)N2C1=CC2=C3C(=C1)[Ir]N1=CC=CC(=C31)B2C1=C(C)C=C(C)C=C1C.FC(F)(F)C1=CC2=C3C(=C1)[Ir]N1=CC=CC(=C31)B2C1=C2C=CC=CC2=CC=C1 Chemical compound CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(N(C4=CC=CC=C4)C4=CC=CC=C4)=C2)[Ir]N2=CC=CC1=C32.CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(N4C5=CC=CC=C5C5=C4C=CC=C5)=C2)[Ir]N2=CC=CC1=C32.CC1=CC2=C(C=C1)N(C1=CC3=C4C(=C1)[Ir]N1=CC=CC(=C41)B3C1=C(C(C)C)C=CC=C1C(C)C)C1=C2C=C(C)C=C1.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)N2C1=CC2=C3C(=C1)[Ir]N1=CC=CC(=C31)B2C1=C(C)C=C(C)C=C1C.FC(F)(F)C1=CC2=C3C(=C1)[Ir]N1=CC=CC(=C31)B2C1=C2C=CC=CC2=CC=C1 ORTJJAJNUNZUBI-UHFFFAOYSA-N 0.000 description 1
- GXDQBGSXDIOHFX-UHFFFAOYSA-N CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(N(C4=CC=CC=C4)C4=CC=CC=C4)=C2)[Pt]2(C4=CC(N(C5=CC=CC=C5)C5=CC=CC=C5)=CC5=C4C4=C(C=CC=N42)B5C2=C(C(C)C)C=CC=C2C(C)C)N2=CC=CC1=C32.CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(N4C5=CC=CC=C5C5=C4C=CC=C5)=C2)[Pt]2(C4=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=CC5=C4C4=C(C=CC=N42)B5C2=C(C(C)C)C=CC=C2C(C)C)N2=CC=CC1=C32.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)N2C1=CC2=C3C(=C1)[Pt]1(C4=CC(N5C6=CC=C(C)C=C6C6=C5C=CC(C)=C6)=CC5=C4C4=C(C=CC=N41)B5C1=C(C)C=C(C)C=C1C)N1=CC=CC(=C31)B2C1=C(C)C=C(C)C=C1C.FC(F)(F)C1=CC2=C3C(=C1)[Pt]1(C4=CC(C(F)(F)F)=CC5=C4C4=C(C=CC=N41)B5C1=C4C=CC=CC4=CC=C1)N1=CC=CC(=C31)B2C1=C2C=CC=CC2=CC=C1 Chemical compound CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(N(C4=CC=CC=C4)C4=CC=CC=C4)=C2)[Pt]2(C4=CC(N(C5=CC=CC=C5)C5=CC=CC=C5)=CC5=C4C4=C(C=CC=N42)B5C2=C(C(C)C)C=CC=C2C(C)C)N2=CC=CC1=C32.CC(C)C1=CC=CC(C(C)C)=C1B1C2=C3C(=CC(N4C5=CC=CC=C5C5=C4C=CC=C5)=C2)[Pt]2(C4=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=CC5=C4C4=C(C=CC=N42)B5C2=C(C(C)C)C=CC=C2C(C)C)N2=CC=CC1=C32.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)N2C1=CC2=C3C(=C1)[Pt]1(C4=CC(N5C6=CC=C(C)C=C6C6=C5C=CC(C)=C6)=CC5=C4C4=C(C=CC=N41)B5C1=C(C)C=C(C)C=C1C)N1=CC=CC(=C31)B2C1=C(C)C=C(C)C=C1C.FC(F)(F)C1=CC2=C3C(=C1)[Pt]1(C4=CC(C(F)(F)F)=CC5=C4C4=C(C=CC=N41)B5C1=C4C=CC=CC4=CC=C1)N1=CC=CC(=C31)B2C1=C2C=CC=CC2=CC=C1 GXDQBGSXDIOHFX-UHFFFAOYSA-N 0.000 description 1
- FINZVTINYHJBMH-IWOQRIGNSA-J CC1=CC(C)=O[Ir]2(O1)/C1=C(\C3=CC=CC=C3)N(C3=CC=CC=C3)C3=C1C1=C(/C=C\C=N/12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)/C1=C/OC3=C1C1=C(/C=C\C=N/12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(C3=CC=CC4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(N3C4=CC=NC=C4C4=C3C=CN=C4)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1 Chemical compound CC1=CC(C)=O[Ir]2(O1)/C1=C(\C3=CC=CC=C3)N(C3=CC=CC=C3)C3=C1C1=C(/C=C\C=N/12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)/C1=C/OC3=C1C1=C(/C=C\C=N/12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(C3=CC=CC4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(N3C4=CC=NC=C4C4=C3C=CN=C4)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1 FINZVTINYHJBMH-IWOQRIGNSA-J 0.000 description 1
- NPHALCZXNSHWSU-DJZACKBQSA-I CC1=CC(C)=O[Ir]2(O1)/C1=C/SC3=C1C1=C(/C=C\C=N/12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(C3=CC=CC=C3)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(N3C4=CC=CC=C4C4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.O=C1O[Ir]2(C3=CC(C4=CC=CC5=C4C=CC=C5)=CC4=C3C3=C(C=CC=N32)N4C2=CC=CC=C2)N2=C1C=CC=C2 Chemical compound CC1=CC(C)=O[Ir]2(O1)/C1=C/SC3=C1C1=C(/C=C\C=N/12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(C3=CC=CC=C3)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC(N3C4=CC=CC=C4C4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.O=C1O[Ir]2(C3=CC(C4=CC=CC5=C4C=CC=C5)=CC4=C3C3=C(C=CC=N32)N4C2=CC=CC=C2)N2=C1C=CC=C2 NPHALCZXNSHWSU-DJZACKBQSA-I 0.000 description 1
- ONUUYJNHMXHOLH-NJJUSXBSSA-I CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)O1.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)S1.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)N1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=CC2=C(C=CC=C2)O1.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=CC2=C(C=CC=C2)S1 Chemical compound CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)O1.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)S1.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)N1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=CC2=C(C=CC=C2)O1.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=CC2=C(C=CC=C2)S1 ONUUYJNHMXHOLH-NJJUSXBSSA-I 0.000 description 1
- AXSZFYCHKLEAAB-NJJUSXBSSA-I CC1=CC(C)=O[Pt]2(O1)C1=C(C3=CC=CC=C3)N(C3=CC=CC=C3)C3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC(N3C4=CC=CC=C4C4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC(N3C4=CC=NC=C4C4=C3C=CN=C4)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=COC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CSC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1 Chemical compound CC1=CC(C)=O[Pt]2(O1)C1=C(C3=CC=CC=C3)N(C3=CC=CC=C3)C3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC(N3C4=CC=CC=C4C4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC(N3C4=CC=NC=C4C4=C3C=CN=C4)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=COC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CSC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1 AXSZFYCHKLEAAB-NJJUSXBSSA-I 0.000 description 1
- FWJKGGVAQHQTAA-YIHIJRQDSA-H CC1=CC(C)=O[Pt]2(O1)C1=CC(C3=C4C=CC=CC4=CC=C3)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)O1.CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)S1.CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=CC2=C(C=CC=C2)O1.CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=CC2=C(C=CC=C2)S1.[C-]#[N+]C1=CC(C#N)=C2C3=C1B(C(C(C)C)(C(C)C)C(C)C)C1=C3N(=CC=C1)[Pt]21OC(C)=CC(C)=O1 Chemical compound CC1=CC(C)=O[Pt]2(O1)C1=CC(C3=C4C=CC=CC4=CC=C3)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)O1.CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)S1.CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=CC2=C(C=CC=C2)O1.CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=CC2=C(C=CC=C2)S1.[C-]#[N+]C1=CC(C#N)=C2C3=C1B(C(C(C)C)(C(C)C)C(C)C)C1=C3N(=CC=C1)[Pt]21OC(C)=CC(C)=O1 FWJKGGVAQHQTAA-YIHIJRQDSA-H 0.000 description 1
- IFWYIMDKBOPIGI-JLUYYOSQSA-H CC1=CC(C)=O[Pt]2(O1)C1=CC(C3=CC=CC4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC(N3C4=CC=CC=C4C4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=NC=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.O=C1O[Pt]2(C3=CN=CC4=C3C3=C(C=CC=N32)N4C2=CC=CC=C2)N2=C1C=CC=C2 Chemical compound CC1=CC(C)=O[Pt]2(O1)C1=CC(C3=CC=CC4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC(N3C4=CC=CC=C4C4=C3C=CC=C4)=CC3=C1C1=C(C=CC=N12)B3C1=C(C2=CC=CC=C2)C=CC=C1C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC=NC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=NC=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.O=C1O[Pt]2(C3=CN=CC4=C3C3=C(C=CC=N32)N4C2=CC=CC=C2)N2=C1C=CC=C2 IFWYIMDKBOPIGI-JLUYYOSQSA-H 0.000 description 1
- XHDNBKXSUFAJPQ-YAHKVBGKSA-K CC1=CC(C)=O[Pt]2(O1)C1=CC(C3=CC=CC=C3)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC2=C(C=C1)N(C1=CC3=C4C(=C1)[Pt]1(C5=CC(N6C7=C(C=C(C)C=C7)C7=C6C=CC(C)=C7)=CC6=C5C5=C(C=CC=N51)B6C1=C(C(C)C)C=CC=C1C(C)C)N1=CC=CC(=C41)B3C1=C(C(C)C)C=CC=C1C(C)C)C1=C2C=C(C)C=C1.O=C1O[Pt@@]2(C3=CC(C4=CC=CC5=C4C=CC=C5)=CC4=C3C3=C(C=CC=N32)N4C2=CC=CC=C2)N2=C1C=CC=C2 Chemical compound CC1=CC(C)=O[Pt]2(O1)C1=CC(C3=CC=CC=C3)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)C1=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=CC3=C1C1=C(C=CC=N12)N3C1=CC=CC=C1.CC1=CC2=C(C=C1)N(C1=CC3=C4C(=C1)[Pt]1(C5=CC(N6C7=C(C=C(C)C=C7)C7=C6C=CC(C)=C7)=CC6=C5C5=C(C=CC=N51)B6C1=C(C(C)C)C=CC=C1C(C)C)N1=CC=CC(=C41)B3C1=C(C(C)C)C=CC=C1C(C)C)C1=C2C=C(C)C=C1.O=C1O[Pt@@]2(C3=CC(C4=CC=CC5=C4C=CC=C5)=CC4=C3C3=C(C=CC=N32)N4C2=CC=CC=C2)N2=C1C=CC=C2 XHDNBKXSUFAJPQ-YAHKVBGKSA-K 0.000 description 1
- COMCJWUULTVDHH-XBGLCSKXSA-K CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)N1C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)N1C=CC3=C1C1=C(C=CC=N12)N3C1=CC=C2C=CC=CC2=C1.CC1=CC=C(C2=C(B3C4=C5C(=CC=C4)[Pt]4(OC(C)=CC(C)=O4)N4=CC=CC3=C54)N(C3=CC=CC=C3)C3=C2C=CC=C3)C=C1.CC1=CC=C2C(=C1)C1=CC(C)=CC=C1[Pt]21N2=CC=CC3=C2C2=C(C=CC=N21)N3C1=CC=CC=C1.CC1=CC=N2C(=C1)C1=CC(C)=CC=N1[Pt]21C2=CC=CC3=C2C2=C(C=CC=C21)N3C1=CC=CC=C1 Chemical compound CC1=CC(C)=O[Pt]2(O1)C1=CC=CC3=C1C1=C(C=CC=N12)N3C1=C(C2=CC=CC=C2)C2=C(C=CC=C2)N1C1=CC=CC=C1.CC1=CC(C)=O[Pt]2(O1)N1C=CC3=C1C1=C(C=CC=N12)N3C1=CC=C2C=CC=CC2=C1.CC1=CC=C(C2=C(B3C4=C5C(=CC=C4)[Pt]4(OC(C)=CC(C)=O4)N4=CC=CC3=C54)N(C3=CC=CC=C3)C3=C2C=CC=C3)C=C1.CC1=CC=C2C(=C1)C1=CC(C)=CC=C1[Pt]21N2=CC=CC3=C2C2=C(C=CC=N21)N3C1=CC=CC=C1.CC1=CC=N2C(=C1)C1=CC(C)=CC=N1[Pt]21C2=CC=CC3=C2C2=C(C=CC=C21)N3C1=CC=CC=C1 COMCJWUULTVDHH-XBGLCSKXSA-K 0.000 description 1
- WAODGUVBNLMTSF-XTPDIVBZSA-N CCC1=C(CC)/C2=C/C3=N4/C(=C\C5=C(CC)C(CC)=C6/C=C7/C(CC)=C(CC)C8=N7[Pt@@]4(N65)N2C1=C8)C(CC)=C3CC Chemical compound CCC1=C(CC)/C2=C/C3=N4/C(=C\C5=C(CC)C(CC)=C6/C=C7/C(CC)=C(CC)C8=N7[Pt@@]4(N65)N2C1=C8)C(CC)=C3CC WAODGUVBNLMTSF-XTPDIVBZSA-N 0.000 description 1
- BIHMYWPOYKLTDK-UHFFFAOYSA-N CCN1C2=C3C4=C1/C(C(F)(F)F)=C\C(C(F)(F)F)=C/4[Ir]N3=CC=C2.CCN1C2=C3C4=C1/C=C(C1=CC=CC=C1)\C=C/4[Ir]1(N3=CC=C2)N2=CC=CN2B(N2C=CC=N2)(N2C=CC=N2)N2C=CC=N21.CCN1C2=C3C4=C1/C=C(N1C5=CC=CC=C5C5=C1C=CC=C5)\C=C/4[Ir]N3=CC=C2.CCN1C2=C3N4/C1=C(C1=CC=CC=C1)\C(C1=CC=CC=C1)=C/4[Ir]N3=CC=C2.[C-]#[N+]/C1=C/C(C#N)=C2/[Ir]N3=CC=CC4=C3C2=C1N4CC Chemical compound CCN1C2=C3C4=C1/C(C(F)(F)F)=C\C(C(F)(F)F)=C/4[Ir]N3=CC=C2.CCN1C2=C3C4=C1/C=C(C1=CC=CC=C1)\C=C/4[Ir]1(N3=CC=C2)N2=CC=CN2B(N2C=CC=N2)(N2C=CC=N2)N2C=CC=N21.CCN1C2=C3C4=C1/C=C(N1C5=CC=CC=C5C5=C1C=CC=C5)\C=C/4[Ir]N3=CC=C2.CCN1C2=C3N4/C1=C(C1=CC=CC=C1)\C(C1=CC=CC=C1)=C/4[Ir]N3=CC=C2.[C-]#[N+]/C1=C/C(C#N)=C2/[Ir]N3=CC=CC4=C3C2=C1N4CC BIHMYWPOYKLTDK-UHFFFAOYSA-N 0.000 description 1
- 241000284156 Clerodendrum quadriloculare Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical group C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229910000799 K alloy Inorganic materials 0.000 description 1
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 1
- 239000004696 Poly ether ether ketone Substances 0.000 description 1
- 239000004697 Polyetherimide Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 239000004734 Polyphenylene sulfide Substances 0.000 description 1
- NRCMAYZCPIVABH-UHFFFAOYSA-N Quinacridone Chemical compound N1C2=CC=CC=C2C(=O)C2=C1C=C1C(=O)C3=CC=CC=C3NC1=C2 NRCMAYZCPIVABH-UHFFFAOYSA-N 0.000 description 1
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- XHCLAFWTIXFWPH-UHFFFAOYSA-N [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] XHCLAFWTIXFWPH-UHFFFAOYSA-N 0.000 description 1
- 125000004062 acenaphthenyl group Chemical group C1(CC2=CC=CC3=CC=CC1=C23)* 0.000 description 1
- CUJRVFIICFDLGR-UHFFFAOYSA-N acetylacetonate Chemical compound CC(=O)[CH-]C(C)=O CUJRVFIICFDLGR-UHFFFAOYSA-N 0.000 description 1
- 150000001339 alkali metal compounds Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 229910003481 amorphous carbon Inorganic materials 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 125000005577 anthracene group Chemical group 0.000 description 1
- 150000008425 anthrones Chemical class 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 125000003828 azulenyl group Chemical group 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 125000006267 biphenyl group Chemical group 0.000 description 1
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 150000001716 carbazoles Chemical class 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- NJRWLESRYZMVRW-UHFFFAOYSA-N carboxy carboxyoxycarbonyl carbonate Chemical compound OC(=O)OC(=O)OC(=O)OC(O)=O NJRWLESRYZMVRW-UHFFFAOYSA-N 0.000 description 1
- HKQOBOMRSSHSTC-UHFFFAOYSA-N cellulose acetate Chemical compound OC1C(O)C(O)C(CO)OC1OC1C(CO)OC(O)C(O)C1O.CC(=O)OCC1OC(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(COC(C)=O)O1.CCC(=O)OCC1OC(OC(=O)CC)C(OC(=O)CC)C(OC(=O)CC)C1OC1C(OC(=O)CC)C(OC(=O)CC)C(OC(=O)CC)C(COC(=O)CC)O1 HKQOBOMRSSHSTC-UHFFFAOYSA-N 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- WDFKMLRRRCGAKS-UHFFFAOYSA-N chloroxine Chemical compound C1=CN=C2C(O)=C(Cl)C=C(Cl)C2=C1 WDFKMLRRRCGAKS-UHFFFAOYSA-N 0.000 description 1
- 125000005578 chrysene group Chemical group 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 125000005583 coronene group Chemical group 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 125000003700 epoxy group Chemical group 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000009975 flexible effect Effects 0.000 description 1
- 125000003914 fluoranthenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC=C4C1=C23)* 0.000 description 1
- 150000008376 fluorenones Chemical class 0.000 description 1
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 150000002240 furans Chemical class 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- JVZRCNQLWOELDU-UHFFFAOYSA-N gamma-Phenylpyridine Natural products C1=CC=CC=C1C1=CC=NC=C1 JVZRCNQLWOELDU-UHFFFAOYSA-N 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- RBTKNAXYKSUFRK-UHFFFAOYSA-N heliogen blue Chemical compound [Cu].[N-]1C2=C(C=CC=C3)C3=C1N=C([N-]1)C3=CC=CC=C3C1=NC([N-]1)=C(C=CC=C3)C3=C1N=C([N-]1)C3=CC=CC=C3C1=N2 RBTKNAXYKSUFRK-UHFFFAOYSA-N 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 150000007857 hydrazones Chemical class 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 150000002460 imidazoles Chemical class 0.000 description 1
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- UEEXRMUCXBPYOV-UHFFFAOYSA-N iridium;2-phenylpyridine Chemical compound [Ir].C1=CC=CC=C1C1=CC=CC=N1.C1=CC=CC=C1C1=CC=CC=N1.C1=CC=CC=C1C1=CC=CC=N1 UEEXRMUCXBPYOV-UHFFFAOYSA-N 0.000 description 1
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229910052745 lead Inorganic materials 0.000 description 1
- ORUIBWPALBXDOA-UHFFFAOYSA-L magnesium fluoride Chemical compound [F-].[F-].[Mg+2] ORUIBWPALBXDOA-UHFFFAOYSA-L 0.000 description 1
- 229910001635 magnesium fluoride Inorganic materials 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 150000002736 metal compounds Chemical class 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 125000004184 methoxymethyl group Chemical group [H]C([H])([H])OC([H])([H])* 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- BBDFECYVDQCSCN-UHFFFAOYSA-N n-(4-methoxyphenyl)-4-[4-(n-(4-methoxyphenyl)anilino)phenyl]-n-phenylaniline Chemical group C1=CC(OC)=CC=C1N(C=1C=CC(=CC=1)C=1C=CC(=CC=1)N(C=1C=CC=CC=1)C=1C=CC(OC)=CC=1)C1=CC=CC=C1 BBDFECYVDQCSCN-UHFFFAOYSA-N 0.000 description 1
- AODWRBPUCXIRKB-UHFFFAOYSA-N naphthalene perylene Chemical group C1=CC=CC2=CC=CC=C21.C1=CC(C2=CC=CC=3C2=C2C=CC=3)=C3C2=CC=CC3=C1 AODWRBPUCXIRKB-UHFFFAOYSA-N 0.000 description 1
- 125000005186 naphthyloxy group Chemical group C1(=CC=CC2=CC=CC=C12)O* 0.000 description 1
- 239000012299 nitrogen atmosphere Substances 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 239000012044 organic layer Substances 0.000 description 1
- 150000002908 osmium compounds Chemical class 0.000 description 1
- 150000007978 oxazole derivatives Chemical class 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 125000003854 p-chlorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1Cl 0.000 description 1
- 238000010422 painting Methods 0.000 description 1
- 125000005582 pentacene group Chemical group 0.000 description 1
- JQQSUOJIMKJQHS-UHFFFAOYSA-N pentaphenyl group Chemical group C1=CC=CC2=CC3=CC=C4C=C5C=CC=CC5=CC4=C3C=C12 JQQSUOJIMKJQHS-UHFFFAOYSA-N 0.000 description 1
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 1
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical group C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 1
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 1
- 150000004986 phenylenediamines Chemical class 0.000 description 1
- 238000000206 photolithography Methods 0.000 description 1
- 125000004592 phthalazinyl group Chemical group C1(=NN=CC2=CC=CC=C12)* 0.000 description 1
- 125000001388 picenyl group Chemical group C1(=CC=CC2=CC=C3C4=CC=C5C=CC=CC5=C4C=CC3=C21)* 0.000 description 1
- 229940081066 picolinic acid Drugs 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 150000003057 platinum Chemical class 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920002530 polyetherether ketone Polymers 0.000 description 1
- 229920001601 polyetherimide Polymers 0.000 description 1
- 239000011112 polyethylene naphthalate Substances 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229920000069 polyphenylene sulfide Polymers 0.000 description 1
- BITYAPCSNKJESK-UHFFFAOYSA-N potassiosodium Chemical compound [Na].[K] BITYAPCSNKJESK-UHFFFAOYSA-N 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- LNKHTYQPVMAJSF-UHFFFAOYSA-N pyranthrene Chemical group C1=C2C3=CC=CC=C3C=C(C=C3)C2=C2C3=CC3=C(C=CC=C4)C4=CC4=CC=C1C2=C34 LNKHTYQPVMAJSF-UHFFFAOYSA-N 0.000 description 1
- VEPOUCHBIJXQFI-UHFFFAOYSA-N pyrazabole Chemical compound [B-]1N2C=CC=[N+]2[B-][N+]2=CC=CN12 VEPOUCHBIJXQFI-UHFFFAOYSA-N 0.000 description 1
- JEXVQSWXXUJEMA-UHFFFAOYSA-N pyrazol-3-one Chemical class O=C1C=CN=N1 JEXVQSWXXUJEMA-UHFFFAOYSA-N 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 238000007788 roughening Methods 0.000 description 1
- 239000004065 semiconductor Substances 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- CIOAGBVUUVVLOB-UHFFFAOYSA-N strontium atom Chemical compound [Sr] CIOAGBVUUVVLOB-UHFFFAOYSA-N 0.000 description 1
- 125000001174 sulfone group Chemical group 0.000 description 1
- 125000000542 sulfonic acid group Chemical group 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000001360 synchronised effect Effects 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000001935 tetracenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC=CC=C4C=C3C=C12)* 0.000 description 1
- 150000007979 thiazole derivatives Chemical class 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- ANRHNWWPFJCPAZ-UHFFFAOYSA-M thionine Chemical compound [Cl-].C1=CC(N)=CC2=[S+]C3=CC(N)=CC=C3N=C21 ANRHNWWPFJCPAZ-UHFFFAOYSA-M 0.000 description 1
- 229930192474 thiophene Natural products 0.000 description 1
- 150000003577 thiophenes Chemical class 0.000 description 1
- RPVGLMKJGQMQSN-UHFFFAOYSA-N tiliquinol Chemical compound C1=CC=C2C(C)=CC=C(O)C2=N1 RPVGLMKJGQMQSN-UHFFFAOYSA-N 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- 239000012780 transparent material Substances 0.000 description 1
- 125000005259 triarylamine group Chemical group 0.000 description 1
- 125000004306 triazinyl group Chemical group 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- ODHXBMXNKOYIBV-UHFFFAOYSA-N triphenylamine Chemical compound C1=CC=CC=C1N(C=1C=CC=CC=1)C1=CC=CC=C1 ODHXBMXNKOYIBV-UHFFFAOYSA-N 0.000 description 1
- 125000006617 triphenylamine group Chemical group 0.000 description 1
- 125000005580 triphenylene group Chemical group 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 229910001935 vanadium oxide Inorganic materials 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 125000005023 xylyl group Chemical group 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/361—Polynuclear complexes, i.e. complexes comprising two or more metal centers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B57/00—Other synthetic dyes of known constitution
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B57/00—Other synthetic dyes of known constitution
- C09B57/10—Metal complexes of organic compounds not being dyes in uncomplexed form
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/321—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3]
- H10K85/322—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3] comprising boron
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/341—Transition metal complexes, e.g. Ru(II)polypyridine complexes
- H10K85/346—Transition metal complexes, e.g. Ru(II)polypyridine complexes comprising platinum
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/341—Transition metal complexes, e.g. Ru(II)polypyridine complexes
- H10K85/348—Transition metal complexes, e.g. Ru(II)polypyridine complexes comprising osmium
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/658—Organoboranes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1014—Carbocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1088—Heterocyclic compounds characterised by ligands containing oxygen as the only heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1092—Heterocyclic compounds characterised by ligands containing sulfur as the only heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1096—Heterocyclic compounds characterised by ligands containing other heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/18—Metal complexes
- C09K2211/185—Metal complexes of the platinum group, i.e. Os, Ir, Pt, Ru, Rh or Pd
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/10—Triplet emission
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/633—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising polycyclic condensed aromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
Definitions
- the present invention relates to an organic electroluminescence element material, an organic electroluminescence element, a display device and an illumination device.
- an emission type electronic display device includes an electroluminescence display (hereinafter, referred to as an ELD).
- ELD electroluminescence display
- a constituent element of ELD includes such as an inorganic electroluminescence element and an organic electroluminescence element (hereinafter, referred to as an organic EL element).
- An inorganic electroluminescence element has been utilized as a flat light source, however, requires a high voltage of alternating current to operate an emission element.
- An organic electroluminescence element is an element provided with a constitution comprising an emission layer containing a emitting substance being sandwiched with a cathode and an anode, and an exciton is generated by an electron and a positive hole being injected into the emission layer to be recombined, resulting emission utilizing light release (fluorescence.phosphorescence) at the time of deactivation of said exciton; the emission is possible at a voltage of approximately a few to a few tens volts, and an organic electroluminescence element is attracting attention with respect to such as superior viewing angle and high visual recognition due to a self-emission type as well as space saving and portability due to a completely solid element of a thin layer type.
- JP-A 63-264692 JP-A refers to Japanese Patent Publication Open to Public Inspection No.
- JP-A 3-255190 an element having an organic emission layer comprising a 8-hydroxyquinoline aluminum complex as a host compound which is doped with quinacridone type dye
- Ikai et al. at The 10th International Workshops on Inorganic and Organic Electroluminescence (EL'00, Hamamatsu) utilized a hole transporting compound as a host of a phosphorescent compound.
- EL'00 Inorganic and Organic Electroluminescence
- M. E. Tompson et al. utilized various types of electron transporting materials as a host of a phosphorescent compound doped with a new iridium complex.
- emission luminance and emission efficiency are significantly improved compared to conventional elements because the emitting light arises from phosphorescence, however, there has been a problem of a poor emission life of the element compared to conventional elements.
- Patent Document 6 WO 02/15645 pamphlet
- Non-Patent Document 1 Inorganic Chemistry, vol. 41, No. 12, pp. 3055-3066
- Non-Patent Document 2 Applied Physics Letters, vol. 79, p. 2082
- Non-Patent Document 3 Applied Physics Letters, vol. 83, p. 3818
- Non-Patent Document 3 New Journal of Chemistry, vol. 26, p. 1171
- This invention has been made in view of these problems, and an object of this invention is to provide an organic EL element material provided with a metal complex having a specific ligand, and an organic EL element, an illumination device and a display device, which have high emission efficiency and long emission life, by utilizing said element material.
- An organic electroluminescence element material comprising a metal complex provided with a ligand represented by Formula (1).
- X 1 , X 2 , X 3 and X 4 are each independently a carbon atom or a nitrogen atom; C 1 and C 2 are carbon atoms; Z 1 in conjunction with C 1 , X 1 and X 3 , and Z 2 in conjunction with C 2 , X 2 and X 4 , are each an atomic group which forms an aromatic hydrocarbon ring or an aromatic heterocyclic ring, respectively; A 1 is a nitrogen atom or a boron atom; R 1 is a substituent group; and a bond between C 1 and X 1 , a bond between C 2 and X 2 , a bond between X 1 and X 3 , and a bond between X 2 and X 4 , are a single bond or a double bond.
- An organic electroluminescence element material comprising a metal complex provided with a partial structure represented by Formula (2).
- C 3 , C 4 , C 5 , C 6 , and C 7 are each independently a carbon atom or a nitrogen atom;
- Z 3 in conjunction with C 3 , C 4 and C 5 is an atomic group which forms an aromatic hydrocarbon ring or an aromatic heterocyclic ring;
- Z 4 in conjunction with C 6 , C 7 and N is an atomic group which forms an aromatic heterocyclic ring;
- a 2 is a nitrogen atom or a boron atom;
- R 2 is a substituent group;
- M 11 is an element of the 8th to 10th groups of the periodic table; and a bond between C 3 and C 4 , a bond between C 4 and C 5 , a bond between C 6 and C 7 , and a bond between C 7 and N, are a single bond or a double bond.
- a 3 is a nitrogen atom or a boron atom
- R 3 is a substituent group
- R 4 and R 5 are substituent groups
- n1 and n2 are each 0, 1 or 2
- M 12 is an element of the 8th to 10th groups of the periodic table.
- An organic electroluminescence element comprising the organic electroluminescence element material of any one of Items 1-7.
- the organic electroluminescence element of Item 8 above wherein the element is provided with at least one emission layer, serving as a constituent layer, and the emission layer comprising the organic electroluminescence element material of any one of Items 1-7.
- This invention has been able to provide an organic EL element material, which is a metal complex provided with a specific ligand, and an organic EL element, an illumination device and a display device having high emission efficiency and long emission life utilizing said organic EL element material.
- FIG. 1 is a schematic drawing to show an example of a display device constituted of an organic EL element.
- FIG. 2 is a schematic drawing of display section A.
- FIG. 3 is an equivalent circuit diagram of an operation circuit constituting an image pixel.
- FIG. 4 is a schematic drawing of a display device according to a passive matrix mode.
- FIG. 5 is a brief schematic drawing of a sealing structure of organic EL element OLED 1-1.
- FIG. 6( a ) is a plane schematic drawing of an illumination device equipped with an organic EL element.
- FIG. 6( b ) is a cross-sectional schematic drawing of an illumination device equipped with an organic EL element.
- an organic EL element material which is a metal complex having a specific ligand, could be obtained by adopting a constitution defined by any one of Items 1-7.
- An organic EL element having high emission efficiency and long emission life could be obtained by utilizing said organic EL element material.
- the display device described in Item 11 and the illumination device described in Item 12 each, exhibit high luminance as well as high durability, could be obtained.
- the inventors of this invention as a result of extensive study in view of the above-described problems, have found that by utilizing a metal complex having a specific ligand such as a metal complex having a ligand represented by aforesaid Formula (1), a metal complex having a partial structure represented by aforesaid Formula (2), and a metal complex having a partial structure represented by aforesaid Formula (3), in an organic EL element, the effects described in this invention can be achieved, that is, an organic EL element exhibiting high emission efficiency and long emission life can be prepared.
- a metal complex having a specific ligand such as a metal complex having a ligand represented by aforesaid Formula (1), a metal complex having a partial structure represented by aforesaid Formula (2), and a metal complex having a partial structure represented by aforesaid Formula (3)
- a metal complex having a specific ligand utilized in this invention is characterized by being provided with a molecular structure in which the ortho-positions of a ligand molecule, which are positioned opposite to the side to form a complex between a center metal and a ligand of a metal complex, each other are connected via one nitrogen atom or one boron atom, in the case of the complex is comprised of a ligand and a center metal.
- a layer containing the aforesaid metal complex is preferably an emission layer and/or a positive hole inhibition layer, and in the case of being contained in an emission layer, by utilizing said complex as an emission dopant in the aforesaid emission layer, elongation of emission life of an organic EL element, which is an effect of this invention, has been achieved.
- each aromatic hydrocarbon group which is formed by Z 1 together with C 1 , X 1 and X 3 and by Z 2 together with C 2 , X 3 and X 4 , includes such as a benzene ring, a biphenyl ring, a naphthalene ring, an azulene ring, an anthracene ring, a phenanthrene ring, a pyrene ring, a chrysene ring, a naphthacene ring, a triphenylene ring, an o-terphenyl ring, a m-terphenyl ring, a p-terphenyl ring, an acenaphthene ring, a coronene ring, a fluorene ring, a fluoranthene ring, a pentacene ring, a perylene ring, a pentaphene ring, a picen
- aforesaid aromatic hydrocarbon group may be provided with a substituent group represented by R 1 in the aforesaid Formula (1).
- each aromatic heterocyclic group which is formed by Z 1 together with C 1 , X 1 and X 3 and by Z 2 together with C 2 , X 3 and X 4 includes such as a furan ring, a thiophene ring, a pyridine ring, a pyridazine ring, a pyrimidine ring, a pyrazine ring, a triazine ring, a benzoimidazole ring, an oxadiazole ring, a triazole ring, an imidazole ring, a pyrazole ring, a thiazole ring, an indole ring, a benzoimidazole ring, a benzothiazole ring, a benzooxazole ring, a quinoxaline ring, a quinazoline ring, a phthalazine ring, a carbazole ring, a carboline ring and
- the aforesaid aromatic heterocyclic group may be provided with a substituent group represented by R 1 in the aforesaid Formula (1).
- a substituent group represented by R 1 includes an alkyl group (such as a methyl group, an ethyl group, an isopropyl group, a hydroxyethyl group, a methoxymethyl group, a trifluoromethyl group and a t-butyl group), a cycloalkyl group (such as a cyclopentyl group and a cyclohexyl group), an aralkyl group (such as a benzyl group and a 2-phenetyl group), an aromatic hydrocarbon ring (such as a phenyl group, a p-chlorophenyl group, a mesityl group, a tolyl group, a xylyl group, a biphenyl group, a naphthyl group, a anthoryl group and a phenanthoryl group), an aromatic heterocyclic ring (such as a furyl group, a thienyl group), an aromatic
- At least one of groups represented by above-described R 1 is preferably the above-described aromatic hydrocarbon group or aromatic heterocyclic group.
- a metal complex is formed by forming a coordinate bond (also referred to as complex formation) between a ligand represented by above-described Formula (1) and a center metal (may be either metal or ion).
- formation of a coordinate bond between the aforesaid ligand and center metal is preferably formation of a coordinate bond or a covalent bond with X 3 and/or X 4 among atoms constituting a ligand represented by aforesaid Formula (1).
- an aromatic hydrocarbon ring which is formed by Z 3 together with C 3 , C 4 and C 5 , is identical with an aromatic hydrocarbon ring which is formed by Z 1 together with C 1 , X 1 and X 3 , in aforesaid Formula (1).
- an aromatic heterocyclic ring which is formed by Z 3 together with C 3 , C 4 and C 5 , is identical with an aromatic heterocyclic ring which is formed by Z 1 together with C 1 , X 1 and X 3 , in aforesaid Formula (1).
- an aromatic heterocyclic ring which is formed by Z 4 together with C 6 , C 7 and N includes such as a pyridine ring, a pyridazine ring, a pyrimidine ring, a pyrazine ring, a triazine ring, a benzoimidazole ring, an oxadiazole ring, a triazole ring, an imidazole ring, a pyrazole ring, a thiazole ring, an indole ring, a benzoimidazole ring, a benzothiazole ring, a benzooxazole ring, a quinoxaline ring, a quinazoline ring, a phthalazine ring, a carbazole ring, a carboline ring and a ring in which at least one carbon atom constituting a carboline ring is further substituted by a nitrogen atom.
- a substituent group represented by R 2 is identical with a substituent group represented by R 1 in aforesaid Formula (1).
- an element of the 8th-10th group in the periodic table represented by M 11 is preferably such as platinum (Pt) and iridium (Ir). Further, in Formula (2), M 11 may be either metal or ion.
- a substituent group represented by R 3 is identical with a substituent group represented by R 1 in aforesaid Formula (1).
- an element of the 8th-10th group in the periodic table represented by M 12 is preferably such as platinum (Pt) and iridium (Ir). Further, in Formula (3), M 12 may be either metal or ion.
- Metal complexes according to an organic EL element material of this invention can be synthesized by applying a method described in such as Organic Letter, vol. 3, No. 16, pp. 2579-2581 (2001), Inorganic Chemistry vol. 30, No. 8, pp. 1685-1687 (1991), J. Am. Chem. Soc., vol. 123, p. 4304 (2001), Inorganic Chemistry vol. 40, No. 7, pp. 1704-1711 (2001), Inorganic Chemistry vol. 41, No. 12, pp. 3055-3066 (2002), New Journal of Chemistry, vol. 26, p. 1171 (2002), Journal of the Chemical Society Perkin Transactions 1, 11, pp. 1505-1510 (1999), and reference documents described in these documents.
- said material is preferably utilized in an emission layer or a positive hole inhibition layer among constituent layers (details will be described later) of the organic EL element. Further, the material is preferably utilized as an emission dopant in an emission layer as described above.
- a mixing ratio of an emission dopant against an emission host as a primary component in an emission layer is preferably adjusted to a range of 0.1-30 weight %.
- plural types of compounds may be utilized in combination as an emission dopant, and the partner to be mixed may be a metal complex having a different structure, and a phosphorescent dopant or a fluorescent dopant having other structures.
- a dopant such as a phosphorescent dopant and a fluorescent dopat
- a metal complex employed as an emission dopant will be described.
- An emission dopant is roughly classified into two types, that is, a fluorescent dopant which emits fluorescence and a phosphorescent dopant which emits phosphorescence.
- a typical example of the former includes cumaline type dye, pyrane type dye, cyanine type dye, croconium type dye, squalium type dye, oxobenzanthrathene type dye, fluoresceine type dye, rhodamine type dye, pyrilium type dye, perillene type dye, stilbene type dye, polythiophene type dye or rare earth complex type fluorescent substances.
- a typical example of the latter (a phosphorescent dopant) is preferably a complex type compound containing metal of the 8th-10th groups of the periodic table, more preferably an iridium compound and an osmium compound and most preferable among them is an iridium compound.
- An emission host means a compound having the largest mixing ratio (weight) in an emission layer comprised of at least two types of compounds, and other compounds are called as a dopant compound (also simply referred to as a dopant).
- a dopant compound also simply referred to as a dopant.
- compound A is a dopant compound
- compound B is a host compound.
- compound A and compound B are dopant compounds and compound C is a host compound.
- An emission host utilized in this invention is preferably a compound having a phosphorescence 0-0 band of a wavelength not longer than that of an emission dopant utilized in combination, and when a compound having a blue emission component of a phosphorescence 0-0 band of not longer than 480 nm is utilized as an emission dopant, an emission host is preferably provided with a phosphorescence 0-0 band of not longer than 450 nm.
- a preferable compound includes typically a compound provided with a basic skeleton such as a carbazole derivative, a triarylamine derivative, an aromatic borane derivative, a nitrogen-containing heterocyclic derivative, a thiophene derivative, a furan derivative and an oligoallylene compound, and having the aforesaid 0-0 band of not longer than 450 nm.
- a basic skeleton such as a carbazole derivative, a triarylamine derivative, an aromatic borane derivative, a nitrogen-containing heterocyclic derivative, a thiophene derivative, a furan derivative and an oligoallylene compound, and having the aforesaid 0-0 band of not longer than 450 nm.
- an emission host of this invention may be either a low molecular weight compound or a polymer compound having a repeating unit, in addition to a low molecular weight compound provided with a polymerizing group such as a vinyl group and an epoxy group (an evaporation polymerizing emission host).
- An emission host is preferably a compound having a positive hole transporting ability and an electron transporting ability, as well as preventing elongation of an emission wavelength and having a high Tg (a glass transition temperature).
- an emission host compounds described in the following Documents are preferable: For example, JP-A Nos. 2001-257076, 2002-308855, 2001-313179, 2002-319491, 2001-357977, 2002-334786, 2002-8860, 2002-334787, 2002-15871, 2002-334788, 2002-43056, 2002-334789, 2002-75645, 2002-338579, 2002-105445, 2002-343568, 2002-141173, 2002-352957, 2002-203683, 2002-363227, 2002-231453, 2003-3165, 2002-234888, 2003-27048, 2002-255934, 2002-260861, 2002-280183, 2002-299060, 2002-302516, 2002-305083, 2002-305084 and 2002-308837. Specific examples of an emission host are shown below; however, this invention is not limited thereto.
- anode/positive hole transport layer/emission layer/positive hole inhibition layer/electron transport layer/cathode anode/electron inhibition layer/emission layer/positive hole inhibition layer/electron transport layer/cathode
- anode/positive hole transport layer/electron inhibition layer/emission layer/positive hole inhibition layer/electron transport layer/cathode anode/anode buffer layer/positive hole transport layer/electron inhibition layer/emission layer/positive hole inhibition layer/electron transport layer/cathode
- anode/anode buffer layer/positive hole transport layer/electron inhibition layer/emission layer/positive hole inhibition layer/electron transport layer/cathode buffer layer/cathode anode/anode buffer layer/positive hole transport layer/electron inhibition layer/emission layer/positive hole inhibition layer/
- an organic EL element material of this invention is preferably utilized in such as a positive hole inhibition layer and an electron inhibition layer, and specifically preferably in a positive hole inhibition layer.
- a metal complex according to this invention may be contained in a state of 100 weight % as a layer constituent component of such as a positive hole inhibition layer and an electron inhibition layer, or may be contained by being mixed with another organic compound (such as compounds utilized in a constituent layer of an organic EL element of this invention).
- the layer thickness of an inhibition layer according to this invention is preferably 3-100 nm and more preferably 5-30 nm.
- a positive hole inhibition layer in a broad meaning, is provided with a function of electron transport layer, being comprised of a material having a function of transporting an electron but a very small ability of transporting a positive hole, and can improve the recombination probability of an electron and a positive hole by inhibiting a positive hole while transporting an electron.
- a positive inhibition layer for example, a positive inhibition layer described in such as JP-A Nos. 11-204258 and 11-204359 and p. 273 of “Organic EL Elements and Idustrialization Front Thereof (Nov. 30, 1998), published by N. T. S Corp.)” is applicable to a positive hole inhibition (hole block) layer according to this invention. Further, a constitution of an electron transport layer described later can be appropriately utilized as a positive hole inhibition layer according to this invention.
- an electron inhibition layer is, in a broad meaning, provided with a function of a positive hole transport layer, being comprised of a material having a function of transporting a positive hole but a very small ability of transporting an electron, and can improve the recombination probability of an electron and a positive hole by inhibiting an electron while transporting a positive hole. Further, a constitution of a positive hole transport layer described later can be appropriately utilized as an electron inhibition layer.
- an organic EL element material of this invention described above in an adjacent layer neighboring to an emission layer, that is in a positive hole inhibition layer and an electron inhibition layer, and specifically preferably in a positive hole inhibition layer.
- a positive hole transport layer contains a material having a function of transporting a positive hole, and in a broad meaning, a positive hole injection layer and an electron inhibition layer are also included in a positive hole transport layer.
- a single layer of or plural layers of a positive hole transport layer may be provided.
- a positive hole transport material is not specifically limited and can be arbitrary selected from those such as generally utilized as a charge injection transporting material of a positive hole in a conventional photoconductive material and those which are well known in the art and utilized in a positive hole injection layer and a positive hole transport layer of an EL element.
- a positive hole transport material is those having any one of a property to inject or transport a positive hole or a barrier property to an electron, and may be either an organic substance or an inorganic substance.
- a triazole derivative an oxadiazole derivative, an imidazole derivative, a polyallylalkane derivative, a pyrazolone derivative, a phenylenediamine derivative, a allylamine derivative, an amino substituted chalcone derivative, an oxazole derivatives, a stilylanthracene derivative, a fluorenone derivative, a hydrazone derivative, a stilubene derivative, a silazane derivative, an aniline type copolymer, or conductive polymer oligomer and specifically preferably such as thiophene oligomer.
- a positive hole transport material those described above can be utilized, however, it is preferable to utilized a poluphlin compound, an aromatic tertiary amine compound and a stirylamine compound, and specifically preferably an aromatic tertiary amine compound.
- Typical examples of an aromatic tertiary amine compound and a styrylamine compound include N,N,N′,N′-tetraphenyl-4,4′-diaminophenyl; N,N′-diphenyl-N,N′-bis(3-methylphenyl)-(1,1′-biphenyl)-4,4′-diamine (TDP); 2,2-bis(4-di-p-tolylaminophenyl)propane; 1,1-bis(4-di-p-tolylaminophenyl)cyclohexane; N,N,N′,N′-tetra-p-tolyl 4,4′-diaminobiphenyl; 1,1-bis(4-di-p-tolylaminophenyl)-4-phenylcyclohexane; bis(4-dimethylamino-2-methyl)phenylmethane; bis(4-di-p-tolylamin
- Pat. No. 5,061,569 such as 4,4′-bis[N-(1-naphthyl)-N-phenylamino]biphenyl (NDP), and 4,4′, 4′′-tris[N-(3-methylphenyl)-N-phenylamino]triphenylamine (MDTDATA), in which three of triphenylamine units are bonded in a star burst form, described in JP-A 4-308688.
- Polymer materials in which these materials are introduced in a polymer chain or constitute the main chain of polymer, can be also utilized.
- an inorganic compound such as a p type-Si and a p type-SiC can be utilized as a positive hole injection material and a positive hole transport material.
- a positive hole transport material preferably has a high Tg.
- This positive hole transport layer can be prepared by forming a thin layer made of the above-described positive hole transport material according to a method well known in the art such as a vacuum evaporation method, a spin coating method, a cast method, an inkjet method and a LB method.
- the layer thickness of a positive hole transport layer is not specifically limited, however, is generally 5-5,000 nm.
- This positive transport layer may have a single layer structure comprised of one or not less than two types of the above described materials.
- An electron transfer layer is comprised of a material having a function to transfer an electron, and an electron injection layer and a positive hole inhibition layer are included in an electron transfer layer in a broad meaning.
- a single layer or plural layers of an electron transfer layer may be provided.
- an electron transfer layer is provided with a function to transmit an electron injected from a cathode to an emission layer, and compounds conventionally well known in the art can be utilized by arbitrarily selection as a material thereof.
- Examples of a material utilized in this electron transfer layer include such as a nitro-substituted fluorene derivative, a diphenylquinone derivative, a thiopyradineoxide derivative, a heterocyclic tetracarbonic acid anhydride such as naphthaleneperylene, carbodiimide, a fluorenylidenemethane derivative, anthraquinonedimethane and anthrone derivatives, and an oxadiazole derivative.
- an electron transfer material include such as a nitro-substituted fluorene derivative, a diphenylquinone derivative, a thiopyradineoxide derivative, a heterocyclic tetracarbonic acid anhydride such as naphthaleneperylene, carbodiimide, a fluorenylidenemethane derivative, anthraquinonedimethane and anthrone derivatives, and an oxadiazole derivative
- a thiazole derivative in which an oxygen atom in the oxadiazole ring of the above-described oxadiazole derivative is substituted by a sulfur atom, and a quinoxaline derivative having a quinoxaline ring which is known as an electron attracting group can be utilized as an electron transfer material.
- Polymer materials in which these materials are introduced in a polymer chain or these materials form the main chain of polymer, can be also utilized.
- a metal complex of a 8-quinolinol derivative such as tris(8-quinolinol)aluminum (Alq), tris(5,7-dichloro-8-quinolinol)aluminum, tris(5,7-dibromo-8-quinolinol)aluminum, tris(2-methyl-8-quinolinol)aluminum, tris(5-methyl-8-quinolinol)aluminum and bis(8-quinolinol)zinc (Znq); and metal complexes in which a central metal of the aforesaid metal complexes is substituted by In, Mg, Cu, Ca, Sn, Ga or Pb, can be also utilized as an electron transfer material.
- metal-free or metal phthalocyanine can be preferably utilized as an electron transfer material.
- distyrylpyradine derivative which has been exemplified as a material of an emission layer, can be also utilized as an electron transfer material, and, similarly to the case of a positive hole injection layer and a positive hole transfer layer, an inorganic semiconductor such as an n-type-Si and an n-type-SiC can be also utilized as an electron transfer material.
- This electron transport layer can be prepared by forming a thin layer made of the above-described electron transport material according to a method well known in the art such as a vacuum evaporation method, a spin coating method, a cast method, an inkjet method and a LB method.
- the layer thickness of an electron transport layer is not specifically limited; however, is generally 5-5,000 nm.
- This electron transport layer may have a single layer structure comprised of one or not less than two types of the above described materials.
- An injection layer is appropriately provided and includes an electron injection layer and a positive hole injection layer, which may be arranged between an anode and an emission layer or a positive transfer layer, and between a cathode and an emission layer or an electron transfer layer, as described above.
- An injection layer is a layer which is arranged between an electrode and an organic layer to decrease an operating voltage and to improve an emission luminance, which is detailed in volume 2, chapter 2 (pp. 123-166) of “Organic EL Elements and Industrialization Front thereof (Nov. 30, 1998, published by N. T. S Corp.)”, and includes a positive hole injection layer (an anode buffer layer) and an electron injection layer (a cathode buffer layer).
- An anode buffer layer (a positive hole injection layer) is also detailed in such as JP-A 9-45479, 9-260062 and 8-288069, and specific examples include such as a phthalocyanine buffer layer comprising such as copper phthalocyanine, an oxide buffer layer comprising such as vanadium oxide, an amorphous carbon buffer layer, and a polymer buffer layer employing conductive polymer such as polythiophene.
- a cathode buffer layer (an electron injection layer) is also detailed in such as JP-A 6-325871, 9-17574 and 10-74586, and specific examples include a metal buffer layer comprising such as strontium and aluminum, an alkali metal compound buffer layer comprising such as lithium fluoride, an alkali earth metal compound buffer layer comprising such as magnesium fluoride, and an oxide buffer layer comprising such as aluminum oxide.
- the above-described buffer layer is preferably a very thin layer, and the layer thickness is preferably in a range of 0.1-100 nm although it depends on a raw material.
- This injection layer can be prepared by forming a thin layer made of the above-described material according to a method well known in the art such as a vacuum evaporation method, a spin coating method, a cast method, an inkjet method and a LB method.
- the layer thickness of an injection layer is not specifically limited; however, is generally 5-5,000 nm.
- This injection layer may have a single layer structure comprised of one or not less than two types of the above described materials.
- anode according to an organic EL element of this invention those comprising metal, alloy, a conductive compound, which is provided with a large work function (not less than 4 eV), and a mixture thereof as an electrode substance are preferably utilized.
- an electrode substance include a conductive transparent material such as metal like Au, CuI, indium tin oxide (ITO), SnO 2 and ZnO.
- a material such as IDIXO (In 2 O 3 —ZnO), which can prepare an amorphous and transparent electrode, may be also utilized.
- these electrode substances may be made into a thin layer by a method such as evaporation or spattering and a pattern of a desired form may be formed by means of photolithography, or in the case of requirement of pattern precision is not so severe (not less than 100 ⁇ m), a pattern may be formed through a mask of a desired form at the time of evaporation or spattering of the above-described substance.
- the transmittance is preferably set to not less than 10% and the sheet resistance as an anode is preferably not more than a few hundreds ⁇ / ⁇ .
- the layer thickness depends on a material, it is generally selected in a range of 10-1,000 nm and preferably of 10-200 nm.
- a cathode metal, alloy, a conductive compound and a mixture thereof, which have a small work function (not more than 4 eV), are utilized as an electrode substance.
- an electrode substance includes such as sodium, sodium-potassium alloy, magnesium, lithium, a magnesium/copper mixture, a magnesium/silver mixture, a magnesium/aluminum mixture, a magnesium/indium mixture, an aluminum/aluminum oxide (Al 2 O 3 ) mixture, indium, a lithium/aluminum mixture and rare earth metal.
- a mixture of electron injecting metal with the second metal which is stable metal having a work function larger than electron injecting metal such as a magnesium/silver mixture, a magnesium/aluminum mixture, a magnesium/indium mixture, an aluminum/aluminum oxide (Al 2 O 3 ) mixture and a lithium/aluminum mixture, and aluminum.
- these electrode substances may be made into a thin layer by a method such as evaporation or spattering.
- the sheet resistance as a cathode is preferably not more than a few hundreds ⁇ / ⁇ and the layer thickness is generally selected in a range of 10-1,000 nm and preferably of 10-200 nm.
- either one of an anode or a cathode of an organic EL element is preferably transparent or translucent to improve the mission luminance.
- Substrate also referred to as Base Plate, Base Material or Support
- a substrate according to an organic EL element of this invention is not specifically limited with respect to types of such as glass and plastics provided being transparent, however, a substrate preferably utilized includes such as glass, quartz and transparent resin film.
- a specifically preferable substrate is resin film capable of providing an organic EL element with a flexible property.
- Resin film includes such as film comprised of polyethylene terephthalate (PET), polyethylene naphthalate (PEN), polyether sulphone (PES), polyether imide, polyether etherketone, polyphenylene sulfide, polyallylate, polyimide, polycarbonate (PC) and cellulose acetate propionate (CAP).
- PET polyethylene terephthalate
- PEN polyethylene naphthalate
- PES polyether sulphone
- polyether imide polyether etherketone
- polyphenylene sulfide polyallylate
- polyimide polyimide
- PC polycarbonate
- CAP cellulose acetate propionate
- an inorganic or organic cover layer or a hybrid cover layer comprising the both may be formed, and the film is preferably provided with a high barrier ability having a vapor transmittance of not more than 0.01 g/m 2 ⁇ day ⁇ atm.
- the taking out efficiency of emission of an organic EL element of this invention at room temperature is preferably not less than 1% and more preferably not less than 2%.
- taking out quantum efficiency (%) photon number emitted out of organic EL element/electron number flown into organic EL element ⁇ 100.
- hue improving filter such as a color filter may be utilized in combination.
- roughening processed film (such as anti-glare film) can be also utilized in combination to decrease emission unevenness.
- the display is comprised of at least two types of organic EL elements having different emission maximum wavelengths, and a preferable example to prepare an organic EL element will now be explained.
- a preparation method of an organic EL element of this invention comprising anode/positive hole injection layer/positive hole transport layer/emission layer/positive hole inhibition layer/electron transport layer/cathode buffer layer/cathode, will be explained.
- a thin layer comprising a desired electrode substance such as an anode electrode substance is formed by means of evaporation or spattering so as to make a layer thickness of not more than 1 ⁇ m and preferably of 10-200 nm, whereby an anode is prepared.
- a desired electrode substance such as an anode electrode substance
- thin layers containing organic substances of such as a positive hole injection layer, a positive hole transport layer, an emission layer, a positive hole inhibition layer and an electron transport layer are formed.
- a thin layer forming method of these layers containing the organic substances includes such as a spin coat method, a cast method, an inkjet method, an evaporation method and a printing method as described before, however, a vacuum evaporation method or a spin coat method is specifically preferable with respect to easy preparation of a homogeneous layer and bare generation of pinholes. Further, a different layer forming method depending on each layer may be applied. In the case of employing an evaporation method in layer formation, the evaporation condition depends on such as the type of a utilized compound, however, is generally appropriately selected in a range of 50-450° C.
- a boat heating temperature 10 ⁇ 6 -10 ⁇ 2 Pa as a vacuum degree, 0.01-50 nm/sec as a deposition rate, ⁇ 50-300° C. as a substrate temperature and 1 nm-5 ⁇ m as a layer thickness.
- a thin layer comprising a cathode electrode substance is formed thereon by means of such as evaporation or spattering so as to make a layer thickness in a range of 50-200 nm to provide a cathode, whereby a desired organic EL element can be prepared.
- This preparation of an organic EL element is preferably carried out with one time evacuation to prepare all through from a positive hole injection layer to a cathode, however, different layer forming method may be also applied by taking out the element on the way. At that time, consideration is necessary such as to perform the operation under a dry inert gas environment.
- a display device of this invention may be either monochromatic or multi-colored.
- a multicolor display device In the case of a multicolor display device, a shadow mask is provided only at the time of emission layer formation, and layers can be formed all over the surface by such as an evaporation method, a cast method, a spin coat method, an inkjet method and a printing method.
- the method is not specifically limited; however, preferable are an evaporation method, an inkjet method and a printing method. And patterning employing a shadow mask is preferred in the case of an evaporation method.
- emission can be observed by application of a voltage of approximately 2-40 V setting an anode to + polarity and a cathode to ⁇ polarity. Further, no current flows and no emission generate at all even when a voltage is applied with a reversed polarity. Further, in the case of alternate current voltage being applied, emission generates only in a state of an anode being + and a cathode being ⁇ .
- the wave shape of alternate current may be arbitrary.
- a multicolor display device can be utilized as a display device, a display and various types of emission light sources.
- a display device and a display full-colored display is possible by employing three types of organic EL elements providing blue, red and green emissions.
- a display device and a display include a TV, a personal computer, a mobile instrument, an AV instrument, a character broadcast display and an information display in a car.
- the display device and the display may be also utilized as a display to playback still images and moving images, and may adopt either a simple matrix (a passive matrix) mode or an active matrix mode when being utilized as a display device for moving image playback.
- An illumination light source includes a home use illumination, a car room illumination, a backlight of a watch or a liquid crystal, a panel advertisement, a signal, a light source of an optical memory medium, a light source for an electrophotographic copier, a light source for an optical telecommunication processor and a light source for a photo-sensor, however, is not limited thereto.
- An organic EL element of this invention can be utilized as an organic EL element provided with a resonator structure, and a utilization purpose of such an organic EL element provided with a resonator structure includes such as a light source for an optical memory medium, a light source for an electrophotographic copier, a light source for a optical telecommunication processor and a light source for a photo-sensor, however, is not limited thereto. Further, the organic EL element may be utilized for the above-described applications by being made to perform laser emission.
- an organic EL element of this invention may be utilized as one type of a lamp like an illumination and an exposure light, and may be also utilized as a display device of a projector of an image projecting type and a display device (a display) of a type to directly view still images and moving images.
- An operating mode in the case of being utilized as a display device for playback of moving images may be either a simple matrix (a passive matrix) mode or an active matrix mode.
- a full-color display device can be prepared by utilizing at least two types of organic EL elements of this invention which emit different emitting colors.
- FIG. 1 is a schematic drawing to show an example of a display device constituted of an organic EL element. It is a schematic drawing of a display, which displays image information by emission of an organic EL element, such as a mobile phone.
- Display 1 is constituted of such as display section A having plural number of pixels and control section B which performs image scanning of display section A based on image information.
- Control section B which is electrically connected to display section A, sends a scanning signal and an image data signal to plural number of pixels based on image information from the outside and pixels of each scanning line successively emit depending on the image data signal by a scanning signal to perform image scanning, whereby image information is displayed on display section A.
- FIG. 2 is a schematic drawing of display section A.
- Display section A is provided with such as a wiring part, which contains plural scanning lines 5 and data lines 6 , and plural pixels 3 on a substrate. Primary part materials of display section A will be explained in the following.
- Scanning lines 5 and plural data lines 6 in a wiring part each are comprised of a conductive material, and scanning lines 5 and data lines 6 are perpendicular in a grid form and are connected to pixels 3 at the right-angled crossing points (details are not shown in the drawing).
- Pixel 3 receives an image data from data line 6 when a scanning signal is applied from scanning line 5 and emits according to the received image data.
- Full-color display device is possible by appropriately arranging pixels having an emission color in a red region, pixels in a green region and pixels in a blue region, side by side on the same substrate.
- FIG. 3 is a schematic drawing of a pixel.
- a pixel is equipped with such as organic EL element 10 , switching transistor 11 , operating transistor 12 and condenser 13 .
- Red, green and blue emitting organic EL elements are utilized as organic EL element 10 for plural pixels, and full-color display device is possible by arranging these side by side on the same substrate.
- an image data signal is applied on the drain of switching transistor 11 via data line 6 from control section B. Then, when a scanning signal is applied on the gate of switching transistor 11 via scanning line 5 from control section B, operation of switching transistor is on to transmit the image data signal applied on the drain to the gates of condenser 13 and operating transistor 12 .
- Operating transistor 12 is on, simultaneously with condenser 13 being charged depending on the potential of an image data signal, by transmission of an image data signal.
- the drain is connected to electric source line 7 and the source is connected to the electrode of organic EL element 10 , and an electric current is supplied from electric source line 7 to organic EL element 10 depending on the potential of an image data applied on the gate.
- emission of each organic EL element 10 of plural pixels 3 is performed by providing switching transistor 11 and operating transistor 12 against each organic EL element 10 of plural pixels 3 .
- Such an emission method is called as an active matrix mode.
- emission of organic EL element 10 may be either emission of plural gradations based on a multiple-valued image data signal having plural number of gradation potentials or on and off of a predetermined emission quantity based on a binary image data signal.
- potential hold of condenser 13 may be either continuously maintained until the next scanning signal application or discharged immediately before the next scanning signal application.
- emission operation is not necessarily limited to the above-described active matrix mode but may be a passive matrix mode in which organic EL element is emitted based on a data signal only when a scanning signal is scanned.
- FIG. 4 is a schematic drawing of a display device based on a passive matrix mode.
- plural number of scanning lines 5 and plural number of image data lines 6 are arranged grid-wise, opposing to each other and sandwiching pixels 3 .
- pixel 3 connected to scanning line 5 applied with said signal emits depending on an image data signal.
- pixel 3 is provided with no active element in a passive matrix mode, decrease of manufacturing cost is possible.
- An organic EL element material of this invention can be also applied to an organic EL element to generate emission of practically white color as an illumination device.
- Plural emission colors are simultaneously emitted by plural number of emission materials to obtain white light by mixing colors.
- a combination of plural emission colors may be either the one, in which three emission maximum wavelengths of three primary colors of blue, green and red are contained, or the other, in which two emission maximum wavelengths, utilizing a relationship of complimentary colors such as blue and yellow, or blue and orange, are contained.
- a combination of emission materials to obtain plural number of emission colors may be either a combination comprising plural number of materials which emit phosphoresce or fluorescence, or a combination of a material which emits phosphoresce or fluorescence and a dye material which emits by light from an emission material as exiting light, however, in a white organic electroluminescence element according to this invention, it is enough only to mix plural emission dopants in combination.
- a mask is provided only at the time of forming such as an emission layer, a positive hole transport layer or an electron transport layer, to only simply arrange the plural emission dopants such as by separately painting through the mask, while other layers are commonly utilized to require no patterning such as a mask.
- an electrode can be formed all over the plane by such as an evaporation method, a cast method, a spin coat method, an inkjet method and a printing method, resulting in improvement of productivity.
- an element itself is white emitting.
- An emission material utilized in an emission layer is not specifically limited, and in the case of a backlight of a liquid crystal display element, any combination by arbitrary selection among platinum complexes according to this invention or emission materials well known in the art can be utilized so as to be fitted to the wavelength range corresponding to CF (color filter) characteristics, whereby white emission can be obtained.
- a white emitting organic EL element of this invention is usefully utilized as one type of a lamp such as a home use illumination, a car room illumination or an exposure light source as various emission light sources or illumination devices, in addition to the aforesaid display device and a display, and is further usefully applied for a display as such as a backlight of a liquid crystal display.
- a backlight of a watch an advertising board, a signal, a light source of an optical memory medium, a light source of an electrophotographic copier, a light source of an optical telecommunication processor and a light source of an optical sensor, and further general home us electric instruments which require a display device.
- emission host materials such as emission host materials, emission dopants and materials employed in formation of a positive hole inhibition layer, which are utilized in any one of Examples 1-6, will be shown.
- the transparent support substrate was washed with isopropyl alcohol by use of ultrasonic waves, followed by being dried with a dry nitrogen gas, and was subjected to UV ozone washing for 5 minutes.
- This transparent support substrate was fixed on a substrate holder of a vacuum evaporation system available on the market, and on the other hand, each of five resistance heating boats made of tantalum was charged with ⁇ -NPD, CBP, Ir-10, BCP and Alq 3 , respectively, which was attached in the vacuum evaporation system (in the first vacuum chamber).
- a resistance heating boat made of tantalum was charged with lithium fluoride and a resistance heating boat made of tungsten was charged with aluminum, respectively, and these boats were attached in the second chamber of the vacuum evaporation system.
- the aforesaid heating boat charged with ⁇ -NPD was heated with an electric current to deposit ⁇ -NPD on a support substrate at a deposition rate of 0.1-0.2 nm/sec so as to make a layer thickness of 25 nm, whereby a positive hole injection/transport layer was formed.
- the aforesaid heating boat charged with CBP and the boat charged with Ir-10 were independently supplied with an electric current to deposit CBP as an emission host and Ir-10 as an emission dopant so as to make a layer thickness of 30 nm while adjusting the deposition rates thereof to 100/7, whereby an emission layer was formed.
- the aforesaid heating boat charged with BCP was heated with an electric current to provide a positive hole inhibition layer having a layer thickness of 10 nm at a deposition rate of 0.1-0.2 nm/sec. Further, the aforesaid heating boat charged with Alq 3 was heated with an electric current to provide an electron transport layer having a layer thickness of 40 nm at a deposition rate of 0.1-0.2 nm/sec.
- a mask which was made of stainless steel and had rectangular holes, was arranged on the electron injection layer by means of remote control from outside of the system.
- a boat charged with lithium fluoride was supplied with an electric current to provide a cathode buffer layer having a layer thickness of 0.5 nm at a deposition rate of 0.01-0.02 nm/sec, and then a boat charged with aluminum was supplied with an electric current to provide a cathode having a layer thickness of 150 nm at a deposition rate of 1-2 nm/sec.
- this organic EL element was transferred into a glove box under a nitrogen atmosphere (a glove box substituted with a nitrogen gas having a high purity of not less than 99.99%) without contacting with the air, and was made into a sealed structure the interior of which is substituted with nitrogen as shown in FIG.
- barium oxide 105 which is a water capturing agent, utilized was high purity barium oxide powder manufactured by Aldrich Corp., which had been pasted in glass sealing can 104 by use of fluorine-containing resin semi-permeable membrane attached with an adhesive (Microtex S-NTF8031Q, manufactured by Nitto Denko Corp.) and prepared in advance.
- an adhesive Microtex S-NTF8031Q, manufactured by Nitto Denko Corp.
- ultraviolet ray curable adhesive 107 was utilized, and the both were adhered with irradiation of ultraviolet rays, whereby a sealed element was prepared.
- 101 is a glass substrate provided with a transparent electrode; 102 is the aforesaid organic EL layer comprising such as a positive hole injection/transport layer, an emission layer and a positive hole inhibition layer and an electron transport layer; and 103 is a cathode.
- Organic EL elements OLED 1-2-1-17 each were prepared in a similar manner to preparation of organic EL element OLED 1-1 described above, except that an emission dopant was changed as shown in table 1.
- Organic EL element OLED 1-18 was prepared in a similar manner to preparation of organic EL element OLED 1-1, except that an emission host was changed to AZ1 from CBP and metal complexes of this invention (shown in the table by compound No.) were utilized as an emission dopant.
- Each of organic EL elements OLED 1-1-1-18 was lighted under a constant current condition of 2.5 mA/cm 2 at room temperature (approximately 23-25° C.), and an emission luminance (L) [cd/m 2 ] immediately after turning on was measured, whereby a quantum efficiency of taking out ( ⁇ ) was calculated.
- CS-1000 produced by Minolta Co., Ltd. was utilized for measurement of emission luminance.
- each of the quantum efficiency of taking out was expressed as a relative value when that of organic EL element OLED 1-1 was set to 100.
- Each of organic EL elements OLED 1-1-1-18 was continuously lighted under a constant current condition of 2.5 mA/cm 2 at room temperature (approximately 23-25° C.), and time to reach a half of the initial luminance ( ⁇ 1/2) was measured. Further, each emission life was expressed as a relative value when that of organic EL element OLED 1-1 was set to 100.
- organic EL elements prepared by employing a metal complex according to this invention as an organic EL element material can achieve high emission efficiency and longer emission life, compared to the comparative elements.
- all emission colors of elements of this invention were blue.
- Organic EL elements OLED 2-1-2-17 were prepared in a similar manner to example 1, except that an emission dopant was changed as described in table 2.
- Organic EL element OLED 2-18 was prepared in a similar manner to preparation of organic EL element OLED 2-1, except that an emission host was changed to AZ1 from CBP and metal complexes of this invention (shown in the table by compound No.) were utilized as an emission dopant.
- any value of each organic EL element sample was expressed as a relative value when the value of OLED 2-1 was set to 100.
- the obtained results are shown in table 2.
- organic EL elements prepared by employing an organic EL element material of this invention as an emission dopant can achieve high emission efficiency and long emission life, compared to the comparative elements.
- all emission colors of elements of this invention were green.
- Organic EL elements OLED 3-1-3-9 were prepared in a similar manner to example 1, except that an emission dopant was changed as described in table 3.
- Organic EL element OLED 3-10 was prepared in a similar manner to preparation of organic EL element OLED 3-1, except that an emission host was changed to AZ1 from CBP and metal complexes of this invention (shown in the table by compound No.) were utilized as an emission dopant.
- organic EL elements employing a compound of this invention as an emission dopant can achieve higher emission efficiency and long emission life, compared to the comparative element.
- all emission colors of elements of this invention were red.
- Organic EL element OLED 4-1 was prepared in a similar manner to organic EL element OLED 2-1 of example 2. Successively, organic EL elements OLED 4-2-4-13 were prepared in a similar manner to organic EL element OLED 4-1, except that a positive hole inhibition material was changed as described in table 4.
- Organic EL element OLED 1-5 of example 1 was utilized as a blue emission element.
- Organic EL element OLED 2-7 of Example 2 was utilized as a green emission element.
- Organic EL element OLED 3-6 of Example 3 was utilized as a red emission element.
- Each of red, green and blue organic EL elements prepared above was arranged parallel on the same substrate to prepare an active matrix mode full-color having a form as described in FIG. 1 , and only display section A of said display device is schematically shown in FIG. 2 . That is, a wiring section containing plural lines of scanning line 5 and data line 6 , and plural pixels 3 (such as a pixel having an emission color of a red region, a pixel of a green region and a pixel of a blue region) arranged parallel are provided on the same substrate, and scanning lines 5 and data lines 6 in a wiring section, which are comprised of a conductive material, respectively, cross each other at a right angle in a grid form to be connected to pixels 3 at the right-angled crossing points (details being not shown in the drawing).
- the aforesaid plural pixels 3 each are operated in an active matrix mode, in which an organic EL element, a switching transistor and an operating transistor are provided corresponding to each emission color, and receive an image data signal from data line 6 when a scanning signal is applied from scanning line 5 to emit based on the received image data.
- Each red, green and blue pixel was appropriately arranged parallel in this manner, whereby a full-color display device was prepared.
- a transparent electrode substrate of example 1 was subjected to patterning of an electrode having an area of 20 mm ⁇ 20 mm, and ⁇ -NPD was deposited thereon at a layer thickness of 25 nm as a positive hole injection/transport layer in a similar manner to example 1; and further the aforesaid heating boat charged with CBP, boat containing I-4 and boat containing Ir-9 were supplied with an electric current to deposit an emission layer having a layer thickness of 30 nm, while adjusting the evaporation rates of CBP as an emission host, Ir-4 and Ir-9 as emission dopants to be 100/5/0.6.
- BCP was deposited at 10 nm to provide a positive hole inhibition layer.
- Alq 3 was deposited at 40 nm to provide an electron transport layer.
- a mask with square holes having a shape nearly same as a transparent electrode made of stainless steel was arranged on an electron injection layer, and 0.5 nm of lithium fluoride as a cathode buffer layer and 150 nm of aluminum as a cathode were deposited.
- FIG. 6 is a schematic drawing of the flat lamp.
- FIG. 6( a ) is a schematic plane view and
- FIG. 6( b ) is a schematic cross-sectional view.
- This invention can provide an organic electroluminescence element material, which is a metal complex having a specific ligand, and an organic EL element, an illumination and a display device, which are provided with high emission efficiency as well as long emission life.
- Said electroluminescence element material is characterized by containing a metal complex having a ligand represented by Formula (1).
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Inorganic Chemistry (AREA)
- Organic Chemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Physics & Mathematics (AREA)
- High Energy & Nuclear Physics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Electroluminescent Light Sources (AREA)
Abstract
Description
| TABLE 1 | ||||
| Quantum | ||||
| Element | Emission | efficiency of | Emission | |
| No. | dopant | taking out | life | Remarks |
| OLED 1-1 | Ir-10 | 100 | 100 | Comparison |
| OLED 1-2 | Ir-12 | 98 | 87 | Comparison |
| OLED 1-3 | |
99 | 80 | Comparison |
| OLED 1-4 | Comparison 2 | 97 | 75 | Comparison |
| OLED 1-5 | I-4 | 119 | 180 | Invention |
| OLED 1-6 | I-6 | 117 | 215 | Invention |
| OLED 1-7 | I-36 | 128 | 177 | Invention |
| OLED 1-8 | I-41 | 123 | 168 | Invention |
| OLED 1-9 | I-67 | 118 | 177 | Invention |
| OLED 1-13 | P-4 | 118 | 177 | Invention |
| OLED 1-14 | P-6 | 117 | 212 | Invention |
| OLED 1-15 | P-13 | 121 | 170 | Invention |
| OLED 1-16 | P-35 | 119 | 166 | Invention |
| OLED 1-17 | P-42 | 119 | 159 | Invention |
| OLED 1-18 | I-68 | 129 | 220 | Invention |
| TABLE 2 | ||||
| Quantum | ||||
| Element | Emission | efficiency of | Emission | |
| No. | dopant | taking out | life | Remarks |
| OLED 2-1 | Ir-1 | 100 | 100 | Comparison |
| OLED 2-2 | I-1 | 112 | 180 | Invention |
| OLED 2-3 | I-8 | 115 | 160 | Invention |
| OLED 2-4 | I-47 | 110 | 155 | Invention |
| OLED 2-5 | I-48 | 112 | 146 | Invention |
| OLED 2-6 | I-50 | 109 | 135 | Invention |
| OLED 2-7 | I-51 | 110 | 139 | Invention |
| OLED 2-8 | I-61 | 110 | 142 | Invention |
| OLED 2-9 | I-69 | 109 | 140 | Invention |
| OLED 2-10 | P-1 | 111 | 176 | Invention |
| OLED 2-11 | P-7 | 114 | 163 | Invention |
| OLED 2-12 | P-45 | 110 | 145 | InventIon |
| OLED 2-13 | P-48 | 110 | 142 | Invention |
| OLED 2-14 | P-5O | 109 | 133 | Invention |
| OLED 2-15 | P-51 | 110 | 142 | Invention |
| OLED 2-16 | P-61 | 110 | 138 | Invention |
| OLED 2-17 | P-66 | 109 | 130 | Invention |
| OLED 2-18 | I-61 | 116 | 181 | Invention |
| TABLE 3 | ||||
| Quantum | ||||
| Element | Emission | efficiency of | Emission | |
| No. | dopant | taking out | life | Remarks |
| OLED 3-1 | Ir-9 | 100 | 100 | Comparison |
| OLED 3-2 | |
94 | 95 | Comparison |
| OLED 3-4 | I-20 | 112 | 160 | Invention |
| OLED 3-5 | I-37 | 112 | 165 | Invention |
| OLED 3-6 | I-70 | 110 | 180 | Invention |
| OLED 3-7 | I-71 | 112 | 168 | Invention |
| OLED 3-8 | P-43 | 110 | 175 | Invention |
| OLED 3-9 | P-44 | 114 | 148 | Invention |
| OLED 3-10 | I-71 | 112 | 185 | Invention |
| TABLE 4 | ||||
| Positive | ||||
| hole | Quantum | |||
| Element | inhibition | efficiency of | Emission | |
| No. | material | taking out | life | Remarks |
| OLED 4-1 | BCP | 100 | 100 | Comparison |
| OLED 4-2 | I-2 | 114 | 155 | Invention |
| OLED 4-3 | I-4 | 114 | 143 | Invention |
| OLED 4-4 | I-10 | 116 | 140 | Invention |
| OLED 4-5 | I-14 | 115 | 130 | Invention |
| OLED 4-6 | I-41 | 113 | 130 | Invention |
| OLED 4-7 | I-61 | 110 | 135 | Invention |
| OLED 4-8 | P-2 | 113 | 150 | Invention |
| OLED 4-9 | P-9 | 115 | 137 | Invention |
| OLED 4-10 | P-4 | 113 | 142 | Invention |
| OLED 4-11 | P-15 | 115 | 134 | Invention |
| OLED 4-12 | P-42 | 112 | 123 | Invention |
| OLED 4-13 | P-61 | 109 | 129 | Invention |
Claims (14)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2004103247 | 2004-03-31 | ||
| JP2004-103247 | 2004-03-31 | ||
| PCT/JP2005/004678 WO2005097940A1 (en) | 2004-03-31 | 2005-03-16 | Organic electroluminescent device material, organic electroluminescent device, display and illuminating device |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20070196687A1 US20070196687A1 (en) | 2007-08-23 |
| US7790890B2 true US7790890B2 (en) | 2010-09-07 |
Family
ID=35125050
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/598,971 Expired - Fee Related US7790890B2 (en) | 2004-03-31 | 2004-03-31 | Organic electroluminescence element material, organic electroluminescence element, display device and illumination device |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US7790890B2 (en) |
| EP (1) | EP1731584A1 (en) |
| JP (1) | JPWO2005097940A1 (en) |
| WO (1) | WO2005097940A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20130341612A1 (en) * | 2011-03-04 | 2013-12-26 | Konica Minolta , Inc. | Organic electroluminescence element |
| US11367841B2 (en) * | 2016-11-08 | 2022-06-21 | Samsung Display Co., Ltd. | Heterocyclic compound and organic electroluminescence device including the same |
Families Citing this family (449)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3919583B2 (en) * | 2002-04-12 | 2007-05-30 | キヤノン株式会社 | Organic light emitting device |
| US20070111024A1 (en) * | 2003-07-02 | 2007-05-17 | Fumino Okuda | Metal complex compound and organic electroluminescence device containing the same |
| US9051344B2 (en) * | 2005-05-06 | 2015-06-09 | Universal Display Corporation | Stability OLED materials and devices |
| WO2007029466A1 (en) * | 2005-09-06 | 2007-03-15 | Konica Minolta Holdings, Inc. | Organic electroluminescent device, display and illuminating device |
| ITMI20060444A1 (en) | 2006-03-13 | 2007-09-14 | Getters Spa | USE OF MAGNESIUM-COPPER COMPOSITIONS FOR MAGNESIUM EVAPORATION AND MAGNESIUM DISPENSERS |
| DE102006017485B4 (en) * | 2006-04-13 | 2014-06-05 | Merck Patent Gmbh | Biphenyl-Metal Complexes - Monomers and Oligomers Triplet emitters for OLED applications |
| DE102007031261A1 (en) | 2007-07-05 | 2009-01-08 | Universtität Regensburg | Luminescent metal complexes with bulky auxiliary ligands |
| JP2009032989A (en) * | 2007-07-27 | 2009-02-12 | Fujifilm Corp | Organic electroluminescent element |
| DE102008013691A1 (en) | 2008-03-11 | 2009-09-17 | Merck Patent Gmbh | Use of compositions of neutral transition metal complexes in opto-electronic devices |
| DE102008015526B4 (en) | 2008-03-25 | 2021-11-11 | Merck Patent Gmbh | Metal complexes |
| DE102008027005A1 (en) | 2008-06-05 | 2009-12-10 | Merck Patent Gmbh | Organic electronic device containing metal complexes |
| DE102008033563A1 (en) | 2008-07-17 | 2010-01-21 | Merck Patent Gmbh | Complexes with Small Singlet-Triplet Energy Intervals for Use in Opto-Electronic Devices (Singlet Harvesting Effect) |
| DE102008036247A1 (en) | 2008-08-04 | 2010-02-11 | Merck Patent Gmbh | Electronic devices containing metal complexes |
| DE102008036982A1 (en) | 2008-08-08 | 2010-02-11 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102008048336A1 (en) | 2008-09-22 | 2010-03-25 | Merck Patent Gmbh | Mononuclear neutral copper (I) complexes and their use for the production of optoelectronic devices |
| DE102008050841B4 (en) | 2008-10-08 | 2019-08-01 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| US8865321B2 (en) | 2008-11-11 | 2014-10-21 | Merck Patent Gmbh | Organic electroluminescent devices |
| DE102009022858A1 (en) | 2009-05-27 | 2011-12-15 | Merck Patent Gmbh | Organic electroluminescent devices |
| DE102008057051B4 (en) | 2008-11-13 | 2021-06-17 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102008057050B4 (en) | 2008-11-13 | 2021-06-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102008063470A1 (en) | 2008-12-17 | 2010-07-01 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102008063490B4 (en) | 2008-12-17 | 2023-06-15 | Merck Patent Gmbh | Organic electroluminescent device and method for adjusting the color locus of a white-emitting electroluminescent device |
| DE102009007038A1 (en) | 2009-02-02 | 2010-08-05 | Merck Patent Gmbh | metal complexes |
| DE102009009277B4 (en) | 2009-02-17 | 2023-12-07 | Merck Patent Gmbh | Organic electronic device, process for its production and use of compounds |
| DE102009011223A1 (en) | 2009-03-02 | 2010-09-23 | Merck Patent Gmbh | metal complexes |
| DE102009012346B4 (en) | 2009-03-09 | 2024-02-15 | Merck Patent Gmbh | Organic electroluminescent device and method for producing the same |
| DE102009013041A1 (en) | 2009-03-13 | 2010-09-16 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009017064A1 (en) | 2009-04-09 | 2010-10-14 | Merck Patent Gmbh | Organic electroluminescent device |
| US8586203B2 (en) * | 2009-05-20 | 2013-11-19 | Universal Display Corporation | Metal complexes with boron-nitrogen heterocycle containing ligands |
| DE102009023154A1 (en) | 2009-05-29 | 2011-06-16 | Merck Patent Gmbh | A composition comprising at least one emitter compound and at least one polymer having conjugation-interrupting units |
| DE102009023155A1 (en) | 2009-05-29 | 2010-12-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP2445976A2 (en) | 2009-06-22 | 2012-05-02 | Merck Patent GmbH | Conducting formulation |
| DE102009031021A1 (en) | 2009-06-30 | 2011-01-05 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009032922B4 (en) | 2009-07-14 | 2024-04-25 | Merck Patent Gmbh | Materials for organic electroluminescent devices, processes for their preparation, their use and electronic device |
| JP5778148B2 (en) | 2009-08-04 | 2015-09-16 | メルク パテント ゲーエムベーハー | Electronic devices containing polycyclic carbohydrates |
| JP5784608B2 (en) | 2009-09-16 | 2015-09-24 | メルク パテント ゲーエムベーハー | Formulations for electronic device manufacturing |
| DE102009041289A1 (en) | 2009-09-16 | 2011-03-17 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102009053645A1 (en) | 2009-11-17 | 2011-05-19 | Merck Patent Gmbh | Materials for organic electroluminescent device |
| DE102009053644B4 (en) | 2009-11-17 | 2019-07-04 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009041414A1 (en) | 2009-09-16 | 2011-03-17 | Merck Patent Gmbh | metal complexes |
| DE102009042693A1 (en) | 2009-09-23 | 2011-03-24 | Merck Patent Gmbh | Materials for electronic devices |
| DE102009048791A1 (en) | 2009-10-08 | 2011-04-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009049587A1 (en) | 2009-10-16 | 2011-04-21 | Merck Patent Gmbh | metal complexes |
| DE102009053382A1 (en) | 2009-11-14 | 2011-05-19 | Merck Patent Gmbh | Materials for electronic devices |
| DE102009053836A1 (en) | 2009-11-18 | 2011-05-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009057167A1 (en) | 2009-12-05 | 2011-06-09 | Merck Patent Gmbh | Electronic device containing metal complexes |
| EP2517537B1 (en) | 2009-12-22 | 2019-04-03 | Merck Patent GmbH | Electroluminescent functional surfactants |
| EP2517278B1 (en) | 2009-12-22 | 2019-07-17 | Merck Patent GmbH | Electroluminescent formulations |
| WO2011076323A1 (en) | 2009-12-22 | 2011-06-30 | Merck Patent Gmbh | Formulations comprising phase-separated functional materials |
| US9178156B2 (en) | 2009-12-23 | 2015-11-03 | Merck Patent Gmbh | Compositions comprising polymeric binders |
| KR20170093267A (en) | 2009-12-23 | 2017-08-14 | 메르크 파텐트 게엠베하 | Compositions comprising organic semiconducting compounds |
| DE102010004803A1 (en) | 2010-01-16 | 2011-07-21 | Merck Patent GmbH, 64293 | Materials for organic electroluminescent devices |
| DE102010005697A1 (en) | 2010-01-25 | 2011-07-28 | Merck Patent GmbH, 64293 | Connections for electronic devices |
| DE102010006377A1 (en) | 2010-01-29 | 2011-08-04 | Merck Patent GmbH, 64293 | Styrene-based copolymers, in particular for use in optoelectronic components |
| KR20110093055A (en) * | 2010-02-11 | 2011-08-18 | 다우어드밴스드디스플레이머티리얼 유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device using the same |
| DE102010009193B4 (en) | 2010-02-24 | 2022-05-19 | MERCK Patent Gesellschaft mit beschränkter Haftung | Composition containing fluorine-fluorine associates, processes for their production, their use and organic electronic devices containing them |
| DE102010009903A1 (en) | 2010-03-02 | 2011-09-08 | Merck Patent Gmbh | Connections for electronic devices |
| DE102010010481A1 (en) | 2010-03-06 | 2011-09-08 | Merck Patent Gmbh | Organic electroluminescent device |
| KR20130020883A (en) | 2010-03-11 | 2013-03-04 | 메르크 파텐트 게엠베하 | Fibers in therapy and cosmetics |
| JP2013522816A (en) | 2010-03-11 | 2013-06-13 | メルク パテント ゲーエムベーハー | Light emitting fiber |
| US9627632B2 (en) | 2010-03-23 | 2017-04-18 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010012738A1 (en) | 2010-03-25 | 2011-09-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010013068A1 (en) | 2010-03-26 | 2011-09-29 | Merck Patent Gmbh | Connections for electronic devices |
| KR101896723B1 (en) | 2010-04-12 | 2018-09-07 | 메르크 파텐트 게엠베하 | Composition and method for preparation of organic electronic devices |
| WO2011128034A1 (en) | 2010-04-12 | 2011-10-20 | Merck Patent Gmbh | Composition having improved performance |
| DE102010014933A1 (en) | 2010-04-14 | 2011-10-20 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010018321A1 (en) | 2010-04-27 | 2011-10-27 | Merck Patent Gmbh | Organic electroluminescent device |
| WO2011137922A1 (en) | 2010-05-03 | 2011-11-10 | Merck Patent Gmbh | Formulations and electronic devices |
| DE102010019306B4 (en) | 2010-05-04 | 2021-05-20 | Merck Patent Gmbh | Organic electroluminescent devices |
| DE102010020044A1 (en) | 2010-05-11 | 2011-11-17 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102010020567A1 (en) | 2010-05-14 | 2011-11-17 | Merck Patent Gmbh | metal complexes |
| EP2576723B1 (en) | 2010-05-27 | 2017-09-20 | Merck Patent GmbH | Compositions comprising quantum dots |
| US9206352B2 (en) | 2010-05-27 | 2015-12-08 | Merck Patent Gmbh | Formulation and method for preparation of organic electronic devices |
| WO2011157339A1 (en) | 2010-06-15 | 2011-12-22 | Merck Patent Gmbh | Metal complexes |
| DE102010024335A1 (en) | 2010-06-18 | 2011-12-22 | Merck Patent Gmbh | Connections for electronic devices |
| DE102010024542A1 (en) | 2010-06-22 | 2011-12-22 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010024897A1 (en) | 2010-06-24 | 2011-12-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| JP4729642B1 (en) * | 2010-07-09 | 2011-07-20 | 富士フイルム株式会社 | Organic electroluminescence device |
| DE102010027218A1 (en) | 2010-07-15 | 2012-01-19 | Merck Patent Gmbh | Organic complexes containing metals |
| DE102010027320A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | Polymeric materials for organic electroluminescent devices |
| DE102010027317A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | metal complexes |
| DE102010027316A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | metal complexes |
| DE102010027319A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | metal complexes |
| CN103026525B (en) | 2010-07-26 | 2016-11-09 | 默克专利有限公司 | Nanocrystal in the devices |
| EP2599141B1 (en) | 2010-07-26 | 2019-12-11 | Merck Patent GmbH | Quantum dots and hosts |
| US9236578B2 (en) | 2010-07-30 | 2016-01-12 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102010033548A1 (en) | 2010-08-05 | 2012-02-09 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010045405A1 (en) | 2010-09-15 | 2012-03-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010046412B4 (en) | 2010-09-23 | 2022-01-13 | Merck Patent Gmbh | metal-ligand coordination compounds |
| DE102010046512A1 (en) | 2010-09-24 | 2012-03-29 | Merck Patent Gmbh | Phosphorus-containing metal complexes |
| DE102010048074A1 (en) | 2010-10-09 | 2012-04-12 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010048498A1 (en) | 2010-10-14 | 2012-04-19 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010048607A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Connections for electronic devices |
| DE102010048608A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN103228647B (en) | 2010-11-24 | 2017-02-15 | 默克专利有限公司 | Materials for organic electroluminescent devices |
| DE102010054316A1 (en) | 2010-12-13 | 2012-06-14 | Merck Patent Gmbh | Substituted tetraarylbenzenes |
| DE102010054525A1 (en) | 2010-12-15 | 2012-04-26 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102011106849A1 (en) | 2010-12-15 | 2012-06-21 | Merck Patent Gmbh | Process for the synthesis of N-N linked and around the N-N bond of rotation-inhibited bis-N-heterocyclic carbenes and their use as ligands for metal complexes |
| DE102010055902A1 (en) | 2010-12-23 | 2012-06-28 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102010055901A1 (en) | 2010-12-23 | 2012-06-28 | Merck Patent Gmbh | Organic electroluminescent device |
| EP2663567B1 (en) | 2011-01-13 | 2016-06-01 | Merck Patent GmbH | Compounds for organic electroluminescent devices |
| DE102012000064A1 (en) | 2011-01-21 | 2012-07-26 | Merck Patent Gmbh | New heterocyclic compound excluding 9,10-dioxa-4b-aza-9a-phospha-indeno(1,2-a)indene and 9,10-dioxa-4b-aza-indeno(1,2-a)indene, useful e.g. as matrix material for fluorescent or phosphorescent emitters of electronic devices |
| US8751777B2 (en) | 2011-01-28 | 2014-06-10 | Honeywell International Inc. | Methods and reconfigurable systems to optimize the performance of a condition based health maintenance system |
| DE102011010841A1 (en) | 2011-02-10 | 2012-08-16 | Merck Patent Gmbh | (1,3) -dioxane-5-one compounds |
| DE102011011104A1 (en) | 2011-02-12 | 2012-08-16 | Merck Patent Gmbh | Substituted dibenzonaphthacenes |
| US9492681B2 (en) | 2011-02-14 | 2016-11-15 | Merck Patent Gmbh | Device and method for treatment of cells and cell tissue |
| DE102011011539A1 (en) | 2011-02-17 | 2012-08-23 | Merck Patent Gmbh | Connections for electronic devices |
| EP2688646A1 (en) | 2011-03-24 | 2014-01-29 | Merck Patent GmbH | Organic ionic functional materials |
| US9469667B2 (en) | 2011-04-04 | 2016-10-18 | Merck Patent Gmbh | Metal complexes |
| CN103477462B (en) | 2011-04-05 | 2016-05-25 | 默克专利有限公司 | Organic electroluminescence device |
| JP5922223B2 (en) | 2011-04-13 | 2016-05-24 | メルク パテント ゲーエムベーハー | Compounds for electronic devices |
| EP2697225B1 (en) | 2011-04-13 | 2015-10-07 | Merck Patent GmbH | Materials for electronic devices |
| US9735371B2 (en) | 2011-04-18 | 2017-08-15 | Merck Patent Gmbh | Compounds for electronic devices |
| JP6215192B2 (en) | 2011-04-18 | 2017-10-18 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescence devices |
| WO2012149992A1 (en) | 2011-05-04 | 2012-11-08 | Merck Patent Gmbh | Device for preserving fresh goods |
| JP6193215B2 (en) | 2011-05-05 | 2017-09-06 | メルク パテント ゲーエムベーハー | Compounds for electronic devices |
| US10177312B2 (en) | 2011-05-05 | 2019-01-08 | Merck Patent Gmbh | Compounds for electronic devices |
| US9496502B2 (en) | 2011-05-12 | 2016-11-15 | Merck Patent Gmbh | Organic ionic compounds, compositions and electronic devices |
| DE102012007810A1 (en) | 2011-05-16 | 2012-11-22 | Merck Patent Gmbh | Electronic device, preferably organic electroluminescent device comprises a transition metal compound exhibiting a transition metal-tin bond |
| DE102011102586A1 (en) | 2011-05-27 | 2012-11-29 | Merck Patent Gmbh | Organic electronic device |
| KR101934135B1 (en) | 2011-06-03 | 2019-04-05 | 메르크 파텐트 게엠베하 | Organic electroluminescence device |
| JP6125492B2 (en) | 2011-06-03 | 2017-05-10 | メルク パテント ゲーエムベーハー | Metal complex |
| EP2726490B1 (en) | 2011-06-28 | 2015-04-29 | Merck Patent GmbH | Metal complexes |
| WO2013017189A1 (en) | 2011-07-29 | 2013-02-07 | Merck Patent Gmbh | Compounds for electronic devices |
| CN103718317B (en) | 2011-08-03 | 2016-11-16 | 默克专利有限公司 | material for electronic device |
| EP2742054B1 (en) | 2011-08-10 | 2016-10-12 | Merck Patent GmbH | Metal complexes |
| DE102012016192A1 (en) | 2011-08-19 | 2013-02-21 | Merck Patent Gmbh | New compounds capable of forming hydrogen bonds are useful in electronic device, e.g. organic electroluminescent device, organic light-emitting transistor and organic light-emitting electrochemical cell |
| KR101914951B1 (en) | 2011-08-22 | 2018-11-05 | 메르크 파텐트 게엠베하 | Organic electroluminescence device |
| KR102077994B1 (en) | 2011-09-21 | 2020-02-17 | 메르크 파텐트 게엠베하 | Carbazole derivatives for organic electroluminescence devices |
| DE102011116165A1 (en) | 2011-10-14 | 2013-04-18 | Merck Patent Gmbh | Benzodioxepin-3-one compounds |
| WO2013056776A1 (en) | 2011-10-20 | 2013-04-25 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| JP6223984B2 (en) | 2011-10-27 | 2017-11-01 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| DE102011117422A1 (en) | 2011-10-28 | 2013-05-02 | Merck Patent Gmbh | Hyperbranched polymers, process for their preparation and their use in electronic devices |
| KR102021162B1 (en) | 2011-11-01 | 2019-09-11 | 메르크 파텐트 게엠베하 | Organic electroluminescent device |
| KR102310368B1 (en) | 2011-11-17 | 2021-10-07 | 메르크 파텐트 게엠베하 | Spirodihydroacridine derivatives and the use thereof as materials for organic electroluminescent devices |
| CN105742499B (en) | 2011-12-12 | 2018-02-13 | 默克专利有限公司 | Compound for electronic device |
| DE102012022880A1 (en) | 2011-12-22 | 2013-06-27 | Merck Patent Gmbh | Electronic device e.g. organic integrated circuits, organic field-effect transistors, organic thin-film transistors, organic light emitting transistors, comprises an organic layer comprising substituted heteroaryl compounds |
| JP2015504884A (en) | 2011-12-27 | 2015-02-16 | メルク パテント ゲーエムベーハー | Metal complexes containing 1,2,3-triazole |
| US9748502B2 (en) | 2012-01-16 | 2017-08-29 | Merck Patent Gmbh | Organic metal complexes |
| WO2013113349A1 (en) | 2012-01-30 | 2013-08-08 | Merck Patent Gmbh | Nanocrystals on fibers |
| KR101605987B1 (en) | 2012-02-14 | 2016-03-23 | 메르크 파텐트 게엠베하 | Spirobifluorene compounds for organic electroluminescent devices |
| KR102268695B1 (en) | 2012-03-15 | 2021-06-23 | 메르크 파텐트 게엠베하 | Electronic devices |
| CN104203955B (en) | 2012-03-23 | 2017-11-17 | 默克专利有限公司 | 9,9 for electroluminescent device,Spiral shell dioxoanthracene derivative |
| US9879177B2 (en) | 2012-05-24 | 2018-01-30 | Merck Patent Gmbh | Metal complexes comprising condensed heteroaromatic rings |
| DE102012011335A1 (en) | 2012-06-06 | 2013-12-12 | Merck Patent Gmbh | Connections for Organic Electronic Devices |
| EP2872589B1 (en) | 2012-07-10 | 2017-07-26 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| JP2015529637A (en) | 2012-07-13 | 2015-10-08 | メルク パテント ゲーエムベーハー | Metal complex |
| US9768391B2 (en) | 2012-07-23 | 2017-09-19 | Merck Patent Gmbh | Derivatives of 2-diarylaminofluorene and organic electronic compounds containing them |
| DE202013012401U1 (en) | 2012-07-23 | 2016-10-12 | Merck Patent Gmbh | Connections and Organic Electronic Devices |
| KR102337198B1 (en) | 2012-07-23 | 2021-12-09 | 메르크 파텐트 게엠베하 | Compounds and organic electroluminescent devices |
| KR102125199B1 (en) | 2012-07-23 | 2020-06-22 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| EP2882763B1 (en) | 2012-08-07 | 2018-08-22 | Merck Patent GmbH | Metal complexes |
| US20150243897A1 (en) | 2012-08-10 | 2015-08-27 | Merck Patent Gmbh | Materials for organic electroluminescence devices |
| EP2898042B1 (en) | 2012-09-18 | 2016-07-06 | Merck Patent GmbH | Materials for electronic devices |
| WO2014044347A1 (en) | 2012-09-20 | 2014-03-27 | Merck Patent Gmbh | Metal complexes |
| EP2906661B1 (en) | 2012-10-11 | 2016-10-26 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| DE102012020167A1 (en) | 2012-10-13 | 2014-04-17 | Eberhard Karls Universität Tübingen | metal complexes |
| EP3806176A1 (en) | 2012-10-31 | 2021-04-14 | Merck Patent GmbH | Electronic device |
| DE102012021650A1 (en) | 2012-11-03 | 2014-05-08 | Eberhard Karls Universität Tübingen | metal complexes |
| WO2014072017A1 (en) | 2012-11-12 | 2014-05-15 | Merck Patent Gmbh | Materials for electronic devices |
| KR102105810B1 (en) | 2012-11-20 | 2020-04-29 | 메르크 파텐트 게엠베하 | Formulation in high-purity solvent for producing electronic devices |
| US10065959B2 (en) | 2012-11-30 | 2018-09-04 | Merck Patent Gmbh | Electronic device |
| EP2941469A2 (en) | 2013-01-03 | 2015-11-11 | Merck Patent GmbH | Materials for electronic devices |
| US20150329772A1 (en) | 2013-01-03 | 2015-11-19 | Merck Patent Gmbh | Materials for Electronic Devices |
| DE102013008189A1 (en) | 2013-05-14 | 2014-12-04 | Eberhard Karls Universität Tübingen | metal complexes |
| GB2515491B (en) * | 2013-06-24 | 2015-11-18 | Cambridge Display Tech Ltd | Phosphorescent light-emitting compounds and their use in light-emitting devices |
| WO2015014427A1 (en) | 2013-07-29 | 2015-02-05 | Merck Patent Gmbh | Electro-optical device and the use thereof |
| WO2015014434A1 (en) | 2013-07-30 | 2015-02-05 | Merck Patent Gmbh | Materials for electronic devices |
| EP3712229A1 (en) | 2013-07-30 | 2020-09-23 | Merck Patent GmbH | Materials for electronic devices |
| CN111848659A (en) | 2013-10-02 | 2020-10-30 | 默克专利有限公司 | Boron-containing compounds for use in OLEDs |
| KR101599965B1 (en) * | 2013-10-08 | 2016-03-04 | 제일모직 주식회사 | Compound, organic optoelectric device and display device |
| KR101759237B1 (en) | 2013-10-08 | 2017-07-18 | 제일모직 주식회사 | Compound, organic optoelectronic device and display device |
| WO2015082037A1 (en) | 2013-12-06 | 2015-06-11 | Merck Patent Gmbh | Compositions containing a polymeric binder which comprises acrylic and/or methacrylic acid ester units |
| WO2015082046A2 (en) | 2013-12-06 | 2015-06-11 | Merck Patent Gmbh | Substituted oxepines |
| CN105980518B (en) | 2013-12-06 | 2019-07-12 | 默克专利有限公司 | Compound and organic electronic device |
| JP6644688B2 (en) | 2013-12-12 | 2020-02-12 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| EP4438693A2 (en) | 2013-12-19 | 2024-10-02 | Merck Patent GmbH | Heterocyclic spiro compounds |
| KR102153043B1 (en) | 2014-01-07 | 2020-09-07 | 삼성전자주식회사 | Organometallic compound and organic light emitting device including the same |
| US9590195B2 (en) * | 2014-02-28 | 2017-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP2927221A1 (en) | 2014-03-31 | 2015-10-07 | Commonwealth Scientific and Industrial Research Organisation | Diamine compounds for phosphorescent diazaborole metal complexes and electroluminescent devices |
| EP2927300B1 (en) | 2014-03-31 | 2016-11-16 | Commonwealth Scientific and Industrial Research Organisation | Phenylenediamine compounds for phosphorescent diazaborole metal complexes |
| JP6789825B2 (en) | 2014-04-30 | 2020-11-25 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| KR101730779B1 (en) | 2014-05-05 | 2017-04-26 | 메르크 파텐트 게엠베하 | Materials for organic light emitting devices |
| DE102015006708A1 (en) | 2014-06-12 | 2015-12-17 | Merck Patent Gmbh | metal complexes |
| DE102014008722B4 (en) | 2014-06-18 | 2024-08-22 | Merck Patent Gmbh | Compositions for electronic devices, formulation containing them, use of the composition, use of the formulation and organic electronic device containing the composition |
| JP6556764B2 (en) | 2014-06-25 | 2019-08-07 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| EP2960315A1 (en) | 2014-06-27 | 2015-12-30 | cynora GmbH | Organic electroluminescent device |
| EP2963044A1 (en) | 2014-06-30 | 2016-01-06 | cynora GmbH | Binuclear metal (I) complexes with tetradentate ligands for optoelectronic applications |
| EP3172775B1 (en) | 2014-07-21 | 2022-04-20 | Merck Patent GmbH | Materials for electronic devices |
| DE102014012818A1 (en) | 2014-08-28 | 2016-03-03 | Eberhard Karls Universität Tübingen | metal complexes |
| JP6695863B2 (en) | 2014-09-05 | 2020-05-20 | メルク パテント ゲーエムベーハー | Formulations and electronics |
| WO2016055557A1 (en) | 2014-10-08 | 2016-04-14 | Cynora Gmbh | Metal complexes with tridentate ligands for optoelectronic applications |
| EP3218368B1 (en) | 2014-11-11 | 2020-11-25 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| WO2016079097A1 (en) | 2014-11-18 | 2016-05-26 | Cynora Gmbh | Copper(i) complexes for optoelectronic applications |
| CN107001334A (en) | 2014-12-12 | 2017-08-01 | 默克专利有限公司 | Organic compound with soluble groups |
| WO2016107663A1 (en) | 2014-12-30 | 2016-07-07 | Merck Patent Gmbh | Formulations and electronic devices |
| JP6827938B2 (en) | 2015-01-30 | 2021-02-10 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| KR102610947B1 (en) | 2015-01-30 | 2023-12-06 | 메르크 파텐트 게엠베하 | Formulations with a low particle content |
| KR102554987B1 (en) | 2015-02-03 | 2023-07-12 | 메르크 파텐트 게엠베하 | Metal complexes |
| CN107431139B (en) | 2015-03-30 | 2020-12-01 | 默克专利有限公司 | Formulations of organic functional materials comprising siloxane solvents |
| EP3307735A1 (en) | 2015-06-10 | 2018-04-18 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US10808170B2 (en) | 2015-06-12 | 2020-10-20 | Merck Patent Gmbh | Esters containing non-aromatic cycles as solvents for OLED formulations |
| KR102643183B1 (en) | 2015-07-15 | 2024-03-04 | 메르크 파텐트 게엠베하 | Compositions Comprising Organic Semiconducting Compounds |
| WO2017012687A1 (en) | 2015-07-22 | 2017-01-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| GB201513037D0 (en) | 2015-07-23 | 2015-09-09 | Merck Patent Gmbh | Phenyl-derived compound for use in organic electronic devices |
| JP6983754B2 (en) | 2015-07-29 | 2021-12-17 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescence devices |
| EP3328841B1 (en) | 2015-07-30 | 2023-10-11 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US10934292B2 (en) | 2015-08-13 | 2021-03-02 | Merck Patent Gmbh | Hexamethylindanes |
| KR102599157B1 (en) | 2015-08-14 | 2023-11-06 | 메르크 파텐트 게엠베하 | Phenoxazine derivatives for organic electroluminescent devices |
| WO2017036573A1 (en) | 2015-08-28 | 2017-03-09 | Merck Patent Gmbh | Compounds for electronic devices |
| US11046884B2 (en) | 2015-08-28 | 2021-06-29 | Merck Patent Gmbh | Formulation of an organic functional material comprising an epoxy group containing solvent |
| DE102015013381A1 (en) | 2015-10-14 | 2017-04-20 | Eberhard Karls Universität Tübingen | metal complexes |
| KR102678422B1 (en) | 2015-10-27 | 2024-06-25 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| JP7106451B2 (en) | 2015-12-10 | 2022-07-26 | メルク パテント ゲーエムベーハー | Formulations containing ketones containing non-aromatic rings |
| DE102015016016A1 (en) | 2015-12-10 | 2017-06-14 | Eberhard Karls Universität Tübingen | metal complexes |
| CN111477768B (en) | 2015-12-15 | 2023-04-07 | 默克专利有限公司 | Aromatic group-containing esters as solvents for organic electronic formulations |
| US11407916B2 (en) | 2015-12-16 | 2022-08-09 | Merck Patent Gmbh | Formulations containing a mixture of at least two different solvents |
| EP3390549B1 (en) | 2015-12-16 | 2022-06-29 | Merck Patent GmbH | Formulations containing a solid solvent |
| JP7051691B2 (en) | 2016-02-05 | 2022-04-11 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| WO2017140404A1 (en) | 2016-02-17 | 2017-08-24 | Merck Patent Gmbh | Formulation of an organic functional material |
| WO2017148564A1 (en) | 2016-03-03 | 2017-09-08 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102016003104A1 (en) | 2016-03-15 | 2017-09-21 | Merck Patent Gmbh | Container comprising a formulation containing at least one organic semiconductor |
| TWI745361B (en) | 2016-03-17 | 2021-11-11 | 德商麥克專利有限公司 | Compounds having spirobifluorene structures |
| CN107286196A (en) * | 2016-03-30 | 2017-10-24 | 上海和辉光电有限公司 | A kind of organometallic complex as electroluminescent organic material |
| JP7444607B2 (en) | 2016-04-11 | 2024-03-06 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Heterocyclic compounds having dibenzofuran and/or dibenzothiophene structures |
| US11643414B2 (en) | 2016-04-29 | 2023-05-09 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TWI731981B (en) | 2016-06-03 | 2021-07-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR20190019138A (en) | 2016-06-16 | 2019-02-26 | 메르크 파텐트 게엠베하 | Formulation of organic functional material |
| WO2017216128A1 (en) | 2016-06-17 | 2017-12-21 | Merck Patent Gmbh | Formulation of an organic functional material |
| TW201815998A (en) | 2016-06-28 | 2018-05-01 | 德商麥克專利有限公司 | Formulation of an organic functional material |
| EP3478698B1 (en) | 2016-06-30 | 2021-01-13 | Merck Patent GmbH | Method for the separation of enantiomeric mixtures from metal complexes |
| TWI745395B (en) | 2016-07-08 | 2021-11-11 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| CN109415344B (en) | 2016-07-14 | 2022-06-03 | 默克专利有限公司 | Metal complexes |
| EP3487864B1 (en) | 2016-07-25 | 2020-04-29 | Merck Patent GmbH | Metal complexes for use as emitters in organic electroluminescence devices |
| WO2018019687A1 (en) | 2016-07-25 | 2018-02-01 | Merck Patent Gmbh | Dinuclear and oligonuclear metal complexes containing tripodal bidentate part ligands and their use in electronic devices |
| JP6980757B2 (en) | 2016-08-04 | 2021-12-15 | メルク パテント ゲーエムベーハー | Formulation of organic functional materials |
| WO2018041769A1 (en) | 2016-08-30 | 2018-03-08 | Merck Patent Gmbh | Binuclear and trinuclear metal complexes composed of two inter-linked tripodal hexadentate ligands for use in electroluminescent devices |
| KR102459000B1 (en) | 2016-09-14 | 2022-10-26 | 메르크 파텐트 게엠베하 | Compounds having a carbazole structure |
| EP3512841B1 (en) | 2016-09-14 | 2023-04-26 | Merck Patent GmbH | Compounds with spirobifluorene-structures |
| CN109715642A (en) | 2016-09-21 | 2019-05-03 | 默克专利有限公司 | PROCESS FOR PRODUCTION OF BINUCLEAR as the illuminator in organic electroluminescence device |
| TWI766884B (en) | 2016-09-30 | 2022-06-11 | 德商麥克專利有限公司 | Compounds having diazadibenzofuran or diazadibenzothiophene structures, process for preparing the same and use thereof |
| KR20240033302A (en) | 2016-09-30 | 2024-03-12 | 메르크 파텐트 게엠베하 | Carbazoles with diazadibenzofurane or diazadibenzothiophene structures |
| TWI764942B (en) | 2016-10-10 | 2022-05-21 | 德商麥克專利有限公司 | Electronic device |
| US11430962B2 (en) | 2016-10-12 | 2022-08-30 | Merck Patent Gmbh | Binuclear metal complexes and electronic devices, in particular organic electroluminescent devices containing said metal complexes |
| JP7064487B2 (en) | 2016-10-12 | 2022-05-10 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Metal complex |
| EP3526226B1 (en) | 2016-10-13 | 2020-07-22 | Merck Patent GmbH | Metal complexes |
| DE102017008794A1 (en) | 2016-10-17 | 2018-04-19 | Merck Patent Gmbh | Materials for use in electronic devices |
| WO2018077769A1 (en) | 2016-10-25 | 2018-05-03 | Merck Patent Gmbh | Metal complexes |
| EP3532566B1 (en) | 2016-10-31 | 2021-04-21 | Merck Patent GmbH | Formulation of an organic functional material |
| EP3532565B1 (en) | 2016-10-31 | 2021-04-21 | Merck Patent GmbH | Formulation of an organic functional material |
| JP2020512273A (en) | 2016-11-02 | 2020-04-23 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| US11440925B2 (en) | 2016-11-08 | 2022-09-13 | Merck Patent Gmbh | Compounds for electronic devices |
| KR102474328B1 (en) | 2016-11-09 | 2022-12-06 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| TWI756292B (en) | 2016-11-14 | 2022-03-01 | 德商麥克專利有限公司 | Compounds having an acceptor group and a donor group |
| KR102580980B1 (en) | 2016-11-17 | 2023-09-20 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| TW201833118A (en) | 2016-11-22 | 2018-09-16 | 德商麥克專利有限公司 | Materials for electronic devices |
| JP7127026B2 (en) | 2016-11-30 | 2022-08-29 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Compound having valerolactam structure |
| EP3548486B1 (en) | 2016-12-05 | 2021-10-27 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TW201831468A (en) | 2016-12-05 | 2018-09-01 | 德商麥克專利有限公司 | Nitrogen-containing heterocycles |
| WO2018104193A1 (en) | 2016-12-05 | 2018-06-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP3552252B1 (en) | 2016-12-06 | 2023-05-17 | Merck Patent GmbH | Preparation process for an electronic device |
| WO2018108760A1 (en) | 2016-12-13 | 2018-06-21 | Merck Patent Gmbh | Formulation of an organic functional material |
| WO2018114744A1 (en) | 2016-12-20 | 2018-06-28 | Merck Patent Gmbh | A white light emitting solid state light source |
| KR102504432B1 (en) | 2016-12-22 | 2023-02-27 | 메르크 파텐트 게엠베하 | A mixture comprising at least two organo-functional compounds |
| US11261291B2 (en) | 2016-12-22 | 2022-03-01 | Merck Patent Gmbh | Materials for electronic devices |
| US20180190915A1 (en) * | 2017-01-03 | 2018-07-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2020504762A (en) | 2017-01-04 | 2020-02-13 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| US10964894B2 (en) | 2017-01-25 | 2021-03-30 | Merck Patent Gmbh | Carbazole derivatives |
| CN110167940A (en) | 2017-01-30 | 2019-08-23 | 默克专利有限公司 | Material for organic electroluminescence device |
| TWI763772B (en) | 2017-01-30 | 2022-05-11 | 德商麥克專利有限公司 | Method for forming an organic element of an electronic device |
| TWI791481B (en) | 2017-01-30 | 2023-02-11 | 德商麥克專利有限公司 | Method for forming an organic electroluminescence (el) element |
| JP7069184B2 (en) | 2017-02-02 | 2022-05-17 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| TW201835075A (en) | 2017-02-14 | 2018-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11393987B2 (en) | 2017-03-01 | 2022-07-19 | Merck Patent Gmbh | Organic electroluminescent device |
| EP3589624A1 (en) | 2017-03-02 | 2020-01-08 | Merck Patent GmbH | Materials for organic electronic devices |
| TW201843143A (en) | 2017-03-13 | 2018-12-16 | 德商麥克專利有限公司 | Compounds containing arylamine structures |
| JP7155142B2 (en) | 2017-03-15 | 2022-10-18 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| CN110446703A (en) | 2017-03-29 | 2019-11-12 | 默克专利有限公司 | Aromatic compounds |
| KR20190131554A (en) | 2017-03-31 | 2019-11-26 | 메르크 파텐트 게엠베하 | Printing method for organic light emitting diodes (OLED) |
| KR102632027B1 (en) | 2017-04-10 | 2024-01-31 | 메르크 파텐트 게엠베하 | Formulation of organic functional materials |
| TW201902891A (en) | 2017-04-13 | 2019-01-16 | 德商麥克專利有限公司 | Composition for organic electronic devices |
| EP3615542B1 (en) | 2017-04-25 | 2023-08-23 | Merck Patent GmbH | Compounds for electronic devices |
| CN110546236A (en) | 2017-05-03 | 2019-12-06 | 默克专利有限公司 | Preparation of organic functional material |
| EP3621971B1 (en) | 2017-05-11 | 2021-06-23 | Merck Patent GmbH | Carbazole-based bodipys for organic electroluminescent devices |
| EP3621970B1 (en) | 2017-05-11 | 2021-01-13 | Merck Patent GmbH | Organoboron complexes for organic electroluminescent devices |
| WO2018215318A1 (en) | 2017-05-22 | 2018-11-29 | Merck Patent Gmbh | Hexacyclic heteroaromatic compounds for electronic devices |
| TW201920343A (en) | 2017-06-21 | 2019-06-01 | 德商麥克專利有限公司 | Materials for electronic devices |
| TW201920598A (en) | 2017-06-23 | 2019-06-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR20200022010A (en) | 2017-06-26 | 2020-03-02 | 메르크 파텐트 게엠베하 | Homogeneous mixture |
| KR102661058B1 (en) | 2017-06-28 | 2024-04-25 | 메르크 파텐트 게엠베하 | Materials for Electronic Devices |
| TWI813576B (en) | 2017-07-03 | 2023-09-01 | 德商麥克專利有限公司 | Formulations with a low content of phenol type impurities |
| KR102717279B1 (en) | 2017-07-05 | 2024-10-15 | 메르크 파텐트 게엠베하 | Composition for organic electronic devices |
| WO2019007867A1 (en) | 2017-07-05 | 2019-01-10 | Merck Patent Gmbh | Composition for organic electronic devices |
| WO2019016184A1 (en) | 2017-07-18 | 2019-01-24 | Merck Patent Gmbh | Formulation of an organic functional material |
| TWI776926B (en) | 2017-07-25 | 2022-09-11 | 德商麥克專利有限公司 | Metal complexes |
| WO2019020654A1 (en) | 2017-07-28 | 2019-01-31 | Merck Patent Gmbh | Spirobifluorene derivatives for use in electronic devices |
| US11744141B2 (en) * | 2017-08-09 | 2023-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN118405981A (en) | 2017-09-08 | 2024-07-30 | 默克专利有限公司 | Material for electronic devices |
| CN111065640B (en) | 2017-09-12 | 2023-04-18 | 默克专利有限公司 | Material for organic electroluminescent device |
| CN111164086A (en) | 2017-10-06 | 2020-05-15 | 默克专利有限公司 | Material for organic electroluminescent device |
| CN108675975A (en) | 2017-10-17 | 2018-10-19 | 默克专利有限公司 | Material for organic electroluminescence device |
| EP3700909B1 (en) | 2017-10-24 | 2024-01-24 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TWI785142B (en) | 2017-11-14 | 2022-12-01 | 德商麥克專利有限公司 | Composition for organic electronic devices |
| KR20200090817A (en) | 2017-11-23 | 2020-07-29 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| TWI820057B (en) | 2017-11-24 | 2023-11-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI838352B (en) | 2017-11-24 | 2024-04-11 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| WO2019115423A1 (en) | 2017-12-13 | 2019-06-20 | Merck Patent Gmbh | Metal complexes |
| EP3724175A1 (en) | 2017-12-15 | 2020-10-21 | Merck Patent GmbH | Substituted aromatic amines for use in organic electroluminescent devices |
| WO2019115573A1 (en) | 2017-12-15 | 2019-06-20 | Merck Patent Gmbh | Formulation of an organic functional material |
| TW201938562A (en) | 2017-12-19 | 2019-10-01 | 德商麥克專利有限公司 | Heterocyclic compounds |
| WO2019121483A1 (en) | 2017-12-20 | 2019-06-27 | Merck Patent Gmbh | Heteroaromatic compounds |
| TWI811290B (en) | 2018-01-25 | 2023-08-11 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| EP3752512B1 (en) | 2018-02-13 | 2023-03-01 | Merck Patent GmbH | Metal complexes |
| CN111712551A (en) | 2018-02-26 | 2020-09-25 | 默克专利有限公司 | Preparation of organic functional material |
| TW201938761A (en) | 2018-03-06 | 2019-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI802656B (en) | 2018-03-06 | 2023-05-21 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| CN111819167A (en) | 2018-03-16 | 2020-10-23 | 默克专利有限公司 | Material for organic electroluminescent device |
| TWI828664B (en) | 2018-03-19 | 2024-01-11 | 愛爾蘭商Udc愛爾蘭責任有限公司 | Metal complexes |
| KR102568783B1 (en) * | 2018-03-22 | 2023-08-22 | 삼성디스플레이 주식회사 | Organometallic compound, organic light emitting device comprising the same and organic emitting apparatus comprising the organic light emitting device |
| EP3802520A1 (en) | 2018-05-30 | 2021-04-14 | Merck Patent GmbH | Composition for organic electronic devices |
| US20220336754A1 (en) | 2018-06-07 | 2022-10-20 | Merck Patent Gmbh | Organic electroluminescence devices |
| TWI835803B (en) | 2018-06-15 | 2024-03-21 | 德商麥克專利有限公司 | Formulation of an organic functional material |
| KR20210031714A (en) | 2018-07-09 | 2021-03-22 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| CN112424169A (en) | 2018-07-20 | 2021-02-26 | 默克专利有限公司 | Material for organic electroluminescent device |
| TWI837167B (en) | 2018-08-28 | 2024-04-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI823993B (en) | 2018-08-28 | 2023-12-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| EP3844243B1 (en) | 2018-08-28 | 2022-06-22 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TWI826522B (en) | 2018-09-12 | 2023-12-21 | 德商麥克專利有限公司 | Electroluminescent devices |
| EP3850055A1 (en) | 2018-09-12 | 2021-07-21 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TW202030902A (en) | 2018-09-12 | 2020-08-16 | 德商麥克專利有限公司 | Electroluminescent devices |
| JP2022502829A (en) | 2018-09-24 | 2022-01-11 | メルク パテント ゲーエムベーハー | Methods for Producing Granular Materials |
| CN112771024A (en) | 2018-09-27 | 2021-05-07 | 默克专利有限公司 | Process for preparing sterically hindered nitrogen-containing heteroaromatics |
| KR20210068054A (en) | 2018-09-27 | 2021-06-08 | 메르크 파텐트 게엠베하 | Compounds that can be used as active compounds in organic electronic devices |
| US20220223801A1 (en) | 2018-10-31 | 2022-07-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2020094539A1 (en) | 2018-11-05 | 2020-05-14 | Merck Patent Gmbh | Compounds that can be used in an organic electronic device |
| KR20210089205A (en) | 2018-11-06 | 2021-07-15 | 메르크 파텐트 게엠베하 | 5,6-diphenyl-5,6-dihydrodibenz[C,E][1,2]azaphosphorine and 6-phenyl-6H-dibenzo[C,E][ as organic electroluminescent materials for OLEDs 1,2]thiazine-5,5-dioxide derivatives and similar compounds |
| TWI846749B (en) | 2018-11-06 | 2024-07-01 | 德商麥克專利有限公司 | Method for forming an organic element of an electronic device and kit of inks |
| KR20210091769A (en) | 2018-11-14 | 2021-07-22 | 메르크 파텐트 게엠베하 | Compounds that can be used in the manufacture of organic electronic devices |
| EP3880682B1 (en) | 2018-11-15 | 2023-06-14 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TW202039493A (en) | 2018-12-19 | 2020-11-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| WO2020148243A1 (en) | 2019-01-16 | 2020-07-23 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US20220098477A1 (en) | 2019-02-11 | 2022-03-31 | Merck Patent Gmbh | Mononuclear iridium complexes containing three ortho-metallated bidentate ligands and optical orientating anistrophy |
| JP2022520284A (en) | 2019-02-18 | 2022-03-29 | メルク パテント ゲーエムベーハー | Compositions for organic electronic devices |
| US20220127286A1 (en) | 2019-03-04 | 2022-04-28 | Merck Patent Gmbh | Ligands for nano-sized materials |
| KR20210137148A (en) | 2019-03-12 | 2021-11-17 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| CN113508117A (en) | 2019-03-20 | 2021-10-15 | 默克专利有限公司 | Material for organic electroluminescent device |
| WO2020193447A1 (en) | 2019-03-25 | 2020-10-01 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| JP2022527591A (en) | 2019-04-11 | 2022-06-02 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Materials for OLED devices |
| WO2020212296A1 (en) | 2019-04-15 | 2020-10-22 | Merck Patent Gmbh | Metal complexes |
| WO2021037401A1 (en) | 2019-08-26 | 2021-03-04 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2021043703A1 (en) | 2019-09-02 | 2021-03-11 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TW202122558A (en) | 2019-09-03 | 2021-06-16 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| CN114450286A (en) | 2019-09-16 | 2022-05-06 | 默克专利有限公司 | Material of organic electroluminescent device |
| CN114342103A (en) | 2019-09-19 | 2022-04-12 | 默克专利有限公司 | Mixture of two host materials and organic electroluminescent device comprising the same |
| WO2021053046A1 (en) | 2019-09-20 | 2021-03-25 | Merck Patent Gmbh | Peri-condensed heterocyclic compounds as materials for electronic devices |
| CN114514628A (en) | 2019-10-22 | 2022-05-17 | 默克专利有限公司 | Material for organic electroluminescent device |
| KR20220090539A (en) | 2019-10-25 | 2022-06-29 | 메르크 파텐트 게엠베하 | Compounds that can be used in organic electronic devices |
| WO2021089450A1 (en) | 2019-11-04 | 2021-05-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TW202134252A (en) | 2019-11-12 | 2021-09-16 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TW202136181A (en) | 2019-12-04 | 2021-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US20230056324A1 (en) | 2019-12-04 | 2023-02-23 | Merck Patent Gmbh | Metal complexes |
| TW202136471A (en) | 2019-12-17 | 2021-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| WO2021122538A1 (en) | 2019-12-18 | 2021-06-24 | Merck Patent Gmbh | Aromatic compounds for organic electroluminescent devices |
| CN114867729A (en) | 2019-12-19 | 2022-08-05 | 默克专利有限公司 | Polycyclic compound for organic electroluminescent device |
| CN115052865A (en) | 2020-01-29 | 2022-09-13 | 默克专利有限公司 | Benzimidazole derivatives |
| EP4110884A1 (en) | 2020-02-25 | 2023-01-04 | Merck Patent GmbH | Use of heterocyclic compounds in an organic electronic device |
| CN115244728A (en) | 2020-03-02 | 2022-10-25 | 默克专利有限公司 | Use of sulfone compounds in organic electronic devices |
| EP4121432A1 (en) | 2020-03-17 | 2023-01-25 | Merck Patent GmbH | Heteroaromatic compounds for organic electroluminescent devices |
| US20230147279A1 (en) | 2020-03-17 | 2023-05-11 | Merck Patent Gmbh | Heterocyclic compounds for organic electroluminescent devices |
| US20230337537A1 (en) | 2020-03-23 | 2023-10-19 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR20220158017A (en) | 2020-03-24 | 2022-11-29 | 메르크 파텐트 게엠베하 | Materials for Electronic Devices |
| KR20220158771A (en) | 2020-03-26 | 2022-12-01 | 메르크 파텐트 게엠베하 | Cyclic compounds for organic electroluminescent devices |
| EP4126870A1 (en) | 2020-04-02 | 2023-02-08 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| CN115362159A (en) | 2020-04-06 | 2022-11-18 | 默克专利有限公司 | Polycyclic compound for organic electroluminescent device |
| WO2021213918A1 (en) | 2020-04-21 | 2021-10-28 | Merck Patent Gmbh | Formulation of an organic functional material |
| CN115700058A (en) | 2020-04-21 | 2023-02-03 | 默克专利有限公司 | Emulsions comprising organic functional materials |
| TW202208594A (en) | 2020-05-27 | 2022-03-01 | 德商麥克專利有限公司 | Materials for electronic devices |
| KR20210152626A (en) * | 2020-06-08 | 2021-12-16 | 삼성디스플레이 주식회사 | Organometallic compound and organic light emitting device including the same |
| US20230234937A1 (en) | 2020-06-18 | 2023-07-27 | Merck Patent Gmbh | Indenoazanaphthalenes |
| EP4169082A1 (en) | 2020-06-23 | 2023-04-26 | Merck Patent GmbH | Method for producing a mixture |
| EP4172164A1 (en) | 2020-06-29 | 2023-05-03 | Merck Patent GmbH | Heteroaromatic compounds for organic electroluminescent devices |
| JP2023530915A (en) | 2020-06-29 | 2023-07-20 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Heterocyclic compounds for organic electroluminescent devices |
| TW202216952A (en) | 2020-07-22 | 2022-05-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| WO2022029096A1 (en) | 2020-08-06 | 2022-02-10 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN116134113A (en) | 2020-08-13 | 2023-05-16 | 默克专利有限公司 | Metal complex |
| WO2022038065A1 (en) | 2020-08-18 | 2022-02-24 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TW202223066A (en) | 2020-08-19 | 2022-06-16 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| EP4222155A1 (en) | 2020-09-29 | 2023-08-09 | Merck Patent GmbH | Mononuclear tripodal hexadentate iridium complexes for use in oleds |
| TW202229215A (en) | 2020-09-30 | 2022-08-01 | 德商麥克專利有限公司 | Compounds for structuring of functional layers of organic electroluminescent devices |
| TW202222748A (en) | 2020-09-30 | 2022-06-16 | 德商麥克專利有限公司 | Compounds usable for structuring of functional layers of organic electroluminescent devices |
| EP4229145A1 (en) | 2020-10-16 | 2023-08-23 | Merck Patent GmbH | Compounds comprising heteroatoms for organic electroluminescent devices |
| US20230389423A1 (en) | 2020-10-16 | 2023-11-30 | Merck Patent Gmbh | Heterocyclic compounds for organic electroluminescent devices |
| US20230422610A1 (en) | 2020-11-10 | 2023-12-28 | Merck Patent Gmbh | Sulfurous compounds for organic electroluminescent devices |
| EP4255905A1 (en) | 2020-12-02 | 2023-10-11 | Merck Patent GmbH | Heterocyclic compounds for organic electroluminescent devices |
| KR20230114756A (en) | 2020-12-08 | 2023-08-01 | 메르크 파텐트 게엠베하 | Ink Systems and Methods for Inkjet Printing |
| WO2022122682A2 (en) | 2020-12-10 | 2022-06-16 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN116724040A (en) | 2020-12-18 | 2023-09-08 | 默克专利有限公司 | Nitrogen-containing compounds for organic electroluminescent devices |
| WO2022129113A1 (en) | 2020-12-18 | 2022-06-23 | Merck Patent Gmbh | Nitrogenous heteroaromatic compounds for organic electroluminescent devices |
| WO2022129116A1 (en) | 2020-12-18 | 2022-06-23 | Merck Patent Gmbh | Indolo[3.2.1-jk]carbazole-6-carbonitrile derivatives as blue fluorescent emitters for use in oleds |
| JP2024502093A (en) | 2021-01-05 | 2024-01-17 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| KR20230137375A (en) | 2021-01-25 | 2023-10-04 | 메르크 파텐트 게엠베하 | Nitrogen compounds for organic electroluminescent devices |
| WO2022184601A1 (en) | 2021-03-02 | 2022-09-09 | Merck Patent Gmbh | Compounds for organic electroluminescent devices |
| WO2022194799A1 (en) | 2021-03-18 | 2022-09-22 | Merck Patent Gmbh | Heteroaromatic compounds for organic electroluminescent devices |
| EP4079742A1 (en) | 2021-04-14 | 2022-10-26 | Merck Patent GmbH | Metal complexes |
| JP2024515366A (en) | 2021-04-23 | 2024-04-09 | メルク パテント ゲーエムベーハー | Organic functional materials formulations |
| WO2022229126A1 (en) | 2021-04-29 | 2022-11-03 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR20240005806A (en) | 2021-04-29 | 2024-01-12 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| EP4330257A1 (en) | 2021-04-30 | 2024-03-06 | Merck Patent GmbH | Nitrogenous heterocyclic compounds for organic electroluminescent devices |
| CN117355364A (en) | 2021-05-21 | 2024-01-05 | 默克专利有限公司 | Method for continuously purifying at least one functional material and device for continuously purifying at least one functional material |
| DE112022003409A5 (en) | 2021-07-06 | 2024-05-23 | MERCK Patent Gesellschaft mit beschränkter Haftung | MATERIALS FOR ORGANIC ELECTROLUMINESCENCE DEVICES |
| CN117730638A (en) | 2021-08-02 | 2024-03-19 | 默克专利有限公司 | Printing method by combining inks |
| EP4402221A1 (en) | 2021-09-14 | 2024-07-24 | Merck Patent GmbH | Boronic heterocyclic compounds for organic electroluminescent devices |
| WO2023052313A1 (en) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materials for electronic devices |
| EP4410071A1 (en) | 2021-09-28 | 2024-08-07 | Merck Patent GmbH | Materials for electronic devices |
| CN118056486A (en) | 2021-09-28 | 2024-05-17 | 默克专利有限公司 | Material for electronic devices |
| DE112022004658A5 (en) | 2021-09-28 | 2024-07-25 | Merck Patent Gmbh | MATERIALS FOR ELECTRONIC DEVICES |
| TW202349760A (en) | 2021-10-05 | 2023-12-16 | 德商麥克專利有限公司 | Method for forming an organic element of an electronic device |
| CN114163480B (en) * | 2021-10-26 | 2024-10-22 | 奥来德(上海)光电材料科技有限公司 | Organic iridium metal complex with long service life and high efficiency and application thereof |
| EP4423209A1 (en) | 2021-10-27 | 2024-09-04 | Merck Patent GmbH | Boronic and nitrogenous heterocyclic compounds for organic electroluminescent devices |
| WO2023094412A1 (en) | 2021-11-25 | 2023-06-01 | Merck Patent Gmbh | Materials for electronic devices |
| EP4442093A1 (en) | 2021-11-30 | 2024-10-09 | Merck Patent GmbH | Compounds having fluorene structures |
| CN118401524A (en) | 2021-12-13 | 2024-07-26 | 默克专利有限公司 | Material for organic electroluminescent device |
| KR20240127395A (en) | 2021-12-21 | 2024-08-22 | 메르크 파텐트 게엠베하 | Electronic devices |
| WO2023117837A1 (en) | 2021-12-21 | 2023-06-29 | Merck Patent Gmbh | Process for preparing deuterated organic compounds |
| KR20240123834A (en) | 2021-12-21 | 2024-08-14 | 메르크 파텐트 게엠베하 | Electronic devices |
| KR20240146680A (en) | 2022-02-09 | 2024-10-08 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| CN118647604A (en) | 2022-02-14 | 2024-09-13 | 默克专利有限公司 | Material for electronic devices |
| CN118696106A (en) | 2022-02-23 | 2024-09-24 | 默克专利有限公司 | Nitrogen-containing heterocyclic compound for organic electroluminescent device |
| WO2023161168A1 (en) | 2022-02-23 | 2023-08-31 | Merck Patent Gmbh | Aromatic hetreocycles for organic electroluminescent devices |
| WO2023213837A1 (en) | 2022-05-06 | 2023-11-09 | Merck Patent Gmbh | Cyclic compounds for organic electroluminescent devices |
| WO2023222559A1 (en) | 2022-05-18 | 2023-11-23 | Merck Patent Gmbh | Process for preparing deuterated organic compounds |
| TW202411366A (en) | 2022-06-07 | 2024-03-16 | 德商麥克專利有限公司 | Method of printing a functional layer of an electronic device by combining inks |
| WO2023247345A1 (en) | 2022-06-20 | 2023-12-28 | Merck Patent Gmbh | Heterocycles for photoelectric devices |
| WO2023247338A1 (en) | 2022-06-20 | 2023-12-28 | Merck Patent Gmbh | Organic heterocycles for photoelectric devices |
| WO2024013004A1 (en) | 2022-07-11 | 2024-01-18 | Merck Patent Gmbh | Materials for electronic devices |
| EP4311849A1 (en) | 2022-07-27 | 2024-01-31 | UDC Ireland Limited | Metal complexes |
| WO2024061948A1 (en) | 2022-09-22 | 2024-03-28 | Merck Patent Gmbh | Nitrogen-containing hetreocycles for organic electroluminescent devices |
| WO2024061942A1 (en) | 2022-09-22 | 2024-03-28 | Merck Patent Gmbh | Nitrogen-containing compounds for organic electroluminescent devices |
| WO2024094592A2 (en) | 2022-11-01 | 2024-05-10 | Merck Patent Gmbh | Nitrogenous heterocycles for organic electroluminescent devices |
| WO2024105066A1 (en) | 2022-11-17 | 2024-05-23 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024126635A1 (en) | 2022-12-16 | 2024-06-20 | Merck Patent Gmbh | Formulation of an organic functional material |
| WO2024132993A1 (en) | 2022-12-19 | 2024-06-27 | Merck Patent Gmbh | Materials for electronic devices |
| WO2024133048A1 (en) | 2022-12-20 | 2024-06-27 | Merck Patent Gmbh | Method for preparing deuterated aromatic compounds |
| WO2024149694A1 (en) | 2023-01-10 | 2024-07-18 | Merck Patent Gmbh | Nitrogenous heterocycles for organic electroluminescent devices |
| WO2024153568A1 (en) | 2023-01-17 | 2024-07-25 | Merck Patent Gmbh | Heterocycles for organic electroluminescent devices |
| WO2024170605A1 (en) | 2023-02-17 | 2024-08-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024184050A1 (en) | 2023-03-07 | 2024-09-12 | Merck Patent Gmbh | Cyclic nitrogen compounds for organic electroluminescent devices |
| WO2024194264A1 (en) | 2023-03-20 | 2024-09-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024218109A1 (en) | 2023-04-20 | 2024-10-24 | Merck Patent Gmbh | Materials for electronic devices |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030068526A1 (en) * | 2000-11-30 | 2003-04-10 | Canon Kabushiki Kaisha | Luminescence device and display apparatus |
| WO2003079736A1 (en) * | 2002-03-18 | 2003-09-25 | Isis Innovation Limited | Phosphorescent dendrimers for use in light-emitting devices |
| JP2003342284A (en) | 2002-05-30 | 2003-12-03 | Canon Inc | Metal coordination compound, light-generating element and display device |
| US20040058194A1 (en) * | 2000-12-22 | 2004-03-25 | Philipp Stossel | Use of boron and aluminium compounds in electronic components |
| JP2005023071A (en) | 2003-06-09 | 2005-01-27 | Hitachi Chem Co Ltd | Metal coordination compound, polymer composition, and organic electroluminescent element using them |
-
2004
- 2004-03-31 US US10/598,971 patent/US7790890B2/en not_active Expired - Fee Related
-
2005
- 2005-03-16 JP JP2006511951A patent/JPWO2005097940A1/en active Pending
- 2005-03-16 WO PCT/JP2005/004678 patent/WO2005097940A1/en not_active Application Discontinuation
- 2005-03-16 EP EP05720929A patent/EP1731584A1/en not_active Withdrawn
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030068526A1 (en) * | 2000-11-30 | 2003-04-10 | Canon Kabushiki Kaisha | Luminescence device and display apparatus |
| US20040058194A1 (en) * | 2000-12-22 | 2004-03-25 | Philipp Stossel | Use of boron and aluminium compounds in electronic components |
| WO2003079736A1 (en) * | 2002-03-18 | 2003-09-25 | Isis Innovation Limited | Phosphorescent dendrimers for use in light-emitting devices |
| JP2003342284A (en) | 2002-05-30 | 2003-12-03 | Canon Inc | Metal coordination compound, light-generating element and display device |
| JP2005023071A (en) | 2003-06-09 | 2005-01-27 | Hitachi Chem Co Ltd | Metal coordination compound, polymer composition, and organic electroluminescent element using them |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20130341612A1 (en) * | 2011-03-04 | 2013-12-26 | Konica Minolta , Inc. | Organic electroluminescence element |
| US9640775B2 (en) * | 2011-03-04 | 2017-05-02 | Konica Minolta, Inc. | Organic electroluminescence element |
| US11367841B2 (en) * | 2016-11-08 | 2022-06-21 | Samsung Display Co., Ltd. | Heterocyclic compound and organic electroluminescence device including the same |
Also Published As
| Publication number | Publication date |
|---|---|
| US20070196687A1 (en) | 2007-08-23 |
| JPWO2005097940A1 (en) | 2008-02-28 |
| EP1731584A1 (en) | 2006-12-13 |
| WO2005097940A1 (en) | 2005-10-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7790890B2 (en) | Organic electroluminescence element material, organic electroluminescence element, display device and illumination device | |
| US8778509B2 (en) | Organic electroluminescence element, display device and lighting device | |
| US7504657B2 (en) | Organic electroluminescent element, display and illuminator | |
| US8133597B2 (en) | Organic electroluminescent device, display and illuminating device | |
| JP4894513B2 (en) | ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE | |
| US9379337B2 (en) | Organic electroluminescent element material, organic electroluminescent element, display device, and lighting device | |
| US9634275B2 (en) | Organic electroluminescent element, display device and illuminating device | |
| US8778506B2 (en) | Organic electroluminescent element, white light emission element, full color display device and lighting device | |
| JP5040077B2 (en) | Organic electroluminescence device | |
| JP4952247B2 (en) | ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE | |
| JP5045100B2 (en) | Organic electroluminescence element material and organic electroluminescence element | |
| US20080303415A1 (en) | Organic Electroluminescence Element, Display and Illuminator | |
| JP5076888B2 (en) | ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE | |
| US7839075B2 (en) | Organic electroluminescent element, illuminator and display | |
| US20090051273A1 (en) | Organic Electroluminescence Element, Image Display Device and Lighting Device | |
| JP2005314663A (en) | Organic electroluminescent element material, organic electroluminescent element, display device and illuminating apparatus | |
| US20180072945A1 (en) | Material for organic electroluminescent elements, organic electroluminescent element, display device and lighting device | |
| JP5104981B2 (en) | Organic electroluminescence element, display device and lighting device | |
| JP4941291B2 (en) | Organic electroluminescence device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: KONICA MINOLTA HOLDINGS, INC., JAPAN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:OSHIYAMA, TOMOHIRO, MR.;KITA, HIROSHI, MR.;KATOH, EISAKU, MR.;AND OTHERS;REEL/FRAME:018263/0132 Effective date: 20060816 |
|
| FEPP | Fee payment procedure |
Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| FEPP | Fee payment procedure |
Free format text: MAINTENANCE FEE REMINDER MAILED (ORIGINAL EVENT CODE: REM.) |
|
| LAPS | Lapse for failure to pay maintenance fees |
Free format text: PATENT EXPIRED FOR FAILURE TO PAY MAINTENANCE FEES (ORIGINAL EVENT CODE: EXP.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| STCH | Information on status: patent discontinuation |
Free format text: PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 |
|
| FP | Lapsed due to failure to pay maintenance fee |
Effective date: 20180907 |