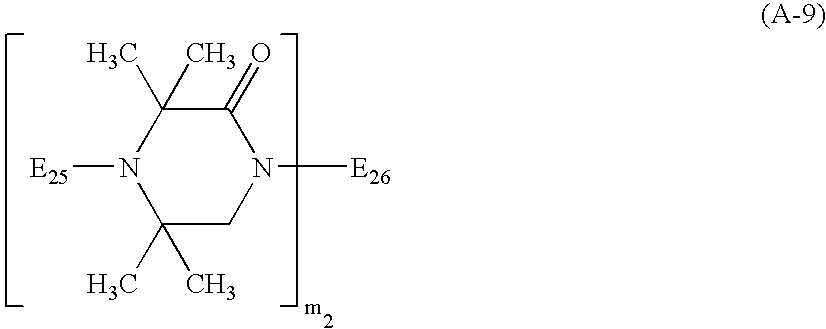US6878761B2 - Synergistic combinations of UV absorbers for pigmented polyolefins - Google Patents
Synergistic combinations of UV absorbers for pigmented polyolefins Download PDFInfo
- Publication number
- US6878761B2 US6878761B2 US10/358,823 US35882303A US6878761B2 US 6878761 B2 US6878761 B2 US 6878761B2 US 35882303 A US35882303 A US 35882303A US 6878761 B2 US6878761 B2 US 6878761B2
- Authority
- US
- United States
- Prior art keywords
- alkyl
- carbon atoms
- bis
- substituted
- phenyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related, expires
Links
- 0 *c1cc([3*])cc([1*])c1-c1nc(-c2ccc(O[7*])cc2O)nc(-c2c(*)cc([4*])cc2[2*])n1.[6*]C Chemical compound *c1cc([3*])cc([1*])c1-c1nc(-c2ccc(O[7*])cc2O)nc(-c2c(*)cc([4*])cc2[2*])n1.[6*]C 0.000 description 28
- KQCCCKRYPQHXJX-YVVBEDTISA-N [3H]N(C)CN([3H])C.[3H][3H].[3H][3H].[3H][3H][3H].[3H][3H][3H] Chemical compound [3H]N(C)CN([3H])C.[3H][3H].[3H][3H].[3H][3H][3H].[3H][3H][3H] KQCCCKRYPQHXJX-YVVBEDTISA-N 0.000 description 3
- YCALTTGEPJHEGN-UHFFFAOYSA-N C.CC1CCC(COC(=O)C2CCC(C)C(O)C2)CC1O Chemical compound C.CC1CCC(COC(=O)C2CCC(C)C(O)C2)CC1O YCALTTGEPJHEGN-UHFFFAOYSA-N 0.000 description 2
- FJZKADZLSJRAQF-UHFFFAOYSA-N CC(=O)OC1CC(C)(C)N(C)C(C)(C)C1 Chemical compound CC(=O)OC1CC(C)(C)N(C)C(C)(C)C1 FJZKADZLSJRAQF-UHFFFAOYSA-N 0.000 description 2
- QDCWMPHJELLHBD-UHFFFAOYSA-N CCC1(C)CC(C)CC(C)(C)C1 Chemical compound CCC1(C)CC(C)CC(C)(C)C1 QDCWMPHJELLHBD-UHFFFAOYSA-N 0.000 description 2
- FZWUOMFXKAPJGR-OJAWKQALSA-N [3H]N([3H])C.[3H][3H].[3H][3H].[3H][3H][3H] Chemical compound [3H]N([3H])C.[3H][3H].[3H][3H].[3H][3H][3H] FZWUOMFXKAPJGR-OJAWKQALSA-N 0.000 description 2
- CRSOQBOWXPBRES-UHFFFAOYSA-N CC(C)(C)C Chemical compound CC(C)(C)C CRSOQBOWXPBRES-UHFFFAOYSA-N 0.000 description 1
- PWOQIZXXTXTRBR-UHFFFAOYSA-N CC(C)(C)C1=CC(C(C)(C)C)=C2OP(OCCN)OC3=C(C(C)(C)C)/C=C(C(C)(C)C)/C=C\3C2=C1.CC(C)(C)C1=CC=C(OP2OCC3(CO2)COP(OC2=CC=C(C(C)(C)C)C=C2C(C)(C)C)OC3)C(C(C)(C)C)=C1.CC1=CC(C(C)(C)C)=C(OP2OCC3(CO2)COP(OC2=C(C(C)(C)C)C=C(C)C=C2C(C)(C)C)OC3)C(C(C)(C)C)=C1.CC1C2=CC(C(C)(C)C)=CC(C(C)(C)C)=C2OP(F)OC2=C(C(C)(C)C)C=C(C(C)(C)C)C=C21.CCCCC(CC)COP1OC2=C(C(C)(C)C)C=C(C(C)(C)C)C=C2C2=C/C(C(C)(C)C)=C/C(C(C)(C)C)=C\2O1.CCCCCCCCCCCCCCCCCCOP1OCC2(CO1)COP(OCCCCCCCCCCCCCCCCCC)OC2.CCOPOC1=C(C(C)(C)C)C=C(C(C)(C)C)C=C1C Chemical compound CC(C)(C)C1=CC(C(C)(C)C)=C2OP(OCCN)OC3=C(C(C)(C)C)/C=C(C(C)(C)C)/C=C\3C2=C1.CC(C)(C)C1=CC=C(OP2OCC3(CO2)COP(OC2=CC=C(C(C)(C)C)C=C2C(C)(C)C)OC3)C(C(C)(C)C)=C1.CC1=CC(C(C)(C)C)=C(OP2OCC3(CO2)COP(OC2=C(C(C)(C)C)C=C(C)C=C2C(C)(C)C)OC3)C(C(C)(C)C)=C1.CC1C2=CC(C(C)(C)C)=CC(C(C)(C)C)=C2OP(F)OC2=C(C(C)(C)C)C=C(C(C)(C)C)C=C21.CCCCC(CC)COP1OC2=C(C(C)(C)C)C=C(C(C)(C)C)C=C2C2=C/C(C(C)(C)C)=C/C(C(C)(C)C)=C\2O1.CCCCCCCCCCCCCCCCCCOP1OCC2(CO1)COP(OCCCCCCCCCCCCCCCCCC)OC2.CCOPOC1=C(C(C)(C)C)C=C(C(C)(C)C)C=C1C PWOQIZXXTXTRBR-UHFFFAOYSA-N 0.000 description 1
- QZWKTQSAWSWQBZ-UHFFFAOYSA-N CC(C)(C)C1=CC(N2N=C3C=CC(Cl)=CC3=N2)=C(O)C(C(C)(C)C)=C1.CC1=CC(N2N=C3C=CC(Cl)=CC3=N2)=C(O)C(C(C)(C)C)=C1.CCC(C)(C)C1=CC(N2N=C3C=CC=CC3=N2)=C(O)C(C(C)(C)CC)=C1.CCCCCCCCOC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1.CCCCCCCCOC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1 Chemical compound CC(C)(C)C1=CC(N2N=C3C=CC(Cl)=CC3=N2)=C(O)C(C(C)(C)C)=C1.CC1=CC(N2N=C3C=CC(Cl)=CC3=N2)=C(O)C(C(C)(C)C)=C1.CCC(C)(C)C1=CC(N2N=C3C=CC=CC3=N2)=C(O)C(C(C)(C)CC)=C1.CCCCCCCCOC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1.CCCCCCCCOC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1 QZWKTQSAWSWQBZ-UHFFFAOYSA-N 0.000 description 1
- PLFCYRVZTAZAES-UHFFFAOYSA-N CC(O)CN1C(C)(C)CC(O)CC1(C)C Chemical compound CC(O)CN1C(C)(C)CC(O)CC1(C)C PLFCYRVZTAZAES-UHFFFAOYSA-N 0.000 description 1
- SFLUSUMPKDADBX-UHFFFAOYSA-M CC.CC.OC1=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=CC(O[Y][Y])=C1 Chemical compound CC.CC.OC1=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=CC(O[Y][Y])=C1 SFLUSUMPKDADBX-UHFFFAOYSA-M 0.000 description 1
- CGUMCOWNSWTCDV-UHFFFAOYSA-N CC.CN1C(C)(C)CC(OC(=O)C(=CC2=CC=CC=C2)C(=O)OC2CC(C)(C)N(C)C(C)(C)C2)CC1(C)C Chemical compound CC.CN1C(C)(C)CC(OC(=O)C(=CC2=CC=CC=C2)C(=O)OC2CC(C)(C)N(C)C(C)(C)C2)CC1(C)C CGUMCOWNSWTCDV-UHFFFAOYSA-N 0.000 description 1
- GIUQVIBQERNEGM-UHFFFAOYSA-N CC1=CC(CC(C)(C)C)=CC(C)=C1O Chemical compound CC1=CC(CC(C)(C)C)=CC(C)=C1O GIUQVIBQERNEGM-UHFFFAOYSA-N 0.000 description 1
- IDURFXVRSDIADZ-UHFFFAOYSA-N CC1=CC=C(C2=NC(C3=CC=C(C)C=C3O)=NC(C3=CC=C(C)C=C3O)=N2)C(O)=C1.CCCCC(CC)COC1=CC=C(C2=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=N2)C(O)=C1.CCCCCCCCOC(=O)C(C)OC1=CC=C(C2=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=N2)C(O)=C1.CCCCOC1=CC=C(C2=NC(C3=CC=C(COCCC)C=C3O)=NC(C3=CC=C(OCCCC)C=C3OCCCC)=N2)C(O)=C1.CCO1CC1=O Chemical compound CC1=CC=C(C2=NC(C3=CC=C(C)C=C3O)=NC(C3=CC=C(C)C=C3O)=N2)C(O)=C1.CCCCC(CC)COC1=CC=C(C2=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=N2)C(O)=C1.CCCCCCCCOC(=O)C(C)OC1=CC=C(C2=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=N2)C(O)=C1.CCCCOC1=CC=C(C2=NC(C3=CC=C(COCCC)C=C3O)=NC(C3=CC=C(OCCCC)C=C3OCCCC)=N2)C(O)=C1.CCO1CC1=O IDURFXVRSDIADZ-UHFFFAOYSA-N 0.000 description 1
- AZLLYSFVTIYEFC-UHFFFAOYSA-N CC1CC(=O)N(C2CC(C)(C)N(C)C(C)(C)C2)C1=O Chemical compound CC1CC(=O)N(C2CC(C)(C)N(C)C(C)(C)C2)C1=O AZLLYSFVTIYEFC-UHFFFAOYSA-N 0.000 description 1
- VDFCWFAFHBUAIP-UHFFFAOYSA-N CC1CN(C)CCN1C.CN1CCN(C)CC1 Chemical compound CC1CN(C)CCN1C.CN1CCN(C)CC1 VDFCWFAFHBUAIP-UHFFFAOYSA-N 0.000 description 1
- BGMRKKAQEXLSPL-UHFFFAOYSA-N CCC(C)(CC(C)C(=O)OC)C(=O)OC Chemical compound CCC(C)(CC(C)C(=O)OC)C(=O)OC BGMRKKAQEXLSPL-UHFFFAOYSA-N 0.000 description 1
- RNTWWGNZUXGTAX-UHFFFAOYSA-N CCC(C)C(C)CC Chemical compound CCC(C)C(C)CC RNTWWGNZUXGTAX-UHFFFAOYSA-N 0.000 description 1
- VNGRFKHOGGDZRI-UHFFFAOYSA-N CCC(O)CN1C(C)(C)CC2(CC1(C)C)OC1(CCCCCCCCCCC1)N(C)C2=O.[H]N1C(C)(C)CC2(CC1(C)C)OC1(CCCCCCCCCCC1)N(CC(C)COC)C2=O Chemical compound CCC(O)CN1C(C)(C)CC2(CC1(C)C)OC1(CCCCCCCCCCC1)N(C)C2=O.[H]N1C(C)(C)CC2(CC1(C)C)OC1(CCCCCCCCCCC1)N(CC(C)COC)C2=O VNGRFKHOGGDZRI-UHFFFAOYSA-N 0.000 description 1
- IGJZIHFHGBCTLK-UHFFFAOYSA-N CCC(O)CNC1CC(C)(C)N(C)C(C)(C)C1 Chemical compound CCC(O)CNC1CC(C)(C)N(C)C(C)(C)C1 IGJZIHFHGBCTLK-UHFFFAOYSA-N 0.000 description 1
- RBACIKXCRWGCBB-UHFFFAOYSA-N CCC1CO1 Chemical compound CCC1CO1 RBACIKXCRWGCBB-UHFFFAOYSA-N 0.000 description 1
- UFGQMLWXAJOXRO-UHFFFAOYSA-N CCCCC(CC)COC1=CC=C(C2=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=N2)C(O)=C1.CCCCCCCCOC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1.COC1=CC(O)=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound CCCCC(CC)COC1=CC=C(C2=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=NC(C3=CC=C(C4=CC=CC=C4)C=C3)=N2)C(O)=C1.CCCCCCCCOC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1.COC1=CC(O)=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1 UFGQMLWXAJOXRO-UHFFFAOYSA-N 0.000 description 1
- PTXIETYFUOWCBX-UHFFFAOYSA-N CCCCC(CC)COCC(O)COC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1.CCCCCCCCOC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1.COC1=CC(O)=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.[H]OC1=CC(OCCOC(=O)C(CC)CCCC)=CC=C1C1=NC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1 Chemical compound CCCCC(CC)COCC(O)COC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1.CCCCCCCCOC1=CC(O)=C(C2=NC(C3=CC=C(C)C=C3C)=NC(C3=C(C)C=C(C)C=C3)=N2)C=C1.COC1=CC(O)=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.[H]OC1=CC(OCCOC(=O)C(CC)CCCC)=CC=C1C1=NC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1 PTXIETYFUOWCBX-UHFFFAOYSA-N 0.000 description 1
- PXKCSKRXWAZGFK-UHFFFAOYSA-N CCCNC1CCCCC1 Chemical compound CCCNC1CCCCC1 PXKCSKRXWAZGFK-UHFFFAOYSA-N 0.000 description 1
- VBILPLAUAHZQCS-UHFFFAOYSA-N CCN(C)C1=NC(N(C)CC)=NC(N(C)CC)=N1 Chemical compound CCN(C)C1=NC(N(C)CC)=NC(N(C)CC)=N1 VBILPLAUAHZQCS-UHFFFAOYSA-N 0.000 description 1
- GIAQEABWKIRQHT-UHFFFAOYSA-N CN(CC(=O)N(C)C1CC(C)(C)N(C)C(C)(C)C1)C1CC(C)(C)N(C)C(C)(C)C1 Chemical compound CN(CC(=O)N(C)C1CC(C)(C)N(C)C(C)(C)C1)C1CC(C)(C)N(C)C(C)(C)C1 GIAQEABWKIRQHT-UHFFFAOYSA-N 0.000 description 1
- AHWDQDMGFXRVFB-UHFFFAOYSA-N CN1C(=O)N(C)C(=O)N(C)C1=O Chemical compound CN1C(=O)N(C)C(=O)N(C)C1=O AHWDQDMGFXRVFB-UHFFFAOYSA-N 0.000 description 1
- LKRRLXWXHJFUKK-UHFFFAOYSA-N CN1C(C)(C)CC(N(C=O)CN(C=O)C2CC(C)(C)N(C)C(C)(C)C2)CC1(C)C Chemical compound CN1C(C)(C)CC(N(C=O)CN(C=O)C2CC(C)(C)N(C)C(C)(C)C2)CC1(C)C LKRRLXWXHJFUKK-UHFFFAOYSA-N 0.000 description 1
- ADKOZMAWHPEEOZ-UHFFFAOYSA-N CN1C(C)(C)CC(N2CN3C(=O)N4CN(C5CC(C)(C)N(C)C(C)(C)C5)CN5C(=O)N(C2)C3C45)CC1(C)C Chemical compound CN1C(C)(C)CC(N2CN3C(=O)N4CN(C5CC(C)(C)N(C)C(C)(C)C5)CN5C(=O)N(C2)C3C45)CC1(C)C ADKOZMAWHPEEOZ-UHFFFAOYSA-N 0.000 description 1
- HIHVSPKCVJGHLO-UHFFFAOYSA-N CN1C(C)(C)CC2(CC1(C)C)OC(C)(C)N(C)C2=O Chemical compound CN1C(C)(C)CC2(CC1(C)C)OC(C)(C)N(C)C2=O HIHVSPKCVJGHLO-UHFFFAOYSA-N 0.000 description 1
- WNOWBRDWVSDLDV-UHFFFAOYSA-N CN1CC(C)(C)N(C)C(C)(C)C1=O Chemical compound CN1CC(C)(C)N(C)C(C)(C)C1=O WNOWBRDWVSDLDV-UHFFFAOYSA-N 0.000 description 1
- VMEASXNKBNLJIE-UHFFFAOYSA-N COC1CC(C)(C)N(CC(C)C)C(C)(C)C1 Chemical compound COC1CC(C)(C)N(CC(C)C)C(C)(C)C1 VMEASXNKBNLJIE-UHFFFAOYSA-N 0.000 description 1
- QBFHEEBFQBHALF-UHFFFAOYSA-N COC1CC(C)(C)N(CC(C)OOCCC(C)=O)C(C)(C)C1 Chemical compound COC1CC(C)(C)N(CC(C)OOCCC(C)=O)C(C)(C)C1 QBFHEEBFQBHALF-UHFFFAOYSA-N 0.000 description 1
- NEIHNYXNOHFAHJ-UHFFFAOYSA-N [H]N(CCCN(CCN(C)CCCNC1=NC(C)=NC(N(C)C2CC(C)(C)N([H])C(C)(C)C2)=N1)C1=NC(N(CCCC)C2CC(C)(C)N([H])C(C)(C)C2)=NC(N(C)C2CC(C)(C)N([H])C(C)(C)C2)=N1)C1=NC(N(CCCC)C2CC(C)(C)N([H])C(C)(C)C2)=NC(N(C)C2CC(C)(C)N([H])C(C)(C)C2)=N1 Chemical compound [H]N(CCCN(CCN(C)CCCNC1=NC(C)=NC(N(C)C2CC(C)(C)N([H])C(C)(C)C2)=N1)C1=NC(N(CCCC)C2CC(C)(C)N([H])C(C)(C)C2)=NC(N(C)C2CC(C)(C)N([H])C(C)(C)C2)=N1)C1=NC(N(CCCC)C2CC(C)(C)N([H])C(C)(C)C2)=NC(N(C)C2CC(C)(C)N([H])C(C)(C)C2)=N1 NEIHNYXNOHFAHJ-UHFFFAOYSA-N 0.000 description 1
- UJRXKQQKEVBLIF-UHFFFAOYSA-N [H]N1C(C)(C)CC(N(C)C2=NC(NCCCN(C)CCN(CCCNC3=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=N3)C3=NC(C)=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=N3)=NC(N(C)C3CC(C)(C)N([H])C(C)(C)C3)=N2)CC1(C)C Chemical compound [H]N1C(C)(C)CC(N(C)C2=NC(NCCCN(C)CCN(CCCNC3=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=N3)C3=NC(C)=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=N3)=NC(N(C)C3CC(C)(C)N([H])C(C)(C)C3)=N2)CC1(C)C UJRXKQQKEVBLIF-UHFFFAOYSA-N 0.000 description 1
- IRYRQJZPACDZCE-UHFFFAOYSA-N [H]N1C(C)(C)CC(N(C)C2=NC(NCCCN(CCN(CCCNC)C3=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=N3)C3=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=N3)=NC(C)=N2)CC1(C)C Chemical compound [H]N1C(C)(C)CC(N(C)C2=NC(NCCCN(CCN(CCCNC)C3=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=N3)C3=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=NC(N(C)C4CC(C)(C)N([H])C(C)(C)C4)=N3)=NC(C)=N2)CC1(C)C IRYRQJZPACDZCE-UHFFFAOYSA-N 0.000 description 1
- WOVYHYBXOXBIGF-UHFFFAOYSA-N [H]N1C(C)(C)CC(N(CCCCCCN(C2=NC(N(CCCC)C3CC(C)(C)N([H])C(C)(C)C3)=NC(N(CCCCCCN(C3=NC(N(CCCC)CCCC)=NC(N(CCCC)CCCC)=N3)C3CC(C)(C)N([H])C(C)(C)C3)C3CC(C)(C)N([H])C(C)(C)C3)=N2)C2CC(C)(C)N([H])C(C)(C)C2)C2=NC(C(CCCC)CCCC)=NC(N(CCCC)CCCC)=N2)CC1(C)C Chemical compound [H]N1C(C)(C)CC(N(CCCCCCN(C2=NC(N(CCCC)C3CC(C)(C)N([H])C(C)(C)C3)=NC(N(CCCCCCN(C3=NC(N(CCCC)CCCC)=NC(N(CCCC)CCCC)=N3)C3CC(C)(C)N([H])C(C)(C)C3)C3CC(C)(C)N([H])C(C)(C)C3)=N2)C2CC(C)(C)N([H])C(C)(C)C2)C2=NC(C(CCCC)CCCC)=NC(N(CCCC)CCCC)=N2)CC1(C)C WOVYHYBXOXBIGF-UHFFFAOYSA-N 0.000 description 1
- KHVVAJCTXMQGBJ-UHFFFAOYSA-N [H]N1C(C)(C)CC2(CC1(C)C)OC(C)(C)N(CC(O)CN1C(C)(C)CC3(CC1(C)C)OC(C)(C)N(CC(O)CN1C(=O)C4(CC(C)(C)N(CC(O)CN5C(=O)C6(CC(C)(C)N([H])C(C)(C)C6)OC5(C)C)C(C)(C)C4)OC1(C)C)C3=O)C2=O Chemical compound [H]N1C(C)(C)CC2(CC1(C)C)OC(C)(C)N(CC(O)CN1C(C)(C)CC3(CC1(C)C)OC(C)(C)N(CC(O)CN1C(=O)C4(CC(C)(C)N(CC(O)CN5C(=O)C6(CC(C)(C)N([H])C(C)(C)C6)OC5(C)C)C(C)(C)C4)OC1(C)C)C3=O)C2=O KHVVAJCTXMQGBJ-UHFFFAOYSA-N 0.000 description 1
- CCJNWUBMBARKAB-UHFFFAOYSA-N [H]N1C(C)(C)CC2(CC1(C)C)OC1(CCCCCCCCCCC1)N(C)C2=O Chemical compound [H]N1C(C)(C)CC2(CC1(C)C)OC1(CCCCCCCCCCC1)N(C)C2=O CCJNWUBMBARKAB-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/16—Elastomeric ethene-propene or ethene-propene-diene copolymers, e.g. EPR and EPDM rubbers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3467—Heterocyclic compounds having nitrogen in the ring having more than two nitrogen atoms in the ring
- C08K5/3472—Five-membered rings
- C08K5/3475—Five-membered rings condensed with carbocyclic rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/0041—Optical brightening agents, organic pigments
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3467—Heterocyclic compounds having nitrogen in the ring having more than two nitrogen atoms in the ring
- C08K5/3477—Six-membered rings
- C08K5/3492—Triazines
Definitions
- the present invention relates to polyolefin compositions which contain a mixture of a hydroxyphenyl benzotriazole and a hydroxyphenyl-s-triazine UV absorber, at least one light stabilizer from the class of sterically hindered amines (HALS) and at least one organic pigment. Further objects are a method for stabilization of pigmented polyolefins and the use of a UV absorber mixture therefore.
- HALS sterically hindered amines
- EP-A-0453 396 discloses that mixtures of hydroxyphenyl-benzotriazole with hydroxybenzophenon or with hydroxyphenyl-s-triazine UV-absorbers lead to synergistic effects which prevent the coatings life time unexpectedly long from degradation.
- FR 2619 814 generically discloses the combined use of oxalamide UV absorbers, particularly in coatings, with benzophenone or benzotriazole UV absorbers, there is however no suggestion in the prior art for the instant combinations in polyolefins.
- GB 2361005 discloses several combinations of UV absorbers in polyolefins, however combinations of benzotriazoles with hydroxyphenyltriazines are not mentioned.
- the combinations of the present invention provide an unexpected synergistic stabilization effect for polyolefin articles.
- the effect is not predictable from the absorption spectra and has so far not been observed with other UV absorber combinations in pigmented polyolefins.
- One aspect of the invention is a polyolefin composition, which comprises
- Polymers of monoolefins and diolefins for example polypropylene, polyisobutylene, polybut-1-ene, poly-4-methylpent-1-ene, polyisoprene or polybutadiene, as well as polymers of cycloolefins, for instance of cyclopentene or norbornene, polyethylene (which optionally can be crosslinked), for example high density polyethylene (HDPE), high density and high molecular weight polyethylene (HDPE-HMW), high density and ultrahigh molecular weight polyethylene (HDPE-UHMW), medium density polyethylene (MDPE), low density polyethylene (LDPE), linear low density polyethylene (LLDPE), (VLDPE) and (ULDPE), metallocene polyethylen (m-PE) in particular m-LLDPE and metallocene poylpropylene (m-PP).
- HDPE high density polyethylene
- HDPE-HMW high density and high molecular weight polyethylene
- Polyolefins i.e. the polymers of monoolefins exemplified in the preceding paragraph, preferably polyethylene and polypropylene, can be prepared by different, and especially by the following, methods:
- polyolefin composition wherein the polyolefin is polypropylene or a copolymer thereof.
- organic pigments examples include azo pigments, anthraquinones, benzimidazolones, dioxazines, phthalocyanines, tetrachloroisoindolinones, quinacridones, isoindolines, perylenes, pyrrolo-pyrroles (diketopyrrolo-pyrrole, such as for example Pigment Red 254).
- a very suitable class is for example the class of diketopyrrolo-pyrrole pigments.
- a particularly preferred inorganic pigment is titanium dioxide, which is often used in combination with an organic pigment.
- the ratio of organic pigment to inorganic pigment can vary in a wide range, typically from 5:95 parts to 95:5 parts by weight.
- the amount of pigment incorporated is typically from 0.1 to 15% preferably from 0.1 to 10% and more preferably from 0.1 to 5% by weight, based on the polymer.
- Typical UV-absorbers of the class of hydroxyphenyl triazines are of formula (I) in which n is 1 or 2;
- Halogen is in all cases fluorine, chlorine, bromine or iodine.
- alkyl examples include methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, 2-ethylbutyl, n-pentyl, isopentyl, 1-methylpentyl, 1,3-dimethylbutyl, n-hexyl, 1-methylhexyl, n-heptyl, isoheptyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl, 3-methylheptyl, n-octyl, 2-ethylhexyl, 1,1,3-trimethylhexyl, 1,1,3,3-tetramethylpentyl, nonyl, decyl, undecyl, 1-methylundecyl, dodecyl.
- alkoxy having up to 12 carbon atoms examples include methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, pentoxy, isopentoxy, hexoxy, heptoxy, octoxy, decyloxy, dodecyloxy.
- alkenoxy examples are propenyloxy, butenyloxy, pentenyloxy and hexenyloxy.
- C 5 -C 12 cycloalkyl examples are cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl and cyclododecyl.
- C 1 -C 4 Alkyl-substituted C 5 -C 12 cycloalkyl is for example methylcyclohexyl or dimethylcyclohexyl.
- OH— and/or C 1 -C 10 alkyl-substituted phenyl is for example methylphenyl, dimethylphenyl, trimethylphenyl, tert-butylphenyl or 3,5-di-tert-butyl-4-hydroxyphenyl.
- Alkoxy-substituted phenyl is for example methoxyphenyl dimethoxyphenyl or trimethoxyphenyl.
- C 7 -C 9 phenylalkyl examples are benzyl and phenylethyl.
- Phenylalkyl which is substituted on the phenyl radical by —OH and/or by alkyl having up to 10 carbon atoms is for example methylbenzyl, dimethylbenzyl, trimethylbenzyl, tert-butylbenzyl or 3,5-di-tert-butyl-4-hydroxybenzyl.
- alkenyl examples are allyl, 2-methallyl, butenyl, pentenyl and hexenyl. Allyl is preferred.
- the carbon atom in position 1 is preferably saturated.
- alkylene examples include methylene, ethylene, propylene, trimethylene, tetramethylene, pentamethylene, 2,2-dimethyltrimethylene, hexamethylene, trimethylhexamethylene, octamethylene and decamethylene.
- Example s of alkenylene are butenylene, pentenylene and hexenylene.
- C 6 -C 12 arylene is preferably phenylene.
- Alkyl interrupted by O is for example —CH 2 —CH 2 —O—CH 2 —CH 3 , —CH 2 —CH 2 —O—CH 3 — or —CH 2 —CH 2 —O—CH 2 —CH 2 —CH 2 —O—CH 2 —CH 3 —. It is preferably derived from polyethlene glycol. A general description is —((CH 2 ) a —O) b —H/CH 3 , wherein a is a number from 1 to 6 and b is a number from 2 to 10.
- C 2 -C 10 oxaalkylene and C 2 -C 10 thiaalkylene can be deduced from the above mentioned alkylene groups by substituting one or more carbon atoms by an oxygen atom or a sulphur atom.
- hydroxyphenyl-triazine UV-absorber is of formula (Ia) wherein
- polyolefin compositions are those, in which, in the compounds of the formula (Ia), the substituents Y 1 are hydrogen, alkyl having 1 to 12 carbon atoms, alkoxy having 1 to 18 carbon atoms, phenyl or halogen, if u is 1, Y 2 is alkyl having 1 to 18 carbon atoms, alkyl which has 1 to 12 carbon atoms and is substituted by hydroxyl, alkoxy having 1 to 18 carbon atoms, —COOY 8 , —CONY 9 Y 10 and/or —OCOY 11 , glycidyl or phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, or, if u is 2, Y 2 is alkylene having 2 to 16 carbon atoms, alkenylene having 4 to 12 carbon atoms, xylylene or alkylene which has 3 to 20 carbon atoms, is interrupted by one or more —O— atoms and/or is substituted
- u is 1.
- Typical individual compounds are the following:
- hydroxyphenyl triazine UV-absorbers are known and partially items of commerce.
- Suitable examples for hydroxyphenyl benzotriazole UV absorbers are compounds of the following structure IIa, IIb or IIc. in the compounds of the formula (IIa),
- R 101 can be hydrogen or alkyl having 1 to 24 carbon atoms, such as methyl, ethyl, propyl, butyl, hexyl, octyl, nonyl, dodecyl, tetradecyl, hexadecyl, octadecyl, nonadecyl and eicosyl and also corresponding branched isomers.
- R 101 can also be cycloalkyl having 5 to 8 carbon atoms, for example cyclopentyl, cyclohexyl and cyclooctyl, or a radical of the formula in which R 104 and R 105 independently of one another are alkyl having in each case 1 to 5 carbon atoms, in particular methyl, or R 104 , together with the C n H 2n+1 ⁇ m radical, forms a cycloalkyl radical having 5 to 12 carbon atoms, for example cyclohexyl, cyclooctyl and cyclodecyl.
- M is a radical of the formula —COOR 106 in which R 106 is not only hydrogen but also alkyl having 1 to 12 carbon atoms or alkoxyalkyl having 1 to 20 carbon atoms in each of the alkyl and alkoxy moieties.
- Suitable alkyl radicals R 106 are those enumerated for R 101 .
- suitable alkoxyalkyl groups are —C 2 H 4 OC 2 H 5 , —C 2 H 4 OC 8 H 17 and —C 4 H 8 OC 4 H 9 .
- R 106 is, for example, benzyl, cumyl, ⁇ -methylbenzyl or phenylbutyl.
- R 102 can also be alkyl having 1 to 18 carbon atoms. Examples of such alkyl radicals are indicated in the definitions of R 101 .
- R 102 can also be phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, for example benzyl, ⁇ -methylbenzyl and cumyl.
- Halogen as a substituent means in all cases fluorine, chlorine, bromine or iodine, preferably chlorine or bromine and more preferably chlorine.
- At least one of the radicals R 101 and R 102 must be other than hydrogen.
- R 103 is also alkyl or alkoxy having in each case 1 to 4 carbon atoms, for example methyl, butyl, methoxy and ethoxy, and also —COOR 106 .
- T is hydrogen or alkyl having 1 to 6 carbon atoms, such as methyl and butyl
- T 1 is not only hydrogen or chlorine, but also alkyl or alkoxy having in each case 1 to 4 carbon atoms, for example methyl, methoxy and butoxy
- T 2 is chlorine or a radical of the formula —OT 3 or —NT 4 T 5
- T 3 is here hydrogen or alkyl having 1 to 18 carbon atoms (cf. the definition of R 101 ).
- These alkyl radicals can be substituted by 1 to 3 hydroxyl groups or by a radical —OCOT 6 .
- T 3 can be alkyl having 3 to 18 carbon atoms (cf.
- R 101 which is interrupted once or several times by —O— or —NT 6 - and is unsubstituted or substituted by hydroxyl or —OCOT 6 .
- T 3 as cycloalkyl are cyclopentyl, cyclohexyl or cyclooctyl.
- T 3 can also be alkenyl having 2 to 18 carbon atoms. Suitable alkenyl radicals are derived from the alkyl radicals enumerated in the definitions of R 101 . These alkenyl radicals can be substituted by hydroxyl.
- T 3 examples are benzyl, phenylethyl, cumyl, ⁇ -methylbenzyl or benzyl.
- T 3 can also be a radical of the formula —CH 2 CH(OH)-T 7 or
- T 4 and T 5 can, independently of one another, be not only hydrogen but also alkyl having 1 to 18 carbon atoms or alkyl which has 3 to 18 carbon atoms and is interrupted once or several times by —O— or —NT 6 -.
- T 4 and T 5 can also be cycloalkyl having 5 to 12 carbon atoms, for example cyclopentyl, cyclohexyl and cyclooctyl.
- T 4 and T 5 as alkenyl groups can be found in the illustrations of T 3 .
- T 4 and T 5 as phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety are benzyl or phenylbutyl.
- substituents can also be hydroxyalkyl having 1 to 3 carbon atoms.
- T 2 is a divalent radical of the formula or —O-T 9 -O—.
- T 6 is alkyl, cycloalkyl, alkenyl, aryl or phenylalkyl; examples of such radicals have already been given above.
- T 7 can be phenyl or hydroxyphenyl and also —CH 2 OT 8 in which T 8 can be one of the alkyl, alkenyl, cycloalkyl, aryl or phenylalkyl radicals enumerated.
- the divalent radical T 9 can be alkylene having 2 to 8 carbon atoms, and such radicals can also be branched. This also applies to the alkenylene and alkynylene radicals T 9 . As well as cyclohexylene, T 9 can also be a radical of the formula —CH 2 CH(OH)CH 2 OT 11 OCH 2 CH(OH)CH 2 — or —CH 2 —C(CH 2 OH) 2 —CH 2 —.
- T 10 is a divalent radical and, in addition to cyclohexylene, is also alkylene which has 2 to 20 carbon atoms and which can be interrupted once or several times by —O—.
- Suitable alkylene radicals are derived from the alkyl radicals mentioned in the definitions of R 101 .
- T 11 is also an alkylene radical. It contains 2 to 8 carbon atoms or, if it is interrupted once or several times by —O—, 4 to 10 carbon atoms. T 11 is also 1,3-cyclohexylene, 1,4-cyclohexylene, 1,3-phenylene or 1,4-phenylene.
- T 6 and T 10 can also be a piperazine ring.
- R 101 is hydrogen or alkyl having 1 to 20 carbon atoms
- R 102 is hydrogen, alkyl having 1 to 18 carbon atoms or phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety
- R 103 is hydrogen, chlorine or alkyl having 1 to 4 carbon atoms.
- R 101 is in the ortho-position relative to the hydroxyl group and is hydrogen or alkyl having 4 to 12 carbon atoms
- R 102 is in the para-position relative to the hydroxyl group and is alkyl having 1 to 6 carbon atoms or cumyl
- R 103 is hydrogen or chlorine.
- UV absorbers of the formulae (I), (Ia), (IIa), (IIb), (IIc) are known per se and are described, together with their preparation, in, for example, WO 96/28431, EP-A-323 408, EP-A-57 160, U.S. Pat. No. 5,736,597 (EP-A-434 608) and U.S. Pat. No. 4,619,956. Preferred meanings of substituents and individual compounds can be deduced from the documents mentioned.
- Particularly preferred compounds are the following:
- the UV-absorbers are typically incorporated in an amount of 0.005 to 5% each by weight based on the polymer.
- the total amount of UV absorber is preferably from 0.01 to 5%, more preferably from 0.05 to 2% and most preferably from 0.05 to 1% by weight, based on the weight of the polyolefin.
- the weight ratio of hydroxyphenyl triazine UV-absorber to hydroxy-phenyl benzotriazole UV-absorber is preferably from 10:1 to 1:10, more preferably from 5:1 to 1:5 and most preferably from 2:1 to 1:2.
- Particularly preferred mixtures are the following:
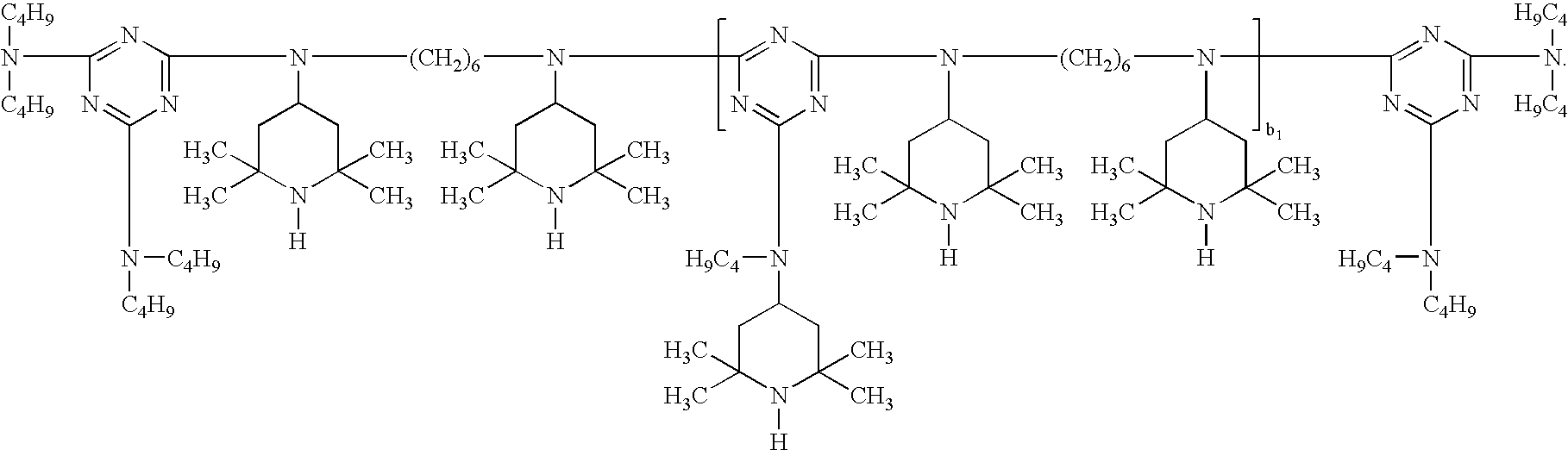
- the sterically hindered amine light stabilizer useful in the instant invention is preferably a compound of formulae (A-1) to (A-10) or of formulae (B-1) to (B-10); ( ⁇ -1) a Compound of the Formula (A-1) in which
- alkyl having up to 30 carbon atoms examples include methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, 2-ethylbutyl, n-pentyl, isopentyl, 1-methylpentyl, 1,3-dimethyl-butyl, n-hexyl, 1-methylhexyl, n-heptyl, isoheptyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl, 3-methylheptyl, n-octyl, 2-ethylhexyl, 1,1,3-trimethylhexyl, 1,1,3,3-tetramethylpentyl, nonyl, decyl, undecyl, 1-methylundecyl, dodecyl, 1,1,3,3,5,5-hexamethylhexyl, tridecyl, te
- E 1 , E 8 , E 12 , E 13 , E 16 , E 18 , E 22 , E 23 , E 25 , E 29 , R 206 , R 213 , R 216 , R 218 , R 230 and R 232 is C 1 -C 4 alkyl, especially methyl.
- R 231 is preferably butyl.
- alkoxy having up to 18 carbon atoms examples include methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, pentoxy, isopentoxy, hexoxy, heptoxy, octoxy, decyloxy, dodecyloxy, tetradecyloxy, hexadecyloxy and octadecyloxy.
- One of the preferred meanings of E 1 is octoxy.
- E 24 is preferably C 1 -C 4 alkoxy and one of the preferred meanings of R 206 is propoxy.
- C 5 -C 12 cycloalkyl examples are cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl and cyclododecyl.
- C 1 -C 4 Alkyl-substituted C 5 -C 12 cycloalkyl is for example methylcyclohexyl or dimethylcyclohexyl.
- C 5 -C 12 cycloalkoxy examples are cyclopentoxy, cyclohexoxy, cycloheptoxy, cyclooctoxy, cyclodecyloxy and cyclododecyloxy.
- C 1 -C 10 alkyl-substituted phenyl is for example methylphenyl, dimethylphenyl, trimethylphenyl, tert-butylphenyl or 3,5-di-tert-butyl-4-hydroxyphenyl.
- C 7 -C 9 phenylalkyl examples are benzyl and phenylethyl.
- Phenylalkyl which is substituted on the phenyl radical by —OH and/or by alkyl having up to 10 carbon atoms is for example methylbenzyl, dimethylbenzyl, trimethylbenzyl, tert-butylbenzyl or 3,5-di-tert-butyl-4-hydroxybenzyl.
- alkenyl having up to 10 carbon atoms examples include allyl, 2-methallyl, butenyl, pentenyl and hexenyl. Allyl is preferred.
- the carbon atom in position 1 is preferably saturated.
- acyl containing not more than 8 carbon atoms are formyl, acetyl, propionyl, butyryl, pentanoyl, hexanoyl, heptanoyl, octanoyl, acryloyl, methacryloyl and benzoyl.
- C 1 -C 8 Alkanoyl, C 3 -C 8 alkenyl and benzoyl are preferred.
- Acetyl and acryloyl are especially preferred.
- alkylene having up to 22 carbon atoms examples include methylene, ethylene, propylene, trimethylene, tetramethylene, pentamethylene, 2,2-dimethyltrimethylene, hexamethylene, trimethylhexamethylene, octamethylene and decamethylene.
- C 3 -C 10 alkylidene is the group
- O 4 —C 10 alkanetetrayl is 1,2,3,4-butanetetrayl.
- C 5 -C 7 cycloalkylene is cyclohexylene.
- C 1 -C 4 alkylenedi(C 5 -C 7 cycloalkylene) is methylenedicyclohexylene.
- phenylenedi(C 1 -C 4 alkylene) is methylene-phenylene-methylene or ethylene-phenylene-ethylene.
- radicals R 201 , R 202 and R 203 together with the nitrogen atoms to which they are attached, form a 5- to 10-membered heterocyclic ring, this ring is for example A 6-membered heterocyclic ring is preferred.
- radicals R 204 and R 205 together with the nitrogen atom to which they are attached, form a 5- to 10-membered heterocyclic ring
- this ring is for example 1-pyrrolidyl, piperidino, morpholino, 1-piperazinyl, 4-methyl-1-piperazinyl, 1-hexahydroazepinyl, 5,5,7-trimethyl-1-homopiperazinyl or 4,5,5,7-tetramethyl-1-homopiperazinyl.
- Morpholino is particularly preferred.
- R 219 and R 223 are phenyl.
- R 226 is preferably a direct bond.
- n 1 , n 2 , n 2 * and n 4 are preferably a number from 2 to 25, in particular 2 to 20.
- n 3 is preferably a number from 1 to 25, in particular 1 to 20.
- b 1 and b 2 are preferably a number from 2 to 25, in particular 2 to 20.
- b 3 and b 4 are preferably a number from 1 to 25, in particular 1 to 20.
- b′ 5 and b′′′ 5 are preferably 3 and b′′ 5 is preferably 2.
- the product (B-6) can be prepared analogously to known processes, for example by reacting a polyamine of formula (B-6-1) with cyanuric chloride in a molar ratio of from 1:2 to 1:4 in the presence of anhydrous lithium carbonate, sodium carbonate or potassium carbonate in an organic solvent such as 1,2-dichloroethane, toluene, xylene, benzene, dioxane or tert-amyl alcohol at a temperature of from ⁇ 20° C. to +10° C., preferably from ⁇ 10° C. to +10° C., in particular from 0° C.
- an organic solvent such as 1,2-dichloroethane, toluene, xylene, benzene, dioxane or tert-amyl alcohol
- the molar ratio of the 2,2,6,6-tetramethyl-4-piperidylamine to polyamine of the formula (B-6-1) employed is for example from 4:1 to 8:1.
- the quantity of the 2,2,6,6-tetramethyl-4-piperidylamine can be added in one portion or in more than one portion at intervals of a few hours.
- the molar ratio of polyamine of the formula (B-6-1) to cyanuric chloride to 2,2,6,6-tetramethyl-4-piperidylamine of the formula (B-6-2) is preferably from 1:3:5 to 1:3:6.
- a further 18 g (0.13 mol) of anhydrous potassium carbonate are added and the mixture is warmed at 60° C. for a further 6 hours.
- the solvent is removed by distillation under a slight vacuum (200 mbar) and replaced by xylene.
- 18.2 g (0.085 mol) of N-(2,2,6,6-tetramethyl-4-piperidyl)butylamine and 5.2 g (0.13 mol) of ground sodium hydroxide are added, the mixture is heated at reflux for 2 hours and, for a further 12 hours, the water formed during the reaction is removed by azeotropic distillation.
- the mixture is filtered.
- the solution is washed with water and dried over Na 2 SO 4 .
- the solvent is evaporated and the residue is dried at 120-130° C. in vacuo (0.1 mbar).
- the desired product is obtained as a colourless resin.
- the product (B-6) can, for example, be represented by a compound of the formula (B-6- ⁇ ), (B-6- ⁇ ) or (B-6- ⁇ ). It can also be in the form of a mixture of these three compounds.
- b 5 is preferably 2 to 20, in particular 2 to 10.
- the sterically hindered amine compounds of component (c) are preferably selected from the group consisting of the following commercial products: DASTIB 845 (RTM), TINUVIN 770 (RTM), TINUVIN 765 (RTM), TINUVIN 144 (RTM), TINUVIN 123 (RTM), TINUVIN 111 (RTM), TINUVIN 783 (RTM), TINUVIN 791 (RTM), MARK LA 52 (RTM), MARK LA 57 (RTM), MARK LA 62 (RTM), MARK LA 67 (RTM), HOSTAVIN N 20 (RTM), HOSTAVIN N 24 (RTM), SANDUVOR 3050 (RTM), DIACETAM 5 (RTM), SUMISORB TM 61 (RTM), UVINUL 4049 (RTM), SANDUVOR PR 31(RTM), GOODRITE UV 3034 (RTM), GOODRITE UV 3150 (RTM), GOODRITE UV 3159 (RTM), GOODRITE 3110 ⁇ 128
- TINUVIN 770 RTM
- TINUVIN 791 RTM
- TINUVIN 622 RTM
- TINUVIN 783 RTM
- CHIMASSORB 944 RTM
- CHIMASSORB 2020 RTM
- CHIMASSORB 119 RTM
- Tinuvin 770 RTM
- TINUVIN 791 RTM
- terminal groups which saturate the free valences in the compounds of the formulae (B-1), (B-3), (B-4), (B-5), (B-6- ⁇ ), (B-6- ⁇ ), (B-6- ⁇ ), (B-7), (B-8-a), (B-8-b) and (B-10) depend on the processes used for their preparation.
- the terminal groups can also be modified after the preparation of the compounds.
- the compounds of the formula (B-1) are prepared by reacting a compound of the formula in which X is, for example, halogen, in particular chlorine, and R 204 and R 205 are as defined above, with a compound of the formula in which R 201 , R 202 and R 203 are as defined above, the terminal group bonded to the diamino radical is hydrogen or and the terminal group bonded to the triazine radical is X or
- X is halogen, it is advantageous to replace this, for example, by —OH or an amino group when the reaction is complete.
- amino groups which may be mentioned are pyrrolidin-1-yl, morpholino, —NH 2 , —N(C 1 -C 8 )alkyl) 2 and —NR(C 1 -C 8 alkyl), in which R is hydrogen or a group of the formula (b-I).
- the compounds of the formula (B-1) also cover compounds of the formula wherein R 201 , R 202 , R 203 , R 204 , R 205 and b 1 are as defined above and R 204 * has one of the meanings of R 204 and R 205 * has one of the meanings of R 205 .
- the terminal group bonded to the silicon atom can be, for example, (R 14 ) 3 Si—O—, and the terminal group bonded to the oxygen can be, for example, —Si(R 14 ) 3 .
- the compounds of the formula (B-3) can also be in the form of cyclic compounds if b 2 is a number from 3 to 10, i.e. the free valences shown in the structural formula then form a direct bond.
- the terminal group bonded to the 2,5-dioxopyrrolidine ring is, for example, hydrogen
- the terminal group bonded to the —C(R 223 )(R 224 )— radical is, for example,
- the terminal group bonded to the carbonyl radical is, for example, and the terminal group bonded to the oxygen radical is, for example,
- the terminal group bonded to the triazine radical is, for example, Cl or a group
- the terminal group bonded to the amino radical is, for example, hydrogen or a group.
- the compounds of the formula (B-7) are prepared, for example, by reacting a compound of the formula in which A 1 is hydrogen or methyl, with a dicarboxylic acid diester of the formula Y—OOC-A 2 -COO—Y, in which Y is, for example, methyl, ethyl or propyl, and A 2 is as defined above, the terminal group bonded to the 2,2,6,6-tetramethyl-4-oxypiperidin-1-yl radical is hydrogen or —CO-A 2 -COO—Y, and the terminal group bonded to the diacyl radical is —O—Y or
- the terminal group bonded to the nitrogen can be, for example, hydrogen and the terminal group bonded to the 2-hydroxypropylene radical can be, for example, a group.
- the terminal group bonded to the dimethylene radical can be, for example, —OH
- the terminal group bonded to the oxygen can be, for example, hydrogen.
- the terminal groups can also be polyether radicals.
- the end group bonded to the —CH 2 — residue can be, for example, hydrogen and the end group bonded to the —CH(CO 2 A 7 ) residue can be, for example, —CH ⁇ CH—COOA 7 .
- Further individual individual compounds are the following: bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate, bis(2,2,6,6-tetramethyl-4-piperidyl)succinate, bis(1,2,2,6,6-pentamethyl-4-piperidyl)sebacate, bis(1-octyloxy-2,2,6,6-tetramethyl-4-piperidyl)sebacate, bis(1,2,2,6,6-pentamethyl-4-piperidyl) n-butyl-3,5-di-tert-butyl-4-hydroxy-benzylmalonate, the condensate of 1-(2-hydroxyethyl)-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, linear or cyclic condensates of N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-tert-octylamino-2,6-dichlor
- the pigment is selected from the class consisting of diketopyrrolo-pyrrole pigments
- the UV absorber is a mixture of Tinuvin 327 (RTM) and Tinuvin 1577 (RTM)
- the sterically hindered amine stabilizer is Tinuvin 791 (RTM) or Tinuvin 770 (RTM)
- the substrate is polypropylene.
- the amount of the sterically hindered amine compound (component (c)) in the polyolefin to be stabilized is preferably 0.005 to 5%, in particular 0.01 to 1% or 0.05 to 1% by weight, based on the weight of the polyolefin.
- the stabilized material may additionally also contain various conventional additives, for example:
- Alkylated monophenols for example 2,6-di-tert-butyl-4-methylphenol, 2-tert-butyl-4,6-di-methylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-butylphenol, 2,6-di-tert-butyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol, 2- ⁇ -methylcyclohexyl)-4,6-dimethylphenol, 2,6-dioctadecyl-4-methylphenol, 2,4,6-tricyclohexylphenol, 2,6-di-tert-butyl-4-methoxymethylphenol, nonylphenols which are linear or branched in the side chains, for example 2,6-di-nonyl-4-methylphenol, 2,4-dimethyl-6-(1′-methylundec-1′-yl)phenol, 2,4-di
- Alkylthiomethylphenols for example 2,4-dioctylthiomethyl-6-tert-butylphenol, 2,4-dioctylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-di-dodecylthiomethyl-4-nonylphenol.
- Hydroquinones and alkylated hydroquinones for example 2,6-di-tert-butyl-4-methoxyphenol, 2,5-di-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-diphenyl-4-octadecyloxyphenol, 2,6-di-tert-butylhydroquinone, 2,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyphenyl stearate, bis(3,5-di-tert-butyl-4-hydroxyphenyl) adipate.
- 2,6-di-tert-butyl-4-methoxyphenol 2,5-di-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-diphenyl-4-
- Tocopherols for example ⁇ -tocopherol, ⁇ -tocopherol, ⁇ -tocopherol, ⁇ -tocopherol and mixtures thereof (vitamin E).
- Hydroxylated thiodiphenyl ethers for example 2,2′-thiobis(6-tert-butyl-4-methylphenol), 2,2′-thiobis(4-octylphenol), 4,4′-thiobis(6-tert-butyl-3-methylphenol), 4,4′-thiobis(6-tert-butyl-2-methylphenol), 4,4′-thiobis(3,6-di-sec-amylphenol), 4,4′-bis(2,6-dimethyl-4-hydroxyphenyl)-disulfide.
- 2,2′-thiobis(6-tert-butyl-4-methylphenol 2,2′-thiobis(4-octylphenol), 4,4′-thiobis(6-tert-butyl-3-methylphenol), 4,4′-thiobis(6-tert-butyl-2-methylphenol), 4,4′-thiobis(3,6-di-sec-amylphenol), 4,4′-bis(2,6-
- Alkylidenebisphenols for example 2,2′-methylenebis(6-tert-butyl-4-methylphenol), 2,2′-methylenebis(6-tert-butyl-4-ethylphenol), 2,2′-methylenebis[4-methyl-6-( ⁇ -methylcyclohexyl)-phenol], 2,2′-methylenebis(4-methyl-6-cyclohexylphenol), 2,2′-methylenebis(6-nonyl-4-methylphenol), 2,2′-methylenebis(4,6-di-tert-butylphenol), 2,2′-ethylidenebis(4,6-di-tert-butyl-phenol), 2,2′-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2′-methylenebis[6-( ⁇ -methylbenzyl)-4-nonylphenol], 2,2′-methylenebis[6-( ⁇ , ⁇ -dimethylbenzyl)-4-n
- O-, N- and S-benzyl compounds for example 3,5,3′,5′-tetra-tert-butyl-4,4′-dihydroxydibenzyl ether, octadecyl-4-hydroxy-3,5-dimethylbenzylmercaptoacetate, tridecyl-4-hydroxy-3,5-di-tert-butylbenzylmercaptoacetate, tris(3,5-di-tert-butyl-4-hydroxybenzyl)amine, bis(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)dithioterephthalate, bis(3,5-di-tert-butyl-4-hydroxybenzyl)sulfide, isooctyl-3,5-di-tert-butyl-4-hydroxybenzylmercaptoacetate.
- Hydroxybenzylated malonates for example dioctadecyl-2,2-bis(3,5-di-tert-butyl-2-hydroxybenzyl)malonate, di-octadecyl-2-(3-tert-butyl-4-hydroxy-5-methylbenzyl)malonate, di-dodecylmercaptoethyl-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate, bis[4-(1,1,3,3-tetramethylbutyl)phenyl]-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate.
- Aromatic hydroxybenzyl compounds for example 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-trimethylbenzene, 1,4-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-2,3,5,6-tetramethylbenzene, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)phenol.
- Triazine compounds for example 2,4-bis(octylmercapto)-6-(3,5-di-tert-butyl-4-hydroxyanilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyanilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,3,5-triazine, 2,4,6-tris-(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazine, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)isocyanurate, 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)isocyanurate, 2,4,6-tris-tris
- Benzylphosphonates for example dimethyl-2,5-di-tert-butyl-4-hydroxybenzylphosphonate, diethyl-3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl-5-tert-butyl-4-hydroxy-3-methylbenzylphosphonate, the calcium salt of the monoethyl ester of 3,5-di-tert-butyl-4-hydroxybenzylphosphonic acid.
- Acylaminophenols for example 4-hydroxylauranilide, 4-hydroxystearanilide, octyl N-(3,5-di-tert-butyl-4-hydroxyphenyl)carbamate.
- esters of ⁇ -(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid with mono- or polyhydric alcohols e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N′-bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[
- esters of ⁇ -(5-tert-butyl-4-hydroxy-3-methylphenyl)propionic acid with mono- or polyhydric alcohols e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N′-bis-(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[
- esters of ⁇ -(3,5-dicyclohexyl-4-hydroxyphenyl)propionic acid with mono- or polyhydric alcohols e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N′-bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
- esters of 3,5-di-tert-butyl-4-hydroxyphenyl acetic acid with mono- or polyhydric alcohols e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N′-bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
- Aminic antioxidants for example N,N′-di-isopropyl-p-phenylenediamine, N,N′-di-sec-butyl-p-phenylenediamine, N,N′-bis(1,4-dimethylpentyl)-p-phenylenediamine, N,N′-bis(1-ethyl-3-methylpentyl)-p-phenylenediamine, N,N′-bis(1-methylheptyl)-p-phenylenediamine, N,N′-dicyclohexyl-p-phenylenediamine, N,N′-diphenyl-p-phenylenediamine, N,N′-bis(2-naphthyl)-p-phenylenediamine, N-isopropyl-N′-phenyl-p-phenylenediamine, N-(1,3-dimethylbutyl)-N′-phenyl-p-
- 2-Hydroxybenzophenones for example the 4-hydroxy, 4-methoxy, 4-octyloxy, 4-decyloxy, 4-dodecyloxy, 4-benzyloxy, 4,2′,4′-trihydroxy and 2′-hydroxy-4,4′-dimethoxy derivatives.
- Esters of substituted and unsubstituted benzoic acids for example 4-tert-butylphenyl salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoyl resorcinol, bis(4-tert-butylbenzoyl)resorcinol, benzoyl resorcinol, 2,4-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxybenzoate, hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, octadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, 2-methyl-4,6-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxybenzoate.
- Acrylates for example ethyl ⁇ -cyano- ⁇ , ⁇ -diphenylacrylate, isooctyl ⁇ -cyano- ⁇ , ⁇ -diphenylacrylate, methyl ⁇ -carbomethoxycinnamate, methyl ⁇ -cyano- ⁇ -methyl-p-methoxycinnamate, butyl ⁇ -cyano-p-methyl- ⁇ -methoxycinnamate, methyl ⁇ -carbomethoxy-p-methoxycinnamate and N-( ⁇ -carbomethoxy- ⁇ -cyanovinyl)-2-methylindoline.
- Nickel compounds for example nickel complexes of 2,2′-thiobis[4-(1,1,3,3-tetramethylbutyl)phenol], such as the 1:1 or 1:2 complex, with or without additional ligands such as n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel dibutyldithiocarbamate, nickel salts of the monoalkyl esters, e.g. the methyl or ethyl ester, of 4-hydroxy-3,5-di-tert-butylbenzylphosphonic acid, nickel complexes of ketoximes, e.g. of 2-hydroxy-4-methylphenylundecylketoxime, nickel complexes of 1-phenyl-4-lauroyl-5-hydroxypyrazole, with or without additional ligands.
- additional ligands such as n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel dibutyldithiocarbamate,
- Oxamides for example 4,4′-dioctyloxyoxanilide, 2,2′-diethoxyoxanilide, 2,2′-dioctyloxy-5,5′-di-tert-butoxanilide, 2,2′-didodecyloxy-5,5′-di-tert-butoxanilide, 2-ethoxy-2′-ethyloxanilide, N,N′-bis(3-dimethylaminopropyl)oxamide, 2-ethoxy-5-tert-butyl-2′-ethoxanilide and its mixture with 2-ethoxy-2′-ethyl-5,4′-di-tert-butoxanilide, mixtures of o- and p-methoxy-disubstituted oxanilides and mixtures of o- and p-ethoxy-disubstituted oxanilides.
- Metal deactivators for example N,N′-diphenyloxamide, N-salicylal-N′-salicyloyl hydrazine, N,N′-bis(salicyloyl)hydrazine, N,N′-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hydrazine, 3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalyl dihydrazide, oxanilide, isophthaloyl dihydrazide, sebacoyl bisphenylhydrazide, N,N′-diacetyladipoyl dihydrazide, N,N′-bis(salicyloyl)oxalyl dihydrazide, N,N′-bis(salicyloyl)thiopropionyl dihydrazide.
- N,N′-diphenyloxamide N
- Phosphites and phosphonites for example triphenyl phosphite, diphenylalkyl phosphites, phenyldialkyl phosphites, tris(nonylphenyl) phosphite, trilauryl phosphite, trioctadecyl phosphite, distearylpentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl) phosphite, diisodecyl pentaerythritol diphosphite, bis(2,4-di-tert-butylphenyl)pentaerythritol diphosphite, bis(2,4-di-cumylphenyl)pentaerythritol diphosphite, bis(2,6-di-tert-butyl-4-methylphenyl)pentaerythritol diphos
- Tris(2,4-di-tert-butylphenyl) phosphite (Irgafose® 168, Ciba-Geigy), tris(nonylphenyl) phosphite,
- Hydroxylamines for example N,N-dibenzylhydroxylamine, N,N-diethylhydroxylamine, N,N-dioctylhydroxylamine, N,N-dilaurylhydroxylamine, N,N-ditetradecylhydroxylamine, N,N-dihexadecylhydroxylamine, N,N-dioctadecylhydroxylamine, N-hexadecyl-N-octadecylhydroxylamine, N-heptadecyl-N-octadecylhydroxylamine, N,N-dialkylhydroxylamine derived from hydrogenated tallow amine.
- Nitrones for example N-benzyl-alpha-phenylnitrone, N-ethyl-alpha-methylnitrone, N-octyl-alpha-heptylnitrone, N-lauryl-alpha-undecylnitrone, N-tetradecyl-alpha-tridecylnitrone, N-hexadecyl-alpha-pentadecylnitrone, N-octadecyl-alpha-heptadecylnitrone, N-hexadecyl-alpha-heptadecylnitrone, N-ocatadecyl-alpha-pentadecyinitrone, N-heptadecyl-alpha-heptadecylnitrone, N-octadecyl-alpha-hexadecylnitrone, nitrone derived from N,N-dialkyl
- Thiosynergists for example dilauryl thiodipropionate or distearyl thiodipropionate.
- Peroxide scavengers for example esters of ⁇ -thiodipropionic acid, for example the lauryl, stearyl, myristyl or tridecyl esters, mercaptobenzimidazole or the zinc salt of 2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide, pentaerythritol tetrakis( ⁇ -dodecylmercapto)propionate.
- esters of ⁇ -thiodipropionic acid for example the lauryl, stearyl, myristyl or tridecyl esters
- mercaptobenzimidazole or the zinc salt of 2-mercaptobenzimidazole zinc dibutyldithiocarbamate
- dioctadecyl disulfide pentaerythritol tetrakis( ⁇ -dodecylmercap
- Basic co-stabilisers for example melamine, polyvinylpyrrolidone, dicyandiamide, triallyl cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides, polyurethanes, alkali metal salts and alkaline earth metal salts of higher fatty acids, for example calcium stearate, zinc stearate, magnesium behenate, magnesium stearate, sodium ricinoleate and potassium palmitate, antimony pyrocatecholate or zinc pyrocatecholate.
- Basic co-stabilisers for example melamine, polyvinylpyrrolidone, dicyandiamide, triallyl cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides, polyurethanes, alkali metal salts and alkaline earth metal salts of higher fatty acids, for example calcium stearate, zinc stearate, magnesium behenate, magnesium stearate, sodium ric
- Nucleating agents for example inorganic substances, such as talcum, metal oxides, such as titanium dioxide or magnesium oxide, phosphates, carbonates or sulfates of, preferably, alkaline earth metals; organic compounds, such as mono- or polycarboxylic acids and the salts thereof, e.g. 4-tert-butylbenzoic acid, adipic acid, diphenylacetic acid, sodium succinate or sodium benzoate; polymeric compounds, such as ionic copolymers (ionomers).
- inorganic substances such as talcum
- metal oxides such as titanium dioxide or magnesium oxide
- phosphates carbonates or sulfates of, preferably, alkaline earth metals
- organic compounds such as mono- or polycarboxylic acids and the salts thereof, e.g. 4-tert-butylbenzoic acid, adipic acid, diphenylacetic acid, sodium succinate or sodium benzoate
- polymeric compounds such as ionic copolymers (
- Fillers and reinforcing agents for example calcium carbonate, silicates, glass fibres, glass bulbs, asbestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxides, carbon black, graphite, wood flour and flours or fibers of other natural products, synthetic fibers.
- additives for example plasticisers, lubricants, emulsifiers, pigments, rheology additives, catalysts, flow-control agents, optical brighteners, flameproofing agents, antistatic agents and blowing agents.
- the above mentioned further stabilizers and additives are usually applied in an amount from 0.01% to 2%.
- Also subject of the invention is therefore a polyolefin composition as described above which contains additionally a further stabilizer selected from the group consisting of a phenolic antioxidant, a phosphite or phosphonite and benzofuranone or indolinone.
- a further stabilizer selected from the group consisting of a phenolic antioxidant, a phosphite or phosphonite and benzofuranone or indolinone.
- the above mentioned components a, b, c and further additives can be incorporated into the polyolefin to be stabilized by known methods, for example before or during shaping or by applying the dissolved or dispersed compounds to the polyolefin, if necessary with subsequent evaporation of the solvent.
- the components can be added to the polyolefin in the form of a powder, granules or a masterbatch, which contains these components in, for example, a concentration of from 2.5 to 25% by weight.
- the components can be melt blended with each other before incorporation in the polyolefin. They can also be added to the polyolefin before or during the polymerization.
- the materials stabilized according to this invention can be used in a wide variety of forms, for example as films, fibres, tapes, moulding compositions or profiles.
- back injection back injection
- slush molding injection molding, co-injection molding, forming, compression
- the polyolefin according to the present invention may be used for the preparation of:
- I-1) Floating devices, marine applications, pontoons, buoys, plastic lumber for decks, piers, boats, kayaks, oars, and beach reinforcements.
- I-2) Automotive applications in particular bumpers, dashboards, battery, rear and front linings, moldings parts under the hood, hat shelf, trunk linings, interior linings, air bag covers, electronic moldings for fittings (lights), panes for dashboards, headlamp glass, instrument panel, exterior linings, upholstery, automotive lights, head lights, parking lights, rear lights, stop lights, interior and exterior trims; door panels; gas tank; glazing front side; rear windows; seat backing, exterior panels, wire insulation, profile extrusion for sealing, cladding, pillar covers, chassis parts, exhaust systems, fuel filter/filler, fuel pumps, fuel tank, body side mouldings, convertible tops, exterior mirrors, exterior trim, fasteners/fixings, front end module, glass, hinges, lock systems, luggage/roof racks, pressed/stamped parts, seals, side impact protection, sound deadener/insulator and sunroof.
- Road traffic devices in particular sign postings, posts for road marking, car accessories, warning triangles, medical cases, helmets, tires.
- I-5) Devices for space applications in particular rockets and satellites, e.g. reentry shields.
- I-6) Devices for architecture and design, mining applications, acoustic quietized systems, street refuges, and shelters.
- Electric appliances in particular washing machines, tumblers, ovens (microwave oven), dish-washers, mixers, and irons.
- shutters e.g. roller shutters
- Hygienic articles in particular diapers (babies, adult incontinence), feminine hygiene articles, shower curtains, brushes, mats, tubs, mobile toilets, tooth brushes, and bed pans.
- Glass substitutes in particular extruded plates, glazing for buildings (monolithic, twin or multiwall), aircraft, schools, extruded sheets, window film for architectural glazing, train, transportation, sanitary articles, and greenhouse.
- Plates (walls, cutting board), extrusion-coating (photographic paper, tetrapack and pipe coating), silos, wood substitute, plastic lumber, wood composites, walls, surfaces, furniture, decorative foil, floor coverings (interior and exterior applications), flooring, duck boards, and tiles.
- IV-1) Plates (walls and cutting board), trays, artificial grass, astroturf, artificial covering for stadium rings (athletics), artificial floor for stadium rings (athletics), and tapes.
- V) Films (packaging, dump, laminating, agriculture and horticulture, greenhouse, mulch, tunnel, silage), bale wrap, swimming pools, waste bags, wallpaper, stretch film, raffia, desalination film, batteries, and connectors.
- VI-2) Storage systems such as boxes (crates), luggage, chest, household boxes, pallets, shelves, tracks, screw boxes, packs, and cans.
- Extrusion coating photo paper, tetrapack, pipe coating
- household articles of any kind e.g. appliances, thermos bottle/clothes hanger
- fastening systems such as plugs, wire and cable clamps, zippers, closures, locks, and snap-closures.
- Support devices articles for the leisure time such as sports and fitness devices, gymnastics mats, ski-boots, inline-skates, skis, big foot, athletic surfaces (e.g. tennis grounds); screw tops, tops and stoppers for bottles, and cans.
- sports and fitness devices gymnastics mats, ski-boots, inline-skates, skis, big foot, athletic surfaces (e.g. tennis grounds); screw tops, tops and stoppers for bottles, and cans.
- Footwear (shoes/shoe-soles), insoles, spats, adhesives, structural adhesives, food boxes (fruit, vegetables, meat, fish), synthetic paper, labels for bottles, couches, artificial joints (human), printing plates (flexographic), printed circuit boards, and display technologies.
- Further aspects of the invention are a method for stabilizing a polyolefin containing at least one organic pigment against degradation induced by light, heat or oxidation, which comprises incorporating into the pigmented polyolefin a stabilizer mixture comprising
- the exposure time corresponding to formation of a carbonyl absorbance of 0.1 is a measure for the stabilizing efficiency of the light stabilizer.
- the values obtained are summarized in Table 1.
- the exposure time corresponding to formation of a carbonyl absorbance of 0.1 is a measure for the stabilizing efficiency of the light stabilizer.
- the values obtained are summarized in Tables 2 and 3.
- the exposure time corresponding to formation of a carbonyl absorbance of 0.1 is a measure for the stabilizing efficiency of the light stabilizer.
- the values obtained are summarized in Tables 4 and 5.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
The instant invention relates to a polyolefin composition which comprises
-
- d) at least one organic pigment
- e) at least one sterically hindered amine light stabilizer and
- f) as UV absorber a mixture of
- a 2-hydroxyphenyl benzotriazole and a 2-hydroxyphenyl-s-triazine. Further objects of the invention are a method for stabilizing pigmented polyolefins and the use of a UV-absorber mixture of a hydroxyphenyl benzotriazole with a hydroxyphenyl-s-triazine for the stabilization of pigmented polyolefins.
Description
The present invention relates to polyolefin compositions which contain a mixture of a hydroxyphenyl benzotriazole and a hydroxyphenyl-s-triazine UV absorber, at least one light stabilizer from the class of sterically hindered amines (HALS) and at least one organic pigment. Further objects are a method for stabilization of pigmented polyolefins and the use of a UV absorber mixture therefore.
The effects of atmospheric oxygen, moisture and, in particular, UV light result in degradation of the polymer material. This manifests itself, for example, in the loss of mechanical strength, changes in shade and finally total breakdown of the polymer article. As is known, it is possible to retard such degradation processes in polyolefins by the use of suitable stabilizers, and there are numerous prior art documents in this field.
In the coatings field, EP-A-0453 396 discloses that mixtures of hydroxyphenyl-benzotriazole with hydroxybenzophenon or with hydroxyphenyl-s-triazine UV-absorbers lead to synergistic effects which prevent the coatings life time unexpectedly long from degradation.
FR 2619 814 generically discloses the combined use of oxalamide UV absorbers, particularly in coatings, with benzophenone or benzotriazole UV absorbers, there is however no suggestion in the prior art for the instant combinations in polyolefins.
GB 2361005 discloses several combinations of UV absorbers in polyolefins, however combinations of benzotriazoles with hydroxyphenyltriazines are not mentioned.
It has now been found that combinations of benzotriazole with hydroxyphenyltriazine UV absorbers in the presence of a sterically hindered amine light stabilizer are capable of substantially preventing the degradation of pigmented polyolefins.
The combinations of the present invention provide an unexpected synergistic stabilization effect for polyolefin articles. The effect is not predictable from the absorption spectra and has so far not been observed with other UV absorber combinations in pigmented polyolefins.
One aspect of the invention is a polyolefin composition, which comprises
- a) at least one organic pigment
- b) at least one sterically hindered amine light stabilizer and
- c) as UV absorber a mixture of
- a 2-hydroxyphenyl benzotriazole and a 2-hydroxyphenyl-s-triazine.
Suitable Polyolefins are Mentioned Below.
- a 2-hydroxyphenyl benzotriazole and a 2-hydroxyphenyl-s-triazine.
1. Polymers of monoolefins and diolefins, for example polypropylene, polyisobutylene, polybut-1-ene, poly-4-methylpent-1-ene, polyisoprene or polybutadiene, as well as polymers of cycloolefins, for instance of cyclopentene or norbornene, polyethylene (which optionally can be crosslinked), for example high density polyethylene (HDPE), high density and high molecular weight polyethylene (HDPE-HMW), high density and ultrahigh molecular weight polyethylene (HDPE-UHMW), medium density polyethylene (MDPE), low density polyethylene (LDPE), linear low density polyethylene (LLDPE), (VLDPE) and (ULDPE), metallocene polyethylen (m-PE) in particular m-LLDPE and metallocene poylpropylene (m-PP).
Polyolefins, i.e. the polymers of monoolefins exemplified in the preceding paragraph, preferably polyethylene and polypropylene, can be prepared by different, and especially by the following, methods:
-
- a) radical polymerisation (normally under high pressure and at elevated temperature).
- b) catalytic polymerisation using a catalyst that normally contains one or more than one metal of groups IVb, Vb, VIb or VIII of the Periodic Table. These metals usually have one or more than one ligand, typically oxides, halides, alcoholates, esters, ethers, amines, alkyls, alkenyls and/or aryls that may be either □- or □-coordinated. These metal complexes may be in the free form or fixed on substrates, typically on activated magnesium chloride, titanium(III) chloride, alumina or silicon oxide. These catalysts may be soluble or insoluble in the polymerisation medium. The catalysts can be used by themselves in the polymerisation or further activators may be used, typically metal alkyls, metal hydrides, metal alkyl halides, metal alkyl oxides or metal alkyloxanes, said metals being elements of groups Ia, IIa and/or IIIa of the Periodic Table. The activators may be modified conveniently with further ester, ether, amine or silyl ether groups. These catalyst systems are usually termed Phillips, Standard Oil Indiana, Ziegler (-Natta), TNZ (DuPont), metallocene or single site catalysts (SSC).
2. Mixtures of the polymers mentioned under 1), for example mixtures of polypropylene with polyisobutylene, polypropylene with polyethylene (for example PP/HDPE, PP/LDPE) and mixtures of different types of polyethylene (for example LDPE/HDPE), metallocene types with conventional types (for example m-PE/PE-LLD, m-PE/PE-LD, m-PP/conventional PP).
Preferred is a polyolefin composition wherein the polyolefin is polypropylene, polyethylene or a copolymer thereof.
Particularly preferred is a polyolefin composition wherein the polyolefin is polypropylene or a copolymer thereof.
All organic pigments described in “Gächter/Müller: Plastics Additives Handbook, 3rd Edition, Hanser Publishers, Munich Vienna New York”, page 647 to 659, point 11.2.1.1 to 11.2.4.2 can be used as component a). Further suitable pigments are mentioned in Plastics Additives Handbook, 5th Edition, edited by H. Zweifel.
Examples of organic pigments are azo pigments, anthraquinones, benzimidazolones, dioxazines, phthalocyanines, tetrachloroisoindolinones, quinacridones, isoindolines, perylenes, pyrrolo-pyrroles (diketopyrrolo-pyrrole, such as for example Pigment Red 254).
Examples of such organic pigments are:
C.I. (Colour Index) Pigment Yellow 93, C.I. Pigment Yellow 95, C.I. Pigment Yellow 138, C.I. Pigment Yellow 139, C.I. Pigment Yellow 155, C.I. Pigment Yellow 162, C.I. Pigment Yellow 168, C.I. Pigment Yellow 180, C.I. Pigment Yellow 183, C.I. Pigment Red 44, C.I. Pigment Red 170, C.I. Pigment Red 202, C.I. Pigment Red 214, C.I. Pigment Red 254, C.I. Pigment Red 264, C.I. Pigment Red 272, C.I. Pigment Red 48:2, C.I. Pigment Red 48:3, C.I. Pigment Red 53:1, C.I. Pigment Red 57:1, C.I. Pigment Green 7, C.I. Pigment Blue 15:1, C.I. Pigment Blue 15:3 and C.I. Pigment Violet 19.
A very suitable class is for example the class of diketopyrrolo-pyrrole pigments.
Mixtures of organic and inorganic pigments are also within the scope of the present invention. A particularly preferred inorganic pigment is titanium dioxide, which is often used in combination with an organic pigment.
When a mixture of an organic and an inorganic pigment is used the ratio of organic pigment to inorganic pigment can vary in a wide range, typically from 5:95 parts to 95:5 parts by weight.
The amount of pigment incorporated is typically from 0.1 to 15% preferably from 0.1 to 10% and more preferably from 0.1 to 5% by weight, based on the polymer.
Typical UV-absorbers of the class of hydroxyphenyl triazines are of formula (I)
in which n is 1 or 2;
in which n is 1 or 2;
- R1, R′1, R2 and R′2, independently of one another, are H, OH, C1-C12alkyl; C2-C6alkenyl; C1-C12alkoxy; C2-C8alkenoxy; halogen; trifluoromethyl; C7-C11phenylalkyl; phenyl; phenyl which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen; phenoxy; or phenoxy which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen;
- R3 and R4, independently of one another, are H, C1-C12alkyl; OR′7; C2-C6alkenyl; C2-C18alkenoxy; halogen; trifluoromethyl; C7-C11phenylalkyl; phenyl; phenyl which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen; phenoxy; or phenoxy which is substituted by C1-C18alkyl, O1-C18alkoxy or halogen;
- R6 is hydrogen, C1-C24alkyl, C5-C12cycloalkyl or C7-C15phenylalkyl;
- R7, in the case where n=1, and R′7, independently of one another, are hydrogen or C1-C18alkyl; or are C1-C12alkyl which is substituted by OH, C1-C18alkoxy, allyloxy, halogen, —COOH, —COOR8, —CONH2, —CONHR9, —CON(R9)(R10), —NH2, —NHR9, —N(R9)(R10), —NHCOR11, —CN, —OCOR11, phenoxy and/or phenoxy which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen; or R7 is C3-C50alkyl which is interrupted by —O— and may be substituted by OH; or R7 is C3-C6 alkenyl; glycidyl; C5-C12cycloalkyl which is substituted by OH, C1-C4alkyl or —OCOR11, C7-C11phenylalkyl which is unsubstituted or substituted by OH, Cl or CH3; —CO—R12 or —SO2—R13;
- R7, in the case where n=2, is C2-C6alkylene, C4-C2alkenylene, xylylene, C3-C20alkylene which is interrupted by O and/or substituted by OH, or is a group of the formula —CH2CH(OH)CH2O—R20—OCH2CH(OH)CH2—, —CO—R21—CO—, —CO—NH—R22—NH—CO— or —(CH2)m—COO—R23—OOC—(CH2)m—, in which m is a number in the range from 1 to 3, or is
- R8 is C1-C18alkyl; C2-C18alkenyl; hydroxyethyl; C3-C50alkyl which is interrupted by O, NH, NR9 or S and/or is substituted by OH; C1-C4alkyl which is substituted by —P(O)(OR14)2, —N(R9)(R10) or —OCOR11 and/or OH; glycidyl; O5-C12cycloalkyl; phenyl; C7-C14alkylphenyl or C7-C11phenylalkyl;
- R9 and R10, independently of one another, are C1-C12alkyl; C3-C12alkoxyalkyl; C4-C16dialkylaminoalkyl or C5-C12cycloalkyl, or R9 and R10 together are C3-C9alkylene or -oxaalkylene or -azaalkylene;
- R11 is C1-C18alkyl; C2-C18alkenyl or phenyl; C2-C12hydroxyalkyl; cyclohexyl; or is C3-C50alkyl which is interrupted by —O— and may be substituted by OH;
- R12 is C1-C18alkyl; C2-C18alkenyl; phenyl; C1-C18alkoxy; C3-C18alkenyloxy; C3-C50alkoxy which is interrupted by O, NH, NR9 or S and/or substituted by OH; cyclohexyloxy; C7-C14alkylphenoxy; C7-C11phenylalkoxy; phenoxy; C1-C12alkylamino; phenylamino; tolylamino or naphthylamino;
- R13 is C1-C12alkyl; phenyl; naphthyl or C7-C14 alkylphenyl;
- R14 is C1-C12alkyl, methylphenyl or phenyl;
- R20 is C2-C10alkylene; C4-C50alkylene which is interrupted by O, phenylene or a -phenylene-X-phenylene-group, in which X is —O—, —S—, —SO2—, —CH2— or —C(CH3)2—;
- R21 is C2-C10alkylene, C2-C10oxaalkylene, C2-C10thiaalkylene, C6-C12arylene or C2-C6alkenylene;
- R22 is C2-C10alkylene, phenylene, tolylene, diphenylenemethane or a
group; and - R23 is C2-C10alkylene or C4-C20alkylene which is interrupted by O.
Halogen is in all cases fluorine, chlorine, bromine or iodine.
Examples of alkyl are methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, 2-ethylbutyl, n-pentyl, isopentyl, 1-methylpentyl, 1,3-dimethylbutyl, n-hexyl, 1-methylhexyl, n-heptyl, isoheptyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl, 3-methylheptyl, n-octyl, 2-ethylhexyl, 1,1,3-trimethylhexyl, 1,1,3,3-tetramethylpentyl, nonyl, decyl, undecyl, 1-methylundecyl, dodecyl.
Examples of alkoxy having up to 12 carbon atoms are methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, pentoxy, isopentoxy, hexoxy, heptoxy, octoxy, decyloxy, dodecyloxy.
Examples of alkenoxy are propenyloxy, butenyloxy, pentenyloxy and hexenyloxy.
Examples of C5-C12cycloalkyl are cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl and cyclododecyl. C5-C8Cycloalkyl, especially cyclohexyl, is preferred.
C1-C4Alkyl-substituted C5-C12cycloalkyl is for example methylcyclohexyl or dimethylcyclohexyl.
OH— and/or C1-C10alkyl-substituted phenyl is for example methylphenyl, dimethylphenyl, trimethylphenyl, tert-butylphenyl or 3,5-di-tert-butyl-4-hydroxyphenyl.
Alkoxy-substituted phenyl is for example methoxyphenyl dimethoxyphenyl or trimethoxyphenyl.
Examples of C7-C9phenylalkyl are benzyl and phenylethyl.
C7-C9Phenylalkyl which is substituted on the phenyl radical by —OH and/or by alkyl having up to 10 carbon atoms is for example methylbenzyl, dimethylbenzyl, trimethylbenzyl, tert-butylbenzyl or 3,5-di-tert-butyl-4-hydroxybenzyl.
Examples of alkenyl are allyl, 2-methallyl, butenyl, pentenyl and hexenyl. Allyl is preferred. The carbon atom in position 1 is preferably saturated.
Examples of alkylene are methylene, ethylene, propylene, trimethylene, tetramethylene, pentamethylene, 2,2-dimethyltrimethylene, hexamethylene, trimethylhexamethylene, octamethylene and decamethylene.
Example s of alkenylene are butenylene, pentenylene and hexenylene.
C6-C12 arylene is preferably phenylene.
Alkyl interrupted by O is for example —CH2—CH2—O—CH2—CH3, —CH2—CH2—O—CH3— or —CH2—CH2—O—CH2—CH2—CH2—O—CH2—CH3—. It is preferably derived from polyethlene glycol. A general description is —((CH2)a—O)b—H/CH3, wherein a is a number from 1 to 6 and b is a number from 2 to 10.
C2-C10 oxaalkylene and C2-C10thiaalkylene can be deduced from the above mentioned alkylene groups by substituting one or more carbon atoms by an oxygen atom or a sulphur atom.
In a specific embodiment of the invention the hydroxyphenyl-triazine UV-absorber is of formula (Ia)
wherein
wherein
- u is 1 or 2 and r is an integer from 1 to 3, the substituents
- Y1 independently of one another are hydrogen, hydroxyl, halogenomethyl, alkyl having 1 to 12 carbon atoms, alkoxy having 1 to 18 carbon atoms, phenyl or halogen,
- if u is 1,
- Y2 is alkyl having 1 to 18 carbon atoms, phenoxy which is unsubstituted or substituted by hydroxyl, alkoxy having 1 to 18 carbon atoms, or halogen, or is substituted by alkyl or alkoxy having in each case 1 to 18 carbon atoms or halogen, alkyl which has 1 to 12 carbon atoms and is substituted by —COOH, —COOY8, —CONH2, —CONHY9, —CONY9Y10, —NH2, —NHY9, —NY9Y10, —NHCOY11, —CN and/or —OCOY11, alkyl which has 4 to 20 carbon atoms, is interrupted by one or more oxygen atoms and is unsubstituted or substituted by hydroxyl or alkoxy having 1 to 12 carbon atoms, alkenyl having 3 to 6 carbon atoms, glycidyl, cyclohexyl which is unsubstituted or substituted by hydroxyl, alkyl having 1 to 4 carbon atoms and/or —OCOY11, phenylalkyl which has 1 to 5 carbon atoms in the alkyl moiety and is unsubstituted or substituted by hydroxyl, chlorine and/or methyl, —COY12 or —SO2Y13, or,
- if u is 2,
- Y2 is alkylene having 2 to 16 carbon atoms, alkenylene having 4 to 12 carbon atoms, xylylene, alkylene which has 3 to 20 carbon atoms, is interrupted by one or more —O— atoms and/or is substituted by hydroxyl, —CH2CH(OH)CH2—O—Y15—OCH2CH(OH)CH2, —CO—Y16—CO—, —CO—NH—Y17—NH—CO— or —(CH2), —CO2—Y18—OCO—(CH2)m, in which
- m is 1, 2 or 3,
- Y8 is alkyl having 1 to 18 carbon atoms, alkenyl having 3 to 18 carbon atoms, alkyl which has 3 to 20 carbon atoms, is interrupted by one or more oxygen or sulfur atoms or —NT6- and/or is substituted by hydroxyl, alkyl which has 1 to 4 carbon atoms and is substituted by —P(O)(OY14)2, —NY9Y10 or —OCOY11 and/or hydroxyl, alkenyl having 3 to 18 carbon atoms, glycidyl, or phenylalkyl having 1 to 5 carbon atoms in the alkyl moiety,
- Y9 and Y10 independently of one another are alkyl having 1 to 12 carbon atoms, alkoxyalkyl having 3 to 12 carbon atoms, dialkylaminoalkyl having 4 to 16 carbon atoms or cyclohexyl having 5 to 12 carbon atoms, or Y9 and Y10 together are alkylene, oxaalkylene or azaalkylene having in each case 3 to 9 carbon atoms,
- Y11 is alkyl having 1 to 18 carbon atoms, alkenyl having 2 to 18 carbon atoms or phenyl,
- Y12 is alkyl having 1 to 18 carbon atoms, alkenyl having 2 to 18 carbon atoms, phenyl, alkoxy having 1 to 12 carbon atoms, phenoxy, alkylamino having 1 to 12 carbon atoms or phenylamino,
- Y13 is alkyl having 1 to 18 carbon atoms, phenyl or alkylphenyl having 1 to 8 carbon atoms in the alkyl radical,
- Y14 is alkyl having 1 to 12 carbon atoms or phenyl,
- Y15 is alkylene having 2 to 10 carbon atoms, phenylene or a group -phenylene-M-phenylene- in which M is —O—, —S—, —SO2—, —CH2— or —C(CH3)2—,
- Y16 is alkylene, oxaalkylene or thiaalkylene having in each case 2 to 10 carbon atoms, phenylene or alkenylene having 2 to 6 carbon atoms,
- Y17 is alkylene having 2 to 10 carbon atoms, phenylene or alkylphenylene having 1 to 11 carbon atoms in the alkyl moiety, and
- Y18 is alkylene having 2 to 10 carbon atoms or alkylene which has 4 to 20 carbon atoms and is interrupted once or several times by oxygen.
Further preferred polyolefin compositions are those, in which, in the compounds of the formula (Ia), the substituents Y1 are hydrogen, alkyl having 1 to 12 carbon atoms, alkoxy having 1 to 18 carbon atoms, phenyl or halogen, if u is 1, Y2 is alkyl having 1 to 18 carbon atoms, alkyl which has 1 to 12 carbon atoms and is substituted by hydroxyl, alkoxy having 1 to 18 carbon atoms, —COOY8, —CONY9Y10 and/or —OCOY11, glycidyl or phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, or, if u is 2, Y2 is alkylene having 2 to 16 carbon atoms, alkenylene having 4 to 12 carbon atoms, xylylene or alkylene which has 3 to 20 carbon atoms, is interrupted by one or more —O— atoms and/or is substituted by hydroxyl, the substituents Y8 to Y11 being as defined above.
Preferably u is 1.
Typical individual compounds are the following:
- 2,4,6-tris(2-hydroxy-4-octyloxyphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis-(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2,4-bis(2-hydroxy-4-propyloxyphenyl)-6-(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(4-methylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-dodecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-tridecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2-[2-hydroxy-4-(2-hydroxy-3-butyloxy-propoxy)phenyl]-4,6-bis(2,4-dimethyl)-1,3,5-triazine, 2-[2-hydroxy-4-(2-hydroxy-3-octyloxy-propyloxy)phenyl]-4,6-bis(2,4-dimethyl)-1,3,5-triazine, 2-[4-(dodecyloxy/tridecyloxy-2-hydroxypropoxy)-2-hydroxy-phenyl]-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2-[2-hydroxy-4-(2-hydroxy-3-dodecyloxy-propoxy)phenyl]-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-hexyloxy)-phenyl-4,6-diphenyl-1,3,5-triazine, 2-(2-hydroxy-4-methoxyphenyl)-4,6-diphenyl-1,3,5-triazine, 2,4,6-tris[2-hydroxy-4-(3-butoxy-2-hydroxy-propoxy)phenyl]-1,3,5-triazine, 2-(2-hydroxy-phenyl)-4-(4-methoxyphenyl)-6-phenyl-1,3,5-triazine, 2-{2-hydroxy-4-[3-(2-ethylhexyl-1-oxy)-2-hydroxypropyloxy]phenyl}-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine.
The hydroxyphenyl triazine UV-absorbers are known and partially items of commerce.
Suitable examples for hydroxyphenyl benzotriazole UV absorbers are compounds of the following structure IIa, IIb or IIc.
in the compounds of the formula (IIa),
in the compounds of the formula (IIa),
- R101 is hydrogen, alkyl having 1 to 24 carbon atoms, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, cycloalkyl having 5 to 8 carbon atoms or a radical of the formula
in which - R104 and R105 independently of one another are alkyl having in each case 1 to 5 carbon atoms, or R104, together with the radical CnH2n+1−m, forms a cycloalkyl radical having 5 to 12 carbon atoms,
- m is 1 or 2, n is an integer from 2 to 20 and
- M is a radical of the formula —COOR106 in which
- R106 is hydrogen, alkyl having 1 to 12 carbon atoms, alkoxyalkyl having in each case 1 to 20 carbon atoms in the alkyl moiety and in the alkoxy moiety or phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety,
- R102 is hydrogen, halogen, alkyl having 1 to 18 carbon atoms, and phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, and
- R103 is hydrogen, chlorine, alkyl or alkoxy having in each case 1 to 4 carbon atoms or —COOR106 in which R106 is as defined above;
in the compounds of the formula (IIb) - T is hydrogen or alkyl having 1 to 6 carbon atoms,
- T1 is hydrogen, chlorine or alkyl or alkoxy having in each case 1 to 4 carbon atoms,
- n is 1 or 2 and,
- if n is 1,
- T2 is chlorine or a radical of the formula —OT3 or
and, - if n is 2, T2 is a radical of the formula
- or —O-T9-O— in which
- T3 is hydrogen, alkyl which has 1 to 18 carbon atoms and is unsubstituted or substituted by 1 to 3 hydroxyl groups or by —OCOT6, alkyl which has 3 to 18 carbon atoms, is interrupted once or several times by —O— or —NT6- and is unsubstituted or substituted by hydroxyl or —OCOT6, cycloalkyl which has 5 to 12 carbon atoms and is unsubstituted or substituted by hydroxyl and/or alkyl having 1 to 4 carbon atoms, alkenyl which has 2 to 18 carbon atoms and is unsubstituted or substituted by hydroxyl, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, or a radical of the formula —CH2CH(OH)-T7 or
- T4 and T5 independently of one another are hydrogen, alkyl having 1 to 18 carbon atoms, alkyl which has 3 to 18 carbon atoms and is interrupted once or several times by —O— or —NT6-, cycloalkyl having 5 to 12 carbon atoms, phenyl, phenyl which is substituted by alkyl having 1 to 4 carbon atoms, alkenyl having 3 to 8 carbon atoms, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety or hydroxyalkyl having 2 to 4 carbon atoms,
- T6 is hydrogen, alkyl having 1 to 18 carbon atoms, cycloalkyl having 5 to 12 carbon atoms, alkenyl having 3 to 8 carbon atoms, phenyl, phenyl which is substituted by alkyl having 1 to 4 carbon atoms, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety,
- T7 is hydrogen, alkyl having 1 to 18 carbon atoms, phenyl which is unsubstituted or substituted by hydroxyl, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, or —CH2OT8,
- T8 is alkyl having 1 to 18 carbon atoms, alkenyl having 3 to 8 carbon atoms, cycloalkyl having 5 to 10 carbon atoms, phenyl, phenyl which is substituted by alkyl having 1 to 4 carbon atoms, or phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety,
- T9 is alkylene having 2 to 8 carbon atoms, alkenylene having 4 to 8 carbon atoms, alkynylene having 4 carbon atoms, cyclohexylene, alkylene which has 2 to 8 carbon atoms and is interrupted once or several times by —O—, or a radical of the formula —CH2CH(OH)CH2OT11OCH2CH(OH)CH2— or —CH2—C(CH2OH)2—CH2—,
- T10 is alkylene which has 2 to 20 carbon atoms and can be interrupted once or several times by —O—, or cyclohexylene,
- T11 is alkylene having 2 to 8 carbon atoms, alkylene which has 2 to 18 carbon atoms and is interrupted once or several times by —O—, 1,3-cyclohexylene, 1,4-cyclohexylene, 1,3-phenylene or 1,4-phenylene, or
- T10 and T6, together with the two nitrogen atoms, are a piperazine ring;
in the compounds of formula (IIc) - R′102 is C1-C12alkyl and k is a number from 1 to 4.
In the compounds of the formula (IIa) R101 can be hydrogen or alkyl having 1 to 24 carbon atoms, such as methyl, ethyl, propyl, butyl, hexyl, octyl, nonyl, dodecyl, tetradecyl, hexadecyl, octadecyl, nonadecyl and eicosyl and also corresponding branched isomers.
Furthermore, in addition to phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, for example benzyl, R101 can also be cycloalkyl having 5 to 8 carbon atoms, for example cyclopentyl, cyclohexyl and cyclooctyl, or a radical of the formula
in which R104 and R105 independently of one another are alkyl having in each case 1 to 5 carbon atoms, in particular methyl, or R104, together with the CnH2n+1−m radical, forms a cycloalkyl radical having 5 to 12 carbon atoms, for example cyclohexyl, cyclooctyl and cyclodecyl. M is a radical of the formula —COOR106 in which R106 is not only hydrogen but also alkyl having 1 to 12 carbon atoms or alkoxyalkyl having 1 to 20 carbon atoms in each of the alkyl and alkoxy moieties. Suitable alkyl radicals R106 are those enumerated for R101. Examples of suitable alkoxyalkyl groups are —C2H4OC2H5, —C2H4OC8H17 and —C4H8OC4H9. As phenylalkyl having 1 to 4 carbon atoms, R106 is, for example, benzyl, cumyl, α-methylbenzyl or phenylbutyl.
in which R104 and R105 independently of one another are alkyl having in each case 1 to 5 carbon atoms, in particular methyl, or R104, together with the CnH2n+1−m radical, forms a cycloalkyl radical having 5 to 12 carbon atoms, for example cyclohexyl, cyclooctyl and cyclodecyl. M is a radical of the formula —COOR106 in which R106 is not only hydrogen but also alkyl having 1 to 12 carbon atoms or alkoxyalkyl having 1 to 20 carbon atoms in each of the alkyl and alkoxy moieties. Suitable alkyl radicals R106 are those enumerated for R101. Examples of suitable alkoxyalkyl groups are —C2H4OC2H5, —C2H4OC8H17 and —C4H8OC4H9. As phenylalkyl having 1 to 4 carbon atoms, R106 is, for example, benzyl, cumyl, α-methylbenzyl or phenylbutyl.
In addition to hydrogen and halogen, for example chlorine and bromine, R102 can also be alkyl having 1 to 18 carbon atoms. Examples of such alkyl radicals are indicated in the definitions of R101. R102 can also be phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, for example benzyl, α-methylbenzyl and cumyl.
Halogen as a substituent means in all cases fluorine, chlorine, bromine or iodine, preferably chlorine or bromine and more preferably chlorine.
At least one of the radicals R101 and R102 must be other than hydrogen.
In addition to hydrogen or chlorine, R103 is also alkyl or alkoxy having in each case 1 to 4 carbon atoms, for example methyl, butyl, methoxy and ethoxy, and also —COOR106.
In the compounds of the formula (IIb) T is hydrogen or alkyl having 1 to 6 carbon atoms, such as methyl and butyl, T1 is not only hydrogen or chlorine, but also alkyl or alkoxy having in each case 1 to 4 carbon atoms, for example methyl, methoxy and butoxy, and, if n is 1, T2 is chlorine or a radical of the formula —OT3 or —NT4T5. T3 is here hydrogen or alkyl having 1 to 18 carbon atoms (cf. the definition of R101). These alkyl radicals can be substituted by 1 to 3 hydroxyl groups or by a radical —OCOT6. Furthermore, T3 can be alkyl having 3 to 18 carbon atoms (cf. the definition of R101) which is interrupted once or several times by —O— or —NT6- and is unsubstituted or substituted by hydroxyl or —OCOT6. Examples of T3 as cycloalkyl are cyclopentyl, cyclohexyl or cyclooctyl. T3 can also be alkenyl having 2 to 18 carbon atoms. Suitable alkenyl radicals are derived from the alkyl radicals enumerated in the definitions of R101. These alkenyl radicals can be substituted by hydroxyl. Examples of T3 as phenylalkyl are benzyl, phenylethyl, cumyl, α-methylbenzyl or benzyl. T3 can also be a radical of the formula —CH2CH(OH)-T7 or
Like T3, T4 and T5 can, independently of one another, be not only hydrogen but also alkyl having 1 to 18 carbon atoms or alkyl which has 3 to 18 carbon atoms and is interrupted once or several times by —O— or —NT6-. T4 and T5 can also be cycloalkyl having 5 to 12 carbon atoms, for example cyclopentyl, cyclohexyl and cyclooctyl. Examples of T4 and T5 as alkenyl groups can be found in the illustrations of T3. Examples of T4 and T5 as phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety are benzyl or phenylbutyl. Finally, these substituents can also be hydroxyalkyl having 1 to 3 carbon atoms.
Like T3, T4 and T5 can, independently of one another, be not only hydrogen but also alkyl having 1 to 18 carbon atoms or alkyl which has 3 to 18 carbon atoms and is interrupted once or several times by —O— or —NT6-. T4 and T5 can also be cycloalkyl having 5 to 12 carbon atoms, for example cyclopentyl, cyclohexyl and cyclooctyl. Examples of T4 and T5 as alkenyl groups can be found in the illustrations of T3. Examples of T4 and T5 as phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety are benzyl or phenylbutyl. Finally, these substituents can also be hydroxyalkyl having 1 to 3 carbon atoms.
In addition to hydrogen, T6 (see above also) is alkyl, cycloalkyl, alkenyl, aryl or phenylalkyl; examples of such radicals have already been given above.
In addition to hydrogen and the phenylalkyl radicals and long-chain alkyl radicals mentioned above, T7 can be phenyl or hydroxyphenyl and also —CH2OT8 in which T8 can be one of the alkyl, alkenyl, cycloalkyl, aryl or phenylalkyl radicals enumerated.
The divalent radical T9 can be alkylene having 2 to 8 carbon atoms, and such radicals can also be branched. This also applies to the alkenylene and alkynylene radicals T9. As well as cyclohexylene, T9 can also be a radical of the formula —CH2CH(OH)CH2OT11OCH2CH(OH)CH2— or —CH2—C(CH2OH)2—CH2—.
T10 is a divalent radical and, in addition to cyclohexylene, is also alkylene which has 2 to 20 carbon atoms and which can be interrupted once or several times by —O—. Suitable alkylene radicals are derived from the alkyl radicals mentioned in the definitions of R101.
T11 is also an alkylene radical. It contains 2 to 8 carbon atoms or, if it is interrupted once or several times by —O—, 4 to 10 carbon atoms. T11 is also 1,3-cyclohexylene, 1,4-cyclohexylene, 1,3-phenylene or 1,4-phenylene.
Together with the two nitrogen atoms, T6 and T10 can also be a piperazine ring.
Preferred is a compound of the formula (IIa), wherein R101 is hydrogen or alkyl having 1 to 20 carbon atoms, R102 is hydrogen, alkyl having 1 to 18 carbon atoms or phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety and R103 is hydrogen, chlorine or alkyl having 1 to 4 carbon atoms.
Particularly preferred is acompound of formula (IIa) in which R101 is in the ortho-position relative to the hydroxyl group and is hydrogen or alkyl having 4 to 12 carbon atoms, R102 is in the para-position relative to the hydroxyl group and is alkyl having 1 to 6 carbon atoms or cumyl and R103 is hydrogen or chlorine.
The UV absorbers of the formulae (I), (Ia), (IIa), (IIb), (IIc) are known per se and are described, together with their preparation, in, for example, WO 96/28431, EP-A-323 408, EP-A-57 160, U.S. Pat. No. 5,736,597 (EP-A-434 608) and U.S. Pat. No. 4,619,956. Preferred meanings of substituents and individual compounds can be deduced from the documents mentioned.
Particularly preferred compounds are the following:
2-(2′-hydroxy-5′-methylphenyl)-benzotriazole, 2-(3′,5′-di-tert-butyl-2′-hydroxyphenyl)benzotriazole, 2-(5′-tert-butyl-2′-hydroxyphenyl)benzotriazole, 2-(2′-hydroxy-5′-(1,1,3,3-tetramethylbutyl)phenyl)benzotriazole, 2-(3′,5′-di-tert-butyl-2′-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3′-tert-butyl-2′-hydroxy-5′-methylphenyl)-5-chloro-benzotriazole, 2-(3′-sec-butyl-5′-tert-butyl-2′-hydroxyphenyl)benzotriazole, 2-(2′-hydroxy-4′-octyloxyphenyl)benzotriazole, 2-(3′,5′-di-tert-amyl-2′-hydroxyphenyl)benzotriazole, 2-(3′,5′-bis-(α,α-dimethylbenzyl)-2′-hydroxyphenyl)-benzotriazole, 2-(3′-tert-butyl-2′-hydroxy-5′-(2-octyloxycarbonylethyl)phenyl)-5-chloro-benzotriazole, 2-(3′-tert-butyl-5′-[2-(2-ethylhexyloxy)-carbonylethyl]-2′-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3′-tert-butyl-2′-hydroxy-5′-(2-methoxycarbonylethyl)phenyl)-5-chloro-benzotriazole, 2-(3′-tert-butyl-2′-hydroxy-5′-(2-methoxycarbonylethyl)phenyl)benzotriazole, 2-(3′-tert-butyl-2′-hydroxy-5′-(2-octyloxycarbonylethyl)phenyl)benzotriazole, 2-(3′-tert-butyl-5′-[2-(2-ethylhexyloxy)carbonylethyl]-2′-hydroxyphenyl)benzotriazole, 2-(3′-dodecyl-2′-hydroxy-5′-methylphenyl)benzotriazole, 2-(3′-tert-butyl-2′-hydroxy-5′-(2-isooctyloxycarbonylethyl)phenyl-benzotriazole, 2,2′-methylene-bis[4-(1,1,3,3-tetramethylbutyl)-6-benzotriazole-2-ylphenol]; the transesterification product of 2-[3′-tert-butyl-5′-(2-methoxycarbonylethyl)-2′-hydroxy-phenyl]-2H-benzotriazole with polyethylene glycol, 300; [R—CH2CH—COO—CH2CH22 where R=3′-tert-butyl-4′-hydroxy-5′-2H-benzotriazol-2-ylphenyl, 2-[2′-hydroxy-3′-(α,α-di-methylbenzyl)-5′-(1,1,3,3-tetramethylbutyl)-phenyl]benzotriazole; 2-[2′-hydroxy-3′-(1,1,3,3-tetramethylbutyl)-5′-(α,α-dimethylbenzyl)-phenyl]benzotriazole.
The UV-absorbers are typically incorporated in an amount of 0.005 to 5% each by weight based on the polymer. The total amount of UV absorber is preferably from 0.01 to 5%, more preferably from 0.05 to 2% and most preferably from 0.05 to 1% by weight, based on the weight of the polyolefin. The weight ratio of hydroxyphenyl triazine UV-absorber to hydroxy-phenyl benzotriazole UV-absorber is preferably from 10:1 to 1:10, more preferably from 5:1 to 1:5 and most preferably from 2:1 to 1:2.
The preparation of compound 101 and analogously substituted triazines is for example disclosed in WO 96/28431.
The sterically hindered amine light stabilizer useful in the instant invention is preferably a compound of formulae (A-1) to (A-10) or of formulae (B-1) to (B-10);
(α-1) a Compound of the Formula (A-1)
in which
(α-1) a Compound of the Formula (A-1)
in which
- E1 is hydrogen, C1-C8alkyl, O−, —OH, —CH2CN, C1-C18alkoxy, C5-C12cycloalkoxy, C3-C6alkenyl,
- C7-C9phenylalkyl unsubstituted or substituted on the phenyl by 1, 2 or 3 C1-C4alkyl; or C1-C8acyl,
- m, is 1, 2 or 4,
- if m1 is 1, E2 is C1-C25alkyl,
- if m1 is 2, E2 is C1-C14alkylene or a group of the formula (a-I)
- wherein E3 is C1-C10 alkyl or C2-C10alkenyl, E4 is C1-C10alkylene, and
- E5 and E6 independently of one another are C1-C4alkyl, cyclohexyl or methylcyclohexyl, and
- if m1 is 4, E2 is C4-C10alkanetetrayl;
(α-2) a Compound of the Formula (A-2)
in which - two of the radicals E7 are —COO—(C1-C20alkyl), and
- two of the radicals E7 are a group of the formula (a-II)
- with E8 having one of the meanings of E1;
(α-3) a Compound of the Formula (A-3)
in which - E9 and E10 together form C2-C14alkylene,
- E11 is hydrogen or a group —Z1—COO-Z2,
- Z1 is C2-C14alkylene, and
- Z2 is C1-C24alkyl, and
- E12 has one of the meanings of E1;
(α-4) a Compound of the Formula (A-4)
wherein - the radicals E13 independently of one another have one of the meanings of E1,
- the radicals E14 independently of one another are hydrogen or C1-C12alkyl, and
- E15 is C1-C10alkylene or C3-C10alkylidene;
(α-5) a Compound of the Formula (A-5)
wherein - the radicals E16 independently of one another have one of the meanings of E1;
(α-6) a Compound of the Formula (A-6)
in which - E17 is C1-C24alkyl, and
- E18 has one of the meanings of E1;
(α-7) a Compound of the Formula (A-7)
in which - E19, E20 and E21 independently of one another are a group of the formula (a-III)
wherein E22 has one of the meanings of E1;
(α-8) a Compound of the Formula (A-8)
wherein - the radicals E23 independently of one another have one of the meanings of E1,
- and E24 is hydrogen, C1-C12alkyl or C1-C12alkoxy;
(α-9) a Compound of the Formula (A-9)
wherein - m2 is 1, 2 or 3,
- E25 has one of the meanings of E1, and
- when m2 is 1, E26 is a group
- when m2 is 2, E26 is C2-C22alkylene, and
- when m2 is 3, E26 is a group of the formula (a-IV)
wherein the radicals E27 independently of one another are C2-C12alkylene, and the radicals E28 independently of one another are C1-C12alkyl or C5-C12cycloalkyl;
(α-10) a Compound of the Formula (A-10)
wherein - the radicals E29 independently of one another have one of the meanings of E1, and
- E30 is C2-C22alkylene, C5-C7cycloalkylene, C1-C4alkylenedi(C5-C7cycloalkylene), phenylene or phenylenedi(C1-C4alkylene);
(β-1) a Compound of the Formula (B-1)
in which - R201; R203, R204 and R205 independently of one another are hydrogen, C1-C12alkyl, C5-C12cycloalkyl, C1-C4-alkyl-substituted C5-C12cycloalkyl, phenyl, phenyl which is substituted by —OH and/or C1-C10alkyl; C7-C9phenylalkyl, C7-C9phenylalkyl which is substituted on the phenyl radical by —OH and/or C1-C10alkyl; or a group of the formula (b-I)
- R202 is C2-C18alkylene, C5-C7cycloalkylene or C1-C4alkylenedi(B5—C7cycloalkylene), or
- the radicals R201, R202 and R203, together with the nitrogen atoms to which they are bonded, perform a 5- to 10-membered heterocyclic ring, or
- R204 and R205, together with the nitrogen atom to which they are bonded, form a 5- to 10-membered heterocyclic ring,
- R206 is hydrogen, C1-C8alkyl, O−, —OH, —CH2CN, C1-C18alkoxy, C5-C12cycloalkoxy, C3-C6alkenyl, C7-C9phenylalkyl unsubstituted or substituted on the phenyl by 1, 2 or 3 C1-C4alkyl; or C1-C8acyl, and
- b1 is a number from 2 to 50,
- with the proviso that at least one of the radicals R201, R203, R204 and R205 is a group of the formula (b-I);
(β-2) a Compound of the Formula (B-2)
wherein - R207 and R211 independently of one another are hydrogen or C1-C12alkyl,
- R208, R209 and R210 independently of one another are C2-C10alkylene, and X1, X2, X3, X4, X5, X6, X7 and X8 independently of one another are a group of the formula (b-II),
in which R212 is hydrogen, C1-C12alkyl, C5-C12cycloalkyl, C1-C4alkyl-substituted C5-C12cycloalkyl, phenyl, —OH— and/or C1-C10alkyl-substituted phenyl, C7-C9phenylalkyl, C7-C9phenylalkyl which is substituted on the phenyl radical by —OH and/or C1-C10alkyl; or a group of the formula (b-I) as defined above, and - R213 has one of the meanings of R206;
(β-3) a Compound of the Formula (B-3)
in which - R214 is C1-C10alkyl, C5-C12cycloalkyl, C1-C4alkyl-substituted C5-C12cycloalkyl, phenyl or C1-C10alkyl-substituted phenyl,
- R215 is C3-C10alkylene,
- R216 has one of the meanings of R206, and
- b2 is a number from 2 to 50;
(β-4) a Compound of the Formula (B-4)
in which - R217 and R221 independently of one another are a direct bond or a —N(X9)—CO—X10—CO—N(X11)— group, where X9 and X11 independently of one another are hydrogen, C1-C8alkyl, C5-C12cycloalkyl, phenyl, C7-C9phenylalkyl or a group of the formula (b-I),
- X10 is a direct bond or C1-C4alkylene,
- R218 has one of the meanings of R206,
- R219, R220, R223 and R224 independently of one another are hydrogen, C1-C30alkyl, C5-C12cycloalkyl or phenyl,
- R222 is hydrogen, C1-C30alkyl, C5-C12cycloalkyl, phenyl, C7-C9phenylalkyl or a group of the formula (b-I), and
- b3 is a number from 1 to 50;
(β-5) a Compound of the Formula (B-5)
in which - R225, R226, R227, R228 and R229 independently of one another are a direct bond or C1-C10alkylene,
- R230 has one of the meanings of R206, and
- b4 is a number from 1 to 50;
(β-6) a product (B-6) obtainable by reacting a product, obtained by reaction of a polyamine of the formula (B-6-1) with cyanuric chloride, with a compound of the formula (B-6-2)
in which - b′5, b″5 and b′″5 independently of one another are a number from 2 to 12,
- R231 is hydrogen, C1-C12alkyl, C5-C12cycloalkyl, phenyl or C7-C9phenylalkyl, and
- R232 has one of the meanings of R206;
(β-7) a Compound of the Formula (B-7)
wherein A1 is hydrogen or C1-C4alkyl, - A2 is a direct bond or C1-C10alkylene, and
- n1 is a number from 2 to 50;
(β-8) at Least One Compound of the Formulae (B-8-a) and (B-8-b)
wherein n2 and n2* are a number from 2 to 50;
(β-9) a Compound of the Formula (B-9)
wherein A3 and A4 independently of one another are hydrogen or C1-C8alkyl, or A3 and A4 together form a C2-C14alkylene group, and - the variables n3 independently of one another are a number from 1 to 50; and
(β-10) a Compound of the Formula (B-10)
wherein n4 is a number from 2 to 50, - A5 is hydrogen or C1-C4alkyl,
- the radicals A6 and A7 independently of one another are C1-C4alkyl or a group of the formula (b-I), with the proviso that at least 50% of the radicals A7 are a group of the formula (b-I).
Examples of alkyl having up to 30 carbon atoms are methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, 2-ethylbutyl, n-pentyl, isopentyl, 1-methylpentyl, 1,3-dimethyl-butyl, n-hexyl, 1-methylhexyl, n-heptyl, isoheptyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl, 3-methylheptyl, n-octyl, 2-ethylhexyl, 1,1,3-trimethylhexyl, 1,1,3,3-tetramethylpentyl, nonyl, decyl, undecyl, 1-methylundecyl, dodecyl, 1,1,3,3,5,5-hexamethylhexyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl, octadecyl, eicosyl, docosyl and triacontyl. One of the preferred definitions of E1, E8, E12, E13, E16, E18, E22, E23, E25, E29, R206, R213, R216, R218, R230 and R232 is C1-C4alkyl, especially methyl. R231 is preferably butyl.
Examples of alkoxy having up to 18 carbon atoms are methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, pentoxy, isopentoxy, hexoxy, heptoxy, octoxy, decyloxy, dodecyloxy, tetradecyloxy, hexadecyloxy and octadecyloxy. One of the preferred meanings of E1 is octoxy. E24 is preferably C1-C4alkoxy and one of the preferred meanings of R206 is propoxy.
Examples of C5-C12cycloalkyl are cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl and cyclododecyl. C5-C8Cycloalkyl, especially cyclohexyl, is preferred.
C1-C4Alkyl-substituted C5-C12cycloalkyl is for example methylcyclohexyl or dimethylcyclohexyl.
Examples of C5-C12cycloalkoxy are cyclopentoxy, cyclohexoxy, cycloheptoxy, cyclooctoxy, cyclodecyloxy and cyclododecyloxy. C5-C8Cycloalkoxy, in particular cyclopentoxy and cyclohexoxy, is preferred.
—OH— and/or C1-C10alkyl-substituted phenyl is for example methylphenyl, dimethylphenyl, trimethylphenyl, tert-butylphenyl or 3,5-di-tert-butyl-4-hydroxyphenyl.
Examples of C7-C9phenylalkyl are benzyl and phenylethyl.
C7-C9Phenylalkyl which is substituted on the phenyl radical by —OH and/or by alkyl having up to 10 carbon atoms is for example methylbenzyl, dimethylbenzyl, trimethylbenzyl, tert-butylbenzyl or 3,5-di-tert-butyl-4-hydroxybenzyl.
Examples of alkenyl having up to 10 carbon atoms are allyl, 2-methallyl, butenyl, pentenyl and hexenyl. Allyl is preferred. The carbon atom in position 1 is preferably saturated.
Examples of acyl containing not more than 8 carbon atoms are formyl, acetyl, propionyl, butyryl, pentanoyl, hexanoyl, heptanoyl, octanoyl, acryloyl, methacryloyl and benzoyl. C1-C8Alkanoyl, C3-C8alkenyl and benzoyl are preferred. Acetyl and acryloyl are especially preferred.
Examples of alkylene having up to 22 carbon atoms are methylene, ethylene, propylene, trimethylene, tetramethylene, pentamethylene, 2,2-dimethyltrimethylene, hexamethylene, trimethylhexamethylene, octamethylene and decamethylene.
An example of O4—C10alkanetetrayl is 1,2,3,4-butanetetrayl.
An example of C5-C7cycloalkylene is cyclohexylene.
An example of C1-C4alkylenedi(C5-C7cycloalkylene) is methylenedicyclohexylene.
An example of phenylenedi(C1-C4alkylene) is methylene-phenylene-methylene or ethylene-phenylene-ethylene.
Where the radicals R201, R202 and R203, together with the nitrogen atoms to which they are attached, form a 5- to 10-membered heterocyclic ring, this ring is for example
A 6-membered heterocyclic ring is preferred.
A 6-membered heterocyclic ring is preferred.
Where the radicals R204 and R205, together with the nitrogen atom to which they are attached, form a 5- to 10-membered heterocyclic ring, this ring is for example 1-pyrrolidyl, piperidino, morpholino, 1-piperazinyl, 4-methyl-1-piperazinyl, 1-hexahydroazepinyl, 5,5,7-trimethyl-1-homopiperazinyl or 4,5,5,7-tetramethyl-1-homopiperazinyl. Morpholino is particularly preferred.
One of the preferred definitions of R219 and R223 is phenyl.
R226 is preferably a direct bond.
n1, n2, n2* and n4 are preferably a number from 2 to 25, in particular 2 to 20.
n3 is preferably a number from 1 to 25, in particular 1 to 20.
b1 and b2 are preferably a number from 2 to 25, in particular 2 to 20.
b3 and b4 are preferably a number from 1 to 25, in particular 1 to 20.
b′5 and b′″5 are preferably 3 and b″5 is preferably 2.
The compounds described above are essentially known and commercially available. All of them can be prepared by known processes.
The preparation of the compounds is disclosed, for example, in U.S. Pat. No. 5,679,733, U.S. Pat. No. 3,640,928, U.S. Pat. No. 4,198,334, U.S. Pat. No. 5,204,473, U.S. Pat. No. 4,619,958, U.S. Pat. No. 4,110,306, U.S. Pat. No. 4,110,334, U.S. Pat. No. 4,689,416, U.S. Pat. No. 4,408,051, SU-A-768,175 (Derwent 88-138,751/20), U.S. Pat. No. 5,049,604, U.S. Pat. No. 4,769,457, U.S. Pat. No. 4,356,307, U.S. Pat. No. 4,619,956, U.S. Pat. No. 5,182,390, GB-A-2,269,819, U.S. Pat. No. 4,292,240, U.S. Pat. No. 5,026,849, U.S. Pat. No. 5,071,981, U.S. Pat. No. 4,547,538, U.S. Pat. No. 4,976,889, U.S. Pat. No. 4,086,204, U.S. Pat. No. 6,046,304, U.S. Pat. No. 4,331,586, U.S. Pat. No. 4,108,829, U.S. Pat. No. 5,051,458, WO-A-94/12,544 (Derwent 94-177,274/22), DD-A-262,439 (Derwent 89-122,983/17), U.S. Pat. No. 4,857,595, U.S. Pat. No. 4,529,760, U.S. Pat. No. 4,477,615, CAS 136,504-96-6, U.S. Pat. No. 4,233,412, U.S. Pat. No. 4,340,534, WO-A-98/51,690 and EP-A-1,803.
The product (B-6) can be prepared analogously to known processes, for example by reacting a polyamine of formula (B-6-1) with cyanuric chloride in a molar ratio of from 1:2 to 1:4 in the presence of anhydrous lithium carbonate, sodium carbonate or potassium carbonate in an organic solvent such as 1,2-dichloroethane, toluene, xylene, benzene, dioxane or tert-amyl alcohol at a temperature of from −20° C. to +10° C., preferably from −10° C. to +10° C., in particular from 0° C. to +10° C., for from 2 to 8 hours, followed by reaction of the resultant product with a 2,2,6,6-tetramethyl-4-piperidylamine of the formula (B-6-2). The molar ratio of the 2,2,6,6-tetramethyl-4-piperidylamine to polyamine of the formula (B-6-1) employed is for example from 4:1 to 8:1. The quantity of the 2,2,6,6-tetramethyl-4-piperidylamine can be added in one portion or in more than one portion at intervals of a few hours.
The molar ratio of polyamine of the formula (B-6-1) to cyanuric chloride to 2,2,6,6-tetramethyl-4-piperidylamine of the formula (B-6-2) is preferably from 1:3:5 to 1:3:6.
The following example indicates one way of preparing a preferred product (B-6-a).
23.6 g (0.128 mol) of cyanuric chloride, 7.43 g (0.0426 mol) of N,N′-bis[3-aminopropyl]ethylenediamine and 18 g (0.13 mol) of anhydrous potassium carbonate are reacted at 5° C. for 3 hours with stirring in 250 ml of 1,2-dichloroethane. The mixture is warmed at room temperature for a further 4 hours. 27.2 g (0.128 mol) of N-(2,2,6,6-tetramethyl-4-piperidyl)butylamine are added and the resultant mixture is warmed at 60° C. for 2 hours. A further 18 g (0.13 mol) of anhydrous potassium carbonate are added and the mixture is warmed at 60° C. for a further 6 hours. The solvent is removed by distillation under a slight vacuum (200 mbar) and replaced by xylene. 18.2 g (0.085 mol) of N-(2,2,6,6-tetramethyl-4-piperidyl)butylamine and 5.2 g (0.13 mol) of ground sodium hydroxide are added, the mixture is heated at reflux for 2 hours and, for a further 12 hours, the water formed during the reaction is removed by azeotropic distillation. The mixture is filtered. The solution is washed with water and dried over Na2SO4. The solvent is evaporated and the residue is dried at 120-130° C. in vacuo (0.1 mbar). The desired product is obtained as a colourless resin.
In general, the product (B-6) can, for example, be represented by a compound of the formula (B-6-α), (B-6-β) or (B-6-γ). It can also be in the form of a mixture of these three compounds.
In the above formulae (B-6-α) to (B-6-γ), b5 is preferably 2 to 20, in particular 2 to 10.
The sterically hindered amine compounds of component (c) are preferably selected from the group consisting of the following commercial products: DASTIB 845 (RTM), TINUVIN 770 (RTM), TINUVIN 765 (RTM), TINUVIN 144 (RTM), TINUVIN 123 (RTM), TINUVIN 111 (RTM), TINUVIN 783 (RTM), TINUVIN 791 (RTM), MARK LA 52 (RTM), MARK LA 57 (RTM), MARK LA 62 (RTM), MARK LA 67 (RTM), HOSTAVIN N 20 (RTM), HOSTAVIN N 24 (RTM), SANDUVOR 3050 (RTM), DIACETAM 5 (RTM), SUMISORB TM 61 (RTM), UVINUL 4049 (RTM), SANDUVOR PR 31(RTM), GOODRITE UV 3034 (RTM), GOODRITE UV 3150 (RTM), GOODRITE UV 3159 (RTM), GOODRITE 3110×128 (RTM), UVINUL 4050 H (RTM), CHIMASSORB 944 (RTM), CHIMASSORB 2020 (RTM), CYASORB UV 3346 (RTM), CYASORB UV 3529 (RTM), DASTIB 1082 (RTM), CHIMASSORB 119 (RTM), UVASIL 299 (RTM), UVASIL 125 (RTM), UVASIL 2000 (RTM), UVINUL 5050H (RTM), LICHTSCHUTZSTOFF UV 31 (RTM), LUCHEM HA B 18 (RTM), MARK LA 63 (RTM), MARK LA 68 (RTM), UVASORB HA 88 (RTM), TINUVIN 622 (RTM), HOSTAVIN N 30 (RTM) and FERRO AM 806 (RTM).
Particularly preferred are TINUVIN 770 (RTM), TINUVIN 791 (RTM), TINUVIN 622 (RTM), TINUVIN 783 (RTM), CHIMASSORB 944 (RTM), CHIMASSORB 2020 (RTM) and CHIMASSORB 119 (RTM).
Most preferred is Tinuvin 770 (RTM) and TINUVIN 791 (RTM).
The meanings of the terminal groups which saturate the free valences in the compounds of the formulae (B-1), (B-3), (B-4), (B-5), (B-6-α), (B-6-β), (B-6-γ), (B-7), (B-8-a), (B-8-b) and (B-10) depend on the processes used for their preparation. The terminal groups can also be modified after the preparation of the compounds.
If the compounds of the formula (B-1) are prepared by reacting a compound of the formula
in which X is, for example, halogen, in particular chlorine, and R204 and R205 are as defined above, with a compound of the formula
in which R201, R202 and R203 are as defined above, the terminal group bonded to the diamino radical is hydrogen or
and the terminal group bonded to the triazine radical is X or
in which X is, for example, halogen, in particular chlorine, and R204 and R205 are as defined above, with a compound of the formula
in which R201, R202 and R203 are as defined above, the terminal group bonded to the diamino radical is hydrogen or
and the terminal group bonded to the triazine radical is X or
If X is halogen, it is advantageous to replace this, for example, by —OH or an amino group when the reaction is complete. Examples of amino groups which may be mentioned are pyrrolidin-1-yl, morpholino, —NH2, —N(C1-C8)alkyl)2 and —NR(C1-C8alkyl), in which R is hydrogen or a group of the formula (b-I).
The compounds of the formula (B-1) also cover compounds of the formula
wherein R201, R202, R203, R204, R205 and b1 are as defined above and R204* has one of the meanings of R204 and R205* has one of the meanings of R205.
wherein R201, R202, R203, R204, R205 and b1 are as defined above and R204* has one of the meanings of R204 and R205* has one of the meanings of R205.
The preparation of this compound is described in Example 10 of U.S. Pat. No. 6,046,304.
In the compounds of the formula (B-3), the terminal group bonded to the silicon atom can be, for example, (R14)3Si—O—, and the terminal group bonded to the oxygen can be, for example, —Si(R14)3.
The compounds of the formula (B-3) can also be in the form of cyclic compounds if b2 is a number from 3 to 10, i.e. the free valences shown in the structural formula then form a direct bond.
In the compounds of the formula (B-4), the terminal group bonded to the 2,5-dioxopyrrolidine ring is, for example, hydrogen, and the terminal group bonded to the —C(R223)(R224)— radical is, for example,
In the compounds of the formula (B-5), the terminal group bonded to the carbonyl radical is, for example,
and the terminal group bonded to the oxygen radical is, for example,
and the terminal group bonded to the oxygen radical is, for example,
In the compounds of the formulae (B-6-α), (B-6-β) and (B-6-γ), the terminal group bonded to the triazine radical is, for example, Cl or a
group, and the terminal group bonded to the amino radical is, for example, hydrogen or a
group.
group, and the terminal group bonded to the amino radical is, for example, hydrogen or a
group.
If the compounds of the formula (B-7) are prepared, for example, by reacting a compound of the formula
in which A1 is hydrogen or methyl, with a dicarboxylic acid diester of the formula Y—OOC-A2-COO—Y, in which Y is, for example, methyl, ethyl or propyl, and A2 is as defined above, the terminal group bonded to the 2,2,6,6-tetramethyl-4-oxypiperidin-1-yl radical is hydrogen or —CO-A2-COO—Y, and the terminal group bonded to the diacyl radical is —O—Y or
in which A1 is hydrogen or methyl, with a dicarboxylic acid diester of the formula Y—OOC-A2-COO—Y, in which Y is, for example, methyl, ethyl or propyl, and A2 is as defined above, the terminal group bonded to the 2,2,6,6-tetramethyl-4-oxypiperidin-1-yl radical is hydrogen or —CO-A2-COO—Y, and the terminal group bonded to the diacyl radical is —O—Y or
In the compounds of the formula (B-8-a), the terminal group bonded to the nitrogen can be, for example, hydrogen and the terminal group bonded to the 2-hydroxypropylene radical can be, for example, a
group.
group.
In the compounds of the formula (B-8-b), the terminal group bonded to the dimethylene radical can be, for example, —OH, and the terminal group bonded to the oxygen can be, for example, hydrogen. The terminal groups can also be polyether radicals.
In the compounds of the formula (B-10), the end group bonded to the —CH2— residue can be, for example, hydrogen and the end group bonded to the —CH(CO2A7) residue can be, for example, —CH═CH—COOA7.
Further individual individual compounds are the following: bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate, bis(2,2,6,6-tetramethyl-4-piperidyl)succinate, bis(1,2,2,6,6-pentamethyl-4-piperidyl)sebacate, bis(1-octyloxy-2,2,6,6-tetramethyl-4-piperidyl)sebacate, bis(1,2,2,6,6-pentamethyl-4-piperidyl) n-butyl-3,5-di-tert-butyl-4-hydroxy-benzylmalonate, the condensate of 1-(2-hydroxyethyl)-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, linear or cyclic condensates of N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-tert-octylamino-2,6-dichloro-1,3,5-triazine, tris(2,2,6,6-tetramethyl-4-piperidyl)nitrilotriacetate, tetrakis(2,2,6,6-tetramethyl-4-piperidyl)-1,2,3,4-butanetetracarboxylate, 1,1′-(1,2-ethanediyl)-bis(3,3,5,5-tetramethylpiperazinone), 4-benzoyl-2,2,6,6-tetramethylpiperidine, 4-stearyloxy-2,2,6,6-tetramethylpiperidine, bis(1,2,2,6,6-pentamethylpiperidyl)-2-n-butyl-2-(2-hydroxy-3,5-di-tert-butylbenzyl)malonate, 3-n-octyl-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-dione, bis(1-octyloxy-2,2,6,6-tetramethylpiperidyl)sebacate, bis(1-octyloxy-2,2,6,6-tetramethylpiperidyl)succinate, linear or cyclic condensates of N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-morpholino-2,6-dichloro-1,3,5-triazine, the condensate of 2-chloro-4,6-bis(4-n-butylamino-2,2,6,6-tetramethylpiperidyl)-1,3,5-triazine and 1,2-bis(3-aminopropylamino)ethane, the condensate of 2-chloro-4,6-di-(4-n-butylamino-1,2,2,6,6-pentamethylpiperidyl)-1,3,5-triazine and 1,2-bis(3-aminopropylamino)ethane, 8-acetyl-3-dodecyl-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]-decane-2,4-dione, 3-dodecyl-1-(2,2,6,6-tetramethyl-4-piperidyl)pyrrolidine-2,5-dione, 3-dodecyl-1-(1,2,2,6,6-pentamethyl-4-piperidyl)pyrrolidine-2,5-dione, a mixture of 4-hexadecyloxy- and 4-stearyloxy-2,2,6,6-tetramethylpiperidine, a condensate of N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-cyclohexylamino-2,6-dichloro-1,3,5-triazine, a condensate of 1,2-bis(3-aminopropylamino)ethane and 2,4,6-trichloro-1,3,5-triazine as well as 4-butylamino-2,2,6,6-tetramethylpiperidine (CAS Reg. No. [136504-96-6]); a condensate of 1,6-hexanediamine and 2,4,6-trichloro-1,3,5-triazine as well as N,N-dibutylamine and 4-butylamino-2,2,6,6-tetramethylpiperidine (CAS Reg. No. [192268-64-7]); N-(2,2,6,6-tetramethyl-4-piperidyl)-n-dodecylsuccinimide, N-(1,2,2,6,6-pentamethyl-4-piperidyl)-n-dodecyl-succinimide, 2-undecyl-7,7,9,9-tetramethyl-1-oxa-3,8-diaza-4-oxo-spiro[4,5]decane, a reaction product of 7,7,9,9-tetramethyl-2-cycloundecyl-1-oxa-3,8-diaza-4-oxospiro-[4,5]decane and epichlorohydrin, 1,1-bis(1,2,2,6,6-pentamethyl-4-piperidyloxycarbonyl)-2-(4-methoxyphenyl)ethene, N,N′-bis-formyl-N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine, a diester of 4-methoxymethylenemalonic acid with 1,2,2,6,6-pentamethyl-4-hydroxypiperidine, poly[methylpropyl-3-oxy-4-(2,2,6,6-tetramethyl-4-piperidyl)]siloxane, a reaction product of maleic acid anhydride-α-olefin copolymer with 2,2,6,6-tetramethyl-4-aminopiperidine or 1,2,2,6,6-pentamethyl-4-aminopiperidine.
In a specific embodiment of the invention the pigment is selected from the class consisting of diketopyrrolo-pyrrole pigments, the UV absorber is a mixture of Tinuvin 327 (RTM) and Tinuvin 1577 (RTM), the sterically hindered amine stabilizer is Tinuvin 791 (RTM) or Tinuvin 770 (RTM) and the substrate is polypropylene.
The amount of the sterically hindered amine compound (component (c)) in the polyolefin to be stabilized is preferably 0.005 to 5%, in particular 0.01 to 1% or 0.05 to 1% by weight, based on the weight of the polyolefin.
The stabilized material may additionally also contain various conventional additives, for example:
1. Antioxidants
1.1. Alkylated monophenols, for example 2,6-di-tert-butyl-4-methylphenol, 2-tert-butyl-4,6-di-methylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-butylphenol, 2,6-di-tert-butyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol, 2-α-methylcyclohexyl)-4,6-dimethylphenol, 2,6-dioctadecyl-4-methylphenol, 2,4,6-tricyclohexylphenol, 2,6-di-tert-butyl-4-methoxymethylphenol, nonylphenols which are linear or branched in the side chains, for example 2,6-di-nonyl-4-methylphenol, 2,4-dimethyl-6-(1′-methylundec-1′-yl)phenol, 2,4-dimethyl-6-(1′-methylheptadec-1′-yl)phenol, 2,4-dimethyl-6-(1′-methyltridec-1′-yl)phenol and mixtures thereof.
1.2. Alkylthiomethylphenols, for example 2,4-dioctylthiomethyl-6-tert-butylphenol, 2,4-dioctylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-di-dodecylthiomethyl-4-nonylphenol.
1.3. Hydroquinones and alkylated hydroquinones, for example 2,6-di-tert-butyl-4-methoxyphenol, 2,5-di-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-diphenyl-4-octadecyloxyphenol, 2,6-di-tert-butylhydroquinone, 2,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyphenyl stearate, bis(3,5-di-tert-butyl-4-hydroxyphenyl) adipate.
1.4. Tocopherols, for example α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol and mixtures thereof (vitamin E).
1.5. Hydroxylated thiodiphenyl ethers, for example 2,2′-thiobis(6-tert-butyl-4-methylphenol), 2,2′-thiobis(4-octylphenol), 4,4′-thiobis(6-tert-butyl-3-methylphenol), 4,4′-thiobis(6-tert-butyl-2-methylphenol), 4,4′-thiobis(3,6-di-sec-amylphenol), 4,4′-bis(2,6-dimethyl-4-hydroxyphenyl)-disulfide.
1.6. Alkylidenebisphenols, for example 2,2′-methylenebis(6-tert-butyl-4-methylphenol), 2,2′-methylenebis(6-tert-butyl-4-ethylphenol), 2,2′-methylenebis[4-methyl-6-(α-methylcyclohexyl)-phenol], 2,2′-methylenebis(4-methyl-6-cyclohexylphenol), 2,2′-methylenebis(6-nonyl-4-methylphenol), 2,2′-methylenebis(4,6-di-tert-butylphenol), 2,2′-ethylidenebis(4,6-di-tert-butyl-phenol), 2,2′-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2′-methylenebis[6-(α-methylbenzyl)-4-nonylphenol], 2,2′-methylenebis[6-(α,α-dimethylbenzyl)-4-nonylphenol], 4,4′-methylenebis(2,6-di-tert-butylphenol), 4,4′-methylenebis(6-tert-butyl-2-methylphenol), 1,1-bis(5-tert-butyl-4-hydroxy-2-methylphenyl)butane, 2,6-bis(3-tert-butyl-5-methyl-2-hydroxybenzyl)-4-methylphenol, 1,1,3-tris(5-tert-butyl-4-hydroxy-2-methylphenyl)butane, 1,1-bis(5-tert-butyl-4-hydroxy-2-methylphenyl)-3-n-dodecylmercaptobutane, ethylene glycol bis[3,3-bis(3′-tert-butyl-4′-hydroxyphenyl)butyrate], bis(3-tert-butyl-4-hydroxy-5-methyl-phenyl)dicyclopentadiene, bis[2-(3′-tert-butyl-2′-hydroxy-5′-methylbenzyl)-6-tert-butyl-4-methylphenyl]terephthalate, 1,1-bis-(3,5-dimethyl-2-hydroxyphenyl)butane, 2,2-bis(3,5-di-tert-butyl-4-hydroxyphenyl)propane, 2,2-bis-(5-tert-butyl-4-hydroxy2-methylphenyl)-4-n-dodecylmercaptobutane, 1,1,5,5-tetra(5-tert-butyl-4-hydroxy-2-methylphenyl)pentane.
1.7. O-, N- and S-benzyl compounds, for example 3,5,3′,5′-tetra-tert-butyl-4,4′-dihydroxydibenzyl ether, octadecyl-4-hydroxy-3,5-dimethylbenzylmercaptoacetate, tridecyl-4-hydroxy-3,5-di-tert-butylbenzylmercaptoacetate, tris(3,5-di-tert-butyl-4-hydroxybenzyl)amine, bis(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)dithioterephthalate, bis(3,5-di-tert-butyl-4-hydroxybenzyl)sulfide, isooctyl-3,5-di-tert-butyl-4-hydroxybenzylmercaptoacetate.
1.8. Hydroxybenzylated malonates, for example dioctadecyl-2,2-bis(3,5-di-tert-butyl-2-hydroxybenzyl)malonate, di-octadecyl-2-(3-tert-butyl-4-hydroxy-5-methylbenzyl)malonate, di-dodecylmercaptoethyl-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate, bis[4-(1,1,3,3-tetramethylbutyl)phenyl]-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate.
1.9. Aromatic hydroxybenzyl compounds, for example 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-trimethylbenzene, 1,4-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-2,3,5,6-tetramethylbenzene, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)phenol.
1.10. Triazine compounds, for example 2,4-bis(octylmercapto)-6-(3,5-di-tert-butyl-4-hydroxyanilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyanilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,3,5-triazine, 2,4,6-tris-(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazine, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)isocyanurate, 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)isocyanurate, 2,4,6-tris-(3,5-di-tert-butyl-4-hydroxyphenylethyl)-1,3,5-triazine, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-hexahydro-1,3,5-triazine, 1,3,5-tris(3,5-dicyclohexyl-4-hydroxybenzyl)isocyanurate.
1.11. Benzylphosphonates, for example dimethyl-2,5-di-tert-butyl-4-hydroxybenzylphosphonate, diethyl-3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl-5-tert-butyl-4-hydroxy-3-methylbenzylphosphonate, the calcium salt of the monoethyl ester of 3,5-di-tert-butyl-4-hydroxybenzylphosphonic acid.
1.12. Acylaminophenols, for example 4-hydroxylauranilide, 4-hydroxystearanilide, octyl N-(3,5-di-tert-butyl-4-hydroxyphenyl)carbamate.
1.13. Esters of β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid with mono- or polyhydric alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N′-bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.14. Esters of β-(5-tert-butyl-4-hydroxy-3-methylphenyl)propionic acid with mono- or polyhydric alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N′-bis-(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane; 3,9-bis[2-{3-(3-tert-butyl-4-hydroxy-5-methylphenyl)propionyloxy}-1,1-dimethylethyl]-2,4,8,10-tetraoxaspiro[5.5]-undecane.
1.15. Esters of β-(3,5-dicyclohexyl-4-hydroxyphenyl)propionic acid with mono- or polyhydric alcohols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N′-bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.16. Esters of 3,5-di-tert-butyl-4-hydroxyphenyl acetic acid with mono- or polyhydric alcohols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N′-bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.17. Amides of β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid e.g. N,N′-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hexamethylenediamide, N,N′-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)trimethylenediamide, N,N′-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hydrazide, N,N′-bis[2-(3-[3,5-di-tert-butyl-4-hydroxyphenylpropionyl]oxy)ethyl]oxamide (Naugard® XL-1, supplied by Uniroyal).
1.18. Ascorbic acid (vitamin C)
1.19. Aminic antioxidants, for example N,N′-di-isopropyl-p-phenylenediamine, N,N′-di-sec-butyl-p-phenylenediamine, N,N′-bis(1,4-dimethylpentyl)-p-phenylenediamine, N,N′-bis(1-ethyl-3-methylpentyl)-p-phenylenediamine, N,N′-bis(1-methylheptyl)-p-phenylenediamine, N,N′-dicyclohexyl-p-phenylenediamine, N,N′-diphenyl-p-phenylenediamine, N,N′-bis(2-naphthyl)-p-phenylenediamine, N-isopropyl-N′-phenyl-p-phenylenediamine, N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine, N-(1-methylheptyl)-N′-phenyl-p-phenylenediamine, N-cyclohexyl-N′-phenyl-p-phenylenediamine, 4-(p-toluenesulfamoyl)diphenylamine, N,N′-dimethyl-N,N′-di-sec-butyl-p-phenylenediamine, diphenylamine, N-allyldiphenylamine, 4-isopropoxydiphenylamine, N-phenyl-1-naphthylamine, N-(4-tert-octylphenyl)-1-naphthylamine, N-phenyl-2-naphthylamine, octylated diphenylamine, for example p,p′-di-tert-octyldiphenylamine, 4-n-butylaminophenol, 4-butyrylaminophenol, 4-nonanoylaminophenol, 4-dodecanoylaminophenol, 4-octadecanoylaminophenol, bis(4-methoxyphenyl)amine, 2,6-di-tert-butyl-4-dimethylaminomethylphenol, 2,4′-diaminodiphenylmethane, 4,4′-diaminodiphenylmethane, N,N,N′,N′-tetramethyl-4,4′-diaminodiphenylmethane, 1,2-bis[(2-methylphenyl)amino]ethane, 1,2-bis(phenylamino)propane, (o-tolyl)biguanide, bis[4-(1′,3′-dimethylbutyl)phenyl]amine, tert-octylated N-phenyl-1-naphthylamine, a mixture of mono- and dialkylated tert-butyl/tert-octyldiphenylamines, a mixture of mono- and dialkylated nonyldiphenylamines, a mixture of mono- and dialkylated dodecyldiphenylamines, a mixture of mono- and dialkylated isopropyl/isohexyldiphenylamines, a mixture of mono- and dialkylated tert-butyldiphenylamines, 2,3-dihydro-3,3-dimethyl-4H-1,4-benzothiazine, phenothiazine, a mixture of mono- and dialkylated tert-butyl/tert-octylphenothiazines, a mixture of mono- and dialkylated tert-octylphenothiazines, N-allylphenothiazine, N,N,N′,N′-tetraphenyl-1,4-diaminobut-2-ene, N,N-bis(2,2,6,6-tetramethylpiperid-4-yl-hexamethylenediamine, bis(2,2,6,6-tetramethylpiperid-4-yl)sebacate, 2,2,6,6-tetramethylpiperidin-4-one, 2,2,6,6-tetramethylpiperidin-4-ol.
2. UV Absorbers and Light Stabilisers
2.2. 2-Hydroxybenzophenones, for example the 4-hydroxy, 4-methoxy, 4-octyloxy, 4-decyloxy, 4-dodecyloxy, 4-benzyloxy, 4,2′,4′-trihydroxy and 2′-hydroxy-4,4′-dimethoxy derivatives.
2.3. Esters of substituted and unsubstituted benzoic acids, for example 4-tert-butylphenyl salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoyl resorcinol, bis(4-tert-butylbenzoyl)resorcinol, benzoyl resorcinol, 2,4-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxybenzoate, hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, octadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, 2-methyl-4,6-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxybenzoate.
2.4. Acrylates, for example ethyl α-cyano-β,β-diphenylacrylate, isooctyl α-cyano-β,β-diphenylacrylate, methyl α-carbomethoxycinnamate, methyl α-cyano-β-methyl-p-methoxycinnamate, butyl α-cyano-p-methyl-β-methoxycinnamate, methyl α-carbomethoxy-p-methoxycinnamate and N-(β-carbomethoxy-β-cyanovinyl)-2-methylindoline.
2.5. Nickel compounds, for example nickel complexes of 2,2′-thiobis[4-(1,1,3,3-tetramethylbutyl)phenol], such as the 1:1 or 1:2 complex, with or without additional ligands such as n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel dibutyldithiocarbamate, nickel salts of the monoalkyl esters, e.g. the methyl or ethyl ester, of 4-hydroxy-3,5-di-tert-butylbenzylphosphonic acid, nickel complexes of ketoximes, e.g. of 2-hydroxy-4-methylphenylundecylketoxime, nickel complexes of 1-phenyl-4-lauroyl-5-hydroxypyrazole, with or without additional ligands.
2.7. Oxamides, for example 4,4′-dioctyloxyoxanilide, 2,2′-diethoxyoxanilide, 2,2′-dioctyloxy-5,5′-di-tert-butoxanilide, 2,2′-didodecyloxy-5,5′-di-tert-butoxanilide, 2-ethoxy-2′-ethyloxanilide, N,N′-bis(3-dimethylaminopropyl)oxamide, 2-ethoxy-5-tert-butyl-2′-ethoxanilide and its mixture with 2-ethoxy-2′-ethyl-5,4′-di-tert-butoxanilide, mixtures of o- and p-methoxy-disubstituted oxanilides and mixtures of o- and p-ethoxy-disubstituted oxanilides.
3. Metal deactivators, for example N,N′-diphenyloxamide, N-salicylal-N′-salicyloyl hydrazine, N,N′-bis(salicyloyl)hydrazine, N,N′-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hydrazine, 3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalyl dihydrazide, oxanilide, isophthaloyl dihydrazide, sebacoyl bisphenylhydrazide, N,N′-diacetyladipoyl dihydrazide, N,N′-bis(salicyloyl)oxalyl dihydrazide, N,N′-bis(salicyloyl)thiopropionyl dihydrazide.
4. Phosphites and phosphonites, for example triphenyl phosphite, diphenylalkyl phosphites, phenyldialkyl phosphites, tris(nonylphenyl) phosphite, trilauryl phosphite, trioctadecyl phosphite, distearylpentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl) phosphite, diisodecyl pentaerythritol diphosphite, bis(2,4-di-tert-butylphenyl)pentaerythritol diphosphite, bis(2,4-di-cumylphenyl)pentaerythritol diphosphite, bis(2,6-di-tert-butyl-4-methylphenyl)pentaerythritol diphosphite, diisodecyloxypentaerythritol diphosphite, bis(2,4-di-tert-butyl-6-methylphenyl)-pentaerythritol diphosphite, bis(2,4,6-tris(tert-butylphenyl)pentaerythritol diphosphite, tristearyl sorbitol triphosphite, tetrakis(2,4-di-tert-butylphenyl) 4,4′-biphenylene diphosphonite, 6-isooctyloxy-2,4,8,10-tetra-tert-butyl-12H-dibenz[d,g]-1,3,2-dioxaphosphocin, bis(2,4-di-tert-butyl-6-methylphenyl)methyl phosphite, bis(2,4-di-tert-butyl-6-methylphenyl)ethyl phosphite, 6-fluoro-2,4,8,10-tetra-tert-butyl-12-methyl-dibenz[d,g]-1,3,2-dioxaphosphocin, 2,2′,2″-nitrilo-[triethyltris(3,3′,5,5′-tetra-tert-butyl-1,1′-biphenyl-2,2′-diyl)phosphite], 2-ethylhexyl(3,3′,5,5′-tetra-tert-butyl-1,1′-biphenyl-2,2′-diyl)phosphite, 5-butyl-5-ethyl-2-(2,4,6-tri-tert-butylphenoxy)-1,3,2-dioxaphosphirane.
The following phosphites are especially preferred:
5. Hydroxylamines, for example N,N-dibenzylhydroxylamine, N,N-diethylhydroxylamine, N,N-dioctylhydroxylamine, N,N-dilaurylhydroxylamine, N,N-ditetradecylhydroxylamine, N,N-dihexadecylhydroxylamine, N,N-dioctadecylhydroxylamine, N-hexadecyl-N-octadecylhydroxylamine, N-heptadecyl-N-octadecylhydroxylamine, N,N-dialkylhydroxylamine derived from hydrogenated tallow amine.
6. Nitrones, for example N-benzyl-alpha-phenylnitrone, N-ethyl-alpha-methylnitrone, N-octyl-alpha-heptylnitrone, N-lauryl-alpha-undecylnitrone, N-tetradecyl-alpha-tridecylnitrone, N-hexadecyl-alpha-pentadecylnitrone, N-octadecyl-alpha-heptadecylnitrone, N-hexadecyl-alpha-heptadecylnitrone, N-ocatadecyl-alpha-pentadecyinitrone, N-heptadecyl-alpha-heptadecylnitrone, N-octadecyl-alpha-hexadecylnitrone, nitrone derived from N,N-dialkylhydroxylamine derived from hydrogenated tallow amine.
7. Thiosynergists, for example dilauryl thiodipropionate or distearyl thiodipropionate.
8. Peroxide scavengers, for example esters of β-thiodipropionic acid, for example the lauryl, stearyl, myristyl or tridecyl esters, mercaptobenzimidazole or the zinc salt of 2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide, pentaerythritol tetrakis(β-dodecylmercapto)propionate.
9. Basic co-stabilisers, for example melamine, polyvinylpyrrolidone, dicyandiamide, triallyl cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides, polyurethanes, alkali metal salts and alkaline earth metal salts of higher fatty acids, for example calcium stearate, zinc stearate, magnesium behenate, magnesium stearate, sodium ricinoleate and potassium palmitate, antimony pyrocatecholate or zinc pyrocatecholate.
10. Nucleating agents, for example inorganic substances, such as talcum, metal oxides, such as titanium dioxide or magnesium oxide, phosphates, carbonates or sulfates of, preferably, alkaline earth metals; organic compounds, such as mono- or polycarboxylic acids and the salts thereof, e.g. 4-tert-butylbenzoic acid, adipic acid, diphenylacetic acid, sodium succinate or sodium benzoate; polymeric compounds, such as ionic copolymers (ionomers). Especially preferred are 1,3:2,4-bis(3′,4′-dimethylbenzylidene)sorbitol, 1,3:2,4-di(paramethyldibenzylidene)sorbitol, and 1,3:2,4-di(benzylidene)sorbitol.
11. Fillers and reinforcing agents, for example calcium carbonate, silicates, glass fibres, glass bulbs, asbestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxides, carbon black, graphite, wood flour and flours or fibers of other natural products, synthetic fibers.
12. Other additives, for example plasticisers, lubricants, emulsifiers, pigments, rheology additives, catalysts, flow-control agents, optical brighteners, flameproofing agents, antistatic agents and blowing agents.
13. Benzofuranones and indolinones, for example those disclosed in U.S. Pat. No. 4,325,863; U.S. Pat. No. 4,338,244; U.S. Pat. No. 5,175,312; U.S. Pat. No. 5,216,052; U.S. Pat. No. 5,252,643; DE-A-4316611; DE-A-4316622; DE-A-4316876; EP-A-0589839 or EP-A-0591102 or 3-[4-(2-acetoxyethoxy)-phenyl]-5,7-di-tert-butylbenzofuran-2-one, 5,7-di-tert-butyl-3-[4-(2-stearoyloxyethoxy)phenyl]-benzofuran-2-one, 3,3′-bis[5,7-di-tert-butyl-3-(4-[2-hydroxyethoxy]phenyl)benzofuran-2-one], 5,7-di-tert-butyl-3-(4-ethoxyphenyl)benzofuran-2-one, 3-(4-acetoxy-3,5-dimethylphenyl)-5,7-di-tert-butylbenzofuran-2-one, 3-(3,5-dimethyl-4-pivaloyloxyphenyl)-5,7-di-tert-butylbenzofuran-2-one, 3-(3,4-dimethylphenyl)-5,7-di-tert-butylbenzofuran-2-one, 3-(2,3-dimethylphenyl)-5,7-di-tert-butylbenzofuran-2-one.
The above mentioned further stabilizers and additives are usually applied in an amount from 0.01% to 2%.
Also subject of the invention is therefore a polyolefin composition as described above which contains additionally a further stabilizer selected from the group consisting of a phenolic antioxidant, a phosphite or phosphonite and benzofuranone or indolinone.
The above mentioned components a, b, c and further additives can be incorporated into the polyolefin to be stabilized by known methods, for example before or during shaping or by applying the dissolved or dispersed compounds to the polyolefin, if necessary with subsequent evaporation of the solvent. The components can be added to the polyolefin in the form of a powder, granules or a masterbatch, which contains these components in, for example, a concentration of from 2.5 to 25% by weight.
If desired, the components can be melt blended with each other before incorporation in the polyolefin. They can also be added to the polyolefin before or during the polymerization.
The materials stabilized according to this invention can be used in a wide variety of forms, for example as films, fibres, tapes, moulding compositions or profiles.
Examples of processing or transformation of the polyolefin according to the present invention are:
Injection blow molding, extrusion, blow molding, rotomolding, in mold decoration (back injection), slush molding, injection molding, co-injection molding, forming, compression molding, pressing, film extrusion (cast film; blown film), fiber spinning (woven, non-woven), drawing (uniaxial, biaxial), annealing, deep drawing, calandering, mechanical transformation, sintering, coextrusion, coating, lamination, crosslinking (radiation, peroxide, silane), vapor deposition, weld together, glue, thermoforming, pipe extrusion, profile extrusion, sheet extrusion, extrusion coating, visbreaking (peroxide, thermal), fiber melt blown, spun bonded, surface treatment (corona discharge, flame, plasma), sterilization (by gamma rays, electron beams), cast polymerization (R&M process, RAM extrusion), gel-coating and tape extrusion.
The polyolefin according to the present invention may be used for the preparation of:
I-1) Floating devices, marine applications, pontoons, buoys, plastic lumber for decks, piers, boats, kayaks, oars, and beach reinforcements.
I-2) Automotive applications, in particular bumpers, dashboards, battery, rear and front linings, moldings parts under the hood, hat shelf, trunk linings, interior linings, air bag covers, electronic moldings for fittings (lights), panes for dashboards, headlamp glass, instrument panel, exterior linings, upholstery, automotive lights, head lights, parking lights, rear lights, stop lights, interior and exterior trims; door panels; gas tank; glazing front side; rear windows; seat backing, exterior panels, wire insulation, profile extrusion for sealing, cladding, pillar covers, chassis parts, exhaust systems, fuel filter/filler, fuel pumps, fuel tank, body side mouldings, convertible tops, exterior mirrors, exterior trim, fasteners/fixings, front end module, glass, hinges, lock systems, luggage/roof racks, pressed/stamped parts, seals, side impact protection, sound deadener/insulator and sunroof.
I-3) Road traffic devices, in particular sign postings, posts for road marking, car accessories, warning triangles, medical cases, helmets, tires.
I-4) Devices for plane, railway, motor car (car, motorbike) including furnishings.
I-5) Devices for space applications, in particular rockets and satellites, e.g. reentry shields.
I-6) Devices for architecture and design, mining applications, acoustic quietized systems, street refuges, and shelters.
II-1) Appliances, cases and coverings in general and electric/electronic devices (personal computer, telephone, handy, printer, television-sets, audio and video devices), flower pots, satellite TV bowl, and panel devices.
II-2) Jacketing for other materials such as steel or textiles.
II-3) Devices for the electronic industry, in particular insulation for plugs, especially computer plugs, cases for electric and electronic parts, printed boards, and materials for electronic data storage such as chips, check cards or credit cards.
II-4) Electric appliances, in particular washing machines, tumblers, ovens (microwave oven), dish-washers, mixers, and irons.
II-5) Covers for lights (e.g. street-lights, lamp-shades).
II-6) Applications in wire and cable (semi-conductor, insulation and cable-jacketing).
II-7) Foils for condensers, refrigerators, heating devices, air conditioners, encapsulating of electronics, semi-conductors, coffee machines, and vacuum cleaners.
III-1) Technical articles such as cogwheel (gear), slide fittings, spacers, screws, bolts, handles, and knobs.
III-2) Rotor blades, ventilators and windmill vanes, solar devices, swimming pools, swimming pool covers, pool liners, pond liners, closets, wardrobes, dividing walls, slat walls, folding walls, roofs, shutters (e.g. roller shutters), fittings, connections between pipes, sleeves, and conveyor belts.
III-3) Sanitary articles, in particular shower cubicles, lavatory seats, covers, and sinks.
III-4) Hygienic articles, in particular diapers (babies, adult incontinence), feminine hygiene articles, shower curtains, brushes, mats, tubs, mobile toilets, tooth brushes, and bed pans.
III-5) Pipes (cross-linked or not) for water, waste water and chemicals, pipes for wire and cable protection, pipes for gas, oil and sewage, guttering, down pipes, and drainage systems.
III-6) Profiles of any geometry (window panes) and siding.
III-7) Glass substitutes, in particular extruded plates, glazing for buildings (monolithic, twin or multiwall), aircraft, schools, extruded sheets, window film for architectural glazing, train, transportation, sanitary articles, and greenhouse.
III-8) Plates (walls, cutting board), extrusion-coating (photographic paper, tetrapack and pipe coating), silos, wood substitute, plastic lumber, wood composites, walls, surfaces, furniture, decorative foil, floor coverings (interior and exterior applications), flooring, duck boards, and tiles.
III-9) Intake and outlet manifolds.
III-10) Cement-, concrete-, composite-applications and covers, siding and cladding, hand rails, banisters, kitchen work tops, roofing, roofing sheets, tiles, and tarpaulins.
IV-1) Plates (walls and cutting board), trays, artificial grass, astroturf, artificial covering for stadium rings (athletics), artificial floor for stadium rings (athletics), and tapes.
IV-2) Woven fabrics continuous and staple, fibers (carpets/hygienic articles/geotextiles/monofilaments; filters; wipes/curtains (shades)/medical applications), bulk fibers (applications such as gown/protection clothes), nets, ropes, cables, strings, cords, threads, safety seat-belts, clothes, underwear, gloves; boots; rubber boots, intimate apparel, garments, swimwear, sportswear, umbrellas (parasol, sunshade), parachutes, paraglides, sails, “balloon-silk”, camping articles, tents, airbeds, sun beds, bulk bags, and bags.
IV-3) Membranes, insulation, covers and seals for roofs, tunnels, dumps, ponds, dumps, walls roofing membranes, geomembranes, swimming pools, curtains (shades)/sun-shields, awnings, canopies, wallpaper, food packing and wrapping (flexible and solid), medical packaging (flexible & solid), airbags/safety belts, arm- and head rests, carpets, centre console, dashboard, cockpits, door, overhead console module, door trim, headliners, interior lighting, interior mirrors, parcel shelf, rear luggage cover, seats, steering column, steering wheel, textiles, and trunk trim.
V) Films (packaging, dump, laminating, agriculture and horticulture, greenhouse, mulch, tunnel, silage), bale wrap, swimming pools, waste bags, wallpaper, stretch film, raffia, desalination film, batteries, and connectors.
VI-1) Food packing and wrapping (flexible and solid), BOPP, BOPET, bottles.
VI-2) Storage systems such as boxes (crates), luggage, chest, household boxes, pallets, shelves, tracks, screw boxes, packs, and cans.
VI-3) Cartridges, syringes, medical applications, containers for any transportation, waste baskets and waste bins, waste bags, bins, dust bins, bin liners, wheely bins, container in general, tanks for water/used water/chemistry/gas/oil/gasoline/diesel; tank liners, boxes, crates, battery cases, troughs, medical devices such as piston, ophthalmic applications, diagnostic devices, and packing for pharmaceuticals blister.
VII-1) Extrusion coating (photo paper, tetrapack, pipe coating), household articles of any kind (e.g. appliances, thermos bottle/clothes hanger), fastening systems such as plugs, wire and cable clamps, zippers, closures, locks, and snap-closures.
VII-2) Support devices, articles for the leisure time such as sports and fitness devices, gymnastics mats, ski-boots, inline-skates, skis, big foot, athletic surfaces (e.g. tennis grounds); screw tops, tops and stoppers for bottles, and cans.
VII-3) Furniture in general, foamed articles (cushions, impact absorbers), foams, sponges, dish clothes, mats, garden chairs, stadium seats, tables, couches, toys, building kits (boards/figures/balls), playhouses, slides, and play vehicles.
VII-4) Materials for optical and magnetic data storage.
VII-5) Kitchen ware (eating, drinking, cooking, storing).
VII-6) Boxes for CD's, cassettes and video tapes; DVD electronic articles, office supplies of any kind (ball-point pens, stamps and ink-pads, mouse, shelves, tracks), bottles of any volume and content (drinks, detergents, cosmetics including perfumes), and adhesive tapes.
VII-7) Footwear (shoes/shoe-soles), insoles, spats, adhesives, structural adhesives, food boxes (fruit, vegetables, meat, fish), synthetic paper, labels for bottles, couches, artificial joints (human), printing plates (flexographic), printed circuit boards, and display technologies.
VII-8) Devices of filled polymers (talc, chalk, china clay (kaolin), wollastonite, pigments, carbon black, TiO2, mica, nanocomposites, dolomite, silicates, glass, asbestos).
Further aspects of the invention are a method for stabilizing a polyolefin containing at least one organic pigment against degradation induced by light, heat or oxidation, which comprises incorporating into the pigmented polyolefin a stabilizer mixture comprising
- a) at least one sterically hindered amine light stabilizer and
- b) as UV absorber a mixture of a 2-hydroxyphenyl benzotriazole and a 2-hydroxyphenyl-s-triazine, and
the use of a mixture of - a) at least one sterically hindered amine light stabilizer and
- b) as UV absorber a mixture of a 2-hydroxyphenyl benzotriazole and a 2-hydroxyphenyl-s-triazine for the stabilization of a polyolefin containing at least one organic pigment.
Definitions and preferences have already been given. They apply also for the method of stabilization and the use of the stabilizer mixture.
The following examples illustrate the invention.
100 parts of unstabilized polypropylene powder (melt flow index 3.8 g/10 minutes, 230° C./2160 g) are homogenized at 200° C. for 10 minutes in a Brabender plastograph with 0.05 parts of pentaerythrityl-tetrakis-3-(3,5-ditert.butyl-4-hydroxyphenyl)-propionate, 0.05 parts of tris-(2,4-di-tert.butylphenyl)-phosphite and the amount of coadditive, pigment and light stabilizer indicated in the table. The material thus obtained is compression molded in a laboratory press between two aluminum foils for 6 minutes at 260° C. to a 0.5 mm thick film which is cooled immediately to room temperature in a water-cooled press. Samples of 60×25 mm are cut out of these 0.5 mm films and exposed in a WEATHER-OMETER Ci 65 (black panel temperature 63±2° C., without water-spraying)
Periodically, these samples are removed from the exposure apparatus and their carbonyl content is measured with an infrared spectrophotometer.
The exposure time corresponding to formation of a carbonyl absorbance of 0.1 is a measure for the stabilizing efficiency of the light stabilizer. The values obtained are summarized in Table 1.
| TABLE 1 |
| Light stability of PP films containing an organic and an inorganic pigment |
| All the formulations contain: 0.1% TINUVIN 770 + |
| 0.1% Mg stearate + 0.25% TiO2(Rutile) |
| Organic pigments and UV absorbers | T0.1: (h) to 0.1 carbonyl absorbance |
|
| 0.25% CHROMOPHTAL | ||
| YELLOW 3G | ||
| Control (no UV absorber) | 770 | |
| 0.10% TINUVIN 327 | 960 | |
| 0.10% TINUVIN 1577 | 840 | |
| 0.05% TINUVIN 327 + 0.05% | 1000 | 900 |
| TINUVIN 1577 | ||
| 0.25% CHROMOPHTAL | ||
| RED BRN | ||
| Control (no UV absorber) | 1750 | |
| 0.10% TINUVIN 327 | 2045 | |
| 0.10% TINUVIN 1577 | 1990 | |
| 0.05% TINUVIN 327 + 0.05% | 2125 | 2018 |
| TINUVIN 1577 | ||
| 0.25% CHROMOPHTAL | ||
| DPP RED BOC | ||
| Control (no UV absorber) | 7140 | |
| 0.10% TINUVIN 327 | 6895 | |
| 0.10% TINUVIN 1577 | 8155 | |
| 0.05% TINUVIN 327 + 0.05% | 9040 | 7525 |
| TINUVIN 1577 | ||
100 parts of unstabilized polypropylene powder (melt flow index 3.4 g/ 10 minutes, 230° C./2160 g) are homogenized at 200° C. for 10 minutes in a Brabender plastograph with 0.05 parts of pentaerythrityl-tetrakis-3-(3,5-ditert.butyl-4-hydroxyphenyl)-propionate, 0.05 parts of tris-(2,4-di-tert.butylphenyl)-phosphite and the amount of coadditive, pigment and light stabilizer indicated in the table. The material thus obtained is compression molded in a laboratory press between two aluminum foils for 6 minutes at 260° C. to a 0.5 mm thick film which is cooled immediately to room temperature in a water-cooled press. Samples of 60×25 mm are cut out of these 0.5 mm films and exposed in a WEATHER-OMETER Ci 65 (black panel temperature 63±2° C., without water-spraying)
Periodically, these samples are removed from the exposure apparatus and their carbonyl content is measured with an infrared spectrophotometer.
The exposure time corresponding to formation of a carbonyl absorbance of 0.1 is a measure for the stabilizing efficiency of the light stabilizer. The values obtained are summarized in Tables 2 and 3.
| TABLE 2 |
| Light stability of PP films containing organic pigments |
| All the formulations contain: 0.1% TINUVIN 770 + |
| 0.1% Ca stearate |
| Pigment and Light stabilization | T0.1 (h) |
|
| 0.25% Chromophtal Yellow 2RLP | ||
| 0.1% TINUVIN 327 | 2073 | |
| 0.1% TINUVIN 1577 | 2340 | |
| 0.05% TINUVIN 327 + 0.05% | 2275 | 2207 |
| TINUVIN 1577 | ||
| 0.25% Chromophtal Yellow | ||
| GT-AD | ||
| 0.1% TINUVIN 327 | 5125 | |
| 0.1% TINUVIN 1577 | 6503 | |
| 0.05% TINUVIN 327 + 0.05% | 5918 | 5814 |
| TINUVIN 1577 | ||
| 0.25% Chromophtal DPP | ||
| Orange TRP | ||
| 0.1% TINUVIN 327 | 3072 | |
| 0.1% TINUVIN 1577 | 4668 | |
| 0.05% TINUVIN 327 + 0.05% | 4019 | 3870 |
| TINUVIN 1577 | ||
| 0.25% Irgazin DPP Rubine TR | ||
| 0.1% TINUVIN 327 | 8565 | |
| 0.1% TINUVIN 1577 | 11868 | |
| 0.05% TINUVIN 327 + 0.05% | 10731 | 10216 |
| TINUVIN 1577 | ||
| 0.25% Cinquasia Red Y RT 759-D | ||
| 0.1% TINUVIN 327 | 7690 | |
| 0.1% TINUVIN 1577 | 8509 | |
| 0.05% TINUVIN 327 + 0.05% | 9285 | 8100 |
| TINUVIN 1577 | ||
| 0.25% Cinquasia Red B RT 195-D | ||
| 0.1% TINUVIN 327 | 4065 | |
| 0.1% TINUVIN 1577 | 6421 | |
| 0.05% TINUVIN 327 + 0.05% | 5898 | 5243 |
| TINUVIN 1577 | ||
| 0.25% IRGALITE Green GFNP | ||
| 0.1% TINUVIN 327 | 4009 | |
| 0.1% TINUVIN 1577 | 6393 | |
| 0.05% TINUVIN 327 + 0.05% | 5561 | 5201 |
| TINUVIN 1577 | ||
| 0.25% Chromophtal Orange GL | ||
| 0.1% TINUVIN 327 | 2851 | |
| 0.1% TINUVIN 1577 | 3388 | |
| 0.05% TINUVIN 327 + 0.05% | 3462 | 3120 |
| TINUVIN 1577 | ||
| 0.25% IRGAZIN Red BPTN | ||
| 0.1% TINUVIN 327 | 4883 | |
| 0.1% TINUVIN 1577 | 4953 | |
| 0.05% TINUVIN 327 + 0.05% | 5034 | 4918 |
| TINUVIN 1577 | ||
| TABLE 3 |
| Light stability of PP films containing an organic and |
| an inorganic pigment |
| All the formulations contain: 0.1% TINUVIN 770 + |
| 0.1% Mg stearate + 0.25% TiO2 |
| Pigment and Light stabilization | T0.1 (h) |
|
| 0.25% Chromophtal | ||
| Yellow GT-AD | ||
| 0.1% TINUVIN 327 | 5837 | |
| 0.1% TINUVIN 1577 | 6052 | |
| 0.05% TINUVIN 327 + 0.05% | 6002 | 5945 |
| TINUVIN 1577 | ||
| 0.25% Chromophtal Orange 2G | ||
| 0.1% TINUVIN 327 | 1614 | |
| 0.1% TINUVIN 1577 | 1739 | |
| 0.05% TINUVIN 327 + 0.05% | 1802 | 1677 |
| TINUVIN 1577 | ||
| 0.25% Chromophtal DPP | ||
| Red BOC | ||
| 0.1% TINUVIN 327 | 4618 | |
| 0.1% TINUVIN 1577 | 8167 | |
| 0.05% TINUVIN 327 + 0.05% | 7897 | 6392 |
| TINUVIN 1577 | ||
| 0.25% Chromophtal Red 2030 | ||
| 0.1% TINUVIN 327 | 3692 | |
| 0.1% TINUVIN 1577 | 5149 | |
| 0.05% TINUVIN 327 + 0.05% | 4928 | 4420 |
| TINUVIN 1577 | ||
| 0.25% Irgazin DPP Rubine TR | ||
| 0.1% TINUVIN 327 | 10087 | |
| 0.1% TINUVIN 1577 | 12202 | |
| 0.05% TINUVIN 327 + 0.05% | 11582 | 11144 |
| TINUVIN 1577 | ||
| 0.25% Cinquasia Red Y | ||
| RT 759-D | ||
| 0.1% TINUVIN 327 | 8417 | |
| 0.1% TINUVIN 1577 | 10155 | |
| 0.05% TINUVIN 327 + 0.05% | 9451 | 9286 |
| TINUVIN 1577 | ||
| 0.25% Irgalite Blue BSP | ||
| 0.1% TINUVIN 327 | 5028 | |
| 0.1% TINUVIN 1577 | 7129 | |
| 0.05% TINUVIN 327 + 0.05% | 6402 | 6078 |
| TINUVIN 1577 | ||
| 0.25% Chromophtal Blue A3R | ||
| 0.1% TINUVIN 327 | 7376 | |
| 0.1% TINUVIN 1577 | 9456 | |
| 0.05% TINUVIN 327 + 0.05% | 9120 | 8416 |
| TINUVIN 1577 | ||
| 0.25% IRGALITE Green GFNP | ||
| 0.1% TINUVIN 327 | 3827 | |
| 0.1% TINUVIN 1577 | 6360 | |
| 0.05% TINUVIN 327 + 0.05% | 5911 | 5094 |
| TINUVIN 1577 | ||
| 0.25% IRGAZIN Red BPTN | ||
| 0.1% TINUVIN 327 | 5279 | |
| 0.1% TINUVIN 1577 | 6525 | |
| 0.05% TINUVIN 327 + 0.05% | 6180 | 5902 |
| TINUVIN 1577 | ||
100 parts of unstabilized polypropylene powder (melt flow index 3.6 g/10 minutes, 230° C./2160 g) are homogenized at 200° C. for 10 minutes in a Brabender plastograph with 0.05 parts of pentaerythrityl-tetrakis-3-(3,5-ditert.butyl-4-hydroxyphenyl)-propionate, 0.05 parts of tris-(2,4-di-tert.butylphenyl)-phosphite, 0.1 parts of Ca stearate and the amount of pigment and light stabilizers indicated in the table. The material thus obtained is compression molded in a laboratory press between two aluminum foils for 6 minutes at 260° C. to a 0.5 mm thick film which is cooled immediately to room temperature in a water-cooled press. Samples of 60×25 mm are cut out of these 0.5 mm films and exposed in a WEATHER-OMETER Ci 65 (black panel temperature 63±2° C., without water-spraying)
Periodically, these samples are removed from the exposure apparatus and their carbonyl content is measured with an infrared spectrophotometer.
The exposure time corresponding to formation of a carbonyl absorbance of 0.1 is a measure for the stabilizing efficiency of the light stabilizer. The values obtained are summarized in Tables 4 and 5.
| TABLE 4 |
| Light stability of PP films containing an organic pigment |
| All the formulations contain: 0.25% Chromophthal DPP Red BOC + |
| 0.1% CHIMASSORB 944 |
| Light stabilization | T0.1 (h) |
|
| 0.1% TINUVIN 327 | 2667 | |
| 0.2% TINUVIN 327 | 2728 | |
| 0.1% TINUVIN 1577 | 3235 | |
| 0.2% TINUVIN 1577 | 3718 | |
| 0.05% TINUVIN 327 + 0.05% | 3284 | 2951 |
| TINUVIN 1577 | ||
| 0.075% TINUVIN 327 + 0.025% | 3081 | 2809 |
| TINUVIN 1577 | ||
| 0.1% TINUVIN 327 + 0.1% | 3588 | 3223 |
| TINUVIN 1577 | ||
| 0.15% TINUVIN 327 + 0.05% | 3008 | 2975 |
| TINUVIN 1577 | ||
| TABLE 5 |
| Light stability of PP films containing an organic pigment |
| All the formulations contain: 0.25% Chromophthal DPP Red BOC + |
| 0.1% TINUVIN 770 |
| Light stabilization | T0.1 (h) |
|
| 0.1% TINUVIN 327 | 3280 | |
| 0.2% TINUVIN 327 | 3976 | |
| 0.1% TINUVIN 1577 | 5969 | |
| 0.2% TINUVIN 1577 | 7255 | |
| 0.05% TINUVIN 327 + 0.05% | 4730 | 4624 |
| TINUVIN 1577 | ||
| 0.075% TINUVIN 327 + 0.025% | 4623 | 3952 |
| TINUVIN 1577 | ||
| 0.1% TINUVIN 327 + 0.1% | 5757 | 5615 |
| TINUVIN 1577 | ||
| 0.15% TINUVIN 327 + 0.05% | 5215 | 4796 |
| TINUVIN 1577 | ||
| Pigments and chemical classes: |
| Trade name | Chemical description | C.I.-designation |
| CHROMOPHTHAL | Disazo condensation | Pigment Yellow 93 |
| Yellow 3G | ||
| CHROMOPHTHAL Red | Disazo condensation | Pigment Red 144 |
| BRN | ||
| CHROMOPHTHAL DPP | Diketopyrrolo-pyrrole | Pigment Red 254 |
| Red BOC | ||
| CHROMOPHTHAL Red | Diketopyrrolo-pyrrole | Pigment Red 254 |
| 2030 | ||
| CHROMOPHTAL Yellow | Isoindolinone | Pigment Yellow 110 |
| 2RLP | ||
| CHROMOPHTAL Yellow | Anthraquinone | Pigment Yellow 199 |
| GT-AD | ||
| CHROMOPHTAL DPP | Diketopyrrolo-pyrrole | Pigment Orange 71 |
| Orange TRP | ||
| IRGAZIN DPP Rubine TR | Diketopyrrolo-pyrrole | Pigment Red 272 |
| CINQUASIA Red Y | Quinacridone | Pigment Violet 19 |
| RT-759-D | ||
| CINQUASIA Red B | Quinacridone | — |
| RT 195-D | ||
| IRGALITE Blue BSP | Cu-phthalocyanine, | Pigment Blue 15:1 |
| stabilized α-form | ||
| CHROMOPHTAL Blue | Anthraquinone | Pigment Blue 60 |
| A3R | ||
| IRGALITE Green GFNP | Cu-phthalocyanine | Pigment Green 7 |
| CHROMOPHTAL Orange | Benzimidazolone | Pigment Orange 64 |
| GL | ||
| IRGAZIN Red BPTN | Perylene | Pigment Red 224 |
| CHROMOPHTAL Orange | Isoindolinone | Pigment Orange 61 |
| 2G | ||
All UV-absorbers and light stabilizers as well as all pigments used are registered trade marks and commercial products of Ciba Specialty Chemicals Corp.
Claims (11)
1. A polyolefin composition which comprises
a) at least one organic pigment
b) at least one sterically hindered amine light stabilizer and
c) as UV absorber a mixture of
a 2-hydroxyphenyl benzotriazole and a 2-hydroxyphenyl-s-triazine wherein the weight ratio of hydroxyphenyl triazine UV-absorber to hydroxyphenyl benzotriazole UV-absorber is from 10:1 to 1:10.
2. A polyolefin composition according to claim 1 wherein the polyolefin is polypropylene, polyethylene or a copolymer thereof.
3. A polyolefin composition according to claim 1 wherein the pigment is selected from the group comprising the classes of azo pigments, anthraquinones, benzimidazolones, dioxazines, phthalocyanines, tetrachloroisoindolinones, quinacridones, isoindolines, perylenes, pyrrolopyrroles.
4. A polyolefin composition according to claim 1 wherein the UV-absorber of the class of hydroxyphenyl-s-triazine is of formula (I)
in which n is 1 or 2;
R1, R′1, R2 and R′2, independently of one another, are H, OH, C1-C12alkyl; C2-C6alkenyl; C1-C12alkoxy; C2-C18alkenoxy; halogen; trifluoromethyl; C7-C11phenylalkyl; phenyl; phenyl which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen; phenoxy; or phenoxy which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen;
R3 and R4, independently of one another, are H, C1-C12alkyl; OR′7; C2-C6alkenyl; C2-C18alkenoxy; halogen; trifluoromethyl; C7-C11phenylalkyl; phenyl; phenyl which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen; phenoxy; or phenoxy which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen;
R6 is hydrogen, C1-C24alkyl, C5-C12cycloalkyl or C7-C15phenylalkyl;
R7, in the case where n=1, and R′7, independently of one another, are hydrogen or C1-C18alkyl; or are C1-C12alkyl which is substituted by OH, C1-C18alkoxy, allyloxy, halogen, —COOH, —COOR6, —CONH2, —CONHR9, —CON(R9)(R10), —NH2, —NHR9, —N(R9)(R10), —NHCOR11, —CN, —OCOR11, phenoxy and/or phenoxy which is substituted by C1-C18alkyl, C1-C18alkoxy or halogen; or R7 is C3-C50alkyl which is interrupted by —O— and may be substituted by OH; or R7 is C3-C6alkenyl; glycidyl; C5-C12cycloalkyl; cyclohexyl which is substituted by OH, C1-C4alkyl or —OCOR11; C7-C11phenylalkyl which is unsubstituted or substituted by OH, Cl or CH3; —CO—R12 or —SO2—R13;
R7, in the case where n=2, is C2-C16alkylene, C4-C12alkenylene, xylylene, C3-C20alkylene which is interrupted by O and/or substituted by OH, or is a group of the formula —CH2CH(OH)CH2O—R20—OCH2CH(OH)CH2—, —CO—R21—CO—, —CO—NH—R22—NH—CO— or —(CH2)m—COO—R23—OOC—(CH2)m—, in which m is a number in the range from 1 to 3, or is
R8 is C1-C18alkyl; C2-C18alkenyl; hydroxyethyl; C3-C50alkyl which is interrupted by O, NH, NR9 or S and/or is substituted by OH; C1-C4alkyl which is substituted by —P(O)(OR14)2, —N(R9)(R10) or —OCOR11 and/or OH; glycidyl; C5-C12cycloalkyl; phenyl; C7-C14alkylphenyl or C7-C11 phenylalkyl;
R9 and R10, independently of one another, are C1-C12alkyl; C3-C12alkoxyalkyl; C4-C16dialkylaminoalkyl or C5-C12cycloalkyl, or R9 and R10 together are C3-C9alkylene or -oxaalkylene or -azaalkylene;
R11 is C1-C18alkyl; C2-C18alkenyl or phenyl; C2-C12hydroxyalkyl; cyclohexyl; or is C3-C50alkyl which is interrupted by —O— and may be substituted by OH;
R12 is C1-C18alkyl; C2-C18alkenyl; phenyl; C1-C18alkoxy; C3-C18alkenyloxy; C3-C50alkoxy which is interrupted by O, NH, NR9 or S and/or substituted by OH; cyclohexyloxy; C7-C14alkylphenoxy; C7-C11phenylalkoxy; phenoxy; C1-C12alkylamino; phenylamino; tolylamino or naphthylamino;
R13 is C1-C12alkyl; phenyl; naphthyl or C7-C14alkylphenyl;
R14 is C1-C12alkyl, methylphenyl or phenyl;
R20 is C2-C10alkylene; C4-C50alkylene which is interrupted by O, phenylene or a -phenylene-X-phenylene- group, in which X is —O—, —S—, —SO2—, —CH2— or —C(CH3)2—;
R21 is C2-C10alkylene, C2-C10oxaalkylene, C2-C10thiaalkylene, C6-C12arylene or C2-C6alkenylene;
group; and
R23 is C2-C10alkylene or C4-C20alkylene which is interrupted by O.
5. A polyolefin composition according to claim 1 wherein the UV-absorber of the class of hydroxyphenyl benzotriazole is of formula IIa, IIb or IIc:
in the compounds of the formula (IIa),
in which
R104 and R105 independently of one another are alkyl having in each case 1 to 5 carbon atoms, or R104, together with the radical CnH2n+1−m, forms a cycloalkyl radical having 5 to 12 carbon atoms,
m is 1 or 2, n is an integer from 2 to 20 and
M is a radical of the formula —COOR106 in which
R106 is hydrogen, alkyl having 1 to 12 carbon atoms, alkoxyalkyl having in each case 1 to 20 carbon atoms in the alkyl moiety and in the alkoxy moiety or phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety,
R102 is hydrogen, halogen, alkyl having 1 to 18 carbon atoms, and phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, and
R103 is hydrogen, chlorine, alkyl or alkoxy having in each case 1 to 4 carbon atoms or —COOR106 in which R106 is as defined above, at least one of the radicals R101 and R102 being other than hydrogen;
in the compounds of the formula (IIb)
T is hydrogen or alkyl having 1 to 6 carbon atoms,
T1 is hydrogen, chlorine or alkyl or alkoxy having in each case 1 to 4 carbon atoms,
n is 1 or 2 and,
if n is 1,
or —O-T9-O— in which
T3 is hydrogen, alkyl which has 1 to 18 carbon atoms and is unsubstituted or substituted by 1 to 3 hydroxyl groups or by —OCOT6, alkyl which has 3 to 18 carbon atoms, is interrupted once or several times by —O— or —NT6- and is unsubstituted or substituted by hydroxyl or —OCOT6, cycloalkyl which has 5 to 12 carbon atoms and is unsubstituted or substituted by hydroxyl and/or alkyl having 1 to 4 carbon atoms, alkenyl which has 2 to 18 carbon atoms and is unsubstituted or substituted by hydroxyl, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, or a radical of the formula —CH2CH(OH)-T7 or
T4 and T5 independently of one another are hydrogen, alkyl having 1 to 18 carbon atoms, alkyl which has 3 to 18 carbon atoms and is interrupted once or several times by —O— or —NT6-, cycloalkyl having 5 to 12 carbon atoms, phenyl, phenyl which is substituted by alkyl having 1 to 4 carbon atoms, alkenyl having 3 to 8 carbon atoms, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety or hydroxyalkyl having 2 to 4 carbon atoms,
T6 is hydrogen, alkyl having 1 to 18 carbon atoms, cycloalkyl having 5 to 12 carbon atoms, alkenyl having 3 to 8 carbon atoms, phenyl, phenyl which is substituted by alkyl having 1 to 4 carbon atoms, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety,
T7 is hydrogen, alkyl having 1 to 18 carbon atoms, phenyl which is unsubstituted or substituted by hydroxyl, phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety, or —CH2OT8,
T8 is alkyl having 1 to 18 carbon atoms, alkenyl having 3 to 8 carbon atoms, cycloalkyl having 5 to 10 carbon atoms, phenyl, phenyl which is substituted by alkyl having 1 to 4 carbon atoms, or phenylalkyl having 1 to 4 carbon atoms in the alkyl moiety,
T9 is alkylene having 2 to 8 carbon atoms, alkenylene having 4 to 8 carbon atoms, alkynylene having 4 carbon atoms, cyclohexylene, alkylene which has 2 to 8 carbon atoms and is interrupted once or several times by —O—, or a radical of the formula —CH2CH(OH)CH2OT11OCH2CH(OH)CH2— or —CH2—C(CH2OH)2—CH2—,
T10 is alkylene which has 2 to 20 carbon atoms and can be interrupted once or several times by —O—, or cyclohexylene,
T11 is alkylene having 2 to 8 carbon atoms, alkylene which has 2 to 18 carbon atoms and is interrupted once or several times by —O—, 1,3-cyclohexylene, 1,4-cyclohexylene, 1,3-phenylene or 1,4-phenylene, or
T10 and T6, together with the two nitrogen atoms, are a piperazine ring;
in the compounds of formula (IIc)
R′102 is C1-C12alkyl and k is a number from 1 to 4.
6. A polyolefin composition according to claim 1 wherein the total amount of UV absorber is from 0.01 to 5% by weight, based on the weight of the polyolefin.
7. A polyolefin composition according to claim 1 wherein the sterically hindered amine light stabilizer is selected from the group consisting of bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate, bis(2,2,6,6-tetramethyl-4-piperidyl)succinate, bis(1,2,2,6,6-pentamethyl-4-piperidyl)sebacate, bis(1-octyloxy-2,2,6,6-tetramethyl-4-piperidyl)sebacate, bis(1,2,2,6,6-pentamethyl-4-piperidyl) n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate, the condensate of 1-(2-hydroxyethyl)-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, linear or cyclic condensates of N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-tert-octylamino-2,6-dichloro-1,3,5-triazine, tris(2,2,6,6-tetramethyl-4-piperidyl)nitrilotriacetate, tetrakis(2,2,6,6-tetramethyl-4-piperidyl)-1,2,3,4-butanetetracarboxylate, 1,1′-(1,2-ethanediyl)-bis(3,3,5,5-tetramethylpiperazinone), 4-benzoyl-2,2,6,6-tetramethylpiperidine, 4-stearyloxy-2,2,6,6-tetramethylpiperidine, bis(1,2,2,6,6-pentamethylpiperidyl)-2-n-butyl-2-(2-hydroxy-3,5-di-tert-butylbenzyl)malonate, 3-n-octyl-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-dione, bis(1-octyloxy-2,2,6,6-tetramethylpiperidyl)sebacate, bis(1-octyloxy-2,2,6,6-tetramethylpiperidyl)succinate, linear or cyclic condensates of N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)-hexamethylenediamine and 4-morpholino-2,6-dichloro-1,3,5-triazine, the condensate of 2-chloro-4,6-bis(4-n-butylamino-2,2,6,6-tetramethylpiperidyl)-1,3,5-triazine and 1,2-bis(3-aminopropylamino)ethane, the condensate of 2-chloro-4,6-di-(4-n-butylamino-1,2,2,6,6-pentamethylpiperidyl)-1,3,5-triazine and 1,2-bis(3-aminopropylamino)ethane, 8-acetyl-3-dodecyl-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-dione, 3-dodecyl-1-(2,2,6,6-tetramethyl-4-piperidyl)pyrrolidine-2,5-dione, 3-dodecyl-1-(1,2,2,6,6-pentamethyl-4-piperidyl)-pyrrolidine-2,5-dione, a mixture of 4-hexadecyloxy- and 4-stearyloxy-2,2,6,6-tetramethylpiperidine, a condensate of N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-cyclohexylamino-2,6-dichloro-1,3,5-triazine, a condensate of 1,2-bis(3-aminopropylamino)ethane and 2,4,6-trichloro-1,3,5-triazine as well as 4-butylamino-2,2,6,6-tetramethylpiperidine (CAS Reg. No. [136504-96-6]); a condensate of 1,6-hexanediamine and 2,4,6-trichloro-1,3,5-triazine as well as N,N-dibutylamine and 4-butylamino-2,2,6,6-tetramethylpiperidine (CAS Reg. No. [192268-64-7]); N-(2,2,6,6-tetramethyl-4-piperidyl)-n-dodecylsuccinimide, N-(1,2,2,6,6-pentamethyl-4-piperidyl)-n-dodecylsuccinimide, 2-undecyl-7,7,9,9-tetramethyl-1-oxa-3,8-diaza-4-oxo-spiro[4,5]decane, a reaction product of 7,7,9,9-tetramethyl-2-cycloundecyl-1-oxa-3,8-diaza-4-oxospiro-[4,5]decane and epichlorohydrin, 1,1-bis-(1,2,2,6,6-pentamethyl-4-piperidyloxycarbonyl)-2-(4-methoxyphenyl)ethene, N,N′-bis-formyl-N,N′-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine, a diester of 4-methoxymethylenemalonic acid with 1,2,2,6,6-pentamethyl-4-hydroxypiperidine, poly[methylpropyl-3-oxy-4-(2,2,6,6-tetramethyl-4-piperidyl)]siloxane, a reaction product of maleic acid anhydride-α-olefin copolymer with 2,2,6,6-tetramethyl-4-aminopiperidine or 1,2,2,6,6-pentamethyl-4-aminopiperidine.
8. A polyolefin composition according to claim 1 wherein the amount of the sterically hindered amine compound (component (c)) is preferably 0.005 to 5% by weight, based on the weight of the polymer.
9. A polyolefin composition according to claim 1 which contains additionally a further stabilizer selected from the group consisting of a phenolic antioxidant, a phosphite or phosphonite and benzofuranone or indolinone.
10. A polyolefin composition according to claim 1 which contains additionally an inorganic pigment.
11. A method for stabilizing a polyolefin containing at least one organic pigment against degradation induced by light, heat or oxidation, which comprises incorporating into the pigmented polyolefin a stabilizer mixture comprising
a) at least one sterically hindered amine light stabilizer and
b) as UV absorber a mixture of a 2-hydroxyphenyl benzotriazole and a 2-hydroxyphenyl-s-triazine wherein the weight ratio of hydroxyphenyl triazine UV-absorber to hydroxyphenyl benzotriazole UV-absorber is from 10:1 to 1:10.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP02405162.5 | 2002-03-04 | ||
| EP02405162 | 2002-03-04 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20040030009A1 US20040030009A1 (en) | 2004-02-12 |
| US6878761B2 true US6878761B2 (en) | 2005-04-12 |
Family
ID=27798954
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/358,823 Expired - Fee Related US6878761B2 (en) | 2002-03-04 | 2003-02-05 | Synergistic combinations of UV absorbers for pigmented polyolefins |
Country Status (19)
| Country | Link |
|---|---|
| US (1) | US6878761B2 (en) |
| EP (1) | EP1342748B1 (en) |
| JP (1) | JP2003261725A (en) |
| KR (1) | KR100902337B1 (en) |
| CN (1) | CN1294193C (en) |
| AR (1) | AR038627A1 (en) |
| AT (1) | ATE406406T1 (en) |
| AU (1) | AU2003200799B2 (en) |
| BR (1) | BR0300500B1 (en) |
| CA (1) | CA2420549C (en) |
| CO (1) | CO5310577A1 (en) |
| DE (1) | DE60323174D1 (en) |
| DK (1) | DK1342748T3 (en) |
| ES (1) | ES2312743T3 (en) |
| IL (1) | IL154706A (en) |
| MX (1) | MX238184B (en) |
| SA (1) | SA03230548B1 (en) |
| TW (1) | TWI293973B (en) |
| ZA (1) | ZA200301683B (en) |
Cited By (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060083940A1 (en) * | 2004-04-30 | 2006-04-20 | Solomon Bekele | Ultraviolet light absorbing composition |
| US20060122293A1 (en) * | 2004-12-03 | 2006-06-08 | Rick Wilk | Ultraviolet light absorber stabilizer combination |
| US20060124904A1 (en) * | 2002-04-12 | 2006-06-15 | Francois Gugumus | Stabilizer mixtures |
| US20060148940A1 (en) * | 2005-01-03 | 2006-07-06 | Board Of Regents, The University Of Texas System | Method for transformation of conventional and commercially important polymers into durable and rechargeable antimicrobial polymeric materials |
| WO2006074455A2 (en) | 2005-01-03 | 2006-07-13 | Board Of Regents, The University Of Texas System | Method for transformation of conventional and commercially important polymers into durable and rechargeable antimicrobial polymeric materials |
| US20070042149A1 (en) * | 2005-08-16 | 2007-02-22 | S.C. Johnson & Son, Inc. | Bottles made from metallocene polypropylene for delivery of fragrances |
| US20070062884A1 (en) * | 2005-08-11 | 2007-03-22 | Board Of Regents, The University Of Texas System | N-halamines compounds as multifunctional additives |
| US20070224161A1 (en) * | 2006-03-27 | 2007-09-27 | Board Of Regents, The University Of Texas System | Compositions and methods for making and using acyclic N-halamine-based biocidal polymeric materials and articles |
| US20080268189A1 (en) * | 2007-03-26 | 2008-10-30 | Board Of Regents, The University Of Texas System | N-Halamine-Based Rechargeable Biofilm-Controlling Tubular Devices, Method of Making and Using |
| US20090074825A1 (en) * | 2007-09-19 | 2009-03-19 | Board Of Regents, The University Of Texas System | Colorants based n-halamines compositions and method of making and using |
| US20090170984A1 (en) * | 2006-03-31 | 2009-07-02 | Basf Se | Composition containing polymers, colouring agents and stabilisers |
| US20090176092A1 (en) * | 2007-09-14 | 2009-07-09 | Fujikura Ltd. | Radiation-resistant resin composition and radiation-proof wire/cable |
| US20100062270A1 (en) * | 2007-01-15 | 2010-03-11 | Ciba Corporation | Tinted clear coatings uv stabilized with 2-hydroxy phenyl triazine |
| WO2010048436A2 (en) * | 2008-10-22 | 2010-04-29 | Microban Products Company | Antimicrobial polymer with anti-yellowing property |
| WO2011018384A1 (en) | 2009-08-11 | 2011-02-17 | Basf Se | Bi - or tricyclic sterically hindered alkoxyamines and process for their preparation |
| US20120145236A1 (en) * | 2009-08-18 | 2012-06-14 | Basf Se | Uv-stabilized photovoltaic module |
| US9179703B2 (en) | 2012-08-28 | 2015-11-10 | Sensor Electronic Technology, Inc. | Ultraviolet system for disinfection |
| US20160208079A1 (en) * | 2013-09-13 | 2016-07-21 | Dow Global Technologies Llc | Peroxide-Crosslinkable Compositions and Processes for Their Manufacture |
| US9707307B2 (en) | 2012-08-28 | 2017-07-18 | Sensor Electronic Technology, Inc. | Ultraviolet system for disinfection |
| US9724441B2 (en) | 2012-08-28 | 2017-08-08 | Sensor Electronic Technology, Inc. | Storage device including target UV illumination ranges |
| US9750830B2 (en) | 2012-08-28 | 2017-09-05 | Sensor Electronic Technology, Inc. | Multi wave sterilization system |
| US9795699B2 (en) | 2012-08-28 | 2017-10-24 | Sensor Electronic Technology, Inc. | Storage device including target UV illumination ranges |
| US9855682B2 (en) | 2011-06-10 | 2018-01-02 | Columbia Insurance Company | Methods of recycling synthetic turf, methods of using reclaimed synthetic turf, and products comprising same |
| US9878061B2 (en) | 2012-08-28 | 2018-01-30 | Sensor Electronic Technology, Inc. | Ultraviolet system for disinfection |
| US9919068B2 (en) | 2012-08-28 | 2018-03-20 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| US9981051B2 (en) | 2012-08-28 | 2018-05-29 | Sensor Electronic Technology, Inc. | Ultraviolet gradient sterilization, disinfection, and storage system |
| US10383964B2 (en) | 2012-08-28 | 2019-08-20 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| US10441670B2 (en) | 2012-08-28 | 2019-10-15 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| US10646603B2 (en) | 2012-08-28 | 2020-05-12 | Sensor Electronic Technology, Inc. | Multi wave sterilization system |
| US10688210B2 (en) | 2012-08-28 | 2020-06-23 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| WO2022207787A1 (en) * | 2021-04-01 | 2022-10-06 | Basf Se | Stabilizer mixture |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040031225A1 (en) * | 2002-08-14 | 2004-02-19 | Gregory Fowler | Water resistant tongue and groove flooring |
| ES2550535T3 (en) * | 2003-03-06 | 2015-11-10 | North-West University | Protective plant cover |
| MY149850A (en) * | 2003-09-29 | 2013-10-31 | Ciba Holding Inc | Stabilization of photochromic systems |
| WO2005054353A1 (en) * | 2003-11-21 | 2005-06-16 | Ciba Specialty Chemicals Holding Inc. | Weatherfast pigmented polystyrene |
| JP4666957B2 (en) | 2004-06-16 | 2011-04-06 | 株式会社Adeka | Polyester resin container with improved weather resistance |
| GB0426742D0 (en) * | 2004-12-06 | 2005-01-12 | Ykk Europ Ltd | A slide fastener |
| DE602006011278D1 (en) * | 2005-02-25 | 2010-02-04 | Basf Se | FLUORINATED COMPOUNDS |
| DE102005061954A1 (en) * | 2005-12-23 | 2007-07-05 | Basf Ag | Recycling of ruthenium from an used ruthenium catalyst comprises treating the catalyst containing ruthenium oxide in a hydrogen stream and treating the carrier material containing ruthenium metal with hydrochloric acid |
| WO2007082842A1 (en) * | 2006-01-13 | 2007-07-26 | Basf Aktiengesellschaft | Stabilizer mixture |
| PT1979406E (en) * | 2006-02-01 | 2013-01-28 | Basf Se | Stabilizer composition for polymers |
| US7789957B2 (en) | 2007-06-06 | 2010-09-07 | Ciba Corporation | Low-dust additive and pigment blends with improved color |
| JP5669584B2 (en) * | 2008-03-12 | 2015-02-12 | ビーエーエスエフ ソシエタス・ヨーロピアBasf Se | Optical film for flat panel display |
| JP5532905B2 (en) * | 2008-12-24 | 2014-06-25 | 住友化学株式会社 | Foaming resin composition and foamed molded article |
| JP2012036270A (en) * | 2010-08-05 | 2012-02-23 | Mitsui Chemicals Inc | 4-methyl-1-pentene-based polymer composition |
| BR112013006994A2 (en) * | 2010-09-30 | 2020-11-24 | Union Carbide Chemicals & Plastic Technology Llc | coated conductor and polymeric composition |
| JP5734826B2 (en) * | 2011-12-20 | 2015-06-17 | 株式会社Adeka | Process for producing olefin resin composition |
| KR101742845B1 (en) * | 2013-09-30 | 2017-06-01 | 주식회사 엘지화학 | Optical film exhibiting excellent blocking property for ultraviolet rays and polarizing plate comprising the same |
| PL2818504T3 (en) * | 2013-06-28 | 2021-03-08 | Borealis Ag | Use of an extrusion processing aid for the production of coloured polyethylene pipes |
| MX2016003734A (en) | 2013-09-27 | 2016-08-04 | Basf Se | Polyolefin compositions for building materials. |
| CN103804952A (en) * | 2014-01-23 | 2014-05-21 | 上海百艳实业有限公司 | Pyrrolopyrroledione pigment composition |
| CN106589514B (en) * | 2015-10-20 | 2020-03-24 | 天罡新材料(廊坊)股份有限公司 | Light stabilizer composition master batch and preparation method and application thereof |
| KR101993033B1 (en) * | 2016-08-02 | 2019-06-26 | (주)비피케미칼 | Flowable additive agent for polymers and method for preparing the same |
| CN107793634A (en) * | 2016-08-29 | 2018-03-13 | 合肥杰事杰新材料股份有限公司 | A kind of light PP alloys with high shading performance and preparation method thereof |
| US20200385607A1 (en) * | 2017-12-27 | 2020-12-10 | Basf Se | A composition comprising methylene malonate monomer and polymer, the preparation thereof and use of the same in flooring applications |
| EP3536742A1 (en) | 2018-03-09 | 2019-09-11 | Polytex Sportbeläge Produktions-GmbH | Artificial turf fiber with uv protection substances |
| KR20210021541A (en) * | 2018-06-21 | 2021-02-26 | 도판 인사츠 가부시키가이샤 | Protective film and sheet |
| CN110862613A (en) * | 2019-12-03 | 2020-03-06 | 江苏林辉塑料制品有限公司 | Anti-aging garbage can and preparation process thereof |
| CN111004428A (en) * | 2019-12-23 | 2020-04-14 | 海南联塑科技实业有限公司 | Special color master batch for polyethylene water supply pipe and preparation method and application thereof |
| US11692085B2 (en) * | 2020-03-18 | 2023-07-04 | University Of South Florida | Geomembranes and methods for making and using the same |
| US12043008B2 (en) * | 2020-10-13 | 2024-07-23 | The Goodyear Tire & Rubber Company | Method for repairing self-sealing tires |
| CN112430393B (en) * | 2020-11-20 | 2022-11-18 | 广州辰东新材料有限公司 | Ultraviolet-resistant thermoplastic polyamide composite material for laser welding and preparation method thereof |
| CN112300546B (en) * | 2020-11-20 | 2022-10-25 | 广州辰东新材料有限公司 | Ultraviolet-resistant thermoplastic polyester composite material for laser welding and preparation method thereof |
| CN118234789A (en) * | 2021-09-14 | 2024-06-21 | 英力士苯领集团股份公司 | High clarity, low haze and UV stable styrene-methyl methacrylate copolymers |
| MX2024003321A (en) * | 2021-09-16 | 2024-04-05 | Basf Se | Stabilizer formulation. |
| CN114213749A (en) * | 2021-11-23 | 2022-03-22 | 金发科技股份有限公司 | Flame-retardant polypropylene material and preparation method and application thereof |
| CN114316471A (en) * | 2021-12-31 | 2022-04-12 | 天津利安隆新材料股份有限公司 | Composite light stabilizer suitable for rigid polyvinyl chloride material, rigid polyvinyl chloride material and preparation method thereof |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4891396A (en) | 1987-08-28 | 1990-01-02 | Sandoz Ltd. | New benztriazolyl compounds |
| US5106891A (en) | 1990-03-30 | 1992-04-21 | Ciba-Geigy Corporation | Light stabilized coating compositions containing a mixture of 2-hydroxyphenylbenzotriazole and 2-hydroxyphenyltriazine |
| EP0704560A1 (en) | 1994-09-30 | 1996-04-03 | Ciba-Geigy Ag | Stabilization of pigmented fiber with a synergistic mixture of hindered amine and UV absorber |
| US5648488A (en) * | 1994-07-27 | 1997-07-15 | Ciba-Geigy Corporation | Compositions stabilized with red-shifted tris-aryl-s-triazines |
| WO1997036880A1 (en) | 1996-04-02 | 1997-10-09 | Ciba Specialty Chemicals Holding Inc. | Amino- and hydroxysubstituted triphenyl-s-triazines as stabilizers |
| US5798147A (en) * | 1994-11-23 | 1998-08-25 | Basf Aktiengesellschaft | Process for coating and printing substrates |
| US6060543A (en) | 1996-09-13 | 2000-05-09 | Ciba Specialty Chemicals Corporation | Stabilizer combination |
| GB2361005A (en) | 2000-04-04 | 2001-10-10 | Ciba Sc Holding Ag | Mixtures of UV absorbers in polyolefins |
| US6545071B1 (en) | 1996-08-22 | 2003-04-08 | Ciba Specialty Chemicals Corporation | Stabilizer mixtures |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2278115B (en) * | 1993-05-17 | 1997-08-06 | Ciba Geigy Ag | 2-(2-Hydroxyphenyl)-1,3-pyrimidine derivatives and their use as stabilizers for coating compositions |
| SE520727C2 (en) * | 1996-03-04 | 2003-08-19 | Ciba Sc Holding Ag | Alkylphenyl bisacylphosphine oxide and photoinitiator mixtures |
| KR100517489B1 (en) * | 1996-04-02 | 2006-05-03 | 시바 스페셜티 케미칼스 홀딩 인크. | Amino- and hydroxy substituted triphenyl-s-triazines as light stabilizers |
| GB2332678B (en) | 1997-12-23 | 2000-09-27 | Ciba Sc Holding Ag | Stabilizer mixtures containing a sterically hindered amine |
-
2003
- 2003-01-01 ZA ZA200301683A patent/ZA200301683B/en unknown
- 2003-02-05 US US10/358,823 patent/US6878761B2/en not_active Expired - Fee Related
- 2003-02-20 CO CO03014529A patent/CO5310577A1/en not_active Application Discontinuation
- 2003-02-24 SA SA3230548A patent/SA03230548B1/en unknown
- 2003-02-25 EP EP03405121A patent/EP1342748B1/en not_active Expired - Lifetime
- 2003-02-25 DK DK03405121T patent/DK1342748T3/en active
- 2003-02-25 ES ES03405121T patent/ES2312743T3/en not_active Expired - Lifetime
- 2003-02-25 DE DE60323174T patent/DE60323174D1/en not_active Expired - Lifetime
- 2003-02-25 AT AT03405121T patent/ATE406406T1/en active
- 2003-02-28 AR ARP030100666A patent/AR038627A1/en not_active Application Discontinuation
- 2003-02-28 CA CA2420549A patent/CA2420549C/en not_active Expired - Fee Related
- 2003-03-02 IL IL154706A patent/IL154706A/en not_active IP Right Cessation
- 2003-03-03 KR KR1020030012956A patent/KR100902337B1/en active IP Right Grant
- 2003-03-03 AU AU2003200799A patent/AU2003200799B2/en not_active Ceased
- 2003-03-03 TW TW092104375A patent/TWI293973B/en not_active IP Right Cessation
- 2003-03-04 JP JP2003057447A patent/JP2003261725A/en active Pending
- 2003-03-04 MX MXPA03001899 patent/MX238184B/en active IP Right Grant
- 2003-03-04 CN CNB031198368A patent/CN1294193C/en not_active Expired - Fee Related
- 2003-03-05 BR BRPI0300500-3A patent/BR0300500B1/en not_active IP Right Cessation
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4891396A (en) | 1987-08-28 | 1990-01-02 | Sandoz Ltd. | New benztriazolyl compounds |
| US5106891A (en) | 1990-03-30 | 1992-04-21 | Ciba-Geigy Corporation | Light stabilized coating compositions containing a mixture of 2-hydroxyphenylbenzotriazole and 2-hydroxyphenyltriazine |
| US5648488A (en) * | 1994-07-27 | 1997-07-15 | Ciba-Geigy Corporation | Compositions stabilized with red-shifted tris-aryl-s-triazines |
| EP0704560A1 (en) | 1994-09-30 | 1996-04-03 | Ciba-Geigy Ag | Stabilization of pigmented fiber with a synergistic mixture of hindered amine and UV absorber |
| US5798147A (en) * | 1994-11-23 | 1998-08-25 | Basf Aktiengesellschaft | Process for coating and printing substrates |
| WO1997036880A1 (en) | 1996-04-02 | 1997-10-09 | Ciba Specialty Chemicals Holding Inc. | Amino- and hydroxysubstituted triphenyl-s-triazines as stabilizers |
| US6545071B1 (en) | 1996-08-22 | 2003-04-08 | Ciba Specialty Chemicals Corporation | Stabilizer mixtures |
| US6060543A (en) | 1996-09-13 | 2000-05-09 | Ciba Specialty Chemicals Corporation | Stabilizer combination |
| GB2361005A (en) | 2000-04-04 | 2001-10-10 | Ciba Sc Holding Ag | Mixtures of UV absorbers in polyolefins |
| US20010039304A1 (en) | 2000-04-04 | 2001-11-08 | Francois Gugumus | Synergistic mixtures of UV-absorbers in polyolefins |
Cited By (51)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060124904A1 (en) * | 2002-04-12 | 2006-06-15 | Francois Gugumus | Stabilizer mixtures |
| US7595008B2 (en) * | 2002-04-12 | 2009-09-29 | Ciba Specialty Chemicals Corporation | Stabilizer mixtures |
| US20060083940A1 (en) * | 2004-04-30 | 2006-04-20 | Solomon Bekele | Ultraviolet light absorbing composition |
| US20060122293A1 (en) * | 2004-12-03 | 2006-06-08 | Rick Wilk | Ultraviolet light absorber stabilizer combination |
| US7541398B2 (en) | 2005-01-03 | 2009-06-02 | Board Of Regents, The University Of Texas System | Method for transformation of conventional and commercially important polymers into durable and rechargeable antimicrobial polymeric materials |
| US20060148940A1 (en) * | 2005-01-03 | 2006-07-06 | Board Of Regents, The University Of Texas System | Method for transformation of conventional and commercially important polymers into durable and rechargeable antimicrobial polymeric materials |
| WO2006074455A2 (en) | 2005-01-03 | 2006-07-13 | Board Of Regents, The University Of Texas System | Method for transformation of conventional and commercially important polymers into durable and rechargeable antimicrobial polymeric materials |
| US10689526B2 (en) | 2005-08-11 | 2020-06-23 | Board Of Regents, The University Of Texas System | N-halamines compounds as multifunctional additives |
| US20070062884A1 (en) * | 2005-08-11 | 2007-03-22 | Board Of Regents, The University Of Texas System | N-halamines compounds as multifunctional additives |
| US10138379B2 (en) | 2005-08-11 | 2018-11-27 | Board Of Regents, The University Of Texas System | N-halamines compounds as multifunctional additives |
| US7416766B2 (en) | 2005-08-16 | 2008-08-26 | S.C. Johnson & Son, Inc. | Bottles made from metallocene polypropylene for delivery of fragrances |
| US20070042149A1 (en) * | 2005-08-16 | 2007-02-22 | S.C. Johnson & Son, Inc. | Bottles made from metallocene polypropylene for delivery of fragrances |
| US8486428B2 (en) | 2006-03-27 | 2013-07-16 | Board Of Regents, The University Of Texas System | Compositions and methods for making and using acyclic N-halamine-based biocidal polymeric materials and articles |
| US20070224161A1 (en) * | 2006-03-27 | 2007-09-27 | Board Of Regents, The University Of Texas System | Compositions and methods for making and using acyclic N-halamine-based biocidal polymeric materials and articles |
| US20090170984A1 (en) * | 2006-03-31 | 2009-07-02 | Basf Se | Composition containing polymers, colouring agents and stabilisers |
| US20100062270A1 (en) * | 2007-01-15 | 2010-03-11 | Ciba Corporation | Tinted clear coatings uv stabilized with 2-hydroxy phenyl triazine |
| US8211361B2 (en) | 2007-03-26 | 2012-07-03 | Board Of Regents, The University Of Texas System | N-halamine-based rechargeable biofilm-controlling tubular devices, method of making and using |
| US20080268189A1 (en) * | 2007-03-26 | 2008-10-30 | Board Of Regents, The University Of Texas System | N-Halamine-Based Rechargeable Biofilm-Controlling Tubular Devices, Method of Making and Using |
| US20090176092A1 (en) * | 2007-09-14 | 2009-07-09 | Fujikura Ltd. | Radiation-resistant resin composition and radiation-proof wire/cable |
| US7977414B2 (en) * | 2007-09-14 | 2011-07-12 | Fujikura Ltd. | Radiation-resistant resin composition and radiation-proof wire/cable |
| US20090074825A1 (en) * | 2007-09-19 | 2009-03-19 | Board Of Regents, The University Of Texas System | Colorants based n-halamines compositions and method of making and using |
| US8367823B2 (en) | 2007-09-19 | 2013-02-05 | Board Of Regents, The University Of Texas System | Colorants based N-halamines compositions and method of making and using |
| WO2010048436A2 (en) * | 2008-10-22 | 2010-04-29 | Microban Products Company | Antimicrobial polymer with anti-yellowing property |
| WO2010048436A3 (en) * | 2008-10-22 | 2010-07-08 | Microban Products Company | Antimicrobial polymer with anti-yellowing property |
| EP2805938A1 (en) | 2009-08-11 | 2014-11-26 | Basf Se | Bi- or tricyclic sterically hindered alkoxyamines and process for their preparation |
| WO2011018384A1 (en) | 2009-08-11 | 2011-02-17 | Basf Se | Bi - or tricyclic sterically hindered alkoxyamines and process for their preparation |
| US20120145236A1 (en) * | 2009-08-18 | 2012-06-14 | Basf Se | Uv-stabilized photovoltaic module |
| US9855682B2 (en) | 2011-06-10 | 2018-01-02 | Columbia Insurance Company | Methods of recycling synthetic turf, methods of using reclaimed synthetic turf, and products comprising same |
| US10172968B2 (en) | 2012-08-28 | 2019-01-08 | Sensor Electronic Technology, Inc. | Storage device including target UV illumination ranges |
| US10272168B2 (en) | 2012-08-28 | 2019-04-30 | Sensor Electronic Technology, Inc. | Storage device including target UV illumination ranges |
| US9750830B2 (en) | 2012-08-28 | 2017-09-05 | Sensor Electronic Technology, Inc. | Multi wave sterilization system |
| US9795699B2 (en) | 2012-08-28 | 2017-10-24 | Sensor Electronic Technology, Inc. | Storage device including target UV illumination ranges |
| US9724441B2 (en) | 2012-08-28 | 2017-08-08 | Sensor Electronic Technology, Inc. | Storage device including target UV illumination ranges |
| US9878061B2 (en) | 2012-08-28 | 2018-01-30 | Sensor Electronic Technology, Inc. | Ultraviolet system for disinfection |
| US9919068B2 (en) | 2012-08-28 | 2018-03-20 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| US9981051B2 (en) | 2012-08-28 | 2018-05-29 | Sensor Electronic Technology, Inc. | Ultraviolet gradient sterilization, disinfection, and storage system |
| US9707307B2 (en) | 2012-08-28 | 2017-07-18 | Sensor Electronic Technology, Inc. | Ultraviolet system for disinfection |
| US9179703B2 (en) | 2012-08-28 | 2015-11-10 | Sensor Electronic Technology, Inc. | Ultraviolet system for disinfection |
| US10849996B2 (en) | 2012-08-28 | 2020-12-01 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| US10688210B2 (en) | 2012-08-28 | 2020-06-23 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| US10383964B2 (en) | 2012-08-28 | 2019-08-20 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| US10441670B2 (en) | 2012-08-28 | 2019-10-15 | Sensor Electronic Technology, Inc. | Storage device including ultraviolet illumination |
| US10478515B2 (en) | 2012-08-28 | 2019-11-19 | Sensor Electronic Technology, Inc. | Multi wave sterilization system |
| US10517976B2 (en) | 2012-08-28 | 2019-12-31 | Sensor Electronic Technology, Inc. | Ultraviolet system for disinfection |
| US10576174B2 (en) | 2012-08-28 | 2020-03-03 | Sensor Electronic Technology, Inc. | Ultraviolet gradient sterilization, disinfection, and storage system |
| US10646603B2 (en) | 2012-08-28 | 2020-05-12 | Sensor Electronic Technology, Inc. | Multi wave sterilization system |
| US10577482B2 (en) | 2013-09-13 | 2020-03-03 | Dow Global Technologies Llc | Peroxide-crosslinkable compositions and processes for their manufacture |
| US20160208079A1 (en) * | 2013-09-13 | 2016-07-21 | Dow Global Technologies Llc | Peroxide-Crosslinkable Compositions and Processes for Their Manufacture |
| US9745447B2 (en) * | 2013-09-13 | 2017-08-29 | Dow Global Technologies Llc | Peroxide-crosslinkable compositions and processes for their manufacture |
| US10221299B2 (en) | 2013-09-13 | 2019-03-05 | Dow Global Technologies Llc | Peroxide-crosslinkable compositions and processes for their manufacture |
| WO2022207787A1 (en) * | 2021-04-01 | 2022-10-06 | Basf Se | Stabilizer mixture |
Also Published As
| Publication number | Publication date |
|---|---|
| AR038627A1 (en) | 2005-01-19 |
| MX238184B (en) | 2006-06-28 |
| JP2003261725A (en) | 2003-09-19 |
| CO5310577A1 (en) | 2003-08-29 |
| KR100902337B1 (en) | 2009-06-12 |
| AU2003200799B2 (en) | 2008-04-24 |
| US20040030009A1 (en) | 2004-02-12 |
| BR0300500A (en) | 2004-08-10 |
| MXPA03001899A (en) | 2003-09-11 |
| CN1294193C (en) | 2007-01-10 |
| TW200408668A (en) | 2004-06-01 |
| DK1342748T3 (en) | 2008-11-24 |
| KR20030072231A (en) | 2003-09-13 |
| EP1342748A1 (en) | 2003-09-10 |
| IL154706A0 (en) | 2003-10-31 |
| EP1342748B1 (en) | 2008-08-27 |
| BR0300500B1 (en) | 2013-03-05 |
| CA2420549A1 (en) | 2003-09-04 |
| DE60323174D1 (en) | 2008-10-09 |
| SA03230548B1 (en) | 2006-11-11 |
| IL154706A (en) | 2006-12-10 |
| CN1442446A (en) | 2003-09-17 |
| ES2312743T3 (en) | 2009-03-01 |
| ATE406406T1 (en) | 2008-09-15 |
| CA2420549C (en) | 2011-07-12 |
| ZA200301683B (en) | 2004-09-06 |
| TWI293973B (en) | 2008-03-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6878761B2 (en) | Synergistic combinations of UV absorbers for pigmented polyolefins | |
| US7595008B2 (en) | Stabilizer mixtures | |
| US6828364B2 (en) | Stabilizer mixtures | |
| US7375149B2 (en) | Stabilization of organic materials | |
| US11180476B2 (en) | Hindered amine light stabilizers | |
| US6747077B2 (en) | Stabilized metallocene polypropylene | |
| US10927236B2 (en) | Additive mixture | |
| US20050288510A1 (en) | Beta-nucleating, light stabilizing agents for polypropylene | |
| US20130216752A1 (en) | Oligomeric light stabilizers with a specific functionalization | |
| US20130053484A1 (en) | Sterically hindered amines | |
| EP2876126A1 (en) | A stabilizer mixture | |
| EP1338622A2 (en) | Stabilizer mixtures | |
| US20240052141A1 (en) | An organic material based shaped article | |
| US20240084104A1 (en) | Additive mixtures |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: CIBA SPECIALTY CHEMICALS CORP., NEW YORK Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:GUGUMUS, FRANCOIS;REEL/FRAME:014112/0632 Effective date: 20030116 |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| REMI | Maintenance fee reminder mailed | ||
| LAPS | Lapse for failure to pay maintenance fees | ||
| STCH | Information on status: patent discontinuation |
Free format text: PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 |
|
| FP | Lapsed due to failure to pay maintenance fee |
Effective date: 20170412 |