US20230053332A1 - Compositions and methods for inhibiting expression of mutant egfr gene - Google Patents
Compositions and methods for inhibiting expression of mutant egfr gene Download PDFInfo
- Publication number
- US20230053332A1 US20230053332A1 US17/733,552 US202217733552A US2023053332A1 US 20230053332 A1 US20230053332 A1 US 20230053332A1 US 202217733552 A US202217733552 A US 202217733552A US 2023053332 A1 US2023053332 A1 US 2023053332A1
- Authority
- US
- United States
- Prior art keywords
- dsrna
- deltaegfr
- cells
- sequence
- nucleotides
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 230000014509 gene expression Effects 0.000 title claims abstract description 139
- 238000000034 method Methods 0.000 title claims abstract description 74
- 230000002401 inhibitory effect Effects 0.000 title claims description 30
- 239000000203 mixture Substances 0.000 title description 139
- 101150039808 Egfr gene Proteins 0.000 title description 11
- 102000001301 EGF receptor Human genes 0.000 claims abstract description 68
- 108060006698 EGF receptor Proteins 0.000 claims abstract description 65
- 229920002477 rna polymer Polymers 0.000 claims abstract description 10
- 125000003729 nucleotide group Chemical group 0.000 claims description 135
- 239000002773 nucleotide Substances 0.000 claims description 122
- 206010028980 Neoplasm Diseases 0.000 claims description 104
- 108090000623 proteins and genes Proteins 0.000 claims description 101
- 108090001005 Interleukin-6 Proteins 0.000 claims description 69
- 230000000692 anti-sense effect Effects 0.000 claims description 66
- 102000004889 Interleukin-6 Human genes 0.000 claims description 65
- 230000000295 complement effect Effects 0.000 claims description 63
- 229940100601 interleukin-6 Drugs 0.000 claims description 56
- 108020004999 messenger RNA Proteins 0.000 claims description 49
- 238000011282 treatment Methods 0.000 claims description 47
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 40
- 239000013598 vector Substances 0.000 claims description 39
- 108091081021 Sense strand Proteins 0.000 claims description 31
- 239000008194 pharmaceutical composition Substances 0.000 claims description 31
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol group Chemical group [C@@H]1(CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)[C@H](C)CCCC(C)C HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 29
- 230000001404 mediated effect Effects 0.000 claims description 26
- 206010018338 Glioma Diseases 0.000 claims description 25
- 201000011510 cancer Diseases 0.000 claims description 25
- 208000032612 Glial tumor Diseases 0.000 claims description 22
- 208000035475 disorder Diseases 0.000 claims description 22
- 230000015556 catabolic process Effects 0.000 claims description 7
- 238000006731 degradation reaction Methods 0.000 claims description 7
- 206010003571 Astrocytoma Diseases 0.000 claims description 5
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical group OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 claims description 4
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 claims description 4
- 208000035657 Abasia Diseases 0.000 claims description 2
- 125000001921 locked nucleotide group Chemical group 0.000 claims description 2
- 208000030883 malignant astrocytoma Diseases 0.000 claims description 2
- PTMHPRAIXMAOOB-UHFFFAOYSA-L phosphoramidate Chemical compound NP([O-])([O-])=O PTMHPRAIXMAOOB-UHFFFAOYSA-L 0.000 claims description 2
- 102000009465 Growth Factor Receptors Human genes 0.000 claims 1
- 108010009202 Growth Factor Receptors Proteins 0.000 claims 1
- 229940116977 epidermal growth factor Drugs 0.000 claims 1
- ONKSSDKXDIVIHK-UHFFFAOYSA-N n,n-didecyldodecanamide Chemical group CCCCCCCCCCCC(=O)N(CCCCCCCCCC)CCCCCCCCCC ONKSSDKXDIVIHK-UHFFFAOYSA-N 0.000 claims 1
- 230000008685 targeting Effects 0.000 abstract description 52
- 210000004027 cell Anatomy 0.000 description 313
- 108020004459 Small interfering RNA Proteins 0.000 description 205
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 120
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 87
- 239000002502 liposome Substances 0.000 description 66
- -1 phosphinates Chemical class 0.000 description 56
- 238000009472 formulation Methods 0.000 description 52
- 230000000694 effects Effects 0.000 description 49
- 239000004094 surface-active agent Substances 0.000 description 46
- 239000003636 conditioned culture medium Substances 0.000 description 44
- 239000003814 drug Substances 0.000 description 39
- 150000002632 lipids Chemical class 0.000 description 39
- 102000005962 receptors Human genes 0.000 description 38
- 108020003175 receptors Proteins 0.000 description 38
- 150000007523 nucleic acids Chemical class 0.000 description 37
- 150000001875 compounds Chemical class 0.000 description 34
- 102000039446 nucleic acids Human genes 0.000 description 34
- 108020004707 nucleic acids Proteins 0.000 description 34
- 239000000243 solution Substances 0.000 description 33
- 239000000839 emulsion Substances 0.000 description 32
- 230000009467 reduction Effects 0.000 description 30
- 229940079593 drug Drugs 0.000 description 29
- 101150101999 IL6 gene Proteins 0.000 description 28
- 108091034117 Oligonucleotide Proteins 0.000 description 28
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 28
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 27
- 238000001262 western blot Methods 0.000 description 27
- 230000004614 tumor growth Effects 0.000 description 26
- 239000007924 injection Substances 0.000 description 25
- 238000002347 injection Methods 0.000 description 25
- 239000002245 particle Substances 0.000 description 25
- 238000001890 transfection Methods 0.000 description 25
- 238000000338 in vitro Methods 0.000 description 24
- 108060001084 Luciferase Proteins 0.000 description 23
- 239000003937 drug carrier Substances 0.000 description 23
- 239000002552 dosage form Substances 0.000 description 22
- 239000003446 ligand Substances 0.000 description 22
- 230000004913 activation Effects 0.000 description 21
- 239000003795 chemical substances by application Substances 0.000 description 21
- 239000004530 micro-emulsion Substances 0.000 description 21
- 229920001223 polyethylene glycol Polymers 0.000 description 21
- 230000028327 secretion Effects 0.000 description 21
- 210000004556 brain Anatomy 0.000 description 20
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 19
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 19
- 239000005090 green fluorescent protein Substances 0.000 description 19
- 239000013642 negative control Substances 0.000 description 19
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 18
- 108020004414 DNA Proteins 0.000 description 18
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 18
- 201000010099 disease Diseases 0.000 description 18
- 230000035515 penetration Effects 0.000 description 18
- 241000699660 Mus musculus Species 0.000 description 17
- 210000001130 astrocyte Anatomy 0.000 description 17
- 238000001727 in vivo Methods 0.000 description 17
- 238000011580 nude mouse model Methods 0.000 description 17
- 102000004169 proteins and genes Human genes 0.000 description 17
- 230000002829 reductive effect Effects 0.000 description 17
- 238000012360 testing method Methods 0.000 description 17
- 102000004890 Interleukin-8 Human genes 0.000 description 16
- 108090001007 Interleukin-8 Proteins 0.000 description 16
- 239000002202 Polyethylene glycol Substances 0.000 description 16
- 238000010521 absorption reaction Methods 0.000 description 16
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 16
- 239000003623 enhancer Substances 0.000 description 16
- XKTZWUACRZHVAN-VADRZIEHSA-N interleukin-8 Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@@H](NC(C)=O)CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N1[C@H](CCC1)C(=O)N1[C@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC(O)=CC=1)C(=O)N[C@H](CO)C(=O)N1[C@H](CCC1)C(N)=O)C1=CC=CC=C1 XKTZWUACRZHVAN-VADRZIEHSA-N 0.000 description 16
- 229940096397 interleukin-8 Drugs 0.000 description 16
- 238000002360 preparation method Methods 0.000 description 16
- 229920001817 Agar Polymers 0.000 description 15
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 15
- 239000005089 Luciferase Substances 0.000 description 15
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 15
- 239000008272 agar Substances 0.000 description 15
- 229940023476 agar Drugs 0.000 description 15
- 235000010419 agar Nutrition 0.000 description 15
- 235000014113 dietary fatty acids Nutrition 0.000 description 15
- 229930195729 fatty acid Natural products 0.000 description 15
- 239000000194 fatty acid Substances 0.000 description 15
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 15
- 239000012528 membrane Substances 0.000 description 15
- 239000012071 phase Substances 0.000 description 15
- 235000018102 proteins Nutrition 0.000 description 15
- 241000124008 Mammalia Species 0.000 description 14
- 241000699670 Mus sp. Species 0.000 description 14
- 208000037273 Pathologic Processes Diseases 0.000 description 14
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 13
- 230000008901 benefit Effects 0.000 description 13
- 235000012000 cholesterol Nutrition 0.000 description 13
- 238000003197 gene knockdown Methods 0.000 description 13
- 230000009054 pathological process Effects 0.000 description 13
- 239000007787 solid Substances 0.000 description 13
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 12
- 239000002253 acid Substances 0.000 description 12
- 230000009368 gene silencing by RNA Effects 0.000 description 12
- 238000001802 infusion Methods 0.000 description 12
- 238000001990 intravenous administration Methods 0.000 description 12
- 239000003921 oil Substances 0.000 description 12
- 102000040430 polynucleotide Human genes 0.000 description 12
- 108091033319 polynucleotide Proteins 0.000 description 12
- 239000002157 polynucleotide Substances 0.000 description 12
- 238000012552 review Methods 0.000 description 12
- 210000003491 skin Anatomy 0.000 description 12
- 238000002965 ELISA Methods 0.000 description 11
- 108700021358 erbB-1 Genes Proteins 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 11
- 150000004665 fatty acids Chemical class 0.000 description 11
- 229940126585 therapeutic drug Drugs 0.000 description 11
- 102000004127 Cytokines Human genes 0.000 description 10
- 108090000695 Cytokines Proteins 0.000 description 10
- 239000003833 bile salt Substances 0.000 description 10
- 239000000872 buffer Substances 0.000 description 10
- 239000002738 chelating agent Substances 0.000 description 10
- 230000008030 elimination Effects 0.000 description 10
- 238000003379 elimination reaction Methods 0.000 description 10
- 239000002609 medium Substances 0.000 description 10
- 230000004048 modification Effects 0.000 description 10
- 238000012986 modification Methods 0.000 description 10
- 150000003839 salts Chemical class 0.000 description 10
- 235000000346 sugar Nutrition 0.000 description 10
- 239000000725 suspension Substances 0.000 description 10
- 239000013603 viral vector Substances 0.000 description 10
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 9
- 241001465754 Metazoa Species 0.000 description 9
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 9
- 125000000217 alkyl group Chemical group 0.000 description 9
- 239000002246 antineoplastic agent Substances 0.000 description 9
- 238000003556 assay Methods 0.000 description 9
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 229940127089 cytotoxic agent Drugs 0.000 description 9
- 239000003995 emulsifying agent Substances 0.000 description 9
- 229960002949 fluorouracil Drugs 0.000 description 9
- 230000012010 growth Effects 0.000 description 9
- 230000005764 inhibitory process Effects 0.000 description 9
- 239000007788 liquid Substances 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 235000019198 oils Nutrition 0.000 description 9
- 230000001105 regulatory effect Effects 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- 229930024421 Adenine Natural products 0.000 description 8
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 8
- 102000009024 Epidermal Growth Factor Human genes 0.000 description 8
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 8
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 8
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 8
- 229960000643 adenine Drugs 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 8
- 125000002091 cationic group Chemical group 0.000 description 8
- 229940104302 cytosine Drugs 0.000 description 8
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 8
- 231100000673 dose–response relationship Toxicity 0.000 description 8
- 150000002148 esters Chemical class 0.000 description 8
- 230000001965 increasing effect Effects 0.000 description 8
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 8
- 239000000546 pharmaceutical excipient Substances 0.000 description 8
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 230000035755 proliferation Effects 0.000 description 8
- 238000011160 research Methods 0.000 description 8
- 239000007929 subcutaneous injection Substances 0.000 description 8
- 239000006228 supernatant Substances 0.000 description 8
- 229940035893 uracil Drugs 0.000 description 8
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 7
- 241000699666 Mus <mouse, genus> Species 0.000 description 7
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 7
- 108700019146 Transgenes Proteins 0.000 description 7
- 239000007864 aqueous solution Substances 0.000 description 7
- 229940093761 bile salts Drugs 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- MWRBNPKJOOWZPW-CLFAGFIQSA-N dioleoyl phosphatidylethanolamine Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(COP(O)(=O)OCCN)OC(=O)CCCCCCC\C=C/CCCCCCCC MWRBNPKJOOWZPW-CLFAGFIQSA-N 0.000 description 7
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 7
- 239000012091 fetal bovine serum Substances 0.000 description 7
- 208000005017 glioblastoma Diseases 0.000 description 7
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 7
- 239000002953 phosphate buffered saline Substances 0.000 description 7
- 238000010254 subcutaneous injection Methods 0.000 description 7
- 238000003786 synthesis reaction Methods 0.000 description 7
- 241000701161 unidentified adenovirus Species 0.000 description 7
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 6
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 101001076408 Homo sapiens Interleukin-6 Proteins 0.000 description 6
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 6
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 6
- 101100383168 Rattus norvegicus Cdkn2b gene Proteins 0.000 description 6
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 6
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 6
- 239000003963 antioxidant agent Substances 0.000 description 6
- 235000006708 antioxidants Nutrition 0.000 description 6
- YZXBAPSDXZZRGB-DOFZRALJSA-N arachidonic acid Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O YZXBAPSDXZZRGB-DOFZRALJSA-N 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- 230000004663 cell proliferation Effects 0.000 description 6
- 230000001413 cellular effect Effects 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 230000005757 colony formation Effects 0.000 description 6
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 6
- KXGVEGMKQFWNSR-UHFFFAOYSA-N deoxycholic acid Natural products C1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)C(O)C2 KXGVEGMKQFWNSR-UHFFFAOYSA-N 0.000 description 6
- 239000003085 diluting agent Substances 0.000 description 6
- 239000000499 gel Substances 0.000 description 6
- 238000001415 gene therapy Methods 0.000 description 6
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 6
- 238000007917 intracranial administration Methods 0.000 description 6
- 229960000485 methotrexate Drugs 0.000 description 6
- 239000002736 nonionic surfactant Substances 0.000 description 6
- 239000002777 nucleoside Substances 0.000 description 6
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 6
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 6
- 150000003904 phospholipids Chemical class 0.000 description 6
- 239000013612 plasmid Substances 0.000 description 6
- 239000003755 preservative agent Substances 0.000 description 6
- 239000011541 reaction mixture Substances 0.000 description 6
- 229940083542 sodium Drugs 0.000 description 6
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 6
- 230000001629 suppression Effects 0.000 description 6
- 229940124597 therapeutic agent Drugs 0.000 description 6
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 6
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-dimethylaminopyridine Substances CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 5
- OPIFSICVWOWJMJ-AEOCFKNESA-N 5-bromo-4-chloro-3-indolyl beta-D-galactoside Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1OC1=CNC2=CC=C(Br)C(Cl)=C12 OPIFSICVWOWJMJ-AEOCFKNESA-N 0.000 description 5
- 239000013607 AAV vector Substances 0.000 description 5
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical class OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 5
- 108010010803 Gelatin Proteins 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 5
- 102100026019 Interleukin-6 Human genes 0.000 description 5
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 5
- 229930182816 L-glutamine Natural products 0.000 description 5
- 108091093037 Peptide nucleic acid Proteins 0.000 description 5
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical class CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 5
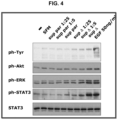
- 108010017324 STAT3 Transcription Factor Proteins 0.000 description 5
- 102100024040 Signal transducer and activator of transcription 3 Human genes 0.000 description 5
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 5
- 229920002472 Starch Polymers 0.000 description 5
- 235000021355 Stearic acid Nutrition 0.000 description 5
- 238000004113 cell culture Methods 0.000 description 5
- 230000004700 cellular uptake Effects 0.000 description 5
- 238000003776 cleavage reaction Methods 0.000 description 5
- 229960003724 dimyristoylphosphatidylcholine Drugs 0.000 description 5
- 238000009826 distribution Methods 0.000 description 5
- 235000019439 ethyl acetate Nutrition 0.000 description 5
- 229940093499 ethyl acetate Drugs 0.000 description 5
- 229920000159 gelatin Polymers 0.000 description 5
- 235000019322 gelatine Nutrition 0.000 description 5
- 235000011852 gelatine desserts Nutrition 0.000 description 5
- 210000003734 kidney Anatomy 0.000 description 5
- 239000010410 layer Substances 0.000 description 5
- 238000011068 loading method Methods 0.000 description 5
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 5
- 239000002105 nanoparticle Substances 0.000 description 5
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 5
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 5
- 229910052698 phosphorus Inorganic materials 0.000 description 5
- 230000002062 proliferating effect Effects 0.000 description 5
- 238000011002 quantification Methods 0.000 description 5
- 230000007017 scission Effects 0.000 description 5
- 239000003381 stabilizer Substances 0.000 description 5
- 238000010186 staining Methods 0.000 description 5
- 235000019698 starch Nutrition 0.000 description 5
- 239000008117 stearic acid Substances 0.000 description 5
- 238000003756 stirring Methods 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 230000009885 systemic effect Effects 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- OQQOAWVKVDAJOI-UHFFFAOYSA-N (2-dodecanoyloxy-3-hydroxypropyl) dodecanoate Chemical compound CCCCCCCCCCCC(=O)OCC(CO)OC(=O)CCCCCCCCCCC OQQOAWVKVDAJOI-UHFFFAOYSA-N 0.000 description 4
- BHQCQFFYRZLCQQ-UHFFFAOYSA-N (3alpha,5alpha,7alpha,12alpha)-3,7,12-trihydroxy-cholan-24-oic acid Natural products OC1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)C(O)C2 BHQCQFFYRZLCQQ-UHFFFAOYSA-N 0.000 description 4
- RUDATBOHQWOJDD-UHFFFAOYSA-N (3beta,5beta,7alpha)-3,7-Dihydroxycholan-24-oic acid Natural products OC1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)CC2 RUDATBOHQWOJDD-UHFFFAOYSA-N 0.000 description 4
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 4
- CITHEXJVPOWHKC-UUWRZZSWSA-N 1,2-di-O-myristoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCC CITHEXJVPOWHKC-UUWRZZSWSA-N 0.000 description 4
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 4
- LVNGJLRDBYCPGB-UHFFFAOYSA-N 1,2-distearoylphosphatidylethanolamine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(COP([O-])(=O)OCC[NH3+])OC(=O)CCCCCCCCCCCCCCCCC LVNGJLRDBYCPGB-UHFFFAOYSA-N 0.000 description 4
- RZRNAYUHWVFMIP-KTKRTIGZSA-N 1-oleoylglycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-KTKRTIGZSA-N 0.000 description 4
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 4
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 4
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 208000003174 Brain Neoplasms Diseases 0.000 description 4
- 239000004380 Cholic acid Substances 0.000 description 4
- 206010009944 Colon cancer Diseases 0.000 description 4
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 4
- 108010092160 Dactinomycin Proteins 0.000 description 4
- 108700024394 Exon Proteins 0.000 description 4
- 102000014150 Interferons Human genes 0.000 description 4
- 108010050904 Interferons Proteins 0.000 description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 4
- 239000005639 Lauric acid Substances 0.000 description 4
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- 108091000080 Phosphotransferase Proteins 0.000 description 4
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 4
- 238000002679 ablation Methods 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 4
- 150000007513 acids Chemical class 0.000 description 4
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 4
- 150000001408 amides Chemical group 0.000 description 4
- 238000010171 animal model Methods 0.000 description 4
- 125000000129 anionic group Chemical group 0.000 description 4
- 239000008346 aqueous phase Substances 0.000 description 4
- 239000011230 binding agent Substances 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 210000003169 central nervous system Anatomy 0.000 description 4
- 229940106189 ceramide Drugs 0.000 description 4
- 210000003679 cervix uteri Anatomy 0.000 description 4
- 235000019416 cholic acid Nutrition 0.000 description 4
- BHQCQFFYRZLCQQ-OELDTZBJSA-N cholic acid Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 BHQCQFFYRZLCQQ-OELDTZBJSA-N 0.000 description 4
- 229960002471 cholic acid Drugs 0.000 description 4
- 230000004087 circulation Effects 0.000 description 4
- 238000004440 column chromatography Methods 0.000 description 4
- 239000004064 cosurfactant Substances 0.000 description 4
- 210000004748 cultured cell Anatomy 0.000 description 4
- 229960000640 dactinomycin Drugs 0.000 description 4
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 4
- KXGVEGMKQFWNSR-LLQZFEROSA-N deoxycholic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 KXGVEGMKQFWNSR-LLQZFEROSA-N 0.000 description 4
- 229960003964 deoxycholic acid Drugs 0.000 description 4
- 239000000975 dye Substances 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 4
- 229960000961 floxuridine Drugs 0.000 description 4
- 239000008273 gelatin Substances 0.000 description 4
- 238000009396 hybridization Methods 0.000 description 4
- 230000001939 inductive effect Effects 0.000 description 4
- 229940079322 interferon Drugs 0.000 description 4
- 238000007913 intrathecal administration Methods 0.000 description 4
- 238000007914 intraventricular administration Methods 0.000 description 4
- 239000008101 lactose Substances 0.000 description 4
- 229960001375 lactose Drugs 0.000 description 4
- 210000004185 liver Anatomy 0.000 description 4
- 239000000314 lubricant Substances 0.000 description 4
- 235000019359 magnesium stearate Nutrition 0.000 description 4
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 4
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 4
- 201000010225 mixed cell type cancer Diseases 0.000 description 4
- 208000029638 mixed neoplasm Diseases 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 125000003835 nucleoside group Chemical group 0.000 description 4
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- 239000012044 organic layer Substances 0.000 description 4
- 230000037361 pathway Effects 0.000 description 4
- 125000004437 phosphorous atom Chemical group 0.000 description 4
- 102000020233 phosphotransferase Human genes 0.000 description 4
- 229940068917 polyethylene glycols Drugs 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 239000011148 porous material Substances 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 108090000765 processed proteins & peptides Proteins 0.000 description 4
- 239000003531 protein hydrolysate Substances 0.000 description 4
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 4
- 238000012216 screening Methods 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 229910052708 sodium Inorganic materials 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 230000000638 stimulation Effects 0.000 description 4
- 239000000454 talc Substances 0.000 description 4
- 235000012222 talc Nutrition 0.000 description 4
- 229910052623 talc Inorganic materials 0.000 description 4
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 4
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 4
- 230000000699 topical effect Effects 0.000 description 4
- 238000013518 transcription Methods 0.000 description 4
- 230000035897 transcription Effects 0.000 description 4
- 239000003981 vehicle Substances 0.000 description 4
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 3
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 description 3
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 3
- AXTGDCSMTYGJND-UHFFFAOYSA-N 1-dodecylazepan-2-one Chemical compound CCCCCCCCCCCCN1CCCCCC1=O AXTGDCSMTYGJND-UHFFFAOYSA-N 0.000 description 3
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 3
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 3
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 3
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- 108010085238 Actins Proteins 0.000 description 3
- 102000007469 Actins Human genes 0.000 description 3
- 208000023275 Autoimmune disease Diseases 0.000 description 3
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 3
- 239000005632 Capric acid (CAS 334-48-5) Substances 0.000 description 3
- 239000005635 Caprylic acid (CAS 124-07-2) Substances 0.000 description 3
- 108090000565 Capsid Proteins Proteins 0.000 description 3
- 201000009030 Carcinoma Diseases 0.000 description 3
- 102100023321 Ceruloplasmin Human genes 0.000 description 3
- 229920002261 Corn starch Polymers 0.000 description 3
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 3
- 241000701022 Cytomegalovirus Species 0.000 description 3
- 229920002307 Dextran Polymers 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- 241001529936 Murinae Species 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 3
- 239000005642 Oleic acid Substances 0.000 description 3
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 3
- 235000021314 Palmitic acid Nutrition 0.000 description 3
- 229930182555 Penicillin Natural products 0.000 description 3
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- 239000012083 RIPA buffer Substances 0.000 description 3
- 108010071390 Serum Albumin Proteins 0.000 description 3
- 102000007562 Serum Albumin Human genes 0.000 description 3
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 3
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 3
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 3
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 230000002776 aggregation Effects 0.000 description 3
- 238000004220 aggregation Methods 0.000 description 3
- 150000001298 alcohols Chemical class 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 150000008051 alkyl sulfates Chemical class 0.000 description 3
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 3
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 229940114079 arachidonic acid Drugs 0.000 description 3
- 235000021342 arachidonic acid Nutrition 0.000 description 3
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 3
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 3
- 239000003613 bile acid Substances 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- 210000000234 capsid Anatomy 0.000 description 3
- 239000001768 carboxy methyl cellulose Substances 0.000 description 3
- 210000000170 cell membrane Anatomy 0.000 description 3
- RUDATBOHQWOJDD-BSWAIDMHSA-N chenodeoxycholic acid Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)CC1 RUDATBOHQWOJDD-BSWAIDMHSA-N 0.000 description 3
- 229960001091 chenodeoxycholic acid Drugs 0.000 description 3
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 3
- 229960004316 cisplatin Drugs 0.000 description 3
- 230000021615 conjugation Effects 0.000 description 3
- 238000013270 controlled release Methods 0.000 description 3
- 239000012043 crude product Substances 0.000 description 3
- 229960004397 cyclophosphamide Drugs 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 229960002086 dextran Drugs 0.000 description 3
- 238000003745 diagnosis Methods 0.000 description 3
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 3
- 108010087914 epidermal growth factor receptor VIII Proteins 0.000 description 3
- 229940071106 ethylenediaminetetraacetate Drugs 0.000 description 3
- 150000002191 fatty alcohols Chemical class 0.000 description 3
- 239000000796 flavoring agent Substances 0.000 description 3
- 238000000684 flow cytometry Methods 0.000 description 3
- 235000013355 food flavoring agent Nutrition 0.000 description 3
- QPJBWNIQKHGLAU-IQZHVAEDSA-N ganglioside GM1 Chemical compound O[C@@H]1[C@@H](O)[C@H](OC[C@H](NC(=O)CCCCCCCCCCCCCCCCC)[C@H](O)\C=C\CCCCCCCCCCCCC)O[C@H](CO)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@]2(O[C@H]([C@H](NC(C)=O)[C@@H](O)C2)[C@H](O)[C@H](O)CO)C(O)=O)[C@@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O3)O)[C@@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](CO)O1 QPJBWNIQKHGLAU-IQZHVAEDSA-N 0.000 description 3
- 238000001476 gene delivery Methods 0.000 description 3
- RZRNAYUHWVFMIP-HXUWFJFHSA-N glycerol monolinoleate Natural products CCCCCCCCC=CCCCCCCCC(=O)OC[C@H](O)CO RZRNAYUHWVFMIP-HXUWFJFHSA-N 0.000 description 3
- 125000003976 glyceryl group Chemical group [H]C([*])([H])C(O[H])([H])C(O[H])([H])[H] 0.000 description 3
- 238000007490 hematoxylin and eosin (H&E) staining Methods 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 239000003112 inhibitor Substances 0.000 description 3
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 3
- 229960004488 linolenic acid Drugs 0.000 description 3
- KQQKGWQCNNTQJW-UHFFFAOYSA-N linolenic acid Natural products CC=CCCC=CCC=CCCCCCCCC(O)=O KQQKGWQCNNTQJW-UHFFFAOYSA-N 0.000 description 3
- 239000006166 lysate Substances 0.000 description 3
- 206010061289 metastatic neoplasm Diseases 0.000 description 3
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 3
- 239000000178 monomer Substances 0.000 description 3
- 229940074096 monoolein Drugs 0.000 description 3
- 210000004877 mucosa Anatomy 0.000 description 3
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 3
- 230000001613 neoplastic effect Effects 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- 239000007764 o/w emulsion Substances 0.000 description 3
- 229960002446 octanoic acid Drugs 0.000 description 3
- 210000001672 ovary Anatomy 0.000 description 3
- 230000002018 overexpression Effects 0.000 description 3
- 238000007911 parenteral administration Methods 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 230000001575 pathological effect Effects 0.000 description 3
- 229940049954 penicillin Drugs 0.000 description 3
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 3
- 229960002340 pentostatin Drugs 0.000 description 3
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 3
- 239000000825 pharmaceutical preparation Substances 0.000 description 3
- 230000026731 phosphorylation Effects 0.000 description 3
- 238000006366 phosphorylation reaction Methods 0.000 description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 3
- 239000013641 positive control Substances 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 3
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 3
- 238000001959 radiotherapy Methods 0.000 description 3
- 230000001177 retroviral effect Effects 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 239000012679 serum free medium Substances 0.000 description 3
- 239000001632 sodium acetate Substances 0.000 description 3
- 235000017281 sodium acetate Nutrition 0.000 description 3
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 3
- 229910000162 sodium phosphate Inorganic materials 0.000 description 3
- IWQPOPSAISBUAH-VOVMJQHHSA-M sodium;2-[[(2z)-2-[(3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetyl-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methylheptanoyl]amino]ethanesulfonate Chemical compound [Na+].C1C[C@@H](O)[C@@H](C)[C@@H]2CC[C@]3(C)[C@@]4(C)C[C@H](C(C)=O)/C(=C(C(=O)NCCS([O-])(=O)=O)/CCCC(C)C)[C@@H]4C[C@@H](O)[C@H]3[C@]21C IWQPOPSAISBUAH-VOVMJQHHSA-M 0.000 description 3
- 229960005322 streptomycin Drugs 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 238000013268 sustained release Methods 0.000 description 3
- 239000012730 sustained-release form Substances 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 229960001603 tamoxifen Drugs 0.000 description 3
- 229960004964 temozolomide Drugs 0.000 description 3
- TUNFSRHWOTWDNC-HKGQFRNVSA-N tetradecanoic acid Chemical compound CCCCCCCCCCCCC[14C](O)=O TUNFSRHWOTWDNC-HKGQFRNVSA-N 0.000 description 3
- 239000002562 thickening agent Substances 0.000 description 3
- 229940104230 thymidine Drugs 0.000 description 3
- 238000011200 topical administration Methods 0.000 description 3
- 231100000588 tumorigenic Toxicity 0.000 description 3
- 230000000381 tumorigenic effect Effects 0.000 description 3
- 230000003827 upregulation Effects 0.000 description 3
- 239000007762 w/o emulsion Substances 0.000 description 3
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 2
- QGVQZRDQPDLHHV-DPAQBDIFSA-N (3s,8s,9s,10r,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthrene-3-thiol Chemical compound C1C=C2C[C@@H](S)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 QGVQZRDQPDLHHV-DPAQBDIFSA-N 0.000 description 2
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical group OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical compound CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 description 2
- JUDOLRSMWHVKGX-UHFFFAOYSA-N 1,1-dioxo-1$l^{6},2-benzodithiol-3-one Chemical compound C1=CC=C2C(=O)SS(=O)(=O)C2=C1 JUDOLRSMWHVKGX-UHFFFAOYSA-N 0.000 description 2
- SLKDGVPOSSLUAI-PGUFJCEWSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine zwitterion Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OCCN)OC(=O)CCCCCCCCCCCCCCC SLKDGVPOSSLUAI-PGUFJCEWSA-N 0.000 description 2
- SNKAWJBJQDLSFF-NVKMUCNASA-N 1,2-dioleoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCC\C=C/CCCCCCCC SNKAWJBJQDLSFF-NVKMUCNASA-N 0.000 description 2
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 description 2
- BIABMEZBCHDPBV-MPQUPPDSSA-N 1,2-palmitoyl-sn-glycero-3-phospho-(1'-sn-glycerol) Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@@H](O)CO)OC(=O)CCCCCCCCCCCCCCC BIABMEZBCHDPBV-MPQUPPDSSA-N 0.000 description 2
- BGRWYRAHAFMIBJ-UHFFFAOYSA-N 1,3-di(propan-2-yl)urea Chemical compound CC(C)NC(=O)NC(C)C BGRWYRAHAFMIBJ-UHFFFAOYSA-N 0.000 description 2
- LDVVTQMJQSCDMK-UHFFFAOYSA-N 1,3-dihydroxypropan-2-yl formate Chemical compound OCC(CO)OC=O LDVVTQMJQSCDMK-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- VSNHCAURESNICA-NJFSPNSNSA-N 1-oxidanylurea Chemical compound N[14C](=O)NO VSNHCAURESNICA-NJFSPNSNSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- DBPWSSGDRRHUNT-CEGNMAFCSA-N 17α-hydroxyprogesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 DBPWSSGDRRHUNT-CEGNMAFCSA-N 0.000 description 2
- SXUXMRMBWZCMEN-UHFFFAOYSA-N 2'-O-methyl uridine Natural products COC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 SXUXMRMBWZCMEN-UHFFFAOYSA-N 0.000 description 2
- LDGWQMRUWMSZIU-LQDDAWAPSA-M 2,3-bis[(z)-octadec-9-enoxy]propyl-trimethylazanium;chloride Chemical compound [Cl-].CCCCCCCC\C=C/CCCCCCCCOCC(C[N+](C)(C)C)OCCCCCCCC\C=C/CCCCCCCC LDGWQMRUWMSZIU-LQDDAWAPSA-M 0.000 description 2
- KSXTUUUQYQYKCR-LQDDAWAPSA-M 2,3-bis[[(z)-octadec-9-enoyl]oxy]propyl-trimethylazanium;chloride Chemical compound [Cl-].CCCCCCCC\C=C/CCCCCCCC(=O)OCC(C[N+](C)(C)C)OC(=O)CCCCCCC\C=C/CCCCCCCC KSXTUUUQYQYKCR-LQDDAWAPSA-M 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- PBUUPFTVAPUWDE-UGZDLDLSSA-N 2-[[(2S,4S)-2-[bis(2-chloroethyl)amino]-2-oxo-1,3,2lambda5-oxazaphosphinan-4-yl]sulfanyl]ethanesulfonic acid Chemical compound OS(=O)(=O)CCS[C@H]1CCO[P@](=O)(N(CCCl)CCCl)N1 PBUUPFTVAPUWDE-UGZDLDLSSA-N 0.000 description 2
- FZWGECJQACGGTI-UHFFFAOYSA-N 2-amino-7-methyl-1,7-dihydro-6H-purin-6-one Chemical compound NC1=NC(O)=C2N(C)C=NC2=N1 FZWGECJQACGGTI-UHFFFAOYSA-N 0.000 description 2
- XIFVTSIIYVGRHJ-UHFFFAOYSA-N 2-n,2-n,4-n,4-n,6-n-pentamethyl-1,3,5-triazine-2,4,6-triamine Chemical compound CNC1=NC(N(C)C)=NC(N(C)C)=N1 XIFVTSIIYVGRHJ-UHFFFAOYSA-N 0.000 description 2
- OHXPGWPVLFPUSM-KLRNGDHRSA-N 3,7,12-trioxo-5beta-cholanic acid Chemical compound C1CC(=O)C[C@H]2CC(=O)[C@H]3[C@@H]4CC[C@H]([C@@H](CCC(O)=O)C)[C@@]4(C)C(=O)C[C@@H]3[C@]21C OHXPGWPVLFPUSM-KLRNGDHRSA-N 0.000 description 2
- WOKDXPHSIQRTJF-UHFFFAOYSA-N 3-[3-[3-[3-[3-[3-[3-[3-[3-(2,3-dihydroxypropoxy)-2-hydroxypropoxy]-2-hydroxypropoxy]-2-hydroxypropoxy]-2-hydroxypropoxy]-2-hydroxypropoxy]-2-hydroxypropoxy]-2-hydroxypropoxy]-2-hydroxypropoxy]propane-1,2-diol Chemical compound OCC(O)COCC(O)COCC(O)COCC(O)COCC(O)COCC(O)COCC(O)COCC(O)COCC(O)COCC(O)CO WOKDXPHSIQRTJF-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 2
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 2
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 2
- OVONXEQGWXGFJD-UHFFFAOYSA-N 4-sulfanylidene-1h-pyrimidin-2-one Chemical compound SC=1C=CNC(=O)N=1 OVONXEQGWXGFJD-UHFFFAOYSA-N 0.000 description 2
- RYVNIFSIEDRLSJ-UHFFFAOYSA-N 5-(hydroxymethyl)cytosine Chemical compound NC=1NC(=O)N=CC=1CO RYVNIFSIEDRLSJ-UHFFFAOYSA-N 0.000 description 2
- NMUSYJAQQFHJEW-UHFFFAOYSA-N 5-Azacytidine Natural products O=C1N=C(N)N=CN1C1C(O)C(O)C(CO)O1 NMUSYJAQQFHJEW-UHFFFAOYSA-N 0.000 description 2
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 2
- LRSASMSXMSNRBT-UHFFFAOYSA-N 5-methylcytosine Chemical compound CC1=CNC(=O)N=C1N LRSASMSXMSNRBT-UHFFFAOYSA-N 0.000 description 2
- UJBCLAXPPIDQEE-UHFFFAOYSA-N 5-prop-1-ynyl-1h-pyrimidine-2,4-dione Chemical compound CC#CC1=CNC(=O)NC1=O UJBCLAXPPIDQEE-UHFFFAOYSA-N 0.000 description 2
- HCGHYQLFMPXSDU-UHFFFAOYSA-N 7-methyladenine Chemical compound C1=NC(N)=C2N(C)C=NC2=N1 HCGHYQLFMPXSDU-UHFFFAOYSA-N 0.000 description 2
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 2
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical compound O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 2
- 229920000856 Amylose Polymers 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 108010006654 Bleomycin Proteins 0.000 description 2
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 2
- 229940045513 CTLA4 antagonist Drugs 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 2
- 229930105110 Cyclosporin A Natural products 0.000 description 2
- 108010036949 Cyclosporine Proteins 0.000 description 2
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 2
- 102000016911 Deoxyribonucleases Human genes 0.000 description 2
- 108010053770 Deoxyribonucleases Proteins 0.000 description 2
- 241000702421 Dependoparvovirus Species 0.000 description 2
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 2
- 201000010915 Glioblastoma multiforme Diseases 0.000 description 2
- 108010035713 Glycodeoxycholic Acid Proteins 0.000 description 2
- WVULKSPCQVQLCU-UHFFFAOYSA-N Glycodeoxycholic acid Natural products C1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(=O)NCC(O)=O)C)C1(C)C(O)C2 WVULKSPCQVQLCU-UHFFFAOYSA-N 0.000 description 2
- 229930186217 Glycolipid Natural products 0.000 description 2
- NYHBQMYGNKIUIF-UUOKFMHZSA-N Guanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NYHBQMYGNKIUIF-UUOKFMHZSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 2
- 101000851181 Homo sapiens Epidermal growth factor receptor Proteins 0.000 description 2
- 241000702617 Human parvovirus B19 Species 0.000 description 2
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 2
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 2
- 229930010555 Inosine Natural products 0.000 description 2
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 2
- 102100034349 Integrase Human genes 0.000 description 2
- 101710203526 Integrase Proteins 0.000 description 2
- 108010064593 Intercellular Adhesion Molecule-1 Proteins 0.000 description 2
- 102000015271 Intercellular Adhesion Molecule-1 Human genes 0.000 description 2
- 108010074328 Interferon-gamma Proteins 0.000 description 2
- 102000000589 Interleukin-1 Human genes 0.000 description 2
- 108010002352 Interleukin-1 Proteins 0.000 description 2
- 241000713666 Lentivirus Species 0.000 description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 2
- 240000007472 Leucaena leucocephala Species 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 101710163270 Nuclease Proteins 0.000 description 2
- REYJJPSVUYRZGE-UHFFFAOYSA-N Octadecylamine Chemical compound CCCCCCCCCCCCCCCCCCN REYJJPSVUYRZGE-UHFFFAOYSA-N 0.000 description 2
- 108700020796 Oncogene Proteins 0.000 description 2
- 239000012124 Opti-MEM Substances 0.000 description 2
- 229930012538 Paclitaxel Natural products 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- MEFKEPWMEQBLKI-AIRLBKTGSA-O S-adenosyl-L-methionine Chemical compound O[C@@H]1[C@H](O)[C@@H](C[S+](CC[C@H]([NH3+])C([O-])=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 MEFKEPWMEQBLKI-AIRLBKTGSA-O 0.000 description 2
- 238000012300 Sequence Analysis Methods 0.000 description 2
- 108091027568 Single-stranded nucleotide Proteins 0.000 description 2
- 229920002125 Sokalan® Polymers 0.000 description 2
- WBWWGRHZICKQGZ-UHFFFAOYSA-N Taurocholic acid Natural products OC1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(=O)NCCS(O)(=O)=O)C)C1(C)C(O)C2 WBWWGRHZICKQGZ-UHFFFAOYSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 241000906446 Theraps Species 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- 108010000134 Vascular Cell Adhesion Molecule-1 Proteins 0.000 description 2
- 102100023543 Vascular cell adhesion protein 1 Human genes 0.000 description 2
- 241000711975 Vesicular stomatitis virus Species 0.000 description 2
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 2
- DSNRWDQKZIEDDB-GCMPNPAFSA-N [(2r)-3-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-2-[(z)-octadec-9-enoyl]oxypropyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C/CCCCCCCC DSNRWDQKZIEDDB-GCMPNPAFSA-N 0.000 description 2
- RLXCFCYWFYXTON-JTTSDREOSA-N [(3S,8S,9S,10R,13S,14S,17R)-3-hydroxy-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-16-yl] N-hexylcarbamate Chemical group C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC(OC(=O)NCCCCCC)[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 RLXCFCYWFYXTON-JTTSDREOSA-N 0.000 description 2
- NONFBHXKNNVFMO-UHFFFAOYSA-N [2-aminoethoxy(tetradecanoyloxy)phosphoryl] tetradecanoate Chemical compound CCCCCCCCCCCCCC(=O)OP(=O)(OCCN)OC(=O)CCCCCCCCCCCCC NONFBHXKNNVFMO-UHFFFAOYSA-N 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- XVIYCJDWYLJQBG-UHFFFAOYSA-N acetic acid;adamantane Chemical compound CC(O)=O.C1C(C2)CC3CC1CC2C3 XVIYCJDWYLJQBG-UHFFFAOYSA-N 0.000 description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 125000000304 alkynyl group Chemical group 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 229960000473 altretamine Drugs 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 2
- 229960001220 amsacrine Drugs 0.000 description 2
- 206010002224 anaplastic astrocytoma Diseases 0.000 description 2
- 239000003945 anionic surfactant Substances 0.000 description 2
- 239000007900 aqueous suspension Substances 0.000 description 2
- 238000011717 athymic nude mouse Methods 0.000 description 2
- 239000012752 auxiliary agent Substances 0.000 description 2
- 229960002756 azacitidine Drugs 0.000 description 2
- 210000000941 bile Anatomy 0.000 description 2
- 230000027455 binding Effects 0.000 description 2
- 229960001561 bleomycin Drugs 0.000 description 2
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 210000000481 breast Anatomy 0.000 description 2
- 201000008275 breast carcinoma Diseases 0.000 description 2
- 229960002092 busulfan Drugs 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 210000002421 cell wall Anatomy 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 2
- 238000002512 chemotherapy Methods 0.000 description 2
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 2
- 229960004630 chlorambucil Drugs 0.000 description 2
- 150000003841 chloride salts Chemical class 0.000 description 2
- 229960001265 ciclosporin Drugs 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 229960001338 colchicine Drugs 0.000 description 2
- 239000000084 colloidal system Substances 0.000 description 2
- 238000010293 colony formation assay Methods 0.000 description 2
- 239000008139 complexing agent Substances 0.000 description 2
- FPUGCISOLXNPPC-IOSLPCCCSA-N cordysinin B Chemical compound CO[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(N)=C2N=C1 FPUGCISOLXNPPC-IOSLPCCCSA-N 0.000 description 2
- 239000008120 corn starch Substances 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 125000000753 cycloalkyl group Chemical group 0.000 description 2
- 229960000684 cytarabine Drugs 0.000 description 2
- 229960003901 dacarbazine Drugs 0.000 description 2
- 229960000975 daunorubicin Drugs 0.000 description 2
- 229960002997 dehydrocholic acid Drugs 0.000 description 2
- CFCUWKMKBJTWLW-UHFFFAOYSA-N deoliosyl-3C-alpha-L-digitoxosyl-MTM Natural products CC=1C(O)=C2C(O)=C3C(=O)C(OC4OC(C)C(O)C(OC5OC(C)C(O)C(OC6OC(C)C(O)C(C)(O)C6)C5)C4)C(C(OC)C(=O)C(O)C(C)O)CC3=CC2=CC=1OC(OC(C)C1O)CC1OC1CC(O)C(O)C(C)O1 CFCUWKMKBJTWLW-UHFFFAOYSA-N 0.000 description 2
- RGLYKWWBQGJZGM-ISLYRVAYSA-N diethylstilbestrol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(\CC)C1=CC=C(O)C=C1 RGLYKWWBQGJZGM-ISLYRVAYSA-N 0.000 description 2
- 229960005160 dimyristoylphosphatidylglycerol Drugs 0.000 description 2
- 208000037765 diseases and disorders Diseases 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- BPHQZTVXXXJVHI-AJQTZOPKSA-N ditetradecanoyl phosphatidylglycerol Chemical compound CCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@@H](O)CO)OC(=O)CCCCCCCCCCCCC BPHQZTVXXXJVHI-AJQTZOPKSA-N 0.000 description 2
- 230000002222 downregulating effect Effects 0.000 description 2
- 230000007783 downstream signaling Effects 0.000 description 2
- 229960004679 doxorubicin Drugs 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 239000013583 drug formulation Substances 0.000 description 2
- 210000001163 endosome Anatomy 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- 229960001904 epirubicin Drugs 0.000 description 2
- 210000002919 epithelial cell Anatomy 0.000 description 2
- ITSGNOIFAJAQHJ-BMFNZSJVSA-N esorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)C[C@H](C)O1 ITSGNOIFAJAQHJ-BMFNZSJVSA-N 0.000 description 2
- 229950002017 esorubicin Drugs 0.000 description 2
- GEIQNJBWHDTVAY-UHFFFAOYSA-N ethyl 3-[(2-ethoxy-2-oxoethyl)-[6-(9h-fluoren-9-ylmethoxycarbonylamino)hexanoyl]amino]propanoate Chemical compound C1=CC=C2C(COC(=O)NCCCCCC(=O)N(CC(=O)OCC)CCC(=O)OCC)C3=CC=CC=C3C2=C1 GEIQNJBWHDTVAY-UHFFFAOYSA-N 0.000 description 2
- KZFYSKDXJHZWRX-UHFFFAOYSA-N ethyl 3-[6-aminohexanoyl-(2-ethoxy-2-oxoethyl)amino]propanoate Chemical compound CCOC(=O)CCN(CC(=O)OCC)C(=O)CCCCCN KZFYSKDXJHZWRX-UHFFFAOYSA-N 0.000 description 2
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 239000013613 expression plasmid Substances 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- 239000003925 fat Substances 0.000 description 2
- 235000019197 fats Nutrition 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 210000001035 gastrointestinal tract Anatomy 0.000 description 2
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 2
- 229960005277 gemcitabine Drugs 0.000 description 2
- 230000030279 gene silencing Effects 0.000 description 2
- 238000012226 gene silencing method Methods 0.000 description 2
- 210000001905 globus pallidus Anatomy 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 125000005456 glyceride group Chemical group 0.000 description 2
- 229940074045 glyceryl distearate Drugs 0.000 description 2
- WVULKSPCQVQLCU-BUXLTGKBSA-N glycodeoxycholic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 WVULKSPCQVQLCU-BUXLTGKBSA-N 0.000 description 2
- 125000003827 glycol group Chemical group 0.000 description 2
- 150000002334 glycols Chemical class 0.000 description 2
- 208000026436 grade III glioma Diseases 0.000 description 2
- 230000003394 haemopoietic effect Effects 0.000 description 2
- 125000001475 halogen functional group Chemical group 0.000 description 2
- 210000003128 head Anatomy 0.000 description 2
- 230000002440 hepatic effect Effects 0.000 description 2
- 125000005842 heteroatom Chemical group 0.000 description 2
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 210000001320 hippocampus Anatomy 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 229920001477 hydrophilic polymer Polymers 0.000 description 2
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 2
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 2
- 229960002899 hydroxyprogesterone Drugs 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- VKOBVWXKNCXXDE-UHFFFAOYSA-N icosanoic acid Chemical compound CCCCCCCCCCCCCCCCCCCC(O)=O VKOBVWXKNCXXDE-UHFFFAOYSA-N 0.000 description 2
- 229960000908 idarubicin Drugs 0.000 description 2
- 229960001101 ifosfamide Drugs 0.000 description 2
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 2
- 238000010166 immunofluorescence Methods 0.000 description 2
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 2
- 239000003701 inert diluent Substances 0.000 description 2
- 238000011221 initial treatment Methods 0.000 description 2
- 239000008011 inorganic excipient Substances 0.000 description 2
- 229960003786 inosine Drugs 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000021995 interleukin-8 production Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 2
- 229960004768 irinotecan Drugs 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 2
- 125000005647 linker group Chemical group 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 229950000547 mafosfamide Drugs 0.000 description 2
- 238000007726 management method Methods 0.000 description 2
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 2
- 229960001924 melphalan Drugs 0.000 description 2
- 229960001428 mercaptopurine Drugs 0.000 description 2
- 230000001394 metastastic effect Effects 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- CFCUWKMKBJTWLW-BKHRDMLASA-N mithramycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1 CFCUWKMKBJTWLW-BKHRDMLASA-N 0.000 description 2
- 229960004857 mitomycin Drugs 0.000 description 2
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 2
- 229960001156 mitoxantrone Drugs 0.000 description 2
- 210000000865 mononuclear phagocyte system Anatomy 0.000 description 2
- 238000010172 mouse model Methods 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 2
- 239000012457 nonaqueous media Substances 0.000 description 2
- 150000003833 nucleoside derivatives Chemical class 0.000 description 2
- 230000009437 off-target effect Effects 0.000 description 2
- 239000008012 organic excipient Substances 0.000 description 2
- 229960001592 paclitaxel Drugs 0.000 description 2
- 125000000913 palmityl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 210000000496 pancreas Anatomy 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- ONTNXMBMXUNDBF-UHFFFAOYSA-N pentatriacontane-17,18,19-triol Chemical compound CCCCCCCCCCCCCCCCC(O)C(O)C(O)CCCCCCCCCCCCCCCC ONTNXMBMXUNDBF-UHFFFAOYSA-N 0.000 description 2
- 230000035699 permeability Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 229960003171 plicamycin Drugs 0.000 description 2
- 229920000771 poly (alkylcyanoacrylate) Polymers 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 229920000768 polyamine Polymers 0.000 description 2
- 229920000223 polyglycerol Polymers 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 2
- 229960004618 prednisone Drugs 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 2
- 229960000624 procarbazine Drugs 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical compound CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 2
- 235000013772 propylene glycol Nutrition 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 230000008707 rearrangement Effects 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 239000012266 salt solution Substances 0.000 description 2
- 238000013207 serial dilution Methods 0.000 description 2
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 2
- 235000012239 silicon dioxide Nutrition 0.000 description 2
- 239000000344 soap Substances 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 2
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 2
- 239000001488 sodium phosphate Substances 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- 238000010532 solid phase synthesis reaction Methods 0.000 description 2
- 238000005063 solubilization Methods 0.000 description 2
- 230000007928 solubilization Effects 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 239000011550 stock solution Substances 0.000 description 2
- 210000003523 substantia nigra Anatomy 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 229920001059 synthetic polymer Polymers 0.000 description 2
- WBWWGRHZICKQGZ-GIHLXUJPSA-N taurocholic acid Chemical compound C([C@@H]1C[C@H]2O)[C@@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@@H]([C@@H](CCC(=O)NCCS(O)(=O)=O)C)[C@@]2(C)[C@H](O)C1 WBWWGRHZICKQGZ-GIHLXUJPSA-N 0.000 description 2
- AWDRATDZQPNJFN-VAYUFCLWSA-N taurodeoxycholic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCS(O)(=O)=O)C)[C@@]2(C)[C@@H](O)C1 AWDRATDZQPNJFN-VAYUFCLWSA-N 0.000 description 2
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 2
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 2
- 229960001278 teniposide Drugs 0.000 description 2
- 229960003604 testosterone Drugs 0.000 description 2
- 150000003568 thioethers Chemical class 0.000 description 2
- 229940113082 thymine Drugs 0.000 description 2
- 229960003087 tioguanine Drugs 0.000 description 2
- 239000012049 topical pharmaceutical composition Substances 0.000 description 2
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 2
- 229960000303 topotecan Drugs 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- ZMANZCXQSJIPKH-UHFFFAOYSA-O triethylammonium ion Chemical compound CC[NH+](CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-O 0.000 description 2
- 229960001099 trimetrexate Drugs 0.000 description 2
- NOYPYLRCIDNJJB-UHFFFAOYSA-N trimetrexate Chemical compound COC1=C(OC)C(OC)=CC(NCC=2C(=C3C(N)=NC(N)=NC3=CC=2)C)=C1 NOYPYLRCIDNJJB-UHFFFAOYSA-N 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- DCXXMTOCNZCJGO-UHFFFAOYSA-N tristearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC DCXXMTOCNZCJGO-UHFFFAOYSA-N 0.000 description 2
- ZDPHROOEEOARMN-UHFFFAOYSA-N undecanoic acid Chemical compound CCCCCCCCCCC(O)=O ZDPHROOEEOARMN-UHFFFAOYSA-N 0.000 description 2
- 125000002948 undecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 229960003048 vinblastine Drugs 0.000 description 2
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- 235000013343 vitamin Nutrition 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- 210000004885 white matter Anatomy 0.000 description 2
- NFLGAXVYCFJBMK-RKDXNWHRSA-N (+)-isomenthone Natural products CC(C)[C@H]1CC[C@@H](C)CC1=O NFLGAXVYCFJBMK-RKDXNWHRSA-N 0.000 description 1
- RVIZTCLKCHZBMR-KWXKLSQISA-N (12z,15z)-1-(dimethylamino)-2-[(9z,12z)-octadeca-9,12-dienoxy]henicosa-12,15-dien-4-one Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCOC(CN(C)C)CC(=O)CCCCCCC\C=C/C\C=C/CCCCC RVIZTCLKCHZBMR-KWXKLSQISA-N 0.000 description 1
- YIMATHOGWXZHFX-WCTZXXKLSA-N (2r,3r,4r,5r)-5-(hydroxymethyl)-3-(2-methoxyethoxy)oxolane-2,4-diol Chemical compound COCCO[C@H]1[C@H](O)O[C@H](CO)[C@H]1O YIMATHOGWXZHFX-WCTZXXKLSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- COAABSMONFNYQH-TTWCUHKNSA-N (2r,3s,4s,5r,6s)-2-(hydroxymethyl)-6-(oxiran-2-ylmethylsulfanyl)oxane-3,4,5-triol Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1SCC1OC1 COAABSMONFNYQH-TTWCUHKNSA-N 0.000 description 1
- FYGDTMLNYKFZSV-URKRLVJHSA-N (2s,3r,4s,5s,6r)-2-[(2r,4r,5r,6s)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2r,4r,5r,6s)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1[C@@H](CO)O[C@@H](OC2[C@H](O[C@H](O)[C@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O FYGDTMLNYKFZSV-URKRLVJHSA-N 0.000 description 1
- VDYVTMXBGOIUMS-KWXKLSQISA-N (6z,9z,29z,32z)-19-[(dimethylamino)methyl]octatriaconta-6,9,29,32-tetraene-18,21-dione Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC(=O)CC(CN(C)C)C(=O)CCCCCCC\C=C/C\C=C/CCCCC VDYVTMXBGOIUMS-KWXKLSQISA-N 0.000 description 1
- VDVMOGXIBBDZNI-DLEQIPTRSA-N (Z)-octadec-9-enoic acid propane-1,2,3-triol Chemical compound OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O VDVMOGXIBBDZNI-DLEQIPTRSA-N 0.000 description 1
- AVZIYOYFVVSTGQ-RBWRNIRVSA-N (z)-octadec-9-enoic acid;propane-1,2,3-triol Chemical compound OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O AVZIYOYFVVSTGQ-RBWRNIRVSA-N 0.000 description 1
- FJXSLZRUXGTLPF-HKIWRJGFSA-N (z)-octadec-9-enoic acid;propane-1,2,3-triol Chemical compound OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O FJXSLZRUXGTLPF-HKIWRJGFSA-N 0.000 description 1
- IIZBNUQFTQVTGU-PTTKHPGGSA-N (z)-octadec-9-enoic acid;propane-1,2,3-triol Chemical compound OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCCCCC\C=C/CCCCCCCC(O)=O IIZBNUQFTQVTGU-PTTKHPGGSA-N 0.000 description 1
- FYADHXFMURLYQI-UHFFFAOYSA-N 1,2,4-triazine Chemical class C1=CN=NC=N1 FYADHXFMURLYQI-UHFFFAOYSA-N 0.000 description 1
- BUOBCSGIAFXNKP-KWXKLSQISA-N 1-[2,2-bis[(9z,12z)-octadeca-9,12-dienyl]-1,3-dioxolan-4-yl]-n,n-dimethylmethanamine Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCC1(CCCCCCCC\C=C/C\C=C/CCCCC)OCC(CN(C)C)O1 BUOBCSGIAFXNKP-KWXKLSQISA-N 0.000 description 1
- PLKOSISDOAHHCI-QYCRHRGJSA-N 1-[2,3-bis[(9z,12z)-octadeca-9,12-dienoxy]propyl]-4-methylpiperazine Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCOCC(OCCCCCCCC\C=C/C\C=C/CCCCC)CN1CCN(C)CC1 PLKOSISDOAHHCI-QYCRHRGJSA-N 0.000 description 1
- JBWYRBLDOOOJEU-UHFFFAOYSA-N 1-[chloro-(4-methoxyphenyl)-phenylmethyl]-4-methoxybenzene Chemical compound C1=CC(OC)=CC=C1C(Cl)(C=1C=CC(OC)=CC=1)C1=CC=CC=C1 JBWYRBLDOOOJEU-UHFFFAOYSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-UHFFFAOYSA-N 1-beta-D-Xylofuranosyl-NH-Cytosine Natural products O=C1N=C(N)C=CN1C1C(O)C(O)C(CO)O1 UHDGCWIWMRVCDJ-UHFFFAOYSA-N 0.000 description 1
- CBXRMKZFYQISIV-UHFFFAOYSA-N 1-n,1-n,1-n',1-n',2-n,2-n,2-n',2-n'-octamethylethene-1,1,2,2-tetramine Chemical compound CN(C)C(N(C)C)=C(N(C)C)N(C)C CBXRMKZFYQISIV-UHFFFAOYSA-N 0.000 description 1
- UHUHBFMZVCOEOV-UHFFFAOYSA-N 1h-imidazo[4,5-c]pyridin-4-amine Chemical compound NC1=NC=CC2=C1N=CN2 UHUHBFMZVCOEOV-UHFFFAOYSA-N 0.000 description 1
- FPUGCISOLXNPPC-UHFFFAOYSA-N 2'-O-Methyladenosine Natural products COC1C(O)C(CO)OC1N1C2=NC=NC(N)=C2N=C1 FPUGCISOLXNPPC-UHFFFAOYSA-N 0.000 description 1
- RFCQJGFZUQFYRF-UHFFFAOYSA-N 2'-O-Methylcytidine Natural products COC1C(O)C(CO)OC1N1C(=O)N=C(N)C=C1 RFCQJGFZUQFYRF-UHFFFAOYSA-N 0.000 description 1
- OVYNGSFVYRPRCG-UHFFFAOYSA-N 2'-O-Methylguanosine Natural products COC1C(O)C(CO)OC1N1C(NC(N)=NC2=O)=C2N=C1 OVYNGSFVYRPRCG-UHFFFAOYSA-N 0.000 description 1
- RFCQJGFZUQFYRF-ZOQUXTDFSA-N 2'-O-methylcytidine Chemical compound CO[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)N=C(N)C=C1 RFCQJGFZUQFYRF-ZOQUXTDFSA-N 0.000 description 1
- OVYNGSFVYRPRCG-KQYNXXCUSA-N 2'-O-methylguanosine Chemical compound CO[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=C(N)NC2=O)=C2N=C1 OVYNGSFVYRPRCG-KQYNXXCUSA-N 0.000 description 1
- SXUXMRMBWZCMEN-ZOQUXTDFSA-N 2'-O-methyluridine Chemical compound CO[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 SXUXMRMBWZCMEN-ZOQUXTDFSA-N 0.000 description 1
- IHPYMWDTONKSCO-UHFFFAOYSA-N 2,2'-piperazine-1,4-diylbisethanesulfonic acid Chemical compound OS(=O)(=O)CCN1CCN(CCS(O)(=O)=O)CC1 IHPYMWDTONKSCO-UHFFFAOYSA-N 0.000 description 1
- QSHACTSJHMKXTE-UHFFFAOYSA-N 2-(2-aminopropyl)-7h-purin-6-amine Chemical compound CC(N)CC1=NC(N)=C2NC=NC2=N1 QSHACTSJHMKXTE-UHFFFAOYSA-N 0.000 description 1
- PIINGYXNCHTJTF-UHFFFAOYSA-N 2-(2-azaniumylethylamino)acetate Chemical group NCCNCC(O)=O PIINGYXNCHTJTF-UHFFFAOYSA-N 0.000 description 1
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 1
- GIUTUZDGHNZVIA-UHFFFAOYSA-N 2-(ethylamino)acetic acid;hydrochloride Chemical compound Cl.CCNCC(O)=O GIUTUZDGHNZVIA-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- RFVNOJDQRGSOEL-UHFFFAOYSA-N 2-hydroxyethyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCO RFVNOJDQRGSOEL-UHFFFAOYSA-N 0.000 description 1
- 125000004200 2-methoxyethyl group Chemical group [H]C([H])([H])OC([H])([H])C([H])([H])* 0.000 description 1
- BVZVICBYYOYVEP-MAZCIEHSSA-N 3-[bis[(9z,12z)-octadeca-9,12-dienyl]amino]propane-1,2-diol Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCN(CC(O)CO)CCCCCCCC\C=C/C\C=C/CCCCC BVZVICBYYOYVEP-MAZCIEHSSA-N 0.000 description 1
- YJCCSLGGODRWKK-NSCUHMNNSA-N 4-Acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid Chemical compound OS(=O)(=O)C1=CC(NC(=O)C)=CC=C1\C=C\C1=CC=C(N=C=S)C=C1S(O)(=O)=O YJCCSLGGODRWKK-NSCUHMNNSA-N 0.000 description 1
- YRNWIFYIFSBPAU-UHFFFAOYSA-N 4-[4-(dimethylamino)phenyl]-n,n-dimethylaniline Chemical compound C1=CC(N(C)C)=CC=C1C1=CC=C(N(C)C)C=C1 YRNWIFYIFSBPAU-UHFFFAOYSA-N 0.000 description 1
- HIQIXEFWDLTDED-UHFFFAOYSA-N 4-hydroxy-1-piperidin-4-ylpyrrolidin-2-one Chemical compound O=C1CC(O)CN1C1CCNCC1 HIQIXEFWDLTDED-UHFFFAOYSA-N 0.000 description 1
- 229940090248 4-hydroxybenzoic acid Drugs 0.000 description 1
- IZZIWIAOVZOBLF-UHFFFAOYSA-N 5-methoxysalicylic acid Chemical compound COC1=CC=C(O)C(C(O)=O)=C1 IZZIWIAOVZOBLF-UHFFFAOYSA-N 0.000 description 1
- ZLAQATDNGLKIEV-UHFFFAOYSA-N 5-methyl-2-sulfanylidene-1h-pyrimidin-4-one Chemical compound CC1=CNC(=S)NC1=O ZLAQATDNGLKIEV-UHFFFAOYSA-N 0.000 description 1
- ROUFCTKIILEETD-UHFFFAOYSA-N 5-nitro-2-[(5-nitropyridin-2-yl)disulfanyl]pyridine Chemical compound N1=CC([N+](=O)[O-])=CC=C1SSC1=CC=C([N+]([O-])=O)C=N1 ROUFCTKIILEETD-UHFFFAOYSA-N 0.000 description 1
- 108091027075 5S-rRNA precursor Proteins 0.000 description 1
- FPCPONSZWYDXRD-UHFFFAOYSA-N 6-(9h-fluoren-9-ylmethoxycarbonylamino)hexanoic acid Chemical compound C1=CC=C2C(COC(=O)NCCCCCC(=O)O)C3=CC=CC=C3C2=C1 FPCPONSZWYDXRD-UHFFFAOYSA-N 0.000 description 1
- KXBCLNRMQPRVTP-UHFFFAOYSA-N 6-amino-1,5-dihydroimidazo[4,5-c]pyridin-4-one Chemical compound O=C1NC(N)=CC2=C1N=CN2 KXBCLNRMQPRVTP-UHFFFAOYSA-N 0.000 description 1
- DCPSTSVLRXOYGS-UHFFFAOYSA-N 6-amino-1h-pyrimidine-2-thione Chemical compound NC1=CC=NC(S)=N1 DCPSTSVLRXOYGS-UHFFFAOYSA-N 0.000 description 1
- QNNARSZPGNJZIX-UHFFFAOYSA-N 6-amino-5-prop-1-ynyl-1h-pyrimidin-2-one Chemical compound CC#CC1=CNC(=O)N=C1N QNNARSZPGNJZIX-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- LOSIULRWFAEMFL-UHFFFAOYSA-N 7-deazaguanine Chemical compound O=C1NC(N)=NC2=C1CC=N2 LOSIULRWFAEMFL-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 108091034151 7SK RNA Proteins 0.000 description 1
- HRYKDUPGBWLLHO-UHFFFAOYSA-N 8-azaadenine Chemical compound NC1=NC=NC2=NNN=C12 HRYKDUPGBWLLHO-UHFFFAOYSA-N 0.000 description 1
- LPXQRXLUHJKZIE-UHFFFAOYSA-N 8-azaguanine Chemical compound NC1=NC(O)=C2NN=NC2=N1 LPXQRXLUHJKZIE-UHFFFAOYSA-N 0.000 description 1
- 229960005508 8-azaguanine Drugs 0.000 description 1
- OYHQOLUKZRVURQ-HZJYTTRNSA-M 9-cis,12-cis-Octadecadienoate Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC([O-])=O OYHQOLUKZRVURQ-HZJYTTRNSA-M 0.000 description 1
- MSSXOMSJDRHRMC-UHFFFAOYSA-N 9H-purine-2,6-diamine Chemical compound NC1=NC(N)=C2NC=NC2=N1 MSSXOMSJDRHRMC-UHFFFAOYSA-N 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 241000710929 Alphavirus Species 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- 102100038778 Amphiregulin Human genes 0.000 description 1
- 108010033760 Amphiregulin Proteins 0.000 description 1
- 101100067974 Arabidopsis thaliana POP2 gene Proteins 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 229920002498 Beta-glucan Polymers 0.000 description 1
- 102400001242 Betacellulin Human genes 0.000 description 1
- 101800001382 Betacellulin Proteins 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 239000004255 Butylated hydroxyanisole Substances 0.000 description 1
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 1
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- QYOVMAREBTZLBT-KTKRTIGZSA-N CCCCCCCC\C=C/CCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO Chemical compound CCCCCCCC\C=C/CCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO QYOVMAREBTZLBT-KTKRTIGZSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 208000005623 Carcinogenesis Diseases 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 102000016918 Complement C3 Human genes 0.000 description 1
- 108010028780 Complement C3 Proteins 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- MIKUYHXYGGJMLM-GIMIYPNGSA-N Crotonoside Natural products C1=NC2=C(N)NC(=O)N=C2N1[C@H]1O[C@@H](CO)[C@H](O)[C@@H]1O MIKUYHXYGGJMLM-GIMIYPNGSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-PSQAKQOGSA-N Cytidine Natural products O=C1N=C(N)C=CN1[C@@H]1[C@@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-PSQAKQOGSA-N 0.000 description 1
- NYHBQMYGNKIUIF-UHFFFAOYSA-N D-guanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(CO)C(O)C1O NYHBQMYGNKIUIF-UHFFFAOYSA-N 0.000 description 1
- 230000004544 DNA amplification Effects 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 239000003109 Disodium ethylene diamine tetraacetate Substances 0.000 description 1
- 241000255581 Drosophila <fruit fly, genus> Species 0.000 description 1
- ZGTMUACCHSMWAC-UHFFFAOYSA-L EDTA disodium salt (anhydrous) Chemical compound [Na+].[Na+].OC(=O)CN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O ZGTMUACCHSMWAC-UHFFFAOYSA-L 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 201000011001 Ebola Hemorrhagic Fever Diseases 0.000 description 1
- UPEZCKBFRMILAV-JNEQICEOSA-N Ecdysone Natural products O=C1[C@H]2[C@@](C)([C@@H]3C([C@@]4(O)[C@@](C)([C@H]([C@H]([C@@H](O)CCC(O)(C)C)C)CC4)CC3)=C1)C[C@H](O)[C@H](O)C2 UPEZCKBFRMILAV-JNEQICEOSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102100038132 Endogenous retrovirus group K member 6 Pro protein Human genes 0.000 description 1
- 102100031780 Endonuclease Human genes 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 101710091045 Envelope protein Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 206010014967 Ependymoma Diseases 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- 229940123457 Free radical scavenger Drugs 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 229920000569 Gum karaya Polymers 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 101800001649 Heparin-binding EGF-like growth factor Proteins 0.000 description 1
- 102400001369 Heparin-binding EGF-like growth factor Human genes 0.000 description 1
- 101000619542 Homo sapiens E3 ubiquitin-protein ligase parkin Proteins 0.000 description 1
- 101100118549 Homo sapiens EGFR gene Proteins 0.000 description 1
- 101100286713 Homo sapiens IL6 gene Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 1
- GRRNUXAQVGOGFE-UHFFFAOYSA-N Hygromycin-B Natural products OC1C(NC)CC(N)C(O)C1OC1C2OC3(C(C(O)C(O)C(C(N)CO)O3)O)OC2C(O)C(CO)O1 GRRNUXAQVGOGFE-UHFFFAOYSA-N 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 201000001916 Hypochondriasis Diseases 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 1
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 1
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 239000004166 Lanolin Substances 0.000 description 1
- 229920002884 Laureth 4 Polymers 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 102000019149 MAP kinase activity proteins Human genes 0.000 description 1
- 108040008097 MAP kinase activity proteins Proteins 0.000 description 1
- 108091054455 MAP kinase family Proteins 0.000 description 1
- 102000043136 MAP kinase family Human genes 0.000 description 1
- 101150018665 MAPK3 gene Proteins 0.000 description 1
- 235000019759 Maize starch Nutrition 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 206010027202 Meningitis bacterial Diseases 0.000 description 1
- NFLGAXVYCFJBMK-UHFFFAOYSA-N Menthone Chemical compound CC(C)C1CCC(C)CC1=O NFLGAXVYCFJBMK-UHFFFAOYSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 208000019695 Migraine disease Diseases 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- 241000714177 Murine leukemia virus Species 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- WTAYIFXKJBMZLY-XZABIIKCSA-N OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O Chemical compound OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO.CCCCCCCC\C=C/CCCCCCCC(O)=O WTAYIFXKJBMZLY-XZABIIKCSA-N 0.000 description 1
- 229910004679 ONO2 Inorganic materials 0.000 description 1
- 201000010133 Oligodendroglioma Diseases 0.000 description 1
- 208000001132 Osteoporosis Diseases 0.000 description 1
- 102000016979 Other receptors Human genes 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 239000007990 PIPES buffer Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 108010067035 Pancrelipase Proteins 0.000 description 1
- 208000002774 Paraproteinemias Diseases 0.000 description 1
- 241000009328 Perro Species 0.000 description 1
- 239000004264 Petrolatum Substances 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical class OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 229920001244 Poly(D,L-lactide) Polymers 0.000 description 1
- 229920002730 Poly(butyl cyanoacrylate) Polymers 0.000 description 1
- 229920002724 Poly(ethyl cyanoacrylate) Polymers 0.000 description 1
- 229920002319 Poly(methyl acrylate) Polymers 0.000 description 1
- 229920002723 Poly(methyl cyanoacrylate) Polymers 0.000 description 1
- 229920002556 Polyethylene Glycol 300 Polymers 0.000 description 1
- 229920002565 Polyethylene Glycol 400 Polymers 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102000007327 Protamines Human genes 0.000 description 1
- 108010007568 Protamines Proteins 0.000 description 1
- 108091008611 Protein Kinase B Proteins 0.000 description 1
- 101710188315 Protein X Proteins 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical class C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 1
- 102000017143 RNA Polymerase I Human genes 0.000 description 1
- 108010013845 RNA Polymerase I Proteins 0.000 description 1
- 102000009572 RNA Polymerase II Human genes 0.000 description 1
- 108010009460 RNA Polymerase II Proteins 0.000 description 1
- 230000006819 RNA synthesis Effects 0.000 description 1
- 230000004570 RNA-binding Effects 0.000 description 1
- 206010037742 Rabies Diseases 0.000 description 1
- 101150062264 Raf gene Proteins 0.000 description 1
- 102000004278 Receptor Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000873 Receptor Protein-Tyrosine Kinases Proteins 0.000 description 1
- 108091005682 Receptor kinases Proteins 0.000 description 1
- 206010063837 Reperfusion injury Diseases 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- 101100123851 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) HER1 gene Proteins 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 206010040070 Septic Shock Diseases 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 239000004141 Sodium laurylsulphate Substances 0.000 description 1
- ABBQHOQBGMUPJH-UHFFFAOYSA-M Sodium salicylate Chemical compound [Na+].OC1=CC=CC=C1C([O-])=O ABBQHOQBGMUPJH-UHFFFAOYSA-M 0.000 description 1
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 1
- 241000934878 Sterculia Species 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- ULUAUXLGCMPNKK-UHFFFAOYSA-N Sulfobutanedioic acid Chemical class OC(=O)CC(C(O)=O)S(O)(=O)=O ULUAUXLGCMPNKK-UHFFFAOYSA-N 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- 208000004732 Systemic Vasculitis Diseases 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 1
- 101710137500 T7 RNA polymerase Proteins 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- 208000034841 Thrombotic Microangiopathies Diseases 0.000 description 1
- 108020004440 Thymidine kinase Proteins 0.000 description 1
- 206010062129 Tongue neoplasm Diseases 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 108091026838 U1 spliceosomal RNA Proteins 0.000 description 1
- 108091026822 U6 spliceosomal RNA Proteins 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 229910052770 Uranium Inorganic materials 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- OIRDTQYFTABQOQ-UHTZMRCNSA-N Vidarabine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O OIRDTQYFTABQOQ-UHTZMRCNSA-N 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 229930003427 Vitamin E Chemical group 0.000 description 1
- 208000008383 Wilms tumor Diseases 0.000 description 1
- LEBBDRXHHNYZIA-LDUWYPJVSA-N [(2s,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] n-[(z)-1,3-dihydroxyoctadec-4-en-2-yl]carbamate Chemical compound CCCCCCCCCCCCC\C=C/C(O)C(CO)NC(=O)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O LEBBDRXHHNYZIA-LDUWYPJVSA-N 0.000 description 1
- QNEPTKZEXBPDLF-JDTILAPWSA-N [(3s,8s,9s,10r,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3-yl] carbonochloridate Chemical compound C1C=C2C[C@@H](OC(Cl)=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 QNEPTKZEXBPDLF-JDTILAPWSA-N 0.000 description 1
- TTWXVHUYMARJHI-KWXKLSQISA-N [(6Z,9Z,29Z,32Z)-20-[(dimethylamino)methyl]octatriaconta-6,9,29,32-tetraen-19-yl] carbamate Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCC(CN(C)C)C(OC(N)=O)CCCCCCCC\C=C/C\C=C/CCCCC TTWXVHUYMARJHI-KWXKLSQISA-N 0.000 description 1
- HCAJCMUKLZSPFT-KWXKLSQISA-N [3-(dimethylamino)-2-[(9z,12z)-octadeca-9,12-dienoyl]oxypropyl] (9z,12z)-octadeca-9,12-dienoate Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC(=O)OCC(CN(C)C)OC(=O)CCCCCCC\C=C/C\C=C/CCCCC HCAJCMUKLZSPFT-KWXKLSQISA-N 0.000 description 1
- HMNZFMSWFCAGGW-XPWSMXQVSA-N [3-[hydroxy(2-hydroxyethoxy)phosphoryl]oxy-2-[(e)-octadec-9-enoyl]oxypropyl] (e)-octadec-9-enoate Chemical compound CCCCCCCC\C=C\CCCCCCCC(=O)OCC(COP(O)(=O)OCCO)OC(=O)CCCCCCC\C=C\CCCCCCCC HMNZFMSWFCAGGW-XPWSMXQVSA-N 0.000 description 1
- YUBGNDHVPXOQEM-UHFFFAOYSA-N [6-[3-[bis(4-methoxyphenyl)-phenylmethoxymethyl]-4-hydroxypyrrolidin-1-yl]-6-oxohexyl]carbamic acid Chemical compound C1=CC(OC)=CC=C1C(C=1C=CC(OC)=CC=1)(C1C(CN(C1)C(=O)CCCCCNC(O)=O)O)OCC1=CC=CC=C1 YUBGNDHVPXOQEM-UHFFFAOYSA-N 0.000 description 1
- KBMUPXLPCMFFOM-UHFFFAOYSA-N [6-[3-hydroxy-4-(hydroxymethyl)pyrrolidin-1-yl]-6-oxohexyl]carbamic acid Chemical compound OCC1CN(C(=O)CCCCCNC(O)=O)CC1O KBMUPXLPCMFFOM-UHFFFAOYSA-N 0.000 description 1
- YKTSYUJCYHOUJP-UHFFFAOYSA-N [O--].[Al+3].[Al+3].[O-][Si]([O-])([O-])[O-] Chemical compound [O--].[Al+3].[Al+3].[O-][Si]([O-])([O-])[O-] YKTSYUJCYHOUJP-UHFFFAOYSA-N 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 229960004150 aciclovir Drugs 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 208000021841 acute erythroid leukemia Diseases 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000005083 alkoxyalkoxy group Chemical group 0.000 description 1
- 125000002877 alkyl aryl group Chemical group 0.000 description 1
- 150000004996 alkyl benzenes Chemical class 0.000 description 1
- 125000005600 alkyl phosphonate group Chemical group 0.000 description 1
- 208000030961 allergic reaction Diseases 0.000 description 1
- UPEZCKBFRMILAV-UHFFFAOYSA-N alpha-Ecdysone Natural products C1C(O)C(O)CC2(C)C(CCC3(C(C(C(O)CCC(C)(C)O)C)CCC33O)C)C3=CC(=O)C21 UPEZCKBFRMILAV-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 125000005122 aminoalkylamino group Chemical group 0.000 description 1
- 239000002280 amphoteric surfactant Substances 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 229940031955 anhydrous lanolin Drugs 0.000 description 1
- 238000005571 anion exchange chromatography Methods 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940124599 anti-inflammatory drug Drugs 0.000 description 1
- 230000001139 anti-pruritic effect Effects 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940037157 anticorticosteroids Drugs 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 239000003908 antipruritic agent Substances 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 239000006286 aqueous extract Substances 0.000 description 1
- 239000012300 argon atmosphere Substances 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 239000003212 astringent agent Substances 0.000 description 1
- 229960000892 attapulgite Drugs 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 201000009904 bacterial meningitis Diseases 0.000 description 1
- 230000037429 base substitution Effects 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- OWMVSZAMULFTJU-UHFFFAOYSA-N bis-tris Chemical compound OCCN(CCO)C(CO)(CO)CO OWMVSZAMULFTJU-UHFFFAOYSA-N 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- 235000019282 butylated hydroxyanisole Nutrition 0.000 description 1
- CZBZUDVBLSSABA-UHFFFAOYSA-N butylated hydroxyanisole Chemical compound COC1=CC=C(O)C(C(C)(C)C)=C1.COC1=CC=C(O)C=C1C(C)(C)C CZBZUDVBLSSABA-UHFFFAOYSA-N 0.000 description 1
- 229940043253 butylated hydroxyanisole Drugs 0.000 description 1
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 1
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 229960003563 calcium carbonate Drugs 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 229960001714 calcium phosphate Drugs 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 235000011132 calcium sulphate Nutrition 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- LDVVMCZRFWMZSG-UHFFFAOYSA-N captan Chemical compound C1C=CCC2C(=O)N(SC(Cl)(Cl)Cl)C(=O)C21 LDVVMCZRFWMZSG-UHFFFAOYSA-N 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 231100000504 carcinogenesis Toxicity 0.000 description 1
- 235000010418 carrageenan Nutrition 0.000 description 1
- 239000000679 carrageenan Substances 0.000 description 1
- 229920001525 carrageenan Polymers 0.000 description 1
- 229940113118 carrageenan Drugs 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 239000003093 cationic surfactant Substances 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 239000013553 cell monolayer Substances 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000033077 cellular process Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920003086 cellulose ether Polymers 0.000 description 1
- 208000015114 central nervous system disease Diseases 0.000 description 1
- 150000001783 ceramides Chemical class 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 150000005829 chemical entities Chemical class 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 239000005289 controlled pore glass Substances 0.000 description 1
- 239000012059 conventional drug carrier Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 210000004087 cornea Anatomy 0.000 description 1
- 210000001653 corpus striatum Anatomy 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 238000009295 crossflow filtration Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- UHDGCWIWMRVCDJ-ZAKLUEHWSA-N cytidine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-ZAKLUEHWSA-N 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-M decanoate Chemical compound CCCCCCCCCC([O-])=O GHVNFZFCNZKVNT-UHFFFAOYSA-M 0.000 description 1
- HABLENUWIZGESP-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O.CCCCCCCCCC(O)=O HABLENUWIZGESP-UHFFFAOYSA-N 0.000 description 1
- STORWMDPIHOSMF-UHFFFAOYSA-N decanoic acid;octanoic acid;propane-1,2,3-triol Chemical compound OCC(O)CO.CCCCCCCC(O)=O.CCCCCCCCCC(O)=O STORWMDPIHOSMF-UHFFFAOYSA-N 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 229940124447 delivery agent Drugs 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 210000004207 dermis Anatomy 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 229960000633 dextran sulfate Drugs 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- 229960001193 diclofenac sodium Drugs 0.000 description 1
- UMGXUWVIJIQANV-UHFFFAOYSA-M didecyl(dimethyl)azanium;bromide Chemical compound [Br-].CCCCCCCCCC[N+](C)(C)CCCCCCCCCC UMGXUWVIJIQANV-UHFFFAOYSA-M 0.000 description 1
- 150000005690 diesters Chemical class 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- OGQYPPBGSLZBEG-UHFFFAOYSA-N dimethyl(dioctadecyl)azanium Chemical compound CCCCCCCCCCCCCCCCCC[N+](C)(C)CCCCCCCCCCCCCCCCCC OGQYPPBGSLZBEG-UHFFFAOYSA-N 0.000 description 1
- PSLWZOIUBRXAQW-UHFFFAOYSA-M dimethyl(dioctadecyl)azanium;bromide Chemical compound [Br-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CCCCCCCCCCCCCCCCCC PSLWZOIUBRXAQW-UHFFFAOYSA-M 0.000 description 1
- UAKOZKUVZRMOFN-JDVCJPALSA-M dimethyl-bis[(z)-octadec-9-enyl]azanium;chloride Chemical compound [Cl-].CCCCCCCC\C=C/CCCCCCCC[N+](C)(C)CCCCCCCC\C=C/CCCCCCCC UAKOZKUVZRMOFN-JDVCJPALSA-M 0.000 description 1
- BPHQZTVXXXJVHI-UHFFFAOYSA-N dimyristoyl phosphatidylglycerol Chemical compound CCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCCCCCCCC BPHQZTVXXXJVHI-UHFFFAOYSA-N 0.000 description 1
- 150000002009 diols Chemical class 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 235000019301 disodium ethylene diamine tetraacetate Nutrition 0.000 description 1
- NAGJZTKCGNOGPW-UHFFFAOYSA-N dithiophosphoric acid Chemical class OP(O)(S)=S NAGJZTKCGNOGPW-UHFFFAOYSA-N 0.000 description 1
- POULHZVOKOAJMA-UHFFFAOYSA-M dodecanoate Chemical compound CCCCCCCCCCCC([O-])=O POULHZVOKOAJMA-UHFFFAOYSA-M 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 229940126534 drug product Drugs 0.000 description 1
- UPEZCKBFRMILAV-JMZLNJERSA-N ecdysone Chemical compound C1[C@@H](O)[C@@H](O)C[C@]2(C)[C@@H](CC[C@@]3([C@@H]([C@@H]([C@H](O)CCC(C)(C)O)C)CC[C@]33O)C)C3=CC(=O)[C@@H]21 UPEZCKBFRMILAV-JMZLNJERSA-N 0.000 description 1
- 230000002900 effect on cell Effects 0.000 description 1
- 229940121647 egfr inhibitor Drugs 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 150000002081 enamines Chemical class 0.000 description 1
- 210000003989 endothelium vascular Anatomy 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940079360 enema for constipation Drugs 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000007071 enzymatic hydrolysis Effects 0.000 description 1
- 238000006047 enzymatic hydrolysis reaction Methods 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 206010015037 epilepsy Diseases 0.000 description 1
- 230000000925 erythroid effect Effects 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- JHFQLFGNGSHVGQ-UHFFFAOYSA-N ethyl 3-[(2-ethoxy-2-oxoethyl)amino]propanoate Chemical compound CCOC(=O)CCNCC(=O)OCC JHFQLFGNGSHVGQ-UHFFFAOYSA-N 0.000 description 1
- JRFPHUDKKRHKHX-UHFFFAOYSA-N ethyl 4-(ethoxycarbonylamino)butanoate Chemical compound CCOC(=O)CCCNC(=O)OCC JRFPHUDKKRHKHX-UHFFFAOYSA-N 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 238000010228 ex vivo assay Methods 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 150000002194 fatty esters Chemical class 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000003818 flash chromatography Methods 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 230000000799 fusogenic effect Effects 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Chemical group CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 229960002963 ganciclovir Drugs 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000037442 genomic alteration Effects 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- UPWGQKDVAURUGE-UHFFFAOYSA-N glycerine monooleate Natural products CCCCCCCCC=CCCCCCCCC(=O)OC(CO)CO UPWGQKDVAURUGE-UHFFFAOYSA-N 0.000 description 1
- 229940074049 glyceryl dilaurate Drugs 0.000 description 1
- 125000005908 glyceryl ester group Chemical group 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 229940029575 guanosine Drugs 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- KWLMIXQRALPRBC-UHFFFAOYSA-L hectorite Chemical compound [Li+].[OH-].[OH-].[Na+].[Mg+2].O1[Si]2([O-])O[Si]1([O-])O[Si]([O-])(O1)O[Si]1([O-])O2 KWLMIXQRALPRBC-UHFFFAOYSA-L 0.000 description 1
- 229910000271 hectorite Inorganic materials 0.000 description 1
- 201000005787 hematologic cancer Diseases 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 1
- 230000003118 histopathologic effect Effects 0.000 description 1
- QRMZSPFSDQBLIX-UHFFFAOYSA-N homovanillic acid Chemical compound COC1=CC(CC(O)=O)=CC=C1O QRMZSPFSDQBLIX-UHFFFAOYSA-N 0.000 description 1
- 102000052611 human IL6 Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 235000003642 hunger Nutrition 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 239000000416 hydrocolloid Substances 0.000 description 1
- 239000008172 hydrogenated vegetable oil Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- GRRNUXAQVGOGFE-NZSRVPFOSA-N hygromycin B Chemical compound O[C@@H]1[C@@H](NC)C[C@@H](N)[C@H](O)[C@H]1O[C@H]1[C@H]2O[C@@]3([C@@H]([C@@H](O)[C@@H](O)[C@@H](C(N)CO)O3)O)O[C@H]2[C@@H](O)[C@@H](CO)O1 GRRNUXAQVGOGFE-NZSRVPFOSA-N 0.000 description 1
- 229940097277 hygromycin b Drugs 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 230000002390 hyperplastic effect Effects 0.000 description 1
- 230000003463 hyperproliferative effect Effects 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 229960000905 indomethacin Drugs 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000002054 inoculum Substances 0.000 description 1
- 229910003480 inorganic solid Inorganic materials 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 239000000138 intercalating agent Substances 0.000 description 1
- 230000035992 intercellular communication Effects 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 230000006662 intracellular pathway Effects 0.000 description 1
- 230000004068 intracellular signaling Effects 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 239000002563 ionic surfactant Substances 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 1
- 229940074928 isopropyl myristate Drugs 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 235000010494 karaya gum Nutrition 0.000 description 1
- 239000000231 karaya gum Substances 0.000 description 1
- 229940039371 karaya gum Drugs 0.000 description 1
- 229940039717 lanolin Drugs 0.000 description 1
- 235000019388 lanolin Nutrition 0.000 description 1
- 229940070765 laurate Drugs 0.000 description 1
- 229940062711 laureth-9 Drugs 0.000 description 1
- 239000008141 laxative Substances 0.000 description 1
- 229940125722 laxative agent Drugs 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 235000001510 limonene Nutrition 0.000 description 1
- 229940087305 limonene Drugs 0.000 description 1
- 229940049918 linoleate Drugs 0.000 description 1
- 238000001638 lipofection Methods 0.000 description 1
- 239000008206 lipophilic material Substances 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 239000003589 local anesthetic agent Substances 0.000 description 1
- 229960005015 local anesthetics Drugs 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 208000019420 lymphoid neoplasm Diseases 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 229930007503 menthone Natural products 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 150000004692 metal hydroxides Chemical class 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 150000001455 metallic ions Chemical class 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 206010027599 migraine Diseases 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 229940042472 mineral oil Drugs 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 239000008185 minitablet Substances 0.000 description 1
- 201000004058 mixed glioma Diseases 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- RZRNAYUHWVFMIP-UHFFFAOYSA-N monoelaidin Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-UHFFFAOYSA-N 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 229940105132 myristate Drugs 0.000 description 1
- XVUQPECVOGMPRU-ZPPAUJSGSA-N n,n-dimethyl-1,2-bis[(9z,12z)-octadeca-9,12-dienoxy]propan-1-amine Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCOC(C)C(N(C)C)OCCCCCCCC\C=C/C\C=C/CCCCC XVUQPECVOGMPRU-ZPPAUJSGSA-N 0.000 description 1
- OZBZDYGIYDRTBV-RSLAUBRISA-N n,n-dimethyl-1,2-bis[(9z,12z,15z)-octadeca-9,12,15-trienoxy]propan-1-amine Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCCOC(C)C(N(C)C)OCCCCCCCC\C=C/C\C=C/C\C=C/CC OZBZDYGIYDRTBV-RSLAUBRISA-N 0.000 description 1
- NFQBIAXADRDUGK-KWXKLSQISA-N n,n-dimethyl-2,3-bis[(9z,12z)-octadeca-9,12-dienoxy]propan-1-amine Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCOCC(CN(C)C)OCCCCCCCC\C=C/C\C=C/CCCCC NFQBIAXADRDUGK-KWXKLSQISA-N 0.000 description 1
- GLGLUQVVDHRLQK-WRBBJXAJSA-N n,n-dimethyl-2,3-bis[(z)-octadec-9-enoxy]propan-1-amine Chemical compound CCCCCCCC\C=C/CCCCCCCCOCC(CN(C)C)OCCCCCCCC\C=C/CCCCCCCC GLGLUQVVDHRLQK-WRBBJXAJSA-N 0.000 description 1
- UKXOXMLXFQEEQJ-KWXKLSQISA-N n,n-dimethyl-2,3-bis[[(9z,12z)-octadeca-9,12-dienyl]sulfanyl]propan-1-amine Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCSCC(CN(C)C)SCCCCCCCC\C=C/C\C=C/CCCCC UKXOXMLXFQEEQJ-KWXKLSQISA-N 0.000 description 1
- PSHKMPUSSFXUIA-UHFFFAOYSA-N n,n-dimethylpyridin-2-amine Chemical compound CN(C)C1=CC=CC=N1 PSHKMPUSSFXUIA-UHFFFAOYSA-N 0.000 description 1
- 229940042880 natural phospholipid Drugs 0.000 description 1
- 239000006199 nebulizer Substances 0.000 description 1
- 210000005170 neoplastic cell Anatomy 0.000 description 1
- 210000001577 neostriatum Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 125000001893 nitrooxy group Chemical group [O-][N+](=O)O* 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 231100000956 nontoxicity Toxicity 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 238000007899 nucleic acid hybridization Methods 0.000 description 1
- 230000000050 nutritive effect Effects 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 239000003883 ointment base Substances 0.000 description 1
- 229940049964 oleate Drugs 0.000 description 1
- 206010073131 oligoastrocytoma Diseases 0.000 description 1
- 229940046166 oligodeoxynucleotide Drugs 0.000 description 1
- 231100000590 oncogenic Toxicity 0.000 description 1
- 230000002246 oncogenic effect Effects 0.000 description 1
- 239000003605 opacifier Substances 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 229910052625 palygorskite Inorganic materials 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 230000003076 paracrine Effects 0.000 description 1
- 102000045222 parkin Human genes 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 239000003961 penetration enhancing agent Substances 0.000 description 1
- JLFNLZLINWHATN-UHFFFAOYSA-N pentaethylene glycol Chemical compound OCCOCCOCCOCCOCCO JLFNLZLINWHATN-UHFFFAOYSA-N 0.000 description 1
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 1
- 229940066842 petrolatum Drugs 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 239000008251 pharmaceutical emulsion Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 238000010587 phase diagram Methods 0.000 description 1
- 229960002895 phenylbutazone Drugs 0.000 description 1
- VYMDGNCVAMGZFE-UHFFFAOYSA-N phenylbutazonum Chemical compound O=C1C(CCCC)C(=O)N(C=2C=CC=CC=2)N1C1=CC=CC=C1 VYMDGNCVAMGZFE-UHFFFAOYSA-N 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 150000003905 phosphatidylinositols Chemical class 0.000 description 1
- 150000004713 phosphodiesters Chemical group 0.000 description 1
- 150000008298 phosphoramidates Chemical class 0.000 description 1
- 150000008300 phosphoramidites Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 208000031223 plasma cell leukemia Diseases 0.000 description 1
- 208000010626 plasma cell neoplasm Diseases 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- ONJQDTZCDSESIW-UHFFFAOYSA-N polidocanol Chemical compound CCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO ONJQDTZCDSESIW-UHFFFAOYSA-N 0.000 description 1
- 229920000729 poly(L-lysine) polymer Polymers 0.000 description 1
- 229920001515 polyalkylene glycol Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920002720 polyhexylacrylate Polymers 0.000 description 1
- 229920002704 polyhistidine Polymers 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 108010055896 polyornithine Proteins 0.000 description 1
- 229920002714 polyornithine Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004804 polysaccharides Chemical class 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920002717 polyvinylpyridine Polymers 0.000 description 1
- 230000023603 positive regulation of transcription initiation, DNA-dependent Effects 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 238000011809 primate model Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 201000008171 proliferative glomerulonephritis Diseases 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 229940048914 protamine Drugs 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- LKUNXBRZDFMZOK-UHFFFAOYSA-N rac-1-monodecanoylglycerol Chemical compound CCCCCCCCCC(=O)OCC(O)CO LKUNXBRZDFMZOK-UHFFFAOYSA-N 0.000 description 1
- GHBFNMLVSPCDGN-UHFFFAOYSA-N rac-1-monooctanoylglycerol Chemical compound CCCCCCCC(=O)OCC(O)CO GHBFNMLVSPCDGN-UHFFFAOYSA-N 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 238000011552 rat model Methods 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000008844 regulatory mechanism Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 125000006853 reporter group Chemical group 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 239000011369 resultant mixture Substances 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 108020004418 ribosomal RNA Proteins 0.000 description 1
- 150000003873 salicylate salts Chemical class 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 201000000980 schizophrenia Diseases 0.000 description 1
- 238000012106 screening analysis Methods 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 230000036303 septic shock Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 229920002545 silicone oil Polymers 0.000 description 1
- RYMZZMVNJRMUDD-HGQWONQESA-N simvastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 RYMZZMVNJRMUDD-HGQWONQESA-N 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 239000012279 sodium borohydride Substances 0.000 description 1
- 229910000033 sodium borohydride Inorganic materials 0.000 description 1
- CDBYLPFSWZWCQE-UHFFFAOYSA-L sodium carbonate Substances [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- NRHMKIHPTBHXPF-TUJRSCDTSA-M sodium cholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 NRHMKIHPTBHXPF-TUJRSCDTSA-M 0.000 description 1
- AJPJDKMHJJGVTQ-UHFFFAOYSA-M sodium dihydrogen phosphate Chemical compound [Na+].OP(O)([O-])=O AJPJDKMHJJGVTQ-UHFFFAOYSA-M 0.000 description 1
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 1
- OABYVIYXWMZFFJ-ZUHYDKSRSA-M sodium glycocholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 OABYVIYXWMZFFJ-ZUHYDKSRSA-M 0.000 description 1
- VMSNAUAEKXEYGP-YEUHZSMFSA-M sodium glycodeoxycholate Chemical compound [Na+].C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 VMSNAUAEKXEYGP-YEUHZSMFSA-M 0.000 description 1
- 229940001584 sodium metabisulfite Drugs 0.000 description 1
- 235000010262 sodium metabisulphite Nutrition 0.000 description 1
- RYYKJJJTJZKILX-UHFFFAOYSA-M sodium octadecanoate Chemical class [Na+].CCCCCCCCCCCCCCCCCC([O-])=O RYYKJJJTJZKILX-UHFFFAOYSA-M 0.000 description 1
- 229960004025 sodium salicylate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- JAJWGJBVLPIOOH-IZYKLYLVSA-M sodium taurocholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 JAJWGJBVLPIOOH-IZYKLYLVSA-M 0.000 description 1
- 229940045946 sodium taurodeoxycholate Drugs 0.000 description 1
- WDFRNBJHDMUMBL-OICFXQLMSA-M sodium;(4r)-4-[(3r,5s,7r,8r,9s,10s,13r,14s,17r)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)CC1 WDFRNBJHDMUMBL-OICFXQLMSA-M 0.000 description 1
- FKJIJBSJQSMPTI-CAOXKPNISA-M sodium;(4r)-4-[(5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-3,7,12-trioxo-1,2,4,5,6,8,9,11,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl]pentanoate Chemical compound [Na+].C1CC(=O)C[C@H]2CC(=O)[C@H]3[C@@H]4CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]4(C)C(=O)C[C@@H]3[C@]21C FKJIJBSJQSMPTI-CAOXKPNISA-M 0.000 description 1
- JGMJQSFLQWGYMQ-UHFFFAOYSA-M sodium;2,6-dichloro-n-phenylaniline;acetate Chemical compound [Na+].CC([O-])=O.ClC1=CC=CC(Cl)=C1NC1=CC=CC=C1 JGMJQSFLQWGYMQ-UHFFFAOYSA-M 0.000 description 1
- YXHRQQJFKOHLAP-FVCKGWAHSA-M sodium;2-[[(4r)-4-[(3r,5r,8r,9s,10s,12s,13r,14s,17r)-3,12-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]ethanesulfonate Chemical compound [Na+].C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 YXHRQQJFKOHLAP-FVCKGWAHSA-M 0.000 description 1
- JJICLMJFIKGAAU-UHFFFAOYSA-M sodium;2-amino-9-(1,3-dihydroxypropan-2-yloxymethyl)purin-6-olate Chemical compound [Na+].NC1=NC([O-])=C2N=CN(COC(CO)CO)C2=N1 JJICLMJFIKGAAU-UHFFFAOYSA-M 0.000 description 1
- RMLUKZWYIKEASN-UHFFFAOYSA-M sodium;2-amino-9-(2-hydroxyethoxymethyl)purin-6-olate Chemical compound [Na+].O=C1[N-]C(N)=NC2=C1N=CN2COCCO RMLUKZWYIKEASN-UHFFFAOYSA-M 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 235000011076 sorbitan monostearate Nutrition 0.000 description 1
- 239000001587 sorbitan monostearate Substances 0.000 description 1
- 229940035048 sorbitan monostearate Drugs 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 210000000278 spinal cord Anatomy 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 238000003153 stable transfection Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 230000037351 starvation Effects 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 150000003432 sterols Chemical class 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000001384 succinic acid Substances 0.000 description 1
- 229940014800 succinic anhydride Drugs 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 150000003445 sucroses Chemical class 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- IIACRCGMVDHOTQ-UHFFFAOYSA-N sulfamic acid Chemical group NS(O)(=O)=O IIACRCGMVDHOTQ-UHFFFAOYSA-N 0.000 description 1
- 150000003456 sulfonamides Chemical group 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 150000003457 sulfones Chemical group 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 150000003505 terpenes Chemical class 0.000 description 1
- 235000007586 terpenes Nutrition 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- TUNFSRHWOTWDNC-UHFFFAOYSA-N tetradecanoic acid Chemical compound CCCCCCCCCCCCCC(O)=O TUNFSRHWOTWDNC-UHFFFAOYSA-N 0.000 description 1
- JYKSTGLAIMQDRA-UHFFFAOYSA-N tetraglycerol Chemical compound OCC(O)CO.OCC(O)CO.OCC(O)CO.OCC(O)CO JYKSTGLAIMQDRA-UHFFFAOYSA-N 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 125000002640 tocopherol group Chemical class 0.000 description 1
- 235000019149 tocopherols Nutrition 0.000 description 1
- 201000006134 tongue cancer Diseases 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 230000002463 transducing effect Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 208000006961 tropical spastic paraparesis Diseases 0.000 description 1
- 230000010415 tropism Effects 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- RUDATBOHQWOJDD-UZVSRGJWSA-N ursodeoxycholic acid Chemical compound C([C@H]1C[C@@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)CC1 RUDATBOHQWOJDD-UZVSRGJWSA-N 0.000 description 1
- 229960001661 ursodiol Drugs 0.000 description 1
- 210000004509 vascular smooth muscle cell Anatomy 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 229960003636 vidarabine Drugs 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 239000011709 vitamin E Chemical group 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 239000008307 w/o/w-emulsion Substances 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 229940124024 weight reducing agent Drugs 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
- UHVMMEOXYDMDKI-JKYCWFKZSA-L zinc;1-(5-cyanopyridin-2-yl)-3-[(1s,2s)-2-(6-fluoro-2-hydroxy-3-propanoylphenyl)cyclopropyl]urea;diacetate Chemical compound [Zn+2].CC([O-])=O.CC([O-])=O.CCC(=O)C1=CC=C(F)C([C@H]2[C@H](C2)NC(=O)NC=2N=CC(=CC=2)C#N)=C1O UHVMMEOXYDMDKI-JKYCWFKZSA-L 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1138—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against receptors or cell surface proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1136—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against growth factors, growth regulators, cytokines, lymphokines or hormones
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering N.A.
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/321—2'-O-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/351—Conjugate
- C12N2310/3515—Lipophilic moiety, e.g. cholesterol
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2320/00—Applications; Uses
- C12N2320/30—Special therapeutic applications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2320/00—Applications; Uses
- C12N2320/30—Special therapeutic applications
- C12N2320/31—Combination therapy
Definitions
- the invention relates to a double-stranded ribonucleic acid (dsRNA) targeting a mutant Epidermal Growth Factor Receptor (EGFR), and methods of using the dsRNA to inhibit expression of mutant EGFR.
- dsRNA double-stranded ribonucleic acid
- EGFR Epidermal Growth Factor Receptor
- the Epidermal Growth Factor Receptor (EGFR) gene is frequently upregulated in carcinomas of the breast, kidney, ovary, cervix, and in squamous cells.
- the upregulation is typically due to gene amplification or overexpression.
- EGFR upregulation in gliomas is most often associated with the rearrangement of the EGFR gene resulting in alterations of its transcript so that such gliomas express both wild-type endogenous EGFR as well as the episomal mutant form.
- ds 2-7 EGFR genomic alterations leading to deletion of exons 2-7 in the EGFR mRNA
- deltaEGFR genomic alterations leading to deletion of exons 2-7 in the EGFR mRNA
- EGFRvIII genomic alterations leading to deletion of exons 2-7 in the EGFR mRNA
- the EGFR gene is amplified in >50% of glioblastomas. This amplification is often associated with expression of deltaEGFR, which conveys enhanced tumor aggressiveness.
- Double-stranded RNA molecules have been shown to block gene expression in a highly conserved regulatory mechanism known as RNA interference (RNAi).
- RNAi RNA interference
- WO 99/32619 (Fire et al.) disclosed the use of a dsRNA of at least 25 nucleotides in length to inhibit the expression of genes in C. elegans.
- dsRNA has also been shown to degrade target RNA in other organisms, including plants (see, e.g., WO 99/53050, Waterhouse et al.; and WO 99/61631, Heifetz et al.), Drosophila (see, e.g., Yang, D., et al., Curr. Biol. (2000) 10:1191-1200), and mammals (see WO 00/44895, Limmer; and DE 101 00 586.5, Kreutzer et al.).
- the invention provides compositions containing double-stranded ribonucleic acid (dsRNA) and methods for inhibiting the expression of a mutant EGFR gene, such as a deltaEGFR gene, in a cell or mammal.
- the invention also provides compositions and methods for treating pathological conditions and diseases caused by the expression of deltaEGFR gene, such as cancer, including glioma.
- the dsRNAs included in the compositions featured herein include a dsRNA having an RNA strand (the antisense strand) having a region which is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and is substantially complementary to at least part of an mRNA transcript of the deltaEGFR gene.
- the dsRNA also targets a wildtype mRNA transcript of the EGFR gene.
- a dsRNA for inhibiting expression of a deltaEGFR gene includes at least two sequences that are complementary to each other.
- the dsRNA includes a sense strand having a first sequence and an antisense strand having a second sequence.
- the antisense strand includes a nucleotide sequence that is substantially complementary to at least part of an mRNA encoding deltaEGFR, and the region of complementarity is less than 30 nucleotides in length, and at least 15 nucleotides in length.
- the dsRNA is 19 to 24, e.g., 19 to 21 nucleotides in length.
- the dsRNA is from about 10 to about 15 nucleotides in length, and in other embodiments the dsRNA is from about 25 to about 30 nucleotides in length.
- the dsRNA upon contacting with a cell expressing deltaEGFR, inhibits the expression of the deltaEGFR gene by at least 20%, at least 25%, at least 30%, at least 35%, or at least 40%, such as when assayed by a method as described herein.
- the deltaEGFR dsRNA is formulated in a stable nucleic acid particle (SNALP).
- the dsRNA molecules featured herein can include a first sequence of the dsRNA that is selected from the group consisting of the sense sequences of Tables 2, 3 and 4, and a second sequence that is selected from the group consisting of the antisense sequences of Tables 2, 3 and 4.
- the dsRNA molecules featured herein can include naturally occurring nucleotides or can include at least one modified nucleotide, such as a 2′-O-methyl modified nucleotide, a nucleotide having a 5′-phosphorothioate group, and a terminal nucleotide linked to a conjugate group, such as a cholesteryl derivative or a vitamin E group.
- the modified nucleotide may be chosen from the group of: a 2′-deoxy-2′-fluoro modified nucleotide, a T-deoxy-modified nucleotide, a locked nucleotide, an abasic nucleotide, 2′-amino-modified nucleotide, 2′-alkyl-modified nucleotide, morpholino nucleotide, a phosphoramidate, and a non-natural base comprising nucleotide.
- such modified sequence will be based on a first sequence of said dsRNA selected from the group consisting of the sense sequences of Tables 2, 3 and 4 and a second sequence selected from the group consisting of the antisense sequences of Tables 2, 3 and 4.
- an interleukin-6 (IL6) dsRNA is also featured in the invention, and the IL-6 dsRNA is capable of decreasing levels of IL6 protein secretion in cultured cells, e.g., human cultured cells.
- the cultured cells are U87- ⁇ EGFR cells.
- the IL-6 dsRNA is capable of decreasing IL6 secretion into culture supernatant by 20%, 30%, 40%, 50%, 60%, 70%, 80%, 85%, 90% or more.
- the IL6 dsRNA is capable of reducing tumor volume in an animal model, such as in a mouse, rat, or primate model.
- a first sequence of an IL6 dsRNA is selected from the group consisting of the sense sequences of Tables 5, 6, 7, and 8
- a second sequence is selected from the group consisting of the antisense sequences of Tables 5, 6, 7, and 8.
- the invention provides a cell containing at least one of the dsRNAs featured in the invention.
- the cell is generally a mammalian cell, such as a human cell.
- the invention provides a pharmaceutical composition for inhibiting the expression of a deltaEGFR gene in an organism, generally a human subject.
- the composition typically includes one or more of the dsRNAs described herein and a pharmaceutically acceptable carrier or delivery vehicle.
- the composition is used for treating cancer, e.g., a glioma.
- the pharmaceutical composition is formulated for administration of a dosage regimen described herein, e.g., not more than once every four weeks, not more than once every three weeks, not more than once every two weeks, or not more than once every week.
- the pharmaceutical composition can be maintained for a month or longer, e.g., one, two, three, or six months, or one year or longer.
- a composition containing a dsRNA featured in the invention is administered with a non-dsRNA therapeutic agent, such as an agent known to treat a cancer, such as a glioma.
- a dsRNA featured in the invention can be administered with, e.g, a chemotherapeutic agent, such as temozolomide, or with radiation therapy.
- the composition further includes a dsRNA having at least two sequences that are complementary to each other, and where a sense strand includes a region of complementarity that is substantially complementary to at least a part of an mRNA encoding an IL6 protein, and where the region of complementarity is less than 30 nucleotides in length and at least 15 nucleotides in length.
- the IL6 dsRNA is 19 to 24, e.g., 19 to 21 nucleotides in length.
- the dsRNA is from about 10 to about 15 nucleotides in length, and in other embodiments the dsRNA is from about 25 to about 30 nucleotides in length.
- a first sequence of the IL6 dsRNA is selected from the group consisting of the sense sequences of Tables 5, 6, 7, and 8
- a second sequence is selected from the group consisting of the antisense sequences of Tables 5, 6, 7, and 8.
- the deltaEGFR dsRNA is administered to a patient, and then a non-dsRNA agent is administered to the patient (or vice versa).
- the deltaEGFR dsRNA and the non-dsRNA therapeutic agent are administered at the same time.
- the deltaEGFR dsRNA is administered with an IL6 dsRNA, such as for the treatment of cancer.
- the patient has a cancer, e.g., a tumor, such as an astrocytic tumor, or a glioma.
- a cancer e.g., a tumor, such as an astrocytic tumor, or a glioma.
- the invention provides a method for inhibiting the expression of a deltaEGFR gene in a cell by performing the following steps:
- the dsRNA that inhibits expression of the deltaEGFR gene also inhibits expression of a wildtype EGFR gene in the cell.
- a dsRNA can inhibit both deltaEGFR expression and wildtype EGFR expression, because the antisense strand has a region of complementarity that is substantially complementary to at least a part of an mRNA encoding deltaEGFR and at least part of an mRNA encoding wildtype EGFR.
- the method is for inhibiting gene expression in a tumor cell.
- the invention provides methods for treating, preventing or managing pathological processes mediated by deltaEGFR expression, e.g., a cancer, such as a glioma, e.g., a glial tumor of the central nervous system, such as a grade I, II, III, or IV glioma.
- a cancer such as a glioma, e.g., a glial tumor of the central nervous system, such as a grade I, II, III, or IV glioma.
- a dsRNA targeting deltaEGFR is used to treat a grade III glioma, such as anaplastic astrocytoma, or a grade IV glioma, such as a glioblastoma multiforme.
- a dsRNA targeting deltaEGFR is used to treat a carcinoma of the breast, ovary, cervix, kidney, or squamous cell.
- the deltaEGFR dsRNA is administered with a second dsRNA, such as an IL6 dsRNA, for treatment of a disorder associated with deltaEGFR expression.
- the IL6 and deltaEGFR dsRNAs can be administered in combination or sequentially.
- an IL6 dsRNA alone is administered to treat a disorder associated with deltaEGFR expression.
- a method featured in the invention can include administering to a patient in need of such treatment, prevention or management a therapeutically or prophylactically effective amount of one or more of the dsRNAs featured in the invention, e.g., one or both of a dsRNA targeting deltaEGFR or IL6.
- the patient has cancer.
- administration of the dsRNA targeting deltaEGFR and/or the dsRNA targeting IL6, alleviates or relieves the severity of at least one symptom of the deltaEGFR-mediated disorder in the patient.
- the invention provides a vector for inhibiting the expression of a deltaEGFR gene in a cell.
- the vector includes at least one regulatory sequence operably linked to a nucleotide sequence that encodes at least one strand of one of a dsRNA featured in the invention.
- the invention provides a vector for inhibiting the expression of an IL6 gene in a cell.
- the vector includes at least one regulatory sequence operably linked to a nucleotide sequence that encodes at least one strand of one of a dsRNA featured in the invention.
- the invention provides a cell containing a vector for inhibiting the expression of a deltaEGFR gene in a cell.
- the vector includes a regulatory sequence operably linked to a nucleotide sequence that encodes at least one strand of one of the deltaEGFR dsRNA featured in the invention.
- the cell also contains a vector for inhibiting expression of an IL6 gene in a cell.
- This vector also has a regulatory sequence operably linked to a nucleotide sequence that encodes at least one strand of an IL6 dsRNA featured in the invention.
- the invention provides a composition containing a deltaEGFR dsRNA, in combination with a second dsRNA targeting a second gene involved in a pathological disease, and useful for treating the disease, e.g., cancer.
- the second dsRNA is a dsRNA targeting IL6.
- FIGS. 1 A and 1 B are graphs showing tumor growth kinetics ( FIG. 1 A ) and tumor volume ( FIG. 1 B ) following injection of cells subcutaneously into the right flank of 4 to 5 weeks-old female athymic nude mice.
- FIGS. 2 A and 2 B show hematoxylin and eosin (H&E) staining of brain cryo-sections from mice.
- FIG. 2 A is H&E staining of brain cryo-sections from nude mice injected intracranially with cells
- FIG. 2 B shows H&E staining of brain cryo-sections of mice injected intracranially with cells either alone or mixed with deltaEGFR over-expressing astrocytes (upper panel).
- the lower panel shows the presence of wtEGFR astrocytes within the tumor by immunofluorescence (GFP IF).
- FIG. 3 A is a graph showing the composition of various tumor samples in 4 to 5 week old mice injected with the indicated cell types, as assayed by X-Gal staining.
- FIG. 3 B is a graph showing the composition of tumor samples as assayed by flow cytometry using Ab-1 (FITC) and Ab-5 (APC) antibodies to stain tumors formed after infection of U87wt mixed with U87delta at the indicated ratios.
- FITC Ab-1
- APC Ab-5
- FIG. 4 is a panel of Western blots showing analysis of EGFR activation and known downstream signaling molecules in U87wt cells stimulated with serial dilutions of U87delta CM or negative control U87Par CM, or positive control EGF ligand.
- FIGS. 5 A- 5 C are Western blots showing the activity of siRNAs targeting deltaEGFR ( FIG. 5 A ), wildtype EGFR ( FIG. 5 B ), or both mutant and wildtype receptors ( FIG. 5 C ).
- Cl, C2, C3, and CA indicate negative controls (untransfected cells).
- FIGS. 6 A- 6 C are Western blots showing the activity of siRNAs targeting deltaFGFR ( FIG. 6 A ) or wildtype EGFR ( FIG. 6 C ).
- FIG. 6 B shows the activity of siRNAs specific for deltaEGFR (AD-13375) or wtEGFR (AD-13377) in U87-deltaEGFR cells or U87-wtEGFR cells, respectively.
- Luc and ( ⁇ ) indicate negative controls (cells transfected with an irrelevant gene siRNA (targeting luciferase) and untransfected cells, respectively).
- FIGS. 7 A and 7 B are Western blots showing dose response activity of siRNA activity in U87-deltaEGFR cells ( FIG. 7 A ) and in U87-wtEGFR cells ( FIG. 7 B ).
- U87-deltaEGFR cells were transfected with siRNAs specific for deltaEGFR, and for both mutant and wildtype receptors.
- U87-wtEGFR cells were transfected with siRNAs specific for wtEGFR, and for both mutant and wildtype receptors.
- C1, C2, C3, C4, C5, and C6 indicate negative controls (untransfected cells).
- FIGS. 8 A- 8 D are Western blots showing durability of the effect of unstabilized ( FIGS. 8 A and 8 B ) and stabilized ( FIGS. 8 C and 8 D ) siRNAs.
- FIGS. 8 A and 8 C U87-deltaEGFR cells were transfected with non-stabilized ( FIG. 8 A ) or stabilized ( FIG. 8 C ) siRNAs.
- FIGS. 8 B and 8 D U87-wtEGFR cells were transfected with unstabilized ( FIG. 8 B ) or stabilized ( FIG. 8 D ) siRNAs. Lysates were prepared and Western blots were performed at the indicated day post-transfection. Luc and ( ⁇ ) indicate negative controls (cells transfected with an irrelevant gene siRNA (targeting luciferase) and untransfected cells, respectively).
- FIGS. 9 A and 9 B are graphs showing the effects of siRNAs on tumorigenicity in mice injected with U87-wtEGFR cells ( FIG. 9 A ) and in mice injected with U87-deltaEGFR cells ( FIG. 9 B ). Lysates were prepared and Western blots were performed at the indicated day post-transfection. Data are shown as mean ⁇ standard deviation (SD).
- FIGS. 10 A and 10 B are graphs showing tumor kinetics ( FIG. 10 A ) and volume ( FIG. 10 B ) in nude mice injected with U87delta cells and then injected intratumorally with 5 mg of deltaEGFR siRNA#1 or irrelevant siRNA (siRNA luc).
- FIG. 11 is the mRNA sequence of IL-6 reported at GenBank Accession No. NM_000600.2 (record dated Jan. 4, 2009, GI No.155369258; SEQ ID NO:274).
- FIGS. 12 A and 12 B are graphs showing screening analysis of stabilized siRNAs designed for IL-6 by ELISA.
- FIG. 12 A demonstrates the effect of the siRNAs on their target cytokines. Specificity of the siRNAs for IL-6 was assessed by quantifying IL-8 levels ( FIG. 12 B ). Values are mean ⁇ SE of 2 independent samples. (“Neg”: siRNA targeting an irrelevant sequence).
- FIGS. 13 A and 13 B are graphs showing dose-response analysis of stabilized IL-6 siRNAs. Quantification of secreted IL-6 ( FIG. 13 A ) and IL-8 ( FIG. 13 B ) in supernatants from U87- ⁇ EGFR cells transfected with 100, 20, 4, and 0.8 nM of different IL-6 siRNAs was performed by ELISA. Values are mean ⁇ SE of 2 independent samples. (“Neg”: siRNA targeting an irrelevant sequence).
- FIG. 14 is a graph illustrating durability analysis of stabilized IL-6 siRNAs. Quantification of secreted IL-6 in supernatants of U87- ⁇ EGFR cells at different days after transfection with the siRNAs was performed by ELISA. (“Neg2”: siRNA targeting an irrelevant sequence).
- FIGS. 15 A and 15 B are two graphs illustrating ex vivo tumorigenicity test of IL-6-specific stabilized siRNAs.
- the graphs show tumor growth kinetics (top) and tumor volume at the end of the experiment (bottom) after injection into nude mice of U87-deltaEGFR cells transfected with stabilized siRNAs against luciferase or GFP, IL-6 (AD-15644 and AD-15660).
- the experimental group included 6 animals. Data are shown as mean +SE.
- FIGS. 16 A and 16 B are graphs illustrating the efficacy of in vivo delivery of IL-6 siRNAs (AD-15644 and AD-15660).
- Tumor growth kinetics FIG. 11 A
- tumor volume at the end of the experiment FIG. 11 B
- Each group included 4 animals. Data are shown as mean+SE.
- FIGS. 17 A and 17 B are graphs illustrating tumor volume (A) and tumor growth kinetics (B) after subcutaneous injection of the indicated cell types into nude mice. Tumor volume ( FIG. 17 A ) was assayed at day 32 after injection.
- FIG. 18 is a graph illustrating quantification of soft agar colony formation of U87wt colonies formed after treatment with normal media (Neg), or U87 ⁇ (ACM (conditioned medium)), U87Par (parental CM), or U87Par-IL6 (parental-IL6) CM (*: p ⁇ 0.05; ** p ⁇ 0.001).
- FIG. 19 is a graph illustrating quantification of soft agar colony formation of U87wt colonies formed after treatment with normal media (negative control, Neg), or with U87 ⁇ cell CM untreated ( ⁇ CM) or pretreated with IL-6 neutralizing antibody (ACM +IL-6 Ab) (**: p ⁇ 0.001).
- FIGS. 20 A and 20 B are graphs illustrating tumor growth kinetics (A) and tumor volume at day 20 (B) after subcutaneous injection of U87wt, U87 ⁇ -Luc siRNA or U87 ⁇ -IL6 siRNA cells only, or U87wt cells mixed with U87 ⁇ -Luc siRNA or U87 ⁇ -IL6 siRNA cells at a ratio of 90:10.
- FIGS. 21 A and 21 B represent the mRNA sequence of wtEGFR (SEQ ID NO:1) (GenBank Accession No. NM_005228; record dated Aug. 24, 2008, GI No. 41327737).
- the underlined nucleotides are deleted in deltaEGFR mRNA sequence.
- the invention provides dsRNAs and methods of using the dsRNAs for inhibiting the expression of a deltaEGFR gene in a cell or a mammal where the dsRNA targets the deltaEGFR gene.
- the dsRNAs featured in the invention target both a deltaEGFR gene and a wildtype EGFR (wtEGFR) gene.
- the invention also provides dsRNAs and methods of using the dsRNAs for inhibiting the expression of an IL6 gene in a cell or a mammal where the dsRNA targets the IL6 gene.
- the invention provides compositions and methods for treating pathological conditions and diseases, such as a cancer, in a mammal caused by the expression of the deltaEGFR or IL6 genes.
- dsRNA directs the sequence-specific degradation of mRNA through a process known as RNA interference (RNAi).
- RNAi RNA interference
- the dsRNAs of the compositions featured herein include an RNA strand (the antisense strand) having a region which is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and is substantially complementary to at least part of an mRNA transcript of the deltaEGFR gene.
- the use of these dsRNAs enables the targeted degradation of mRNAs of genes that are implicated in replication or maintenance of cancer cells in mammals.
- Very low dosages of deltaEGFR or IL6 dsRNAs in particular can specifically and efficiently mediate RNAi, resulting in significant inhibition of expression of the deltaEGFR and IL6 genes.
- dsRNAs targeting deltaEGFR alone, or targeting both deltaEGFR and wtEGFR can specifically and efficiently mediate RNAi, resulting in significant inhibition of expression of one or both of the deltaEGFR or EGFR genes.
- methods and compositions including these dsRNAs are useful for treating pathological processes that can be mediated by down regulating deltaEGFR and EGFR, such as in the treatment of cancer.
- dsRNAs targeting IL6 can also specifically and efficiently mediate RNAi, resulting in significant inhibition of expression of an IL6 gene.
- methods and compositions including these dsRNAs are useful for treating pathological processes that can be mediated by down regulating IL6, such as in the treatment of cancer.
- compositions featured in the invention also include a dsRNA having an antisense strand having a region of complementarity which is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and which is substantially complementary to at least part of an RNA transcript of the deltaEGFR or IL6 gene.
- compositions containing the deltaEGFR dsRNA and a pharmaceutically acceptable carrier are featured in the invention.
- G,” “C,” “A,” “T” and “U” each generally stand for a nucleotide that contains guanine, cytosine, adenine, thymidine and uracil as a base, respectively.
- ribonucleotide or “nucleotide” can also refer to a modified nucleotide, as further detailed below, or a surrogate replacement moiety.
- guanine, cytosine, adenine, thymidine, and uracil may be replaced by other moieties without substantially altering the base pairing properties of an oligonucleotide comprising a nucleotide bearing such replacement moiety.
- a nucleotide comprising inosine as its base may base pair with nucleotides containing adenine, cytosine, or uracil.
- nucleotides containing uracil, guanine, or adenine may be replaced in the nucleotide sequences of dsRNA featured in the invention by a nucleotide containing, for example, inosine.
- adenine and cytosine anywhere in the oligonucleotide can be replaced with guanine and uracil, respectively to form G-U Wobble base pairing with the target mRNA. Sequences containing such replacement moieties are suitable for the compositions and methods featured in the invention.
- deltaEGFR refers to an in-frame deletion of exons 2-7 from the EGFR gene.
- deltaEGFR is also known as “de 2-7 EGFR” (Nishikawa et al. “A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity” Proc. Natl. Acad. Sci. USA 91:7727-7731, 1994), “EGFR-de2-7”, “EGFR*”, “ ⁇ EGFR”, and “EGFRvIII.”
- the sequence of deltaEGFR is equivalent to the sequence shown at FIGS. 21 A and 21 B carrying a deletion of nucleotides 335 through 1135.
- wild-type EGFR refers to a non-mutant EGFR gene (e.g., an endogenous EGFR gene) in a cell, such as in a non-transformed, or non-cancerous cell in a human.
- EGFR is also known as EC2.7.10.1 (Epidermal Growth Factor Receptor Precursor), ERBB (Receptor Protein Tyrosine Kinase ErbB1), ERBB1, HER1, PIG61 (cell proliferation-inducing protein 61), mENA, avian erythroblastic leukemia viral (v-erb-b) oncogene homolog, and cell growth inhibiting protein 40.
- Interleukin-6 refers to an IL-6 gene (e.g., an endogenous IL-6 gene) in a cell, such as in a non-transformed, or non-cancerous cell in a human.
- IL-6 is also known as Interleukin 6; IFNB2 (Interferon beta 2, or interferon, beta 2); BSF-2 (B-cell stimulatory factor 2); BSF2; CDF (CTL differentiation factor); HGF (hybridoma growth factor); HSF.
- IFNB2 Interferon beta 2, or interferon, beta 2
- BSF-2 B-cell stimulatory factor 2
- BSF2 BSF2
- CDF CTL differentiation factor
- HGF hybrida growth factor
- HSF hybridothelial growth factor
- target sequence of a dsRNA refers to a contiguous portion of the nucleotide sequence of an mRNA molecule formed during the transcription of the target gene, e.g., a deltaEGFR gene or an IL-6 gene, including mRNA that is a product of RNA processing of a primary transcription product.
- strand comprising a sequence refers to an oligonucleotide comprising a chain of nucleotides that is described by the sequence referred to using the standard nucleotide nomenclature.
- the term “complementary,” when used to describe a first nucleotide sequence in relation to a second nucleotide sequence, refers to the ability of an oligonucleotide or polynucleotide comprising the first nucleotide sequence to hybridize and form a duplex structure under certain conditions with an oligonucleotide or polynucleotide comprising the second nucleotide sequence, as will be understood by the skilled person.
- Such conditions can, for example, be stringent conditions, where stringent conditions may include: 400 mM NaCl, 40 mM PIPES pH 6.4, 1 mM EDTA, 50° C. or 70° C. for 12-16 hours followed by washing.
- sequences can be referred to as “fully complementary” with respect to each other herein.
- first sequence is referred to as “substantially complementary” with respect to a second sequence herein
- the two sequences can be fully complementary, or they may form one or more, but generally not more than 4, 3 or 2 mismatched base pairs upon hybridization, while retaining the ability to hybridize under the conditions most relevant to their ultimate application.
- a dsRNA comprising one oligonucleotide 21 nucleotides in length and another oligonucleotide 23 nucleotides in length, wherein the longer oligonucleotide comprises a sequence of 21 nucleotides that is fully complementary to the shorter oligonucleotide, may yet be referred to as “fully complementary” for the purposes described herein.
- “Complementary” sequences may also include, or be formed entirely from, non-Watson-Crick base pairs and/or base pairs formed from non-natural and modified nucleotides, insofar as the above requirements with respect to their ability to hybridize are fulfilled.
- Such non-Watson-Crick base pairs includes, but not limited to, G:U Wobble or Hoogstein base pairing.
- a polynucleotide that is “substantially complementary to at least part of” a messenger RNA (mRNA) refers to a polynucleotide that is substantially complementary to a contiguous portion of an mRNA of interest (e.g., encoding deltaEGFR or IL6).
- mRNA messenger RNA
- a polynucleotide is complementary to at least a part of a deltaEGFR mRNA if the sequence is substantially complementary to a non-interrupted portion of an mRNA encoding deltaEGFR.
- a polynucleotide is complementary to at least a part of a wtEGFR mRNA if the sequence is substantially complementary to a non-interrupted portion of an mRNA encoding wtEGFR.
- double-stranded RNA refers to a complex of ribonucleic acid molecules, having a duplex structure comprising two anti-parallel and substantially complementary, as defined above, nucleic acid strands.
- the two strands forming the duplex structure may be different portions of one larger RNA molecule, or they may be separate RNA molecules.
- the connecting RNA chain is referred to as a “hairpin loop.”
- the connecting structure is referred to as a “linker.”
- the RNA strands may have the same or a different number of nucleotides.
- a dsRNA may comprise one or more nucleotide overhangs.
- nucleotide overhang refers to the unpaired nucleotide or nucleotides that protrude from the duplex structure of a dsRNA when a 3′-end of one strand of the dsRNA extends beyond the 5′-end of the other strand, or vice versa.
- “Blunt” or “blunt end” means that there are no unpaired nucleotides at that end of the dsRNA, i.e., no nucleotide overhang.
- a “blunt ended” dsRNA is a dsRNA that is double-stranded over its entire length, i.e., no nucleotide overhang at either end of the molecule.
- antisense strand refers to the strand of a dsRNA which includes a region that is substantially complementary to a target sequence.
- region of complementarity refers to the region on the antisense strand that is substantially complementary to a sequence, for example a target sequence, as defined herein. Where the region of complementarity is not fully complementary to the target sequence, the mismatches may be in the internal or terminal regions of the molecule. Generally, the most tolerated mismatches are in the terminal regions, e.g., within 6, 5, 4, 3, or 2 nucleotides of the 5′ and/or 3′ terminus.
- sense strand refers to the strand of a dsRNA that includes a region that is substantially complementary to a region of the antisense strand.
- identity is the relationship between two or more polynucleotide sequences, as determined by comparing the sequences. Identity also means the degree of sequence relatedness between polynucleotide sequences, as determined by the match between strings of such sequences. While there exist a number of methods to measure identity between two polynucleotide sequences, the term is well known to skilled artisans (see, e.g., Sequence Analysis in Molecular Biology, von Heinje, G., Academic Press (1987); and Sequence Analysis Primer, Gribskov., M. and Devereux, J., eds., M. Stockton Press, New York (1991)).
- “Substantially identical,” as used herein, means there is a very high degree of homology (e.g., 100% sequence identity) between the sense strand of the dsRNA and the corresponding part of the target gene.
- dsRNA having greater than 90%, or 95% sequence identity may be used in the present invention, and thus sequence variations that might be expected due to genetic mutation, strain polymorphism, or evolutionary divergence can be tolerated.
- the dsRNA is typically 100% complementary to the target RNA, but in some embodiments, the dsRNA may contain single or multiple base-pair random mismatches between the RNA and the target gene.
- SNALP refers to a stable nucleic acid-lipid particle.
- a SNALP represents a vesicle of lipids coating a reduced aqueous interior comprising a nucleic acid such as an iRNA agent or a plasmid from which an iRNA agent is transcribed.
- SNALPs are described, e.g., in U.S. Patent Application Publication Nos. 20060240093, 20070135372, and USSN 61/045,228 filed Apr. 15, 2008. These applications are hereby incorporated by reference.
- dsRNA “Introducing into a cell,” when referring to a dsRNA, means facilitating uptake or absorption into the cell, as is understood by those skilled in the art. Absorption or uptake of dsRNA can occur through unaided diffusive or active cellular processes, or by auxiliary agents or devices. The meaning of this term is not limited to cells in vitro; a dsRNA may also be “introduced into a cell,” wherein the cell is part of a living organism. In such instance, introduction into the cell will include the delivery to the organism.
- dsRNA can be injected into a tissue site or administered systemically. In vivo delivery can also be by a beta-glucan delivery system, such as those described in U.S. Pat.
- the terms “silence,” “inhibit the expression of,” “down-regulate the expression of,” “suppress the expression of,” and the like, insofar as they refer to a deltaEGFR or IL6 gene refer to the at least partial suppression of expression of the deltaEGFR or IL6 gene, as manifested by a reduction of the amount of deltaEGFR or IL6 mRNA which may be isolated or detected from a first cell or group of cells in which the deltaEGFR or IL6 gene is transcribed and which has or have been treated such that the expression of the deltaEGFR or IL6 gene is inhibited, as compared to a second cell or group of cells substantially identical to the first cell or group of cells but which has or have not been so treated (control cells).
- the degree of inhibition is usually expressed in terms of
- the degree of inhibition may be given in terms of a reduction of a parameter that is functionally linked to gene expression, e.g., the amount of protein encoded by the deltaEGFR or IL6 gene which is secreted by a cell, or the number of cells displaying a certain phenotype, e.g., apoptosis.
- gene silencing may be determined in any cell expressing the target gene, either constitutively or by genomic engineering, and by any appropriate assay.
- the assays provided in the Examples below shall serve as such reference.
- deltaEGFR gene silencing may be determined in U87-deltaEGFR (Nishikawa et al., PNAS 91:7727-7731, 1994) or U87-wtEGFR (Nagana et al., Cancer Research 56:5079-5086, 1996) cells.
- expression of the deltaEGFR gene or IL6 gene is suppressed by at least about 20%, 25%, 30%, 35%, 40%, 45%, or 50% by administration of a double-stranded oligonucleotide featured in the invention.
- the deltaEGFR or IL6 gene is suppressed by at least about 60%, 70%, or 80% by administration of the double-stranded oligonucleotide featured in the invention.
- the deltaEGFR gene is suppressed by at least about 85%, 90%, or 95% by administration of the double-stranded oligonucleotide featured in the invention.
- Table 4, for example, and FIGS. 7 - 9 indicate a range of inhibition of expression obtained in in vitro and ex vivo assays using various deltaEGFR dsRNA molecules at various concentrations.
- the terms “treat,” “treatment,” and the like refer to relief from or alleviation of pathological processes mediated by deltaEGFR or IL6 gene expression. Insofar as they relate to any of the other conditions recited herein below (other than pathological processes mediated by deltaEGFR expression), the terms “treat,” “treatment,” and the like mean to relieve or alleviate at least one symptom associated with such condition, or to slow or reverse the progression of such condition, such as the slowing and progression of glioma.
- the phrases “therapeutically effective amount” and “prophylactically effective amount” refer to an amount that provides a therapeutic benefit in the treatment, prevention, or management of pathological processes mediated by deltaEGFR or IL6 expression or an overt symptom of pathological processes mediated by deltaEGFR or IL6 expression.
- the specific amount that is therapeutically effective can be readily determined by an ordinary medical practitioner, and may vary depending on factors known in the art, such as, for example, the type of pathological processes mediated by deltaEGFR or IL6 expression, the patient's history and age, the stage of pathological processes mediated by deltaEGFR or IL6 expression, and the administration of other anti-pathological processes mediated by deltaEGFR or IL6 expression agents.
- a “pharmaceutical composition” comprises a pharmacologically effective amount of a dsRNA and a pharmaceutically acceptable carrier.
- pharmaceutically effective amount refers to that amount of an RNA effective to produce the intended pharmacological, therapeutic or preventive result. For example, if a given clinical treatment is considered effective when there is at least a 25% reduction in a measurable parameter associated with a disease or disorder, a therapeutically effective amount of a drug for the treatment of that disease or disorder is the amount necessary to effect at least a 25% reduction in that parameter.
- pharmaceutically acceptable carrier refers to a carrier for administration of a therapeutic agent.
- Such carriers include, but are not limited to, saline, buffered saline, dextrose, water, glycerol, ethanol, and combinations thereof.
- the term specifically excludes cell culture medium.
- pharmaceutically acceptable carriers include, but are not limited to pharmaceutically acceptable excipients such as inert diluents, disintegrating agents, binding agents, lubricating agents, sweetening agents, flavoring agents, coloring agents and preservatives.
- suitable inert diluents include sodium and calcium carbonate, sodium and calcium phosphate, and lactose, while corn starch and alginic acid are suitable disintegrating agents.
- Binding agents may include starch and gelatin, while the lubricating agent, if present, will generally be magnesium stearate, stearic acid or talc. If desired, the tablets may be coated with a material such as glyceryl monostearate or glyceryl distearate, to delay absorption in the gastrointestinal tract.
- a “transformed cell” is a cell into which a vector has been introduced from which a dsRNA molecule may be expressed.
- Double-Stranded Ribonucleic Acid dsRNA
- the invention provides double-stranded ribonucleic acid (dsRNA) molecule for inhibiting expression of a deltaEGFR gene in a cell or mammal, e.g., in a human having a cancer, such as a glioma, where the dsRNA includes an antisense strand having a region of complementarity which is complementary to at least a part of an mRNA formed in the expression of the deltaEGFR gene, and where the region of complementarity is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and where said dsRNA, upon contact with a cell expressing said deltaEGFR gene, inhibits the expression of said deltaEGFR gene by at least 30% as assayed by, for example, a PCR or branched DNA (bDNA)-based method, or by a protein-based method, such as by Western blot.
- dsRNA double-stranded ribonucleic acid
- Expression of the deltaEGFR gene can be reduced by at least 30% when measured by an assay as described in the Examples below.
- the expression of wtEGFR may also be reduced by at least 30%, e.g., as assayed by a method described herein, and the level of reduced expression of deltaEGFR and wtEGFR may be different.
- the reduction in deltaEGFR or wtEGFR expression can also be assayed by measuring protein levels, such as by Western blot analysis.
- the invention provides a double-stranded ribonucleic acid (dsRNA) molecule for inhibiting expression of an IL6 gene in a cell or mammal, e.g., in a human having a cancer, such as a glioma, where the dsRNA includes an antisense strand having a region of complementarity which is complementary to at least a part of an mRNA formed in the expression of the IL6 gene, and where the region of complementarity is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and where the dsRNA, upon contact with a cell expressing the IL6 gene, inhibits the expression of the gene by at least 30% as assayed by, for example, a PCR or branched DNA (bDNA)-based method, or by a protein-based method, such as by Western blot.
- dsRNA double-stranded ribonucleic acid
- a dsRNA featured in the invention includes two RNA strands that are sufficiently complementary to hybridize to form a duplex structure.
- One strand of the dsRNA includes a region of complementarity that is substantially complementary, and generally fully complementary, to a target sequence, derived from the sequence of an mRNA formed during the expression of the target gene
- the other strand includes a region that is complementary to the antisense strand, such that the two strands hybridize and form a duplex structure when combined under suitable conditions.
- the region of the antisense strand that is substantially complementary to a sequence of a deltaEGFR mRNA is also substantially complementary to a wtEGFR mRNA.
- the duplex structure of a dsRNA featured herein is between 15 and 30, more generally between 18 and 25, yet more generally between 19 and 24, and most generally between 19 and 21 base pairs in length.
- the region of complementarity to the target sequence is between 15 and 30, more generally between 18 and 25, yet more generally between 19 and 24, and most generally between 19 and 21 nucleotides in length.
- the dsRNA is between 10 and 15 nucleotides in length, and in other embodiments, the dsRNA is between 25 and 30 nucleotides in length.
- the dsRNA featured in the invention may further include one or more single-stranded nucleotide overhangs.
- the dsRNA can be synthesized by standard methods known in the art as further discussed below, e.g., by use of an automated DNA synthesizer, such as are commercially available from, for example, Biosearch, Applied Biosystems, Inc.
- the deltaEGFR gene is a human deltaEGFR gene
- the wtEGFR gene is a human wtEGFR gene.
- the first sequence is a sense strand of the dsRNA that includes a sense sequence from Tables 2 or 3
- the second sequence is an antisense strand that includes an antisense sequence from Tables 2 or 3.
- Alternative antisense agents that target elsewhere in the target sequence provided in Tables 2 or 3 can readily be determined using the target sequence and the flanking deltaEGFR sequence.
- the dsRNA targeting deltaEGFR will include at least two nucleotide sequences selected from the groups of sequences provided in Tables 2 or 3. One of the two sequences is complementary to the other of the two sequences, with one of the sequences being substantially complementary to a sequence of an mRNA generated in the expression of the deltaEGFR gene.
- the dsRNA will include two oligonucleotides, where one oligonucleotide is described as the sense strand in Tables 2 or 3 and the second oligonucleotide is described as the antisense strand in Tables 2 or 3.
- the dsRNA will target an IL-6 gene, e.g., a human IL-6 gene.
- the first sequence of the dsRNA is a sense strand that includes a sense sequence from Tables 5-8
- the second sequence is an antisense strand that includes an antisense sequence from Tables 5-8.
- Alternative antisense agents that target elsewhere in the target sequence provided in Tables 5-8 can readily be determined using the target sequence and the flanking IL-6 sequence.
- a dsRNA targeting IL-6 will include at least two nucleotide sequences selected from the groups of sequences provided in Tables 5-8. One of the two sequences is complementary to the other of the two sequences, with one of the sequences being substantially complementary to a sequence of an mRNA generated in the expression of the IL-6 gene.
- the dsRNA will include two oligonucleotides, where one oligonucleotide is described as the sense strand in Tables 6-10 and the second oligonucleotide is described as the antisense strand in Tables 6-10.
- the IL-6 dsRNA does not have a sense or antisense strand consisting of the sequences shown in Tables 6A or 6B of WO 2007/064846.
- the dsRNA does not consist of the sequence of SEQ ID NO:1 of US2008/0234218, and its complementary sequence of SEQ NO:2; the sequence of SEQ ID NO:3 of US2008/0234218, and its complementary sequence of SEQ NO:4; or the sequence of SEQ ID NO:5 of US2008/0234218, and its complementary sequence of SEQ NO:6.
- the skilled person is well aware that dsRNAs having a duplex structure of between 20 and 23, but specifically 21, base pairs have been hailed as particularly effective in inducing RNA interference (Elbashir et al., EMBO 2001, 20:6877-6888). However, others have found that shorter or longer dsRNAs can be effective as well.
- the dsRNAs featured in the invention can include at least one strand of a length of minimally 21 nt.
- dsRNAs having one of the sequences of Tables 2 or 3, or 5-8 minus only a few nucleotides on one or both ends may be similarly effective as compared to the dsRNAs described above.
- dsRNAs having a partial sequence of at least 15, 16, 17, 18, 19, 20, or more contiguous nucleotides from one of the sequences of Tables 2, 3 and 5-8, and differing in their ability to inhibit the expression of the respective target genes, e.g., as measured by a FACS assay as described herein below by not more than 5, 10, 15, 20, 25, or 30% inhibition from a dsRNA comprising the full sequence are contemplated by the invention.
- dsRNAs that cleave within the desired target sequence can readily be made using the corresponding deltaEGFR or IL6 antisense sequence and a complementary sense sequence.
- the dsRNAs provided in Tables 2 and 3 identify a site in a deltaEGFR mRNA and the wtEGFR sequence that is susceptible to RNAi based cleavage
- the dsRNAs provided in Tables 5-8 identify a site in an IL6 mRNA susceptible to RNAi based cleavage
- the present invention further provides dsRNAs that target within the sequence targeted by one of the other agents featured in the invention.
- a second dsRNA is said to target within the sequence of a first dsRNA if the second dsRNA cleaves the message anywhere within the mRNA that is complementary to the antisense strand of the first dsRNA.
- Such a second dsRNA will generally consist of at least 15 contiguous nucleotides from one of the sequences provided in Tables 2, 3 or 5-8, coupled to an additional nucleotide sequence taken from the region contiguous to the selected sequence in the target gene, e.g., the deltaEGFR gene, the wtEGFR gene, or the IL6 gene.
- the last 15 nucleotides of SEQ ID NO:2 combined with the next six nucleotides from the target deltaEGFR gene produces a single strand agent of 21 nucleotides that is based on one of the sequences provided in Tables 2 and 3.
- the dsRNA featured in the invention can contain one or more mismatches to the target sequence.
- the dsRNA contains no more than 3 mismatches. If the antisense strand of the dsRNA contains mismatches to a target sequence, it is preferable that the area of mismatch not be located in the center of the region of complementarity. If the antisense strand of the dsRNA contains mismatches to the target sequence, it is preferable that the mismatch be restricted to 5 nucleotides from either end, for example 5, 4, 3, 2, or 1 nucleotide from either the 5′ or 3′ end of the region of complementarity.
- the dsRNA generally does not contain any mismatch within the central 13 nucleotides.
- the methods described within the invention can be used to determine whether a dsRNA containing a mismatch to a target sequence is effective in inhibiting the expression of the target gene, e.g., a deltaEGFR gene or an IL6 gene. Consideration of the efficacy of dsRNAs with mismatches in inhibiting expression of the target gene is important, especially if the particular region of complementarity in the target gene is known to have polymorphic sequence variation within the population.
- At least one end of the dsRNA has a single-stranded nucleotide overhang of 1 to 4, generally 1 or 2 nucleotides.
- dsRNAs having at least one nucleotide overhang have unexpectedly superior inhibitory properties than their blunt-ended counterparts.
- the present inventors have discovered that the presence of only one nucleotide overhang strengthens the interference activity of the dsRNA, without affecting its overall stability.
- dsRNA having only one overhang has proven particularly stable and effective in vivo, as well as in a variety of cells, cell culture mediums, blood, and serum.
- the single-stranded overhang is located at the 3′-terminal end of the antisense strand or, alternatively, at the 3′-terminal end of the sense strand.
- the dsRNA may also have a blunt end, generally located at the 5′-end of the antisense strand.
- Such dsRNAs have improved stability and inhibitory activity, thus allowing administration at low dosages, i.e., less than 5 mg/kg body weight of the recipient per day.
- the antisense strand of the dsRNA has a 1-10 nucleotide overhang at the 3′-end and/or the 5′ end.
- the sense strand of the dsRNA has a 1-10 nucleotide overhang at the 3′ end and/or the 5′ end. In another embodiment, one or more of the nucleotides in the overhang is replaced with a nucleoside thiophosphate.
- the dsRNA is chemically modified to enhance stability.
- the nucleic acids featured in the invention may be synthesized and/or modified by methods well established in the art, such as those described in “Current protocols in nucleic acid chemistry,” Beaucage, S.L. et al. (Edrs.), John Wiley & Sons, Inc., New York, N.Y., USA, which is hereby incorporated herein by reference.
- Specific examples of dsRNA compounds useful in this invention include dsRNAs containing modified backbones or no natural internucleoside linkages.
- dsRNAs having modified backbones include those that retain a phosphorus atom in the backbone and those that do not have a phosphorus atom in the backbone.
- modified dsRNAs that do not have a phosphorus atom in their internucleoside backbone can also be considered to be oligonucleosides.
- Modified dsRNA backbones include, for example, phosphorothioates, chiral phosphorothioates, phosphorodithioates, phosphotriesters, aminoalkylphosphotriesters, methyl and other alkyl phosphonates including 3′-alkylene phosphonates and chiral phosphonates, phosphinates, phosphoramidates including 3′-amino phosphoramidate and aminoalkylphosphoramidates, thionophosphoramidates, thionoalkylphosphonates, thionoalkylphosphotriesters, and boranophosphates having normal 3′-5′ linkages, 2′-5′ linked analogs of these, and those) having inverted polarity wherein the adjacent pairs of nucleoside units are linked 3′-5′ to 5′-3′ or 2′-5′ to 5′-2′.
- Various salts, mixed salts and free acid forms are also included.
- Modified dsRNA backbones that do not include a phosphorus atom have backbones that are formed by short chain alkyl or cycloalkyl internucleoside linkages, mixed heteroatoms and alkyl or cycloalkyl internucleoside linkages, or ore or more short chain heteroatomic or heterocyclic internucleoside linkages.
- morpholino linkages formed in part from the sugar portion of a nucleoside
- siloxane backbones sulfide, sulfoxide and sulfone backbones
- formacetyl and thioformacetyl backbones methylene formacetyl and thioformacetyl backbones
- alkene containing backbones sulfamate backbones
- sulfonate and sulfonamide backbones amide backbones; and others having mixed N, O, S and CH 2 component parts.
- both the sugar and the internucleoside linkage, i.e., the backbone, of the nucleotide units are replaced with novel groups.
- the base units are maintained for hybridization with an appropriate nucleic acid target compound.
- a dsRNA mimetic that has been shown to have excellent hybridization properties, is referred to as a peptide nucleic acid (PNA).
- PNA peptide nucleic acid
- the sugar backbone of a dsRNA is replaced with an amide containing backbone, in particular an aminoethylglycine backbone.
- the nucleobases are retained and are bound directly or indirectly to aza nitrogen atoms of the amide portion of the backbone. Representative U.S.
- PNA compounds include, but are not limited to, U.S. Pat. Nos. 5,539,082; 5,714,331; and 5,719,262, each of which is incorporated herein by reference. Further teaching of PNA compounds can be found in Nielsen et al., Science, 1991, 254, 1497-1500.
- Most embodiments featured in the invention include dsRNAs with phosphorothioate backbones and oligonucleosides with heteroatom backbones, and in particular —CH 2 —NH—CH 2 —, —CH 2 —N(CH 3 )—O—CH 2 —[known as a methylene (methylimino) or MMI backbone], —CH 2 —O—N(CH 3 )—CH 2 —, —CH 2 —N(CH 3 )—N(CH 3 )—CH 2 —and —N(CH 3 )—CH 2 —CH 2 —[wherein the native phosphodiester backbone is represented as —O—P—O—CH 2 —] of the above-referenced U.S.
- the dsRNAs featured herein have morpholino backbone structures of the above-referenced U.S. Pat. No. 5,034,506.
- Modified dsRNAs may also contain one or more substituted sugar moieties.
- the dsRNAs featured herein can have one of the following at the 2′ position: OH; F; O—, S—, or N—alkyl; O—, S—, or N-alkenyl; O—, S— or N-alkynyl; or O-alkyl-O-alkyl, wherein the alkyl, alkenyl and alkynyl may be substituted or unsubstituted C 1 to C 10 alkyl or C 2 to C 10 alkenyl and alkynyl.
- Exemplary suitable modifications include O[(CH 2 ) n O] m CH 3 , O(CH 2 ) n OCH 3 , O(CH 2 ) n NH 2 , O(CH 2 ) n CH 3 , O(CH 2 ) n ONH 2 , and O(CH 2 ) n ON[(CH 2 ) n CH 3 )] 2 , where n and m are from 1 to about 10.
- dsRNAs include one of the following at the 2′ position: C 1 to C 10 lower alkyl, substituted lower alkyl, alkaryl, aralkyl, O-alkaryl or O-aralkyl, SH, SCH 3 , OCN, Cl, Br, CN, CF 3 , OCF 3 , SOCH 3 , SO 2 CH 3 , ONO 2 , NO 2 , N 3 , NH 2 , heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalkylamino, substituted silyl, an RNA cleaving group, a reporter group, an intercalator, a group for improving the pharmacokinetic properties of a dsRNA, or a group for improving the pharmacodynamic properties of a dsRNA, and other substituents having similar properties.
- the modification includes a 2′-methoxyethoxy (2′-O—CH 2 CH 2 OCH 3 , also known as 2′-O-(2-methoxyethyl) or 2′-MOE) (Martin et al., Helv. Chim. Acta, 1995, 78:486-504) i.e., an alkoxy-alkoxy group.
- 2′-dimethylaminooxyethoxy i.e., a O(CH 2 ) 2 ON(CH 3 ) 2 group, also known as 2′-DMAOE, as described in examples hereinbelow
- 2′-dimethylaminoethoxyethoxy also known in the art as 2′-O-dimethylaminoethoxyethyl or 2′-DMAEOE
- 2′-O—CH 2 —O—CH 2 —N(CH 2 ) 2 also described in examples hereinbelow.
- modifications include 2′-methoxy (2′-OCH 3 ), 2′-aminopropoxy (2′-OCH 2 CH 2 CH 2 NH 2 ) and 2′-fluoro (2′-F). Similar modifications may also be made at other positions on the dsRNA, particularly the 3′ position of the sugar on the 3′ terminal nucleotide or in 2′-5′ linked dsRNAs and the 5′ position of 5′ terminal nucleotide. DsRNAs may also have sugar mimetics such as cyclobutyl moieties in place of the pentofuranosyl sugar. Representative U.S. patents that teach the preparation of such modified sugar structures include, but are not limited to, U.S. Pat. Nos.
- DsRNAs may also include nucleobase (often referred to in the art simply as “base”) modifications or substitutions.
- base include the purine bases adenine (A) and guanine (G), and the pyrimidine bases thymine (T), cytosine (C) and uracil (U).
- Modified nucleobases include other synthetic and natural nucleobases such as 5-methylcytosine (5-me-C), 5-hydroxymethyl cytosine, xanthine, hypoxanthine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine and guanine, 2-propyl and other alkyl derivatives of adenine and guanine, 2-thiouracil, 2-thiothymine and 2-thiocytosine, 5-halouracil and cytosine, 5-propynyl uracil and cytosine, 6-azo uracil, cytosine and thymine, 5-uracil (pseudouracil), 4-thiouracil, 8-halo, 8-amino, 8-thiol, 8-thioalkyl, 8-hydroxyl anal other 8-substituted adenines and guanines, 5-halo, particularly 5-bromo, 5-trifluoromethyl and other 5-substi
- nucleobases include those disclosed in U.S. Pat. No. 3,687,808, those disclosed in The Concise Encyclopedia Of Polymer Science And Engineering, pages 858-859, Kroschwitz, J. L, ed. John Wiley & Sons, 1990, these disclosed by Englisch et al., Angewandte Chemie, International Edition, 1991, 30, 613, and those disclosed by Sanghvi, Y S., Chapter 15, DsRNA Research and Applications, pages 289-302, Crooke, S. T. and Lebleu, B., Ed., CRC Press, 1993. Certain of these nucleobases are particularly useful for increasing the binding affinity of the oligomeric compounds featured in the invention.
- 5-substituted pyrimidines include 5-substituted pyrimidines, 6-azapyrimidines and N-2, N-6 and 0-6 substituted purines, including 2-aminopropyladenine, 5-propynyluracil and 5-propynylcytosine.
- 5-methylcytosine substitutions have been shown to increase nucleic acid duplex stability by 0.6-1.2° C. (Sanghvi, Y. S., Crooke, S. T. and Lebleu, B., Eds., DsRNA Research and Applications, CRC Press, Boca Raton, 1993, pp. 276-278) and are exemplary base substitutions, even more particularly when combined with 2′-O-methoxyethyl sugar modifications.
- dsRNAs featured in the invention involves chemically linking to the dsRNA one or more moieties or conjugates which enhance the activity, cellular distribution or cellular uptake of the dsRNA.
- moieties include but are not limited to lipid moieties such as a cholesterol moiety (Letsinger et al., Proc. Natl. Acid. Sci. USA, 1989, 86: 6553-6556), cholic acid (Manoharan et al., Biorg. Med. Chem. Let., 1994, 4:1053-1060), a thioether, e.g., beryl-S-tritylthiol (Manoharan et al., Ann. N.Y. Acad.
- Acids Res., 1990, 18:3777-3783 a polyamine or a polyethylene glycol chain (Manoharan et al., Nucleosides & Nucleotides, 1995, 14:969-973), or adamantane acetic acid (Manoharan et al., Tetrahedron Lett., 1995, 36:3651-3654), a palmityl moiety (Mishra et al., Biochim. Biophys. Acta, 1995, 1264:229-237), or an octadecylamine or hexylamino-carbonyloxycholesterol moiety (Crooke et al., J. Pharmacol. Exp. Ther., 1996, 277:923-937).
- dsRNA compounds which are chimeric compounds. “Chimeric” dsRNA compounds or “chimeras,” in the context of this invention, are dsRNA compounds, particularly dsRNAs, which contain two or more chemically distinct regions, each made up of at least one monomer unit, i.e., a nucleotide in the case of a dsRNA compound.
- dsRNAs typically contain at least one region wherein the dsRNA is modified so as to confer upon the dsRNA increased resistance to nuclease degradation, increased cellular uptake, and/or increased binding affinity for the target nucleic acid.
- An additional region of the dsRNA may serve as a substrate for enzymes capable of cleaving RNA:DNA or RNA:RNA hybrids.
- RNase H is a cellular endonuclease which cleaves the RNA strand of an RNA:DNA duplex. Activation of RNase H, therefore, results in cleavage of the RNA target, thereby greatly enhancing the efficiency of dsRNA inhibition of gene expression.
- a thioether e.g., hexyl-S-tritylthiol (Manoharan et al., Ann. N.Y. Acad. Sci., 1992, 660:306; Manoharan et al., Bioorg. Med. Chem. Let., 1993, 3:2765), a thiocholesterol (Oberhauser et al., Nucl.
- Typical conjugation protocols involve the synthesis of dsRNAs bearing an aminolinker at one or more positions of the sequence. The amino group is then reacted with the molecule being conjugated using appropriate coupling or activating reagents. The conjugation reaction may be performed either with the dsRNA still bound to the solid support or following cleavage of the dsRNA in solution phase. Purification of the dsRNA conjugate by HPLC typically affords the pure conjugate.
- dsRNA molecules featured in the invention are expressed from transcription units inserted into DNA or RNA vectors (see, e.g., Couture, A, et al., TIG. (1996), 12:5-10; Skillern, A., et al., International PCT Publication No. WO 00/22113, Conrad, International PCT Publication No. WO 00/22114, and Conrad, U.S. Pat. No. 6,054,299).
- These transgenes can be introduced as a linear construct, a circular plasmid, or a viral vector, which can be incorporated and inherited as a transgene integrated into the host genome.
- the transgene can also be constructed to permit it to be inherited as an extrachromosomal plasmid (Gassmann, et al., Proc. Natl. Acad. Sci. USA (1995) 92:1292).
- a dsRNA can be transcribed by promoters on two separate expression vectors and co-transfected into a target cell.
- each individual strand of the dsRNA can be transcribed by promoters both of which are located on the same expression plasmid.
- a dsRNA is expressed as an inverted repeat joined by a linker polynucleotide sequence such that the dsRNA has a stem and loop structure.
- the recombinant dsRNA expression vectors are generally DNA plasmids or viral vectors.
- dsRNA expressing viral vectors can be constructed based on, but not limited to, adeno-associated virus (for a review, see Muzyczka, et al., Curr. Topics Micro. Immunol. (1992) 158:97-129)); adenovirus (see, for example, Berkner, et al., BioTechniques (1998) 6:616), Rosenfeld et al. (1991, Science 252:431-434), and Rosenfeld et al. (1992), Cell 68:143-155)); or alphavirus as well as others known in the art.
- adeno-associated virus for a review, see Muzyczka, et al., Curr. Topics Micro. Immunol. (1992) 158:97-129
- adenovirus see, for example, Berkner, et al., BioTechniques (1998) 6
- Retroviruses have been used to introduce a variety of genes into many different cell types, including epithelial cells, in vitro and/or in vivo (see, e.g., Eglitis, et al., Science (1985) 230:1395-1398; Danos and Mulligan, Proc. Natl. Acad. Sci. USA (1998) 85:6460-6464; Wilson et al., 1988, Proc. Natl. Acad. Sci. USA 85:3014-3018; Armentano et al., 1990, Proc. Natl. Acad. Sci. USA 87:61416145; Huber et al., 1991, Proc. Natl. Acad. Sci.
- Recombinant retroviral vectors capable of transducing and expressing genes inserted into the genome of a cell can be produced by transfecting the recombinant retroviral genome into suitable packaging cell lines such as PA317 and Psi-CRIP (Comette et al., 1991, Human Gene Therapy 2:5-10; Cone et al., 1984, Proc. Natl. Acad. Sci. USA 81:6349).
- Recombinant adenoviral vectors can be used to infect a wide variety of cells and tissues in susceptible hosts (e.g., rat, hamster, dog, and chimpanzee) (Hsu et al., 1992, J. Infectious Disease, 166:769), and also have the advantage of not requiring mitotically active cells for infection.
- susceptible hosts e.g., rat, hamster, dog, and chimpanzee
- Any viral vector capable of accepting the coding sequences for the dsRNA molecule(s) to be expressed can be used, for example vectors derived from adenovirus (AV); adeno-associated virus (AAV); retroviruses (e.g, lentiviruses (LV), Rhabdoviruses, murine leukemia virus); herpes virus, and the like.
- AV adenovirus
- AAV adeno-associated virus
- retroviruses e.g, lentiviruses (LV), Rhabdoviruses, murine leukemia virus
- herpes virus and the like.
- the tropism of viral vectors can be modified by pseudotyping the vectors with envelope proteins or other surface antigens from other viruses, or by substituting different viral capsid proteins, as appropriate.
- AAV vectors which express different capsid protein serotypes are within the skill in the art; see, e.g., Rabinowitz J E et al. (2002), J Virol 76:791-801, the entire disclosure of which is herein incorporated by reference.
- Viral vectors can be derived from AV and AAV.
- the dsRNA featured in the invention is expressed as two separate, complementary single-stranded RNA molecules from a recombinant AAV vector having, for example, either the U6 or H1 RNA promoters, or the cytomegalovirus (CMV) promoter.
- CMV cytomegalovirus
- a suitable AV vector for expressing the dsRNA featured in the invention a method for constructing the recombinant AV vector, and a method for delivering the vector into target cells, are described in Xia H et al. (2002), Nat. Biotech. 20: 1006-1010.
- Suitable AAV vectors for expressing the dsRNA featured in the invention, methods for constructing the recombinant AV vector, and methods for delivering the vectors into target cells are described in Samulski R et al. (1987), J. Virol. 61: 3096-3101; Fisher K J et al. (1996), J. Virol, 70: 520-532; Samulski R et al. (1989), J. Virol. 63: 3822-3826; U.S. Pat. Nos. 5,252,479; 5,139,941; International Patent Application No. WO 94/13788; and International Patent Application No. WO 93/24641, the entire disclosures of which are herein incorporated by reference.
- the promoter driving dsRNA expression in either a DNA plasmid or viral vector featured in the invention may be a eukaryotic RNA polymerase I (e.g., ribosomal RNA promoter), RNA polymerase II (e.g., CMV early promoter or actin promoter or U1 snRNA promoter) or generally RNA polymerase HI promoter (e.g., U6 snRNA or 7SK RNA promoter) or a prokaryotic promoter, for example the T7 promoter, provided the expression plasmid also encodes T7 RNA polymerase required for transcription from a T7 promoter.
- RNA polymerase I e.g., ribosomal RNA promoter
- RNA polymerase II e.g., CMV early promoter or actin promoter or U1 snRNA promoter
- RNA polymerase HI promoter e.g., U6 snRNA or 7SK RNA promoter
- the promoter can also direct transgene expression to the pancreas (see, e.g., the insulin regulatory sequence for pancreas (Bucchini et al., 1986, Proc. Natl. Acad. Sci. USA 83:2511-2515)).
- expression of the transgene can be precisely regulated, for example, by using an inducible regulatory sequence and expression systems such as a regulatory sequence that is sensitive to certain physiological regulators, e.g., circulating glucose levels, or hormones (Docherty et al., 1994, FASEB J. 8:20-24).
- inducible expression systems suitable for the control of transgene expression in cells or in mammals include regulation by ecdysone, by estrogen, progesterone, tetracycline, chemical inducers of dimerization, and isopropyl-beta-D1-thiogalactopyranoside (EPTG).
- ETG isopropyl-beta-D1-thiogalactopyranoside
- recombinant vectors capable of expressing dsRNA molecules are delivered as described below, and persist in target cells.
- viral vectors can be used that provide for transient expression of dsRNA molecules.
- Such vectors can be repeatedly administered as necessary. Once expressed, the dsRNAs bind to target RNA and modulate its function or expression. Delivery of dsRNA expressing vectors can be systemic, such as by intravenous or intramuscular administration, by administration to target cells ex-planted from the patient followed by reintroduction into the patient, or by any other means that allows for introduction into a desired target cell.
- dsRNA expression DNA plasmids are typically transfected into target cells as a complex with cationic lipid carriers (e.g., Oligofectamine) or non-cationic lipid-based carriers (e.g., Transit-TKOTM).
- cationic lipid carriers e.g., Oligofectamine
- Transit-TKOTM non-cationic lipid-based carriers
- Multiple lipid transfections for dsRNA-mediated knockdowns targeting different regions of a single target gene or multiple target genes over a period of a week or more are also contemplated by the invention.
- Successful introduction of vectors into host cells can be monitored using various known methods. For example, transient transfection can be signaled with a reporter, such as a fluorescent marker, such as Green Fluorescent Protein (GFP). Stable transfection of cells ex vivo can be ensured using markers that provide the transfected cell with resistance to specific environmental factors (e.g., antibiotics and drugs), such as hygromycin B resistance.
- the deltaEGFR- and IL6-specific dsRNA molecules can also be inserted into vectors and used as gene therapy vectors for human patients.
- Gene therapy vectors can be delivered to a subject by, for example, intravenous injection, local administration (see U.S. Pat. No. 5,328,470) or by stereotactic injection (see e.g., Chen et al. (1994) Proc. Natl. Acad. Sci. USA 91:3054-3057).
- the pharmaceutical preparation of the gene therapy vector can include the gene therapy vector in an acceptable diluent, or can include a slow release matrix in which the gene delivery vehicle is imbedded.
- the pharmaceutical preparation can include one or more cells which produce the gene delivery system.
- the invention provides pharmaceutical compositions containing a dsRNA, as described herein, and a pharmaceutically acceptable carrier.
- the pharmaceutical composition containing the dsRNA is useful for treating a disease or disorder associated with the expression or activity of the deltaEGFR gene and/or the IL6 gene, such as pathological processes mediated by deltaEGFR or IL6 expression.
- Such pharmaceutical compositions are formulated based on the mode of delivery.
- a composition formulated for direct delivery into the brain parenchyma e.g., by infusion into the brain, such as by continuous pump infusion.
- Another example is a composition formulated for intraventricular or intrathecal delivery into the cerebrospinal fluid, e.g., by bolus or continuous pump infusion.
- Another example is a compositions formulated for systemic administration via parenteral delivery, e.g., by intravenous (IV) delivery.
- the pharmaceutical composition may be administered once daily, or the dsRNA may be administered as two, three, or more sub-doses at appropriate intervals throughout the day or even using continuous infusion or delivery through a controlled release formulation. In that case, the dsRNA contained in each sub-dose must be correspondingly smaller in order to achieve the total daily dosage.
- the dosage unit can also be compounded for delivery over several days, e.g., using a conventional sustained release formulation which provides sustained release of the dsRNA over a several day period. Sustained release formulations are well known in the art and are particularly useful for delivery of agents at a particular site, such as could be used with the agents featured in the invention. In this embodiment, the dosage unit contains a corresponding multiple of the daily dose.
- dsRNA dsRNA on target RNA levels
- target RNA levels e.g., deltaEGFR levels (or both deltaEGFR and wtEGFR levels) or IL6 levels
- deltaEGFR levels or both deltaEGFR and wtEGFR levels
- IL6 levels IL6 levels
- the present invention includes pharmaceutical compositions that can be delivered by injection directly into the brain.
- the injection can be by stereotactic injection into the brain tumor directly, or into a particular region of the brain (e.g., into white matter, such as the corona radiata, or the substantia nigra, cortex, hippocampus, striatum, or globus pallidus), or the dsRNA can be delivered into multiple regions of the central nervous system (e.g., into multiple regions of the brain, and/or into the spinal cord).
- the dsRNA can also be delivered into diffuse regions of the brain (e.g., diffuse delivery to the cortex of the brain).
- a dsRNA targeting deltaEGFR or IL-6 can be delivered by way of a cannula or other delivery device having one end implanted in a tissue, e.g., the brain, e.g., the white matter, such as the corona radiata, or the substantia nigra, cortex, hippocampus, striatum, corpus callosum or globus pallidus of the brain.
- the cannula or other delivery device has one end implanted into a tumor in the brain.
- the cannula can be connected to a reservoir of the dsRNA composition.
- the flow or delivery can be mediated by a pump.
- a pump and reservoir are implanted in an area distant from the tissue, e.g., in the abdomen, and delivery is effected by a conduit leading from the pump or reservoir to the site of release.
- Infusion of the dsRNA composition into the brain can be over several hours or for several days, e.g., for 1, 2, 3, 5, or 7 days or more.
- Devices for delivery to the brain are described, for example, in U.S. Pat. Nos. 6,093,180, and 5,814,014.
- the pump is externalized (not implanted). Infusion of the dsRNA composition into the brain can be over several hours or for several days up to approximately 7 days, e.g., for 1, 2, 3, 5, or 7 days.
- the present invention also includes pharmaceutical compositions and formulations which include the dsRNA compounds featured in the invention.
- the pharmaceutical compositions may be administered in a number of ways depending upon whether local or systemic treatment is desired and upon the area to be treated. Administration may be topical (e.g., by transdermal patch), pulmonary, e.g., by inhalation or insufflation of powders or aerosols, including by nebulizer; intratracheal, intranasal, epidermal and transdermal, oral or parenteral.
- Parenteral administration includes intravenous, intraarterial, subcutaneous, intraperitoneal or intramuscular injection or infusion; subdermal, e.g., via an implanted device; or intracranial, e.g., by intraparenchymal, intrathecal or intraventricular, administration.
- compositions and formulations for topical administration may include transdermal patches, ointments, lotions, creams, gels, drops, suppositories, sprays, liquids and powders.
- Conventional pharmaceutical carriers, aqueous, powder or oily bases, thickeners and the like may be necessary or desirable.
- Coated condoms, gloves and the like may also be useful.
- Suitable topical formulations include those in which the dsRNAs featured in the invention are in admixture with a topical delivery agent such as lipids, liposomes, fatty acids, fatty acid esters, steroids, chelating agents and surfactants.
- the particles typically have a mean diameter of about 50 nm to about 150 nm, more typically about 60 nm to about 130 nm, more typically about 70 nm to about 110 nm, most typically about 70 to about 90 nm, and are substantially nontoxic.
- the nucleic acids when present in the nucleic acid-lipid particles are resistant in aqueous solution to degradation with a nuclease. Nucleic acid-lipid particles and their method of preparation are disclosed in, e.g., U.S. Pat. Nos. 5,976,567; 5,981,501; 6,534,484; 6,586,410; 6,815,432; and PCT Publication No. WO 96/40964.
- the lipid to drug ratio (mass/mass ratio) (e.g., lipid to dsRNA ratio) will be in the range of from about 1:1 to about 50:1, from about 1:1 to about 25:1, from about 3:1 to about 15:1, from about 4:1 to about 10:1, from about 5:1 to about 9:1, or about 6:1 to about 9:1.
- the cationic lipid may be, for example, N,N-dioleyl-N,N-dimethylammonium chloride (DODAC), N,N-distearyl-N,N-dimethylammonium bromide (DDAB), N-(I-(2,3-dioleoyloxy)propyl)-N,N,N-trimethylammonium chloride (DOTAP), N-(I-(2,3-dioleyloxy)propyl)-N,N,N-trimethylammonium chloride (DOTMA), N,N-dimethyl-2,3-dioleyloxy)propylamine (DODMA), 1,2-DiLinoleyloxy-N,N-dimethylaminopropane (DLinDMA), 1,2-Dilinolenyloxy-N,N-dimethylaminopropane (DLenDMA), 1,2-Dilinoleylcarbamoyloxy-3-dimethylamino
- the conjugated lipid that inhibits aggregation of particles may be, for example, a polyethyleneglycol (PEG)-lipid including, without limitation, a PEG-diacylglycerol (DAG), a PEG-dialkyloxypropyl (DAA), a PEG-phospholipid, a PEG-ceramide (Cer), or a mixture thereof.
- the PEG-DAA conjugate may be, for example, a PEG-dilauryloxypropyl (Ci 2 ), a PEG-dimyristyloxypropyl (Ci 4 ), a PEG-dipalmityloxypropyl (Ci 6 ), or a PEG-distearyloxypropyl (Ci 8 ).
- the conjugated lipid that prevents aggregation of particles may be from 0 mol % to about 20 mol % or about 2 mol % of the total lipid present in the particle.
- the nucleic acid-lipid particle further includes cholesterol at, e.g., about 10 mol % to about 60 mol % or about 48 mol % of the total lipid present in the particle.
- the lipidoid ND98 ⁇ 4HCl (MW 1487) (Formula 1), Cholesterol (Sigma-Aldrich), and PEG-Ceramide C16 (Avanti Polar Lipids) can be used to prepare lipid-siRNA nanoparticles (i.e., LNP01 particles).
- Stock solutions of each in ethanol can be prepared as follows: ND98, 133 mg/mL; Cholesterol, 25 mg/mL, PEG-Ceramide C16, 100 mg/mL.
- the ND98, Cholesterol, and PEG-Ceramide C16 stock solutions can then be combined in a, e.g., 42:48:10 molar ratio.
- the combined lipid solution can be mixed with aqueous siRNA (e.g., in sodium acetate pH 5) such that the final ethanol concentration is about 35-45% and the final sodium acetate concentration is about 100-300 mM.
- aqueous siRNA e.g., in sodium acetate pH 5
- Lipid-siRNA nanoparticles typically form spontaneously upon mixing.
- the resultant nanoparticle mixture can be extruded through a polycarbonate membrane (e.g., 100 nm cut-off) using, for example, a thermobarrel extruder, such as Lipex Extruder (Northern Lipids, Inc). In some cases, the extrusion step can be omitted.
- Ethanol removal and simultaneous buffer exchange can be accomplished by, for example, dialysis or tangential flow filtration.
- Buffer can be exchanged with, for example, phosphate buffered saline (PBS) at about pH 7, e.g., about pH 6.9, about pH 7.0, about pH 7.1, about pH 7.2, about pH 7.3, or about pH 7.4.
- PBS phosphate buffered saline
- LNP01 formulations are described, e.g., in International Application Publication No. WO 2008/042973, which is hereby incorporated by reference.
- Formulations prepared by either the standard or extrusion-free method can be characterized in similar manners.
- formulations are typically characterized by visual inspection. They should be whitish translucent solutions free from aggregates or sediment. Particle size and particle size distribution of lipid-nanoparticles can be measured by light scattering using, for example, a Malvern Zetasizer Nano ZS (Malvern, USA). Particles should be about 20-300 nm, such as 40-100 nm in size. The particle size distribution should be unimodal.
- the total siRNA concentration in the formulation, as well as the entrapped fraction is estimated using a dye exclusion assay.
- the particle size is at least 30 nm, at least 40 nm, at least 50 nm, at least 60 nm, at least 70 nm, at least 80 nm, at least 90 nm, at least 100 nm, at least 110 nm, and at least 120 nm.
- the suitable range is typically about at least 50 nm to about at least 110 nm, about at least 60 nm to about at least 100 nm, or about at least 80 nm to about at least 90 nm.
- compositions and formulations for oral administration include powders or granules, microparticulates, nanoparticulates, suspensions or solutions in water or non-aqueous media, capsules, gel capsules, sachets, tablets or minitablets. Thickeners, flavoring agents, diluents, emulsifiers, dispersing aids or binders may be desirable.
- oral formulations are those in which dsRNAs featured in the invention are administered in conjunction with one or more penetration enhancers surfactants and chelators.
- Suitable surfactants include fatty acids and/or esters or salts thereof, bile acids and/or salts thereof.
- Suitable bile acids/salts include chenodeoxycholic acid (CDCA) and ursodeoxychenodeoxycholic acid (UDCA), cholic acid, dehydrocholic acid, deoxycholic acid, glucholic acid, glycholic acid, glycodeoxycholic acid, taurocholic acid, taurodeoxycholic acid, sodium tauro-24,25-dihydro-fusidate and sodium glycodihydrofusidate.
- DCA chenodeoxycholic acid
- UDCA ursodeoxychenodeoxycholic acid
- cholic acid dehydrocholic acid
- deoxycholic acid deoxycholic acid
- glucholic acid glycholic acid
- glycodeoxycholic acid taurocholic acid
- taurodeoxycholic acid sodium tauro-24,25-dihydro-fusidate and sodium glycodihydrofusidate.
- Suitable fatty acids include arachidonic acid, undecanoic acid, oleic acid, lauric acid, caprylic acid, capric acid, myristic acid, palmitic acid, stearic acid, linoleic acid, linolenic acid, dicaprate, tricaprate, monoolein, dilaurin, glyceryl 1-monocaprate, 1-dodecylazacycloheptan-2-one, an acylcarnitine, an acylcholine, or a monoglyceride, a diglyceride or a pharmaceutically acceptable salt thereof (e.g., sodium).
- arachidonic acid arachidonic acid, undecanoic acid, oleic acid, lauric acid, caprylic acid, capric acid, myristic acid, palmitic acid, stearic acid, linoleic acid, linolenic acid, dicaprate, tricaprate, monoolein, dilaurin, gly
- combinations of penetration enhancers are used, for example, fatty acids/salts in combination with bile acids/salts.
- One exemplary combination is the sodium salt of lauric acid, capric acid and UDCA.
- Further penetration enhancers include polyoxyethylene-9-lauryl ether, polyoxyethylene-20-cetyl ether.
- DsRNAs featured in the invention may be delivered orally, in granular form including sprayed dried particles, or complexed to form micro or nanoparticles.
- DsRNA complexing agents include poly-amino acids; polyimines; polyacrylates; polyalkylacrylates, polyoxethanes, polyalkylcyanoacrylates; cationized gelatins, albumins, starches, acrylates, polyethyleneglycols (PEG) and starches; polyalkylcyanoacrylates; DEAE-derivatized polyimines, pollulans, celluloses and starches.
- Suitable complexing agents include chitosan, N-trimethylchitosan, poly-L-lysine, polyhistidine, polyornithine, polyspermines, protamine, polyvinylpyridine, polythiodiethylaminomethylethylene P(TDAE), polyaminostyrene (e.g., p-amino), poly(methylcyanoacrylate), poly(ethylcyanoacrylate), poly(butylcyanoacrylate), poly(isobutylcyanoacrylate), poly(isohexylcynaoacrylate), DEAE-methacrylate, DEAE-hexylacrylate, DEAE-acrylamide, DEAE-albumin and DEAE-dextran, polymethylacrylate, polyhexylacrylate, poly(D,L-lactic acid), poly(DL-lactic-co-glycolic acid (PLGA), alginate, and polyethyleneglycol (PEG).
- TDAE polythiodiethylamino
- compositions and formulations for parenteral, intraparenchymal (into the brain), intrathecal, intraventricular or intrahepatic administration may include sterile aqueous solutions which may also contain buffers, diluents and other suitable additives such as, but not limited to, penetration enhancers, carrier compounds and other pharmaceutically acceptable carriers or excipients.
- compositions featured in the invention include, but are not limited to, solutions, emulsions, and liposome-containing formulations. These compositions may be generated from a variety of components that include, but are not limited to, preformed liquids, self-emulsifying solids and self-emulsifying semisolids. Particularly perfered are formulations that target the liver when treating hepatic disorders such as hepatic carcinoma.
- the pharmaceutical formulations may be prepared according to conventional techniques well known in the pharmaceutical industry. Such techniques include the step of bringing into association the active ingredients with the pharmaceutical carrier(s) or excipient(s). In general, the formulations are prepared by uniformly and intimately bringing into association the active ingredients with liquid carriers or finely divided solid carriers or both, and then, if necessary, shaping the product.
- compositions may be formulated into any of many possible dosage forms such as, but not limited to, tablets, capsules, gel capsules, liquid syrups, soft gels, suppositories, and enemas.
- the compositions may also be formulated as suspensions in aqueous, non-aqueous or mixed media.
- Aqueous suspensions may further contain substances which increase the viscosity of the suspension including, for example, sodium carboxymethylcellulose, sorbitol and/or dextran.
- the suspension may also contain stabilizers.
- compositions may be prepared and formulated as emulsions.
- Emulsions are typically heterogenous systems of one liquid dispersed in another in the form of droplets usually exceeding 0.1 ⁇ mu ⁇ m in diameter (Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p. 199; Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., Volume 1, p.
- Emulsions are often biphasic systems comprising two immiscible liquid phases intimately mixed and dispersed with each other.
- emulsions may be of either the water-in-oil (w/o) or the oil-in-water (o/w) variety.
- Emulsions may contain additional components in addition to the dispersed phases, and the active drug which may be present as a solution in either the aqueous phase, oily phase or itself as a separate phase.
- compositions such as emulsifiers, stabilizers, dyes, and anti-oxidants may also be present in emulsions as needed.
- Pharmaceutical emulsions may also be multiple emulsions that are comprised of more than two phases such as, for example, in the case of oil-in-water-in-oil (o/w/o) and water-in-oil-in-water (w/o/w) emulsions.
- Such complex formulations often provide certain advantages that simple binary emulsions do not.
- Multiple emulsions in which individual oil droplets of an o/w emulsion enclose small water droplets constitute a w/o/w emulsion.
- a system of oil droplets enclosed in globules of water stabilized in an oily continuous phase provides an o/w/o emulsion.
- Emulsions are characterized by little or no thermodynamic stability. Often, the dispersed or discontinuous phase of the emulsion is well dispersed into the external or continuous phase and maintained in this form through the means of emulsifiers or the viscosity of the formulation. Either of the phases of the emulsion may be a semisolid or a solid, as is the case of emulsion-style ointment bases and creams. Other means of stabilizing emulsions entail the use of emulsifiers that may be incorporated into either phase of the emulsion.
- Emulsifiers may broadly be classified into four categories: synthetic surfactants, naturally occurring emulsifiers, absorption bases, and finely dispersed solids (Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p. 199).
- Synthetic surfactants also known as surface active agents, have found wide applicability in the formulation of emulsions and have been reviewed in the literature (Rieger, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p. 285; Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), Marcel Dekker, Inc., New York, N.Y., 1988, volume 1, p. 199).
- Surfactants are typically amphiphilic and comprise a hydrophilic and a hydrophobic portion.
- HLB hydrophile/lipophile balance
- surfactants may be classified into different classes based on the nature of the hydrophilic group: nonionic, anionic, cationic and amphoteric (Rieger, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p. 285).
- Naturally occurring emulsifiers used in emulsion formulations include lanolin, beeswax, phosphatides, lecithin and acacia.
- Absorption bases possess hydrophilic properties such that they can soak up water to form w/o emulsions yet retain their semisolid consistencies, such as anhydrous lanolin and hydrophilic petrolatum. Finely divided solids have also been used as good emulsifiers especially in combination with surfactants and in viscous preparations.
- polar inorganic solids such as heavy metal hydroxides, nonswelling clays such as bentonite, attapulgite, hectorite, kaolin, montmorillonite, colloidal aluminum silicate and colloidal magnesium aluminum silicate, pigments and nonpolar solids such as carbon or glyceryl tristearate.
- non-emulsifying materials are also included in emulsion formulations and contribute to the properties of emulsions. These include fats, oils, waxes, fatty acids, fatty alcohols, fatty esters, humectants, hydrophilic colloids, preservatives and antioxidants (Block, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p. 335; Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p. 199).
- Hydrophilic colloids or hydrocolloids include naturally occurring gums and synthetic polymers such as polysaccharides (for example, acacia, agar, alginic acid, carrageenan, guar gum, karaya gum, and tragacanth), cellulose derivatives (for example, carboxymethylcellulose and carboxypropylcellulose), and synthetic polymers (for example, carbomers, cellulose ethers, and carboxyvinyl polymers). These disperse or swell in water to form colloidal solutions that stabilize emulsions by forming strong interfacial films around the dispersed-phase droplets and by increasing the viscosity of the external phase.
- polysaccharides for example, acacia, agar, alginic acid, carrageenan, guar gum, karaya gum, and tragacanth
- cellulose derivatives for example, carboxymethylcellulose and carboxypropylcellulose
- synthetic polymers for example, carbomers, cellulose ethers, and
- Antioxidants used may be free radical scavengers such as tocopherols, alkyl gallates, butylated hydroxyanisole, butylated hydroxytoluene, or reducing agents such as ascorbic acid and sodium metabisulfite, and antioxidant synergists such as citric acid, tartaric acid, and lecithin.
- free radical scavengers such as tocopherols, alkyl gallates, butylated hydroxyanisole, butylated hydroxytoluene, or reducing agents such as ascorbic acid and sodium metabisulfite
- antioxidant synergists such as citric acid, tartaric acid, and lecithin.
- Emulsion formulations for oral delivery have been very widely used because of ease of formulation, as well as efficacy from an absorption and bioavailability standpoint (Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p.
- compositions of dsRNAs and nucleic acids are formulated as microemulsions.
- a microemulsion may be defined as a system of water, oil and amphiphile which is a single optically isotropic and thermodynamically stable liquid solution (Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p. 245).
- microemulsions are systems that are prepared by first dispersing an oil in an aqueous surfactant solution and then adding a sufficient amount of a fourth component, generally an intermediate chain-length alcohol to form a transparent system.
- microemulsions have also been described as thermodynamically stable, isotropically clear dispersions of two immiscible liquids that are stabilized by interfacial films of surface-active molecules (Leung and Shah, in: Controlled Release of Drugs: Polymers and Aggregate Systems, Rosoff, M., Ed., 1989, VCH Publishers, New York, pages 185-215).
- Microemulsions commonly are prepared via a combination of three to five components that include oil, water, surfactant, cosurfactant and electrolyte.
- microemulsion is of the water-in-oil (w/o) or an oil-in-water (o/w) type is dependent on the properties of the oil and surfactant used and on the structure and geometric packing of the polar heads and hydrocarbon tails of the surfactant molecules (Schott, in Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton, Pa., 1985, p. 271).
- microemulsions offer the advantage of solubilizing water-insoluble drugs in a formulation of thermodynamically stable droplets that are formed spontaneously.
- Surfactants used in the preparation of microemulsions include, but are not limited to, ionic surfactants, non-ionic surfactants, Brij 96, polyoxyethylene oleyl ethers, polyglycerol fatty acid esters, tetraglycerol monolaurate (ML310), tetraglycerol monooleate (MO310), hexaglycerol monooleate (PO310), hexaglycerol pentaoleate (PO500), decaglycerol monocaprate (MCA750), decaglycerol monooleate (MO750), decaglycerol sequioleate (SO750), decaglycerol decaoleate (DAO750), alone or in combination with cosurfactants.
- ionic surfactants non-ionic surfactants
- Brij 96 polyoxyethylene oleyl ethers
- polyglycerol fatty acid esters tetraglycerol monolaurate (ML310),
- the cosurfactant usually a short-chain alcohol such as ethanol, 1-propanol, and 1-butanol, serves to increase the interfacial fluidity by penetrating into the surfactant film and consequently creating a disordered film because of the void space generated among surfactant molecules.
- Microemulsions may, however, be prepared without the use of cosurfactants and alcohol-free self-emulsifying microemulsion systems are known in the art.
- the aqueous phase may typically be, but is not limited to, water, an aqueous solution of the drug, glycerol, PEG300, PEG400, polyglycerols, propylene glycols, and derivatives of ethylene glycol.
- the oil phase may include, but is not limited to, materials such as Captex 300, Captex 355, Capmul MCM, fatty acid esters, medium chain (C8-C12) mono, di, and tri-glycerides, polyoxyethylated glyceryl fatty acid esters, fatty alcohols, polyglycolized glycerides, saturated polyglycolized C8-C10 glycerides, vegetable oils and silicone oil.
- materials such as Captex 300, Captex 355, Capmul MCM, fatty acid esters, medium chain (C8-C12) mono, di, and tri-glycerides, polyoxyethylated glyceryl fatty acid esters, fatty alcohols, polyglycolized glycerides, saturated polyglycolized C8-C10 glycerides, vegetable oils and silicone oil.
- Microemulsions are particularly of interest from the standpoint of drug solubilization and the enhanced absorption of drugs.
- Lipid based microemulsions both o/w and w/o have been proposed to enhance the oral bioavailability of drugs, including peptides (Constantinides et al., Pharmaceutical Research, 1994, 11, 1385-1390; Ritschel, Meth. Find. Exp. Clin. Pharmacol., 1993, 13, 205).
- Microemulsions afford advantages of improved drug solubilization, protection of drug from enzymatic hydrolysis, possible enhancement of drug absorption due to surfactant-induced alterations in membrane fluidity and permeability, ease of preparation, ease of oral administration over solid dosage forms, improved clinical potency, and decreased toxicity (Constantinides et al., Pharmaceutical Research, 1994, 11, 1385; Ho et al., J. Pharm. Sci., 1996, 85, 138-143). Often microemulsions may form spontaneously when their components are brought together at ambient temperature. This may be particularly advantageous when formulating thermolabile drugs, peptides or dsRNAs. Microemulsions have also been effective in the transdermal delivery of active components in both cosmetic and pharmaceutical applications. It is expected that the microemulsion compositions and formulations will facilitate the increased systemic absorption of dsRNAs and nucleic acids from the gastrointestinal tract, as well as improve the local cellular uptake of dsRNAs and nucleic acids.
- Microemulsions may also contain additional components and additives such as sorbitan monostearate (Grill 3), Labrasol, and penetration enhancers to improve the properties of the formulation and to enhance the absorption of the dsRNAs and nucleic acids featured herein.
- Penetration enhancers used in the microemulsions may be classified as belonging to one of five broad categories—surfactants, fatty acids, bile salts, chelating agents, and non-chelating non-surfactants (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, p. 92). Each of these classes has been discussed above.
- liposome means a vesicle composed of amphiphilic lipids arranged in a spherical bilayer or bilayers.
- Liposomes are unilamellar or multilamellar vesicles which have a membrane formed from a lipophilic material and an aqueous interior. The aqueous portion contains the composition to be delivered. Cationic liposomes possess the advantage of being able to fuse to the cell wall. Non-cationic liposomes, although not able to fuse as efficiently with the cell wall, are taken up by macrophages in vivo.
- lipid vesicles In order to cross intact mammalian skin, lipid vesicles must pass through a series of fine pores, each with a diameter less than 50 nm, under the influence of a suitable transdermal gradient. Therefore, it is desirable to use a liposome which is highly deformable and able to pass through such fine pores.
- liposomes obtained from natural phospholipids are biocompatible and biodegradable; liposomes can incorporate a wide range of water and lipid soluble drugs; liposomes can protect encapsulated drugs in their internal compartments from metabolism and degradation (Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y., volume 1, p. 245).
- Important considerations in the preparation of liposome formulations are the lipid surface charge, vesicle size and the aqueous volume of the liposomes.
- Liposomes are useful for the transfer and delivery of active ingredients to the site of action. Because the liposomal membrane is structurally similar to biological membranes, when liposomes are applied to a tissue, the liposomes start to merge with the cellular membranes and as the merging of the liposome and cell progresses, the liposomal contents are emptied into the cell where the active agent may act.
- Liposomes present several advantages over other formulations. Such advantages include reduced side-effects related to high systemic absorption of the administered drug, increased accumulation of the administered drug at the desired target, and the ability to administer a wide variety of drugs, both hydrophilic and hydrophobic, into the skin.
- liposomes to deliver agents including high-molecular weight DNA into the skin.
- Compounds including analgesics, antibodies, hormones and high-molecular weight DNAs have been administered to the skin. The majority of applications resulted in the targeting of the upper epidermis
- Liposomes fall into two broad classes. Cationic liposomes are positively charged liposomes which interact with the negatively charged DNA molecules to form a stable complex. The positively charged DNA/liposome complex binds to the negatively charged cell surface and is internalized in an endosome. Due to the acidic pH within the endosome, the liposomes are ruptured, releasing their contents into the cell cytoplasm (Wang et al., Biochem. Biophys. Res. Commun., 1987, 147, 980-985).
- liposomal composition includes phospholipids other than naturally-derived phosphatidylcholine.
- Neutral liposome compositions can be formed from dimyristoyl phosphatidylcholine (DMPC) or dipalmitoyl phosphatidylcholine (DPPC).
- Anionic liposome compositions generally are formed from dimyristoyl phosphatidylglycerol, while anionic fusogenic liposomes are formed primarily from dioleoyl phosphatidylethanolamine (DOPE).
- DOPE dioleoyl phosphatidylethanolamine
- Another type of liposomal composition is formed from phosphatidylcholine (PC) such as, for example, soybean PC, and egg PC.
- PC phosphatidylcholine
- Another type is formed from mixtures of phospholipid and/or phosphatidylcholine and/or cholesterol.
- Non-ionic liposomal systems have also been examined to determine their utility in the delivery of drugs to the skin, in particular systems comprising non-ionic surfactant and cholesterol.
- Non-ionic liposomal formulations comprising NovasomeTM I (glyceryl dilaurate/cholesterol/po- lyoxyethylene-10-stearyl ether) and NovasomeTM II (glyceryl distearate/cholesterol/polyoxyethylene-10-stearyl ether) were used to deliver cyclosporin-A into the dermis of mouse skin. Results indicated that such non-ionic liposomal systems were effective in facilitating the deposition of cyclosporin-A into different layers of the skin (Hu et al. S.T.P.Pharma. Sci., 1994, 4, 6, 466).
- Liposomes also include “sterically stabilized” liposomes, a term which, as used herein, refers to liposomes comprising one or more specialized lipids that, when incorporated into liposomes, result in enhanced circulation lifetimes relative to liposomes lacking such specialized lipids.
- sterically stabilized liposomes are those in which part of the vesicle-forming lipid portion of the liposome (A) comprises one or more glycolipids, such as monosialoganglioside G M 1, or (B) is derivatized with one or more hydrophilic polymers, such as a polyethylene glycol (PEG) moiety.
- PEG polyethylene glycol
- liposomes comprising one or more glycolipids are known in the art.
- Liposomes comprising (1) sphingomyelin and (2) the ganglioside G M 1 or a galactocerebroside sulfate ester.
- U.S. Pat. No. 5,543,152 discloses liposomes comprising sphingomyelin. Liposomes comprising 1,2-sn-dimyristoylphosphat-idylcholine are disclosed in WO 97/13499 (Lim et al).
- liposomes comprising lipids derivatized with one or more hydrophilic polymers, and methods of preparation thereof, are known in the art.
- Sunamoto et al. (Bull. Chem. Soc. Jpn., 1980, 53, 2778) described liposomes comprising a nonionic detergent, 2C 12 15G, that contains a PEG moiety.
- Ilium et al. (FEBS Lett., 1984, 167, 79) noted that hydrophilic coating of polystyrene particles with polymeric glycols results in significantly enhanced blood half-lives.
- Synthetic phospholipids modified by the attachment of carboxylic groups of polyalkylene glycols (e.g., PEG) are described by Sears (U.S. Pat.
- Liposomes having covalently bound PEG moieties on their external surface are described in European Patent No. EP 0 445 131 B1 and WO 90/04384 to Fisher.
- Liposome compositions containing 1-20 mole percent of PE derivatized with PEG, and methods of use thereof, are described by Woodle et al. (U.S. Pat. Nos. 5,013,556 and 5,356,633) and Martin et al. (U.S. Pat. No. 5,213,804 and European Patent No. EP 0 496 813 B1).
- Liposomes comprising a number of other lipid-polymer conjugates are disclosed in WO 91/05545 and U.S. Pat. No.
- WO 96/40062 to Thierry et al. discloses methods for encapsulating high molecular weight nucleic acids in liposomes.
- U.S. Pat. No. 5,264,221 to Tagawa et al. discloses protein-bonded liposomes and asserts that the contents of such liposomes may include a dsRNA.
- U.S. Pat. No. 5,665,710 to Rahman et al. describes certain methods of encapsulating oligodeoxynucleotides in liposomes.
- WO 97/04787 to Love et al. discloses liposomes comprising dsRNAs targeted to the raf gene.
- Transfersomes are yet another type of liposomes, and are highly deformable lipid aggregates which are attractive candidates for drug delivery vehicles. Transfersomes may be described as lipid droplets which are so highly deformable that they are easily able to penetrate through pores which are smaller than the droplet. Transfersomes are adaptable to the environment in which they are used, e.g., they are self-optimizing (adaptive to the shape of pores in the skin), self-repairing, frequently reach their targets without fragmenting, and often self-loading. To make transfersomes it is possible to add surface edge-activators, usually surfactants, to a standard liposomal composition. Transfersomes have been used to deliver serum albumin to the skin. The transfersome-mediated delivery of serum albumin has been shown to be as effective as subcutaneous injection of a solution containing serum albumin.
- HLB hydrophile/lipophile balance
- Nonionic surfactants find wide application in pharmaceutical and cosmetic products and are usable over a wide range of pH values. In general their HLB values range from 2 to about 18 depending on their structure.
- Nonionic surfactants include nonionic esters such as ethylene glycol esters, propylene glycol esters, glyceryl esters, polyglyceryl esters, sorbitan esters, sucrose esters, and ethoxylated esters.
- Nonionic alkanolamides and ethers such as fatty alcohol ethoxylates, propoxylated alcohols, and ethoxylated/propoxylated block polymers are also included in this class.
- the polyoxyethylene surfactants are the most popular members of the nonionic surfactant class.
- Anionic surfactants include carboxylates such as soaps, acyl lactylates, acyl amides of amino acids, esters of sulfuric acid such as alkyl sulfates and ethoxylated alkyl sulfates, sulfonates such as alkyl benzene sulfonates, acyl isethionates, acyl taurates and sulfosuccinates, and phosphates.
- the most important members of the anionic surfactant class are the alkyl sulfates and the soaps.
- Cationic surfactants include quaternary ammonium salts and ethoxylated amines. The quaternary ammonium salts are the most used members of this class.
- amphoteric surfactants include acrylic acid derivatives, substituted alkylamides, N-alkylbetaines and phosphatides.
- the present invention employs various penetration enhancers to effect the efficient delivery of nucleic acids, particularly dsRNAs, to the skin of animals.
- nucleic acids particularly dsRNAs
- Most drugs are present in solution in both ionized and nonionized forms. However, usually only lipid soluble or lipophilic drugs readily cross cell membranes. It has been discovered that even non-lipophilic drugs may cross cell membranes if the membrane to be crossed is treated with a penetration enhancer. In addition to aiding the diffusion of non-lipophilic drugs across cell membranes, penetration enhancers also enhance the permeability of lipophilic drugs.
- Penetration enhancers may be classified as belonging to one of five broad categories, i.e., surfactants, fatty acids, bile salts, chelating agents, and non-chelating non-surfactants (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, p.92). Each of the above mentioned classes of penetration enhancers are described below in greater detail.
- surfactants are chemical entities which, when dissolved in an aqueous solution, reduce the surface tension of the solution or the interfacial tension between the aqueous solution and another liquid, with the result that absorption of dsRNAs through the mucosa is enhanced.
- these penetration enhancers include, for example, sodium lauryl sulfate, polyoxyethylene-9-lauryl ether and polyoxyethylene-20-cetyl ether) (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, p.92); and perfluorochemical emulsions, such as FC-43. Takahashi et al., J. Pharm. Pharmacol., 1988, 40, 252).
- Fatty acids Various fatty acids and their derivatives which act as penetration enhancers include, for example, oleic acid, lauric acid, capric acid (n-decanoic acid), myristic acid, palmitic acid, stearic acid, linoleic acid, linolenic acid, dicaprate, tricaprate, monoolein (1-monooleoyl-rac-glycerol), dilaurin, caprylic acid, arachidonic acid, glycerol 1-monocaprate, 1-dodecylazacycloheptan-2-one, acylcarnitines, acylcholines, C 1-10 alkyl esters thereof (e.g., methyl, isopropyl and t-butyl), and mono- and di-glycerides thereof (i.e., oleate, laurate, caprate, myristate, palmitate, stearate, linoleate, etc.) (Lee e
- Bile salts The physiological role of bile includes the facilitation of dispersion and absorption of lipids and fat-soluble vitamins (Brunton, Chapter 38 in: Goodman & Gilman's The Pharmacological Basis of Therapeutics, 9th Ed., Hardman et al. Eds., McGraw-Hill, New York, 1996, pp. 934-935).
- bile salts includes any of the naturally occurring components of bile as well as any of their synthetic derivatives.
- Suitable chelating agents include but are not limited to disodium ethylenediaminetetraacetate (EDTA), citric acid, salicylates (e.g., sodium salicylate, 5-methoxysalicylate and homovanilate), N-acyl derivatives of collagen, laureth-9 and N-amino acyl derivatives of beta-diketones (enamines)(Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, page 92; Muranishi, Critical Reviews in Therapeutic Drug Carrier Systems, 1990, 7, 1-33; Buur et al., J. Control Rel., 1990, 14, 43-51).
- EDTA disodium ethylenediaminetetraacetate
- citric acid e.g., citric acid
- salicylates e.g., sodium salicylate, 5-methoxysalicylate and homovanilate
- N-acyl derivatives of collagen e.g., laureth-9 and N-amino acyl derivatives of
- This class of penetration enhancers include, for example, unsaturated cyclic ureas, 1-alkyl- and 1-alkenylazacyclo-alkanone derivatives (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, page 92); and non-steroidal anti-inflammatory agents such as diclofenac sodium, indomethacin and phenylbutazone (Yamashita et al., J. Pharm. Pharmacol., 1987, 39, 621-626).
- compositions also incorporate carrier compounds in the formulation.
- carrier compound or “carrier” can refer to a nucleic acid, or analog thereof, which is inert (i.e., does not possess biological activity per se) but is recognized as a nucleic acid by in vivo processes that reduce the bioavailability of a nucleic acid having biological activity by, for example, degrading the biologically active nucleic acid or promoting its removal from circulation.
- the coadministration of a nucleic acid and a carrier compound typically with an excess of the latter substance, can result in a substantial reduction of the amount of nucleic acid recovered in the liver, kidney or other extracirculatory reservoirs, presumably due to competition between the carrier compound and the nucleic acid for a common receptor.
- the recovery of a partially phosphorothioate dsRNA in hepatic tissue can be reduced when it is coadministered with polyinosinic acid, dextran sulfate, polycytidic acid or 4-acetamido-4′isothiocyano-stilbene-2,2′-disulfonic acid (Miyao et al., DsRNA Res. Dev., 1995, 5, 115-121; Takakura et al., DsRNA & Nucl. Acid Drug Dev., 1996, 6, 177-183.
- a “pharmaceutical carrier” or “excipient” is a pharmaceutically acceptable solvent, suspending agent or any other pharmacologically inert vehicle for delivering one or more nucleic acids to an animal.
- the excipient may be liquid or solid and is selected, with the planned manner of administration in mind, so as to provide for the desired bulk, consistency, etc., when combined with a nucleic acid and the other components of a given pharmaceutical composition.
- Typical pharmaceutical carriers include, but are not limited to, binding agents (e.g., pregelatinized maize starch, polyvinylpyrrolidone or hydroxypropyl methylcellulose, etc.); fillers (e.g., lactose and other sugars, microcrystalline cellulose, pectin, gelatin, calcium sulfate, ethyl cellulose, polyacrylates or calcium hydrogen phosphate, etc.); lubricants (e.g., magnesium stearate, talc, silica, colloidal silicon dioxide, stearic acid, metallic stearates, hydrogenated vegetable oils, corn starch, polyethylene glycols, sodium benzoate, sodium acetate, etc.); disintegrants (e.g., starch, sodium starch glycolate, etc.); and wetting agents (e.g., sodium lauryl sulphate, etc).
- binding agents e.g., pregelatinized maize starch, polyvinylpyrrolidone or hydroxypropy
- compositions suitable organic or inorganic excipient suitable for non-parenteral administration, which do not deleteriously react with nucleic acids, can also be used to formulate the compositions.
- suitable pharmaceutically acceptable carriers include, but are not limited to, water, salt solutions, alcohols, polyethylene glycols, gelatin, lactose, amylose, magnesium stearate, talc, silicic acid, viscous paraffin, hydroxymethylcellulose, polyvinylpyrrolidone and the like.
- Formulations for topical administration of nucleic acids may include sterile and non-sterile aqueous solutions, non-aqueous solutions in common solvents such as alcohols, or solutions of the nucleic acids in liquid or solid oil bases.
- the solutions may also contain buffers, diluents and other suitable additives.
- Pharmaceutically acceptable organic or inorganic excipients suitable for non-parenteral administration which do not deleteriously react with nucleic acids can be used.
- compositions featured in the invention may additionally contain other adjunct components conventionally found in pharmaceutical compositions, at their art-established usage levels.
- the compositions may contain additional, compatible, pharmaceutically-active materials such as, for example, antipruritics, astringents, local anesthetics or anti-inflammatory agents, or may contain additional materials useful in physically formulating various dosage forms of the compositions, such as dyes, flavoring agents, preservatives, antioxidants, opacifiers, thickening agents and stabilizers.
- additional materials useful in physically formulating various dosage forms of the compositions such as dyes, flavoring agents, preservatives, antioxidants, opacifiers, thickening agents and stabilizers.
- such materials when added, should not unduly interfere with the biological activities of the components of the compositions.
- Aqueous suspensions may contain substances which increase the viscosity of the suspension including, for example, sodium carboxymethylcellulose, sorbitol and/or dextran.
- the suspension may also contain stabilizers.
- compositions featured in the invention include (a) one or more dsRNA compounds and (b) one or more other chemotherapeutic agents which function by a non-RNAi mechanism.
- chemotherapeutic agents include but are not limited to temozolomide, daunorubicin, daunomycin, dactinomycin, doxorubicin, epirubicin, idarubicin, esorubicin, bleomycin, mafosfamide, ifosfamide, cytosine arabinoside, bis-chloroethylnitrosurea, busulfan, mitomycin C, actinomycin D, mithramycin, prednisone, hydroxyprogesterone, testosterone, tamoxifen, dacarbazine, procarbazine, hexamethylmelamine, pentamethylmelamine, mitoxantrone, amsacrine, chlorambucil, methylcyclohexyl
- chemotherapeutic agents may be used individually (e.g., 5-FU and oligonucleotide), sequentially (e.g., 5-FU and oligonucleotide for a period of time followed by MTX and oligonucleotide), or in combination with one or more other such chemotherapeutic agents (e.g., 5-FU, MTX and oligonucleotide, or 5-FU, radiotherapy and oligonucleotide).
- 5-FU and oligonucleotide e.g., 5-FU and oligonucleotide
- sequentially e.g., 5-FU and oligonucleotide for a period of time followed by MTX and oligonucleotide
- one or more other such chemotherapeutic agents e.g., 5-FU, MTX and oligonucleotide, or 5-FU, radiotherapy and oligonucleotide.
- Anti-inflammatory drugs including but not limited to nonsteroidal anti-inflammatory drugs and corticosteroids, and antiviral drugs, including but not limited to ribivirin, vidarabine, acyclovir and ganciclovir, may also be combined in compositions featured in the invention. See, generally, The Merck Manual of Diagnosis and Therapy, 15th Ed., Berkow et al., eds., 1987, Rahway, N.J., pages 2499-2506 and 46-49, respectively). Other non-RNAi chemotherapeutic agents are also within the scope of this invention. Two or more combined compounds may be used together or sequentially.
- Toxicity and therapeutic efficacy of such compounds can be determined by standard pharmaceutical procedures in cell cultures or experimental animals, e.g., for determining the LD50 (the dose lethal to 50% of the population) and the ED50 (the dose therapeutically effective in 50% of the population).
- the dose ratio between toxic and therapeutic effects is the therapeutic index and it can be expressed as the ratio LD50/ED50.
- Compounds that exhibit high therapeutic indices are generally preferred.
- a dose may be formulated in animal models to achieve a circulating plasma concentration range of the compound or, when appropriate, of the polypeptide product of a target sequence (e.g., achieving a decreased concentration of the polypeptide) that includes the IC50 (i.e., the concentration of the test compound which achieves a half-maximal inhibition of symptoms) as determined in cell culture.
- a target sequence e.g., achieving a decreased concentration of the polypeptide
- the IC50 i.e., the concentration of the test compound which achieves a half-maximal inhibition of symptoms
- levels in plasma may be measured, for example, by high performance liquid chromatography.
- the dsRNAs featured in the invention can be administered in combination with other known agents effective in treatment of pathological processes mediated by deltaEGFR or IL6 expression.
- the administering physician can adjust the amount and timing of dsRNA administration on the basis of results observed using standard measures of efficacy known in the art or described herein.
- a composition containing a dsRNA targeting deltaEGFR can be used to treat a grade III glioma, such as anaplastic astrocytoma, or a grade IV glioma, such as a glioblastoma multiforme.
- the glioma can be an ependymoma, astrocytoma, oligodendroglioma, or a mixed glioma, such as an oligoastrocytoma.
- a composition containing a dsRNA targeting a mutant EGFR, e.g., deltaEGFR or an IL6, is used to treat a carcinoma of the breast, ovary, cervix, kidney, or a squamous cell.
- the dsRNA targeting deltaEGFR can also target wtEGFR.
- a composition containing a dsRNA targeting a mutant EGFR, e.g., a deltaEGFR or an IL6 may also be used to treat other tumors and cancers, such as breast cancer, lung cancer, head and neck cancer, brain cancer, abdominal cancer, colon cancer, colorectal cancer, esophagus cancer, gastrointestinal cancer, tongue cancer, neuroblastoma, osteosarcoma, ovarian cancer, pancreatic cancer, prostate cancer, cervical cancer (e.g., squamous carcinoma of the cervix), lymphoid tumor, retinoblastoma, Wilm's tumor, multiple myeloma and for the treatment of skin cancer, like melanoma, for the treatment of lymphomas and blood cancer.
- the compositions featured herein can be used to treat a tumor of the brain or spine.
- a dsRNA targeting deltaEGFR or IL6 may be used to treat a proliferative disorder or differentiative disorder.
- cellular proliferative and/or differentiative disorders include cancer, e.g., carcinoma, sarcoma, metastatic disorders or hematopoietic neoplastic disorders, e.g., leukemias.
- a metastatic tumor can arise from a multitude of primary tumor types, including those of prostate, colon, lung, breast and liver origin.
- the terms “cancer,” “hyperproliferative,” and “neoplastic” refer to cells having the capacity for autonomous growth, i.e., an abnormal state or condition characterized by rapidly proliferating cell growth.
- administration of a dsRNA targeting deltaEGFR can be administered in combination with a chemotherapeutic agent, such as temozolomide, deoxycoformycin, cisplatin, cyclophosphamide, 5-fluorouracil, adriamycin, daunorubicin, tamoxifen aunorubicin, daunomycin, dactinomycin, doxorubicin, epirubicin, idarubicin, esorubicin, bleomycin, mafosfamide, ifosfamide, cytosine arabinoside, bis-chloroethylnitrosurea, busulfan, mitomycin C, actinomycin D, mithramycin, prednisone, hydroxyprogesterone, testosterone, tamoxifen, dacarbazine, procarbazine, hexamethylmelamine, pentamethylmelamine, mitoxantrone, ams
- a deltaEGFR dsRNA is administered in combination with at least one additional therapeutic agent, such as a second dsRNA targeting a different nucleic acid, e.g., an IL6 dsRNA, for the treatment of a condition or a symptom of a condition, such as for the treatment of a cancer.
- the second therapteutic agent is a chemotherapeutic agent.
- the dsRNA and an additional therapeutic agent can be administered in the same combination, e.g., intracranially or parenterally, or the additional therapeutic agent can be administered as part of a separate composition, e.g., intracranially or parenterally, or by another method described herein.
- Treatment with a dsRNA targeting deltaEGFR can also be performed in combination with radiation therapy, including external beam radiation, such as for treatment of tumors of the brain.
- a dsRNA featured herein may be administered before or after a surgical procedure to treat a cancer (e.g., to remove a tumor), such as resection of a brain tumor.
- the invention also relates to the use of a dsRNA targeting IL6 and compositions containing at least one such dsRNA, for the treatment of a IL6 or a deltaEGFR-mediated disorder or disease.
- an IL-6 dsRNA featured in the invention may be used to treat a hematological disorder, such as plasma cell dyscrasia, leukemia or lymphoma; proliferative glomerulonephritis; an inflammatory disease, such as rheumatoid arthritis, or an inflammatory bowel disease, such as Crohn's disease or ulcerative colitis; diabetes; septic shock; bacterial infections; viral infections, including HIV-1 infections; osteoporosis; autoimmune disorders, such as chronic immune deficiency syndrome or autoimmune deficiency syndrome (AIDS); neural disorders, such as multiple sclerosis, HTLV1-associated myelopathy or bacterial meningitis, systemic lupus erythematosus and vasculitis-associated central nervous system diseases; or other
- An IL-6 dsRNA featured in the invention may also be used to prevent allograft rejection or xenograft rejection and ischemia/reperfusion injury in solid organ or tissue transplantation.
- an IL-6 dsRNA can be administered to prevent rejection of a transplanted organ, such as a transplanted kidney, liver, lung, pancrease, heart, small bowel, cornea, epithelial cells, vascular endothelium, vascular smooth muscle cells, myocardium and passenger leukocytes resident in the organ at the time of transplantation.
- Treatment with a dsRNA targeting IL-6 can be performed in combination with a second dsRNA also targeting IL-6, and which targets a different sequence than a first dsRNA targeting IL-6.
- a dsRNA targeting IL-6 can also be administered in combination with one or more dsRNAs targeting other cytokines, immunomodulatory or immunoeffector genes, such as the C3 (complement component 3) gene, ICAM1 (intercellular adhesion molecule 1), VCAM-1 (vascular cell adhesion molecule 1), IFN-gamma (interferon gamma), IL-1 (interleukin-1), IL-8 (interleukin-8), TNF-alpha (tumor necrosis factor-alpha), CD80, CD86, MHC-II (major histocombatibility complex-II), MHC-I (major histocombatibility complex-I), CD28, CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) or PV-B19 (parvovirus B19
- Patients can be administered a therapeutic amout of dsRNA, such as 0.01 mg/kg, 0.02 mg/kg, 0.1 mg/kg, 0.5 mg/kg, 1.0 mg/kg, 1.5 mg/kg, 2.0 mg/kg, or 2.5 mg/kg dsRNA.
- the dsRNA can be administered by intracranial infusion over a period of time, such as over a 30 minute, 1 hour, 2 hour, 3 hour or 4 hour period.
- the administration is repeated, for example, on a regular basis, such as biweekly (i.e., every two weeks) for one month, two months, three months, four months or longer.
- the treatments can be administered on a less frequent basis.
- Intracranial infusion can be continous.
- Administration of the dsRNA can reduce target RNA and protein levels, e.g., deltaEGFR or IL-6 levels, in the cerebrospinal fluid of the patient by at least 20%, 25%, 30%, 40%, 50%, 60%, 70%, 80% or 90% or more.
- the dsRNA can be administered by intravenous infusion over a period of time, such as over a 5 minute, 10 minute, 15 minute, 20 minute, or 25 minute period.
- the administration is repeated, for example, on a regular basis, such as biweekly (i.e., every two weeks) for one month, two months, three months, four months or longer.
- the treatments can be administered on a less frequent basis.
- administration can be repeated once per month, for six months or a year or longer.
- Administration of the dsRNA can reduce deltaEGFR levels in the blood or urine of the patient by at least 20%, 25%, 30%, 40%, 50%, 60%, 70%, 80% or 90% or more.
- patients Before administration of a full dose of the dsRNA, patients can be administered a smaller dose, such as 5% of the total dose, and monitored for adverse effects, such as an allergic reaction. Patients can be monitored for adverse effects depending on the formulation. For example, if the dsRNA is formulated in a lipid, the patient can be administered a smaller dose, and then monitored for elevated lipid levels or blood pressure. In another example, the patient can be monitored for unwanted immunostimulatory effects, such as increased cytokine (e.g., TNF-alpha or INF-alpha) levels.
- cytokine e.g., TNF-alpha or INF-alpha
- a patient in need of a deltaEGFR dsRNA can be identified by taking a family history.
- a healthcare provider such as a doctor, nurse, or family member, can take a family history before prescribing or administering a deltaEGFR dsRNA.
- the composition may be administered by any means known in the art including, but not limited to oral or parenteral routes, including intracranial (e.g., intraventricular, intraparenchymal and intrathecal), intravenous, intramuscular, subcutaneous, transdermal, airway (aerosol), nasal, rectal, and topical (including buccal and sublingual) administration.
- intracranial e.g., intraventricular, intraparenchymal and intrathecal
- intravenous intramuscular
- subcutaneous e.g., transdermal
- transdermal e.g., pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary pulmonary a
- such reagent may be obtained from any supplier of reagents for molecular biology at a quality/purity standard for application in molecular biology.
- RNAs Single-stranded RNAs were produced by solid phase synthesis on a scale of 1 ⁇ mole using an Expedite 8909 synthesizer (Applied Biosystems, Appleratechnik GmbH, Darmstadt, Germany) and controlled pore glass (CPG, 500 ⁇ , Proligo Biochemie GmbH, Hamburg, Germany) as solid support.
- RNA and RNA containing 2′-O-methyl nucleotides were generated by solid phase synthesis employing the corresponding phosphoramidites and 2′-O-methyl phosphoramidites, respectively (Proligo Biochemie GmbH, Hamburg, Germany).
- RNA synthesis For the synthesis of 3′-cholesterol-conjugated siRNAs (herein referred to as -Chol-3′), an appropriately modified solid support is used for RNA synthesis.
- the modified solid support is prepared as follows:
- Fmoc-6-amino-hexanoic acid (9.12 g, 25.83 mmol) is dissolved in dichloromethane (50 mL) and cooled with ice.
- Diisopropylcarbodiimde (3.25 g, 3.99 mL, 25.83 mmol) is added to the solution at 0° C. It is then followed by the addition of Diethyl-azabutane-1,4-dicarboxylate (5 g, 24.6 mmol) and dimethylamino pyridine (0.305 g, 2.5 mmol). The solution is brought to room temperature and stirred further for 6 h. Completion of the reaction is ascertained by TLC.
- reaction mixture is concentrated under vacuum and ethyl acetate is added to precipitate diisopropyl urea.
- the suspension is filtered.
- the filtrate is washed with 5% aqueous hydrochloric acid, 5% sodium bicarbonate and water.
- the combined organic layer is dried over sodium sulfate and concentrated to give the crude product which is purified by column chromatography (50% EtOAC/Hexanes) to yield 11.87 g (88%) of AB.
- the hydrochloride salt of 3-[(6-Amino-hexanoyl)-ethoxycarbonylmethyl-amino]-propionic acid ethyl ester AC (4.7 g, 14.8 mmol) is taken up in dichloromethane. The suspension is cooled to 0° C. on ice. To the suspension diisopropylethylamine (3.87 g, 5.2 mL, 30 mmol) is added. To the resulting solution cholesteryl chloroformate (6.675 g, 14.8 mmol) is added. The reaction mixture is stirred overnight. The reaction mixture is diluted with dichloromethane and ished with 10% hydrochloric acid. The product is purified by flash chromatography (10.3 g, 92%).
- Potassium t-butoxide (1.1 g, 9.8 mmol) is slurried in 30 mL of dry toluene. The mixture is cooled to 0° C. on ice and 5 g (6.6 mmol) of diester AD is added slowly with stirring within 20 mins. The temperature is kept below 5° C. during the addition. The stirring is continued for 30 mins at 0° C.
- Diol AF (1.25 gm 1.994 mmol) is dried by evaporating with pyridine (2 ⁇ 5 mL) in vacuo.
- the reaction is carried out at room temperature overnight.
- the reaction is quenched by the addition of methanol.
- the reaction mixture is concentrated under vacuum and to the residue dichloromethane (50 mL) is added.
- the organic layer is washed with 1M aqueous sodium bicarbonate.
- the organic layer is dried over anhydrous sodium sulfate, filtered and concentrated.
- the residual pyridine is removed by evaporating with toluene.
- the solution is agitated briefly using a wrist-action shaker (5 mins).
- Long chain alkyl amine-CPG (LCAA-CPG) (1.5 g, 61 mM) is added.
- the suspension is agitated for 2 h.
- the CPG is filtered through a sintered funnel and washed with acetonitrile, dichloromethane and ether successively. Unreacted amino groups are masked using acetic anhydride/pyridine.
- the achieved loading of the CPG is measured by taking UV measurement (37 mM/g).
- nucleotide monomers used in nucleic acid sequence representation. It will be understood that these monomers, when present in an oligonucleotide, are mutually linked by 5′-3′-phosphodiester bonds.
- Nucleotide(s) A adenosine C cytidine G guanosine T thymidine U uridine N any nucleotide (G, A, C, or T) a 2′-O-methyladenosine c 2′-O-methylcytidine g 2′-O-methylguanosine u 2′-O-methyluridine sT phosphorothioate linkage
- Example 2 ⁇ EGFR Enhances Tumorigenicity of wt EGFR Over-Expressing Cells after Subcutaneous Injection into Nude Mice
- Ink4/Arf ⁇ / ⁇ astrocytes engineered to over-express wt and deltaEGFR.
- Ink4/Arf ⁇ / ⁇ deltaEGFR astrocytes are tumorigenic upon intracranial injection in nude mice.
- Ink4/Arf ⁇ / ⁇ , wtEGFR astrocytes require the introduction of EGF to elicit this effect (Bachoo et al., Cancer Cell 1:269-77, 2002).
- deltaEGFR over-expressing astrocytes might be able to promote the tumorigenicity of wtEGFR over-expressing astrocytes if the cells were co-injected.
- these cells were tagged with nuclear GFP and injected either alone or mixed with deltaEGFR over-expressing astrocytes ( FIG. 2 B ).
- tumor size at day 22 after injection was significantly bigger in mice co-injected with 90% wtEGFR and 10% deltaEGFR Ink4/Arf ⁇ / ⁇ murine astrocytes than with 10% of deltaEGFR Ink4/Arf ⁇ / ⁇ astrocytes alone.
- wtEGFR astrocytes did not form tumors. These results not only demonstrated that there was tumor growth potentiation when wtEGFR astrocytes were mixed with deltaEGFR astrocytes, but also showed the presence of wtEGFR astrocytes within the tumor by immunofluorescence (GFP IF) ( FIG. 2 B , lower panel). Interestingly, a small number of GFP positive cells were detected in mice injected with wtEGFR astrocytes only, which may represent dormant cells that could be activated upon activation of the receptor.
- Example 4 Analysis of Tumor Composition by X-Gal Staining and Flow Cytometry
- X-Gal staining was performed to detect LacZ-tagged U87delta cells.
- Representative images of X-Gal stained tumors obtained after injection of admixed U87wt and U87delta cells show a significant number of U87delta cells (LacZ positive) among U87wt cells (LacZ negative).
- the large tumors that resulted from an initial inoculum ratio of 90% U87wt combined with 10% U87delta cells resulted in a final composition of 51.5% U87wt and 48.5% U87delta cells, while tumors from mice injected with 99% U87wt plus 1% U87delta were composed of 58.7% U87wt and a 41.3% U87delta cells.
- the small tumors formed by the mixture of U87wt with U87 ⁇ K or U87Parental cells were predominantly composed of U87wt cells ( FIG. 3 A ).
- Example 5 Treatment of U87wt Cells with U87delta Conditioned Media (CM) Activates EGFR.
- CM Conditioned Media
- conditioned media was collected from 48h-starved deltaEGFR cells and used to stimulate U87wt cells, also starved 48h.
- Western blot analysis of EGFR activation and known signaling molecules downstream of the receptor was performed on lysates of U87wt cells stimulated for 15 minutes with serial dilutions of U87delta CM, negative control U87Par CM or positive control EGF ligand.
- Membranes were interrogated with anti-pTyr monoclonal antibody (4G10) to check the activation of the EGFR, and with phospho-specific antibodies directed to the major known transduction proteins involved in tumorigenesis in GBMs (gliobastoma multiformes): Akt, ERK1/2 (a.k.a., MAPK) and STAT3.
- 4G10 anti-pTyr monoclonal antibody
- phospho-specific antibodies directed to the major known transduction proteins involved in tumorigenesis in GBMs (gliobastoma multiformes): Akt, ERK1/2 (a.k.a., MAPK) and STAT3.
- FIG. 4 shows that these pathways were activated in response to the U87 ⁇ CM stimulation, as shown by the increase of the phosphorylated forms of those proteins in a dose-dependent manner.
- CM from U87Par cells failed to activate EGFR or any of these pathways.
- the level of phosphorylation of these proteins, except EGFR is at the same extent for the undiluted conditioned medium and the high dose of EGF that was used, indicating that activation of EGFR downstream signaling is efficiently mimicked by factors secreted from U87 ⁇ .
- Activation of these pathways was also elicited in part by abundant IL-6 produced and secreted by the deltaEGFR-expressing cells.
- wtEGFR phosphorylation was observed to be significantly higher (p ⁇ 0.05) in mixed tumors than in tumors obtained after injection of U87wt alone (0.576 ⁇ 0.166 vs 0.19 ⁇ 0.007), while no differences in phosphorylation were detected for ⁇ EGFR in the mixed tumors compared to the tumors generated from injection of U87 ⁇ alone (1.215 ⁇ 0.225 vs 1.179 ⁇ 0.260).
- CM was tested for the ability to induce the release of EGFR ligands expressed on the surface of U87wt cells.
- U87wt cells were stimulated with serum-free medium, recombinant EGF and U87 ⁇ CM, and then these media were collected to analyze by ELISA changes in EGFR ligand concentration after the stimulation. None of the tested ligands showed any significant change in concentration, indicating that EGFR is not stimulated by soluble factors shed from the membrane of wtEGFR cells.
- Example 7 IL-6 is Over-Expressed in U87Delta Cells and in deltaEGFR-Positive GBM Clinical Samples
- a cytokine array was used to qualitatively detect 79 human cytokines and growth factors in supernatants of cultured cells.
- IL-6 was found to be significantly upregulated in U87 ⁇ cells compared to the other U87 cell lines.
- an ELISA assay was performed on supernatants from the different U87 cell lines collected after 48 hours starvation.
- the present inventors observed that in vitro treatment of cells expressing wtEGFR with conditioned media from cells overexpressing deltaEGFR resulted in activation of STAT3, Akt, Erk1/2 and wtEGFR. In vivo tumor growth potentiation was also observed when wtEGFR overexpressing cells were mixed with deltaEGFR expressing cells, but not when those cells were mixed with cells with normal levels of wtEGFR or overexpressing a dead kinase version of deltaEGFR. Based on these observations, siRNA technology was used to knock-down either wt or deltaEGFR to assess the effect of specific receptor ablation on tumorigenicity and contribution to heterogeneity. As shown below, siRNAs specific for deltaEGFR or wtEGFR were able to reduce tumor growth after subcutaneous injection of ex vivo transfected cells.
- U87-wtEGFR Nagane et al, Cancer Research, 56: 5079-5086, 1996) and U87-deltaEGFR (Nishikawa et al, PNAS, 91: 7727-7731, 1994) cells were used as test cell lines to assess the specificity of these siRNA molecules.
- U87-deltaEGFR cells are recombinant cells expressing an EGFR gene deleted for exons 2-7 as described in Nishikawa et al. (1994).
- U87-wtEGFR cells are recombinant cells expressing a wtEGFR gene as described in Nishikawa et al. (1994).
- DMEM medium Cells were seeded in 24 well plates at 48,000 cells per well in DMEM medium (Cellgro) supplemented with 10% fetal bovine serum and L-Glutamine. The following day, siRNAs were transfected at 100 nM, 10 nM and 1 nM concentrations using LipofectamineTM 2000 (Invitrogen) and OptiMEM (Gibco). Twenty four hours after transfection, the medium was changed to DMEM supplemented with 10% fetal bovine serum, penicillin/streptomycin and L-Glutamine.
- protein lysates were prepared using RIPA buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.5, 1 mM EDTA, 1% NP-40, 0.1% SDS and 0.5% sodium deoxycholate) supplemented with protease inhibitors (Roche) and 5 ⁇ g of protein were resolved on 12% NuPAGE Bis-Tris acrylamide gels (Invitrogen). Gels were blotted onto nitrocellulose membranes, blocked with 5% milk in TBS-Tween and probed with anti-EGFR monoclonal antibody, C13, which recognizes both wt and mutant EGFR (BD).
- RIPA buffer 150 mM NaCl, 50 mM Tris-HCl pH 7.5, 1 mM EDTA, 1% NP-40, 0.1% SDS and 0.5% sodium deoxycholate
- protease inhibitors Roche
- Membrane bound C13 antibody was detected with HRP-conjugated anti-mouse IgG (Dako) followed by chemiluminiscence. Nitrocellulose membranes were also probed with anti-actin antibody as a positive control for protein loading.
- siRNAs determined to be specific and able to knock-down the expression of the receptor for which they were designed were tested again in U87-deltaEGFR and U87-wtEGFR to determine the minimal effective dose to achieve receptor expression knock-down.
- Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 100, 25, 5 and 1 nM concentrations using LipofectamineTM 2000. Twenty four hours after transfection, medium was changed and two days after protein lysates were obtained as described previously. Cells non-transfected as well as transfected with a siRNA against GFP or Luciferase protein were included as negative controls. Receptor expression was analyzed by western blot as described previously.
- siRNAs determined to be robust in the ablation of deltaEGFR or wtEGFR expression, when transfected at low concentration, were tested for suppression durability using U87-deltaEGFR and U87-wtEGFR cell lines.
- U87-wtEGFR or 0.5 ⁇ 10 6 U87-deltaEGFR transfected cells were resuspended in 100 ⁇ l PBS.
- Cells were injected subcutaneously into the right flank of Nu/Nu mice using a 1 ml syringe with a 26 G needle. Tumor volume was measured starting at day 5 after injection and was calculated using the formula 0.5 ⁇ L ⁇ W 2 . Mice were euthanized when tumor volume reached 1500 mm 3 .
- Protein lysates were prepared from the remaining cells of the injection using RIPA buffer supplemented with protease inhibitors. Receptor expression was analyzed by western blot as described previously.
- DeltaEGFR-specific siRNAs Western blot analysis demonstrated that 7 of 8 deltaEGFR-specific siRNAs were capable of complete elimination of the mutant receptor expressed in U87-deltaEGFR cells when tested at 100 nM (AL-DP-6901-6907) while three (AL-DP-6902, -6903, -6906) were able to effect a modest receptor reduction as low as 1 nM concentration (Table 4 and FIG. 4 A ).
- One siRNA, AL-DP-6908 had no effect.
- each deltaEGFR-specific siRNA was tested against U87-wtEGFR cells. Only one of the siRNAs (AL-DP-6906) that reduced deltaEGFR expression was also able to reduce wtEGFR expression.
- wtEGFR-specific siRNAs As above, western blot analysis was used to demonstrate that 5 of 8 wtEGFR-specific siRNAs were capable of complete elimination of the wt receptor expressed in U87-wtEGFR cells when tested at 100 nM (AL-DP-6918-6921 and -6923), while two (AL-DP-6919 and -6920) were able to effect a modest receptor reduction as low as 1 nM concentration (Table 4 and FIG. 5 B ). Three siRNAs (AL-DP-6917, -6922, and -6924) had little or no effect. To show specificity for the wt receptor, each active wtEGFR-specific siRNA (AL-DP-6918-6921 and -6923) was tested against U87-deltaEGFR cells with none showing reduction of the mutant receptor.
- Wt and deltaEGFR-specific siRNAs As above, western blot analysis was used to demonstrate the specificity of 8 siRNAs designed to knock-down the expression of both wt and deltaEGFR. Of this series, three siRNAs were able to simultaneously knock-down both receptors (AL-DP-6913, -6915, and -6916) albeit the effect was stronger for suppressing deltaEGFR (Table 4 and FIG. 5 C ).
- FIGS. 5 A- 5 C two samples were run for each treatment condition. Membranes were blotted with c13 antibody to detect EGFR and beta-actin antibody to confirm equivalent loading between lanes.
- DeltaEGFR-specific siRNAs Western blot analysis demonstrated that 8 of 8 deltaEGFR-specific siRNAs were capable of reducing mutant receptor expression in U87-deltaEGFR cells when tested at 25 nM (Table 4 and FIGS. 6 A-B ). To show specificity for the mutant receptor, deltaEGFR-specific siRNAs (AD-15416, AD-15417, AD-13375, AD-15418) were tested against U87-wtEGFR cells. None of siRNAs tested resulted in reduced wtEGFR expression.
- wtEGFR-specific siRNAs Western blot analysis demonstrated that 4 of 8 wtEGFR-specific siRNAs strongly suppress wtEGFR protein expression (AD-16177, AD-16178, AD-16179 and AD-13377), while 2 of these 8 siRNAs were able to moderately reduce wtEGFR protein expression (AD-13376 and AD-13378) (Table 4 and FIG. 6 C ). Two of the 8 siRNAs had little or no effect on wtEGFR protein levels (AD-16180 and AD-16181).
- FIGS. 6 A- 6 C two samples were run for each treatment condition. Membranes were blotted with c13 antibody to detect EGFR and beta-actin antibody to confirm equivalent loading between lanes.
- Non-Stabilized siRNAs are non-Stabilized siRNAs:
- DeltaEGFR-specific siRNAs Western blot analysis demonstrate that the four deltaEGFR-specific siRNAs tested (AL-DP-6901-6903, and -6905) were capable of complete elimination of the mutant receptor expressed in U87-deltaEGFR cells when transfected at 25 nM, nearly complete elimination at 5 nM and partial elimination at 1 nM ( FIG. 7 A ).
- wtEGFR-specific siRNAs As above, western blot analysis was used to demonstrate that 4 of 4 wtEGFR-specific siRNAs (AL-DP-6918-6921) were capable to varying degrees of wt receptor elimination in U87-wtEGFR cells ( FIG. 7 B ).
- Wt and deltaEGFR-specific siRNAs AL-DP-6913 and AL-DP-6916 were able to suppress expression of both wt and deltaEGFR protein in a dose-dependent manner ( FIGS. 7 A-B ).
- FIGS. 7 A and 7 B two samples were run for each treatment condition. Membranes were blotted with c13 antibody to detect EGFR.
- Non-Stabilized siRNAs are non-Stabilized siRNAs:
- DeltaEGFR-specific siRNAs Western blot analysis demonstrated that 4 of 4 deltaEGFR-specific siRNAs analyzed (AL-DP-6901, AL-DP-6902, AL-DP-6903, AL-DP-6905) were capable of complete elimination of mutant receptor expression in U87-deltaEGFR cells at 7 days after siRNA transfection, while siRNA AL-DP-6905 was capable of durable suppression as far as 10 days after siRNA transfection ( FIG. 8 A ).
- wtEGFR-specific siRNAs As above, western blot analysis was used to demonstrate that 2 of 2 wtEGFR-specific siRNAs analyzed (AL-DP-6920, AL-DP-6921) were capable of complete wt receptor expression elimination in U87-wtEGFR cells out to 7 days after siRNA transfection; however, for both siRNAs, receptor levels were restored to control levels by 10 days post transfection ( FIG. 8 B ).
- DeltaEGFR-specific siRNAs Western blot analysis demonstrated that 3 of 5 deltaEGFR-specific siRNAs analyzed (AD-15416, AD15417, AD-13374, AD-13375, AD-15418) were capable of complete elimination of mutant receptor expression in U87-deltaEGFR cells 5 days post transfection, while siRNA AD-15416 was capable of durable and complete suppression as far as 7 days post transfection ( FIG. 8 C ). Receptor expression levels were completely restored by day 12 post transfection for all stabilized siRNAs tested.
- wtEGFR-specific siRNAs As above, western blot analysis was used to demonstrate that AD-13377 wtEGFR-specific siRNA was capable of complete wt receptor protein elimination in U87-wtEGFR cells out to 7 days after transfection ( FIG. 8 D ). However, receptor levels were restored to control levels by 10 days post transfection.
- Non-Stabilized siRNAs are non-Stabilized siRNAs:
- U87-deltaEGFR cells were transfected with 100 nM siRNA and injected subcutaneously into nude mice. Treatment with siRNAs AL-DP-6901 and AL-DP-6902 ( FIG. 9 B ) resulted in a substantial reduction of U87-deltaEGFR tumor growth. As a negative control, cells non transfected or transfected with an irrelevant GFP siRNA were included in the study ( FIGS. 9 B ). In both of these groups, substantial tumor growth occurred.
- wtEGFR-specific siRNAs U87-wtEGFR cells were transfected with 25 nM siRNA concentration and injected subcutaneously into nude mice. Treatment with siRNAs AL-DP-6920 and AL-DP-6921 ( FIG. 9 A ) resulted in ablation of U87-wtEGFR tumor growth. As a negative control, cells non transfected or transfected with an irrelevant GFP siRNA were included in the study ( FIG. 9 A ). In both of these groups, substantial tumor growth occurred.
- each treatment group included six animals.
- siRNAs were designed to be specific to IL-6 (AD-15637 to AD-15660) and were synthesized.
- the sequences of the siRNAs and their target position on the IL-6 mRNA (GenBank Accession No. NM_000600.2, version Jan. 4, 2009) ( FIG. 11 ) are provided in Table 5.
- DMEM medium Cells were seeded in 24 well plates at 48,000 cells per well in DMEM medium (Cellgro) supplemented with 10% fetal bovine serum and L-Glutamine. The following day, siRNAs were transfected at 100 nM using LipofectamineTM 2000 (InvitrogenTM) and Opti-MEM® (GibcoTM). Cells non-transfected as well as transfected with an siRNA specific for GFP or Luciferase were included as negative controls. Forty-eight hours after transfection, the medium was changed to DMEM-serum-free supplemented with penicillin/streptomycin and L-Glutamine after washing the cells with serum-free medium.
- IL-6/IL-8 Quantification of IL-6/IL-8 in supernatants was assessed by ELISA. Briefly, 96-well plates (MaxiSorp, Nunc) were coated overnight at room temperature with the capture antibody diluted in PBS. The following day, the plates were blocked in blocking buffer composed of PBS containing 1% BSA and 5% sucrose. The standards (recombinant human IL-6 and IL-8) and the samples were diluted in diluent buffer (1X TBS, 0.5% BSA, 0.05% Tween-20) and incubated 2 hours at room temperature.
- diluent buffer (1X TBS, 0.5% BSA, 0.05% Tween-20
- the plates were then washed with PBS 0.05% Tween-20 and incubated with the biotinylated detection antibody and then with streptavidin-HRP (Biosource) both diluted in diluent buffer.
- the HRP activity was determined by using Tetramethylbenzidine (Sigma) as substrate.
- the enzymatic reaction was stopped with 1 N sulfuric acid and the absorbance was measured at 450 nm with wavelength correction set to 540 nm using a Tecan Genios Pro microplate reader. The absorbance readings were converted using a four parameter logistic curve.
- siRNAs determined to be specifically able to knock-down the secretion of IL-6 were tested again in U87- ⁇ EGFR to determine the minimal effective dose to achieve cytokine secretion knock-down.
- Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 100, 20, 4 and 0.8 nM concentrations using LipofectamineTM 2000. Forty-eight hours after transfection the medium was changed to serum-free medium and twenty-four hours later the supernatants were collected and centrifuged as described previously. Cells non-transfected as well as transfected with an siRNA against GFP or Luciferase were included as negative controls.
- IL-6 or IL-8 secretion was analyzed by ELISA.
- Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 25 nM concentration using LipofectamineTM 2000.
- Supernatants were collected at days 3, 7, and 11 or 14 days post-transfection (in each case after 24 hours serum-starvation). Cells non-transfected as well as transfected with an siRNA against GFP or Luciferase were included as negative controls.
- IL-6 secretion was analyzed by ELISA.
- In vitro proliferation test Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 25 nM concentration using LipofectamineTM 2000. 48 hours after transfection the cells were trypsinized, counted, and seeded at the same density in larger dishes to allow them to grow. The cell proliferation was evaluated by counting the cells at day 4 and at day 6-8 as indicated. Three independent samples were counted for each treatment/time point.
- U87- ⁇ EGFR cells were transfected with the different siRNAs (AD-15644 and AD-15660) and then injected subcutaneously into nude mice. Briefly, 1.3 ⁇ 10 6 cells were seeded in 10 cm plates and one day after they were transfected either with a control siRNA or with a specific siRNA at the concentration of 25 nM with LipofectamineTM 2000. The following day, the medium was changed and cells were split into larger dishes. Transfected U87- ⁇ EGFR cells were injected subcutaneously into the right flank of Nu/Nu mice using a 1 ml syringe with a 26 G needle.
- mice 5 ⁇ 10 5 cells resuspended in 100 ⁇ l of PBS were injected into each mouse. Tumor volume was measured starting at day 5 after injection and was calculated using the formula 0.5 ⁇ L ⁇ W 2 . Mice were euthanized according to our animal protocol when tumor volume reached 1500 mm 3 .
- In vivo siRNA delivery One million cells of U87-wtEGFR mixed with U87- ⁇ EGFR in a ratio 90:10% respectively were injected subcutaneously into 4 to 5 weeks-old female nude mice. Treatment of tumors was started after 13 days when the tumor volume reached approximately 80 mm3. JetPEI/siRNA complexes were prepared following manufacturer instructions (Polyplus Transfection, Illkirch, France) in 5% Glucose at N/P ratio of 15 and 70 ⁇ l of the complex was injected intratumorally at a dose of 10 ⁇ g siRNA/mouse every two days. Tumor volumes were measured every second day from the commencement of siRNA delivery through day 21 of treatment.
- siRNAs designed against IL-6 were capable of reducing IL-6 secretion in U87- ⁇ EGFR cells when tested at 100 nM (Table 9, and FIGS. 12 A and 12 B ).
- siRNAs AD-15647, AD-15651, AD-15654, and AD-15656 were unable to reduce IL-6 secretion in U87- ⁇ EGFR cells ( FIG. 12 A ).
- the strongest effect was obtained with AD-15658 (99.09%), and the weakest with AD-15642 (34.1%), with an average 82.4% reduction compared with cells transfected with the control siRNA.
- IL-8 concentration was tested in the 15 samples ( FIG. 12 B ) where strongest reduction of IL-6 secretion was observed.
- IL-8 production was significantly reduced in 11 of the analyzed samples.
- AD-15641 there was a significant increase in IL-8 secretion.
- Two siRNAs designed against IL-6 (AD-15644, AD-15650), showed no significant effect on the IL-8 secretion in U87- ⁇ EGFR cells demonstrating specificity for IL-6.
- AD-15660 caused a moderate increase in IL-8 production.
- ELISA analysis demonstrated that the 5 IL-6-specific siRNAs tested (AD-15644, -15649, -15650, -15657 and -15660) reduced significantly the secretion of IL-6 when transfected into U87- ⁇ EGFR cells at doses as low as 0.8 nM ( FIG. 13 A ).
- the concentration of IL-8 was also measured in these samples ( FIG. 13 B ) showing that siRNAs AD-15650 and AD-15660 caused the least significant reduction in IL-8 secretion when compared to control siRNA.
- siRNA AD-15644 and AD-15660 were capable of maximally reducing IL-6 secretion in U87- ⁇ EGFR cells as far as day 7 after siRNA transfection. At day 14 these two siRNAs were still able to suppress IL-6 expression.
- siRNA AD-15650 was capable of suppression of IL-6 secretion only until day three, and AD-15657 only until day 7 ( FIG. 14 ).
- U87- ⁇ EGFR cells were transfected with 25 nM siRNA and injected subcutaneously into nude mice. Treatment with siRNAs AD-15644 and AD-15660 resulted in no substantial reduction of U87- ⁇ EGFR tumor growth ( FIGS. 15 A and 15 B ). As a negative control, cells non transfected or transfected with an irrelevant Luciferase siRNA were included in the study. In both groups, substantial tumor growth occurred.
- FIGS. 9 A and 9 B Two siRNAs (AD-15644 and AD-15660) that demonstrated specificity for IL-6 and showed durable, low dose knock-down were chosen for in vivo studies.
- Nude mice were injected subcutaneously with 1 ⁇ 10 6 U87wt or U87 ⁇ cells or with U87wt+U87 ⁇ (90:10%) ( FIGS. 9 A and 9 B ) and monitored until tumors reached 80 mm 3 whereupon ten micrograms of AD-15644 or AD-15660 siRNA against IL-6 or an siRNA against Luciferase gene were injected intratumorally every two days. Tumor growth kinetics ( FIG. 16 A ) and tumor volume ( FIG.
- U87Parental cells which lack the ability to enhance U87wt tumor growth ( FIG. 1 A ), were engineered to over-express IL-6 (U87Par-IL6).
- U87wt alone or mixed with U87 ⁇ , U87Parental or U87Par-IL6 were injected subcutaneously into nude mice (1 ⁇ 10 6 total cells) at a ratio of 90:10% ( FIG. 17 A ) and resultant tumor volumes were measured over 32 days.
- mice were injected with 10% of the total cell number (1 ⁇ 10 5 cells) of U87 ⁇ , U87Parental and U87Par-IL6 cells. As shown in FIG.
- U87Par-IL6 did not grow faster than U87Parental. When they were mixed with U87wt cells, tumor growth kinetics were much faster for the U87wt+U87Par-IL6 mixture than for U87wt+U87Par demonstrating a paracrine tumor enhancement effect mediated by IL-6 secretion in these composite tumors. Even though U87Parental and U87Par-IL6 grew more slowly than U87 ⁇ , we observed a potent tumor enhancement when U87wt were injected with U87Par-IL6 with tumor volumes being nearly double that of U87wt tumors by the end of the experiment ( FIG. 17 B ).
- CM generated from U87Parental-IL6 was able to potently enhanced U87wt colony formation in the in vitro soft agar colony formation assays ( FIG. 18 ).
- U 87 Parental-IL6 CM was able to enhance U87wt colony formation with the same efficiency as U87 ⁇ CM (p>0.05).
- U87Parental CM was unable to enhance colony formation, and no significant differences were found when U87wt cells were treated with U87Parental or normal media (p>0.05).
- the bottom layer of agar was prepared by mixing equal volumes of 1.2% agar (USB Corporation) and 2 ⁇ DMEM/20% FBS solutions. Two ml of the resulting 0.6% agar/1X DMEM/10% FBS solution was added to each well of 6 well/plates and let to solidify at room temperature.
- the upper layer containing 2.5 ⁇ 10 3 U87wt cells was prepared by mixing equal volumes of 1.2% agar, 2 ⁇ DMEM/20%FBS and conditioned media or normal media. Plates were kept at room temperature until top agar solidified and treatment media was added on top of the agar. Plates were placed at 37° C/5% CO 2 for three weeks. Once a week, media on top of the agar was replaced with fresh media. Every treatment was performed in triplicate. After three weeks, plates were stained with 0.005% crystal violet solution and pictures were taken using a digital camera illuminated with white light. Colonies were counted using Image Pro-Analyzer 6.2 Software.
- CM generated from U87 ⁇ cells transfected with siRNA against the irrelevant luciferase gene resulted in a significant increase in colony number (** p ⁇ 0.01).
- CM generated from U87 ⁇ cells transfected with a mixture of the IL-6 siRNAs AD-15644 and AD-15660 resulted in a reduction in soft agar colony number to levels comparable to normal growth media.
- U87 ⁇ cells were transfected with a 25 nM dose of a combination of IL-6 siRNAs AD-15644 and AD-15660, or an siRNA against the irrelevant luciferase gene as a negative control.
- 1.3 ⁇ 10 6 U87 ⁇ cells were seeded in 10 cm plates and the following day were transfected using 5 LipofectamineTM 2000 (Invitrogen) and a mixture of IL-6 siRNAs AD-15644 and AD-15660 at a concentration of 12.5 nM each, or an siRNA against the luciferase gene at a concentration of 25 nM. After 18 hours of transfection, the media was changed and cells were partitioned into larger plates.
- the transfected U87 ⁇ cells (10 5 cells per mouse, 10%) were injected subcutaneously into nude mice alone or mixed with U87wt cells (9 ⁇ 10 5 cells per 10 mouse, 90%). Tumors were measured starting at day 5 after injection and volumes were calculated as described above.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Endocrinology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
The invention relates to a double-stranded ribonucleic acid (dsRNA) targeting a mutant Epidermal Growth Factor Receptor (EGFR), and methods of using the dsRNA to inhibit expression of mutant EGFR.
Description
- This application claims the benefit of U.S. Provisional Application No. 61/093,620, filed Sep. 2, 2008, U.S. Provisional Application No. 61/095,487, filed Sep. 9, 2008, U.S. Provisional Application No. 61/147,668, filed Jan. 27, 2009, U.S. Provisional Application No. 61/147,680, filed Jan. 27, 2009, and U.S. Provisional Application No. 61/166,488, filed Apr. 3, 2009. Each of these provisional applications is incorporated herein by reference in its entirety.
- The invention relates to a double-stranded ribonucleic acid (dsRNA) targeting a mutant Epidermal Growth Factor Receptor (EGFR), and methods of using the dsRNA to inhibit expression of mutant EGFR.
- The Epidermal Growth Factor Receptor (EGFR) gene is frequently upregulated in carcinomas of the breast, kidney, ovary, cervix, and in squamous cells. The upregulation is typically due to gene amplification or overexpression. EGFR upregulation in gliomas is most often associated with the rearrangement of the EGFR gene resulting in alterations of its transcript so that such gliomas express both wild-type endogenous EGFR as well as the episomal mutant form. The most common of the rearrangements are genomic alterations leading to deletion of exons 2-7 in the EGFR mRNA (called ds 2-7 EGFR, deltaEGFR, EGFR-de2-7, or EGFRvIII), which causes an in-frame truncation of 801 bp in the extracellular domain of the molecule. The EGFR gene is amplified in >50% of glioblastomas. This amplification is often associated with expression of deltaEGFR, which conveys enhanced tumor aggressiveness.
- Double-stranded RNA molecules (dsRNA) have been shown to block gene expression in a highly conserved regulatory mechanism known as RNA interference (RNAi). WO 99/32619 (Fire et al.) disclosed the use of a dsRNA of at least 25 nucleotides in length to inhibit the expression of genes in C. elegans. dsRNA has also been shown to degrade target RNA in other organisms, including plants (see, e.g., WO 99/53050, Waterhouse et al.; and WO 99/61631, Heifetz et al.), Drosophila (see, e.g., Yang, D., et al., Curr. Biol. (2000) 10:1191-1200), and mammals (see WO 00/44895, Limmer; and DE 101 00 586.5, Kreutzer et al.).
- The invention provides compositions containing double-stranded ribonucleic acid (dsRNA) and methods for inhibiting the expression of a mutant EGFR gene, such as a deltaEGFR gene, in a cell or mammal. The invention also provides compositions and methods for treating pathological conditions and diseases caused by the expression of deltaEGFR gene, such as cancer, including glioma. The dsRNAs included in the compositions featured herein include a dsRNA having an RNA strand (the antisense strand) having a region which is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and is substantially complementary to at least part of an mRNA transcript of the deltaEGFR gene. In some embodiments, the dsRNA also targets a wildtype mRNA transcript of the EGFR gene.
- In one embodiment, a dsRNA for inhibiting expression of a deltaEGFR gene includes at least two sequences that are complementary to each other. The dsRNA includes a sense strand having a first sequence and an antisense strand having a second sequence. The antisense strand includes a nucleotide sequence that is substantially complementary to at least part of an mRNA encoding deltaEGFR, and the region of complementarity is less than 30 nucleotides in length, and at least 15 nucleotides in length. Generally, the dsRNA is 19 to 24, e.g., 19 to 21 nucleotides in length. In some embodiments the dsRNA is from about 10 to about 15 nucleotides in length, and in other embodiments the dsRNA is from about 25 to about 30 nucleotides in length. The dsRNA, upon contacting with a cell expressing deltaEGFR, inhibits the expression of the deltaEGFR gene by at least 20%, at least 25%, at least 30%, at least 35%, or at least 40%, such as when assayed by a method as described herein. In one embodiment, the deltaEGFR dsRNA is formulated in a stable nucleic acid particle (SNALP).
- For example, the dsRNA molecules featured herein can include a first sequence of the dsRNA that is selected from the group consisting of the sense sequences of Tables 2, 3 and 4, and a second sequence that is selected from the group consisting of the antisense sequences of Tables 2, 3 and 4. The dsRNA molecules featured herein can include naturally occurring nucleotides or can include at least one modified nucleotide, such as a 2′-O-methyl modified nucleotide, a nucleotide having a 5′-phosphorothioate group, and a terminal nucleotide linked to a conjugate group, such as a cholesteryl derivative or a vitamin E group. Alternatively, the modified nucleotide may be chosen from the group of: a 2′-deoxy-2′-fluoro modified nucleotide, a T-deoxy-modified nucleotide, a locked nucleotide, an abasic nucleotide, 2′-amino-modified nucleotide, 2′-alkyl-modified nucleotide, morpholino nucleotide, a phosphoramidate, and a non-natural base comprising nucleotide. Generally, such modified sequence will be based on a first sequence of said dsRNA selected from the group consisting of the sense sequences of Tables 2, 3 and 4 and a second sequence selected from the group consisting of the antisense sequences of Tables 2, 3 and 4.
- In one aspect, an interleukin-6 (IL6) dsRNA is also featured in the invention, and the IL-6 dsRNA is capable of decreasing levels of IL6 protein secretion in cultured cells, e.g., human cultured cells. In one embodiment, the cultured cells are U87-ΔEGFR cells. In another embodiment, the IL-6 dsRNA is capable of decreasing IL6 secretion into culture supernatant by 20%, 30%, 40%, 50%, 60%, 70%, 80%, 85%, 90% or more. In yet another embodiment, the IL6 dsRNA is capable of reducing tumor volume in an animal model, such as in a mouse, rat, or primate model. In another embodiment, a first sequence of an IL6 dsRNA is selected from the group consisting of the sense sequences of Tables 5, 6, 7, and 8, a second sequence is selected from the group consisting of the antisense sequences of Tables 5, 6, 7, and 8.
- In another aspect, the invention provides a cell containing at least one of the dsRNAs featured in the invention. The cell is generally a mammalian cell, such as a human cell.
- In yet another aspect, the invention provides a pharmaceutical composition for inhibiting the expression of a deltaEGFR gene in an organism, generally a human subject. The composition typically includes one or more of the dsRNAs described herein and a pharmaceutically acceptable carrier or delivery vehicle. In one embodiment, the composition is used for treating cancer, e.g., a glioma.
- In another embodiment, the pharmaceutical composition is formulated for administration of a dosage regimen described herein, e.g., not more than once every four weeks, not more than once every three weeks, not more than once every two weeks, or not more than once every week. In another embodiment, the pharmaceutical composition can be maintained for a month or longer, e.g., one, two, three, or six months, or one year or longer.
- In another embodiment, a composition containing a dsRNA featured in the invention, e.g., a dsRNA targeting deltaEGFR, is administered with a non-dsRNA therapeutic agent, such as an agent known to treat a cancer, such as a glioma. For example, a dsRNA featured in the invention can be administered with, e.g, a chemotherapeutic agent, such as temozolomide, or with radiation therapy.
- In one embodiment, the composition further includes a dsRNA having at least two sequences that are complementary to each other, and where a sense strand includes a region of complementarity that is substantially complementary to at least a part of an mRNA encoding an IL6 protein, and where the region of complementarity is less than 30 nucleotides in length and at least 15 nucleotides in length. Generally, the IL6 dsRNA is 19 to 24, e.g., 19 to 21 nucleotides in length. In some embodiments, the dsRNA is from about 10 to about 15 nucleotides in length, and in other embodiments the dsRNA is from about 25 to about 30 nucleotides in length. In another embodiment, a first sequence of the IL6 dsRNA is selected from the group consisting of the sense sequences of Tables 5, 6, 7, and 8, a second sequence is selected from the group consisting of the antisense sequences of Tables 5, 6, 7, and 8.
- In another aspect, the deltaEGFR dsRNA is administered to a patient, and then a non-dsRNA agent is administered to the patient (or vice versa). In one embodiment, the deltaEGFR dsRNA and the non-dsRNA therapeutic agent are administered at the same time. In another embodiment, the deltaEGFR dsRNA is administered with an IL6 dsRNA, such as for the treatment of cancer.
- In certain embodiments, the patient has a cancer, e.g., a tumor, such as an astrocytic tumor, or a glioma.
- In one aspect, the invention provides a method for inhibiting the expression of a deltaEGFR gene in a cell by performing the following steps:
-
- (a) introducing into the cell a double-stranded ribonucleic acid (dsRNA), wherein the dsRNA includes at least two sequences that are complementary to each other. The dsRNA has a sense strand having a first sequence and an antisense strand having a second sequence; the antisense strand has a region of complementarity that is substantially complementary to at least a part of a mRNA encoding deltaEGFR, and where the region of complementarity is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and where the dsRNA, upon contact with a cell expressing the deltaEGFR, inhibits expression of the deltaEGFR gene by at least 40%;
- and
-
- (b) maintaining the cell produced in step (a) for a time sufficient to obtain degradation of the mRNA transcript of deltaEGFR gene, thereby inhibiting expression of the deltaEGFR gene in the cell.
- In one embodiment, the dsRNA that inhibits expression of the deltaEGFR gene also inhibits expression of a wildtype EGFR gene in the cell. Typically, such a dsRNA can inhibit both deltaEGFR expression and wildtype EGFR expression, because the antisense strand has a region of complementarity that is substantially complementary to at least a part of an mRNA encoding deltaEGFR and at least part of an mRNA encoding wildtype EGFR.
- In another embodiment, the method is for inhibiting gene expression in a tumor cell.
- In another aspect, the invention provides methods for treating, preventing or managing pathological processes mediated by deltaEGFR expression, e.g., a cancer, such as a glioma, e.g., a glial tumor of the central nervous system, such as a grade I, II, III, or IV glioma. For example, a dsRNA targeting deltaEGFR is used to treat a grade III glioma, such as anaplastic astrocytoma, or a grade IV glioma, such as a glioblastoma multiforme. In other embodiments, a dsRNA targeting deltaEGFR is used to treat a carcinoma of the breast, ovary, cervix, kidney, or squamous cell. In one embodiment, the deltaEGFR dsRNA is administered with a second dsRNA, such as an IL6 dsRNA, for treatment of a disorder associated with deltaEGFR expression. The IL6 and deltaEGFR dsRNAs can be administered in combination or sequentially. In yet another embodiment, an IL6 dsRNA alone is administered to treat a disorder associated with deltaEGFR expression.
- A method featured in the invention can include administering to a patient in need of such treatment, prevention or management a therapeutically or prophylactically effective amount of one or more of the dsRNAs featured in the invention, e.g., one or both of a dsRNA targeting deltaEGFR or IL6. In one embodiment the patient has cancer. In another embodiment, administration of the dsRNA targeting deltaEGFR and/or the dsRNA targeting IL6, alleviates or relieves the severity of at least one symptom of the deltaEGFR-mediated disorder in the patient.
- In another aspect, the invention provides a vector for inhibiting the expression of a deltaEGFR gene in a cell. In one embodiment, the vector includes at least one regulatory sequence operably linked to a nucleotide sequence that encodes at least one strand of one of a dsRNA featured in the invention.
- In another aspect, the invention provides a vector for inhibiting the expression of an IL6 gene in a cell. In one embodiment, the vector includes at least one regulatory sequence operably linked to a nucleotide sequence that encodes at least one strand of one of a dsRNA featured in the invention.
- In yet another aspect, the invention provides a cell containing a vector for inhibiting the expression of a deltaEGFR gene in a cell. The vector includes a regulatory sequence operably linked to a nucleotide sequence that encodes at least one strand of one of the deltaEGFR dsRNA featured in the invention. In one embodiment, the cell also contains a vector for inhibiting expression of an IL6 gene in a cell. This vector also has a regulatory sequence operably linked to a nucleotide sequence that encodes at least one strand of an IL6 dsRNA featured in the invention.
- In yet another aspect, the invention provides a composition containing a deltaEGFR dsRNA, in combination with a second dsRNA targeting a second gene involved in a pathological disease, and useful for treating the disease, e.g., cancer. In one embodiment, the second dsRNA is a dsRNA targeting IL6.
- The details of one or more embodiments of the invention are set forth in the description below. Other features, objects, and advantages of the invention will be apparent from the description and the drawings, and from the claims.
-
FIGS. 1A and 1B are graphs showing tumor growth kinetics (FIG. 1A ) and tumor volume (FIG. 1B ) following injection of cells subcutaneously into the right flank of 4 to 5 weeks-old female athymic nude mice. -
FIGS. 2A and 2B show hematoxylin and eosin (H&E) staining of brain cryo-sections from mice.FIG. 2A is H&E staining of brain cryo-sections from nude mice injected intracranially with cells, andFIG. 2B shows H&E staining of brain cryo-sections of mice injected intracranially with cells either alone or mixed with deltaEGFR over-expressing astrocytes (upper panel). The lower panel shows the presence of wtEGFR astrocytes within the tumor by immunofluorescence (GFP IF). -
FIG. 3A is a graph showing the composition of various tumor samples in 4 to 5 week old mice injected with the indicated cell types, as assayed by X-Gal staining.FIG. 3B is a graph showing the composition of tumor samples as assayed by flow cytometry using Ab-1 (FITC) and Ab-5 (APC) antibodies to stain tumors formed after infection of U87wt mixed with U87delta at the indicated ratios. -
FIG. 4 is a panel of Western blots showing analysis of EGFR activation and known downstream signaling molecules in U87wt cells stimulated with serial dilutions of U87delta CM or negative control U87Par CM, or positive control EGF ligand. -
FIGS. 5A-5C are Western blots showing the activity of siRNAs targeting deltaEGFR (FIG. 5A ), wildtype EGFR (FIG. 5B ), or both mutant and wildtype receptors (FIG. 5C ). Cl, C2, C3, and CA indicate negative controls (untransfected cells). -
FIGS. 6A-6C are Western blots showing the activity of siRNAs targeting deltaFGFR (FIG. 6A ) or wildtype EGFR (FIG. 6C ).FIG. 6B shows the activity of siRNAs specific for deltaEGFR (AD-13375) or wtEGFR (AD-13377) in U87-deltaEGFR cells or U87-wtEGFR cells, respectively. Luc and (−) indicate negative controls (cells transfected with an irrelevant gene siRNA (targeting luciferase) and untransfected cells, respectively). -
FIGS. 7A and 7B are Western blots showing dose response activity of siRNA activity in U87-deltaEGFR cells (FIG. 7A ) and in U87-wtEGFR cells (FIG. 7B ). U87-deltaEGFR cells were transfected with siRNAs specific for deltaEGFR, and for both mutant and wildtype receptors. U87-wtEGFR cells were transfected with siRNAs specific for wtEGFR, and for both mutant and wildtype receptors. C1, C2, C3, C4, C5, and C6 indicate negative controls (untransfected cells). -
FIGS. 8A-8D are Western blots showing durability of the effect of unstabilized (FIGS. 8A and 8B ) and stabilized (FIGS. 8C and 8D ) siRNAs. InFIGS. 8A and 8C , U87-deltaEGFR cells were transfected with non-stabilized (FIG. 8A ) or stabilized (FIG. 8C ) siRNAs. InFIGS. 8B and 8D , U87-wtEGFR cells were transfected with unstabilized (FIG. 8B ) or stabilized (FIG. 8D ) siRNAs. Lysates were prepared and Western blots were performed at the indicated day post-transfection. Luc and (−) indicate negative controls (cells transfected with an irrelevant gene siRNA (targeting luciferase) and untransfected cells, respectively). -
FIGS. 9A and 9B are graphs showing the effects of siRNAs on tumorigenicity in mice injected with U87-wtEGFR cells (FIG. 9A ) and in mice injected with U87-deltaEGFR cells (FIG. 9B ). Lysates were prepared and Western blots were performed at the indicated day post-transfection. Data are shown as mean±standard deviation (SD). -
FIGS. 10A and 10B are graphs showing tumor kinetics (FIG. 10A ) and volume (FIG. 10B ) in nude mice injected with U87delta cells and then injected intratumorally with 5 mg ofdeltaEGFR siRNA# 1 or irrelevant siRNA (siRNA luc). -
FIG. 11 is the mRNA sequence of IL-6 reported at GenBank Accession No. NM_000600.2 (record dated Jan. 4, 2009, GI No.155369258; SEQ ID NO:274). -
FIGS. 12A and 12B are graphs showing screening analysis of stabilized siRNAs designed for IL-6 by ELISA.FIG. 12A demonstrates the effect of the siRNAs on their target cytokines. Specificity of the siRNAs for IL-6 was assessed by quantifying IL-8 levels (FIG. 12B ). Values are mean±SE of 2 independent samples. (“Neg”: siRNA targeting an irrelevant sequence). -
FIGS. 13A and 13B are graphs showing dose-response analysis of stabilized IL-6 siRNAs. Quantification of secreted IL-6 (FIG. 13A ) and IL-8 (FIG. 13B ) in supernatants from U87-ΔEGFR cells transfected with 100, 20, 4, and 0.8 nM of different IL-6 siRNAs was performed by ELISA. Values are mean±SE of 2 independent samples. (“Neg”: siRNA targeting an irrelevant sequence). -
FIG. 14 is a graph illustrating durability analysis of stabilized IL-6 siRNAs. Quantification of secreted IL-6 in supernatants of U87-ΔEGFR cells at different days after transfection with the siRNAs was performed by ELISA. (“Neg2”: siRNA targeting an irrelevant sequence). -
FIGS. 15A and 15B are two graphs illustrating ex vivo tumorigenicity test of IL-6-specific stabilized siRNAs. The graphs show tumor growth kinetics (top) and tumor volume at the end of the experiment (bottom) after injection into nude mice of U87-deltaEGFR cells transfected with stabilized siRNAs against luciferase or GFP, IL-6 (AD-15644 and AD-15660). The experimental group included 6 animals. Data are shown as mean +SE. -
FIGS. 16A and 16B are graphs illustrating the efficacy of in vivo delivery of IL-6 siRNAs (AD-15644 and AD-15660). Tumor growth kinetics (FIG. 11A ) and tumor volume at the end of the experiment (FIG. 11B ) was reduced in tumors treated with IL-6 specific siRNA AD-15660, but not in tumors treated with a siRNA against luciferase. Each group included 4 animals. Data are shown as mean+SE. -
FIGS. 17A and 17B are graphs illustrating tumor volume (A) and tumor growth kinetics (B) after subcutaneous injection of the indicated cell types into nude mice. Tumor volume (FIG. 17A ) was assayed at day 32 after injection. -
FIG. 18 is a graph illustrating quantification of soft agar colony formation of U87wt colonies formed after treatment with normal media (Neg), or U87Δ (ACM (conditioned medium)), U87Par (parental CM), or U87Par-IL6 (parental-IL6) CM (*: p<0.05; ** p<0.001). -
FIG. 19 is a graph illustrating quantification of soft agar colony formation of U87wt colonies formed after treatment with normal media (negative control, Neg), or with U87Δ cell CM untreated (ΔCM) or pretreated with IL-6 neutralizing antibody (ACM +IL-6 Ab) (**: p<0.001). -
FIGS. 20A and 20B are graphs illustrating tumor growth kinetics (A) and tumor volume at day 20 (B) after subcutaneous injection of U87wt, U87Δ-Luc siRNA or U87Δ-IL6 siRNA cells only, or U87wt cells mixed with U87Δ-Luc siRNA or U87Δ-IL6 siRNA cells at a ratio of 90:10. -
FIGS. 21A and 21B represent the mRNA sequence of wtEGFR (SEQ ID NO:1) (GenBank Accession No. NM_005228; record dated Aug. 24, 2008, GI No. 41327737). - The underlined nucleotides are deleted in deltaEGFR mRNA sequence.
- The invention provides dsRNAs and methods of using the dsRNAs for inhibiting the expression of a deltaEGFR gene in a cell or a mammal where the dsRNA targets the deltaEGFR gene. In some embodiments, the dsRNAs featured in the invention target both a deltaEGFR gene and a wildtype EGFR (wtEGFR) gene. The invention also provides dsRNAs and methods of using the dsRNAs for inhibiting the expression of an IL6 gene in a cell or a mammal where the dsRNA targets the IL6 gene. The invention provides compositions and methods for treating pathological conditions and diseases, such as a cancer, in a mammal caused by the expression of the deltaEGFR or IL6 genes. dsRNA directs the sequence-specific degradation of mRNA through a process known as RNA interference (RNAi).
- The dsRNAs of the compositions featured herein include an RNA strand (the antisense strand) having a region which is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and is substantially complementary to at least part of an mRNA transcript of the deltaEGFR gene. The use of these dsRNAs enables the targeted degradation of mRNAs of genes that are implicated in replication or maintenance of cancer cells in mammals. Very low dosages of deltaEGFR or IL6 dsRNAs in particular can specifically and efficiently mediate RNAi, resulting in significant inhibition of expression of the deltaEGFR and IL6 genes. Using cell-based and animal assays, the present inventors have demonstrated that dsRNAs targeting deltaEGFR alone, or targeting both deltaEGFR and wtEGFR, can specifically and efficiently mediate RNAi, resulting in significant inhibition of expression of one or both of the deltaEGFR or EGFR genes. Thus, methods and compositions including these dsRNAs are useful for treating pathological processes that can be mediated by down regulating deltaEGFR and EGFR, such as in the treatment of cancer.
- Using cell-based and animal assays, the present inventors have also demonstrated that dsRNAs targeting IL6 can also specifically and efficiently mediate RNAi, resulting in significant inhibition of expression of an IL6 gene. Thus, methods and compositions including these dsRNAs are useful for treating pathological processes that can be mediated by down regulating IL6, such as in the treatment of cancer.
- The methods and compositions containing the deltaEGFR or IL6 dsRNA featured in the invention are useful for treating pathological processes mediated by deltaEGFR or IL6 expression, e.g., cancer, such as glioma.
- The following detailed description discloses how to make and use the compositions containing dsRNAs to inhibit the expression of the deltaEGFR or IL6 genes, as well as compositions and methods for treating diseases and disorders caused by the expression of these genes, such as leukemia. The pharmaceutical compositions featured in the invention include a dsRNA having an antisense strand comprising a region of complementarity which is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and is substantially complementary to at least part of an RNA transcript of the deltaEGFR or IL6 gene, together with a pharmaceutically acceptable carrier. The compositions featured in the invention also include a dsRNA having an antisense strand having a region of complementarity which is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and which is substantially complementary to at least part of an RNA transcript of the deltaEGFR or IL6 gene.
- Accordingly, in some aspects, pharmaceutical compositions containing the deltaEGFR dsRNA and a pharmaceutically acceptable carrier, methods of using the compositions to inhibit expression of the deltaEGFR gene, and methods of using the pharmaceutical compositions to treat diseases caused by expression of the deltaEGFR gene are featured in the invention.
- In other aspects, pharmaceutical compositions containing the IL6 dsRNA and a pharmaceutically acceptable carrier, methods of using the compositions to inhibit expression of an IL6 gene, and methods of using the pharmaceutical compositions to treat diseases caused by expression of the IL6 gene are featured in the invention.
- For convenience, the meaning of certain terms and phrases used in the specification, examples, and appended claims, are provided below. If there is an apparent discrepancy between the usage of a term in other parts of this specification and its definition provided in this section, the definition in this section shall prevail.
- “G,” “C,” “A,” “T” and “U” each generally stand for a nucleotide that contains guanine, cytosine, adenine, thymidine and uracil as a base, respectively. However, it will be understood that the term “ribonucleotide” or “nucleotide” can also refer to a modified nucleotide, as further detailed below, or a surrogate replacement moiety. The skilled person is well aware that guanine, cytosine, adenine, thymidine, and uracil may be replaced by other moieties without substantially altering the base pairing properties of an oligonucleotide comprising a nucleotide bearing such replacement moiety. For example, without limitation, a nucleotide comprising inosine as its base may base pair with nucleotides containing adenine, cytosine, or uracil. Hence, nucleotides containing uracil, guanine, or adenine may be replaced in the nucleotide sequences of dsRNA featured in the invention by a nucleotide containing, for example, inosine. In another example, adenine and cytosine anywhere in the oligonucleotide can be replaced with guanine and uracil, respectively to form G-U Wobble base pairing with the target mRNA. Sequences containing such replacement moieties are suitable for the compositions and methods featured in the invention.
- As used herein, “deltaEGFR” refers to an in-frame deletion of exons 2-7 from the EGFR gene. deltaEGFR is also known as “de 2-7 EGFR” (Nishikawa et al. “A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity” Proc. Natl. Acad. Sci. USA 91:7727-7731, 1994), “EGFR-de2-7”, “EGFR*”, “ΔEGFR”, and “EGFRvIII.” The sequence of deltaEGFR is equivalent to the sequence shown at
FIGS. 21A and 21B carrying a deletion of nucleotides 335 through 1135. - As used herein, “wild-type EGFR” (“wtEGFR”) refers to a non-mutant EGFR gene (e.g., an endogenous EGFR gene) in a cell, such as in a non-transformed, or non-cancerous cell in a human. EGFR is also known as EC2.7.10.1 (Epidermal Growth Factor Receptor Precursor), ERBB (Receptor Protein Tyrosine Kinase ErbB1), ERBB1, HER1, PIG61 (cell proliferation-inducing protein 61), mENA, avian erythroblastic leukemia viral (v-erb-b) oncogene homolog, and cell growth inhibiting protein 40. The sequence of four alternative wildtype EGFR mRNA transcripts can be found at Genbank Accession Numbers NM_005228.3 (record dated Aug. 24, 2008, GI No. 41327737; see
FIGS. 21A and 21B ), NM 201282.1 (record dated Aug. 24, 2008, GI No. 41327731), NM_201283.1 (record dated Aug. 24, 2008, GI No. 41327733), and NM_201284.1 (record dated Aug. 24, 2008, GI No. 41327735). - As used herein “Interleukin-6” (“IL-6”) refers to an IL-6 gene (e.g., an endogenous IL-6 gene) in a cell, such as in a non-transformed, or non-cancerous cell in a human.
- IL-6 is also known as
Interleukin 6; IFNB2 (Interferon beta 2, or interferon, beta 2); BSF-2 (B-cell stimulatory factor 2); BSF2; CDF (CTL differentiation factor); HGF (hybridoma growth factor); HSF. The mRNA sequence of IL-6 is at GenBank Accession No. NM_000600.2 (FIG. 11 ) (record dated Jan. 4, 2009, GI No. 155369258). - As used herein, “target sequence” of a dsRNA refers to a contiguous portion of the nucleotide sequence of an mRNA molecule formed during the transcription of the target gene, e.g., a deltaEGFR gene or an IL-6 gene, including mRNA that is a product of RNA processing of a primary transcription product.
- As used herein, the term “strand comprising a sequence” refers to an oligonucleotide comprising a chain of nucleotides that is described by the sequence referred to using the standard nucleotide nomenclature.
- As used herein, and unless otherwise indicated, the term “complementary,” when used to describe a first nucleotide sequence in relation to a second nucleotide sequence, refers to the ability of an oligonucleotide or polynucleotide comprising the first nucleotide sequence to hybridize and form a duplex structure under certain conditions with an oligonucleotide or polynucleotide comprising the second nucleotide sequence, as will be understood by the skilled person. Such conditions can, for example, be stringent conditions, where stringent conditions may include: 400 mM NaCl, 40 mM PIPES pH 6.4, 1 mM EDTA, 50° C. or 70° C. for 12-16 hours followed by washing. Other conditions, such as physiologically relevant conditions as may be encountered inside an organism, can apply. The skilled person will be able to determine the set of conditions most appropriate for a test of complementarity of two sequences in accordance with the ultimate application of the hybridized nucleotides.
- This includes base-pairing of the oligonucleotide or polynucleotide comprising the first nucleotide sequence to the oligonucleotide or polynucleotide comprising the second nucleotide sequence over the entire length of the first and second nucleotide sequence. Such sequences can be referred to as “fully complementary” with respect to each other herein. However, where a first sequence is referred to as “substantially complementary” with respect to a second sequence herein, the two sequences can be fully complementary, or they may form one or more, but generally not more than 4, 3 or 2 mismatched base pairs upon hybridization, while retaining the ability to hybridize under the conditions most relevant to their ultimate application. However, where two oligonucleotides are designed to form, upon hybridization, one or more single stranded overhangs, such overhangs shall not be regarded as mismatches with regard to the determination of complementarity. For example, a dsRNA comprising one
oligonucleotide 21 nucleotides in length and another oligonucleotide 23 nucleotides in length, wherein the longer oligonucleotide comprises a sequence of 21 nucleotides that is fully complementary to the shorter oligonucleotide, may yet be referred to as “fully complementary” for the purposes described herein. - “Complementary” sequences, as used herein, may also include, or be formed entirely from, non-Watson-Crick base pairs and/or base pairs formed from non-natural and modified nucleotides, insofar as the above requirements with respect to their ability to hybridize are fulfilled. Such non-Watson-Crick base pairs includes, but not limited to, G:U Wobble or Hoogstein base pairing.
- The terms “complementary,” “fully complementary” and “substantially complementary” herein may be used with respect to the base matching between the sense strand and the antisense strand of a dsRNA, or between the antisense strand of a dsRNA and a target sequence, as will be understood from the context of their use.
- As used herein, a polynucleotide that is “substantially complementary to at least part of” a messenger RNA (mRNA) refers to a polynucleotide that is substantially complementary to a contiguous portion of an mRNA of interest (e.g., encoding deltaEGFR or IL6). For example, a polynucleotide is complementary to at least a part of a deltaEGFR mRNA if the sequence is substantially complementary to a non-interrupted portion of an mRNA encoding deltaEGFR. Similarly, a polynucleotide is complementary to at least a part of a wtEGFR mRNA if the sequence is substantially complementary to a non-interrupted portion of an mRNA encoding wtEGFR.
- The term “double-stranded RNA” or “dsRNA,” as used herein, refers to a complex of ribonucleic acid molecules, having a duplex structure comprising two anti-parallel and substantially complementary, as defined above, nucleic acid strands. The two strands forming the duplex structure may be different portions of one larger RNA molecule, or they may be separate RNA molecules. Where the two strands are part of one larger molecule, and therefore are connected by an uninterrupted chain of nucleotides between the 3′-end of one strand and the 5′end of the respective other strand forming the duplex structure, the connecting RNA chain is referred to as a “hairpin loop.” Where the two strands are connected covalently by means other than an uninterrupted chain of nucleotides between the 3′-end of one strand and the 5′end of the respective other strand forming the duplex structure, the connecting structure is referred to as a “linker.” The RNA strands may have the same or a different number of nucleotides. The maximum number of base pairs is the number of nucleotides in the shortest strand of the dsRNA minus any overhangs that are present in the duplex. In addition to the duplex structure, a dsRNA may comprise one or more nucleotide overhangs.
- As used herein, a “nucleotide overhang” refers to the unpaired nucleotide or nucleotides that protrude from the duplex structure of a dsRNA when a 3′-end of one strand of the dsRNA extends beyond the 5′-end of the other strand, or vice versa. “Blunt” or “blunt end” means that there are no unpaired nucleotides at that end of the dsRNA, i.e., no nucleotide overhang. A “blunt ended” dsRNA is a dsRNA that is double-stranded over its entire length, i.e., no nucleotide overhang at either end of the molecule.
- The term “antisense strand” refers to the strand of a dsRNA which includes a region that is substantially complementary to a target sequence. As used herein, the term “region of complementarity” refers to the region on the antisense strand that is substantially complementary to a sequence, for example a target sequence, as defined herein. Where the region of complementarity is not fully complementary to the target sequence, the mismatches may be in the internal or terminal regions of the molecule. Generally, the most tolerated mismatches are in the terminal regions, e.g., within 6, 5, 4, 3, or 2 nucleotides of the 5′ and/or 3′ terminus.
- The term “sense strand,” as used herein, refers to the strand of a dsRNA that includes a region that is substantially complementary to a region of the antisense strand.
- The term “identity” is the relationship between two or more polynucleotide sequences, as determined by comparing the sequences. Identity also means the degree of sequence relatedness between polynucleotide sequences, as determined by the match between strings of such sequences. While there exist a number of methods to measure identity between two polynucleotide sequences, the term is well known to skilled artisans (see, e.g., Sequence Analysis in Molecular Biology, von Heinje, G., Academic Press (1987); and Sequence Analysis Primer, Gribskov., M. and Devereux, J., eds., M. Stockton Press, New York (1991)). “Substantially identical,” as used herein, means there is a very high degree of homology (e.g., 100% sequence identity) between the sense strand of the dsRNA and the corresponding part of the target gene. However, dsRNA having greater than 90%, or 95% sequence identity may be used in the present invention, and thus sequence variations that might be expected due to genetic mutation, strain polymorphism, or evolutionary divergence can be tolerated. The dsRNA is typically 100% complementary to the target RNA, but in some embodiments, the dsRNA may contain single or multiple base-pair random mismatches between the RNA and the target gene.
- As used herein, the term “SNALP” refers to a stable nucleic acid-lipid particle. A SNALP represents a vesicle of lipids coating a reduced aqueous interior comprising a nucleic acid such as an iRNA agent or a plasmid from which an iRNA agent is transcribed. SNALPs are described, e.g., in U.S. Patent Application Publication Nos. 20060240093, 20070135372, and
USSN 61/045,228 filed Apr. 15, 2008. These applications are hereby incorporated by reference. - “Introducing into a cell,” when referring to a dsRNA, means facilitating uptake or absorption into the cell, as is understood by those skilled in the art. Absorption or uptake of dsRNA can occur through unaided diffusive or active cellular processes, or by auxiliary agents or devices. The meaning of this term is not limited to cells in vitro; a dsRNA may also be “introduced into a cell,” wherein the cell is part of a living organism. In such instance, introduction into the cell will include the delivery to the organism. For example, for in vivo delivery, dsRNA can be injected into a tissue site or administered systemically. In vivo delivery can also be by a beta-glucan delivery system, such as those described in U.S. Pat. Nos. 5,032,401 and 5,607,677, and U.S. Publication No. 2005/0281781. U.S. Pat. Nos. 5,032,401 and 5,607,677, and U.S. Publication No. 2005/0281781 are hereby incorporated by reference in their entirety. In vitro introduction into a cell includes methods known in the art such as electroporation and lipofection.
- The terms “silence,” “inhibit the expression of,” “down-regulate the expression of,” “suppress the expression of,” and the like, insofar as they refer to a deltaEGFR or IL6 gene, refer to the at least partial suppression of expression of the deltaEGFR or IL6 gene, as manifested by a reduction of the amount of deltaEGFR or IL6 mRNA which may be isolated or detected from a first cell or group of cells in which the deltaEGFR or IL6 gene is transcribed and which has or have been treated such that the expression of the deltaEGFR or IL6 gene is inhibited, as compared to a second cell or group of cells substantially identical to the first cell or group of cells but which has or have not been so treated (control cells). The degree of inhibition is usually expressed in terms of
-
- Alternatively, the degree of inhibition may be given in terms of a reduction of a parameter that is functionally linked to gene expression, e.g., the amount of protein encoded by the deltaEGFR or IL6 gene which is secreted by a cell, or the number of cells displaying a certain phenotype, e.g., apoptosis. In principle, gene silencing may be determined in any cell expressing the target gene, either constitutively or by genomic engineering, and by any appropriate assay. However, when a reference is needed in order to determine whether a given dsRNA inhibits the expression of a deltaEGFR gene or an IL6 gene by a certain degree and therefore is encompassed by the instant invention, the assays provided in the Examples below shall serve as such reference. For example, deltaEGFR gene silencing may be determined in U87-deltaEGFR (Nishikawa et al., PNAS 91:7727-7731, 1994) or U87-wtEGFR (Nagana et al., Cancer Research 56:5079-5086, 1996) cells.
- In some embodiments, expression of the deltaEGFR gene or IL6 gene is suppressed by at least about 20%, 25%, 30%, 35%, 40%, 45%, or 50% by administration of a double-stranded oligonucleotide featured in the invention. In some embodiments, the deltaEGFR or IL6 gene is suppressed by at least about 60%, 70%, or 80% by administration of the double-stranded oligonucleotide featured in the invention. In some embodiments, the deltaEGFR gene is suppressed by at least about 85%, 90%, or 95% by administration of the double-stranded oligonucleotide featured in the invention. Table 4, for example, and
FIGS. 7-9 indicate a range of inhibition of expression obtained in in vitro and ex vivo assays using various deltaEGFR dsRNA molecules at various concentrations. - As used herein in the context of deltaEGFR or IL6 expression, the terms “treat,” “treatment,” and the like, refer to relief from or alleviation of pathological processes mediated by deltaEGFR or IL6 gene expression. Insofar as they relate to any of the other conditions recited herein below (other than pathological processes mediated by deltaEGFR expression), the terms “treat,” “treatment,” and the like mean to relieve or alleviate at least one symptom associated with such condition, or to slow or reverse the progression of such condition, such as the slowing and progression of glioma.
- As used herein, the phrases “therapeutically effective amount” and “prophylactically effective amount” refer to an amount that provides a therapeutic benefit in the treatment, prevention, or management of pathological processes mediated by deltaEGFR or IL6 expression or an overt symptom of pathological processes mediated by deltaEGFR or IL6 expression. The specific amount that is therapeutically effective can be readily determined by an ordinary medical practitioner, and may vary depending on factors known in the art, such as, for example, the type of pathological processes mediated by deltaEGFR or IL6 expression, the patient's history and age, the stage of pathological processes mediated by deltaEGFR or IL6 expression, and the administration of other anti-pathological processes mediated by deltaEGFR or IL6 expression agents.
- As used herein, a “pharmaceutical composition” comprises a pharmacologically effective amount of a dsRNA and a pharmaceutically acceptable carrier. As used herein, “pharmacologically effective amount,” “therapeutically effective amount” or simply “effective amount” refers to that amount of an RNA effective to produce the intended pharmacological, therapeutic or preventive result. For example, if a given clinical treatment is considered effective when there is at least a 25% reduction in a measurable parameter associated with a disease or disorder, a therapeutically effective amount of a drug for the treatment of that disease or disorder is the amount necessary to effect at least a 25% reduction in that parameter.
- The term “pharmaceutically acceptable carrier” refers to a carrier for administration of a therapeutic agent. Such carriers include, but are not limited to, saline, buffered saline, dextrose, water, glycerol, ethanol, and combinations thereof. The term specifically excludes cell culture medium. For drugs administered orally, pharmaceutically acceptable carriers include, but are not limited to pharmaceutically acceptable excipients such as inert diluents, disintegrating agents, binding agents, lubricating agents, sweetening agents, flavoring agents, coloring agents and preservatives. Suitable inert diluents include sodium and calcium carbonate, sodium and calcium phosphate, and lactose, while corn starch and alginic acid are suitable disintegrating agents. Binding agents may include starch and gelatin, while the lubricating agent, if present, will generally be magnesium stearate, stearic acid or talc. If desired, the tablets may be coated with a material such as glyceryl monostearate or glyceryl distearate, to delay absorption in the gastrointestinal tract.
- As used herein, a “transformed cell” is a cell into which a vector has been introduced from which a dsRNA molecule may be expressed.
- II. Double-Stranded Ribonucleic Acid (dsRNA)
- In one embodiment, the invention provides double-stranded ribonucleic acid (dsRNA) molecule for inhibiting expression of a deltaEGFR gene in a cell or mammal, e.g., in a human having a cancer, such as a glioma, where the dsRNA includes an antisense strand having a region of complementarity which is complementary to at least a part of an mRNA formed in the expression of the deltaEGFR gene, and where the region of complementarity is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and where said dsRNA, upon contact with a cell expressing said deltaEGFR gene, inhibits the expression of said deltaEGFR gene by at least 30% as assayed by, for example, a PCR or branched DNA (bDNA)-based method, or by a protein-based method, such as by Western blot. Expression of the deltaEGFR gene can be reduced by at least 30% when measured by an assay as described in the Examples below. The expression of wtEGFR may also be reduced by at least 30%, e.g., as assayed by a method described herein, and the level of reduced expression of deltaEGFR and wtEGFR may be different. The reduction in deltaEGFR or wtEGFR expression can also be assayed by measuring protein levels, such as by Western blot analysis.
- In one embodiment, the invention provides a double-stranded ribonucleic acid (dsRNA) molecule for inhibiting expression of an IL6 gene in a cell or mammal, e.g., in a human having a cancer, such as a glioma, where the dsRNA includes an antisense strand having a region of complementarity which is complementary to at least a part of an mRNA formed in the expression of the IL6 gene, and where the region of complementarity is less than 30 nucleotides in length, generally 19-24 nucleotides in length, and where the dsRNA, upon contact with a cell expressing the IL6 gene, inhibits the expression of the gene by at least 30% as assayed by, for example, a PCR or branched DNA (bDNA)-based method, or by a protein-based method, such as by Western blot.
- A dsRNA featured in the invention, e.g., a dsRNA targeting deltaEGFR or IL6 mRNA, includes two RNA strands that are sufficiently complementary to hybridize to form a duplex structure. One strand of the dsRNA (the antisense strand) includes a region of complementarity that is substantially complementary, and generally fully complementary, to a target sequence, derived from the sequence of an mRNA formed during the expression of the target gene, the other strand (the sense strand) includes a region that is complementary to the antisense strand, such that the two strands hybridize and form a duplex structure when combined under suitable conditions. Optionally, the region of the antisense strand that is substantially complementary to a sequence of a deltaEGFR mRNA is also substantially complementary to a wtEGFR mRNA.
- Generally, the duplex structure of a dsRNA featured herein is between 15 and 30, more generally between 18 and 25, yet more generally between 19 and 24, and most generally between 19 and 21 base pairs in length. Similarly, the region of complementarity to the target sequence is between 15 and 30, more generally between 18 and 25, yet more generally between 19 and 24, and most generally between 19 and 21 nucleotides in length. In some embodiments, the dsRNA is between 10 and 15 nucleotides in length, and in other embodiments, the dsRNA is between 25 and 30 nucleotides in length. The dsRNA featured in the invention may further include one or more single-stranded nucleotide overhangs. The dsRNA can be synthesized by standard methods known in the art as further discussed below, e.g., by use of an automated DNA synthesizer, such as are commercially available from, for example, Biosearch, Applied Biosystems, Inc. In one embodiment, the deltaEGFR gene is a human deltaEGFR gene, and the wtEGFR gene is a human wtEGFR gene. In specific embodiments, the first sequence is a sense strand of the dsRNA that includes a sense sequence from Tables 2 or 3, and the second sequence is an antisense strand that includes an antisense sequence from Tables 2 or 3. Alternative antisense agents that target elsewhere in the target sequence provided in Tables 2 or 3 can readily be determined using the target sequence and the flanking deltaEGFR sequence.
- The dsRNA targeting deltaEGFR will include at least two nucleotide sequences selected from the groups of sequences provided in Tables 2 or 3. One of the two sequences is complementary to the other of the two sequences, with one of the sequences being substantially complementary to a sequence of an mRNA generated in the expression of the deltaEGFR gene. As such, the dsRNA will include two oligonucleotides, where one oligonucleotide is described as the sense strand in Tables 2 or 3 and the second oligonucleotide is described as the antisense strand in Tables 2 or 3.
- In some embodiments, the dsRNA will target an IL-6 gene, e.g., a human IL-6 gene. In certain embodiments, the first sequence of the dsRNA is a sense strand that includes a sense sequence from Tables 5-8, and the second sequence is an antisense strand that includes an antisense sequence from Tables 5-8. Alternative antisense agents that target elsewhere in the target sequence provided in Tables 5-8 can readily be determined using the target sequence and the flanking IL-6 sequence.
- A dsRNA targeting IL-6 will include at least two nucleotide sequences selected from the groups of sequences provided in Tables 5-8. One of the two sequences is complementary to the other of the two sequences, with one of the sequences being substantially complementary to a sequence of an mRNA generated in the expression of the IL-6 gene. As such, the dsRNA will include two oligonucleotides, where one oligonucleotide is described as the sense strand in Tables 6-10 and the second oligonucleotide is described as the antisense strand in Tables 6-10.
- In certain embodiments, the IL-6 dsRNA does not have a sense or antisense strand consisting of the sequences shown in Tables 6A or 6B of WO 2007/064846. In other embodiments, the dsRNA does not consist of the sequence of SEQ ID NO:1 of US2008/0234218, and its complementary sequence of SEQ NO:2; the sequence of SEQ ID NO:3 of US2008/0234218, and its complementary sequence of SEQ NO:4; or the sequence of SEQ ID NO:5 of US2008/0234218, and its complementary sequence of SEQ NO:6.
- The skilled person is well aware that dsRNAs having a duplex structure of between 20 and 23, but specifically 21, base pairs have been hailed as particularly effective in inducing RNA interference (Elbashir et al., EMBO 2001, 20:6877-6888). However, others have found that shorter or longer dsRNAs can be effective as well. In the embodiments described above, by virtue of the nature of the oligonucleotide sequences provided in Tables 2 and 3, and 5-8, the dsRNAs featured in the invention can include at least one strand of a length of minimally 21 nt. It can be reasonably expected that shorter dsRNAs having one of the sequences of Tables 2 or 3, or 5-8 minus only a few nucleotides on one or both ends may be similarly effective as compared to the dsRNAs described above. Hence, dsRNAs having a partial sequence of at least 15, 16, 17, 18, 19, 20, or more contiguous nucleotides from one of the sequences of Tables 2, 3 and 5-8, and differing in their ability to inhibit the expression of the respective target genes, e.g., as measured by a FACS assay as described herein below by not more than 5, 10, 15, 20, 25, or 30% inhibition from a dsRNA comprising the full sequence, are contemplated by the invention. Further, dsRNAs that cleave within the desired target sequence can readily be made using the corresponding deltaEGFR or IL6 antisense sequence and a complementary sense sequence.
- In addition, the dsRNAs provided in Tables 2 and 3 identify a site in a deltaEGFR mRNA and the wtEGFR sequence that is susceptible to RNAi based cleavage, and the dsRNAs provided in Tables 5-8 identify a site in an IL6 mRNA susceptible to RNAi based cleavage As such, the present invention further provides dsRNAs that target within the sequence targeted by one of the other agents featured in the invention. As used herein, a second dsRNA is said to target within the sequence of a first dsRNA if the second dsRNA cleaves the message anywhere within the mRNA that is complementary to the antisense strand of the first dsRNA. Such a second dsRNA will generally consist of at least 15 contiguous nucleotides from one of the sequences provided in Tables 2, 3 or 5-8, coupled to an additional nucleotide sequence taken from the region contiguous to the selected sequence in the target gene, e.g., the deltaEGFR gene, the wtEGFR gene, or the IL6 gene. For example, the last 15 nucleotides of SEQ ID NO:2 combined with the next six nucleotides from the target deltaEGFR gene produces a single strand agent of 21 nucleotides that is based on one of the sequences provided in Tables 2 and 3.
- The dsRNA featured in the invention can contain one or more mismatches to the target sequence. In one embodiment, the dsRNA contains no more than 3 mismatches. If the antisense strand of the dsRNA contains mismatches to a target sequence, it is preferable that the area of mismatch not be located in the center of the region of complementarity. If the antisense strand of the dsRNA contains mismatches to the target sequence, it is preferable that the mismatch be restricted to 5 nucleotides from either end, for example 5, 4, 3, 2, or 1 nucleotide from either the 5′ or 3′ end of the region of complementarity. For example, for a 23 nucleotide dsRNA strand which is complementary to a region of the deltaEGFR gene, the dsRNA generally does not contain any mismatch within the central 13 nucleotides. The methods described within the invention can be used to determine whether a dsRNA containing a mismatch to a target sequence is effective in inhibiting the expression of the target gene, e.g., a deltaEGFR gene or an IL6 gene. Consideration of the efficacy of dsRNAs with mismatches in inhibiting expression of the target gene is important, especially if the particular region of complementarity in the target gene is known to have polymorphic sequence variation within the population.
- In one embodiment, at least one end of the dsRNA has a single-stranded nucleotide overhang of 1 to 4, generally 1 or 2 nucleotides. dsRNAs having at least one nucleotide overhang have unexpectedly superior inhibitory properties than their blunt-ended counterparts. Moreover, the present inventors have discovered that the presence of only one nucleotide overhang strengthens the interference activity of the dsRNA, without affecting its overall stability. dsRNA having only one overhang has proven particularly stable and effective in vivo, as well as in a variety of cells, cell culture mediums, blood, and serum. Generally, the single-stranded overhang is located at the 3′-terminal end of the antisense strand or, alternatively, at the 3′-terminal end of the sense strand. The dsRNA may also have a blunt end, generally located at the 5′-end of the antisense strand. Such dsRNAs have improved stability and inhibitory activity, thus allowing administration at low dosages, i.e., less than 5 mg/kg body weight of the recipient per day. In one embodiment, the antisense strand of the dsRNA has a 1-10 nucleotide overhang at the 3′-end and/or the 5′ end. In another embodiment, the sense strand of the dsRNA has a 1-10 nucleotide overhang at the 3′ end and/or the 5′ end. In another embodiment, one or more of the nucleotides in the overhang is replaced with a nucleoside thiophosphate.
- In yet another embodiment, the dsRNA is chemically modified to enhance stability. The nucleic acids featured in the invention may be synthesized and/or modified by methods well established in the art, such as those described in “Current protocols in nucleic acid chemistry,” Beaucage, S.L. et al. (Edrs.), John Wiley & Sons, Inc., New York, N.Y., USA, which is hereby incorporated herein by reference. Specific examples of dsRNA compounds useful in this invention include dsRNAs containing modified backbones or no natural internucleoside linkages. As defined in this specification, dsRNAs having modified backbones include those that retain a phosphorus atom in the backbone and those that do not have a phosphorus atom in the backbone. For the purposes of this specification, and as sometimes referenced in the art, modified dsRNAs that do not have a phosphorus atom in their internucleoside backbone can also be considered to be oligonucleosides.
- Modified dsRNA backbones include, for example, phosphorothioates, chiral phosphorothioates, phosphorodithioates, phosphotriesters, aminoalkylphosphotriesters, methyl and other alkyl phosphonates including 3′-alkylene phosphonates and chiral phosphonates, phosphinates, phosphoramidates including 3′-amino phosphoramidate and aminoalkylphosphoramidates, thionophosphoramidates, thionoalkylphosphonates, thionoalkylphosphotriesters, and boranophosphates having normal 3′-5′ linkages, 2′-5′ linked analogs of these, and those) having inverted polarity wherein the adjacent pairs of nucleoside units are linked 3′-5′ to 5′-3′ or 2′-5′ to 5′-2′. Various salts, mixed salts and free acid forms are also included.
- Representative U.S. patents that teach the preparation of the above phosphorus-containing linkages include, but are not limited to, U.S. Pat. Nos. 3,687,808; 4,469,863; 4,476,301; 5,023,243; 5,177,195; 5,188,897; 5,264,423; 5,276,019; 5,278,302; 5,286,717; 5,321,131; 5,399,676; 5,405,939; 5,453,496; 5,455,233; 5,466,677; 5,476,925; 5,519,126; 5,536,821; 5,541,316; 5,550,111; 5,563,253; 5,571,799; 5,587,361; and 5,625,050, each of which is incorporated herein by reference
- Modified dsRNA backbones that do not include a phosphorus atom have backbones that are formed by short chain alkyl or cycloalkyl internucleoside linkages, mixed heteroatoms and alkyl or cycloalkyl internucleoside linkages, or ore or more short chain heteroatomic or heterocyclic internucleoside linkages. These include those having morpholino linkages (formed in part from the sugar portion of a nucleoside); siloxane backbones; sulfide, sulfoxide and sulfone backbones; formacetyl and thioformacetyl backbones; methylene formacetyl and thioformacetyl backbones; alkene containing backbones; sulfamate backbones; methyleneimino and methylenehydrazino backbones; sulfonate and sulfonamide backbones; amide backbones; and others having mixed N, O, S and CH2 component parts.
- Representative U.S. patents that teach the preparation of the above oligonucleosides include, but are not limited to, U.S. Pat. Nos. 5,034,506; 5,166,315; 5,185,444; 5,214,134; 5,216,141; 5,235,033; 5,64,562; 5,264,564; 5,405,938; 5,434,257; 5,466,677; 5,470,967; 5,489,677; 5,541,307; 5,561,225; 5,596,086; 5,602,240; 5,608,046; 5,610,289; 5,618,704; 5,623,070; 5,663,312; 5,633,360; 5,677,437; and, 5,677,439, each of which is incorporated herein by reference.
- In other suitable dsRNA mimetics, both the sugar and the internucleoside linkage, i.e., the backbone, of the nucleotide units are replaced with novel groups. The base units are maintained for hybridization with an appropriate nucleic acid target compound. One such oligomeric compound, a dsRNA mimetic that has been shown to have excellent hybridization properties, is referred to as a peptide nucleic acid (PNA). In PNA compounds, the sugar backbone of a dsRNA is replaced with an amide containing backbone, in particular an aminoethylglycine backbone. The nucleobases are retained and are bound directly or indirectly to aza nitrogen atoms of the amide portion of the backbone. Representative U.S. patents that teach the preparation of PNA compounds include, but are not limited to, U.S. Pat. Nos. 5,539,082; 5,714,331; and 5,719,262, each of which is incorporated herein by reference. Further teaching of PNA compounds can be found in Nielsen et al., Science, 1991, 254, 1497-1500.
- Most embodiments featured in the invention include dsRNAs with phosphorothioate backbones and oligonucleosides with heteroatom backbones, and in particular —CH2—NH—CH2—, —CH2—N(CH3)—O—CH2—[known as a methylene (methylimino) or MMI backbone], —CH2—O—N(CH3)—CH2—, —CH2—N(CH3)—N(CH3)—CH2—and —N(CH3)—CH2—CH2—[wherein the native phosphodiester backbone is represented as —O—P—O—CH2—] of the above-referenced U.S. Pat. No. 5,489,677, and the amide backbones of the above-referenced U.S. Pat. No. 5,602,240. In some embodiments, the dsRNAs featured herein have morpholino backbone structures of the above-referenced U.S. Pat. No. 5,034,506.
- Modified dsRNAs may also contain one or more substituted sugar moieties. The dsRNAs featured herein can have one of the following at the 2′ position: OH; F; O—, S—, or N—alkyl; O—, S—, or N-alkenyl; O—, S— or N-alkynyl; or O-alkyl-O-alkyl, wherein the alkyl, alkenyl and alkynyl may be substituted or unsubstituted C1 to C10 alkyl or C2 to C10 alkenyl and alkynyl. Exemplary suitable modifications include O[(CH2)nO]mCH3, O(CH2)nOCH3, O(CH2)nNH2, O(CH2)nCH3, O(CH2)nONH2, and O(CH2)nON[(CH2)nCH3)]2, where n and m are from 1 to about 10. In other embodiments, dsRNAs include one of the following at the 2′ position: C1 to C10 lower alkyl, substituted lower alkyl, alkaryl, aralkyl, O-alkaryl or O-aralkyl, SH, SCH3, OCN, Cl, Br, CN, CF3, OCF3, SOCH3, SO2CH3, ONO2, NO2, N3, NH2, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalkylamino, substituted silyl, an RNA cleaving group, a reporter group, an intercalator, a group for improving the pharmacokinetic properties of a dsRNA, or a group for improving the pharmacodynamic properties of a dsRNA, and other substituents having similar properties. In some embodiments, the modification includes a 2′-methoxyethoxy (2′-O—CH2CH2OCH3, also known as 2′-O-(2-methoxyethyl) or 2′-MOE) (Martin et al., Helv. Chim. Acta, 1995, 78:486-504) i.e., an alkoxy-alkoxy group. Another exemplary modification is 2′-dimethylaminooxyethoxy, i.e., a O(CH2)2ON(CH3)2 group, also known as 2′-DMAOE, as described in examples hereinbelow, and 2′-dimethylaminoethoxyethoxy (also known in the art as 2′-O-dimethylaminoethoxyethyl or 2′-DMAEOE), i.e., 2′-O—CH2—O—CH2—N(CH2)2, also described in examples hereinbelow.
- Other modifications include 2′-methoxy (2′-OCH3), 2′-aminopropoxy (2′-OCH2CH2CH2NH2) and 2′-fluoro (2′-F). Similar modifications may also be made at other positions on the dsRNA, particularly the 3′ position of the sugar on the 3′ terminal nucleotide or in 2′-5′ linked dsRNAs and the 5′ position of 5′ terminal nucleotide. DsRNAs may also have sugar mimetics such as cyclobutyl moieties in place of the pentofuranosyl sugar. Representative U.S. patents that teach the preparation of such modified sugar structures include, but are not limited to, U.S. Pat. Nos. 4,981,957; 5,118,800; 5,319,080; 5,359,044; 5,393,878; 5,446,137; 5,466,786; 5,514,785; 5,519,134; 5,567,811; 5,576,427; 5,591,722; 5,597,909; 5,610,300; 5,627,053; 5,639,873; 5,646,265; 5,658,873; 5,670,633; and 5,700,920, certain of which are commonly owned with the instant application, and each of which is incorporated herein by reference in its entirety.
- DsRNAs may also include nucleobase (often referred to in the art simply as “base”) modifications or substitutions. As used herein, “unmodified” or “natural” nucleobases include the purine bases adenine (A) and guanine (G), and the pyrimidine bases thymine (T), cytosine (C) and uracil (U). Modified nucleobases include other synthetic and natural nucleobases such as 5-methylcytosine (5-me-C), 5-hydroxymethyl cytosine, xanthine, hypoxanthine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine and guanine, 2-propyl and other alkyl derivatives of adenine and guanine, 2-thiouracil, 2-thiothymine and 2-thiocytosine, 5-halouracil and cytosine, 5-propynyl uracil and cytosine, 6-azo uracil, cytosine and thymine, 5-uracil (pseudouracil), 4-thiouracil, 8-halo, 8-amino, 8-thiol, 8-thioalkyl, 8-hydroxyl anal other 8-substituted adenines and guanines, 5-halo, particularly 5-bromo, 5-trifluoromethyl and other 5-substituted uracils and cytosines, 7-methylguanine and 7-methyladenine, 8-azaguanine and 8-azaadenine, 7-deazaguanine and 7-daazaadenine and 3-deazaguanine and 3-deazaadenine. Further nucleobases include those disclosed in U.S. Pat. No. 3,687,808, those disclosed in The Concise Encyclopedia Of Polymer Science And Engineering, pages 858-859, Kroschwitz, J. L, ed. John Wiley & Sons, 1990, these disclosed by Englisch et al., Angewandte Chemie, International Edition, 1991, 30, 613, and those disclosed by Sanghvi, Y S.,
Chapter 15, DsRNA Research and Applications, pages 289-302, Crooke, S. T. and Lebleu, B., Ed., CRC Press, 1993. Certain of these nucleobases are particularly useful for increasing the binding affinity of the oligomeric compounds featured in the invention. These include 5-substituted pyrimidines, 6-azapyrimidines and N-2, N-6 and 0-6 substituted purines, including 2-aminopropyladenine, 5-propynyluracil and 5-propynylcytosine. 5-methylcytosine substitutions have been shown to increase nucleic acid duplex stability by 0.6-1.2° C. (Sanghvi, Y. S., Crooke, S. T. and Lebleu, B., Eds., DsRNA Research and Applications, CRC Press, Boca Raton, 1993, pp. 276-278) and are exemplary base substitutions, even more particularly when combined with 2′-O-methoxyethyl sugar modifications. - Representative U.S. patents that teach the preparation of certain of the above noted modified nucleobases as well as other modified nucleobases include, but are not limited to, the above noted U.S. Pat. No. 3,687,808, as well as U.S. Pat. Nos. 4,845,205; 5,130,30; 5,134,066; 5,175,273; 5,367,066; 5,432,272; 5,457,187; 5,459,255; 5,484,908; 5,502,177; 5,525,711; 5,552,540; 5,587,469; 5,594,121, 5,596,091; 5,614,617; and 5,681,941, each of which is herein incorporated by reference, and U.S. Pat. No. 5,750,692, also incorporated herein by reference.
- Another modification of the dsRNAs featured in the invention involves chemically linking to the dsRNA one or more moieties or conjugates which enhance the activity, cellular distribution or cellular uptake of the dsRNA. Such moieties include but are not limited to lipid moieties such as a cholesterol moiety (Letsinger et al., Proc. Natl. Acid. Sci. USA, 1989, 86: 6553-6556), cholic acid (Manoharan et al., Biorg. Med. Chem. Let., 1994, 4:1053-1060), a thioether, e.g., beryl-S-tritylthiol (Manoharan et al., Ann. N.Y. Acad. Sci., 1992, 660:306-309; Manoharan et al., Biorg. Med. Chem. Let., 1993, 3:2765-2770), a thiocholesterol (Oberhauser et al., Nucl. Acids Res., 1992, 20:533-538), an aliphatic chain, e.g., dodecandiol or undecyl residues (Saison-Behmoaras et al., EMBO J, 1991, 10:1111-1118; Kabanov et al., FEBS Lett., 1990, 259:327-330; Svinarchuk et al., Biochimie, 1993, 75:49-54), a phospholipid, e.g., di-hexadecyl-rac-glycerol or triethyl-
ammonium 1,2-di-O-hexadecyl-rac-glycero-3-Hphosphonate (Manoharan et al., Tetrahedron Lett., 1995, 36:3651-3654; Shea et al., Nucl. Acids Res., 1990, 18:3777-3783), a polyamine or a polyethylene glycol chain (Manoharan et al., Nucleosides & Nucleotides, 1995, 14:969-973), or adamantane acetic acid (Manoharan et al., Tetrahedron Lett., 1995, 36:3651-3654), a palmityl moiety (Mishra et al., Biochim. Biophys. Acta, 1995, 1264:229-237), or an octadecylamine or hexylamino-carbonyloxycholesterol moiety (Crooke et al., J. Pharmacol. Exp. Ther., 1996, 277:923-937). - Representative U.S. patents that teach the preparation of such dsRNA conjugates include, but are not limited to, U.S. Pat. Nos. 4,828,979; 4,948,882; 5,218,105; 5,525,465; 5,541,313; 5,545,730; 5,552,538; 5,578,717, 5,580,731; 5,591,584; 5,109,124; 5,118,802; 5,138,045; 5,414,077; 5,486,603; 5,512,439; 5,578,718; 5,608,046; 4,587,044; 4,605,735; 4,667,025; 4,762,779; 4,789,737; 4,824,941; 4,835,263; 4,876,335; 4,904,582; 4,958,013; 5,082,830; 5,112,963; 5,214,136; 5,082,830; 5,112,963; 5,214,136; 5,245,022; 5,254,469; 5,258,506; 5,262,536; 5,272,250; 5,292,873; 5,317,098; 5,371,241, 5,391,723; 5,416,203, 5,451,463; 5,510,475; 5,512,667; 5,514,785; 5,565,552; 5,567,810; 5,574,142; 5,585,481; 5,587,371; 5,595,726; 5,597,696; 5,599,923; 5,599,928 and 5,688,941, each of which is herein incorporated by reference.
- It is not necessary for all positions in a given compound to be uniformly modified, and in fact more than one of the aforementioned modifications may be incorporated in a single compound or even at a single nucleoside within a dsRNA. The present invention also includes dsRNA compounds which are chimeric compounds. “Chimeric” dsRNA compounds or “chimeras,” in the context of this invention, are dsRNA compounds, particularly dsRNAs, which contain two or more chemically distinct regions, each made up of at least one monomer unit, i.e., a nucleotide in the case of a dsRNA compound. These dsRNAs typically contain at least one region wherein the dsRNA is modified so as to confer upon the dsRNA increased resistance to nuclease degradation, increased cellular uptake, and/or increased binding affinity for the target nucleic acid. An additional region of the dsRNA may serve as a substrate for enzymes capable of cleaving RNA:DNA or RNA:RNA hybrids. By way of example, RNase H is a cellular endonuclease which cleaves the RNA strand of an RNA:DNA duplex. Activation of RNase H, therefore, results in cleavage of the RNA target, thereby greatly enhancing the efficiency of dsRNA inhibition of gene expression. Consequently, comparable results can often be obtained with shorter dsRNAs when chimeric dsRNAs are used, compared to phosphorothioate deoxydsRNAs hybridizing to the same target region. Cleavage of the RNA target can be routinely detected by gel electrophoresis and, if necessary, associated nucleic acid hybridization techniques known in the art.
- In certain instances, the dsRNA may be modified by a non-ligand group. A number of non-ligand molecules have been conjugated to dsRNAs in order to enhance the activity, cellular distribution or cellular uptake of the dsRNA, and procedures for performing such conjugations are available in the scientific literature. Such non-ligand moieties have included lipid moieties, such as cholesterol (Letsinger et al., Proc. Natl. Acad. Sci. USA, 1989, 86:6553), cholic acid (Manoharan et al., Bioorg. Med. Chem. Lett., 1994, 4:1053), a thioether, e.g., hexyl-S-tritylthiol (Manoharan et al., Ann. N.Y. Acad. Sci., 1992, 660:306; Manoharan et al., Bioorg. Med. Chem. Let., 1993, 3:2765), a thiocholesterol (Oberhauser et al., Nucl. Acids Res., 1992, 20:533), an aliphatic chain, e.g., dodecandiol or undecyl residues (Saison-Behmoaras et al., EMBO J., 1991, 10:111; Kabanov et al., FEBS Lett., 1990, 259:327; Svinarchuk et al., Biochimie, 1993, 75:49), a phospholipid, e.g., di-hexadecyl-rac-glycerol or
triethylammonium 1,2-di-O-hexadecyl-rac-glycero-3-H-phosphonate (Manoharan et al., Tetrahedron Lett., 1995, 36:3651; Shea et al., Nucl. Acids Res., 1990, 18:3777), a polyamine or a polyethylene glycol chain (Manoharan et al., Nucleosides & Nucleotides, 1995, 14:969), or adamantane acetic acid (Manoharan et al., Tetrahedron Lett., 1995, 36:3651), a palmityl moiety (Mishra et al., Biochim. Biophys. Acta, 1995, 1264:229), or an octadecylamine or hexylamino-carbonyl-oxycholesterol moiety (Crooke et al., J. Pharmacol. Exp. Ther., 1996, 277:923). Representative United States patents that teach the preparation of such dsRNA conjugates have been listed above. Typical conjugation protocols involve the synthesis of dsRNAs bearing an aminolinker at one or more positions of the sequence. The amino group is then reacted with the molecule being conjugated using appropriate coupling or activating reagents. The conjugation reaction may be performed either with the dsRNA still bound to the solid support or following cleavage of the dsRNA in solution phase. Purification of the dsRNA conjugate by HPLC typically affords the pure conjugate. - Vector Encoded dsRNAs
- In another aspect, dsRNA molecules featured in the invention, e.g., deltaEGFR and IL6 dsRNAs, are expressed from transcription units inserted into DNA or RNA vectors (see, e.g., Couture, A, et al., TIG. (1996), 12:5-10; Skillern, A., et al., International PCT Publication No. WO 00/22113, Conrad, International PCT Publication No. WO 00/22114, and Conrad, U.S. Pat. No. 6,054,299). These transgenes can be introduced as a linear construct, a circular plasmid, or a viral vector, which can be incorporated and inherited as a transgene integrated into the host genome. The transgene can also be constructed to permit it to be inherited as an extrachromosomal plasmid (Gassmann, et al., Proc. Natl. Acad. Sci. USA (1995) 92:1292).
- The individual strands of a dsRNA can be transcribed by promoters on two separate expression vectors and co-transfected into a target cell. Alternatively each individual strand of the dsRNA can be transcribed by promoters both of which are located on the same expression plasmid. In one embodiment, a dsRNA is expressed as an inverted repeat joined by a linker polynucleotide sequence such that the dsRNA has a stem and loop structure.
- The recombinant dsRNA expression vectors are generally DNA plasmids or viral vectors. dsRNA expressing viral vectors can be constructed based on, but not limited to, adeno-associated virus (for a review, see Muzyczka, et al., Curr. Topics Micro. Immunol. (1992) 158:97-129)); adenovirus (see, for example, Berkner, et al., BioTechniques (1998) 6:616), Rosenfeld et al. (1991, Science 252:431-434), and Rosenfeld et al. (1992), Cell 68:143-155)); or alphavirus as well as others known in the art. Retroviruses have been used to introduce a variety of genes into many different cell types, including epithelial cells, in vitro and/or in vivo (see, e.g., Eglitis, et al., Science (1985) 230:1395-1398; Danos and Mulligan, Proc. Natl. Acad. Sci. USA (1998) 85:6460-6464; Wilson et al., 1988, Proc. Natl. Acad. Sci. USA 85:3014-3018; Armentano et al., 1990, Proc. Natl. Acad. Sci. USA 87:61416145; Huber et al., 1991, Proc. Natl. Acad. Sci. USA 88:8039-8043; Ferry et al., 1991, Proc. NatI. Acad. Sci. USA 88:8377-8381; Chowdhury et al., 1991, Science 254:1802-1805; van Beusechem. et al., 1992, Proc. Nad. Acad. Sci. USA 89:7640-19 ; Kay et al., 1992, Human Gene Therapy 3:641-647; Dai et al., 1992, Proc. Natl.Acad. Sci. USA 89:10892-10895; Hwu et al., 1993, J. Immunol. 150:4104-4115; U.S. Patent No. 4,868,116; U.S. Patent No. 4,980,286; PCT Application WO 89/07136; PCT Application WO 89/02468; PCT Application WO 89/05345; and PCT Application WO 92/07573). Recombinant retroviral vectors capable of transducing and expressing genes inserted into the genome of a cell can be produced by transfecting the recombinant retroviral genome into suitable packaging cell lines such as PA317 and Psi-CRIP (Comette et al., 1991, Human Gene Therapy 2:5-10; Cone et al., 1984, Proc. Natl. Acad. Sci. USA 81:6349). Recombinant adenoviral vectors can be used to infect a wide variety of cells and tissues in susceptible hosts (e.g., rat, hamster, dog, and chimpanzee) (Hsu et al., 1992, J. Infectious Disease, 166:769), and also have the advantage of not requiring mitotically active cells for infection.
- Any viral vector capable of accepting the coding sequences for the dsRNA molecule(s) to be expressed can be used, for example vectors derived from adenovirus (AV); adeno-associated virus (AAV); retroviruses (e.g, lentiviruses (LV), Rhabdoviruses, murine leukemia virus); herpes virus, and the like. The tropism of viral vectors can be modified by pseudotyping the vectors with envelope proteins or other surface antigens from other viruses, or by substituting different viral capsid proteins, as appropriate.
- For example, lentiviral vectors featured in the invention can be pseudotyped with surface proteins from vesicular stomatitis virus (VSV), rabies, Ebola, Mokola, and the like. AAV vectors featured in the invention can be made to target different cells by engineering the vectors to express different capsid protein serotypes. For example, an AAV vector expressing a
serotype 2 capsid on aserotype 2 genome is calledAAV 2/2. Thisserotype 2 capsid gene in theAAV 2/2 vector can be replaced by aserotype 5 capsid gene to produce anAAV 2/5 vector. Techniques for constructing AAV vectors which express different capsid protein serotypes are within the skill in the art; see, e.g., Rabinowitz J E et al. (2002), J Virol 76:791-801, the entire disclosure of which is herein incorporated by reference. - Selection of recombinant viral vectors suitable for use in the invention, methods for inserting nucleic acid sequences for expressing the dsRNA into the vector, and methods of delivering the viral vector to the cells of interest are within the skill in the art. See, for example, Domburg R (1995), Gene Therap. 2: 301-310; Eglitis M A (1988), Biotechniques 6: 608-614; Miller A D (1990), Hum Gene Therap. 1: 5-14; Anderson W F (1998), Nature 392: 25-30; and Rubinson D A et al., Nat. Genet. 33: 401-406, the entire disclosures of which are herein incorporated by reference.
- Viral vectors can be derived from AV and AAV. In one embodiment, the dsRNA featured in the invention is expressed as two separate, complementary single-stranded RNA molecules from a recombinant AAV vector having, for example, either the U6 or H1 RNA promoters, or the cytomegalovirus (CMV) promoter.
- A suitable AV vector for expressing the dsRNA featured in the invention, a method for constructing the recombinant AV vector, and a method for delivering the vector into target cells, are described in Xia H et al. (2002), Nat. Biotech. 20: 1006-1010.
- Suitable AAV vectors for expressing the dsRNA featured in the invention, methods for constructing the recombinant AV vector, and methods for delivering the vectors into target cells are described in Samulski R et al. (1987), J. Virol. 61: 3096-3101; Fisher K J et al. (1996), J. Virol, 70: 520-532; Samulski R et al. (1989), J. Virol. 63: 3822-3826; U.S. Pat. Nos. 5,252,479; 5,139,941; International Patent Application No. WO 94/13788; and International Patent Application No. WO 93/24641, the entire disclosures of which are herein incorporated by reference.
- The promoter driving dsRNA expression in either a DNA plasmid or viral vector featured in the invention may be a eukaryotic RNA polymerase I (e.g., ribosomal RNA promoter), RNA polymerase II (e.g., CMV early promoter or actin promoter or U1 snRNA promoter) or generally RNA polymerase HI promoter (e.g., U6 snRNA or 7SK RNA promoter) or a prokaryotic promoter, for example the T7 promoter, provided the expression plasmid also encodes T7 RNA polymerase required for transcription from a T7 promoter. The promoter can also direct transgene expression to the pancreas (see, e.g., the insulin regulatory sequence for pancreas (Bucchini et al., 1986, Proc. Natl. Acad. Sci. USA 83:2511-2515)).
- In addition, expression of the transgene can be precisely regulated, for example, by using an inducible regulatory sequence and expression systems such as a regulatory sequence that is sensitive to certain physiological regulators, e.g., circulating glucose levels, or hormones (Docherty et al., 1994, FASEB J. 8:20-24). Such inducible expression systems, suitable for the control of transgene expression in cells or in mammals include regulation by ecdysone, by estrogen, progesterone, tetracycline, chemical inducers of dimerization, and isopropyl-beta-D1-thiogalactopyranoside (EPTG). A person skilled in the art would be able to choose the appropriate regulatory/promoter sequence based on the intended use of the dsRNA transgene.
- Generally, recombinant vectors capable of expressing dsRNA molecules are delivered as described below, and persist in target cells. Alternatively, viral vectors can be used that provide for transient expression of dsRNA molecules. Such vectors can be repeatedly administered as necessary. Once expressed, the dsRNAs bind to target RNA and modulate its function or expression. Delivery of dsRNA expressing vectors can be systemic, such as by intravenous or intramuscular administration, by administration to target cells ex-planted from the patient followed by reintroduction into the patient, or by any other means that allows for introduction into a desired target cell.
- dsRNA expression DNA plasmids are typically transfected into target cells as a complex with cationic lipid carriers (e.g., Oligofectamine) or non-cationic lipid-based carriers (e.g., Transit-TKO™). Multiple lipid transfections for dsRNA-mediated knockdowns targeting different regions of a single target gene or multiple target genes over a period of a week or more are also contemplated by the invention. Successful introduction of vectors into host cells can be monitored using various known methods. For example, transient transfection can be signaled with a reporter, such as a fluorescent marker, such as Green Fluorescent Protein (GFP). Stable transfection of cells ex vivo can be ensured using markers that provide the transfected cell with resistance to specific environmental factors (e.g., antibiotics and drugs), such as hygromycin B resistance.
- The deltaEGFR- and IL6-specific dsRNA molecules can also be inserted into vectors and used as gene therapy vectors for human patients. Gene therapy vectors can be delivered to a subject by, for example, intravenous injection, local administration (see U.S. Pat. No. 5,328,470) or by stereotactic injection (see e.g., Chen et al. (1994) Proc. Natl. Acad. Sci. USA 91:3054-3057). The pharmaceutical preparation of the gene therapy vector can include the gene therapy vector in an acceptable diluent, or can include a slow release matrix in which the gene delivery vehicle is imbedded. Alternatively, where the complete gene delivery vector can be produced intact from recombinant cells, e.g., retroviral vectors, the pharmaceutical preparation can include one or more cells which produce the gene delivery system.
- III. Pharmaceutical Compositions Containing dsRNA
- In one embodiment, the invention provides pharmaceutical compositions containing a dsRNA, as described herein, and a pharmaceutically acceptable carrier. The pharmaceutical composition containing the dsRNA is useful for treating a disease or disorder associated with the expression or activity of the deltaEGFR gene and/or the IL6 gene, such as pathological processes mediated by deltaEGFR or IL6 expression. Such pharmaceutical compositions are formulated based on the mode of delivery. One example is a composition formulated for direct delivery into the brain parenchyma, e.g., by infusion into the brain, such as by continuous pump infusion. Another example is a composition formulated for intraventricular or intrathecal delivery into the cerebrospinal fluid, e.g., by bolus or continuous pump infusion. Another example is a compositions formulated for systemic administration via parenteral delivery, e.g., by intravenous (IV) delivery.
- The pharmaceutical compositions featured herein are administered in dosages sufficient to inhibit expression of the target gene, e.g, the deltaEGFR or IL6 gene. In general, a suitable dose of dsRNA will be in the range of 0.01 to 200.0 milligrams per kilogram body weight of the recipient per day, generally in the range of 0.02 to 50 mg per kilogram body weight per day. For example, the dsRNA can be administered at 0.01 mg/kg, 0.05 mg/kg, 0.1 mg/kg, 0.5 mg/kg, 1 mg/kg, 2 mg/kg, 3 mg/kg, 10 mg/kg, 20 mg/kg, 30 mg/kg, 40 mg/kg, or 50 mg/kg per single dose. The pharmaceutical composition may be administered once daily, or the dsRNA may be administered as two, three, or more sub-doses at appropriate intervals throughout the day or even using continuous infusion or delivery through a controlled release formulation. In that case, the dsRNA contained in each sub-dose must be correspondingly smaller in order to achieve the total daily dosage. The dosage unit can also be compounded for delivery over several days, e.g., using a conventional sustained release formulation which provides sustained release of the dsRNA over a several day period. Sustained release formulations are well known in the art and are particularly useful for delivery of agents at a particular site, such as could be used with the agents featured in the invention. In this embodiment, the dosage unit contains a corresponding multiple of the daily dose.
- The effect of a single dose of dsRNA on target RNA levels, e.g., deltaEGFR levels (or both deltaEGFR and wtEGFR levels) or IL6 levels, is long lasting, such that subsequent doses are administered at not more than 3, 4, or 5 day intervals, or at not more than 1, 2, 3, or 4 week intervals.
- The present invention includes pharmaceutical compositions that can be delivered by injection directly into the brain. The injection can be by stereotactic injection into the brain tumor directly, or into a particular region of the brain (e.g., into white matter, such as the corona radiata, or the substantia nigra, cortex, hippocampus, striatum, or globus pallidus), or the dsRNA can be delivered into multiple regions of the central nervous system (e.g., into multiple regions of the brain, and/or into the spinal cord). The dsRNA can also be delivered into diffuse regions of the brain (e.g., diffuse delivery to the cortex of the brain).
- In one embodiment, a dsRNA targeting deltaEGFR or IL-6 can be delivered by way of a cannula or other delivery device having one end implanted in a tissue, e.g., the brain, e.g., the white matter, such as the corona radiata, or the substantia nigra, cortex, hippocampus, striatum, corpus callosum or globus pallidus of the brain. In one embodiment, the cannula or other delivery device has one end implanted into a tumor in the brain. The cannula can be connected to a reservoir of the dsRNA composition. The flow or delivery can be mediated by a pump. In one embodiment, a pump and reservoir are implanted in an area distant from the tissue, e.g., in the abdomen, and delivery is effected by a conduit leading from the pump or reservoir to the site of release. Infusion of the dsRNA composition into the brain can be over several hours or for several days, e.g., for 1, 2, 3, 5, or 7 days or more. Devices for delivery to the brain are described, for example, in U.S. Pat. Nos. 6,093,180, and 5,814,014. In another embodiment, the pump is externalized (not implanted). Infusion of the dsRNA composition into the brain can be over several hours or for several days up to approximately 7 days, e.g., for 1, 2, 3, 5, or 7 days.
- The skilled artisan will appreciate that certain factors may influence the dosage and timing required to effectively treat a subject, including but not limited to the severity of the disease or disorder, previous treatments, the general health and/or age of the subject, and other diseases present. Moreover, treatment of a subject with a therapeutically effective amount of a composition can include a single treatment or a series of treatments. Estimates of effective dosages and in vivo half-lives for the individual dsRNAs encompassed by the invention can be made using conventional methodologies or on the basis of in vivo testing using an appropriate animal model, as described elsewhere herein.
- Advances in mouse genetics have generated a number of mouse models for the study of various human diseases, such as pathological processes mediated by deltaEGFR or IL6 expression. Such models are used for in vivo testing of dsRNA, as well as for determining a therapeutically effective dose.
- The present invention also includes pharmaceutical compositions and formulations which include the dsRNA compounds featured in the invention. The pharmaceutical compositions may be administered in a number of ways depending upon whether local or systemic treatment is desired and upon the area to be treated. Administration may be topical (e.g., by transdermal patch), pulmonary, e.g., by inhalation or insufflation of powders or aerosols, including by nebulizer; intratracheal, intranasal, epidermal and transdermal, oral or parenteral. Parenteral administration includes intravenous, intraarterial, subcutaneous, intraperitoneal or intramuscular injection or infusion; subdermal, e.g., via an implanted device; or intracranial, e.g., by intraparenchymal, intrathecal or intraventricular, administration.
- Pharmaceutical compositions and formulations for topical administration may include transdermal patches, ointments, lotions, creams, gels, drops, suppositories, sprays, liquids and powders. Conventional pharmaceutical carriers, aqueous, powder or oily bases, thickeners and the like may be necessary or desirable. Coated condoms, gloves and the like may also be useful. Suitable topical formulations include those in which the dsRNAs featured in the invention are in admixture with a topical delivery agent such as lipids, liposomes, fatty acids, fatty acid esters, steroids, chelating agents and surfactants. Suitable lipids and liposomes include neutral (e.g., dioleoylphosphatidyl DOPE ethanolamine, dimyristoylphosphatidyl choline DMPC, distearolyphosphatidyl choline) negative (e.g., dimyristoylphosphatidyl glycerol DMPG) and cationic (e.g., dioleoyltetramethylaminopropyl DOTAP and dioleoylphosphatidyl ethanolamine DOTMA). DsRNAs featured in the invention may be encapsulated within liposomes or may form complexes thereto, in particular to cationic liposomes. Alternatively, dsRNAs may be complexed to lipids, in particular to cationic lipids. Suitable fatty acids and esters include but are not limited to arachidonic acid, oleic acid, eicosanoic acid, lauric acid, caprylic acid, capric acid, myristic acid, palmitic acid, stearic acid, linoleic acid, linolenic acid, dicaprate, tricaprate, monoolein, dilaurin, glyceryl 1-monocaprate, 1-dodecylazacycloheptan-2-one, an acylcarnitine, an acylcholine, or a C1-10 alkyl ester (e.g., isopropylmyristate IPM), monoglyceride, diglyceride or pharmaceutically acceptable salt thereof. Topical formulations are described in detail in U.S. Pat. No. 6,747,014, which is incorporated herein by reference in its entirety.
- In one embodiment, a deltaEGFR or an IL-6 dsRNA featured in the invention is fully encapsulated in the lipid formulation (e.g., to form a SPLP, pSPLP, SNALP, or other nucleic acid-lipid particle). As used herein, the term “SNALP” refers to a stable nucleic acid-lipid particle, including SPLP. As used herein, the term “SPLP” refers to a nucleic acid-lipid particle comprising plasmid DNA encapsulated within a lipid vesicle. SNALPs and SPLPs typically contain a cationic lipid, a non-cationic lipid, and a lipid that prevents aggregation of the particle (e.g., a PEG-lipid conjugate). SNALPs and SPLPs are extremely useful for systemic applications, as they exhibit extended circulation lifetimes following intravenous (i.v.) injection and accumulate at distal sites (e.g., sites physically separated from the administration site). SPLPs include “pSPLP,” which include an encapsulated condensing agent-nucleic acid complex as set forth in PCT Publication No. WO 00/03683. The particles typically have a mean diameter of about 50 nm to about 150 nm, more typically about 60 nm to about 130 nm, more typically about 70 nm to about 110 nm, most typically about 70 to about 90 nm, and are substantially nontoxic. In addition, the nucleic acids when present in the nucleic acid-lipid particles are resistant in aqueous solution to degradation with a nuclease. Nucleic acid-lipid particles and their method of preparation are disclosed in, e.g., U.S. Pat. Nos. 5,976,567; 5,981,501; 6,534,484; 6,586,410; 6,815,432; and PCT Publication No. WO 96/40964.
- In one embodiment, the lipid to drug ratio (mass/mass ratio) (e.g., lipid to dsRNA ratio) will be in the range of from about 1:1 to about 50:1, from about 1:1 to about 25:1, from about 3:1 to about 15:1, from about 4:1 to about 10:1, from about 5:1 to about 9:1, or about 6:1 to about 9:1.
- The cationic lipid may be, for example, N,N-dioleyl-N,N-dimethylammonium chloride (DODAC), N,N-distearyl-N,N-dimethylammonium bromide (DDAB), N-(I-(2,3-dioleoyloxy)propyl)-N,N,N-trimethylammonium chloride (DOTAP), N-(I-(2,3-dioleyloxy)propyl)-N,N,N-trimethylammonium chloride (DOTMA), N,N-dimethyl-2,3-dioleyloxy)propylamine (DODMA), 1,2-DiLinoleyloxy-N,N-dimethylaminopropane (DLinDMA), 1,2-Dilinolenyloxy-N,N-dimethylaminopropane (DLenDMA), 1,2-Dilinoleylcarbamoyloxy-3-dimethylaminopropane (DLin-DAC), 1,2-Dilinoleyoxy-3-(dimethylamino)acetoxypropane (DLin-DAC), 1,2-Dilinoleyoxy-3-morpholinopropane (DLin-MA), 1,2-Dilinoleoyl-3-dimethylaminopropane (DLinDAP), 1,2-Dilinoleylthio-3-dimethylaminopropane (DLin-S-DMA), 1-Linoleoyl-2-linoleyloxy-3-dimethylaminopropane (DLin-2-DMAP), 1,2-Dilinoleyloxy-3-trimethylaminopropane chloride salt (DLin-TMA.C1), 1,2-Dilinoleoyl-3-trimethylaminopropane chloride salt (DLin-TAP.C1), 1,2-Dilinoleyloxy-3-(N-methylpiperazino)propane (DLin-MPZ), or 3-(N,N-Dilinoleylamino)-1,2-propanediol (DLinAP), 3-(N,N-Dioleylamino)-1,2-propanedio (DOAP), 1,2-Dilinoleyloxo-3-(2-N,N-dimethylamino)ethoxypropane (DLin-EG-DMA), 2,2-Dilinoleyl-4-dimethylaminomethyl-[1,3]-dioxolane (DLin-K-DMA), or a mixture thereof. The cationic lipid may comprise from about 20 mol % to about 50 mol % or about 40 mol % of the total lipid present in the particle.
- The non-cationic lipid may be an anionic lipid or a neutral lipid including, but not limited to, distearoylphosphatidylcholine (DSPC), dioleoylphosphatidylcholine (DOPC), dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylglycerol (DOPG), dipalmitoylphosphatidylglycerol (DPPG), dioleoyl-phosphatidylethanolamine (DOPE), palmitoyloleoylphosphatidylcholine (POPC), palmitoyloleoyl- phosphatidylethanolamine (POPE), dioleoyl-phosphatidylethanolamine 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (DOPE-mal), dipalmitoyl phosphatidyl ethanolamine (DPPE), dimyristoylphosphoethanolamine (DMPE), distearoyl-phosphatidyl-ethanolamine (DSPE), 16-O-monomethyl PE, 16-O-dimethyl PE, 18-1 -trans PE, 1-stearoyl-2-oleoyl-phosphatidyethanolamine (SOPE), cholesterol, or a mixture thereof. The non-cationic lipid may be from about 5 mol % to about 90 mol %, about 10 mol %, or about 58 mol % if cholesterol is included, of the total lipid present in the particle.
- The conjugated lipid that inhibits aggregation of particles may be, for example, a polyethyleneglycol (PEG)-lipid including, without limitation, a PEG-diacylglycerol (DAG), a PEG-dialkyloxypropyl (DAA), a PEG-phospholipid, a PEG-ceramide (Cer), or a mixture thereof. The PEG-DAA conjugate may be, for example, a PEG-dilauryloxypropyl (Ci2), a PEG-dimyristyloxypropyl (Ci4), a PEG-dipalmityloxypropyl (Ci6), or a PEG-distearyloxypropyl (Ci8). The conjugated lipid that prevents aggregation of particles may be from 0 mol % to about 20 mol % or about 2 mol % of the total lipid present in the particle.
- In some embodiments, the nucleic acid-lipid particle further includes cholesterol at, e.g., about 10 mol % to about 60 mol % or about 48 mol % of the total lipid present in the particle.
- In one embodiment, the lipidoid ND98·4HCl (MW 1487) (Formula 1), Cholesterol (Sigma-Aldrich), and PEG-Ceramide C16 (Avanti Polar Lipids) can be used to prepare lipid-siRNA nanoparticles (i.e., LNP01 particles). Stock solutions of each in ethanol can be prepared as follows: ND98, 133 mg/mL; Cholesterol, 25 mg/mL, PEG-Ceramide C16, 100 mg/mL. The ND98, Cholesterol, and PEG-Ceramide C16 stock solutions can then be combined in a, e.g., 42:48:10 molar ratio. The combined lipid solution can be mixed with aqueous siRNA (e.g., in sodium acetate pH 5) such that the final ethanol concentration is about 35-45% and the final sodium acetate concentration is about 100-300 mM. Lipid-siRNA nanoparticles typically form spontaneously upon mixing. Depending on the desired particle size distribution, the resultant nanoparticle mixture can be extruded through a polycarbonate membrane (e.g., 100 nm cut-off) using, for example, a thermobarrel extruder, such as Lipex Extruder (Northern Lipids, Inc). In some cases, the extrusion step can be omitted. Ethanol removal and simultaneous buffer exchange can be accomplished by, for example, dialysis or tangential flow filtration. Buffer can be exchanged with, for example, phosphate buffered saline (PBS) at about
pH 7, e.g., about pH 6.9, about pH 7.0, about pH 7.1, about pH 7.2, about pH 7.3, or about pH 7.4. - LNP01 formulations are described, e.g., in International Application Publication No. WO 2008/042973, which is hereby incorporated by reference.
- Formulations prepared by either the standard or extrusion-free method can be characterized in similar manners. For example, formulations are typically characterized by visual inspection. They should be whitish translucent solutions free from aggregates or sediment. Particle size and particle size distribution of lipid-nanoparticles can be measured by light scattering using, for example, a Malvern Zetasizer Nano ZS (Malvern, USA). Particles should be about 20-300 nm, such as 40-100 nm in size. The particle size distribution should be unimodal. The total siRNA concentration in the formulation, as well as the entrapped fraction, is estimated using a dye exclusion assay. A sample of the formulated siRNA can be incubated with an RNA-binding dye, such as Ribogreen (Molecular Probes) in the presence or absence of a formulation disrupting surfactant, e.g., 0.5% Triton-X100. The total siRNA in the formulation can be determined by the signal from the sample containing the surfactant, relative to a standard curve. The entrapped fraction is determined by subtracting the “free” siRNA content (as measured by the signal in the absence of surfactant) from the total siRNA content. Percent entrapped siRNA is typically >85%. For SNALP formulation, the particle size is at least 30 nm, at least 40 nm, at least 50 nm, at least 60 nm, at least 70 nm, at least 80 nm, at least 90 nm, at least 100 nm, at least 110 nm, and at least 120 nm. The suitable range is typically about at least 50 nm to about at least 110 nm, about at least 60 nm to about at least 100 nm, or about at least 80 nm to about at least 90 nm.
- Compositions and formulations for oral administration include powders or granules, microparticulates, nanoparticulates, suspensions or solutions in water or non-aqueous media, capsules, gel capsules, sachets, tablets or minitablets. Thickeners, flavoring agents, diluents, emulsifiers, dispersing aids or binders may be desirable. In some embodiments, oral formulations are those in which dsRNAs featured in the invention are administered in conjunction with one or more penetration enhancers surfactants and chelators. Suitable surfactants include fatty acids and/or esters or salts thereof, bile acids and/or salts thereof.
- Suitable bile acids/salts include chenodeoxycholic acid (CDCA) and ursodeoxychenodeoxycholic acid (UDCA), cholic acid, dehydrocholic acid, deoxycholic acid, glucholic acid, glycholic acid, glycodeoxycholic acid, taurocholic acid, taurodeoxycholic acid, sodium tauro-24,25-dihydro-fusidate and sodium glycodihydrofusidate. Suitable fatty acids include arachidonic acid, undecanoic acid, oleic acid, lauric acid, caprylic acid, capric acid, myristic acid, palmitic acid, stearic acid, linoleic acid, linolenic acid, dicaprate, tricaprate, monoolein, dilaurin, glyceryl 1-monocaprate, 1-dodecylazacycloheptan-2-one, an acylcarnitine, an acylcholine, or a monoglyceride, a diglyceride or a pharmaceutically acceptable salt thereof (e.g., sodium). In some embodiments, combinations of penetration enhancers are used, for example, fatty acids/salts in combination with bile acids/salts. One exemplary combination is the sodium salt of lauric acid, capric acid and UDCA. Further penetration enhancers include polyoxyethylene-9-lauryl ether, polyoxyethylene-20-cetyl ether. DsRNAs featured in the invention may be delivered orally, in granular form including sprayed dried particles, or complexed to form micro or nanoparticles. DsRNA complexing agents include poly-amino acids; polyimines; polyacrylates; polyalkylacrylates, polyoxethanes, polyalkylcyanoacrylates; cationized gelatins, albumins, starches, acrylates, polyethyleneglycols (PEG) and starches; polyalkylcyanoacrylates; DEAE-derivatized polyimines, pollulans, celluloses and starches. Suitable complexing agents include chitosan, N-trimethylchitosan, poly-L-lysine, polyhistidine, polyornithine, polyspermines, protamine, polyvinylpyridine, polythiodiethylaminomethylethylene P(TDAE), polyaminostyrene (e.g., p-amino), poly(methylcyanoacrylate), poly(ethylcyanoacrylate), poly(butylcyanoacrylate), poly(isobutylcyanoacrylate), poly(isohexylcynaoacrylate), DEAE-methacrylate, DEAE-hexylacrylate, DEAE-acrylamide, DEAE-albumin and DEAE-dextran, polymethylacrylate, polyhexylacrylate, poly(D,L-lactic acid), poly(DL-lactic-co-glycolic acid (PLGA), alginate, and polyethyleneglycol (PEG). Oral formulations for dsRNAs and their preparation are described in detail in U.S. Pat. No. 6,887,906, US Pubin. No. 20030027780, and U.S. Pat. No. 6,747,014, each of which is incorporated herein by reference in their entirety.
- Compositions and formulations for parenteral, intraparenchymal (into the brain), intrathecal, intraventricular or intrahepatic administration may include sterile aqueous solutions which may also contain buffers, diluents and other suitable additives such as, but not limited to, penetration enhancers, carrier compounds and other pharmaceutically acceptable carriers or excipients.
- Pharmaceutical compositions featured in the invention include, but are not limited to, solutions, emulsions, and liposome-containing formulations. These compositions may be generated from a variety of components that include, but are not limited to, preformed liquids, self-emulsifying solids and self-emulsifying semisolids. Particularly perfered are formulations that target the liver when treating hepatic disorders such as hepatic carcinoma.
- The pharmaceutical formulations, which may conveniently be presented in unit dosage form, may be prepared according to conventional techniques well known in the pharmaceutical industry. Such techniques include the step of bringing into association the active ingredients with the pharmaceutical carrier(s) or excipient(s). In general, the formulations are prepared by uniformly and intimately bringing into association the active ingredients with liquid carriers or finely divided solid carriers or both, and then, if necessary, shaping the product.
- The compositions may be formulated into any of many possible dosage forms such as, but not limited to, tablets, capsules, gel capsules, liquid syrups, soft gels, suppositories, and enemas. The compositions may also be formulated as suspensions in aqueous, non-aqueous or mixed media. Aqueous suspensions may further contain substances which increase the viscosity of the suspension including, for example, sodium carboxymethylcellulose, sorbitol and/or dextran. The suspension may also contain stabilizers.
- Emulsions
- The compositions may be prepared and formulated as emulsions. Emulsions are typically heterogenous systems of one liquid dispersed in another in the form of droplets usually exceeding 0.1 ·mu·m in diameter (Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,
volume 1, p. 199; Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,Volume 1, p. 245; Block in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,volume 2, p. 335; Higuchi et al., in Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton, Pa., 1985, p. 301). Emulsions are often biphasic systems comprising two immiscible liquid phases intimately mixed and dispersed with each other. In general, emulsions may be of either the water-in-oil (w/o) or the oil-in-water (o/w) variety. When an aqueous phase is finely divided into and dispersed as minute droplets into a bulk oily phase, the resulting composition is called a water-in-oil (w/o) emulsion. Alternatively, when an oily phase is finely divided into and dispersed as minute droplets into a bulk aqueous phase, the resulting composition is called an oil-in-water (o/w) emulsion. Emulsions may contain additional components in addition to the dispersed phases, and the active drug which may be present as a solution in either the aqueous phase, oily phase or itself as a separate phase. Pharmaceutical excipients such as emulsifiers, stabilizers, dyes, and anti-oxidants may also be present in emulsions as needed. Pharmaceutical emulsions may also be multiple emulsions that are comprised of more than two phases such as, for example, in the case of oil-in-water-in-oil (o/w/o) and water-in-oil-in-water (w/o/w) emulsions. Such complex formulations often provide certain advantages that simple binary emulsions do not. Multiple emulsions in which individual oil droplets of an o/w emulsion enclose small water droplets constitute a w/o/w emulsion. Likewise a system of oil droplets enclosed in globules of water stabilized in an oily continuous phase provides an o/w/o emulsion. - Emulsions are characterized by little or no thermodynamic stability. Often, the dispersed or discontinuous phase of the emulsion is well dispersed into the external or continuous phase and maintained in this form through the means of emulsifiers or the viscosity of the formulation. Either of the phases of the emulsion may be a semisolid or a solid, as is the case of emulsion-style ointment bases and creams. Other means of stabilizing emulsions entail the use of emulsifiers that may be incorporated into either phase of the emulsion. Emulsifiers may broadly be classified into four categories: synthetic surfactants, naturally occurring emulsifiers, absorption bases, and finely dispersed solids (Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,
volume 1, p. 199). - Synthetic surfactants, also known as surface active agents, have found wide applicability in the formulation of emulsions and have been reviewed in the literature (Rieger, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,
volume 1, p. 285; Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), Marcel Dekker, Inc., New York, N.Y., 1988,volume 1, p. 199). Surfactants are typically amphiphilic and comprise a hydrophilic and a hydrophobic portion. The ratio of the hydrophilic to the hydrophobic nature of the surfactant has been termed the hydrophile/lipophile balance (HLB) and is a valuable tool in categorizing and selecting surfactants in the preparation of formulations. Surfactants may be classified into different classes based on the nature of the hydrophilic group: nonionic, anionic, cationic and amphoteric (Rieger, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,volume 1, p. 285). - Naturally occurring emulsifiers used in emulsion formulations include lanolin, beeswax, phosphatides, lecithin and acacia. Absorption bases possess hydrophilic properties such that they can soak up water to form w/o emulsions yet retain their semisolid consistencies, such as anhydrous lanolin and hydrophilic petrolatum. Finely divided solids have also been used as good emulsifiers especially in combination with surfactants and in viscous preparations. These include polar inorganic solids, such as heavy metal hydroxides, nonswelling clays such as bentonite, attapulgite, hectorite, kaolin, montmorillonite, colloidal aluminum silicate and colloidal magnesium aluminum silicate, pigments and nonpolar solids such as carbon or glyceryl tristearate.
- A large variety of non-emulsifying materials are also included in emulsion formulations and contribute to the properties of emulsions. These include fats, oils, waxes, fatty acids, fatty alcohols, fatty esters, humectants, hydrophilic colloids, preservatives and antioxidants (Block, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,
volume 1, p. 335; Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,volume 1, p. 199). - Hydrophilic colloids or hydrocolloids include naturally occurring gums and synthetic polymers such as polysaccharides (for example, acacia, agar, alginic acid, carrageenan, guar gum, karaya gum, and tragacanth), cellulose derivatives (for example, carboxymethylcellulose and carboxypropylcellulose), and synthetic polymers (for example, carbomers, cellulose ethers, and carboxyvinyl polymers). These disperse or swell in water to form colloidal solutions that stabilize emulsions by forming strong interfacial films around the dispersed-phase droplets and by increasing the viscosity of the external phase.
- Since emulsions often contain a number of ingredients such as carbohydrates, proteins, sterols and phosphatides that may readily support the growth of microbes, these formulations often incorporate preservatives. Commonly used preservatives included in emulsion formulations include methyl paraben, propyl paraben, quaternary ammonium salts, benzalkonium chloride, esters of p-hydroxybenzoic acid, and boric acid. Antioxidants are also commonly added to emulsion formulations to prevent deterioration of the formulation. Antioxidants used may be free radical scavengers such as tocopherols, alkyl gallates, butylated hydroxyanisole, butylated hydroxytoluene, or reducing agents such as ascorbic acid and sodium metabisulfite, and antioxidant synergists such as citric acid, tartaric acid, and lecithin.
- The application of emulsion formulations via dermatological, oral and parenteral routes and methods for their manufacture have been reviewed in the literature (Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,
volume 1, p. 199). Emulsion formulations for oral delivery have been very widely used because of ease of formulation, as well as efficacy from an absorption and bioavailability standpoint (Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,volume 1, p. 245; Idson, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,volume 1, p. 199). Mineral-oil base laxatives, oil-soluble vitamins and high fat nutritive preparations are among the materials that have commonly been administered orally as o/w emulsions. - In one embodiment, the compositions of dsRNAs and nucleic acids are formulated as microemulsions. A microemulsion may be defined as a system of water, oil and amphiphile which is a single optically isotropic and thermodynamically stable liquid solution (Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,
volume 1, p. 245). Typically microemulsions are systems that are prepared by first dispersing an oil in an aqueous surfactant solution and then adding a sufficient amount of a fourth component, generally an intermediate chain-length alcohol to form a transparent system. Therefore, microemulsions have also been described as thermodynamically stable, isotropically clear dispersions of two immiscible liquids that are stabilized by interfacial films of surface-active molecules (Leung and Shah, in: Controlled Release of Drugs: Polymers and Aggregate Systems, Rosoff, M., Ed., 1989, VCH Publishers, New York, pages 185-215). Microemulsions commonly are prepared via a combination of three to five components that include oil, water, surfactant, cosurfactant and electrolyte. Whether the microemulsion is of the water-in-oil (w/o) or an oil-in-water (o/w) type is dependent on the properties of the oil and surfactant used and on the structure and geometric packing of the polar heads and hydrocarbon tails of the surfactant molecules (Schott, in Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton, Pa., 1985, p. 271). - The phenomenological approach utilizing phase diagrams has been extensively studied and has yielded a comprehensive knowledge, to one skilled in the art, of how to formulate microemulsions (Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,
volume 1, p. 245; Block, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,volume 1, p. 335). Compared to conventional emulsions, microemulsions offer the advantage of solubilizing water-insoluble drugs in a formulation of thermodynamically stable droplets that are formed spontaneously. - Surfactants used in the preparation of microemulsions include, but are not limited to, ionic surfactants, non-ionic surfactants, Brij 96, polyoxyethylene oleyl ethers, polyglycerol fatty acid esters, tetraglycerol monolaurate (ML310), tetraglycerol monooleate (MO310), hexaglycerol monooleate (PO310), hexaglycerol pentaoleate (PO500), decaglycerol monocaprate (MCA750), decaglycerol monooleate (MO750), decaglycerol sequioleate (SO750), decaglycerol decaoleate (DAO750), alone or in combination with cosurfactants. The cosurfactant, usually a short-chain alcohol such as ethanol, 1-propanol, and 1-butanol, serves to increase the interfacial fluidity by penetrating into the surfactant film and consequently creating a disordered film because of the void space generated among surfactant molecules. Microemulsions may, however, be prepared without the use of cosurfactants and alcohol-free self-emulsifying microemulsion systems are known in the art. The aqueous phase may typically be, but is not limited to, water, an aqueous solution of the drug, glycerol, PEG300, PEG400, polyglycerols, propylene glycols, and derivatives of ethylene glycol. The oil phase may include, but is not limited to, materials such as
Captex 300, Captex 355, Capmul MCM, fatty acid esters, medium chain (C8-C12) mono, di, and tri-glycerides, polyoxyethylated glyceryl fatty acid esters, fatty alcohols, polyglycolized glycerides, saturated polyglycolized C8-C10 glycerides, vegetable oils and silicone oil. - Microemulsions are particularly of interest from the standpoint of drug solubilization and the enhanced absorption of drugs. Lipid based microemulsions (both o/w and w/o) have been proposed to enhance the oral bioavailability of drugs, including peptides (Constantinides et al., Pharmaceutical Research, 1994, 11, 1385-1390; Ritschel, Meth. Find. Exp. Clin. Pharmacol., 1993, 13, 205). Microemulsions afford advantages of improved drug solubilization, protection of drug from enzymatic hydrolysis, possible enhancement of drug absorption due to surfactant-induced alterations in membrane fluidity and permeability, ease of preparation, ease of oral administration over solid dosage forms, improved clinical potency, and decreased toxicity (Constantinides et al., Pharmaceutical Research, 1994, 11, 1385; Ho et al., J. Pharm. Sci., 1996, 85, 138-143). Often microemulsions may form spontaneously when their components are brought together at ambient temperature. This may be particularly advantageous when formulating thermolabile drugs, peptides or dsRNAs. Microemulsions have also been effective in the transdermal delivery of active components in both cosmetic and pharmaceutical applications. It is expected that the microemulsion compositions and formulations will facilitate the increased systemic absorption of dsRNAs and nucleic acids from the gastrointestinal tract, as well as improve the local cellular uptake of dsRNAs and nucleic acids.
- Microemulsions may also contain additional components and additives such as sorbitan monostearate (Grill 3), Labrasol, and penetration enhancers to improve the properties of the formulation and to enhance the absorption of the dsRNAs and nucleic acids featured herein. Penetration enhancers used in the microemulsions may be classified as belonging to one of five broad categories—surfactants, fatty acids, bile salts, chelating agents, and non-chelating non-surfactants (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, p. 92). Each of these classes has been discussed above.
- Liposomes
- There are many organized surfactant structures besides microemulsions that have been studied and used for the formulation of drugs. These include monolayers, micelles, bilayers and vesicles. Vesicles, such as liposomes, have attracted great interest because of their specificity and the duration of action they offer from the standpoint of drug delivery. As used in the present invention, the term “liposome” means a vesicle composed of amphiphilic lipids arranged in a spherical bilayer or bilayers.
- Liposomes are unilamellar or multilamellar vesicles which have a membrane formed from a lipophilic material and an aqueous interior. The aqueous portion contains the composition to be delivered. Cationic liposomes possess the advantage of being able to fuse to the cell wall. Non-cationic liposomes, although not able to fuse as efficiently with the cell wall, are taken up by macrophages in vivo.
- In order to cross intact mammalian skin, lipid vesicles must pass through a series of fine pores, each with a diameter less than 50 nm, under the influence of a suitable transdermal gradient. Therefore, it is desirable to use a liposome which is highly deformable and able to pass through such fine pores.
- Further advantages of liposomes include; liposomes obtained from natural phospholipids are biocompatible and biodegradable; liposomes can incorporate a wide range of water and lipid soluble drugs; liposomes can protect encapsulated drugs in their internal compartments from metabolism and degradation (Rosoff, in Pharmaceutical Dosage Forms, Lieberman, Rieger and Banker (Eds.), 1988, Marcel Dekker, Inc., New York, N.Y.,
volume 1, p. 245). Important considerations in the preparation of liposome formulations are the lipid surface charge, vesicle size and the aqueous volume of the liposomes. - Liposomes are useful for the transfer and delivery of active ingredients to the site of action. Because the liposomal membrane is structurally similar to biological membranes, when liposomes are applied to a tissue, the liposomes start to merge with the cellular membranes and as the merging of the liposome and cell progresses, the liposomal contents are emptied into the cell where the active agent may act.
- Liposomal formulations have been the focus of extensive investigation as the mode of delivery for many drugs. There is growing evidence that for topical administration, liposomes present several advantages over other formulations. Such advantages include reduced side-effects related to high systemic absorption of the administered drug, increased accumulation of the administered drug at the desired target, and the ability to administer a wide variety of drugs, both hydrophilic and hydrophobic, into the skin.
- Several reports have detailed the ability of liposomes to deliver agents including high-molecular weight DNA into the skin. Compounds including analgesics, antibodies, hormones and high-molecular weight DNAs have been administered to the skin. The majority of applications resulted in the targeting of the upper epidermis
- Liposomes fall into two broad classes. Cationic liposomes are positively charged liposomes which interact with the negatively charged DNA molecules to form a stable complex. The positively charged DNA/liposome complex binds to the negatively charged cell surface and is internalized in an endosome. Due to the acidic pH within the endosome, the liposomes are ruptured, releasing their contents into the cell cytoplasm (Wang et al., Biochem. Biophys. Res. Commun., 1987, 147, 980-985).
- Liposomes which are pH-sensitive or negatively-charged, entrap DNA rather than complex with it. Since both the DNA and the lipid are similarly charged, repulsion rather than complex formation occurs. Nevertheless, some DNA is entrapped within the aqueous interior of these liposomes. pH-sensitive liposomes have been used to deliver DNA encoding the thymidine kinase gene to cell monolayers in culture. Expression of the exogenous gene was detected in the target cells (Zhou et al., Journal of Controlled Release, 1992, 19, 269-274).
- One major type of liposomal composition includes phospholipids other than naturally-derived phosphatidylcholine. Neutral liposome compositions, for example, can be formed from dimyristoyl phosphatidylcholine (DMPC) or dipalmitoyl phosphatidylcholine (DPPC). Anionic liposome compositions generally are formed from dimyristoyl phosphatidylglycerol, while anionic fusogenic liposomes are formed primarily from dioleoyl phosphatidylethanolamine (DOPE). Another type of liposomal composition is formed from phosphatidylcholine (PC) such as, for example, soybean PC, and egg PC. Another type is formed from mixtures of phospholipid and/or phosphatidylcholine and/or cholesterol.
- Several studies have assessed the topical delivery of liposomal drug formulations to the skin. Application of liposomes containing interferon to guinea pig skin resulted in a reduction of skin herpes sores while delivery of interferon via other means (e.g., as a solution or as an emulsion) were ineffective (Weiner et al., Journal of Drug Targeting, 1992, 2, 405-410). Further, an additional study tested the efficacy of interferon administered as part of a liposomal formulation to the administration of interferon using an aqueous system, and concluded that the liposomal formulation was superior to aqueous administration (du Plessis et al., Antiviral Research, 1992, 18, 259-265).
- Non-ionic liposomal systems have also been examined to determine their utility in the delivery of drugs to the skin, in particular systems comprising non-ionic surfactant and cholesterol. Non-ionic liposomal formulations comprising Novasome™ I (glyceryl dilaurate/cholesterol/po- lyoxyethylene-10-stearyl ether) and Novasome™ II (glyceryl distearate/cholesterol/polyoxyethylene-10-stearyl ether) were used to deliver cyclosporin-A into the dermis of mouse skin. Results indicated that such non-ionic liposomal systems were effective in facilitating the deposition of cyclosporin-A into different layers of the skin (Hu et al. S.T.P.Pharma. Sci., 1994, 4, 6, 466).
- Liposomes also include “sterically stabilized” liposomes, a term which, as used herein, refers to liposomes comprising one or more specialized lipids that, when incorporated into liposomes, result in enhanced circulation lifetimes relative to liposomes lacking such specialized lipids. Examples of sterically stabilized liposomes are those in which part of the vesicle-forming lipid portion of the liposome (A) comprises one or more glycolipids, such as
monosialoganglioside G M1, or (B) is derivatized with one or more hydrophilic polymers, such as a polyethylene glycol (PEG) moiety. While not wishing to be bound by any particular theory, it is thought in the art that, at least for sterically stabilized liposomes containing gangliosides, sphingomyelin, or PEG-derivatized lipids, the enhanced circulation half-life of these sterically stabilized liposomes derives from a reduced uptake into cells of the reticuloendothelial system (RES) (Allen et al., FEBS Letters, 1987, 223, 42; Wu et al., Cancer Research, 1993, 53, 3765). - Various liposomes comprising one or more glycolipids are known in the art.
- Papahadjopoulos et al. (Ann. N.Y. Acad. Sci., 1987, 507, 64) reported the ability of
monosialoganglioside G M1, galactocerebroside sulfate and phosphatidylinositol to improve blood half-lives of liposomes. These findings were expounded upon by Gabizon et al. (Proc. Natl. Acad. Sci. U.S.A., 1988, 85, 6949). U.S. Pat. No. 4,837,028 and WO 88/04924, both to Allen et al., disclose liposomes comprising (1) sphingomyelin and (2) theganglioside G M1 or a galactocerebroside sulfate ester. U.S. Pat. No. 5,543,152 (Webb et al.) discloses liposomes comprising sphingomyelin. Liposomes comprising 1,2-sn-dimyristoylphosphat-idylcholine are disclosed in WO 97/13499 (Lim et al). - Many liposomes comprising lipids derivatized with one or more hydrophilic polymers, and methods of preparation thereof, are known in the art. Sunamoto et al. (Bull. Chem. Soc. Jpn., 1980, 53, 2778) described liposomes comprising a nonionic detergent, 2C1215G, that contains a PEG moiety. Ilium et al. (FEBS Lett., 1984, 167, 79) noted that hydrophilic coating of polystyrene particles with polymeric glycols results in significantly enhanced blood half-lives. Synthetic phospholipids modified by the attachment of carboxylic groups of polyalkylene glycols (e.g., PEG) are described by Sears (U.S. Pat. Nos. 4,426,330 and 4,534,899). Klibanov et al. (FEBS Lett., 1990, 268, 235) described experiments demonstrating that liposomes comprising phosphatidylethanolamine (PE) derivatized with PEG or PEG stearate have significant increases in blood circulation half-lives. Blume et al. (Biochimica et Biophysica Acta, 1990, 1029, 91) extended such observations to other PEG-derivatized phospholipids, e.g., DSPE-PEG, formed from the combination of distearoylphosphatidylethanolamine (DSPE) and PEG. Liposomes having covalently bound PEG moieties on their external surface are described in European Patent No.
EP 0 445 131 B1 and WO 90/04384 to Fisher. Liposome compositions containing 1-20 mole percent of PE derivatized with PEG, and methods of use thereof, are described by Woodle et al. (U.S. Pat. Nos. 5,013,556 and 5,356,633) and Martin et al. (U.S. Pat. No. 5,213,804 and European Patent No.EP 0 496 813 B1). Liposomes comprising a number of other lipid-polymer conjugates are disclosed in WO 91/05545 and U.S. Pat. No. 5,225,212 (both to Martin et al.) and in WO 94/20073 (Zalipsky et al.) Liposomes comprising PEG-modified ceramide lipids are described in WO 96/10391 (Choi et al). U.S. Pat. No. 5,540,935 (Miyazaki et al.) and U.S. Pat. No. 5,556,948 (Tagawa et al.) describe PEG-containing liposomes that can be further derivatized with functional moieties on their surfaces. - A limited number of liposomes comprising nucleic acids are known in the art. WO 96/40062 to Thierry et al. discloses methods for encapsulating high molecular weight nucleic acids in liposomes. U.S. Pat. No. 5,264,221 to Tagawa et al. discloses protein-bonded liposomes and asserts that the contents of such liposomes may include a dsRNA. U.S. Pat. No. 5,665,710 to Rahman et al. describes certain methods of encapsulating oligodeoxynucleotides in liposomes. WO 97/04787 to Love et al. discloses liposomes comprising dsRNAs targeted to the raf gene.
- Transfersomes are yet another type of liposomes, and are highly deformable lipid aggregates which are attractive candidates for drug delivery vehicles. Transfersomes may be described as lipid droplets which are so highly deformable that they are easily able to penetrate through pores which are smaller than the droplet. Transfersomes are adaptable to the environment in which they are used, e.g., they are self-optimizing (adaptive to the shape of pores in the skin), self-repairing, frequently reach their targets without fragmenting, and often self-loading. To make transfersomes it is possible to add surface edge-activators, usually surfactants, to a standard liposomal composition. Transfersomes have been used to deliver serum albumin to the skin. The transfersome-mediated delivery of serum albumin has been shown to be as effective as subcutaneous injection of a solution containing serum albumin.
- Surfactants find wide application in formulations such as emulsions (including microemulsions) and liposomes. The most common way of classifying and ranking the properties of the many different types of surfactants, both natural and synthetic, is by the use of the hydrophile/lipophile balance (HLB). The nature of the hydrophilic group (also known as the “head”) provides the most useful means for categorizing the different surfactants used in formulations (Rieger, in Pharmaceutical Dosage Forms, Marcel Dekker, Inc., New York, N.Y., 1988, p. 285).
- If the surfactant molecule is not ionized, it is classified as a nonionic surfactant. Nonionic surfactants find wide application in pharmaceutical and cosmetic products and are usable over a wide range of pH values. In general their HLB values range from 2 to about 18 depending on their structure. Nonionic surfactants include nonionic esters such as ethylene glycol esters, propylene glycol esters, glyceryl esters, polyglyceryl esters, sorbitan esters, sucrose esters, and ethoxylated esters. Nonionic alkanolamides and ethers such as fatty alcohol ethoxylates, propoxylated alcohols, and ethoxylated/propoxylated block polymers are also included in this class. The polyoxyethylene surfactants are the most popular members of the nonionic surfactant class.
- If the surfactant molecule carries a negative charge when it is dissolved or dispersed in water, the surfactant is classified as anionic. Anionic surfactants include carboxylates such as soaps, acyl lactylates, acyl amides of amino acids, esters of sulfuric acid such as alkyl sulfates and ethoxylated alkyl sulfates, sulfonates such as alkyl benzene sulfonates, acyl isethionates, acyl taurates and sulfosuccinates, and phosphates. The most important members of the anionic surfactant class are the alkyl sulfates and the soaps.
- If the surfactant molecule carries a positive charge when it is dissolved or dispersed in water, the surfactant is classified as cationic. Cationic surfactants include quaternary ammonium salts and ethoxylated amines. The quaternary ammonium salts are the most used members of this class.
- If the surfactant molecule has the ability to carry either a positive or negative charge, the surfactant is classified as amphoteric. Amphoteric surfactants include acrylic acid derivatives, substituted alkylamides, N-alkylbetaines and phosphatides.
- The use of surfactants in drug products, formulations and in emulsions has been reviewed (Rieger, in Pharmaceutical Dosage Forms, Marcel Dekker, Inc., New York, N.Y., 1988, p. 285).
- Penetration Enhancers
- In one embodiment, the present invention employs various penetration enhancers to effect the efficient delivery of nucleic acids, particularly dsRNAs, to the skin of animals. Most drugs are present in solution in both ionized and nonionized forms. However, usually only lipid soluble or lipophilic drugs readily cross cell membranes. It has been discovered that even non-lipophilic drugs may cross cell membranes if the membrane to be crossed is treated with a penetration enhancer. In addition to aiding the diffusion of non-lipophilic drugs across cell membranes, penetration enhancers also enhance the permeability of lipophilic drugs.
- Penetration enhancers may be classified as belonging to one of five broad categories, i.e., surfactants, fatty acids, bile salts, chelating agents, and non-chelating non-surfactants (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, p.92). Each of the above mentioned classes of penetration enhancers are described below in greater detail.
- Surfactants: In connection with the present invention, surfactants (or “surface-active agents”) are chemical entities which, when dissolved in an aqueous solution, reduce the surface tension of the solution or the interfacial tension between the aqueous solution and another liquid, with the result that absorption of dsRNAs through the mucosa is enhanced. In addition to bile salts and fatty acids, these penetration enhancers include, for example, sodium lauryl sulfate, polyoxyethylene-9-lauryl ether and polyoxyethylene-20-cetyl ether) (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, p.92); and perfluorochemical emulsions, such as FC-43. Takahashi et al., J. Pharm. Pharmacol., 1988, 40, 252).
- Fatty acids: Various fatty acids and their derivatives which act as penetration enhancers include, for example, oleic acid, lauric acid, capric acid (n-decanoic acid), myristic acid, palmitic acid, stearic acid, linoleic acid, linolenic acid, dicaprate, tricaprate, monoolein (1-monooleoyl-rac-glycerol), dilaurin, caprylic acid, arachidonic acid, glycerol 1-monocaprate, 1-dodecylazacycloheptan-2-one, acylcarnitines, acylcholines, C1-10 alkyl esters thereof (e.g., methyl, isopropyl and t-butyl), and mono- and di-glycerides thereof (i.e., oleate, laurate, caprate, myristate, palmitate, stearate, linoleate, etc.) (Lee et al., Critical Reviews in Therapeutic Drug Carryier Systems, 1991, p.92; Muranishi, Critical Reviews in Therapeutic Drug Carrier Systems, 1990, 7, 1-33; El Hariri et al., J. Pharm. Pharmacol., 1992, 44, 651-654).
- Bile salts: The physiological role of bile includes the facilitation of dispersion and absorption of lipids and fat-soluble vitamins (Brunton, Chapter 38 in: Goodman & Gilman's The Pharmacological Basis of Therapeutics, 9th Ed., Hardman et al. Eds., McGraw-Hill, New York, 1996, pp. 934-935). Various natural bile salts, and their synthetic derivatives, act as penetration enhancers. Thus the term “bile salts” includes any of the naturally occurring components of bile as well as any of their synthetic derivatives. Suitable bile salts include, for example, cholic acid (or its pharmaceutically acceptable sodium salt, sodium cholate), dehydrocholic acid (sodium dehydrocholate), deoxycholic acid (sodium deoxycholate), glucholic acid (sodium glucholate), glycholic acid (sodium glycocholate), glycodeoxycholic acid (sodium glycodeoxycholate), taurocholic acid (sodium taurocholate), taurodeoxycholic acid (sodium taurodeoxycholate), chenodeoxycholic acid (sodium chenodeoxycholate), ursodeoxycholic acid (UDCA), sodium tauro-24,25-dihydro-fusidate (STDHF), sodium glycodihydrofusidate and polyoxyethylene-9-lauryl ether (POE) (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, page 92; Swinyard, Chapter 39 In: Remington's Pharmaceutical Sciences, 18th Ed., Gennaro, ed., Mack Publishing, Co., Easton, Pa., 1990, pages 782-783; Muranishi, Critical Reviews in Therapeutic Drug Carrier Systems, 1994, 7, 1-33; Yamamoto et al., J. Pharm. Exp. Ther., 1992, 263, 25; Yamashita et al., J. Pharm. Sci., 1990, 79, 579-583).
- Chelating Agents: Chelating agents, as used in connection with the present invention, can be defined as compounds that remove metallic ions from solution by forming complexes therewith, with the result that absorption of dsRNAs through the mucosa is enhanced. With regards to their use as penetration enhancers in the present invention, chelating agents have the added advantage of also serving as DNase inhibitors, as most characterized DNA nucleases require a divalent metal ion for catalysis and are thus inhibited by chelating agents (Jarrett, J. Chromatogr., 1993, 618, 315-339). Suitable chelating agents include but are not limited to disodium ethylenediaminetetraacetate (EDTA), citric acid, salicylates (e.g., sodium salicylate, 5-methoxysalicylate and homovanilate), N-acyl derivatives of collagen, laureth-9 and N-amino acyl derivatives of beta-diketones (enamines)(Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, page 92; Muranishi, Critical Reviews in Therapeutic Drug Carrier Systems, 1990, 7, 1-33; Buur et al., J. Control Rel., 1990, 14, 43-51).
- Non-chelating non-surfactants: As used herein, non-chelating non-surfactant penetration enhancing compounds can be defined as compounds that demonstrate insignificant activity as chelating agents or as surfactants but that nonetheless enhance absorption of dsRNAs through the alimentary mucosa (Muranishi, Critical Reviews in Therapeutic Drug Carrier Systems, 1990, 7, 1-33). This class of penetration enhancers include, for example, unsaturated cyclic ureas, 1-alkyl- and 1-alkenylazacyclo-alkanone derivatives (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems, 1991, page 92); and non-steroidal anti-inflammatory agents such as diclofenac sodium, indomethacin and phenylbutazone (Yamashita et al., J. Pharm. Pharmacol., 1987, 39, 621-626).
- Agents that enhance uptake of dsRNAs at the cellular level may also be added to the pharmaceutical and other compositions featured in the invention. For example, cationic lipids, such as lipofectin (Junichi et al, U.S. Pat. No. 5,705,188), cationic glycerol derivatives, and polycationic molecules, such as polylysine (Lollo et al., PCT Application WO 97/30731), are also known to enhance the cellular uptake of dsRNAs.
- Other agents may be utilized to enhance the penetration of the administered nucleic acids, including glycols such as ethylene glycol and propylene glycol, pyrrols such as 2-pyrrol, azones, and terpenes such as limonene and menthone.
- Carriers
- Certain compositions also incorporate carrier compounds in the formulation. As used herein, “carrier compound” or “carrier” can refer to a nucleic acid, or analog thereof, which is inert (i.e., does not possess biological activity per se) but is recognized as a nucleic acid by in vivo processes that reduce the bioavailability of a nucleic acid having biological activity by, for example, degrading the biologically active nucleic acid or promoting its removal from circulation. The coadministration of a nucleic acid and a carrier compound, typically with an excess of the latter substance, can result in a substantial reduction of the amount of nucleic acid recovered in the liver, kidney or other extracirculatory reservoirs, presumably due to competition between the carrier compound and the nucleic acid for a common receptor. For example, the recovery of a partially phosphorothioate dsRNA in hepatic tissue can be reduced when it is coadministered with polyinosinic acid, dextran sulfate, polycytidic acid or 4-acetamido-4′isothiocyano-stilbene-2,2′-disulfonic acid (Miyao et al., DsRNA Res. Dev., 1995, 5, 115-121; Takakura et al., DsRNA & Nucl. Acid Drug Dev., 1996, 6, 177-183.
- Excipients
- In contrast to a carrier compound, a “pharmaceutical carrier” or “excipient” is a pharmaceutically acceptable solvent, suspending agent or any other pharmacologically inert vehicle for delivering one or more nucleic acids to an animal. The excipient may be liquid or solid and is selected, with the planned manner of administration in mind, so as to provide for the desired bulk, consistency, etc., when combined with a nucleic acid and the other components of a given pharmaceutical composition. Typical pharmaceutical carriers include, but are not limited to, binding agents (e.g., pregelatinized maize starch, polyvinylpyrrolidone or hydroxypropyl methylcellulose, etc.); fillers (e.g., lactose and other sugars, microcrystalline cellulose, pectin, gelatin, calcium sulfate, ethyl cellulose, polyacrylates or calcium hydrogen phosphate, etc.); lubricants (e.g., magnesium stearate, talc, silica, colloidal silicon dioxide, stearic acid, metallic stearates, hydrogenated vegetable oils, corn starch, polyethylene glycols, sodium benzoate, sodium acetate, etc.); disintegrants (e.g., starch, sodium starch glycolate, etc.); and wetting agents (e.g., sodium lauryl sulphate, etc).
- Pharmaceutically acceptable organic or inorganic excipient suitable for non-parenteral administration, which do not deleteriously react with nucleic acids, can also be used to formulate the compositions. Suitable pharmaceutically acceptable carriers include, but are not limited to, water, salt solutions, alcohols, polyethylene glycols, gelatin, lactose, amylose, magnesium stearate, talc, silicic acid, viscous paraffin, hydroxymethylcellulose, polyvinylpyrrolidone and the like.
- Formulations for topical administration of nucleic acids may include sterile and non-sterile aqueous solutions, non-aqueous solutions in common solvents such as alcohols, or solutions of the nucleic acids in liquid or solid oil bases. The solutions may also contain buffers, diluents and other suitable additives. Pharmaceutically acceptable organic or inorganic excipients suitable for non-parenteral administration which do not deleteriously react with nucleic acids can be used.
- Suitable pharmaceutically acceptable excipients include, but are not limited to, water, salt solutions, alcohol, polyethylene glycols, gelatin, lactose, amylose, magnesium stearate, talc, silicic acid, viscous paraffin, hydroxymethylcellulose, polyvinylpyrrolidone and the like.
- Other Components
- The compositions featured in the invention may additionally contain other adjunct components conventionally found in pharmaceutical compositions, at their art-established usage levels. Thus, for example, the compositions may contain additional, compatible, pharmaceutically-active materials such as, for example, antipruritics, astringents, local anesthetics or anti-inflammatory agents, or may contain additional materials useful in physically formulating various dosage forms of the compositions, such as dyes, flavoring agents, preservatives, antioxidants, opacifiers, thickening agents and stabilizers. However, such materials, when added, should not unduly interfere with the biological activities of the components of the compositions. The formulations can be sterilized and, if desired, mixed with auxiliary agents, e.g., lubricants, preservatives, stabilizers, wetting agents, emulsifiers, salts for influencing osmotic pressure, buffers, colorings, flavorings and/or aromatic substances and the like which do not deleteriously interact with the nucleic acid(s) of the formulation.
- Aqueous suspensions may contain substances which increase the viscosity of the suspension including, for example, sodium carboxymethylcellulose, sorbitol and/or dextran. The suspension may also contain stabilizers.
- In some embodiments, pharmaceutical compositions featured in the invention include (a) one or more dsRNA compounds and (b) one or more other chemotherapeutic agents which function by a non-RNAi mechanism. Examples of such chemotherapeutic agents include but are not limited to temozolomide, daunorubicin, daunomycin, dactinomycin, doxorubicin, epirubicin, idarubicin, esorubicin, bleomycin, mafosfamide, ifosfamide, cytosine arabinoside, bis-chloroethylnitrosurea, busulfan, mitomycin C, actinomycin D, mithramycin, prednisone, hydroxyprogesterone, testosterone, tamoxifen, dacarbazine, procarbazine, hexamethylmelamine, pentamethylmelamine, mitoxantrone, amsacrine, chlorambucil, methylcyclohexylnitrosurea, nitrogen mustards, melphalan, cyclophosphamide, 6-mercaptopurine, 6-thioguanine, cytarabine, 5-azacytidine, hydroxyurea, deoxycoformycin, 4-hydroxyperoxycyclophosphor- amide, 5-fluorouracil (5-FU), 5-fluorodeoxyuridine (5-FUdR), methotrexate (MTX), colchicine, taxol, vincristine, vinblastine, etoposide (VP-16), trimetrexate, irinotecan, topotecan, gemcitabine, teniposide, cisplatin and diethylstilbestrol (DES). See, generally, The Merck Manual of Diagnosis and Therapy, 15th Ed. 1987, pp. 1206-1228, Berkow et al., eds., Rahway, N.J. When used with the dsRNAs featured in the invention, such chemotherapeutic agents may be used individually (e.g., 5-FU and oligonucleotide), sequentially (e.g., 5-FU and oligonucleotide for a period of time followed by MTX and oligonucleotide), or in combination with one or more other such chemotherapeutic agents (e.g., 5-FU, MTX and oligonucleotide, or 5-FU, radiotherapy and oligonucleotide). Anti-inflammatory drugs, including but not limited to nonsteroidal anti-inflammatory drugs and corticosteroids, and antiviral drugs, including but not limited to ribivirin, vidarabine, acyclovir and ganciclovir, may also be combined in compositions featured in the invention. See, generally, The Merck Manual of Diagnosis and Therapy, 15th Ed., Berkow et al., eds., 1987, Rahway, N.J., pages 2499-2506 and 46-49, respectively). Other non-RNAi chemotherapeutic agents are also within the scope of this invention. Two or more combined compounds may be used together or sequentially.
- Toxicity and therapeutic efficacy of such compounds can be determined by standard pharmaceutical procedures in cell cultures or experimental animals, e.g., for determining the LD50 (the dose lethal to 50% of the population) and the ED50 (the dose therapeutically effective in 50% of the population). The dose ratio between toxic and therapeutic effects is the therapeutic index and it can be expressed as the ratio LD50/ED50. Compounds that exhibit high therapeutic indices are generally preferred.
- The data obtained from cell culture assays and animal studies can be used in formulation a range of dosage for use in humans. The dosage of compositions featured in the invention lies generally within a range of circulating concentrations that include the ED50 with little or no toxicity. The dosage may vary within this range depending upon the dosage form employed and the route of administration utilized. For any compound used in the methods featured in the invention, the therapeutically effective dose can be estimated initially from cell culture assays. A dose may be formulated in animal models to achieve a circulating plasma concentration range of the compound or, when appropriate, of the polypeptide product of a target sequence (e.g., achieving a decreased concentration of the polypeptide) that includes the IC50 (i.e., the concentration of the test compound which achieves a half-maximal inhibition of symptoms) as determined in cell culture. Such information can be used to more accurately determine useful doses in humans. Levels in plasma may be measured, for example, by high performance liquid chromatography.
- In addition to their administration, as discussed above, the dsRNAs featured in the invention can be administered in combination with other known agents effective in treatment of pathological processes mediated by deltaEGFR or IL6 expression. In any event, the administering physician can adjust the amount and timing of dsRNA administration on the basis of results observed using standard measures of efficacy known in the art or described herein.
- Methods for Treating Diseases Caused by Expression of DeltaEGFR or IL6 genes
- The invention relates in particular to the use of a dsRNA targeting deltaEGFR or IL6 and compositions containing at least one such dsRNA, for the treatment of a deltaEGFR-mediated disorder or disease. For example, a dsRNA targeting a deltaEGFR gene can be useful for the treatment of a proliferative disorder, such as cancer, such as glioma, e.g., a glial tumor of the central nervous system, such as a grade I, II, III, or IV glioma. For example, a composition containing a dsRNA targeting deltaEGFR can be used to treat a grade III glioma, such as anaplastic astrocytoma, or a grade IV glioma, such as a glioblastoma multiforme. The glioma can be an ependymoma, astrocytoma, oligodendroglioma, or a mixed glioma, such as an oligoastrocytoma. A composition containing a dsRNA targeting a mutant EGFR, e.g., deltaEGFR or an IL6, is used to treat a carcinoma of the breast, ovary, cervix, kidney, or a squamous cell. The dsRNA targeting deltaEGFR can also target wtEGFR.
- A composition containing a dsRNA targeting a mutant EGFR, e.g., a deltaEGFR or an IL6, may also be used to treat other tumors and cancers, such as breast cancer, lung cancer, head and neck cancer, brain cancer, abdominal cancer, colon cancer, colorectal cancer, esophagus cancer, gastrointestinal cancer, tongue cancer, neuroblastoma, osteosarcoma, ovarian cancer, pancreatic cancer, prostate cancer, cervical cancer (e.g., squamous carcinoma of the cervix), lymphoid tumor, retinoblastoma, Wilm's tumor, multiple myeloma and for the treatment of skin cancer, like melanoma, for the treatment of lymphomas and blood cancer. The compositions featured herein can be used to treat a tumor of the brain or spine.
- A dsRNA targeting deltaEGFR or IL6 may be used to treat a proliferative disorder or differentiative disorder. Examples of cellular proliferative and/or differentiative disorders include cancer, e.g., carcinoma, sarcoma, metastatic disorders or hematopoietic neoplastic disorders, e.g., leukemias. A metastatic tumor can arise from a multitude of primary tumor types, including those of prostate, colon, lung, breast and liver origin. As used herein, the terms “cancer,” “hyperproliferative,” and “neoplastic” refer to cells having the capacity for autonomous growth, i.e., an abnormal state or condition characterized by rapidly proliferating cell growth. These terms are meant to include all types of cancerous growths or oncogenic processes, metastatic tissues or malignantly transformed cells, tissues, or organs, irrespective of histopathologic type or stage of invasiveness. Proliferative disorders also include hematopoietic neoplastic disorders, including diseases involving hyperplastic/neoplastic cells of hematopoictic origin, e.g., arising from myeloid, lymphoid or erythroid lineages, or precursor cells thereof.
- The invention further relates to the use of a dsRNA or a pharmaceutical composition thereof, e.g., for treating a cancer, in combination with other pharmaceuticals and/or other therapeutic methods, e.g., with known pharmaceuticals and/or known therapeutic methods, such as, for example, those which are currently employed for treating these disorders. In one example, administration of a dsRNA targeting deltaEGFR can be administered in combination with a chemotherapeutic agent, such as temozolomide, deoxycoformycin, cisplatin, cyclophosphamide, 5-fluorouracil, adriamycin, daunorubicin, tamoxifen aunorubicin, daunomycin, dactinomycin, doxorubicin, epirubicin, idarubicin, esorubicin, bleomycin, mafosfamide, ifosfamide, cytosine arabinoside, bis-chloroethylnitrosurea, busulfan, mitomycin C, actinomycin D, mithramycin, prednisone, hydroxyprogesterone, testosterone, tamoxifen, dacarbazine, procarbazine, hexamethylmelamine, pentamethylmelamine, mitoxantrone, amsacrine, chlorambucil, methylcyclohexylnitrosurea, nitrogen mustards, melphalan, cyclophosphamide, 6-mercaptopurine, 6-thioguanine, cytarabine, 5-azacytidine, hydroxyurea, deoxycoformycin, 4-hydroxyperoxycyclophosphoramide, 5-fluorouracil (5-FU), 5-fluorodeoxyuridine (5-FUdR), methotrexate (MTX), colchicine, taxol, vincristine, vinblastine, etoposide (VP-16), trimetrexate, irinotecan, topotecan, gemcitabine, teniposide, cisplatin and diethylstilbestrol (DES). See, generally, The Merck Manual of Diagnosis and Therapy, 15th Ed. 1987, pp. 1206-1228, Berkow et al., eds., Rahway, N.J. When used with the dsRNAs featured in the invention, such chemotherapeutic agents may be used individually, sequentially (e.g., dsRNA for a period of time, followed by chemotherapy), or in combination with one or more other such agents (e.g., chemotherapy and dsRNA). Two or more combined compounds may be used together or sequentially.
- In one embodiment, a deltaEGFR dsRNA is administered in combination with at least one additional therapeutic agent, such as a second dsRNA targeting a different nucleic acid, e.g., an IL6 dsRNA, for the treatment of a condition or a symptom of a condition, such as for the treatment of a cancer. In some embodiments, the second therapteutic agent is a chemotherapeutic agent.
- The dsRNA and an additional therapeutic agent can be administered in the same combination, e.g., intracranially or parenterally, or the additional therapeutic agent can be administered as part of a separate composition, e.g., intracranially or parenterally, or by another method described herein.
- Treatment with a dsRNA targeting deltaEGFR can also be performed in combination with radiation therapy, including external beam radiation, such as for treatment of tumors of the brain. A dsRNA featured herein may be administered before or after a surgical procedure to treat a cancer (e.g., to remove a tumor), such as resection of a brain tumor.
- The invention also relates to the use of a dsRNA targeting IL6 and compositions containing at least one such dsRNA, for the treatment of a IL6 or a deltaEGFR-mediated disorder or disease. For example, an IL-6 dsRNA featured in the invention may be used to treat a hematological disorder, such as plasma cell dyscrasia, leukemia or lymphoma; proliferative glomerulonephritis; an inflammatory disease, such as rheumatoid arthritis, or an inflammatory bowel disease, such as Crohn's disease or ulcerative colitis; diabetes; septic shock; bacterial infections; viral infections, including HIV-1 infections; osteoporosis; autoimmune disorders, such as chronic immune deficiency syndrome or autoimmune deficiency syndrome (AIDS); neural disorders, such as multiple sclerosis, HTLV1-associated myelopathy or bacterial meningitis, systemic lupus erythematosus and vasculitis-associated central nervous system diseases; or other disorders of the central nervous system, including Alzheimer's disease, hypochondria, epilepsy, migraine, pain, Parkin's disease or schizophrenia.
- An IL-6 dsRNA featured in the invention may also be used to prevent allograft rejection or xenograft rejection and ischemia/reperfusion injury in solid organ or tissue transplantation. For example, an IL-6 dsRNA can be administered to prevent rejection of a transplanted organ, such as a transplanted kidney, liver, lung, pancrease, heart, small bowel, cornea, epithelial cells, vascular endothelium, vascular smooth muscle cells, myocardium and passenger leukocytes resident in the organ at the time of transplantation.
- Treatment with a dsRNA targeting IL-6 can be performed in combination with a second dsRNA also targeting IL-6, and which targets a different sequence than a first dsRNA targeting IL-6. A dsRNA targeting IL-6 can also be administered in combination with one or more dsRNAs targeting other cytokines, immunomodulatory or immunoeffector genes, such as the C3 (complement component 3) gene, ICAM1 (intercellular adhesion molecule 1), VCAM-1 (vascular cell adhesion molecule 1), IFN-gamma (interferon gamma), IL-1 (interleukin-1), IL-8 (interleukin-8), TNF-alpha (tumor necrosis factor-alpha), CD80, CD86, MHC-II (major histocombatibility complex-II), MHC-I (major histocombatibility complex-I), CD28, CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) or PV-B19 (parvovirus B19). As stated above, the IL6 dsRNA can also be administered in combination with a dsRNA targeting deltaEGFR, and optionally, also targeting wtEGFR, such as for the treatment of a deltaEGFR mediated disease, such as a cancer.
- Patients can be administered a therapeutic amout of dsRNA, such as 0.01 mg/kg, 0.02 mg/kg, 0.1 mg/kg, 0.5 mg/kg, 1.0 mg/kg, 1.5 mg/kg, 2.0 mg/kg, or 2.5 mg/kg dsRNA. The dsRNA can be administered by intracranial infusion over a period of time, such as over a 30 minute, 1 hour, 2 hour, 3 hour or 4 hour period. The administration is repeated, for example, on a regular basis, such as biweekly (i.e., every two weeks) for one month, two months, three months, four months or longer. After an initial treatment regimen, the treatments can be administered on a less frequent basis. For example, after administration biweekly for three months, administration can be repeated once per month, for six months or a year or longer. Intracranial infusion can be continous. Administration of the dsRNA can reduce target RNA and protein levels, e.g., deltaEGFR or IL-6 levels, in the cerebrospinal fluid of the patient by at least 20%, 25%, 30%, 40%, 50%, 60%, 70%, 80% or 90% or more. Alternatively, the dsRNA can be administered by intravenous infusion over a period of time, such as over a 5 minute, 10 minute, 15 minute, 20 minute, or 25 minute period. The administration is repeated, for example, on a regular basis, such as biweekly (i.e., every two weeks) for one month, two months, three months, four months or longer. After an initial treatment regimen, the treatments can be administered on a less frequent basis. For example, after administration biweekly for three months, administration can be repeated once per month, for six months or a year or longer. Administration of the dsRNA can reduce deltaEGFR levels in the blood or urine of the patient by at least 20%, 25%, 30%, 40%, 50%, 60%, 70%, 80% or 90% or more.
- Before administration of a full dose of the dsRNA, patients can be administered a smaller dose, such as 5% of the total dose, and monitored for adverse effects, such as an allergic reaction. Patients can be monitored for adverse effects depending on the formulation. For example, if the dsRNA is formulated in a lipid, the patient can be administered a smaller dose, and then monitored for elevated lipid levels or blood pressure. In another example, the patient can be monitored for unwanted immunostimulatory effects, such as increased cytokine (e.g., TNF-alpha or INF-alpha) levels.
- Many EGFR- and IL6-associated diseases and disorders are hereditary. Therefore, a patient in need of a deltaEGFR dsRNA can be identified by taking a family history. A healthcare provider, such as a doctor, nurse, or family member, can take a family history before prescribing or administering a deltaEGFR dsRNA.
- Owing to the inhibitory effects on deltaEGFR expression, and of the inhibitory effects of IL6 overexpression, a composition according to the invention or a pharmaceutical composition prepared therefrom can enhance the quality of life of a subject.
- Methods for Inhibiting Expression of a DeltaEGFR or IL6 Gene
- In yet another aspect, the invention provides a method for inhibiting expression of a deltaEGFR gene in a mammal. The method includes administering a composition featured in the invention to the mammal such that expression of the target deltaEGFR gene and, optionally, a wtEGFR gene is decreased or silenced. In one aspect, the invention provides a method for inhibiting expression of an IL6 gene in a mammal. The method includes administering a composition featured in the invention to the mammal such that expression of the target IL6 gene is decreased or silenced.
- When the organism to be treated is a mammal such as a human, the composition may be administered by any means known in the art including, but not limited to oral or parenteral routes, including intracranial (e.g., intraventricular, intraparenchymal and intrathecal), intravenous, intramuscular, subcutaneous, transdermal, airway (aerosol), nasal, rectal, and topical (including buccal and sublingual) administration. In certain embodiments, the compositions are administered by intravenous infusion or injection. In other embodiments, the compositions are administered by intracranial infusion or injection.
- Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. Although methods and materials similar or equivalent to those described herein can be used in the practice or testing of the dsRNAs and methods featured in the invention, suitable methods and materials are described below. All publications, patent applications, patents, and other references mentioned herein are incorporated by reference in their entirety. In case of conflict, the present specification, including definitions, will control. In addition, the materials, methods, and examples are illustrative only and not intended to be limiting.
- Source of reagents
- Where the source of a reagent is not specifically given herein, such reagent may be obtained from any supplier of reagents for molecular biology at a quality/purity standard for application in molecular biology.
- siRNA synthesis
- Single-stranded RNAs were produced by solid phase synthesis on a scale of 1 μmole using an Expedite 8909 synthesizer (Applied Biosystems, Applera Deutschland GmbH, Darmstadt, Germany) and controlled pore glass (CPG, 500Å, Proligo Biochemie GmbH, Hamburg, Germany) as solid support. RNA and RNA containing 2′-O-methyl nucleotides were generated by solid phase synthesis employing the corresponding phosphoramidites and 2′-O-methyl phosphoramidites, respectively (Proligo Biochemie GmbH, Hamburg, Germany). These building blocks were incorporated at selected sites within the sequence of the oligoribonucleotide chain using standard nucleoside phosphoramidite chemistry such as described in Current protocols in nucleic acid chemistry, Beaucage, S.L. et al. (Edrs.), John Wiley & Sons, Inc., New York, N.Y., USA. Phosphorothioate linkages were introduced by replacement of the iodine oxidizer solution with a solution of the Beaucage reagent (Chruachem Ltd, Glasgow, UK) in acetonitrile (1%). Further ancillary reagents were obtained from Mallinckrodt Baker (Griesheim, Germany).
- Deprotection and purification of the crude oligoribonucleotides by anion exchange HPLC were carried out according to established procedures. Yields and concentrations were determined by UV absorption of a solution of the respective RNA at a wavelength of 260 nm using a spectral photometer (DU 640B, Beckman Coulter GmbH, UnterschleiBheim, Germany). Double stranded RNA was generated by mixing an equimolar solution of complementary strands in annealing buffer (20 mM sodium phosphate, pH 6.8; 100 mM sodium chloride), heated in a water bath at 85 - 90° C. for 3 minutes and cooled to room temperature over a period of 3 - 4 hours. The annealed RNA solution was stored at −20 ° C. until use.
- For the synthesis of 3′-cholesterol-conjugated siRNAs (herein referred to as -Chol-3′), an appropriately modified solid support is used for RNA synthesis. The modified solid support is prepared as follows:
-
- A 4.7 M aqueous solution of sodium hydroxide (50 mL) is added into a stirred, ice-cooled solution of ethyl glycinate hydrochloride (32.19 g, 0.23 mole) in water (50 mL). Then, ethyl acrylate (23.1 g, 0.23 mole) is added and the mixture is stirred at room temperature until completion of the reaction is ascertained by TLC. After 19 h the solution is partitioned with dichloromethane (3×100 mL). The organic layer is dried with anhydrous sodium sulfate, filtered and evaporated. The residue is distilled to afford AA (28.8 g, 61%).
-
- Fmoc-6-amino-hexanoic acid (9.12 g, 25.83 mmol) is dissolved in dichloromethane (50 mL) and cooled with ice. Diisopropylcarbodiimde (3.25 g, 3.99 mL, 25.83 mmol) is added to the solution at 0° C. It is then followed by the addition of Diethyl-azabutane-1,4-dicarboxylate (5 g, 24.6 mmol) and dimethylamino pyridine (0.305 g, 2.5 mmol). The solution is brought to room temperature and stirred further for 6 h. Completion of the reaction is ascertained by TLC. The reaction mixture is concentrated under vacuum and ethyl acetate is added to precipitate diisopropyl urea. The suspension is filtered. The filtrate is washed with 5% aqueous hydrochloric acid, 5% sodium bicarbonate and water. The combined organic layer is dried over sodium sulfate and concentrated to give the crude product which is purified by column chromatography (50% EtOAC/Hexanes) to yield 11.87 g (88%) of AB.
-
- 3- {Ethoxycarbonylmethyl-[6-(9H-fluoren-9-ylmethoxycarbonylamino)-hexanoyl]-amino}-propionic acid ethyl ester AB (11.5 g, 21.3 mmol) is dissolved in 20% piperidine in dimethylformamide at 0° C. The solution is continued stirring for 1 h. The reaction mixture is concentrated under vacuum, water is added to the residue, and the product is extracted with ethyl acetate. The crude product is purified by conversion into its hydrochloride salt.
-
- The hydrochloride salt of 3-[(6-Amino-hexanoyl)-ethoxycarbonylmethyl-amino]-propionic acid ethyl ester AC (4.7 g, 14.8 mmol) is taken up in dichloromethane. The suspension is cooled to 0° C. on ice. To the suspension diisopropylethylamine (3.87 g, 5.2 mL, 30 mmol) is added. To the resulting solution cholesteryl chloroformate (6.675 g, 14.8 mmol) is added. The reaction mixture is stirred overnight. The reaction mixture is diluted with dichloromethane and ished with 10% hydrochloric acid. The product is purified by flash chromatography (10.3 g, 92%).
-
- Potassium t-butoxide (1.1 g, 9.8 mmol) is slurried in 30 mL of dry toluene. The mixture is cooled to 0° C. on ice and 5 g (6.6 mmol) of diester AD is added slowly with stirring within 20 mins. The temperature is kept below 5° C. during the addition. The stirring is continued for 30 mins at 0° C. and 1 mL of glacial acetic acid is added, immediately followed by 4 g of NaH2PO4·H2O in 40 mL of water The resultant mixture is extracted twice with 100 mL of dichloromethane each and the combined organic extracts are washed twice with 10 mL of phosphate buffer each, dried, and evaporated to dryness. The residue is dissolved in 60 mL of toluene, cooled to 0° C. and extracted with three 50 mL portions of cold pH 9.5 carbonate buffer. The aqueous extracts are adjusted to
pH 3 with phosphoric acid, and extracted with five 40 mL portions of chloroform which are combined, dried and evaporated to dryness. The residue is purified by column chromatography using 25% ethylacetate/hexane to afford 1.9 g of b-ketoester (39%). -
- Methanol (2 mL) is added dropwise over a period of 1 h to a refluxing mixture of b-ketoester AE (1.5 g, 2.2 mmol) and sodium borohydride (0.226 g, 6 mmol) in tetrahydrofuran (10 mL). Stirring is continued at reflux temperature for 1 h. After cooling to room temperature, 1 N HCl (12.5 mL) is added, the mixture is extracted with ethylacetate (3×40 mL). The combined ethylacetate layer is dried over anhydrous sodium sulfate and concentrated under vacuum to yield the product which is purified by column chromatography (10% MeOH/CHCl3) (89%).
-
- Diol AF (1.25 gm 1.994 mmol) is dried by evaporating with pyridine (2×5 mL) in vacuo. Anhydrous pyridine (10 mL) and 4,4′-dimethoxytritylchloride (0.724 g, 2.13 mmol) are added with stirring. The reaction is carried out at room temperature overnight. The reaction is quenched by the addition of methanol. The reaction mixture is concentrated under vacuum and to the residue dichloromethane (50 mL) is added. The organic layer is washed with 1M aqueous sodium bicarbonate. The organic layer is dried over anhydrous sodium sulfate, filtered and concentrated. The residual pyridine is removed by evaporating with toluene. The crude product is purified by column chromatography (2% MeOH/Chloroform, Rf=0.5 in 5% MeOH/CHCl3) (1.75 g, 95%).
-
- Compound AG (1.0 g, 1.05 mmol) is mixed with succinic anhydride (0.150 g, 1.5 mmol) and DMAP (0.073 g, 0.6 mmol) and dried in a vacuum at 40° C. overnight. The mixture is dissolved in anhydrous dichloroethane (3 mL), triethylamine (0.318 g, 0.440 mL, 3.15 mmol) is added and the solution is stirred at room temperature under argon atmosphere for 16 h. It is then diluted with dichloromethane (40 mL) and washed with ice cold aqueous citric acid (5 wt %, 30 mL) and water (2×0 mL). The organic phase is dried over anhydrous sodium sulfate and concentrated to dryness. The residue is used as such for the next step.
- Cholesterol derivatised CPG AI
- Succinate AH (0.254 g, 0.242 mmol) is dissolved in a mixture of dichloromethane/acetonitrile (3:2, 3 mL). To that solution DMAP (0.0296 g, 0.242 mmol) in acetonitrile (1.25 mL), 2,2′-Dithio-bis(5-nitropyridine) (0.075 g, 0.242 mmol) in acetonitrile/dichloroethane (3:1, 1.25 mL) are added successively. To the resulting solution triphenylphosphine (0.064 g, 0.242 mmol) in acetonitrile (0.6 ml) is added. The reaction mixture turned bright orange in color. The solution is agitated briefly using a wrist-action shaker (5 mins). Long chain alkyl amine-CPG (LCAA-CPG) (1.5 g, 61 mM) is added. The suspension is agitated for 2 h. The CPG is filtered through a sintered funnel and washed with acetonitrile, dichloromethane and ether successively. Unreacted amino groups are masked using acetic anhydride/pyridine. The achieved loading of the CPG is measured by taking UV measurement (37 mM/g).
- The synthesis of siRNAs bearing a 5′-12-dodecanoic acid bisdecylamide group (herein referred to as “5′-C32-”) or a 5′-cholesteryl derivative group (herein referred to as “5′-Chol-”) is performed as described in WO 2004/065601, except that, for the cholesteryl derivative, the oxidation step is performed using the Beaucage reagent in order to introduce a phosphorothioate linkage at the 5′-end of the nucleic acid oligomer.
- Nucleic acid sequences are represented below using standard nomenclature, and specifically the abbreviations of Table 1.
-
TABLE 1 Abbreviations of nucleotide monomers used in nucleic acid sequence representation. It will be understood that these monomers, when present in an oligonucleotide, are mutually linked by 5′-3′-phosphodiester bonds. Abbreviation Nucleotide(s) A adenosine C cytidine G guanosine T thymidine U uridine N any nucleotide (G, A, C, or T) a 2′-O- methyladenosine c 2′-O- methylcytidine g 2′-O- methylguanosine u 2′-O-methyluridine sT phosphorothioate linkage - U87MG (U87Par) cells were engineered to over-express wtEGFR (U87wt), mutant EGFR (de 2-7EGFR, deltaEGFR, ΔEGFR or EGFRvIII) (U87Δ) or the kinase-deficient AEGFR (U87ΔK). Cell lines that were mixed with U87wt were engineered to express the LacZ gene and so could be distinguished by X-Gal staining. Cell populations expressing equivalent levels of over-expressed receptors were selected by FACS. 1×106U87wt cells (
U87wt 100%), or these cells mixed with U87Par, U87ΔK or U87Δ in ratios 90-10% or 99-1%, were injected subcutaneously into the right flank of 4 to 5 weeks-old female athymic nude mice and tumor volume was measured periodically. Tumor growth kinetics (FIG. 1A ) and tumor volume at the end of the experiment (FIG. 1B ) were measured. - A strong tumor growth enhancement was observed when U87wt cells were co-injected with U87Δ at either 90-10% or 99-1% ratios. This tumor potentiation was not observed when U87wt cells were injected together with U87Par or U87AK, corroborating our hypothesis that deltaEGFR induces enhanced proliferation, and that the enhanced proliferation is dependent on the kinase activation of deltaEGFR. Tumor volumes at
day 21 after injection of U87wt +U87delta cells were significantly bigger than the theoretical volume of the sum of two different population volumes. - 0.5×106 cells (100%) were injected intracranially into nude mice using a guide-screw system as described by Lal S. et al. (J Neurosurg 92(2):326-333, 2000). Time matched mice were sacrificed and brains were removed, fixed in 4% PFA and embedded in OCT. H&E stain was performed on 6 μm cryo-sections in order to compare tumor size between U87wt, U87Δ and mixtures 90-10% or 99-1% of U87wt and U87Δ, respectively. Similar to the results in
FIGs. 1A and 1B , a strong tumor growth potentiation was observed when U87wt cells were mixed with U87Δ. This effect was more evident when injections were performed with 99% U87wt with 1% U87Δ (FIG. 2A ). No tumor enhancement was observed when U87wt cells were mixed with U87Par or U87ΔK, confirming the role of catalytically active ΔEGFR in the tumorigenic growth promotion of glioma cells expressing wtEGFR. These results indicated that U87Δ not only can enhance heterogeneous tumor growth subcutaneously, but also intracranially. - To confirm that these results were not cell line specific, the trans-proliferation model was further tested with murine Ink4/Arf−/− astrocytes engineered to over-express wt and deltaEGFR. Previously it had been shown that Ink4/Arf−/− deltaEGFR astrocytes are tumorigenic upon intracranial injection in nude mice. However Ink4/Arf−/−, wtEGFR astrocytes require the introduction of EGF to elicit this effect (Bachoo et al., Cancer Cell 1:269-77, 2002). It was therefore hypothesized that deltaEGFR over-expressing astrocytes might be able to promote the tumorigenicity of wtEGFR over-expressing astrocytes if the cells were co-injected. In order to demonstrate the presence of wtEGFR astrocytes within the tumor, these cells were tagged with nuclear GFP and injected either alone or mixed with deltaEGFR over-expressing astrocytes (
FIG. 2B ). As shown in the upper panel ofFIG. 2B , tumor size atday 22 after injection was significantly bigger in mice co-injected with 90% wtEGFR and 10% deltaEGFR Ink4/Arf−/− murine astrocytes than with 10% of deltaEGFR Ink4/Arf−/− astrocytes alone. It was also confirmed that wtEGFR astrocytes did not form tumors. These results not only demonstrated that there was tumor growth potentiation when wtEGFR astrocytes were mixed with deltaEGFR astrocytes, but also showed the presence of wtEGFR astrocytes within the tumor by immunofluorescence (GFP IF) (FIG. 2B , lower panel). Interestingly, a small number of GFP positive cells were detected in mice injected with wtEGFR astrocytes only, which may represent dormant cells that could be activated upon activation of the receptor. - To determine the relative cell type composition of the mixed U87 xenografts, X-Gal staining was performed to detect LacZ-tagged U87delta cells. Representative images of X-Gal stained tumors obtained after injection of admixed U87wt and U87delta cells show a significant number of U87delta cells (LacZ positive) among U87wt cells (LacZ negative). Specifically, the large tumors that resulted from an initial inoculum ratio of 90% U87wt combined with 10% U87delta cells resulted in a final composition of 51.5% U87wt and 48.5% U87delta cells, while tumors from mice injected with 99% U87wt plus 1% U87delta were composed of 58.7% U87wt and a 41.3% U87delta cells. In contrast, the small tumors formed by the mixture of U87wt with U87ΔK or U87Parental cells were predominantly composed of U87wt cells (
FIG. 3A ). Notably, the absolute tumor volume attributable to U87 wt cells was approximately 3-10-fold greater in tumors resulting from an injection of U87wt plus U87delta cells than in tumors resulting from an injection of U87wt cells alone, or U87wt with U87ΔK or U87Parental cells, demonstrating the dramatic growth effect of U87Δ cells on U87wt cells within the same tumor. - To more accurately quantify tumor composition, differential fluorescence activated cell sorting analysis was performed on single cell suspensions of subcutaneous xenografted mixed tumors stained with two different antibodies against EGFR, one antibody that recognizes the wt and mutant receptor (Ab-1) and another antibody that only recognizes the wt receptor (Ab-5). Using this approach, tumors generated 24 days post injection of 90% U87wt plus 10% U87delta cells were shown to consist of 52.8±17.3% (mean±SD) wt and 47.2±17.3% deltaEGF receptors (
FIG. 3B ), confirming the LacZ staining results. This further illustrates that DEGFR-expressing cells do not exert an overwhelmingly dominant growth advantage in heterogeneous tumors containing amplified levels of wtEGFR, but rather stimulate the latter to grow more robustly. - To analyze the effect of deltaEGFR cells on wtEGFR activation, conditioned media was collected from 48h-starved deltaEGFR cells and used to stimulate U87wt cells, also starved 48h. Western blot analysis of EGFR activation and known signaling molecules downstream of the receptor was performed on lysates of U87wt cells stimulated for 15 minutes with serial dilutions of U87delta CM, negative control U87Par CM or positive control EGF ligand. Membranes were interrogated with anti-pTyr monoclonal antibody (4G10) to check the activation of the EGFR, and with phospho-specific antibodies directed to the major known transduction proteins involved in tumorigenesis in GBMs (gliobastoma multiformes): Akt, ERK1/2 (a.k.a., MAPK) and STAT3.
-
FIG. 4 shows that these pathways were activated in response to the U87Δ CM stimulation, as shown by the increase of the phosphorylated forms of those proteins in a dose-dependent manner. In contrast, CM from U87Par cells failed to activate EGFR or any of these pathways. Notably the level of phosphorylation of these proteins, except EGFR, is at the same extent for the undiluted conditioned medium and the high dose of EGF that was used, indicating that activation of EGFR downstream signaling is efficiently mimicked by factors secreted from U87Δ. Activation of these pathways was also elicited in part by abundant IL-6 produced and secreted by the deltaEGFR-expressing cells. - Similarly, wtEGFR phosphorylation was observed to be significantly higher (p<0.05) in mixed tumors than in tumors obtained after injection of U87wt alone (0.576±0.166 vs 0.19±0.007), while no differences in phosphorylation were detected for ΔEGFR in the mixed tumors compared to the tumors generated from injection of U87Δ alone (1.215±0.225 vs 1.179±0.260).
- To determine whether the activation of these intracellular pathways is dependent on the kinase activity of EGFR, the effect of Δ CM in the presence of the EGFR inhibitor AG1478 was analyzed. Pre-treating U87wt cells with this inhibitor for 30 minutes prior to Δ CM stimulation, completely abolished the activation of EGFR. Moreover, it was also observed that activation of Akt and ERK was maintained at the level of untreated cells. Conversely, the activation of STAT3 caused by exposure to the Δ CM was not affected by blocking the activity of EGFR with this inhibitor. These results suggested that at least two (or more) soluble factors exist in the Δ CM, producing different effects on target U87wt cells: (i) activation of EGFR and its intracellular signaling surrogates (Akt, ERK), via receptor kinase activity, and (ii) STAT3 activation independent of EGFR stimulation.
- The results obtained with A CM would suggested that U87Δ cells secrete one or more factors responsible for EGFR activation in wtEGFR cells. In order to test whether these were known ligands for the EGFR, neutralization using an EGFR ligand trap was performed. This recombinant ligand-binding protein consisted of the extracellular portion of the EGFR bound to a human Fc fragment and bound the known EGFR ligands with high affinity. Pre-incubation of recombinant EGF with 10 μg/m1 of the ligand trap reduced the activation of EGFR as well as Akt and ERK, however, no effect on either EGFR or these two downstream pathways was observed when Δ CM was pretreated with the ligand trap. As expected, STAT3 activation was not affected in the presence of the ligand trap. To further demonstrate that EGFR ligands were not involved in the transactivation of U87wt by Δ CM, ELISA was used to quantify the concentrations of EGF, TGF-a, amphiregulin, HB-EGF and betacellulin in U87Δ CM as well as in control U87Parental, U87wt and U87ΔK CM. All of these tested EGFR ligands were either undetectable or showed no significant increase in expression in Δ CM when compared to the conditioned media from the other cell lines. Given the possibility that active soluble EGFR ligands may be released from the surface of the target cells by proteolytic cleavage of membrane-anchored precursors (Sanderson et al., 2006), A CM was tested for the ability to induce the release of EGFR ligands expressed on the surface of U87wt cells. U87wt cells were stimulated with serum-free medium, recombinant EGF and U87Δ CM, and then these media were collected to analyze by ELISA changes in EGFR ligand concentration after the stimulation. None of the tested ligands showed any significant change in concentration, indicating that EGFR is not stimulated by soluble factors shed from the membrane of wtEGFR cells. Confirmation of these results was achieved by incubation of A CM with neutralizing antibodies against each EGFR ligand; these also failed to block the ability of the CM to activate EGFR, while each antibody tested was able to block the activity of the respective recombinant ligand. In summary, U87Δ cells do not produce a detectable EGF family ligand activity, pointing to other factors driving inter-cellular activation of wtEGFR.
- To identify soluble factors expressed by U87Δ cells that could potentially mediate intercellular communication with and promote the proliferation of U87wt cells, a cytokine array was used to qualitatively detect 79 human cytokines and growth factors in supernatants of cultured cells. With this approach, IL-6 was found to be significantly upregulated in U87Δ cells compared to the other U87 cell lines. To further quantify IL-6 upregulation in U87Δ CM, an ELISA assay was performed on supernatants from the different U87 cell lines collected after 48 hours starvation. The values obtained (pg/ml/4×105 cells) illustrate a 13-fold increase of IL-6 secretion for U87Δ (3813±2) compared to U87Parental cells (299±25), while no significant increase was detected with U87wt (567±85) or U87ΔK (355±75) CM.
- Nineteen GBM tumor samples, U87 cell lines and one normal brain sample were analyzed for ΔEGFR and IL-6 RNA expression by real time PCR. As expected, U87Δ demonstrated a significant higher (p<0.001) expression of IL-6, while no significant differences in IL-6 expression were observed between the rest of U87 cell lines. Notably, we observed a very significant correlation between ΔEGFR and IL-6 expression (p=0.0034) in the GBM tumor samples. All tumor samples that presented ΔEGFR expression also showed high IL-6 expression (8/8), while only three tumors that did not show ΔEGFR expression over-expressed IL-6 (3/11).
- The present inventors observed that in vitro treatment of cells expressing wtEGFR with conditioned media from cells overexpressing deltaEGFR resulted in activation of STAT3, Akt, Erk1/2 and wtEGFR. In vivo tumor growth potentiation was also observed when wtEGFR overexpressing cells were mixed with deltaEGFR expressing cells, but not when those cells were mixed with cells with normal levels of wtEGFR or overexpressing a dead kinase version of deltaEGFR. Based on these observations, siRNA technology was used to knock-down either wt or deltaEGFR to assess the effect of specific receptor ablation on tumorigenicity and contribution to heterogeneity. As shown below, siRNAs specific for deltaEGFR or wtEGFR were able to reduce tumor growth after subcutaneous injection of ex vivo transfected cells.
- In vitro siRNA Screening:
- siRNAs non-stabilized (Table 2) and stabilized (Table 3), designed to be specific (i) to wtEGFR, (ii) to deltaEGFR, and (iii) to both receptors, were synthesized. U87-wtEGFR (Nagane et al, Cancer Research, 56: 5079-5086, 1996) and U87-deltaEGFR (Nishikawa et al, PNAS, 91: 7727-7731, 1994) cells were used as test cell lines to assess the specificity of these siRNA molecules. The term “deltaEGFR,” as used in this example, refers to an EGFR gene construct deleted for exons 2-7. U87-deltaEGFR cells are recombinant cells expressing an EGFR gene deleted for exons 2-7 as described in Nishikawa et al. (1994). U87-wtEGFR cells are recombinant cells expressing a wtEGFR gene as described in Nishikawa et al. (1994).
- Cells were seeded in 24 well plates at 48,000 cells per well in DMEM medium (Cellgro) supplemented with 10% fetal bovine serum and L-Glutamine. The following day, siRNAs were transfected at 100 nM, 10 nM and 1 nM concentrations using Lipofectamine™ 2000 (Invitrogen) and OptiMEM (Gibco). Twenty four hours after transfection, the medium was changed to DMEM supplemented with 10% fetal bovine serum, penicillin/streptomycin and L-Glutamine.
- Cells non-transfected as well as transfected with a siRNA specific for GFP or Luciferase protein were included as negative controls.
- Three days after transfection, protein lysates were prepared using RIPA buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.5, 1 mM EDTA, 1% NP-40, 0.1% SDS and 0.5% sodium deoxycholate) supplemented with protease inhibitors (Roche) and 5μg of protein were resolved on 12% NuPAGE Bis-Tris acrylamide gels (Invitrogen). Gels were blotted onto nitrocellulose membranes, blocked with 5% milk in TBS-Tween and probed with anti-EGFR monoclonal antibody, C13, which recognizes both wt and mutant EGFR (BD). Membrane bound C13 antibody was detected with HRP-conjugated anti-mouse IgG (Dako) followed by chemiluminiscence. Nitrocellulose membranes were also probed with anti-actin antibody as a positive control for protein loading.
- In vitro Specificity Test:
- Specificity for the receptor for which each siRNA candidate was designed was assessed by transfecting siRNAs at 100 nM dose in the U87 cell line that expresses the other receptor. Expression knock-down was assessed by western blot as previously described.
- To exclude that any reduction in the cytokine synthesis was caused by off-target effects of the siRNAs, the concentration of both IL-6 and IL-8 was measured in the samples where an siRNA was found to have a strong effect on the expression of IL-6, and compared to non-transfected cells as well as cells transfected with an siRNA against GFP or Luciferase
- Cells non-transfected as well as transfected with an siRNA against GFP or Luciferase protein were included as negative controls.
- In vitro Dose-Response Analysis:
- siRNAs determined to be specific and able to knock-down the expression of the receptor for which they were designed were tested again in U87-deltaEGFR and U87-wtEGFR to determine the minimal effective dose to achieve receptor expression knock-down. Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 100, 25, 5 and 1 nM concentrations using
Lipofectamine™ 2000. Twenty four hours after transfection, medium was changed and two days after protein lysates were obtained as described previously. Cells non-transfected as well as transfected with a siRNA against GFP or Luciferase protein were included as negative controls. Receptor expression was analyzed by western blot as described previously. - In Vitro Durability Test:
- siRNAs determined to be robust in the ablation of deltaEGFR or wtEGFR expression, when transfected at low concentration, were tested for suppression durability using U87-deltaEGFR and U87-wtEGFR cell lines.
- Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 25 nM concentration using
Lipofectamine™ 2000. Protein lysates were obtained using RIPA buffer supplemented with protease inhibitors atdays - Ex vivo Experiments:
- To determine the effect of specific receptor knock-down on tumorigenicity, cells were transfected and then injected subcutaneously into nude mice.
- Briefly, 25 cm2 plates were seeded with 1.3×106 cells and one day after they were transfected with 25 nM or 100 nM siRNA using
Lipofectamine™ 2000. The following day, the medium was changed and cells were split into larger dishes when they were almost confluent. - Cells non-transfected as well as transfected with a siRNA against GFP or Luciferase protein were included as negative controls.
- One million U87-wtEGFR or 0.5×106 U87-deltaEGFR transfected cells were resuspended in 100 μl PBS. Cells were injected subcutaneously into the right flank of Nu/Nu mice using a 1 ml syringe with a 26 G needle. Tumor volume was measured starting at
day 5 after injection and was calculated using the formula 0.5×L×W2. Mice were euthanized when tumor volume reached 1500 mm3. - Protein lysates were prepared from the remaining cells of the injection using RIPA buffer supplemented with protease inhibitors. Receptor expression was analyzed by western blot as described previously.
- Results
- In Vitro siRNA Screening (Table 4):
- Non-Stabilized siRNAs:
- DeltaEGFR-specific siRNAs: Western blot analysis demonstrated that 7 of 8 deltaEGFR-specific siRNAs were capable of complete elimination of the mutant receptor expressed in U87-deltaEGFR cells when tested at 100 nM (AL-DP-6901-6907) while three (AL-DP-6902, -6903, -6906) were able to effect a modest receptor reduction as low as 1 nM concentration (Table 4 and
FIG. 4A ). One siRNA, AL-DP-6908, had no effect. To show specificity for the mutant receptor, each deltaEGFR-specific siRNA was tested against U87-wtEGFR cells. Only one of the siRNAs (AL-DP-6906) that reduced deltaEGFR expression was also able to reduce wtEGFR expression. - wtEGFR-specific siRNAs: As above, western blot analysis was used to demonstrate that 5 of 8 wtEGFR-specific siRNAs were capable of complete elimination of the wt receptor expressed in U87-wtEGFR cells when tested at 100 nM (AL-DP-6918-6921 and -6923), while two (AL-DP-6919 and -6920) were able to effect a modest receptor reduction as low as 1 nM concentration (Table 4 and
FIG. 5B ). Three siRNAs (AL-DP-6917, -6922, and -6924) had little or no effect. To show specificity for the wt receptor, each active wtEGFR-specific siRNA (AL-DP-6918-6921 and -6923) was tested against U87-deltaEGFR cells with none showing reduction of the mutant receptor. - Wt and deltaEGFR-specific siRNAs: As above, western blot analysis was used to demonstrate the specificity of 8 siRNAs designed to knock-down the expression of both wt and deltaEGFR. Of this series, three siRNAs were able to simultaneously knock-down both receptors (AL-DP-6913, -6915, and -6916) albeit the effect was stronger for suppressing deltaEGFR (Table 4 and
FIG. 5C ). - In
FIGS. 5A-5C , two samples were run for each treatment condition. Membranes were blotted with c13 antibody to detect EGFR and beta-actin antibody to confirm equivalent loading between lanes. - Stabilized siRNAs:
- DeltaEGFR-specific siRNAs: Western blot analysis demonstrated that 8 of 8 deltaEGFR-specific siRNAs were capable of reducing mutant receptor expression in U87-deltaEGFR cells when tested at 25 nM (Table 4 and
FIGS. 6A-B ). To show specificity for the mutant receptor, deltaEGFR-specific siRNAs (AD-15416, AD-15417, AD-13375, AD-15418) were tested against U87-wtEGFR cells. None of siRNAs tested resulted in reduced wtEGFR expression. - wtEGFR-specific siRNAs: Western blot analysis demonstrated that 4 of 8 wtEGFR-specific siRNAs strongly suppress wtEGFR protein expression (AD-16177, AD-16178, AD-16179 and AD-13377), while 2 of these 8 siRNAs were able to moderately reduce wtEGFR protein expression (AD-13376 and AD-13378) (Table 4 and
FIG. 6C ). Two of the 8 siRNAs had little or no effect on wtEGFR protein levels (AD-16180 and AD-16181). - In
FIGS. 6A-6C , two samples were run for each treatment condition. Membranes were blotted with c13 antibody to detect EGFR and beta-actin antibody to confirm equivalent loading between lanes. - In Vitro Dose-Response Analysis:
- Non-Stabilized siRNAs:
- DeltaEGFR-specific siRNAs: Western blot analysis demonstrate that the four deltaEGFR-specific siRNAs tested (AL-DP-6901-6903, and -6905) were capable of complete elimination of the mutant receptor expressed in U87-deltaEGFR cells when transfected at 25 nM, nearly complete elimination at 5 nM and partial elimination at 1 nM (
FIG. 7A ). - wtEGFR-specific siRNAs: As above, western blot analysis was used to demonstrate that 4 of 4 wtEGFR-specific siRNAs (AL-DP-6918-6921) were capable to varying degrees of wt receptor elimination in U87-wtEGFR cells (
FIG. 7B ). - Wt and deltaEGFR-specific siRNAs: AL-DP-6913 and AL-DP-6916 were able to suppress expression of both wt and deltaEGFR protein in a dose-dependent manner (
FIGS. 7A-B ). - In
FIGS. 7A and 7B , two samples were run for each treatment condition. Membranes were blotted with c13 antibody to detect EGFR. - In Vitro Durability Test:
- Non-Stabilized siRNAs:
- DeltaEGFR-specific siRNAs: Western blot analysis demonstrated that 4 of 4 deltaEGFR-specific siRNAs analyzed (AL-DP-6901, AL-DP-6902, AL-DP-6903, AL-DP-6905) were capable of complete elimination of mutant receptor expression in U87-deltaEGFR cells at 7 days after siRNA transfection, while siRNA AL-DP-6905 was capable of durable suppression as far as 10 days after siRNA transfection (
FIG. 8A ). - wtEGFR-specific siRNAs: As above, western blot analysis was used to demonstrate that 2 of 2 wtEGFR-specific siRNAs analyzed (AL-DP-6920, AL-DP-6921) were capable of complete wt receptor expression elimination in U87-wtEGFR cells out to 7 days after siRNA transfection; however, for both siRNAs, receptor levels were restored to control levels by 10 days post transfection (
FIG. 8B ). - Stabilized siRNAs:
- DeltaEGFR-specific siRNAs: Western blot analysis demonstrated that 3 of 5 deltaEGFR-specific siRNAs analyzed (AD-15416, AD15417, AD-13374, AD-13375, AD-15418) were capable of complete elimination of mutant receptor expression in U87-
deltaEGFR cells 5 days post transfection, while siRNA AD-15416 was capable of durable and complete suppression as far as 7 days post transfection (FIG. 8C ). Receptor expression levels were completely restored byday 12 post transfection for all stabilized siRNAs tested. - wtEGFR-specific siRNAs: As above, western blot analysis was used to demonstrate that AD-13377 wtEGFR-specific siRNA was capable of complete wt receptor protein elimination in U87-wtEGFR cells out to 7 days after transfection (
FIG. 8D ). However, receptor levels were restored to control levels by 10 days post transfection. - Ex Vivo Experiments:
- Non-Stabilized siRNAs:
- DeltaEGFR-specific siRNAs: U87-deltaEGFR cells were transfected with 100 nM siRNA and injected subcutaneously into nude mice. Treatment with siRNAs AL-DP-6901 and AL-DP-6902 (
FIG. 9B ) resulted in a substantial reduction of U87-deltaEGFR tumor growth. As a negative control, cells non transfected or transfected with an irrelevant GFP siRNA were included in the study (FIGS. 9B ). In both of these groups, substantial tumor growth occurred. - wtEGFR-specific siRNAs: U87-wtEGFR cells were transfected with 25 nM siRNA concentration and injected subcutaneously into nude mice. Treatment with siRNAs AL-DP-6920 and AL-DP-6921 (
FIG. 9A ) resulted in ablation of U87-wtEGFR tumor growth. As a negative control, cells non transfected or transfected with an irrelevant GFP siRNA were included in the study (FIG. 9A ). In both of these groups, substantial tumor growth occurred. - In
FIGS. 9A and 9B , each treatment group included six animals. - U87delta cells were injected into nude mice and at days 13 (approximately 160 mm3) and 16, 5 mg of
deltaEGFR siRNA# 1 or irrelevant siRNA (siRNA luc) were administrated intratumorally using JetPei (Polyplus) following the manufacturer's instructions followed by the monitoring of tumor growth as above. These initial results illustrate modest tumor reduction can be achieved in vivo with siRNAs targeting deltaEGFRFIGS. 10A and 10B . -
TABLE 2 Sequences of dsRNAs tested for deltaEGFR and wtEGFR gene expression inhibiting activity Target position of 5′ base of sense strand (see FIGS. 21A SEQ SEQ Duplex and ID Sense strand sequence ID Antisense strand name 21B) Specificity NO: (5′-3′) NO: sequence (5′-3′) AL-DP-6901 320 deltaEGFR 2 UGGAGGAAAAGAAAGGUAATT 3 UUACCUUUCUUUUCCUCCATT AL-DP-6902 321 deltaEGFR 4 GGAGGAAAAGAAAGGUAAUTT 5 AUUACCUUUCUUUUCCUCCTT AL-DP-6903 322 deltaEGFR 6 GAGGAAAAGAAAGGUAAUUTT 7 AAUUACCUUUCUUUUCCUCTT AL-DP-6904 319 deltaEGFR 8 CUGGAGGAAAAGAAAGGUATT 9 UACCUUUCUUUUCCUCCAGTT AL-DP-6905 323 deltaEGFR 10 AGGAAAAGAAAGGUAAUUATT 11 UAAUUACCUUUCUUUUCCUTT AL-DP-6906 324 deltaEGFR 12 GGAAAAGAAAGGUAAUUAUTT 13 AUAAUUACCUUUCUUUUCCTT AL-DP-6907 325 deltaEGFR 14 GAAAGGUAAUUAUGUGGUGTT 15 CACCACAUAAUUACCUUUCTT AL-DP-6908 329 deltaEGFR 16 AGAAAGGUAAUUAUGUGGUTT 17 ACCACAUAAUUACCUUUCUTT AL-DP-6909 40 deltaEGFR 18 ACGGUGUGAGCGCCCGACGTT 19 CGUCGGGCGCUCACACCGUTT and wtEGFR AL-DP-6910 1150 deltaEGFR 20 ACAGAUCACGGCUCGUGCGTT 21 CGCACGAGCCGUGAUCUGUTT and wtEGFR AL-DP-6911 1156 deltaEGFR 22 CACGGCUCGUGCGUCCGAGTT 23 CUCGGACGCACGAGCCGUGTT and wtEGFR AL-DP-6912 117 deltaEGFR 24 CGACAGGCCACCUCGUCGGTT 25 CCGACGAGGUGGCCUGUCGTT and wtEGFR AL-DP-6913 1147 deltaEGFR 26 GUGACAGAUCACGGCUCGUTT 27 ACGAGCCGUGAUCUGUCACTT and wtEGFR AL-DP-6914 129 deltaEGFR 28 UCGUCGGCGUCCGCCCGAGTT 29 CUCGGGCGGACGCCGACGATT and wtEGFR AL-DP-6915 197 deltaEGFR 30 CCGUCCAGUAUUGAUCGGGTT 31 CCCGAUCAAUACUGGACGGTT and wtEGFR AL-DP-6916 1146 deltaEGFR 32 GGUGACAGAUCACGGCUCGTT 33 CGAGCCGUGAUCUGUCACCTT and wtEGFR AL-DP-6917 997 wtEGFR 34 UGCCGCAAAUUCCGAGACGTT 35 CGUCUCGGAAUUUGCGGCATT AL-DP-6918 683 wtEGFR 36 GCGCCGUGCGGUUCAGCAATT 37 UUGCUGAACCGCACGGCGCTT AL-DP-6919 999 wtEGFR 38 CCGCAAAUUCCGAGACGAATT 39 UUCGUCUCGGAAUUUGCGGTT AL-DP-6920 337 wtEGFR 40 UGCCAAGGCACGAGUAACATT 41 UGUUACUCGUGCCUUGGCATT AL-DP-6921 569 wtEGFR 42 GAGGAAAUAUGUACUACGATT 43 UCGUAGUACAUAUUUCCUCTT AL-DP-6922 668 wtEGFR 44 AGGAAAUCCUGCAUGGCGCTT 45 GCGCCAUGCAGGAUUUCCUTT AL-DP-6923 677 wtEGFR 46 UGCAUGGCGCCGUGCGGUUTT 47 AACCGCACGGCGCCAUGCATT AL-DP-6924 732 wtEGFR 48 CCAGUGGCGGGACAUAGUCTT 49 GACUAUGUCCCGCCACUGGTT -
TABLE 3 Sequences of dsRNAs with stabilizing modifications tested for deltaEGFR and wtEGFR gene expression inhibiting activity Target position of 5′ base of sense strand (see FIGS. SEQ SEQ Duplex 21A and ID Sense strand sequence ID Antisense strand name 21B) NO: (5′-3′) NO: sequence (5′-3′) AD- 320 50 uGGAGGAAAAGAAAGGuAATsT 51 UuACCUUUCUUUUCCUCcATsT 15416 AD- 321 52 GGAGGAAAAGAAAGGuAAuTsT 53 AUuACCUUUCUUUUCCUCCTsT 15417 AD- 322 54 GAGGAAAAGAAAGGuAAuuTsT 55 AAUuACCUUUCUUUUCCUCTsT 13373 AD- 319 56 cuGGAGGAAAAGAAAGGuATsT 57 uACCUUUCUUUUCCUCcAGTsT 13374 AD- 323 58 AGGAAAAGAAAGGuAAuuATsT 59 uAAUUACCUUUCUUUUCCUTST 13375 AD- 324 60 GGAAAAGAAAGGuAAuuAuTsT 61 AuAAUuACCUUUCUUUUCCTST 15418 AD- 325 62 GAAAGGuAAuuAuGuGGuGTsT 63 cACcAcAuAAUuACCUUUCTsT 15419 AD- 329 64 AGAAAGGuAAuuAuGuGGuTsT 65 ACcAcAuAAUuACCUUUCUTsT 15420 AD- 997 66 uGccGcAAAuuccGAGAcGTsT 67 CGUCUCGGAAUUUGCGGcATsT 16177 AD- 683 68 GcGccGuGcGGuucAGcAATsT 69 UUGCUGAACCGcACGGCGCTsT 13376 AD- 999 70 ccGcAAAuuccGAGAcGAATsT 71 UUCGUCUCGGAAUUuGCGGTsT 16178 AD- 337 72 uGccAAGGcAcGAGuAAcATsT 73 UGUuACUCGUGCCUUGGcATsT 16179 AD- 569 74 GAGGAAAuAuGuAcuAcGATsT 75 UCGuAGuAcAuAUUUCCUCTsT 13377 AD- 668 76 AGGAAAuccuGcAuGGcGcTsT 77 GCGCcAUGcAGGAUUUCCUTsT 16180 AD- 677 78 uGcAuGGcGccGuGcGGuuTsT 79 AACCGcACGGCGCcAUGcATsT 13378 AD- 732 80 ccAGuGGcGGGAcAuAGucTsT 81 GACuAUGUCCCGCcACUGGTsT 16181 -
TABLE 4 Summary of the western blot results of the knock-down of delta or wtEGFR expression after transfection of non-stabilized or stabilized siRNAs. U87- U87- Unmodified delta U87-wt Durability Modified delta U87-wt Durability siRNA Specificity EGFR EGFR (days) siRNA EGFR EGFR (days) AL-DP-6901 deltaEGFR + − 7-10 AD-15416 + − 7-12 AL-DP-6902 deltaEGFR + − 7-10 AD-15417 + − 7-12 AL-DP-6903 deltaEGFR + − 7-10 AD-13373 + x x AL-DP-6904 deltaEGFR + − x AD-13374 + x 5-7 AL-DP-6905 deltaEGFR + − 10-14 AD-13375 + − 5-7 AL-DP-6906 deltaEGFR + + x AD-15418 + − 7-12 AL-DP-6907 deltaEGFR + x x AD-15419 + x x AL-DP-6908 deltaEGFR - x x AD-15420 + x x AL-DP-6909 both +/− + x none na na na AL-DP-6910 both + − x none na na na AL-DP-6911 both − − x none na na na AL-DP-6912 both − − x none na na na AL-DP-6913 both + + 7-10 none na na na AL-DP-6914 both − − x none na na na AL-DP-6915 both + + x none na na na AL-DP-6916 both + + 7-10 none na na na AL-DP-6917 wtEGFR x +/− x AD-16177 − +/− x AL-DP-6918 wtEGFR − + x AD-13376 x +/− x AL-DP-6919 wtEGFR − + x AD-16178 − + x AL-DP-6920 wtEGFR − + 7-10 AD-16179 − + x AL-DP-6921 wtEGFR − + 7-10 AD-13377 − + ≥7 AL-DP-6922 wtEGFR x − x AD-16180 x − x AL-DP-6923 wtEGFR − + x AD-13378 x − x AL-DP-6924 wtEGFR x − x AD-16181 x − x − = no reduction of expression + = reduction of expression +/− = small reduction of expression x = not determined na = not available - PCT/US2009/055745
- Methods
- In vitro siRNA screening: U87-ΔEGFR (Nishikawa et al, PNAS 91: 7727-7731, 1994) cells over-express the IL-6 cytokine, and the importance of IL-6 secretion was underscored by demonstrating that the in vivo growth of wtEGFR-expressing cells could be enhanced when mixed with parental glioma cells engineered to overexpress IL-6 (see Example 10 below).
- To further test the role of IL-6 in enhanced cell proliferation, 24 stabilized siRNAs were designed to be specific to IL-6 (AD-15637 to AD-15660) and were synthesized. The sequences of the siRNAs and their target position on the IL-6 mRNA (GenBank Accession No. NM_000600.2, version Jan. 4, 2009) (
FIG. 11 ) are provided in Table 5. - Cells were seeded in 24 well plates at 48,000 cells per well in DMEM medium (Cellgro) supplemented with 10% fetal bovine serum and L-Glutamine. The following day, siRNAs were transfected at 100 nM using Lipofectamine™ 2000 (Invitrogen™) and Opti-MEM® (Gibco™). Cells non-transfected as well as transfected with an siRNA specific for GFP or Luciferase were included as negative controls. Forty-eight hours after transfection, the medium was changed to DMEM-serum-free supplemented with penicillin/streptomycin and L-Glutamine after washing the cells with serum-free medium. After twenty-four hours of serum-starvation, supernatants were collected, centrifuged to remove cell debris, and either analyzed immediately or frozen at −80° C. Quantification of IL-6/IL-8 in supernatants was assessed by ELISA. Briefly, 96-well plates (MaxiSorp, Nunc) were coated overnight at room temperature with the capture antibody diluted in PBS. The following day, the plates were blocked in blocking buffer composed of PBS containing 1% BSA and 5% sucrose. The standards (recombinant human IL-6 and IL-8) and the samples were diluted in diluent buffer (1X TBS, 0.5% BSA, 0.05% Tween-20) and incubated 2 hours at room temperature. The plates were then washed with PBS 0.05% Tween-20 and incubated with the biotinylated detection antibody and then with streptavidin-HRP (Biosource) both diluted in diluent buffer. The HRP activity was determined by using Tetramethylbenzidine (Sigma) as substrate. The enzymatic reaction was stopped with 1 N sulfuric acid and the absorbance was measured at 450 nm with wavelength correction set to 540 nm using a Tecan Genios Pro microplate reader. The absorbance readings were converted using a four parameter logistic curve.
- In vitro specificity test: To exclude that any reduction in the cytokine synthesis was caused by off-target effects of the siRNAs, the concentration of both IL-6 and IL-8 was measured in the samples where an siRNA was found to have a strong effect on the expression of IL-6, and compared to non-transfected cells as well as cells transfected with an siRNA against GFP or Luciferase.
- In vitro dose-response analysis: siRNAs determined to be specifically able to knock-down the secretion of IL-6 were tested again in U87-ΔEGFR to determine the minimal effective dose to achieve cytokine secretion knock-down. Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 100, 20, 4 and 0.8 nM concentrations using
Lipofectamine™ 2000. Forty-eight hours after transfection the medium was changed to serum-free medium and twenty-four hours later the supernatants were collected and centrifuged as described previously. Cells non-transfected as well as transfected with an siRNA against GFP or Luciferase were included as negative controls. IL-6 or IL-8 secretion was analyzed by ELISA. - In vitro durability test: siRNAs determined to be robust in the ablation of IL-6 secretion, when transfected at low concentration, were tested for suppression durability using U87-ΔEGFR cells. Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 25 nM concentration using
Lipofectamine™ 2000. Supernatants were collected atdays - In vitro proliferation test: Cells were seeded in 24 well plates at 48,000 cells per well and siRNAs were transfected the following day at 25 nM concentration using
Lipofectamine™ 2000. 48 hours after transfection the cells were trypsinized, counted, and seeded at the same density in larger dishes to allow them to grow. The cell proliferation was evaluated by counting the cells atday 4 and at day 6-8 as indicated. Three independent samples were counted for each treatment/time point. - Ex vivo experiments: To determine the effect of specific IL-6 knock-down on tumorigenicity, U87-ΔEGFR cells were transfected with the different siRNAs (AD-15644 and AD-15660) and then injected subcutaneously into nude mice. Briefly, 1.3×106 cells were seeded in 10 cm plates and one day after they were transfected either with a control siRNA or with a specific siRNA at the concentration of 25 nM with
Lipofectamine™ 2000. The following day, the medium was changed and cells were split into larger dishes. Transfected U87-ΔEGFR cells were injected subcutaneously into the right flank of Nu/Nu mice using a 1 ml syringe with a 26 G needle. 5×105 cells resuspended in 100 μl of PBS were injected into each mouse. Tumor volume was measured starting atday 5 after injection and was calculated using the formula 0.5×L×W2. Mice were euthanized according to our animal protocol when tumor volume reached 1500 mm3. - In vivo siRNA delivery: One million cells of U87-wtEGFR mixed with U87-ΔEGFR in a ratio 90:10% respectively were injected subcutaneously into 4 to 5 weeks-old female nude mice. Treatment of tumors was started after 13 days when the tumor volume reached approximately 80 mm3. JetPEI/siRNA complexes were prepared following manufacturer instructions (Polyplus Transfection, Illkirch, France) in 5% Glucose at N/P ratio of 15 and 70 μl of the complex was injected intratumorally at a dose of 10 μg siRNA/mouse every two days. Tumor volumes were measured every second day from the commencement of siRNA delivery through
day 21 of treatment. - Results
- In vitro siRNA Screening (Table 9):
- ELISA analysis demonstrated that 20 of 24 siRNAs designed against IL-6 were capable of reducing IL-6 secretion in U87-ΔEGFR cells when tested at 100 nM (Table 9, and
FIGS. 12A and 12B ). Only siRNAs AD-15647, AD-15651, AD-15654, and AD-15656 were unable to reduce IL-6 secretion in U87-ΔEGFR cells (FIG. 12A ). The strongest effect was obtained with AD-15658 (99.09%), and the weakest with AD-15642 (34.1%), with an average 82.4% reduction compared with cells transfected with the control siRNA. IL-8 concentration was tested in the 15 samples (FIG. 12B ) where strongest reduction of IL-6 secretion was observed. IL-8 production was significantly reduced in 11 of the analyzed samples. In one case (AD-15641) there was a significant increase in IL-8 secretion. Two siRNAs designed against IL-6 (AD-15644, AD-15650), showed no significant effect on the IL-8 secretion in U87-ΔEGFR cells demonstrating specificity for IL-6. AD-15660 caused a moderate increase in IL-8 production. - In vitro dose-response analysis: The five siRNAs that showed the least non-specific effects were selected, transfected into U87-ΔEGFR cells in serial 1:5 dilutions starting at 100 nM and compared to a control siRNA at 100 nM.
- ELISA analysis demonstrated that the 5 IL-6-specific siRNAs tested (AD-15644, -15649, -15650, -15657 and -15660) reduced significantly the secretion of IL-6 when transfected into U87-ΔEGFR cells at doses as low as 0.8 nM (
FIG. 13A ). The concentration of IL-8 was also measured in these samples (FIG. 13B ) showing that siRNAs AD-15650 and AD-15660 caused the least significant reduction in IL-8 secretion when compared to control siRNA. - In Vitro Durability Test:
- ELISA analysis demonstrated that two out of four IL-6-specific siRNAs analyzed (AD-15644 and AD-15660) were capable of maximally reducing IL-6 secretion in U87-ΔEGFR cells as far as
day 7 after siRNA transfection. Atday 14 these two siRNAs were still able to suppress IL-6 expression. siRNA AD-15650 was capable of suppression of IL-6 secretion only until day three, and AD-15657 only until day 7 (FIG. 14 ). - In Vitro Proliferation Test:
- The proliferation of U87-ΔEGFR transfected with siRNAs AD-15644, -15650, and -15660 was monitored in vitro for 6 days, and compared with cells non transfected or transfected with a control siRNA. Only AD-15644 and AD-15660 had no effect on cell proliferation, while AD-15650 almost completely blocked cell proliferation in vitro.
- Ex Vivo Experiments:
- U87-ΔEGFR cells were transfected with 25 nM siRNA and injected subcutaneously into nude mice. Treatment with siRNAs AD-15644 and AD-15660 resulted in no substantial reduction of U87-ΔEGFR tumor growth (
FIGS. 15A and 15B ). As a negative control, cells non transfected or transfected with an irrelevant Luciferase siRNA were included in the study. In both groups, substantial tumor growth occurred. - In Vivo siRNA Delivery:
- Two siRNAs (AD-15644 and AD-15660) that demonstrated specificity for IL-6 and showed durable, low dose knock-down were chosen for in vivo studies. Nude mice were injected subcutaneously with 1×106 U87wt or U87Δ cells or with U87wt+U87Δ (90:10%) (
FIGS. 9A and 9B ) and monitored until tumors reached 80 mm3 whereupon ten micrograms of AD-15644 or AD-15660 siRNA against IL-6 or an siRNA against Luciferase gene were injected intratumorally every two days. Tumor growth kinetics (FIG. 16A ) and tumor volume (FIG. 16B ) at the end of the experiment was reduced in tumors treated with IL-6 specific siRNA AD-15660, but not in tumors treated with an siRNA against luciferase or with AD-15644 siRNA. These results illustrate that the emergence of the ΔEGFR oncogene during gliomagenesis not only conveys a cell intrinsic growth potential but also establishes a cell extrinsic potentiation loop to neighboring cells expressing the amplified antecedent genetic lesion. These results also illustrate a potential therapeutic use of IL-6 siRNA to inhibit the tumor enhancement conferred by ΔEGFR on cells over-expressing wtEGFR and demonstrate a role for this cytokine in driving glioma heterogeneity. - To determine whether the over-expression of IL-6 was a mediator of U87wt tumor enhancement through its secretion from U87Δ cells, U87Parental cells, which lack the ability to enhance U87wt tumor growth (
FIG. 1A ), were engineered to over-express IL-6 (U87Par-IL6). U87wt alone or mixed with U87Δ, U87Parental or U87Par-IL6 were injected subcutaneously into nude mice (1×106 total cells) at a ratio of 90:10% (FIG. 17A ) and resultant tumor volumes were measured over 32 days. As controls, mice were injected with 10% of the total cell number (1×105 cells) of U87Δ, U87Parental and U87Par-IL6 cells. As shown inFIG. 17A and 17B , U87Par-IL6 did not grow faster than U87Parental. When they were mixed with U87wt cells, tumor growth kinetics were much faster for the U87wt+U87Par-IL6 mixture than for U87wt+U87Par demonstrating a paracrine tumor enhancement effect mediated by IL-6 secretion in these composite tumors. Even though U87Parental and U87Par-IL6 grew more slowly than U87Δ, we observed a potent tumor enhancement when U87wt were injected with U87Par-IL6 with tumor volumes being nearly double that of U87wt tumors by the end of the experiment (FIG. 17B ). In accordance with the previous results, CM generated from U87Parental-IL6 was able to potently enhanced U87wt colony formation in the in vitro soft agar colony formation assays (FIG. 18 ). U87Parental-IL6 CM was able to enhance U87wt colony formation with the same efficiency as U87Δ CM (p>0.05). In contrast, U87Parental CM was unable to enhance colony formation, and no significant differences were found when U87wt cells were treated with U87Parental or normal media (p>0.05). - Two siRNAs targeting IL-6 (AD-15644 and AD-15660) were studied in an in vitro soft agar colony formation assay (
FIG. 19 ). 5×105 U87Δ cells, U87Δ cells transfected with 25 nM luciferase siRNA or U87Δ cells transfected with 25 nM of a mixture of AD-15644 and AD-15660 (12.5 nM each) were plated in 10 cm2 dishes with 10 ml DMEM supplemented with 10% FBS, penicillin/streptomycin and L-Glutamine, and media was collected after 48 hours. Conditioned medium (CM) or normal medium was filtered and used in the upper layer of agar as well as on top of the agar. Briefly, the bottom layer of agar was prepared by mixing equal volumes of 1.2% agar (USB Corporation) and 2× DMEM/20% FBS solutions. Two ml of the resulting 0.6% agar/1X DMEM/10% FBS solution was added to each well of 6 well/plates and let to solidify at room temperature. The upper layer containing 2.5×103 U87wt cells was prepared by mixing equal volumes of 1.2% agar, 2× DMEM/20%FBS and conditioned media or normal media. Plates were kept at room temperature until top agar solidified and treatment media was added on top of the agar. Plates were placed at 37° C/5% CO2 for three weeks. Once a week, media on top of the agar was replaced with fresh media. Every treatment was performed in triplicate. After three weeks, plates were stained with 0.005% crystal violet solution and pictures were taken using a digital camera illuminated with white light. Colonies were counted using Image Pro-Analyzer 6.2 Software. - CM generated from U87Δ cells transfected with siRNA against the irrelevant luciferase gene (A CM Luc siRNA) resulted in a significant increase in colony number (** p<0.01). In contrast, CM generated from U87Δ cells transfected with a mixture of the IL-6 siRNAs AD-15644 and AD-15660 (Δ CM IL-6 siRNA) resulted in a reduction in soft agar colony number to levels comparable to normal growth media. These results demonstrate that IL-6 produced from U87Δ cells has an important role in the promotion of U87wt cell proliferation, and that siRNAs targeting IL-6 inhibit this promotion of U87wt cell proliferation.
- To study the effect of IL-6 secretion from U87Δ cells on tumor growth enhancement, U87Δ cells were transfected with a 25 nM dose of a combination of IL-6 siRNAs AD-15644 and AD-15660, or an siRNA against the irrelevant luciferase gene as a negative control. 1.3× 106 U87Δ cells were seeded in 10 cm plates and the following day were transfected using 5 Lipofectamine™ 2000 (Invitrogen) and a mixture of IL-6 siRNAs AD-15644 and AD-15660 at a concentration of 12.5 nM each, or an siRNA against the luciferase gene at a concentration of 25 nM. After 18 hours of transfection, the media was changed and cells were partitioned into larger plates. The transfected U87Δ cells (105 cells per mouse, 10%) were injected subcutaneously into nude mice alone or mixed with U87wt cells (9×105 cells per 10 mouse, 90%). Tumors were measured starting at
day 5 after injection and volumes were calculated as described above. - As shown in
FIGS. 14A and 14B , there was no significant difference in tumor volume between U87Δ cells transfected with IL-6 siRNAs or with luciferase siRNA when injected alone (p>0.05). However, tumor volume was significantly reduced when U87wt cells were 15 mixed with U87Δ cells transfected with IL-6 siRNAs compared to U87Δ cells transfected with luciferase siRNA (p<0.05). Tumor volumes obtained at the end of the experiment when U87Δ cells were transfected with IL-6 siRNAs and mixed with U87wt were similar to the sum of the volumes obtained after injection of U87wt (90%) and U87A (10%) indicating a reduction in the proliferation enhancement of U87wt cells induced by U87Δ cells (FIGS. 20A and 20B ). This reduction of U87wt contribution to tumor volume was confirmed when we analyzed the cell composition by flow cytometry. A significant reduction in the proportion of U87wt cells was detected in mixed tumors where IL-6 was knocked-down (41.8±2.73% U87wt cells in U87wt+U87Δ-Luciferase siRNA tumors vs. 32.87±2.28% U87wt cells in U87wt+U87Δ-IL6 siRNA tumors). These results illustrate a potential therapeutic use of IL-6 25 siRNAs to inhibit the tumor enhancement conferred by ΔEGFR on cells over-expressing wtEGFR. -
TABLE 5 Modified siRNAs targeting IL-6. Target position of 5′ base of sense strand s SEQ Sense (S) AS SEQ Antisense (AS) Duplex (see Oligo ID Oligo Sequence Oligo ID Oligo Sequence Name FIG. 11) Name NO: (5′ to 3′) Name NO: (5′ to 3′) AD- 803 25860 82 AAAAGuAuGAGcGuuAGGAdTsdT 25861 83 UCCuAACGCUcAuACUUUUdTsdT 15637 AD- 223 25862 84 uGAcAAAcAAAuucGGuAcdTsdT 25863 85 GuACCGAAUUUGUUUGUcAdTsdT 15638 AD- 802 25864 86 uAAAAGuAuGAGcGuuAGGdTsdT 25865 87 CCuAACGCUcAuACUUUuAdTsdT 15639 AD- 804 25866 88 AAAGuAuGAGCGuuAGGAcdTsdT 25867 89 GUCCuAACGCUcAuACDUUdTsdT 15640 AD- 234 25868 90 uucGGuAcAuccucGAcGGdTsdT 25869 91 CCGUCGAGGAUGUACCGAAdTsdT 15641 AD- 235 25870 92 ucGGuAcAuccucGAcGGcdTsdT 25871 93 GCCGUCGAGGAUGUACCGAdTsdT 15642 AD- 222 25872 94 UuGAcAAAcAAAuucGGuAdTsdT 25873 95 uACCGAAUUUGUUUGUCAAdTsdT 15643 AD- 809 25874 96 AuGAGCGuuAGGAcAcuAudTsdT 25875 97 AuAGUGUCCUAACGCUcAUdTsdT 15644 AD- 231 25876 98 AAAuucGGuAcAuccucGAdTsdT 25877 99 UCGAGGAUGUACCGAAUUUdTsdT 15645 AD- 425 25878 100 GAGuuuGAGGUAuAcCuAGdTsdT 25879 101 CuAGGuAuACCUcAAACUCdTsdT 15646 AD- 542 25880 102 AAucuAGAuGcAAuAAccAdTsdT 25881 103 UGGUuAUUGcAUCuAGAUUdTsdT 15647 AD- 805 25882 104 AAGuAuGAGcGuuAGGAcAdTsdT 25883 105 UGUCCuAACGCUcAuACUUdTsdT 15648 AD- 806 25884 106 AGuAuGAGcGuuAGGAcAcdTsdT 25885 107 GUGUCCUAACGCUcAuACUdTsdT 15649 AD- 1009 25886 108 AGuGuAGGcuuAccucAAAdTsdT 25887 109 UUGAGGuAAGCCuAcACDdTsdT 15650 AD- 422 25888 110 uuGGAGuuuGAGGuAuAccdTsdT 25889 111 GGuAuACCUcAAACUCCAAdTsdT 15651 AD- 225 25890 112 AcAAAcAAAuucGGuAcAudTsdT 25891 113 AUGuACCGAAUUUGUUUGUdTsdT 15652 AD- 808 25892 114 uAuGAGcGuuAGGAcAcuAdTsdT 25893 115 uAGUGUCCuAACGCUcAuAdTsdT 15653 AD- 210 25894 116 cuucAGAAcGAAuuGAcAAdTsdT 25895 117 UUGUcAAUUCGUUCUGAAGdTsdT 15654 AD- 680 25896 118 CuGAGGGcucuucGGcAAAdTsdT 25897 119 UUUGCCGAAGAGCCCUCAGdTsdT 15655 AD- 636 25898 120 cucAucucAuucuGcGcAGdTsdT 25899 121 CUGCGCAGAAUGAGAUGAGdTsdT 15656 AD- 1004 25900 122 UGGAAAGuGuAGGcuuAccdTsdT 25901 123 GGuAAGCCUACACUUUCcAdTsdT 15657 AD- 691 25902 124 UCGGcAAAuGuAGcAuGGGdTsdT 25903 125 CCcAUGCUACAUUUGCCGAdTsdT 15658 AD- 543 25904 126 AucuAGAuGcAAuAAccAcdTsdT 25905 127 GUGGUuAUUGcAUCuAGAUdTsdT 15659 AD- 811 25906 128 GAGcGuuAGGAcAcuAuuudTsdT 25907 129 AAAuAGUGUCCuAACGCUCdTsdT 15660 -
TABLE 6 Unmodified siRNAs targeting IL-6. Position of 5′ base of sense strand on transcript SEQ Sense (S) SEQ Antisense (AS) (see ID Oligo Sequence ID Oligo Sequence FIG. 11) NO: (5′ to 3′) NO: (5′ to 3′) 803 130 AAAAGUAUGAGCGUUAGGA 131 UCCUAACGCUCAUACUUUU 223 132 UGACAAACAAAUUCGGUAC 133 GUACCGAAUUUGUUUGUCA 802 134 UAAAAGUAUGAGCGUUAGG 135 CCUAACGCUCAUACUUUUA 804 136 AAAGUAUGAGCGUUAGGAC 137 GUCCUAACGCUCAUACUUU 234 138 UUCGGUACAUCCUCGACGG 139 CCGUCGAGGAUGUACCGAA 235 140 UCGGUACAUCCUCGACGGC 141 GCCGUCGAGGAUGUACCGA 222 142 UUGACAAACAAAUUCGGUA 143 UACCGAAUUUGUUUGUCAA 809 144 AUGAGCGUUAGGACACUAU 145 AUAGUGUCCUAACGCUCAU 231 146 AAAUUCGGUACAUCCUCGA 147 UCGAGGAUGUACCGAAUUU 425 148 GAGUUUGAGGUAUACCUAG 149 CUAGGUAUACCUCAAACUC 542 150 AAUCUAGAUGCAAUAACCA 151 UGGUUAUUGCAUCUAGAUU 805 152 AAGUAUGAGCGUUAGGACA 153 UGUCCUAACGCUCAUACUU 806 154 AGUAUGAGCGUUAGGACAC 155 GUGUCCUAACGCUCAUACU 1009 156 AGUGUAGGCUUACCUCAAA 157 UUUGAGGUAAGCCUACACU 422 158 UUGGAGUUUGAGGUAUACC 159 GGUAUACCUCAAACUCCAA 225 160 ACAAACAAAUUCGGUACAU 161 AUGUACCGAAUUUGUUUGU 808 162 UAUGAGCGUUAGGACACUA 163 UAGUGUCCUAACGCUCAUA 210 164 CUUCAGAACGAAUUGACAA 165 UUGUCAAUUCGUUCUGAAG 680 166 CUGAGGGCUCUUCGGCAAA 167 UUUGCCGAAGAGCCCUCAG 636 168 CUCAUCUCAUUCUGCGCAG 169 CUGCGCAGAAUGAGAUGAG 1004 170 UGGAAAGUGUAGGCUUACC 171 GGUAAGCCUACACUUUCCA 691 172 UCGGCAAAUGUAGCAUGGG 173 CCCAUGCUACAUUUGCCGA 543 174 AUCUAGAUGCAAUAACCAC 175 GUGGUUAUUGCAUCUAGAU 811 176 GAGCGUUAGGACACUAUUU 177 AAAUAGUGUCCUAACGCUC -
TABLE 7 siRNAs targeting IL-6 and modified with 3′ dinucleotide (NN) overhang. Position of 5′ base of sense strand on transcript SEQ Sense (S) SEQ Antisense (AS) (see ID Oligo Sequence ID Oligo Sequence FIG. 11) NO: (5′ to 3′) NO: (5′ to 3′) 803 178 AAAAGUAUGAGCGUUAGGANN 179 UCCUAACGCUCAUACUUUUNN 223 180 UGACAAACAAAUUCGGUACNN 181 GUACCGAAUUUGUUUGUCANN 802 182 UAAAAGUAUGAGCGUUAGGNN 183 CCUAACGCUCAUACUUUUANN 804 184 AAAGUAUGAGCGUUAGGACNN 185 GUCCUAACGCUCAUACUUUNN 234 186 UUCGGUACAUCCUCGACGGNN 187 CCGUCGAGGAUGUACCGAANN 235 188 UCGGUACAUCCUCGACGGCNN 189 GCCGUCGAGGAUGUACCGANN 222 190 UUGACAAACAAAUUCGGUANN 191 UACCGAAUUUGUUUGUCAANN 809 192 AUGAGCGUUAGGACACUAUNN 193 AUAGUGUCCUAACGCUCAUNN 231 194 AAAUUCGGUACAUCCUCGANN 195 UCGAGGAUGUACCGAAUUUNN 425 196 GAGUUUGAGGUAUACCUAGNN 197 CUAGGUAUACCUCAAACUCNN 542 198 AAUCUAGAUGCAAUAACCANN 199 UGGUUAUUGCAUCUAGAUUNN 805 200 AAGUAUGAGCGUUAGGACANN 201 UGUCCUAACGCUCAUACUUNN 806 202 AGUAUGAGCGUUAGGACACNN 203 GUGUCCUAACGCUCAUACUNN 1009 204 AGUGUAGGCUUACCUCAAANN 205 UUUGAGGUAAGCCUACACUNN 422 206 UUGGAGUUUGAGGUAUACCNN 207 GGUAUACCUCAAACUCCAANN 225 208 ACAAACAAAUUCGGUACAUNN 209 AUGUACCGAAUUUGUUUGUNN 808 210 UAUGAGCGUUAGGACACUANN 211 UAGUGUCCUAACGCUCAUANN 210 212 CUUCAGAACGAAUUGACAANN 213 UUGUCAAUUCGUUCUGAAGNN 680 214 CUGAGGGCUCUUCGGCAAANN 215 UUUGCCGAAGAGCCCUCAGNN 636 216 CUCAUCUCAUUCUGCGCAGNN 217 CUGCGCAGAAUGAGAUGAGNN 1004 218 UGGAAAGUGUAGGCUUACCNN 219 GGUAAGCCUACACUUUCCANN 691 220 UCGGCAAAUGUAGCAUGGGNN 221 CCCAUGCUACAUUUGCCGANN 543 222 AUCUAGAUGCAAUAACCACNN 223 GUGGUUAUUGCAUCUAGAUNN 811 224 GAGCGUUAGGACACUAUUUNN 225 AAAUAGUGUCCUAACGCUCNN -
TABLE 8 siRNAs targeting IL-6 and modified with 3′ dithymidine (dTdT) overhang. Position of 5′ base of sense strand on transcript SEQ Sense (S) SEQ Antisense (AS) (see ID Oligo Sequence ID Oligo Sequence FIG. 11) NO: (5′ to 3′) NO: (5′ to 3′) 803 226 AAAAGUAUGAGCGUUAGGAdTdT 227 UCCUAACGCUCAUACUUUUdTdT 223 228 UGACAAACAAAUUCGGUACdTdT 229 GUACCGAAUUUGUUUGUCAdTdT 802 230 UAAAAGUAUGAGCGUUAGGdTdT 231 CCUAACGCUCAUACUUUUAdTdT 804 232 AAAGUAUGAGCGUUAGGACdTdT 233 GUCCUAACGCUCAUACUUUdTdT 234 234 UUCGGUACAUCCUCGACGGdTdT 235 CCGUCGAGGAUGUACCGAAdTdT 235 236 UCGGUACAUCCUCGACGGCdTdT 237 GCCGUCGAGGAUGUACCGAdTdT 222 238 UUGACAAACAAAUUCGGUAdTdT 239 UACCGAAUUUGUUUGUCAAdTdT 809 240 AUGAGCGUUAGGACACUAUdTdT 241 AUAGUGUCCUAACGCUCAUdTdT 231 242 AAAUUCGGUACAUCCUCGAdTdT 243 UCGAGGAUGUACCGAAUUUdTdT 425 244 GAGUUUGAGGUAUACCUAGdTdT 245 CUAGGUAUACCUCAAACUCdTdT 542 246 AAUCUAGAUGCAAUAACCAdTdT 247 UGGUUAUUGCAUCUAGAUUdTdT 805 248 AAGUAUGAGCGUUAGGACAdTdT 249 UGUCCUAACGCUCAUACUUdTdT 806 250 AGUAUGAGCGUUAGGACACdTdT 251 GUGUCCUAACGCUCAUACUdTdT 1009 252 AGUGUAGGCUUACCUCAAAdTdT 253 UUUGAGGUAAGCCUACACUdTdT 422 254 UUGGAGUUUGAGGUAUACCdTdT 255 GGUAUACCUCAAACUCCAAdTdT 225 256 ACAAACAAAUUCGGUACAUdTdT 257 AUGUACCGAAUUUGUUUGUdTdT 808 258 UAUGAGCGUUAGGACACUAdTdT 259 UAGUGUCCUAACGCUCAUAdTdT 210 260 CUUCAGAACGAAUUGACAAdTdT 261 UUGUCAAUUCGUUCUGAAGdTdT 680 262 CUGAGGGCUCUUCGGCAAAdTdT 263 UUUGCCGAAGAGCCCUCAGdTdT 636 264 CUCAUCUCAUUCUGCGCAGdTdT 265 CUGCGCAGAAUGAGAUGAGdTdT 1004 266 UGGAAAGUGUAGGCUUACCdTdT 267 GGUAAGCCUACACUUUCCAdTdT 691 268 UCGGCAAAUGUAGCAUGGGdTdT 269 CCCAUGCUACAUUUGCCGAdTdT 543 270 AUCUAGAUGCAAUAACCACdTdT 271 GUGGUUAUUGCAUCUAGAUdTdT 811 272 GAGCGUUAGGACACUAUUUdTdT 273 AAAUAGUGUCCUAACGCUCdTdT -
TABLE 9 Effect of IL-6 siRNAs on IL-6 and IL-8 gene expression. Durability siRNA IL-6 IL-8 (days) AD-15637 + + x AD-15638 + + x AD-15639 + + x AD-15640 + + x AD-15641 + − x AD-15642 +/− x x AD-15643 + x x AD-15644 + − ≥14 AD-15645 + x x AD-15646 + x x AD-15647 − x x AD-15648 + + x AD-15649 + + x AD-15650 + − 3-7 AD-15651 − x x AD-15652 + + x AD-15653 + + x AD-15654 − x x AD-15655 + x x AD-15656 − x x AD-15657 + + 7-14 AD-15658 + + x AD-15659 + + x AD-15660 + − ≥14 − = no reduction of expression + = reduction of expression +/− = small reduction of expression x = not determined - Other embodiments are in the claims.
Claims (25)
1. A double-stranded ribonucleic acid (dsRNA), wherein said dsRNA comprises at least two sequences that are complementary to each other and wherein a sense strand comprises a first sequence and an antisense strand comprises a second sequence comprising a region of complementarity which is substantially complementary to at least a part of a mRNA encoding an delta-Epidermal Growth Factor Receptor (deltaEGFR) and comprising at least 15 contiguous nucleotides differing by no more than 3 nucleotides from any one of the antisense sequences listed in Tables 2 or 3, and wherein said region of complementarity is less than 30 nucleotides in length.
2. The dsRNA of claim 1 , wherein said dsRNA comprises at least one modified nucleotide.
3. The dsRNA of claim 2 , wherein
(a) said at least one of modified nucleotide is chosen from the group of: a 2′-O-methyl modified nucleotide, a nucleotide comprising a 5′ phosphorothioate group, and a terminal nucleotide linked to a cholesteryl derivative or dodecanoic acid bisdecylamide group; and/or
(b) said at least one modified nucleotide is chosen from the group of: a 2′-deoxy-2′-fluoro modified nucleotide, a 2′-deoxy-modified nucleotide, a locked nucleotide, an abasic nucleotide, 2′-amino-modified nucleotide, 2′-alkyl-modified nucleotide, morpholino nucleotide, a phosphoramidate, and a non-natural base comprising nucleotide.
4. (canceled)
5. The dsRNA of claim 1 , wherein
(a) the region of complementary is at least 15 nucleotides in length; and/or
(b) the region of complementarity is between 19 and 21 nucleotides in length.
6. (canceled)
7. The dsRNA of claim 1 , wherein
(a) the dsRNA comprises a sense strand consisting of a sense strand sequence selected from Tables 2 and 3, and an antisense strand consisting of an antisense sequence selected from Tables 2 and 3. and/or
(b) the sense strand and the antisense strand are also substantially complementary to at least a part of an mRNA encoding a wildtype Epidermal Growth Factor Receptor (EGFR).
8. (canceled)
9. The dsRNA of claim 1 , wherein the sense strand comprises a nucleotide sequence of SEQ ID NO:2, and the antisense strand comprises a nucleotide sequence of SEQ ID NO:3.
10. A cell containing the dsRNA of claim 1 .
11. A pharmaceutical composition for inhibiting expression of a deltaEGFR gene comprising the dsRNA of claim 1 .
12. The pharmaceutical composition of claim 11 , further comprising an IL6 dsRNA comprising at least two sequences that are complementary to each other and wherein a sense strand comprises a first sequence and an antisense strand comprises a second sequence which is substantially complementary to at least a part of an mRNA encoding an Interleukin-6 (IL6) and comprising at least 15 contiguous nucleotides differing by no more than 3 nucleotides from any one of the antisense sequences listed in Tables 5-8, and wherein said region of complementarity is less than 30 nucleotides in length.
13. The pharmaceutical composition of claim 12 , wherein the IL6 dsRNA comprises a sense strand comprising a sequence selected from Tables 5-8, and an antisense sequence comprising an antisense sequence selected from Tables 5-8.
14. A method of inhibiting deltaEGFR expression in a cell, the method comprising:
(a) introducing into the cell the dsRNA of claim 1 ; and
(b) maintaining the cell produced in step (a) for a time sufficient to obtain degradation of the mRNA transcript of the deltaEGFR gene, thereby inhibiting expression of the deltaEGFR gene in the cell.
15. A method of treating a disorder mediated by deltaEGFR expression comprising administering to a human in need of such treatment a therapeutically effective amount of the dsRNA of claim 1 .
16. The method of claim 15 , wherein
(a) the human has cancer;
(b) the human has a tumor;
(c) the human has an astrocytic tumor; and/or
(d) the human has a glioma.
17.-19. (canceled)
20. The method of claim 15 , further comprising administering a therapeutically effective amount of an IL6 dsRNA comprising at least two sequences that are complementary to each other and wherein a sense strand comprises a first sequence and an antisense strand comprises a second sequence which is substantially complementary to at least a part of an mRNA encoding an IL6 and comprising at least 15 contiguous nucleotides differing by no more than 3 nucleotides from any one of the antisense sequences listed in Tables 5-8, and wherein said region of complementarity is less than 30 nucleotides in length.
21. A vector comprising a nucleotide sequence that encodes at least one strand of the dsRNA of claim 1 .
22. The vector of claim 21 , wherein
(a) the region of complementarity is at least 15 nucleotides in length; and/or
(b) the region of complementarity is 19 to 21 nucleotides in length.
23. (canceled)
24. A cell comprising the vector of claim 21 .
25. A double-stranded ribonucleic acid (dsRNA), wherein said dsRNA comprises at least two sequences that are complementary to each other and wherein a sense strand comprises a first sequence and an antisense strand comprises a second sequence comprising a region of complementarity which is substantially complementary to at least a part of a mRNA encoding IL-6 and comprising at least 15 contiguous nucleotides differing by no more than 3 nucleotides from any one of the antisense sequences listed in Tables 5-8, and wherein said region of complementarity is less than 30 nucleotides in length.
26. The dsRNA of claim 25 , wherein the dsRNA comprises a sense strand comprising a sequence selected from Tables 5-8, and an antisense sequence comprising an antisense sequence selected from Tables 5-8.
27. A method of treating a disorder mediated by IL-6 expression comprising administering to a human in need of such treatment a therapeutically effective amount of the dsRNA of claim 25 .
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US17/733,552 US20230053332A1 (en) | 2008-09-02 | 2022-04-29 | Compositions and methods for inhibiting expression of mutant egfr gene |
Applications Claiming Priority (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US9362008P | 2008-09-02 | 2008-09-02 | |
| US9548708P | 2008-09-09 | 2008-09-09 | |
| US14766809P | 2009-01-27 | 2009-01-27 | |
| US14768009P | 2009-01-27 | 2009-01-27 | |
| US16648809P | 2009-04-03 | 2009-04-03 | |
| PCT/US2009/055745 WO2010028054A1 (en) | 2008-09-02 | 2009-09-02 | Compositions and methods for inhibiting expression of mutant egfr gene |
| US201113061569A | 2011-07-21 | 2011-07-21 | |
| US13/659,315 US9212364B2 (en) | 2008-09-02 | 2012-10-24 | Compositions and methods for inhibiting expression of mutant EGFR gene |
| US14/936,059 US9957507B2 (en) | 2008-09-02 | 2015-11-09 | Compositions and methods for inhibiting expression of mutant EGFR gene |
| US15/942,126 US20190010502A1 (en) | 2008-09-02 | 2018-03-30 | Compositions and methods for inhibiting expression of mutant egfr gene |
| US201916519868A | 2019-07-23 | 2019-07-23 | |
| US16/807,828 US20200377893A1 (en) | 2008-09-02 | 2020-03-03 | Compositions and methods for inhibiting expression of mutant egfr gene |
| US17/733,552 US20230053332A1 (en) | 2008-09-02 | 2022-04-29 | Compositions and methods for inhibiting expression of mutant egfr gene |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/807,828 Continuation US20200377893A1 (en) | 2008-09-02 | 2020-03-03 | Compositions and methods for inhibiting expression of mutant egfr gene |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20230053332A1 true US20230053332A1 (en) | 2023-02-23 |
Family
ID=41268470
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/061,569 Active US8318693B2 (en) | 2008-09-02 | 2009-09-02 | Compositions and methods for inhibiting expression of mutant EGFR gene |
| US13/659,315 Active US9212364B2 (en) | 2008-09-02 | 2012-10-24 | Compositions and methods for inhibiting expression of mutant EGFR gene |
| US14/936,059 Active US9957507B2 (en) | 2008-09-02 | 2015-11-09 | Compositions and methods for inhibiting expression of mutant EGFR gene |
| US15/942,126 Abandoned US20190010502A1 (en) | 2008-09-02 | 2018-03-30 | Compositions and methods for inhibiting expression of mutant egfr gene |
| US16/807,828 Abandoned US20200377893A1 (en) | 2008-09-02 | 2020-03-03 | Compositions and methods for inhibiting expression of mutant egfr gene |
| US17/733,552 Abandoned US20230053332A1 (en) | 2008-09-02 | 2022-04-29 | Compositions and methods for inhibiting expression of mutant egfr gene |
Family Applications Before (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/061,569 Active US8318693B2 (en) | 2008-09-02 | 2009-09-02 | Compositions and methods for inhibiting expression of mutant EGFR gene |
| US13/659,315 Active US9212364B2 (en) | 2008-09-02 | 2012-10-24 | Compositions and methods for inhibiting expression of mutant EGFR gene |
| US14/936,059 Active US9957507B2 (en) | 2008-09-02 | 2015-11-09 | Compositions and methods for inhibiting expression of mutant EGFR gene |
| US15/942,126 Abandoned US20190010502A1 (en) | 2008-09-02 | 2018-03-30 | Compositions and methods for inhibiting expression of mutant egfr gene |
| US16/807,828 Abandoned US20200377893A1 (en) | 2008-09-02 | 2020-03-03 | Compositions and methods for inhibiting expression of mutant egfr gene |
Country Status (3)
| Country | Link |
|---|---|
| US (6) | US8318693B2 (en) |
| EP (3) | EP2690175B1 (en) |
| WO (1) | WO2010028054A1 (en) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2690175B1 (en) | 2008-09-02 | 2016-12-28 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for combined inhibition of mutant EGFR gene and IL-6 expression |
| US20120114710A1 (en) * | 2009-05-18 | 2012-05-10 | Lynn Kirkpatrick | Carbon nanotubes complexed with multiple bioactive agents and methods related thereto |
| US20140155462A1 (en) * | 2011-04-22 | 2014-06-05 | Dicerna Pharmaceuticals, Inc. | Methods and compositions for the specific inhibitions of egfr by double-stranded rna |
| AU2013216361B2 (en) | 2012-02-02 | 2017-09-07 | The University Of British Columbia | Combination therapy for cancer using HSP27 inhibitor and EGFR tyrosine kinase inhibitors or anti-folates |
| EP3314022A4 (en) * | 2015-06-23 | 2019-05-01 | Abbott Molecular Inc. | Egfr assay |
| MA45349A (en) | 2016-04-01 | 2019-02-06 | Avidity Biosciences Llc | EGFR NUCLEIC ACIDS AND THEIR USES |
| MA45470A (en) | 2016-04-01 | 2019-02-06 | Avidity Biosciences Llc | KRAS NUCLEIC ACIDS AND THEIR USES |
| MA45469A (en) | 2016-04-01 | 2019-02-06 | Avidity Biosciences Llc | BETA-CATENIN NUCLEIC ACIDS AND THEIR USES |
| MA45328A (en) | 2016-04-01 | 2019-02-06 | Avidity Biosciences Llc | NUCLEIC ACID-POLYPEPTIDE COMPOSITIONS AND USES THEREOF |
| MX2020005860A (en) | 2017-12-06 | 2020-09-09 | Avidity Biosciences Inc | Compositions and methods of treating muscle atrophy and myotonic dystrophy. |
| TW202144572A (en) | 2020-03-19 | 2021-12-01 | 美商亞維代堤生物科學公司 | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
Family Cites Families (191)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US564562A (en) | 1896-07-21 | Joseph p | ||
| US513030A (en) | 1894-01-16 | Machine for waxing or coating paper | ||
| US3687808A (en) | 1969-08-14 | 1972-08-29 | Univ Leland Stanford Junior | Synthetic polynucleotides |
| US4522808A (en) | 1980-08-15 | 1985-06-11 | Societe Anonyme Dite: L'oreal | Anti-sunburn compositions containing 2-phenyl-indole derivatives |
| US4469863A (en) | 1980-11-12 | 1984-09-04 | Ts O Paul O P | Nonionic nucleic acid alkyl and aryl phosphonates and processes for manufacture and use thereof |
| US4426330A (en) | 1981-07-20 | 1984-01-17 | Lipid Specialties, Inc. | Synthetic phospholipid compounds |
| US4534899A (en) | 1981-07-20 | 1985-08-13 | Lipid Specialties, Inc. | Synthetic phospholipid compounds |
| US5023243A (en) | 1981-10-23 | 1991-06-11 | Molecular Biosystems, Inc. | Oligonucleotide therapeutic agent and method of making same |
| US4476301A (en) | 1982-04-29 | 1984-10-09 | Centre National De La Recherche Scientifique | Oligonucleotides, a process for preparing the same and their application as mediators of the action of interferon |
| JPS5927900A (en) | 1982-08-09 | 1984-02-14 | Wakunaga Seiyaku Kk | Oligonucleotide derivative and its preparation |
| FR2540122B1 (en) | 1983-01-27 | 1985-11-29 | Centre Nat Rech Scient | NOVEL COMPOUNDS COMPRISING A SEQUENCE OF OLIGONUCLEOTIDE LINKED TO AN INTERCALATION AGENT, THEIR SYNTHESIS PROCESS AND THEIR APPLICATION |
| US4605735A (en) | 1983-02-14 | 1986-08-12 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| US4948882A (en) | 1983-02-22 | 1990-08-14 | Syngene, Inc. | Single-stranded labelled oligonucleotides, reactive monomers and methods of synthesis |
| US4824941A (en) | 1983-03-10 | 1989-04-25 | Julian Gordon | Specific antibody to the native form of 2'5'-oligonucleotides, the method of preparation and the use as reagents in immunoassays or for binding 2'5'-oligonucleotides in biological systems |
| US4587044A (en) | 1983-09-01 | 1986-05-06 | The Johns Hopkins University | Linkage of proteins to nucleic acids |
| US5118800A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | Oligonucleotides possessing a primary amino group in the terminal nucleotide |
| US5118802A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | DNA-reporter conjugates linked via the 2' or 5'-primary amino group of the 5'-terminal nucleoside |
| US5550111A (en) | 1984-07-11 | 1996-08-27 | Temple University-Of The Commonwealth System Of Higher Education | Dual action 2',5'-oligoadenylate antiviral derivatives and uses thereof |
| FR2567892B1 (en) | 1984-07-19 | 1989-02-17 | Centre Nat Rech Scient | NOVEL OLIGONUCLEOTIDES, THEIR PREPARATION PROCESS AND THEIR APPLICATIONS AS MEDIATORS IN DEVELOPING THE EFFECTS OF INTERFERONS |
| US5258506A (en) | 1984-10-16 | 1993-11-02 | Chiron Corporation | Photolabile reagents for incorporation into oligonucleotide chains |
| US5430136A (en) | 1984-10-16 | 1995-07-04 | Chiron Corporation | Oligonucleotides having selectably cleavable and/or abasic sites |
| US5367066A (en) | 1984-10-16 | 1994-11-22 | Chiron Corporation | Oligonucleotides with selectably cleavable and/or abasic sites |
| US4828979A (en) | 1984-11-08 | 1989-05-09 | Life Technologies, Inc. | Nucleotide analogs for nucleic acid labeling and detection |
| FR2575751B1 (en) | 1985-01-08 | 1987-04-03 | Pasteur Institut | NOVEL ADENOSINE DERIVATIVE NUCLEOSIDES, THEIR PREPARATION AND THEIR BIOLOGICAL APPLICATIONS |
| US5405938A (en) | 1989-12-20 | 1995-04-11 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5185444A (en) | 1985-03-15 | 1993-02-09 | Anti-Gene Deveopment Group | Uncharged morpolino-based polymers having phosphorous containing chiral intersubunit linkages |
| US5034506A (en) | 1985-03-15 | 1991-07-23 | Anti-Gene Development Group | Uncharged morpholino-based polymers having achiral intersubunit linkages |
| US5235033A (en) | 1985-03-15 | 1993-08-10 | Anti-Gene Development Group | Alpha-morpholino ribonucleoside derivatives and polymers thereof |
| US5166315A (en) | 1989-12-20 | 1992-11-24 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US4762779A (en) | 1985-06-13 | 1988-08-09 | Amgen Inc. | Compositions and methods for functionalizing nucleic acids |
| US4980286A (en) | 1985-07-05 | 1990-12-25 | Whitehead Institute For Biomedical Research | In vivo introduction and expression of foreign genetic material in epithelial cells |
| ATE68013T1 (en) | 1985-07-05 | 1991-10-15 | Whitehead Biomedical Inst | EXPRESSION OF FOREIGN GENETIC MATERIAL IN EPITHELIAL CELLS. |
| US5139941A (en) | 1985-10-31 | 1992-08-18 | University Of Florida Research Foundation, Inc. | AAV transduction vectors |
| US5317098A (en) | 1986-03-17 | 1994-05-31 | Hiroaki Shizuya | Non-radioisotope tagging of fragments |
| JPS638396A (en) | 1986-06-30 | 1988-01-14 | Wakunaga Pharmaceut Co Ltd | Poly-labeled oligonucleotide derivative |
| US4920016A (en) | 1986-12-24 | 1990-04-24 | Linear Technology, Inc. | Liposomes with enhanced circulation time |
| US4837028A (en) | 1986-12-24 | 1989-06-06 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5276019A (en) | 1987-03-25 | 1994-01-04 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US5264423A (en) | 1987-03-25 | 1993-11-23 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US4904582A (en) | 1987-06-11 | 1990-02-27 | Synthetic Genetics | Novel amphiphilic nucleic acid conjugates |
| AU598946B2 (en) | 1987-06-24 | 1990-07-05 | Howard Florey Institute Of Experimental Physiology And Medicine | Nucleoside derivatives |
| EP0633318A1 (en) | 1987-09-11 | 1995-01-11 | Whitehead Institute For Biomedical Research | Transduced fibroblasts and uses therefor |
| US5585481A (en) | 1987-09-21 | 1996-12-17 | Gen-Probe Incorporated | Linking reagents for nucleotide probes |
| US5188897A (en) | 1987-10-22 | 1993-02-23 | Temple University Of The Commonwealth System Of Higher Education | Encapsulated 2',5'-phosphorothioate oligoadenylates |
| US4924624A (en) | 1987-10-22 | 1990-05-15 | Temple University-Of The Commonwealth System Of Higher Education | 2,',5'-phosphorothioate oligoadenylates and plant antiviral uses thereof |
| US5525465A (en) | 1987-10-28 | 1996-06-11 | Howard Florey Institute Of Experimental Physiology And Medicine | Oligonucleotide-polyamide conjugates and methods of production and applications of the same |
| DE3738460A1 (en) | 1987-11-12 | 1989-05-24 | Max Planck Gesellschaft | MODIFIED OLIGONUCLEOTIDS |
| JP2914692B2 (en) | 1987-12-11 | 1999-07-05 | ホワイトヘツド・インスチチユート・フオー・バイオメデイカル・リサーチ | Endothelial cell genetic modification |
| DE68927996T2 (en) | 1988-02-05 | 1997-12-04 | Hughes Howard Med Inst | MODIFIED HEPATOCYTES AND THEIR USE |
| US5082830A (en) | 1988-02-26 | 1992-01-21 | Enzo Biochem, Inc. | End labeled nucleotide probe |
| WO1989009221A1 (en) | 1988-03-25 | 1989-10-05 | University Of Virginia Alumni Patents Foundation | Oligonucleotide n-alkylphosphoramidates |
| US5278302A (en) | 1988-05-26 | 1994-01-11 | University Patents, Inc. | Polynucleotide phosphorodithioates |
| US5109124A (en) | 1988-06-01 | 1992-04-28 | Biogen, Inc. | Nucleic acid probe linked to a label having a terminal cysteine |
| US5216141A (en) | 1988-06-06 | 1993-06-01 | Benner Steven A | Oligonucleotide analogs containing sulfur linkages |
| US5175273A (en) | 1988-07-01 | 1992-12-29 | Genentech, Inc. | Nucleic acid intercalating agents |
| US5262536A (en) | 1988-09-15 | 1993-11-16 | E. I. Du Pont De Nemours And Company | Reagents for the preparation of 5'-tagged oligonucleotides |
| GB8824593D0 (en) | 1988-10-20 | 1988-11-23 | Royal Free Hosp School Med | Liposomes |
| US5512439A (en) | 1988-11-21 | 1996-04-30 | Dynal As | Oligonucleotide-linked magnetic particles and uses thereof |
| US5457183A (en) | 1989-03-06 | 1995-10-10 | Board Of Regents, The University Of Texas System | Hydroxylated texaphyrins |
| US5599923A (en) | 1989-03-06 | 1997-02-04 | Board Of Regents, University Of Tx | Texaphyrin metal complexes having improved functionalization |
| US5328470A (en) | 1989-03-31 | 1994-07-12 | The Regents Of The University Of Michigan | Treatment of diseases by site-specific instillation of cells or site-specific transformation of cells and kits therefor |
| US5391723A (en) | 1989-05-31 | 1995-02-21 | Neorx Corporation | Oligonucleotide conjugates |
| US4958013A (en) | 1989-06-06 | 1990-09-18 | Northwestern University | Cholesteryl modified oligonucleotides |
| US5032401A (en) | 1989-06-15 | 1991-07-16 | Alpha Beta Technology | Glucan drug delivery system and adjuvant |
| US5451463A (en) | 1989-08-28 | 1995-09-19 | Clontech Laboratories, Inc. | Non-nucleoside 1,3-diol reagents for labeling synthetic oligonucleotides |
| US5134066A (en) | 1989-08-29 | 1992-07-28 | Monsanto Company | Improved probes using nucleosides containing 3-dezauracil analogs |
| US5254469A (en) | 1989-09-12 | 1993-10-19 | Eastman Kodak Company | Oligonucleotide-enzyme conjugate that can be used as a probe in hybridization assays and polymerase chain reaction procedures |
| US5591722A (en) | 1989-09-15 | 1997-01-07 | Southern Research Institute | 2'-deoxy-4'-thioribonucleosides and their antiviral activity |
| US5356633A (en) | 1989-10-20 | 1994-10-18 | Liposome Technology, Inc. | Method of treatment of inflamed tissues |
| US5225212A (en) | 1989-10-20 | 1993-07-06 | Liposome Technology, Inc. | Microreservoir liposome composition and method |
| US5013556A (en) | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5399676A (en) | 1989-10-23 | 1995-03-21 | Gilead Sciences | Oligonucleotides with inverted polarity |
| US5264562A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences, Inc. | Oligonucleotide analogs with novel linkages |
| ATE269870T1 (en) | 1989-10-24 | 2004-07-15 | Isis Pharmaceuticals Inc | 2'-MODIFIED OLIGONUCLEOTIDES |
| US5264564A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences | Oligonucleotide analogs with novel linkages |
| US5292873A (en) | 1989-11-29 | 1994-03-08 | The Research Foundation Of State University Of New York | Nucleic acids labeled with naphthoquinone probe |
| US5177198A (en) | 1989-11-30 | 1993-01-05 | University Of N.C. At Chapel Hill | Process for preparing oligoribonucleoside and oligodeoxyribonucleoside boranophosphates |
| US5130302A (en) | 1989-12-20 | 1992-07-14 | Boron Bilogicals, Inc. | Boronated nucleoside, nucleotide and oligonucleotide compounds, compositions and methods for using same |
| US5486603A (en) | 1990-01-08 | 1996-01-23 | Gilead Sciences, Inc. | Oligonucleotide having enhanced binding affinity |
| US5578718A (en) | 1990-01-11 | 1996-11-26 | Isis Pharmaceuticals, Inc. | Thiol-derivatized nucleosides |
| US5670633A (en) | 1990-01-11 | 1997-09-23 | Isis Pharmaceuticals, Inc. | Sugar modified oligonucleotides that detect and modulate gene expression |
| US5587361A (en) | 1991-10-15 | 1996-12-24 | Isis Pharmaceuticals, Inc. | Oligonucleotides having phosphorothioate linkages of high chiral purity |
| US5459255A (en) | 1990-01-11 | 1995-10-17 | Isis Pharmaceuticals, Inc. | N-2 substituted purines |
| US5681941A (en) | 1990-01-11 | 1997-10-28 | Isis Pharmaceuticals, Inc. | Substituted purines and oligonucleotide cross-linking |
| US5646265A (en) | 1990-01-11 | 1997-07-08 | Isis Pharmceuticals, Inc. | Process for the preparation of 2'-O-alkyl purine phosphoramidites |
| US5587470A (en) | 1990-01-11 | 1996-12-24 | Isis Pharmaceuticals, Inc. | 3-deazapurines |
| US5214136A (en) | 1990-02-20 | 1993-05-25 | Gilead Sciences, Inc. | Anthraquinone-derivatives oligonucleotides |
| AU7579991A (en) | 1990-02-20 | 1991-09-18 | Gilead Sciences, Inc. | Pseudonucleosides and pseudonucleotides and their polymers |
| US5321131A (en) | 1990-03-08 | 1994-06-14 | Hybridon, Inc. | Site-specific functionalization of oligodeoxynucleotides for non-radioactive labelling |
| US5470967A (en) | 1990-04-10 | 1995-11-28 | The Dupont Merck Pharmaceutical Company | Oligonucleotide analogs with sulfamate linkages |
| US5665710A (en) | 1990-04-30 | 1997-09-09 | Georgetown University | Method of making liposomal oligodeoxynucleotide compositions |
| GB9009980D0 (en) | 1990-05-03 | 1990-06-27 | Amersham Int Plc | Phosphoramidite derivatives,their preparation and the use thereof in the incorporation of reporter groups on synthetic oligonucleotides |
| DK0455905T3 (en) | 1990-05-11 | 1998-12-07 | Microprobe Corp | Dipsticks for nucleic acid hybridization assays and method for covalent immobilization of oligonucleotides |
| US5602240A (en) | 1990-07-27 | 1997-02-11 | Ciba Geigy Ag. | Backbone modified oligonucleotide analogs |
| US5618704A (en) | 1990-07-27 | 1997-04-08 | Isis Pharmacueticals, Inc. | Backbone-modified oligonucleotide analogs and preparation thereof through radical coupling |
| US5541307A (en) | 1990-07-27 | 1996-07-30 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogs and solid phase synthesis thereof |
| US5138045A (en) | 1990-07-27 | 1992-08-11 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5688941A (en) | 1990-07-27 | 1997-11-18 | Isis Pharmaceuticals, Inc. | Methods of making conjugated 4' desmethyl nucleoside analog compounds |
| US5608046A (en) | 1990-07-27 | 1997-03-04 | Isis Pharmaceuticals, Inc. | Conjugated 4'-desmethyl nucleoside analog compounds |
| US5218105A (en) | 1990-07-27 | 1993-06-08 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5610289A (en) | 1990-07-27 | 1997-03-11 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogues |
| US5489677A (en) | 1990-07-27 | 1996-02-06 | Isis Pharmaceuticals, Inc. | Oligonucleoside linkages containing adjacent oxygen and nitrogen atoms |
| US5623070A (en) | 1990-07-27 | 1997-04-22 | Isis Pharmaceuticals, Inc. | Heteroatomic oligonucleoside linkages |
| JPH0874B2 (en) | 1990-07-27 | 1996-01-10 | アイシス・ファーマシューティカルス・インコーポレーテッド | Nuclease-resistant, pyrimidine-modified oligonucleotides that detect and modulate gene expression |
| US5677437A (en) | 1990-07-27 | 1997-10-14 | Isis Pharmaceuticals, Inc. | Heteroatomic oligonucleoside linkages |
| US5245022A (en) | 1990-08-03 | 1993-09-14 | Sterling Drug, Inc. | Exonuclease resistant terminally substituted oligonucleotides |
| IL113519A (en) | 1990-08-03 | 1997-11-20 | Sterling Winthrop Inc | Oligonucleoside sequences of from about 6 to about 200 bases having a three atom internucleoside linkage, their preparation and pharmaceutical compositions for inhibiting gene expression containing said oligonucleosides |
| US5512667A (en) | 1990-08-28 | 1996-04-30 | Reed; Michael W. | Trifunctional intermediates for preparing 3'-tailed oligonucleotides |
| US5214134A (en) | 1990-09-12 | 1993-05-25 | Sterling Winthrop Inc. | Process of linking nucleosides with a siloxane bridge |
| US5561225A (en) | 1990-09-19 | 1996-10-01 | Southern Research Institute | Polynucleotide analogs containing sulfonate and sulfonamide internucleoside linkages |
| AU662298B2 (en) | 1990-09-20 | 1995-08-31 | Gilead Sciences, Inc. | Modified internucleoside linkages |
| US5432272A (en) | 1990-10-09 | 1995-07-11 | Benner; Steven A. | Method for incorporating into a DNA or RNA oligonucleotide using nucleotides bearing heterocyclic bases |
| ES2115662T3 (en) | 1990-10-31 | 1998-07-01 | Cell Genesys Inc | GENETIC MODIFICATION OF ENDOTHELIAL CELLS. |
| KR930702373A (en) | 1990-11-08 | 1993-09-08 | 안토니 제이. 페이네 | Addition of Multiple Reporter Groups to Synthetic Oligonucleotides |
| GB9100304D0 (en) | 1991-01-08 | 1991-02-20 | Ici Plc | Compound |
| JP3220180B2 (en) | 1991-05-23 | 2001-10-22 | 三菱化学株式会社 | Drug-containing protein-bound liposomes |
| US5539082A (en) | 1993-04-26 | 1996-07-23 | Nielsen; Peter E. | Peptide nucleic acids |
| US5714331A (en) | 1991-05-24 | 1998-02-03 | Buchardt, Deceased; Ole | Peptide nucleic acids having enhanced binding affinity, sequence specificity and solubility |
| US5719262A (en) | 1993-11-22 | 1998-02-17 | Buchardt, Deceased; Ole | Peptide nucleic acids having amino acid side chains |
| US5371241A (en) | 1991-07-19 | 1994-12-06 | Pharmacia P-L Biochemicals Inc. | Fluorescein labelled phosphoramidites |
| US5571799A (en) | 1991-08-12 | 1996-11-05 | Basco, Ltd. | (2'-5') oligoadenylate analogues useful as inhibitors of host-v5.-graft response |
| DE59208572D1 (en) | 1991-10-17 | 1997-07-10 | Ciba Geigy Ag | Bicyclic nucleosides, oligonucleotides, processes for their preparation and intermediates |
| US5594121A (en) | 1991-11-07 | 1997-01-14 | Gilead Sciences, Inc. | Enhanced triple-helix and double-helix formation with oligomers containing modified purines |
| US5252479A (en) | 1991-11-08 | 1993-10-12 | Research Corporation Technologies, Inc. | Safe vector for gene therapy |
| US5484908A (en) | 1991-11-26 | 1996-01-16 | Gilead Sciences, Inc. | Oligonucleotides containing 5-propynyl pyrimidines |
| US5359044A (en) | 1991-12-13 | 1994-10-25 | Isis Pharmaceuticals | Cyclobutyl oligonucleotide surrogates |
| US5565552A (en) | 1992-01-21 | 1996-10-15 | Pharmacyclics, Inc. | Method of expanded porphyrin-oligonucleotide conjugate synthesis |
| US5595726A (en) | 1992-01-21 | 1997-01-21 | Pharmacyclics, Inc. | Chromophore probe for detection of nucleic acid |
| FR2687679B1 (en) | 1992-02-05 | 1994-10-28 | Centre Nat Rech Scient | OLIGOTHIONUCLEOTIDES. |
| DE4203923A1 (en) | 1992-02-11 | 1993-08-12 | Henkel Kgaa | METHOD FOR PRODUCING POLYCARBOXYLATES ON A POLYSACCHARIDE BASE |
| US5633360A (en) | 1992-04-14 | 1997-05-27 | Gilead Sciences, Inc. | Oligonucleotide analogs capable of passive cell membrane permeation |
| US5434257A (en) | 1992-06-01 | 1995-07-18 | Gilead Sciences, Inc. | Binding compentent oligomers containing unsaturated 3',5' and 2',5' linkages |
| US5587308A (en) | 1992-06-02 | 1996-12-24 | The United States Of America As Represented By The Department Of Health & Human Services | Modified adeno-associated virus vector capable of expression from a novel promoter |
| EP0577558A2 (en) | 1992-07-01 | 1994-01-05 | Ciba-Geigy Ag | Carbocyclic nucleosides having bicyclic rings, oligonucleotides therefrom, process for their preparation, their use and intermediates |
| US5272250A (en) | 1992-07-10 | 1993-12-21 | Spielvogel Bernard F | Boronated phosphoramidate compounds |
| US5478745A (en) | 1992-12-04 | 1995-12-26 | University Of Pittsburgh | Recombinant viral vector system |
| US5574142A (en) | 1992-12-15 | 1996-11-12 | Microprobe Corporation | Peptide linkers for improved oligonucleotide delivery |
| JP3351476B2 (en) | 1993-01-22 | 2002-11-25 | 三菱化学株式会社 | Phospholipid derivatives and liposomes containing the same |
| US5476925A (en) | 1993-02-01 | 1995-12-19 | Northwestern University | Oligodeoxyribonucleotides including 3'-aminonucleoside-phosphoramidate linkages and terminal 3'-amino groups |
| WO1994018987A1 (en) | 1993-02-19 | 1994-09-01 | Nippon Shinyaku Co., Ltd. | Drug composition containing nucleic acid copolymer |
| US5395619A (en) | 1993-03-03 | 1995-03-07 | Liposome Technology, Inc. | Lipid-polymer conjugates and liposomes |
| GB9304618D0 (en) | 1993-03-06 | 1993-04-21 | Ciba Geigy Ag | Chemical compounds |
| ES2107205T3 (en) | 1993-03-30 | 1997-11-16 | Sanofi Sa | ANALOGS OF ACICLIC NUCLEOSIDES AND OLIGONUCLEOTIDE SEQUENCES THAT CONTAIN THEM. |
| HU9501974D0 (en) | 1993-03-31 | 1995-09-28 | Sterling Winthrop Inc | Oligonucleotides with amide linkages replacing phosphodiester linkages |
| DE4311944A1 (en) | 1993-04-10 | 1994-10-13 | Degussa | Coated sodium percarbonate particles, process for their preparation and detergent, cleaning and bleaching compositions containing them |
| US5502177A (en) | 1993-09-17 | 1996-03-26 | Gilead Sciences, Inc. | Pyrimidine derivatives for labeled binding partners |
| US5540935A (en) | 1993-12-06 | 1996-07-30 | Nof Corporation | Reactive vesicle and functional substance-fixed vesicle |
| US5457187A (en) | 1993-12-08 | 1995-10-10 | Board Of Regents University Of Nebraska | Oligonucleotides containing 5-fluorouracil |
| US5446137B1 (en) | 1993-12-09 | 1998-10-06 | Behringwerke Ag | Oligonucleotides containing 4'-substituted nucleotides |
| US5519134A (en) | 1994-01-11 | 1996-05-21 | Isis Pharmaceuticals, Inc. | Pyrrolidine-containing monomers and oligomers |
| US5596091A (en) | 1994-03-18 | 1997-01-21 | The Regents Of The University Of California | Antisense oligonucleotides comprising 5-aminoalkyl pyrimidine nucleotides |
| US5627053A (en) | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| US5625050A (en) | 1994-03-31 | 1997-04-29 | Amgen Inc. | Modified oligonucleotides and intermediates useful in nucleic acid therapeutics |
| US6054299A (en) | 1994-04-29 | 2000-04-25 | Conrad; Charles A. | Stem-loop cloning vector and method |
| WO2000022114A1 (en) | 1998-10-09 | 2000-04-20 | Ingene, Inc. | PRODUCTION OF ssDNA $i(IN VIVO) |
| US5525711A (en) | 1994-05-18 | 1996-06-11 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Pteridine nucleotide analogs as fluorescent DNA probes |
| US5543152A (en) | 1994-06-20 | 1996-08-06 | Inex Pharmaceuticals Corporation | Sphingosomes for enhanced drug delivery |
| US5597696A (en) | 1994-07-18 | 1997-01-28 | Becton Dickinson And Company | Covalent cyanine dye oligonucleotide conjugates |
| US5580731A (en) | 1994-08-25 | 1996-12-03 | Chiron Corporation | N-4 modified pyrimidine deoxynucleotides and oligonucleotide probes synthesized therewith |
| US5597909A (en) | 1994-08-25 | 1997-01-28 | Chiron Corporation | Polynucleotide reagents containing modified deoxyribose moieties, and associated methods of synthesis and use |
| US5820873A (en) | 1994-09-30 | 1998-10-13 | The University Of British Columbia | Polyethylene glycol modified ceramide lipids and liposome uses thereof |
| DE69532767T2 (en) | 1994-11-28 | 2004-08-12 | Thomas Jefferson University | A peptide comprising a sequence from a fusion junction in a mutant EGF type III receptor |
| WO1996033761A1 (en) | 1995-04-28 | 1996-10-31 | Medtronic, Inc. | Intraparenchymal infusion catheter system |
| US5981501A (en) | 1995-06-07 | 1999-11-09 | Inex Pharmaceuticals Corp. | Methods for encapsulating plasmids in lipid bilayers |
| AU723163B2 (en) | 1995-06-07 | 2000-08-17 | Tekmira Pharmaceuticals Corporation | Lipid-nucleic acid particles prepared via a hydrophobic lipid-nucleic acid complex intermediate and use for gene transfer |
| US5756122A (en) | 1995-06-07 | 1998-05-26 | Georgetown University | Liposomally encapsulated nucleic acids having high entrapment efficiencies, method of manufacturer and use thereof for transfection of targeted cells |
| US7422902B1 (en) | 1995-06-07 | 2008-09-09 | The University Of British Columbia | Lipid-nucleic acid particles prepared via a hydrophobic lipid-nucleic acid complex intermediate and use for gene transfer |
| EP0843555B1 (en) | 1995-08-01 | 2003-08-27 | Isis Pharmaceuticals, Inc. | Liposomal oligonucleotide compositions |
| US5858397A (en) | 1995-10-11 | 1999-01-12 | University Of British Columbia | Liposomal formulations of mitoxantrone |
| US5994316A (en) | 1996-02-21 | 1999-11-30 | The Immune Response Corporation | Method of preparing polynucleotide-carrier complexes for delivery to cells |
| US5735814A (en) | 1996-04-30 | 1998-04-07 | Medtronic, Inc. | Techniques of treating neurodegenerative disorders by brain infusion |
| US6887906B1 (en) | 1997-07-01 | 2005-05-03 | Isispharmaceuticals, Inc. | Compositions and methods for the delivery of oligonucleotides via the alimentary canal |
| US6506559B1 (en) | 1997-12-23 | 2003-01-14 | Carnegie Institute Of Washington | Genetic inhibition by double-stranded RNA |
| EP2267138B1 (en) | 1998-04-08 | 2016-06-08 | Commonwealth Scientific and Industrial Research Organization | Methods and means for obtaining modified phenotypes |
| AR020078A1 (en) | 1998-05-26 | 2002-04-10 | Syngenta Participations Ag | METHOD FOR CHANGING THE EXPRESSION OF AN OBJECTIVE GENE IN A PLANT CELL |
| ATE237312T1 (en) | 1998-07-20 | 2003-05-15 | Protiva Biotherapeutics Inc | NUCLEIC ACID COMPLEXES ENCAPSULATED IN LIPOSOMES |
| AU6430599A (en) | 1998-10-09 | 2000-05-01 | Cytogenix, Inc. | Enzymatic synthesis of ssdna |
| DE19956568A1 (en) | 1999-01-30 | 2000-08-17 | Roland Kreutzer | Method and medicament for inhibiting the expression of a given gene |
| EP1156812A4 (en) | 1999-02-23 | 2004-09-29 | Isis Pharmaceuticals Inc | Multiparticulate formulation |
| DE10100586C1 (en) | 2001-01-09 | 2002-04-11 | Ribopharma Ag | Inhibiting gene expression in cells, useful for e.g. treating tumors, by introducing double-stranded complementary oligoRNA having unpaired terminal bases |
| WO2003070912A2 (en) * | 2001-06-06 | 2003-08-28 | Sirna Therapeutics, Inc. | RNA INTERFERENCE MEDIATED INHIBITION OF EPIDERMAL GROWTH FACTOR RECEPTOR GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| DE10302421A1 (en) | 2003-01-21 | 2004-07-29 | Ribopharma Ag | New double-stranded interfering RNA, useful for inhibiting hepatitis C virus, has one strand linked to a lipophilic group to improve activity and eliminate the need for transfection auxiliaries |
| CN101291653B (en) | 2003-07-16 | 2012-06-27 | 普洛体维生物治疗公司 | Lipid encapsulated interfering rna |
| GB0327726D0 (en) | 2003-11-28 | 2003-12-31 | Isis Innovation | Method |
| US7740861B2 (en) | 2004-06-16 | 2010-06-22 | University Of Massachusetts | Drug delivery product and methods |
| KR20060119412A (en) | 2005-05-20 | 2006-11-24 | 아주대학교산학협력단 | Sirna for inhibiting il-6 expression and composition containing them |
| US8101741B2 (en) | 2005-11-02 | 2012-01-24 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| CN101426913A (en) | 2005-11-30 | 2009-05-06 | 因特拉迪格姆公司 | Compositions and methods of using siRNA to knockdown gene expression and to improve solid organ and cell transplantation |
| WO2008042973A2 (en) | 2006-10-03 | 2008-04-10 | Alnylam Pharmaceuticals, Inc. | Lipid containing formulations |
| WO2008109350A2 (en) * | 2007-03-02 | 2008-09-12 | Mdrna, Inc. | Nucleic acid compounds for inhibiting il6 gene expression and uses thereof |
| EP2690175B1 (en) | 2008-09-02 | 2016-12-28 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for combined inhibition of mutant EGFR gene and IL-6 expression |
-
2009
- 2009-09-02 EP EP13179293.9A patent/EP2690175B1/en active Active
- 2009-09-02 EP EP09792177.9A patent/EP2331690B1/en active Active
- 2009-09-02 EP EP16205971.1A patent/EP3208337A1/en not_active Withdrawn
- 2009-09-02 WO PCT/US2009/055745 patent/WO2010028054A1/en active Application Filing
- 2009-09-02 US US13/061,569 patent/US8318693B2/en active Active
-
2012
- 2012-10-24 US US13/659,315 patent/US9212364B2/en active Active
-
2015
- 2015-11-09 US US14/936,059 patent/US9957507B2/en active Active
-
2018
- 2018-03-30 US US15/942,126 patent/US20190010502A1/en not_active Abandoned
-
2020
- 2020-03-03 US US16/807,828 patent/US20200377893A1/en not_active Abandoned
-
2022
- 2022-04-29 US US17/733,552 patent/US20230053332A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| EP3208337A1 (en) | 2017-08-23 |
| WO2010028054A8 (en) | 2010-04-29 |
| US20120022132A1 (en) | 2012-01-26 |
| US20130184329A1 (en) | 2013-07-18 |
| US8318693B2 (en) | 2012-11-27 |
| US9212364B2 (en) | 2015-12-15 |
| US20200377893A1 (en) | 2020-12-03 |
| EP2331690A1 (en) | 2011-06-15 |
| US9957507B2 (en) | 2018-05-01 |
| US20160194645A1 (en) | 2016-07-07 |
| EP2690175B1 (en) | 2016-12-28 |
| US20190010502A1 (en) | 2019-01-10 |
| EP2331690B1 (en) | 2016-01-13 |
| EP2690175A2 (en) | 2014-01-29 |
| EP2690175A3 (en) | 2014-05-07 |
| WO2010028054A1 (en) | 2010-03-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12031133B2 (en) | GNAQ targeted dsRNA compositions and methods for inhibiting expression | |
| US20230257740A1 (en) | Compositions and methods for inhibiting expression of transthyretin | |
| US20230053332A1 (en) | Compositions and methods for inhibiting expression of mutant egfr gene | |
| US8912316B2 (en) | Compositions and methods for inhibiting expression of CD45 gene | |
| US20120016006A1 (en) | Compositions And Methods For Increasing Cellular Uptake Of RNAi Via SID-1 | |
| AU2019216630A1 (en) | Compositions and methods for inhibiting expression of transthyretin |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: DOCKETED NEW CASE - READY FOR EXAMINATION |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NON FINAL ACTION MAILED |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |