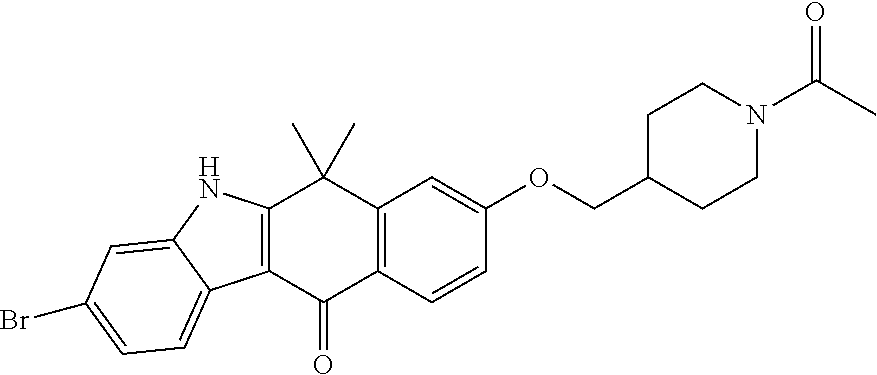
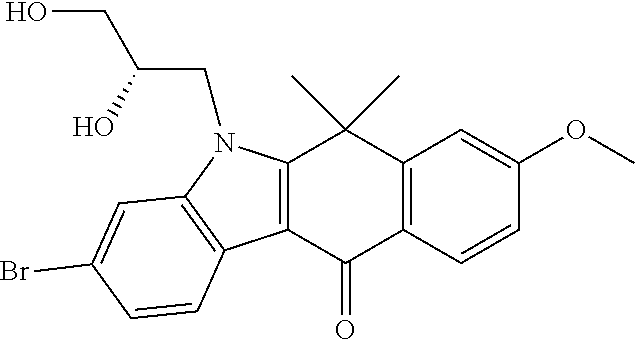
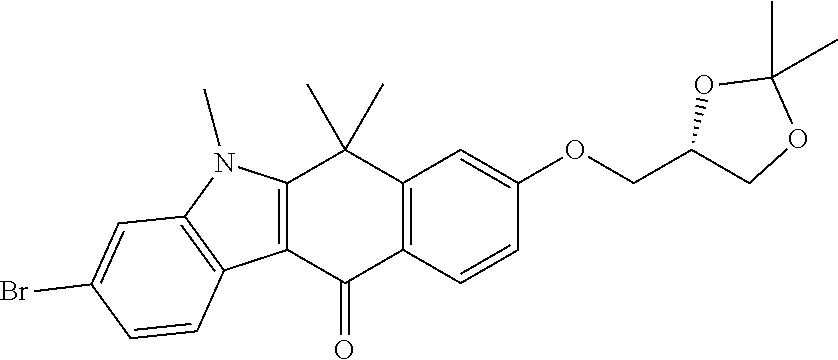
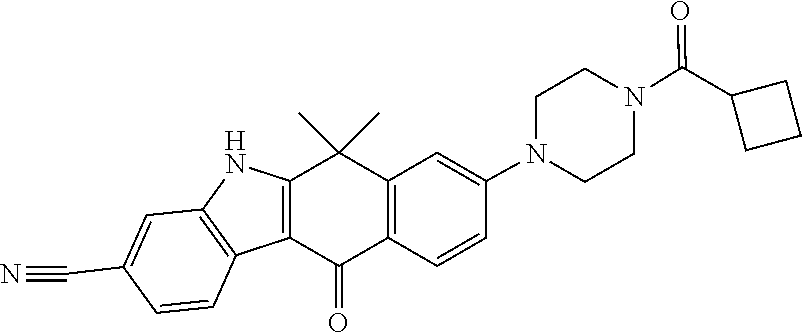
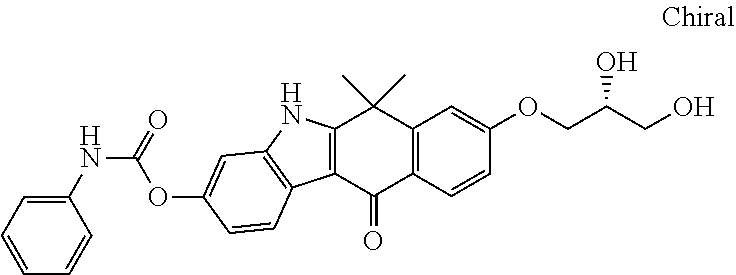
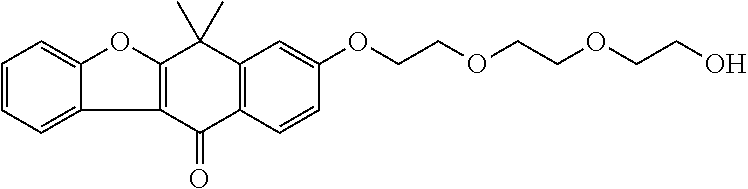
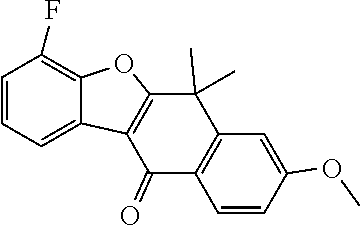
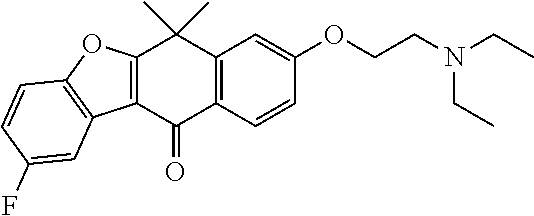
US20220306578A1 - Tetracyclic compound - Google Patents
Tetracyclic compound Download PDFInfo
- Publication number
- US20220306578A1 US20220306578A1 US16/862,125 US202016862125A US2022306578A1 US 20220306578 A1 US20220306578 A1 US 20220306578A1 US 202016862125 A US202016862125 A US 202016862125A US 2022306578 A1 US2022306578 A1 US 2022306578A1
- Authority
- US
- United States
- Prior art keywords
- group
- substituted
- alkyl
- hydroxy
- amino
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 558
- 150000003839 salts Chemical class 0.000 claims abstract description 48
- 239000012453 solvate Substances 0.000 claims abstract description 39
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 34
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 29
- 201000011510 cancer Diseases 0.000 claims abstract description 26
- 238000011282 treatment Methods 0.000 claims abstract description 19
- 230000003920 cognitive function Effects 0.000 claims abstract description 15
- 206010027476 Metastases Diseases 0.000 claims abstract description 11
- 230000009401 metastasis Effects 0.000 claims abstract description 11
- 238000011321 prophylaxis Methods 0.000 claims abstract description 7
- 229940122531 Anaplastic lymphoma kinase inhibitor Drugs 0.000 claims abstract description 5
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 claims description 450
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 268
- 125000001424 substituent group Chemical group 0.000 claims description 267
- -1 hydroxycarbonyl group Chemical group 0.000 claims description 239
- 125000000217 alkyl group Chemical group 0.000 claims description 230
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims description 200
- 125000003545 alkoxy group Chemical group 0.000 claims description 193
- 125000005843 halogen group Chemical group 0.000 claims description 158
- 238000000034 method Methods 0.000 claims description 157
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 115
- 125000004390 alkyl sulfonyl group Chemical group 0.000 claims description 108
- 125000004453 alkoxycarbonyl group Chemical group 0.000 claims description 104
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 claims description 99
- 125000003277 amino group Chemical group 0.000 claims description 99
- 125000000041 C6-C10 aryl group Chemical group 0.000 claims description 96
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 claims description 64
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 claims description 63
- 125000002252 acyl group Chemical group 0.000 claims description 62
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 62
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 50
- 125000001072 heteroaryl group Chemical group 0.000 claims description 46
- 125000004043 oxo group Chemical group O=* 0.000 claims description 45
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 claims description 35
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 claims description 34
- 125000004414 alkyl thio group Chemical group 0.000 claims description 32
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 22
- 125000006594 (C1-C3) alkylsulfony group Chemical group 0.000 claims description 20
- 125000004432 carbon atom Chemical group C* 0.000 claims description 20
- 125000005196 alkyl carbonyloxy group Chemical group 0.000 claims description 18
- 229910052757 nitrogen Inorganic materials 0.000 claims description 18
- 125000004448 alkyl carbonyl group Chemical group 0.000 claims description 17
- 125000005278 alkyl sulfonyloxy group Chemical group 0.000 claims description 17
- 208000035475 disorder Diseases 0.000 claims description 17
- 239000003814 drug Substances 0.000 claims description 17
- 125000005553 heteroaryloxy group Chemical group 0.000 claims description 15
- 125000005161 aryl oxy carbonyl group Chemical group 0.000 claims description 13
- 125000005129 aryl carbonyl group Chemical group 0.000 claims description 12
- 125000003302 alkenyloxy group Chemical group 0.000 claims description 10
- 125000004644 alkyl sulfinyl group Chemical group 0.000 claims description 10
- 125000004397 aminosulfonyl group Chemical group NS(=O)(=O)* 0.000 claims description 10
- 239000008194 pharmaceutical composition Substances 0.000 claims description 10
- 229910052717 sulfur Inorganic materials 0.000 claims description 10
- 239000004480 active ingredient Substances 0.000 claims description 9
- 229910052799 carbon Inorganic materials 0.000 claims description 9
- 125000004104 aryloxy group Chemical group 0.000 claims description 8
- ORTFAQDWJHRMNX-UHFFFAOYSA-N hydroxidooxidocarbon(.) Chemical group O[C]=O ORTFAQDWJHRMNX-UHFFFAOYSA-N 0.000 claims description 8
- 229910052760 oxygen Inorganic materials 0.000 claims description 8
- 125000004739 (C1-C6) alkylsulfonyl group Chemical group 0.000 claims description 7
- 125000006636 (C3-C8) cycloalkylcarbonyl group Chemical group 0.000 claims description 7
- 125000005193 alkenylcarbonyloxy group Chemical group 0.000 claims description 7
- 239000003937 drug carrier Substances 0.000 claims description 6
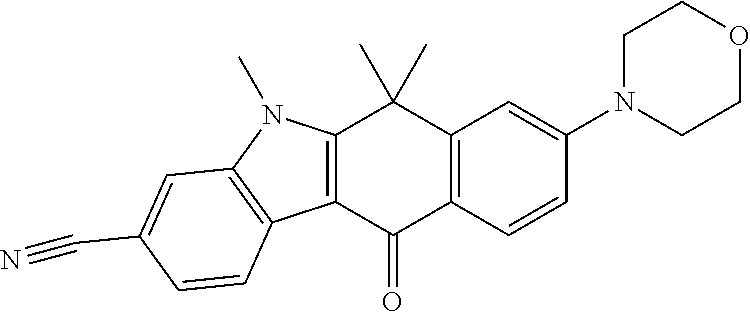
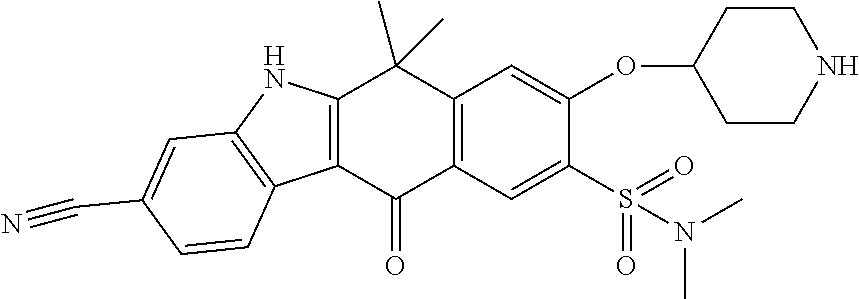
- FEBVWLCMSGQWIR-UHFFFAOYSA-N 9-ethynyl-6,6-dimethyl-8-morpholin-4-yl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(C#C)=C1N1CCOCC1 FEBVWLCMSGQWIR-UHFFFAOYSA-N 0.000 claims description 5
- 229910052805 deuterium Inorganic materials 0.000 claims description 5
- 125000005143 heteroarylsulfonyl group Chemical group 0.000 claims description 5
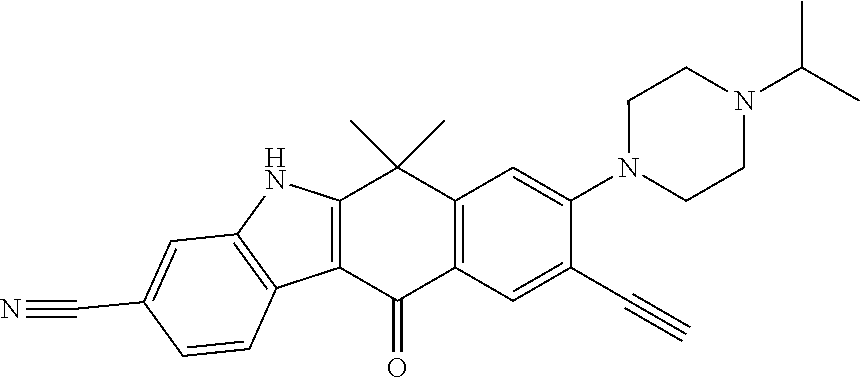
- OJAQYCZSPRPJGR-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-(4-propan-2-ylpiperazin-1-yl)-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(C(C)C)CCN1C1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 OJAQYCZSPRPJGR-UHFFFAOYSA-N 0.000 claims description 4
- IYFGVOZZJBVKEU-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-(4-pyrrolidin-1-ylpiperidin-1-yl)-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N(CC1)CCC1N1CCCC1 IYFGVOZZJBVKEU-UHFFFAOYSA-N 0.000 claims description 4
- AKAVFRAMRYYUBK-UHFFFAOYSA-N 8-[2-(tert-butylamino)ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNC(C)(C)C)=C3NC2=C1 AKAVFRAMRYYUBK-UHFFFAOYSA-N 0.000 claims description 4
- 125000004391 aryl sulfonyl group Chemical group 0.000 claims description 4
- GXWLTJGZFHXMSX-UHFFFAOYSA-N 6,6,9-trimethyl-8-(4-morpholin-4-ylpiperidin-1-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound CC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCC1N1CCOCC1 GXWLTJGZFHXMSX-UHFFFAOYSA-N 0.000 claims description 3
- LDPMDKZJARLSKX-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-(1-propan-2-ylpiperidin-4-yl)-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(C(C)C)CCC1C1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 LDPMDKZJARLSKX-UHFFFAOYSA-N 0.000 claims description 3
- AKVSDZYZDIOJIN-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-9-(4-propan-2-ylpiperazin-1-yl)-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(C(C)C)CCN1C1=CC=C2C(C)(C)C(NC=3C4=CC=C(C=3)C#N)=C4C(=O)C2=C1 AKVSDZYZDIOJIN-UHFFFAOYSA-N 0.000 claims description 3
- LLGFEJJPLZCCQL-UHFFFAOYSA-N 6,6-dimethyl-8-[1-(oxetan-3-yl)piperidin-4-yl]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1C(CC1)CCN1C1COC1 LLGFEJJPLZCCQL-UHFFFAOYSA-N 0.000 claims description 3
- CVXLHDNZWSLSQC-UHFFFAOYSA-N 6,6-dimethyl-8-[4-(oxetan-3-yl)piperazin-1-yl]-11-oxo-9-prop-1-ynyl-5h-benzo[b]carbazole-3-carbonitrile Chemical compound CC#CC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCN1C1COC1 CVXLHDNZWSLSQC-UHFFFAOYSA-N 0.000 claims description 3
- OUQRQZVDADPPCN-UHFFFAOYSA-N 6,6-dimethyl-8-[4-(oxetan-3-yl)piperazin-1-yl]-11-oxo-9-propyl-5h-benzo[b]carbazole-3-carbonitrile Chemical compound CCCC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCN1C1COC1 OUQRQZVDADPPCN-UHFFFAOYSA-N 0.000 claims description 3
- ZKUDBTZORJCEGR-UHFFFAOYSA-N 8-(4-cyclobutylpiperazin-1-yl)-6,6-dimethyl-11-oxo-9-prop-1-ynyl-5h-benzo[b]carbazole-3-carbonitrile Chemical compound CC#CC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCN1C1CCC1 ZKUDBTZORJCEGR-UHFFFAOYSA-N 0.000 claims description 3
- FKCYTIXKYHKHGS-UHFFFAOYSA-N 8-(4-cyclobutylpiperazin-1-yl)-6,6-dimethyl-11-oxo-9-propyl-5h-benzo[b]carbazole-3-carbonitrile Chemical compound CCCC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCN1C1CCC1 FKCYTIXKYHKHGS-UHFFFAOYSA-N 0.000 claims description 3
- RIKQYICGJAXSMT-UHFFFAOYSA-N 8-(4-cyclobutylpiperazin-1-yl)-9-cyclopropyl-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(C2CCC2)CCN1C=1C=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=1C1CC1 RIKQYICGJAXSMT-UHFFFAOYSA-N 0.000 claims description 3
- NNOQDCPCPZUPJA-UHFFFAOYSA-N 8-(4-cyclobutylpiperazin-1-yl)-9-ethyl-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound CCC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCN1C1CCC1 NNOQDCPCPZUPJA-UHFFFAOYSA-N 0.000 claims description 3
- FLLVBTUETQQQPR-UHFFFAOYSA-N 8-(4-cyclobutylpiperazin-1-yl)-9-ethynyl-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(C#C)=C1N(CC1)CCN1C1CCC1 FLLVBTUETQQQPR-UHFFFAOYSA-N 0.000 claims description 3
- BKRSEGHJIYGHPJ-UHFFFAOYSA-N 9-(2-cyclopropylethynyl)-6,6-dimethyl-8-[4-(oxetan-3-yl)piperazin-1-yl]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(C2COC2)CCN1C=1C=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=1C#CC1CC1 BKRSEGHJIYGHPJ-UHFFFAOYSA-N 0.000 claims description 3
- RUHPGASDSDOKTE-UHFFFAOYSA-N 9-bromo-6,6-dimethyl-8-[4-(oxetan-3-yl)piperazin-1-yl]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(Br)=C1N(CC1)CCN1C1COC1 RUHPGASDSDOKTE-UHFFFAOYSA-N 0.000 claims description 3
- FBLIRAHZHGXUFB-UHFFFAOYSA-N 9-bromo-8-(4-cyclobutylpiperazin-1-yl)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(Br)=C1N(CC1)CCN1C1CCC1 FBLIRAHZHGXUFB-UHFFFAOYSA-N 0.000 claims description 3
- XOHOLGXNXSPLQL-UHFFFAOYSA-N 9-bromo-8-(4-cyclopropylpiperazin-1-yl)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(Br)=C1N(CC1)CCN1C1CC1 XOHOLGXNXSPLQL-UHFFFAOYSA-N 0.000 claims description 3
- BTTUTLAQSFGRAD-UHFFFAOYSA-N 9-chloro-6,6-dimethyl-8-(4-morpholin-4-ylpiperidin-1-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(Cl)=C1N(CC1)CCC1N1CCOCC1 BTTUTLAQSFGRAD-UHFFFAOYSA-N 0.000 claims description 3
- XDTMZEAHVJVNJG-UHFFFAOYSA-N 9-ethyl-6,6-dimethyl-8-[4-(oxetan-3-yl)piperazin-1-yl]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound CCC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCN1C1COC1 XDTMZEAHVJVNJG-UHFFFAOYSA-N 0.000 claims description 3
- RFOCMACJDQBEMY-UHFFFAOYSA-N 9-ethynyl-6,6-dimethyl-11-oxo-8-(4-pyrrolidin-1-ylpiperidin-1-yl)-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(C#C)=C1N(CC1)CCC1N1CCCC1 RFOCMACJDQBEMY-UHFFFAOYSA-N 0.000 claims description 3
- COBQNQCQHCRNNB-UHFFFAOYSA-N 9-ethynyl-6,6-dimethyl-8-(4-methylsulfonylpiperazin-1-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(C#C)=C1N1CCN(S(C)(=O)=O)CC1 COBQNQCQHCRNNB-UHFFFAOYSA-N 0.000 claims description 3
- OIYDABDXSYUHNK-UHFFFAOYSA-N 9-ethynyl-6,6-dimethyl-8-[4-(oxetan-3-yl)piperazin-1-yl]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC(C#C)=C1N(CC1)CCN1C1COC1 OIYDABDXSYUHNK-UHFFFAOYSA-N 0.000 claims description 3
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 3
- 201000003803 Inflammatory myofibroblastic tumor Diseases 0.000 claims description 3
- 206010067917 Inflammatory myofibroblastic tumour Diseases 0.000 claims description 3
- 208000032004 Large-Cell Anaplastic Lymphoma Diseases 0.000 claims description 3
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 3
- 206010029260 Neuroblastoma Diseases 0.000 claims description 3
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 3
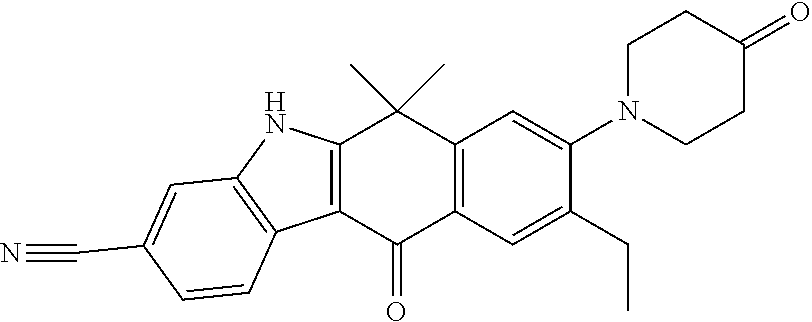
- KDGFLJKFZUIJMX-UHFFFAOYSA-N alectinib Chemical compound CCC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCC1N1CCOCC1 KDGFLJKFZUIJMX-UHFFFAOYSA-N 0.000 claims description 3
- 201000004101 esophageal cancer Diseases 0.000 claims description 3
- 201000005202 lung cancer Diseases 0.000 claims description 3
- 208000020816 lung neoplasm Diseases 0.000 claims description 3
- 206010073478 Anaplastic large-cell lymphoma Diseases 0.000 claims description 2
- 125000004431 deuterium atom Chemical group 0.000 claims 2
- 102100033793 ALK tyrosine kinase receptor Human genes 0.000 abstract description 25
- 201000010099 disease Diseases 0.000 abstract description 16
- 230000005856 abnormality Effects 0.000 abstract description 2
- 238000006243 chemical reaction Methods 0.000 description 159
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 147
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 120
- 238000004128 high performance liquid chromatography Methods 0.000 description 119
- 238000004458 analytical method Methods 0.000 description 114
- 230000014759 maintenance of location Effects 0.000 description 113
- 230000002194 synthesizing effect Effects 0.000 description 80
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 78
- 239000002904 solvent Substances 0.000 description 73
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 66
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 61
- 238000005160 1H NMR spectroscopy Methods 0.000 description 54
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 51
- 239000000243 solution Substances 0.000 description 51
- 235000019439 ethyl acetate Nutrition 0.000 description 50
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 49
- 239000000203 mixture Substances 0.000 description 48
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 43
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 42
- 230000002829 reductive effect Effects 0.000 description 38
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 36
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 35
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 33
- 125000003118 aryl group Chemical group 0.000 description 33
- 239000002585 base Substances 0.000 description 33
- 239000003054 catalyst Substances 0.000 description 33
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 31
- 239000012044 organic layer Substances 0.000 description 30
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 28
- 238000001914 filtration Methods 0.000 description 28
- DTQVDTLACAAQTR-UHFFFAOYSA-N trifluoroacetic acid Substances OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 27
- 101710168331 ALK tyrosine kinase receptor Proteins 0.000 description 24
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 24
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 24
- 239000002274 desiccant Substances 0.000 description 24
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 24
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 24
- ZHGNHOOVYPHPNJ-UHFFFAOYSA-N Amigdalin Chemical compound FC(F)(F)C(=O)OCC1OC(OCC2OC(OC(C#N)C3=CC=CC=C3)C(OC(=O)C(F)(F)F)C(OC(=O)C(F)(F)F)C2OC(=O)C(F)(F)F)C(OC(=O)C(F)(F)F)C(OC(=O)C(F)(F)F)C1OC(=O)C(F)(F)F ZHGNHOOVYPHPNJ-UHFFFAOYSA-N 0.000 description 21
- 238000009835 boiling Methods 0.000 description 21
- 125000006239 protecting group Chemical group 0.000 description 21
- 238000003786 synthesis reaction Methods 0.000 description 21
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 20
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 20
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical class Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 19
- 239000003795 chemical substances by application Substances 0.000 description 19
- 239000003446 ligand Substances 0.000 description 19
- KDLHZDBZIXYQEI-UHFFFAOYSA-N palladium Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 19
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 19
- 125000006274 (C1-C3)alkoxy group Chemical group 0.000 description 18
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 18
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 18
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 18
- 230000015572 biosynthetic process Effects 0.000 description 18
- 125000004122 cyclic group Chemical group 0.000 description 18
- 238000010898 silica gel chromatography Methods 0.000 description 18
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 16
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 16
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 16
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 16
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 16
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 16
- 229910000024 caesium carbonate Inorganic materials 0.000 description 16
- 238000002360 preparation method Methods 0.000 description 16
- 229910052938 sodium sulfate Inorganic materials 0.000 description 16
- 235000011152 sodium sulphate Nutrition 0.000 description 16
- 239000002253 acid Substances 0.000 description 15
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 15
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 14
- 239000003153 chemical reaction reagent Substances 0.000 description 14
- CXNIUSPIQKWYAI-UHFFFAOYSA-N xantphos Chemical compound C=12OC3=C(P(C=4C=CC=CC=4)C=4C=CC=CC=4)C=CC=C3C(C)(C)C2=CC=CC=1P(C=1C=CC=CC=1)C1=CC=CC=C1 CXNIUSPIQKWYAI-UHFFFAOYSA-N 0.000 description 14
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 13
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 description 13
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 13
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 13
- 238000003756 stirring Methods 0.000 description 13
- HZNVUJQVZSTENZ-UHFFFAOYSA-N 2,3-dichloro-5,6-dicyano-1,4-benzoquinone Chemical compound ClC1=C(Cl)C(=O)C(C#N)=C(C#N)C1=O HZNVUJQVZSTENZ-UHFFFAOYSA-N 0.000 description 12
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 12
- 229910000027 potassium carbonate Inorganic materials 0.000 description 12
- 239000000843 powder Substances 0.000 description 12
- 229910000029 sodium carbonate Inorganic materials 0.000 description 12
- 239000007787 solid Substances 0.000 description 12
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical class OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 11
- 238000010511 deprotection reaction Methods 0.000 description 11
- SKTCDJAMAYNROS-UHFFFAOYSA-N methoxycyclopentane Chemical compound COC1CCCC1 SKTCDJAMAYNROS-UHFFFAOYSA-N 0.000 description 11
- 125000004433 nitrogen atom Chemical group N* 0.000 description 11
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 10
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical class CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 10
- AHVYPIQETPWLSZ-UHFFFAOYSA-N N-methyl-pyrrolidine Natural products CN1CC=CC1 AHVYPIQETPWLSZ-UHFFFAOYSA-N 0.000 description 10
- 239000007864 aqueous solution Substances 0.000 description 10
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 10
- 239000012153 distilled water Substances 0.000 description 10
- 229910052731 fluorine Inorganic materials 0.000 description 10
- WRIKHQLVHPKCJU-UHFFFAOYSA-N sodium bis(trimethylsilyl)amide Chemical compound C[Si](C)(C)N([Na])[Si](C)(C)C WRIKHQLVHPKCJU-UHFFFAOYSA-N 0.000 description 10
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 9
- 125000006555 (C3-C5) cycloalkyl group Chemical group 0.000 description 9
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 9
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 9
- 230000003197 catalytic effect Effects 0.000 description 9
- 125000001153 fluoro group Chemical group F* 0.000 description 9
- 239000011968 lewis acid catalyst Substances 0.000 description 9
- YNESATAKKCNGOF-UHFFFAOYSA-N lithium bis(trimethylsilyl)amide Chemical compound [Li+].C[Si](C)(C)[N-][Si](C)(C)C YNESATAKKCNGOF-UHFFFAOYSA-N 0.000 description 9
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 9
- 235000019341 magnesium sulphate Nutrition 0.000 description 9
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 9
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 9
- 239000000126 substance Substances 0.000 description 9
- 239000008096 xylene Substances 0.000 description 9
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 8
- KZMGYPLQYOPHEL-UHFFFAOYSA-N Boron trifluoride etherate Chemical compound FB(F)F.CCOCC KZMGYPLQYOPHEL-UHFFFAOYSA-N 0.000 description 8
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 8
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 8
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 8
- 229910052801 chlorine Inorganic materials 0.000 description 8
- XHQZXHMRBXBPEL-UHFFFAOYSA-N eaton reagent Chemical compound CS(O)(=O)=O.O1P(O2)(=O)OP3(=O)OP1(=O)OP2(=O)O3 XHQZXHMRBXBPEL-UHFFFAOYSA-N 0.000 description 8
- 238000000605 extraction Methods 0.000 description 8
- 230000002401 inhibitory effect Effects 0.000 description 8
- ZCSHNCUQKCANBX-UHFFFAOYSA-N lithium diisopropylamide Chemical compound [Li+].CC(C)[N-]C(C)C ZCSHNCUQKCANBX-UHFFFAOYSA-N 0.000 description 8
- MZRVEZGGRBJDDB-UHFFFAOYSA-N n-Butyllithium Substances [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 8
- 150000002825 nitriles Chemical class 0.000 description 8
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 8
- IUBQJLUDMLPAGT-UHFFFAOYSA-N potassium bis(trimethylsilyl)amide Chemical compound C[Si](C)(C)N([K])[Si](C)(C)C IUBQJLUDMLPAGT-UHFFFAOYSA-N 0.000 description 8
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 8
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 8
- 238000007363 ring formation reaction Methods 0.000 description 8
- 239000012312 sodium hydride Substances 0.000 description 8
- 229910000104 sodium hydride Inorganic materials 0.000 description 8
- 229940124597 therapeutic agent Drugs 0.000 description 8
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 8
- QAEDZJGFFMLHHQ-UHFFFAOYSA-N trifluoroacetic anhydride Chemical compound FC(F)(F)C(=O)OC(=O)C(F)(F)F QAEDZJGFFMLHHQ-UHFFFAOYSA-N 0.000 description 8
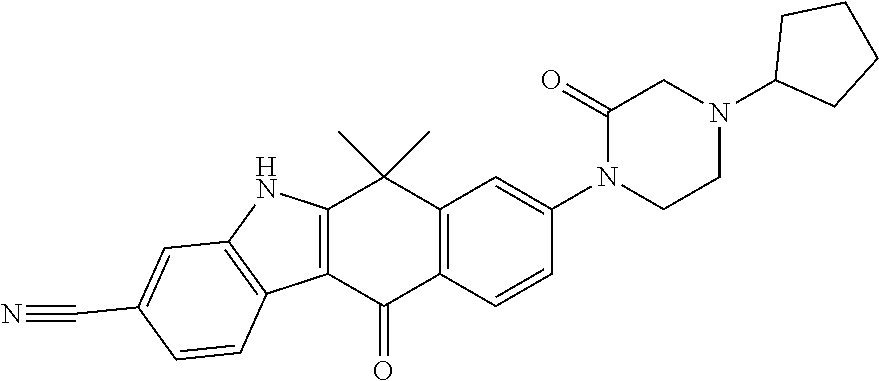
- RPRHWNHZMVOTGU-UHFFFAOYSA-N (3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl) trifluoromethanesulfonate Chemical compound O=C1C2=CC=C(OS(=O)(=O)C(F)(F)F)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 RPRHWNHZMVOTGU-UHFFFAOYSA-N 0.000 description 7
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 7
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 7
- YNHIGQDRGKUECZ-UHFFFAOYSA-L PdCl2(PPh3)2 Substances [Cl-].[Cl-].[Pd+2].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-L 0.000 description 7
- 125000001309 chloro group Chemical group Cl* 0.000 description 7
- 125000000524 functional group Chemical group 0.000 description 7
- 229910000160 potassium phosphate Inorganic materials 0.000 description 7
- 235000011009 potassium phosphates Nutrition 0.000 description 7
- 238000012746 preparative thin layer chromatography Methods 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 7
- COIOYMYWGDAQPM-UHFFFAOYSA-N tri(ortho-tolyl)phosphine Substances CC1=CC=CC=C1P(C=1C(=CC=CC=1)C)C1=CC=CC=C1C COIOYMYWGDAQPM-UHFFFAOYSA-N 0.000 description 7
- 125000004455 (C1-C3) alkylthio group Chemical group 0.000 description 6
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 description 6
- OISVCGZHLKNMSJ-UHFFFAOYSA-N 2,6-dimethylpyridine Chemical compound CC1=CC=CC(C)=N1 OISVCGZHLKNMSJ-UHFFFAOYSA-N 0.000 description 6
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 6
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 6
- RHQDFWAXVIIEBN-UHFFFAOYSA-N Trifluoroethanol Chemical compound OCC(F)(F)F RHQDFWAXVIIEBN-UHFFFAOYSA-N 0.000 description 6
- RBYGDVHOECIAFC-UHFFFAOYSA-L acetonitrile;palladium(2+);dichloride Chemical compound [Cl-].[Cl-].[Pd+2].CC#N.CC#N RBYGDVHOECIAFC-UHFFFAOYSA-L 0.000 description 6
- 125000004429 atom Chemical group 0.000 description 6
- MUALRAIOVNYAIW-UHFFFAOYSA-N binap Chemical compound C1=CC=CC=C1P(C=1C(=C2C=CC=CC2=CC=1)C=1C2=CC=CC=C2C=CC=1P(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 MUALRAIOVNYAIW-UHFFFAOYSA-N 0.000 description 6
- 230000037396 body weight Effects 0.000 description 6
- 239000012267 brine Substances 0.000 description 6
- 150000001733 carboxylic acid esters Chemical group 0.000 description 6
- 238000001816 cooling Methods 0.000 description 6
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 6
- SHFJWMWCIHQNCP-UHFFFAOYSA-M hydron;tetrabutylazanium;sulfate Chemical compound OS([O-])(=O)=O.CCCC[N+](CCCC)(CCCC)CCCC SHFJWMWCIHQNCP-UHFFFAOYSA-M 0.000 description 6
- KMAKOBLIOCQGJP-UHFFFAOYSA-N indole-3-carboxylic acid Chemical compound C1=CC=C2C(C(=O)O)=CNC2=C1 KMAKOBLIOCQGJP-UHFFFAOYSA-N 0.000 description 6
- 150000007529 inorganic bases Chemical class 0.000 description 6
- 239000012299 nitrogen atmosphere Substances 0.000 description 6
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 6
- 230000000069 prophylactic effect Effects 0.000 description 6
- 229920006395 saturated elastomer Polymers 0.000 description 6
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 6
- 239000003643 water by type Substances 0.000 description 6
- UGOMMVLRQDMAQQ-UHFFFAOYSA-N xphos Chemical compound CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 UGOMMVLRQDMAQQ-UHFFFAOYSA-N 0.000 description 6
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Substances CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 5
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 5
- ZEMZPXWZVTUONV-UHFFFAOYSA-N 2-(2-dicyclohexylphosphanylphenyl)-n,n-dimethylaniline Chemical compound CN(C)C1=CC=CC=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 ZEMZPXWZVTUONV-UHFFFAOYSA-N 0.000 description 5
- VBKIEGIMQUUFOU-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]acetic acid Chemical compound O=C1C2=CC=C(OCC(O)=O)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 VBKIEGIMQUUFOU-UHFFFAOYSA-N 0.000 description 5
- AMPWXZFRDSJWSN-UHFFFAOYSA-N 8-hydroxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(O)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 AMPWXZFRDSJWSN-UHFFFAOYSA-N 0.000 description 5
- FEJUGLKDZJDVFY-UHFFFAOYSA-N 9-borabicyclo[3.3.1]nonane Substances C1CCC2CCCC1B2 FEJUGLKDZJDVFY-UHFFFAOYSA-N 0.000 description 5
- GSNUFIFRDBKVIE-UHFFFAOYSA-N DMF Natural products CC1=CC=C(C)O1 GSNUFIFRDBKVIE-UHFFFAOYSA-N 0.000 description 5
- IDKFJAWYYLNKGO-UHFFFAOYSA-N [5-bis[3,5-bis(trifluoromethyl)phenyl]phosphanyl-9,9-dimethylxanthen-4-yl]-bis[3,5-bis(trifluoromethyl)phenyl]phosphane Chemical compound C=12OC3=C(P(C=4C=C(C=C(C=4)C(F)(F)F)C(F)(F)F)C=4C=C(C=C(C=4)C(F)(F)F)C(F)(F)F)C=CC=C3C(C)(C)C2=CC=CC=1P(C=1C=C(C=C(C=1)C(F)(F)F)C(F)(F)F)C1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1 IDKFJAWYYLNKGO-UHFFFAOYSA-N 0.000 description 5
- SPEUIVXLLWOEMJ-UHFFFAOYSA-N acetaldehyde dimethyl acetal Natural products COC(C)OC SPEUIVXLLWOEMJ-UHFFFAOYSA-N 0.000 description 5
- FKOASGGZYSYPBI-UHFFFAOYSA-K bis(trifluoromethylsulfonyloxy)alumanyl trifluoromethanesulfonate Chemical compound [Al+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F FKOASGGZYSYPBI-UHFFFAOYSA-K 0.000 description 5
- NYENCOMLZDQKNH-UHFFFAOYSA-K bis(trifluoromethylsulfonyloxy)bismuthanyl trifluoromethanesulfonate Chemical compound [Bi+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F NYENCOMLZDQKNH-UHFFFAOYSA-K 0.000 description 5
- 239000006227 byproduct Substances 0.000 description 5
- 150000001721 carbon Chemical group 0.000 description 5
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 5
- XMPZTFVPEKAKFH-UHFFFAOYSA-P ceric ammonium nitrate Chemical compound [NH4+].[NH4+].[Ce+4].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O XMPZTFVPEKAKFH-UHFFFAOYSA-P 0.000 description 5
- 229910052802 copper Inorganic materials 0.000 description 5
- 239000010949 copper Substances 0.000 description 5
- 239000012043 crude product Substances 0.000 description 5
- CNXMDTWQWLGCPE-UHFFFAOYSA-N ditert-butyl-(2-phenylphenyl)phosphane Chemical compound CC(C)(C)P(C(C)(C)C)C1=CC=CC=C1C1=CC=CC=C1 CNXMDTWQWLGCPE-UHFFFAOYSA-N 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 125000004185 ester group Chemical group 0.000 description 5
- 125000005842 heteroatom Chemical group 0.000 description 5
- 125000000623 heterocyclic group Chemical group 0.000 description 5
- 230000007062 hydrolysis Effects 0.000 description 5
- 238000006460 hydrolysis reaction Methods 0.000 description 5
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 5
- 229910052744 lithium Inorganic materials 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 150000007530 organic bases Chemical class 0.000 description 5
- 229910052763 palladium Inorganic materials 0.000 description 5
- 239000000651 prodrug Substances 0.000 description 5
- 229940002612 prodrug Drugs 0.000 description 5
- 238000000926 separation method Methods 0.000 description 5
- VNFWTIYUKDMAOP-UHFFFAOYSA-N sphos Chemical compound COC1=CC=CC(OC)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 VNFWTIYUKDMAOP-UHFFFAOYSA-N 0.000 description 5
- HXJUTPCZVOIRIF-UHFFFAOYSA-N sulfolane Chemical compound O=S1(=O)CCCC1 HXJUTPCZVOIRIF-UHFFFAOYSA-N 0.000 description 5
- 125000001412 tetrahydropyranyl group Chemical group 0.000 description 5
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical class CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 5
- AHZJKOKFZJYCLG-UHFFFAOYSA-K trifluoromethanesulfonate;ytterbium(3+) Chemical compound [Yb+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F AHZJKOKFZJYCLG-UHFFFAOYSA-K 0.000 description 5
- CSRZQMIRAZTJOY-UHFFFAOYSA-N trimethylsilyl iodide Substances C[Si](C)(C)I CSRZQMIRAZTJOY-UHFFFAOYSA-N 0.000 description 5
- 239000003981 vehicle Substances 0.000 description 5
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 4
- CBJKFCBCSGBQSD-UHFFFAOYSA-N 8-methoxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OC)=C3NC2=C1 CBJKFCBCSGBQSD-UHFFFAOYSA-N 0.000 description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 4
- 238000005727 Friedel-Crafts reaction Methods 0.000 description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 4
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 4
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical class [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 4
- 150000008065 acid anhydrides Chemical class 0.000 description 4
- 125000003342 alkenyl group Chemical group 0.000 description 4
- 125000004694 alkoxyaminocarbonyl group Chemical group 0.000 description 4
- 235000019270 ammonium chloride Nutrition 0.000 description 4
- 229920002678 cellulose Polymers 0.000 description 4
- 239000001913 cellulose Substances 0.000 description 4
- GBRBMTNGQBKBQE-UHFFFAOYSA-L copper;diiodide Chemical compound I[Cu]I GBRBMTNGQBKBQE-UHFFFAOYSA-L 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- 150000003997 cyclic ketones Chemical class 0.000 description 4
- 229910001873 dinitrogen Inorganic materials 0.000 description 4
- 229910052736 halogen Inorganic materials 0.000 description 4
- 150000002367 halogens Chemical class 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 239000000543 intermediate Substances 0.000 description 4
- 229940098779 methanesulfonic acid Drugs 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 4
- 239000007800 oxidant agent Substances 0.000 description 4
- PENAXHPKEVTBLF-UHFFFAOYSA-L palladium(2+);prop-1-ene;dichloride Chemical class [Pd+]Cl.[Pd+]Cl.[CH2-]C=C.[CH2-]C=C PENAXHPKEVTBLF-UHFFFAOYSA-L 0.000 description 4
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 4
- 229920000137 polyphosphoric acid Polymers 0.000 description 4
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 4
- 230000008707 rearrangement Effects 0.000 description 4
- 239000001488 sodium phosphate Substances 0.000 description 4
- 229910000162 sodium phosphate Inorganic materials 0.000 description 4
- 239000008107 starch Substances 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 4
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 4
- BWHDROKFUHTORW-UHFFFAOYSA-N tritert-butylphosphane Chemical compound CC(C)(C)P(C(C)(C)C)C(C)(C)C BWHDROKFUHTORW-UHFFFAOYSA-N 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 3
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 description 3
- VLSDXINSOMDCBK-BQYQJAHWSA-N (E)-1,1'-azobis(N,N-dimethylformamide) Chemical compound CN(C)C(=O)\N=N\C(=O)N(C)C VLSDXINSOMDCBK-BQYQJAHWSA-N 0.000 description 3
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical compound C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 description 3
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- UOXJNGFFPMOZDM-UHFFFAOYSA-N 2-[di(propan-2-yl)amino]ethylsulfanyl-methylphosphinic acid Chemical compound CC(C)N(C(C)C)CCSP(C)(O)=O UOXJNGFFPMOZDM-UHFFFAOYSA-N 0.000 description 3
- CFMZSMGAMPBRBE-UHFFFAOYSA-N 2-hydroxyisoindole-1,3-dione Chemical compound C1=CC=C2C(=O)N(O)C(=O)C2=C1 CFMZSMGAMPBRBE-UHFFFAOYSA-N 0.000 description 3
- UXBFRNQTNRAQNS-UHFFFAOYSA-N 8-(2-aminoethoxy)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(OCCN)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 UXBFRNQTNRAQNS-UHFFFAOYSA-N 0.000 description 3
- AMKGKYQBASDDJB-UHFFFAOYSA-N 9$l^{2}-borabicyclo[3.3.1]nonane Chemical compound C1CCC2CCCC1[B]2 AMKGKYQBASDDJB-UHFFFAOYSA-N 0.000 description 3
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 3
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 description 3
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 description 3
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- 239000012359 Methanesulfonyl chloride Substances 0.000 description 3
- 238000005481 NMR spectroscopy Methods 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 3
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 3
- 238000003477 Sonogashira cross-coupling reaction Methods 0.000 description 3
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 3
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 3
- 230000003213 activating effect Effects 0.000 description 3
- 125000001931 aliphatic group Chemical group 0.000 description 3
- 150000001345 alkine derivatives Chemical group 0.000 description 3
- 150000001350 alkyl halides Chemical class 0.000 description 3
- 239000002168 alkylating agent Substances 0.000 description 3
- 229940100198 alkylating agent Drugs 0.000 description 3
- 125000000304 alkynyl group Chemical group 0.000 description 3
- 150000001491 aromatic compounds Chemical class 0.000 description 3
- SIKJAQJRHWYJAI-UHFFFAOYSA-N benzopyrrole Natural products C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 3
- 125000000609 carbazolyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 238000006473 carboxylation reaction Methods 0.000 description 3
- 239000003638 chemical reducing agent Substances 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 125000000753 cycloalkyl group Chemical group 0.000 description 3
- FAMRKDQNMBBFBR-BQYQJAHWSA-N diethyl azodicarboxylate Substances CCOC(=O)\N=N\C(=O)OCC FAMRKDQNMBBFBR-BQYQJAHWSA-N 0.000 description 3
- HSUGRBWQSSZJOP-RTWAWAEBSA-N diltiazem Chemical compound C1=CC(OC)=CC=C1[C@H]1[C@@H](OC(C)=O)C(=O)N(CCN(C)C)C2=CC=CC=C2S1 HSUGRBWQSSZJOP-RTWAWAEBSA-N 0.000 description 3
- 238000004821 distillation Methods 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 125000001041 indolyl group Chemical group 0.000 description 3
- 238000003402 intramolecular cyclocondensation reaction Methods 0.000 description 3
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- QARBMVPHQWIHKH-UHFFFAOYSA-N methanesulfonyl chloride Chemical compound CS(Cl)(=O)=O QARBMVPHQWIHKH-UHFFFAOYSA-N 0.000 description 3
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 3
- DAOUXLWTJKRSSU-UHFFFAOYSA-N n-(2-bromo-5-cyanophenyl)methanesulfonamide Chemical compound CS(=O)(=O)NC1=CC(C#N)=CC=C1Br DAOUXLWTJKRSSU-UHFFFAOYSA-N 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 229910052698 phosphorus Inorganic materials 0.000 description 3
- 125000000587 piperidin-1-yl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 230000002062 proliferating effect Effects 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 239000000741 silica gel Substances 0.000 description 3
- 229910002027 silica gel Inorganic materials 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 239000007858 starting material Substances 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 3
- ITMCEJHCFYSIIV-UHFFFAOYSA-N triflic acid Chemical compound OS(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-N 0.000 description 3
- 125000006595 (C1-C3) alkylsulfinyl group Chemical group 0.000 description 2
- 125000004454 (C1-C6) alkoxycarbonyl group Chemical group 0.000 description 2
- 125000006592 (C2-C3) alkenyl group Chemical group 0.000 description 2
- 125000006593 (C2-C3) alkynyl group Chemical group 0.000 description 2
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 description 2
- NDOVLWQBFFJETK-UHFFFAOYSA-N 1,4-thiazinane 1,1-dioxide Chemical compound O=S1(=O)CCNCC1 NDOVLWQBFFJETK-UHFFFAOYSA-N 0.000 description 2
- HJTVAAODOXTIDT-UHFFFAOYSA-N 2-(2-hydroxypropan-2-yl)-1h-indole-6-carbonitrile Chemical compound C1=C(C#N)C=C2NC(C(C)(O)C)=CC2=C1 HJTVAAODOXTIDT-UHFFFAOYSA-N 0.000 description 2
- SBASXUCJHJRPEV-UHFFFAOYSA-N 2-(2-methoxyethoxy)ethanol Chemical compound COCCOCCO SBASXUCJHJRPEV-UHFFFAOYSA-N 0.000 description 2
- HTFNVAVTYILUCF-UHFFFAOYSA-N 2-[2-ethoxy-4-[4-(4-methylpiperazin-1-yl)piperidine-1-carbonyl]anilino]-5-methyl-11-methylsulfonylpyrimido[4,5-b][1,4]benzodiazepin-6-one Chemical compound CCOc1cc(ccc1Nc1ncc2N(C)C(=O)c3ccccc3N(c2n1)S(C)(=O)=O)C(=O)N1CCC(CC1)N1CCN(C)CC1 HTFNVAVTYILUCF-UHFFFAOYSA-N 0.000 description 2
- WHLOIJQDEQPQFS-UHFFFAOYSA-N 2-[[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]acetyl]amino]ethyl 2-methylprop-2-enoate Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCC(=O)NCCOC(=O)C(=C)C)=C3NC2=C1 WHLOIJQDEQPQFS-UHFFFAOYSA-N 0.000 description 2
- LDLCZOVUSADOIV-UHFFFAOYSA-N 2-bromoethanol Chemical compound OCCBr LDLCZOVUSADOIV-UHFFFAOYSA-N 0.000 description 2
- XWKFPIODWVPXLX-UHFFFAOYSA-N 2-methyl-5-methylpyridine Natural products CC1=CC=C(C)N=C1 XWKFPIODWVPXLX-UHFFFAOYSA-N 0.000 description 2
- WRMNZCZEMHIOCP-UHFFFAOYSA-N 2-phenylethanol Chemical compound OCCC1=CC=CC=C1 WRMNZCZEMHIOCP-UHFFFAOYSA-N 0.000 description 2
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 2
- GGDYGOHFANSCSU-UHFFFAOYSA-N 3-bromo-8-methoxy-6,6-dimethyl-5h-benzo[b]carbazol-11-one Chemical compound BrC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OC)=C3NC2=C1 GGDYGOHFANSCSU-UHFFFAOYSA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- CSDQQAQKBAQLLE-UHFFFAOYSA-N 4-(4-chlorophenyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Chemical compound C1=CC(Cl)=CC=C1C1C(C=CS2)=C2CCN1 CSDQQAQKBAQLLE-UHFFFAOYSA-N 0.000 description 2
- CFKMVGJGLGKFKI-UHFFFAOYSA-N 4-chloro-m-cresol Chemical compound CC1=CC(O)=CC=C1Cl CFKMVGJGLGKFKI-UHFFFAOYSA-N 0.000 description 2
- MGZPAZZMHQXDEC-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-(piperidin-4-ylmethoxy)-5h-benzo[b]carbazole-3-carbonitrile;hydrochloride Chemical compound Cl.C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCC1CCNCC1 MGZPAZZMHQXDEC-UHFFFAOYSA-N 0.000 description 2
- UUAPUUVYUFBVPN-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-[(2-phenyl-1,3-dioxan-5-yl)oxy]-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OC(CO1)COC1C1=CC=CC=C1 UUAPUUVYUFBVPN-UHFFFAOYSA-N 0.000 description 2
- SFHYNDMGZXWXBU-LIMNOBDPSA-N 6-amino-2-[[(e)-(3-formylphenyl)methylideneamino]carbamoylamino]-1,3-dioxobenzo[de]isoquinoline-5,8-disulfonic acid Chemical compound O=C1C(C2=3)=CC(S(O)(=O)=O)=CC=3C(N)=C(S(O)(=O)=O)C=C2C(=O)N1NC(=O)N\N=C\C1=CC=CC(C=O)=C1 SFHYNDMGZXWXBU-LIMNOBDPSA-N 0.000 description 2
- XKNWKUDWLZEPBU-UHFFFAOYSA-N 7-methoxy-1,1-dimethyl-3,4-dihydronaphthalen-2-one Chemical compound C1CC(=O)C(C)(C)C2=CC(OC)=CC=C21 XKNWKUDWLZEPBU-UHFFFAOYSA-N 0.000 description 2
- DOGCBTCESYMHKI-UHFFFAOYSA-N 8-(2-bromoethoxy)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(OCCBr)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 DOGCBTCESYMHKI-UHFFFAOYSA-N 0.000 description 2
- GLSLTGYOLWHGHV-UHFFFAOYSA-N 8-(4-hydroxypiperidin-1-yl)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCC(O)CC1 GLSLTGYOLWHGHV-UHFFFAOYSA-N 0.000 description 2
- INSGBIIBCRFLFF-UHFFFAOYSA-N 8-[tert-butyl(dimethyl)silyl]oxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)O[Si](C)(C)C(C)(C)C)=C3NC2=C1 INSGBIIBCRFLFF-UHFFFAOYSA-N 0.000 description 2
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 239000007848 Bronsted acid Substances 0.000 description 2
- 208000005623 Carcinogenesis Diseases 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- 229920002261 Corn starch Polymers 0.000 description 2
- RGSFGYAAUTVSQA-UHFFFAOYSA-N Cyclopentane Chemical compound C1CCCC1 RGSFGYAAUTVSQA-UHFFFAOYSA-N 0.000 description 2
- 230000004544 DNA amplification Effects 0.000 description 2
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 2
- GKQLYSROISKDLL-UHFFFAOYSA-N EEDQ Chemical compound C1=CC=C2N(C(=O)OCC)C(OCC)C=CC2=C1 GKQLYSROISKDLL-UHFFFAOYSA-N 0.000 description 2
- 239000001856 Ethyl cellulose Substances 0.000 description 2
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 2
- 108090000368 Fibroblast growth factor 8 Proteins 0.000 description 2
- XPDWGBQVDMORPB-UHFFFAOYSA-N Fluoroform Chemical compound FC(F)F XPDWGBQVDMORPB-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 239000007818 Grignard reagent Substances 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 102100030335 Midkine Human genes 0.000 description 2
- 108010092801 Midkine Proteins 0.000 description 2
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 2
- NFHFRUOZVGFOOS-UHFFFAOYSA-N Pd(PPh3)4 Substances [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 125000004656 alkyl sulfonylamino group Chemical group 0.000 description 2
- 230000029936 alkylation Effects 0.000 description 2
- 238000005804 alkylation reaction Methods 0.000 description 2
- 150000003863 ammonium salts Chemical class 0.000 description 2
- 238000006254 arylation reaction Methods 0.000 description 2
- 229910052788 barium Inorganic materials 0.000 description 2
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 2
- 229940092714 benzenesulfonic acid Drugs 0.000 description 2
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 2
- 125000002619 bicyclic group Chemical group 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- ILAHWRKJUDSMFH-UHFFFAOYSA-N boron tribromide Chemical compound BrB(Br)Br ILAHWRKJUDSMFH-UHFFFAOYSA-N 0.000 description 2
- XJHCXCQVJFPJIK-UHFFFAOYSA-M caesium fluoride Chemical compound [F-].[Cs+] XJHCXCQVJFPJIK-UHFFFAOYSA-M 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 230000036952 cancer formation Effects 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- 230000021523 carboxylation Effects 0.000 description 2
- 231100000504 carcinogenesis Toxicity 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 239000007810 chemical reaction solvent Substances 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- IJOOHPMOJXWVHK-UHFFFAOYSA-N chlorotrimethylsilane Chemical compound C[Si](C)(C)Cl IJOOHPMOJXWVHK-UHFFFAOYSA-N 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- DOBRDRYODQBAMW-UHFFFAOYSA-N copper(i) cyanide Chemical compound [Cu+].N#[C-] DOBRDRYODQBAMW-UHFFFAOYSA-N 0.000 description 2
- 239000008120 corn starch Substances 0.000 description 2
- 238000001212 derivatisation Methods 0.000 description 2
- 150000001975 deuterium Chemical group 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- CTSPAMFJBXKSOY-UHFFFAOYSA-N ellipticine Chemical compound N1=CC=C2C(C)=C(NC=3C4=CC=CC=3)C4=C(C)C2=C1 CTSPAMFJBXKSOY-UHFFFAOYSA-N 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 150000002081 enamines Chemical group 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 235000019325 ethyl cellulose Nutrition 0.000 description 2
- 229920001249 ethyl cellulose Polymers 0.000 description 2
- FAMRKDQNMBBFBR-UHFFFAOYSA-N ethyl n-ethoxycarbonyliminocarbamate Chemical compound CCOC(=O)N=NC(=O)OCC FAMRKDQNMBBFBR-UHFFFAOYSA-N 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 235000019634 flavors Nutrition 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 150000004795 grignard reagents Chemical class 0.000 description 2
- 230000002140 halogenating effect Effects 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N hydrazine Substances NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- 150000002429 hydrazines Chemical class 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 125000003453 indazolyl group Chemical class N1N=C(C2=C1C=CC=C2)* 0.000 description 2
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 2
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 239000010410 layer Substances 0.000 description 2
- DLEDOFVPSDKWEF-UHFFFAOYSA-N lithium butane Chemical compound [Li+].CCC[CH2-] DLEDOFVPSDKWEF-UHFFFAOYSA-N 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 159000000003 magnesium salts Chemical class 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- XQSHBPPIDMBFFE-UHFFFAOYSA-N methyl 2-[2-(6-cyano-1-methylsulfonylindol-2-yl)propan-2-yl]-4-methoxybenzoate Chemical compound COC(=O)C1=CC=C(OC)C=C1C(C)(C)C1=CC2=CC=C(C#N)C=C2N1S(C)(=O)=O XQSHBPPIDMBFFE-UHFFFAOYSA-N 0.000 description 2
- PTEMPYDJJRBFHW-UHFFFAOYSA-N methyl 4-methoxy-2-(2-methyl-4-trimethylsilylbut-3-yn-2-yl)benzoate Chemical compound COC(=O)C1=CC=C(OC)C=C1C(C)(C)C#C[Si](C)(C)C PTEMPYDJJRBFHW-UHFFFAOYSA-N 0.000 description 2
- PPVUJRVFOIYJFF-UHFFFAOYSA-N methyl 4-methoxy-2-(2-methylbut-3-yn-2-yl)benzoate Chemical compound COC(=O)C1=CC=C(OC)C=C1C(C)(C)C#C PPVUJRVFOIYJFF-UHFFFAOYSA-N 0.000 description 2
- GFMFQOWZSKLOFG-UHFFFAOYSA-N methyl 4-methoxy-2-(3-trimethylsilylprop-2-ynyl)benzoate Chemical compound COC(=O)C1=CC=C(OC)C=C1CC#C[Si](C)(C)C GFMFQOWZSKLOFG-UHFFFAOYSA-N 0.000 description 2
- GDOPTJXRTPNYNR-UHFFFAOYSA-N methyl-cyclopentane Natural products CC1CCCC1 GDOPTJXRTPNYNR-UHFFFAOYSA-N 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 125000002950 monocyclic group Chemical group 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- PSHKMPUSSFXUIA-UHFFFAOYSA-N n,n-dimethylpyridin-2-amine Chemical compound CN(C)C1=CC=CC=N1 PSHKMPUSSFXUIA-UHFFFAOYSA-N 0.000 description 2
- KEQQONPVAQQBHG-UHFFFAOYSA-N n-[4-(dimethylamino)pyridin-1-ium-1-yl]sulfonyl-1-[(2-methylpropan-2-yl)oxy]methanimidate Chemical compound C[N+](C)=C1C=CN(S(=O)(=O)[N-]C(=O)OC(C)(C)C)C=C1 KEQQONPVAQQBHG-UHFFFAOYSA-N 0.000 description 2
- LQNUZADURLCDLV-UHFFFAOYSA-N nitrobenzene Chemical compound [O-][N+](=O)C1=CC=CC=C1 LQNUZADURLCDLV-UHFFFAOYSA-N 0.000 description 2
- 150000004965 peroxy acids Chemical class 0.000 description 2
- 239000008177 pharmaceutical agent Substances 0.000 description 2
- 239000011574 phosphorus Substances 0.000 description 2
- 102000005162 pleiotrophin Human genes 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- NTTOTNSKUYCDAV-UHFFFAOYSA-N potassium hydride Chemical compound [KH] NTTOTNSKUYCDAV-UHFFFAOYSA-N 0.000 description 2
- 229910000105 potassium hydride Inorganic materials 0.000 description 2
- 239000012286 potassium permanganate Substances 0.000 description 2
- 229920001592 potato starch Polymers 0.000 description 2
- VVWRJUBEIPHGQF-UHFFFAOYSA-N propan-2-yl n-propan-2-yloxycarbonyliminocarbamate Chemical compound CC(C)OC(=O)N=NC(=O)OC(C)C VVWRJUBEIPHGQF-UHFFFAOYSA-N 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- AOJFQRQNPXYVLM-UHFFFAOYSA-N pyridin-1-ium;chloride Chemical compound [Cl-].C1=CC=[NH+]C=C1 AOJFQRQNPXYVLM-UHFFFAOYSA-N 0.000 description 2
- PTMBWNZJOQBTBK-UHFFFAOYSA-N pyridin-4-ylmethanol Chemical compound OCC1=CC=NC=C1 PTMBWNZJOQBTBK-UHFFFAOYSA-N 0.000 description 2
- YAAWASYJIRZXSZ-UHFFFAOYSA-N pyrimidine-2,4-diamine Chemical group NC1=CC=NC(N)=N1 YAAWASYJIRZXSZ-UHFFFAOYSA-N 0.000 description 2
- 125000002112 pyrrolidino group Chemical group [*]N1C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000006722 reduction reaction Methods 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- UKLNMMHNWFDKNT-UHFFFAOYSA-M sodium chlorite Chemical compound [Na+].[O-]Cl=O UKLNMMHNWFDKNT-UHFFFAOYSA-M 0.000 description 2
- 229960002218 sodium chlorite Drugs 0.000 description 2
- JVBXVOWTABLYPX-UHFFFAOYSA-L sodium dithionite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])=O JVBXVOWTABLYPX-UHFFFAOYSA-L 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 125000004434 sulfur atom Chemical group 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- JXAPVPFUQDGOLJ-UHFFFAOYSA-N tert-butyl 4-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)methyl]piperidine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)CCC1CC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 JXAPVPFUQDGOLJ-UHFFFAOYSA-N 0.000 description 2
- WPSISCGXTXYKTJ-UHFFFAOYSA-N tert-butyl 4-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]piperidine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)CCC1OC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 WPSISCGXTXYKTJ-UHFFFAOYSA-N 0.000 description 2
- WLXXXDCHYNARBD-UHFFFAOYSA-N tert-butyl 4-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]acetyl]piperazine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)CCN1C(=O)COC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 WLXXXDCHYNARBD-UHFFFAOYSA-N 0.000 description 2
- CWXPZXBSDSIRCS-UHFFFAOYSA-N tert-butyl piperazine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCNCC1 CWXPZXBSDSIRCS-UHFFFAOYSA-N 0.000 description 2
- NHGXDBSUJJNIRV-UHFFFAOYSA-M tetrabutylammonium chloride Chemical compound [Cl-].CCCC[N+](CCCC)(CCCC)CCCC NHGXDBSUJJNIRV-UHFFFAOYSA-M 0.000 description 2
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 229910052723 transition metal Inorganic materials 0.000 description 2
- 150000003624 transition metals Chemical class 0.000 description 2
- 230000005945 translocation Effects 0.000 description 2
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 description 2
- 125000004044 trifluoroacetyl group Chemical group FC(C(=O)*)(F)F 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 description 2
- VCGRFBXVSFAGGA-UHFFFAOYSA-N (1,1-dioxo-1,4-thiazinan-4-yl)-[6-[[3-(4-fluorophenyl)-5-methyl-1,2-oxazol-4-yl]methoxy]pyridin-3-yl]methanone Chemical compound CC=1ON=C(C=2C=CC(F)=CC=2)C=1COC(N=C1)=CC=C1C(=O)N1CCS(=O)(=O)CC1 VCGRFBXVSFAGGA-UHFFFAOYSA-N 0.000 description 1
- CYPYTURSJDMMMP-WVCUSYJESA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].[Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 CYPYTURSJDMMMP-WVCUSYJESA-N 0.000 description 1
- UKSZBOKPHAQOMP-SVLSSHOZSA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 UKSZBOKPHAQOMP-SVLSSHOZSA-N 0.000 description 1
- SSJXIUAHEKJCMH-PHDIDXHHSA-N (1r,2r)-cyclohexane-1,2-diamine Chemical compound N[C@@H]1CCCC[C@H]1N SSJXIUAHEKJCMH-PHDIDXHHSA-N 0.000 description 1
- HXOYWCSTHVTLOW-UHFFFAOYSA-N (2,2-dimethyl-1,3-dioxolan-4-yl)methanamine Chemical compound CC1(C)OCC(CN)O1 HXOYWCSTHVTLOW-UHFFFAOYSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- HNVIQLPOGUDBSU-OLQVQODUSA-N (2s,6r)-2,6-dimethylmorpholine Chemical compound C[C@H]1CNC[C@@H](C)O1 HNVIQLPOGUDBSU-OLQVQODUSA-N 0.000 description 1
- RPYIPFXHIKXRKS-UHFFFAOYSA-N (3-bromophenyl)hydrazine;hydron;chloride Chemical compound Cl.NNC1=CC=CC(Br)=C1 RPYIPFXHIKXRKS-UHFFFAOYSA-N 0.000 description 1
- 125000006559 (C1-C3) alkylamino group Chemical group 0.000 description 1
- 125000006603 (C1-C3) alkylaminosulfonyl group Chemical group 0.000 description 1
- 125000006596 (C1-C3) alkylcarbonyloxy group Chemical group 0.000 description 1
- 125000004738 (C1-C6) alkyl sulfinyl group Chemical group 0.000 description 1
- 125000006700 (C1-C6) alkylthio group Chemical group 0.000 description 1
- MIOPJNTWMNEORI-GMSGAONNSA-N (S)-camphorsulfonic acid Chemical compound C1C[C@@]2(CS(O)(=O)=O)C(=O)C[C@@H]1C2(C)C MIOPJNTWMNEORI-GMSGAONNSA-N 0.000 description 1
- OVKIDXBGVUQFFC-UHFFFAOYSA-N 1,1-dioxothiolan-3-amine Chemical group NC1CCS(=O)(=O)C1 OVKIDXBGVUQFFC-UHFFFAOYSA-N 0.000 description 1
- AUYBSFAHQLKXSW-UHFFFAOYSA-N 1,2-dichloroethane;3-(ethyliminomethylideneamino)-n,n-dimethylpropan-1-amine;hydrochloride Chemical compound Cl.ClCCCl.CCN=C=NCCCN(C)C AUYBSFAHQLKXSW-UHFFFAOYSA-N 0.000 description 1
- ULTHEAFYOOPTTB-UHFFFAOYSA-N 1,4-dibromobutane Chemical compound BrCCCCBr ULTHEAFYOOPTTB-UHFFFAOYSA-N 0.000 description 1
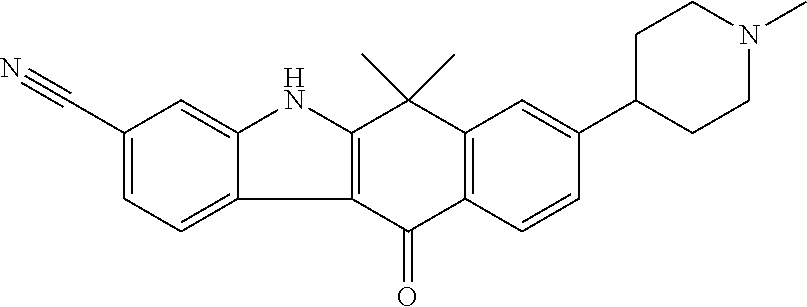
- OBUZDBBGOPBWSG-UHFFFAOYSA-N 1,6,6-trimethyl-11-oxo-8-piperidin-4-yl-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=2C(C)=CC(C#N)=CC=2NC(C(C2=C3)(C)C)=C1C(=O)C2=CC=C3C1CCNCC1 OBUZDBBGOPBWSG-UHFFFAOYSA-N 0.000 description 1
- HBAIZGPCSAAFSU-UHFFFAOYSA-N 1-(2-hydroxyethyl)imidazolidin-2-one Chemical compound OCCN1CCNC1=O HBAIZGPCSAAFSU-UHFFFAOYSA-N 0.000 description 1
- 125000005851 1-(N-(alkoxycarbonyl)amino)ethyl group Chemical group 0.000 description 1
- 125000005846 1-(alkanoyloxy)ethyl group Chemical group 0.000 description 1
- 125000005848 1-(alkoxycarbonyloxy)ethyl group Chemical group 0.000 description 1
- RBYCZVYQQLVQRA-UHFFFAOYSA-N 1-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethyl]piperidine-4-carboxamide Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCCN1CCC(C(N)=O)CC1 RBYCZVYQQLVQRA-UHFFFAOYSA-N 0.000 description 1
- FOZVXADQAHVUSV-UHFFFAOYSA-N 1-bromo-2-(2-bromoethoxy)ethane Chemical compound BrCCOCCBr FOZVXADQAHVUSV-UHFFFAOYSA-N 0.000 description 1
- YZUPZGFPHUVJKC-UHFFFAOYSA-N 1-bromo-2-methoxyethane Chemical compound COCCBr YZUPZGFPHUVJKC-UHFFFAOYSA-N 0.000 description 1
- HXGFHANKSAZRNL-UHFFFAOYSA-N 1-bromo-8-methoxy-6,6-dimethyl-5,11-dihydrobenzo[b]carbazole;3-bromo-8-methoxy-6,6-dimethyl-5,11-dihydrobenzo[b]carbazole Chemical compound BrC1=CC=C2C(CC3=CC=C(C=C3C3(C)C)OC)=C3NC2=C1.C1=CC(Br)=C2C(CC3=CC=C(C=C3C3(C)C)OC)=C3NC2=C1 HXGFHANKSAZRNL-UHFFFAOYSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- WGCYRFWNGRMRJA-UHFFFAOYSA-N 1-ethylpiperazine Chemical compound CCN1CCNCC1 WGCYRFWNGRMRJA-UHFFFAOYSA-N 0.000 description 1
- DFPYXQYWILNVAU-UHFFFAOYSA-N 1-hydroxybenzotriazole Chemical compound C1=CC=C2N(O)N=NC2=C1.C1=CC=C2N(O)N=NC2=C1 DFPYXQYWILNVAU-UHFFFAOYSA-N 0.000 description 1
- 125000005847 1-methyl-1-(alkanoyloxy)-ethyl group Chemical group 0.000 description 1
- 125000005849 1-methyl-1-(alkoxycarbonyloxy)ethyl group Chemical group 0.000 description 1
- UCNGGGYMLHAMJG-UHFFFAOYSA-N 1-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole Chemical compound C1=NN(C)C=C1B1OC(C)(C)C(C)(C)O1 UCNGGGYMLHAMJG-UHFFFAOYSA-N 0.000 description 1
- BAUWRHPMUVYFOD-UHFFFAOYSA-N 1-methylpiperidin-4-ol Chemical compound CN1CCC(O)CC1 BAUWRHPMUVYFOD-UHFFFAOYSA-N 0.000 description 1
- ZZAKLGGGMWORRT-UHFFFAOYSA-N 1-methylsulfonylpiperazine Chemical compound CS(=O)(=O)N1CCNCC1 ZZAKLGGGMWORRT-UHFFFAOYSA-N 0.000 description 1
- 125000001637 1-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C(*)=C([H])C([H])=C([H])C2=C1[H] 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- WHKWMTXTYKVFLK-UHFFFAOYSA-N 1-propan-2-ylpiperazine Chemical compound CC(C)N1CCNCC1 WHKWMTXTYKVFLK-UHFFFAOYSA-N 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- VUZNLSBZRVZGIK-UHFFFAOYSA-N 2,2,6,6-Tetramethyl-1-piperidinol Chemical group CC1(C)CCCC(C)(C)N1O VUZNLSBZRVZGIK-UHFFFAOYSA-N 0.000 description 1
- ZFFMLCVRJBZUDZ-UHFFFAOYSA-N 2,3-dimethylbutane Chemical group CC(C)C(C)C ZFFMLCVRJBZUDZ-UHFFFAOYSA-N 0.000 description 1
- IFNWESYYDINUHV-UHFFFAOYSA-N 2,6-dimethylpiperazine Chemical compound CC1CNCC(C)N1 IFNWESYYDINUHV-UHFFFAOYSA-N 0.000 description 1
- QAXLTZBBDSDRPW-UHFFFAOYSA-N 2-(diethylamino)ethanethiol;hydron;chloride Chemical compound Cl.CCN(CC)CCS QAXLTZBBDSDRPW-UHFFFAOYSA-N 0.000 description 1
- XNWFRZJHXBZDAG-UHFFFAOYSA-N 2-METHOXYETHANOL Chemical compound COCCO XNWFRZJHXBZDAG-UHFFFAOYSA-N 0.000 description 1
- ZYOQAYBBSMUFEV-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]-n-(2,3-dihydroxypropyl)acetamide Chemical compound O=C1C2=CC=C(OCC(=O)NCC(O)CO)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 ZYOQAYBBSMUFEV-UHFFFAOYSA-N 0.000 description 1
- NAQYMURKBCLJCX-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]-n-(2-cyanoethyl)-n-methylacetamide Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCC(=O)N(CCC#N)C)=C3NC2=C1 NAQYMURKBCLJCX-UHFFFAOYSA-N 0.000 description 1
- VYYUYKDVQXBDAY-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]-n-(2-cyanoethyl)acetamide Chemical compound O=C1C2=CC=C(OCC(=O)NCCC#N)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 VYYUYKDVQXBDAY-UHFFFAOYSA-N 0.000 description 1
- ZSPKHUPNUPTGOB-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]-n-(2-hydroxyethyl)acetamide Chemical compound O=C1C2=CC=C(OCC(=O)NCCO)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 ZSPKHUPNUPTGOB-UHFFFAOYSA-N 0.000 description 1
- ZHSVGGLZOMQPJF-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]-n-[(2,2-dimethyl-1,3-dioxolan-4-yl)methyl]acetamide Chemical compound O1C(C)(C)OCC1CNC(=O)COC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 ZHSVGGLZOMQPJF-UHFFFAOYSA-N 0.000 description 1
- CTDWJXKBFBTCBU-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]-n-methylacetamide Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCC(=O)NC)=C3NC2=C1 CTDWJXKBFBTCBU-UHFFFAOYSA-N 0.000 description 1
- METUHGKGBJAKIH-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]acetamide Chemical compound O=C1C2=CC=C(OCC(N)=O)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 METUHGKGBJAKIH-UHFFFAOYSA-N 0.000 description 1
- BGLYSKVZXDMUDZ-UHFFFAOYSA-N 2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethylurea Chemical compound O=C1C2=CC=C(OCCNC(N)=O)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 BGLYSKVZXDMUDZ-UHFFFAOYSA-N 0.000 description 1
- UTPHRZRWMXLNIK-UHFFFAOYSA-N 2-[di(propan-2-yl)amino]ethanethiol;hydron;chloride Chemical compound Cl.CC(C)N(C(C)C)CCS UTPHRZRWMXLNIK-UHFFFAOYSA-N 0.000 description 1
- QLIBJPGWWSHWBF-UHFFFAOYSA-N 2-aminoethyl methacrylate Chemical compound CC(=C)C(=O)OCCN QLIBJPGWWSHWBF-UHFFFAOYSA-N 0.000 description 1
- ICSNLGPSRYBMBD-UHFFFAOYSA-N 2-aminopyridine Chemical group NC1=CC=CC=N1 ICSNLGPSRYBMBD-UHFFFAOYSA-N 0.000 description 1
- MQENOLZCEXMCNX-UHFFFAOYSA-N 2-bromo-8-[2-(diethylamino)ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=C(Br)C=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCN(CC)CC)=C3NC2=C1 MQENOLZCEXMCNX-UHFFFAOYSA-N 0.000 description 1
- FVIUYRLNDNTDLA-UHFFFAOYSA-N 2-bromo-8-hydroxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(O)C=C2C(C)(C)C2=C1C1=CC(Br)=C(C#N)C=C1N2 FVIUYRLNDNTDLA-UHFFFAOYSA-N 0.000 description 1
- MJKLBTQOVWRLJO-UHFFFAOYSA-N 2-bromo-8-methoxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=C(Br)C=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OC)=C3NC2=C1 MJKLBTQOVWRLJO-UHFFFAOYSA-N 0.000 description 1
- JUIKUQOUMZUFQT-UHFFFAOYSA-N 2-bromoacetamide Chemical compound NC(=O)CBr JUIKUQOUMZUFQT-UHFFFAOYSA-N 0.000 description 1
- JBKINHFZTVLNEM-UHFFFAOYSA-N 2-bromoethoxy-tert-butyl-dimethylsilane Chemical compound CC(C)(C)[Si](C)(C)OCCBr JBKINHFZTVLNEM-UHFFFAOYSA-N 0.000 description 1
- PGFIHORVILKHIA-UHFFFAOYSA-N 2-bromopyrimidine Chemical compound BrC1=NC=CC=N1 PGFIHORVILKHIA-UHFFFAOYSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- YMDNODNLFSHHCV-UHFFFAOYSA-N 2-chloro-n,n-diethylethanamine Chemical compound CCN(CC)CCCl YMDNODNLFSHHCV-UHFFFAOYSA-N 0.000 description 1
- BITBMHVXCILUEX-UHFFFAOYSA-N 2-chloroethylurea Chemical compound NC(=O)NCCCl BITBMHVXCILUEX-UHFFFAOYSA-N 0.000 description 1
- 125000004732 2-ethylbutylthio group Chemical group C(C)C(CS*)CC 0.000 description 1
- DLFIXPTUMHDGCB-UHFFFAOYSA-N 2-fluoroethyl methanesulfonate Chemical compound CS(=O)(=O)OCCF DLFIXPTUMHDGCB-UHFFFAOYSA-N 0.000 description 1
- YEDUAINPPJYDJZ-UHFFFAOYSA-N 2-hydroxybenzothiazole Chemical compound C1=CC=C2SC(O)=NC2=C1 YEDUAINPPJYDJZ-UHFFFAOYSA-N 0.000 description 1
- AMSDWLOANMAILF-UHFFFAOYSA-N 2-imidazol-1-ylethanol Chemical compound OCCN1C=CN=C1 AMSDWLOANMAILF-UHFFFAOYSA-N 0.000 description 1
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 1
- WBBPRCNXBQTYLF-UHFFFAOYSA-N 2-methylthioethanol Chemical compound CSCCO WBBPRCNXBQTYLF-UHFFFAOYSA-N 0.000 description 1
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 description 1
- WFCSWCVEJLETKA-UHFFFAOYSA-N 2-piperazin-1-ylethanol Chemical compound OCCN1CCNCC1 WFCSWCVEJLETKA-UHFFFAOYSA-N 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- SALIGPAVMVFEJN-UHFFFAOYSA-N 2h-benzotriazol-4-yloxy(tripyrrolidin-1-yl)phosphanium Chemical compound C1CCCN1[P+](N1CCCC1)(N1CCCC1)OC1=CC=CC2=NNN=C12 SALIGPAVMVFEJN-UHFFFAOYSA-N 0.000 description 1
- MCZYEFODKAZWIH-UHFFFAOYSA-N 3-(chloromethyl)-3-methyloxetane Chemical compound ClCC1(C)COC1 MCZYEFODKAZWIH-UHFFFAOYSA-N 0.000 description 1
- VPGIXMGIEOUWPH-UHFFFAOYSA-N 3-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)sulfanyl]propanoic acid Chemical compound O=C1C2=CC=C(SCCC(O)=O)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 VPGIXMGIEOUWPH-UHFFFAOYSA-N 0.000 description 1
- UAGGUNDAQDTCFW-UHFFFAOYSA-N 3-amino-4-bromobenzonitrile Chemical compound NC1=CC(C#N)=CC=C1Br UAGGUNDAQDTCFW-UHFFFAOYSA-N 0.000 description 1
- QJKIIGMDUFPDQX-UHFFFAOYSA-N 3-bromo-8-hydroxy-6,6-dimethyl-5h-benzo[b]carbazol-11-one Chemical compound O=C1C2=CC=C(O)C=C2C(C)(C)C2=C1C1=CC=C(Br)C=C1N2 QJKIIGMDUFPDQX-UHFFFAOYSA-N 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 1
- DKIDEFUBRARXTE-UHFFFAOYSA-N 3-mercaptopropanoic acid Chemical compound OC(=O)CCS DKIDEFUBRARXTE-UHFFFAOYSA-N 0.000 description 1
- XCEGFNRUBBVHJT-UHFFFAOYSA-N 3-methylsulfonylpyrrolidine Chemical compound CS(=O)(=O)C1CCNC1 XCEGFNRUBBVHJT-UHFFFAOYSA-N 0.000 description 1
- ROADCYAOHVSOLQ-UHFFFAOYSA-N 3-oxetanone Chemical compound O=C1COC1 ROADCYAOHVSOLQ-UHFFFAOYSA-N 0.000 description 1
- ZREYEONHFGJPJC-UHFFFAOYSA-N 3h-pyrazolo[3,4-c]isoquinoline Chemical class C1=CC=C2C3=CNN=C3N=CC2=C1 ZREYEONHFGJPJC-UHFFFAOYSA-N 0.000 description 1
- OABUKBBBSMNNPM-UHFFFAOYSA-N 4,4-difluoropiperidin-1-ium;chloride Chemical compound Cl.FC1(F)CCNCC1 OABUKBBBSMNNPM-UHFFFAOYSA-N 0.000 description 1
- WFHPXSHLCFHEIA-UHFFFAOYSA-N 4,6,11-tris(2-methylpropyl)-1,4,6,11-tetraza-5-phosphabicyclo[3.3.3]undecane Chemical group C1CN(CC(C)C)P2N(CC(C)C)CCN1CCN2CC(C)C WFHPXSHLCFHEIA-UHFFFAOYSA-N 0.000 description 1
- DPBWFNDFMCCGGJ-UHFFFAOYSA-N 4-Piperidine carboxamide Chemical compound NC(=O)C1CCNCC1 DPBWFNDFMCCGGJ-UHFFFAOYSA-N 0.000 description 1
- YNVQQJLIVYOTGC-UHFFFAOYSA-N 4-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)methyl]piperidine-1-sulfonamide Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1CC1CCN(S(N)(=O)=O)CC1 YNVQQJLIVYOTGC-UHFFFAOYSA-N 0.000 description 1
- KVCQTKNUUQOELD-UHFFFAOYSA-N 4-amino-n-[1-(3-chloro-2-fluoroanilino)-6-methylisoquinolin-5-yl]thieno[3,2-d]pyrimidine-7-carboxamide Chemical compound N=1C=CC2=C(NC(=O)C=3C4=NC=NC(N)=C4SC=3)C(C)=CC=C2C=1NC1=CC=CC(Cl)=C1F KVCQTKNUUQOELD-UHFFFAOYSA-N 0.000 description 1
- XYOXIERJKILWCG-UHFFFAOYSA-N 4-chlorobutanamide Chemical compound NC(=O)CCCCl XYOXIERJKILWCG-UHFFFAOYSA-N 0.000 description 1
- SALKOMRDFREPDS-UHFFFAOYSA-N 4-cyclopentylpiperazin-2-one Chemical compound C1CNC(=O)CN1C1CCCC1 SALKOMRDFREPDS-UHFFFAOYSA-N 0.000 description 1
- GQPIVVPQPRWBML-UHFFFAOYSA-N 4-methylpiperazine-1-sulfonic acid Chemical compound CN1CCN(S(O)(=O)=O)CC1 GQPIVVPQPRWBML-UHFFFAOYSA-N 0.000 description 1
- YYBXNWIRMJXEQJ-UHFFFAOYSA-N 4-piperidin-4-ylmorpholine Chemical compound C1CNCCC1N1CCOCC1 YYBXNWIRMJXEQJ-UHFFFAOYSA-N 0.000 description 1
- STWODXDTKGTVCJ-UHFFFAOYSA-N 4-pyrrolidin-1-ylpiperidine Chemical compound C1CCCN1C1CCNCC1 STWODXDTKGTVCJ-UHFFFAOYSA-N 0.000 description 1
- GTXHOTRVAAKSDB-UHFFFAOYSA-N 5,6,6-trimethyl-8-(1-methylsulfonylpiperidin-4-yl)oxy-11-oxobenzo[b]carbazole-3-carbonitrile Chemical compound C12=CC=C(C#N)C=C2N(C)C(C(C2=C3)(C)C)=C1C(=O)C2=CC=C3OC1CCN(S(C)(=O)=O)CC1 GTXHOTRVAAKSDB-UHFFFAOYSA-N 0.000 description 1
- NTWNAWYIQMZTHL-UHFFFAOYSA-N 5,6,6-trimethyl-8-(oxan-4-yloxy)-11-oxobenzo[b]carbazole-3-carbonitrile Chemical compound C12=CC=C(C#N)C=C2N(C)C(C(C2=C3)(C)C)=C1C(=O)C2=CC=C3OC1CCOCC1 NTWNAWYIQMZTHL-UHFFFAOYSA-N 0.000 description 1
- QQWUGDVOUVUTOY-UHFFFAOYSA-N 5-chloro-N2-[2-methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl]phenyl]-N4-(2-propan-2-ylsulfonylphenyl)pyrimidine-2,4-diamine Chemical compound COC1=CC(N2CCC(CC2)N2CCN(C)CC2)=CC=C1NC(N=1)=NC=C(Cl)C=1NC1=CC=CC=C1S(=O)(=O)C(C)C QQWUGDVOUVUTOY-UHFFFAOYSA-N 0.000 description 1
- 125000006163 5-membered heteroaryl group Chemical group 0.000 description 1
- HNEFKQIXMRCGAG-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-(1,2,3,6-tetrahydropyridin-4-yl)-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1C1=CCNCC1 HNEFKQIXMRCGAG-UHFFFAOYSA-N 0.000 description 1
- SOZIXUQMDJGAGF-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-(2-oxo-2-piperazin-1-ylethoxy)-5h-benzo[b]carbazole-3-carbonitrile;hydrochloride Chemical compound Cl.C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCC(=O)N1CCNCC1 SOZIXUQMDJGAGF-UHFFFAOYSA-N 0.000 description 1
- DNFRGZYTEXALJG-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-(4-oxopiperidin-1-yl)-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCC(=O)CC1 DNFRGZYTEXALJG-UHFFFAOYSA-N 0.000 description 1
- RDJKJVDXCOBOAM-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-(pyridin-4-ylmethoxy)-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCC1=CC=NC=C1 RDJKJVDXCOBOAM-UHFFFAOYSA-N 0.000 description 1
- JKONYOLRCISFQZ-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-[(1-propan-2-ylpiperidin-4-yl)methyl]-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(C(C)C)CCC1CC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 JKONYOLRCISFQZ-UHFFFAOYSA-N 0.000 description 1
- AQJFHKREVMBYDS-RTBURBONSA-N 6,6-dimethyl-11-oxo-8-[(2r,3r)-2,3,4-trihydroxybutoxy]-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(OC[C@@H](O)[C@H](O)CO)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 AQJFHKREVMBYDS-RTBURBONSA-N 0.000 description 1
- KYPWTWMEHIVGCT-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-[2-(2-oxoimidazolidin-1-yl)ethoxy]-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCCN1CCNC1=O KYPWTWMEHIVGCT-UHFFFAOYSA-N 0.000 description 1
- UPPXIQZQDMJSNM-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-[2-(3-oxopiperazin-1-yl)ethoxy]-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCCN1CCNC(=O)C1 UPPXIQZQDMJSNM-UHFFFAOYSA-N 0.000 description 1
- KVYXHXPMHSJWIS-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-piperazin-1-yl-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCNCC1 KVYXHXPMHSJWIS-UHFFFAOYSA-N 0.000 description 1
- VQZFCYOTZLCUGN-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-piperidin-1-yl-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCCCC1 VQZFCYOTZLCUGN-UHFFFAOYSA-N 0.000 description 1
- IUHJBYHMCIDSSE-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-piperidin-4-yloxy-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OC1CCNCC1 IUHJBYHMCIDSSE-UHFFFAOYSA-N 0.000 description 1
- QYQCJAHENYTBKW-UHFFFAOYSA-N 6,6-dimethyl-11-oxo-8-pyrimidin-2-yloxy-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OC1=NC=CC=N1 QYQCJAHENYTBKW-UHFFFAOYSA-N 0.000 description 1
- ZXSWOBLFKBUDKN-UHFFFAOYSA-N 6,6-dimethyl-8-(1-methylpiperidin-4-yl)oxy-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(C)CCC1OC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 ZXSWOBLFKBUDKN-UHFFFAOYSA-N 0.000 description 1
- PWZSVTGWDRQZLJ-UHFFFAOYSA-N 6,6-dimethyl-8-(1-methylpyrazol-4-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=NN(C)C=C1C1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 PWZSVTGWDRQZLJ-UHFFFAOYSA-N 0.000 description 1
- LYCUMSBGCYJMTO-UHFFFAOYSA-N 6,6-dimethyl-8-(1-methylsulfonylpiperidin-4-yl)oxy-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OC1CCN(S(C)(=O)=O)CC1 LYCUMSBGCYJMTO-UHFFFAOYSA-N 0.000 description 1
- BMMNKHFAPLBGME-UHFFFAOYSA-N 6,6-dimethyl-8-(2-methylsulfanylethoxy)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCSC)=C3NC2=C1 BMMNKHFAPLBGME-UHFFFAOYSA-N 0.000 description 1
- ONWPAIFOMYMSNM-UHFFFAOYSA-N 6,6-dimethyl-8-(2-methylsulfanylethoxy)-5-(2-methylsulfanylethyl)-11-oxobenzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCSC)=C3N(CCSC)C2=C1 ONWPAIFOMYMSNM-UHFFFAOYSA-N 0.000 description 1
- HPDQOHWWQIMFRK-UHFFFAOYSA-N 6,6-dimethyl-8-(2-methylsulfinylethoxy)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCS(=O)C)=C3NC2=C1 HPDQOHWWQIMFRK-UHFFFAOYSA-N 0.000 description 1
- KZQGYRDZWNEIRF-UHFFFAOYSA-N 6,6-dimethyl-8-(2-methylsulfonylethoxy)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(OCCS(C)(=O)=O)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 KZQGYRDZWNEIRF-UHFFFAOYSA-N 0.000 description 1
- LAVQFOCBUCDALE-UHFFFAOYSA-N 6,6-dimethyl-8-(2-morpholin-4-ylethoxy)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCCN1CCOCC1 LAVQFOCBUCDALE-UHFFFAOYSA-N 0.000 description 1
- NNVBRQCREDQPDL-UHFFFAOYSA-N 6,6-dimethyl-8-(3-methylsulfonylpyrrolidin-1-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCC(S(C)(=O)=O)C1 NNVBRQCREDQPDL-UHFFFAOYSA-N 0.000 description 1
- KNNQVOYZNULLOT-UHFFFAOYSA-N 6,6-dimethyl-8-(4-methylsulfonylpiperazin-1-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCN(S(C)(=O)=O)CC1 KNNQVOYZNULLOT-UHFFFAOYSA-N 0.000 description 1
- OVRQGEYZDUHJNZ-UHFFFAOYSA-N 6,6-dimethyl-8-(4-morpholin-4-ylpiperidin-1-yl)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N(CC1)CCC1N1CCOCC1 OVRQGEYZDUHJNZ-UHFFFAOYSA-N 0.000 description 1
- NXIPGGIDAQWUDH-UHFFFAOYSA-N 6,6-dimethyl-8-(oxan-4-yloxy)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OC1CCOCC1 NXIPGGIDAQWUDH-UHFFFAOYSA-N 0.000 description 1
- ANGXOLUWLHRSIP-UHFFFAOYSA-N 6,6-dimethyl-8-(oxetan-3-yloxy)-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OC1COC1 ANGXOLUWLHRSIP-UHFFFAOYSA-N 0.000 description 1
- BYZMIAAEARRQMS-AWEZNQCLSA-N 6,6-dimethyl-8-[(2s)-2-methylpiperazin-1-yl]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C[C@H]1CNCCN1C1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 BYZMIAAEARRQMS-AWEZNQCLSA-N 0.000 description 1
- SAUDPQDAHFQMRI-UHFFFAOYSA-N 6,6-dimethyl-8-[(3-methyloxetan-3-yl)methoxy]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C=1C=C2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C2=CC=1OCC1(C)COC1 SAUDPQDAHFQMRI-UHFFFAOYSA-N 0.000 description 1
- UFYUKJCQJLCQDZ-UHFFFAOYSA-N 6,6-dimethyl-8-[2-(methylsulfamoylamino)ethoxy]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNS(=O)(=O)NC)=C3NC2=C1 UFYUKJCQJLCQDZ-UHFFFAOYSA-N 0.000 description 1
- OQTFIZMLXPZEOD-UHFFFAOYSA-N 6,6-dimethyl-8-[2-[1-(oxetan-3-yl)piperidin-4-yl]ethoxy]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCCC(CC1)CCN1C1COC1 OQTFIZMLXPZEOD-UHFFFAOYSA-N 0.000 description 1
- SAPAFVSRVQDNRS-UHFFFAOYSA-N 6,6-dimethyl-8-[[1-(oxetan-3-yl)piperidin-4-yl]methoxy]-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCC(CC1)CCN1C1COC1 SAPAFVSRVQDNRS-UHFFFAOYSA-N 0.000 description 1
- BQCOOKAOMXIBEQ-UHFFFAOYSA-N 6,6-dimethyl-8-morpholin-4-yl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCOCC1 BQCOOKAOMXIBEQ-UHFFFAOYSA-N 0.000 description 1
- DWUBVULEMQOCPO-UHFFFAOYSA-N 6-benzylpyrrolo[3,4-b]pyridine-5,7-dione Chemical compound O=C1C2=NC=CC=C2C(=O)N1CC1=CC=CC=C1 DWUBVULEMQOCPO-UHFFFAOYSA-N 0.000 description 1
- XEAPZXNZOJGVCZ-UHFFFAOYSA-N 7-methoxy-3,4-dihydro-1h-naphthalen-2-one Chemical compound C1CC(=O)CC2=CC(OC)=CC=C21 XEAPZXNZOJGVCZ-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- NDJWJLLTWGQCAB-UHFFFAOYSA-N 8-(1,1-dioxo-1,4-thiazinan-4-yl)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCS(=O)(=O)CC1 NDJWJLLTWGQCAB-UHFFFAOYSA-N 0.000 description 1
- HVGPHZKFZGPADU-UHFFFAOYSA-N 8-(1,3-dihydroxypropan-2-yloxy)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(OC(CO)CO)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 HVGPHZKFZGPADU-UHFFFAOYSA-N 0.000 description 1
- HOCLYLJMXSGTPI-UHFFFAOYSA-N 8-(1-acetylpiperidin-4-yl)oxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(C(=O)C)CCC1OC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 HOCLYLJMXSGTPI-UHFFFAOYSA-N 0.000 description 1
- CYHZDISHAYTGPY-UHFFFAOYSA-N 8-(2,3-dihydroxypropylsulfanyl)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(SCC(O)CO)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 CYHZDISHAYTGPY-UHFFFAOYSA-N 0.000 description 1
- CBPWNRXFJCPEJV-UHFFFAOYSA-N 8-(2-hydroxyethoxy)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(OCCO)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 CBPWNRXFJCPEJV-UHFFFAOYSA-N 0.000 description 1
- LKMNSQYOBIULLE-UHFFFAOYSA-N 8-(2-imidazol-1-ylethoxy)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCCN1C=CN=C1 LKMNSQYOBIULLE-UHFFFAOYSA-N 0.000 description 1
- ORNJWBNEXKNLAS-UHFFFAOYSA-N 8-(2-methoxyethoxy)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCOC)=C3NC2=C1 ORNJWBNEXKNLAS-UHFFFAOYSA-N 0.000 description 1
- CYJRNFFLTBEQSQ-UHFFFAOYSA-N 8-(3-methyl-1-benzothiophen-5-yl)-N-(4-methylsulfonylpyridin-3-yl)quinoxalin-6-amine Chemical compound CS(=O)(=O)C1=C(C=NC=C1)NC=1C=C2N=CC=NC2=C(C=1)C=1C=CC2=C(C(=CS2)C)C=1 CYJRNFFLTBEQSQ-UHFFFAOYSA-N 0.000 description 1
- LVPWLHCEMYAHKW-UHFFFAOYSA-N 8-(4-cyclopentyl-2-oxopiperazin-1-yl)-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N(C(C1)=O)CCN1C1CCCC1 LVPWLHCEMYAHKW-UHFFFAOYSA-N 0.000 description 1
- BVBOVYUYNPNIRI-UHFFFAOYSA-N 8-[(2,2-dimethyl-1,3-dioxolan-4-yl)methoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O1C(C)(C)OCC1COC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 BVBOVYUYNPNIRI-UHFFFAOYSA-N 0.000 description 1
- UNFWQVRBBMXECC-CQSZACIVSA-N 8-[(2r)-2,3-dihydroxypropoxy]-5,6,6-trimethyl-11-oxobenzo[b]carbazole-3-carbonitrile Chemical compound C12=CC=C(C#N)C=C2N(C)C2=C1C(=O)C1=CC=C(OC[C@H](O)CO)C=C1C2(C)C UNFWQVRBBMXECC-CQSZACIVSA-N 0.000 description 1
- LDSJXFYRUQVQKV-CYBMUJFWSA-N 8-[(2r)-2,3-dihydroxypropoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(OC[C@H](O)CO)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 LDSJXFYRUQVQKV-CYBMUJFWSA-N 0.000 description 1
- RGAKWABSXVEWNC-KRWDZBQOSA-N 8-[(2s)-4-cyclobutyl-2-methylpiperazin-1-yl]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C([C@@H](N(CC1)C=2C=C3C(C)(C)C4=C(C5=CC=C(C=C5N4)C#N)C(=O)C3=CC=2)C)N1C1CCC1 RGAKWABSXVEWNC-KRWDZBQOSA-N 0.000 description 1
- ZQERSSFUQHTCEV-GASCZTMLSA-N 8-[(3r,5s)-3,5-dimethylpiperazin-1-yl]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1[C@@H](C)N[C@@H](C)CN1C1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 ZQERSSFUQHTCEV-GASCZTMLSA-N 0.000 description 1
- DBOBTHXUUFEUIX-UHFFFAOYSA-N 8-[1-(2-fluoroethyl)piperidin-4-yl]oxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OC1CCN(CCF)CC1 DBOBTHXUUFEUIX-UHFFFAOYSA-N 0.000 description 1
- BZTCDKPKAWSRPT-UHFFFAOYSA-N 8-[1-(2-hydroxyethyl)piperidin-4-yl]oxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OC1CCN(CCO)CC1 BZTCDKPKAWSRPT-UHFFFAOYSA-N 0.000 description 1
- CHQQCONVEPPVNT-UHFFFAOYSA-N 8-[1-(2-methoxyethyl)piperidin-4-yl]oxy-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(CCOC)CCC1OC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 CHQQCONVEPPVNT-UHFFFAOYSA-N 0.000 description 1
- XBLAPMPMKLEWHJ-UHFFFAOYSA-N 8-[2-(1,1-dioxo-1,4-thiazinan-4-yl)ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1OCCN1CCS(=O)(=O)CC1 XBLAPMPMKLEWHJ-UHFFFAOYSA-N 0.000 description 1
- YYHCEHNATNSMFB-UHFFFAOYSA-N 8-[2-(2-methoxyethoxy)ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCOCCOC)=C3NC2=C1 YYHCEHNATNSMFB-UHFFFAOYSA-N 0.000 description 1
- CAICRDOKZLIPFG-UHFFFAOYSA-N 8-[2-(4-ethylpiperazin-1-yl)ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1CN(CC)CCN1CCOC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 CAICRDOKZLIPFG-UHFFFAOYSA-N 0.000 description 1
- VUXJQPQSZJIJDX-UHFFFAOYSA-N 8-[2-(butan-2-ylamino)ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNC(C)CC)=C3NC2=C1 VUXJQPQSZJIJDX-UHFFFAOYSA-N 0.000 description 1
- BEDSGOCODTYQEM-UHFFFAOYSA-N 8-[2-(diethylamino)ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCN(CC)CC)=C3NC2=C1 BEDSGOCODTYQEM-UHFFFAOYSA-N 0.000 description 1
- MIUASMOUZQAEET-UHFFFAOYSA-N 8-[2-(diethylamino)ethylsulfanyl]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)SCCN(CC)CC)=C3NC2=C1 MIUASMOUZQAEET-UHFFFAOYSA-N 0.000 description 1
- DGOGMPGOZQDNKT-UHFFFAOYSA-N 8-[2-(dimethylamino)ethylsulfanyl]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)SCCN(C)C)=C3NC2=C1 DGOGMPGOZQDNKT-UHFFFAOYSA-N 0.000 description 1
- VWPAOMJENNNHCI-UHFFFAOYSA-N 8-[2-(ethylamino)ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNCC)=C3NC2=C1 VWPAOMJENNNHCI-UHFFFAOYSA-N 0.000 description 1
- QSYXOEHBHZYUKF-UHFFFAOYSA-N 8-[2-[(1-hydroxy-2-methylpropan-2-yl)amino]ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNC(C)(CO)C)=C3NC2=C1 QSYXOEHBHZYUKF-UHFFFAOYSA-N 0.000 description 1
- JTXKWWFAJPRLCE-UHFFFAOYSA-N 8-[2-[bis(2-hydroxyethyl)amino]ethoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(OCCN(CCO)CCO)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 JTXKWWFAJPRLCE-UHFFFAOYSA-N 0.000 description 1
- QWQZWLXXZOGTCN-UHFFFAOYSA-N 8-[2-[di(propan-2-yl)amino]ethylsulfanyl]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)SCCN(C(C)C)C(C)C)=C3NC2=C1 QWQZWLXXZOGTCN-UHFFFAOYSA-N 0.000 description 1
- VHMRNOAYMFYWDI-UHFFFAOYSA-N 8-[4-(2-hydroxyethyl)piperazin-1-yl]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N1CCN(CCO)CC1 VHMRNOAYMFYWDI-UHFFFAOYSA-N 0.000 description 1
- AFHMLHTVDDTUBE-UHFFFAOYSA-N 8-[4-(4,4-difluoropiperidin-1-yl)piperidin-1-yl]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1=C2C(C)(C)C=3NC4=CC(C#N)=CC=C4C=3C(=O)C2=CC=C1N(CC1)CCC1N1CCC(F)(F)CC1 AFHMLHTVDDTUBE-UHFFFAOYSA-N 0.000 description 1
- BZAFIEFQZPDXNQ-KDURUIRLSA-N 8-[4-[(2r,6s)-2,6-dimethylmorpholin-4-yl]piperidin-1-yl]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound C1[C@@H](C)O[C@@H](C)CN1C1CCN(C=2C=C3C(C)(C)C4=C(C5=CC=C(C=C5N4)C#N)C(=O)C3=CC=2)CC1 BZAFIEFQZPDXNQ-KDURUIRLSA-N 0.000 description 1
- OCFAYZVRMHXEEH-KRWDZBQOSA-N 8-[[(4s)-2,2-dimethyl-1,3-dioxolan-4-yl]methoxy]-5,6,6-trimethyl-11-oxobenzo[b]carbazole-3-carbonitrile Chemical compound C12=CC=C(C#N)C=C2N(C)C(C(C2=C3)(C)C)=C1C(=O)C2=CC=C3OC[C@H]1COC(C)(C)O1 OCFAYZVRMHXEEH-KRWDZBQOSA-N 0.000 description 1
- BVBOVYUYNPNIRI-INIZCTEOSA-N 8-[[(4s)-2,2-dimethyl-1,3-dioxolan-4-yl]methoxy]-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O1C(C)(C)OC[C@@H]1COC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 BVBOVYUYNPNIRI-INIZCTEOSA-N 0.000 description 1
- LUHODGMMIKUPBW-UHFFFAOYSA-N 8-ethenyl-6,6-dimethyl-11-oxo-5h-benzo[b]carbazole-3-carbonitrile Chemical compound O=C1C2=CC=C(C=C)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 LUHODGMMIKUPBW-UHFFFAOYSA-N 0.000 description 1
- OTMRRKAZQMDSOX-UHFFFAOYSA-N 8-methoxy-5,6,6-trimethyl-11-oxobenzo[b]carbazole-3-carbonitrile Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OC)=C3N(C)C2=C1 OTMRRKAZQMDSOX-UHFFFAOYSA-N 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- BWKDAAFSXYPQOS-UHFFFAOYSA-N Benzaldehyde glyceryl acetal Chemical compound O1CC(O)COC1C1=CC=CC=C1 BWKDAAFSXYPQOS-UHFFFAOYSA-N 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 238000007024 Blaise reaction Methods 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- PXIKBWOMDNIXGE-YWEHKCAJSA-N C(C)(C)(C)[SiH2]OC([C@@H]1[C@H](OC(O1)(C)C)COC=1C=CC2=C(C(C=3NC4=CC(=CC=C4C=3C2=O)C#N)(C)C)C=1)(C)C Chemical compound C(C)(C)(C)[SiH2]OC([C@@H]1[C@H](OC(O1)(C)C)COC=1C=CC2=C(C(C=3NC4=CC(=CC=C4C=3C2=O)C#N)(C)C)C=1)(C)C PXIKBWOMDNIXGE-YWEHKCAJSA-N 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 229910021595 Copper(I) iodide Inorganic materials 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 239000004287 Dehydroacetic acid Substances 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- 239000003109 Disodium ethylene diamine tetraacetate Substances 0.000 description 1
- ZGTMUACCHSMWAC-UHFFFAOYSA-L EDTA disodium salt (anhydrous) Chemical compound [Na+].[Na+].OC(=O)CN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O ZGTMUACCHSMWAC-UHFFFAOYSA-L 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 208000022072 Gallbladder Neoplasms Diseases 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 230000010558 Gene Alterations Effects 0.000 description 1
- 208000034951 Genetic Translocation Diseases 0.000 description 1
- 201000005409 Gliomatosis cerebri Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- ZIXGXMMUKPLXBB-UHFFFAOYSA-N Guatambuinine Natural products N1C2=CC=CC=C2C2=C1C(C)=C1C=CN=C(C)C1=C2 ZIXGXMMUKPLXBB-UHFFFAOYSA-N 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000818543 Homo sapiens Tyrosine-protein kinase ZAP-70 Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical class Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 102000003746 Insulin Receptor Human genes 0.000 description 1
- 108010001127 Insulin Receptor Proteins 0.000 description 1
- 229930194542 Keto Natural products 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- 229930182821 L-proline Natural products 0.000 description 1
- 229920002884 Laureth 4 Polymers 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 238000006751 Mitsunobu reaction Methods 0.000 description 1
- 239000004368 Modified starch Substances 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 1
- HSHXDCVZWHOWCS-UHFFFAOYSA-N N'-hexadecylthiophene-2-carbohydrazide Chemical compound CCCCCCCCCCCCCCCCNNC(=O)c1cccs1 HSHXDCVZWHOWCS-UHFFFAOYSA-N 0.000 description 1
- ZKGNPQKYVKXMGJ-UHFFFAOYSA-N N,N-dimethylacetamide Chemical compound CN(C)C(C)=O.CN(C)C(C)=O ZKGNPQKYVKXMGJ-UHFFFAOYSA-N 0.000 description 1
- 125000005850 N-(alkoxycarbonyl)aminomethyl group Chemical group 0.000 description 1
- AYCPARAPKDAOEN-LJQANCHMSA-N N-[(1S)-2-(dimethylamino)-1-phenylethyl]-6,6-dimethyl-3-[(2-methyl-4-thieno[3,2-d]pyrimidinyl)amino]-1,4-dihydropyrrolo[3,4-c]pyrazole-5-carboxamide Chemical compound C1([C@H](NC(=O)N2C(C=3NN=C(NC=4C=5SC=CC=5N=C(C)N=4)C=3C2)(C)C)CN(C)C)=CC=CC=C1 AYCPARAPKDAOEN-LJQANCHMSA-N 0.000 description 1
- GXCLVBGFBYZDAG-UHFFFAOYSA-N N-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amine Chemical compound CN(CCC1=CNC2=C1C=CC=C2)CC=C GXCLVBGFBYZDAG-UHFFFAOYSA-N 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229920002701 Polyoxyl 40 Stearate Polymers 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- SUYXJDLXGFPMCQ-INIZCTEOSA-N SJ000287331 Natural products CC1=c2cnccc2=C(C)C2=Nc3ccccc3[C@H]12 SUYXJDLXGFPMCQ-INIZCTEOSA-N 0.000 description 1
- 101100482117 Saimiri sciureus THBD gene Proteins 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 235000005764 Theobroma cacao ssp. cacao Nutrition 0.000 description 1
- 235000005767 Theobroma cacao ssp. sphaerocarpum Nutrition 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 229910021626 Tin(II) chloride Inorganic materials 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 1
- 102100021125 Tyrosine-protein kinase ZAP-70 Human genes 0.000 description 1
- 208000023915 Ureteral Neoplasms Diseases 0.000 description 1
- 206010046392 Ureteric cancer Diseases 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- MQMGEXKUFJKUBN-NXEZZACHSA-N [(4R,5R)-5-(2-tert-butylsilyloxypropan-2-yl)-2,2-dimethyl-1,3-dioxolan-4-yl]methanol Chemical compound C(C)(C)(C)[SiH2]OC([C@H]1[C@H](OC(O1)(C)C)CO)(C)C MQMGEXKUFJKUBN-NXEZZACHSA-N 0.000 description 1
- SRKDUHUULIWXFT-LLVKDONJSA-N [(4r)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl 4-methylbenzenesulfonate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)OC[C@@H]1OC(C)(C)OC1 SRKDUHUULIWXFT-LLVKDONJSA-N 0.000 description 1
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 1
- 239000012346 acetyl chloride Substances 0.000 description 1
- 239000003377 acid catalyst Substances 0.000 description 1
- 238000005903 acid hydrolysis reaction Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- YBCVMFKXIKNREZ-UHFFFAOYSA-N acoh acetic acid Chemical compound CC(O)=O.CC(O)=O YBCVMFKXIKNREZ-UHFFFAOYSA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 150000001263 acyl chlorides Chemical class 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 125000005042 acyloxymethyl group Chemical group 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000004466 alkoxycarbonylamino group Chemical group 0.000 description 1
- 125000005206 alkoxycarbonyloxymethyl group Chemical group 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- 125000005210 alkyl ammonium group Chemical group 0.000 description 1
- 125000005336 allyloxy group Chemical group 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- CBTVGIZVANVGBH-UHFFFAOYSA-N aminomethyl propanol Chemical compound CC(C)(N)CO CBTVGIZVANVGBH-UHFFFAOYSA-N 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- MXMOTZIXVICDSD-UHFFFAOYSA-N anisoyl chloride Chemical compound COC1=CC=C(C(Cl)=O)C=C1 MXMOTZIXVICDSD-UHFFFAOYSA-N 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 229940054051 antipsychotic indole derivative Drugs 0.000 description 1
- 239000000010 aprotic solvent Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 239000012300 argon atmosphere Substances 0.000 description 1
- 150000004982 aromatic amines Chemical class 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical group 0.000 description 1
- 150000001502 aryl halides Chemical class 0.000 description 1
- CBHOOMGKXCMKIR-UHFFFAOYSA-N azane;methanol Chemical compound N.OC CBHOOMGKXCMKIR-UHFFFAOYSA-N 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 125000002047 benzodioxolyl group Chemical group O1OC(C2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 150000001558 benzoic acid derivatives Chemical class 0.000 description 1
- 125000005874 benzothiadiazolyl group Chemical group 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- AGSPXMVUFBBBMO-UHFFFAOYSA-N beta-aminopropionitrile Chemical compound NCCC#N AGSPXMVUFBBBMO-UHFFFAOYSA-N 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- PFYXSUNOLOJMDX-UHFFFAOYSA-N bis(2,5-dioxopyrrolidin-1-yl) carbonate Chemical compound O=C1CCC(=O)N1OC(=O)ON1C(=O)CCC1=O PFYXSUNOLOJMDX-UHFFFAOYSA-N 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- WTEOIRVLGSZEPR-UHFFFAOYSA-N boron trifluoride Chemical compound FB(F)F WTEOIRVLGSZEPR-UHFFFAOYSA-N 0.000 description 1
- 238000009937 brining Methods 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000004063 butyryl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000001046 cacaotero Nutrition 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 150000001734 carboxylic acid salts Chemical class 0.000 description 1
- 239000004203 carnauba wax Substances 0.000 description 1
- 235000013869 carnauba wax Nutrition 0.000 description 1
- 238000010531 catalytic reduction reaction Methods 0.000 description 1
- 230000007541 cellular toxicity Effects 0.000 description 1
- KXZJHVJKXJLBKO-UHFFFAOYSA-N chembl1408157 Chemical compound N=1C2=CC=CC=C2C(C(=O)O)=CC=1C1=CC=C(O)C=C1 KXZJHVJKXJLBKO-UHFFFAOYSA-N 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 229960002242 chlorocresol Drugs 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical class OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000006482 condensation reaction Methods 0.000 description 1
- 230000006552 constitutive activation Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- LSXDOTMGLUJQCM-UHFFFAOYSA-M copper(i) iodide Chemical compound I[Cu] LSXDOTMGLUJQCM-UHFFFAOYSA-M 0.000 description 1
- OPQARKPSCNTWTJ-UHFFFAOYSA-L copper(ii) acetate Chemical compound [Cu+2].CC([O-])=O.CC([O-])=O OPQARKPSCNTWTJ-UHFFFAOYSA-L 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 150000001907 coumarones Chemical class 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 229930003836 cresol Natural products 0.000 description 1
- KTEIFNKAUNYNJU-GFCCVEGCSA-N crizotinib Chemical compound O([C@H](C)C=1C(=C(F)C=CC=1Cl)Cl)C(C(=NC=1)N)=CC=1C(=C1)C=NN1C1CCNCC1 KTEIFNKAUNYNJU-GFCCVEGCSA-N 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 1
- 125000006254 cycloalkyl carbonyl group Chemical group 0.000 description 1
- SHQSVMDWKBRBGB-UHFFFAOYSA-N cyclobutanone Chemical compound O=C1CCC1 SHQSVMDWKBRBGB-UHFFFAOYSA-N 0.000 description 1
- 125000006637 cyclobutyl carbonyl group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000006640 cycloheptyl carbonyl group Chemical group 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000006639 cyclohexyl carbonyl group Chemical group 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000006641 cyclooctyl carbonyl group Chemical group 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000006638 cyclopentyl carbonyl group Chemical group 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000006255 cyclopropyl carbonyl group Chemical group [H]C1([H])C([H])([H])C1([H])C(*)=O 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- DEZRYPDIMOWBDS-UHFFFAOYSA-N dcm dichloromethane Chemical compound ClCCl.ClCCl DEZRYPDIMOWBDS-UHFFFAOYSA-N 0.000 description 1
- 238000006114 decarboxylation reaction Methods 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 235000019258 dehydroacetic acid Nutrition 0.000 description 1
- JEQRBTDTEKWZBW-UHFFFAOYSA-N dehydroacetic acid Chemical compound CC(=O)C1=C(O)OC(C)=CC1=O JEQRBTDTEKWZBW-UHFFFAOYSA-N 0.000 description 1
- 229940061632 dehydroacetic acid Drugs 0.000 description 1
- PGRHXDWITVMQBC-UHFFFAOYSA-N dehydroacetic acid Natural products CC(=O)C1C(=O)OC(C)=CC1=O PGRHXDWITVMQBC-UHFFFAOYSA-N 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 125000005131 dialkylammonium group Chemical group 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 125000001664 diethylamino group Chemical group [H]C([H])([H])C([H])([H])N(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- JMRYOSQOYJBDOI-UHFFFAOYSA-N dilithium;di(propan-2-yl)azanide Chemical compound [Li+].CC(C)[N-]C(C)C.CC(C)N([Li])C(C)C JMRYOSQOYJBDOI-UHFFFAOYSA-N 0.000 description 1
- 125000006263 dimethyl aminosulfonyl group Chemical group [H]C([H])([H])N(C([H])([H])[H])S(*)(=O)=O 0.000 description 1
- VAYGXNSJCAHWJZ-UHFFFAOYSA-N dimethyl sulfate Chemical compound COS(=O)(=O)OC VAYGXNSJCAHWJZ-UHFFFAOYSA-N 0.000 description 1
- UXGNZZKBCMGWAZ-UHFFFAOYSA-N dimethylformamide dmf Chemical compound CN(C)C=O.CN(C)C=O UXGNZZKBCMGWAZ-UHFFFAOYSA-N 0.000 description 1
- 235000019301 disodium ethylene diamine tetraacetate Nutrition 0.000 description 1
- IIRVGTWONXBBAW-UHFFFAOYSA-M disodium;dioxido(oxo)phosphanium Chemical compound [Na+].[Na+].[O-][P+]([O-])=O IIRVGTWONXBBAW-UHFFFAOYSA-M 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- UJONYAVMBYXBJQ-UHFFFAOYSA-N ditert-butyl-[2-(2-methylphenyl)phenyl]phosphane Chemical compound CC1=CC=CC=C1C1=CC=CC=C1P(C(C)(C)C)C(C)(C)C UJONYAVMBYXBJQ-UHFFFAOYSA-N 0.000 description 1
- UZZWBUYVTBPQIV-UHFFFAOYSA-N dme dimethoxyethane Chemical compound COCCOC.COCCOC UZZWBUYVTBPQIV-UHFFFAOYSA-N 0.000 description 1
- CETRZFQIITUQQL-UHFFFAOYSA-N dmso dimethylsulfoxide Chemical compound CS(C)=O.CS(C)=O CETRZFQIITUQQL-UHFFFAOYSA-N 0.000 description 1
- WNAHIZMDSQCWRP-UHFFFAOYSA-N dodecane-1-thiol Chemical compound CCCCCCCCCCCCS WNAHIZMDSQCWRP-UHFFFAOYSA-N 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 150000002085 enols Chemical class 0.000 description 1
- 230000007515 enzymatic degradation Effects 0.000 description 1
- 238000010931 ester hydrolysis Methods 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 125000003754 ethoxycarbonyl group Chemical group C(=O)(OCC)* 0.000 description 1
- ZCLGVXACCAZJOX-UHFFFAOYSA-N ethyl 3-chloropropanoate Chemical compound CCOC(=O)CCCl ZCLGVXACCAZJOX-UHFFFAOYSA-N 0.000 description 1
- 235000010944 ethyl methyl cellulose Nutrition 0.000 description 1
- 239000001761 ethyl methyl cellulose Substances 0.000 description 1
- QHSHDVYEJKLXLB-UHFFFAOYSA-N ethyl n-(2-chloroethyl)carbamate Chemical compound CCOC(=O)NCCCl QHSHDVYEJKLXLB-UHFFFAOYSA-N 0.000 description 1
- LXWYCMOLRDQCPN-UHFFFAOYSA-N ethyl n-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethyl]carbamate Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNC(=O)OCC)=C3NC2=C1 LXWYCMOLRDQCPN-UHFFFAOYSA-N 0.000 description 1
- 125000006125 ethylsulfonyl group Chemical group 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- OJCSPXHYDFONPU-UHFFFAOYSA-N etoac etoac Chemical compound CCOC(C)=O.CCOC(C)=O OJCSPXHYDFONPU-UHFFFAOYSA-N 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 201000010175 gallbladder cancer Diseases 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 125000006343 heptafluoro propyl group Chemical group 0.000 description 1
- DMEGYFMYUHOHGS-UHFFFAOYSA-N heptamethylene Natural products C1CCCCCC1 DMEGYFMYUHOHGS-UHFFFAOYSA-N 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000003104 hexanoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000006038 hexenyl group Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical class I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- 125000002632 imidazolidinyl group Chemical group 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 125000005945 imidazopyridyl group Chemical group 0.000 description 1
- 150000002466 imines Chemical group 0.000 description 1
- 150000002475 indoles Chemical class 0.000 description 1
- 125000003406 indolizinyl group Chemical group C=1(C=CN2C=CC=CC12)* 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- HVTICUPFWKNHNG-UHFFFAOYSA-N iodoethane Chemical compound CCI HVTICUPFWKNHNG-UHFFFAOYSA-N 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- RBTARNINKXHZNM-UHFFFAOYSA-K iron trichloride Chemical compound Cl[Fe](Cl)Cl RBTARNINKXHZNM-UHFFFAOYSA-K 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- FMKOJHQHASLBPH-UHFFFAOYSA-N isopropyl iodide Chemical compound CC(C)I FMKOJHQHASLBPH-UHFFFAOYSA-N 0.000 description 1
- 125000005956 isoquinolyl group Chemical group 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 238000011813 knockout mouse model Methods 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 238000002514 liquid chromatography mass spectrum Methods 0.000 description 1
- UBJFKNSINUCEAL-UHFFFAOYSA-N lithium;2-methylpropane Chemical compound [Li+].C[C-](C)C UBJFKNSINUCEAL-UHFFFAOYSA-N 0.000 description 1
- WGOPGODQLGJZGL-UHFFFAOYSA-N lithium;butane Chemical compound [Li+].CC[CH-]C WGOPGODQLGJZGL-UHFFFAOYSA-N 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 229960003511 macrogol Drugs 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- IUYHWZFSGMZEOG-UHFFFAOYSA-M magnesium;propane;chloride Chemical compound [Mg+2].[Cl-].C[CH-]C IUYHWZFSGMZEOG-UHFFFAOYSA-M 0.000 description 1
- 150000004701 malic acid derivatives Chemical class 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- BCVXHSPFUWZLGQ-UHFFFAOYSA-N mecn acetonitrile Chemical compound CC#N.CC#N BCVXHSPFUWZLGQ-UHFFFAOYSA-N 0.000 description 1
- NRVFDGZJTPCULU-UHFFFAOYSA-N meda Chemical compound Cl.CN(C)CCS NRVFDGZJTPCULU-UHFFFAOYSA-N 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- COTNUBDHGSIOTA-UHFFFAOYSA-N meoh methanol Chemical compound OC.OC COTNUBDHGSIOTA-UHFFFAOYSA-N 0.000 description 1
- 238000001465 metallisation Methods 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 description 1
- KXENUXLNAPNGAC-UHFFFAOYSA-N methyl 2-(bromomethyl)-4-methoxybenzoate Chemical compound COC(=O)C1=CC=C(OC)C=C1CBr KXENUXLNAPNGAC-UHFFFAOYSA-N 0.000 description 1
- VUQUOGPMUUJORT-UHFFFAOYSA-N methyl 4-methylbenzenesulfonate Chemical compound COS(=O)(=O)C1=CC=C(C)C=C1 VUQUOGPMUUJORT-UHFFFAOYSA-N 0.000 description 1
- 125000006261 methyl amino sulfonyl group Chemical group [H]N(C([H])([H])[H])S(*)(=O)=O 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- MBABOKRGFJTBAE-UHFFFAOYSA-N methyl methanesulfonate Chemical compound COS(C)(=O)=O MBABOKRGFJTBAE-UHFFFAOYSA-N 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- OIRDBPQYVWXNSJ-UHFFFAOYSA-N methyl trifluoromethansulfonate Chemical compound COS(=O)(=O)C(F)(F)F OIRDBPQYVWXNSJ-UHFFFAOYSA-N 0.000 description 1
- NQMRYBIKMRVZLB-UHFFFAOYSA-N methylamine hydrochloride Chemical compound [Cl-].[NH3+]C NQMRYBIKMRVZLB-UHFFFAOYSA-N 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 125000006216 methylsulfinyl group Chemical group [H]C([H])([H])S(*)=O 0.000 description 1
- 235000019426 modified starch Nutrition 0.000 description 1
- PJUIMOJAAPLTRJ-UHFFFAOYSA-N monothioglycerol Chemical compound OCC(O)CS PJUIMOJAAPLTRJ-UHFFFAOYSA-N 0.000 description 1
- GLGNSAPAWZUDRT-UHFFFAOYSA-N morpholine-4-sulfonic acid Chemical compound OS(=O)(=O)N1CCOCC1 GLGNSAPAWZUDRT-UHFFFAOYSA-N 0.000 description 1
- 125000006518 morpholino carbonyl group Chemical group [H]C1([H])OC([H])([H])C([H])([H])N(C(*)=O)C1([H])[H] 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 229940124303 multikinase inhibitor Drugs 0.000 description 1
- AQCGBJIHZWZHFQ-UHFFFAOYSA-N n-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethyl]-2,2,2-trifluoroacetamide Chemical compound O=C1C2=CC=C(OCCNC(=O)C(F)(F)F)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 AQCGBJIHZWZHFQ-UHFFFAOYSA-N 0.000 description 1
- JBVFMIKHPRYXDF-UHFFFAOYSA-N n-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethyl]acetamide Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNC(=O)C)=C3NC2=C1 JBVFMIKHPRYXDF-UHFFFAOYSA-N 0.000 description 1
- FAIJVPSZRFZWPP-UHFFFAOYSA-N n-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethyl]methanesulfonamide Chemical compound O=C1C2=CC=C(OCCNS(C)(=O)=O)C=C2C(C)(C)C2=C1C1=CC=C(C#N)C=C1N2 FAIJVPSZRFZWPP-UHFFFAOYSA-N 0.000 description 1
- 125000006606 n-butoxy group Chemical group 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- WOOWBQQQJXZGIE-UHFFFAOYSA-N n-ethyl-n-propan-2-ylpropan-2-amine Chemical compound CCN(C(C)C)C(C)C.CCN(C(C)C)C(C)C WOOWBQQQJXZGIE-UHFFFAOYSA-N 0.000 description 1
- 125000003506 n-propoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 125000006093 n-propyl sulfinyl group Chemical group 0.000 description 1
- 125000006124 n-propyl sulfonyl group Chemical group 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- VWBWQOUWDOULQN-UHFFFAOYSA-N nmp n-methylpyrrolidone Chemical compound CN1CCCC1=O.CN1CCCC1=O VWBWQOUWDOULQN-UHFFFAOYSA-N 0.000 description 1
- 125000005246 nonafluorobutyl group Chemical group FC(F)(F)C(F)(F)C(F)(F)C(F)(F)* 0.000 description 1
- 238000007339 nucleophilic aromatic substitution reaction Methods 0.000 description 1
- 238000007344 nucleophilic reaction Methods 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 229940100688 oral solution Drugs 0.000 description 1
- 229940100692 oral suspension Drugs 0.000 description 1
- 125000001715 oxadiazolyl group Chemical group 0.000 description 1
- LMYJGUNNJIDROI-UHFFFAOYSA-N oxan-4-ol Chemical compound OC1CCOCC1 LMYJGUNNJIDROI-UHFFFAOYSA-N 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- OJEOJUQOECNDND-UHFFFAOYSA-N oxetan-3-amine Chemical group NC1COC1 OJEOJUQOECNDND-UHFFFAOYSA-N 0.000 description 1
- 125000003566 oxetanyl group Chemical group 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- BSCHIACBONPEOB-UHFFFAOYSA-N oxolane;hydrate Chemical compound O.C1CCOC1 BSCHIACBONPEOB-UHFFFAOYSA-N 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- MUJIDPITZJWBSW-UHFFFAOYSA-N palladium(2+) Chemical compound [Pd+2] MUJIDPITZJWBSW-UHFFFAOYSA-N 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 239000003182 parenteral nutrition solution Substances 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 125000006340 pentafluoro ethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 125000002255 pentenyl group Chemical group C(=CCCC)* 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 239000003444 phase transfer catalyst Substances 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N phenylbenzene Natural products C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 1
- 229940067107 phenylethyl alcohol Drugs 0.000 description 1
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Natural products O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 1
- 125000003170 phenylsulfonyl group Chemical group C1(=CC=CC=C1)S(=O)(=O)* 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- 150000003016 phosphoric acids Chemical class 0.000 description 1
- 125000004437 phosphorous atom Chemical group 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- IWELDVXSEVIIGI-UHFFFAOYSA-N piperazin-2-one Chemical compound O=C1CNCCN1 IWELDVXSEVIIGI-UHFFFAOYSA-N 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- HDOWRFHMPULYOA-UHFFFAOYSA-N piperidin-4-ol Chemical compound OC1CCNCC1 HDOWRFHMPULYOA-UHFFFAOYSA-N 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 239000010773 plant oil Substances 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 229940099429 polyoxyl 40 stearate Drugs 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 1
- NNFCIKHAZHQZJG-UHFFFAOYSA-N potassium cyanide Chemical compound [K+].N#[C-] NNFCIKHAZHQZJG-UHFFFAOYSA-N 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- JKANAVGODYYCQF-UHFFFAOYSA-N prop-2-yn-1-amine Chemical compound NCC#C JKANAVGODYYCQF-UHFFFAOYSA-N 0.000 description 1
- YORCIIVHUBAYBQ-UHFFFAOYSA-N propargyl bromide Chemical compound BrCC#C YORCIIVHUBAYBQ-UHFFFAOYSA-N 0.000 description 1
- 125000001501 propionyl group Chemical group O=C([*])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- ZDYVRSLAEXCVBX-UHFFFAOYSA-N pyridinium p-toluenesulfonate Chemical compound C1=CC=[NH+]C=C1.CC1=CC=C(S([O-])(=O)=O)C=C1 ZDYVRSLAEXCVBX-UHFFFAOYSA-N 0.000 description 1
- YEYHFKBVNARCNE-UHFFFAOYSA-N pyrido[2,3-b]pyrazine Chemical class N1=CC=NC2=CC=CN=C21 YEYHFKBVNARCNE-UHFFFAOYSA-N 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- XELRMPRLCPFTBH-UHFFFAOYSA-N quinazoline-2,4-diamine Chemical class C1=CC=CC2=NC(N)=NC(N)=C21 XELRMPRLCPFTBH-UHFFFAOYSA-N 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- 125000005493 quinolyl group Chemical group 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 1
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 238000006476 reductive cyclization reaction Methods 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical class OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 1
- 125000005920 sec-butoxy group Chemical group 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- BHRZNVHARXXAHW-UHFFFAOYSA-N sec-butylamine Chemical compound CCC(C)N BHRZNVHARXXAHW-UHFFFAOYSA-N 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- XGVXKJKTISMIOW-ZDUSSCGKSA-N simurosertib Chemical compound N1N=CC(C=2SC=3C(=O)NC(=NC=3C=2)[C@H]2N3CCC(CC3)C2)=C1C XGVXKJKTISMIOW-ZDUSSCGKSA-N 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- BEOOHQFXGBMRKU-UHFFFAOYSA-N sodium cyanoborohydride Chemical compound [Na+].[B-]C#N BEOOHQFXGBMRKU-UHFFFAOYSA-N 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 235000011150 stannous chloride Nutrition 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical class OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- QAHVHSLSRLSVGS-UHFFFAOYSA-N sulfamoyl chloride Chemical compound NS(Cl)(=O)=O QAHVHSLSRLSVGS-UHFFFAOYSA-N 0.000 description 1
- 238000005849 sulfamoylation reaction Methods 0.000 description 1
- 150000003459 sulfonic acid esters Chemical class 0.000 description 1
- 150000003460 sulfonic acids Chemical class 0.000 description 1
- 230000006103 sulfonylation Effects 0.000 description 1
- 238000005694 sulfonylation reaction Methods 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 125000006253 t-butylcarbonyl group Chemical group [H]C([H])([H])C(C(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 150000003892 tartrate salts Chemical class 0.000 description 1
- FMLPQHJYUZTHQS-QMMMGPOBSA-N tert-butyl (3s)-3-methylpiperazine-1-carboxylate Chemical compound C[C@H]1CN(C(=O)OC(C)(C)C)CCN1 FMLPQHJYUZTHQS-QMMMGPOBSA-N 0.000 description 1
- CHJLEVNKMJADLL-KRWDZBQOSA-N tert-butyl (3s)-4-(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)-3-methylpiperazine-1-carboxylate Chemical compound C[C@H]1CN(C(=O)OC(C)(C)C)CCN1C1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 CHJLEVNKMJADLL-KRWDZBQOSA-N 0.000 description 1
- YBNJZIDYXCGAPX-UHFFFAOYSA-N tert-butyl 4-(2-hydroxyethyl)piperidine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCC(CCO)CC1 YBNJZIDYXCGAPX-UHFFFAOYSA-N 0.000 description 1
- VRWAWMKRGXNRQK-UHFFFAOYSA-N tert-butyl 4-(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)-3,6-dihydro-2h-pyridine-1-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCC(C=2C=C3C(C)(C)C4=C(C5=CC=C(C=C5N4)C#N)C(=O)C3=CC=2)=C1 VRWAWMKRGXNRQK-UHFFFAOYSA-N 0.000 description 1
- VHCAXUOGYAJVGO-UHFFFAOYSA-N tert-butyl 4-(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)piperazine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)CCN1C1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 VHCAXUOGYAJVGO-UHFFFAOYSA-N 0.000 description 1
- VVDCRJGWILREQH-UHFFFAOYSA-N tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,6-dihydro-2h-pyridine-1-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCC(B2OC(C)(C)C(C)(C)O2)=C1 VVDCRJGWILREQH-UHFFFAOYSA-N 0.000 description 1
- CTEDVGRUGMPBHE-UHFFFAOYSA-N tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCC(CO)CC1 CTEDVGRUGMPBHE-UHFFFAOYSA-N 0.000 description 1
- KFIVHOWYXWKCON-UHFFFAOYSA-N tert-butyl 4-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxymethyl]piperidine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)CCC1COC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 KFIVHOWYXWKCON-UHFFFAOYSA-N 0.000 description 1
- PWQLFIKTGRINFF-UHFFFAOYSA-N tert-butyl 4-hydroxypiperidine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCC(O)CC1 PWQLFIKTGRINFF-UHFFFAOYSA-N 0.000 description 1
- PDTZMULNKGUIEJ-UHFFFAOYSA-N tert-butyl 4-methylidenepiperidine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCC(=C)CC1 PDTZMULNKGUIEJ-UHFFFAOYSA-N 0.000 description 1
- GPTXCAZYUMDUMN-UHFFFAOYSA-N tert-butyl n-(2-hydroxyethyl)carbamate Chemical compound CC(C)(C)OC(=O)NCCO GPTXCAZYUMDUMN-UHFFFAOYSA-N 0.000 description 1
- NODGDKIATNMZCU-UHFFFAOYSA-N tert-butyl n-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethyl]-n-ethylcarbamate Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCN(CC)C(=O)OC(C)(C)C)=C3NC2=C1 NODGDKIATNMZCU-UHFFFAOYSA-N 0.000 description 1
- SJDBWAHQISMZCO-UHFFFAOYSA-N tert-butyl n-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethyl]carbamate Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNC(=O)OC(C)(C)C)=C3NC2=C1 SJDBWAHQISMZCO-UHFFFAOYSA-N 0.000 description 1
- YBNMKRDBFKKGOM-UHFFFAOYSA-N tert-butyl n-[2-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)oxy]ethylsulfamoyl]carbamate Chemical compound N#CC1=CC=C2C(C(=O)C3=CC=C(C=C3C3(C)C)OCCNS(=O)(=O)NC(=O)OC(C)(C)C)=C3NC2=C1 YBNMKRDBFKKGOM-UHFFFAOYSA-N 0.000 description 1
- OPSQZIGTEBZROY-UHFFFAOYSA-N tert-butyl n-ethyl-n-(2-hydroxyethyl)carbamate Chemical compound OCCN(CC)C(=O)OC(C)(C)C OPSQZIGTEBZROY-UHFFFAOYSA-N 0.000 description 1
- ILMRJRBKQSSXGY-UHFFFAOYSA-N tert-butyl(dimethyl)silicon Chemical group C[Si](C)C(C)(C)C ILMRJRBKQSSXGY-UHFFFAOYSA-N 0.000 description 1
- FUFQUTSKNXAJEY-UHFFFAOYSA-N tert-butyl-[4-[(3-cyano-6,6-dimethyl-11-oxo-5h-benzo[b]carbazol-8-yl)methyl]piperidin-1-yl]sulfonylcarbamic acid Chemical compound C1CN(S(=O)(=O)N(C(O)=O)C(C)(C)C)CCC1CC1=CC=C(C(=O)C=2C3=CC=C(C=C3NC=2C2(C)C)C#N)C2=C1 FUFQUTSKNXAJEY-UHFFFAOYSA-N 0.000 description 1
- YBRBMKDOPFTVDT-UHFFFAOYSA-N tert-butylamine Chemical compound CC(C)(C)N YBRBMKDOPFTVDT-UHFFFAOYSA-N 0.000 description 1
- BCNZYOJHNLTNEZ-UHFFFAOYSA-N tert-butyldimethylsilyl chloride Chemical compound CC(C)(C)[Si](C)(C)Cl BCNZYOJHNLTNEZ-UHFFFAOYSA-N 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- WHRNULOCNSKMGB-UHFFFAOYSA-N tetrahydrofuran thf Chemical compound C1CCOC1.C1CCOC1 WHRNULOCNSKMGB-UHFFFAOYSA-N 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 125000005958 tetrahydrothienyl group Chemical group 0.000 description 1
- 125000004632 tetrahydrothiopyranyl group Chemical group S1C(CCCC1)* 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- WROMPOXWARCANT-UHFFFAOYSA-N tfa trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.OC(=O)C(F)(F)F WROMPOXWARCANT-UHFFFAOYSA-N 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 150000003557 thiazoles Chemical class 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 125000005505 thiomorpholino group Chemical group 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- AXZWODMDQAVCJE-UHFFFAOYSA-L tin(II) chloride (anhydrous) Chemical compound [Cl-].[Cl-].[Sn+2] AXZWODMDQAVCJE-UHFFFAOYSA-L 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- YONPGGFAJWQGJC-UHFFFAOYSA-K titanium(iii) chloride Chemical compound Cl[Ti](Cl)Cl YONPGGFAJWQGJC-UHFFFAOYSA-K 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 125000004306 triazinyl group Chemical group 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- TUQOTMZNTHZOKS-UHFFFAOYSA-N tributylphosphine Chemical compound CCCCP(CCCC)CCCC TUQOTMZNTHZOKS-UHFFFAOYSA-N 0.000 description 1
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 125000001889 triflyl group Chemical group FC(F)(F)S(*)(=O)=O 0.000 description 1
- 125000000025 triisopropylsilyl group Chemical group C(C)(C)[Si](C(C)C)(C(C)C)* 0.000 description 1
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- CWMFRHBXRUITQE-UHFFFAOYSA-N trimethylsilylacetylene Chemical group C[Si](C)(C)C#C CWMFRHBXRUITQE-UHFFFAOYSA-N 0.000 description 1
- MHNHYTDAOYJUEZ-UHFFFAOYSA-N triphenylphosphane Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 MHNHYTDAOYJUEZ-UHFFFAOYSA-N 0.000 description 1
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 1
- 230000000381 tumorigenic effect Effects 0.000 description 1
- 238000002525 ultrasonication Methods 0.000 description 1
- 201000011294 ureter cancer Diseases 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 125000003774 valeryl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 239000011592 zinc chloride Substances 0.000 description 1
- 235000005074 zinc chloride Nutrition 0.000 description 1
- GTLDTDOJJJZVBW-UHFFFAOYSA-N zinc cyanide Chemical compound [Zn+2].N#[C-].N#[C-] GTLDTDOJJJZVBW-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
- C07D209/58—[b]- or [c]-condensed
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/34—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide
- A61K31/343—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide condensed with a carbocyclic ring, e.g. coumaran, bufuralol, befunolol, clobenfurol, amiodarone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/404—Indoles, e.g. pindolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4178—1,3-Diazoles not condensed 1,3-diazoles and containing further heterocyclic rings, e.g. pilocarpine, nitrofurantoin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4523—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems
- A61K31/454—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. pimozide, domperidone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/506—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5377—1,4-Oxazines, e.g. morpholine not condensed and containing further heterocyclic rings, e.g. timolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/54—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame
- A61K31/541—Non-condensed thiazines containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/7056—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing five-membered rings with nitrogen as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
- C07D209/80—[b, c]- or [b, d]-condensed
- C07D209/82—Carbazoles; Hydrogenated carbazoles
- C07D209/88—Carbazoles; Hydrogenated carbazoles with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to carbon atoms of the ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D307/78—Benzo [b] furans; Hydrogenated benzo [b] furans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D307/92—Naphthofurans; Hydrogenated naphthofurans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D333/52—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D333/76—Dibenzothiophenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/10—Spiro-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/02—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains two hetero rings
- C07D491/10—Spiro-condensed systems
- C07D491/107—Spiro-condensed systems with only one oxygen atom as ring hetero atom in the oxygen-containing ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F7/00—Compounds containing elements of Groups 4 or 14 of the Periodic Table
- C07F7/02—Silicon compounds
- C07F7/08—Compounds having one or more C—Si linkages
- C07F7/0834—Compounds having one or more O-Si linkage
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H15/00—Compounds containing hydrocarbon or substituted hydrocarbon radicals directly attached to hetero atoms of saccharide radicals
- C07H15/26—Acyclic or carbocyclic radicals, substituted by hetero rings
Definitions
- the present invention relates to tetracyclic compounds, salts or solvates thereof. More specifically, the present invention relates to the tetracyclic compounds and provides a medicament, pharmaceutical compositions comprising the compounds, ALK inhibitors, and pharmaceuticals for the prophylaxis or treatment of the diseases including cancer, cancer metastasis, depression or cognitive function disorder comprising the compounds. Furthermore, the present invention relates to a method for the treatment of the diseases comprising administering to the patient who is in need of the treatment of the disease the compounds described herein, salts or solvates thereof in an effective amount for the treatment of the diseases, and use of the tetracyclic compounds for the preparation of the pharmaceutical composition.
- Anaplastic Lymphoma Kinase is one of the receptor tyrosine kinases belonging to insulin receptor family (Non-Patent Document Nos. 1 and 2).
- Non-Patent Document 1 For example, in lung cancer, ALK forms EML4-ALK due to chromosomal translocation, leading to constitutive activation of tyrosine kinase, and it acquires a tumorigenic activity (Non-Patent Document 1).
- ALK translocation were reported in systemic anaplastic large cell lymphoma (ALCL) and inflammatory myofibroblastic tumors (IMTs) (Non-Patent Document Nos. 3 and 4), and esophageal cancer (Non-Patent Document 5). It was also found that active point mutation (approximately 10%) or gene amplification of ALK is involved in oncogenesis of neuroblastoma (Non-Patent Document Nos. 6 and 7).
- Non-Patent Document Nos. 8 and 9 both a ligand for ALK.
- Non-Patent Document 10 and Patent Document 1 an inhibitor for ALK is useful as an anti-depression agent or as a preventive or therapeutic agent for cognitive function disorders.
- a compound having an inhibitory activity on ALK will be very useful for the prevention and treatment of cancer, depression and cognitive function disorders, etc.
- ALK inhibiting material there are some compounds among multi-kinase inhibitors which have an inhibitory activity on ALK as one of their activities.
- c-MET mesenchymal-epithelial transition factor
- ALK PF02341066 having a 2-aminopyridine structure
- FAK a compound which has a 2,4-diaminopyrimidine structure
- ZAP70 a compound which has a 2,4-diaminopyrimidine structure
- IGF-1R and ALK etc.
- NVP-TAE684 As an inhibitor for FAK, ZAP70, IGF-1R and ALK, etc., NVP-TAE684 having a 2,4-diaminopyrimidine structure was reported (Patent Document 3 and Non-Patent Document 13).
- Patent Document 4 2,4-diaminopyrimidines and 2,4-diaminoquinazolines
- Patent Document 5 pyridopyrazines
- Patent Document 6 pyrazolo [3,4-C] isoquinolines
- Patent Document 7 thiazoles
- Patent Document 8 tricyclic compounds
- Patent Document 9 indazoles
- tetracyclic compounds comprising carbazole structure like ellipticine are known.
- Non-Patent Document 15 Non-Patent Document 15
- the present invention is to provide ALK-inhibiting compounds having a novel structure.
- object of the present invention is to provide a pharmaceuticals for the prophylaxis or treatment comprising the ALK-inhibiting compounds that is effective for prophylaxising or treating a disease accompanied by abnormality in ALK, for example, cancer, cancer metastasis, depression and cognitive function disorder.
- the tetracyclic compounds, a medicament and a pharmaceutical composition comprising the compounds, etc. shown below are provided.
- a 1 , A 2 , A 3 , A 4 , A 7 , A 8 , A 9 and A 10 all represent C, or any one of A 2 , A 3 , A 4 , A 7 , A 8 and A 9 represents N (with the proviso that, when it represents N, no substituent group exists therefor) and the remainings represent C;
- a 5 is selected from NR 5 , O and S;
- R 1 and R 10 each independently represent [1] a hydrogen atom, [2] a cyano group,
- R 2 is selected from the group consisting of:
- R 3A [1] a C 6-10 aryl group, [2] a C 1-8 alkoxy group, [3] a 5- to 14-membered heteroaryl group, or [4] a C 6-10 aryl sulfonyl group,
- R 3B [1] a hydroxy group, [2] a C 1-8 alkoxy group, [3] a C 6-10 aryl (C 0-8 alkyl) aminocarbonyl group, [4] a (C 1-8 alkyl) m3d -amino group, or [5] a halogen atom,
- R 3C [1] a C 1-8 alkyl group which may be substituted by halogen atom(s), or [2] a C 1-8 alkoxy group,
- R 4 is selected from the group consisting of:
- R 5 is selected from the group consisting of:
- R 5A [1] a hydroxycarbonyl group, [2] a C 1-8 alkoxycarbonyl group, [3] a hydroxy group, [4] a C 1-8 alkoxy group, [5] a (C 1-8 alkyl) m5 -amino group, [6] a C 6-10 aryl group, or [7] a C 1-8 alkylthio group,
- R 6 and R 6′ are each independently selected from the group consisting of:
- R 6 and R 6′ are taken together with the carbon atoms to which they are bound to form:
- R 7 is selected from the group consisting of:
- R 7A [1] a (C 1-8 alkyl) m7a -amino group, [2] a hydroxy, [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by C 1-8 alkyl group(s),
- R 8 is selected from the group consisting of:
- R 8A [1] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R 8A1 , [2] a (C 1-8 alkyl) m8a -amino group which may be substituted by a halogen atom, and [3] a hydroxy group,
- R 8A1 [1] a C 1-8 alkyl group, [2] a C 1-8 alkylsulfonyl group, [3] a (C 1-8 alkyl) m8b -aminosulfonyl group, [4] an oxo group, [5] a C 1-8 alkoxycarbonyl, or [6] a C 1-8 alkoxycarbonyl (C 0-8 alkyl) aminosulfonyl,
- R 8B1 [1] a C 3-8 cycloalkyl group, [2] a hydroxy group, or [3] a C 1-8 alkoxy group(s),
- R 8B2 [1] a halogen atom, [2] a C 1-8 alkyl group, [3] an oxo group, [4] a hydroxy group, or [5] a deuterium atom,
- R 8B3 a (C 1-8 alkyl) m8f -amino group
- R 8B4 [1] a C 3-8 cycloalkyl group, or [2] a hydroxy group
- R 8C [1] a hydroxy group, [2] a (C 1-8 alkyl) m8h -amino group which may be substituted by substituent(s) selected from the group consisting of ⁇ 1> a (C 1-8 alkyl) m8i -aminosulfonyl group, ⁇ 2> a C 1-8 alkylsulfonyl group, ⁇ 3> a C 1-8 alkoxycarbonyl group and
- R 8D [1] a C 1-8 alkyl group which may be substituted by one or more R 8D1 , [2] a hydroxy group, [3] a C 1-8 alkylsulfonyl group, or [4] a C 1-8 alkoxycarbonyl group,
- R 8D1 [1] a hydroxy group, or [2] a C 1-8 alkoxy group,
- R 8H [1] a hydroxy group, or [2] a 4- to 10-membered heterocycloalkyl group,
- R 8F1 [1] a hydroxy group, [2] a C 1-8 alkoxy group, or [3] a halogen atom,
- R 8F2 [1] a 4- to 10-membered heterocycloalkyl group, [2] a C 1-8 alkoxycarbonyl group, or [3] a C 1-8 alkylsulfonyl group,
- R 8G [1] a hydroxycarbonyl group, [2] a hydroxy group, or [3] a (C 1-8 alkyl) m8l3 -amino group,
- (21) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group which may be substituted by C 1-8 alkyl group(s),
- R 9 is selected from the group consisting of:
- R 9A [1] a C 3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R 9A , [3] a hydroxy group, [4] a C 1-8 alkoxy group, or [5] a hydroxycarbonyl group,
- R 9A1 [1] a C 1-8 alkyl group, [2] a C 3-8 cycloalkyl group, or [3] a 4- to 10-membered heterocycloalkyl group,
- R 9B [1] a (C 1-8 alkyl) m9a -amino group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more group R 9B1
- R 9B1 [1] a C 3-8 cycloalkyl group, or [2] a 4- to 10-membered heterocycloalkyl group,
- R 9C [1] a C 1-8 alkoxy group, [2] a (C 1-8 alkyl) m9b -amino group which may be substituted by C 6-10 aryl group(s), [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R 9C1 , [4] a C 3-8 cycloalkyl group, [5] a hydroxy group,
- R 9C1 [1] a C 3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group, or [3] an oxo group,
- R 9D [1] a C 1-8 alkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s), [2] a C 3-8 cycloalkyl group, [3] a 4- to 10-membered heterocycloalkyl group, or [4] a C 1-6 alkylsulfonyl group, or [5] a C 1-8 alkoxycarbonyl group,
- R 9E [1] a halogen atom, [2] a hydroxy group, [3] a hydroxycarbonyl group, or [4] a C 1-8 alkyl group which may be substituted by hydroxy group(s), or [5] a C 1-8 alkoxy group,
- R 9G [1] a hydroxy group, [2] a hydroxycarbonyl group, [3] a C 6-10 aryl group which may be substituted by C 1-8 alkoxy group(s), [4] a (C 1-8 alkyl) m9g1 -amino group, [5] a C 1-8 alkoxy group which may be substituted by one or more R 9G 1, [6] a 5- to 14-membered heteroaryl group, or [7] a 4- to 10-membered heterocycloalkyloxy group which may be substituted by C 1-8 alkyl group(s),
- R 9G1 [1] a C 1-8 alkoxy group, or [2] a hydroxycarbonyl group,
- a 1 , A 2 , A 3 , A 4 , A 7 , A 8 , A 9 and A 10 all represent C, or any one of A 2 , A 3 , A 4 , A 7 , A 8 and A 9 represents N (with the proviso that, when it represents N, no substituent group exists therefor) and the remainings represent C;
- a 5 is selected from NR 5 , O and S;
- R 1 represents [1] a hydrogen atom, [2] a cyano group, or [3] a halogen atom;
- R 2 is selected from the group consisting of:
- R 3A [1] a C 6-10 aryl group, [2] a C 1-8 alkoxy group, [3] a 5- to 14-membered heteroaryl group, or [4] a C 6-10 aryl sulfonyl group,
- R 3B [1] a hydroxy group, [2] a C 1-8 alkoxy group, [3] a C 6-10 aryl (C 0-8 alkyl) aminocarbonyl group, [4] a (C 1-8 alkyl) m3a -amino group, or [5] a halogen atom,
- (21) a C 6-10 aryloxycarbonyl (C 0-8 alkyl) amino group which may be substituted by C 1-8 alkyl group(s) which may be substituted by halogen atom(s),
- R 4 is selected from the group consisting of:
- R 5 is selected from the group consisting of:
- R 5A [1] a hydroxycarbonyl group, [2] a C 1-8 alkoxycarbonyl group, [3] a hydroxy group, [4] a C 1-8 alkoxy group, [5] a (C 1-8 alkyl) m5 -amino group, or [6], a C 1-8 alkylthio group,
- R 6 and R 6′ are each independently:
- R 6 and R 6′ are taken together with the carbon atoms to which they are bound to form,
- R 7 is selected from the group consisting of:
- R 7A [1] a (C 1-8 alkyl) m7a -amino group, or [2] a hydroxy group,
- R 8 is selected from the group consisting of:
- R 8A1 [1] a C 1-8 alkyl group, [2] a C 1-8 alkylsulfonyl group, [3] a (C 1-8 alkyl) m8b -aminosulfonyl group, or [4] an oxo group,
- R 8B1 [1] a C 3-8 cycloalkyl group, [2] a hydroxy group, or [3] C 1-8 alkoxy group which may be substituted by C 1-8 alkoxy group(s),
- R 8B2 [1] a halogen atom, [2] a C 1-8 alkyl group, [3] an oxo group, or [4] a hydroxy group,
- R 8B3 a (C 1-8 alkyl) m8f -amino group
- R 8B4 [1] a C 3-8 cycloalkyl group, or [2] a hydroxy group
- R 8C [1] a hydroxy group, [2] a (C 1-8 alkyl) m8h -amino group which may be substituted by substituent(s) selected from the group consisting of ⁇ 1> a (C 1-8 alkyl) m8i -aminosulfonyl group and ⁇ 2> a C 1-8 alkylsulfonyl group, or [3] a C 1-8 alkylsulfonyl group,
- R 8D [1] a C 1-8 alkyl group which may be substituted by one or more R 8D1 , [2] a hydroxy group, or [3] a C 1-8 alkylsulfonyl group,
- R 8D1 [1] a hydroxy group, or [2] a C 1-8 alkoxy group,
- R 8F1 [1] a hydroxy group, [2] a C 1-8 alkoxy group, or [3] a halogen atom,
- R 8F2 [1] a 4- to 10-membered heterocycloalkyl group, [2] a C 1-8 alkoxycarbonyl group, or [3] a C 1-8 alkylsulfonyl group,
- R 8G [1] a hydroxycarbonyl group, [2] a hydroxy group, or [3] a (C 1-8 alkyl) m8l3 -amino group,
- (21) a C 1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s);
- R 9 is selected from the group consisting of:
- R 9A [1] a C 3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R 9A , [3] a hydroxy group, or [4] a C 1-8 alkoxy group,
- R 9A1 [1] a C 1-8 alkyl group, [2] a C 3-8 cycloalkyl group, or [3] a 4- to 10-membered heterocycloalkyl group,
- R 9C [1] a C 1-8 alkoxy group, [2] a (C 1-8 alkyl) m9b -amino group which may be substituted by C 6-10 aryl group(s), [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R 9C1 , [4] a C 3-8 cycloalkyl group, [5] a hydroxy group, or [6] a hydroxycarbonyl group,
- R 9C1 [1] a C 3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group, or [3] an oxo group,
- R 9D [1] a C 1-8 alkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s), [2] a C 3-8 cycloalkyl group, [3] a 4- to 10-membered heterocycloalkyl group, or [4] a C 1-6 alkylsulfonyl group,
- R 9E [1] a halogen atom, [2] a hydroxy group, [3] a hydroxycarbonyl group, or [4] a C 1-8 alkyl group which may be substituted by hydroxy group(s),
- R 9G [1] a hydroxy group, [2] a hydroxycarbonyl group, [3] a C 6-10 aryl group which may be substituted by C 1-8 alkoxy group(s), [4] a (C 1-8 alkyl) m9g1 -amino group, [5] a C 1-8 alkoxy group which may be substituted by one or more R 9G 1, or [6] a 5- to 14-membered heteroaryl group,
- R 9G1 [1] a C 1-8 alkoxy group, or [2] a hydroxycarbonyl group,
- R 10 represents [1] a hydrogen atom, or [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s)].
- a compound according to claim 1 or salt or solvate thereof, which said compound is selected from the group consisting of:
- the compounds of the present invention or salts or salvates thereof have an excellent activity of inhibiting ALK, excellent stability in organisms, and excellent solubility in water, and therefore are useful as a prophylactic or therapeutic agent for proliferative disorders (in particular, therapeutic agent).
- the compounds of the present invention or salts salts or solvates thereof are useful as a prophylactic or therapeutic agent (in particular, therapeutic agent) for various diseases such as cancers including leukemia (acute myelogenous leukemia, chronic myelogenous leukemia, acute lymphatic leukemia, chronic lymphatic leukemia and the like), malignant lymphoma (Hodgkin lymphoma, non-Hodgkin lymphoma and the like), brain tumor, neuroblastoma, gliomatosis, thyroid cancer, myelodysplastic syndrome, head and neck cancer, esophageal cancer, stomach cancer, colon cancer, colorectal cancer, breast cancer, ovarian cancer, lung cancer, pancreatic cancer, liver cancer, gall bladder cancer, skin
- the compounds of the present invention are useful as a prophylactic or therapeutic agent (in particular, therapeutic agent) for infiltration/metastasis of solid tumors. Still further, the compounds of the present invention are useful as a prophylactic or therapeutic agent for other diseases that are related with ALK, for example, depression or a cognitive function disorder.
- the method of the present invention comprises a step of administering a pharmaceutically effective amount of the pharmaceutical composition comprising the compounds of the present invention or salts or solvates thereof to a patient who is in need of such treatment or suffers from such diseases or conditions.
- the “halogen atom” means a fluorine atom, a chlorine atom, a bromine atom, an iodine atom and the like.
- the preferred halogen atom includes a fluorine atom, a chlorine atom and a bromine atom.
- the halogen atom when the halogen atom is a substituent group for an alkyl group or a group which comprises the alkyl as at least a part of the group (e.g., alkoxy, alkenyl, unsaturated carbocycle, unsaturated heterocycle and the like), the preferred halogen atom includes a fluorine atom.
- examples thereof include a trifluoromethyl group, a pentafluoroethyl group, a heptafluoropropyl group, a nonafluorobutyl group, a trifluoromethoxy group, a pentafluoroethoxy group, a heptafluoropropoxy group, a nonafluorobutoxy group, a trifluoroacetyl group, a pentafluoropropionyl group, a heptafluorobutyryl group and a nonafluoropentanoyl group.
- the “C 1-8 alkyl group” means a monovalent group which is derived by removing any one of hydrogen atoms from a linear or branched aliphatic hydrocarbon having 1 to 8 carbon atoms. Specifically, examples thereof include a methyl group, an ethyl group, an isopropyl group, a butyl group, a n-butyl group, an isobutyl group, a sec-butyl group, a t-butyl group, a pentyl group, an isopentyl group, a 2,3-dimethyl propyl group, a hexyl group, a 2,3-dimethyl hexyl group, a 1,1-dimethyl pentyl group, a heptyl group and an octyl group.
- it is a C 1-6 alkyl group, more preferably a C 1-5 alkyl group, still more preferably a C 1-4 alkyl group, and still
- the “C 1-8 alkyl group which may be substituted” means an unsubstituted C 1-8 alkyl group or a C 1-8 alkyl group of which at least one hydrogen atom on the alkyl group is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the alkyl group may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 1-8 alkyl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) for C 1-6 alkyl group and a C 1-4 alkyl group, and 1 to 2 substituent(s) for a C 1-3 alkyl group.
- the “C 2-8 alkenyl group” means a monovalent group wherein at least one double bond (two adjacent SP2 carbon atoms) is comprised in a linear or branched aliphatic hydrocarbon group having 1 to 8 carbon atoms.
- Specific examples of the C 2-8 alkenyl group include a vinyl group, an allyl group, a 1-propenyl group, a 2-propenyl group, a 1-butenyl group, a 2-butenyl group (including both cis and trans), a 3-butenyl group, a pentenyl group and a hexenyl group.
- it is a C 2-6 alkenyl group, more preferably a C 2-5 alkenyl group, still more preferably a C 2-4 alkenyl group, and still even more preferably a C 2-3 alkenyl group.
- C 2-8 alkenyl group which may be substituted means the unsubstituted C 2-8 alkenyl group described above or a C 2-8 alkenyl group of which at least one hydrogen atom on the alkenyl group is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the single-bonded carbon atom may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 2-8 alkenyl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) for a C 2-6 alkenyl group and a C 2-4 alkenyl group, 1 to 2 substituent(s) for a C 2-3 alkenyl group.
- the “C 2-8 alkynyl group” means a monovalent group wherein at least one triple bond (two adjacent SP carbon atoms) is comprised in a linear or branched aliphatic hydrocarbon group having 1 to 8 carbon atoms.
- Specific examples of the C 2-8 alkynyl group include an ethynyl group, a 1-propynyl group, a propargyl group and a 3-butynyl group.
- it is a C 2-6 alkynyl group, more preferably a C 2-5 alkynyl group, still more preferably a C 2-4 alkynyl group, and still even more preferably a C 2-3 alkynyl group.
- C 2-8 alkynyl group which may be substituted means the unsubstituted C 2-8 alkynyl group described above or a C 2-8 alkynyl group of which at least one hydrogen atom on the alkynyl group is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the single-bonded carbon atom may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 2-8 alkynyl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) for a C 2-6 alkynyl group and a C 2-4 alkynyl group, and 1 to 2 substituent(s) for C 2-3 alkynyl group.
- the “C 3-8 cycloalkyl group” means an aliphatic hydrocarbon group in cyclic form. Preferably, it includes a C 3-6 cycloalkyl group. Specifically, examples thereof include a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, a cycloheptyl group and a cyclooctyl group. Preferably, it is a C 3-6 cycloalkyl group.
- C 3-8 cycloalkyl group which may be substituted means the unsubstituted C 3-8 cycloalkyl group described above or a C 3-8 cycloalkyl group of which at least one hydrogen atom is substituted by a defined substituent group(s).
- each substituent group can be the same or different from each other.
- the single-bonded carbon atom may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 3-8 cycloalkyl group which may be substituted by 1 to 3 substituent(s).
- the “4- to 10-membered heterocycloalkyl group” means a saturated or partially unsaturated heterocyclic group which consists of 4 to 10 ring-constituting atoms and comprises 1 to 3 hetero atoms that are selected from O, S and N.
- the heterocycloalkyl group can be a monocyclic, a bicyclic or a spirocyclic type heterocycloalkyl group.
- examples thereof include an oxetanyl group, a tetrahydrofuryl group, a tetrahydrothienyl group, a tetrahydropyranyl group, a pyrrolidino group, a pyrrolidinyl group, a piperidino group, a piperidinyl group, a piperazino group, a piperazinyl group, a morpholino group, a morpholinyl group, a tetrahydrothiopyranyl group, a thiomorpholino group, an imidazolidinyl group, a 1,3-dioxolanyl group, a tetrahydropyranyl group, a 1,3-dioxanyl group, a 1,2,3,6-tetrahydropyridinyl group, a 1,4-Dioxa-8-aza-spiro[4.5]decanyl group, and a 1-oxa
- the “4- to 10-membered heterocycloalkyl group which may be substituted” means the unsubstituted 4- to 10-membered heterocycloalkyl group described above or a 4- to 10-membered heterocycloalkyl group of which at least one hydrogen atom on the heterocycloalkyl group is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the alkyl group may be substituted by a cyclic substituent group through a spiro bond.
- it is a 4-to 10-membered heterocycloalkyl group which may be substituted by 1 to 4 substituent(s).
- substituent(s) for a 4- to 8-membered heterocycloalkyl group is 1 to 4 substituent(s) for a 4- to 8-membered heterocycloalkyl group, and 1 to 3 substituent(s) for a 4- to 6-membered heterocycloalkyl group.
- substituent is an oxo group
- 2 oxo group can combine with the same sulfur atom.
- salt is formed, 2 alkyl group can combine with the same nitrogen atom.
- the “C 6-10 aryl group” means a monovalent aromatic hydrocarbon ring.
- Specific examples of the C 6-10 aryl group include a phenyl group, a 1-naphthyl group and a 2-naphthyl group.
- it is a C 6 aryl group or a C 10 aryl group.
- C 6-10 aryl group which may be substituted means the unsubstituted C 6-10 aryl group described above or a C 6-10 aryl group of which at least one hydrogen atom is substituted by a defined substituent group(s).
- each substituent group can be the same or different from each other.
- it is a C 6-10 aryl group which may be substituted by 1 to 3 substituent(s).
- the “5- to 14-membered heteroaryl group” means an aromatic cyclic group comprising one or more hetero atoms among 5 to 14 ring-constituting atoms.
- the cycle can be a monocyclic or bicyclic heteroaryl group fused to a benzene ring or a monocyclic heteroaryl ring.
- a furyl group a thienyl group, a pyrrolyl group, an imidazolyl group, a pyrazolyl group, a thiazolyl group, an isothiazolyl group, an oxazolyl group, an isooxazolyl group, an oxadiazolyl group, a thiadiazolyl group, a triazolyl group, a tetrazolyl group, a pyridyl group, a pyrimidyl group, a pyridazinyl group, a pyrazinyl group, a triazinyl group, a benzofuranyl group, a benzothienyl group, a benzothiadiazolyl group, a benzothiazolyl group, a benzoxazolyl group, a benzoxadiazolyl group, a benzoimidazolyl group, an indolyl group, an iso
- the “5- to 14-membered heteroaryl group which may be substituted” means the unsubstituted 5- to 14-membered ring heteroaryl group described above or a 5- to 14-membered ring heteroaryl group of which at least one hydrogen atom on the heteroaryl group is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a 5- to 14-membered heteroaryl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) or 1 to 2 substituent(s) for a 5- to 6-membered heteroaryl group.
- the “C 1-8 alkanoyl group” means a C 1-8 alkyl-C(O)— group, and the C 1-8 alkyl group is described above. Specifically, examples thereof include acetyl, propionyl, butyryl, isobutyryl, pentanoyl, tert-butylcarbonyl and a hexanoyl group. Preferably, it is a C 1-6 alkanoyl group, and more preferably a C 1-3 alkanoyl group.
- C 1-8 alkanoyl group which may be substituted means the unsubstituted C 1-8 alkanoyl group described above or a C 1-8 alkanoyl group of which at least one hydrogen atom on the alkanoyl group is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a C 1-8 alkanoyl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 2 substituent(s) for a C 1-6 alkanoyl group and a C 1-3 alkanoyl group.
- the “C 3-8 cycloalkylcarbonyl group” means a C 3-8 cycloalkyl-C(O)— group, and the C 3-8 cycloalkyl group is described above. Specifically, examples thereof include a cyclopropylcarbonyl group, a cyclobutylcarbonyl group, a cyclopentylcarbonyl group, a cyclohexylcarbonyl group, a cycloheptylcarbonyl group and a cyclooctylcarbonyl group.
- the “4- to 10-membered heterocycloalkylcarbonyl group” means a 4- to 10-membered heterocycloalkyl-CO— group, and the 4- to 10-membered heterocycloalkyl is described above.
- the “4- to 10-membered heterocycloalkylcarbonyl group which may be substituted” means the unsubstituted 4- to 10-membered heterocycloalkylcarbonyl group described above or a 4- to 10-membered heterocycloalkylcarbonyl group in which at least one hydrogen atom of the heterocycloalkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the heterocycloalkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a 4-to 10-membered heterocycloalkylcarbonyl group which may be substituted by 1 to 3 substituent(s).
- aminocarbonyl group which may be substituted means an unsubstituted aminocarbonyl group or an aminocarbonyl group in which one or two hydrogen atoms on the nitrogen atom are substituted by a defined substituent(s). When two substituent groups are present, each substituent group can be the same or different from each other.
- C 3-8 cycloalkyl (C 0-8 alkyl) aminocarbonyloxy group means a C 3-8 cycloalkyl-NHC(O)O— group or a C 3-8 cycloalkyl-N(C 1-8 alkyl) C(O)O— group, and the C 3-8 cycloalkyl group is described above.
- examples thereof include a cyclopropylaminocarbonyloxy group, a cyclobutylaminocarbonyloxy group, a cyclopentylaminocarbonyloxy group, a cyclohexylaminocarbonyloxy group, a cyclopropyl(N-methyl)aminocarbonyloxy group, and a cyclobutyl(N-methyl)aminocarbonyloxy group.
- (C 1-8 alkyl) x -aminocarbonyl group means a NH 2 C(O)— group, a (C 1-8 alkyl)NH—C(O)— group, or a (C 1-8 alkyl) 2 N—C(O)— group.
- examples thereof include a N-methyl-aminocarbonyl group, N-ethyl-aminocarbonyl group, N-n-buthyl-aminocarbonyl group, a N,N-dimethyl-aminocarbonyl group.
- the “(C 1-8 alkyl) x -aminocarbonyl group which may be substituted” means an unsubstituted (C 1-8 alkyl) x -aminocarbonyl group described above or an (C 1-8 alkyl) x -aminocarbonyl group in which at least one hydrogen atom on the nitrogen atom or the alkyl moiety are substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the “C 6-10 aryl(C 0-8 alkyl)aminocarbonyl group” means a C 6-10 aryl-NHC(O)— group, or a C 6-10 aryl-N(C 1-8 alkyl)-C(O)— group. Specifically, examples thereof include a phenyl-NHC(O)— group, or a phenyl-(N-methyl)-aminocarbonyl group, wherein the C 6-10 aryl group and C 1-8 alkyl are described above. Specifically, examples thereof include a phenylaminocarbonylamino group and a phenylaminocarbonyl(N-methyl)amino group.
- the “4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group” means a carbonyl group to which a 4- to 10-membered nitrogen-containing heterocycloalkyl group is bonded.
- the 4- to 10-membered nitrogen-containing heterocycloalkyl group i.e., 4- to 10-membered heterocycloalkyl group comprising a nitrogen atom(s)
- it is bonded to the carbonyl group via a nitrogen atom that is comprised in the heterocycloalkyl ring.
- the 4- to 10-membered nitrogen-containing heterocycloalkyl group examples include a pyrrolidinyl group, an imidazolidinnyl group, a morpholino group, a thiomorphorino group, a piperazino group and a piperidino group.
- examples thereof include a pyrrolidinocarbonyl group, a piperidinocarbonyl group, a piperazinocarbonyl group and a morpholinocarbonyl group.
- the “4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group, which may be substituted” means the unsubstituted 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group as described above or a 4- to 10-membered heterocycloalkylcarbonyl group in which at least one hydrogen atom of the heterocycloalkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the heterocycloalkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by 1 to 3 substituent(s).
- the “4- to 10-membered heterocycloalkyl (C 0-8 alkyl) aminocarbonyl group” means 4- to 10-membered heterocycloalkyl NHC(O)— group, or a 4- to 10-membered heterocycloalkyl N(C 1-8 alkyl)-C(O)— group.
- examples thereof include a oxetan-3-yl amide group, and a (1,1-dioxo-tetrahydro-thiophen-3-yl)-amide group.
- the “4- to 10-membered heterocycloalkyl (C 0-8 alkyl) aminocarbonyl group which may be substituted by one or more oxo groups” means the unsubstituted 4- to 10-membered heterocycloalkylaminocarbonyl group described above or the 4- to 10-membered heterocycloalkylaminocarbonyl group in which the heterocycloalkyl moiety is substituted by at least one oxo group.
- C 6-10 arylsulfonyl group means a C 6-10 aryl-S(O) 2 — group and the C 6-10 aryl group is described above. Specifically, examples thereof include a phenylsulfonyl group.
- the “5- to 14-membered heteroarylsulfonyl group” means a 5- to 14-membered heteroaryl-S(O) 2 — group, and the 5- to 14-membered heteroaryl is described above. Specifically, examples thereof include a imidazol-sulfonyl group.
- (C 1-8 alkyl) x -amino group means an amino group, a NH(C 1-8 alkyl) group, or a N(C 1-8 alkyl) 2 - group.
- examples thereof include amino, methylamino, ethylamino, butylamino, isopropylamino, dimethylamino and diethylamino.
- it is a C 1-3 alkylamino group.
- (C 1-8 alkyl) x -amino group which may be substituted means an unsubstituted (C 1-8 alkyl) x -amino group or an amino group in which one or two hydrogen atoms on the nitrogen atom or the alkyl moiety are substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- C 1-8 alkylcarbonyl (C 0-8 alkyl) amino group means a C 1-8 alkyl-C(O)—NH— group or a C 1-8 alkyl-C(O)—N(C 1-8 alkyl)- group, and the C 1-8 alkyl is described above. Specifically, examples thereof include a methylcarbonylamino group, an ethylcarbonylamino group, a propylcarbonylamino group and a butylcarbonylamino group.
- C 1-8 alkylcarbonyl (C 0-8 alkyl) amino group which may be substituted means the unsubstituted C 1-8 alkylcarbonyl (C 0-8 alkyl) amino group described above or the C 1-8 alkylcarbonyl (C 0-8 alkyl) amino group in which at least one hydrogen atoms of the terminal alkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 1-8 alkylcarbonyl (C 0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- C 6-10 arylcarbonyl (C 0-8 alkyl) amino group means a C 6-10 aryl-C(O)—NH— group or a C 6-10 aryl-C(O)—N(C 1-8 alkyl)- group and the C 6-10 aryl group and the C 1-8 alkyl group are described above. Specifically, examples thereof include a phenylcarbonylamino group.
- C 6-10 arylcarbonyl (C 0-8 alkyl) amino group which may be substituted means the unsubstituted C 6-10 arylcarbonyl (C 0-8 alkyl) amino group described above or the C 6-10 arylcarbonyl (C 0-8 alkyl) amino group in which at least one hydrogen atoms of the aryl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a C 6-10 arylcarbonyl (C 0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- (C 1-8 alkyl) x -aminocarbonyl (C 0-8 alkyl) amino group means a NH 2 C(O)NH— group, a (C 1-8 alkyl)NHC(O)NH— group, a NH 2 C(O)N(C 1-8 alkyl)- group, or a (C 1-8 alkyl)NHC(O)N(C 1-8 alkyl)- group, and the C 1-8 alkyl is described above.
- examples thereof include aminocarbonyl-(N-methyl)amino, and (N-methyl)aminocarbonyl-(N′-methyl)amino.
- the “(C 1-8 alkyl) x -aminocarbonyl (C 0-8 alkyl) amino group which may be substituted” means an unsubstituted (C 1-8 alkyl) x -aminocarbonyl (C 0-8 alkyl) amino group, or a (C 1-8 alkyl) x -aminocarbonyl (C 0-8 alkyl) amino group in which at least one hydrogen atom on the nitrogen atom or the alkyl moiety is substituted by a defined substituent.
- it is a (C 1-8 alkyl) x -aminocarbonyl (C 0-8 alkyl) amino group which may be substituted by a phenyl group.
- C 1-8 alkylsulfonylamino group means a C 1-8 alkyl-S(O) 2 —NH— group and the C 1-6 alkyl group is described above. Specifically, examples thereof include a methylsulfonylamino group and an ethylsulfonylamino group.
- (C 1-8 alkyl) x -aminosulfonyl(C 0-8 alkyl)amino group means a NH 2 S(O) 2 NH— group, a NH(C 1-8 alkyl)-S(O) 2 NH— group, or a N(C 1-8 alkyl) 2 -S(O) 2 NH— group, a NH 2 S(O) 2 N (C 1-8 alkyl) x - group, a NH(C 1-8 alkyl)-S(O) 2 (C 1-8 alkyl)N— group, or a N(C 1-8 alkyl) 2 -S(O) 2 (C 1-8 alkyl)N— group, and the C 1-8 alkyl group is described above. Specifically, examples thereof include a methylamino-sulfonylamino group and a dimethylamino-sulfonylamino group.
- the “C 1-8 alkoxy group” means a C 1-8 alkyl-O— group. Specifically, examples thereof include a methoxy group, an ethoxy group, a 1-propoxy group, a 2-propoxy group, a n-butoxy group, an i-butoxy group, a sec-butoxy group, a t-butoxy group, a 1-pentyloxy group, a 2-pentyloxy group, a 3-pentyloxy group, a 2-methyl-1-butyloxy group, a 3-methyl-1-butyloxy group, a 2-methyl-2-butyloxy group, a 3-methyl-2-butyloxy group, a 2,2-dimethyl-1-propyloxy group, a 1-hexyloxy group, a 2-hexyloxy group, a 3-hexyloxy group, a 2-methyl-1-pentyloxy group, a 3-methyl-1-pentyloxy group, a 4-methyl-1-pentyloxy group,
- the “C 1-8 alkoxy group which may be substituted” means an unsubstituted C 1-8 alkoxy group or a C 1-8 alkoxy group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 1-8 alkoxy group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) for C 1-6 alkoxy group and a C 1-4 alkoxy group, and 1 to 2 substituent(s) for a C 1-3 alkoxy group.
- the “C 1-8 alkoxycarbonyl group” means a C 1-8 alkyl-O—C(O)— group and the C 1-8 alkyl group is described above. Specifically, examples thereof include a methoxycarbonyl group, an ethoxycarbonyl group, a n-propoxycarbonyl group and an i-propoxycarbonyl group. Preferably, it is a C 1-6 alkoxycarbonyl group, and more preferably a C 1-3 alkoxycarbonyl group.
- C 1-8 alkoxycarbonyl group which may be substituted means the unsubstituted C 1-8 alkoxycarbonyl group described above or a C 1-8 alkoxycarbonyl group of which at least one hydrogen atom is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the alkyl moiety of the alkoxycarbonyl group may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 1-8 alkoxycarbonyl group which may be substituted by 1 to 3 substituent(s).
- the “C 0-8 alkoxy (C 0-8 alkyl) aminocarbonyl group” means a HO—NH—C(O)— group, a C 1-8 alkyl-NH—C(O)— group, a HO—N(C 1-8 alkyl)-C(O)— group, or a C 1-8 alkyl-N(C 1-8 alkyl)-C(O)— group, and has a C 1-8 alkoxy group or a C 1-8 alkyl group as described above.
- examples thereof include a methoxyaminocarbonyl group, an ethoxyaminocarbonyl group, a n-propoxyaminocarbonyl group and an i-propoxyaminocarbonyl group.
- it is a C 1-6 alkoxyaminocarbonyl group, and more preferably a C 1-3 alkoxyaminocarbonyl group.
- C 0-8 alkoxy (C 0-8 alkyl) aminocarbonyl group which may be substituted means the unsubstituted hydroxyaminocarbonyl group described above, or a C 1-8 alkoxyaminocarbonyl group, a hydroxy (C 1-8 alkyl) aminocarbonyl group or a C 1-8 alkoxy (C 1-8 alkyl) aminocarbonyl group, wherein at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 1-8 alkoxy aminocarbonyl group which may be substituted by 1 to 3 substituent(s).
- the “4- to 10-membered heterocycloalkyloxy group” means a 4- to 10-membered heterocycloalkyl-O— group, and the 4- to 10-membered heterocycloalkyl is described above.
- the “4- to 10-membered heterocycloalkyloxy group which may be substituted” means the unsubstituted 4- to 10-membered heterocycloalkyloxy group described above or a 4- to 10-membered heterocycloalkyloxy group in which at least one hydrogen atom of the heterocycloalkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the heterocycloalkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a 4-to 10-membered heterocycloalkyloxy group which may be substituted by 1 to 3 substituent(s).
- C 6-10 aryloxy group means a C 6-10 aryl-O— group, and the C 6-10 aryl group is described above.
- the “5- to 14-membered heteroaryloxy group” means a 5- to 14-membered heteroaryl-O— group, and the 5- to 14-membered heteroaryl is described above. Specifically, examples thereof include a pyrimidinyloxy group.
- C 1-8 alkylcarbonyloxy group means a C 1-8 alkyl-C(O)—O— group, and the C 1-8 alkyl is described above. Specifically, examples thereof include a methylcarbonyloxy group, an ethylcarbonyloxy group and a propylcarbonyloxy group.
- C 2-8 alkenylcarbonyloxy group means a C 2-8 alkenyl-C(O)—O— group, and the C 2-8 alkenyl is described above. Specifically, examples thereof include a 2-methyl-2-butenoyloxy group.
- the “4- to 10-membered heterocycloalkylcarbonyloxy group” means a 4- to 10-membered heterocycloalkyl-C(O)—O— group, and the 4- to 10-membered heterocycloalkyl is described above.
- (C 1-8 alkyl) x -aminocarbonyloxy group means a NH 2 C(O)—O— group, a NH(C 1-8 alkyl)-C(O)—O— group, or a N(C 1-8 alkyl) 2 -C(O)—O— group.
- examples thereof include a methylamino-carbonyloxy group, an ethylamino-carbonyloxy group and a propylamino-carbonyloxy group.
- the “(C 1-8 alkyl) x -aminocarbonyloxy group which may be substituted” means an unsubstituted (C 1-8 alkyl) x -aminocarbonyloxy group or a (C 1-8 alkyl) x -aminocarbonyloxy group group in which one or two hydrogen atoms on the nitrogen atom or the alkyl moiety are substituted by a defined substituent(s). When two substituent groups are present, each substituent group can be the same or different from each other.
- the “4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group” means the 4- to 10-membered nitrogen-containing heterocycloalkyl-S(O) 2 — group described above. Specifically, examples thereof include a morphorino-sulfonyl group.
- the “4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted” means the unsubstituted 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group described above or the 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group in which at least one hydrogen atom of the 4- to 10-membered nitrogen-containing heterocycloalkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a 4-to 10-membered nitrogen-containing heterocycloalkylsulfonyl which may be substituted by 1 to 3 substituent(s).
- the “4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group” means the 4- to 10-membered nitrogen-containing heterocycloalkyl-S(O) 2 —O— group described above. Specifically, examples thereof include a morphorino-sulfonyloxy group and a piperadino-sulfonyloxy group.
- the “4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group which may be substituted” means the unsubstituted 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group described above or the 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group in which at least one hydrogen atom of the 4- to 10-membered nitrogen-containing heterocycloalkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a 4-to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy which may be substituted by 1 to 3 substituent(s).
- C 1-8 alkylsulfonyloxy group means a C 1-8 alkyl-S(O) 2 —O— group, and the C 1-8 alkyl is described above.
- C 1-8 alkylsulfonyloxy group which may be substituted means the unsubstituted C 1-8 alkylsulfonyloxy group described above or a C 1-8 alkylsulfonyloxy group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 1-8 alkylsulfonyloxy group which may be substituted by 1 to 3 substituent(s).
- examples thereof include a trifluoromethylsulfonyloxy group.
- (C 1-8 alkyl) x -aminosulfonyloxy group wherein x is a symbol defined in claims, means a NH 2 S(O) 2 — group, a N(C 1-8 alkyl)S(O) 2 — group, or a N(C 1-8 alkyl) 2 S(O) 2 — group. Specifically, examples thereof include a N-methylaminosulfonyloxy group.
- C 1-8 alkylthio group means a C 1-8 alkyl-S— group, and the C 1-8 alkyl group is described above. Examples thereof include methylthio, ethylthio, n-propylthio, i-propylthio, n-butylthio, s-butylthio, i-butylthio, t-butylthio, n-pentylthio, 3-methylbutylthio, 2-methylbutylthio, 1-methylbutylthio, 1-ethylpropylthio, n-hexylthio, 4-methylpentylthio, 3-methylpentylthio, 2-methylpentylthio, 1-methylpentylthio, 3-ethylbutylthio, and 2-ethylbutylthio and the like.
- it is a C 1-6 alkylthio group, and more preferably a C 1-3 alky
- C 1-8 alkylthio group which may be substituted means an unsubstituted C 1-8 alkylthio group or a C 1-8 alkylthio group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a C 1-8 alkylthio group which may be substituted by 1 to 3 substituent(s).
- the “C 1-8 alkylsulfonyl group” means a C 1-8 alkyl-S(O) 2 — group, and the C 1-8 alkyl group is described above. Specifically, examples thereof include a methylsulfonyl group, an ethylsulfonyl group and a n-propylsulfonyl group. Preferably, it is a C 1-6 alkylsulfonyl group, and more preferably a C 1-3 alkylsulfonyl group.
- the “C 1-8 alkylsulfinyl group” means a C 1-8 alkyl-S(O)— group, and the C 1-8 alkyl group is described above. Specifically, examples thereof include a methylsulfinyl group, an ethylsulfinyl group and a n-propylsulfinyl group. Preferably, it is a C 1-6 alkylsulfinyl group, and more preferably a C 1-3 alkylsulfinyl group.
- C 1-8 alkylsulfonyl group which may be substituted means the unsubstituted C 1-8 alkylsulfonyl group described above or a C 1-8 alkylsulfonyl group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a C 1-8 alkylsulfonyl group which may be substituted by 1 to 3 substituent(s).
- C 1-8 alkylsulfinyl group which may be substituted means the unsubstituted C 1-8 alkylsulfinyl group described above or a C 1-8 alkylsulfinyl group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a C 1-8 alkylsulfinyl group which may be substituted by 1 to 3 substituent(s).
- the “4- to 10-membered heterocycloalkylsulfonyl group” means a 4- to 10-membered heterocycloalkyl-S(O) 2 — group, and the 4- to 10-membered heterocycloalkyl is described above.
- the “4- to 10-membered heterocycloalkylsulfonyl group which may be substituted” means the unsubstituted 4- to 10-membered heterocycloalkylsulfonyl group described above or a 4- to 10-membered heterocycloalkylsulfonyl group in which at least one hydrogen atom of the heterocycloalkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- the heterocycloalkyl moiety may be substituted by a cyclic substituent group through a spiro bond.
- it is a 4-to 10-membered heterocycloalkylsulfonyl group which may be substituted by 1 to 3 substituent(s).
- (C 1-8 alkyl) x -aminosulfonyl group means a NH 2 —S(O) 2 — group, a C 1-8 alkylamino-S(O) 2 — group, or a (C 1-8 alkyl) 2 amino-S (O) 2 — group and the C 1-8 alkyl is described above. Specifically, examples thereof include an aminosulfonyl group, a methylaminosulfonyl group and a dimethylaminosulfonyl group.
- the “(C 1-8 alkyl) x -aminosulfonyl group which may be substituted” means an unsubstituted aminosulfonyl group or a (C 1-8 alkyl) x -aminosulfonyl group in which one or two hydrogen atoms on the nitrogen atom or the alkyl moiety are substituted by a defined substituent(s). When two substituent groups are present, each substituent group can be the same or different from each other.
- C 1-8 alkoxycarbonyl (C 0-8 alkyl) amino group means a C 1-8 alkoxy-C(O)—NH— group or a C 1-8 alkoxy-C(O)—N(C 1-8 alkyl)- group, wherein the C 1-8 alkoxy group and C 1-8 alkyl) are described above. Specifically, examples thereof include a methoxycarbmamoyl group and an N-ethylcarbonyl-N-methyl-amino group.
- C 1-8 alkoxycarbony(C 0-8 alkyl) amino group which may be substituted means the unsubstituted C 1-8 alkoxycarbony(C 0-8 alkyl) amino group described above, or a C 1-8 alkoxycarbony(C 0-8 alkyl) amino group, wherein at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a C 1-8 alkoxycarbony(C 0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- C 1-8 alkoxycarbonyl (C 0-8 alkyl) aminosulfonyl group means a C 1-8 alkoxy-C(O)—NHS(O) 2 — group or a C 1-8 alkoxy-C(O)—N(C 1-8 alkyl)S(O) 2 — group, wherein the C 1-8 alkoxy group and C 1-8 alkyl group are described above. Specifically, examples thereof include a methoxy carbonyl aminosulfonyl group and an ethoxycarbonyl-N-methyl-aminosulfonyl group.
- C 6-10 aryloxycarbonyl (C 0-8 alkyl) amino group means a C 6-10 aryl-O—C(O)—NH— group or a C 6-10 aryl-O—C(O)—N(C 1-8 alkyl)- group, wherein the C 6-10 aryl group and C 1-8 alkyl are described above. Specifically, examples thereof include a phenyloxycarbonylamino group and a N-methyl-N-phenyloxycarbonyl-amino group.
- C 6-10 aryloxycarbonyl (C 0-8 alkyl) amino group which may be substituted means the unsubstituted C 6-10 aryloxycarbonyl (C 0-8 alkyl) amino group described above or the C 6-10 aryloxycarbonyl (C 0-8 alkyl) amino group in which at least one hydrogen atoms of the aryl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a C 6-10 aryloxycarbonyl (C 0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- C 6-10 aryl (C 0-8 alkyl) aminocarbonyl (C 0-8 alkyl) amino group means a C 6-10 aryl NHC(O)NH— group, a C 6-10 aryl-N(C 1-8 alkyl)-C(O)NH— group, a C 6-10 aryl-N(C 1-8 alkyl)-C(O)N(C 1-8 alkyl)- group, or a C 6-10 aryl-NH—C(O)N(C 1-8 alkyl)- group, wherein the C 6-10 aryl group and C 1-8 alkyl are described above. Specifically, examples thereof include a phenylaminocarbonylamino group and a phenylaminocarbonyl(N-methyl)amino group.
- C 6-10 aryl (C 0-8 alkyl) aminocarbonyl (C 0-8 alkyl) amino group which may be substituted means the unsubstituted C 6-10 aryl (C 0-8 alkyl) aminocarbonyl (C 0-8 alkyl) amino group described above or the C 6-10 aryl (C 0-8 alkyl) aminocarbonyl (C 0-8 alkyl) amino group in which at least one hydrogen atoms of the aryl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a C 6-10 aryl (C 0-8 alkyl) aminocarbonyl (C 0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- C 6-10 aryl (C 0-8 alkyl) aminocarbonyloxy group means a C 6-10 aryl-NHC(O)—O—, or a C 6-10 aryl-N(C 1-8 alkyl)-C(O)—O— group, wherein the C 6-10 aryl group and C 1-8 alkyl are described above. Specifically, examples thereof include a phenylaminocarbonyloxy group and a phenylaminocarbonyl(N-methyl)amino carbonyloxy group.
- C 6-10 aryl (C 0-8 alkyl) aminocarbonyloxy group which may be substituted means the unsubstituted C 6-10 aryl (C 0-8 alkyl) aminocarbonyloxy group described above or the C 6-10 aryl (C 0-8 alkyl) aminocarbonyloxy group in which at least one hydrogen atoms of the aryl moiety is substituted by a defined substituent(s).
- each substituent group can be the same or different from each other.
- it is a C 6-10 aryl (C 0-8 alkyl) aminocarbonyloxy group which may be substituted by 1 to 3 substituent(s).
- C 1-8 alkylsulfonyl (C 0-8 alkyl) amino group means a C 1-8 alkyl-S(O) 2 —NH— group, or a C 1-8 alkyl-S(O) 2 —N(C 1-8 alkyl)- group. Specifically, examples thereof include a methylsulphonylamino group and a methylsulphonyl-(N-methyl)amino group.
- C 2-8 alkenyloxy group means a C 2-8 alkenyl-O— group, wherein the C 2-8 alkenyl group is described above.
- Specific examples of the C 2-8 alkenyloxy group include a vinyloxy group and a allyloxy group.
- all of A 1 , A 2 , A 3 , A 4 , A 7 , A 8 , A 9 and A 10 are C, or any one of A 2 , A 4 , A 7 and A 9 is N and the remainings are C (with the proviso that, when A 2 , A 4 , A 7 or A 9 is N, they do not have a substituent group R 2 , R 4 , R 7 or R 9 ).
- all of them are C, or A 4 is N while the remainings are C, even more preferably, all of them are C, or A 4 , A 7 , and A 9 is N and the remainings are C (with the proviso that, when A 4 , A 7 , or A 9 is N, they do not have a substituent group R 4 , R 7 , R 9 ).
- a 5 is preferably NR 5 or O, more preferably NR 5 , even more preferably NH.
- R 1 is preferably
- R 10 is preferably a hydrogen atom.
- R 2 is preferably
- R 4 is preferably
- R 5 is preferably
- R 5A is preferably
- R 6 and R 6′ are preferably
- R 7 is preferably
- R 7A is preferably
- R 3 is preferably
- R 3 is more preferably
- R 3 is still more preferably
- R 3 is still even more preferably
- R 3A is preferably
- R 3B is preferably
- R 3C is preferably
- a C 1-5 alkyl group which may be substituted by 1-11 halogen atom(s), [2] a C 1-5 alkoxy group, more preferably [1] a C 1-4 alkyl group which may be substituted by 1-9 halogen atom(s), [2] a C 1-3 alkoxy group.
- R 8 is preferably
- R 5A is preferably
- [8A-1] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-4 R 8A1 substituent(s),
- [8A-2] a (C 1-5 alkyl) m8a -amino group (m8a:0 ⁇ 2) which may be substituted by 1-11 halogen atom(s), and
- R 8B1 is preferably
- R 8B1 is preferably
- R 8B2 is preferably
- R 8B3 is preferably
- R 8B4 is preferably
- R 8C is preferably
- R 8D1 is preferably
- R 8D1 is preferably
- R 8H is preferably
- R 8E is preferably
- R 8E1 is preferably
- R 8E2 is preferably
- R 8E3 is preferably
- R 8E4 is preferably
- R 8E6 is preferably
- R 8E7 is preferably
- R 8F is preferably
- R 8F1 is preferably
- R 8F2 is preferably
- R 8G is preferably
- R 9 is preferably
- R 9A is preferably [9A-1] a C 3-6 cycloalkyl group, [9A-2] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-3 R 9A1 substituent(s), [9A-3] a hydroxy group, or [9A-4] a C 1-6 alkoxy group, More preferably [9A-1] a 4- to 6-membered heterocycloalkyl group which may be substituted by a R 9A1 substituent, [9A-2] a hydroxy group, or [9A-3] a C 1-3 alkoxy group,
- R 9A1 is preferably
- R 9C is preferably
- R 9C1 is preferably
- R 9D is preferably
- [9D-1] a C 1-5 alkyl group which may be substituted by one or two 4- to 6-membered heterocycloalkyl group(s), [9D-2] a C 3-5 cycloalkyl group, [9D-3] a 4- to 6-membered heterocycloalkyl group, or [9D-4] a C 1-3 alkylsulfonyl group; More preferably [9D-1] a C 1-5 alkyl group which may be substituted by a 4- to 6-membered heterocycloalkyl group, [9D-2] a 4- to 6-membered heterocycloalkyl group, or [9D-3] a methylsulfonyl group;
- R 9E is preferably
- R 9F is preferably
- R 9G is preferably
- R 9G1 is preferably
- a 5 is NH, while the remaining are C, R 3 is a cyano group, R 6 and R 6′ are methyl, R 8 is (1) a hydrogen atom, (2) a C 1-8 alkyl group which may be substituted by one or more R 8A , (3) a C 2-8 alkenyl group, (4) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R 8B , (5) a 5- to 14-membered heteroaryl group which may be substituted by a C 1-8 alkyl group, (6) a (C 1-8 alkyl) m8g -aminocarbonyl group which may be substituted by one or more R C , (7) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R 8D , (8) a hydroxycarbonyl group, (9) a C 0-8 alkoxy (C 0-8 alkyl) aminocarbonyl group
- a 5 is NH while the remaining are C
- R 3 is a cyano group
- R 6 and R 6′ are methyl groups
- R 8 is (1) a hydrogen atom, (2) a C 1-8 alkyl group which may be substituted by one or more R 8A , (3) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R 8B , or (4) a C 1-8 alkoxy group which may be substituted by one or more R 8E
- R 9 is (1) a hydrogen atom, (2) a C 1-8 alkyl group which may be substituted by one or more R 9A , (3) a C 2-8 alkynyl group which may be substituted by one or more R 9C , (4) a C 3-8 cycloalkyl group, or (5) a halogen atom
- R 1 , R 2 , R 5 , R 7 and R 10 are an hydrogen atom.
- examples of the salts of the compounds that are represented by the Formula (I) include hydrochloric acid salt, hydrobromic acid salt, hydriodic acid salt, phosphoric acid salt, phosphonic acid salt, sulfuric acid salt, sulfonic acid salt such as methanesulfonic acid salt, p-toluene sulfonic acid salt and the like, carboxylic acid salt such as acetic acid salt, citric acid salt, malic acid salt, tartaric acid salt, succinic acid salt, salicylic acid salt and the like, or alkali metal salt such as sodium salt, potassium salt and the like, alkaline earth metal salt such as magnesium salt, calcium salt and the like, ammonium salt such as ammonium salt, alkyl ammonium salt, dialkyl ammonium salt and trialkyl ammonium salt tetraalkyl ammonium salt.
- the salts are pharmaceutically acceptable salts. These salts are produced by brining the compounds described above in contact with an acid
- the compounds that are represented by the Formula (I) or salts thereof can be an anhydride or a solvate such as a hydrate and the like.
- solvate (d) indicates a phenomenon by which solute molecules or ions contained in a solution strongly attract neighboring solvent molecules to form a huge group of molecules.
- the solvent is water, it is called “hydrate (d).”
- the solvate can be any one of a hydrate and a non-hydrate.
- the solvates are pharmaceutically acceptable solvates.
- alcohol for example, methanol, ethanol, n-propanol
- dimethylformamide and the like can be used.
- the compounds of the present invention and salts thereof may be present in several tautomer forms, for example, enol and imine form, keto and enamine form, and a mixture thereof.
- a tautomer is present as a mixture of tautomeric set.
- one type of tautomer is generally present in dominant ratio.
- the present invention includes all types of tautomer of the compounds of the present invention.
- the present invention includes all types of stereoisomer of the compounds of the present invention that are represented by the Formula (I) (for example, enantiomer, diastereomer (including cis and trans geometric isomer)), racemate of the isomer and a mixture thereof.
- the compounds having the Formula (I) of the present invention may have one or more asymmetric center, and the present invention includes a racemic mixture, a diastereomer mixture and enantiomer of such compound.
- the compounds of the present invention When the compounds of the present invention are obtained in free form, they can be converted into a salt, a hydrate or solvate thereof which can be formed from the compounds according to a method generally known in the art.
- the compounds of the present invention when obtained in the form of a salt, hydrate or solvate of the compounds, they can be converted to free form according to a method generally known in the art.
- the present invention include all isotopes of compounds that are represented by the Formula (I).
- the isotopes of the compounds of the present invention indicate the compounds of the present invention in which at least one atom is substituted by an atom with the same atomic number (i.e., number of protons) but with different mass number (sum of the number of protons and the number of neutrons).
- Example of the isotopes that are included in the compounds of the present invention includes a hydrogen atom, a carbon atom, a nitrogen atom, an oxygen atom, a phosphorus atom, a sulfur atom, a fluorine atom, a chlorine atom and the like, and 2 H, 3 H, 13 C, 14 C, 15 N, 17 O, 18 O, 31 P, 32 P, 35 S, 18 F, 36 Cl and the like are included.
- a radioisotope which decays by emitting radiation for example 3 H and 14 C, are useful for determining the distribution of a pharmaceutical agent or a compound in a living tissue, etc.
- Isotopes of the compounds of the present invention can be converted by replacing a chemical reagent used for synthesis with a chemical reagent comprising a corresponding radioisotope according to a method generally known in the art.
- the compounds (I) of the present invention can be administered in the form of prodrug.
- prodrug indicates the derivatives of the compounds having the Formula (I) that can be converted to the compounds having the Formula (I) or salts or solvates thereof after administration by enzymatic or non-enzymatic degradation under a physiological condition.
- the prodrug can be in inactive form when it is administered to a patient. However, in organisms, it converts to the compounds having the Formula (I) and present therein in the active form.
- the prodrug converts into desired drug form at specific pH or by an enzymatic action.
- Conventional prodrug is a compound having a hydrolyzable ester residue which produces a free acid in organisms.
- hydrolyzable ester residue include a residue having a carboxyl moiety of which free hydrogen (for example, a free hydrogen in a carboxyl group when Y in the Formula (I) has a carboxyl group) is replaced by a C 1-4 alkyl group, a C 2-7 alkanoyloxymethyl group, a 1-(alkanoyloxy)ethyl group having 4 to 9 carbon atoms, a 1-methyl-1-(alkanoyloxy)-ethyl group having 5 to 10 carbon atoms, an alkoxycarbonyloxymethyl group having 3 to 5 carbon atoms, a 1-(alkoxycarbonyloxy)ethyl group having 4 to 7 carbon atoms, a 1-methyl-1-(alkoxycarbonyloxy)eth
- the compounds having the Formula (I) of the present invention can be produced by the method described below, for example.
- method of preparing the compounds of the present invention is not limited thereto.
- order of the reaction step like introduction of a substituent group, etc. can be changed.
- the compounds of the present invention are novel compounds which have not been described in literatures, they can be prepared according to a chemical method that is generally known in the art.
- reacting compounds that are used for the preparation commercially available ones can be used or they can be produced according to a method that is generally known in the art depending on necessity.
- a 1 to A 10 and R 1 to R 10 are as defined in the Formula (I).
- PR 1 to PR 10 are the same as R 1 to R 10 that are defined in the Formula (I) or represent a group which can be converted to R 1 to R 1I according to modification or deprotection of a functional group.
- PG represents a protecting group (for example, methyl, ethyl, t-butyl, benzyl, substituted benzyl, acetyl, t-butoxycarbonyl, benzyloxycarbonyl, methanesulfonyl, trifluoromethanesulfonyl, trimethylsilyl, triethylsilyl, triisopropylsilyl, t-butyldimethylsilyl, tetrahydropyranyl and the like).
- the preparation can be carried out by using means such as protection and deprotection of a functional group, etc.
- LG represents a leaving group such as fluorine, chlorine, bromine, iodine, methanesulfonate, trifluoromethanesulfonate and the like, which can be applied for the reaction described above.
- the step can be carried out by reacting cyclic ketone derivative Ia with an alkylating agent corresponding to R 6 and R 6′ in the presence of a base.
- an alkylating agent corresponding to R 6 and R 6′ for example, it can be carried out in view of the method described in Journal of the American Chemical Society, 115(23), 10628-36; 1993 and Organic Letters, 9(24), 5027-5029; 2007, etc.
- the reaction is carried out in a solvent under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent, in the presence or the absence of a catalyst.
- R 6 and R 6′ are atomic groups other than a hydrogen atom, the reaction order can be optionally selected, and separation and purification can be carried out at each step or the reaction can be carried out continuously.
- alkylating agent examples thereof include an alkyl halide such as Mel, ethyl iodide, 2-iodopropane, 1,4-dibromobutane, 1,1′-oxybis (2-bromoethane) and the like, dimethyl sulfate, and sulfonic acid ester such as methylmethanesulfonate, methyl tosylate and methyltrifluoromethanesulfonate.
- it is an alkyl halide such as Mel and the like.
- the catalyst examples thereof include a phase transfer catalyst such as tetrabutylammonium chloride and tetrabutylammonium hydrogen sulfate.
- the base examples thereof include an inorganic base such as sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, potassium hydride, calcium hydride and the like or an organic base such as t-BuOK, t-BuONa, pyridine, TEA, DIPEA, LDA, LiHMDS and n-BuLi.
- it is potassium hydroxide, potassium t-butoxy, or sodium t-butoxy.
- examples thereof include toluene, xylene, n-hexane, cyclohexane, DMF, DMA, EtOAc, DMSO, dichloromethane, carbon tetrachloride, THF, dioxane, acetonitrile, water, methanol, ethanol and a mixture thereof.
- it is a mixture solvent of water-THF or THF.
- the reaction consists of a step of producing phenyl hydrazone and a step of sigmatropic rearrangement. Separation and purification can be carried out at each step or the reaction can be carried out continuously. Further, according to the structure of aryl hydrazine, which is a reacting material of this reaction step, mixture of a position isomer can be obtained as a reaction product. Such position isomer can be separated from each other or used as a mixture for the next reaction step.
- examples thereof include formic acid, acetic acid, methanesulfonic acid, p-toluenesulfonic acid, benzenesulfonic acid, TFA, hydrochloric acid, sulfuric acid and pyridinium p-toluenesulfonate.
- it is acetic acid, sulfuric acid, or TFA.
- examples thereof include toluene, xylene, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME, TFE, diethylene glycol, triethylene glycol and a mixture thereof.
- This step is carried out by applying an oxidizing agent to a substrate in a solvent in the presence or absence of a catalyst under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent.
- a reaction condition the method described in Journal of Medicinal Chemistry, 51(13), 3814-3824; 2008, etc. can be considered.
- DDQ peracid such as, mCPBA and the like, cerium ammonium nitrate (IV) (CAN), permanganate such as potassium permanganate, barium permanganate and the like, sodium chlorite, hydrogen peroxide, or N-hydroxyphthalimide and the like
- peracid such as, mCPBA and the like, cerium ammonium nitrate (IV) (CAN)
- permanganate such as potassium permanganate, barium permanganate and the like, sodium chlorite, hydrogen peroxide, or N-hydroxyphthalimide and the like
- reaction solvent used for the reaction examples thereof include water, t-butanol, acetonitrile, THF, dichloromethane, ethyl acetate and a mixture thereof.
- it is THF.
- Step II-1 Step II-2
- the step can be carried out by reacting with an alkylating agent corresponding to R 6 and R 6′ in a solvent under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent, in the presence of abase.
- an alkylating agent corresponding to R 6 and R 6′ in a solvent under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent, in the presence of abase.
- ester hydrolysis step of carboxylic acid ester IId is an ester hydrolysis step of carboxylic acid ester IId.
- This step can be carried out by hydrolysis in an aqueous solvent at the reaction temperature of 0° C. to boiling point of the solvent in the presence of an inorganic base, for example in view of the method described in Tetrahedron Lett. 3529, 1977.
- it can be carried out according to a method in which hydrolysis is carried out in the presence of an acid, in view of the method described in J. Am. Chem. Soc, 1977, 99, 2353, for example.
- the inorganic base examples thereof include sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate and cesium carbonate.
- it is sodium hydroxide or potassium hydroxide.
- water, methanol, ethanol, tetrahydrofuran, dioxane, and the like can be used alone or in a combination thereof.
- it is methanol comprising water or ethanol comprising water.
- the acid which can be used for acid hydrolysis hydrochloric acid, sulfuric acid, trifluoroacetic acid and methanesulfonic acid can be used alone in a combination thereof.
- it is sulfuric acid.
- This step can be carried out by S N Ar reaction in which carboxylic acid ester or nitrile is reacted with aromatic compound IIf having a leaving group in the presence of a base. It can be carried out in view of the method described in J. Am. Chem. Soc. 2000, 122, 712-713. Alternatively, it can be also carried out according to a method in which carboxylic acid ester or nitrile is reacted with aromatic compound IIf having a leaving group in the presence of a catalyst, a ligand and abase. For example, it can be carried out in view of the method described in Org. Lett, 2008, 10(8), 1545, J. Org. Chem. 2003, 68, 8003 and Angew. Chem. Int. Ed. 2003, 42, 5051, etc.
- sodium phosphate, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, LiHMDS, NaHMDS, LDA, lithium dicyclohexylamide, lithium 2,2,6,6-tetramethyl pyrrolidide, KHMDS, t-BuONa, t-BuOK and the like can be used.
- it is NaHMDS, KHMDS, or t-BuONa.
- the catalyst ligand, or catalyst-ligand complex which are used for the reaction, palladium acetate, Pd 2 (dba) 3 , ⁇ -allyl palladium chloride dimer, PdCl 2 (CH 3 CN) 2 , trialkylproazaphosphatrane, ⁇ P(t-Bu) 3 PdBr ⁇ 2 , PPh 3 , P(o-tol) 3 , BINAP, DPPF, P(t-Bu) 3 , DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene, 1,3-diallyldihydroimidazolium salt and the like can be used, for example.
- it is triisobutylproazaphosphat
- This step can be carried out by hydrolysis in the presence of an acid under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and the reaction conditions include that described in, for example, Tetrahedron, 64(36), 8464-8475; 2008, etc.
- the acid itself can be used as a solvent or diluted with other solvent.
- it can be carried out by hydrolysis in the presence of an inorganic base under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and the reaction condition described in, for example, Bioorganic & Medicinal Chemistry Letters, 18(2), 749-754; 2008, etc. can be employed.
- the reaction consists of the hydrolysis of nitrile IIb to acid amide and further conversion into carboxylic acid. Separation and purification can be carried out at each step or the reaction can be carried out continuously.
- examples thereof include methanesulfonic acid, p-toluenesulfonic acid, benzenesulfonic acid, trifluoroacetic acid, hydrochloric acid and sulfuric acid.
- examples thereof include toluene, xylene, dioxane, dimethoxyethane, diethylene glycol, triethylene glycol, TFE and the like and a mixture thereof.
- examples thereof include sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate and cesium carbonate.
- 290-292 can be used, for example.
- the method of activating carboxylic acid examples thereof include a method of converting into an acid chloride by using thionyl chloride, oxalyl chloride, phosphorus oxychloride, etc. or a method of converting into an active ester by using CDI.
- thionyl chloride or CDI is used.
- the activated carboxylic acid itself can be subjected to separation and purification, or can be used continuously for the next reaction.
- the method of producing an enolate of malonic acid monoester a combination of magnesium salt like magnesium chloride, etc.
- an organic base such as TEA, DIPEA and the like can be also added to the reaction system.
- the solvent examples thereof include toluene, xylene, MeCN, THF, CPME, MTBE, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME, and the like and a mixture thereof.
- it is MeCN, THF or DME.
- This step can be carried out by so-called Blaise reaction in which 2-halo carboxylic acid ester is reacted with nitrile in the presence of activated zinc powder under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and the reaction method described in, for example, SYNTHESIS 2004, No. 16, pp 2629-2632x. can be used.
- the method of activating zinc powder a method in which acid washing and drying are carried out in advance, or a method in which a catalytic amount of an acid such as methanesulfonic acid, etc. is included in the reaction system can be employed.
- This step can be carried out by nucleophilic aromatic substitution reaction in which an aromatic nitro compound having a leaving group is reacted with ⁇ -ketoester IIg in the presence of a base under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and the reaction method include that described in, for example, Synlett, (5), 883-885; 2004 and Tetrahedron, 38(23), 3479-83; 1982.
- sodium phosphate, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, LiHMDS, NaHMDS, LDA, lithium dicyclohexylamide, lithium 2,2,6,6-tetramethylpyrrolidide, KHMDS, t-BuOK, t-BuONa and the like can be used.
- it is potassium carbonate, cesium carbonate, t-BuOK, or t-BuONa.
- examples thereof include toluene, xylene, MeCN, THF, CPME, MTBE, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME, acetone, methylethyl ketone, and a mixture thereof.
- it is THF, DMF, DMA, NMP or a mixture thereof.
- the present step can be also carried out in the presence of a catalyst and a base, as described in Step III-5 or Journal of Organic Chemistry, 72(14), 5337-5341; 2007.
- This reaction can be carried out by reacting ⁇ -ketoester IIIa with a reducing agent under the condition of a reaction temperature of 0° C. to boiling point of the solvent to reduce the nitro group.
- the condition generally used for reduction of a nitro group for example, iron as exemplified in Synthesis, (18), 2943-2952, 2008, zinc as exemplified in Tetrahedron, 64(40), 9607-9618, 2008, titanium (III) chloride as exemplified in Organic & Biomolecular Chemistry, 3(2), 213-215, 2005, tin (II) chloride as exemplified in Journal of Organic Chemistry, 58(19), 5209-5220,1993, sodium hydrosulphite as exemplified in Gazzetta Chimica Italiana, 121(11), 499-504, 1991, and catalytic reduction condition as exemplified in Synlett, (17), 2689-2691, 2008, etc.
- the reducing agent is iron or sodium hydrosulphite.
- an ester protecting group of indole-3-carboxylic acid ester IIb is a step of deprotecting an ester protecting group of indole-3-carboxylic acid ester IIb.
- an ester protecting group a methyl group, an ethyl group, a t-butyl group, a benzyl group, a substituted benzyl group and the like can be used. Preferably, it is a t-butyl.
- examples thereof include a method described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4 th edition, John Wiley & Sons 2007), and it can be appropriately used according to each reaction condition.
- TMSI t-butyl
- TMSCl t-butyl
- BF 3 .OEt 2 a deprotection condition
- examples thereof include toluene, xylene, diethyl ether, THF, CPME, MTBE, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME, TFE and the like and a mixture thereof.
- it is THF or TFE.
- a mixed acid anhydride is formed by using acetic anhydride, trifluoroacetic anhydride and the like, or acid chloride is formed by using thionyl chloride, oxalyl chloride, phosphorus oxychloride and the like, which results in activiation of the carboxylic acid.
- acetic anhydride or trifluoroacetic anhydride is used.
- the reaction is carried out in the absence or presence of a solvent.
- examples thereof include toluene, xylene, diethyl ether, THF, CPME, MTBE, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME and the like and a mixture thereof.
- it is THF, DMF, DMA or DME.
- an organic base such as TEA, DIPEA, pyridine and the like can be used.
- the cyclization is carried out without a catalyst or with Bronsted acid or Lewis acid catalyst (Heterocycles 1999, 51, 2127).
- the Lewis acid catalyst examples thereof include aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate and BF 3 .OEt 2 .
- it is BF 3 .OEt 2 .
- the step can be carried out by reacting an aromatic acylamide compound having a leaving group with ⁇ -ketoester IIg in the presence of a base, a catalyst, and a ligand under the condition of a reaction temperature of 0° C. to boiling point of the solvent, followed by deprotection of an acyl protecting group.
- a reaction temperature 0° C. to boiling point of the solvent
- deprotection of an acyl protecting group examples thereof include a method described in Journal of Organic Chemistry 2007, 72, 9329-9334 and Organic Letters 10(4), 625-628, 2008.
- the metal catalyst copper (I) iodide and palladium acetate can be used.
- (S)-proline tri t-butylphosphine, bis (t-butyl) (2′-methyl[1,1′-biphenyl]-2-yl)phosphine and the like can be used.
- the base which is used for the reaction sodium phosphate, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, LiHMDS, NaHMDS, LDA, lithium dicyclohexylamide, lithium 2,2,6,6-tetramethylpyrrolidide, potassium hexamethyldisilazide, t-BuONa, t-BuOK and the like can be used.
- the cyclization can be carried out based on an oxidative method.
- a reaction condition described in Angewandte Chemie, International Edition, 47(38), 7230-7233; 2008 can be also employed, for example.
- This step can be carried out in the same manner as Step III-1 and III-2 or Step III-5 and III-6.
- examples thereof include a method described in Organic Letters, 11(1), 221-224; 2009.
- the reaction is carried out in an appropriate solvent in the presence of a palladium catalyst and a ligand (or a complex thereof) with or without a base and a copper catalyst.
- Example of the copper catalyst used for the reaction include copper iodide.
- sodium phosphate, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, TEA, DIPEA and the like can be used.
- it is cesium carbonate, TEA or DIPEA.
- This step corresponds to a tandem Friedel-Crafts reaction in which acylation at 3-position of di-substituted indole derivative IVb is carried out in the presence of Lewis acid catalyst under the condition of a reaction temperature of 0° C. to boiling point of the solvent, followed by intramolecular cyclization.
- Lewis acid catalyst examples thereof include aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate and BF 3 .OEt 2 .
- it is aluminum chloride.
- This step consists of deprotection of carboxylic acid ester comprised in di-substituted indole derivative IVc and subsequent intramolecular cyclization at 3-position of the indole either in catalytic or non-catalytic manner.
- deprotection examples thereof include a method described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4 th edition, John Wiley & Sons 2007), and it can be appropriately used according to the type of each protecting group.
- an activated indole derivative is used for the reaction, cyclization occurs more easily so that the reaction can be carried out in a non-catalytic manner.
- the cylclization can be also carried out by using a condensing agent such as polyphosphoric acid, methanesulfonic acid-phosphorus pentoxide (Eaton reagent) and the like.
- a condensing agent such as polyphosphoric acid, methanesulfonic acid-phosphorus pentoxide (Eaton reagent) and the like.
- carboxylic acid is first converted into carboxylic acid chloride, a mixed acid anhydride and the like under the same condition as defined in Step III-4 and the cyclization is carried out under Friedel-Crafts condition in the presence of Lewis acid catalyst.
- Lewis acid catalyst used for the reaction examples thereof include aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate and BF 3 .OEt 2 .
- the reaction is catalytically carried out in the presence of a base with combination of a transition metal catalyst and a ligand, and the condition described in J. Am. Chem. Soc. 2000, 122, 1360-1370 and Journal of Organic Chemistry (2003), 68(25), 9865-9866 can be used, for example.
- the base used for the reaction examples thereof include t-BuONa, t-BuOK, LiHMDS, NaHMDS, potassium phosphate, sodium carbonate, potassium carbonate and cesium carbonate.
- the catalyst and a ligand (or a catalyst-ligand complex), palladium acetate, Pd 2 (dba) 3 , ⁇ -allylpalladium chloride dimer, PdCl 2 (CH 3 CN) 2 , PdCl 2 (PPh 3 ) 2 , trialkylproazaphosphatrane, ⁇ P(t-Bu) 3 PdBr ⁇ 2 , PPh 3 , P(o-tol) 3 , BINAP, DPPF, P(t-Bu) 3 , DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene 1,3-diallyldihydroimidazolium salt and the like can be used.
- a 5 is O or S
- a t-butyl group, a benzyl group and a substituted benzyl group can be used as a protecting group.
- a 5 is O
- a t-butyldimethylsilyl group and a tetrahydropyranyl group can be used.
- it is NH
- a t-butoxycarbonyl group, a benzyloxycarbonyl group, a methanesulfonyl group, a trifluoroacetyl group and the like can be used.
- examples thereof include a method described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4 th edition, John Wiley & Sons 2007), and it can be appropriately used according to the type of each protecting group.
- the reaction can be carried out under condition of using an acid catalyst or dehydrating condition.
- an acid catalyst or dehydrating condition for example, the reaction condition described in Acta Pharmaceutica Hungarica (2003), 73(3), 171-178 can be employed.
- a protecting group for hydroxyl group it can be carried out simultaneously with the deprotection of Step V-2, as described in Heterocycles, 26(7), 1863-71; 1987.
- a combination of an organic base and an acid anhydride such as trifluoromethanesulfonic acid and the like can be used.
- This step is carried out by applying an oxidizing agent to a substrate in a solvent in the presence or absence of a catalyst under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent.
- a reaction condition the method described in Journal of Medicinal Chemistry, 51(13), 3814-3824; 2008, etc. can be employed.
- DDQ peracid such as, mCPBA and the like, cerium ammonium nitrate (IV) (CAN), permanganate such as potassium permanganate, barium permanganate and the like, sodium chlorite, hydrogen peroxide, N-hydroxyphthalimide and the like
- peracid such as, mCPBA and the like
- permanganate such as potassium permanganate, barium permanganate and the like
- sodium chlorite hydrogen peroxide
- hydrogen peroxide N-hydroxyphthalimide and the like
- solvent used for the reaction examples thereof include water, t-butanol, acetonitrile, tetrahydrofuran, dichloromethane, ethyl acetate and a mixture thereof.
- the reaction can be carried out in the presence of a base, and the condition include that described in Synthesis, (2), 155-7; 1988, for example.
- the base examples thereof include n-butyl lithium, sodium methylate and triethylamine.
- reaction for the synthesis of an aromatic carboxylic acid It is a reaction for the synthesis of an aromatic carboxylic acid.
- the reaction can be carried out by metallization like addition of lithium or magnesium based on exchange between halogen and metal in the presence of a base, followed by carboxylation using carbonate gas, dry ice, etc.
- the reaction condition as described in Journal of Organic Chemistry (2008), 73(19), 7785-7788 can be employed.
- the base n-butyl lithium, s-butyl lithium, t-butyl lithium, a Grignard reagent, and various ate complexes can be used.
- carboxylation condition using a transition metal catalyst can be also employed.
- This step corresponds to intramolecular cyclization at 3-position of di-substituted benzothiophene derivative VId either in catalytic or non-catalytic manner.
- the reaction condition as described in Journal of the American Chemical Society, 130(23), 7286-7299; 2008 can be employed.
- the reaction can be carried out by using a condensing agent such as polyphosphoric acid, methanesulfonic acid-phosphorus pentoxide (Eaton reagent) and the like.
- carboxylic acid is first converted into carboxylic acid chloride, a mixed acid anhydride and the like and the cyclization is carried out under Friedel-Crafts condition in the presence of Lewis acid catalyst.
- the Lewis acid catalyst used for the reaction examples thereof include aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate and BF 3 .OEt 2 .
- Q 1 and Q 2 represent any substituent group which constitutes PR 1 to PR 4 and R 6 to PR 10 .
- a protecting group for an aromatic hydroxyl group.
- a methyl group, a t-butyl group, a benzyl group, a substituted benzyl group, a t-butyldimethylsilyl group, a tetrahydropyranyl group and the like can be used.
- it is a methyl group.
- examples thereof include a method described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4 th edition, John Wiley & Sons 2007), and it can be appropriately used according to the type of each protecting group.
- reaction conditions can be used selectively for the deprotection depending on reactivity. Examples thereof include heating in the presence of pyridine hydrochloric acid salt, heating in the presence of a solvent with dodecane thiol and sodium methylate and heating in the presence of a solvent with anhydrous lithium halide, boron tribromide, TMSI and the like.
- the reaction is carried out by reacting with a reacting reagent such as trifluoromethanesulfonic acid and the like in the presence of a base with or without a solvent under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent.
- a reacting reagent such as trifluoromethanesulfonic acid and the like
- a base with or without a solvent under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent.
- TEA, DIPEA, pyridine, 2,6-lutidine, dimethylaminopyridine and the like can be used.
- pyridine is used without any solvent.
- the obtained trifluoromethanesulfonic acid ester VIId is a good leaving group and can be used for various derivatization.
- the reaction is carried out by reacting with a reacting reagent such as sulfamoyl chloride and the like in the presence of a base with a solvent under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent.
- a reacting reagent such as sulfamoyl chloride and the like
- sodium hydride, TEA, DIPEA, pyridine, 2,6-lutidine, dimethylaminopyridine and the like can be used.
- it is sodium hydride.
- the obtained sulfamic acid ester VIIe is a substrate for the thiaFries rearrangement of Step VII-5 and can be used for various derivatization.
- This step corresponds to rearrangement of a sulfamoyl group to a neighboring position in the presence of a Lewis acid catalyst under the condition of a reaction temperature of 0° C. to boiling point of the solvent when the neighboring position of the sulfamic acid ester is unsubstituted (i.e., C—H), i.e., a reaction called thiaFries rearrangement.
- a Lewis acid catalyst i.e., aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate, BF 3 .OEt 2 and the like can be used.
- it is aluminum chloride.
- a reagent having an appropriate leaving group such as alkyl halide and the like is subjected to nucleophilic reaction with the hydroxyl group of compound VIIb in the presence of an appropriate base to form an ether bond.
- an appropriate base examples thereof include an inorganic base such as sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, potassium hydride, calcium hydride and the like or an organic base such as pyridine, TEA, DIPEA and the like.
- aryl halide, aryl borate and the like as a reagent having a leaving group, formation of an diaryl ether bond can be also achieved and used.
- a catalyst such as copper powder, copper acetate, copper iodide and the like or a ligand such as phenanthroline, trans-1,2-cyclohexanediamine and the like can be used.
- reaction for forming a bond between aryl and a hetero atom by using compound VIIg having a leaving group is carried out in an appropriate solvent inert to the reaction, in the presence of a base.
- a leaving group LG a halogen, triflate and the like can be used.
- the solvent examples thereof include toluene, xylene, n-hexane, cyclohexane, DMF, DMA, EtOAc, DMSO, NMP, THF, DME, dioxane, acetonitrile and the like and a mixture thereof.
- the base to be used for the reaction examples thereof include t-BuONa, t-BuOK, LiHMDS, NaHMDS, KHMDS, potassium phosphate, sodium carbonate, potassium carbonate and cesium carbonate.
- This step can be also carried out by using a catalyst and a ligand.
- the catalyst and a ligand (or a catalyst-ligand complex), palladium acetate, Pd 2 (dba) 3 , ⁇ -allylpalladium chloride dimer, PdCl 2 (CH 3 CN) 2 , PdCl 2 (PPh 3 ) 2 , trialkylproazaphosphatrane, ⁇ P(t-Bu) 3 PdBr ⁇ 2 , PPh 3 , P(o-tol) 3 , BINAP, DPPF, P(t-Bu) 3 , DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene, 1,3-diallyldihydroimidazolium salt and the like can be used, for example.
- Step VII-7 When the reaction product of Step VII-7 is thio ether VIIh, it is possible to obtain sulfoxide or sulfone compound VIIj by oxidation with m-chloro perbenzoic acid, oxone, TEMPO and the like.
- reaction for forming a bond between aryl and SP 2 carbon or a bond between aryl and SP 3 carbon in which compound VIIg having a leaving group is used.
- the reaction is carried out in an appropriate solvent inert to the reaction, in the presence of a base.
- a leaving group LG a halogen, triflate and the like can be used.
- the solvent examples thereof include toluene, xylene, n-hexane, cyclohexane, DMF, DMA, EtOAc, DMSO, NMP, THF, DME, dioxane, acetonitrile, water, isopropanol and the like and a mixture thereof.
- the base to be used for the reaction examples thereof include t-BuONa, t-BuOK, LiHMDS, NaHMDS, KHMDS, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, TEA and DIPEA.
- This step can be also carried out by using a catalyst and a ligand.
- the catalyst and a ligand (or a catalyst-ligand complex), palladium acetate, Pd 2 (dba) 3 , ⁇ -allylpalladium chloride dimer, PdCl 2 (CH 3 CN) 2 , PdCl 2 (PPh 3 ) 2 , trialkylproazaphosphatrane, ⁇ P(t-Bu) 3 PdBr ⁇ 2 , PPh 3 , P(o-tol) 3 , BINAP, DPPF, P(t-Bu) 3 , DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene, 1,3-diallyldihydroimidazolium salt and the like can be used, for example.
- Step VIIg It is a carboxylation reaction using compound VIIg having a leaving group.
- the reaction is carried out by reacting with formic acid (or a synthetic equivalent thereof) in an appropriate solvent inert to the reaction, in the presence of a base and a catalyst.
- a base and a catalyst As for the leaving group LG, a halogen, triflate and the like can be used.
- the solvent and the catalyst can be selected and used in the same manner as Step VII-9.
- the reaction can be carried out by dehydrating condensation reaction using various amines such as ammonia, primary amines, secondary amines, hydrazines, substituted hydrazines and the like.
- the reaction is carried out in the presence of an acid halogenating agent or a dehydrating condensing agent in an aprotic solvent under the condition of a reaction temperature of ⁇ 20° C. to boiling point of the solvent, with or without an active esterifying agent and a base.
- examples thereof include oxalyl chloride and thionyl chloride.
- examples thereof include HOBt, di(N-succinimidyl) carbonate and carbonyl diimidazole.
- examples thereof include TEA, DIPEA and DBU.
- examples thereof include DMF, DMA, DCM, acetone, THF, dioxane, DME, ethyl acetate, MeCN, and a mixture thereof.
- the reaction is carried out by reacting terminal alkyne in an appropriate solvent in the presence of a base and a catalyst with or without a catalytic amount of a copper reagent, and the reaction is referred to as Sonogashira reaction.
- the reagents and the condition for the reaction are as defined in Step IV-1 and Step IV-3.
- examples thereof include a method disclosed in Tetrahedron, 63(43), 10671-10683; 2007. Specifically, by having secondary amines and the like in a reaction system and using propargyl bromide as an alkyne, a propargyl amine can be introduced.
- the reaction can be carried out by adding CN ⁇ source in an appropriate solvent in the presence of a copper, zinc or palladium catalyst, with or without a ligand, in view of the reaction condition shown in Organic Letters, 10(23), 5325-5328; 2008, Tetrahedron Letters, 49(32), 4693-4694; 2008 and Bioorganic & Medicinal Chemistry, 16(13), 6489-6500; 2008.
- the CN ⁇ -source copper (I) cyanide, zinc (II) cyanide, iron (III) hexacyanide, sodium cyanide, potassium cyanide and the like can be used.
- Some of the starting materials for the present invention are novel compounds, and they can be easily synthesized in the same manner as known reacting compounds or according to the method well known to a skilled person in the art.
- the pharmaceutical composition of the present invention comprises a pharmaceutically acceptable carrier, in addition to the compound that is selected as being useful for the invention.
- pharmaceutically acceptable carrier means one or more type of appropriate solid or liquid vehicle, diluent or an encapsulating material which is suitable for administration to mammals.
- accepted means that it does not cause any reaction to substantially reduce the pharmaceutical efficacy of a composition under normal condition for use, and the components of the composition and the subject compound can be mixed well with each other.
- the pharmaceutically acceptable carrier should have substantially high purity and substantially low toxicity so that it can be suitably administered to a subject to be treated, preferably an animal, and more preferably a mammal.
- materials which can be used as a pharmaceutically acceptable carrier examples thereof include sugars such as lactose, glucose, sucrose, and the like; starch such as corn starch, potato starch and the like; cellulose and cellulose derivatives such as sodium carboxy methyl cellulose, ethyl cellulose, methyl cellulose and the like; tragacanth rubber powder; malt; gelatin; talc; solid lubricating agent such as stearic acid or magnesium stearate and the like; calcium sulfate; vegetable oils such as peanut oil, cotton seed oil, sesame oil, olive oil, corn oil, plant oil, cacao oil, and the like; polyhydric alcohols such as propylene glycol, glycerin, sorbitol, mannitol, polyethylene glycol and the like; alginic acid; an emulsifying agent such as TWEEN; humectant such as lecithin and the like; colorant; flavor; tabletting agent; stabilizer, anti-oxidant; pre
- composition of the present invention is used as an ALK inhibitor or a therapeutic or prophylactic agent for a proliferative disorder, or used against depression or cognitive function disorder, as an administration route, oral, rectal, parenteral (intravenous, intramuscular, subcutaneous), intracisternal, intravaginal, intraperitoneal, intrabladder, topical (drop, powder, ointment, gel or cream) administration or administration via inhalation (mouth or nasal spray) and the like can be considered.
- parenteral intravenous, intramuscular, subcutaneous
- intracisternal intravaginal
- intraperitoneal intrabladder
- topical drop, powder, ointment, gel or cream
- administration or administration via inhalation mouth or nasal spray
- the administration form examples thereof include a tablet, a capsule, a granule, powder, a pill, an aqueous or non-aqueous oral solution and suspension, and a parenteral solution which is filled in a container suitable to be divided into several small dosages.
- the administration form can be modified for various administration routes including subcutaneous transplant which gives controlled release of a drug compound.
- the aforementioned preparation is prepared according to a method generally known in the art by using additives such as a vehicle, a lubricating agent (i.e., coating agent), a binding agent, a disintegrating agent, a stabilizing agent, a corrigent for taste and smell, a diluent and the like.
- starch such as starch, potato starch, corn starch, lactose, crystalline cellulose and calcium hydrogen phosphate.
- examples thereof include ethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose, shellac, talc, carnauba wax and paraffin.
- binding agent examples thereof include polyvinyl pyrrolidone, Macrogol and the compounds described above as a vehicle.
- examples thereof include the compounds described as a vehicle in the above and a chemically modified starch or cellulose such as sodium croscarmellose, sodium carboxymethyl starch and crosslinked polyvinyl pyrrolidone.
- examples thereof include paraoxy benzoic acid esters such as methyl paraben, propyl paraben and the like; alcohols such as chlorobutanol, benzyl alcohol, phenylethyl alcohol and the like; benzalkonium chloride; phenols such as phenol, cresol and the like; thimerosal; dehydroacetic acid; and sorbic acid.
- paraoxy benzoic acid esters such as methyl paraben, propyl paraben and the like
- alcohols such as chlorobutanol, benzyl alcohol, phenylethyl alcohol and the like
- benzalkonium chloride phenols such as phenol, cresol and the like
- thimerosal dehydroacetic acid
- sorbic acid examples thereof include paraoxy benzoic acid esters such as methyl paraben, propyl paraben and the like; alcohols such as chlorobutanol, benzyl alcohol, phenylethyl alcohol and the like
- a corrigent for taste and smell examples thereof include a sweetener, an acid tasting agent, a flavor and the like that are commonly used in the art.
- a solvent to prepare a liquid preparation examples thereof include ethanol, phenol, chlorocresol, purified water and distilled water.
- examples thereof include polysorbate 80, polyoxyl 40 stearate and lauromacrogol.
- the pharmaceutical composition of the present invention When the pharmaceutical composition of the present invention is used as an ALK inhibitor or a therapeutic or prophylactic agent for a proliferative disorder, or used against depression or cognitive function disorder, the use amount of the compounds of the present invention or salts or solvates thereof varies depending on symptom, age, body weight, relative health state of a subject, administration of other drug compounds, administration method and the like.
- the amount which is generally effective for a patient is, in an effective component (i.e., the compound of the present invention that is represented by the Formula (I)), preferably 0.001 to 1000 mg per 1 kg body weight per day, more preferably 0.01 to 300 mg per 1 kg body weight per day in case of an orally administered agent, and dosage per day is preferably in the range of 1 to 800 mg for an adult patient with normal body weight.
- an effective component i.e., the compound of the present invention that is represented by the Formula (I)
- dosage per day is preferably in the range of 1 to 800 mg for an adult patient with normal body weight.
- a parenterally administered agent it is preferably 0.001 to 1000 mg per 1 kg body weight per day, and more preferably, 0.01 to 300 mg per 1 kg body weight per day. It is preferable to administer them once a day or in divided dosages a day, depending on symptom of a subject to be treated.
- NMR analysis was carried out by using JNM-EX270 (270 MHz, manufactured by JEOL), JNM-GSX400 (400 MHz, manufactured by JEOL), or 400 MR (400 MHz, manufactured by Varian). NMR data was expressed in ppm (parts per million; 6), while it was compared with the deuterium lock signal obtained from a sample solvent.
- the measurement was carried out by using JMS-DX303 or JMS-SX/SX102A (both manufactured by JEOL).
- Measurement was carried out by using Micromass (ZMD, manufactured by Micromass) equipped with 996-600E gradient high performance liquid chromatography (manufactured by Waters) or Micromass (ZQ, manufactured by Micromass) equipped with 2525 gradient high performance liquid chromatography (manufactured by Waters).
- ZMD manufactured by Micromass
- ZQ manufactured by Micromass
- the reaction was carried out by using a snap cap reaction vial together with an ExplorerTM (manufactured by CEM Microwave Technology) or an initiator (manufactured by Biotage).
- Maximum output setting includes cooling of the reaction vessel by air in order to avoid temperature increase caused by microwave irradiation.
- a functional group was protected using a protecting group to produce a target molecule, and the protecting group was removed later, if desired. Selection, addition and removal of a protecting group were carried out according to the method described in the literature [Greene and Wuts, “Protective Groups in Organic Synthesis” (4 th edition, John Wiley & Sons 2007)], for example.
- the crude product obtained from the above i.e., mixture of A3-1 and A3-2 was dissolved in a mixture solvent of THF (450 ml) and distilled water (50 ml), added once with DDQ (115 g, 509 mmol), and then stirred at room temperature for 1 hr.
- the reaction mixture was diluted with CPME 3 L, and the organic layer was washed three times with 0.5 N aqueous solution of sodium hydroxide (1 L) and twice with distilled water (1 L) in order and dried over anhydrous sodium sulfate.
- the organic layer was concentrated to 500 ml under reduced pressure.
- the precipitated product was collected by filtration and washed with a small amount of CPME to obtain the title compound as a yellow crystal (48 g, 40%).
- the organic layer was washed with brine and dried over magnesium sulfate.
- the drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (yellow oily substance, 226 mg, 75%).
- the drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (yellow oily substance, 524 mg, 75%).
- reaction solution was added with water, extracted with ethyl acetate. The organic layer was washed with an brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (white solid, 152 mg, 62%).
- the drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were added with tetrahydrofuran (100 ml), water (400 l) and sodium hydride (540 mg, 15.5 mmol), and stirred at room temperature for 16 hr.
- saturated aqueous solution of ammonium chloride 200 ml was added followed by extraction with ethyl acetate.
- the organic layer was dried over sodium sulfate.
- the drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane/ethyl acetate) to obtain the title compound (2.48 g, 90%).
- the organic layer was washed once with an aqueous solution of disodium EDTA (200 mL) and twice with saturated brine (200 mL) in order, and dried over anhydrous sodium sulfate.
- the organic layer was concentrated under reduced pressure to yield a product, which was suspended and washed with a small amount of CPME to obtain the title compound as a colorless crystal (6.58 g, 73%).
- the combined organic layer was washed twice with distilled water (100 mL) and once with saturated brine (100 mL) in order, and dried over anhydrous sodium sulfate.
- the organic layer was concentrated under reduced pressure to yield a product, which was suspended and washed with a small amount of CPME to obtain the title compound as a colorless crystal (5.91 g, 93%).
- the organic layer was washed with saturated brine and dried over sodium sulfate.
- the drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (37 mg, 76%).
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Molecular Biology (AREA)
- Psychiatry (AREA)
- Oncology (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Hospice & Palliative Care (AREA)
- Pain & Pain Management (AREA)
- Hematology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
- Plural Heterocyclic Compounds (AREA)
- Saccharide Compounds (AREA)
- Indole Compounds (AREA)
- Furan Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Steroid Compounds (AREA)
- Pyridine Compounds (AREA)
Abstract
A compound represented by the general Formula (I) below, or a salt or solvate thereof, which is useful as an ALK inhibitor, and is useful for prophylaxis or treatment of a disease accompanied by abnormality in ALK, for example, cancer, cancer metastasis, depression or cognitive function disorder:
-
- (meanings of the symbols that are included in the formula are as given in the specification).
Description
- This application is a Continuation of U.S. application Ser. No. 15/221,926, filed Jul. 28, 2016, which is a Continuation of U.S. application Ser. No. 14/619,242, filed Feb. 11, 2015, which is a Divisional of U.S. application Ser. No. 13/377,300, which is the U.S. National Stage application of PCT/JP2010/059785, filed Jun. 9, 2010, which claims priority from Japanese application JP 2009-139691, filed Jun. 10, 2009.
- The present invention relates to tetracyclic compounds, salts or solvates thereof. More specifically, the present invention relates to the tetracyclic compounds and provides a medicament, pharmaceutical compositions comprising the compounds, ALK inhibitors, and pharmaceuticals for the prophylaxis or treatment of the diseases including cancer, cancer metastasis, depression or cognitive function disorder comprising the compounds. Furthermore, the present invention relates to a method for the treatment of the diseases comprising administering to the patient who is in need of the treatment of the disease the compounds described herein, salts or solvates thereof in an effective amount for the treatment of the diseases, and use of the tetracyclic compounds for the preparation of the pharmaceutical composition.
- Anaplastic Lymphoma Kinase (ALK) is one of the receptor tyrosine kinases belonging to insulin receptor family (Non-Patent Document Nos. 1 and 2).
- It was reported that, due to gene alteration of ALK (translocation, point mutation and gene amplification), an abnormal activation of ALK is eventually involved in oncogenesis.
- For example, in lung cancer, ALK forms EML4-ALK due to chromosomal translocation, leading to constitutive activation of tyrosine kinase, and it acquires a tumorigenic activity (Non-Patent Document 1). In addition, the ALK translocation were reported in systemic anaplastic large cell lymphoma (ALCL) and inflammatory myofibroblastic tumors (IMTs) (Non-Patent Document Nos. 3 and 4), and esophageal cancer (Non-Patent Document 5). It was also found that active point mutation (approximately 10%) or gene amplification of ALK is involved in oncogenesis of neuroblastoma (Non-Patent Document Nos. 6 and 7).
- On the other hand, it was also reported in tumors activated by pleiotrophin (PTN) or midkine (MK) (Non-Patent Document Nos. 8 and 9), both a ligand for ALK.
- Further, from the study using ALK knock-out mouse, it was suggested that an inhibitor for ALK is useful as an anti-depression agent or as a preventive or therapeutic agent for cognitive function disorders (Non-Patent Document 10 and Patent Document 1).
- Therefore, a compound having an inhibitory activity on ALK will be very useful for the prevention and treatment of cancer, depression and cognitive function disorders, etc.
- Meanwhile, as an ALK inhibiting material, there are some compounds among multi-kinase inhibitors which have an inhibitory activity on ALK as one of their activities. For example, as an inhibitor for c-MET (mesenchymal-epithelial transition factor) and ALK, PF02341066 having a 2-aminopyridine structure was reported (Patent Document 2, Non-Patent Document Nos. 11 and 12). As an inhibitor for FAK, ZAP70, IGF-1R and ALK, etc., NVP-TAE684 having a 2,4-diaminopyrimidine structure was reported (Patent Document 3 and Non-Patent Document 13). In addition, 2,4-diaminopyrimidines and 2,4-diaminoquinazolines (Patent Document 4), pyridopyrazines (Patent Document 5), pyrazolo [3,4-C] isoquinolines (Patent Document 6), thiazoles (Patent Document 7), tricyclic compounds (Patent Document 8), and indazoles (Patent Document 9) and the like have been reported.
- However, the tetracyclic compounds of the present invention are not disclosed in any of the documents described above.
- As a tetracyclic compound exhibiting an anti-tumor activity, tetracyclic compounds comprising carbazole structure like ellipticine are known.
- However, their action mechanism is based on interaction with DNA to exhibit cell toxicity (Non-Patent Document 15), and there is no description at all regarding the activity of inhibiting ALK by the tetracyclic compounds.
-
- [Patent Document 1] WO 2007/023310 A2
- [Patent Document 2] WO 2006/021884 A2
- [Patent Document 3] WO 2004/080980 A1
- [Patent Document 4] WO 2009/008371 A1
- [Patent Document 5] WO 2007/130468 A2
- [Patent Document 6] WO 2005/009389 A2
- [Patent Document 7] WO 2005/097765 A1
- [Patent Document 8] WO 2008/021369 A2
- [Patent Document 9] WO 2009/013126 A1
- [Non-Patent Document 1] Proc Natl Acad Sci USA, Vol. 101, pages 13306-13311, 2004
- [Non-Patent Document 2] Nature, Vol. 448, pages 561-566, 2007
- [Non-Patent Document 3] Blood, Vol. 72, pages 234-240, 1988
- [Non-Patent Document 4] Cancer Res, Vol. 59, pages 2776-2780, 1999
- [Non-Patent Document 5] World J Gastroenterol, Vol. 12, pages 7104-7112, 2006
- [Non-Patent Document 6] Nature, Vol. 455, pages 930-935, 2008
- [Non-Patent Document 7] Nature, Vol. 455, pages 971-974, 2008
- [Non-Patent Document 8] J Biol Chem, Vol. 276, pages 16772-16779, 2001
- [Non-Patent Document 9] J Biol Chem, Vol. 277, pages 35990-35999, 2002
- [Non-Patent Document 10] Neuropsychopharmacology, Vol. 33, pages 685-700, 2008
- [Non-Patent Document 11] Proc Am Assoc Cancer Res (AACR) 2006, 47: Abst LB-271
- [Non-Patent Document 12] Proc Am Assoc Cancer Res (AACR) 2006, 47: Abst LB-273
- [Non-Patent Document 13] Proc Natl Acad Sci USA Vol. 104, pages 270-275, 2007
- [Non-Patent Document 14] Current Organic Chemistry, Vol. 5, Issue No. 5, pages 507-518, 2001
- [Non-Patent Document 15] Current Medicinal Chemistry: Anti-Cancer Agents, Vol. 4, Issue No. 2, pages 149-172, 2004
- The present invention is to provide ALK-inhibiting compounds having a novel structure. In addition, object of the present invention is to provide a pharmaceuticals for the prophylaxis or treatment comprising the ALK-inhibiting compounds that is effective for prophylaxising or treating a disease accompanied by abnormality in ALK, for example, cancer, cancer metastasis, depression and cognitive function disorder.
- As a result of extensive studies by the inventors of the present invention, it was found that the tetracyclic compounds that are represented by the following Formula (1) with a structure clearly different from any other existing pharmaceutical compounds have an excellent ALK-inhibiting activity, are useful for the treatment and prophylaxis of the diseases including cancer, cancer metastasis, depression and cognitive function disorder, and have a remarkable efficacy against said diseases. Accordingly, the present invention was completed.
- Thus, according to one aspect of the present invention, the tetracyclic compounds, a medicament and a pharmaceutical composition comprising the compounds, etc. shown below are provided.
- [1] A compound or salt or solvate thereof represented by Formula (I):
- [wherein,
- A1, A2, A3, A4, A7, A8, A9 and A10 all represent C, or any one of A2, A3, A4, A7, A8 and A9 represents N (with the proviso that, when it represents N, no substituent group exists therefor) and the remainings represent C;
- A5 is selected from NR5, O and S;
- R1 and R10 each independently represent [1] a hydrogen atom, [2] a cyano group,
- [3] a halogen atom or [4] a 4- to 10-membered heterocycloalkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s);
- R2 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group,
- (3) a C2-8 alkenyl group,
- (4) a C2-8 alkynyl group,
- (5) a cyano group,
- (6) a halogen atom,
- (7) a (C1-8 alkyl)m2-amino group which may be substituted by C1-8 alkylsulfonyl group(s),
-
- m2: 0˜2, and
- (8) a nitro group;
-
- R3 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by [1] halogen atom(s), [2]hydroxy group(s) or [3] C1-8 alkoxy group(s),
- (3) a C6-10 aryl group,
- (4) a cyano group,
- (5) a C1-8 alkanoyl group which may be substituted by C6-10 aryl group(s),
- (6) a (C1-8 alkyl)m3a-aminocarbonyl group which may be substituted by one or more R3A,
- R3A: [1] a C6-10 aryl group, [2] a C1-8 alkoxy group, [3] a 5- to 14-membered heteroaryl group, or [4] a C6-10 aryl sulfonyl group,
- m3a: 0˜2,
- (7) a hydroxycarbonyl group,
- (8) a C1-8 alkoxycarbonyl group which may be substituted by [1] hydroxy group(s) or [2] C1-8 alkoxy group(s),
- (9) a halogen atom,
- (10) a (C1-8 alkyl)m3b-amino group which may be substituted by C6-10 aryl group(s),
-
- m3b: 0˜2,
- (11) a C1-8 alkylcarbonyl (C0-8 alkyl) amino group which may be substituted by [1]C6-10 aryl group(s) or [2] C6-10 aryloxy group(s),
- (12) a C6-10 arylcarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
- (13) a (C1-8 alkyl)m3c-aminocarbonyl (C0-8 alkyl) amino group which may be substituted by C6-10 aryl group(s),
-
- m3c: 0˜2,
- (14) a nitro group,
- (15) a hydroxy group,
- (16) a C1-8 alkoxy group which may be substituted by one or more R3B
- R3B: [1] a hydroxy group, [2] a C1-8 alkoxy group, [3] a C6-10 aryl (C0-8 alkyl) aminocarbonyl group, [4] a (C1-8 alkyl)m3d-amino group, or [5] a halogen atom,
-
- m3d: 0˜2,
- (17) a 4- to 10-membered heterocycloalkyloxy group,
- (18) a 5- to 14-membered heteroaryloxy group,
- (19) a (C1-8 alkyl)m3e-aminocarbonyloxy group which may be substituted by C6-10 aryl group(s)
-
- m3e: 0˜2,
- (20) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group,
- (21) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s),
- (22) a C1-8 alkylthio group,
- (23) a C1-8 alkylsulfonyl group which may be substituted by C6-10 aryl group(s),
- (24) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s) which may be substituted by C1-8 alkoxy group(s),
- (25) a C1-8 alkoxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkoxy group(s),
- (26) a C6-10 aryloxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
- (27) a C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group which may be substituted by one or more R3C,
- R3C: [1] a C1-8 alkyl group which may be substituted by halogen atom(s), or [2] a C1-8 alkoxy group,
- (28) a C3-8 cycloalkyl (C0-8 alkyl) aminocarbonyloxy group, and
- (29) a C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkyl group and [2] a C1-8 alkoxy group;
- R4 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by halogen atom(s),
- (3) a C2-8 alkenyl group,
- (4) a C2-8 alkynyl group,
- (5) a C3-8 cycloalkyl group,
- (6) a cyano group,
- (7) an aminocarbonyl group,
- (8) a (C1-8 alkyl)m4a-aminocarbonyl group,
-
- m4a: 1˜2,
- (9) a hydroxycarbonyl group,
- (10) a C1-8 alkoxycarbonyl group,
- (11) a halogen atom,
- (12) a (C1-8 alkyl)m4b-amino group,
-
- m4b: 0˜2,
- (13) a hydroxy group, and
- (14) a C1-8 alkoxy group which may be substituted by hydroxy group(s);
- R5 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by one or more RA,
- R5A: [1] a hydroxycarbonyl group, [2] a C1-8 alkoxycarbonyl group, [3] a hydroxy group, [4] a C1-8 alkoxy group, [5] a (C1-8 alkyl)m5-amino group, [6] a C6-10 aryl group, or [7] a C1-8 alkylthio group,
-
- m5: 0˜2,
- (3) a C2-8 alkenyl group,
- (4) a C2-8 alkynyl group,
- (5) a C3-8 cycloalkyl group, and
- (6) a C1-8 alkylsulfonyl group;
- R6 and R6′ are each independently selected from the group consisting of:
- (1) a C1-8 alkyl group which may be substituted by halogen atom(s),
- (2) a C2-8 alkenyl group, and
- (3) a C2-8 alkynyl group; or
- R6 and R6′ are taken together with the carbon atoms to which they are bound to form:
- (4) a C3-8 cycloalkyl group, or
- (5) a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl C6-10 aryl sulfonyl group(s) which may be substituted by C1-8 alkyl group(s);
- R7 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a halogen atom,
- (3) a C1-8 alkoxy group which may be substituted by one or more R7A,
- R7A: [1] a (C1-8 alkyl)m7a-amino group, [2] a hydroxy, [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl group(s),
- m7a: 0˜2,
- (4) a C1-8 alkylsulfonyl group,
- (5) a nitro group, and
- (6) a hydroxyl group;
- R8 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by one or more R8A,
- R8A: [1] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8A1, [2] a (C1-8 alkyl)m8a-amino group which may be substituted by a halogen atom, and [3] a hydroxy group,
-
- m8a:0˜2,
- R8A1: [1] a C1-8 alkyl group, [2] a C1-8 alkylsulfonyl group, [3] a (C1-8 alkyl)m8b-aminosulfonyl group, [4] an oxo group, [5] a C1-8 alkoxycarbonyl, or [6] a C1-8 alkoxycarbonyl (C0-8 alkyl) aminosulfonyl,
- m8b: 0˜2,
- (3) a C2-8 alkenyl group,
- (4) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B
- R8B:
- <1> a C1-8 alkyl group which may be substituted by one or more R8B1,
<2> a C2-8 alkenyl group,
<3> a C2-8 alkynyl group,
<4> a C3-8 cycloalkyl group which may be substituted by [1] cyano group(s) or [2] C1-8 alkyl group(s),
<5> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B2
<6> a C1-8 alkoxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkoxy group and [2] a C3-8 cycloalkyl group,
<7> a C1-8 alkoxycarbonyl group,
<8> a C1-8 alkylsulfonyl group,
<9> a 5- to 14-membered heteroarylsulfonyl group,
<10> an oxo group,
<11> a cyano group,
<12> a C1-8 alkanoyl group which may be substituted by one or more R8B3
<13> a C3-8 cycloalkylcarbonyl group,
<14> a (C1-8 alkyl)m8c-aminosulfonyl group,
<15> a C1-8 alkylsulfonyl (C0-8 alkyl) amino group,
<16> a (C1-8 alkyl)m8a-amino group which may be substituted by one or more R8B4,
<17> a hydroxy group,
<18> a (C1-8 alkyl)m8e-aminocarbonyl group, or
<19> a C1-8 alkoxycarbonyl (C0-8 alkyl) amino group -
- m8c: 0˜2
- m8d: 0˜2
- m8e: 0˜2
- R8B1 [1] a C3-8 cycloalkyl group, [2] a hydroxy group, or [3] a C1-8 alkoxy group(s),
- R8B2: [1] a halogen atom, [2] a C1-8 alkyl group, [3] an oxo group, [4] a hydroxy group, or [5] a deuterium atom,
- R8B3: a (C1-8 alkyl)m8f-amino group,
-
- m8f: 0˜2,
- R8B4: [1] a C3-8 cycloalkyl group, or [2] a hydroxy group,
- (5) a 5- to 14-membered heteroaryl group which may be substituted by a C1-8 alkyl group,
- (6) a (C1-8 alkyl)m8g-aminocarbonyl group which may be substituted by one or more R8C,
-
- m8g: 0˜2,
- R8C:[1] a hydroxy group, [2] a (C1-8 alkyl)m8h-amino group which may be substituted by substituent(s) selected from the group consisting of <1> a (C1-8 alkyl)m8i-aminosulfonyl group, <2> a C1-8 alkylsulfonyl group, <3> a C1-8 alkoxycarbonyl group and
- <4> a C1-8 alkoxycarbonyl(C0-8 alkyl) aminosulfonyl group, [3] a C1-8 alkylsulfonyl group, or [4] a C1-8 alkoxy group which may be substituted by a hydroxy group,
-
- m8h: 0˜2,
- m8i: 0˜2,
- (7) a 4- to 10-membered heterocycloalkyl (C0-8 alkyl) aminocarbonyl group which may be substituted by oxo group(s),
- (8) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8D
- R8D: [1] a C1-8 alkyl group which may be substituted by one or more R8D1, [2] a hydroxy group, [3] a C1-8 alkylsulfonyl group, or [4] a C1-8 alkoxycarbonyl group,
- R8D1: [1] a hydroxy group, or [2] a C1-8 alkoxy group,
- (9) a hydroxycarbonyl group,
- (10) a C0-8 alkoxy (C0-8 alkyl) aminocarbonyl group which may be substituted by hydroxy group(s),
- (11) a halogen atom,
- (12) a (C1-8 alkyl)m8j-amino group which may be substituted by one or more R8H,
-
- m8j: 0˜2,
- R8H: [1] a hydroxy group, or [2] a 4- to 10-membered heterocycloalkyl group,
- (13) a hydroxyl group,
- (14) a C1-8 alkoxy group which may be substituted by one or more R8E,
- R8E:
- <1> a hydroxy group,
<2> halogen atom,
<3> a hydroxycarbonyl group,
<4> a C1-8 alkoxycarbonyl group,
<5> a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8E1
<6> a (C1-8 alkyl)m8k1-amino group which may be substituted by one or more R8E2 -
- m8k1: 0˜2,
<7> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8E3
<8> a 5- to 14-membered heteroaryl group,
<9> a (C1-8 alkyl)m8k2-aminocarbonyl group which may be substituted by one or more R8E6 - m8k2: 0˜2,
<10> a C1-8 alkoxy group which may be substituted by one or more R8E7,
<11> a C1-8 alkylthio group,
<12> a C1-8 alkylsulfinyl group,
<13> a C1-8 alkylsulfonyl group,
- m8k1: 0˜2,
- R8E1:
- <1> a C1-8 alkoxycarbonyl group,
<2> a C1-8 alkanoyl group,
<3> a C1-8 alkylsulfonyl group, 5<4> a (C1-8 alkyl)m8k3-aminosulfonyl group, -
- m8k3: 0˜2, or
<5> a 4- to 10-membered heterocycloalkyl group,
- m8k3: 0˜2, or
- R8E2:
- <1> a hydroxy group,
<2> a C1-8 alkoxycarbonyl group which may be substituted by halogen atom(s),
<3> a C3-8 cycloalkyl group which may be substituted by C1-8 alkyl group(s) which may be substituted by hydroxy group(s),
<4> a C1-8 alkanoyl group which may be substituted by substituent(s) selected from the group consisting of [1] a (C1-8 alkyl)m8k4-amino group and [2] a halogen atom(s), -
- m8k4: 0˜2,
<5> a (C1-8 alkyl)m8k5-aminocarbonyl group, - m8k5: 0˜2,
<6> a C1-8 alkylsulfonyl group,
<7> a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s),
<8> a (C1-8 alkyl)m8k6-aminosulfonyl group which may be substituted by C1-8 alkoxycarbonyl group(s), - m8k6: 0˜2, or
- m8k4: 0˜2,
- R8E3:
- <1> a C1-8 alkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a hydroxy group and [2] a C1-8 alkylcarbonyloxy group,
<2> a C1-8 alkylcarbonyloxy group,
<3> a hydroxy group,
<4> a C3-8 cycloalkyl group,
<5> a C1-8 alkoxy group,
<6> a C1-8 alkoxycarbonyl group,
<7> a C1-8 alkylsulfonyl group,
<8> a (C1-8 alkyl)m8k8-aminocarbonyl group -
- m8k8: 0˜2,
<9> a C1-8 alkanoyl group which may be substituted by hydroxy group(s),
<10> an oxo group, or
<11> a 4- to 10-membered heterocycloalkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkanoyl group, [2] a C1-8 alkoxycarbonyl group and [3] a C1-8 alkylsulfonyl group,
- m8k8: 0˜2,
- R8E6:
- <1> a C2-8 alkenylcarbonyloxy group,
<2> a hydroxy group,
<3> a cyano group,
<4> a (C1-8 alkyl)m8k9-amino group which may be substituted by hydroxy group(s) -
- m8k9: 0˜2,
<5> a C1-8 alkoxy group which may be substituted by hydroxy group(s),
<6> a C1-8 alkylcarbonyloxy group,
<7> a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl group(s), or
<8> a 5- to 14-membered heteroaryl group,
- m8k9: 0˜2,
- R8E7:
- <1> a hydroxy group, or
<2> a C1-8 alkoxy group which may be substituted by hydroxy group(s), - (15) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by one or more R8F
- R8F:
- <1> a C1-8 alkyl group which may be substituted by one or more R8F1
<2> a C3-8 cycloalkyl group,
<3> a C1-8 alkanoyl group which may be substituted by halogen atom(s),
<4> a C1-8 alkylcarbonyloxy group,
<5> a C1-8 alkoxycarbonyl group,
<6> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8F2
<7> a C1-8 alkyl sulfonyl group,
<8> a hydroxy group, or
[9] a C6-10 aryl group, - R8F1: [1] a hydroxy group, [2] a C1-8 alkoxy group, or [3] a halogen atom,
- R8F2: [1] a 4- to 10-membered heterocycloalkyl group, [2] a C1-8 alkoxycarbonyl group, or [3] a C1-8 alkylsulfonyl group,
- (16) a 5- to 14-membered heteroaryloxy group,
- (17) a 4- to 10-membered heterocycloalkylcarbonyloxy group,
- (18) a (C1-8 alkyl)m8l1-aminosulfonyloxy group,
-
- m8l1: 0˜2,
- (19) a C1-8 alkyl thio group which may be substituted by [1] (C1-8 alkyl)m8l2-amino group(s), [2] hydroxy group(s) or [3] hydroxycarbonyl group(s),
-
- m8l2: 0˜2,
- (20) a C1-8 alkylsulfonyl group which may be substituted by one or more R8G,
- R8G: [1] a hydroxycarbonyl group, [2] a hydroxy group, or [3] a (C1-8 alkyl)m8l3-amino group,
-
- m8l3: 0˜2,
- (21) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group which may be substituted by C1-8 alkyl group(s),
- (22) a C2-8 alkenyloxy group, and
- (23) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s);
- R9 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by one or more R9A,
- R9A: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9A, [3] a hydroxy group, [4] a C1-8 alkoxy group, or [5] a hydroxycarbonyl group,
- R9A1: [1] a C1-8 alkyl group, [2] a C3-8 cycloalkyl group, or [3] a 4- to 10-membered heterocycloalkyl group,
- (3) a C2-8 alkenyl group which may be substituted by one or more R9B,
- R9B: [1] a (C1-8 alkyl)m9a-amino group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more group R9B1
- R9B1:[1] a C3-8 cycloalkyl group, or [2] a 4- to 10-membered heterocycloalkyl group,
-
- m9a: 0˜2,
- (4) a C2-8 alkynyl group which may be substituted by one or more R9C,
- R9C: [1] a C1-8 alkoxy group, [2] a (C1-8 alkyl)m9b-amino group which may be substituted by C6-10 aryl group(s), [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9C1, [4] a C3-8 cycloalkyl group, [5] a hydroxy group,
- [6] a hydroxycarbonyl group, or [7] a C1-8 alkyloxycarbonyl group,
-
- m9b: 0˜2,
- R9C1: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group, or [3] an oxo group,
- (5) a C3-8 cycloalkyl group,
- (6) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9D
- R9D: [1] a C1-8 alkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s), [2] a C3-8 cycloalkyl group, [3] a 4- to 10-membered heterocycloalkyl group, or [4] a C1-6 alkylsulfonyl group, or [5] a C1-8 alkoxycarbonyl group,
- (7) a C6-10 aryl group which may be substituted by one or more R9E
- R9E: [1] a halogen atom, [2] a hydroxy group, [3] a hydroxycarbonyl group, or [4] a C1-8 alkyl group which may be substituted by hydroxy group(s), or [5] a C1-8 alkoxy group,
- (8) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s),
- (9) a cyano group,
- (10) a C1-8 alkanoyl group,
- (11) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by C1-8 alkyl group(s),
- (12) a halogen atom,
- (13) a (C1-8 alkyl)m9c-amino group which may be substituted by one or more R9F
-
- m9c: 0˜2,
- (14) a C1-8 alkylcarbonyl(C0-8 alkyl)amino group which may be substituted by (C1-s alkyl)m9a-amino group(s),
-
- m9d: 0˜2,
- (15) a C1-8 alkylsulfonyl(C0-8 alkyl)amino group,
- (16) a (C1-8 alkyl)m9e-aminosulfonyl(C0-8 alkyl)amino group,
-
- m9e: 0˜2,
- (17) a nitro group,
- (18) a hydroxy group,
- (19) a C1-8 alkoxy group which may be substituted by one or more R9G
- R9G: [1] a hydroxy group, [2] a hydroxycarbonyl group, [3] a C6-10 aryl group which may be substituted by C1-8 alkoxy group(s), [4] a (C1-8 alkyl)m9g1-amino group, [5] a C1-8 alkoxy group which may be substituted by one or more R9G1, [6] a 5- to 14-membered heteroaryl group, or [7] a 4- to 10-membered heterocycloalkyloxy group which may be substituted by C1-8 alkyl group(s),
- m9g1: 0˜2,
- R9G1: [1] a C1-8 alkoxy group, or [2] a hydroxycarbonyl group,
- (20) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by
- [1] 4- to 10-membered heterocycloalkyl group(s), or [2] C1-8 alkoxycarbonyl group(s),
- (21) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s),
- (22) a C1-8 alkylthio group which may be substituted by (C1-8 alkyl)m9f-amino group(s),
-
- m9f: 0˜2,
- (23) a C1-8 alkylsulfonyl group which may be substituted by (C1-8 alkyl)m9g-amino group(s),
-
- m9g: 0˜2,
- (24) a (C1-8 alkyl)m9h-aminosulfonyl group,
-
- m9h: 0˜2,
- (25) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s), and (26) a hydroxycarbonyl group].
- [2] The compound according to the above [1], or a salt or solvate thereof, wherein R3 is a cyano group or a halogen atom.
[3] The compound according to the above [1], or a salt or solvate thereof, wherein A5 is NR5 and R5 is a hydrogen atom.
[4] The compound according to the above [1], or a salt or solvate thereof, wherein all of the A1, A2, A3, A4, A7, A8, A9 and A10 are a carbon atom.
[5] The compound according to claim 1, or a salt or solvate thereof, wherein: A1, A2, A3, A4, A7, A8, A9 and A10 all represent C, or any one of A2, A3, A4, A7, A8 and A9 represents N (with the proviso that, when it represents N, no substituent group exists therefor) and the remainings represent C; - A5 is selected from NR5, O and S;
- R1 represents [1] a hydrogen atom, [2] a cyano group, or [3] a halogen atom;
- R2 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group,
- (3) a cyano group,
- (4) a halogen atom, and
- (5) a (C1-8 alkyl)m2-amino group which may be substituted by C1-8 alkylsulfonyl group(s),
-
- m2: 0˜2;
- R3 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by halogen atom(s),
- (3) a cyano group,
- (4) a (C1-8 alkyl)m3a-aminocarbonyl group which may be substituted by one or more R3A,
- R3A: [1] a C6-10 aryl group, [2] a C1-8 alkoxy group, [3] a 5- to 14-membered heteroaryl group, or [4] a C6-10 aryl sulfonyl group,
-
- m3a: 0˜2,
- (5) a hydroxycarbonyl group,
- (6) a C1-8 alkoxycarbonyl group which may be substituted by hydroxy group(s),
- (7) a halogen atom,
- (8) a (C1-8 alkyl)m3b-amino group which may be substituted by C6-10 aryl group(s),
-
- m3b: 0˜2,
- (9) a C1-8 alkylcarbonyl (C0-8 alkyl) amino group which may be substituted by [1]C6-10 aryl group(s) or [2] C6-10 aryloxy group(s),
- (10) a C6-10 arylcarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
- (11) a nitro group,
- (12) a hydroxy group,
- (13) a C1-8 alkoxy group which may be substituted by one or more R3B
- R3B: [1] a hydroxy group, [2] a C1-8 alkoxy group, [3] a C6-10 aryl (C0-8 alkyl) aminocarbonyl group, [4] a (C1-8 alkyl)m3a-amino group, or [5] a halogen atom,
-
- m3d: 0˜2,
- (14) a 4- to 10-membered heterocycloalkyloxy group,
- (15) a 5- to 14-membered heteroaryloxy group,
- (16) a (C1-8 alkyl)m3e-aminocarbonyloxy group which may be substituted by C6-10 aryl group(s),
-
- m3e: 0˜2,
- (17) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group,
- (18) a C1-8 alkylthio group,
- (19) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s) which may be substituted by C1-8 alkoxy group(s),
- (20) a C1-8 alkoxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkoxy group(s),
- (21) a C6-10 aryloxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
- (22) a C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkoxy group(s),
- (23) a C3-8 cycloalkyl (C0-8 alkyl) aminocarbonyloxy group, and
- (24) a C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkyl group and [2] a C1-8 alkoxy group;
- R4 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by halogen atom(s),
- (3) a C3-8 cycloalkyl group,
- (4) a cyano group,
- (5) an aminocarbonyl group,
- (6) a hydroxycarbonyl group,
- (7) a halogen atom,
- (8) a (C1-8 alkyl)m4b-amino group,
-
- m4b: 0˜2,
- (9) a hydroxy group, and
- (10) a C1-8 alkoxy group which may be substituted by hydroxy group(s);
- R5 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by one or more R5A,
- R5A: [1] a hydroxycarbonyl group, [2] a C1-8 alkoxycarbonyl group, [3] a hydroxy group, [4] a C1-8 alkoxy group, [5] a (C1-8 alkyl)m5-amino group, or [6], a C1-8 alkylthio group,
-
- m5: 0˜2, and
- (3) a C1-8 alkylsulfonyl group;
- R6 and R6′ are each independently:
- (1) a C1-8 alkyl group, or
- R6 and R6′ are taken together with the carbon atoms to which they are bound to form,
- (2) a C3-8 cycloalkyl group, or
- (3) a 4- to 10-membered heterocycloalkyl group;
- R7 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a halogen atom, and
- (3) a C1-8 alkoxy group which may be substituted by one or more R7A,
- R7A: [1] a (C1-8 alkyl)m7a-amino group, or [2] a hydroxy group,
-
- m7a:0-2;
- R8 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by one or more R8A, R5A: [1] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8A1, [2] a (C1-8 alkyl)m8a-amino group which may be substituted by a halogen atom, and [3] a hydroxy group,
-
- m8a:0˜2,
- R8A1: [1] a C1-8 alkyl group, [2] a C1-8 alkylsulfonyl group, [3] a (C1-8 alkyl)m8b-aminosulfonyl group, or [4] an oxo group,
-
- m8b: 0˜2,
- (3) a C2-8 alkenyl group,
- (4) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B,
- R8B:
- <1> a C1-8 alkyl group which may be substituted by one or more R8B1
<2> a C2-8 alkynyl group,
<3> a C3-8 cycloalkyl group which may be substituted by [1] cyano group(s) or [2] C1-8 alkyl group(s),
<4> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B2
<5> a C1-8 alkoxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkoxy group and [2] a C3-8 cycloalkyl group,
<6> a C1-8 alkylsulfonyl group,
<7> an oxo group,
<8> a cyano group,
<9> a C1-8 alkanoyl group which may be substituted by one or more R8B3
<10> a C3-8 cycloalkylcarbonyl group,
<11> a (C1-8 alkyl)m8c-aminosulfonyl group,
<12> a C1-8 alkylsulfonyl (C0-8 alkyl) amino group,
<13> a (C1-8 alkyl)m8d-amino group which may be substituted by one or more R8B4,
<14> a hydroxy group, or
<15> a (C1-8 alkyl)m8e-aminocarbonyl group, -
- m8c: 0˜2,
- m8d: 0˜2,
- m8e: 0˜2,
- R8B1: [1] a C3-8 cycloalkyl group, [2] a hydroxy group, or [3] C1-8 alkoxy group which may be substituted by C1-8 alkoxy group(s),
- R8B2: [1] a halogen atom, [2] a C1-8 alkyl group, [3] an oxo group, or [4] a hydroxy group,
- R8B3: a (C1-8 alkyl)m8f-amino group,
-
- m8f: 0˜2,
- R8B4: [1] a C3-8 cycloalkyl group, or [2] a hydroxy group,
- (5) a 5- to 14-membered heteroaryl group which may be substituted by a C1-8 alkyl group,
- (6) a (C1-8 alkyl)m8g-aminocarbonyl group which may be substituted by one or more R8C,
-
- m8g: 0˜2,
- R8C:[1] a hydroxy group, [2] a (C1-8 alkyl)m8h-amino group which may be substituted by substituent(s) selected from the group consisting of <1> a (C1-8 alkyl)m8i-aminosulfonyl group and <2> a C1-8 alkylsulfonyl group, or [3] a C1-8 alkylsulfonyl group,
-
- m8h: 0˜2,
- m8i: 0˜2,
- (7) a 4- to 10-membered heterocycloalkyl (C0-8 alkyl) aminocarbonyl group which may be substituted by oxo group(s),
- (8) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8D,
- R8D: [1] a C1-8 alkyl group which may be substituted by one or more R8D1, [2] a hydroxy group, or [3] a C1-8 alkylsulfonyl group,
- R8D1: [1] a hydroxy group, or [2] a C1-8 alkoxy group,
- (9) a hydroxycarbonyl group,
- (10) a C0-8 alkoxy (C0-8 alkyl) aminocarbonyl group which may be substituted by hydroxy group(s),
- (11) a halogen atom,
- (12) a (C1-8 alkyl)m8j-amino group which may be substituted by 4- to 10-membered heterocycloalkyl group(s),
-
- m8j: 0˜2,
- (13) a hydroxyl group,
- (14) a C1-8 alkoxy group which may be substituted by one or more R8E, R8E:
- <1> a hydroxy group,
<2> a C1-8 alkoxycarbonyl group,
<3> a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8E1
<4> a (C1-8 alkyl)m8k1-amino group which may be substituted by one or more R8E2 -
- m8k1: 0˜2,
<5> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8E3
<6> a 5- to 14-membered heteroaryl group,
<7> a (C1-8 alkyl)m8k2-aminocarbonyl group which may be substituted by one or more R8E6 - m8k2: 0˜2,
<8> a C1-8 alkoxy group which may be substituted by one or more R8E7,
<9> a C1-8 alkylthio group,
<10> a C1-8 alkylsulfinyl group, or
<11> a C1-8 alkylsulfonyl group,
- m8k1: 0˜2,
- R8E1:
- <1> a C1-8 alkoxycarbonyl group,
<2> a C1-8 alkanoyl group,
<3> a C1-8 alkylsulfonyl group,
<4> a (C1-8 alkyl)mk3-aminosulfonyl group -
- m8k3: 0˜2, or
<5> a 4- to 10-membered heterocycloalkyl group,
- m8k3: 0˜2, or
- R8E2:
- <1> a hydroxy group,
<2> a C1-8 alkoxycarbonyl group,
<3> a C3-8 cycloalkyl group which may be substituted by C1-8 alkyl group(s) which may be substituted by hydroxy group(s),
<4> a C1-8 alkanoyl group which may be substituted by substituent(s) selected from the group consisting of [1] a (C1-8 alkyl)mk4-amino group and [2] a halogen atom, -
- m8k4: 0˜2,
<5> a (C1-8 alkyl)m8k5-aminocarbonyl group, - m8k5: 0˜2,
<6> a C1-8 alkylsulfonyl group,
<7> a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s),
<8> a (C1-8 alkyl)m8k6-aminosulfonyl group, - m8k6: 0˜2, or
- m8k4: 0˜2,
- R8E3:
- <1> a C1-8 alkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a hydroxy group and [2] a C1-8 alkylcarbonyloxy group,
<2> a hydroxy group,
<3> a C3-8 cycloalkyl group,
<4> a C1-8 alkylsulfonyl group,
<5> a (C1-8 alkyl)m8k8-aminocarbonyl group, -
- m8k8: 0˜2,
<6> a C1-8 alkanoyl group which may be substituted by hydroxy group(s),
<7> an oxo group, or
<8> a 4- to 10-membered heterocycloalkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkanoyl group, and [2] a C1-8 alkylsulfonyl group,
- m8k8: 0˜2,
- R8E6:
- <1> a C2-8 alkenylcarbonyloxy group,
<2> a hydroxy group,
<3> a cyano group,
<4> a (C1-8 alkyl)m8k9-amino group which may be substituted by hydroxy group(s), -
- m8k9: 0˜2,
<5> a C1-8 alkoxy group which may be substituted by hydroxy group(s),
<6> a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl group(s), or
<7> a 5- to 14-membered heteroaryl group,
- m8k9: 0˜2,
- R8E7:
- <1> a hydroxy group, or
<2> a C1-8 alkoxy group which may be substituted by hydroxy group(s), - (15) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by one or more R8F:
- R8F:
- <1> a C1-8 alkyl group which may be substituted by one or more R8F1
<2> a C3-8 cycloalkyl group,
<3> a C1-8 alkanoyl group which may be substituted by halogen atom(s),
<4> a C1-8 alkoxycarbonyl group,
<5> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8F2
<6> a C1-8 alkyl sulfonyl group, or
<7> a hydroxy group, - R8F1: [1] a hydroxy group, [2] a C1-8 alkoxy group, or [3] a halogen atom,
- R8F2: [1] a 4- to 10-membered heterocycloalkyl group, [2] a C1-8 alkoxycarbonyl group, or [3] a C1-8 alkylsulfonyl group,
- (16) a 5- to 14-membered heteroaryloxy group,
- (17) a (C1-8 alkyl)m8l1-aminosulfonyloxy group,
-
- m8l1: 0˜2,
- (18) a C1-8 alkylthio group which may be substituted by (C1-8 alkyl)m8l2-amino group(s),
-
- m8l2: 0˜2,
- (19) a C1-8 alkylsulfonyl group which may be substituted by one or more R8G,
- R8G: [1] a hydroxycarbonyl group, [2] a hydroxy group, or [3] a (C1-8 alkyl)m8l3-amino group,
-
- m8l3: 0˜2,
- (20) a C2-8 alkenyloxy group, and
- (21) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s);
- R9 is selected from the group consisting of:
- (1) a hydrogen atom,
- (2) a C1-8 alkyl group which may be substituted by one or more R9A,
- R9A: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9A, [3] a hydroxy group, or [4] a C1-8 alkoxy group,
- R9A1: [1] a C1-8 alkyl group, [2] a C3-8 cycloalkyl group, or [3] a 4- to 10-membered heterocycloalkyl group,
- (3) a C2-8 alkenyl group,
- (4) a C2-8 alkynyl group which may be substituted by one or more R9C,
- R9C: [1] a C1-8 alkoxy group, [2] a (C1-8 alkyl)m9b-amino group which may be substituted by C6-10 aryl group(s), [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9C1, [4] a C3-8 cycloalkyl group, [5] a hydroxy group, or [6] a hydroxycarbonyl group,
-
- m9b: 0˜2,
- R9C1: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group, or [3] an oxo group,
- (5) a C3-8 cycloalkyl group,
- (6) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9D
- R9D: [1] a C1-8 alkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s), [2] a C3-8 cycloalkyl group, [3] a 4- to 10-membered heterocycloalkyl group, or [4] a C1-6 alkylsulfonyl group,
- (7) a C6-10 aryl group which may be substituted by one or more R9E
- R9E: [1] a halogen atom, [2] a hydroxy group, [3] a hydroxycarbonyl group, or [4] a C1-8 alkyl group which may be substituted by hydroxy group(s),
- (8) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s),
- (9) a cyano group,
- (10) a C1-8 alkanoyl group,
- (11) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by C1-8 alkyl group(s),
- (12) a halogen atom,
- (13) a (C1-8 alkyl)m9c-amino group,
-
- m9c: 0˜2,
- (14) a C1-8 alkylcarbonyl(C0-8 alkyl)amino group which may be substituted by (C1-8 alkyl)m9d-amino group(s),
-
- m9d: 0˜2,
- (15) a C1-8 alkylsulfonyl(C0-8 alkyl)amino group,
- (16) a (C1-8 alkyl)m9e-aminosulfonyl(C0-8 alkyl)amino group,
-
- m9e: 0˜2,
- (17) a nitro group,
- (18) a hydroxy group,
- (19) a C1-8 alkoxy group which may be substituted by one or more R9G
- R9G: [1] a hydroxy group, [2] a hydroxycarbonyl group, [3] a C6-10 aryl group which may be substituted by C1-8 alkoxy group(s), [4] a (C1-8 alkyl)m9g1-amino group, [5] a C1-8 alkoxy group which may be substituted by one or more R9G1, or [6] a 5- to 14-membered heteroaryl group,
-
- m9g1: 0˜2,
- R9G1: [1] a C1-8 alkoxy group, or [2] a hydroxycarbonyl group,
- (20) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by 4- to 10-membered heterocycloalkyl group(s),
- (21) a C1-8 alkylthio group which may be substituted by (C1-8 alkyl)m9f-amino group(s),
-
- m9f: 0˜2,
- (22) a C1-8 alkylsulfonyl group which may be substituted by (C1-8 alkyl)m9g-amino group(s),
-
- m9g: 0˜2,
- (23) a (C1-8 alkyl)m9h-aminosulfonyl group,
-
- m9h: 0˜2, and
- (24) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s);
- R10 represents [1] a hydrogen atom, or [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s)].
- [6] A compound according to claim 1, or salt or solvate thereof, which said compound is selected from the group consisting of:
- 9-(4-isopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-9-prop-1-ynyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-cyclopropylethynyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 6,6-dimethyl-8-(1-oxetan-3-yl-piperidin-4-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-bromo-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-bromo-8-(4-cyclopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-chloro-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-9-prop-1-ynyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 6,6,9-trimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-ethyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-ethynyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 8-(4-cyclobutyl-piperazin-1-yl)-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-ethynyl-6,6-dimethyl-11-oxo-8-(4-pyrrolidin-1-yl-piperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 6,6-dimethyl-11-oxo-8-(4-pyrrolidin-1-yl-piperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 8-(4-cyclobutyl-piperazin-1-yl)-9-ethynyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-9-propyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 8-(1-isopropyl-piperidin-4-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 8-(4-isopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 8-(4-cyclobutyl-piperazin-1-yl)-9-cyclopropyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 8-(2-tert-butylamino-ethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-ethynyl-8-(4-methanesulfonyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 9-bromo-8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
- 6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-9-propyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; and
- 9-ethynyl-6,6-dimethyl-8-morpholin-4-yl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
[7] A medicament comprising as an active ingredient the compound according to any one of the above [1] to [5], or a salt or solvate thereof.
[8] An ALK inhibitor comprising as an active ingredient the compound according to any one of the above [1] to [5], or a salt or solvate thereof.
[9] A pharmaceutical for the prophylaxis or treatment of cancer, cancer metastasis, depression or cognitive function disorder, comprising as an active ingredient the compound according to any one of the above [1] to [5], or a salt or solvate thereof.
[10] A pharmaceutical composition comprising the compound according to any one of the above [1] to [5], or a salt or solvate thereof and a pharmaceutically acceptable carrier(s).
[11] A method of treating a patient suffering from the disease including cancer, cancer metastasis, depression or cognitive function disorder, comprising administering to the patient who is in need of the treatment of the disease the compound described in any one of the above [1] to [5], salt or solvate thereof in an effective amount for the treatment of the disease.
[12] Use of the compound described in any one of the above [1] to [5], salt or solvate thereof in the manufacture of a pharmaceutical.
[13] The use according to above [11] in the manufacture of a pharmaceutical composition for the treatment or prophylaxis of the disease of mammals including human, wherein the disease is related with ALK activity. - The compounds of the present invention or salts or salvates thereof have an excellent activity of inhibiting ALK, excellent stability in organisms, and excellent solubility in water, and therefore are useful as a prophylactic or therapeutic agent for proliferative disorders (in particular, therapeutic agent). Further, the compounds of the present invention or salts salts or solvates thereof are useful as a prophylactic or therapeutic agent (in particular, therapeutic agent) for various diseases such as cancers including leukemia (acute myelogenous leukemia, chronic myelogenous leukemia, acute lymphatic leukemia, chronic lymphatic leukemia and the like), malignant lymphoma (Hodgkin lymphoma, non-Hodgkin lymphoma and the like), brain tumor, neuroblastoma, gliomatosis, thyroid cancer, myelodysplastic syndrome, head and neck cancer, esophageal cancer, stomach cancer, colon cancer, colorectal cancer, breast cancer, ovarian cancer, lung cancer, pancreatic cancer, liver cancer, gall bladder cancer, skin cancer, malignant melanoma, kidney cancer, renal pelvis-ureter cancer, bladder cancer, uterine cancer, testicle cancer, prostate cancer, and the like. Further, the compounds of the present invention are useful as a prophylactic or therapeutic agent (in particular, therapeutic agent) for infiltration/metastasis of solid tumors. Still further, the compounds of the present invention are useful as a prophylactic or therapeutic agent for other diseases that are related with ALK, for example, depression or a cognitive function disorder.
- The method of the present invention comprises a step of administering a pharmaceutically effective amount of the pharmaceutical composition comprising the compounds of the present invention or salts or solvates thereof to a patient who is in need of such treatment or suffers from such diseases or conditions.
- Hereinbelow, the compounds of the present invention, the method of preparing the same, and the pharmaceutical agent comprising the same will be explained.
- According to the present invention, the “halogen atom” means a fluorine atom, a chlorine atom, a bromine atom, an iodine atom and the like. According to the present invention, when the halogen atom is a substituent group for an aromatic carbon ring, an aromatic heterocycle and the like, the preferred halogen atom includes a fluorine atom, a chlorine atom and a bromine atom. According to the present invention, when the halogen atom is a substituent group for an alkyl group or a group which comprises the alkyl as at least a part of the group (e.g., alkoxy, alkenyl, unsaturated carbocycle, unsaturated heterocycle and the like), the preferred halogen atom includes a fluorine atom. Specifically, examples thereof include a trifluoromethyl group, a pentafluoroethyl group, a heptafluoropropyl group, a nonafluorobutyl group, a trifluoromethoxy group, a pentafluoroethoxy group, a heptafluoropropoxy group, a nonafluorobutoxy group, a trifluoroacetyl group, a pentafluoropropionyl group, a heptafluorobutyryl group and a nonafluoropentanoyl group.
- The “C1-8 alkyl group” means a monovalent group which is derived by removing any one of hydrogen atoms from a linear or branched aliphatic hydrocarbon having 1 to 8 carbon atoms. Specifically, examples thereof include a methyl group, an ethyl group, an isopropyl group, a butyl group, a n-butyl group, an isobutyl group, a sec-butyl group, a t-butyl group, a pentyl group, an isopentyl group, a 2,3-dimethyl propyl group, a hexyl group, a 2,3-dimethyl hexyl group, a 1,1-dimethyl pentyl group, a heptyl group and an octyl group. Preferably, it is a C1-6 alkyl group, more preferably a C1-5 alkyl group, still more preferably a C1-4 alkyl group, and still even more preferably a C1-3 alkyl group.
- The “C1-8 alkyl group which may be substituted” means an unsubstituted C1-8 alkyl group or a C1-8 alkyl group of which at least one hydrogen atom on the alkyl group is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the alkyl group may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C1-8 alkyl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) for C1-6 alkyl group and a C1-4 alkyl group, and 1 to 2 substituent(s) for a C1-3 alkyl group.
- The “C2-8 alkenyl group” means a monovalent group wherein at least one double bond (two adjacent SP2 carbon atoms) is comprised in a linear or branched aliphatic hydrocarbon group having 1 to 8 carbon atoms. Specific examples of the C2-8 alkenyl group include a vinyl group, an allyl group, a 1-propenyl group, a 2-propenyl group, a 1-butenyl group, a 2-butenyl group (including both cis and trans), a 3-butenyl group, a pentenyl group and a hexenyl group. Preferably, it is a C2-6 alkenyl group, more preferably a C2-5 alkenyl group, still more preferably a C2-4 alkenyl group, and still even more preferably a C2-3 alkenyl group.
- The “C2-8 alkenyl group which may be substituted” means the unsubstituted C2-8 alkenyl group described above or a C2-8 alkenyl group of which at least one hydrogen atom on the alkenyl group is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the single-bonded carbon atom may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C2-8 alkenyl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) for a C2-6 alkenyl group and a C2-4 alkenyl group, 1 to 2 substituent(s) for a C2-3 alkenyl group.
- The “C2-8 alkynyl group” means a monovalent group wherein at least one triple bond (two adjacent SP carbon atoms) is comprised in a linear or branched aliphatic hydrocarbon group having 1 to 8 carbon atoms. Specific examples of the C2-8 alkynyl group include an ethynyl group, a 1-propynyl group, a propargyl group and a 3-butynyl group. Preferably, it is a C2-6 alkynyl group, more preferably a C2-5 alkynyl group, still more preferably a C2-4 alkynyl group, and still even more preferably a C2-3 alkynyl group.
- The “C2-8 alkynyl group which may be substituted” means the unsubstituted C2-8 alkynyl group described above or a C2-8 alkynyl group of which at least one hydrogen atom on the alkynyl group is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the single-bonded carbon atom may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C2-8 alkynyl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) for a C2-6 alkynyl group and a C2-4 alkynyl group, and 1 to 2 substituent(s) for C2-3 alkynyl group.
- The “C3-8 cycloalkyl group” means an aliphatic hydrocarbon group in cyclic form. Preferably, it includes a C3-6 cycloalkyl group. Specifically, examples thereof include a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, a cyclohexyl group, a cycloheptyl group and a cyclooctyl group. Preferably, it is a C3-6 cycloalkyl group.
- The “C3-8 cycloalkyl group which may be substituted” means the unsubstituted C3-8 cycloalkyl group described above or a C3-8 cycloalkyl group of which at least one hydrogen atom is substituted by a defined substituent group(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the single-bonded carbon atom may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C3-8 cycloalkyl group which may be substituted by 1 to 3 substituent(s).
- The “4- to 10-membered heterocycloalkyl group” means a saturated or partially unsaturated heterocyclic group which consists of 4 to 10 ring-constituting atoms and comprises 1 to 3 hetero atoms that are selected from O, S and N. The heterocycloalkyl group can be a monocyclic, a bicyclic or a spirocyclic type heterocycloalkyl group. Specifically, examples thereof include an oxetanyl group, a tetrahydrofuryl group, a tetrahydrothienyl group, a tetrahydropyranyl group, a pyrrolidino group, a pyrrolidinyl group, a piperidino group, a piperidinyl group, a piperazino group, a piperazinyl group, a morpholino group, a morpholinyl group, a tetrahydrothiopyranyl group, a thiomorpholino group, an imidazolidinyl group, a 1,3-dioxolanyl group, a tetrahydropyranyl group, a 1,3-dioxanyl group, a 1,2,3,6-tetrahydropyridinyl group, a 1,4-Dioxa-8-aza-spiro[4.5]decanyl group, and a 1-oxa-8-aza-spiro[4.5]decanyl group. Preferably, it is a 4- to 8-membered heterocycloalkyl group, more preferably, 4- to 6-membered heterocycloalkyl group.
- The “4- to 10-membered heterocycloalkyl group which may be substituted” means the unsubstituted 4- to 10-membered heterocycloalkyl group described above or a 4- to 10-membered heterocycloalkyl group of which at least one hydrogen atom on the heterocycloalkyl group is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the alkyl group may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a 4-to 10-membered heterocycloalkyl group which may be substituted by 1 to 4 substituent(s). More preferably, it is 1 to 4 substituent(s) for a 4- to 8-membered heterocycloalkyl group, and 1 to 3 substituent(s) for a 4- to 6-membered heterocycloalkyl group. When the substituent is an oxo group, 2 oxo group can combine with the same sulfur atom. When the salt is formed, 2 alkyl group can combine with the same nitrogen atom.
- The “C6-10 aryl group” means a monovalent aromatic hydrocarbon ring. Specific examples of the C6-10 aryl group include a phenyl group, a 1-naphthyl group and a 2-naphthyl group. Preferably, it is a C6 aryl group or a C10 aryl group.
- The “C6-10 aryl group which may be substituted” means the unsubstituted C6-10 aryl group described above or a C6-10 aryl group of which at least one hydrogen atom is substituted by a defined substituent group(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C6-10 aryl group which may be substituted by 1 to 3 substituent(s).
- The “5- to 14-membered heteroaryl group” means an aromatic cyclic group comprising one or more hetero atoms among 5 to 14 ring-constituting atoms. The cycle can be a monocyclic or bicyclic heteroaryl group fused to a benzene ring or a monocyclic heteroaryl ring. Specific examples thereof include a furyl group, a thienyl group, a pyrrolyl group, an imidazolyl group, a pyrazolyl group, a thiazolyl group, an isothiazolyl group, an oxazolyl group, an isooxazolyl group, an oxadiazolyl group, a thiadiazolyl group, a triazolyl group, a tetrazolyl group, a pyridyl group, a pyrimidyl group, a pyridazinyl group, a pyrazinyl group, a triazinyl group, a benzofuranyl group, a benzothienyl group, a benzothiadiazolyl group, a benzothiazolyl group, a benzoxazolyl group, a benzoxadiazolyl group, a benzoimidazolyl group, an indolyl group, an isoindolyl group, an indazolyl group, a quinolyl group, an isoquinolyl group, a cinnolinyl group, a quinazolinyl group, a quinoxalinyl group, a benzodioxolyl group, an indolizinyl group, an imidazopyridyl group and the like. Preferably, it is a 5- to 6-membered heteroaryl group.
- The “5- to 14-membered heteroaryl group which may be substituted” means the unsubstituted 5- to 14-membered ring heteroaryl group described above or a 5- to 14-membered ring heteroaryl group of which at least one hydrogen atom on the heteroaryl group is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a 5- to 14-membered heteroaryl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) or 1 to 2 substituent(s) for a 5- to 6-membered heteroaryl group.
- The “C1-8 alkanoyl group” means a C1-8 alkyl-C(O)— group, and the C1-8 alkyl group is described above. Specifically, examples thereof include acetyl, propionyl, butyryl, isobutyryl, pentanoyl, tert-butylcarbonyl and a hexanoyl group. Preferably, it is a C1-6 alkanoyl group, and more preferably a C1-3 alkanoyl group.
- The “C1-8 alkanoyl group which may be substituted” means the unsubstituted C1-8 alkanoyl group described above or a C1-8 alkanoyl group of which at least one hydrogen atom on the alkanoyl group is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C1-8 alkanoyl group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 2 substituent(s) for a C1-6 alkanoyl group and a C1-3 alkanoyl group.
- The “C3-8 cycloalkylcarbonyl group” means a C3-8 cycloalkyl-C(O)— group, and the C3-8 cycloalkyl group is described above. Specifically, examples thereof include a cyclopropylcarbonyl group, a cyclobutylcarbonyl group, a cyclopentylcarbonyl group, a cyclohexylcarbonyl group, a cycloheptylcarbonyl group and a cyclooctylcarbonyl group.
- The “4- to 10-membered heterocycloalkylcarbonyl group” means a 4- to 10-membered heterocycloalkyl-CO— group, and the 4- to 10-membered heterocycloalkyl is described above.
- The “4- to 10-membered heterocycloalkylcarbonyl group which may be substituted” means the unsubstituted 4- to 10-membered heterocycloalkylcarbonyl group described above or a 4- to 10-membered heterocycloalkylcarbonyl group in which at least one hydrogen atom of the heterocycloalkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the heterocycloalkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a 4-to 10-membered heterocycloalkylcarbonyl group which may be substituted by 1 to 3 substituent(s).
- The “aminocarbonyl group which may be substituted” means an unsubstituted aminocarbonyl group or an aminocarbonyl group in which one or two hydrogen atoms on the nitrogen atom are substituted by a defined substituent(s). When two substituent groups are present, each substituent group can be the same or different from each other.
- The “C3-8 cycloalkyl (C0-8 alkyl) aminocarbonyloxy group” means a C3-8 cycloalkyl-NHC(O)O— group or a C3-8 cycloalkyl-N(C1-8 alkyl) C(O)O— group, and the C3-8 cycloalkyl group is described above. Specifically, examples thereof include a cyclopropylaminocarbonyloxy group, a cyclobutylaminocarbonyloxy group, a cyclopentylaminocarbonyloxy group, a cyclohexylaminocarbonyloxy group, a cyclopropyl(N-methyl)aminocarbonyloxy group, and a cyclobutyl(N-methyl)aminocarbonyloxy group.
- The “(C1-8 alkyl)x-aminocarbonyl group”, wherein x is a symbol defined in claims, means a NH2C(O)— group, a (C1-8 alkyl)NH—C(O)— group, or a (C1-8 alkyl)2N—C(O)— group. Specifically, examples thereof include a N-methyl-aminocarbonyl group, N-ethyl-aminocarbonyl group, N-n-buthyl-aminocarbonyl group, a N,N-dimethyl-aminocarbonyl group.
- The “(C1-8 alkyl)x-aminocarbonyl group which may be substituted” means an unsubstituted (C1-8 alkyl)x-aminocarbonyl group described above or an (C1-8 alkyl)x-aminocarbonyl group in which at least one hydrogen atom on the nitrogen atom or the alkyl moiety are substituted by a defined substituent(s). When plural substituent groups are present, each substituent group can be the same or different from each other.
- The “C6-10 aryl(C0-8 alkyl)aminocarbonyl group” means a C6-10 aryl-NHC(O)— group, or a C6-10 aryl-N(C1-8 alkyl)-C(O)— group. Specifically, examples thereof include a phenyl-NHC(O)— group, or a phenyl-(N-methyl)-aminocarbonyl group, wherein the C6-10 aryl group and C1-8 alkyl are described above. Specifically, examples thereof include a phenylaminocarbonylamino group and a phenylaminocarbonyl(N-methyl)amino group.
- The “4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group” means a carbonyl group to which a 4- to 10-membered nitrogen-containing heterocycloalkyl group is bonded. Herein, the 4- to 10-membered nitrogen-containing heterocycloalkyl group (i.e., 4- to 10-membered heterocycloalkyl group comprising a nitrogen atom(s)) means a heterocycloalkyl group which consists of 4 to 10 ring-constituting atoms and comprises at least one nitrogen atom as a hetero atom. Preferably, it is bonded to the carbonyl group via a nitrogen atom that is comprised in the heterocycloalkyl ring. Specific examples of the 4- to 10-membered nitrogen-containing heterocycloalkyl group include a pyrrolidinyl group, an imidazolidinnyl group, a morpholino group, a thiomorphorino group, a piperazino group and a piperidino group. As for the 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group, examples thereof include a pyrrolidinocarbonyl group, a piperidinocarbonyl group, a piperazinocarbonyl group and a morpholinocarbonyl group.
- The “4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group, which may be substituted” means the unsubstituted 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group as described above or a 4- to 10-membered heterocycloalkylcarbonyl group in which at least one hydrogen atom of the heterocycloalkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the heterocycloalkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by 1 to 3 substituent(s).
- The “4- to 10-membered heterocycloalkyl (C0-8 alkyl) aminocarbonyl group” means 4- to 10-membered heterocycloalkyl NHC(O)— group, or a 4- to 10-membered heterocycloalkyl N(C1-8 alkyl)-C(O)— group. Specifically, examples thereof include a oxetan-3-yl amide group, and a (1,1-dioxo-tetrahydro-thiophen-3-yl)-amide group.
- The “4- to 10-membered heterocycloalkyl (C0-8 alkyl) aminocarbonyl group which may be substituted by one or more oxo groups” means the unsubstituted 4- to 10-membered heterocycloalkylaminocarbonyl group described above or the 4- to 10-membered heterocycloalkylaminocarbonyl group in which the heterocycloalkyl moiety is substituted by at least one oxo group.
- The “C6-10 arylsulfonyl group” means a C6-10 aryl-S(O)2— group and the C6-10 aryl group is described above. Specifically, examples thereof include a phenylsulfonyl group.
- The “5- to 14-membered heteroarylsulfonyl group” means a 5- to 14-membered heteroaryl-S(O)2— group, and the 5- to 14-membered heteroaryl is described above. Specifically, examples thereof include a imidazol-sulfonyl group.
- The “(C1-8 alkyl)x-amino group”, wherein x is a symbol defined in claims, means an amino group, a NH(C1-8 alkyl) group, or a N(C1-8 alkyl)2- group. Specifically, examples thereof include amino, methylamino, ethylamino, butylamino, isopropylamino, dimethylamino and diethylamino. Preferably, it is a C1-3 alkylamino group.
- The “(C1-8 alkyl)x-amino group which may be substituted” means an unsubstituted (C1-8 alkyl)x-amino group or an amino group in which one or two hydrogen atoms on the nitrogen atom or the alkyl moiety are substituted by a defined substituent(s). When two substituent groups are present, each substituent group can be the same or different from each other.
- The “C1-8 alkylcarbonyl (C0-8 alkyl) amino group” means a C1-8 alkyl-C(O)—NH— group or a C1-8 alkyl-C(O)—N(C1-8 alkyl)- group, and the C1-8 alkyl is described above. Specifically, examples thereof include a methylcarbonylamino group, an ethylcarbonylamino group, a propylcarbonylamino group and a butylcarbonylamino group.
- The “C1-8 alkylcarbonyl (C0-8 alkyl) amino group which may be substituted” means the unsubstituted C1-8 alkylcarbonyl (C0-8 alkyl) amino group described above or the C1-8 alkylcarbonyl (C0-8 alkyl) amino group in which at least one hydrogen atoms of the terminal alkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C1-8 alkylcarbonyl (C0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- The “C6-10 arylcarbonyl (C0-8 alkyl) amino group” means a C6-10 aryl-C(O)—NH— group or a C6-10 aryl-C(O)—N(C1-8 alkyl)- group and the C6-10 aryl group and the C1-8 alkyl group are described above. Specifically, examples thereof include a phenylcarbonylamino group.
- The “C6-10 arylcarbonyl (C0-8 alkyl) amino group which may be substituted” means the unsubstituted C6-10 arylcarbonyl (C0-8 alkyl) amino group described above or the C6-10 arylcarbonyl (C0-8 alkyl) amino group in which at least one hydrogen atoms of the aryl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C6-10 arylcarbonyl (C0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- The “(C1-8 alkyl)x-aminocarbonyl (C0-8 alkyl) amino group”, wherein x is a symbol defined in claims, means a NH2C(O)NH— group, a (C1-8 alkyl)NHC(O)NH— group, a NH2C(O)N(C1-8 alkyl)- group, or a (C1-8 alkyl)NHC(O)N(C1-8 alkyl)- group, and the C1-8 alkyl is described above. Specifically, examples thereof include aminocarbonyl-(N-methyl)amino, and (N-methyl)aminocarbonyl-(N′-methyl)amino.
- The “(C1-8 alkyl)x-aminocarbonyl (C0-8 alkyl) amino group which may be substituted” means an unsubstituted (C1-8 alkyl)x-aminocarbonyl (C0-8 alkyl) amino group, or a (C1-8 alkyl)x-aminocarbonyl (C0-8 alkyl) amino group in which at least one hydrogen atom on the nitrogen atom or the alkyl moiety is substituted by a defined substituent. Preferably, it is a (C1-8 alkyl)x-aminocarbonyl (C0-8 alkyl) amino group which may be substituted by a phenyl group.
- The “C1-8 alkylsulfonylamino group” means a C1-8 alkyl-S(O)2—NH— group and the C1-6 alkyl group is described above. Specifically, examples thereof include a methylsulfonylamino group and an ethylsulfonylamino group.
- The “(C1-8 alkyl)x-aminosulfonyl(C0-8 alkyl)amino group”, wherein x is a symbol defined in claims, means a NH2S(O)2NH— group, a NH(C1-8 alkyl)-S(O)2NH— group, or a N(C1-8 alkyl)2-S(O)2NH— group, a NH2S(O)2N (C1-8 alkyl)x- group, a NH(C1-8 alkyl)-S(O)2 (C1-8 alkyl)N— group, or a N(C1-8 alkyl)2-S(O)2 (C1-8 alkyl)N— group, and the C1-8 alkyl group is described above. Specifically, examples thereof include a methylamino-sulfonylamino group and a dimethylamino-sulfonylamino group.
- The “C1-8 alkoxy group” means a C1-8 alkyl-O— group. Specifically, examples thereof include a methoxy group, an ethoxy group, a 1-propoxy group, a 2-propoxy group, a n-butoxy group, an i-butoxy group, a sec-butoxy group, a t-butoxy group, a 1-pentyloxy group, a 2-pentyloxy group, a 3-pentyloxy group, a 2-methyl-1-butyloxy group, a 3-methyl-1-butyloxy group, a 2-methyl-2-butyloxy group, a 3-methyl-2-butyloxy group, a 2,2-dimethyl-1-propyloxy group, a 1-hexyloxy group, a 2-hexyloxy group, a 3-hexyloxy group, a 2-methyl-1-pentyloxy group, a 3-methyl-1-pentyloxy group, a 4-methyl-1-pentyloxy group, a 2-methyl-2-pentyloxy group, a 3-methyl-2-pentyloxy group, a 4-methyl-2-pentyloxy group, a 2-methyl-3-pentyloxy group, a 3-methyl-3-pentyloxy group, a 2,3-dimethyl-1-butyloxy group, a 3,3-dimethyl-1-butyloxy group, a 2,2-dimethyl-1-butyloxy group, a 2-ethyl-1-butyloxy group, a 3,3-dimethyl-2-butyloxy group, a 2,3-dimethyl-2-butyloxy group and a 1-methyl-cyclopropylmethoxy group. Preferably, it is a C1-6 alkoxy group, more preferably a C1-5 alkoxy group, still more preferably a C1-4 alkoxy group, and still even more preferably a C1-3 alkoxy group.
- The “C1-8 alkoxy group which may be substituted” means an unsubstituted C1-8 alkoxy group or a C1-8 alkoxy group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C1-8 alkoxy group which may be substituted by 1 to 3 substituent(s). More preferably, it is 1 to 3 substituent(s) for C1-6 alkoxy group and a C1-4 alkoxy group, and 1 to 2 substituent(s) for a C1-3 alkoxy group.
- The “C1-8 alkoxycarbonyl group” means a C1-8 alkyl-O—C(O)— group and the C1-8 alkyl group is described above. Specifically, examples thereof include a methoxycarbonyl group, an ethoxycarbonyl group, a n-propoxycarbonyl group and an i-propoxycarbonyl group. Preferably, it is a C1-6 alkoxycarbonyl group, and more preferably a C1-3 alkoxycarbonyl group.
- The “C1-8 alkoxycarbonyl group which may be substituted” means the unsubstituted C1-8 alkoxycarbonyl group described above or a C1-8 alkoxycarbonyl group of which at least one hydrogen atom is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the alkyl moiety of the alkoxycarbonyl group may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C1-8 alkoxycarbonyl group which may be substituted by 1 to 3 substituent(s).
- The “C0-8 alkoxy (C0-8 alkyl) aminocarbonyl group” means a HO—NH—C(O)— group, a C1-8 alkyl-NH—C(O)— group, a HO—N(C1-8 alkyl)-C(O)— group, or a C1-8 alkyl-N(C1-8 alkyl)-C(O)— group, and has a C1-8 alkoxy group or a C1-8 alkyl group as described above. Specifically, examples thereof include a methoxyaminocarbonyl group, an ethoxyaminocarbonyl group, a n-propoxyaminocarbonyl group and an i-propoxyaminocarbonyl group. Preferably, it is a C1-6 alkoxyaminocarbonyl group, and more preferably a C1-3 alkoxyaminocarbonyl group.
- The “C0-8 alkoxy (C0-8 alkyl) aminocarbonyl group which may be substituted” means the unsubstituted hydroxyaminocarbonyl group described above, or a C1-8 alkoxyaminocarbonyl group, a hydroxy (C1-8 alkyl) aminocarbonyl group or a C1-8 alkoxy (C1-8 alkyl) aminocarbonyl group, wherein at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C1-8 alkoxy aminocarbonyl group which may be substituted by 1 to 3 substituent(s).
- The “4- to 10-membered heterocycloalkyloxy group” means a 4- to 10-membered heterocycloalkyl-O— group, and the 4- to 10-membered heterocycloalkyl is described above.
- The “4- to 10-membered heterocycloalkyloxy group which may be substituted” means the unsubstituted 4- to 10-membered heterocycloalkyloxy group described above or a 4- to 10-membered heterocycloalkyloxy group in which at least one hydrogen atom of the heterocycloalkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the heterocycloalkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a 4-to 10-membered heterocycloalkyloxy group which may be substituted by 1 to 3 substituent(s).
- The “C6-10 aryloxy group” means a C6-10 aryl-O— group, and the C6-10 aryl group is described above.
- The “5- to 14-membered heteroaryloxy group” means a 5- to 14-membered heteroaryl-O— group, and the 5- to 14-membered heteroaryl is described above. Specifically, examples thereof include a pyrimidinyloxy group.
- The “C1-8 alkylcarbonyloxy group” means a C1-8 alkyl-C(O)—O— group, and the C1-8 alkyl is described above. Specifically, examples thereof include a methylcarbonyloxy group, an ethylcarbonyloxy group and a propylcarbonyloxy group.
- The “C2-8 alkenylcarbonyloxy group” means a C2-8 alkenyl-C(O)—O— group, and the C2-8 alkenyl is described above. Specifically, examples thereof include a 2-methyl-2-butenoyloxy group.
- The “4- to 10-membered heterocycloalkylcarbonyloxy group” means a 4- to 10-membered heterocycloalkyl-C(O)—O— group, and the 4- to 10-membered heterocycloalkyl is described above.
- The “(C1-8 alkyl)x-aminocarbonyloxy group”, wherein x is a symbol defined in claims, means a NH2C(O)—O— group, a NH(C1-8 alkyl)-C(O)—O— group, or a N(C1-8 alkyl)2-C(O)—O— group. Specifically, examples thereof include a methylamino-carbonyloxy group, an ethylamino-carbonyloxy group and a propylamino-carbonyloxy group.
- The “(C1-8 alkyl)x-aminocarbonyloxy group which may be substituted” means an unsubstituted (C1-8 alkyl)x-aminocarbonyloxy group or a (C1-8 alkyl)x-aminocarbonyloxy group group in which one or two hydrogen atoms on the nitrogen atom or the alkyl moiety are substituted by a defined substituent(s). When two substituent groups are present, each substituent group can be the same or different from each other.
- The “4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group” means the 4- to 10-membered nitrogen-containing heterocycloalkyl-S(O)2— group described above. Specifically, examples thereof include a morphorino-sulfonyl group.
- The “4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted” means the unsubstituted 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group described above or the 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group in which at least one hydrogen atom of the 4- to 10-membered nitrogen-containing heterocycloalkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a 4-to 10-membered nitrogen-containing heterocycloalkylsulfonyl which may be substituted by 1 to 3 substituent(s).
- The “4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group” means the 4- to 10-membered nitrogen-containing heterocycloalkyl-S(O)2—O— group described above. Specifically, examples thereof include a morphorino-sulfonyloxy group and a piperadino-sulfonyloxy group.
- The “4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group which may be substituted” means the unsubstituted 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group described above or the 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group in which at least one hydrogen atom of the 4- to 10-membered nitrogen-containing heterocycloalkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a 4-to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy which may be substituted by 1 to 3 substituent(s).
- The “C1-8 alkylsulfonyloxy group” means a C1-8 alkyl-S(O)2—O— group, and the C1-8 alkyl is described above.
- The “C1-8 alkylsulfonyloxy group which may be substituted” means the unsubstituted C1-8 alkylsulfonyloxy group described above or a C1-8 alkylsulfonyloxy group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C1-8 alkylsulfonyloxy group which may be substituted by 1 to 3 substituent(s). Specifically, examples thereof include a trifluoromethylsulfonyloxy group.
- The “(C1-8 alkyl)x-aminosulfonyloxy group” wherein x is a symbol defined in claims, means a NH2S(O)2— group, a N(C1-8 alkyl)S(O)2— group, or a N(C1-8 alkyl)2S(O)2— group. Specifically, examples thereof include a N-methylaminosulfonyloxy group.
- The “C1-8 alkylthio group” means a C1-8 alkyl-S— group, and the C1-8 alkyl group is described above. Examples thereof include methylthio, ethylthio, n-propylthio, i-propylthio, n-butylthio, s-butylthio, i-butylthio, t-butylthio, n-pentylthio, 3-methylbutylthio, 2-methylbutylthio, 1-methylbutylthio, 1-ethylpropylthio, n-hexylthio, 4-methylpentylthio, 3-methylpentylthio, 2-methylpentylthio, 1-methylpentylthio, 3-ethylbutylthio, and 2-ethylbutylthio and the like. Preferably, it is a C1-6 alkylthio group, and more preferably a C1-3 alkylthio group.
- The “C1-8 alkylthio group which may be substituted” means an unsubstituted C1-8 alkylthio group or a C1-8 alkylthio group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the alkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a C1-8 alkylthio group which may be substituted by 1 to 3 substituent(s).
- The “C1-8 alkylsulfonyl group” means a C1-8 alkyl-S(O)2— group, and the C1-8 alkyl group is described above. Specifically, examples thereof include a methylsulfonyl group, an ethylsulfonyl group and a n-propylsulfonyl group. Preferably, it is a C1-6 alkylsulfonyl group, and more preferably a C1-3 alkylsulfonyl group.
- The “C1-8 alkylsulfinyl group” means a C1-8 alkyl-S(O)— group, and the C1-8 alkyl group is described above. Specifically, examples thereof include a methylsulfinyl group, an ethylsulfinyl group and a n-propylsulfinyl group. Preferably, it is a C1-6 alkylsulfinyl group, and more preferably a C1-3 alkylsulfinyl group.
- The “C1-8 alkylsulfonyl group which may be substituted” means the unsubstituted C1-8 alkylsulfonyl group described above or a C1-8 alkylsulfonyl group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C1-8 alkylsulfonyl group which may be substituted by 1 to 3 substituent(s).
- The “C1-8 alkylsulfinyl group which may be substituted” means the unsubstituted C1-8 alkylsulfinyl group described above or a C1-8 alkylsulfinyl group in which at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C1-8 alkylsulfinyl group which may be substituted by 1 to 3 substituent(s).
- The “4- to 10-membered heterocycloalkylsulfonyl group” means a 4- to 10-membered heterocycloalkyl-S(O)2— group, and the 4- to 10-membered heterocycloalkyl is described above.
- The “4- to 10-membered heterocycloalkylsulfonyl group which may be substituted” means the unsubstituted 4- to 10-membered heterocycloalkylsulfonyl group described above or a 4- to 10-membered heterocycloalkylsulfonyl group in which at least one hydrogen atom of the heterocycloalkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. In addition, the heterocycloalkyl moiety may be substituted by a cyclic substituent group through a spiro bond. Preferably, it is a 4-to 10-membered heterocycloalkylsulfonyl group which may be substituted by 1 to 3 substituent(s).
- The “(C1-8 alkyl)x-aminosulfonyl group”, wherein x is a symbol defined in claims, means a NH2—S(O)2— group, a C1-8 alkylamino-S(O)2— group, or a (C1-8 alkyl)2amino-S (O)2— group and the C1-8 alkyl is described above. Specifically, examples thereof include an aminosulfonyl group, a methylaminosulfonyl group and a dimethylaminosulfonyl group.
- The “(C1-8 alkyl)x-aminosulfonyl group which may be substituted” means an unsubstituted aminosulfonyl group or a (C1-8 alkyl)x-aminosulfonyl group in which one or two hydrogen atoms on the nitrogen atom or the alkyl moiety are substituted by a defined substituent(s). When two substituent groups are present, each substituent group can be the same or different from each other.
- The “C1-8 alkoxycarbonyl (C0-8 alkyl) amino group” means a C1-8 alkoxy-C(O)—NH— group or a C1-8 alkoxy-C(O)—N(C1-8 alkyl)- group, wherein the C1-8 alkoxy group and C1-8 alkyl) are described above. Specifically, examples thereof include a methoxycarbmamoyl group and an N-ethylcarbonyl-N-methyl-amino group.
- The “C1-8 alkoxycarbony(C0-8 alkyl) amino group which may be substituted” means the unsubstituted C1-8 alkoxycarbony(C0-8 alkyl) amino group described above, or a C1-8 alkoxycarbony(C0-8 alkyl) amino group, wherein at least one hydrogen atom of the alkyl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C1-8 alkoxycarbony(C0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- The “C1-8 alkoxycarbonyl (C0-8 alkyl) aminosulfonyl group” means a C1-8 alkoxy-C(O)—NHS(O)2— group or a C1-8 alkoxy-C(O)—N(C1-8 alkyl)S(O)2— group, wherein the C1-8 alkoxy group and C1-8 alkyl group are described above. Specifically, examples thereof include a methoxy carbonyl aminosulfonyl group and an ethoxycarbonyl-N-methyl-aminosulfonyl group.
- The “C6-10 aryloxycarbonyl (C0-8 alkyl) amino group” means a C6-10 aryl-O—C(O)—NH— group or a C6-10 aryl-O—C(O)—N(C1-8 alkyl)- group, wherein the C6-10 aryl group and C1-8 alkyl are described above. Specifically, examples thereof include a phenyloxycarbonylamino group and a N-methyl-N-phenyloxycarbonyl-amino group.
- The “C6-10 aryloxycarbonyl (C0-8 alkyl) amino group which may be substituted” means the unsubstituted C6-10 aryloxycarbonyl (C0-8 alkyl) amino group described above or the C6-10 aryloxycarbonyl (C0-8 alkyl) amino group in which at least one hydrogen atoms of the aryl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C6-10 aryloxycarbonyl (C0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- The “C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group” means a C6-10 aryl NHC(O)NH— group, a C6-10 aryl-N(C1-8 alkyl)-C(O)NH— group, a C6-10 aryl-N(C1-8 alkyl)-C(O)N(C1-8 alkyl)- group, or a C6-10 aryl-NH—C(O)N(C1-8 alkyl)- group, wherein the C6-10 aryl group and C1-8 alkyl are described above. Specifically, examples thereof include a phenylaminocarbonylamino group and a phenylaminocarbonyl(N-methyl)amino group.
- The “C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group which may be substituted” means the unsubstituted C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group described above or the C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group in which at least one hydrogen atoms of the aryl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group which may be substituted by 1 to 3 substituent(s).
- The “C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group” means a C6-10 aryl-NHC(O)—O—, or a C6-10 aryl-N(C1-8 alkyl)-C(O)—O— group, wherein the C6-10 aryl group and C1-8 alkyl are described above. Specifically, examples thereof include a phenylaminocarbonyloxy group and a phenylaminocarbonyl(N-methyl)amino carbonyloxy group.
- The “C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group which may be substituted” means the unsubstituted C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group described above or the C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group in which at least one hydrogen atoms of the aryl moiety is substituted by a defined substituent(s). When two or more substituent groups are present, each substituent group can be the same or different from each other. Preferably, it is a C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group which may be substituted by 1 to 3 substituent(s).
- The “C1-8 alkylsulfonyl (C0-8 alkyl) amino group” means a C1-8 alkyl-S(O)2—NH— group, or a C1-8 alkyl-S(O)2—N(C1-8 alkyl)- group. Specifically, examples thereof include a methylsulphonylamino group and a methylsulphonyl-(N-methyl)amino group.
- The “C2-8 alkenyloxy group” means a C2-8 alkenyl-O— group, wherein the C2-8 alkenyl group is described above. Specific examples of the C2-8 alkenyloxy group include a vinyloxy group and a allyloxy group.
- Preferably, all of A1, A2, A3, A4, A7, A8, A9 and A10 are C, or any one of A2, A4, A7 and A9 is N and the remainings are C (with the proviso that, when A2, A4, A7 or A9 is N, they do not have a substituent group R2, R4, R7 or R9). More preferably, all of them are C, or A4 is N while the remainings are C, even more preferably, all of them are C, or A4, A7, and A9 is N and the remainings are C (with the proviso that, when A4, A7, or A9 is N, they do not have a substituent group R4, R7, R9).
- A5 is preferably NR5 or O, more preferably NR5, even more preferably NH.
- R1 is preferably
- [1] a hydrogen atom,
[2] a halogen atom,
and more preferably
[1] a hydrogen atom,
[2] a fluorine atom,
[3] a chlorine atom. - R10 is preferably a hydrogen atom.
- R2 is preferably
- [1] a hydrogen atom,
[2] a C1-5 alkyl group,
[3] a cyano,
[4] a halogen atom,
and more preferably
[1] a hydrogen atom,
[2] a C1-3 alkyl group,
[3] a fluorine atom,
[4] a chlorine atom,
[5] a bromine atom.
And even more preferably
[1] a hydrogen atom,
[2] a halogen atom,
Still more preferably
[1] a hydrogen atom, - R4 is preferably
- [1] a hydrogen atom,
[2] a C1-5 alkyl group which may be substituted by 1-11 halogen atom(s),
[3] a C3-6 cycloalkyl group,
[4] a cyano,
[5] a halogen atom,
[6] a (C1-3 alkyl)m4b-amino group (m4b: 0˜2),
[7] a hydroxy,
[8] a C1-5 alkoxy group which may be substituted by 1-4 hydroxy(s),
and more preferably
[1] a hydrogen atom,
[2] a C1-3 alkyl group which may be substituted by 1-7 halogen atom(s),
[3] a C3-5 cycloalkyl group,
[4] a cyano,
[5] a florine atom,
[6] a bromine atom,
[7] an amino (C1-3 alkyl)m4b-amino group,
[8] a hydroxy,
[9] a C1-3 alkoxy group which may be substituted by 1-2 hydroxy(s).
Even more preferably
[1] a hydrogen atom,
[2] a C1-8 alkyl group which may be substituted by at least one halogen atom,
[3] a C3-8 cycloalkyl group,
[4] a cyano,
[5] a halogen atom,
[6] a hydroxy,
[7] a C1-8 alkoxy group which may be substituted by a hydroxy,
Still more preferably
[1] a hydrogen atom,
[2] a halogen atom, - R5 is preferably
- [1] a hydrogen atom,
[2] a C1-5 alkyl group which may be substituted by 1-5 R5A substituent(s),
[3] a C1-5 alkylsulfonyl group,
and more preferably
[1] a hydrogen atom,
[2] a C1-3 alkyl group which may be substituted by 1-3 R5A substituent(s),
[3] a C1-3 alkylsulfonyl group.
Even more preferably
[1] a hydrogen atom,
[2] a C1-8 alkyl group,
Still more preferably
[1] a hydrogen atom. - R5A is preferably
- [1] a C1-5 alkoxycarbonyl group,
[2] a hydroxy,
[3] a C1-5 alkoxy group,
[4] a (C1-5 alkyl)m5-amino group (m5: 0˜2),
[5] a C6 aryl,
[6] a C1-8 alkylthio group,
and more preferably
[1] a C1-3 alkoxycarbonyl group,
[2] a hydroxy,
[3] a C1-3 alkoxy group,
[4] a (C1-3 alkyl)m5-amino group (m5: 0˜2),
[5] a C1-3 alkylthio group,
even more preferably
[1] a hydroxy,
[2] a C1-5 alkoxy group,
[3] a (C1-5 alkyl)m5-amino group (m5: 0˜2),
[4] a C1-5 alkylthio group. - R6 and R6′ are preferably
- [1] a C1-8 alkyl group,
taken together with carbon atoms to which they are bound to form
[2] a C3-8 cycloalkyl group,
[3] a 4- to 10-membered heterocycloalkyl group,
more preferably
[1] a C1-3 alkyl group,
taken together with carbon atoms to which they are bound to form
[2] a C3-6 cycloalkyl group,
[3] a 4- to 6-membered heterocycloalkyl group,
even more preferably
[1] a methyl,
taken together with carbon atoms to which they are bound to form
[2] a cyclopentane,
[3] a tetrahydropyran,
[4] or a piperidine. - R7 is preferably
- [1] a hydrogen atom,
[2] a fluorine atom,
[3] a bromine atom,
[4] a chlorine atom,
[5] a C1-5 alkoxy group which may be substituted by 1-4 R7A substituent(s),
and more preferably
[1] a hydrogen atom,
[2] a halogen atom,
and even more preferably
[1] a hydrogen atom,
[2] a fluorine atom,
[3] a bromine atom,
[4] a chlorine atom,
and still more preferably
[1] a hydrogen atom. - R7A is preferably
- [1] a (C1-5 alkyl)m7-amino group (m7: 0˜2),
[2] a hydroxy,
[3] a 4- to 6-membered heterocycloalkyl group which may be substituted by C1-5 alkyl group(s), and more preferably
[1] a (C1-3 alkyl)m7-amino group (m7: 2),
[2] a hydroxy,
[3] a 4- to 6-membered heterocycloalkyl group which may be substituted by C1-3 alkyl group(s). - R3 is preferably
- [1] a hydrogen atom,
[2] a C1-5 alkyl group which may be substituted by 1-11 halogen atom(s),
[3] a cyano,
[4] a (C1-5 alkyl)m3a-aminocarbonyl group (m3a: 0˜2) which may be substituted by 1-5 R3A substituents,
[5] a hydroxycarbonyl,
[6] a C1-5 alkylcarbonyl group which may be substituted by 1-4 hydroxy(s),
[7] a halogen atom,
[8] a (C1-3 alkyl)m3b-amino group (m3b: 0˜2) which may be substituted by 1-2 C6 aryl(s),
[9] a C1-5 alkyl carbonyl (C0-3 alkyl) amino group which may be substituted by 1-2 C6 aryl(s) or 1-2 C6 aryloxy(s),
[10] a C6 arylcarbonyl (C0-3 alkyl) amino group which may be substituted by 1-5 C1-3 alkyl group(s) which may be substituted by 1-7 halogen atom(s),
[11] a (C1-3 alkyl)m3c-aminocarbonyl(C-3 alkyl) amino group (m3c: 0˜1) which may be substituted by a C6 aryl,
[12] a nitro,
[13] a hydroxy,
[14] a C1-5 alkoxy group which may be substituted by 1-4 R3B(s),
[15] a 4- to 6-membered heterocycloalkyloxy group,
[16] a 6-membered heteroaryloxy,
[17] a (C1-5 alkyl)m3e-aminocarbonyloxy group (m3e: 0˜2) which may be substituted by 1-3 C6 aryl(s),
[18] a 4- to 6-membered nitrogen-containing heterocycloalkylaminocarbonyl group,
[19] a C1-5 alkylthio group,
[20] a 5- to 6-membered heteroaryl group which may be substituted by 1-4 C1-5 alkyl group(s) which may be substituted by 1-3 C1-5 alkoxy group(s),
[21] a C1-3 alkoxycarbonyl (C0-3 alkyl) amino group which may be substituted by a C1-3 alkoxy group,
[22] a C6 aryloxycarbonyl (C0-3 alkyl) amino group which may be substituted by 1-3 C1-3 alkyl group(s) which may be substituted by 1-9 halogen atom(s),
[23] a C6 aryloxycarbonyl (C0-3 alkyl) aminocarbonyl (C0-3 alkyl)amino group which may be substituted by 1-3 R3C,
[24] a C3-6 cycloalkyl (C0-3 alkyl) aminocarbonyloxy group, and
[25] a C6 aryl (C0-3 alkyl) aminocarbonyloxy group which may be substituted by 1-3 substituent(s) selected from the group consisting of a C1-5 alkyl group and a C1-5 alkoxy group(s). - R3 is more preferably
- [1] a hydrogen atom,
[2] a C1-3 alkyl group which may be substituted by 1-7 halogen atom(s),
[3] a cyano,
[4] a (C1-4 alkyl)m3a-aminocarbonyl group (m3a: 0˜1) which may be substituted by 1-4 R3A substituents,
[5] a hydroxycarbonyl,
[6] a halogen atom,
[7] a C1-4 alkyl carbonyl (C1-3 alkyl) amino group which may be substituted by 1-2 C6 aryl(s) or 1-2 C6 aryloxy(s),
[8] a C6 arylcarbonyl (C0-3 alkyl) amino group which may be substituted by a C1-3 alkyl group which may be substituted by 1-7 halogen atom(s),
[9] a nitro,
[10] a hydroxy,
[11] a C1-4 alkoxy group which may be substituted by 1-3 R3B substituent(s),
[12] a 4-membered heterocycloalkyloxy group,
[13] a (C1-3 alkyl)m3e-aminocarbonyloxy group (m3e:1) which may be substituted by a C6 aryl(s),
[14] a 6-membered nitrogen-containing heterocycloalkylaminocarbonyl group,
[15] a C1-3 alkylthio group,
[16] a 5-membered heteroaryl group which may be substituted by a C1-5 alkyl group which may be substituted by a C1-3 alkoxy group,
[17] a C6 aryloxycarbonyl (C0-3 alkyl) aminocarbonyl (C0-3 alkyl)amino group which may be substituted by a R3C substituent,
[18] a C6 cycloalkyl (C0-2 alkyl) aminocarbonyloxy group, and
[19] a C6 aryl (C0-3 alkyl) aminocarbonyloxy group which may be substituted by 1-2 substituent(s) selected from the group consisting of a C1-4 alkyl group and a C1-3 alkoxy group. - R3 is still more preferably
- [1] a hydrogen atom,
[2] a cyano,
[3] a halogen atom, - R3 is still even more preferably
- [1] a cyano,
[2] a halogen atom. - R3A is preferably
- [1] a C6 aryl,
[2] a C1-5 alkoxy group,
[3] a 5- or 6-membered heteroaryl group,
[4] a C6 arylsulfonyl. - R3B is preferably
- [1] a hydroxy,
[2] a C1-5 alkoxy group,
[3] a C6 aryl (C0-3 alkyl) aminocarbonyl group,
[4] a (C1-3 alkyl)m3d-amino group (m3d: 0˜2),
[5] a halogen atom,
more preferably
[1] a hydroxy,
[2] a C1-5 alkoxy group. - R3C is preferably
- [1] a C1-5 alkyl group which may be substituted by 1-11 halogen atom(s),
[2] a C1-5 alkoxy group,
more preferably
[1] a C1-4 alkyl group which may be substituted by 1-9 halogen atom(s),
[2] a C1-3 alkoxy group. - R8 is preferably
- [1] a hydrogen atom,
[2] a C1-5 alkyl group which may be substituted by 1-5 R5A substituent(s),
[3] a C2-8 alkenyl group,
[4] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-4 R8B substituent(s),
[5] a 5- to 6-membered heteroaryl group which may be substituted by 1-4 C1-8 alkyl group(s),
[6] a (C1-5 alkyl)m8g-aminocarbonyl group (m8g: 0˜2) which may be substituted by 1-3 R8C substituent(s),
[7] a 4- to 6-membered heterocycloalkyl (C0-3 alkyl) aminocarbonyl group which may be substituted by 1-2 oxo group(s),
[8] a 4- to 6-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by 1-4 R8D substituent(s),
[9] a hydroxycarbonyl,
[10] a C1-5 alkoxy (C0-3 alkyl) aminocarbonyl group which may be substituted by 1-3 hydroxy group(s),
[11] a halogen atom,
[12] a (C1-5 alkyl)m8j-amino group (m8j:0-2) which may be substituted by 1-2 R8H substituent(s),
[13] a hydroxyl,
[14] a C1-5 alkoxy group which may be substituted by 1-4 R8E substituent(s),
[15] a 4- to 6-membered heterocycloalkyloxy group which may be substituted by 1-5 R8F substituent(s),
[16] a 6-membered heteroaryloxy group,
[17] a (C1-5 alkyl)m8l1-aminosulfonyloxy group (m8l1:0-2),
[18] a C1-5 alkyl thio group which may be substituted by 1-4 R8I substituent(s),
[19] a C1-5 alkylsulfonyl group which may be substituted by 1-4 R8G substituent(s),
[20] a 6-membered heterocycloalkylsulfonyl group which may be substituted by a C1-3 alkyl group,
[21] a C2-8 alkenyloxy group, and
[22] a C1-3 alkylsulfonyloxy group which may be substituted by 1-7 halogen atom(s). And more preferably
[1] a hydrogen atom,
[2] a C1-3 alkyl group which may be substituted by 1-3 R5A substituent(s),
[3] a C2-4 alkenyl group,
[4] a 6-membered heterocycloalkyl group which may be substituted by 1-3 R8B substituent(s),
[5] a 5- to 6-membered heteroaryl group which may be substituted by 1-2 C1-3 alkyl group(s),
[6] a (C1-3 alkyl)m8g-aminocarbonyl group (m8g: 0˜2) which may be substituted by 1-2 RC substituent(s),
[7] a 4- to 6-membered heterocycloalkyl (C0-1 alkyl) aminocarbonyl group which may be substituted by 1-2 oxo group(s),
[8] a 6-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by 1-2 R8D substituent(s),
[9] a hydroxycarbonyl,
[10] a C1-3 alkoxy (C0-3 alkyl) aminocarbonyl group which may be substituted by 1-2 hydroxy group(s),
[11] a bromine atom,
[12] a (C1-3 alkyl)m8j-amino group (m8j:0-2) which may be substituted by 1-2 R8H substituent(s),
[13] a hydroxyl,
[14] a C1-5 alkoxy group which may be substituted by 1-3 R8E substituent(s),
[15] a 4- to 6-membered heterocycloalkyloxy group which may be substituted by 1-3 R8F substituent(s),
[16] a 6-membered heteroaryloxy group,
[17] a (C1-3 alkyl)m8l1-aminosulfonyloxy group (m8l1:0-2),
[18] a C1-3 alkyl thio group which may be substituted by 1-2 R8I substituent(s),
[19] a C1-3 alkylsulfonyl group which may be substituted by 1-2 R8G substituent(s),
[20] a 6-membered heterocycloalkylsulfonyl group which may be substituted by a C1-3 alkyl group,
[21] a C2-3 alkenyloxy group, and
[22] a trifluoromethylsulfonyloxy group, Even more preferably
[1] a hydrogen atom,
[2] a C1-3 alkyl group which may be substituted by 1-3 R5A substituent(s),
[3] a 6-membered heterocycloalkyl group which may be substituted by 1-3 R8B substituent(s),
[4] a 5- to 6-membered heteroaryl group which may be substituted by 1-2 C1-3 alkyl group(s),
[5] a 4- to 6-membered heterocycloalkyl (C0-1 alkyl) aminocarbonyl group which may be substituted by 1-2 oxo group(s),
[6] a 6-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by 1-2 R8D substituent(s),
[7] a (C1-3 alkyl)m8j-amino group (m8j:0-2) which may be substituted by 1-2 R8H substituent(s),
[8] a hydroxyl,
[9] a C1-5 alkoxy group which may be substituted by 1-3 R8E substituent(s),
[10] a 4- to 6-membered heterocycloalkyloxy group which may be substituted by 1-3 R8F substituent(s),
[11] a 6-membered heteroaryloxy group,
[12] a C1-3 alkyl thio group which may be substituted by 1-2 R8I substituent(s),
[13] a C1-3 alkylsulfonyl group which may be substituted by 1-2 R8G substituent(s), and
[14] a C2-3 alkenyloxy group, further preferably
[1] a hydrogen atom,
[2] a C1-3 alkyl group which may be substituted by 1-3 R5A substituent(s),
[3] a 6-membered heterocycloalkyl group which may be substituted by 1-3 R8B substituent(s),
[4] a (C1-3 alkyl)m8j-amino group (m8j:0-2) which may be substituted by 1-2 R8H substituent(s),
[5] a C1-5 alkoxy group which may be substituted by 1-3 R8E substituent(s), and
[6] a 4- to 6-membered heterocycloalkyloxy group which may be substituted by 1-3 R8F substituent(s), Still more preferably
[1] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B described below, Still even more preferably
[1] a 4- to 10-membered heterocycloalkyl group which may be substituted by at least one halogen atom, C1-8 alkyl group, or an oxo. - R5A is preferably
- [8A-1] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-4 R8A1 substituent(s),
[8A-2] a (C1-5 alkyl)m8a-amino group (m8a:0˜2) which may be substituted by 1-11 halogen atom(s), and
[8A-3] a hydroxy; -
- R8A is more preferably
[8A-1] a 6-membered heterocycloalkyl group which may be substituted by 1-2 R8A1 substituent(s),
[8A-2] a (C1-3 alkyl)m8a-amino group (m8a:0˜2) which may be substituted by 1-11 halogen atom(s), and
[8A-3] a hydroxy; R8A1 is preferably
[8A1-1] a C1-5 alkyl group,
[8A1-2] a C1-5 alkylsulfonyl group,
[8A1-3] a (C1-5 alkyl)m8b-aminosulfonyl group (m8b: 0˜2), or
[8A1-4] an oxo group,
more preferably
[8A1-1] a C1-3 alkyl group,
[8A1-2] a C1-3 alkylsulfonyl group, or
[8A1-3] a (C1-3 alkyl)m8b-aminosulfonyl group (m8b: 0),
- R8A is more preferably
- R8B1 is preferably
- [8B-1] a C1-6 alkyl group which may be substituted by 1-13 R8B1 substituent(s),
[8B-2] a C2-6 alkynyl group,
[8B-3] a C3-6 cycloalkyl group which may be substituted by [1] cyano(s) or [2] C1-6 alkyl group(s),
[8B-4] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-10 R8B2 substituent(s),
[8B-5] a C1-6 alkoxy group which may be substituted by 1-5 substituent(s) selected from the group consisting of [1] a C1-5 alkoxy group and [2] a C3-6 cycloalkyl group,
[8B-6] a C1-5 alkoxycarbonyl group,
[8B-7] a C1-5 alkylsulfonyl group,
[8B-8] a 5- to 6-membered heteroarylsulfonyl group,
[8B-9] a cyano,
[8B-10] a C1-6 alkanoyl group which may be substituted by 1-2 R8B3 substituent(s),
[8B-11] a C3-8 cycloalkylcarbonyl group,
[8B-12] a (C1-5 alkyl)m8c-aminosulfonyl group (m8c:0-2),
[8B-13] a C1-6 alkylsulfonyl (C0-6 alkyl) amino group,
[8B-14] a (C1-5 alkyl)m8a-amino group (m8d:0-2) which may be substituted by 1-3 R8B4 substituent(s),
[8B-15] a hydroxy,
[8B-16] a (C1-6 alkyl)m8e-aminocarbonyl group (m8e:0-2), or
[8B-17] a C1-4 alkoxycarbonylamino group more preferably
[8B-1] a C1-5 alkyl group which may be substituted by 1-3 R8B1,
[8B-2] a C2-5 alkynyl group,
[8B-3] a C3-5 cycloalkyl group which may be substituted by [1] a cyano or [2] a C1-6 alkyl group,
[8B-4] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-8 R8B2 substituent(s),
[8B-5] a C1-5 alkoxy group which may be substituted by 1-2 substituent(s) selected from the group consisting of [1] a C1-3 alkoxy group and [2] a C3-6 cycloalkyl group,
[8B-6] a C1-3 alkylsulfonyl group,
[8B-7] a cyano,
[8B-8] a C1-6 alkanoyl group which may be substituted by a R8B3 substituent,
[8B-9] a C3-5 cycloalkylcarbonyl group,
[8B-10] a (C1-3 alkyl)m8c-aminosulfonyl group (m8c:1-2),
[8B-11] a C1-3 alkylsulfonyl (C0-3 alkyl) amino group,
[8B-12] a (C1-5 alkyl)m8a-amino group (m8d:0-1) which may be substituted by 1-2 R8B4 substituent(s),
[8B-13] a hydroxy, or
[8B-14] a (C1-3 alkyl)m8e-aminocarbonyl group (m8e:0-1). - R8B1 is preferably
- [8B1-1] a C3-6 cycloalkyl group,
[8B1-2] a hydroxy,
[8B1-3] a C1-8 alkoxy group which may be substituted by 1-2 C1-5 alkoxy group(s), or
[8B1-4] a cyano, More preferably
[8B1-1] a C3-5 cycloalkyl group,
[8B1-2] a hydroxy,
[8B1-3] a C1-8 alkoxy group which may be substituted by 1 C1-3 alkoxy group, or
[8B1-4] a cyano, - R8B2 is preferably
- [8B2-1] a halogen atom,
[8B2-2] a C1-6 alkyl group,
[8B2-3] an oxo,
[8B2-4] a hydroxy, or
[8B2-5] a deuterium atom,
more preferably
[8B2-1] a fluorine atom,
[8B2-2] a C1-3 alkyl group,
[8B2-3] an oxo, or
[8B2-4] a hydroxyl. - R8B3 is preferably
- [8B3-1] a (C1-6 alkyl)m8f-amino group (m8f:0-2),
more preferably
[8B3-1] a (C1-3 alkyl)m8f-amino group (m8f: 2). - R8B4 is preferably
- [8B4-1] a C3-6 cycloalkyl group, or
[8B4-2] a hydroxy; - R8C is preferably
- [8C-1] a hydroxyl,
[8C-2] a (C1-3 alkyl)m8h-amino group (m8h:0-1) which may be substituted by a (C1-3 alkyl)m8i-aminosulfonyl group (m8i:0-2),
[8C-3] a C1-3 alkylsulfonyl group, - R8D1 is preferably
- [8D-1] a C1-6 alkyl group which may be substituted by a R8D1 substituent,
[8D-2] a hydroxy group,
[8D-3] a C1-3 alkylsulfonyl group, or
[8D-4] a C1-4 alkoxycarbonyl group; - R8D1 is preferably
- [8D1-1] a hydroxy group, or
[8D1-2] a C1-3 alkoxy group; - R8H is preferably
- [8H-1] a 4- to 6-membered heterocycloalkyl group,
- R8E is preferably
- [8E-1] a hydroxy group,
[8E-2] a C1-8 alkoxy group which may be substituted by 1-2 R8E7 substituent(s),
[8E-3] a C1-3 alkylsulfonyl group,
[8E-4] a C1-4 alkoxycarbonyl group,
[8E-5] a 4- to 6-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by 1-2 R8E1 substituent(s),
[8E-6] a (C1-5 alkyl)m8k1-amino group (m8k1: 0˜2) which may be substituted by a R8E2 substituent,
[8E-7] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-4 R8E3 substituent(s),
[8E-8] a 5- to 6-membered heteroaryl group,
[8E-9] a (C1-6 alkyl)m8k2-aminocarbonyl group (m8k2: 0˜2) which may be substituted by 1-2 R8E6 substituent(s),
[8E-10] a C1-5 alkoxy group which may be substituted by a R8E7 substituent,
[8E-11] a C1-3 alkylthio group,
[8E-12] a C1-3 alkylsulfinyl group,
[8E-13] a C1-5 alkylsulfonyl group,
[8E-14] a C1-3 alkylsulfonyl (C0-8 alkyl) amino group,
[8E-15] a 4- to 6-membered heterocycloalkylsulfonyl (C0-3 alkyl) amino group which may be substituted by 1-3 C1-5 alkyl group(s); -
- more preferably
[8E-1] a (C1-3 alkyl)m8k1-amino group (m8k1: 2) which may be substituted by one or more R8E2
[8E-2] a C1-8 alkoxy group which may be substituted by one or more R8E7
[8E-3] a C1-3 alkylsulfonyl group,
[8E-4] a (C1-5 alkyl)m8k1-amino group (m8k1: 0˜2) which may be substituted by a R8E2 substituent,
[8E-5] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-4 R8E3 substituent(s),
[8E-6] a 4- to 6-membered heterocycloalkylsulfonylamino group which may be substituted by 1-2 C1-3 alkyl group(s);
- more preferably
- R8E1 is preferably
- [8E1-1] a C1-4 alkoxycarbonyl group,
[8E1-2] a C1-3 alkanoyl group, 2 0 [8E1-3] a C1-5 alkylsulfonyl group,
[8E1-4] a (C1-3 alkyl)m8k3-aminosulfonyl group (m8k3: 0˜2),
[8E1-5] a 4- to 6-membered heterocycloalkyl group; - R8E2 is preferably
- [8E2-1] a hydroxy group,
[8E2-2] a C1-6 alkoxycarbonyl group,
[8E2-3] a C3-6 cycloalkyl group which may be substituted by a C1-8 alkyl group which may be substituted by a hydroxy,
[8E1-4] a C1-5 alkanoyl group which may be substituted by 1-3 substituent(s) selected from the group consisting of [1] a (C1-3 alkyl)m8k4-amino group (m8k4: 0˜2) and [2] a halogen atom,
[8E2-5] a (C1-3 alkyl)m8k5-aminocarbonyl group (m8k5: 0˜2),
[8E2-6] a C1-3 alkylsulfonyl group,
[8E2-7] a (C1-3 alkyl)m8k6-aminosulfonyl group (m8k6: 0˜1) which may be substituted by a C1-4 alkoxycarbonyl group.
more preferably
[8E2-1] a hydroxy group. - R8E3 is preferably
- [8E3-1] a C1-6 alkyl group which may be substituted by 1-3 substituent(s) selected from the group consisting of [1] a hydroxy group or [2] a C1-3 alkylcarbonyloxy group,
[8E3-2] a C1-4 alkylcarbonyloxy group,
[8E3-3] a hydroxy group,
[8E3-4] a C3-5 cycloalkyl group,
[8E3-5] a C1-4 alkoxycarbonyl group,
[8E3-6] a C1-5 alkylsulfonyl group,
[8E3-7] a (C1-3 alkyl)m8k8-aminocarbonyl group (m8k8: 0˜2),
[8E3-8] a C1-3 alkanoyl group which may be substituted by a hydroxy,
[8E3-9] an oxo group, or
[8E3-10] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-3 substituent(s) selected from the group consisting of [1] a C1-3 alkanoyl group, [2] a C1-4 alkoxycarbonyl group, or [3] a C1-3 alkylsulfonyl group; -
- more preferably
[8E3-1] a (C1-3 alkyl)m8k8-aminocarbonyl group (m8k8: 0˜2), or
[8E3-2] an oxo group;
- more preferably
- R8E4 is preferably
- [8E4-1] a 4- to 6-membered heterocycloalkyl group,
[8E4-2] a C1-3 alkanoyl group,
[8E4-3] a C1-3 alkoxycarbonyl group,
[8E4-4] a C1-3 alkylsulfonyl group,
[8E4-5] a C1-3 alkylaminosulfonyl group; - R8E6 is preferably
- [8E6-1] a C2-3 alkenylcarbonyloxy group,
[8E6-2] a hydroxy group,
[8E6-3] a cyano,
[8E6-4] a (C1-3 alkyl)m89-amino group (m8k9: 0˜2) which may be substituted by 1-2 hydroxy group(s),
[8E6-5] a C1-3 alkoxy group which may be substituted by a hydroxy,
[8E6-6] a C1-4 alkylcarbonyloxy group,
[8E6-7] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-3 C1-8 alkyl group(s),
[8E6-8] a 5- to 6-membered heteroaryl group; - R8E7 is preferably
- [8E7-1] a hydroxy group,
[8E7-2] a C1-3 alkoxy group which may be substituted by a hydroxy; - R8F is preferably
- [8F-1] a C1-5 alkyl group which may be substituted by 1-3 R8F1 substituent(s),
[8F-2] a C3-6 cycloalkyl group,
[8F-3] a C1-3 alkanoyl group which may be substituted by 1-7 halogen atom(s),
[8F-4] a C1-5 alkylcarbonyloxy group,
[8F-5] a C1-5 alkoxycarbonyl group,
[8F-6] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-3 R8F2 substituent(s),
[8F-7] a C1-5 alkylsulfonyl group, or
[8F-8] a hydroxy group; More preferably
[8F-1] a C1-3 alkyl group which may be substituted by a R8F substituent,]
[8F-2] a C3-5 cycloalkyl group,
[8F-3] a 4- to 6-membered heterocycloalkyl group which may be substituted by a R8F2 substituent,
[8F-4] a C1-3 alkylsulfonyl group, - R8F1 is preferably
- [8F1-1] a hydroxy group,
[8F1-2] a C1-8 alkoxy group, or
[8F1-3] a halogen atom; - R8F2 is preferably
- [8F2-1] a 4- to 6-membered heterocycloalkyl group,
[8F2-2] a C1-5 alkoxycarbonyl group, or
[8F2-3] a C1-3 alkylsulfonyl group, - R8G is preferably
- [8G-1] a hydroxycarbonyl group,
[8G-2] a hydroxy group, or
[8G-3] a (C1-5 alkyl)m8l3-amino group (m8l3: 0˜2), - R9 is preferably
- [1] a hydrogen atom,
[2] a C1-8 alkyl group which may be substituted by 1-8 R9A substituent(s),
[3] a C2-6 alkenyl group,
[4] a C2-8 alkynyl group which may be substituted by 1-6 R9C substituent(s),
[5] a C3-6 cycloalkyl group,
[6] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-5 R9D substituent(s),
[7] a C6 aryl group which may be substituted by 1-2 R9E substituent(s),
[8] a 5- to 6-membered heteroaryl group which may be substituted by 1-3 C1-5 alkyl group(s),
[9] a cyano,
[10] a C1-6 alkanoyl group,
[11] a 4- to 6-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by a C1-5 alkyl group,
[12] a halogen atom,
[13] a (C1-4 alkyl)m9c-amino group (m9c: 0˜2) which may be substituted by a R9F substituent,
[14] a hydroxy,
[15] a C1-6 alkoxy group which may be substituted by 1-5 R9G substituent(s),
[16] a 4- to 6-membered heterocycloalkyloxy group which may be substituted by one or two 4- to 6-membered heterocycloalkyl group(s),
[17] a C1-5 alkylthio group which may be substituted by (C1-3 alkyl)m9f-amino group(s) (m9f: 0˜2),
[18] a C1-5 alkylsulfonyl group which may be substituted by (C-3 alkyl)m9g-amino group(s) (m9g: 0˜2),
[19] a (C1-3 alkyl)m9h-aminosulfonyl group (m9h: 0˜2),
[20] a 4- to 6-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by a C1-3 alkyl group; More preferably
[1] a hydrogen atom,
[2] a C1-6 alkyl group which may be substituted by a R9A substituent,
[3] a C2-5 alkenyl group,
[4] a C2-8 alkynyl group which may be substituted by a R9C substituent,
[5] a C3-6 cycloalkyl group,
[6] a 4- to 6-membered heterocycloalkyl group which may be substituted by a R9D substituent,
[7] a 5- to 6-membered heteroaryl group which may be substituted by a C1-5 alkyl group,
[8] a cyano,
[9] a C1-3 alkanoyl group,
[10] a halogen atom,
[11] a (C1-4 alkyl)m9b-n amino group (m9b: 0),
[12] a hydroxy,
[13] a C1-6 alkoxy group which may be substituted by 1-3 R9G substituent(s),
[14] a 4- to 6-membered heterocycloalkyloxy group which may be substituted by a 4- to 6-membered heterocycloalkyl group, Even more preferably
[1] a hydrogen atom,
[2] a C1-8 alkyl group which may be substituted by one or more R9A substituent(s),
[3] a C2-8 alkenyl group which may be substituted by one or more R9B substituent(s),
[4] a C2-8 alkynyl group which may be substituted by one or more R9C substituent(s),
[5] a C3-8 cycloalkyl group,
[6] a halogen atom,
Still more preferably
[1] a hydrogen atom,
[2] a C1-8 alkyl group which may be substituted by one or more R9A substituent(s),
[3] a C2-8 alkynyl group which may be substituted by one or more R9C substituent(s).
R9A is preferably
[9A-1] a C3-6 cycloalkyl group,
[9A-2] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-3 R9A1 substituent(s),
[9A-3] a hydroxy group, or
[9A-4] a C1-6 alkoxy group, More preferably
[9A-1] a 4- to 6-membered heterocycloalkyl group which may be substituted by a R9A1 substituent,
[9A-2] a hydroxy group, or
[9A-3] a C1-3 alkoxy group, - R9A1 is preferably
- [9A1-1] a C1-5 alkyl group,
[9A1-2] a C3-5 cycloalkyl group, or
[9A1-3] a 4- to 6-membered heterocycloalkyl group,
More preferably
[9A1-1] a C1-3 alkyl group,
[9A1-2] a C3 cycloalkyl group, or
[9A1-3] a 4-membered heterocycloalkyl group, - R9C is preferably
- [9C-1] a C1-8 alkoxy group,
[9C-2] a (C1-5 alkyl)m9b-amino group (m9b: 0˜2) which may be substituted by 1-2 C6 aryl group(s),
[9C-3] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-3 R9C1 substituent(s),
[9C-4] a C3-6 cycloalkyl group,
[9C-5] a hydroxy group, or
[9C-6] a hydroxycarbonyl group; More preferably
[9C-1] a C1-5 alkoxy group,
[9C-2] a (C1-3 alkyl)m9b-amino group (m9b: 2) which may be substituted by 1-2 C6 aryl group(s),
[9C-3] a 4- to 6-membered heterocycloalkyl group which may be substituted by 1-2 R91 substituent(s),
[9C-4] a C3-5 cycloalkyl group,
[9C-5] a hydroxy group. - R9C1 is preferably
- [9C1-1] a C3-5 cycloalkyl group,
[9C1-2] a 4- to 6-membered heterocycloalkyl group, or
[9C1-3] an oxo group, More preferably
[9C1-1] a C3 cycloalkyl group,
[9C1-2] a 4- to 6-membered heterocycloalkyl group, or
[9C1-3] an oxo group, - R9D is preferably
- [9D-1] a C1-5 alkyl group which may be substituted by one or two 4- to 6-membered heterocycloalkyl group(s),
[9D-2] a C3-5 cycloalkyl group,
[9D-3] a 4- to 6-membered heterocycloalkyl group, or
[9D-4] a C1-3 alkylsulfonyl group; More preferably
[9D-1] a C1-5 alkyl group which may be substituted by a 4- to 6-membered heterocycloalkyl group,
[9D-2] a 4- to 6-membered heterocycloalkyl group, or
[9D-3] a methylsulfonyl group; - R9E is preferably
- [9E-1] a halogen atom,
[9E-2] a hydroxy group,
[9E-3] a hydroxycarbonyl group, or
[9E-4] a C1-3 alkyl group which may be substituted by a hydroxy; - R9F is preferably
- [9F-1] a C1-3 alkylsulfonyl group,
[9F-2] a (C1-3 alkyl)m9f1-aminosulfonyl group (m9f1: 0˜2), or
[9F-3] a C1-3 alkanoyl group which may be substituted by (C1-3 alkyl)m9f2-amino group(s) (m9f2: 0˜2), - R9G is preferably
- [9G-1] a hydroxy group,
[9G-2] a hydroxycarbonyl group,
[9G-3] a C6 aryl group which may be substituted by C1-3 alkoxy group(s),
[9G-4] a (C1-3 alkyl)m9gl-amino group (m9g1: 0˜2),
[9G-5] a C1-5 alkoxy group which may be substituted by 1-3 R9G1 substituent(s), or
[9G-6] a 5- to 6-membered heteroaryl group; More preferably
[9G-1] a hydroxy group,
[9G-2] a (C1-3 alkyl)m9g1-amino group (m9g1: 0˜2),
[9G-3] a C1-3 alkoxy group which may be substituted by a R9G substituent, or
[9G-4] a 5- to 6-membered heteroaryl group; - R9G1 is preferably
- [9G1-1] a C1-3 alkoxy group, or
[9G1-2] a hydroxycarbonyl group. - Preferably, A5 is NH, while the remaining are C, R3 is a cyano group, R6 and R6′ are methyl, R8 is (1) a hydrogen atom, (2) a C1-8 alkyl group which may be substituted by one or more R8A, (3) a C2-8 alkenyl group, (4) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B, (5) a 5- to 14-membered heteroaryl group which may be substituted by a C1-8 alkyl group, (6) a (C1-8 alkyl)m8g-aminocarbonyl group which may be substituted by one or more RC, (7) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8D, (8) a hydroxycarbonyl group, (9) a C0-8 alkoxy (C0-8 alkyl) aminocarbonyl group which may be substituted by one or more hydroxy group(s), (10) a halogen atom, (11) a hydroxy group, (12) a C1-8 alkoxy group which may be substituted by one or more RE, (13) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by one or more R8F, (14) an aminosulfonyloxy group which may be substituted by one or more C1-8 alkyl group(s), (15) a C1-8 alkyl thio group which may be substituted by a (C1-8 alkyl)-amino group, or (16) a C1-8 alkylsulfonyl group which may be substituted by R8G, R9 is (1) a hydrogen atom, (2) a C1-8 alkyl group which may be substituted by one or more R9A, (3) a C2-8 alkenyl group which may be substituted by one or more R9B, (4) a C2-8 alkynyl group which may be substituted by one or more R9C, (5) a C3-8 cycloalkyl group, (6) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9D, (7) a C6-aryl group which may be substituted by one or more R9E, (8) a 5- to 14-membered heteroaryl group which may be substituted by a C1-8 alkyl group, (9) a cyano group, (10) a C1-8 alkanoyl group, (11) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by a C1-8 alkyl group, (12) a halogen atom, (13) a (C1-8 alkyl)m9e-amino group which may be substituted by one or more R9F, (14) a C1-8 alkylsulfonylamino group, (15) a nitro group, (16) a hydroxy group, (17) a C1-8 alkoxy group which may be substituted by one or more R9G, (18) a C1-8 alkyl thio group which may be substituted by a (C1-8 alkyl)m9f-amino group, (19) a C1-8 alkylsulfonyl group which may be substituted by a (C1-8 alkyl)m9g-amino group, (20) a (C1-8 alkyl)m9h-aminosulfonyl group, or (21) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by a C1-8 alkyl group, and R1, R2, R5, R7 and R10 are defined above.
- More preferably, A5 is NH while the remaining are C, R3 is a cyano group, R6 and R6′ are methyl groups, R8 is (1) a hydrogen atom, (2) a C1-8 alkyl group which may be substituted by one or more R8A, (3) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B, or (4) a C1-8 alkoxy group which may be substituted by one or more R8E, R9 is (1) a hydrogen atom, (2) a C1-8 alkyl group which may be substituted by one or more R9A, (3) a C2-8 alkynyl group which may be substituted by one or more R9C, (4) a C3-8 cycloalkyl group, or (5) a halogen atom, and R1, R2, R5, R7 and R10 are an hydrogen atom.
- According to the present invention, examples of the salts of the compounds that are represented by the Formula (I) include hydrochloric acid salt, hydrobromic acid salt, hydriodic acid salt, phosphoric acid salt, phosphonic acid salt, sulfuric acid salt, sulfonic acid salt such as methanesulfonic acid salt, p-toluene sulfonic acid salt and the like, carboxylic acid salt such as acetic acid salt, citric acid salt, malic acid salt, tartaric acid salt, succinic acid salt, salicylic acid salt and the like, or alkali metal salt such as sodium salt, potassium salt and the like, alkaline earth metal salt such as magnesium salt, calcium salt and the like, ammonium salt such as ammonium salt, alkyl ammonium salt, dialkyl ammonium salt and trialkyl ammonium salt tetraalkyl ammonium salt. Preferably, the salts are pharmaceutically acceptable salts. These salts are produced by brining the compounds described above in contact with an acid or a base which can be used for the production of a pharmaceutical product.
- According to the present invention, the compounds that are represented by the Formula (I) or salts thereof can be an anhydride or a solvate such as a hydrate and the like. Herein, the term “solvate (d)” indicates a phenomenon by which solute molecules or ions contained in a solution strongly attract neighboring solvent molecules to form a huge group of molecules. When the solvent is water, it is called “hydrate (d).” The solvate can be any one of a hydrate and a non-hydrate. Preferably, the solvates are pharmaceutically acceptable solvates. For the non-hydrate, alcohol (for example, methanol, ethanol, n-propanol), dimethylformamide and the like can be used.
- The compounds of the present invention and salts thereof may be present in several tautomer forms, for example, enol and imine form, keto and enamine form, and a mixture thereof. In a solution, a tautomer is present as a mixture of tautomeric set. In case of solid form, one type of tautomer is generally present in dominant ratio. In this regard, even if only one type of tautomer is described, the present invention includes all types of tautomer of the compounds of the present invention.
- The present invention includes all types of stereoisomer of the compounds of the present invention that are represented by the Formula (I) (for example, enantiomer, diastereomer (including cis and trans geometric isomer)), racemate of the isomer and a mixture thereof. For example, the compounds having the Formula (I) of the present invention may have one or more asymmetric center, and the present invention includes a racemic mixture, a diastereomer mixture and enantiomer of such compound.
- When the compounds of the present invention are obtained in free form, they can be converted into a salt, a hydrate or solvate thereof which can be formed from the compounds according to a method generally known in the art.
- Further, when the compounds of the present invention are obtained in the form of a salt, hydrate or solvate of the compounds, they can be converted to free form according to a method generally known in the art.
- The present invention include all isotopes of compounds that are represented by the Formula (I). The isotopes of the compounds of the present invention indicate the compounds of the present invention in which at least one atom is substituted by an atom with the same atomic number (i.e., number of protons) but with different mass number (sum of the number of protons and the number of neutrons). Example of the isotopes that are included in the compounds of the present invention includes a hydrogen atom, a carbon atom, a nitrogen atom, an oxygen atom, a phosphorus atom, a sulfur atom, a fluorine atom, a chlorine atom and the like, and 2H, 3H, 13C, 14C, 15N, 17O, 18O, 31P, 32P, 35S, 18F, 36Cl and the like are included. In particular, a radioisotope which decays by emitting radiation, for example 3H and 14C, are useful for determining the distribution of a pharmaceutical agent or a compound in a living tissue, etc. On the other hand, a stable isotope does not degrade and remains in almost the same amount without exhibiting radioactivity, and therefore can be safely used. Isotopes of the compounds of the present invention can be converted by replacing a chemical reagent used for synthesis with a chemical reagent comprising a corresponding radioisotope according to a method generally known in the art.
- Further, the compounds (I) of the present invention can be administered in the form of prodrug. Herein, the term “prodrug” indicates the derivatives of the compounds having the Formula (I) that can be converted to the compounds having the Formula (I) or salts or solvates thereof after administration by enzymatic or non-enzymatic degradation under a physiological condition. The prodrug can be in inactive form when it is administered to a patient. However, in organisms, it converts to the compounds having the Formula (I) and present therein in the active form.
- For example, the prodrug converts into desired drug form at specific pH or by an enzymatic action. Conventional prodrug is a compound having a hydrolyzable ester residue which produces a free acid in organisms. Examples of such hydrolyzable ester residue include a residue having a carboxyl moiety of which free hydrogen (for example, a free hydrogen in a carboxyl group when Y in the Formula (I) has a carboxyl group) is replaced by a C1-4 alkyl group, a C2-7 alkanoyloxymethyl group, a 1-(alkanoyloxy)ethyl group having 4 to 9 carbon atoms, a 1-methyl-1-(alkanoyloxy)-ethyl group having 5 to 10 carbon atoms, an alkoxycarbonyloxymethyl group having 3 to 5 carbon atoms, a 1-(alkoxycarbonyloxy)ethyl group having 4 to 7 carbon atoms, a 1-methyl-1-(alkoxycarbonyloxy)ethyl group having 5 to 8 carbon atoms, a N-(alkoxycarbonyl)aminomethyl having 3 to 9 carbon atoms, a 1-(N-(alkoxycarbonyl)amino)ethyl group having 4 to 10 carbon atoms, a 3-phthalidyl group, a 4-crotonolactonyl group, a y-butyrolacton-4-yl group, a di-N,N—(C1-2)alkylamino(C2-3)alkyl group (for example, N,N-dimethylaminoethyl group), a carbamoyl(C1-2)alkyl group, a N,N-di(C1-2)alkylcarbamoyl-(C1-2)alkyl group, a piperidino(C2-3)alkyl group, a pyrrolidino(C2-3)alkyl group, or a morpholino(C2-3) alkyl group, but not limited thereto.
- The compounds having the Formula (I) of the present invention can be produced by the method described below, for example. However, method of preparing the compounds of the present invention is not limited thereto. Further, depending on necessity, order of the reaction step like introduction of a substituent group, etc. can be changed. Although the compounds of the present invention are novel compounds which have not been described in literatures, they can be prepared according to a chemical method that is generally known in the art. Still further, as for the reacting compounds that are used for the preparation, commercially available ones can be used or they can be produced according to a method that is generally known in the art depending on necessity.
- In the following reaction schemes showing the reaction step, A1 to A10 and R1 to R10 are as defined in the Formula (I). PR1 to PR10 are the same as R1 to R10 that are defined in the Formula (I) or represent a group which can be converted to R1 to R1I according to modification or deprotection of a functional group.
- Other abbreviated symbols described in the following reaction schemes have the general meanings that can be understood by a skilled person in the art.
- PG represents a protecting group (for example, methyl, ethyl, t-butyl, benzyl, substituted benzyl, acetyl, t-butoxycarbonyl, benzyloxycarbonyl, methanesulfonyl, trifluoromethanesulfonyl, trimethylsilyl, triethylsilyl, triisopropylsilyl, t-butyldimethylsilyl, tetrahydropyranyl and the like). In the preparation method described below, when a defined group is subjected to undesirable chemical modification under a condition for implementing the method, the preparation can be carried out by using means such as protection and deprotection of a functional group, etc.
- Herein, regarding selection, addition and removal of a protecting group include the methods described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4th edition, John Wiley & Sons 2007), and they can be suitably employed according to each reaction condition.
- LG represents a leaving group such as fluorine, chlorine, bromine, iodine, methanesulfonate, trifluoromethanesulfonate and the like, which can be applied for the reaction described above.
- In addition, abbreviated symbols that are typically used to describe the general synthetic method and examples below and names of the chemical reagents and solvents corresponding to the chemical formulae are listed in the following.
- 9-BBN 9-borabicyclo[3.3.1]nonane
- AcOH acetic acid
- BINAP 2,2′-bis (diphenylphosphino)-1,1′-binaphthyl
- BF3OEt2 trifluoroboron etherate
- t-BuOK potassium t-butoxy
- n-BuLi n-butyl lithium
- t-BuONa sodium t-butoxy
- CDI carbonyl diimidazole
- CPME c-pentylmethyl ether
- DBU 1,8-diazabicyclo[5.4.0]-7-undecene
- DCM dichloromethane
- DEAD diethyl azodicarboxylate
- DDQ 2,3-dichloro-5,6-dicyano-p-benzoquinone
- DIPEA N,N-diisopropylethylamine
- DMA N,N-dimethylacetamide
- DME dimethoxyethane
- DMF N,N-dimethyl formamide
- DMSO dimethyl sulfoxide
- DPPF bis (diphenylphosphino)ferrocene
- EDC 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
- EtOAc ethyl acetate
- HOBt 1-hydroxybenzotriazole
- KHMDS potassium hexamethyldisilazide
- LDA lithium diisopropylamide
- LiHMDS lithium hexamethyldisilazide
- MeCN acetonitrile
- Mel methyl iodide
- MeOH methanol
- MTBE t-butylmethyl ether
- NaHMDS sodium hexamethyldisilazide
- NMP N-methylpyrrolidone
- Pd2(dba)3 tris (dibenzylideneacetone) dipalladium (O)
- Pd(OAc)2 palladium acetate
- PdCl2(CH3CN)2 dichloro(bisacetonitrile) palladium (II)
- PdCl2(PPh3)2 dichlorobis (triphenylphosphine) palladium (II)
- Pd(PPh3)4 tetrakis (triphenylphosphine) palladium (O)
- P(t-Bu)3 tri t-butylphosphine
- PPh3 triphenylphosphine
- P(o-tol)3 tri o-tolylphosphine
- TEA triethylamine
- TEMPO 2,2,6,6-tetramethylpiperidin-1-oxyl
- TFA trifluoroacetic acid
- TFAA trifluoroacetic anhydride
- TFE trifluoroethanol
- THF tetrahydrofuran
- TMAD 1,1′-azobis (N,N-dimethylformamide)
- TMSC trimethylsilyl chloride
- TMSI trimethylsilyl iodide
- DavePhos 2-dicyclohexylphosphino-2′-(N,N-dimethylamino)biphenyl
- JohnPhos 2-(di-t-butylphosphino)biphenyl
- c-Hexyl JohnPhos 2-(dicyclohexylphosphino)biphenyl
- S-Phos 2′,6′-dimethoxy-2-(dicyclohexylphosphino)biphenyl
- X-Phos 2′,4′,6′-triisopropyl-2-(dicyclohexylphosphino)biphenyl
- t-ButylX-Phos 2′,4′,6′-triisopropyl-2-(di-t-butylphosphino)biphenyl
- Xantphos 4,5′-bis (diphenylphosphino)-9,9′-dimethylxanthene
- This is one of the methods for producing the compounds of the Formula (I) in which A5 is N and R5 is H.
- It is an alkylation step of a cyclic ketone derivative Ia. The step can be carried out by reacting cyclic ketone derivative Ia with an alkylating agent corresponding to R6 and R6′ in the presence of a base. For example, it can be carried out in view of the method described in Journal of the American Chemical Society, 115(23), 10628-36; 1993 and Organic Letters, 9(24), 5027-5029; 2007, etc. The reaction is carried out in a solvent under the condition of a reaction temperature of −20° C. to boiling point of the solvent, in the presence or the absence of a catalyst. When R6 and R6′ are atomic groups other than a hydrogen atom, the reaction order can be optionally selected, and separation and purification can be carried out at each step or the reaction can be carried out continuously.
- As for the alkylating agent, examples thereof include an alkyl halide such as Mel, ethyl iodide, 2-iodopropane, 1,4-dibromobutane, 1,1′-oxybis (2-bromoethane) and the like, dimethyl sulfate, and sulfonic acid ester such as methylmethanesulfonate, methyl tosylate and methyltrifluoromethanesulfonate. Preferably, it is an alkyl halide such as Mel and the like. As for the catalyst, examples thereof include a phase transfer catalyst such as tetrabutylammonium chloride and tetrabutylammonium hydrogen sulfate. Preferably, it is tetrabutylammonium hydrogen sulfate. As for the base, examples thereof include an inorganic base such as sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, potassium hydride, calcium hydride and the like or an organic base such as t-BuOK, t-BuONa, pyridine, TEA, DIPEA, LDA, LiHMDS and n-BuLi. Preferably, it is potassium hydroxide, potassium t-butoxy, or sodium t-butoxy. As for the solvent, examples thereof include toluene, xylene, n-hexane, cyclohexane, DMF, DMA, EtOAc, DMSO, dichloromethane, carbon tetrachloride, THF, dioxane, acetonitrile, water, methanol, ethanol and a mixture thereof. Preferably, it is a mixture solvent of water-THF or THF.
- It is the synthesis of carbazole skeleton Id according to Fischer method. This step is generally carried out by using cyclic ketone Ib in the presence of hydrazine compound Ic and an acid in a solvent or by using an acid as a solvent under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and also can be carried out in view of the method described in Journal of Heterocyclic Chemistry, 28(2), 321-3; 1991 and Bioorganic & Medicinal Chemistry Letters (2008), 18(24), 6479-6481. Further, when the reaction proceeds slowly, a zinc chloride catalyst and the like can be also used in view of the reaction condition disclosed in Organic Letters (2006), 8(3), 367-370. The reaction consists of a step of producing phenyl hydrazone and a step of sigmatropic rearrangement. Separation and purification can be carried out at each step or the reaction can be carried out continuously. Further, according to the structure of aryl hydrazine, which is a reacting material of this reaction step, mixture of a position isomer can be obtained as a reaction product. Such position isomer can be separated from each other or used as a mixture for the next reaction step.
- As for the acid used for the reaction, examples thereof include formic acid, acetic acid, methanesulfonic acid, p-toluenesulfonic acid, benzenesulfonic acid, TFA, hydrochloric acid, sulfuric acid and pyridinium p-toluenesulfonate. Preferably, it is acetic acid, sulfuric acid, or TFA. As for the solvent, examples thereof include toluene, xylene, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME, TFE, diethylene glycol, triethylene glycol and a mixture thereof.
- It is a step of oxidation at benzyl at 11-position of carbazole skeleton Id. This step is carried out by applying an oxidizing agent to a substrate in a solvent in the presence or absence of a catalyst under the condition of a reaction temperature of −20° C. to boiling point of the solvent. As for the reaction condition, the method described in Journal of Medicinal Chemistry, 51(13), 3814-3824; 2008, etc. can be considered.
- As for the oxidizing agent and the catalyst used for the reaction, DDQ, peracid such as, mCPBA and the like, cerium ammonium nitrate (IV) (CAN), permanganate such as potassium permanganate, barium permanganate and the like, sodium chlorite, hydrogen peroxide, or N-hydroxyphthalimide and the like can be used alone or in a combination thereof. Preferably, it is DDQ or N-hydroxyphthalimide. As for the reaction solvent used for the reaction, examples thereof include water, t-butanol, acetonitrile, THF, dichloromethane, ethyl acetate and a mixture thereof. Preferably, it is THF.
- It is an exemplary method of producing 3-ketoester intermediate IIg, which is used for constructing the skeleton of the compounds that are represented by the Formula (I).
- It is an alkylation step at a position of carboxylic acid ester IIc or nitrile IIa. The step can be carried out by reacting with an alkylating agent corresponding to R6 and R6′ in a solvent under the condition of a reaction temperature of −20° C. to boiling point of the solvent, in the presence of abase. For example, it can be carried out in view of the method described in J. Org. Chem., 2007, 72 (25), 9541-9549 and European Journal of Organic Chemistry (21), 3449-3462, etc. The reagents and the condition for the reaction are the same as those described for Step I-1.
- It is an ester hydrolysis step of carboxylic acid ester IId. This step can be carried out by hydrolysis in an aqueous solvent at the reaction temperature of 0° C. to boiling point of the solvent in the presence of an inorganic base, for example in view of the method described in Tetrahedron Lett. 3529, 1977. Alternatively, it can be carried out according to a method in which hydrolysis is carried out in the presence of an acid, in view of the method described in J. Am. Chem. Soc, 1977, 99, 2353, for example. As for the inorganic base, examples thereof include sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate and cesium carbonate. Preferably, it is sodium hydroxide or potassium hydroxide. As for the solvent, water, methanol, ethanol, tetrahydrofuran, dioxane, and the like can be used alone or in a combination thereof. Preferably, it is methanol comprising water or ethanol comprising water. As for the acid which can be used for acid hydrolysis, hydrochloric acid, sulfuric acid, trifluoroacetic acid and methanesulfonic acid can be used alone in a combination thereof. Preferably, it is sulfuric acid.
- It is a direct (hetero)arylation step at a position of carboxylic acid ester or nitrile. This step can be carried out by SNAr reaction in which carboxylic acid ester or nitrile is reacted with aromatic compound IIf having a leaving group in the presence of a base. It can be carried out in view of the method described in J. Am. Chem. Soc. 2000, 122, 712-713. Alternatively, it can be also carried out according to a method in which carboxylic acid ester or nitrile is reacted with aromatic compound IIf having a leaving group in the presence of a catalyst, a ligand and abase. For example, it can be carried out in view of the method described in Org. Lett, 2008, 10(8), 1545, J. Org. Chem. 2003, 68, 8003 and Angew. Chem. Int. Ed. 2003, 42, 5051, etc.
- As for the base used for the reaction, sodium phosphate, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, LiHMDS, NaHMDS, LDA, lithium dicyclohexylamide, lithium 2,2,6,6-tetramethyl pyrrolidide, KHMDS, t-BuONa, t-BuOK and the like can be used. Preferably, it is NaHMDS, KHMDS, or t-BuONa. As for the catalyst, ligand, or catalyst-ligand complex which are used for the reaction, palladium acetate, Pd2(dba)3, π-allyl palladium chloride dimer, PdCl2(CH3CN)2, trialkylproazaphosphatrane, {P(t-Bu)3PdBr}2, PPh3, P(o-tol)3, BINAP, DPPF, P(t-Bu)3, DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene, 1,3-diallyldihydroimidazolium salt and the like can be used, for example. Preferably, it is triisobutylproazaphosphatrane.
- It is a step of hydrolyzing nitrile IIb to carboxylic acid. This step can be carried out by hydrolysis in the presence of an acid under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and the reaction conditions include that described in, for example, Tetrahedron, 64(36), 8464-8475; 2008, etc. For the reaction, the acid itself can be used as a solvent or diluted with other solvent. Alternatively, it can be carried out by hydrolysis in the presence of an inorganic base under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and the reaction condition described in, for example, Bioorganic & Medicinal Chemistry Letters, 18(2), 749-754; 2008, etc. can be employed.
- The reaction consists of the hydrolysis of nitrile IIb to acid amide and further conversion into carboxylic acid. Separation and purification can be carried out at each step or the reaction can be carried out continuously.
- As for the acid which is used for the reaction, examples thereof include methanesulfonic acid, p-toluenesulfonic acid, benzenesulfonic acid, trifluoroacetic acid, hydrochloric acid and sulfuric acid. As for the solvent, examples thereof include toluene, xylene, dioxane, dimethoxyethane, diethylene glycol, triethylene glycol, TFE and the like and a mixture thereof. As for the inorganic base, examples thereof include sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate and cesium carbonate.
- It is a step of converting carboxylic acid IIe to β-ketoester. According to this step, the carboxylic acid as a reacting material is converted into an acid chloride, active ester and the like by an action of an activating agent in a solvent under the condition of a reaction temperature of 0° C. to boiling point of the solvent. Thereafter, the acid chloride or active ester is reacted with enolate of malonic acid monoester under the condition of a reaction temperature of 0° C. to boiling point of the solvent to give a target compound through decarboxylation. As for the reaction condition, a method described in J. Chem. Soc. Perkin Trans. 1 1988, 2345-2352 and Synthesis 1993, 290-292 can be used, for example. As for the method of activating carboxylic acid, examples thereof include a method of converting into an acid chloride by using thionyl chloride, oxalyl chloride, phosphorus oxychloride, etc. or a method of converting into an active ester by using CDI. Preferably, thionyl chloride or CDI is used. The activated carboxylic acid itself can be subjected to separation and purification, or can be used continuously for the next reaction. As for the method of producing an enolate of malonic acid monoester, a combination of magnesium salt like magnesium chloride, etc. and malonic acid monoester (and a salt thereof) or a Grignard reagent like i-propyl magnesium chloride, etc. and malonic acid monoester (and a salt thereof), etc. can be used. In order to improve the reaction yield, an organic base such as TEA, DIPEA and the like can be also added to the reaction system. As for the solvent, examples thereof include toluene, xylene, MeCN, THF, CPME, MTBE, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME, and the like and a mixture thereof. Preferably, it is MeCN, THF or DME.
- It is a step of converting nitrile IIb to β-ketoester. This step can be carried out by so-called Blaise reaction in which 2-halo carboxylic acid ester is reacted with nitrile in the presence of activated zinc powder under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and the reaction method described in, for example, SYNTHESIS 2004, No. 16, pp 2629-2632x. can be used. As for the method of activating zinc powder, a method in which acid washing and drying are carried out in advance, or a method in which a catalytic amount of an acid such as methanesulfonic acid, etc. is included in the reaction system can be employed.
- It is an exemplary method of preparing compound IIIh from intermediate IIg that is obtained from Preparation method II.
- This step can be carried out by nucleophilic aromatic substitution reaction in which an aromatic nitro compound having a leaving group is reacted with β-ketoester IIg in the presence of a base under the condition of a reaction temperature of 0° C. to boiling point of the solvent, and the reaction method include that described in, for example, Synlett, (5), 883-885; 2004 and Tetrahedron, 38(23), 3479-83; 1982.
- As for the base used for the reaction, sodium phosphate, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, LiHMDS, NaHMDS, LDA, lithium dicyclohexylamide, lithium 2,2,6,6-tetramethylpyrrolidide, KHMDS, t-BuOK, t-BuONa and the like can be used. Preferably, it is potassium carbonate, cesium carbonate, t-BuOK, or t-BuONa. As for the solvent, examples thereof include toluene, xylene, MeCN, THF, CPME, MTBE, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME, acetone, methylethyl ketone, and a mixture thereof. Preferably, it is THF, DMF, DMA, NMP or a mixture thereof.
- Further, the present step can be also carried out in the presence of a catalyst and a base, as described in Step III-5 or Journal of Organic Chemistry, 72(14), 5337-5341; 2007.
- It is a reductive cyclization step to form an indole ring following the reduction of a nitro group. This reaction can be carried out by reacting β-ketoester IIIa with a reducing agent under the condition of a reaction temperature of 0° C. to boiling point of the solvent to reduce the nitro group. As for the reducing agent used for the reaction, the condition generally used for reduction of a nitro group, for example, iron as exemplified in Synthesis, (18), 2943-2952, 2008, zinc as exemplified in Tetrahedron, 64(40), 9607-9618, 2008, titanium (III) chloride as exemplified in Organic & Biomolecular Chemistry, 3(2), 213-215, 2005, tin (II) chloride as exemplified in Journal of Organic Chemistry, 58(19), 5209-5220,1993, sodium hydrosulphite as exemplified in Gazzetta Chimica Italiana, 121(11), 499-504, 1991, and catalytic reduction condition as exemplified in Synlett, (17), 2689-2691, 2008, etc. can be employed. Preferably, the reducing agent is iron or sodium hydrosulphite.
- It is a step of deprotecting an ester protecting group of indole-3-carboxylic acid ester IIb. As an example of an ester protecting group, a methyl group, an ethyl group, a t-butyl group, a benzyl group, a substituted benzyl group and the like can be used. Preferably, it is a t-butyl. As for the deprotection, examples thereof include a method described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4th edition, John Wiley & Sons 2007), and it can be appropriately used according to each reaction condition. When the ester protecting group is a t-butyl, as a deprotection condition, TMSI, TMSCl, and BF3.OEt2 can be used. As for the solvent, examples thereof include toluene, xylene, diethyl ether, THF, CPME, MTBE, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME, TFE and the like and a mixture thereof. Preferably, it is THF or TFE.
- It is a step of cyclizing indole-3-carboxylic acid IIIc to carbazole based on Friedel-Crafts reaction. According to the reaction, a mixed acid anhydride is formed by using acetic anhydride, trifluoroacetic anhydride and the like, or acid chloride is formed by using thionyl chloride, oxalyl chloride, phosphorus oxychloride and the like, which results in activiation of the carboxylic acid. Preferably, acetic anhydride or trifluoroacetic anhydride is used. The reaction is carried out in the absence or presence of a solvent. As for the solvent, examples thereof include toluene, xylene, diethyl ether, THF, CPME, MTBE, NMP, DMF, DMA, DMSO, sulfolane, dioxane, DME and the like and a mixture thereof. Preferably, it is THF, DMF, DMA or DME. Further, an organic base such as TEA, DIPEA, pyridine and the like can be used.
- Thereafter, under the condition of a reaction temperature of 0° C. to boiling point of the solvent, the cyclization is carried out without a catalyst or with Bronsted acid or Lewis acid catalyst (Heterocycles 1999, 51, 2127). As for the Lewis acid catalyst, examples thereof include aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate and BF3.OEt2. Preferably, it is BF3.OEt2. Depending on the type of a substituent group, it is also possible to carry out the reaction by applying methanesulfonic acid-phosphorus pentoxide (Eaton reagent), polyphosphoric acid and the like to indole-3-carboxylic acid ester IIIb without undergoing Step 111-3.
- The step can be carried out by reacting an aromatic acylamide compound having a leaving group with β-ketoester IIg in the presence of a base, a catalyst, and a ligand under the condition of a reaction temperature of 0° C. to boiling point of the solvent, followed by deprotection of an acyl protecting group. Examples thereof include a method described in Journal of Organic Chemistry 2007, 72, 9329-9334 and Organic Letters 10(4), 625-628, 2008. As for the metal catalyst, copper (I) iodide and palladium acetate can be used. As for the ligand, (S)-proline, tri t-butylphosphine, bis (t-butyl) (2′-methyl[1,1′-biphenyl]-2-yl)phosphine and the like can be used. As for the base which is used for the reaction, sodium phosphate, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, LiHMDS, NaHMDS, LDA, lithium dicyclohexylamide, lithium 2,2,6,6-tetramethylpyrrolidide, potassium hexamethyldisilazide, t-BuONa, t-BuOK and the like can be used.
- It is a step of reacting an aromatic amino compound with β-ketoester IIg to form an enamine intermediate followed by catalytic cyclization. Examples thereof include a method described in Journal of Organic Chemistry, 68(15), 6011-6019; 2003 and European Journal of Organic Chemistry, (24), 3977-3980; 2007.
- Alternatively, the cyclization can be carried out based on an oxidative method. For example, a reaction condition described in Angewandte Chemie, International Edition, 47(38), 7230-7233; 2008 can be also employed, for example.
- It is a step of synthesizing 1,3-diketone based on cyclization of β-keto ester IIg. As for the condition and reagents for the reaction, a method described in Bioorganic & Medicinal Chemistry Letters, 18(2), 568-570; 2008 wherein β-keto ester IIg is reacted in an solvent in the presence of Bronsted acid catalyst or Lewis acid catalyst, or a method of using a condensing agent such as methanesulfonic acid-phosphorus pentoxide (Eaton reagent), polyphosphoric acid and the like can be employed.
- This step can be carried out in the same manner as Step III-1 and III-2 or Step III-5 and III-6.
- An exemplary method of producing compound IIIh wherein formula Iva is employed as a starting material.
- It is a step of constructing di-substituted indole derivatives based on Sonogashira reaction in which a terminal alkyne is reacted with aromatic amine derivative IVa having a leaving group at ortho position in the presence of a base and a catalyst with or without a catalytic amount of a copper reagent. Specifically, examples thereof include a method described in Organic Letters, 11(1), 221-224; 2009. The reaction is carried out in an appropriate solvent in the presence of a palladium catalyst and a ligand (or a complex thereof) with or without a base and a copper catalyst. Example of the copper catalyst used for the reaction include copper iodide. As an example of the catalyst and the ligand (or a complex thereof), palladium acetate, Pd2(dba)3, r-allyl palladium chloride dimer, PdCl2(CH3CN)2, PdCl2(PPh3)2, trialkylproazaphosphatrane, {P(t-Bu)3PdBr}2, PPh3, P(o-tol)3, BINAP, DPPF, P(t-Bu)3, DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene, 1,3-diallyldihydroimidazolium salt and the like can be used. As for the base used for the reaction, sodium phosphate, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, TEA, DIPEA and the like can be used. Preferably, it is cesium carbonate, TEA or DIPEA.
- This step corresponds to a tandem Friedel-Crafts reaction in which acylation at 3-position of di-substituted indole derivative IVb is carried out in the presence of Lewis acid catalyst under the condition of a reaction temperature of 0° C. to boiling point of the solvent, followed by intramolecular cyclization. As for the catalyst used for the reaction, examples thereof include aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate and BF3.OEt2. Preferably, it is aluminum chloride.
- This step consists of deprotection of carboxylic acid ester comprised in di-substituted indole derivative IVc and subsequent intramolecular cyclization at 3-position of the indole either in catalytic or non-catalytic manner. As for the deprotection, examples thereof include a method described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4th edition, John Wiley & Sons 2007), and it can be appropriately used according to the type of each protecting group. When an activated indole derivative is used for the reaction, cyclization occurs more easily so that the reaction can be carried out in a non-catalytic manner. Further, the cylclization can be also carried out by using a condensing agent such as polyphosphoric acid, methanesulfonic acid-phosphorus pentoxide (Eaton reagent) and the like. Alternatively, it is also possible that carboxylic acid is first converted into carboxylic acid chloride, a mixed acid anhydride and the like under the same condition as defined in Step III-4 and the cyclization is carried out under Friedel-Crafts condition in the presence of Lewis acid catalyst. As for the Lewis acid catalyst used for the reaction, examples thereof include aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate and BF3.OEt2.
- It is one of the methods for constructing the skeleton of the compounds having the Formula (I) in which A5 is O, S or NH.
- It is a step of arylation of cyclic ketone derivative Ib using aromatic compound Va having a leaving group. The reaction is catalytically carried out in the presence of a base with combination of a transition metal catalyst and a ligand, and the condition described in J. Am. Chem. Soc. 2000, 122, 1360-1370 and Journal of Organic Chemistry (2003), 68(25), 9865-9866 can be used, for example. As for the base used for the reaction, examples thereof include t-BuONa, t-BuOK, LiHMDS, NaHMDS, potassium phosphate, sodium carbonate, potassium carbonate and cesium carbonate. As for the catalyst and a ligand (or a catalyst-ligand complex), palladium acetate, Pd2(dba)3, π-allylpalladium chloride dimer, PdCl2(CH3CN)2, PdCl2(PPh3)2, trialkylproazaphosphatrane, {P(t-Bu)3PdBr}2, PPh3, P(o-tol)3, BINAP, DPPF, P(t-Bu)3, DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene 1,3-diallyldihydroimidazolium salt and the like can be used.
- It is a step of deprotecting a protecting group. When A5 is O or S, a t-butyl group, a benzyl group and a substituted benzyl group can be used as a protecting group. When A5 is O, a t-butyldimethylsilyl group and a tetrahydropyranyl group can be used. When it is NH, a t-butoxycarbonyl group, a benzyloxycarbonyl group, a methanesulfonyl group, a trifluoroacetyl group and the like can be used. As for the deprotection, examples thereof include a method described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4th edition, John Wiley & Sons 2007), and it can be appropriately used according to the type of each protecting group.
- It is a cyclization step of cyclic α-aryl ketone derivative Vc to a benzofuran derivative, benzothiophene or indole Vd. The reaction can be carried out under condition of using an acid catalyst or dehydrating condition. For example, the reaction condition described in Acta Pharmaceutica Hungarica (2003), 73(3), 171-178 can be employed. In addition, depending on the type of a protecting group for hydroxyl group, it can be carried out simultaneously with the deprotection of Step V-2, as described in Heterocycles, 26(7), 1863-71; 1987. With respect to the condition for dehydration, a combination of an organic base and an acid anhydride such as trifluoromethanesulfonic acid and the like can be used.
- It is a step of oxidation at benzyl at 11-position of tetracyclic compound Vd. This step is carried out by applying an oxidizing agent to a substrate in a solvent in the presence or absence of a catalyst under the condition of a reaction temperature of −20° C. to boiling point of the solvent. As for the reaction condition, the method described in Journal of Medicinal Chemistry, 51(13), 3814-3824; 2008, etc. can be employed.
- As for the oxidizing agent and the catalyst used for the reaction, DDQ, peracid such as, mCPBA and the like, cerium ammonium nitrate (IV) (CAN), permanganate such as potassium permanganate, barium permanganate and the like, sodium chlorite, hydrogen peroxide, N-hydroxyphthalimide and the like can be used alone or in a combination thereof. As for the solvent used for the reaction, examples thereof include water, t-butanol, acetonitrile, tetrahydrofuran, dichloromethane, ethyl acetate and a mixture thereof.
- It is an exemplary method of constructing the skeleton of the compounds that are represented by the Formula (I) in which A5 is S.
- It is a reaction to construct a benzothiophene ring based on the reaction between ylide VIa having a thiol at ortho position and acyl chloride VIb. The reaction can be carried out in the presence of a base, and the condition include that described in Synthesis, (2), 155-7; 1988, for example. As for the base, examples thereof include n-butyl lithium, sodium methylate and triethylamine.
- It is a reaction for the synthesis of an aromatic carboxylic acid. The reaction can be carried out by metallization like addition of lithium or magnesium based on exchange between halogen and metal in the presence of a base, followed by carboxylation using carbonate gas, dry ice, etc. The reaction condition as described in Journal of Organic Chemistry (2008), 73(19), 7785-7788 can be employed. As for the base, n-butyl lithium, s-butyl lithium, t-butyl lithium, a Grignard reagent, and various ate complexes can be used. Alternatively, as described in e-EROS Encyclopedia of Reagents for Organic Synthesis 2001 (electronic edition; https://www3.interscience.wiley.com/cgi-bin/mrwhome/104554785/HOME), carboxylation condition using a transition metal catalyst can be also employed.
- This step corresponds to intramolecular cyclization at 3-position of di-substituted benzothiophene derivative VId either in catalytic or non-catalytic manner. For example, the reaction condition as described in Journal of the American Chemical Society, 130(23), 7286-7299; 2008 can be employed. The reaction can be carried out by using a condensing agent such as polyphosphoric acid, methanesulfonic acid-phosphorus pentoxide (Eaton reagent) and the like. Alternatively, it is also possible that carboxylic acid is first converted into carboxylic acid chloride, a mixed acid anhydride and the like and the cyclization is carried out under Friedel-Crafts condition in the presence of Lewis acid catalyst. As for the Lewis acid catalyst used for the reaction, examples thereof include aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate and BF3.OEt2.
- To the functional groups PR1 to PR10 in the Formula (I) of the present invention, various substituent groups can be introduced based on a method of converting and modifying a functional group that is well known to a skilled person in the pertinent art. Hereinbelow, representative examples of functional group conversion will be explained. Further, although the following reaction scheme is specific in that examples of PR8 and PR9 are given for the tetracyclic compound that is already constructed, it can be also carried out to an intermediate during any steps explained in Preparation methods I to VI above or to a final compound. Further, it can be carried out at any substitution position of PR1 to PR4 and R6 to PR10.
- In the following formula, Q1 and Q2 represent any substituent group which constitutes PR1 to PR4 and R6 to PR10.
- It is a step of deprotecting a protecting group for an aromatic hydroxyl group. As an example of the protecting group, a methyl group, a t-butyl group, a benzyl group, a substituted benzyl group, a t-butyldimethylsilyl group, a tetrahydropyranyl group and the like can be used. Preferably, it is a methyl group. As for the deprotection, examples thereof include a method described in “Protective Groups in Organic Synthesis” (Greene and Wuts, 4th edition, John Wiley & Sons 2007), and it can be appropriately used according to the type of each protecting group. When a methyl group is used as a protecting group, various reaction conditions can be used selectively for the deprotection depending on reactivity. Examples thereof include heating in the presence of pyridine hydrochloric acid salt, heating in the presence of a solvent with dodecane thiol and sodium methylate and heating in the presence of a solvent with anhydrous lithium halide, boron tribromide, TMSI and the like.
- It is one of the methods for introducing a substituent group based on formation of ether bond with an aromatic hydroxyl group. For the formation of an ether bond, Mitsunobu reaction described in a known literature (Mitsunobu, et. al., Synthesis, Vol. 1, page 1, 1981) or a similar method can be used. Specifically, the reaction is carried out in the presence of a phosphorus compound and an azo compound in a solvent under the condition of a reaction temperature of −78° C. to boiling point of the solvent. As for the phosphorus compound, examples thereof include PPh3 and tri-n-butylphosphine. As for the azo compound, examples thereof include DEAD, TMAD and diisopropyl azodicarboxylic acid. Also, by using them in any combination, the target compound can be obtained.
- It is a step of carrying out trifluoromethane sulfonylation on an aromatic hydroxyl group. The reaction is carried out by reacting with a reacting reagent such as trifluoromethanesulfonic acid and the like in the presence of a base with or without a solvent under the condition of a reaction temperature of −20° C. to boiling point of the solvent. As for the base used for the reaction, TEA, DIPEA, pyridine, 2,6-lutidine, dimethylaminopyridine and the like can be used. Preferably, pyridine is used without any solvent. The obtained trifluoromethanesulfonic acid ester VIId is a good leaving group and can be used for various derivatization.
- It is a step of obtaining sulfamic acid ester by carrying out sulfamoylation on an aromatic hydroxyl group. The reaction is carried out by reacting with a reacting reagent such as sulfamoyl chloride and the like in the presence of a base with a solvent under the condition of a reaction temperature of −20° C. to boiling point of the solvent. As for the base used for the reaction, sodium hydride, TEA, DIPEA, pyridine, 2,6-lutidine, dimethylaminopyridine and the like can be used. Preferably, it is sodium hydride. The obtained sulfamic acid ester VIIe is a substrate for the thiaFries rearrangement of Step VII-5 and can be used for various derivatization.
- This step corresponds to rearrangement of a sulfamoyl group to a neighboring position in the presence of a Lewis acid catalyst under the condition of a reaction temperature of 0° C. to boiling point of the solvent when the neighboring position of the sulfamic acid ester is unsubstituted (i.e., C—H), i.e., a reaction called thiaFries rearrangement. As for the catalyst used for the reaction, aluminum chloride, aluminum triflate, bismuth triflate, ytterbium triflate, BF3.OEt2 and the like can be used. Preferably, it is aluminum chloride.
-
- It is another step of introducing a substituent group based on formation of an ether bond. According to the present step, a reagent having an appropriate leaving group such as alkyl halide and the like is subjected to nucleophilic reaction with the hydroxyl group of compound VIIb in the presence of an appropriate base to form an ether bond. As for the base, examples thereof include an inorganic base such as sodium carbonate, potassium carbonate, cesium carbonate, sodium hydride, potassium hydride, calcium hydride and the like or an organic base such as pyridine, TEA, DIPEA and the like.
- Further, by using aryl halide, aryl borate and the like as a reagent having a leaving group, formation of an diaryl ether bond can be also achieved and used. When reactivity is not satisfactory, a catalyst such as copper powder, copper acetate, copper iodide and the like or a ligand such as phenanthroline, trans-1,2-cyclohexanediamine and the like can be used.
- It is a reaction for forming a bond between aryl and a hetero atom by using compound VIIg having a leaving group. The reaction is carried out in an appropriate solvent inert to the reaction, in the presence of a base. As for the leaving group LG, a halogen, triflate and the like can be used. As for the solvent, examples thereof include toluene, xylene, n-hexane, cyclohexane, DMF, DMA, EtOAc, DMSO, NMP, THF, DME, dioxane, acetonitrile and the like and a mixture thereof. As for the base to be used for the reaction, examples thereof include t-BuONa, t-BuOK, LiHMDS, NaHMDS, KHMDS, potassium phosphate, sodium carbonate, potassium carbonate and cesium carbonate. This step can be also carried out by using a catalyst and a ligand. As for the catalyst and a ligand (or a catalyst-ligand complex), palladium acetate, Pd2(dba)3, π-allylpalladium chloride dimer, PdCl2(CH3CN)2, PdCl2(PPh3)2, trialkylproazaphosphatrane, {P(t-Bu)3PdBr}2, PPh3, P(o-tol)3, BINAP, DPPF, P(t-Bu)3, DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene, 1,3-diallyldihydroimidazolium salt and the like can be used, for example.
- When the reaction product of Step VII-7 is thio ether VIIh, it is possible to obtain sulfoxide or sulfone compound VIIj by oxidation with m-chloro perbenzoic acid, oxone, TEMPO and the like.
-
- It is a reaction for forming a bond between aryl and SP2 carbon or a bond between aryl and SP3 carbon in which compound VIIg having a leaving group is used. The reaction is carried out in an appropriate solvent inert to the reaction, in the presence of a base. As for the leaving group LG, a halogen, triflate and the like can be used. As for the solvent, examples thereof include toluene, xylene, n-hexane, cyclohexane, DMF, DMA, EtOAc, DMSO, NMP, THF, DME, dioxane, acetonitrile, water, isopropanol and the like and a mixture thereof. As for the base to be used for the reaction, examples thereof include t-BuONa, t-BuOK, LiHMDS, NaHMDS, KHMDS, potassium phosphate, sodium carbonate, potassium carbonate, cesium carbonate, TEA and DIPEA. This step can be also carried out by using a catalyst and a ligand. As for the catalyst and a ligand (or a catalyst-ligand complex), palladium acetate, Pd2(dba)3, π-allylpalladium chloride dimer, PdCl2(CH3CN)2, PdCl2(PPh3)2, trialkylproazaphosphatrane, {P(t-Bu)3PdBr}2, PPh3, P(o-tol)3, BINAP, DPPF, P(t-Bu)3, DavePhos, JohnPhos, c-Hexyl JohnPhos, S-Phos, X-Phos, t-ButylX-Phos, Xantphos, 4,5-bis[bis (3,5-bistrifluoromethylphenyl)phosphanyl]-9,9-dimethyl-9H-xanthene, 1,3-diallyldihydroimidazolium salt and the like can be used, for example.
- It is a carboxylation reaction using compound VIIg having a leaving group. The reaction is carried out by reacting with formic acid (or a synthetic equivalent thereof) in an appropriate solvent inert to the reaction, in the presence of a base and a catalyst. As for the leaving group LG, a halogen, triflate and the like can be used. The solvent and the catalyst can be selected and used in the same manner as Step VII-9.
- It is an amidation reaction using carboxylic acid VIIm. Specifically, the reaction can be carried out by dehydrating condensation reaction using various amines such as ammonia, primary amines, secondary amines, hydrazines, substituted hydrazines and the like. The reaction is carried out in the presence of an acid halogenating agent or a dehydrating condensing agent in an aprotic solvent under the condition of a reaction temperature of −20° C. to boiling point of the solvent, with or without an active esterifying agent and a base.
- As for the acid halogenating agent, examples thereof include oxalyl chloride and thionyl chloride. As for the dehydrating condensing agent, examples thereof include 1,3-dicyclohexylcarbodiimide (DCC), 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ), bromo-tris (pyrrolidino)-phosphonium hexafluorophosphate (PyBrOP), EDC and (benzotriazolyloxy)tripyrrolidino-phosphonium=hexafluorophosphate (PyBOP). As for the active esterifying agent, examples thereof include HOBt, di(N-succinimidyl) carbonate and carbonyl diimidazole. As for the base, examples thereof include TEA, DIPEA and DBU. As for the solvent, examples thereof include DMF, DMA, DCM, acetone, THF, dioxane, DME, ethyl acetate, MeCN, and a mixture thereof.
-
- It is a step of forming a bond between aryl and SP carbon using compound VIIo having a leaving group. The reaction is carried out by reacting terminal alkyne in an appropriate solvent in the presence of a base and a catalyst with or without a catalytic amount of a copper reagent, and the reaction is referred to as Sonogashira reaction. The reagents and the condition for the reaction are as defined in Step IV-1 and Step IV-3. As a variant of Sonogashira reaction, examples thereof include a method disclosed in Tetrahedron, 63(43), 10671-10683; 2007. Specifically, by having secondary amines and the like in a reaction system and using propargyl bromide as an alkyne, a propargyl amine can be introduced.
-
- It is a reaction of forming a bond between aryl and CN by using compound VIIo having a leaving group. The reaction can be carried out by adding CN− source in an appropriate solvent in the presence of a copper, zinc or palladium catalyst, with or without a ligand, in view of the reaction condition shown in Organic Letters, 10(23), 5325-5328; 2008, Tetrahedron Letters, 49(32), 4693-4694; 2008 and Bioorganic & Medicinal Chemistry, 16(13), 6489-6500; 2008. As for the CN−-source, copper (I) cyanide, zinc (II) cyanide, iron (III) hexacyanide, sodium cyanide, potassium cyanide and the like can be used.
- Some of the starting materials for the present invention are novel compounds, and they can be easily synthesized in the same manner as known reacting compounds or according to the method well known to a skilled person in the art.
- Hereinabove, examples of a method of preparing the compounds having the Formula (I) according to the present invention are described. However, separation and purification of the target compounds that are described in detail in each reaction step can be performed by applying common chemical treatments such as extraction, concentration, removal by distillation, crystallization, filtration, recrystallization, various chromatography, etc.
- The pharmaceutical composition of the present invention comprises a pharmaceutically acceptable carrier, in addition to the compound that is selected as being useful for the invention. In the present specification, the term “pharmaceutically acceptable carrier” means one or more type of appropriate solid or liquid vehicle, diluent or an encapsulating material which is suitable for administration to mammals. In the present specification, the term “acceptable” means that it does not cause any reaction to substantially reduce the pharmaceutical efficacy of a composition under normal condition for use, and the components of the composition and the subject compound can be mixed well with each other. The pharmaceutically acceptable carrier should have substantially high purity and substantially low toxicity so that it can be suitably administered to a subject to be treated, preferably an animal, and more preferably a mammal.
- As the materials which can be used as a pharmaceutically acceptable carrier, examples thereof include sugars such as lactose, glucose, sucrose, and the like; starch such as corn starch, potato starch and the like; cellulose and cellulose derivatives such as sodium carboxy methyl cellulose, ethyl cellulose, methyl cellulose and the like; tragacanth rubber powder; malt; gelatin; talc; solid lubricating agent such as stearic acid or magnesium stearate and the like; calcium sulfate; vegetable oils such as peanut oil, cotton seed oil, sesame oil, olive oil, corn oil, plant oil, cacao oil, and the like; polyhydric alcohols such as propylene glycol, glycerin, sorbitol, mannitol, polyethylene glycol and the like; alginic acid; an emulsifying agent such as TWEEN; humectant such as lecithin and the like; colorant; flavor; tabletting agent; stabilizer, anti-oxidant; preservative; pyrogen-free water; aqueous isotonic solution and phosphate buffer solution.
- When the pharmaceutical composition of the present invention is used as an ALK inhibitor or a therapeutic or prophylactic agent for a proliferative disorder, or used against depression or cognitive function disorder, as an administration route, oral, rectal, parenteral (intravenous, intramuscular, subcutaneous), intracisternal, intravaginal, intraperitoneal, intrabladder, topical (drop, powder, ointment, gel or cream) administration or administration via inhalation (mouth or nasal spray) and the like can be considered. As for the administration form, examples thereof include a tablet, a capsule, a granule, powder, a pill, an aqueous or non-aqueous oral solution and suspension, and a parenteral solution which is filled in a container suitable to be divided into several small dosages. In addition, the administration form can be modified for various administration routes including subcutaneous transplant which gives controlled release of a drug compound.
- The aforementioned preparation is prepared according to a method generally known in the art by using additives such as a vehicle, a lubricating agent (i.e., coating agent), a binding agent, a disintegrating agent, a stabilizing agent, a corrigent for taste and smell, a diluent and the like.
- As a vehicle, examples thereof include starch such as starch, potato starch, corn starch, lactose, crystalline cellulose and calcium hydrogen phosphate.
- As a coating agent, examples thereof include ethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose, shellac, talc, carnauba wax and paraffin.
- As a binding agent, examples thereof include polyvinyl pyrrolidone, Macrogol and the compounds described above as a vehicle.
- As a disintegrating agent, examples thereof include the compounds described as a vehicle in the above and a chemically modified starch or cellulose such as sodium croscarmellose, sodium carboxymethyl starch and crosslinked polyvinyl pyrrolidone.
- As a stabilizing agent, examples thereof include paraoxy benzoic acid esters such as methyl paraben, propyl paraben and the like; alcohols such as chlorobutanol, benzyl alcohol, phenylethyl alcohol and the like; benzalkonium chloride; phenols such as phenol, cresol and the like; thimerosal; dehydroacetic acid; and sorbic acid.
- As a corrigent for taste and smell, examples thereof include a sweetener, an acid tasting agent, a flavor and the like that are commonly used in the art.
- Further, as a solvent to prepare a liquid preparation, examples thereof include ethanol, phenol, chlorocresol, purified water and distilled water.
- As a surface active agent or an emulsifying agent, examples thereof include polysorbate 80, polyoxyl 40 stearate and lauromacrogol.
- When the pharmaceutical composition of the present invention is used as an ALK inhibitor or a therapeutic or prophylactic agent for a proliferative disorder, or used against depression or cognitive function disorder, the use amount of the compounds of the present invention or salts or solvates thereof varies depending on symptom, age, body weight, relative health state of a subject, administration of other drug compounds, administration method and the like. For example, the amount which is generally effective for a patient (i.e., warm-blooded animal, in particular human) is, in an effective component (i.e., the compound of the present invention that is represented by the Formula (I)), preferably 0.001 to 1000 mg per 1 kg body weight per day, more preferably 0.01 to 300 mg per 1 kg body weight per day in case of an orally administered agent, and dosage per day is preferably in the range of 1 to 800 mg for an adult patient with normal body weight. In case of a parenterally administered agent, it is preferably 0.001 to 1000 mg per 1 kg body weight per day, and more preferably, 0.01 to 300 mg per 1 kg body weight per day. It is preferable to administer them once a day or in divided dosages a day, depending on symptom of a subject to be treated.
- Hereinbelow, the present invention will be explained in greater detail in view of the following examples. However, the present invention is not limited by the examples. NMR analysis
- NMR analysis was carried out by using JNM-EX270 (270 MHz, manufactured by JEOL), JNM-GSX400 (400 MHz, manufactured by JEOL), or 400 MR (400 MHz, manufactured by Varian). NMR data was expressed in ppm (parts per million; 6), while it was compared with the deuterium lock signal obtained from a sample solvent.
- The measurement was carried out by using JMS-DX303 or JMS-SX/SX102A (both manufactured by JEOL).
- Measurement was carried out by using Micromass (ZMD, manufactured by Micromass) equipped with 996-600E gradient high performance liquid chromatography (manufactured by Waters) or Micromass (ZQ, manufactured by Micromass) equipped with 2525 gradient high performance liquid chromatography (manufactured by Waters).
- One of the following conditions that are described in the Table 1 below was taken as a condition for high performance liquid chromatography.
-
TABLE 1 Analysis Flow Rate Detection Condition Apparatus Column used Column Temperature Mobile phase, Gradient (mL/min) Wavelength A ZMD Cadenza CD-C18 35 deg. A) 0.05% TFA, H2O B) 0.05% 1.5 210-400 nm (Intakt) 3.0 mm I.D. × TFA, MeCN PDA total 30 mm, 3 um (A/B): 95/5 => 0/100(3.5 min) => 0/100(1 min) B ZMD Cadenza CD-C18 35 deg. A) 0.05% TFA, H2O B) 0.05% 1.0 210-400 nm (Intakt) 3.0 mm I.D. × TFA, MeCN PDA total 30 mm, 3 um (A/B): 95/5 => 0/100(9.5 min) => 0/100(2.5 min) C ZQ Chromolith Flash RP- Room Temp. A) 10 mM AcONH4, H2O B) MeOH 2.0 210-400 nm 18e (Merck KGaA) (A/B): 95/5 => 0/100(3 min) => PDA total 4.6 mm I.D. × 25 mm 0/100(2 min) D ZQ Chromolith Flash RP- Room Temp. A) 10 mM AcONH4, H2O B) MeCN 2.0 210-400 nm 18e (Merck KGaA) (A/B): 95/5 => 0/100(3 min) => PDA total 4.6 mm I.D. × 25 mm 0/100(2 min) F ZQ Cadenza CD-C18 35 deg. A) 0.05% TFA, H2O B) 0.05% 1.5 210-400 nm (Intakt) 3.0 mm I.D. × TFA, MeCN PDA total 30 mm, 3 um (A/B): 95/5 => 0/100(3.5 min) => 0/100(1 min) H ZQ Cadenza CD-C18 35 deg. A) 0.05% TFA, H2O B) 0.05% 1.0 210-400 nm (Intakt) 3.0 mm I.D. × TFA, MeCN PDA total 30 mm, 3 um (A/B): 95/5 => 0/100(9.5 min) => 0/100(2.5 min) I ZQ Ascentis Express C18 Room Temp. A) 10 mM AcONH4, H2O B) MeOH 1.0 210-400 nm (Sigma Aldrich) (A/B): 95/5 => 0/100(9.5 min) => PDA total 2.1 mm I.D. × 50 mm 0/100(1 min) S ZQ Sunfire C18 (Waters) Room Temp. A) 0.05% TFA, H2O B) 0.05% 4.0 200-400 nm 4.5 mm I.D. × 50 mm, 5 um TFA, MeCN PDA total (A/B): 90/10 => 5/95(3.5 min) => 90/10(1 min) => 90/10(0.5 min) T ZQ Sunfire C18 (Waters) Room Temp. A) 0.05% TFA, H2O B) 0.05% 4.0 200-400 nm 4.5 mm I.D. × 50 mm, 5 um TFA, MeCN PDA total (A/B): 90/10 => 5/95(2 min) => 5/95(1.5 min) => 90/10(1.0 min) => 90/10(0.5 min) U ZQ WAKOsil 3C18 AR, (Wako Room Temp. A) 0.05% TFA, H2O B) 0.05% 2.0 210-400 nm Pure Chemical Industries, TFA, MeCN PDA total Ltd.) 4.6 mm I.D. × 30 mm (A/B): 90/10 => 90/10(0.2 min) => 5/95(3.1 min) => 5/95(1.4 min) W ZMD Sunfire C18 (Waters) Room Temp. A) 0.05% TFA, H2O B) 0.05% 4.0 200-400 nm 4.5 mm I.D. × 50 mm, 5 um TFA, MeCN PDA total (A/B): 90/10 => 5/95(3.5 min) => 90/10(1 min) => 90/10(0.5 min) Y ZMD Sunfire C18 (Waters) Room Temp. A) 0.05% TFA, H2O B) 0.05% 2.0 210-400 nm 4.5 mm I.D. × 50 mm, 7 um TFA, MeCN PDA total (A/B): 90/10 => 0/100(3.5 min) => 0/100(1 min) - The reaction was carried out by using a snap cap reaction vial together with an Explorer™ (manufactured by CEM Microwave Technology) or an initiator (manufactured by Biotage). Maximum output setting includes cooling of the reaction vessel by air in order to avoid temperature increase caused by microwave irradiation.
- Commercially available reagents were obtained and used without any further purification. The room temperature indicates the temperature range of between about 20 to 25° C. All the non-aqueous reaction was carried out in anhydrous solvent under nitrogen or argon atmosphere. For concentration under reduced pressure or removal of a solvent by distillation, a rotary evaporator was used.
- For preparing the compounds, when there is a possibility of having an undesirable side reaction, a functional group was protected using a protecting group to produce a target molecule, and the protecting group was removed later, if desired. Selection, addition and removal of a protecting group were carried out according to the method described in the literature [Greene and Wuts, “Protective Groups in Organic Synthesis” (4th edition, John Wiley & Sons 2007)], for example.
- Compound A2
-
- 7-Methoxy-3,4-dihydro-1H-naphthalen-2-one (Compound A1, 209 g, 1.18 mol), tetrabutylammonium hydrogen sulfate (40 g, 0.118 mol) and methyl iodide (162 g, 2.60 mol) were suspended in THF (500 ml) at room temperature. Under stirring, the mixture was added with 50% aqueous solution of potassium hydroxide (400 g) over 5 min. Reflux occurred as the inner temperature rapidly increases. Once the inner temperature stopped to increase, stirring was continued for 45 min. The reaction solution was diluted with distilled water (1 L) and extracted twice with CPME (1.5 L). The combined organic layer was washed (distilled water 1 L×3), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting crude product was recrystallized with MeOH (1 L) and distilled water (500 ml) to obtain the title compound as a colorless needle-like crystal (177 g, 73%).
- 1H-NMR (400 MHz, CDCl3) δ: 1.43 (6H, s), 2.65 (2H, t, 12 Hz), 3.02 (2H, t, 12 Hz), 3.79 (3H, s), 6.74 (1H, m), 6.87 (1H, m), 7.24 (1H, m).
- LCMS: m/z 205 [M+H]+
- Compound A3-1, Compound A3-2
-
- 7-Methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 66.2 g, 324 mmol) and 3-bromophenylhydrazine hydrochloric acid salt (71.0 g, 318 mmol) were dissolved in AcOH (350 ml) and refluxed under stirring for 6 hr. The reaction solvent was removed by distillation under reduced pressure to obtain the crude product as a mixture of the title compound A3-1 and A3-2.
- Compound A4
-
- The crude product obtained from the above (i.e., mixture of A3-1 and A3-2) was dissolved in a mixture solvent of THF (450 ml) and distilled water (50 ml), added once with DDQ (115 g, 509 mmol), and then stirred at room temperature for 1 hr. The reaction mixture was diluted with CPME 3 L, and the organic layer was washed three times with 0.5 N aqueous solution of sodium hydroxide (1 L) and twice with distilled water (1 L) in order and dried over anhydrous sodium sulfate. The organic layer was concentrated to 500 ml under reduced pressure. The precipitated product was collected by filtration and washed with a small amount of CPME to obtain the title compound as a yellow crystal (48 g, 40%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 1.73 (6H, s), 3.90 (3H, s), 7.06-7.09 (1H, m), 7.32-7.38 (2H, m), 7.65-7.66 (1H, m), 8.09-8.17 (2H, m), 12.32 (1H, br. s).
- LCMS: m/z 370, 372 [M+H]+
- Compound AA1
-
- To the THF (16 ml) solution of 2-bromomethyl-4-methoxy-benzoic acid methyl ester (961 mg, 4.09 mmol), triphenylphosphine (107 mg, 0.1 eq.), cesium carbonate (1.87 g, 1.4 eq.), copper iodide (59 mg, 0.076 eq.) and tris (dibenzylideneacetone) dipalladium (86 mg, 0.023 eq.) were added, degassed, flushed with nitrogen gas, added with trimethylsilylacetylene (734 μl, 1.3 eq.), and then stirred overnight at 55° C. To the reaction solution, saturated aqueous solution of ammonium chloride was added followed by extraction with ethyl acetate. The organic layer was washed with brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (brown oily substance, 606 mg, 54%).
- 1H-NMR (400 MHz, CDCl3) δ: 7.93 (1H, d, J=8.8 Hz), 7.33 (1H, d, J=2.6 Hz), 6.78 (1H, dd, J=8.8, 2.6 Hz), 4.09 (2H, s), 3.86 (3H, s), 3.84 (3H, s), 0.14 (9H, s).
- LCMS: m/z 277 [M+H]+
- HPLC retention time: 3.30 min (analysis condition U)
- Compound AA2
-
- To the toluene (4 ml) solution of 4-methoxy-2-(3-trimethylsilanyl-prop-2-ynyl)-benzoic acid methyl ester (Compound AA1, 273 mg, 0.988 mmol), sodium bis (trimethylsilyl) amide (2.1 ml, 1.9 m solution, 4 eq.) and iodomethane (308 μl, 5 eq.) were added at −78° C. After allowing the reaction temperature to increase to the room temperature, the mixture was stirred for 2 hr. To the reaction solution, saturated aqueous solution of ammonium chloride was added followed by extraction with ethyl acetate. The organic layer was washed with brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (yellow oily substance, 226 mg, 75%).
- 1H-NMR (400 MHz, CDCl3) δ: 7.45 (1.0H, d, J=8.4 Hz), 7.09 (1.1H, d, J=2.6 Hz), 6.75 (1H, m), 3.84 (3H, s), 3.82 (3H, s), 1.70 (6H, s), 0.14 (9H, s)
- LCMS: m/z 305 [M+H]+
- HPLC retention time: 3.38 min (analysis condition U)
- Compound AA3
-
- To the THF (18 ml) solution of 2-(1,1-dimethyl-3-trimethylsilanylprop-2-ynyl)-4-methoxy-benzoic acid methyl ester (Compound AA2, 912 mg, 3 mmol), tetrabutylammonium fluoride (2.061 g, 2.6 eq.) was added, and then stirred for 3 hr at room temperature. To the reaction solution, saturated aqueous solution of ammonium chloride was added followed by extraction with ethyl acetate. The organic layer was washed with brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (yellow oily substance, 524 mg, 75%).
- 1H-NMR (400 MHz, CDCl3) δ: 7.44 (1H, d, J=8.4 Hz), 7.05 (1H, d, J=2.3 Hz), 6.76 (1H, dd, J=8.4, 2.3 Hz), 3.84 (3H, s), 3.82 (3H, s), 1.73 (6H, s)
- LCMS: m/z 223 [M+H]+
- HPLC retention time: 2.55 min (analysis condition U)
- Compound AA4
-
- To the DMF (2 ml) solution of 2-(1,1-dimethylprop-2-ynyl)-4-methoxy-benzoic acid methyl ester (Compound AA3, 134 mg, 0.577 mmol) and N-(2-bromo-5-cyanophenyl)methanesulfonamide (Compound AA5, 167 mg, 1.05 eq.), copper iodide (9 mg, 0.08 eq.) and TEA (129 μl, 1.6 eq.) were added, degassed and flushed with nitrogen gas, added with dicholorobis (triphenylphosphine) palladium (20 mg, 0.05 eq.), and then degassed and flushed again with nitrogen gas. After stirring for 2 hr at 90° C., the reaction solution was added with water, extracted with ethyl acetate. The organic layer was washed with an brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (white solid, 152 mg, 62%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.19 (1H, dd, J=0.6, 0.6 Hz), 7.84 (1H, dd, J=8.0, 0.6 Hz), 7.67 (1H, dd, J=8.0, 1.3 Hz), 7.13 (1H, d, J=8.4 Hz), 6.99 (1H, s), 6.96 (1H, br.s), 6.85 (1H, dd, J=8.4, 2.5 Hz), 3.78 (3H, s), 3.12 (3H, s), 3.09 (3H, br. s), 1.89 (6H, s).
- LCMS: m/z 427 [M+H]+
- HPLC retention time: 2.77 min (analysis condition U)
- Compound AA5
-
- To a mixture of 3-amino-4-bromo-benzonitrile (1.98 g, 10 mmol), TEA (5.06 g, 50 mmol), and methylene chloride (50 ml), mesyl chloride (2.71 ml, 35 mmol) was added at 0° C. and the mixture was stirred at room temperature for 30 min. Water was added to the reaction solution, which was then extracted with dichloromethane. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were added with tetrahydrofuran (100 ml), water (400 l) and sodium hydride (540 mg, 15.5 mmol), and stirred at room temperature for 16 hr. To the reaction solution, saturated aqueous solution of ammonium chloride (200 ml) was added followed by extraction with ethyl acetate. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane/ethyl acetate) to obtain the title compound (2.48 g, 90%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 9.82 (1H, s), 7.87 (1H, d, J=4 Hz), 7.75 (1H, d, J=8 Hz), 7.70 (1H, dd, J=8 Hz, 4 Hz), 3.14 (3H, s)
- HPLC retention time: 1.63 min (analysis condition U)
- Compound AA6
-
- To N-(2-bromo-5-cyanophenyl)methanesulfonamide (Compound AA5, 230 mg, 1 mmol), 3-methyl-2-butyn-3-ol (0.15 ml, 1.5 mmol), X-Phos (72 mg, 15% mol), PdCl2(CH3CN)2 (13 mg, 5% mol) and cesium carbonate (390 mg, 2 mmol), DMA (2 ml) was added, and the mixture was stirred at 100° C. for 3 hr. Water and 5 N hydrochloric acid solution were added to the reaction solution, which was then extracted with ethyl acetate. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane/ethyl acetate) to obtain the title compound (130 mg, 75%).
- 1H-NMR (400 MHz, CDCl3) δ: 8.76 (1H, s), 7.68 (1H, s), 7.60 (1H, d, J=8 Hz), 7.32 (1H, dd, J=8 Hz, 4 Hz), 6.37 (1H, m), 1.93 (1H, s), 1.70 (6H, s)
- LCMS: m/z 201 [M+H]+
- HPLC retention time: 2.12 min (analysis condition U)
- Compound A5-1
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was synthesized from Compound A4.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.30 (1H, s), 10.21 (1H, s), 8.06-8.11 (1H, m), 8.01-8.05 (1H, m), 7.62-7.66 (1H, m), 7.32-7.37 (1H, m), 7.08-7.12 (1H, m), 6.84-6.90 (1H, m), 1.69 (6H, s).
- LCMS: m/z 356, 358 [M+H]+
- HPLC retention time: 2.30 min (analysis condition U)
- Compound A5-2
-
- (Method 1) 3-Bromo-8-methoxy-6,6-dimethyl-5,6-dihydrobenzo[b]carbazol-11-one (Compound A4, 10.45 g, 28.2 mmol) and copper (I) cyanide (5.0 g, 50.2 mmol) were dissolved in NMP (100 ml), followed by stirring at 170° C. for 17 hr. The reaction mixture was suspended in ethyl acetate (500 mL) and distilled water (200 mL). The insoluble matters were removed by Celite filtration and washed twice with ethyl acetate (300 mL×2). The organic layer was washed once with an aqueous solution of disodium EDTA (200 mL) and twice with saturated brine (200 mL) in order, and dried over anhydrous sodium sulfate. The organic layer was concentrated under reduced pressure to yield a product, which was suspended and washed with a small amount of CPME to obtain the title compound as a colorless crystal (6.58 g, 73%).
- (Method 2) To the THF (5.6 ml) solution of 2-[1-(6-cyano-1-methanesulfonyl-1H-indol-2-yl)-1-methylethyl]-4-methoxy-benzoic acid methyl ester (Compound AA4, 138 mg, 0.324 mmol), tetrabutylammonium fluoride (514 mg, 6 eq.) was added, and the mixture was stirred at room temperature overnight. Thereafter, 2 M aqueous solution of sodium hydroxide (5.6 ml) was added to the mixture, which was then stirred for 4 hr, added with 1 M HCl, and extracted with ethyl acetate. The organic layer was washed with brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were dissolved in ethyl acetate (10 ml) and added with e 4 M HCl and ethyl acetate solution (10 ml) followed by stirring at room temperature for 30 min. The residues obtained after concentration of the reaction solution under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (89.2 mg, 62%).
- (Method 3) To nitrobenzene (5 ml) and aluminum chloride (400 mg, 3 mmol), 4-methoxybenzoyl chloride (400 mg, 2.3 mmol) was added. After stirring for 30 min at room temperature, 2-(1-hydroxy-1-methyl-ethyl)-1H-indole-6-carbonitrile (Compound AA6, 200 mg, 1 mmol) was added followed by stirring at room temperature for 3 hr. Water was added to the reaction solution, which was then extracted with ethyl acetate. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane/ethyl acetate) to obtain the title compound (127 mg, 40%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 1.71 (6H, s), 3.89 (3H, s), 7.07-7.09 (1H, m), 7.34 (1H, s), 7.58-7.60 (1H, m), 7.99 (1H, s), 8.14-8.16 (1H, m), 8.30-8.32 (1H, m), 12.32 (1H, br. s),
- LCMS: m/z 317 [M+H]+
- HPLC retention time: 2.56 min (analysis condition U)
- Compound A6
-
- 8-Methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A5-2, 6.58 g, 20.8 mmol) was dissolved in pyridine hydrochloric acid salt (25.0 g), and stirred at 170° C. for 13 hr. The reaction mixture was partitioned in ethyl acetate (400 mL) and distilled water (400 mL), and the aqueous layer was extracted one more time with ethyl acetate (400 mL). The combined organic layer was washed twice with distilled water (100 mL) and once with saturated brine (100 mL) in order, and dried over anhydrous sodium sulfate. The organic layer was concentrated under reduced pressure to yield a product, which was suspended and washed with a small amount of CPME to obtain the title compound as a colorless crystal (5.91 g, 93%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 1.73 (6H, s), 6.87-6.90 (1H, m), 7.11 (1H, s), 7.57-7.59 (1H, m), 7.97 (1H, s), 8.04-8.06 (1H, m), 8.29-8.31 (1H, m), 10.27 (1H, s), 12.66 (1H, br. s),
- LCMS: m/z 303 [M+H]+
- Compound A7-1
-
- 8-Hydroxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A6, 30 mg, 0.099 mmol) was dissolved in THF (1 mL), added with 4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester (40 mg, 2 eq.), triphenylphosphine (52 mg, 2 eq.), and diisopropyl azodicarboxlyate (43 μL, 2 eq.) in order, and stirred at room temperature for 4 hr. The reaction solution was poured to water, and then extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (37 mg, 76%).
- 1H-NMR (400 MHz, CDCl3) δ: 9.44 (1H, s), 8.77 (1H, d, J=7.8 Hz), 8.62 (1H, d, J=8.2 Hz), 8.00 (1H, s), 7.81 (1H, d, J=8.2 Hz), 7.34 (1H, s), 7.26 (1H, d, J=7.8 Hz), 4.85-4.93 (1H, m), 3.96-4.04 (2H, m), 3.60-3.70 (2H, m), 2.19-2.32 (2H, m), 1.89-2.15 (8H, m), 1.74 (9H, s)
- LCMS: m/z 430 [M+H]+
- HPLC retention time: 4.09 min (analysis condition W)
- Compound A7-2
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and 1-(2-hydroxy-ethyl)-imidazolidin-2-one.
- LCMS: m/z 415 [M+H]+
- HPLC retention time: 2.96 min (analysis condition W)
- Compound A7-3
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and (2-hydroxy-ethyl)-carbamic acid tert-butyl ester.
- LCMS: m/z 346 [M+H]+
- HPLC retention time: 2.40 min (analysis condition W)
- Compound A7-4
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and 2-methylthioethanol.
- LCMS: m/z 451 [M+H]+
- HPLC retention time: 4.23 min (analysis condition W)
- Compound A7-5
-
- The title compound was obtained as a by-product of the synthesis of Compound A7-4.
- LCMS: m/z 377 [M+H]+
- HPLC retention time: 3.75 min (analysis condition W)
- Compound A7-6
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and tetrahydropyran-4-ol.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.72 (1H, br.s), 8.32 (1H, d, 8.5H z), 8. (1H, d, 8.5 Hz), 8.01 (1H, s), 7.61 (1H, d, 8.5 Hz), 7.38 (1H, s), 7.15 (1H, d, 8.5 Hz), 4.86-4.81 (1H, m), 3.93-3.88 (2H, m), 3.58-3.52 (2H, m), 2.06-2.00 (2H, m), 1.85 (6H, s), 1.69-1.60 (2H, m)
- LCMS: m/z 387 [M+H]+
- HPLC retention time: 3.47 min (analysis condition W)
- Compound A7-7
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and pyridin-4-yl-methanol.
- LCMS: m/z 394 [M+H]+
- HPLC retention time: 2.56 min (analysis condition W)
- Compound A7-8
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and 2-methoxyethanol.
- 1H-NMR (400 MHz, DMSO-d6) δ: 11.69 (1H, br. s), 8.27 (1H, d, 7.9 Hz), 8. (1H, d, 8.5 Hz), 7.95 (1H, s), 7.55 (1H, d, 7.9 Hz), 7.32 (1H, d, 2.4 Hz), 7.05 (1 H, d, 8.5 Hz), 4.22 (2H, t, 4.3 Hz), 3.67 (2H, t, 4.3 Hz), 1.72 (6H, s)
- LCMS: m/z 361 [M+H]+
- HPLC retention time: 3.38 min (analysis condition W)
- Compound A7-9
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and 2-(2-methoxyethoxy)ethanol.
- LCMS: m/z 405 [M+H]+
- HPLC retention time: 3.32 min (analysis condition W)
- Compound A7-10
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and 3-chloromethyl-3-methyloxetane.
- LCMS: m/z 387 [M+H]+
- HPLC retention time: 2.23 min (analysis condition S)
- Compound A7-11-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and ethyl-(2-hydroxy-ethyl)-carbamic acid tert-butyl ester.
- LCMS: m/z 474 [M+H]+
- HPLC retention time: 2.93 min (analysis condition U)
- Compound A7-11-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound A7-11-1.
- LCMS: m/z 374 [M+H]+
- HPLC retention time: 1.35 min (analysis condition U)
- Compound A7-12
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and 2-bromo-ethanol.
- LCMS: m/z 437 [M+H]+
- HPLC retention time: 2.93 min (analysis condition U)
- Compound A7-13-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and 2-phenyl-[1,3]dioxan-5-ol.
- LCMS: m/z 465 [M+H]+
- HPLC retention time: 4.10 min (analysis condition W)
- Compound A7-13-2
-
- Anhydrous ferric trichloride (56 mg, 5 eq.) was added to the dichloromethane (2 mL) suspension of 6,6-dimethyl-11-oxo-8-(2-phenyl-[1,3]dioxan-5-yloxy)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A7-13-1, 13 mg, 0.028 mmol), and stirred at room temperature for 1 hr. The reaction solution was added to water, and then extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by high performance liquid chromatography to obtain the title compound (7 mg, 46%).
- LCMS: m/z 377 [M+H]+
- HPLC retention time: 2.70 min (analysis condition W)
- Compound A7-14-1
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and toluene-4-sulfonic acid (R)-2,2-dimethyl-[1,3]dioxolan-4-ylmethyl ester.
- LCMS: m/z 417 [M+H]+
- HPLC retention time: 3.47 min (analysis condition Y)
- Compound A7-14-2
-
- To the solution of THF and water (4: 1, 1 mL) of 8-(2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A14-1, 30 mg, 0.07 mmol), camphor sulfonic acid (36 mg, 0.14 mmol) was added at room temperature. After stirring at room temperature for 38 hr, the mixture was extracted with ethyl acetate. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (dichloromethane/methanol) to obtain the title compound (white solid, 28 mg, 72%).
- 1H-NMR (300 MHz, DMSO-d6) σppm; 12.7 (s, 1H), 8.31 (d, 1H, J=8.01 Hz), 8.15 (d, 1H, J=8.77 Hz), 8.00 (s, 1H), 7.60 (d, 1H, J=8.01 Hz), 7.12 (s, 1H), 7.09 (d, 1H, J=8.77 Hz), 4.46 (m, 1H), 4.15 (m, 3H), 3.78 (m, 1H), 1.76 (s, 6H), 1.38 (s, 3H), 1.32 (s, 3H)
- LCMS: m/z 377 [M+H]+
- HPLC retention time: 1.80 min (analysis condition U)
- Compound A7-14-3
-
- Under the same conditions as the method for synthesizing Compound B3-4, the title compound was prepared as a crude product from Compound A7-14-1.
- Compound A7-14-4
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound A7-14-3 (303 mg, 98%).
- LCMS: m/z 484 [M+H]+
- HPLC retention time: 2.08 min (analysis condition D)
- Compound A7-15-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and [(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-methanol.
- LCMS: m/z 516 [M+H]+
- HPLC retention time: 3.97 min (analysis condition Y)
- Compound A7-15-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound A7-15-1.
- LCMS: m/z 407 [M+H]+
- HPLC retention time: 1.73 min (analysis condition U)
- Compound A7-16
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and 1-methylpiperidin-4-ol.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.75 (1H, s), 8.32 (1H, d, J=7.9 Hz), 8.14 (1H, d, J=9.8 Hz), 8.00 (1H, s), 7.60 (1H, d, J=7.9 Hz), 7.34 (1H, s), 7.11 (1H, d, J=9.1 Hz), 4.62 (1H, m), 2.64 (2H, m), 2.23 (2H, m), 2.21 (s, 3H), 1.99 (2H, m), 1.77 (s, 6H), 1.73 (2H, m).
- LCMS: m/z 400 [M+H]+
- HPLC retention time: 1.42 min (analysis condition S)
- Compound A7-17
-
- 8-Hydroxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A6, 25 mg, 0.083 mmol) was dissolved in N,N-dimethylacetamide (1 mL), added with 2-chloroethyldiethylamine (16 mg, 1.1 eq.) and cesium carbonate (54 mg, 2 eq.) in order and stirred at 100° C. for 4 hr. The reaction solution was poured over water and extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by amino silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (11 mg, 32%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.32 (1H, d, J=8.2 Hz), 8.15 (1H, d, J=8.7 Hz), 8.01 (1H, s), 7.61 (1H, d, J=8.2 Hz), 7.35 (1H, d, J=1.8 Hz), 7.09 (1H, dd, J=8.7, 1.8 Hz), 4.19 (2H, t, J=5.9 Hz), 2.83 (2H, t, J=5.9 Hz), 2.58 (4H, q, J=7.0 Hz), 1.78 (6H, s), 1.00 (6H, t, J=7.0 Hz)
- LCMS: m/z 402 [M+H]+
- HPLC retention time: 2.52 min (analysis condition W)
- Compound A7-18
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and 2-chloroethylacetamide.
- LCMS: m/z 388 [M+H]+
- HPLC retention time: 2.91 min (analysis condition W)
- Compound A7-19
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and ethyl-2-chloroethylcarbamate.
- LCMS: m/z 418 [M+H]+
- HPLC retention time: 3.35 min (analysis condition W)
- Compound A7-20
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and 2-chloroethylurea.
- LCMS: m/z 399 [M+H]+
- HPLC retention time: 2.80 min (analysis condition W)
- Compound A7-21
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and toluene-4-sulfonic acid oxetan-3-yl ester.
- LCMS: m/z 359 [M+H]+
- HPLC retention time: 2.00 min (analysis condition S)
- Compound A7-22
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and 2-bromopyrimidine.
- LCMS: m/z 381 [M+H]+
- HPLC retention time: 2.00 min (analysis condition S)
- Compound A7-23
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and 3-chloro-propionic acid ethyl ester.
- LCMS: m/z 389 [M+H]+
- HPLC retention time: 3.37 min (analysis condition U)
- Compound A7-24
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and 2-bromoethanol.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.75 (1H, br.s), 8.32 (1H, d, J=8.2 Hz), 8.17 (1H, d, J=8.6 Hz), 8.01 (1H, s), 7.61 (1H, dd, J=8.2, 1.4 Hz), 7.40 (1H, d, J=2.2 Hz), 7.12 (1H, dd, J=8.6, 2.2 Hz), 4.50 (2H, t, J=5.3 Hz), 3.88 (2H, t, J=5.3 Hz), 1.77 (6H, s).
- LCMS: m/z 409, 411 [M+H]+
- HPLC retention time: 2.48 min (analysis condition S)
- Compound A7-25
-
- Under nitrogen atmosphere, 3-cyano-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound A6, 85 mg, 0.28 mmol) and triphenylphosphine (150 mg, 2 eq.) were added with THF (2 ml), and then further added dropwise with 4-hydroxymethyl-piperidine-1-carboxylic acid tert-butyl ester (120 mg, 2 eq.) and 2.19 N toluene solution of diethyl azodicarboxylic acid (0.26 mL, 2 eq.). The resultant was stirred at room temperature for 12 hr under nitrogen atmosphere. The residues obtained after concentrating the reaction solution under reduced pressure were purified by silica gel column chromatography (ethyl acetate/dichloromethane) to obtain 4-(3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (white powder, 120 mg).
- To the resulting compound, 4 N hydrochloric acid and dioxane solution was added under cooling. After stirring at room temperature for 2 hr, the solvent was removed under nitrogen stream. Then, the residues were washed with diethyl ether and then subjected to azeotropic treatment with toluene, followed by drying under vacuum and filtration to obtain the title compound (79 mg).
- LCMS: m/z 399 [M+H]+
- HPLC retention time: 2.22 min (analysis condition C)
- Compound A8-1
-
- THF (0.5 mL) and TFA (0.5 mL) were added to 4-(3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxy)-piperidine-1-carboxylic acid tert-butyl ester (Compound A7-1, 35 mg, 0.072 mmol), and the mixture was stirred at room temperature until Compound A7-1 disappears. The reaction solution was concentrated under reduced pressure and the residue was desalinated by using anion exchanger PL StratoSpheres (trademark) PL-HCO3 MP to obtain the title compound (37 mg, 76%).
- 1H-NMR (400 MHz, CD3OD) δ: 8.38 (1H, d, J=7.9 Hz), 8.24 (1H, d, J=8.5 Hz), 7.85 (1H, s), 7.53 (1H, d, J=7.9 Hz), 7.27 (1H, s), 7.09 (1H, d, J=8.5 Hz), 4. 67-4.76 (1H, m), 3.07-3.20 (2H, m), 2.77-2.87 (2H, m), 2.03-2.15 (2H, m), 1.80 (6H, s), 1.69-1.77 (2H, m)
- LCMS: m/z 386 [M+H]+
- HPLC retention time: 2.51 min (analysis condition W)
- Compound A8-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound A7-3.
- LCMS: m/z 346 [M+H]+
- HPLC retention time: 2.40 min (analysis condition W)
- Compound A8-3
-
- Under the same conditions as the method for synthesizing Compound B3-8, the title compound was prepared from Compound A7-5.
- LCMS: m/z 409 [M+H]+
- HPLC retention time: 3.13 min (analysis condition W)
- Compound A8-4
-
- The title compound was obtained as a by-product of the synthesis of Compound A8-3.
- LCMS: m/z 393 [M+H]+
- HPLC retention time: 2.87 min (analysis condition W)
- Compound A8-5
-
- Under the same conditions as the method for synthesizing Compound A10-1, the title compound was prepared from Compound A7-6.
- LCMS: m/z 401 [M+H]+
- HPLC retention time: 2.72 min (analysis condition S)
- Compound A8-6-1
-
- 8-Methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A5-2, 50 mg, 0.158 mmol) was dissolved in CH3CN (1 mL), added with NBS (56 mg, 2 eq.), and stirred at 80° C. overnight. The reaction solution was added to water, and then extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were added with MeOH and the solid remained after dissolution was filtered to obtain the target compound (yellow powder, 20 mg, 38%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.92 (1H, s), 8.50 (1H, s), 8.16 (1H, d, J=8.5 Hz), 8.14 (1H, s), 7.36 (1H, d, J=2.4 Hz), 7.11 (1H, dd, J=8.5, 2.4 Hz), 3.92 (3H, s), 1.78 (6H, s).
- LCMS: m/z 395, 397 [M+H]+
- HPLC retention time: 2.57 min (analysis condition S)
- Compound A8-6-2
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound A8-6-1.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.46 (1H, s), 8.10 (1H, s), 8.05 (1H, d, J=8.6 Hz), 7.13 (1H, d, J=2.1 Hz), 6.89 (1H, dd, J=8.5, 2.1 Hz), 1.71 (6H, s).
- LCMS: m/z 381, 383 [M+H]+
- HPLC retention time: 2.10 min (analysis condition S)
- Compound A8-6-3
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A8-6-2.
- 1H-NMR (400 MHz, CD3OD) δ: 8.53 (1H, d, J=0.5 Hz), 8.20 (1H, d, J=8.7 Hz), 7.88 (1H, d, J=0.5 Hz), 7.28 (1H, d, J=2.3 Hz), 7.05 (1H, dd, J=8.9, 2.5 Hz), 4.24 (2H, t, J=5.7 Hz), 2.96 (2H, t, J=5.7 Hz), 2.70 (4H, q, J=7.1 Hz), 1.79 (6H, s), 1.12 (6H, t, J=7.2 Hz).
- LCMS: m/z 480, 482 [M+H]+
- HPLC retention time: 1.73 min (analysis condition S)
- Compound A8-7
-
- (3-Cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxy)-ethyl acetate ester (Compound A7-23, 180 mg, 0.464 mmol) and potassium hydroxide (130 mg, 2.32 mmol) were dissolved in THF (10 ml) and water (1.8 mL), and stirred at 70° C. for 2 hr. After cooling to room temperature, the mixture was extracted with dichloromethane. Water layer () was adjusted to be acidic by using 1 N hydrochloric acid, and the precipitated solid was filtered and washed several times with water to obtain the title compound (white solid, 130 mg, 78%).
- 1H-NMR (300 MHz, DMSO) σppm 13.09 (s, 1H), 8.31 (d, 1H, J=8.1 Hz), 8.11 (d, 1H, J=8.4 Hz), 8.01 (s, 1H), 7.58 (d, 1H, J=7.8 Hz), 7.25 (d, 1H, J=2.1 Hz), 6.97 (d, 1H, J=8.4 Hz), 4.51 (s, 2H), 1.73 (s, 6H)
- LCMS: m/z 361 [M+H]+
- HPLC retention time: 2.97 min (analysis condition U)
- Compound A8-8
-
- Under the same conditions as the method for synthesizing Compound A8-17, the title compound was prepared from Compound A7-24 and morpholine.
- 1H-NMR (500 MHz, CD3OD+CDCl3) a ppm; 8.4 (d, 1H, J=8.2 Hz), 8.3 (d, 1H, J=8.7 Hz), 7.8 (s, 1H), 7.5 (dd, 1H, J=1.1 Hz, J=8.2 Hz), 7.2 (d, 1H, J=2.3 Hz), 7.0 (dd, 1H, J=2.2 Hz, J=8.7 Hz), 4.2 (t, 2H, J=5.3 Hz), 3.7 (t, 4H, J=4.5 Hz), 2.9 (t, 2H, J=5.3 Hz), 2.6 (t, 4H, J=4.5 Hz), 1.8 (s, 6H)
- LCMS: m/z 416 [M+H]+
- HPLC retention time: 2.40 min (analysis condition U)
- Compound A8-9
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A7-24 and thiomorpholine-1,1-dioxide.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.72 (1H, s), 8.31 (1H, d, 8.5 Hz), 8.15 (1H, d, 8.5 Hz), 8.00 (1H, s), 7.60 (1H, d, 8.5 Hz), 7.36 (1H, d, 1.8 Hz), 7.10 (1H, dd, 1.8, 8.5), 4.25 (2H, t, 5.5 Hz), 3.06-3.33 (8H, m), 2.97 (2H, t, 5.5), 1.77 (6H, s)
- LCMS: m/z 464 [M+H]+
- HPLC retention time: 2.70 min (analysis condition W)
- Compound A8-10
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A7-24 and tert-butylamine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.71 (1H, s), 8.32 (1H, d, 7.9 Hz), 8.15 (1H, d, 9.1 Hz), 8.07 (1 d, 1.8 Hz), 7.60 (1H, dd, 1.8, 7.9 Hz), 7.35 (1H, d, 2.4 Hz), 7.09 (1H, dd, 2.4, 9.1 Hz), 4.16 (2H, t, 6.1 Hz), 2.91 (2H, t, 6.1 Hz), 1.77 (6H, s), 1.08 (9H, s)
- LCMS: m/z 402 [M+H]+
- HPLC retention time: 2.55 min (analysis condition W)
- Compound A8-11
-
- Under the same conditions as the method for synthesizing Compound A8-17, the title compound was prepared from Compound A7-24 and sec-butylamine.
- LCMS: m/z 402 [M+H]+
- HPLC retention time: 1.88 min (analysis condition U)
- Compound A8-12
-
- Under the same conditions as the method for synthesizing Compound A8-17, the title compound was prepared from Compound A7-24 and 2-amino-2-methyl-propan-1-ol.
- 1H-NMR (300 MHz, DMSO-d6) σppm; 12.65 (brs, 1H), 8.31 (d, 1H, J=8.0 Hz), 8.15 (d, 1H, J=8.8 Hz), 7.99 (s, 1H), 7.59 (d, 1H, J=8.0 Hz), 7.34 (d, 1H, J=2.3 Hz), 7.08 (dd, 1H, J=2.2 Hz, J=8.8 Hz), 4.58 (brs, 1H), 4.16 (t, 2H, J=5.7 Hz), 3.20 (s, 2H), 2.88 (t, 2H, J=5.7 Hz), 1.76 (s, 6H), 0.97 (s, 6H)
- LCMS: m/z 418 [M+H]+
- HPLC retention time: 2.47 min (analysis condition U)
- Compound A8-13
-
- Under the same conditions as the method for synthesizing Compound A8-17, the title compound was prepared from Compound A7-24 and 1-ethyl-piperazine.
- LCMS: m/z 443 [M+H]+
- HPLC retention time: 1.68 min (analysis condition U)
- Compound A8-14
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A6 and 2-imidazol-1-yl-ethanol.
- 1H-NMR (300 MHz, DMSO-d6) a ppm; 12.71 (s, 1H), 8.31 (d, 1H, J=8.3 Hz), 8.14 (d, 1H, J=8.8 Hz), 7.99 (s, 1H), 7.73 (s, 1H), 7.60 (d, 1H, J=8.3 Hz), 7.34 (s, 1H), 7.29 (s, 1H), 7.09 (d, 1H, J=8.8 Hz), 6.91 (s, 1H), 4.20 (s, 4H), 1.76 (s, 6H)
- LCMS: m/z 387 [M+H]+
- HPLC retention time: 1.77 min (analysis condition U)
- Compound A8-15
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A7-24 and 2-(2-hydroxy-ethylamino)-ethanol.
- LCMS: m/z 434 [M+H]+
- HPLC retention time: 2.40 min (analysis condition U)
- Compound A8-16
-
- Under the same conditions as the method for synthesizing Compound A8-17, the title compound was prepared from Compound A7-24 and piperidine-4-carboxylic acid amide.
- LCMS: m/z 457 [M+H]+
- HPLC retention time: 1.28 min (analysis condition S)
- Compound A8-17
-
- To DMF solution (5 mL) of 8-(2-bromo-ethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A7-24, 30 mg, 0.07 mmol), piperazin-2-one (44.9 mg, 0.35 mmol) and N,N-diisopropylethylamine (0.061 mL, 0.35 mmol) were added at room temperature and stirred at 80° C. for 18 hr. After cooling to room temperature, the mixture was extracted with ethyl acetate washed with saturated brine. The organic layer was dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by preparative TLC (dichloromethane/methanol) to obtain the title compound (white solid, 24 mg, 80%).
- 1H-NMR (300 MHz, DMSO-d6) σppm; 12.71 (s, 1H), 8.32 (d, 1H, J=8.4 Hz), 8.15 (d, 1H, J=8.8 Hz), 8.00 (s, 1H), 7.75 (s, 1H), 7.60 (d, 1H, J=8.4 Hz), 7.37 (d, 1H, J=2.3 Hz), 7.09 (dd, 1H, J=2.3 Hz, J=8.8 Hz), 4.27 (t, 2H, J=5.7 Hz), 3.19 (m, 2H), 3.08 (s, 2H), 2.83 (t, 2H, J=5.7 Hz), 2.70 (t, 2H, J=5.7 Hz), 1.8 (s, 6H)
- LCMS: m/z 429 [M+H]+
- HPLC retention time: 1.29 min (analysis condition S)
- Compound A8-18
-
- The title compound was obtained as a by-product of the synthesis of Compound C1-2.
- LCMS: m/z 495 [M+H]+
- HPLC retention time: 2.00 min (analysis condition S)
- Compound A8-19
-
- The title compound was obtained as a by-product of the synthesis of Compound C1-4.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.77 (6H, s), 2.16 (3H, s), 2.34 (4H, m), 3.08 (4H, m), 3.35 (2H, m), 4.19 (2H, t, 5.34 Hz), 7.09 (1H, dd, 8.77 Hz, 2.99 Hz), 7.37 (1H, bs, 1.91 Hz), 7.59 (2H, m), 8.01 (1H, s), 8.16 (1H, d, 8.40 Hz), 8.32 (1H, d, 8.01 Hz), 12.7 (1H, s).
- LCMS: m/z 501 [M+H]+
- HPLC retention time: 1.43 min (analysis condition S)
- Compound A8-20
-
- 6,6-Dimethyl-11-oxo-8-(piperidin-4-ylmethoxy)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloric acid salt (Compound A7-25, 30 mg, 0.075 mmol) and oxetan-3-one (38 mg, 7 eq.) were dissolved in acetic acid (0.2 ml), THF (1 ml) and methanol (1 ml), added with sodium cyanoborohydride (33 mg, 7 eq.) at room temperature, and stirred overnight. The reaction solution was added with water, and then extracted with ethyl acetate. The solution was dried over sodium sulfate and the solvent was removed under vacuum and the resulting residues were purified by preparative TLC (chloroform: 2 N ammonia methanol=9: 1) to obtain the target compound (15 mg).
- LCMS: m/z 456 [M+H]+
- HPLC retention time: 2.78 min (analysis condition C)
- Compound A8-21
-
- Under the same conditions as the method for synthesizing Compound A7-25, and Compound A8-20, the title compound was prepared from Compound A6 and 4-(2-hydroxy-ethyl)-piperidine-1-carboxylic acid tert-butyl ester (15 mg).
- LCMS: m/z 470 [M+H]+
- HPLC retention time: 2.85 min (analysis condition C)
- Compound A9-1
-
- Trifluoroacetic acid salt of 8-(2-amino-ethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A8-2, 19 mg, 0.044 mmol) was suspended in dichloromethane (0.5 mL), added with diisopropylethylamine (0.0157 mL, 2 eq.) and methanesulfonyl chloride (0.0034 mL, 1 eq.), and then stirred at room temperature for 2 hr. The reaction solution was added to water, and then extracted with dichloromethane. After washing with saturated brine, the organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were separated by silica gel preparative TLC (ethyl acetate 100%) to obtain the target compound (5.5 mg, 29%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.47 (1H, d, J=8.2 Hz), 8.32 (1H, d, J=8.7 Hz), 8.16 (1H, s), 7.76 (1H, d, J=8.2 Hz), 7.53-7.46 (2H, m), 7.26 (1H, d, J=8.7 Hz), 4.39-4.33 (2H, m), 3.58-3.51 (2H, m), 3.12 (3H, s), 1.93 (6H, s)
- LCMS: m/z 424 [M+H]+
- HPLC retention time: 3.10 min (analysis condition W)
- Compound A9-2
-
- The title compound was obtained as a by-product of the synthesis of Compound A9-1.
- LCMS: m/z 442 [M+H]+
- HPLC retention time: 3.45 min (analysis condition W)
- Compound A9-3-1
-
- Trifluoroacetic acid salt of 8-(2-amino-ethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A8-2, 20 mg, 0.044 mmol) was dissolved in pyridine (0.5 mL), added with N-(tert-butoxycarbonyl)-N-[4-(dimethyl azaniumylidene)-1,4-dihydropyridin-1-yl sulfonyl]azanide (13.5 mg, 1 eq.), and then stirred at room temperature for 14 hr. The reaction solution was added to water, and then extracted with ethyl acetate. After washing with saturated brine, the organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were separated by silica gel preparative TLC (ethyl acetate) to obtain the title compound (16.1 mg, 68%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.74 (1H, s), 10.94 (1H, s), 8.33 (1H, d, J=8.5 Hz), 8.16 (1H, d, J=9.1 Hz), 8.02 (1H, s), 7.84 (1H, br.s), 7.62 (1H, d, J=7.9 Hz), 7.36 (1H, s), 7.10 (1H, d, J=7.9 Hz). 4.24-4.18 (2H, in), 1.78 (6H, s), 1.32 (9H, s)
- LCMS: m/z 525 [M+H]+
- HPLC retention time: 3.48 min (analysis condition W)
- Compound A9-3-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound A9-3-1.
- LCMS: m/z 425 [M+H]+
- HPLC retention time: 2.95 min (analysis condition W)
- Compound A9-4
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A8-1 and methanesulfonyl chloride.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.72 (1H, s), 8.30 (1H, d, J=8.5 Hz), 8.14 (1H, d, J=8.5 Hz), 8.00 (1H, s), 7.59 (1H, d, J=7.9 Hz), 7.38 (1H, s), 7.13 (1H, d, J=8.5 Hz), 4.81 (1H, s), 3.39-3.38 (2H, m), 3.19-3.13 (2H, m), 2.93 (3H, s), 2.11-2.04 (2H, m), 1.83-1.75 (8H, m).
- LCMS: m/z 464 [M+H]+
- HPLC retention time: 3.41 min (analysis condition U)
- Compound A9-5
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound A8-1 and 1-bromo-2-methoxy-ethane.
- 1H-NMR (400 MHz, CDCl3) δ: 8.48-8.53 (1H, m), 8.32-8.38 (1H, m), 7.74-7.77 (1H, m), 7.50-7.55 (1H, m), 7.07-7.10 (1H, m), 6.95-7.00 (1H, m), 4.43-4.51 (1H, m), 3.53 (2H, t, J=5.6 Hz), 3.36 (3H, s), 2.77-2.87 (2H, m), 2.62 (2H, t, J=5.6 Hz), 2.35-2.47 (2H, m), 2.02-2.12 (2H, m), 1.78-1.95 (2H, m), 1.82 (6H, s).
- LCMS: m/z 444 [M+H]+
- HPLC retention time: 2.00 min (analysis condition U)
- Compound A9-6-2
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A8-1 and (2-bromoethoxy)-tert-butyldimethylsilane, followed by treatment with tetrabutylammonium fluoride.
- LCMS: m/z 430 [M+H]+
- HPLC retention time: 1.45 min (analysis condition S)
- Compound A9-7
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A8-1 and methanesulfonic acid 2-fluoroethyl ester.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.67 (2H, m), 1.76 (6H, s), 2.01 (2H, m), 2.37 (2H, t, 11.0 Hz), 2.61 (1H, t, 4.20 Hz), 2.70 (1H, t, 4.58), 2.78 (2H, m), 4.46 (1H, t, 4.58 Hz), 4.62 (2H, t, 5.34 Hz), 7.10 (1H, dd, 9.16 Hz, 2.29 Hz), 7.34 (1H, bs, 1.53 Hz), 7.60 (1H, dd, 8.40 Hz, 1.53 Hz), 7.99 (1H, s), 8.13 (1H, d, 8.39 Hz), 8.30 (1H, d, 8.39 Hz), 12.7 (1H, s).
- LCMS: m/z 432 [M+H]+
- HPLC retention time: 1.52 min (analysis condition S)
- Compound A9-8
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A8-1 and acetyl chloride.
- LCMS: m/z 428 [M+H]+
- HPLC retention time: 1.91 min (analysis condition S)
- Compound A9-9
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A6 and 2-bromo-acetamide.
- LCMS: m/z 360 [M+H]+
- HPLC retention time: 2.83 min (analysis condition U)
- Compound A9-10
-
- (3-Cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxy)-acetic acid (Compound A8-7, 30 mg, 0.0838 mmol), methylamine hydrochloric acid salt (28.1 mg, 0.417 mmol), EDC (32 mg, 0.167 mmol) and HOBT (0.023 mg, 0.167 mmol) were dissolved in DMF (1 mL), and added with diisopropylethylamine (0.145 mL, 0.833 mmol) at room temperature. After stirring at room temperature for 18 hr, water was added and the extraction was carried out with ethyl acetate. After washing with saturated brine, the organic layer was dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were dissolved in dichloromethane, added with diethyl ether, and the precipitated title compound was obtained (white solid, 19.7 mg, 63%).
- 1H-NMR (300 MHz, DMSO) a ppm 12.73 (s, 1H), 8.33 (d, 1H, J=8.1 Hz), 8.17 (d, 1H, J=8.7 Hz), 8.13 (s, 1H), 8.00 (s, 1H), 7.62 (d, 1H, J=8.1 Hz), 7.39 (d, 1H, J=2.4 Hz), 7.11 (dd, 1H, J=8.7 Hz, 2.4 Hz), 4.64 (s, 2H), 3.17 (d, 1H, J=5.4 Hz), 2.69 (d, 1H, J=4.5 Hz), 1.76 (s, 6H)
- LCMS: m/z 374 [M+H]+
- HPLC retention time: 2.43 min (analysis condition U)
- Compound A9-11
-
- Under the same conditions as the method for synthesizing Compound A9-10, the title compound was prepared from Compound A8-7 and C-(2,2-dimethyl-[1,3]dioxolan-4-yl)-methylamine.
- LCMS: m/z 474 [M+H]+
- HPLC retention time: 2.20 min (analysis condition U)
- Compound A9-12
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound A9-11.
- LCMS: m/z 434 [M+H]+
- HPLC retention time: 1.72 min (analysis condition U)
- Compound A9-13
-
- Under the same conditions as the method for synthesizing Compound A9-10, the title compound was prepared from Compound A8-7 and 2-methyl-acrylic acid 2-amino-ethyl ester.
- LCMS: m/z 472 [M+H]+
- HPLC retention time: 3.30 min (analysis condition U)
- Compound A9-14
-
- 2-Methyl-acrylic acid 2-[2-(3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxy)-acetylamino]-ethyl ester (Compound A9-13, 40 mg, 0.085 mmol) was dissolved in a mixture solvent of methanol (2 mL) and water (2 mL), added with potassium hydroxide (48 mg, 0.85 mmol), and then stirred at room temperature for 18 hr. After the neutralization with 1 N hydrochloric acid, the reaction solution was concentrated under reduced pressure. The resulting residues were purified by amino silica gel to obtain the title compound (white solid, 8.9 mg, 26%).
- 1H-NMR (300 MHz, DMSO) a ppm 12.75 (s, 1H), 8.32 (d, 1H, J=8.1 Hz), 8.17-8.13 (m, 2 Hz), 7.99 (s, 1H), 7.60 (d, 1H, J=8.1 Hz), 7.38 (d, 1H, J=1.8 Hz), 7.11 (dd, 1H, J=2.1 Hz, 8.7 Hz), 4.72 (t, 1H, J=5.7 Hz), 4.65 (s, 1H), 3.48 (dd, 2H, J=12.0 Hz, 6.0 Hz), 3.26 (dd, 2H, J=12.0 Hz, 6.0 Hz), 1.76 (s, 6H)
- LCMS: m/z 404 [M+H]+
- HPLC retention time: 2.83 min (analysis condition U)
- Compound A9-15-1
-
- (3-Cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxy)-acetic acid (Compound A8-7, 30 mg, 0.083 mmol), piperazine-1-carboxylic acid tert-butyl ester (31 mg, 2 eq.), and HOBt (30 mg, 3 eq.) were dissolved in 0.5 ml DMF, added with EDC (48 mg, 3 eq.), and stirred at room temperature overnight. Thereafter, the solvent was removed under reduced pressure and the resulting residues were purified by preparative TLC to obtain the title compound (20 mg).
- LCMS: m/z 527, 471, 427[M−H]−
- HPLC retention time: 2.77 min (analysis condition C)
- Compound A9-15-2
-
- 4-[2-(3-Cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxy)-acetyl]-piperazine-1-carboxylic acid tert-butyl ester (Compound A9-15-1, 20 mg) was added with 4 N hydrochloric acid and dioxane solution (1 ml), and stirred in an water bath at 10° C. for 4 hr. Water was added to the reaction solution and the resulting precipitates were filtered and dried to obtain the title compound (15 mg, white powder).
- LCMS: m/z 429 [M+H]+
- HPLC retention time: 0.81 min (analysis condition I)
- Compound A9-16
-
- (3-Cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxy)-acetic acid (Compound A8-7, 30 mg, 0.083 mmol), 3-aminopropionitrile (12 mg, 2 eq.) and HOBt (30 mg, 3 eq.) were dissolved in 0.5 ml DMF, added with EDC (48 mg, 3 eq.), and stirred at room temperature overnight. Thereafter, the solvent was removed under reduced pressure and the resulting residues were purified by preparative TLC to obtain the title compound (23 mg).
- LCMS: m/z 411 [M+H]+
- HPLC retention time: 2.27 min (analysis condition C)
- Compound A9-17
-
- (3-Cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yloxy)-acetic acid (Compound A8-7, 30 mg, 0.083 mmol), N-methyl-3-aminopropionitrile (14 mg, 2 eq.) and HOBt (30 mg, 3 eq.) were dissolved in 0.5 ml DMF, added with EDC (48 mg, 3 eq.), and stirred at room temperature overnight. Thereafter, the solvent was removed under reduced pressure and the resulting residues were purified by preparative TLC to obtain the title compound (7 mg).
- LCMS: m/z 411 [M+H]+
- HPLC retention time: 2.33 min (analysis condition C)
- Compound A10
-
- The DMF solution of 8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A6, 100 mg, 0.331 mmol), imidazole (67.5 mg, 3 eq.) and tert-butylchlorodimethylsilane (92.4 mg, 1.5 eq.) was stirred overnight at room temperature. To the reaction solution, saturated aqueous solution of sodium hydrogen carbonate was added followed by extraction with tert-butylmethyl ether. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (white solid, 170 mg, 100%).
- LCMS: m/z 417 [M+H]+
- HPLC retention time: 3.38 min (analysis condition S)
- Compound A10-1
-
- To the THF solution of triphenylphosphine (260 mg, 3 eq.), azodicarboxylic acid diisopropyl ester (0.195 ml, 3 eq.) was added and the mixture was stirred at room temperature for 1 hr. Thereafter, 8-(tert-butyldimethylsilanyloxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A10, 138 mg, 0.331 mmol) and methanol (1 ml) were added and stirred overnight. The reaction solution was purified by HPLC to obtain the target compound (44.8 mg, 41%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.44 (1H, d, J=8.1 Hz), 8.33 (1H, s), 8.14 (1H, d, J=8.7 Hz), 7.66 (1H, dd, J=8.2, 1.1 Hz), 7.39 (1H, d, J=2.3 Hz), 7.09 (1H, dd, J=8.7, 2.3 Hz), 4.17 (3H, s), 3.92 (3H, s), 1.88 (6H, s).
- LCMS: m/z 331 [M+H]+
- HPLC retention time: 2.35 min (analysis condition S)
- Compound A10-2
-
- Under the same conditions as the method for synthesizing Compound B3-4, the title compound was prepared from Compound A9-4.
- LCMS: m/z 478 [M+H]+
- HPLC retention time: 2.68 min (analysis condition U)
- Compound B1
-
- 8-Hydroxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A6, 550 mg, 0.189 mmol) was dissolved in pyridine (18 mL), added with anhydrous trifluoromethanesulfonic acid (0.758 ml, 3 eq.), and stirred at room temperature for 30 min. The reaction solution was added to water and then extracted with dichloromethane. The organic layer was dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (white powder, 641 mg, 81%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.89 (1H, br. s), 8.36 (1H, d, J=8.8 Hz), 8.31 (1H, dd, J=8.1, 0.7 Hz), 8.11 (1H, d, J=2.3 Hz), 8.04 (1H, dd, J=1.5, 0.7 Hz), 7.65-7.60 (2H, m). 1.76 (6H, s)
- LCMS: m/z 435 [M+H]+
- HPLC retention time: 3.10 min (analysis condition U)
- Compound B2-1
-
- Trifluoro-methanesulfonic acid 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl ester (Compound B1, 40 mg, 0.0921 mmol) was dissolved in NMP (1 ml) and added with 1-isopropylpiperazine (236 mg, 20 eq.). The mixture was stirred at 120° C. for 3 hr. After cooling to room temperature, purification was carried out by HPLC to obtain the target compound (white powder, 12.8 mg, 34%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.30 (1H, d, 8.1 Hz), 8.03 (1H, d, 8.6 Hz), 7.98 (1H, s), 7.56 (1H, d, 8.6 Hz), 7.21 (1H, s), 7.04 (1H, d, 9.1 Hz), 3.40-3.37 (4H, m), 2.73-2.65 (1H, m), 2.61-2.58 (4H, m), 1.75 (6H, s), 1.02 (6H, d, 6.6 Hz)
- LCMS: m/z 413 [M+H]+
- Compound B2-2
-
- According to the same method as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and N-(2-hydroxyethyl)piperazine.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.30 (1H, d, 8.1 Hz), 8.03 (1H, d, 8.7 Hz), 7.99 (1H, s), 7.58 (1H, d, 7.9 Hz), 7.21 (1H, s), 7.04 (1H, d, 8.7 Hz), 4.50-4.46 (1H, br m), 3.59-3.53 (2H, m), 3.39-3.35 (4H, m), 2.59-2.56 (4H, m), 2.45 (2H, t, 6.1 Hz), 1.76 (6H, s)
- LCMS: m/z 415 [M+H]+
- HPLC retention time: 1.27 min (analysis condition S)
- Compound B2-3
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and morpholine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.62 (1H, br. s), 8.29 (1H, d, 8.2 Hz), 8.04 (1H, d, 9.0 Hz), 7.96 (1H, s), 7.56 (1H, d, 8.2 Hz), 7.22 (1H, s), 7.04 (1H, d, 9.0 Hz), 3.77-3.75 (4H, m), 3.35-3.30 (4H, m), 1.74 (6H, s)
- LCMS: m/z 372 [M+H]+
- HPLC retention time: 2.45 min (analysis condition U)
- Compound B2-4
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and 4-pyrrolidin-1-yl-piperidine.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.30 (1H, d, 8.1 Hz), 8.01 (1H, d, 8.7 Hz), 7.97 (1H, s), 7.56 (1H, d, 8.6 Hz), 7.20 (1H, s), 3.94-3.90 (2H, m), 3.30-3.28 (4H, m), 2.95 (2H, t, 11.8 Hz), 2.24-2.20 (1H, m), 1.95-1.91 (2H, m), 1.75 (6H, s), 1.70-1.66 (4H, m), 1.54-1.52 (2H, m)
- LCMS: m/z 439 [M+H]+
- Compound B2-5-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and piperazine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 471 [M+H]+
- HPLC retention time: 2.67 min (analysis condition S)
- Compound B2-5-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B2-5-1.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.32 (1H, d, 8.5 Hz), 8.03 (1H, d, 9.1 Hz), 7.99 (1H, s), 7.59 (1H, dd, 8.2, 1.5 Hz), 7.20 (1H, d, 2.4 Hz), 7.04 (1H, dd, 8.8, 2.1 Hz), 3.32-3.30 (4H, m), 2.88-2.87 (4H, m), 1.77 (6H, s)
- LCMS: m/z 371 [M+H]+
- Compound B2-6
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and piperidine.
- LCMS: m/z 370 [M+H]+
- HPLC retention time: 2.40 min (analysis condition U)
- Compound B2-7-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and piperidin-4-ol.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.30 (1H, d, 8.1 Hz), 8.01 (1H, d, 8.7 Hz), 7.97 (1H, s), 7.56 (1H, d, 7.7 Hz), 7.19 (1H, s), 7.04 (1H, d, 10.6 Hz), 4.76-4.71 (1H, br m), 3.81-3.75 (3H, m), 3.08 (2H, t, 10.2 Hz), 1.86-1.82 (2H, m), 1.75 (6H, s), 1.49-1.42 (2H, m)
- LCMS: m/z 386 [M+H]+
- Compound B2-7-2
-
- 8-(4-Hydroxy-piperidin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound B2-7-1, 210 mg, 0.545 mmol), was dissolved in the DCM (2 mL) and DMF (0.6 mL) mixture solvent, added with 1,1,1-triacetoxy-1,1-dihydro-1,2-benzoiodoxol-3 (1H)-one (300 mg, 1.3 eq.), and the mixture was stirred at room temperature for 2 hr. To the reaction solution, 0.25 mol/L aqueous solution of sodium thiosulfate, saturated sodium bicarbonate solution and CPME were added followed by further stirring at room temperature for 1 hr. The reaction solution was filtered and the filtrate was subjected to liquid seperation. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (yellowish white powder, 109 mg, 52%).
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 2.17 min (analysis condition U)
- Compound B2-8
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and 1-methanesulfonylpiperazine.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.66 (1H, br.s), 8.31 (1H, d, J=8.2 Hz), 8.06 (1H, d, J=8.7 Hz), 7.99 (1H, s), 7.59 (1H, d, J=8.2 Hz), 7.30 (1H, d, J=1.8 Hz), 7.09 (1H, dd, J=8.7, 1.8 Hz), 3.53 (4H, t, J=4.8 Hz), 3.27 (4H, t, J=4.8 Hz), 2.94 (3H, s), 1.77 (6H, s).
- LCMS: m/z 449 [M+H]+
- HPLC retention time: 1.98 min (analysis condition S)
- Compound B2-9
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and 3-methanesulfonylpyrrolidine.
- LCMS: m/z 434 [M+H]+
- HPLC retention time: 1.83 min (analysis condition S)
- Compound B2-10
-
- Trifluoro-methanesulfonic acid 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl ester (Compound B1, 30 mg, 0.069 mmol) was dissolved in 1,4-dioxane (1 mL), added with thiomorpholine 1,1-dioxide (19 mg, 2 eq.), Pd2(dba)3 (6.3 mg, 0.1 eq.), BINAP (8.6 mg, 0.2 eq.) and K3PO4 (29 mg, 2 eq.), and stirred at 100° C. overnight. The reaction solution was added to water, and then extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (white powder, 2.1 mg, 7%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.29 (1H, d, J=8.6 Hz), 8.07 (1H, d, J=8.9 Hz), 8.00 (1H, s), 7.55 (1H, dd, J=8.5, 1.7 Hz), 7.34 (1H, d, J=2.0 Hz), 7.15 (1H, dd, J=9.1, 2.7 Hz), 4.01 (4H, s), 3.16 (4H, s), 1.77 (6H, s).
- LCMS: m/z 420 [M+H]+
- HPLC retention time: 1.80 min (analysis condition S)
- Compound B2-11
-
- Under the same conditions as the method for synthesizing Compound B2-10, the title compound was prepared from Compound B1 and 4-cyclopentylpiperazin-2-one.
- LCMS: m/z 453 [M+H]+
- HPLC retention time: 1.30 min (analysis condition S)
- Compound B2-12
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and 4-piperidin-4-yl morpholine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.73 (1H, s), 8.27-8.31 (1H, m), 7.98-8.02 (1H, m), 7.95-7.97 (1H, m), 7.53-7.58 (1H, m), 7.17-7.21 (1H, m), 6.99-7.05 (1H, m), 3.97-4.05 (2H, m), 3.53-3.59 (4H, m), 2.80-2.90 (2H, m), 2.43-2.51 (4H, m), 2.31-2.40 (1H, m), 1.83-1.92 (2H, m), 1.74 (6H, s), 1.39-1.52 (2H, m)
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 1.73 min (analysis condition U)
- Compound B2-13
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-7-2 and 4,4-difluoropiperidine hydrochloric acid salt.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.59 (1H, s), 8.25-8.32 (1H, m), 7.97-8.02 (1H, m), 7.96 (1H, s), 7.52-7.59 (1H, m), 7.16-7.21 (1H, m), 6.99-7.05 (1H, mz), 4.00-4.09 (2H, m), 3.55-3.62 (2H, m), 2.79-2.90 (2H, m), 2.55-2.67 (4H, m), 1.78-1.98 (5H, m), 1.74 (6H, s), 1.44-1.58 (2H, m)
- LCMS: m/z 489 [M+H]+
- HPLC retention time: 1.88 min (analysis condition U)
- Compound B2-14
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-7-2 and (2R,6S)-2,6-dimethylmorpholine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.60 (1H, s), 8.25-8.31 (1H, m), 7.97-8.02 (1H, m), 7.95 (1H, s), 7.51-7.58 (1H, m), 7.18 (1H, s), 6.99-7.05 (1H, m), 3.96-4. 06 (2H, m), 3.45-3.55 (2H, m), 2.80-2.91 (2H, m), 2.72-2.79 (2H, m), 2.29-2.41 (1H, m), 1.70-1.90 (10H, m), 1.40-1.53 (2H, m), 1.03 (6H, d, 6.3 Hz)
- LCMS: m/z 483 [M+H]+
- HPLC retention time: 1.83 min (analysis condition U)
- Compound B2-15
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound B1 and 2,6-dimethylpiperazine.
- LCMS: m/z 399 [M+H]+
- HPLC retention time: 1.76 min (analysis condition U)
- Compound B2-16-1
-
- Under the same conditions as the method for synthesizing Compound B2-10, the title compound was prepared from Compound B1 and (S)-3-methylpiperazine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 485 [M+H]+
- HPLC retention time: 3.97 min (analysis condition W)
- Compound B2-16-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound 2-16-1.
- LCMS: m/z 385 [M+H]+
- HPLC retention time: 2.43 min (analysis condition W)
- Compound B2-16-3
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-16-2 and cyclobutanone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.31 (1H, d, 8 Hz), 8.03 (1H, d, 12 Hz), 7.98 (1H, s), 7.59 (1H, d, 12 Hz), 7.13 (1H, s), 6.98 (1H, d, 8 Hz), 4.35-4.28 (1H, m), 3.70 (1H, d, 12 Hz), 3.02 (1H, ddd, 12, 12, 4 Hz), 2.87 (1H, d, 8 Hz), 2.74-2.67 (2H, m), 2.08-1.99 (2H, m), 1.92-1.64 (10H, m), 1.70-1.62 (2H, m), 1.12 (3H, d, 8 Hz)
- LCMS: m/z 439 [M+H]+
- HPLC retention time: 2.59 min (analysis condition W)
- Compound B2-17
-
- Trifluoro-methanesulfonic acid 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl ester (Compound B1, 25 mg, 0.057 mmol) was dissolved in dimethoxyethane (0.5 mL), added with 2-diethylaminoethanethiol hydrochloric acid salt (19.6 mg, 2 eq.), Pd2(dba)3 (2.6 mg, 0.05 eq.), Xantphos (3.3 mg, 0.1 eq.) and DIPEA (0.06 mg, 6 eq.), and the mixture was stirred at 160° C. for 30 min. The reaction solution was added to water, extracted with ethyl acetate, and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (dichloromethane/methanol) to obtain the target compound (white amorphous, 22.4 mg, 93%).
- 1H-NMR (400 MHz, CDCl3) δ: 9.60 (1H, s), 8.53-8.48 (1H, m), 8.32 (1H, d, J=8.4 Hz), 7.77 (1H, s), 7.53-7.50 (2H, m), 7.38-7.35 (1H, m), 3.18-3.12 (2H, m), 2.81-2.75 (2H, m), 2.65-2.57 (4H, m), 1.76 (6H, s), 1.08-1.04 (6H, m)
- LCMS: m/z 418 [M+H]+
- HPLC retention time: 2.10 min (analysis condition U)
- Compound B2-18
-
- Under the same conditions as the method for synthesizing Compound B2-17, the title compound was prepared from Compound B1 and 2-diisopropylaminoethanethiol hydrochloric acid salt.
- LCMS: m/z 446 [M+H]+
- HPLC retention time: 2.22 min (analysis condition U)
- Compound B2-19
-
- Under the same conditions as the method for synthesizing Compound B2-17, the title compound was prepared from Compound B1 and 2-dimethylaminoethanethiol hydrochloric acid salt.
- LCMS: m/z 390 [M+H]+
- HPLC retention time: 1.98 min (analysis condition U)
- Compound B2-20
-
- Under the same conditions as the method for synthesizing Compound B2-17, the title compound was prepared from Compound B1 and 3-mercaptopropionic acid.
- LCMS: m/z 391 [M+H]+
- HPLC retention time: 2.45 min (analysis condition U)
- Compound B2-21
-
- Under the same conditions as the method for synthesizing Compound B2-17, the title compound was prepared from Compound B1 and 3-mercaptopropane-1,2-diol.
- LCMS: m/z 393 [M+H]+
- HPLC retention time: 2.15 min (analysis condition U)
- Compound B2-22-1
-
- To trifluoro-methanesulfonic acid 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl ester (Compound B1, 7.80 g, 18.0 mmol), 4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester (6.11 g, 19.8 mmol, 1.1 eq.), Pd(PPh3)2Cl2 (630 mg, 0.898 mmol, 0.05 eq.), and sodium carbonate (5.71 g, 53.9 mmol, 3.0 eq.), DME (125 ml) and water (25 ml) were added. The mixture was subjected to reduced pressure under ultrasonication treatment, followed by flushing with nitrogen gas. This procedure was repeated five times and then degassed. After further stirring at 80° C. for 2 hr under nitrogen atmosphere, the mixture was cooled to room temperature, added with water (250 ml), and further stirred for 30 min. The precipitates were filtered and washed with water (50 ml). They were further washed with CH3CN (50 ml) to obtain the target compound as a crude product (gray powder, 7.54 g, 90%).
- LCMS: m/z 468 [M+H]+
- HPLC retention time: 2.90 min (analysis condition S)
- Compound B2-22-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B2-22-1.
- LCMS: m/z 368 [M+H]+
- HPLC retention time: 1.47 min (analysis condition S)
- Compound B2-23
-
- Under the same conditions as the method for synthesizing Compound B2-22-1, the title compound was prepared from Compound B1 and 1-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole.
- LCMS: m/z 367 [M+H]+
- HPLC retention time: 2.42 min (analysis condition U)
- Compound B2-24
-
- Under nitrogen atmosphere, trifluoro-methanesulfonic acid 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl ester (Compound B1, 1.00 g, 2.302 mmol) was added with n-propanol (20 mL), potassium vinyltrifluoroborate (854 mg, 3.0 eq.), dichloro-((bis-diphenylphosphino)ferrocenyl)palladium (217 mg, 0.1 eq.) and triethylamine (1.11 ml, 3.0 eq.) in order and the resultant was stirred at 60° C. for 4 hr. Upon the completion of the reaction, water was added to the reaction solution. The resulting precipitates were filtered and washed with distilled water, and the residues were dried to obtain the title compound (666 mg, 80%).
- 1H-NMR (400 MHz, CDCl3) δ: 8.90 (1H, s), 8.55 (1H, d, J=7.9 Hz), 8.40 (1H, d, J=8.5 Hz), 7.79 (1H, s), 7.58-7.61 (3H, m), 6.85 (1H, dd, J=17.7, 11.0 Hz), 5.95 (1H, d, J=17.1 Hz), 5.46 (1H, d, J=11.0 Hz), 1.84 (6H, s)
- LCMS: m/z 313 [M+H]+
- HPLC retention time: 3.75 min (analysis condition W)
- Compound B2-25-1
-
- 4-Methylene-piperidine-1-carboxylic acid tert-butyl ester (409 mg, 2.07 mmol, 1.2 eq.) was dissolved in THF (2 ml), added under nitrogen atmosphere with 9-BBN (0.5 M THF solution, 4.83 ml, 2.42 mmol, 1.4 eq.) and then stirred at 60° C. for 1 hr. Thereafter, 9-BBN (0.5 M THF solution, 5.52 ml, 2.77 mmol, 1.6 eq.) was further added and the mixture was stirred at 60° C. for 1 hr. The resulting mixture was cooled to room temperature, added with cesium fluoride (1.31 g, 8.60 mmol, 5.0 eq.), and stirred at room temperature for 30 min.
- To the solution obtained from the above, DMF (18 ml) suspension comprising trifluoro-methanesulfonic acid 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl ester (Compound B1, 750 mg, 1.73 mmol) and dichloro-((bisdiphenylphosphino)ferrocenyl)palladium (70.5 mg, 0.0863 mmol, 0.05 eq.) was added, and the mixture was stirred at 100° C. for 3 hr. After cooling to the room temperature, water (50 ml) was added, followed by extraction with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain 4-(3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl methyl)-piperidine-1-carboxylic acid tert-butyl ester (yellow powder, 763 mg, 91%).
- LCMS: m/z 484 [M+H]+
- HPLC retention time: 2.97 min (analysis condition S)
- Compound B2-25-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B2-25-1.
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 1.40 min (analysis condition S)
- Compound B2-26-1
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B2-25-2 and N-(tert-butoxycarbonyl)-N-[4-(dimethylazaniumylidene)-1,4-dihydropyridin-1-yl sulfonyl]azanide (CAS No. 872496-91-8).
- LCMS: m/z 563 [M+H]+
- HPLC retention time: 2.63 min (analysis condition S)
- Compound B2-26-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B2-26-1.
- LCMS: m/z 463 [M+H]+
- HPLC retention time: 2.10 min (analysis condition S)
- Compound B2-27
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-25-2 and acetone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.80 (1H, s), 8.32 (1H, d, 7.9 Hz), 8.12 (1H, d, 7.9 Hz), 8.01 (1H, s), 7.65 (1H, s), 7.61 (1H, d, 9.1 Hz), 7.30 (1H, d, 7.9 Hz), 2.75 (2H, d, 11.0 Hz), 2.65 (3H, q, 6.5 Hz), 2.04 (2H, t, 11.0 Hz), 1.77 (6H, s), 1.60-1.57 (3H, m), 1.22 (2H, t, 11.6 Hz), 0.94 (6H, d, 6.7 Hz)
- LCMS: m/z 426 [M+H]+
- Compound B2-28
-
- Under nitrogen atmosphere, to the dimethyl formamide (3 ml) solution comprising trifluoro-methanesulfonic acid 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl ester (Compound B1, 150 mg, 0.345 mmol), lithium formate monohydrate (90 mg, 5.0 eq.), 4,5-bis (diphenylphosphino)-9,9-dimethylxanthene (Xantphos) (20 mg, 0.1 eq.), Pd2(dba)3 (32 mg, 0.1 eq.), lithium chloride (88 mg, 6.0 eq.), N,N-diisopropylethylamine (241 μl, 4.0 eq.), and acetic anhydride (131 μl, 4.0 eq.) were added, and the mixture was stirred at 80° C. for 15 hr. Upon the completion of the reaction, ethyl acetate was added to the reaction solution. The organic layer was washed in order with 1 M hydrochloric acid, distilled water, and brine. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (88 mg, 76%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 13.17 (1H, s), 8.35 (1H, d, J=7.9 Hz), 8.34 (1H, s), 8.23 (1H, d, J=7.9 Hz), 8.07 (1H, s), 8.02 (1H, d, J=9.1 Hz), 7.64 (1H, d, J=7.9 Hz), 1.80 (6H, s)
- LCMS: m/z 331 [M+H]+
- HPLC retention time: 3.08 min (analysis condition W)
- Compound B2-29
-
- To the THF (24 ml) and distilled water (6 ml) suspension of 6,6-dimethyl-11-oxo-8-vinyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound B2-24, 600 mg, 1.920 mmol), t-butanol solution of osmium tetraoxide (192 μl, 0.1 eq.) and sodium meta periodate (821 mg, 2.0 eq.) were added and the mixture was stirred at room temperature for 3 hr. Aqueous solution of sodium thiosulfate (0.3 M) was added to the solution, which was then extracted with an ethyl acetate. The organic layer was washed with 10% aqueous solution of disodium ethylenediamine tetraacetic acid. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (470 mg, 77%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.95 (1H, s), 10.20 (1H, s), 8.48 (1H, s), 8.42 (1H, d, J=8.5 Hz), 8.36 (1H, d, J=8.5 Hz), 8.07 (1H, s), 8.02 (1H, d, J=7.9 Hz), 7.67 (1H, d, J=7.9 Hz), 1.85 (6H, s)
- LCMS: m/z 315 [M+H]+
- HPLC retention time: 3.38 min (analysis condition W)
- Compound B3-1
-
- Under the same conditions as the method for synthesizing Compound A10-1, the title compound was prepared from Compound B2-3.
- LCMS: m/z 386 [M+H]+
- HPLC retention time: 2.62 min (analysis condition U)
- Compound B3-2-1
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B2-5-2 and N-(tert-butoxycarbonyl)-N-[4-(dimethylazaniumylidene)-1,4-dihydropyridin-1-yl sulfonyl]azanide (CAS No. 872496-91-8).
- LCMS: m/z 550 [M+H]+
- HPLC retention time: 2.39 min (analysis condition S)
- Compound B3-2-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B3-2-1.
- LCMS: m/z 450 [M+H]+
- HPLC retention time: 1.82 min (analysis condition S)
- Compound B3-3
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B2-5-2 and dimethylsulfamoyl chloride.
- LCMS: m/z 478 [M+H]+
- HPLC retention time: 2.45 min (analysis condition S)
- Compound B3-4
-
- To the DMF suspension of 4-(3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl)-piperazine-1-sulfonic acid amide (Compound B3-2-2, 20 mg, 0.04 mmol) and sodium hydride (21.4 mg, 12 eq.), iodomethane (28 μl, 10 eq.) was added and stirred at room temperature overnight. Water was added to the reaction solution, followed by filtration to obtain the target compound (25.8 mg, 100%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.43 (1H, d, J=8.2 Hz), 8.31 (1H, s), 8.03 (1H, d, J=8.9 Hz), 7.64 (1H, dd, J=8.1, 1.3 Hz), 7.30 (1H, d, J=2.0 Hz), 7.08 (1H, dd, J=8.9, 2.0 Hz), 4.16 (3H, s), 3.43-3.53 (4H, t, J=4.7 Hz), 3.26-3.41 (4H, s), 2.82 (6H, s), 1.87 (6H, s).
- LCMS: m/z 492 [M+H]+
- HPLC retention time: 2.69 min (analysis condition S)
- Compound B3-5
-
- The title compound was obtained as a by-product of the synthesis of Compound F5-36.
- LCMS: m/z 411 [M+H]+
- HPLC retention time: 1.31 min (analysis condition S)
- Compound B3-6
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-5-2 and cyclobutanone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.29 (1H, d, J=8.2 Hz), 8.01 (1H, d, J=8.8 Hz), 7.96 (1H, s), 7.55 (1H, d, J=8.2 Hz), 7.19 (1H, d, J=2.2 Hz), 7.03 (1H, dd, J=2.4, 8.8 Hz), 2.71-2.75 (1H, m), 2.37-2.39 (4H, m), 1.98-2.00 (2H, m), 1.77-1.85 (2H, m), 1.74 (6H, s), 1.63-1.68 (2H, m).
- LCMS: m/z 425 [M+H]+
- HPLC retention time: 1.80 min (analysis condition U)
- Compound B3-7
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-5-2 and 3-oxetanone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.29 (1H, dd, J=8.2, 0.59 Hz), 8.02 (1H, d, J=9.0 Hz), 7.97 (1H, d, J=0.59 Hz), 7.56 (1H, dd, J=8.0, 1.4 Hz), 7.22 (1H, d, J=2.3 Hz), 7.04 (1H, dd, J=8.8, 2.2 Hz), 4.56-4.59 (2H, m), 4.47-4.50 (2H, m), 3.43-3.48 (1H, m), 3.39-3.42 (4H, m), 2.40-2.42 (4H, m), 1.74 (6H, s)
- LCMS: m/z 427 [M+H]+
- HPLC retention time: 1.67 min (analysis condition U)
- Compound B3-8
-
- 8-(2-Diethylamino-ethylsulfanyl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound B2-17, 16.8 mg, 0.0402 mmol) was dissolved in methanol (1.5 mL), added with oxone (54.3 mg, 2.2 eq.) which had been dissolved in water (0.5 mL), and then stirred at room temperature for 2 hr. The reaction solution was concentrated, extracted with ethyl acetate, washed with saturated sodium hydrogen carbonate, and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (dichloromethane/methanol) to obtain the target compound (white solid, 5.8 mg, 32%).
- 1H-NMR (400 MHz, CDCl3) δ: 9.29 (1H, s), 8.61 (1H, d, J=8.2 Hz), 8.52 (1H, d, J=8.0 Hz), 8.21 (1H, s), 8.01 (1H, d, J=8.2 Hz), 7.81 (1H, s), 7.61 (1H, d, J=8.2 Hz), 3.33 (2H, t, J=7.4 Hz), 2.95 (2H, t, J=7.4 Hz), 2.41 (4H, q, J=7.2 Hz), 1.86 (6H, s), 0.89 (4H, t, J=7.1 Hz)
- LCMS: m/z 450 [M+H]+
- HPLC retention time: 2.05 min (analysis condition U)
- Compound B3-9
-
- Under the same conditions as the method for synthesizing Compound B3-8, the title compound was prepared from Compound B2-18.
- LCMS: m/z 478 [M+H]+
- HPLC retention time: 2.18 min (analysis condition U)
- Compound B3-10
-
- Under the same conditions as the method for synthesizing Compound B3-8, the title compound was prepared from Compound B2-19.
- LCMS: m/z 422 [M+H]+
- HPLC retention time: 2.03 min (analysis condition U)
- Compound B3-11
-
- Under the same conditions as the method for synthesizing Compound B3-8, the title compound was prepared from Compound B2-20.
- LCMS: m/z 423 [M+H]+
- HPLC retention time: 2.28 min (analysis condition U)
- Compound B3-12
-
- Under the same conditions as the method for synthesizing Compound B3-8, the title compound was prepared from Compound B2-21.
- LCMS: m/z 425 [M+H]+
- HPLC retention time: 2.17 min (analysis condition U)
- Compound B3-13-1
-
- 4-(3-Cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl)-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester (Compound B2-22-1, 16.2 g, 34.6 mmol) was dissolved in THF (800 ml) and methanol (230 ml), added with 10 wt % Pd/C (3.2 g), and stirred under hydrogen atmosphere for 19 hr. The solid was filtered through Celite, eluted with a mixture solvent (400 ml; THF/methanol=4/1), and concentrated under reduced pressure. The residues were dissolved in ethyl acetate (400 ml), and then washed with 1% aqueous solution of N-acetylcysteine, saturated aqueous solution of NaHCO3 and saturated brine. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues were concentrated under reduced pressure to obtain the title compound as a crude product (white powder, 14.0 g, 86%).
- LCMS: m/z 470 [M+H]+
- HPLC retention time: 2.88 min (analysis condition S)
- Compound B3-13-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B3-13-1.
- LCMS: m/z 370 [M+H]+
- HPLC retention time: 1.30 min (analysis condition S)
- Compound B3-14
-
- To the THF (1 ml) solution of 6,6-dimethyl-11-oxo-8-vinyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound B2-24, 20 mg, 0.064 mmol), t-butanol solution of osmium tetraoxide (19 μl, 0.3 eq.) and 50% aqueous solution of N-methylmorpholine-N-oxide (30 μl, 2.0 eq.) were added and the mixture was stirred at room temperature for 3 hr. To the reaction solution, 10% aqueous solution of disodium ethylenediamine tetraacetic acid was added, followed by extraction with ethyl acetate. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by high performance liquid chromatography to obtain the title compound (21 mg, 63%).
- 1H-NMR (400 MHz, CD3OD) δ: 8.41 (1H, d, J=7.9 Hz), 8.29 (1H, d, J=7.9 Hz), 7.87 (1H, s), 7.86 (1H, s), 7.57 (1H, d, J=7.9 Hz), 7.52 (1H, d, J=6.7 Hz), 4.85 (1H, dd, J=7.0, 4.6 Hz), 3.73 (1H, dd, J=11.3, 4.6 Hz), 3.68 (1H, dd, J=11.3, 7.0 Hz), 1.83 (6H, s)
- LCMS: m/z 347 [M+H]+
- HPLC retention time: 2.68 min (analysis condition W)
- Compound B3-15
-
- To the tetrahydrofuran (1 ml) solution of 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-carboxylic acid (Compound B2-28, 15 mg, 0.045 mmol), morpholine (6 μl, 1.5 eq.), hexafluorophosphoric acid uronium 2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3-tetramethylmethane aminium (HATU) (26 mg, 1.5 eq.), and N,N-diisopropylethylamine (24 μl, 3.0 eq.) were added and the mixture was stirred at room temperature for 3 hr. The reaction solution was filtered to remove insoluble matters and the residues obtained after concentration under reduced pressure were purified by high performance liquid chromatography to obtain the title compound (11 mg, 55%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.85 (1H, s), 8.33 (1H, d, J=8.5 Hz), 8.27 (1H, d, J=7.9 Hz), 8.03 (1H, s), 7.92 (1H, s), 7.63 (1H, d, J=8.5 Hz), 7.54 (1H, d, J=7.9 Hz), 3.52-3.77 (6H, m), 3.30-3.42 (2H, m), 1.79 (6H, s)
- LCMS: m/z 400 [M+H]+
- HPLC retention time: 2.96 min (analysis condition W)
- Compound B3-16
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and 1-methanesulfonylpiperazine.
- LCMS: m/z 477 [M+H]+
- HPLC retention time: 3.03 min (analysis condition W)
- Compound B3-17
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and piperidin-4-ol.
- LCMS: m/z 414 [M+H]+
- HPLC retention time: 2.75 min (analysis condition W)
- Compound B3-18
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and 2-aminopropane-1,3-diol.
- LCMS: m/z 404 [M+H]+
- HPLC retention time: 2.60 min (analysis condition W)
- Compound B3-19
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and 2-methanesulfonylethylamine.
- LCMS: m/z 436 [M+H]+
- HPLC retention time: 2.87 min (analysis condition W)
- Compound B3-20
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and (1,1-dioxotetrahydrothiophen-3-yl)amine
- LCMS: m/z 448 [M+H]+
- HPLC retention time: 1.70 min (analysis condition S)
- Compound B3-21
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and (R)-(+)-3-amino-1,2-propanediol.
- LCMS: m/z 404 [M+H]+
- HPLC retention time: 1.38 min (analysis condition S)
- Compound B3-22
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and N,N-diethanolamine.
- LCMS: m/z 418 [M+H]+
- HPLC retention time: 1.35 min (analysis condition S)
- Compound B3-23
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and oxetan-3-yl amine.
- LCMS: m/z 386 [M+H]+
- HPLC retention time: 1.63 min (analysis condition S)
- Compound B3-24
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and 2-aminooxy-ethanol.
- LCMS: m/z 390 [M+H]+
- HPLC retention time: 1.54 min (analysis condition S)
- Compound B3-25-1
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and (2-amino-ethyl)-carbamic acid tert-butyl ester.
- LCMS: m/z 473 [M+H]+
- HPLC retention time: 2.08 min (analysis condition S)
- Compound B3-25-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B3-25-1.
- LCMS: m/z 373 [M+H]+
- HPLC retention time: 1.19 min (analysis condition S)
- Compound B3-25-3
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B3-25-2.
- LCMS: m/z 451 [M+H]+
- HPLC retention time: 1.62 min (analysis condition S)
- Compound B3-26
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and 2-methylamino-ethanol.
- LCMS: m/z 388 [M+H]+
- HPLC retention time: 1.53 min (analysis condition S)
- Compound B3-27-1
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B3-25-2 and N-(tert-butoxycarbonyl)-N-[4-(dimethylazaniumylidene)-1,4-dihydropyridin-1-yl sulfonyl]azanide (CAS No. 872496-91-8).
- LCMS: m/z 552 [M+H]+
- HPLC retention time: 2.03 min (analysis condition S)
- Compound B3-27-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B3-27-1.
- LCMS: m/z 452 [M+H]+
- HPLC retention time: 1.57 min (analysis condition S)
- Compound B3-28
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and 2-piperazin-1-yl ethanol.
- LCMS: m/z 443 [M+H]+
- HPLC retention time: 1.75 min (analysis condition U)
- Compound B3-29
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and 1-tert-butylpiperazine.
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 1.88 min (analysis condition U)
- Compound B3-30
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and 1-(2-methoxyethyl)piperazine.
- LCMS: m/z 457 [M+H]+
- HPLC retention time: 1.83 min (analysis condition U)
- Compound B3-31-1
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound B2-28 and piperazine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 499 [M+H]+
- HPLC retention time: 2.63 min (analysis condition U)
- Compound B3-31-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B3-31-1.
- LCMS: m/z 399 [M+H]+
- HPLC retention time: 1.78 min (analysis condition U)
- Compound B3-32
-
- To the THF (1 ml) solution of Compound B2-29 (30 mg, 0.095 mmol), morpholine (6 μl, 1.5 eq.) and sodium triacetoxyborohydride (81 mg, 2.0 eq.) were added and stirred at room temperature for 1 hr. The reaction solution was filtered to remove insoluble matters, and the residues obtained after concentration under reduced pressure were purified by high performance liquid chromatography to obtain the title compound (19 mg, 50%).
- 1H-NMR (400 MHz, CD3OD) δ: 8.41 (1H, d, 7.9 Hz), 8.27 (1H, d, 8.5 Hz), 7.87 (1 s), 7.81 (1H, s), 7.56 (1H, d, 8.5 Hz), 7.49 (1H, d, 7.9 Hz), 3.71 (4H, t, 4.6 Hz), 3.68 (2H, s), 2.51 (4H, t, 4.6 Hz), 1.82 (6H, s)
- LCMS: m/z 386 [M+H]+
- HPLC retention time: 2.41 min (analysis condition W)
- Compound B3-33
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-29 and 1-methylpiperazine.
- 1H-NMR (400 MHz, CD3OD) δ: 8.41 (1H, d, 7.9 Hz), 8.26 (1H, d, 7.9 Hz), 7.88 (1 s), 7.81 (1H, s), 7.56 (1H, d, 7.9 Hz), 7.48 (1H, d, 7.9 Hz), 3.70 (2H, s), 2.42-2.78 (8H, m), 2.31 (3H, s), 1.82 (6H, s)
- LCMS: m/z 399 [M+H]+
- HPLC retention time: 2.30 min (analysis condition W)
- Compound B3-34
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-29 and thiomorpholine 1,1-dioxide.
- LCMS: m/z 434 [M+H]+
- HPLC retention time: 2.75 min (analysis condition W)
- Compound B3-35
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-29 and 1-methanesulfonylpiperazine.
- 1H-NMR (400 MHz, CD3OD) δ: 8.41 (1H, d, 8.5 Hz), 8.28 (1H, d, 7.9 Hz), 7.87 (1 s), 7.80 (1H, s), 7.56 (1H, d, 8.5 Hz), 7.50 (1H, d, 7.9 Hz), 3.73 (2H, s), 3.24-3.28 (4H, m), 2.85 (3H, s), 2.59-2.65 (4H, m), 1.82 (6H, s)
- LCMS: m/z 463 [M+H]+
- HPLC retention time: 2.47 min (analysis condition W)
- Compound B3-36
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-29 and piperazine-1-sulfonic acid dimethylamide.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.79 (1H, s), 8.32 (1H, d, 7.9 Hz), 8.18 (1H, d, 7.9 Hz), 8.00 (1s), 7.77 (1H, s), 7.60 (1H, d, 7.9 Hz), 7.46 (1H, d, 7.9 Hz), 3.67 (2H, s), 3.18-3.23 (4H, m), 2.76 (6H, s), 2.45-2.50 (4H, m), 1.77 (6H, s)
- LCMS: m/z 492 [M+H]+
- HPLC retention time: 2.58 min (analysis condition W)
- Compound B3-37
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B2-29 and 2,2,2-trifluoroethylamine.
- LCMS: m/z 398 [M+H]+
- HPLC retention time: 2.73 min (analysis condition W)
- Compound B3-38
-
- The by-product obtained from the synthesis of Compound B3-37 was purified by high performance liquid chromatography to obtain the target compound.
- LCMS: m/z 317 [M+H]+
- HPLC retention time: 2.91 min (analysis condition W)
- Compound B4-1
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B3-13-2 and cyclobutanone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.73 (1H, s), 8.28-8.33 (1H, m), 8.09-8.14 (1H, m), 7.99 (1H, s), 7.72 (1H, s), 7.56-7.62 (1H, m), 7.34-7.41 (1H, m), 3.52-3.64 (2H, m), 2.85-2.95 (2H, m), 2.56-2.75 (2H, m), 1.91-2.04 (2H, m), 1.56-1.84 (14H, m)
- LCMS: m/z 424 [M+H]+
- HPLC retention time: 1.87 min (analysis condition U)
- Compound B4-2
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B3-13-2 and mesyl chloride.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.77 (1H, s), 8.31 (1H, d, 8.6 Hz), 8.15 (1H, d, 8.2 Hz), 8.00 (1H, s), 7.77 (1H, s), 7.59 (1H, d, 7.3 Hz), 7.42 (1H, d, 8.6 Hz), 3.74-3.70 (1H, m), 2.93 (3H, s), 2.86-2.77 (4H, m), 1.93-1.87 (4H, m), 1.77 (6.0H, s)
- LCMS: m/z 448 [M+H]+
- HPLC retention time: 2.37 min (analysis condition S)
- Compound B4-3-1
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B3-13-2 and N-(tert-butoxycarbonyl)-N-[4-(dimethylazaniumylidene)-1,4-dihydropyridine-1-yl-sulfonyl]azanide.
- LCMS: m/z 549 [M+H]+
- HPLC retention time: 2.72 min (analysis condition S)
- Compound B4-3-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B4-3-1.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.78 (1H, s), 8.29 (1H, d, 7.9 Hz), 8.14 (1H, d, 8.5 Hz), 7.97 (1H, s), 7.76 (1H, s), 7.55 (1H, d, 8.5 Hz), 7.41 (1H, d, 7.9 Hz), 6.79 (2H, s), 3.63 (2H, d, 12.2 Hz), 2.80-2.73 (1H, m), 2.70-2.64 (2H, m), 1.96-1.93 (2H, m), 1.87-1.81 (2H, m), 1.77 (6H, s)
- LCMS: m/z 449 [M+H]+
- HPLC retention time: 2.03 min (analysis condition S)
- Compound B4-4
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B3-13-2 and 2-oxooxazolidine-3-sulfonic acid methylamide.
- LCMS: m/z 463 [M+H]+
- HPLC retention time: 2.40 min (analysis condition S)
- Compound B4-5
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B3-13-2 and dimethylsulfamoyl chloride.
- LCMS: m/z 477 [M+H]+
- HPLC retention time: 2.65 min (analysis condition S)
- Compound B4-6
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B3-13-2 and iodomethane.
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 1.50 min (analysis condition S)
- Compound B4-7
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B3-13-2 and acetone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.77 (1H, s), 8.32 (1H, d, 7.9 Hz), 8.13 (1H, d, 7.9 Hz), 8.01 (1H, s), 7.73 (1H, s), 7.61 (1H, d, 9.1 Hz), 7.39 (1H, d, 9.8 Hz), 2.93 (2H, d, 11.0 Hz), 2.77-2.71 (1H, m), 2.67-2.62 (1H, m), 2.25 (2H, t, 10.1 Hz), 1.80-1.73 (10H, m), 1.02 (6H, d, 6.7 Hz)
- LCMS: m/z 412 [M+H]+
- HPLC retention time: 1.60 min (analysis condition S)
- Compound B4-8
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound B3-13-2 and oxetan-3-one.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.74 (1H, s), 8.32 (1H, d, 7.9 Hz), 8.13 (1H, d, 7.9 Hz), 8.00 (1H, s), 7.74 (1H, s), 7.61 (1H, d, 9.8 Hz), 7.40 (1H, d, 7.9 Hz), 4.56 (2H, t, 6.7 Hz), 4.46 (2H, t, 6.1 Hz), 3.46-3.39 (1H, m), 2.85-2.82 (2H, m), 2.71-2.64 (1H, m), 1.92-1.86 (2H, m), 1.82-1.79 (4H, m), 1.77 (6H, s)
- LCMS: m/z 426 [M+H]+
- HPLC retention time: 1.53 min (analysis condition S)
- Sulfuric Acid Salt of Compound B4-8
- 6,6-Dimethyl-8-(1-oxetan-3-yl-piperidin-4-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile was dissolved at 80° C. in a mixture of 5 volumes of DMA and 1.4 volumes of 2 N sulfuric acid. After cooling to room temperature, 15 volumes of acetone were added dropwise, and the precipitated solids were filtered and dried to obtain sulfuric acid salt of 6,6-dimethyl-8-(1-oxetan-3-yl-piperidin-4-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.81 (1H, s), 10.26 (1H, br. s), 8.33 (1H, d, 8.3 Hz), 8.21 (1H, d, 8.3 Hz), 8.04 (1H, s), 7.75 (1H, s), 7.63 (1H, d, 8.3 Hz), 7.41 (1H, d, 8.3 Hz), 4.85-4.70 (4H, m), 4.50-4.40 (1H, br. s), 3.60-3.00 (6H, br. m), 2.20-2.10 (2H, m), 2.05-1.90 (2H, m), 1.79 (6H, s)
- LCMS: m/z 426 [M+H]+
- Compound B4-9
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound B-3-13-2 and ethylisocyanate.
- LCMS: m/z 441 [M+H]+
- HPLC retention time: 2.20 min (analysis condition S)
- Compound B4-10
-
- According to the method disclosed in Journal of Organic Chemistry, 2003, page 115, 6,6-dimethyl-11-oxo-8-piperidin-4-yl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound B3-13-2, 10 mg, 0.027 mmol) was reacted with 3-(imidazole-1-sulfonyl)-1-methyl-3H-imidazol-1-ium (19 mg, 2 eq.). After removing the solvent, the residues were purified by liquid chromatography to obtain the title compound (3 mg).
- LCMS: m/z 500 [M+H]+
- HPLC retention time: 2.80 min (analysis condition C)
- Compound CC1
-
- To the dichloromethane (2 ml) solution of 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 200 mg, 0.980 mmol), chlorosulfonic acid (110 μl, 1.70 eq.) was added and the mixture was stirred at room temperature for 2 hr. To the reaction solution, oxalyl chloride (297 μl, 3.0 eq.) and N,N-dimethyl formamide (45 μl, 0.6 eq.) were added in three divided portions, and the mixture was stirred at room temperature for 30 min. The reaction solution was concentrated under reduced pressure to obtain the title compound (295 mg). Since the title compound is unstable, its structure was identified in the next step.
- Compound CC2-1
-
- The THF (4 ml) solution of 3-methoxy-5,5-dimethyl-6-oxo-5,6,7,8-tetrahydro-naphthalen-2-sulfonyl chloride (Compound CC1, 295 mg, 0.974 mmol) was cooled to 0° C., and the tetrafuran (1 ml) solution combining pyrrolidine (121 μl, 1.5 eq.) and triethylamine (272 μl, 2 eq.) was added dropwise thereto over 2 min. The mixture was stirred at 0° C. until Compound CC-1 disappears. The reaction solution was added with distilled water and extracted with ethyl acetate. The organic layer was washed with 10% aqueous solution of disodium ethylenediamine tetraacetic acid. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (246 mg, 75%).
- 1H-NMR (400 MHz, CDCl3) δ: 7.76 (1H, s), 6.93 (1H, s), 3.95 (3H, s), 3.37-3.46 (4H, m), 3.09 (0.0H, t, J=6.9 Hz), 2.69 (0.0H, t, J=6.9 Hz), 1.82-1.91 (4H, m), 1.47 (6H, s)
- LCMS: m/z 338 [M+H]+
- HPLC retention time: 3.21 min (analysis condition W)
- Compound CC2-2
-
- Under the same conditions as the method for synthesizing Compound CC2-1, the title compound was prepared from Compound CC and N-methylpiperazine.
- LCMS: m/z 367 [M+H]+
- HPLC retention time: 2.22 min (analysis condition Y)
- Compound CC3-1
-
- Under the same conditions as the method for synthesizing Compound E2-1, the title compound was prepared from Compound CC2-1.
- LCMS: m/z 436 [M+H]+
- HPLC retention time: 3.76 min (analysis condition W)
- Compound CC3-2
-
- Under the same conditions as the method for synthesizing Compound A3-1, the title compound was prepared from Compound CC2-2.
- LCMS: m/z 519 [M+H]+
- HPLC retention time: 2.99 min (analysis condition Y)
- Compound CC4-1
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound CC3-1.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.86 (1H, s), 8.60 (1H, s), 8.30 (1H, d, J=8.5 Hz), 8.01 (1H, s), 7.60 (1H, s), 7.59 (1H, d, J=8.5 Hz), 4.09 (3H, s), 3.21-3.42 (4H, m), 1.72-1.90 (10H, m)
- LCMS: m/z 450 [M+H]+
- HPLC retention time: 3.40 min (analysis condition W)
- Compound CC4-2
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound CC3-2.
- LCMS: m/z 532, 534 [M+H]+
- HPLC retention time: 2.18 min (analysis condition U)
- Compound CC-4-3
-
- Under the same conditions as the method for synthesizing Compound A5-2, the title compound was prepared from Compound CC4-2.
- LCMS: m/z 479 [M+H]+
- HPLC retention time: 1.93 min (analysis condition U)
- Compound C1-1
-
- 8-Hydroxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound A6, 50 mg, 0.165 mmol) was dissolved in DMF (1.5 mL), added with sodium hydride (13 mg, 2.0 eq.) and dimethylsulfamoyl chloride (0.02 mL, 1.2 eq.), and then stirred at room temperature for 1 hr. Water was added to the reaction solution, which was then extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues were concentrated under reduced pressure to obtain the target compound (yellowish white powder, 62 mg, 92%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.87 (1H, s), 8.40-8.30 (2H, m), 8.05 (1H, s), 7.82 (1H, d, J=1.8 Hz), 7.64 (1H, d, J=7.9 Hz), 7.50 (1H, dd, J=8.5, 2.4 Hz), 2.96 (6H, s), 1.81 (6H, s)
- LCMS: m/z 410 [M+H]+
- HPLC retention time: 2.38 min (analysis condition S)
- Compound C1-2
-
- According to the same method as the method for synthesizing Compound A8-17, the title compound was prepared as a crude product from Compound A6 and Compound A8-18-0.
- Compound C1-4
-
- According to the same method as the method for synthesizing Compound A8-17, the title compound was prepared as a crude product from Compound A6 and Compound A8-19-0.
- Compound C2-1
-
- To dimethyl-sulfamic acid 3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl ester (Compound C1-1, 250 mg, 0.610 mmol), aluminum chloride (1.0 M, nitromethane solution (1.8 mL, 3.0 eq.)) was added and the mixture was stirred at 160° C. for 10 min under irradiation with microwave. Water was added to the reaction solution, which was then extracted with dichloromethane. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (dichloromethane/methanol) to obtain the target compound (yellowish white powder, 99 mg, 40%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.78 (1H, s), 11.72 (1H, s), 8.50 (1H, s), 8.32 (1H, d, J=8.5 Hz), 8.02 (1H, s), 7.62 (1H, d, J=7.9 Hz), 7.25 (1H, s), 2.80 (6H, s), 1.75 (6H, s).
- LCMS: m/z 410 [M+H]+
- HPLC retention time: 2.00 min (analysis condition S)
- Compound C2-2
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound CC4-1.
- LCMS: m/z 436 [M+H]+
- HPLC retention time: 3.32 min (analysis condition W)
- Compound C2-3
-
- Under the same conditions as the method for synthesizing Compound C2-1, the title compound was prepared from Compound C1-2.
- LCMS: m/z 452 [M+H]+
- HPLC retention time: 1.89 min (analysis condition S)
- Compound C2-4
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound CC4-3.
- LCMS: m/z 465 [M+H]+
- HPLC retention time: 1.87 min (analysis condition U)
- Compound C3-1
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound C2-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 13.05 (1H, s), 8.67 (1H, s), 8.32 (1H, d, J=8.2 Hz), 8.06 (2H, m), 7.67 (1H, dd, J=7.9, 1.3 Hz), 2.79 (6H, s), 1.84 (6H, s).
- LCMS: m/z 542 [M+H]+
- HPLC retention time: 2.67 min (analysis condition S)
- Compound C3-2
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound C2-2.
- LCMS: m/z 568 [M+H]+
- HPLC retention time: 4.00 min (analysis condition W)
- Compound C4-1
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound C2-1 and 1-bromo-2-methoxy-ethane.
- 1H-NMR (300 MHz, DMSO-d6) σppm; 12.8 (s, 1H), 8.58 (s, 1H), 8.31 (d, 1H, J=8.4 Hz), 8.03 (s, 1H), 7.62 (m, 2H), 4.47 (m, 2H), 3.75 (m, 2H), 3.32 (s, 3H), 2.27 (s, 6H), 1.83 (s, 6H)
- LCMS: m/z 468 [M+H]+
- HPLC retention time: 2.68 min (analysis condition U)
- Compound C4-2
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound C2-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.57 (1H, s), 8.29 (1H, d, J=8.4 Hz), 8.02 (1H, s), 7.70-7.60 (2H, m), 4.37 (2H, t, J=6.3 Hz), 2.84 (2H, m), 2.80 (6H, s), 2.64-2.53 (4H, m), 1.83 (6H, s), 0.98 (6H, t, J=7.1 Hz).
- LCMS: m/z 509 [M+H]+
- HPLC retention time: 1.55 min (analysis condition S)
- Compound C4-3
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound C2-1 and iodomethane.
- LCMS: m/z 424 [M+H]+
- HPLC retention time: 2.17 min (analysis condition S)
- Compound C4-4
-
- Under the same conditions as the method for synthesizing Compound A7-1 and Compound A8-1, the title compound was prepared from Compound C2-1 and 4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 493 [M+H]+
- HPLC retention time: 1.49 min (analysis condition S)
- Compound C4-5
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound C2-1 and 2-morpholin-4-yl-ethanol.
- LCMS: m/z 523 [M+H]+
- HPLC retention time: 1.64 min (analysis condition S)
- Compound C4-6
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound C2-1 and 2-(1,1-dioxothiomorpholino)ethanol.
- LCMS: m/z 571 [M+H]+
- HPLC retention time: 1.75 min (analysis condition S)
- Compound C4-7
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound C2-1 and 1-ethyl-piperidin-4-ol.
- LCMS: m/z 521 [M+H]+
- HPLC retention time: 1.52 min (analysis condition S)
- Compound C4-8
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound C3-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.61 (1H, s), 8.30 (1H, d, J=8.1 Hz), 8. 04 (1H, s), 7.87 (1H, s), 7.62 (1H, dd, J=8.2, 1.8 Hz), 3.17-3.06 (2H, m), 2.75-2.70 (6H, s), 2.67-2.58 (2H, m), 1.81 (6H, s), 1.02 (6H, d, J=6.4 Hz).
- LCMS: m/z 520 [M+H]+
- HPLC retention time: 1.52 min (analysis condition S)
- Compound C4-9
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound C3-1 and 2-piperazin-1-yl-ethanol.
- LCMS: m/z 522 [M+H]+
- HPLC retention time: 1.40 min (analysis condition S)
- Compound C4-10
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound C3-1 and morpholine.
- LCMS: m/z 479 [M+H]+
- HPLC retention time: 2.22 min (analysis condition S)
- Compound C4-11
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound C3-1 and piperazine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 578 [M+H]+
- HPLC retention time: 2.72 min (analysis condition S)
- Compound C4-12
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from C4-11.
- 1H-NMR (270 MHz, CD3OD) δ: 8.78 (1H, s), 8.39 (1H, dd, J=8.2, 0.7 Hz), 7.88 (1H, m), 7.75 (1.1H, s), 7.55 (1H, dd, J=8.2, 1.5 Hz), 3.15 (4H, m), 3.04 (4H, m), 2.82 (s, 6H), 1.85 (6H, s)
- LCMS: m/z 478 [M+H]+
- HPLC retention time: 1.43 min (analysis condition S)
- Compound C4-13
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound C3-2 and 4-(1-pyrrolidyl)-piperidine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.83 (1H, s), 8.64 (1H, s), 8.32 (1H, d, 8.2 Hz), 8.03 (1H, s), 7.80 (1H, s), 7.63 (1H, d, 8.2 Hz), 2.87-2.94 (4H, m), 1.94-1.99 (4H, m), 1.80 (6H, s), 1.58-1.76 (10H, m)
- LCMS: m/z 572 [M+H]+
- HPLC retention time: 2.81 min (analysis condition W)
- Compound C4-14
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound C2-3.
- LCMS: m/z 551 [M+H]+
- HPLC retention time: 1.46 min (analysis condition S)
- Compound C4-15
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound C2-3 and tetrahydropyran-4-ol.
- LCMS: m/z 536 [M+H]+
- HPLC retention time: 2.05 min (analysis condition S)
- Compound C4-16
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound C2-4 and tetrahydropyran-4-ol.
- LCMS: m/z 549 [M+H]+
- HPLC retention time: 2.03 min (analysis condition U)
- Compound C4-17
-
- Under the same conditions as the method for synthesizing Compound A7-1, the target compound was prepared from Compound C2-3.
- LCMS: m/z 564 [M+H]+
- HPLC retention time: 1.20 min (analysis condition S)
- Compound C5
-
- The title compound was obtained as a by-product of the synthesis of Compound C4-3.
- LCMS: m/z 438 [M+H]+
- HPLC retention time: 2.29 min (analysis condition S)
- Compound D0-1-1
-
- Tetrabutylammonium nitrate (2.47 g, 1.07 eq.) was dissolved in dichloromethane, and added with trifluoromethanesulfonic anhydride (1.33 ml, 1.07 eq.) at 0° C. The mixture was stirred for 1 hr, added with DCM solution of 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 1.55 g, 7.59 mmol), and then stirred at 0° C. for 2 hr and 30 min. The reaction solution was added to saturated aqueous solution of sodium hydrogen carbonate and then extracted with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (pale yellow solid, 1.144 g, 60%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 7.79 (1H, s), 7.28 (1H, s), 3.95 (3H, s), 3.06 (2H, t, J=6.9 Hz), 2.64 (2H, t, J=6.9 Hz), 1.41 (6H, s).
- HPLC retention time: 2.03 min (analysis condition S)
- Compound D0-1-2
-
- The title compound was obtained as a by-product of the synthesis of Compound D0-1-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 7.44 (1H, d, J=8.6 Hz), 7.23 (1H, d, J=8.6 Hz), 3.84 (3H, s), 3.07 (2H, t, J=6.9 Hz), 2.65 (2H, t, J=6.9 Hz), 1.35 (6H, s)
- HPLC retention time: 2.15 min (analysis condition S)
- Compound D0-2-1
-
- Under the same conditions as the method for synthesizing Compound A3-1, the title compound was prepared from Compound D0-1-1.
- LCMS: m/z 401, 403 [M+H]+
- HPLC retention time: 3.07 min (analysis condition S)
- Compound D0-2-2
-
- Under the same conditions as the method for synthesizing Compound A3-1, the title compound was prepared from Compound D0-1-2.
- LCMS: m/z 401, 403 [M+H]+
- HPLC retention time: 3.10 min (analysis condition S)
- Compound D0-3-1
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound D0-2-1.
- LCMS: m/z 415, 417 [M+H]+
- HPLC retention time: 3.07 min (analysis condition S)
- Compound D0-3-2
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound D0-2-2.
- LCMS: m/z 415, 417 [M+H]+
- HPLC retention time: 2.72 min (analysis condition S)
- Compound D0-4-1
-
- Under the same conditions as the method for synthesizing Compound A5-2, the title compound was prepared from Compound D0-3-1.
- LCMS: m/z 362 [M+H]+
- HPLC retention time: 2.35 min (analysis condition S)
- Compound D0-4-2
-
- Under the same conditions as the method for synthesizing Compound A5-2, the title compound was prepared from Compound D0-3-2.
- LCMS: m/z 362 [M+H]+
- HPLC retention time: 2.35 min (analysis condition S)
- Compound D0-5-1
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound D0-4-1.
- LCMS: m/z 348 [M+H]+
- HPLC retention time: 2.28 min (analysis condition S)
- Compound D0-5-2
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound D0-4-2.
- LCMS: m/z 348 [M+H]+
- HPLC retention time: 2.23 min (analysis condition S)
- Compound D1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound D0-5-1 and 1-methylpiperidin-4-ol.
- LCMS: m/z 445 [M+H]+
- HPLC retention time: 1.64 min (analysis condition S)
- Compound D2
-
- 6,6-Dimethyl-8-(1-methyl-piperidin-4-yl oxy)-9-nitro-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound D1, 83 mg, 0.19 mmol) was dissolved in ethanol, added with aqueous solution of ammonium acetate and aqueous solution of titanium (III) chloride, and then the mixture was stirred at room temperature for 45 min. The reaction solution was added to saturated aqueous solution of sodium hydrogen carbonate and then extracted with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues were concentrated under reduced pressure to obtain the title compound (yellow solid, 60 mg, 78%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.61 (1H, br. s), 8.28-8.34 (1H, m), 7.94-8.00 (1H, m), 7.57 (1H, dd, J=8.2, 1.4 Hz), 7.46 (1H, s), 7.19 (1H, s), 4.93 (1.8H, s), 4.65 (1.0H, s), 4.06-4.15 (1H, m), 3.34 (5.7H, s), 3.16-3.18 (2H, m), 2.55-2.67 (2H, m), 2.17-2.33 (5H, m), 1.89-2.07 (2H, m), 1.65-1.81 (8H, m)
- LCMS: m/z 415 [M+H]+
- HPLC retention time: 1.12 min (analysis condition S)
- Compound D3-1
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound D2 and methanesulfonyl chloride.
- LCMS: m/z 493 [M+H]+
- HPLC retention time: 1.43 min (analysis condition S)
- Compound D3-2
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound D2 and dimethylsulfamoyl chloride.
- 1H-NMR (270 MHz, CD3OD) δ: 8.34-8.42 (2. OH, m), 7.85 (1.0H, s), 7.47-7.58 (1.0H, m), 7.32 (1.0H, s), 4.73-4.89 (1H, m), 2.75-2.91 (8H, m), 2.38-2.52 (2H, m), 2.34 (3H, s), 2.06-2.21 (2H, m), 1.87-2.05 (2H, m), 1.80 (6H, s).
- LCMS: m/z 522 [M+H]+
- HPLC retention time: 1.66 min (analysis condition S)
- Compound D3-3
-
- Under the same conditions as the method for synthesizing Compound A9-10, the title compound was prepared from Compound D2 and N,N-dimethylglycine.
- LCMS: m/z 500 [M+H]+
- HPLC retention time: 1.31 min (analysis condition S)
- Compound E1
-
- 7-Methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 2.0 g, 9.791 mmol) was dissolved in CH3CN (40 mL), added with NBS (1.92 g, 1.1 eq.), and the mixture was stirred at room temperature for 2.5 hr. The reaction solution was added to water (40 mL), and the precipitated solid was filtered to obtain the title compound (white powder, 2.55 g, 92%).
- 1H-NMR (270 MHz, CDCl3) δ: 7.36 (1H, s), 6.84 (1H, s), 3.91 (3H, s), 3.02 (2H, t, J=6.8 Hz), 2.66 (2H, t, J=6.8 Hz), 1.42 (6H, s).
- LCMS: m/z 283, 285 [M+H]+
- HPLC retention time: 2.67 min (analysis condition S)
- Compound E2-1
-
- 6-Bromo-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound E1, 7.89 g, 27.85 mmol) and 3-hydrazino-benzonitrile (4.45 g, 1.2 eq.) were dissolved in TFA (250 mL), and stirred at 100° C. for 2 hr. TFA was removed under reduced pressure and the residues were added with saturated aqueous solution of NaHCO3 (500 mL), followed by extraction with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were added with ethyl acetate. After stirring at room temperature, the precipitated solid was separated by filtration (Compound E2-2). The filtrate was concentrated under reduced pressure to obtain the title compound as a mixture with E2-2 (yellowish white powder, 2.65 g).
- LCMS: m/z 381, 383 [M+H]+
- HPLC retention time: 3.03 min (analysis condition S)
- Compound E2-2
-
- The title compound was obtained as a by-product of the synthesis of Compound E2-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 11.70 (1H, s), 7.69 (1H, dd, J=8.1, 0.8 Hz), 7.55 (1H, s), 7.48 (1H, dd, J=7.4, 0.8 Hz), 7.27 (1H, s), 7.22 (1H, dd, J=8.1, 7.4 Hz), 4.23 (2H, s), 3.91 (3H, s), 1.70 (6H, s).
- LCMS: m/z 381, 383 [M+H]+
- HPLC retention time: 2.92 min (analysis condition S)
- Compound E2-3, Compound E2-4
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared (as a mixture) from Compound E1.
- Compound E3-1-1
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound E2-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.82 (1H, s), 8.30 (2H, s+d), 8.03 (1H, s), 7.61 (1H, dd, J=8.2, 1.4 Hz), 7.49 (1H, s), 4.04 (3H, s), 1.81 (6H, s).
- LCMS: m/z 395, 397 [M+H]+
- HPLC retention time: 2.77 min (analysis condition S)
- Compound E3-1-2
-
- The title compound was obtained as a by-product of the synthesis of Compound E3-1-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.84 (1H, s), 8.31 (1H, s), 7.86 (1H, dd, J=8.2, 0.9 Hz), 7.70 (1H, d, J=7.1 Hz), 7.47 (1H, s), 7.43 (1H, t, J=7.8 Hz), 4.04 (3H, s), 1.81 (6H, s).
- LCMS: m/z 395, 397 [M+H]+
- HPLC retention time: 2.42 min (analysis condition S)
- Compound E3-1-3
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound E2-3 and Compound E2-4 (mixture).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.42 (1H, s), 8.28 (1H, s), 8.09 (1H, d, J=8.2 Hz), 7.68 (1H, d, J=1.6 Hz), 7.47 (1H, s), 7.39 (1H, dd, J=8.3, 1.7 Hz), 4.03 (3H, s), 1.78 (6H, s).
- LCMS: m/z 448, 450, 452 [M+H]+
- HPLC retention time: 2.93 min (analysis condition S)
- Compound E3-2
-
- 9-Bromo-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound E3-1-1, 1.0 g, 2.53 mmol) was dissolved in NMP (10 mL), added with NaOMe (683 mg, 5 eq.) and 1-dodecanethiol (3.0 mL, 5 eq.), and stirred at 160° C. for 1 hr. The reaction solution was added to 0.5N aqueous solution of hydrochloric acid, and then extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were added with MeOH, and the solid remaining after dissolution was filtered to obtain the title compound (yellow powder, 1.88 g, 65%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.77 (1H, s), 11.13 (1H, d, J=2.4 Hz), 8.31 (1H, dd, J=7.9, 2.4 Hz), 8.25 (1H, d, J=3.0 Hz), 8.01 (1H, s), 7.61 (1H, d, J=7.9 Hz), 7.28 (1H, d, J=2.4 Hz), 1.74 (6H, s).
- LCMS: m/z 381, 383 [M+H]+
- HPLC retention time: 2.40 min (analysis condition S)
- Compound E3-3
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound E3-2 and 2-bromopropane.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.77 (1H, s), 8.29 (2H, s+d), 8.01 (1H, s), 7.60 (1H, d, J=8.1 Hz), 7.50 (1H, s), 5.03 (1H, m), 1.79 (6H, s), 1.36 (6H, d, J=5.9 Hz).
- LCMS: m/z 423, 425 [M+H]+
- HPLC retention time: 2.98 min (analysis condition S)
- Compound E4-1
-
- Under the same conditions as the method for synthesizing Compound A5-2, the title compound was prepared from Compound E3-1-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.88 (1H, br. s), 8.43 (1H, s), 8.30 (1H, d, J=8.2 Hz), 8.05 (1H, d, J=0.5 Hz), 7.65-7.62 (2H, m), 4.11 (3H, s), 1.84 (6H, s).
- LCMS: m/z 342 [M+H]+
- HPLC retention time: 2.23 min (analysis condition S)
- Compound E4-2-1
-
- 9-Bromo-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound E3-1-1, 50 mg, 0.13 mmol), bis (acetonitrile)dichloropalladium (II) (1.64 mg, 0.05 eq.), XPhos (9.05 mg, 0.15 eq.), cesium carbonate (185 mg, 4.5 eq.) and 3-methyl-1-butyn-1-ol (18.6 μl, 1.5 eq.) were dissolved in acetonitrile and stirred at 85° C. for 2 hr. The reaction solution was added to water, and then extracted with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by HPLC to obtain the title compound (brown solid, 21.3 mg, 42%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.29 (1H, d, J=8.1 Hz), 8.11 (1H, s), 8.00 (1H, s), 7.57 (1H, d, J=8.1 Hz), 7.40 (1H, s), 5.50 (1H, s), 3.95 (3H, s), 2.54 (1H, s), 1.79 (6H, s), 1.49 (6H, s).
- LCMS: m/z 399 [M+H]+
- HPLC retention time: 2.10 min (analysis condition S)
- Compound E4-2-2
-
- 9-(3-Hydroxy-3-methyl-but-1-ynyl)-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound E4-2-1, 21.3 mg, 0.05 mmol) and sodium hydride (3.2 mg, 1.5 eq.) were dissolved in THF, and the mixture was stirred overnight at 50° C. Water was added to the reaction solution and the residues obtained after concentration under reduced pressure were purified by HPLC to obtain the title compound (brown solid, 9.6 mg, 31%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.26 (1H, d, J=8.2 Hz), 8.16 (1H, s), 7.97 (1H, s), 7.53 (1H, d, J=8.2 Hz), 7.41 (1H, s), 4.32 (1H, s), 4.00 (3H, s), 1.79 (6H, s).
- LCMS: m/z 341 [M+H]+
- HPLC retention time: 2.27 min (analysis condition S)
- Compound E4-3
-
- 9-Bromo-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound E3-1-1, 50 mg, 0.13 mmol), [1,1′-bis (diphenylphosphino)ferrocene]palladium (II) dichloride dichloromethane complex (1: 1) (10.3 mg, 0.1 eq.), TEA (53 μl, 3 eq.) and potassium vinyltrifluoroborate (51 mg, 3 eq.) were dissolved in n-propanol and the mixture was stirred at 60° C. for 5 days. The reaction solution was added to water and then extracted with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (brown powder, 25 mg, 19%).
- LCMS: m/z 343 [M+H]+
- HPLC retention time: 2.55 min (analysis condition S)
- Compound E4-4
-
- Under the same conditions as the method for synthesizing Compound B2-17, the title compound was prepared from Compound E3-1-1.
- LCMS: m/z 448 [M+H]+
- HPLC retention time: 2.05 min (analysis condition U)
- Compound E4-5
-
- Under the same conditions as the method for synthesizing Compound B2-17, the title compound was prepared from Compound E3-1-1 and sodium salt of propane-2-thiol.
- LCMS: m/z 391 [M+H]+
- HPLC retention time: 2.98 min (analysis condition U)
- Compound E4-6
-
- Under the same conditions as the method for synthesizing Compound B2-10, the title compound was prepared from Compound E3-1-1 and 1-methylpiperazine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.25 (1H, d, J=7.8 Hz), 7.93 (1H, s), 7.65 (1H, s), 7.50 (1H, d, J=6.8 Hz), 7.25 (1H, s), 3.93 (3H, s), 3.02 (4H, br), 2.22 (3H, s), 1.73 (6H, s).
- LCMS: m/z 415 [M+H]+
- HPLC retention time: 1.80 min (analysis condition U)
- Compound E4-7-1
-
- To 9-bromo-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound E3-1-1, 300 mg, 0.759 mmol), 4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester (282 mg, 0.911 mmol, 1.2 eq.), Pd(PPh3)2Cl2 (26.6 mg, 0.0379 mmol, 0.05 eq.) and sodium carbonate (241 mg, 2.28 mmol, 3.0 eq.), DME (5 ml) and water (1 ml) were added. The mixture was subjected to reduced pressure under ultrasonication treatment, followed by flushing with nitrogen gas. This procedure was repeated five times and then degassed. The mixture was stirred at 80° C. for 80 min under nitrogen atmosphere. Pd(PPh3)2Cl2 (26.6 mg, 0.0379 mmol, 0.05 eq.) was added and the mixture was further stirred at 80° C. for 20 min. Then, the mixture was cooled to room temperature, and added with water and ethyl acetate. The insoluble matters were filtered through Celite. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration, followed by concentration under reduced pressure to obtain the title compound as a crude product (gray powder).
- LCMS: m/z 498 [M+H]+
- HPLC retention time: 2.85 min (analysis condition S)
- Compound E4-7-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B4-4-1.
- LCMS: m/z 368 [M+H]+
- HPLC retention time: 1.27 min (analysis condition S)
- Compound E4-8-1
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound B4-7-1.
- LCMS: m/z 500 [M+H]+
- HPLC retention time: 4.18 min (analysis condition W)
- Compound E4-8-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound B4-8-1.
- LCMS: m/z 400 [M+H]+
- HPLC retention time: 1.35 min (analysis condition S)
- Compound E4-9-1
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound E3-3.
- LCMS: m/z 526 [M+H]+
- HPLC retention time: 3.13 min (analysis condition S)
- Compound E4-9-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound E4-9-1.
- LCMS: m/z 426 [M+H]+
- HPLC retention time: 1.40 min (analysis condition S)
- Compound E4-10
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound E3-1-1 and potassium cyclopropyltrifluoroborate.
- LCMS: m/z 357 [M+H]+
- HPLC retention time: 2.62 min (analysis condition S)
- Compound E4-11
-
- Under the same conditions as the method for synthesizing Compound B2-28, the title compound was prepared from Compound E3-1-1.
- LCMS: m/z 361 [M+H]+
- HPLC retention time: 1.68 min (analysis condition S)
- Compound E5-1
-
- The ethyl acetate suspension of 8-methoxy-6,6-dimethyl-11-oxo-9-vinyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound E4-3, 25 mg, 0.07 mmol) and palladium carbon (25 mg) were stirred at room temperature for 1 hr under hydrogen atmosphere. The reaction solution was filtered through Celite. The filtrate was concentrated under reduced pressure and the resulting residues were purified by high performance liquid chromatography to obtain the title compound (white solid, 3.2 mg, 13%).
- LCMS: m/z 345 [M+H]+
- HPLC retention time: 2.62 min (analysis condition S)
- Compound E5-2
-
- Under the same conditions as the method for synthesizing Compound B3-8, the title compound was prepared from Compound E4-4.
- LCMS: m/z 480 [M+H]+
- HPLC retention time: 1.97 min (analysis condition U)
- Compound E5-3
-
- Under the same conditions as the method for synthesizing Compound B3-8, the title compound was prepared from Compound E4-5.
- LCMS: m/z 423 [M+H]+
- HPLC retention time: 2.40 min (analysis condition U)
- Compound E5-4
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound E4-8-2 and acetone.
- LCMS: m/z 442 [M+H]+
- HPLC retention time: 1.48 min (analysis condition S)
- Compound E5-5
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound E4-7-2 and oxetan-3-one.
- LCMS: m/z 454 [M+H]+
- HPLC retention time: 1.32 min (analysis condition S)
- Compound E5-6
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound E4-9-2 and oxetan-3-one.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.71 (1H, s), 8.31 (1H, d, J=8.2 Hz), 7.99 (1H, s), 7.94 (1H, s), 7.58 (1H, d, J=7.6 Hz), 7.33 (1H, s), 5.84 (1.0H, m), 4.95 (1H, m), 4.56 (4H, dt, J=17.4, 6.3 Hz), 3.56 (1H, m), 3.01 (2H, br), 1.78 (6H, s), 1.34 (6H, d, J=5.9 Hz).
- LCMS: m/z 482 [M+H]+
- HPLC retention time: 1.43 min (analysis condition S)
- Compound E5-7
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound E4-11 and 1-isopropylpiperazine.
- LCMS: m/z 471 [M+H]+
- HPLC retention time: 1.18 min (analysis condition S)
- Compound E5-8
-
- Under the same conditions as the method for synthesizing Compound B3-15, the title compound was prepared from Compound E4-11 and morpholine.
- LCMS: m/z 430 [M+H]+
- HPLC retention time: 1.68 min (analysis condition S)
- Compound E6-1
-
- To the mixture of 9-ethynyl-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound E4-2, 27 mg, 0.079 mmol), palladium (II) chloride (2.0 mg, 0.14 eq.), copper (II) chloride (25.0 mg, 2.2 eq.), and sodium acetate (14.1 mg, 2.13 eq.), methanol (1.5 mL) was added, and then the mixture was stirred at room temperature for 2 days under carbon monoxide atmosphere. The mixture was extracted with water and ethyl acetate and the insoluble matters were filtered off. The organic layer was washed with brine and dried over magnesium sulfate. The residues obtained after filtration and concentration under reduced pressure were washed with dichloromethane to obtain the title compound (13.9 mg, 44%).
- LCMS: m/z 399 [M+H]+
- HPLC retention time: 2.81 min (analysis condition F)
- Compound E6-2
-
- (3-Cyano-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-9-yl)-propynoic acid methyl ester (Compound E6-1, 15.2 mg, 0.038 mmol) was dissolved in a mixture solvent of methanol (1.5 mL) and THF (0.5 mL), added with 2 N aqueous solution of potassium hydroxide (5 drops), and then stirred at room temperature overnight. 0.5 N Hydrochloric acid was added to the reaction solution, which was then extracted with ethyl acetate. The organic layer was washed with brine and dried over magnesium sulfate. The solids obtained after filtration and concentration under reduced pressure were washed with dichloromethane and purified by HPLC to obtain the title compound (white solid, 9.6 mg, 66%).
- LCMS: m/z 385 [M+H]+
- HPLC retention time: 2.35 min (analysis condition F)
- Compound E6-3
-
- 9-(3-Hydroxy-3-methyl-but-1-ynyl)-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound E4-2-1, 21.0 mg, 0.0527 mmol) was dissolved in ethanol (15 mL) and N,N-dimethylacetamide (2 mL), added with 10% Pd/C (6.7 mg), and then stirred at room temperature overnight under hydrogen atmosphere. The reaction solution was filtered and concentrated under reduced pressure. The resulting residues were diluted with ethyl acetate, washed with brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The resulting solid was washed with dichloromethane to obtain the title compound (yellow powder, 16.9 mg, 80%).
- LCMS: m/z 403 [M+H]+
- HPLC retention time: 5.39 min (analysis condition H)
- Compound F1-1
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound E3-2 and 4-trifluoromethanesulfonyloxy-piperidine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 564, 566 [M+H]+
- HPLC retention time: 3.30 min (analysis condition S)
- Compound F1-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound F1-1.
- LCMS: m/z 464, 466 [M+H]+
- HPLC retention time: 1.52 min (analysis condition S)
- Compound F1-3
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound F1-2 and methanesulfonyl chloride.
- LCMS: m/z 542, 544 [M+H]+
- HPLC retention time: 2.57 min (analysis condition S)
- Compound F1-4
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound E3-2 and tetrahydropyran-4-ol.
- LCMS: m/z 465, 467 [M+H]+
- HPLC retention time: 2.70 min (analysis condition S)
- Compound F2
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound E3-2.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.99 (1H, s), 8.51 (1H, s), 8.31 (1H, dd, J=8.2, 0.7 Hz), 8.17 (1H, s), 8.07 (1H, s), 7.67 (1H, dd, J=8.2, 1.4 Hz), 1.81 (6H, s).
- LCMS: m/z 513, 515 [M+H]+
- HPLC retention time: 3.13 min (analysis condition S)
- Compound F3-1
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F1-2 and oxetan-3-one.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.29 (1H, d, 8 Hz), 8.29 (1H, s), 8.01 (1H, s), 7.60 (1H, d, 8 Hz), 7.55 (1H, s), 5.00-4.95 (1H, m), 4.55 (2H, dd, 8, 8 Hz), 4.44 (2H, dd, 8, 8 Hz), 2.52-2.46 (1H, m), 2.33-2.29 (2H, m), 1.96-1.94 (2H, m), 1.79 (8H, br. s)
- LCMS: m/z 519, 521 [M+H]+
- HPLC retention time: 2.78 min (analysis condition W)
- Compound F3-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and 4-pyrrolidin-1-yl-piperidine.
- LCMS: m/z 517, 519 [M+H]+
- HPLC retention time: 1.70 min (analysis condition S)
- Compound F3-3
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and 1-methanesulfonylpiperazine.
- LCMS: m/z 527, 529 [M+H]+
- HPLC retention time: 2.48 min (analysis condition S)
- Compound F3-4
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and morpholine.
- LCMS: m/z 450, 452 [M+H]+
- HPLC retention time: 2.65 min (analysis condition S)
- Compound F3-5
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and 2-piperazin-1-yl ethanol.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.26 (2.0H, s+d), 7.97 (1H, s), 7.54 (1H, d, J=8.7 Hz), 7.43 (1H, s), 4.45 (1H, t, J=5.4 Hz), 3.55 (2H, q, J=5.8 Hz), 3.17 (4H, br), 2.66 (2H, br), 1.76 (6H, s).
- LCMS: m/z 493, 495 [M+H]+
- HPLC retention time: 1.43 min (analysis condition S)
- Compound F3-6-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and piperidin-4-yl-carbamic acid tert-butyl ester.
- LCMS: m/z 563, 565 [M+H]+
- HPLC retention time: 3.05 min (analysis condition S)
- Compound F3-6-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound F3-6-1.
- LCMS: m/z 463, 465 [M+H]+
- HPLC retention time: 1.47 min (analysis condition S)
- Compound F3-7
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and piperidin-4-ol.
- LCMS: m/z 464, 466 [M+H]+
- HPLC retention time: 2.25 min (analysis condition S)
- Compound F3-8
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and 1-isopropylpiperazine.
- LCMS: m/z 491, 493 [M+H]+
- HPLC retention time: 1.58 min (analysis condition S)
- Compound F3-9
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and piperazine.
- 1H-NMR (DMSO-d6) δ: 8.30-8.24 (2H, m), 8.00 (1H, s), 7.63-7.58 (1H, m), 7.37 (1H, s), 3.10-3.01 (4H, m), 2.91-2.85 (4H, m), 1.76 (6H, s)
- LCMS: m/z 449, 451 [M+H]+
- HPLC retention time: 1.45 min (analysis condition S)
- Compound F3-10
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and piperazine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 549, 551 [M+H]+
- HPLC retention time: 4.61 min (analysis condition W)
- Compound F3-11
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound F2 and 4-piperidin-4-yl morpholine.
- 1H-NMR (DMSO-d6) δ: 8.30-8.24 (2H, m), 8.00 (1H, s), 7.59 (1H, d, J=8.2 Hz), 7.42 (1H, s), 3.66-3.45 (6H, m), 2.80 (2H, t, J=11.1 Hz), 2.38-2.28 (1H, m), 1.96-1.87 (2H, m), 1.75 (6H, s), 1.66-1.56 (2H, m)
- LCMS: m/z 533, 535 [M+H]+
- HPLC retention time: 1.53 min (analysis condition S)
- Compound F4-1-1
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound F3-1.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.30 (1H, d, 8 Hz), 8.17 (1H, s), 8.01 (1H, s), 7.60 (1H, d, 8 Hz), 7.50 (1H, s), 4.87-4.83 (1H, m), 4.55 (2H, dd, 4, 4 Hz), 4.45 (2H, dd, 4, 4 Hz), 3.44 (1H, ddd, 4, 4, 4 Hz), 2.33-2.24 (2H, m), 1.99-1.91 (2H, m), 1.78 (8H, br. s)
- LCMS: m/z 466 [M+H]+
- HPLC retention time: 2.67 min (analysis condition W)
- Compound F4-1-2
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F4-1-1.
- LCMS: m/z 470 [M+H]+
- HPLC retention time: 2.74 min (analysis condition W)
- Compound F4-2
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound F3-6-2 and methanesulfonyl chloride.
- LCMS: m/z 541, 543 [M+H]+
- HPLC retention time: 2.37 min (analysis condition S)
- Compound F4-3
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F3-9 and 1-oxetan-3-one.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.83 (1H, br. s), 8.31-8.32 (1H, m), 8.27-8.29 (1H, m), 8.01-8.04 (1H, m), 7.59-7.64 (1H, m), 7.48 (1H, s), 4.59 (2H, dd, J=6.3, 6.3 Hz), 4.48 (2H, dd, J=6.3, 6.3 Hz), 3.52 (1H, t, J=6.3 Hz), 3.12-3.25 (4H, m), 2.44-2.54 (4H, m), 1.78 (6H, s).
- LCMS: m/z 505, 507 [M+H]+
- HPLC retention time: 1.45 min (analysis condition S)
- Hydrochloric Acid Salt of Compound F4-3
- 9-Bromo-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile was added with DMSO and 6 N hydrochloric acid (1.05 eq.) and dissolved therein. After freeze-drying, crystallization was performed by using ethanol comprising 25% water to obtain monohydrochloric acid salt of 9-bromo-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.91 (1H, br.s), 11.70 (1H, br. s), 8.32-8.29 (2H, m), 8.04 (1H, s), 7.64-7.62 (1H, m), 7.52 (1H, s), 4.89-4.62 (4H, br. m), 3.66-3.39 (1H, m), 3.31-3.05 (8H, br. m), 1.81 (6H, s)
- LCMS: m/z 505, 507 [M+H]+
- Compound F4-4
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound F3-9 and 1-bromo-2-(2-methoxyethoxy)ethane.
- LCMS: m/z 551, 553 [M+H]+
- HPLC retention time: 2.80 min (analysis condition W)
- Compound F4-5
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F3-9 and tetrahydropyran-4-one.
- LCMS: m/z 533, 535 [M+H]+
- HPLC retention time: 2.67 min (analysis condition W)
- Compound F4-6
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F3-9 and tetrahydrothiopyran-4-one.
- LCMS: m/z 549, 551 [M+H]+
- HPLC retention time: 2.86 min (analysis condition W)
- Compound F4-7
-
- Under the same conditions as the method for synthesizing Compound B3-8, the title compound was prepared from Compound F4-6.
- LCMS: m/z 581, 583 [M+H]+
- HPLC retention time: 2.66 min (analysis condition W)
- Compound F4-8
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound F3-9 and bromomethylcyclopropane.
- LCMS: m/z 503, 505 [M+H]+
- HPLC retention time: 2.81 min (analysis condition W)
- Compound F4-9
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F3-9 and (1-ethoxy-cyclopropoxy)-trimethyl-silane.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.22-8.30 (2H, m), 8.00 (1H, s), 7.56 (1H, d, J=7.9 Hz), 7.43 (1H, s), 3.30 (1H, d, J=5.8 Hz), 3.11 (4H, s), 2.75 (4H, s), 1.75 (6H, s), 0.47 (2H, d, J=5.8 Hz), 0.34 (2H, d, J=5.8 Hz)
- LCMS: m/z 489, 491 [M+H]+
- HPLC retention time: 1.68 min (analysis condition S)
- Compound F4-10
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F3-9 and cyclobutanone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.23-8.29 (2H, m), 8.00 (1H, s), 7.55 (1H, d, 7.9 Hz), 7.45 (1H, s), 4.04-4.15 (1H, m), 3.10-3.20 (4H, m), 2.39-2.48 (4H, m), 1.97-2.06 (2H, m), 1.78-1.88 (2H, m), 1.77 (6H, s), 1.61-1.72 (2H, m)
- LCMS: m/z 503, 505 [M+H]+
- HPLC retention time: 2.78 min (analysis condition W)
- Compound F5-1
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound F3-3.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.78 (1H, s), 8.31 (1H, dd, J=8.1, 0.7 Hz), 8.19 (1H, s), 8.02 (1H, dd, J=1.4, 0.7 Hz), 7.61 (1H, dd, J=8.2, 1.4 Hz), 7.33 (1H, s), 4.55 (1H, s), 3.43 (4H, br), 2.98 (3H, s), 1.79 (6H, s).
- LCMS: m/z 473 [M+H]+
- HPLC retention time: 2.27 min (analysis condition S)
- Compound F5-2
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound F4-2.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.98 (1H, s), 8.30 (1H, d, J=8.1 Hz), 8. (1H, s), 8.02 (1H, s), 7.61 (1H, d, J=7.9 Hz), 7.23 (2H, s+d), 4.55 (1H, s), 3.79 (2H, brd), 2.95 (4H, br), 1.96 (2H, brd), 1.78 (3H, s), 1.65 (2H, brd).
- LCMS: m/z 487 [M+H]+
- HPLC retention time: 2.15 min (analysis condition S)
- Compound F5-3
-
- Under the same conditions as the method for synthesizing Compound A5-2, the target compound was prepared from Compound F3-2.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.33 (1H, d, J=1.3 Hz), 8.27 (1H, dd, J=7.7, 1.3 Hz), 8.00 (1H, s), 7.57 (1H, d, J=7.7 Hz), 7.40 (1H, s), 3.74 (2H, m), 3.19-3.33 (1H, m), 2.98-3.12 (2H, m), 2.35-2.62 (2H, m), 2.11-2.29 (2H, m), 1.89-2.06 (2H, m), 1.78 (6H, s), 1.54-1.70 (6H, m).
- LCMS: m/z 464 [M+H]+
- HPLC retention time: 1.55 min (analysis condition S)
- Compound F5-4
-
- Under the same conditions as the method for synthesizing Compound E4-2-1 and Compound E4-2-2, the title compound was prepared from Compound F3-2.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.29 (1H, d, J=8.2 Hz), 8.14 (1H, s), 8.00 (1H, s), 7.58 (1H, dd, J=8.1, 1.3 Hz), 7.24 (1H, s), 4.50 (1H, s), 3.70-3.83 (2H, m), 3.34-3.48 (1H, m), 2.83-2.98 (2H, m), 2.45-2.58 (2H, m), 2.10-2.23 (2H, m), 1.90-2.03 (2H, m), 1.76 (6H, s), 1.51-1.74 (6H, m).
- LCMS: m/z 463 [M+H]+
- HPLC retention time: 1.60 min (analysis condition S)
- Compound F5-5
-
- Under the same conditions as the method for synthesizing Compound E4-2-1 and Compound E4-2-2, the title compound was prepared from Compound F3-4.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.82 (1H, s), 8.31 (1H, d, J=7.9 Hz), 8.18 (1H, s), 8.02 (1H, s), 7.61 (1H, d, J=7.9 Hz), 7.28 (1H, s), 4.53 (1H, s), 3.80 (4 H, s), 3.36 (4H, s), 1.79 (6H, s).
- LCMS: m/z 396 [M+H]+
- HPLC retention time: 2.32 min (analysis condition S)
- Compound F5-6
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F3-4 and 3-dimethylaminopropyne.
- 1H-NMR (270 MHz, CDCl3) δ: 8.52 (1H, d, J=7.8 Hz), 8.47 (1H, s), 7.76 (1H, s), 7.56 (1H, d, J=7.8 Hz), 7.03 (1H, s), 3.92 (4H, m), 3.55 (2H, s), 3.39 (4H, m), 2.37 (6H, s), 1.83 (6H, s)
- LCMS: m/z 453 [M+H]+
- Compound F5-7
-
- To 9-bromo-6,6-dimethyl-8-morpholin-4-yl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound F3-4, 30 mg, 0.067 mmol), 3-bromopropyne (0.01 ml, 0.13 mmol), morpholine (0.029 ml, 0.33 mmol), X-Phos (4.8 mg, 15% mol), PdCl2 (CH3CN)2 (0.9 mg, 5% mol) and cesium carbonate (87 mg, 0.27 mmol), acetonitrile (2 ml) was added and the mixture was stirred at 80° C. for 2 hr. The reaction solution was added to water, and then extracted with dichloromethane. The organic layer was dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (dichloromethane/methanol) to obtain the target compound (pale brown solid, 18 mg, 64%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.29 (1H, d, J=7.8 Hz), 8.14 (1H, s), 8.00 (1H, s), 7.59 (1H, d, J=7.8 Hz), 7.27 (1H, s), 3.79 (4H, m), 3.64 (4H, m), 3.61 (2H, s), 3.33 (4H, m), 2.56 (4H, m), 1.77 (6H, s)
- LCMS: m/z 495 [M+H]+
- Compound F5-8
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F3-4 and 1-pentyne.
- LCMS: m/z 438 [M+H]+
- HPLC retention time: 2.88 min (analysis condition S)
- Compound F5-9
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F3-4 and 3-methoxypropyne.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.30 (1H, d, J=7.8 Hz), 8.15 (1H, s), 8.01 (1H, s), 7.60 (1H, d, J=7.8 Hz), 7.28 (1H, s), 4.41 (2H, s), 3.79 (4H, m), 3.37 (3H, s), 3.34 (4H, m), 1.78 (6H, s)
- LCMS: m/z 440 [M+H]+
- Compound F5-10
-
- Under the same conditions as the method for synthesizing Compound F5-7, the title compound was prepared from Compound F3-4 and 3-bromopropyne and 4-cyclopropylpiperazine.
- LCMS: m/z 534 [M+H]+
- HPLC retention time: 1.40 min (analysis condition S)
- Compound F5-11
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F3-4 and 1-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.29 (1H, d, J=7.8 Hz), 8.22 (1H, s), 8.09 (1H, s), 7.99 (1H, s), 7.95 (1H, s), 7.56-7.61 (1H, m), 7.36 (1H, s), 3.90 (3H, s), 3.73 (4H, s), 2.95 (4H, s), 1.77 (6H, s).
- LCMS: m/z 452 [M+H]+
- HPLC retention time: 2.18 min (analysis condition U)
- Compound F5-12
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F3-4 and potassium cyclopropyltrifluoroborate.
- 1H-NMR (270 MHz, CD3OD+CDCl3) δ: 8.45 (1H, d, J=7.8 Hz), 7.83 (2H, m), 7.54 (1H, d, J=7.8 Hz), 7.20 (1H, s), 3.96 (4H, m), 3.24 (4H, m), 2.25 (1H, m), 1.80 (6H, s), 1.09 (2H, m), 0.93 (2H, m)
- LCMS: m/z 412 [M+H]+
- Compound F5-13
-
- Under the same conditions as the method for synthesizing Compound B2-24, the title compound was prepared from Compound F3-4 and potassium vinyltrifluoroborate.
- LCMS: m/z 398 [M+H]+
- HPLC retention time: 2.67 min (analysis condition U)
- Compound F5-14
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound F3-8.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.73 (1H, s), 8.31 (1H, d, J=9.1 Hz), 8.16 (1H, d, J=1.2 Hz), 8.00 (1H, s), 7.60 (1H, d, J=7.9 Hz), 7.25 (1H, s), 4.50 (1H, d, J=1.8 Hz), 2.72 (1H, m), 2.65 (4H, s), 1.78 (6H, s), 1.04 (6H, d, J=5.5 Hz).
- LCMS: m/z 437 [M+H]+
- HPLC retention time: 1.48 min (analysis condition S)
- Compound F5-15-1
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F3-10 and potassium cyclopropyltrifluoroborate.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.55 (1H, s), 8.28-8.25 (1H, m), 7.98-7.95 (1H, m), 7.62 (1H, s), 7.32 (1H, s), 3.56-3.53 (4 h, m), 3.09-3.07 (4H, m), 2.22-2.18 (1H, m), 1.73 (6H, br s), 1.44 (9H, s), 1.08-1.05 (2H, m), 0.77-0.76 (2H, m)
- LCMS: m/z 511 [M+H]+
- HPLC retention time: 4.50 min (analysis condition W)
- Compound F5-15-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound F5-15-1.
- LCMS: m/z 411 [M+H]+
- HPLC retention time: 2.67 min (analysis condition W)
- Compound F5-16
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound F4-3.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.77 (1H, br. s), 8.31 (1H, d, J=8.2 Hz), 8.16 (1H, s), 8.02 (1H, s), 7.61 (1H, dd, J=8.2, 1.3 Hz), 7.27 (1H, s), 4.59 (2H, dd, J=6.6, 6.6 Hz), 4.51 (1H, s), 4.49 (2H, dd, J=6.6, 6.6 Hz), 3.51 (1H, t, J=6.6 Hz), 3.35-3.43 (4H, m), 2.43-2.50 (4H, s), 1.78 (6H, s).
- LCMS: m/z 451 [M+H]+
- HPLC retention time: 1.40 min (analysis condition S)
- Compound F5-17
-
- According to the same method as the method for synthesizing Compound A5-2, the title compound was prepared from Compound F4-3.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.84 (1H, br. s), 8.36 (1H, s), 8.32-8.29 (1H, d, 8.08 Hz), 8.04 (1H, s), 7.65-7.62 (1H, d, 8.08 Hz), 7.44 (1H, s), 4.62-4.57 (2H, m), 4.52-4.47 (2H, m), 3.81-3.78 (2H, t, 4.61 Hz), 3.57-3.50 (1H, m), 3.43 (4H, m) 2.51 (4H, m), 1.80 (6H, s)
- LCMS: m/z 452 [M+H]+
- Compound F5-18
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and 3-methoxypropyne.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.77 (1H, br. s), 8.32-8.29 (1H, d, 8.08 Hz), 8.13 (1H, s), 8.01 (1H, s), 7.62-7.59 (1H, d, 8.08 Hz), 7.27 (1H, s), 4.62-4.57 (2H, m), 4.52-4.47 (2H, m), 4.39 (2H, s), 3.53-3.47 (1H, m), 3.38 (4H, m), 3.36 (3H, s), 2.51 (4H, m), 1.77 (6H, s)
- LCMS: m/z 495 [M+H]+
- Compound F5-19
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and dimethyl-prop-2-ynylamine.
- LCMS: m/z 508 [M+H]+
- HPLC retention time: 1.07 min (analysis condition S)
- Compound F5-20
-
- Under the same conditions as the method for synthesizing Compound F5-7, the title compound was prepared from Compound F4-3, 3-bromopropyne and 4-oxetan-3-yl-piperazine.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.30 (1H, d, J=7.8 Hz), 8.12 (1H, s), 8.00 (1H, s), 7.59 (1H, d, J=7.8 Hz), 7.26 (1H, s), 4.60-4.42 (8H, m), 3.61 (2H, s), 3.60-3.30 (6H, m), 2.60-2.30 (12H, m), 1.77 (6H, s)
- LCMS: m/z 605 [M+H]+
- Compound F5-21
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and cyclopentylacetylene.
- LCMS: m/z 519 [M+H]+
- HPLC retention time: 1.80 min (analysis condition S)
- Compound F5-22
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and propyne.
- 1H-NMR (400 MHz, CD3OD) δ: 8.37 (1H, d, J=8.2 Hz), 8.18 (1H, s), 7.84 (1H, s), 7.53 (1H, d, J=8.2 Hz), 7.19 (1H, s), 4.70-4.77 (2H, m), 4.62-4.68 (2H, m), 3.57-3.63 (1H, m), 3.38-3.45 (4H, m), 2.54-2.61 (4H, m), 2.10 (3H, s), 1.79 (6H, s)
- LCMS: m/z 465 [M+H]+
- HPLC retention time: 1.90 min (analysis condition U)
- Compound F5-23
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the TMS complex of the title compound was prepared from Compound F4-3 and trimethylprop-2-ynyloxysilane. By treating the resulting TMS complex with tetrabutylammonium fluoride, the title compound was obtained.
- LCMS: m/z 481 [M+H]+
- HPLC retention time: 1.30 min (analysis condition S)
- Compound F5-24
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and 4-methylpent-1-yne.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.75 (1H, br. s), 8.32-8.29 (1H, d, 8.08 Hz), 8.08 (1H, s), 8.01 (1H, s), 7.62-7.59 (1H, m), 7.23 (1H, s), 4.61-4.57 (2H, m), 4.51-4.46 (2H, m), 3.51-3.47 (1H, m), 3.37 (4H, m), 2.46 (4H, m), 2.41-2.39 (2H, d, 5.94 Hz), 1.92-1.80 (1H, m), 1.77 (6H, s), 1.04 (3H, s), 1.01 (3H, s)
- LCMS: m/z 507 [M+H]+
- Compound F5-25
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and ethynylcyclopropane.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.74 (1H, br. s), 8.32-8.29 (1H, d, 8.08 Hz), 8.05 (1H, s), 8.00 (1H, s), 7.62-7.58 (1H, m), 7.21 (1H, s), 4.62-4.57 (2H, m), 4.51-4.47 (2H, m), 3.53-3.48 (1H, m), 3.34 (4H, m), 2.46 (4H, m), 1.76 (6H, s), 1.64-1.58 (1H, m), 0.97-0.89 (2H, m), 0.76-0.70 (2H, m)
- LCMS: m/z 491 [M+H]+
- Compound F5-26
-
- Under the same conditions as the method for synthesizing Compound F5-7, the title compound was prepared from Compound F4-3, 3-bromopropyne and morpholine.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.29 (1H, d, J=7.8 Hz), 8.13 (1H, s), 8.02 (1H, s), 7.59 (1H, d, J=7.8 Hz), 7.25 (1H, s), 4.61-4.48 (4H, m), 3.64-3.32 (11H, m), 2.60-2.40 (8H, m), 1.78 (6H, s)
- LCMS: m/z 550 [M+H]+
- Compound F5-27
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and 1-pentyne. 1H-NMR (400 MHz, DMSO-d6) δ: 12.72 (1H, br. s), 8.28 (1H, d, 8.1 Hz), 8.06 (1H, s), 7.98 (1H, s), 7.58 (1H, d, 8.1 Hz), 7.21 (1H, s), 4.60-4.43 (4H, m), 3.53-3.44 (1H, m), 3.39-3.32 (2H, m), 1.75 (6H, s), 1.60-1.53 (4H, m), 1.01 (3H, t, 7.3 Hz)
- LCMS: m/z 493 [M+H]+
- HPLC retention time: 2.17 min (analysis condition U)
- Compound F5-28
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and 5-methylhex-1-yne.
- LCMS: m/z 521 [M+H]+
- HPLC retention time: 2.37 min (analysis condition U)
- Compound F5-29
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and 3-diethylaminopropyne.
- LCMS: m/z 536 [M+H]+
- HPLC retention time: 1.13 min (analysis condition S)
- Compound F5-30
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and 3-benzyl-3-ethylaminopropyne.
- LCMS: m/z 584 [M+H]+
- HPLC retention time: 1.32 min (analysis condition S)
- Compound F5-31
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-3 and 3-(1,1-dioxo-λ6-thiomorpholin-4-yl)-propyne.
- LCMS: m/z 598 [M+H]+
- HPLC retention time: 1.35 min (analysis condition S)
- Compound F5-32
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F4-3 and 2-isopropenyl-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane.
- 1H-NMR (270 MHz, CD3OD+CDCl3) δ: 8.44 (1H, d, J=7.8 Hz), 8.09 (1H, s), 7.83 (1H, s), 7.54 (1H, d, J=7.8 Hz), 7.18 (1H, s), 5.24-5.20 (2H, m), 4.81-4.68 (4H, m), 3.66 (1H, m), 3.30 (4H, m), 2.57 (4H, m), 2.21 (3H, s), 1.82 (6H, s)
- LCMS: m/z 467 [M+H]+
- Compound F5-33
-
- Under the same conditions as the method for synthesizing Compound F5-47, the title compound was prepared from Compound F4-3.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.71 (1H, br. s), 8.33-8.31 (1H, d, 8.08 Hz), 8.01 (1H, s), 7.97 (1H, s), 7.62-7.59 (1H, m), 7.32 (1H, s), 4.61-4.57 (2H, m), 4.51-4.47 (2H, m), 3.55-3.49 (1H, m), 3.05 (4H, m), 2.47 (4H, m), 2.33 (3H, s), 1.76 (6H, s)
- LCMS: m/z 441 [M+H]+
- Compound F5-34
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F5-15-2 and oxetan-3-one.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.32-8.29 (1H, m), 8.00-7.99 (1H, m), 7.62-7.58 (2H, m), 7.32-7.31 (1H, m), 4.61-4.57 (2H, m), 4.52-4.49 (2H, m), 3.53 (1H, br. s), 3.18 (4H, br. s), 1.75 (6H, s), 1.25-1.23 (1H, m), 1.09-1.04 (2H, m), 0.79-0.75 (2H, m)
- LCMS: m/z 467 [M+H]+
- HPLC retention time: 2.74 min (analysis condition W)
- Compound F5-35
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F4-3 and 1-methyl-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole.
- LCMS: m/z 507 [M+H]+
- HPLC retention time: 1.75 min (analysis condition U)
- Compound F5-36-1
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F3-2.
- LCMS: m/z 621 [M+H]+
- HPLC retention time: 2.58 min (analysis condition U)
- Compound F5-36-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound F5-36-1.
- LCMS: m/z 520 [M+H]+
- HPLC retention time: 1.82 min (analysis condition U)
- Compound F5-37
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound F4-9.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.76 (1H, br. s), 8.31 (1H, d, J=8.1 Hz), 8.15 (1H, s), 8.01 (1H, s), 7.61 (1H, dd, J=8.1, 1.5 Hz), 7.24 (1H, s), 4.52 (1H, s), 3.28-3.36 (4H, m), 3.17 (1H, d, J=5.3 Hz), 2.70-2.77 (4H, m), 1.76 (6H, s), 0.47 (2H, d, J=5.3 Hz), 0.36 (2H, d, J=5.3 Hz).
- LCMS: m/z 435 [M+H]+
- HPLC retention time: 1.57 min (analysis condition S)
- Compound F5-38
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-9 and propyne.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.76 (1H, br. s), 8.31-8.28 (1H, d, 8.08 Hz), 8.06 (1H, s), 8.00 (1H, s), 7.60-7.57 (1H, m), 7.19 (1H, s), 3.29 (4H, m), 2.74 (4H, m), 2.55 (1H, m), 2.13 (3H, s), 1.75 (6H, s), 0.51-0.43 (2H, m), 0.38-0.32 (2H, m)
- LCMS: m/z 449 [M+H]+
- Compound F5-39
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F4-9 and phenylboric acid.
- LCMS: m/z 487 [M+H]+
- HPLC retention time: 2.15 min (analysis condition U)
- Compound F5-40
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F4-9 and pyridine-3-boric acid.
- LCMS: m/z 488 [M+H]+
- HPLC retention time: 1.53 min (analysis condition U)
- Compound F5-41
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F4-9 and thiophene-2-boric acid.
- LCMS: m/z 493 [M+H]+
- HPLC retention time: 2.13 min (analysis condition U)
- Compound F5-42
-
- Under the same conditions as the method for synthesizing Compound F5-47, the title compound was prepared from Compound F4-9.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.71 (1H, br. s), 8.33-8.30 (1H, d, 8.08 Hz), 8.00 (1H, s), 7.96 (1H, s), 7.61-7.58 (1H, m), 7.29 (1H, s), 2.97 (4H, m), 2.73 (4H, m), 2.56 (1H, m), 2.34 (3H, s), 1.76 (6H, s), 1.64-1.58 (1H, m), 0.50-0.44 (2H, m), 0.37-0.32 (2H, m)
- LCMS: m/z 425 [M+H]+
- Compound F5-43
-
- Under nitrogen atmosphere, to the MeCN (8 ml) suspension of 9-bromo-8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound F4-10, 200 mg, 0.397 mmol), ethynyltriisopropylsilane (268 mg, 3.0 eq.), 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (Xphos) (39 mg, 0.2 eq.), Pd(CH3CN)2Cl2 (11 mg, 0.1 eq.) and cesium carbonate (518 mg, 4.0 eq.) were added and the mixture was stirred and heated under reflux condition until the reaction is completed. Upon the completion of the reaction, distilled water was added to the reaction solution, which was then extracted with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/methanol) to obtain 8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-9-[(triisopropylsilanyl)-ethynyl]-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (179 mg, 74%).
- To the THF (6 ml) solution of the obtained compound (179 mg, 0.295 mmol), 1 M THF solution (710 l) of tetrabutylammonium fluoride was added and the mixture was stirred until the reaction is completed. Upon the completion of the reaction, ethyl acetate was added to the reaction solution, which was then washed with distilled water and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were washed with a mixture solvent of ethanol and distilled water to obtain the title compound (67 mg, 92%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.85 (1H, s), 8.31 (1H, d, 7.9 Hz), 8.20 (1H, s), 8.03 (1H, s), 7.62 (1H, d, 7.9 Hz), 7.35 (1H, s), 4.62 (1H, s), 3.94-4.03 (2H, m), 3.79-3.89 (1H, m), 3.48-3.54 (2H, m), 3.27-3.38 (2H, m), 2.96-3.16 (2H, m), 2.30-2.41 (2H, m), 2.16-2.26 (2H, m), 1.72-1.85 (8H, m)
- LCMS: m/z 449 [M+H]+
- HPLC retention time: 2.69 min (analysis condition W)
- Compound F5-44
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-10 under propyne gas atmosphere.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.71 (1H, s), 8.30 (1H, d, 7.9 Hz), 8.06 (1H, s), 8.00 (1H, s), 7.59 (1H, d, 7.9 Hz), 7.20 (1H, s), 2.75-2.83 (1H, m), 2.40-2.48 (4H, m), 2.11 (3H, s), 1.97-2.06 (2H, m), 1.76 (6H, s), 1.62-1.71 (2H, m)
- LCMS: m/z 463 [M+H]+
- HPLC retention time: 2.80 min (analysis condition W)
- Compound F5-45
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F4-10 and ethynylcyclobutane.
- LCMS: m/z 503 [M+H]+
- HPLC retention time: 1.85 min (analysis condition S)
- Compound F5-46
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F5-15-2 and cyclobutanone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.23 (1H, d, 8 Hz), 7.92 (1H, br. s), 7.59 (1H, s), 7.47 (1H, br. d, 8 Hz), 7.28 (1H, s), 3.12 (4H, br. s), 2.80 (1H, dddd, 8, 8, 8, 8 Hz), 2.20-2.13 (1H, m), 2.01 (2H, br. s), 1.86-1.68 (10H, m), 1.05 (2H, d, 8 Hz), 0.76 (2H, d, 4 Hz)
- LCMS: m/z 465 [M+H]+
- HPLC retention time: 2.79 min (analysis condition W)
- Hydrochloric Acid Salt of Compound F5-46
- 8-(4-Cyclobutyl-piperazin-1-yl)-9-cyclopropyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile was added with DMSO and 6 N hydrochloric acid (1.05 eq.) and dissolved therein. After freeze-drying, crystallization was performed by using ethanol comprising 25% water to obtain monohydrochloric acid salt of 8-(4-cyclobutyl-piperazin-1-yl)-9-cyclopropyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.81 (1H, s), 10.64 (1H, br. s), 8.32-8.29 (1H, m), 8.01 (1H, s), 7.67 (1H, s), 7.61-7.60 (1H, m), 7.33 (1H, s), 4.00-3.39 (6H, m), 3.28-3.02 (3H, m), 2.45-2.05 (5H, m), 1.83-1.77 (8H, m), 1.09-1.07 (2H, m), 0.81-0.80 (2H, m)
- LCMS: m/z 465 [M+H]+
- Compound F5-47
-
- Under nitrogen atmosphere, to the N,N-dimethyl formamide (1.5 ml) solution of 9-bromo-8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound F4-10, 50 mg, 0.099 mmol), trimethyl boroxine (12 mg, 0.1 eq.), tetrakis triphenylphosphine palladium (39 mg, 0.2 eq.), and potassium carbonate (41 mg, 3.0 eq.) were added, and the mixture was stirred at 100° C. for 24 hr. Upon the completion of the reaction, distilled water was added to the reaction solution, which was then extracted with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/methanol) to obtain the title compound (25 mg, 58%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.67 (1H, s), 8.31 (1H, d, 7.9 Hz), 7.98 (1H, s), 7.95 (1H, s), 7.59 (1H, d, 7.9 Hz), 7.30 (1H, s), 2.96-3.04 (4H, m), 2.76-2.84 (1H, m), 2.39-2.48 (4H, m), 2.32 (3H, s), 1.78-1.87 (2H, m), 1.75 (6H, s), 1.63-1.71 (2H, m)
- LCMS: m/z 439 [M+H]+
- HPLC retention time: 2.66 min (analysis condition W)
- Compound F5-48
-
- Under the same conditions as the method for synthesizing Compound E4-7-1, the title compound was prepared from Compound F4-10 and 2-isopropenyl-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane.
- LCMS: m/z 465 [M+H]+
- HPLC retention time: 1.63 min (analysis condition S)
- Compound F5-49
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound F3-11.
- LCMS: m/z 479 [M+H]+
- HPLC retention time: 1.90 min (analysis condition U)
- Compound F5-50
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound F3-11 and propyne gas.
- 1H-NMR (270 MHz, CD3OD+CDCl3) δ: 8.40 (1H, d, J=7.8 Hz), 8.24 (1H, s), 7.84 (1H, s), 7.54 (1H, d, J=7.8 Hz), 7.14 (1H, s), 4.01-3.96 (2H, m), 3.78 (4H, m), 2.88-2.84 (2H, m), 2.68 (4H, m), 2.16-1.73 (5H, m), 2.16 (3H, s), 1.80 (6H, s)
- LCMS: m/z 493 [M+H]+
- Compound F5-51
-
- Under the same conditions as the method for synthesizing Compound F5-47, the title compound was prepared from Compound F3-11
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.70 (1H, br. s), 8.33-8.30 (1H, d, 8.08 Hz), 8.00 (1H, s), 7.95 (1H, s), 7.61-7.58 (1H, m), 7.28 (1H, s), 3.60 (4H, m), 3.32-3.26 (2H, m), 2.79-2.69 (2H, m), 2.32 (3H, s), 1.95-1.90 (2H, m), 1.74 (6H, s), 1.65-1.52 (2H, m),
- LCMS: m/z 469 [M+H]+
- Methanesulfonic Acid Salt of Compound F5-51
- 6,6,9-Trimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile was added with DMSO and 2 N methanesulfonic acid (1.05 eq.) and dissolved therein. After freeze-drying, crystallization was performed with ethanol to obtain methanesulfonic acid salt of 6,6,9-trimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.72 (1H, br.s), 9.60 (1H, br. s), 8.33-8.31 (1H, d, 9.8 Hz), 8.01 (1H, s), 7.99 (1H, s), 7.61-7.59 (1H, m), 7.31 (1H, s), 4.07-4.04 (2H, m), 3.73-3.67 (2H, m), 3.55-3.40 (8H, m), 3.32-3.26 (1H, m), 2.70-2.60 (2H, m), 2.34 (3H, s), 2.30 (3H, s), 1.95-1.90 (2H, m), 1.75 (6H, s)
- LCMS: m/z 469 [M+H]+
- Compound F6-1
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound F5-36-2 and acetone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.68 (1H, br. s), 8.30 (1H, d, 8.1 Hz), 7.98 (1H, s), 7.82 (1H, s), 7.58 (1H, d, 8.1 Hz), 7.20 (1H, s), 5.85 (1H, s), 3.56-3.44 (2H, m), 3.21-3.14 (2H, m), 2.77-2.66 (5H, m), 2.12-2.09 (1H, m), 1.98-1.88 (2H, m), 1.74 (6H, s), 1.70-1.63 (1H, m), 1.58-1.45 (2H, m), 1.09-1.00 (6H, m)
- LCMS: m/z 563 [M+H]+
- HPLC retention time: 1.90 min (analysis condition U)
- Compound F6-2
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound F5-36-2.
- LCMS: m/z 598 [M+H]+
- HPLC retention time: 1.52 min (analysis condition S)
- Compound F6-3
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-10.
- LCMS: m/z 538 [M+H]+
- HPLC retention time: 1.32 min (analysis condition S)
- Compound F6-4
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-16.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.70 (1H, br. s), 8.29 (1H, d, 8.0 Hz), 8.03-7.94 (2H, m), 7.59-7.55 (1H, m), 7.38 (1H, s), 4.59-4.47 (4H, m), 3.53-5.47 (1H, m), 3.03-2.97 (2H, m), 2.73-2.62 (2H, m), 1.74 (6H, s), 1.29-1.98 (3H, m)
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 1.92 min (analysis condition U)
- Hydrochloric Acid Salt of Compound F6-4
- 9-Ethyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile was added with DMSO and 6 N hydrochloric acid (1.05 eq.) and dissolved therein. After freeze-drying, crystallization was performed with ethanol comprising 25% water to obtain monohydrochloric acid salt of 9-ethyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.83 (1H, br.s), 11.59 (1H, br. s), 8.33-8.31 (1H, m), 8.09 (1H, s), 8.02 (1H, s), 7.63-7.61 (1H, m), 7.39 (1H, s), 4.91-4.60 (4H, br. m), 3.58-3.40 (1H, m), 3.31-3.05 (8H, br. m), 2.73 (2H, q, J=7.3), 1.81 (6H, s), 1.29 (3H, t, J=7.3)
- LCMS: m/z 455 [M+H]+
- Compound F6-5
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-18.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.73 (1H, br. s), 8.33-8.30 (1H, d, 8.08 Hz), 8.01 (1H, s), 8.00 (1H, s), 7.62-7.59 (1H, d, 8.08 Hz), 7.42 (1H, s), 4.61-4.56 (2H, m), 4.51-4.46 (2H, m), 3.53-3.47 (1H, m), 3.42-3.37 (2H, m), 3.02 (4H, m), 2.75-2.68 (2H, m), 2.51 (4H, m), 1.93-1.82 (2H, m), 1.76 (6H, s)
- LCMS: m/z 499 [M+H]+
- Compound F6-6
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-20.
- LCMS: m/z 609 [M+H]+
- HPLC retention time: 1.00 min (analysis condition S)
- Compound F6-7
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-21.
- LCMS: m/z 523 [M+H]+
- HPLC retention time: 1.92 min (analysis condition S)
- Compound F6-8
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-22.
- 1H-NMR (270 mHz DMSO-d6) δ: 12.75 (1H, s), 8.30 (1H, d, J=8.2 Hz), 8.01-7.97 (2H, m), 7.59 (1H, d, J=7.1 Hz), 7.38 (1H, s), 4.51 (4H, dt, J=27.7, 6.3 Hz), 3.55-3.49 (1H, m), 3.02-2.96 (4H, m), 2.63 (2H, t, J=7.3 Hz), 2.47-2.41 (4H, m), 1.73 (6H, s), 1.70-1.61 (2H, m), 0.94 (3H, t, J=7.4 Hz).
- LCMS: m/z 469 [M+H]+
- HPLC retention time: 1.57 min (analysis condition S)
- Compound F6-9
-
- The title compound was obtained as a by-product of the synthesis of Compound F6-8.
- LCMS: m/z 485 [M+H]+
- HPLC retention time: 1.61 min (analysis condition S)
- Compound F6-10
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-23.
- LCMS: m/z 499 [M+H]+
- HPLC retention time: 1.42 min (analysis condition S)
- Compound F6-11
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-26.
- LCMS: m/z 554 [M+H]+
- HPLC retention time: 1.50 min (analysis condition U)
- Compound F6-12
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-27.
- LCMS: m/z 497 [M+H]+
- HPLC retention time: 2.25 min (analysis condition U)
- Compound F6-13
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound F5-23 and 2-bromopropane.
- 1H-NMR (270 MHz, CD3OD+CDCl3) δ: 8.40 (1H, d, J=7.8 Hz), 8.32 (1H, s), 7.84 (1H, s), 7.53 (1H, d, J=7.8 Hz), 7.18 (1H, s), 4.80-4.68 (4H, m), 4.46 (2H, s), 3.95 (1H, m), 3.64 (1H, m), 3.46 (4H, m), 2.62 (4H, m), 1.82 (6H, s), 1.24 (6H, d, J=7.0 Hz)
- LCMS: m/z 523 [M+H]+
- Compound F6-14
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-32.
- 1H-NMR (270 MHz, CD3OD+CDCl3) δ: 8.44 (1H, d, J=7.8 Hz), 8.27 (1H, s), 7.84 (1H, s), 7.54 (1H, d, J=7.8 Hz), 7.36 (1H, s), 4.82-4.70 (4H, m), 3.68 (1H, m), 3.45 (1H, m), 3.13-3.09 (4H, m), 2.64-2.62 (4H, m), 1.81 (6H, s), 1.31 (6H, d, J=7.0 Hz)
- LCMS: m/z 469 [M+H]+
- Compound F6-15
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-37.
- LCMS: m/z 439 [M+H]+
- HPLC retention time: 1.98 min (analysis condition U)
- Compound F6-16
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-38.
- LCMS: m/z 453 [M+H]+
- HPLC retention time: 1.63 min (analysis condition S)
- Compound F6-17
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-43.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.80 (1H, s), 8.32 (1H, d, 7.9 Hz), 8.10 (1H, s), 8.02 (1H, s), 7.62 (1H, d, 7.9 Hz), 7.38 (1H, s), 3.78-3.88 (1H, m), 3.79-3.89 (1H, m), 3.48-3.54 (2H, m), 3.40-3.47 (2H, m), 3.30-3.39 (2H, m), 3.02-3.24 (4H, m), 2.73 (2H, q, 7.3 Hz), 2.30-2.41 (2H, m), 2.17-2.26 (2H, m), 1.71-1.86 (8H, m), 1.29 (3H, t, 7.3 Hz)
- LCMS: m/z 453 [M+H]+
- HPLC retention time: 2.76 min (analysis condition W)
- Methanesulfonic Acid Salt of Compound F6-17
- 8-(4-Cyclobutyl-piperazin-1-yl)-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile was dissolved in 6 volumes of DMF at room temperature and added dropwise with aqueous solution of methanesulfonic acid (2 M, 1.05 eq.). The resulting solution was added dropwise to 60 volumes of acetonitrile, and the precipitated solid was filtered and dried to obtain monomethanesulfonic acid salt of 8-(4-cyclobutyl-piperazin-1-yl)-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.75 (1H, s), 8.31 (1H, J=8.4 Hz), 8.07 (1H, s), 8.01 (1H, s), 7.59 (1H, d, J=7.9 Hz), 7.38 (1H, s), 3.58-2.84 (10H, m), 2.71 (2H, q, J=7.5 Hz), 2.34 (3H, s), 2.20-2.04 (4H, m), 1.76-1.68 (8H, m), 1.26 (3H, t, J=7.5 Hz)
- FABMS: m/z 453 [M+H]+
- Compound F6-18
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-44.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.69 (1H, s), 8.31 (1H, d, 7.9 Hz), 8.01 (1H, s), 7.99 (1H, s), 7.60 (1H, d, 7.9 Hz), 7.39 (1H, s), 2.92-3.02 (4H, m), 2.75-2.84 (1H, m), 2.65 (2H, t, 7.3 Hz), 2.38-2.48 (4H, m), 1.96-2.06 (2H, m), 1.78-1.87 (2H, m), 1.75 (6H, s), 1.62-1.73 (4H, m), 0.97 (3H, t, 7.3 Hz)
- LCMS: m/z 467 [M+H]+
- HPLC retention time: 2.96 min (analysis condition W)
- Compound F6-19
-
- Under the same conditions as the method for synthesizing Compound B3-13, the title compound was prepared from Compound F5-48.
- LCMS: m/z 467 [M+H]+
- HPLC retention time: 1.67 min (analysis condition S)
- Compound F6-20
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-49.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.70 (1H, s), 8.32 (1H, d, J=7.9 Hz), 8.04 (1H, s), 8.00 (1H, s), 7.61 (1H, d, J=8.5 Hz), 7.34 (1H, s), 3.64-3.57 (4H, m), 3.27-3.18 (2H, m), 2.82-2.66 (4H, m), 2.39-2.28 (1H, m), 1.96-1.87 (2H, m), 1.76 (6H, s), 1.69-1.53 (2H, m), 1.29 (3H, t, J=7.3 Hz)
- LCMS: m/z 483 [M+H]+
- HPLC retention time: 1.98 min (analysis condition U)
- Hydrochloric Acid Salt of Compound F6-20
- 9-Ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile was dissolved in a mixture solution of methylethyl ketone (10 volumes), water (4 volumes) and acetic acid (3 volumes) at 60° C. To the dissolved solution, hydrochloric acid (2 N) was added dropwise (1 volume). After stirring at 60° C. for 30 min, ethanol (25 volume) was added dropwise. The precipitated solid was filtered and dried to obtain monohydrochloric acid salt of 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.78 (1H, s), 10.57 (1H, br. s), 8.30 (1H, J=8.4 Hz), 8.05 (1H, s), 7.99 (1H, s), 7.59 (1H, d, J=7.9 Hz), 7.36 (1H, s), 4.02-3.99 (2H, m), 3.84-3.78 (2H, m), 3.51-3.48 (2H, m), 3.15-3.13 (1H, s), 2.83-2.73 (2H, s), 2.71-2.67 (2H, s), 2.23-2.20 (2H, m), 1.94-1.83 (2H, m), 1.75 (6H, s), 1.27 (3H, t, J=7.5 Hz)
- FABMS: m/z 483 [M+H]+
- Compound F6-21
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound F5-50.
- 1H-NMR (270 MHz, CD3OD+CDCl3) δ: 8.41 (1H, d, J=7.8 Hz), 8.14 (1H, s), 7.84 (1H, s), 7.53 (1H, d, J=7.8 Hz), 7.31 (1H, s), 3.77 (4H, m), 3.32 (2H, m), 2.86-2.66 (8H, m), 2.43-2.05 (3H, m), 1.79 (6H, s), 1.79-1.66 (4H, m), 1.02 (3H, t, J=7.3 Hz)
- LCMS: m/z 497 [M+H]+
- Compound G2
-
- 2-Hydrazinopyridine (1.3 g, 11.8 mmol) and 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 2.4 g, 11.8 mmol) were dissolved in NMP (60 mL), and stirred at 190° C. for 48 hr. The reaction solution was diluted with ethyl acetate, washed with water and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane/ethyl acetate) to obtain the target compound (white solid, 101 mg, 3%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 11.53 (1H, s), 8.16-8.12 (1H, m), 7.84 (1H, d, J=7.8 Hz), 7.23 (1H, d, J=8.4 Hz), 7.11 (1H, s), 7.03-6.98 (1H, m), 6.85-6.81 (1H, m), 3.96 (2H, s), 3.77 (3H, s), 1.64 (6H, s)
- LCMS: m/z 279 [M+H]+
- HPLC retention time: 2.08 min (analysis condition U)
- Compound G3
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound G2.
- 1H-NMR (400 MHz, CDCl3) δ: 12.95 (1H, br. s), 8.78 (1H, d, J=7.8 Hz), 8.52 (1H, d, J=4.9 Hz), 8.41 (1H, d, J=8.8 Hz), 7.37 (1H, dd, J=7.7, 5.0 Hz), 7.15 (1H, s), 7.04-7.00 (1H, m), 3.94 (3H, s), 1.98 (6H, s)
- LCMS: m/z 293 [M+H]+
- HPLC retention time: 2.13 min (analysis condition U)
- Compound G4
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound G3.
- 1H-NMR (400 MHz, CDCl3) δ: 8.66 (1H, d, J=7.7 Hz), 8.29 (1H, d, J=4.9 Hz), 8.23 (1H, d, J=13.8 Hz), 7.29 (1H, dd, J=7.7, 5.0 Hz), 7.12 (1H, s), 6.93 (1H, d, J=8.6 Hz), 1.71 (6H, s)
- LCMS: m/z 279 [M+H]+
- HPLC retention time: 1.72 min (analysis condition U)
- Compound G5
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound G4.
- 1H-NMR (400 MHz, CDCl3) δ: 8.81 (1H, d, J=7.8 Hz), 8.60-8.52 (2H, m), 7.55 (1H, s), 7.46-7.40 (2H, m), 2.01 (6H, s)
- LCMS: m/z 411 [M+H]+
- HPLC retention time: 1.75 min (analysis condition U)
- Compound G6
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound G5 and 4-pyrrolidin-1-yl-piperidine.
- 1H-NMR (400 MHz, CDCl3) δ: 13.12 (1H, s), 8.78 (1H, d, J=7.8 Hz), 8.49 (1H, d, J=4.9 Hz), 8.29 (1H, d, J=8.8 Hz), 7.34 (1H, dd, J=7.7, 5.0 Hz), 7.06-6.98 (2H, m), 3.96-3.88 (2H, m), 3.02-3.92 (2H, m), 2.69-2.60 (4H, m), 2.32-2.23 (1H, m), 2.09-2.00 (4H, m), 1.92 (6H, s), 1.26-1.19 (4H, m)
- LCMS: m/z 415 [M+H]+
- HPLC retention time: 1.57 min (analysis condition U)
- Compound H1
-
- To the dichloromethane (70 ml) solution of 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 3 g, 14.7 mmol), acetic anhydride (1.7 ml, 1.2 eq.) and aluminum chloride-nitrobenzene solution (1 M, 44 ml, 3 eq.) was added at 0° C., and stirred for 3 hr. Thereafter, the reaction solution was added with saturated aqueous solution of sodium hydrogen carbonate and extracted twice with dichloromethane. The organic layer was washed with brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound as a crude product.
- Compound H2-1
-
- Under the same conditions as the method for synthesizing Compound A3-1, the title compound was prepared from Compound H1 and (3-bromo-phenyl)-hydrazine.
- LCMS: m/z 398 [M+H]+
- HPLC retention time: 3.97 min (analysis condition Y)
- Compound H2-2
-
- The title compound was obtained as a by-product of the synthesis of Compound H2-1.
- LCMS: m/z 398 [M+H]+
- HPLC retention time: 3.97 min (analysis condition Y)
- Compound H3
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound H2.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.80 (6H, s), 2.58 (3H, s), 4.06 (3H, s), 7.38 (1H, dd, 8.39 Hz, 1.91 Hz), 7.51 (1H, s), 7.67 (1H, bs, 1.53 Hz), 8.10 (1H, d, 8.39 Hz), 8.41 (1H, s), 12.3 (1H, s)
- LCMS: m/z 412, 414 [M+H]+
- HPLC retention time: 2.73 min (analysis condition U)
- Compound H4
-
- According to the same method as the method for synthesizing Compound A5-2, the title compound was prepared from Compound H3.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.83 (6H, s), 2.58 (3H, s), 4.07 (3H, s), 7.53 (1H, s), 7.61 (1H, d, 8.01 Hz), 8.03 (1H, s), 8.31 (1H, d, 8.77 Hz), 8.42 (1H, s), 12.8 (1H, s).
- LCMS: m/z 359 [M+H]+
- HPLC retention time: 2.47 min (analysis condition U)
- Compound H5
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound H4.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.77 (6H, s), 2.75 (3H, s), 7.43 (1H, s), 7.63 (1H, d, 8.01 Hz), 8.02 (1H, s), 8.32 (1H, d, 8.01 Hz), 8.67 (1H, s), 12.2 (1H, s), 12.8 (1H, s).
- LCMS: m/z 345 [M+H]+
- HPLC retention time: 2.27 min (analysis condition S)
- Compound H6-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound H5 and tetrahydropyran-4-ol.
- LCMS: m/z 429 [M+H]+
- HPLC retention time: 2.48 min (analysis condition U)
- Compound H6-2
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound H5.
- LCMS: m/z 444 [M+H]+
- HPLC retention time: 2.05 min (analysis condition U)
- Compound H7
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound H5.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.83 (6H, s), 2.74 (3H, s), 7.68 (1H, dd, 8.01 Hz, 1.53 Hz), 8.08 (2H, s), 8.33 (1H, d, 8.77 Hz), 8.79 (1H, s), 12.9 (1H, s).
- Compound H8-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound H7 and 4-pyrrolidin-1-yl-piperidine. 1H-NMR (300 MHz, DMSO-d6) δ: 1.65 (2H, m), 1.69 (4H, s), 1.79 (6H, s), 1.95 (2H, m), 2.18 (1H, m), 2.54 (4H, s), 2.59 (3H, s), 2.93 (2H, t, 11.8 Hz), 3.37 (2H, m), 7.36 (1H, s), 7.60 (1H, d, 8.01), 8.01 (1H, s), 8.13 (1H, s), 8.30 (1H, d, 8.39), 12.7 (1H, s).
- LCMS: m/z 481 [M+H]+
- HPLC retention time: 2.03 min (analysis condition U)
- Compound H8-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound H7.
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 2.02 min (analysis condition U)
- Compound H8-3
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound H7 and morpholine.
- LCMS: m/z 414 [M+H]+
- HPLC retention time: 2.11 min (analysis condition S)
- Compound H8-4
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound H7 and 4-piperidin-4-yl-morpholine.
- LCMS: m/z 497 [M+H]+
- HPLC retention time: 1.45 min (analysis condition S)
- Compound H8-5
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound H7 and piperazine.
- LCMS: m/z 413 [M+H]+
- HPLC retention time: 1.71 min (analysis condition U)
- Compound H9-1
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound H8-5 and cyclobutanone.
- LCMS: m/z 467 [M+H]+
- HPLC retention time: 1.82 min (analysis condition U)
- Compound H9-2
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound H8-5 and tetrahydropyran-4-one.
- LCMS: m/z 497 [M+H]+
- HPLC retention time: 1.76 min (analysis condition U)
- Compound H9-3
-
- To the anhydrous THF solution (0.5 mL) of 9-acetyl-6,6-dimethyl-11-oxo-8-piperazin-1-yl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound H8-5, 25 mg, 0.06 mmol), 3-chloro-3-methyl-but-1-yne (0.013 mL, 0.12 mmol), copper (I) chloride (0.6 mg, 0.006 mmol) and triethylamine (0.017 mL, 0.12 mmol) were added at room temperature. After stirring for 30 min, the mixture was added with water and extracted with ethyl acetate. The organic layer was dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by amino silica gel column chromatography (dichloromethane/methanol) to obtain the title compound (white solid, 9.8 mg, 35%).
- LCMS: m/z 479 [M+H]+
- HPLC retention time: 1.88 min (analysis condition U)
- Compound I1-1
-
- 7-Methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 3.37 g, 16.5 mmol) was dissolved in CH3CN (82 mL), added with NCS (2.42 g, 1.1 eq.) and stirred at 90° C. for 1.5 hr. The reaction solution was extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed and the target compound was obtained after concentration under reduced pressure (yellow oily substance, 4.45 g).
- 1H-NMR (400 MHz, CDCl3) δ: 7.16 (1H, s), 6.85 (1H, s), 3.90 (3H, s), 3.00 (2H, t, J=6.8 Hz), 2.65 (2H, t, J=6.8 Hz), 1.42 (6H, s).
- LCMS: m/z 239 [M+H]+
- HPLC retention time: 2.80 min (analysis condition U)
- Compound I1-2
-
- 6-Chloro-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound I1-1, 4.45 g, 16.5 mmol) and 3-hydrazinobenzonitrile (2.63 g, 1.2 eq.) were dissolved in TFA (91 mL), and stirred at 90° C. for 3 hr. According to the concentration under reduced pressure, TFA was removed and the residues were added with saturated aqueous solution of NaHCO3, followed by extraction with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were added with ethyl acetate. After stirring at room temperature, the precipitated solid was separated by filtration. The filtrate was concentrated under reduced pressure to obtain the title compound as a mixture with I1-3 (red powder, 6.46 g).
- Compound I1-3
-
- The title compound was obtained as a by-product of the synthesis of Compound I1-2.
- 1H-NMR (400 MHz, DMSO-d6) δ: 11.66 (1H, s), 7.65-7.69 (1H, m), 7.44-7.48 (1H, m), 7.39 (1H, s), 7.29 (1H, s), 7.17-7.23 (1H, m), 4.21 (2H, s), 3.91 (3H, s), 1.69 (6H, s).
- LCMS: m/z 337 [M+H]+
- HPLC retention time: 3.15 min (analysis condition U)
- Compound I2-1
-
- 1-Chloro-4-fluoro-2-methoxy-benzene (4.3 g, 26.78 mmol) and isobutyronitrile (9.61 mL, 4.0 eq.) were dissolved in toluene (9.0 mL), added with KHMDS (80 mL, 0.5 M toluene solution) and stirred at 65° C. for 2 hr. The reaction solution was cooled to room temperature, added with aqueous solution of 1 N hydrochloric acid and then extracted with MTBE. The organic layer was washed with saturated brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane/ethyl acetate) to obtain the title compound (1.72 g, 31%).
- 1H-NMR (270 MHz, CDCl3) δ: 7.37 (1H, d, J=8.4 Hz), 7.05 (1H, d, J=2.1 Hz), 6.97 (1H, dd, J=8.2, 2.1 Hz), 3.95 (3H, s), 1.73 (6H, s).
- HPLC retention time: 2.33 min (analysis condition S)
- Compound I2-2
-
- Under the same conditions as the method for synthesizing Compound K3, the title compound was prepared from Compound I2-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 7.42 (1H, d, J=8.1 Hz), 6.92 (1H, d, J=2.1 Hz), 6.86 (1H, dd, J=8.2, 2.3 Hz), 4.01 (2H, q, J=7.1 Hz), 3.87 (3H, s), 3.43 (2H, s), 1.44 (6H, s), 1.12 (3H, t, J=7.2 Hz).
- LCMS: m/z 299, 301 [M+H]+
- HPLC retention time: 2.52, 3.05 min (analysis condition S)
- Compound I2-3
-
- Under the same conditions as the method for synthesizing Compound K4, the title compound was obtained as a crude product from Compound I2-2.
- Compound I2-4
-
- Under the same conditions as the method for synthesizing Compound K5, the title compound was obtained from Compound I2-3.
- LCMS: m/z 397, 399 [M+H]+
- HPLC retention time: 2.83 min (analysis condition S)
- Compound I3
-
- (Method 1) Under the same conditions as the method for synthesizing Compound A4, the title compound was obtained from Compound I1-2.
- (Method 2) Under the same conditions as the method for synthesizing Compound L8-1, the title compound was obtained from Compound I2-4.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.79 (1H, s), 8.27-8.31 (1H, m), 8.12 (1H, s), 8.00-8.02 (1H, m), 7.58-7.63 (1H, m), 7.51 (1H, s), 4.03 (3H, s), 1.80 (6H, s).
- LCMS: m/z 351 [M+H]+
- HPLC retention time: 2.87 min (analysis condition U)
- Compound I4
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound I3.
- LCMS: m/z 337 [M+H]+
- HPLC retention time: 2.47 min (analysis condition U)
- Compound I5
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound I4.
- LCMS: m/z 469 [M+H]+
- HPLC retention time: 3.40 min (analysis condition U)
- Compound I6-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound I5 and 4-pyrrolidin-1-yl-piperidine.
- LCMS: m/z 473 [M+H]+
- HPLC retention time: 2.25 min (analysis condition U)
- Compound I6-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound I5.
- LCMS: m/z 447 [M+H]+
- HPLC retention time: 2.30 min (analysis condition U)
- Compound I6-3
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from 15 and morpholine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.79 (1H, s), 8.28 (1H, d, 8.0 Hz), 8.09 (1H, s), 8.00 (1H, s), 7.59 (1H, d, 8.0 Hz), 7.45 (1H, s), 3.75-3.81 (4H, m), 3.13-3.19 (4H, m), 1.76 (6H, s)
- LCMS: m/z 406 [M+H]+
- HPLC retention time: 2.88 min (analysis condition U)
- Compound I6-4
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound I5 and 4-piperidin-4-yl-morpholine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.75 (1H, s), 8.28 (1H, d, 8.0 Hz), 8.07 (1H, s), 8.00 (1H, s), 7.59 (1H, d, 8.0 Hz), 7.41 (1H, s), 3.55-3.62 (4H, m), 3.47-3.56 (4H, m), 2.75-2.86 (2H, m), 2.45-2.55 (4H, m), 2.28-2.39 (1H, m), 1.86-1.96 (2H, m), 1.76 (6H, s), 1.52-1.66 (2H, m)
- LCMS: m/z 489 [M+H]+
- HPLC retention time: 1.97 min (analysis condition U)
- Compound I6-5-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound I5 and piperidin-4-yl-carbamic acid tert-butyl ester.
- LCMS: m/z 519 [M+H]+
- HPLC retention time: 3.27 min (analysis condition U)
- Compound I6-5-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound I6-5-1.
- LCMS: m/z 419 [M+H]+
- HPLC retention time: 2.12 min (analysis condition U)
- Compound I6-6
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound I5 and piperazine.
- LCMS: m/z 405 [M+H]+
- HPLC retention time: 1.87 min (analysis condition U)
- Compound I7-1
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound I6-5-2.
- LCMS: m/z 497 [M+H]+
- HPLC retention time: 2.62 min (analysis condition U)
- Compound I7-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound I5 and 1-oxetan-3-yl-piperazine.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.78 (1H, s), 8.27-8.31 (1H, m), 8.07-8.09 (1H, s), 7.99-8.02 (1H, m), 7.59-7.62 (1H, m), 7.44-7.46 (1H, s), 4.54-4.60 (2H, m), 4.44-4.51 (2H, m), 3.47-3.55 (1H, m), 3.16-3.24 (4H, m), 2.40-2.55 (4H, m), 1.77 (6H, s)
- LCMS: m/z 461 [M+H]+
- HPLC retention time: 2.13 min (analysis condition U)
- Compound I7-3
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound I5 and 1-cyclopropylpiperazine.
- LCMS: m/z 445 [M+H]+
- HPLC retention time: 1.97 min (analysis condition U)
- Compound I7-4
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound I6-6 and cyclobutanone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.78 (1H, s), 8.29 (1H, d, 8.5 Hz), 8.08 (1H, s), 8.01 (1H, s), 7.60 (1H, d, 8.5 Hz), 7.44 (1H, s), 3.17-3.15 (4H, m), 2.83-2.76 (1H, m), 2.47-2.44 (4H, m), 2.04-1.97 (2H, m), 1.82 (2H, t, 9.8 Hz), 1.77 (6H, s), 1.70-1.63 (2H, m)
- LCMS: m/z 459, 461 [M+H]+
- HPLC retention time: 1.63 min (analysis condition S)
- Compound J2
-
- Under the same conditions as the method for synthesizing Compound A2, the title compound was prepared from 6-methoxy-3,4-dihydro-H-naphthalen-2-one and iodomethane.
- LCMS: m/z 205 [M+H]+
- HPLC retention time: 1.54 min (analysis condition S)
- Compound J3-1
-
- Under the same conditions as the method for synthesizing Compound E2-1, the title compound was prepared from Compound J2 and 3-hydrazino-benzonitrile.
- LCMS: m/z 303 [M+H]+
- HPLC retention time: 2.73 min (analysis condition S)
- Compound J3-2
-
- Compound J3-2 was obtained as a by-product of the synthesis of Compound J3-1.
- LCMS: m/z 303 [M+H]+
- HPLC retention time: 2.67 min (analysis condition S)
- Compound J4
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound J3-1 and Compound J3-2 (mixture).
- 1H-NMR (DMSO-d6) δ: 12.79 (1H, s), 8.33 (1H, d, J=8.2 Hz), 8.02 (1H, s), 7.81 (1H, d, J=8.6 Hz), 7.69 (1H, d, J=3.0 Hz), 7.63 (1H, dd, J=8.3, 1.4 Hz), 7.28 (1H, dd, J=8.7, 3.0 Hz), 3.87 (3H, s), 1.74 (6H, s).
- LCMS: m/z 317 [M+H]+
- HPLC retention time: 2.25 min (analysis condition S)
- Compound J5
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound J4.
- 1H-NMR (DMSO-d6) δ: 12.75 (1H, s), 9.77 (1H, s), 8.32 (1H, dd, J=8.2, 0.7 Hz), 8.01 (1H, s), 7.68 (1H, d, J=8.6 Hz), 7.62 (1H, dd, J=8.2, 1.4 Hz), 7.58 (1H, d, J=2.8 Hz), 7.10 (1H, dd, J=8.6, 2.8 Hz), 1.72 (6H, s).
- LCMS: m/z 303 [M+H]+
- HPLC retention time: 1.75 min (analysis condition S)
- Compound J6
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound J5.
- 1H-NMR (DMSO-d6) δ: 12.95 (1H, s), 8.31 (1H, d, J=8.2 Hz), 8.15 (2H, m), 8.05 (1H, s), 7.87 (1H, dd, J=9.0, 2.7 Hz), 7.65 (1H, d, J=8.2 Hz), 1.80 (6H, s).
- LCMS: m/z 435 [M+H]+
- HPLC retention time: 2.75 min (analysis condition S)
- Compound J7-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound J4 and isopropanol.
- LCMS: m/z 345 [M+H]+
- HPLC retention time: 3.87 min (analysis condition W)
- Compound J7-2-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound J5.
- LCMS: m/z 486 [M+H]+
- HPLC retention time: 4.15 min (analysis condition W)
- Compound J7-2-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound J7-2-1.
- LCMS: m/z 386 [M+H]+
- HPLC retention time: 2.48 min (analysis condition W)
- Compound J7-2-3
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound J7-2-2 and oxetan-3-one.
- LCMS: m/z 442 [M+H]+
- HPLC retention time: 2.61 min (analysis condition W)
- Compound J7-3
-
- Under the same conditions as the method for synthesizing Compound B2-10, the title compound was prepared from Compound J6 and 4-pyrrolidin-1-yl-piperidine.
- 1H-NMR (270 MHz, DMSO-d6) δ: 13.12 (1H, s), 8.32 (1H, d, J=8.1 Hz), 8.01 (1H, s), 7.72 (1H, d, J=8.7 Hz), 7.68 (1H, d, J=2.6 Hz), 7.62 (1H, dd, J=8.2, 1.2 Hz), 7.38 (1H, dd, J=9.1, 2.8 Hz), 3.90 (2H, d, J=11.5 Hz), 2.76 (2H, t, J=12.2 Hz), 2.14 (2H, d, J=10.9 Hz), 1.91 (4H, br), 1.74 (6H, s).
- LCMS: m/z 439 [M+H]+
- HPLC retention time: 1.35 min (analysis condition S)
- Compound J7-4
-
- Under the same conditions as the method for synthesizing Compound B2-10, the title compound was prepared from Compound J6 and 1-isopropyl-piperazine. 1H-NMR (270 MHz, DMSO-d6) δ: 12.80 (1H, s), 8.33 (1H, d, J=7.6 Hz), 8. 02 (1H, s), 7.66 (3H, m), 7.33 (1H, d, J=8.2 Hz), 3.21 (4H, br), 2.66 (5H, m), 1.72 (6H, s), 1.02 (6H, d, J=6.3 Hz).
- LCMS: m/z 413 [M+H]+
- HPLC retention time: 1.38 min (analysis condition S)
- Compound J7-5
-
- Under the same conditions as the method for synthesizing Compound B2-10, the title compound was prepared from Compound J6 and pyrrolidine.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.24 (1H, d, J=8.1 Hz), 7.91 (1H, s), 7.59 (1H, d, J=8.6 Hz), 7.45 (1H, d, J=7.9 Hz), 7.30 (1H, d, J=2.6 Hz), 6.85 (1H, dd, J=8.6, 2.8 Hz), 3.31 (4H, t, J=6.3 Hz), 1.99 (4H, t, J=6.2 Hz), 1.67 (6H, s).
- LCMS: m/z 356 [M+H]+
- HPLC retention time: 2.38 min (analysis condition S)
- Compound J7-6
-
- Under the same conditions as the method for synthesizing Compound B2-10, the title compound was prepared from Compound J6 and (S)-2-pyrrolidin-1-yl methyl-pyrrolidine.
- LCMS: m/z 439 [M+H]+
- HPLC retention time: 1.50 min (analysis condition S)
- Compound J7-7
-
- Under the same conditions as the method for synthesizing Compound B2-10, the title compound was prepared from Compound J6 and piperazine.
- LCMS: m/z 371 [M+H]+
- HPLC retention time: 1.31 min (analysis condition S)
- Compound J7-8
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound J6.
- LCMS: m/z 369 [M+H]+
- HPLC retention time: 2.16 min (analysis condition S)
- Compound J7-9
-
- Under the same conditions as the method for synthesizing Compound E4-2-2, the title compound was prepared from Compound J7-8.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.31 (1H, d, J=8.1 Hz), 8.23 (1H, d, J=1.8 Hz), 8.02 (1H, d, J=1.3 Hz), 7.93 (1H, d, J=8.2 Hz), 7.78 (1H, dd, J=8.2, 1.8 Hz), 7.61 (1H, dd, J=8.1, 1.3 Hz), 4.31 (1H, s), 1.77 (6H, s).
- LCMS: m/z 311 [M+H]+
- HPLC retention time: 2.40 min (analysis condition S)
- Compound J7-10-1
-
- Under the same conditions as the method for synthesizing Compound B2-22-1, the title compound was prepared from Compound J6.
- LCMS: m/z 468 [M+H]+
- HPLC retention time: 2.90 min (analysis condition S)
- Compound J7-10-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound J7-10-1.
- LCMS: m/z 368 [M+H]+
- HPLC retention time: 1.27 min (analysis condition S)
- Compound J7-11-1
-
- Under the same conditions as the method for synthesizing Compound B2-25-1 and Compound B2-25-2, the title compound was prepared from Compound J6.
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 1.42 min (analysis condition S)
- Compound J7-11-2
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound J7-11-1 and acetone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.79 (1H, s), 8.33 (1H, d, 7.9 Hz), 8.01 (1H, s), 7.98 (1H, d, 1.8 Hz), 7.79 (1H, d, 7.9 Hz), 7.61 (1H, d, 7.9 Hz), 7.51-7.49 (1H, m), 2.74 (2H, d, 11.0 Hz), 2.64-2.60 (3H, m), 2.04 (2H, t, 10.7 Hz), 1.77 (6H, s), 1.60-1.51 (3H, m), 1.23-1.14 (2H, m), 0.94 (6H, d, 6.7 Hz)
- LCMS: m/z 426 [M+H]+
- Compound J7-12
-
- 9-Hydroxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound J5, 30 mg, 0.099 mmol), 4-bromo-butyric acid methyl ester (24.9 μl, 0.198 mmol) and cesium carbonate (64.5 mg, 0.198 mmol) were dissolved in DMA (0.20 ml) and stirred at room temperature for 4 hr. Water was added to the reaction solution, which was then extracted with diethyl ether. The organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. The yellow solid obtained after concentration under reduced pressure was purified by silica gel column chromatography (methylene chloride/MeOH) to obtain 4-(3-cyano-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-9-yl oxy)-butyric acid methyl ester as an intermediate. The intermediate was dissolved in MeOH (0.50 ml), added with aqueous solution of sodium hydroxide (6 mol/l) and stirred at room temperature for 30 min. The reaction solution was added with hydrochloric acid (3 mol/), extracted with diethyl ether. The organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. After concentration under reduced pressure, white solid was obtained, which was then washed with methylene chloride to obtain the title compound (19.0 mg, 70%).
- LCMS: m/z 389 [M+H]+
- HPLC retention time: 2.39 min (analysis condition F)
- Compound J7-13
-
- Under the same conditions as the method for synthesizing Compound J7-12, Compound J5 and 5-bromo-pentanoic acid methyl ester were reacted to obtain the target compound (19.5 mg, 64%).
- LCMS: m/z 403 [M+H]+
- HPLC retention time: 2.49 min (analysis condition F)
- Compound J7-14
-
- Under the same conditions as the method for synthesizing Compound J7-12, Compound J5 and 6-bromo-hexanoic acid ethyl ester were reacted to obtain the target compound (19.6 mg, 66%).
- LCMS: m/z 417 [M+H]+
- HPLC retention time: 2.61 min (analysis condition F)
- Compound J7-15
-
- Under the same conditions as the method for synthesizing Compound A7-1, Compound JJ2 and 3-(2-hydroxy-ethoxy)-propionic acid tert-butyl ester were reacted to obtain 3-[2-(3-bromo-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-9-yl oxy)-ethoxy]-propionic acid tert-butyl ester.
- The resultant was dissolved in DMA (0.30 ml), added with copper cyanide (25.5 mg, 0.285 mmol), and stirred at 200° C. for 1 hr under irradiation with microwave. The reaction solution was diluted with ethyl acetate, washed with saturated brine and dried over anhydrous magnesium sulfate. The residues obtained after concentration under reduced pressure were dissolved in methylene chloride (0.75 ml). The solution was added with TFA (250 l) and stirred at room temperature for 5 min. Thereafter, the residues obtained from the reaction solution after concentration under reduced pressure were purified by silica gel column chromatography (methylene chloride/MeOH) to obtain the title compound (5.6 mg, 14%).
- LCMS: m/z 419 [M+H]+
- HPLC retention time: 2.31 min (analysis condition F)
- Compound J7-16
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound J5 and pyridin-4-yl-methanol (pale yellow solid, 6.1 mg, 31%).
- LCMS: m/z 394 [M+H]+
- HPLC retention time: 1.97 min (analysis condition F)
- Compound J7-17
-
- Under the same conditions as the method for synthesizing Compound JJ3-1, the title compound was prepared from Compound J5 and pyridin-3-yl-methanol (pale yellow solid, 7.9 mg, 38%).
- LCMS: m/z 394 [M+H]+
- HPLC retention time: 1.99 min (analysis condition F)
- Compound J8-1
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound J7-7 and oxetan-3-one.
- LCMS: m/z 427 [M+H]+
- HPLC retention time: 1.31 min (analysis condition S)
- Compound J8-2
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound J7-7 and (1-ethoxycyclopropoxy)trimethylsilane.
- LCMS: m/z 411 [M+H]+
- HPLC retention time: 1.39 min (analysis condition S)
- Compound J8-3
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound J7-10-2.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.81 (1H, s), 8.33 (1H, d, 7.9 Hz), 8.26 (1H, d, 2.4 Hz), 8.01 (1H, s), 7.88-7.81 (2H, m), 7.61 (1H, d, 7.9 Hz), 6.36 (1H, s), 3.93 (2H, d, 3.0 Hz), 3.45 (2H, t, 5.8 Hz), 2.97 (3H, s), 2.73-2.70 (2H, m), 1.78 (6H, s)
- LCMS: m/z 446 [M+H]+
- HPLC retention time: 2.15 min (analysis condition S)
- Compound J8-4
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound J7-10-2 and acetone.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.82 (1H, s), 8.33 (1H, d, 7.9 Hz), 8.22 (1H, d, 1.8 Hz), 8.02 (1H, s), 7.84 (1H, d, 8.5 Hz), 7.78 (1H, dd, 8.2, 2.1 Hz), 7.62 (1H, d, 7.9 Hz), 6.32 (1H, t, 3.7 Hz), 3.23-3.20 (2H, m), 2.83-2.76 (1H, m), 2.72 (2H, t, 5.5 Hz), 2.56-2.54 (2H, m), 1.78 (6H, s), 1.06 (6H, d, 6.7 Hz)
- LCMS: m/z 410 [M+H]+
- HPLC retention time: 1.38 min (analysis condition S)
- Compound J8-5
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound J7-10-2 and oxetan-3-one.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.81 (1H, br. s), 8.34 (1H, d, J=8.2 Hz), 8.22 (1H, d, J=1.8 Hz), 8.03 (1H, s), 7.76-7.90 (2H, m), 7.64 (1H, dd, J=8.2, 1.8 Hz), 6.25-6.34 (1H, m), 4.60 (2H, dd, J=6.6, 6.0 Hz), 4.52 (2H, dd, J=6.6, 6.0 Hz), 3.57 (1H, t, J=6.0 Hz), 3.03 (2H, m), 2.55 (4H, m), 1.77 (6H, s).
- LCMS: m/z 424 [M+H]+
- HPLC retention time: 1.34 min (analysis condition S)
- Compound J8-6
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound J7-10-2 and (1-ethoxycyclopropoxy)trimethylsilane.
- LCMS: m/z 408 [M+H]+
- HPLC retention time: 1.36 min (analysis condition S)
- Compound J9-1
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound J7-10-1.
- LCMS: m/z 414, 470 [M+H]+
- HPLC retention time: 2.83 min (analysis condition S)
- Compound J9-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound J9-1.
- LCMS: m/z 370 [M+H]+
- HPLC retention time: 1.30 min (analysis condition S)
- Compound J9-3
-
- Under the same conditions as the method for synthesizing Compound A9-1, the title compound was prepared from Compound J9-2 and 2-bromopropane.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.83 (1H, s), 8.34 (2H, d, J=8.1 Hz), 8.05 (2H, m), 7.82 (1H, d, J=8.1 Hz), 7.61 (2H, m), 3.02 (2H, br), 2.42 (2H, br), 1.76 (6H, s), 1.06 (6H, d, J=6.4 Hz).
- LCMS: m/z 412 [M+H]+
- HPLC retention time: 1.45 min (analysis condition S)
- Compound J9-4
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound J8-5.
- LCMS: m/z 426 [M+H]+
- HPLC retention time: 1.26 min (analysis condition S)
- Compound J9-5
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound J8-6.
- LCMS: m/z 410 [M+H]+
- HPLC retention time: 1.43 min (analysis condition S)
- Compound JJ1
-
- 6-Methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound J2, 2.15 g, 10.5 mmol) and 3-bromophenylhydrazine hydrochloric acid salt (3.11 g, 1.3 eq.) were dissolved in acetic acid (12 mL), and stirred at 100° C. for 2.5 hr under nitrogen atmosphere. After cooling, the reaction solution was added with ethyl acetate, washed with water, saturated aqueous solution of sodium hydrogen carbonate, and saturated brine, and dried over magnesium sulfate. After the filtration, it was concentrated under reduced pressure. The resulting residues were dissolved in THF (30 mL) and water (3 mL), added with DDQ (5.96 g, 2.5 eq.) at 0° C., and then stirred at room temperature overnight. The reaction solution was added with MTBE, washed with 0.5 N aqueous solution of sodium hydroxide and saturated brine, and dried over magnesium sulfate. After filtration and the concentration under reduced pressure, the resulting residues were washed with MTBE to obtain the title compound (brown solid, 1.80 g, 46%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.4 (1H, s), 8.12 (1H, d, J=8.6 Hz), 7.79 (1H, d, J=8.9 Hz), 7.67-7.68 (2H, m), 7.40 (1H, dd, J=1.7, 8.6 Hz), 7.26 (1H, dd, J=2.6, 8.9 Hz), 3.86 (3H, s), 1.72 (6H, s),
- LCMS: m/z 370 [M+H]+
- HPLC retention time: 6.45 min (analysis condition H)
- Compound JJ2
-
- 3-Bromo-9-methoxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound JJ, 1.50 g, 4.05 mmol) and pyridinium chloride (15.2 g, 32.5 eq.) were stirred at 160° C. for 12 hr under nitrogen atmosphere. After cooling, water and ethyl acetate were added and the resulting suspension was filtered. The organic layer was washed with water and saturated brine and dried over magnesium sulfate. After filtration and the concentration under reduced pressure, the resulting residues were washed with MTBE to obtain the title compound (brown solid, 1.47 g, 100%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.4 (1H, s), 9.71 (1H, s), 8.11 (1H, d, J=8.2 Hz), 7.64-7.68 (2H, m), 7.57 (1H, d, J=3.0 Hz), 7.38 (1H, dd, J=1.7, 8.2 Hz), 7.07 (1H, dd, J=3.0, 8.6 Hz), 1.69 (6H, s),
- LCMS: m/z 356 [M+H]+
- HPLC retention time: 2.52 min (analysis condition F)
- Compound JJ3-1
-
- Under nitrogen atmosphere, 3-bromo-9-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound JJ2, 356 mg, 1.00 mmol) and triphenylphosphine (317 mg, 1.2 eq.) were added with THF (3 ml), followed by dropwise addition of ((S)-2,2-dimethyl-[1,3]dioxolan-4-yl)-methanol (148 μl, 1.2 eq.) and diisopropyl azodicarboxylic acid (252 μl, 1.3 eq.). The mixture was then stirred at 50° C. for 2 hr. After cooling, the reaction solution was added with ethyl acetate, washed with brine and dried over magnesium sulfate. After filtration and the concentration under reduced pressure, the resulting residues were purified by silica gel column chromatography (ethyl acetate/dichloromethane) to yield the solid, which was then washed with dichloromethane to obtain the title compound (white powder, 241.6 mg, 51%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.4 (1H, s), 8.12 (1H, d, J=8.2 Hz), 7.79 (1H, d, J=8.9 Hz), 7.67-7.69 (2H, m), 7.40 (1H, dd, J=1.8, 8.2 Hz), 7.28 (1H, dd, J=3.0, 8.9 Hz), 4.41-4.48 (1H, m), 4.06-4.17 (2H, m), 3.79-3.85 (1H, m), 1.72 (3H, s), 1.38 (3H, s), 1.33 (3H, s),
- LCMS: m/z 470 [M+H]+
- HPLC retention time: 3.08 min (analysis condition F)
- Compound JJ3-2
-
- 3-Bromo-9-((S)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound JJ3-1, 18.7 mg, 0.0398 mmol) was dissolved in methanol (1 mL) and THF (0.3 mL), added with 1N hydrochloric acid (5 drops) and stirred at 50° C. for 1 hr. After cooling, the reaction solution was concentrated under reduced pressure, and the resulting residues were added with dichloromethane, and the solid was separated by filtration to obtain the title compound (yellow powder, 16.8 mg, 98%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.43 (1H, s), 8.12 (1H, d, J=8.6 Hz), 7.78 (1H, d, J=8.9 Hz), 7.67-7.70 (2H, m), 7.40 (2H, dd, J=1.8, 8.6 Hz), 7.27 (2H, dd, J=2.8, 8.9 Hz), 4.43 (2H, brs), 4.12 (1H, dd, J=9.9, 4.3 Hz), 3.96 (1H, dd, J=9.7, 6.1 Hz), 3.85 (1H, dd, J=9.9, 5.6 Hz), 3.48 (2H, d, J=5.6 Hz), 1.72 (6H, s),
- LCMS: m/z 430 [M+H]+
- HPLC retention time: 2.02 min (analysis condition F)
- Compound JJ4-1
-
- To the mixture of 3-bromo-9-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound JJ3-1, 33.2 mg, 0.0706 mmol) and sodium hydride (60% in oil, 6.4 mg, 2.3 eq.), DMA (0.55 mL) and methyl iodide (0.015 mL, 3.4 eq.) were added under nitrogen atmosphere at 0° C., and the mixture was stirred at room temperature overnight. The reaction solution was added with water and extracted with ethyl acetate. The organic layer was washed with brine and dried over magnesium sulfate. After filtration and the concentration under reduced pressure, the resulting solid was washed with MTBE to obtain the title compound (white solid, 31.2 mg, 91%).
- LCMS: m/z 484 [M+H]+
- HPLC retention time: 3.34 min (analysis condition F)
- Compound JJ4-2
-
- Under the same conditions as the method for synthesizing Compound JJ3-2, the title compound was prepared from Compound JJ4-1 (yellow solid, 13.3 mg, 83%).
- LCMS: m/z 444 [M+H]+
- HPLC retention time: 2.47 min (analysis condition F)
- Compound JJ5
-
- Under the same conditions as the method for synthesizing Compound A7-1, (3-bromo-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-9-yl oxy)-acetic acid methyl ester was prepared from Compound JJ2 and hydroxy-acetic acid methyl ester. The resultant was dissolved in MeOH (0.35 ml), added with aqueous solution of sodium hydroxide (6 mol/l), and stirred at room temperature for 10 min. The reaction solution was added with hydrochloric acid (3 mol/l), extracted with diethyl ether and dried over anhydrous magnesium sulfate. After the concentration under reduced pressure, white solid was obtained, which was then washed with methylene chloride to obtain the title compound (11.2 mg, 48%).
- LCMS: m/z 414 [M+H]+
- HPLC retention time: 2.50 min (analysis condition F)
- Compound JJ6
-
- 3-Bromo-9-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound JJ2, 20 mg, 0.056 mmol), 4-bromo-butyric acid methyl ester (7.0 μl, 0.056 mmol) and cesium carbonate (36.6 mg, 0.112 mmol) were dissolved in DMA (0.09 ml), and then stirred at room temperature for 1 hr. Thereafter, 4-bromo-butyric acid methyl ester (7.0 μl, 0.056 mmol) was added thereto and the mixture was stirred at room temperature for 3 hr, followed by further stirring at 45° C. for 30 min. The reaction solution was added with water, extracted with diethyl ether and dried over anhydrous magnesium sulfate. After the concentration under reduced pressure, the resulting residues were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain 4-(3-bromo-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-9-yl oxy)-butyric acid methyl ester. This compound was dissolved in MeOH (0.50 ml), added with aqueous solution of sodium hydroxide (6 mol/l), and then stirred at room temperature for 10 min. The reaction solution was added with hydrochloric acid (3 mol/), extracted with diethyl ether, and dried over anhydrous magnesium sulfate and concentrated under reduced pressure to obtain white solid. This white solid was washed with methylene chloride to obtain the title compound (6.1 mg, 25%).
- LCMS: m/z 442 [M+H]+
- HPLC retention time: 2.65 min (analysis condition F)
- Compound JJ7-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound (white solid, 111.5 mg, 65%) was prepared from Compound JJ2 and [(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-methanol.
- LCMS: m/z 614 [M+H]+
- HPLC retention time: 4.04 min (analysis condition F)
- Compound JJ7-2
-
- 3-Bromo-9-[(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound JJ7-1, 13.7 mg, 0.0223 mmol) was dissolved in THF (0.15 mL) and methanol (0.1 mL), added with 0.5 M sulfuric acid (0.05 mL), and then stirred at 60° C. for 3 hr. After cooling, the reaction solution was added with saturated aqueous solution of sodium hydrogen carbonate and extracted with ethyl acetate. The organic layer was washed with brine and dried over magnesium sulfate. After filtration and the concentration under reduced pressure, the resulting solid was washed with dichloromethane to obtain the title compound (white solid, 8.4 mg, 82%).
- LCMS: m/z 460 [M+H]+
- HPLC retention time: 2.18 min (analysis condition F)
- Compound JJ8-1
-
- According to the same method as the method for synthesizing Compound A5-2, the title compound (11.1 mg, 50%) was prepared from Compound JJ7-1 and [(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-methanol.
- LCMS: m/z 561 [M+H]+
- HPLC retention time: 3.84 min (analysis condition F)
- Compound JJ8-2
-
- Under the same conditions as the method for synthesizing Compound JJ7-2, the title compound was prepared from 9-[(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound JJ8-1) (white solid, 7.8 mg, 97%).
- LCMS: m/z 407 [M+H]+
- HPLC retention time: 1.92 min (analysis condition F)
- Compound JJ9-1
-
- 3-Bromo-9-((S)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound JJ3-1, 49.5 mg, 0.105 mmol) and copper cyanide (90%, 35.3 mg, 3.4 eq.) were added with DMA (0.5 mL), and the mixture was irradiated with microwave at 200° C. for 1 hr under nitrogen atmosphere. After cooling, the reaction solution was added with water and extracted with ethyl acetate. The insoluble matters were separated off by filtration, and the organic layer was washed with brine and dried over magnesium sulfate. After filtration and the concentration under reduced pressure, the resulting residues were purified by preparative TLC (methanol/dichloromethane) to obtain the title compound (white solid, 8.5 mg, 22%).
- LCMS: m/z 377 [M+H]+
- HPLC retention time: 2.02 min (analysis condition F)
- Compound JJ9-2
-
- The title compound was obtained as a by-product of the synthesis of Compound JJ9-1 (white solid, 24.8 mg, 57%).
- LCMS: m/z 417 [M+H]+
- HPLC retention time: 2.81 min (analysis condition F)
- Compound JJ9-3
-
- Under the same conditions as the method for synthesizing Compound JJ4-1, the title compound was prepared from 9-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound JJ9-2) (17.0 mg, 84%).
- LCMS: m/z 431 [M+H]+
- HPLC retention time: 3.00 min (analysis condition F)
- Compound JJ9-4
-
- Under the same conditions as the method for synthesizing Compound JJ3-2, the title compound was prepared from 9-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-5,6,6-trimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (JJ9-3) (white solid, 12.1 mg, 90%).
- LCMS: m/z 391 [M+H]+
- HPLC retention time: 2.13 min (analysis condition F)
- Compound JJ10-1
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound JJ2 and benzyl bromide (18.2 mg, 61%).
- LCMS: m/z 446 [M+H]+
- HPLC retention time: 2.68 min (analysis condition D)
- Compound JJ10-2
-
- The title compound was obtained as a by-product of the synthesis of Compound JJ10-1 (5.3 mg, 21%).
- LCMS: m/z 536 [M+H]+
- HPLC retention time: 3.17 min (analysis condition D)
- Compound JJ10-3
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared by reacting Compound JJ2 and (4-methoxyphenyl)-methanol (7.5 mg, 28%).
- LCMS: m/z 476 [M+H]+
- HPLC retention time: 2.70 min (analysis condition D)
- Compound K2
-
- To the THF suspension of potassium tert-butoxide (15.35 g, 3 eq.), (3-bromo-4-methoxyphenyl)acetonitrile (Compound K1, 10 g, 0.044 mmol) was added, and then stirred at 0° C. for 1 hr. Then, iodomethane (8.26 ml, 3 eq.) was added and the mixture was stirred at room temperature for 1 hr. To the reaction solution, saturated aqueous solution of ammonium chloride and water were added followed by extraction with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (colorless oily substance, 11.24 g, 100%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 7.69 (1H, d, J=2.5 Hz), 7.50 (1H, dd, J=8.6, 2.5 Hz), 7.16 (1H, d, J=8.6 Hz), 3.86 (3H, s), 1.67 (6H, s).
- HPLC retention time: 2.30 min (analysis condition S)
- Compound K3
-
- To the THF suspension of zinc (5.72 g, 2 eq.), methanesulfonic acid (25.6 μl, 0.01 eq.) was added, and then stirred at 80° C. for 10 min. Then, the THF solution of 2-(3-bromo-4-methoxy-phenyl)-2-methyl-propionitrile (10 g, 39.35 mmol) was added, followed by addition of bromoethyl acetate (11.07 ml, 1.6 eq.) over 1 hr. The mixture was further stirred for 30 min. To the reaction solution, 4 M hydrochloric acid was added, and stirred at room temperature overnight. After extraction with ethyl acetate, the organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (orange oily substance, 9.74 g, 72%).
- 1H-NMR (270 MHz, CDCl3) δ: 7.46 (1H, d, J=2.5 Hz), 7.16 (1H, dd, J=8.6, 2.5 Hz), 6.89 (1H, d, J=8.6 Hz), 4.17-4.08 (2H, m), 3.90 (3H, s), 3.26 (2H, s), 1.49 (6H, s), 1.23 (3H, t, J=7.2 Hz).
- LCMS: m/z 343, 345 [M+H]+
- HPLC retention time: 2.64 min (analysis condition S)
- Compound K4
-
- 4-(3-Bromo-4-methoxy-phenyl)-4-methyl-3-oxo-pentanoic acid ethyl ester (Compound K3, 10.3 g, 30.01 mmol) was dissolved in DMF (80 mL), added with cesium carbonate (24.4 g, 2.5 eq.) and 4-chloro-3-nitro-benzonitrile (7.12 g, 1.3 eq.), and then stirred at 45° C. for 4 hr. The reaction solution was added to 1 N aqueous solution of hydrochloric acid, and extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration, and after concentration under reduced pressure the title compound was obtained as a crude product (yellow oily substance).
- LCMS: m/z 489, 491 [M+H]+
- HPLC retention time: 2.85, 3.20 min (analysis condition S)
- Compound K5
-
- 4-(3-Bromo-4-methoxy-phenyl)-2-(4-cyano-2-nitro-phenyl)-4-methyl-3-oxo-pentanoic acid ethyl ester (Compound K4), which had been obtained from the above, was dissolved in THF (140 mL) and water (70 mL), added with Na2S2O4 (26.13 g, 5.0 eq.) and stirred at 50° C. overnight. The reaction solution was added to saturated brine and extracted with ethyl acetate. The organic layer was washed with 1 M aqueous solution of potassium carbonate and saturated brine in order, and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by crystallization in MeCN (80 ml) to obtain the title compound (yellow solid, 8.20 g, 62%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.15 (1H, s), 8.07 (1H, d, J=8.4 Hz), 7.94 (1H, s), 7.51 (1H, dd, J=8.5, 1.2 Hz), 7.33 (1H, d, J=2.1 Hz), 7.03 (1H, dd, J=8.7, 2.4 Hz), 6.96 (1H, d, J=8.4 Hz), 3.97 (2H, q, J=7.3 Hz), 3.78 (3H, s), 1.80 (6H, s), 1.09 (3H, t, J=7.2 Hz).
- LCMS: m/z 441, 443 [M+H]+
- HPLC retention time: 2.85 min (analysis condition S)
- Compound K6
-
- Phosphorus pentoxide-methanesulfonic acid (12 mL) was added with 2-[1-(3-bromo-4-methoxy-phenyl)-1-methyl-ethyl]-6-cyano-1H-indole-3-carboxylic acid ethyl ester (Compound K5, 1.0 g, 2.27 mmol), and the mixture was stirred at room temperature for 20 min. The reaction solution was diluted with MeCN (20 mL), poured into water (20 mL), and the precipitated solid was filtered to obtain the title compound (yellow solid, 763 mg, 85%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.84 (1H, s), 8.32 (1H, d, J=8.1 Hz), 8. (1H, s), 8.03 (1H, s), 7.77 (1H, s), 7.64 (1H, dd, J=8.2, 1.4 Hz), 3.97 (3H, s), 1.75 (6H, s).
- LCMS: m/z 395, 397 [M+H]+
- HPLC retention time: 2.58 min (analysis condition S)
- Compound K7-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound K6 and 4-pyrrolidin-1-yl-piperidine.
- LCMS: m/z 469 [M+H]+
- HPLC retention time: 1.37 min (analysis condition S)
- Compound K7-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound K6 and 4-piperidin-4-yl-morpholine.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.70 (1H, s), 8.31 (1H, d, J=8.2 Hz), 7.99 (1H, s), 7.63 (1H, s), 7.60 (1H, dd, J=8.2, 1.2 Hz), 7.16 (1H, s), 3.89 (3H, s), 3.64 (2H, brd), 2.72 (2H, brd), 1.91 (2H, brd), 1.73 (6H, s), 1.57 (2H, brd).
- LCMS: m/z 485 [M+H]+
- HPLC retention time: 1.33 min (analysis condition S)
- Compound K7-3
-
- Under the same conditions as the method for synthesizing Compound B2-1, the target compound was prepared from Compound K6 and piperazine.
- LCMS: m/z 401 [M+H]+
- HPLC retention time: 1.31 min (analysis condition S)
- Compound K7-4
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound K6 and morpholine.
- LCMS: m/z 402 [M+H]+
- HPLC retention time: 2.10 min (analysis condition S)
- Compound K8
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound K7-3 and cyclobutanone.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.70 (1H, br. s), 8.31 (1H, d, J=8.2 Hz), 8.00 (1H, s), 7.64 (1H, s), 7.61 (1H, dd, J=8.1, 1.3 Hz), 7.16 (1H, s), 3.88 (3H, s), 3.60 (1H, t, J=6.2 Hz), 3.10-3.25 (4H, m), 2.77 (1H, t, J=7.1 Hz), 2.35-2.51 (4H, m), 1.74 (6H, s), 1.58-2.08 (6H, m).
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 1.45 min (analysis condition S)
- Compound K9-1
-
- The title compound was obtained as a by-product of the synthesis of Compound K7-1.
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 1.22 min (analysis condition S)
- Compound K9-2
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound K7-2.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.65 (1H, s), 9.61 (1H, s), 8.30 (1H, d, J=8.2 Hz), 7.98 (1H, s), 7.59-7.56 (2H, m), 7.10 (1H, s), 3.71 (2H, brd, J=11.2 Hz), 3.60 (4H, m), 2.66 (2H, m), 1.88 (2H, brd, J=9.7 Hz), 1.71 (6H, s), 1.57 (2H, brd).
- LCMS: m/z 471 [M+H]+
- HPLC retention time: 1.20 min (analysis condition S)
- Compound K9-3
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound K8.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.66 (1H, br. s), 9.67 (1H, s), 8.31 (1H, d, J=8.2 Hz), 7.98 (1H, s), 7.56-7.60 (2H, m), 7.09 (1H, s), 3.10-3.24 (4H, m), 2.77 (1H, t, J=7.5 Hz), 2.37-2.49 (4H, m), 1.52-2.07 (6H, m), 1.72 (6H, s).
- LCMS: m/z 441 [M+H]+
- HPLC retention time: 1.31 min (analysis condition S)
- Compound K9-4
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound K7-4.
- LCMS: m/z 388 [M+H]+
- HPLC retention time: 1.67 min (analysis condition S)
- Compound K10-1
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound K9-2 and 2-bromopropane.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.68 (1H, s), 8.30 (1H, d, J=8.1 Hz), 7.99 (1H, s), 7.60 (2H, m), 7.14 (1H, s), 4.72-4.63 (2H, m), 3.71 (2H, brd, J=10.7 Hz), 3.59 (6H, m), 2.68 (2H, t, J=12.9 Hz), 2.27 (2H, brd), 1.90 (2H, brd), 1.73 (6H, s), 1.56 (2H, br), 1.34 (6H, d, J=5.9 Hz).
- LCMS: m/z 513 [M+H]+
- HPLC retention time: 1.48 min (analysis condition S)
- Compound K10-2
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound K9-3 and 2-iodopropane.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.29 (1H, d, J=8.1 Hz), 7.98 (1H, s), 7.56-7.63 (2H, m), 7.14 (1H, s), 4.62-4.74 (1H, m), 3.10-3.26 (4H, m), 2.69-2.85 (1H, m), 2.35-2.48 (4H, m), 1.57-2.08 (6H, m), 1.73 (6H, s), 1.32 (6H, d, J=6.1 Hz).
- LCMS: m/z 483 [M+H]+
- HPLC retention time: 1.65 min (analysis condition S)
- Compound K10-3
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound K9-4 and 1-bromo-2-methoxyethane.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.67 (1H, s), 8.30 (1H, d, 7.9 Hz), 7.98 (1H, s), 7.64 (1, s), 7.58 (1H, d, 7.9 Hz), 7.16 (1H, s), 4.18-4.22 (2H, m), 3.72-3.80 (6H, m), 3.35 (3H, s), 3.18-3.24 (4H, s), 1.74 (1H, s)
- LCMS: m/z 446 [M+H]+
- HPLC retention time: 3.23 min (analysis condition W)
- Compound K10-4
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound K9-4 and 1-bromo-2-(2-methoxyethoxy)ethane.
- LCMS: m/z 490 [M+H]+
- HPLC retention time: 3.16 min (analysis condition W)
- Compound K10-5
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound K9-4 and 3-mesyloxytetrahydrofurane.
- LCMS: m/z 458 [M+H]+
- HPLC retention time: 3.20 min (analysis condition W)
- Compound K10-6
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound K9-4 and 2-bromopropane.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.70 (1H, br. s), 8.32-8.29 (1H, d, 8.08 Hz), 8.00 (1H, s), 7.63 (1H, s), 7.62-7.59 (1H, d, 8.08 Hz), 7.16 (1H, s), 4.75-4.66 (1H, m), 3.77 (4H, m) 3.19 (4H, m), 1.74 (6H, s), 1.35 (3H, s), 1.33 (3H, s)
- LCMS: m/z 430 [M+H]+
- Compound K10-7
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound K9-4 and 2-bromoethanol.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.71 (1H, br. s), 8.33-8.30 (1H, d, 8.08 Hz), 8.00 (1H, s), 7.63 (1H, s), 7.62-7.59 (1H, d, 8.08 Hz), 7.16 (1H, s), 4.13-4.09 (2H, t, 4.61 Hz), 3.81-3.78 (2H, t, 4.61 Hz), 3.78 (4H, m) 3.23 (4H, m), 1.75 (6H, s)
- LCMS: m/z 432 [M+H]+
- Compound L2-1
-
- (4-Hydroxy-3-methoxy-phenyl)-ethyl acetate ester (Compound L1-1, 3.0 g, 14.27 mmol) was dissolved in DMF (70 mL), added with 2-iodopropane (2.9 mL, 2.0 eq.) and potassium carbonate (3.94 g, 2.0 eq.), and stirred at 80° C. overnight. The reaction solution was added to water and extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (yellow oily substance, 2.61 g, 73%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 6.88 (2H, m), 6.74 (1H, dd, J=8.1, 2.1 Hz), 4.52-4.43 (1H, m), 4.07 (2H, q, J=7.1 Hz), 3.72 (3H, s), 3.56 (2H, s), 1.23 (6H, d, J=6.1 Hz), 1.18 (3H, t, J=7.1 Hz).
- LCMS: m/z 253 [M+H]+
- HPLC retention time: 2.18 min (analysis condition S)
- Compound L2-2
-
- (4-Hydroxy-3-methoxy-phenyl)-acetic acid (Compound L1-2, 1.5 g, 8.23 mmol) was dissolved in DMF (30 mL), added with 2-iodopropane (3.3 mL, 4.0 eq.) and potassium carbonate (4.55 g, 4.0 eq.), and stirred at 80° C. overnight. The reaction solution was added to water and extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (yellow oily substance, 1.21 g, 55%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 6.87 (2H, s+d), 6.73 (1H, dd, J=8.1, 2.1 Hz), 4.94-4.84 (1H, m), 4.52-4.43 (1H, m), 3.72 (3H, s), 3.52 (2H, s), 1.23 (6H, d, J=6.1 Hz), 1.18 (6H, d, J=6.1 Hz).
- LCMS: m/z 267 [M+H]+
- HPLC retention time: 2.40 min (analysis condition S)
- Compound L3-1
-
- Under the same conditions as the method for synthesizing Compound K2, the title compound was prepared from Compound L2-1.
- 1H-NMR (270 MHz, DMSO-d6) δ: 6.90-6.76 (3H, m), 4.53-4.44 (1H, m), 4.06 (2H, q, J=7.1 Hz), 3.73 (3H, s), 1.47 (6H, s), 1.23 (6H, d, J=6.1 Hz), 1.12 (3H, t, J=7.0 Hz).
- LCMS: m/z 281 [M+H]+
- HPLC retention time: 2.57 min (analysis condition S)
- Compound L3-2
-
- Under the same conditions as the method for synthesizing Compound K2, the title compound was prepared from Compound L2-2.
- 1H-NMR (270 MHz, DMSO-d6) δ: 6.88 (1H, d, J=8.2 Hz), 6.79 (2H, m), 4. 94-4.84 (1H, m), 4.53-4.44 (1H, m), 3.72 (3H, s), 1.45 (6H, s), 1.23 (6H, d, J=6.1 Hz), 1.12 (6H, d, J=6.3 Hz).
- LCMS: m/z 295 [M+H]+
- HPLC retention time: 2.75 min (analysis condition S)
- Compound L4
-
- 2-(4-Isopropoxy-3-methoxy-phenyl)-2-methyl-propionic acid ethyl ester (Compound L3-1, 1.45 g, 5.17 mmol) was dissolved in THF (13 mL) and EtOH (13 mL), added with 1 N aqueous solution of sodium hydroxide (10.3 mL, 2.0 eq.), and stirred at 80° C. overnight. The reaction solution was added to water and extracted with ethyl acetate. The aqueous layer was acidified by using 1 N aqueous solution of hydrochloric acid, extracted with ethyl acetate, washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (white solid, 1.10 g, 84%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.26 (1H, s), 6.90-6.80 (3H, m), 4.49 (1H, m), 3.73 (3H, s), 1.45 (6H, s), 1.23 (6H, d, J=6.1 Hz).
- LCMS: m/z 253 [M+H]+
- HPLC retention time: 1.83 min (analysis condition S)
- Compound L5
-
- 2-(4-Isopropoxy-3-methoxy-phenyl)-2-methyl-propionic acid (Compound L4, 1.4 g, 5.55 mmol) was added with thionyl chloride (10 mL), and then stirred at room temperature for 5 hr. According to the concentration under reduced pressure, unreacted thionyl chloride was removed to obtain corresponding acid chloride. To MeCN (40 mL), malonic acid monoethyl ester potassium salt (1.98 g, 2.1 eq.), triethylamine (2.47 mL, 3.2 eq.), and magnesium chloride (1.32 g, 2.5 eq.) were added and the mixture was stirred at room temperature for 2 hr. To the reaction solution, MeCN (15 mL) solution of the acid chloride prepared from the above was added dropwise. Upon the completion of the dropwise addition, the mixture was further stirred at room temperature for overnight. MeCN was removed by distillation and concentrated under reduced pressure, and the resulting residues were added with 1 N aqueous solution of hydrochloric acid, extracted with toluene, washed with saturated brine, and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (yellow oily substance, 1.45 g, 81%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 6.94 (1H, d, J=8.2 Hz), 6.76 (2H, m), 4.56-4.47 (1H, m), 4.00 (2H, q, J=7.1 Hz), 3.74 (3H, s), 3.38 (2H, s), 1.41 (6H, s), 1.24 (6H, d, J=6.1 Hz), 1.12 (3H, t, J=7.3 Hz).
- LCMS: m/z 323 [M+H]+
- HPLC retention time: 2.45, 3.03 min (analysis condition S)
- Compound L6
-
- Under the same conditions as the method for synthesizing Compound K4, the title compound was prepared from Compound L5.
- 1H-NMR (270 MHz, DMSO-d6) δ: 8.35 (1H, d, J=1.8 Hz), 8.14 (1H, dd, J=8.2, 1.9 Hz), 7.67 (1H, d, J=8.2 Hz), 6.68 (1H, d, J=8.4 Hz), 6.59 (1H, dd, J=8.4, 2.0 Hz), 6.45 (1H, d, J=2.1 Hz), 5.44 (1H, s), 4.43 (1H, m), 4.09 (2H, q, J=7.1 Hz), 3.53 (3H, s), 1.59 (3H, s), 1.35 (3H, s), 1.24 (6H, d×2), 1.13 (3H, t, J=7.1 Hz).
- LCMS: m/z 469 [M+H]+
- HPLC retention time: 2.85, 3.10 min (analysis condition S)
- Compound L7
-
- Under the same conditions as the method for synthesizing Compound K5, the title compound was prepared from Compound L6.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.04 (1H, s), 8.05 (1H, d, J=8.4 Hz), 7.93 (1H, s), 7.49 (1H, dd, J=8.4, 1.5 Hz), 6.79 (2H, m), 6.54 (1H, dd, J=8.3, 1.9 Hz), 4.43 (1H, t, J=6.1 Hz), 3.94 (2H, q, J=7.0 Hz), 3.65 (3H, s), 1.81 (6H, s), 1.21 (6H, d, J=5.9 Hz), 1.05 (3H, t, J=7.1 Hz).
- LCMS: m/z 421 [M+H]+
- HPLC retention time: 2.82 min (analysis condition S)
- Compound L8-1
-
- 6-Cyano-2-[1-(4-isopropoxy-3-methoxy-phenyl)-1-methyl-ethyl]-1H-indole-3-carboxylic acid ethyl ester (Compound L7, 1.25 g, 2.97 mmol) was dissolved in MeCN (18 mL), added with methanesulfonic acid (3.75 mL), and then stirred at 50° C. for 8 hr. Hexane was added to the reaction solution, and the precipitated solid was filtered to obtain the title compound (yellow solid, 185 mg, 19%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.67 (1H, s), 8.30 (1H, d, J=8.2 Hz), 7.99 (1H, s), 7.59 (2H, m), 7.28 (1H, s), 3.93 (3H, s), 1.75 (6H, s).
- LCMS: m/z 333 [M+H]+
- HPLC retention time: 1.73 min (analysis condition S)
- Compound L8-2
-
- To the filtrate obtained from the synthesis of Compound L8-1, water was added and the extraction was carried out with ethyl acetate. The resultant was washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the concentration was performed under reduced pressure to obtain the target compound (red amorphous, 830 mg, 75%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.72 (1H, s), 8.31 (1H, d, J=8.4 Hz), 8.01 (1H, d, J=0.7 Hz), 7.66 (1H, s), 7.61 (1H, dd, J=8.2, 1.4 Hz), 7.33 (1H, s), 4.65 (1H, m), 3.93 (3H, s), 1.77 (6H, s), 1.32 (6H, d, J=6.1 Hz).
- LCMS: m/z 375 [M+H]+
- HPLC retention time: 2.38 min (analysis condition S)
- Compound L9
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound L8-2.
- 1H-NMR (270 MHz, DMSO-d6) δ: 12.69 (1H, s), 9.69 (1H, s), 8.30 (1H, d, J=8.1 Hz), 7.99 (1H, s), 7.65 (1H, s), 7.60 (1H, dd, J=8.2, 1.2 Hz), 7.17 (1H, s), 4.64 (1H, m), 1.69 (6H, s), 1.32 (6H, d, J=6.1 Hz).
- LCMS: m/z 361 [M+H]+
- HPLC retention time: 2.20 min (analysis condition S)
- Compound L10-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound L9 and 1-cyclobutylpiperidin-4-ol. 1H-NMR (270 MHz, CDCl3) δ: 9.31 (1H, br. s), 8.54-8.50 (1H, d, 8.08 Hz), 7.90 (1H, s), 7.77 (1H, s), 7.59-7.55 (1H, m), 7.09 (1H, s), 4.70-4.61 (1H, m), 4.52-4.43 (1H, m), 2.79-2.73 (1H, m), 2.70-2.60 (2H, m), 2.25-2.16 (2H, m), 2.09-1.99 (4H, m), 1.98-1.88 (4H, m), 1.77 (6H, s), 1.72-1.58 (2H, m), 1.39 (3H, s), 1.37 (3H, s)
- LCMS: m/z 498 [M+H]+
- Compound L10-2
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound L9 and (S)-1-cyclobutylpyrrolidin-3-ol.
- 1H-NMR (270 MHz, CDCl3) δ: 10.63 (1H, br. s), 8.51-8.48 (1H, d, 8.08 Hz), 7.89 (1H, s), 7.85 (1H, s), 7.55-7.51 (1H, m), 6.99 (1H, s), 5.03-4.97 (1H, m), 4.71-4.62 (1H, m), 3.07-292 (2H, m), 2.84-2.73 (2H, m), 2.64-2.53 (1H, m), 2.36-2.23 (2H, m), 2.10-1.97 (2H, m), 1.83-1.67 (2H, m), 1.78 (6H, s), 1.53-1.46 (2H, m), 1.39 (3H, s), 1.37 (3H, s)
- LCMS: m/z 484 [M+H]+
- Compound M1
-
- To the THF (300 ml) solution of 7-methoxy-3,4-dihydro-1H-naphthalen-2-one (Compound A1, 0.5 g, 2.84 mmol), sodium hydride (36.4 mg, 2.2 eq.) was added at 0° C. After stirring for 20 min, 1,4-dibromobutane (0.74 ml, 1.2 eq.) was added dropwise thereto, and the mixture was stirred at 80° C. for 4 hr. To the reaction solution, saturated aqueous solution of ammonium chloride was added followed by extraction with ethyl acetate. The organic layer was washed with saturated aqueous solution of ammonium chloride and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (yellow solid, 0.31 g, 47%).
- 1H-NMR (CDCl3, 300 MHz) δ: 1.79-1.92 (6H, m), 2.42-2.27 (m, 2H), 3.03 (t, 2H, J=6.5 Hz), 3.81 (t, 2H, J=6.5 Hz), 3.81 (s, 3H), 6.73 (dd, 1H, J=2.7 Hz, 8.0 Hz), 6.83 (d, 1H, J=2.7 Hz), 7.09 (d, 1H, J=8.0 Hz)
- LCMS: m/z 231 [M+H]+
- Compound M2
-
- Under the same conditions as the method for synthesizing Compound A3-1, the title compound was prepared from Compound M1 and (3-bromo-phenyl)-hydrazine.
- LCMS: m/z 380, 382 [M+H]+
- HPLC retention time: 2.90 min (analysis condition Y)
- Compound M3
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound M2.
- 1H-NMR (CDCl3, 300 MHz) δ: 2.11-2.51 (8H, m), 3.91 (s, 3H), 6.98 (dd, 1H, J=2.3 Hz, 8.8 Hz), 7.01 (d, 1H, J=2.3 Hz), 7.41 (dd, 1H, J=1.5 Hz, 8.4 Hz), 7.57 (d, 1H, J=1.5 Hz), 8.30 (d, 1H, J=8.4 Hz), 8.35 (d, 1H, J=8.8 Hz), 8.69 (s, 1H)
- LCMS: m/z 396, 398 [M+H]+
- Compound M4
-
- Under the same conditions as the method for synthesizing Compound A5-2, the title compound was prepared from Compound M3.
- 1H-NMR (DMSO-d6, 300 MHz) δ: 2.14-2.37 (m, 8H), 3.90 (s, 3H), 7.05-7.10 (m, 2H), 7.60 (dd, 1H, J=1.5 Hz, 8.4 Hz), 7.95 (s, 1H), 8.13 (d, 1H, J=9.5 Hz), 8.30 (d, 1H, J=8.4 Hz), 12.24 (s, 1H)
- LCMS: m/z 343 [M+H]+
- Compound M5
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound M4.
- 1H-NMR (DMSO-d6, 300 MHz) δ: 2.06-2.39 (m, 8H), 6.87 (dd, 1H, J=1.9 Hz, 8.8 Hz), 6.90 (d, 1H, J=1.9 Hz), 7.57 (dd, 1H, J=1.1 Hz, 8.0 Hz), 7.95 (s, 1H), 8.02 (d, 1H, J=8.8 Hz), 8.30 (d, 1H, J=8.0 Hz), 10.29 (s, 1H), 12.25 (s, 1H)
- LCMS: m/z 329 [M+H]+
- Compound M6-1
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared as a crude product from Compound M5 and toluene-4-sulfonic acid (R)-2,2-dimethyl-[1,3]dioxolan-4-yl methyl ester.
- Compound M6-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound M6-1.
- LCMS: m/z 403 [M+H]+
- HPLC retention time: 2.88 min (analysis condition U)
- Compound N1
-
- To the THF (300 ml) solution of 7-methoxy-3,4-dihydro-1H-naphthalen-2-one (Compound A1, 20 g, 0.11 mol), sodium hydride (9.9 g, 3.7 eq.) was added at 0° C. After stirring for 10 min, 1-bromo-2-(2-bromo-ethoxy)-ethane (19 ml, 12 eq.) was added dropwise thereto, and the mixture was stirred at 80° C. for 3 hr. To the reaction solution, saturated aqueous solution of ammonium chloride was added and the extraction was carried out twice with ethyl acetate. The organic layer was dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (white solid, 13 g, 51%).
- 1H-NMR (300 MHz, CDCl3) δ: 2.07 (4H, m), 2.70 (t, 2H, 6.8 Hz), 3.12 (t, 2H, 6.8 Hz), 3.81 (s, 3H), 3.89 (m, 4H), 6.75 (dd, 1H, 2.6 Hz, 8.3 Hz), 6.9 (d, 1H, 2.6 Hz), 7.0 (d, 1H, 8.3 Hz)
- LCMS: m/z 247 [M+H]+
- Compound N2-1, Compound N2-2
-
- Under the same conditions as the method for synthesizing Compound A3-1, the title compound was prepared as a mixture from Compound N1.
- Compound N3
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound N2-1.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.9 (2H, m), 2.4 (m, 2H), 3.9 (s, 3H), 4.0 (m, 2H), 4.2 (m, 2H), 7.1 (dd, 1H, 2.2 Hz, 8.7 Hz), 7.3 (m, 2H), 7.8 (d, 1H, 2.2 Hz), 8.1 (d, 2H, 8.7 Hz), 11.8 (s, 1H)
- LCMS: m/z 413 (M+1)+
- Compound N4
-
- Under the same conditions as the method for synthesizing Compound A5-2, the title compound was prepared from Compound N3.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.9 (m, 2H), 2.4 (m, 2H), 3.9 (s, 3H), 4.0 (m, 2H), 4.1 (m, 2H), 7.1 (dd, 1H, 2.2 Hz, 8.7 Hz), 7.4 (d, 1H, 2.2 Hz), 7.6 (dd, 1H, 1.5 Hz, 8.3 Hz), 8.0 (s, 1H), 8.1 (d, 1H, 8.7 Hz), 8.3 (d, 1H, 8.3 Hz), 12.2 (s, 1H)
- LCMS: m/z 359 [M+H]+
- HPLC retention time: 2.80 min (analysis condition U)
- Compound N5
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound N4.
- 1H-NMR (300 MHz, DMSO-d6) d ppm 2.0 (m, 2H), 2.3 (m, 2H), 4.0 (m, 2H), 4.1 (m, 2H), 6.9 (dd, 1H, 1.9 Hz, 8.3 Hz), 7.3 (d, 1H, 1.9 Hz), 7.6 (dd, 1H, 1.5 Hz, 8.3 Hz), 8.0 (s, 1H), 8.1 (d, 1H, 8.3 Hz), 8.3 (d, 1H, 8.3 Hz), 10.3 (s, 1H), 12.2 (s, 1H)
- LCMS: m/z 345 [M+H]+
- HPLC retention time: 2.37 min (analysis condition U)
- Compound N6-1-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound N6-2 and (S)-2,2-dimethyl-4-p-tolyloxymethyl-[1,3]dioxolane.
- LCMS: m/z 459 [M+H]+
- HPLC retention time: 2.93 min (analysis condition Y)
- Compound N6-1-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound N6-1-1.
- LCMS: m/z 419 [M+H]+
- HPLC retention time: 1.52 min (analysis condition S)
- Compound N6-2
-
- Under the same conditions as the method for synthesizing Compound A7-1 and Compound A8-1, the title compound was prepared from Compound N5.
- LCMS: m/z 428 [M+H]+
- HPLC retention time: 1.38 min (analysis condition S)
- Compound N6-3
-
- Under the same conditions as the method for synthesizing Compound A8-17, the title compound was prepared from Compound N5.
- LCMS: m/z 458 [M+H]+
- HPLC retention time: 1.33 min (analysis condition S)
- Compound N6-4
-
- Under the same conditions as the method for synthesizing Compound A8-17, the title compound was prepared from Compound N5.
- LCMS: m/z 472 [M+H]+
- HPLC retention time: 1.41 min (analysis condition S)
- Compound N6-5
-
- Under the same conditions as the method for synthesizing Compound A8-17, the title compound was prepared from Compound N5.
- LCMS: m/z 506 [M+H]+
- HPLC retention time: 1.53 min (analysis condition S)
- Compound N6-6
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound N6-2.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.02 (3H, t, 7.25 Hz), 1.18 (2H, m), 1.71 (2H, m), 1.97 (4H, m), 2.27 (2H, m), 2.38 (3H, m), 2.71 (2H, m), 4.03 (2H, m), 4.21 (2H, m), 4.66 (1H, s), 7.13 (1H, dd, 8.77 Hz, 1.91 Hz), 7.39 (1H, bs, 1.91 Hz), 7.60 (1H, d, 8.40 Hz), 8.07 (1H, s), 8.15 (1H, d, 8.40 Hz), 8.37 (1H, d, 8.01 Hz), 12.2 (1 H, s).
- LCMS: m/z 456 [M+H]+
- HPLC retention time: 1.48 min (analysis condition S)
- Compound N7
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound N5.
- LCMS: m/z 477 [M+H]+
- HPLC retention time: 3.58 min (analysis condition Y)
- Compound N8-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound N7 and 4-pyrrolidin-1-yl-piperidine.
- LCMS: m/z 481 [M+H]+
- HPLC retention time: 1.75 min (analysis condition U)
- Compound N8-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound N7 and 4-piperidin-4-yl-morpholine.
- LCMS: m/z 497 [M+H]+
- HPLC retention time: 1.70 min (analysis condition U)
- Compound O1
-
- Under the same conditions as the method for synthesizing Compound E-1, the title compound was prepared from Compound N1.
- 1H-NMR (300 MHz, DMSO-d6) δ: 2.01 (4H, m), 2.66 (2H, t, 6.87 Hz), 3.08 (2H, t, 6.87 Hz), 3.62 (2H, m), 3.78 (2H, m), 3.87 (3H, s), 7.00 (1H, s), 7.43 (1H, s)
- Compound O2
-
- Under the same conditions as the method for synthesizing Compound E2-1, the title compound was prepared as a crude product from Compound O1.
- Compound O3
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound O2.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.95 (2H, d, 14.87 Hz), 2.55 (2H, m), 4.04 (2H, m), 4.09 (3H, s), 4.22 (2H, m), 7.51 (1H, s), 7.63 (1H, dd, 8.01 Hz, 1.53 Hz), 8.09 (1H, s), 8.30 (1H, s), 8.36 (1H, d, 8.01 Hz), 12.3 (1H, s).
- LCMS: m/z 437,439 [M+H]+
- HPLC retention time: 2.65 min (analysis condition U)
- Compound O4
-
- Under the same conditions as the method for synthesizing Compound O5-3, the title compound was prepared from Compound O3.
- LCMS: m/z 377 [M+H]+
- HPLC retention time: 2.29 min (analysis condition S)
- Compound O5-1
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound O4.
- LCMS: m/z 363 [M+H]+
- HPLC retention time: 1.88 min (analysis condition S)
- Compound O5-2
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound O5-1.
- LCMS: m/z 495 [M+H]+
- HPLC retention time: 3.47 min (analysis condition Y)
- Compound O5-3
-
- To the THF (0.9 ml) solution of 9-bromo-6-tetrahydropyran-8-pyrrolidinopiperidin-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (Compound O8-1, 90 mg, 0.161 mmol), THF solution of n-butyl lithium (2 M solution, 0.241 ml, 3 eq.) was added at −78° C. After stirring for 30 min, THF (1 ml) solution of N-fluorobenzenesulfonimide (152 mg, 3 eq.) was added dropwise thereto. After rising to room temperature, the mixture was stirred for 18 hr. To the reaction solution, water was added and the extraction was carried out with ethyl acetate. The organic layer was washed with brine and dried over magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by high performance chromatography to obtain the target compound (white solid, 0.44 mg, 0.5%).
- 1H-NMR (CDCl3+CD3OD, 300 MHz) δ: 1.75-1.94 (m, 11H), 2.02-2.01 (m, 2H), 2.30-2.27 (m, 1H), 2.75-2.72 (m, 2H), 2.90-3.00 (m, 2H), 3.61-3.47 (m, 4H), 4.01-3.90 (m, 4H), 7.08 (dd, 1H, J=1, 2 Hz, 8.4 Hz), 7.29 (dd, 1H, J=1, 5 Hz, 8.1 Hz), 7.68 (d, 1H, J=12.9 Hz), 7.72 (s, 1H), 8.22 (d, 1H, J=8.4 Hz)
- LCMS: m/z 499 [M+H]+
- HPLC retention time: 1.95 min (analysis condition U)
- Compound O5-4
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound O5-2 and 1-cyclobutylpiperazine.
- LCMS: m/z 485 [M+H]+
- HPLC retention time: 1.97 min (analysis condition U)
- Compound O6-1
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound O3.
- LCMS: m/z 423, 425 [M+H]+
- HPLC retention time: 2.30 min (analysis condition U)
- Compound O6-2
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound O6-1.
- LCMS: m/z 555, 557 [M+H]+
- HPLC retention time: 3.13 min (analysis condition U)
- Compound O7-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound O6-2 and piperazine.
- LCMS: m/z 491, 493 [M+H]+
- HPLC retention time: 1.88 min (analysis condition U)
- Compound O7-2
-
- To the dichloromethane (5 mL) solution of 9-bromo-11-oxo-8-(piperazin-1-yl)-2′,3′,5,5′,6′,11-hexahydrospiro[benzo[b]carbazole-6,4′-pyran]-3-carbonitrile (Compound 07-1, 250 mg, 0.509 mmol) and mono-tert-butyl ester carbonic anhydride (122 mg, 0.560 mmol), triethylamine (0.21 mL, 1.53 mmol) was added at 0° C., and stirred at room temperature for 1 hr. The reaction mixture was concentrated under reduced pressure and the residues were purified by silica gel column chromatography (methanol/dichloromethane) to obtain the target compound as a white solid (212 mg, 70%).
- 1H-NMR (300 MHz, DMSO-d6) d ppm: 1.44 (9H, s), 1.97 (2H, m), 2.44 (2H, m), 1.35 (4H, m), 3.54 (4H, m), 4.06 (2H, m), 4.18 (2H, m), 7.57 (1H, s), 7.63 (1H, dd, 8.01 Hz, 1.52 Hz), 8.08 (1H, d, 1.52 Hz), 8.31 (1H, s), 8.36 (1H, d, 8.01 Hz), 12.3 (1H, s)
- LCMS: m/z 591, 593 [M+H]+
- HPLC retention time: 3.23 min (analysis condition T)
- Compound O7-3
-
- Under the same conditions as the method for synthesizing Compound O9-1, the title compound was prepared from Compound O7-2.
- LCMS: m/z 551 [M+H]+
- HPLC retention time: 3.92 min (analysis condition Y)
- Compound O7-4
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound O7-3.
- LCMS: 451 m/z [M+H]+
- HPLC retention time: 1.87 min (analysis condition U)
- Compound O7-5
-
- LCMS: m/z 537 [M+H]+
- HPLC retention time: 3.82 min (analysis condition Y)
- Compound O8-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound O6-2 and 4-pyrrolidin-1-yl-piperidine.
- LCMS: m/z 559, 561 [M+H]+
- HPLC retention time: 2.05 min (analysis condition U)
- Compound O8-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound O6-2 and 1-cyclobutylpiperazine.
- LCMS: m/z 547 [M+H]+
- HPLC retention time: 1.61 min (analysis condition S)
- Compound O8-3
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound O6-2 and 4-piperidin-4-yl-morpholine.
- LCMS: m/z 575, 577 [M+H]+
- HPLC retention time: 1.95 min (analysis condition U)
- Compound O8-4
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound O7-1 and oxetan-3-one.
- LCMS: m/z 547, 549 [M+H]+
- HPLC retention time: 1.43 min (analysis condition S)
- Compound O8-5
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound O6-2 and 1-tert-butylpiperazine.
- LCMS: 547, 549 m/z [M+H]+
- HPLC retention time: 2.07 min (analysis condition U)
- Compound O9-1
-
- 9-Bromo-11-oxo-8-(4-(pyrrolidin-1-yl)piperidin-1-yl)-2′,3′,5,5′,6′,11-hexahydrospiro[benzo[b]carbazole-6,4′-pyran]-3-carbonitrile (Compound O8-1, 100 mg, 0.170 mmol), tin tributyl(1-propynyl) (0.082 mL, 0.268 mmol), bis(acetonitrile) palladium dichloride (II) (2.64 mg, 0.00895 mmol), X-Phos (12.8 mg, 0.0269 mmol), and cesium carbonate (262.4 mg, 0.806 mmol) were suspended in acetonitrile (1 mL), and then stirred at 80° C. for 2 hr. The reaction mixture was cooled to room temperature followed by addition of water and extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (dichloromethane/methanol) to obtain the target compound (pale yellow solid, 3.8 mg, 4.1%).
- 1H-NMR (300 MHz, DMSO) σppm 12.20 (bs, 1H), 8.35 (d, 1H, J=8.1 Hz), 8.06 (s, 1H), 8.06 (d, 1H, J=10.8 Hz), 7.58 (d, 1H, J=8.4 Hz), 7.29 (s, 1H), 4.25-4.23 (m, 2H), 4.02-3.98 (m, 2H), 3.78 (d, 2H, J=11.4 Hz), 2.93 (t, 2H, J=11.1 Hz), 2.55 (s, 1H), 2.45-2.28 (m, 2H), 2.24-2.05 (m, 4H), 2.08-1.81 (m, 4H), 1.75-1.50 (m, 7H)
- LCMS: m/z 519 [M+H]+
- HPLC retention time: 1.98 min (analysis condition U)
- Compound O9-2
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound O8-1.
- LCMS: m/z 505 [M+H]+
- HPLC retention time: 1.92 min (analysis condition U)
- Compound O9-3
-
- Under the same conditions as the method for synthesizing Compound A5-2, the title compound was prepared from Compound O8-1.
- LCMS: 506 m/z [M+H]+
- HPLC retention time: 1.87 min (analysis condition U)
- Compound O9-4
-
- Under the same conditions as the method for synthesizing Compound E4-2-1, the title compound was prepared from Compound O8-1.
- LCMS: m/z 563 [M+H]+
- HPLC retention time: 1.92 min (analysis condition U)
- Compound O9-5
-
- Under the same conditions as the method for synthesizing Compound O9-1, the title compound was prepared from Compound O8-2.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.6 (m, 2H), 1.8 (m, 2H), 1.9 (m, 4H), 2.1 (s, 3H), 2.4 (m, 6H), 2.8 (m, 1H), 3.4 (m, 4H), 4.0 (m, 2H), 4.1 (m, 2H), 7.3 (s, 1H), 7.6 (d, 1H, 8.0 Hz), 8.0 (m, 2H), 8.3 (d, 1H, 8.0 Hz), 12.2 (s, 1H)
- LCMS: m/z 505 [M+H]+
- HPLC retention time: 2.03 min (analysis condition U)
- Compound O9-6
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from Compound O8-2.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.66 (2H, m), 1.83 (2H, t, 8.77 Hz), 1.99 (4H, m), 2.41 (6H, m), 2.79 (1H, t, 7.63 Hz), 3.35 (4H, m), 4.01 (2H, m), 4.27 (2H, m), 4.51 (1H, s), 7.33 (1H, s), 7.54 (1H, m), 8.03 (1H, s), 8.16 (1H, s), 8.32 (1H, d, 8.40 Hz), 12.3 (1H, s).
- LCMS: m/z 491 [M+H]+
- HPLC retention time: 1.95 min (analysis condition U)
- Compound O9-7
-
- Under the same conditions as the method for synthesizing Compound O9-1, the title compound was prepared from Compound O8-3.
- 1H-NMR (300 MHz, DMSO-d6) δ: 1.57 (2H, m), 1.95 (4H, m), 2.14 (3H, s), 2.37 (3H, m), 3.35 (4H, m), 2.83 (2H, t, 12.6 Hz), 3.56 (4H, s), 3.86 (2H, d, 11.8 Hz), 4.04 (2H, m), 4.17 (2H, m), 7.31 (1H, s), 7.61 (1H, d, 8.01 Hz), 8.06 (1H, s), 8.07 (1H, s), 8.36 (1H, d, 8.01 Hz), 12.3 (1H, s).
- LCMS: m/z 535 [M+H]+
- HPLC retention time: 1.95 min (analysis condition U)
- Compound O9-8
-
- Under the same conditions as the method for synthesizing Compound F5-43, the title compound was prepared from compound 08-3.
- LCMS: m/z 521 [M+H]+
- HPLC retention time: 1.90 min (analysis condition U)
- Compound O9-9
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound O7-4 and oxetan-3-one.
- LCMS: m/z 507 [M+H]+
- HPLC retention time: 1.43 min (analysis condition S)
- Compound O10-1-1
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound O7-5.
- LCMS: m/z 541 [M+H]+
- HPLC retention time: 3.08 min (analysis condition S)
- Compound O10-1-2
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound O10-1-1.
- LCMS: m/z 441 [M+H]+
- HPLC retention time: 1.42 min (analysis condition S)
- Compound O10-2
-
- According to the same method as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound O9-8.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.23-8.21 (1H, m), 8.02-8.00 (1H, m), 7.88-7.86 (1H, m), 7.39-7.36 (2H, m), 4.63-4.59 (2H, m), 3.89-3.85 (2H, m), 3.60-3.56 (6H, m), 3.22-3.19 (4H, m), 2.76-2.68 (4H, m), 2.37-2.32 (3H, m), 1.92-1.88 (2H, m), 1.75-1.72 (2H, m), 1.61-1.57 (2H, m), 1.27-1.25 (3H, m)
- LCMS: m/z 525 [M+H]+
- HPLC retention time: 1.48 min (analysis condition S)
- Compound O10-3
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound O10-1-2 and oxetan-3-one.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.26 (1H, s), 8.39 (1H, d, 7.9 Hz), 8.09-8.07 (2H, m), 7.63 (1H, d, 8.5 Hz), 7.51 (1H, s), 4.60-4.50 (4H, m), 4.20-4.09 (4H, m), 3.56-3.51 (1H, m), 3.07-3.05 (4H, m), 2.76-2.70 (2H, m), 2.44-2.40 (2H, m), 2.02-1.98 (2H, m), 1.29-1.26 (4H, m)
- LCMS: m/z 497 [M+H]+
- HPLC retention time: 1.42 min (analysis condition S)
- Compound O10-4
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound O10-1-2 and cyclobutanone.
- LCMS: m/z 495 [M+H]+
- HPLC retention time: 1.57 min (analysis condition S)
- Compound P1 (intermediate) 8-Methoxy-6,6-dimethyl-2-nitro-6,11-dihydro-5H-benzo[b]carbazole
- Under the same conditions as the method for synthesizing Compound A3-1, the title compound was prepared from Compound A2 and 4-nitrophenylhydrazine.
- LCMS: m/z 323 [M+H]+
- HPLC retention time: 4.08 min (analysis condition W)
- Compound P2 (intermediate)
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound P1.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.85 (1H, s), 9.03 (1H, d, J=1.9 Hz), 8.17-8.20 (2H, m), 7.71 (1H, d, J=9.1 Hz), 7.38 (1H, d, J=2.4 Hz), 7.12 (1H, dd, J=8.5, 2.4 Hz), 3.93 (3H, s), 1.79 (6H, s)
- LCMS: m/z 337 [M+H]+
- HPLC retention time: 3.55 min (analysis condition W)
- Compound P3 (intermediate)
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound P2.
- LCMS: m/z 323 [M+H]+
- HPLC retention time: 3.11 min (analysis condition W)
- Compound P4 (intermediate)
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound P3.
- 1H-NMR (400 MHz, CDCl3) δ: 9.40 (1H, s), 9.37 (1H, s), 8.41 (1H, d, J=8.5 Hz), 8.24 (1H, d, J=11.0 Hz), 7.51 (1H, d, J=8.5 Hz), 7.13 (1H, s), 7.03 (1H, d, J=9.1 Hz), 4.61-4.71 (1H, m), 3.69-3.84 (2H, m), 3.35-3.49 (2H, m), 1.94-2.10 (2H, m), 1.75-1.93 (8H, m), 1.50 (9H, s)
- LCMS: m/z 506 [M+H]+
- HPLC retention time: 4.17 min (analysis condition W)
- Compound P5
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound P6.
- 1H-NMR (400 MHz, CD3OD) δ: 8.23 (1H, d, J=8.5 Hz), 7.68 (1H, d, J=2.4 Hz), 7.26 (1H, d, J=8.5 Hz), 7.24 (1H, d, J=2.4 Hz), 7.06 (1H, dd, J=8.5, 2.4 Hz), 6.80 (1H, dd, J=8.5, 2.4 Hz), 4.64-4.71 (1H, m), 3.06-3.15 (2H, m), 2.73-2.83 (2H, m), 2.02-2.13 (2H, m), 1.67-1.82 (8H, m)
- LCMS: m/z 506 [M+H]+
- HPLC retention time: 4.17 min (analysis condition W)
- Compound P6 (intermediate)
-
- To the ethanol (8 ml) suspension of 4-(6,6-dimethyl-2-nitro-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-8-yl oxy)-piperidine-1-carboxylic acid tert-butyl ester (Compound P4,103 mg, 0.204 mmol), iron powder (228 mg, 20 eq.), ammonium chloride (109 mg, 10 eq.), and distilled water (4 ml) were added and the mixture was stirred at 90° C. for 30 min. Upon the completion of the reaction, insoluble matters were filtered off, and the filtrate was extracted with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (115 mg, 57%).
- LCMS: m/z 476 [M+H]+
- HPLC retention time: 2.82 min (analysis condition W)
- Compound P7 (intermediate)
-
- To the pyridine (2 ml) solution of 4-(2-amino-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-8-yl oxy)-piperidine-1-carboxylic acid tert-butyl ester (Compound P6, 50 mg, 0.105 mmol), mesyl chloride (9 μl, 1.2 eq.) was added and stirred at room temperature for 30 min. Upon the completion of the reaction, the reaction solution was concentrated under reduced pressure to obtain the title compound as an unpurified product.
- LCMS: m/z 554 [M+H]+
- HPLC retention time: 3.60 min (analysis condition W)
- Compound P8
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound P7.
- 1H-NMR (400 MHz, CD3OD) δ: 8.25 (1H, d, J=8.5 Hz), 8.16 (1H, d, J=1.8 Hz), 7.46 (1H, d, J=9.1 Hz), 7.27-7.29 (2H, m), 7.09 (1H, dd, J=9.1, 1.8 Hz), 4.67-4.75 (1H, m), 3.09-3.18 (2H, m), 2.95 (3H, s), 2.77-2.87 (2H, m), 1.70-1.84 (8H, m)
- LCMS: m/z 454 [M+H]+
- HPLC retention time: 2.22 min (analysis condition W)
- Compound Q3 (intermediate)
-
- Under the same conditions as the method for synthesizing Compound A3-1, the title compound was prepared from Compound A2 and 3-cyano-4-fluorophenylhydrazine.
- LCMS: m/z 321 [M+H]+
- HPLC retention time: 4.13 min (analysis condition W)
- Compound Q4 (intermediate)
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound Q3.
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.89 (1H, s), 8.16 (1H, d, J=8.5 Hz), 8.07 (1H, d, J=4.9 Hz), 8.04 (1H, d, J=9.8 Hz), 7.36 (1H, d, J=2.4 Hz), 7.10 (1H, dd, J=8.5, 2.4 Hz), 3.91 (3H, s), 1.78 (3H, s)
- LCMS: m/z 335 [M+H]+
- HPLC retention time: 3.61 min (analysis condition W)
- Compound Q5 (intermediate)
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound Q4.
- LCMS: m/z 321 [M+H]+
- HPLC retention time: 3.16 min (analysis condition W)
- Compound Q6 (intermediate)
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound Q5.
- LCMS: m/z 504 [M+H]+
- HPLC retention time: 4.25 min (analysis condition W)
- Compound Q7
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound Q5.
- 1H-NMR (400 MHz, CD3OD) δ: 8.25 (1H, d, J=8.5 Hz), 8.09 (1H, d, J=9.8 Hz), 7.83 (1H, d, J=5.5 Hz), 7.30 (1H, d, J=2.4 Hz), 7.09 (1H, dd, J=8.5, 2.4 Hz), 4.26 (2H, t, J=5.7 Hz), 2.98 (2H, t, J=5.7 Hz), 2.72 (4H, q, J=7.2 Hz), 1.81 (6H, s), 1.13 (6H, t, J=7.2 Hz)
- LCMS: m/z 420 [M+H]+
- HPLC retention time: 2.65 min (analysis condition W)
- Compound Q8
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from Compound Q6.
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.11 (1H, d, J=8.5 Hz), 7.98 (1H, d, J=5.5 Hz), 7.96 (1H, d, J=9.8 Hz), 7.29 (1H, s), 7.08 (1H, d, J=8.5 Hz), 4.58-4.69 (1H, m), 2.93-3.05 (2H, m), 2.60-2.69 (2H, m), 1.94-2.03 (2H, m), 1.74 (6H, s), 1.45-1.57 (2H, m)
- LCMS: m/z 404 [M+H]+
- HPLC retention time: 2.67 min (analysis condition W)
- Compound R2
-
- 3-Amino-2-fluoro-benzonitrile (100 mg, 0.735 mmol) was dissolved in water (0.94 mL), added with conc. hydrochloric acid (0.74 mL) at 0° C., and then further added with an aqueous solution (0.294 mL) of sodium nitrite (61 mg, 0.882 mmol). The resulting mixture was stirred at 0° C. for 1 hr. To the reaction mixture, conc. hydrochloric acid solution (0.94 mL) of tin chloride (321 mg, 1.69 mmol) was added and stirred at room temperature for 1 hr. Thereafter, the reaction solution was neutralized with aqueous solution of sodium hydroxide, and extracted with dichloromethane. The organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate. The drying agent was removed by filtration and the residues were obtained after concentration under reduced pressure to give the target compound as a crude product.
- Compound R3
-
- Under the same conditions as the method for synthesizing Compound E2-1, the title compound was prepared as a crude product from Compound A2 and Compound R2.
- Compound R4
-
- Under the same conditions as the method for synthesizing Compound A4, the title compound was prepared from Compound R3.
- LCMS: m/z 335 [M+H]+
- HPLC retention time: 2.70 min (analysis condition U)
- Compound R5
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound R4.
- LCMS: m/z 321 [M+H]+
- HPLC retention time: 2.32 min (analysis condition U)
- Compound R6
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound R5.
- LCMS: m/z 420 [M+H]+
- HPLC retention time: 1.51 min (analysis condition S)
- Compound R7
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound R5.
- LCMS: m/z 453 [M+H]+
- HPLC retention time: 3.82 min (analysis condition Y)
- Compound R8-1
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound R7 and 4-pyrrolidin-1-yl-piperidine.
- LCMS: m/z 457 [M+H]+
- HPLC retention time: 2.10 min (analysis condition U)
- Compound R8-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound R7.
- LCMS: m/z 431 [M+H]+
- HPLC retention time: 2.07 min (analysis condition U)
- Compound R9-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared as a crude product from Compound R5 and (R)-(−)-2,2-dimethyl-1,3-dioxolan-4-methanol.
- Compound R9-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound R9-1 (9.9 mg, 80%).
- LCMS: m/z 395 [M+H]+
- HPLC retention time: 2.38 min (analysis condition C)
- Compound S1-1
-
- Under the same conditions as the method for synthesizing Compound A3-1 and Compound A4, the title compound was prepared as a crude product from Compound A2 and (3-chlorophenyl)-hydrazine hydrochloric acid salt.
- Compound S1-2
-
- 7-Methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 99.1 mg, 0.485 mmol) and (3-chloro-4-methyl-phenyl)hydrazine hydrochloric acid salt (100.4 mg, 1.1 eq.) were dissolved in TFA (1 mL) and the mixture was irradiated with microwave at 80° C. for 10 min under nitrogen atmosphere. After cooling, the reaction solution was added with ethyl acetate, washed with water, saturated aqueous solution of sodium hydrogen carbonate and saturated brine and dried over magnesium sulfate. After filtration and concentration under reduced pressure, the residues obtained therefrom were dissolved in THF (2 mL) and water (0.2 mL), added with DDQ (125.7 mg, 1.1 eq.), and stirred at room temperature overnight. The reaction solution was added with the mixture solvent of hexane and ethyl acetate, and the starting-point components were removed by dry type silica gel column. The eluent was concentrated under reduced pressure, and the resulting residues were purified by preparative TLC (methanol/dichloromethane) to obtain the title compound (19.4 mg, 12%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.2 (1H, s), 8.15 (1H, d, J=8.8 Hz), 8.12 (1H, s), 7.52 (1H, s), 7.32 (1H, s), 7.07 (1H, dd, J=2.4, 8.8 Hz), 3.90 (3H, s), 2. (3H, s), 1.73 (6H, s),
- LCMS: m/z 340 [M+H]+
- HPLC retention time: 2.80 min (analysis condition F)
- Compound S1-3
-
- According to the same method as the method for synthesizing Compound A3-1, the title compound was prepared from Compound A2 and (3-chloro-2-fluoro-phenyl)-hydrazine.
- LCMS: m/z 344,346 [M+H]+
- HPLC retention time: 2.68 min (analysis condition S)
- Compound S1-4
-
- 6-Bromo-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound E1, 0.2 g, 0.71 mmol) and 3-chlorophenylhydrazine hydrochloric acid salt (0.17 g, 1.3 eq.) were dissolved in acetic acid (0.5 mL). Under nitrogen atmosphere, the reaction solution was stirred at 90° C. for 8 hr. After cooling to room temperature, the reaction solution was added with ethyl acetate, washed with water, saturated aqueous solution of sodium hydrogen carbonate and saturated brine and dried over magnesium sulfate. After filtration and concentration under reduced pressure, the residues obtained therefrom were dissolved in THF (3 mL) comprising 10% water, added with DDQ (227 mg, 3 eq.) at room temperature, and the mixture was stirred at room temperature for 2 hr. The reaction solution was added with the mixture liquid of THF/diethyl ether (1: 1) and washed with 0.5 N aqueous solution of sodium hydroxide and saturated brine. After drying with sodium sulfate, the mixture was filtered and the resulting residues obtained after concentration under reduced pressure were washed with the mixture liquid of hexane/diethyl ether (1: 1) to obtain the title compound (brown powder, 86 mg).
- LCMS: m/z 404, 406, 408 [M+H]+
- HPLC retention time: 3.02 min (analysis condition C)
- Compound 52-1
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound S1-1.
- LCMS: m/z 312 [M+H]+
- HPLC retention time: 4.18 min (analysis condition H)
- Compound S2-2
-
- 3-Chloro-8-methoxy-2,6,6-trimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S1-2, 18.9 mg, 0.0556 mmol) and pyridinium chloride (220 mg, 34 eq.) were stirred at 185° C. for 2.5 hr. After cooling, the reaction solution was added with water and ethyl acetate, and the organic layer was washed with water and saturated brine, dried over magnesium sulfate, filtered and concentrated under reduced pressure to obtain the title compound as a crude product.
- Compound S2-3
-
- 3-Chloro-4-fluoro-8-methoxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound 51-3, 220.0 mg, 0.640 mmol) and pyridinium chloride (800 mg, 6.922 mmol) were mixed with each other, heated to 160° C., and then stirred for 20 hr. The reaction solution was added with water. As a result, black solid was obtained as a precipitate, which was then filtered and subjected to purification by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (139.4 mg, 66%).
- LCMS: m/z 330 [M+H]+
- HPLC retention time: 2.60 min (analysis condition F)
- Compound S2-4
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound S1-4.
- LCMS: m/z 390, 392, 394 [M+H]+
- HPLC retention time: 2.75 min (analysis condition C)
- Compound S3
-
- 3-Chloro-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S2-1, 10 mg, 0.03207 mmol) was dissolved in DMF (0.1 mL), added with (2-chloroethyl)diethylamine (5.5 mg, 0.03207 mmol) and cesium carbonate (20.9 mg, 0.06414 mmol), and stirred at 80° C. for 2 hr. The reaction solution was added to water and extracted with ethyl acetate. The organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by NH silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (11.6 mg, 76%).
- LCMS: m/z 411 [M+H]+
- HPLC retention time: 4.49 min (analysis condition H)
- Compound S4
-
- Crude product of Compound S2-2 was dissolved in THF (0.4 mL) under nitrogen atmosphere, together with THF (0.2 mL) solution of triphenylphosphine (18.9 mg, 1.3 eq.) and [(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-methanol (17 mg, 1.2 eq.). DEAD (40% toluene solution, 0.0031 mL, 1.2 eq.) was added to the solution, which was then stirred at room temperature for 40 min and at 40° C. for 4 hr. The reaction solution was added with triphenylphosphine (18.9 mg, 1.3 eq.) and DEAD (40% toluene solution, 0.002 mL, 0.8 eq.) and stirred at 40° C. overnight. The reaction solution was added with ethyl acetate, washed with water and saturated brine, dried over magnesium sulfate, and filtered. The residues obtained after concentration under reduced pressure were purified by praparative TLC (ethyl acetate/hexane) to obtain the crude product of 8-[(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-3-chloro-2,6,6-trimethyl-5,6-dihydro-benzo[b]carbazol-11-one (12.6 mg).
- The resultant was dissolved in THF (0.15 mL) and methanol (0.03 mL) under nitrogen atmosphere, added with 0.5 M sulfuric acid (0.05 mL) and stirred at 60° C. for 3 hr. After cooling, diethyl ether was added and sodium hydrogen carbonate (8.4 mg) and water were further added thereto. The organic layer was washed with saturated brine. The aqueous layer was extracted with ethyl acetate, and the combined organic layer was dried over magnesium sulfate, and filtered. The solid obtained from the concentration under reduced pressure was washed with dichloromethane to obtain the target compound (white solid, 5.3 mg, 22%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.18 (1H, s), 8.14 (1H, d, J=8.8 Hz), 8.12 (1H, s), 7.52 (1H, s), 7.31 (1H, d, J=2.4 Hz), 7.06 (1H, dd, J=2.4, 8.8 Hz), 4.78 (1H, d, J=5.9 Hz), 4.60 (1H, d, J=5.9 Hz), 4.52 (1H, t, J=5.4 Hz), 4.18-4.22 (1H, m), 4.02-4.06 (1H, m), 3.85-3.95 (1H, m), 3.50-3.60 (2H, m), 3.40-3.46 (1H, m), 2.45 (3H, s), 1.73 (3H, s),
- LCMS: m/z 430 [M+H]+
- HPLC retention time: 2.27 min (analysis condition F)
- Compound S5
-
- The title compound was obtained as a by-product of the synthesis of Compound S6.
- LCMS: m/z 358 [M+H]+
- HPLC retention time: 3.16 min (analysis condition F)
- Compound S6
-
- 3-Chloro-4-fluoro-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S2-3, 20.0 mg, 0.061 mmol) was dissolved in THF (0.25 mL), added with ((S)-2,2-dimethyl-[1,3]dioxolan-4-yl)-methanol (9.8 μL, 0.079 mmol), triphenylphosphine (20.7 mg, 0.079 mmol) and diethyl azodicarboxylic acid (35.9 μl, 0.079 mmol), and then stirred at 40° C. for 5 hr. The reaction solution was concentrated under reduced pressure, and the resulting residues were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the intermediate, 3-chloro-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-4-fluoro-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one. This compound was dissolved in THF (0.10 mL) and MeOH (0.08 ml), added with sulfuric acid (0.5 M, 0.045 ml), and then stirred at 60° C. for 1 hr. The reaction solution was added with saturated aqueous solution of sodium hydrogen carbonate, extracted with ethyl acetate and dried over anhydrous magnesium sulfate. The yellow solid obtained after concentration under reduced pressure was washed with methylene chloride/hexane solvent and filtered to obtain the title compound (4.3 mg, 18%).
- LCMS: m/z 404 [M+H]+
- HPLC retention time: 2.34 min (analysis condition F)
- Compound S7-1
-
- Under nitrogen atmosphere, 9-bromo-3-chloro-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S2-4, 76 mg, 0.2 mmol) and triphenylphosphine (69 mg, 1.3 eq.) were added with THF (2 ml), and ((S)-2,2-dimethyl-[1,3]dioxolan-4-yl)-methanol (35 mg, 1.3 eq.) and 2.19 N toluene solution (118 μL, 1.3 eq.) of diethyl azodicarboxylic acid were added dropwise thereto, followed by stirring at 50° C. for 2 hr. After cooling, the reaction solution was added with ethyl acetate, washed with brine, dried over sodium sulfate and filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/dichloromethane) to give a solid, which was then washed with dichloromethane to obtain the title compound (brown powder, 53 mg).
- LCMS: m/z 504, 506, 508 [M+H]+
- HPLC retention time: 3.17 min (analysis condition C)
- Compound S7-2
-
- 3-Chloro-9-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S7-1, 56 mg, 0.11 mmol) was dissolved in methanol (5 mL), added with 1 N hydrochloric acid (0.2 ml), and stirred at 50° C. for 2 hr. After cooling, the reaction solution was concentrated under reduced pressure and the resulting residues were added with methanol to obtain a precipitated solid, which was then filtered to obtain the title compound (white powder, 26 mg).
- LCMS: m/z 464, 466, 468 [M+H]+
- HPLC retention time: 2.77 min (analysis condition C)
- Compound S7-3
-
- Under nitrogen atmosphere, 9-bromo-3-chloro-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S2-4, 112 mg, 0.29 mmol) and triphenylphosphine (227 mg, 3 eq.) were added with THF (2 ml), and ((R)-2,2-dimethyl-[1,3]dioxolan-4-yl)-methanol (114 mg, 3 eq.) and 2.19 N toluene solution (0.4 mL, 3 eq.) of diethyl azodicarboxylic acid were added dropwise thereto, followed by stirring at 40° C. for 12 hr under nitrogen atmosphere. The residues obtained from the reaction solution after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/dichloromethane) to obtain the title compound (white powder, 100 mg).
- LCMS: m/z 504, 506, 508 [M+H]+
- HPLC retention time: 3.15 min (analysis condition C)
- Compound S7-4
-
- Under the same conditions as the method for synthesizing Compound S7-2, the title compound was prepared from Compound S7-3.
- LCMS: m/z 464, 466, 468 [M+H]+
- HPLC retention time: 2.77 min (analysis condition C)
- Compound S8-1
-
- 9-Bromo-3-chloro-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S7-1, 30 mg, 0.06 mmol) was dissolved in the mixture solvent of water dioxane (1: 1) (0.5 mL), added with tris(benzylidenacetone dipalladium)chloroform complex (3.1 mg, 0.05 eq.), 2-di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl (2.5 mg, 0.1 eq.) and KOH (0.5 N aqueous solution 180 μL, 1.5 eq.), and stirred at 60° C. for 12 hr. After cooling, the reaction solution was concentrated under reduced pressure, and the resulting residues were purified by HPLC to obtain the title compound (white solid, 4.6 mg).
- LCMS: m/z 442, 444 [M+H]+
- HPLC retention time: 2.78 min (analysis condition C)
- Compound S8-2
-
- Under the same conditions as the method for synthesizing Compound S7-2, the title compound was prepared from Compound S8-1.
- LCMS: m/z 402, 404 [M+H]+
- HPLC retention time: 0.90 min (analysis condition I)
- Compound S9-1
-
- 9-Bromo-3-chloro-8-methoxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S1-4, 150 mg, 0.37 mMol) was dissolved in NMP, added with CuCN (100 mg, 3 eq.), and stirred at 210° C. for 1.5 hr under irradiation with microwave. After cooling, the reaction solution was added with water and ethyl acetate, and the precipitated solid was filtered to remove the solvent. The obtained residues were dissolved in DMF (1 ml), added with sodium azide (100 mg, 8 eq.) and ammonium chloride (5 mg), and then stirred at 120° C. for 24 hr in a sealed tube. After adding water, the insoluble matters were filtered and purified by HPLC to obtain the title compound (6.5 mg).
- LCMS: m/z 371 [M+H]+
- HPLC retention time: 2.22 min (analysis condition C)
- Compound S9-2
-
- The title compound was obtained as an intermediate for the synthesis of Compound S9-1.
- LCMS: m/z 380, 382 [M+H]+
- HPLC retention time: 2.38 min (analysis condition C)
- Compound S10
-
- To the mixture of 9-bromo-3-chloro-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S7-1, 50 mg, 0.1 mmol), bis(acetonitrile) palladium (II) dichloride (2.6 mg, 0.01 eq.), cesium carbonate (195 mg, 6 eq.) and 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (14.3 mg, 0.03 eq.), acetonitrile (2 mL) was added and stirred at 80° C. for 12 hr. Tar-like residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain 3-chloro-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-9-(3-hydroxy-3-methyl-but-1-ynyl)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (brown powder, 105 mg).
- The resulting 3-chloro-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-9-(3-hydroxy-3-methyl-but-1-ynyl)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (20 mg, 0.04 mmol) was dissolved in methanol (3 mL), added with 1 N hydrochloric acid (1 ml), and stirred at room temperature for 12 hr. After cooling, the reaction solution was concentrated under reduced pressure, and the resulting residues were washed with methylene chloride to obtain the title compound (pale yellow powder, 5.2 mg).
- LCMS: m/z 468, 470 [M+H]+
- HPLC retention time: 2.70 min (analysis condition C)
- Compound S11-1
-
- 3-Chloro-9-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound S7-2, 17 mg, 37 μmol) was dissolved in DMA, added with CuCN (17 mg, 5 eq.), and stirred at 220° C. for 2 hr under irradiation with microwave. After cooling, the reaction solution was added with ethyl acetate, and the precipitated solid was filtered to remove the solvent. The resulting residues were purified by HPLC to obtain the title compound (4 mg).
- LCMS: m/z 409, 411 [M+H]+
- HPLC retention time: 2.65 min (analysis condition C)
- Compound S11-2
-
- The title compound was obtained as a by-product of the synthesis of Compound S11-1.
- LCMS: m/z 337, 339 [M+H]+
- HPLC retention time: 2.35 min (analysis condition C)
- Compound T1-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and (S)-tetrahydro-furan-3-ol.
- LCMS: m/z 426 [M+H]+
- HPLC retention time: 2.08 min (analysis condition D)
- Compound T1-2
-
- According to the same method as the method for synthesizing Compound A5-2, the title compound was prepared from Compound T1-1.
- LCMS: m/z 373 [M+H]+
- HPLC retention time: 1.98 min (analysis condition A)
- Compound T2-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and (R)-tetrahydro-furan-3-ol.
- LCMS: m/z 426 [M+H]+
- HPLC retention time: 6.12 min (analysis condition H)
- Compound T2-2
-
- According to the same method as the method for synthesizing Compound A5-2, the title compound was prepared from Compound T2-1.
- LCMS: m/z 373 [M+H]+
- HPLC retention time: 2.00 min (analysis condition D)
- Compound T3-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and tetrahydro-pyran-4-ol.
- LCMS: m/z 440 [M+H]+
- HPLC retention time: 8.07 min (analysis condition H)
- Compound T3-2
-
- According to the same method as the method for synthesizing Compound A10-2, the title compound was prepared from Compound T3-1.
- LCMS: m/z 454 [M+H]+
- HPLC retention time: 6.88 min (analysis condition H)
- Compound T4-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and 5-phenyl-[1,3]dioxan-2-ol.
- LCMS: m/z 518 [M+H]+
- HPLC retention time: 2.68 min (analysis condition D)
- Compound T4-2
-
- According to the same method as the method for synthesizing Compound A7-13-2, the title compound was prepared from Compound T4-1.
- LCMS: m/z 430 [M+H]+
- HPLC retention time: 4.64 min (analysis condition H)
- Compound T5-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and 4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 539 [M+H]+
- HPLC retention time: 2.72 min (analysis condition D)
- Compound T5-2
-
- According to the same method as the method for synthesizing Compound A10-1, the title compound was prepared from Compound T5-1.
- LCMS: m/z 553 [M+H]+
- HPLC retention time: 2.93 min (analysis condition D)
- Compound T5-3
-
- According to the same method as the method for synthesizing Compound A8-1, the title compound was prepared from Compound T5-2.
- LCMS: m/z 453 [M+H]+
- HPLC retention time: 1.98 min (analysis condition D)
- Compound T5-4
-
- According to the same method as the method for synthesizing Compound A9-7, the title compound was prepared from Compound T5-3 and methanesulfonyl chloride.
- LCMS: m/z 531 [M+H]+
- HPLC retention time: 2.38 min (analysis condition D)
- Compound T5-5
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T5-3 and acetic anhydride.
- LCMS: m/z 482 [M+H]+
- HPLC retention time: 2.10 min (analysis condition D)
- Compound T6-1
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T6-2 and trifluoroacetic anhydride.
- LCMS: m/z 535 [M+H]+
- HPLC retention time: 2.53 min (analysis condition D)
- Compound T6-2
-
- 3-Bromo-6,6-dimethyl-8-[1-(2,2,2-trifluoro-acetyl)-piperidin-4-yloxy]-5,6-dihydro-benzo[b]carbazol-11-one (Compound T6-1, 28.0 mg, 52.3 mol) was dissolved in THF (1.00 mL) and methanol (0.50 mL), added with aqueous solution of potassium hydroxide (1.00 mL, 20 wt %), and stirred at room temperature for 1 hr. The reaction solution was added to water, and extracted with mixture solution of chloroform and methanol, and dried over sodium sulfate. Then, after filtering and concentration under reduced pressure, 3-bromo-6,6-dimethyl-8-(piperidin-4-yloxy)-5,6-dihydro-benzo[b]carbazol-11-one was obtained as a crude product.
- LCMS: m/z 439 [M+H]+
- HPLC retention time: 1.83 min (analysis condition D)
- Compound T6-3
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T6-2 and mesyl chloride.
- LCMS: m/z 517 [M+H]+
- HPLC retention time: 2.23 min (analysis condition D)
- Compound T6-4
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T6-2 and acetic anhydride.
- LCMS: m/z 496 [M+H]+
- HPLC retention time: 2.27 min (analysis condition D)
- Compound T7-1
-
- The title compound was obtained as a by-product of the synthesis of Compound T4-1.
- LCMS: m/z 398 [M+H]+
- HPLC retention time: 3.18 min (analysis condition F)
- Compound T7-2
-
- According to the same method as the method for synthesizing Compound A10-2, the title compound was prepared from Compound T7-1.
- LCMS: m/z 413 [M+H]+
- HPLC retention time: 2.70 min (analysis condition D)
- Compound T8-1
-
- According to the same method as the method for synthesizing Compound A10-2, the title compound was prepared from Compound T4-1.
- LCMS: m/z 532 [M+H]+
- HPLC retention time: 2.90 min (analysis condition D)
- Compound T8-2
-
- According to the same method as the method for synthesizing Compound A7-13-2, the title compound was prepared from Compound T4-1.
- LCMS: m/z 444 [M+H]+
- HPLC retention time: 1.90 min (analysis condition D)
- Compound T9
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A5-1 and (N-(2-chloro-ethyl)-acetamide.
- LCMS: m/z 441 [M+H]+
- HPLC retention time: 1.92 min (analysis condition D)
- Compound T10
- 3-Bromo-6,6-dimethyl-8-(oxetan-3-yl oxy)-5,6-dihydro-benzo[b]carbazol-11-one
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A5-1 and toluene-4-sulfonic acid oxetan-3-yl ester.
- LCMS: m/z 412 [M+H]+
- HPLC retention time: 2.17 min (analysis condition D)
- Compound T11
-
- Under nitrogen atmosphere, tetrahydro-furo[3,4-d][1,3,2]dioxathiol 2,2-dioxide (71.5 mg, 0.420 mmol) was dissolved in DMF (1.40 mL), added with 3-bromo-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound A5-1, 50.0 mg, 0.140 mmol) and cesium carbonate (228 mg, 0.700 mmol), and stirred at 80° C. for 15 hr. Subsequently, sulfuric acid (0.10 mL, 18 M), THF (3.00 mL) and water (0.50 mL) were added to the mixture, which was then stirred at room temperature for 24 hr and further at 60° C. for 24 hr. The reaction solution was added to water, extracted with ethyl acetate, washed with water, saturated aqueous solution of sodium bicarbonate, and saturated brine, and dried over sodium sulfate. After filtering and concentration under reduced pressure, the resulting residues were washed with dichloromethane and purified by NH silica gel column chromatography (ethyl acetate/THF) to obtain the target compound (44.7 mg, 72%).
- LCMS: m/z 442 [M+H]+
- HPLC retention time: 1.98 min (analysis condition D)
- Compound T12-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and methyl 2,3,4-tri-O-acetyl-a-D-glucopyranoside.
- LCMS: m/z 658 [M+H]+
- HPLC retention time: 2.38 min (analysis condition D)
- Compound T12-2
-
- Under nitrogen atmosphere, to acetic acid (2S,3R,4S,5R,6R)-4,5-diacetoxy-6-(3-bromo-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-8-yl oxymethyl)-2-methoxy-tetrahydro-pyran-3-yl ester (Compound T12-1, 34.0 mg, 51.63 μmol), methanol solution (2.50 mL, 2 M) of ammonia was added, and the mixture was stirred at room temperature for 21 hr. The reaction solution was concentrated under reduced pressure, and the resulting resides were washed with diethyl ether to obtain the target compound (25.7 mg, 94%).
- LCMS: m/z 532 [M+H]+
- HPLC retention time: 2.42 min (analysis condition D)
- Compound T13-1
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A5-1 and bromo-acetic acid tert-butyl ester.
- LCMS: m/z 470 [M+H]+
- HPLC retention time: 2.53 min (analysis condition D)
- Compound T13-2
-
- According to the same method as the method for synthesizing Compound A8-1, the title compound was prepared from Compound T13-1.
- LCMS: m/z 414 [M+H]+
- HPLC retention time: 1.50 min (analysis condition D)
- Compound T13-3
-
- Under nitrogen atmosphere, (3-azide-1,1-diethyl-propoxy)-trimethyl-silane (16.6 mg, 72.42 μmol) was dissolved in toluene (0.48 mL), added with 3-bromo-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-8-yl oxy)-acetic acid (20.0 mg, 48.28 μmol) and Molecular Sieves 4 angstrom, and the mixture was stirred at room temperature for 5 min. Thereafter, the mixture was added with trimethylphosphine (10.2 μL, 96.56 μmol) and stirred at 80° C. for 22 hr. The reaction solution was added to hydrochloric acid (1 M), extracted with ethyl acetate, washed with water, saturated aqueous solution of sodium bicarbonate, saturated brine and dried over sodium sulfate. After filtering and concentration under reduced pressure, the resulting residues were purified by silica gel column chromatography (methanol/dichloromethane) to obtain the target compound (0.7 mg, 3%).
- LCMS: m/z 527 [M+H]+
- HPLC retention time: 2.93 min (analysis condition D)
- Compound T13-4
-
- According to the same method as the method for synthesizing Compound A9-10, the title compound was prepared from Compound T13-2 and 1-(tert-butoxycarbonyl)piperazine.
- LCMS: m/z 582 [M+H]+
- HPLC retention time: 2.32 min (analysis condition D)
- Compound T13-5
-
- According to the same method as the method for synthesizing Compound A8-1, the title compound was prepared from Compound T13-4.
- LCMS: m/z 482 [M+H]+
- HPLC retention time: 1.75 min (analysis condition D)
- Compound T13-6
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T13-5 and methanesulfonyl chloride.
- LCMS: m/z 560 [M+H]+
- HPLC retention time: 2.00 min (analysis condition D)
- Compound T13-7
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T13-5 and isopropylsulfonyl chloride.
- LCMS: m/z 588 [M+H]+
- HPLC retention time: 2.47 min (analysis condition D)
- Compound T13-8
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T13-5 and acetic anhydride.
- LCMS: m/z 524 [M+H]+
- HPLC retention time: 1.85 min (analysis condition D)
- Compound T13-9
-
- According to the same method as the method for synthesizing Compound B3-32, the title compound was prepared from Compound T13-5 and 3-oxetanone.
- LCMS: m/z 538 [M+H]+
- HPLC retention time: 1.88 min (analysis condition D)
- Compound T13-10
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T13-5 and 2-oxo-oxazolidine-3-sulfonic acid methylamide.
- LCMS: m/z 575 [M+H]+
- HPLC retention time: 2.29 min (analysis condition A)
- Compound T14-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and 1-(tert-butoxycarbonyl)-4-(hydroxymethyl)piperidine.
- LCMS: m/z 553 [M+H]+
- HPLC retention time: 2.80 min (analysis condition D)
- Compound T14-2
-
- According to the same method as the method for synthesizing Compound A8-1, the title compound was prepared from Compound T14-1.
- LCMS: m/z 454 [M+H]+
- HPLC retention time: 1.90 min (analysis condition D)
- Compound T14-3
-
- According to the same method as the method for synthesizing Compound B3-32, the title compound was prepared from Compound T14-2 and 3-oxetanone.
- 1H-NMR (400 MHz, CDCl3) δ: 9.24 (1H, s), 8.37 (1H, d, 8.8 Hz), 8.30 (1H, d, 8.3 Hz), 7.57 (1H, d, 1.5 Hz), 7.41 (1H, dd, 8.3, 1.5 Hz), 7.08 (1H, d, 2.4 Hz), 6.98 (1H, dd, 8.8, 2.4 Hz) 4.60-4.95 (7H, m), 3.93 (2H, d, 5.9 Hz), 3.50 (1H, m), 2.83 (2H, d, 11.2 Hz), 1.89 (4H, m), 1.78 (6H, s),
- LCMS: m/z 509 [M+H]+
- HPLC retention time: 2.10 min (analysis condition A)
- Compound T14-4
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T14-2 and acetic anhydride.
- LCMS: m/z 495 [M+H]+
- HPLC retention time: 2.53 min (analysis condition A)
- Compound T14-5
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T14-2 and methanesulfonyl chloride.
- LCMS: m/z 531 [M+H]+
- HPLC retention time: 2.30 min (analysis condition D)
- Compound T14-6
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T14-2 and isopropylsulfonyl chloride.
- LCMS: m/z 559 [M+H]+
- HPLC retention time: 2.58 min (analysis condition D)
- Compound T14-7
-
- According to the same method as the method for synthesizing Compound B3-32, the title compound was prepared from Compound T14-2 and 3-oxo-azetidine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 608 [M+H]+
- HPLC retention time: 2.29 min (analysis condition A)
- Compound T14-8
-
- According to the same method as the method for synthesizing Compound A8-1, the title compound was prepared from Compound T14-7.
- LCMS: m/z 508 [M+H]+
- HPLC retention time: 1.90 min (analysis condition A)
- Compound T14-9
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T14-8 and mesyl chloride.
- LCMS: m/z 586 [M+H]+
- HPLC retention time: 2.06 min (analysis condition A)
- Compound T14-10
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T14-8 and acetic anhydride.
- LCMS: m/z 550 [M+H]+
- HPLC retention time: 2.53 min (analysis condition A)
- Compound T15-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and N-(tert-butoxycarbonyl)-4-piperidine ethanol.
- LCMS: m/z 567 [M+H]+
- HPLC retention time: 2.29 min (analysis condition D)
- Compound T15-2
-
- According to the same method as the method for synthesizing Compound A8-1, the title compound was prepared from Compound T15-1.
- LCMS: m/z 467 [M+H]+
- HPLC retention time: 1.95 min (analysis condition D)
- Compound T15-3
-
- According to the same method as the method for synthesizing Compound B3-32, the title compound was prepared from Compound T15-2 and 3-oxetanone.
- LCMS: m/z 523 [M+H]+
- HPLC retention time: 2.28 min (analysis condition D)
- Compound T16-1
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and 3-hydroxy-1-oxa-8-aza-spiro[4.5]decane-8-carboxylic acid tert-butyl ester.
- LCMS: m/z 595 [M+H]+
- HPLC retention time: 3.08 min (analysis condition A)
- Compound T16-2
-
- According to the same method as the method for synthesizing Compound A8-1, the title compound was prepared from Compound T16-1.
- LCMS: m/z 496 [M+H]+
- HPLC retention time: 1.99 min (analysis condition A)
- Compound T16-3
-
- According to the same method as the method for synthesizing Compound A9-1, the title compound was prepared from Compound T16-2 and mesyl chloride.
- LCMS: m/z 573 [M+H]+
- HPLC retention time: 2.56 min (analysis condition A)
- Compound T16-4
-
- According to the same method as the method for synthesizing Compound B3-32, the title compound was prepared from Compound T16-2 and 3-oxetanone.
- LCMS: m/z 551 [M+H]+
- HPLC retention time: 2.01 min (analysis condition A)
- Compound T17-1
-
- Under nitrogen atmosphere, 4-[1,3]dithian-2-ylidene-piperidine-1-carboxylicacid tert-butyl ester (100 g, 0.332 mmol) was dissolved in dichloromethane (2.50 mL), added with trifluoromethanesulfonic acid (30.8 μL, 0.348 mmol) at −20° C., and stirred at room temperature for 30 min. The reaction solution was cooled to −70° C., and then added dropwise with the dichloromethane (2.50 mL) solution of 3-bromo-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound A5-1, 177 mg, 0.498 mmol) and triethylamine (78.6 μL, 0.564 mmol). Thereafter, triethylamine hydrotrifluoric acid salt (262 μL, 1.610 mmol) and 1,3-dibromo-5,5-dimethylhydantoin (460 mg, 1.610 mmol) were added thereto and stirred at −70° C. for 1 hr. The reaction solution was added to aqueous solution of sodium hydroxide (1 M), extracted with ethyl acetate, washed with water, saturated aqueous solution of sodium bicarbonate, saturated brine and dried over sodium sulfate. After filtering and concentration under reduced pressure, the resulting residues were purified by silica gel column chromatography (ethyl acetate/hexane) and aminosilica gel column chromatography (ethyl acetate/hexane) to obtain the target compound (42.0 mg, 25%).
- LCMS: m/z 511 [M+H]+
- HPLC retention time: 6.34 min (analysis condition B)
- Compound T17-2
-
- According to the same method as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A17-1 and (S)-(+)-2,2-dimethyl-1,3-dioxolan-4-methanol.
- LCMS: m/z 625 [M+H]+
- HPLC retention time: 3.41 min (analysis condition A)
- Compound T17-3
-
- According to the same method as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound T17-2.
- LCMS: m/z 585 [M+H]+
- HPLC retention time: 2.44 min (analysis condition A)
- Compound T18-1
-
- 3-Bromo-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound A5-1, 18.0 mg, 50.5 μmol) was dissolved in DMF (0.18 mL), added with toluene-4-sulfonic acid (R)-2,2-dimethyl-[1,3]dioxolan-4-yl methyl ester (14.5 mg, 0.0505 mmol) and potassium carbonate (10.0 mg, 0.07575 mmol), and the mixture was stirred at 70° C. for 3 days. The reaction solution was added to water, extracted with ethyl acetate, and the organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by preparative TLC (methylene chloride/methanol) to obtain the title compound (16.6 mg, 70%).
- LCMS: m/z 470 [M+H]+
- HPLC retention time: 3.01 min (analysis condition F)
- Compound T18-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound T18-1.
- LCMS: m/z 430 [M+H]+
- HPLC retention time: 4.72 min (analysis condition H)
- Compound T19-1-1
-
- Under the same conditions as the method for synthesizing Compound A10-1, the title compound was prepared from Compound A4.
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 2.84 min (analysis condition D)
- Compound T19-1
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound T19-1-1.
- LCMS: m/z 370 [M+H]+
- HPLC retention time: 2.40 min (analysis condition D)
- Compound T19-2
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound A5-1 (9.8 mg, 36%).
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 1.96 min (analysis condition D)
- Compound T19-3
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound T19-1.
- LCMS: m/z 469 [M+H]+
- HPLC retention time: 2.09 min (analysis condition D)
- Compound T20
-
- Under the same conditions as the method for synthesizing Compound A7-17, Compound A5-1 and methyl 5-bromovalerate were reacted, added with 1 N NaOH (140 L), and then stirred at room temperature for 2 hr. The reaction mixture was added with 2 N HCl (70 μL), and concentrated under reduced pressure. The resulting residues were purified by preparative TLC (methylene chloride:methanol=15:1) to obtain 7 mg (55%).
- LCMS: m/z 456 [M+H]+
- HPLC retention time: 5.88 min (analysis condition H)
- Compound T21
-
- Under the same conditions as the method for synthesizing Compound T20, the title compound was prepared from the reaction between Compound A5-1 and toluene-4-sulfonic acid (R)-5-oxo-tetrahydrofuran-2-yl methyl ester.
- LCMS: m/z 471 [M+H]+
- HPLC retention time: 4.57 min (analysis condition H)
- Compound T22-0
-
- To THF (50 mL), NaH (1.41 g, 0.032 mmol) was added at room temperature, followed by addition of ((4R,5R)-5-hydroxymethyl-2,2-dimethyl-[1,3]dioxolan-4-yl)-methanol (5.0 g, 0.031 mmol) at room temperature. The mixture was stirred at room temperature for 1 hr. After that, TBSCl (5.11 g, 0.034 mmol) was added at room temperature and stirred at room temperature overnight. The reaction solution was added with saturated aqueous solution of sodium hydrogen carbonate and extracted with ethyl acetate. The organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (8.21 g, 96%).
- 1H-NMR (270 MHz, DMSO-d6) δ: 3.64-4.98 (6H, m), 2.37 (1H, m), 1.41 (3H, s), 1.40 (3H, s), 0.90 (9H, s), 0.08 (6H, s)
- Compound T22-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound A5-1 and [5-(tert-butyldimethylsilanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-methanol (Compound T22-0) (704 mg, 80%).
- LCMS: m/z 614 [M+H]+
- HPLC retention time: 4.00 min (analysis condition F)
- Compound T22-1-1
-
- Under nitrogen atmosphere, to the DMF (0.4 mL) suspension of 3-bromo-8-[(1R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound T22-1, 50.3 mg, 0.0818 mmol) and copper (I) iodide (34 mg), sodium methoxide (1 M methanol solution, 0.82 mL, 0.818 mmol) was added and the mixture was stirred for 6 hr and 45 min at ambient temperature of 90° C. After cooling to room temperature, the reaction mixture was added with diethyl ether and ethyl acetate, and the insoluble matters were removed by Celite filtration. The concentrated residues were added with diethyl ether, hexane, ethyl acetate and water, and then the mixture was extracted twice with diethyl ether. The organic layer was washed with water and subsequently with brine, dried over sodium sulfate and concentrated under reduced pressure. The resulting crude product was purified by preparative TLC (Merck60 F254, 0.5 mm) {solution for elution: hexane/ethyl acetate (1: 2)} to obtain the title compound (colorless oily substance, 22.6 mg, 55%).
- 1H-NMR (270 MHz, CDCl3) δ: 8.44-8.38 (1H, b), 8.39 (1H, d, 8.6 Hz), 8.31 (1H, d, 8.2 Hz), 7.60 (1H, d, 1.3 Hz), 7.44 (1H, dd, 8.2 Hz, 1.3 Hz), 7.12 (1H, d, 2.3 Hz), 7.02 (1H, dd, 8.6 Hz, 2.3 Hz), 4.41-4.10 (4H, m), 4.00-3.88 (1H, m), 3.86-3.76 (1H, m), 1.78 (6H, s), 1.50 (3H, s), 1.49 (3H, s)
- LCMS: m/z 500 [M+H]+
- HPLC retention time: 2.85 min (analysis condition C)
- Compound T22-1-2
-
- The title compound was obtained as a by-product of the synthesis of T22-1-1 (white solid, 17.8 mg, 40%).
- 1H-NMR (270 MHz, CDCl3) δ: 8.92-8.80 (1H, b), 8.40 (1H, d, 8.9 Hz), 8.31 (1H, d, 8.6 Hz), 7.58 (1H, d, 1.7 Hz), 7.43 (1H, dd, 8.6 Hz, 1.7 Hz), 7.14 (1H, d, 2.3 Hz), 7.02 (1H, dd, 8.9 Hz, 2.3 Hz), 4.51-4.38 (1H, m), 4.34-4.16 (4H, m), 2.13 (3H, s), 1.78 (6H, s), 1.50 (6H, s)
- LCMS: m/z 542 [M+H]+
- HPLC retention time: 3.00 min (analysis condition C)
- Compound T22-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound T22-1 (2.83 g, 95%).
- LCMS: m/z 460 [M+H]+
- HPLC retention time: 4.50 min (analysis condition H)
- Compound T22-3
-
- Under the same conditions as the method for synthesizing Compound B3-4, the title compound was prepared from Compound T22-1.
- LCMS: m/z 628 [M+H]+
- HPLC retention time: 4.74 min (analysis condition F)
- Compound T22-4
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound T22-3.
- LCMS: m/z 475 [M+H]+
- HPLC retention time: 4.86 min (analysis condition H)
- Compound T22-5
-
- Under nitrogen atmosphere, 3-bromo-8-[(4R,5R)-5-(tert-butyldimethylsilanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound T22-1, 40.0 mg, 65.2 μmol) was dissolved in DMF (0.20 mL), added at 0° C. with methyl bromoacetate (30.5 μL, 134.5 μmol) and sodium hydride (4.5 mg, 132 μmol), and then stirred at room temperature for 2 hr. The residues obtained from the reaction solution after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (44.5 mg, 85%).
- LCMS: m/z 686 [M+H]+
- HPLC retention time: 3.35 min (analysis condition D)
- Compound T22-6
-
- {3-Bromo-8-[(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy]-6,6-dimethyl-11-oxo-6,11-dihydro-benzo[b]carbazol-5-yl}-acetic acid methyl ester (Compound T22-5, 40 mg, 60.0 μmol) was dissolved in the mixture solvent of methanol (120 l) and water (30 l), added with lithium hydroxide monohydrate (10 mg, 240 μmol), and then stirred 40° C. for 15 min. The residues obtained from the reaction solution after concentration under reduced pressure were purified by silica gel column chromatography (methylene chloride/methanol) to obtain the target compound (35.2 mg, 96%).
- LCMS: m/z 672 [M+H]+
- HPLC retention time: 3.41 min (analysis condition D)
- Compound T22-7
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound T22-6 (6.2 mg, 31%).
- LCMS: m/z 518 [M+H]+
- HPLC retention time: 1.30 min (analysis condition D)
- Compound T22-8
-
- [3-Bromo-6,6-dimethyl-11-oxo-8-((2R,3R)-2,3,4-trihydroxy-butoxy)-6,11-dihydro-benzo[b]carbazol-5-yl]-acetic acid (Compound T22-6, 15.0 mg, 29.0 μmol) was dissolved in methanol (0.30 mL), added with trimethylsilyldiazomethane (0.10 mL), and then stirred at room temperature for 1 hr. The residues obtained from the reaction solution after concentration under reduced pressure were purified by silica gel column chromatography (methylene chloride/methanol) to obtain the target compound (15.2 mg, 96%).
- LCMS: m/z 532 [M+H]+
- HPLC retention time: 1.80 min (analysis condition D)
- Compound T23-1
-
- Under the same conditions as the method for synthesizing Compound T18-1 and Compound T18-2, the title compound was prepared from Compound A5-1 and toluene-4-sulfonic acid (R)-2,2-dimethyl-[1,3]dioxolan-4-yl methyl ester.
- LCMS: m/z 366 [M+H]+
- HPLC retention time: 4.50 min (analysis condition H)
- Compound T23-2
-
- Under the same conditions as the method for synthesizing Compound T18-1 and Compound T18-2, the title compound was prepared from Compound A4 and toluene-4-sulfonic acid (S)-2,2-dimethyl-[1,3]dioxolan-4-yl methyl ester.
- LCMS: m/z 366 [M+H]+
- HPLC retention time: 4.50 min (analysis condition H)
- Compound T24-1
-
- Under nitrogen atmosphere, to the DMF (1 mL) suspension of 3-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound T18-1, 112.2 mg, 0.239 mmol) and sodium hydride (60%) (19 mg, 0.477 mmol), cooled in an ice bath, methyl iodide (37 mL, 0.596 mmol) was added. The reaction mixture was stirred at room temperature for 45 min, and then added with saturated aqueous solution of ammonium chloride and saturated aqueous solution of sodium thiosulfate under ice cooling. The mixture was extracted twice with ethyl acetate/diethyl ether/hexane. The organic layer was washed with water and subsequently with aqueous solution of ammonium chloride, dried over sodium sulfate, and then concentrated under reduced pressure. The resulting crude product was purified by flash column chromatography {Merck Kieselgel60, solution for elution: hexane/ethyl acetate (1: 1)} to obtain the title compound (white solid, 107.3 mg, 93%).
- 1H-NMR (270 MHz, CDCl3) δ: 8.41 (1H, d, 8.6 Hz), 8.35 (1H, d, 8.9 Hz), 7.56 (1H, d, 1.7 Hz), 7.46 (1H, dd, 8.6 Hz, 1.7 Hz), 7.14 (1H, d, 2.3 Hz), 7.00 (1H, dd, 8.9 Hz, 2.3 Hz), 4.60-4.49 (1H, m), 4.20-3.90 (4H, m), 4.03 (3H, s), 1.88 (6H, s), 1.50 (3H, s), 1.43 (3H, s)
- LCMS: m/z 484 [M+H]+
- HPLC retention time: 6.59 min (analysis condition B)
- Compound T24-2
-
- Under nitrogen atmosphere, to the THF (0.15 mL)-MeOH (0.1 mL) solution of 3-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-5,6,6-trimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound T24-1, 15.5 mg, 0.0320 mmol), 0.5 M aqueous solution of sulfuric acid (128 μL, 0.0640 mmol) was added at room temperature. The reaction mixture was stirred at ambient temperature of 55° C. for 2 hr, cooled to room temperature, and then added with diethyl ether and subsequently with sodium hydrogen carbonate (11 mg). The mixture was extracted twice with diethyl ether/ethyl acetate, and the organic layer was washed with brine, dried over sodium sulfate and concentrated under reduced pressure to obtain the title compound (white solid, 11.9 mg, 84%).
- 1H-NMR (270 MHz, CD3OD) δ: 8.26 (1H, d, 8.6 Hz), 8.20 (1H, d, 8.9 Hz), 7.77 (1H, d, 1.7 Hz), 7.42 (1H, dd, 8.6 Hz, 1.7 Hz), 7.33 (1H, d, 2.3 Hz), 7.09 (1H, dd, 8.9 Hz, 2.3 Hz), 4.26-3.96 (3H, m), 4.10 (3H, s), 3.74-3.66 (1H, m), 1.92 (6H, s)
- LCMS: m/z 444 [M+H]+
- HPLC retention time: 4.65 min (analysis condition B)
- Compound T25
-
- Under the same conditions as the method for synthesizing Compound A3-1 and Compound A4, the title compound was prepared from 3,4-dihydro-1H-naphthalen-2-one (560 mg).
- LCMS: m/z 340 [M+H]+
- HPLC retention time: 4.57 min (analysis condition H)
- Compound T26-1
-
- Under nitrogen atmosphere, 3-bromo-8-[(4R,5R)-5-(tert-butyldimethylsilanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound T22-1, 300 mg, 0.47 mmol), sodium iodide (147 mg, 0.94 mmol) and copper iodide (9.40 mg, 0.047 mmol) were dissolved in dioxane (1.00 ml), added with (1R,2R)—N,N,N′,N′-tetramethyl-cyclohexane-1,2-diamine (15.4 μl, 0.094 mmol), and then stirred at 110° C. for 16 hr. The residues obtained from the reaction solution after concentration under reduced pressure were purified by silica gel column chromatography (methylene chloride/methanol) to obtain the title compound (220 mg, 70%).
- LCMS: m/z 662 [M+H]+
- HPLC retention time: 3.40 min (analysis condition D)
- Compound T26-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound T26-1 (17.0 mg, 90%).
- LCMS: m/z 508 [M+H]+
- HPLC retention time: 1.77 min (analysis condition D)
- Compound T27-1
-
- To the mixture of 6-bromo-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound E1, 410 mg, 1.44 mmol), tetrakistriphenylphosphine palladium (80 mg, 0.05 eq.) and sodium carbonate (614 mg, 4 eq.), toluene (3 mL) and water (1 ml) were added and then stirred at room temperature and at 90° C. for 3 hr. The mixture was extracted by adding water and diethyl ether, and the organic layer was washed with brine, and dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain 6-(2-fluoro-4-methoxy-phenyl)-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (white solid, 320 mg).
- Thus-obtained 6-(2-fluoro-4-methoxy-phenyl)-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (320 mg, 0.1 mmol) and 3-bromophenylhydrazine (0.29 g, 1.3 eq.) were dissolved in acetic acid (1 mL), and stirred under nitrogen atmosphere at 90° C. for 8 hr. After cooling, the reaction solution was added with ethyl acetate, washed with water, saturated aqueous solution of sodium hydrogen carbonate, and saturated brine, dried over magnesium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were dissolved in THF (3 mL) comprising 10% water, added with DDQ (227 mg, 3 eq.) at room temperature, and stirred at room temperature for 2 hr. To the reaction solution, the mixture solution of THF/diethyl ether (1: 1) was added, and the reaction solution was washed with 0.5 N aqueous solution of sodium hydroxide and saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (red solid, 75 mg).
- LCMS: m/z 494, 496 [M+H]+
- HPLC retention time: 3.10 min (analysis condition C)
- Compound T27-2
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound T27-1.
- LCMS: m/z 464, 466 [M+H]+
- HPLC retention time: 2.68 min (analysis condition C)
- Compound U5
-
- 2-Bromo-3-nitro-benzonitrile (Compound U1, 678 mg, 2.987 mmol) was dissolved in ethanol (20.9 mL) and water (8.96 mL), added with acetic acid (2.39 mL, 41.81 mmol) andiron (1.17 g, 20.91 mmol), and stirred at 60° C. for 18 hr. The reaction solution was poured into aqueous solution of sodium hydroxide (1 M), extracted with ethyl acetate, washed with water and saturated brine, dried over sodium sulfate, and then filtered. After concentration under reduced pressure, 3-amino-2-bromo-benzonitrile (Compound U2) was obtained as a crude product.
- The crude product obtained from the above was dissolved in 12 M aqueous solution of hydrochloric acid (4.00 mL), added slowly at 0° C. with aqueous solution in which sodium nitrite (247 mg, 3.584 mmol) is dissolved in water (3.58 mL), and then the mixture was stirred at 0° C. for 30 min. Under light-shielding conditions, aqueous solution in which tin chloride dihydrate (2.02 g, 8.961 mmol) is dissolved in 12 M aqueous solution of hydrochloric acid (4.00 mL) was slowly added to the reaction solution at 0° C., and then the mixture was stirred at 0° C. for 1 hr. The reaction solution was poured into 5 M aqueous solution of sodium hydroxide, extracted with ethyl acetate, washed with saturated brine, dried over sodium sulfate, and filtered. After concentration under reduced pressure, 2-bromo-3-hydrazino-benzonitrile (Compound U3) was obtained as a crude product. Under nitrogen atmosphere, the above crude product and 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 462 mg, 2.260 mmol) were added with TFA (6.78 mL) and stirred at 100° C. for 2 hr. After cooling, the reaction solution was poured into saturated aqueous solution of sodium bicarbonate, extracted with ethyl acetate, washed with water and saturated brine, dried over sodium sulfate, and filtered. After concentration under reduced pressure, 4-bromo-8-methoxy-6,6-dimethyl-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U4) was obtained as a crude product. The above crude product was dissolved in THF (10.0 mL) and water (1.00 mL), added with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (1.54 g, 6.780 mmol), and stirred at room temperature for 20 hr. The reaction solution was poured into 1 M aqueous solution of sodium hydroxide, extracted with cyclopentylmethyl ether, washed with 1 M aqueous solution of sodium hydroxide, water and saturated brine, dried over sodium sulfate, and filtered. The residues obtained after concentration under reduced pressure were washed with cyclopentylmethyl ether to obtain the title compound (460 mg, 52%).
- LCMS: m/z 395 [M+H]+
- HPLC retention time: 2.25 min (analysis condition D)
- Compound U6
-
- 4-Bromo-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U5, 325 mg, 0.822 mmol) was added with pyridine hydrochloride salt (3.80 g, 32.89 mmol) and stirred at 160° C. for 28 hr. The reaction solution was poured into water, extracted with ethyl acetate, washed with water, dried over sodium sulfate, and filtered. After concentration under reduced pressure, the title compound was obtained as a crude product.
- LCMS: m/z 381 [M+H]+
- HPLC retention time: 1.92 min (analysis condition D)
- Compound U7-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from the reaction between Compound U6 and (R)-(−)-2,2-dimethyl-1,3-dioxolane-4-methanol (354 mg, 87%).
- LCMS: m/z 495 [M+H]+
- HPLC retention time: 2.35 min (analysis condition D)
- Compound U7-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound U7-1.
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 2.40 min (analysis condition C)
- Compound U8-2
-
- Under the same conditions as the method for synthesizing Compound U7-1 and Compound U7-2, the title compound was prepared from the reaction between Compound U6 and (S)-(+)-2,2-dimethyl-1,3-dioxolane-4-methanol (4.5 mg, 29%).
- LCMS: m/z 455 [M+H]+
- HPLC retention time: 2.37 min (analysis condition C)
- Compound U8-3-1
-
- Under nitrogen atmosphere, 4-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U6, 20.0 mg, 40.37 μmol) was dissolved in DMA (0.35 mL), added with copper (I) cyanide (18.1 mg, 201.9 μmol), and stirred at 200° C. for 1 hr under irradiation with microwave. The reaction solution was poured into water, extracted with ethyl acetate, washed with water and saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound as a crude product.
- LCMS: m/z 442 [M+H]+
- HPLC retention time: 2.30 min (analysis condition D)
- Compound U8-3-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound U8-3-1 (9.5 mg, 59%).
- LCMS: m/z 402 [M+H]+
- HPLC retention time: 2.40 min (analysis condition D)
- Compound U8-4-1
-
- Under the same conditions as the method for synthesizing Compound U9, the title compound was prepared as a crude product from Compound U8-1 (9.5 mg, 59%).
- LCMS: m/z 433 [M+H]+
- HPLC retention time: 2.34 min (analysis condition A)
- Compound U8-4-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound U8-4-1 (crude product) (9.7 mg, 52%).
- LCMS: m/z 393 [M+H]+
- HPLC retention time: 1.69 min (analysis condition A)
- Compound U8-4-3
-
- Under nitrogen atmosphere, 8-((R)-2,3-dihydroxy-propoxy)-4-hydroxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U8-4-2, 8.0 mg, 20.39 μmol) was dissolved in methanol (2.0 mL) and chloroform (2.00 mL), added with trimethylsilyldiazomethane (diethyl ether solution, 2 M, 15.3 μL, 30.58 μmol) and diisopropylethylamine (0.05 mL), and then stirred at room temperature for 31 hr. The residues obtained from the reaction solution after concentration under reduced pressure were purified by silica gel column chromatography (methanol/dichloromethane) to obtain the title compound (5.1 mg, 62%).
- LCMS: m/z 407 [M+H]+
- HPLC retention time: 3.74 min (analysis condition A)
- Compound U8-5-1
-
- Under nitrogen atmosphere, 4-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U8-1, 25.0 mg, 50.47 μmol) was dissolved in DMF (0.75 mL), added with copper iodide (I) (48.0 mg, 252.3 μmol) and difluoro-fluorosulfonyl-acetic acid methyl ester (31.9 μL, 252.3 μmol), and then stirred at 100° C. for 2 days. The reaction solution was poured into hydrochloric acid (1 M), extracted with ethyl acetate, washed with water, saturated aqueous solution of sodium bicarbonate and saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound as a crude product.
- LCMS: m/z 485 [M+H]+
- HPLC retention time: 2.88 min (analysis condition A)
- Compound U8-5-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound U8-5-1 (4.0 mg, 30%).
- LCMS: m/z 445 [M+H]+
- HPLC retention time: 2.17 min (analysis condition A)
- Compound U8-6-1
-
- Under nitrogen atmosphere, 2-cyclopropyl-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane (13.2 mg, 78.73 82 mol) and potassium phosphate (212.27 mg, 212.0 μmol) were dissolved in water (0.20 mL), and the mixture was stirred at room temperature for 15 min. To the reaction solution, 4-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U8-1, 30.0 mg, 60.56 82 mol), palladium acetate (1.36 mg, 6.056 82 mol), and tricyclohexylphosphine (toluene solution, 20 wt %, 17.0 mg, 12.11 82 mol) were added and the mixture was stirred at 80° C. for 24 hr. The reaction solution was poured into hydrochloric acid (1 M), extracted with ethyl acetate, washed with saturated aqueous solution of sodium bicarbonate and saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (13.6 mg, 49%).
- LCMS: m/z 457 [M+H]+
- HPLC retention time: 2.38 min (analysis condition D)
- Compound U8-6-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound U8-6-1.
- LCMS: m/z 417 [M+H]+
- HPLC retention time: 2.42 min (analysis condition A)
- Compound U8-7-1
-
- Under nitrogen atmosphere, 4-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U8-1, 30.0 mg, 60.56 82 mol) and lithium chloride (7.70 mg, 181.7 82 mol) were dissolved in DMF (1.00 mL), added with tetramethyl tin (12.5 μL, 90.84 82 mol), tetrakistriphenylphosphine palladium (3.50 mg, 6.056 82 mol) and tricyclohexylphosphine (toluene solution, 20 wt %, 17.0 mg, 3.028 82 mol), and the mixture was stirred at 100° C. for 24 hr. The reaction solution was poured into hydrochloric acid (1 M), extracted with ethyl acetate, washed with water, saturated aqueous solution of sodium bicarbonate and saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain 8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-4,6,6-trimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile as a crude product (20.9 mg, 80%).
- Compound U8-7-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound U8-7-1.
- LCMS: m/z 391 [M+H]+
- HPLC retention time: 1.82 min (analysis condition A)
- Compound U8-8-1
-
- Under nitrogen atmosphere, 4-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U8-1, 30.0 mg, 60.56 82 mol), palladium acetate (1.36 mg, 6.056 82 mol), 1,1′-bis(diphenylphosphino)ferrocene (3.36 mg, 6.056 82 mol), imidazole (4.12 mg, 60.56 mol) and tert-potassium butoxy (10.2 mg, 90.84 mol) were dissolved in formamide (3.00 mL) and the mixture was stirred at 180° C. for 5 min under irradiation with microwave. The reaction solution was poured into water, extracted with ethyl acetate, washed with water and saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (methanol/dichloromethane) to obtain the target compound (7.6 mg, 27%).
- LCMS: m/z 460 [M+H]+
- HPLC retention time: 1.82 min (analysis condition A)
- Compound U8-8-2
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound U8-8-1.
- LCMS: m/z 421 [M+H]+
- HPLC retention time: 1.57 min (analysis condition A)
- Compound U8-8-3
-
- The title compound was obtained as a by-product of the synthesis of Compound U8-8-2.
- LCMS: m/z 420 [M+H]+
- HPLC retention time: 1.27 min (analysis condition A)
- Compound U9
-
- Under nitrogen atmosphere, 4-bromo-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U5, 10.0 mg, 25.30 82 mol), X-phos (1.07 mg, 2.530 82 mol), sodium hydroxide (4.36 mg, 75.90 82 mol) and Pd2dba3.CHCl3 (1.31 mg, 1.265 82 mol) were dissolved in dioxane (0.50 mL) and water (0.50 mL) and the mixture was stirred at 100° C. for 1 hr. The reaction solution was poured into hydrochloric acid (1 M), extracted with ethyl acetate, washed with saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) and washed with dichloromethane to obtain the title compound (5.4 mg, 64%).
- LCMS: m/z 333 [M+H]+
- HPLC retention time: 1.62 min (analysis condition D)
- Compound U10-1
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from the reaction between Compound U9 and (S)-(+)-2,2-dimethyl-1,3-dioxolane-4-methanol.
- LCMS: m/z 407 [M+H]+
- HPLC retention time: 2.06 min (analysis condition A)
- Compound U10-2
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from the reaction between Compound U9 and (R)-(−)-2,2-dimethyl-1,3-dioxolane-4-methanol.
- LCMS: m/z 407 [M+H]+
- HPLC retention time: 2.06 min (analysis condition A)
- Compound U11
-
- Under nitrogen atmosphere, 4-bromo-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound U5, 25.0 mg, 63.25 82 mol), copper iodide (2.41 mg, 12.65 82 mol), sodium azide (20.6 mg, 316.3 82 mol), (1S,2S)—N,N′-dimethyl-cyclohexane-1,2-diamine (2.70 mg, 18.98 82 mol), and sodium ascorbate (1.25 mg, 6.325 82 mol) were dissolved in ethanol (0.70 mL) and water (0.30 mL) and the mixture was stirred at 100° C. for 2 hr. The reaction solution was poured into aqueous solution of sodium hydroxide (1 M), extracted with ethyl acetate, washed with water and saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (methanol/dichloromethane) to obtain the title compound (5.6 mg, 27%).
- LCMS: m/z 332 [M+H]+
- HPLC retention time: 2.16 min (analysis condition A)
- Compound V2
-
- Under nitrogen atmosphere, the acetic acid (1 mL) suspension of 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 101.0 mg, 0.495 mmol) and (3-fluoro-phenyl)-hydrazine hydrochloric acid salt (Compound VI, 96.5 mg, 0.593 mmol) was stirred at ambient temperature of 95° for 3.75 hr. After cooling to room temperature, the reaction mixture was added with water (1 mL) and hexane/ethyl acetate (15: 1) (0.5 mL), and stirred at room temperature for 15 min. The solid was filtered, washed with hexane/ethyl acetate (15: 1), and then dried under reduced pressure to obtain the title compound (beige powder, 72.7 mg, 50%).
- 1H-NMR (270 MHz, CDCl3) δ: 7.92-7.82 (1H, b), 7.47 (1H, dd, 8.9 Hz, 5.6 Hz), 7.10-7.03 (2H, m), 6.95-6.81 (2H, m), 4.05 (2H, s), 3.86 (3H, s), 1.67 (6H, s)
- Compound V3
-
- Under nitrogen atmosphere, to the THF (1.8 mL)-water (0.18 mL) solution of 3-fluoro-8-methoxy-6,6-dimethyl-6,11-dihydro-5H-benzo[b]carbazole (Compound V2, 72.4 mg, 0.245 mmol), DDQ (122.4 mg, 0.539 mmol) was added and the mixture was stirred at room temperature for 5 hr. The reaction mixture was added with diethyl ether and 0.5 N aqueous solution of sodium hydroxide (2 mL), and the resulting mixture was extracted twice with diethyl ether. The organic layer was washed twice with 0.5 N aqueous solution of sodium hydroxide (2 mL) and subsequently twice with brine (2 mL), and dried over sodium sulfate. After concentration under reduced pressure, hexane/ethyl acetate (5: 1) and diethyl ether were added to the obtained crude product, and the solid was triturated. After removing the supernatant and drying under reduced pressure, the title compound was obtained (yellow solid, 57.0 mg, 75%).
- 1H-NMR (270 MHz, CDCl3) δ: 8.54-8.44 (1H, b), 8.43-8.33 (2H, m), 7.16-6.98 (4H, m), 3.93 (3H, s), 1.77 (6H, s)
- Compound V4
-
- Mixture of 3-fluoro-8-methoxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound V3, 56.6 mg, 0.183 mmol) and pyridinium chloride (0.65 g) was stirred at ambient temperature of 160° C. for 12 hr. The reaction mixture was cooled to room temperature, added with ethyl acetate and water, and the resulting mixture was extracted four times with ethyl acetate. The organic layer was washed with water three times, dried over sodium sulfate, and concentrated under reduced pressure. The obtained crude product was used for the next step without further purification (brown solid, 61.6 mg, 100%).
- 1H-NMR (270 MHz, CD3OD) δ: 8.20 (1H, dd, 8.9 Hz, 5.3 Hz), 8.15 (1H, d, 9.6 Hz), 7.17 (1H, dd, 9.6 Hz, 2.3 Hz), 7.12 (1H, d, 2.3 Hz), 7.05-6.95 (1H, m), 6.88 (1H, dd, 8.9 Hz, 2.3 Hz), 1.74 (6H, s)
- Compound V5-1
-
- Under nitrogen atmosphere, to the THF (1.5 mL) solution of 3-fluoro-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound V4, 0.183 mmol), (4S,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl[1,3]dioxolan-4-ol (75.9 mg, 0.275 mmol) and triphenylphosphine (72 mg, 0.275 mmol), toluene solution (125 μL, 0.275 mmol) of DEAD was added dropwise at room temperature. The reaction mixture was stirred at ambient temperature of 40° C. for 7 hr. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure, and the resulting crude product was purified by preparative TLC (Merck 60 F254, 0.5 mm) {solution for elution: hexane/ethyl acetate (3: 1)} to obtain the title compound (pale orange amorphous, 54.1 mg, 53.4%).
- 1H-NMR (270 MHz, CDCl3) δ: 8.54-8.45 (1H, b), 8.42-8.33 (2H, m), 7.17-6.99 (4H, m), 4.41-4.27 (2H, m), 4.25-4.15 (1H, m), 4.06-3.96 (1H, m), 3.96-3.88 (1H, m), 3.83-3.74 (1H, m), 1.76 (3H, s), 1.75 (3H, s), 1.48 (3H, s), 1.47 (3H, s), 0.87 (9H, s), 0.092 (6H, s)
- Compound V5-2
-
- Under nitrogen atmosphere, to the THF (0.3 mL)-MeOH (0.1 mL) solution of 8-[(1R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-3-fluoro-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound V5-1, 52.8 mg, 0.0954 mmol), 0.5 M aqueous solution of sulfuric acid (0.19 mL, 0.0954 mmol) was added at room temperature. The reaction mixture was stirred at ambient temperature of 55° C. for 4 hr, cooled to room temperature, and then added with diethyl ether, sodium hydrogen carbonate (20 mg) and water in order. The mixture was extracted twice with diethyl ether and subsequently twice with ethyl acetate, and the organic layer was washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The resulting crude product was washed with dichloromethane, and dried under reduced pressure to obtain the title compound (white powder, 29.9 mg, 78%).
- 1H-NMR (270 MHz, CD3OD) δ: 8.24 (1H, d, 8.9 Hz), 8.19 (1H, dd, 8.6 Hz, 5.3 Hz), 7.30 (1H, d, 2.3 Hz), 7.18 (1H, dd, 9.2 Hz, 2.3 Hz), 7.09 (1H, dd, 8.9 Hz, 2.3 Hz), 7.06-6.96 (1H, m), 4.32-4.22 (1H, m), 4.21-4.12 (1H, m), 4.11-4.02 (1H, m), 3.84-3.75 (1H, m), 3.74-3.61 (2H, m), 1.77 (6H, s)
- LCMS: m/z 400 [M+H]+
- HPLC retention time: 4.02 min (analysis condition H)
- Compound W2
-
- To the toluene suspension of sodium t-butoxide (700 mg, 2.5 eq.), 8-methoxy-3,4-dihydro-1H-naphthalen-2-one (Compound W1, 500 mg, 2.9 mmol) was added dropwise at 0° C. After 15 minutes, the solution turned into blackish green color. The mixture solution was added dropwise with methyl iodide (1.03 g, 2.5 eq.) and stirred at 15° C. overnight. Brown solid precipitated. The reaction solution was added to saturated aqueous solution of ammonium chloride/diethyl ether under stirring and cooling. After that, the solution was extracted with diethyl ether, and dried over sodium sulfate. After removing the solvent under reduced pressure, the resulting residues were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain 8-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (350 mg).
- Thus-obtained 8-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (250 mg, 1.23 mmol) and 3-cyanophenylhydrazine (0.2 g, 1.2 eq.) were dissolved in trifluoroacetic acid (1 mL), and stirred at 120° C. for 1 hr under irradiation with microwave. After cooling, the reaction solution was added with ethyl acetate, washed with water, saturated aqueous solution of sodium hydrogen carbonate, and saturated brine, dried over magnesium sulfate, and filtered. The residues obtained after concentration under reduced pressure were dissolved in THF (3 mL) comprising 10% water and added at room temperature with DDQ (227 mg, 3 eq.). The mixture was then stirred at room temperature for 2 hr. The reaction solution was added with mixture solution of THF/diethyl ether (1: 1), washed with 0.5 N aqueous solution of sodium hydroxide and saturated brine, dried over sodium sulfate, and then filtered. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain 7-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (brown solid, 54 mg).
- LCMS: m/z 317 [M+H]+
- HPLC retention time: 1.00 min (analysis condition I)
- Compound W3
-
- Under the same conditions as Compound A6, the title compound was prepared from Compound W2.
- LCMS: m/z 316 [M+H]+
- HPLC retention time: 0.93 min (analysis condition I)
- Compound W4-1
-
- Under nitrogen atmosphere, 7-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbzaole-3-carbonitrile (Compound W3, 15 mg, 0.05 mmol) and triphenylphosphine (40 mg, 3 eq.) were added with THF (1 ml), further added dropwise with ((R)-2,2-dimethyl-[1,3]dioxolan-4-yl)-methanol (20 mg, 3 eq.) and 2.19 N toluene solution of diethyl azodicarboxylate (68 μL, 3 eq.), and the mixture was stirred at 50° C. for 2 hr. After cooling, the reaction solution was added with ethyl acetate, washed with brine, dried over sodium sulfate, and filtered. The residues obtained after concentration under reduced pressure were purified by preparative TLC (ethyl acetate/dichloromethane), and the resulting solid was washed with dichloromethane to obtain the target compound (brown powder, 5 mg).
- LCMS: m/z 417 [M+H]+
- HPLC retention time: 1.04 min (analysis condition I)
- Compound W4-2
-
- Under the same conditions as Compound S7-2, the title compound was prepared from Compound W4-1.
- LCMS: m/z 377 [M+H]+
- HPLC retention time: 0.88 min (analysis condition I)
- Compound X1
-
- 7-Methoxy-3,4-dihydro-1H-naphthalen-2-one (Compound A1, 100 mg, 0.568 mmol) was dissolved in toluene (4 mL), added with NaH (60% in oil, 68 mg, 3 eq.), and stirred at room temperature for 10 min. The mixture solution was added with bis-(2-iodo-ethyl)-p-toluenesulfonamide (172 mg, 0.568 mmol), and stirred at 70° C. for 2 hr under nitrogen stream. After cooling, the reaction solution was added to saturated aqueous solution of ammonium chloride, extracted with ethyl acetate, washed with saturated brine, and then dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane: ethyl acetate/3: 1) to obtain the title compound (colorless oily substance, 62 mg, 33%).
- LCMS: m/z 400 [M+H]+
- HPLC retention time: 2.02 min (analysis condition B)
- Compound X2
-
- 1,1-Spiro-4-piperidine-N-paratoluenesulfonyl-7-methoxy-3,4-dihydro-1H-naphthalen-2-one (Compound X1, 400 mg, 1.0 mmol) and phenylhydrazine (217 mg, 1.5 eq.) were dissolved in acetic acid (6 mL), and the mixture was stirred at 120° C. for 4 hr under nitrogen atmosphere. After cooling, the reaction solution was added to water, extracted with ethyl acetate, washed with saturated brine, and then dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane:ethyl acetate/4: 1) to obtain the title compound (brown solid, 185 mg, 43%).
- LCMS: m/z 473 [M+H]+
- HPLC retention time: 7.23 min (analysis condition B)
- Compound X3
-
- 6,6-Spiro-4-piperidine-N-paratoluenesulfonyl-8-methoxy-5,6-dihydro-5H-benzo[b]carbazole (Compound X2,400 mg, 0.848 mmol) and DDQ (770 mg, 4 eq.) were dissolved in THF (10 mL) and water (2 mL), and then the mixture was stirred at 50° C. for 5 hr. After cooling, the reaction solution was added to saturated aqueous solution of sodium hydrogen carbonate, extracted with ethyl acetate, washed with saturated brine, and then dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (hexane: ethyl acetate/3: 1) to give a solid, which was then washed with ethyl ether to obtain the title compound (yellow solid, 86 mg, 21%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 11.9 (1H, s), 8.22 (2H, m), 7.75 (2H, d), 7.60 (4H, m) 7.30 (2H, m), 7.11 (1H, d), 3.81 (2H, m), 3.68 (3H, s), 3.62 (2H, m), 2.49 (3H, s), 2.21 (2H, m), 2.10 (2H, m),
- LCMS: m/z 487 [M+H]+
- HPLC retention time: 6.05 min (analysis condition B)
- Compound X4
-
- Mixture of 6,6-spiro-4-piperidine-N-paratoluenesulfonyl-8-methoxy-5,6-dihydro-benzo[b]carbazol-11-one (Compound X3, 35 mg, 0.072 mmol) and pyridine hydrochloride salt (800 mg) was stirred in a sealing tube at 160° C. for 10 hr. After cooling, the reaction solution was added to water, extracted with ethyl acetate, washed with saturated brine, and then dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (dichloromethane:methanol/4: 1) to obtain the title compound (yellow solid, 30 mg, 98%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 8.20 (1H, m), 8.10 (1H, m), 7.53 (1H, m), 7.25 (3H, m), 6.80 (1H, m), 3.60 (2H, m), 3.45 (2H, m), 2.52 (2H, m), 2.05 (2H, m).
- LCMS: m/z 319 [M+H]+
- HPLC retention time: 2.86 min (analysis condition B)
- Compound X5
-
- 6,6-Spiro-4-piperidine-8-hydroxy-5,6-dihydro-benzo[b]carbazol-11-one (Compound X4, 30 mg, 0.094 mmol), diethylaminoethanol (22 mg, 2 eq.), triphenylphosphine (50 mg, 2 eq.) and DIAD (39 mg, 2 eq.) were dissolved in THF (4 mL) and the mixture was stirred at room temperature for 4 hr. The reaction solution was added to water, extracted with ethyl acetate, washed with saturated brine, and then dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (dichloromethane:methanol/4: 1) to obtain the title compound (yellow oily substance, 6.8 mg, 17%).
- LCMS: m/z 418 [M+H]+
- HPLC retention time: 2.75 min (analysis condition B)
- Compound Y2
-
- Under nitrogen atmosphere, the acetic acid (1 mL) suspension of 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 92.3 mg, 0.452 mmol) and (3,4-dichlorophenyl)hydrazine hydrochloric acid salt (Compound Y1, 96.5 mg, 0.452 mmol) was stirred at ambient temperature of 90° C. for 3.5 hr. After cooling to room temperature, the reaction mixture was added with diethyl ether and water, and the resulting mixture was extracted twice with diethyl ether. The organic layer was washed three times with water, dried over sodium sulfate, and concentrated under reduced pressure. The resulting crude product was purified by flash column chromatography {Merck Kieselgel60, solution for elution: hexane/ethyl acetate (4: 1)} to obtain the title compound (pale yellow solid, 62.1 mg, 40%).
- 1H-NMR (270 MHz, CDCl3) δ: 7.92-7.84 (1H, b), 7.62 (1H, s), 7.46 (1H, s), 7.05 (1H, d, 2.6), 6.84 (1H, dd, 8.6 Hz, 2.6 Hz), 4.01 (2H, s), 3.86 (3H, s), 1.67 (6H, s)
- Compound Y3
-
- Under nitrogen atmosphere, to the 1,4-dioxane (1.7 mL)-water (0.1 mL) solution of 2,3-dichloro-8-methoxy-6,6-dimethyl-6,11-dihydro-5H-benzo[b]carbazole (Compound Y2, 61.0 mg, 0.176 mmol), DDQ (120 mg, 0.529 mmol) was added and the mixture was stirred at room temperature for 16 hr and 15 min. The reaction mixture was purified by flash column chromatography {Merck Kieselgel60, solution for elution: hexane/ethyl acetate (2: 1)} to obtain the title compound (pale orange solid, 16.7 mg, 26%).
- 1H-NMR (270 MHz, CDCl3) δ: 8.55 (1H, s), 8.42-8.36 (1H, b), 8.39 (1H, d, 8.6 Hz), 7.54 (1H, s), 7.08 (1H, d, 2.3 Hz), 7.03 (1H, dd, 8.6 Hz, 2.3 Hz), 3.93 (3H, s), 1.76 (6H, s)
- Compound Y4
-
- Mixture of 2,3-dichloro-8-methoxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound Y3, 16.5 mg, 0.0457 mmol) and pyridinium chloride (0.2 g) was stirred at ambient temperature of 160° C. for 7 hr. The reaction mixture was cooled to room temperature and added with ethyl acetate and water. The mixture was extracted three times with ethyl acetate. The organic layer was washed twice with water, dried over sodium sulfate, and concentrated under reduced pressure. The resulting crude product was used for the next step without further purification (brown solid, 14.8 mg, 94%).
- 1H-NMR (270 MHz, CD3OD) δ: 8.34 (1H, s), 8.14 (1H, d, 8.6 Hz), 7.61 (1H, s), 7.10 (1H, d, 2.3 Hz), 6.89 (1H, dd, 8.6 Hz, 2.3 Hz), 1.75 (1H, s)
- Compound Y5-1
-
- Under nitrogen atmosphere, to the THF (0.3 mL) solution of 2,3-dichloro-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound Y4, 12.9 mg, 0.0373 mmol), (4S,5R)-5-(tert-butyldimethylsilanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-ol (15.5 mg, 0.0559 mmol) and triphenylphosphine (14.7 mg, 0.0559 mmol), toluene solution (25.4 μL, 0.0559 mmol) of DEAD was added dropwise at room temperature. The reaction mixture was stirred at ambient temperature of 40° C. for 4 hr. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure, and the resulting crude product was purified by preparative TLC (Merck 60 F254, 0.5 mm) {solution for elution: hexane/ethyl acetate (3: 1)} to obtain the title compound (white solid, 15.1 mg, 67%).
- 1H-NMR (270 MHz, CDCl3) δ: 8.55 (1H, s), 8.44-8.37 (1H, b), 8.37 (1H, d, 8.6 Hz), 7.54 (1H, s), 7.15 (1H, d, 2.6 Hz), 7.03 (1H, dd, 8.6 Hz, 2.6 Hz), 4.41-4.26 (2H, m), 4.25-4.15 (1H, m), 4.06-3.86 (2H, m), 3.83-3.73 (1H, m), 1.76 (3H, s), 1.75 (3H, s), 1.48 (3H, s), 1.47 (3H, s), 0.90 (9H, s), 0.092 (6H, s)
- Compound Y5-2
-
- Under nitrogen atmosphere, to the THF (0.2 mL)-MeOH (0.1 mL) solution of 8-[(1R,5R)-5-(tert-butyldimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-2,3-dichloro-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (Compound Y5-1, 14.6 mg, 0.0242 mmol), 0.5 M aqueous solution of sulfuric acid (96.6 μL, 0.0483 mmol) was added at room temperature. The reaction mixture was stirred at ambient temperature of 55° C. for 3 hr, cooled to room temperature, and then added with diethyl ether and sodium hydrogen carbonate (10 mg) in order. The mixture was extracted twice with diethyl ether, and the organic layer was washed with water and brine, dried over sodium sulfate, and concentrated under reduced pressure. The resulting crude product was washed with dichloromethane, and dried under reduced pressure to obtain the title compound (white solid, 8.3 mg, 76%).
- 1H-NMR (270 MHz, CD3OD) δ: 8.35 (1H, s), 8.24 (1H, d, 8.9 Hz), 7.62 (1H, s), 7.31 (1H, d, 2.3 Hz), 7.10 (1H, dd, 8.9 Hz, 2.3 Hz), 4.31-4.23 (1H, m), 4.12-4.12 (1H, m), 4.11-4.02 (1H, m), 3.84-3.74 (1H, m), 3.73-3.61 (1H, m), 1.78 (6H, s)
- LCMS: m/z 450 [M+H]+
- HPLC retention time: 4.92 min (analysis condition H)
- Compound Z3
-
- 2-(2-Bromo-5-methoxyphenyl)-2-methyl-propinoic acid (1.5 g, 5.5 mmol) was dissolved in methylene chloride (15 mL), added with oxalyl chloride (1.5 mL) and dimethylformamide (2 micro liter) at room temperature, and the mixture was stirred at room temperature for 30 min. After removing the solvent, the residues were dissolved in toluene, added at room temperature with 2-[(triphenyl-5-phosphanyl)-methyl]-benzenethiol hydrobromide (2.56 g, 5.5 mmol) and triethylamine (2.27 mL), and then the mixture was refluxed under heating for 30 min. Thereafter, the mixture was cooled to 0° C., added with lithium hexamethyldisilazide (1 M tetrahydrofuran solution, 5.5 mL), and refluxed under heating for 24 hr. The reaction mixture was extracted with ethyl acetate and washed with water. The organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (0.55 g, 28%).
- 1H-NMR (270 MHz, CDCl3) δ: 6.61 (1H, s), 3.37 (3H, s), 1.83 (6H, s)
- Compound Z4
-
- 2-[1-(2-Bromo-5-methoxy-phenyl)-1-methylethyl]-benzo[b]thiophene (Compound Z3, 40 mg, 0.11 mmol) was dissolved in tetrahydrofuran (0.5 mL), cooled to −78° C., added with n-butyl lithium (1.57 M, hexane solution, 0.07 mL), and the mixture was stirred for 10 min. The reaction mixture was added with dry ice and then maintained for 1 hr. After that, the mixture was added with 0.5 N hydrochloric acid, extracted with ethyl acetate and washed with water. The organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (22 mg, 55%).
- 1H-NMR (270 MHz, CDCl3) δ: 7.46 (1H, d), 7.44 (1H, d), 6.92 (s, 1H), 6.70 (d, 1H), 3.84 (s, 3H), 1.89 (6H, s)
- Compound Z5
-
- To 2-(1-benzo[b]thiophen-2-yl-1-methylethyl)-4-methoxy-benzoic acid (Compound Z4, 68 mg, 0.22 mmol), polyphosphoric acid (3.5 g) was added, and the mixture was stirred for 1 hr at 100° C. under heating. The mixture was added with water, extracted with ethyl acetate and washed with water. The organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (41 mg, 63%).
- LCMS: m/z 309 [M+H]+
- HPLC retention time: 2.89 min (analysis condition C)
- Compound Z6
-
- Under the same conditions as Compound A6, the title compound was prepared from Compound Z5.
- LCMS: m/z 295 [M+H]+
- HPLC retention time: 2.91 min (analysis condition F)
- Compound Z7
-
- According to the same method as the method for synthesizing Compound A7-17, the title compound was prepared from Compound Z6.
- LCMS: m/z 394 [M+H]+
- HPLC retention time: 5.06 min (analysis condition F)
- Compound Z9
-
- To 4-bromo-benzene-1,3-diol (Compound Z8, 20 g, 105.8 mmol) and 3,4-dihydro-2H-pyran (38.6 mL), pyridium paratoluenesulfonate (266 mg) was added, and the mixture was stirred at 50° C. for 1 hr. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (31.82 mg, 84%).
- LCMS: m/z 358 [M+H]+
- HPLC retention time: 3.15 min (analysis condition C)
- Compound Z10
-
- To 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 10 g), 2-bromo-1,3-dihydroxytetrahydropyranbenzene (Compound Z9, 20.98 g), sodium t-butoxide (5.88 g), palladium acetate (550 mg) and tri-t-butylphosphonium tetrafluoroborate (710 mg), toluene (40 mL) was added and the mixture was stirred and heated at 70° C. under nitrogen atmosphere for 6 hr. After cooling, the reaction mixture was added with methanol (38 mL) and trifluoroacetic acid (14.54 mL) at room temperature, and then stirred at room temperature overnight. To the resulting residues, methylene chloride and saturated dipotassium hydrogen phosphate were added and the organic layer was washed with saturated brine. Thereafter, the organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (5.53 g, 36%).
- LCMS: m/z 312 [M+H]+
- HPLC retention time: 2.39 min (analysis condition F)
- Compound Z11
-
- 3-(2,4-Dihydroxy-phenyl)-7-methoxy-1,1-dimethyl-3,4-dihydro-TH-naphthalen-2-one (Compound Z10, 5.53 g) was dissolved in methylene chloride (40 mL), and added with trifluoromethanesulfonic anhydride (2.98 mL) at room temperature. After cooling to 5° C., diisopropylethylamine (9.25 mL) and trifluoromethanesulfonic anhydride (4.47 mL) were added thereto. To the reaction mixture, methylene chloride and saturated dipotassium hydrogen phosphate were added and the organic layer was washed with saturated brine. Thereafter, the organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (4.82 g, 64%).
- LCMS: m/z 427 [M+H]+
- HPLC retention time: 8.95 min (analysis condition H)
- Compound Z12
-
- Trifluoromethanesulfonic acid 8-methoxy-6,6-dimethyl-6,11-dihydro-benzo[b]naphth[2,3-d]furan-3-yl ester (Compound Z11, 4.82 g) was dissolved in acetonitrile (48 mL) and water (24 mL), added with sodium chlorite (2.55 g) and N-hydroxyphthalimide (369 mg), and then the mixture was stirred at 40° C. for 1 hr. The reaction mixture was added with methylene chloride and the organic layer was washed with saturated brine. Thereafter, the organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (2.80 g, 56%).
- LCMS: m/z 441 [M+H]+
- HPLC retention time: 8.02 min (analysis condition H)
- Compound Z13
-
- Under the same conditions as Compound A6, the title compound was prepared as a crude product from Compound Z12.
- Compound Z14
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was obtained as a crude product from Compound Z13 and [5-(tert-butyldimethylsilanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-methanol (Compound T22-0).
- Compound Z15
-
- Trifluoro-methanesulfonic acid 8-[(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6 dimethyl-11-oxo-6,11-dihydro-benzo[b]naphth[2,3-d]furan-3-yl ester (Compound Z14, 24 mg) was dissolved in DMF (0.5 mL), added with zinc (II) cyanide (8.2 mg) and palladium tetrakistriphenylphosphine (2.0 mg), and the mixture was stirred under heating at 200° C. for 20 min with microwave irradiation. To the reaction mixture, ethyl acetate was added and the organic layer was washed with saturated brine. Thereafter, the organic layer was dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (15 mg).
- LCMS: m/z 562 [M+H]+
- HPLC retention time: 4.14 min (analysis condition F)
- Compound Z16
-
- Under the same conditions as Compound S7-2, the title compound was prepared from Compound Z15.
- LCMS: m/z 408 [M+H]+
- HPLC retention time: 4.51 min (analysis condition H)
- Compound K7-5
-
- Under the same conditions as the method for synthesizing Compound B2-22-1, the title compound was prepared from Compound K6 and 4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester.
- LCMS: m/z 498 [M+H]+
- HPLC retention time: 4.24 min (analysis condition W)
- Compound K7-6
-
- Under the same conditions as the method for synthesizing Compound A8-1, the title compound was prepared from K7-5.
- LCMS: m/z 398 [M+H]+
- HPLC retention time: 2.57 min (analysis condition W)
- Compound K8-1
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound K7-6 and cyclobutanone.
- LCMS: m/z 452 [M+H]+
- HPLC retention time: 2.72 min (analysis condition W)
- Compound K8-2
-
- Under the same conditions as the method for synthesizing Compound B3-13-1, the title compound was prepared from Compound K8-1.
- LCMS: m/z 454 [M+H]+
- HPLC retention time: 2.76 min (analysis condition W)
- Compound K9-5
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound K8-2.
- LCMS: m/z 440 [M+H]+
- HPLC retention time: 2.57 min (analysis condition W)
- Compound K10-8
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound K9-5 and isopropyl iodide.
- LCMS: m/z 482 [M+H]+
- HPLC retention time: 1.74 min (analysis condition S)
- The compounds described in the following Tables 2-3 were synthesized from the intermediates of Compound K or Compound L by alkylation of hydroxyl group according to Mitsunobu reaction used for preparing Compound A7-1 or the method used for the synthesis of Compound A7-17 (described in the Table).
-
TABLE 2 Exam- HPLC Reten- ple Comp. Condi- tion No. No. Structure Compound Name tion Time m/z Method 750 K10-9 6,6-Dimethyl-8-(4- morpholin-4-yl- piperidin-1-yl)-11- oxo-9-(tetrahydro- pyran-4-yloxy)- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 1.85 555 A7-1 751 K10-10 9-(2-Methoxy- ethoxy)-5-(2- methoxy-ethyl)- 6,6-dimethyl-8-(4- morpholin-4-yl- piperidin-1-yl)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile S 1.50 587 A7-17 752 K10-11 9-(2-Methoxy- ethoxy)-6,6- dimethyl-8-(4- morpholin-4-yl- piperidin-1-yl)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile S 1.37 529 A7-17 753 K10-12 9-Ethoxy-6,6- dimethyl-8-(4- morpholin-4-yl- piperidin-1-yl)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile U 1.95 499 A7-17 754 K10-13 6,6-Dimethyl-8-(4- morpholin-4-yl- piperidin-1-yl)-11- oxo-9-(tetrahydro- furan-3-yloxy)- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.40 541 A7-1 755 K10-14 9-(2-Diethylamino- ethoxy)-6,6- dimethyl-8-(4- morpholin-4-yl- piperidin-1-yl)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile S 1.13 570 A7-17 756 K10-15 8-(4-Cyclobutyl- piperazin-1-yl)-9- (2-methoxy- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.45 499 A7-17 757 K10-16 8-(4-Cyclobutyl- piperazin-1-yl)- 5,6,6-trimethyl-11- oxo-9-(tetrahydro- furan-3-yloxy)- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.98 525 A7-1 758 K10-17 8-(4-Cyclobutyl- piperazin-1-yl)-9- (1-ethyl-propoxy)- 5,6,6-trimethyl-11- oxo-6,11-dihydro- 5H-benzo[b]carbazole- 3-carbonitrile S 2.43 525 A7-1 759 K10-18 8-(4-Cyclobutyl- piperazin-1-yl)-6,6- dimethyl-11-oxo-9- (tetrahydro-furan- 3-yloxy)-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.92 511 A7-1 760 K10-19 8-(4-Cyclobutyl- piperazin-1-yl)-6,6- dimethyl-11-oxo-9- (tetrahydro-pyran- 4-yloxy)-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 1.97 525 A7-1 761 K10-20 8-(4-Cyclobutyl- piperazin-1-yl)-9- (1-ethyl-propoxy)- 6,6-dimethyl-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile S 1.82 511 A7-1 762 L10-3 9-Isopropoxy-8-(2- methoxy-ethoxy)- 5-(2-methoxy- ethyl)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 2.68 477 A7-17 763 L10-4 9-Isopropoxy-8-(2- methoxy-ethoxy)- 6,6-dimethyl-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile S 2.68 419 A7-17 -
TABLE 3 Exam- HPLC Reten- ple Comp. Condi- tion No. No. Structure Compound Name tion Time m/z Method 764 L10-5 8-(1-Cyclobutyl- piperidin-4- ylmethoxy)-9- isopropoxy-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.65 512 A7-1 765 L10-6 9-Isopropoxy-8-(1- isopropyl-piperidin- 4-ylmethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.62 500 A7-1 766 L10-7 8-(1-Cyclobutyl- piperidin-3-yloxy)- 9-isopropoxy-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.65 498 A7-1 767 L10-8 9-Isopropoxy-8-(1- isopropyl-piperidin- 3-yloxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.59 486 A7-1 768 L10-9 8-(2-Diethylamino- ethoxy)-9- isopropoxy-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.07 460 A7-17 769 L10-10 9-Isopropoxy-6,6- dimethyl-11-oxo-8- (pyridin-4-yloxy)- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.07 438 A7-17 770 L10-11 9-Isopropoxy-6,6- dimethyl-11-oxo-8- vinyloxy-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.77 387 A7-17 771 L10-12 9-Isopropoxy-6,6- dimethyl-11-oxo-8- (tetrahydro-furan- 3-yloxy)-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 2.45 431 A7-1 - The compounds described in the following Table 4 were synthesized from the intermediates of Compound B according to the method described in the Table.
-
TABLE 4 Exam- HPLC Reten- ple Comp. Condi- tion No. No. Structure Compound Name tion Time m/z Method 772 B3-39 8-((3R,5S)-4- Cyclobutyl-3,5- dimethyl-piperazin-1- yl)-6,6-dimethyl-11- oxo-6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile U 1.90 453 B3-32 773 B3-40 8-((3R,5S)-4-Ethyl- 3,5-dimethyl-piperazin- 1-yl)-6,6-dimethyl-11- oxo-6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile U 1.82 427 B3-32 774 B2-30 6,6-Dimethyl-8-[4-(1- methyl-piperidin-4-yl)- piperazin-1-yl]-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile U 1.58 468 B2-1 775 B3-41 8-(4- Cyclobutanecarbonyl- piperazin-1-yl)-6,6- dimethyl-11-oxo-6,11- dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.35 453 A9-10 776 B3-42 8-(4- Cyclopropanecarbonyl- piperazin-1-yl)-6,6- dimethyl-11-oxo-6,11- dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.22 439 A9-10 - Compound E6-4
-
- Under the same conditions as the method for synthesizing Compound E3-2, the title compound was prepared from Compound E5-1.
- LCMS: m/z 331 [M+H]+
- HPLC retention time: 3.42 min (analysis condition W)
- Compound E7
-
- Under the same conditions as the method for synthesizing Compound B1, the title compound was prepared from Compound E6-4.
- LCMS: m/z 463 [M+H]+
- HPLC retention time: 4.39 min (analysis condition W)
- Compound E8-1
-
- The title compound was prepared from Compound E7 and piperazine in the same manner as the method for synthesizing Compound B2-1.
- LCMS: m/z 399 [M+H]+
- HPLC retention time: 1.88 min (analysis condition U)
- Compound E8-2
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound E7 and 2-(S)-methylpiperazine.
- LCMS: m/z 413 [M+H]+
- HPLC retention time: 2.76 min (analysis condition W)
- Compound E8-3
-
- Under the same conditions as the method for synthesizing Compound B2-1, the title compound was prepared from Compound E7 and cis-2,6-dimethylpiperazine.
- LCMS: m/z 427 [M+H]+
- HPLC retention time: 2.00 min (analysis condition U)
- Compound E8-4
- Compound 7 was converted in the same manner as Compound B2-22-1 and Compound 2, and subsequently subjected to reductive amination in the same manner as Compound B3-32 to obtain the title compound.
- LCMS: m/z 450 [M+H]+
- HPLC retention time: 2.12 min (analysis condition U)
- Compound E9-1
-
- Under the same conditions as the method for synthesizing Compound B3-32, the title compound was prepared from Compound E8-2 and cyclobutanone.
- LCMS: m/z 467 [M+H]+
- HPLC retention time: 2.90 min (analysis condition W)
- The compounds described in the following Table 5 were prepared by acylation from Compound E8-1 in the same manner as the method for synthesizing Compound A9-10.
-
TABLE 5 Reten- Example Comp. HPLC tion m/z No. No. Structure Compound Name Condition Time 784 E9-2 8-(4- Cyclopropanecarbonyl- piperazin-1-yl)-9-ethyl- 6,6-dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.58 467 785 E9-3 8-(4- Cyclobutanecarbonyl- piperazin-1-yl)-9-ethyl- 6,6-dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.74 481 786 E9-4 8-[4-(2- Dimethylamino-acetyl)- piperazin-1-yl]-9-ethyl- 6,6-dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile U 1.98 484 787 E9-5 9-Ethyl-8-(4-isobutyryl- piperazin-1-yl)-6,6- dimethyl-11-oxo-6,11- dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.67 469 788 E9-6 8-(4-Acetyl-piperazin- 1-yl)-9-ethyl-6,6- dimethyl-11-oxo-6,11- dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.35 441 789 E9-7 8-(4- Cyclopentanecarbonyl- piperazin-1-yl)-9-ethyl- 6,6-dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile S 2.87 495 790 E9-8 8-(4- Cyclohexanecarbonyl- piperazin-1-yl)-9-ethyl- 6,6-dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile S 2.97 509 - Compound E9-9
-
- 9-Ethyl-6,6-dimethyl-11-oxo-8-piperazin-1-yl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (45 mg) and cyclohexanone (25 mg) were suspended in chloroform (2 ml), added with trimethylsilyl cyanide (30 mg) and zinc iodide (5 mg), and the mixture was stirred at 60° C. for 17 hrs. The reaction mixture was diluted with ethyl acetate (20 ml) and the organic layer was washed with 1000 brine solution and concentrated under reduced pressure. The resulting residues were purified by silica gel column (dichloromethane/methanol (=99/1)) to obtain the title compound (12 mg, yield 30%).
- LCMS: m/z 506 [M+H]+
- HPLC retention time: 3.00 min (analysis condition U)
- The compounds described in the following Table 6 were synthesized from Compound E8-1 or Compound PR10-1 in the same manner as the method for Compound E9-9.
-
TABLE 6 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 792 E9-10 8-[4-(1-Cyano- cyclobutyl)-piperazin- 1-yl]-9-ethyl-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.88 478 793 PR11-20 8-(4-Cyano-4- hydroxy-piperazin-1- yl)-9-ethyl-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile Y 3.05 439 794 PR11-21 8-(4-Cyano-4- morpholine-4-yl- piperidine-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H-benzo[b]carbazole- 3-carbonitrile Y 3.35 508 - With respect to the compounds described in the following Table 7, Compound F2 was subjected to amination in the same manner as Compound B2-1. Subsequently, the preparation was carried out by reductive amination in the same manner as the method for Compound B3-32.
-
TABLE 7 Exam- HPLC Reten- ple Comp. Condi- tion No. No. Structure Compound Name tion Time m/z Method 795 F3-12 9-Bromo-8-((3R,5S)- 3,5-dimethyl- piperazin-1-yl)-6,6- dimethyl-11-oxo-6,11- dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.05 477, 479 B2-1 796 F4-11 9-Bromo-8-((3R,5S)- 4-cyclobutyl-3,5- dimethyl-piperazin-1- yl)-6,6-dimethyl-11- oxo-6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile U 2.28 531, 533 B3-32 - Compound PR1
-
- 2-(4-Bromophenyl)-2-methylpropanoic acid (30 g), PPh3 (5.0 g), potassium vinyltrifluoroborate (24.8 g), potassium carbonate (51.2 g), and palladium acetate (1.43 g) were dissolved in 1-propanol (198 ml) and distilled water (99 ml). After deaeration, the mixture was stirred under reflux for 6 hrs under nitrogen atmosphere. Insoluble matters were removed by filtration and washed with 1-propanol (210 ml). The filtrate was then concentrated under reduced pressure. Concentrated residues were partitioned between CPME (300 ml) and distilled water (150 ml, comprising 4.17 ml of ethylenediamine). The organic layer was removed and the aqueous layer was adjusted to pH 5 by using 2 N hydrochloric acid. The aqueous layer was extracted with a mixture of isopropyl acetate (240 ml) and heptane (240 ml). The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. Ethanol (300 ml) was added thereto for suspending and washing the resultant. The solid was removed by Celite filtration, and the filtrate was concentrated under reduced pressure to obtain the title compound (21.7 g, 93%).
- 1H-NMR (400 MHz CDCl3) δ ppm 7.49-7.34 (4H, m), 6.69 (1H, dd, J=17.6, 11.0 Hz), 5.72 (1H, d, J=17.6 Hz), 5.23 (1H, d, 11.0 Hz), 1.59 (s, 6H)
- HPLC retention time: 2.05 min (analysis condition S)
- Compound PR2
-
- 2-(4-Vinylphenyl)-2-methylpropanoic acid (58 g) was dissolved in ethanol, and then stirred for 3 hrs under atmospheric hydrogen pressure in the presence of 10% palladium carbon (5.8 g). The catalyst was removed by filtration, and the filtrate was concentrated to obtain a crude product, which was then suspended and washed with hexane to give the title compound (56.5 g, 94.8%).
- 1H-NMR (270 MHz DMSO-d6) δ ppm 12.28 (1H, s), 7.27-7.22 (2H, m), 7.18-7.14 (2H, m), 2.56 (2H, q, J=7.6 Hz), 1.45 (6H, s), 1.16 (3H, t, J=7.6 Hz)
- LCMS: m/z 193 [M+H]+
- HPLC retention time: 2.18 min (analysis condition S)
- Compound PR3
-
- 2-(4-Ethylphenyl)-2-methylpropanoic acid (58.1 g, 302.2 mmol) was dissolved in acetic acid (175 ml), added with N-iodosuccinimide (71.4 g, 317.3 mmol, 1.05 eq.) and conc. sulfuric acid (75 ml) at 0° C. Thereafter, the mixture was stirred at room temperature for 2 hrs. After cooling the reaction solution to 0° C., 10% aqueous solution of sodium hydrogen sulfite (100 ml) was added and the mixture was stirred for 1 hr. H2O (450 ml) was added to the mixture and the precipitated solid was filtered to obtain the title compound as a crude product. Ethanol (150 ml) and 10% aqueous solution of sodium hydrogen sulfite (50 ml) were added to the crude product, and the mixture was dissolved under heating at 50° C. After confirming the dissolution, the solution was cooled to room temperature, added with H2O (300 ml), and then stirred at 0° C. for 1 hr. The precipitated solid was filtered to obtain the title compound (95.8 g, 99%).
- 1H-NMR (270 MHz DMSO-d6) δ ppm 12.46 (1H, s), 7.70 (1H, d, J=1.8 Hz), 7.32 (1H, dd, J=8.1, 1.8 Hz), 7.26 (1H, d, J=8.1 Hz), 2.64 (2H, q, J=7.5 Hz), 1.43 (6H, s), 1.12 (3H, t, J=7.5 Hz)
- HPLC retention time: 2.53 min (analysis condition S)
- Compound PR4
-
- Mono-tert-butyl malonic acid (72.5 g) was dissolved in DME (360 ml), added with TEA (189 ml) and magnesium chloride (29.63 g) and the mixture was stirred for 2 hrs. In a separate vessel, CDI (52.75 g) was added to the DME (360 ml) solution of 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid (90 g) and stirred at room temperature for 1 hr to prepare a solution. This solution was then added dropwise to the aforementioned mixture, and the resulting solution was washed with DME (90 ml) and stirred at 70° C. for 3 hrs. The reaction mixture was diluted with isopropyl acetate (225 ml) and heptane (225 ml), and the organic layer was washed with 2 N hydrochloric acid (684 ml), 0.17 N hydrochloric acid (540 ml), 15% aqueous solution of ammonium chloride (540 ml), 1 N aqueous solution of sodium hydroxide (540 ml) and 15% brine (540 ml) in order. The organic layer was concentrated under reduced pressure to obtain the title compound as a crude product, which was used for the next step without further purification.
- 1H-NMR (270 MHz DMSO-d6) δ: 7.64 (1H, d, J=2.0 Hz), 7.30 (1H, d, J=8.1 Hz), 7.24 (1H, d, J=8.0, 2.0 Hz), 3.32 (2H, s), 2.65 (2H, q, J=7.4 Hz), 1.40 (6H, s), 1.34 (9H, s), 1.13 (3H, t, J=7.4 Hz)
- Compound PR5-1
-
- 4-(4-Ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl (117.76 g) was dissolved in DMF (471 ml) and added with cesium carbonate (276.5 g). DMF solution (176.6 ml) of 4-chloro-3-nitrobenzonitrile (63.9 g) was added dropwise thereto (washed with DMF 58.8 ml), and the mixture was stirred at 35° C. for 6 hrs. To the mixture, THF (588.8 ml), ethyl acetate (588.8 ml), acetic acid (72.87 ml) and distilled water (588.8 ml) were added for distribution, and the aqueous layer was removed. The organic layer was added with THF (588.8 ml) and water (588.8 ml), and under stirring sodium hydrosulfite (80%, 147.76 g) was added in small portions and the mixture was stirred at room temperature for 3 hrs. After removing the aqueous layer, the organic layer was washed with 15% brine (588.8 ml). The organic layer was added with 1 N hydrochloric acid (94.2 ml), stirred for 1 hr, and then added with 1 N aqueous solution of sodium hydroxide (329.7 ml). The aqueous layer was removed and the organic layer was concentrated under reduced pressure. The concentrated residues were dissolved in ethanol (824.3 ml) and added dropwise with distilled water (247.3 ml). The resulting precipitated crystals were filtered and collected, washed with water: ethanol (=1: 2 mixture solution, 588.8 ml), and then dried to obtain the title compound (98.12 g, two-step 63.5%).
- 1H-NMR (270 MHz DMSO-d6) δ: 12.04 (1H, br. s), 8.01 (1H, d, J=8.4 Hz), 7.91 (1H, d, J=0.8 Hz), 7.55 (1H, d, J=1.8 Hz), 7.49 (1H, dd, J=1.5, 8.4 Hz), 7.16 (1H, d, J=8.1 Hz), 7.07 (1H, dd, J=2.0, 8.1 Hz), 2.58 (2H, q, J=7.4 Hz), 1.79 (6H, s), 1.23 (9H, s), 1.06 (3H, t, J=7.4 Hz)
- LCMS: m/z 459, 515 [M+H]+
- Compound PR5-2
-
- The title compound was prepared from monomethyl malonate and 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid in the same manner as the method for Compound PR4 and Compound PR5-1.
- 1H-NMR (270 MHz DMSO-D6) 6: 12.20 (s, 1H), 8.06-8.03 (m, 1H), 7.95-7.94 (m, 1H), 7.58-7.57 (m, 1H), 7.53-7.49 (m, 1H), 7.17-7.14 (m, 1H), 7.06-7.02 (m, 1H), 3.46 (s, 3H), 2.65-2.56 (q, 2H, J=7.5 Hz), 1.78 (s, 6H), 1.12-1.07 (t, 3H, J=7.5 Hz)
- LCMS: m/z 473 [M+H]+
- Compound PR6
-
- Tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylic acid (390.5 g), 4-morpholin-4-yl piperidine (158 g), and 1,3-bis-(2,6-diisopropylphenyl)-imidazoyl-2-ylidene (allyl) palladium (II) chloride (8.83 g) were dissolved in a mixture of NaHMDS (1.9 M, THF solution 1.32 L) and DME (1.95 L) under nitrogen stream, and the mixture was stirred at 40° C. for 1 hr. The reaction mixture was then partitioned between isopropyl acetate (1.95 L) and 20% aqueous solution of ammonium chloride (1.95 L). The organic layer was washed twice with 10% brine (1.56 L), and then concentrated under reduced pressure. The resulting residues were dissolved in a mixture of DME (3.9 L) and water (78.1 ml), added with N-acetylcysteine (12.39 g), and stirred at 45° C. for 1 hr. After that, the insoluble matters were filtered and washed with DME (1.95 L). The filtrate was concentrated under reduced pressure. The resulting residues were dissolved in acetone (5.5 L) and added with the solution in which pyridinium chloride (96.5 g) is dissolved in acetone (195 ml) and ethanol (78 ml). The precipitated solid was filtered, collected, washed with acetone (1.95 L) and dried to obtain the title compound (373 g, 83%).
- 1H-NMR (400 MHz DMSO-D6) 6: 12.03 (1H, s), 10.75-10.88 (1H, m), 7.99 (1 H, d, J=8.3 Hz), 7.93 (1H, d, J=1.3 Hz), 7.46 (1H, dd, J=1.3, 8.1 Hz), 7.10 (1H, d, J=7.9 Hz), 6.88 (1H, dd, J=1.7, 7.9 Hz), 6.79 (1H, d, J=1.7 Hz), 3.91-4.01 (2H, m), 3.76-3.87 (2H, m), 3.37-3.46 (2H, m), 3.22 (1H, m), 2.94-3.11 (4H, m), 2.57 (2H, q, J=7.5 Hz), 2.45-2.53 (2H, m), 2.09-2.16 (2H, m), 1.80 (6H, s), 1.71-1.77 (2H, m), 1.19 (9H, s), 1.14 (3H, t, J=7.5 Hz)
- LCMS: m/z 557 [M+H]+
- Compound PR7
-
- Tert-butyl 6-cyano-2-(2-(4-ethyl-3-(4-morpholin-4-yl-piperidin-1-yl)phenyl)propan-2-yl)-1H-indole-3-carboxylic acid hydrochloric acid salt (1400 g) was suspended in TFE (7 L) under nitrogen stream, and added dropwise with TMSCl (554 ml) at 8° C. After stirring for 3 hrs, the reaction solution was added with acetone (5.6 L) and aqueous solution of NaOH (1 N, 4.39 L), and 10% aqueous solution of K2HPO4 (1.4 L) was added thereto for neutralization. The precipitated solid was filtered and collected, washed twice with a mixture solution of water: acetone (=1: 1, 2.8 L), and dried to obtain the title compound (1061 g, 96.6%).
- 1H-NMR (270 MHz DMSO-D6) 6: 11.95 (1H, s), 11.92 (1H, bs), 8.04 (1H, d, J=8.4 Hz), 7.89 (1H, d, J=1.3 Hz), 7.44 (1H, J=dd, 1.3, 8.4 Hz), 7.00 (1H, d, J=8.4 Hz), 6.88 (1H, d, J=1.8 Hz), 6.71 (1H, dd, J=2.2, 7.9 Hz), 3.50-3.55 (4H, m), 2.92-2.96 (2H, m), 2.54 (2H, q, 7.5 Hz), 2.39-2.50 (6H, m), 2.15-2.22 (1H, m), 1.74-1.85 (8H, m), 1.43-1.52 (2H, m), 1.13 (3H, t, J=7.5 Hz)
- LCMS: m/z 501 [M+H]+
- HPLC retention time: 1.53 min (analysis condition U)
- Compound F6-20
-
- 6-Cyano-2-(2-(4-ethyl-3-(4-morpholin-4-yl-piperidin-1-yl)phenyl)propan-2-yl)-1H-indole-3-carboxylic acid (500 g) was dissolved in a mixture of DMA (9.4 L), acetic anhydride (270 ml) and DIPEA (1170 ml) under nitrogen stream. The mixture was stirred at 90° C. for 1 hr. After cooling to room temperature, the mixture was added with methanol (3.525 L) and subsequently with distilled water (5.875 L). The precipitated solid was filtered, collected, washed twice with the mixture solution (methanol:water=3:5, 1.41 L), and then dried to obtain the title compound (389.6 g, 85%).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.70 (1H, s), 8.32 (1H, d, J=7.9 Hz), 8.04 (1H, s), 8.00 (1H, s), 7.61 (1H, d, J=8.5 Hz), 7.34 (1H, s), 3.64-3.57 (4H, m), 3.27-3.18 (2H, m), 2.82-2.66 (4H, m), 2.39-2.28 (1H, m), 1.96-1.87 (2H, m), 1.76 (6H, s), 1.69-1.53 (2H, m), 1.29 (3H, t, J=7.3 Hz)
- LCMS: m/z 483 [M+H]+
- HPLC retention time: 1.98 min (analysis condition U)
- Hydrochloric Acid Salt of Compound F6-20
- 9-Ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (400 g) was dissolved in a mixture solvent of methylethyl ketone (4.8 L), acetic acid (1.44 L) and distilled water (1.68 L) at room temperature. The resulting solution was added dropwise to the mixture of ethanol (12 L) and 2 N hydrochloric acid (0.8 L). The precipitated solid was filtered, washed with ethanol (2 L), and dried to obtain hydrochloric acid salt of Compound F6-20 (357 g).
- 1H-NMR (400 MHz, DMSO-d6) δ: 12.83 (1H, s), 10.78 (1H, s), 8.32 (1H, d, J=8.1 Hz), 8.06 (1H, s), 8.01 (1H, s), 7.61 (1H, d, J=8.1 Hz), 7.37 (1H, s), 4.02 (2H, m), 3.85 (2H, m), 3.51 (2H, m), 3.34 (1H, m), 3.32 (2H, m), 3.15 (2H, m), 2.81 (2H, dd, J=11.98, 11.7 Hz), 2.72 (2H, q, J=7.5 Hz), 2.23 (2H, m), 1.89 (2H, m), 1.77 (6H, s), 1.29 (3H, t, J=7.5 Hz) FABMS: m/z 483.4 [M+H]+
- Compound F6-22
-
- From the filtrate solution obtained from the synthesis of Compound F6-20, the title compound was obtained.
- 1H-NMR (400 MHz DMSO-D6) 6: 12.56 (1H, s), 8.32 (1H, d, J=7.9 Hz), 7.96 (1H, s), 7.45-7.59 (3H, m), 3.55-3.62 (4H, m), 3.36-3.50 (2H, m), 2.75-2.86 (2H, m), 2.71 (2H, q, J=7.5 Hz), 2.45-2.56 (4H, m), 2.27-2.38 (1H, m), 1.73-1.84 (2H, m), 1.69 (6H, s), 1.43-1.58 (2H, m), 1.21 (3H, t, J=7.5 Hz).
- LCMS: m/z 483 [M+H]+
- HPLC retention time: 1.52 min (analysis condition U)
- Compound PR8
-
- Tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylic acid (11 g) was dissolved in Eaton's reagent (200 g) and stirred at room temperature for 30 min. The reaction solution was diluted with acetonitrile (200 ml) and distilled water (400 ml). The precipitated solid was collected by filtration, washed with distilled water, and then dried. The crude product was dissolved in DMA (45 ml), diluted with acetonitrile (20 ml) and distilled water (18 ml), and re-precipitated to obtain the title compound (6.62 g, 70%).
- 1H-NMR (400 MHz DMSO-D6) 6: 12.79 (1H, s), 8.32-8.29 (2H, m), 8.06 (1H, s), 8.01 (1H, s), 7.62 (1H, dd, J=1.3, 7.9 Hz), 2.78 (2H, q, J=7.5 Hz), 1.75 (6H, s), 1.20 (3H, t, J=7.5 Hz)
- LCMS: m/z 441 [M+H]+
- HPLC retention time: 3.17 min (analysis condition U)
- Compound PR9-1
-
- The dioxane solution (50 ml) of 9-ethyl-6,6-dimethyl-8-iodo-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (5.0 g), 1,4-dioxa-8-aza-spiro[4.5]decane (2.08 ml), Pd2(dba)3 (520 mg), and S-Phos (963 mg) was flushed with nitrogen gas, added with NaHMDS (1M, THF solution 40 ml), and stirred at 60° C. for 1 hr. The resulting mixture was diluted with ethyl acetate (200 ml). The organic layer was washed three times with 10% brine, and then concentrated under reduced pressure to obtain the title compound as a crude product. This crude product was used for the next step without further purification.
- LCMS: m/z 456 [M+H]+
- HPLC retention time: 2.78 min (analysis condition U)
- Compound PR9-2
-
- As a by-product of Compound PR9-1, the target compound was obtained according to silica gel column separation of Example 810.
- LCMS: m/z 315 [M+H]+
- HPLC retention time: 2.77 min (analysis condition U)
- Compound PR10-1
-
- 8-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile, which had been prepared in Example 809, was dissolved in THF (10 ml), added with 5 N hydrochloric acid (50 ml), and the mixture was stirred for 17 hrs. The reaction mixture was neutralized with 5 N aqueous solution of sodium hydroxide and diluted with ethyl acetate (200 ml). The organic layer was washed with 10% brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (dichloromethane/methanol=99/1 to 90/10) to obtain the title compound (2.9 g, two step yield 64%).
- 1H-NMR (400 MHz DMSO-D6) 6: 12.70 (1H, s), 8.32 (1H, d, J=8.4), 8.07 (1H, s), 7.99 (1H, s), 7.60 (1H, dd, J=1.3, 7.9 Hz), 7.42 (1H, s), 3.28 (4H, t, J=5.7), 2.80 (q, 2H, J=7.5 Hz), 2.55 (4H, t, J=5.7), 1.75 (6H, s), 1.31 (3H, t, J=7.5 Hz)
- LCMS: m/z 412 [M+H]+
- HPLC retention time: 2.57 min (analysis condition U)
- Compound PR11-1
-
- 9-Ethyl-6,6-dimethyl-11-oxo-8-(4-oxopiperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (30 mg) and 2-ketopiperazine (10 mg) were dissolved in THF (2 ml), added with sodium triacetoxy borohydride (30 mg), and the mixture was stirred at 30° C. for 6 hrs. The reaction mixture was diluted with ethyl acetate (20 ml). The organic layer was washed with 10% brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (dichloromethane/methanol=99/1 to 90/10) to obtain the title compound (11.5 mg, yield 32%).
- LCMS: m/z 496 [M+H]+
- HPLC retention time: 1.90 min (analysis condition U)
- Compound PR11-2
-
- As a by-product of Compound PR11-1, the target compound was obtained.
- LCMS: m/z 414 [M+H]+
- HPLC retention time: 2.13 min (analysis condition S)
- The compounds described in the following Tables 8-10 were synthesized by introducing a corresponding amine to 9-ethyl-6,6-dimethyl-8-iodo-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile according to the method used for the synthesis of Compound PR9-1. Although the relevant literatures are not entirely known, some amines in which a tertiary alkyl group is attached to the nitrogen atom were prepared according to the method described in Journal of Medicinal Chemistry, 45 (14), 3143-3160, 2002. Alternatively, the preparation was carried out by introducing a corresponding amine to 9-ethyl-6,6-dimethyl-11-oxo-8-(4-oxopiperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile based on the method that is used for the synthesis of Compound PR11-1 (i.e., reductive amination).
-
TABLE 8 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 813 PR9-3 8-(4-tert-Butyl- piperazin-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.63 455 814 PR9-4 9-Ethyl-6,6- dimethyl-11-oxo-8- (4-pyrrolidin-1-yl- piperidin-1-yl)- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.13 467 815 PR9-5 9-Ethyl-8-(4- isopropyl- piperazin-1-yl)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.08 441 816 PR9-6 9-Ethyl-6,6- dimethyl-8-(4- methyl-piperazin- 1-yl)-11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 1.97 413 817 PR9-7 9-Ethyl-8-(4-ethyl- piperazin-1-yl)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.03 427 818 PR9-8 9-Ethyl-6,6- dimethyl-8- morpholin-4-yl-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile U 2.65 400 819 PR11-3 8-[4-((2R,6S)-2,6- Dimethyl- morpholin-4-yl)- piperidin-1-yl]-9- ethyl-6,6-dimethyl- 11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.65 511 820 PR11-4 8- [1,4′]Bipiperidinyl- 1′-yl-9-ethyl-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.63 481 821 PR11-5 8-(4,4-Difluoro- [1,4′]bipiperidinyl- 1′-yl)-9-ethyl-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.70 517 822 PR11-6 8-(4-Azetidin-1-yl- piperidin-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.55 453 823 PR11-7 9-Ethyl-8-(4- hydroxy- [1,4′]bipiperidinyl- 1′-yl)-6,6-dimethyl- 11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 1.95 497 824 PR9-9 8-(4-Cyclopropyl- 4-hydroxy- piperidin-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.53 454 825 PR11-8 9-Ethyl-8-(4- fluoro- [1,4′]bipiperidinyl- 1′-yl)-6,6-dimethyl- 11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.13 499 826 PR9-10 9-Ethyl-6,6- dimethyl-8-(3- morpholin-4-yl- azetidin-1-yl)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile S 1.48 455 -
TABLE 9 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 827 PR9-11 9-Ethyl-6,6- dimethyl-11-oxo-8- (3-piperidin-1-yl- azetidin-1-yl)-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.57 453 828 PR9-12 9-Ethyl-6,6- dimethyl-8-[4-(1- methyl-piperidin-4- yl)-piperazin-1-yl]- 11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 1.70 496 829 PR9-13 9-Ethyl-6,6- dimethyl-8-[4-(4- methyl-piperazin- 1-yl)-piperidin-1- yl]-1l-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 1.73 496 830 PR11-9 8-(4-Cyclopentyl- piperazin-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.12 467 831 PR11-10 9-Ethyl-8-[4-(2- hydroxy- ethylamino)- piperidin-1-yl]-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 1.90 457 832 PR-11-11 9-Ethyl-8-[4-(3- hydroxy- propylamino)- piperidin-1-yl]-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile S 1.90 471 833 PR9-14 9-Ethyl-6,6- dimethyl-8-(4- methyl-4- morpholine-4-yl- piperidin-1-yl)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile U 2.00 497 834 PR9-15 9-Ethyl-8-[4-(1- ethyl-cyclobutyl)- piperazine-1-yl]- 6,6-dimethyl-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile Y 2.25 481 835 PR9-16 9-Ethyl-8-(4- ethyl-4- morpholine-4-yl- piperidine-1-yl)- 6,6-dimethyl-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile Y 2.17 511 836 PR9-17 9-Ethyl-8-(4- isopropyl-4- morpholine-4-yl- piperidine-1-yl)- 6,6-dimethyl-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile U 2.12 525 837 PR9-18 9-Ethyl-6,6- dimethyl-11-oxo-8- [4-(1-propyl- cyclobutyl)- piperazine-1-yl]- 6,11-dihydro-5H- benzo[b]carbazole- 3-carbonitrile U 2.28 495 838 PR9-19 9-Ethyl-8-[4-(1- isopropyl- cyclobutyl)- piperazine-1-yl]- 6,6-dimethyl-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile U 2.25 495 839 PR9-20 9-Ethyl-6,6- dimethyl-8-(2- morpholine-4-yl- ethylamino)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-carbonitrile Y 1.85 443 840 PR9-21 9-Ethyl-6,6- dimethyl-11-oxo-6- (2-piperidine-1-yl- ethylamino)-6,11- dihydro-5H- benzo[b]carbazole- 3-carbonitrile Y 1.85 441 -
TABLE 10 Ex- HPLC Re- ample Comp. Con- tention No. No. Structure Compound Name dition Time m/z 841 PR11-12 8-(4-Amino- piperidine-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H- benzo[b]carbazole-3- carbonitrile U 1.92 413 842 PR11-13 9-Ethyl-6,6- dimethyl-8-(4- 2,2,3,3,5,5,6,6-d8- morpholine-4-yl- piperidine-1-yl)-1 1-oxo-6,11- dihydro-5H-benzo [b]carbazole-3- carbonitrile U 1.98 491 843 PR9-22 9-Ethyl-8-[4-(2- methoxy-ethoxy)- piperidine-1-yl]6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile Y 3.23 472 844 PR9-23 8-[4-(2-Ethoxy- ethoxy)-piperidine-1- yl]-9-ethyl-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile Y 3.35 486 845 PR9-24 8-(4- Cyclopropylmethoxy- piperidine-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H- benzo[b]carbazole-3- carbonitrile Y 3.50 468 846 PR9-25 8-[4-(2-Ethoxy- ethoxy)-piperidine-1- yl]-9-ethyl-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile Y 4.00 524 847 PR11-14 8-(4- Cyclopropylamino- piperidine-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H- benzo[b]carbazole-3- carbonitrile Y 2.33 495 848 PR11-15 8-(4- Cyclopropylamino- piperidine-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H- benzo[b]carbazole-3- carbonitrile Y 2.15 481 849 PR11-16 8-[4- Cyclopropylmethoxy- amino)-piperidine-1- yl]-9-ethyl-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile Y 2.15 467 850 PR11-17 8-(4- Cyclopropylamino- piperidine-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H- benzo[b]carbazole-3- carbonitrile Y 2.08 453 851 PR11-18 8-(4- Cyclopropylamino- piperidine-1-yl)-9- ethyl-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H- benzo[b]carbazole-3- carbonitrile Y 2.13 467 852 PR11-19 8-[4- Cyclohexylmethl- amino)-piperidine-1- yl]-9-ethyl-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazole-3- carbonitrile Y 2.42 509 - Compound PR11-22
-
- 9-ethyl-6,6-dimethyl-11-oxo-8-(4-oxopiperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (30 mg) and hydroxylamine hydrochloric acid salt (10 mg) were dissolved in ethanol (5 ml) and stirred at 60° C. for 6 hrs. The reaction mixture was diluted with ethyl acetate (20 ml). The organic layer was washed with 10% brine and concentrated under reduced pressure. The resulting residues were purified by silica gel column (dichloromethane/methanol=99/1 to 90/10) to obtain the title compound (23.5 mg, yield 74%).
- LCMS: m/z 427 [M+H]+
- HPLC retention time: 3.08 min (analysis condition Y)
- Compound PR10-2
-
- 9-Ethyl-6,6-dimethyl-8-(2-morpholin-4-yl-ethylamino)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (10 mg) was dissolved in DMF (1 ml), added with K2CO3 (10 mg) and 1-(2-chloroethyl)morpholine (8 mg), and then stirred at 90° C. for 17 hrs. The reaction mixture was diluted with ethyl acetate (10 ml). The organic layer was washed with 10% brine and concentrated under reduced pressure. The resulting residues were purified by silica gel column (dichloromethane/methanol=99/1 to 90/10) to obtain the title compound (6.4 mg, yield 58%).
- LCMS: m/z 556 [M+H]+
- HPLC retention time: 1.78 min (analysis condition Y)
- Compound F7
-
- 9-Ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (400 mg) was dissolved in trifuloroethanol (80 ml), added with 30% hydrogen peroxide solution (0.8 ml), and the mixture was stirred at 60° C. for 17 hrs. The reaction mixture was concentrated to 30 ml and diluted with water (20 ml). The precipitated matter was collected by filtration and dried to obtain the title compound (375 mg, yield 90%).
- LCMS: m/z 499 [M+H]+
- HPLC retention time: 2.05 min (analysis condition U)
- Compound FR1
-
- 4-(4-Ethyl-3-iodo-phenyl)-4-methyl-3-oxo-pentanoic acid tert-butyl ester (1.00 g, 2.40 mmol) was dissolved in NMP (4 ml), added with cesium carbonate (1.56 g, 4.80 mmol, 2.0 eq.), and the mixture was stirred for 5 min. The NMP solution (2 ml) of 4-chloro-3-nitro-benzonitrile (542 mg, 2.88 mmol, 1.2 eq.) was added thereto, and the mixture was stirred at 50° C. for 64 hrs under nitrogen atmosphere. After cooling to room temperature, ethyl acetate (20 ml) was added and the organic layer was washed with saturated aqueous solution of ammonium chloride (20 ml). The organic layer was further washed with saturated brine and dried over sodium sulfate. The drying agent was removed by filtration and the residues obtained after concentration under reduced pressure were purified by silica gel column chromatography (ethyl acetate/hexane) to obtain the title compound (white amorphous, 320 mg, 26%).
- LCMS: m/z 516 [M+H]+
- Compound FR2
-
- 6-Cyano-2-[1-(4-ethyl-3-iodophenyl)-1-methyl-ethyl]-benzofuran-3-carboxylic acid tert-butyl ester was converted to obtain the title compound in the same manner as the method for Compound PR6.
- LCMS: m/z 558 [M+H]+
- Compound FR3
-
- To obtain the title compound, 6-Cyano-2-{1-[4-ethyl-3-(4-morpholin-4-yl-piperidin-1-yl)-phenyl]-1-methyl-ethyl}-benzofuran-3-carboxylic acid tert-butyl ester was deprotected by using trimethylsilyl iodide in the same manner as the method for Compound PR7.
- LCMS: m/z 502 [M+H]+
- Compound FR4
-
- 6-Cyano-2-{1-[4-ethyl-3-(4-morpholin-4-yl-piperidin-1-yl)-phenyl]-1-methyl-ethyl}-benzofuran-3-carboxylic acid hydroiodic acid salt was converted in the same method as Example 805 to obtain the target compound.
- LCMS: m/z 484 [M+H]+
- Compound LB1
-
- The title compound was prepared from 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid by carrying out amination in the same manner as the method for synthesizing Compound PR6.
- LCMS: m/z 361 [M+H]+
- Compound LB2
-
- 2-[4-Ethyl-3-(4-morpholin-4-yl-piperidin-1-yl)-phenyl]-2-methyl-propionic acid was converted in the same manner as the method for synthesizing Compound PR5-1 to obtain the title compound.
- LCMS: m/z 658 [M+H]+
- HPLC retention time: 2.76 min (analysis condition U)
- Compound LB3
-
- 2-{1-[4-Ethyl-3-(4-morpholin-4-yl-piperidin-1-yl)-phenyl]-1-methyl-ethyl}-6-iodo-1H-indole-3-carboxylic acid tert-butyl ester was deprotected in the same manner as the method for preparing Compound PR7 to obtain the title compound.
- LCMS: m/z 602 [M+H]+
- HPLC retention time: 2.17 min (analysis condition U)
- Compound LB4
-
- 2-{1-[4-Ethyl-3-(4-morpholin-4-yl-piperidin-1-yl)-phenyl]-1-methyl-ethyl}-6-iodo-1H-indole-3-carboxylic acid was converted in the same manner as Example 805 to obtain the title compound.
- LCMS: m/z 584 [M+H]+
- HPLC retention time: 2.25 min (analysis condition U)
- The compounds described in the following Table 11 were converted and prepared from 9-ethyl-3-iodo-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-5,6-dihydro-benzo[b]carbazol-11-one according to the method described in the Table.
-
TABLE 11 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z Method 864 LB5-1 N-[9-Ethyl-6,6- dimethyl-8-(4- morpholine-4-yl- piperidine-1-yl)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-yl]-benzamide Y 1.78 577 B2-10 865 LB5-2 9-Ethyl-3- ethylsulfanyl-6,6- dimethyl-8-(4- morpholine-4-yl- piperidine-1-yl)-5,6- dihydro- benzo[b]carbazole- 11-one Y 2.28 518 B2-17 866 LB5-3 N-[9-Ethyl-6,6- dimethyl-8-(4- morpholine-4-yl- piperidine-1-yl)-11- oxo-6,11-dihydro- 5H- benzo[b]carbazole- 3-yl]-acetamide Y 1.50 515 B2-10 - Compound AZT1
-
- (2-Fluoropyridin-4-yl) methanol (1g) was dissolved in DCM (40 ml), added with TEA (3.3 ml) and mesyl chloride (0.67 ml), and the mixture was stirred at 0° C. for 1 hr. The mixture was concentrated and then purified by silica gel column (n-hexane/ethyl acetate=4/1) to obtain the title compound (1.18 g, 77%).
- 1H-NMR (270 MHz DMSO-d6) δ: 3.21 (3H, s), 5.38 (2H, s), 7.22 (1H, s), 7.39 (1H, d, J=5.0), 8.29 (1H, d, J=5.0)
- Compound AZ2
-
- To the DMF (28 ml) solution of methanesulfonic acid (2-fluoropyridin-4-yl)methyl ester (1.16 g), sodium cyanide (0.42 g) was added and the mixture was stirred at 80° C. for 1 hr. The mixture was diluted with ethyl acetate (100 ml), and washed with 15% brine and distilled water in order. The organic layer was concentrated and purified by silica gel column (n-hexane/ethyl acetate=5/1) to obtain the title compound (278 mg, 36%).
- 1H-NMR (270 MHz DMSO-d6) δ: 4.22 (2H, s), 7.18 (1H, s), 7.36 (1H, d, J=5.0), 8.27 (1H, d, J=5.0)
- Compound AZ3
-
- The title compound was prepared from (2-fluoropyridin-4-yl) acetonitrile in the same manner as the method for Compound K2.
- 1H-NMR (270 MHz DMSO-d6) δ: 1.72 (6H, s), 7.34 (1H, s), 7.53 (1H, d, J=5.3), 8.31 (1H, d, J=5.3)
- Compound AZ4
-
- The title compound was prepared from (2-fluoropyridin-4-yl) 2-methylpropionitrile in the same manner as the method for Compound K3.
- 1H-NMR (270 MHz DMSO-d6) δ: 1.13 (3H, t, J=7.3), 1.48 (6H, s), 3.57 (2H, s), 4.01 (2H, q, J=7.3), 7.12 (1H, s), 7.25 (1H, d, J=5.3), 8.22 (1H, d, J=5.3)
- Compound AZ5
-
- The title compound was prepared from 4-(2-fluoropyridin-4-yl)-4-methyl-3-oxopentanoic acid ethyl ester in the same manner as the method for Compound K4 and Compound K5.
- LCMS: m/z 352 [M+H]+
- 1H-NMR (270 MHz DMSO-d6) δ: 1.05 (3H, t, J=7.3), 1.82 (6H, s), 3.98 (2H, q, J=7.3), 6.99-7.02 (2H, m), 7.16 (1H, dd, J=8.4, 1.5), 7.97 (1H, s), 8.05-8.11 (2H, m)
- Compound AZ6
-
- 6-Cyano-2-[1-(2-fluoropyridin-4-yl)-1-methyl-ethyl]-1H-indole-3-carboxylic acid ethyl ester (110 mg) was dissolved in NMP (3.3 ml), added with 4-morpholin-4-yl-piperidine (319 mg), and stirred in a sealing tube at 120° C. for 1 hr. The reaction mixture was diluted with ethyl acetate (50 ml) and washed with 15% brine and distilled water in order. The organic layer was concentrated and purified by silica gel column (DCM/methanol=20/1) to obtain the title compound (120 mg, 76%).
- LCMS: m/z 502 [M+H]+
- Compound AZ7-1
-
- 6-Cyano-2-[1-(2-(4-morpholin-4-yl-piperidin-1-yl))pyridin-4-yl)-1-methyl-ethyl]-1H-indole-3-carboxylic acid ethyl ester (110 mg) was dissolved in Eaton's reagent (2.5 ml) and stirred at 55° C. for 17 hrs. The reaction mixture was neutralized with saturated aqueous solution of sodium bicarbonate. The precipitated matters were collected by filtration, and then washed with water to obtain the title compound (72 mg, 70%).
- LCMS: m/z 474 [M+H]+
- HPLC retention time: 1.17 min (analysis condition U)
- 1H-NMR (270 MHz DMSO-d6) δ: 1.38 (2H, m), 1.75 (6H, s), 1.88 (2H, m), 2.44 (5H, m), 2.94 (2H, m), 3.57 (4H, m), 4.58 (2H, m), 7.10 (1H, s), 7.32 (1H, s), 7.75 (1 H, d, J=8.4), 8.00 (2H, m), 8.15 (1H, d, J=8.4), 8.85 (1H, s), 12.3 (1H, s)
- Compound AZ7-2
-
- 5,5-Dimethyl-3-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-pyrido[4,3-b]carbazole-8-carboxylic acid amide (54 mg) was dissolved in DMF (1 ml), added with thionyl chloride (25 μL), and the mixture was stirred at room temperature for 1 hr. The reaction mixture was diluted with water. The precipitated matters were collected by filtration to obtain the title compound (25 mg, 49%).
- LCMS: m/z 456 [M+H]+
- HPLC retention time: 1.55 min (analysis condition U)
- 1H-NMR (270 MHz DMSO-d6) δ: 1.36 (2H, m), 1.76 (6H, s), 1.89 (2H, m), 2.44 (5H, m), 2.95 (2H, m), 3.57 (4H, m) 4.58 (2H, m), 7.10 (1H, s), 7.59 (1H, d, J=8.0), 7.99 (1H, s), 8.29 (1H, d, J=8.0), 8.86 (1H, s), 12.7 (1H, s)
- The compounds described in the following Tables 12-13 were synthesized by introducing a corresponding amino group to 6-cyano-2-[1-(2-fluoropyridin-4-yl)-1-methyl-ethyl]-1H-indole-3-carboxylic acid ethyl ester and forming a ring according to the method that is used for the synthesis of Compound AZ7-1. Furthermore, the preparation was carried out by converting the substituent group at position 3 from a carboxamide group to a cyano group according to the method that is used for the synthesis of Compound AZ7-2.
-
TABLE 12 Ex- HPLC Re- ample Comp. Con- tention No. No. Structure Compound Name dition Time m/z 875 AZ7-3 3-[4-((2R,6S)-2,6- Dimethyl-morpholine- 4-yl)-piperidine-1- yl]-5,5-dimethyl-11- oxo-6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.32 502 876 AZ7-4 3-[4-((2R,6S)-2,6- Dimethyl-morpholine- 4-yl)-piperidine-1- yl]-5,5-dimethyl-11- oxo-6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile U 1.70 484 877 AZ7-5 3-[1,4′]Biperidinyl- 1′-yl-5,5-dimethyl- 11-oxo-6,11-dihydro- 5H-pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.30 472 878 AZ7-6 5,5-Dimethyl-3-(4- methyl-4-morpholine- 4-yl-piperidine-1-yl)- 11-oxo-6,11-dihydro- 5H-pyrido[4,3- b]carbazole-8- carboxylic acid amide Y 1.00 488 879 AZ7-7 5,5-Dimethyl-3-(4- methyl-4-morpholine- 4-yl-piperidine-1-yl)- 11-oxo-6,11-dihydro- 5H-pyrido[4,3- b]carbazole-8- carbonitrile Y 1.62 470 880 AZ7-8 3-(4-Cyclobutyl- piperazine-1-yl)-5,5- dimethyl-11-oxo- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide Y 1.22 444 881 AZ7-9 3-(4-Cyclobutyl- piperazine-1-yl)-5,5- dimethyl-11-oxo- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile Y 1.55 426 882 AZ7-10 3-[1,4′]Biperidinyl- 1′-yl-5,5-dimethyl- 11-oxo-6,11-dihydro- 5H-pyrido[4,3- b]carbazole-8- carbonitrile Y 1.73 454 883 AZ7-11 5,5-Dimethyl-3- morpholine-4-yl-11- oxo-6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.33 391 884 AZ7-12 5,5-Dimethyl-3- morpholine-4-yl-11- oxo-6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile U 1.77 373 885 AZ7-13 3-[4-(1-Ethyl- cyclobutyl)- piperazine-1-yl]5,5- dimethyl-11-oxo- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile Y 1.93 464 886 AZ7-14 3-(4-Ethyl-4- morpholine-4-yl- piperidine-1-yl)-5,5- dimethyl-11-oxo- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide Y 1.18 502 887 AZ7-15 3-(4-Ethyl-4- morpholine-4-yl- piperidine-1-yl)-5,5- dimethyl-11-oxo- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile Y 1.78 484 888 AZ7-16 3-[4-(1-Ethyl- cyclobutyl)- piperazine-1-yl]5,5- dimethyl-11-oxo- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide Y 1.25 472 -
TABLE 13 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 889 AZ7-17 3-(4-Isopropyl- 4-morpholine-4- yl-piperidine-1- yl)-5,5-dimethyl- 11-oxo-6,11- dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.27 516 890 AZ7-18 5,5-Dimethyl-3- (4-morpholine-4- yl-4-propyl- piperidine-1-yl)- 11-oxo-6,11- dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.36 516 891 AZ7-19 5,5-Dimethyl-3- (4-morpholine-4- yl-4-propyl- piperidine-1-yl)- 11-oxo-6,11- dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile U 1.68 498 892 AZ7-20 3-(4-Isopropyl- 4-morpholine-4- yl-piperidine-1- yl)-5,5-dimethyl- 11-oxo-6,11- dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile U 1.60 498 893 AZ7-21 5,5-Dimethyl- 11-oxo-3-[4-(1- propyl- cyclobutyl)- piperazine-1-yl]- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.58 486 894 AZ7-22 5,5-Dimethyl- 11-oxo-3-[4-(1- propyl- cyclobutyl)- piperazine-1-yl]- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile U 1.97 468 - The compounds described in the following Table 14 were synthesized from (2-chloro-3-fluoropyridin-4-yl)methanol according to the method that is used for the synthesis of Compound AZ1 to AZ7-2.
-
TABLE 14 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 895 AZ7-23 4-Fluoro-5,5- dimethyl-3-(4- morpholin-4-yl- piperidin-1-yl)-11- oxo-6,11-dihydro- 5H-pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.37 492 896 AZ7-24 4-Fluoro-5,5- dimethyl-3-(4- morpholin-4-yl- piperidin-1-yl)-11- oxo-6,11-dihydro- 5H-pyrido[4,3- b]carbazole-8- carbonitrile U 1.14 474 897 AZ7-25 4-Fluoro-5,5- dimethyl-11-oxo-3- [4-(3-oxo- piperazin-1-yl)- piperidin-1-yl]-6,11- dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.32 505 898 AZ7-26 3- [1,4′]Bipiperidinyl- 1′-yl-4-fluoro-5,5- dimethyl-11-oxo- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carboxylic acid amide U 1.45 490 899 AZ7-27 3- [1,4′]Bipiperidinyl- 1′-yl-4-fluoro-5,5- dimethyl-11-oxo- 6,11-dihydro-5H- pyrido[4,3- b]carbazole-8- carbonitrile U 1.87 472 - Compound BZ1
-
- 4-Chloro-2-cyanopyridine (1 g) was dissolved in hydrazine monohydrate (1 ml) and 1,4-dioxane (10 ml), and stirred overnight under reflux. The reaction solution was diluted with water (30 ml) and extracted repeatedly with ethyl acetate. The organic layer was concentrated to obtain the title compound as a crude product, which was used for the next step without further purification.
- LCMS: m/z 135 [M+H]+
- Compound BZ2-1
-
- According to the method used for synthesizing Compound A3-1, the intermediate was prepared from 2-cyano-4-hydrazinopyridine and 7-methoxy-1,1-dimethyl-3,4 dihydro-1H-naphthalen-2-one. Without any purification, the intermediate was subjected to oxidation according to the method used for synthesizing Compound A4 to obtain the title compound.
- LCMS: m/z 318 [M+H]+
- HPLC retention time: 2.10 min (analysis condition U)
- Compound BZ2-2
-
- According to the method used for synthesizing Compound A3-1, the intermediate was prepared from 2-chloro-4-hydrazinopyridine and 7-methoxy-1,1-dimethyl-3,4 dihydro-1H-naphthalen-2-one. Without any purification, the intermediate was subjected to oxidation according to the method used for synthesizing Compound A4 to obtain the title compound.
- LCMS: m/z 327, 329 [M+H]+
- HPLC retention time: 1.80 min (analysis condition S)
- Compound CZ1
-
- According to the method used for synthesizing Compound A3-1, the intermediate was prepared from 2-bromo-6-hydrazinopyridine and 7-methoxy-1,1-dimethyl-3,4 dihydro-1H-naphthalen-2-one. Without any purification, the intermediate was subjected to oxidation according to the method used for synthesizing Compound A4 to obtain the title compound.
- LCMS: m/z 371, 373 [M+H]+
- HPLC retention time: 2.85 min (analysis condition U)
- Compound CZ2
-
- According to the method 1 for Compound A5-2, 2-bromo-8-methoxy-10,10-dimethyl-10,11-dihydro-1,11-diaza-benzo[b]fluoren-5-one was subjected to cyanation to obtain the title compound.
- LCMS: m/z 318 [M+H]+
- HPLC retention time: 2.35 min (analysis condition U)
- Compound CZ3
-
- According to the method used for synthesizing Compound A6, 8-methoxy-10,10-dimethyl-5-oxo-10,11-dihydro-5H-1,11-diaza-benzo[b]fluorene-2-carbonitrile was subjected to demethylation to obtain the title compound.
- LCMS: m/z 304 [M+H]+
- HPLC retention time: 1.72 min (analysis condition S)
- Compound CZ4
-
- According to the method used for synthesizing Compound B1, 8-hydroxy-10,10-dimethyl-5-oxo-10,11-dihydro-5H-1,11-diaza-benzo[b]fluorene-2-carbonitrile was subjected to trifluoromethanesulfone esterification to obtain the title compound.
- LCMS: m/z 436 [M+H]+
- HPLC retention time: 3.32 min (analysis condition Y)
- Compound CZ5-1
-
- According to the method used for synthesizing Compound B2-1, trifuloromethanesulfonic acid 2-cyano-10,10-dimethyl-5-oxo-10,11-dihydro-5H-1,11-diaza-benzo[b]fluoren-8-yl ester was introduced with piperazine to obtain the title compound.
- LCMS: m/z 372 [M+H]+
- HPLC retention time: 1.17 min (analysis condition S)
- Compound CZ5-2
-
- According to the method used for synthesizing Compound B2-1, trifuloromethanesulfonic acid 2-cyano-10,10-dimethyl-5-oxo-10,11-dihydro-5H-1,11-diaza-benzo[b]fluoren-8-yl ester was introduced with 4-morpholin-4-yl piperidine to obtain the title compound.
- LCMS: m/z 456 [M+H]+
- HPLC retention time: 1.68 min (analysis condition U)
- Compound CZ6
-
- According to the method used for synthesizing Compound B3-32, 10,10-dimethyl-5-oxo-8-piperazin-1-yl-10,11-dihydro-5H-1,11-diaza-benzo[b]fluorene-2-carbonitrile was subjected to reductive amination with cyclobutanone to obtain the title compound.
- LCMS: m/z 426 [M+H]+
- HPLC retention time: 1.60 min (analysis condition U)
- Compound DZ1
-
- 6-Bromo-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (1 g) was dissolved in acetonitrile (50 ml), added with PdCl2(CH3CN)2 (45 mg), X-phos (168 mg), CsCO3 (1.2 g) and trimethylsilylacetylene (0.9 ml), and the mixture was stirred at 85° C. for 2 hrs. The reaction mixture was diluted with ethyl acetate (100 ml). The organic layer was washed twice with 10% brine and concentrated under reduced pressure. The resulting residues were dissolved in THF (10 ml), added with the THF solution (4 ml) comprising tetrabutylammonium fluoride and stirred at room temperature for 1 hr. The reaction mixture was diluted with ethyl acetate (100 ml). The organic layer was washed twice with 10% brine and concentrated under reduced pressure. The resulting residues were purified by silica gel column (n-hexane/ethyl acetate=9/1) to obtain the title compound (346 mg, two step yield 43%).
- LCMS: m/z 229 [M+H]+
- Compound DZ2
-
- 6-Ethynyl-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (346 mg) was dissolved in ethanol: THF (=2: 1 mixture solvent, 20 ml), added with 10% Pd/C (170 mg), and then the mixture was stirred at room temperature for 1 hr under hydrogen atmosphere. The catalyst was removed by filtration and the organic layer was concentrated under reduced pressure to obtain the title compound (322 mg, yield 91%).
- LCMS: m/z 233 [M+H]+
- Compound DZ3
-
- According to the method used for synthesizing Compound A3-1, the intermediate was prepared from 2-bromo-6-hydrazinopyridine and 6-ethyl-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one. Without any purification, the intermediate was subjected to oxidation according to the method used for synthesizing Compound A4 to obtain the title compound.
- LCMS: m/z 399, 401 [M+H]+
- HPLC retention time: 3.35 min (analysis condition Y)
- Compound DZ4
-
- According to the method 1 for Compound A5-2, 2-bromo-7-ethyl-8-methoxy-10,10-dimethyl-10,11-dihydro-1,11-diaza-benzo[b]fluoren-5-one was subjected to cyanation to obtain the title compound.
- LCMS: m/z 346 [M+H]+
- HPLC retention time: 3.05 min (analysis condition Y)
- Compound DZ5
-
- According to the method used for synthesizing Compound A5, 7-ethyl-8-methoxy-10,10-dimethyl-5-oxo-10,11-dihydro-5H-1,11-diaza-benzo[b]fluorene-2-carbonitrile was subjected to demethylation to obtain the title compound.
- LCMS: m/z 332 [M+H]+
- HPLC retention time: 2.60 min (analysis condition Y)
- Compound DZ6-1
-
- According to the method used for synthesizing Compound B1, 7-ethyl-8-hydroxy-10,10-dimethyl-5-oxo-10,11-dihydro-5H-1,11-diaza-benzo[b]fluorene-2-carbonitrile was subjected to trifluoromethanesulfone esterification to obtain the title compound.
- LCMS: m/z 464 [M+H]+
- HPLC retention time: 3.50 min (analysis condition Y)
- The compounds described in the following Table 15 were prepared from trifuloro-methanesulfonic acid 2-cyano-7-ethyl-10,10-dimethyl-5-oxo-10,11-dihydro-5H-1,11-diaza-benzo[b]fluoren-8-yl ester and corresponding amine according to the method that is used for the synthesis of Compound B2-10. The compounds of Example 919 and Example 920 were obtained as a by-product of the reaction.
-
TABLE 15 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 916 DZ7-1 8-(4-Cyclobutyl- piperazine-1-yl)-7-ethyl- 10,10-dimethyl-5-oxo- 10,11-dihydro-5H-1,11- diaza-benzo[b]fluorene- 2-carbonitrile Y 1.83 454 917 DZ7-2 7-Ethyl-10,10-dimethyl- 8-(4-morpholine-4-yl- piperidine-1-yl)-5-oxo- 10,11-dihydro-5H-1,11- diaza-benzo[b]fluorene- 2-carbonitrile Y 1.85 484 918 DZ7-3 7-Ethyl-10,10-dimethyl- 8-morpholine-4-yl-5- oxo-10,11-dihydro-5H- 1,11-diaza- benzo[b]fluorene-2- carbonitrile Y 3.02 401 919 DZ7-4 7-Ethyl-10,10-dimethyl- 5-oxo-10,11-dihydro-5H- 1,11-diaza- benzo[b]fluorene-2- carbonitrile Y 3.07 316 920 DZ7-5 7-Ethyl-10,10-dimethyl- 2-(morpholine-4- carbonyl)-8-morpholine- 4-yl-10,11-dihydro-1,11- diaza-benzo[b]fluorene- 5-one Y 2.70 489 - Compound DZ6-2
-
- According to the method used for synthesizing Compound A7-17, 7-ethyl-8-hydroxy-10,10-dimethyl-5-oxo-10,11-dihydro-5H-1,11-diaza-benzo[b]fluorene-2-carbonitrile was alkylated to obtain the title compound.
- LCMS: m/z 530 [M+H]+
- HPLC retention time: 1.38 min (analysis condition Y)
- Compound EZ1
-
- 2-Bromo-6-methoxypyridine (7.0 g), ethyl isobutyrate (4.75 g), tri t-butylphosphine (300 mg) and Pd2(dba)3 (680 mg) were dissolved in toluene (200 ml) under nitrogen atmosphere, added with THF solution of LiHMDS (1.6 M, 24 ml), and the mixture was stirred at 100° C. for 6 hrs. The reaction mixture was diluted with ethyl acetate (300 ml), and washed three times with 15% brine (200 ml). The organic layer was concentrated under reduced pressure and the resulting residues were purified by silica gel column (n-hexane/ethyl acetate=4/1) to obtain the title compound (5.353 g, yield 60%).
- LCMS: m/z 224 [M+H]+
- Compound EZ2
-
- 2-(6-Methoxy-pyridin-2-yl)-2-methyl-propionic acid ethyl ester (5.33 g) was dissolved in methanol (200 ml), added with 5 N aqueous solution of potassium hydroxide (25 ml), and then stirred under reflux. The reaction mixture was concentrated and neutralized with 2N hydrochloric acid. The precipitated matters were collected by filtration and dried to obtain the title compound (3.55 g).
- LCMS: m/z 196 [M+H]+
- Compound EZ3
-
- The title compound was synthesized from 2-(6-methoxy-pyridin-2-yl)-2-methyl-propionic acid and mono-tert-butyl malonic acid according to the method used for the synthesis of Compound PR4. The resultant was used for the next step without further purification.
- Compound EZ4-1
-
- According to the method that is used for the preparation of Compound PR5-1, the title compound was synthesized from 4-(6-methoxy-pyridin-2-yl)-4-methyl-3-oxo-pentanoic acid tert-butyl ester and 4-chloro-3-nitrobenzonitrile
- LCMS: m/z 392 [M+H]+
- Compound EZ4-2
-
- According to the method that is used for the preparation of Compound FR1, the title compound was synthesized from 4-(6-methoxy-pyridin-2-yl)-4-methyl-3-oxo-pentanoic acid tert-butyl ester and 4-chloro-3-nitrobenzonitrile.
- LCMS: m/z 393 [M+H]+
- Compound EZ5-1
-
- According to the method that is used for the preparation of Compound PR7, the title compound was synthesized from 6-cyano-2-[1-(6-methoxy-pyridin-2-yl)-1-methyl-ethyl]-1H-indole-3-carboxylic acid tert-butyl ester.
- LCMS: m/z 336 [M+H]+
- Compound EZ5-2
-
- According to the method that is used for the preparation of Compound PR7, the title compound was synthesized from 6-cyano-2-[1-(6-methoxy-pyridin-2-yl)-1-methyl-ethyl]-benzofuran-3-carboxylic acid tert-butyl ester.
- LCMS: m/z 337 [M+H]+
- Compound EZ6-1
-
- According to the method that is used for the preparation of Compound AZ7-1, the title compound was synthesized from 6-cyano-2-[1-(6-methoxy-pyridin-2-yl)-1-methyl-ethyl]-1H-indole-3-carboxylic acid.
- LCMS: m/z 336 [M+H]+
- HPLC retention time: 1.98 min (analysis condition S)
- Compound EZ6-2
-
- According to the method that is used for the preparation of Compound AZ7-1, the title compound was synthesized from 6-cyano-2-[1-(6-methoxy-pyridin-2-yl)-1-methyl-ethyl]-benzofuran-3-carboxylic acid.
- LCMS: m/z 337 [M+H]+
- HPLC retention time: 2.38 min (analysis condition S)
- Compound EZ7-1
-
- According to the method that is used for the preparation of Compound AZ7-2, the title compound was synthesized from 2-methoxy-11,11-dimethyl-5-oxo-10,11-dihydro-5H-pyrido[2,3-b]carbazole-8-carboxylic acid amide.
- LCMS: m/z 318 [M+H]+
- HPLC retention time: 2.60 min (analysis condition S)
- Compound EZ7-2
-
- According to the method that is used for the preparation of Compound AZ7-2, the title compound was synthesized from 2-methoxy-11,11-dimethyl-5-oxo-5,11-dihydro-benzo[4,5]furo[3,2-g]quinoline-8-carboxylic acid amide.
- LCMS: m/z 319 [M+H]+
- HPLC retention time: 3.18 min (analysis condition S)
- Compound EZ8-1
-
- According to the method that is used for the preparation of Compound A5, 2-methoxy-11,11-dimethyl-5-oxo-10,11-dihydro-5H-pyrido[2,3-b]carbazole-8-carbonitrile was demethylated to synthesize the title compound.
- LCMS: m/z 304 [M+H]+
- HPLC retention time: 1.70 min (analysis condition U)
- Compound EZ8-2
-
- According to the method that is used for the preparation of Compound A5, 2-methoxy-11,11-dimethyl-5-oxo-5,11-dihydro-benzo[4,5]furo[3,2-g]quinoline-8-carbonitrile was demethylated to synthesize the title compound.
- LCMS: m/z 305 [M+H]+
- HPLC retention time: 2.17 min (analysis condition U)
- The compounds described in the following Table 16 were synthesized from 2-hydroxy-11,11-dimethyl-5-oxo-10,11-dihydro-5H-pyrido[2,3-b]carbazole-8-carbonitrile or from 2-hydroxy-11,11-dimethyl-5-oxo-5,11-dihydro-benzo[4,5]furo[3,2-g]quinoline-8-carbonitrile according to the method described in the Table.
-
TABLE 16 Ex- HPLC Re- ample Comp. Con- tention No. No. Structure Compound Name dition Time m/z Method 935 EZ9-1 Trifluoro- methanesulfonic acid 8-cyano- 11,11-dimethyl-5- oxo-10,11- dihydro-5H- pyrido[2,3- b]carbazol-2-yl ester U 2.93 436 B1 936 EZ9-2 11,11-Dimethyl- 5-oxo-2- (tetrahydro-pyran- 4-yloxy)-10,11- dihydro-5H- pyrido[2,3- b]carbazole-8- carbonitrile U 2.57 388 A7-1 937 EZ9-3 2-(2- Diethylamino- ethoxy)-11,11- dimethyl-5-oxo- 10,11-dihydro-5H- pyrido[2,3- b]carbazole-8- carbonitrile Y 1.63 403 A7-17 938 EZ9-4 2-(2- Diethylamino- ethoxy)-10-(2- diethylamino- ethyl)-11,11- dimethyl-5-oxo- 10,11-dihydro-5H- pyrido[2,3- b]carbazole-8- carbonitrile Y 1.82 502 A7-17 939 EZ9-5 2-(2- Diethylamino- ethoxy)-11,11- dimethyl-5-oxo- 5,11-dihydro- benzo[4,5]furo[3,2- g]quinoline-8- carbonitrile Y 1.77 404 A7-17 - The compounds described in the following Table 17 were synthesized from Compound W3 and corresponding halide by alkylation of hydroxyl group according to the method that is used for the synthesis of Compound A7-17.
-
TABLE 17 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 940 W4-3 7-(2-Dimethylamino- ethoxy)-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H-benzo[b]carbazole- 3-carbonitrile 1 0.96 374.0 941 W4-4 7-(3-Dimethylamino- propoxy)-6,6-dimethyl- 11-oxo-6,11-dihydro- 5H-benzo[b]carbazole- 3-carbonitrile 1 0.92 388.0 - The compounds described in the following Table 18 were synthesized according to the method shown below. Specifically, Compound GT1-1 was prepared from Compound J2 and phenylhydrazine according to the method that is used for the synthesis of Compound A3 and Compound A4. Subsequently, in accordance with the methylation carried out in the same manner as Compound A10-1, Compound GT1-2 was prepared.
- The compounds described in the following Table 19 were synthesized according to the method shown below. Specifically, Compound GT2-1 was prepared from Compound A2 and phenylhydrazine according to the method that is used for the synthesis of Compound A3 and Compound A4.
- Subsequently, by carrying out the alkylation in the same manner as Compound A10-1, Compound GT2-2 and Compound GT2-8 were prepared.
- To obtain the compounds of the Table, chemical conversion of Compound GT2-1 or the 5-alkylate of Compound GT2-1 was achieved by using in combination the functional group modifications (e.g., demethylation according to the method used for the preparation of Compound A6 and subsequent introduction of a functional group, etc.) as explained before and described in the Table.
-
TABLE 19 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z Method 944 GT2-1 8-Methoxy-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 2.30 292.0 A3 A4 945 GT2-2 8-Methoxy-5,6,6- trimethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 2.55 306.0 A10-1 946 GT2-3 8-(2- Diethylamino- ethoxy)-5,6,6- trimethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 1.90 391.0 A6 A7-17 A10-1 947 GT2-4 8-((R)-2,3- Dihydroxy- propoxy)-5,6,6- trimethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 1.90 366.0 A6 A7-17 A10-1 948 GT2-5 8-(2- Diethylamino- ethoxy)-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one F 1.93 377.3 A6 A7-17 949 GT2-6 8-(2- Diethylamino- ethoxy)-6,6- dimethyl-5- propyl-5,6- dihydro- benzo[b]carbazol- 11-one A 2.09 419.0 A6 A7-17 A10-1 950 GT2-7 5-Benzyl-8-(2- diethylamino- ethoxy)-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one B 4.83 467.3 A6 A7-17 A10-1 951 GT2-8 5-Ethyl-8- methoxy-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one F 2.94 320.0 A10-1 952 GT2-9 8-(2- Diethylamino- ethoxy)-5-ethyl- 6,6-dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one F 2.16 405.0 A6 A7-17 A10-1 953 GT2-10 8-(2- Diethylamino- ethoxy)-5- isopropyl-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 2.02 419.0 A6 A7-17 A10-1 954 GT2-11 8-((R)-2,3- Dihydroxy- propoxy)-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one C 2.17 352.2 A6 A7-17 A7- 14-2 955 GT2-12 5- Methanesulfonyl- 8-methoxy-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one C 2.81 370.1 A9-1 956 GT2-13 8-(2- Diethylamino- ethoxy)-5- methanesulfonyl- 6,6-dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one F 2.20 455.1 A6 A7-17 A9-1 957 GT2-14 8-(2- Diethylamino- ethoxy)-5-(2- hydroxy-ethyl)- 6,6-dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one H 3.73 421.0 A6 A7-17 A10-1 958 GT2-15 6,6-Dimethyl-8- ((2R,3R)-2,3,4- trihydroxy- butoxy)-5,6- dihydro- benzo[b]carbazol- 11-one H 3.73 382.4 A7-1 A7- 14-2 - The compounds described in the following Table 20 were synthesized according to the method shown below. Specifically, by using Compound A2 and phenylhydrazine having a corresponding substituent group, 2 (or 3)-substituted-8-methoxy-6,6-dimethyl-6,11-dihydro-5H-benzo[b]carbazol-11-one was prepared according to the method that is used for the synthesis of Compound A3 and Compound A4. Subsequently, to obtain the compounds of the Table, chemical conversion of the above compounds was achieved by using in combination the functional group modifications as explained before and described in the Table.
-
TABLE 20 Exam- HPLC Reten- ple Comp. Condi- tion No. No. Structure Compound Name tion Time m/z Method 959 GT3-1 8-Methoxy-3,6,6- trimethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 2.39 306.0 A3 A4 960 GT3-2 8-(2-Diethylamino- ethoxy)-3,5,6,6- tetramethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 1.97 405.0 A6 A7-17 A10-1 961 GT3-3 8-(2-Diethylamino- ethoxy)-6,6- dimethyl-3-nitro- 5,6-dihydro- benzo[b]carbazol- 11-one B 3.86 422.2 A6 A7-17 962 GT3-4 8-(2-Diethylamino- ethoxy)-6,6- dimethyl-3- trifluoromethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 2.03 445.0 A6 A7-17 963 GT3-5 8-(2-Diethylamino- ethoxy)-5-(2- diethylamino- ethyl)-6,6- dimethyl-3-nitro- 5,6-dihydro- benzo[b]carbazol- 11-one F 1.74 521.3 A6 A7-17 A10-1 964 GT3-6 2,6,6-Trimethyl-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 5,6-dihydro- benzo[b]carbazol- 11-one F 2.03 396.0 A6 A7-17 A7- 14-2 965 GT3-7 2-Fluoro-6,6- dimethyl-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 5,6-dihydro- benzo[b]carbazol- 11-one F 1.99 400.0 A6 A7-17 A7-14-2 966 GT3-8 2-Chloro-6,6- dimethyl-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 5,6-dihydro- benzo[b]carbazol- 11-one F 2.14 416.0 A6 A7-17 A7- 14-2 967 GT3-9 6,6-Dimethyl-11- oxo-8-((2R,3R)- 2,3,4-trihydroxy- butoxy)-6,11- dihydro-5H- benzo[b]carbazole- 2-carbonitrile F 1.90 407.4 A6 A7-17 A7- 14-2 968 GT3-10 6,6-Dimethyl-3- trifluoromethoxy-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 5,6-dihydro- benzo[b]carbazol- 11-one F 2.31 466.4 A6 A7-17 A7- 14-2 969 GT3-11 8-((R)-2,3- Dihydroxy- propoxy)-6,6- dimethyl-3- trifluoromethoxy- 5,6-dihydro- benzo[b]carbazol- 11-one F 2.43 436.4 A6 A7-17 A7- 14-2 970 GT3-12 8-((R)-2,3- Dihydroxy- propoxy)-3- methoxy-5,6,6- trimethyl-5,6- dihydro- benzo[b]carbazol- 11-one B 3.79 396.5 A6 A7-17 A10-1 A7- 14-2 971 GT3-13 8-((R)-2,3- Dihydroxy- propoxy)-3- methoxy-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one B 3.40 382.4 A6 A7-17 A7- 14-2 - The compounds described in the following Table 21 were synthesized according to the method shown below. Specifically, by using Compound E1 and phenylhydrazine having a corresponding substituent group, 9-bromo-1-chloro-8-methoxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one or 9-bromo-8-methoxy-6,6-dimethyl-3-trifuloromethoxy-5,6-dihydro-benzo[b]carbazol-11-one was prepared according to the method used for the synthesis of Compound A3 and Compound A4. Subsequently, to obtain the compounds of the Table, chemical conversion of the above compounds was achieved by using in combination the functional group modifications as explained before and described in the Table.
-
TABLE 21 Exam- HPLC Reten- ple Comp. Condi- tion No. No. Structure Compound Name tion Time m/z Method 972 GT4-1 9-Bromo-8-((R)- 2,3-dihydroxy- propoxy)-6,6- dimethyl-3- trifluoromethoxy- 5,6-dihydro- benzo[b]carbazol- 11-one C 2.68 514.0 A6 A7-17 A7- 14-2 973 GT4-2 9-Bromo-1- chloro-8-((R)-2,3- dihydroxy- propoxy)-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one C 2.58 464.0 A6 A7-17 A7- 14-2 - The compounds described in the following Tables 22-23 were synthesized according to the method shown below. Specifically, catalytic reduction of Compound GT3-3 was carried out according to the method used for the preparation of Compound D2 to prepare Compound GT5-1.
- Reductive alkylation of Compound GT5-1 was carried out according to the method used for the preparation of Compound B3-32 for the introduction of a methyl group or a benzyl group (Compound GT5-2, Compound GT5-3).
- Catalytic reduction of Compound GT5-3 was carried out according to the method used for the preparation of Compound D2, and then processed to prepare Compound GT5-4.
- The resulting amino derivatives of Compound GT5-1 to 4 were reacted with corresponding acyl chloride, isocynate, or chloroformate according to the method used for the preparation of Compound A9-1 to obtain the compounds of the Table.
-
TABLE 22 Exam- Reten- ple Comp. HPLC tion No. No. Structure Compound Name Condition Time m/z Method 974 GT5-1 3-Amino-8-(2- diethylamino- ethoxy)-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 1.15 392.3 D2 975 GT5-2 8-(2-Diethylamino- ethoxy)-3- dimethylamino-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one A 1.18 420.2 B3-32 976 GT5-3 3-(Benzyl-methyl- amino)-8-(2- diethylamino- ethoxy)-6,6- dimethyl-5,6- dihydro- benzo[b]carbazol- 11-one B 3.05 496.4 B3-32 977 GT5-4 8-(2-Diethylamino- ethoxy)-6,6- dimethyl-3- methylamino-5,6- dihydro- benzo[b]carbazol- 11-one B 2.46 406.3 B3-32 D2 978 GT5-5 Pentanoic acid [8- (2-diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]amide C 2.52 476.5 A9-1 979 GT5-6 N-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-2,2-dimethyl- A 1.74 476.4 A9-1 propionamide 980 GT5-7 [8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-carbamic acid 2-methoxy-ethyl A 1.55 494.3 A9-1 ester 981 GT5-8 1-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-3-phenyl-urea B 3.79 511.3 A9-1 982 GT5-9 N-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-2-phenyl- acetamide B 3.81 510.4 A9-1 983 GT5-10 N-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-3- trifluoromethyl- benzamide B 4.47 564.4 A9-1 984 GT5-11 1-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-3-(3- trifluoromethyl- B 4.55 579.4 A9-1 phenyl)-urea 985 GT5-12 [8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-carbamic acid 3-trifluoromethyl- H 5.17 580.1 A9-1 phenyl ester 986 GT5-13 N-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-2-phenoxy- acetamide C 2.57 526.1 A9-1 987 GT5-14 1-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-1-methyl-3- phenyl-urea B 3.83 525.6 A9-1 -
TABLE 23 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z Method 988 GT5-15 1-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-1-methyl-3- (3-trifluoromethyl- phenyl)-urea B 4.58 593.4 A9-1 989 GT5-16 3-Benzyl-1-[8-(2- diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-1-methyl- urea B 3.81 539.4 A9-1 990 GT5-17 N-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-N-methyl-3- trifluoromethyl- benzamide B 415 578.3 A9-1 991 GT5-18 N-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]N-methyl-2- phenoxy- acetamide C 262 540.4 A9-1 992 GT5-19 3-(4-tert-Butyl- phenyl)-1-[8-(2- diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-1-methyl- F 2.45 581.6 A9-1 urea 993 GT5-20 1-[8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]-3-(4- methoxy-phenyl)- B 377 555.4 A9-1 1-methyl-urea 994 GT5-21 [8-(2- Diethylamino- ethoxy)-6,6- dimethyl-11-oxo- 6,11-dihydro-5H- benzo[b]carbazol- 3-yl]carbamic acid phenyl ester A 1.89 512.2 A9-1 - The compounds described in the following Table 24 were synthesized according to the method shown below. Specifically, having Compound T22-1 as a starting material, 8-[(4R,5R)-5-(tert-butyl-dimethylsilanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6-dimethyl-5,6-dihydro-11-oxo-benzo[b]carbazole-3-carboxylic acid was prepared according to the method that is used for the preparation of Compound B2-28.
- The resulting carboxylic acid was subjected to dehydrating condensation with corresponding amine, alcohol according to the method that is used for the preparation of Compound A9-10. Subsequently, deprotection was carried out according to the method used for the preparation of Compound T22-1-1 and Compound T22-1-2 to obtain the compounds described in the Table.
-
TABLE 24 Exam- HPLC Reten- ple Comp. Condi- tion No. No. Structure Compound Name tion Time m/z Method 995 GT6-1 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid phenylamide A 1.79 501.0 A9-10 996 GT6-2 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid dimethylamide D 1.33 453.0 A9-10 997 GT6-3 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid 2- hydroxy-ethyl ester A 1.40 470.0 A9-10 998 GT6-4 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid butylamide D 1.55 481.0 A9-10 999 GT6-5 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid (2-methoxy-ethyl)-amide D 1.30 483.0 A9-10 1000 GT6-6 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid methylamide D 1.24 439.0 A9-10 1001 GT6-7 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid benzylamide D 1.58 515.0 A9-10 1002 GT6-8 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid amide D 1.18 425.0 A9-10 1003 GT6-9 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihyrdo-5H-benzo[b]carbazole- 3-carboxylic acid pyridin-4ylamide D 1.40 502.0 A9-10 1004 GT6-10 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid D 1.01 426.0 E2-28 1005 GT6-11 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6-,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid pyridin-2-ylamide D 1.52 502.0 A9-10 1006 GT6-12 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carboxylic acid pyridin-3-ylamide D 1.34 502.0 A9-10 1007 GT6-13 6,6-Dimethyl-11-oxo-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6,11- dihydro-5H-benzo[b]carbazole- 3-carbolic acid phenethyl-amide D 1.67 529.0 A9-10 1008 GT6-14 N-[6,6-Dimethyl-11-oxo-8- ((2R,3R)-2,3,4-trihydroxy- butoxy)-6,11-dihydro- 5H-benzo[b]carbazole-3- carbonyl]- benzenesulfonamide D 1.23 565.0 A9-10 - To the compounds described in the following Table 25, a hydroxyl group was introduced from Compound T17-3 according to the method described in JACS 2006, Vol. 128, page 10964. Subsequently, deprotection was carried out according to the method used for Compound A7-14-2 and Compound T22-2 to obtain the compounds shown below.
-
TABLE 25 Exam- Reten- ple Comp. HPLC tion No. No. Structure Compound Name Condition Time m/z 1009 GT7-1 8-((R)-2,3-Dihydroxy-propoxyl-3- hydroxy-6,6-dimethyl-5,6-dihydro- benzo[b]carbazol-11-one B 259 368.4 1010 GT7-2 8-((R)-2,3-Dihydroxy-propoxy-3- hydroxy-5,6,6-trimethyl-5,6-dihydro- benzo[b]carbazol-11-one C 1.90 382.4 - The compounds described in the following Table 26 were prepared by alkylation according to the method used for the preparation of Compound A7-1 from Compound GT7-1 or Compound GT7-2, or by carbamation according to the method used for the preparation of A9-1.
-
TABLE 26 Exam- HPLC Reten- ple Comp. Condi- tion No. No. Structure Compound Name tion Time m/z Method 1011 GT8-1 8-((R)-2,3-Dihydroxy-propoxy)- 3-ethoxy-5,6,6-trimethyl-5,6- dihydro-benzo[b]carbazol-11- one C 2.43 410.5 A7-1 1012 GT8-2 8-((R)-2,3-Dihydroxy-propoxy)- 3-ethoxy-6,6-dimethyl-5,6- dihydro-benzo[b]carbazol-11- one C 2.30 396.5 A7-1 1013 GT8-3 8-((R)-2,3-Dihydroxy-propoxy)- 5,6,6-trimethyl-3-(oxetan-3- yloxy)-5,6-dihydro-benzo[b] carbazol-11-one B 1.72 438.5 A7-1 1014 GT8-4 Phenyl-carbamic acid 8-((R)- 2,3-dihydroxy-propoxy)- 6,6-dimethyl- 11-oxo-6,11-dihydro-5H- benzo[b]carbazol-3-yl ester B 4.23 487.0 A9-1 - Compound GT9-1
-
- 8-((S)-2,2-Dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (20.0 mg, 0.048 mmol), ammonium chloride (1.28 mg, 0.024 mmol) and NaN3 (6.24 mg, 0.096 mmol) were dissolved in DMF, and the mixture was stirred at 120° C. for 14 hrs. NaN3 (6.24 mg, 0.096 mmol) was further added to the mixture, which was then stirred at 120° C. for 30 hrs. The reaction solution was added with 1 N aqueous solution of hydrochloric acid and extracted with ethyl acetate. The organic layer was washed with saturated brine and concentrated under reduced pressure. The resultant solid obtained after concentration was washed with hexane: ethyl acetate=1: 1 to obtain 8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-3-(2H-tetrazol-5-yl)-5,6-dihydro-benzo[b]carbazol-11-one as a white solid.
- The product was suspended in MeOH (1.0 ml), added with 1 N aqueous solution of hydrochloric acid, and then stirred at 60° C. for 1 hr and 30 min. After cooling to room temperature, the reaction solution was concentrated under reduced pressure, and the resulting solid was washed with DCM to obtain the title compound as a pale yellow solid (13.4 mg, 66.3%).
- LCMS: m/z 420 [M+H]+
- Compound GT9-2
-
- 3-Bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (20.0 mg, 0.043 mmol), thiophene-3-boronic acid (10.9 mg, 0.085 mmol), K3PO4 (40 mg) and Pd (PPh3)4 (9.9 mg, 0.0086 mmol) were dissolved in DMA (0.8 ml) and water (0.2 ml), and stirred at 140° C. for 10 min under microwave irradiation. The reaction solution was diluted with ethyl acetate and washed with saturated brine. The organic layer was concentrated under reduced pressure, and the resulting residues were purified by column chromatography (hexane/ethyl acetate) to obtain 8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-3-thiphen-3-yl-5,6-dihydro-benzo[b]carbazol-11-one.
- This product was suspended in MeOH (1.0 ml), added with 1 N hydrochloric acid, and then stirred at 60° C. for 1 hr and 30 min. The reaction solution was concentrated under reduced pressure, and the resulting solid was washed with DCM to obtain the title compound as a yellow solid (12.6 mg, 67.1%).
- LCMS: m/z 434 [M+H]+
- Using the combination of Compound T18-1 and corresponding boronic acid or the combination of (S)-8-((2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)-6,6-dimethyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5H-benzo[b]carbazol-11 (6H)-one and corresponding bromide, the reaction was carried out in the same manner as Compound GT9-2 to obtain the compounds of the following Table 27.
-
TABLE 27 Exam- Reten- ple Comp. HPLC tion No. No. Structure Compound Name Condition Time m/z 1017 GT9-3 8-((R)-2,3-Dihydroxy-propoxy)-6,6- dimethyl-3-thiophen-2-yl-5,6-dihydro- benzo[b]carbazol-11-one B 4.59 494.0 1018 GT9-4 8-((R)-2,3-Dihydroxy-propoxy)-6,6- dimethyl-3-(1H-pyrazol-4-yl)-5,6 dihydro-benzo[b]carbazol-11-one B 4.04 418.0 1019 GT9-5 8-((R)-2,3-Dihydroxy-propoxy)-6,6- dimethyl-3-(2H-pyrazol-3-yl)-5,6- dihydro-benzo[b]carbazol-11-one A 1.51 418.0 1020 GT9-6 8-((R)-2,3-Dihydroxy-propoxy)-6,6- dimethyl-3-thiazol-5-yl-5,6-dihydro- benzo[b]carbazol-11-one F 1.97 435.0 1021 GT9-7 8-((R)-2,3-Dihydroxy-propoxy)-3-(3H- imidazol-4-yl)-6,6-dimethyl-5,6-dihydro- benzo[b]carbazol-11-one H 2.91 418.0 - Compound GT10-1
-
- Preparation was carried out in the same manner as Compound N6-1-2.
- LCMS: m/z 434 [M+H]+
- HPLC retention time: 1.56 min (analysis condition A)
- The compounds described in the following Table 28—were synthesized according to the method shown below. Specifically, by using the method for the preparation of Compound Z10, Z11, Z12 and Z13, 8-hydroxy-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one was prepared from Compound A2 and bromophenol. To the resulting compound, a side chain or a synthetic equivalent thereof was introduced according to Mitsunobu reaction that is used for the preparation of Compound A7-1 or the method that is used for A7-17, etc. After that, if necessary, functional group modification such as deprotection, etc. was carried out to prepare the compounds listed below.
-
TABLE 28 Exam- Reten- ple Comp. HPLC tion No. No. Structure Compound Name Condition Time m/z 1023 GT11-1 (R)-5-(6,6-Dimethyl-11-oxo-6,11- dihydro-benzo[b]naphtho[2,3-d]furan- 8-yloxy)-4-hydroxy-pentanoic acid H 5.37 395.0 1024 GT11-2 (R)-5-(6,6-Dimethyl-11-oxo-6,11- dihydro-benzo[b]naphtho[2,3-d]furan- 8-yloxymethyl)-pyrrolidin-2-one H 5.50 376.0 1025 GT11-3 8-(3-Hydroxy-propoxy)-6,6-dimethyl- 6H-benzo[b]naphtho[2,3-d]furan-11- one H 5.97 337.0 1026 GT11-4 8-(3-Ethyl-3-hydroxy-pentyloxy)-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one H 9.29 393.0 1027 GT11-5 (6,6-Dimethyl-11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d]furan-8-yloxy)- acetic acid H 5.65 336.0 1028 GT11-6 4-(6,6-Dimethyl-11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d]furan-8-yloxy)- butyric acid H 6.15 365.0 1029 GT11-7 5-(6,6-Dimethyl-11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d]furan-8-yloxy)- pentanoic acid H 6.44 379.0 1030 GT11-8 6-(6,6-Dimethyl-11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d]furan-8-yloxy)- hexanoic acid H 6.77 393.0 1031 GT11-9 2-(6,6-Dimethyl-11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d]furan-8-yloxy)- N,N-diethyl-acetamide H 6.39 392.0 1032 GT11-10 6,6-Dimethyl-8-(2-morpholin-4-yl- ethoxy)-6H-benzo[b]naphtho[2,3- d]furan-11-one H 4.45 392.0 1033 GT11-11 8-(2-Dimethylamino-ethoxy)-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one H 4.59 350.0 -
TABLE 29 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1034 GT11-12 8-((S)-2,3-Dihydroxy-propoxy)-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one F 2.40 353.0 1035 GT11-13 8-((R)-2,3-Dihydroxy-propoxy)-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one H 5.07 353.0 1036 GT11-14 6,6-Dimethyl-8-(2-pyrrolidin-1-yl- ethoxy)-6H-benzo[b]naphtho[2,3- d]furan-11-one C 3.03 375.9 1037 GT11-15 6,6-Dimethyl-8-(2-piperidin-1-yl- ethoxy)-6H-benzo[b]naphtho[2,3- d]furan-11-one C 3.15 389.9 1038 GT11-16 8-(3-Dimethylamino-propoxy)-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one C 3.30 364.2 1039 GT11-17 8-(2-Azepan-1-yl-ethoxy)-6,6-dimethyl- 6H-benzo[b]naphtho[2,3-d]furan-11- one F 2.35 404.3 1040 GT11-18 (6,6-Dimethyl-11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d]furan-8-yloxy)- acetic acid methyl ester D 2.38 351.0 1041 GT11-19 2-(6,6-Dimethyl-11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d]furan-8-yloxy)- N-(2-morpholin-4-yl-ethyl)-acetamide D 2.03 450.0 1042 GT11-20 6,6-Dimethyl-8-(2-piperazin-1-yl- ethoxy)-6H-benzo[b]naphtho[2,3- d]furan-11-one C 2.81 391.2 1043 GT11-21 8-[2-(4-Methanesulfonyl-piperazin-1- yl)-ethoxy]-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one F 2.21 469.1 1044 GT11-22 2-(6,6-Dimethyl-11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d]furan-8-yloxy)- acetamide D 1.95 336.0 1045 GT11-23 4-[2-(6,6-Dimethyl-11-oxo-6,11- dihydro-benzo[b]naphtho[2,3-d]furan- 8-yloxy)-ethyl]-piperazine-1-carboxylic acidamide F 2.04 434.0 1046 GT11-24 N-(2,3-Dihydroxy-propyl)-2-(6,6- dimethyl-11-oxo-6,11-dihydro- benzo[b]naptho[2,3-d]furan-8-yloxy)- acetamide D 1.82 410.0 -
TABLE 30 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1047 GT11-25 8-[2-(4-Acetyl-piperazin-1-yl)- ethoxy]-6,6-dimethyl-6H- benzo[b]naphtho [2,3-d]furan-11-one F 2.10 433.1 1048 GT11-26 4-[2-(6,6-Dimethyl-11-oxo-6,11- dihydro-benzo[b]naphtho[2,3-d] furan-8-yloxy)-acetyl]- piperazine-1-carboxylic acid tert-butyl ester D 2.53 505.0 1049 GT11-27 8-[2-(2-Hydroxy-ethoxy)- ethoxy]-6,6-dimethyl-6H- benzo[b]naphtho[2,3- d]furan-11-one H 5.62 367.0 1050 GT11-28 8-{2-[2-(2-Hydroxy-ethoxy)- ethoxy]-ethoxy}-6,6- dimethyl-6H-benzo[b]naphtho [2,3-d]furan-11-one H 5.64 411.0 1051 GT11-29 8-(2-Imidazol-1-yl-ethoxy)-6,6- dimethyl-6H-benzo[b] naphtho[2,3-d]furan-11-one F 2.05 373.1 1052 GT11-30 2-(6,6-Dimethyl-11-oxo- 6,11-dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)- N-(2-pyridin-4-yl-ethyl)- acetamide D 2.10 441.0 1053 GT11-31 N-(2-Dimethylamino-ethyl)- 2-(6,6-dimethyl-11-oxo- 6,11-dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)- acetamide D 2.01 407.0 1054 GT11-32 2-(6,6-Dimethyl-11-oxo- 6,11-dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)- N-[2-(2-hydroxy-ethoxy)- ethyl]-acetamide D 1.88 424.0 1055 GT11-33 6,6-Dimethyl-8-[2-(4-methyl- piperazin-1-yl)-ethoxy]- 6H-benzo[b]naphtho [2,3-d]furan-11-one F 405.2 1056 GT11-34 6,6-Dimethyl-8-((2R,3R)-2,3,4- trihydroxy-butoxy)-6H- benzo[b]naphtho [2,3-d]furan-11-one F 2.21 383.0 1057 GT11-35 8-((R)-2-Hydroxy-3- piperidin-1-yl-propoxy)-6,6- dimethyl-6H- benzo[b]naphtho [2,3-d]furan-11-one F 2.25 420.0 1058 GT11-36 6,6-Dimethyl-8-(2-oxo-2- piperazin-1-yl-ethoxy)- 6H-benzo[b]naphtho[2,3- d]furan-11-one D 1.92 405.0 1059 GT11-37 8-{2-[4-(2-Hydroxy-acetyl)- piperazin-1- yl]-ethoxy}-6,6-dimethyl-6H- benzo[b]naphtho [2,3-d]furan-11-one F 2.06 449.1 1060 GT11-38 8-((S)-2-Hydroxy-3-piperidin-1-yl- propoxy)-6,6-dimethyl-6H- benzo[b]naptho[2,3-d]furan-11-one F 2.24 420.0 indicates data missing or illegible when filed -
TABLE 31 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1061 GT11-39 8-[2-((R)-2,3-Dihydroxy- propylamino)- ethoxy]-6,6-dimethyl-6H- benzo[b]naphtho [2,3-d]furan-11-one H 4.18 1062 GT11-40 8-((S)-4,5-Dihydroxy- pentyloxy)-6,6- dimethyl-6H-benzo[b] naphtho[2,3-d]furan-11-one F 2.56 381.0 1063 GT11-41 4-[2-(6,6-Dimethyl-11-oxo-6,11- dihydro-benzo[b]naphtho [2,3-d]furan- 8-yloxy)-ethyl]- piperazin-2-one F 2.06 405.0 1064 GT11-42 2-(6,6-Dimethyl-11-oxo- 6,11-dihydro- benzo[b]naphtho[2,3-d] furan-8-yloxy)- N-piperidin-4-yl-acetamide D 419.0 1065 GT11-43 N-{2-[Bis-(2-hydroxy- ethyl)-amino]- ethyl}-2-(6,6-dimethyl-11-oxo- 6,11-dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)-acetamide D 1.83 467.0 1066 GT11-44 N-(3-Dimethylamino-propyl)- 2-(6,6-dimethyl-11-oxo- 6,11-dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)- acetamide D 2.08 422.0 1067 GT11-45 N-(2-Diethylamino-ethyl)- 2-(6,6-dimethyl-11-oxo-6,11- dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)- acetamide D 2.10 1068 GT11-46 6,6-Dimethyl-8-(pyrimidin- 2-yloxy)-6H-benzo[b]naphtho [2,3-d]furan-11-one H 6.35 357.0 1069 GT11-47 8-(2-Ethylamino-ethoxy)- 6,6-dimethyl- 6H-benzo[b]naphtho [2,3-d]furan-11-one F 2.15 350.0 1070 GT11-48 1-[2-(6,6-Dimethyl-11-oxo- 6,11-dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)-ethyl]- piperazin-2-one H 4.42 405.0 1071 GT11-49 4-[2-(6,6-Dimethyl-11-oxo- 6,11-dihydro-benzo[b] naphtho[2,3-d]furan- 8-yloxy)-ethyl]-1- methyl-piperazin-2-one H 4.33 419.0 1072 GT11-50 2-(6,6-Dimethyl-11-oxo- 6,11-dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)- N-(2-piperazin-1-yl-ethyl)- acetamide A 3.99 448.0 1073 GT11-51 2-Dimethylamino- N-[2-(6,6-dimethyl- 11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d] furan-8-yloxy)- ethyl]-acetamide D 2.13 408.0 1074 GT11-52 4-[2-(6,6-Dimethyl-11-oxo- 6,11-dihydro-benzo[b]naptho [2,3-d]furan-8-yloxy)-ethyl]- 1,1-dimethyl-3-oxo- piperazin-1-ium; chloride H 4.47 indicates data missing or illegible when filed -
TABLE 32 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1075 GT12-53 8-{2-[4-(2-Hydroxy-ethyl)- piperazin-1-yl]-ethoxy}-6,6- dimethyl-6H-benzo[b] naphtho[2,3-d]furan-11-one A 1.75 435.0 1076 GT12-54 1-[2-(6,6-Dimethyl-11-oxo- 6,11-dihydro-benzo[b]naphtho [2,3-d]furan-8-yloxy)-ethyl]- 4-methyl-piperazin-2-one B 4.09 419.0 1077 GT12-55 6,6-Dimethyl-8-(3-piperazin-1-yl- propoxy)-6H-benzo[b]naphtho[2,3- d]furan-11-one A 1.75 405.0 1078 GT12-56 8-{2-[4-((S)-2,3-Dihydroxy- propyl)-piperazin-1-yl]-ethoxy}- 6,6-dimethyl-6H-benzo[b]naphtho [2,3-d]furan-11-one A 1.72 465.0 1079 GT12-57 8-[2-(2-Hydroxy-1,1- dimethyl-ethylamino)-ethoxy]- 6,6-dimethyl-6H-benzo[b] naphtho[2,3-d]furan-11-one 2.57 394.0 1080 GT12-58 8-{2-[Bis-(2-hydroxy-ethyl)- amino]-ethoxy}-6,6- dimethyl-6H-benzo[b]naphtho [2,3-d]furan-11-one 2.77 410.0 1081 GT12-59 8-[2-(3-Hydroxy-piperidin-1-yl)- ethoxy]-6,6-dimethyl-6H- benzo[b]naphtho[2,3- d]furan-11-one 2.88 406.0 1082 GT12-60 8-[2-(2-Hydroxymethyl- pyrrolidin-1-yl)- ethoxy]-6,6-dimethyl-6H- benzo[b]naphtho [2,3-d]furan-11-one 2.82 406.0 1083 GT12-61 8-{2-[Ethyl-(2-hydroxy- ethyl)-amino]- ethoxy}-6,6-dimethyl-6H- benzol[b]naphtho [2,3-d]furan-11-one 2.85 394.0 1084 GT12-62 6,6-Dimethyl-8-(3-methyl- oxetan-3- ylmethoxy)-6H- benzo[b]naphtho[2,3- d]furan-11-one 3.03 363.0 1085 GT12-63 8-[2-(1-Hydroxymethyl- cycloperrtylamino)-ethoxy]- 6,6-dimethyl- 6H-benzo[b]naphtho [2,3-d]furan-11-one 2.72 420.0 1086 GT12-64 8-(4-Hydroxy-pyrrolidin- 2-ylmethoxy)-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d] furan-11-one Y 2.96 378.0 1087 GT12-65 6,6-Dimethyl-8-(piperidin- 3-yloxy)-6H-benzo[b]naphtho [2,3-d]furan-11-one Y 3.03 362.0 indicates data missing or illegible when filed - The compounds described in the following Table 33 were synthesized according to the method shown below. From Compound A2 and 2-bromophenol having a fluorine atom at corresponding position, 8-methoxy-6H-benzo[b]naphtho[2,3-d]furan-11-one having a fluorine atom at corresponding position (Compound GT12-1, GT12-2, GT12-5 and GT12-7) was prepared according to the method used for the preparation of Compound Z10, Z11 and Z12. Further, demethylation was carried out according to the method used for the preparation of Compound A6 to obtain 8-hydroxy-6H-benzo[b]naphtho[2,3-d]furan-11-one which has a fluorine atom at corresponding position. Thereafter, according to Mitsunobu reaction used for the preparation of Compound A7-1 or the alkylation method used for the preparation of Compound A7-17, a corresponding side chain was introduced and, if necessary, functional group modification such as deprotection, etc. was carried out to prepare the compounds listed below.
-
TABLE 33 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1088 GT12-1 3-Fluoro-8-methoxy-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one A 3.02 311.0 1089 GT12-2 4-Fluoro-8-methoxy-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one A 3.00 311.0 1090 GT12-3 8-(2-Diethylamino-ethoxy)-3-fluoro-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one B 4.48 396.0 1091 GT12-4 3-Fluoro-6,6-dimethyl-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6H- benzo[b]naphtho[2,3-d]furan-11-one H 4.91 401.4 1092 GT12-5 2-Fluoro-8-methoxy-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one 3.01 311.0 1093 GT12-6 8-(2-Diethylamino-ethoxy)-2-fluoro-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one 2.09 395.0 1094 GT12-7 1-Fluoro-8-methoxy-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one B 6.26 311.0 1095 GT12-8 8-((R)-2,3-Dihydroxy-propoxy)-2- fluoro-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one 2.22 371.0 1096 GT12-9 8-(2-Diethylamino-ethoxy)-1-fluoro-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one B 4.20 396.0 1097 GT12-10 8-((R)-2,3-Dihydroxy-propoxy)-3- fluoro-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one B 371.0 1098 GT12-11 8-((R)-2,3-Dihydroxy-propoxy)-4- fluoro-6,6-dimethyl-6H- benzo[d]naphtho[2,3-d]furan-11-one D 1.80 371.0 1099 GT12-12 8-((R)-2,3-Dihydroxy-propoxy)-1- fluoro-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one D 1.85 371.0 indicates data missing or illegible when filed - The compounds described in the following Table 34 were synthesized according to the method shown below. 8-Hydroxy-6H-benzo[b]naphtho[2,3-d]furan-11-one was transformed into trifuloromethanesulfonic acid ester according to the method used for the preparation of Compound BT. Subsequently, by carrying out the method used for the preparation of Compound B2-1 or Compound B2-18, Compound GT13-1, Compound GT13-2 and Compound GT13-3 were prepared. Compound GT13-3 was oxidized according to the method used for the preparation of Compound B3-8 to prepare Compound 13-4.
-
TABLE 34 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z Method 1100 GT13-1 6,6-Dimethyl-8-morpholin-4-yl-6H- benzo[b]naphtho[2,3-d]furan-11-one F 3.04 348.2 B1, B2-1 1101 GT13-2 6,6-Dimethyl-8-(4-methyl-piperazin-1- yl)-6H-benzo[b]naphtho[2,3-d]furan- 11-one F 2.13 361.3 B1, B2-1 1102 GT13-3 8-(2-Diisopropylamino-ethylsulfanyl)- 6,6-dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one 3.45 422.0 1103 GT13-4 8-(2-Diisopropylamino- ethanesulfonyl)-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one 3.23 454.0 indicates data missing or illegible when filed - The compounds described in the following Table 35 were synthesized according to the method shown below. Specifically, a side chain was introduced to Compound Z13 to prepare Compound GT13-5 according to the method that is used for the preparation of Compound A7-17. Further Compound GT13-5 or trifuloromethanesulfonic acid ester of Compound Z14 was hydrolyzed to prepare Compound GT13-6 and Compound GT13-7 according to the method used for the preparation of Compound T20.
-
TABLE 35 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z Method 1104 GT13-5 Trifluoro-methanesulfonic acid 8-(2-diethylamino-ethoxy)- 6,6-dimethyl-11-oxo-6,11- dihydro-benzo[b]naphtho [2,3-d]furan-3-yl ester H 5.37 526.0 A7-17 1105 GT13-6 8-(2-Diethylamino-ethoxy)- 3-hydroxy-6,6-dimethyl-6H- benzo[b]naphtho[2,3- d]furan-11-one B 3.40 384.0 T20 1106 GT13-7 3-Hydroxy-6,6-dimethyl- 8-((2R,3R)-2,3,4-trihydroxy- butoxy)-6H-benzo[b]naphtho [2,3-d]furan-11-one F 1.87 399.0 T20 - The compounds described in the following Table 36 were prepared by subjecting Compound GT13-6 or GT13-7 to Mitsunobu reaction that is used for the preparation of Compound A7-1 to introduce a corresponding side chain or a synthetic equivalent. After that, by carrying out deprotection, if necessary, the compounds listed below were prepared.
-
TABLE 36 HPLC Re- Exam- Comp. Con- tention Me- ple No. No. Structure Compound Name dition Time m/z thod 1107 GT13-8 8-(2-Diethylamino- ethoxy)-3-methoxy- 6,6-dimethyl-6H- benzo[b]naphtho[2,3- d]furan-11-one H 4.72 408.0 A7-1 1108 GT13-9 3-Methoxy-6,6- dimethyl-8-((2R,3R)- 2,3,4-trihydroxy- butoxy)-6H -benzo[b] naphtho[2,3-d] furan-11-one B 4.23 413.2 A7-1 1109 GT13-10 8-(2-Diethylamino- ethoxy)-3-ethoxy- 6,6-dimethyl-6H- benzo[b]naphtho [2,3-d]furan- 11-one H 5.10 422.0 A7-1 1110 GT13-11 8-(2-Diethylamino- ethoxy)-6,6-dimethyl- 3-propoxy-6H-benzo [b]naphtho[2,3-d] furan-1-one H 5.63 436.0 A7-1 1111 GT13-12 3-(2-Hydroxy- ethoxy)-6,6-dimethyl- 8-((2R,3R)-2,3,4- trihydroxy-butoxy)- 6H-benzo[b]naphtho [2,3-d]furan-11-one F 1.83 443.0 A7-1 1112 GT13-13 3-(3-Hydroxy- propoxy)-6,6- dimethyl-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 6H-benzo[b]naphtho [2,3-d]furan-11-one F 1.94 457.0 A7-1 1113 GT13-14 3-(4-Hydroxy- butoxy)-6,6-dimethyl- 8-((2R,3R)-2,3,4- trihydroxy-butoxy)- 6H-benzo[b]naphtho [2,3-d]furan-11-one F 2.01 471.0 A7-1 1114 GT13-15 3-Isopropoxy-6,6- dimethyl-8-((2R,3R)- 2,3,4-trihydroxy- butoxy)-6H-benzo [b]naphtho[2,3-d] furan-11-one H 5.40 441.0 A7-1 1115 GT13-16 3-(2-Methoxy- ethoxy)-6,6- dimethyl-8-((2R,3R)- 2,3,4-trihydroxy- butoxy)-6H-benzo [b]naphtho[2,3-d] furan-11-one F 2.17 457.0 A7-1 1116 GT13-17 3-(3-Methoxy- propoxy)-6,6-dimethyl- 8-((2R,3R)-2,3,4- trihydroxy-butoxy)-6H- benzo[b]naphtho[2,3-d] furan-11-one F 2.34 471.0 A7-1 1117 GT13-18 3-(4-Methoxy- butoxy)-6,6- dimethyl-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 6H-benzo[b]naphtho [2,3-d]furan-11-one F 2.45 485.0 A7-1 1118 GT13-19 3-((S)-2,3- Dihydroxy-propoxy)- 6,6-dimethyl-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 6H-benzo[b] naphtho[2,3-d] furan-11-one F 1.69 473.0 A7-1 A7- 14-1 1119 GT13-20 3-((R)-2,3- Dihydroxy-propoxy)- 6,6-dimethyl-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 6H-benzo[b]naphtho [2,3-d]furan-11-one F 1.69 473.0 A7-1 A7- 14-1 - The compounds described in the following Table 37 were prepared from Compound GTT3-7 according to carbamation that is used for the preparation of Compound A9-1.
-
TABLE 37 HPLC Re- Exam- Comp. Con- tention Me- ple No. No. Structure Compound Name dition Time m/z thod 1120 GT13-21 (4-Methoxy-phenyl)- carbamic acid 6,6- dimethyl-11-oxo-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 6,11-dihydro- benzo[b]naphtho [2,3-d]furan-3-yl ester B 4.34 548.2 1121 GT13-22 (3-Methoxy-phenyl)- carbamic acid 6,6- dimethyl-11-oxo-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 6,11-dihydro-benzo [b]naphtho[2,3-d] furan-3-yl ester B 4.69 548.2 1122 GT13-23 (2-Methoxy-phenyl)- carbamic acid 6,6- dimethyl-11-oxo-8- ((2R,3R)-2,3,4- trihydroxy-butoxy)- 6,11-dihydro-benzo [b]naphtho[2,3-d] furan-3-yl ester B 548.3 1123 GT13-24 Phenyl-carbamic acid 6,6-dimethyl-11- oxo-8-((2R,3R)-2,3,4- trihydroxy- butoxy)-6,11-dihydro- benzo[b]naphtho [2,3-d]furan-3-yl ester B 4.67 518.2 1124 GT13-25 Cyclohexyl-carbamic acid 6,6-dimethyl-11- oxo-8-((2R,3R)-2,3,4- trihydroxy-butoxy)- 6,11-dihydro-benzo [b]naphtho[2,3-d] furan-3-yl ester B 4.94 524.2 1125 GT13-26 Benzyl-carbamic acid 6,6-dimethyl-11- oxo-8-((2R,3R)-2,3,4- trihydroxy-butoxy)- 6,11-dihydro-benzo [b]naphtho[2,3-d] furan-3-yl ester A 2.08 532.3 1126 GT13-27 Methyl-phenyl-carbamic acid 6,6-dimethyl-11- oxo-8-((2R,3R)-2,3,4- trihydroxy-butoxy)-6,11- dihydro-benzo[b]naphtho [2,3-d]furan-3-ylester B 4.88 532.3 indicates data missing or illegible when filed - The compounds described in the following Table 38 were prepared from corresponding intermediates by alkylation and carbamation based on the method described in the Table.
-
TABLE 38 HPLC Re- Exam- Comp. Con- tention Me- ple No. No. Structure Compound Name dition Time m/z thod 1127 GT13-28 3-(2-Diethylamino- ethoxy)-8-methoxy- 6,6-dimethyl-6H- benzo[b]naphtho[2,3- d]furan-11-one H 4.65 408.0 A7-17 1128 GT13-29 2-[8-((R)-2,3- Dihydroxy-propoxy)- 6,6-dimethyl-11-oxo- 6,11-dihydro-benzo [b]naphtho[2,3-d] furan-3-yloxy]- N-phenyl-acetamide B 4.70 502.0 A7-17 A8-1 T13-3 1129 GT13-30 (4-tert-Butyl-phenyl)- carbamic acid 8- ((R)-2,3-dihydroxy- propoxy)-6,6-dimethyl- 11-oxo-6,11-dihydro- benzo[b]naphtho[2,3-d] furan-3-yl ester B 6.19 544.3 1130 GT13-31 (2-ferf-Butyl-phenyl)- carbamic acid 8-((R)- 2,3-dihydroxy-propoxy)- 6,6-dimethyl-11-oxo- 6,11-dihydro-benzo[b] naphtho[2,3-d]furan- 3-yl ester B 5.74 544.3 1131 GT13-32 (5-tert-Butyl-2- methoxy-phenyl)- carbamic acid 8-((R)- 2,3-dihydroxy- propoxy)-6,6-dimethyl- 11-oxo-6,11-dihydro- benzo[b]naphtho [2,3-d]furan- 3-yl ester B 6.52 574.3 1132 GT13-33 6,6-Dimethyl-3- (pyrimidin-2-yloxy)- 8-((2R,3R)-2,3,4- trihydroxy-butoxy)- 6H-benzo[b]naphtho [2,3-d]furan-11-one H 4.14 477.0 A7-17 indicates data missing or illegible when filed - Compound GT14-1
-
- Compound BZ2-2 was demethylated according to the method used for the preparation of Compound A6, and subsequently introduced with a substituent group and deprotected according to the method used for the preparation of Compound A7-14-1 and A7-14-2.
- LCMS: m/z 386 [M+H]+
- HPLC retention time: 3.02 min (analysis condition B)
- Compound GT15-1
-
- 2-Iodo-pyridin-3-ol (50 mg, 0.226 mmol), K2CO3 (62 mg, 0.452 mmol) and DMF (2 ml) were added with para-methoxybenzyl chloride (46 μL, 0.339 mmol), and the mixture was stirred overnight at 45° C. The resultant was added with water, and then extracted with ethyl acetate. The organic layer was washed with saturated brine, and the resulting residues obtained after concentration under reduced pressure were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (21 mg, 27%).
- LCMS: m/z 342 [M+H]+
- HPLC retention time: 3.44 min (analysis condition Y)
- Compound GT15-2
-
- Toluene (0.5 ml) was added to 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 36 mg), 2-iodo-3-(4-methoxy-benzyloxy)-pyridine (Compound GT15-1, 50 mg), sodium t-butoxide (35.3 mg), Pd2dba3 (13.5 mg) and Xantphos (17 mg), and the mixture was stirred and heated at 80° C. for 2.5 hrs under nitrogen atmosphere. After cooling, the reaction mixture was diluted with ethyl acetate and subjected to Celite filtration. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (27 mg, 44%).
- LCMS: m/z 419 [M+H]+
- HPLC retention time: 3.31 min (analysis condition Y)
- Compound GT15-3
-
- To the mixture of 7-methoxy-3-[3-(4-methoxy-benzyloxy)-pyridin-2-yl]-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound GT15-2, 21 mg) and ethyl acetate (0.8 ml), sulfuric acid (0.2 ml) was added. The mixture was stirred and heated at 70° C. for 5 hrs. After cooling, the reaction mixture was neutralized with 2 N aqueous solution of sodium hydroxide. The mixture was diluted with ethyl acetate. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (7 mg, 50%).
- LCMS: m/z 280 [M+H]+
- HPLC retention time: 2.71 min (analysis condition Y)
- Compound GT15-4
-
- 8-Methoxy-10,10-dimethyl-5,10-dihydro-11-oxa-4-aza-benzo[b]fluorene (Compound GT15-3, 22 mg) was dissolved in MeCN (0.26 ml) and water (0.13 ml), added with sodium chlorite (14 mg) and N-hydroxyphthalimide (2.6 mg), and the mixture was stirred at 40° C. for 1 hr. The reaction mixture was diluted with ethyl acetate. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (16 mg, 70%).
- LCMS: m/z 294 [M+H]+
- HPLC retention time: 2.85 min (analysis condition Y)
- Compound GT15-5
-
- Mixture of 8-methoxy-10,10-dimethyl-10H-11-oxa-4-aza-benzo[b]fluoren-5-one (Compound GT15-4, 25 mg) and pyridine hydrochloric acid salt (492 mg) was stirred and heated at 178° C. overnight. The reaction mixture was cooled, and added with water. The mixture was neutralized with saturated aqueous solution of sodium bicarbonate and extracted with DCM. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (DCM/MeOH) to obtain the title compound (13 mg, 54%).
- LCMS: m/z 280 [M+H]+
- HPLC retention time: 2.30 min (analysis condition Y)
- Compound GT15-6
-
- In the same manner as Compound A7-1, the title compound was synthesized from Compound GT15-5 and Compound T22-0 (29 mg, 50%).
- LCMS: m/z 538 [M+H]+
- HPLC retention time: 3.64 min (analysis condition Y)
- Compound GT15-7
-
- To the mixture of 8-[(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-10,10-dimethyl-10H-11-oxa-4-aza-benzo[b]fluoren-5-one (Compound GT15-6, 27 mg), MeOH (0.1 ml) and THF (0.3 ml), 0.5 N sulfuric acid (0.1 ml) was added, and the mixture was stirred and heated at 55 to 60° C. for 4 hrs. The reaction mixture was neutralized with saturated aqueous solution of sodium bicarbonate. The resulting solid was filtered and washed with diethyl ether. The filtrate was extracted with the mixture solution of DCM and MeOH (DCM:MeOH=10:1). The organic layer was washed with saturated brine, and dried over magnesium sulfate. The filtered solid and the residues obtained after concentration under reduced pressure were combined and purified by silica gel column (DCM/MeOH) to obtain the title compound (5.4 mg, 28%).
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 2.02 min (analysis condition Y)
- Compound GT15-8
-
- To 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 924 mg), 4-iodo-3-methoxymethoxy-pyridine (1 g), sodium t-butoxide (906 mg), Pd2dba3 (173 mg) and S-Phos (185 mg), toluene (19 ml) was added, and the mixture was stirred and heated at 70° C. for 2 hrs under nitrogen atmosphere. The reaction mixture was diluted with ethyl acetate and filtered through Celite. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (610 mg, 47%).
- LCMS: m/z 342 [M+H]+
- HPLC retention time: 2.60 min (analysis condition Y)
- Compound GT15-9
-
- Mixture of 7-methoxy-3-(3-methoxymethoxy-pyridin-4-yl)-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound GT15-8, 430 mg) and 4 N hydrochloric acid dioxane solution (5 ml) was heated and stirred at room temperature for 1.5 hrs. The reaction mixture was neutralized with 2 N aqueous solution of sodium hydroxide. The resulting mixture was extracted with the mixture of DCM and MeOH (DCM:MeOH=9:1). The residues obtained after concentration under reduced pressure were purified by silica gel column (DCM/MeOH) to obtain the title compound (280 mg, 75%).
- LCMS: m/z 298 [M+H]+
- HPLC retention time: 2.41 min (analysis condition Y)
- Compound GT15-10
-
- Mixture of 3-(3-hydroxy-pyridin-4-yl)-7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound GT15-9, 270 mg) and methanesulfonic acid (1 ml) was stirred and heated at 110° C. for 0.5 hrs. After cooling, the reaction mixture was neutralized with 2 N aqueous solution of sodium hydroxide. The resulting mixture was extracted with the mixture of DCM and MeOH (DCM:MeOH=9: 1). The organic layer was concentrated under reduced pressure, and the resulting residues were purified by silica gel column (DCM/MeOH) to obtain the title compound (110 mg, 43%).
- LCMS: m/z 280 [M+H]+
- HPLC retention time: 2.53 min (analysis condition Y)
- Compound GT15-11
-
- 8-Methoxy-10,10-dimethyl-5,10-dihydro-11-oxa-2-aza-benzo[b]fluorene (Compound GT15-10, 20 mg) was dissolved in acetonitrile (0.2 ml) and water (0.15 ml), added with sodium chlorite (16 mg) and N-hydroxyphthalimide (2.3 mg), and the mixture was stirred at 40° C. for 40 min. To the reaction mixture, ethyl acetate was added. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (12 mg, 57%).
- LCMS: m/z 294 [M+H]+
- HPLC retention time: 2.51 min (analysis condition Y)
- Compound GT15-12
-
- DCM (0.34 ml) solution of 8-methoxy-10,10-dimethyl-10H-11-oxa-2-aza-benzo[b]fluoren-5-one (Compound GT15-11, 10 mg) was cooled to −78° C., added with the DCM solution (0.17 ml) of 1.0 M BBr3, and the mixture was stirred at room temperature overnight. To the reaction mixture, water and saturated aqueous solution of sodium bicarbonate were added, and the solid produced therefrom was filtered off. The filtrate was extracted with the mixture solution of DCM and MeOH (DCM:MeOH=9: 1). The organic layer was washed with saturated brine. The filtered solid and the residues obtained after concentration under reduced pressure were combined to obtain the title compound (9.5 mg, 99%).
- LCMS: m/z 280 [M+H]+
- HPLC retention time: 2.50 min (analysis condition Y)
- Compound GT15-13
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound GT15-12 and Compound T22-0 (38 mg, 66%).
- LCMS: m/z 538 [M+H]+
- HPLC retention time: 3.55 min (analysis condition Y)
- Compound GT15-14
-
- Under the same conditions as the method for synthesizing Compound GT15-7, the title compound was prepared from Compound GT15-13 (2.1 mg, 84%).
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 1.70 min (analysis condition Y)
- Compound GT15-15
-
- Under the same conditions as the method for synthesizing Compound G15-1, the title compound was prepared from 3-bromo-pyridin-2-ol (740 mg, 88%).
- LCMS: m/z 295 [M+H]+
- HPLC retention time: 2.86 min (analysis condition Y)
- Compound GT15-16
-
- To 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 845 mg), 3-bromo-2-(4-methoxy-benzyloxy)-pyridine (Compound GT15-15, 1.46 g), sodium t-butoxide (597 mg), palladium acetate (18.6 mg) and tri-tert-butylphosphine tetrafluoroboric acid (21 mg), toluene (10 ml) and THF (2 ml) were added and the mixture was stirred and heated at 90° C. for 2.5 hrs under nitrogen atmosphere. After cooling, the reaction mixture was added with saturated aqueous solution of ammonium chloride, and then extracted with ethyl acetate. The organic layer was washed with saturated brine, and the residues obtained after concentration under reduced pressure were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (140 mg, 8%).
- LCMS: m/z 419 [M+H]+
- HPLC retention time: 3.50 min (analysis condition Y)
- Compound GT15-17
-
- Under the same conditions as the method for synthesizing Compound GT15-3, the title compound was prepared from Compound GT15-16 (49 mg, 52%).
- LCMS: m/z 294 [M+H]+
- HPLC retention time: 3.39 min (analysis condition Y)
- Compound GT15-18
-
- Under the same conditions as the method for synthesizing Compound GT15-5, the title compound was prepared from Compound GT15-17 (6.5 mg, 51%).
- LCMS: m/z 280 [M+H]+
- HPLC retention time: 3.10 min (analysis condition Y)
- Compound GT15-19
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound GT15-18 and Compound T22-0 (4.5 mg, 11%).
- LCMS: m/z 538 [M+H]+
- HPLC retention time: 3.88 min (analysis condition Y)
- Compound GT15-20
-
- Under the same conditions as the method for synthesizing Compound GT15-7, the title compound was prepared from Compound GT15-19 (7.9 mg, 51%).
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 2.57 min (analysis condition Y)
- Compound GT15-21
-
- To 7-methoxy-1,1-dimethyl-3,4-dihydro-1H-naphthalen-2-one (Compound A2, 2.5 g), 3-bromo-4-chloro-pyridine (2 g), sodium t-butoxide (3 g), Pd2dba3 (476 mg), and S-Phos (512 mg), toluene (20 ml) was added, and the mixture was stirred and heated at 100° C. overnight under nitrogen atmosphere. After cooling, the reaction mixture was diluted with ethyl acetate and filtered through Celite. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine. Thereafter, the organic layer was dried over sodium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (112 mg, 4%).
- LCMS: m/z 280 [M+H]+
- HPLC retention time: 2.46 min (analysis condition Y)
- Compound GT15-22
-
- Under the same conditions as the method for synthesizing Compound GT15-3, the title compound was prepared from Compound GT15-21 (49 mg, 52%).
- LCMS: m/z 294 [M+H]+
- HPLC retention time: 2.30 min (analysis condition Y)
- Compound GT15-23
-
- Under the same conditions as the method for synthesizing Compound GT15-12, the title compound was prepared from Compound GT15-22 (110 mg, 77%).
- LCMS: m/z 280 [M+H]+
- HPLC retention time: 1.95 min (analysis condition Y)
- Compound GT15-24
-
- Under the same conditions as the method for synthesizing Compound A7-1, the title compound was prepared from Compound GT15-23 and Compound T22-0 (38 mg, 49%).
- LCMS: m/z 538 [M+H]+
- HPLC retention time: 3.40 min (analysis condition Y)
- Compound GT15-25
-
- Under the same conditions as the method for synthesizing Compound GT15-7, the title compound was prepared from Compound GT15-24 (17 mg, 72%).
- LCMS: m/z 384 [M+H]+
- HPLC retention time: 1.48 min (analysis condition Y)
- Compound GT16-1
-
- To the mixture of 1-(2-bromo-4-methoxyphenyl)-ethanone (300 mg) dissolved in THF solution (3 ml), MeMgBr (3 M THF solution, 0.52 ml) was added at 0° C. under nitrogen atmosphere. Then, the mixture was stirred at room temperature for 6 hrs. The reaction mixture was added with saturated aqueous solution of ammonium chloride and extracted with ethyl acetate. The organic layer was washed with saturated brine, and the residues obtained after concentration under reduced pressure were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (220 mg, 69%).
- 1H-NMR (CDCL3) δ: 7.55 (1H, d), 7.14 (1H, d), 6.83 (1H, dd), 3.79 (3H, s), 2.72 (1H, s), 1.73 (6H, s)
- Compound GT16-2
-
- Mixture comprising 2-(2-bromo-4-methoxyphenyl)-propan-2-ol (100 mg), 2,3-benzofuran (0.19 ml) and polyphosphoric acid (1 g) was stirred and heated at 90° C. for 30 min. The reaction mixture was added with water and extracted with DCM. The residues obtained after concentration under reduced pressure were purified by silica gel column (DCM/hexane) to obtain the title compound (143 mg, 51%).
- 1H-NMR (CDCL3) δ: 7.4-7.5 (1H, m), 7.3-7.4 (2H, m), 7.1-7.25 (3H, m), 6.87 (1H, dd), 6.42 (1H, s) 3.79 (3H, s), 1.84 (6H, s)
- Compound GT16-3
-
- To the mixture comprising 2-[1-(2-bromo-4-methoxyphenyl)-1-methylethyl]benzofuran (140 mg) and THF (2 ml), n-butyl lithium (2.5 M solution, 0.17 ml) was added at −78° C. under nitrogen atmosphere. The mixture was stirred for 20 min. The resulting reaction mixture was flushed with carbon dioxide gas for 15 min. Then, the reaction mixture was added with saturated aqueous solution of ammonium chloride and extracted with ethyl acetate. The organic layer was washed with water and saturated brine, and the residues obtained after concentration under reduced pressure were purified by silica gel column (DCM/MeOH) to obtain the title compound (68 mg, 54%).
- LCMS: m/z 311 [M+H]+
- HPLC retention time: 2.92 min (analysis condition Y)
- Compound GT16-4
-
- To the DCM solution (1 ml) of 2-(1-benzofuran-2-yl-1-methylethyl)-5-methoxy benzoic acid (63 mg), trifuloroacetic anhydride (0.03 ml) was added at room temperature under nitrogen atmosphere. The mixture was stirred for 30 min. The reaction mixture was then added with water and extracted with DCM. The organic layer was dried over sodium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column (DCM) to obtain the title compound (50 mg, 84%).
- LCMS: m/z 293 [M+H]+
- HPLC retention time: 3.49 min (analysis condition Y)
- Compound GT16-5
-
- Under the same conditions as the method for synthesizing Compound A6, the title compound was prepared from Compound GT16-4.
- LCMS: m/z 279 [M+H]+
- HPLC retention time: 3.05 min (analysis condition Y)
- Compound GT16-6
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound GT16-5.
- LCMS: m/z 378 [M+H]+
- HPLC retention time: 2.41 min (analysis condition Y)
- Compound GT16-7
-
- Under the same conditions as the method for synthesizing Compound A7-17, the title compound was prepared from Compound GT16-5 and toluene-4-sulfonic acid (R)-2,2-dimethyl-[1,3]dioxolan-4-yl methyl ester.
- LCMS: m/z 393 [M+H]+
- HPLC retention time: 3.22 min (analysis condition Y)
- Compound GT16-8
-
- Under the same conditions as the method for synthesizing Compound A7-14-2, the title compound was prepared from Compound GT16-7.
- LCMS: m/z 353 [M+H]+
- HPLC retention time: 2.83 min (analysis condition Y)
- Compound GT16-9
-
- In the same manner as Compound GT9-2, the title compound was synthesized from Compound GT16-5.
- LCMS: m/z 383 [M+H]+
- HPLC retention time: 7.11 min (analysis condition H)
- Compound GT16-10
-
- In the same manner as Compound GT9-2, the title compound was synthesized from Compound GT16-5.
- LCMS: m/z 369 [M+H]+
- HPLC retention time: 6.97 min (analysis condition H)
- The compounds described in the following Table 39 were synthesized according to the method shown below. Specifically, Compound GT17-1 was prepared from 8-methoxy-1,1-dimethyl-3,4-dihydro-1H naphthalen-2-one and bromophenol by following the method that is used for the preparation of Compound Z10, Z11 and Z12. Compound GT17-1 was demethylated according to the method used for the preparation of Compound A6, and as a result 7-hydroxy-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one was prepared. The resulting hydroxy compound was subjected to the alkylation according to the method used for the preparation of A7-1, or Mitsunobu reaction used for the preparation of Compound A7-17 for introducing a corresponding side chain or a synthetic equivalent thereof. Thereafter, if necessary, functional group modification was carried out to prepare Compound GT17-2 and Compound GT17-3.
-
TABLE 39 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1169 GT17-1 7-Methoxy-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one Y 13.42 293.0 1170 GT17-2 7-(2-Diethylamino-ethoxy)-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one Y 9.92 378.0 1171 GT17-3 7-((R)-2,3-Dihydroxy-propoxy)-6,6- dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one Y 11.68 353.0 - The compounds described in the following Table 40 were synthesized according to the method shown below.
- By using the method used for the preparation of Compound Z10, Z11 and Z12, Compound GT18-1 was prepared from Compound M1 and bromophenol. Further, according to the method used for the preparation of Compound A6, Compound GT18-1 was demethylated to prepare 8-hydroxy-1H-spiro[benzo[b]naphtho[2,3-d]furan-6,1′-cyclopentan]-11-one, which was then introduced with a side chain based on the alkylation that is used for the preparation of Compound A7-1. As a result, Compound GT18-2 was prepared.
- The following spiro compounds were prepared from 7-methoxy-3,4-dihydro-H-naphthalen-2-one and corresponding dibromide in the same manner as above.
-
TABLE 40 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1172 GT18-1 8-methoxy-11H- spiro[benzo[b]naphtho[2,3-d] furan-6,1′-cyclopentane]-11-one Y 10.00 319.0 1173 GT18-2 8-(2-diethylamino-ethoxy)-11H- spiro[benzo[b]naphtho[2,3-d] furan-6,1′-cyclopentane]-11-one C 2.19 404.0 1174 GT18-3 8-(2-diethylamino-ethoxy)-11H- spiro[benzo[b]naphtho[2,3-d] furan-6,1′-cyclohexane]-11-one C 3.28 418.2 1175 GT18-4 8-((2R,3R)-2,3,4- trihydroxybutoxy-11H- spiro[benzo[b]naphtho[2,3-d] furan-6,1′-cyclohexane]-11-one A 2.26 423.2 1176 GT18-5 8-methoxy-11H- spiro[benzo[b]naphtho[2,3-d] furan-6,1′-cyclobutane]-11-one Y 9.00 305.0 1177 GT18-6 8-(2-diethylamino-ethoxy)- 2′,3′,5′,6′-tetrahydro-11H- spiro[benzo[b]naphtho[2,3-d] furan-6,4′-pyran]-11-one B 4.05 420.3 - Compound GT19-1
-
- According to the method described before, the preparation was carried out by using 7-methoxy-1,1-dimethyl-3,4-dihydro-1H naphthalen-2-one and 2-bromo-5-trifulorophenol.
- LCMS: m/z 446 [M+H]+
- HPLC retention time: 3.25 min (analysis condition C)
- Compound GT19-2
-
- By carrying out Suzuki coupling of Compound GT23-5 and a corresponding boronic acid reagent based on the method that is used for the preparation of Compound GT9-2, the title compound was prepared.
- LCMS: m/z 454 [M+H]+
- HPLC retention time: 2.67 min (analysis condition F)
- Compound GT20-1
-
- 3-Bromo-8-hydroxy-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (72.2 mg, 0.203 mmol), 2-phenylethanethiol (0.0297 ml, 0.221 mmol), Pd2dba3 (9.3 mg, 0.0102 mmol), Xantphos (11.6 mg, 0.020 mmol) and ethyl diisopropylamine (0.068 ml, 0.40 mmol) were dissolved in dioxane (0.6 ml), and the mixture was stirred at 110° C. for 16 hrs under nitrogen atmosphere. Water and ethyl acetate were added to the mixture to give a suspension, which was then filtered. The organic layer was washed with water and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane). The resulting residues were dissolved in THF (4 ml), and the supernatant liquid (2 ml) was taken and added with water (1 ml) and OXONE (99 mg). The resulting mixture was stirred at room temperature overnight. The reaction solution was partitioned between water and ethyl acetate. The organic layer was washed with brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (37.1 mg).
- LCMS: m/z 446 [M+H]+
- HPLC retention time: 2.51 min (analysis condition F)
- Compound GT20-2
- Compound GT20-3
-
- 8-Hydroxy-6,6-dimethyl-3-(2-phenyl-ethanesulfonyl)-5,6-dihydro-benzo[b]carbazol-11-one (30 mg, 0.0673 mmol), [(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]methanol (22.3 mg, 0.0808 mmol), and PPh3 (23 mg, 0.0875 mmol) were dissolved in THF (0.5 ml), added with DIAD (0.0169 ml, 0.0808 mmol), and the mixture was stirred at 50° C. overnight. After cooling, the reaction solution was filtered and concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane). The resulting residues were dissolved in THF (0.4 ml) and water (0.13 ml), added with camphor sulfonic acid (28.1 mg, 0.121 mmol), and then subjected to microwave irradiation at 80° C. for 15 min under nitrogen atmosphere. Ethyl acetate was added to the resultant. The organic layer was washed with saturated aqueous solution of sodium hydrocarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by thin layer chromatography (MeOH/DCM) to obtain Compound GT20-2 (10.5 mg) and Compound GT20-3 (2.4 mg).
- Compound GT20-2
- LCMS: m/z 550 [M+H]+
- HPLC retention time: 2.20 min (analysis condition F)
- Compound GT20-3
- LCMS: m/z 488 [M+H]+
- HPLC retention time: 3.13 min (analysis condition F)
- Compound GT20-4
-
- The DMA solution (0.6 ml) comprising Compound GT23-2 (59.6 mg, 0.167 mmol), sodium methanethiolate (77 mg, 1.10 mmol), Pd2dba3 (23.7 mg, 0.0259 mmol) and Xantphos (29.7 mg, 0.0513 mmol) was subjected to microwave irradiation at 180° C. for 30 min under nitrogen atmosphere. The reaction solution was partitioned between aqueous solution of potassium dihydrophosphoric acid and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane and MeOH/DCM). The resulting solids were dissolved in THF (1 ml) and water (0.5 ml), and then added with OXONE (101.4 mg). The resulting mixture was stirred at room temperature for 2 hrs. The reaction solution was partitioned between water and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were suspended and washed with MTBE. The resulting solid was dissolved in THF (0.4 ml), and added with PPh3 (37 mg, 0.141 mmol), [(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-methanol (39.0 mg, 0.141 mmol) and DEAD (2.2 M toluene solution, 0.064 ml, 0.141 mmol), and the mixture was stirred at 40° C. for 4 hrs under nitrogen atmosphere. The reaction solution was partitioned between water and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (MeOH/DCM). Thus-obtained product was dissolved in THF (0.25 ml) and MeOH (0.05 ml), added with 0.5 M sulfuric acid (0.1 ml), and the mixture was stirred at 60° C. for 5 hrs. After cooling, the mixture was added with diethyl ether and sodium hydrocarbonate (13 mg, 0.15 mmol). The separated aqueous layer was filtered, concentrated under reduced pressure, and suspended and purified with MeOH to obtain the title compound as a white solid (10.4 mg, 14%).
- LCMS: m/z 460 [M+H]+
- HPLC retention time: 1.71 min (analysis condition F)
- Compound GT20-5
-
- 3-Bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-5,6-dihydro-benzo[b]carbazol-11-one (47.3 mg, 0.101 mmol), sodium methanethiolate (34.6 mg, 0.493 mmol), Pd2(dba)3 (13.1 mg, 0.0413 mmol), and Xantphos (17.9 mg, 0.0309 mmol) were dissolved in DMA (0.5 ml) and subjected to microwave irradiation at 200° C. for 30 min under nitrogen atmosphere. The resultant was partitioned between aqueous solution of potassium dihydrophosphoric acid and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by thin layer chromatography (ethyl acetate/DCM). The resulting solid was dissolved in THF (0.23 ml) and MeOH (0.06 ml), and then added with 0.5 M sulfuric acid (0.12 ml). The resulting mixture was stirred at 60° C. for 2 hrs. The reaction solution was diluted with diethyl ether and neutralized with sodium hydrocarbonate (15.5 mg, 0.185 mmol). Thereafter, the solution was partitioned between brine and ethyl acetate. The organic layer was concentrated under reduced pressure. The resulting residues were added with diethyl ether. The precipitated solid was filtered to obtain the title compound as a white solid (15.8 mg, 39%).
- LCMS: m/z 398 [M+H]+
- HPLC retention time: 4.46 min (analysis condition H)
- Compound GT20-5
-
- 3-Bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-5,6-dihydrobenzo[b]carbazol-11-one (47 mg, 0.10 mmol), bis (pinacolate)diborone (33 mg, 0.13 mmol), Pd (dppf)2C2. DCM (8.2 mg, 0.010 mmol) and potassium acetate (294 mg, 0.3 mmol) were dissolved in dioxane (0.6 ml), and the mixture was stirred at 100° C. overnight under nitrogen atmosphere. The resultant was partitioned between aqueous solution of potassium dihydrophosphoric acid and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/DCM) to obtain 8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-6,6-dimethyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-5,6-dihydro-benzo[b]carbazol-11-one (30.2 mg). The product (11 mg) was dissolved in DMA (0.4 ml), added with 2-bromothiazole (0.0038 ml, 0.0428 mmol), Pd (PPh3)4 (5.3 mg, 0.00459 mmol), potassium phosphate (27.4 mg, 0.129 mmol) and water (0.1 ml), and the mixture was subjected to microwave irradiation at 140° C. for 7 min under nitrogen atmosphere. The resultant was partitioned between aqueous solution of potassium dihydrophosphoric acid and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by thin layer chromatography (MeOH/DCM). The resulting solid was dissolved in MeOH (1 ml), and then added with 1 N HCl (3 drops). The resulting mixture was stirred at 60° C. for 2 hrs. The reaction solution was concentrated under reduced pressure, and the residues obtained therefrom were suspended and washed with DCM/hexane (2/1) followed by drying to obtain the title compound as a yellow solid (8.7 mg).
- LCMS: m/z 435 [M+H]+
- HPLC retention time: 1.76 min (analysis condition A)
- The compounds described in the following Table 41 were also synthesized in the same manner.
-
TABLE 41 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1185 GT20-6 8-((R)-2,3-Dihydroxy- propoxy)-3-(1-methoxymethyl- 1H-midazol-2-yl)-6,6- dimethyl-5,6-dihydro- benzo[b]carbazol-11-one B 2.63 462.0 1186 GT20-7 8-((R)-2,3-Dihydroxy- propoxy)-3-(1H- imidazol-2-yl)-6,6- dimethyl-5,6-dihydro- benzo[b]carbazol-11-one B 2.45 418.5 - Compound GT20-8
- Compound GT20-9
-
- 3-Bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-5,6-dihydrobenzo[b]carbazol-11-one (200.2 mg, 0.426 mmol), palladium acetate (II) (19 mg, 0.0848 mmol), hexacarbonyl molybdenum (115.5 mg, 0.438 mmol) and tris(o-tolyl)phosphine (52.5 mg, 0.172 mmol) were dissolved in THF (1.3 ml) and ethanol (0.075 ml), added with DBU (0.195 ml), and subjected to microwave irradiation at 160° C. for 15 min under nitrogen atmosphere. The resulting reaction solution was partitioned between aqueous solution of potassium dihydrophosphoric acid and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were dissolved in ethanol (10 ml) and THF (3 ml), added with 2 N KOH (2 ml), and stirred at room temperature for 2 hrs, at 50° C. overnight and at 70° C. for 2 hrs. The reaction solution was partitioned between aqueous solution of potassium dihydrophosphoric acid and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were suspended and purified with MTBE/hexane (1/1) (155.4 mg). The THF solution (1.5 ml) of the product (109 mg) was added with TEA (0.052 ml, 0.373 mmol) and ethyl chloroformate (0.029 ml, 0.303 mmol) under ice cooling, and the mixture was stirred at 0° C. for 2 hrs. Subsequently, ethanol (1 ml) and sodium borohydride (75.7 mg, 2.0 mmol) were added to the mixture, which was then stirred at room temperature for 2 hrs. The reaction solution was partitioned between aqueous solution of potassium dihydrophosphoric acid and ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (MeOH/DCM) (35.2 mg). Thus-obtained solid (9.6 mg) was dissolved in MeOH (1 ml), added with 1 N HCl (3 drops), and the mixture was stirred at 60° C. for 90 min. After cooling and concentration under reduced pressure, the resultant was purified by TLC (MeOH/DCM) to obtain Compound GT20-8 (6.2 mg, white solid) and Compound GT20-9 (4.3 mg, white solid).
- Compound GT20-8
- LCMS: m/z 396 [M+H]+
- HPLC retention time: 1.66 min (analysis condition A)
- Compound GT20-9
- LCMS: m/z 382 [M+H]+
- HPLC retention time: 1.37 min (analysis condition A)
- Compound GT21-1
-
- To the DMF solution (4 ml) of trifuloro-methanesulfonic acid 6,6-dimethyl-1-oxo-6,11-dihydro-benzo[b]naphtho[2,3-d]furan-8-yl ester (300 mg), 2,2-dimethyl-4-vinyl-[1,3]dioxolane (469 mg) and PdCl2(PPh3)2 (103 mg), sodium hydrocarbonate (184 mg) was added, and the mixture was stirred at 100° C. overnight under nitrogen atmosphere. The reaction mixture was diluted with ethyl acetate. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (130 mg, 46%).
- LCMS: m/z 389 [M+H]+
- HPLC retention time: 3.28 min (analysis condition Y)
- Compound GT21-2
-
- To the MeOH solution (5 ml) of 8-[(E)-2-(2,2-dimethyl-[1,3]dioxolan-4-yl)-vinyl]-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one (125 mg), 10% Pd—C (25 mg) was added and the mixture was stirred overnight at room temperature under hydrogen atmosphere. The catalyst was removed by filtration. The residues obtained after concentration under reduced pressure were purified by HPLC to obtain the title compound (35 mg, 31%).
- LCMS: m/z 351 [M+H]+
- HPLC retention time: 1.79 min (analysis condition Y)
- Compound GT21-3
-
- To trifuloro-methanesulfonic acid 6,6-dimethyl-11-oxo-6,11-dihydro-benzo[b]naphtho[2,3-d]furan-8-yl ester (100 mg), benzhydrylideneamine (0.05 ml), cesium carbonate (110 mg), palladium acetate (2 mg) and BINAP (7 mg), THF (2 ml) was added. The mixture was stirred and heated at 65° C. overnight under nitrogen atmosphere, and the reaction mixture was diluted with ethyl acetate. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (16 mg, 23%).
- LCMS: m/z 278 [M+H]+
- HPLC retention time: 2.52 min (analysis condition Y)
- Compound GT21-4
-
- The mixture comprising 8-amino-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one (50 mg), (R)-4-iodomethyl-2,2-dimethyl-[1,3]dioxolane (104 mg), potassium carbonate (150 mg) and DMF (2 ml) was stirred and heated at 160° C. for 2 days under nitrogen atmosphere. The reaction mixture was diluted with ethyl acetate. The organic layer was washed with water and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane). To the compound (71 mg) obtained therefrom, THF (1 ml) and conc. hydrochloric acid (8 drops) were added and the mixture was stirred at room temperature for 1 hr. The reaction mixture was added with saturated aqueous solution of sodium bicarbonate and then diluted with ethyl acetate. The organic layer was washed with saturated aqueous solution of sodium bicarbonate and saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (34 mg, 51%).
- LCMS: m/z 392 [M+H]+
- HPLC retention time: 3.11 min (analysis condition Y)
- Compound GT21-5
-
- In the same manner as Compound A7-14-2, Compound GT21-4 was deprotected to obtain the title compound.
- LCMS: m/z 352 [M+H]+
- HPLC retention time: 2.26 min (analysis condition Y)
- Compound GT22-1
-
- To the mixture of trifluoro-methanesulfonic acid 8-(2-diethylamino-ethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-benzo[b]naphtho[2,3-d]furan-3-yl ester (15 mg), which had been obtained in the same manner as Compound A7-25, tris(1-methyl-3-oxo-1-butenyloxy) iron (III) (1 mg), NMP (0.3 ml) and THF (0.3 ml), n-PrMgBr (0.88 M, THF solution, 0.291 ml) and zinc chloride (0.5 M THF solution, 0.114 ml) were added at 0° C. under nitrogen atmosphere, and the mixture was stirred at 0° C. for 10 min. The reaction mixture was added with water and extracted with ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain the title compound (4.5 mg, 38%).
- LCMS: m/z 420 [M+H]+
- HPLC retention time: 5.77 min (analysis condition H)
- Compound GT22-2
-
- In the same manner as Compound GT22-2, the title compound was synthesized.
- LCMS: m/z 406 [M+H]+
- HPLC retention time: 5.12 min (analysis condition B)
- Compound GT23-1
-
- 8-Methoxy-6,6-dimethyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-6H-benzo[b]naphtho[2,3-d]furan-11-one (10.3 mg), which had been synthesized from Compound Z12 under the same conditions as the method for synthesizing Compound GT20-5, was mixed with copper (II) bromide (16.5 mg), MeOH (0.5 ml) and water (0.25 ml), and the mixture was stirred and heated at 70° C. for 2 hrs. DCM was added to the reaction solution for extraction. The organic layer was concentrated and purified by silica gel column to obtain the title compound (9.4 mg).
- LCMS: m/z 371 [M+H]+
- HPLC retention time: 7.55 min (analysis condition B)
- Compound GT23-2
-
- In the same manner as Compound GT15-5, 3-bromo-8-methoxy-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one was deprotected to obtain the title compound.
- LCMS: m/z 357 [M+H]+
- HPLC retention time: 2.82 min (analysis condition A)
- The compounds described in the following Table 42 were synthesized from Compound GT23-2 according to the method given in the Table.
-
TABLE 42 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z Method 1197 GT23-3 3-Bromo-6,6-dimethyl- 8-((2R,3R)- 2,3,4-trihydroxy-butoxy)- 6H-benzo[b]naphtho [2,3-d]furan-11-one D 2.30 461.0 1198 GT23-4 3-Bromo-8- ((R)-2,3-dihydroxy- propoxy)- 6,6-dimethyl-6H- benzo[b]naphtho [2,3-d]furan-11-one A 5.42 431.0 1199 GT23-5 3-Bromo-8-(2-diethylamino- ethoxy)-6,6-dimethyl-6H- benzo[b]naphtho [2,3-d]furan-11-one H 5.37 456.0 A7-17 indicates data missing or illegible when filed - Compound GT24-1
-
- To 3-bromo-8-((S)-2,2-dimethyl-[1,3]dioxolan-4-ylmethoxy)-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one (15 mg, 0.032 mmol), CuI (6.2 mg, 0.032 mmol), NaI (9.6 mg, 0.064 mmol) and trans-N,N′-dimethylcyclohexane-1,2-diamine (0.01 ml) were added and the mixture was stirred for 48 hrs under nitrogen atmosphere. The reaction solution was diluted with ethyl acetate. The organic layer was washed with saturated brine, and then concentrated under reduced pressure. The resulting residues were purified by silica gel column (ethyl acetate/hexane) to obtain 8-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy)-3-iodo-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one (16 mg, 97%), which was then deprotected according to the method of A-14-2 to give the title compound.
- LCMS: m/z 479 [M+H]+
- HPLC retention time: 4.26 min (analysis condition A)
- Compound GT24-2
-
- In the same manner as Compound GT24-1, 3-iodo-8-[(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one was synthesized from 3-bromo-8-[(4R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-[1,3]dioxolan-4-yl methoxy]-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one. Subsequently, according to the same method as Compound T22-2, deprotection was carried out to obtain the title compound.
- Compound GT25-1
-
- By using the method for preparing Compound B1, trifuloro-methanesulfonic acid 6,6-dimethyl-11-oxo-6,11-dihydro-benzo[b]naphtho[2,3-d]furan-8-yl ester was prepared from 8-hydroxy-6,6-dimethyl-6H-benzo[b]naphtho[2,3-d]furan-11-one. This trifuloromethanesulfonic acid ester (205 mg), (2R)-1-[(1R)-1-[bis (1,1-dimethylethyl)phosphino]ethyl]-2-(dicyclohexylphosphino)ferrocene (13 mg), palladium acetate (6 mg), 2-trimethylsilanyl-ethanethiol (90 μL) and potassium carbonate (85 mg) were reacted in DME to obtain a product (120 mg). To the benzyl alcohol solution (90 μL) of the product (50 mg), DCM solution of N-chlorosuccinimide (90 mg) was added, and the mixture was stirred at room temperature. The reaction solution was partitioned between water and ethyl acetate, and the organic layer was concentrated under reduced pressure. To the DCM solution of thus-obtained white solid, N-methylpiperazine (10 μL) was added and the mixture was stirred. The residues obtained after removing the solvent by distillation were purified by TLC to obtain the title compound as a white solid (6 mg).
- Compound GT26-1
-
- To the THF solution (1000 ml) of 2-bromo-5-methoxy-benzoic acid methyl ester (20 g, 81.6 mmol), the THF suspension (50 ml) of LAH (4.07 g, 102 mmol) was added under ice cooling. The mixture was stirred for 30 min under ice cooling. The reaction solution was partitioned between saturated aqueous solution of Na2SO4 and ethyl acetate. The organic layer was washed with saturated brine and concentrated under reduced pressure. The resulting residues were dissolved in DCM (200 ml), and added with TEA (12.51 ml, 89.76 mmol) and MsCl (6.63 ml, 85.68 mmol) under ice cooling, followed by stirring overnight at room temperature. The reaction mixture was diluted with DCM, and washed in order with 10% aqueous solution of citric acid, saturated aqueous solution of NaHCO3 and saturated brine. The residues obtained after concentration under reduced pressure were dissolved in DMF (100 ml), and added with the DMF (500 ml) solution of NaCN (40 g, 81.6 mmol) under ice cooling. After stirring for 2 hrs under ice cooling, the reaction mixture was extracted with ether, washed with saturated brine and dried over magnesium sulfate. The residues obtained after concentration under reduced pressure were purified by silica gel column (hexane:ethyl acetate=10:1) to obtain the title compound (12.1 g, 67%).
- 1H-NMR (400 MHz, CDCl3) δ 3.82 (s, 3H), 6.77 (d, 1H), 7.07 (s, 1H), 7.47 (d, 1H)
-
- 2-Bromo-5-methoxy-phenyl)-acetonitrile (12.2 g, 53.97 mmol) was dissolved in toluene (50 ml), and added with tetrabutylammonium bromide (3.55 g, 10.79 mmol), dibromoethane (7.05 ml, 80.95 mmol) and 50% aqueous solution of NaOH (50 ml) at room temperature. The mixture was stirred at room temperature for 4 hrs. The reaction mixture was added with water and extracted with ethyl acetate. The residues obtained after concentration under reduced pressure were purified by silica gel column (hexane: ethyl acetate) to obtain the title compound (11.18 g, 82%).
- 1H-NMR (400 MHz, CDCl3) δ 1.33 (t, 1H), 1.76 (t, 1H), 3.79 (s, 3H), 6.75-6.79 (m, 1H), 6.89 (d, 1H), 7.47 (d, 1H)
-
- 1-(2-Bromo-5-methoxy-phenyl)-cyclopropane carbonitrile (3.0 g, 11.9 mmol) was dissolved in ethylene glycol (30 ml). After adding KOH (2.1 g, 33.3 mmol) thereto, the mixture was stirred and heated at 180° C. for 7 hrs. After cooling, the reaction mixture was added with 1 N HCl (90 ml). The reaction mixture was extracted with ether, washed with water and saturated brine and dried over magnesium sulfate. After concentration under reduced pressure, the title compound was obtained (12.3 g, 72%).
- LCMS: m/z 272 [M+H]+
- HPLC retention time: 2.03 min (analysis condition Y)
- Compound GT26-4
-
- To the DCM solution (6 ml) of 1-(2-bromo-5-methoxy-phenyl)-cyclopropane carboxylic acid (0.3 g, 1.1 mmol), DMF (2 drops) and oxalyl chloride (0.23 ml, 2.5 mmol) were added at room temperature, and the mixture was stirred at room temperature for 2 hrs. The residues obtained from the reaction solution after concentration under reduced pressure were dissolved in toluene (6 ml), added with (2-hydroxybenzyl)triphenylphosphonium bromide (0.605 g, 1.32 mmol) and TEA (0.46 ml, 3.3 mmol), and the resulting mixture was stirred and heated at 100° C. overnight. The residues obtained after concentration under reduced pressure were purified by silica gel column (hexane: DCM) to obtain the title compound (0.309 g, 81%).
- LCMS: m/z 343 [M+H]+
- HPLC retention time: 3.55 min (analysis condition Y)
- Compound GT26-5
-
- To the THF solution (3 ml) of 2-[1-(2-bromo-5-methoxy-phenyl)-cyclopropyl]-benzofuran (0.259 g, 0.75 mmol), n-BuLi was added at −78° C., and the mixture was stirred at −78° C. for 20 min. Thereafter, the mixture was flushed with carbon dioxide gas. The reaction mixture was added with saturated solution of NH4Cl and extracted with ethyl acetate. The residues obtained after concentration under reduced pressure were purified by silica gel column (DCM:MeOH) to obtain the title compound (0.163 g, 70%).
- LCMS: m/z 309 [M+H]+
- HPLC retention time: 2.67 min (analysis condition Y)
- Compound GT26-6
-
- To the DCM solution (10 ml) of 2-(1-benzofuran-2-yl-cyclopropyl)-4-methoxy-benzoic acid (1.0 g, 3.24 mmol), trifuloroacetic acid anhydride (0.45 ml, 3.24 mmol) was added at −78° C., and the mixture was stirred at −78° C. for 10 min, at −50° C. for 10 min, and at −30° C. for 20 min. Thereafter, the mixture was added with water and extracted with DCM. The residues obtained after concentration under reduced pressure were washed with DCM and hexane to obtain the title compound (0.163 g, 70%).
- LCMS: m/z 291 [M+H]+
- HPLC retention time: 2.90 min (analysis condition Y)
- Compound GT26-7
-
- In the same manner as Compound A6 and Compound A7-17, the title compound was obtained from 8-methoxy-11H-spiro[benzo[d]naphtho[2,3-b]furan-6,1′-cyclopropan]-11-one.
- LCMS: m/z 376 [M+H]+
- HPLC retention time: 1.65 min (analysis condition Y)
- Compound GT27-1
-
- (2-Bromo-5-methoxy-phenyl)-acetic acid methyl ester (3.51 g, 13.5 mmol) was dissolved in DMF (4.5 ml), and added with NaH (2.1 g, 67.7 mmol). Subsequently, 15-crown-5 (1.38 ml, 6.8 mmol) and EtI (5.5 ml, 67.7 mmol) cooled to 0° C. were added to the mixture. The mixture was diluted with ethyl acetate and washed with water and saturated brine. The residues obtained after concentration under reduced pressure were purified by silica gel column (hexane-ethyl acetate). Then, the resultant was dissolved in ethanol (80 ml) and water (80 ml), added with KOH (91 g), and stirred at 140° C. The reaction solution was extracted with ethyl acetate, and washed with water and saturated brine. After concentration under reduced pressure, the target compound was obtained (10.62 g, 78%).
- LCMS: m/z 301 [M+H]+
- HPLC retention time: 3.07 min (analysis condition Y)
- Compound GT27-2
-
- Compound GT27-1 (0.5 g, 1.66 mmol) was dissolved in DCM (10 ml), added with DMF (2 drops) and oxalyl chloride (0.28 ml, 3.32 mmol), and the mixture was stirred for 2 hrs. The reaction mixture obtained after concentration under reduced pressure was dissolved in toluene (5 ml), added with DMAP (406 mg, 3.32 mmol), and the mixture was heated under reflux. The reaction mixture was extracted with ethyl acetate, washed with 1 N HCl, and saturated brine. The residues obtained after concentration under reduced pressure were purified by silica gel column (hexane-ethyl acetate) to obtain the target compound (0.36 g, 86%).
- LCMS: m/z 393 [M+H]+
- HPLC retention time: 3.03 min (analysis condition Y)
- Compound GT27-3
-
- Compound GT27-2 (0.118 g, 0.302 mmol) was dissolved in carbon tetrachloride (3 ml), added with N-bromosuccinimide (54 mg, 0.302 mmol), and the mixture was heated under reflux. The reaction mixture was concentrated under reduced pressure and the resulting residues were purified by silica gel column (ethyl acetate-hexane). The product was dissolved in toluene (3 ml), added with PPh3 (77 mg, 0.302 mmol), and the mixture was heated under reflux. The reaction mixture was concentrated under reduced pressure to obtain the target compound (130 mg, 57%).
- Compound GT27-4
-
- To the toluene solution (3 ml) of Compound GT27-3 (0.14 g, 0.137 mmol), toluene solution (0.16 ml, 0.164 mmol) of 1 M LiHMDS was added. The mixture was heated and stirred for 4 hrs. The reaction mixture was concentrated under reduced pressure and the resulting residues were purified by silica gel column (ethyl acetate: hexane) to obtain the title compound (28 mg, 35%).
- LCMS: m/z 373 [M+H]+
- HPLC retention time: 2.73 min (analysis condition Y)
- Compound GT27-5
-
- In the same manner as Compound A7-17, the title compound was obtained from Compound GT27-4.
- LCMS: m/z 407 [M+H]+
- HPLC retention time: 1.92 min (analysis condition Y)
- Compound GT27-6
-
- In the same manner as Compound A7-14-1 and Compound A7-14-2, the title compound was obtained from Compound GT27-4.
- LCMS: m/z 381 [M+H]+
- HPLC retention time: 2.38 min (analysis condition Y)
- The compounds described in the following Table 43 were synthesized according to the method shown below. According to the method used for the preparation of Compound Z10, Z11 and Z12, 3-chloro-8-methoxy-6H-benzo[b]naphtho[2,3-d]furan-11-one was prepared from Compound A2 and 2-bromo-5-chlorophenol. Subsequently, demethylation was carried out according to the method that is used for the preparation of Compound A6, and thus 3-chloro-8-hydroxy-6H-benzo[b]naphtho[2,3-d]furan-11-one was obtained. Thereafter, according to Mitsunobu reaction that is used for the preparation of Compound A7-1 or the alkylation method that is used for the preparation of A7-17, a corresponding side chain was introduced and, if necessary, functional group modification such as deprotection, etc. was carried out to prepare the compounds listed below.
-
TABLE 43 Example Comp. HPLC Retention No. No. Structure Compound Name Condition Time m/z 1216 GT28-1 3-Chloro-8-(2-diethylamino-ethoxy)- 6,6-dimethyl-6H-benzo[b]naphtho[2,3- d]furan-11-one F 2.52 412.0 1217 GT28-2 3-Chloro-6,6-dimethyl-8-((2R,3R)- 2,3,4-trihydroxy-butoxy)-6H- benzo[b]naphtho[2,3-d]furan-11-one H 5.39 417.0 1218 GT28-3 3-Chloro-8-((R)-2,3-dihydroxy- propoxy)-6,6-dimethyl-6H- benzo[b]naphtho[2,3-d]furan-11-one H 5.77 387.0 - ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the detection of phosphorylation of the biotinylated peptide, time-resolved fluorescence measurement was performed using an anti-phosphorylated tyrosine antibody labeled with europium cryptate and streptavidin conjugated to XL665, i.e., an allophycocyanin derivative. From the inhibition ratio compared to the control group that does not comprise a test compound, 50% inhibitory concentration (i.e., IC50 value) was calculated.
- The test compounds were serially diluted with dimethyl sulfoxide, further diluted with phosphate buffered saline which is free of any Ca2, Mg2+ (×50 dilution), and 10 μL of the resulting solution was aliquoted in a 96-well plate. Human lymphoma cell line KARPAS-299 was prepared in RPMI-1640 medium to which 10% bovine fetal serum was added to give a cell suspension with the cell density of 10,000 cells/190 μL. The resulting cell suspension was aliquoted to the plate (190 μL per well) to which the test compound had been already added, and the plate was kept in a 5% carbon dioxide gas incubator at 37° C. Ninety-six hours later, 10 μL of WST-8 (manufactured by Dojindo Laboratories) was added to each well, and subsequently the absorbance was measured at 450 nm. From the ratio of inhibition on cell growth which had been obtained from the addition of a test compound compared to the control group with no addition, 50% growth inhibitory concentration (i.e., IC50 value) of the test compound was calculated. The results are summarized in Tables 44-49.
-
TABLE 44 Inhibitory activity ALK-inhibiting on Karpas-299 cell Examples activity IC50(μM) growth IC50(μM) 123(Compound B2-27) 0.00228 0.0138 177(Compound B4-7) 0.00084 0.0105 178(Compound B4-8) 0.00153 0.0214 304(Compound F5-11) 0.00081 0.0061 338(Compound F5-43) 0.00032 0.0086 341(Compound F5-46) 0.01056 0.0289 364(Compound F6-18) 0.00177 0.0231 366(Compound F6-20) 0.0053 0.0093 372(Compound G6) 0.03074 0.1682 380(Compound H6-2) 0.00053 0.0062 429(Compound J7-10-2) 0.00083 0.0303 543(Compound O8-5) 0.00032 0.03 550(Compound O9-7) 0.00090 0.0044 735(Compound Z7) 0.09385 1.1924 516(Compound N6-2) 0.003906748 0.0248 725(Compound X5) 0.687683357 2.8765 882(Compound AZ7-10) 0.000493765 0.005769 916(Compound DZ7-1) 0.001836659 0.357381 937(Compound EZ9-3) 0.006473484 0.056914 939(Compound EZ9-5) 0.399865279 13.421227 1175(Compound GT18-4) 0.093 2.012 -
TABLE 45 Example ALK-inhibiting No. Compound activity IC50(μM) 13 A7-1 0.052707597 14 A7-2 0.006159417 38 A7-20 0.026183852 39 A7-21 0.017713716 40 A7-22 0.030434111 41 A7-23 0.029469872 45 A8-2 0.008009528 47 A8-4 0.010253392 51 A8-6-3 0.097920152 52 A8-7 0.045959643 55 A8-10 0.00673264 57 A8-12 0.003594618 63 A8-18 0.016005139 65 A8-20 0.0029 67 A9-1 0.004943 70 A9-3-2 0.007649647 73 A9-6-2 0.001398207 74 A9-7 0.0034607 76 A9-9 0.017148495 78 A9-11 0.051123952 79 A9-12 0.017501168 83 A9-15-2 0.0035 84 A9-16 0.08468 90 B2-1 0.033572 100 B2-9 0.016225317 101 B2-10 0.039433518 102 B2-11 0.072607257 104 B2-13 0.001681324 109 B2-16-3 0.000980809 117 B2-23 0.005436966 118 B2-24 0.014834642 122 B2-26-2 0.007278245 124 B2-28 0.059632226 128 B3-2-2 0.003183521 130 B3-4 0.063798146 135 B3-9 0.01492317 137 B3-11 0.071084446 141 B3-14 0.011893599 142 B3-15 0.030133825 143 B3-16 0.027324427 146 B3-19 0.010369469 147 B3-20 0.026851192 149 B3-22 0.272356381 150 B3-23 0.023088404 151 B3-24 0.003610645 157 B3-27-2 0.002114607 158 B3-28 0.042375341 159 B3-29 0.006002322 165 B3-34 0.006783031 166 B3-35 0.003473067 168 B3-37 0.011859342 179 B4-9 0.002000975 187 CC4-2 0.096115639 189 C1-1 0.051102036 206 C4-9 0.005101172 210 C4-13 0.008752733 212 C4-15 0.009616778 226 D1 0.000991134 227 D2 0.003611773 228 D3-1 0.006279559 245 E4-5 0.009450575 256 E5-2 0.00133756 264 E6-2 0.006668071 265 E6-3 0.008113087 268 F1-3 0.005054399 277 F3-6-2 0.000167996 283 F4-1-1 0.001625048 286 F4-3 0.000951804 290 F4-7 0.001133931 293 F4-10 0.002098847 298 F5-5 0.002385717 300 F5-7 0.002575475 306 F5-13 0.002051837 314 F5-20 0.000996109 319 F5-25 0.000881378 322 F5-28 0.01227125 331 F5-36-2 0.001778367 334 F5-39 0.014824288 -
TABLE 46 Example ALK-inhibiting No. Compound activity IC50(μM) 340 F5-45 0.000579745 346 F5-51 0.002610782 350 F6-4 0.00715425 353 F6-7 0.020276801 355 F6-9 0.001092627 358 F6-12 0.015047658 359 F6-13 0.000399685 389 H9-3 0.002622129 403 I6-4 0.000391036 407 I7-1 0.001863642 421 J7-3 0.015290853 422 J7-4 0.004631153 423 J7-5 0.012009506 424 J7-6 0.001570404 426 J7-8 0.001170682 431 J7-11-2 0.01172814 435 J7-15 0.02319 437 J7-17 0.007091939 438 J8-1 0.012517614 443 J8-6 0.00396 455 JJ5 0.862941682 458 JJ7-2 0.028993627 461 JJ9-1 0.004337558 465 JJ10-1 0.492725332 472 K6 0.029284532 486 K10-5 0.000589765 501 L10-2 0.00160 508 M6-2 0.006136762 517 N6-3 0.03272871 519 N6-5 0.026853329 531 O5-4 0.00431 546 O9-3 0.00086 571 Q8 0.005719259 579 R8-2 0.000769618 591 S4 1.664818863 599 S8-2 0.04064 601 S9-2 0.000456356 607 T2-1 0.432812267 618 T6-1 0.614075453 621 T6-4 0.341433432 628 T11 0.271479209 630 T12-2 0.15422 633 T13-3 0.16211 637 T13-7 0.16821 639 T13-9 0.16189 645 T14-5 0.41327 650 T14-10 0.18923 654 T16-1 0.01951 657 T16-4 0.07941 668 T21 0.8521 671 T22-1-1 0.151061541 678 T22-7 2.8135 679 T22-8 0.583 686 T26-2 0.08320 702 U8-6-2 0.00260 704 U8-7-2 0.00604 706 U8-8-2 0.35976 707 U8-8-3 0.84884 709 U10-1 0.55215 711 U11 0.00193 720 W4-2 0.13445 730 Y5-2 0.554738402 751 K10-10 0.0085 753 K10-12 0.0022 755 K10-14 0.0118 758 K10-17 0.1422 760 K10-19 0.0015 762 L10-3 0.0099 770 L10-11 0.0231 776 B3-42 0.0042 786 E9-4 0.0004 790 E9-8 0.0075 796 F4-11 0.0003 822 PR11-6 0.0003 823 PR11-7 0.0003 824 PR9-9 0.0142 829 PR9-13 0.0007 832 PR11-11 0.0006 846 PR9-25 0.021743738 847 PR11-14 0.001890642 -
TABLE 47 Example ALK-inhibiting No. Compound activity IC50(μM) 849 PR11-16 0.000813047 864 LB5-1 0.424843491 866 LB5-3 2.398295 875 AZ7-3 0.113911239 892 AZ7-20 0.009369855 893 AZ7-21 0.142933634 920 DZ7-5 0.326374265 938 EZ9-4 0.300760062 949 GT2-6 1.3255 956 GT2-13 0.1617 960 GT3-2 5.9473 962 GT3-4 1.0829 963 GT3-5 4.224 967 GT3-9 0.8981 970 GT3-12 1.6214 972 GT4-1 0.29 973 GT4-2 0.104 981 GT5-8 2.9743 982 GT5-9 21.3078 983 GT5-10 3.2 994 GT5-21 1.2466 995 GT6-1 12.9519 996 GT6-2 12.9704 997 GT6-3 0.575 998 GT6-4 4.3855 999 GT6-5 3.9 1000 GT6-6 5.4 1001 GT6-7 3.7 1004 GT6-10 0.9 1005 GT6-11 1.4385 1007 GT6-13 0.7526 1008 GT6-14 4.8429 1013 GT8-3 0.93 1017 GT9-3 0.3785 1019 GT9-5 0.77 1030 GT11-8 5.9346 1031 GT11-9 7.7947 1034 GT11-12 2.076 1035 GT11-13 1.6274 1039 GT11-17 0.7938 1042 GT11-20 0.5083 1043 GT11-21 2.2822 1047 GT11-25 1.9038 1048 GT11-26 5.3708 1050 GT11-28 3.2813 1051 GT11-29 1.811 1052 GT11-30 4.0931 1054 GT11-32 7.0451 1059 GT11-37 2.7739 1060 GT11-38 1.1587 1061 GT11-39 1.0914 1065 GT11-43 3.7028 1066 GT11-44 3.1203 1072 GT11-50 3.3428 1073 GT11-51 2.547 1074 GT11-52 1.2588 1081 GT11-59 1.0586 1083 GT11-61 0.7928 1085 GT11-63 0.9013 1086 GT11-64 0.3127 1087 GT11-65 0.206 1090 GT12-3 0.8541 1096 GT12-9 5.7571 1102 GT13-3 0.4209 1105 GT13-6 0.3894 1113 GT13-14 0.1571 1114 GT13-15 0.7 1117 GT13-18 2.2 1118 GT13-19 0.5 1119 GT13-20 0.42 1125 GT13-26 0.028 1126 GT13-27 3.0645 1127 GT13-28 5.6311 1128 GT13-29 17.4641 1129 GT13-30 0.51 1130 GT13-31 0.54 1164 GT16-5 0.4149 1177 GT18-6 0.7527 1185 GT20-6 1.8 -
TABLE 48 Inhibitory activity Example on Karpas-299 cell No. Compound growth IC50(μM) 15 A7-3 0.1138 17 A7-5 0.6268 19 A7-7 0.3293 21 A7-9 0.2037 22 A7-10 0.3031 25 A7-12 0.1119 46 A8-3 0.0866 56 A8-11 0.0677 58 A8-13 0.0226 60 A8-15 0.2322 61 A8-16 0.0345 62 A8-17 0.1269 64 A8-19 0.0726 66 A8-21 0.1050 68 A9-2 0.1372 72 A9-5 0.0523 93 B2-4 0.0365 138 B3-12 1.4358 154 B3-25-3 0.7298 155 B3-26 1.3613 160 B3-30 0.2282 163 B3-32 0.0652 167 B3-36 0.0390 174 B4-4 0.0812 229 D3-2 0.9700 230 D3-3 0.1320 244 E4-4 0.1090 257 E5-3 0.1895 260 E5-6 0.0527 273 F3-3 0.0162 287 F4-4 0.0071 289 F4-6 0.0291 291 F4-8 0.0221 292 F4-9 0.0650 294 F5-1 0.0091 297 F5-4 0.0018 301 F5-8 0.0297 302 F5-9 0.0043 303 F5-10 0.0135 309 F5-15-2 0.0098 310 F5-16 0.0042 315 F5-21 0.0663 316 F5-22 0.0066 323 F5-29 0.0076 325 F5-31 0.0727 326 F5-32 0.0240 335 F5-40 0.0256 336 F5-41 0.1491 339 F5-44 0.0060 348 F6-2 0.0295 351 F6-5 0.0274 352 F6-6 0.0364 357 F6-11 0.0776 359 F6-13 0.0079 420 J7-2-3 0.0295 434 J7-14 0.5567 446 J9-3 0.0532 467 JJ10-3 6.0632 488 K10-7 0.0518 518 N6-4 0.1224 562 P5 27.7670 605 T1-1 1.8669 636 T13-6 1.2901 640 T13-10 1.3775 642 T14-2 0.6324 646 T14-6 1.9418 649 T14-9 1.08 656 T16-3 2.25 672 T22-1-2 1.7820 680 T23-1 4.2526 681 T23-2 7.0799 688 T27-2 0.9970 689 U5 0.1217 695 U8-3-2 0.6773 698 U8-4-3 1.10 700 U8-5-2 0.3573 708 U9 0.4070 710 U10-2 0.94 -
TABLE 49 Inhibitory activity Example on Karpas-299 cell No. Compound growth IC50(μM) 764 L10-5 0.019 766 L10-7 0.037 767 L10-8 0.024 769 L10-10 0.159 773 B3-40 0.022 787 E9-5 0.041 792 E9-9 0.004 793 PR11-20 0.020313 794 PR11-21 0.06439 827 PR9-11 0.036 839 PR9-20 0.018772 844 PR9-23 0.020492 845 PR9-24 0.067888 850 PR11-17 0.005766 852 PR11-19 0.034632 865 LB5-2 1.287666 878 AZ7-6 1.126471 896 AZ7-24 0.054 935 EZ9-1 16.635 941 W4-4 0.116 976 GT5-3 1.868 979 GT5-6 8.231 980 GT5-7 17.135 984 GT5-11 1.957 985 GT5-12 19.989 986 GT5-13 1.332 987 GT5-14 3.787 990 GT5-17 2.359 991 GT5-18 4.255 993 GT5-20 6.081 1020 GT9-6 2.655 1115 GT13-16 7.875 1123 GT13-24 3.951 1124 GT13-25 5.511 1131 GT13-32 2.501 1132 GT13-33 10.887
Claims (16)
1. A compound or salt or solvate thereof represented by Formula (I):
wherein,
A1, A2, A3, A4, A7, A8, A9 and A10 all represent C, or any one of A2, A3, A4, A7, A8 and A9 represents N (with the proviso that, when it represents N, no substituent group exists therefor) and the remainings represent C;
A5 is selected from NR5, O and S;
R1 and R10 each independently represent [1] a hydrogen atom, [2] a cyano group, [3] a halogen atom or [4] a 4-to 10-membered heterocycloalkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s);
R2 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group,
(3) a C2-8 alkenyl group,
(4) a C2-8 alkynyl group,
(5) a cyano group,
(6) a halogen atom,
(7) a (C1-8 alkyl)m2-amino group which may be substituted by C1-8 alkylsulfonyl group(s),
m2: 0˜2, and
(8) a nitro group;
R3 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by [1] halogen atom(s), [2] hydroxy group(s) or [3] C1-8 alkoxy group(s),
(3) a C6-10 aryl group,
(4) a cyano group,
(5) a C1-8 alkanoyl group which may be substituted by C6-10 aryl group(s),
(6) a (C1-8 alkyl)m3a-aminocarbonyl group which may be substituted by one or more R3A,
R3A: [1] a C6-10 aryl group, [2] a C1-8 alkoxy group, [3] a 5- to 14-membered heteroaryl group, or [4] a C6-10 arylsulfonyl group,
m3a: 0˜2,
(7) a hydroxycarbonyl group,
(8) a C1-8 alkoxycarbonyl group which may be substituted by [1] hydroxy group(s) or [2] C1-8 alkoxy group(s),
(9) a halogen atom,
(10) a (C1-8 alkyl)m3b-amino group which may be substituted by C6-10 aryl group(s),
m3b: 0˜2,
(11) a C1-8 alkylcarbonyl (C0-8 alkyl) amino group which may be substituted by [1] C6-10 aryl group(s) or [2] C6-10 aryloxy group(s),
(12) a C6-10 arylcarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
(13) a (C1-8 alkyl)m3c-aminocarbonyl (C0-8 alkyl) amino group which may be substituted by C6-10 aryl group(s),
m3c: 0˜2,
(14) a nitro group,
(15) a hydroxy group,
(16) a C1-8 alkoxy group which may be substituted by one or more R3B,
R3B: [1] a hydroxy group, [2] a C1-8 alkoxy group, [3] a C6-10 aryl (C0-8 alkyl) aminocarbonyl group, [4] a (C1-8 alkyl)m3d-amino group, or [5] a halogen atom,
m3d: 0˜2,
(17) a 4- to 10-membered heterocycloalkyloxy group,
(18) a 5- to 14-membered heteroaryloxy group,
(19) a (C1-8 alkyl)m3e-aminocarbonyloxy group which may be substituted by C6-10 aryl group(s)
m3e: 0˜2,
(20) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group,
(21) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s),
(22) a C1-8 alkylthio group,
(23) a C1-8 alkylsulfonyl group which may be substituted by C6-10 aryl group(s),
(24) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s) which may be substituted by C1-8 alkoxy group(s),
(25) a C1-8 alkoxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkoxy group(s),
(26) a C6-10 aryloxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
(27) a C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group which may be substituted by one or more R3C,
R3C: [1] a C1-8 alkyl group which may be substituted by halogen atom(s), or [2] a C1-8 alkoxy group,
(28) a C3-8 cycloalkyl (C0-8 alkyl) aminocarbonyloxy group, and
(29) a C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkyl group and [2] a C1-8 alkoxy group;
R4 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by halogen atom(s),
(3) a C2-8 alkenyl group,
(4) a C2-8 alkynyl group,
(5) a C3-8 cycloalkyl group,
(6) a cyano group,
(7) an aminocarbonyl group,
(8) a (C1-8 alkyl)m4a-aminocarbonyl group,
m4a: 1˜2,
(9) a hydroxycarbonyl group,
(10) a C1-8 alkoxycarbonyl group,
(11) a halogen atom,
(12) a (C1-8 alkyl)m4b-amino group,
m4b: 0˜2,
(13) a hydroxy group, and
(14) a C1-8 alkoxy group which may be substituted by hydroxy group(s);
R5 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R5A,
R5A: [1] a hydroxycarbonyl group, [2] a C1-8 alkoxycarbonyl group, [3] a hydroxy group, [4] a C1-8 alkoxy group, [5] a (C1-8 alkyl)m5-amino group, [6] a C6-10 aryl group, or [7] a C1-8 alkylthio group,
m5: 0˜2,
(3) a C2-8 alkenyl group,
(4) a C2-8 alkynyl group,
(5) a C3-8 cycloalkyl group, and
(6) a C1-8 alkylsulfonyl group;
R6 and R6′ are each independently selected from the group consisting of:
(1) a C1-8 alkyl group which may be substituted by halogen atom(s),
(2) a C2-8 alkenyl group, and
(3) a C2-8 alkynyl group; or
R6 and R6′ are taken together with the carbon atoms to which they are bound to form:
(4) a C3-8 cycloalkyl group, or
(5) a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl C6-10 aryl sulfonyl group(s) which may be substituted by C1-8 alkyl group(s);
R7 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a halogen atom,
(3) a C1-8 alkoxy group which may be substituted by one or more R7A,
R7A: [1] a (C1-8 alkyl)m7a-amino group, [2] a hydroxy, [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl group(s),
m7a: 0˜2,
(4) a C1-8 alkylsulfonyl group,
(5) a nitro group, and
(6) a hydroxyl group;
R8 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R8A,
R8A: [1] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8A1, [2] a (C1-8 alkyl)m8a-amino group which may be substituted by a halogen atom, or [3] a hydroxy group,
m8a:0˜2,
R8A1: [1] a C1-8 alkyl group, [2] a C1-8 alkylsulfonyl group, [3] a (C1-8 alkyl)m8b-aminosulfonyl group, [4] an oxo group, [5] a C1-8 alkoxycarbonyl, or [6] a C1-8 alkoxycarbonyl (C0-8 alkyl) aminosulfonyl,
m8b: 0˜2,
(3) a C2-8 alkenyl group,
(4) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B,
R8B:
<1> a C1-8 alkyl group which may be substituted by one or more R8B1,
<2> a C2-8 alkeynyl group,
<3> a C2-8 alkynyl group,
<4> a C3-8 cycloalkyl group which may be substituted by [1] cyano group(s) or [2] C1-8 alkyl group(s),
<5> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B2,
<6> a C1-8 alkoxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkoxy group and [2] a C3-8 cycloalkyl group,
<7> a C1-8 alkoxycarbonyl group,
<8> a C1-8 alkylsulfonyl group,
<9> a 5- to 14-membered heteroarylsulfonyl group,
<10> an oxo group,
<11> a cyano group,
<12> a C1-8 alkanoyl group which may be substituted by one or more R8B3,
<13> a C3-8 cycloalkylcarbonyl group,
<14> a (C1-8 alkyl)m8c-aminosulfonyl group,
<15> a C1-8 alkylsulfonyl (C0-8 alkyl) amino group,
<16> a (C1-8 alkyl)m8d-amino group which may be substituted by one or more R8B4,
<17> a hydroxy group,
<18> a (C1-8 alkyl)m8e-aminocarbonyl group, or
<19> a C1-8 alkoxycarbonyl (C0-8 alkyl) amino group
m8c: 0˜2
m8d: 0˜2
m8e: 0˜2
R8B1: [1] a C3-8 cycloalkyl group, [2] a hydroxy group, or [3] a C1-8 alkoxy group(s),
R8B2: [1] a halogen atom, [2] a C1-8 alkyl group, [3] an oxo group, [4] a hydroxy group, or [5] a deuterium atom,
R8B3: a (C1-8 alkyl)m8f-amino group,
m8f: 0˜2,
R8B4: [1] a C3-8 cycloalkyl group, or [2] a hydroxy group,
(5) a 5- to 14-membered heteroaryl group which may be substituted by a C1-8 alkyl group,
(6) a (C1-8 alkyl)m8g-aminocarbonyl group which may be substituted by one or more R8C,
m8g: 0˜2,
R8C:[1] a hydroxy group, [2] a (C1-8 alkyl)m8h-amino group which may be substituted by substituent(s) selected from the group consisting of <1> a (C1-8 alkyl)m8i-aminosulfonyl group, <2> a C1-8 alkylsulfonyl group, <3> a C1-8 alkoxycarbonyl group and 5<4> a C1-8 alkoxycarbonyl(C0-8 alkyl) aminosulfonyl group, [3] a C1-8 alkylsulfonyl group, or [4] a C1-8 alkoxy group which may be substituted by a hydroxy group,
m8h: 0˜2,
m8i: 0˜2,
(7) a 4- to 10-membered heterocycloalkyl (C0-8 alkyl) aminocarbonyl group which may be substituted by oxo group(s),
(8) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8D,
R8D: [1] a C1-8 alkyl group which may be substituted by one or more R8D1, [2] a hydroxy group, [3] a C1-8 alkylsulfonyl group, or [4] a C1-8 alkoxycarbonyl group,
R8D1: [1] a hydroxy group, or [2] a C1-8 alkoxy group,
(9) a hydroxycarbonyl group,
(10) a C0-8 alkoxy (C0-8 alkyl) aminocarbonyl group which may be substituted by hydroxy group(s),
(11) a halogen atom,
(12) a (C1-8 alkyl)m8j-amino group which may be substituted by one or more R8H,
m8j: 0˜2,
R8H: [1] a hydroxy group, or [2] a 4- to 10-membered heterocycloalkyl group,
(13) a hydroxyl group,
(14) a C1-8 alkoxy group which may be substituted by one or more R8E,
R8E:
<1> a hydroxy group,
<2> halogen atom,
<3> a hydroxycarbonyl group,
<4> a C1-8 alkoxycarbonyl group,
<5> a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8E1,
<6> a (C1-8 alkyl)m8k1-amino group which may be substituted by one or more R8E2,
m8k1: 0˜2,
<7> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8E3,
<8> a 5- to 14-membered heteroaryl group,
<9> a (C1-8 alkyl)m8k2-aminocarbonyl group which may be substituted by one or more R8E6,
m8k2: 0˜2,
<10> a C1-8 alkoxy group which may be substituted by one or more R8E7,
<11> a C1-8 alkylthio group,
<12> a C1-8 alkylsulfinyl group,
<13> a C1-8 alkylsulfonyl group,
R8E1:
<1> a C1-8 alkoxycarbonyl group,
<2> a C1-8 alkanoyl group,
<3> a C1-8 alkylsulfonyl group,
<4> a (C1-8 alkyl)m8k3-aminosulfonyl group,
m8k3: 0˜2, or
<5> a 4- to 10-membered heterocycloalkyl group,
R8E2:
<1> a hydroxy group,
<2> a C1-8 alkoxycarbonyl group which may be substituted by halogen atom(s),
<3> a C3-8 cycloalkyl group which may be substituted by C1-8 alkyl group(s) which may be substituted by hydroxy group(s),
<4> a C1-8 alkanoyl group which may be substituted by substituent(s) selected from the group consisting of [1] a (C1-8 alkyl)m8k4-amino group and [2] a halogen atom(s),
m8k4: 0˜2,
<5> a (C1-8 alkyl)m8k5-aminocarbonyl group,
m8k5: 0˜2,
<6> a C1-8 alkylsulfonyl group,
<7> a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s),
<8> a (C1-8 alkyl)m8k6-aminosulfonyl group which may be substituted by C1-8 alkoxycarbonyl group(s),
m8k6: 0˜2, or
R8E3:
<1> a C1-8 alkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a hydroxy group and [2] a C1-8 alkylcarbonyloxy group,
<2> a C1-8 alkylcarbonyloxy group,
<3> a hydroxy group,
<4> a C3-8 cycloalkyl group,
<5> a C1-8 alkoxy group,
<6> a C1-8 alkoxycarbonyl group,
<7> a C1-8 alkylsulfonyl group,
<8> a (C1-8 alkyl)m8k8-aminocarbonyl group
m8k8: 0˜2,
<9> a C1-8 alkanoyl group which may be substituted by hydroxy group(s),
<10> an oxo group, or
<11> a 4- to 10-membered heterocycloalkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkanoyl group, [2] a C1-8 alkoxycarbonyl group and [3] a C1-8 alkylsulfonyl group,
R8E6:
<1> a C2-8 alkenylcarbonyloxy group,
<2> a hydroxy group,
<3> a cyano group,
<4> a (C1-8 alkyl)m8k9-amino group which may be substituted by hydroxy group(s)
m8k9: 0˜2,
<5> a C1-8 alkoxy group which may be substituted by hydroxy group(s),
<6> a C1-8 alkylcarbonyloxy group,
<7> a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl group(s), or
<8> a 5- to 14-membered heteroaryl group,
R8E7:
<1> a hydroxy group, or
<2> a C1-8 alkoxy group which may be substituted by hydroxy group(s),
(15) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by one or more R8F,
R8F:
<1> a C1-8 alkyl group which may be substituted by one or more R8F1,
<2> a C3-8 cycloalkyl group,
<3> a C1-8 alkanoyl group which may be substituted by halogen atom(s),
<4> a C1-8 alkylcarbonyloxy group,
<5> a C1-8 alkoxycarbonyl group,
<6> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8F2,
<7> a C1-8 alkyl sulfonyl group,
<8> a hydroxy group, or
[9] a C6-10 aryl group,
R8F1: [1] a hydroxy group, [2] a C1-8 alkoxy group, or [3] a halogen atom,
R8F2: [1] a 4- to 10-membered heterocycloalkyl group, [2] a C1-8 alkoxycarbonyl group, or [3] a C1-8 alkylsulfonyl group,
(16) a 5- to 14-membered heteroaryloxy group,
(17) a 4- to 10-membered heterocycloalkylcarbonyloxy group,
(18) a (C1-8 alkyl)m8l1-aminosulfonyloxy group,
m8l1: 0˜2,
(19) a C1-8 alkyl thio group which may be substituted by [1] (C1-8 alkyl)m8l2-amino group(s), [2] hydroxy group(s) or [3] hydroxycarbonyl group(s),
m8l2: 0˜2,
(20) a C1-8 alkylsulfonyl group which may be substituted by one or more R8G,
R8G: [1] a hydroxycarbonyl group, [2] a hydroxy group, or [3] a (C1-8 alkyl)m8l3-amino group,
m8l3: 0˜2,
(21) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group which may be substituted by C1-8 alkyl group(s),
(22) a C2-8 alkenyloxy group, and
(23) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s);
R9 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R9A,
R9A: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9A1, [3] a hydroxy group, [4] a C1-8 alkoxy group, or [5] a hydroxycarbonyl group,
R9A1: [1] a C1-8 alkyl group, [2] a C3-8 cycloalkyl group, or [3] a 4- to 10-membered heterocycloalkyl group,
(3) a C2-8 alkenyl group which may be substituted by one or more R9B,
R9B: [1] a (C1-8 alkyl)m9a-amino group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more group R9B1,
R9B1:[1] a C3-8 cycloalkyl group, or [2] a 4- to 10-membered heterocycloalkyl group,
m9a: 0˜2,
(4) a C2-8 alkynyl group which may be substituted by one or more R9C,
R9C: [1] a C1-8 alkoxy group, [2] a (C1-8 alkyl)m9b-amino group which may be substituted by C6-10 aryl group(s), [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9C1, [4] a C3-8 cycloalkyl group, [5] a hydroxy group, [6] a hydroxycarbonyl group, or [7] a C1-8 alkyloxycarbonyl group,
m9b: 0˜2,
R9C1: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group, or [3] an oxo group,
(5) a C3-8 cycloalkyl group,
(6) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9D,
R9D: [1] a C1-8 alkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s), [2] a C3-8 cycloalkyl group, [3] a 4- to 10-membered heterocycloalkyl group, or [4] a C1-6 alkylsulfonyl group, or [5] a C1-8 alkoxycarbonyl group,
(7) a C6-10 aryl group which may be substituted by one or more R9E,
R9E: [1] a halogen atom, [2] a hydroxy group, [3] a hydroxycarbonyl group, or [4] a C1-8 alkyl group which may be substituted by hydroxy group(s), or [5] a C1-8 alkoxy group,
(8) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s),
(9) a cyano group,
(10) a C1-8 alkanoyl group,
(11) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by C1-8 alkyl group(s),
(12) a halogen atom,
(13) a (C1-8 alkyl)m9c-amino group which may be substituted by one or more R9F,
m9c: 0˜2,
(14) a C1-8 alkylcarbonyl(C0-8 alkyl)amino group which may be substituted by (C1-8 alkyl)m9d-amino group(s),
m9d: 0˜2,
(15) a C1-8 alkylsulfonyl(C0-8 alkyl)amino group,
(16) a (C1-8 alkyl)m9e-aminosulfonyl(C0-8 alkyl)amino group,
m9e: 0˜2,
(17) a nitro group,
(18) a hydroxy group,
(19) a C1-8 alkoxy group which may be substituted by one or more R9G,
R9G: [1] a hydroxy group, [2] a hydroxycarbonyl group, [3] a C6-10 aryl group which may be substituted by C1-8 alkoxy group(s), [4] a (C1-8 alkyl)m9g1-amino group, [5] a C1-8 alkoxy group which may be substituted by one or more R9G1, [6] a 5- to 14-membered heteroaryl group, or [7] a 4- to 10-membered heterocycloalkyloxy group which may be substituted by C1-8 alkyl group(s),
m9g1: 0˜2,
R9G1: [1] a C1-8 alkoxy group, or [2] a hydroxycarbonyl group,
(20) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by [1] 4- to 10-membered heterocycloalkyl group(s), or [2] C1-8 alkoxycarbonyl group(s),
(21) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s),
(22) a C1-8 alkylthio group which may be substituted by (C1-8 alkyl)m9f-amino group(s),
m9f: 0˜2,
(23) a C1-8 alkylsulfonyl group which may be substituted by (C1-8 alkyl)m9g-amino group(s),
m9g: 0˜2,
(24) a (C1-8 alkyl)m9h-aminosulfonyl group,
m9h: 0˜2,
(25) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s), and
(26) a hydroxycarbonyl group.
2. The compound according to claim 1 , or a salt or solvate thereof, wherein R3 is a cyano group or a halogen atom.
3. The compound according to claim 1 , or a salt or solvate thereof, wherein A5 is NR5 and R5 is a hydrogen atom.
4. The compound according to claim 1 , or a salt or solvate thereof, wherein all of the A1, A2, A3, A4, A7, A8, A9 and A10 are a carbon atom.
5. The compound according to claim 1 , or a salt or solvate thereof, wherein:
A1, A2, A3, A4, A7, A8, A9 and A10 all represent C, or any one of A2, A3, A4, A7, A8 and A9 represents N (with the proviso that, when it represents N, no substituent group exists therefor) and the remainings represent C;
A5 is selected from NR5, O and S;
R1 represents [1] a hydrogen atom, [2] a cyano group, or [3] a halogen atom;
R2 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group,
(3) a cyano group,
(4) a halogen atom, and
(5) a (C1-8 alkyl)m2-amino group which may be substituted by C1-8 alkylsulfonyl group(s),
m2: 0˜2;
R3 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by halogen atom(s),
(3) a cyano group,
(4) a (C1-8 alkyl)m3a-aminocarbonyl group which may be substituted by one or more R3A,
R3A: [1] a C6-10 aryl group, [2] a C1-8 alkoxy group, [3] a 5- to 14-membered heteroaryl group, or [4] a C6-10 aryl sulfonyl group,
m3a: 0˜2,
(5) a hydroxycarbonyl group,
(6) a C1-8 alkoxycarbonyl group which may be substituted by hydroxy group(s),
(7) a halogen atom,
(8) a (C1-8 alkyl)m3b-amino group which may be substituted by C6-10 aryl group(s),
m3b: 0˜2,
(9) a C1-8 alkylcarbonyl (C0-8 alkyl) amino group which may be substituted by [1] C6-10 aryl group(s) or [2] C6-10 aryloxy group(s),
(10) a C6-10 arylcarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
(11) a nitro group,
(12) a hydroxy group,
(13) a C1-8 alkoxy group which may be substituted by one or more R3B,
R3B: [1] a hydroxy group, [2] a C1-8 alkoxy group, [3] a C6-10 aryl (C0-8 alkyl) aminocarbonyl group, [4] a (C1-8 alkyl)m3d-amino group, or [5] a halogen atom,
m3d: 0˜2,
(14) a 4- to 10-membered heterocycloalkyloxy group,
(15) a 5- to 14-membered heteroaryloxy group,
(16) a (C1-8 alkyl)m3e-aminocarbonyloxy group which may be substituted by C6-10 aryl group(s),
m3e: 0˜2,
(17) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group,
(18) a C1-8 alkylthio group,
(19) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s) which may be substituted by C1-8 alkoxy group(s),
(20) a C1-8 alkoxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkoxy group(s),
(21) a C6-10 aryloxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
(22) a C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkoxy group(s),
(23) a C3-8 cycloalkyl (C0-8 alkyl) aminocarbonyloxy group, and
(24) a C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkyl group and [2] a C1-8 alkoxy group;
R4 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by halogen atom(s),
(3) a C3-8 cycloalkyl group,
(4) a cyano group,
(5) an aminocarbonyl group,
(6) a hydroxycarbonyl group,
(7) a halogen atom,
(8) a (C1-8 alkyl)m4b-amino group,
m4b: 0˜2,
(9) a hydroxy group, and
(10) a C1-8 alkoxy group which may be substituted by hydroxy group(s);
R5 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R5A,
R5A: [1] a hydroxycarbonyl group, [2] a C1-8 alkoxycarbonyl group, [3] a hydroxy group, [4] a C1-8 alkoxy group, [5] a (C1-8 alkyl)m5-amino group, or [6] a C1-8 alkylthio group,
m5: 0˜2, and
(3) a C1-8 alkylsulfonyl group;
R6 and R6′ are each independently:
(1) a C1-8 alkyl group, or
R6 and R6′ are taken together with the carbon atoms to which they are bound to form,
(2) a C3-8 cycloalkyl group, or
(3) a 4- to 10-membered heterocycloalkyl group;
R7 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a halogen atom, and
(3) a C1-8 alkoxy group which may be substituted by one or more R7A,
R7A: [1] a (C1-8 alkyl)m7a-amino group, or [2] a hydroxy group,
m7a:0-2;
R8 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R8A,
R8A: [1] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8A1, [2] a (C1-8 alkyl)m8a-amino group which may be substituted by a halogen atom, or [3] a hydroxy group,
m8a:0˜2,
R8A1: [1] a C1-8 alkyl group, [2] a C1-8 alkylsulfonyl group, [3] a (C1-8 alkyl)m8b-aminosulfonyl group, or [4] an oxo group,
m8b: 0˜2,
(3) a C2-8 alkenyl group,
(4) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B,
R8B:
<1> a C1-8 alkyl group which may be substituted by one or more R8B1,
<2> a C2-8 alkynyl group,
<3> a C3-8 cycloalkyl group which may be substituted by [1] cyano group(s) or [2] C1-8 alkyl group(s),
<4> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B2,
<5> a C1-8 alkoxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkoxy group and [2] a C3-8 cycloalkyl group,
<6> a C1-8 alkylsulfonyl group,
<7> an oxo group,
<8> a cyano group,
<9> a C1-8 alkanoyl group which may be substituted by one or more R8B3,
<10> a C3-8 cycloalkylcarbonyl group,
<11> a (C1-8 alkyl)m8c-aminosulfonyl group,
<12> a C1-8 alkylsulfonyl (C0-8 alkyl) amino group,
<13> a (C1-8 alkyl)m8d-amino group which may be substituted by one or more R8B4,
<14> a hydroxy group, or
<15> a (C1-8 alkyl)m8e-aminocarbonyl group,
m8c: 0˜2,
m8d: 0˜2,
m8e: 0˜2,
R8B1: [1] a C3-8 cycloalkyl group, [2] a hydroxy group, or [3] C1-8 alkoxy group which may be substituted by C1-8 alkoxy group(s),
R8B2: [1] a halogen atom, [2] a C1-8 alkyl group, [3] an oxo group, or [4] a hydroxy group,
R8B3: a (C1-8 alkyl)m8f-amino group,
m8f: 0˜2,
R8B4: [1] a C3-8 cycloalkyl group, or [2] a hydroxy group,
(5) a 5- to 14-membered heteroaryl group which may be substituted by a C1-8 alkyl group,
(6) a (C1-8 alkyl)m8g-aminocarbonyl group which may be substituted by one or more R8C,
m8g: 0˜2,
R8C:[1] a hydroxy group, [2] a (C1-8 alkyl)m8h-amino group which may be substituted by substituent(s) selected from the group consisting of <1> a (C1-8 alkyl)m8i-aminosulfonyl group and <2> a C1-8 alkylsulfonyl group, or [3] a C1-8 alkylsulfonyl group,
m8h: 0˜2,
m8i: 0˜2,
(7) a 4- to 10-membered heterocycloalkyl (C0-8 alkyl) aminocarbonyl group which may be substituted by oxo group(s),
(8) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8D,
R8D: [1] a C1-8 alkyl group which may be substituted by one or more R8D1, [2] a hydroxy group, or [3] a C1-8 alkylsulfonyl group,
R8D1: [1] a hydroxy group, or [2] a C1-8 alkoxy group,
(9) a hydroxycarbonyl group,
(10) a C0-8 alkoxy (C0-8 alkyl) aminocarbonyl group which may be substituted by hydroxy group(s),
(11) a halogen atom,
(12) a (C1-8 alkyl)m8j-amino group which may be substituted by 4- to 10-membered heterocycloalkyl group(s),
m8j: 0˜2,
(13) a hydroxyl group,
(14) a C1-8 alkoxy group which may be substituted by one or more R8E,
R8E:
<1> a hydroxy group,
<2> a C1-8 alkoxycarbonyl group,
<3> a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8E1,
<4> a (C1-8 alkyl)m8k1-amino group which may be substituted by one or more R8E2,
m8k1: 0˜2,
<5> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8E3,
<6> a 5- to 14-membered heteroaryl group,
<7> a (C1-8 alkyl)m8k2-aminocarbonyl group which may be substituted by one or more R8E6
m8k2: 0˜2,
<8> a C1-8 alkoxy group which may be substituted by one or more R8E7,
<9> a C1-8 alkylthio group,
<10> a C1-8 alkylsulfinyl group, or
<11> a C1-8 alkylsulfonyl group,
R8E1:
<1> a C1-8 alkoxycarbonyl group,
<2> a C1-8 alkanoyl group,
<3> a C1-8 alkylsulfonyl group,
<4> a (C1-8 alkyl)m8k3-aminosulfonyl group
m8k3: 0˜2, or
<5> a 4- to 10-membered heterocycloalkyl group,
R8E2:
<1> a hydroxy group,
<2> a C1-8 alkoxycarbonyl group,
<3> a C3-8 cycloalkyl group which may be substituted by C1-8 alkyl group(s) which may be substituted by hydroxy group(s),
<4> a C1-8 alkanoyl group which may be substituted by substituent(s) selected from the group consisting of [1] a (C1-8 alkyl)m8k4-amino group and [2] a halogen atom,
m8k4: 0˜2,
<5> a (C1-8 alkyl)m8k5-aminocarbonyl group,
m8k5: 0˜2,
<6> a C1-8 alkylsulfonyl group,
<7> a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s),
<8> a (C1-8 alkyl)m8k6-aminosulfonyl group,
m8k6: 0˜2, or
R8E3:
<1> a C1-8 alkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a hydroxy group and [2] a C1-8 alkylcarbonyloxy group,
<2> a hydroxy group,
<3> a C3-8 cycloalkyl group,
<4> a C1-8 alkylsulfonyl group,
<5> a (C1-8 alkyl)m8k8-aminocarbonyl group,
m8k8: 0˜2,
<6> a C1-8 alkanoyl group which may be substituted by hydroxy group(s),
<7> an oxo group, or
<8> a 4- to 10-membered heterocycloalkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkanoyl group, and [2] a C1-8 alkylsulfonyl group,
R8E6:
<1> a C2-8 alkenylcarbonyloxy group,
<2> a hydroxy group,
<3> a cyano group,
<4> a (C1-8 alkyl)m8k9-amino group which may be substituted by hydroxy group(s),
m8k9: 0˜2,
<5> a C1-8 alkoxy group which may be substituted by hydroxy group(s),
<6> a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl group(s), or
<7> a 5- to 14-membered heteroaryl group,
R8E7:
<1> a hydroxy group, or
<2> a C1-8 alkoxy group which may be substituted by hydroxy group(s),
(15) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by one or more R8F:
R8F:
<1> a C1-8 alkyl group which may be substituted by one or more R8F1,
<2> a C3-8 cycloalkyl group,
<3> a C1-8 alkanoyl group which may be substituted by halogen atom(s),
<4> a C1-8 alkoxycarbonyl group,
<5> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8F2,
<6> a C1-8 alkyl sulfonyl group, or
<7> a hydroxy group,
R8F1: [1] a hydroxy group, [2] a C1-8 alkoxy group, or [3] a halogen atom,
R8F2: [1] a 4- to 10-membered heterocycloalkyl group, [2] a C1-8 alkoxycarbonyl group, or [3] a C1-8 alkylsulfonyl group,
(16) a 5- to 14-membered heteroaryloxy group,
(17) a (C1-8 alkyl)m8l1-aminosulfonyloxy group,
m8l1: 0˜2,
(18) a C1-8 alkylthio group which may be substituted by (C1-8 alkyl)m8l2-amino group(s),
m8l2: 0˜2,
(19) a C1-8 alkylsulfonyl group which may be substituted by one or more R8G,
R8G: [1] a hydroxycarbonyl group, [2] a hydroxy group, or [3] a (C1-8 alkyl)m813-amino group,
m8l3: 0˜2,
(20) a C2-8 alkenyloxy group, and
(21) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s);
R9 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R9A,
R9A: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9A1, [3] a hydroxy group, or [4] a C1-8 alkoxy group,
R9A1: [1] a C1-8 alkyl group, [2] a C3-8 cycloalkyl group, or [3] a 4- to 10-membered heterocycloalkyl group,
(3) a C2-8 alkenyl group,
(4) a C2-8 alkynyl group which may be substituted by one or more R9C,
R9C: [1] a C1-8 alkoxy group, [2] a (C1-8 alkyl)m9b-amino group which may be substituted by C6-10 aryl group(s), [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9C1, [4] a C3-8 cycloalkyl group, [5] a hydroxy group, or [6] a hydroxycarbonyl group,
m9b: 0˜2,
R9C1: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group, or [3] an oxo group,
(5) a C3-8 cycloalkyl group,
(6) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9D,
R9D: [1] a C1-8 alkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s), [2] a C3-8 cycloalkyl group, [3] a 4- to 10-membered heterocycloalkyl group, or [4] a C1-6 alkylsulfonyl group,
(7) a C6-10 aryl group which may be substituted by one or more R9E,
R9E: [1] a halogen atom, [2] a hydroxy group, [3] a hydroxycarbonyl group, or [4] a C1-8 alkyl group which may be substituted by hydroxy group(s),
(8) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s),
(9) a cyano group,
(10) a C1-8 alkanoyl group,
(11) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by C1-8 alkyl group(s),
(12) a halogen atom,
(13) a (C1-8 alkyl)m9c-amino group,
m9c: 0˜2,
(14) a C1-8 alkylcarbonyl(C0-8 alkyl)amino group which may be substituted by (C1-8 alkyl)m9d-amino group(s),
m9d: 0˜2,
(15) a C1-8 alkylsulfonyl(C0-8 alkyl)amino group,
(16) a (C1-8 alkyl)m9e-aminosulfonyl(C0-8 alkyl)amino group,
m9e: 0˜2,
(17) a nitro group,
(18) a hydroxy group,
(19) a C1-8 alkoxy group which may be substituted by one or more R9G,
R9G: [1] a hydroxy group, [2] a hydroxycarbonyl group, [3] a C6-10 aryl group which may be substituted by C1-8 alkoxy group(s), [4] a (C1-8 alkyl)m9g1-amino group, [5] a C1-8 alkoxy group which may be substituted by one or more R9G1, or [6] a 5- to 14-membered heteroaryl group,
m9g1: 0˜2,
R9G1: [1] a C1-8 alkoxy group, or [2] a hydroxycarbonyl group,
(20) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by 4- to 10-membered heterocycloalkyl group(s),
(21) a C1-8 alkylthio group which may be substituted by (C1-8 alkyl)m9f-amino group(s),
m9f: 0˜2,
(22) a C1-8 alkylsulfonyl group which may be substituted by (C1-8 alkyl)m9g-amino group(s),
m9g: 0˜2,
(23) a (C1-8 alkyl)m9h-aminosulfonyl group,
m9h: 0˜2, and
(24) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s);
R10 represents [1] a hydrogen atom, or [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s)].
6. A compound or salt or solvate thereof, which said compound is selected from the group consisting of:
9-(4-isopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-9-prop-1-ynyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-cyclopropylethynyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
6,6-dimethyl-8-(1-oxetan-3-yl-piperidin-4-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-bromo-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-bromo-8-(4-cyclopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-chloro-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-9-prop-1-ynyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
6,6,9-trimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-ethyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-ethynyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
8-(4-cyclobutyl-piperazin-1-yl)-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-ethynyl-6,6-dimethyl-11-oxo-8-(4-pyrrolidin-1-yl-piperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
6,6-dimethyl-11-oxo-8-(4-pyrrolidin-1-yl-piperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
8-(4-cyclobutyl-piperazin-1-yl)-9-ethynyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-9-propyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
8-(1-isopropyl-piperidin-4-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
8-(4-isopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
8-(4-cyclobutyl-piperazin-1-yl)-9-cyclopropyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
8-(2-tert-butylamino-ethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-ethynyl-8-(4-methanesulfonyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
9-bromo-8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile;
6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-9-propyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; and
9-ethynyl-6,6-dimethyl-8-morpholin-4-yl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
7. A medicament comprising as an active ingredient the compound according to claim 1 , or a salt or solvate thereof.
8. An ALK inhibitor comprising as an active ingredient the compound according to claim 1 , or a salt or solvate thereof.
9. A pharmaceutical for the prophylaxis or treatment of cancer, cancer metastasis, depression or cognitive function disorder, comprising as an active ingredient the compound according to claim 1 , or a salt or solvate thereof.
10. A pharmaceutical composition comprising the compound according to claim 1 , or a salt or solvate thereof and a pharmaceutically acceptable carrier(s).
11. A method of treating or preventing cancer, cancer metastasis, depression or cognitive function disorder in a subject in need thereof comprising administering an effective amount of a pharmaceutical for the treatment of cancer, cancer metastasis, depression or cognitive function disorder, comprising as an active ingredient a compound represented by Formula (I):
wherein,
A5 is selected from NR5, O and S;
A4, A7, and A9 represent CR4, CR7, and CR9 respectively, or wherein A4 is N and A7 and A9 represent CR7 and CR9 respectively;
R1 and R10 each independently represent [1] a hydrogen atom, [2] a cyano group, [3] a halogen atom or [4] a 4- to 10-membered heterocycloalkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s);
R2 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group,
(3) a C2-8 alkenyl group,
(4) a C2-8 alkynyl group,
(5) a cyano group,
(6) a halogen atom,
(7) a (C1-8 alkyl)m2-amino group which may be substituted by C1-8 alkylsulfonyl group(s),
m2: 0˜2, and
(8) a nitro group;
R3 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by [1] halogen atom(s), [2] hydroxy group(s) or [3] C1-8 alkoxy group(s),
(3) a C6-10 aryl group,
(4) a cyano group,
(5) a C1-8 alkanoyl group which may be substituted by C6-10 aryl group(s),
(6) a (C1-8 alkyl)m3a-aminocarbonyl group which may be substituted by one or more R3A,
R3A: [1] a C6-10 aryl group, [2] a C1-8 alkoxy group, [3] a 5- to 14-membered heteroaryl group, or [4] a C6-10 arylsulfonyl group,
m3a: 0˜2,
(7) a hydroxycarbonyl group,
(8) a C1-8 alkoxycarbonyl group which may be substituted by [1] hydroxy group(s) or [2] C1-8 alkoxy group(s),
(9) a halogen atom,
(10) a (C1-8 alkyl)m3b-amino group which may be substituted by C6-10 aryl group(s),
m3b: 0˜2,
(11) a C1-8 alkylcarbonyl (C0-8 alkyl) amino group which may be substituted by [1] C6-10 aryl group(s) or [2] C6-10 aryloxy group(s),
(12) a C6-10 arylcarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
(13) a (C1-8 alkyl)m3c-aminocarbonyl (C0-8 alkyl) amino group which may be substituted by C6-10 aryl group(s),
m3c: 0˜2,
(14) a nitro group,
(15) a hydroxy group,
(16) a C1-8 alkoxy group which may be substituted by one or more R3B,
R3B: [1] a hydroxy group, [2] a C1-8 alkoxy group, [3] a C6-10 aryl (C0-8 alkyl) aminocarbonyl group, [4] a (C1-8 alkyl)m3a-amino group, or [5] a halogen atom,
m3d: 0˜2,
(17) a 4- to 10-membered heterocycloalkyloxy group,
(18) a 5- to 14-membered heteroaryloxy group,
(19) a (C1-8 alkyl)m3e-aminocarbonyloxy group which may be substituted by C6-10 aryl group(s)
m3e: 0˜2,
(20) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group,
(21) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s),
(22) a C1-8 alkylthio group,
(23) a C1-8 alkylsulfonyl group which may be substituted by C6-10 aryl group(s),
(24) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s) which may be substituted by C1-8 alkoxy group(s),
(25) a C1-8 alkoxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkoxy group(s),
(26) a C6-10 aryloxycarbonyl (C0-8 alkyl) amino group which may be substituted by C1-8 alkyl group(s) which may be substituted by halogen atom(s),
(27) a C6-10 aryl (C0-8 alkyl) aminocarbonyl (C0-8 alkyl) amino group which may be substituted by one or more R3C,
R3C: [1] a C1-8 alkyl group which may be substituted by halogen atom(s), or [2] a C1-8 alkoxy group,
(28) a C3-8 cycloalkyl (C0-8 alkyl) aminocarbonyloxy group, and
(29) a C6-10 aryl (C0-8 alkyl) aminocarbonyloxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkyl group and [2] a C1-8 alkoxy group;
R4 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by halogen atom(s),
(3) a C2-8 alkenyl group,
(4) a C2-8 alkynyl group,
(5) a C3-8 cycloalkyl group,
(6) a cyano group,
(7) an aminocarbonyl group,
(8) a (C1-8 alkyl)m4a-aminocarbonyl group,
m4a: 1˜2,
(9) a hydroxycarbonyl group,
(10) a C1-8 alkoxycarbonyl group,
(11) a halogen atom,
(12) a (C1-8 alkyl)m4b-amino group,
m4b: 0˜2,
(13) a hydroxy group, and
(14) a C1-8 alkoxy group which may be substituted by hydroxy group(s);
R5 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R5A,
R5A: [1] a hydroxycarbonyl group, [2] a C1-8 alkoxycarbonyl group, [3] a hydroxy group, [4] a C1-8 alkoxy group, [5] a (C1-8 alkyl)m5-amino group, [6] a C6-10 aryl group, or [7] a C1-8 alkylthio group,
m5: 0˜2,
(3) a C2-8 alkenyl group,
(4) a C2-8 alkynyl group,
(5) a C3-8 cycloalkyl group, and
(6) a C1-8 alkylsulfonyl group;
R6 and R6′ are each independently selected from the group consisting of:
(1) a C1-8 alkyl group which may be substituted by halogen atom(s),
(2) a C2-8 alkenyl group, and
(3) a C2-8 alkynyl group; or
R6 and R6′ are taken together with the carbon atoms to which they are bound to form:
(4) a C3-8 cycloalkyl group, or
(5) a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl C6-10 aryl sulfonyl group(s) which may be substituted by C1-8 alkyl group(s);
R7 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a halogen atom,
(3) a C1-8 alkoxy group which may be substituted by one or more R7A,
R7A: [1] a (C1-8 alkyl)m7a-amino group, [2] a hydroxy, [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl group(s),
m7a: 0˜2,
(4) a C1-8 alkylsulfonyl group,
(5) a nitro group, and
(6) a hydroxyl group;
R8 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R7A,
R8A: [1] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8A1, [2] a (C1-8 alkyl)m8a-amino group which may be substituted by a halogen atom, or [3] a hydroxy group,
m8a:0˜2,
R8A1: [1] a C1-8 alkyl group, [2] a C1-8 alkylsulfonyl group, [3] a (C1-8 alkyl)m8b-aminosulfonyl group, [4] an oxo group, [5] a C1-8 alkoxycarbonyl, or [6] a C1-8 alkoxycarbonyl (C0-8 alkyl) aminosulfonyl,
m8b: 0˜2,
(3) a C2-8 alkenyl group,
(4) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B,
R8B:
<1> a C1-8 alkyl group which may be substituted by one or more R8B1,
<2> a C2-8 alkeynyl group,
<3> a C2-8 alkynyl group,
<4> a C3-8 cycloalkyl group which may be substituted by [1] cyano group(s) or [2] C1-8 alkyl group(s),
<5> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8B2,
<6> a C1-8 alkoxy group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkoxy group and [2] a C3-8 cycloalkyl group,
<7> a C1-8 alkoxycarbonyl group,
<8> a C1-8 alkylsulfonyl group,
<9> a 5- to 14-membered heteroarylsulfonyl group,
<10> an oxo group,
<11> a cyano group,
<12> a C1-8 alkanoyl group which may be substituted by one or more R8B3,
<13> a C3-8 cycloalkylcarbonyl group,
<14> a (C1-8 alkyl)m8c-aminosulfonyl group,
<15> a C1-8 alkylsulfonyl (C0-8 alkyl) amino group,
<16> a (C1-8 alkyl)m8d-amino group which may be substituted by one or more R8B4,
<17> a hydroxy group,
<18> a (C1-8 alkyl)m8e-aminocarbonyl group, or
<19> a C1-8 alkoxycarbonyl (C0-8 alkyl) amino group
m8c: 0˜2
m8d: 0˜2
m8e: 0˜2
R8B1: [1] a C3-8 cycloalkyl group, [2] a hydroxy group, or [3] a C1-8 alkoxy group(s),
R8B2: [1] a halogen atom, [2] a C1-8 alkyl group, [3] an oxo group, [4] a hydroxy group, or [5] a deuterium atom,
R8B3: a (C1-8 alkyl)m8f-amino group,
m8f: 0˜2,
R8B4: [1] a C3-8 cycloalkyl group, or [2] a hydroxy group,
(5) a 5- to 14-membered heteroaryl group which may be substituted by a C1-8 alkyl group,
(6) a (C1-8 alkyl)m8g-aminocarbonyl group which may be substituted by one or more R8C,
m8g:0-2,
R8C:[1] a hydroxy group, [2] a (C1-8 alkyl)m8h-amino group which may be substituted by substituent(s) selected from the group consisting of <1> a (C1-8 alkyl)m8i-aminosulfonyl group, <2> a C1-8 alkylsulfonyl group, <3> a C1-8 alkoxycarbonyl group and <4> a C1-8 alkoxycarbonyl(C0-8 alkyl) aminosulfonyl group, [3] a C1-8 alkylsulfonyl group, or [4] a C1-8 alkoxy group which may be substituted by a hydroxy group,
m8h: 0˜2,
m8i: 0˜2,
(7) a 4- to 10-membered heterocycloalkyl (C0-8 alkyl) aminocarbonyl group which may be substituted by oxo group(s),
(8) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8D,
R8D: [1] a C1-8 alkyl group which may be substituted by one or more R8D1, [2] a hydroxy group, [3] a C1-8 alkylsulfonyl group, or [4] a C1-8 alkoxycarbonyl group,
R8D1: [1] a hydroxy group, or [2] a C1-8 alkoxy group,
(9) a hydroxycarbonyl group,
(10) a C0-8 alkoxy (C0-8 alkyl) aminocarbonyl group which may be substituted by hydroxy group(s),
(11) a halogen atom,
(12) a (C1-8 alkyl)m8j-amino group which may be substituted by one or more R8H,
m8j: 0˜2,
R8H: [1] a hydroxy group, or [2] a 4- to 10-membered heterocycloalkyl group,
(13) a hydroxyl group,
(14) a C1-8 alkoxy group which may be substituted by one or more R8E,
R8E:
<1> a hydroxy group,
<2> halogen atom,
<3> a hydroxycarbonyl group,
<4> a C1-8 alkoxycarbonyl group,
<5> a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by one or more R8E1,
<6> a (C1-8 alkyl)m8k1-amino group which may be substituted by one or more R8E2,
m8k1: 0˜2,
<7> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8E3,
<8> a 5- to 14-membered heteroaryl group,
<9> a (C1-8 alkyl)m8k2-aminocarbonyl group which may be substituted by one or more R8E6,
m8k2: 0˜2,
<10> a C1-8 alkoxy group which may be substituted by one or more R8E7,
<11> a C1-8 alkylthio group,
<12> a C1-8 alkylsulfinyl group,
<13> a C1-8 alkylsulfonyl group,
R8E1:
<1> a C1-8 alkoxycarbonyl group,
<2> a C1-8 alkanoyl group,
<3> a C1-8 alkylsulfonyl group, 25<4> a (C1-8 alkyl)m8k3-aminosulfonyl group,
m8k3: 0˜2, or
<5> a 4- to 10-membered heterocycloalkyl group,
R8E2:
<1> a hydroxy group,
<2> a C1-8 alkoxycarbonyl group which may be substituted by halogen atom(s),
<3> a C3-8 cycloalkyl group which may be substituted by C1-8 alkyl group(s) which may be substituted by hydroxy group(s),
<4> a C1-8 alkanoyl group which may be substituted by substituent(s) selected from the group consisting of [1] a (C1-8 alkyl)mk4-amino group and [2] a halogen atom(s),
m8k4: 0˜2,
<5> a (C1-8 alkyl)m8k5-aminocarbonyl group,
m8k5: 0˜2,
<6> a C1-8 alkylsulfonyl group,
<7> a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s),
<8> a (C1-8 alkyl)m8k6-aminosulfonyl group which may be substituted by C1-8 alkoxycarbonyl group(s),
m8k6: 0˜2, or
R8E3:
<1> a C1-8 alkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a hydroxy group and [2] a C1-8 alkylcarbonyloxy group,
<2> a C1-8 alkylcarbonyloxy group,
<3> a hydroxy group,
<4> a C3-8 cycloalkyl group,
<5> a C1-8 alkoxy group,
<6> a C1-8 alkoxycarbonyl group,
<7> a C1-8 alkylsulfonyl group,
<8> a (C1-8 alkyl)m8k8-aminocarbonyl group,
m8k8: 0˜2,
<9> a C1-8 alkanoyl group which may be substituted by hydroxy group(s),
<10> an oxo group, or
<11> a 4- to 10-membered heterocycloalkyl group which may be substituted by substituent(s) selected from the group consisting of [1] a C1-8 alkanoyl group, [2] a C1-8 alkoxycarbonyl group and [3] a C1-8 alkylsulfonyl group,
R8E6:
<1> a C2-8 alkenylcarbonyloxy group,
<2> a hydroxy group,
<3> a cyano group,
<4> a (C1-8 alkyl)m8k9-amino group which may be substituted by hydroxy group(s)
m8k9: 0˜2,
<5> a C1-8 alkoxy group which may be substituted by hydroxy group(s),
<6> a C1-8 alkylcarbonyloxy group,
<7> a 4- to 10-membered heterocycloalkyl group which may be substituted by C1-8 alkyl group(s), or
<8> a 5- to 14-membered heteroaryl group,
R8E7:
<1> a hydroxy group, or
<2> a C1-8 alkoxy group which may be substituted by hydroxy group(s),
(15) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by one or more R8F,
R8F:
<1> a C1-8 alkyl group which may be substituted by one or more R8F1,
<2> a C3-8 cycloalkyl group,
<3> a C1-8 alkanoyl group which may be substituted by halogen atom(s),
<4> a C1-8 alkylcarbonyloxy group,
<5> a C1-8 alkoxycarbonyl group,
<6> a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R8F2,
<7> a C1-8 alkyl sulfonyl group,
<8> a hydroxy group, or
[9] a C6-10 aryl group,
R8F1: [1] a hydroxy group, [2] a C1-8 alkoxy group, or [3] a halogen atom,
R8F2: [1] a 4- to 10-membered heterocycloalkyl group, [2] a C1-8 alkoxycarbonyl group, or [3] a C1-8 alkylsulfonyl group,
(16) a 5- to 14-membered heteroaryloxy group,
(17) a 4- to 10-membered heterocycloalkylcarbonyloxy group,
(18) a (C1-8 alkyl)m8l1-aminosulfonyloxy group,
m8l1: 0˜2,
(19) a C1-8 alkyl thio group which may be substituted by [1] (C1-8 alkyl) m8l2-amino group(s), [2] hydroxy group(s) or [3] hydroxycarbonyl group(s),
m8l2: 0˜2,
(20) a C1-8 alkylsulfonyl group which may be substituted by one or more R8G,
R8G: [1] a hydroxycarbonyl group, [2] a hydroxy group, or [3] a (C1-8 alkyl)m13-amino group,
m8l3: 0˜2,
(21) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyloxy group which may be substituted by C1-8 alkyl group(s),
(22) a C2-8 alkenyloxy group, and
(23) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s);
R9 is selected from the group consisting of:
(1) a hydrogen atom,
(2) a C1-8 alkyl group which may be substituted by one or more R9A,
R9A: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9A, [3] a hydroxy group, [4] a C1-8 alkoxy group, or [5] a hydroxycarbonyl group,
R9A1: [1] a C1-8 alkyl group, [2] a C3-8 cycloalkyl group, or [3] a 4- to 10-membered heterocycloalkyl group,
(3) a C2-8 alkenyl group which may be substituted by one or more R9B,
R9B: [1] a (C1-8 alkyl)m9a-amino group, [2] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more group R9B1,
R9B1:[1] a C3-8 cycloalkyl group, or [2] a 4- to 10-membered heterocycloalkyl group,
m9a: 0˜2,
(4) a C2-8 alkynyl group which may be substituted by one or more R9C,
R9C: [1] a C1-8 alkoxy group, [2] a (C1-8 alkyl)m9b-amino group which may be substituted by C6-10 aryl group(s), [3] a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9C1, [4] a C3-8 cycloalkyl group, [5] a hydroxy group, [6] a hydroxycarbonyl group, or [7] a C1-8 alkyloxycarbonyl group,
m9b: 0˜2,
R9C1: [1] a C3-8 cycloalkyl group, [2] a 4- to 10-membered heterocycloalkyl group, or [3] an oxo group,
(5) a C3-8 cycloalkyl group,
(6) a 4- to 10-membered heterocycloalkyl group which may be substituted by one or more R9D,
R9D: [1] a C1-8 alkyl group which may be substituted by 4- to 10-membered heterocycloalkyl group(s), [2] a C3-8 cycloalkyl group, [3] a 4- to 10-membered heterocycloalkyl group, or [4] a C1-6 alkylsulfonyl group, or [5] a C1-8 alkoxycarbonyl group,
(7) a C6-10 aryl group which may be substituted by one or more R9E,
R9E: [1] a halogen atom, [2] a hydroxy group, [3] a hydroxycarbonyl group, or [4] a C1-8 alkyl group which may be substituted by hydroxy group(s), or [5] a C1-8 alkoxy group,
(8) a 5- to 14-membered heteroaryl group which may be substituted by C1-8 alkyl group(s),
(9) a cyano group,
(10) a C1-8 alkanoyl group,
(11) a 4- to 10-membered nitrogen-containing heterocycloalkylcarbonyl group which may be substituted by C1-8 alkyl group(s),
(12) a halogen atom,
(13) a (C1-8 alkyl)m9c-amino group which may be substituted by one or more R9F,
m9c: 0˜2,
R9F: [9F-1] a C1-3 alkylsulfonyl group, [9F-2] a (C1-3 alkyl)m9f1-aminosulfonyl group (m9f1: 0˜2), or [9F-3] a C1-3 alkanoyl group which may be substituted by (C1-3 alkyl)m9f2-amino group(s) (m9f2: 0˜2),
(14) a C1-8 alkylcarbonyl(C0-8 alkyl)amino group which may be substituted by (C1-8 alkyl)m9d-amino group(s),
m9d: 0˜2,
(15) a C1-8 alkylsulfonyl(C0-8 alkyl)amino group,
(16) a (C1-8 alkyl)m9e-aminosulfonyl(C0-8 alkyl)amino group,
m9e: 0˜2,
(17) a nitro group,
(18) a hydroxy group,
(19) a C1-8 alkoxy group which may be substituted by one or more R9G,
R9G: [1] a hydroxy group, [2] a hydroxycarbonyl group, [3] a C6-10 aryl group which may be substituted by C1-8 alkoxy group(s), [4] a (C1-8 alkyl)m9g1-amino group, [5] a C1-8 alkoxy group which may be substituted by one or more R9G1, [6] a 5- to 14-membered heteroaryl group, or [7] a 4- to 10-membered heterocycloalkyloxy group which may be substituted by C1-8 alkyl group(s),
m9g1: 0˜2,
R9G1: [1] a C1-8 alkoxy group, or [2] a hydroxycarbonyl group,
(20) a 4- to 10-membered heterocycloalkyloxy group which may be substituted by [1] 4- to 10-membered heterocycloalkyl group(s), or [2] C1-8 alkoxycarbonyl group(s),
(21) a C1-8 alkylsulfonyloxy group which may be substituted by halogen atom(s),
(22) a C1-8 alkylthio group which may be substituted by (C1-8 alkyl)m9f-amino group(s),
m9f: 0˜2,
(23) a C1-8 alkylsulfonyl group which may be substituted by (C1-8 alkyl)m9g-amino group(s),
m9g: 0˜2,
(24) a (C1-8 alkyl)m9h-aminosulfonyl group,
m9h: 0˜2,
(25) a 4- to 10-membered nitrogen-containing heterocycloalkylsulfonyl group which may be substituted by C1-8 alkyl group(s), and
(26) a hydroxycarbonyl group,
or a salt or solvate thereof.
12. The method of claim 11 , wherein the method comprises treating cancer or cancer metastasis in the subject in need thereof.
13. The method of claim 12 , wherein the cancer is selected from the group consisting of lung cancer, anaplastic large cell lymphoma, inflammatory myofibroblastic tumor, esophageal cancer, and neuroblastoma.
14. The method of claim 11 , wherein the method comprises treating depression or cognitive function disorder in the subject in need thereof.
15. The method of claim 11 , wherein the pharmaceutical comprises as an active ingredient a compound selected from the group consisting of 9-(4-isopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-9-prop-1-ynyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-cyclopropylethynyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 6,6-dimethyl-8-(1-oxetan-3-yl-piperidin-4-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-bromo-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-bromo-8-(4-cyclopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-chloro-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-9-prop-1-ynyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 6,6,9-trimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-ethyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-ethynyl-6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 8-(4-cyclobutyl-piperazin-1-yl)-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-ethynyl-6,6-dimethyl-11-oxo-8-(4-pyrrolidin-1-yl-piperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 6,6-dimethyl-11-oxo-8-(4-pyrrolidin-1-yl-piperidin-1-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 8-(4-cyclobutyl-piperazin-1-yl)-9-ethynyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-9-propyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 8-(1-isopropyl-piperidin-4-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 8-(4-isopropyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 8-(4-cyclobutyl-piperazin-1-yl)-9-cyclopropyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 8-(2-tert-butylamino-ethoxy)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-ethynyl-8-(4-methanesulfonyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 9-bromo-8-(4-cyclobutyl-piperazin-1-yl)-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; 6,6-dimethyl-8-(4-oxetan-3-yl-piperazin-1-yl)-11-oxo-9-propyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile; and 9-ethynyl-6,6-dimethyl-8-morpholin-4-yl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile, or a salt or solvate thereof.
16. The method of claim 11 , wherein the pharmaceutical comprises as an active ingredient 9-ethynyl-6,6-dimethyl-8-morpholin-4-yl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile, or a salt or solvate thereof.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/862,125 US20220306578A1 (en) | 2009-06-10 | 2020-04-29 | Tetracyclic compound |
| US17/860,589 US20230142119A1 (en) | 2009-06-10 | 2022-07-08 | Tetracyclic compound |
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009-139691 | 2009-06-10 | ||
| JP2009139691 | 2009-06-10 | ||
| PCT/JP2010/059785 WO2010143664A1 (en) | 2009-06-10 | 2010-06-09 | Tetracyclic compound |
| US201113377300A | 2011-12-09 | 2011-12-09 | |
| US14/619,242 US9440922B2 (en) | 2009-06-10 | 2015-02-11 | Tetracyclic compound |
| US15/221,926 US20160340308A1 (en) | 2009-06-10 | 2016-07-28 | Tetracyclic compound |
| US16/239,839 US20200017442A1 (en) | 2009-06-10 | 2019-01-04 | Tetracyclic compound |
| US16/862,125 US20220306578A1 (en) | 2009-06-10 | 2020-04-29 | Tetracyclic compound |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/239,839 Continuation US20200017442A1 (en) | 2009-06-10 | 2019-01-04 | Tetracyclic compound |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/860,589 Continuation US20230142119A1 (en) | 2009-06-10 | 2022-07-08 | Tetracyclic compound |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20220306578A1 true US20220306578A1 (en) | 2022-09-29 |
Family
ID=43308919
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/377,300 Active 2031-04-21 US9126931B2 (en) | 2009-06-10 | 2010-06-09 | Tetracyclic compound |
| US14/619,242 Active US9440922B2 (en) | 2009-06-10 | 2015-02-11 | Tetracyclic compound |
| US15/221,926 Abandoned US20160340308A1 (en) | 2009-06-10 | 2016-07-28 | Tetracyclic compound |
| US16/239,839 Abandoned US20200017442A1 (en) | 2009-06-10 | 2019-01-04 | Tetracyclic compound |
| US16/862,125 Abandoned US20220306578A1 (en) | 2009-06-10 | 2020-04-29 | Tetracyclic compound |
| US17/860,589 Pending US20230142119A1 (en) | 2009-06-10 | 2022-07-08 | Tetracyclic compound |
Family Applications Before (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/377,300 Active 2031-04-21 US9126931B2 (en) | 2009-06-10 | 2010-06-09 | Tetracyclic compound |
| US14/619,242 Active US9440922B2 (en) | 2009-06-10 | 2015-02-11 | Tetracyclic compound |
| US15/221,926 Abandoned US20160340308A1 (en) | 2009-06-10 | 2016-07-28 | Tetracyclic compound |
| US16/239,839 Abandoned US20200017442A1 (en) | 2009-06-10 | 2019-01-04 | Tetracyclic compound |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/860,589 Pending US20230142119A1 (en) | 2009-06-10 | 2022-07-08 | Tetracyclic compound |
Country Status (40)
| Country | Link |
|---|---|
| US (6) | US9126931B2 (en) |
| EP (4) | EP2441753B1 (en) |
| JP (2) | JP4588121B1 (en) |
| KR (1) | KR101351120B1 (en) |
| CN (1) | CN102459172B (en) |
| AR (1) | AR077025A1 (en) |
| AU (1) | AU2010259588B2 (en) |
| BR (1) | BRPI1011649B1 (en) |
| CA (1) | CA2764653C (en) |
| CL (1) | CL2011002433A1 (en) |
| CO (1) | CO6430460A2 (en) |
| CR (1) | CR20110492A (en) |
| CY (2) | CY1117752T1 (en) |
| DK (3) | DK2975024T3 (en) |
| EC (1) | ECSP12011573A (en) |
| ES (3) | ES2759510T3 (en) |
| FR (1) | FR17C1019I2 (en) |
| HK (1) | HK1165794A1 (en) |
| HR (3) | HRP20160605T1 (en) |
| HU (4) | HUE046402T2 (en) |
| IL (1) | IL216817A (en) |
| LT (3) | LT2975024T (en) |
| LU (1) | LUC00022I2 (en) |
| MA (1) | MA33418B1 (en) |
| MX (1) | MX2011013306A (en) |
| MY (1) | MY159850A (en) |
| NL (1) | NL300876I2 (en) |
| NO (2) | NO2975024T3 (en) |
| NZ (1) | NZ597477A (en) |
| PE (1) | PE20121065A1 (en) |
| PL (3) | PL2441753T3 (en) |
| PT (3) | PT3345903T (en) |
| RS (1) | RS58855B1 (en) |
| RU (2) | RU2725140C2 (en) |
| SG (1) | SG175707A1 (en) |
| SI (3) | SI3345903T1 (en) |
| TW (2) | TWI624457B (en) |
| UA (1) | UA107796C2 (en) |
| WO (1) | WO2010143664A1 (en) |
| ZA (1) | ZA201106938B (en) |
Families Citing this family (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2441753B1 (en) * | 2009-06-10 | 2016-03-30 | Chugai Seiyaku Kabushiki Kaisha | Tetracyclic compound |
| SG187614A1 (en) * | 2010-08-20 | 2013-03-28 | Chugai Pharmaceutical Co Ltd | Composition containing tetracyclic compound |
| JP5006987B2 (en) * | 2010-11-22 | 2012-08-22 | 中外製薬株式会社 | Medicine containing tetracyclic compound |
| WO2013151584A1 (en) * | 2011-10-31 | 2013-10-10 | The Methodist Hospital Research Institute | Compound comprising a mao targeting/ seeker moiety for treating human gliomas |
| EA025859B1 (en) | 2012-03-06 | 2017-02-28 | Сефалон, Инк. | Fused bicyclic 2,4-diaminopyrimidine derivative as a dual alk and fak inhibitor |
| US10668075B2 (en) * | 2012-09-25 | 2020-06-02 | Chugai Seiyaku Kabushiki Kaisha | RET inhibitor |
| MX2015008187A (en) | 2012-12-20 | 2016-02-05 | Concert Pharmaceuticals Inc | Deuterated alk inhibitors. |
| WO2014121654A1 (en) * | 2013-02-05 | 2014-08-14 | 山东轩竹医药科技有限公司 | Tetrazocyclokinase inhibitor |
| EP2981526B1 (en) | 2013-04-02 | 2021-11-03 | Oxular Acquisitions Limited | Intermediates useful in the preparation of naphthylurea derivatives useful as kinase inhibitors |
| CN104109168B (en) * | 2013-04-20 | 2017-02-15 | 山东轩竹医药科技有限公司 | Tetra-cyclo-kinase inhibitor |
| CN104130241A (en) * | 2013-05-05 | 2014-11-05 | 山东轩竹医药科技有限公司 | Tetrocycle kinase inhibitor |
| CN104177332A (en) * | 2013-05-20 | 2014-12-03 | 中国科学院上海药物研究所 | Amido substituted indolonaphthalenone derivatives and medicinal uses thereof |
| CN104177342B (en) * | 2013-05-21 | 2018-01-05 | 中国科学院上海药物研究所 | The indoles and naphthalene ketone derivant and its medical usage of heterocyclic radical substitution |
| CN105579459B (en) * | 2013-06-01 | 2018-03-13 | 山东轩竹医药科技有限公司 | Four and ring class anaplastic lymphoma kinase inhibitor |
| CN104230960B (en) * | 2013-06-06 | 2017-02-15 | 山东轩竹医药科技有限公司 | Four-ring anaplastic lymphoma kinase inhibitor |
| WO2014203152A1 (en) | 2013-06-18 | 2014-12-24 | Novartis Ag | Pharmaceutical combinations |
| CN104804016B (en) * | 2014-01-23 | 2017-06-20 | 山东轩竹医药科技有限公司 | Four and ring class anaplastic lymphoma kinase inhibitor |
| BR112016021206B1 (en) | 2014-04-25 | 2022-07-12 | Chugai Seiyaku Kabushiki Kaisha | PHARMACEUTICAL COMPOSITION CONTAINING HIGH DOSE TETRACYCLIC COMPOUND AND ITS PRODUCTION METHOD |
| EP3135671B1 (en) * | 2014-04-25 | 2019-09-18 | Chugai Seiyaku Kabushiki Kaisha | Novel crystal of tetracyclic compound |
| RU2720680C2 (en) * | 2014-06-18 | 2020-05-12 | Ф. Хоффманн-Ля Рош Аг | New pharmaceutical composition containing nonionic surfactants |
| TWI831347B (en) * | 2014-08-08 | 2024-02-01 | 日商中外製藥股份有限公司 | Solid dispersion containing amorphous body of tetracyclic compound and preparation |
| ES2796349T3 (en) | 2014-09-29 | 2020-11-26 | Hainan Xuanzhu Pharma Co Ltd | Polycyclic anaplastic lymphoma kinase inhibitor |
| CN105566307B (en) * | 2014-10-11 | 2019-04-23 | 中国科学院上海药物研究所 | The indoles and naphthalene ketone derivant, preparation method, medical composition and its use that heterocycle replaces |
| CN104402862B (en) * | 2014-11-12 | 2016-10-05 | 苏州明锐医药科技有限公司 | Ai Li is for the preparation method of Buddhist nun |
| US11077093B2 (en) | 2015-01-16 | 2021-08-03 | Chugai Seiyaku Kabushiki Kaisha | Combination drug |
| US9573932B2 (en) * | 2015-03-02 | 2017-02-21 | Yong Xu | Synthesis of intermediates in the preparation of ALK inhibitor |
| WO2016145288A1 (en) * | 2015-03-11 | 2016-09-15 | International Flavors & Fragrances Inc. | Processes for the preparation of unsaturated malonates |
| US10259816B2 (en) | 2015-04-24 | 2019-04-16 | Guangzhou Maxinovel Pharmaceuticals Co., Ltd. | Condensed-ring pyrimidylamino derivative, preparation method therefor, and intermediate, pharmaceutical composition and applications thereof |
| US10508082B2 (en) | 2015-09-23 | 2019-12-17 | Dana-Farber Cancer Institute, Inc. | Substituted 6,11-dihydro-5H-benzo[b]carbazoles as inhibitors of ALK and SRPK |
| CN105777710B (en) * | 2016-04-05 | 2018-09-04 | 湖南欧亚药业有限公司 | A kind of Ai Le replaces the synthetic method of Buddhist nun |
| CN106946650B (en) * | 2017-03-01 | 2021-06-08 | 南京远淑医药科技有限公司 | Synthetic method of intermediate of erlotinib hydrochloride |
| CN106928184B (en) * | 2017-04-21 | 2019-09-20 | 湖南博奥德药业有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun |
| CN107033124B (en) * | 2017-04-21 | 2019-09-20 | 湖南博奥德药业有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun |
| CN106928125A (en) * | 2017-04-21 | 2017-07-07 | 湖南博奥德生物医药技术开发有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun's intermediate |
| CN106995433A (en) * | 2017-04-21 | 2017-08-01 | 湖南博奥德生物医药技术开发有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun |
| CN106892860B (en) * | 2017-04-21 | 2019-08-02 | 湖南博奥德药业有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun's intermediate |
| CN107129488A (en) * | 2017-04-21 | 2017-09-05 | 湖南博奥德生物医药技术开发有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun |
| CN106928185B (en) * | 2017-04-21 | 2019-09-20 | 湖南博奥德药业有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun |
| CN107033125B (en) * | 2017-04-21 | 2019-09-20 | 湖南博奥德药业有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun |
| WO2018202487A1 (en) | 2017-05-04 | 2018-11-08 | Basf Se | Substituted 5-(haloalkyl)-5-hydroxy-isoxazoles for combating phytopathogenic fungi |
| EP3649116A1 (en) | 2017-07-05 | 2020-05-13 | Fresenius Kabi Oncology Limited | A process for preparing alectinib or a pharmaceutically acceptable salt thereof |
| JP7054134B2 (en) * | 2017-12-07 | 2022-04-13 | 国立大学法人京都大学 | Benzo [b] carbazole compound and imaging using it |
| JP7565078B2 (en) * | 2017-12-13 | 2024-10-10 | 上海科技大学 | ALC protein degraders and their use in cancer therapy |
| CN108178743A (en) * | 2018-02-08 | 2018-06-19 | 安庆奇创药业有限公司 | A kind of Ai Le replaces the preparation method of Buddhist nun's key intermediate |
| KR102548191B1 (en) * | 2018-04-09 | 2023-06-28 | 상하이테크 유니버시티 | Targeted proteolytic compounds, their antitumor applications, their intermediates and applications of intermediates |
| WO2019211868A1 (en) * | 2018-04-30 | 2019-11-07 | Msn Laboratories Private Limited, R&D Center | Improved process for the preparation of 9-ethyl-6,6-dimethyl-8-[4-(morpholin-4-yl) piperidin-1-yl]-11-oxo-6,11-dihydro-5h-benzo[b]carbazole-3-carbonitrile hydrochloride |
| CA3104995A1 (en) | 2018-06-29 | 2020-01-02 | Chugai Seiyaku Kabushiki Kaisha | Pharmaceutical composition comprising poorly soluble basic agent |
| TWI825163B (en) * | 2018-09-04 | 2023-12-11 | 日商中外製藥股份有限公司 | Method for producing tetracyclic compound |
| US20220409731A1 (en) * | 2018-09-27 | 2022-12-29 | Dana-Farber Cancer Institute, Inc. | Degraders that target alk and therapeutic uses thereof |
| CN109438218B (en) * | 2018-10-23 | 2021-04-09 | 成都艾必克医药科技有限公司 | Synthesis method of hydrochloric acid neritinib intermediate 2- (4-ethyl-3-iodophenyl) -2-methylpropanoic acid |
| CN109384664B (en) * | 2018-11-20 | 2021-04-13 | 成都正善达生物医药科技有限公司 | Preparation method of erlotinib intermediate |
| EP3556754A1 (en) | 2018-12-07 | 2019-10-23 | Fresenius Kabi iPSUM S.r.l. | Process for the preparation of alectinib |
| EP3903828A4 (en) | 2018-12-21 | 2022-10-05 | Daiichi Sankyo Company, Limited | Combination of antibody-drug conjugate and kinase inhibitor |
| CN111349012B (en) * | 2018-12-21 | 2023-01-10 | 上海复星星泰医药科技有限公司 | Preparation method of halogenated aromatic compound and intermediate thereof |
| KR20210142154A (en) | 2019-03-21 | 2021-11-24 | 옹쎄오 | DBAIT molecules in combination with kinase inhibitors for the treatment of cancer |
| CN110590739A (en) * | 2019-09-20 | 2019-12-20 | 中国药科大学 | Preparation method of erlotinib |
| AU2020378630A1 (en) | 2019-11-08 | 2022-05-26 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for the treatment of cancers that have acquired resistance to kinase inhibitors |
| WO2021148581A1 (en) | 2020-01-22 | 2021-07-29 | Onxeo | Novel dbait molecule and its use |
| AU2022215105A1 (en) | 2021-01-29 | 2023-07-20 | Chugai Seiyaku Kabushiki Kaisha | Drug composition for treating pediatric cancers |
| EP4320153A1 (en) | 2021-04-09 | 2024-02-14 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for the treatment of anaplastic large cell lymphoma |
| CN115340523B (en) * | 2021-05-12 | 2023-12-15 | 盛世泰科生物医药技术(苏州)股份有限公司 | Compound with ALK inhibitory activity and preparation method and application thereof |
| JPWO2023074785A1 (en) | 2021-10-28 | 2023-05-04 | ||
| WO2023161233A1 (en) * | 2022-02-22 | 2023-08-31 | Synthon B.V. | Solid forms of alectinib and alectinib salts |
| CN115677659B (en) * | 2022-10-11 | 2024-03-22 | 枣庄市润安制药新材料有限公司 | Preparation method of aletinib |
Family Cites Families (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0892090A (en) * | 1994-07-26 | 1996-04-09 | Tanabe Seiyaku Co Ltd | Medicinal composition |
| DE69505470T2 (en) | 1994-08-04 | 1999-05-12 | F. Hoffmann-La Roche Ag, Basel | Pyrrolocarbazole |
| JPH08291285A (en) * | 1995-04-21 | 1996-11-05 | Fuji Photo Film Co Ltd | Photosensitive composition and element using the composition |
| YU48398A (en) | 1996-05-01 | 2000-12-28 | Eli Lily And Company | Halo-supstituted protein kinase c inhibitors |
| JP2002544275A (en) * | 1999-05-14 | 2002-12-24 | ジ・オーストラリアン・ナショナル・ユニバーシティー | Compounds and methods of treatment |
| CN100398519C (en) * | 2003-02-28 | 2008-07-02 | 伊诺泰克制药公司 | Tetracyclic benzamide derivatives and methods of use thereof |
| EP1454992B1 (en) * | 2003-03-07 | 2006-05-31 | Istituto Nazionale Per Lo Studio E La Cura Dei Tumori | Anaplastic lymphoma kinase assay, reagents and compositions thereof |
| GB0305929D0 (en) | 2003-03-14 | 2003-04-23 | Novartis Ag | Organic compounds |
| AU2004259012C1 (en) | 2003-07-23 | 2012-08-02 | Exelixis, Inc. | Anaplastic lymphoma kinase modulators and methods of use |
| US7378414B2 (en) * | 2003-08-25 | 2008-05-27 | Abbott Laboratories | Anti-infective agents |
| BRPI0416801A (en) * | 2003-11-21 | 2007-01-09 | Novartis Ag | 1h-imidazoquinoline derivatives as protein synase inhibitors |
| US7687524B2 (en) | 2003-12-12 | 2010-03-30 | Merck Frosst Canada & Co. | Cathepsin cysteine protease inhibitors |
| CA2560195A1 (en) | 2004-03-19 | 2005-09-29 | Speedel Experimenta Ag | Organic compounds |
| WO2005097765A1 (en) | 2004-03-31 | 2005-10-20 | Exelixis, Inc. | Anaplastic lymphoma kinase modulators and methods of use |
| ME01788B (en) | 2004-08-26 | 2011-02-28 | Pfizer | Enantiomerically pure aminoheteroaryl compounds as protein kinase inhibitors |
| US7091202B2 (en) * | 2004-09-15 | 2006-08-15 | Bristol-Myers Squibb Company | 4-arylspirocycloalkyl-2-aminopyrimidine carboxamide KCNQ potassium channel modulators |
| GB0517329D0 (en) | 2005-08-25 | 2005-10-05 | Merck Sharp & Dohme | Stimulation of neurogenesis |
| US20080292608A1 (en) * | 2005-11-07 | 2008-11-27 | Irm Llc | Compounds and Compositions as Ppar Modulators |
| US7601716B2 (en) | 2006-05-01 | 2009-10-13 | Cephalon, Inc. | Pyridopyrazines and derivatives thereof as ALK and c-Met inhibitors |
| US8063225B2 (en) | 2006-08-14 | 2011-11-22 | Chembridge Corporation | Tricyclic compound derivatives useful in the treatment of neoplastic diseases, inflammatory disorders and immunomodulatory disorders |
| EP2222647B1 (en) * | 2006-10-23 | 2015-08-05 | Cephalon, Inc. | Fused bicyclic derivatives of 2,4-diaminopyrimidine as alk and c-met inhibitors |
| US8546394B2 (en) | 2007-04-17 | 2013-10-01 | Bristol-Myers Squibb Company | Substituted [1,2,4]triazolo[4,3-A]pyrazine 11-beta-hydroxysteroid dehydrogenase inhibitors |
| TWI389893B (en) | 2007-07-06 | 2013-03-21 | Astellas Pharma Inc | Di (arylamino) ary1 compound |
| PT2176231T (en) | 2007-07-20 | 2016-12-09 | Nerviano Medical Sciences Srl | Substituted indazole derivatives active as kinase inhibitors |
| CA2707308C (en) * | 2007-11-30 | 2016-08-02 | Newlink Genetics Corporation | Ido inhibitors |
| CN102574830A (en) | 2009-05-07 | 2012-07-11 | 阿斯利康(瑞典)有限公司 | Substituted 1-cyanoethylheterocyclylcarboxamide compounds 750 |
| GB0910046D0 (en) | 2009-06-10 | 2009-07-22 | Glaxosmithkline Biolog Sa | Novel compositions |
| EP2441753B1 (en) * | 2009-06-10 | 2016-03-30 | Chugai Seiyaku Kabushiki Kaisha | Tetracyclic compound |
| US8609097B2 (en) | 2009-06-10 | 2013-12-17 | Hoffmann-La Roche Inc. | Use of an anti-Tau pS422 antibody for the treatment of brain diseases |
| SG187614A1 (en) * | 2010-08-20 | 2013-03-28 | Chugai Pharmaceutical Co Ltd | Composition containing tetracyclic compound |
| US10668075B2 (en) * | 2012-09-25 | 2020-06-02 | Chugai Seiyaku Kabushiki Kaisha | RET inhibitor |
| EP3135671B1 (en) * | 2014-04-25 | 2019-09-18 | Chugai Seiyaku Kabushiki Kaisha | Novel crystal of tetracyclic compound |
| BR112016021206B1 (en) * | 2014-04-25 | 2022-07-12 | Chugai Seiyaku Kabushiki Kaisha | PHARMACEUTICAL COMPOSITION CONTAINING HIGH DOSE TETRACYCLIC COMPOUND AND ITS PRODUCTION METHOD |
| RU2720680C2 (en) * | 2014-06-18 | 2020-05-12 | Ф. Хоффманн-Ля Рош Аг | New pharmaceutical composition containing nonionic surfactants |
| TWI831347B (en) * | 2014-08-08 | 2024-02-01 | 日商中外製藥股份有限公司 | Solid dispersion containing amorphous body of tetracyclic compound and preparation |
-
2010
- 2010-06-09 EP EP10786195.7A patent/EP2441753B1/en active Active
- 2010-06-09 SI SI201031961T patent/SI3345903T1/en unknown
- 2010-06-09 HU HUE18158255A patent/HUE046402T2/en unknown
- 2010-06-09 MA MA34519A patent/MA33418B1/en unknown
- 2010-06-09 AR ARP100102015A patent/AR077025A1/en active IP Right Grant
- 2010-06-09 HU HUE15180863A patent/HUE039167T2/en unknown
- 2010-06-09 DK DK15180863.1T patent/DK2975024T3/en active
- 2010-06-09 DK DK18158255T patent/DK3345903T3/en active
- 2010-06-09 KR KR1020117031143A patent/KR101351120B1/en active Protection Beyond IP Right Term
- 2010-06-09 JP JP2010526082A patent/JP4588121B1/en active Active
- 2010-06-09 SI SI201031676T patent/SI2975024T1/en unknown
- 2010-06-09 RU RU2015156881A patent/RU2725140C2/en active
- 2010-06-09 LT LTEP15180863.1T patent/LT2975024T/en unknown
- 2010-06-09 NZ NZ597477A patent/NZ597477A/en unknown
- 2010-06-09 BR BRPI1011649-4A patent/BRPI1011649B1/en active IP Right Grant
- 2010-06-09 TW TW105101212A patent/TWI624457B/en active
- 2010-06-09 EP EP15180863.1A patent/EP2975024B1/en active Active
- 2010-06-09 MY MYPI2011004483A patent/MY159850A/en unknown
- 2010-06-09 PL PL10786195.7T patent/PL2441753T3/en unknown
- 2010-06-09 PE PE2011002060A patent/PE20121065A1/en active IP Right Grant
- 2010-06-09 LT LT18158255T patent/LT3345903T/en unknown
- 2010-06-09 PL PL15180863T patent/PL2975024T3/en unknown
- 2010-06-09 CA CA2764653A patent/CA2764653C/en active Active
- 2010-06-09 EP EP19201621.0A patent/EP3613729A1/en active Pending
- 2010-06-09 ES ES18158255T patent/ES2759510T3/en active Active
- 2010-06-09 WO PCT/JP2010/059785 patent/WO2010143664A1/en active Application Filing
- 2010-06-09 US US13/377,300 patent/US9126931B2/en active Active
- 2010-06-09 MX MX2011013306A patent/MX2011013306A/en active IP Right Grant
- 2010-06-09 AU AU2010259588A patent/AU2010259588B2/en active Active
- 2010-06-09 PT PT181582552T patent/PT3345903T/en unknown
- 2010-06-09 RU RU2011154150A patent/RU2585622C3/en active Protection Beyond IP Right Term
- 2010-06-09 CN CN201080025574.4A patent/CN102459172B/en active Active
- 2010-06-09 PL PL18158255T patent/PL3345903T3/en unknown
- 2010-06-09 ES ES15180863T patent/ES2668775T3/en active Active
- 2010-06-09 EP EP18158255.2A patent/EP3345903B1/en active Active
- 2010-06-09 PT PT107861957T patent/PT2441753E/en unknown
- 2010-06-09 SI SI201031199A patent/SI2441753T1/en unknown
- 2010-06-09 SG SG2011072295A patent/SG175707A1/en unknown
- 2010-06-09 RS RS20110548A patent/RS58855B1/en unknown
- 2010-06-09 HU HUE10786195A patent/HUE028278T2/en unknown
- 2010-06-09 DK DK10786195.7T patent/DK2441753T3/en active
- 2010-06-09 ES ES10786195.7T patent/ES2575084T3/en active Active
- 2010-06-09 TW TW099118688A patent/TWI531367B/en active
- 2010-06-09 PT PT151808631T patent/PT2975024T/en unknown
- 2010-06-09 NO NO15180863A patent/NO2975024T3/no unknown
- 2010-09-06 UA UAA201200237A patent/UA107796C2/en unknown
- 2010-09-07 JP JP2010199935A patent/JP5628603B2/en active Active
-
2011
- 2011-09-19 CR CR20110492A patent/CR20110492A/en unknown
- 2011-09-22 ZA ZA2011/06938A patent/ZA201106938B/en unknown
- 2011-09-30 CL CL2011002433A patent/CL2011002433A1/en unknown
- 2011-12-07 IL IL216817A patent/IL216817A/en active IP Right Grant
-
2012
- 2012-01-04 EC EC2012011573A patent/ECSP12011573A/en unknown
- 2012-01-06 CO CO12002119A patent/CO6430460A2/en active IP Right Grant
- 2012-06-29 HK HK12106400.7A patent/HK1165794A1/en unknown
-
2015
- 2015-02-11 US US14/619,242 patent/US9440922B2/en active Active
-
2016
- 2016-06-02 CY CY20161100490T patent/CY1117752T1/en unknown
- 2016-06-06 HR HRP20160605TT patent/HRP20160605T1/en unknown
- 2016-07-28 US US15/221,926 patent/US20160340308A1/en not_active Abandoned
-
2017
- 2017-06-01 FR FR17C1019C patent/FR17C1019I2/en active Active
- 2017-06-02 LT LTPA2017017C patent/LTC2441753I2/en unknown
- 2017-06-02 NL NL300876C patent/NL300876I2/en unknown
- 2017-06-06 HU HUS1700026C patent/HUS1700026I1/en unknown
- 2017-06-12 NO NO2017026C patent/NO2017026I2/en unknown
- 2017-06-14 LU LU00022C patent/LUC00022I2/fr unknown
- 2017-07-12 CY CY2017026C patent/CY2017026I2/en unknown
-
2018
- 2018-04-18 HR HRP20180615TT patent/HRP20180615T1/en unknown
-
2019
- 2019-01-04 US US16/239,839 patent/US20200017442A1/en not_active Abandoned
- 2019-11-07 HR HRP20192022TT patent/HRP20192022T1/en unknown
-
2020
- 2020-04-29 US US16/862,125 patent/US20220306578A1/en not_active Abandoned
-
2022
- 2022-07-08 US US17/860,589 patent/US20230142119A1/en active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230142119A1 (en) | Tetracyclic compound | |
| JP5006987B2 (en) | Medicine containing tetracyclic compound | |
| ES2289274T3 (en) | DERIVATIVES OF PIRROL-2,5-DIONA AND ITS USE AS INHIBITORS OF GSK-3. | |
| CN107556244B (en) | Fused ring compound, pharmaceutical composition and application thereof | |
| WO2023205595A2 (en) | Egfr inhibitors in cancer treatment | |
| WO2022174765A1 (en) | Fused ring compound as wee-1 inhibitor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: FINAL REJECTION MAILED |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |