US20160222086A1 - Conjugated Factor VIII Molecules - Google Patents
Conjugated Factor VIII Molecules Download PDFInfo
- Publication number
- US20160222086A1 US20160222086A1 US14/831,515 US201514831515A US2016222086A1 US 20160222086 A1 US20160222086 A1 US 20160222086A1 US 201514831515 A US201514831515 A US 201514831515A US 2016222086 A1 US2016222086 A1 US 2016222086A1
- Authority
- US
- United States
- Prior art keywords
- fviii
- peg
- factor viii
- domain
- fviii molecule
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/96—Stabilising an enzyme by forming an adduct or a composition; Forming enzyme conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/745—Blood coagulation or fibrinolysis factors
- C07K14/755—Factors VIII, e.g. factor VIII C (AHF), factor VIII Ag (VWF)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/36—Blood coagulation or fibrinolysis factors
- A61K38/37—Factors VIII
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/548—Phosphates or phosphonates, e.g. bone-seeking
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/04—Antihaemorrhagics; Procoagulants; Haemostatic agents; Antifibrinolytic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Definitions
- the present invention relates to conjugated coagulation Factor VIII molecules.
- the present invention relates to conjugated Factor VIII molecules having a modified circulatory half-life.
- Haemophilia A is an inherited bleeding disorder caused by deficiency or dysfunction of coagulation Factor VIII (FVIII) activity.
- the clinical manifestation is not on primary haemostasis—formation of the blood clot occurs normally—but the clot is unstable due to a lack of secondary thrombin formation.
- the disease is treated by intravenous injection of coagulation Factor FVIII which is either isolated from blood or produced recombinantly.
- the present invention relates to a B domain truncated Factor VIII molecule with a modified circulatory half-life, said molecule being covalently conjugated with a hydrophilic polymer via an O-linked oligosaccharide in the truncated B domain, wherein Factor VIII activation results in removal of the covalently conjugated side group.
- the present invention furthermore relates to methods for obtaining such molecules, use of such molecules and pharmaceutical compositions comprising such molecules.
- conjugated Factor VIII molecule with modified circulatory half-life, wherein the conjugated side group (e.g. hydraphilic polymer) is removed upon activation.
- the molecules according to the invention are preferably homogenous in structure—at least with regard to position of the hydrophilic polymer in the truncated B-domain—and preferably have an advantageous safety profile.
- relatively simple methods for obtaining such molecules are furthermore provided herein.
- activated Factor VIII molecules according to the invention are similar to endogenous activated Factor VIII.
- FVIII/Factor VIII is a large, complex glycoprotein that primarily is produced by hepatocytes.
- FVIII consists of 2351 amino acids, including signal peptide, and contains several distinct domains, as defined by homology. There are three A-domains, a unique B-domain, and two C-domains. The domain order can be listed as NH2-A1-A2-B-A3-C1-C2-COOH.
- FVIII circulates in plasma as two chains, separated at the B-A3 border. The chains are connected by bivalent metal ion-bindings.
- the A1-A2-B chain is termed the heavy chain (HC) while the A3-C1-C2 is termed the light chain (LC).
- Endogenous Factor VIII molecules circulate in vivo as a pool of molecules with B domains of various sizes. What probably occurs in vivo is a gradual enzymatic removal of the B domain resulting in a pool of molecules with B-domains of various sizes. It is generally believed that cleavage at position 740, by which the last part of the B-domain is removed, occurs in connection with thrombin activation. However, it cannot be ruled out that a Factor VIII variant in which e.g. the cleavage site at position 740 has been impaired may be active.
- Vector VIII or “FVIII” as used herein refers to a human plasma glycoprotein that is a member of the intrinsic coagulation pathway and is essential to blood coagulation.
- “Native FVIII” is the full length human FVIII molecule as shown in SEQ ID NO. 1 (amino acid 1-2332). The B-domain spans amino acids 741-1648 in SEQ ID NO 1.
- SEQ ID NO 1 ATRRYYLGAVELSWDYMQSDLGELPVDARFPPRVPKSFPFNTSVVYKKTL FVEFTDHLFNIAKPRPPWMGLLGPTIQAEVYDTVVITLKNMASHPVSLHA VGVSYWKASEGAEYDDQTSQREKEDDKVFPGGSHTYVWQVLKENGPMASD PLCLTYSYLSHVDLVKDLNSGLIGALLVCREGSLAKEKTQTLHKFILLFA VFDEGKSWHSETKNSLMQDRDAASARAWPKMHTVNGYVNRSLPGLIGCHR KSVYWHVIGMGTTPEVHSIFLEGHTFLVRNHRQASLEISPITFLTAQTLL MDLGQFLLFCHISSHQHDGMEAYVKVDSCPEEPQLRMKNNEEAEDYDDDL TDSEMDVVRFDDDNSPSFIQIRSVAKKHPKTWVHYIAAEEEDWDYAPLVL APDDRSYKSQYLNNGPQRIGRKYKKVRFM
- the Factor VIII molecules according to the present invention are B domain truncated Factor FVIII molecules wherein the remaining domains correspond to the sequence as set forth in amino acid no 1-740 and 1649-2332 in SEQ ID NO. 1. It follows that molecules according to the invention are recombinant molecules produced in transformed host cells, preferably of mammalian origin. However, the remaining domains (i.e. the three A-domains and the two C-domains) may differ slightly e.g. about 1%, 2%, 3%, 4% or 5% from the amino acid sequence as set forth in SEQ ID NO 1 (amino acids 1-740 and 1649-2332).
- the Factor VIII molecules according to the invention comprise other post-translational modifications in e.g. the truncated B-domain and/or in one or more of the other domains of the molecules.
- These other post-translational modifications may be in the form of various molecules conjugated to the Factor VIII molecule according to the invention such as e.g. polymeric compounds, peptidic compounds, fatty acid derived compounds, etc.
- Factor VIII molecules according to the present invention regardless of whether they are modified outside the B domain or not, have other posttranslational modifications or not, all have Factor VIII activity, meaning the ability to function in the coagulation cascade in a manner functionally similar or equivalent to FVIII, induce the formation of FXa via interaction with FIXa on an activated platelet, and support the formation of a blood clot.
- the activity can be assessed in vitro by techniques well known in the art such as e.g. clot analysis, endogenous thrombin potential analysis, etc.
- Factor VIII molecules according to the present invention have FVIII activity being at least about 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, and 100% or even more than 100% of that of native human FVIII.
- B domain The B-domain in Factor VIII spans amino acids 741-1648 in SEQ ID NO 1.
- the B-domain is cleaved at several different sites, generating large heterogeneity in circulating plasma FVIII molecules.
- the exact function of the heavily glycosylated B-domain is unknown. What is known is that the domain is dispensable for FVIII activity in the coagulation cascade. This apparent lack of function is supported by the fact that B domain deleted/truncated FVIII appears to have in vivo properties identical to those seen for full length native FVIII. That being said there are indications that the B-domain may reduce the association with the cell membrane, at least under serum free conditions.
- B domain truncated/deleted Factor VIII molecule Endogenous full length FVIII is synthesized as a single-chain precursor molecule. Prior to secretion, the precursor is cleaved into the heavy chain and the light chain. Recombinant B domain-deleted FVIII can be produced from two different strategies. Either the heavy chain without the B-domain and the light chain are synthesized individually as two different polypeptide chains (two-chain strategy) or the B-domain deleted FVIII is synthesized as a single precursor polypeptide chain (single-chain strategy) that is cleaved into the heavy and light chains in the same way as the full-length FVIII precursor.
- the heavy and light chain moieties are normally separated by a linker.
- the sequence of the linker is preferable derived from the FVIII B-domain.
- the linker must comprise a recognition site for the protease that separates the B domain-deleted FVIII precursor polypeptide into the heavy and light chain.
- amino acid 1644-1648 constitutes this recognition site.
- the thrombin site leading to removal of the linker on activation of B domain-deleted FVIII is located in the heavy chain.
- the size and amino acid sequence of the linker is unlikely to influence its removal from the remaining FVIII molecule by thrombin activation.
- Deletion of the B domain is an advantage for production of FVIII. Nevertheless, parts of the B domain can be included in the linker without reducing the productivity.
- the negative effect of the B domain on productivity has not been attributed to any specific size or sequence of the B domain.
- the truncated B-domain may contain several O-glycosylation sites.
- the molecule comprises only one, alternatively two, three or four O-linked oligosaccharides in the truncated B-domain.
- the truncated B domain comprises only one potential O-glycosylation site and the hydrophilic polymer is covalently conjugated to this O-glycosylation site.
- the O-linked oligosaccharides in the B-domain truncated molecules according to the invention may be attached to O-glycosylation sites that were either artificially created by recombinant means and/or by exposure of “hidden” O-glycosylation sites by truncation of the B-domain.
- such molecules may be made by designing a B-domain trunctated Factor VIII amino acid sequence and subsequently subjecting the amino acid sequence to an in silico analysis predicting the probability of O-glycosylation sites in the truncated B-domain.
- Suitable host cells for producing recombinant Factor VIII protein are preferably of mammalian origin in order to ensure that the molecule is glycosylated.
- the cells are mammalian cells, more preferably an established mammalian cell line, including, without limitation, CHO (e.g., ATCC CCL 61), COS-1 (e.g., ATCC CRL 1650), baby hamster kidney (BHK), and HEK293 (e.g., ATCC CRL 1573; Graham et al., J. Gen. Virol. 36:59-72, 1977) cell lines.
- a preferred BHK cell line is the tk-ts13 BHK cell line (Waechter and Baserga, Proc.Natl.Acad.Sci.USA 79:1106-1110, 1982), hereinafter referred to as BHK 570 cells.
- the BHK 570 cell line is available from the American Type Culture Collection, 12301 Parklawn Dr., Rockville, Md. 20852, under ATCC accession number CRL 10314.
- a tk-ts13 BHK cell line is also available from the ATCC under accession number CRL 1632.
- a preferred CHO cell line is the CHO K1 cell line available from ATCC under accession number CC161 as well as cell lines CHO-DXB11 and CHO-DG44.
- Suitable cell lines include, without limitation, Rat Hep I (Rat hepatoma; ATCC CRL 1600), Rat Hep II (Rat hepatoma; ATCC CRL 1548), TCMK (ATCC CCL 139), Human lung (ATCC HB 8065), NCTC 1469 (ATCC CCL 9.1); DUKX cells (CHO cell line) (Urlaub and Chasin, Proc. Natl. Acad. Sci. USA 77:4216-4220, 1980) (DUKX cells also being referred to as DXB11 cells), and DG44 (CHO cell line) (Cell, 33: 405, 1983, and Somatic Cell and Molecular Genetics 12: 555, 1986).
- the cells may be mutant or recombinant cells, such as, e.g., cells that express a qualitatively or quantitatively different spectrum of enzymes that catalyze post-translational modification of proteins (e.g., glycosylation enzymes such as glycosyl transferases and/or glycosidases, or processing enzymes such as propeptides) than the cell type from which they were derived.
- DUKX cells CHO cell line
- HEK293, COS Chinese Hamster Ovary (CHO) cells
- Baby Hamster Kidney (BHK) and myeloma cells
- Chinese Hamster Ovary (CHO) cells are preferred cells.
- the inventors of the present invention have thus shown that it is possible to activate “hidden” O-glycosylation sites in the Factor VIII B-domain by truncating the B-domain. While not wishing to be bound by any theory, this phenomenon could be attributable to the tertiary structure of the molecule in the truncated B-domain being altered. “Hidden” O-glycosylation sites are thus “made accessible” to glycosylation in the truncated B-domain.
- One advantage of this approach is the provision of recombinant molecules with an advantageous safety profile with respect to e.g. allergenicity.
- Another advantage could be that it may represent a simpler approach of obtaining B-domain truncated variants with an O-linked oligosaccharide in the B-domain due to the inherent abundance of glycosylation sites in the B-domain as it has previously proven difficult to engineer artificial O-glycosylation sites in recombinant proteins.
- the length of the B domain in the wt FVIII molecule is about 907 amino acids.
- the length of the truncated B domain in molecules according to the present invention may vary from about 10 amino acids to about 700 acids, such as e.g. about 12-500 amino acids, 12-400 amino acids, 12-300 amino acids, 12-200 amino acids, 15-100 amino acids, 15-75 amino acids, 15-50 amino acids, 15-45 amino acids, 20-45 amino acids, 20-40 amino acids, or 20-30 amino acids.
- the truncated B-domain may comprise fragments of the heavy chain and/or the light chain and/or an artificially introduced sequence that is not found in the wt FVIII molecule.
- the terms “B-domain truncated” and “B-domain deleted” may be used interchangeably herein.
- Modified circulatory half life Molecules according to the present invention have a modified circulatory half-life compared to the wild type Factor VIII molecule, preferably an increased circulatory half-life.
- Circulatory half-life is preferably increased at least 10%, preferably at least 15%, preferably at least 20%, preferably at least 25%, preferably at least 30%, preferably at least 35%, preferably at least 40%, preferably at least 45%, preferably at least 50%, preferably at least 55%, preferably at least 60%, preferably at least 65%, preferably at least 70%, preferably at least 75%, preferably at least 80%, preferably at least 85%, preferably at least 90%, preferably at least 95%, preferably at least 100%, more preferably at least 125%, more preferably at least 150%, more preferably at least 175%, more preferably at least 200%, and most preferably at least 250% or 300%. Even more preferably, such molecules have a circulatory half-life that is increased at least 400%, 500%, 600%, or even
- the modifying group/hydrophilic polymer according to the present invention is preferably non-naturally occurring.
- the “non-naturally occurring modifying group” is a polymeric modifying group, in which at least one polymeric moiety is non-naturally occurring.
- the non-naturally occurring modifying group is a modified carbohydrate.
- the locus of functionalization with the modifying group is selected such that it does not prevent the “modified sugar” from being added enzymatically to a polypeptide.
- “Modified sugar” also refers to any glycosyl mimetic moiety that is functionalized with a modifying group and which is a substrate for a natural or modified enzyme, such as a glycosyltransferase.
- the polymeric modifying group added to a polypeptide can alter a property of such polypeptide, for example, its bioavailability, biological activity or its half-life in the body.
- Exemplary polymers according to the invention include water-soluble polymers that can be linear or branched and can include one or more independently selected polymeric moieties, such as poly(alkylene glycol) and derivatives thereof.
- the polymeric modifying group according to the invention may include a water-soluble polymer, e.g. poly(ethylene glycol) and derivatives thereof (PEG, m-PEG), poly(propylene glycol) and derivatives thereof (PPG, m-PPG) and the like.
- water-soluble refers to moieties that have some detectable degree of solubility in water. Methods to detect and/or quantify water solubility are well known in the art.
- Exemplary water-soluble polymers according to the invention include peptides, saccharides, poly(ethers), poly(amines), poly(carboxylic acids) and the like. Peptides can have mixed sequences and be composed of a single amino acid, e.g., poly(lysine).
- An exemplary polysaccharide is poly(sialic acid).
- An exemplary poly(ether) is poly(ethylene glycol), e.g., m-PEG.
- Poly(ethylene imine) is an exemplary polyamine
- poly(acrylic) acid is a representative poly(carboxylic acid).
- the polymer backbone of the water-soluble polymer according to the invention can be poly(ethylene glycol) (i.e. PEG).
- PEG in connection with the present invention includes poly(ethylene glycol) in any of its forms, including alkoxy PEG, difunctional PEG, multiarmed PEG, forked PEG, branched PEG, pendent PEG (i.e. PEG or related polymers having one or more functional groups pendent to the polymer backbone), or PEG with degradable linkages therein.
- the polymer backbone can be linear or branched.
- Branched polymer backbones are generally known in the art.
- a branched polymer has a central branch core moiety and a plurality of linear polymer chains linked to the central branch core.
- PEG is commonly used in branched forms that can be prepared by addition of ethylene oxide to various polyols, such as glycerol, pentaerythritol and sorbitol.
- the central branch moiety can also be derived from several amino acids, such as lysine or cysteine.
- the branched poly(ethylene glycol) can be represented in general form as R(-PEG-OH)m in which R represents the core moiety, such as glycerol or pentaerythritol, and m represents the number of arms.
- R represents the core moiety, such as glycerol or pentaerythritol
- m represents the number of arms.
- Multi-armed PEG molecules such as those described in U.S. Pat. No. 5,932,462, which is incorporated by reference herein in its entirety, can also be used as the polymer backbone.
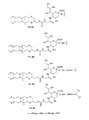
- FIGS. 8A-D show a representative branched PEG polymer of use in embodiments of the invention, referred to herein as “SA-glycerol-PEG.”
- FIG. 8A shows an exemplary SA-glycerol-PEG component of CMP-SA-glycerol-PEG or of a SA-glycerol-PEG linked to a glycan or an amino acid of a polypeptide.
- FIG. 8B shows the SA-glycerol-PEG moiety linked to a glycan or polypeptide through a Gal residue.
- FIG. 8C shows the SA-glycerol-PEG moiety linked to a glycan or polypeptide through a Gal-GalNAc residue.
- FIG. 8A shows an exemplary SA-glycerol-PEG component of CMP-SA-glycerol-PEG or of a SA-glycerol-PEG linked to a glycan or an amino acid of a polypeptide.
- FIG. 8D shows the SA-glycerol-PEG moiety linked to an amino acid of a polypeptide through a Gal-GalNAc moiety.
- AA is threonine or serine.
- AA is converted to an O-linked glycosylation site by deletion of the B-domain of the FVIII polypeptide.
- the discussion regarding the molecular weight of the polymer herein below under this definition is generally applicable to the branched PEG shown in FIG. 8D .
- the index “n” represents any integer providing a linear (and thus a branched) m-PEG of the desired molecular weight.
- n is selected such that the linear m-PEG moiety is about 20 KDa to about 40 KDa, for example, about 20 KDa, about 30 KDa or about 40 KDa. Integers corresponding to these m-PEG molecular weights correspond to about 400 (e.g. about 455) to about 900 (e.g. about 910). Accordingly, “n” is selected to provide a branched PEG that is about 40 KDa to about 80 KDa, e.g., about 40 KDa, about 50 KDa, about 60 KDa, about 70 KDa, or about 80 KDa.
- polymers are also suitable for the invention.
- Polymer backbones that are non-peptidic and water-soluble, are particularly useful in the invention.
- suitable polymers include, but are not limited to, other poly(alkylene glycols), such as poly(propylene glycol) (“PPG”), copolymers of ethylene glycol and propylene glycol and the like, poly(oxyethylated polyol), poly(olefinic alcohol), poly(vinylpyrrolidone), poly(hydroxypropylmethacrylamide), poly([alpha]-hydroxy acid), poly(vinyl alcohol), polyphosphazene, polyoxazoline, poly(N-acryloylmorpholine), such as described in U.S. Pat. No. 5,629,384, which is incorporated by reference herein in its entirety, as well as copolymers, terpolymers, and mixtures thereof.
- PPG poly(propylene glycol)
- PPG poly(propylene glycol)
- each chain of the polymer backbone can vary, it is typically in the range of from about 100 Da to about 160,000 Da, such as e.g., from about 5,000 Da to about 100,000 Da. More specifically, the size of each conjugated hydrophilic polymer according to the present invention may vary from about 500 Da to about 80,000 Da, such as e.g. about 1000 Da to about 80,000 Da; about 2000 Da to about 70,000 Da; about 5000 to about 70,000 Da; about 5000 to about 60,000 Da; about 10,000 to about 70,000 Da; about 20,000 to about 60,000 Da; about 30,000 to about 60,000 Da; about 30,000 to about 50,000 Da; or about 30,000 to about 40,000 Da. It should be understood that these sizes represent estimates rather than exact measures.
- the molecules according to the invention are conjugated with a heterogeneous population of hydrophilic polymers, such as e.g. PEG of a size of e.g. 10,000, 40,000, or 80,000 Da+/ ⁇ about 5000, about 4000, about 3000, about 2000, or about 1000 Da.
- a heterogeneous population of hydrophilic polymers such as e.g. PEG of a size of e.g. 10,000, 40,000, or 80,000 Da+/ ⁇ about 5000, about 4000, about 3000, about 2000, or about 1000 Da.
- N-linked oligosaccharide Both N-glycans and O-glycans are attached to proteins by the cells producing the protein.
- the cellular N-glycosylation machinery recognizes and glycosylates N-glycosylation signals (N—X-S/T motifs) in the amino acid chain, as the nascent protein is translocated from the ribosome to the endoplasmic reticulum (Kiely et al. 1976; Glabe et al. 1980).
- O-glycans are attached to specific O-glycosylation sites in the amino acid chain, but the motifs triggering O-glycosylation are much more heterogeneous than the N-glycosylation signals, and our ability to predict O-glycosylation sites in amino acid sequences is still inadequate (Julenius et al. 2004).
- the construction of artificial O-glycosylation sites it is thus associated with some uncertainty.
- the general assumption is that the native FVIII molecule does not contain any O-glycosylation sites, and the skilled man would therefore expect that at least one artificial O-glycosylation site would have to be constructed and inserted into the B domain in connection with practicing the present invention.
- the O-linked oligosaccharide in a truncated Factor VIII B domain may thus be covalently linked to a naturally occurring O-linked glycosylation sequence or an O-linked glycosylation sequence which has been artificially constructed by recombinant techniques.
- the O-linked oligosaccharide is linked to a naturally occurring O-linked glycosylation sequence which is not exposed to glycosylation in the wild type Factor VIII molecule but is becoming accessible to O-glycosylation as a consequence of truncation of the B domain.
- An example thereof is shown in the examples and in SEQ ID NO 2 (the truncated B-domain corresponds to amino acids 742-763). It is plausible that the “hidden” O-glycosylation site in SEQ ID NO 2 will also become glycosylated even if the B-domain is truncated at a somewhat different place, i.e. if the truncated B domain is somewhat shorter (e.g.
- Glyco-PEGylation of O-linked oligosaccharide The biosynthesis of O-glycans can be modified and terminated with the addition of sialic acid residues relatively early in biosynthesis.
- Certain sialyltransferase enzymes are capable of acting on GalNAc ⁇ -Ser/Thr, or early O-glycan core subtypes after Core 1 GalT action.
- T antigen is associated with the presence of the Gal ⁇ 1-3GalNAc ⁇ -Ser/Thr disaccharide.
- Production of these structures involves a competition among glycosyltransferases for the same substrate and thus the expression levels and subcellular distributions of glycosyltransferases within the Golgi apparatus determines the structural outcome in O-glycan biosynthesis and diversification. As illustrated in FIG. 1 , only the Gal ⁇ 1-3GalNAc ⁇ -Ser/Thr disaccharide is amenable for glycoPEGylation.
- the available amount of this structure may be greatly enhanced through treatment of the protein with sialidase or Corel GalT or a combination thereof.
- sialidase or Corel GalT or a combination thereof.
- the Sialic acid PEG is added to the native structure through an ⁇ 3 bond to the Gal ⁇ 1-3GalNAc ⁇ -Ser/Thr disaccharide of the target protein ( FIG. 1 ).
- hydrophilic polymers can also be attached to O-linked oligosaccharides.
- the basic requirement for enzymatically conjugating other hydrophilic polymers to FVIII via the O-glycan is the ability to couple them to the glycyl-Sialic acid derivative via the free amino group as disclosed in WO03031464. This may be achieved through a large variety of coupling chemistries known to those skilled in the art.
- activated biocompatible polymer includes polyalkylene oxides such as without limitation polyethylene glycol (PEG), 2-(methacryloyloxy)ethyl phosphorylcholine (mPC) polymers (as described in WO03062290), dextrans, colominic acids or other carbohydrate based polymers, polymers of amino acids or of specific peptides sequences, biotin derivatives, polyvinyl alcohol (PVA), polycarboxylates, polyvinylpyrrolidone, polyethylene-co-maleic acid anhydride, polystyrene-co-malic acid anhydride, polyoxazoline, poly-acryloylmorpholine, heparin, albumin, celluloses, hydrolysates of chitosan, starches such as hydroxyethyl-starches and hydroxy propyl-starches, glycogen, agaroses and derivatives thereof, guar gum, pullulan, inulin, xanthan gum, carrageenan
- compositions comprising Factor VIIII molecules according to the present invention suitable for parenteral administration, such as e.g. ready-to-use sterile aqueous compositions or dry sterile compositions that can be reconstituted in e.g. water or an aqueous buffer.
- the compositions according to the invention may comprise various pharmaceutically acceptable excipients, stabilizers, etc.
- Additional ingredients in such compositions may include wetting agents, emulsifiers, antioxidants, bulking agents, tonicity modifiers, chelating agents, metal ions, oleaginous vehicles, proteins (e.g., human serum albumin, gelatine or proteins) and a zwitterion (e.g., an amino acid such as betaine, taurine, arginine, glycine, lysine and histidine).
- proteins e.g., human serum albumin, gelatine or proteins
- a zwitterion e.g., an amino acid such as betaine, taurine, arginine, glycine, lysine and histidine.
- Such additional ingredients should not adversely affect the overall stability of the pharmaceutical formulation of the present invention.
- Parenteral administration may be performed by subcutaneous, intramuscular, intraperitoneal or intravenous injection by means of a syringe, optionally a pen-like syringe.
- parenteral administration can be performed by means of an infusion pump.
- a composition which may be a solution or suspension for the administration of the FVIII compound in the form of a nasal or pulmonal spray.
- the pharmaceutical compositions containing the FVIII compound of the invention may also be adapted to transdermal administration, e.g. by needle-free injection or from a patch, optionally an iontophoretic patch, or transmucosal, e.g. buccal, administration.
- the present invention thus relates to a B-domain truncated Factor VIII molecule with a modified circulatory half-life, said molecule being covalently conjugated with a hydrophilic polymer via an O-linked oligosaccharide in the truncated B domain, wherein Factor VIII activation (activation of the molecule) results in removal of the covalently conjugated hydrophilic polymer.
- the hydrophilic polymer is PEG.
- the size of the PEG polymer may vary from about 10,000 to about 160,000 Da; such as 10,000 to 80,000 Da, such as e.g. about 10,000; 15,000, 20,000; 25,000; 30,000; 35,000; 40,000; 45,000; 50,000; 55,000; 60,000; 65,000, 70,000; 75,000; or 80,000 Da.
- the O-linked oligosaccharide is attached to an O-glycosylation site that is made by truncation of the B-domain and not by inserting an artificial O-glycosylation site that is not found in the wt FVIII molecule.
- the molecule according to the present invention comprises the amino acid sequence as set forth in SEQ ID NO 2.
- Such molecules have a unique feature in that the activated FVIII molecule is identical to the native active FVIII molecule. This feature appears to have advantageous properties in safety assessments.
- the present invention also relates to pharmaceutical compositions comprising molecules according to the present invention.
- the present invention furthermore relates to a method of obtaining a molecule according to the present invention, wherein said method comprises conjugating a B-domain truncated Factor VIII molecule with a hydrophilic polymer, such as e.g. a PEG group, via an O-linked oligosaccharide in the truncated B domain.
- a hydrophilic polymer such as e.g. a PEG group
- the present invention relates to a method of treatment of a haemophilic disease comprising administering to a patient in need thereof a therapeutically effective amount of a molecule according to the invention.
- treatment refers to the medical therapy of any human or other animal subject in need thereof. Said subject is expected to have undergone physical examination by a medical practitioner, who has given a tentative or definitive diagnosis which would indicate that the use of said specific treatment is beneficial to the health of said human or other animal subject.
- the timing and purpose of said treatment may vary from one individual to another, according to the status quo of the subject's health.
- said treatment may be prophylactic, palliative, symptomatic and/or curative.
- the present invention relates to use of a molecule according to the invention as a medicament as well as use of a molecule according to the invention for manufacture of a medicament for treatment of haemophilia.
- the present invention relates to a method of engineering a B-domain truncated Factor VIII molecule according to the present invention, said method comprising (i) truncating the B-domain and optionally subjecting the amino acid sequence of this truncated Factor VIII molecule to an analysis identifying potential O-linked glycosylation sites, (ii) producing the molecule in a suitable host cell and (iii) selecting molecules having O-linked glycans in the truncated B-domain.
- the size of the conjugated groups is sometimes referred to as “K”, which is herein meant to be equivalent to KDa (kilo Dalton).
- FIG. 1 Schematic drawing of glycol PEGylation process of O-linked oligosaccharides. The figure does not represent an exhaustive list of possible ways to arrive at the products obtained in the examples.
- FIG. 2 Ion-exchange chromatography of the reaction mixture on Source 15Q (A). SDS-PAGE with molecular markers (left) of collected fraction (B).
- FIG. 3 Purification of the capped product on superdex 200 size-exclusion chromatography.
- FIGS. 4A-B Clotting activity of O-glycoPEGylated rFVIII using various aPTT reagents.
- Figure A shows the ration between the clotting activity and the chromogenic activity.
- Figure B shows the specific clotting activity.
- FIG. 5 In vivo effects (time to occlusion) in FVIII KO mice of 40K-PEG-[O]—N8.
- FIG. 6 Flow diagram showing the process steps involved in production of glycoPEGylated Factor FVIII according to the invention.
- FIG. 7 Schematic representation of a Factor VIII molecule according to the present invention produced in the Examples.
- FIGS. 8A-D show a representative branched PEG polymer of use in embodiments of the invention, referred to herein as “SA-glycerol-PEG.”
- FIG. 8A shows an exemplary SA-glycerol-PEG component of CMP-SA-glycerol-PEG or of a SA-glycerol-PEG linked to a glycan or an amino acid of a polypeptide.
- FIG. 8B shows the SA-glycerol-PEG moiety linked to a glycan or polypeptide through a Gal residue.
- FIG. 8C shows the SA-glycerol-PEG moiety linked to a glycan or polypeptide through a Gal-GalNAc residue.
- FIG. 8D shows the SA-glycerol-PEG moiety linked to an amino acid of a polypeptide through a Gal-GalNAc moiety.
- SEQ ID NO 2 An example of the amino acid sequence of a B-domain deleted Factor VIII molecule is given in SEQ ID NO 2.
- This polypeptide may also be referred to as “N8”.
- This molecule comprises a 21 amino acid residue linker sequence (SFSQNSRHP S QNPPVLKRHQR (SEQ ID NO 3)—the underlined S is the Serine residue with the O-glygan that is pegylated in Example 2).
- Factor VIII molecules according to the present invention may in the Examples be referred to in various ways—but all references to Factor VIII molecules refer to Factor VIII molecules according to the invention, or alternatively Factor VIII molecules in the process of being converted to Factor VIII molecules according to the invention.
- SEQ ID NO 2 ATRRYYLGAVELSWDYMQSDLGELPVDARFPPRVPKSFPFNTSVVYKKTL FVEFTDHLFNIAKPRPPWMGLLGPTIQAEVYDTVVITLKNMASHPVSLHA VGVSYWKASEGAEYDDQTSQREKEDDKVFPGGSHTYVWQVLKENGPMASD PLCLTYSYLSHVDLVKDLNSGLIGALLVCREGSLAKEKTQTLHKFILLFA VFDEGKSWHSETKNSLMQDRDAASARAWPKMHTVNGYVNRSLPGLIGCHR KSVYWHVIGMGTTPEVHSIFLEGHTFLVRNHRQASLEISPITFLTAQTLL MDLGQFLLFCHISSHQHDGMEAYVKVDSCPEEPQLRMKNNEEAEDYDDDL TDSEMDVVRFDDDNSPSFIQIRSVAKKHPKTWVHYIAAEEEDWDYAPLVL APDDRSYKSQYLNNGPQRIGRKYKKVRFM
- a mammalian expression plasmid encoding B-domain deleted Factor VIII having an amino acid sequence as set forth in SEQ ID NO 2 was constructed.
- the plasmid is encoding Factor VIII heavy chain comprising amino acid 1-740 of full length human Factor VIII and Factor VIII light chain comprising amino acid 1649-2332 of full length human Factor VIII.
- the heavy and light chain sequences are connected by a 21 amino acid linker with the sequence of amino acid 741-750 and 1638-1648 of full length human Factor VIII.
- Chinese hamster ovary (CHO) cells were transfected with the BDD Factor VIII coding plasmid and selected with the dihydrofolate reductase system eventually leading to a clonal suspension producer cell cultivated in animal component-free medium.
- the first step in the process is the inoculation of a cell vial, from a working cell bank vial, into a chemically defined and animal component free growth medium. Initially after thawing, the cells are incubated in a T-flask. One or two days after thawing, the cells are transferred to a shaker flask, and the culture volume is expanded by successive dilutions in order to keep the cell density between 0.2-3.0 ⁇ 10 6 cells/ml. The next step is the transfer of the shaker flask culture into seed bioreactors. The culture volume is here further expanded before the final transfer to the production bioreactor. The same chemically defined and animal component free medium is used for all the inoculum expansion steps.
- the medium is supplemented with components that increase the product concentration.
- the cells are cultured in a repeated batch process with a cycle time of three days. At harvest, 80-90% of the culture volume is transferred to a harvest tank. The remaining culture fluid is then diluted with fresh medium, in order to obtain the initial cell density, and then a new growth period is initiated.
- the harvest batch is clarified by centrifugation and filtration and transferred to a holding tank before initiation of the purification process.
- a buffer is added to the cell free harvest in the holding tank to stabilise pH.
- a four step purification procedure was used including a concentration step on a Capto MMC column, an immunoabsorbent chromatography step, an anionic exchange chromatography and finally a gel filtration step.
- the column was washed with 75 ml of buffer A followed by wash with 75 ml of buffer A containing 1.5 M NaCl.
- F25 A monoclonal antibody against Factor VIII has been developed (Kjalke Eur J Biochem 234 773). By epitope mapping (results not shown) this antibody, F25, was found to recognise the far C-terminal sequence of the heavy chain from amino acid residue 725 to 740.
- the F25 antibody was coupled to NHS-activated Sepharose 4 FF (GE Healthcare, Bio-Sciences AB, Uppsala, Sweden) at a density of 2.4 mg per ml of gel essentially as described by the manufacturer.
- a column (1 ⁇ 10 cm) of Macro-Prep 25Q Support Bio-Rad Laboratories, Hercules, Calif., USA
- the pool from the previous step was diluted 10 times with buffer A and pumped onto the column with a flow of 2 ml/min.
- the column was washed with 85% buffer A/15% buffer B at a flow of 2 ml/min and Factor VIII was eluted with a linear gradient from 15% buffer B to 70% buffer B over 120 ml at a flow of 2 ml/min.
- Fractions of 2 ml were collected and assayed for Factor VIII activity (CoA-test).
- Factor VIII containing fractions were pooled and normally a pool volume of around 36 ml was obtained.
- the cell line used for manufacture of N8 is a recombinant Chinese hamster ovary (CHO) cell line stably transfected with expression plasmid #814 F8-500 in pTSV7 consisting of the pTSV7 expression vector with an insert containing cDNA encoding the F8-500 protein.
- N8 is herein meant to correspond to a protein having an amino acid sequence as listed in SEQ ID NO 2.
- the F8-500 protein (N8) consists of the FVIII signal peptide (amino acids ⁇ 19 to ⁇ 1) followed by the FVIII heavy chain without the B domain (amino acids 1-740), a 21 amino acid linker (SFSQNSRHPSQNPPVLKRHQR), and the FVIII light chain (amino acids 1649-2332 of wild-type human FVIII).
- the sequence of the 21 amino acid linker is derived from the FVIII B domain and consists of amino acids 741-750 and 1638-1648 of full length FVIII.
- CHO cells were transfected with 814 F8-500 in pTSV7 and selected with the dihydrofolate reductase system eventually leading to a clonal suspension producer cell cultivated in animal component-free medium.
- a production run is initiated by thawing a working cell bank vial and expanding the cells until transfer to a production bioreactor. The same chemically defined and animal component free medium is used for all the inoculum expansion steps. After transfer to the production bioreactor, the medium is supplemented with components that increase the product concentration.
- the cells are cultured in a repeated batch process with a cycle time of three days. At harvest, 80-90% of the culture volume is transferred to a harvest tank.
- the remaining culture fluid is then diluted with fresh medium, in order to obtain the initial cell density, and then a new growth period is initiated.
- the harvest batch is clarified by centrifugation and filtration and transferred to a holding tank before initiation of the purification process.
- a buffer is added to the cell free harvest in the holding tank to stabilize pH.
- the recombinant Factor VIII molecules obtained in Example 1 are conjugated with polyethylenglycol (PEG) using the following procedure:
- a buffer containing 50 mM MES, 50 mM CaCl2, 150 mM NaCl, 20% glycerol, pH 6.0 was found to be a suitable reaction buffer.
- Reaction buffer composition Precipitate % Aggregate 10 mM Histidine, 260 mM Glycine, 1% YES n. d. Sucrose, 10 mM CaCl2 50 mM HEPES, 10 mM CaCl2, 150 mM YES n. d. NaCl, pH 7; 50 mM MES, 10 mM CaCl2, 150 mM YES n. d.
- Recombinant FVIII which had been purified as described above was concentrated in reaction buffer either by ion exchange on a Poros 50 HQ column using step elution, on a Sartorius Vivaspin (PES) filter, 10 kDa cut-off or on an Amicon 10 kDa MWCO PES filter to a concentration of 6-10 mg/mL.
- the glycoPEGylation of FVIII was initiated by mixing Factor VIII (BDD) ( ⁇ 4.7 mg/mL final) with Sialidase ( A.
- reaction buffer 50 mM MES, 50 mM CaCl2, 150 mM NaCl, 20% glycerol, 0.5 mM antipain, pH 6.0.
- the reaction mixture was incubated at 32° C. until a conversion yield of ⁇ 20-30% of total.
- the sample was diluted with Buffer A (25 mM Tris, 5 mM CaCl 2 , 20 mM NaCl, 20% glycerol, pH 7.5) and applied onto a Source 15Q column (1 cm id ⁇ 6 cm, 4.7 mL, 1 mL/min, 280 nm).
- Buffer A 25 mM Tris, 5 mM CaCl 2 , 20 mM NaCl, 20% glycerol, pH 7.5
- Buffer B 25 mM Tris, 5 mM CaCl 2 , 1 M NaCl, 20% glycerol, pH 7.5.
- FIG. 2 shows ion-exchange chromatography of the reaction mixture on Source 15Q.
- the pooled fraction of Factor VIII-SA-glycerol-PEG-40 kDa (1.0 mg/mL final) was mixed with CMP-SA (2,000 mol eq) and MBP-SBD-ST3Gal3 (400 mU/mL) in reaction buffer 50 mM MES, 20 mM CaCl2, 150 mM NaCl, 10 mM MnCl2, 20% glycerol, pH 6.0 and incubated at 32° C. for 11 hours.
- the resulting capped, glycoPEGylated Factor VIII-SA-glycerol-PEG-40 kDa was separated from CMP-SA and ST3GalIII by gel-filtration on a Superdex 200 column (10 cm id ⁇ 300 mm; 280 nm) equilibrated with 50 mM MES, 50 mM CaCl2, 150 mM NaCl, 10% glycerol, pH 6.0; flow rate of 0.25 mL/min.
- the product Factor VIII-SA-glycerol-PEG-40 kDa elutes at 38 min.
- FIG. 3 shows purification of the capped product using Superdex 200 size-exclusion chromatography. The peak fraction was collected, aliquoted and subjected to subsequent analysis.
- the purpose of the capping procedure is to reduce in vivo clearance of the conjugated Factor VIII molecule.
- the activity of the O-glycoPEGylated rFVIII obtained in Example 2 was evaluated in a chromogenic FVIII assay using Coatest SP reagents (Chromogenix) as follows: rFVIII samples and calibrator (the 7th international FVIII standard from NIBSC) were diluted in Coatest assay buffer (50 mM Tris, 150 mM NaCl, 1% BSA, pH 7.3, with preservative). Fifty ⁇ l of samples, standards, and buffer negative control were added to 96-well microtiter plates (Nunc) in duplicates.
- the Factor IXa/Factor X reagent, the phospholipid reagent and CaCl 2 from the Coatest SP kit were mixed 5:1:3 (vol:vol:vol) and 75 ⁇ l of this added to the wells. After 15 min incubation at room temperature 50 ⁇ l of the Factor Xa substrate 5-2765/thrombin inhibitor 1-2581 mix was added and the reactions incubated 10 min at room temperature before 25 ⁇ l 1 M citric acid, pH 3, was added. The absorbance at 415 nm was measured on a Spectramax microtiter plate reader (Molecular Devices) with absorbance at 620 nm used as reference wavelength.
- the value for the negative control was subtracted from all samples and a calibration curve prepared by linear regression of the absorbance values plotted vs. FVIII concentration.
- the specific activity was calculated by dividing the activity of the samples with the protein concentration determined by size exclusion HPLC by integrating the light chain peak in the HPLC chromatogram, i.e. the PEG-moiety was not included.
- the data in table 2 demonstrate that the specific chromogenic activity was maintained for the O-glycoPEGylated rFVIII compounds, meaning that Factor VIII activity appear to be retained in the PEGylated variants.
- rFVIII The activity of the O-glycoPEGylated rFVIII was further evaluated in FVIII clotting assay.
- rFVIII samples were diluted in HBS/BSA (20 mM hepes, 150 mM NaCl, pH 7.4 with 1% BSA) to approximately 10 U/ml followed by 10-fold dilution in FVIII-deficient plasma containing VWF (Dade Behring).
- the samples and a calibrated plasma standard HemosIL Calibration Plasma from Instrumentation Laboratory
- the clotting time was measured on an ACL9000 instrument (Instrumentation laboratory) using the single factor program, where samples/standards were mixed with equal volumes of FVIII-deficient plasma with VWF (Dade Behring), calcium and aPTT reagents, and the clotting time measured.
- reagents the following were used: Synthasil (HemosIL, Instrumentation Laboratory), Actin FS (Activated PTT Reagent, Dade Behring) Stago (STA® PTT-A, Stago), and dAPPTin (DAPPTIN®TC, Technoclone).
- the activities of the samples were calculated based on a semi-log plot of clotting time versus concentration of the calibrator.
- the clotting activity ( FIG. 4 ) of the O-glycoPEGyated rFVIII compounds was decreased to various extend depending on the PEG size and the aPTT reagents used.
- Synthasil or dAPPTin as aPTT reagents resulted in a gradual decrease in clotting activity with PEG-size.
- Stago's aPTT reagent a 50% lower specific clotting activity was observed for all three O-glycoPEGylated N8 compounds evaluated.
- Actin FS was used as aPTT reagent a specific clotting activity around 10,000 IU/mg was maintained.
- the data indicates that the aPTT assay is influenced by the presence of a PEG moiety, however, using a selected aPTT reagents e.g. Actin FS the specific clotting activity of rFVIII is not impaired upon O-glycoPEGylation.
- aPTT reagents e.g. Actin FS the specific clotting activity of rFVIII is not impaired upon O-glycoPEGylation.
- the co-factor activity of thrombin-activated rFVIII or PEG-rFVIII was characterized by determining the kinetic parameters of FIXa-catalyzed FX activation in the presence of phospholipids and thrombin-activated rFVIII or PEG-rFVIII.
- FVIIIa activity assay FIXa-cofactor activity assay
- reciprocal titrations of FIXa and FVIIIa against a fixed concentration (0.1 nM) of rFVIIIa or FIXa, respectively were performed to obtain apparent affinity of FIXa for rFVIIIa (K 1/2FIXa ) and functional FVIIIa concentration.
- the Michaelis constant (k m ) and turn-over number (k cat ) of FX activation were obtained from titrations of FX against a fixed concentration of FIXa-FVIIIa complex.
- FIXa-cofactor activity assays was carried out as follows: Thrombin-activated rFVIII and PEG-rFVIII variants were prepared freshly for each test by incubating rFVIII (usually 0.7 nM, 1 U/mL) with 5 nM human ⁇ -thrombin for exactly 30 seconds at 37° C.
- the rate of FX activation was quantified by subsampling the activation reaction above into a prepared mixture of FIXa, phospholipid vesicles (Phospholipid TGT from Rossix [Mölndal, Sweden]), hirudin, Pefabloc Xa and CaCl 2 ; FX activation was initiated by addition of FX and allowed to proceed for either 30 seconds or 60 seconds at 37° C. Activation was stopped by dilution of the FX activation reaction into ice cold buffer containing EDTA. Using a FXa specific chromogenic substrate, the concentration of FXa was quantified by reading absorbance at 405 nM in an ELISA reader.
- a reference curve prepared using purified FXa was used to convert absorbance to FXa concentration.
- the turn-over number of FIXa-rFVIIIa complexes assembled from activated rFVIII or PEG-rFVIII variants was used to convert the rate of FX activation to rFVIIIa concentration.
- the rate of thrombin-catalyzed rFVIII activation was measured by quantifying the initial (0 to 3 min) formation of rFVIIIa in a mixture containing 0.7 nM rFVIII or PEG-rFVIII and 0.13 nM human ⁇ -thrombin. Formation of FVIIIa was linear in time. The rate of FVIIIa activation was expressed as moles rFVIIIa formed per minute per mole of rFVIII initially present (v/[rFVIII] 0 ).
- O-linked glycoPEGylation of rFVIII did not affect the rate of thrombin-catalyzed rFVIII activation or the k m or k cat of FIXa-catalyzed activation of FX in the presence of activated rFVIII (see Table 3). Furthermore, O-linked glycoPEGylation did not affect the apparent K d of rFVIIIa-FIXa interaction (K 1/2FIXa ).
- FIGS. 4A-B show clotting activity of O-glycoPEGylated rFVIII using various aPTT reagents.
- BDD-FVIII BDD-FVIII-10K PEG (O-glycan, 0129-0000-1005), BDD-FVIII-40K PEG (O-glycan, 0129-0000-1003), BDD-FVIII-2 ⁇ 40K PEG (O and N-glycan 0129-0000-1008-1A), BDD-FVIII-80K PEG (N-glycan, 0129-0000-1012, 0-glycan 0129-0000-1009).
- FVIII KO mice Factor VIII knock out mice were bred at Taconic M&B, based on exon 16 KO in C57B1/6 background. A mixture of male and female (app.1:1) with an approximate weight of 25 g and age range of 19-26 weeks were employed. The mice were not fully back-crossed. No FVIII is detected in this mouse strain.
- mice were given single i.v. injections of 280 IU/kg in the tail vein with the compounds listed above. If a mouse was dosed peri-veneously, the mouse was exchanged with another mouse. After dosing, orbital plexus blood samples were collected from pre-dose until 64 hours after dosing using non-coated capillary glass tubes. Three samples were taken from each mouse, and 2, 3 or 4 samples were collected at each time point. Blood was stabilised in sodium citrate (9:1) and diluted in FVIII COA SP buffer (1:4) before centrifugation for 5 minutes at 4000 g. Plasma obtained from diluted blood was frozen at dry ice at kept at ⁇ 80° C. before quantitative analysis by means of FVIII chromogenic activity and/or FVIII antigen analysis.
- the FVIII chromogenic activity was determined by the use of reagents from the Coatest SP kit (Chromogenix). Diluted plasma samples, calibrators (ILS calibration plasma) in Coatest SP-buffer, and buffer negative control (50 ⁇ l) were added to 96-well microtiter plates (Nunc) in duplicates.
- the Factor IXa/Factor X reagent, the phospholipid reagent and CaCl2 from the Coatest SP kit were mixed 5:1:3 (vol:vol:vol) and 75 l of this added to the wells.
- the FVIII antigen assay was a commercial available ELISA kit from Diagnostica Stago (Asserachrom VIII:CAg) using two monoclonal antibodies directed against the light chain of human FVIII. Calibrators (dilutions of the compounds) or plasma samples were diluted at least 50-fold in coatest SP dilution buffer supplied by the kit were applied to the precoated wells and the ELISA performed according to the manufactures instructions. The values used for reporting the pharmacokinetic study are based on the standard curve made from the compounds themselves.
- NCA non-compartmental methods
- GlycoPEGylation of BDD-FVIII increased the T1 ⁇ 2 1.3-2.1 fold as compared to BDD-FVIII after i.v. administration of 280 IU/kg to FVIII KO mice.
- An increasing T1 ⁇ 2 was observed as the size of the PEG group was increased in the range of 10 KDa to 80 KDa PEG.
- mice were anesthetized and placed on a heating pad (37° C.) to maintain body temperature.
- the carotid artery was exposed and a flow-probe (0.5PSB Nanoprobe) that measures blood flow by ultrasound was placed around the artery.
- the injury an iron-mediated chemical oxidation
- the filter paper was removed after 3 min.
- the artery was then washed three times with 0.9% NaCl and finally Surgilube (an acoustic coupler) was applied in order to displace air in the flow-probe and secure an optimised measurement of the blood flow.
- mice treated with 40 KDa-PEG-[O]—N8 occlusion was observed 24 hours after dosing whereas only 67% of the mice treated with Advate occluded. After 72 hours occlusion was still seen in 63% of the mice treated with 40 KDa-PEG-[O]—N8, whereas no occlusion was observed 60 and 72 hours after administration of Advate.
- the FeCl3 induced injury was made 5 min (acute effect), 24, 48, 60, and 72 hours after dosing 280 IU/kg 40 KDa-PEG-[O]—N8, 280 IU/kg Advate, or vehicle.
- the blood flow (ml/min) was recorded for 25 min after removal of FeCl3, and subsequently the time to occlusion was determined.
- Mean and SEM of 6-10 mice per group are shown. Time to occlusion between the different groups was compared using Kruskal-Wallis test including Dunn's post test. * : p ⁇ 0.05; **: p ⁇ 0.01.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Zoology (AREA)
- Epidemiology (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Hematology (AREA)
- Wood Science & Technology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- Microbiology (AREA)
- Toxicology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Immunology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Diabetes (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Other Resins Obtained By Reactions Not Involving Carbon-To-Carbon Unsaturated Bonds (AREA)
Abstract
The present invention relates to B-domain truncated Factor VIII molecules with a modified circulatory half-life, said molecule being covalently conjugated with a hydrophilic polymer. The invention furthermore relates to methods for obtaining such molecules as well as use of such molecules.
Description
- This application is a continuation of U.S. patent application Ser. No. 14/272,726, filed May 8, 2014 (Notice of Allowance Received), which is a continuation of U.S. patent application Ser. No. 12/597,473, filed Mar. 1, 2010 (Abandoned), which is a 35 U.S.C. §371 national stage application of International Patent Application PCT/US2009/035339 (published as WO 2009/108806), filed Feb. 26, 2009; this application further claims priority under 35 U.S.C §119 of U.S. Provisional Application 61/032,006, filed Feb. 27, 2008; the contents of all above-named applications are incorporated herein by reference.
- The present invention relates to conjugated coagulation Factor VIII molecules. In particular, the present invention relates to conjugated Factor VIII molecules having a modified circulatory half-life.
- The instant application contains a Sequence Listing which has been submitted in ASCII format via EFS-Web and is hereby incorporated by reference in its entirety. Said ASCII copy, created on Aug. 19, 2015, is named 7982US02_SeqList_ST25.txt and is 33,925 bytes in size.
- Haemophilia A is an inherited bleeding disorder caused by deficiency or dysfunction of coagulation Factor VIII (FVIII) activity. The clinical manifestation is not on primary haemostasis—formation of the blood clot occurs normally—but the clot is unstable due to a lack of secondary thrombin formation. The disease is treated by intravenous injection of coagulation Factor FVIII which is either isolated from blood or produced recombinantly.
- Current treatment recommendations are moving from traditional on-demand treatment towards prophylaxis. The circulatory half-life of endogenous FVIII is 12-14 hours and prophylactic treatment is thus to be performed several times a week in order to obtain a virtually symptom-free life for the patients. IV administration is for many, especially children and young persons, associated with significant inconvenience and/or pain. There is thus a need in the art for novel Factor VIII products with Factor VIII activity that are preferably homogenous in structure, preferably safe and preferably having a significantly prolonged circulatory half-life in order to reduce the number of Factor VIII administration per week. There is furthermore a need in the art for relatively simple methods for obtaining and producing such molecules.
- PEGylation of Factor VIII in order to prolong circulatory half-life is known in the art. It has however been an obstacle to obtain safe products having a homogenous structure as well as a significantly improved circulatory half-life. The available methods of producing conjugated Factor VIII molecules are often laborious, and/or tend to result in low yields and/or products that are not homogenous in structure. The use of artificially engineered O-linked glycosylation sites for obtaining therapeutic proteins having a prolonged circulatory half-life of therapeutic proteins has been suggested in WO2008011633, however, no conjugated Factor VIII molecules are disclosed therein.
- In a first aspect, the present invention relates to a B domain truncated Factor VIII molecule with a modified circulatory half-life, said molecule being covalently conjugated with a hydrophilic polymer via an O-linked oligosaccharide in the truncated B domain, wherein Factor VIII activation results in removal of the covalently conjugated side group.
- In other aspects, the present invention furthermore relates to methods for obtaining such molecules, use of such molecules and pharmaceutical compositions comprising such molecules.
- What is thus provided is a conjugated Factor VIII molecule with modified circulatory half-life, wherein the conjugated side group (e.g. hydraphilic polymer) is removed upon activation. The molecules according to the invention are preferably homogenous in structure—at least with regard to position of the hydrophilic polymer in the truncated B-domain—and preferably have an advantageous safety profile. Likewise, relatively simple methods for obtaining such molecules are furthermore provided herein. Preferably, activated Factor VIII molecules according to the invention are similar to endogenous activated Factor VIII.
- Factor VIII molecules: FVIII/Factor VIII is a large, complex glycoprotein that primarily is produced by hepatocytes. FVIII consists of 2351 amino acids, including signal peptide, and contains several distinct domains, as defined by homology. There are three A-domains, a unique B-domain, and two C-domains. The domain order can be listed as NH2-A1-A2-B-A3-C1-C2-COOH. FVIII circulates in plasma as two chains, separated at the B-A3 border. The chains are connected by bivalent metal ion-bindings. The A1-A2-B chain is termed the heavy chain (HC) while the A3-C1-C2 is termed the light chain (LC).
- Endogenous Factor VIII molecules circulate in vivo as a pool of molecules with B domains of various sizes. What probably occurs in vivo is a gradual enzymatic removal of the B domain resulting in a pool of molecules with B-domains of various sizes. It is generally believed that cleavage at position 740, by which the last part of the B-domain is removed, occurs in connection with thrombin activation. However, it cannot be ruled out that a Factor VIII variant in which e.g. the cleavage site at position 740 has been impaired may be active.
- “Factor VIII” or “FVIII” as used herein refers to a human plasma glycoprotein that is a member of the intrinsic coagulation pathway and is essential to blood coagulation. “Native FVIII” is the full length human FVIII molecule as shown in SEQ ID NO. 1 (amino acid 1-2332). The B-domain spans amino acids 741-1648 in
SEQ ID NO 1. -
SEQ ID NO 1: ATRRYYLGAVELSWDYMQSDLGELPVDARFPPRVPKSFPFNTSVVYKKTL FVEFTDHLFNIAKPRPPWMGLLGPTIQAEVYDTVVITLKNMASHPVSLHA VGVSYWKASEGAEYDDQTSQREKEDDKVFPGGSHTYVWQVLKENGPMASD PLCLTYSYLSHVDLVKDLNSGLIGALLVCREGSLAKEKTQTLHKFILLFA VFDEGKSWHSETKNSLMQDRDAASARAWPKMHTVNGYVNRSLPGLIGCHR KSVYWHVIGMGTTPEVHSIFLEGHTFLVRNHRQASLEISPITFLTAQTLL MDLGQFLLFCHISSHQHDGMEAYVKVDSCPEEPQLRMKNNEEAEDYDDDL TDSEMDVVRFDDDNSPSFIQIRSVAKKHPKTWVHYIAAEEEDWDYAPLVL APDDRSYKSQYLNNGPQRIGRKYKKVRFMAYTDETFKTREAIQHESGILG PLLYGEVGDTLLIIFKNQASRPYNIYPHGITDVRPLYSRRLPKGVKHLKD FPILPGEIFKYKWTVTVEDGPTKSDPRCLTRYYSSFVNMERDLASGLIGP LLICYKESVDQRGNQIMSDKRNVILFSVFDENRSWYLTENIQRFLPNPAG VQLEDPEFQASNIMHSINGYVFDSLQLSVCLHEVAYWYILSIGAQTDFLS VFFSGYTFKHKMVYEDTLTLFPFSGETVFMSMENPGLWILGCHNSDFRNR GMTALLKVSSCDKNTGDYYEDSYEDISAYLLSKNNAIEPRSFSQNSRHPS TRQKQFNATTIPENDIEKTDPWFAHRTPMPKIQNVSSSDLLMLLRQSPTP HGLSLSDLQEAKYETFSDDPSPGAIDSNNSLSEMTHFRPQLHHSGDMVFT PESGLQLRLNEKLGTTAATELKKLDFKVSSTSNNLISTIPSDNLAAGTDN TSSLGPPSMPVHYDSQLDTTLFGKKSSPLTESGGPLSLSEENNDSKLLES GLMNSQESSWGKNVSSTESGRLFKGKRAHGPALLTKDNALFKVSISLLKT NKTSNNSATNRKTHIDGPSLLIENSPSVWQNILESDTEFKKVTPLIHDRM LMDKNATALRLNHMSNKTTSSKNMEMVQQKKEGPIPPDAQNPDMSFFKML FLPESARWIQRTHGKNSLNSGQGPSPKQLVSLGPEKSVEGQNFLSEKNKV VVGKGEFTKDVGLKEMVFPSSRNLFLTNLDNLHENNTHNQEKKIQEEIEK KETLIQENVVLPQIHTVTGTKNFMKNLFLLSTRQNVEGSYDGAYAPVLQD FRSLNDSTNRTKKHTAHFSKKGEEENLEGLGNQTKQIVEKYACTTRISPN TSQQNFVTQRSKRALKQFRLPLEETELEKRIIVDDTSTQWSKNMKHLTPS TLTQIDYNEKEKGAITQSPLSDCLTRSHSIPQANRSPLPIAKVSSFPSIR PIYLTRVLFQDNSSHLPAASYRKKDSGVQESSHFLQGAKKNNLSLAILTL EMTGDQREVGSLGTSATNSVTYKKVENTVLPKPDLPKTSGKVELLPKVHI YQKDLFPTETSNGSPGHLDLVEGSLLQGTEGAIKWNEANRPGKVPFLRVA TESSAKTPSKLLDPLAWDNHYGTQIPKEEWKSQEKSPEKTAFKKKDTILS LNACESNHAIAAINEGQNKPEIEVTWAKQGRTERLCSQNPPVLKRHQREI TRTTLQSDQEEIDYDDTISVEMKKEDFDIYDEDENQSPRSFQKKTRHYFI AAVERLWDYGMSSSPHVLRNRAQSGSVPQFKKVVFQEFTDGSFTQPLYRG ELNEHLGLLGPYIRAEVEDNIMVTFRNQASRPYSFYSSLISYEEDQRQGA EPRKNFVKPNETKTYFWKVQHHMAPTKDEFDCKAWAYFSDVDLEKDVHSG LIGPLLVCHTNTLNPAHGRQVTVQEFALFFTIFDETKSWYFTENMERNCR APCNIQMEDPTFKENYRFHAINGYIMDTLPGLVMAQDQRIRWYLLSMGSN ENIHSIHFSGHVFTVRKKEEYKMALYNLYPGVFETVEMLPSKAGIWRVEC LIGEHLHAGMSTLFLVYSNKCQTPLGMASGHIRDFQITASGQYGQWAPKL ARLHYSGSINAWSTKEPFSWIKVDLLAPMIIHGIKTQGARQKFSSLYISQ FIIMYSLDGKKWQTYRGNSTGTLMVFFGNVDSSGIKHNIFNPPIIARYIR LHPTHYSIRSTLRMELMGCDLNSCSMPLGMESKAISDAQITASSYFTNMF ATWSPSKARLHLQGRSNAWRPQVNNPKEWLQVDFQKTMKVTGVTTQGVKS LLTSMYVKEFLISSSQDGHQWTLFFQNGKVKVFQGNQDSFTPVVNSLDPP LLTRYLRIHPQSWVHQIALRMEVLGCEAQDLY - The Factor VIII molecules according to the present invention are B domain truncated Factor FVIII molecules wherein the remaining domains correspond to the sequence as set forth in amino acid no 1-740 and 1649-2332 in SEQ ID NO. 1. It follows that molecules according to the invention are recombinant molecules produced in transformed host cells, preferably of mammalian origin. However, the remaining domains (i.e. the three A-domains and the two C-domains) may differ slightly e.g. about 1%, 2%, 3%, 4% or 5% from the amino acid sequence as set forth in SEQ ID NO 1 (amino acids 1-740 and 1649-2332). In particular, it is plausible that amino acid modifications (substitutions, deletions, etc.) are introduced in the remaining domains e.g. in order to modify the binding capacity of Factor VIII with various other components such as e.g. vW factor, LPR, various receptors, other coagulation factors, cell surfaces, etc. Furthermore, it is plausible that the Factor VIII molecules according to the invention comprise other post-translational modifications in e.g. the truncated B-domain and/or in one or more of the other domains of the molecules. These other post-translational modifications may be in the form of various molecules conjugated to the Factor VIII molecule according to the invention such as e.g. polymeric compounds, peptidic compounds, fatty acid derived compounds, etc.
- Factor VIII molecules according to the present invention, regardless of whether they are modified outside the B domain or not, have other posttranslational modifications or not, all have Factor VIII activity, meaning the ability to function in the coagulation cascade in a manner functionally similar or equivalent to FVIII, induce the formation of FXa via interaction with FIXa on an activated platelet, and support the formation of a blood clot. The activity can be assessed in vitro by techniques well known in the art such as e.g. clot analysis, endogenous thrombin potential analysis, etc. Factor VIII molecules according to the present invention have FVIII activity being at least about 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, and 100% or even more than 100% of that of native human FVIII.
- B domain: The B-domain in Factor VIII spans amino acids 741-1648 in
SEQ ID NO 1. The B-domain is cleaved at several different sites, generating large heterogeneity in circulating plasma FVIII molecules. The exact function of the heavily glycosylated B-domain is unknown. What is known is that the domain is dispensable for FVIII activity in the coagulation cascade. This apparent lack of function is supported by the fact that B domain deleted/truncated FVIII appears to have in vivo properties identical to those seen for full length native FVIII. That being said there are indications that the B-domain may reduce the association with the cell membrane, at least under serum free conditions. - B domain truncated/deleted Factor VIII molecule: Endogenous full length FVIII is synthesized as a single-chain precursor molecule. Prior to secretion, the precursor is cleaved into the heavy chain and the light chain. Recombinant B domain-deleted FVIII can be produced from two different strategies. Either the heavy chain without the B-domain and the light chain are synthesized individually as two different polypeptide chains (two-chain strategy) or the B-domain deleted FVIII is synthesized as a single precursor polypeptide chain (single-chain strategy) that is cleaved into the heavy and light chains in the same way as the full-length FVIII precursor.
- In a B domain-deleted FVIII precursor polypeptide, the heavy and light chain moieties are normally separated by a linker. To minimize the risk of introducing immunogenic epitopes in the B domain-deleted FVIII, the sequence of the linker is preferable derived from the FVIII B-domain. The linker must comprise a recognition site for the protease that separates the B domain-deleted FVIII precursor polypeptide into the heavy and light chain. In the B domain of full length FVIII, amino acid 1644-1648 constitutes this recognition site. The thrombin site leading to removal of the linker on activation of B domain-deleted FVIII is located in the heavy chain. Thus, the size and amino acid sequence of the linker is unlikely to influence its removal from the remaining FVIII molecule by thrombin activation. Deletion of the B domain is an advantage for production of FVIII. Nevertheless, parts of the B domain can be included in the linker without reducing the productivity. The negative effect of the B domain on productivity has not been attributed to any specific size or sequence of the B domain.
- The truncated B-domain may contain several O-glycosylation sites. However, according to a preferred embodiment, the molecule comprises only one, alternatively two, three or four O-linked oligosaccharides in the truncated B-domain.
- According to a preferred embodiment, the truncated B domain comprises only one potential O-glycosylation site and the hydrophilic polymer is covalently conjugated to this O-glycosylation site.
- The O-linked oligosaccharides in the B-domain truncated molecules according to the invention may be attached to O-glycosylation sites that were either artificially created by recombinant means and/or by exposure of “hidden” O-glycosylation sites by truncation of the B-domain. In both cases, such molecules may be made by designing a B-domain trunctated Factor VIII amino acid sequence and subsequently subjecting the amino acid sequence to an in silico analysis predicting the probability of O-glycosylation sites in the truncated B-domain. Molecules with a relatively high probability of having such glycosylation sites can be synthesized in a suitable host cell followed by analysis of the glycosylation pattern and subsequent selection of molecules having O-linked glycosylation in the truncated B-domain. Suitable host cells for producing recombinant Factor VIII protein are preferably of mammalian origin in order to ensure that the molecule is glycosylated. In practicing the present invention, the cells are mammalian cells, more preferably an established mammalian cell line, including, without limitation, CHO (e.g., ATCC CCL 61), COS-1 (e.g., ATCC CRL 1650), baby hamster kidney (BHK), and HEK293 (e.g., ATCC CRL 1573; Graham et al., J. Gen. Virol. 36:59-72, 1977) cell lines. A preferred BHK cell line is the tk-ts13 BHK cell line (Waechter and Baserga, Proc.Natl.Acad.Sci.USA 79:1106-1110, 1982), hereinafter referred to as BHK 570 cells. The BHK 570 cell line is available from the American Type Culture Collection, 12301 Parklawn Dr., Rockville, Md. 20852, under ATCC accession number CRL 10314. A tk-ts13 BHK cell line is also available from the ATCC under accession number CRL 1632. A preferred CHO cell line is the CHO K1 cell line available from ATCC under accession number CC161 as well as cell lines CHO-DXB11 and CHO-DG44.
- Other suitable cell lines include, without limitation, Rat Hep I (Rat hepatoma; ATCC CRL 1600), Rat Hep II (Rat hepatoma; ATCC CRL 1548), TCMK (ATCC CCL 139), Human lung (ATCC HB 8065), NCTC 1469 (ATCC CCL 9.1); DUKX cells (CHO cell line) (Urlaub and Chasin, Proc. Natl. Acad. Sci. USA 77:4216-4220, 1980) (DUKX cells also being referred to as DXB11 cells), and DG44 (CHO cell line) (Cell, 33: 405, 1983, and Somatic Cell and Molecular Genetics 12: 555, 1986). Also useful are 3T3 cells, Namalwa cells, myelomas and fusions of myelomas with other cells. In some embodiments, the cells may be mutant or recombinant cells, such as, e.g., cells that express a qualitatively or quantitatively different spectrum of enzymes that catalyze post-translational modification of proteins (e.g., glycosylation enzymes such as glycosyl transferases and/or glycosidases, or processing enzymes such as propeptides) than the cell type from which they were derived. DUKX cells (CHO cell line) are especially preferred.
- Currently preferred cells are HEK293, COS, Chinese Hamster Ovary (CHO) cells, Baby Hamster Kidney (BHK) and myeloma cells, in particular Chinese Hamster Ovary (CHO) cells.
- The inventors of the present invention have thus shown that it is possible to activate “hidden” O-glycosylation sites in the Factor VIII B-domain by truncating the B-domain. While not wishing to be bound by any theory, this phenomenon could be attributable to the tertiary structure of the molecule in the truncated B-domain being altered. “Hidden” O-glycosylation sites are thus “made accessible” to glycosylation in the truncated B-domain. One advantage of this approach is the provision of recombinant molecules with an advantageous safety profile with respect to e.g. allergenicity. Another advantage could be that it may represent a simpler approach of obtaining B-domain truncated variants with an O-linked oligosaccharide in the B-domain due to the inherent abundance of glycosylation sites in the B-domain as it has previously proven difficult to engineer artificial O-glycosylation sites in recombinant proteins.
- The length of the B domain in the wt FVIII molecule is about 907 amino acids. The length of the truncated B domain in molecules according to the present invention may vary from about 10 amino acids to about 700 acids, such as e.g. about 12-500 amino acids, 12-400 amino acids, 12-300 amino acids, 12-200 amino acids, 15-100 amino acids, 15-75 amino acids, 15-50 amino acids, 15-45 amino acids, 20-45 amino acids, 20-40 amino acids, or 20-30 amino acids. The truncated B-domain may comprise fragments of the heavy chain and/or the light chain and/or an artificially introduced sequence that is not found in the wt FVIII molecule. The terms “B-domain truncated” and “B-domain deleted” may be used interchangeably herein.
- Modified circulatory half life: Molecules according to the present invention have a modified circulatory half-life compared to the wild type Factor VIII molecule, preferably an increased circulatory half-life. Circulatory half-life is preferably increased at least 10%, preferably at least 15%, preferably at least 20%, preferably at least 25%, preferably at least 30%, preferably at least 35%, preferably at least 40%, preferably at least 45%, preferably at least 50%, preferably at least 55%, preferably at least 60%, preferably at least 65%, preferably at least 70%, preferably at least 75%, preferably at least 80%, preferably at least 85%, preferably at least 90%, preferably at least 95%, preferably at least 100%, more preferably at least 125%, more preferably at least 150%, more preferably at least 175%, more preferably at least 200%, and most preferably at least 250% or 300%. Even more preferably, such molecules have a circulatory half-life that is increased at least 400%, 500%, 600%, or even 700% relative to the circulatory half-life of the wild type FVIII.
- Hydrophilic polymer: The modifying group/hydrophilic polymer according to the present invention is preferably non-naturally occurring. In one example, the “non-naturally occurring modifying group” is a polymeric modifying group, in which at least one polymeric moiety is non-naturally occurring. In another example, the non-naturally occurring modifying group is a modified carbohydrate. The locus of functionalization with the modifying group is selected such that it does not prevent the “modified sugar” from being added enzymatically to a polypeptide. “Modified sugar” also refers to any glycosyl mimetic moiety that is functionalized with a modifying group and which is a substrate for a natural or modified enzyme, such as a glycosyltransferase.
- The polymeric modifying group added to a polypeptide can alter a property of such polypeptide, for example, its bioavailability, biological activity or its half-life in the body. Exemplary polymers according to the invention include water-soluble polymers that can be linear or branched and can include one or more independently selected polymeric moieties, such as poly(alkylene glycol) and derivatives thereof. The polymeric modifying group according to the invention may include a water-soluble polymer, e.g. poly(ethylene glycol) and derivatives thereof (PEG, m-PEG), poly(propylene glycol) and derivatives thereof (PPG, m-PPG) and the like.
- The term “water-soluble” refers to moieties that have some detectable degree of solubility in water. Methods to detect and/or quantify water solubility are well known in the art. Exemplary water-soluble polymers according to the invention include peptides, saccharides, poly(ethers), poly(amines), poly(carboxylic acids) and the like. Peptides can have mixed sequences and be composed of a single amino acid, e.g., poly(lysine). An exemplary polysaccharide is poly(sialic acid). An exemplary poly(ether) is poly(ethylene glycol), e.g., m-PEG. Poly(ethylene imine) is an exemplary polyamine, and poly(acrylic) acid is a representative poly(carboxylic acid).
- The polymer backbone of the water-soluble polymer according to the invention can be poly(ethylene glycol) (i.e. PEG). The term PEG in connection with the present invention includes poly(ethylene glycol) in any of its forms, including alkoxy PEG, difunctional PEG, multiarmed PEG, forked PEG, branched PEG, pendent PEG (i.e. PEG or related polymers having one or more functional groups pendent to the polymer backbone), or PEG with degradable linkages therein.
- The polymer backbone can be linear or branched. Branched polymer backbones are generally known in the art. Typically, a branched polymer has a central branch core moiety and a plurality of linear polymer chains linked to the central branch core. PEG is commonly used in branched forms that can be prepared by addition of ethylene oxide to various polyols, such as glycerol, pentaerythritol and sorbitol. The central branch moiety can also be derived from several amino acids, such as lysine or cysteine. In one example, the branched poly(ethylene glycol) can be represented in general form as R(-PEG-OH)m in which R represents the core moiety, such as glycerol or pentaerythritol, and m represents the number of arms. Multi-armed PEG molecules, such as those described in U.S. Pat. No. 5,932,462, which is incorporated by reference herein in its entirety, can also be used as the polymer backbone.
-
FIGS. 8A-D show a representative branched PEG polymer of use in embodiments of the invention, referred to herein as “SA-glycerol-PEG.”FIG. 8A shows an exemplary SA-glycerol-PEG component of CMP-SA-glycerol-PEG or of a SA-glycerol-PEG linked to a glycan or an amino acid of a polypeptide.FIG. 8B shows the SA-glycerol-PEG moiety linked to a glycan or polypeptide through a Gal residue.FIG. 8C shows the SA-glycerol-PEG moiety linked to a glycan or polypeptide through a Gal-GalNAc residue.FIG. 8D shows the SA-glycerol-PEG moiety linked to an amino acid of a polypeptide through a Gal-GalNAc moiety. In various embodiments, AA is threonine or serine. In an exemplary embodiment, AA is converted to an O-linked glycosylation site by deletion of the B-domain of the FVIII polypeptide. The discussion regarding the molecular weight of the polymer herein below under this definition is generally applicable to the branched PEG shown inFIG. 8D . InFIGS. 8A-D , the index “n” represents any integer providing a linear (and thus a branched) m-PEG of the desired molecular weight. In various embodiments, “n” is selected such that the linear m-PEG moiety is about 20 KDa to about 40 KDa, for example, about 20 KDa, about 30 KDa or about 40 KDa. Integers corresponding to these m-PEG molecular weights correspond to about 400 (e.g. about 455) to about 900 (e.g. about 910). Accordingly, “n” is selected to provide a branched PEG that is about 40 KDa to about 80 KDa, e.g., about 40 KDa, about 50 KDa, about 60 KDa, about 70 KDa, or about 80 KDa. - Many other polymers are also suitable for the invention. Polymer backbones that are non-peptidic and water-soluble, are particularly useful in the invention. Examples of suitable polymers include, but are not limited to, other poly(alkylene glycols), such as poly(propylene glycol) (“PPG”), copolymers of ethylene glycol and propylene glycol and the like, poly(oxyethylated polyol), poly(olefinic alcohol), poly(vinylpyrrolidone), poly(hydroxypropylmethacrylamide), poly([alpha]-hydroxy acid), poly(vinyl alcohol), polyphosphazene, polyoxazoline, poly(N-acryloylmorpholine), such as described in U.S. Pat. No. 5,629,384, which is incorporated by reference herein in its entirety, as well as copolymers, terpolymers, and mixtures thereof.
- Although the molecular weight of each chain of the polymer backbone can vary, it is typically in the range of from about 100 Da to about 160,000 Da, such as e.g., from about 5,000 Da to about 100,000 Da. More specifically, the size of each conjugated hydrophilic polymer according to the present invention may vary from about 500 Da to about 80,000 Da, such as e.g. about 1000 Da to about 80,000 Da; about 2000 Da to about 70,000 Da; about 5000 to about 70,000 Da; about 5000 to about 60,000 Da; about 10,000 to about 70,000 Da; about 20,000 to about 60,000 Da; about 30,000 to about 60,000 Da; about 30,000 to about 50,000 Da; or about 30,000 to about 40,000 Da. It should be understood that these sizes represent estimates rather than exact measures. According to a preferred embodiment, the molecules according to the invention are conjugated with a heterogeneous population of hydrophilic polymers, such as e.g. PEG of a size of e.g. 10,000, 40,000, or 80,000 Da+/− about 5000, about 4000, about 3000, about 2000, or about 1000 Da.
- O-linked oligosaccharide: Both N-glycans and O-glycans are attached to proteins by the cells producing the protein. The cellular N-glycosylation machinery recognizes and glycosylates N-glycosylation signals (N—X-S/T motifs) in the amino acid chain, as the nascent protein is translocated from the ribosome to the endoplasmic reticulum (Kiely et al. 1976; Glabe et al. 1980).
- Likewise, O-glycans are attached to specific O-glycosylation sites in the amino acid chain, but the motifs triggering O-glycosylation are much more heterogeneous than the N-glycosylation signals, and our ability to predict O-glycosylation sites in amino acid sequences is still inadequate (Julenius et al. 2004). The construction of artificial O-glycosylation sites it is thus associated with some uncertainty. The general assumption is that the native FVIII molecule does not contain any O-glycosylation sites, and the skilled man would therefore expect that at least one artificial O-glycosylation site would have to be constructed and inserted into the B domain in connection with practicing the present invention.
- The O-linked oligosaccharide in a truncated Factor VIII B domain may thus be covalently linked to a naturally occurring O-linked glycosylation sequence or an O-linked glycosylation sequence which has been artificially constructed by recombinant techniques.
- According to a preferred embodiment of the present invention, the O-linked oligosaccharide is linked to a naturally occurring O-linked glycosylation sequence which is not exposed to glycosylation in the wild type Factor VIII molecule but is becoming accessible to O-glycosylation as a consequence of truncation of the B domain. An example thereof is shown in the examples and in SEQ ID NO 2 (the truncated B-domain corresponds to amino acids 742-763). It is plausible that the “hidden” O-glycosylation site in
SEQ ID NO 2 will also become glycosylated even if the B-domain is truncated at a somewhat different place, i.e. if the truncated B domain is somewhat shorter (e.g. 1, 2, 3, 4, or 5 amino acids shorter than SEQ ID NO 2) or longer (such as e.g. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, or 50 amino acids) compared toSEQ ID NO 2. This approach by activating a “hidden” O-glycosylation site by truncation of a B-domain rather than creation of an artificial O-glycosylation site has the advantage of creating a molecule with an advantageous safety profile (i.e. reduced allergenicity, etc.). Other O-glycosylation sites in the Factor VIII B-domain may likewise become activated by truncating the molecules in different ways. - Glyco-PEGylation of O-linked oligosaccharide: The biosynthesis of O-glycans can be modified and terminated with the addition of sialic acid residues relatively early in biosynthesis. Certain sialyltransferase enzymes are capable of acting on GalNAcα-Ser/Thr, or early O-glycan core subtypes after
Core 1 GalT action. The term T antigenis associated with the presence of the Galβ1-3GalNAcα-Ser/Thr disaccharide. Production of these structures involves a competition among glycosyltransferases for the same substrate and thus the expression levels and subcellular distributions of glycosyltransferases within the Golgi apparatus determines the structural outcome in O-glycan biosynthesis and diversification. As illustrated inFIG. 1 , only the Galβ1-3GalNAcα-Ser/Thr disaccharide is amenable for glycoPEGylation. - However, the available amount of this structure may be greatly enhanced through treatment of the protein with sialidase or Corel GalT or a combination thereof. As a result of the glycoPEGylation process the Sialic acid PEG is added to the native structure through an α3 bond to the Galβ1-3GalNAcα-Ser/Thr disaccharide of the target protein (
FIG. 1 ). - Other hydrophilic polymers can also be attached to O-linked oligosaccharides. The basic requirement for enzymatically conjugating other hydrophilic polymers to FVIII via the O-glycan is the ability to couple them to the glycyl-Sialic acid derivative via the free amino group as disclosed in WO03031464. This may be achieved through a large variety of coupling chemistries known to those skilled in the art. Examples of activated biocompatible polymer includes polyalkylene oxides such as without limitation polyethylene glycol (PEG), 2-(methacryloyloxy)ethyl phosphorylcholine (mPC) polymers (as described in WO03062290), dextrans, colominic acids or other carbohydrate based polymers, polymers of amino acids or of specific peptides sequences, biotin derivatives, polyvinyl alcohol (PVA), polycarboxylates, polyvinylpyrrolidone, polyethylene-co-maleic acid anhydride, polystyrene-co-malic acid anhydride, polyoxazoline, poly-acryloylmorpholine, heparin, albumin, celluloses, hydrolysates of chitosan, starches such as hydroxyethyl-starches and hydroxy propyl-starches, glycogen, agaroses and derivatives thereof, guar gum, pullulan, inulin, xanthan gum, carrageenan, pectin, alginic acid hydrolysates, other bio-polymers and any equivalents thereof.
- Pharmaceutical composition: A pharmaceutical composition is herein preferably meant to encompass compositions comprising Factor VIIII molecules according to the present invention suitable for parenteral administration, such as e.g. ready-to-use sterile aqueous compositions or dry sterile compositions that can be reconstituted in e.g. water or an aqueous buffer. The compositions according to the invention may comprise various pharmaceutically acceptable excipients, stabilizers, etc.
- Additional ingredients in such compositions may include wetting agents, emulsifiers, antioxidants, bulking agents, tonicity modifiers, chelating agents, metal ions, oleaginous vehicles, proteins (e.g., human serum albumin, gelatine or proteins) and a zwitterion (e.g., an amino acid such as betaine, taurine, arginine, glycine, lysine and histidine). Such additional ingredients, of course, should not adversely affect the overall stability of the pharmaceutical formulation of the present invention. Parenteral administration may be performed by subcutaneous, intramuscular, intraperitoneal or intravenous injection by means of a syringe, optionally a pen-like syringe. Alternatively, parenteral administration can be performed by means of an infusion pump. A further option is a composition which may be a solution or suspension for the administration of the FVIII compound in the form of a nasal or pulmonal spray. As a still further option, the pharmaceutical compositions containing the FVIII compound of the invention may also be adapted to transdermal administration, e.g. by needle-free injection or from a patch, optionally an iontophoretic patch, or transmucosal, e.g. buccal, administration.
- In a first aspect the present invention thus relates to a B-domain truncated Factor VIII molecule with a modified circulatory half-life, said molecule being covalently conjugated with a hydrophilic polymer via an O-linked oligosaccharide in the truncated B domain, wherein Factor VIII activation (activation of the molecule) results in removal of the covalently conjugated hydrophilic polymer.
- According to one embodiment, the hydrophilic polymer is PEG. The size of the PEG polymer may vary from about 10,000 to about 160,000 Da; such as 10,000 to 80,000 Da, such as e.g. about 10,000; 15,000, 20,000; 25,000; 30,000; 35,000; 40,000; 45,000; 50,000; 55,000; 60,000; 65,000, 70,000; 75,000; or 80,000 Da. Preferably, the O-linked oligosaccharide is attached to an O-glycosylation site that is made by truncation of the B-domain and not by inserting an artificial O-glycosylation site that is not found in the wt FVIII molecule.
- According to a particularly preferred embodiment, the molecule according to the present invention comprises the amino acid sequence as set forth in
SEQ ID NO 2. Such molecules have a unique feature in that the activated FVIII molecule is identical to the native active FVIII molecule. This feature appears to have advantageous properties in safety assessments. - The present invention also relates to pharmaceutical compositions comprising molecules according to the present invention.
- The present invention furthermore relates to a method of obtaining a molecule according to the present invention, wherein said method comprises conjugating a B-domain truncated Factor VIII molecule with a hydrophilic polymer, such as e.g. a PEG group, via an O-linked oligosaccharide in the truncated B domain. It follows that the present invention also relates to molecules obtained by or obtainable by such methods.
- In another aspect, the present invention relates to a method of treatment of a haemophilic disease comprising administering to a patient in need thereof a therapeutically effective amount of a molecule according to the invention.
- The term “treatment”, as used herein, refers to the medical therapy of any human or other animal subject in need thereof. Said subject is expected to have undergone physical examination by a medical practitioner, who has given a tentative or definitive diagnosis which would indicate that the use of said specific treatment is beneficial to the health of said human or other animal subject. The timing and purpose of said treatment may vary from one individual to another, according to the status quo of the subject's health. Thus, said treatment may be prophylactic, palliative, symptomatic and/or curative.
- In yet another aspect, the present invention relates to use of a molecule according to the invention as a medicament as well as use of a molecule according to the invention for manufacture of a medicament for treatment of haemophilia.
- In a final aspect, the present invention relates to a method of engineering a B-domain truncated Factor VIII molecule according to the present invention, said method comprising (i) truncating the B-domain and optionally subjecting the amino acid sequence of this truncated Factor VIII molecule to an analysis identifying potential O-linked glycosylation sites, (ii) producing the molecule in a suitable host cell and (iii) selecting molecules having O-linked glycans in the truncated B-domain.
- In the figures, the size of the conjugated groups is sometimes referred to as “K”, which is herein meant to be equivalent to KDa (kilo Dalton).
-
FIG. 1 : Schematic drawing of glycol PEGylation process of O-linked oligosaccharides. The figure does not represent an exhaustive list of possible ways to arrive at the products obtained in the examples. -
FIG. 2 : Ion-exchange chromatography of the reaction mixture onSource 15Q (A). SDS-PAGE with molecular markers (left) of collected fraction (B). -
FIG. 3 : Purification of the capped product onsuperdex 200 size-exclusion chromatography. -
FIGS. 4A-B : Clotting activity of O-glycoPEGylated rFVIII using various aPTT reagents. Figure A shows the ration between the clotting activity and the chromogenic activity. Figure B shows the specific clotting activity. -
FIG. 5 : In vivo effects (time to occlusion) in FVIII KO mice of 40K-PEG-[O]—N8. -
FIG. 6 : Flow diagram showing the process steps involved in production of glycoPEGylated Factor FVIII according to the invention. -
FIG. 7 : Schematic representation of a Factor VIII molecule according to the present invention produced in the Examples. -
FIGS. 8A-D :FIGS. 8A-D show a representative branched PEG polymer of use in embodiments of the invention, referred to herein as “SA-glycerol-PEG.”FIG. 8A shows an exemplary SA-glycerol-PEG component of CMP-SA-glycerol-PEG or of a SA-glycerol-PEG linked to a glycan or an amino acid of a polypeptide.FIG. 8B shows the SA-glycerol-PEG moiety linked to a glycan or polypeptide through a Gal residue.FIG. 8C shows the SA-glycerol-PEG moiety linked to a glycan or polypeptide through a Gal-GalNAc residue.FIG. 8D shows the SA-glycerol-PEG moiety linked to an amino acid of a polypeptide through a Gal-GalNAc moiety. - An example of the amino acid sequence of a B-domain deleted Factor VIII molecule is given in
SEQ ID NO 2. This polypeptide may also be referred to as “N8”. This molecule comprises a 21 amino acid residue linker sequence (SFSQNSRHPSQNPPVLKRHQR (SEQ ID NO 3)—the underlined S is the Serine residue with the O-glygan that is pegylated in Example 2). - Factor VIII molecules according to the present invention may in the Examples be referred to in various ways—but all references to Factor VIII molecules refer to Factor VIII molecules according to the invention, or alternatively Factor VIII molecules in the process of being converted to Factor VIII molecules according to the invention.
-
SEQ ID NO 2: ATRRYYLGAVELSWDYMQSDLGELPVDARFPPRVPKSFPFNTSVVYKKTL FVEFTDHLFNIAKPRPPWMGLLGPTIQAEVYDTVVITLKNMASHPVSLHA VGVSYWKASEGAEYDDQTSQREKEDDKVFPGGSHTYVWQVLKENGPMASD PLCLTYSYLSHVDLVKDLNSGLIGALLVCREGSLAKEKTQTLHKFILLFA VFDEGKSWHSETKNSLMQDRDAASARAWPKMHTVNGYVNRSLPGLIGCHR KSVYWHVIGMGTTPEVHSIFLEGHTFLVRNHRQASLEISPITFLTAQTLL MDLGQFLLFCHISSHQHDGMEAYVKVDSCPEEPQLRMKNNEEAEDYDDDL TDSEMDVVRFDDDNSPSFIQIRSVAKKHPKTWVHYIAAEEEDWDYAPLVL APDDRSYKSQYLNNGPQRIGRKYKKVRFMAYTDETFKTREAIQHESGILG PLLYGEVGDTLLIIFKNQASRPYNIYPHGITDVRPLYSRRLPKGVKHLKD FPILPGEIFKYKWTVTVEDGPTKSDPRCLTRYYSSFVNMERDLASGLIGP LLICYKESVDQRGNQIMSDKRNVILFSVFDENRSWYLTENIQRFLPNPAG VQLEDPEFQASNIMHSINGYVFDSLQLSVCLHEVAYWYILSIGAQTDFLS VFFSGYTFKHKMVYEDTLTLFPFSGETVFMSMENPGLWILGCHNSDFRNR GMTALLKVSSCDKNTGDYYEDSYEDISAYLLSKNNAIEPRSFSQNSRHPS QNPPVLKRHQREITRTTLQSDQEEIDYDDTISVEMKKEDFDIYDEDENQS PRSFQKKTRHYFIAAVERLWDYGMSSSPHVLRNRAQSGSVPQFKKVVFQE FTDGSFTQPLYRGELNEHLGLLGPYIRAEVEDNIMVTFRNQASRPYSFYS SLISYEEDQRQGAEPRKNFVKPNETKTYFWKVQHHMAPTKDEFDCKAWAY FSDVDLEKDVHSGLIGPLLVCHTNTLNPAHGRQVTVQEFALFFTIFDETK SWYFTENMERNCRAPCNIQMEDPTFKENYRFHAINGYIMDTLPGLVMAQD QRIRWYLLSMGSNENIHSIHFSGHVFTVRKKEEYKMALYNLYPGVFETVE MLPSKAGIWRVECLIGEHLHAGMSTLFLVYSNKCQTPLGMASGHIRDFQI TASGQYGQWAPKLARLHYSGSINAWSTKEPFSWIKVDLLAPMIIHGIKTQ GARQKFSSLYISQFIIMYSLDGKKWQTYRGNSTGTLMVFFGNVDSSGIKH NIFNPPIIARYIRLHPTHYSIRSTLRMELMGCDLNSCSMPLGMESKAISD AQITASSYFTNMFATWSPSKARLHLQGRSNAWRPQVNNPKEWLQVDFQKT MKVTGVTTQGVKSLLTSMYVKEFLISSSQDGHQWTLFFQNGKVKVFQGNQ DSFTPVVNSLDPPLLTRYLRIHPQSWVHQIALRMEVLGCEAQDLY - Using Factor VIII cDNA a mammalian expression plasmid encoding B-domain deleted Factor VIII having an amino acid sequence as set forth in
SEQ ID NO 2 was constructed. The plasmid is encoding Factor VIII heavy chain comprising amino acid 1-740 of full length human Factor VIII and Factor VIII light chain comprising amino acid 1649-2332 of full length human Factor VIII. The heavy and light chain sequences are connected by a 21 amino acid linker with the sequence of amino acid 741-750 and 1638-1648 of full length human Factor VIII. Chinese hamster ovary (CHO) cells were transfected with the BDD Factor VIII coding plasmid and selected with the dihydrofolate reductase system eventually leading to a clonal suspension producer cell cultivated in animal component-free medium. - The first step in the process is the inoculation of a cell vial, from a working cell bank vial, into a chemically defined and animal component free growth medium. Initially after thawing, the cells are incubated in a T-flask. One or two days after thawing, the cells are transferred to a shaker flask, and the culture volume is expanded by successive dilutions in order to keep the cell density between 0.2-3.0×106 cells/ml. The next step is the transfer of the shaker flask culture into seed bioreactors. The culture volume is here further expanded before the final transfer to the production bioreactor. The same chemically defined and animal component free medium is used for all the inoculum expansion steps. After transfer to the production bioreactor, the medium is supplemented with components that increase the product concentration. In the production bioreactor the cells are cultured in a repeated batch process with a cycle time of three days. At harvest, 80-90% of the culture volume is transferred to a harvest tank. The remaining culture fluid is then diluted with fresh medium, in order to obtain the initial cell density, and then a new growth period is initiated.
- The harvest batch is clarified by centrifugation and filtration and transferred to a holding tank before initiation of the purification process. A buffer is added to the cell free harvest in the holding tank to stabilise pH.
- By the end of the production run, cells are collected and frozen down, in order to make an end of production cell bank. This cell bank is tested for mycoplasma, sterility and viral contamination.
- For the isolation of B-domain-deleted Factor VIII from cell culture media, a four step purification procedure was used including a concentration step on a Capto MMC column, an immunoabsorbent chromatography step, an anionic exchange chromatography and finally a gel filtration step. Typically the following procedure was used: 11 litre of sterile filtered medium was pumped onto at column (1.6×12 cm) of Capto MMC (GE Healthcare, Sweden) equilibrated in buffer A: 20 mM imidazole, 10 mM CaCl2, 50 mM NaCl, 0.02
% Tween 80, pH=7.5 at a flow of 15 ml/min. The column was washed with 75 ml of buffer A followed by wash with 75 ml of buffer A containing 1.5 M NaCl. The protein was eluted with 20 mM imidazole, 10 mM CaCl2, 0.02% Tween 80, 2.5 M NaCl, 8 M ethyleneglycol, pH=7.5 at a flow of 1 ml/min. Fractions of 8 ml were collected and assayed for Factor VIII activity (CoA-test). Factor VIII containing fractions were pooled and normally a pool volume of around 50 ml was obtained. - A monoclonal antibody against Factor VIII has been developed (Kjalke Eur J Biochem 234 773). By epitope mapping (results not shown) this antibody, F25, was found to recognise the far C-terminal sequence of the heavy chain from amino acid residue 725 to 740. The F25 antibody was coupled to NHS-activated
Sepharose 4 FF (GE Healthcare, Bio-Sciences AB, Uppsala, Sweden) at a density of 2.4 mg per ml of gel essentially as described by the manufacturer. The pool from the previous step was diluted 10 times with 20 mM imidazole, 10 mM CaCl2, 0.02% Tween 80, pH=7.3 and applied to the F25 Sepharose column (1.6×9.5 cm) equilibrated with 20 mM imidazole, 10 mM CaCl2, 150 mM NaCl, 0.02% Tween 80, 1 M glycerol pH=7.3 at a flow of 0.5 ml/min. The column was washed with equilibration buffer until the UV signal was constant and then with 20 mM imidazole, 10 mM CaCl2, 0.65 M NaCl, pH=7.3 until the UV signal was constant again. Factor VIII was eluted with 20 mM imidazole, 10 mM CaCl2, 0.02% Tween 80, 2.5 M NaCl, 50% ethyleneglycol, pH=7.3 at a flow of 1 ml/min. Fractions of 1 ml were collected and assayed for Factor VIII activity (CoA-test). Factor VIII containing fractions were pooled and normally a pool volume of around 25 ml was obtained. - A buffer A: 20 mM imidazole, 10 mM CaCl2, 0.02
% Tween 80, 1 M glycerol, pH=7.3 and a buffer B: 20 mM imidazole, 10 mM CaCl2, 0.02% Tween 80, 1 M glycerol, 1 M NaCl, pH=7.3 was prepared for the ion-exchange step. A column (1×10 cm) of Macro-Prep 25Q Support (Bio-Rad Laboratories, Hercules, Calif., USA) was equilibrated with 85% buffer A/15% Buffer B at a flow of 2 ml/min. The pool from the previous step was diluted 10 times with buffer A and pumped onto the column with a flow of 2 ml/min. The column was washed with 85% buffer A/15% buffer B at a flow of 2 ml/min and Factor VIII was eluted with a linear gradient from 15% buffer B to 70% buffer B over 120 ml at a flow of 2 ml/min. Fractions of 2 ml were collected and assayed for Factor VIII activity (CoA-test). Factor VIII containing fractions were pooled and normally a pool volume of around 36 ml was obtained. - The pool from the previous step was applied to a
Superdex 200, prep grade (GE Healthcare, Bio-Sciences AB, Uppsala, Sweden) column (2.6×60 cm) equilibrated and eluted at 1 ml/min with 20 mM imidazole, 10 mM CaCl2, 0.02% Tween 80, 1 M glycerol, 150 mM NaCl, pH=7.3. Fractions of 3 ml were collected and assayed for Factor VIII activity (CoA-test). Factor VIII containing fractions were pooled and normally a pool volume of around 57 ml was obtained. The pool containing Factor VIII was store at −80° C. - With the use of the above four-step purification procedure an overall yield of approximately 15% was obtained as judged by CoA activity and ELISA measurements.
- The cell line used for manufacture of N8 is a recombinant Chinese hamster ovary (CHO) cell line stably transfected with expression plasmid #814 F8-500 in pTSV7 consisting of the pTSV7 expression vector with an insert containing cDNA encoding the F8-500 protein. “N8” is herein meant to correspond to a protein having an amino acid sequence as listed in
SEQ ID NO 2. Starting at the N-terminus, the F8-500 protein (N8) consists of the FVIII signal peptide (amino acids −19 to −1) followed by the FVIII heavy chain without the B domain (amino acids 1-740), a 21 amino acid linker (SFSQNSRHPSQNPPVLKRHQR), and the FVIII light chain (amino acids 1649-2332 of wild-type human FVIII). The sequence of the 21 amino acid linker is derived from the FVIII B domain and consists of amino acids 741-750 and 1638-1648 of full length FVIII. - CHO cells were transfected with 814 F8-500 in pTSV7 and selected with the dihydrofolate reductase system eventually leading to a clonal suspension producer cell cultivated in animal component-free medium. A production run is initiated by thawing a working cell bank vial and expanding the cells until transfer to a production bioreactor. The same chemically defined and animal component free medium is used for all the inoculum expansion steps. After transfer to the production bioreactor, the medium is supplemented with components that increase the product concentration. In the production bioreactor the cells are cultured in a repeated batch process with a cycle time of three days. At harvest, 80-90% of the culture volume is transferred to a harvest tank. The remaining culture fluid is then diluted with fresh medium, in order to obtain the initial cell density, and then a new growth period is initiated. The harvest batch is clarified by centrifugation and filtration and transferred to a holding tank before initiation of the purification process. A buffer is added to the cell free harvest in the holding tank to stabilize pH.
- The recombinant Factor VIII molecules obtained in Example 1 are conjugated with polyethylenglycol (PEG) using the following procedure:
- For the glycoPEGylation of the recombinant Factor VIII molecules obtained in Example 1 to be efficient a FVIII concentration >5 mg/ml is preferred. Since FVIII is not normally soluble at the concentration a screening of selected buffer compositions was conducted (see some of these results in table 1).
- Based on these considerations, a buffer containing 50 mM MES, 50 mM CaCl2, 150 mM NaCl, 20% glycerol, pH 6.0 was found to be a suitable reaction buffer.
-
TABLE 1 Evaluation of impact of reaction conditions on FVIII solubility and aggregation. Reaction buffer composition Precipitate % Aggregate 10 mM Histidine, 260 mM Glycine, 1% YES n. d. Sucrose, 10 mM CaCl2 50 mM HEPES, 10 mM CaCl2, 150 mM YES n. d. NaCl, pH 7;50 mM MES, 10 mM CaCl2, 150 mM YES n. d. NaCl, pH 6.0 50 mM MES, 50 mM CaCl2, 150 mM NO 8 NaCl, pH 6.0 50 mM MES, 50 mM CaCl2, 150 mM NO 5 NaCl, 10% glycerol, pH 6.0 50 mM MES, 50 mM CaCl2, 150 mM NO 1.0-1.7 NaCl, 20% glycerol, pH 6.0 - Recombinant FVIII which had been purified as described above was concentrated in reaction buffer either by ion exchange on a
Poros 50 HQ column using step elution, on a Sartorius Vivaspin (PES) filter, 10 kDa cut-off or on anAmicon 10 kDa MWCO PES filter to a concentration of 6-10 mg/mL. The glycoPEGylation of FVIII was initiated by mixing Factor VIII (BDD) (˜4.7 mg/mL final) with Sialidase (A. urifaciens) (159 mU/mL), CMP-SA-glycerol-PEG-40 kDa (5 mol.eq.) and MBP-ST3Gal1 (540 mU) in reaction buffer (50 mM MES, 50 mM CaCl2, 150 mM NaCl, 20% glycerol, 0.5 mM antipain, pH 6.0). The reaction mixture was incubated at 32° C. until a conversion yield of ˜20-30% of total. - Following the incubation the sample was diluted with Buffer A (25 mM Tris, 5 mM CaCl2, 20 mM NaCl, 20% glycerol, pH 7.5) and applied onto a
Source 15Q column (1 cm id×6 cm, 4.7 mL, 1 mL/min, 280 nm). The bound material was washed with Buffer A and eluted using a step gradient with Buffer B (25 mM Tris, 5 mM CaCl2, 1 M NaCl, 20% glycerol, pH 7.5). GlycoPEGylated Factor VIII-(O)-SA-glycerol-PEG-40 kDa was eluted from the column at ˜25% Buffer B.FIG. 2 shows ion-exchange chromatography of the reaction mixture onSource 15Q. - In order to block free galactose moieties which had been exposed on the N-glycans during the sialidase treatment the pooled fraction of Factor VIII-SA-glycerol-PEG-40 kDa (1.0 mg/mL final) was mixed with CMP-SA (2,000 mol eq) and MBP-SBD-ST3Gal3 (400 mU/mL) in
reaction buffer 50 mM MES, 20 mM CaCl2, 150 mM NaCl, 10 mM MnCl2, 20% glycerol, pH 6.0 and incubated at 32° C. for 11 hours. - The resulting capped, glycoPEGylated Factor VIII-SA-glycerol-PEG-40 kDa was separated from CMP-SA and ST3GalIII by gel-filtration on a
Superdex 200 column (10 cm id×300 mm; 280 nm) equilibrated with 50 mM MES, 50 mM CaCl2, 150 mM NaCl, 10% glycerol, pH 6.0; flow rate of 0.25 mL/min. The product Factor VIII-SA-glycerol-PEG-40 kDa elutes at 38 min.FIG. 3 shows purification of the cappedproduct using Superdex 200 size-exclusion chromatography. The peak fraction was collected, aliquoted and subjected to subsequent analysis. - The purpose of the capping procedure is to reduce in vivo clearance of the conjugated Factor VIII molecule.
- The activity of the O-glycoPEGylated rFVIII obtained in Example 2 was evaluated in a chromogenic FVIII assay using Coatest SP reagents (Chromogenix) as follows: rFVIII samples and calibrator (the 7th international FVIII standard from NIBSC) were diluted in Coatest assay buffer (50 mM Tris, 150 mM NaCl, 1% BSA, pH 7.3, with preservative). Fifty μl of samples, standards, and buffer negative control were added to 96-well microtiter plates (Nunc) in duplicates. The Factor IXa/Factor X reagent, the phospholipid reagent and CaCl2 from the Coatest SP kit were mixed 5:1:3 (vol:vol:vol) and 75 μl of this added to the wells. After 15 min incubation at
room temperature 50 μl of the Factor Xa substrate 5-2765/thrombin inhibitor 1-2581 mix was added and the reactions incubated 10 min at room temperature before 25 μl 1 M citric acid,pH 3, was added. The absorbance at 415 nm was measured on a Spectramax microtiter plate reader (Molecular Devices) with absorbance at 620 nm used as reference wavelength. The value for the negative control was subtracted from all samples and a calibration curve prepared by linear regression of the absorbance values plotted vs. FVIII concentration. The specific activity was calculated by dividing the activity of the samples with the protein concentration determined by size exclusion HPLC by integrating the light chain peak in the HPLC chromatogram, i.e. the PEG-moiety was not included. The data in table 2 demonstrate that the specific chromogenic activity was maintained for the O-glycoPEGylated rFVIII compounds, meaning that Factor VIII activity appear to be retained in the PEGylated variants. -
TABLE 2 Specific chromogenic activity of O-glycoPEGylated rFVIII with different PEG group sizes. Specific chromogenic rFVIII compound activity (IU/mg) rFVIII 11819 ± 727 (5) 10 KDa-PEG-[O]-rFVIII Approx 8331 (1) 40 KDa-PEG-[O]-rFVIII 9760 ± 886 (8) 80 KDa-PEG-[O]-rFVIII 12129 ± 2643 (3) Data are mean and standard deviations of the numbers of independent determinations noted in parentheses - The activity of the O-glycoPEGylated rFVIII was further evaluated in FVIII clotting assay. rFVIII samples were diluted in HBS/BSA (20 mM hepes, 150 mM NaCl, pH 7.4 with 1% BSA) to approximately 10 U/ml followed by 10-fold dilution in FVIII-deficient plasma containing VWF (Dade Behring). The samples and a calibrated plasma standard (HemosIL Calibration Plasma from Instrumentation Laboratory) were subsequently diluted in HBS/BSA to four (samples) or six (calibrator) different concentrations. The clotting time was measured on an ACL9000 instrument (Instrumentation laboratory) using the single factor program, where samples/standards were mixed with equal volumes of FVIII-deficient plasma with VWF (Dade Behring), calcium and aPTT reagents, and the clotting time measured. As reagents the following were used: Synthasil (HemosIL, Instrumentation Laboratory), Actin FS (Activated PTT Reagent, Dade Behring) Stago (STA® PTT-A, Stago), and dAPPTin (DAPPTIN®TC, Technoclone). The activities of the samples were calculated based on a semi-log plot of clotting time versus concentration of the calibrator.
- The clotting activity (
FIG. 4 ) of the O-glycoPEGyated rFVIII compounds (control, 10, 40, and 80 kDA PEG, respectively) was decreased to various extend depending on the PEG size and the aPTT reagents used. Using Synthasil or dAPPTin as aPTT reagents resulted in a gradual decrease in clotting activity with PEG-size. With Stago's aPTT reagent, a 50% lower specific clotting activity was observed for all three O-glycoPEGylated N8 compounds evaluated. When Actin FS was used as aPTT reagent a specific clotting activity around 10,000 IU/mg was maintained. The data indicates that the aPTT assay is influenced by the presence of a PEG moiety, however, using a selected aPTT reagents e.g. Actin FS the specific clotting activity of rFVIII is not impaired upon O-glycoPEGylation. - Incorporation of activated FVIII into the FIXa-FVIIIa complex enhances the catalytic efficiency of FIXa-catalyzed FX activation five orders of magnitude (van Dieij en et al. (1981) J Biol Chem 256:3433) and characterization of FIXa-FVIIIa complex assembly and FX activation kinetics is a sensitive measure of the functional integrity of FVIIIa molecules. The co-factor activity of thrombin-activated rFVIII or PEG-rFVIII was characterized by determining the kinetic parameters of FIXa-catalyzed FX activation in the presence of phospholipids and thrombin-activated rFVIII or PEG-rFVIII. Using the FVIIIa activity assay (FIXa-cofactor activity assay), reciprocal titrations of FIXa and FVIIIa against a fixed concentration (0.1 nM) of rFVIIIa or FIXa, respectively, were performed to obtain apparent affinity of FIXa for rFVIIIa (K1/2FIXa) and functional FVIIIa concentration. The Michaelis constant (km) and turn-over number (kcat) of FX activation were obtained from titrations of FX against a fixed concentration of FIXa-FVIIIa complex.
- The FIXa-cofactor activity assays was carried out as follows: Thrombin-activated rFVIII and PEG-rFVIII variants were prepared freshly for each test by incubating rFVIII (usually 0.7 nM, 1 U/mL) with 5 nM human α-thrombin for exactly 30 seconds at 37° C. Subsequently, the rate of FX activation was quantified by subsampling the activation reaction above into a prepared mixture of FIXa, phospholipid vesicles (Phospholipid TGT from Rossix [Mölndal, Sweden]), hirudin, Pefabloc Xa and CaCl2; FX activation was initiated by addition of FX and allowed to proceed for either 30 seconds or 60 seconds at 37° C. Activation was stopped by dilution of the FX activation reaction into ice cold buffer containing EDTA. Using a FXa specific chromogenic substrate, the concentration of FXa was quantified by reading absorbance at 405 nM in an ELISA reader. A reference curve prepared using purified FXa was used to convert absorbance to FXa concentration. The turn-over number of FIXa-rFVIIIa complexes assembled from activated rFVIII or PEG-rFVIII variants was used to convert the rate of FX activation to rFVIIIa concentration.
- The rate of thrombin-catalyzed rFVIII activation was measured by quantifying the initial (0 to 3 min) formation of rFVIIIa in a mixture containing 0.7 nM rFVIII or PEG-rFVIII and 0.13 nM human α-thrombin. Formation of FVIIIa was linear in time. The rate of FVIIIa activation was expressed as moles rFVIIIa formed per minute per mole of rFVIII initially present (v/[rFVIII]0).
- O-linked glycoPEGylation of rFVIII did not affect the rate of thrombin-catalyzed rFVIII activation or the km or kcat of FIXa-catalyzed activation of FX in the presence of activated rFVIII (see Table 3). Furthermore, O-linked glycoPEGylation did not affect the apparent Kd of rFVIIIa-FIXa interaction (K1/2FIXa).
-
FIGS. 4A-B show clotting activity of O-glycoPEGylated rFVIII using various aPTT reagents. - Data are shown as the ratio between the clotting activity and the chromogenic activity (
FIG. 4A ) or as the specific clotting activity (FIG. 4B ). Mean and standard deviations of values from three independent experiments are shown. -
TABLE 3 Rate of rFVIII activation and kinetic constants of FX activation by FIXa Rate of FVIII FX Activation FVIII molecule activation K1/2FIXa Km kcat 10−3 × nM nM s−1 min−1 rFVIII 10.4 ± 1.9 0.88 ± 0.46 7.9 ± 1.7 4.5 ± 1.9 40 K-PEG-[O]-rFVIII 9.9 ± 3.8 0.42 ± 0.02 6.4 ± 0.8 4.7 ± 0.2 80 K-PEG-[O]-rFVIII 9.8 ± 3.4 1.11 ± 0.12 8.2 ± 0.6 3.7 ± 0.4 Data are mean and standard deviations of 3-6 measurements. - The pharmacokinetics of BDD-FVIII glycoPEGylated with various PEG sizes was studied following i.v. administration of 280 IU/kg to FVIII KO mice.
- The following compounds were studied: BDD-FVIII, BDD-FVIII-10K PEG (O-glycan, 0129-0000-1005), BDD-FVIII-40K PEG (O-glycan, 0129-0000-1003), BDD-FVIII-2×40K PEG (O and N-glycan 0129-0000-1008-1A), BDD-FVIII-80K PEG (N-glycan, 0129-0000-1012, 0-glycan 0129-0000-1009).
- Factor VIII knock out (FVIII KO) mice were bred at Taconic M&B, based on exon 16 KO in C57B1/6 background. A mixture of male and female (app.1:1) with an approximate weight of 25 g and age range of 19-26 weeks were employed. The mice were not fully back-crossed. No FVIII is detected in this mouse strain.
- The mice were given single i.v. injections of 280 IU/kg in the tail vein with the compounds listed above. If a mouse was dosed peri-veneously, the mouse was exchanged with another mouse. After dosing, orbital plexus blood samples were collected from pre-dose until 64 hours after dosing using non-coated capillary glass tubes. Three samples were taken from each mouse, and 2, 3 or 4 samples were collected at each time point. Blood was stabilised in sodium citrate (9:1) and diluted in FVIII COA SP buffer (1:4) before centrifugation for 5 minutes at 4000 g. Plasma obtained from diluted blood was frozen at dry ice at kept at −80° C. before quantitative analysis by means of FVIII chromogenic activity and/or FVIII antigen analysis.
- The FVIII chromogenic activity was determined by the use of reagents from the Coatest SP kit (Chromogenix). Diluted plasma samples, calibrators (ILS calibration plasma) in Coatest SP-buffer, and buffer negative control (50 μl) were added to 96-well microtiter plates (Nunc) in duplicates. The Factor IXa/Factor X reagent, the phospholipid reagent and CaCl2 from the Coatest SP kit were mixed 5:1:3 (vol:vol:vol) and 75 l of this added to the wells. After 15 min incubation at room temperature 50 l of the Factor Xa substrate 5-2765/thrombin inhibitor 1-2581 mix was added and the reactions incubated 10 min at room temperature before 25
μl 2% citric acid was added. The absorbance at 405 nm was measured on a Spectramax microtiter plate reader (Molecular Devices). FVIII activity in the plasma samples was calculated from the calibration curve made by dilutions of the calibrated international plasma standard (ILS). - The FVIII antigen assay was a commercial available ELISA kit from Diagnostica Stago (Asserachrom VIII:CAg) using two monoclonal antibodies directed against the light chain of human FVIII. Calibrators (dilutions of the compounds) or plasma samples were diluted at least 50-fold in coatest SP dilution buffer supplied by the kit were applied to the precoated wells and the ELISA performed according to the manufactures instructions. The values used for reporting the pharmacokinetic study are based on the standard curve made from the compounds themselves.
- Pharmacokinetic analysis was carried out by non-compartmental methods (NCA) of data using ILS as calibrator (data based on chromogenic activity), using the compounds themselves as calibrator (data based on ELISA). From the data the following parameters were estimated: Cmax (maximum concentration, after i.v. administration this is at the first sampling time point), Tmax (time of maximum concentration, after i.v. administration this is the first time point), AUCO-∞ (area under the curve from
time 0 to infinity), T½, (terminal half-live), CL (clearance) and Vss (volume of distribution at steady state). All calculations were performed using WinNonlin Pro version 4.1. - After i.v. injection of 280 IU/Kg BDD-FVIII, BDD-FVIII-10 KDa PEG, BDD-FVIII-40 KDa PEG, BDD-FVIII-2×40 KDa PEG and BDD-FVIII-80 KDa PEG to FVIII KO mice, the half-life increased along with increasing PEG size in the range of 7.8 h (BDD-FVIII) to 15-16 h (Table 4), which corresponds to a 2-fold increase. Similarly, the clearance was reduced and the MRT increased with increasing PEG sizes (Table 4).
-
TABLE 4 Pharmacokinetic parameters estimates of FVIII glycoPEGylated with different sizes of PEG after i.v. administration to FVIII KO mice based on chromogenic activity (BDD: B-domain deleted). CL Prolon- Dose T½ (ml/h/ MRT gation Compound (IU/kg) (h) kg) (h) (fold) BDD-FVIII 280 6.7-9.3 8.1-10 9.9-11 1 BDD- FVIII 10 KDa PEG280 10 8.5 16 1.3 (O-glycan) BDD-FVIII-2x40 KDa PEG 280 13 5.8 19 1.9-2.1 BDD- FVIII 40 KDa PEG(O-glycan) 280 15-16 3.6-3.8 20-22 1.7 BDD- FVIII 80 KDa PEG280 15 6.4 21 1.9 (O-glycan) - GlycoPEGylation of BDD-FVIII increased the T½ 1.3-2.1 fold as compared to BDD-FVIII after i.v. administration of 280 IU/kg to FVIII KO mice. An increasing T½ was observed as the size of the PEG group was increased in the range of 10 KDa to 80 KDa PEG.
- The duration of action of 40K-PEG-[O]—N8 vs. recombinant FVIII (Advate) was investigated in a FeCl3 induced injury model in haemophilia A (F8-KO) mice.
- Mice were anesthetized and placed on a heating pad (37° C.) to maintain body temperature. The carotid artery was exposed and a flow-probe (0.5PSB Nanoprobe) that measures blood flow by ultrasound was placed around the artery. The injury (an iron-mediated chemical oxidation) was induced by applying a filter paper (2×5 mm) briefly soaked in a 10% FeCl3 solution around the exposed carotid artery. The filter paper was removed after 3 min. The artery was then washed three times with 0.9% NaCl and finally Surgilube (an acoustic coupler) was applied in order to displace air in the flow-probe and secure an optimised measurement of the blood flow. Blood flow (ml/min) was recorded for 25 min after removing the FeCl3 saturated filter paper and the time to occlusion was determined by measuring the time (in min) from removal of FeCl3 saturated filter paper until the blood flow was 0 ml/min. If occlusion did not occur after 25 min the occlusion time was reported as 25 min even though no occlusion occurred during the observation period. F8-KO mice (n=6-10) were treated with Advate (280 U/kg), 40K-PEG-[O]—N8 (280 U/kg), or vehicle. The FeCl3 induced injury was made 5 min (acute effect) or 24, 48, 60, and 72 hours after dosing. The blood flow (ml/min) was recorded for 25 min after removal of FeCl3, and subsequently the time to occlusion was determined.
- No occlusion occurred in vehicle treated F8-KO mice, whereas occlusion occurred in all mice treated with 40 KDa-PEG-[O]—N8 and
Advate 5 min after dosing (acute effect) with a mean occlusion time of 4.3±0.4 min and 5.2±0.7 min, respectively. In 40 KDa-PEG-[O]—N8 treated F8-KO mice the average occlusion time increased to 13.8±3.4 min at 72 hours after dosing. In contrast the Advate treated F8-KO mice had an occlusion time of 13.0±3.4 min and 15.9±2 9 min after 24 and 48 hours, respectively. Importantly no occlusions were observed 60 and 72 hours after administration of Advate. In all mice treated with 40 KDa-PEG-[O]—N8 occlusion was observed 24 hours after dosing whereas only 67% of the mice treated with Advate occluded. After 72 hours occlusion was still seen in 63% of the mice treated with 40 KDa-PEG-[O]—N8, whereas no occlusion was observed 60 and 72 hours after administration of Advate. - The FeCl3 induced injury was made 5 min (acute effect), 24, 48, 60, and 72 hours after dosing 280 IU/
kg 40 KDa-PEG-[O]—N8, 280 IU/kg Advate, or vehicle. The blood flow (ml/min) was recorded for 25 min after removal of FeCl3, and subsequently the time to occlusion was determined. At 60 and 72 hours after dosing no occlusion occurred in mice dosed with Advate. Mean and SEM of 6-10 mice per group are shown. Time to occlusion between the different groups was compared using Kruskal-Wallis test including Dunn's post test. * : p<0.05; **: p<0.01. - In conclusion, the haemostatic effect of 40 KDa-PEG-[O]—N8 is significantly prolonged compared to Advate in a FeCl3 induced injury model in F8-KO mice.
Claims (13)
1. A Factor VIII molecule with a modified circulatory half-life comprising a FVIII molecule with a truncated B-domain consisting of amino acids 741-760 of SEQ ID N0:2, wherein the FVIII molecule is covalently conjugated to a hydrophilic polymer via an O-linked oligosaccharide on a serine residue corresponding to position 750 of SEP ID N0:2.
2. The FVIII molecule of claim 1 made by a process comprising: a) transfecting a mammalian host cell with a vector encoding the FVIII molecule of claim 1 ; b) culturing the host cell of step (a) under conditions suitable to express the FVIII molecule in the host cell; c) harvesting the FVIII molecule from the host cell culture of step (b); and d) covalently conjugating the FVIII molecule with the hydrophilic polymer via an O-linked oligosaccharide on the serine residue corresponding to position 750 of SEQ ID N0:2.
3. The FVIII molecule of claim 1 , wherein the hydrophilic polymer is a polysaccharide.
4. The FVIII molecule of claim 1 , wherein the hydrophilic polymer is heparin.
5. The FVIII molecule of claim 2 , wherein the mammalian host cell is a CHO cell.
6. A pharmaceutical composition comprising the FVIII molecule of claim 1 .
7. A pharmaceutical composition comprising the FVIII molecule of claim 2 .
8. A method of treating a haemophilic disease comprising administering to a patient in need thereof a therapeutically effective amount of the pharmaceutical composition of claim 6 .
9. A method of treating a haemophilic disease comprising administering to a patient in need thereof a therapeutically effective amount of the pharmaceutical composition of claim 7 .
10. A method according to claim 8 , wherein the pharmaceutical composition is administered subcutaneously.
11. A method according to claim 9 , wherein the pharmaceutical composition is administered intravenously.
12. The FVIII molecule of claim 2 , wherein the hydrophilic polymer is a polysaccharide.
13. The FVIII molecule of claim 2 , wherein the hydrophilic polymer is heparin.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/831,515 US20160222086A1 (en) | 2008-02-27 | 2015-08-20 | Conjugated Factor VIII Molecules |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US3200608P | 2008-02-27 | 2008-02-27 | |
| PCT/US2009/035339 WO2009108806A1 (en) | 2008-02-27 | 2009-02-26 | Conjugated factor viii molecules |
| US59747310A | 2010-03-01 | 2010-03-01 | |
| US14/272,726 US9150848B2 (en) | 2008-02-27 | 2014-05-08 | Conjugated factor VIII molecules |
| US14/831,515 US20160222086A1 (en) | 2008-02-27 | 2015-08-20 | Conjugated Factor VIII Molecules |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/272,726 Continuation US9150848B2 (en) | 2008-02-27 | 2014-05-08 | Conjugated factor VIII molecules |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20160222086A1 true US20160222086A1 (en) | 2016-08-04 |
Family
ID=42634664
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/597,473 Abandoned US20130189239A1 (en) | 2008-02-27 | 2009-02-26 | Conjugated Factor VIII Molecules |
| US13/759,261 Active US8536126B2 (en) | 2008-02-27 | 2013-02-05 | Conjugated factor VIII molecules |
| US14/272,726 Active US9150848B2 (en) | 2008-02-27 | 2014-05-08 | Conjugated factor VIII molecules |
| US14/831,515 Abandoned US20160222086A1 (en) | 2008-02-27 | 2015-08-20 | Conjugated Factor VIII Molecules |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/597,473 Abandoned US20130189239A1 (en) | 2008-02-27 | 2009-02-26 | Conjugated Factor VIII Molecules |
| US13/759,261 Active US8536126B2 (en) | 2008-02-27 | 2013-02-05 | Conjugated factor VIII molecules |
| US14/272,726 Active US9150848B2 (en) | 2008-02-27 | 2014-05-08 | Conjugated factor VIII molecules |
Country Status (16)
| Country | Link |
|---|---|
| US (4) | US20130189239A1 (en) |
| EP (3) | EP2257311B1 (en) |
| JP (3) | JP5619630B2 (en) |
| KR (1) | KR101582841B1 (en) |
| CN (3) | CN103497246B (en) |
| AU (1) | AU2009219232B2 (en) |
| CA (1) | CA2715465C (en) |
| DK (1) | DK2257311T3 (en) |
| ES (1) | ES2476690T3 (en) |
| IL (1) | IL207188A (en) |
| MX (1) | MX2010009154A (en) |
| PL (1) | PL2257311T3 (en) |
| RU (1) | RU2573587C2 (en) |
| TW (1) | TWI425953B (en) |
| WO (1) | WO2009108806A1 (en) |
| ZA (1) | ZA201005556B (en) |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8637007B2 (en) | 2006-12-15 | 2014-01-28 | Baxter International Inc. | Factor VIIa-polysialic acid conjugate having prolonged in vivo half-life |
| EP2417155B1 (en) * | 2009-04-06 | 2013-06-19 | Novo Nordisk A/S | Targeted delivery of factor viii proteins to platelets |
| US8642737B2 (en) | 2010-07-26 | 2014-02-04 | Baxter International Inc. | Nucleophilic catalysts for oxime linkage |
| US8809501B2 (en) | 2009-07-27 | 2014-08-19 | Baxter International Inc. | Nucleophilic catalysts for oxime linkage |
| JP5908401B2 (en) | 2009-07-27 | 2016-04-26 | バクスター・インターナショナル・インコーポレイテッドBaxter International Incorp0Rated | Blood coagulation protein complex |
| EP3093029A1 (en) | 2009-07-27 | 2016-11-16 | Baxalta GmbH | Blood coagulation protein conjugates |
| RU2533619C2 (en) | 2009-07-27 | 2014-11-20 | Лайпоксен Текнолоджиз Лимитед | Glycopolysialylation of proteins, which are not blood clotting proteins |
| PL3326643T3 (en) * | 2009-12-06 | 2021-10-25 | Bioverativ Therapeutics Inc. | Factor viii-fc chimeric and hybrid polypeptides, and methods of use thereof |
| CN105153313A (en) * | 2010-02-16 | 2015-12-16 | 诺沃—诺迪斯克有限公司 | Factor viii fusion protein |
| JP5914363B2 (en) * | 2010-02-16 | 2016-05-11 | ノヴォ ノルディスク アー/エス | Factor VIII molecule with reduced VWF binding |
| US9234192B2 (en) | 2010-02-16 | 2016-01-12 | Novo Nordisk A/S | Conjugated proteins |
| US20130012684A1 (en) | 2010-02-16 | 2013-01-10 | Novo Nordisk A/S | Purification Method |
| US20130183280A1 (en) * | 2010-07-15 | 2013-07-18 | Novo Nordisk A/S | Stabilized factor viii variants |
| WO2012035050A2 (en) | 2010-09-15 | 2012-03-22 | Novo Nordisk A/S | Factor viii variants having a decreased cellular uptake |
| US9062115B2 (en) | 2010-09-22 | 2015-06-23 | Novo Nordisk A/S | Therapeutic factor VIII antibodies |
| US20130345403A1 (en) | 2010-12-16 | 2013-12-26 | Novo Nordisk A/S | Aqueous factor viii solution |
| DK2654794T3 (en) | 2010-12-22 | 2020-06-08 | Baxalta GmbH | MATERIALS AND PROCEDURES FOR CONJUGING A WATER SOLUBLE FAT ACID DERIVATIVE TO A PROTEIN |
| US20130337537A1 (en) | 2011-03-02 | 2013-12-19 | Novo Nordisk A/S | Coagulation factor-targeting to tlt-1 on activated platelets |
| JP6292718B2 (en) | 2011-07-01 | 2018-03-14 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | Method for separating monomeric polypeptides from aggregated polypeptides |
| BR112014006684A2 (en) | 2011-09-23 | 2017-03-28 | Novo Nordisk As | glucagon analogs |
| ES2600081T3 (en) * | 2011-10-18 | 2017-02-07 | Csl Behring Gmbh | Use of sulfated glycosaminoglycans to improve the bioavailability of Factor VIII |
| CA2851057A1 (en) | 2011-10-18 | 2013-04-25 | Csl Behring Gmbh | Combined use of a sulfated glycosaminoglycan and a hyaluronidase for improving the bioavailability of factor viii |
| GEP201706716B (en) | 2012-04-16 | 2017-08-10 | Cantab Biopharmaceuticals Patents Ltd | Optimised subcutaneous therapeutic agents |
| CN104411716B (en) * | 2012-04-24 | 2018-09-07 | 诺和诺德股份有限公司 | Compounds suitable for treatment of haemophilia |
| EP2841091A1 (en) * | 2012-04-24 | 2015-03-04 | Novo Nordisk A/S | Pharmaceutical composition suitable for treatment of haemophilia |
| SI2986313T1 (en) | 2013-04-18 | 2019-09-30 | Novo Nordisk A/S | Stable, protracted glp-1/glucagon receptor co-agonists for medical use |
| AR101060A1 (en) * | 2014-02-12 | 2016-11-23 | Novo Nordisk As | FVIII CONJUGATES |
| US10570184B2 (en) | 2014-06-04 | 2020-02-25 | Novo Nordisk A/S | GLP-1/glucagon receptor co-agonists for medical use |
| EP3177317B1 (en) | 2014-08-04 | 2020-03-18 | CSL Limited | Factor viii formulation |
| CN107406493B (en) | 2015-03-06 | 2021-08-13 | 康诺贝林伦瑙有限公司 | Modified von willebrand factor with improved half-life |
| WO2016198521A1 (en) * | 2015-06-10 | 2016-12-15 | Novo Nordisk A/S | Fviii fusion proteins |
| BR112018008319A2 (en) | 2015-11-05 | 2018-10-30 | Novo Nordisk A/S | fviii formulation |
| JP2019510022A (en) * | 2015-12-03 | 2019-04-11 | バクスアルタ インコーポレイテッド | Factor VIII with extended half-life and reduced ligand binding |
| CN110177804A (en) * | 2016-11-16 | 2019-08-27 | 拜尔健康护理有限责任公司 | The Factor IX of red blood cell targeting and the method for using it |
| CN114072420B (en) | 2019-07-04 | 2024-06-11 | 康诺贝林伦瑙有限公司 | Truncated Von Willebrand Factor (VWF) for increasing the in vitro stability of coagulation factor VIII |
| US20220348637A1 (en) | 2019-11-11 | 2022-11-03 | CSL Behring Lengnau AG | Polypeptides for inducing tolerance to factor viii |
Family Cites Families (406)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1479268A (en) | 1973-07-05 | 1977-07-13 | Beecham Group Ltd | Pharmaceutical compositions |
| US4179337A (en) | 1973-07-20 | 1979-12-18 | Davis Frank F | Non-immunogenic polypeptides |
| GB1451798A (en) | 1973-08-02 | 1976-10-06 | Ici Ltd | Prostanoic acid derivatives |
| CH596313A5 (en) | 1975-05-30 | 1978-03-15 | Battelle Memorial Institute | |
| US4385260A (en) | 1975-09-09 | 1983-05-24 | Beckman Instruments, Inc. | Bargraph display |
| US4414147A (en) | 1981-04-17 | 1983-11-08 | Massachusetts Institute Of Technology | Methods of decreasing the hydrophobicity of fibroblast and other interferons |
| JPS57206622A (en) | 1981-06-10 | 1982-12-18 | Ajinomoto Co Inc | Blood substitute |
| US4451566A (en) | 1981-12-04 | 1984-05-29 | Spencer Donald B | Methods and apparatus for enzymatically producing ethanol |
| US4438253A (en) | 1982-11-12 | 1984-03-20 | American Cyanamid Company | Poly(glycolic acid)/poly(alkylene glycol) block copolymers and method of manufacturing the same |
| EP0263533A3 (en) | 1983-03-01 | 1990-05-30 | C.R.C. Compagnia di Ricerca Chimica S.p.A. | A method for preparing the cytidine monophosphate of 5-acetamido-3,5-dideoxy-d-glycero-d-galactononulosaminic acid |
| DE3308806A1 (en) | 1983-03-12 | 1984-09-13 | Basf Ag, 6700 Ludwigshafen | FUNGICIDAL AGENTS, SUBSTITUTED GLUCOPYRANOSYLAMINE AND METHOD FOR CONTROLLING FUNGI |
| JPS59172425A (en) | 1983-03-18 | 1984-09-29 | Nippon Chemiphar Co Ltd | Novel blood coagulation factor derivative, its preparation and blood coagulation promoting agent containing the same |
| US4496689A (en) | 1983-12-27 | 1985-01-29 | Miles Laboratories, Inc. | Covalently attached complex of alpha-1-proteinase inhibitor with a water soluble polymer |
| US4565653A (en) | 1984-03-30 | 1986-01-21 | Pfizer Inc. | Acyltripeptide immunostimulants |
| US4879236A (en) | 1984-05-16 | 1989-11-07 | The Texas A&M University System | Method for producing a recombinant baculovirus expression vector |
| US4970300A (en) | 1985-02-01 | 1990-11-13 | New York University | Modified factor VIII |
| US4675414A (en) | 1985-03-08 | 1987-06-23 | The United States Of America As Represented By The Secretary Of The Navy | Maleimidomethyl-carbonate polyethers |
| GR860984B (en) | 1985-04-17 | 1986-08-18 | Zymogenetics Inc | Expression of factor vii and ix activities in mammalian cells |
| JP2524586B2 (en) | 1985-06-26 | 1996-08-14 | シタス コーポレイション | Solubilization of proteins for pharmaceutical compositions utilizing polymer conjugation |
| US5206344A (en) | 1985-06-26 | 1993-04-27 | Cetus Oncology Corporation | Interleukin-2 muteins and polymer conjugation thereof |
| JPS6238172A (en) | 1985-08-12 | 1987-02-19 | 株式会社 高研 | Production of anti-thrombotic medical material |
| SE451849B (en) | 1985-12-11 | 1987-11-02 | Svenska Sockerfabriks Ab | VIEW TO SYNTHETIZE GYCLOSIDIC BINDINGS AND USE OF THIS RECEIVED PRODUCTS |
| IT1213029B (en) | 1986-01-30 | 1989-12-07 | Bracco Ind Chimica Spa | PARAMAGNETIC METAL ION CHELATES. |
| US4767702A (en) | 1986-02-06 | 1988-08-30 | Cohenford Menashi A | Paper strip assay for neisseria species |
| US5272066A (en) | 1986-03-07 | 1993-12-21 | Massachusetts Institute Of Technology | Synthetic method for enhancing glycoprotein stability |
| WO1987005330A1 (en) | 1986-03-07 | 1987-09-11 | Michel Louis Eugene Bergh | Method for enhancing glycoprotein stability |
| US4925796A (en) | 1986-03-07 | 1990-05-15 | Massachusetts Institute Of Technology | Method for enhancing glycoprotein stability |
| US4902505A (en) | 1986-07-30 | 1990-02-20 | Alkermes | Chimeric peptides for neuropeptide delivery through the blood-brain barrier |
| IL82834A (en) | 1987-06-09 | 1990-11-05 | Yissum Res Dev Co | Biodegradable polymeric materials based on polyether glycols,processes for the preparation thereof and surgical artiicles made therefrom |
| US5153265A (en) | 1988-01-20 | 1992-10-06 | Cetus Corporation | Conjugation of polymer to colony stimulating factor-1 |
| US4847325A (en) | 1988-01-20 | 1989-07-11 | Cetus Corporation | Conjugation of polymer to colony stimulating factor-1 |
| GB8810808D0 (en) | 1988-05-06 | 1988-06-08 | Wellcome Found | Vectors |
| US5169933A (en) | 1988-08-15 | 1992-12-08 | Neorx Corporation | Covalently-linked complexes and methods for enhanced cytotoxicity and imaging |
| US5874261A (en) | 1988-09-02 | 1999-02-23 | The Trustees Of The University Of Pennsylvania | Method for the purification of glycosyltransferases |
| US5218092A (en) | 1988-09-29 | 1993-06-08 | Kyowa Hakko Kogyo Co., Ltd. | Modified granulocyte-colony stimulating factor polypeptide with added carbohydrate chains |
| JPH0276894U (en) | 1988-11-30 | 1990-06-13 | ||
| US5104651A (en) | 1988-12-16 | 1992-04-14 | Amgen Inc. | Stabilized hydrophobic protein formulations of g-csf |
| US5047335A (en) | 1988-12-21 | 1991-09-10 | The Regents Of The University Of Calif. | Process for controlling intracellular glycosylation of proteins |
| US6166183A (en) | 1992-11-30 | 2000-12-26 | Kirin-Amgen, Inc. | Chemically-modified G-CSF |
| EP0401384B1 (en) | 1988-12-22 | 1996-03-13 | Kirin-Amgen, Inc. | Chemically modified granulocyte colony stimulating factor |
| EP0853121B1 (en) | 1988-12-23 | 2007-03-28 | Genentech, Inc. | Human DNase |
| AU634517B2 (en) | 1989-01-19 | 1993-02-25 | Pharmacia & Upjohn Company | Somatotropin analogs |
| WO1990008823A1 (en) | 1989-01-31 | 1990-08-09 | The Upjohn Company | Somatotropin analogs |
| US5194376A (en) | 1989-02-28 | 1993-03-16 | University Of Ottawa | Baculovirus expression system capable of producing foreign gene proteins at high levels |
| US5059535A (en) | 1989-04-12 | 1991-10-22 | Chembiomed, Ltd. | Process for the separation and purification of sialyl transferases |
| US5324844A (en) | 1989-04-19 | 1994-06-28 | Enzon, Inc. | Active carbonates of polyalkylene oxides for modification of polypeptides |
| US5122614A (en) | 1989-04-19 | 1992-06-16 | Enzon, Inc. | Active carbonates of polyalkylene oxides for modification of polypeptides |
| ES2247656T3 (en) | 1989-04-19 | 2006-03-01 | Enzon, Inc. | A PROCESS TO FORM A MODIFIED POLYPEPTIDE THAT INCLUDES A POLYPEPTIDE AND A POLYCHYLENE OXIDE. |
| US5166322A (en) | 1989-04-21 | 1992-11-24 | Genetics Institute | Cysteine added variants of interleukin-3 and chemical modifications thereof |
| US5342940A (en) | 1989-05-27 | 1994-08-30 | Sumitomo Pharmaceuticals Company, Limited | Polyethylene glycol derivatives, process for preparing the same |
| US5672683A (en) | 1989-09-07 | 1997-09-30 | Alkermes, Inc. | Transferrin neuropharmaceutical agent fusion protein |
| US5182107A (en) | 1989-09-07 | 1993-01-26 | Alkermes, Inc. | Transferrin receptor specific antibody-neuropharmaceutical or diagnostic agent conjugates |
| US5154924A (en) | 1989-09-07 | 1992-10-13 | Alkermes, Inc. | Transferrin receptor specific antibody-neuropharmaceutical agent conjugates |
| US5527527A (en) | 1989-09-07 | 1996-06-18 | Alkermes, Inc. | Transferrin receptor specific antibody-neuropharmaceutical agent conjugates |
| US5977307A (en) | 1989-09-07 | 1999-11-02 | Alkermes, Inc. | Transferrin receptor specific ligand-neuropharmaceutical agent fusion proteins |
| US5032519A (en) | 1989-10-24 | 1991-07-16 | The Regents Of The Univ. Of California | Method for producing secretable glycosyltransferases and other Golgi processing enzymes |
| US5312808A (en) | 1989-11-22 | 1994-05-17 | Enzon, Inc. | Fractionation of polyalkylene oxide-conjugated hemoglobin solutions |
| IL96477A0 (en) | 1989-12-01 | 1991-08-16 | Amgen Inc | Megakaryocyte production |
| SE465222C5 (en) | 1989-12-15 | 1998-02-10 | Pharmacia & Upjohn Ab | A recombinant human factor VIII derivative and process for its preparation |
| US5595900A (en) | 1990-02-14 | 1997-01-21 | The Regents Of The University Of Michigan | Methods and products for the synthesis of oligosaccharide structures on glycoproteins, glycolipids, or as free molecules, and for the isolation of cloned genetic sequences that determine these structures |
| US5324663A (en) | 1990-02-14 | 1994-06-28 | The Regents Of The University Of Michigan | Methods and products for the synthesis of oligosaccharide structures on glycoproteins, glycolipids, or as free molecules, and for the isolation of cloned genetic sequences that determine these structures |
| DE4009630C2 (en) | 1990-03-26 | 1995-09-28 | Reinhard Prof Dr Dr Brossmer | CMP-activated fluorescent sialic acids and processes for their preparation |
| EP0481038B1 (en) | 1990-04-16 | 2002-10-02 | The Trustees Of The University Of Pennsylvania | Saccharide compositions, methods and apparatus for their synthesis |
| US5583042A (en) | 1990-04-16 | 1996-12-10 | Neose Pharmaceuticals, Inc. | Apparatus for the synthesis of saccharide compositions |
| GB9107846D0 (en) | 1990-04-30 | 1991-05-29 | Ici Plc | Polypeptides |
| US5951972A (en) | 1990-05-04 | 1999-09-14 | American Cyanamid Company | Stabilization of somatotropins and other proteins by modification of cysteine residues |
| US5399345A (en) | 1990-05-08 | 1995-03-21 | Boehringer Mannheim, Gmbh | Muteins of the granulocyte colony stimulating factor |
| US5219564A (en) | 1990-07-06 | 1993-06-15 | Enzon, Inc. | Poly(alkylene oxide) amino acid copolymers and drug carriers and charged copolymers based thereon |
| CU22302A1 (en) | 1990-09-07 | 1995-01-31 | Cigb | Codifying nucleotidic sequence for a protein of the external membrane of neisseria meningitidis and the use of that protein in preparing vaccines. |
| JPH06502987A (en) | 1990-07-10 | 1994-04-07 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | O-glycosylated IFN-alpha |
| DE4028800A1 (en) | 1990-09-11 | 1992-03-12 | Behringwerke Ag | GENETIC SIALYLATION OF GLYCOPROTEINS |
| US5529914A (en) | 1990-10-15 | 1996-06-25 | The Board Of Regents The Univeristy Of Texas System | Gels for encapsulation of biological materials |
| US5410016A (en) | 1990-10-15 | 1995-04-25 | Board Of Regents, The University Of Texas System | Photopolymerizable biodegradable hydrogels as tissue contacting materials and controlled-release carriers |
| US5492821A (en) | 1990-11-14 | 1996-02-20 | Cargill, Inc. | Stabilized polyacrylic saccharide protein conjugates |
| US5164374A (en) | 1990-12-17 | 1992-11-17 | Monsanto Company | Use of oligosaccharides for treatment of arthritis |
| US5861374A (en) | 1991-02-28 | 1999-01-19 | Novo Nordisk A/S | Modified Factor VII |
| CA2103546C (en) | 1991-02-28 | 2002-10-01 | Kathleen L. Berkner | Modified factor vii |
| US5833982A (en) | 1991-02-28 | 1998-11-10 | Zymogenetics, Inc. | Modified factor VII |
| US5788965A (en) | 1991-02-28 | 1998-08-04 | Novo Nordisk A/S | Modified factor VII |
| US5278299A (en) | 1991-03-18 | 1994-01-11 | Scripps Clinic And Research Foundation | Method and composition for synthesizing sialylated glycosyl compounds |
| WO1992016555A1 (en) | 1991-03-18 | 1992-10-01 | Enzon, Inc. | Hydrazine containing conjugates of polypeptides and glycopolypeptides with polymers |
| AU668505B2 (en) | 1991-03-18 | 1996-05-09 | Scripps Research Institute, The | Oligosaccharide enzyme substrates and inhibitors: methods and compositions |
| US5212075A (en) | 1991-04-15 | 1993-05-18 | The Regents Of The University Of California | Compositions and methods for introducing effectors to pathogens and cells |
| WO1992021029A1 (en) | 1991-05-10 | 1992-11-26 | Genentech, Inc. | Selecting ligand agonists and antagonists |
| SE9201544L (en) | 1991-05-31 | 1992-12-01 | Ciba Geigy Ag | MAKE SUBSTANTIAL GYCOSYL TRANSFER PHASES |
| GB2256197B (en) | 1991-05-31 | 1995-11-22 | Ciba Geigy Ag | Yeast as host for expression of heterologous glycosyl transferase enzymes |
| US5352670A (en) | 1991-06-10 | 1994-10-04 | Alberta Research Council | Methods for the enzymatic synthesis of alpha-sialylated oligosaccharide glycosides |
| US5374655A (en) | 1991-06-10 | 1994-12-20 | Alberta Research Council | Methods for the synthesis of monofucosylated oligosaccharides terminating in di-N-acetyllactosaminyl structures |
| KR950014915B1 (en) | 1991-06-19 | 1995-12-18 | 주식회사녹십자 | Asialoglycoprotein-conjugated compounds |
| US5281698A (en) | 1991-07-23 | 1994-01-25 | Cetus Oncology Corporation | Preparation of an activated polymer ester for protein conjugation |
| EP0863154A1 (en) | 1991-10-12 | 1998-09-09 | The Regents Of The University Of California | Use of thiol redox proteins for reducing protein intramolecular disulfide bonds, for improving the quality of cereal products, dough and baked goods |
| US6319695B1 (en) | 1991-10-15 | 2001-11-20 | The Scripps Research Insitute | Production of fucosylated carbohydrates by enzymatic fucosylation synthesis of sugar nucleotides; and in situ regeneration of GDP-fucose |
| WO1993008842A1 (en) | 1991-11-08 | 1993-05-13 | Somatogen, Inc. | Hemoglobins as drug delivery agents |
| US5384249A (en) | 1991-12-17 | 1995-01-24 | Kyowa Hakko Kogyo Co., Ltd. | α2→3 sialyltransferase |
| IT1260468B (en) | 1992-01-29 | 1996-04-09 | METHOD FOR MAINTAINING THE ACTIVITY OF PROTEOLYTIC ENZYMES MODIFIED WITH POLYETHYLENGLYCOL | |
| US5858751A (en) | 1992-03-09 | 1999-01-12 | The Regents Of The University Of California | Compositions and methods for producing sialyltransferases |
| US5962294A (en) | 1992-03-09 | 1999-10-05 | The Regents Of The University Of California | Compositions and methods for the identification and synthesis of sialyltransferases |
| WO1993018157A1 (en) | 1992-03-09 | 1993-09-16 | The Regents Of The University Of California | Compositions and methods for the identification and synthesis of sialyltransferases |
| WO1993018787A1 (en) | 1992-03-25 | 1993-09-30 | New York University | Trans-sialidase and methods of use and making thereof |
| US6037452A (en) | 1992-04-10 | 2000-03-14 | Alpha Therapeutic Corporation | Poly(alkylene oxide)-Factor VIII or Factor IX conjugate |
| JPH0686684A (en) | 1992-05-26 | 1994-03-29 | Monsanto Co | Synthesis of sialo conjugation |
| US5614184A (en) | 1992-07-28 | 1997-03-25 | New England Deaconess Hospital | Recombinant human erythropoietin mutants and therapeutic methods employing them |
| JPH08503125A (en) | 1992-08-07 | 1996-04-09 | プロジェニクス・ファーマスーティカルス・インコーポレーテッド | CD4-gamma2 and CD4-IgG2 immunoconjugates complexed with non-peptidyl components and uses thereof |
| WO1994004193A1 (en) | 1992-08-21 | 1994-03-03 | Enzon, Inc. | Novel attachment of polyalkylene oxides to bio-effecting substances |
| JP3979678B2 (en) | 1992-08-24 | 2007-09-19 | サントリー株式会社 | Novel glycosyltransferase, gene encoding the same, and method for producing the enzyme |
| AU5098193A (en) | 1992-09-01 | 1994-03-29 | Berlex Laboratories, Inc. | Glycolation of glycosylated macromolecules |
| US5308460A (en) | 1992-10-30 | 1994-05-03 | Glyko, Incorporated | Rapid synthesis and analysis of carbohydrates |
| US6361977B1 (en) | 1992-11-24 | 2002-03-26 | S. Christopher Bauer | Methods of using multivariant IL-3 hematopoiesis fusion protein |
| JP2845698B2 (en) | 1992-11-25 | 1999-01-13 | オルガノ株式会社 | Method and apparatus for measuring anions contained in condensate in condensate circulation system |
| NZ250375A (en) | 1992-12-09 | 1995-07-26 | Ortho Pharma Corp | Peg hydrazone and peg oxime linkage forming reagents and protein derivatives |
| NO934477L (en) | 1992-12-09 | 1994-06-10 | Ortho Pharma Corp | PEG hydrazone and PEG oxime-binding reagents and protein derivatives thereof |
| CA2110543A1 (en) | 1992-12-09 | 1994-06-10 | David E. Wright | Peg hydrazone and peg oxime linkage forming reagents and protein derivatives thereof |
| US5298643A (en) | 1992-12-22 | 1994-03-29 | Enzon, Inc. | Aryl imidate activated polyalkylene oxides |
| WO1994015625A1 (en) | 1993-01-15 | 1994-07-21 | Enzon, Inc. | Factor viii - polymeric conjugates |
| US5349001A (en) | 1993-01-19 | 1994-09-20 | Enzon, Inc. | Cyclic imide thione activated polyalkylene oxides |
| US5321095A (en) | 1993-02-02 | 1994-06-14 | Enzon, Inc. | Azlactone activated polyalkylene oxides |
| US5202413A (en) | 1993-02-16 | 1993-04-13 | E. I. Du Pont De Nemours And Company | Alternating (ABA)N polylactide block copolymers |
| US6180134B1 (en) | 1993-03-23 | 2001-01-30 | Sequus Pharmaceuticals, Inc. | Enhanced ciruclation effector composition and method |
| SG49117A1 (en) | 1993-03-29 | 1998-05-18 | Kyowa Hakko Kogyo Kk | Alfa -1, 3-fucosyltransferase |
| US5409817A (en) | 1993-05-04 | 1995-04-25 | Cytel, Inc. | Use of trans-sialidase and sialyltransferase for synthesis of sialylα2→3βgalactosides |
| US5374541A (en) | 1993-05-04 | 1994-12-20 | The Scripps Research Institute | Combined use of β-galactosidase and sialyltransferase coupled with in situ regeneration of CMP-sialic acid for one pot synthesis of oligosaccharides |
| CA2162478A1 (en) | 1993-05-14 | 1994-11-24 | Shawn A. Defrees | Sialyl lex analogues as inhibitors of cellular adhesion |
| AU6663294A (en) | 1993-05-14 | 1994-12-12 | Upjohn Company, The | Cloned dna encoding a udp-galnac:polypeptide,n-acetylgalactos aminyltransferase |
| EP0699075B1 (en) | 1993-05-21 | 2001-12-12 | Zymogenetics, Inc. | MODIFIED FACTOR VII for inhibiting vascular restenosis and platelet deposition |
| WO1994028024A1 (en) | 1993-06-01 | 1994-12-08 | Enzon, Inc. | Carbohydrate-modified polymer conjugates with erythropoietic activity |
| US5621039A (en) | 1993-06-08 | 1997-04-15 | Hallahan; Terrence W. | Factor IX- polymeric conjugates |
| DE4325317C2 (en) | 1993-07-29 | 1998-05-20 | Univ Dresden Tech | Process for the radioactive labeling of immunoglobulins |
| CN1057534C (en) | 1993-08-17 | 2000-10-18 | 柯瑞英-艾格公司 | Erythropoietin analogs |
| JPH0770195A (en) | 1993-08-23 | 1995-03-14 | Yutaka Mizushima | Sugar-modified interferon |
| US6485930B1 (en) | 1993-09-15 | 2002-11-26 | The Scripps Research Institute | Mannosyl transfer with regeneration of GDP-mannose |
| EP0775711B1 (en) | 1993-09-22 | 2003-03-05 | Ajinomoto Co., Inc. | Peptide having antithrombotic activity and process for producing the same |
| US5874075A (en) | 1993-10-06 | 1999-02-23 | Amgen Inc. | Stable protein: phospholipid compositions and methods |
| US5919455A (en) | 1993-10-27 | 1999-07-06 | Enzon, Inc. | Non-antigenic branched polymer conjugates |
| US5643575A (en) | 1993-10-27 | 1997-07-01 | Enzon, Inc. | Non-antigenic branched polymer conjugates |
| US5446090A (en) | 1993-11-12 | 1995-08-29 | Shearwater Polymers, Inc. | Isolatable, water soluble, and hydrolytically stable active sulfones of poly(ethylene glycol) and related polymers for modification of surfaces and molecules |
| US5443953A (en) | 1993-12-08 | 1995-08-22 | Immunomedics, Inc. | Preparation and use of immunoconjugates |
| US5369017A (en) | 1994-02-04 | 1994-11-29 | The Scripps Research Institute | Process for solid phase glycopeptide synthesis |
| JP3516272B2 (en) | 1994-02-10 | 2004-04-05 | 株式会社成和化成 | Cosmetic base material |
| US5605793A (en) | 1994-02-17 | 1997-02-25 | Affymax Technologies N.V. | Methods for in vitro recombination |
| US5837458A (en) | 1994-02-17 | 1998-11-17 | Maxygen, Inc. | Methods and compositions for cellular and metabolic engineering |
| US5492841A (en) | 1994-02-18 | 1996-02-20 | E. I. Du Pont De Nemours And Company | Quaternary ammonium immunogenic conjugates and immunoassay reagents |
| AU2118195A (en) | 1994-03-07 | 1995-09-25 | Dendritech Inc. | Bioactive and/or targeted dendrimer conjugates |
| IL113010A (en) * | 1994-03-31 | 1999-10-28 | Pharmacia & Upjohn Ab | Pharmaceutical formulation comprising factor viii with an activity of at least 500iu/ml and an enhancer for improved subcutaneous intramuscular or intradermal administration |
| US5432059A (en) | 1994-04-01 | 1995-07-11 | Specialty Laboratories, Inc. | Assay for glycosylation deficiency disorders |
| US5646113A (en) | 1994-04-07 | 1997-07-08 | Genentech, Inc. | Treatment of partial growth hormone insensitivity syndrome |
| US5629384A (en) | 1994-05-17 | 1997-05-13 | Consiglio Nazionale Delle Ricerche | Polymers of N-acryloylmorpholine activated at one end and conjugates with bioactive materials and surfaces |
| US5545553A (en) | 1994-09-26 | 1996-08-13 | The Rockefeller University | Glycosyltransferases for biosynthesis of oligosaccharides, and genes encoding them |
| WO1996010089A1 (en) | 1994-09-29 | 1996-04-04 | Ajinomoto Co., Inc. | Modification of peptide and protein |
| US5824784A (en) | 1994-10-12 | 1998-10-20 | Amgen Inc. | N-terminally chemically modified protein compositions and methods |
| US5834251A (en) | 1994-12-30 | 1998-11-10 | Alko Group Ltd. | Methods of modifying carbohydrate moieties |
| US5932462A (en) | 1995-01-10 | 1999-08-03 | Shearwater Polymers, Inc. | Multiarmed, monofunctional, polymer for coupling to molecules and surfaces |
| IL116730A0 (en) | 1995-01-13 | 1996-05-14 | Amgen Inc | Chemically modified interferon |
| EP0820520B1 (en) | 1995-04-11 | 2002-08-14 | Neose Technologies, Inc. | Improved enzymatic synthesis of oligosaccharides |
| US5922577A (en) | 1995-04-11 | 1999-07-13 | Cytel Corporation | Enzymatic synthesis of glycosidic linkages |
| US5728554A (en) | 1995-04-11 | 1998-03-17 | Cytel Corporation | Enzymatic synthesis of glycosidic linkages |
| US5876980A (en) | 1995-04-11 | 1999-03-02 | Cytel Corporation | Enzymatic synthesis of oligosaccharides |
| US6030815A (en) | 1995-04-11 | 2000-02-29 | Neose Technologies, Inc. | Enzymatic synthesis of oligosaccharides |
| US5695760A (en) | 1995-04-24 | 1997-12-09 | Boehringer Inglehiem Pharmaceuticals, Inc. | Modified anti-ICAM-1 antibodies and their use in the treatment of inflammation |
| CA2227326A1 (en) | 1995-05-15 | 1996-11-21 | Philip Dehazya | Carbohydrate-mediated coupling of peptides to immunoglobulins |
| US6015555A (en) | 1995-05-19 | 2000-01-18 | Alkermes, Inc. | Transferrin receptor specific antibody-neuropharmaceutical or diagnostic agent conjugates |
| US5824864A (en) | 1995-05-25 | 1998-10-20 | Pioneer Hi-Bred International, Inc. | Maize gene and protein for insect control |
| US5858752A (en) | 1995-06-07 | 1999-01-12 | The General Hospital Corporation | Fucosyltransferase genes and uses thereof |
| AU6255096A (en) | 1995-06-07 | 1996-12-30 | Mount Sinai School Of Medicine Of The City University Of New York, The | Pegylated modified proteins |
| US6127153A (en) | 1995-06-07 | 2000-10-03 | Neose Technologies, Inc. | Method of transferring at least two saccharide units with a polyglycosyltransferase, a polyglycosyltransferase and gene encoding a polyglycosyltransferase |
| US6251864B1 (en) | 1995-06-07 | 2001-06-26 | Glaxo Group Limited | Peptides and compounds that bind to a receptor |
| US5672662A (en) | 1995-07-07 | 1997-09-30 | Shearwater Polymers, Inc. | Poly(ethylene glycol) and related polymers monosubstituted with propionic or butanoic acids and functional derivatives thereof for biotechnical applications |
| AU6597996A (en) | 1995-07-27 | 1997-02-26 | Cytec Technology Corp. | Synthetic cationic polymers as promoters for ASA sizing |
| US5770420A (en) | 1995-09-08 | 1998-06-23 | The Regents Of The University Of Michigan | Methods and products for the synthesis of oligosaccharide structures on glycoproteins, glycolipids, or as free molecules, and for the isolation of cloned genetic sequences that determine these structures |
| WO1997011178A1 (en) | 1995-09-21 | 1997-03-27 | Genentech, Inc. | Human growth hormone variants |
| SE9503380D0 (en) | 1995-09-29 | 1995-09-29 | Pharmacia Ab | Protein derivatives |
| US5716812A (en) | 1995-12-12 | 1998-02-10 | The University Of British Columbia | Methods and compositions for synthesis of oligosaccharides, and the products formed thereby |
| WO1997021822A2 (en) | 1995-12-12 | 1997-06-19 | The University Of British Columbia | Methods and compositions for synthesis of oligosaccharides using mutant glycosidase enzymes |
| CA2165041C (en) | 1995-12-12 | 2005-07-05 | The University Of British Columbia | Methods and compositions for synthesis of oligosaccharides, and the products formed thereby |
| JP3065925B2 (en) | 1996-01-30 | 2000-07-17 | 日清製油株式会社 | Active oxygen species scavenger and anti-fading agent |
| ATE380820T1 (en) | 1996-03-08 | 2007-12-15 | Univ Michigan | MURINE ALPHA(1,3)-FUCOSYLTRANSFERASE (FUC-TVII) |
| CA2262994A1 (en) | 1996-08-02 | 1998-02-12 | Ortho-Mcneil Pharmaceutical, Inc. | Polypeptides having a single covalently bound n-terminal water-soluble polymer |
| US6495365B1 (en) | 1996-08-13 | 2002-12-17 | Fujisawa Pharmaceutical Co., Ltd. | Hematopoietic stem cell proliferating agents |
| US20020064546A1 (en) | 1996-09-13 | 2002-05-30 | J. Milton Harris | Degradable poly(ethylene glycol) hydrogels with controlled half-life and precursors therefor |
| JP2001502005A (en) | 1996-10-10 | 2001-02-13 | サイテル コーポレイション | Purification of carbohydrates using ultrafiltration, reverse osmosis and nanofiltration |
| EP0964690B1 (en) | 1996-10-15 | 2003-07-09 | The Liposome Company, Inc. | Peptide-lipid conjugates, liposomes and liposomal drug delivery |
| NZ335628A (en) | 1996-11-08 | 2000-10-27 | Neose Technologies Inc | Improved expression vectors comprising a nucleic acid construct with tac and gal bacterial promoters |
| JP2001507215A (en) | 1997-01-16 | 2001-06-05 | サイテル コーポレイション | Practical in vitro sialylation of recombinant glycoproteins |
| ATE200030T1 (en) | 1997-01-29 | 2001-04-15 | Polymasc Pharmaceuticals Plc | PEGYLATION PROCEDURE |
| DE19709787A1 (en) | 1997-03-11 | 1998-09-17 | Bayer Ag | Oligosaccaride and their derivatives as well as a chemo-enzymatic process for their production |
| US5945314A (en) | 1997-03-31 | 1999-08-31 | Abbott Laboratories | Process for synthesizing oligosaccharides |
| AU7266698A (en) | 1997-04-30 | 1998-11-24 | Enzon, Inc. | Polyalkylene oxide-modified single chain polypeptides |
| JPH10307356A (en) | 1997-05-08 | 1998-11-17 | Konica Corp | Silver halide emulsion and silver halide photographic sensitive material using the same |
| US6183738B1 (en) | 1997-05-12 | 2001-02-06 | Phoenix Pharamacologics, Inc. | Modified arginine deiminase |
| US6075134A (en) | 1997-05-15 | 2000-06-13 | The Regents Of The University Of California | Glycoconjugates and methods |
| WO1998055630A2 (en) | 1997-06-06 | 1998-12-10 | The Governors Of The University Of Alberta | Alpha-1,3-fucosyltransferase of helicobacter pylori |
| PT994903E (en) | 1997-06-24 | 2005-10-31 | Genentech Inc | METHODS AND COMPOSITIONS FOR GALACTOSILED GLICOPROTEINS |
| AU8269898A (en) | 1997-06-27 | 1999-01-19 | Regents Of The University Of California, The | Drug targeting of a peptide radiopharmaceutical through the primate blood-brain barrier in vivo with a monoclonal antibody to the human insulin receptor |
| US20030027257A1 (en) | 1997-08-21 | 2003-02-06 | University Technologies International, Inc. | Sequences for improving the efficiency of secretion of non-secreted protein from mammalian and insect cells |
| WO1999013063A1 (en) | 1997-09-09 | 1999-03-18 | Nycomed Imaging As | Factor vii fragments and analogs thereof and their use in the treatment of blood clottng disorders |
| DE69840412D1 (en) | 1997-10-31 | 2009-02-12 | Genentech Inc | METHODS AND COMPOSITIONS CONTAINING GLYCOPROTEIN GLYCOR FORMS |
| CA2312843A1 (en) | 1997-12-01 | 1999-06-10 | Neose Technologies Inc. | Enzymatic synthesis of gangliosides |
| EP0924298A1 (en) | 1997-12-18 | 1999-06-23 | Stichting Instituut voor Dierhouderij en Diergezondheid (ID-DLO) | Protein expression in baculovirus vector expression systems |
| PT1053019E (en) | 1998-01-07 | 2004-04-30 | Debio Rech Pharma Sa | POLY (ETHYLENE-GLYCOL) ACETYLATERAL DEGRADAVAS AND GELS AND CONJUGATES DERIVED FROM THE SAME |
| AU2559799A (en) | 1998-01-22 | 1999-08-09 | Genentech Inc. | Antibody fragment-polymer conjugates and humanized anti-il-8 monoclonal antibodies and uses of same |
| US6362254B2 (en) | 1998-03-12 | 2002-03-26 | Shearwater Corporation | Poly(ethylene glycol) derivatives with proximal reactive groups |
| JP2002507577A (en) | 1998-03-25 | 2002-03-12 | スローン−ケッタリング インスティトュート フォア キャンサー リサーチ | Trimeric antigenic O-linked glycoconjugate, production method thereof and use thereof |
| ES2434961T5 (en) | 1998-04-20 | 2018-01-18 | Roche Glycart Ag | Antibody glycosylation engineering to improve antibody-dependent cell cytotoxicity |
| ES2309618T3 (en) | 1998-04-28 | 2008-12-16 | Laboratoires Serono Sa | CONJUGATES OF LHRH-PEG ANALOGS. |
| US20030166525A1 (en) | 1998-07-23 | 2003-09-04 | Hoffmann James Arthur | FSH Formulation |
| PT1656952E (en) | 1998-10-16 | 2014-02-21 | Biogen Idec Inc | Polyalkylene glycol conjugates of interferon beta-1a and uses thereof |
| US7304150B1 (en) | 1998-10-23 | 2007-12-04 | Amgen Inc. | Methods and compositions for the prevention and treatment of anemia |
| US6660843B1 (en) * | 1998-10-23 | 2003-12-09 | Amgen Inc. | Modified peptides as therapeutic agents |
| CN1325443A (en) | 1998-10-30 | 2001-12-05 | 诺沃奇梅兹有限公司 | Glycosylated proteins having reduced allergenicity |
| DE69939549D1 (en) | 1998-11-13 | 2008-10-23 | Henrick Clausen | UDP-GALACTOSE: BETA-I (N) -ACETYL-GLUCOSAMINE BETA1,3 GALACTOSYL TRANSFERASES, BETA3GAL-T5 |
| DE19852729A1 (en) | 1998-11-16 | 2000-05-18 | Werner Reutter | Recombinant glycoproteins, processes for their preparation, medicaments containing them and their use |
| AU773845B2 (en) | 1998-11-18 | 2004-06-10 | Neose Technologies, Inc. | Low cost manufacture of oligosaccharides |
| US6465220B1 (en) | 1998-12-21 | 2002-10-15 | Glycozym Aps | Glycosylation using GalNac-T4 transferase |
| AU2618500A (en) | 1999-01-29 | 2000-08-18 | Amgen, Inc. | Gcsf conjugates |
| US6503744B1 (en) | 1999-02-01 | 2003-01-07 | National Research Council Of Canada | Campylobacter glycosyltransferases for biosynthesis of gangliosides and ganglioside mimics |
| US6949372B2 (en) | 1999-03-02 | 2005-09-27 | The Johns Hopkins University | Engineering intracellular sialylation pathways |
| CA2370469A1 (en) | 1999-04-22 | 2000-11-02 | Astrazeneca Ab | Assay for detecting phospho-n-acetylmuramyl-pentapeptide translocase activity |
| JO2291B1 (en) | 1999-07-02 | 2005-09-12 | اف . هوفمان لاروش ايه جي | Erythopintin derivatives |
| US6261805B1 (en) | 1999-07-15 | 2001-07-17 | Boyce Thompson Institute For Plant Research, Inc. | Sialyiation of N-linked glycoproteins in the baculovirus expression vector system |
| AU6357900A (en) | 1999-07-20 | 2001-02-05 | Amgen, Inc. | Hyaluronic acid-protein conjugates, pharmaceutical compositions and related methods |
| US6642038B1 (en) | 1999-09-14 | 2003-11-04 | Genzyme Glycobiology Research Institute, Inc. | GlcNAc phosphotransferase of the lysosomal targeting pathway |
| US6716626B1 (en) | 1999-11-18 | 2004-04-06 | Chiron Corporation | Human FGF-21 nucleic acids |
| AU1804201A (en) | 1999-12-02 | 2001-06-12 | Zymogenetics Inc. | Methods for targeting cells that express fibroblast growth receptor-3 or-2 |
| US6348558B1 (en) | 1999-12-10 | 2002-02-19 | Shearwater Corporation | Hydrolytically degradable polymers and hydrogels made therefrom |
| MXPA02006215A (en) | 1999-12-22 | 2003-10-15 | Nektar Therapeutics Al Corp | Method for the preparation of 1benzotriazolyl carbonate esters of poly(ethylene glycol). |
| EP1270642B1 (en) | 1999-12-24 | 2011-08-31 | Kyowa Hakko Kirin Co., Ltd. | Branched polyalkylene glycols |
| IL150314A0 (en) | 1999-12-30 | 2002-12-01 | Maxygen Aps | Improved lysosomal enzymes and lysosomal enzyme activators |
| US6555660B2 (en) | 2000-01-10 | 2003-04-29 | Maxygen Holdings Ltd. | G-CSF conjugates |
| CZ20022727A3 (en) | 2000-01-10 | 2002-11-13 | Maxygen Holdings Ltd | Polypeptide conjugate, process of its preparation and pharmaceutical preparation |
| US6646110B2 (en) | 2000-01-10 | 2003-11-11 | Maxygen Holdings Ltd. | G-CSF polypeptides and conjugates |
| JP2003521930A (en) | 2000-02-11 | 2003-07-22 | マキシゲン・エイピーエス | Factor VII or Factor VIIa-like molecule |
| AU2001231531A1 (en) | 2000-02-11 | 2001-08-20 | Maxygen Aps | Follicle stimulating hormones |
| AU2001232337A1 (en) | 2000-02-18 | 2001-08-27 | Kanagawa Academy Of Science And Technology | Pharmaceutical composition, reagent and method for intracerebral delivery of pharmaceutically active ingredient or labeling substance |
| US20010041683A1 (en) | 2000-03-09 | 2001-11-15 | Schmitz Harold H. | Cocoa sphingolipids, cocoa extracts containing sphingolipids and methods of making and using same |
| DE60120650T2 (en) | 2000-03-16 | 2007-05-31 | The Regents Of The University Of California, Oakland | CHEMOSELECTIVE ANNOUNCEMENT BY USING A PHOSPHINE |
| US6586398B1 (en) | 2000-04-07 | 2003-07-01 | Amgen, Inc. | Chemically modified novel erythropoietin stimulating protein compositions and methods |
| JP4455802B2 (en) | 2000-05-03 | 2010-04-21 | ノボ ノルディスク ヘルス ケア アクチェンゲゼルシャフト | Human VII coagulation factor variant |
| US6905683B2 (en) | 2000-05-03 | 2005-06-14 | Novo Nordisk Healthcare A/G | Human coagulation factor VII variants |
| US7338932B2 (en) | 2000-05-11 | 2008-03-04 | Glycozym Aps | Methods of modulating functions of polypeptide GalNAc-transferases and of screening test substances to find agents herefor, pharmaceutical compositions comprising such agents and the use of such agents for preparing medicaments |
| JP2004528001A (en) | 2000-05-12 | 2004-09-16 | ネオーズ テクノロジーズ, インコーポレイテッド | Fucosylated recombinant glycopeptides in vitro |
| RU2281116C2 (en) | 2000-05-15 | 2006-08-10 | Ф.Хоффманн-Ля Рош Аг | Novel pharmaceutical composition |
| NZ522847A (en) | 2000-05-16 | 2004-11-26 | Bolder Biotechnology Inc | Methods for refolding proteins containing free cysteine residues |
| KR100787073B1 (en) | 2000-06-28 | 2007-12-21 | 글리코파이, 인크. | Methods for producing modified glycoproteins |
| CA2412882A1 (en) | 2000-06-30 | 2002-01-10 | Maxygen Aps | Peptide extended glycosylated polypeptides |
| US6423826B1 (en) | 2000-06-30 | 2002-07-23 | Regents Of The University Of Minnesota | High molecular weight derivatives of vitamin K-dependent polypeptides |
| KR100396983B1 (en) | 2000-07-29 | 2003-09-02 | 이강춘 | Highly reactive branched polymer and proteins or peptides conjugated with the polymer |
| WO2002013873A2 (en) | 2000-08-17 | 2002-02-21 | Synapse Technologies, Inc. | P97-active agent conjugates and their methods of use |
| AU2001283740A1 (en) | 2000-08-17 | 2002-02-25 | University Of British Columbia | Chemotherapeutic agents conjugated to p97 and their methods of use in treating neurological tumours |
| US20020151471A1 (en) | 2000-10-02 | 2002-10-17 | Pingel Hans Kurt | Factor VII glycoforms |
| US20020142964A1 (en) | 2000-11-02 | 2002-10-03 | Nissen Torben Lauesgaard | Single-chain polypeptides |
| BR0113091A (en) | 2000-11-27 | 2003-07-08 | Rmf Dictagene Sa | Process for the multiplication of chemically synthesized polypeptides and process for the preparation of biologically active proteins |
| US20030165849A1 (en) | 2000-11-28 | 2003-09-04 | Biliang Zhang | Methods and reagents for introducing a sulfhydryl group into the 5'-terminus of RNA |
| AU3323002A (en) | 2000-12-20 | 2002-07-01 | Hoffmann La Roche | Erythropoietin conjugates |
| WO2002049673A2 (en) | 2000-12-20 | 2002-06-27 | F. Hoffmann-La Roche Ag | Conjugates of erythropoietin (pep) with polyethylene glycol (peg) |
| US7892730B2 (en) | 2000-12-22 | 2011-02-22 | Sagres Discovery, Inc. | Compositions and methods for cancer |
| US6531121B2 (en) | 2000-12-29 | 2003-03-11 | The Kenneth S. Warren Institute, Inc. | Protection and enhancement of erythropoietin-responsive cells, tissues and organs |
| PA8536201A1 (en) | 2000-12-29 | 2002-08-29 | Kenneth S Warren Inst Inc | PROTECTION AND IMPROVEMENT OF CELLS, FABRICS AND ORGANS RESPONDING TO Erythropoietin |
| SE0004932D0 (en) | 2000-12-31 | 2000-12-31 | Apbiotech Ab | A method for mixed mode adsorption and mixed mode adsorbents |
| HUP0303251A2 (en) | 2001-02-27 | 2003-12-29 | Maxygen Aps | Interferon beta-like molecules |
| US7235638B2 (en) | 2001-03-22 | 2007-06-26 | Novo Nordisk Healthcare A/G | Coagulation factor VII derivatives |
| JP2004529640A (en) | 2001-03-22 | 2004-09-30 | ノボ ノルディスク ヘルス ケア アクチェンゲゼルシャフト | Coagulation factor VII derivative |
| EP1383785B1 (en) | 2001-05-03 | 2011-03-16 | Merck Patent GmbH | Recombinant tumor specific antibody and use thereof |
| WO2002092147A2 (en) | 2001-05-11 | 2002-11-21 | Aradigm Corporation | Optimization of the molecular properties and formulation of proteins delivered by inhalation |
| US7271150B2 (en) | 2001-05-14 | 2007-09-18 | United States Of America, Represented By The Secretary, Department Of Health And Human Services | Modified growth hormone |
| KR100961859B1 (en) | 2001-07-11 | 2010-06-09 | 맥시겐 홀딩스 엘티디 | G-csf conjugate |
| KR100453877B1 (en) | 2001-07-26 | 2004-10-20 | 메덱스젠 주식회사 | METHOD OF MANUFACTURING Ig-FUSION PROTEINS BY CONCATAMERIZATION, TNFR/Fc FUSION PROTEINS MANUFACTURED BY THE METHOD, DNA CODING THE PROTEINS, VECTORS INCLUDING THE DNA, AND CELLS TRANSFORMED BY THE VECTOR |
| CA2455347A1 (en) | 2001-08-01 | 2003-02-13 | Neose Technologies, Inc. | Neutral glycosphingolipids and glycosyl-sphingosines and methods for isolating the same |
| JP2005500058A (en) | 2001-08-17 | 2005-01-06 | ネオーズ テクノロジーズ, インコーポレイテッド | Chemoenzymatic synthesis of sialylated oligosaccharides. |
| US7842677B2 (en) | 2001-08-29 | 2010-11-30 | Seneb Biosciences, Inc. | Synthetic ganglioside derivatives and compositions thereof |
| US6930086B2 (en) | 2001-09-25 | 2005-08-16 | Hoffmann-La Roche Inc. | Diglycosylated erythropoietin |
| US7052868B2 (en) | 2001-09-27 | 2006-05-30 | Novo Nordisk Healthcare A/G | Human coagulation factor VII polypeptides |
| US7157277B2 (en) | 2001-11-28 | 2007-01-02 | Neose Technologies, Inc. | Factor VIII remodeling and glycoconjugation of Factor VIII |
| US7226903B2 (en) | 2001-10-10 | 2007-06-05 | Neose Technologies, Inc. | Interferon beta: remodeling and glycoconjugation of interferon beta |
| NZ532027A (en) | 2001-10-10 | 2008-09-26 | Neose Technologies Inc | Remodeling and glycoconjugation of peptides |
| US7179617B2 (en) | 2001-10-10 | 2007-02-20 | Neose Technologies, Inc. | Factor IX: remolding and glycoconjugation of Factor IX |
| US7399613B2 (en) | 2001-10-10 | 2008-07-15 | Neose Technologies, Inc. | Sialic acid nucleotide sugars |
| US7173003B2 (en) | 2001-10-10 | 2007-02-06 | Neose Technologies, Inc. | Granulocyte colony stimulating factor: remodeling and glycoconjugation of G-CSF |
| US7265085B2 (en) | 2001-10-10 | 2007-09-04 | Neose Technologies, Inc. | Glycoconjugation methods and proteins/peptides produced by the methods |
| US7265084B2 (en) | 2001-10-10 | 2007-09-04 | Neose Technologies, Inc. | Glycopegylation methods and proteins/peptides produced by the methods |
| US7439043B2 (en) | 2001-10-10 | 2008-10-21 | Neose Technologies, Inc. | Galactosyl nucleotide sugars |
| US7214660B2 (en) | 2001-10-10 | 2007-05-08 | Neose Technologies, Inc. | Erythropoietin: remodeling and glycoconjugation of erythropoietin |
| US7795210B2 (en) | 2001-10-10 | 2010-09-14 | Novo Nordisk A/S | Protein remodeling methods and proteins/peptides produced by the methods |
| US7696163B2 (en) | 2001-10-10 | 2010-04-13 | Novo Nordisk A/S | Erythropoietin: remodeling and glycoconjugation of erythropoietin |
| US8008252B2 (en) | 2001-10-10 | 2011-08-30 | Novo Nordisk A/S | Factor VII: remodeling and glycoconjugation of Factor VII |
| US7297511B2 (en) | 2001-10-10 | 2007-11-20 | Neose Technologies, Inc. | Interferon alpha: remodeling and glycoconjugation of interferon alpha |
| US7125843B2 (en) | 2001-10-19 | 2006-10-24 | Neose Technologies, Inc. | Glycoconjugates including more than one peptide |
| US6784154B2 (en) | 2001-11-01 | 2004-08-31 | University Of Utah Research Foundation | Method of use of erythropoietin to treat ischemic acute renal failure |
| CA2468295C (en) | 2001-11-28 | 2010-08-03 | Neose Technologies, Inc. | Glycoprotein remodeling using endoglycanases |
| US7473680B2 (en) | 2001-11-28 | 2009-01-06 | Neose Technologies, Inc. | Remodeling and glycoconjugation of peptides |
| US7368108B2 (en) | 2001-11-28 | 2008-05-06 | Neose Technologies, Inc. | Glycopeptide remodeling using amidases |
| US8048408B2 (en) | 2002-01-16 | 2011-11-01 | Biocompatibles Uk Limited | Polymer conjugates |
| US20060035224A1 (en) | 2002-03-21 | 2006-02-16 | Johansen Jack T | Purification methods for oligonucleotides and their analogs |
| WO2003093448A2 (en) | 2002-05-03 | 2003-11-13 | Neose Technologies, Inc. | Recombinant glycosyltransferase fusion proteins |
| MXPA04012496A (en) | 2002-06-21 | 2005-09-12 | Novo Nordisk Healthcare Ag | Pegylated factor vii glycoforms. |
| ATE503498T1 (en) | 2002-06-21 | 2011-04-15 | Novo Nordisk Healthcare Ag | PEGYLATED GLYCOFORMS OF FACTOR VII |
| DE10232916B4 (en) | 2002-07-19 | 2008-08-07 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Apparatus and method for characterizing an information signal |
| JP2005533525A (en) | 2002-07-23 | 2005-11-10 | ニオズ テクノロジーズ インコーポレイテッド | Synthesis of oligosaccharides, glycolipids, and glycoproteins using bacterial glycosyltransferases |
| CN1671722A (en) | 2002-08-01 | 2005-09-21 | 加拿大国家研究院 | Campylobacter glycans and glycopeptides |
| KR20050039839A (en) | 2002-08-02 | 2005-04-29 | 글락소스미스클라인 바이오로지칼즈 에스.에이. | Vaccine compositions comprising l2 and/or l3 immunotype lipooligosaccharides from lgtb- neisseria minigitidis |
| CA2497777A1 (en) | 2002-09-05 | 2004-03-18 | The General Hospital Corporation | Modified asialo-interferons and uses thereof |
| WO2004022004A2 (en) | 2002-09-06 | 2004-03-18 | Bayer Pharmaceuticals Corporation | Modified glp-1 receptor agonists and their pharmacological methods of use |
| ZA200502320B (en) | 2002-09-20 | 2006-10-25 | Pharmacia Corp | Process for decreasing aggregate levels of pegylated protein |
| AU2003266214A1 (en) | 2002-09-25 | 2004-04-19 | Novo Nordisk A/S | Human coagulation factor vii polypeptides |
| AU2003266931B2 (en) | 2002-09-30 | 2010-01-21 | Bayer Healthcare Llc | FVII or FVIIa variants having increased clotting activity |
| US20040062748A1 (en) | 2002-09-30 | 2004-04-01 | Mountain View Pharmaceuticals, Inc. | Polymer conjugates with decreased antigenicity, methods of preparation and uses thereof |
| BR0315178A (en) | 2002-10-09 | 2005-08-16 | Neose Technologies Inc | Erythropoietin: remodeling and glycoconjugation of erythropoietin |
| WO2004042075A2 (en) | 2002-11-08 | 2004-05-21 | Glycozym Aps | Inactivated ga1 - nac - transferases, methods for inhibitors of such transferases and their use |
| JP4412461B2 (en) | 2002-11-20 | 2010-02-10 | 日油株式会社 | Modified bio-related substance, production method thereof and intermediate |
| US7459436B2 (en) | 2002-11-22 | 2008-12-02 | Hoffmann-La Roche Inc. | Treatment of disturbances of iron distribution |
| US20050064540A1 (en) | 2002-11-27 | 2005-03-24 | Defrees Shawn Ph.D | Glycoprotein remodeling using endoglycanases |
| EP1424344A1 (en) | 2002-11-29 | 2004-06-02 | Aventis Behring Gesellschaft mit beschränkter Haftung | Modified cDNA factor VIII and its derivates |
| PT1428878E (en) | 2002-12-13 | 2008-11-18 | Bioceuticals Arzneimittel Ag | Process for the production and purification of erythropoietin |
| TWI406672B (en) | 2002-12-26 | 2013-09-01 | Mountain View Pharmaceuticals | Polymer conjugates of interferon-beta with enhanced biological potency |
| US7041635B2 (en) | 2003-01-28 | 2006-05-09 | In2Gen Co., Ltd. | Factor VIII polypeptide |
| US8537814B2 (en) | 2003-01-31 | 2013-09-17 | Qwest Communications International Inc. | Configurable network interface device and systems and methods for its use |
| CA2517369C (en) | 2003-02-26 | 2013-06-04 | Nektar Therapeutics Al, Corporation | Polymer-factor viii moiety conjugates |
| JP2006523211A (en) | 2003-03-14 | 2006-10-12 | ネオス テクノロジーズ インコーポレイテッド | Branched water-soluble polymers and their composites |
| EP1603954A4 (en) | 2003-03-18 | 2006-04-12 | Neose Technologies Inc | Activated forms of water-soluble polymers |
| WO2004093823A2 (en) | 2003-03-19 | 2004-11-04 | Eli Lilly And Company | Polyethelene glycol link glp-1 compounds |
| US8791070B2 (en) | 2003-04-09 | 2014-07-29 | Novo Nordisk A/S | Glycopegylated factor IX |
| WO2004091499A2 (en) | 2003-04-09 | 2004-10-28 | Neose Technologies, Inc. | Intracellular formation of peptide conjugates |
| CA2522345A1 (en) | 2003-04-09 | 2004-11-18 | Neose Technologies, Inc. | Glycopegylation methods and proteins/peptides produced by the methods |
| WO2006127896A2 (en) | 2005-05-25 | 2006-11-30 | Neose Technologies, Inc. | Glycopegylated factor ix |
| US7718363B2 (en) | 2003-04-25 | 2010-05-18 | The Kenneth S. Warren Institute, Inc. | Tissue protective cytokine receptor complex and assays for identifying tissue protective compounds |
| ATE497783T1 (en) | 2003-05-06 | 2011-02-15 | Syntonix Pharmaceuticals Inc | CLOTTING FACTOR VII-FC CHIMERIC PROTEINS FOR THE TREATMENT OF HEMOSTATIC DISEASES |
| TWI353991B (en) | 2003-05-06 | 2011-12-11 | Syntonix Pharmaceuticals Inc | Immunoglobulin chimeric monomer-dimer hybrids |
| AU2004240553A1 (en) | 2003-05-09 | 2004-12-02 | Neose Technologies, Inc. | Compositions and methods for the preparation of human growth hormone glycosylation mutants |
| WO2004101597A2 (en) | 2003-05-13 | 2004-11-25 | Frutarom Ltd. | Methods for the reduction of disulfide bonds |
| US7074755B2 (en) | 2003-05-17 | 2006-07-11 | Centocor, Inc. | Erythropoietin conjugate compounds with extended half-lives |
| EP1481985A1 (en) | 2003-05-28 | 2004-12-01 | Innogenetics N.V. | Modified hepatitis C virus (HCV) NS3 for medical treatment |
| GB0315457D0 (en) | 2003-07-01 | 2003-08-06 | Celltech R&D Ltd | Biological products |
| US9005625B2 (en) | 2003-07-25 | 2015-04-14 | Novo Nordisk A/S | Antibody toxin conjugates |
| JP2007501811A (en) | 2003-08-08 | 2007-02-01 | ノボ ノルディスク ヘルス ケア アクチェンゲゼルシャフト | Use of galactose oxidase for selective chemical conjugation of a retarder molecule to a protein of therapeutic interest. |
| WO2005014024A2 (en) | 2003-08-08 | 2005-02-17 | Fresenius Kabi Deutschland Gmbh | Conjugates of a polymer and a protein linked by an oxime linking group |
| US20060198819A1 (en) | 2003-08-08 | 2006-09-07 | Novo Nordisk Healthcare A/G | Use of galactose oxidase for selective chemical conjugation of protractor molecules to proteins of therapeutic interest |
| BRPI0409650A (en) | 2003-09-09 | 2006-04-25 | Warren Pharmaceuticals Inc | methods for regulating hematocrit and human levels, artificial erythropoietin products, methods for preparing an erythropoietin product and for treating anemia in patients at risk of tissue damage, and, pharmaceutical composition |
| US7524813B2 (en) | 2003-10-10 | 2009-04-28 | Novo Nordisk Health Care Ag | Selectively conjugated peptides and methods of making the same |
| US20080305992A1 (en) | 2003-11-24 | 2008-12-11 | Neose Technologies, Inc. | Glycopegylated erythropoietin |
| ES2445948T3 (en) | 2003-11-24 | 2014-03-06 | Ratiopharm Gmbh | Glycopegylated Erythropoietin |
| US8633157B2 (en) | 2003-11-24 | 2014-01-21 | Novo Nordisk A/S | Glycopegylated erythropoietin |
| US7956032B2 (en) | 2003-12-03 | 2011-06-07 | Novo Nordisk A/S | Glycopegylated granulocyte colony stimulating factor |
| CA2549409C (en) | 2003-12-03 | 2013-10-29 | Neose Technologies, Inc. | Glycopegylated granulocyte colony stimulating factor |
| US20080015142A1 (en) | 2003-12-03 | 2008-01-17 | Defrees Shawn | Glycopegylated Follicle Stimulating Hormone |
| US20060040856A1 (en) | 2003-12-03 | 2006-02-23 | Neose Technologies, Inc. | Glycopegylated factor IX |
| MXPA06006023A (en) | 2003-12-03 | 2006-08-23 | Neose Technologies Inc | Glycopegylated factor ix. |
| US20080318850A1 (en) | 2003-12-03 | 2008-12-25 | Neose Technologies, Inc. | Glycopegylated Factor Ix |
| WO2005070138A2 (en) | 2004-01-08 | 2005-08-04 | Neose Technologies, Inc. | O-linked glycosylation of peptides |
| WO2005067601A2 (en) | 2004-01-09 | 2005-07-28 | Neose Technologies, Inc. | Vectors for recombinant protein expression in e.coli |
| US20070105770A1 (en) | 2004-01-21 | 2007-05-10 | Novo Nordisk A/S | Transglutaminase mediated conjugation of peptides |
| AU2005208897B2 (en) | 2004-01-26 | 2011-05-19 | Ratiopharm Gmbh | Branched polymeric sugars and nucleotides thereof |
| ES2674129T3 (en) | 2004-02-12 | 2018-06-27 | Archemix Llc | Aptamer-based therapeutic agents useful in the treatment of complement-related disorders |
| CA2557782A1 (en) | 2004-03-17 | 2005-10-06 | Eli Lilly And Company | Glycol linked fgf-21 compounds |
| WO2005111225A1 (en) | 2004-05-04 | 2005-11-24 | Novo Nordisk Health Care Ag | O-linked glycoforms of polypeptides and method to manufacture them |
| US20070037966A1 (en) | 2004-05-04 | 2007-02-15 | Novo Nordisk A/S | Hydrophobic interaction chromatography purification of factor VII polypeptides |
| JP4251399B2 (en) | 2004-05-21 | 2009-04-08 | 独立行政法人産業技術総合研究所 | Screening method for peptides to which O-linked sugar chains are added |
| US20080206810A1 (en) | 2004-06-03 | 2008-08-28 | Neose Technologies, Inc. | Truncated St6galnaci Polypeptides and Nucleic Acids |
| EP1765992A4 (en) | 2004-06-03 | 2009-01-07 | Neose Technologies Inc | Truncated galnact2 polypeptides and nucleic acids |
| MXPA06015234A (en) | 2004-06-30 | 2007-11-22 | Nektar Therapeutics Al Corp | Polymer-factor ix moiety conjugates. |
| AU2005260664A1 (en) | 2004-06-30 | 2006-01-12 | Egen Corporation | Pegylated interferon alpha-1b |
| WO2006014466A2 (en) | 2004-07-02 | 2006-02-09 | The Kenneth S. Warren Institute, Inc. | Novel carbamylated epo and method for its production |
| EP1771190A4 (en) | 2004-07-02 | 2009-07-22 | Kenneth S Warren Inst Inc | Method of producing fully carbamylated erythropoietin |
| US20080300173A1 (en) | 2004-07-13 | 2008-12-04 | Defrees Shawn | Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1] |
| WO2006020372A2 (en) | 2004-07-23 | 2006-02-23 | Neose Technologies, Inc. | Enzymatic modification of glycopeptides |
| SE0401951D0 (en) | 2004-07-29 | 2004-07-29 | Amersham Biosciences Ab | Chromatography method |
| EP1778838A2 (en) | 2004-08-02 | 2007-05-02 | Novo Nordisk Health Care AG | Conjugation of fvii |
| US20060024286A1 (en) | 2004-08-02 | 2006-02-02 | Paul Glidden | Variants of tRNA synthetase fragments and uses thereof |
| ES2346072T3 (en) | 2004-08-17 | 2010-10-08 | Csl Behring Gmbh | DEPENDENT MODIFIED POLYPEPTIDES OF VITAMIN K. |
| WO2006031811A2 (en) | 2004-09-10 | 2006-03-23 | Neose Technologies, Inc. | Glycopegylated interferon alpha |
| US20080108557A1 (en) | 2004-09-29 | 2008-05-08 | Novo Nordisk Healthcare A/G | Modified Proteins |
| CA2585758C (en) | 2004-10-29 | 2017-08-01 | Neose Technologies, Inc. | Remodeling and glycopegylation of fibroblast growth factor (fgf) |
| SI3130601T1 (en) * | 2004-11-12 | 2020-11-30 | Bayer Healthcare Llc | Site-directed modification of fviii |
| WO2006066258A2 (en) | 2004-12-17 | 2006-06-22 | Neose Technologies, Inc. | Lipoconjugation of peptides |
| US8080391B2 (en) | 2004-12-22 | 2011-12-20 | Ambrx, Inc. | Process of producing non-naturally encoded amino acid containing high conjugated to a water soluble polymer |
| WO2006074279A1 (en) | 2005-01-06 | 2006-07-13 | Neose Technologies, Inc. | Glycoconjugation using saccharyl fragments |
| CA2593682C (en) | 2005-01-10 | 2016-03-22 | Neose Technologies, Inc. | Glycopegylated granulocyte colony stimulating factor |
| WO2006078645A2 (en) | 2005-01-19 | 2006-07-27 | Neose Technologies, Inc. | Heterologous polypeptide expression using low multiplicity of infection of viruses |
| PA8660701A1 (en) | 2005-02-04 | 2006-09-22 | Pfizer Prod Inc | SMALL AGONISTS AND THEIR USES |
| ATE527371T1 (en) | 2005-03-24 | 2011-10-15 | Biogenerix Ag | EXPRESSION OF SOLUBLE, ACTIVE, EUKARYOTIC GLUCOSYL TRANSFERASES IN PROKARYOTIC ORGANISMS |
| US20060246544A1 (en) | 2005-03-30 | 2006-11-02 | Neose Technologies,Inc. | Manufacturing process for the production of peptides grown in insect cell lines |
| JP2008534559A (en) * | 2005-04-01 | 2008-08-28 | ノボ ノルディスク ヘルス ケア アクチェンゲゼルシャフト | Blood coagulation FVIII analogue |
| EP2386571B1 (en) | 2005-04-08 | 2016-06-01 | ratiopharm GmbH | Compositions and methods for the preparation of protease resistant human growth hormone glycosylation mutants |
| CA2606935A1 (en) | 2005-05-05 | 2006-11-16 | Pulmatrix Inc. | Ultrasonic aerosol generator |
| ATE483027T1 (en) | 2005-05-11 | 2010-10-15 | Eth Zuerich | RECOMBINANT N-GLYCOSYLATED PROTEINS FROM PROKARYONTIC CELLS |
| WO2006127910A2 (en) | 2005-05-25 | 2006-11-30 | Neose Technologies, Inc. | Glycopegylated erythropoietin formulations |
| US20110003744A1 (en) | 2005-05-25 | 2011-01-06 | Novo Nordisk A/S | Glycopegylated Erythropoietin Formulations |
| WO2006134173A2 (en) | 2005-06-17 | 2006-12-21 | Novo Nordisk Health Care Ag | Selective reduction and derivatization of engineered proteins comprising at least one non-native cysteine |
| US20070105755A1 (en) | 2005-10-26 | 2007-05-10 | Neose Technologies, Inc. | One pot desialylation and glycopegylation of therapeutic peptides |
| AU2006280932A1 (en) | 2005-08-19 | 2007-02-22 | Novo Nordisk A/S | Glycopegylated factor VII and factor Vila |
| JP5690047B2 (en) | 2005-09-14 | 2015-03-25 | ノボ ノルディスク ヘルス ケア アーゲー | Human coagulation factor VII polypeptide |
| US20090048440A1 (en) | 2005-11-03 | 2009-02-19 | Neose Technologies, Inc. | Nucleotide Sugar Purification Using Membranes |
| DE102006009437A1 (en) | 2006-03-01 | 2007-09-13 | Bioceuticals Arzneimittel Ag | G-CSF liquid formulation |
| US7645860B2 (en) | 2006-03-31 | 2010-01-12 | Baxter Healthcare S.A. | Factor VIII polymer conjugates |
| EP2010222A1 (en) | 2006-03-31 | 2009-01-07 | Baxter International Inc. | Pegylated factor viii |
| EP2213733A3 (en) | 2006-05-24 | 2010-12-29 | Novo Nordisk Health Care AG | Factor IX analogues having prolonged in vivo half life |
| WO2008011633A2 (en) * | 2006-07-21 | 2008-01-24 | Neose Technologies, Inc. | Glycosylation of peptides via o-linked glycosylation sequences |
| ITMI20061624A1 (en) | 2006-08-11 | 2008-02-12 | Bioker Srl | SINGLE-CONJUGATE SITE-SPECIFIC OF G-CSF |
| ES2531934T3 (en) | 2006-09-01 | 2015-03-20 | Novo Nordisk Health Care Ag | Modified glycoproteins |
| US8969532B2 (en) | 2006-10-03 | 2015-03-03 | Novo Nordisk A/S | Methods for the purification of polypeptide conjugates comprising polyalkylene oxide using hydrophobic interaction chromatography |
| HUE036502T2 (en) | 2006-10-04 | 2018-07-30 | Novo Nordisk As | Glycerol linked pegylated sugars and glycopeptides |
| WO2008073620A2 (en) | 2006-11-02 | 2008-06-19 | Neose Technologies, Inc. | Manufacturing process for the production of polypeptides expressed in insect cell-lines |
| PT2144923E (en) | 2007-04-03 | 2013-05-15 | Biogenerix Ag | Methods of treatment using glycopegylated g-csf |
| US20090053167A1 (en) | 2007-05-14 | 2009-02-26 | Neose Technologies, Inc. | C-, S- and N-glycosylation of peptides |
| CN101778937A (en) * | 2007-06-04 | 2010-07-14 | 诺和诺德公司 | o-linked glycosylation using n-acetylglucosaminyl transferases |
| EP2170919B8 (en) | 2007-06-12 | 2016-01-20 | ratiopharm GmbH | Improved process for the production of nucleotide sugars |
| US8207112B2 (en) | 2007-08-29 | 2012-06-26 | Biogenerix Ag | Liquid formulation of G-CSF conjugate |
| EP2242505A4 (en) | 2008-01-08 | 2012-03-07 | Biogenerix Ag | Glycoconjugation of polypeptides using oligosaccharyltransferases |
| EP2417155B1 (en) * | 2009-04-06 | 2013-06-19 | Novo Nordisk A/S | Targeted delivery of factor viii proteins to platelets |
-
2009
- 2009-02-26 CN CN201310180825.1A patent/CN103497246B/en active Active
- 2009-02-26 US US12/597,473 patent/US20130189239A1/en not_active Abandoned
- 2009-02-26 KR KR1020107018763A patent/KR101582841B1/en active IP Right Grant
- 2009-02-26 RU RU2010137743/10A patent/RU2573587C2/en active
- 2009-02-26 EP EP09714286.3A patent/EP2257311B1/en active Active
- 2009-02-26 JP JP2010548875A patent/JP5619630B2/en active Active
- 2009-02-26 ES ES09714286.3T patent/ES2476690T3/en active Active
- 2009-02-26 PL PL09714286T patent/PL2257311T3/en unknown
- 2009-02-26 MX MX2010009154A patent/MX2010009154A/en active IP Right Grant
- 2009-02-26 CN CN2009801067383A patent/CN101965200B/en active Active
- 2009-02-26 CA CA2715465A patent/CA2715465C/en active Active
- 2009-02-26 AU AU2009219232A patent/AU2009219232B2/en active Active
- 2009-02-26 EP EP13150934.1A patent/EP2626080A3/en not_active Withdrawn
- 2009-02-26 EP EP13150933.3A patent/EP2626079A3/en not_active Withdrawn
- 2009-02-26 DK DK09714286.3T patent/DK2257311T3/en active
- 2009-02-26 WO PCT/US2009/035339 patent/WO2009108806A1/en active Application Filing
- 2009-02-26 CN CN201310180846.3A patent/CN103497247A/en not_active Withdrawn
- 2009-02-27 TW TW098106523A patent/TWI425953B/en active
-
2010
- 2010-07-25 IL IL207188A patent/IL207188A/en active IP Right Grant
- 2010-08-04 ZA ZA2010/05556A patent/ZA201005556B/en unknown
-
2013
- 2013-02-05 US US13/759,261 patent/US8536126B2/en active Active
- 2013-09-26 JP JP2013200218A patent/JP5933503B2/en active Active
-
2014
- 2014-05-08 US US14/272,726 patent/US9150848B2/en active Active
- 2014-06-12 JP JP2014121288A patent/JP5927237B2/en active Active
-
2015
- 2015-08-20 US US14/831,515 patent/US20160222086A1/en not_active Abandoned
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9150848B2 (en) | Conjugated factor VIII molecules | |
| EP2536753B1 (en) | Factor viii molecules with reduced vwf binding | |
| US20160264645A1 (en) | Stabilized Factor VIII Variants | |
| AU2013204960B2 (en) | Conjugated factor VII molecules | |
| TWI535454B (en) | Conjugated factor viii molecules |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: EXPRESSLY ABANDONED -- DURING EXAMINATION |