US20140163688A1 - Malleolar Replacement Devices - Google Patents
Malleolar Replacement Devices Download PDFInfo
- Publication number
- US20140163688A1 US20140163688A1 US14/177,732 US201414177732A US2014163688A1 US 20140163688 A1 US20140163688 A1 US 20140163688A1 US 201414177732 A US201414177732 A US 201414177732A US 2014163688 A1 US2014163688 A1 US 2014163688A1
- Authority
- US
- United States
- Prior art keywords
- prosthesis
- ankle
- proximal portion
- human
- distal portion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/42—Joints for wrists or ankles; for hands, e.g. fingers; for feet, e.g. toes
- A61F2/4202—Joints for wrists or ankles; for hands, e.g. fingers; for feet, e.g. toes for ankles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws, setting implements or the like
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/84—Fasteners therefor or fasteners being internal fixation devices
- A61B17/86—Pins or screws or threaded wires; nuts therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/30772—Apertures or holes, e.g. of circular cross section
- A61F2002/30784—Plurality of holes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/42—Joints for wrists or ankles; for hands, e.g. fingers; for feet, e.g. toes
- A61F2/4202—Joints for wrists or ankles; for hands, e.g. fingers; for feet, e.g. toes for ankles
- A61F2002/4205—Tibial components
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/42—Joints for wrists or ankles; for hands, e.g. fingers; for feet, e.g. toes
- A61F2/4202—Joints for wrists or ankles; for hands, e.g. fingers; for feet, e.g. toes for ankles
- A61F2002/421—Fibular components, e.g. fibular-malleolar shields
Definitions
- FIG. 2 is a perspective view of the ankle joint of FIG. 1 , with a break being shown in the fibula bone.
- the insert 106 has at least one hole 108 and preferably a plurality of holes 108 that will allow screws 110 (see FIG. 7 ) to attach the prosthesis 100 to the fibula.
- a plurality of holes 108 is preferable, in that it allows for the insert 106 , and the prosthesis 100 in general, to be attached at varying angles and elevations, depending on a particular fracture or on other characteristics, such as the age or gender of the patient.
Landscapes
- Health & Medical Sciences (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
- Surgical Instruments (AREA)
Abstract
A prosthesis and kit for replacing an ankle joint, and methods of applying the devices or systems. The prosthesis is an intramedullary device directed towards replacement of either of the tibia or fibula bone, wherein the prosthesis is a replacement for the lateral malleolus or the medial malleolus, respectively.
Description
- The present application is a divisional application of U.S. patent application Ser. No. 13/178,208, filed on 7 Jul. 2011, and entitled “Malleolar Replacement Devices,” which claims the benefit of U.S. Provisional Application, Ser. No. 61/362,122, filed on 7 Jul. 2010, which are both incorporated by reference herein in their entireties.
- The invention relates to ankle replacement prostheses and systems, as well as associated surgical instruments and procedures. The present invention is more specifically directed towards intramedullary ankle joint replacements.
- Until the early- to mid-1970's, patients with injured or diseased ankle joints commonly resulting from rheumatism, or degenerative or traumatic arthritis, had few options when their ankle joints failed. The most common procedure to help these patients regain some use of their ankle was obliteration of the joint by fusion, a procedure that is still commonly used today. Fusion, however, rendered the ankle stiff and generally immobile relative to the lower leg, resulting in limited use and additional stresses on the knee and hip joints.
- Probably the first reported use of a total ankle prosthesis was by Buckholz in 1969. The medical community recognized that such ankle replacement led to largely increased use of the ankle joint because the replacement permitted ankle ranges of motion which generally attempted to mimic the natural human joint. Since that time, ankle replacement prostheses have become increasingly common in use and improved in design.
- Ankle fractures are particularly common in people having bone disease, such as osteoporosis. Geriatrics, particularly women, are very susceptible to ankle fractures, and the prognosis after fracture is generally poor, even with the use of a prosthesis. In general, currently used prostheses do not afford the necessary flexibility required for an ankle joint and recovery can be slow and arduous. The fusing together of bones or bone segments required and carried out with prior prostheses limits the ability of the ankle joint to completely heal properly, particularly with those who may have had limited mobility prior to the ankle fracture.
- Stability and weight bearing are other issues that are more important when replacing an ankle joint as opposed to other joints. For example, hip, shoulder, or knee joints are not required to bear the load that is supported by an ankle joint. Consequently replacement devices for these other joints do not necessarily translate to possible replacement joints for an ankle joint.
- The present invention is directed towards a prosthesis and kit for replacing an ankle joint, and methods of applying the devices or systems. The prosthesis is an intramedullary device directed towards replacement of a portion of either a human tibia or fibula bone, wherein the prosthesis is a replacement for the lateral malleolus or the medial malleolus, respectively.
- The device has a first end that is inserted into the intramedullary canal of either the fibula or tibia. A second end of the device is shaped and configured to assimilate the shape of the lateral or medial malleolus, respectively. The device will be secured to the respective tibia or fibula. Likewise, a system could comprise two devices, wherein one is directed towards each of the tibia and fibula.
- The invention also contemplates methods of installing or inserting the device, wherein the particular malleolus is resected, sufficiently or completely so that the device will replicate the contours of the bone once inserted. The first end of the device is inserted into the intramedullary canal and secured to the bone.
-
FIG. 1 is a perspective view of an ankle joint. -
FIG. 2 is a perspective view of the ankle joint ofFIG. 1 , with a break being shown in the fibula bone. -
FIG. 3A is a perspective view of an ankle replacement device according to the present invention. -
FIG. 3B is a second perspective view of the device ofFIG. 3A . -
FIG. 4 is a side view of the device ofFIG. 3A . -
FIG. 5 is a view of the ankle ofFIG. 2 showing an incision being made in the skin for eventual insertion of a prosthesis as shown inFIG. 3A . -
FIG. 6 demonstrates a prosthesis being inserted into the incision ofFIG. 5 to determine a properly sized prosthesis. -
FIG. 7 demonstrates the ankle ofFIG. 2 being resected to prepare the ankle for placement of the device ofFIG. 3A within the ankle. -
FIG. 8 depict the device ofFIG. 3A being inserted into the fibula. -
FIG. 9 depicts the device ofFIG. 3A being affixed to the ankle. -
FIG. 10 is a perspective view of the ankle ofFIG. 1 , with a break being shown in the tibia bone. -
FIG. 11A is a perspective view of a second ankle replacement device according to the present invention. -
FIG. 11B is second perspective view of the device ofFIG. 11A . -
FIG. 12 is a side view of the device ofFIG. 11A . -
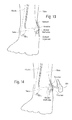
FIG. 13 is a view of the ankle ofFIG. 10 showing an incision being made in the skin for eventual insertion of a prosthesis as shown inFIG. 11A . -
FIG. 14 demonstrates a prosthesis being inserted into the incision ofFIG. 13 to determine a properly sized prosthesis. -
FIG. 15 demonstrates the ankle ofFIG. 10 being resected to prepare the ankle for placement of the device ofFIG. 11A within the ankle. -
FIG. 16 depicts the device ofFIG. 11A being inserted into the ankle. -
FIG. 17 depicts the device ofFIG. 11A being affixed to the ankle. - Although the disclosure hereof is detailed and exact to enable those skilled in the art to practice the invention, the physical embodiments herein disclosed merely exemplify the invention which may be embodied in other specific structures. While the preferred embodiment has been described, the details may be changed without departing from the invention, which is defined by the claims.
-
FIG. 1 depicts a normal ankle joint, free of fracture. The ankle generally consists of the distal ends of the fibula and tibia bones, which are connected to the talus bone. The fibula bone comprises the lateral malleolus, which is connected to the talus by way of the lateral ligament. The tibia bone comprises the medial malleolus, which is connected to the talus by way of the deltoid ligament. The tibia and fibula are connected two one another by way of the syndesmotic ligament. - If undue stress is put on the ankle joint, the joint may fracture, with either the fibula or tibia fracturing, or possibly both. Often a fracture will form at the proximal end of respective malleolus, e.g. the lateral or medial malleolus. Such a fracture of the lateral malleolus is shown in
FIG. 2 , wherein the fracture is shown on the fibula at the proximal end of the lateral malleolus. -
FIGS. 3A-4 depict aprosthesis 100 according to the present invention to address a fracture, as shown, inFIG. 2 . Theprosthesis 100 generally comprises aproximal portion 102 and adistal portion 104. Theproximal portion 102 comprises aninsert 106 adapted to be positioned within the intramedullary canal of the fibula. Theproximal portion 102 preferably has a smaller diameter than thedistal portion 104, so that when theinsert 106 is inserted into the intramedullary canal, there is a definite distance that theprosthesis 100 may be inserted into the intramedullary canal. Theinsert 106 can be of any shape, e.g. a post, or wedge or multiple posts or wedges, that will allow theinsert 106 to be properly inserted and affixed within the intramedullary canal. Theinsert 106 has at least onehole 108 and preferably a plurality ofholes 108 that will allow screws 110 (seeFIG. 7 ) to attach theprosthesis 100 to the fibula. A plurality ofholes 108 is preferable, in that it allows for theinsert 106, and theprosthesis 100 in general, to be attached at varying angles and elevations, depending on a particular fracture or on other characteristics, such as the age or gender of the patient. - Still referring to
FIGS. 3A-4 , thedistal portion 104 generally forms abody 112 that is shaped and sized to follow the contours of the lateral malleolus. Thebody 112 also has anopening 114, and preferably a plurality ofopenings 114. Theopenings 114 are generally used during the implantation of the prosthesis as an insertion guide when positioning the prosthesis. Theopenings 114 may also receivescrews 110 so that thedistal portion 104 may also be attached to the joint by way of screws 110 (seeFIG. 7 ). As with theinsert 106, it is preferable for thebody 112 to have a plurality ofopenings 114 so that theprosthesis 100 can be positioned at varying angles and elevations. A throughbore 116 may also be located on the body, which can receive a pin for syndesmotic fixation, if necessary. - The
prosthesis 100 is also designed to provide protection for the ankle and surrounding tendons once theprosthesis 100 is inserted. For example, a flange or groove 118 is located in thebody 112, which is intended to protect the peroneal tendon once the prosthesis is properly positioned. The peroneal tendon will rest within thegroove 118, thereby allowing the groove to act as a shield for the tendon. Thebody 112 may have agroove 118 on either the right side or the left side of thebody 112, or both sides of thebody 112, which will allow the prosthesis to be used for a right or left ankle repair. -
FIGS. 5-9 depict theprosthesis 100 being secured to the fibula bone. Initially, a doctor, surgeon, or radiologist will take a radiograph or X-ray of the ankle to assist with making a template for the ankle and to assist in properly sizing a prosthesis to be used in the ankle repair. -
FIG. 5 shows a doctor or surgeon preparing the fractured ankle ofFIG. 2 for insertion of theprosthesis 100. An incision over the lateral malleolus will be cut into the skin of the ankle to thereby expose the malleolus. The tendons, e.g. the peroneal tendon, will be mobilized by the surgeon. -
FIG. 6 show aprosthesis 100 being inserted into the incision. Theprosthesis 100 is used as a trial implant to determine the appropriate size for aprosthesis 100 that will eventually be inserted into the incision. The use of a trial implant will also assist in determining the necessary level of bone resection that will be required for the fractured/comminuted bone. -
FIG. 7 demonstrates the bone being resected for insertion of theprosthesis 100. An oscillating saw is used to cut the bone at the levels of the trial implant (seeFIG. 6 ). The bone is resected so that once theprosthesis 100 is positioned, it will follow the contours of the native lateral malleolus. Similarly, the intramedullary canal will be resected so that it will be shaped to receive theproximal portion 102 of the prosthesis. The resected bone fragments will be detached from the ligamentous and tendon attachments so that the properly sized and configured cavity will remain within the ankle joint. The relevant canal, e.g. the endosteal canal, will be enlarged with reamers on the lateral malleolus to insure proper alignment within the cavity. The amount of bone material that will be resected will depend on the size and severity of the fracture. -
FIG. 8 shows theproximal portion 102 of theprosthesis 100 being inserted into the intramedullary canal so that it may be affixed to the fibula. Theprosthesis 100 will be inserted so that it is properly affixed to the fibula, but also to protect the peroneal tendon with the use of the posterior groove 118 (seeFIG. 4 ). The tendon will sit within thegroove 118, thereby allowing thegroove 118 to protect the tendon. Similarly, as shown inFIG. 8 , enough of the lateral malleolus remains around theprosthesis 100 so that theprosthesis 100 is retained properly, which will prevent the prosthesis from unnecessarily moving from side to side once positioned in the ankle. - Once properly inserted, the prosthesis will mimic the shape and contour of a portion of the fibula, particularly the lateral malleolus, as shown in
FIG. 9 . Theprosthesis 100 than can be secured to the anklejoint using screws 110. Preferably, theprosthesis 100 is locked in place by securing one or more, e.g. two,screws 110 laterally through fibula, the syndesmosis, and locking the screws to the tibia. Alignment guides may be used to assist insertion of the screws.Screws 110 will also be used to secure thedistal portion 104 properly within the lateral malleolus. The resultant arrangement allows for a repaired ankle that will closely resemble the fibula bone prior to fracture, thereby decreasing the amount of time needed for recovery and increasing the chance that the patient will recover mobility and stability of the ankle. - As noted above, a fracture may also occur in the tibia as opposed to, or in addition to, the fibula. Such a fracture is depicted in
FIG. 10 . Such a fracture typically happens at the proximal end of the medial malleolus.FIGS. 11A-12 show aprosthesis 200 according to the present invention for addressing fractures as shown inFIG. 10 . Theprosthesis 200 is similar to theprosthesis 100 described above inFIG. 3A-4 , except that theprosthesis 200 is directed towards a fracture of the tibia as opposed to the fibula. That is, theprosthesis 200 is designed to be shaped according to the contours of the medial malleolus as opposed to the lateral malleolus. - Still referring to
FIGS. 11A-12 , the prosthesis comprises aproximal portion 202 and adistal portion 204. The proximal portion comprises aninsert 206 that will be inserted into the intramedullary canal of the tibia. As with theprosthesis 100, theproximal portion 202 preferably has a smaller diameter than thedistal portion 204, so that when theinsert 206 is inserted into the intramedullary canal, there is a definite distance that theprosthesis 200 may be inserted into the intramedullary canal. Theinsert 206 can be of any shape, e.g. a post or wedge or multiple posts or wedges, that will allow theinsert 206 to be properly inserted and affixed within the intramedullary canal. Theinsert 206 has ahole 208 or plurality ofholes 208 for attachment to the tibia by way of screws 210 (seeFIG. 13 ). A plurality ofholes 208 is preferable, in that it allows for theinsert 206, and theprosthesis 200 in general, to be attached at varying angles and elevations, depending on a particular fracture or on other characteristics, such as the age or gender of the patient. - Still referring to
FIGS. 9A-10 , thedistal portion 204 of theprosthesis 200 generally forms abody 212 that is shaped and sized to follow the contours of the medial malleolus. Thebody 212 also has anopening 214, and preferably a plurality ofopenings 214. Theopenings 214 are generally used during the implantation of the prosthesis as an insertion guide when positioning the prosthesis. Theopenings 214 may also receivescrews 210 so that thedistal portion 204 of theprosthesis 200 may also be attached to the ankle joint by way of screws 210 (seeFIG. 7 ). As with theinsert 206, it is preferable for thebody 212 to have a plurality ofopenings 214 so that theprosthesis 100 can be positioned at varying angles and elevations. A through bore 216 may also be located on the body, which can receive a pin for syndesmotic fixation, if necessary. - The
prosthesis 200 is also designed to provide protection for the ankle and surrounding tendons, e.g. posterior tibial tendon, once theprosthesis 200 is inserted. For example, a flange or groove 218 is located on thebody 212, which is intended to protect the posterior tibial tendon once the prosthesis is properly positioned. The posterior tibial tendon will rest within thegroove 218, thereby allowing the groove to act as a shield for the tendon. Thebody 112 may have agroove 218 on either the right side or the left side of thebody 212, or both sides of thebody 212, which will allow the prosthesis to be used for a right or left ankle repair. -
FIGS. 13-17 depict theprosthesis 200 being secured to the tibia bone. Initially, a doctor, surgeon, or radiologist will take a radiograph or X-ray of the ankle to assist with making a template for the ankle and to assist in properly sizing a prosthesis to be used in the ankle repair. -
FIG. 13 shows a doctor or surgeon preparing the fractured ankle ofFIG. 10 for insertion of theprosthesis 200. An incision over the medial malleolus will be cut into the skin of the ankle to thereby expose the malleolus. The tendons, e.g. the posterior tibial tendon, will be mobilized by the surgeon. -
FIG. 14 show aprosthesis 200 being inserted into the incision. Theprosthesis 200 is used as a trial implant to determine the appropriate size for aprosthesis 200 that will eventually be inserted into the incision. The use of a trial implant will also assist in determining the necessary level of bone resection that will be required for the fractured/comminuted bone. - Referring to
FIG. 15 , the tibia bone is resected so that once theprosthesis 200 is positioned within the intramedullary canal, it will follow the contours of the native medial malleolus. An oscillating saw is used to cut the bone at the levels of the trial implant (seeFIG. 14 ). In the same fashion, the intramedullary canal will be resected so that it will be shaped to properly receive theproximal portion 202 of the prosthesis. The resected bone fragments will be detached from the ligamentous and tendon attachments so that the properly sized and configured cavity will remain within the ankle joint. The relevant canal, e.g. the endosteal canal, will be enlarged with reamers on the medial malleolus to insure proper alignment within the cavity. The amount of bone material that will be resected will depend on the size and severity of the fracture. -
FIG. 16 shows theproximal portion 202 of theprosthesis 200 being inserted into the intramedullary canal so that it may be affixed to the tibia. Once properly inserted, theprosthesis 200 will mimic the shape and contour of a portion of the tibia, particularly the medial malleolus, as shown inFIG. 17 . Theprosthesis 200 than can be secured to the anklejoint using screws 210. One ormore screws 210, e.g. threescrews 210, preferably with thescrews 210 being in the form of offset screws, will pass through the medial mallelolar cortex, through the intramedullary canal, and ending in the distal side of the tibia, e.g. the tibial metaphysic. The arrangement helps to promote boney in-growth into the prosthesis, thereby increasing the recovery and stability of the repaired ankle. The resultant arrangement allows for a repaired ankle that will closely resemble the tibia bone prior to fracture, thereby decreasing the amount of time needed for recovery and increasing the chance that the patient will recover mobility and stability of the ankle. - The
prosthesis 200 will be inserted so that it is properly affixed to the tibia, but also to protect the posterior tibial tendon with the use of the posterior groove 218 (seeFIG. 12 ). The tendon will sit within thegroove 218, thereby allowing thegroove 218 to protect the tendon and also to prevent the prosthesis from unnecessarily moving from side to side once positioned in the ankle. - As such, the present invention is directed towards a prosthesis generally comprising a proximal portion, that will be inserted into the intramedullary canal of a specific bone of the ankle joint, and a distal portion that is shaped and designed to replicate the malleolus section of the particular bone that the prosthesis is used in connection with. By using the prostheses to replicate the shape and form of the bone prior to fracture, these prostheses increase the stability of the ankle joint and also decrease the recovery time, as the ankle joint is capable of bearing weight sooner than prior art devices. Similarly, the intramedullary design also promotes healing and recovery, in that it fosters grafting of the prosthesis to the bone.
- The prostheses of the present invention may be made of any suitable biocompatible material. Preferably the prostheses are made of a material that will help with in bone growth. A porous material, e.g. sintered titanium, is one preferred material. For example, the
prosthesis - It should also be understood that, if necessary, the present invention contemplates a kit that will include both a
prosthesis 100 for use with the fibula and aprosthesis 200 for use with the tibia. However, one of the advantages of the present invention over the prior art is that it is not necessary that both the fibula and tibia be resected if one of the bones is not fractured. The prostheses are inserted and attached independently from one another, which also provides for a more efficient reconstruction process for the ankle joint, since alignment of separate prostheses for the fibula and tibia during surgery is not necessary. Likewise, it should be understood that the use ofscrews screws screw 210 or other fastening device will fall within the scope of the present invention. - The foregoing is considered as illustrative only of the principles of the invention. Furthermore, since numerous modifications and changes will readily occur to those skilled in the art, it is not desired to limit the invention to the exact construction and operation shown and described. While the preferred embodiment has been described, the details may be changed without departing from the invention, which is defined by the claims.
Claims (15)
1. An ankle prosthesis comprising:
a proximal portion adapted for insertion into an intramedullary canal of one of a human tibia and a human fibula; and
a distal portion coupled to the proximal portion, the distal portion shaped similarly to a malleolus of the one of a human tibia and a human fibula, wherein said distal portion further includes a groove configured to receive a tendon associated with a human ankle.
2. The ankle prosthesis of claim 1 further comprising at least one hole located in said distal portion, said at least one hole capable of receiving means for attaching the prosthesis to the ankle.
3. The ankle prosthesis of claim 2 further comprising a plurality of holes located in said distal portion, said holes capable of receiving means for attaching the prosthesis to the ankle.
4. The ankle prosthesis of claim 1 further comprising at least one hole located in said proximal portion, said at least one hole capable of receiving means for attaching the prosthesis to the one of a human tibia and a human fibula.
5. The ankle prosthesis of claim 4 , further comprising a plurality of holes located in said proximal portion, said holes capable of receiving means for attaching the prosthesis to the one of a human tibia and a human fibula.
6. The ankle prosthesis of claim 1 wherein the distal portion has a larger cross-sectional diameter than said proximal portion.
7. The ankle prosthesis of claim 6 , wherein the proximal portion comprises an insert that defines a distance that the proximal end may be inserted into the intramedullary canal.
8. The ankle prosthesis of claim 1 , wherein the distal portion is coupled to the proximal portion by being integrally formed therewith.
9. The ankle prosthesis of claim 1 , wherein the proximal portion is adapted for insertion into the intramedullary canal of the human tibia and the tendon comprises the posterior tibial tendon.
10. The ankle prosthesis of claim 1 , wherein the proximal portion is adapted for insertion into the intramedullary canal of the human fibula and the tendon comprises the peroneal tendon.
11. The ankle prosthesis of claim 1 , wherein the distal portion comprises a through bore configured to receive a pin for syndesmotic fixation to the ankle.
12. The ankle prosthesis of claim 1 , wherein the proximal portion and distal portion comprise a porous material.
13. The ankle prosthesis of claim 12 , wherein the porous material comprises titanium.
14. The ankle prosthesis of claim 13 , wherein the titanium is sintered titanium.
15. The ankle prosthesis of claim 13 , wherein the titanium comprises a titanium porous coating.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/177,732 US20140163688A1 (en) | 2010-07-07 | 2014-02-11 | Malleolar Replacement Devices |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US36212210P | 2010-07-07 | 2010-07-07 | |
| US13/178,208 US8647391B2 (en) | 2010-07-07 | 2011-07-07 | Malleolar replacement devices |
| US14/177,732 US20140163688A1 (en) | 2010-07-07 | 2014-02-11 | Malleolar Replacement Devices |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/178,208 Division US8647391B2 (en) | 2010-07-07 | 2011-07-07 | Malleolar replacement devices |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20140163688A1 true US20140163688A1 (en) | 2014-06-12 |
Family
ID=45441559
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/178,208 Active US8647391B2 (en) | 2010-07-07 | 2011-07-07 | Malleolar replacement devices |
| US14/177,732 Abandoned US20140163688A1 (en) | 2010-07-07 | 2014-02-11 | Malleolar Replacement Devices |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/178,208 Active US8647391B2 (en) | 2010-07-07 | 2011-07-07 | Malleolar replacement devices |
Country Status (2)
| Country | Link |
|---|---|
| US (2) | US8647391B2 (en) |
| WO (1) | WO2012006434A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11278416B2 (en) | 2019-11-14 | 2022-03-22 | Howmedica Osteonics Corp. | Concentric keel TKA |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9974588B2 (en) | 2012-12-27 | 2018-05-22 | Wright Medical Technology, Inc. | Ankle replacement system and method |
| US9480571B2 (en) | 2012-12-27 | 2016-11-01 | Wright Medical Technology, Inc. | Ankle replacement system and method |
| US10080573B2 (en) | 2012-12-27 | 2018-09-25 | Wright Medical Technology, Inc. | Ankle replacement system and method |
| AU2013270628B2 (en) | 2012-12-27 | 2015-02-05 | Wright Medical Technology, Inc. | Ankle replacement system and method |
| US9918724B2 (en) | 2012-12-27 | 2018-03-20 | Wright Medical Technology, Inc. | Ankle replacement system and method |
| JP6410792B2 (en) | 2013-03-14 | 2018-10-24 | ライト メディカル テクノロジー インコーポレイテッドWright Medical Technology, Inc. | Ankle joint replacement system and method |
| TW201707653A (en) * | 2015-08-18 | 2017-03-01 | Pao Nan Biotech Co Ltd | Apparatus for preventing resected fibula sections from bone mergence and connection capable of effectively preventing resected fibula from rigid re-mergence |
| US20170056188A1 (en) * | 2015-08-25 | 2017-03-02 | Wright Medical Technology, Inc. | Total ankle talar prosthesis with anchor holes and grooves |
| US10314712B2 (en) * | 2015-11-30 | 2019-06-11 | Jonathan Fisher | Transcortal bone joint fusion system |
| AU2016398429B2 (en) | 2016-03-23 | 2019-09-12 | Wright Medical Technology, Inc | Fixation apparatus and method for total ankle replacement |
| EP3435926A4 (en) * | 2016-03-28 | 2020-08-05 | Wright Medical Technology, Inc. | Anterior resurfacing talar plate |
| CN111759542A (en) * | 2020-06-29 | 2020-10-13 | 北京力达康科技有限公司 | Fibula prosthesis based on 3D printing |
| US12114872B2 (en) | 2021-03-30 | 2024-10-15 | Wright Medical Technology, Inc. | Alignment guide, systems, and methods |
| US11872137B2 (en) | 2021-06-15 | 2024-01-16 | Wright Medical Technology, Inc. | Unicompartmental ankle prosthesis |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3991425A (en) * | 1975-11-20 | 1976-11-16 | Minnesota Mining And Manufacturing Company | Prosthetic bone joint devices |
| US4546501A (en) * | 1982-09-28 | 1985-10-15 | Gustilo Ramon B | Hip prosthesis |
| US5248313A (en) * | 1991-04-17 | 1993-09-28 | Greene Bruce L | Fibular intramedullary rod |
| US20090105840A1 (en) * | 2007-10-18 | 2009-04-23 | Inbone Technologies, Inc. | Fibular stiffener and bony defect replacer |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3709218A (en) | 1970-04-24 | 1973-01-09 | W Halloran | Combination intramedullary fixation and external bone compression apparatus |
| FR2797178B1 (en) | 1999-08-05 | 2002-02-22 | Tornier Sa | MALLEOLAR IMPLANT FOR PARTIAL OR TOTAL ANKLE PROSTHESIS AND ANCILLARY MATERIAL FOR PLACING SUCH AN IMPLANT |
| US6673116B2 (en) | 1999-10-22 | 2004-01-06 | Mark A. Reiley | Intramedullary guidance systems and methods for installing ankle replacement prostheses |
| US7267678B2 (en) | 2003-09-30 | 2007-09-11 | Robert J. Medoff | Intramedullary implant for fracture fixation |
| FR2896404B1 (en) * | 2006-01-24 | 2008-02-29 | Tornier Sas | SURGICAL INSTRUMENTATION ASSEMBLY FOR POSTING AN ANKLE PROSTHESIS |
-
2011
- 2011-07-07 WO PCT/US2011/043207 patent/WO2012006434A1/en active Application Filing
- 2011-07-07 US US13/178,208 patent/US8647391B2/en active Active
-
2014
- 2014-02-11 US US14/177,732 patent/US20140163688A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3991425A (en) * | 1975-11-20 | 1976-11-16 | Minnesota Mining And Manufacturing Company | Prosthetic bone joint devices |
| US4546501A (en) * | 1982-09-28 | 1985-10-15 | Gustilo Ramon B | Hip prosthesis |
| US5248313A (en) * | 1991-04-17 | 1993-09-28 | Greene Bruce L | Fibular intramedullary rod |
| US20090105840A1 (en) * | 2007-10-18 | 2009-04-23 | Inbone Technologies, Inc. | Fibular stiffener and bony defect replacer |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11278416B2 (en) | 2019-11-14 | 2022-03-22 | Howmedica Osteonics Corp. | Concentric keel TKA |
Also Published As
| Publication number | Publication date |
|---|---|
| US8647391B2 (en) | 2014-02-11 |
| US20120185057A1 (en) | 2012-07-19 |
| WO2012006434A1 (en) | 2012-01-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8647391B2 (en) | Malleolar replacement devices | |
| US20210121297A1 (en) | Devices And Methods For Metatarsophalangeal Arthroplasty Procedures | |
| KR102566656B1 (en) | Platform Fracture Fixed Implant | |
| US20100057133A1 (en) | Tibia-talus-calcaneus (T-T-C) locking plate | |
| AU2014239941B2 (en) | Intramedullary ankle technique and system | |
| US20040254646A1 (en) | Provisional coupling mechanism | |
| WO2009032101A2 (en) | Tibia-talus-calcaneus (t-t-c) locking plate | |
| KR102140052B1 (en) | Osteotomy device with an in-vitro alignment component | |
| Papagelopoulos et al. | Total knee arthroplasty in patients with pre-existing fracture deformity | |
| US20140358242A1 (en) | Implant augment | |
| US11273045B2 (en) | Motion toe systems and methods | |
| RU2591534C1 (en) | Set for hip replacement | |
| US10595915B2 (en) | Bone implant devices, instruments and methods of use | |
| KR20200068040A (en) | Universal osteotomy device | |
| Kanzaki et al. | Flexible hinge silicone implant with or without titanium grommets for arthroplasty of the first metatarsophalangeal joint | |
| RU224986U1 (en) | DIAPHYSAL FEMORAL PLATE | |
| Öztürkmen et al. | The use of a cementless modular stem in the treatment of subtrochanteric femoral fractures in conjunction with ipsilateral coxarthrosis | |
| Malhotra et al. | Proximal femoral fracture in ankylosed hip treated with primary total hip arthroplasty: Technical tips with report of two cases | |
| CN209107548U (en) | A kind of lockplate suitable for around femoral prosthesis | |
| Lee et al. | The mobility total ankle replacement: techniques and pitfalls | |
| HIGHLANDER | Primary Total Ankle Total Talus Replacement | |
| Vishwanathan | Implantology of Fracture of the Distal Humerus | |
| Fink | Revision Arthroplasty in Periprosthetic Fractures of the Knee | |
| WO2022192250A1 (en) | Toe implant assemblies, kits, surgical methods, and methods of manufacturing | |
| Fitzpatrick | Fracture fixation-the weird. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: GLOBAL ORTHOPAEDIC SOLUTIONS LLC, CALIFORNIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:ABIDI, NICHOLAS A.;BORKOWSKI, JONATHAN;REEL/FRAME:032544/0500 Effective date: 20111210 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |