US20130303307A1 - Golf ball - Google Patents
Golf ball Download PDFInfo
- Publication number
- US20130303307A1 US20130303307A1 US13/891,277 US201313891277A US2013303307A1 US 20130303307 A1 US20130303307 A1 US 20130303307A1 US 201313891277 A US201313891277 A US 201313891277A US 2013303307 A1 US2013303307 A1 US 2013303307A1
- Authority
- US
- United States
- Prior art keywords
- acid
- golf ball
- salt
- carboxylic acid
- hardness
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- VQTUBCCKSQIDNK-UHFFFAOYSA-N C=C(C)C Chemical compound C=C(C)C VQTUBCCKSQIDNK-UHFFFAOYSA-N 0.000 description 1
- CRSOQBOWXPBRES-UHFFFAOYSA-N CC(C)(C)C Chemical compound CC(C)(C)C CRSOQBOWXPBRES-UHFFFAOYSA-N 0.000 description 1
- NNPPMTNAJDCUHE-UHFFFAOYSA-N CC(C)C Chemical compound CC(C)C NNPPMTNAJDCUHE-UHFFFAOYSA-N 0.000 description 1
- GETQZCLCWQTVFV-UHFFFAOYSA-N CN(C)C Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B37/00—Solid balls; Rigid hollow balls; Marbles
- A63B37/0003—Golf balls
- A63B37/005—Cores
- A63B37/0051—Materials other than polybutadienes; Constructional details
- A63B37/0054—Substantially rigid, e.g. metal
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B37/00—Solid balls; Rigid hollow balls; Marbles
- A63B37/0003—Golf balls
- A63B37/005—Cores
- A63B37/0051—Materials other than polybutadienes; Constructional details
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B37/00—Solid balls; Rigid hollow balls; Marbles
- A63B37/0003—Golf balls
- A63B37/005—Cores
- A63B37/006—Physical properties
- A63B37/0062—Hardness
- A63B37/0063—Hardness gradient
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B37/00—Solid balls; Rigid hollow balls; Marbles
- A63B37/0003—Golf balls
- A63B37/007—Characteristics of the ball as a whole
- A63B37/0072—Characteristics of the ball as a whole with a specified number of layers
- A63B37/0074—Two piece balls, i.e. cover and core
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/0025—Crosslinking or vulcanising agents; including accelerators
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/09—Carboxylic acids; Metal salts thereof; Anhydrides thereof
- C08K5/098—Metal salts of carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/14—Peroxides
Definitions
- the present invention relates to a golf ball traveling a great distance on driver shots, in particular, an improvement of a core of a golf ball.
- a method for improving flight distance on driver shots for example, there are methods of using a core having high resilience and using a core having a hardness distribution in which the hardness increases toward the surface of the core from the center thereof.
- the former method has an effect of enhancing an initial speed
- the latter method has an effect of a higher launch angle and a lower spin rate.
- a golf ball having a higher launch angle and a low spin rate travels a great distance.
- Japanese Patent Publications Nos. S61-37178 A, S61-113475 A, S61-253079 A, 2008-212681 A, 2008-523952 A and 2009-119256 A disclose a technique of enhancing resilience of the core.
- Japanese Patent Publication No. S61-37178 A and S61-113475 A disclose a solid golf ball having an inner core where zinc acrylate as a co-crosslinking agent, palmitic acid, stearic acid, or myristic acid as a co-crosslinking activator, zinc oxide as another co-crosslinking activator, and a reaction rate retarder are blended.
- Japanese Patent Publication No. S61-253079 A discloses a solid golf ball formed from a rubber composition containing an ⁇ , ⁇ -unsaturated carboxylic acid in an amount of 15 parts to 35 parts by weight, a metal compound to react with the ⁇ , ⁇ -unsaturated carboxylic acid and form a salt thereof in an amount of 7 parts to 60 parts by weight, and a high fatty acid metal salt in an amount of 1 part to 10 parts by weight with respect to 100 parts by weight of a base rubber.
- Japanese Patent Publication No. 2008-212681 A discloses a golf ball comprising, as a component, a molded and crosslinked product obtained from a rubber composition essentially comprising a base rubber, a filler, an organic peroxide, an ⁇ , ⁇ -unsaturated carboxylic acid and/or a metal salt thereof, a copper salt of a saturated or unsaturated fatty acid.
- Japanese Patent Publication No. 2008-523952 T discloses a golf ball, or a component thereof, molded from a composition comprising a base elastomer selected from the group consisting of polybutadiene and mixtures of polybutadiene with other elastomers, at least one metallic salt of an unsaturated monocarboxylic acid, a free radical initiator, and a non-conjugated diene monomer.
- Japanese Patent Publication No. 2009-119256 A discloses a method of manufacturing a golf ball, comprising preparing a masterbatch of an unsaturated carboxylic acid and/or a metal salt thereof by mixing the unsaturated carboxylic acid and/or the metal salt thereof with a rubber material ahead, using the masterbatch to prepare a rubber composition containing the rubber material, and employing a heated and molded product of the rubber composition as a golf ball component, wherein the masterbatch of the unsaturated carboxylic acid and/or the metal salt thereof comprises; (A) from 20 wt % to 100 wt % of a modified polybutadiene obtained by modifying a polybutadiene having a vinyl content of from 0 to 2%, a cis-1,4 bond content of at least 80% and active terminals, the active terminal being modified with at least one type of alkoxysilane compound, and (B) from 80 wt % to 0 wt % of a diene rubber other than (A
- Japanese Patent Publications Nos. H6-154357 A, 2008-194471 A, 2008-194473 A and 2010-253268 A disclose a core having a hardness distribution.
- Japanese Patent Publication No. H6-154357 A discloses a two-piece golf ball comprising a core formed of a rubber composition containing a base rubber, a co-crosslinking agent, and an organic peroxide, and a cover covering said core, wherein the core has the following hardness distribution according to JIS-C type hardness meter readings: (1) hardness at center: 58-73, (2) hardness at 5 to 10 mm from center: 65-75, (3) hardness at 15 mm from center: 74-82, (4) surface hardness: 76-84, wherein hardness (2) is almost constant within the above range, and the relation (1) ⁇ (2) ⁇ (3)(4) is satisfied.
- Japanese Patent Publication No. 2008-194471 A discloses a solid golf ball comprising a solid core and a cover layer that encases the core, wherein the solid core is formed of a rubber composition composed of 100 parts by weight of a base rubber that includes from 60 to 100 parts by weight of a polybutadiene rubber having a cis-1,4 bond content of at least 60% and synthesized using a rare-earth catalyst, from 0.1 to 5 parts by weight of an organosulfur compound, an unsaturated carboxylic acid or a metal salt thereof, an inorganic filler, and an antioxidant; the solid core has a deformation from 2.0 mm to 4.0 mm, when applying a load from an initial load of 10 kgf to a final load of 130 kgf and has the hardness distribution shown in the following table.
- Japanese Patent Publication No. 2008-194473 A discloses a solid golf ball comprising a solid core and a cover layer that encases the core, wherein the solid core is formed of a rubber composition composed of 100 parts by weight of a base rubber that includes from 60 to 100 parts by weight of a polybutadiene rubber having a cis-1,4 bond content of at least 60% and synthesized using a rare-earth catalyst, from 0.1 to 5 parts by weight of an organosulfur compound, an unsaturated carboxylic acid or a metal salt thereof, and an inorganic filler; the solid core has a deformation from 2.0 mm to 4.0 mm, when applying a load from an initial load of 10 kgf to a final load of 130 kgf and has the hardness distribution shown in the following table.
- Japanese Patent Publication No. 2010-253268 A discloses a multi-piece solid golf ball comprising a core, an envelope layer encasing the core, an intermediate layer encasing the envelope layer, and a cover which encases the intermediate layer and has formed on a surface thereof a plurality of dimples, wherein the core is formed primarily of a rubber material and has a hardness which gradually increases from a center to a surface thereof, the hardness difference in JIS-C hardness units between the core center and the core surface being at least 15 and, letting (I) be the average value for cross-sectional hardness at a position about 15 mm from the core center and at the core center and letting (II) be the cross-sectional hardness at a position about 7.5 mm from the core center, the hardness difference (I)-(II) in JIS-C units being within ⁇ 2; and the envelope layer, intermediate layer and cover have hardness which satisfy the condition: cover hardness>intermediate layer hardness>envelope layer hard
- An object of the present invention is to provide a golf ball traveling a great flight distance on driver shots.
- the present invention provides a golf ball comprising a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) an acid and/or a salt thereof, provided that the rubber composition further contains (e) a metal compound in the case of containing only (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent, wherein (d) the acid and/or the salt thereof has a relative solubility parameter ranging from 0.45 to 1.10 to (a) the base rubber and (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof.
- the spherical core has hardness distribution where the hardness increases linearly or almost linearly from the center of the core toward the surface thereof.
- the spherical core having hardness distribution where the hardness increase linearly or almost linearly from the center of the core toward the surface thereof, with a high degree of the outer-hard inner-soft structure reduces the spin rate on driver shots, thereby providing a greater flight distance on driver shots.
- the reason why the core has hardness distribution where the hardness increases linearly or almost linearly from the center of the core toward the surface thereof is considered as follows.
- the metal salt of (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms blended in the rubber composition is considered to form an ion cluster in the core thereby crosslinking the rubber molecules with metals.
- (d) the acid and/or the salt thereof having the relative solubility parameter satisfying a predetermined value to (a) the base rubber and (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof into this rubber composition (d) the acid and/or the salt thereof exchanges a cation with the ion cluster formed from the metal salt of (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms, thereby breaking the metal crosslinking by the metal salt of the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms.
- This cation exchange reaction easily occurs at the core central part where the temperature is high, and less occurs toward the core surface.
- the internal temperature of the core is high at the core central part and decreases toward the core surface, since reaction heat from a curing reaction of the rubber composition accumulates at the core central part.
- the breaking of the metal crosslinking by (d) the acid and/or the salt thereof easily occurs at the core central part, but less occurs toward the surface.
- FIG. 1 is a partially cutaway view of the golf ball of the preferred embodiment of the present invention.
- FIG. 2 is a graph showing the hardness distribution of the core
- FIG. 3 is a graph showing the hardness distribution of the core
- FIG. 4 is a graph showing the hardness distribution of the core
- FIG. 5 is a graph showing the hardness distribution of the core
- FIG. 6 is a graph showing the hardness distribution of the core
- FIG. 7 is a graph showing the hardness distribution of the core
- FIG. 8 is a graph showing the hardness distribution of the core
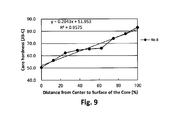
- FIG. 9 is a graph showing the hardness distribution of the core.
- FIG. 10 is a graph showing the hardness distribution of the core
- FIG. 11 is a graph showing the hardness distribution of the core
- FIG. 12 is a graph showing the hardness distribution of the core
- FIG. 13 is a graph showing the hardness distribution of the core
- FIG. 14 is a graph showing the hardness distribution of the core
- FIG. 15 is a graph showing the hardness distribution of the core
- FIG. 16 is a graph showing the hardness distribution of the core
- FIG. 17 is a graph showing the hardness distribution of the core
- FIG. 18 is a graph showing the hardness distribution of the core
- FIG. 19 is a graph showing the hardness distribution of the core.
- FIG. 20 is a graph showing the hardness distribution of the core.
- the present invention provides a golf ball comprising a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) an acid and/or a salt thereof, provided that the rubber composition further contains (e) a metal compound in the case of containing only (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking initiator, wherein (d) the acid and/or the salt thereof has a relative solubility parameter (hereinafter, sometimes may be merely referred to as “RS value”) ranging from 0.45 to 1.10 to (a) the base rubber and (b) the ⁇ , ⁇ -unsaturated carboxylic
- the base rubber used in the present invention will be described.
- natural rubber and/or synthetic rubber can be used.
- polybutadiene rubber, natural rubber, polyisoprene rubber, styrene polybutadiene rubber, ethylene-propylene-diene rubber (EPDM), or the like can be used.
- EPDM ethylene-propylene-diene rubber
- These rubbers may be used solely or two or more of these rubbers may be used in combination.
- Typically preferred of them is the high cis-polybutadiene having a cis-1,4 bond in a proportion of 40% or more, more preferably 80% or more, even more preferably 90% or more in view of its superior resilience property.
- the high-cis polybutadiene preferably has a 1,2-vinyl bond in a content of 2 mass % or less, more preferably 1.7 mass % or less, and even more preferably 1.5 mass % or less. If the content of 1,2-vinyl bond is excessively high, the resilience may be lowered.
- the high-cis polybutadiene is preferably one synthesized using a rare earth element catalyst.
- a neodymium catalyst which employs a neodymium compound of a lanthanum series rare earth element compound, is used, a polybutadiene rubber having a high content of a cis-1,4 bond and a low content of a 1,2-vinyl bond is obtained with excellent polymerization activity.
- a polybutadiene rubber is particularly preferred.
- the high-cis polybutadiene preferably has a Mooney viscosity (ML 1+4 (100° C.)) of 30 or more, more preferably 32 or more, even more preferably 35 or more, and preferably has a Mooney viscosity (ML 1+4 (100° C.)) of 140 or less, more preferably 120 or less, even more preferably 100 or less, and most preferably 80 or less.
- Mooney viscosity (ML 1+4 (100° C.)) in the present invention is a value measured according to JIS K6300 using an L rotor under the conditions of: a preheating time of 1 minute; a rotor revolution time of 4 minutes; and a temperature of 100° C.
- the high-cis polybutadiene preferably has a molecular weight distribution Mw/Mn (Mw: weight average molecular weight, Mn: number average molecular weight) of 2.0 or more, more preferably 2.2 or more, even more preferably 2.4 or more, and most preferably 2.6 or more, and preferably has a molecular weight distribution Mw/Mn of 6.0 or less, more preferably 5.0 or less, even more preferably 4.0 or less, and most preferably 3.4 or less. If the molecular weight distribution (Mw/Mn) of the high-cis polybutadiene is excessively low, the processability deteriorates.
- Mw/Mn molecular weight distribution
- the molecular weight distribution (Mw/Mn) of the high-cis polybutadiene is excessively high, the resilience may be lowered. It is noted that the measurement of the molecular weight distribution is conducted by gel permeation chromatography (“HLC-8120GPC”, manufactured by Tosoh Corporation) using a differential refractometer as a detector under the conditions of column: GMHHXL (manufactured by Tosoh Corporation), column temperature: 40° C., and mobile phase: tetrahydrofuran, and calculated by converting based on polystyrene standard.
- HSC-8120GPC gel permeation chromatography
- the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof will be described.
- the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof is blended as a co-crosslinking agent in the rubber composition and has an action of crosslinking a rubber molecule by graft polymerization to a base rubber molecular chain.
- the rubber composition used in the present invention contains only the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent, the rubber composition further contains (e) a metal compound as an essential component.
- Neutralizing the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms with the metal compound in the rubber composition provides substantially the same effect as using the metal salt of the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms. Further, in the case of using the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and the metal salt thereof in combination, (e) the metal compound may be used as an optional component.
- the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms includes, for example, acrylic acid, methacrylic acid, fumaric acid, maleic acid, crotonic acid, and the like.
- Examples of the metals constituting the metal salts of the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms include: monovalent metal ions such as sodium, potassium, lithium or the like; divalent metal ions such as magnesium, calcium, zinc, barium, cadmium or the like; trivalent metal ions such as aluminum ion or the like; and other metal ions such as tin, zirconium or the like.
- monovalent metal ions such as sodium, potassium, lithium or the like
- divalent metal ions such as magnesium, calcium, zinc, barium, cadmium or the like
- trivalent metal ions such as aluminum ion or the like
- other metal ions such as tin, zirconium or the like.
- the above metal ions can be used solely or as a mixture of at least two of them. Of these metal ions, divalent metal ions such as magnesium, calcium, zinc, barium, cadmium or the like are preferable.
- the divalent metal salts of the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms easily generates a metal crosslinking between the rubber molecules.
- zinc acrylate is preferable, because zinc acrylate enhances the resilience of the resultant golf ball.
- the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof may be used solely or in combination at least two of them.
- the content of (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof is preferably 15 parts by mass or more, more preferably 20 parts by mass or more, and is preferably 50 parts by mass or less, more preferably 45 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber. If the content of (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof is less than 15 parts by mass, the content of (c) the crosslinking initiator which will be described below must be increased in order to obtain the appropriate hardness of the constituting member formed from the rubber composition, which tends to cause the lower resilience.
- the crosslinking initiator is blended in order to crosslink (a) the base rubber component.
- an organic peroxide is preferred.
- the organic peroxide include organic peroxides such as dicumyl peroxide, 1,1-bis(t-butylperoxy)-3,3,5-trimethylcyclohexane, 2,5-dimethyl-2,5-di(t-butylperoxy)hexane, and di-t-butyl peroxide. These organic peroxides may be used solely or two or more of these organic peroxides may be used in combination. Dicumyl peroxide is preferably used of the organic peroxides.
- the content of (c) the crosslinking initiator is preferably 0.2 part by mass or more, and more preferably 0.5 part by mass or more, and is preferably 5.0 parts by mass or less, and more preferably 2.5 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber. If the content of (c) the crosslinking initiator is less than 0.2 part by mass, the constituting member formed from the rubber composition becomes too soft, and thus the golf ball may have the lower resilience. If the content of (c) the crosslinking initiator exceeds 5.0 parts by mass, the amount of (b) the co-crosslinking agent must be decreased in order to obtain the appropriate hardness of the constituting member formed from the rubber composition, resulting in the insufficient resilience and lower durability of the golf ball.
- RS value of (d) the acid and/or the salt thereof to (a) the base rubber and (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof falls within the above range, the compatibility of (d) the acid and/or the salt thereof to (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt is enhanced, resulting in the greater effect of breaking the metal crosslinking.
- RS value is preferably 0.45 or more, more preferably 0.48 or more, even more preferably 0.52 or more, and is preferably 1.10 or less, more preferably 1.05 or less.
- RS value of the acid constituting the salt may fall within the above range, and RS value of the salt itself may be without the above range. It is noted that (d) the acid and/or the salt thereof used in the present invention does not include the metal salt of (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms used as the co-crosslinking agent.
- RS value employed in the present invention is an index that shows to which the compatibility of the acid and/or the salt thereof added as (d) component is higher, (a) the base rubber or (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof that constitute the core. That is, RS value that is closer to 1 indicates that the compatibility of (d) component to (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof is higher.
- RS value is calculated from each SP value of (a) the base rubber, (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof, and the acid and/or the salt thereof added as (d) component.
- each SP value of (a) the base rubber, (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof, and the acid and/or the salt thereof added as (d) component should be calculated.
- SP value (at) means at defined by the following equations.
- ⁇ ⁇ ⁇ d ⁇ F di V
- ⁇ ⁇ ⁇ p ⁇ F pi 2 V
- ⁇ ⁇ ⁇ h ⁇ E hi V
- ⁇ t 2 ⁇ d 2 + ⁇ p 2 + ⁇ h 2
- V means a volume V (cm 3 /mole) according to Fedors
- Fdi, Fpi, and Ehi are solubility parameter components by the method of Hoftyzer and Van Krevelen.
- ⁇ d is a London dispersion component
- ⁇ p is a dipole moment component
- ⁇ h is hydrogen bonding component.
- the method for calculating SP values are described in “Properties of Polymers, chapter 7 (D. W. VANKREVELEN, Publisher: ELSEVIER, Published year: Third impression 2003)” in detail.
- Fdi, Fpi, Ehi and V of main functional groups are shown in table 3.
- a three-dimensional graph with ad on X axis, ⁇ P on y axis, and ah on z axis is prepared by plotting (a) component, (b) component and (c) component.
- the points of (a) component, (b) component, and (c) component are represented by A point (x A , y A , z A ), B point (x B , y B , Z B ), and P point (x P , y P , Z P ), respectively.
- the acid and/or the salt thereof may include any one of an aliphatic acid and/or a salt thereof and an aromatic acid and/or a salt thereof, as long as it exchanges the cation component with the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof.
- the acid and/or the salt thereof preferably includes a protonic acid and/or a salt thereof.
- the protonic acid includes oxo acids such as a carboxylic acid, a sulfonic acid, and a phosphoric acid or the like; and hydroacids such as hydrochloric acid, hydrofluoric acid or the like.
- Preferred of the acids is an oxo acid, and more preferred is a carboxylic acid.
- the numerical value after the names of compounds is RS value when using polybutadiene as (a) the base rubber and zinc acrylate as (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof.
- the protonic acid when used as (d) the acid and/or the salt thereof, the protonic acid preferably has a proton bond dissociation energy of 270 kcal/mole or less. If the protonic acid has the proton bond dissociation energy of 270 kcal/mole or less, the effect of breaking the metal crosslinking is enhanced. In this light, the proton bond dissociation energy is preferably 270 kcal/mole or less, and more preferably 265 kcal/mole or less. The proton bond dissociation energy is preferably 200 kcal/mole or more, and more preferably 210 kcal/mole or more.
- the bond dissociation energy of (d) the acid and/or the salt thereof is calculated from the energies of ground states before and after the dissociation.
- the bond dissociation energy is determined by subtracting the energy of the ground state before the dissociation (RCOOH) from the energy of the ground state after the dissociation (RCOO ⁇ +H + ).
- the energy of RCOOH, RCOO ⁇ , and H + can be calculated by a density functional theory (b3lyp/6-31g*).
- a cation component of the salt of (d) the acid may be any one of a metal ion, an ammonium ion and an organic cation.
- the metal ions include, for example: monovalent metal ions of sodium, potassium, lithium, silver, and the like; divalent metal ions of magnesium, calcium, zinc, barium, cadmium, copper, cobalt, nickel, manganese, and the like; trivalent metal ions of aluminum, iron, and the like; and other ions of tin, zirconium, titanium, and the like.
- the cation component of the salt of the carboxylic acid preferably includes zinc ion.
- the cation component may be used solely or as a mixture of two or more of them.
- the organic cation includes a cation having a carbon chain.
- the organic cation includes, for example, without limitation, an organic ammonium ion.
- the organic ammonium ion includes, for example: primary ammonium ions such as stearyl ammonium ion, hexyl ammonium ion, octyl ammonium ion, and 2-ethylhexyl ammonium ion; secondary ammonium ions such as dodecyl(lauryl) ammonium ion and octadecyl(stearyl) ammonium ion; tertiary ammonium ions such as trioctyl ammonium ion; and quaternary ammonium ions such as dioctyldimethyl ammonium ion and distearyldimethyl ammonium ion.
- the organic cation component may be used solely or as a mixture of two or more of them.
- the content of (d) the acid and/or the salt thereof is preferably 2 parts by mass or more, more preferably 2.2 parts by mass or more, even more preferably 2.4 parts by mass or more, and is preferably less than 40 parts by mass, more preferably 35 parts by mass or less, even more preferably 30 parts by mass or less. If the content is too little, the effect of adding (d) the acid and/or the salt thereof is not sufficient, and thus the degree of the outer-hard and inner-soft structure may be lowered. If the content is too much, the resilience of the core may be lowered, since the hardness of the resultant core may be lowered as a whole.
- the surface of zinc acrylate used as the co-crosslinking agent is treated with (d) the acid and/or the salt thereof to improve the dispersibility to the rubber.
- the amount of (d) the acid and/or the salt thereof used as a surface treating agent is included in the content of (d) component. For example, if 25 parts by mass of zinc acrylate whose surface treatment amount with (d) the acid and/or the salt thereof is 10 mass % is used, the amount of (d) the acid and/or the salt thereof is 2.5 parts by mass and the amount of zinc acrylate is 22.5 parts by mass. Thus, 2.5 parts by mass is counted as the content of (d) component.
- the content of (d) the acid and/or the salt thereof is preferably determined by the kind and the combination of the acid and/or the salt thereof. Particularly, the content of (d) the acid and/or the salt thereof is preferably determined by the carbon number and the combination of the acid and/or the salt thereof. It is conceivable that the action of breaking the metal crosslinking by (d) the acid and/or the salt thereof is affected by the number of moles of the acid and/or the salt thereof to be added. Concurrently, the acid and/or the salt thereof acts as a plasticizer for the spherical core. If the blending amount (mass) of the acid and/or the salt thereof to be added increases, the entire core is softened.
- This plasticizing effect is affected by the blending amount (mass) of the acid and/or the salt thereof to be added.
- the number of moles of the acid and/or the salt thereof to be added is made larger by using the acid and/or the salt thereof having less carbon atoms (small molecular weight) compared to using the acid and/or the salt thereof having larger carbon atoms (large molecular weight). That is, the acid and/or the salt thereof having less carbon atoms can enhance the effect of breaking the metal crosslinking, while suppressing softening the entire spherical core by the plasticizing effect.
- the content of the carboxylic acid having 1 to 14 carbon atoms and/or a salt thereof is preferably 2 parts by mass or more, more preferably 2.2 parts by mass or more, even more preferably 2.4 parts by mass or more, and is preferably 20 parts by mass or less, more preferably 18 parts by mass or less, even more preferably 16 parts by mass or less with respect to 100 parts by mass of (a) the base rubber.
- the carbon number of the salt of the carboxylic acid having 1 to 14 carbon atoms is the carbon number of the carboxylic acid component, and the carbon number of the organic cation is not included.
- the content of the carboxylic acid having 15 to 30 carbon atoms and/or the salt thereof is preferably 5 parts by mass or more, more preferably 6 parts by mass or more, even more preferably 7 parts by mass or more, and is preferably less than 40 parts by mass, more preferably 35 parts by mass or less, even more preferably 30 parts by mass or less.
- the carbon number of the salt of the carboxylic acid having 15 to 30 carbon atoms is the carbon number of the carboxylic acid component, and the carbon number of the organic cation is not included.
- the rubber composition used in the present invention contains only the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent
- the rubber composition further contains (e) a metal compound as an essential component.
- the metal compound is not limited as long as it can neutralize (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms in the rubber composition.
- the metal compound includes, for example, metal hydroxides such as magnesium hydroxide, zinc hydroxide, calcium hydroxide, sodium hydroxide, lithium hydroxide, potassium hydroxide, copper hydroxide, and the like; metal oxides such as magnesium oxide, calcium oxide, zinc oxide, copper oxide, and the like; metal carbonates such as magnesium carbonate, zinc carbonate, calcium carbonate, sodium carbonate, lithium carbonate, potassium carbonate, and the like.
- the metal compound preferably includes a divalent metal compound, more preferably includes a zinc compound. The divalent metal compound reacts with the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms, thereby forming a metal crosslinking. Use of the zinc compound provides a golf ball with excellent resilience.
- These metal compounds may be used solely or as a mixture of at least two of them.
- the rubber composition used in the preset invention preferably further contains (f) an organic sulfur compound.
- an organic sulfur compound is not particularly limited, as long as it is an organic compound having a sulfur atom in the molecule thereof.
- Examples thereof include an organic compound having a thiol group (—SH), a polysulfide bond having 2 to 4 sulfur atoms (—S—S—, —S—S—S—, or —S—S—S—S—), or a metal salt thereof (—SM, —S-M-S—, —S-M-S—S—, —S—S-M-S—S—, —S-M-S—S—S—, or the like; M is a metal atom).
- the organic sulfur compound may be any one of aliphatic compounds (aliphatic thiol, aliphatic thiocarboxylic acid, aliphatic dithiocarboxylic acid, aliphatic polysulfides, or the like), heterocyclic compounds, alicyclic compounds (alicyclic thiol, alicyclic thiocarboxylic acid, alicyclic dithiocarboxylic acid, alicyclic polysulfides, or the like), and aromatic compounds.
- aliphatic compounds aliphatic thiol, aliphatic thiocarboxylic acid, aliphatic dithiocarboxylic acid, aliphatic polysulfides, or the like
- heterocyclic compounds alicyclic compounds (alicyclic thiol, alicyclic thiocarboxylic acid, alicyclic dithiocarboxylic acid, alicyclic polysulfides, or the like)
- aromatic compounds alicyclic compounds (alicyclic
- the organic sulfur compound includes, for example, thiophenols, thionaphthols, polysulfides, thiocarboxylic acids, dithiocarboxylic acids, sulfenamides, thiurams, dithiocarbamates, and thiazoles. From the aspect of the larger hardness distribution of the core, (f) the organic sulfur compound preferably includes, organic compounds having a thiol group (—SH) or a metal salt thereof, more preferably thiophenols, thionaphthols, or a metal salt thereof.
- —SH thiol group
- metal salts examples include salts of monovalent metals such as sodium, lithium, potassium, copper (I), and silver (I), and salts of divalent metals such as zinc, magnesium, calcium, strontium, barium, titanium (II), manganese (II), iron (II), cobalt (II), nickel(II), zirconium(II), and tin (II).
- monovalent metals such as sodium, lithium, potassium, copper (I), and silver (I
- divalent metals such as zinc, magnesium, calcium, strontium, barium, titanium (II), manganese (II), iron (II), cobalt (II), nickel(II), zirconium(II), and tin (II).
- thiophenols include, for example, thiophenol; thiophenols substituted with a fluoro group, such as 4-fluorothiophenol, 2,5-difluorothiophenol, 2,4,5-trifluorothiophenol, 2,4,5,6-tetrafluorothiophenol, pentafluorothiophenol and the like; thiophenols substituted with a chloro group, such as 2-chlorothiophenol, 4-chlorothiophenol, 2,4-dichlorothiophenol, 2,5-dichlorothiophenol, 2,6-dichlorothiophenol, 2,4,5-trichlorothiophenol, 2,4,5,6-tetrachlorothiophenol, pentachlorothiophenol and the like; thiophenols substituted with a bromo group, such as 4-bromothiophenol, 2,5-dibromothiophenol, 2,4,5-tribromothiophenol, 2,4,5,6-tetrabrom
- naphthalenethiols examples include 2-naphthalenethiol, 1-naphthalenethiol, 2-chloro-1-naphthalenethiol, 2-bromo-1-naphthalenethiol, 2-fluoro-1-naphthalenethiol, 2-cyano-1-naphthalenethiol, 2-acetyl-1-naphthalenethiol, 1-chloro-2-naphthalenethiol, 1-bromo-2-naphthalenethiol, 1-fluoro-2-naphthalenethiol, 1-cyano-2-naphthalenethiol, and 1-acetyl-2-naphthalenethiol and metal salts thereof.
- Preferable examples include 1-naphthalenethiol, 2-naphthalenethiol and zinc salt thereof.
- the sulfenamide based organic sulfur compound includes, for example, N-cyclohexyl-2-benzothiazole sulfenamide, N-oxydiethylene-2-benzothiazole sulfenamide, and N-t-butyl-2-benzothiazole sulfenamide.
- the thiuram based organic sulfur compound includes, for example, tetramethylthiuram monosulfide, tetramethylthiuram disulfide, tetraethylthiuram disulfide, tetrabutylthiuram disulfide, and dipentamethylenethiuram tetrasulfide.
- the dithiocarbamates include, for example, zinc dimethyldithiocarbamate, zinc diethyldithiocarbamate, zinc dibutyldithiocarbamate, zinc ethylphenyl dithiocarbamate, sodium dimethyldithiocarbamate, sodium diethyldithiocarbamate, copper (II) dimethyldithiocarbate, iron (III) dimethyldithiocarbamate, selenium diethyldithiocarbamate, and tellurium diethyldithiocarbamate.
- the thiazole based organic sulfur compound includes, for example, 2-mercaptobenzothiazole (MBT), dibenzothiazyl disulfide (MBTS), sodium salt, zinc salt, copper salt, or cyclohexylamine salt of 2-mercaptobenzothiazole, 2-(2,4-dinitrophenyl) mercaptobenzothiazole, and 2-(2,6-diethyl-4-morpholinothio)benzothiazole.
- MBT 2-mercaptobenzothiazole
- MBTS dibenzothiazyl disulfide
- sodium salt zinc salt
- copper salt copper salt
- 2-(2,6-diethyl-4-morpholinothio)benzothiazole 2-(2,6-diethyl-4-morpholinothio)benzothiazole.
- the organic sulfur compound can be used solely or as a mixture of at least two of them.
- the content of (f) the organic sulfur compound is preferably 0.05 part by mass or more, more preferably 0.1 part by mass or more, and is preferably 5.0 parts by mass or less, more preferably 2.0 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber. If the content of (f) the organic sulfur compound is less than 0.05 part by mass, the effect of adding (f) the organic sulfur compound cannot be obtained and thus the resilience of the golf ball may not improve. If the content of (f) the organic sulfur compound exceeds 5.0 parts by mass, the compression deformation amount of the obtained golf ball becomes large and thus the resilience may be lowered.
- the rubber composition used in the present invention can include additives such as a pigment, a filler for adjusting weight or the like, an antioxidant, a peptizing agent, and a softener where necessary. Further, as described above, if the rubber composition used in the present invention contains only the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms as the crosslinking agent, the rubber composition preferably contains (e) the metal compound.
- Examples of the pigment blended in the rubber composition include a white pigment, a blue pigment, and a purple pigment.
- a white pigment titanium oxide is preferably used.
- the type of titanium oxide is not particularly limited, but rutile type is preferably used because of the high opacity.
- the blending amount of titanium oxide is preferably 0.5 part by mass or more, and more preferably 2 parts by mass or more, and is preferably 8 parts by mass or less, and more preferably 5 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber.
- the rubber composition contains both a white pigment and a blue pigment.
- the blue pigment is blended in order to cause white color to be vivid, and examples thereof include ultramarine blue, cobalt blue, and phthalocyanine blue.
- Examples of the purple pigment include anthraquinone violet, dioxazine violet, and methyl violet.
- the blending amount of the blue pigment is preferably 0.001 part by mass or more, and more preferably 0.05 part by mass or more, and is preferably 0.2 part by mass or less, and more preferably 0.1 part by mass or less, with respect to 100 parts by mass of (a) the base rubber. If the blending amount of the blue pigment is less than 0.001 part by mass, blueness is insufficient, and the color looks yellowish. If the blending amount of the blue pigment exceeds 0.2 part by mass, blueness is excessively strong, and a vivid white appearance is not provided.
- the filler blended in the rubber composition is used as a weight adjusting agent for mainly adjusting the weight of the golf ball obtained as an final product.
- the filler may be blended where necessary.
- the filler includes, for example, inorganic fillers such as zinc oxide, barium sulfate, calcium carbonate, magnesium oxide, tungsten powder, molybdenum powder, or the like.
- the content of the filler is preferably 0.5 part by mass or more, more preferably 1 part by mass or more, and is preferably 30 parts by mass or less, more preferably 25 parts by mass or less, even more preferably 20 parts by mass or less. If the content of the filler is less than 0.5 part by mass, it is difficult to adjust the weight, while if the content of the filler exceeds 30 parts by mass, the weight ratio of the rubber component is reduced and thus the resilience tends to be lowered.
- the blending amount of the antioxidant is preferably 0.1 part by mass or more and 1 part by mass or less, with respect to 100 parts by mass of (a) the base rubber.
- the blending amount of the peptizing agent is preferably 0.1 part by mass or more and 5 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber.
- the rubber composition used in the present invention is obtained by mixing and kneading (a) the base rubber, (b) the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof, (c) the crosslinking initiator, (d) the acid and/or the salt thereof, and other additives where necessary.
- the kneading can be conducted, without any limitation, with a well-known kneading machine such as a kneading roll, a banbury mixer, a kneader, or the like.
- the spherical core of the golf ball of the present invention can be obtained by molding the rubber composition after kneaded.
- the temperature for molding the spherical core is preferably 120° C. or more, more preferably 150° C. or more, even more preferably 160° C. or more, and is preferably 170° C. or less. If the molding temperature exceeds 170° C., the surface hardness of the core tends to decrease.
- the molding pressure preferably ranges from 2.9 MPa to 11.8 MPa.
- the molding time preferably ranges from 10 minutes to 60 minutes.
- the spherical core is such that R 2 of a linear approximation curve obtained by the least square method is 0.93 or higher. If R 2 is 0.93 or more, the linearity of the core hardness distribution is enhanced, thus the spin rate on driver shots decreases, resulting in the improved flying performance.
- the hardness of the spherical core is JIS-C hardness measured at nine points obtained by dividing a radius of the spherical core into equal parts having 12.5% interval. That is, JIS-C hardness is measured at nine points, namely at distances of 0% (core center), 12.5%, 25%, 37.5%, 50%, 62.5%, 75%, 87.5%, 100% (core surface) from the core center. Next, the measurement results are plotted to make a graph having JIS-C hardness as a vertical axis and distances (%) from the core center as a horizontal axis.
- R 2 of a linear approximation curve obtained from this graph by the least square method is preferably 0.93 or higher.
- R 2 of the linear approximation curve obtained by the least square method indicates the linearity of the obtained plot.
- R 2 of 0.93 or more means that the core has the hardness distribution where the hardness increases linearly or almost linearly. If the core having the hardness distribution where the hardness increases linearly or almost linearly is used for the golf ball, the spin rate on driver shots decrease. As a result, the flight distance on driver shots increases.
- R 2 of the linear approximation curve is preferably 0.94 or more. The higher linearity provides a greater flight distance on driver shots.
- the spherical core preferably has a hardness difference (Hs ⁇ Ho) between a surface hardness Hs and a center hardness Ho of 20 or more, more preferably 25 or more, even more preferably 30 or more, and preferably has a hardness difference of 80 or less, more preferably 70 or less, even more preferably 60 or less in JIS-C hardness. If the hardness difference between the center hardness and the surface hardness is large, the golf ball having a great flight distance due to the high launch angle and low spin rate is obtained.
- Hs ⁇ Ho hardness difference
- the spherical core preferably has a center hardness Ho of 30 or more, more preferably 35 or more, and even more preferably 40 or more in JIS-C hardness. If the center hardness Ho is less than 30 in JIS-C hardness, the core becomes too soft and thus the resilience may be lowered. Further, the spherical core preferably has a center hardness Ho of 70 or less, more preferably 65 or less, even more preferably 60 or less in JIS-C hardness. If the center hardness Ho exceeds 70 in JIS-C hardness, the core becomes too hard and thus the shot feeling tends to be lowered.
- the spherical core preferably has a surface hardness Hs of 76 or more, more preferably 78 or more, even more preferably 80 or more, and preferably has a surface hardness Hs of 100 or less, more preferably 95 or less in JIS-C hardness. If the surface hardness is 76 or more in JIS-C hardness, the spherical core does not become excessively soft, and thus the better resilience is obtained. Further, if the surface hardness of the spherical core is 100 or less in JIS-C hardness, the spherical core does not become excessively hard, and thus the better shot feeling is obtained.
- the spherical core preferably has a diameter of 34.8 mm or more, more preferably 36.8 mm or more, and even more preferably 38.8 mm or more, and preferably has a diameter of 42.2 mm or less, more preferably 41.8 mm or less, and even more preferably 41.2 mm or less, and most preferably 40.8 mm or less.
- the spherical core has a diameter of 34.8 mm or more, the thickness of the cover does not become too thick and thus the resilience becomes better. On the other hand, if the spherical core has a diameter of 42.2 mm or less, the thickness of the cover does not become too thin, and hence a protection ability of the cover is sufficiently provided.
- a compression deformation amount (shrinking deformation amount of the spherical core along the compression direction) of the spherical core when applying a load from an initial load of 98N to a final load of 1275N is preferably 2.0 mm or more, more preferably 2.8 mm or more, and is preferably 6.0 mm or less, more preferably 5.0 mm or less. If the compression deformation amount is 2.0 mm or more, the shot feeling of the golf ball becomes better. If the compression deformation amount is 6.0 mm or less, the resilience of the golf ball becomes better.
- the golf ball cover of the present invention is formed from a cover composition comprising a resin component.
- the resin component include, for example, an ionomer rein; a thermoplastic polyurethane elastomer having a commercial name of “Elastollan (e.g. “Elastollan XNY85A”)” commercially available from BASF Japan Ltd; a thermoplastic polyamide elastomer having a commercial name of “Pebax (e.g. “Pebax 2533”)” commercially available from Arkema K.
- the ionomer resin includes a product prepared by neutralizing at least a part of carboxyl groups in the binary copolymer composed of an olefin and an ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms with a metal ion, a product prepared by neutralizing at least a part of carboxyl groups in the ternary copolymer composed of an olefin, an ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms, and an ⁇ , ⁇ -unsaturated carboxylic acid ester with a metal ion, or a mixture of those.
- the olefin preferably includes an olefin having 2 to 8 carbon atoms.
- Examples of the olefin are ethylene, propylene, butene, pentene, hexene, heptene, and octene.
- the olefin more preferably includes ethylene.
- Examples of the ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms are acrylic acid, methacrylic acid, fumaric acid, maleic acid and crotonic acid. Acrylic acid and methacrylic acid are particularly preferred.
- the ⁇ , ⁇ -unsaturated carboxylic acid ester examples include methyl ester, ethyl ester, propyl ester, n-butyl ester, isobutyl ester of acrylic acid, methacrylic acid, fumaric acid, maleic acid or the like.
- acrylic acid ester and methacrylic acid ester are preferable.
- the ionomer resin preferably includes the metal ion-neutralized product of the binary copolymer composed of ethylene-(meth)acrylic acid and the metal ion-neutralized product of the ternary copolymer composed of ethylene, (meth)acrylic acid, and (meth)acrylic acid ester.
- ionomer resins include trade name “Himilan® (e.g. the binary copolymerized ionomer such as Himilan 1555 (Na), Himilan 1557 (Zn), Himilan 1605 (Na), Himilan 1706 (Zn), Himilan 1707 (Na), Himilan AM7311 (Mg); and the ternary copolymerized ionomer such as Himilan 1856 (Na), Himilan 1855 (Zn))” commercially available from Du Pont-Mitsui Polychemicals Co., Ltd.
- Himilan® e.g. the binary copolymerized ionomer such as Himilan 1555 (Na), Himilan 1557 (Zn), Himilan 1605 (Na), Himilan 1706 (Zn), Himilan 1707 (Na), Himilan AM7311 (Mg)
- Himilan 1856 Na
- Himilan 1855 Zn
- examples include “Surlyn (registered trademark) (e.g. the binary copolymerized ionomer such as Surlyn 8945 (Na), Surlyn 9945 (Zn), Surlyn 8140 (Na), Surlyn 8150 (Na), Surlyn 9120 (Zn), Surlyn 9150 (Zn), Surlyn 6910 (Mg), Surlyn 6120 (Mg), Surlyn 7930 (Li), Surlyn 7940 (Li), Surlyn AD8546 (Li); and the ternary copolymerized ionomer such as Surlyn 8120 (Na), Surlyn 8320 (Na), Surlyn 9320 (Zn), Surlyn 6320 (Mg), HPF 1000 (Mg), HPF 2000 (Mg))” commercially available from E.I. du Pont de Nemours and Company.
- the binary copolymerized ionomer such as Surlyn 8945 (Na), Surlyn 9945
- examples include “lotek (registered trademark) (e.g. the binary copolymerized ionomer such as lotek 8000 (Na), lotek 8030 (Na), lotek 7010 (Zn), lotek 7030 (Zn); and the ternary copolymerized ionomer such as lotek 7510 (Zn), lotek 7520 (Zn))” commercially available from ExxonMobil Chemical Corporation.
- the binary copolymerized ionomer such as lotek 8000 (Na), lotek 8030 (Na), lotek 7010 (Zn), lotek 7030 (Zn); and the ternary copolymerized ionomer such as lotek 7510 (Zn), lotek 7520 (Zn)
- Na, Zn, Li, and Mg described in the parentheses after the trade names indicate metal types of neutralizing metal ions for the ionomer resins.
- the ionomer resin may be used solely or as a mixture of two or more of them.
- the cover composition constituting the cover of the golf ball of the present invention preferably includes, as a resin component, a thermoplastic polyurethane elastomer or an ionomer rein.
- a thermoplastic polyurethane elastomer or an ionomer rein it is preferred to use a thermoplastic styrene elastomer together.
- the content of the polyurethane or ionomer resin in the resin component of the cover composition is preferably 50 mass % or more, more preferably 60 mass % or more, even more preferably 70 mass % or more.
- the cover composition may further contain a pigment component such as a white pigment (for example, titanium oxide), a blue pigment, and a red pigment; a weight adjusting agent such as zinc oxide, calcium carbonate, and barium sulfate; a dispersant; an antioxidant; an ultraviolet absorber; a light stabilizer; a fluorescent material or a fluorescent brightener; and the like, as long as they do not impair the effect of the present invention.
- a pigment component such as a white pigment (for example, titanium oxide), a blue pigment, and a red pigment
- a weight adjusting agent such as zinc oxide, calcium carbonate, and barium sulfate
- a dispersant such as zinc oxide, calcium carbonate, and barium sulfate
- an antioxidant such as an antioxidant
- an ultraviolet absorber for example, a light stabilizer
- a fluorescent material or a fluorescent brightener for example, a fluorescent material or a fluorescent brightener
- the amount of the white pigment (for example, titanium oxide) is preferably 0.5 part or more, more preferably 1 part or more, and the content of the white pigment is preferably 10 parts or less, more preferably 8 parts or less, with respect to 100 parts of the resin component constituting the cover by mass. If the amount of the white pigment is 0.5 part by mass or more, it is possible to impart the opacity to the resultant cover. Further, if the amount of the white pigment is more than 10 parts by mass, the durability of the resultant cover may deteriorate.
- the slab hardness of the cover composition is preferably set in accordance with the desired performance of the golf balls.
- the cover composition preferably has a slab hardness of 50 or more, more preferably 55 or more, and preferably has a slab hardness of 80 or less, more preferably 70 or less in shore D hardness. If the cover composition has a slab hardness of 50 or more, the obtained golf ball has a high launch angle and low spin rate on driver shots and iron shots, and thus the flight distance improves. If the cover composition has a slab hardness of 80 or less, the golf ball excellent in durability is obtained.
- the cover composition preferably has a slab hardness of less than 50, and preferably has a slab hardness of 20 or more, more preferably 25 or more in shore D hardness. If the cover composition has a slab hardness of less than 50, the flight distance on driver shots can be improved by the core of the present invention, as well as the obtained golf ball readily stops on the green due to the high spin rate on approach shots. If the cover composition has a slab hardness of 20 or more, the abrasion resistance improves. In case of a plurality of cover layers, the slab hardness of the cover composition constituting each layer can be identical or different, as long as the slab hardness of each layer is within the above range.
- An embodiment for molding a cover is not particularly limited, and includes an embodiment which comprises injection molding the cover composition directly onto the core, or an embodiment which comprises molding the cover composition into a hollow-shell, covering the core with a plurality of the hollow-shells and subjecting the core with a plurality of the hollow shells to the compression-molding (preferably an embodiment which comprises molding the cover composition into a half hollow-shell, covering the core with the two half hollow-shells, and subjecting the core with the two half hollow-shells to the compression-molding).
- molding of the half shell can be performed by either compression molding method or injection molding method, and the compression molding method is preferred.
- the compression-molding of the cover composition into half shell can be carried out, for example, under a pressure of 1 MPa or more and 20 MPa or less at a temperature of ⁇ 20° C. or more and 70° C. or less relative to the flow beginning temperature of the cover composition.
- a half shell having a uniform thickness can be formed.
- Examples of a method for molding the cover using half shells include compression molding by covering the core with two half shells.
- the compression molding of half shells into the cover can be carried out, for example, under a pressure of 0.5 MPa or more and 25 MPa or less at a temperature of ⁇ 20° C. or more and 70° C. or less relative to the flow beginning temperature of the cover composition.
- the cover composition extruded in the pellet form beforehand may be used for injection molding or the materials such as the base resin components and the pigment may be dry blended, followed by directly injection molding the blended material. It is preferred to use upper and lower molds having a spherical cavity and pimples for forming a cover, wherein a part of the pimples also serves as a retractable hold pin.
- the core is charged and held with the protruding hold pin, the cover composition which has been heated and melted is charged, and then cooled to obtain a cover.
- the cover composition heated and melted at the temperature ranging from 200° C. to 250° C. is charged into a mold held under the pressure of 9 MPa to 15 MPa for 0.5 to 5 seconds, and after cooling for 10 to 60 seconds, the mold is opened and the golf ball with the cover molded is taken out from the mold.
- the concave portions called “dimple” are usually formed on the surface of the cover.
- the total number of the dimples is preferably 200 or more and 500 or less. If the total number is less than 200, the dimple effect is hardly obtained. On the other hand, if the total number exceeds 500, the dimple effect is hardly obtained because the size of the respective dimples is small.
- the shape (shape in a plan view) of dimples includes, for example, without limitation, a circle, polygonal shapes such as roughly triangular shape, roughly quadrangular shape, roughly pentagonal shape, roughly hexagonal shape, and another irregular shape. The shape of the dimples is employed solely or at least two of them may be used in combination.
- the cover of the golf ball preferably has a thickness of 4.0 mm or less, more preferably 3.0 mm or less, even more preferably 2.0 mm or less. If the thickness of the cover is 4.0 mm or less, the resilience and shot feeling of the obtained golf ball become better.
- the cover of the golf ball preferably has a thickness of 0.3 mm or more, more preferably 0.5 mm or more, and even more preferably 0.8 mm or more, and most preferably 1.0 mm or more. If the thickness of the cover is less than 0.3 mm, the durability and the wear resistance of the cover may deteriorate. If the cover has a plurality of layers, it is preferred that the total thickness of the cover layers falls within the above range.
- the mold is opened and the golf ball body is taken out from the mold, and as necessary, the golf ball body is preferably subjected to surface treatments such as deburring, cleaning, and sandblast.
- a paint film or a mark may be formed.
- the paint film preferably has a thickness of, but not limited to, 5 ⁇ m or larger, and more preferably 7 ⁇ m or larger, and preferably has a thickness of 50 ⁇ m or smaller, and more preferably 40 ⁇ m or smaller, even more preferably 30 ⁇ m or smaller. If the thickness is smaller than 5 ⁇ m, the paint film is easy to wear off due to continued use of the golf ball, and if the thickness is larger than 50 ⁇ m, the effect of the dimples is reduced, resulting in lowering flying performance of the golf ball.
- a compression deformation amount of the golf ball (shrinking amount of the golf ball in the compression direction thereof) when applying a load from an initial load of 98 N to a final load of 1275 N to the golf ball is preferably 2.0 mm or more, more preferably 2.4 mm or more, even more preferably 2.5 mm or more, most preferably 2.8 mm or more, and is preferably 5.0 mm or less, more preferably 4.5 mm or less, even more preferably 3.6 mm or less. If the compression deformation amount is 2.0 mm or more, the golf ball does not become excessively hard, and thus exhibits the good shot feeling. On the other hand, if the compression deformation amount is 5.0 mm or less, the resilience is enhanced.
- FIG. 1 is a partially cutaway sectional view showing the golf ball 2 according to the preferable embodiment of the present invention.
- the golf ball 2 comprises a spherical core 4 , and a cover 12 covering the spherical core 4 .
- Plurality of dimples 14 are formed on a surface of the cover. Other portions than dimples 14 on the surface of the golf ball 2 are referred to as land 16 .
- the golf ball 2 is provided with a paint layer and a mark layer outside the cover 12 , but these layers are not depicted.
- the spherical core preferably has a single layered structure. Unlike the multi-layered structure, the spherical core of the single layered structure does not have an energy loss at the interface of the multi-layered structure when hitting, and thus has an enhanced resilience.
- the cover has a structure of at least one layer, for example a single layered structure, or a multi-layered structure of at least two layers.
- the golf ball of the present invention includes, for example, a two-piece golf ball comprising a spherical core and a single layered cover disposed around the spherical core, a multi-piece golf ball comprising a spherical core, and at least two cover layers disposed around the spherical core (including the three-piece golf ball), and a wound golf ball comprising a spherical core, a rubber thread layer which is formed around the spherical core, and a cover disposed over the rubber thread layer.
- the present invention can be suitably applied to any one of the above golf balls.
- a compression deformation amount of the core or golf ball (a shrinking amount of the core or golf ball in the compression direction thereof), when applying a load from an initial load of 98N to a final load of 1275N to the core or golf ball, was measured.
- a 198.4 g of metal cylindrical object was allowed to collide with each core or golf ball at a speed of 40 m/sec, and the speeds of the cylindrical object and the core or golf ball before and after the collision were measured. Based on these speeds and the mass of each object, coefficient of restitution for each core or golf ball was calculated. The measurement was conducted by using twelve samples for each core or golf ball, and the average value was regarded as the coefficient of restitution for the core or golf ball. In Tables 4 to 6, the coefficient of restitution is shown as the difference from that of golf ball (core) No. 17.
- Sheets with a thickness of about 2 mm were produced by injection molding the cover composition, and stored at 23° C. for two weeks. Three or more of these sheets were stacked on one another so as not to be affected by the measuring substrate on which the sheets were placed, and the hardness of the stack was measured with a type P1 auto loading durometer manufactured by Kobunshi Keiki Co., Ltd., provided with a Shore D type spring hardness tester prescribed in ASTM-D2240.
- a type P1 auto loading durometer manufactured by Kobunshi Keiki Co., Ltd., provided with a JIS-C type spring hardness tester was used to measure the hardness of the spherical core.
- the hardness measured at the surface of the spherical core was adopted as the surface hardness of the spherical core.
- the spherical core was cut into two hemispheres to obtain a cut plane, and the hardness was measured at the central point and at predetermined distances from the central point of the cut plane.
- the core hardness was measured at 4 points at predetermined distances from the central point of the cut plane of the core.
- the core hardness was calculated by averaging the hardness measured at 4 points.
- a metal-headed W#1 driver (XXIO S, loft: 11°, manufactured by SRI Sports Limited) was installed on a swing robot M/C manufactured by Golf Laboratories, Inc.
- a golf ball was hit at a head speed of 40 m/sec, and the flight distance (the distance from the launch point to the stop point) and the spin rate right after hitting the golf ball were measured. This measurement was conducted twelve times for each golf ball, and the average value was adopted as the measurement value for the golf ball.
- a sequence of photographs of the hit golf ball were taken for measuring the spin rate (rpm) right after hitting the golf ball.
- the flight distance and the spin rate is shown as the difference from that of golf ball (core) No. 17.
- the rubber compositions having formulations shown in Tables 4 to 6 were kneaded and heat-pressed in upper and lower molds, each having a hemispherical cavity, at 170° C. for 20 minutes to prepare spherical cores having a diameter of 39.8 mm.
- Zinc oxide “Ginrei R” manufactured by Toho Zinc Co., Ltd.
- Barium sulfate “Barium sulfate BD” manufactured by Sakai Chemical Industry Co., Ltd., adjustment was made such that the finally obtained golf ball had a mass of 45.4 g.
- 2-thionaphthol manufactured by Tokyo Chemical Industry Co., Ltd.
- 4-acetoxybenzoic acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 98.0% Lauric acid: manufactured by Tokyo Chemical Industry Co., Ltd.
- Cyclohexane carboxylic acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 98.0% Dimethylaminobenzoic acid: manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 98.0% Benzoic acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 98.0% Chlorobenzoic acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 99.0% Decanoic acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 98.0% Octanoic acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 98.0 % Dodecanedioic acid: manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 98.0% Suberic acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 99.0% 2-propylvaleric acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Purity 99.0% Neodecanoic acid manufactured by Wako Pure Chemical Industries
- Purity 100.0% 2-heptylundecanoic acid manufactured by Tokyo Chemical Industry Co., Ltd.
- Cover materials shown in Table 7 were mixed with a twin-screw kneading extruder to prepare the cover compositions in the pellet form.
- the cover compositions obtained above were injection-molded onto the spherical cores to produce the golf balls having the spherical core and the cover covering the spherical core.
- the golf balls comprising a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an ⁇ , ⁇ -unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) an acid and/or a salt thereof travel a great flight distance on driver shots, respectively.
- the golf ball of the present invention travels a great flight distance on driver shots.
- This application is based on Japanese Patent applications No. 2012-109717 filed on May 11, 2012 the contents of which are hereby incorporated by reference.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Physical Education & Sports Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
The present invention provides a golf ball having a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) an acid and/or a salt thereof, provided that the rubber composition further contains (e) a metal compound in the case of containing only (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent, wherein (d) the acid and/or the salt thereof has a relative solubility parameter to (a) the base rubber and (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof ranging from 0.45 to 1.10.
Description
- The present invention relates to a golf ball traveling a great distance on driver shots, in particular, an improvement of a core of a golf ball.
- As a method for improving flight distance on driver shots, for example, there are methods of using a core having high resilience and using a core having a hardness distribution in which the hardness increases toward the surface of the core from the center thereof. The former method has an effect of enhancing an initial speed, and the latter method has an effect of a higher launch angle and a lower spin rate. A golf ball having a higher launch angle and a low spin rate travels a great distance.
- For example, Japanese Patent Publications Nos. S61-37178 A, S61-113475 A, S61-253079 A, 2008-212681 A, 2008-523952 A and 2009-119256 A disclose a technique of enhancing resilience of the core. Japanese Patent Publication No. S61-37178 A and S61-113475 A disclose a solid golf ball having an inner core where zinc acrylate as a co-crosslinking agent, palmitic acid, stearic acid, or myristic acid as a co-crosslinking activator, zinc oxide as another co-crosslinking activator, and a reaction rate retarder are blended.
- Japanese Patent Publication No. S61-253079 A discloses a solid golf ball formed from a rubber composition containing an α,β-unsaturated carboxylic acid in an amount of 15 parts to 35 parts by weight, a metal compound to react with the α,β-unsaturated carboxylic acid and form a salt thereof in an amount of 7 parts to 60 parts by weight, and a high fatty acid metal salt in an amount of 1 part to 10 parts by weight with respect to 100 parts by weight of a base rubber.
- Japanese Patent Publication No. 2008-212681 A discloses a golf ball comprising, as a component, a molded and crosslinked product obtained from a rubber composition essentially comprising a base rubber, a filler, an organic peroxide, an α,β-unsaturated carboxylic acid and/or a metal salt thereof, a copper salt of a saturated or unsaturated fatty acid.
- Japanese Patent Publication No. 2008-523952 T discloses a golf ball, or a component thereof, molded from a composition comprising a base elastomer selected from the group consisting of polybutadiene and mixtures of polybutadiene with other elastomers, at least one metallic salt of an unsaturated monocarboxylic acid, a free radical initiator, and a non-conjugated diene monomer.
- Japanese Patent Publication No. 2009-119256 A discloses a method of manufacturing a golf ball, comprising preparing a masterbatch of an unsaturated carboxylic acid and/or a metal salt thereof by mixing the unsaturated carboxylic acid and/or the metal salt thereof with a rubber material ahead, using the masterbatch to prepare a rubber composition containing the rubber material, and employing a heated and molded product of the rubber composition as a golf ball component, wherein the masterbatch of the unsaturated carboxylic acid and/or the metal salt thereof comprises; (A) from 20 wt % to 100 wt % of a modified polybutadiene obtained by modifying a polybutadiene having a vinyl content of from 0 to 2%, a cis-1,4 bond content of at least 80% and active terminals, the active terminal being modified with at least one type of alkoxysilane compound, and (B) from 80 wt % to 0 wt % of a diene rubber other than (A) the above rubber component [the figures are represented by wt % in the case that a total amount of (A) and (B) equal to 100 wt %] and (C) an unsaturated carboxylic acid and/or a metal salt thereof.
- For example, Japanese Patent Publications Nos. H6-154357 A, 2008-194471 A, 2008-194473 A and 2010-253268 A disclose a core having a hardness distribution. Japanese Patent Publication No. H6-154357 A discloses a two-piece golf ball comprising a core formed of a rubber composition containing a base rubber, a co-crosslinking agent, and an organic peroxide, and a cover covering said core, wherein the core has the following hardness distribution according to JIS-C type hardness meter readings: (1) hardness at center: 58-73, (2) hardness at 5 to 10 mm from center: 65-75, (3) hardness at 15 mm from center: 74-82, (4) surface hardness: 76-84, wherein hardness (2) is almost constant within the above range, and the relation (1)<(2)<(3)(4) is satisfied.
- Japanese Patent Publication No. 2008-194471 A discloses a solid golf ball comprising a solid core and a cover layer that encases the core, wherein the solid core is formed of a rubber composition composed of 100 parts by weight of a base rubber that includes from 60 to 100 parts by weight of a polybutadiene rubber having a cis-1,4 bond content of at least 60% and synthesized using a rare-earth catalyst, from 0.1 to 5 parts by weight of an organosulfur compound, an unsaturated carboxylic acid or a metal salt thereof, an inorganic filler, and an antioxidant; the solid core has a deformation from 2.0 mm to 4.0 mm, when applying a load from an initial load of 10 kgf to a final load of 130 kgf and has the hardness distribution shown in the following table.
-
TABLE 1 Hardness distribution in solid core Shore D harness Center 30 to 48 Region located 4 mm from center 34 to 52 Region located 8 mm from center 40 to 58 Region located 12 mm from center (Q) 43 to 61 Region located 2 to 3 mm inside of 36 to 54 surface (R) Surface (S) 41 to 59 Hardness difference [(Q) − (S)] 1 to 10 Hardness difference [(S) − (R)] 3 to 10 - Japanese Patent Publication No. 2008-194473 A discloses a solid golf ball comprising a solid core and a cover layer that encases the core, wherein the solid core is formed of a rubber composition composed of 100 parts by weight of a base rubber that includes from 60 to 100 parts by weight of a polybutadiene rubber having a cis-1,4 bond content of at least 60% and synthesized using a rare-earth catalyst, from 0.1 to 5 parts by weight of an organosulfur compound, an unsaturated carboxylic acid or a metal salt thereof, and an inorganic filler; the solid core has a deformation from 2.0 mm to 4.0 mm, when applying a load from an initial load of 10 kgf to a final load of 130 kgf and has the hardness distribution shown in the following table.
-
TABLE 2 Hardness distribution in solid core Shore D harness Center 25 to 45 Region located 5 to 10 mm from center 39 to 58 Region located 15 mm from center 36 to 55 Surface (S) 55 to 75 Hardness difference 20 to 50 between center and surface - Japanese Patent Publication No. 2010-253268 A discloses a multi-piece solid golf ball comprising a core, an envelope layer encasing the core, an intermediate layer encasing the envelope layer, and a cover which encases the intermediate layer and has formed on a surface thereof a plurality of dimples, wherein the core is formed primarily of a rubber material and has a hardness which gradually increases from a center to a surface thereof, the hardness difference in JIS-C hardness units between the core center and the core surface being at least 15 and, letting (I) be the average value for cross-sectional hardness at a position about 15 mm from the core center and at the core center and letting (II) be the cross-sectional hardness at a position about 7.5 mm from the core center, the hardness difference (I)-(II) in JIS-C units being within ±2; and the envelope layer, intermediate layer and cover have hardness which satisfy the condition: cover hardness>intermediate layer hardness>envelope layer hardness.
- An object of the present invention is to provide a golf ball traveling a great flight distance on driver shots.
- The present invention provides a golf ball comprising a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) an acid and/or a salt thereof, provided that the rubber composition further contains (e) a metal compound in the case of containing only (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent, wherein (d) the acid and/or the salt thereof has a relative solubility parameter ranging from 0.45 to 1.10 to (a) the base rubber and (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof. The present invention is configured as described above and thus provides the spherical core having a high degree of an outer-hard and inner soft structure, which reduces a spin rate and provides a greater flight distance on driver shots.
- In the preferable embodiment of the present invention, the spherical core has hardness distribution where the hardness increases linearly or almost linearly from the center of the core toward the surface thereof. The spherical core having hardness distribution where the hardness increase linearly or almost linearly from the center of the core toward the surface thereof, with a high degree of the outer-hard inner-soft structure reduces the spin rate on driver shots, thereby providing a greater flight distance on driver shots. The reason why the core has hardness distribution where the hardness increases linearly or almost linearly from the center of the core toward the surface thereof is considered as follows. The metal salt of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms blended in the rubber composition is considered to form an ion cluster in the core thereby crosslinking the rubber molecules with metals. By blending (d) the acid and/or the salt thereof having the relative solubility parameter satisfying a predetermined value to (a) the base rubber and (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof into this rubber composition, (d) the acid and/or the salt thereof exchanges a cation with the ion cluster formed from the metal salt of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms, thereby breaking the metal crosslinking by the metal salt of the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms. This cation exchange reaction easily occurs at the core central part where the temperature is high, and less occurs toward the core surface. When molding a core, the internal temperature of the core is high at the core central part and decreases toward the core surface, since reaction heat from a curing reaction of the rubber composition accumulates at the core central part. In other words, the breaking of the metal crosslinking by (d) the acid and/or the salt thereof easily occurs at the core central part, but less occurs toward the surface. As a result, it is conceivable that since a crosslinking density in the core increases from the center of the core toward the surface thereof, the core hardness increases linearly or almost linearly from the center of the core toward the surface thereof. Additionally, in the present invention, it is conceivable that since the value of the relative solubility parameter falls within the range from 0.45 to 1.10, the compatibility of (d) the acid and/or the salt thereof to the metal salt of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms is enhanced, and the cation exchange reaction occurs more easily, thereby increasing the effect of breaking the metal crosslinking.
- According to the present invention, it is possible to provide a golf ball traveling a great flight distance.
-
FIG. 1 is a partially cutaway view of the golf ball of the preferred embodiment of the present invention; -
FIG. 2 is a graph showing the hardness distribution of the core; -
FIG. 3 is a graph showing the hardness distribution of the core; -
FIG. 4 is a graph showing the hardness distribution of the core; -
FIG. 5 is a graph showing the hardness distribution of the core; -
FIG. 6 is a graph showing the hardness distribution of the core; -
FIG. 7 is a graph showing the hardness distribution of the core; -
FIG. 8 is a graph showing the hardness distribution of the core; -
FIG. 9 is a graph showing the hardness distribution of the core; -
FIG. 10 is a graph showing the hardness distribution of the core; -
FIG. 11 is a graph showing the hardness distribution of the core; -
FIG. 12 is a graph showing the hardness distribution of the core; -
FIG. 13 is a graph showing the hardness distribution of the core; -
FIG. 14 is a graph showing the hardness distribution of the core; -
FIG. 15 is a graph showing the hardness distribution of the core; -
FIG. 16 is a graph showing the hardness distribution of the core; -
FIG. 17 is a graph showing the hardness distribution of the core; -
FIG. 18 is a graph showing the hardness distribution of the core; -
FIG. 19 is a graph showing the hardness distribution of the core; and -
FIG. 20 is a graph showing the hardness distribution of the core. - The present invention provides a golf ball comprising a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) an acid and/or a salt thereof, provided that the rubber composition further contains (e) a metal compound in the case of containing only (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking initiator, wherein (d) the acid and/or the salt thereof has a relative solubility parameter (hereinafter, sometimes may be merely referred to as “RS value”) ranging from 0.45 to 1.10 to (a) the base rubber and (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof.
- First, (a) the base rubber used in the present invention will be described. As (a) the base rubber used in the present invention, natural rubber and/or synthetic rubber can be used. For example, polybutadiene rubber, natural rubber, polyisoprene rubber, styrene polybutadiene rubber, ethylene-propylene-diene rubber (EPDM), or the like can be used. These rubbers may be used solely or two or more of these rubbers may be used in combination. Typically preferred of them is the high cis-polybutadiene having a cis-1,4 bond in a proportion of 40% or more, more preferably 80% or more, even more preferably 90% or more in view of its superior resilience property.
- The high-cis polybutadiene preferably has a 1,2-vinyl bond in a content of 2 mass % or less, more preferably 1.7 mass % or less, and even more preferably 1.5 mass % or less. If the content of 1,2-vinyl bond is excessively high, the resilience may be lowered.
- The high-cis polybutadiene is preferably one synthesized using a rare earth element catalyst. When a neodymium catalyst, which employs a neodymium compound of a lanthanum series rare earth element compound, is used, a polybutadiene rubber having a high content of a cis-1,4 bond and a low content of a 1,2-vinyl bond is obtained with excellent polymerization activity. Such a polybutadiene rubber is particularly preferred.
- The high-cis polybutadiene preferably has a Mooney viscosity (ML1+4 (100° C.)) of 30 or more, more preferably 32 or more, even more preferably 35 or more, and preferably has a Mooney viscosity (ML1+4 (100° C.)) of 140 or less, more preferably 120 or less, even more preferably 100 or less, and most preferably 80 or less. It is noted that the Mooney viscosity (ML1+4 (100° C.)) in the present invention is a value measured according to JIS K6300 using an L rotor under the conditions of: a preheating time of 1 minute; a rotor revolution time of 4 minutes; and a temperature of 100° C.
- The high-cis polybutadiene preferably has a molecular weight distribution Mw/Mn (Mw: weight average molecular weight, Mn: number average molecular weight) of 2.0 or more, more preferably 2.2 or more, even more preferably 2.4 or more, and most preferably 2.6 or more, and preferably has a molecular weight distribution Mw/Mn of 6.0 or less, more preferably 5.0 or less, even more preferably 4.0 or less, and most preferably 3.4 or less. If the molecular weight distribution (Mw/Mn) of the high-cis polybutadiene is excessively low, the processability deteriorates. If the molecular weight distribution (Mw/Mn) of the high-cis polybutadiene is excessively high, the resilience may be lowered. It is noted that the measurement of the molecular weight distribution is conducted by gel permeation chromatography (“HLC-8120GPC”, manufactured by Tosoh Corporation) using a differential refractometer as a detector under the conditions of column: GMHHXL (manufactured by Tosoh Corporation), column temperature: 40° C., and mobile phase: tetrahydrofuran, and calculated by converting based on polystyrene standard.
- Next, (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof will be described. (b) The α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof is blended as a co-crosslinking agent in the rubber composition and has an action of crosslinking a rubber molecule by graft polymerization to a base rubber molecular chain. In the case that the rubber composition used in the present invention contains only the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent, the rubber composition further contains (e) a metal compound as an essential component. Neutralizing the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms with the metal compound in the rubber composition provides substantially the same effect as using the metal salt of the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms. Further, in the case of using the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and the metal salt thereof in combination, (e) the metal compound may be used as an optional component.
- The α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms includes, for example, acrylic acid, methacrylic acid, fumaric acid, maleic acid, crotonic acid, and the like.
- Examples of the metals constituting the metal salts of the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms include: monovalent metal ions such as sodium, potassium, lithium or the like; divalent metal ions such as magnesium, calcium, zinc, barium, cadmium or the like; trivalent metal ions such as aluminum ion or the like; and other metal ions such as tin, zirconium or the like. The above metal ions can be used solely or as a mixture of at least two of them. Of these metal ions, divalent metal ions such as magnesium, calcium, zinc, barium, cadmium or the like are preferable. Use of the divalent metal salts of the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms easily generates a metal crosslinking between the rubber molecules. Especially, as the divalent metal salt, zinc acrylate is preferable, because zinc acrylate enhances the resilience of the resultant golf ball. The α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof may be used solely or in combination at least two of them.
- The content of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof is preferably 15 parts by mass or more, more preferably 20 parts by mass or more, and is preferably 50 parts by mass or less, more preferably 45 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber. If the content of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof is less than 15 parts by mass, the content of (c) the crosslinking initiator which will be described below must be increased in order to obtain the appropriate hardness of the constituting member formed from the rubber composition, which tends to cause the lower resilience. On the other hand, if the content of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof exceeds 50 parts by mass, the constituting member formed from the rubber composition becomes excessively hard, which tends to cause the lower shot feeling.
- (c) The crosslinking initiator is blended in order to crosslink (a) the base rubber component. As (c) the crosslinking initiator, an organic peroxide is preferred. Specific examples of the organic peroxide include organic peroxides such as dicumyl peroxide, 1,1-bis(t-butylperoxy)-3,3,5-trimethylcyclohexane, 2,5-dimethyl-2,5-di(t-butylperoxy)hexane, and di-t-butyl peroxide. These organic peroxides may be used solely or two or more of these organic peroxides may be used in combination. Dicumyl peroxide is preferably used of the organic peroxides.
- The content of (c) the crosslinking initiator is preferably 0.2 part by mass or more, and more preferably 0.5 part by mass or more, and is preferably 5.0 parts by mass or less, and more preferably 2.5 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber. If the content of (c) the crosslinking initiator is less than 0.2 part by mass, the constituting member formed from the rubber composition becomes too soft, and thus the golf ball may have the lower resilience. If the content of (c) the crosslinking initiator exceeds 5.0 parts by mass, the amount of (b) the co-crosslinking agent must be decreased in order to obtain the appropriate hardness of the constituting member formed from the rubber composition, resulting in the insufficient resilience and lower durability of the golf ball.
- Next, (d) the acid and/or the salt thereof will be described. It is though that (d) the acid and/or the salt thereof has an action of breaking the metal crosslinking by the metal salt of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms, in the center part of the core, when molding the core. In particular, if RS value of (d) the acid and/or the salt thereof to (a) the base rubber and (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof falls within the above range, the compatibility of (d) the acid and/or the salt thereof to (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt is enhanced, resulting in the greater effect of breaking the metal crosslinking. In this light, RS value is preferably 0.45 or more, more preferably 0.48 or more, even more preferably 0.52 or more, and is preferably 1.10 or less, more preferably 1.05 or less. If the salt is used as (d) component, RS value of the acid constituting the salt may fall within the above range, and RS value of the salt itself may be without the above range. It is noted that (d) the acid and/or the salt thereof used in the present invention does not include the metal salt of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms used as the co-crosslinking agent.
- RS value employed in the present invention is an index that shows to which the compatibility of the acid and/or the salt thereof added as (d) component is higher, (a) the base rubber or (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof that constitute the core. That is, RS value that is closer to 1 indicates that the compatibility of (d) component to (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof is higher. RS value is calculated from each SP value of (a) the base rubber, (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof, and the acid and/or the salt thereof added as (d) component.
- Hereinafter, a method of calculating RS value will be described. To calculate RS value, firstly, each SP value of (a) the base rubber, (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof, and the acid and/or the salt thereof added as (d) component should be calculated. SP value (at) means at defined by the following equations.
-
- In the equations, V means a volume V (cm3/mole) according to Fedors, and Fdi, Fpi, and Ehi are solubility parameter components by the method of Hoftyzer and Van Krevelen. Herein, δd is a London dispersion component, δp is a dipole moment component, and δh is hydrogen bonding component. The method for calculating SP values are described in “Properties of Polymers, chapter 7 (D. W. VANKREVELEN, Publisher: ELSEVIER, Published year: Third impression 2003)” in detail. Fdi, Fpi, Ehi and V of main functional groups are shown in table 3.
-
TABLE 3 Kind of Fdi Fpi Ehi V atomic group J1/2•cm2/3•mol−1 J1/2•cm2/3•mol−1 J/mol cm3/mol —CH3 420 0 0 33.5 —CH2— 270 0 0 16.1 80 0 0 −1.0 −70 0 0 −19.2 ═CH2 400 0 0 28.5 ═CH— 200 0 0 13.5 70 0 0 —5.5 Phenyl 1430 110 0 71.4 Phenylene 1270 110 0 52.4 (o,m,p) —F (220) — — 18.0 —Cl 450 550 400 24.0 —Br (550) — — 30.0 —CN 430 1100 2500 24.0 —OH 210 500 20000 10.0 —O— 100 400 3000 3.8 —COH 470 800 4500 22.3 (aldehyde) —CO— 290 770 2000 10.8 —COOH 530 420 10000 28.5 —COO— 390 490 7000 18.0 HCOO— 530 — — 32.5 (formate) —NH2 280 — 8400 19.2 —NH— 160 210 3100 4.5 20 800 5000 −9.0 —NO2 500 1070 1500 24.0 (aliphatic) —S— 440 — — 12 ═PO4— 740 1890 13000 28.0 - Based on the obtained SP value, a three-dimensional graph with ad on X axis, σP on y axis, and ah on z axis is prepared by plotting (a) component, (b) component and (c) component. Herein, the points of (a) component, (b) component, and (c) component are represented by A point (xA, yA, zA), B point (xB, yB, ZB), and P point (xP, yP, ZP), respectively. To the line AB connecting A point and B point, a perpendicular line is drawn from P point to determine the intersection H. Assuming that the length of the line segment AB is 1, RS value is defined as the distance from the point A to the point H. RS value is calculated by the equation described below.
-
- (d) The acid and/or the salt thereof may include any one of an aliphatic acid and/or a salt thereof and an aromatic acid and/or a salt thereof, as long as it exchanges the cation component with the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof.
- (d) The acid and/or the salt thereof preferably includes a protonic acid and/or a salt thereof. The protonic acid includes oxo acids such as a carboxylic acid, a sulfonic acid, and a phosphoric acid or the like; and hydroacids such as hydrochloric acid, hydrofluoric acid or the like. Preferred of the acids is an oxo acid, and more preferred is a carboxylic acid. As (d) the acid and/or the salt thereof, 4-acetoxybenzoic acid (0.99), dimethylaminobenzoic acid (0.96), benzoic acid (0.90), cyclohexane carboxylic acid (0.90), suberic acid (0.88), dodecanedioic acid (0.74), octanoic acid (0.61), 2-propylpentanoic acid (0.60), neodecanoic acid (0.54), lauric acid (0.54), decanoic acid (0.53), chlorobenzoic acid (0.52), myristic acid (0.50), 2-hexyldecanoic acid (0.47), stearic acid (0.46), oleic acid (0.46), 2-heptylundecanoic acid (0.45) are exemplified. The numerical value after the names of compounds is RS value when using polybutadiene as (a) the base rubber and zinc acrylate as (b) the α,γ-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof.
- When the protonic acid and/or the salt thereof is used as (d) the acid and/or the salt thereof, the protonic acid preferably has a proton bond dissociation energy of 270 kcal/mole or less. If the protonic acid has the proton bond dissociation energy of 270 kcal/mole or less, the effect of breaking the metal crosslinking is enhanced. In this light, the proton bond dissociation energy is preferably 270 kcal/mole or less, and more preferably 265 kcal/mole or less. The proton bond dissociation energy is preferably 200 kcal/mole or more, and more preferably 210 kcal/mole or more.
- The bond dissociation energy of (d) the acid and/or the salt thereof is calculated from the energies of ground states before and after the dissociation. For example, in case of a carboxylic acid, the bond dissociation energy is determined by subtracting the energy of the ground state before the dissociation (RCOOH) from the energy of the ground state after the dissociation (RCOO−+H+). The energy of RCOOH, RCOO−, and H+ can be calculated by a density functional theory (b3lyp/6-31g*).
- A cation component of the salt of (d) the acid may be any one of a metal ion, an ammonium ion and an organic cation. The metal ions include, for example: monovalent metal ions of sodium, potassium, lithium, silver, and the like; divalent metal ions of magnesium, calcium, zinc, barium, cadmium, copper, cobalt, nickel, manganese, and the like; trivalent metal ions of aluminum, iron, and the like; and other ions of tin, zirconium, titanium, and the like. The cation component of the salt of the carboxylic acid preferably includes zinc ion. The cation component may be used solely or as a mixture of two or more of them.
- The organic cation includes a cation having a carbon chain. The organic cation includes, for example, without limitation, an organic ammonium ion. The organic ammonium ion includes, for example: primary ammonium ions such as stearyl ammonium ion, hexyl ammonium ion, octyl ammonium ion, and 2-ethylhexyl ammonium ion; secondary ammonium ions such as dodecyl(lauryl) ammonium ion and octadecyl(stearyl) ammonium ion; tertiary ammonium ions such as trioctyl ammonium ion; and quaternary ammonium ions such as dioctyldimethyl ammonium ion and distearyldimethyl ammonium ion. The organic cation component may be used solely or as a mixture of two or more of them.
- The content of (d) the acid and/or the salt thereof is preferably 2 parts by mass or more, more preferably 2.2 parts by mass or more, even more preferably 2.4 parts by mass or more, and is preferably less than 40 parts by mass, more preferably 35 parts by mass or less, even more preferably 30 parts by mass or less. If the content is too little, the effect of adding (d) the acid and/or the salt thereof is not sufficient, and thus the degree of the outer-hard and inner-soft structure may be lowered. If the content is too much, the resilience of the core may be lowered, since the hardness of the resultant core may be lowered as a whole. There are cases where the surface of zinc acrylate used as the co-crosslinking agent is treated with (d) the acid and/or the salt thereof to improve the dispersibility to the rubber. In the case of using zinc acrylate whose surface is treated with (d) the acid and/or the salt thereof, in the present invention, the amount of (d) the acid and/or the salt thereof used as a surface treating agent is included in the content of (d) component. For example, if 25 parts by mass of zinc acrylate whose surface treatment amount with (d) the acid and/or the salt thereof is 10 mass % is used, the amount of (d) the acid and/or the salt thereof is 2.5 parts by mass and the amount of zinc acrylate is 22.5 parts by mass. Thus, 2.5 parts by mass is counted as the content of (d) component.
- The content of (d) the acid and/or the salt thereof is preferably determined by the kind and the combination of the acid and/or the salt thereof. Particularly, the content of (d) the acid and/or the salt thereof is preferably determined by the carbon number and the combination of the acid and/or the salt thereof. It is conceivable that the action of breaking the metal crosslinking by (d) the acid and/or the salt thereof is affected by the number of moles of the acid and/or the salt thereof to be added. Concurrently, the acid and/or the salt thereof acts as a plasticizer for the spherical core. If the blending amount (mass) of the acid and/or the salt thereof to be added increases, the entire core is softened. This plasticizing effect is affected by the blending amount (mass) of the acid and/or the salt thereof to be added. In view of those actions, on the same blending amount (mass), the number of moles of the acid and/or the salt thereof to be added is made larger by using the acid and/or the salt thereof having less carbon atoms (small molecular weight) compared to using the acid and/or the salt thereof having larger carbon atoms (large molecular weight). That is, the acid and/or the salt thereof having less carbon atoms can enhance the effect of breaking the metal crosslinking, while suppressing softening the entire spherical core by the plasticizing effect.
- For example, if a carboxylic acid having 1 to 14 carbon atoms and/or a salt thereof is used as (d) the acid and/or the salt thereof, the content of the carboxylic acid having 1 to 14 carbon atoms and/or a salt thereof is preferably 2 parts by mass or more, more preferably 2.2 parts by mass or more, even more preferably 2.4 parts by mass or more, and is preferably 20 parts by mass or less, more preferably 18 parts by mass or less, even more preferably 16 parts by mass or less with respect to 100 parts by mass of (a) the base rubber. The carbon number of the salt of the carboxylic acid having 1 to 14 carbon atoms is the carbon number of the carboxylic acid component, and the carbon number of the organic cation is not included.
- For example, if a carboxylic acid having 15 to 30 carbon atoms and/or a salt thereof is used as (d) the acid and/or the salt thereof, the content of the carboxylic acid having 15 to 30 carbon atoms and/or the salt thereof is preferably 5 parts by mass or more, more preferably 6 parts by mass or more, even more preferably 7 parts by mass or more, and is preferably less than 40 parts by mass, more preferably 35 parts by mass or less, even more preferably 30 parts by mass or less. The carbon number of the salt of the carboxylic acid having 15 to 30 carbon atoms is the carbon number of the carboxylic acid component, and the carbon number of the organic cation is not included.
- In the case that the rubber composition used in the present invention contains only the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent, the rubber composition further contains (e) a metal compound as an essential component. (e) The metal compound is not limited as long as it can neutralize (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms in the rubber composition. (e) The metal compound includes, for example, metal hydroxides such as magnesium hydroxide, zinc hydroxide, calcium hydroxide, sodium hydroxide, lithium hydroxide, potassium hydroxide, copper hydroxide, and the like; metal oxides such as magnesium oxide, calcium oxide, zinc oxide, copper oxide, and the like; metal carbonates such as magnesium carbonate, zinc carbonate, calcium carbonate, sodium carbonate, lithium carbonate, potassium carbonate, and the like. (e) The metal compound preferably includes a divalent metal compound, more preferably includes a zinc compound. The divalent metal compound reacts with the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms, thereby forming a metal crosslinking. Use of the zinc compound provides a golf ball with excellent resilience. (e) These metal compounds may be used solely or as a mixture of at least two of them.
- The rubber composition used in the preset invention preferably further contains (f) an organic sulfur compound. In the present invention, by using (f) the organic sulfur compound and (d) the acid and/or the salt thereof in combination for the core rubber composition, the degree of the outer-hard and inner-soft structure of the core can be controlled, while maintaining approximate linearity of the core hardness distribution. (f) The organic sulfur compound is not particularly limited, as long as it is an organic compound having a sulfur atom in the molecule thereof. Examples thereof include an organic compound having a thiol group (—SH), a polysulfide bond having 2 to 4 sulfur atoms (—S—S—, —S—S—S—, or —S—S—S—S—), or a metal salt thereof (—SM, —S-M-S—, —S-M-S—S—, —S—S-M-S—S—, —S-M-S—S—S—, or the like; M is a metal atom). Furthermore, (f) the organic sulfur compound may be any one of aliphatic compounds (aliphatic thiol, aliphatic thiocarboxylic acid, aliphatic dithiocarboxylic acid, aliphatic polysulfides, or the like), heterocyclic compounds, alicyclic compounds (alicyclic thiol, alicyclic thiocarboxylic acid, alicyclic dithiocarboxylic acid, alicyclic polysulfides, or the like), and aromatic compounds. (f) The organic sulfur compound includes, for example, thiophenols, thionaphthols, polysulfides, thiocarboxylic acids, dithiocarboxylic acids, sulfenamides, thiurams, dithiocarbamates, and thiazoles. From the aspect of the larger hardness distribution of the core, (f) the organic sulfur compound preferably includes, organic compounds having a thiol group (—SH) or a metal salt thereof, more preferably thiophenols, thionaphthols, or a metal salt thereof. Examples of the metal salts are salts of monovalent metals such as sodium, lithium, potassium, copper (I), and silver (I), and salts of divalent metals such as zinc, magnesium, calcium, strontium, barium, titanium (II), manganese (II), iron (II), cobalt (II), nickel(II), zirconium(II), and tin (II).
- Examples of the thiophenols include, for example, thiophenol; thiophenols substituted with a fluoro group, such as 4-fluorothiophenol, 2,5-difluorothiophenol, 2,4,5-trifluorothiophenol, 2,4,5,6-tetrafluorothiophenol, pentafluorothiophenol and the like; thiophenols substituted with a chloro group, such as 2-chlorothiophenol, 4-chlorothiophenol, 2,4-dichlorothiophenol, 2,5-dichlorothiophenol, 2,6-dichlorothiophenol, 2,4,5-trichlorothiophenol, 2,4,5,6-tetrachlorothiophenol, pentachlorothiophenol and the like; thiophenols substituted with a bromo group, such as 4-bromothiophenol, 2,5-dibromothiophenol, 2,4,5-tribromothiophenol, 2,4,5,6-tetrabromothiophenol, pentabromothiophenol and the like; thiophenols substituted with an iodo group, such as 4-iodothiophenol, 2,5-diiodothiophenol, 2,4,5-triiodothiophenol, 2,4,5,6-tetraiodothiophenol, pentaiodothiophenol and the like; or a metal salt thereof. As the metal salt, zinc salt is preferred.
- Examples of the naphthalenethiols (thionaphthols) are 2-naphthalenethiol, 1-naphthalenethiol, 2-chloro-1-naphthalenethiol, 2-bromo-1-naphthalenethiol, 2-fluoro-1-naphthalenethiol, 2-cyano-1-naphthalenethiol, 2-acetyl-1-naphthalenethiol, 1-chloro-2-naphthalenethiol, 1-bromo-2-naphthalenethiol, 1-fluoro-2-naphthalenethiol, 1-cyano-2-naphthalenethiol, and 1-acetyl-2-naphthalenethiol and metal salts thereof. Preferable examples include 1-naphthalenethiol, 2-naphthalenethiol and zinc salt thereof.
- The sulfenamide based organic sulfur compound includes, for example, N-cyclohexyl-2-benzothiazole sulfenamide, N-oxydiethylene-2-benzothiazole sulfenamide, and N-t-butyl-2-benzothiazole sulfenamide. The thiuram based organic sulfur compound includes, for example, tetramethylthiuram monosulfide, tetramethylthiuram disulfide, tetraethylthiuram disulfide, tetrabutylthiuram disulfide, and dipentamethylenethiuram tetrasulfide. The dithiocarbamates include, for example, zinc dimethyldithiocarbamate, zinc diethyldithiocarbamate, zinc dibutyldithiocarbamate, zinc ethylphenyl dithiocarbamate, sodium dimethyldithiocarbamate, sodium diethyldithiocarbamate, copper (II) dimethyldithiocarbate, iron (III) dimethyldithiocarbamate, selenium diethyldithiocarbamate, and tellurium diethyldithiocarbamate. The thiazole based organic sulfur compound includes, for example, 2-mercaptobenzothiazole (MBT), dibenzothiazyl disulfide (MBTS), sodium salt, zinc salt, copper salt, or cyclohexylamine salt of 2-mercaptobenzothiazole, 2-(2,4-dinitrophenyl) mercaptobenzothiazole, and 2-(2,6-diethyl-4-morpholinothio)benzothiazole.
- (f) The organic sulfur compound can be used solely or as a mixture of at least two of them.
- The content of (f) the organic sulfur compound is preferably 0.05 part by mass or more, more preferably 0.1 part by mass or more, and is preferably 5.0 parts by mass or less, more preferably 2.0 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber. If the content of (f) the organic sulfur compound is less than 0.05 part by mass, the effect of adding (f) the organic sulfur compound cannot be obtained and thus the resilience of the golf ball may not improve. If the content of (f) the organic sulfur compound exceeds 5.0 parts by mass, the compression deformation amount of the obtained golf ball becomes large and thus the resilience may be lowered.
- The rubber composition used in the present invention can include additives such as a pigment, a filler for adjusting weight or the like, an antioxidant, a peptizing agent, and a softener where necessary. Further, as described above, if the rubber composition used in the present invention contains only the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the crosslinking agent, the rubber composition preferably contains (e) the metal compound.
- Examples of the pigment blended in the rubber composition include a white pigment, a blue pigment, and a purple pigment. As the white pigment, titanium oxide is preferably used. The type of titanium oxide is not particularly limited, but rutile type is preferably used because of the high opacity. The blending amount of titanium oxide is preferably 0.5 part by mass or more, and more preferably 2 parts by mass or more, and is preferably 8 parts by mass or less, and more preferably 5 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber.
- It is also preferred that the rubber composition contains both a white pigment and a blue pigment. The blue pigment is blended in order to cause white color to be vivid, and examples thereof include ultramarine blue, cobalt blue, and phthalocyanine blue. Examples of the purple pigment include anthraquinone violet, dioxazine violet, and methyl violet.
- The blending amount of the blue pigment is preferably 0.001 part by mass or more, and more preferably 0.05 part by mass or more, and is preferably 0.2 part by mass or less, and more preferably 0.1 part by mass or less, with respect to 100 parts by mass of (a) the base rubber. If the blending amount of the blue pigment is less than 0.001 part by mass, blueness is insufficient, and the color looks yellowish. If the blending amount of the blue pigment exceeds 0.2 part by mass, blueness is excessively strong, and a vivid white appearance is not provided.
- The filler blended in the rubber composition is used as a weight adjusting agent for mainly adjusting the weight of the golf ball obtained as an final product. The filler may be blended where necessary. The filler includes, for example, inorganic fillers such as zinc oxide, barium sulfate, calcium carbonate, magnesium oxide, tungsten powder, molybdenum powder, or the like. The content of the filler is preferably 0.5 part by mass or more, more preferably 1 part by mass or more, and is preferably 30 parts by mass or less, more preferably 25 parts by mass or less, even more preferably 20 parts by mass or less. If the content of the filler is less than 0.5 part by mass, it is difficult to adjust the weight, while if the content of the filler exceeds 30 parts by mass, the weight ratio of the rubber component is reduced and thus the resilience tends to be lowered.
- The blending amount of the antioxidant is preferably 0.1 part by mass or more and 1 part by mass or less, with respect to 100 parts by mass of (a) the base rubber. In addition, the blending amount of the peptizing agent is preferably 0.1 part by mass or more and 5 parts by mass or less, with respect to 100 parts by mass of (a) the base rubber.
- The rubber composition used in the present invention is obtained by mixing and kneading (a) the base rubber, (b) the α,δ-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof, (c) the crosslinking initiator, (d) the acid and/or the salt thereof, and other additives where necessary. The kneading can be conducted, without any limitation, with a well-known kneading machine such as a kneading roll, a banbury mixer, a kneader, or the like.
- The spherical core of the golf ball of the present invention can be obtained by molding the rubber composition after kneaded. The temperature for molding the spherical core is preferably 120° C. or more, more preferably 150° C. or more, even more preferably 160° C. or more, and is preferably 170° C. or less. If the molding temperature exceeds 170° C., the surface hardness of the core tends to decrease. The molding pressure preferably ranges from 2.9 MPa to 11.8 MPa. The molding time preferably ranges from 10 minutes to 60 minutes.
- In a preferable embodiment, when the hardness is measured at nine points obtained by dividing a radius of the spherical core into equal parts having 12.5% interval and the hardness is plotted against distance (%) from the center of the spherical core, the spherical core is such that R2 of a linear approximation curve obtained by the least square method is 0.93 or higher. If R2 is 0.93 or more, the linearity of the core hardness distribution is enhanced, thus the spin rate on driver shots decreases, resulting in the improved flying performance.
- The hardness of the spherical core is JIS-C hardness measured at nine points obtained by dividing a radius of the spherical core into equal parts having 12.5% interval. That is, JIS-C hardness is measured at nine points, namely at distances of 0% (core center), 12.5%, 25%, 37.5%, 50%, 62.5%, 75%, 87.5%, 100% (core surface) from the core center. Next, the measurement results are plotted to make a graph having JIS-C hardness as a vertical axis and distances (%) from the core center as a horizontal axis. In the present invention, R2 of a linear approximation curve obtained from this graph by the least square method is preferably 0.93 or higher. R2 of the linear approximation curve obtained by the least square method indicates the linearity of the obtained plot. In the present invention, R2 of 0.93 or more means that the core has the hardness distribution where the hardness increases linearly or almost linearly. If the core having the hardness distribution where the hardness increases linearly or almost linearly is used for the golf ball, the spin rate on driver shots decrease. As a result, the flight distance on driver shots increases. R2 of the linear approximation curve is preferably 0.94 or more. The higher linearity provides a greater flight distance on driver shots.
- The spherical core preferably has a hardness difference (Hs−Ho) between a surface hardness Hs and a center hardness Ho of 20 or more, more preferably 25 or more, even more preferably 30 or more, and preferably has a hardness difference of 80 or less, more preferably 70 or less, even more preferably 60 or less in JIS-C hardness. If the hardness difference between the center hardness and the surface hardness is large, the golf ball having a great flight distance due to the high launch angle and low spin rate is obtained.
- The spherical core preferably has a center hardness Ho of 30 or more, more preferably 35 or more, and even more preferably 40 or more in JIS-C hardness. If the center hardness Ho is less than 30 in JIS-C hardness, the core becomes too soft and thus the resilience may be lowered. Further, the spherical core preferably has a center hardness Ho of 70 or less, more preferably 65 or less, even more preferably 60 or less in JIS-C hardness. If the center hardness Ho exceeds 70 in JIS-C hardness, the core becomes too hard and thus the shot feeling tends to be lowered.
- The spherical core preferably has a surface hardness Hs of 76 or more, more preferably 78 or more, even more preferably 80 or more, and preferably has a surface hardness Hs of 100 or less, more preferably 95 or less in JIS-C hardness. If the surface hardness is 76 or more in JIS-C hardness, the spherical core does not become excessively soft, and thus the better resilience is obtained. Further, if the surface hardness of the spherical core is 100 or less in JIS-C hardness, the spherical core does not become excessively hard, and thus the better shot feeling is obtained.
- The spherical core preferably has a diameter of 34.8 mm or more, more preferably 36.8 mm or more, and even more preferably 38.8 mm or more, and preferably has a diameter of 42.2 mm or less, more preferably 41.8 mm or less, and even more preferably 41.2 mm or less, and most preferably 40.8 mm or less.
- If the spherical core has a diameter of 34.8 mm or more, the thickness of the cover does not become too thick and thus the resilience becomes better. On the other hand, if the spherical core has a diameter of 42.2 mm or less, the thickness of the cover does not become too thin, and hence a protection ability of the cover is sufficiently provided.
- When the spherical core has a diameter from 34.8 mm to 42.2 mm, a compression deformation amount (shrinking deformation amount of the spherical core along the compression direction) of the spherical core when applying a load from an initial load of 98N to a final load of 1275N is preferably 2.0 mm or more, more preferably 2.8 mm or more, and is preferably 6.0 mm or less, more preferably 5.0 mm or less. If the compression deformation amount is 2.0 mm or more, the shot feeling of the golf ball becomes better. If the compression deformation amount is 6.0 mm or less, the resilience of the golf ball becomes better.
- The golf ball cover of the present invention is formed from a cover composition comprising a resin component. Examples of the resin component include, for example, an ionomer rein; a thermoplastic polyurethane elastomer having a commercial name of “Elastollan (e.g. “Elastollan XNY85A”)” commercially available from BASF Japan Ltd; a thermoplastic polyamide elastomer having a commercial name of “Pebax (e.g. “Pebax 2533”)” commercially available from Arkema K. K.; a thermoplastic polyester elastomer having a commercial name of “Hytrel” commercially available from Du Pont-Toray Co., Ltd.; and a thermoplastic styrene elastomer having a commercial name of “Rabalon” commercially available from Mitsubishi Chemical Corporation; and the like.
- The ionomer resin includes a product prepared by neutralizing at least a part of carboxyl groups in the binary copolymer composed of an olefin and an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms with a metal ion, a product prepared by neutralizing at least a part of carboxyl groups in the ternary copolymer composed of an olefin, an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms, and an α,β-unsaturated carboxylic acid ester with a metal ion, or a mixture of those. The olefin preferably includes an olefin having 2 to 8 carbon atoms. Examples of the olefin are ethylene, propylene, butene, pentene, hexene, heptene, and octene. The olefin more preferably includes ethylene. Examples of the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms are acrylic acid, methacrylic acid, fumaric acid, maleic acid and crotonic acid. Acrylic acid and methacrylic acid are particularly preferred. Examples of the α,β-unsaturated carboxylic acid ester include methyl ester, ethyl ester, propyl ester, n-butyl ester, isobutyl ester of acrylic acid, methacrylic acid, fumaric acid, maleic acid or the like. In particular, acrylic acid ester and methacrylic acid ester are preferable. Of these, the ionomer resin preferably includes the metal ion-neutralized product of the binary copolymer composed of ethylene-(meth)acrylic acid and the metal ion-neutralized product of the ternary copolymer composed of ethylene, (meth)acrylic acid, and (meth)acrylic acid ester.
- Specific examples of the ionomer resins include trade name “Himilan® (e.g. the binary copolymerized ionomer such as Himilan 1555 (Na), Himilan 1557 (Zn), Himilan 1605 (Na), Himilan 1706 (Zn), Himilan 1707 (Na), Himilan AM7311 (Mg); and the ternary copolymerized ionomer such as Himilan 1856 (Na), Himilan 1855 (Zn))” commercially available from Du Pont-Mitsui Polychemicals Co., Ltd.
- Further, examples include “Surlyn (registered trademark) (e.g. the binary copolymerized ionomer such as Surlyn 8945 (Na), Surlyn 9945 (Zn), Surlyn 8140 (Na), Surlyn 8150 (Na), Surlyn 9120 (Zn), Surlyn 9150 (Zn), Surlyn 6910 (Mg), Surlyn 6120 (Mg), Surlyn 7930 (Li), Surlyn 7940 (Li), Surlyn AD8546 (Li); and the ternary copolymerized ionomer such as Surlyn 8120 (Na), Surlyn 8320 (Na), Surlyn 9320 (Zn), Surlyn 6320 (Mg), HPF 1000 (Mg), HPF 2000 (Mg))” commercially available from E.I. du Pont de Nemours and Company.
- Further, examples include “lotek (registered trademark) (e.g. the binary copolymerized ionomer such as lotek 8000 (Na), lotek 8030 (Na), lotek 7010 (Zn), lotek 7030 (Zn); and the ternary copolymerized ionomer such as lotek 7510 (Zn), lotek 7520 (Zn))” commercially available from ExxonMobil Chemical Corporation.
- It is noted that Na, Zn, Li, and Mg described in the parentheses after the trade names indicate metal types of neutralizing metal ions for the ionomer resins. The ionomer resin may be used solely or as a mixture of two or more of them.
- The cover composition constituting the cover of the golf ball of the present invention preferably includes, as a resin component, a thermoplastic polyurethane elastomer or an ionomer rein. In case of using the ionomer rein, it is preferred to use a thermoplastic styrene elastomer together. The content of the polyurethane or ionomer resin in the resin component of the cover composition is preferably 50 mass % or more, more preferably 60 mass % or more, even more preferably 70 mass % or more.
- In the present invention, the cover composition may further contain a pigment component such as a white pigment (for example, titanium oxide), a blue pigment, and a red pigment; a weight adjusting agent such as zinc oxide, calcium carbonate, and barium sulfate; a dispersant; an antioxidant; an ultraviolet absorber; a light stabilizer; a fluorescent material or a fluorescent brightener; and the like, as long as they do not impair the effect of the present invention.
- The amount of the white pigment (for example, titanium oxide) is preferably 0.5 part or more, more preferably 1 part or more, and the content of the white pigment is preferably 10 parts or less, more preferably 8 parts or less, with respect to 100 parts of the resin component constituting the cover by mass. If the amount of the white pigment is 0.5 part by mass or more, it is possible to impart the opacity to the resultant cover. Further, if the amount of the white pigment is more than 10 parts by mass, the durability of the resultant cover may deteriorate.
- The slab hardness of the cover composition is preferably set in accordance with the desired performance of the golf balls. For example, in case of a so-called distance golf ball which focuses on a flight distance, the cover composition preferably has a slab hardness of 50 or more, more preferably 55 or more, and preferably has a slab hardness of 80 or less, more preferably 70 or less in shore D hardness. If the cover composition has a slab hardness of 50 or more, the obtained golf ball has a high launch angle and low spin rate on driver shots and iron shots, and thus the flight distance improves. If the cover composition has a slab hardness of 80 or less, the golf ball excellent in durability is obtained. Further, in case of a so-called spin golf ball which focuses on controllability, the cover composition preferably has a slab hardness of less than 50, and preferably has a slab hardness of 20 or more, more preferably 25 or more in shore D hardness. If the cover composition has a slab hardness of less than 50, the flight distance on driver shots can be improved by the core of the present invention, as well as the obtained golf ball readily stops on the green due to the high spin rate on approach shots. If the cover composition has a slab hardness of 20 or more, the abrasion resistance improves. In case of a plurality of cover layers, the slab hardness of the cover composition constituting each layer can be identical or different, as long as the slab hardness of each layer is within the above range.
- An embodiment for molding a cover is not particularly limited, and includes an embodiment which comprises injection molding the cover composition directly onto the core, or an embodiment which comprises molding the cover composition into a hollow-shell, covering the core with a plurality of the hollow-shells and subjecting the core with a plurality of the hollow shells to the compression-molding (preferably an embodiment which comprises molding the cover composition into a half hollow-shell, covering the core with the two half hollow-shells, and subjecting the core with the two half hollow-shells to the compression-molding).
- When molding the cover in a compression molding method, molding of the half shell can be performed by either compression molding method or injection molding method, and the compression molding method is preferred. The compression-molding of the cover composition into half shell can be carried out, for example, under a pressure of 1 MPa or more and 20 MPa or less at a temperature of −20° C. or more and 70° C. or less relative to the flow beginning temperature of the cover composition. By performing the molding under the above conditions, a half shell having a uniform thickness can be formed. Examples of a method for molding the cover using half shells include compression molding by covering the core with two half shells. The compression molding of half shells into the cover can be carried out, for example, under a pressure of 0.5 MPa or more and 25 MPa or less at a temperature of −20° C. or more and 70° C. or less relative to the flow beginning temperature of the cover composition. By performing the molding under the above conditions, a golf ball cover having a uniform thickness can be formed.
- In the case of directly injection molding the cover composition, the cover composition extruded in the pellet form beforehand may be used for injection molding or the materials such as the base resin components and the pigment may be dry blended, followed by directly injection molding the blended material. It is preferred to use upper and lower molds having a spherical cavity and pimples for forming a cover, wherein a part of the pimples also serves as a retractable hold pin. When molding the cover by injection molding, the core is charged and held with the protruding hold pin, the cover composition which has been heated and melted is charged, and then cooled to obtain a cover. For example, it is preferred that the cover composition heated and melted at the temperature ranging from 200° C. to 250° C. is charged into a mold held under the pressure of 9 MPa to 15 MPa for 0.5 to 5 seconds, and after cooling for 10 to 60 seconds, the mold is opened and the golf ball with the cover molded is taken out from the mold.
- The concave portions called “dimple” are usually formed on the surface of the cover. The total number of the dimples is preferably 200 or more and 500 or less. If the total number is less than 200, the dimple effect is hardly obtained. On the other hand, if the total number exceeds 500, the dimple effect is hardly obtained because the size of the respective dimples is small. The shape (shape in a plan view) of dimples includes, for example, without limitation, a circle, polygonal shapes such as roughly triangular shape, roughly quadrangular shape, roughly pentagonal shape, roughly hexagonal shape, and another irregular shape. The shape of the dimples is employed solely or at least two of them may be used in combination.
- In the present invention, the cover of the golf ball preferably has a thickness of 4.0 mm or less, more preferably 3.0 mm or less, even more preferably 2.0 mm or less. If the thickness of the cover is 4.0 mm or less, the resilience and shot feeling of the obtained golf ball become better. The cover of the golf ball preferably has a thickness of 0.3 mm or more, more preferably 0.5 mm or more, and even more preferably 0.8 mm or more, and most preferably 1.0 mm or more. If the thickness of the cover is less than 0.3 mm, the durability and the wear resistance of the cover may deteriorate. If the cover has a plurality of layers, it is preferred that the total thickness of the cover layers falls within the above range.
- After the cover is molded, the mold is opened and the golf ball body is taken out from the mold, and as necessary, the golf ball body is preferably subjected to surface treatments such as deburring, cleaning, and sandblast. If desired, a paint film or a mark may be formed. The paint film preferably has a thickness of, but not limited to, 5 μm or larger, and more preferably 7 μm or larger, and preferably has a thickness of 50 μm or smaller, and more preferably 40 μm or smaller, even more preferably 30 μm or smaller. If the thickness is smaller than 5 μm, the paint film is easy to wear off due to continued use of the golf ball, and if the thickness is larger than 50 μm, the effect of the dimples is reduced, resulting in lowering flying performance of the golf ball.
- When the golf ball of the present invention has a diameter in a range from 40 mm to 45 mm, a compression deformation amount of the golf ball (shrinking amount of the golf ball in the compression direction thereof) when applying a load from an initial load of 98 N to a final load of 1275 N to the golf ball is preferably 2.0 mm or more, more preferably 2.4 mm or more, even more preferably 2.5 mm or more, most preferably 2.8 mm or more, and is preferably 5.0 mm or less, more preferably 4.5 mm or less, even more preferably 3.6 mm or less. If the compression deformation amount is 2.0 mm or more, the golf ball does not become excessively hard, and thus exhibits the good shot feeling. On the other hand, if the compression deformation amount is 5.0 mm or less, the resilience is enhanced.
- The golf ball construction is not limited, as long as the golf ball of the present invention comprises a spherical core and at least one cover layer covering the spherical core.
FIG. 1 is a partially cutaway sectional view showing thegolf ball 2 according to the preferable embodiment of the present invention. Thegolf ball 2 comprises aspherical core 4, and acover 12 covering thespherical core 4. Plurality ofdimples 14 are formed on a surface of the cover. Other portions thandimples 14 on the surface of thegolf ball 2 are referred to asland 16. Thegolf ball 2 is provided with a paint layer and a mark layer outside thecover 12, but these layers are not depicted. - The spherical core preferably has a single layered structure. Unlike the multi-layered structure, the spherical core of the single layered structure does not have an energy loss at the interface of the multi-layered structure when hitting, and thus has an enhanced resilience. The cover has a structure of at least one layer, for example a single layered structure, or a multi-layered structure of at least two layers. The golf ball of the present invention includes, for example, a two-piece golf ball comprising a spherical core and a single layered cover disposed around the spherical core, a multi-piece golf ball comprising a spherical core, and at least two cover layers disposed around the spherical core (including the three-piece golf ball), and a wound golf ball comprising a spherical core, a rubber thread layer which is formed around the spherical core, and a cover disposed over the rubber thread layer. The present invention can be suitably applied to any one of the above golf balls.
- Hereinafter, the present invention will be described in detail by way of example. The present invention is not limited to examples described below. Various changes and modifications can be made without departing from the spirit and scope of the present invention.
- A compression deformation amount of the core or golf ball (a shrinking amount of the core or golf ball in the compression direction thereof), when applying a load from an initial load of 98N to a final load of 1275N to the core or golf ball, was measured.
- A 198.4 g of metal cylindrical object was allowed to collide with each core or golf ball at a speed of 40 m/sec, and the speeds of the cylindrical object and the core or golf ball before and after the collision were measured. Based on these speeds and the mass of each object, coefficient of restitution for each core or golf ball was calculated. The measurement was conducted by using twelve samples for each core or golf ball, and the average value was regarded as the coefficient of restitution for the core or golf ball. In Tables 4 to 6, the coefficient of restitution is shown as the difference from that of golf ball (core) No. 17.
- Sheets with a thickness of about 2 mm were produced by injection molding the cover composition, and stored at 23° C. for two weeks. Three or more of these sheets were stacked on one another so as not to be affected by the measuring substrate on which the sheets were placed, and the hardness of the stack was measured with a type P1 auto loading durometer manufactured by Kobunshi Keiki Co., Ltd., provided with a Shore D type spring hardness tester prescribed in ASTM-D2240.
- A type P1 auto loading durometer manufactured by Kobunshi Keiki Co., Ltd., provided with a JIS-C type spring hardness tester was used to measure the hardness of the spherical core. The hardness measured at the surface of the spherical core was adopted as the surface hardness of the spherical core. The spherical core was cut into two hemispheres to obtain a cut plane, and the hardness was measured at the central point and at predetermined distances from the central point of the cut plane. The core hardness was measured at 4 points at predetermined distances from the central point of the cut plane of the core. The core hardness was calculated by averaging the hardness measured at 4 points.
- A metal-headed
W# 1 driver (XXIO S, loft: 11°, manufactured by SRI Sports Limited) was installed on a swing robot M/C manufactured by Golf Laboratories, Inc. A golf ball was hit at a head speed of 40 m/sec, and the flight distance (the distance from the launch point to the stop point) and the spin rate right after hitting the golf ball were measured. This measurement was conducted twelve times for each golf ball, and the average value was adopted as the measurement value for the golf ball. A sequence of photographs of the hit golf ball were taken for measuring the spin rate (rpm) right after hitting the golf ball. In Tables 4 to 6, the flight distance and the spin rate is shown as the difference from that of golf ball (core) No. 17. - The rubber compositions having formulations shown in Tables 4 to 6 were kneaded and heat-pressed in upper and lower molds, each having a hemispherical cavity, at 170° C. for 20 minutes to prepare spherical cores having a diameter of 39.8 mm.
-
TABLE 4 Golf ball No. 1 2 3 4 5 6 Rubber composition BR730 100 100 100 100 100 100 (parts by mass) Sanceler SR 34 29 31 31 31 30 Zinc oxide 5 5 5 5 5 5 Barium sulfate *1) *1) *1) *1) *1) *1) 2-thionaphthol 0.1 0.32 0.1 0.1 0.1 0.1 4-acetoxybenzoic acid 5.1 — — — — — Lauric acid — 7.0 — — — — Cyclohexane carboxylic acid — — 5.0 — — — Dimethylaminobenzoic acid — — — 5.0 — — Benzoic acid — — — — 5.0 — Chlorobenzoic acid — — — — 5.0 Dicumyl peroxide 0.8 0.8 0.8 0.8 0.8 0.8 Relative solubility parameter 0.99 0.53 0.90 0.96 0.90 1.03 Bond dissociation energy (kcal/mole) 248.7 257.3 256.3 256.5 251.1 245.5 Core hardness Center hardness 45.1 50.3 46.5 45.6 45.9 47.3 distribution 12.5% point hardness 51.0 55.0 51.3 50.7 51.8 55.1 (JIS-C) 25% point hardness 57.7 59.8 58.0 58.5 59.0 62.0 37.5% point hardness 61.4 62.9 61.5 63.3 64.2 65.1 50% point hardness 63.6 63.9 62.5 64.4 66.5 65.8 62.5% point hardness 62.7 66.7 68.9 67.4 65.4 69.7 75% point hardness 70.0 75.1 76.0 78.0 77.7 76.1 87.5% point hardness 75.4 77.9 75.2 79.6 83.0 75.2 Surface hardness 82.9 81.9 81.4 83.5 89.3 81.7 Surface hardness-center hardness 37.9 31.7 34.9 37.9 43.4 34.4 R2 of approximated curve 0.96 0.98 0.98 0.98 0.96 0.95 Slope of approximated curve 0.33 0.31 0.34 0.38 0.41 0.31 Core coefficient of restitution −0.004 −0.011 0.002 −0.009 0.004 −0.009 Core compression deformation amount (mm) 4.0 4.3 4.2 3.9 4.1 4.1 Cover composition A A A A A A Cover hardness (Shore D) 65 65 65 65 65 65 Cover thickness (mm) 1.5 1.5 1.5 1.5 1.5 1.5 Ball Driver spin rate (rpm) −80 −70 −100 −130 −120 −80 Driver flying distance (m) 2.0 3.3 2.8 3.5 3.9 1.1 Coefficient of restitution −0.004 −0.011 0.002 −0.009 0.001 −0.010 Compression deformation amount (mm) 3.3 3.6 3.5 3.2 3.4 3.3 -
TABLE 5 Golf ball No. 7 8 9 10 11 12 Rubber composition BR730 100 100 100 100 100 100 (parts by mass) Sanceler SR 31 29 32 29 29 29 Zinc oxide 5 5 5 5 5 5 Barium sulfate *1) *1) *1) *1) *1) *1) 2-thionaphthol 0.32 0.32 0.32 0.32 0.32 0.32 Decanoic acid 5.0 — — — — — Octanoic acid — 2.5 — — — — Dodecanedioic acid — — 4.0 — — — Suberic acid — — — 3.0 — — 2-propylvaleric acid — — — — 2.5 — Neodecanoic acid — — — — 9.0 Dicumyl peroxide 0.8 0.8 0.8 0.8 0.8 0.8 Relative solubility parameter 0.57 0.61 0.74 0.88 0.60 0.56 Bond dissociation energy (kcal/mole) 257.3 255.9 256.6 255.4 257.0 257.3 Core hardness Center hardness 47.9 50.4 46.6 47.1 45.3 48.0 distribution 12.5% point hardness 54.2 56.2 54.8 56.2 52.5 53.6 (JIS-C) 25% point hardness 60.4 62.3 61.1 62.6 60.6 60.4 37.5% point hardness 63.6 64.5 63.7 65.5 63.6 63.4 50% point hardness 64.4 65.5 65.1 66.8 64.2 63.9 62.5% point hardness 67.2 66.1 65.9 67.0 63.8 65.1 75% point hardness 75.8 74.1 73.2 72.2 72.5 74.2 87.5% point hardness 79.3 77.9 77.0 75.9 75.6 76.7 Surface hardness 81.7 83.0 84.1 83.6 81.9 82.0 Surface hardness-center hardness 33.8 32.7 37.5 36.6 36.6 34.0 R2 of approximated curve 0.97 0.96 0.95 0.93 0.94 0.96 Slope of approximated curve 0.33 0.29 0.32 0.30 0.32 0.31 Core coefficient of restitution 0.016 0.004 −0.015 −0.013 0.002 0.003 Core compression deformation amount (mm) 4.0 4.5 4.1 4.1 4.3 4.2 Cover composition A A A A A A Cover hardness (Shore D) 65 65 65 65 65 65 Cover thickness (mm) 1.5 1.5 1.5 1.5 1.5 1.5 Ball Driver spin rate (rpm) −100 −90 −70 −60 −80 −95 Driver flying distance (m) 4.0 2.7 1.5 1.3 2.8 4.0 Coefficient of restitution 0.016 0.005 −0.015 −0.013 0.002 0.003 Compression deformation amount (mm) 3.3 3.8 3.4 3.4 3.6 3.5 -
TABLE 6 Golf ball No. 13 14 15 16 17 18 19 Rubber composition BR730 100 100 100 100 100 100 100 (parts by mass) Sanceler SR 29 29 29 31 23 32 28 Zinc oxide 5 5 5 5 5 5 5 Barium sulfate *1) *1) *1) *1) *1) *1) *1) 2-thionaphthol 0.32 0.32 0.32 0.32 — 0.1 0.32 2-heptylundecanoic acid 10 — — — — — — Zinc stearate — — 30 40 — — — Zinc octanoate — 5.0 — — — — — 4-aminobenzoic acid — — — — — 5.8 — Behenic acid — — — — — — 20.0 Dicumyl peroxide 0.8 0.8 0.8 0.8 0.8 0.8 0.8 Relative solubility parameter 0.45 0.61 0.46 0.46 — 1.13 0.43 Bond dissociation energy (kcal/mole) 251.4 255.9 257.3 257.3 — 255.6 257.3 Core hardness Center hardness 49.4 47.6 57.6 60.3 55.0 49.1 52.9 distribution 12.5% point hardness 56.5 54.2 59.9 61.6 61.4 57.1 56.6 (JIS-C) 25% point hardness 63.5 57.9 62.6 63.7 65.4 63.1 59.8 37.5% point hardness 66.3 60.5 64.1 64.9 67.1 66.4 61.1 50% point hardness 67.4 62.5 66.1 66.3 67.4 67.4 64.1 62.5% point hardness 67.2 68.5 68.7 67.1 67.2 67.9 68.3 75% point hardness 73.7 75.1 71.7 68.5 71.3 72.6 72.6 87.5% point hardness 78.0 78.5 72.1 66.9 73.4 74.6 73.2 Surface hardness 81.7 81.6 78.0 69.6 80.2 80.0 77.1 Surface hardness-center hardness 32.3 34.1 20.4 9.3 25.2 30.9 24.2 R2 of approximated curve 0.95 0.99 0.98 0.91 0.90 0.93 0.99 Slope of approximated curve 0.29 0.34 0.19 0.09 0.20 0.26 0.24 Core coefficient of restitution 0.018 −0.007 0.002 −0.016 0.000 −0.018 −0.003 Core compression deformation amount (mm) 4.1 4.6 4.0 4.4 4.2 4.0 4.2 Cover composition A A A A A A A Cover hardness (Shore D) 65 65 65 65 65 65 65 Cover thickness (mm) 1.5 1.5 1.5 1.5 1.5 1.5 1.5 Ball Driver spin rate (rpm) −95 −110 −30 60 0 −20 −40 Driver flying distance (m) 4.2 4.0 1.4 −2.1 0.0 −2.0 0.3 Coefficient of restitution 0.018 −0.007 0.002 −0.016 0.000 −0.018 −0.003 Compression deformation amount (mm) 3.4 3.9 3.3 3.7 3.5 3.3 3.5 *1) As to an amount of barium sulfate, adjustment was made such that the golf ball had a mass of 45.4 g. BR730: a high-cis polybutadiene (cis-1,4 bond content = 96 mass %, 1,2-vinyl bond content = 1.3 mass %, Moony viscosity (ML1+4 (100° C.) = 55, molecular weight distribution (Mw/Mn) = 3) available from JSR Corporation Sanceler SR: zinc acrylate (product of 10 mass % stearic acid coating) available from Sanshin Chemical Industry Co., Ltd. Zinc oxide: “Ginrei R” manufactured by Toho Zinc Co., Ltd. Barium sulfate: “Barium sulfate BD” manufactured by Sakai Chemical Industry Co., Ltd., adjustment was made such that the finally obtained golf ball had a mass of 45.4 g. 2-thionaphthol: manufactured by Tokyo Chemical Industry Co., Ltd. 4-acetoxybenzoic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 98.0% Lauric acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 98.0% Cyclohexane carboxylic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 98.0% Dimethylaminobenzoic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 98.0% Benzoic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 98.0% Chlorobenzoic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 99.0% Decanoic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 98.0% Octanoic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 98.0 % Dodecanedioic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 98.0% Suberic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 99.0% 2-propylvaleric acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 99.0% Neodecanoic acid: manufactured by Wako Pure Chemical Industries Purity 100.0% 2-heptylundecanoic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 95.0% Zinc stearate: manufactured by Wako Pure Chemical Industries Purity 95.0% Zinc octanoate: manufactured by Mitsuwa Chemicals Co., Ltd. Purity 99.0% 4-aminobenzoic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 99.0% Dicumyl peroxide: “Percumyl (Registered trade mark) D (dicumyl peroxide)” manufactured by NOF CORPORATION Behenic acid: manufactured by Tokyo Chemical Industry Co., Ltd. Purity 80.0% - Cover materials shown in Table 7 were mixed with a twin-screw kneading extruder to prepare the cover compositions in the pellet form. The extruding conditions of the cover composition were a screw diameter of 45 mm, a screw rotational speed of 200 rpm, and screw L/D=35, and the mixtures were heated to 150 to 230° C. at the die position of the extruder. The cover compositions obtained above were injection-molded onto the spherical cores to produce the golf balls having the spherical core and the cover covering the spherical core.
-
TABLE 7 Cover composition No. A Himilan 1605 50 Himilan 1706 50 Titanium oxide 4 Slab hardness (shore D) 65 Formulation: parts by mass Himilan 1605: Sodium ion neutralized ethylene-methacrylic acid copolymer ionomer resin available from Du Pont-Mitsui Polychemicals Co., Ltd Himilan 1706: Zinc ion neutralized ethylene-methacrylic acid copolymer ionomer resin available from Du Pont-Mitsui Polychemicals Co., Ltd - As apparent from the results in Tables 4 to 6, the golf balls comprising a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) an acid and/or a salt thereof travel a great flight distance on driver shots, respectively.
- The golf ball of the present invention travels a great flight distance on driver shots. This application is based on Japanese Patent applications No. 2012-109717 filed on May 11, 2012 the contents of which are hereby incorporated by reference.
Claims (19)
1. A golf ball having a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) an acid and/or a salt thereof, provided that the rubber composition further contains (e) a metal compound in the case of containing only (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent, wherein (d) the acid and/or the salt thereof has a relative solubility parameter ranging from 0.45 to 1.10 to (a) the base rubber and (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof.
2. The golf ball according to claim 1 , wherein (d) the acid and/or the salt thereof has a relative solubility parameter of 0.48 or more.
3. The golf ball according to claim 1 , wherein (d) the acid and/or the salt thereof has a relative solubility parameter of 0.52 or more.
4. The golf ball according to claim 1 , wherein (d) the acid and/or the salt thereof has a relative solubility parameter of 1.05 or less.
5. The golf ball according to claim 1 , wherein (d) the acid and/or the salt thereof has a proton bond dissociation energy of 270 kcal/mole or less.
6. The golf ball according to claim 1 , wherein (d) the acid and/or the salt thereof is a carboxylic acid and/or a salt thereof.
7. The golf ball according to claim 1 , wherein the rubber composition contains (d) the acid and/or the salt thereof in a content of 2 parts by mass or more and less than 40 parts by mass with respect to 100 parts by mass of (a) the base rubber.
8. The golf ball according to claim 1 , wherein the rubber composition further contains (f) an organic sulfur compound.
9. The golf ball according to claim 8 , wherein (f) the organic sulfur compound includes at least one compound selected from the group consisting of thiophenols, diphenyl disulfides, thionaphthols, thiuram disulfides, and metal salts thereof.
10. The golf ball according to claim 8 , wherein the rubber composition contains (f) the organic sulfur compound in a content ranging from 0.05 part to 5 parts by mass with respect to 100 parts by mass of (a) the base rubber.
11. The golf ball according to claim 1 , wherein the rubber composition contains (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof in a content ranging from 15 parts by mass to 50 parts by mass with respect to 100 parts by mass of (a) the base rubber.
12. The golf ball according to claim 1 , wherein the rubber composition contains the metal salt of (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent.
13. The golf ball according to claim 1 , wherein the spherical core has a hardness difference of 20 or more in JIS-C hardness between a surface hardness and a center hardness thereof.
14. The golf ball according to claim 1 , wherein the spherical core is such that R2 of a linear approximation curve obtained from a least square method is 0.93 or higher, when JIS-C hardness, which is measured at nine points obtained by dividing a radius of the spherical core into equal parts having 12.5% intervals therebetween, is plotted against distance (%) from a core center.
15. A golf ball having a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) a base rubber, (b) an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, and (d) a carboxylic acid and/or a salt thereof, provided that the rubber composition further contains (e) a metal compound in the case of containing only (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent, wherein (d) the carboxylic acid and/or the salt thereof has a relative solubility parameter ranging from 0.48 to 1.05 to (a) the base rubber and (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and/or the metal salt thereof.
16. The golf ball according to claim 15 , wherein (d) the carboxylic acid and/or the salt thereof has a proton bond dissociation energy of 270 kcal/mole or less.
17. The golf ball according to claim 15 , wherein the rubber composition contains (d) the carboxylic acid and/or the salt thereof in a content of 2 parts by mass or more and less than 40 parts by mass with respect to 100 parts by mass of (a) the base rubber.
18. The golf ball according to claim 15 , wherein the rubber composition further contains (f) an organic sulfur compound.
19. The golf ball according to claim 15 , wherein (d) the carboxylic acid and/or the salt thereof includes at least one compound selected from the group consisting of 4-acetoxybenzoic acid, dimethylaminobenzoic acid, benzoic acid, cyclohexane carboxylic acid, suberic acid, dodecanedioic acid, octanoic acid, 2-propylpentanoic acid, neodecanoic acid, lauric acid, decanoic acid, chlorobenzoic acid, myristic acid and salts thereof.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-109717 | 2012-05-11 | ||
| JP2012109717A JP6053317B2 (en) | 2012-05-11 | 2012-05-11 | Golf ball |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20130303307A1 true US20130303307A1 (en) | 2013-11-14 |
Family
ID=49549029
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/891,277 Abandoned US20130303307A1 (en) | 2012-05-11 | 2013-05-10 | Golf ball |
Country Status (2)
| Country | Link |
|---|---|
| US (1) | US20130303307A1 (en) |
| JP (1) | JP6053317B2 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120142453A1 (en) * | 2010-12-03 | 2012-06-07 | Sri Sports Limited | Golf ball |
| US20120172150A1 (en) * | 2010-12-29 | 2012-07-05 | Sri Sports Limited | Golf ball |
| US20120172149A1 (en) * | 2010-12-29 | 2012-07-05 | Sri Sports Limited | Golf ball |
| US20130172111A1 (en) * | 2011-12-28 | 2013-07-04 | Dunlop Sports Co., Ltd. | Golf ball |
| US9061181B2 (en) | 2012-05-17 | 2015-06-23 | Dunlop Sports Co. Ltd. | Golf ball |
| US9162112B2 (en) | 2011-11-15 | 2015-10-20 | Dunlop Sports Co. Ltd. | Golf ball |
| US9364720B2 (en) | 2010-12-29 | 2016-06-14 | Dunlop Sports Co. Ltd. | Golf ball |
| US10265585B2 (en) | 2010-12-03 | 2019-04-23 | Sumitomo Rubber Industries, Ltd. | Golf ball |
| US10279221B2 (en) | 2015-03-02 | 2019-05-07 | Bridgestone Sports Co., Ltd. | Golf ball |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6596842B2 (en) * | 2015-03-02 | 2019-10-30 | ブリヂストンスポーツ株式会社 | Golf ball and manufacturing method thereof |
| JP6787048B2 (en) * | 2016-10-31 | 2020-11-18 | 住友ゴム工業株式会社 | Golf ball |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4688801A (en) * | 1985-09-23 | 1987-08-25 | Pony Ind Inc | Production of homogeneous molded golf balls |
| US20040110906A1 (en) * | 2002-11-21 | 2004-06-10 | Koichi Fujisawa | Rubber composition for golf ball |
| US20050170910A1 (en) * | 2004-02-04 | 2005-08-04 | Bridgestone Sports Co., Ltd. | Golf Ball |
| US20060128900A1 (en) * | 2004-12-15 | 2006-06-15 | Bridgestone Sports Co., Ltd. | Solid golf ball |
| US20070129174A1 (en) * | 2005-12-05 | 2007-06-07 | Bridgestone Sports Co., Ltd. | Solid golf ball |
| US20080020864A1 (en) * | 2006-01-04 | 2008-01-24 | Bridgestone Sports Co., Ltd. | Golf ball |
| US20080076603A1 (en) * | 2006-01-04 | 2008-03-27 | Bridgestone Sports Co., Ltd. | Golf ball |
| US20080214324A1 (en) * | 2007-03-02 | 2008-09-04 | Bridgestone Sports Co., Ltd. | Golf ball |
| US20080312008A1 (en) * | 2007-02-13 | 2008-12-18 | Bridgestone Sports Co., Ltd. | Solid golf ball |
| US20090111612A1 (en) * | 2007-10-31 | 2009-04-30 | Bridgestone Sports Co., Ltd. | Three-piece solid golf ball |
| US7838584B2 (en) * | 2006-08-11 | 2010-11-23 | Bridgestone Sports Co., Ltd. | Golf ball |
| US20100298067A1 (en) * | 2009-05-21 | 2010-11-25 | Bridgestone Sports Co., Ltd. | Golf ball |
| US20110053709A1 (en) * | 2007-07-03 | 2011-03-03 | Brian Comeau | Negative hardness gradient cores made of polyalkenamer rubber for golf balls |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0696651B2 (en) * | 1985-05-13 | 1994-11-30 | 株式会社ブリヂストン | Rubber composition for golf ball |
| JP4082895B2 (en) * | 2001-12-04 | 2008-04-30 | Sriスポーツ株式会社 | Solid golf balls |
| JP2003320054A (en) * | 2002-04-30 | 2003-11-11 | Bridgestone Sports Co Ltd | Golf ball |
| JP2005218618A (en) * | 2004-02-05 | 2005-08-18 | Sumitomo Rubber Ind Ltd | Solid golf ball |
| US8242195B2 (en) * | 2009-07-31 | 2012-08-14 | Bridgestone Sports Co., Ltd. | Golf ball |
-
2012
- 2012-05-11 JP JP2012109717A patent/JP6053317B2/en active Active
-
2013
- 2013-05-10 US US13/891,277 patent/US20130303307A1/en not_active Abandoned
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4688801A (en) * | 1985-09-23 | 1987-08-25 | Pony Ind Inc | Production of homogeneous molded golf balls |
| US20040110906A1 (en) * | 2002-11-21 | 2004-06-10 | Koichi Fujisawa | Rubber composition for golf ball |
| US20050170910A1 (en) * | 2004-02-04 | 2005-08-04 | Bridgestone Sports Co., Ltd. | Golf Ball |
| US20060128900A1 (en) * | 2004-12-15 | 2006-06-15 | Bridgestone Sports Co., Ltd. | Solid golf ball |
| US20070129174A1 (en) * | 2005-12-05 | 2007-06-07 | Bridgestone Sports Co., Ltd. | Solid golf ball |
| US20080076603A1 (en) * | 2006-01-04 | 2008-03-27 | Bridgestone Sports Co., Ltd. | Golf ball |
| US20080020864A1 (en) * | 2006-01-04 | 2008-01-24 | Bridgestone Sports Co., Ltd. | Golf ball |
| US7838584B2 (en) * | 2006-08-11 | 2010-11-23 | Bridgestone Sports Co., Ltd. | Golf ball |
| US20080312008A1 (en) * | 2007-02-13 | 2008-12-18 | Bridgestone Sports Co., Ltd. | Solid golf ball |
| US20080214324A1 (en) * | 2007-03-02 | 2008-09-04 | Bridgestone Sports Co., Ltd. | Golf ball |
| US20110053709A1 (en) * | 2007-07-03 | 2011-03-03 | Brian Comeau | Negative hardness gradient cores made of polyalkenamer rubber for golf balls |
| US20090111612A1 (en) * | 2007-10-31 | 2009-04-30 | Bridgestone Sports Co., Ltd. | Three-piece solid golf ball |
| US20100298067A1 (en) * | 2009-05-21 | 2010-11-25 | Bridgestone Sports Co., Ltd. | Golf ball |
Non-Patent Citations (1)
| Title |
|---|
| Compressions by any other name - J. Dalton * |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120142453A1 (en) * | 2010-12-03 | 2012-06-07 | Sri Sports Limited | Golf ball |
| US10265585B2 (en) | 2010-12-03 | 2019-04-23 | Sumitomo Rubber Industries, Ltd. | Golf ball |
| US9566476B2 (en) * | 2010-12-03 | 2017-02-14 | Dunlop Sports Co. Ltd. | Golf ball |
| US9486673B2 (en) * | 2010-12-29 | 2016-11-08 | Dunlop Sports Co. Ltd. | Golf ball |
| US20120172150A1 (en) * | 2010-12-29 | 2012-07-05 | Sri Sports Limited | Golf ball |
| US20120172149A1 (en) * | 2010-12-29 | 2012-07-05 | Sri Sports Limited | Golf ball |
| US9364720B2 (en) | 2010-12-29 | 2016-06-14 | Dunlop Sports Co. Ltd. | Golf ball |
| US9409058B2 (en) * | 2010-12-29 | 2016-08-09 | Dunlop Sports Co. Ltd. | Golf ball |
| US9162112B2 (en) | 2011-11-15 | 2015-10-20 | Dunlop Sports Co. Ltd. | Golf ball |
| US20130172111A1 (en) * | 2011-12-28 | 2013-07-04 | Dunlop Sports Co., Ltd. | Golf ball |
| US9174092B2 (en) * | 2011-12-28 | 2015-11-03 | Dunlop Sports Co. Ltd. | Golf ball |
| US9873022B2 (en) | 2011-12-28 | 2018-01-23 | Dunlop Sports Co. Ltd. | Golf ball |
| US9061181B2 (en) | 2012-05-17 | 2015-06-23 | Dunlop Sports Co. Ltd. | Golf ball |
| US10279221B2 (en) | 2015-03-02 | 2019-05-07 | Bridgestone Sports Co., Ltd. | Golf ball |
Also Published As
| Publication number | Publication date |
|---|---|
| JP6053317B2 (en) | 2016-12-27 |
| JP2013236672A (en) | 2013-11-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9283442B2 (en) | Golf ball | |
| EP2537888B1 (en) | Golf ball | |
| US20130303307A1 (en) | Golf ball | |
| US9061181B2 (en) | Golf ball | |
| US9873022B2 (en) | Golf ball | |
| US9162112B2 (en) | Golf ball | |
| EP2537887B1 (en) | Golf ball | |
| US9457234B2 (en) | Golf ball | |
| US9533195B2 (en) | Golf ball | |
| US9694243B2 (en) | Golf ball | |
| US9884226B2 (en) | Golf ball | |
| US9238161B2 (en) | Golf ball | |
| US11376476B2 (en) | Golf ball | |
| US11065510B2 (en) | Golf ball | |
| US10590259B2 (en) | Golf ball | |
| US10232224B2 (en) | Golf ball | |
| JP7552343B2 (en) | Golf balls | |
| JP2023177977A (en) | Golf ball | |
| JP2023177978A (en) | Golf ball | |
| KR20230168117A (en) | Golf ball |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: DUNLOP SPORTS CO., LTD., JAPAN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:SAKAMINE, RYOTA;MIKURA, CHIEMI;SHINDO, AYAKA;AND OTHERS;SIGNING DATES FROM 20130304 TO 20130305;REEL/FRAME:030404/0988 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |