US20130292414A1 - Dispensing Device - Google Patents
Dispensing Device Download PDFInfo
- Publication number
- US20130292414A1 US20130292414A1 US13/886,090 US201313886090A US2013292414A1 US 20130292414 A1 US20130292414 A1 US 20130292414A1 US 201313886090 A US201313886090 A US 201313886090A US 2013292414 A1 US2013292414 A1 US 2013292414A1
- Authority
- US
- United States
- Prior art keywords
- dispensing device
- dispensing
- fluid ounces
- internal volume
- substance
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 239000000126 substance Substances 0.000 claims abstract description 34
- 239000012530 fluid Substances 0.000 claims description 112
- 239000000463 material Substances 0.000 claims description 19
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 claims description 10
- 239000003814 drug Substances 0.000 claims description 9
- 239000006071 cream Substances 0.000 claims description 8
- 229910045601 alloy Inorganic materials 0.000 claims description 7
- 239000000956 alloy Substances 0.000 claims description 7
- 238000003825 pressing Methods 0.000 claims description 7
- 239000000853 adhesive Substances 0.000 claims description 6
- 230000001070 adhesive effect Effects 0.000 claims description 6
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 claims description 5
- 235000020186 condensed milk Nutrition 0.000 claims description 5
- 239000003921 oil Substances 0.000 claims description 5
- 229920000642 polymer Polymers 0.000 claims description 5
- 239000006210 lotion Substances 0.000 claims description 4
- 239000000843 powder Substances 0.000 claims description 4
- 239000000344 soap Substances 0.000 claims description 4
- 235000013409 condiments Nutrition 0.000 claims description 3
- 230000007246 mechanism Effects 0.000 claims description 3
- 239000011800 void material Substances 0.000 claims description 3
- 239000002390 adhesive tape Substances 0.000 claims description 2
- 229910052943 magnesium sulfate Inorganic materials 0.000 claims description 2
- 235000019341 magnesium sulphate Nutrition 0.000 claims description 2
- 235000010746 mayonnaise Nutrition 0.000 claims description 2
- 239000008268 mayonnaise Substances 0.000 claims description 2
- 229960005489 paracetamol Drugs 0.000 claims description 2
- 239000011888 foil Substances 0.000 claims 1
- 238000003860 storage Methods 0.000 description 17
- 210000003811 finger Anatomy 0.000 description 13
- 235000015067 sauces Nutrition 0.000 description 12
- 210000003813 thumb Anatomy 0.000 description 12
- 239000003973 paint Substances 0.000 description 9
- 239000004909 Moisturizer Substances 0.000 description 8
- 239000000499 gel Substances 0.000 description 8
- 230000001333 moisturizer Effects 0.000 description 8
- -1 stucco Substances 0.000 description 8
- 239000007921 spray Substances 0.000 description 7
- 239000000052 vinegar Substances 0.000 description 7
- 239000012528 membrane Substances 0.000 description 6
- 239000002453 shampoo Substances 0.000 description 6
- 241000282472 Canis lupus familiaris Species 0.000 description 5
- 241000255925 Diptera Species 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 235000015071 dressings Nutrition 0.000 description 5
- 235000015110 jellies Nutrition 0.000 description 5
- 239000008274 jelly Substances 0.000 description 5
- 235000008960 ketchup Nutrition 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 235000021400 peanut butter Nutrition 0.000 description 5
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- 235000019198 oils Nutrition 0.000 description 4
- 239000004753 textile Substances 0.000 description 4
- 240000008415 Lactuca sativa Species 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 235000015220 hamburgers Nutrition 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000007769 metal material Substances 0.000 description 3
- 239000003595 mist Substances 0.000 description 3
- 238000004806 packaging method and process Methods 0.000 description 3
- 239000002304 perfume Substances 0.000 description 3
- 235000012045 salad Nutrition 0.000 description 3
- 230000000087 stabilizing effect Effects 0.000 description 3
- 235000021419 vinegar Nutrition 0.000 description 3
- 244000056139 Brassica cretica Species 0.000 description 2
- 235000003351 Brassica cretica Nutrition 0.000 description 2
- 235000003343 Brassica rupestris Nutrition 0.000 description 2
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 2
- 102000000536 PPAR gamma Human genes 0.000 description 2
- 108010016731 PPAR gamma Proteins 0.000 description 2
- 102100027913 Peptidyl-prolyl cis-trans isomerase FKBP1A Human genes 0.000 description 2
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 2
- 108010006877 Tacrolimus Binding Protein 1A Proteins 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 230000001028 anti-proliverative effect Effects 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 206010003246 arthritis Diseases 0.000 description 2
- QKSKPIVNLNLAAV-UHFFFAOYSA-N bis(2-chloroethyl) sulfide Chemical compound ClCCSCCCl QKSKPIVNLNLAAV-UHFFFAOYSA-N 0.000 description 2
- 235000008429 bread Nutrition 0.000 description 2
- 229910052804 chromium Inorganic materials 0.000 description 2
- 239000011651 chromium Substances 0.000 description 2
- 229910017052 cobalt Inorganic materials 0.000 description 2
- 239000010941 cobalt Substances 0.000 description 2
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 230000005484 gravity Effects 0.000 description 2
- 235000012907 honey Nutrition 0.000 description 2
- 230000036512 infertility Effects 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 235000010460 mustard Nutrition 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 2
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 2
- 235000014438 salad dressings Nutrition 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- 239000004332 silver Substances 0.000 description 2
- 229960002930 sirolimus Drugs 0.000 description 2
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 229910052718 tin Inorganic materials 0.000 description 2
- 239000011135 tin Substances 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 244000144927 Aloe barbadensis Species 0.000 description 1
- 235000002961 Aloe barbadensis Nutrition 0.000 description 1
- 229940122361 Bisphosphonate Drugs 0.000 description 1
- 244000025254 Cannabis sativa Species 0.000 description 1
- 235000012766 Cannabis sativa ssp. sativa var. sativa Nutrition 0.000 description 1
- 235000012765 Cannabis sativa ssp. sativa var. spontanea Nutrition 0.000 description 1
- 235000002568 Capsicum frutescens Nutrition 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 229920000298 Cellophane Polymers 0.000 description 1
- DQEFEBPAPFSJLV-UHFFFAOYSA-N Cellulose propionate Chemical compound CCC(=O)OCC1OC(OC(=O)CC)C(OC(=O)CC)C(OC(=O)CC)C1OC1C(OC(=O)CC)C(OC(=O)CC)C(OC(=O)CC)C(COC(=O)CC)O1 DQEFEBPAPFSJLV-UHFFFAOYSA-N 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 206010011224 Cough Diseases 0.000 description 1
- 102400001368 Epidermal growth factor Human genes 0.000 description 1
- 101800003838 Epidermal growth factor Proteins 0.000 description 1
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 1
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- YACHGFWEQXFSBS-UHFFFAOYSA-N Leptomycin B Natural products OC(=O)C=C(C)CC(C)C(O)C(C)C(=O)C(C)C=C(C)C=CCC(C)C=C(CC)C=CC1OC(=O)C=CC1C YACHGFWEQXFSBS-UHFFFAOYSA-N 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 108010006519 Molecular Chaperones Proteins 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 229920002292 Nylon 6 Polymers 0.000 description 1
- 229920002302 Nylon 6,6 Polymers 0.000 description 1
- 229910052778 Plutonium Inorganic materials 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 229920002367 Polyisobutene Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 229940079156 Proteasome inhibitor Drugs 0.000 description 1
- 229920000297 Rayon Polymers 0.000 description 1
- 240000003768 Solanum lycopersicum Species 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 1
- 239000004809 Teflon Substances 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- CBPNZQVSJQDFBE-FUXHJELOSA-N Temsirolimus Chemical compound C1C[C@@H](OC(=O)C(C)(CO)CO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 CBPNZQVSJQDFBE-FUXHJELOSA-N 0.000 description 1
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 1
- 229910052770 Uranium Inorganic materials 0.000 description 1
- 240000001717 Vaccinium macrocarpon Species 0.000 description 1
- 235000012545 Vaccinium macrocarpon Nutrition 0.000 description 1
- 235000002118 Vaccinium oxycoccus Nutrition 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- FJWGYAHXMCUOOM-QHOUIDNNSA-N [(2s,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6s)-4,5-dinitrooxy-2-(nitrooxymethyl)-6-[(2r,3r,4s,5r,6s)-4,5,6-trinitrooxy-2-(nitrooxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-3,5-dinitrooxy-6-(nitrooxymethyl)oxan-4-yl] nitrate Chemical compound O([C@@H]1O[C@@H]([C@H]([C@H](O[N+]([O-])=O)[C@H]1O[N+]([O-])=O)O[C@H]1[C@@H]([C@@H](O[N+]([O-])=O)[C@H](O[N+]([O-])=O)[C@@H](CO[N+]([O-])=O)O1)O[N+]([O-])=O)CO[N+](=O)[O-])[C@@H]1[C@@H](CO[N+]([O-])=O)O[C@@H](O[N+]([O-])=O)[C@H](O[N+]([O-])=O)[C@H]1O[N+]([O-])=O FJWGYAHXMCUOOM-QHOUIDNNSA-N 0.000 description 1
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 1
- 229920001893 acrylonitrile styrene Polymers 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- SSNQAUBBJYCSMY-UHFFFAOYSA-N aigialomycin A Natural products C12OC2CC(O)C(O)C(=O)C=CCC(C)OC(=O)C=2C1=CC(OC)=CC=2O SSNQAUBBJYCSMY-UHFFFAOYSA-N 0.000 description 1
- 235000013334 alcoholic beverage Nutrition 0.000 description 1
- 229920000180 alkyd Polymers 0.000 description 1
- 235000011399 aloe vera Nutrition 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 229940062331 androgel Drugs 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000003712 anti-aging effect Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- 235000021420 balsamic vinegar Nutrition 0.000 description 1
- 230000003796 beauty Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000012867 bioactive agent Substances 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 1
- 150000004663 bisphosphonates Chemical class 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 235000014121 butter Nutrition 0.000 description 1
- 235000009120 camo Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 229920006217 cellulose acetate butyrate Polymers 0.000 description 1
- 229920001727 cellulose butyrate Polymers 0.000 description 1
- 229920003086 cellulose ether Polymers 0.000 description 1
- 229920006218 cellulose propionate Polymers 0.000 description 1
- 239000004568 cement Substances 0.000 description 1
- 235000005607 chanvre indien Nutrition 0.000 description 1
- 235000013351 cheese Nutrition 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 235000008504 concentrate Nutrition 0.000 description 1
- 235000009508 confectionery Nutrition 0.000 description 1
- 239000008162 cooking oil Substances 0.000 description 1
- 235000004634 cranberry Nutrition 0.000 description 1
- 235000015142 cultured sour cream Nutrition 0.000 description 1
- 239000000824 cytostatic agent Chemical class 0.000 description 1
- 230000001085 cytostatic effect Effects 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 239000003822 epoxy resin Substances 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 229960005167 everolimus Drugs 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 230000001815 facial effect Effects 0.000 description 1
- 235000013410 fast food Nutrition 0.000 description 1
- 235000013861 fat-free Nutrition 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 239000011094 fiberboard Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- 235000013882 gravy Nutrition 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 1
- 210000004247 hand Anatomy 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 239000011487 hemp Substances 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- SSNQAUBBJYCSMY-KNTMUCJRSA-N hypothemycin Chemical compound O([C@@H](C)C\C=C/C(=O)[C@@H](O)[C@@H](O)C[C@H]1O[C@@H]11)C(=O)C=2C1=CC(OC)=CC=2O SSNQAUBBJYCSMY-KNTMUCJRSA-N 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 239000003317 industrial substance Substances 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000002262 irrigation Effects 0.000 description 1
- 238000003973 irrigation Methods 0.000 description 1
- 235000015094 jam Nutrition 0.000 description 1
- 239000011133 lead Substances 0.000 description 1
- YACHGFWEQXFSBS-XYERBDPFSA-N leptomycin B Chemical compound OC(=O)/C=C(C)/C[C@H](C)[C@@H](O)[C@H](C)C(=O)[C@H](C)/C=C(\C)/C=C/C[C@@H](C)/C=C(/CC)\C=C\[C@@H]1OC(=O)C=C[C@@H]1C YACHGFWEQXFSBS-XYERBDPFSA-N 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 235000004213 low-fat Nutrition 0.000 description 1
- 239000010721 machine oil Substances 0.000 description 1
- 239000003120 macrolide antibiotic agent Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 235000012054 meals Nutrition 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 229940051866 mouthwash Drugs 0.000 description 1
- 229940049337 neosporin Drugs 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229960003753 nitric oxide Drugs 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 239000005022 packaging material Substances 0.000 description 1
- 235000012771 pancakes Nutrition 0.000 description 1
- 239000000123 paper Substances 0.000 description 1
- 235000013550 pizza Nutrition 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- OYEHPCDNVJXUIW-UHFFFAOYSA-N plutonium atom Chemical compound [Pu] OYEHPCDNVJXUIW-UHFFFAOYSA-N 0.000 description 1
- 229920001490 poly(butyl methacrylate) polymer Polymers 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920002432 poly(vinyl methyl ether) polymer Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920002239 polyacrylonitrile Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920006216 polyvinyl aromatic Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 229920001290 polyvinyl ester Polymers 0.000 description 1
- 229920001289 polyvinyl ether Polymers 0.000 description 1
- 229920006215 polyvinyl ketone Polymers 0.000 description 1
- 229920006214 polyvinylidene halide Polymers 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- SCUZVMOVTVSBLE-UHFFFAOYSA-N prop-2-enenitrile;styrene Chemical compound C=CC#N.C=CC1=CC=CC=C1 SCUZVMOVTVSBLE-UHFFFAOYSA-N 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 239000003207 proteasome inhibitor Substances 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 229910052761 rare earth metal Inorganic materials 0.000 description 1
- 150000002910 rare earth metals Chemical class 0.000 description 1
- 239000002964 rayon Substances 0.000 description 1
- 238000005057 refrigeration Methods 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 229910052706 scandium Inorganic materials 0.000 description 1
- SIXSYDAISGFNSX-UHFFFAOYSA-N scandium atom Chemical compound [Sc] SIXSYDAISGFNSX-UHFFFAOYSA-N 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 239000008257 shaving cream Substances 0.000 description 1
- 229920005573 silicon-containing polymer Polymers 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 235000013555 soy sauce Nutrition 0.000 description 1
- 235000014268 sports nutrition Nutrition 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000000021 stimulant Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 229920003051 synthetic elastomer Polymers 0.000 description 1
- 239000005061 synthetic rubber Substances 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 229960001967 tacrolimus Drugs 0.000 description 1
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 1
- 229960000235 temsirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-UHFFFAOYSA-N temsirolimus Natural products C1CC(O)C(OC)CC1CC(C)C1OC(=O)C2CCCCN2C(=O)C(=O)C(O)(O2)C(C)CCC2CC(OC)C(C)=CC=CC=CC(C)CC(C)C(=O)C(OC)C(O)C(C)=CC(C)C(=O)C1 QFJCIRLUMZQUOT-UHFFFAOYSA-N 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 229920001187 thermosetting polymer Polymers 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 229940034610 toothpaste Drugs 0.000 description 1
- 239000000606 toothpaste Substances 0.000 description 1
- 231100000167 toxic agent Toxicity 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 229940072651 tylenol Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- DNYWZCXLKNTFFI-UHFFFAOYSA-N uranium Chemical compound [U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U][U] DNYWZCXLKNTFFI-UHFFFAOYSA-N 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 229940117958 vinyl acetate Drugs 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000011345 viscous material Substances 0.000 description 1
- 239000004636 vulcanized rubber Substances 0.000 description 1
- 235000012773 waffles Nutrition 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
- CGTADGCBEXYWNE-JUKNQOCSSA-N zotarolimus Chemical compound N1([C@H]2CC[C@@H](C[C@@H](C)[C@H]3OC(=O)[C@@H]4CCCCN4C(=O)C(=O)[C@@]4(O)[C@H](C)CC[C@H](O4)C[C@@H](/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C3)OC)C[C@H]2OC)C=NN=N1 CGTADGCBEXYWNE-JUKNQOCSSA-N 0.000 description 1
- 229950009819 zotarolimus Drugs 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D83/00—Containers or packages with special means for dispensing contents
- B65D83/0055—Containers or packages provided with a flexible bag or a deformable membrane or diaphragm for expelling the contents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D75/00—Packages comprising articles or materials partially or wholly enclosed in strips, sheets, blanks, tubes, or webs of flexible sheet material, e.g. in folded wrappers
- B65D75/28—Articles or materials wholly enclosed in composite wrappers, i.e. wrappers formed by associating or interconnecting two or more sheets or blanks
- B65D75/30—Articles or materials enclosed between two opposed sheets or blanks having their margins united, e.g. by pressure-sensitive adhesive, crimping, heat-sealing, or welding
- B65D75/32—Articles or materials enclosed between two opposed sheets or blanks having their margins united, e.g. by pressure-sensitive adhesive, crimping, heat-sealing, or welding one or both sheets or blanks being recessed to accommodate contents
- B65D75/321—Both sheets being recessed
- B65D75/322—Both sheets being recessed and forming one compartment
-
- A—HUMAN NECESSITIES
- A47—FURNITURE; DOMESTIC ARTICLES OR APPLIANCES; COFFEE MILLS; SPICE MILLS; SUCTION CLEANERS IN GENERAL
- A47K—SANITARY EQUIPMENT NOT OTHERWISE PROVIDED FOR; TOILET ACCESSORIES
- A47K5/00—Holders or dispensers for soap, toothpaste, or the like
- A47K5/06—Dispensers for soap
- A47K5/12—Dispensers for soap for liquid or pasty soap
- A47K5/1201—Dispensers for soap for liquid or pasty soap hand-carried
-
- A—HUMAN NECESSITIES
- A47—FURNITURE; DOMESTIC ARTICLES OR APPLIANCES; COFFEE MILLS; SPICE MILLS; SUCTION CLEANERS IN GENERAL
- A47K—SANITARY EQUIPMENT NOT OTHERWISE PROVIDED FOR; TOILET ACCESSORIES
- A47K5/00—Holders or dispensers for soap, toothpaste, or the like
- A47K5/06—Dispensers for soap
- A47K5/12—Dispensers for soap for liquid or pasty soap
- A47K5/122—Dispensers for soap for liquid or pasty soap using squeeze bottles or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
- A61J1/05—Containers specially adapted for medical or pharmaceutical purposes for collecting, storing or administering blood, plasma or medical fluids ; Infusion or perfusion containers
- A61J1/06—Ampoules or carpules
- A61J1/067—Flexible ampoules, the contents of which are expelled by squeezing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J7/00—Devices for administering medicines orally, e.g. spoons; Pill counting devices; Arrangements for time indication or reminder for taking medicine
- A61J7/0015—Devices specially adapted for taking medicines
- A61J7/0053—Syringes, pipettes or oral dispensers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/0005—Components or details
- B05B11/0027—Means for neutralising the actuation of the sprayer ; Means for preventing access to the sprayer actuation means
- B05B11/0032—Manually actuated means located downstream the discharge nozzle for closing or covering it, e.g. shutters
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/0005—Components or details
- B05B11/0062—Outlet valves actuated by the pressure of the fluid to be sprayed
- B05B11/007—Outlet valves actuated by the pressure of the fluid to be sprayed being opened by deformation of a sealing element made of resiliently deformable material, e.g. flaps, skirts, duck-bill valves
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/0005—Components or details
- B05B11/0062—Outlet valves actuated by the pressure of the fluid to be sprayed
- B05B11/0072—A valve member forming part of an outlet opening
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/01—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use characterised by the means producing the flow
- B05B11/04—Deformable containers producing the flow, e.g. squeeze bottles
- B05B11/048—Deformable containers producing the flow, e.g. squeeze bottles characterised by the container, e.g. this latter being surrounded by an enclosure, or the means for deforming it
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D35/00—Pliable tubular containers adapted to be permanently or temporarily deformed to expel contents, e.g. collapsible tubes for toothpaste or other plastic or semi-liquid material; Holders therefor
- B65D35/02—Body construction
- B65D35/10—Body construction made by uniting or interconnecting two or more components
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D47/00—Closures with filling and discharging, or with discharging, devices
- B65D47/04—Closures with discharging devices other than pumps
- B65D47/20—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge
- B65D47/2018—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure
- B65D47/2031—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure the element being formed by a slit, narrow opening or constrictable spout, the size of the outlet passage being able to be varied by increasing or decreasing the pressure
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D75/00—Packages comprising articles or materials partially or wholly enclosed in strips, sheets, blanks, tubes, or webs of flexible sheet material, e.g. in folded wrappers
- B65D75/52—Details
- B65D75/58—Opening or contents-removing devices added or incorporated during package manufacture
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D75/00—Packages comprising articles or materials partially or wholly enclosed in strips, sheets, blanks, tubes, or webs of flexible sheet material, e.g. in folded wrappers
- B65D75/52—Details
- B65D75/58—Opening or contents-removing devices added or incorporated during package manufacture
- B65D75/5855—Peelable seals
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D75/00—Packages comprising articles or materials partially or wholly enclosed in strips, sheets, blanks, tubes, or webs of flexible sheet material, e.g. in folded wrappers
- B65D75/52—Details
- B65D75/58—Opening or contents-removing devices added or incorporated during package manufacture
- B65D75/5861—Spouts
- B65D75/5872—Non-integral spouts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D83/00—Containers or packages with special means for dispensing contents
- B65D83/04—Containers or packages with special means for dispensing contents for dispensing annular, disc-shaped, or spherical or like small articles, e.g. tablets or pills
- B65D83/0409—Containers or packages with special means for dispensing contents for dispensing annular, disc-shaped, or spherical or like small articles, e.g. tablets or pills the dispensing means being adapted for delivering one article, or a single dose, upon each actuation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D83/00—Containers or packages with special means for dispensing contents
- B65D83/04—Containers or packages with special means for dispensing contents for dispensing annular, disc-shaped, or spherical or like small articles, e.g. tablets or pills
- B65D83/0481—Containers or packages with special means for dispensing contents for dispensing annular, disc-shaped, or spherical or like small articles, e.g. tablets or pills the articles passing through a small opening or passage, without additional dispensing devices and without retaining means for the following article
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D85/00—Containers, packaging elements or packages, specially adapted for particular articles or materials
- B65D85/70—Containers, packaging elements or packages, specially adapted for particular articles or materials for materials not otherwise provided for
- B65D85/72—Containers, packaging elements or packages, specially adapted for particular articles or materials for materials not otherwise provided for for edible or potable liquids, semiliquids, or plastic or pasty materials
-
- A—HUMAN NECESSITIES
- A47—FURNITURE; DOMESTIC ARTICLES OR APPLIANCES; COFFEE MILLS; SPICE MILLS; SUCTION CLEANERS IN GENERAL
- A47K—SANITARY EQUIPMENT NOT OTHERWISE PROVIDED FOR; TOILET ACCESSORIES
- A47K5/00—Holders or dispensers for soap, toothpaste, or the like
- A47K5/06—Dispensers for soap
- A47K5/12—Dispensers for soap for liquid or pasty soap
- A47K5/1211—Dispensers for soap for liquid or pasty soap using pressure on soap, e.g. with piston
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D2221/00—Small packaging specially adapted for product samples, single-use packages or échantillons
Definitions
- dispensing devices which can contain at least one substance that can be removed from the devices by deforming at least a portion of the device.
- a substance can be squished, extruded, emptied and/or poured out.
- the dispensing devices can comprise an enclosure, a circumferential edge portion, a dispensing port, and a squeezable portion.
- the dispensing devices can include internal volumes which can house the at least one substance either together or independently.
- the dispensing device can include a top portion including a dispensing port and a bottom portion including an inset groove.
- the circumferential edge portion can be a ring, securing mechanism, border, or rim and can protrude from the device or can be formed as a part of the device without protruding.
- the at least one substance is dispensed from the dispensing device by squeezing at least a portion of the squeezable enclosure.
- the dispensing device can be formed partially or completely from compressible material wherein the compressible material contains at least one polymer.
- the dispensing device can have any shape that can be at least partially deformed to remove the substance from the internal volume.
- the dispensing device can be substantially spherical.
- the dispensing device can also comprise a substantially circular or oval cross-section.
- FIG. 1 illustrates the dispensing device with a dispensing port.
- FIG. 2 illustrates the dispensing device when the dispensing port lid is open.
- FIG. 3 illustrates a cross-sectional view of the dispensing device.
- FIG. 4 illustrates the dispensing device where the dispensing port lid can be an adhesive alloy or tape.
- FIG. 5 illustrates the dispensing device where the dispensing port lid can be a pressure actuated slit.
- FIG. 6 illustrates pressure applied to the inset groove on the dispensing device thereby opening the actuated slit of FIG. 5 .
- FIG. 7 illustrates an embodiment where a person is using the dispensing device as described herein to squish ketchup on a hamburger.
- FIG. 8A illustrates a storage compartment for the storage of the dispensing devices.
- FIG. 8B illustrates a storage compartment for the storage of the dispensing devices with a hopper attached.
- FIG. 9A illustrates a dispensing device with a tearable side port.
- FIG. 9B illustrates the dispensing device where there is a spray nozzle or a one way nozzle inside the enclosure.
- FIG. 10A illustrates a dispensing device with a spreader hinged to the top of the dispensing device, which also acts as the dispensing port lid, and appendages extending from the circumferential edge portion.
- FIG. 10B illustrates a side view of a dispensing device with a spreader hinged to the top of the dispensing device, which also acts like the dispensing port lid, and appendages extending from the circumferential edge portion.
- FIG. 11A illustrates a perspective view of a dispensing device with a rectangular circumferential edge portion and rectangular enclosure.
- FIG. 11B illustrates the top view of the dispensing device illustrated in FIG. 11A .
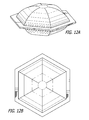
- FIG. 12A illustrates a perspective view of a dispensing device with a hexagonal circumferential edge portion and hexagonal enclosure.
- FIG. 12B illustrates the top view of the dispensing device illustrated in FIG. 12A .
- dispensing devices configured to contain at least one substance.
- the dispensing devices can be formed in such a manner that the substance(s) can be emptied from the devices by applying pressure to and/or deforming at least a portion of the device. Upon applying pressure and/or deforming at least a portion of a device, the substance(s) can be said to be squished, extruded or emptied out.
- the substances can be poured out.
- the dispensing device can comprise an enclosure including an internal volume, a circumferential edge portion and a dispensing port. The at least one substance can be housed within the internal volume.
- a dispensing device can be formed from two halves or portions, a top portion and a bottom portion, along the circumferential edge portion.
- the top portion can have a dispensing port and the bottom portion can have an inset groove.
- the top portion can be formed of rigid and/or non-deformable material and the bottom portion can be formed of a compressible and/or deformable material.
- top portion and the bottom portion can be independently sealed along the circumferential edge portion and the top portion can have an inset groove opposite the side with the dispensing port, and the bottom portion can have a dispensing port opposite the side with the inset groove thereby creating two dispensing devices in one.
- dispensing device 100 comprises a dispensing port lid 102 and a circumferential edge portion 104 .
- Dispensing device 100 can also include a top enclosure portion 106 and a bottom enclosure portion 108 .
- Dispensing device 100 in open position can be held upside down and stabilized by the use of fingers at the circumferential edge portion 104 and bottom enclosure portion 108 while applying pressure at inset groove 110 thereby emptying the contents.
- the packaging can be convenient in that it can allow a person to open and use dispensing device 100 with one hand. It is especially easy to use for those with physical ailments such as but not limited to arthritis. The use of one hand to open and use dispensing device 100 can be particularly convenient for people trying to multi-task.
- dispensing device 100 can include a sealed slit 502 that can be unsealed and opened by applying pressure 604 to bottom enclosure portion 108 .
- sealed slit 502 unseals and opens to form a slit dispensing port 602 .
- dispensing device 100 comprises two portions that can be halves, top half 302 and bottom half 304 . In some embodiments, the two portions do not need to be halves. For example, a top portion can be larger than a bottom portion or vice versa.
- FIG. 3 illustrates a cross sectional view of dispensing device 100 in which imaginary line 306 is drawn to represent where circumferential edge portion 104 would be. Bottom half 304 can contain inset groove 110 and top half 302 can contain dispensing port 112 .
- dispensing device 100 can contain a pierceable membrane located inside dispensing device 100 at imaginary line 306 to keep the contents in bottom half 304 separated from the contents in top half 302 .
- a piercer (not illustrated) inside dispensing device 100 , connected to inset groove 110 on the interior of the device. Once pressure is applied to at least a portion of inset groove 110 the piercer can pierce through a pierceable membrane.
- the pierceable membrane can be located anywhere in dispensing device 100 .
- the peirceble membrane can be located in dispensing device 100 at circumferential edge portion 104 to separate the top half 302 from the bottom half 304 .
- Dispensing device 100 can have any shape that can hold at least one substance within internal volume.
- dispensing device 100 can be substantially spherical.
- Dispensing device 100 can also comprise a substantially circular cross-section.
- a substantially spherical shape can allow the enclosure to be print on and to be easily readable and seen from all angles.
- the enclosure can include one or more linear portions. For example, it can include 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 linear portions.
- the enclosure can be any polygonal shape such as but not limited to a triangle, a square, a rectangle, a pentagon, a hexagon, a heptagon, an octagon, a nonagon, or a decagon.
- the enclosure can also be non-polygonal for example, a circle or oval.
- Circumferential edge portion 104 can be a ring, securing mechanism, border, or rim. Circumferential edge portion 104 can include one or more linear portions. For example, it can include 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 linear portions. Circumferential edge portion 104 can be a polygonal shape such as but not limited to a triangle, a square, a rectangle, a pentagon, a hexagon, a heptagon, an octagon, a nonagon, or a decagon. Circumferential edge portion 104 can also be non-polygonal for example, a circle or oval.
- FIG. 11A illustrates a side view of a dispensing device with a rectangular circumferential edge portion and substantially rectangular enclosure. A top view of this substantially rectangular dispensing device is illustrated in FIG. 11B .
- FIG. 12A illustrates a side view of a dispensing device with a hexagonal circumferential edge portion and substantially hexagonal enclosure. A top view of this substantially hexagonal dispensing device is illustrated in FIG. 12B .
- Dispensing device 100 can be formed in a shape to allow multiple devices to be stacked in storage compartment 800 as illustrated by FIG. 8 .
- Storage compartment lid 802 can be removed and multiple dispensing devices can be stacked in storage compartment 800 .
- First dispensing device 804 easily rests at the bottom of storage compartment 800 .
- Second dispensing device 806 can easily be stacked on first dispensing device 804 by inset groove 110 of second dispensing device 806 lying on top of dispensing port lid 102 of first dispensing device 804 . This continues as inset groove 110 of third dispensing device 808 lies on top of dispensing port lid 102 of second dispensing device 806 .
- Dispensing device 804 can easily be removed at opening 810 of storage compartment 800 .
- Storage compartment 800 can hold anywhere from 1 to 100 dispensing devices. For example, there can be 1-10, 10-20, 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, 80-90, or 90-100 dispensing devices in storage compartment 800 .
- Dispensing devices are stored in hopper 902 where the removal of one dispensing device allows through gravity for the other dispensing devices to funnel down as illustrated in FIG. 8B .
- Storage compartment 800 can include a loader device or hopper 902 .
- Hopper 902 can hold one or more devices that can feed into the top of storage compartment 800 .
- the loading of devices can be by gravity as a device is removed from the bottom of storage compartment 800 .
- a hopper can house 1-10, 10-20, 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, 80-90, 90-100, 100-500, 500-1,000, or 1,000-5,000 or more devices.
- dispensing device 100 allows for clear advertising space. Even while stacked substantially all of top enclosure portion 106 and bottom enclosure portion 108 can be used for advertising space by the product housed by dispensing device 100 and/or can be clearly seen through storage compartment 800 allowing great marketability of the product. This can be very beneficial in attracting consumers of all types. Further, the stackability and ease of removing one dispensing device from storage compartment 800 without touching any of the other dispensing devices allows for a state of sterility among the dispensing devices remaining in storage compartment 800 . This application could be extremely useful in hospitals or doctor offices where sterility can be important.
- Dispensing port lid 102 can be any device, substance, or shape that can allow for sealing of the at least one substance within the internal volume of a device.
- Dispensing port lid 102 can include, but is not limited to caps, lids, snap caps, peel caps, pressure actuated slits, spray actuated nozzles, mist nozzles, adhesive tape, or adhesive alloy.
- FIG. 4 illustrates adhesive alloy dispensing port 402 which can be peeled or pierced open.
- the alloy can be but is not limited to aluminum, bismuth, chromium, cobalt, copper, gallium, gold, indium, iron, lead, magnesium, mercury, nickel, potassium, plutonium, rare earth alloys, scandium, silver, sodium, titanium, tin, uranium, zinc, or zirconium.
- FIG. 5 illustrates pressure actuated slit dispensing port 502 . Pressure applied to inset groove 110 can cause the pressure actuated slit to tear thereby allowing the contents of dispensing device 100 to be squished out.
- appendage 702 can be connected to top enclosure portion 106 and bottom enclosure portion 108 at or near circumferential edge 104 and serve as a second dispensing port. Appendage 702 can be torn along the perforated edge 706 to allow at least one substance from the enclosure to be squished out. Appendage 702 can allow for a more controlled placement of the at least one substance to the desired area.
- nozzle 704 is situated in opening 708 of dispensing device 100 .
- Nozzle 704 can be but is not limited to a spray nozzle, a mist nozzle, or a one-way nozzle.
- Dispensing port lid 102 is configured to cover nozzle 704 when the dispensing device is in the closed position.
- Top enclosure portion 106 of dispensing device 100 can be made of slightly harder compressible material in relation to bottom enclosure portion 108 to allow ideal pressure conditions for the functioning of nozzle 704 .
- spreader 710 can be actuated using hinge 712 .
- Spreader 710 can function as a lid for dispensing device 100 in the closed position.
- Spreader 706 can act as a knife at distal end 714 to spread the at least one substance housed in dispensing device 100 .
- Distal end 714 can have any shape that allows spreading of the housed substance.
- FIG. 10B illustrates dispensing device 100 with spreader 706 in the open position as reflected by dispensing port 112 .
- the at least one substance can be squished onto the desired area and spread over an area by spreader 706 at distal end 714 .
- the circumferential edge portion 104 can extend outwards on the right and left side to form ear-like appendages 708 and 708 ′.
- Ear-like appendages 708 and 708 ′ on dispensing device 100 can be easier for people with bigger hands and physical aliments to control.
- Ear-like appendages 708 and 708 ′ can allow for increased stability while holding dispensing device 100 and applying pressure to inset groove 108 in order to squish out the at least one substance.
- Ear-like appendages 708 and 708 ′ can have any shape that aids in using and/or holding dispensing device 100 .
- Dispensing device 100 can be made of a polymer, metal, textile or a combination thereof. Further, top enclosure portion 106 and bottom enclosure portion 108 can be formed of the same or different materials.
- Polymeric materials can include but are not limited to thermosets, thermoplastics, solidified gels, tar, stucco, resin, rubber, vulcanized rubber, synthetic rubber, cement, silicone polymers, polyolefins, polyisobutylene, acrylic polymers, ethylene-co-vinylacetate, polybutylmethacrylate, vinyl halide polymers (for example, polyvinyl chloride), polyvinyl ethers (for example, polyvinyl methyl ether), polyvinylidene halides, polyacrylonitrile, polyvinyl ketones, polyvinyl aromatics, polyvinyl esters, acrylonitrile-styrene copolymers, ABS resins, ethylene-vinyl acetate copolymers, polyamides (for example, Nylon 66 and
- Metal or metallic materials can include but are not limited to aluminum, copper, tin, steel, iron, titanium, gold, silver, cobalt, chromium, or alloys or combinations thereof.
- Textile materials can include but are not limited to paper, caroboard, fiberboard, cotton, hemp, other polymeric textiles, and combinations thereof.
- dispensing device 100 can be formed of a combination or combinations of polymeric materials, metallic materials, and textile materials.
- the compressible material can be aseptic.
- the material can be aseptic in order to prevent the contents in dispensing device 100 from spoiling. This allows for the contents to be refrigerated only upon opening of dispensing device 100 . For example, where the contents is sweetened condensed milk, the sweetened condensed milk would remain unspoiled due to compressible material which is aseptic thereby allowing the sweetened condensed milk to be fresh upon opening of dispensing device 100 and only require refrigeration after opening.
- the embodiment can also have compressible material which is free of Bisphenol A (BPA).
- BPA Bisphenol A
- Compressible material free of BPA is safer for the environment and humans.
- BPA is an industrial chemical which has been found in plastic bottles and metal-based cans since the 1960's. Toxicity reports have shown that BPA can have negative effects on the brain, behavior, and prostate glad in fetuses, infants, and young children. As such dispensing device 100 can provide a safer way to package edible contents in comparison with other packaging materials that are not BPA free.
- the internal volume can be but is not limited to a void, an encasement, an empty space, or housing.
- the internal void is an encasement containing at least one substance.
- the at least one substance can be in the form of a fluid or viscous substance.
- it could be a liquid, solid, powder, cream, gel, polymer, or semi-solid.
- the substance can be a condiment, such as but not limited to ketchup, mustard, mayonnaise (regular, light, low-fat, fat-free), relish, sweet relish, oil, vinegar, salad dressing, peanut butter, jelly, jam, sweetened condensed milk, grey poupon, BBQ sauce, honey mustard sauce, spicy wing sauce, A1 steak sauce, cocktail sauce, tarter sauce, jellied cranberry sauce, brown and white gravy, pizza tomato sauce, hot sauce, Tabasco sauce, sriracha hot chili sauce, soy sauce, teriyaki sauce, balsamic vinegar, honey, nutella, frosting, cooking oil, liquid pancake batter, liquid waffle batter, soft butter, sour cream, cream cheese and coffee creamer.
- Dispensing device 100 offers an innovative enhancement to the way current condiment packaging controls the consumer; dispensing device 100 gives the piloting controls back so the consumer can “land” the liquid anywhere they want, using just one hand.
- the substance can be related to personal care such as but not limited to body lotion, hand lotion, aftershave, soap, hand sanitizer, bath salts, powder, gel, body oil, cream, shampoo, conditioner, sample shampoos, hair gels, body soaps/washes, moisturizer, shaving cream, toothpaste, facial soap, anti-aging cream, mouth wash, aspracream, soothing gels or creams, suntan lotion, sample intensive conditioners, and sexual lubricant.
- TSA Transportation Security Administration
- the substances can be packaged into dispensing device 100 for single-use in hotels, cruise lines, resorts, hotel chains, fast food restaurants, and airport lounge retail stores.
- the substance can be a medicament, therapeutic agent, and/or drug which may or may not include an active agent.
- a medicament can include any compound or drug having a therapeutic effect in an animal.
- anti-proliferatives including, but not limited to, macrolide antibiotics including FKBP-12 binding compounds, estrogens, chaperone inhibitors, protease inhibitors, protein-tyrosine kinase inhibitors, leptomycin B, peroxisome proliferator-activated receptor gamma ligands (PPAR ⁇ ), hypothemycin, nitric oxide, bisphosphonates, epidermal growth factor inhibitors, antibodies, proteasome inhibitors, antibiotics, anti-inflammatories, anti-sense nucleotides and transforming nucleic acids.
- macrolide antibiotics including FKBP-12 binding compounds, estrogens, chaperone inhibitors, protease inhibitors, protein-tyrosine kinase inhibitors, leptomycin B, peroxisome proliferator-activated receptor
- Drugs can also refer to bioactive agents including anti-proliferative compounds, cytostatic compounds, toxic compounds, anti-inflammatory compounds, chemotherapeutic agents, analgesics, antibiotics, protease inhibitors, statins, nucleic acids, polypeptides, growth factors and delivery vectors including recombinant micro-organisms, liposomes, and the like.
- bioactive agents including anti-proliferative compounds, cytostatic compounds, toxic compounds, anti-inflammatory compounds, chemotherapeutic agents, analgesics, antibiotics, protease inhibitors, statins, nucleic acids, polypeptides, growth factors and delivery vectors including recombinant micro-organisms, liposomes, and the like.
- Exemplary FKBP-12 binding agents include sirolimus (rapamycin), tacrolimus, everolimus, temsirolimus and zotarolimus.
- a medicament can be baby Acetaminophen (TYLENOL®), single-use androgel, single-use NEOSPORIN®, single-use burn cream soother, single-use aloe vera, single-use arthritis cream, single-use cough syrup, hydrogen peroxide, sanitizer, or wound irrigation (ER surgery).
- TYLENOL® baby Acetaminophen
- NEOSPORIN® single-use NEOSPORIN®
- single-use burn cream soother single-use aloe vera
- single-use arthritis cream single-use cough syrup
- hydrogen peroxide sanitizer
- wound irrigation ER surgery
- the substance can be related to health and beauty products such as, but not limited to, perfume, cologne, make-up, foundation, and nail polish remover.
- the substance can be related to sports nutrition such as, but not limited to, GU energy gel, hammer gel, liquid Gatorade concentrate, Gatorade fuel pouch, accel gel, protein powder, energy boosts, and stimulants.
- sports nutrition such as, but not limited to, GU energy gel, hammer gel, liquid Gatorade concentrate, Gatorade fuel pouch, accel gel, protein powder, energy boosts, and stimulants.
- the substance can be related to industrial use such as machine oils or machine lubricants.
- the substance can be alcoholic beverages (which are mixable or not), JELL-O® shots, paint, mosquito/bug spray repellant or cake frosting for cake decorating.
- dispensing devices as described herein can have an internal volume of 3.0 fluid ounces and in another 6.0 fluid ounces.
- the internal volume can be anywhere from 0.5 fluid ounces to about 1.5 liters.
- the internal volume can be about 0.5 to about 1.0 fluid ounces, about 1.0 to about 2.0 fluid ounces, about 2.0 to about 3.0 fluid ounces, about 3.0 to about 4.0 fluid ounces, about 4.0 to about 5.0 fluid ounces, about 5.0 to about 6.0 fluid ounces, about 6.0 fluid ounces to about 7.0 fluid ounces, about 7.0 fluid ounces to about 8.0 fluid ounces, about 8.0 fluid ounces to about 9.0 fluid ounces, about 9.0 fluid ounces to about 10.0 fluid ounces, about 10.0 fluid ounces to about 11.0 fluid ounces, about 11.0 fluid ounces to about 12.0 fluid ounces, about 12.0 fluid ounces to about 13.0 fluid ounces, about 13.0 fluid ounces to about 14.0 fluid
- the dispensing device is used to apply ketchup onto a hamburger bun.
- the person first takes the dispensing device and opens the dispensing port lid.
- the dispensing port lid is a snap cap.
- the person then turns the dispensing device upside down while stabilizing it in the open position using the middle finger and index finger at the circumferential edge portion and squeezable enclosure. Any fingers can be used to stabilize the dispensing device.
- the thumb applies pressure onto the inset groove of the dispensing device in order to squish out the ketchup. The burger can then be enjoyed.
- the dispensing device is used to create an oil-vinegar dressing.
- the dispensing device contains a pierceable membrane at imaginary line 306 located at the circumferential edge portion to separate the top enclosure portion of the dispensing device from the bottom enclosure portion of the dispensing device.
- the top enclosure portion of the dispensing device contains the dispensing port and the bottom enclosure portion of the dispensing device contains the inset groove.
- the top enclosure portion houses the vinegar while the bottom enclosure portion houses the oil.
- the pressure applied to the inset groove allows a piercer located inside the enclosure at the inset groove to pierce through the pierceable membrane thereby allowing the oil and vinegar to come into contact with one another and mix.
- the dispensing device can then be given a little shake and the dispensing port lid opened. Pressure can then be applied again while the dispensing device is open to empty out the oil-vinegar dressing onto the salad. The salad can then be enjoyed anytime anywhere with the convenient instant oil-vinegar dressing on hand. No longer does one have to worry about how to store their dressing and worry about spillage.
- the dispensing device is used to apply moisturizer to the skin.
- the person first takes the dispensing device and opens the dispensing port lid.
- the dispensing port lid is a peel cap.
- the person then turns the dispensing device upside down while stabilizing it in the open position using the middle finger and index finger at the circumferential edge portion and enclosure.
- the thumb then applies pressure onto the inset groove of the dispensing device in order to squish out the moisturizer onto the desired area.
- the dispensing device is for a single use and is thrown out upon removal of all the moisturizer. This is extremely convenient for camping trips, trips in general, and for the average women on the go whose purse is too heavy to carry a bulky store brought moisturizer and desires that easy single use moisturizer to moisturize her skin during the day.
- the advantage is also having a different scented moisturizer for each day of the week. There is no monotony but only variety.
- the dispensing device is used to make peanut butter and jelly sandwiches on a camping trip.
- Both dispensing devices have a spreader hinged to the top enclosure portion of the dispensing device.
- the spreader acts as both a knife and a snap cap to cover the dispensing port.
- the spreader is snapped open and the dispensing device turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb.
- the peanut butter or jelly, depending on which dispensing device is used first, is then squished onto the bread.
- the spreader then acts as a knife and spreads the peanut butter and jelly all over the bread.
- the spreader snaps back onto the dispensing port of the dispensing device to reseal the dispensing device. This is particularly beneficial for the wilderness because being able to reseal the dispensing devices will keep the smell of food intact and not attract bears and other animals.
- the dispensing device is used to store cologne or perfume for travel or everyday use when on the go.
- a 0.5 fluid ounce dispensing device contains men's cologne.
- the desired amount of cologne can be applied to the sponge applicator by the amount of pressure exerted by the thumb on the inset groove.
- the dispensing device is turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb and then the cologne gets absorbed by the sponge applicator.
- the dispensing port lid is then opened and the cologne applied onto the desired area by the sponge applicator.
- the sponge applicator can be provided separately opposed to being situated inside the enclosure.
- the dispensing device is used to apply paint to a desired area. This is convenient for the painter who wants to do a touch up or wants to paint without the bulkiness of paint cans.
- the 6.0 fluid ounce dispensing device contains the desired paint.
- the desired amount of paint can be applied to the sponge applicator by the amount of pressure exerted by the thumb on the inset groove.
- the dispensing device is turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb and then the paint gets absorbed by the sponge applicator.
- the dispensing port lid is then opened and the paint can be applied onto the desired area by the sponge applicator.
- the sponge applicator can be provided separately opposed to being situated inside the enclosure.
- the dispensing device is used to apply makeup/foundation to the face. This is convenient for the woman who wishes to try different foundations, for instance one day she could be tanner than the next without the sun.
- the dispensing device is a 2.0 fluid ounces dispensing device with desired foundation shade.
- the desired amount of foundation can be applied to the sponge applicator by the amount of pressure exerted by the thumb on the inset groove.
- the dispensing device is turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb and then the foundation gets absorbed by the sponge applicator.
- the dispensing port lid is then opened and the foundation applied onto the desired area by the sponge applicator.
- the sponge applicator can be provided separately opposed to being situated inside the enclosure.
- the dispensing device is used to wash a pet.
- the dispensing device is 2.0 fluid ounces and contains animal safe shampoo for dogs.
- the dispensing device has a brush hinged to the top of the squeezable enclosure of the dispensing device.
- the brush acts as both a brush and a snap cap to cover the dispensing port of the dispensing device.
- the bristles of the brush point upwards from the dispensing port so that the non-bristle side is used to reseal the dispensing device and act as a dispensing port lid.
- the brush is snapped open and the dispensing device turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb.
- the shampoo is then squished onto the dog.
- the brush can then be used to brush the shampoo through the wet hair of the dog. Water can then be poured on the dog to wash away the shampoo.
- the brush snaps back onto the dispensing port of the dispensing device to reseal the dispensing device. This is particularly beneficial as the dog can be bathed anywhere where there is water.
- the brush can also be provided separately and a dispensing port lid such as a snap cap used instead of the brush.
- the dispensing device is used to spray mosquito repellant. This is convenient for camping trips and travel even in your own back yard during those beautiful summer nights.
- the dispensing device is a 3.0 fluid ounces dispensing device with mosquito repellant.
- the dispensing port lid is opened and the dispensing device turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb and the mosquito repellant gets sprayed out onto the desired area.
- the dispensing port lid can then be closed to reseal the dispensing device for later use.
Landscapes
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Composite Materials (AREA)
- Hematology (AREA)
- Pharmacology & Pharmacy (AREA)
- Containers And Packaging Bodies Having A Special Means To Remove Contents (AREA)
Abstract
Dispensing devices are described which can contain at least one substance(s) that can be squished, emptied, extruded, and/or poured out. The dispensing devices can comprise an enclosure, a circumferential edge portion, a dispensing port, and a squeezable portion.
Description
- This application claims priority to U.S. provisional patent application No. 61/641,477 filed on May 2, 2012 and U.S. provisional patent application No. 61/656,456 filed on Jun. 6, 2012, the disclosures each of which are incorporated herein by reference in their entirety.
- Described herein generally are dispensing devices which can contain at least one substance that can be removed from the devices by deforming at least a portion of the device. In some embodiments, a substance can be squished, extruded, emptied and/or poured out. The dispensing devices can comprise an enclosure, a circumferential edge portion, a dispensing port, and a squeezable portion. The dispensing devices can include internal volumes which can house the at least one substance either together or independently.
- The dispensing device can include a top portion including a dispensing port and a bottom portion including an inset groove. The circumferential edge portion can be a ring, securing mechanism, border, or rim and can protrude from the device or can be formed as a part of the device without protruding.
- Methods are also described wherein the at least one substance is dispensed from the dispensing device by squeezing at least a portion of the squeezable enclosure. The dispensing device can be formed partially or completely from compressible material wherein the compressible material contains at least one polymer.
- The dispensing device can have any shape that can be at least partially deformed to remove the substance from the internal volume. In one embodiment, the dispensing device can be substantially spherical. The dispensing device can also comprise a substantially circular or oval cross-section.
-
FIG. 1 illustrates the dispensing device with a dispensing port. -
FIG. 2 illustrates the dispensing device when the dispensing port lid is open. -
FIG. 3 illustrates a cross-sectional view of the dispensing device. -
FIG. 4 illustrates the dispensing device where the dispensing port lid can be an adhesive alloy or tape. -
FIG. 5 illustrates the dispensing device where the dispensing port lid can be a pressure actuated slit. -
FIG. 6 illustrates pressure applied to the inset groove on the dispensing device thereby opening the actuated slit ofFIG. 5 . -
FIG. 7 illustrates an embodiment where a person is using the dispensing device as described herein to squish ketchup on a hamburger. -
FIG. 8A illustrates a storage compartment for the storage of the dispensing devices. -
FIG. 8B illustrates a storage compartment for the storage of the dispensing devices with a hopper attached. -
FIG. 9A illustrates a dispensing device with a tearable side port. -
FIG. 9B illustrates the dispensing device where there is a spray nozzle or a one way nozzle inside the enclosure. -
FIG. 10A illustrates a dispensing device with a spreader hinged to the top of the dispensing device, which also acts as the dispensing port lid, and appendages extending from the circumferential edge portion. -
FIG. 10B illustrates a side view of a dispensing device with a spreader hinged to the top of the dispensing device, which also acts like the dispensing port lid, and appendages extending from the circumferential edge portion. -
FIG. 11A illustrates a perspective view of a dispensing device with a rectangular circumferential edge portion and rectangular enclosure. -
FIG. 11B illustrates the top view of the dispensing device illustrated inFIG. 11A . -
FIG. 12A illustrates a perspective view of a dispensing device with a hexagonal circumferential edge portion and hexagonal enclosure. -
FIG. 12B illustrates the top view of the dispensing device illustrated inFIG. 12A . - Described herein are dispensing devices configured to contain at least one substance. The dispensing devices can be formed in such a manner that the substance(s) can be emptied from the devices by applying pressure to and/or deforming at least a portion of the device. Upon applying pressure and/or deforming at least a portion of a device, the substance(s) can be said to be squished, extruded or emptied out. In one embodiment, the substances can be poured out. In one embodiment the dispensing device can comprise an enclosure including an internal volume, a circumferential edge portion and a dispensing port. The at least one substance can be housed within the internal volume.
- In one embodiment, a dispensing device can be formed from two halves or portions, a top portion and a bottom portion, along the circumferential edge portion. The top portion can have a dispensing port and the bottom portion can have an inset groove. Further, the top portion can be formed of rigid and/or non-deformable material and the bottom portion can be formed of a compressible and/or deformable material.
- In another embodiment, the top portion and the bottom portion can be independently sealed along the circumferential edge portion and the top portion can have an inset groove opposite the side with the dispensing port, and the bottom portion can have a dispensing port opposite the side with the inset groove thereby creating two dispensing devices in one.
- In one embodiment, as illustrated in
FIG. 1 , dispensingdevice 100 comprises adispensing port lid 102 and acircumferential edge portion 104.Dispensing device 100 can also include atop enclosure portion 106 and abottom enclosure portion 108. Dispensingdevice 100 in open position, as illustrated inFIG. 2 , can be held upside down and stabilized by the use of fingers at thecircumferential edge portion 104 andbottom enclosure portion 108 while applying pressure atinset groove 110 thereby emptying the contents. The packaging can be convenient in that it can allow a person to open and usedispensing device 100 with one hand. It is especially easy to use for those with physical ailments such as but not limited to arthritis. The use of one hand to open and usedispensing device 100 can be particularly convenient for people trying to multi-task. - In another embodiment, as illustrated in
FIGS. 5 and 6 , dispensingdevice 100 can include a sealedslit 502 that can be unsealed and opened by applyingpressure 604 tobottom enclosure portion 108. Whenpressure 604 is applied tobottom enclosure portion 108, sealedslit 502 unseals and opens to form aslit dispensing port 602. - In one embodiment, as illustrated in
FIG. 3 , dispensingdevice 100 comprises two portions that can be halves,top half 302 andbottom half 304. In some embodiments, the two portions do not need to be halves. For example, a top portion can be larger than a bottom portion or vice versa.FIG. 3 illustrates a cross sectional view of dispensingdevice 100 in whichimaginary line 306 is drawn to represent wherecircumferential edge portion 104 would be.Bottom half 304 can containinset groove 110 andtop half 302 can contain dispensingport 112. In one particular embodiment, dispensingdevice 100 can contain a pierceable membrane located inside dispensingdevice 100 atimaginary line 306 to keep the contents inbottom half 304 separated from the contents intop half 302. For instance, there can be a piercer (not illustrated) inside dispensingdevice 100, connected toinset groove 110 on the interior of the device. Once pressure is applied to at least a portion ofinset groove 110 the piercer can pierce through a pierceable membrane. The pierceable membrane can be located anywhere in dispensingdevice 100. In a preferred embodiment the peirceble membrane can be located in dispensingdevice 100 atcircumferential edge portion 104 to separate thetop half 302 from thebottom half 304. -
Dispensing device 100 can have any shape that can hold at least one substance within internal volume. For example, dispensingdevice 100 can be substantially spherical.Dispensing device 100 can also comprise a substantially circular cross-section. A substantially spherical shape can allow the enclosure to be print on and to be easily readable and seen from all angles. The enclosure can include one or more linear portions. For example, it can include 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 linear portions. The enclosure can be any polygonal shape such as but not limited to a triangle, a square, a rectangle, a pentagon, a hexagon, a heptagon, an octagon, a nonagon, or a decagon. The enclosure can also be non-polygonal for example, a circle or oval. -
Circumferential edge portion 104 can be a ring, securing mechanism, border, or rim.Circumferential edge portion 104 can include one or more linear portions. For example, it can include 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 linear portions.Circumferential edge portion 104 can be a polygonal shape such as but not limited to a triangle, a square, a rectangle, a pentagon, a hexagon, a heptagon, an octagon, a nonagon, or a decagon.Circumferential edge portion 104 can also be non-polygonal for example, a circle or oval. - As an example,
FIG. 11A illustrates a side view of a dispensing device with a rectangular circumferential edge portion and substantially rectangular enclosure. A top view of this substantially rectangular dispensing device is illustrated inFIG. 11B .FIG. 12A illustrates a side view of a dispensing device with a hexagonal circumferential edge portion and substantially hexagonal enclosure. A top view of this substantially hexagonal dispensing device is illustrated inFIG. 12B . -
Dispensing device 100 can be formed in a shape to allow multiple devices to be stacked instorage compartment 800 as illustrated byFIG. 8 .Storage compartment lid 802 can be removed and multiple dispensing devices can be stacked instorage compartment 800. First dispensingdevice 804 easily rests at the bottom ofstorage compartment 800.Second dispensing device 806 can easily be stacked onfirst dispensing device 804 byinset groove 110 ofsecond dispensing device 806 lying on top of dispensingport lid 102 offirst dispensing device 804. This continues asinset groove 110 ofthird dispensing device 808 lies on top of dispensingport lid 102 ofsecond dispensing device 806.Dispensing device 804 can easily be removed at opening 810 ofstorage compartment 800.Storage compartment 800 can hold anywhere from 1 to 100 dispensing devices. For example, there can be 1-10, 10-20, 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, 80-90, or 90-100 dispensing devices instorage compartment 800. - In another embodiment, dispensing devices are stored in
hopper 902 where the removal of one dispensing device allows through gravity for the other dispensing devices to funnel down as illustrated inFIG. 8B .Storage compartment 800 can include a loader device orhopper 902.Hopper 902 can hold one or more devices that can feed into the top ofstorage compartment 800. The loading of devices can be by gravity as a device is removed from the bottom ofstorage compartment 800. A hopper can house 1-10, 10-20, 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, 80-90, 90-100, 100-500, 500-1,000, or 1,000-5,000 or more devices. - The ease of packaging and stackability of dispensing
device 100 allows for clear advertising space. Even while stacked substantially all oftop enclosure portion 106 andbottom enclosure portion 108 can be used for advertising space by the product housed by dispensingdevice 100 and/or can be clearly seen throughstorage compartment 800 allowing great marketability of the product. This can be very beneficial in attracting consumers of all types. Further, the stackability and ease of removing one dispensing device fromstorage compartment 800 without touching any of the other dispensing devices allows for a state of sterility among the dispensing devices remaining instorage compartment 800. This application could be extremely useful in hospitals or doctor offices where sterility can be important. However, this application can also be beneficial among other establishments as well as people in today's age are very cautious about what they touch and what contaminants they come in contact with. For instance, people don't want someone else's germs on their dispensing device. Thus the ease in stackability and ability to remove one dispensing device without touching the other is very attractive to many individuals who are worried about becoming sick of fearful of encountering contaminants. - Dispensing
port lid 102 can be any device, substance, or shape that can allow for sealing of the at least one substance within the internal volume of a device. Dispensingport lid 102 can include, but is not limited to caps, lids, snap caps, peel caps, pressure actuated slits, spray actuated nozzles, mist nozzles, adhesive tape, or adhesive alloy.FIG. 4 illustrates adhesivealloy dispensing port 402 which can be peeled or pierced open. The alloy can be but is not limited to aluminum, bismuth, chromium, cobalt, copper, gallium, gold, indium, iron, lead, magnesium, mercury, nickel, potassium, plutonium, rare earth alloys, scandium, silver, sodium, titanium, tin, uranium, zinc, or zirconium.FIG. 5 illustrates pressure actuatedslit dispensing port 502. Pressure applied toinset groove 110 can cause the pressure actuated slit to tear thereby allowing the contents of dispensingdevice 100 to be squished out. - In one embodiment, as illustrated in
FIG. 9A ,appendage 702, can be connected totop enclosure portion 106 andbottom enclosure portion 108 at or nearcircumferential edge 104 and serve as a second dispensing port.Appendage 702 can be torn along theperforated edge 706 to allow at least one substance from the enclosure to be squished out.Appendage 702 can allow for a more controlled placement of the at least one substance to the desired area. - In another embodiment, as illustrated in
FIG. 9B ,nozzle 704 is situated in opening 708 of dispensingdevice 100.Nozzle 704 can be but is not limited to a spray nozzle, a mist nozzle, or a one-way nozzle. Dispensingport lid 102 is configured to covernozzle 704 when the dispensing device is in the closed position.Top enclosure portion 106 of dispensingdevice 100 can be made of slightly harder compressible material in relation tobottom enclosure portion 108 to allow ideal pressure conditions for the functioning ofnozzle 704. - In yet another embodiment, as illustrated in
FIG. 10A ,spreader 710 can be actuated usinghinge 712.Spreader 710 can function as a lid for dispensingdevice 100 in the closed position.Spreader 706 can act as a knife atdistal end 714 to spread the at least one substance housed in dispensingdevice 100.Distal end 714 can have any shape that allows spreading of the housed substance.FIG. 10B illustrates dispensingdevice 100 withspreader 706 in the open position as reflected by dispensingport 112. The at least one substance can be squished onto the desired area and spread over an area byspreader 706 atdistal end 714. - In a further embodiment, as illustrated by
FIG. 10A thecircumferential edge portion 104 can extend outwards on the right and left side to form ear-like appendages like appendages device 100 can be easier for people with bigger hands and physical aliments to control. Ear-like appendages dispensing device 100 and applying pressure toinset groove 108 in order to squish out the at least one substance. Ear-like appendages dispensing device 100. -
Dispensing device 100 can be made of a polymer, metal, textile or a combination thereof. Further,top enclosure portion 106 andbottom enclosure portion 108 can be formed of the same or different materials. Polymeric materials can include but are not limited to thermosets, thermoplastics, solidified gels, tar, stucco, resin, rubber, vulcanized rubber, synthetic rubber, cement, silicone polymers, polyolefins, polyisobutylene, acrylic polymers, ethylene-co-vinylacetate, polybutylmethacrylate, vinyl halide polymers (for example, polyvinyl chloride), polyvinyl ethers (for example, polyvinyl methyl ether), polyvinylidene halides, polyacrylonitrile, polyvinyl ketones, polyvinyl aromatics, polyvinyl esters, acrylonitrile-styrene copolymers, ABS resins, ethylene-vinyl acetate copolymers, polyamides (for example, Nylon 66 and polycaprolactam), alkyd resins, polycarbonates, polyoxymethylenes, polyimides, polyethers, epoxy resins, polyurethanes, rayon, cellulose, cellulose acetate, cellulose butyrate, cellulose acetate butyrate, cellophane, cellulose nitrate, cellulose propionate, cellulose ethers, carboxymethyl cellulose, polytetrafluororethylene (for example, Teflon), combinations thereof, and the like. Metal or metallic materials can include but are not limited to aluminum, copper, tin, steel, iron, titanium, gold, silver, cobalt, chromium, or alloys or combinations thereof. Textile materials can include but are not limited to paper, caroboard, fiberboard, cotton, hemp, other polymeric textiles, and combinations thereof. - In other embodiments, dispensing
device 100 can be formed of a combination or combinations of polymeric materials, metallic materials, and textile materials. - In yet another embodiment, the compressible material can be aseptic. The material can be aseptic in order to prevent the contents in dispensing
device 100 from spoiling. This allows for the contents to be refrigerated only upon opening of dispensingdevice 100. For example, where the contents is sweetened condensed milk, the sweetened condensed milk would remain unspoiled due to compressible material which is aseptic thereby allowing the sweetened condensed milk to be fresh upon opening of dispensingdevice 100 and only require refrigeration after opening. - Further, the embodiment can also have compressible material which is free of Bisphenol A (BPA). Compressible material free of BPA is safer for the environment and humans. BPA is an industrial chemical which has been found in plastic bottles and metal-based cans since the 1960's. Toxicity reports have shown that BPA can have negative effects on the brain, behavior, and prostate glad in fetuses, infants, and young children. As
such dispensing device 100 can provide a safer way to package edible contents in comparison with other packaging materials that are not BPA free. - The internal volume can be but is not limited to a void, an encasement, an empty space, or housing.
- In another embodiment the internal void is an encasement containing at least one substance. The at least one substance can be in the form of a fluid or viscous substance. For example it could be a liquid, solid, powder, cream, gel, polymer, or semi-solid.
- In one embodiment the substance can be a condiment, such as but not limited to ketchup, mustard, mayonnaise (regular, light, low-fat, fat-free), relish, sweet relish, oil, vinegar, salad dressing, peanut butter, jelly, jam, sweetened condensed milk, grey poupon, BBQ sauce, honey mustard sauce, spicy wing sauce, A1 steak sauce, cocktail sauce, tarter sauce, jellied cranberry sauce, brown and white gravy, pizza tomato sauce, hot sauce, Tabasco sauce, sriracha hot chili sauce, soy sauce, teriyaki sauce, balsamic vinegar, honey, nutella, frosting, cooking oil, liquid pancake batter, liquid waffle batter, soft butter, sour cream, cream cheese and coffee creamer.
Dispensing device 100 offers an innovative enhancement to the way current condiment packaging controls the consumer; dispensingdevice 100 gives the piloting controls back so the consumer can “land” the liquid anywhere they want, using just one hand. - In yet another embodiment the substance can be related to personal care such as but not limited to body lotion, hand lotion, aftershave, soap, hand sanitizer, bath salts, powder, gel, body oil, cream, shampoo, conditioner, sample shampoos, hair gels, body soaps/washes, moisturizer, shaving cream, toothpaste, facial soap, anti-aging cream, mouth wash, aspracream, soothing gels or creams, suntan lotion, sample intensive conditioners, and sexual lubricant. These substances can be packaged into dispensing
device 100 at a certain travel size approved by the Transportation Security Administration (TSA). Further, the substances can be packaged into dispensingdevice 100 for single-use in hotels, cruise lines, resorts, hotel chains, fast food restaurants, and airport lounge retail stores. - In even yet another embodiment the substance can be a medicament, therapeutic agent, and/or drug which may or may not include an active agent. A medicament can include any compound or drug having a therapeutic effect in an animal. Exemplary, non limiting examples include anti-proliferatives including, but not limited to, macrolide antibiotics including FKBP-12 binding compounds, estrogens, chaperone inhibitors, protease inhibitors, protein-tyrosine kinase inhibitors, leptomycin B, peroxisome proliferator-activated receptor gamma ligands (PPARγ), hypothemycin, nitric oxide, bisphosphonates, epidermal growth factor inhibitors, antibodies, proteasome inhibitors, antibiotics, anti-inflammatories, anti-sense nucleotides and transforming nucleic acids. Drugs can also refer to bioactive agents including anti-proliferative compounds, cytostatic compounds, toxic compounds, anti-inflammatory compounds, chemotherapeutic agents, analgesics, antibiotics, protease inhibitors, statins, nucleic acids, polypeptides, growth factors and delivery vectors including recombinant micro-organisms, liposomes, and the like. Exemplary FKBP-12 binding agents include sirolimus (rapamycin), tacrolimus, everolimus, temsirolimus and zotarolimus.
- In one embodiment, a medicament can be baby Acetaminophen (TYLENOL®), single-use androgel, single-use NEOSPORIN®, single-use burn cream soother, single-use aloe vera, single-use arthritis cream, single-use cough syrup, hydrogen peroxide, sanitizer, or wound irrigation (ER surgery).
- In another embodiment the substance can be related to health and beauty products such as, but not limited to, perfume, cologne, make-up, foundation, and nail polish remover.
- In yet another embodiment the substance can be related to sports nutrition such as, but not limited to, GU energy gel, hammer gel, liquid Gatorade concentrate, Gatorade fuel pouch, accel gel, protein powder, energy boosts, and stimulants.
- In a further embodiment the substance can be related to industrial use such as machine oils or machine lubricants.
- In yet another embodiment the substance can be alcoholic beverages (which are mixable or not), JELL-O® shots, paint, mosquito/bug spray repellant or cake frosting for cake decorating.
- In another embodiment, dispensing devices as described herein can have an internal volume of 3.0 fluid ounces and in another 6.0 fluid ounces. The internal volume can be anywhere from 0.5 fluid ounces to about 1.5 liters. The internal volume can be about 0.5 to about 1.0 fluid ounces, about 1.0 to about 2.0 fluid ounces, about 2.0 to about 3.0 fluid ounces, about 3.0 to about 4.0 fluid ounces, about 4.0 to about 5.0 fluid ounces, about 5.0 to about 6.0 fluid ounces, about 6.0 fluid ounces to about 7.0 fluid ounces, about 7.0 fluid ounces to about 8.0 fluid ounces, about 8.0 fluid ounces to about 9.0 fluid ounces, about 9.0 fluid ounces to about 10.0 fluid ounces, about 10.0 fluid ounces to about 11.0 fluid ounces, about 11.0 fluid ounces to about 12.0 fluid ounces, about 12.0 fluid ounces to about 13.0 fluid ounces, about 13.0 fluid ounces to about 14.0 fluid ounces, about 14.0 fluid ounces to about 15.0 fluid ounces, about 15.0 fluid ounces to about 16.0 fluid ounces, about 16.0 fluid ounces to about 17.0 fluid ounces, about 17.0 fluid ounces to about 18.0 fluid ounces, about 18.0 fluid ounces to about 19.0 fluid ounces, about 19.0 fluid ounces to about 20.0 fluid ounces, about 21.0 fluid ounces to about 22.0 fluid ounces, about 22.0 fluid ounces to about 23.0 fluid ounces, about 23.0 fluid ounces to about 24.0 fluid ounces, about 24.0 fluid ounces to about 25.0 fluid ounces, about 25.0 fluid ounces to about 26.0 fluid ounces, about 26.0 fluid ounces to about 27.0 fluid ounces, about 27.0 fluid ounces to about 28.0 fluid ounces, about 28.0 fluid ounces to about 29.0 fluid ounces, about 29.0 fluid ounces to about 30.0 fluid ounces, about 30.0 fluid ounces to about 31.0 fluid ounces, about 31.0 fluid ounces to about 32.0 fluid ounces, about 32.0 fluid ounces to about 33.0 fluid ounces, about 33.0 fluid ounces to about 34.0 fluid ounces, about 34.0 fluid ounces to about 35.0 fluid ounces, about 35.0 fluid ounces to about 36.0 fluid ounces, about 36.0 fluid ounces to about 37.0 fluid ounces, about 37.0 fluid ounces to about 38.0 fluid ounces, about 38.0 fluid ounces to about 39.0 fluid ounces, about 39.0 fluid ounces to about 40.0 fluid ounces, about 40.0 fluid ounces to about 41.0 fluid ounces, about 41.0 fluid ounces to about 42.0 fluid ounces, about 42.0 fluid ounces to about 43.0 fluid ounces, about 43.0 fluid ounces to about 44.0 fluid ounces, about 44.0 fluid ounces to about 45.0 fluid ounces, about 45.0 fluid ounces to about 46.0 fluid ounces, about 46.0 fluid ounces to about 47.0 fluid ounces, about 47.0 fluid ounces to about 48.0 fluid ounces, about 48.0 fluid ounces to about 49.0 fluid ounces, about 49.0 fluid ounces to about 50.0 fluid ounces, about 50.0 fluid ounces to about 51.0 fluid ounces.
- As illustrated, in
FIG. 7 , the dispensing device is used to apply ketchup onto a hamburger bun. The person first takes the dispensing device and opens the dispensing port lid. The dispensing port lid is a snap cap. The person then turns the dispensing device upside down while stabilizing it in the open position using the middle finger and index finger at the circumferential edge portion and squeezable enclosure. Any fingers can be used to stabilize the dispensing device. The thumb then applies pressure onto the inset groove of the dispensing device in order to squish out the ketchup. The burger can then be enjoyed. - The dispensing device is used to create an oil-vinegar dressing. The dispensing device contains a pierceable membrane at
imaginary line 306 located at the circumferential edge portion to separate the top enclosure portion of the dispensing device from the bottom enclosure portion of the dispensing device. The top enclosure portion of the dispensing device contains the dispensing port and the bottom enclosure portion of the dispensing device contains the inset groove. The top enclosure portion houses the vinegar while the bottom enclosure portion houses the oil. Once a person is ready to eat their salad they can create a delicious oil-vinegar based dressing by keeping the dispensing port lid intact turning the dispensing device upside down and stabilizing it in their fingers while applying pressure to the inset groove with the thumb. The pressure applied to the inset groove allows a piercer located inside the enclosure at the inset groove to pierce through the pierceable membrane thereby allowing the oil and vinegar to come into contact with one another and mix. The dispensing device can then be given a little shake and the dispensing port lid opened. Pressure can then be applied again while the dispensing device is open to empty out the oil-vinegar dressing onto the salad. The salad can then be enjoyed anytime anywhere with the convenient instant oil-vinegar dressing on hand. No longer does one have to worry about how to store their dressing and worry about spillage. - The dispensing device is used to apply moisturizer to the skin. The person first takes the dispensing device and opens the dispensing port lid. The dispensing port lid is a peel cap. The person then turns the dispensing device upside down while stabilizing it in the open position using the middle finger and index finger at the circumferential edge portion and enclosure. The thumb then applies pressure onto the inset groove of the dispensing device in order to squish out the moisturizer onto the desired area. The dispensing device is for a single use and is thrown out upon removal of all the moisturizer. This is extremely convenient for camping trips, trips in general, and for the average women on the go whose purse is too heavy to carry a bulky store brought moisturizer and desires that easy single use moisturizer to moisturize her skin during the day. The advantage is also having a different scented moisturizer for each day of the week. There is no monotony but only variety.
- The dispensing device is used to make peanut butter and jelly sandwiches on a camping trip. There are two 3.0 fluid ounce dispensing devices; one contains peanut butter and the other jelly. Both dispensing devices have a spreader hinged to the top enclosure portion of the dispensing device. The spreader acts as both a knife and a snap cap to cover the dispensing port. The spreader is snapped open and the dispensing device turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb. The peanut butter or jelly, depending on which dispensing device is used first, is then squished onto the bread. The spreader then acts as a knife and spreads the peanut butter and jelly all over the bread. The spreader snaps back onto the dispensing port of the dispensing device to reseal the dispensing device. This is particularly beneficial for the wilderness because being able to reseal the dispensing devices will keep the smell of food intact and not attract bears and other animals.
- The dispensing device is used to store cologne or perfume for travel or everyday use when on the go. A 0.5 fluid ounce dispensing device contains men's cologne. There is a sponge applicator inside the enclosure at the dispensing port. The desired amount of cologne can be applied to the sponge applicator by the amount of pressure exerted by the thumb on the inset groove. The dispensing device is turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb and then the cologne gets absorbed by the sponge applicator. The dispensing port lid is then opened and the cologne applied onto the desired area by the sponge applicator. The sponge applicator can be provided separately opposed to being situated inside the enclosure.
- The dispensing device is used to apply paint to a desired area. This is convenient for the painter who wants to do a touch up or wants to paint without the bulkiness of paint cans. The 6.0 fluid ounce dispensing device contains the desired paint. There is a sponge applicator inside the enclosure at the dispensing port. The desired amount of paint can be applied to the sponge applicator by the amount of pressure exerted by the thumb on the inset groove. The dispensing device is turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb and then the paint gets absorbed by the sponge applicator. The dispensing port lid is then opened and the paint can be applied onto the desired area by the sponge applicator. The sponge applicator can be provided separately opposed to being situated inside the enclosure.
- The dispensing device is used to apply makeup/foundation to the face. This is convenient for the woman who wishes to try different foundations, for instance one day she could be tanner than the next without the sun. The dispensing device is a 2.0 fluid ounces dispensing device with desired foundation shade. There is a sponge applicator inside the enclosure at the dispensing port. The desired amount of foundation can be applied to the sponge applicator by the amount of pressure exerted by the thumb on the inset groove. The dispensing device is turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb and then the foundation gets absorbed by the sponge applicator. The dispensing port lid is then opened and the foundation applied onto the desired area by the sponge applicator. The sponge applicator can be provided separately opposed to being situated inside the enclosure.
- The dispensing device is used to wash a pet. The dispensing device is 2.0 fluid ounces and contains animal safe shampoo for dogs. The dispensing device has a brush hinged to the top of the squeezable enclosure of the dispensing device. The brush acts as both a brush and a snap cap to cover the dispensing port of the dispensing device. The bristles of the brush point upwards from the dispensing port so that the non-bristle side is used to reseal the dispensing device and act as a dispensing port lid. The brush is snapped open and the dispensing device turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb. The shampoo is then squished onto the dog. The brush can then be used to brush the shampoo through the wet hair of the dog. Water can then be poured on the dog to wash away the shampoo. The brush snaps back onto the dispensing port of the dispensing device to reseal the dispensing device. This is particularly beneficial as the dog can be bathed anywhere where there is water. The brush can also be provided separately and a dispensing port lid such as a snap cap used instead of the brush.
- The dispensing device is used to spray mosquito repellant. This is convenient for camping trips and travel even in your own back yard during those beautiful summer nights. The dispensing device is a 3.0 fluid ounces dispensing device with mosquito repellant. There is a spray mist nozzle situated inside the enclosure at the dispensing port. The dispensing port lid is opened and the dispensing device turned upside down and stabilized by the index and middle finger. Pressure is then applied to the inset groove of the dispensing device with the thumb and the mosquito repellant gets sprayed out onto the desired area. The dispensing port lid can then be closed to reseal the dispensing device for later use.
- Unless otherwise indicated, all numbers expressing quantities of ingredients, properties such as molecular weight, reaction conditions, and so forth used in the specification and claims are to be understood as being modified in all instances by the term “about.” Accordingly, unless indicated to the contrary, the numerical parameters set forth in the specification and attached claims are approximations that may vary depending upon the desired properties sought to be obtained by the present invention. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should at least be construed in light of the number of reported significant digits and by applying ordinary rounding techniques. Notwithstanding that the numerical ranges and parameters setting forth the broad scope of the invention are approximations, the numerical values set forth in the specific examples are reported as precisely as possible. Any numerical value, however, inherently contains certain errors necessarily resulting from the standard deviation found in their respective testing measurements.
- The terms “a,” “an,” “the” and similar referents used in the context of describing the invention (especially in the context of the following claims) are to be construed to cover both the singular and the plural, unless otherwise indicated herein or clearly contradicted by context. Recitation of ranges of values herein is merely intended to serve as a shorthand method of referring individually to each separate value falling within the range. Unless otherwise indicated herein, each individual value is incorporated into the specification as if it were individually recited herein. All methods described herein can be performed in any suitable order unless otherwise indicated herein or otherwise clearly contradicted by context. The use of any and all examples, or exemplary language (e.g., “such as”) provided herein is intended merely to better illuminate the invention and does not pose a limitation on the scope of the invention otherwise claimed. No language in the specification should be construed as indicating any non-claimed element essential to the practice of the invention.
- Groupings of alternative elements or embodiments of the invention disclosed herein are not to be construed as limitations. Each group member may be referred to and claimed individually or in any combination with other members of the group or other elements found herein. It is anticipated that one or more members of a group may be included in, or deleted from, a group for reasons of convenience and/or patentability. When any such inclusion or deletion occurs, the specification is deemed to contain the group as modified thus fulfilling the written description of all Markush groups used in the appended claims.
- Certain embodiments of this invention are described herein, including the best mode known to the inventors for carrying out the invention. Of course, variations on these described embodiments will become apparent to those of ordinary skill in the art upon reading the foregoing description. The inventor expects skilled artisans to employ such variations as appropriate, and the inventors intend for the invention to be practiced otherwise than specifically described herein. Accordingly, this invention includes all modifications and equivalents of the subject matter recited in the claims appended hereto as permitted by applicable law. Moreover, any combination of the above-described elements in all possible variations thereof is encompassed by the invention unless otherwise indicated herein or otherwise clearly contradicted by context.
- In closing, it is to be understood that the embodiments of the invention disclosed herein are illustrative of the principles of the present invention. Other modifications that may be employed are within the scope of the invention. Thus, by way of example, but not of limitation, alternative configurations of the present invention may be utilized in accordance with the teachings herein. Accordingly, the present invention is not limited to that precisely as shown and described.
Claims (25)
1. A dispensing device comprising:
an enclosure including an internal volume;
a circumferential edge portion;
a dispensing port; and
a squeezable portion.
2. A dispensing device according to claim 1 , wherein the internal volume is a void.
3. A dispensing device according to claim 1 , wherein the internal volume is an encasement.
4. A dispensing device according to claim 3 , wherein the encasement contains at least one substance.
5. A dispensing device according to claim 4 , wherein the at least one substance is a condiment, mayonnaise, sweetened condensed milk, lotion, soap, bath salts, powder, gel, body oil, cream, or medicament.
6. A dispensing device according to claim 5 , wherein the medicament is baby Acetaminophen.
7. A dispensing device according to claim 1 , wherein the circumferential edge portion is a ring, securing mechanism, border, or rim.
8. A dispensing device according to claim 7 , wherein the circumferential edge portion separates the dispensing device into two portions, a top portion and a bottom portion.
9. A dispensing device according to claim 8 , wherein the top portion comprises a dispensing port.
10. A dispensing device according to claim 8 , wherein the bottom portion comprises an inset groove.
11. A dispensing device according to claim 1 , wherein the dispensing port is covered by a dispensing port lid.
12. A dispensing device according to claim 11 , wherein the dispensing port lid is a cap, lid, snap cap, peel cap, pressure actuated slit, adhesive tape, or adhesive foil.
13. A dispensing device according to claim 12 , wherein the dispensing port lid is a cap.
14. A dispensing device according to claim 12 , wherein the dispensing port lid is an adhesive strip.
15. A dispensing device according to claim 12 , wherein the dispensing port lid is an adhesive alloy.
16. A dispensing device according to claim 4 , wherein the at least one substance is dispensed from the dispensing device by applying pressure to the squeezable portion.
17. A dispensing device according to claim 1 , wherein the dispensing device is formed from a compressible material.
18. A dispensing device according to claim 17 , wherein the compressible material comprises at least one polymer.
19. A dispensing device according to claim 17 , wherein the compressible material is aseptic.
20. A dispensing device according to claim 17 , wherein the compressible material is free of Bisphenol A (BPA).
21. A dispensing device according to claim 1 , wherein the dispensing device is substantially spherical.
22. A dispensing device according to claim 21 , wherein the dispensing device comprises a substantially circular cross-section.
23. A dispensing device according to claim 1 , wherein the internal volume is about 3.0 fluid ounces.
24. A dispensing device according to claim 1 , wherein the internal volume is about 6.0 fluid ounces.
25. A dispensing device according to claim 1 , wherein the internal volume is about 0.5 to about 1.0 fluid ounces, about 1.0 to about 2.0 fluid ounces, about 2.0 to about 3.0 fluid ounces, about 3.0 to about 4.0 fluid ounces, about 4.0 to about 5.0 fluid ounces, and about 5.0 to about 6.0 fluid ounces.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/886,090 US20130292414A1 (en) | 2012-05-02 | 2013-05-02 | Dispensing Device |
| US14/555,046 US20150083750A1 (en) | 2012-05-02 | 2014-11-26 | Dispensing Device |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261641477P | 2012-05-02 | 2012-05-02 | |
| US201261656456P | 2012-06-06 | 2012-06-06 | |
| US13/886,090 US20130292414A1 (en) | 2012-05-02 | 2013-05-02 | Dispensing Device |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/555,046 Continuation US20150083750A1 (en) | 2012-05-02 | 2014-11-26 | Dispensing Device |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20130292414A1 true US20130292414A1 (en) | 2013-11-07 |
Family
ID=49511767
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/886,090 Abandoned US20130292414A1 (en) | 2012-05-02 | 2013-05-02 | Dispensing Device |
| US14/555,046 Abandoned US20150083750A1 (en) | 2012-05-02 | 2014-11-26 | Dispensing Device |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/555,046 Abandoned US20150083750A1 (en) | 2012-05-02 | 2014-11-26 | Dispensing Device |
Country Status (1)
| Country | Link |
|---|---|
| US (2) | US20130292414A1 (en) |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140299625A1 (en) * | 2013-04-02 | 2014-10-09 | Natura Cosméticos S.A. | Single dose fluid dispenser package |
| US20170151362A1 (en) * | 2013-10-15 | 2017-06-01 | Vapor Communications, Inc. | Table top scent dispensing device and method |
| US9708117B1 (en) | 2016-02-11 | 2017-07-18 | The Procter & Gamble Company | Packaged product |
| EP3205602A1 (en) * | 2016-02-11 | 2017-08-16 | The Procter & Gamble Company | Packaged product |
| WO2017161419A1 (en) * | 2016-03-23 | 2017-09-28 | Mashblox Pty Ltd | Feeding aid and method of use |
| US20170313494A1 (en) * | 2014-11-07 | 2017-11-02 | Fimtech As | Dosage device |
| WO2019040907A1 (en) * | 2017-08-24 | 2019-02-28 | Sutherland Spencer D | Dispensing device |
| US20200054108A1 (en) * | 2017-04-11 | 2020-02-20 | Ojip, Llc | Device for applying and removing nail polish |
| US20200189313A1 (en) * | 2018-12-12 | 2020-06-18 | Wilfred Cormier | All in the bag art |
| US10704182B2 (en) | 2016-02-11 | 2020-07-07 | The Procter & Gamble Company | Method of washing |
| US10952568B2 (en) * | 2019-02-18 | 2021-03-23 | Sanibeads, Llc | Wearable sanitizer dispenser |
| US20210177214A1 (en) * | 2019-02-18 | 2021-06-17 | Sanibeads, Llc. | Wearable sanitizer dispenser |
| US11084053B2 (en) * | 2017-11-14 | 2021-08-10 | Gemmytec (Shanghai) Co., Ltd. | Portable pump by which liquid may be horizontally pressed out |
| US20220031126A1 (en) * | 2020-07-31 | 2022-02-03 | Marcel H. Lussier | Toothpaste and solution capsule storage and delivery systems |
| US20220061367A1 (en) * | 2020-09-02 | 2022-03-03 | Charles Johnson | Method of Making Butter-Based Hot Sauce |
| US11344163B2 (en) * | 2019-02-18 | 2022-05-31 | Sanibeads, Llc | Wearable sanitizer dispenser |
| US11419984B2 (en) * | 2014-02-12 | 2022-08-23 | Sanofi-Aventis Deutschland Gmbh | Compressible reservoir for liquid medicament |
| US11484047B2 (en) * | 2020-06-05 | 2022-11-01 | Daniel Elisea | Beverageware seasoning packet |
| USD970009S1 (en) | 2022-07-06 | 2022-11-15 | Elliott Bohler | Ophthalmic fluid dispenser |
| US11591200B2 (en) * | 2020-09-14 | 2023-02-28 | Christopher Robles | Package opener, dispenser, and related methods |
| USD986730S1 (en) | 2020-09-24 | 2023-05-23 | Mccormick & Company, Inc. | Package |
| USD995288S1 (en) | 2020-09-24 | 2023-08-15 | Mccormick & Company, Inc. | Package |
| USD995287S1 (en) | 2020-09-24 | 2023-08-15 | Mccormick & Company, Inc. | Package |
| US11772877B2 (en) * | 2018-10-17 | 2023-10-03 | Coradin Sas | Device for packaging at least one fluid |
| USD1003708S1 (en) | 2020-09-24 | 2023-11-07 | Mccormick & Company, Inc. | Package |
| WO2024037962A1 (en) * | 2022-08-16 | 2024-02-22 | advima Beratungs- und Dienstleistungs GmbH | Set consisting of a non-woven hygiene wipe and a liquid container |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10159823B2 (en) | 2015-06-26 | 2018-12-25 | C. R. Bard, Inc. | Topical substance application device including applicator |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US770215A (en) * | 1902-07-24 | 1904-09-13 | Louis Blatz | Containing and dispensing vessel. |
| US3862684A (en) * | 1971-04-26 | 1975-01-28 | Karlsruhe Augsburg Iweka | Aseptic packing container and method of making and filling it |
| US4072249A (en) * | 1975-03-03 | 1978-02-07 | Landstingens Inkopscentral | Container suitable for smaller quantities of fluid or semi-fluid substances |
| US5839609A (en) * | 1997-08-27 | 1998-11-24 | Colgate-Palmolive Company | Thermoformed pack with ridge valve |
| US6322271B1 (en) * | 1998-11-04 | 2001-11-27 | The Procter & Gamble Company | Applicator for applying and distributing substances to target surfaces |
| US20060011666A1 (en) * | 2004-07-15 | 2006-01-19 | Patrice Wurtz | Pipette/applicator |
| US20100125097A1 (en) * | 2008-11-19 | 2010-05-20 | Mark David Soll | Compositions comprising an aryl pyrazole and/or a formamidine, methods and uses thereof |
-
2013
- 2013-05-02 US US13/886,090 patent/US20130292414A1/en not_active Abandoned
-
2014
- 2014-11-26 US US14/555,046 patent/US20150083750A1/en not_active Abandoned
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US770215A (en) * | 1902-07-24 | 1904-09-13 | Louis Blatz | Containing and dispensing vessel. |
| US3862684A (en) * | 1971-04-26 | 1975-01-28 | Karlsruhe Augsburg Iweka | Aseptic packing container and method of making and filling it |
| US4072249A (en) * | 1975-03-03 | 1978-02-07 | Landstingens Inkopscentral | Container suitable for smaller quantities of fluid or semi-fluid substances |
| US5839609A (en) * | 1997-08-27 | 1998-11-24 | Colgate-Palmolive Company | Thermoformed pack with ridge valve |
| US6322271B1 (en) * | 1998-11-04 | 2001-11-27 | The Procter & Gamble Company | Applicator for applying and distributing substances to target surfaces |
| US20060011666A1 (en) * | 2004-07-15 | 2006-01-19 | Patrice Wurtz | Pipette/applicator |
| US20100125097A1 (en) * | 2008-11-19 | 2010-05-20 | Mark David Soll | Compositions comprising an aryl pyrazole and/or a formamidine, methods and uses thereof |
Cited By (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140299625A1 (en) * | 2013-04-02 | 2014-10-09 | Natura Cosméticos S.A. | Single dose fluid dispenser package |
| US20170151362A1 (en) * | 2013-10-15 | 2017-06-01 | Vapor Communications, Inc. | Table top scent dispensing device and method |
| US11419984B2 (en) * | 2014-02-12 | 2022-08-23 | Sanofi-Aventis Deutschland Gmbh | Compressible reservoir for liquid medicament |
| US20170313494A1 (en) * | 2014-11-07 | 2017-11-02 | Fimtech As | Dosage device |
| US10773874B2 (en) * | 2014-11-07 | 2020-09-15 | Fimtech As | Dosage device |
| US10704182B2 (en) | 2016-02-11 | 2020-07-07 | The Procter & Gamble Company | Method of washing |
| EP3205599A1 (en) * | 2016-02-11 | 2017-08-16 | The Procter and Gamble Company | Packaged product |
| US9708117B1 (en) | 2016-02-11 | 2017-07-18 | The Procter & Gamble Company | Packaged product |
| WO2017139422A1 (en) * | 2016-02-11 | 2017-08-17 | The Procter & Gamble Company | Packaged product |
| EP3205602A1 (en) * | 2016-02-11 | 2017-08-16 | The Procter & Gamble Company | Packaged product |
| WO2017139424A1 (en) * | 2016-02-11 | 2017-08-17 | The Procter & Gamble Company | Packaged product |
| US20190090669A1 (en) * | 2016-03-23 | 2019-03-28 | Mashblox Pty Ltd | Feeding aid and method of use |
| JP2019512371A (en) * | 2016-03-23 | 2019-05-16 | マッシュブロックス ピーティーワイ リミテッドMashblox Pty Ltd | Feeding aids and methods of use |
| WO2017161419A1 (en) * | 2016-03-23 | 2017-09-28 | Mashblox Pty Ltd | Feeding aid and method of use |
| US20200054108A1 (en) * | 2017-04-11 | 2020-02-20 | Ojip, Llc | Device for applying and removing nail polish |
| US11690434B2 (en) * | 2017-04-11 | 2023-07-04 | Ojip, Llc | Device for applying and removing nail polish |
| CN111194292A (en) * | 2017-08-24 | 2020-05-22 | 斯潘塞·D·萨瑟兰 | Dispensing device |
| WO2019040907A1 (en) * | 2017-08-24 | 2019-02-28 | Sutherland Spencer D | Dispensing device |
| JP2020532477A (en) * | 2017-08-24 | 2020-11-12 | サザランド,スペンサー,ディー. | Distribution device |
| EP3672886A4 (en) * | 2017-08-24 | 2021-05-19 | Spencer D. Sutherland | Dispensing device |
| US11084053B2 (en) * | 2017-11-14 | 2021-08-10 | Gemmytec (Shanghai) Co., Ltd. | Portable pump by which liquid may be horizontally pressed out |
| US11772877B2 (en) * | 2018-10-17 | 2023-10-03 | Coradin Sas | Device for packaging at least one fluid |
| US20200189313A1 (en) * | 2018-12-12 | 2020-06-18 | Wilfred Cormier | All in the bag art |
| US10952568B2 (en) * | 2019-02-18 | 2021-03-23 | Sanibeads, Llc | Wearable sanitizer dispenser |
| US20210177214A1 (en) * | 2019-02-18 | 2021-06-17 | Sanibeads, Llc. | Wearable sanitizer dispenser |
| US11344163B2 (en) * | 2019-02-18 | 2022-05-31 | Sanibeads, Llc | Wearable sanitizer dispenser |
| US11666183B2 (en) * | 2019-02-18 | 2023-06-06 | Sanibeads, Llc | Wearable sanitizer dispenser |
| US11484047B2 (en) * | 2020-06-05 | 2022-11-01 | Daniel Elisea | Beverageware seasoning packet |
| US20220031126A1 (en) * | 2020-07-31 | 2022-02-03 | Marcel H. Lussier | Toothpaste and solution capsule storage and delivery systems |
| US20220061367A1 (en) * | 2020-09-02 | 2022-03-03 | Charles Johnson | Method of Making Butter-Based Hot Sauce |
| US11591200B2 (en) * | 2020-09-14 | 2023-02-28 | Christopher Robles | Package opener, dispenser, and related methods |
| USD995287S1 (en) | 2020-09-24 | 2023-08-15 | Mccormick & Company, Inc. | Package |
| USD995288S1 (en) | 2020-09-24 | 2023-08-15 | Mccormick & Company, Inc. | Package |
| USD986730S1 (en) | 2020-09-24 | 2023-05-23 | Mccormick & Company, Inc. | Package |
| USD1003708S1 (en) | 2020-09-24 | 2023-11-07 | Mccormick & Company, Inc. | Package |
| USD970009S1 (en) | 2022-07-06 | 2022-11-15 | Elliott Bohler | Ophthalmic fluid dispenser |
| WO2024037962A1 (en) * | 2022-08-16 | 2024-02-22 | advima Beratungs- und Dienstleistungs GmbH | Set consisting of a non-woven hygiene wipe and a liquid container |
Also Published As
| Publication number | Publication date |
|---|---|
| US20150083750A1 (en) | 2015-03-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20150083750A1 (en) | Dispensing Device | |
| CA2552716C (en) | Package for a perishable product | |
| US8528736B2 (en) | Frangible container with hinge cover | |
| RU2459753C2 (en) | Dispenser and method of its making | |
| AU734380B2 (en) | A two-part storage container | |
| US8839982B1 (en) | Dispensing capsule with dual independent dispensing chambers | |
| AU2010250997B2 (en) | Non-resealable thermoformed packaging for liquid or pasty substances | |
| EP1201562A2 (en) | Compartmentalized storage system | |
| DK2539240T3 (en) | LIGHT WEIGHT SINGLE DOSAGE CONTAINER | |
| AU2006243387A1 (en) | Squeezable container for dairy products | |
| CA2790320C (en) | Disposable dual chamber container | |
| US20210139216A1 (en) | Dispensing device | |
| US11801974B2 (en) | Dispenser tips and methods of use | |
| JP3172582U (en) | Multi-room packaging bag that can adjust the discharge amount of cosmetic contents | |
| US20100318041A1 (en) | Method and apparatus for single-use cream or liquid applicator | |
| US20220234792A1 (en) | Dual opening dispensing package | |
| AU2013100302A4 (en) | Sickness Bags, Sickness Packs and Wellness Kits | |
| WO2004099028A1 (en) | Flexible multichamber mixing container | |
| US12128202B1 (en) | Disposable topical application system | |
| SE1530183A1 (en) | Tube for a product in paste or semi liquid form and a cap used together with a tube | |
| JP2024115989A (en) | Containers for cosmetics, etc. | |
| WO2005039992A1 (en) | Container of flexible and flaccid material | |
| AU2005202605B2 (en) | A disposable sealed canister adapted for holding a solution for injection | |
| WO2019173592A1 (en) | Dual opening dispensing package | |
| CA2534164A1 (en) | Method and apparatus for dispensing a measured amount of a product |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: SQUISH PAK LLC, CALIFORNIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:SUTHERLAND, SPENCER D.;REEL/FRAME:031672/0884 Effective date: 20131121 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |