US20080251427A1 - Flotation Separation Device and Method - Google Patents
Flotation Separation Device and Method Download PDFInfo
- Publication number
- US20080251427A1 US20080251427A1 US12/101,376 US10137608A US2008251427A1 US 20080251427 A1 US20080251427 A1 US 20080251427A1 US 10137608 A US10137608 A US 10137608A US 2008251427 A1 US2008251427 A1 US 2008251427A1
- Authority
- US
- United States
- Prior art keywords
- slurry
- flotation separation
- sparger unit
- sparger
- gas
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B03—SEPARATION OF SOLID MATERIALS USING LIQUIDS OR USING PNEUMATIC TABLES OR JIGS; MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS
- B03D—FLOTATION; DIFFERENTIAL SEDIMENTATION
- B03D1/00—Flotation
- B03D1/14—Flotation machines
- B03D1/16—Flotation machines with impellers; Subaeration machines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F23/00—Mixing according to the phases to be mixed, e.g. dispersing or emulsifying
- B01F23/20—Mixing gases with liquids
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F23/00—Mixing according to the phases to be mixed, e.g. dispersing or emulsifying
- B01F23/20—Mixing gases with liquids
- B01F23/23—Mixing gases with liquids by introducing gases into liquid media, e.g. for producing aerated liquids
- B01F23/232—Mixing gases with liquids by introducing gases into liquid media, e.g. for producing aerated liquids using flow-mixing means for introducing the gases, e.g. baffles
- B01F23/2323—Mixing gases with liquids by introducing gases into liquid media, e.g. for producing aerated liquids using flow-mixing means for introducing the gases, e.g. baffles by circulating the flow in guiding constructions or conduits
- B01F23/23231—Mixing gases with liquids by introducing gases into liquid media, e.g. for producing aerated liquids using flow-mixing means for introducing the gases, e.g. baffles by circulating the flow in guiding constructions or conduits being at least partially immersed in the liquid, e.g. in a closed circuit
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F25/00—Flow mixers; Mixers for falling materials, e.g. solid particles
- B01F25/30—Injector mixers
- B01F25/31—Injector mixers in conduits or tubes through which the main component flows
- B01F25/313—Injector mixers in conduits or tubes through which the main component flows wherein additional components are introduced in the centre of the conduit
- B01F25/3133—Injector mixers in conduits or tubes through which the main component flows wherein additional components are introduced in the centre of the conduit characterised by the specific design of the injector
- B01F25/31331—Perforated, multi-opening, with a plurality of holes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F25/00—Flow mixers; Mixers for falling materials, e.g. solid particles
- B01F25/30—Injector mixers
- B01F25/31—Injector mixers in conduits or tubes through which the main component flows
- B01F25/313—Injector mixers in conduits or tubes through which the main component flows wherein additional components are introduced in the centre of the conduit
- B01F25/3133—Injector mixers in conduits or tubes through which the main component flows wherein additional components are introduced in the centre of the conduit characterised by the specific design of the injector
- B01F25/31333—Rotatable injectors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B03—SEPARATION OF SOLID MATERIALS USING LIQUIDS OR USING PNEUMATIC TABLES OR JIGS; MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS
- B03D—FLOTATION; DIFFERENTIAL SEDIMENTATION
- B03D1/00—Flotation
- B03D1/02—Froth-flotation processes
- B03D1/028—Control and monitoring of flotation processes; computer models therefor
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B03—SEPARATION OF SOLID MATERIALS USING LIQUIDS OR USING PNEUMATIC TABLES OR JIGS; MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS
- B03D—FLOTATION; DIFFERENTIAL SEDIMENTATION
- B03D1/00—Flotation
- B03D1/08—Subsequent treatment of concentrated product
- B03D1/082—Subsequent treatment of concentrated product of the froth product, e.g. washing
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B03—SEPARATION OF SOLID MATERIALS USING LIQUIDS OR USING PNEUMATIC TABLES OR JIGS; MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS
- B03D—FLOTATION; DIFFERENTIAL SEDIMENTATION
- B03D1/00—Flotation
- B03D1/14—Flotation machines
- B03D1/1487—Means for cleaning or maintenance
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B03—SEPARATION OF SOLID MATERIALS USING LIQUIDS OR USING PNEUMATIC TABLES OR JIGS; MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS
- B03D—FLOTATION; DIFFERENTIAL SEDIMENTATION
- B03D1/00—Flotation
- B03D1/14—Flotation machines
- B03D1/16—Flotation machines with impellers; Subaeration machines
- B03D1/22—Flotation machines with impellers; Subaeration machines with external blowers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B03—SEPARATION OF SOLID MATERIALS USING LIQUIDS OR USING PNEUMATIC TABLES OR JIGS; MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS
- B03D—FLOTATION; DIFFERENTIAL SEDIMENTATION
- B03D1/00—Flotation
- B03D1/14—Flotation machines
- B03D1/24—Pneumatic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B03—SEPARATION OF SOLID MATERIALS USING LIQUIDS OR USING PNEUMATIC TABLES OR JIGS; MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS
- B03D—FLOTATION; DIFFERENTIAL SEDIMENTATION
- B03D1/00—Flotation
- B03D1/14—Flotation machines
- B03D1/24—Pneumatic
- B03D1/247—Mixing gas and slurry in a device separate from the flotation tank, i.e. reactor-separator type
Definitions
- Flotation separators are used extensively throughout the minerals industry to partition and recover the constituent species within slurries.
- a slurry is a mixture of liquids (usually water) with various species having varying degrees of hydrophobicity.
- the species could be insoluble particulate matter such as coal, metals, clay, sand, etc. or soluble elements or compounds in solution.
- Flotation separators work on the principle that the various species within the slurry interact differently with bubbles formed in the slurry. Gas bubbles introduced into the slurry attach, either through physical or chemical means, to one or more of the hydrophobic species of the slurry.
- the bubble-hydrophobic species agglomerates are sufficiently buoyant to lift away from the remaining constituents and are removed for further processing to concentrate and recover the adhered species.
- Various methods used to achieve this process typically require significant energy to inject gas into the slurry and form a bubble dispersion.
- a flotation separation system for partitioning a slurry that includes a hydrophobic species which can adhere to gas bubbles formed in the slurry.
- the flotation separation system comprises a flotation separation cell that includes a sparger unit and a separation tank.
- the sparger unit has a slurry inlet for receiving slurry and a gas inlet to receive gas with at least enough pressure to allow bubbles to form in the slurry within the sparger unit.
- the sparger unit includes a sparging mechanism constructed to disperse gas bubbles within the slurry.
- the sparging mechanism sparges the gas bubbles to form a bubble dispersion so as to cause adhesion of the hydrophobic species to the gas bubbles substantially within the sparger unit while causing a pressure drop of about 10 psig or less across the sparging mechanism.
- the sparger unit includes a slurry outlet to discharge the slurry and the bubble dispersion into the separation tank.
- the separation tank has sufficient capacity to allow the bubble dispersion to form a froth at the top of the separation tank.
- Various embodiments of the flotation separation system can include a center well that surrounds the sparging unit.
- the sparging mechanism of the sparger unit includes a high-shear element to help shear the bubbles formed in the slurry into a bubble dispersion.
- the high-shear element can include rotating high-shear elements or a combination of rotating and static high-shear elements. Rotating high shear elements can comprise a series of rotating elements along the length of the sparging unit.
- the high-shear element can alternatively comprise a series of grooved discs pressed together to form channels from the gas inlets to the slurry with gas passing through the channels to reach the slurry. Other possible embodiments and variations are discussed in more detail herein.
- FIG. 1 is a perspective view of a flotation separation cell with one sparger unit
- FIG. 2 is a perspective view of a flotation separation cell with three sparger units
- FIG. 3 is an embodiment of a sparger unit
- FIG. 4 is a view of an embodiment of a sparger unit showing the rotating high-shear element of the sparging mechanism
- FIG. 5 is a view of an embodiment of a sparger unit showing the rotating and static high-shear elements of the sparging mechanism
- FIG. 6A is a view of an embodiment of a sparger unit in which the sparging mechanism has gas inlets along its length;
- FIG. 6B is a view of the sparging mechanism of the sparger unit of FIG. 6A ;
- FIG. 6C is a close up of a check valve of a gas inlet of FIG. 6A ;
- FIG. 6D is a gas inlet of FIG. 6A ;
- FIG. 6E is a different view of the gas inlet of FIG. 6D ;
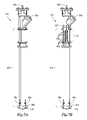
- FIG. 7A is an embodiment of a sparger unit that does not use an electric motor
- FIG. 7B is a view of the sparger unit of FIG. 7A showing the sparging mechanism with the high shear element comprising a series of grooved discs;
- FIG. 7C is a view of the high shear element of FIG. 7B ;
- FIG. 7D is a view of the high shear element of FIG. 7B without the grooved discs
- FIG. 7E is a view of a grooved disc of FIG. 7B ;
- FIG. 8 is a view of an alternative embodiment of the grooved discs of FIG. 7B ;
- FIG. 9A is an embodiment of a sparger unit with a cleaning system for the sparging unit
- FIG. 9B is a close up of the sparger unit of FIG. 9A without the grooved discs;
- FIG. 9C is an exploded view of the sparger unit of FIG. 9A ;
- FIG. 10 is a sparger unit in which the sparging mechanism is a high frequency linear displacement device
- FIG. 11 is a view of an embodiment of a sparger unit showing a sparger mechanism having multiple banks of rotating high shear elements
- FIG. 12 is a representation of some of the control systems for a flotation separation cell
- FIG. 13 shows a flotation separation system that comprises a series of flotation separation cells in a modular vertical arrangement
- FIG. 14 shows a flotation separation system that comprises a series of flotation separation cells in a staggered horizontal arrangement
- FIG. 15 is a graph plotting the recovery of a target species versus process rate and retention time for various circuit configurations
- FIG. 16A shows a flotation separation system in which a flotation separation cell discharges slurry from the underflow removal port to the inlet of a conventional flotation cell;
- FIG. 16B shows a flotation separation system in which a flotation separation cell discharges slurry from the underflow removal port to the inlet of a column flotation cell;
- FIG. 17A shows an embodiment of a flotation separation cell that incorporates a center well
- FIG. 17B shows the center well shown in FIG. 17A showing the sparger unit within the center well
- FIG. 18A shows a different embodiment of a flotation separation cell in which the center well liquid level is maintained by adjusting the size of the orifices at the end of the center well based on pressure sensor readings;
- FIG. 18B shows a different embodiment of a flotation separation cell in which the liquid level in the center well is maintained by adjusting the inflow of slurry to the flotation separation cell;
- FIG. 18C shows a different embodiment of a flotation separation system comprising a number of flotation separation cells in series in which the liquid level in the center well for each flotation separation cell is maintained by adjusting the inflow of slurry to each flotation separation cell;
- FIG. 19 is a perspective view of a flotation separation cell with four sparger units that feed slurry from the bottom of the separation tank;
- FIG. 20 is a perspective view of a flotation separation cell with four sparger units that feed slurry through the sidewalls of the separation tank;
- FIG. 21 is a perspective view of a flotation separation cell in which the underflow removal port leaves through the side of the separation tank.
- Flotation separation is commonly used in the minerals industry to separate mineral species in suspension in liquid slurries. Such mineral species are often suspended with a mixture of unwanted constituent species. Flotation separators currently in common use require an extensive application of large amounts of energy for pressurizing gas, pressuring the slurry, increasing the flow velocity of the slurry, and/or maintaining the slurry in suspension.
- a flotation separation system comprises at least one flotation separation cell 10 in a hydraulic system for the partitioning and recovery of the constituents of a slurry.
- the flotation separation cell 10 comprises at least one sparger unit 12 in which gas is introduced into the slurry.
- the sparger unit 12 includes a sparging mechanism 42 for sparging gas into a bubble dispersion within the slurry.
- the sparging mechanism 42 is configured such that slurry flow through it is substantially unrestricted.
- the effective open area in the sparging mechanism 42 is substantially the same as the effective open area in the sparger unit 12 upstream and downstream of the sparging mechanism 42 .
- the pressure drop across the sparging mechanism 42 is about 10 psig or less.
- the sparger unit 12 feeds the slurry and bubble dispersion mixture to a separation tank 14 .
- the separation tank 14 comprises an overflow launder 16 , an underflow removal port 18 , and a froth washing system 20 .
- the overflow launder connects to an overflow drain 22 .
- the flotation separation cell 10 may be supported by legs 24 or by any other means required by the particular application.
- the flotation separation cell 10 may even be placed directly on the floor if warranted by the design of the facility to which the flotation separation cell 10 is installed.
- the separation tank 14 requires no additional equipment within the tank to assist in froth formation (as discussed in more detail below) or to maintain the slurry in suspension. This represents a further energy savings in the overall operation as compared to conventional flotation separation systems, column flotation separation systems, and packed column flotation separation systems.
- the operation of the flotation separation system is presented in more detail below.
- the flotation slurries typically include hydrophobic and hydrophilic species. Flotation separation takes advantage of the differing hydrophobicity of these species.
- the hydrophobic species within the slurry tend to selectively adhere to the bubbles while hydrophilic species tend to remain in suspension. Sparging, or breaking up, the bubbles into a bubble dispersion of many smaller bubbles increases the available bubble surface area for hydrophobic species adhesion.
- the bubbles, with the adhered hydrophobic species tend to rise above the slurry and form a froth in the separation tank 14 that is easily separated from the remainder of the slurry for further processing to recover the adhered hydrophobic species.
- Flotation separation systems are typically part of larger hydraulic systems that process slurry over a number of steps.
- the liquid portion of the slurry is typically water.
- the chemistry of the slurry is often adjusted with additives to assist in recovering a target component depending on the constituent species of the slurry.
- Surface tension modifying reagents also known as frothers, are often added to slurries to assist in bubble formation.
- frothers including alcohols, glycols, Methylisobutyl Carbinol (MIBC), and various blends.
- collectors include fuel oil, fatty acids, xanthates, various amines, etc.
- oxidized coal tends to be less hydrophobic and is more difficult to recover from a slurry than unoxidized coal.
- Chemical additives called extenders, are used to increase their hydrophobicity. Examples of extenders are diesel fuels and other fuel oils.
- depressants are used to reduce the hydrophobicity of a species. For example, in the recovery of iron ore, various types of starches are used to depress the bubble adhesion response of iron ore so that only silica can be floated in the froth from the slurry. If the depressants are not added, a portion of the iron ore will also adhere to bubbles and float within the froth.
- R is the recovery of a particular species
- k is the reaction rate of adhesion of a species to a bubble
- T is the retention time of the slurry in the flotation separation system.
- An increase in either parameter provides a corresponding increase in recovery, R.
- the reaction rate, k, for a process is indicative of the speed at which the flotation separation will proceed and can be a function of several parameters including, but not limited to, gas introduction rate, bubble size, species size, and chemistry.
- the reaction rate, k is increased when these parameters are adjusted to maximize the probability that a hydrophobic species will collide with and adhere to a bubble and to reduce the probability that a hydrophobic species will detach from a bubble.
- the probability of attachment is controlled by the surface chemistry of both the species and the bubbles in the process stream and is increased when the probability of a collision between a hydrophobic species and a bubble increases.
- the probability of collision is directly proportional to the concentration of hydrophobic species within the sparging region.
- the probability of detachment is controlled by the hydrodynamics of the flotation separation cell.
- aeration of the slurry prior to its introduction to a separation tank is the preferred method of sparging as this ensures that the maximum amount of floatable species is concentrated within the sparging unit to obtain a high recovery of the hydrophobic species.
- the embodiments described herein aim to increase the reaction rate, k, which means that a lower retention time, T, and thereby a smaller separation tank, can be used to obtain a suitable recovery, R.
- reaction rate, k, of Equation [1] is increased by forcing the bubble-particle contact with high particle and air bubble concentrations and imparting significant energy within the bubble/particle contacting zone.
- Recovery, R can also be represented in turbulent systems described herein as a function of the bubble concentration, C b , particle concentration, C p , and specific energy input, E, as follows:
- the embodiments disclosed herein efficiently pre-aerate slurry in the sparger units 12 of the flotation separation cell 10 prior to injection of the slurry and gas mixture into the separation tank 14 .
- Slurry introduced into the sparger unit 12 passes through a sparging mechanism 42 , described in more detail below.
- the sparging mechanism 42 sparges the gas in the slurry into a bubble dispersion creating a relatively large surface area for hydrophobic species attachment within the sparger unit 12 such that hydrophobic species adhesion to bubbles occurs substantially in the sparger unit 12 before the slurry and the bubble dispersion is discharged into the separation tank 14 .
- the sparger assembly 30 is operated at a very high air fraction (>40%), insuring that the bubble concentration (C b ) is maximized.
- the design of the sparging mechanism 42 in the sparger unit 12 is such that maximum energy is imparted to the slurry for the sole purpose of bubble-particle contacting. As a result, the contact time is reduced by several orders of magnitude over prior art column and conventional flotation separators. After contacting, the slurry is discharged to the separation tank 14 for phase separation (slurry and froth) and froth washing (if used). Since phase separation is a relatively quick process, the overall separation tank 14 size is significantly reduced.
- the sparging mechanism 42 is configured such that slurry flow through it is substantially unrestricted.
- the effective open area in the sparging mechanism 42 is substantially the same as the effective open area in the sparger unit 12 upstream and downstream of the sparging mechanism 42 . This ensures a low pressure drop across the sparging mechanism 42 that allows for a lower pressure and flow rate of slurry through the sparger unit 12 and represents a significant energy savings for the flotation separation system.
- the pressure drop across the sparging mechanism 42 is about 10 psig or less. Nevertheless, the embodiments depicted herein are able to operate with pressure drops of about 1 psig or less.
- the flotation separation cell 10 does not require the slurry to be introduced at a high velocity and/or a high pressure.
- the slurry may be pumped under pressure into the sparger unit 12 if the hydraulics of the flotation separation system require, but this need only be sufficient to provide enough hydraulic pressure for the slurry to flow through the flotation separation system.
- Slurry can be introduced into the flotation separation cell 10 at the slurry inlet of the sparger unit 12 at a hydraulic pressure of about 25 psig or less.
- the embodiments depicted herein are able to operate at slurry introduction hydraulic pressures of 2 psig or less.
- the relatively low hydraulic pressure gradient that the slurry must overcome represents an energy savings during the operation of the flotation separation cell 10 .
- the hydraulics of a flotation separation cell 10 can be adjusted in various embodiments by, for example, adjusting the height of the sparger units 12 in relation to the height of the slurry in the separation tank 14 or by adjusting the entry point of slurry to the flotation separation cell 10 .
- the sparging mechanisms 42 do not require gas to be introduced at a high pressure.
- the gas introduction pressure need only be high enough to form bubbles in the slurry and the sparging mechanisms 42 described herein will sparge the bubbles into effective bubble dispersions.
- the low pressure and flow requirements for both slurry and gas introduction represent significant energy savings when compared to conventional flotation separation systems, column flotation separation systems, and packed column flotation separation systems.
- the separation tank 14 is only required to provide time for the slurry and bubble phases to separate.
- a smaller separation tank 14 can be utilized without additional equipment in the separation tank when compared to conventional flotation separation systems, column flotation separation systems, and packed column flotation separation systems.
- the smaller and simpler flotation separation cell 10 allows for greater flexibility in designing flotation separation systems for particular applications. Energy is also not consumed to maintain the slurry in suspension in the separation tank 14 .
- the separation tank 14 is used solely for froth separation, and does not require any additional equipment to maintain the slurry in suspension, the embodiments described herein are able to maintain a relatively deep froth in the separation tank 14 with no additional turbulence imparted to the separation tank 14 . Therefore, unlike with conventional flotation separation systems, the addition of wash water from the froth washing system 20 (described in more detail below) to clean the froth does not affect the retention time of the froth in the separation tank 14 . It is therefore possible to have effective froth washing in the flotation separation systems described herein.
- the overall energy input is reduced. While a compressor may be used to introduce gas into the flotation separation system, because the sparging mechanism 42 operates at atmospheric pressure a compressor is not required to overcome the hydrostatic system head. Instead, a simple blower can be used, providing energy and maintenance savings. The energy reduction, of course, implies reduced operating costs. Finally, the smaller separation tank 14 requirements reduce equipment and installation costs. Structural steel requirements are significantly less due to the reduction in tank weight and live load. The space requirement is less than that required for equivalent conventional column flotation separation. Shipping and installation is also simplified since the units can be shipped fully assembled and installed without field welding.
- FIG. 2 shows how the flotation separation cell 10 a can be designed with multiple sparger units 12 a, in this case three, with an appropriately sized separation tank 14 a.
- a feed manifold distributor 26 a having distributor pipes 28 a may be used to evenly distribute slurry to each sparger unit 12 a.
- each sparger unit 12 b comprises a sparger assembly 30 b that allows for the passage of feed slurry to a separation tank ( 14 and 14 a in FIGS. 1 and 2 ).
- the size of the sparger assembly 30 b is dictated by the size of the flotation separation system in which the sparger unit 12 b is installed and is primarily intended to direct the slurry discharge to an appropriate location within the separation tank 14 .
- the slurry should be discharged low enough in the separation tank 14 so as to not interfere with froth formation at the top of the separation tank 14 .
- Slurry is introduced into the sparger unit 12 b through the slurry inlet 38 b and passes through a sparging mechanism 42 b.
- the sparging mechanism 42 b is configured such that slurry flow through it is substantially unrestricted.
- the effective open area in the sparging mechanism 42 b is substantially the same as the effective open area in the sparger unit 12 b upstream and downstream of the sparging mechanism 42 b.
- the pressure drop across the sparging mechanism 42 b is about 10 psig or less.
- the sparging mechanism 42 b comprises a rotating high-shear element 32 b attached to a rotating shaft 34 b that is powered by an electric motor 36 b.
- the slurry may be gravity fed if there is enough hydraulic pressure to ensure that the slurry will flow through the flotation separation system. If the hydraulics of the system requires the slurry to be pumped, the slurry need only be pumped with sufficient pressure to ensure passage of the slurry through the flotation separation system. Nevertheless, the sparger unit 12 b will function well over a broad range of slurry flow rates and pressures. Slurry can be introduced into the slurry inlet 38 b of the sparger unit 12 b at a hydraulic pressure of about 25 psig or less. The sparger unit 12 b can operate at a slurry hydraulic pressure of about 2 psig or less.
- Gas typically air
- gas inlets 40 b that are supplied from a gas injection system (discussed in more detail below).
- the passing slurry flow immediately shears the gas to form bubbles as the gas enters the sparger unit 12 b through the gas inlets 40 b.
- the gas need not be at a high pressure for effective bubble formation in the slurry. Even at high slurry feed rates, the gas flow and pressure needs only be high enough to allow bubble formation in the slurry.
- the bubbles are sheared into smaller bubbles as the slurry passes through the sparging mechanism 42 b and forms a fine bubble dispersion within the slurry.
- the formation of the bubble dispersion within the sparger unit 12 b exposes a larger volume of slurry to the surface of the bubbles. This increases the incidences of hydrophobic species collision with the bubbles and increases the probability of adhesion of a hydrophobic species to a bubble. In the embodiment depicted in FIGS. 3 and 4 , this gas shearing is aided with the rotating high-shear element 32 b.
- the rotating high-shear element 32 b is intended to shear gas bubbles only and is not intended to agitate or mix the entire slurry volume, therefore, the electric motor 36 b need only be large enough to drive the rotating high-shear element 32 b. This represents a significant energy savings over flotation separation systems that require agitation of the slurry for bubble shearing.
- the creation of the bubble dispersion with the sparger unit 12 b exposes the entire volume of slurry to the surface of a bubble. Therefore the bulk of the adhesion of a hydrophobic species to a bubble is likely to occur within the sparger assembly 30 b, in and downstream of the sparging mechanism 42 b.
- the slurry and the bubble dispersion is discharged into a separation tank ( 14 and 14 a in FIGS. 1 and 2 ) through a slurry outlet 51 b.
- the velocity of slurry discharge is adjusted by changing the location of the distributor plate 44 b using adjustment bolts 46 b.
- the sparger assembly 30 c can contain opposing static vanes 48 c to increase the shearing of gas bubbles in the sparging mechanism 42 c.
- the rotating high-shear elements 32 b and 32 c, as shown in FIGS. 4 and 5 , and the static vanes 48 c shown only in FIG. 5 are for example purposes only and that other configurations of rotating high-shear elements and static vanes are possible and intended to be covered herein.
- the gas inlets 40 b and 40 c are situated upstream of the sparging mechanisms 42 b and 42 c.
- the embodiment of sparging mechanism 42 d depicted in FIGS. 6A and 6B has gas inlets 40 d over the length of the sparging mechanism 42 d.
- the gas inlets 40 d are supplied by gas from an outer sleeve 45 d that connects to the gas injection system (discussed in more detail below) through a hose connection 47 d.
- the gas inlets 40 d are shown in more detail in FIGS. 6C through 6E and comprise an elastomeric check valve 49 d that prevents the backflow of slurry into the outer sleeve 45 d.
- the rotating high shear elements 32 b and 32 c and the static vanes 48 c in the sparging mechanisms 42 b and 42 c serve to break up the bubbles formed at the gas inlets 40 b and 40 c into smaller bubbles to increase the cumulative surface area.
- Variations of air sparging units are possible in which the gas is introduced to the slurry through the sparging mechanisms such that the bubbles formed are of an appropriate size to form a bubble dispersion.
- the top of the sparger unit 12 e comprises a gas supply coupling 50 e to the gas injection system (discussed in more detail below). Gas is supplied through a gas supply tube 52 e to the sparging mechanism 42 e.
- the bottom of the supply tube 52 e ends in a series of slots 56 e that define the length of the sparging mechanism 42 e.
- the sparging mechanism 42 e comprises a series of discs 58 e that are stacked up to at least the length of the slots 56 e in the gas supply tube 52 e.
- Each disc 58 e has a series of grooves 60 e that run from the slots 56 e in the gas supply tube 52 e to the outer edge of the disc 58 e.
- the grooves 60 e define channels for the gas to mix with the passing slurry.
- each groove 60 e acts as a gas inlet for the sparger unit 12 e.
- the number and size of the grooves 60 e and the thickness and the number of the discs 58 e are determined by the particular application. The smaller the grooves 60 e, the smaller the bubbles formed when the passing flow of slurry sparges the gas.
- the smaller gas bubbles created by the sparging mechanism 42 e in this embodiment are of an appropriate size to form a bubble dispersion. Therefore the grooves 60 e also serve as the high shear element of this embodiment of sparger unit 12 e. This sparger unit 12 e requires even less energy to operate than the embodiments presented earlier.
- the sparging mechanism 42 e is configured such that slurry flow through it is substantially unrestricted.
- the effective open area in the sparging mechanism 42 e is substantially the same as the effective open area in the sparger unit 12 e upstream and downstream of the sparging mechanism 42 e.
- the pressure drop across the sparging mechanism 42 e is about 10 psig or less.
- the sparger units 12 e can be easily disconnected from the gas injection system (discussed in more detail below) and water, gas, or another cleaning agent can be forced through the grooves 60 e to facilitate cleaning of the sparging mechanism 42 e.
- the discs 58 e may be made from metal, plastic, polyurethane, ceramics, or any other material that would be appropriate for the particular application. While the discs 58 e depicted in FIGS. 7A though 7 E have grooves 60 e on only one side, FIG. 8 shows a disc 58 f having grooves 60 f on both sides.
- the sparger units 12 g shown in FIGS. 9A through 9C are a variation of the sparger unit 12 e of FIG. 7A .
- This embodiment incorporates a cleaning mechanism for the sparging mechanisms 42 g.
- the sparger unit 12 g includes an inner gas supply tube 52 g connected by a gas supply coupling 50 g to the gas injection system (discussed in more detail below).
- a cleaning fluid coupling 53 g allows for the introduction of a cleaning fluid into the sparger unit 12 g.
- the fluid could be water, compressed gas, or other fluid that could be fed at high pressure to clear debris or clean out the grooves on the discs 58 g during routine maintenance or as needed.
- the embodiment of sparger unit 12 h shown in FIG. 10 shows the sparging mechanism 42 h comprising a high frequency displacement device 54 h.
- gas is introduced to the sparger unit 12 h similar to the embodiment shown earlier, but other gas injection mechanisms are possible.
- the high frequency displacement device 54 h generates a high frequency vibration at the high shear element 32 h that sparges bubbles formed by the gas inlets (not shown) as the bubbles pass the sparging mechanism 42 h. This vibration shears the bubbles to create the fine bubble dispersion in the slurry.
- the sparging mechanism 42 h is configured such that slurry flow through it is substantially unrestricted.
- the effective open area in the sparging mechanism 42 h is substantially the same as the effective open area in the sparger unit 12 h upstream and downstream of the sparging mechanism 42 h.
- the pressure drop across the sparging mechanism 42 h is about 10 psig or less.
- the sparging mechanism 42 i shown in FIG. 11 comprises a series of rotating high shear elements 32 i that serve to further break up and shear introduced gas into fine bubbles.
- the blades of the high shear elements 32 i have openings cut into them to further shear the bubbles.
- the stacked rotating high shear elements 32 i increase the amount of sparging each unit volume of slurry is exposed to as it moves through the sparger unit 12 i.
- the energy input into the sparger unit 12 i is for shearing introduced gas into a fine bubble dispersion and not for agitating the slurry.
- the sparger unit 12 i could also incorporate static vanes as shown for example in FIG. 5 to increase the shearing of gas bubbles in the sparging mechanism.
- the embodiment shown in FIG. 11 shows the outlets 51 i from the sparger unit 12 i as holes cut into the side of the sparger assembly 30 i.
- the flotation separation cell 10 j shows three sparger units 12 j, but the operation described is applicable to any number of sparger units 12 j.
- a flotation separation cell having only one sparger unit would not require a feed manifold distributor as shown in FIG. 12 .
- Slurry is fed to the feed manifold distributor 26 j from upstream operations in which the flotation separation cell 10 j is installed.
- the slurry may be pumped under pressure into the sparger unit if the system hydraulics require, but this need only be sufficient to provide enough hydraulic pressure for the slurry to flow through the flotation separation cell 10 j.
- Slurry can be introduced into the flotation separation cell 10 j at the slurry inlet 38 j of the sparger unit 12 j at a hydraulic pressure of about 25 psig or less.
- the feed manifold distributor 26 j evenly distributes slurry to the slurry inlets 38 j of the sparger units 12 j through distributor pipes 28 j.
- the pressure drop across the sparging mechanisms of the sparger units 12 j is about 10 psig or less.
- Gas typically air
- the gas injection system 62 j consists of a pressure regulator 64 j, a gas flow meter 66 j, a flow regulating valve 70 j, and a gas manifold distributor 72 j.
- the gas manifold distributor 72 j connects the gas injection system to the sparger units 12 j.
- a low-pressure gas blower (not shown) would preferably supply gas to the gas injection system 62 j.
- compressed gas tanks (not shown) or gas compressors (not shown) can be employed.
- the operation of sparger units 12 j is as previously described.
- the slurry and the bubble dispersion are discharged into the separation tank 14 j, which allows for the separation of the floatable and non-floatable hydrophobic species.
- a froth of bubbles with adhered floatable hydrophobic species forms above the slurry at the top the separation tank 14 j.
- the froth can be removed from the top of the separation tank for further processing.
- the froth overflows the separation tank into a product launder 16 j.
- the froth overflow is discharged from the product launder 16 j through the overflow drain 22 j for further processing.
- the rate of underflow discharge is controlled through a control valve 74 j that is actuated based on a signal provided by a process controller 76 j.
- the output of the process controller 76 j is proportional to an input signal derived from a pressure sensor 78 j located on the side of the separation tank 14 j.
- various other level control systems can be employed such as pumps, sand gates, and overflow weir systems.
- the froth at the top of the separation tank is washed with the froth washing system 20 j.
- Water or any other cleaning liquid used for froth washing is controlled by the froth washing control system 80 j.
- clean water is evenly distributed across the top of the froth using a perforated wash pan.
- the froth washing system 20 j can comprise rings of perforated pipe (not shown).
- the flow of wash water is controlled using a flow meter 82 j and a flow control valve 84 j.
- a pilot scale flotation separation system similar to the flotation separation cell depicted in FIG. 1 is currently in operation.
- the pilot flotation separation cell comprises a separation tank that is 48 inches in diameter and about 60 inches deep and has a single sparger unit that is about 4 inches in diameter.
- the sparger unit processes coal slurry at the rate of about 600 gpm.
- the sparging mechanism is similar to the embodiment depicted in FIG. 4 .
- the high shear element of the sparger unit rotates at about 1,200 rpm. Gas is introduced at the gas inlets at about 60 scfm. Slurry enters the sparging mechanism by gravity and has been measured at the sparging mechanism to have a hydraulic pressure of less than 1 psig.
- slurry fills the separation tank up to 3 feet from the bottom with froth filling an additional 2 feet above the slurry.
- the froth is washed with clean water using clean water sprayed over the top of the froth through an arrangement of perforated pipes at a rate of up to 60 gpm.
- the flotation response of several coal types were investigated including the Amburgy, Hazard No. 4, Red Ash, Gilbert and Pocahontas No. 3 seams.
- the ash content of the flotation feed averaged 52%, by weight.
- Combustible recovery ranged from 30% to 78% depending on operating parameters.
- the average combustible recovery for a single-stage of treatment was approximately 60% with a product ash content of 6%.
- an average combustible recovery of between 40% and 50% was achievable while treating Red Ash, Gilbert, or Pocahontas No. 3 coal seams.
- the product ash averaged less than 4% by weight.
- R is a function of the number of perfect mixers (N) for a system with a constant process rate (k) and retention time ( ⁇ ).
- N the number of perfect mixers
- k the number of perfect mixers
- ⁇ retention time
- each cell contains a mixing element that is used to disperse air and maintain the solids in suspension.
- each cell behaves “almost” as a single perfectly mixed tank.
- a perfectly mixed tank has an equal concentration of material at any location in the system. Therefore, a portion of the feed material has an opportunity to immediately short circuit to the tailings discharge point. In a system using a single large cell, this would imply a loss in recovery.
- discharging to a second tank another opportunity exists to collect the floatable material.
- the third and fourth cell in the series Of course, at some point, the law of diminishing returns applies. In conventional flotation systems, this is typically after four or five cells in series. However, the recovery gain with each cell requires additional energy.
- the in-series arrangements shown for example in FIGS. 13 and 14 reduce the inadvertent bypass of feed slurry from individual flotation separation cells 10 j.
- the slurry that leaves through the underflow nozzle 18 j of one separation tank 14 j is redirected to the sparger units 12 j of the next flotation separation cell 10 j.
- This arrangement increases the particulate recovery from a slurry stream.
- the flotation separation cells 10 j can be placed in a modular vertical arrangement (as in FIG. 13 ), a staggered horizontal arrangement (as in FIG. 14 ), or any arrangement that allows for a sufficient hydraulic pressure to convey the slurry from cell to cell. If such a configuration is not possible in the particular application, the slurry could be pumped to each subsequent cell in the series.
- the number of required flotation separation cells 10 j will be dependent on the specific application.
- any of the embodiments herein it is also possible to divert a portion of the slurry discharge from the underflow removal port 18 or the overflow drain 22 back to the initial sparger unit 12 (or the feed manifold distributor 26 a in flotation separation systems with more than one sparger unit 12 a ). This would serve to recycle any chemical additives used to promote frothing and would reduce the materials cost of operation.
- a portion of the discharge from the underflow removal port 18 j or the overflow drain (not shown) from the last flotation separation cell 10 j can be diverted back to the feed manifold distributor 26 j of the first flotation separation cell 10 j.
- a conventional flotation separation system that processes 3,000 gpm of coal slurry may typically comprise 6-8 separation tanks in series, with each separation tank containing a 20-30 HP motor to turn impellers to mix the slurry in the tanks, for a total of about 200 HP for mechanical agitation. Such a conventional system would require an additional 150 HP to power the air blower system for sparging gas.
- a typical column flotation separation system that processes 3,000 gpm of coal slurry requires slurry recirculation pumps that could require around 200 HP to operate.
- a packed column flotation separation systems of similar 3,000 gpm capacity typically would have similar requirements to a typical column flotation system with about 200 HP for recirculation pumps and about 200 HP for air compressors.
- a flotation separation system as described herein for processing 3,000 gpm of coal slurry comprising three flotation separation cells in series, each cell having a single sparger unit with sparging mechanisms that comprise a series of rotating high shear elements (similar to those shown in FIG. 11 ) would require significantly less energy.
- the energy required to power each sparger unit in such a system would be around 20 HP for a total of 60 HP for all three sparger units.
- the energy required by the gas supply system would be about 70 HP for all three sparger units.
- Each separation tank in such a configuration would be about 11 feet in diameter and about 6 feet deep. This represents a significant savings in energy consumption and material requirements.
- the small footprint required for the flotation separation cell 10 j suggests that it can be used to relieve the loading on existing conventional flotation cells 85 j as shown for example in FIG. 16A .
- slurry that has been processed in the flotation separation cell 10 j and discharged through the underflow removal port 18 j is fed to the inlet 86 j of a conventional flotation cell 85 j.
- Collected froth from the flotation separation cell's 10 j overflow launder 16 j and overflow drain 22 j is combined with product collected from the conventional flotation cell's 85 j discharge 87 j.
- the reduced loading to the conventional flotation cell 85 j leads to an overall increase in its performance and an improved overall recovery percentage of the hydrophobic species from flotation separation.
- a flotation separation cell 10 j can be located upstream of an existing column flotation cell 88 j.
- slurry that has been processed in the flotation separation cell 10 j and discharged through the underflow removal port 18 j is fed to the inlet 89 j of a conventional column flotation cell 88 j.
- Collected froth from the flotation separation cell's 10 j overflow launder 16 j and overflow drain 22 j is combined with product collected from the column flotation cell's 88 j discharge 91 j.
- the reduced loading to the column flotation cell 88 j leads to an overall increase in its performance and an improved overall recovery percentage of the hydrophobic species from flotation separation.
- FIG. 17A The pilot scale test indicated that there would be additional benefit to the flotation separation systems disclosed herein if a center well 90 k were to be incorporated in the separation tank 14 k, as shown in FIG. 17A .
- the center well 90 k fits around the outside of the sparger unit 12 k and comprises a tube that runs the height of the separation tank 14 k. Outlets 92 k near the bottom of the center well 90 k allow for the slurry discharged from the sparger unit 12 k to enter the separation tank 14 k.
- the purpose of the center well 90 k is to ensure that the sparger assembly within the center well 90 k remains submerged below the liquid level and to aid in efficient bubble formation and promote efficient bubble/particle interaction.
- the center well 90 k liquid level will be at the same level as that of the surrounding separation tank 14 k. However, at higher flows, the level within the center well 90 k will be higher than that of the surrounding separation tank 14 k. The higher level ensures that there is no chance for air to coalesce within the sparger unit 12 k and ultimately reduces burping and inefficient contacting within the sparger unit 12 k.
- the liquid level in the center well 90 k can be determined by reading a low-pressure pressure gauge (not shown) that is installed on the slurry inlet 38 k. In order to ensure that the center well 90 k stays full, the center well 90 k must be engineered such that it flushes just slightly slower than it fills. Only a positive pressure is required to indicate that the center well 90 k is full.
- Level control in the center well can be maintained in several ways as shown in FIGS. 18A through 18C .
- the center well 90 l is constructed such that the size of the outlets 92 l can be continuously adjusted.
- a low-pressure gauge 94 l installed at the slurry inlet 38 l monitors the pressure in sparger unit 12 l.
- a PID control loop 96 l adjusts the outlet 92 l size in response to changes in the pressure readings—an increase in pressure above a preset limit will trigger the PID control loop 96 l to increase the outlet 92 l size to allow more slurry to leave the sparger unit 12 l and the center well 90 l; a decrease in pressure below a preset limit will trigger the PID control loop 96 l to decrease the outlet 92 l size which will retain more slurry in the center well 90 l and keep the sparger unit 12 l submerged.
- direct level control of the level of the separation tank 14 l could be performed by using a PID process controller to throttle the outflow from the underflow nozzle 18 l based on pressure readings in the separation tank 14 l. While this method will ensure a consistent level in the separation tank 14 l, it would not ensure that there is sufficient pressure within the center well 90 l.
- FIG. 18B A simpler control scheme is shown in FIG. 18B that negates the need for a control mechanism to be placed within the separation tank 14 m.
- the center well 90 m level is maintained by controlling the flow from the inflow to the flotation separation system by automating a make-up valve 98 m through a PID control loop 96 m such that a low pressure reading from the low-pressure pressure gauge 94 m triggers additional liquid, and hence flow, to be routed to the separation cell 10 m.
- This method can be easily applied to a series of separation tanks 10 n, as shown in FIG. 18C .
- a second PID control loop 100 n controls the underflow nozzle 18 n of the previous separation cell 10 n in the series.
- FIG. 19 shows a flotation separation cell 10 o in which the slurry enters the sparger units 12 o from underneath the separation tank 14 o.
- a feed manifold distributor 26 o distributes slurry to each sparger unit 12 o through distributor pipes 28 o to the sparging mechanisms 42 o. Gas is supplied to the sparger units as described above.
- the electric motors 36 o that power the rotating high-shear element (not shown) via rotating shafts 34 o are located above the separation tank 14 o.
- the electric motors 36 o are supported in place with a support ring 90 o. Slurry passes up through the sparging mechanism 42 o and into the separation tank 14 o.
- FIG. 20 shows an embodiment of a flotation separation cell 10 p in which the sparger units 12 p are located on the side of the separation tank 14 p.
- the feed manifold distributor 26 p is shown feeding the sparger units 12 p from underneath the separation tank 14 p.
- the feed manifold distributor 26 p can also be located above the separation tank 14 p as shown in earlier embodiments.
- the underflow removal port 18 q does not need to be located at the bottom of the flotation separation cell 10 q.
- the embodiment shown in FIG. 21 shows how the underflow removal port 18 q can remove slurry from the side of the separation tank 14 q.
- the underflow removal port 18 q has a right angle bend directed towards the bottom of the separation tank 14 q to allow for a uniform withdrawal of slurry from the bottom of the separation tank 14 q.
- the slurry can be withdrawn from the underflow removal port 18 q by gravity as a drain or with a pump, sand gates, an overflow weir system, or any other appropriate mechanism.
Landscapes
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biotechnology (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dispersion Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Physical Water Treatments (AREA)
Abstract
Description
- This application takes priority from U.S.
provisional application 60/911,327 filed Apr. 12, 2007, which is incorporated herein by reference. - Flotation separators are used extensively throughout the minerals industry to partition and recover the constituent species within slurries. A slurry is a mixture of liquids (usually water) with various species having varying degrees of hydrophobicity. The species could be insoluble particulate matter such as coal, metals, clay, sand, etc. or soluble elements or compounds in solution. Flotation separators work on the principle that the various species within the slurry interact differently with bubbles formed in the slurry. Gas bubbles introduced into the slurry attach, either through physical or chemical means, to one or more of the hydrophobic species of the slurry. The bubble-hydrophobic species agglomerates are sufficiently buoyant to lift away from the remaining constituents and are removed for further processing to concentrate and recover the adhered species. Various methods used to achieve this process typically require significant energy to inject gas into the slurry and form a bubble dispersion.
- A flotation separation system is provided for partitioning a slurry that includes a hydrophobic species which can adhere to gas bubbles formed in the slurry. The flotation separation system comprises a flotation separation cell that includes a sparger unit and a separation tank. The sparger unit has a slurry inlet for receiving slurry and a gas inlet to receive gas with at least enough pressure to allow bubbles to form in the slurry within the sparger unit. The sparger unit includes a sparging mechanism constructed to disperse gas bubbles within the slurry. The sparging mechanism sparges the gas bubbles to form a bubble dispersion so as to cause adhesion of the hydrophobic species to the gas bubbles substantially within the sparger unit while causing a pressure drop of about 10 psig or less across the sparging mechanism. The sparger unit includes a slurry outlet to discharge the slurry and the bubble dispersion into the separation tank. The separation tank has sufficient capacity to allow the bubble dispersion to form a froth at the top of the separation tank. Various embodiments of the flotation separation system can include a center well that surrounds the sparging unit.
- In one embodiment, the sparging mechanism of the sparger unit includes a high-shear element to help shear the bubbles formed in the slurry into a bubble dispersion. The high-shear element can include rotating high-shear elements or a combination of rotating and static high-shear elements. Rotating high shear elements can comprise a series of rotating elements along the length of the sparging unit. The high-shear element can alternatively comprise a series of grooved discs pressed together to form channels from the gas inlets to the slurry with gas passing through the channels to reach the slurry. Other possible embodiments and variations are discussed in more detail herein.
- Those skilled in the art will realize that this invention is capable of embodiments that are different from those shown and that details of the devices and methods can be changed in various manners without departing from the scope of this invention. Accordingly, the drawings and descriptions are to be regarded as including such equivalent embodiments as do not depart from the spirit and scope of this invention.
- For a more complete understanding and appreciation of this invention, and its many advantages, reference will be made to the following detailed description taken in conjunction with the accompanying drawings.
-
FIG. 1 is a perspective view of a flotation separation cell with one sparger unit; -
FIG. 2 is a perspective view of a flotation separation cell with three sparger units; -
FIG. 3 is an embodiment of a sparger unit; -
FIG. 4 is a view of an embodiment of a sparger unit showing the rotating high-shear element of the sparging mechanism; -
FIG. 5 is a view of an embodiment of a sparger unit showing the rotating and static high-shear elements of the sparging mechanism; -
FIG. 6A is a view of an embodiment of a sparger unit in which the sparging mechanism has gas inlets along its length; -
FIG. 6B is a view of the sparging mechanism of the sparger unit ofFIG. 6A ; -
FIG. 6C is a close up of a check valve of a gas inlet ofFIG. 6A ; -
FIG. 6D is a gas inlet ofFIG. 6A ; -
FIG. 6E is a different view of the gas inlet ofFIG. 6D ; -
FIG. 7A is an embodiment of a sparger unit that does not use an electric motor; -
FIG. 7B is a view of the sparger unit ofFIG. 7A showing the sparging mechanism with the high shear element comprising a series of grooved discs; -
FIG. 7C is a view of the high shear element ofFIG. 7B ; -
FIG. 7D is a view of the high shear element ofFIG. 7B without the grooved discs; -
FIG. 7E is a view of a grooved disc ofFIG. 7B ; -
FIG. 8 is a view of an alternative embodiment of the grooved discs ofFIG. 7B ; -
FIG. 9A is an embodiment of a sparger unit with a cleaning system for the sparging unit; -
FIG. 9B is a close up of the sparger unit ofFIG. 9A without the grooved discs; -
FIG. 9C is an exploded view of the sparger unit ofFIG. 9A ; -
FIG. 10 is a sparger unit in which the sparging mechanism is a high frequency linear displacement device; -
FIG. 11 is a view of an embodiment of a sparger unit showing a sparger mechanism having multiple banks of rotating high shear elements; -
FIG. 12 is a representation of some of the control systems for a flotation separation cell; -
FIG. 13 shows a flotation separation system that comprises a series of flotation separation cells in a modular vertical arrangement; -
FIG. 14 shows a flotation separation system that comprises a series of flotation separation cells in a staggered horizontal arrangement; -
FIG. 15 is a graph plotting the recovery of a target species versus process rate and retention time for various circuit configurations; -
FIG. 16A shows a flotation separation system in which a flotation separation cell discharges slurry from the underflow removal port to the inlet of a conventional flotation cell; -
FIG. 16B shows a flotation separation system in which a flotation separation cell discharges slurry from the underflow removal port to the inlet of a column flotation cell; -
FIG. 17A shows an embodiment of a flotation separation cell that incorporates a center well; -
FIG. 17B shows the center well shown inFIG. 17A showing the sparger unit within the center well; -
FIG. 18A shows a different embodiment of a flotation separation cell in which the center well liquid level is maintained by adjusting the size of the orifices at the end of the center well based on pressure sensor readings; -
FIG. 18B shows a different embodiment of a flotation separation cell in which the liquid level in the center well is maintained by adjusting the inflow of slurry to the flotation separation cell; -
FIG. 18C shows a different embodiment of a flotation separation system comprising a number of flotation separation cells in series in which the liquid level in the center well for each flotation separation cell is maintained by adjusting the inflow of slurry to each flotation separation cell; -
FIG. 19 is a perspective view of a flotation separation cell with four sparger units that feed slurry from the bottom of the separation tank; -
FIG. 20 is a perspective view of a flotation separation cell with four sparger units that feed slurry through the sidewalls of the separation tank; and -
FIG. 21 is a perspective view of a flotation separation cell in which the underflow removal port leaves through the side of the separation tank. - Referring to the drawings, some of the reference numerals are used to designate the same or corresponding parts through several of the embodiments and figures shown and described. Corresponding parts are denoted in different embodiments with the addition of lowercase letters. Variations of corresponding parts in form or function that are depicted in the figures are described. It will be understood that variations in the embodiments can generally be interchanged without deviating from the invention.
- Flotation separation is commonly used in the minerals industry to separate mineral species in suspension in liquid slurries. Such mineral species are often suspended with a mixture of unwanted constituent species. Flotation separators currently in common use require an extensive application of large amounts of energy for pressurizing gas, pressuring the slurry, increasing the flow velocity of the slurry, and/or maintaining the slurry in suspension.
- However, effective flotation separation is possible with the embodiments depicted herein without the need for high energy consumption. In one embodiment, shown in
FIG. 1 , a flotation separation system comprises at least oneflotation separation cell 10 in a hydraulic system for the partitioning and recovery of the constituents of a slurry. Theflotation separation cell 10 comprises at least onesparger unit 12 in which gas is introduced into the slurry. Thesparger unit 12 includes asparging mechanism 42 for sparging gas into a bubble dispersion within the slurry. Thesparging mechanism 42 is configured such that slurry flow through it is substantially unrestricted. The effective open area in thesparging mechanism 42 is substantially the same as the effective open area in thesparger unit 12 upstream and downstream of thesparging mechanism 42. This ensures a low pressure drop across thesparging mechanism 42 that allows for a lower pressure and flow rate of slurry through thesparger unit 12 and represents a significant energy savings for the flotation separation system. The pressure drop across thesparging mechanism 42 is about 10 psig or less. The operation of various embodiments ofsparger units 12 is described in further detail below. - The
sparger unit 12 feeds the slurry and bubble dispersion mixture to aseparation tank 14. Theseparation tank 14 comprises an overflow launder 16, anunderflow removal port 18, and afroth washing system 20. The overflow launder connects to anoverflow drain 22. Theflotation separation cell 10 may be supported bylegs 24 or by any other means required by the particular application. Theflotation separation cell 10 may even be placed directly on the floor if warranted by the design of the facility to which theflotation separation cell 10 is installed. Theseparation tank 14 requires no additional equipment within the tank to assist in froth formation (as discussed in more detail below) or to maintain the slurry in suspension. This represents a further energy savings in the overall operation as compared to conventional flotation separation systems, column flotation separation systems, and packed column flotation separation systems. The operation of the flotation separation system is presented in more detail below. - The flotation slurries typically include hydrophobic and hydrophilic species. Flotation separation takes advantage of the differing hydrophobicity of these species. When bubbles of gas are introduced into the slurry, the hydrophobic species within the slurry tend to selectively adhere to the bubbles while hydrophilic species tend to remain in suspension. Sparging, or breaking up, the bubbles into a bubble dispersion of many smaller bubbles increases the available bubble surface area for hydrophobic species adhesion. The bubbles, with the adhered hydrophobic species, tend to rise above the slurry and form a froth in the
separation tank 14 that is easily separated from the remainder of the slurry for further processing to recover the adhered hydrophobic species. In the embodiment shown inFIG. 1 removal of the froth is accomplished by overflowing the froth from theseparation tank 14 into the overflow launder 16 and draining the collected froth through theoverflow drain 22 to downstream processes. The species not adhered to the froth remain in the slurry and are discharged through theunderflow removal port 18 for further processing. Further processing can include a subsequent stage of froth formation to catch hydrophobic species that for whatever reason were not captured in the preceding step. - Flotation separation systems are typically part of larger hydraulic systems that process slurry over a number of steps. The liquid portion of the slurry is typically water. The chemistry of the slurry is often adjusted with additives to assist in recovering a target component depending on the constituent species of the slurry. Surface tension modifying reagents, also known as frothers, are often added to slurries to assist in bubble formation. There are many types of frothers, including alcohols, glycols, Methylisobutyl Carbinol (MIBC), and various blends.
- Sometimes the target species for recovery from the slurry are naturally hydrophobic, for example coal. But in slurries in which the target species are not hydrophobic, chemicals additives, also known as collectors, are introduced to chemically activate them. Collectors include fuel oil, fatty acids, xanthates, various amines, etc.
- Some target species are quasi-hydrophobic. For example, oxidized coal tends to be less hydrophobic and is more difficult to recover from a slurry than unoxidized coal. Chemical additives, called extenders, are used to increase their hydrophobicity. Examples of extenders are diesel fuels and other fuel oils.
- Chemical additives called depressants are used to reduce the hydrophobicity of a species. For example, in the recovery of iron ore, various types of starches are used to depress the bubble adhesion response of iron ore so that only silica can be floated in the froth from the slurry. If the depressants are not added, a portion of the iron ore will also adhere to bubbles and float within the froth.
- Because the pH of the slurry can affect froth formation, other chemical additives are introduced to modify the pH of the slurry. Acids or bases are added as needed to adjust the pH depending on the composition of the slurry.
- In mineral flotation, the recovery of a particular species is predominantly controlled and proportional to two parameters: reaction rate and retention time. Recovery can be generally represented by the following equation:
-
R=kT [1] - Where R is the recovery of a particular species, k is the reaction rate of adhesion of a species to a bubble, and T is the retention time of the slurry in the flotation separation system. An increase in either parameter provides a corresponding increase in recovery, R. The reaction rate, k, for a process is indicative of the speed at which the flotation separation will proceed and can be a function of several parameters including, but not limited to, gas introduction rate, bubble size, species size, and chemistry. The reaction rate, k, is increased when these parameters are adjusted to maximize the probability that a hydrophobic species will collide with and adhere to a bubble and to reduce the probability that a hydrophobic species will detach from a bubble. The probability of attachment is controlled by the surface chemistry of both the species and the bubbles in the process stream and is increased when the probability of a collision between a hydrophobic species and a bubble increases. The probability of collision is directly proportional to the concentration of hydrophobic species within the sparging region. The probability of detachment is controlled by the hydrodynamics of the flotation separation cell. As such, aeration of the slurry prior to its introduction to a separation tank is the preferred method of sparging as this ensures that the maximum amount of floatable species is concentrated within the sparging unit to obtain a high recovery of the hydrophobic species. The embodiments described herein aim to increase the reaction rate, k, which means that a lower retention time, T, and thereby a smaller separation tank, can be used to obtain a suitable recovery, R.
- In the embodiments disclosed herein, the reaction rate, k, of Equation [1] is increased by forcing the bubble-particle contact with high particle and air bubble concentrations and imparting significant energy within the bubble/particle contacting zone. Recovery, R, can also be represented in turbulent systems described herein as a function of the bubble concentration, Cb, particle concentration, Cp, and specific energy input, E, as follows:
-
R∝CbCpE [2] - The embodiments disclosed herein efficiently pre-aerate slurry in the
sparger units 12 of theflotation separation cell 10 prior to injection of the slurry and gas mixture into theseparation tank 14. Slurry introduced into thesparger unit 12 passes through asparging mechanism 42, described in more detail below. Thesparging mechanism 42 sparges the gas in the slurry into a bubble dispersion creating a relatively large surface area for hydrophobic species attachment within thesparger unit 12 such that hydrophobic species adhesion to bubbles occurs substantially in thesparger unit 12 before the slurry and the bubble dispersion is discharged into theseparation tank 14. This approach ensures that bubbles are generated in the presence of the slurry prior to any dilution with wash water (if used), thus maintaining the maximum particle concentration (Cp). Additionally, thesparger assembly 30 is operated at a very high air fraction (>40%), insuring that the bubble concentration (Cb) is maximized. Finally, the design of thesparging mechanism 42 in thesparger unit 12 is such that maximum energy is imparted to the slurry for the sole purpose of bubble-particle contacting. As a result, the contact time is reduced by several orders of magnitude over prior art column and conventional flotation separators. After contacting, the slurry is discharged to theseparation tank 14 for phase separation (slurry and froth) and froth washing (if used). Since phase separation is a relatively quick process, theoverall separation tank 14 size is significantly reduced. - The
sparging mechanism 42 is configured such that slurry flow through it is substantially unrestricted. The effective open area in thesparging mechanism 42 is substantially the same as the effective open area in thesparger unit 12 upstream and downstream of thesparging mechanism 42. This ensures a low pressure drop across thesparging mechanism 42 that allows for a lower pressure and flow rate of slurry through thesparger unit 12 and represents a significant energy savings for the flotation separation system. The pressure drop across thesparging mechanism 42 is about 10 psig or less. Nevertheless, the embodiments depicted herein are able to operate with pressure drops of about 1 psig or less. - As the bulk of the hydrophobic species adhesion to a bubble occurs in the
sparging unit 12, theflotation separation cell 10 does not require the slurry to be introduced at a high velocity and/or a high pressure. The slurry may be pumped under pressure into thesparger unit 12 if the hydraulics of the flotation separation system require, but this need only be sufficient to provide enough hydraulic pressure for the slurry to flow through the flotation separation system. Slurry can be introduced into theflotation separation cell 10 at the slurry inlet of thesparger unit 12 at a hydraulic pressure of about 25 psig or less. The embodiments depicted herein are able to operate at slurry introduction hydraulic pressures of 2 psig or less. - The relatively low hydraulic pressure gradient that the slurry must overcome represents an energy savings during the operation of the
flotation separation cell 10. The hydraulics of aflotation separation cell 10 can be adjusted in various embodiments by, for example, adjusting the height of thesparger units 12 in relation to the height of the slurry in theseparation tank 14 or by adjusting the entry point of slurry to theflotation separation cell 10. - Similarly, the sparging
mechanisms 42, described in more detail below, do not require gas to be introduced at a high pressure. The gas introduction pressure need only be high enough to form bubbles in the slurry and thesparging mechanisms 42 described herein will sparge the bubbles into effective bubble dispersions. The low pressure and flow requirements for both slurry and gas introduction represent significant energy savings when compared to conventional flotation separation systems, column flotation separation systems, and packed column flotation separation systems. - As has been already discussed, with an increase in the rate of reaction provided by the method of pre-aeration, there is a corresponding decrease in the required retention time for a given application. Therefore the same flotation recovery can be obtained in a smaller volume than with prior art systems. As the bubble and species attachment substantially occurs in close proximity to the
sparging mechanism 42 in thesparger units 12, described in more detail below, and not within theseparation tank 14 itself, theseparation tank 14 is only required to provide time for the slurry and bubble phases to separate. Asmaller separation tank 14 can be utilized without additional equipment in the separation tank when compared to conventional flotation separation systems, column flotation separation systems, and packed column flotation separation systems. The smaller and simplerflotation separation cell 10 allows for greater flexibility in designing flotation separation systems for particular applications. Energy is also not consumed to maintain the slurry in suspension in theseparation tank 14. - Because the
separation tank 14 is used solely for froth separation, and does not require any additional equipment to maintain the slurry in suspension, the embodiments described herein are able to maintain a relatively deep froth in theseparation tank 14 with no additional turbulence imparted to theseparation tank 14. Therefore, unlike with conventional flotation separation systems, the addition of wash water from the froth washing system 20 (described in more detail below) to clean the froth does not affect the retention time of the froth in theseparation tank 14. It is therefore possible to have effective froth washing in the flotation separation systems described herein. - As the energy input to the system is focused specifically on creating fine bubbles and not in maintaining the particles in suspension, the overall energy input is reduced. While a compressor may be used to introduce gas into the flotation separation system, because the
sparging mechanism 42 operates at atmospheric pressure a compressor is not required to overcome the hydrostatic system head. Instead, a simple blower can be used, providing energy and maintenance savings. The energy reduction, of course, implies reduced operating costs. Finally, thesmaller separation tank 14 requirements reduce equipment and installation costs. Structural steel requirements are significantly less due to the reduction in tank weight and live load. The space requirement is less than that required for equivalent conventional column flotation separation. Shipping and installation is also simplified since the units can be shipped fully assembled and installed without field welding. - Depending on the operational requirements of the system to which the flotation separation system is installed,
FIG. 2 shows how theflotation separation cell 10 a can be designed withmultiple sparger units 12 a, in this case three, with an appropriatelysized separation tank 14 a. Afeed manifold distributor 26 a havingdistributor pipes 28 a may be used to evenly distribute slurry to eachsparger unit 12 a. - In one embodiment of the sparger unit best understood by comparing
FIGS. 3 and 4 , eachsparger unit 12 b comprises asparger assembly 30 b that allows for the passage of feed slurry to a separation tank (14 and 14 a inFIGS. 1 and 2 ). The size of thesparger assembly 30 b is dictated by the size of the flotation separation system in which thesparger unit 12 b is installed and is primarily intended to direct the slurry discharge to an appropriate location within theseparation tank 14. The slurry should be discharged low enough in theseparation tank 14 so as to not interfere with froth formation at the top of theseparation tank 14. - Slurry is introduced into the
sparger unit 12 b through theslurry inlet 38 b and passes through asparging mechanism 42 b. As has been already discussed, thesparging mechanism 42 b is configured such that slurry flow through it is substantially unrestricted. The effective open area in thesparging mechanism 42 b is substantially the same as the effective open area in thesparger unit 12 b upstream and downstream of thesparging mechanism 42 b. The pressure drop across thesparging mechanism 42 b is about 10 psig or less. - In the embodiments depicted in
FIG. 3 and 4 , thesparging mechanism 42 b comprises a rotating high-shear element 32 b attached to arotating shaft 34 b that is powered by anelectric motor 36 b. The slurry may be gravity fed if there is enough hydraulic pressure to ensure that the slurry will flow through the flotation separation system. If the hydraulics of the system requires the slurry to be pumped, the slurry need only be pumped with sufficient pressure to ensure passage of the slurry through the flotation separation system. Nevertheless, thesparger unit 12 b will function well over a broad range of slurry flow rates and pressures. Slurry can be introduced into theslurry inlet 38 b of thesparger unit 12 b at a hydraulic pressure of about 25 psig or less. Thesparger unit 12 b can operate at a slurry hydraulic pressure of about 2 psig or less. - Gas (typically air) is introduced to the
sparger unit 12 b throughgas inlets 40 b that are supplied from a gas injection system (discussed in more detail below). The passing slurry flow immediately shears the gas to form bubbles as the gas enters thesparger unit 12 b through thegas inlets 40 b. The gas need not be at a high pressure for effective bubble formation in the slurry. Even at high slurry feed rates, the gas flow and pressure needs only be high enough to allow bubble formation in the slurry. - The bubbles are sheared into smaller bubbles as the slurry passes through the
sparging mechanism 42 b and forms a fine bubble dispersion within the slurry. The formation of the bubble dispersion within thesparger unit 12 b exposes a larger volume of slurry to the surface of the bubbles. This increases the incidences of hydrophobic species collision with the bubbles and increases the probability of adhesion of a hydrophobic species to a bubble. In the embodiment depicted inFIGS. 3 and 4 , this gas shearing is aided with the rotating high-shear element 32 b. The rotating high-shear element 32 b is intended to shear gas bubbles only and is not intended to agitate or mix the entire slurry volume, therefore, theelectric motor 36 b need only be large enough to drive the rotating high-shear element 32 b. This represents a significant energy savings over flotation separation systems that require agitation of the slurry for bubble shearing. - The creation of the bubble dispersion with the
sparger unit 12 b exposes the entire volume of slurry to the surface of a bubble. Therefore the bulk of the adhesion of a hydrophobic species to a bubble is likely to occur within thesparger assembly 30 b, in and downstream of thesparging mechanism 42 b. - Once the slurry has passed though
sparging mechanism 42 b, the slurry and the bubble dispersion is discharged into a separation tank (14 and 14 a inFIGS. 1 and 2 ) through aslurry outlet 51b. The velocity of slurry discharge is adjusted by changing the location of thedistributor plate 44 b usingadjustment bolts 46 b. - As shown in the embodiment depicted in
FIG. 5 , thesparger assembly 30 c can contain opposingstatic vanes 48 c to increase the shearing of gas bubbles in thesparging mechanism 42 c. It will be appreciated that the rotating high-shear elements FIGS. 4 and 5 , and thestatic vanes 48 c shown only inFIG. 5 are for example purposes only and that other configurations of rotating high-shear elements and static vanes are possible and intended to be covered herein. - In the embodiments shown in
FIGS. 4 and 5 , thegas inlets sparging mechanisms sparging mechanism 42 d depicted inFIGS. 6A and 6B hasgas inlets 40 d over the length of thesparging mechanism 42 d. Thegas inlets 40 d are supplied by gas from anouter sleeve 45 d that connects to the gas injection system (discussed in more detail below) through ahose connection 47 d. Thegas inlets 40 d are shown in more detail inFIGS. 6C through 6E and comprise anelastomeric check valve 49d that prevents the backflow of slurry into theouter sleeve 45 d. - The rotating
high shear elements static vanes 48 c in thesparging mechanisms gas inlets - As can best be understood by comparing the alternate arrangement in
FIGS. 7A through 7E , the top of thesparger unit 12 e comprises agas supply coupling 50 e to the gas injection system (discussed in more detail below). Gas is supplied through agas supply tube 52 e to thesparging mechanism 42 e. The bottom of thesupply tube 52 e ends in a series ofslots 56 e that define the length of thesparging mechanism 42 e. In this embodiment, thesparging mechanism 42 e comprises a series ofdiscs 58 e that are stacked up to at least the length of theslots 56 e in thegas supply tube 52 e. Eachdisc 58 e has a series ofgrooves 60 e that run from theslots 56 e in thegas supply tube 52 e to the outer edge of thedisc 58 e. When thediscs 58 e are stacked on top of each other, thegrooves 60 e define channels for the gas to mix with the passing slurry. In this embodiment eachgroove 60 e acts as a gas inlet for thesparger unit 12 e. The number and size of thegrooves 60 e and the thickness and the number of thediscs 58 e are determined by the particular application. The smaller thegrooves 60 e, the smaller the bubbles formed when the passing flow of slurry sparges the gas. The smaller gas bubbles created by thesparging mechanism 42 e in this embodiment are of an appropriate size to form a bubble dispersion. Therefore thegrooves 60 e also serve as the high shear element of this embodiment ofsparger unit 12 e. Thissparger unit 12 e requires even less energy to operate than the embodiments presented earlier. - Nevertheless, the
sparging mechanism 42 e is configured such that slurry flow through it is substantially unrestricted. The effective open area in thesparging mechanism 42 e is substantially the same as the effective open area in thesparger unit 12 e upstream and downstream of thesparging mechanism 42 e. The pressure drop across thesparging mechanism 42 e is about 10 psig or less. - The
sparger units 12 e can be easily disconnected from the gas injection system (discussed in more detail below) and water, gas, or another cleaning agent can be forced through thegrooves 60 e to facilitate cleaning of thesparging mechanism 42 e. Thediscs 58 e may be made from metal, plastic, polyurethane, ceramics, or any other material that would be appropriate for the particular application. While thediscs 58 e depicted inFIGS. 7A though 7E havegrooves 60 e on only one side,FIG. 8 shows adisc 58f having grooves 60 f on both sides. - The
sparger units 12 g shown inFIGS. 9A through 9C are a variation of thesparger unit 12 e ofFIG. 7A . This embodiment incorporates a cleaning mechanism for thesparging mechanisms 42 g. As can be best understood by comparingFIGS. 9A through 9C , thesparger unit 12 g includes an innergas supply tube 52 g connected by agas supply coupling 50 g to the gas injection system (discussed in more detail below). A cleaningfluid coupling 53 g allows for the introduction of a cleaning fluid into thesparger unit 12 g. The fluid could be water, compressed gas, or other fluid that could be fed at high pressure to clear debris or clean out the grooves on thediscs 58 g during routine maintenance or as needed. - The embodiment of
sparger unit 12 h shown inFIG. 10 shows thesparging mechanism 42 h comprising a highfrequency displacement device 54 h. In this embodiment gas is introduced to thesparger unit 12 h similar to the embodiment shown earlier, but other gas injection mechanisms are possible. The highfrequency displacement device 54 h generates a high frequency vibration at thehigh shear element 32 h that sparges bubbles formed by the gas inlets (not shown) as the bubbles pass thesparging mechanism 42 h. This vibration shears the bubbles to create the fine bubble dispersion in the slurry. Nevertheless, thesparging mechanism 42 h is configured such that slurry flow through it is substantially unrestricted. The effective open area in thesparging mechanism 42 h is substantially the same as the effective open area in thesparger unit 12 h upstream and downstream of thesparging mechanism 42 h. The pressure drop across thesparging mechanism 42 h is about 10 psig or less. - As shown in
FIG. 11 , other embodiments ofsparger units 12 i are possible in which thesparging mechanism 42 i extends across the length of thesparger assembly 30 i. These embodiments function similarly to thesparger unit 12 b shown and described inFIG. 4 above, however any of the other embodiments described above would work equally well. Thesparging mechanism 42 i shown inFIG. 11 comprises a series of rotatinghigh shear elements 32 i that serve to further break up and shear introduced gas into fine bubbles. In this embodiment, the blades of thehigh shear elements 32 i have openings cut into them to further shear the bubbles. The stacked rotatinghigh shear elements 32 i increase the amount of sparging each unit volume of slurry is exposed to as it moves through thesparger unit 12 i. As with the embodiments discussed above, the energy input into thesparger unit 12 i is for shearing introduced gas into a fine bubble dispersion and not for agitating the slurry. Thesparger unit 12 i could also incorporate static vanes as shown for example inFIG. 5 to increase the shearing of gas bubbles in the sparging mechanism. The embodiment shown inFIG. 11 shows theoutlets 51 i from thesparger unit 12 i as holes cut into the side of thesparger assembly 30 i. - Regardless of the embodiment of
sparger unit 12 j used, the operation of the flotation separation system is demonstrated in theflotation separation cell 10 j depicted inFIG. 12 . Theflotation separation cell 10 j shows threesparger units 12 j, but the operation described is applicable to any number ofsparger units 12 j. A flotation separation cell having only one sparger unit (for example as shown inFIG. 1 ) would not require a feed manifold distributor as shown inFIG. 12 . - Slurry is fed to the
feed manifold distributor 26 j from upstream operations in which theflotation separation cell 10 j is installed. As has already been discussed, the slurry may be pumped under pressure into the sparger unit if the system hydraulics require, but this need only be sufficient to provide enough hydraulic pressure for the slurry to flow through theflotation separation cell 10 j. Slurry can be introduced into theflotation separation cell 10 j at theslurry inlet 38 j of thesparger unit 12 j at a hydraulic pressure of about 25 psig or less. Thefeed manifold distributor 26 j evenly distributes slurry to theslurry inlets 38 j of thesparger units 12 j throughdistributor pipes 28 j. The pressure drop across the sparging mechanisms of thesparger units 12 j is about 10 psig or less. - Gas, typically air, is supplied to the
sparger units 12 j from thegas injection system 62 j. As discussed earlier, gas introduction pressure need only be high enough to allow bubbles to form in the slurry. Thegas injection system 62 j consists of apressure regulator 64 j, agas flow meter 66 j, aflow regulating valve 70 j, and agas manifold distributor 72 j. Thegas manifold distributor 72 j connects the gas injection system to thesparger units 12 j. A low-pressure gas blower (not shown) would preferably supply gas to thegas injection system 62 j. Alternatively, compressed gas tanks (not shown) or gas compressors (not shown) can be employed. - The operation of
sparger units 12 j is as previously described. The slurry and the bubble dispersion are discharged into theseparation tank 14 j, which allows for the separation of the floatable and non-floatable hydrophobic species. A froth of bubbles with adhered floatable hydrophobic species forms above the slurry at the top theseparation tank 14 j. The froth can be removed from the top of the separation tank for further processing. In one embodiment, the froth overflows the separation tank into a product launder 16 j. The froth overflow is discharged from the product launder 16 j through theoverflow drain 22 j for further processing. - Non-floatable hydrophobic species, heavier particles that do not adhere to the froth, and any hydrophobic species that for whatever reason do not adhere to the froth fall to the bottom of the
separation tank 14 j and are drained through theunderflow removal port 18 j for further processing. The rate of underflow discharge is controlled through acontrol valve 74 j that is actuated based on a signal provided by aprocess controller 76 j. The output of theprocess controller 76 j is proportional to an input signal derived from apressure sensor 78 j located on the side of theseparation tank 14 j. Alternatively, various other level control systems can be employed such as pumps, sand gates, and overflow weir systems. - The froth at the top of the separation tank is washed with the
froth washing system 20 j. Water or any other cleaning liquid used for froth washing is controlled by the frothwashing control system 80 j. In thefroth washing system 20 j, clean water is evenly distributed across the top of the froth using a perforated wash pan. Alternatively, thefroth washing system 20 j can comprise rings of perforated pipe (not shown). The flow of wash water is controlled using aflow meter 82 j and aflow control valve 84 j. - A pilot scale flotation separation system similar to the flotation separation cell depicted in
FIG. 1 is currently in operation. The pilot flotation separation cell comprises a separation tank that is 48 inches in diameter and about 60 inches deep and has a single sparger unit that is about 4 inches in diameter. The sparger unit processes coal slurry at the rate of about 600 gpm. The sparging mechanism is similar to the embodiment depicted inFIG. 4 . The high shear element of the sparger unit rotates at about 1,200 rpm. Gas is introduced at the gas inlets at about 60 scfm. Slurry enters the sparging mechanism by gravity and has been measured at the sparging mechanism to have a hydraulic pressure of less than 1 psig. During normal operating conditions, slurry fills the separation tank up to 3 feet from the bottom with froth filling an additional 2 feet above the slurry. The froth is washed with clean water using clean water sprayed over the top of the froth through an arrangement of perforated pipes at a rate of up to 60 gpm. - The flotation response of several coal types were investigated including the Amburgy, Hazard No. 4, Red Ash, Gilbert and Pocahontas No. 3 seams. For the Amburgy and Hazard No. 4 seams (
FIG. 5 ), the ash content of the flotation feed averaged 52%, by weight. Combustible recovery ranged from 30% to 78% depending on operating parameters. The average combustible recovery for a single-stage of treatment was approximately 60% with a product ash content of 6%. Similarly, an average combustible recovery of between 40% and 50% was achievable while treating Red Ash, Gilbert, or Pocahontas No. 3 coal seams. For these coals, the product ash averaged less than 4% by weight. The lower feed ash (i.e., 18%) for these seams resulted in a slightly lower range of combustible recovery. This finding is not unexpected given that as the feed ash decreases, the amount of floatable coal increases for a given volume flow and retention time. - While hydrophobic species adhesion to the bubble dispersion in the
sparger units 12 j allows for a high recovery of hydrophobic species in the slurry, not all of the hydrophobic species in the slurry will adhere to a bubble. Furthermore, there is a reduction in bubble surface area at the interface of the froth and the slurry in theseparation tank 14 j that leads some adhered hydrophobic species to fall off and be lost to theunderflow nozzle 18 j. As has been already discussed, the flotation separation system described herein requires a smaller separation tank size than conventional flotation separation systems. As shown inFIGS. 13 and 14 , this allows for severalflotation separation cells 10 j to be easily combined in-series to negate the effects of mixing and hydrophobic species bypass of the bubble dispersion. - The fundamental principle favoring a tank-in-series approach is simple and well known: for an equivalent retention time, a series of perfectly mixed tanks will provide a higher recovery than a single cell. This point is illustrated by the following equation:
-
- where the change in recovery, R, is a function of the number of perfect mixers (N) for a system with a constant process rate (k) and retention time (τ). As shown in
FIG. 15 , increasing the number of mixers in series, at a constant value of kτ, results in an increase in recovery. For example, for a kτ value of 4, changing from one perfectly mixed tank to four cells in series results in an increased recovery of nearly 15%. - This concept can be understood by examining the basic operation of a conventional flotation cell. Each cell contains a mixing element that is used to disperse air and maintain the solids in suspension. As a result, each cell behaves “almost” as a single perfectly mixed tank. By definition, a perfectly mixed tank has an equal concentration of material at any location in the system. Therefore, a portion of the feed material has an opportunity to immediately short circuit to the tailings discharge point. In a system using a single large cell, this would imply a loss in recovery. However, by discharging to a second tank, another opportunity exists to collect the floatable material. Likewise, this is also true with the third and fourth cell in the series. Of course, at some point, the law of diminishing returns applies. In conventional flotation systems, this is typically after four or five cells in series. However, the recovery gain with each cell requires additional energy.
- Based on the same principle, the in-series arrangements shown for example in
FIGS. 13 and 14 reduce the inadvertent bypass of feed slurry from individualflotation separation cells 10 j. In such modular in-series arrangements, the slurry that leaves through theunderflow nozzle 18 j of oneseparation tank 14 j is redirected to thesparger units 12 j of the nextflotation separation cell 10 j. This arrangement increases the particulate recovery from a slurry stream. Theflotation separation cells 10 j can be placed in a modular vertical arrangement (as inFIG. 13 ), a staggered horizontal arrangement (as inFIG. 14 ), or any arrangement that allows for a sufficient hydraulic pressure to convey the slurry from cell to cell. If such a configuration is not possible in the particular application, the slurry could be pumped to each subsequent cell in the series. The number of requiredflotation separation cells 10 j will be dependent on the specific application. - In any of the embodiments herein, it is also possible to divert a portion of the slurry discharge from the
underflow removal port 18 or theoverflow drain 22 back to the initial sparger unit 12 (or thefeed manifold distributor 26 a in flotation separation systems with more than onesparger unit 12 a). This would serve to recycle any chemical additives used to promote frothing and would reduce the materials cost of operation. Similarly, in the embodiments shown inFIGS. 13 and 14 , a portion of the discharge from theunderflow removal port 18 j or the overflow drain (not shown) from the lastflotation separation cell 10 j can be diverted back to thefeed manifold distributor 26 j of the firstflotation separation cell 10 j. - The energy requirements of the flotation separation systems described herein are orders of magnitude lower than conventional flotation separation systems, column flotation separation systems, and packed column flotation separation systems for processing a similar amount of slurry with comparable recovery results. A conventional flotation separation system that processes 3,000 gpm of coal slurry may typically comprise 6-8 separation tanks in series, with each separation tank containing a 20-30 HP motor to turn impellers to mix the slurry in the tanks, for a total of about 200 HP for mechanical agitation. Such a conventional system would require an additional 150 HP to power the air blower system for sparging gas. A typical column flotation separation system that processes 3,000 gpm of coal slurry requires slurry recirculation pumps that could require around 200 HP to operate. An additional 200 HP would be required to operate the air compressors for sparging bubbles. A packed column flotation separation systems of similar 3,000 gpm capacity typically would have similar requirements to a typical column flotation system with about 200 HP for recirculation pumps and about 200 HP for air compressors.
- In contrast, a flotation separation system as described herein for processing 3,000 gpm of coal slurry, comprising three flotation separation cells in series, each cell having a single sparger unit with sparging mechanisms that comprise a series of rotating high shear elements (similar to those shown in
FIG. 11 ) would require significantly less energy. The energy required to power each sparger unit in such a system would be around 20 HP for a total of 60 HP for all three sparger units. The energy required by the gas supply system would be about 70 HP for all three sparger units. Each separation tank in such a configuration would be about 11 feet in diameter and about 6 feet deep. This represents a significant savings in energy consumption and material requirements. - The small footprint required for the
flotation separation cell 10 j suggests that it can be used to relieve the loading on existingconventional flotation cells 85 j as shown for example inFIG. 16A . In such an arrangement, slurry that has been processed in theflotation separation cell 10 j and discharged through theunderflow removal port 18 j is fed to theinlet 86 j of aconventional flotation cell 85 j. Collected froth from the flotation separation cell's 10 j overflow launder 16 j and overflow drain 22 j is combined with product collected from the conventional flotation cell's 85j discharge 87 j. As a significant portion of the hydrophobic species in the slurry has been removed by theflotation separation cell 10 j, the reduced loading to theconventional flotation cell 85 j leads to an overall increase in its performance and an improved overall recovery percentage of the hydrophobic species from flotation separation. - Similarly, as shown in
FIG. 16B , aflotation separation cell 10 j can be located upstream of an existingcolumn flotation cell 88 j. In such an arrangement, slurry that has been processed in theflotation separation cell 10 j and discharged through theunderflow removal port 18 j is fed to theinlet 89j of a conventionalcolumn flotation cell 88 j. Collected froth from the flotation separation cell's 10 j overflow launder 16 j and overflow drain 22 j is combined with product collected from the column flotation cell's 88j discharge 91 j. As a significant portion of the hydrophobic species in the slurry has been removed by theflotation separation cell 10 j, the reduced loading to thecolumn flotation cell 88 j leads to an overall increase in its performance and an improved overall recovery percentage of the hydrophobic species from flotation separation. - The pilot scale test indicated that there would be additional benefit to the flotation separation systems disclosed herein if a center well 90 k were to be incorporated in the
separation tank 14 k, as shown inFIG. 17A . As can be best understood by comparingFIGS. 17A and 17B , the center well 90 k fits around the outside of thesparger unit 12 k and comprises a tube that runs the height of theseparation tank 14 k.Outlets 92 k near the bottom of the center well 90 k allow for the slurry discharged from thesparger unit 12 k to enter theseparation tank 14 k. - The purpose of the center well 90 k is to ensure that the sparger assembly within the center well 90 k remains submerged below the liquid level and to aid in efficient bubble formation and promote efficient bubble/particle interaction. At low flows, the center well 90 k liquid level will be at the same level as that of the surrounding
separation tank 14 k. However, at higher flows, the level within the center well 90 k will be higher than that of the surroundingseparation tank 14 k. The higher level ensures that there is no chance for air to coalesce within thesparger unit 12 k and ultimately reduces burping and inefficient contacting within thesparger unit 12 k. The liquid level in the center well 90 k can be determined by reading a low-pressure pressure gauge (not shown) that is installed on theslurry inlet 38 k. In order to ensure that the center well 90 k stays full, the center well 90 k must be engineered such that it flushes just slightly slower than it fills. Only a positive pressure is required to indicate that the center well 90 k is full. - Level control in the center well can be maintained in several ways as shown in
FIGS. 18A through 18C . As shown inFIG. 18A , the center well 90 l is constructed such that the size of the outlets 92 l can be continuously adjusted. A low-pressure gauge 94 l installed at the slurry inlet 38 l monitors the pressure in sparger unit 12 l. A PID control loop 96 l adjusts the outlet 92 l size in response to changes in the pressure readings—an increase in pressure above a preset limit will trigger the PID control loop 96 l to increase the outlet 92 l size to allow more slurry to leave the sparger unit 12 l and the center well 90 l; a decrease in pressure below a preset limit will trigger the PID control loop 96 l to decrease the outlet 92 l size which will retain more slurry in the center well 90 l and keep the sparger unit 12 l submerged. It was contemplated that direct level control of the level of the separation tank 14 l could be performed by using a PID process controller to throttle the outflow from the underflow nozzle 18 l based on pressure readings in the separation tank 14 l. While this method will ensure a consistent level in the separation tank 14 l, it would not ensure that there is sufficient pressure within the center well 90 l. - A simpler control scheme is shown in
FIG. 18B that negates the need for a control mechanism to be placed within theseparation tank 14 m. In essence, the center well 90 m level is maintained by controlling the flow from the inflow to the flotation separation system by automating a make-upvalve 98 m through aPID control loop 96 m such that a low pressure reading from the low-pressure pressure gauge 94 m triggers additional liquid, and hence flow, to be routed to theseparation cell 10 m. - This method can be easily applied to a series of
separation tanks 10 n, as shown inFIG. 18C . For the next cell in series for flotation separation systems that comprise a series offlotation separation cells 10 n, a secondPID control loop 100 n controls theunderflow nozzle 18 n of theprevious separation cell 10 n in the series. These embodiments require only automation of theunderflow nozzle 18 n as per accepted industrial practice. - Other designs of flotation separation cells are also possible.
FIG. 19 shows a flotation separation cell 10 o in which the slurry enters the sparger units 12 o from underneath the separation tank 14 o. A feed manifold distributor 26 o distributes slurry to each sparger unit 12 o through distributor pipes 28 o to the sparging mechanisms 42 o. Gas is supplied to the sparger units as described above. The electric motors 36 o that power the rotating high-shear element (not shown) via rotating shafts 34 o are located above the separation tank 14 o. The electric motors 36 o are supported in place with a support ring 90 o. Slurry passes up through the sparging mechanism 42 o and into the separation tank 14 o. -
FIG. 20 shows an embodiment of aflotation separation cell 10 p in which thesparger units 12 p are located on the side of theseparation tank 14 p. In this embodiment thefeed manifold distributor 26 p is shown feeding thesparger units 12 p from underneath theseparation tank 14 p. Thefeed manifold distributor 26 p can also be located above theseparation tank 14 p as shown in earlier embodiments. - The underflow removal port 18 q does not need to be located at the bottom of the
flotation separation cell 10 q. The embodiment shown inFIG. 21 shows how the underflow removal port 18 q can remove slurry from the side of theseparation tank 14 q. The underflow removal port 18 q has a right angle bend directed towards the bottom of theseparation tank 14 q to allow for a uniform withdrawal of slurry from the bottom of theseparation tank 14 q. The slurry can be withdrawn from the underflow removal port 18 q by gravity as a drain or with a pump, sand gates, an overflow weir system, or any other appropriate mechanism. - This invention has been described with reference to several preferred embodiments. Many modifications and alterations will occur to others upon reading and understanding the preceding specification. It is intended that the invention be construed as including all such alterations and modifications in so far as they come within the scope of the appended claims or the equivalents of these claims.
Claims (65)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/101,376 US8960443B2 (en) | 2007-04-12 | 2008-04-11 | Flotation separation device and method |
| US14/587,173 US10478830B2 (en) | 2007-04-12 | 2014-12-31 | Flotation separation device and method |
| US16/687,537 US10898904B2 (en) | 2007-04-12 | 2019-11-18 | Flotation separation device |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US91132707P | 2007-04-12 | 2007-04-12 | |
| US12/101,376 US8960443B2 (en) | 2007-04-12 | 2008-04-11 | Flotation separation device and method |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/587,173 Continuation US10478830B2 (en) | 2007-04-12 | 2014-12-31 | Flotation separation device and method |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20080251427A1 true US20080251427A1 (en) | 2008-10-16 |
| US8960443B2 US8960443B2 (en) | 2015-02-24 |
Family
ID=39852744
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/101,376 Active 2029-09-23 US8960443B2 (en) | 2007-04-12 | 2008-04-11 | Flotation separation device and method |
| US14/587,173 Active 2031-10-04 US10478830B2 (en) | 2007-04-12 | 2014-12-31 | Flotation separation device and method |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/587,173 Active 2031-10-04 US10478830B2 (en) | 2007-04-12 | 2014-12-31 | Flotation separation device and method |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US8960443B2 (en) |
| CN (1) | CN101622074B (en) |
| AU (1) | AU2008240254B2 (en) |
| BR (1) | BRPI0810649B1 (en) |
| CA (1) | CA2676776C (en) |
| MX (2) | MX336785B (en) |
| WO (1) | WO2008128044A1 (en) |
| ZA (1) | ZA200907086B (en) |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100122421A1 (en) * | 2008-11-19 | 2010-05-20 | Samsung Electronics Co., Ltd. | Washing machine and control method thereof |
| DE102008062197A1 (en) * | 2008-12-13 | 2010-06-17 | Voith Patent Gmbh | Flotation method for removing impurities from diluted-fibrous suspension in flotation container, involves subjecting stream of fibrous suspension with compressed air jet during or after supply of gas at preset pressure |
| US20120145606A1 (en) * | 2009-06-24 | 2012-06-14 | Siemens Aktiengesellschaft | Pneumatic Flotation Machine and Flotation Method |
| CN103433150A (en) * | 2013-07-25 | 2013-12-11 | 山东煤机装备集团有限公司 | Selective cutting and mixing flotation device |
| WO2014043205A1 (en) * | 2012-09-14 | 2014-03-20 | Valerio Thomas A | System and method for iron ore byproduct processing |
| WO2015057246A1 (en) * | 2013-10-17 | 2015-04-23 | Eriez Manufacturing Co. | Improved air-assisted separation system |
| WO2016100704A1 (en) * | 2014-12-17 | 2016-06-23 | Eriez Manufacturing Co. | Multi-stage fluidized-bed flotation separator |
| WO2018067649A1 (en) * | 2016-10-04 | 2018-04-12 | Cidra Corporate Services Inc. | Hybrid - flotation recovery of mineral bearing ores |
| US20180272360A1 (en) * | 2015-03-16 | 2018-09-27 | Johannes Gideon Andries Swanepoel | Flotation cell |
| WO2019227109A1 (en) * | 2018-05-21 | 2019-11-28 | Mintek | Froth flotation apparatus |
| WO2020025850A1 (en) | 2018-08-01 | 2020-02-06 | Outotec (Finland) Oy | Flotation cell |
| WO2020025852A1 (en) | 2018-08-01 | 2020-02-06 | Outotec (Finland) Oy | Flotation cell |
| WO2020025849A1 (en) | 2018-08-01 | 2020-02-06 | Outotec (Finland) Oy | Flotation cell |
| US20200179948A1 (en) * | 2017-07-04 | 2020-06-11 | Outotec (Finland) Oy | Froth collection launder |
| US10864486B2 (en) * | 2016-01-29 | 2020-12-15 | Richard LADOUCEUR | Rotary gas bubble ejector |
| CN112248189A (en) * | 2020-10-27 | 2021-01-22 | 广州元玛高新材料技术研究有限公司 | Forming method and forming equipment for inorganic artificial stone blocks |
| US10898904B2 (en) * | 2007-04-12 | 2021-01-26 | Eriez Manufacturing Co. | Flotation separation device |
| CN113550367A (en) * | 2021-09-22 | 2021-10-26 | 江苏玖泰电力实业有限公司 | Dredger slurry charging and plugging spray head device |
| EP3829773B1 (en) * | 2018-08-01 | 2024-11-06 | Metso Finland Oy | Flotation cell |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103316777A (en) * | 2013-07-05 | 2013-09-25 | 河南东大矿业股份有限公司 | Method for realizing multi-path transferring of mineralized bubbles of flotation machine |
| CN105344496A (en) * | 2015-11-20 | 2016-02-24 | 上海迈亚投资有限公司 | Digital intelligent test flotation column |
| AU2017331824B2 (en) | 2016-09-21 | 2022-10-27 | 2678380 Ontario Inc. | Method and apparatus for direct recovery of mineral values as a bubble-solids aggregate |
| MX2020009852A (en) * | 2018-03-23 | 2020-10-15 | Smidth As F L | Flotation machine apparatus and method of using the same. |
| EP3885021B1 (en) * | 2020-03-27 | 2024-10-09 | Bauer Resources GmbH | Method and treatment plant for cleaning contaminated material |
| CN113457853B (en) * | 2021-06-23 | 2022-09-16 | 湖南柿竹园有色金属有限责任公司 | Unpowered gas stirring type flotation device and flotation method |
| CN117861870A (en) * | 2024-03-12 | 2024-04-12 | 北矿科技股份有限公司 | Flotation equipment and flotation system |
Citations (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1157176A (en) * | 1914-02-27 | 1915-10-19 | Edward William Culver | Separation of metallic sulfids from ores. |
| US1226062A (en) * | 1916-05-31 | 1917-05-15 | Herbert C Colburn | Ore-flotation process. |
| US1468226A (en) * | 1919-01-03 | 1923-09-18 | Colburn Flotation & Engineerin | Mixing apparatus |
| US2938629A (en) * | 1955-07-28 | 1960-05-31 | Smith Douglass Company Inc | Concentration of comminuted materials |
| US3271293A (en) * | 1963-05-03 | 1966-09-06 | Cities Service Athabasca Inc | Process and apparatus for stripping solids from bituminous sand |
| US3539000A (en) * | 1967-08-05 | 1970-11-10 | Bergwerksverband Gmbh | Classification by flotation |
| US4214982A (en) * | 1977-08-27 | 1980-07-29 | J. M. Voith Gmbh | Process and device for removing printer's ink from a fiber suspension |
| US4226706A (en) * | 1979-08-09 | 1980-10-07 | Envirotech Corporation | Dispersed air flotation machine |
| US4287137A (en) * | 1979-01-08 | 1981-09-01 | Shionogi & Co., Ltd. | Vane-type fluid impeller and method of aerating a liquid |
| US4328107A (en) * | 1980-11-28 | 1982-05-04 | Synergo, Inc. | Process and apparatus for forming dispersions |
| US4399028A (en) * | 1982-06-14 | 1983-08-16 | The Black Clawson Company | Froth flotation apparatus and method |
| US4521349A (en) * | 1983-01-20 | 1985-06-04 | A. R. Wilfley And Sons, Inc. | Fluid diffuser for gases and liquids |
| US4545892A (en) * | 1985-04-15 | 1985-10-08 | Alberta Energy Company Ltd. | Treatment of primary tailings and middlings from the hot water extraction process for recovering bitumen from tar sand |
| US4639313A (en) * | 1985-07-05 | 1987-01-27 | The Deister Concentrator Company | Floatation apparatus for concentration of minerals from high water content aqueous slurries |
| US4674888A (en) * | 1984-05-06 | 1987-06-23 | Komax Systems, Inc. | Gaseous injector for mixing apparatus |
| US4752383A (en) * | 1986-08-05 | 1988-06-21 | The United States Of America As Represented By The Secretary Of The Interior | Bubble generator |
| US4783268A (en) * | 1987-12-28 | 1988-11-08 | Alberta Energy Company, Ltd. | Microbubble flotation process for the separation of bitumen from an oil sands slurry |
| US4871448A (en) * | 1988-06-14 | 1989-10-03 | Gosudarstvenny Proektno-Konstruktorsky I Experimentalny Institut Po Obogatitelnomu Oborudovaniju | Mechanical flotation machine |
| US4938865A (en) * | 1986-09-25 | 1990-07-03 | University Of Newcastle Research Assoc., Ltd. | Column flotation method and apparatus |
| US4940534A (en) * | 1989-07-20 | 1990-07-10 | J. M. Huber Corporation | Froth flotation column |
| US4971685A (en) * | 1989-04-11 | 1990-11-20 | The United States Of America As Represented By The Secretary Of The Interior | Bubble injected hydrocyclone flotation cell |
| US4981582A (en) * | 1988-01-27 | 1991-01-01 | Virginia Tech Intellectual Properties, Inc. | Process and apparatus for separating fine particles by microbubble flotation together with a process and apparatus for generation of microbubbles |
| US4997549A (en) * | 1989-09-19 | 1991-03-05 | Advanced Processing Technologies, Inc. | Air-sparged hydrocyclone separator |
| US5028315A (en) * | 1989-12-11 | 1991-07-02 | The Black Clawson Company | Froth flotation apparatus and method |
| US5096572A (en) * | 1990-03-12 | 1992-03-17 | Board Of Control Of Michigan Tech. University | Froth flotation |
| US5122261A (en) * | 1990-09-26 | 1992-06-16 | Hollingsworth Clinton A | Concentration of minerals |
| US5167798A (en) * | 1988-01-27 | 1992-12-01 | Virginia Tech Intellectual Properties, Inc. | Apparatus and process for the separation of hydrophobic and hydrophilic particles using microbubble column flotation together with a process and apparatus for generation of microbubbles |
| US5188726A (en) * | 1989-07-26 | 1993-02-23 | University Of Newcastle Research Associates Ltd. | Method of operating a plurality of minerals separation flotation cells |
| US5294003A (en) * | 1990-09-26 | 1994-03-15 | Hollingsworth Clinton A | Process for concentration of minerals |
| JPH06154656A (en) * | 1992-11-20 | 1994-06-03 | Hirobumi Onari | Floating separator for suspended matter in liquid |
| US5460270A (en) * | 1993-08-20 | 1995-10-24 | Alberta Energy Company Ltd. | Oil sand extraction process with in-line middlings aeration and recycle |
| US5520818A (en) * | 1989-12-06 | 1996-05-28 | The University Of Toronto Innovations Foundation | Method for effecting gas-liquid contact |
| US5591328A (en) * | 1990-11-23 | 1997-01-07 | Atomaer Pty. Ltd. | Gas particle formation |
| US5667018A (en) * | 1996-01-02 | 1997-09-16 | Fmc Corporation | Fire control foam distribution system for use in distributing foam beneath a passenger boarding bridge |
| US5672267A (en) * | 1995-06-06 | 1997-09-30 | Multotec Cyclones (Pty) Limited | Flotation column with constant feed arrangement |
| US5676823A (en) * | 1996-03-07 | 1997-10-14 | Baker Hughes Incorporated | Sparger system including jet stream aerator |
| US5693263A (en) * | 1995-04-26 | 1997-12-02 | Cominco Engineering Services Ltd. | Sparger for producing gas bubbles in a liquid |
| US5702612A (en) * | 1995-07-20 | 1997-12-30 | University Of Kentucky Research Foundation | Method and apparatus for flotation separation |
| US5855769A (en) * | 1994-02-14 | 1999-01-05 | Commonwealth Scientific And Industrial Research Organisation | Apparatus and method for selective separation of hydrophobic material |
| US5928125A (en) * | 1997-06-09 | 1999-07-27 | Inter-Citic Envirotec, Inc. | Centrifugal flotation cell with rotating drum |
| US6004386A (en) * | 1995-06-21 | 1999-12-21 | Revtech Industries, Inc. | Apparatus for creating gas-liquid interfacial contact conditions for highly efficient mass transfer |
| US6092667A (en) * | 1997-12-09 | 2000-07-25 | Multotec Process Equipment Limited | Method and apparatus for aeration of liquids or slurries |
| US6126836A (en) * | 1997-06-09 | 2000-10-03 | Inter-Citic Mineral Technologies, Inc. | Centrifugal flotation cell with rotating feed |
| US20020000412A1 (en) * | 2000-03-10 | 2002-01-03 | Craddock Mark Norman Paul | Apparatus and process for recovering a desired fraction of a raw material |
| US6372140B2 (en) * | 1998-10-15 | 2002-04-16 | Red Valve Co., Inc. | Diffused aeration method |
| US20040150121A1 (en) * | 2003-02-05 | 2004-08-05 | Armstrong Richard James | Gas entrainer |
| US6793079B2 (en) * | 2002-11-27 | 2004-09-21 | University Of Illinois | Method and apparatus for froth flotation |
| US20040245151A1 (en) * | 2001-10-04 | 2004-12-09 | Lilja Launo Leo | Flotation mechanism and cell |
| US20050284818A1 (en) * | 2004-06-28 | 2005-12-29 | Patterson Stanley A | Column flotation cell for enhanced recovery of minerals such as phosphates by froth flotation |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3650510A (en) * | 1970-04-03 | 1972-03-21 | Denver Brick & Pipe Co | Mixing and aerating apparatus for plastics |
| US4031006A (en) * | 1976-03-12 | 1977-06-21 | Swift And Company Limited | Vortex coagulation means and method for wastewater clarification |
| DE3606747A1 (en) | 1986-03-01 | 1987-09-03 | Kloeckner Humboldt Deutz Ag | Apparatus for introducing gas into liquids |
| DE3634903A1 (en) | 1986-10-14 | 1988-04-28 | Voith Gmbh J M | Flotation device |
| DE3808154A1 (en) | 1988-03-11 | 1989-09-21 | Voith Gmbh J M | Injector having bubble divider |
| US5660718A (en) | 1993-02-10 | 1997-08-26 | M.D. Research Company Pty, Ltd. | Method and apparatus for separation by flotation |
| AUPP584698A0 (en) * | 1998-09-11 | 1998-10-08 | Jameson, Graeme John | Internal recycle apparatus and process for flotation column cells |
| AUPQ563800A0 (en) * | 2000-02-15 | 2000-03-09 | University Of Newcastle Research Associates Limited, The | Improved froth flotation process and apparatus |
| WO2001062392A1 (en) | 2000-02-23 | 2001-08-30 | Baker Hughes Incorporated | Sparging nozzle assembly for aerated reaction vessels and method for operating such vessels |
| AU2003901207A0 (en) * | 2003-03-17 | 2003-04-03 | Outokumpu Oyj | Auxiliary agitator for a floatation device |
| FI122973B (en) * | 2005-06-17 | 2012-09-28 | Metso Paper Inc | Injector for flotation cell, nozzle part in injector for flotation cell, flotation cell and method for mixing fiber suspension strip and air with each other in injector for flotation cell |
-
2008
- 2008-04-11 MX MX2013004523A patent/MX336785B/en unknown
- 2008-04-11 US US12/101,376 patent/US8960443B2/en active Active
- 2008-04-11 MX MX2009009309A patent/MX2009009309A/en active IP Right Grant
- 2008-04-11 WO PCT/US2008/060035 patent/WO2008128044A1/en active Application Filing
- 2008-04-11 CA CA2676776A patent/CA2676776C/en active Active
- 2008-04-11 CN CN200880006174.1A patent/CN101622074B/en active Active
- 2008-04-11 BR BRPI0810649A patent/BRPI0810649B1/en active IP Right Grant
- 2008-04-11 AU AU2008240254A patent/AU2008240254B2/en active Active
-
2009
- 2009-10-12 ZA ZA200907086A patent/ZA200907086B/en unknown
-
2014
- 2014-12-31 US US14/587,173 patent/US10478830B2/en active Active
Patent Citations (50)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1157176A (en) * | 1914-02-27 | 1915-10-19 | Edward William Culver | Separation of metallic sulfids from ores. |
| US1226062A (en) * | 1916-05-31 | 1917-05-15 | Herbert C Colburn | Ore-flotation process. |
| US1468226A (en) * | 1919-01-03 | 1923-09-18 | Colburn Flotation & Engineerin | Mixing apparatus |
| US2938629A (en) * | 1955-07-28 | 1960-05-31 | Smith Douglass Company Inc | Concentration of comminuted materials |
| US3271293A (en) * | 1963-05-03 | 1966-09-06 | Cities Service Athabasca Inc | Process and apparatus for stripping solids from bituminous sand |
| US3539000A (en) * | 1967-08-05 | 1970-11-10 | Bergwerksverband Gmbh | Classification by flotation |
| US4214982A (en) * | 1977-08-27 | 1980-07-29 | J. M. Voith Gmbh | Process and device for removing printer's ink from a fiber suspension |
| US4287137A (en) * | 1979-01-08 | 1981-09-01 | Shionogi & Co., Ltd. | Vane-type fluid impeller and method of aerating a liquid |
| US4226706A (en) * | 1979-08-09 | 1980-10-07 | Envirotech Corporation | Dispersed air flotation machine |
| US4328107A (en) * | 1980-11-28 | 1982-05-04 | Synergo, Inc. | Process and apparatus for forming dispersions |
| US4399028A (en) * | 1982-06-14 | 1983-08-16 | The Black Clawson Company | Froth flotation apparatus and method |
| US4521349A (en) * | 1983-01-20 | 1985-06-04 | A. R. Wilfley And Sons, Inc. | Fluid diffuser for gases and liquids |
| US4674888A (en) * | 1984-05-06 | 1987-06-23 | Komax Systems, Inc. | Gaseous injector for mixing apparatus |
| US4545892A (en) * | 1985-04-15 | 1985-10-08 | Alberta Energy Company Ltd. | Treatment of primary tailings and middlings from the hot water extraction process for recovering bitumen from tar sand |
| US4639313A (en) * | 1985-07-05 | 1987-01-27 | The Deister Concentrator Company | Floatation apparatus for concentration of minerals from high water content aqueous slurries |
| US4752383A (en) * | 1986-08-05 | 1988-06-21 | The United States Of America As Represented By The Secretary Of The Interior | Bubble generator |
| US4938865A (en) * | 1986-09-25 | 1990-07-03 | University Of Newcastle Research Assoc., Ltd. | Column flotation method and apparatus |
| US4783268A (en) * | 1987-12-28 | 1988-11-08 | Alberta Energy Company, Ltd. | Microbubble flotation process for the separation of bitumen from an oil sands slurry |
| US4981582A (en) * | 1988-01-27 | 1991-01-01 | Virginia Tech Intellectual Properties, Inc. | Process and apparatus for separating fine particles by microbubble flotation together with a process and apparatus for generation of microbubbles |
| US5167798A (en) * | 1988-01-27 | 1992-12-01 | Virginia Tech Intellectual Properties, Inc. | Apparatus and process for the separation of hydrophobic and hydrophilic particles using microbubble column flotation together with a process and apparatus for generation of microbubbles |
| US4871448A (en) * | 1988-06-14 | 1989-10-03 | Gosudarstvenny Proektno-Konstruktorsky I Experimentalny Institut Po Obogatitelnomu Oborudovaniju | Mechanical flotation machine |
| US4971685A (en) * | 1989-04-11 | 1990-11-20 | The United States Of America As Represented By The Secretary Of The Interior | Bubble injected hydrocyclone flotation cell |
| US4940534A (en) * | 1989-07-20 | 1990-07-10 | J. M. Huber Corporation | Froth flotation column |
| US5188726A (en) * | 1989-07-26 | 1993-02-23 | University Of Newcastle Research Associates Ltd. | Method of operating a plurality of minerals separation flotation cells |
| US4997549A (en) * | 1989-09-19 | 1991-03-05 | Advanced Processing Technologies, Inc. | Air-sparged hydrocyclone separator |
| US5520818A (en) * | 1989-12-06 | 1996-05-28 | The University Of Toronto Innovations Foundation | Method for effecting gas-liquid contact |
| US5028315A (en) * | 1989-12-11 | 1991-07-02 | The Black Clawson Company | Froth flotation apparatus and method |
| US5096572A (en) * | 1990-03-12 | 1992-03-17 | Board Of Control Of Michigan Tech. University | Froth flotation |
| US5122261A (en) * | 1990-09-26 | 1992-06-16 | Hollingsworth Clinton A | Concentration of minerals |
| US5294003A (en) * | 1990-09-26 | 1994-03-15 | Hollingsworth Clinton A | Process for concentration of minerals |
| US5591328A (en) * | 1990-11-23 | 1997-01-07 | Atomaer Pty. Ltd. | Gas particle formation |
| JPH06154656A (en) * | 1992-11-20 | 1994-06-03 | Hirobumi Onari | Floating separator for suspended matter in liquid |
| US5460270A (en) * | 1993-08-20 | 1995-10-24 | Alberta Energy Company Ltd. | Oil sand extraction process with in-line middlings aeration and recycle |
| US5855769A (en) * | 1994-02-14 | 1999-01-05 | Commonwealth Scientific And Industrial Research Organisation | Apparatus and method for selective separation of hydrophobic material |
| US5693263A (en) * | 1995-04-26 | 1997-12-02 | Cominco Engineering Services Ltd. | Sparger for producing gas bubbles in a liquid |
| US5672267A (en) * | 1995-06-06 | 1997-09-30 | Multotec Cyclones (Pty) Limited | Flotation column with constant feed arrangement |
| US6004386A (en) * | 1995-06-21 | 1999-12-21 | Revtech Industries, Inc. | Apparatus for creating gas-liquid interfacial contact conditions for highly efficient mass transfer |
| US5702612A (en) * | 1995-07-20 | 1997-12-30 | University Of Kentucky Research Foundation | Method and apparatus for flotation separation |
| US5667018A (en) * | 1996-01-02 | 1997-09-16 | Fmc Corporation | Fire control foam distribution system for use in distributing foam beneath a passenger boarding bridge |
| US5676823A (en) * | 1996-03-07 | 1997-10-14 | Baker Hughes Incorporated | Sparger system including jet stream aerator |
| US5928125A (en) * | 1997-06-09 | 1999-07-27 | Inter-Citic Envirotec, Inc. | Centrifugal flotation cell with rotating drum |
| US6126836A (en) * | 1997-06-09 | 2000-10-03 | Inter-Citic Mineral Technologies, Inc. | Centrifugal flotation cell with rotating feed |
| US6092667A (en) * | 1997-12-09 | 2000-07-25 | Multotec Process Equipment Limited | Method and apparatus for aeration of liquids or slurries |
| US6372140B2 (en) * | 1998-10-15 | 2002-04-16 | Red Valve Co., Inc. | Diffused aeration method |
| US20020000412A1 (en) * | 2000-03-10 | 2002-01-03 | Craddock Mark Norman Paul | Apparatus and process for recovering a desired fraction of a raw material |
| US20040245151A1 (en) * | 2001-10-04 | 2004-12-09 | Lilja Launo Leo | Flotation mechanism and cell |
| US6793079B2 (en) * | 2002-11-27 | 2004-09-21 | University Of Illinois | Method and apparatus for froth flotation |
| US20050051465A1 (en) * | 2002-11-27 | 2005-03-10 | Khan Latif A. | Method for froth flotation |
| US20040150121A1 (en) * | 2003-02-05 | 2004-08-05 | Armstrong Richard James | Gas entrainer |
| US20050284818A1 (en) * | 2004-06-28 | 2005-12-29 | Patterson Stanley A | Column flotation cell for enhanced recovery of minerals such as phosphates by froth flotation |
Cited By (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10898904B2 (en) * | 2007-04-12 | 2021-01-26 | Eriez Manufacturing Co. | Flotation separation device |
| US8695139B2 (en) * | 2008-11-19 | 2014-04-15 | Samsung Electronics Co., Ltd. | Washing machine including a bubble generator and control method thereof |
| US20100122421A1 (en) * | 2008-11-19 | 2010-05-20 | Samsung Electronics Co., Ltd. | Washing machine and control method thereof |
| DE102008062197A1 (en) * | 2008-12-13 | 2010-06-17 | Voith Patent Gmbh | Flotation method for removing impurities from diluted-fibrous suspension in flotation container, involves subjecting stream of fibrous suspension with compressed air jet during or after supply of gas at preset pressure |
| US20120145606A1 (en) * | 2009-06-24 | 2012-06-14 | Siemens Aktiengesellschaft | Pneumatic Flotation Machine and Flotation Method |
| US8678193B2 (en) * | 2009-06-24 | 2014-03-25 | Siemens Aktiengesellschaft | Pneumatic flotation machine and flotation method |
| WO2014043205A1 (en) * | 2012-09-14 | 2014-03-20 | Valerio Thomas A | System and method for iron ore byproduct processing |
| CN103433150A (en) * | 2013-07-25 | 2013-12-11 | 山东煤机装备集团有限公司 | Selective cutting and mixing flotation device |
| WO2015057246A1 (en) * | 2013-10-17 | 2015-04-23 | Eriez Manufacturing Co. | Improved air-assisted separation system |
| US10994284B2 (en) * | 2014-12-17 | 2021-05-04 | Eriez Manufacturing Co. | Multi-stage fluidized-bed flotation separator |
| US20180050346A1 (en) * | 2014-12-17 | 2018-02-22 | Eriez Manufacturing Co. | Multi-Stage Fluidized-Bed Flotation Separator |
| CN107206394A (en) * | 2014-12-17 | 2017-09-26 | 埃里埃兹制造公司 | Multistage fluidized bed flotation separator |
| WO2016100704A1 (en) * | 2014-12-17 | 2016-06-23 | Eriez Manufacturing Co. | Multi-stage fluidized-bed flotation separator |
| AU2015364496B2 (en) * | 2014-12-17 | 2019-06-27 | Eriez Manufacturing Co. | Multi-stage fluidized-bed flotation separator |
| RU2693791C2 (en) * | 2014-12-17 | 2019-07-04 | Эриез Мануфэкчуринг Ко. | Multi-stage fluidized-bed flotation separator |
| US10828648B2 (en) * | 2015-03-16 | 2020-11-10 | Johannes Gideon Andries Swanepoel | Flotation cell |
| AU2016231908B2 (en) * | 2015-03-16 | 2020-09-03 | Johannes Gideon Andries Swanepoel | Flotation cell |
| US20180272360A1 (en) * | 2015-03-16 | 2018-09-27 | Johannes Gideon Andries Swanepoel | Flotation cell |
| US10864486B2 (en) * | 2016-01-29 | 2020-12-15 | Richard LADOUCEUR | Rotary gas bubble ejector |
| US10974257B2 (en) | 2016-10-04 | 2021-04-13 | Cidra Corporate Services Llc | Hybrid-flotation recovery of mineral bearing ores |
| WO2018067649A1 (en) * | 2016-10-04 | 2018-04-12 | Cidra Corporate Services Inc. | Hybrid - flotation recovery of mineral bearing ores |
| US20200179948A1 (en) * | 2017-07-04 | 2020-06-11 | Outotec (Finland) Oy | Froth collection launder |
| US10828647B2 (en) * | 2017-07-04 | 2020-11-10 | Outotec (Finland) Oy | Froth collection launder |
| WO2019227109A1 (en) * | 2018-05-21 | 2019-11-28 | Mintek | Froth flotation apparatus |
| US12023687B2 (en) | 2018-05-21 | 2024-07-02 | Mintek | Froth flotation apparatus |
| AU2019273043B2 (en) * | 2018-05-21 | 2024-05-23 | Mintek | Froth flotation apparatus |
| WO2020025850A1 (en) | 2018-08-01 | 2020-02-06 | Outotec (Finland) Oy | Flotation cell |
| US20210323002A1 (en) * | 2018-08-01 | 2021-10-21 | Metso Outotec Finland Oy | Flotation Cell |
| EP3829776A4 (en) * | 2018-08-01 | 2022-04-13 | Metso Outotec Finland Oy | Flotation cell |
| EP3829777A4 (en) * | 2018-08-01 | 2022-04-13 | Metso Outotec Finland Oy | Flotation cell |
| EP3829774A4 (en) * | 2018-08-01 | 2022-04-13 | Metso Outotec Finland Oy | Flotation cell |
| WO2020025849A1 (en) | 2018-08-01 | 2020-02-06 | Outotec (Finland) Oy | Flotation cell |
| WO2020025852A1 (en) | 2018-08-01 | 2020-02-06 | Outotec (Finland) Oy | Flotation cell |
| EP3829773B1 (en) * | 2018-08-01 | 2024-11-06 | Metso Finland Oy | Flotation cell |
| CN112248189A (en) * | 2020-10-27 | 2021-01-22 | 广州元玛高新材料技术研究有限公司 | Forming method and forming equipment for inorganic artificial stone blocks |
| CN113550367A (en) * | 2021-09-22 | 2021-10-26 | 江苏玖泰电力实业有限公司 | Dredger slurry charging and plugging spray head device |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2008240254B2 (en) | 2012-11-08 |
| WO2008128044A1 (en) | 2008-10-23 |
| BRPI0810649B1 (en) | 2019-01-29 |
| ZA200907086B (en) | 2010-07-28 |
| CA2676776C (en) | 2015-03-31 |
| US8960443B2 (en) | 2015-02-24 |
| BRPI0810649A2 (en) | 2014-11-04 |
| US20150108044A1 (en) | 2015-04-23 |
| MX2009009309A (en) | 2010-07-29 |
| CA2676776A1 (en) | 2008-10-23 |
| AU2008240254A1 (en) | 2008-10-23 |
| CN101622074B (en) | 2014-10-22 |
| CN101622074A (en) | 2010-01-06 |
| MX336785B (en) | 2016-02-02 |
| US10478830B2 (en) | 2019-11-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10478830B2 (en) | Flotation separation device and method | |
| AU596924B2 (en) | Improved froth flotation method and apparatus | |
| US7624877B2 (en) | Separate size flotation device | |
| US20160089679A1 (en) | Automated system of froth flotation columns with aerators injection nozzles and process thereof | |
| EP3038757B1 (en) | Method and apparatus for treating a feed stream for a flotation device | |
| SE469877B (en) | flotation | |
| EA039769B1 (en) | Flotation line | |
| CA2518853C (en) | Auxiliary agitator for a flotation device | |
| US20210308695A1 (en) | Flotation line | |
| US10898904B2 (en) | Flotation separation device | |
| CA2117620C (en) | Membrane washing apparatus for flotation device | |
| US4226706A (en) | Dispersed air flotation machine | |
| US10882057B2 (en) | Apparatus for direct recovery of mineral values as a bubble-solids aggregate | |
| KR200494191Y1 (en) | Bubble Generator for Water Treatment System | |
| FI115447B (en) | Flotation plant and method | |
| CA2462740C (en) | Method for froth flotation | |
| AU2004222669B2 (en) | A separate size flotation device | |
| CA2371840A1 (en) | Agitated counter current flotation apparatus | |
| GB1582193A (en) | Dispersed air flotation machine | |
| WO2019008216A1 (en) | A froth flotation method and a froth flotation arrangement | |
| PL162937B1 (en) | Pneumatic flotator |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: ERIEZ MANUFACTURING CO., PENNSYLVANIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:MANKOSA, MICHAEL J.;KOHMUENCH, JAISEN;YAN, ERIC S.;AND OTHERS;REEL/FRAME:020789/0840 Effective date: 20080411 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 4TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1551) Year of fee payment: 4 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 8TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1552); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Year of fee payment: 8 |