US20040029908A1 - Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same - Google Patents
Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same Download PDFInfo
- Publication number
- US20040029908A1 US20040029908A1 US10/426,659 US42665903A US2004029908A1 US 20040029908 A1 US20040029908 A1 US 20040029908A1 US 42665903 A US42665903 A US 42665903A US 2004029908 A1 US2004029908 A1 US 2004029908A1
- Authority
- US
- United States
- Prior art keywords
- compound
- hydroxy
- substance
- salt
- formula
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [1*]C1CCC(/C=C(\C)C2OC(=O)C3CCCN3C(=O)C(=O)C3(O)OC(C(OC)CC(C)C/C(C)=C/C([3*])C(=O)cc(C)C2C)C(OC)CC3C)CC1OC Chemical compound [1*]C1CCC(/C=C(\C)C2OC(=O)C3CCCN3C(=O)C(=O)C3(O)OC(C(OC)CC(C)C/C(C)=C/C([3*])C(=O)cc(C)C2C)C(OC)CC3C)CC1OC 0.000 description 27
- AOPMJTXVTVQAGD-AMASXYNMSA-N C=CCC1/C=C(\C)CC(C)CC(OC)C2OC(O)(C(=O)C(=O)N3CCCC3C(=O)OC(/C(C)=C/C3CCC(O)C(OC)C3)C(C)C(O)CC1=O)C(C)CC2OC Chemical compound C=CCC1/C=C(\C)CC(C)CC(OC)C2OC(O)(C(=O)C(=O)N3CCCC3C(=O)OC(/C(C)=C/C3CCC(O)C(OC)C3)C(C)C(O)CC1=O)C(C)CC2OC AOPMJTXVTVQAGD-AMASXYNMSA-N 0.000 description 1
- QJJXYPPXXYFBGM-FWWCNTFXSA-N C=CCC1/C=C(\C)CC(C)CC(OC)C2OC(O)(C(=O)C(=O)N3CCCCC3C(=O)OC(/C(C)=C/C3CCC(O)C(OC)C3)C(C)C(O)CC1=O)C(C)CC2OC Chemical compound C=CCC1/C=C(\C)CC(C)CC(OC)C2OC(O)(C(=O)C(=O)N3CCCCC3C(=O)OC(/C(C)=C/C3CCC(O)C(OC)C3)C(C)C(O)CC1=O)C(C)CC2OC QJJXYPPXXYFBGM-FWWCNTFXSA-N 0.000 description 1
- ZDQSOHOQTUFQEM-AMASXYNMSA-N CCC1/C=C(\C)CC(C)CC(OC)C2OC(O)(C(=O)C(=O)N3CCCCC3C(=O)OC(/C(C)=C/C3CCC(O)C(OC)C3)C(C)C(O)CC1=O)C(C)CC2OC Chemical compound CCC1/C=C(\C)CC(C)CC(OC)C2OC(O)(C(=O)C(=O)N3CCCCC3C(=O)OC(/C(C)=C/C3CCC(O)C(OC)C3)C(C)C(O)CC1=O)C(C)CC2OC ZDQSOHOQTUFQEM-AMASXYNMSA-N 0.000 description 1
- UBQBKOBGHRTZRG-WAFMTNMZSA-N COC1CC(/C=C(\C)C2OC(=O)C3CCCCN3C(=O)C(=O)C3(O)OC(C(OC)CC(C)C/C(C)=C/C(C)C(=O)CC(O)C2C)C(OC)CC3C)CCC1O Chemical compound COC1CC(/C=C(\C)C2OC(=O)C3CCCCN3C(=O)C(=O)C3(O)OC(C(OC)CC(C)C/C(C)=C/C(C)C(=O)CC(O)C2C)C(OC)CC3C)CCC1O UBQBKOBGHRTZRG-WAFMTNMZSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H17/00—Compounds containing heterocyclic radicals directly attached to hetero atoms of saccharide radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/12—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains three hetero rings
- C07D498/18—Bridged systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/01—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing oxygen
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/20—Bacteria; Culture media therefor
- C12N1/205—Bacterial isolates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P17/00—Preparation of heterocyclic carbon compounds with only O, N, S, Se or Te as ring hetero atoms
- C12P17/18—Preparation of heterocyclic carbon compounds with only O, N, S, Se or Te as ring hetero atoms containing at least two hetero rings condensed among themselves or condensed with a common carbocyclic ring system, e.g. rifamycin
- C12P17/188—Heterocyclic compound containing in the condensed system at least one hetero ring having nitrogen atoms and oxygen atoms as the only ring heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P19/00—Preparation of compounds containing saccharide radicals
- C12P19/44—Preparation of O-glycosides, e.g. glucosides
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12R—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES C12C - C12Q, RELATING TO MICROORGANISMS
- C12R2001/00—Microorganisms ; Processes using microorganisms
- C12R2001/01—Bacteria or Actinomycetales ; using bacteria or Actinomycetales
- C12R2001/465—Streptomyces
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12R—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES C12C - C12Q, RELATING TO MICROORGANISMS
- C12R2001/00—Microorganisms ; Processes using microorganisms
- C12R2001/01—Bacteria or Actinomycetales ; using bacteria or Actinomycetales
- C12R2001/465—Streptomyces
- C12R2001/55—Streptomyces hygroscopicus
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S435/00—Chemistry: molecular biology and microbiology
- Y10S435/8215—Microorganisms
- Y10S435/822—Microorganisms using bacteria or actinomycetales
- Y10S435/886—Streptomyces
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S435/00—Chemistry: molecular biology and microbiology
- Y10S435/8215—Microorganisms
- Y10S435/822—Microorganisms using bacteria or actinomycetales
- Y10S435/886—Streptomyces
- Y10S435/898—Streptomyces hygroscopicus
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Oncology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Virology (AREA)
- Biomedical Technology (AREA)
- Communicable Diseases (AREA)
- Transplantation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Saccharide Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
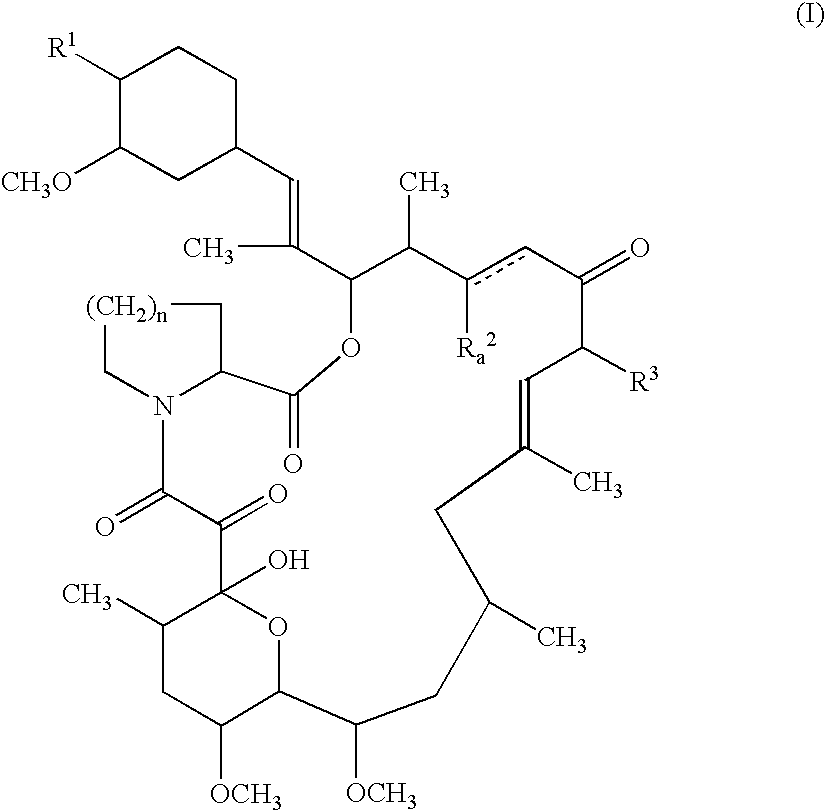
This invention relates to tricyclo compounds useful for treatment and prevention of resistance by transplanation, graft-versus-host diseases by medulla ossium transplanation, autoimmune diseases, infectious diseases, and the like, which can be represented by the formula:
to a process for their production, to a pharmaceutical composition containing the same and to a use thereof.
Description
- This invention relates to novel tricyclo compounds having pharmacological activities, to a process for their production and to a pharmaceutical composition containing the same.
- More particularly, it relates to novel tricyclo compounds, which have pharmacological activities such as immunosuppressive activity, antimicrobial activity, and the like, to a process for their production, to a pharmaceutical composition containing the same and to a use thereof.
- Accordingly, one object of this invention is to provide a novel tricyclo compounds, which are useful for treatment and prevention of resistance by transplantation, graft-versus-host diseases by medulla ossium transplantation, autoimmune diseases, infectious diseases, and the like.
- Another object of this invention is to provide a process for production of the tricyclo compounds by fermentation processes and synthetic processes.
- A further object of this invention is to provide a pharmaceutical composition containing, as active ingredients, the tricyclo compounds.
- Still further object of this invention is to provide a use of the tricyclo compounds for manufacturing a medicament for treating and preventing resistance by transplantation, graft-versus-host diseases by medulla ossium transplantation, autoimmune diseases, infectious diseases, and the like.
- With respect to the present invention, it is to be noted that this invention is originated from and based on the first and new discovery of new certain specific compounds, FR-900506, FR-900520, FR-900523 and FR-900525 substances. In more detail, the FR-900506, FR-900520, FR-900523 and FR-900525 substances were firstly and new isolated in pure form from culture broths obtained by fermentation of new species belonging to genus Streptomyces.
- And, as a result of an extensive study for elucidation of chemical structures of the FR-900506, FR-900520, FR-900523 and FR-900525 substances, the inventors of this invention have succeeded in determining the chemical structures thereof and in producing the tricyclo compounds of this invention.
-
- wherein R 1 is hydroxy or protected hydroxy, R2 is hydrogen, hydroxy or protected hydroxy, R3 is methyl, ethyl, propyl or allyl, n is an integer of 1 or 2, and the symbol of a line and dotted line is a single bond or a double bond, and salts thereof.
- Among the object compound (I) , the following four specific compounds were found to be produced by fermentation. (1) The compound (I) wherein R 1 and R2 are each hydroxy, R3 is allyl, n is an integer of 2, and the symbol of a line and dotted line is a single bond, which is entitled to the FR-900506 substance;
- (2) The compound (I) wherein R 1 and R2 are each hydroxy, R3 is ethyl, n is an integer of 2, and the symbol of a line and dotted line is a single bond, which is entitled to the FR-900520 substance (another name: the WS 7238A substance);
- (3) The compound (I) wherein R 1 and R2 are each hydroxy, R3 is methyl, n is an integer of 2, and the symbol of a line and dotted line is a single bond, which is entitled to the FR-900523 substance (another name: the WS 7238B substance); and
- (4) The compound (I) wherein R 1 and R2 are each hydroxy, R3 is allyl, n is an integer of 1, and the symbol of a line and dotted line is a single bond, which is entitled to the FR-900525 substance.
- With respect to the tricyclo compounds (I) of this invention, it is to be understood that there may be one or more conformer(s) or stereoisomeric pairs such as optical and geometrical isomers due to asymmetric carbon atom(s) and double bond(s) , and such isomers are also included within a scope of this invention.
- According to this invention, the object tricyclo compounds (I) can be prepared by the following processes.
-
- [II] Synthetic Processes:
-
-
-
-
-
-
- in which R 1, R2, R3, n and the symbol of a line and dotted line are each as defined above, Ra 1 and Ra 2 are each protected hydroxy, Rb −is protected carboxy(lower)alkyl-carbamoyloxy, Rc 1 is carboxy(lower)alkylcarbamoyloxy, and Rb −is a leaving group.
- Particulars of the above definitions and the preferred embodiments thereof are explained in detail as follows.
- The term “lower” used in the specification is intended to mean 1 to 6 carbon atoms, unless otherwise indicated.
- Suitable hydroxy-protective group in the “protected hydroxy” may include:
- 1-(lower alkylthio) (lower, alkyl such as lower alkylthiomethyl (e.g. methylthiomethyl, ethylthiomethyl, propylthiomethyl, isopropylthiomethyl, butylthiomethyl, isobutylthiomethyl, hexylthiomethyl, etc.), and the like, in which the preferred one may be C 1-C4alkylthiomethyl and the most preferred one may be methylthiomethyl;
- trisubstituted silyl such as tri(lower)alkylsilyl (e.g. trimethylsilyl, triethylsilyl, tributylsilyl, tert-butyl-dimethylsilyl, tri-tert-butylsilyl, etc.), lower alkyl-diarylsilyl (e.g. methyl-diphenylsilyl, ethyl-diphenylsilyl, propyl-diphenylsilyl, tert-butyl-diphenylsilyl, etc.), and the like, in which the preferred one may be tri(C 1-C4)alkylsilyl and C1-C4 alkyl-diphenylsilyl, and the most preferred one may be tert-butyl-dimethylsilyl and tert-butyl-diphenylsilyl;
- acyl such as aliphatic acyl, aromatic acyl and aliphatic acyl substituted with aromatic group, which are derived from carboxylic, sulfonic and carbamic acids; and the like.
- The aliphatic acyl may include lower alkanoyl which may have one or more suitable substituent(s) such as carboxy (e.g. formyl, acetyl, propionyl, butyryl, isobutyryl, valeryl, isovaleryl, pivaloyl, hexanoyl, carboxvacetyl, carboxyoropionyl, carboxybutyryl, carboxyhexanoyl, etc.), cyclo (lower)alkyloxy (lower) alkanoyl which may have one or more suitable substituent(s) such as lower alkyl (e.g. cyclopropyloxyacetyl, cyclobutyloxypropionyl, cycloheptyl-oxybutyryl, menthyloxyaceyl, menthyloxypropionyl, menthyloxybutyryl, menthyloxyheptanoyl, menthyloxyhexanoyl, etc.), camphorsulfonyl, lower alkylcarbamoyl having one or more suitable substituent(s) such as carboxy and a protected carboxy, for example, carboxy(lower)alkylcarbamoyl (e.g. carboxymethylcarbamoyl, carboxyethylcarbamoyl, carboxy-propylcarbamoyl, carboxybutylcarbamoyl, carboxypentyl- carbamoyl, carboxyhexylcarbamoyl, etc.), protected carboxy(lower)alkylcarbamoyl such as tri(lower)alkyl-silyl (lower) alkoxycarbonyl(lower)alkylcarbamoyl (e.g. trimethyl silyl methoxy carbonyl ethyl carbamoyl, trimethyl silyl ethoxy carbonyl propyl carbamoyl, triethyl silyl ethoxy carbonyl propyl carbamoyl, tert-butyl dimethyl silyl ethoxy carbonyl propyl carbamoyl, trimethyl silyl propoxy carbonyl butyl carbamoyl, etc.), and the like.
- The aromatic acyl may include aroyl which may have one or more suitable substiuent(s) such as nitro (e.g. benzoyl, toluoyl, xyloyl, naphthoyl, nitrobenzoyl, dinitrobenzoyl, nitronaphthoyl, etc.), arenesulfonyl which may have one or more suitable substituent(s) such as halogen (e.g. benzenesulfonyl, toluenesulfonyl, xylenesulfonyl, naphthalenesulfonyl, fluorobenzenesulfonyl, chlorobenzenesulfonyl bromobenzenesulfonyl, iodobenzenesulfonyl, etc.), and the like.
- The aliphatic acyl substituted with aromatic group may include ar (lower) alkanoyl which may have one or more suitable substituent(s) such as lower alkoxy and trihalo (lower) alkyl (e.g. phenylacetyl, phenylpropionyl, phenylbutyrvl, 2-trifluoromethyl-2-methoxy-2-phenylacetyl, 2-ethyl-2-trifluoromethyl-2-phenylacetyl, 2-trifluoromethyl-2-propoxy-2-phenylacetyl, etc.), and the like.
- The more preferred acyl group thus defined may be C 1-C4 alkanoyl which may have carboxy, cyclo (C5-C6)-alkyloxy(C1-C4)alkanoyl having two (C1-C4)alkyl groups on the cycloalkyl moiety, camphorsulfonyl, carboxy(C1-C4)-alkylcarbamoyl, tri(C1-C4) alkylsilyl(C1-C4)alkoxycarbonyl-(C1-C4)alkylcarbamoyl, benzoyl which may have one or two nitro, benzenesulfonyl having halogen, phenyl(C1-C4)alkanoyl having C1-C4alkoxy and trihalo(C1-C4)alkyl, and the most preferred one may be acetyl, carboxypropionyl, menthyloxyacetyl, camphorsulfonyl, benzoyl, nitrobenzoyl, dinitrobenzoyl, iodobenzenesulfonyl and 2-trifluoromethyl-2-methoxy-2-phenylacetyl.
- Suitable “protected carboxy (lower) alkylcarbamoyl” and “carboxy (lower) alkylcarbamoyl” moieties of the “protected carboxy (lower) alkylcarbamoyloxy” and “carboxy (lower) alkyl-carbamoyloxy” groups may include the same as those exemplified in the explanation of the hydroxy-protective group mentioned above.
- Suitable “leaving group” may include hydroxy, acyloxy in which the acyl moiety may be those as exemplified above, and the like.
- The processes for production of the tricyclo compounds (I) of this invention are explained in detail in the following.
- [I] Fermentation Processes:
- The FR-900506, FR-900520, FR-900523 and FR-900525 substances of this invention can be produced by fermentation of FR-900506, FR-900520, FR-900523 and/or FR-900525 substance(s)-producing strains belonging to the genus Streptomyces such as Streptomyces tsukubaensis No. 9993 and Streptomyces hygroscopicus subsp. yakushimaensis No. 7238 in a nutrient medium.
- Particulars of microorganisms used for the production of the FR-900506, FR-900520, FR-900523 and FR-900525 substances are explained in the following.
- [A] The FP-900506, FR-900520 and FR-900525 substances of this invention can be produced by fermentation of a FR-900506, FR-900520 and/or FR-900525 substance(s)-producing strain belonging to the genus Streptomyces such as Streptomyces tsukubaensis No. 9993 in a nutrient medium.
- The microorganism which can be used for the production of the FR-900506, FR-900520 and/or FR-900525 substances is FR-900506, FR-900520 and/or FR-900525 substance(s)-producing strain belonging to the genus Streptomyces, among which Streptomyces tsukubaensis No. 9993 has been newly isolated from a soil sample collected at Toyosato-cho, Tsukuba-gun, Ibaraki Prefecture, Japan.
- A lyophilized sample of the newly isolated Streptomyces tsukubaensis No. 9993 has been deposited with the Fermentation Research Institute, Agency of Industrial Science and Technology (No. 1-3, Higashi 1-chome, Yatabemachi Tsukuba-gun, Ibaraki Prefecture, Japan) under the deposit number of FERM P-7886 (deposited date: Oct. 5th, 1984), and then converted to Budapest Treaty route of the same depository on Oct. 19, 1985 under the new deposit number of FERM BP-927.
- It is to be understood that the production of the novel FR-900506, FR-900520 and/or FR-900525 substance(s) is not limited to the use of the particular organism described herein, which is given for the illustrative purpose only. This invention also includes the use of any mutants which are capable of producing the FR-900506, FR-900520 and/or FR-900525 substances including natural mutants as well as artificial mutants which can be produced from the described organism by conventional means such as irradiation of X-rays, ultra-violet radiation, treatment with N-methyl-N′-nitro-N-nitrosoguanidine, 2-aminopurine, and the like.
- The Streptomyces tsukubaensis No. 9993 has the following morphological, cultural, biological and physiological characteristics.
- [1] Morphological Characteristics:
- The methods described by Shirling and Gottlieb (Shirling, E. B. and D. Gottlieb: Methods for characterization of Streptomyces species. International Journal of Systematic Bacteriology, 16, 313 - 340, 1966) were employed principally for this taxonomic study.
- Morphological observations were made with light and electron microscopes on cultures grown at 30° C. for 14 days on oatmeal agar, yeast-malt extract aqar and inorganic salts-starch agar. The mature sporophores formed Rectiflexibiles with 10 to 50 or more than 50 spores in each chain. The spores were oblong or cylindrical, 0.5 - 0.7× 0.7−0.8 μm in size by electron microscopic observation. Spore surfaces were smooth.
- [2] Cultural Characteristics:
- Cultural characteristics were observed on ten kinds of media described by Shirling and Gottlieb as mentioned above, and by Waksman (Waksman, S. A.: The actinomycetes, vol. 2: Classification, identification and description of genera and species. The Williams and Wilkins Co., Baltimore, 1961).
- The incubation was made at 300° C. for 14 days. The color names used in this study were based on Guide to Color Standard (manual published by Nippon Shikisai Kenkyusho, Tokyo) . Colonies belonged to the gray color series when grown on oatmeal agar, yeast-malt extract agar and inorganic salts-starch agar. Soluble pigment was produced in yeast-malt extract agar but not in other media. The results are shown in Table 1.
TABLE 1 Cultural Characteristics of Strain No. 9993 and Streptomyces misakiensis IFO 12891 Cultural characteristics Medium No. 9993 IFO 12891 Oatmeal Agar G Moderate Moderate A Gray Grayish White R Pale Pink Colorless S None None Yeast-Malt G Moderate Moderate Extract Agar A Light Gray Grayish White R Dull Reddish Orange Light Brown S Dull Reddish Orange None Inorganic Salts- G Moderate Moderate Starch Agar A Pale Yellow Orange to Grayish White Light Gray R Dark Orange Pale Yellowish Brown S None None Glucose- G Poor Moderate Asparagine A White Grayish White Agar R Pale Brown Pale Yellowish Brown S None Pale Brown Glycerin- G Moderate Moderate Asparagine A Pale Pink to White Grayish White Agar R Pale Pink Pale Yellowish Brown S None Pale Brown Czapek Agar G Poor Abundant A None Grayish White R Pale Pink Dark Orange to Dark Brown S None None Nutrient Agar G Poor Poor A White, Poor White R Colorless Colorless S None None Potato-Dextrose G Poor Moderate Agar A None Yellowish Gray R Pale Pink Brown S None None Tyrosine Agar G Moderate Moderate A White Grayish White to Light Gray R Dull Reddish Orange Dark Orange to Black S None None Peptone-Yeast G Poor Poor Extract-Iron A None None Agar R Colorless Colorless S None None - The cell wall analysis was performed by the methods of Becker et al. (Becker, B., M. P. Lechevalier, R. E. Gordon and H. A. Lechevalier: Rapid differentiation between Nocardia and Streptomyces by paper chromatography of whole cell hydrolysates: Appl. Microbiol., 12, 421-423, 1961 and Yamaguchi (Yamaguchi, T.: Comparison of the cell wall composition of morphologically distinct actinomycetes: J. Bacteriol., 89, 444-453, 1965). Analysis of whole cell hydrolysates of the strain No. 9993 showed the presence of LL-diaminopimelic acid. Accordingly, the cell wall of this strain is believed to be of type I.
- [3] Biological and Physiological Properties:
- Physiological properties of the strain No. 9993 were determined according to the methods described by Shirling and Gottlieb as mentioned above. The results are shown in Table 2. Temperature range and optimum temperature for growth were determines on yeast-malt extract agar using a temperature gradient incubator (made by Toyo Kagaku Sangyo Co., Ltd.). Temperature range for growth was from 18 to 35° C. with optimum temperature at 28° C. Milk peptonization and gelatin liquefaction were positive. Melanoid pigment production was negative.
TABLE 2 Physiological Properties of Strain No. 9993 and Streptomyces misakiensis IFO 12891 Physiological properties No. 9993 IFO 12891 Temperature Range for Growth 18° C.-35° C. 12° C.-35° C. Optimum Temperature 28° C. 28° C. Nitrate Reduction Negative Negative Starch Hydrolysis Negative Positive Milk Coagulation Negative Negative Milk Peptonization Positive Weakly Positive Melanin Production Negative Negative Gelatin Liquefaction Positive Negative H2S Production Negative Negative NaCl Tolerance (%) ≦3% 3%<, <5% - Utilization G. carbon sources was examined according to the methods of Pridham and Gottlieb (Pridham, T. G. and D. Gottlieb: The utilization of carbon compounds by some Actinomycetales as an aid for species determination: J. Bacteriol., 56, 107—114, 1948). The growth was observed after 14 days incubation at 30° C.
- Summarized carbon sources utilization of this strain is shown in Table 3. Glycerin, maltose and sodium succinate could be utilized by the strain No. 9993. Further, doubtful utilization of D-glucose, sucrose, D-mannose and salicin was also observed.
TABLE 3 Carbon Sources Utilization of Strain No. 9993 and Streptomyces misakiensis IFO 12891 Carbon Sources No. 9993 IFO 12891 D-Glucose ± − Sucrose ± − Glycerin + − D-Xylose − − D-Fructose − − Lactose − − Maltose + − Rhamnose − − Raffinose − − D-Galactose − + L-Arabinose − − D-Mannose ± − D-Trehalose − − Inositol − − D-Mannitol − − Inulin − + Cellulose − − Salicin ± − Chitin − ± Sodium Citrate − − Sodium Succinate + − Sodium Acetate − − - Microscopic studies and cell wall composition analysis of the strain No. 9993 indicate that this strain belongs to the genus Streptomyces Waksman and Henrici 1943.
- Accordingly, a comparison of this strain was made with various Streptomyces species in the light of the published descriptions [International Journal of Systematic Bacteriology, 18, 69 to 189, 279 to 392 (1968) and 19, 391 to 512 (1969), and BergyIs Manual of Determinative Bacteriology 8th Edition (1974)].
- As a result of the comparison, the strain No. 9993 is considered to resemble Streptomyces aburaviensis Nishimura et. al., Streptomyces avellaneus Baldacci and Grein and Streptomyces misakiensis Nakamura. Therefore, the cultural characteristics of the strain No. 9993 were compared with the corresponding Streptomyces aburaviensis IFO 12830, Streptomyces avellaneus IFO 13451 and Streptomyces misakiensis IFO 12891. As a result, the strain No. 9993 was the most similar to Streptomyces misakiensis IFO 12891. Therefore, the strain No. 9993 was further compared with Streptomyces misakiensis IFO 12891 as shown in the above Tables 1, 2 and 3. From further comparison, the strain No. 9993 could be differentiated from Streptomyces misakiensis IFO 12891 in the following points, and therefore the strain No. 9993 is considered to be a new species of Streptomyces and has been designated as Streptomyces tsukubaensis sp. nov., referring to the soil, collected at Tsukuba-gun, from which the organism was isolated.
- Difference from Streptomyces misakiensis IFO 12891
- Cultural characteristics of the strain No. 9993 are different from the Streptomyces misakiensis IFO 12891 on oatmeal agar, yeast-malt extract agar, glucose-asparagine agar, Czapek agar and potato-dextrose agar.
- Starch hydrolysis of the strain No. 9993 is negative, but that of the Streptomyces misakiensis IFO 12891 is positive.
- Gelatin liquefaction of the strain No. 9993 is positive, but that of the Streptomyces misakiensis IFO 12891 is negative.
- In carbon sources utilization, the strain No. 9993 can utilize glycerin, maltose and sodium succinate, but the Streptomyces misakiensis IFO 12891 can not utilize them. And, the strain No. 9993 can not utilize D-galactose and inulin, but the Streptomyces misakiensis IFO 12891 can utilize them.
- PRODUCTION OF FR-900506, FR-900520 AND FR-900525 SUBSTANCES
- The novel FR-900506, FR-900520 and FR-900525 substances of this invention can be produced by culturing a FR-900506, FR-900520 and/or FR-900525 substance(s)-producing strain belonging to the genus Streptomyces (e.g. Streptomyces tsukubaensis No. 9993, FERM BP-927) in a nutrient medium.
- In general, the FR-900506, FR-900520 and/or FR-900525 substance(s) can be produced by culturing the FR-900506, FR-900520 and/or FR-900525 substance(s)-producing strain in an aqueous nutrient medium containing sources of assimilable carbon and nitrogen, preferably under aerobic conditions (e.g. shaking culture, submerged culture, etc.).
- The preferred sources of carbon in the nutrient medium are carbohydrates such as glucose, xylose, galactose, glycerin, starch, dextrin, and the like. Other sources which may be included are maltose, rhamnose, raffinose, arabinose, mannose, salicin, sodium succinate, and the like.
- The preferred sources of nitrogen are yeast extract, peptone, gluten meal, cottonseed meal, soybean meal, corn steep liquor, dried yeast, wheat germ, feather meal, peanut powder etc., as well as inorganic and organic nitrogen compounds such as ammonium salts (e.g. ammonium nitrate, ammonium sulfate, ammonium phosphate, etc.), urea, amino acid, and the like.
- The carbon and nitrogen sources, though advantageously employed in combination, need not be used in their pure form, because less pure materials which contain traces of growth factors and considerable quantities of mineral nutrients, are also suitable for use. When desired, there may be added to the medium mineral salts such as sodium or calcium carbonate, sodium or potassium phosphate, sodium or potassium chloride, sodium or potassium iodide, magnesium salts, copper salts, cobalt salt and the like. If necessary, especially when the culture medium foams seriously, a defoaming agent, such as liquid paraffin, fatty oil, plant oil, mineral oil or silicone may be added.
- As the conditions for the production of the FR-900506, FR-900520 and FR-900525 substances in massive amounts, submerged aerobic cultural conditions are preferred therefor. For the production in small amounts, a shaking or surface culture in a flask or bottle is employed. Furthermore, when the growth is carried out in large tanks, it is preferable to use the vegetative form of the organism for inoculation in the production tanks in order to avoid growth lag in the process of production of the FR-900506, FR-900520 and FR-900525 substances. Accordingly, it is desirable first to produce a vegetative inoculum of the organism by inoculating a relatively small quantity of culture medium with spores or mycelia of the organism and culturing said inoculated medium, and then to transfer the cultured vegetative inoculum aseptically to large tanks. The medium, in which the vegetative inoculum is produced, is substantially the same as or different from the medium utilized for the production of the FR-900506, FR-900520 and FR-900525 substances.
- Agitation and aeration of the culture mixture may be accomplished in a variety of ways. Agitation may be provided by a propeller or similar mechanical agitation equipment, by revolving or shaking the fermentor, by various pumping equipment or by the passage of sterile air through the medium. Aeration may be effected by passing sterile air through the fermentation mixture.
- The fermentation is usually conducted a temperature between about 20° C. and 40° C., preferably 25-35° C., for a period of about 50 hours to 150 hours, which may be varied according to fermentation conditions and scales.
- Thus produced FR-900506, FR-900520 and/or FR-900525 substance(s) can be recovered from the culture medium by conventional means which are commonly used for the recovery of other known biologically active substances. The FR-900506, FR-900520 and FR-900525 substances produced are found in the cultured mycelium and filtrate, and accordingly the FR-900506, FR-900520 and FR-900525 substances can be isolated and purified from the mycelium and the filtrate, which are obtained by filtering or centrifuging the cultured broth, by a conventional method such as concentration under reduced pressure, lyophilization, extraction with a conventional solvent, pH adjustment, treatment with a conventional resin (e.g. anion or cation exchange resin, non-ionic adsorption resin, etc.), treatment with a conventional adsorbent (e.g. activated charcoal, silicic acid, silica gel, cellulose, alumina, etc.), crystallization, recrystallization, and the like.
- The FR-900506, FR-900520 and FR-900525 substances produced according to the aforementioned process possess the following physical and chemical properties.
- FR-900506 Substance
- (1) Form and Color:
- white powder
- (2) Elemental Analysis:
C: 64.72%, H: 8.78%, N: 1.59% 64.59% 8.74% 1.62% - (3) Color Reaction:
- Positive: cerium sulfate reaction, sulfuric acid reaction, Ehrlich reaction, Dragendorff reaction and iodine vapor reaction Negative: ferric chloride reaction, ninhydrin reaction and Molish reaction
- (4) Solubility:
- Soluble: methanol, ethanol, acetone, ethyl acetate, chloroform, diethyl ether and benzene
- Sparingly Soluble: hexanl, petroleum ether
- Insoluble: water
- (5) Melting Point: 85 —90 ° C.
- (6) Specific Rotation: [α] d 23 : −73° (c=0.8, CHCl3)
- (7) Ultraviolet Absorption Spectrum:
- end absorption
-
-
- the chart of which being shown in FIG. 1,
- (10) 1H Nuclear Magnetic Resonance Spectrum:
- the chart of which being shown in FIG. 2,
- (11) Thin Layer Chromatography:
Developing Stationary Phase Solvent Rf Values silica gel plate chloroform: 0.58 methanol (10:1, v/v) ethyl acetate 0.52 - (12) Property of the Substance:
- neutral substance
- With regard to the FR-900506 substance, it is to be noted that in case of measurements of 13C and 1H nuclear magnetic resonance spectra, this substance showed pairs of the signals in various chemical shifts.
- The FR-900506 substance thus characterized further possesses the following properties.
- (i) The measurements of 13C Nuclear Magnetic Resonance Spectra at 25° C. and 60° C. revealed the fact that the intensities of each pair of the various signals therein were changed.
- (ii) The measurements of the thin layer chromatography and the high performance liquid chromatography revealed that the FR-900506 substance occurs as a single spot in the thin layer chromatography and a single peak in the high performance liquid chromatography, respectively.
- This white powder of the FR-900506 substance could be transformed into a form of crystals by recrystallization thereof from acetonitrile, which possess the following physical and chemical properties.
- (1) Form and Color:
- colorless prisms
- (2) Elemental Analysis: C: 64.30 %, H: 8.92 %, WI: 1.77 % 64.20 %, 8.86 %, 1.72 %,
- (3) Melting Point:
C: 64.30%, H: 8.92%, N: 1.77% 64.20%, 8.86%, 1.72%, - 127 - 129 ° C.
- (4) Specific Rotation: [α] D 23: −84.4° (c=1.02, CHCl3)
-
- 6 (ppm, CDC 3) :{211.98 (s) 196.28 (s) 168.97 (s) 15211.74 (s), 193.56 (s), 168.81 (s), 164.85 (s) 5138.76 (s) 135.73 (d) 165.97 (s), 139.51 (s), 135.63 (d), 132.38 (s) 130.39 (d) i122.82 (d) _131.90 (s),l130.17 (d), 122.96 (d)f 116.43 (t)f, 97.19 (s) 84.29 (d), 198.63 (s), 77.84 (d) 77.52 (d) 69.89 (d) 78.21 (d), 76.97 (d), 69.00 (d), (56.63 (d) 52.97 (d) X48.76 (t) 154.87 (d) , 52.82 (d), t48.31 (t), 240.21 (d) 31.62 (t), 30.72 (t), l40.54 (d), 24.56 (t), l21.12 (t) i20.33 (q) t20.86 (t), t19.74 (q), 16.17 (q) 15.88 (q) 13.89 (q) 16.10 (q), 15.75 (q), .14.05 (q), 9.64 (q) 9.96 (q), the chart of which being shown in FIG. 3,
- (6) 1H Nuclear Magnetic Resonance Spectrum:
- the chart of which being shown in FIG. 4.
- Other physical and chemical properties, that is, the color reaction, solubility, ultraviolet absorption spectrum, infrared absorption spectrum, thin layer chromatography and property of the substance of the colorless prisms of the FR-900506 substance were the same as those for the white powder of the same under the identical conditions.
-
- 17-Allyl-1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methyvinyl]-23-25-dimethoxy- 13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone
- FR-900520 Substance
- The physical and chemical properties are mentioned later.
- FR-900525 Substance
- (1) Form and Color:
- white powder
- (2) Elemental Analysis:
- C: 65.17 % H: 8.53 %, N: 1.76 %
- (3) Color Reaction:
- Positive: cerium sulfate reaction, sulfuric acid reaction, Ehrlich reaction, Dragendorff reaction and iodine vapor reaction
- Negative: ferric chloride reaction, ninhydrin reaction and Molish reaction
- (4) Solubility:
- Soluble: methanol, ethanol, acetone, ethyl acetate, chloroform, diethyl ether and benzene
- Sparingly Soluble: hexane, petroleum ether
- Insoluble: water
- (5) Melting Point:
- 85 - 89 ° C.
- (6) Specific Rotation:
- [α] D 23 : -88° (c=1.0, CHCl3)
- (7) Ultraviolet Absorption Spectrum:
- end absorption
-
-
- 6(ppm, CDC1 3) :212.61 (s) 188.57 (s) 168.76 (s) 1211.87 (s), 191.12 (s), 170.18 (s), 163.11 (s) 140.28 (s) 5135.62 (d) l161.39 (s), 139.37 (s) ,i135.70 (d) 132.28 (s) 130.09 (d) >122.50 (d) 131.34 (s), 130.00 (d) ,123.23 (d), 116.48 (t), i99.16 (s) 84.42 (d) 199.11 (s), 184.48 (d), 78.60 (d) p76.73 (d) i59.97 (d) 479.86 (d), i77.33 (d), 60.45 (d), 57.52 (q), 56.56 (q) 56.14 (q) 56.48 (q), 55.97 (q), 5 3.45 (d) 449.15 (t) 48.46 (t) 553.26 (d), 449.73 (t), 447.62 (t) 44.47 (t) 541.40 (d) 35.19 (d) 445.23 (t) , 040.4 0 (d), 35. 11 (d) >33 1 0 (d) 13 2 .81 (t) 31.53 (t) 3 34. 17 (d) 3 32.2:29 (t) g31.33 (t), 30.80 (t) 28.60 (t), 26.03 (d) 30.66 (t), 26.98 (d) 25.43 (t) 118.93 (q) 114.09 (q) 224.40 (t), 220.5 7 (q) , 113.95 (q) 9.85 (q) l10.00 (q)
- the chart of which being shown in FIG. 5,
- (10) H Nuclear Magnetic Resonance Spectrum::
- the chart of which being shown in FIG. 6,
- (11) Thin Layer Chromatography:
Developing Stationary Phase Solvent Rf Value silica gel plate ethyl acetate 0.34 - (12) Property of the Substance:
- neutral substance
- With regard to the FR-900525 substance, it is to be noted that in case of measurements of 13C and 1II nuclear magnetic resonance spectra, this substance showed pairs of the signals in various chemical shifts, however, in case of measurements of the thin layer chromatography and the high performance liquid chromatography, the FR-900525 substance showed a single spot in the thin layer chromatography and a single peak in the high performance liquid chromatography, respectively.
-
- 16-Allyl-1, 13-dihydroxy-1I- [2- (4-hydroxy-3-methoxycyclohexyl) -l-methylvinyl -22,24-dimethoxy-12,18,20, 2 6-tetramethyl-l0 , 27-dioxa-4-azatricyclo-(21.3.1.04 ,8 heptacos-17-ene-2 3,9 , l5-tetraone [B] The FR-900520 and FR-900523 substances of this invention can be produced by fermentation of FR-900520 and/or FR-900523 substance(s)-producing strain belonging to the genus Streptomyces such as Streptomyces hygroscopicus subsp. vakushimaensis No. 7238 in a nutrient medium.
- The microorganism which can be used for the production of the FR-900520 and/or FR-900523 substances is FR-900520 and/or FR-900523 substance(s)-producing strain belonging to the genus Streptomyces, among which Streptomyces hygroscodicus subsp. vakushimaensis No. 7238 has been newly isolated from a soil sample collected at Yakushima, Kagoshima Prefecture, Japan.
- A lyophilized sample of the NEWLY isolated Streptomyces hygroscoricus subsp. vakushimaensis No. 7238 has been deposited with the Fermentation Research Institute, Agency of Industrial Science and Technology (No.1-3, Higashi 1-chome, Yatabemachi, Tsukuba-gun, Ibaraki Prefecture, Japan) under the number of FERM P-8043 (deposited date: Jan. 12th, 1985), and then converted to Budapest Treaty route of the same depository on Oct. 19, 1985 under the new deposit number of FERM BP-928.
- It is to be understood that the production of the novel FR-900520 and FR-900523 substances is not limited to the use of the particular organism described herein, which is given for the illustrative purpose only. This invention also includes the use of any mutants which are capable of producing the FR-900520 and/or FR-900523 substance(s) including natural mutants as well as artificial mutants which can be produced from the described organism by conventional means such as irradiation of X-rays, ultra-violet radiation, treatment with N-methyl-N′-nitro-N-nitrosoguanidine, 2-aminopurine, and the like.
- The Streptomyces hygroscoricus subsp. vakushimaensis No. 7238 has the following morphological, cultural, biological and physiological characteristics.
- [1] Morphological Characteristics:
- The methods described by Shirling and Gottlieb (Shirling, E. B. and D. Gottlieb: Methods for characterization of Streptomyces species. International Journal of Systematic Bacteriology, 16, 313 - 340, 1966) were employed principally for this taxonomic study.
- Morphological observations were made with light and electron microscopes on cultures grown at 30° C. for 14 days on oatmeal agar, yeast-malt extract acar and inorganic salts-starch agar. The mature sporophores were moderately short and formed RetinaculiaDerti and SDirales with about 20 spores in each chain. Hygroscopic spore mass were seen in the aerial mycelia on oatmeal agar and inorganic salts-starch agar. Surface irregularities on spores were intermediate between very short, thick spines and warts.
- [2] Cultural Characteristics:
- Cultural characteristics were observed on ten kinds of media described by Shirling and Gottlieb as mentioned above, and by Waksman (Waksman, S. A.: The actinomycetes, vol. 2: Classification, identification and description of genera and species. The Williams and Wilkins Co., Baltimore, 1961).
- The incubation was made at 30° C. for 14 days. The color names used in this study were based on Guide to Color Standard (manual published by Nippon Shikisai Kenkyusho, Tokyo) . Colonies belonged to the gray color series when grown on oatmeal agar, yeast-malt extract agar and inorganic salts-starch agar. Soluble pigment was not produced in the examined media. The results are shown in Table 4.
TABLE 4 Cultural Characteristics of Strain No. 7238, Streptomyces antimycoticus IFO 12839 and Streptomyces hygroscopicus subsp. glebosus IFO 13786 Cultural Characteristics Medium No. 7238 IFO 12839 IFO 13786 Oatmeal Agar G Poor Poor Poor A Grayish Yellow Brown Grayish Yellow Brown Grayish Yellow Brown R Pale Yellow Pale Yellow Pale Yellow S None None None Yeast-Malt G Moderate Abundant Moderate Extract Agar A Grayish White Gray Gray R Pale Yellowish Brown Pale Yellowish Brown Dark Orange S None None None Inorganic Salts- G Moderate Moderate Moderate Starch Agar A Gray to Black Gray Light Gray R Pale Yellow Orange Yellowish Gray Pale Yellow Orange S None None None Glucose- G Moderate Moderate Moderate Asparagine A Grayish White Gray White Agar R Pale Yellow Orange Pale Yellow Orange Pale Yellow Orange S None None None Glycerin- G Moderate Moderate Moderate Asparagine A White Gray Light Gray Agar R Yellowish Gray Yellowish Gray Grayish Yellow Brown S None None None Czapek Agar G Moderate Moderate Moderate A Grayish White Grayish White White R Pale Yellowish Brown Pale Yellowish Brown Pale Yellowish Brown S None None None Nutrient Agar G Moderate Moderate Moderate A Grayish White Grayish White None R Pale Yellow Pale Yellow Pale Yellow S None None None Potato-Dextrose G Moderate Moderate Moderate Agar A White, Poor Pale Reddish Brown Pale Pink to White R Pale Yellow Orange Pale Yellow Orange Pale Yellowish Brown S None None None Tyrosine Agar G Moderate Moderate Moderate A White Grayish White Gray to Black R Pale Yellowish Brown Brown Pale Yellowish Brown S None Brown None Peptone-Yeast G Moderate Moderate Moderate Extract-Iron A None Grayish White None Agar R Pale Yellow Pale Yellow Colorless S None None None - The cell wall analysis was performed by the methods of Becker et al. (Becker, B., M. P. Lechevalier, R. E. Gordon and H. A. Lechevalier: Rapid differentiation between Nocardia and Streptomyces by paper chromatography of whole cell hydrolysates: Appl. Microbiol., 12, 421-423, 1964) and Yamaguchi (Yamaguchi, T.: Comparison of the cell wall composition of morphologically distinct actinomycetes: J. Bacteriol., 89, 444-453, 1965). Analysis of whole cell hydrolysates of the strain No. 7238 showed the presence of LL-diaminopimelic acid. Accordingly, the cell wall of this strain is believed to be of type I.
- [3] Biological and Physiological Properties:
- Physiological properties of the strain No. 7238 were determined according to the methods described by Shirling and Gottlieb as mentioned above. The results are shown in Table 5. Temperature range and optimum temperature for growth were determined on yeast-malt extract agar using a temperature gradient incubator (made by Toyo Kagaku Sangyo Co., Ltd.). Temperature range for growth was from 18 to 36° C. with optimum temperature at 28° C.. Starch hydrolysis and gelatin liquefaction were positive. No melanoid pigment was produced.
TABLE 5 Physiological Properties of Strain No. 7238, Streptomyces antimycoticus IFO 12839 and Streptomyces hygroscopicus subsp. glebosus IFO 13786 Physiological properties No. 7238 IFO 12839 IFO 13786 Temperature Range 18° C.-36° C. 18° C.-38° C. 16° C.-35° C. for Growth Optimum Temperature 28° C. 28° C. 27° C. Nitrate Reduction Negative Negative Negative Starch Hydrolysis Positive Positive Positive Milk Coagulation Negative Negative Negative Milk Peptonization Negative Negative Positive Melanin Production Negative Negative Negative Gelatin Liquefaction Positive Positive Positive H2S Production Negative Negative Negative Urease Activity Negative Negative Negative NaCl Tolerance (%) 7%<, <10% 7%<, <10% 5%<, <7% - Utilization of carbon sources was examined according to the methods of Pridham and Gottlieb (Pridham, T. G. and D. Gottlieb: The utilization of carbon compounds by some Actinomycetales as an aid for species determination: J. Bacteriol., 56, 107-114, 1948). The growth was observed after 14 days incubation at 30° C.
- Summarized carbon sources utilization of this strain is shown in Table 6. D-Glucose, sucrose, lactose, maltose, D-trehalose, inositol, inulin and salicin could be utilized by the strain No. 7238.
TABLE 6 Carbon Sources Utilization of Strain No. 7238, Streptomyces antimycoticus IFO 12839 and Streptomyces hygroscopicus subsp. glebosus IFO 13786 Carbon Sources No. 7238 IFO 12839 IFO 13786 D-Glucose + + + Sucrose + + + Glycerin − + + D-Xylose − ± + D-Fructose − + + Lactose + + − Maltose + − + Rhamnose − + − Raffinose − + + D-Galactose − + + L-Arabinose − ± ± D-Mannose − + + D-Trehalose + ± + Inositol + + + D-Mannitol − + + Inulin + + − Cellulose ± − − Salicin + + − Chitin ± − − Sodium Citrate − − ± Sodium Succinate − + + Sodium Acetate − − − - Microscopic studies and cell wall composition analysis of the strain No. 7238 indicate that this strain belongs to the genus Streptomyces Waksman and Henrici 1943.
- Accordingly, a comparison of this strain was made with various Streptomyces species in the light of the published descriptions [International Journal of Systematic Bacteriology, 18, 69 to 189, 279 to 392 (1968) and 19, 391 to 512 (1969), and Bergy's Manual of Determinative Bacteriology 8th Edition (1974)].
- As a result of the comparison, the strain No. 7238 is considered to resemble Streptomyces antimycoticus Waksman 1957 and Streptomyces hygroscopicus subsp. glebosus Ohmori, et. al. 1962. Therefore, the cultural characteristics of the strain No. 7238 were further compared with the corresponding Streptomyces antimycoticus IFC 12839 and Streptomyces hygroscodicus subsp. glebosus IFO 13786 as shown in the above Tables 4, 5 and 6. From further comparison, the strain No. 7238 could be differentiated from Streptomyces antimycoticus IFO 12839 and Streptomyces hygroscorius subsp. alebosus IFO 13786 in the following points.
- (i) Difference from Streptomyces antimycoticus IFO 12839
- Cultural characteristics of the strain No. 7238 are different from the Streptomyces antimycoticus IFO 12839 on yeast-malt extract agar, glucose-asparagine agar, glycerin-asparagine agar, potato-dextrose agar and tyrosine agar.
- In carbon sources utilization, the strain No. 7238 can utilize maltose, but the Streptomyces antimycoticus IFO 12839 can not utilize it. And, the strain No. 7238 can not utilize glycerin, D-fructose, rhamnose, raffinose, D-galactose, D-mannose, mannitol and sodium succinate, but the Streptomyces antimycoticus IFO 12839 can utilize them.
- (ii) Difference from Streptomyces hygroscopicus subsp. glebosus IFO 13786
- Cultural characteristics of the strain No. 7238 are different from the Streptomyces hygroscoricus subsp. glebosus IFO 13786 on yeast-malt extract agar, potato-dextrose agar and tyrosine agar.
- Milk peptonization of the strain No. 7238 is negative, but that of the Streptomyces hygroscoiicus subsp. glebosus IFO 13786 is positive. The strain No. 7238 can grow in the presence of 7% NaCl, but the Streptomyces hygroscopicus subsp. glebosus IFO 13786 can not grow under the same condition.
- In carbon sources utilization, the strain No. 7238 can utilize lactose, inulin and salicin, but the Streptomyces hygroscoricus subsp. glebosus IFO 13786 can not utilize them. And, the strain No. 7238 can not utilize glycerin, D-xylose, D-fructose, raffinose, D-galactose, D-mannose, mannitol and sodium succinate, but the Streptomyces hyqroscocicus subsp. glebosus IFO 13786 can utilize them.
- However, the strain No. 7238 forms hygroscopic spore mass in the aerial mycelia on oatmeal agar and inorganic salts-starch agar, and further morphological and cultural characteristics of the strain No. 7238 are similar to the Streptomyces hygroscopicus subsp. glebosus IFO 13786. Therefore, the strain No. 7238 is considered to belong to Streptomyces hygroscocicus, but the strain No. 7238 is different from the Streptomyces hygroscomicus subsp. glebosus IFO 13786, though this known strain is the most similar to the strain No. 7238 in Streptomyces hygroscodicus subspecies. From the above facts, the strain No. 7238 is considered to be a new subspecies of Streptomyces hygroscomicus and has been designated as Streptomyces hygroscorius subsp. vakushimaensis subsp. nov., referring to the soil collected at Yakushima, from which the organism was isolated.
- The novel FR-900520 and/or FR-900523 substance(s) can be produced by culturing FR-900520 and/or FR-900523 substance(s)-producing strain belonging to the genus Streptomyces (e.g. Streptomyces hygrosconicus subsp. yakushimaensis No. 7238, FERM BP-928) in a nutrient medium.
- In general, the FR-900520 and/or FR-900523 substance(s) can be produced by culturing the FR-900520 and/or FR-900523 substance(s)-producing strain in an aqueous nutrient medium containing sources of assimilable carbon and nitrogen, preferably under aerobic conditions (e.g. shaking culture, submerged culture, etc.).
- The preferred sources of carbon in the nutrient medium are carbohydrates such as glucose, sucrose, lactose, glycerin, starch, dextrin, and the like. Other sources which may be included are maltose, D-trehalose, inositol, inulin, salicin, and the like.
- The preferred sources of nitrogen are yeast extract, peptone, gluten meal, cottonseed meal, soybean meal, corn steep liquor, dried yeast, wheat germ, feather meal, peanut powder etc., as well as inorganic and organic nitrogen compounds such as ammonium salts (e.g. ammonium nitrate, ammonium sulfate, ammonium phosphate, etc.) , urea, amino acid, and the like.
- The carbon and nitrogen sources, though advantageously employed in combination, need not be used in their pure form, because less pure materials which contain traces of growth factors and considerable quantities of mineral nutrients, are also suitable for use. When desired, there may be added to the medium mineral salts such as sodium or calcium carbonate, sodium or potassium phosphate, sodium or potassium chloride, sodium or potassium iodide, magnesium salts, copper salts, cobalt salt and the like. If necessary, especially when the culture medium foams seriously, a defoaming agent, such as liquid paraffin, fatty oil, plant oil, mineral oil or silicone may be added.
- As the conditions :or the production of the FR-900520 and FR-900523 substances in massive amounts, submerged aerobic cultural conditions are preferred therefor. For the production in small amounts, a shaking or surface culture in a flask or bottle is employed. Furthermore, when the growth is carried out in large tanks, it is preferable to use the vegetative form of the organism for inoculation in the production tanks in order to avoid growth lag in the process of production of the FR-900520 and FR-900523 substances. Accordingly, it is desirable first to produce a vegetative inoculum of the organism by inoculating a relatively small quantity of culture medium with spores or mycelia of the organism and culturing said inoculated medium, and then to transfer the cultured vegetative inoculum aseptically to large tanks. The medium, in which the vegetative inoculum is produced, is substantially the same as or different from the medium utilized for the production of the FR-900520 and FR-900523 substances.
- Agitation and aeration of the culture mixture may be accomplished in a variety of ways. Agitation may be provided by a propeller or similar mechanical agitation equipment, by revolving or shaking the fermentor, by various pumping equipment or by the passage of sterile air through the medium. Aeration may be effected by passing sterile air through the fermentation mixture.
- The fermentation is usually conducted at a temperature between about 20° C. and 400C, preferably 25-35° C., for a period of about 50 hours to 150 hours, which may be varied according to fermentation conditions and scales.
- Thus produced FR-900520 and/or FR-900523 substance(s) can be recovered from the culture medium by conventional means which are commonly used for the recovery of other known biologically active substances. The FR-900520 and FR-900523 substances produced are mainly found in the cultured mycelium, and accordingly the FR-900520 and FR-900523 substances can be isolated and purified from the mycelium, which are obtained by filtering or centrifuging the cultured broth, by a conventional method such as concentration under reduced pressure, lyophilization, extraction with a conventional solvent, pH adjustment, treatment with a conventional resin (e.g. anion or cation exchange resin, non-ionic adsorption resin, etc.), treatment with a conventional adsorbent (e.g. activated charcoal, silicic acid, silica gel, cellulose, alumina, etc.), crystallization, recrystallization, and the like.
- Particularly, the FR-900520 substance and the FR-900523 substance can be separated by dissolving the materials containing both products produced by fermentation in an appropriate solvent such as ethyl acetate, n-hexane, and the like, and then by subjecting said solution to chromatography, for example, on silica gel in a column with an appropriate organic solvent such as ethyl acetate and n-hexane, or a mixture thereof. And each of the FR-900520 substance and the FR-900523 substance thus separated can be further purified by a conventional method, for example, recrystallization re-chromatography, high performance liquid chromatography, and the like.
- FR-900520 Substance
- (1) Form and Color:
- colorless plates
- (2) Elemental Analysis:
- C: 64.81 %, HI: b.82 %, N: 1.55 %
- (3) Color Reaction:
- Positive: cerium sulfate reaction, sulfuric acid reaction, Ehrlich reaction, Dragendorff reaction and iodine vapor reaction
- Negative: ferric chloride reaction, ninhydrin reaction and Molish reaction
- (4) Solubility:
- Soluble: methanol, ethanol, acetone, ethyl acetate, chloroform, diethyl ether and benzene
- Sparingly Soluble: n-hexane, petroleum ether
- Insoluble: water
- (5) Melting Point:
- 163 - 165° C.
- (6) Specific Rotation:
- [α] D 23: -84.10 (c=1.0, CHCl3)
- (7) Ultraviolet Absorption Spectrum:
- end absorption
-
-
- (10) H Nuclear Magnetic Resonance Spectrum:
- the chart of which being shown in FIG. 8,
- (11) Thin Layer Chromatography:
Developing Stationary Phase Solvent Rf Values silica gel plate chloroform: 0.38 methanol (20:1, v/v) ethyl acetate 0.51 - (12) Property of the Substance:
- neutral substance
- With regard to the FR-900520 substance, it is to be noted that in case of measurements of 13C and 1H nuclear magnetic resonance spectra, this substance shows pairs of the signals in various chemical shifts, however, in case of measurements of the thin layer chromatography and the high performance liquid chromatography, the FR-900520 substance showed a single spot in the thin layer chromatography and a single peak in the high performance liquid chromatography, respectively.
-
- 17-Ethyl-1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylvinyl)-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo-[ 22.3.1.04,9) octacos-18-ene-2,3,10,16-tetraone
- FR-900523 Substance
- (1) Form and Color:
- colorless needles
- (2) Elemental Analysis:
- C: 64.57 %, H: 8.84 %, N: 1.81 %
- (3) Color Reaction:
- Positive: cerium sulfate reaction, sulfuric acid reaction, Ehrlich reaction, Dragendorff reaction and iodine vapor reaction
- Negative: ferric chloride reaction and ninhydrin reaction
- (4) Solubility:
- Soluble: methanol, ethanol, acetone, ethyl acetate, chloroform, diethyl ether and benzene
- Sparingly Soluble: n-hexane and petroleum ether
- Insoluble: water
- (5) Melting Point:
- 152 - 154 ° C.
- (6) Specific Rotation:
- [α] D 23 : −73.00 (C=0.65, CHCl3)
- (7) Ultraviolet Absorption Spectrum:
- and absorption
-
- (10) 1H Nuclear Magnetic Resonance Spectrum:
- the chart of which being shown in FIG. 10,
- (11) Thin Layer Chromatography:
Developing Stationary Phase Solvent Rf Values silica gel plate chloroform: 0.38 methanol (20:1, v/v) ethyl acetate 0.51 - (12) Property of the Substance:
- neutral substance
- With regard to the FR-900523 substance, it is to be noted that in case of measurements of 13C and 1H nuclear magnetic resonance spectra, this substance shows pairs of the signals in various chemical shifts, however, in case of measurements of the thin layer chromatography and the high performance liquid chromatography, the FR-900523 substance showed a single spot in the thin layer chromatography and a single peak in the high performance liquid chromatography, respectively.
-
- 1,14-Dihydroxy-12-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylvinyl-[-23,25-dimethoxy-13,19,17,21,27-pentamethyl-11,28-dioxa-4-azatricyclo[22.3.1.0 4,9]-octacos-18-ene-2,3,10,16-tetraone
- [III] Synthetic Processes:
- (1) Process 1: (Introduction of Hydroxy-Protective Group)
- The compound (lb) or a salt thereof can be prepared by introducing a hydroxy-protective group into the compound (Ia) or a salt thereof.
- Suitable introducing agent of the hydroxy-protective group used in this reaction may be a conventional one such as di(lower)alkyl sulfoxide, for example, lower alkyl methyl sulfoxide (e.g. dimethyl sulfoxide, ethyl methyl sulfoxide, propyl methyl sulfoxide, isopropyl methyl sulfoxide, butyl methyl sulfoxide, isobutyl methyl sulfoxide, hexyl methyl sulfoxide, etc.), trisubstituted silyl compound such as tri(lower)alkylsilyl halide (e.g. trimethylsilyl chloride, triethylsilyl bromide, tributylsilyl chloride, tert-butyl-dimethylsilyl chloride, etc.), lower alkyl-diarylsilyl halide (e.g. methyl-diphenylsilyl chloride, ethyl-diphenylsilyl bromide, propyl-ditolylsilyl chloride, tert-butyl-diphenylsilyl chloride, etc.), and acylating agent which is capable of introducing the acyl group as mentioned before such as carboxylic acid, sulfonic acid, carbamic acid and their reactive derivative, for example, an acid halide, an acid anhydride, an activated amide, an activated ester, isocyanate, and the like. Preferable example of such reactive derivative may include acid chloride, acid bromide, a mixed acid anhydride with an acid such as substituted phosphoric acid (e.g. dialkylphosphoric acid, phenylphosphoric acid, diphenylphosphoric acid, dibenzylphosphoric acid, halogenated phosphoric acid, etc.) , dialkylphosphorous acid, sulfurous acid, thiosulfuric acid, sulfuric acid, alkyl carbonate (e.g. methyl carbonate, ethyl carbonate, propyl carbonate, etc.) , aliphatic carboxylic acid (e.g. pivalic acid, pentanoic acid, isopentanoic acid, 2-ethylbutyric acid, trichloroacetic trifluoroacetic acid, etc.) , aromatic carboxylic acid (e.g. benzoic acid, etc.), a symmetrical acid anhydride, an activated acid amide with a heterocyclic compound containing imino function such as imidazole, 4-substituted imidazole, dimethylpyrazole, triazole and tetrazole, an activated ester (e.g. p-nitrophenyl ester, 2,4-dinitrophenyl ester, trichlorophenyl ester, pentachlorophenyl ester, mesylphenyl ester, phenylazophenyl ester, phenyl thioester, p-nitrophenyl thioester, p-cresyl thioester, carboxymethyl thioester, pyridyl ester, piperidinyl ester, 8-quinolyl thioester, or an ester with a N-hydroxy compound such as N,N-dimethylhydroxylamine, 1-hydroxy-2-( 1H)-pyridone, N-hydroxysuccinimide, N-hydroxyphthalimide, 1-hydroxybenzotriazole, 1-hydroxy-6-chlorobenzotriazole, etc.), isocyanate, and the like.
- In this reaction, in case that the di(lower)alkyl sulfoxide is used as an introducing agent of the hydroxy-protective group, the reaction is usually conducted in the presence of lower alkanoic anhydride such as acetic anhydride.
- Further, in case that the trisubstituted silyl compound is used as an introducing agent of the hydroxy-protective group, the reaction is preferable conducted in the presence of a conventional condensing agent such as imidazole, and the like.
- Still further, in case that the acylating agent is used as an introducing agent of the hydroxy-protective group, the reaction is preferably conducted in the presence of an organic or inorganic base such as alkali metal (e.g. lithium, sodium, potassium, etc.), alkaline earth metal (e.g. calcium, etc.), alkali metal hydride (e.g. sodium hydride, etc.) , alkaline earth metal hydride (e.g. calcium hydride, etc.), alkali metal hydroxide (e.g. sodium hydroxide, potassium hydroxide, etc.), alkali metal carbonate (e.g. sodium carbonate, potassium carbonate, etc.), alkali metal hydrogen carbonate (e.g. sodium hydrogen carborate, potassium hydrogen carbonate, etc.), alkali metal alkoxide (e.g. sodium methoxide, sodium ethoxide, potassium tert-butoxide, etc.), alkali metal alkanoic acid (e.g. sodium acetate, etc.), trialkylamine (e.g. triethylamine, etc.), pyridine compound (e.g. pyridine, lutidine, picoline, 4-N,N-dimethylaminopyridine, etc.), quinoline, and the like.
- In case that the acylating agent is used in a free form or its salt in this reaction, the reaction is preferably conducted in the presence of a conventional condensing agent such as a carbodiimide compound [e.g. N,N′-dicyclohexyl- carbodiimide, N-cyclohexyl-N′-(4-diethylaminocyclohexyl)- carbodiimide, N,N′-diethylcarbodiimide, N,N′-diisopropyl- carbodiimide, N-ethyl-N′- (3-dimethylaminopropyl) - carbodiimide, etc.], a ketenimine compound (e.g. N,N′-carbonylbis(2-methylimidazole), pentamethyleneketene-N-cyclohexylimine, diphenylketene-N-cyclohexylimine, etc.); an olefinic or acetylenic ether compounds (e.g. ethoxyacetylene, B-cyclovinylethyl ether), a sulfonic acid ester of N-hydroxybenzotriazole derivative [e.g. 1- (4-chlorobenzenesulfonyloxy) 6-chloro-1H-benzotriazole, etc.], and the like.
- The reaction is usually conducted in a conventional solvent which does not adversely influence the reaction such as water, acetone, dichloromethane, alcohol (e.g. methanol, ethanol, etc.) , tetrahydrofuran, pyridine, benzene, N,N-dimethylformamide, etc., or a mixture thereof, and further in case that the base or the introducing agent of the hydroxy-protective group is in liquid, it can also be used as a solvent.
- The reaction temperature is not critical and the reaction is usually conducted under from cooling to heating.
- This process includes, within a scope thereof, a case that during the reaction, the hydroxy group for R 2 of the compound (Ia) may occasionally be transformed into the corresponding protected hydroxy group in the object compound (Ib).
-
-
- wherein R 2 is hydroxy.
- (2) Process 2: (Introduction of Hydroxy-Protective Group)
- The compound (Id) or a salt thereof can be prepared by introducing a hydroxy-protective group into the compound (Ic) or a salt thereof.
- The reaction can be conducted by substantially the same method as that of Process 1, and therefore the reaction conditions (e.g. base, condensing agent, solvent, reaction temperature, etc.) are referred to those of Process 1.
- This process includes, within a scope thereof, a case that during the reaction, the hydroxy group for R 1 of the compound (Ic) may frequently be transformed into the corresponding protected hydroxy group in the object compound (Id).
- (3) Process 3: (Formation of Double Bond)
- The compound (If) or a salt thereof can be prepared by reacting the compound (Ie) or a salt thereof with a base.
- Suitable base to be used in this reaction may include one as exemplified in Process 1.
- This reaction can also be conducted by reacting the compound (Ie), where R 2 is hydroxy, with an acylating agent in the presence of a base.
- The reaction is usually conducted in a conventional solvent which does not adversely influence the reaction such as water, acetone, dichloromethane, alcohol (e.g. methanol, ethanol, propanol, etc.), tetrahydrofuran, pyridine, N,N-dimethylformamide, etc., or a mixture thereof, and further in case that the base is in liquid, it can also be used as a solvent.
- The reaction temperature is not critical and the reaction is usually conducted under from cooling to heating.
- (4) Process 4: (Oxidation of Hydroxyethylene Group)
- The compound (Ih) or a salt thereof can be prepared by oxidizing the compound (Ig) or a salt thereof.
- The oxidizing agent to be used in this reaction may include di(lower)alkyl sulfoxide such as those given in Process 1.
- This reaction is usually conducted in the presence of lower alkanoic anhydride such as acetic anhydride in a conventional solvent which does not adversely influence the reaction such as acetone, dichloromethane, ethyl acetate, tetrahydrofuran, pyridine, N,N-dimethylformamide, etc., or a mixture thereof, and further in case that the lower alkanoic anhydride is in liquid, it can also be used as a solvent.
- The reaction temperature is not critical and the reaction is usually conducted under from cooling to heating.
- This process includes, within a scope thereof, a case that during the reaction the hydroxy group for R 1 of the starting compound (Ig) may occasionally be transformed into 1-(lower alkylthio) (lower) alkyloxy group in the object compound (Ih).
- (5) Process 5:(Reduction of Allyl Group)
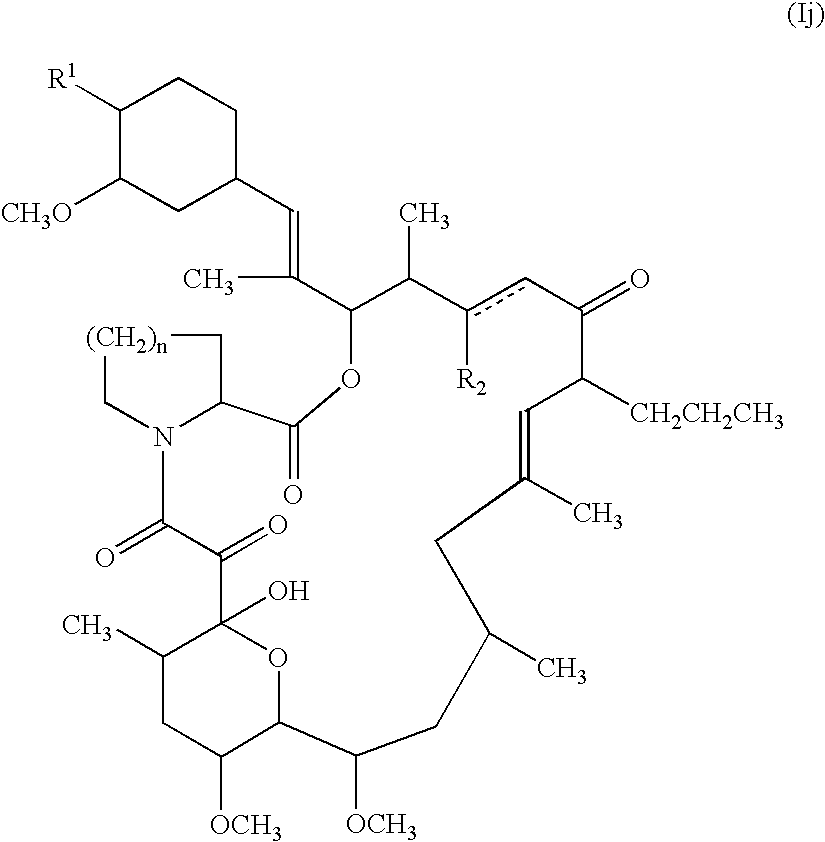
- The compound (Ij) or a salt thereof can be obtained by reducing the compound (Ii) or a salt thereof.
- Reduction in this process can be conducted by a conventional method which is capable of reducing an allyl group to a propyl group, such ascatalytic reduction, or the like.
- Suitable catalysts used in catalytic reduction are conventional ones such as platinum catalysts (e.g. platinum plate, spongy platinum, platinum black, colloidal platinum, platinum oxide, platinum wire, etc.) , palladium catalysts (e.g. spongy palladium, palladium black, palladium oxide, palladium on carbon, colloidal palladium, palladium on barium sulfate, palladium on barium carbonate, etc.) , nickel catalysts (e.g. reduced nickel, nickel oxide, Raney nickel, etc.) , cobalt catalysts (e.g. reduced cobalt, Raney cobalt, etc.), iron catalysts (e.g. reduced iron, Raney iron, etc.), copper catalysts (e.q. reduced copper, Raney copper, Ullman copper, etc.), and the. like.
- The reduction is usually conducted in a conventional solvent which does not adversely influence the reaction such as water, methanol, ethanol, propanol, pyridine, ethyl acetate, N,N-dimethylformamide, dichloromethane, or a mixture thereof.
- The reaction temperature of this reduction is not critical and the reaction is usually conducted under from cooling to warming.
- (6) Process 6: (Removal of the carboxy-protective group)
- The compound (IL) or a salt thereof can be prepared by removing the carboxy-protective group from the compound (Ik) or a salt thereof.
- The removal reaction in this process can be conducted in a conventional manner which is capable of transforming a tri(lower)alkylsilyl(lower)alkoxycarbonyl group to a carboxy group, that is, in the presence of tetra (lower) alkylammonium fluoride (e.g. tetrabutylammonium fluoride, etc.), potassium fluoride, hydrogen fluoride, and the like.
- This reaction is usually conducted in a conventional solvent which does not adversely influence the reaction such as tetrahydrofuran, and the like.
- The reaction temperature is not critical and the reaction is usually conducted under from cooling to warming.
- The object tricyclo compounds (I) obtained according to the synthetic processes 1 to 6 as explained above can be isolated and purified in a conventional manner, for example, extraction, precipitation, fractional crystallization, recrystallization, chromatography, and the like.
- Suitable salts of the compounds (I) and (Ia) to (Il) may include pharamaceutically acceptable salts such as basic salts, for example, alkali metal salt (e.g. sodium salt, potassium salt, etc.), alkaline earth metal salt (e.g. calcium salt, magnesium salt, etc.), ammonium salt, amine salt (e.g. triethylamine salt, N-benzyl-N-methylamine salt, etc.) and other conventional organic salts.
- It is to be noted that in the aforementioned reactions in the synthetic processes 1 to 6 or the post-treatment of the reaction mixture therein, the conformer and/or stereo isomer(s) due to asymmetric carbon atom(s) or double bond(s) of the starting and object compounds may occasionally be transformed into the other conformer and/or stereoisomer(s), and such cases are also included within the scope of the present invention.
- The tricyclo compounds (I) of the present invention possess pharmacological activities such as immunosuppressive activity, antimicrobial activity, and the like, and therefore are useful for the treatment and prevention of the resistance by transplantation of organs or tissues such as heart, kidney, liver, medulla ossium, skin, etc., graft-versus-host diseases by medulla ossium transplantation, autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, Aashimoto's thyroiditis, multiple sclerosis, myasthenia travis, type I diabetes, uveitis, etc., infectious diseases caused by pathogenic microorganisms, and the like.
- As examples for showing such pharmacological activities, some pharmacological test data of the tricyclo compounds are illustrated in the following.
- Test 1
- Suppression of Tricyclo Compounds (I) in vitro Mixed Lymphocyte Reaction (MLR)
- The MLR test was performed in microtiter plates, with each well containing 5 ×10 5 C57BL/6 responder cells (H-2b ), 5 ×105 mitomycin C treated (25μg/ml mitomycin C at 37° C. for 30 minutes and washed three times with RPMI 1640 medium) BALB/C stimulator cells (H-2d) in 0.2 ml RPMI 1640 medium supplemented with 10% fetal calf serum, 2mM sodium hydrogen carbonate, penicillin (50 unit/ml) and streptomycin (50 μg/ml) . The cells were incubated at 37 ° C. in humidified atmosphere of 5% carbon dioxide and 95% of air for 68 hours and pulsed with 3H-thymidine (0.5 μCi) 4 hours before the cells were collected. The object compound of this invention was dissolved in ethanol and further diluted in RPMI 1640 medium and added to the cultures to give final concentrations of 0.1 μg/ml or less.
- The results are shown in Tables 7 to 10. The tricyclo compounds of the present invention suppressed mouse MLR.
TABLE 7 Effect of the FR-900506 Substance on MLR FR-900506 concentration Radioactivities (ng/ml) (mean C.P.M. ± S.E.) Suppression (%) IC50(ng/ml) 2.5 54 ± 4 99.5 1.25 168 ± 23 98.3 0.625 614 ± 57 93.8 0.313 3880 ± 222 60.9 0.26 0.156 5490 ± 431 44.7 0.078 7189 ± 365 27.6 0 9935 ± 428 0 -
TABLE 8 Effect of FR-900520 Substance on MLR FR-900520 concentration Radioactivities Suppression IC50 (ng/ml) (mean C.P.M. ± S.E.) (%) (ng/ml) 100 175 ± 16 99.2 10 515 ± 55 97.8 1 2744 ± 527 88.1 0.38 0.500 9434 ± 1546 59.2 0.25 14987 ± 1786 35.1 0 23106 ± 1652 0 -
TABLE 9 Effect of FR-900523 Substance on MLR FR-900523 concentration Radioactivities Suppression IC50 (ng/ml) (mean C.P.M. ± S.E.) (%) (ng/ml) 100 25 ± 12 99.9 10 156 ± 37 99.3 1 5600 ± 399 75.8 0.5 0.500 11624 ± 395 49.7 0.250 17721 ± 1083 23.3 0 23106 ± 1052 0 -
TABLE 10 Effect of the FR-900525 Substance on MLR FR-900525 concentration Radioactivities Suppression IC50 (ng/ml) (mean C.P.M. ± S.E.) (%) (ng/ml) 100 469 ± 56 97.0 10 372 ± 32 97.6 5 828 ± 369 94.7 1.55 2.5 3564 ± 512 77.4 1.2 10103 ± 421 35.8 0 15741 ± 411 0 - Test 2 Antimicrobial activities of Tricyclo Compounds (I)
- Antimicrobial activities of the tricyclo compounds (I) against various fungi were determined by a serial agar dilution method in a Sabouraud agar. Minimum inhibitory concentrations (MIC) were expressed in terms of μg/ml after incubation at 30° C. for 24 hours.
- Tricyclo compounds of the present invention showed antimicrobial activities against fungi, for example, Asperaillus fumicatus IFO 5840 and Fusarium oxysporum IFO 5942 as described in the following Tables 11 and 12.
TABLE 11 MIC values (μg/ml) of Tricyclo Compounds (I) against Aspergillus fumigatus IFO 5840 Substances MIC (μg/ml) FR-900506 0.025 FR-900520 0.1 FR-900523 0.3 FR-900525 0.5 -
TABLE 12 MIC values (μg/ml) of Tricyclo Compounds (I) of against Fusarium oxysporum Substances MIC (μg/ml) FR-900506 0.05 FR-900525 1 - Test 3
- Effect of Tricyclo Compounds (I) on Skin Allograft Survival in Rats
- Vental allografts from donor (Fischer) rats were grafted onto the lateral thoracic area of recipient (WKA) rats. The dressings were removed on day 5. The grafts were inspected daily until rejection which was defined as more than 90% necrosis of the graft epitherium.
- The FR-900506 substance was dissolved in olive oil and administered intramuscularly for 14 consecutive days, beginning at the day of transplantation.
- As shown in Table 13, all skin allografts were rejected within 8 days in rats treated with olive oil intramuscularly for 14 consecutive days, but daily treatment with the FR-900506 substance clearly prolonged skin allograft survival.
TABLE 13 Effect of FR-900506 Substance on Skin Allograft Survival Number of Skin Allograft Dose (mg/kg) Animals Survival Day Control — 11 7, 7, 7, 7, 7, 7, 8, 8, 8, 8, 8 (olive oil) FR-900506 1 8 19, 19, 19, 20, 21, 21, 22, 22 Substance 3.2 6 22, 23, 23, 26, 27, 35 10 5 56, 61, 82, 85, 89 - Test 4
- Effect of Tricyclo Compounds (I) on Type II Collagen-Induced-Arthritis in Rats
- Collagen was dissolved in cold 0.01 M acetic acid at a concentration of 2 mg/ml. The solution was emulsified in an equal volume of incomplete Freund's adjuvant. A total volume of 0.5 ml of the cold emulsion was injected intradermally at several sites on the back and one or two sites into the tail of female Lewis rats. The FR-900506 substance was dissolved in olive oil and administered orally. Control rats immunized with same amount of type II collagen received oral administrations of olive oil alone. Incidences of the arthritis were observed.
- The test results are shown in Table 14. The inflammatory polyarthritis was induced in all rats treated with olive oil for 14 days starting on the same day as the type II collagen immunization.
- Daily treatment with the FR-900506 substance for 14 days gave complete suppression of arthritis induction during an observation period of 3 weeks.
TABLE 14 Effect of FR-900506 Substance on Type II Collagen-induced-Arthritis in Rats Incidence of Dose (mg/kg per day) Arthritis Control — 5/5 (olive oil) FR-900506 3.2 0/5 Substance - Test 5
- Effect of Tricylo Compounds (I) on Experimental Allergic Encephalomvelytis (EAE) in SJL/J Mice
- Spinal cord homogenate was prepared from SJL/J mice. The spinal cords were removed by insufflation, mixed with an approximately equal volume of water and homogenized at 4° C.. An equal volume of this cold homogenate (10 mg/ml) was emulsified with complete Freund's adjuvant (CFA) containing 0.6 mg/ml of Mycobacterium tuberculosis H37RA.
- EAE was induced by two injections of 0.2 ml of spinal cord-CFA emulsion into SJL/J mice on day 0 and day 13. All mice used in these tests were evaluated and scored daily for clinical signs of EAE.
- The severity of EAE was scored according to the following criteria: grade 1-decreased tail tone: grade 2- a clumsy gait: grade 3- weakness of one or more limb: grade 4- paraplegia or hemiplegia.
- The FR-900506 substance was dissolved in olive oil and administered orally for 19 days starting on day 0 (the day of first immunization). As shown in Table 15, the FR-900506 substance clearly prevented the development of clinical signs of EAE.
TABLE 15 Effect of FR-900506 Substance on Experimental Allergic Encephalomyelytis in SJL/J Mice Number of Animals Dose (mg/kg) with Disease at Day 24 Control — 10/10 (olive oil) FR-900506 32 0/5 Substance - Test 6
- Effect of Tricyclo Compounds (I) on Local Graft-versus-Host Reaction (GvHR) in Mice
- The viable spleen cells (1 ×10 cells) from C57BL/6 donors were injected subcutaneously into the right hind foot pad of BDF 1 mice to induce local GvHR. The mice were killed 7 days later and both right (injected paw) and left (uninjected paw) popliteal lymph nodes (PLN) were weighed. The GVHR was expressed as the weight difference between right and left PLN.
- The FR-900506 substance was dissolved in olive oil and administered orally for five days starting on the same day as sensitization.
- ED 50 Value of the FR-900506 substance for prevention of the local graft-versus-host reaction was 19 mg/kg.
- Test 7
- Acute toxicities of Tricyclo Compounds (I)
- Test on acute toxicities of the FR-900506, FR-900520, FR-900523 and FR-900525 substances in ddY mice by intraperitoneal injection were conducted, and the dead at dose of 100 mg/kg could not be observed in each case.
- Test 8
- Effect of Tricyclo Compounds (I) on Antibody Formation in mice (Assay for the haemagglutination test)
- Male BDF 1 mice 6 to 8 weeks of age were used for this test. The mice were immunized on day 0 with 1 ×10 sheep erythrocytes (SRBC) intraperitoneally. Serum aggulutinin titers were determined for each individual animal with sedimentation of erythrocytes by serial two fold dilutions. The titers are expressed as -log2
- The FR-900506 substance was administered orally for 5 days from one day before immunization to day 3.
- The results of each experiments were evaluated by means of Student's t-test as shown in Table 16.
TABLE 16 Effect of FR-900506 Substance on antibody formation in mice Dose Number Haemagglutination (mg/kg) of Animals Mean log2 titer ± SE ED50 (mg/kg) Control — 5 8.2 ± 0.2 — FR-900506 10 5 7.6 ± 0.4 32 5 6.0 ± 0.45 16.9 100 5 4.4 ± 0.75 - Test 9
- Effect of Tricyclo Compounds (I) on in vivo Plaque Forming Cell (PFC) Response in Mice
- Male C3H/He mice 6 to 7 weeks of age were used for this 1 test. The mice were immunized on day 0 with 0.2 ml of 1 ×10 8 washed sheep erythrocytes intravenously. Spleens were removed on day 4 and spleen cells were incubated in the presence of SRBC as described by Cunningham and Szenberg (1968). Tests were evaluated by enumeration of direct generated plaque forming cells in the presence of complement. Suspensions of spleen cells were counted with a Microcellcounter CC-130 (Sysmex, Japan) and PFC results were calculated as PFC/106 recovered cells.
- The FR-900506 substance was administered orally for 4 days starting from the immunization.
- The results of each experiment were evaluated by means of Student's t-test as shown in Table 17.
TABLE 17 Effect of FR-900506 Substance on in vivo PFC Response in Mice Dose Number (mg/kg) of Animals PFC/106 cells ED50 (mg/kg) Control — 5 5054 ± 408 — FR-900506 3.2 5 3618 ± 476 10 5 1382 ± 243 5.9 32 5 657 ± 41 100 5 278 ± 41 - Test 10
- Effect of Tricyclo Compounds (I) on Contact-Delayed Hypersensitivity in mice
- Female ICR mice were used for this test. A tenth of a 7% (w/v) solution of picryl chloride in ethanol prepared at the time of the experiment was applied to the ventral surface of previously shaved animals. Such a sensitization was performed twice at intervals of 7 days. Seven days later, the thickness of the ears were measured with an ordinary engineer's micrometer and a 1% (w/v) solution of picryl chloride in olive oil was painted to both surfaces of each ear. The inflammation was evaluated 24 hours later by measuring the both of ears. The results were expressed as the average increase in thickness of the ears measured in units of 10 −3cm.
- The FR-900506 substance was injected for 14 days orally, starting from the first sensitization.
- Statistical significance was evaluated by Student's t-test as shown in Table 18.
TABLE 18 Effect of FR-900506 Substance on contact-delayed hypersensitivity in mice Dose Increase of ears at 24 hours (mg/kg) Number of Animals after challenge (× 10−3 cm) Control — 5 15.5 ± 0.8 FR-900506 32 5 6.4 ± 1.6 100 5 2.2 ± 1.5 - Test 11
- Effect of Tricyclo Compounds (I) on Delayed Type Hypersensitivity (DTH) Response to methylated bovine serum albumin (MBSA)
- Female BDF1 mice were used for this test. The mice were sensitized with a subcutaneous injection of 0.1 ml emulsion consisting of equal volume of MBSA (2 mg/ml) and Freund's incomplete adjuvant(FIA). Seven days later, a 0.05 ml challenge dose of 0.4 mq/ml MIBSA in saline was injected into the plantar region of the right hind foot and 0.05 ml saline into the left hind foot to act as a control. Twenty four hours after challenge, both hind feet were measured with a dial gauge and the mean challenge in footpad thickness was measured.
- The FR-900506 substance was injected for 8 days orally, starting from the sensitization.
- Statistical significance was evaluated by Student's t-test as shown in Table 19.
TABLE 19 Effect of FR-900506 Substance on DTH to MBSA in mice Dose Footpad Swelling ED50 (mg/kg) Number of Animals (× 10−3 cm) (mg/kg) Control — 5 57.4 ± 3.4 — FR-900506 10 5 31.0 ± 4.4 32 5 20.8 ± 4.4 18.4 100 5 7.8 ± 1.6 - Test 12
- Inhibition of Interleukin-2 (IL-2) Production by Tricyclo Compounds (I)
- The mixed lymphocyte reaction (MILR) was performed in microtiter plates, with each well containing 5 ×10 5 C57BL/6 responder cells (H-2b), 5 ×105 mitomycin C treated (25 μg/ml mitomycin C at 37° C. for 30 minutes and washed three times pith RPMI 1640 medium) BALB/C stimulator cells (H-2b) in 0.2 ml RPMI 1640 medium supplemented with 10% fetal calf serum, 2mM sodium hydrogen carbonate, penicillin 20 (50 unites/ml) and streptomycin (50 μg/ml) . The cells were incubated at 37° C. in humidified atmosphere of 5% carbon dioxide and 95% of air for 48 hours and the supernatant was collected. The FR-900506 substance was dissolved in ethanol and further diluted in RPMI 1640 medium and added to the cultures to give final concentrations of 0.1 μg/ml or less.
- The IL-2 activity was measured according to the method described by S. Gillis et. al. [Journal of Inununology, Vol. 120, page 2027, (1978)]. The IL-2 dependent CTLL-2 cell line was used to quantitate IL-2 activity. Cells (5 ×10 3/well) were cultured at 37° C. for 24 hours with various dilutions of IL-2 containing supernatant from MLR. The uptake of 3H-thymidine was measured by pulsing cultures with 0.5 uCi of 1T-thymidine for 6 hours. The unit value was calculated by dilution analysis of test sample and was compared with a laboratory standard preparation in which 100 units are equivalent to the amount of IL-2 necessary to achieve 50% proliferation of CTLL-2 cells.
- As shown in Table 20, the FR-900506 substance of the present invention inhibited the production of Il-2 from mouse MLR.
TABLE 20 Effect of the FR-900506 Substance on IL-2 Production FR-900506 concentration IL-2 (nM) (units/ml) Inhibition % 10 0 100 1 0 100 0.3 0.2 99.5 0.1 25.0 34.4 0 38.1 — - The FR-900506 substance also inhibited lymphokine production such as interleukin 3 and interferones, especially y-interferone, in the supernatant of MLR.
- The pharmaceutical composition of this invention can be used in the form of a pharmaceutical preparation, for example, in solid, semisolid or liquid form, which contains the tricyclo compounds (I) of the present invention, as an active ingredient, in admixture with an organic or inorganic carrier or excipient suitable for external, enteral or parenteral applications. The active ingredient may be compounded, for example, with the usual non-toxic, pharmaceutically acceptable carriers for tablets, pellets, capsules, suppositories, solutions, emulsions, suspensions, and any other form suitable for use. The carriers which can be used are water, glucose, lactose, gum acacia, gelatin, mannitol, starch paste, magnesium trisilicate, talc, corn starch, keratin, colloidal silica, potato starch, urea and other carriers suitable for use in manufacturing preparations, in solid, semisolid, or liquid form, and in addition auxiliary, stabilizing, thickening and coloring agents and perfumes may be used. The active object compound is included in the pharmaceutical composition in an amount sufficient to produce the desired effect upon the process or condition of diseases.
- For applying this composition to human, it is preferable to apply it by parenteral or enteral administration. While the dosage of therapeutically effective amount of the tricyclo compounds (I) varies from (continued to the next page) and also depends upon the age and condition of each individual patient to be treated, a daily dose of about 0.01-1000 mg, preferably 0.1-500 mg and more preferably 0.5-100 mg, of the active ingredient is generally given for treating diseases, and an average single dose of about 0.5 mg, 1 mg, 5 mg, 10 mg, 50 mg, 100 mg, 250 mg and 500 mg is generally administered.
- The following examples are given for the purpose of illustrating the present invention.
- Isolation of Streptomyces tsukubaensis No. 9993
- Streptomyces tsukubaensis No. 9993 was isolated by using dilution plate techniques as shown in the following.
- About one gram soil which was collected at Toyosato-cho, Tsukuba Gun, Ibaraki Prefecture, Japan, was added to a sterile test tube and the volume made up to 5 ml with sterile water. The mixture was then blended for 10 second by a tube buzzer and kept on 10 minutes. The supernatant was sequentially diluted by 100 fold with sterile water. The diluted solution (0.1 ml) was spread on Czapek agar supplemented with thiamine hydrochloride (saccharose 30 g, sodium nitrate 3 g, dipotassium phosphate 1 g, magnesium sulfate 0.5 g, potassium chloride 0.5 g, ferrous sulfate 0.01 g, thiamine hydrochloride 0.1 g, agar 20 g, taD water 1000 ml; pH 7.2) in a Petri dish. The growing colonies developed on the plates after 21 days incubation at 30° C. were transferred to slants (yeast-malt extract acar (ISP-medium 2)], and cultured for 10 days at 30° C.. Among of the colonies isolated, the Streptomyces tsukubaensis No. 9993 could be found.
- Fermentation
- A culture medium (160 ml) containing glycerin (1%) soluble starch (1 %), glucose (0.5%), cottonseed meal (0.2%), dried yeast (0.5%) , corn steep liquor (0.5%) and calcium carbonate (0.2%) (adjusted to pH 6.5) was poured, into each of twenty 500 ml-Erlenmeyer flasks and sterilized at 120° C. for 30 minutes. A loopful of slant culture of Streptomyces tsukubaensis No.9993, FERM BP-927 was inoculated to each of the media and cultured at 300° C. for 4 days on a rotary shaker. The resultant culture was inoculated to a medium containing soluble starch (4.5%), corn steep liquor (1%), dried yeast (1%), calcium carbonate (0.1%) and Adekanol (defoaming agent, Trade Mark, maker; Asahi Denka Co.) (0.1%) (150 liters) in a 200-liter jar-fermentor, which had been sterilized at 120° C. for 20 minutes in advance, and cultured at 30° C. for 4 days under aeration of 150 liters/minutes and agitation of 250 rpm.
- Isolation and Purification
- The cultured broth thus obtained was filtered with an aid of diatomaseous earth (5 kg) . The mycelial cake was extracted with methanol (50 liters) , yielding 50 liters of the extract. The methanol extract from mycelium and the filtrate were combined and passed through a column of a non-ionic adsorption resin “Diaion HP-20” (Trade Mark, maker Mitsubishi Chemical Industries Ltd.) ( 10 liters) . After washing with water (30 liters) and aqueous methanol (30 liters), elution was carried out with methanol. The eluate was evaporated under reduced pressure to give residual water (2 liters) . This residue was extracted with ethyl acetate (2 liters). The ethyl acetate extract was concentrated under reduced pressure to give an oily residue. The oily residue was mixed with twice weight of acidic silica gel (special silica gel grade 12, maker Fuji Devison Co.), and this mixture was slurried in ethyl acetate. After evaporating the solvent, the resultant dry powder was subjected to column chromatography of the same acid silica gel (800 ml) which was packed with n-hexane. The column was developed with n-hexane (3 liters) , a mixture of n-hexane and ethyl acetate (9:1 v/v, 3 liters and 4:1 v/v, 3 liters) and ethyl acetate (3 liters) . The fractions containing the object compound were collected and concentrated under reduced pressure to give an oily residue. The oily residue was dissolved in a mixture of n-hexane and ethyl acetate (1:1 v/v, 30 ml) and subjected to column chromatography of silica gel (maker Merck Co., Ltd. 230 - 400 mesh) (500 ml) packed with the same solvents system.
- Elution was carried out with a mixture of n-hexane and ethyl acetate (1:1 v/v, 2 liters and 1:2 v/v, 1.5 liters). Fractions containing the first object compound were collected and concentrated under reduced pressure to give a yellowish oil. The oily residue was mixed twice weight of acidic silica gel and this mixture was slurried in ethyl acetate. After evaporating the solvent, the resultant dry powder was chromatographed on acidic silica gel packed and developed with n-hexane. Fractions containing the object compound were collected and concentrated under reduced pressure to give crude FR-900506 substance (1054 mg) in the form of white powder. 100 mg Of this crude product was subjected to high performance liquid chromatography. Elution was carried out using a column (80φ×500 mm) with Lichrosorb SI 60 (Trade Mark, made by Merck & Co.) as a carrier. This chromatography was monitored by UV detector at 230 nm and mobile phase was a mixture of methylene chloride and dioxane (85:15 v/v) under flow rate of 5 ml/minute., and the active fractions were collected and evaporated. This high performance chromatography was repeated again, and 14 mg of the purified FR-900506 substance was obtained as white powder.
- Further, elution was continually carried out with ethyl acetate (1.5 liters), and: fractions containing the second object compound were collected and concentrated under reduced pressure to give crude FR-900525 substance (30 mg) in the form of yellowish oil.
- Fermentation
- A preculture medium (100 ml) containing glycerin (1%), corn starch (1%), glucose (0.5%), cottenseed meal (1%), corn steep liquor (0.5%), dried yeast (0.5%) and calcium carbonate (0.2%) at pH 6.5 was poured into a 500 ml-Erlenmeyer flask and sterilized at 120° C. for 30 minutes. A loopful of slant culture of Streptomyces tsukubaensis No. 9993 was inoculated to the medium and cultured at 30° C. for four days. The resultant culture was transferred to the same preculture medium (20 liters) in 30 liters jar-fermentor which had been sterilized at 120° C. for 30 minutes in advance. After the culture was incubated at 30° C. for 2 days, 16 liters of the preculture was inoculated to a fermentation medium (1600 liters) containing soluble starch (4.5%), corn steep liquor (1%), dried yeast (1%), calcium carbonate (0.1%) and Adekanol (defoaming agent, Trade Mark, maker Asahi Denka Co.) (0.1%) at pH 6.8 in 2 ton tank which had been sterilized at 120° C. for 30 minutes in advance and cultured at 30° C. for 4 days.
- Isolation and Purification
- The cultured broth thus obtained was filtered with an aid of diatomaseous earth (25 kg). The mycelial cake was extracted with acetone (500 liters), yielding 500 liters of the extract. The acetone extract from mycelium and the filtrate (1350 liters) were combined and passed through a column of a non-ionic adsorption resin “Diaion, HP-20” (Trade Mark, maker Mitsubishi Chemical Industries Ltd.) (100 liters). After washing with water (300 liters) and 50% aqueous acetone (300 liters), elution was carried out with 75% aqueous acetone. The eluate was evaporated under reduced pressure to give residual water (300 liters). This residue was extracted with ethyl acetate (20 liters) three times. The ethyl acetate extract was concentrated under reduced pressure to give an oily residue. The oily residue was mixed with twice weight of acidic silica gel (special silica gel grade 12, maker Fuji Devison Co.) , and this mixture was slurried in ethyl acetate. After evaporating the solvent, the resultant dry powder was subjected to column chromatography of the same acidic silica gel (8 liters) which was packed with n-hexane. The column was developed with n-hexane (30 liters), a mixture of n-hexane and ethyl acetate (4:1 v/v, 30 liters) and ethyl acetate (30 liters). The fractions containing the object compound were collected and concentrated under reduced pressure to give an oily residue. The oily residue was mixed with twice weight of acidic silica gel and this mixture was slurried in ethyl acetate. After evaporating the solvent, the resultant dry powder was rechromatographed on acidic silica gel (3.5 liters) packed with n-hexane. The column was developed with n-hexane (10 liters), a mixture of n-hexane and ethyl acetate (4:1 v/v, 10 liters) and ethyl acetate (10 liters). Fractions containing the object compound were collected and concentrated under reduced pressure to give a yellowish oil. The oily residue was dissolved in a mixture of n-hexane and ethyl acetate (1:1 v/v, 300 ml) and subjected to column chromatography of silica gel (maker Merck Co., Ltd. 230-400 mesh) (2 liters) packed with the same solvents system. Elution was curried out with a mixture of n-hexane and ethyl acetate (1:1 v/v, 10 liters and 1:2 v/v 6 liters) and ethyl acetate (6 liters).
- Fractions containing the first object compound were collected and concentrated under reduced pressure to give FR-900506 substance in the form of white powder (34 g). This white powder was dissolved in acetonitrile and concentrated under reduced pressure. This concentrate was kept at 5° C. overnight and prisms (22.7 g) were obtained. Recrystallization from the same solvent gave purified FR-900506 substance (13.6 g) as colorless prisms.
- Further, fractions containing the second object compound were collected and concentrated under reduced pressure to give crude FR-900525 substance (314 mg) in the form of yellowish powder.
- Fermentation
- A culture medium (160 ml) containing glycerin (1%), corn starch (1%), glucose (0.5%), cottonseed meal (1%), dried yeast (0.5%), corn steep liquor (0.5%) and calcium carbonate (0.2%) (adjusted to pH 6.5) was poured into each of ten 500 ml-Erlenmever flasks and sterilized at 120° C. for 30 minutes. A loopful of slant culture of Streptomyces tsukubaensis No. 9993 was inoculated to each of the medium and cultured at 30° C. for 4 days on a rotary shaker. The resultant culture was inoculated to a medium containing soluble starch 15%) , peanut powder (0.5%) , dried yeast (0.5%), gluten meal (0.5%), calcium carbonate (0.1%) and Adekanol (deforming agent, Trade Mark, maker Asasi Denka Co.) (0.1%) (150 liters) in a 200-liter jar-fermentor, which had been sterilized at 120° C. for 20 minutes in advance, and cultured at 30° C. for 4 days under aeration of 150 liters/minutes and agitation of 250 rpm.
- Isolation and Purification
- The cultured broth thus obtained was filtered with an aid of diatomaseous earth (5 kg). The mycelial cake was extracted with acetone (50 liters), yielding 50 liters of the extract. The acetone extract from mycelium and the filtrate (135 liters) were combined and passed through a column of a non-ionic adsorption resin “Diaion HP-20” (Trade Mark, maker Mitsubishi Chemical Industries Ltd.) (10 liters). After washing with water (30 liters) and 50% aqueous acetone (30 liters), elution was carried out with 75% aqueous acetone. The eluate (30 liters) was evaporated under reduced pressure to give residual water (2 liters). This residue was extracted with ethyl acetate (2 liters) three times. The ethyl acetate extract was concentrated under reduced pressure to give an oily residue. The oily residue was mixed with twice weight of acidic silica gel (special silica gel grade 12, maker Fuji Devison Co.), and this mixture was slurried in ethyl acetate. After evaporating the solvent, the resultant dry powder was subjected to column chromatography of the same acidic silica gel (800 ml) which was packed with n-hexane. The column was developed with n-hexane (3 liters) , a mixture of n-hexane and ethyl acetate (4:1 v/v, 3 liters) and ethyl acetate (3 liters). The fractions containing the object compound were collected and concentrated under reduced pressure to give an oily residue. The oily residue was dissolved in a mixture of n-hexane and ethyl acetate (1:1 v/v, 30 ml) and subjected to column chromatography of silica gel (maker Merck Co., Ltd. 230-400 mesh) (500 ml) packed with the same solvents system. Elution was carried out with a mixture of n-hexane and ethyl acetate (1:1 v/v, 2 liters and 1:2 v/v, 1.5 liters) and ethyl Acetate (1.5 liters)
- Fractions containing the first object compound were collected and concentrated under reduced pressure to give crude FR-900506 substance (3 g) in the form of yellowish powder.
- Further, fractions containing the second object compound were collected and concentrated under reduced pressure to give an oily residue. This oily residue was rechromatographed with silica gel to give a yellowish oil. The oily residue was mixed with twice weight of acidic silica gel and this mixture was slurried in ethyl acetate. After evaporating the solvent, the resultant dry powder was chromatographed on acidic silica gel (100 ml) packed and developed with n-hexane. Fractions containing the object compound were collected and concentrated under reduced pressure to give FR-900525 substance in the form of pale yellowish powder (380 mg). This powder was dissolved in a mixture of n-hexane and ethyl acetate (1:2 v/v, 5 ml) and subjected to acidic silica gel (special silica gel grade 922, maker Fuji Devison Co.) (100 ml) packed and washed with the same solvent system. Elution was carried out with ethyl acetate. The active fractions were collected and evaporated under reduced pressure to give the purified FR-900525 substance (230 mg) in the form of white powder.
- Isolation of Streptomyces hygrosconicus subso. vakushimaensis No. 7238
- Streptomyces hygroscomicus subsp. vakushimaensis No. 7238 was isolated by using dilution plate techniques as shown in the following.
- About one gram soil which was collected at Yakushima, Kagoshima Prefecture, Japan, was added to a sterile test tube and the volume made up to 5 ml with sterile water. The mixture was then blended for 10 seconds by a tube buzzer and. kept on 10 minutes. The supernatant was sequentially diluted by 100 fold with sterile water. The diluted solution (0.1 ml) was spread on Czapek agar supplemented with thiamine hydrochloride (saccharose 30 g, sodium nitrate 3 g, dipotassium phosphate 1 g, magnesium sulfate 0.5 g, potassium chloride 0.5 g, ferrous sulfate 0.01 g, thiamine hydrochloride 0.1 g, agar 20 g, tap water 1000 ml; pH 7.2) in a Petri dish. The growing colonies developed on the plates after 21 days incubation at 30° C. were transferred to slants (yeast-malt extract agar (ISP-medium 2)], and cultured for 10 days at 30° C.. Among of the colonies isolated, the Streptomyces hygroscomicus subsp. vakushimaensis No. 7238 could be found.
- Fermentation
- A culture medium (160 ml) containing glycerin (1%), soluble starch (1 %), glucose (0.5%), cottonseed meal (0.5%), dried yeast (0.5%), corn steep liquor (0.5%) and calcium carbonate (0.2%) (adjusted to pH 6.5) was poured into each of twenty 500 ml-Erlenmeyer flasks and sterilized at 120° C. for 30 minutes. A loopful of slant culture of Streptcmyces hygroscopicus subsp. vakushimaensis No. 7238, FERM BP-928 was inoculated to each of the media and cultured at 30° C. for 4 days on a rotary shaker. The resultant culture was inoculated to a medium containing glucose (4.5%) , corn steep liquor (1%) , dried yeast (1%) , gluten meal (1%), wheat germ (0.5%) , calcium carbonate (0.1%) and Adekanol (defoaming agent, Trade Mark, maker Asahi Denka Co.) (0.1%) (150 liters) in a 200-liter ,ar-fermentor, which had been sterilized at 120° C. for 20 minutes in advance, and cultured at 30° C. for 4 days under aeration 150 liters/minutes and agitation of 250 rpm.
- Isolation and Purification
- The cultured broth thus obtained was filtered with an aid of diatomaseous earth (5 kg) . The mycelial cake was extracted with acetone (50 liters) , yielding 50 liters of the extract. The acetone extract from mycelium and the filtrate (135 liters) were combined and passed through a column of a non-ionic adsorption resin “Diaion HP-20” (Trade Mark, maker Mitsubishi Chemical Industries Ltd.) ( 10 liters). After washing with water (30 liters) and aqueous acetone (30 liters), elution was carried out with acetone. The eluate was evaporated under reduced pressure to give residual water (2 liters). This residue was extracted with ethyl acetate (4 liters). The ethyl acetate extract was concentrated under reduced pressure to give an oily residue. The oily residue was mixed with twice weight of acidic silica gel (special silica gel grade 12, maker Fuji Devison Co.) , and this mixture was slurried in ethyl acetate. After evaporating the solvent, the resultant dry powder was subjected to column chromatography of the same acid silica gel (800 ml) which was packed with n-hexane. The column was developed with n-hexane (3 liters) , a mixture of n-hexane and ethyl acetate (4:1v/v, 3 liters) and ethyl acetate (3 liters). The fractions containing the FR-900520 and FR-90052 substances were collected and concentrated under reduced pressure to give an oily residue. The oily residue was dissolved in a mixture of n-hexane and ethyl acetane (1:1 v/v, 50 ml) and subjected to column chromatography of 2 silica gel (maker Merck Co., Ltd. 70 - 230 mesh) (1000 ml) packed with the same solvents system. Elution was carried out with a mixture of n-hexane and ethyl acetate (1:1 v/v, 3 liters and 1:2 v/v, 3 liters) and ethyl acetate (3 liters). Fractions containing the object compounds were collected and concentrated under reduced pressure to give a yellowish powder (4.5 g) . This powder was dissolved in methanol (20 ml) and mixed with water (10ml). The mixture was chromatographed on a reverse phase silica gel “YMC” (60-200 mesh) (500ml) (Trade Mark, maker Yamamura Chemical institute) packed and developed with a mixture of methanol and water (4:1 v/v).
- Fractions containing the FR-900520 substances were collected and concentrated under reduced pressure to give crude product of the FR-900520 substance (1.8 g) in the form of pale yellowish powder. This powder was dissolved in a small amount of diethyl,l ether. After standing overnight, the precipitated crystals were collected by filtration, washed with diethyl ether and then dried under reduced pressure. Recrystallization from diethyl ether gave 600 mg of the purified FR-900520 substance in the form of colorless plates.
- The chromatography of the reverse phase silica gel was carried on with the same solvents system, and the subsequent fractions containing he FR-900523 substance were collected and then concentrated under reduced pressure to give crude product of the Fr-900522 substance ( 0.51 g) in the form of pale yellowish powder. This crude product was dissolved in acetonitrile (3 ml) and subjected to a reverse phase silica gel “YMC” (70 ml) packed and developed with a mixture of acetonitrile, tetrahydrofuran and 50 mM phosphate buffer solution (pH 2.0) (3:2:5, v/v). Fractions containing the object compound were collected and were extracted with ethyl acetate. This extract was concentrated under reduced pressure to give a yellowish white powder (190 mg). The yellowish white powder was chromatographed again on a reverse phase silica (el “YMC” to give white powder (80 mg). This white powder was dissolved in a small amount of dimethyl ether and allowed to stand overnight at room temperature to give 56mg of crystals. Recrystallization from diethyl ether gave 34mg of the FR-900523 substance in the norm of colorless needles.
- To a solution of the FR-900506 substance (10.4 mg) in dichloromethane (0.2 ml) were added pyridine (0.1 ml) and acetic anhydride (0.05 ml) at room temperature, and the mixture was stirred for 5 hours. The solvent was removed from the reaction mixture under reduced pressure. The residue was subjected to silica gel thin layer chromatography (developing solvent: diethyl ether and dichloromethane, 1:2 v/v) to give 12-[2-(4-acetoxy-3-methoxycyclohexyl)-1-methylvinyl[-17-allyl-1,14-dihydroxy-2d3,25-dimethoxy-i3,19,21,27-tetramethyl-[,8-dioxa-4-azatricyclo(22.3.1.0 4,9)octacos-18-ene-2,3,10,16-tetraone (6.0 mg).
- IR ν(CHCl 3) 3520, 1728, 1705(sh), 1640, 1095 cm−
- To a solution of the FR-900506 substance (52.5 mg) in dichloromethane (1 ml) were added pyridine (0.5 ml) and acetic anhydride (0.3 ml) at room temperature, and the mixture was stirred at room temperature for 9 hours. The solvent was removed from the reaction mixture under reduced pressure. The residue was subjected to silica gel thin layer chromatography (developing solvent: diethyl ether and hexane, 3:1 v/v) to give 14-acetoxy-12-[2-(4-acetoxy-3-methoxycyclohexyl)-1-methylvinyl]-17-allyl-1-hydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone (48.0 mg) and (5.4 mg), respectively.
- Former Compound
- IR ν(CHCl 3): 1730, 1720(sh), 1640 cm−1
- Latter Compound
- IR ν(CHCl 3): 1730, 1690, 1640, 1627 cm−1
- To a solution of the FR-900506 substance (9..7 mg) in dichloromethane (0.2 ml) and pyridine (0.1 ml) was added benzoyl chloride (50 ul) at room temperature, and the mixture was stirred at room temperature or 2 hours. The solvent was removed from the reaction mixture under reduced pressure to give a crude oil. This oil was purified on silica gel thin layer chromatography (developing solvent: diethyl ether and hexane, 2:1 v/v) to afford 17-allyl-12-[2-(4-benzoyloxy-3-methoxycyclohexyl)-1-methylvinyl]-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone (8.0 mg).
- IR ν(CHCl 3): 3500, 1735(sh), 1710, 1640, 1600 cm−1
- To a solution of the FR-90056 substance (30.5 mg) in pyridine (1 ml) was added p-nitrobenzoyl chloride (ca. 100 mg), and the mixture was stirred at room temperature for 2 hours. The reaction mixture was diluted with ethyl acetate, and washed with a saturated aqueous sodium hydrogen carbonate, water 1N-hydrochloric acid, water, a saturated aqueous sodium hydrogen carbonate, water and an aqueous sodium chloride, successively, and then dried. The resulting solution was concentrated under reduced pressure, and the residue was purified on silica gel column chromatography to give 17-allyl-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-12-[2-[4-(p-nitrobenzoyloxy)-3-methoxycyclohexyl]-1-methyvinyl]-11,28-dioxa-4-azatricyclo[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone (37.7 mg).
- IR ν(CHCl 3): 1720, 1640, 1610, 1530-1520 cm−1
- 17-Allyl-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-12-[2-[4-(3,5-dinitrobenzoyloxy)-3-methoxycyclohexyl]-1-methylvinyl]-11,28-dioxa-4-azatricyclo-(22.3.1.0 4,9)octacos-13-ene-2,3,10,16-tetraone (36.0 mg) was obtained by reacting the FR-90056 substance 930.6 mg) with 3,5-dinitrobenzoyl chloride (33 mg) in pyridine (0.5 ml) in accordance with a similar manner to that of Example 8.
- 17-Allyl-1,14-dihydroxy-23,25-dimethoxy-12-[2-[4-(2-l menthyloxyacetoxy)-3-methoxycyclohexyl]-1-methylvinyl]-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo-[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone (50.9 mg) was obtained by reacting the FR-900506 substance (48 mg) with 2-l-methyloxyacetyl chloride (0.08 ml) in pyridine (0.5 ml) in accordance with a similar manner to that of Example 8.
- To a solution of (-)-2-trifluoromethyl-2-methoxy-2-phenylacetic acid (51 mg) in ethyl acetate (10 ml) was added at room temperature N,N′-dicyclohexylcarbodiimide (47 mg). After stirring for 1.5 hours at room temperature, then the FR-900506 substance (25.0 mg) and 4-(N,N-dimethylamino)-pyridine (11 mg) were added, followed by stirring at room temperature for 3.5 hours. The resulting solution was concentrated to provide a residue, which was taken up in diethyl ether and then washed successively with hydrochloric acid, an aqueous sodium hydrogen carbonate and an aqueous sodium chloride. The organic layer was dried over sodium sulfate and concentrated to provide a residue, which was chromatographed on silica gel (developing solvent: dichloromethane and diethyl ether, 10:1 v/v) to give 17-allyl-12-[2-[4-[(-)-2-trifluoromethyl-2-methoxy-2-phenylacetoxy]-3-methoxycyclohexyl]-1-methylvinyl]-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone (6.5 mg) and 17-allyl-14-[(-)-2-trifluoromethyl-2-methoxy-2-phenylacetoxy]-12-[2-[4-[(-)-2-trifluoromethyl-2-methoxy-2-phenylacetoxy]-3-methoxycyclohexyl]-1-methyvinyl]-1-hydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo-[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetraone (20.2 mg). dimethoxy-13 19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo-[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetraone (20.2 mg).
- Former Compound
- IR ν(neat): 3510, 1750, 1730(sh), 1710, 1652, 1500 cm −1
- Latter Compound
- IR ν(neat): 1750, 1720, 1652, 1500 cm −1
- To a stirred solution of the FR-900506 substance (248 mg) in pyridine (7 ml) were added succinic anhydride (145 mg) and 4-(N,N-dimethylamino)pyridine (7 mg), and the resulting mixture was stirred at room temperature for 18 hours. The reaction mixture was concentrated under reduced pressure and the residue was subjected to chromatography on silica gel (20 g) with ethyl acetate to give 17-allyl-12-[2-[ 4-(3-carboxypropionyloxy)-3-methoxycyclohexvl]-l-methylvinyl-[-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-l, 128-dioxa-4-azatricycloxy-cyclo f 22.3.1. 09]octacos-18-ene-2,3,10,16-tetraone (90 mg).
- IR ν(CHCl 3): 3500, 3100-2300, 1720, 1705(sh), 1635 cm −1
- To a solution of the FR-900506 substance (100.7 mg) in pyridine (3 ml) was added p-iodobenzenesulfonyl chloride (500 mg), and the mixture was stirred at room temperature for 36 hours. The solution was diluted with ethyl acetate and washed with a saturated aqueous sodium hydrogen carbonate, water and an aqueous sodium chloride. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure. The residue was chromatographed on silica gel (developing solvent: diethyl ether and hexane, 3:1 v/v) to give 17-allyl-1,14-dihydroxy-12 - t2 - 4-(p-iodbenzenesulfonyloxy) -3-methoxycyclohexyl]1-methylvinyl-[]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone ( 61 mg) and 17-allyl-1-hydroxy-12-[2-[4-(p-iodobenzenesulfonyloxv)-3-methoxycyclohexvl]-l-methylyin-i1]azatricyclo [22.3.1.04,9]octacosa-14,18-diene-2,3,10,16-tetraone (12 mg), respectively.
- Former Compound
- IR ν(CHCl 3): 3470, 1730, 1717, 1692, 1635, 1568 cm−1
-
- 17-Allyl-12-[2-(4-d-camphorsulfonyloxy-3-methoxycyclohexyl]-.1-methylvinyl] -1, l4-dihydroxy-,3,25-5 dimethoxy-13,1.9,21,27-tetramethyl-11,28-dioxa-4-azatricyclo(22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone (34 mg) was obtained by reacting the FR-900506 substance (27 mg) with d-camphorsulfonyl chloride (97 mg) in pyridine (0.6 ml) in accordance with a similar manner to that of Example 3.
- IR ν(neat): 3500, 1747, 1720(sh), 1710(sh), 1655 c −1
- To a stirred solution of the FR-900506 substance (89.7 mg) in dichloromethane (3 ml) were added imidazole (118 mg) and tert-butyl-diphenylsilyl chloride (52.2 mg) After the mixture was stirred at room temperature for 2 hours, the reaction mixture was diluted with a saturated aqueous ammonium chloride and extracted three times with diethyl ether. The extract was washed with water and an aqueous sodium chloride, dried over sodium sulfate, and then concentrated under reduced pressure. The residue was purified on silica gel column chromatography (developing solvent: ethyl acetate and hexane, 1:3 v/v) to give 17-allyl-12-[2-(4-tert-butyl-diphenysilyloxy-3-methoxycyclohexyl)-1-melthyvinyl)-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.10 4,9]octacos-18-ene-2,3,10,16-tetraone (107 mg).
- 17-Allyl-12-[2-(4-tert-butyl-dimethylsilyloxy-3-methoxycyclohexyl) -1-methylvinyl-]1,14-dihydoxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9-octacos-18-ene-2,3,10,16-tetraone (85 mg) was obtained by reacting the FR-900506 substance (80 mg) with tert-butyl-dimethylsilyl chloride (17 mg) in the presence of imidazole (15 mg) in N,N-dimethylformamide (1 ml) in accordance with a similar manner to that of Example 15.
- IRν(CHCl 3): 1735, 720(sh), 1700, 1640 cm −1
- To a solution of the FR-900506 substance (100 mg) in dimethyl sulfoxide (1.5 ml) was added acetic anhydride (1.5 ml) and he mixture was stored at room temperature for 14 hours. The reaction mixture was diluted with ethyl acetate and washed with a saturated aqueous sodium hydrogen carbonate, water and an aqueous sodium chloride. The organic layer was dried over sodium sulfate, filtered and then concentrated under reduced pressure. The residue was subjected to thin layer chromatography on silica gel (developing solvent: diethyl ether) to give 17-allyl-1,14-dihydroxy-23,25-dimethoxy-1 ,19,21,27-tetramethyl-12-[2-( 4-methylthiomethoxy-3-methoxycyclohexyl)-1-methylvinyl]-28-dioxa-4-azatricyclo-cl o 2.1 07, ]octacosa-14,18-diene-,2,3,0,16-retraone (51 mg) , 17-allyl-1-hydroxy-12-2-(4-hydroxy-3-methoxycyclohexyl)-1-methylvinyl-[-23,25- dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacosa-14,18-diene-2,3,10,16-tetrazone (18 mg) and 17-allyl-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-12-[2-(4- methylthiomethoxy-3-methoxycyclohexyl)-l-methylvinyl)- 11,28-dioxa-4-azatricyclo([22.3.1.04,9]octacos-18-ene-2,3,10,16-tetraone (10 mg), respectively.
- First Compound
- IR ν(CHCl 3): 3470, 1730, 1635, 1630(sh), 1580(sh) cm−1
- Second Compound
- IR ν(CHCl 3): 1728, 1640, 1090 cm−1
- Third Compound
- IR ν(CHCl 3): 3480, 1735, 1710, 1640 cm−1
- To a solution of 17-allyl-12-[2-(4-tert-butyl-dimethylsilyloxy-3-methoxycyclohexyl)-l-methylvinyl]-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11, 28-dioxa-4-azatricyclo[22.3.1 49] octacos-18-ene-12r,3f1O,16-tetraone (39.9 mg) in pyridine (1.5 ml) was added acetic anhydride (0.5 ml), and the mixture was stirred at room temperature for 6 hours. The solvent was removed from the reaction mixture under reduced pressure to give a crude oil, which was purified on silica gel thin layer chromatography (developing solvent: diethyl ether and hexane, 1:1 v/v) to afford 14-acetoxy-17-allyl-12-(2-(4-tert-butyl-dimethylsilyloxy-3-methoxycyclohexyl)-1-methyvinyl]-1-hydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.104,9]octacos-18-ene-2,3,10,16-tetraone (26.5 mg).
- IR ν(CHCl 3): 1728, 1715(sh), 1635 cm−1
- 14-Acetoxy-17-allyl-12-[2-(4-tert-butyl- diphhenylsilyloxy-3-methoxycyclohexyl) -1-methylvinyl]-1-hydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo(22.3..09] octacos-18-ene-2,3,10,16-tetraone (10 mg) was obtained by reacting 17-allyl-12-(2-(4-tert-butyl-diphenylsilyloxy-3-methoxycyclohexyl) -1-methylvinyl]-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl- 11,28-dioxa-4-azatricyclol[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone (10.6 mg) with acetic anhydride (0.1 mg) in pyridine (0.2 ml) in accordance with a similar manner to that of Example 18.
- IR ν(CHCl 3) : 3500, 1730, 1720(sh), 1660(sh), 1640, 1620(sh), 1100 cm−1
- To a solution of 14-acetoxy-17-allyl-12-(2-(4-tert-butyl-diphenylsilyloxy-3-methoxycyclohexyl)-1-methylvinyl]-l-hydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo (2.3.10 4,9]octacos-18-ene-2,3,10,16-tetraone (43.8 mg) in tetrahydrofuran (1.5 ml) was added potassium carbonate (ca 100 mg) at room temperature and the mixture was stirred at the same temperature for 3 hours. The reaction mixture was diluted with diethyl ether and the resulting solution was washed with a saturated aqueous ammonium chloride, water and an aqueous sodium chloride successively, and dried over sodium sulfate. The result solution was concentrated under reduced pressure and the residue was purified on silica gel thin layer chromatography (developing solvent: diethyl ether and hexane, 3:2 v/v) to give 17-allyl-12-[2-(4-tert-butyl-diphenylsilyloxy-3-methoxycyclohexyl)-1-methylvinyl-[-l-hydroxy-22,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacosa-14,18-diene-2,3,10,16-tetraone (30 mg).
- IR ν(CHCl 3) : 1733, 1720(sh), 1685, 1640(sh), 1620 cm−1
- A solution of the FR-900506 substance (50 mg) in ethyl acetate (2 ml) was subjected to catalytic reduction using 10% Palladium on carbon (10 mg) under atmospheric pressure at room temperature for 20 minutes. The reaction mixture was filtered and the filtrate was evaporated to dryness, which was purified on thin layer chromatography. Development with a mixture of chloroform and acetone (5:1 v/v) gave 1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxycyclohexyl) -1-methylvinyl]-23,25-dimethoxy-13,19, 21,27-tetramethyl-17-propyl-11,28-dioxa-4-azatricyclo(22.3.1.0 4,9 ]9]octacos-18-ene-2,3,10,16-tetraone (50.0 mg).
- IR ν(CHCl 3): 3480, 1735(sh), 1717, 1700, 1650(sh), 1625 cm−1
- White powder of crude FR-900506 substance (1 g) obtained by a similar fermentation process to Example 1 was dissolved in acetonitrile (5 ml) and subjected to high performance liquid chromatography (HPLC) using Shimazu LC4A (Trade Mark made by Shimazu Seisaku-sho). Steel column (25 mm inside diameter, 250 mm length) packed with YMC-S343 (ODS) (Trade Mark, made by Shimakyu Co., Ltd.) was used at a flow rate of 12 ml/min. Mobile phase was an aqueous mixture of 28% acetonitrile 10% n-butanol, 0.075% phosphoric acid, 3.75 mM sodium dodecyl sulfate (SDS) and detection was carried out using Hitachi UV-recorder at 210 nm. One hundred μl of the sample was injected each time and the HPLC was repeated 50 times so that all the sample could be subjected to the column. Each eluate with a retention time of 85 min. to 90 min. was collected and extracted with an equal volume of ethyl acetate (3.6 liters). The ethyl acetate layer was separated and washed with an aqueous sodium hydrogen carbonate (1%, 2 liters) and concentrated in vacuo to a small amount. SDS crystallized on concentration was removed by filtration. Crude powder obtained was dissolved in acetonitrile at a concentration of 100 mg/ml and applied again to HPLC. Mobile phase was an aqueous mixture of 12.5% acetonitrile, 9.75% n-butanol, 0.075% phosphoric acid, 2.75 mM EDS. The column was eluted at a flow rate of 10 ml/min. The eluates with a retention time of 131 min. to 143 min. were collected and extracted with equal volume of ethyl acetate. The solvent layer was separated and washed with 1% aqueous sodium hydrogen carbonate and concentrated in vacuo to a small volume. SDS crystallized on concentration was removed by filtration.
- Crude powder thus obtained was dissolved in a small amount of ethyl acetate and subjected to column chromatography using silica gel (10 ml) (Kiesel gel, 230-400 mesh, maker: Merck Co., Ltd.). The column was washed with a mixture cf n-hexane and ethyl acetate (30 ml) (1:1 v/v) and a mixture of n-hexane and ethyl acetate (60 ml) (1:2 v/v). Elution was carried out using ethyl acetate and fractionated (each fraction: 3 ml). Fractions 19 to 24 were collected and concentrated in vacuo to dryness to give FR-900520 substance (24 mg).
- A solution of the FR-900506 substance (310 mg) 2-trimethylsilylethyl 4-isocyanatobutyrate (350 mg) and triethylamine (6 drops) in anhydrous benzene (4 ml) was heated at 50° C. with stirring for 2 hours. The reaction mixture was allowed to stand at room temperature overnight. The mixture was concentrated to dryness under reduced pressure to leave a residue, which was chromatographed on silica gel in chloroform. Elution was carried out with chloroform to give 17-allyl-12-(2-(3-methoxy-4-{3-(2-trimethylsilylethoxycarbonyl)propylcarbamoyloxy}cyclohexyl]-1-methylvinyl-1,1,4-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo(22.2.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone (99 mg). This product was treated with tetra(n-butyl)ammonium fluoride (0.12 m mole) ir tetrahydrofuran (2.5 ml) at room temperature or 20 minutes. The reaction mixture was concentrated to dryness under reduced pressure to leave a residue, which was purified with preparative thin layer chromatography on silica gel. Elution with a mixture of chloroform and methanol (5:1) gave 17-allyl-12-12-{4-(3-carboxypropylcarbamoyloxy)-3-methoxycyclohexyl-[-methylvinyl]-1,14-dihydroxy-23,25-dimethoxy-13,19, 21, 27-tetramethyl-11,28-dioxa-4-azatricyclo-[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetraone (18.1 mg).
- Ir ν(CHCl 3) 3550-3100, 2870, 2750-2400, 1730, 1690, 1630 cm−1
Claims (19)
3. A compound of claim 2 , wherein R3 is allyl.
4 . A compound of claim 3 , wherein R1 hydroxy, 1-(lower alkythio) (lower)alkoxy, trisubstituted silyloxy or acyloxy.
5. A compound of claim 4 , wherein R1 is hydroxy, lower alkylthiomethoxy, tri(lower)alkylsilyloxy, lower alkyl-diarylsilyloxy, lower alkanoyloxy which may have carboxy, cyclo(lower)alkoxy(lower)alkanoyloxy which may have two lower alkyl groups on the cycloalkyl moiety, camphorsulfonyloxy, carboxy(lower)alkylcarbamoyloxy, tri(lower)alkylsilyl(lower)alkoxycarbonyl(lower)-alkylcarbamoyloxy, aroyloxy which may have one or two nitro, arenesulfonyloxy which may have halogen or ar(lower)alkanoyloxy which may have lower alkoxy and trihalo(lower)alkyl, and R2 is hydroxy or lower alkanoyloxy.
6. A compound of claim 5 , which is 17-allyl-1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxycyclohexyl) -1-methylvinyl[-23,25-dimethoxy-13,19,21, 27-tetramethyl-11, 28-dioxa-4-azatricyclo-[22.3. 1.04,9octacos-18-ene-2,3,10,16-tetraone.
7. A compound of claim 5 , wherein R1 is lower alkanoyloxy and R2 is hydroxy or lower alkanoyloxy.
8. A compound of claim 7 , which is 12-[2- (4-acetoxy-3-methoxycyclohexyl) -1-methylvinyl]-17-allyl-1, 14-dihydroxy-2.3, 25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9)-octacos-18-ene-2,3,10,16-tetraone.
9. A compound of claim 7 , which is 14-acetoxy-12- [2-(4-acetoxy-3-methoxycyclohexyl)-1-methylvinyl]-17-allyl-l-hydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo-[22.3.1.04,9-octacos-18-ene-2,3,10,16-tetraone.
10. A compound of claim 2 , which is 17-ethyl-1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxycyclohexyl)-i-methylvinyl)-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo-[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetraone.
11. A compound of claim 2 , which is 1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylvinyl]-23,25-dimethoxy-13,19,17,21,27-pentamethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]-octacos-18-ene-2,3,10,16-tetraone.
12. A compound of claim 1 , wherein R1 is hydroxy, lower alkylthiomethoxy, lower alkanoyloxy or arenesulfonyloxy which may have halogen, R2 is hydrogen or hydroxy, n is an integer of 2 and the symbol of a line and dotted line is a double bond.
13. A compound of claim 1 , which is 16-allyl-1,13-dihydroxy-11-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylvinyl]-22,24-dimethoxy-12,18,20,26-tetramethyl-10,27-dioxa-4-azatricyclo-[21.3.1.04,8]heptacos-17-ene-2,3,9,15-tetraone.
14. A process for production of the compound of the formula:
wherein R1 is hydroxy or protected hydroxy, R2 is hydrogen, hydroxy or protected hydroxy, R3 is methyl, ethyl, propyl or allyl, n is an integer of 1 or 2, and the symbol of a line and dotted line is a single bond or a double bond, and salt thereof, which comprises
(a) culturing a FR-900506, FR-900520 and/or FR-900525 substance(s)-producing strain belonging to the genus Streptomyces in a nutrient medium and recovering the FR-900506, FR-900520 and/or FR-900525 substance(s) to R3 the FR-900506, FR-900520 and/or FR-900525 substance(s);
(b) culturing a FR-900520 and/or FR-900523 substance(s)-producing strain belonging to the genus Streptomyces in a nutrient medium and recovering the FR-900520 and/or FR-900523 substance(s) to give the FR-900520 and/or FR-900523 substance(s);
(c) introducing a hydroxy-protective group into a compound of the formula:
wherein R2, R3 n and the symbol of a line and dotted line are each as defined above, to give a compound of the formula:
a
wherein R2, R3, n and the symbol of a line and dotted line are each as defiend above, and R1 is protected hydroxy, or a salt thereof;
(d) introducing a hydroxy-protective group into a compound of the formula:
wherein R1, R3, n and the symbol of a line and the dotted line are each as defined above, or a salt thereof, to give a compound of the formula:
wherein R1, R3, n and the symbol of a line and dotted line are each as defined above, and Ra 2 is protected hydroxy, or a salt thereof;
(3) reacting a compound of the formula:
wherein R1, R2 and n are each as defined above, and Rb 2 is a leaving group, or a salt thereof with a base, to give a compound of the formula:
wherein R1, R3 and n are each as defined above, or a salt thereof;
(f) oxidizing a compound of the formula:
wherein R1, R3 and n are each as defined above, or a salt thereof, to give a compound of the formula:
wherein R1, R3 and n are each as defiend above, or a salt thereof; and
(g) reducing a compound of the formula:
wherein R1, R2, n and the symbol of a line and dotted line are each as defined above, or a salt thereof, to give a compound of the formula:
wherein R1, R2, n and the symbol of a line and dotted line are each as defined above, or a salt thereof, and (h) removing a carboxy-protective group from a compound of the formula:
wherein R2, R3, n and the symbol of a line and dotted line are each as defined above, and Rb 1 is protected carboxy(lower)alkylcarbamoyloxy, or a salt thereof to give a compound of the formula:
wherein R2, R3, n and the symbol of a line and dotted line are each as defined above, and Rc 1 is carboxy(lower)alkylcarbamoyloxy, or a salt thereof.
15. A pharmaceutical composition containing tricyclo compounds of claim 1 , as active ingredients, in association with a pharmaceutically acceptable, substantially non-toxic carrier or excipient.
16. A use of tricyclo compounds of claim 1 for manufacture of medicament for treating or preventing resistance by transplantation, autoimmune diseases and infectious diseases.
17. A method for treating or preventing resistance by transplantation, autoimmune diseases and infectious diseases by administering tricyclo compounds of claim 1
18. A biologically pure culture of the microorganism Streptomyces tsukubaensis No. 9993.
19. A biologically pure culture of the microorganisms Streptomyces hygrosconicus subsp. yakushimaensis No. 7238.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/426,659 US20040029908A1 (en) | 1984-12-03 | 2003-05-01 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US10/983,710 US20050124646A1 (en) | 1984-12-03 | 2004-11-09 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
Applications Claiming Priority (17)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB8430455 | 1984-12-03 | ||
| GB848430455A GB8430455D0 (en) | 1984-12-03 | 1984-12-03 | Fr-900506 substance |
| GB858502869A GB8502869D0 (en) | 1985-02-05 | 1985-02-05 | Ws 7238 substances |
| GB8502869 | 1985-02-05 | ||
| GB858508420A GB8508420D0 (en) | 1985-04-01 | 1985-04-01 | Fr-900506 & fr-900525 substances |
| GB8508420 | 1985-04-01 | ||
| US06/799,855 US4894366A (en) | 1984-12-03 | 1985-11-20 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US06/868,749 US4929611A (en) | 1984-12-03 | 1986-05-30 | Method for immunosuppression |
| US07/491,205 US5110811A (en) | 1984-12-03 | 1990-03-09 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US82438492A | 1992-01-23 | 1992-01-23 | |
| US08/450,412 US5624842A (en) | 1984-12-03 | 1995-05-25 | Strain of Streptomyces for the production of tricyclo compounds |
| US08/753,950 US5830717A (en) | 1984-12-03 | 1996-12-03 | Process for production of tricyclo compounds with streptomyces |
| US09/084,099 US6028097A (en) | 1984-03-12 | 1998-05-26 | Method of immunosuppression |
| US09/411,630 US6201005B1 (en) | 1984-12-03 | 1999-10-01 | Method for inhibiting lymphokine production |
| US09/721,650 US6482845B1 (en) | 1984-12-03 | 2000-11-27 | Method for inhibiting interleukin-2 production using tricyclo compounds |
| US10/247,305 US20030170831A1 (en) | 1984-12-03 | 2002-09-20 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US10/426,659 US20040029908A1 (en) | 1984-12-03 | 2003-05-01 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/247,305 Continuation US20030170831A1 (en) | 1984-12-03 | 2002-09-20 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/983,710 Continuation US20050124646A1 (en) | 1984-12-03 | 2004-11-09 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040029908A1 true US20040029908A1 (en) | 2004-02-12 |
Family
ID=27262535
Family Applications (15)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US06/799,855 Expired - Lifetime US4894366A (en) | 1984-03-12 | 1985-11-20 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US06/868,749 Expired - Lifetime US4929611A (en) | 1984-03-12 | 1986-05-30 | Method for immunosuppression |
| US07/395,798 Expired - Lifetime US4956352A (en) | 1984-12-03 | 1989-08-17 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US07/491,205 Expired - Lifetime US5110811A (en) | 1984-03-12 | 1990-03-09 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US08/450,412 Expired - Lifetime US5624842A (en) | 1984-03-12 | 1995-05-25 | Strain of Streptomyces for the production of tricyclo compounds |
| US08/450,639 Expired - Lifetime US5496727A (en) | 1984-12-03 | 1995-05-25 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US08/545,172 Expired - Fee Related US5565559A (en) | 1984-12-03 | 1995-10-19 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US08/753,950 Expired - Lifetime US5830717A (en) | 1984-03-12 | 1996-12-03 | Process for production of tricyclo compounds with streptomyces |
| US09/084,099 Expired - Fee Related US6028097A (en) | 1984-03-12 | 1998-05-26 | Method of immunosuppression |
| US09/411,630 Expired - Fee Related US6201005B1 (en) | 1984-12-03 | 1999-10-01 | Method for inhibiting lymphokine production |
| US09/721,650 Expired - Fee Related US6482845B1 (en) | 1984-12-03 | 2000-11-27 | Method for inhibiting interleukin-2 production using tricyclo compounds |
| US10/247,305 Abandoned US20030170831A1 (en) | 1984-12-03 | 2002-09-20 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US10/422,901 Abandoned US20030229115A1 (en) | 1984-12-03 | 2003-04-25 | Tricyclo compounds, a process for their production and pharmaceutical composition containing the same |
| US10/426,659 Abandoned US20040029908A1 (en) | 1984-12-03 | 2003-05-01 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US10/983,710 Abandoned US20050124646A1 (en) | 1984-12-03 | 2004-11-09 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
Family Applications Before (13)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US06/799,855 Expired - Lifetime US4894366A (en) | 1984-03-12 | 1985-11-20 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US06/868,749 Expired - Lifetime US4929611A (en) | 1984-03-12 | 1986-05-30 | Method for immunosuppression |
| US07/395,798 Expired - Lifetime US4956352A (en) | 1984-12-03 | 1989-08-17 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US07/491,205 Expired - Lifetime US5110811A (en) | 1984-03-12 | 1990-03-09 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US08/450,412 Expired - Lifetime US5624842A (en) | 1984-03-12 | 1995-05-25 | Strain of Streptomyces for the production of tricyclo compounds |
| US08/450,639 Expired - Lifetime US5496727A (en) | 1984-12-03 | 1995-05-25 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US08/545,172 Expired - Fee Related US5565559A (en) | 1984-12-03 | 1995-10-19 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US08/753,950 Expired - Lifetime US5830717A (en) | 1984-03-12 | 1996-12-03 | Process for production of tricyclo compounds with streptomyces |
| US09/084,099 Expired - Fee Related US6028097A (en) | 1984-03-12 | 1998-05-26 | Method of immunosuppression |
| US09/411,630 Expired - Fee Related US6201005B1 (en) | 1984-12-03 | 1999-10-01 | Method for inhibiting lymphokine production |
| US09/721,650 Expired - Fee Related US6482845B1 (en) | 1984-12-03 | 2000-11-27 | Method for inhibiting interleukin-2 production using tricyclo compounds |
| US10/247,305 Abandoned US20030170831A1 (en) | 1984-12-03 | 2002-09-20 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US10/422,901 Abandoned US20030229115A1 (en) | 1984-12-03 | 2003-04-25 | Tricyclo compounds, a process for their production and pharmaceutical composition containing the same |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/983,710 Abandoned US20050124646A1 (en) | 1984-12-03 | 2004-11-09 | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
Country Status (24)
| Country | Link |
|---|---|
| US (15) | US4894366A (en) |
| EP (1) | EP0184162B1 (en) |
| JP (6) | JPH0372483A (en) |
| KR (5) | KR930010704B1 (en) |
| CN (1) | CN1013687B (en) |
| AT (1) | ATE104984T1 (en) |
| AU (1) | AU592067B2 (en) |
| CA (1) | CA1338491C (en) |
| CY (1) | CY1912A (en) |
| DE (2) | DE3587806T2 (en) |
| DK (1) | DK169550B1 (en) |
| ES (1) | ES8705038A1 (en) |
| FI (2) | FI87803C (en) |
| GR (1) | GR852904B (en) |
| HK (1) | HK18596A (en) |
| HU (1) | HU195250B (en) |
| IE (1) | IE62865B1 (en) |
| IL (2) | IL92345A (en) |
| LU (1) | LU90317I2 (en) |
| MX (1) | MX9202943A (en) |
| NL (1) | NL960023I2 (en) |
| NO (1) | NO168372C (en) |
| NZ (1) | NZ214407A (en) |
| PT (1) | PT81589B (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110212988A1 (en) * | 2008-10-08 | 2011-09-01 | Hironori Masui | Tacrolimus preparation for external applications |
Families Citing this family (311)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4894366A (en) * | 1984-12-03 | 1990-01-16 | Fujisawa Pharmaceutical Company, Ltd. | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US5254562A (en) * | 1984-12-03 | 1993-10-19 | Fujisawa Pharmaceutical Company, Ltd. | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US5266692A (en) * | 1984-12-03 | 1993-11-30 | Fujisawa Pharmaceutical Co., Ltd. | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| GB8608080D0 (en) * | 1986-04-02 | 1986-05-08 | Fujisawa Pharmaceutical Co | Solid dispersion composition |
| ATE98700T1 (en) * | 1987-06-05 | 1994-01-15 | Fujisawa Pharmaceutical Co | ANTI-FR-900506 SUBSTANCES ANTIBODIES AND HIGHLY SENSITIVE ENZYME IMMUNOASSAY PROCEDURE. |
| DE3737523A1 (en) * | 1987-11-05 | 1989-05-18 | Bayer Ag | USE OF SUBSTITUTED HYDROXYPIPERIDINES AS ANTIVIRAL AGENTS |
| DE3838035C2 (en) * | 1987-11-09 | 1994-03-24 | Sandoz Ag | New use of 11,28-dioxa-4-azatricyclo [22.3.1.O.4,...,... 9] octacos-18-ene derivatives |
| US5366971A (en) * | 1987-11-09 | 1994-11-22 | Sandoz Ltd. | Use of 11,28-dioxa-4-azatricyclo[22.3.1.04,9 ]octacos-18-ene derivatives and pharmaceutical compositions containing them |
| AT400808B (en) * | 1987-11-09 | 1996-03-25 | Sandoz Ag | Use of tricyclic compounds for producing topical pharmaceuticals |
| DE3844904C2 (en) * | 1987-11-09 | 1997-01-30 | Sandoz Ag | New use of 11,28-dioxa-4-azatricyclo- [22.3.1.0 · 4 ·, · 9 ·] octacos-18-ene derivatives |
| DK175235B1 (en) * | 1987-11-09 | 2004-07-19 | Novartis Ag | New use of azatricyclo derivatives and pharmaceutical compositions containing them |
| JP2799208B2 (en) * | 1987-12-09 | 1998-09-17 | フアイソンズ・ピーエルシー | Macrocyclic compound |
| GB8728820D0 (en) * | 1987-12-09 | 1988-01-27 | Fisons Plc | Compounds |
| AT407957B (en) * | 1987-12-17 | 2001-07-25 | Novartis Erfind Verwalt Gmbh | Novel use of 11,28-dioxa-4- azatricyclo(22.3.1.04,9)octacos-18-ene derivatives and pharmaceutical preparations containing them |
| EP0323865A1 (en) * | 1988-01-07 | 1989-07-12 | Merck & Co. Inc. | Novel immunosuppressant agent |
| US5290772A (en) * | 1988-06-29 | 1994-03-01 | Merck & Co., Inc. | Immunosuppressant agent |
| EP0349061B1 (en) * | 1988-06-29 | 1995-03-29 | Merck & Co. Inc. | Immunosuppressant agent |
| US4981792A (en) * | 1988-06-29 | 1991-01-01 | Merck & Co., Inc. | Immunosuppressant compound |
| DE68925080T2 (en) * | 1988-08-01 | 1996-05-15 | Fujisawa Pharmaceutical Co | FR-901154 and FR-901155 derivatives, process for their preparation |
| US5202258A (en) * | 1988-08-05 | 1993-04-13 | Merck & Co., Inc. | Immunosuppressant-producing culture |
| EP0356399A3 (en) * | 1988-08-26 | 1991-03-20 | Sandoz Ag | Substituted 4-azatricyclo (22.3.1.04.9) octacos-18-ene derivatives, their preparation and pharmaceutical compositions containing them |
| EP0358508A3 (en) * | 1988-09-08 | 1991-03-20 | Merck & Co. Inc. | Novel immunosuppressant compound |
| US4980466A (en) * | 1988-10-12 | 1990-12-25 | Merck & Co., Inc. | Hydroxide mediated FK-506 rearrangement product |
| CA1316916C (en) * | 1988-10-12 | 1993-04-27 | David Askin | Hydroxide mediated fk-506 rearrangement product |
| DE68904037T2 (en) * | 1988-10-12 | 1993-06-03 | Merck & Co Inc | METHOD FOR TRANSFERRING FK-506 WITH THE PARTICIPATION OF HYDROXIDE. |
| GB2225576B (en) * | 1988-11-29 | 1992-07-01 | Sandoz Ltd | Substituted azatricyclo derivatives and metabolites,their preparation and pharmaceutical compositions containing them |
| US4975372A (en) * | 1989-01-13 | 1990-12-04 | Merck & Co., Inc. | Microbial transformation product of L-683,590 |
| US5268370A (en) * | 1989-01-13 | 1993-12-07 | Merck & Co., Inc. | Microbial transformation product of L-679,934 |
| EP0378317A3 (en) * | 1989-01-13 | 1990-11-28 | Merck & Co. Inc. | Microbial transformation product of l-679,934 |
| EP0378320A3 (en) * | 1989-01-13 | 1990-11-28 | Merck & Co. Inc. | Microbial transformation product |
| EP0388152B1 (en) * | 1989-03-15 | 1994-10-12 | Merck & Co. Inc. | Process for producing an immunosuppressant agent (demethimmunomycin) using a mutant strain of a microorganism |
| EP0388153B1 (en) * | 1989-03-15 | 1994-12-28 | Merck & Co. Inc. | Immunosuppressant agent |
| US5272068A (en) * | 1989-03-15 | 1993-12-21 | Merck & Co., Inc. | Process for producing immunosuppressant agent L-683942 by fermentation |
| US4940797A (en) * | 1989-03-23 | 1990-07-10 | Merck & Co., Inc. | Process for synthesis of FK-506 C10-C18 intermediates |
| US5155228A (en) * | 1989-03-23 | 1992-10-13 | Merck & Co., Inc. | FK-506 C10-C18 process intermediates |
| CA2014841A1 (en) * | 1989-04-21 | 1990-10-21 | Byron H. Arison | Macrolides having immunosuppressive activity |
| US5068323A (en) * | 1989-04-21 | 1991-11-26 | Merck & Co., Inc. | Thermally re-arranged FK-506 derivatives having immunosuppressant activity |
| US5057608A (en) * | 1989-04-21 | 1991-10-15 | Merck & Co., Inc. | Immunoregulants, immunosuppressants, process to make ring expanded macrolide related to FK-506/FK-520 |
| US5270187A (en) * | 1989-05-05 | 1993-12-14 | Merck & Co., Inc. | Microbial transformation product |
| EP0396400A1 (en) * | 1989-05-05 | 1990-11-07 | Merck & Co. Inc. | Microbial transformation product |
| US4987139A (en) * | 1989-05-05 | 1991-01-22 | Merck & Co., Inc. | FK-520 microbial transformation product |
| IE64214B1 (en) * | 1989-06-06 | 1995-07-26 | Fujisawa Pharmaceutical Co | Macrolides for the treatment of reversible obstructive airways diseases |
| CA2018710A1 (en) * | 1989-06-13 | 1990-12-13 | Shieh-Shung T. Chen | L-683,590 microbial transformation product |
| US5138052A (en) * | 1989-06-13 | 1992-08-11 | Merck & Co., Inc. | L-683,590 microbial transformation product |
| EP0402931A1 (en) * | 1989-06-14 | 1990-12-19 | Sandoz Ltd. | Heteroatoms-containing tricyclic compounds |
| US5164525A (en) * | 1989-06-30 | 1992-11-17 | Merck & Co., Inc. | Synthetic process for fk-506 type macrolide intermediates |
| US5235066A (en) * | 1989-06-30 | 1993-08-10 | Merck & Co., Inc. | FK-506 type macrolide intermediate |
| EP0413532A3 (en) * | 1989-08-18 | 1991-05-15 | Fisons Plc | Macrocyclic compounds |
| EP0487593A1 (en) * | 1989-08-18 | 1992-06-03 | FISONS plc | Macrocyclic compounds |
| US5011943A (en) * | 1989-08-28 | 1991-04-30 | Merck Frosst Canada, Inc. | FK-506 C10 -C24 process intermediates |
| GR1001225B (en) * | 1989-09-14 | 1993-06-30 | Fisons Plc | Novel macrocyclic compositions and new application method thereof |
| US5215995A (en) * | 1989-10-16 | 1993-06-01 | Fujisawa Pharmaceutical Co., Ltd. | Hair revitalizing agent |
| DE69021833T2 (en) * | 1989-11-09 | 1996-03-21 | Sandoz Ag | Tricyclic compounds containing heteroatoms. |
| IE904050A1 (en) * | 1989-11-13 | 1991-05-22 | Merck & Co Inc | Aminomacrolides and derivatives having immunosuppressive¹activity |
| US5208228A (en) * | 1989-11-13 | 1993-05-04 | Merck & Co., Inc. | Aminomacrolides and derivatives having immunosuppressive activity |
| GB8925797D0 (en) * | 1989-11-15 | 1990-01-04 | Fisons Plc | Compositions |
| EP0444829A3 (en) * | 1990-02-27 | 1992-06-03 | Fisons Plc | Immunosuppressive compounds |
| US5064835A (en) * | 1990-03-01 | 1991-11-12 | Merck & Co., Inc. | Hydroxymacrolide derivatives having immunosuppressive activity |
| US5260301A (en) * | 1990-03-01 | 1993-11-09 | Fujisawa Pharmaceutical Co., Ltd. | Pharmaceutical solution containing FK-506 |
| WO1991013899A1 (en) * | 1990-03-12 | 1991-09-19 | Fujisawa Pharmaceutical Co., Ltd. | Tricyclo compounds |
| US5296489A (en) * | 1990-03-13 | 1994-03-22 | Fisons | Immunosuppressive macrocyclic compounds |
| IE910847A1 (en) * | 1990-03-13 | 1991-09-25 | Fisons Plc | Immunosuppressive macrocyclic compounds |
| CA2040551A1 (en) * | 1990-04-30 | 1991-10-31 | Mark T. Goulet | Deoxymacrolide derivatives having immunosuppressive activity |
| WO1991017754A1 (en) * | 1990-05-11 | 1991-11-28 | Fujisawa Pharmaceutical Co., Ltd. | Methods for treating and preventing inflammation of mucosa and blood vessels using fk 506 and related compounds |
| US5643901A (en) * | 1990-06-11 | 1997-07-01 | Fujisawa Pharmaceutical Co., Ltd. | Medicament for treating idiopathic thrombocytopenic purpura |
| EP0533930A1 (en) * | 1990-06-11 | 1993-03-31 | Fujisawa Pharmaceutical Co., Ltd. | Use of a macrolide compound such as fk 506 for manufacturing a medicament for treating idiopathic thrombocytopenic purpura and basedow's disease |
| GB9014136D0 (en) * | 1990-06-25 | 1990-08-15 | Fujisawa Pharmaceutical Co | Tricyclo compounds,a process for their production and a pharmaceutical composition containing the same |
| US5342935A (en) * | 1990-06-25 | 1994-08-30 | Merck & Co., Inc. | Antagonists of immunosuppressive macrolides |
| US5210030A (en) * | 1990-06-25 | 1993-05-11 | Merck & Co., Inc. | Process for selectively acylating immunomycin |
| CA2044846A1 (en) * | 1990-06-25 | 1991-12-26 | Thomas R. Beattie | Antagonists of immunosuppressive macrolides |
| US5190950A (en) * | 1990-06-25 | 1993-03-02 | Merck & Co., Inc. | Antagonists of immunosuppressive macrolides |
| GB9014681D0 (en) * | 1990-07-02 | 1990-08-22 | Fujisawa Pharmaceutical Co | Tricyclo compounds,a process for their production and a pharmaceutical composition containing the same |
| MY110418A (en) * | 1990-07-02 | 1998-05-30 | Novartis Ag | Heteroatoms-containing tricyclic compounds. |
| EP0466365A3 (en) * | 1990-07-03 | 1992-04-15 | Merck & Co. Inc. | Novel immunosuppressant fermentation products of a microorganism |
| GB2245891A (en) * | 1990-07-09 | 1992-01-15 | Fujisawa Pharmaceutical Co | Tricyclo compounds |
| GB2246350A (en) * | 1990-07-23 | 1992-01-29 | Fujisawa Pharmaceutical Co | Tricyclo compounds |
| US5089517A (en) * | 1990-08-03 | 1992-02-18 | The Board Of Trustees Of The Leland Stanford Junior University | Neuroprotection by indolactam v and derivatives thereof |
| WO1992003441A1 (en) * | 1990-08-18 | 1992-03-05 | Fisons Plc | Macrocyclic compounds |
| GB2247620A (en) * | 1990-09-07 | 1992-03-11 | Fujisawa Pharmaceutical Co | The use of macrolide compounds for cytomegalovirus infection |
| CA2051872A1 (en) * | 1990-09-24 | 1992-03-25 | Kevin M. Byrne | Directed biosynthesis process for prolyl-immunomycin |
| GB2248184A (en) * | 1990-09-28 | 1992-04-01 | Fujisawa Pharmaceutical Co | New use of macrolide compounds for active oxygen-mediated diseases |
| US5143918A (en) * | 1990-10-11 | 1992-09-01 | Merck & Co., Inc. | Halomacrolides and derivatives having immunosuppressive activity |
| US5233036A (en) * | 1990-10-16 | 1993-08-03 | American Home Products Corporation | Rapamycin alkoxyesters |
| US5348966A (en) * | 1990-10-24 | 1994-09-20 | Fujisawa Pharmaceutical Co., Ltd. | Method for treating pyoderma and Sezary's syndrome using FK 506 and related compounds |
| CA2054128A1 (en) * | 1990-10-29 | 1992-04-30 | Kevin M. Byrne | Process for the production of analogues of immunomycin |
| PH31204A (en) * | 1990-11-02 | 1998-05-05 | Fujisawa Pharmaceutical Co | Pharmaceutical composition. |
| CA2054983A1 (en) * | 1990-11-08 | 1992-05-09 | Sotoo Asakura | Suspendible composition and process for preparing the same |
| GB2249787A (en) * | 1990-11-19 | 1992-05-20 | Fujisawa Pharmaceutical Co | Lactone compounds |
| GB9027471D0 (en) * | 1990-12-19 | 1991-02-06 | Fujisawa Pharmaceutical Co | Novel compound |
| US5116756A (en) * | 1991-01-28 | 1992-05-26 | Merck & Co., Inc. | Process for producing FK-506 |
| US5194378A (en) * | 1991-01-28 | 1993-03-16 | Merck & Co., Inc. | Process for producing fk-506 |
| WO1992013862A1 (en) * | 1991-02-05 | 1992-08-20 | Fujisawa Pharmaceutical Co., Ltd. | Lactone compounds |
| US5147877A (en) * | 1991-04-18 | 1992-09-15 | Merck & Co. Inc. | Semi-synthetic immunosuppressive macrolides |
| DE69231644T2 (en) * | 1991-04-26 | 2001-05-23 | Fujisawa Pharmaceutical Co., Ltd. | USE OF MACROLID COMPOUNDS FOR EYE DISEASES |
| US5565560A (en) * | 1991-05-13 | 1996-10-15 | Merck & Co., Inc. | O-Aryl,O-alkyl,O-alkenyl and O-alkynylmacrolides having immunosuppressive activity |
| US5262533A (en) * | 1991-05-13 | 1993-11-16 | Merck & Co., Inc. | Amino O-aryl macrolides having immunosuppressive activity |
| US5162334A (en) * | 1991-05-13 | 1992-11-10 | Merck & Co., Inc. | Amino O-alkyl, O-alkenyl and O-alkynlmacrolides having immunosuppressive activity |
| US5250678A (en) * | 1991-05-13 | 1993-10-05 | Merck & Co., Inc. | O-aryl, O-alkyl, O-alkenyl and O-alkynylmacrolides having immunosuppressive activity |
| EP0528452A1 (en) * | 1991-06-19 | 1993-02-24 | Merck & Co. Inc. | Immunomycin enzymatic and/or microbial methylation products |
| US5225403A (en) * | 1991-06-25 | 1993-07-06 | Merck & Co., Inc. | C-21 hydroxylated FK-506 antagonist |
| US5149701A (en) * | 1991-08-01 | 1992-09-22 | Merck & Co., Inc. | C-31 methylated FR-900520 cyclic hemiketal immunosuppressant agents |
| US5198358A (en) * | 1991-08-28 | 1993-03-30 | Merck & Co., Inc. | Microorganism for producing C-31 desmethyl FR-900520 cyclic hemiketal immunosuppressant agent |
| US5273979A (en) * | 1991-08-01 | 1993-12-28 | Merck & Co., Inc. | C-31 desmethyl FR-900520 cyclic hemiketal immunosuppressant agent |
| US5189042A (en) * | 1991-08-22 | 1993-02-23 | Merck & Co. Inc. | Fluoromacrolides having immunosuppressive activity |
| US5708002A (en) * | 1991-09-05 | 1998-01-13 | Abbott Laboratories | Macrocyclic immunomodulators |
| CA2103453A1 (en) * | 1991-09-05 | 1993-03-06 | Jay R. Luly | Macrocyclic immunomodulators |
| US5252732A (en) * | 1991-09-09 | 1993-10-12 | Merck & Co., Inc. | D-heteroaryl, O-alkylheteroaryl, O-alkenylheteroaryl and O-alkynylheteroarylmacrolides having immunosuppressive activity |
| US5247076A (en) * | 1991-09-09 | 1993-09-21 | Merck & Co., Inc. | Imidazolidyl macrolides having immunosuppressive activity |
| US5208241A (en) * | 1991-09-09 | 1993-05-04 | Merck & Co., Inc. | N-heteroaryl, n-alkylheteroaryl, n-alkenylheteroaryl and n-alkynylheteroarylmacrolides having immunosuppressive activity |
| ZA926812B (en) | 1991-09-09 | 1993-04-28 | Merck & Co Inc | O-heteroaryl,o-alkylheteroaryl,o-alkenylheteroaryl and o-alkynylheteroaryl macrolides having immunosupressive activity |
| US5164495A (en) * | 1991-09-18 | 1992-11-17 | Abbott Laboratories | Method for preparing a dicarboxylic acid half-acid ester of FK506 |
| US5221625A (en) * | 1992-01-10 | 1993-06-22 | Merck & Co., Inc. | Cyclcic FR-900520 microbial biotransformation agent |
| WO1993014780A1 (en) * | 1992-01-28 | 1993-08-05 | Abraham Karpas | The use of a compound for the manufacture of a medicament for the treatment of hiv infection |
| JP3140228B2 (en) * | 1992-02-17 | 2001-03-05 | ファイザー製薬株式会社 | Novel macrocyclic lactone and its producing bacteria |
| JP2564765B2 (en) * | 1992-03-02 | 1996-12-18 | ファイザー・インコーポレーテッド | Fluoro sugar derivatives of macrolide antibiotics |
| DE69330747T2 (en) * | 1992-03-02 | 2002-05-29 | Pfizer Inc., New York | DESOSAMINO DERIVATIVES OF MACROLIDES AS IMMUNOSUPPRESSIVE AND ANTIFUNGAL AGENTS |
| JP2563080B2 (en) * | 1992-03-02 | 1996-12-11 | フアイザー・インコーポレイテツド | Sugar derivative of macrolide |
| ATE159725T1 (en) * | 1992-03-02 | 1997-11-15 | Pfizer | 2-AMINO SUGAR MACROLIDE DERIVATIVES |
| EP0562853B1 (en) | 1992-03-27 | 1996-06-05 | American Home Products Corporation | 29-Demethoxyrapamycin for inducing immunosuppression |
| CA2091194A1 (en) * | 1992-04-08 | 1993-10-09 | Richard D. Connell | 2-oxo-ethyl derivatives as immunosuppressants |
| HUT66531A (en) * | 1992-05-07 | 1994-12-28 | Sandoz Ag | Heteroatoms-containing tricyclic compounds, pharmaceutical prepns contg. them and process for producing them |
| WO1993025533A1 (en) * | 1992-06-05 | 1993-12-23 | Abbott Laboratories | Methods and reagents for the determination of immunosuppressive agents |
| US5284877A (en) * | 1992-06-12 | 1994-02-08 | Merck & Co., Inc. | Alkyl and alkenyl macrolides having immunosuppressive activity |
| US5284840A (en) * | 1992-06-12 | 1994-02-08 | Merck & Co., Inc. | Alkylidene macrolides having immunosuppressive activity |
| US5264355A (en) * | 1992-07-02 | 1993-11-23 | Merck & Co., Inc. | Methlating enzyme from streptomyces MA6858 |
| ZA935112B (en) * | 1992-07-17 | 1994-02-08 | Smithkline Beecham Corp | Rapamycin derivatives |
| US5324644A (en) * | 1992-07-28 | 1994-06-28 | Merck & Co., Inc. | Process for producing immunosuppressant agent |
| CA2142197C (en) * | 1992-08-12 | 2004-06-22 | Masakazu Kobayashi | Monoclonal antibody recognizing fk506-binding protein method for assaying fk506-binding protein level, and kit therefor |
| US5365948A (en) * | 1992-08-21 | 1994-11-22 | J & W Mcmichael Software Inc. | Method for use in treating a patient with FK 506 to prevent an adverse immune response |
| US5268282A (en) * | 1992-09-28 | 1993-12-07 | Merck & Co., Inc. | Cyclic FR-900520 microbial biotransformation agent |
| US5283183A (en) * | 1992-09-28 | 1994-02-01 | Merck & Co., Inc. | Cyclic FR-900520 microbial biotransformation agent |
| US5290689A (en) * | 1992-09-28 | 1994-03-01 | Merck & Co., Inc. | New cyclic FR-900520 microbial biotransformation agent |
| US5268281A (en) * | 1992-09-28 | 1993-12-07 | Merck & Co., Inc. | Cyclic FR-900520 microbial biotransformation agent |
| US5318895A (en) * | 1992-10-05 | 1994-06-07 | Merck & Co., Inc. | Aspergillus niger mutants |
| GB9227055D0 (en) * | 1992-12-29 | 1993-02-24 | Fujisawa Pharmaceutical Co | New use |
| DE4300478C2 (en) * | 1993-01-11 | 1998-05-20 | Eos Electro Optical Syst | Method and device for producing a three-dimensional object |
| EP0690713A4 (en) * | 1993-03-17 | 1996-04-03 | Abbott Lab | Substituted alicyclic amine-containing macrocyclic immunomodulators |
| AT408520B (en) * | 1993-05-27 | 2001-12-27 | Novartis Erfind Verwalt Gmbh | Pharmaceutical formulations |
| MY110603A (en) * | 1993-05-27 | 1998-08-29 | Novartis Ag | Tetrahydropyran derivatives |
| CH686761A5 (en) * | 1993-05-27 | 1996-06-28 | Sandoz Ag | Pharmaceutical formulations. |
| GB2279006A (en) * | 1993-06-03 | 1994-12-21 | Fujisawa Pharmaceutical Co | Treatment of amyotrophic lateral sclerosis |
| JPH06345646A (en) * | 1993-06-08 | 1994-12-20 | Fujisawa Pharmaceut Co Ltd | Lotion preparation |
| US5352783A (en) * | 1993-06-09 | 1994-10-04 | Merck & Co., Inc. | Microbial transformation product having immunosuppressive activity |
| US5359060A (en) * | 1993-07-06 | 1994-10-25 | Pfizer, Inc. | Phosponated derivatives of macrolides |
| EP0711298A1 (en) * | 1993-07-30 | 1996-05-15 | Abbott Laboratories | Activated macrolactams having immunomodulatory activities |
| GB2281294A (en) * | 1993-08-23 | 1995-03-01 | Fujisawa Pharmaceutical Co | Process for producing half esters of the macrolide FK506 |
| US5616588A (en) | 1993-09-30 | 1997-04-01 | American Home Products Corporation | Rapamycin formulation for IV injection |
| UA41884C2 (en) * | 1993-11-05 | 2001-10-15 | Амерікан Хоум Продактс Корпорейшн | method for the isolation of rapacimin from acid, basic and non polar neutral admixtures being present in the concentrate of extract of fermentation broth of mother liquors |
| US5898029A (en) * | 1994-04-12 | 1999-04-27 | The John Hopkins University | Direct influences on nerve growth of agents that interact with immunophilins in combination with neurotrophic factors |
| US5880280A (en) * | 1994-06-15 | 1999-03-09 | Merck & Co., Inc. | Aryl, alkyl, alkenyl and alkynylmacrolides having immunosuppressive activity |
| US5693648A (en) * | 1994-09-30 | 1997-12-02 | Merck & Co., Inc. | O-aryl, O-alkyl, O-alkenyl and O-alkynyl-macrolides having immunosuppressive activity |
| MX9702699A (en) | 1994-10-26 | 1997-06-28 | Novartis Ag | Pharmaceutical compositions. |
| AR004480A1 (en) * | 1995-04-06 | 1998-12-16 | Amico Derin C D | ASCOMICINE COMPOUNDS HAVING ANTI-INFLAMMATORY ACTIVITY, PROCEDURE TO PREPARE THEM, USE OF SUCH COMPOUNDS TO PREPARE PHARMACEUTICAL AGENTS AND PHARMACEUTICAL COMPOSITIONS INCLUDING THEM |
| US5616595A (en) * | 1995-06-07 | 1997-04-01 | Abbott Laboratories | Process for recovering water insoluble compounds from a fermentation broth |
| DK0851753T3 (en) * | 1995-09-19 | 2004-03-15 | Fujisawa Pharmaceutical Co | aerosol |
| ATE222502T1 (en) | 1996-07-30 | 2002-09-15 | Novartis Ag | COMBINATION PREPARATION WITH AN INHIBITANT EFFECT ON TRANSPLANT REJECTION, AUTOIMMUNE DISEASES AND INFLAMMATION, CONTAINING CYCLOSPORIN A AND 40-0-(2-HYDROXYETHYL)-RAPAMYCIN |
| KR100244164B1 (en) * | 1997-07-15 | 2000-03-02 | 김용옥 | Water soluble polymer takurolimus conjugate compounds and its manufacturing process |
| US6562620B2 (en) * | 1997-09-19 | 2003-05-13 | Mcgill University | Medium to promote islet cell survival |
| US20030129215A1 (en) | 1998-09-24 | 2003-07-10 | T-Ram, Inc. | Medical devices containing rapamycin analogs |
| US6015815A (en) | 1997-09-26 | 2000-01-18 | Abbott Laboratories | Tetrazole-containing rapamycin analogs with shortened half-lives |
| US6890546B2 (en) | 1998-09-24 | 2005-05-10 | Abbott Laboratories | Medical devices containing rapamycin analogs |
| US7399480B2 (en) | 1997-09-26 | 2008-07-15 | Abbott Laboratories | Methods of administering tetrazole-containing rapamycin analogs with other therapeutic substances using medical devices |
| US8257725B2 (en) | 1997-09-26 | 2012-09-04 | Abbott Laboratories | Delivery of highly lipophilic agents via medical devices |
| US8057816B2 (en) | 1997-09-26 | 2011-11-15 | Abbott Laboratories | Compositions and methods of administering paclitaxel with other drugs using medical devices |
| US8257726B2 (en) | 1997-09-26 | 2012-09-04 | Abbott Laboratories | Compositions, systems, kits, and methods of administering rapamycin analogs with paclitaxel using medical devices |
| US8394398B2 (en) | 1997-09-26 | 2013-03-12 | Abbott Laboratories | Methods of administering rapamycin analogs with anti-inflammatories using medical devices |
| US7357942B2 (en) | 1997-09-26 | 2008-04-15 | Abbott Laboratories | Compositions, systems, and kits for administering zotarolimus and paclitaxel to blood vessel lumens |
| ES2192832T3 (en) * | 1998-02-23 | 2003-10-16 | Fujisawa Pharmaceutical Co | USE OF MACROLID COMPOUNDS TO TREAT GLAUCOMA. |
| AUPP223198A0 (en) * | 1998-03-06 | 1998-04-02 | Fujisawa Pharmaceutical Co., Ltd. | New use |
| RU2214244C9 (en) | 1998-03-26 | 2020-07-29 | Астеллас Фарма Инк. | Sustained-release preparations |
| SK462001A3 (en) | 1998-07-17 | 2002-02-05 | Agouron Pharma | Compounds, compositions, and methods for stimulating neuronal growth and elongation |
| US8257724B2 (en) | 1998-09-24 | 2012-09-04 | Abbott Laboratories | Delivery of highly lipophilic agents via medical devices |
| US7455853B2 (en) | 1998-09-24 | 2008-11-25 | Abbott Cardiovascular Systems Inc. | Medical devices containing rapamycin analogs |
| US7960405B2 (en) | 1998-09-24 | 2011-06-14 | Abbott Laboratories | Compounds and methods for treatment and prevention of diseases |
| EP1117801B1 (en) | 1998-10-02 | 2006-11-29 | Kosan Biosciences, Inc. | Polyketide synthase enzymes and recombinant dna constructs therefor, for the production of compounds related to fk-506 and fk-520 |
| DE19853487A1 (en) | 1998-11-19 | 2000-05-25 | Fumapharm Ag Muri | Use of dialkyl fumarate for treating transplant rejection and autoimmune disease |
| GB9826656D0 (en) | 1998-12-03 | 1999-01-27 | Novartis Ag | Organic compounds |
| US6121257A (en) * | 1999-03-31 | 2000-09-19 | Abbott Laboratories | Sulfamate containing macrocyclic immunomodulators |
| EP1165575A1 (en) * | 1999-03-31 | 2002-01-02 | Abbott Laboratories | Phosphate containing macrocyclic immunomodulators |
| US7572788B2 (en) | 1999-04-30 | 2009-08-11 | The Regents Of The University Of Michigan | Compositions and methods relating to novel compounds and targets thereof |
| US20060025388A1 (en) | 1999-04-30 | 2006-02-02 | Glick Gary D | Compositions and methods relating to novel compounds and targets thereof |
| US20050113460A1 (en) * | 1999-04-30 | 2005-05-26 | The Regents Of The University Of Michigan | Compositions and methods relating to novel compounds and targets thereof |
| ATE433752T1 (en) * | 1999-04-30 | 2009-07-15 | Univ Michigan | USE OF BENZODIAZEPINES TO TREAT AUTOIMMUNE DISEASES, INFLAMMATION, NEOPLASIA, VIRAL INFECTIONS AND ATHEROSCLEROSIS |
| US20040176358A1 (en) * | 1999-04-30 | 2004-09-09 | The Regents Of The University Of Michigan | Compositions and methods relating to novel compounds and targets thereof |
| US20030119029A1 (en) * | 1999-04-30 | 2003-06-26 | Regents Of The University Of Michigan | Compositions and methods relating to novel benzodiazepine compounds and targets thereof |
| US7063857B1 (en) | 1999-04-30 | 2006-06-20 | Sucampo Ag | Use of macrolide compounds for the treatment of dry eye |
| GB9917158D0 (en) * | 1999-07-21 | 1999-09-22 | Fujisawa Pharmaceutical Co | New use |
| US20030018044A1 (en) * | 2000-02-18 | 2003-01-23 | Peyman Gholam A. | Treatment of ocular disease |
| US6471980B2 (en) | 2000-12-22 | 2002-10-29 | Avantec Vascular Corporation | Intravascular delivery of mycophenolic acid |
| US7018405B2 (en) | 2000-12-22 | 2006-03-28 | Avantec Vascular Corporation | Intravascular delivery of methylprednisolone |
| AR033151A1 (en) * | 2001-04-12 | 2003-12-03 | Sucampo Pharmaceuticals Inc | AGENT FOR THE TOPICAL OPHTHALMIC TREATMENT OF OCULAR INFLAMMATORY DISEASES |
| US6656460B2 (en) | 2001-11-01 | 2003-12-02 | Yissum Research Development | Method and composition for dry eye treatment |
| US7452692B2 (en) * | 2002-02-13 | 2008-11-18 | Teva Gyógyszergyár Zártkörüen Müködö Részvénytársaság | Method for extracting a macrolide from biomatter |
| CA2475383C (en) | 2002-02-13 | 2009-12-22 | Biogal Gyogyszergyar Rt. | Method for extracting a macrolide from biomatter |
| WO2003072026A2 (en) * | 2002-02-22 | 2003-09-04 | 3M Innovative Properties Company | Method of reducing and treating uvb-induced immunosuppression |
| AU2002321821A1 (en) * | 2002-06-28 | 2004-01-19 | Biocon Limited | Solid state fermentation and fed batch for the production of an immunosuppressant |
| JP2005537854A (en) | 2002-09-06 | 2005-12-15 | アボット・ラボラトリーズ | Medical device comprising a hydration inhibitor |
| DE60331367D1 (en) | 2002-12-30 | 2010-04-01 | Angiotech Int Ag | ACTIVE COMPOSITION OF FAST GELING POLYMERIC COMPOSITION |
| AU2003209664A1 (en) * | 2003-02-10 | 2004-08-30 | Biocon Limited | Solid state fermentation and fed batch for the production of an immunosuppressant |
| DE04758730T1 (en) * | 2003-03-31 | 2005-06-23 | Biogal Gyogyszergyar Rt. | Crystallization and purification of macrolides |
| US20060169199A1 (en) * | 2003-03-31 | 2006-08-03 | Vilmos Keri | Crystallization and purification of macrolides |
| KR100486016B1 (en) * | 2003-07-09 | 2005-04-29 | 주식회사종근당 | The solid dispersion and manufacturing process for the rapid dissolution of tacrolimus |
| EP1558622B1 (en) * | 2003-07-24 | 2007-11-14 | TEVA Gyógyszergyár Zártkörüen Müködö Részvénytársaság | Method of purifying macrolides |
| WO2005011796A1 (en) * | 2003-08-05 | 2005-02-10 | Kaneka Corporation | Stent to be placed in vivo |
| CA2537041C (en) | 2003-08-29 | 2012-04-03 | Lifecycle Pharma A/S | Modified release compositions comprising tacrolimus |
| ATE531368T1 (en) | 2003-08-29 | 2011-11-15 | Veloxis Pharmaceuticals As | MODIFIED RELEASE COMPOSITIONS CONTAINING TACROLIMUS |
| TW200517114A (en) | 2003-10-15 | 2005-06-01 | Combinatorx Inc | Methods and reagents for the treatment of immunoinflammatory disorders |
| DE602004018547D1 (en) * | 2003-10-17 | 2009-01-29 | Ranbaxy Lab Ltd | PREPARATION OF TACROLIMUS (FK-506) USING NEW STREPTOMYCES SPECIES |
| CA2546347A1 (en) | 2003-11-21 | 2005-06-09 | Combinatorx, Incorporated | Methods and reagents for the treatment of inflammatory disorders |
| CA2548297C (en) * | 2003-12-05 | 2011-06-14 | Biocon Limited | Process for the purification of macrolides |
| KR100485877B1 (en) * | 2003-12-30 | 2005-04-28 | 종근당바이오 주식회사 | Microorganism producing tacrolimus and mass-productive method of tacrolimus using the same |
| US20050176080A1 (en) * | 2004-02-10 | 2005-08-11 | Vani Bodepudi | Hapten, immunogens and derivatives of ascomycin useful for preparation of antibodies and immunoassays |
| WO2005089855A1 (en) | 2004-03-19 | 2005-09-29 | Abbott Laboratories | Multiple drug delivery from a balloon and a prosthesis |
| US8431145B2 (en) | 2004-03-19 | 2013-04-30 | Abbott Laboratories | Multiple drug delivery from a balloon and a prosthesis |
| CA2562805C (en) * | 2004-04-12 | 2014-03-11 | Biocon Limited | Process for the production of macrolides using a novel strain, streptomyces sp. bicc 7522 |
| US20050272723A1 (en) * | 2004-04-27 | 2005-12-08 | The Regents Of The University Of Michigan | Methods and compositions for treating diseases and conditions associated with mitochondrial function |
| US20090275099A1 (en) * | 2004-04-27 | 2009-11-05 | Regents Of The University Of Michigan | Methods and compositions for treating diseases and conditions associated with mitochondrial function |
| JP2006014722A (en) * | 2004-06-02 | 2006-01-19 | Keio Gijuku | Gene marker and its use |
| US20110104186A1 (en) | 2004-06-24 | 2011-05-05 | Nicholas Valiante | Small molecule immunopotentiators and assays for their detection |
| EP1768692B8 (en) * | 2004-07-01 | 2015-06-17 | Yale University | Targeted and high density drug loaded polymeric materials |
| US20060014677A1 (en) * | 2004-07-19 | 2006-01-19 | Isotechnika International Inc. | Method for maximizing efficacy and predicting and minimizing toxicity of calcineurin inhibitor compounds |
| US20060052369A1 (en) * | 2004-09-07 | 2006-03-09 | The Regents Of The University Of Michigan | Compositions and methods relating to novel compounds and targets thereof |
| WO2006031664A1 (en) * | 2004-09-10 | 2006-03-23 | Ivax Pharmaceuticals S.R.O. | Process for isolation of crystalline tacrolimus |
| JP2008512125A (en) * | 2004-09-10 | 2008-04-24 | アイバックス ファーマシューティカルズ スポレツノスト エス ルチェニム オメゼニム | Method for isolating macrolide compounds |
| GT200500282A (en) * | 2004-10-12 | 2006-05-04 | HETEROATOMOS CONTAINING TRICYCLE COMPOUNDS. | |
| ITMI20042098A1 (en) * | 2004-11-03 | 2005-02-03 | Antibioticos Spa | PROCESS FOR TACROLIMUS PURIFICATION |
| JP2007519758A (en) * | 2004-12-01 | 2007-07-19 | テバ ジョジセルジャール ザ−トケルエン ムケド レ−スベニュタ−ルシャシャ−グ | Method for producing crystalline macrolide |
| EP1835889A1 (en) * | 2004-12-15 | 2007-09-26 | Elan Pharma International Limited | Nanoparticulate tacrolimus formulations |
| MX2007005867A (en) * | 2004-12-22 | 2007-07-04 | Teva Gyogyszergyar Zartkoeruen Mukoedo Reszvenytarsasag | Method of purifying macrolides. |
| CA2593019A1 (en) * | 2005-01-03 | 2006-07-13 | The Regents Of The University Of Michigan | Compositions and methods relating to novel compounds and targets thereof |
| TW200635934A (en) * | 2005-01-05 | 2006-10-16 | Teva Gyogyszergyar Zartkoruen Mukodo Reszvenytarsasag | Amorphous tacrolimus and preparation thereof |
| US20090162868A1 (en) * | 2005-01-13 | 2009-06-25 | Yusuke Tanigawara | Gene Markers and Utilization of the Same |
| EP1868663B1 (en) | 2005-03-23 | 2011-11-16 | Abbott Laboratories | Delivery of highly lipophilic agents via medical devices |
| JP5242374B2 (en) | 2005-03-23 | 2013-07-24 | アボット・ラボラトリーズ | Composition and method of administering rapamycin analogs using medical devices for long-term efficacy |
| AU2006309291B2 (en) | 2005-06-01 | 2010-05-27 | The Regents Of The University Of Michigan | Unsolvated benzodiazepine compositions and methods |
| CN1876822B (en) * | 2005-06-06 | 2010-05-12 | 上海市农药研究所 | Tacrolimus generation strain and production method thereof |
| WO2007013017A1 (en) * | 2005-07-29 | 2007-02-01 | Ranbaxy Laboratories Limited | A process for purification of macrolides |
| ITMI20051549A1 (en) * | 2005-08-05 | 2007-02-06 | Antibioticos Spa | PURIFICATION OF TACROLIMUS ON VEGETABLE DIMORIGINE SUPPORTS |
| WO2007029082A2 (en) * | 2005-09-05 | 2007-03-15 | Ranbaxy Laboratories Limited | An improved fermentation process for preparing ascomycin |
| US20080318289A1 (en) * | 2005-10-05 | 2008-12-25 | Parveen Kumar | Fermentation Processes for the Preparation of Tacrolimus |
| US20070105844A1 (en) * | 2005-10-26 | 2007-05-10 | Regents Of The University Of Michigan | Therapeutic compositions and methods |
| NZ568254A (en) | 2005-11-01 | 2011-06-30 | Univ Michigan | 1,4-Benzodiazepine-2,5-diones for regulating cell death and treating autoimmune diseases |
| US8067433B2 (en) | 2005-11-09 | 2011-11-29 | Zalicus Inc. | Methods, compositions, and kits for the treatment of ophthalmic disorders |
| US7622477B2 (en) | 2006-02-28 | 2009-11-24 | Cordis Corporation | Isomers and 42-epimers of rapamycin alkyl ether analogs, methods of making and using the same |
| US7678901B2 (en) | 2006-02-28 | 2010-03-16 | Wyeth | Rapamycin analogs containing an antioxidant moiety |
| US20080000834A1 (en) * | 2006-03-15 | 2008-01-03 | Ladislav Cvak | Process for purifying Tacrolimus |
| US8022188B2 (en) * | 2006-04-24 | 2011-09-20 | Abbott Laboratories | Immunosuppressant binding antibodies and methods of obtaining and using same |
| US7759338B2 (en) * | 2006-04-27 | 2010-07-20 | The Regents Of The University Of Michigan | Soluble 1,4 benzodiazepine compounds and stable salts thereof |
| EP2037741B1 (en) | 2006-06-09 | 2013-11-27 | The Regents Of The University Of Michigan | Benzodiazepine derivatives for use in the treatment of immune, inflammatory and proliferative disorders |
| CA2660804A1 (en) | 2006-08-31 | 2008-03-06 | Astellas Pharma Inc. | Reverse targeting lipid vesicle |
| US20080161324A1 (en) * | 2006-09-14 | 2008-07-03 | Johansen Lisa M | Compositions and methods for treatment of viral diseases |
| TW200837067A (en) * | 2006-11-06 | 2008-09-16 | Teva Gyogyszergyar Zartkoruen Mukodo Reszvenytarsasag | Ascomycin and pimecrolimus having reduced levels of desmethylascomycin and 32-deoxy-32-epichloro-desmethylascomycin respectively, and methods for preparation thereof |
| US9044391B2 (en) * | 2007-01-10 | 2015-06-02 | Board Of Regents, The University Of Texas System | Enhanced delivery of immunosuppressive drug compositions for pulmonary delivery |
| US10265407B2 (en) | 2007-02-15 | 2019-04-23 | Yale University | Modular nanodevices for smart adaptable vaccines |
| US20100151436A1 (en) * | 2007-03-02 | 2010-06-17 | Fong Peter M | Methods for Ex Vivo Administration of Drugs to Grafts Using Polymeric Nanoparticles |
| WO2008112553A1 (en) | 2007-03-09 | 2008-09-18 | The Regents Of The University Of Michigan | Compositions and methods relating to novel compounds and targets thereof |
| ES2634153T3 (en) | 2007-05-30 | 2017-09-26 | Veloxis Pharmaceuticals A/S | Once-daily oral dosage form comprising tacrolimus |
| US12083103B2 (en) | 2007-05-30 | 2024-09-10 | Veloxis Pharmaceuticals, Inc. | Tacrolimus for improved treatment of transplant patients |
| KR100891313B1 (en) | 2007-08-17 | 2009-03-31 | (주) 제노텍 | Method of extraction and yield-up of tricyclo compounds by adding a solid adsorbent resin as their carrier in fermentation medium |
| WO2009036175A2 (en) | 2007-09-14 | 2009-03-19 | The Regents Of The University Of Michigan | F1f0-atpase inhibitors and related methods |
| KR20100083819A (en) | 2007-11-06 | 2010-07-22 | 더 리젠츠 오브 더 유니버시티 오브 미시간 | Benzodiazepinone compounds useful in the treatment of skin conditions |
| US8921642B2 (en) * | 2008-01-11 | 2014-12-30 | Massachusetts Eye And Ear Infirmary | Conditional-stop dimerizable caspase transgenic animals |
| KR101003042B1 (en) | 2008-03-17 | 2010-12-21 | 종근당바이오 주식회사 | Method for refining of high purity of Tacrolimus |
| MX2010011068A (en) * | 2008-04-08 | 2010-11-04 | Amyris Biotechnologies Inc | Expression of heterologous sequences. |
| US9549918B2 (en) | 2008-05-30 | 2017-01-24 | Veloxis Pharmaceuticals A/S | Stabilized tacrolimus composition |
| US8173621B2 (en) | 2008-06-11 | 2012-05-08 | Gilead Pharmasset Llc | Nucleoside cyclicphosphates |
| US20100092479A1 (en) * | 2008-08-18 | 2010-04-15 | Combinatorx (Singapore) Pte. Ltd. | Compositions and methods for treatment of viral diseases |
| WO2010030891A2 (en) | 2008-09-11 | 2010-03-18 | The Regents Of The University Of Michigan | Aryl guanidine f1f0-atpase inhibitors and related methods |
| KR100910165B1 (en) | 2008-09-18 | 2009-07-30 | (주) 제노텍 | Purification method of lactone compounds containing unsaturated alkyl group by extraction with silver ion solution |
| US8716263B2 (en) | 2008-12-23 | 2014-05-06 | Gilead Pharmasset Llc | Synthesis of purine nucleosides |
| AR074897A1 (en) | 2008-12-23 | 2011-02-23 | Pharmasset Inc | NUCLEOSID PHOSPHORAMIDATES |
| KR20110098849A (en) | 2008-12-23 | 2011-09-01 | 파마셋 인코포레이티드 | Nucleoside analogs |
| US8604023B2 (en) | 2009-04-17 | 2013-12-10 | The Regents Of The University Of Michigan | 1,4-benzodiazepinone compounds and their use in treating cancer |
| CN101712686B (en) * | 2009-06-22 | 2012-08-29 | 鲁南制药集团股份有限公司 | Method for separating and purifying tacrolimus in fermentation liquor |
| EP2272963A1 (en) * | 2009-07-09 | 2011-01-12 | LEK Pharmaceuticals d.d. | Process for Preparation of Tacrolimus |
| US8673897B2 (en) | 2009-09-18 | 2014-03-18 | The Regents Of The University Of Michigan | Benzodiazepinone compounds and methods of treatment using same |
| CA2780287C (en) | 2009-11-17 | 2015-03-17 | The Regents Of The University Of Michigan | 1,4-benzodiazepine-2,5-diones and related compounds with therapeutic properties |
| JP5856063B2 (en) | 2009-11-17 | 2016-02-09 | ザ リージェンツ オブ ザ ユニバーシティ オブ ミシガン | 1,4-Benzodiazepine-2,5-dione and related compounds having therapeutic properties |
| US20110130711A1 (en) * | 2009-11-19 | 2011-06-02 | Follica, Inc. | Hair growth treatment |
| US8951595B2 (en) | 2009-12-11 | 2015-02-10 | Abbott Cardiovascular Systems Inc. | Coatings with tunable molecular architecture for drug-coated balloon |
| US8480620B2 (en) | 2009-12-11 | 2013-07-09 | Abbott Cardiovascular Systems Inc. | Coatings with tunable solubility profile for drug-coated balloon |
| TW201139457A (en) | 2010-03-31 | 2011-11-16 | Pharmasset Inc | Stereoselective synthesis of phosphorus containing actives |
| US20110318277A1 (en) | 2010-06-25 | 2011-12-29 | APT Pharmaceuticals, Inc. University of Maryland, Baltimore | Tacrolimus compositions for aerosol administration |
| KR101261131B1 (en) | 2010-08-24 | 2013-05-06 | 이화여자대학교 산학협력단 | Novel tacrolimus derivative, neuroprotective composition comprising the same, immunosuppressive composition comprising the same, method of manufacturing the same and strain for manufacturing the same |
| MA34586B1 (en) | 2010-08-25 | 2013-10-02 | Medis Lab | MICRONIZED TACROLIMUS CRYSTALLINE PARTICLES WITH MODIFIED SURFACE AND PHARMACEUTICAL COMPOSITIONS THEREOF |
| US10117411B2 (en) | 2010-10-06 | 2018-11-06 | Dow Agrosciences Llc | Maize cytoplasmic male sterility (CMS) C-type restorer RF4 gene, molecular markers and their use |
| AU2012229236B2 (en) | 2011-03-11 | 2017-05-18 | Beth Israel Deaconess Medical Center, Inc. | Anti-CD40 antibodies and uses thereof |
| US9597385B2 (en) | 2012-04-23 | 2017-03-21 | Allertein Therapeutics, Llc | Nanoparticles for treatment of allergy |
| NZ723459A (en) | 2013-03-14 | 2017-12-22 | Alkermes Pharma Ireland Ltd | Prodrugs of fumarates and their use in treating various diseases |
| US8669281B1 (en) | 2013-03-14 | 2014-03-11 | Alkermes Pharma Ireland Limited | Prodrugs of fumarates and their use in treating various diseases |
| EP3760223A1 (en) | 2013-04-03 | 2021-01-06 | N-Fold Llc | Nanoparticle composition for desensitizing a subject to peanut allergens |
| US9814703B2 (en) | 2013-11-14 | 2017-11-14 | The Board Of Trustees Of The Leland Stanford Junior University | Methods for treating cancer by activation of BMP signaling |
| CN104650112B (en) * | 2013-11-18 | 2018-07-31 | 山东新时代药业有限公司 | The preparation method of tacrolimus 8- propyl analogs |
| UY35927A (en) | 2013-12-31 | 2015-07-31 | Dow Agrosciences Llc | ? GENE RESTORATOR RF3 TYPE S OF MALE CYLOPLASM MATIC STERILITY (CMS), MOLECULAR MARKERS AND ITS USES ?. |
| CA2935801A1 (en) | 2014-01-13 | 2015-07-16 | Amplyx Pharmaceuticals, Inc. | Antifungal compounds |
| CA2935167C (en) | 2014-01-16 | 2022-02-22 | Musc Foundation For Research Development | Targeted nanocarriers for the administration of immunosuppressive agents |
| JP6337135B2 (en) | 2014-02-24 | 2018-06-06 | アルカーメス ファーマ アイルランド リミテッド | Sulfonamide and sulfinamide prodrugs of fumarate and their use in the treatment of various diseases |
| KR101694879B1 (en) | 2014-08-01 | 2017-01-12 | 주식회사 인트론바이오테크놀로지 | Non-immuno suppressive FK506 analogues with neuroregenerative activity and the use thereof |
| EP3246036B1 (en) | 2014-10-28 | 2021-10-13 | Koushi Yamaguchi | Medicine for improving pregnancy-induced hypertension syndrome |
| CA2994859A1 (en) | 2015-08-19 | 2017-02-23 | Vivus, Inc. | Pharmaceutical formulations |
| CA3002789A1 (en) | 2015-09-04 | 2017-03-09 | Primatope Therapeutics Inc. | Humanized anti-cd40 antibodies and uses thereof |
| CN106074367A (en) * | 2016-07-20 | 2016-11-09 | 中山大学中山眼科中心 | Containing FK506 compounds/dimeric pharmaceutical composition of FKBP albumen and preparation method thereof |
| CN108384819B (en) * | 2017-02-03 | 2021-06-25 | 上海医药工业研究院 | Culture medium for fermenting tacrolimus and fermentation method |
| RU2686779C1 (en) * | 2018-07-26 | 2019-04-30 | Общество с ограниченной ответственностью "Изварино Фарма" | Strain of streptomyces tsukubensis - producer of tacrolimus and a method for preparing tacrolimus |
| JP7465806B2 (en) | 2018-08-10 | 2024-04-11 | 晃史 山口 | Drugs for treating humoral immune-related disorders in the mother and fetus |
| WO2020076738A2 (en) * | 2018-10-12 | 2020-04-16 | Bellicum Pharmaceuticals, Inc | Protein-binding compounds |
| WO2020117134A1 (en) | 2018-12-04 | 2020-06-11 | İlko İlaç Sanayi̇ Ve Ti̇caret A.Ş. | Stable tacrolimus ointment formulation for topical treatment of skin conditions |
| KR102135527B1 (en) * | 2018-12-18 | 2020-07-20 | 이문수 | The socks |
| JPWO2020129348A1 (en) | 2018-12-18 | 2021-11-04 | 晃史 山口 | Drugs to improve infertility / recurrent pregnancy loss or pregnancy |
| GR1009790B (en) | 2019-03-20 | 2020-08-03 | Φαρματεν Α.Β.Ε.Ε. | Prolonged release formulation comprising tacrolimus |
| WO2022187379A1 (en) | 2021-03-03 | 2022-09-09 | Sana Biotechnology, Inc. | Immunosuppressive therapies for use with cardiomyocyte cell therapies, and associated methods and compositions |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4894366A (en) * | 1984-12-03 | 1990-01-16 | Fujisawa Pharmaceutical Company, Ltd. | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3244592A (en) * | 1962-06-09 | 1966-04-05 | Arai Tadashi | Ascomycin and process for its production |
| JPS54110000A (en) * | 1978-02-17 | 1979-08-29 | Kaken Pharmaceut Co Ltd | Novel streptovaricin c derivative |
| US4309504A (en) * | 1980-01-28 | 1982-01-05 | Eli Lilly And Company | Process for preparing narasin |
| US4654334A (en) * | 1980-10-08 | 1987-03-31 | International Minerals & Chemical Corp. | Manganese-containing antibiotic agents |
| US4415669A (en) * | 1981-12-07 | 1983-11-15 | Merck & Co., Inc. | Substance and process for its production |
| US4390546A (en) * | 1981-12-07 | 1983-06-28 | Merck & Co., Inc. | Antiparasitic macrolide from a strain of Streptomyces hygroscopicus |
| US4510317A (en) * | 1983-07-28 | 1985-04-09 | Hoffmann-La Roche Inc. | Antibiotic X-14934A |
| GB8430455D0 (en) * | 1984-12-03 | 1985-01-09 | Fujisawa Pharmaceutical Co | Fr-900506 substance |
| US5266692A (en) * | 1984-12-03 | 1993-11-30 | Fujisawa Pharmaceutical Co., Ltd. | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| US5254562A (en) * | 1984-12-03 | 1993-10-19 | Fujisawa Pharmaceutical Company, Ltd. | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
| DK175235B1 (en) * | 1987-11-09 | 2004-07-19 | Novartis Ag | New use of azatricyclo derivatives and pharmaceutical compositions containing them |
| US5956352A (en) * | 1992-04-24 | 1999-09-21 | Digital Equipment Corporation | Adjustable filter for error detecting and correcting system |
-
1985
- 1985-11-20 US US06/799,855 patent/US4894366A/en not_active Expired - Lifetime
- 1985-11-26 IE IE297185A patent/IE62865B1/en not_active IP Right Cessation
- 1985-11-28 CA CA000496479A patent/CA1338491C/en not_active Expired - Lifetime
- 1985-11-29 FI FI854731A patent/FI87803C/en not_active IP Right Cessation
- 1985-11-29 AU AU50596/85A patent/AU592067B2/en not_active Expired
- 1985-11-29 DK DK556285A patent/DK169550B1/en not_active IP Right Cessation
- 1985-11-30 DE DE3587806T patent/DE3587806T2/en not_active Expired - Lifetime
- 1985-11-30 CY CY191285A patent/CY1912A/en unknown
- 1985-11-30 DE DE1995175026 patent/DE19575026I2/en active Active
- 1985-11-30 EP EP85115202A patent/EP0184162B1/en not_active Expired - Lifetime
- 1985-11-30 AT AT8585115202T patent/ATE104984T1/en active
- 1985-12-02 ES ES549478A patent/ES8705038A1/en not_active Expired
- 1985-12-02 NO NO85854833A patent/NO168372C/en not_active IP Right Cessation
- 1985-12-02 HU HU854600A patent/HU195250B/en unknown
- 1985-12-02 KR KR1019850009022A patent/KR930010704B1/en not_active IP Right Cessation
- 1985-12-02 GR GR852904A patent/GR852904B/el unknown
- 1985-12-02 PT PT81589A patent/PT81589B/en unknown
- 1985-12-02 NZ NZ214407A patent/NZ214407A/en unknown
- 1985-12-03 CN CN85109492A patent/CN1013687B/en not_active Expired
- 1985-12-03 IL IL92345A patent/IL92345A/en not_active IP Right Cessation
- 1985-12-03 IL IL77222A patent/IL77222A/en not_active IP Right Cessation
-
1986
- 1986-05-30 US US06/868,749 patent/US4929611A/en not_active Expired - Lifetime
- 1986-11-07 FI FI864527A patent/FI85977C/en not_active IP Right Cessation
-
1989
- 1989-08-17 US US07/395,798 patent/US4956352A/en not_active Expired - Lifetime
-
1990
- 1990-03-09 US US07/491,205 patent/US5110811A/en not_active Expired - Lifetime
- 1990-07-31 JP JP2204518A patent/JPH0372483A/en active Granted
- 1990-07-31 JP JP2204519A patent/JPH0720970B2/en not_active Expired - Lifetime
-
1992
- 1992-06-17 MX MX9202943A patent/MX9202943A/en unknown
-
1993
- 1993-09-01 KR KR1019930017416A patent/KR930010705B1/en not_active IP Right Cessation
- 1993-09-01 KR KR1019930017418A patent/KR930010707B1/en not_active IP Right Cessation
- 1993-09-01 KR KR1019930017419A patent/KR930010708B1/en not_active IP Right Cessation
- 1993-09-01 KR KR1019930017417A patent/KR930010706B1/en not_active IP Right Cessation
-
1994
- 1994-09-01 JP JP6208445A patent/JP2746134B2/en not_active Expired - Lifetime
-
1995
- 1995-05-25 US US08/450,412 patent/US5624842A/en not_active Expired - Lifetime
- 1995-05-25 US US08/450,639 patent/US5496727A/en not_active Expired - Lifetime
- 1995-10-19 US US08/545,172 patent/US5565559A/en not_active Expired - Fee Related
-
1996
- 1996-02-01 HK HK18596A patent/HK18596A/en not_active IP Right Cessation
- 1996-10-07 NL NL960023C patent/NL960023I2/en unknown
- 1996-12-03 US US08/753,950 patent/US5830717A/en not_active Expired - Lifetime
-
1997
- 1997-04-18 JP JP9101673A patent/JP2828091B2/en not_active Expired - Fee Related
-
1998
- 1998-05-26 US US09/084,099 patent/US6028097A/en not_active Expired - Fee Related
- 1998-05-28 JP JP10146822A patent/JP2976966B2/en not_active Expired - Fee Related
- 1998-11-11 LU LU90317C patent/LU90317I2/en unknown
-
1999
- 1999-04-14 JP JP10603999A patent/JP3211891B2/en not_active Expired - Fee Related
- 1999-10-01 US US09/411,630 patent/US6201005B1/en not_active Expired - Fee Related
-
2000
- 2000-11-27 US US09/721,650 patent/US6482845B1/en not_active Expired - Fee Related
-
2002
- 2002-09-20 US US10/247,305 patent/US20030170831A1/en not_active Abandoned
-
2003
- 2003-04-25 US US10/422,901 patent/US20030229115A1/en not_active Abandoned
- 2003-05-01 US US10/426,659 patent/US20040029908A1/en not_active Abandoned
-
2004
- 2004-11-09 US US10/983,710 patent/US20050124646A1/en not_active Abandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4894366A (en) * | 1984-12-03 | 1990-01-16 | Fujisawa Pharmaceutical Company, Ltd. | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110212988A1 (en) * | 2008-10-08 | 2011-09-01 | Hironori Masui | Tacrolimus preparation for external applications |
| US8575189B2 (en) | 2008-10-08 | 2013-11-05 | Takata Seiyaku Co., Ltd. | Tacrolimus preparation for external applications |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6482845B1 (en) | Method for inhibiting interleukin-2 production using tricyclo compounds | |
| EP0349061B1 (en) | Immunosuppressant agent | |
| US5254562A (en) | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same | |
| EP0349049A2 (en) | Immunosuppressant compound | |
| EP0627009B1 (en) | Macrocyclic lactones and a productive strain thereof | |
| US5266692A (en) | Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same | |
| EP0378321A2 (en) | Microbial transformation product of L-683-590 | |
| US5290772A (en) | Immunosuppressant agent | |
| EP0378317A2 (en) | Microbial transformation product of L-679,934 | |
| EP0378320A2 (en) | Microbial transformation product |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO PAY ISSUE FEE |