US20030197183A1 - Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds - Google Patents
Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds Download PDFInfo
- Publication number
- US20030197183A1 US20030197183A1 US10/366,295 US36629503A US2003197183A1 US 20030197183 A1 US20030197183 A1 US 20030197183A1 US 36629503 A US36629503 A US 36629503A US 2003197183 A1 US2003197183 A1 US 2003197183A1
- Authority
- US
- United States
- Prior art keywords
- compound
- compounds
- layer
- ocf
- bis
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- FSEXLNMNADBYJU-UHFFFAOYSA-N 2-phenylquinoline Chemical class C1=CC=CC=C1C1=CC=C(C=CC=C2)C2=N1 FSEXLNMNADBYJU-UHFFFAOYSA-N 0.000 title claims abstract description 23
- OXPDQFOKSZYEMJ-UHFFFAOYSA-N 2-phenylpyrimidine Chemical class C1=CC=CC=C1C1=NC=CC=N1 OXPDQFOKSZYEMJ-UHFFFAOYSA-N 0.000 title claims abstract description 17
- 150000001875 compounds Chemical class 0.000 title claims description 99
- 150000002504 iridium compounds Chemical class 0.000 title description 19
- 150000005359 phenylpyridines Chemical class 0.000 title description 3
- CBHCDHNUZWWAPP-UHFFFAOYSA-N pecazine Chemical compound C1N(C)CCCC1CN1C2=CC=CC=C2SC2=CC=CC=C21 CBHCDHNUZWWAPP-UHFFFAOYSA-N 0.000 claims description 48
- 239000003446 ligand Substances 0.000 claims description 31
- VQGHOUODWALEFC-UHFFFAOYSA-N 2-phenylpyridine Chemical compound C1=CC=CC=C1C1=CC=CC=N1 VQGHOUODWALEFC-UHFFFAOYSA-N 0.000 claims description 25
- -1 poly(N-vinyl carbazole) Polymers 0.000 claims description 14
- 229910052757 nitrogen Inorganic materials 0.000 claims description 11
- 229910052801 chlorine Inorganic materials 0.000 claims description 10
- 230000005525 hole transport Effects 0.000 claims description 10
- 229910052739 hydrogen Inorganic materials 0.000 claims description 10
- 239000003085 diluting agent Substances 0.000 claims description 8
- 229910052799 carbon Inorganic materials 0.000 claims description 7
- JVZRCNQLWOELDU-UHFFFAOYSA-N gamma-Phenylpyridine Natural products C1=CC=CC=C1C1=CC=NC=C1 JVZRCNQLWOELDU-UHFFFAOYSA-N 0.000 claims description 6
- 229920003227 poly(N-vinyl carbazole) Polymers 0.000 claims description 5
- ZVFQEOPUXVPSLB-UHFFFAOYSA-N 3-(4-tert-butylphenyl)-4-phenyl-5-(4-phenylphenyl)-1,2,4-triazole Chemical compound C1=CC(C(C)(C)C)=CC=C1C(N1C=2C=CC=CC=2)=NN=C1C1=CC=C(C=2C=CC=CC=2)C=C1 ZVFQEOPUXVPSLB-UHFFFAOYSA-N 0.000 claims description 4
- 239000007983 Tris buffer Substances 0.000 claims description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 4
- 229910052782 aluminium Inorganic materials 0.000 claims description 4
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 claims description 4
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 4
- 229920000548 poly(silane) polymer Polymers 0.000 claims description 4
- ODHXBMXNKOYIBV-UHFFFAOYSA-N triphenylamine Chemical compound C1=CC=CC=C1N(C=1C=CC=CC=1)C1=CC=CC=C1 ODHXBMXNKOYIBV-UHFFFAOYSA-N 0.000 claims description 4
- VFUDMQLBKNMONU-UHFFFAOYSA-N 9-[4-(4-carbazol-9-ylphenyl)phenyl]carbazole Chemical group C12=CC=CC=C2C2=CC=CC=C2N1C1=CC=C(C=2C=CC(=CC=2)N2C3=CC=CC=C3C3=CC=CC=C32)C=C1 VFUDMQLBKNMONU-UHFFFAOYSA-N 0.000 claims description 3
- 239000004305 biphenyl Substances 0.000 claims description 3
- XZCJVWCMJYNSQO-UHFFFAOYSA-N butyl pbd Chemical compound C1=CC(C(C)(C)C)=CC=C1C1=NN=C(C=2C=CC(=CC=2)C=2C=CC=CC=2)O1 XZCJVWCMJYNSQO-UHFFFAOYSA-N 0.000 claims description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 3
- 150000003513 tertiary aromatic amines Chemical class 0.000 claims description 3
- STTGYIUESPWXOW-UHFFFAOYSA-N 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline Chemical compound C=12C=CC3=C(C=4C=CC=CC=4)C=C(C)N=C3C2=NC(C)=CC=1C1=CC=CC=C1 STTGYIUESPWXOW-UHFFFAOYSA-N 0.000 claims description 2
- RIKNNBBGYSDYAX-UHFFFAOYSA-N 2-[1-[2-(4-methyl-n-(4-methylphenyl)anilino)phenyl]cyclohexyl]-n,n-bis(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C(=CC=CC=1)C1(CCCCC1)C=1C(=CC=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 RIKNNBBGYSDYAX-UHFFFAOYSA-N 0.000 claims description 2
- OGGKVJMNFFSDEV-UHFFFAOYSA-N 3-methyl-n-[4-[4-(n-(3-methylphenyl)anilino)phenyl]phenyl]-n-phenylaniline Chemical compound CC1=CC=CC(N(C=2C=CC=CC=2)C=2C=CC(=CC=2)C=2C=CC(=CC=2)N(C=2C=CC=CC=2)C=2C=C(C)C=CC=2)=C1 OGGKVJMNFFSDEV-UHFFFAOYSA-N 0.000 claims description 2
- DHDHJYNTEFLIHY-UHFFFAOYSA-N 4,7-diphenyl-1,10-phenanthroline Chemical compound C1=CC=CC=C1C1=CC=NC2=C1C=CC1=C(C=3C=CC=CC=3)C=CN=C21 DHDHJYNTEFLIHY-UHFFFAOYSA-N 0.000 claims description 2
- YGBCLRRWZQSURU-UHFFFAOYSA-N 4-[(diphenylhydrazinylidene)methyl]-n,n-diethylaniline Chemical compound C1=CC(N(CC)CC)=CC=C1C=NN(C=1C=CC=CC=1)C1=CC=CC=C1 YGBCLRRWZQSURU-UHFFFAOYSA-N 0.000 claims description 2
- PGDARWFJWJKPLY-UHFFFAOYSA-N 4-[2-[3-[4-(diethylamino)phenyl]-2-phenyl-1,3-dihydropyrazol-5-yl]ethenyl]-n,n-diethylaniline Chemical compound C1=CC(N(CC)CC)=CC=C1C=CC1=CC(C=2C=CC(=CC=2)N(CC)CC)N(C=2C=CC=CC=2)N1 PGDARWFJWJKPLY-UHFFFAOYSA-N 0.000 claims description 2
- KBXXZTIBAVBLPP-UHFFFAOYSA-N 4-[[4-(diethylamino)-2-methylphenyl]-(4-methylphenyl)methyl]-n,n-diethyl-3-methylaniline Chemical compound CC1=CC(N(CC)CC)=CC=C1C(C=1C(=CC(=CC=1)N(CC)CC)C)C1=CC=C(C)C=C1 KBXXZTIBAVBLPP-UHFFFAOYSA-N 0.000 claims description 2
- ZOKIJILZFXPFTO-UHFFFAOYSA-N 4-methyl-n-[4-[1-[4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]cyclohexyl]phenyl]-n-(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)C1(CCCCC1)C=1C=CC(=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 ZOKIJILZFXPFTO-UHFFFAOYSA-N 0.000 claims description 2
- 125000000590 4-methylphenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])[H] 0.000 claims description 2
- PMPVIKIVABFJJI-UHFFFAOYSA-N Cyclobutane Chemical compound C1CCC1 PMPVIKIVABFJJI-UHFFFAOYSA-N 0.000 claims description 2
- 235000010290 biphenyl Nutrition 0.000 claims description 2
- JGOAZQAXRONCCI-SDNWHVSQSA-N n-[(e)-benzylideneamino]aniline Chemical compound C=1C=CC=CC=1N\N=C\C1=CC=CC=C1 JGOAZQAXRONCCI-SDNWHVSQSA-N 0.000 claims description 2
- KWYHDKDOAIKMQN-UHFFFAOYSA-N N,N,N',N'-tetramethylethylenediamine Chemical compound CN(C)CCN(C)C KWYHDKDOAIKMQN-UHFFFAOYSA-N 0.000 claims 1
- MILUBEOXRNEUHS-UHFFFAOYSA-N iridium(3+) Chemical class [Ir+3] MILUBEOXRNEUHS-UHFFFAOYSA-N 0.000 abstract description 13
- 150000005360 2-phenylpyridines Chemical class 0.000 abstract description 8
- 239000010410 layer Substances 0.000 description 90
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 42
- 239000000203 mixture Substances 0.000 description 35
- YMWUJEATGCHHMB-DICFDUPASA-N dichloromethane-d2 Chemical compound [2H]C([2H])(Cl)Cl YMWUJEATGCHHMB-DICFDUPASA-N 0.000 description 34
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 33
- 239000000463 material Substances 0.000 description 29
- TVIVIEFSHFOWTE-UHFFFAOYSA-K tri(quinolin-8-yloxy)alumane Chemical compound [Al+3].C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1 TVIVIEFSHFOWTE-UHFFFAOYSA-K 0.000 description 26
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 24
- 229910052741 iridium Inorganic materials 0.000 description 22
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 20
- 150000002503 iridium Chemical class 0.000 description 18
- 239000007787 solid Substances 0.000 description 17
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 14
- 0 [1*]*1c([2*])c([3*])c([4*])n(C)c1-c1c([5*])c([6*])c([7*])c([8*])c1C Chemical compound [1*]*1c([2*])c([3*])c([4*])n(C)c1-c1c([5*])c([6*])c([7*])c([8*])c1C 0.000 description 13
- 239000000243 solution Substances 0.000 description 13
- 238000004293 19F NMR spectroscopy Methods 0.000 description 12
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 12
- 229910052751 metal Inorganic materials 0.000 description 11
- 239000002184 metal Substances 0.000 description 11
- 239000000377 silicon dioxide Substances 0.000 description 11
- 229910021638 Iridium(III) chloride Inorganic materials 0.000 description 10
- 239000000758 substrate Substances 0.000 description 10
- DANYXEHCMQHDNX-UHFFFAOYSA-K trichloroiridium Chemical compound Cl[Ir](Cl)Cl DANYXEHCMQHDNX-UHFFFAOYSA-K 0.000 description 10
- 238000005160 1H NMR spectroscopy Methods 0.000 description 9
- 239000000284 extract Substances 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- 238000005401 electroluminescence Methods 0.000 description 7
- 238000005481 NMR spectroscopy Methods 0.000 description 6
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 239000013078 crystal Substances 0.000 description 6
- 150000002739 metals Chemical class 0.000 description 6
- 238000000034 method Methods 0.000 description 6
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 5
- 125000003545 alkoxy group Chemical group 0.000 description 5
- 230000001815 facial effect Effects 0.000 description 5
- GRVDJDISBSALJP-UHFFFAOYSA-N methyloxidanyl Chemical compound [O]C GRVDJDISBSALJP-UHFFFAOYSA-N 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 125000004429 atom Chemical group 0.000 description 4
- 229920001940 conductive polymer Polymers 0.000 description 4
- 238000000151 deposition Methods 0.000 description 4
- 239000010408 film Substances 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 125000001153 fluoro group Chemical group F* 0.000 description 4
- 238000010791 quenching Methods 0.000 description 4
- 238000004467 single crystal X-ray diffraction Methods 0.000 description 4
- 125000001424 substituent group Chemical group 0.000 description 4
- HXFVJCBIRYKDRY-UHFFFAOYSA-N 1,2-dichloroethane;hexane Chemical class ClCCCl.CCCCCC HXFVJCBIRYKDRY-UHFFFAOYSA-N 0.000 description 3
- UDGYLQTZGJGKPC-UHFFFAOYSA-N 2-(4-fluorophenyl)-5-(trifluoromethyl)pyridine Chemical compound C1=CC(F)=CC=C1C1=CC=C(C(F)(F)F)C=N1 UDGYLQTZGJGKPC-UHFFFAOYSA-N 0.000 description 3
- NFYOPUPSCJTLPQ-UHFFFAOYSA-N FC1=CC2=C(C=C1)C1=CC=C(C(F)(F)F)C=N1[Ir]21C2=CC(Br)=CC=C2C2=N1C=C(Br)C=C2 Chemical compound FC1=CC2=C(C=C1)C1=CC=C(C(F)(F)F)C=N1[Ir]21C2=CC(Br)=CC=C2C2=N1C=C(Br)C=C2 NFYOPUPSCJTLPQ-UHFFFAOYSA-N 0.000 description 3
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000002800 charge carrier Substances 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000001704 evaporation Methods 0.000 description 3
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 3
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 3
- 239000012044 organic layer Substances 0.000 description 3
- 238000010992 reflux Methods 0.000 description 3
- KZJPVUDYAMEDRM-UHFFFAOYSA-M silver;2,2,2-trifluoroacetate Chemical compound [Ag+].[O-]C(=O)C(F)(F)F KZJPVUDYAMEDRM-UHFFFAOYSA-M 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 238000002207 thermal evaporation Methods 0.000 description 3
- 239000010409 thin film Substances 0.000 description 3
- OKDGRDCXVWSXDC-UHFFFAOYSA-N 2-chloropyridine Chemical class ClC1=CC=CC=N1 OKDGRDCXVWSXDC-UHFFFAOYSA-N 0.000 description 2
- SCFYKVOWPXBYCK-UHFFFAOYSA-N BO1[Ir](C)(C)O(B)[Ir]1(C)[La] Chemical compound BO1[Ir](C)(C)O(B)[Ir]1(C)[La] SCFYKVOWPXBYCK-UHFFFAOYSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 238000002441 X-ray diffraction Methods 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 229910052788 barium Inorganic materials 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 239000002322 conducting polymer Substances 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 230000008025 crystallization Effects 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 238000001194 electroluminescence spectrum Methods 0.000 description 2
- 238000000921 elemental analysis Methods 0.000 description 2
- XYIBRDXRRQCHLP-UHFFFAOYSA-N ethyl acetoacetate Chemical compound CCOC(=O)CC(C)=O XYIBRDXRRQCHLP-UHFFFAOYSA-N 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 238000004770 highest occupied molecular orbital Methods 0.000 description 2
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 2
- UEEXRMUCXBPYOV-UHFFFAOYSA-N iridium;2-phenylpyridine Chemical compound [Ir].C1=CC=CC=C1C1=CC=CC=N1.C1=CC=CC=C1C1=CC=CC=N1.C1=CC=CC=C1C1=CC=CC=N1 UEEXRMUCXBPYOV-UHFFFAOYSA-N 0.000 description 2
- YOLNUNVVUJULQZ-UHFFFAOYSA-J iridium;tetrachloride Chemical compound [Cl-].[Cl-].[Cl-].[Cl-].[Ir] YOLNUNVVUJULQZ-UHFFFAOYSA-J 0.000 description 2
- 238000004020 luminiscence type Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 150000001455 metallic ions Chemical class 0.000 description 2
- 229910003455 mixed metal oxide Inorganic materials 0.000 description 2
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical class OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 2
- 229920000767 polyaniline Polymers 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 239000011241 protective layer Substances 0.000 description 2
- 230000000171 quenching effect Effects 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000005215 recombination Methods 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 239000012453 solvate Substances 0.000 description 2
- 238000000859 sublimation Methods 0.000 description 2
- 230000008022 sublimation Effects 0.000 description 2
- 229910052723 transition metal Inorganic materials 0.000 description 2
- 150000003624 transition metals Chemical class 0.000 description 2
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 2
- 238000001771 vacuum deposition Methods 0.000 description 2
- POILWHVDKZOXJZ-ARJAWSKDSA-M (z)-4-oxopent-2-en-2-olate Chemical compound C\C([O-])=C\C(C)=O POILWHVDKZOXJZ-ARJAWSKDSA-M 0.000 description 1
- PPUIIGZBBURFML-UHFFFAOYSA-N 2-(2-fluorophenyl)-5-(trifluoromethyl)pyridine Chemical compound FC1=CC=CC=C1C1=CC=C(C(F)(F)F)C=N1 PPUIIGZBBURFML-UHFFFAOYSA-N 0.000 description 1
- SEWNWACDICHTTP-UHFFFAOYSA-N 2-(3,5-difluorophenyl)-5-(trifluoromethyl)pyridine Chemical compound FC1=CC(F)=CC(C=2N=CC(=CC=2)C(F)(F)F)=C1 SEWNWACDICHTTP-UHFFFAOYSA-N 0.000 description 1
- WNCPHYQOEBGTSR-UHFFFAOYSA-N 2-(3-methoxyphenyl)-5-(trifluoromethyl)pyridine Chemical compound COC1=CC=CC(C=2N=CC(=CC=2)C(F)(F)F)=C1 WNCPHYQOEBGTSR-UHFFFAOYSA-N 0.000 description 1
- HHYIUGQFXUVOPD-UHFFFAOYSA-N 2-[3-(trifluoromethyl)phenyl]quinoline Chemical compound FC(F)(F)C1=CC=CC(C=2N=C3C=CC=CC3=CC=2)=C1 HHYIUGQFXUVOPD-UHFFFAOYSA-N 0.000 description 1
- KGWDRUMNLRKFSV-UHFFFAOYSA-N 2-[4-(trifluoromethoxy)phenyl]-5-(trifluoromethyl)pyridine Chemical compound C1=CC(OC(F)(F)F)=CC=C1C1=CC=C(C(F)(F)F)C=N1 KGWDRUMNLRKFSV-UHFFFAOYSA-N 0.000 description 1
- UNCQVRBWJWWJBF-UHFFFAOYSA-N 2-chloropyrimidine Chemical class ClC1=NC=CC=N1 UNCQVRBWJWWJBF-UHFFFAOYSA-N 0.000 description 1
- OFUFXTHGZWIDDB-UHFFFAOYSA-N 2-chloroquinoline Chemical class C1=CC=CC2=NC(Cl)=CC=C21 OFUFXTHGZWIDDB-UHFFFAOYSA-N 0.000 description 1
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N 2-ethoxyethanol Chemical compound CCOCCO ZNQVEEAIQZEUHB-UHFFFAOYSA-N 0.000 description 1
- 229940093475 2-ethoxyethanol Drugs 0.000 description 1
- XSKLNXHHATVCSA-UHFFFAOYSA-N 3-chloro-2-(2-fluorophenyl)-5-(trifluoromethyl)pyridine Chemical compound FC1=CC=CC=C1C1=NC=C(C(F)(F)F)C=C1Cl XSKLNXHHATVCSA-UHFFFAOYSA-N 0.000 description 1
- MVIXNQZIMMIGEL-UHFFFAOYSA-N 4-methyl-n-[4-[4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]phenyl]-n-(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)C=1C=CC(=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 MVIXNQZIMMIGEL-UHFFFAOYSA-N 0.000 description 1
- RTEQSCVHPJOKJG-UHFFFAOYSA-N 5-(trifluoromethyl)-2-[3-(trifluoromethyl)phenyl]pyridine Chemical compound N1=CC(C(F)(F)F)=CC=C1C1=CC=CC(C(F)(F)F)=C1 RTEQSCVHPJOKJG-UHFFFAOYSA-N 0.000 description 1
- YDCKPVSNPLMYRB-UHFFFAOYSA-N 5-bromo-2-(4-bromophenyl)pyridine Chemical compound C1=CC(Br)=CC=C1C1=CC=C(Br)C=N1 YDCKPVSNPLMYRB-UHFFFAOYSA-N 0.000 description 1
- UHBIKXOBLZWFKM-UHFFFAOYSA-N 8-hydroxy-2-quinolinecarboxylic acid Chemical compound C1=CC=C(O)C2=NC(C(=O)O)=CC=C21 UHBIKXOBLZWFKM-UHFFFAOYSA-N 0.000 description 1
- WEKSDEUQNRSYNE-UHFFFAOYSA-N C.C1=CC=C(C2=CC=CC=N2)C=C1.CC.CC.C[Y].C[Y].ClC1=NC=CC=C1.OB(O)C1=CC=CC=C1.[Pd] Chemical compound C.C1=CC=C(C2=CC=CC=N2)C=C1.CC.CC.C[Y].C[Y].ClC1=NC=CC=C1.OB(O)C1=CC=CC=C1.[Pd] WEKSDEUQNRSYNE-UHFFFAOYSA-N 0.000 description 1
- JBJBUFXQKQZNNX-UHFFFAOYSA-N C1=CC=C(C2=CC=CC=N2)C=C1.C1=CC=N2[Ir]C3=C(C=CC=C3)C2=C1.CC.CC.C[Y].C[Y] Chemical compound C1=CC=C(C2=CC=CC=N2)C=C1.C1=CC=N2[Ir]C3=C(C=CC=C3)C2=C1.CC.CC.C[Y].C[Y] JBJBUFXQKQZNNX-UHFFFAOYSA-N 0.000 description 1
- BCIPGGDABZHRBK-UHFFFAOYSA-N CCOC1CC(C)O[Ir]2(O1)C1=C(C=CC(F)=C1)C1=CC=C(C(F)(F)F)C=N12 Chemical compound CCOC1CC(C)O[Ir]2(O1)C1=C(C=CC(F)=C1)C1=CC=C(C(F)(F)F)C=N12 BCIPGGDABZHRBK-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 229940126062 Compound A Drugs 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NFHFRUOZVGFOOS-UHFFFAOYSA-N Pd(PPh3)4 Substances [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 1
- 206010034972 Photosensitivity reaction Diseases 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 1
- 229910052772 Samarium Inorganic materials 0.000 description 1
- 238000000944 Soxhlet extraction Methods 0.000 description 1
- 238000006069 Suzuki reaction reaction Methods 0.000 description 1
- 241001126918 Sycon Species 0.000 description 1
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 1
- FHBFEMJVNJOSGN-UHFFFAOYSA-N [H]O1[Ir]2(C3=C(C=CC(F)=C3)C3=CC=C(C(F)(F)F)C=N32)O([H])[Ir]12C1=C(C=CC(F)=C1)C1=CC=C(C(F)(F)F)C=N12 Chemical compound [H]O1[Ir]2(C3=C(C=CC(F)=C3)C3=CC=C(C(F)(F)F)C=N32)O([H])[Ir]12C1=C(C=CC(F)=C1)C1=CC=C(C(F)(F)F)C=N12 FHBFEMJVNJOSGN-UHFFFAOYSA-N 0.000 description 1
- 229910052768 actinide Inorganic materials 0.000 description 1
- 150000001255 actinides Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 229940111121 antirheumatic drug quinolines Drugs 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 150000001543 aryl boronic acids Chemical class 0.000 description 1
- 150000003851 azoles Chemical class 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 229920000547 conjugated polymer Polymers 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 150000001893 coumarin derivatives Chemical class 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- SPWVRYZQLGQKGK-UHFFFAOYSA-N dichloromethane;hexane Chemical class ClCCl.CCCCCC SPWVRYZQLGQKGK-UHFFFAOYSA-N 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 238000000295 emission spectrum Methods 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- RBTKNAXYKSUFRK-UHFFFAOYSA-N heliogen blue Chemical compound [Cu].[N-]1C2=C(C=CC=C3)C3=C1N=C([N-]1)C3=CC=CC=C3C1=NC([N-]1)=C(C=CC=C3)C3=C1N=C([N-]1)C3=CC=CC=C3C1=N2 RBTKNAXYKSUFRK-UHFFFAOYSA-N 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052747 lanthanoid Inorganic materials 0.000 description 1
- 150000002602 lanthanoids Chemical class 0.000 description 1
- 239000002346 layers by function Substances 0.000 description 1
- 239000012263 liquid product Substances 0.000 description 1
- PQXKHYXIUOZZFA-UHFFFAOYSA-M lithium fluoride Inorganic materials [Li+].[F-] PQXKHYXIUOZZFA-UHFFFAOYSA-M 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 238000004776 molecular orbital Methods 0.000 description 1
- 239000012452 mother liquor Substances 0.000 description 1
- 229910052755 nonmetal Inorganic materials 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 150000002902 organometallic compounds Chemical class 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 230000036211 photosensitivity Effects 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 239000012264 purified product Substances 0.000 description 1
- 150000003222 pyridines Chemical class 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 150000003248 quinolines Chemical class 0.000 description 1
- 229910052761 rare earth metal Inorganic materials 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- KZUNJOHGWZRPMI-UHFFFAOYSA-N samarium atom Chemical compound [Sm] KZUNJOHGWZRPMI-UHFFFAOYSA-N 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000012265 solid product Substances 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000002230 thermal chemical vapour deposition Methods 0.000 description 1
- 150000004867 thiadiazoles Chemical class 0.000 description 1
- 238000000427 thin-film deposition Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
- 238000002061 vacuum sublimation Methods 0.000 description 1
- 238000007740 vapor deposition Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/26—Radicals substituted by halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/28—Radicals substituted by singly-bound oxygen or sulphur atoms
- C07D213/30—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/61—Halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/62—Oxygen or sulfur atoms
- C07D213/63—One oxygen atom
- C07D213/68—One oxygen atom attached in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/04—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, directly attached to the ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/26—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F15/00—Compounds containing elements of Groups 8, 9, 10 or 18 of the Periodic Table
- C07F15/0006—Compounds containing elements of Groups 8, 9, 10 or 18 of the Periodic Table compounds of the platinum group
- C07F15/0033—Iridium compounds
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/341—Transition metal complexes, e.g. Ru(II)polypyridine complexes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/341—Transition metal complexes, e.g. Ru(II)polypyridine complexes
- H10K85/342—Transition metal complexes, e.g. Ru(II)polypyridine complexes comprising iridium
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1014—Carbocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/18—Metal complexes
- C09K2211/185—Metal complexes of the platinum group, i.e. Os, Ir, Pt, Ru, Rh or Pd
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
Definitions
- This invention relates to electroluminescent complexes of iridium(III) with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines. It also relates to electronic devices in which the active layer includes an electroluminescent Ir(III) complex.
- Organic electronic devices that emit light such as light-emitting diodes that make up displays, are present in many different kinds of electronic equipment.
- an organic active layer is sandwiched between two electrical contact layers. At least one of the electrical contact layers is light-transmitting so that light can pass through the electrical contact layer.
- the organic active layer emits light through the light-transmitting electrical contact layer upon application of electricity across the electrical contact layers.
- organic electroluminescent compounds As the active component in light-emitting diodes. Simple organic molecules such as anthracene, thiadiazole derivatives, and coumarin derivatives are known to show electroluminescence. Semiconductive conjugated polymers have also been used as electroluminescent components, as has been disclosed in, for example, Friend et al., U.S. Pat. No. 5,247,190, Heeger et al., U.S. Pat. No. 5,408,109, and Nakano et al., Published European Patent Application 443 86 1. Complexes of 8-hydroxyquinolate with trivalent metal ions, particularly aluminum, have been extensively used as electroluminescent components, as has been disclosed in, for example, Tang et al., U.S. Pat. No. 5,552,678.
- the present invention is directed to an iridium compound (generally referred as “Ir(III) compounds”) having at least two 2-phenylpyridine ligands in which there is at least one fluorine or fluorinated group on the ligand.
- the iridium compound has the following First Formula:
- L′ a bidentate ligand or a monodentate ligand, and is not a phenylpyridine, phenylpyrimidine, or phenylquinoline; with the proviso that:
- L′′ a monodentate ligand, and is not a phenylpyridine, and phenylpyrimidine, or phenylquinoline;
- L a , L b and L c are alike or different from each other and each of L a , L b and L c has structure (I) below:
- adjacent pairs of R 1 -R 4 and R 5 -R 8 can be joined to form a five- or six-membered ring
- the present invention is directed to substituted 2-phenylpyridine, phenylpyrimidine, and phenylquinoline precursor compounds from which the above Ir(III) compounds are made.
- the precursor compounds have a structure (II) or (III) below:
- R 1 -R 8 are as defined in structure (I) above, and R 9 is H.
- the present invention is directed to an organic electronic device having at least one emitting layer comprising the above Ir(III) compound, or combinations of the above Ir(III) compounds.
- the term “compound” is intended to mean an electrically uncharged substance made up of molecules that further consist of atoms, wherein the atoms cannot be separated by physical means.
- ligand is intended to mean a molecule, ion, or atom that is attached to the coordination sphere of a metallic ion.
- complex when used as a noun, is intended to mean a compound having at least one metallic ion and at least one ligand.
- group is intended to mean a part of a compound, such a substituent in an organic compound or a ligand in a complex.
- the term “facial” is intended to mean one isomer of a complex, Ma 3 b 3 , having octahedral geometry, in which the three “a” groups are all adjacent, i.e. at the corners of one face of the octahedron.
- the term “meridional” is intended to mean one isomer of a complex, Ma 3 b 3 , having octahedral geometry, in which the three “a” groups occupy three positions such that two are trans to each other.
- the phrase “adjacent to,” when used to refer to layers in a device, does not necessarily mean that one layer is immediately next to another layer.
- adjacent R groups is used to refer to R groups that are next to each other in a chemical formula (i.e., R groups that are on atoms joined by a bond).
- photoactive refers to any material that exhibits electroluminescence and/or photosensitivity.
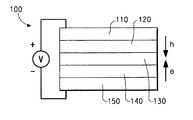
- FIG. 1 is a schematic diagram of a light-emitting device (LED).
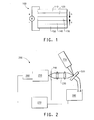
- FIG. 2 is a schematic diagram of an LED testing apparatus.
- the Ir(III) compounds of the invention have the First Formula Ir(III)L a L b L c x L′ y above.
- Ir(III) compounds are frequently referred to as cyclometalated complexes: Ir(III) compounds having the following Second Formula is also frequently referred to as a bis-cyclometalated complex.:
- y, z, L a , L b , L′, and L′′ are as defined in the First Formula above.
- Ir(III) compounds having the following Third Formula is also frequently referred to as a tris-cyclometalated complex.:
- L a , L b and L c are as defined in the First Formula described above.
- the preferred cyclometalated complexes are neutral and non-ionic, and can be sublimed intact. Thin films of these materials obtained via vacuum deposition exhibit good to excellent electroluminescent properties.
- Introduction of fluorine substituents into the ligands on the iridium atom increases both the stability and volatility of the complexes. As a result, vacuum deposition can be carried out at lower temperatures and decomposition of the complexes can be avoided. Introduction of fluorine substituents into the ligands can often reduce the non-radiative decay rate and the self-quenching phenomenon in the solid state. These reductions can lead to enhanced luminescence efficiency. Variation of substituents with electron-donating and electron-withdrawing properties allows for fine-tuning of electroluminescent properties of the compound and hence optimization of the brightness and efficiency in an electroluminescent device.
- compounds that can exhibit electroluminescence include those of compounds of the Second Formula IrL a L b L′ y L′′ z above, and the Third Formula IrL a L b L c above, where all L a , L b , and L c in the Third Formula are phenylpyridines, phenylpyrimidines, or phenylquinolines.
- R 1 -R 8 groups of structures (I) and (II), and the R 10 -R 19 groups of structure (III) above may be chosen from conventional substitutents for organic compounds, such as alkyl, alkoxy, halogen, nitro, and cyano groups, as well as fluoro, fluorinated alkyl and fluorinated alkoxy groups.
- the groups can be partially or fully fluorinated (perfluorinated).
- the nitrogen-containing ring can be a pyridine ring, a pyrimidine or a quinoline. It is preferred that at least one fluorinated substituent is on the nitrogen-containing ring; most preferably CF 3 .
- any conventional ligands known to transition metal coordination chemistry is suitable as the L′ and L′′ ligands.
- bidentate ligands include compounds having two coordinating groups, such as ethylenediamine and acetylacetonate, which may be substituted.
- monodentate ligands include chloride and nitrate ions and mono-amines. It is preferred that the iridium complex be neutral and sublimable. If a single bidentate ligand is used, it should have a net charge of minus one ( ⁇ 1). If two monodentate ligands are used, they should have a combined net charge of minus one ( ⁇ 1).
- the bis-cyclometalated complexes can be useful in preparing tris-cyclometalated complexes where the ligands are not all the same.
- the iridium compound has the Third Formula IrL a L b L c as described above.
- These more preferred compounds frequently exhibit a facial geometry, as determined by single crystal X-ray diffraction, in which the nitrogen atoms coordinated to the iridium are trans with respect to carbon atoms coordinated to the iridium.
- These more preferred compounds have the following Fourth Formula:
- L a has structure (I) above.
- the compounds can also exhibit a meridional geometry in which two of the nitrogen atoms coordinated to the iridium are trans to each other. These compounds have the following Fifth Formula:
- L a has structure (1) above.
- Examples compounds of the Second Formula IrL a L b L′ y L′′ z above include compounds 1-n, 1-o, 11-w and 1-x, respectively having structure (IV), (V), (VI), (IX) and (X) below:
- the iridium complexes of the Third Formula IrL a L b L c above are generally prepared from the appropriate substituted 2-phenylpyridine, phenylpyrimidine, or phenylquinoline.
- the substituted 2-phenylpyridines, phenylpyrimidines, and phenylquinolines, as shown in Structure (II) above, are prepared, in good to excellent yield, using the Suzuki coupling of the substituted 2-chloropyridine, 2-chloropyrimidine or 2-chloroquinoline with arylboronic acid as described in 0. Lohse, P.Thevenin, E. Waldvogel Synlett, 1999, 45-48. This reaction is illustrated for the pyridine derivative, where X and Y represent substituents, in Equation (1) below:
- the 2-phenylpyridines, pyrimidines, and quinolines thus prepared are used for the synthesis of the cyclometalated iridium complexes.
- a convenient one-step method has been developed employing commercially available iridium trichloride hydrate and silver trifluoroacetate.
- the reactions are generally carried out with an excess of 2-phenylpyridine, pyrimidine, or quinoline, without a solvent, in the presence of 3 equivalents of AgOCOCF 3 . This reaction is illustrated for a 2-phenylpyridine in Equation (2) below:
- the tris-cyclometalated iridium complexes were isolated, purified, and fully characterized by elemental analysis, 1 H and 19 F NMR spectral data, and, for compounds 1-b, 1-c, and 1-e, single crystal X-ray diffraction. In some cases, mixtures of isomers are obtained. Often the mixture can be used without isolating the individual isomers.
- the iridium complexes having the Second Formula IrL a L b L′ y L′′ z above may, in some cases, be isolated from the reaction mixture using the same synthetic procedures as preparing those having Third Formula IrL a L b L c above.
- the complexes can also be prepared by first preparing an intermediate iridium dimer having structure VII below:
- B H, CH 3 , or C 2 H 5 .
- L a , L b ,L c , and L d can be the same or different from each other and each of L a , L b ,L c , and L d has structure (I) above.
- the iridium dimers can generally be prepared by first reacting iridium trichloride hydrate with the 2-phenylpyridine, phenylpyrimidine or phenylquinoline, and adding NaOB.
- One particularly useful iridium dimer is the hydroxo iridium dimer, having structure VIII below:
- This intermediate can be used to prepare compound 1-p by the addition of ethyl acetoacetate.
- the present invention also relates to an electronic device comprising at least one photoactive layer positioned between two electrical contact layers, wherein the at least one layer of the device includes the iridium complex of the invention.
- Devices frequently have additional hole transport and electron transport layers.
- a typical structure is shown in FIG. 1.
- the device 100 has an anode layer 110 and a cathode layer 150 .
- Adjacent to the anode is a layer 120 comprising hole transport material.
- Adjacent to the cathode is a layer 140 comprising an electron transport material.
- Between the hole transport layer and the electron transport layer is the photoactive layer 130 .
- the photoactive layer 130 can be a light-emitting layer that is activated by an applied voltage (such as in a light-emitting diode or light-emitting electrochemical cell), a layer of material that responds to radiant energy and generates a signal with or without an applied bias voltage (such as in a photodetector).
- an applied voltage such as in a light-emitting diode or light-emitting electrochemical cell
- a layer of material that responds to radiant energy and generates a signal with or without an applied bias voltage
- Examples of photodetectors include photoconductive cells, photoresistors, photoswitches, phototransistors, and phototubes, and photovoltaic cells, as these terms are describe in Markus, John, Electronics and Nucleonics Dictionary, 470 and 476 (McGraw-Hill, Inc. 1966).
- the iridium compounds of the invention are particularly useful as the photoactive material in layer 130 , or as electron transport material in layer 140 .
- the iridium complexes of the invention are used as the light-emitting material in diodes. It has been found that in these applications, the fluorinated compounds of the invention do not need to be in a solid matrix diluent in order to be effective.
- a layer that is greater than 20% by weight iridium compound, based on the total weight of the layer, up to 100% iridium compound, can be used as the emitting layer.
- the iridium compound is generally present in a small amount, usually less than 20% by weight, preferably less than 10% by weight, based on the total weight of the layer.
- the iridium complexes may be present in more than one isomeric form, or mixtures of different complexes may be present. It will be understood that in the above discussion of OLEDs, the term “the iridium compound” is intended to encompass mixtures of compounds and/or isomers.
- the HOMO (highest occupied molecular orbital) of the hole transport material should align with the work function of the anode
- the LUMO (lowest un-occupied molecular orbital) of the electron transport material should align with the work function of the cathode.
- Chemical compatibility and sublimation temp of the materials are also important considerations in selecting the electron and hole transport materials.
- the other layers in the OLED can be made of any materials which are known to be useful in such layers.
- the anode 110 is an electrode that is particularly efficient for injecting positive charge carriers. It can be made of, for example materials containing a metal, mixed metal, alloy, metal oxide or mixed-metal oxide, or it can be a conducting polymer. Suitable metals include the Group 11 metals, the metals in Groups 4, 5, and 6, and the Group 8-10 transition metals. If the anode is to be light-transmitting, mixed-metal oxides of Groups 12, 13 and 14 metals, such as indium-tin-oxide, are generally used.
- the IUPAC numbering system is used throughout, where the groups from the Periodic Table are numbered from left to right as I-18 (CRC Handbook of Chemistry and Physics, 81 st Edition, 2000).
- the anode 110 may also comprise an organic material such as polyaniline as described in “Flexible light-emitting diodes made from soluble conducting polymer,” Nature vol. 357, pp 477-479 (Jun. 11, 1992). At least one of the anode and cathode should be at least partially transparent to allow the generated light to be observed.
- Examples of hole transport materials for layer 120 have been summarized for example, in Kirk-Othmer Encyclopedia of Chemical Technology, Fourth Edition, Vol. 18, p. 837-860, 1996, by Y. Wang. Both hole transporting molecules and polymers can be used.
- hole transporting molecules are: N,N′-diphenyl-N,N′-bis(3-methylphenyl)-[1,1′-biphenyl]-4,4′-diamine (TPD), 1,1-bis[(di-4-tolylamino) phenyl]cyclohexane (TAPC), N,N′-bis(4-methylphenyl)-N,N′-bis(4-ethylphenyl)-[1,1′-(3,3′-dimethyl)biphenyl]-4,4′-diamine (ETPD), tetrakis-(3-methylphenyl)-N,N,N′,N′-2,5-phenylenediamine (PDA), a-phenyl-4-N,N-diphenylaminostyrene (TPS), p-(diethylamino)-benzaldehyde diphenylhydrazone (DEH), triphenylamine (TPA),
- hole transporting polymers are polyvinylcarbazole, (phenylmethyl)polysilane, and polyaniline. It is also possible to obtain hole transporting polymers by doping hole transporting molecules such as those mentioned above into polymers such as polystyrene and polycarbonate.
- Examples of electron transport materials for layer 140 include metal chelated oxinoid compounds, such as tris(8-hydroxyquinolato)aluminum (Alq 3 ); phenanthroline-based compounds, such as 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (DDPA) or 4,7-diphenyl-1,10-phenanthroline (DPA), and azole compounds such as 2-(4-biphenylyl)-5-(4-t-butylphenyl)-1,3,4-oxadiazole (PBD) and 3-(4-biphenylyl)-4-phenyl-5-(4-t-butylphenyl)-1,2,4-triazole (TAZ).
- Layer 140 can function both to facilitate electron transport, and also serve as a buffer layer or confinement layer to prevent quenching of the exciton at layer interfaces. Preferably, this layer promotes electron mobility and reduces exciton quenching.
- the cathode 150 is an electrode that is particularly efficient for injecting electrons or negative charge carriers.
- the cathode can be any metal or nonmetal having a lower work function than the anode.
- Materials for the cathode can be selected from alkali metals of Group 1 (e.g., Li, Cs), the Group 2 (alkaline earth) metals, the Group 12 metals, including the rare earth elements and lanthanides, and the actinides. Materials such as aluminum, indium, calcium, barium, samarium and magnesium, as well as combinations, can be used.
- Li-containing organometallic compounds can also be deposited between the organic layer and the cathode layer to lower the operating voltage.
- inorganic anode layer 110 may be surface treated to increase charge carrier transport efficiency.
- the choice of materials for each of the component layers is preferably determined by balancing the goals of providing a device with high device efficiency.
- each functional layer may be made up of more than one layer.
- the device can be prepared by sequentially vapor depositing the individual layers on a suitable substrate.
- Substrates such as glass and polymeric films can be used.
- Conventional vapor deposition techniques can be used, such as thermal evaporation, chemical vapor deposition, and the like.
- the organic layers can be coated from solutions or dispersions in suitable solvents, using any conventional coating technique.
- the different layers will have the following range of thicknesses: anode 110 , 500-5000 ⁇ , preferably 1000-2000 ⁇ ; hole transport layer 120 , 50-1000 ⁇ , preferably 200-800 ⁇ ; light-emitting layer 130 , 10-1000 ⁇ , preferably 100-800 ⁇ ; electron transport layer 140 , 50-1000 ⁇ , preferably 200-800 ⁇ ; cathode 150 , 200-10000 ⁇ , preferably 300-5000 ⁇ .
- the location of the electron-hole recombination zone in the device, and thus the emission spectrum of the device, can be affected by the relative thickness of each layer.
- the thickness of the electron-transport layer should be chosen so that the electron-hole recombination zone is in the light-emitting layer.
- the desired ratio of layer thicknesses will depend on the exact nature of the materials used.
- the efficiency of devices made with the iridium compounds of the invention can be further improved by optimizing the other layers in the device.
- more efficient cathodes such as Ca, Ba or LiF can be used.
- Shaped substrates and novel hole transport materials that result in a reduction in operating voltage or increase quantum efficiency are also applicable.
- Additional layers can also be added to tailor the energy levels of the various layers and facilitate electroluminescence.
- the iridium complexes of the invention often are phosphorescent and photoluminescent and may be useful in applications other than OLEDs.
- organometallic complexes of iridium have been used as oxygen sensitive indicators, as phosphorescent indicators in bioassays, and as catalysts.
- the bis cyclometalated complexes can be used to sythesize tris cyclometalated complexes where the third ligand is the same or different.
- This example illustrates the preparation of the 2-phenylpyridines and 2-phenylpyrimidines which are used to form the iridium compounds.
- This example illustrates the preparation of iridium compounds of the Fourth Formula fac-Ir(L a ) 3 above.
- the solid was vigorously stirred under reflux with 30 mL of 1,2-dichloroethane and aqueous NaOH (2.2 g in 8 mL of water) for 6 hours.
- the organic solvent was evaporated from the mixture to leave a suspension of an orange solid in the aqueous phase.
- the orange solid was separated by filtration, thoroughly washed with water, and dried under vacuum to produce 0.94 g (95%) of the iridium hydroxo dimer (spectroscopically pure).
- This example illustrates the preparation of bis-cyclometalated complexes from an iridium dimer.
- This mer-complex was prepared in a manner similar to compound 1-w, using the trifluoroacetate dicyclometalated intermediate, compound 1-x, and 2-(4-fluorophenyl)-5-trifluoromethylpyridine.
- 19 F NMR (CD 2 Cl 2 , 20° C.), ⁇ : ⁇ 63.30 (s, 3F), ⁇ 63.34 (s, 3F), ⁇ 63.37 (s, 3F), ⁇ 108.9 (ddd, 1F), ⁇ 109.0 (ddd, 1F), ⁇ 109.7 (ddd, 1F).
- Thin film OLED devices including a hole transport layer (HT layer), electroluminescent layer (EL layer) and at least one electron transport layer (ET layer) were fabricated by the thermal evaporation technique.
- An Edward Auto 306 evaporator with oil diffusion pump was used.
- the base vacuum for all of the thin film deposition was in the range of 10-6 torr.
- the deposition chamber was capable of depositing five different films without the need to break up the vacuum.
- ITO indium tin oxide
- the substrate was first patterned by etching away the unwanted ITO area with 1N HCl solution, to form a first electrode pattern.
- Polyimide tape was used as the mask.
- the patterned ITO substrates were then cleaned ultrasonically in aqueous detergent solution.
- the substrates were then rinsed with distilled water, followed by isopropanol, and then degreased in toluene vapor for ⁇ 3 hours.
- the cleaned, patterned ITO substrate was then loaded into the vacuum chamber and the chamber was pumped down to 10 ⁇ 6 torr. The substrate was then further cleaned using an oxygen plasma for about 5-10 minutes. After cleaning, multiple layers of thin films were then deposited sequentially onto the substrate by thermal evaporation. Finally, patterned metal electrodes of Al were deposited through a mask. The thickness of the film was measured during deposition using a quartz crystal monitor (Sycon STC-200). All film thickness reported in the Examples are nominal, calculated assuming the density of the material deposited to be one. The completed OLED device was then taken out of the vacuum chamber and characterized immediately without encapsulation.
- the OLED samples were characterized by measuring their (1) current-voltage (I-V) curves, (2) electroluminescence radiance versus voltage, and (3) electroluminescence spectra versus voltage.
- the apparatus used, 200 is shown in FIG. 2.
- the I-V curves of an OLED sample, 220 were measured with a Keithley Source-Measurement Unit Model 237 , 280 .
- the electroluminescence radiance (in the unit of Cd/m 2 ) vs. voltage was measured with a Minolta LS-110 luminescence meter, 210 , while the voltage was scanned using the Keithley SMU.
- the electroluminescence spectrum was obtained by collecting light using a pair of lenses, 230 , through an electronic shutter, 240 , dispersed through a spectrograph, 250 , and then measured with a diode array detector, 260 . All three measurements were performed at the same time and controlled by a computer, 270 .
- the efficiency of the device at certain voltage is determined by dividing the electroluminescence radiance of the LED by the current density needed to run the device. The unit is in Cd/A.
- the peak efficiency is the best indication of the value of the electroluminescent compound in a device. It gives a measure of how many electrons have to be input into a device in order to get a certain number of photons out (radiance). It is a fundamentally important number, which reflects the intrinsic efficiency of the light-emitting material. It is also important for practical applications, since higher efficiency means that fewer electrons are needed in order to achieve the same radiance, which in turn means lower power consumption. Higher efficiency devices also tend to have longer lifetimes, since a higher proportion of injected electrons are converted to photons, instead of generating heat or causing an undesirable chemical side reactions.
- iridium complexes of the invention have much higher peak efficiencies than the parent-tris(2-phenylpyridine) iridium complex. Those complexes with lower efficiencies may also find utility as phosphorescent or photoluminescent materials, or as catalysts, as discussed above.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Crystallography & Structural Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Electroluminescent Light Sources (AREA)
- Pyridine Compounds (AREA)
- Quinoline Compounds (AREA)
Abstract
The present invention is generally directed to electroluminescent Ir(III) compounds, the substituted 2-phenylpyridines, phenylpyrimidines, and phenylquinolines that are used to make the Ir(III) compounds, and devices that are made with the Ir(III) compounds.
Description
- 1. Field of the Invention
- This invention relates to electroluminescent complexes of iridium(III) with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines. It also relates to electronic devices in which the active layer includes an electroluminescent Ir(III) complex.
- 2. Description of the Related Art
- Organic electronic devices that emit light, such as light-emitting diodes that make up displays, are present in many different kinds of electronic equipment. In all such devices, an organic active layer is sandwiched between two electrical contact layers. At least one of the electrical contact layers is light-transmitting so that light can pass through the electrical contact layer. The organic active layer emits light through the light-transmitting electrical contact layer upon application of electricity across the electrical contact layers.
- It is well known to use organic electroluminescent compounds as the active component in light-emitting diodes. Simple organic molecules such as anthracene, thiadiazole derivatives, and coumarin derivatives are known to show electroluminescence. Semiconductive conjugated polymers have also been used as electroluminescent components, as has been disclosed in, for example, Friend et al., U.S. Pat. No. 5,247,190, Heeger et al., U.S. Pat. No. 5,408,109, and Nakano et al., Published European Patent Application 443 86 1. Complexes of 8-hydroxyquinolate with trivalent metal ions, particularly aluminum, have been extensively used as electroluminescent components, as has been disclosed in, for example, Tang et al., U.S. Pat. No. 5,552,678.
- Burrows and Thompson have reported that fac-tris(2-phenylpyridine) iridium can be used as the active component in organic light-emitting devices. ( Appl. Phys. Lett. 1999, 75, 4.) The performance is maximized when the iridium compound is present in a host conductive material. Thompson has further reported devices in which the active layer is poly(N-vinyl carbazole) doped with fac-tris[2-(4′,5′-difluorophenyl)pyridine-C′2,N]iridium(III). (Polymer Preprints 2000, 41(1), 770.)
- However, there is a continuing need for electroluminescent compounds having improved efficiency.
- The present invention is directed to an iridium compound (generally referred as “Ir(III) compounds”) having at least two 2-phenylpyridine ligands in which there is at least one fluorine or fluorinated group on the ligand. The iridium compound has the following First Formula:
- IrLaLbLc xL′yL″z (First Formula)
- where:
- x=0 or 1, y=0, 1 or 2, and z=0 or 1, with the proviso that:
- x=0 or y+z=0 and
- when y=2 then z=0;
- L′=a bidentate ligand or a monodentate ligand, and is not a phenylpyridine, phenylpyrimidine, or phenylquinoline; with the proviso that:
- when L′ is a monodentate ligand, y+z=2, and
- when L′ is a bidentate ligand, z=0;
- L″=a monodentate ligand, and is not a phenylpyridine, and phenylpyrimidine, or phenylquinoline; and
-
- wherein:
- adjacent pairs of R 1-R4 and R5-R8 can be joined to form a five- or six-membered ring,
- at least one of R 1-R8 is selected from F, CnF2n+1, OCnF2n+1, and OCF2X, where n=1-6 and X=H, Cl, or Br, and
- A=C or N, provided that when A=N, there is no R 1.
-
-
- where:
- at least one of R 10-R19 is selected from F, CnF2n+1, OCnF2n+1, and OCF2X, where n=1-6 and X=H, Cl, or Br, and R20 is H.
- It is understood that there is free rotation about the phenyl-pyridine, phenyl-pyrimidine and the phenyl-quinoline bonds. However, for the discussion herein, the compounds will be described in terms of one orientation.
- In another embodiment, the present invention is directed to an organic electronic device having at least one emitting layer comprising the above Ir(III) compound, or combinations of the above Ir(III) compounds.
- As used herein, the term “compound” is intended to mean an electrically uncharged substance made up of molecules that further consist of atoms, wherein the atoms cannot be separated by physical means. The term “ligand” is intended to mean a molecule, ion, or atom that is attached to the coordination sphere of a metallic ion. The term “complex”, when used as a noun, is intended to mean a compound having at least one metallic ion and at least one ligand. The term “group” is intended to mean a part of a compound, such a substituent in an organic compound or a ligand in a complex. The term “facial” is intended to mean one isomer of a complex, Ma 3b3, having octahedral geometry, in which the three “a” groups are all adjacent, i.e. at the corners of one face of the octahedron. The term “meridional” is intended to mean one isomer of a complex, Ma3b3, having octahedral geometry, in which the three “a” groups occupy three positions such that two are trans to each other. The phrase “adjacent to,” when used to refer to layers in a device, does not necessarily mean that one layer is immediately next to another layer. On the other hand, the phrase “adjacent R groups,” is used to refer to R groups that are next to each other in a chemical formula (i.e., R groups that are on atoms joined by a bond). The term “photoactive” refers to any material that exhibits electroluminescence and/or photosensitivity.
- FIG. 1 is a schematic diagram of a light-emitting device (LED).
- FIG. 2 is a schematic diagram of an LED testing apparatus.
- The Ir(III) compounds of the invention have the First Formula Ir(III)L aLbLc xL′y above.
- The above Ir(III) compounds are frequently referred to as cyclometalated complexes: Ir(III) compounds having the following Second Formula is also frequently referred to as a bis-cyclometalated complex.:
- IrLaLbL′yL″z (Second Formula)
- where:
- y, z, L a, Lb, L′, and L″ are as defined in the First Formula above.
- Ir(III) compounds having the following Third Formula is also frequently referred to as a tris-cyclometalated complex.:
- IrLaLbLc (Third Formula)
- where:
- L a, Lb and Lc are as defined in the First Formula described above.
- The preferred cyclometalated complexes are neutral and non-ionic, and can be sublimed intact. Thin films of these materials obtained via vacuum deposition exhibit good to excellent electroluminescent properties. Introduction of fluorine substituents into the ligands on the iridium atom increases both the stability and volatility of the complexes. As a result, vacuum deposition can be carried out at lower temperatures and decomposition of the complexes can be avoided. Introduction of fluorine substituents into the ligands can often reduce the non-radiative decay rate and the self-quenching phenomenon in the solid state. These reductions can lead to enhanced luminescence efficiency. Variation of substituents with electron-donating and electron-withdrawing properties allows for fine-tuning of electroluminescent properties of the compound and hence optimization of the brightness and efficiency in an electroluminescent device.
- While not wishing to be bound by theory, it is believed that the emission from the iridium compounds is ligand-based, resulting from metal-to-ligand charge transfer. Therefore, compounds that can exhibit electroluminescence include those of compounds of the Second Formula IrL aLbL′yL″z above, and the Third Formula IrLaLbLc above, where all La, Lb, and Lc in the Third Formula are phenylpyridines, phenylpyrimidines, or phenylquinolines. The R1-R8 groups of structures (I) and (II), and the R10-R19 groups of structure (III) above may be chosen from conventional substitutents for organic compounds, such as alkyl, alkoxy, halogen, nitro, and cyano groups, as well as fluoro, fluorinated alkyl and fluorinated alkoxy groups. The groups can be partially or fully fluorinated (perfluorinated). Preferred iridium compounds have all R1-R8 and R10-R19 substituents selected from fluoro, perfluorinated alkyl (CnF2n+1) and perfluorinated alkoxy groups (OCnF2n+1), where the perfluorinated alkyl and alkoxy groups have 1-6 carbon atoms, or a group of the formula OCF2X, where X=H, Cl, or Br.
- It has been found that the electroluminescent properties of the cyclometalated iridium complexes are poorer when any one or more of the R 1-R8 and R10-R19 groups is a nitro group. Therefore, it is preferred that none of the R1-R8 and R10-R19 groups is a nitro group.
- The nitrogen-containing ring can be a pyridine ring, a pyrimidine or a quinoline. It is preferred that at least one fluorinated substituent is on the nitrogen-containing ring; most preferably CF 3.
- Any conventional ligands known to transition metal coordination chemistry is suitable as the L′ and L″ ligands. Examples of bidentate ligands include compounds having two coordinating groups, such as ethylenediamine and acetylacetonate, which may be substituted. Examples of monodentate ligands include chloride and nitrate ions and mono-amines. It is preferred that the iridium complex be neutral and sublimable. If a single bidentate ligand is used, it should have a net charge of minus one (−1). If two monodentate ligands are used, they should have a combined net charge of minus one (−1). The bis-cyclometalated complexes can be useful in preparing tris-cyclometalated complexes where the ligands are not all the same.
- In a preferred embodiment, the iridium compound has the Third Formula IrL aLbLc as described above.
- In a more preferred embodiment, L a=Lb=Lc. These more preferred compounds frequently exhibit a facial geometry, as determined by single crystal X-ray diffraction, in which the nitrogen atoms coordinated to the iridium are trans with respect to carbon atoms coordinated to the iridium. These more preferred compounds have the following Fourth Formula:
- fac−Ir(La)3 (Fourth Formula)
- where L a has structure (I) above.
- The compounds can also exhibit a meridional geometry in which two of the nitrogen atoms coordinated to the iridium are trans to each other. These compounds have the following Fifth Formula:
- mer−Ir(La)3 (Fifth Formula)
- where L a has structure (1) above.
- Examples of compounds of the Fourth Formula and Fifth Formula above are given in Table I below:
TABLE 1 Com- pound A R1 R2 R3 R4 R5 R6 R7 R8 Formula 1-a C H H CF3 H H H H H Fourth 1-b C H H CF3 H H H F H Fourth 1-c C H H CF3 H F H H H Fourth 1-d C H H H H F H H H Fourth 1-e C H H CF3 H H CF3 H H Fourth 1-f C H H H H H CF3 H H Fourth 1-g C H H H H H H F H Fourth 1-h C Cl H CF3 H H H H H Fourth 1-i C H H CF3 H H H OCH3 H Fourth 1-j C H H CF3 H H F H H Fourth 1-k C H H NO2 H H CF3 H H Fourth 1-l C H H CF3 H H H OCF3 H Fourth 1-m N — CF3 H H H H F H Fourth 1-q C H H CF3 H H OCH3 H H Fourth 1-r C H OCH3 H H H H CF3 H Fourth 1-s C H H H H F H F H Fourth and Fifth 1-t C H H CF3 H H F H F Fifth 1-u C H H CF3 H F H F H Fifth 1-v C H H CF3 H H H F H Fifth -
- The iridium complexes of the Third Formula IrL aLbLc above are generally prepared from the appropriate substituted 2-phenylpyridine, phenylpyrimidine, or phenylquinoline. The substituted 2-phenylpyridines, phenylpyrimidines, and phenylquinolines, as shown in Structure (II) above, are prepared, in good to excellent yield, using the Suzuki coupling of the substituted 2-chloropyridine, 2-chloropyrimidine or 2-chloroquinoline with arylboronic acid as described in 0. Lohse, P.Thevenin, E. Waldvogel Synlett, 1999, 45-48. This reaction is illustrated for the pyridine derivative, where X and Y represent substituents, in Equation (1) below:
- Examples of 2-phenylpyridine and 2-phenylpyrimidine compounds, having structure (II) above, are given in Table 2 below:
TABLE 2 Compound A R1 R2 R3 R4 R5 R6 R7 R8 R9 2-a C H H CF3 H F H H H H 2-b C H H CF3 H H CF3 H H H 2-c C H H NO2 H H CF3 H H H 2-d C H H CF3 H H F H H H 2-e C H H CF3 H H H CH3O H H 2-f C Cl H CF3 H H H H H H 2-g C H H H CF3 H H F H H 2-h N — H H H H H F H H 2-i C H H CF3 H H H CF3O H H 2-j N — CF3 H H F H H H H 2-k C H H CF3 H H H F H H 2-l C CF3 H H H H H H H H 2-m C Cl H CF3 H H H F H H 2-n C CF3 H H H H H F H H 2-o C CF3 H H H H H CH3O H H 2-p C Cl H CF3 H H H CH3O H H 2-q N — CF3 H H H H F H H 2-r C Cl H CF3 H H H H H F 2-s C H H CF3 H H H H H H 2-t C Cl H H H F H H H H 2-v C H H CF3 H H CH3O H H H 2-w C H CH3O H H H H CF3 H H 2-x C H H H H H F F H H 2-y C H H CF3 H H F H F H 2-z C H H CF3 H F H F H H 2-aa C H H Br H H H Br H H - One example of a substituted 2-phenylquinoline compound, having structure (III) above, is compound 2-u, which has R 17=CF3 and R10-R16 and R18-R20=H.
- The 2-phenylpyridines, pyrimidines, and quinolines thus prepared are used for the synthesis of the cyclometalated iridium complexes. A convenient one-step method has been developed employing commercially available iridium trichloride hydrate and silver trifluoroacetate. The reactions are generally carried out with an excess of 2-phenylpyridine, pyrimidine, or quinoline, without a solvent, in the presence of 3 equivalents of AgOCOCF 3. This reaction is illustrated for a 2-phenylpyridine in Equation (2) below:
- The tris-cyclometalated iridium complexes were isolated, purified, and fully characterized by elemental analysis, 1H and 19F NMR spectral data, and, for compounds 1-b, 1-c, and 1-e, single crystal X-ray diffraction. In some cases, mixtures of isomers are obtained. Often the mixture can be used without isolating the individual isomers.
- The iridium complexes having the Second Formula IrL aLbL′yL″z above, may, in some cases, be isolated from the reaction mixture using the same synthetic procedures as preparing those having Third Formula IrLaLbLc above. The complexes can also be prepared by first preparing an intermediate iridium dimer having structure VII below:
- wherein:
- B=H, CH 3, or C2H5, and
- L a, Lb,Lc, and Ld can be the same or different from each other and each of La, Lb,Lc, and Ld has structure (I) above.
- The iridium dimers can generally be prepared by first reacting iridium trichloride hydrate with the 2-phenylpyridine, phenylpyrimidine or phenylquinoline, and adding NaOB.
-
- This intermediate can be used to prepare compound 1-p by the addition of ethyl acetoacetate.
- Electronic Device
- The present invention also relates to an electronic device comprising at least one photoactive layer positioned between two electrical contact layers, wherein the at least one layer of the device includes the iridium complex of the invention. Devices frequently have additional hole transport and electron transport layers. A typical structure is shown in FIG. 1. The
device 100 has ananode layer 110 and acathode layer 150. Adjacent to the anode is alayer 120 comprising hole transport material. Adjacent to the cathode is alayer 140 comprising an electron transport material. Between the hole transport layer and the electron transport layer is thephotoactive layer 130. - Depending upon the application of the
device 100, thephotoactive layer 130 can be a light-emitting layer that is activated by an applied voltage (such as in a light-emitting diode or light-emitting electrochemical cell), a layer of material that responds to radiant energy and generates a signal with or without an applied bias voltage (such as in a photodetector). Examples of photodetectors include photoconductive cells, photoresistors, photoswitches, phototransistors, and phototubes, and photovoltaic cells, as these terms are describe in Markus, John, Electronics and Nucleonics Dictionary, 470 and 476 (McGraw-Hill, Inc. 1966). - The iridium compounds of the invention are particularly useful as the photoactive material in
layer 130, or as electron transport material inlayer 140. Preferably the iridium complexes of the invention are used as the light-emitting material in diodes. It has been found that in these applications, the fluorinated compounds of the invention do not need to be in a solid matrix diluent in order to be effective. A layer that is greater than 20% by weight iridium compound, based on the total weight of the layer, up to 100% iridium compound, can be used as the emitting layer. This is in contrast to the non-fluorinated iridium compound, tris(2-phenylpyridine) iridium (III), which was found to achieve maximum efficiency when present in an amount of only 6-8% by weight in the emitting layer. This was necessary to reduce the self-quenching effect. Additional materials can be present in the emitting layer with the iridium compound. For example, a fluorescent dye may be present to alter the color of emission. A diluent may also be added. The diluent can be a polymeric material, such as poly(N-vinyl carbazole) and polysilane. It can also be a small molecule, such as 4,4′-N,N′-dicarbazole biphenyl or tertiary aromatic amines. When a diluent is used, the iridium compound is generally present in a small amount, usually less than 20% by weight, preferably less than 10% by weight, based on the total weight of the layer. - In some cases the iridium complexes may be present in more than one isomeric form, or mixtures of different complexes may be present. It will be understood that in the above discussion of OLEDs, the term “the iridium compound” is intended to encompass mixtures of compounds and/or isomers.
- To achieve a high efficiency LED, the HOMO (highest occupied molecular orbital) of the hole transport material should align with the work function of the anode, the LUMO (lowest un-occupied molecular orbital) of the electron transport material should align with the work function of the cathode. Chemical compatibility and sublimation temp of the materials are also important considerations in selecting the electron and hole transport materials.
- The other layers in the OLED can be made of any materials which are known to be useful in such layers. The
anode 110, is an electrode that is particularly efficient for injecting positive charge carriers. It can be made of, for example materials containing a metal, mixed metal, alloy, metal oxide or mixed-metal oxide, or it can be a conducting polymer. Suitable metals include the Group 11 metals, the metals in Groups 4, 5, and 6, and the Group 8-10 transition metals. If the anode is to be light-transmitting, mixed-metal oxides of Groups 12, 13 and 14 metals, such as indium-tin-oxide, are generally used. The IUPAC numbering system is used throughout, where the groups from the Periodic Table are numbered from left to right as I-18 (CRC Handbook of Chemistry and Physics, 81st Edition, 2000). Theanode 110 may also comprise an organic material such as polyaniline as described in “Flexible light-emitting diodes made from soluble conducting polymer,” Nature vol. 357, pp 477-479 (Jun. 11, 1992). At least one of the anode and cathode should be at least partially transparent to allow the generated light to be observed. - Examples of hole transport materials for
layer 120 have been summarized for example, in Kirk-Othmer Encyclopedia of Chemical Technology, Fourth Edition, Vol. 18, p. 837-860, 1996, by Y. Wang. Both hole transporting molecules and polymers can be used. Commonly used hole transporting molecules are: N,N′-diphenyl-N,N′-bis(3-methylphenyl)-[1,1′-biphenyl]-4,4′-diamine (TPD), 1,1-bis[(di-4-tolylamino) phenyl]cyclohexane (TAPC), N,N′-bis(4-methylphenyl)-N,N′-bis(4-ethylphenyl)-[1,1′-(3,3′-dimethyl)biphenyl]-4,4′-diamine (ETPD), tetrakis-(3-methylphenyl)-N,N,N′,N′-2,5-phenylenediamine (PDA), a-phenyl-4-N,N-diphenylaminostyrene (TPS), p-(diethylamino)-benzaldehyde diphenylhydrazone (DEH), triphenylamine (TPA), bis[4-(N,N-diethylamino)-2-methylphenyl](4-methylphenyl)methane (MPMP), 1-phenyl-3-[p-(diethylamino)styryl]-5-[p-(diethylamino)phenyl] pyrazoline (PPR or DEASP), 1,2-trans-bis(9H-carbazol-9-yl)cyclobutane (DCZB), N,N,N′,N′-tetrakis(4-methyl-phenyl)-(1,1′-biphenyl)-4,4′-diamine (TTB), and porphyrinic compounds, such as copper phthalocyanine. Commonly used hole transporting polymers are polyvinylcarbazole, (phenylmethyl)polysilane, and polyaniline. It is also possible to obtain hole transporting polymers by doping hole transporting molecules such as those mentioned above into polymers such as polystyrene and polycarbonate. - Examples of electron transport materials for
layer 140 include metal chelated oxinoid compounds, such as tris(8-hydroxyquinolato)aluminum (Alq3); phenanthroline-based compounds, such as 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (DDPA) or 4,7-diphenyl-1,10-phenanthroline (DPA), and azole compounds such as 2-(4-biphenylyl)-5-(4-t-butylphenyl)-1,3,4-oxadiazole (PBD) and 3-(4-biphenylyl)-4-phenyl-5-(4-t-butylphenyl)-1,2,4-triazole (TAZ).Layer 140 can function both to facilitate electron transport, and also serve as a buffer layer or confinement layer to prevent quenching of the exciton at layer interfaces. Preferably, this layer promotes electron mobility and reduces exciton quenching. - The
cathode 150, is an electrode that is particularly efficient for injecting electrons or negative charge carriers. The cathode can be any metal or nonmetal having a lower work function than the anode. Materials for the cathode can be selected from alkali metals of Group 1 (e.g., Li, Cs), the Group 2 (alkaline earth) metals, the Group 12 metals, including the rare earth elements and lanthanides, and the actinides. Materials such as aluminum, indium, calcium, barium, samarium and magnesium, as well as combinations, can be used. Li-containing organometallic compounds can also be deposited between the organic layer and the cathode layer to lower the operating voltage. - It is known to have other layers in organic electronic devices. For example, there can be a layer (not shown) between the
conductive polymer layer 120 and theactive layer 130 to facilitate positive charge transport and/or band-gap matching of the layers, or to function as a protective layer. Similarly, there can be additional layers (not shown) between theactive layer 130 and thecathode layer 150 to facilitate negative charge transport and/or band-gap matching between the layers, or to function as a protective layer. Layers that are known in the art can be used. In addition, any of the above-described layers can be made of two or more layers. Alternatively, some or all ofinorganic anode layer 110, theconductive polymer layer 120, theactive layer 130, andcathode layer 150, may be surface treated to increase charge carrier transport efficiency. The choice of materials for each of the component layers is preferably determined by balancing the goals of providing a device with high device efficiency. - It is understood that each functional layer may be made up of more than one layer.
- The device can be prepared by sequentially vapor depositing the individual layers on a suitable substrate. Substrates such as glass and polymeric films can be used. Conventional vapor deposition techniques can be used, such as thermal evaporation, chemical vapor deposition, and the like. Alternatively, the organic layers can be coated from solutions or dispersions in suitable solvents, using any conventional coating technique. In general, the different layers will have the following range of thicknesses:
anode 110, 500-5000 Å, preferably 1000-2000 Å;hole transport layer 120, 50-1000 Å, preferably 200-800 Å; light-emittinglayer 130, 10-1000 Å, preferably 100-800 Å;electron transport layer 140, 50-1000 Å, preferably 200-800 Å;cathode 150, 200-10000 Å, preferably 300-5000 Å. The location of the electron-hole recombination zone in the device, and thus the emission spectrum of the device, can be affected by the relative thickness of each layer. Thus the thickness of the electron-transport layer should be chosen so that the electron-hole recombination zone is in the light-emitting layer. The desired ratio of layer thicknesses will depend on the exact nature of the materials used. - It is understood that the efficiency of devices made with the iridium compounds of the invention, can be further improved by optimizing the other layers in the device. For example, more efficient cathodes such as Ca, Ba or LiF can be used. Shaped substrates and novel hole transport materials that result in a reduction in operating voltage or increase quantum efficiency are also applicable. Additional layers can also be added to tailor the energy levels of the various layers and facilitate electroluminescence.
- The iridium complexes of the invention often are phosphorescent and photoluminescent and may be useful in applications other than OLEDs. For example, organometallic complexes of iridium have been used as oxygen sensitive indicators, as phosphorescent indicators in bioassays, and as catalysts. The bis cyclometalated complexes can be used to sythesize tris cyclometalated complexes where the third ligand is the same or different.
- The following examples illustrate certain features and advantages of the present invention. They are intended to be illustrative of the invention, but not limiting. All percentages are by weight, unless otherwise indicated.
- This example illustrates the preparation of the 2-phenylpyridines and 2-phenylpyrimidines which are used to form the iridium compounds.
- The general procedure used was described in O. Lohse, P. Thevenin, E. Waldvogel Synlett, 1999, 45-48. In a typical experiment, a mixture of 200 ml of degassed water, 20 g of potassium carbonate, 150 ml of 1,2-dimethoxyethane, 0.5 g of Pd(PPh3)4, 0.05 mol of a substituted 2-chloropyridine (quinoline or pyrimidine) and 0.05 mol of a substituted phenylboronic acid was refluxed (80-90° C.) for 16-30 h. The resulting reaction mixture was diluted with 300 ml of water and extracted with CH2Cl2 (2×100 ml). The combined organic layers were dried over MgSO4, and the solvent removed by vacuum. The liquid products were purified by fractional vacuum distillation. The solid materials were recrystallized from hexane. The typical purity of isolated materials was >98%. The starting materials, yields, melting and boiling points of the new materials are given in Table 3. NMR data and analytical data are given in Table 4.
TABLE 3 Preparation of 2-Phenyl Pyridines, Phenylpyrimidines and Phenylquinolines Compound Yield in % B.p./mm Hg (m.p.) in ° C. 2-s 70 — 2-a 72 — 2-b 48 — 2-u 75 (76-78) 2-c 41 (95-96) 2-d 38 (39-40) 2-e 55 74.5/0.1 2-g 86 71-73/0.07 2-t 65 77-78/0.046 2-k 50 (38-40) 2-m 80 72-73/0.01 2-f 22 52-33/0.12 2-v 63 95-96/13 2-w 72 2-x 35 61-62/0.095 2-y 62 (68-70) 2-z 42 66-67/0.06 (58-60) 2-aa 60 -
TABLE 4 Properties of 2-Phenyl Pyridines, Phenylpyrimidines and Phenylquinolines Analysis %, found Compound 1H NMR 19F NMR (calc.) or MS (M+) 2-s 7.48 (3H), −62.68 C, 64.50 (64.57) 7.70 (1H), H, 3.49 (3.59) 7.83 (1H), N, 6.07 (6.28) 7.90 (2H), 8.75 (1H) 2-a 7.19 (1H), −60.82 (3F, s), C, 59.56 (59.75) 7.30 (1H), −116.96 (1F, m) H, 3.19 (2.90) 7.43 (1H), N, 5.52 (5.81) 7.98 (2H), 8.07 (1H) 9.00 (1H) 2-b 7.58 (1H), −62.75 (3F, s), C, 53.68 (53.60) 7.66 (1H), −63.10 (3F, s) H, 2.61 (2.40) 7.88 (1H), N, 4.53 (4.81) 8.03 (1H), 8.23 (1H), 8.35 (1H) 8.99 (1H) 2-u 7.55 (1H), −62.89 (s) C, 69.17 (70.33) 7.63 (1H), H, 3.79 (3.66) 7.75 (2H), N, 4.88 (5.12) 7.89 (2H), 8.28 (2H), 8.38 (1H), 8.50 (1H) 2-c 7.53 (1H), −62.14 (s) C, 53.83 (53.73) 7.64 (1H), H, 2.89 (2.61) 7.90 (1H), N, 9.99 (10.44) 8.18 (1H), 8.30 (1H), 8.53 (1H), 9.43 (1H) 2-d 7.06 (1H), −62.78 (3F, s), C, 59.73 (59.75) 7.48 (1H), −112.61 (1F, m) H, 2.86 (2.90) 7.81 (3H), N, 5.70 (5.81) 8.01 (1H), 8.95 (1H), 2-e 3.80 (3H) −62.63 (s) C, 61.66 (61.90) 6.93 (2H), H, 3.95 (4.04) 7.68 (1H), N, 5.53 (5.38) 7.85 (1H), 7.96 (2H), 8.82 (1H), 2-g 2.70 (3H) −114.03 (m) C, 76.56 (77.00) 7.10 (3H), H, 5.12 (5.30) 7.48 (1H), N, 5.43 (7.50) 7.60 (1H), 8.05 (2H), 2-t 7.10 (2H), −62.73 (3F, s) C, 50.51 (52.17) 7.35 (2H), −113.67 (1F, m) H, 1.97 (2.17) 7.96 (1H), N, 5.09 (5.07) 8.78 (1H), 2-k 7.08 (2H), −62.75 (3F, s) C, 60.39 (59.75), 7.62 (1H), −111.49 (m) H, 3.38 (2.90), 7.90 (3H), N, 5.53 (5.51) 8.80 (1H), 2-m 7.10 (2H), −62.63 (3F, s) C, 52.13 (52.17) 7.80 (2H), −111.24 (m) H, 2.16 (2.17) 8.00 (1H), N, 4.85 (5.07) 8.75 (1H), 2-f 7.55 (3H), −62.57 (s) 257 (M+, 7.77 (2H), C12H7F3ClN+), 8.06 (1H), 222 (M—Cl) 8.87 (1H) 2-v 3.8 (3H), −62.70 ppm C, 61.66 (61.37), 6.95 (1H), H, 3.98 (3.67), 7.30 (1H), N, 5.53 (5.48) 7.50 (1H), 7.58 (1H), 7.75 (1H), 7.90 (1H), 8.87 (1H) 2-w 8.54 (1H, d), −63.08 (3F, s) 8.21 (2H, d), 7.70 (2H, d), 7.24 (1H, s), 6.82 (1H, dd), 3.91 (3H, s) 2-x 6.9 (2H, m), −109.70 (1F, m), 7.18 (2H, m), −113.35 (1F, m). 7.68 (2H, m), 7.95 (1H, m), 8.65 (1H, m); 6.94 (1H), −62.72 (3F, s), 7.62 (2H), −109.11 (2F, m) 7.82 (1H), 8.03 (1H), 8.96 (1H); 2-z 6.85 (1H), −62.80 (3F, s), 6.93 (1H), −107.65 (1F, m), 7.80, 7.90, −112.45 (1F, m). 8.05 (3H), 8.89 (1H); 2-aa 7.70 (3H, m), 7.85 (3H, m), 7.80, 7.90, 8.85 (1H, m). - This example illustrates the preparation of iridium compounds of the Fourth Formula fac-Ir(L a)3 above.
- In a typical experiment, a mixture of IrCl 3.nH2O (53-55% Ir), AgOCOCF3 (3.1 equivalents per Ir), 2-arylpyridine (excess), and (optionally) a small amount of water was vigorously stirred under N2 at 180-195° C. (oil bath) for 2-8 hours. The resulting mixture was thoroughly extracted with CH2Cl2 until the extracts were colorless. The extracts were filtered through a silica column to produce a clear yellow solution. Evaporation of this solution gave a residue which was treated with methanol to produce colored crystalline tris-cyclometalated Ir complexes. The complexes were separated by filtration, washed with methanol, dried under vacuum, and (optionally) purified by crystallization, vacuum sublimation, or Soxhlet extraction. Yields: 10-82%. All materials were characterized by NMR spectroscopic data and elemental analysis, and the results are given in Table 5 below. Single-crystal X-ray structures were obtained for three complexes of the series.
- Compound 1-b
- A mixture of IrCl 3.nH2O (54% Ir; 508 mg), 2-(4-fluorophenyl)-5-trifluoromethylpyridine, compound kk (2.20 g), AgOCOCF3 (1.01 g), and water (1 mL) was vigorously stirred under a flow of N2 as the temperature was slowly (30 min) brought up to 185° C. (oil bath). After 2 hours at 185-190° C. the mixture solidified. The mixture was cooled down to room temperature. The solids were extracted with dichloromethane until the extracts decolorized. The combined dichloromethane solutions were filtered through a short silica column and evaporated. After methanol (50 mL) was added to the residue the flask was kept at −10° C. overnight. The yellow precipitate of the tris-cyclometalated complex, compound b, was separated, washed with methanol, and dried under vacuum. Yield: 1.07 g (82%). X-Ray quality crystals of the complex were obtained by slowly cooling its warm solution in 1,2-dichloroethane.
- Compound 1-e
- A mixture of IrCl 3.nH2O (54% Ir; 504 mg), 2-(3-trifluoromethylphenyl)-5-trifluoromethylpyridine, compound bb (1.60 g), and AgOCOCF3 (1.01 g) was vigorously stirred under a flow of N2 as the temperature was slowly (15 min) brought up to 192° C. (oil bath). After 6 hours at 190-195° C. the mixture solidified. The mixture was cooled down to room temperature. The solids were placed on a silica column which was then washed with a large quantity of dichloromethane. The residue after evaporation of the filtrate was treated with methanol to produce yellow solid. The solid was collected and purified by extraction with dicilloromethane in a 25-mL micro-Soxhlet extractor. The yellow precipitate of the tris-cyclometalated complex, compound e, was separated, washed with methanol, and dried under vacuum. Yield: 0.59 g (39%). X-Ray quality crystals of the complex were obtained from hot 1,2-dichloroethane.
- Compound 1-d
- A mixture of IrCl 3.nH2O (54% Ir; 508 mg), 2-(2-fluorophenyl)-5-trifluoromethylpyridine, compound aa (1.53 g), and AgOCOCF3 (1.01 g) was vigorously stirred under a flow of N2 at 190-195° C. (oil bath) for 6 h 15 min. The mixture was cooled down to room temperature and then extracted with hot 1,2-dichloroethane. The extracts were filtered through a short silica column and evaporated. Treatment of the residue with methanol (20 mL) resulted in precipitation of the desired product, compound d, which was separated by filtration, washed with methanol, and dried under vacuum. Yield: 0.63 g (49%). X-Ray quality crystals of the complex were obtained from dichloromethane/methanol.
- Compound 1-i
- A mixture of IrCl 3.nH2O (54% Ir; 503 mg), 2-(4-trifluoromethoxyphenyl)-5-trifluoromethylpyridine, compound ee (2.00 g), and AgOCOCF3 (1.10 g) was vigorously stirred under a flow of N2 at 190-195° C. (oil bath) for 2 h 45 min. The mixture was cooled down to room temperature and then extracted with dichloromethane. The extracts were filtered through a short silica column and evaporated. Treatment of the residue with methanol (20 mL) resulted in precipitation of the desired product, compound i, which was separated by filtration, washed with methanol, and dried under vacuum. The yield was 0.86 g. Additionally, 0.27 g of the complex was obtained by evaporating the mother liquor and adding petroleum ether to the residue. Overall yield: 1.13 g (72%).
- Compound 1-q
- A mixture of IrCl 3.nH2O (54% Ir; 530 mg), 2-(3-methoxyphenyl)-5-trifluoromethylpyridine (2.50 g), AgOCOCF3 (1.12 g), and water (1 mL) was vigorously stirred under a flow of N2 as the temperature was slowly (30 min) brought up to 185° C. (oil bath). After 1 hour at 185° C. the mixture solidified. The mixture was cooled down to room temperature. The solids were extracted with dichloromethane until the extracts decolorized. The combined dichloromethane solutions were filtered through a short silica column and evaporated. The residue was washed with hexanes and then recrystallized from 1,2-dichloroethane-hexanes (twice). Yield: 0.30 g. 19F NMR (CD2Cl2, 20° C.), δ: −63 (s). 1NMR (CD2Cl2, 20° C.), δ: 8.1 (1H), 7.9 (1H), 7.8 (1H), 7.4 (11H), 6.6 (2H), 4.8 (3H). X-Ray quality crystals of the complex (1,2-dichloroethane, hexane solvate) were obtained from 1,2-dichloroethane-hexanes. This facial complex was orange-photoluminescent.
- Compounds 1-a, 1-c, 1-f through 1-h, 1-i through 1-m, and 1-r were similarly prepared. In the preparation of compound 1-j, a mixture of isomers was obtained with the fluorine in either the R 6 or R8 position.
TABLE 5 Analysis NMR Compound (calcd (found) (CD2Cl2, 25° C.) 1-a C: 50.3 (50.1) 1H: 6.8 (1H), 6.9 (1H), 7.0 (1H), 7.8 H: 2.5 (2.7) (2H), 7.95 (1H), 8.1 (1H) N: 4.9 (4.9) 19F: −63.4 Cl: 0.0 (0.2) 1-b C: 47.4 (47.3) 1H: 6.4 (1H), 6.75 (1H), 7.7 (1H), 7.8 H: 2.0 (2.1) (1H), 7.95 (1H), 8.05 (1H) N: 4.6 (4.4) 19F: −63.4 (s); −109.5 (ddd) 1-c C: 47.4 (47.2) 1H: 6.6 (1H), 6.7 (1H), 6.9 (1H), 7.8 H: 2.0 (2.0) (1H), 8.0 (1H), 8.6 (1H) N: 4.6 (4.5) 19F: −63.5 (s); −112.8 (ddd) 1-d C: 55.9 (56.1) 1H: 6.6 (2H), 6.8 (1H), 7.0 (1H), 7.6 H: 3.0 (3.2) (1H), 7.7 (1H), 8.4 (1H) N: 5.9 (5.8) 19F: −115.0 (ddd) 1-e C: 44.1 (43.3) 1H: 6.9 (1H), 7.1 (1H), 7.8 (1H), 8.0 H: 1.7 (2.1) (2H), 8.2 (1H) N: 3.9 (3.6) 19F: −63.0 (1F), −63.4 (1F) 1-f C: 50.4 (50.5) 1H: 6.9 (1H), 7.1 (2H), 7.6 (1H), 7.8 H: 2.5 (2.7) (1H), 7.9 (1H), 8.1 (1H) N: 4.9 (4.9) 19F: −62.4 1-g C: 55.9 (56.3) 1H: 6.4 (1H), 6.7 (1H), 7.0 (1H), 7.6 H: 3.0 (3.2) (1H), 7.7 (2H), 7.9 (1H) N: 5.9 (6.0) 19F: −112.6 (ddd) 1-h C: 51.0 (45.2) 1H: 6.8 (1H), 6.95 (1H), 7.05 (1H), 7.7 H: 2.1 (2.3) (1H), 8.0 (1H), 8.9 (1H) N: 4.9 (4.2) 19F: −63.3 1-i C: 49.4 (49.3) 1H: 3.6 (3H), 6.3 (1H), 6.6 (1H), 7.7 H: 2.9 (2.8) (2H), 7.85 (1H), 7.95 (1H) N: 4.4 (4.4) 19F: −63.2 1-j C: 47.4 (47.4) 1H: 6.7 (m), 7.1 (m), 7.5 (m), 7.6 (m), H: 2.0 (2.3) 7.7 (m), 8.0 (m), 8.2 (m) N: 4.6 (4.7) 19F: 8 s resonances (−63.0-−63.6) and 8 ddd resonances (−92.2-−125.5) 1-k C: 43.5 (44.0) 1H: 6.9 (1H), 7.15 (1H), 8.1 (1H); 8.3 H: 1.8 (2.1) (1H), 8.45 (1H), 8.6 (1H) N: 8.5 (8.4) 19F: −62.9 1-l C: 42.2 (42.1) 1H: 6.5 (1H), 6.7 (1H), 7.75 (1H), 7.85 H: 16. (1.8) (1H), 8.0 (1H), 8.1 (1H) N: 3.8 (3.7) 19F: −58.1 (1F), −63.4 (1F) - This example illustrates the preparation of iridium complexes of the Second Formula IrL aLbLC xL′yL″z above,
- Compound 1-n
- A mixture of IrCl 3.nH2O (54% Ir; 510 mg), 2-(3-trifluoromethylphenyl)-quinoline (1.80 g), and silver trifluoroacetate (1.10 g) was vigorously stirred at 190-195° C. for 4 hours. The resulting solid was chromatographed on silica with dichloromethane to produce a mixture of the dicyclometalated complex and the unreacted ligand. The latter was removed from the mixture by extraction with warm hexanes. After the extracts became colorless the hexane-insoluble solid was collected and dried under vacuum. The yield was 0.29 g. 19F NMR: −63.5 (s, 6F), −76.5 (s, 3F). The structure of this complex was established by a single crystal X ray diffraction study.
- Compound 1-o
- A mixture of IrCl 3.nH2O (54% Ir; 500 mg), 2-(2-fluorophenyl)-3-chloro-5-trifluoromethylpyridine (2.22 g), water (0.3 mL), and silver trifluoroacetate (1.00 g) was stirred at 190° C. for 1.5 hours. The solid product was chromatographed on silica with dichloromethane to produce 0.33 g of a 2:1 co-crystallized adduct of the dicyclometalated aqua trifluoroacetato complex, compound 1-p, and the unreacted ligand. 19F NMR: −63.0 (9F), −76.5 (3F), −87.7 (2F), −114.4 (1F). The co-crystallized phenylpyridine ligand was removed by recrystallization from dichloromethane-hexanes. The structures of both the adduct and the complex were established by a single crystal X-ray diffraction study.
- This example illustrates the preparation of an hydroxo iridium dimer, having structure (VIII) above.
- A mixture of IrCl 3.nH2O (54% Ir; 510 mg), 2-(4-fluorophenyl)-5-trifluoromethylpyridine (725 mg), water (5 mL), and 2-ethoxyethanol (20 mL) was vigorously stirred under reflux for 4.5 hours. After a solution of NaOH (2.3 g) in water (5 mL) was added, followed by 20 mL of water, the mixture was stirred under reflux for 2 hours. The mixture was cooled down to room temperature, diluted with 50 mL of water, and filtered. The solid was vigorously stirred under reflux with 30 mL of 1,2-dichloroethane and aqueous NaOH (2.2 g in 8 mL of water) for 6 hours. The organic solvent was evaporated from the mixture to leave a suspension of an orange solid in the aqueous phase. The orange solid was separated by filtration, thoroughly washed with water, and dried under vacuum to produce 0.94 g (95%) of the iridium hydroxo dimer (spectroscopically pure). 1H NMR (CD2Cl2): −1.0 (s, 11H, IrOH), 5.5 (dd, 2H), 6.6 (dt, 2H), 7.7 (dd, 2H), 7.9 (dd, 2H), 8.0 (d, 2H), 9.1 (d, 2H). 19F NMR (CD2Cl2): −62.5 (s, 3F), −109.0 (ddd, IF).
- This example illustrates the preparation of bis-cyclometalated complexes from an iridium dimer.
- Compound 1-p
- A mixture of the iridium hydroxo dimer (100 mg) from Example 4, ethyl acetoacetate (0.075 mL; 4-fold excess), and dichloromethane (4 mL) was stirred at room temperature overnight. The solution was filtered through a short silica plug and evaporated to give an orange-yellow solid which was washed with hexanes and dried. The yield of the complex was 109 mg (94%). 1H NMR (CD2Cl2): 1.1 (t, CH3), 3.9 (dm, CH2), 4.8 (s, CH3COCH), 5.9 (m), 6.7 (m), 7.7 (m), 8.0 (m), 8.8 (d). 19F NMR (CD2Cl2): −63.1 (s, 3F), −63.2 (s, 3F), −109.1 (ddd, IF), −109.5 (ddd). Analysis: Calcd: C, 44.9; H, 2.6; N, 3.5. Found: C, 44.4; H, 2.6; N, 3.3.
- Compound 1-w
- A solution of hydroxo iridium dimer from Example 4 (0.20 g) in THF (6 mL) was treated with 50 mg of trifluoroacetic acid, filtered through a short silica plug, evaporated to ca. 0.5 mL, treated with hexanes (8 mL), and left overnight. The yellow crystalline solid was separated, washed with hexanes, and dried under vacuum. Yield (1:1 THF solvate): 0.24 g (96%). 19F NMR (CD2Cl2, 20° C.), δ: −63.2 (s, 3F), −76.4 (s, 3F), −107.3 (ddd, 1F). 1H NMR (CD2Cl2, 20° C.), δ: 9.2 (br s, 1H), 8.2 (dd, 1H), 8.1 (d, 1H), 7.7 (m, 1H), 6.7 (m, 1H), 5.8 (dd, 1H), 3.7 (m, 2H, THF), 1.8 (m, 2H, THF).
- Compound 1-x
- A mixture of the trifluoroacetate intermediate, compound 1-w (75 mg), and 2-(4-bromophenyl)-5-bromopyridine (130 mg) was stirred under N 2 at 150-155° C. for 30 min. The resulting solid was cooled to room temperature and dissolved in CH2Cl2. The resulting solution was filtered through silica gel and evaporated. The residue was washed several times with warm hexanes and dried under vacuum to leave a yellow, yellow-photoluminescent solid. Yield: 74 mg (86%). 19F NMR (CD2Cl2, 20° C.), δ: −63.1 (s, 3F), −63.3 (s, 3F), −108.8 (ddd, 1F), −109.1 (ddd, 1F). 1HNMR (CD2Cl2, 20° C.), δ: 8.2 (s), 7.9 (m), 7.7(m), 7.0 (d), 6.7 (m), 6.2 (dd), 6.0 (dd). The complex was meridional, with the nitrogens of the fluorinated ligands being trans, as confirmed by X-ray analysis.
- This example illustrates the preparation of iridium compounds of the Fifth Formula mer-Ir(L a)3 above.
- Compound 1-s
- This complex was synthesized in a manner similar to compound 1-n. According to the NMR, TLC, and TGA data, the result was an approximately 1:1 mixture of the facial and meridional isomers.
- Compound 1-t
- A mixture of IrCl 3.nH2O (54% Ir; 0.40 g), 2-(3,5-difluorophenyl)-5-trifluoromethylpyridine (1.40 g), AgOCOCF3 (0.81 g), and water (0.5 mL) was vigorously stirred under a flow of N2 as the temperature was slowly (30-40 min) brought up to 165° C. (oil bath). After 40 min at 165° C. the mixture solidified. The mixture was cooled down to room temperature. The solids were extracted with dichloromethane until the extracts decolorized. The combined dichloromethane solutions were filtered through a short silica column and evaporated. The residue was thoroughly washed with hexanes and dried under vacuum. Yield: 0.53 g (49%). 19F NMR (CD2Cl2, 20° C.), δ: −63.55 (s, 3F), −63.57 (s, 3F), −63.67 (s, 3F), −89.1 (t, 1F), −100.6 (t, 1F), −102.8 (dd, 1F), −118.6 (ddd, 1F), −119.3 (ddd, 1F), −123.3 (ddd, 1F). 1H NMR (CD2Cl2, 20° C.), δ: 8.4 (s), 8.1 (m), 7.9 (m), 7.6 (s), 7.5 (m), 6.6 (m), 6.4 (m). The complex was meridional, as was also confirmed by X-ray analysis.
- Compound 1-u
- This complex was prepared and isolated similarly to compound 1-q, then purified by crystallization from 1,2-dichloroethane-hexanes. The yield of the purified product was 53%. The complex is mer, as follows from the NMR data. 19F NMR (CD2Cl2, 20° C.), δ: −63.48 (s, 3F), −63.52 (s, 6F), −105.5 (ddd, 1F), −105.9 (ddd, 1F), −106.1 (ddd, 1F), −107.4 (t, 1F), −107.9 (t, 1F), −109.3 (t, 1F). 1H NMR (CD2Cl2, 20° C.), δ: 8.6 (m), 8.3 (s), 8.2 (s), 8.1 (m), 7.9 (m), 7.6 (m), 6.6 (m), 6.4 (m), 6.0 (m), 5.8 (m).
- Compound 1-v
- This mer-complex was prepared in a manner similar to compound 1-w, using the trifluoroacetate dicyclometalated intermediate, compound 1-x, and 2-(4-fluorophenyl)-5-trifluoromethylpyridine. 19F NMR (CD2Cl2, 20° C.), δ: −63.30 (s, 3F), −63.34 (s, 3F), −63.37 (s, 3F), −108.9 (ddd, 1F), −109.0 (ddd, 1F), −109.7 (ddd, 1F). 1H NMR (CD2Cl2, 20° C.), δ: 8.3-7.6 (m), 6.7 (m), 6.6 (dd), 6.3 (dd), 6.0 (dd). This yellow-luminescent merisional complex isomerised to the green luminescent facial isomer, compound 1-b, upon sublimation at 1 atm.
- This example illustrates the formation of OLEDs using the iridium complexes of the invention.
- Thin film OLED devices including a hole transport layer (HT layer), electroluminescent layer (EL layer) and at least one electron transport layer (ET layer) were fabricated by the thermal evaporation technique. An Edward Auto 306 evaporator with oil diffusion pump was used. The base vacuum for all of the thin film deposition was in the range of 10-6 torr. The deposition chamber was capable of depositing five different films without the need to break up the vacuum.
- An indium tin oxide (ITO) coated glass substrate was used, having an ITO layer of about 1000-2000 Å. The substrate was first patterned by etching away the unwanted ITO area with 1N HCl solution, to form a first electrode pattern. Polyimide tape was used as the mask. The patterned ITO substrates were then cleaned ultrasonically in aqueous detergent solution. The substrates were then rinsed with distilled water, followed by isopropanol, and then degreased in toluene vapor for ˜3 hours.
- The cleaned, patterned ITO substrate was then loaded into the vacuum chamber and the chamber was pumped down to 10 −6 torr. The substrate was then further cleaned using an oxygen plasma for about 5-10 minutes. After cleaning, multiple layers of thin films were then deposited sequentially onto the substrate by thermal evaporation. Finally, patterned metal electrodes of Al were deposited through a mask. The thickness of the film was measured during deposition using a quartz crystal monitor (Sycon STC-200). All film thickness reported in the Examples are nominal, calculated assuming the density of the material deposited to be one. The completed OLED device was then taken out of the vacuum chamber and characterized immediately without encapsulation.
- A summary of the device layers and thicknesses is given in Table 6. In all cases the anode was ITO as discussed above, and the cathode was Al having a thickness in the range of 700-760 A. In some of the samples, a two-layer electron transport layer was used. The layer indicated first was applied adjacent to the EL layer.
TABLE 6 HT layer EL layer ET layer Sample (Thickness, Å) (Thickness, Å) (Thickness, Å) Comparative MPMP (528) Ir(ppy)3 DDPA (106) + (408) Alq3 (320) 1 MPMP (520) Compound 1-b DDPA (125) + (499) Alq3 (365) 2 MPMP (541) Compound 1-b DDPA (407) (580) 3 MPMP (540) Compound 1-e DDPA (112) + (499) Alq3 (340) 4 MPMP (525) Compound 1-k DDPA (106) (406) Alq3 (341) 5 MPMP (570) Compound 1-i DDPA (107) + (441) Alq3 (339) 6 MPMP (545) Compound 1-j DDPA (111) + (462) Alq3 (319) 7 MPMP (643) Compound 1-g DDPA (112) + (409) Alq3 (361) 8 MPMP (539) Compound 1-f DDPA (109) + (430) Alq3 (318) 9 MPMP (547) Compound 1-a DDPA (105) + (412) Alq3 (300) 10 MPMP (532) Compound 1-h DDPA (108) + (457) Alq3 (306) 11 MPMP (603) Compound 1-d DDPA (111) + (415) Alq3 (303) 12 MPMP (551) Compound 1-c DDPA (106) + (465) Alq3 (313) 13 MPMP (520) Compound 1-l DDPA (410) (405) 14 MPMP (504) Compound 1-b DDPA (393) (400) 15 MPMP (518) Compound 1-b DDPA (418) (153) 16 MPMP (556) Compound 1-m DDPA (430) (416) 17 MPMP (520) Compound 1-n DDPA (420) (419) 18 MPMP (511) Compound 1-o DDPA (413) (412) 19 MPMP (527) Compound 1-p DDPA (412) (425) 20 MPMP (504) Compound 1-q DPA (407) (417) 21 MPMP (525) Compound 1-t DPA (416) (419) 22 MPMP (520) Compound 1-u DPA (405) (421) - The OLED samples were characterized by measuring their (1) current-voltage (I-V) curves, (2) electroluminescence radiance versus voltage, and (3) electroluminescence spectra versus voltage. The apparatus used, 200, is shown in FIG. 2. The I-V curves of an OLED sample, 220, were measured with a Keithley Source-
Measurement Unit Model 237, 280. The electroluminescence radiance (in the unit of Cd/m2) vs. voltage was measured with a Minolta LS-110 luminescence meter, 210, while the voltage was scanned using the Keithley SMU. The electroluminescence spectrum was obtained by collecting light using a pair of lenses, 230, through an electronic shutter, 240, dispersed through a spectrograph, 250, and then measured with a diode array detector, 260. All three measurements were performed at the same time and controlled by a computer, 270. The efficiency of the device at certain voltage is determined by dividing the electroluminescence radiance of the LED by the current density needed to run the device. The unit is in Cd/A. - The results are given in Table 7 below:
TABLE 7 ElectroluminescentProperties of Iridium Compounds Peak Efficiency at Peak Approximate Radiance, peak radiance, efficiency, Peak Wave- Sample Cd/m2 Cd/A Cd/A lengths, nm Comparative 540 0.39 0.48 522 at 22 V 1 1400 3.4 11 525 at 21 V 2 1900 5.9 13 525 at 25 V 3 830 1.7 13.5 525 at 18 V 4 7.6 0.005 0.13 521 at 27 V 5 175 0.27 1.8 530, 563 at 25 V 6 514 1.5 2.2 560 at 20 V 7 800 0.57 1.9 514 at 26 V 8 1200 0.61 2 517 at 28 V 9 400 1.1 4 545 at 18 V 10 190 2.3 3.3 575 at 16 V 11 1150 1.2 3.8 506, 526 at 25 V 12 340 0.49 2.1 525 at 20 V 13 400 3 5 520 at 21 V 14 1900 5 9 525 15 2500 6 11 525 16 100 0.17 0.2 560 at 27 V 17 3.5 0.005 0.014 575 at 28 V 18 30 0.08 0.16 590 at 26 V 19 2000 6 8 532 at 21 V 20 350 0.60 1.6 595 at 26 V 21 1200 5 545 at 22 V 22 80 1 540 at 19 V - The peak efficiency is the best indication of the value of the electroluminescent compound in a device. It gives a measure of how many electrons have to be input into a device in order to get a certain number of photons out (radiance). It is a fundamentally important number, which reflects the intrinsic efficiency of the light-emitting material. It is also important for practical applications, since higher efficiency means that fewer electrons are needed in order to achieve the same radiance, which in turn means lower power consumption. Higher efficiency devices also tend to have longer lifetimes, since a higher proportion of injected electrons are converted to photons, instead of generating heat or causing an undesirable chemical side reactions. Most of the iridium complexes of the invention have much higher peak efficiencies than the parent-tris(2-phenylpyridine) iridium complex. Those complexes with lower efficiencies may also find utility as phosphorescent or photoluminescent materials, or as catalysts, as discussed above.
Claims (22)
1. An organic electronic device comprising an emitting layer wherein at least 20% by weight of the emitting layer comprises at least one compound having a formula below:
IrLaLbLc xL′yL″z,
where:
x=0 or 1, y=0, 1 or 2, and z=0 or 1, with the proviso that:
x=0 or y+z=0 and
when y=2then z=0;
L′=a bidentate ligand or a monodentate ligand, and is not a phenylpyridine, phenylpyrimidine, or phenylquinoline; with the proviso that:
when L′ is a monodentate ligand, y+z=2, and when L′ is a bidentate ligand, z=0;
L″=a monodentate ligand, and is not a phenylpyridine, and phenylpyrimidine, or phenylquinoline; and
La, Lb and Lc are alike or different from each other and each of La, Lb and Lc has structure (I) below:
wherein:
adjacent pairs of R1-R4 and R5-R8 can be joined to form a five- or six-membered ring,
at least one of R1-R8 is selected from F, CnF2n+1, OCnF2n+1, and OCF2X, where n=1-6 and X=H, Cl, or Br, and
A=C or N, provided that when A=N, there is no R1.
2. The device of claim 1 wherein x=1, y=0, and z=0.
3. The device of claim 2 wherein A=C and none of R1-R8 is selected from nitro.
4. The device of claim 1 wherein R3 is CF3.
5. The device of claim 4 wherein at least one of R5-R8 is selected from F, CnF2n+1, OCnF2n+1, and OCF2X, where n=1-6 and X=H, Cl, or Br.
6. The device of claim 2 wherein A=C, R3=CF3, R7=F, and R1, R2, R4-R6 and R8=H.
7. The device of claim 2 wherein A=C, R3 and R6=CF3, and R1, R2, R4, R5, R7 and R8=H.
8. The device of claim 2 wherein A=C, R3=CF3, R6 and R8=F, and R1, R2, R4, R5, and R7=H.
10. An organic electronic device comprising an emitting layer wherein the emitting layer comprises a diluent and less than 20% by weight of at least one compound that has a formula below:
IrLaLbLc,
where:
La, Lb and Lc are alike or different from each other and each of La, Lb and Lc has structure (I) below:
wherein:
adjacent pairs of R1-R4 and R5-R8 can be joined to form a five- or six-membered ring,
at least one of R1-R8 is selected from F, CnF2n+1, OCnF2n+1, and OCF2X, where n=1-6 and X=H, Cl, or Br, and A=C or N, provided that when A=N, there is no R1.
11. The device of claim 10 wherein the diluent is selected from poly(N-vinyl carbazole), polysilane, 4,4′-N,N′-dicarbazole biphenyl, and tertiary aromatic amines.
12. The device of claim 1 , further comprising a hole transport layer selected from N,N′-diphenyl-N,N′-bis(3-methylphenyl)-[1,1′-biphenyl]-4,4′-diamine (TPD), 1,1-bis[(di-4-tolylamino) phenyl]cyclohexane (TAPC), N,N′-bis(4-methylphenyl)-N,N′-bis(4-ethylphenyl)-[1,1′-(3,3′-dimethyl)biphenyl]-4,4′-diamine (ETPD), tetrakis-(3-methylphenyl)-N,N,N′,N′-2,5-phenylenediamine (PDA), -phenyl-4-N,N-diphenylaminostyrene (TPS), p-(diethylamino)-benzaldehyde diphenylhydrazone (DEH), triphenylamine (TPA), bis[4-(N,N-diethylamino)-2-methylphenyl](4-methylphenyl)methane (MPMP), 1-phenyl-3-[p-(diethylamino)styryl]-5-[p-(diethylamino)phenyl] pyrazoline (PPR or DEASP), 1,2-trans-bis(9H-carbazol-9-yl)cyclobutane (DCZB), N,N,N′,N′-tetrakis(4-methylphenyl)-(1, I′-biphenyl)-4,4′-diamine (TTB), porphyrinic compounds, and combinations thereof.
13. The device of claim 1 , further comprising an electron transport layer selected from tris(8-hydroxyquinolato)aluminum, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (DDPA), 4,7-diphenyl-1,10-phenanthroline (DPA), 2-(4-biphenylyl)-5-(4-t-butylphenyl)-1,3,4-oxadiazole (PBD), 3-(4-biphenylyl)-4-phenyl-5-(4-t-butylphenyl)-1,2,4-triazole (TAZ), and combinations thereof.
14. A compound having a formula selected from fac-Ir(L)3, mer-Ir(L)3, and combinations thereof, where L is selected from group 1-a through 1-m and 1-q through 1-v, as shown in Table 1, and has structure (I) below:
16. An organic electronic device comprising an emitting layer that comprises a compound selected from the following (i) and (ii):
(i) a compound having a formula selected from fac-Ir(L)3, mer-Ir(L)3, and combinations thereof, where L is a group selected from 1-a through 1-m and 1-q through 1-v, as shown in Table 1 and has structure (I) below:
wherein:
adjacent pairs of R1-R4 and R5-R8 can be joined to form a five- or six-membered ring,
at least one of R1-R8 is selected from F, CnF2n+1, OCnF2n+1, and OCF2X, where n=1-6 and X=H, Cl, or Br, and
A=C or N, provided that when A N, there is no R1;
(ii) a compound having one of structures (IV), (V), (VI), (IX), and (X) below:
17. The device of claim 16 wherein the emitting layer further comprises a diluent.
18. The device of claim 17 wherein the diluent is selected from poly(N-vinyl carbazole), polysilane, 4,4′-N,N′-dicarbazole biphenyl, and tertiary aromatic amines.
19. A compound selected from compounds 2-a through 2-aa as shown in Table 2, having structure (II) below:
21. A compound having structure VII below:
wherein:
B=H, CH3, or C2H5;
La, Lb, Lc, and Ld are the same or different from each other; and
each of La, Lb, Lc, and Ld has structure (I) below:
wherein:
adjacent pairs of R1-R4 and R5-R8 can be joined to form a five- or six-membered ring,
at least one of R1-R8 is selected from F, CnF2n+1a, OCnF2n+1, and OCF2X, where n=1-6 and X=H, Cl, or Br, and
A=C or N, provided that when A=N, there is no R1.
22. The compound of claim 21 wherein:
La=Lb=Lc=Ld;
B=H;
R3=CF3;
R7=F;
R1, R2, R4-R6 and R8=H.
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/366,295 US20030197183A1 (en) | 2000-06-30 | 2003-02-13 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/459,946 US6979739B2 (en) | 2000-06-30 | 2003-06-12 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/720,967 US7132681B2 (en) | 2000-06-30 | 2003-11-24 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/720,954 US7161172B2 (en) | 2000-06-30 | 2003-11-24 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/774,286 US7476452B2 (en) | 2000-06-30 | 2004-02-06 | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| US10/983,119 US20050095457A1 (en) | 2000-06-30 | 2004-11-05 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made such compounds |
| US11/315,741 US7816016B1 (en) | 2003-02-13 | 2005-12-22 | Electroluminescent iridium compounds and devices made therefrom |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US21536200P | 2000-06-30 | 2000-06-30 | |
| US22427300P | 2000-08-10 | 2000-08-10 | |
| US09/879,014 US20020121638A1 (en) | 2000-06-30 | 2001-06-12 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/366,295 US20030197183A1 (en) | 2000-06-30 | 2003-02-13 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/879,014 Continuation US20020121638A1 (en) | 2000-06-30 | 2001-06-12 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
Related Child Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/459,946 Division US6979739B2 (en) | 2000-06-30 | 2003-06-12 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/720,954 Division US7161172B2 (en) | 2000-06-30 | 2003-11-24 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/720,967 Division US7132681B2 (en) | 2000-06-30 | 2003-11-24 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US76829804A Continuation-In-Part | 2000-06-30 | 2004-01-30 | |
| US10/774,286 Continuation-In-Part US7476452B2 (en) | 2000-06-30 | 2004-02-06 | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| US10/983,119 Continuation US20050095457A1 (en) | 2000-06-30 | 2004-11-05 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made such compounds |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030197183A1 true US20030197183A1 (en) | 2003-10-23 |
Family
ID=26909953
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/879,014 Abandoned US20020121638A1 (en) | 2000-06-30 | 2001-06-12 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/366,295 Abandoned US20030197183A1 (en) | 2000-06-30 | 2003-02-13 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/459,946 Expired - Lifetime US6979739B2 (en) | 2000-06-30 | 2003-06-12 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/720,954 Expired - Lifetime US7161172B2 (en) | 2000-06-30 | 2003-11-24 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/983,119 Abandoned US20050095457A1 (en) | 2000-06-30 | 2004-11-05 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made such compounds |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/879,014 Abandoned US20020121638A1 (en) | 2000-06-30 | 2001-06-12 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/459,946 Expired - Lifetime US6979739B2 (en) | 2000-06-30 | 2003-06-12 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/720,954 Expired - Lifetime US7161172B2 (en) | 2000-06-30 | 2003-11-24 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US10/983,119 Abandoned US20050095457A1 (en) | 2000-06-30 | 2004-11-05 | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made such compounds |
Country Status (12)
| Country | Link |
|---|---|
| US (5) | US20020121638A1 (en) |
| EP (4) | EP1431288A3 (en) |
| JP (4) | JP5174310B2 (en) |
| KR (1) | KR100838010B1 (en) |
| CN (1) | CN100438123C (en) |
| AT (1) | ATE335386T1 (en) |
| AU (2) | AU2001271550B2 (en) |
| CA (1) | CA2411624A1 (en) |
| DE (1) | DE60121950T2 (en) |
| IL (1) | IL153319A0 (en) |
| TW (1) | TW593623B (en) |
| WO (1) | WO2002002714A2 (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030173896A1 (en) * | 2002-02-14 | 2003-09-18 | Vladimir Grushin | Electroluminescent iridium compounds with phosphinoalkoxides and phenylpyridines or phenylpyrimidines and devices made with such compounds |
| US20030189216A1 (en) * | 2002-03-08 | 2003-10-09 | Canon Kabushiki Kaisha | Organic light emitting device |
| US20050037233A1 (en) * | 2000-06-30 | 2005-02-17 | Dobbs Kerwin D. | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| US20050112401A1 (en) * | 2003-11-25 | 2005-05-26 | Samsung Sdi Co., Ltd. | Organic electroluminescent display device having superior characteristics at high temperature |
| US20060041126A1 (en) * | 2002-10-30 | 2006-02-23 | Ciba Specialty Chemicals Holding Inc. | Electroluminescent device |
| US20080192802A1 (en) * | 2004-10-15 | 2008-08-14 | Koninklijke Philips Electronics, N.V. | Colour Switching Temperature Indicator |
| US20090010305A1 (en) * | 2004-10-15 | 2009-01-08 | Koninklijke Philips Electronics, N.V. | Temperature Indicator |
| US7781075B2 (en) | 2005-09-07 | 2010-08-24 | Au Optronics Corporation | Organic light-emitting material and organic light-emitting device |
| US7816016B1 (en) | 2003-02-13 | 2010-10-19 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds and devices made therefrom |
| US20110163665A1 (en) * | 2003-12-02 | 2011-07-07 | Semiconductor Energy Laboratory Co., Ltd. | Organometal complex and light-emitting element using the same |
| US20120238047A1 (en) * | 2005-02-16 | 2012-09-20 | Bawendi Moungi G | Light emitting device including semiconductor nanocrystals |
| JP2017171671A (en) * | 2011-10-06 | 2017-09-28 | 株式会社半導体エネルギー研究所 | Iridium complex |
| US9923148B2 (en) | 2002-10-30 | 2018-03-20 | Udc Ireland Limited | Electroluminescent device |
| US9978960B2 (en) | 2013-06-14 | 2018-05-22 | Semiconductor Energy Laboratory Co., Ltd. | Organometallic iridium complex, light-emitting element, light-emitting device, and lighting device |
| CN115216291A (en) * | 2014-10-08 | 2022-10-21 | 环球展览公司 | Organic electroluminescent material and device |
Families Citing this family (1236)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6830828B2 (en) | 1998-09-14 | 2004-12-14 | The Trustees Of Princeton University | Organometallic complexes as phosphorescent emitters in organic LEDs |
| US7001536B2 (en) | 1999-03-23 | 2006-02-21 | The Trustees Of Princeton University | Organometallic complexes as phosphorescent emitters in organic LEDs |
| KR100794975B1 (en) * | 1999-12-01 | 2008-01-16 | 더 트러스티즈 오브 프린스턴 유니버시티 | Complexes of form l2mx as phosphorescent dopants for organic leds |
| US6821645B2 (en) | 1999-12-27 | 2004-11-23 | Fuji Photo Film Co., Ltd. | Light-emitting material comprising orthometalated iridium complex, light-emitting device, high efficiency red light-emitting device, and novel iridium complex |
| JP4701191B2 (en) * | 1999-12-27 | 2011-06-15 | 富士フイルム株式会社 | Light emitting device material, light emitting device and novel iridium complex comprising orthometalated iridium complex |
| KR100721656B1 (en) | 2005-11-01 | 2007-05-23 | 주식회사 엘지화학 | Organic electronic devices |
| US7132681B2 (en) | 2000-06-30 | 2006-11-07 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US20020121638A1 (en) | 2000-06-30 | 2002-09-05 | Vladimir Grushin | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US6946688B2 (en) | 2000-06-30 | 2005-09-20 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US7306856B2 (en) * | 2000-07-17 | 2007-12-11 | Fujifilm Corporation | Light-emitting element and iridium complex |
| US6962755B2 (en) * | 2000-07-17 | 2005-11-08 | Fuji Photo Film Co., Ltd. | Light emitting element and azole compound |
| US6939624B2 (en) * | 2000-08-11 | 2005-09-06 | Universal Display Corporation | Organometallic compounds and emission-shifting organic electrophosphorescence |
| JP5241053B2 (en) * | 2000-08-11 | 2013-07-17 | ザ、トラスティーズ オブ プリンストン ユニバーシティ | Organometallic compounds and radiation-transfer organic electrophosphors |
| US7153592B2 (en) * | 2000-08-31 | 2006-12-26 | Fujitsu Limited | Organic EL element and method of manufacturing the same, organic EL display device using the element, organic EL material, and surface emission device and liquid crystal display device using the material |
| JP4067286B2 (en) * | 2000-09-21 | 2008-03-26 | 富士フイルム株式会社 | Light emitting device and iridium complex |
| JP4154138B2 (en) | 2000-09-26 | 2008-09-24 | キヤノン株式会社 | Light emitting element, display device and metal coordination compound |
| JP4154139B2 (en) * | 2000-09-26 | 2008-09-24 | キヤノン株式会社 | Light emitting element |
| JP4460743B2 (en) * | 2000-09-29 | 2010-05-12 | 富士フイルム株式会社 | Method for producing iridium complex or tautomer thereof |
| IL154960A0 (en) | 2000-10-10 | 2003-10-31 | Du Pont | Polymers having attached luminescent metal complexes and devices made with sych polymers |
| KR100798561B1 (en) * | 2000-11-30 | 2008-01-28 | 캐논 가부시끼가이샤 | Luminescent Element and Display |
| EP1349435B8 (en) * | 2000-11-30 | 2018-09-19 | Canon Kabushiki Kaisha | Luminescent element and display |
| JP4154145B2 (en) * | 2000-12-01 | 2008-09-24 | キヤノン株式会社 | Metal coordination compound, light emitting device and display device |
| DE10104426A1 (en) | 2001-02-01 | 2002-08-08 | Covion Organic Semiconductors | Process for the production of high-purity, tris-ortho-metallated organo-iridium compounds |
| WO2002064700A1 (en) * | 2001-02-14 | 2002-08-22 | Sanyo Electric Co., Ltd. | Organic electroluminescence device, lumincescent material, and organic compound |
| US7998595B2 (en) | 2001-02-14 | 2011-08-16 | Sanyo Electric Co., Ltd. | Organic electroluminescent device, luminescent material and organic compound |
| DE10109027A1 (en) * | 2001-02-24 | 2002-09-05 | Covion Organic Semiconductors | Rhodium and iridium complexes |
| JP4307000B2 (en) | 2001-03-08 | 2009-08-05 | キヤノン株式会社 | Metal coordination compound, electroluminescent element and display device |
| JP4438042B2 (en) | 2001-03-08 | 2010-03-24 | キヤノン株式会社 | Metal coordination compound, electroluminescent element and display device |
| SG92833A1 (en) * | 2001-03-27 | 2002-11-19 | Sumitomo Chemical Co | Polymeric light emitting substance and polymer light emitting device using the same |
| US7067202B2 (en) * | 2001-06-15 | 2006-06-27 | Sanyo Electric Co., Ltd. | Luminescent organometallic compound and light emitting device |
| JP2003007469A (en) * | 2001-06-25 | 2003-01-10 | Canon Inc | Light emitting element and display equipment |
| US7037598B2 (en) | 2001-08-07 | 2006-05-02 | Fuji Photo Film Co., Ltd. | Light-emitting element and novel iridium complexes |
| JP5135660B2 (en) * | 2001-09-27 | 2013-02-06 | コニカミノルタホールディングス株式会社 | Organic electroluminescence device |
| US6835469B2 (en) * | 2001-10-17 | 2004-12-28 | The University Of Southern California | Phosphorescent compounds and devices comprising the same |
| US7320833B2 (en) | 2001-11-07 | 2008-01-22 | E.I. Du Pont De Nemours And Company | Electroluminescent platinum compounds and devices made with such compounds |
| US7250512B2 (en) * | 2001-11-07 | 2007-07-31 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds having red-orange or red emission and devices made with such compounds |
| US7166368B2 (en) | 2001-11-07 | 2007-01-23 | E. I. Du Pont De Nemours And Company | Electroluminescent platinum compounds and devices made with such compounds |
| IL158314A0 (en) * | 2001-12-26 | 2004-05-12 | Du Pont | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| DE10215010A1 (en) | 2002-04-05 | 2003-10-23 | Covion Organic Semiconductors | Rhodium and iridium complexes |
| WO2003092334A1 (en) * | 2002-04-26 | 2003-11-06 | Nippon Hoso Kyokai | Phosphorescent polymer compound, light emitting material and organic electroluminescent (el) device using the compound |
| DE10223337A1 (en) * | 2002-05-25 | 2003-12-04 | Covion Organic Semiconductors | Process for the production of high-purity, tris-orthometallized organo-iridium compounds |
| US7090929B2 (en) | 2002-07-30 | 2006-08-15 | E.I. Du Pont De Nemours And Company | Metallic complexes covalently bound to conjugated polymers and electronic devices containing such compositions |
| JP4343838B2 (en) * | 2002-08-16 | 2009-10-14 | ザ ユニバーシティ オブ サザン カリフォルニア | Organic light emitting materials and devices |
| US6916554B2 (en) * | 2002-11-06 | 2005-07-12 | The University Of Southern California | Organic light emitting materials and devices |
| US7098060B2 (en) * | 2002-09-06 | 2006-08-29 | E.I. Du Pont De Nemours And Company | Methods for producing full-color organic electroluminescent devices |
| JP4115788B2 (en) * | 2002-09-17 | 2008-07-09 | 日本放送協会 | ORGANIC LIGHT EMITTING MATERIAL, ORGANIC LIGHT EMITTING ELEMENT AND DISPLAY USING THE SAME |
| US7317047B2 (en) | 2002-09-24 | 2008-01-08 | E.I. Du Pont De Nemours And Company | Electrically conducting organic polymer/nanoparticle composites and methods for use thereof |
| US20040124504A1 (en) | 2002-09-24 | 2004-07-01 | Che-Hsiung Hsu | Electrically conducting organic polymer/nanoparticle composites and methods for use thereof |
| US7371336B2 (en) | 2002-09-24 | 2008-05-13 | E.I. Du Pont Nemours And Company | Water dispersible polyanilines made with polymeric acid colloids for electronics applications |
| WO2004029128A2 (en) | 2002-09-24 | 2004-04-08 | E.I. Du Pont De Nemours And Company | Water dispersible polythiophenes made with polymeric acid colloids |
| US7462298B2 (en) | 2002-09-24 | 2008-12-09 | E.I. Du Pont De Nemours And Company | Water dispersible polyanilines made with polymeric acid colloids for electronics applications |
| KR100520937B1 (en) * | 2002-12-03 | 2005-10-17 | 엘지전자 주식회사 | Phenyl pyridine - iridium metal complex compounds for organic electroluminescent device, process for preparing them and organic electroluminescent device using them |
| GB0230076D0 (en) * | 2002-12-24 | 2003-01-29 | Elam T Ltd | Electroluminescent materials and devices |
| KR100509603B1 (en) * | 2002-12-28 | 2005-08-22 | 삼성에스디아이 주식회사 | Red emitting compound and organic electroluminescence device |
| KR101162933B1 (en) | 2003-04-15 | 2012-07-05 | 메르크 파텐트 게엠베하 | Mixtures of matrix materials and organic semiconductors capable of emission, use of the same and electronic components containing said mixtures |
| US7029765B2 (en) * | 2003-04-22 | 2006-04-18 | Universal Display Corporation | Organic light emitting devices having reduced pixel shrinkage |
| DE10320103A1 (en) * | 2003-05-05 | 2004-12-02 | Basf Ag | Process for the preparation of phenylpyridine metal complexes and use of such complexes in OLEDs |
| NL1023679C2 (en) * | 2003-06-17 | 2004-12-20 | Tno | Light-emitting diode. |
| KR100682858B1 (en) * | 2003-06-26 | 2007-02-15 | 삼성에스디아이 주식회사 | Organometallic compound and organic electroluminescent device employing the same |
| US8592614B2 (en) | 2003-07-07 | 2013-11-26 | Merck Patent Gmbh | Mixtures of organic emissive semiconductors and matrix materials, their use and electronic components comprising said materials |
| TWI287567B (en) * | 2003-07-30 | 2007-10-01 | Chi Mei Optoelectronics Corp | Light-emitting element and iridium complex |
| US7686978B2 (en) | 2003-09-24 | 2010-03-30 | E. I. Du Pont De Nemours And Company | Method for the application of active materials onto active surfaces and devices made with such methods |
| US8048535B2 (en) | 2003-09-24 | 2011-11-01 | Fujifiilm Corporation | Electroluminescent device |
| US7087320B2 (en) * | 2003-11-04 | 2006-08-08 | Eastman Kodak Company | Organic element for electroluminescent devices |
| US20050100657A1 (en) * | 2003-11-10 | 2005-05-12 | Macpherson Charles D. | Organic material with a region including a guest material and organic electronic devices incorporating the same |
| DE602004005486T2 (en) * | 2003-11-10 | 2007-11-29 | E.I. Dupont De Nemours And Co., Wilmington | PROCESS FOR THE FORMATION OF ORGANIC LAYERS WITH A REGION WITH A GUEST MATERIAL AND ELECTRONIC ORGANIC COMPONENTS THEREWITH |
| US20060139342A1 (en) * | 2004-12-29 | 2006-06-29 | Gang Yu | Electronic devices and processes for forming electronic devices |
| WO2005053051A1 (en) | 2003-11-25 | 2005-06-09 | Merck Patent Gmbh | Organic electroluminescent element |
| DE10356099A1 (en) | 2003-11-27 | 2005-07-07 | Covion Organic Semiconductors Gmbh | Organic electroluminescent element |
| US7084425B2 (en) | 2003-12-05 | 2006-08-01 | Eastman Kodak Company | Organic electroluminescent devices |
| DE10357315A1 (en) | 2003-12-05 | 2005-07-07 | Covion Organic Semiconductors Gmbh | Organic electroluminescent element |
| KR100806989B1 (en) * | 2003-12-22 | 2008-02-25 | 히다치 가세고교 가부시끼가이샤 | Method of luminescence and chemical substance for luminescence |
| EP1555269B1 (en) * | 2004-01-13 | 2006-07-05 | LG Electronics Inc. | Phenyl pyridine-iridium metal complex compounds for organic electroluminescent device, process for preparing the compounds, and organic electroluminescent device using the compounds |
| KR100657892B1 (en) | 2004-02-11 | 2006-12-14 | 삼성에스디아이 주식회사 | Organic electroluminescence device |
| GB0403322D0 (en) * | 2004-02-14 | 2004-03-17 | Elam T Ltd | Electroluminescent materials and devices |
| US7365230B2 (en) | 2004-02-20 | 2008-04-29 | E.I. Du Pont De Nemours And Company | Cross-linkable polymers and electronic devices made with such polymers |
| US7208233B2 (en) | 2004-03-16 | 2007-04-24 | Eastman Kodak Company | Organic element for electroluminescent devices |
| US7351358B2 (en) | 2004-03-17 | 2008-04-01 | E.I. Du Pont De Nemours And Company | Water dispersible polypyrroles made with polymeric acid colloids for electronics applications |
| EP2292583A3 (en) | 2004-03-31 | 2011-08-31 | E. I. du Pont de Nemours and Company | Triarylamine compounds for use as charge transport materials |
| US8084145B2 (en) * | 2004-04-02 | 2011-12-27 | Semiconductor Energy Laboratory Co., Ltd. | Organometallic complex, light emitting element using the complex, light emitting device using the element, and electric apparatus using the device |
| US8147962B2 (en) | 2004-04-13 | 2012-04-03 | E. I. Du Pont De Nemours And Company | Conductive polymer composites |
| US7154114B2 (en) * | 2004-05-18 | 2006-12-26 | Universal Display Corporation | Cyclometallated iridium carbene complexes for use as hosts |
| US7393599B2 (en) | 2004-05-18 | 2008-07-01 | The University Of Southern California | Luminescent compounds with carbene ligands |
| US7445855B2 (en) * | 2004-05-18 | 2008-11-04 | The University Of Southern California | Cationic metal-carbene complexes |
| US7655323B2 (en) * | 2004-05-18 | 2010-02-02 | The University Of Southern California | OLEDs utilizing macrocyclic ligand systems |
| US7491823B2 (en) | 2004-05-18 | 2009-02-17 | The University Of Southern California | Luminescent compounds with carbene ligands |
| US7279704B2 (en) | 2004-05-18 | 2007-10-09 | The University Of Southern California | Complexes with tridentate ligands |
| US7601436B2 (en) | 2004-05-18 | 2009-10-13 | The University Of Southern California | Carbene metal complexes as OLED materials |
| US7534505B2 (en) * | 2004-05-18 | 2009-05-19 | The University Of Southern California | Organometallic compounds for use in electroluminescent devices |
| US7598388B2 (en) | 2004-05-18 | 2009-10-06 | The University Of Southern California | Carbene containing metal complexes as OLEDs |
| JP4496357B2 (en) * | 2004-06-04 | 2010-07-07 | 独立行政法人産業技術総合研究所 | Fluorine-substituted iridium complex and light emitting material using the same |
| JP4886177B2 (en) * | 2004-06-08 | 2012-02-29 | キヤノン株式会社 | Oriented film of organometallic complex with pores |
| KR101292376B1 (en) * | 2004-06-09 | 2013-08-01 | 이 아이 듀폰 디 네모아 앤드 캄파니 | Organometallic compounds and devices made with such compounds |
| CN1305883C (en) * | 2004-06-17 | 2007-03-21 | 复旦大学 | Iridium complex containing aromatic amine and its preparation process and use |
| KR100730115B1 (en) * | 2004-06-23 | 2007-06-19 | 삼성에스디아이 주식회사 | Iridium compound and organic electroluminescence display employing the same |
| KR100738053B1 (en) * | 2004-06-29 | 2007-07-10 | 삼성에스디아이 주식회사 | Iridium complexes having heteroatom linking group and organic electroluminescence device using the same |
| US7316756B2 (en) | 2004-07-27 | 2008-01-08 | Eastman Kodak Company | Desiccant for top-emitting OLED |
| KR100669718B1 (en) | 2004-07-29 | 2007-01-16 | 삼성에스디아이 주식회사 | Organic electroluminescence device |
| US20060040139A1 (en) | 2004-08-18 | 2006-02-23 | Norman Herron | Electronic devices made with metal Schiff base complexes |
| US7402345B2 (en) | 2004-09-14 | 2008-07-22 | E.I. Du Pont De Nemours And Company | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| US9040170B2 (en) * | 2004-09-20 | 2015-05-26 | Global Oled Technology Llc | Electroluminescent device with quinazoline complex emitter |
| ITMN20040024A1 (en) * | 2004-09-24 | 2004-12-24 | Pe Srl | LABELING MACHINE FOR LABELS INTENDED FOR THERMAL Shrinking |
| JP2006128624A (en) | 2004-09-29 | 2006-05-18 | Canon Inc | Light emitting element |
| US7449601B2 (en) | 2004-12-16 | 2008-11-11 | E. I. Du Pont De Nemours And Company | Catalysts useful for catalyzing the coupling of arylhalides with arylboronic acids |
| US7273939B1 (en) | 2004-12-22 | 2007-09-25 | E. I. Du Pont De Nemours And Company | Methods of making tris(N-aryl benzimidazoles)benzenes and their use in electronic devices |
| US8063230B1 (en) | 2004-12-22 | 2011-11-22 | E. I. Du Pont De Nemours And Company | Tris(N-aryl benzimidazole)benzenes and their use in electronic devices |
| US7230107B1 (en) | 2004-12-29 | 2007-06-12 | E. I. Du Pont De Nemours And Company | Metal quinoline complexes |
| US7838127B1 (en) | 2004-12-29 | 2010-11-23 | E. I. Du Pont De Nemours And Company | Metal quinoline complexes |
| US8950328B1 (en) | 2004-12-29 | 2015-02-10 | E I Du Pont De Nemours And Company | Methods of fabricating organic electronic devices |
| US20060141135A1 (en) * | 2004-12-29 | 2006-06-29 | Jian Wang | Processes for forming layers for electronic devices using heating elements |
| US7524923B1 (en) | 2004-12-30 | 2009-04-28 | Dupont Displays, Inc. | Suzuki polycondensation for preparing aryl polymers from dihalide monomers |
| US7723546B1 (en) | 2004-12-30 | 2010-05-25 | E. I. Du Pont De Nemours And Company | Arylamine compounds and their use in electronic devices |
| KR101334442B1 (en) | 2004-12-30 | 2013-12-02 | 이 아이 듀폰 디 네모아 앤드 캄파니 | Derivatized 3,4-Alkylenedioxythiophene Monomers, Methods of Making Them, and Use Thereof |
| US7469638B2 (en) * | 2004-12-30 | 2008-12-30 | E.I. Du Pont De Nemours And Company | Electronic devices and processes for forming the same |
| JP5258302B2 (en) | 2004-12-30 | 2013-08-07 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Organic electronic devices and methods |
| US8217181B2 (en) | 2004-12-30 | 2012-07-10 | E. I. Du Pont De Nemours And Company | Dihalogen indolocarbazole monomers and poly (indolocarbazoles) |
| US7584701B2 (en) * | 2004-12-30 | 2009-09-08 | E.I. Du Pont De Nemours And Company | Processes for printing layers for electronic devices and printing apparatuses for performing the processes |
| US7732062B1 (en) | 2004-12-30 | 2010-06-08 | E. I. Du Pont De Nemours And Company | Charge transport layers and organic electron devices comprising same |
| US7781550B1 (en) | 2004-12-30 | 2010-08-24 | E. I. Du Pont De Nemours And Company | Charge transport compositions and their use in electronic devices |
| EP1859656B1 (en) | 2004-12-30 | 2013-07-17 | E.I. Du Pont De Nemours And Company | Organometallic complexes |
| WO2006074062A2 (en) | 2004-12-30 | 2006-07-13 | E.I. Dupont De Nemours And Company | Encapsulation tool and methods |
| US7563392B1 (en) | 2004-12-30 | 2009-07-21 | E.I. Du Pont De Nemours And Company | Organic conductive compositions and structures |
| US7759428B1 (en) | 2004-12-30 | 2010-07-20 | Dupont Displays, Inc. | Conjugated heteroaryl-containing polymers |
| US20060228466A1 (en) * | 2004-12-30 | 2006-10-12 | Gang Yu | Solution dispense and patterning process and apparatus |
| US8691667B1 (en) | 2004-12-30 | 2014-04-08 | E. I. Du Pont De Nemours And Company | Method and apparatus for depositing a pattern on a substrate |
| US7781588B1 (en) | 2004-12-30 | 2010-08-24 | E.I. Du Pont De Nemours And Company | Acridan monomers and polymers |
| WO2006078427A2 (en) | 2004-12-30 | 2006-07-27 | E.I. Dupont De Nemours And Company | Device patterning using irradiation |
| US7268006B2 (en) | 2004-12-30 | 2007-09-11 | E.I. Du Pont De Nemours And Company | Electronic device including a guest material within a layer and a process for forming the same |
| US7736540B1 (en) | 2004-12-30 | 2010-06-15 | E. I. Du Pont De Nemours And Company | Organic compositions for depositing onto fluorinated surfaces |
| JP2008527629A (en) | 2004-12-30 | 2008-07-24 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | How to condition getter materials |
| US8017223B2 (en) | 2004-12-30 | 2011-09-13 | E. I. Du Pont De Nemours And Company | Containment structure and method |
| US7670506B1 (en) | 2004-12-30 | 2010-03-02 | E. I. Du Pont De Nemours And Company | Photoactive compositions for liquid deposition |
| US7811624B1 (en) | 2004-12-30 | 2010-10-12 | Dupont Displays, Inc. | Self-assembled layers for electronic devices |
| US20060146079A1 (en) * | 2004-12-30 | 2006-07-06 | Macpherson Charles D | Process and apparatus for forming an electronic device |
| US20060154180A1 (en) | 2005-01-07 | 2006-07-13 | Kannurpatti Anandkumar R | Imaging element for use as a recording element and process of using the imaging element |
| KR101234226B1 (en) | 2005-02-03 | 2013-02-18 | 삼성디스플레이 주식회사 | Organometallic complexes and organic electroluminescence device using the same |
| JP5130606B2 (en) * | 2005-02-25 | 2013-01-30 | コニカミノルタホールディングス株式会社 | ORGANIC ELECTROLUMINESCENCE ELEMENT, ITS MANUFACTURING METHOD, DISPLAY DEVICE, AND LIGHTING DEVICE |
| US8889266B2 (en) * | 2005-03-17 | 2014-11-18 | Semiconductor Energy Laboratory Co., Ltd. | Organometallic complex, and light-emitting element, light-emitting device and electronic-device using the organometallic complex |
| WO2006106842A1 (en) * | 2005-03-31 | 2006-10-12 | Idemitsu Kosan Co., Ltd. | Transition metal complex compound and organic electroluminescence element using the same |
| US9070884B2 (en) | 2005-04-13 | 2015-06-30 | Universal Display Corporation | Hybrid OLED having phosphorescent and fluorescent emitters |
| US8057916B2 (en) * | 2005-04-20 | 2011-11-15 | Global Oled Technology, Llc. | OLED device with improved performance |
| TWI282250B (en) * | 2005-04-21 | 2007-06-01 | Au Optronics Corp | Light emission material and organic electroluminescent device using the same |
| US20060240281A1 (en) * | 2005-04-21 | 2006-10-26 | Eastman Kodak Company | Contaminant-scavenging layer on OLED anodes |
| KR100611885B1 (en) * | 2005-04-21 | 2006-08-11 | 삼성에스디아이 주식회사 | Organic metal compounds which the compound for host and the compound for dopant were connected, organic electroluminescence display devices using the compounds and method for preparation of the devices |
| KR100732823B1 (en) * | 2005-04-21 | 2007-06-27 | 삼성에스디아이 주식회사 | Organic metal compounds which the compounds for host and the compounds for dopant were connected, organic electroluminescence display devices using the compounds and method for preparation of the devices |
| US8586204B2 (en) | 2007-12-28 | 2013-11-19 | Universal Display Corporation | Phosphorescent emitters and host materials with improved stability |
| DE102005027548A1 (en) * | 2005-06-14 | 2006-12-21 | Basf Ag | Process for the isomerization of cyclometallated, carbene ligand-containing transition metal complexes |
| CN101595532B (en) | 2005-06-28 | 2013-07-31 | E.I.内穆尔杜邦公司 | Buffer compositions |
| KR101356296B1 (en) | 2005-06-28 | 2014-02-06 | 이 아이 듀폰 디 네모아 앤드 캄파니 | High Work Function Transparent Conductors |
| KR101300029B1 (en) * | 2005-06-30 | 2013-08-29 | 코닌클리즈케 필립스 일렉트로닉스 엔.브이. | Electro luminescent metal complexes |
| CN100362006C (en) * | 2005-09-16 | 2008-01-16 | 中国科学院长春应用化学研究所 | Dendritic iridium complex and organic electroluminescent device therewith |
| DE102005047397A1 (en) * | 2005-10-04 | 2007-04-12 | Consortium für elektrochemische Industrie GmbH | Phosphorescent mixtures |
| CN100358971C (en) * | 2005-10-11 | 2008-01-02 | 友达光电股份有限公司 | Organic light-emitting material and organic light-emitting device |
| US8956738B2 (en) * | 2005-10-26 | 2015-02-17 | Global Oled Technology Llc | Organic element for low voltage electroluminescent devices |
| JP4802671B2 (en) * | 2005-11-10 | 2011-10-26 | 凸版印刷株式会社 | Organic EL device with low molecular organic thin film |
| JP4769551B2 (en) * | 2005-11-10 | 2011-09-07 | 凸版印刷株式会社 | Coating type low molecular carrier transportable material, light emitting material and ink |
| US20070122657A1 (en) * | 2005-11-30 | 2007-05-31 | Eastman Kodak Company | Electroluminescent device containing a phenanthroline derivative |
| US9666826B2 (en) * | 2005-11-30 | 2017-05-30 | Global Oled Technology Llc | Electroluminescent device including an anthracene derivative |
| KR101478004B1 (en) * | 2005-12-05 | 2015-01-02 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Organometallic Complex and Light-Emitting Element, Light-Emitting Device and Electronic Device using the Same |
| US8173995B2 (en) | 2005-12-23 | 2012-05-08 | E. I. Du Pont De Nemours And Company | Electronic device including an organic active layer and process for forming the electronic device |
| US8440324B2 (en) | 2005-12-27 | 2013-05-14 | E I Du Pont De Nemours And Company | Compositions comprising novel copolymers and electronic devices made with such compositions |
| US7795653B2 (en) * | 2005-12-27 | 2010-09-14 | E. I. Du Pont De Nemours And Company | Electronic device including space-apart radiation regions and a process for forming the same |
| US7807992B2 (en) | 2005-12-28 | 2010-10-05 | E.I. Du Pont De Nemours And Company | Organic electronic device having dual emitter dopants |
| EP2412699A1 (en) | 2005-12-28 | 2012-02-01 | E.I. Du Pont De Nemours And Company | Compositions comprising novel compounds and electronic devices made with such compositions |
| US7960717B2 (en) * | 2005-12-29 | 2011-06-14 | E.I. Du Pont De Nemours And Company | Electronic device and process for forming same |
| US7838627B2 (en) | 2005-12-29 | 2010-11-23 | E. I. Du Pont De Nemours And Company | Compositions comprising novel compounds and polymers, and electronic devices made with such compositions |
| EP1974590B1 (en) | 2006-01-18 | 2020-03-04 | LG Display Co., Ltd. | Oled having stacked organic light-emitting units |
| US8470208B2 (en) | 2006-01-24 | 2013-06-25 | E I Du Pont De Nemours And Company | Organometallic complexes |
| US8216680B2 (en) | 2006-02-03 | 2012-07-10 | E I Du Pont De Nemours And Company | Transparent composite conductors having high work function |
| EP2243785B1 (en) | 2006-02-10 | 2016-02-03 | Universal Display Corporation | Phosphorescent condensed ring carbene metal complexes for use in organic light emitting devices |
| US20070207345A1 (en) * | 2006-03-01 | 2007-09-06 | Eastman Kodak Company | Electroluminescent device including gallium complexes |
| TWI423982B (en) * | 2006-03-21 | 2014-01-21 | Semiconductor Energy Lab | Organometallic complex and light emitting element, light emitting device, and electronic device using the organometallic complex |
| JP4785594B2 (en) * | 2006-03-31 | 2011-10-05 | キヤノン株式会社 | Method for producing iridium complex, organic electroluminescence element and display device |
| CN100422285C (en) * | 2006-04-06 | 2008-10-01 | 友达光电股份有限公司 | Luminescent layer of organic electroluminescent device and organic electroluminescent materials |
| US9118020B2 (en) * | 2006-04-27 | 2015-08-25 | Global Oled Technology Llc | Electroluminescent devices including organic eil layer |
| WO2007130047A1 (en) | 2006-05-08 | 2007-11-15 | Eastman Kodak Company | Oled electron-injecting layer |
| US7737277B2 (en) | 2006-05-08 | 2010-06-15 | E.I. Du Pont De Nemours And Company | Electroluminescent bis-cyclometalled iridium compounds and devices made with such compounds |
| US7675228B2 (en) | 2006-06-14 | 2010-03-09 | E.I. Du Pont De Nemours And Company | Electroluminescent iridium compounds with silylated, germanylated, and stannylated ligands, and devices made with such compounds |
| KR101073232B1 (en) * | 2006-11-07 | 2011-10-12 | 쇼와 덴코 가부시키가이샤 | Iridium complex compound, organic electroluminescent device obtained by using the same, and uses of the device |
| EP2097938B1 (en) | 2006-12-28 | 2019-07-17 | Universal Display Corporation | Long lifetime phosphorescent organic light emitting device (oled) structures |
| US8062553B2 (en) | 2006-12-28 | 2011-11-22 | E. I. Du Pont De Nemours And Company | Compositions of polyaniline made with perfuoropolymeric acid which are heat-enhanced and electronic devices made therewith |
| US8153029B2 (en) | 2006-12-28 | 2012-04-10 | E.I. Du Pont De Nemours And Company | Laser (230NM) ablatable compositions of electrically conducting polymers made with a perfluoropolymeric acid applications thereof |
| US20080166566A1 (en) * | 2006-12-29 | 2008-07-10 | Shiva Prakash | Process for forming an organic light-emitting diode and devices made by the process |
| US20080191172A1 (en) | 2006-12-29 | 2008-08-14 | Che-Hsiung Hsu | High work-function and high conductivity compositions of electrically conducting polymers |
| DE102007002714A1 (en) | 2007-01-18 | 2008-07-31 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| US9130177B2 (en) | 2011-01-13 | 2015-09-08 | Universal Display Corporation | 5-substituted 2 phenylquinoline complexes materials for light emitting diode |
| JP5638246B2 (en) | 2007-03-08 | 2014-12-10 | ユニバーサル ディスプレイ コーポレイション | Phosphorescent material |
| US20130032785A1 (en) | 2011-08-01 | 2013-02-07 | Universal Display Corporation | Materials for organic light emitting diode |
| US20080284318A1 (en) * | 2007-05-17 | 2008-11-20 | Deaton Joseph C | Hybrid fluorescent/phosphorescent oleds |
| US20080284317A1 (en) * | 2007-05-17 | 2008-11-20 | Liang-Sheng Liao | Hybrid oled having improved efficiency |
| US8241526B2 (en) | 2007-05-18 | 2012-08-14 | E I Du Pont De Nemours And Company | Aqueous dispersions of electrically conducting polymers containing high boiling solvent and additives |
| WO2008143113A1 (en) | 2007-05-18 | 2008-11-27 | Semiconductor Energy Laboratory Co., Ltd. | Organometallic complex, composition and light emitting element including the organometallic complex |
| JP5127300B2 (en) | 2007-05-28 | 2013-01-23 | キヤノン株式会社 | Fluorene compound, organic light emitting device using the same, and display device |
| JP5053713B2 (en) | 2007-05-30 | 2012-10-17 | キヤノン株式会社 | Phosphorescent material, organic electroluminescent element and image display device using the same |
| WO2008149828A1 (en) * | 2007-06-05 | 2008-12-11 | Semiconductor Energy Laboratory Co., Ltd. | Organometallic complex, and light-emitting materia light-emitting element, light-emitting device and electronic device |
| JP5008470B2 (en) | 2007-06-18 | 2012-08-22 | キヤノン株式会社 | Organic electroluminescence device |
| US8034465B2 (en) * | 2007-06-20 | 2011-10-11 | Global Oled Technology Llc | Phosphorescent oled having double exciton-blocking layers |
| EP3345983B1 (en) | 2007-07-05 | 2020-08-26 | UDC Ireland Limited | Compounds containing at least one disilyl compound selected from disilylcarbazoles, disilyldibenzofurans, disilyldibenzothiophenes, disilyldibenzophospholes, disilyldibenzothiophene s-oxides and disilyldibenzothiophene s, s-dioxides |
| TWI531567B (en) | 2007-08-08 | 2016-05-01 | 環球展覽公司 | Organic electroluminescent materials and devices |
| TWI501943B (en) | 2007-08-08 | 2015-10-01 | Universal Display Corp | Single triphenylene chromophores in phosphorescent light emitting diodes |
| JP2009076865A (en) | 2007-08-29 | 2009-04-09 | Fujifilm Corp | Organic electroluminescence device |
| JP5311785B2 (en) | 2007-09-13 | 2013-10-09 | キヤノン株式会社 | Organic light emitting device and display device |
| KR101548382B1 (en) | 2007-09-14 | 2015-08-28 | 유디씨 아일랜드 리미티드 | Organic electroluminescence device |
| CN101848882B (en) * | 2007-09-20 | 2015-04-29 | 巴斯夫欧洲公司 | Electroluminescent device |
| JP5438941B2 (en) | 2007-09-25 | 2014-03-12 | ユー・ディー・シー アイルランド リミテッド | Organic electroluminescence device |
| KR20090032250A (en) * | 2007-09-27 | 2009-04-01 | 엘지디스플레이 주식회사 | Red phosphorescence compound and organic electroluminescence device using the same |
| WO2009047147A1 (en) | 2007-10-02 | 2009-04-16 | Basf Se | Use of acridine derivatives as matrix materials and/or an electron blocker in oleds |
| US8067100B2 (en) | 2007-10-04 | 2011-11-29 | Universal Display Corporation | Complexes with tridentate ligands |
| US8383249B2 (en) * | 2007-10-04 | 2013-02-26 | Universal Display Corporation | Complexes with tridentate ligands |
| KR20100094475A (en) | 2007-10-26 | 2010-08-26 | 이 아이 듀폰 디 네모아 앤드 캄파니 | Process and materials for making contained layers and devices made with same |
| DE102007053771A1 (en) | 2007-11-12 | 2009-05-14 | Merck Patent Gmbh | Organic electroluminescent devices |
| JP5489445B2 (en) | 2007-11-15 | 2014-05-14 | 富士フイルム株式会社 | Thin film field effect transistor and display device using the same |
| US8319214B2 (en) | 2007-11-15 | 2012-11-27 | Fujifilm Corporation | Thin film field effect transistor with amorphous oxide active layer and display using the same |
| JP5082800B2 (en) * | 2007-11-27 | 2012-11-28 | コニカミノルタホールディングス株式会社 | Manufacturing method of organic EL element |
| WO2009073245A1 (en) | 2007-12-06 | 2009-06-11 | Universal Display Corporation | Light-emitting organometallic complexes |
| JP5438955B2 (en) | 2007-12-14 | 2014-03-12 | ユー・ディー・シー アイルランド リミテッド | Platinum complex compound and organic electroluminescence device using the same |
| US20090162612A1 (en) * | 2007-12-19 | 2009-06-25 | Hatwar Tukaram K | Oled device having two electron-transport layers |
| WO2009082043A1 (en) * | 2007-12-21 | 2009-07-02 | Dongwoo Fine-Chem Co., Ltd. | Iridium complex and organic electroluminescent device |
| WO2009085344A2 (en) | 2007-12-28 | 2009-07-09 | Universal Display Corporation | Dibenzothiophene-containing materials in phosphorescent light emitting diodes |
| US8221905B2 (en) | 2007-12-28 | 2012-07-17 | Universal Display Corporation | Carbazole-containing materials in phosphorescent light emitting diodes |
| US20090191427A1 (en) * | 2008-01-30 | 2009-07-30 | Liang-Sheng Liao | Phosphorescent oled having double hole-blocking layers |
| US8040053B2 (en) | 2008-02-09 | 2011-10-18 | Universal Display Corporation | Organic light emitting device architecture for reducing the number of organic materials |
| JP5243972B2 (en) | 2008-02-28 | 2013-07-24 | ユー・ディー・シー アイルランド リミテッド | Organic electroluminescence device |
| JP4555358B2 (en) | 2008-03-24 | 2010-09-29 | 富士フイルム株式会社 | Thin film field effect transistor and display device |
| DE102008015526B4 (en) | 2008-03-25 | 2021-11-11 | Merck Patent Gmbh | Metal complexes |
| DE102008017591A1 (en) | 2008-04-07 | 2009-10-08 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| JP4531836B2 (en) | 2008-04-22 | 2010-08-25 | 富士フイルム株式会社 | Organic electroluminescent device, novel platinum complex compound and novel compound that can be a ligand |
| JP4531842B2 (en) | 2008-04-24 | 2010-08-25 | 富士フイルム株式会社 | Organic electroluminescence device |
| TW201016325A (en) * | 2008-05-19 | 2010-05-01 | Du Pont | Apparatus and method for solution coating thin layers |
| DE102008027005A1 (en) | 2008-06-05 | 2009-12-10 | Merck Patent Gmbh | Organic electronic device containing metal complexes |
| US8324800B2 (en) * | 2008-06-12 | 2012-12-04 | Global Oled Technology Llc | Phosphorescent OLED device with mixed hosts |
| KR102174125B1 (en) | 2008-06-20 | 2020-11-06 | 오엘이디워크스 게엠베하 | Cyclic phosphazene compounds and use thereof in organic light emitting diodes |
| JP5609022B2 (en) * | 2008-06-23 | 2014-10-22 | 住友化学株式会社 | Polymer compound containing residue of metal complex and device using the same |
| US8440326B2 (en) | 2008-06-30 | 2013-05-14 | Universal Display Corporation | Hole transport materials containing triphenylene |
| DE102008033943A1 (en) | 2008-07-18 | 2010-01-21 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| DE102008036982A1 (en) | 2008-08-08 | 2010-02-11 | Merck Patent Gmbh | Organic electroluminescent device |
| US8247088B2 (en) * | 2008-08-28 | 2012-08-21 | Global Oled Technology Llc | Emitting complex for electroluminescent devices |
| WO2010027583A1 (en) | 2008-09-03 | 2010-03-11 | Universal Display Corporation | Phosphorescent materials |
| CN102203977B (en) | 2008-09-04 | 2014-06-04 | 通用显示公司 | White phosphorescent organic light emitting devices |
| EP2161272A1 (en) | 2008-09-05 | 2010-03-10 | Basf Se | Phenanthrolines |
| US9034483B2 (en) | 2008-09-16 | 2015-05-19 | Universal Display Corporation | Phosphorescent materials |
| KR101804084B1 (en) | 2008-09-25 | 2017-12-01 | 유니버셜 디스플레이 코포레이션 | Organoselenium materials and their uses in organic light emitting devices |
| JP5661635B2 (en) | 2008-10-07 | 2015-01-28 | オスラム オプト セミコンダクターズ ゲゼルシャフト ミット ベシュレンクテル ハフツングOsram Opto Semiconductors GmbH | Siloles substituted with fused ring systems and their use in organic electronics |
| DE102008050841B4 (en) | 2008-10-08 | 2019-08-01 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| US8053770B2 (en) | 2008-10-14 | 2011-11-08 | Universal Display Corporation | Emissive layer patterning for OLED |
| US8865321B2 (en) | 2008-11-11 | 2014-10-21 | Merck Patent Gmbh | Organic electroluminescent devices |
| DE102009022858A1 (en) | 2009-05-27 | 2011-12-15 | Merck Patent Gmbh | Organic electroluminescent devices |
| DE102008056688A1 (en) | 2008-11-11 | 2010-05-12 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN103396455B (en) | 2008-11-11 | 2017-03-01 | 通用显示公司 | Phosphorescent emitters |
| JP5448030B2 (en) * | 2008-11-19 | 2014-03-19 | 新日鐵住金株式会社 | Ultrasonic flaw detection method and apparatus |
| JP2010153820A (en) | 2008-11-21 | 2010-07-08 | Fujifilm Corp | Organic electroluminescent element |
| US8778511B2 (en) | 2008-12-12 | 2014-07-15 | Universal Display Corporation | OLED stability via doped hole transport layer |
| US8815415B2 (en) | 2008-12-12 | 2014-08-26 | Universal Display Corporation | Blue emitter with high efficiency based on imidazo[1,2-f] phenanthridine iridium complexes |
| DE102008063490B4 (en) | 2008-12-17 | 2023-06-15 | Merck Patent Gmbh | Organic electroluminescent device and method for adjusting the color locus of a white-emitting electroluminescent device |
| DE102008063470A1 (en) | 2008-12-17 | 2010-07-01 | Merck Patent Gmbh | Organic electroluminescent device |
| CN102265425B (en) * | 2008-12-27 | 2015-07-29 | E.I.内穆尔杜邦公司 | For preventing equipment and the method for splatter for continuous printing |
| US8766239B2 (en) | 2008-12-27 | 2014-07-01 | E I Du Pont De Nemours And Company | Buffer bilayers for electronic devices |
| US8785913B2 (en) | 2008-12-27 | 2014-07-22 | E I Du Pont De Nemours And Company | Buffer bilayers for electronic devices |
| US9067947B2 (en) | 2009-01-16 | 2015-06-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| DE102009005290A1 (en) | 2009-01-20 | 2010-07-22 | Merck Patent Gmbh | Connections for electronic devices |
| JP2010182449A (en) | 2009-02-03 | 2010-08-19 | Fujifilm Corp | Organic electroluminescent display device |
| JP2010186723A (en) | 2009-02-13 | 2010-08-26 | Fujifilm Corp | Organic el device and method of manufacturing the same |
| DE102009009277B4 (en) | 2009-02-17 | 2023-12-07 | Merck Patent Gmbh | Organic electronic device, process for its production and use of compounds |
| KR101705213B1 (en) | 2009-02-26 | 2017-02-09 | 노발레드 게엠베하 | Quinone compounds as dopants in organic electronics |
| DE102009011223A1 (en) | 2009-03-02 | 2010-09-23 | Merck Patent Gmbh | metal complexes |
| JP2010232163A (en) | 2009-03-03 | 2010-10-14 | Fujifilm Corp | Method of manufacturing light-emitting display device, light-emitting display device, and light-emitting display |
| JP2010205650A (en) | 2009-03-05 | 2010-09-16 | Fujifilm Corp | Organic el display device |
| DE102009012346B4 (en) | 2009-03-09 | 2024-02-15 | Merck Patent Gmbh | Organic electroluminescent device and method for producing the same |
| WO2010105140A2 (en) | 2009-03-12 | 2010-09-16 | E. I. Du Pont De Nemours And Company | Electrically conductive polymer compositions for coating applications |
| KR101031314B1 (en) | 2009-03-23 | 2011-04-29 | 한국세라믹기술원 | Mercury ion detection sensor using phosphorescence, mercury ion detection sensor array comprising the same and method of preparing the same |
| DE102009014513A1 (en) | 2009-03-23 | 2010-09-30 | Merck Patent Gmbh | Organic electroluminescent device |
| US8722205B2 (en) | 2009-03-23 | 2014-05-13 | Universal Display Corporation | Heteroleptic iridium complex |
| US11910700B2 (en) | 2009-03-23 | 2024-02-20 | Universal Display Corporation | Heteroleptic iridium complexes as dopants |
| US8709615B2 (en) | 2011-07-28 | 2014-04-29 | Universal Display Corporation | Heteroleptic iridium complexes as dopants |
| KR20120001734A (en) | 2009-03-27 | 2012-01-04 | 후지필름 가부시키가이샤 | Coating solution for organic electroluminescent element |
| JP5624784B2 (en) * | 2009-03-31 | 2014-11-12 | 株式会社半導体エネルギー研究所 | Derivatives having heteroaromatic rings, light-emitting elements, light-emitting devices, lighting devices, and electronic devices using derivatives having heteroaromatic rings |
| TWI680132B (en) | 2009-04-06 | 2019-12-21 | 美商環球展覽公司 | Metal complex comprising novel ligand structures |
| DE102009017064A1 (en) | 2009-04-09 | 2010-10-14 | Merck Patent Gmbh | Organic electroluminescent device |
| CN102395628B (en) | 2009-04-21 | 2016-01-20 | E.I.内穆尔杜邦公司 | Conductive polymer compositions and the film obtained by it |
| JP2012524834A (en) | 2009-04-24 | 2012-10-18 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Conductive polymer composition and film made therefrom |
| TWI638807B (en) | 2009-04-28 | 2018-10-21 | 環球展覽公司 | Iridium complex with methyl-d3 substitution |
| TWI541234B (en) | 2009-05-12 | 2016-07-11 | 環球展覽公司 | 2-azatriphenylene materials for organic light emitting diodes |
| US8586203B2 (en) | 2009-05-20 | 2013-11-19 | Universal Display Corporation | Metal complexes with boron-nitrogen heterocycle containing ligands |
| DE102009023156A1 (en) | 2009-05-29 | 2010-12-02 | Merck Patent Gmbh | Polymers containing substituted indenofluorene derivatives as a structural unit, process for their preparation and their use |
| DE102009023154A1 (en) | 2009-05-29 | 2011-06-16 | Merck Patent Gmbh | A composition comprising at least one emitter compound and at least one polymer having conjugation-interrupting units |
| CN102482574B (en) | 2009-06-18 | 2014-09-24 | 巴斯夫欧洲公司 | Phenanthroazole compounds as hole transporting materials for electro luminescent devices |
| US20120104380A1 (en) | 2009-06-22 | 2012-05-03 | Merck Patent Gmbh | Conducting formulation |
| DE102009031021A1 (en) | 2009-06-30 | 2011-01-05 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009032922B4 (en) | 2009-07-14 | 2024-04-25 | Merck Patent Gmbh | Materials for organic electroluminescent devices, processes for their preparation, their use and electronic device |
| KR101675176B1 (en) | 2009-07-27 | 2016-11-10 | 이 아이 듀폰 디 네모아 앤드 캄파니 | Process and materials for making contained layers and devices made with same |
| WO2011013626A1 (en) | 2009-07-31 | 2011-02-03 | 富士フイルム株式会社 | Vapor deposition material for organic device and method for manufacturing organic device |
| US8581262B2 (en) | 2009-08-04 | 2013-11-12 | Merck Patent Gmbh | Electronic devices comprising multi cyclic hydrocarbons |
| US20110193066A1 (en) * | 2009-08-13 | 2011-08-11 | E. I. Du Pont De Nemours And Company | Current limiting element for pixels in electronic devices |
| EP2471889A4 (en) * | 2009-08-27 | 2013-06-26 | Sumitomo Chemical Co | Metal complex composition and complex polymer |
| EP2471800B1 (en) | 2009-08-27 | 2014-01-15 | National Institute of Advanced Industrial Science And Technology | Iridium complex and light emitting material formed from same |
| JP5779318B2 (en) | 2009-08-31 | 2015-09-16 | ユー・ディー・シー アイルランド リミテッド | Organic electroluminescence device |
| JP5473506B2 (en) | 2009-09-14 | 2014-04-16 | ユー・ディー・シー アイルランド リミテッド | Color filter and light emitting display element |
| JP5657243B2 (en) | 2009-09-14 | 2015-01-21 | ユー・ディー・シー アイルランド リミテッド | Color filter and light emitting display element |
| DE102009041289A1 (en) | 2009-09-16 | 2011-03-17 | Merck Patent Gmbh | Organic electroluminescent device |
| CN102498120B (en) | 2009-09-16 | 2016-06-08 | 默克专利有限公司 | For manufacturing the preparation of electronic device |
| DE102009053645A1 (en) | 2009-11-17 | 2011-05-19 | Merck Patent Gmbh | Materials for organic electroluminescent device |
| DE102009053644B4 (en) | 2009-11-17 | 2019-07-04 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009048791A1 (en) | 2009-10-08 | 2011-04-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2011049904A2 (en) | 2009-10-19 | 2011-04-28 | E. I. Du Pont De Nemours And Company | Triarylamine compounds for electronic applications |
| WO2011049953A2 (en) | 2009-10-19 | 2011-04-28 | E. I. Du Pont De Nemours And Company | Triarylamine compounds for electronic applications |
| US8545996B2 (en) | 2009-11-02 | 2013-10-01 | The University Of Southern California | Ion-pairing soft salts based on organometallic complexes and their applications in organic light emitting diodes |
| JP2011100944A (en) | 2009-11-09 | 2011-05-19 | Fujifilm Corp | Organic electroluminescent element |
| DE102009052428A1 (en) | 2009-11-10 | 2011-05-12 | Merck Patent Gmbh | Connection for electronic devices |
| DE102009053382A1 (en) | 2009-11-14 | 2011-05-19 | Merck Patent Gmbh | Materials for electronic devices |
| DE102009053836A1 (en) | 2009-11-18 | 2011-05-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US8580394B2 (en) | 2009-11-19 | 2013-11-12 | Universal Display Corporation | 3-coordinate copper(I)-carbene complexes |
| US8617720B2 (en) | 2009-12-21 | 2013-12-31 | E I Du Pont De Nemours And Company | Electroactive composition and electronic device made with the composition |
| EP2517275B1 (en) | 2009-12-22 | 2018-11-07 | Merck Patent GmbH | Formulations comprising phase-separated functional materials |
| WO2011076326A1 (en) | 2009-12-22 | 2011-06-30 | Merck Patent Gmbh | Electroluminescent functional surfactants |
| JP5968786B2 (en) | 2009-12-22 | 2016-08-10 | メルク パテント ゲーエムベーハー | Electroluminescence formulation |
| EP2517273B1 (en) | 2009-12-23 | 2019-04-03 | Merck Patent GmbH | Compositions comprising organic semiconducting compounds |
| CN107573484A (en) | 2009-12-23 | 2018-01-12 | 默克专利有限公司 | Composition including polymer-binder |
| DE102010004803A1 (en) | 2010-01-16 | 2011-07-21 | Merck Patent GmbH, 64293 | Materials for organic electroluminescent devices |
| US8288187B2 (en) | 2010-01-20 | 2012-10-16 | Universal Display Corporation | Electroluminescent devices for lighting applications |
| DE102010005697A1 (en) | 2010-01-25 | 2011-07-28 | Merck Patent GmbH, 64293 | Connections for electronic devices |
| DE102010006377A1 (en) | 2010-01-29 | 2011-08-04 | Merck Patent GmbH, 64293 | Styrene-based copolymers, in particular for use in optoelectronic components |
| DE102010009193B4 (en) | 2010-02-24 | 2022-05-19 | MERCK Patent Gesellschaft mit beschränkter Haftung | Composition containing fluorine-fluorine associates, processes for their production, their use and organic electronic devices containing them |
| US9156870B2 (en) | 2010-02-25 | 2015-10-13 | Universal Display Corporation | Phosphorescent emitters |
| DE102010009903A1 (en) | 2010-03-02 | 2011-09-08 | Merck Patent Gmbh | Connections for electronic devices |
| US9175211B2 (en) | 2010-03-03 | 2015-11-03 | Universal Display Corporation | Phosphorescent materials |
| DE102010010481A1 (en) | 2010-03-06 | 2011-09-08 | Merck Patent Gmbh | Organic electroluminescent device |
| US9373807B2 (en) | 2010-03-11 | 2016-06-21 | Merck Patent Gmbh | Radiative fibers |
| US9539438B2 (en) | 2010-03-11 | 2017-01-10 | Merck Patent Gmbh | Fibers in therapy and cosmetics |
| CN102812573B (en) | 2010-03-23 | 2015-09-30 | 默克专利有限公司 | For the material of organic electroluminescence device |
| DE102010012738A1 (en) | 2010-03-25 | 2011-09-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP2550690B1 (en) | 2010-03-25 | 2018-12-26 | Universal Display Corporation | Solution processable doped triarylamine hole injection materials |
| DE102010013068A1 (en) | 2010-03-26 | 2011-09-29 | Merck Patent Gmbh | Connections for electronic devices |
| JP6073216B2 (en) | 2010-04-12 | 2017-02-01 | メルク パテント ゲーエムベーハー | Compositions and methods for making organic electronic devices |
| US9379323B2 (en) | 2010-04-12 | 2016-06-28 | Merck Patent Gmbh | Composition having improved performance |
| DE102010014933A1 (en) | 2010-04-14 | 2011-10-20 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010018321A1 (en) | 2010-04-27 | 2011-10-27 | Merck Patent Gmbh | Organic electroluminescent device |
| WO2011136755A1 (en) | 2010-04-28 | 2011-11-03 | Universal Display Corporation | Depositing premixed materials |
| US8968887B2 (en) | 2010-04-28 | 2015-03-03 | Universal Display Corporation | Triphenylene-benzofuran/benzothiophene/benzoselenophene compounds with substituents joining to form fused rings |
| CN105949177B (en) | 2010-05-03 | 2019-02-01 | 默克专利有限公司 | Preparation and electronic device |
| DE102010019306B4 (en) | 2010-05-04 | 2021-05-20 | Merck Patent Gmbh | Organic electroluminescent devices |
| DE102010020044A1 (en) | 2010-05-11 | 2011-11-17 | Merck Patent Gmbh | Organic electroluminescent device |
| WO2011147521A1 (en) | 2010-05-27 | 2011-12-01 | Merck Patent Gmbh | Down conversion |
| JP6309269B2 (en) | 2010-05-27 | 2018-04-11 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツングMerck Patent Gesellschaft mit beschraenkter Haftung | Formulations and methods for preparing organic electronic devices |
| US10190043B2 (en) | 2010-05-27 | 2019-01-29 | Merck Patent Gmbh | Compositions comprising quantum dots |
| US8673458B2 (en) | 2010-06-11 | 2014-03-18 | Universal Display Corporation | Delayed fluorescence OLED |
| US8742657B2 (en) | 2010-06-11 | 2014-06-03 | Universal Display Corporation | Triplet-Triplet annihilation up conversion (TTA-UC) for display and lighting applications |
| US9142792B2 (en) | 2010-06-18 | 2015-09-22 | Basf Se | Organic electronic devices comprising a layer comprising at least one metal organic compound and at least one metal oxide |
| WO2011157790A1 (en) | 2010-06-18 | 2011-12-22 | Basf Se | Organic electronic devices comprising a layer of a dibenzofurane compound and a 8-hydroxyquinolinolato earth alkaline metal, or alkali metal complex |
| DE102010024335A1 (en) | 2010-06-18 | 2011-12-22 | Merck Patent Gmbh | Connections for electronic devices |
| KR101877581B1 (en) | 2010-06-18 | 2018-07-11 | 유디씨 아일랜드 리미티드 | Organic electronic devices comprising a layer of a pyridine compound and a 8-hydroxyquinolinolato earth alkaline metal, or alkali metal complex |
| DE102010024542A1 (en) | 2010-06-22 | 2011-12-22 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010024897A1 (en) | 2010-06-24 | 2011-12-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| JP5882318B2 (en) | 2010-07-26 | 2016-03-09 | メルク パテント ゲーエムベーハー | Nanocrystals in devices |
| EP2599141B1 (en) | 2010-07-26 | 2019-12-11 | Merck Patent GmbH | Quantum dots and hosts |
| WO2012016074A1 (en) | 2010-07-29 | 2012-02-02 | University Of Southern California | Co-deposition methods for the fabrication of organic optoelectronic devices |
| KR101877582B1 (en) | 2010-07-30 | 2018-07-12 | 메르크 파텐트 게엠베하 | Organic electroluminescent device |
| DE102010033080A1 (en) | 2010-08-02 | 2012-02-02 | Merck Patent Gmbh | Polymers with structural units that have electron transport properties |
| DE102010033548A1 (en) | 2010-08-05 | 2012-02-09 | Merck Patent Gmbh | Materials for electronic devices |
| JP5299381B2 (en) * | 2010-08-18 | 2013-09-25 | コニカミノルタ株式会社 | Manufacturing method of organic EL element |
| KR101753172B1 (en) | 2010-08-20 | 2017-07-04 | 유니버셜 디스플레이 코포레이션 | Bicarbazole compounds for oleds |
| DE102010045369A1 (en) | 2010-09-14 | 2012-03-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010045405A1 (en) | 2010-09-15 | 2012-03-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010046412B4 (en) | 2010-09-23 | 2022-01-13 | Merck Patent Gmbh | metal-ligand coordination compounds |
| KR20130124494A (en) | 2010-09-29 | 2013-11-14 | 바스프 에스이 | Security element |
| CN103153974A (en) | 2010-10-07 | 2013-06-12 | 巴斯夫欧洲公司 | Phenanthro[9,10-B]furans for electronic applications |
| US9079872B2 (en) | 2010-10-07 | 2015-07-14 | Basf Se | Phenanthro[9, 10-B]furans for electronic applications |
| US8932734B2 (en) | 2010-10-08 | 2015-01-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| DE102010048074A1 (en) | 2010-10-09 | 2012-04-12 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010048497A1 (en) | 2010-10-14 | 2012-04-19 | Merck Patent Gmbh | Formulations for organic electroluminescent devices |
| DE102010048498A1 (en) | 2010-10-14 | 2012-04-19 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010048607A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Connections for electronic devices |
| DE102010048608A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US8269317B2 (en) | 2010-11-11 | 2012-09-18 | Universal Display Corporation | Phosphorescent materials |
| JP5980796B2 (en) | 2010-11-24 | 2016-08-31 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| US20120138906A1 (en) | 2010-12-07 | 2012-06-07 | The University of Southern California USC Stevens Institute for Innovation | Capture agents for unsaturated metal complexes |
| US8362246B2 (en) | 2010-12-13 | 2013-01-29 | Basf Se | Bispyrimidines for electronic applications |
| EP2651904B1 (en) | 2010-12-13 | 2014-11-12 | Basf Se | Bispyrimidines for electronic applications |
| DE102010054316A1 (en) | 2010-12-13 | 2012-06-14 | Merck Patent Gmbh | Substituted tetraarylbenzenes |
| DE102010054525A1 (en) | 2010-12-15 | 2012-04-26 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102010055901A1 (en) | 2010-12-23 | 2012-06-28 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102010055902A1 (en) | 2010-12-23 | 2012-06-28 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102010056151A1 (en) | 2010-12-28 | 2012-06-28 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| JP6022478B2 (en) | 2011-01-13 | 2016-11-09 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| US10008677B2 (en) | 2011-01-13 | 2018-06-26 | Universal Display Corporation | Materials for organic light emitting diode |
| DE102012000064A1 (en) | 2011-01-21 | 2012-07-26 | Merck Patent Gmbh | New heterocyclic compound excluding 9,10-dioxa-4b-aza-9a-phospha-indeno(1,2-a)indene and 9,10-dioxa-4b-aza-indeno(1,2-a)indene, useful e.g. as matrix material for fluorescent or phosphorescent emitters of electronic devices |
| US8415031B2 (en) | 2011-01-24 | 2013-04-09 | Universal Display Corporation | Electron transporting compounds |
| US8751777B2 (en) | 2011-01-28 | 2014-06-10 | Honeywell International Inc. | Methods and reconfigurable systems to optimize the performance of a condition based health maintenance system |
| DE102011010841A1 (en) | 2011-02-10 | 2012-08-16 | Merck Patent Gmbh | (1,3) -dioxane-5-one compounds |
| DE102011011104A1 (en) | 2011-02-12 | 2012-08-16 | Merck Patent Gmbh | Substituted dibenzonaphthacenes |
| JP6351974B2 (en) | 2011-02-14 | 2018-07-04 | メルク パテント ゲーエムベーハー | Devices and methods for treatment of cells and cellular tissues |
| DE102011011539A1 (en) | 2011-02-17 | 2012-08-23 | Merck Patent Gmbh | Connections for electronic devices |
| TWI560191B (en) | 2011-02-23 | 2016-12-01 | Universal Display Corp | Novel tetradentate platinum complexes |
| US9005772B2 (en) | 2011-02-23 | 2015-04-14 | Universal Display Corporation | Thioazole and oxazole carbene metal complexes as phosphorescent OLED materials |
| US8563737B2 (en) | 2011-02-23 | 2013-10-22 | Universal Display Corporation | Methods of making bis-tridentate carbene complexes of ruthenium and osmium |
| US8748011B2 (en) | 2011-02-23 | 2014-06-10 | Universal Display Corporation | Ruthenium carbene complexes for OLED material |
| US8492006B2 (en) | 2011-02-24 | 2013-07-23 | Universal Display Corporation | Germanium-containing red emitter materials for organic light emitting diode |
| US8883322B2 (en) | 2011-03-08 | 2014-11-11 | Universal Display Corporation | Pyridyl carbene phosphorescent emitters |
| WO2012124622A1 (en) | 2011-03-14 | 2012-09-20 | 東レ株式会社 | Light-emitting element material and light-emitting element |
| WO2012126566A1 (en) | 2011-03-24 | 2012-09-27 | Merck Patent Gmbh | Organic ionic functional materials |
| US9806270B2 (en) | 2011-03-25 | 2017-10-31 | Udc Ireland Limited | 4H-imidazo[1,2-a]imidazoles for electronic applications |
| JP6072760B2 (en) | 2011-03-25 | 2017-02-01 | ユー・ディー・シー アイルランド リミテッド | 4H-imidazo [1,2-a] imidazole for electronics applications |
| CN103477462B (en) | 2011-04-05 | 2016-05-25 | 默克专利有限公司 | Organic electroluminescence device |
| US8580399B2 (en) | 2011-04-08 | 2013-11-12 | Universal Display Corporation | Substituted oligoazacarbazoles for light emitting diodes |
| US9768385B2 (en) | 2011-04-13 | 2017-09-19 | Merck Patent Gmbh | Compounds for electronic devices |
| JP6271417B2 (en) | 2011-04-13 | 2018-01-31 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| KR101979469B1 (en) | 2011-04-18 | 2019-05-16 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| WO2012143079A1 (en) | 2011-04-18 | 2012-10-26 | Merck Patent Gmbh | Compounds for electronic devices |
| WO2012149992A1 (en) | 2011-05-04 | 2012-11-08 | Merck Patent Gmbh | Device for preserving fresh goods |
| US10056549B2 (en) | 2011-05-05 | 2018-08-21 | Merck Patent Gmbh | Compounds for electronic devices |
| CN103503188B (en) | 2011-05-05 | 2016-08-31 | 默克专利有限公司 | compound for electronic device |
| US8432095B2 (en) | 2011-05-11 | 2013-04-30 | Universal Display Corporation | Process for fabricating metal bus lines for OLED lighting panels |
| US8927308B2 (en) | 2011-05-12 | 2015-01-06 | Universal Display Corporation | Method of forming bus line designs for large-area OLED lighting |
| US9391288B2 (en) | 2011-05-12 | 2016-07-12 | Toray Industries, Inc. | Light emitting device material and light emitting device |
| WO2012152366A1 (en) | 2011-05-12 | 2012-11-15 | Merck Patent Gmbh | Organic ionic compounds, compositions and electronic devices |
| US9212197B2 (en) | 2011-05-19 | 2015-12-15 | Universal Display Corporation | Phosphorescent heteroleptic phenylbenzimidazole dopants |
| US8795850B2 (en) | 2011-05-19 | 2014-08-05 | Universal Display Corporation | Phosphorescent heteroleptic phenylbenzimidazole dopants and new synthetic methodology |
| US8748012B2 (en) | 2011-05-25 | 2014-06-10 | Universal Display Corporation | Host materials for OLED |
| US10158089B2 (en) | 2011-05-27 | 2018-12-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10079349B2 (en) | 2011-05-27 | 2018-09-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| DE102011102586A1 (en) | 2011-05-27 | 2012-11-29 | Merck Patent Gmbh | Organic electronic device |
| CN103619986B (en) | 2011-06-03 | 2017-02-08 | 默克专利有限公司 | Organic electroluminescence device |
| KR20190029768A (en) | 2011-06-08 | 2019-03-20 | 유니버셜 디스플레이 코포레이션 | Heteroleptic iridium carbene complexes and light emitting device using them |
| US8659036B2 (en) | 2011-06-17 | 2014-02-25 | Universal Display Corporation | Fine tuning of emission spectra by combination of multiple emitter spectra |
| DE102011104745A1 (en) | 2011-06-17 | 2012-12-20 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US8884316B2 (en) | 2011-06-17 | 2014-11-11 | Universal Display Corporation | Non-common capping layer on an organic device |
| WO2013008835A1 (en) * | 2011-07-12 | 2013-01-17 | 株式会社日立製作所 | Material for forming organic light-emitting layer, coating liquid for forming organic light-emitting element, organic light-emitting element and light source device, and method for manufacturing same |
| US9252377B2 (en) | 2011-07-14 | 2016-02-02 | Universal Display Corporation | Inorganic hosts in OLEDs |
| US9397310B2 (en) | 2011-07-14 | 2016-07-19 | Universal Display Corporation | Organice electroluminescent materials and devices |
| US9023420B2 (en) | 2011-07-14 | 2015-05-05 | Universal Display Corporation | Composite organic/inorganic layer for organic light-emitting devices |
| EP2737553A1 (en) | 2011-07-25 | 2014-06-04 | Merck Patent GmbH | Copolymers with functionalized side chains |
| US9783564B2 (en) | 2011-07-25 | 2017-10-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US8409729B2 (en) | 2011-07-28 | 2013-04-02 | Universal Display Corporation | Host materials for phosphorescent OLEDs |
| KR102197165B1 (en) | 2011-07-29 | 2021-01-04 | 메르크 파텐트 게엠베하 | Compounds for electronic devices |
| CN103718317B (en) | 2011-08-03 | 2016-11-16 | 默克专利有限公司 | material for electronic device |
| US8926119B2 (en) | 2011-08-04 | 2015-01-06 | Universal Display Corporation | Extendable light source with variable light emitting area |
| US8552420B2 (en) | 2011-08-09 | 2013-10-08 | Universal Display Corporation | OLED light panel with controlled brightness variation |
| DE102012016192A1 (en) | 2011-08-19 | 2013-02-21 | Merck Patent Gmbh | New compounds capable of forming hydrogen bonds are useful in electronic device, e.g. organic electroluminescent device, organic light-emitting transistor and organic light-emitting electrochemical cell |
| US9493698B2 (en) | 2011-08-31 | 2016-11-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| IN2014KN00846A (en) | 2011-09-21 | 2015-10-02 | Merck Patent Gmbh | |
| EP2764558B1 (en) | 2011-10-06 | 2019-02-27 | Merck Patent GmbH | Organic electroluminescent device |
| JP2013093541A (en) | 2011-10-06 | 2013-05-16 | Udc Ireland Ltd | Organic electroluminescent element and compound and material for organic electroluminescent element usable therefor, and luminescent device, display device and lighting device using the element |
| JP2013084732A (en) | 2011-10-07 | 2013-05-09 | Udc Ireland Ltd | Organic field light-emitting element and light-emitting material for the same, and light-emitting device, display device and illuminating device |
| DE102011116165A1 (en) | 2011-10-14 | 2013-04-18 | Merck Patent Gmbh | Benzodioxepin-3-one compounds |
| EP2768808B1 (en) | 2011-10-20 | 2017-11-15 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| KR101404240B1 (en) | 2011-10-25 | 2014-06-09 | 울산대학교 산학협력단 | Phosphorescent emitting material comprising vinyl-type polynorbornene copolymers and highly efficient phosphorescent organic light-emitting diode devices using the same |
| JP6223984B2 (en) | 2011-10-27 | 2017-11-01 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| DE102011117422A1 (en) | 2011-10-28 | 2013-05-02 | Merck Patent Gmbh | Hyperbranched polymers, process for their preparation and their use in electronic devices |
| KR102021162B1 (en) | 2011-11-01 | 2019-09-11 | 메르크 파텐트 게엠베하 | Organic electroluminescent device |
| JP2013118349A (en) | 2011-11-02 | 2013-06-13 | Udc Ireland Ltd | Organic electroluminescent element, material for organic electroluminescent element, and light emitting device, display device and illumination device which employ said organic electroluminescent element |
| CN103917542B (en) | 2011-11-10 | 2018-03-27 | Udc 爱尔兰有限责任公司 | 4H imidazos [1,2 a] imidazoles for electronic application |
| US8652656B2 (en) | 2011-11-14 | 2014-02-18 | Universal Display Corporation | Triphenylene silane hosts |
| US9193745B2 (en) | 2011-11-15 | 2015-11-24 | Universal Display Corporation | Heteroleptic iridium complex |
| KR102115018B1 (en) | 2011-11-17 | 2020-05-26 | 메르크 파텐트 게엠베하 | Spirodihydroacridine derivatives and the use thereof as materials for organic electroluminescent devices |
| US9217004B2 (en) | 2011-11-21 | 2015-12-22 | Universal Display Corporation | Organic light emitting materials |
| TWI606051B (en) | 2011-11-22 | 2017-11-21 | Udc愛爾蘭有限公司 | Organic electroluminescent element, material for organic electroluminescent element, and light emitting device, display device and illumination device, using the element, and compound used in the element |
| JP5913938B2 (en) | 2011-11-30 | 2016-05-11 | 富士フイルム株式会社 | Light diffusing transfer material, method of forming light diffusing layer, and method of manufacturing organic electroluminescent device |
| US9512355B2 (en) | 2011-12-09 | 2016-12-06 | Universal Display Corporation | Organic light emitting materials |
| KR101961613B1 (en) | 2011-12-12 | 2019-03-25 | 메르크 파텐트 게엠베하 | Compounds for electronic devices |
| US20130146875A1 (en) | 2011-12-13 | 2013-06-13 | Universal Display Corporation | Split electrode for organic devices |
| ES2648364T3 (en) | 2011-12-19 | 2018-01-02 | Inoviscoat Gmbh | Luminous elements with an electroluminescent arrangement as well as a procedure for the production of a luminous element |
| DE102012022880A1 (en) | 2011-12-22 | 2013-06-27 | Merck Patent Gmbh | Electronic device e.g. organic integrated circuits, organic field-effect transistors, organic thin-film transistors, organic light emitting transistors, comprises an organic layer comprising substituted heteroaryl compounds |
| US8987451B2 (en) | 2012-01-03 | 2015-03-24 | Universal Display Corporation | Synthesis of cyclometallated platinum(II) complexes |
| US9461254B2 (en) | 2012-01-03 | 2016-10-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9163174B2 (en) | 2012-01-04 | 2015-10-20 | Universal Display Corporation | Highly efficient phosphorescent materials |
| KR102012047B1 (en) | 2012-01-06 | 2019-08-19 | 유니버셜 디스플레이 코포레이션 | Highly efficient phosphorescent materials |
| US8969592B2 (en) | 2012-01-10 | 2015-03-03 | Universal Display Corporation | Heterocyclic host materials |
| US10211413B2 (en) | 2012-01-17 | 2019-02-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP5981770B2 (en) | 2012-01-23 | 2016-08-31 | ユー・ディー・シー アイルランド リミテッド | Organic electroluminescence device, charge transport material for organic electroluminescence device, and light emitting device, display device and illumination device using the device |
| KR20140123555A (en) | 2012-01-30 | 2014-10-22 | 메르크 파텐트 게엠베하 | Nanocrystals on fibers |
| JP5978843B2 (en) | 2012-02-02 | 2016-08-24 | コニカミノルタ株式会社 | Iridium complex compound, organic electroluminescence device material, organic electroluminescence device, lighting device and display device |
| JP6118034B2 (en) | 2012-02-06 | 2017-04-19 | ユー・ディー・シー アイルランド リミテッド | ORGANIC ELECTROLUMINESCENT ELEMENT, COMPOUND USABLE FOR THE SAME, ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, AND LIGHT EMITTING DEVICE, DISPLAY DEVICE AND LIGHTING DEVICE USING THE ELEMENT |
| EP2814906B1 (en) | 2012-02-14 | 2016-10-19 | Merck Patent GmbH | Spirobifluorene compounds for organic electroluminescent devices |
| US9118017B2 (en) | 2012-02-27 | 2015-08-25 | Universal Display Corporation | Host compounds for red phosphorescent OLEDs |
| US9054323B2 (en) | 2012-03-15 | 2015-06-09 | Universal Display Corporation | Secondary hole transporting layer with diarylamino-phenyl-carbazole compounds |
| WO2013135352A1 (en) | 2012-03-15 | 2013-09-19 | Merck Patent Gmbh | Electronic devices |
| US9386657B2 (en) | 2012-03-15 | 2016-07-05 | Universal Display Corporation | Organic Electroluminescent materials and devices |
| WO2013139431A1 (en) | 2012-03-23 | 2013-09-26 | Merck Patent Gmbh | 9,9'-spirobixanthene derivatives for electroluminescent devices |
| US8723209B2 (en) | 2012-04-27 | 2014-05-13 | Universal Display Corporation | Out coupling layer containing particle polymer composite |
| US9184399B2 (en) | 2012-05-04 | 2015-11-10 | Universal Display Corporation | Asymmetric hosts with triaryl silane side chains |
| US9773985B2 (en) | 2012-05-21 | 2017-09-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| DE102012011335A1 (en) | 2012-06-06 | 2013-12-12 | Merck Patent Gmbh | Connections for Organic Electronic Devices |
| US9670404B2 (en) | 2012-06-06 | 2017-06-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9502672B2 (en) | 2012-06-21 | 2016-11-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP5445629B2 (en) * | 2012-06-27 | 2014-03-19 | コニカミノルタ株式会社 | Manufacturing method of organic EL element |
| US9725476B2 (en) | 2012-07-09 | 2017-08-08 | Universal Display Corporation | Silylated metal complexes |
| KR102161955B1 (en) | 2012-07-10 | 2020-10-06 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| US9231218B2 (en) | 2012-07-10 | 2016-01-05 | Universal Display Corporation | Phosphorescent emitters containing dibenzo[1,4]azaborinine structure |
| CN108191870A (en) | 2012-07-10 | 2018-06-22 | Udc 爱尔兰有限责任公司 | For benzimidazole simultaneously [1,2-A] benzimidizole derivatives of electronic application |
| US9540329B2 (en) | 2012-07-19 | 2017-01-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9059412B2 (en) | 2012-07-19 | 2015-06-16 | Universal Display Corporation | Transition metal complexes containing substituted imidazole carbene as ligands and their application in OLEDs |
| KR102284234B1 (en) | 2012-07-23 | 2021-07-30 | 메르크 파텐트 게엠베하 | Derivatives of 2-diarylaminofluorene and organic electronic compounds containing them |
| KR102337198B1 (en) | 2012-07-23 | 2021-12-09 | 메르크 파텐트 게엠베하 | Compounds and organic electroluminescent devices |
| CN104507932B (en) | 2012-07-23 | 2016-12-07 | 默克专利有限公司 | Material for organic electroluminescence device |
| KR102696532B1 (en) | 2012-07-23 | 2024-08-19 | 메르크 파텐트 게엠베하 | Fluorenes and electronic devices containing them |
| US9663544B2 (en) | 2012-07-25 | 2017-05-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6299223B2 (en) | 2012-07-25 | 2018-03-28 | 東レ株式会社 | Light emitting device material and light emitting device |
| US9318710B2 (en) | 2012-07-30 | 2016-04-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102265997B1 (en) | 2012-08-10 | 2021-06-16 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescence devices |
| US9978958B2 (en) | 2012-08-24 | 2018-05-22 | Universal Display Corporation | Phosphorescent emitters with phenylimidazole ligands |
| WO2014030666A1 (en) | 2012-08-24 | 2014-02-27 | コニカミノルタ株式会社 | Transparent electrode, electronic device, and method for manufacturing transparent electrode |
| US8952362B2 (en) | 2012-08-31 | 2015-02-10 | The Regents Of The University Of Michigan | High efficiency and brightness fluorescent organic light emitting diode by triplet-triplet fusion |
| US10957870B2 (en) | 2012-09-07 | 2021-03-23 | Universal Display Corporation | Organic light emitting device |
| JP6239624B2 (en) | 2012-09-18 | 2017-11-29 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| EP3318566B1 (en) | 2012-09-20 | 2020-06-24 | UDC Ireland Limited | Azadibenzofurans for electronic applications |
| US9287513B2 (en) | 2012-09-24 | 2016-03-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9312505B2 (en) | 2012-09-25 | 2016-04-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9252363B2 (en) | 2012-10-04 | 2016-02-02 | Universal Display Corporation | Aryloxyalkylcarboxylate solvent compositions for inkjet printing of organic layers |
| US9741942B2 (en) | 2012-10-11 | 2017-08-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN103044490B (en) * | 2012-10-16 | 2015-10-28 | 中科院广州化学有限公司 | A kind of novel phenyl cinnolines class complex of iridium and preparation method thereof and application |
| EP2915199B1 (en) | 2012-10-31 | 2021-03-31 | Merck Patent GmbH | Electronic device |
| KR102080365B1 (en) | 2012-11-06 | 2020-02-21 | 유디씨 아일랜드 리미티드 | Phenoxasiline based compounds for electronic application |
| US8692241B1 (en) | 2012-11-08 | 2014-04-08 | Universal Display Corporation | Transition metal complexes containing triazole and tetrazole carbene ligands |
| US9748500B2 (en) | 2015-01-15 | 2017-08-29 | Universal Display Corporation | Organic light emitting materials |
| US9634264B2 (en) | 2012-11-09 | 2017-04-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9685617B2 (en) | 2012-11-09 | 2017-06-20 | Universal Display Corporation | Organic electronuminescent materials and devices |
| US8946697B1 (en) | 2012-11-09 | 2015-02-03 | Universal Display Corporation | Iridium complexes with aza-benzo fused ligands |
| EP2917198B1 (en) | 2012-11-12 | 2018-05-16 | Merck Patent GmbH | Materials for electronic devices |
| US10069090B2 (en) | 2012-11-20 | 2018-09-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9190623B2 (en) | 2012-11-20 | 2015-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6407877B2 (en) | 2012-11-20 | 2018-10-17 | メルク パテント ゲーエムベーハー | Formulations in high purity solvents for the manufacture of electronic devices |
| US9512136B2 (en) | 2012-11-26 | 2016-12-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9166175B2 (en) | 2012-11-27 | 2015-10-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP2926385B1 (en) | 2012-11-30 | 2020-08-05 | Merck Patent GmbH | Electronic device |
| US9196860B2 (en) | 2012-12-04 | 2015-11-24 | Universal Display Corporation | Compounds for triplet-triplet annihilation upconversion |
| KR102143741B1 (en) | 2012-12-05 | 2020-08-12 | 메르크 파텐트 게엠베하 | Electronic apparatus having an oxygen ion pump |
| US9209411B2 (en) | 2012-12-07 | 2015-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN103087113B (en) * | 2012-12-21 | 2015-09-02 | 南京邮电大学 | One class boracic heteronuclear complex of iridium and its preparation method and application |
| EP2939283A1 (en) | 2012-12-28 | 2015-11-04 | Merck Patent GmbH | Composition comprising polymeric organic semiconducting compounds |
| KR102197749B1 (en) | 2013-01-03 | 2021-01-04 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| US11631816B2 (en) | 2013-01-03 | 2023-04-18 | Merck Patent Gmbh | Electronic device |
| JP2016506414A (en) | 2013-01-03 | 2016-03-03 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| US10400163B2 (en) | 2013-02-08 | 2019-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10367154B2 (en) | 2013-02-21 | 2019-07-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US8927749B2 (en) | 2013-03-07 | 2015-01-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9419225B2 (en) | 2013-03-14 | 2016-08-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6377718B2 (en) | 2013-03-20 | 2018-08-22 | ユー・ディー・シー アイルランド リミテッド | Azabenzimidazolecarbene complex as a high efficiency booster in OLED |
| KR101553590B1 (en) | 2013-03-22 | 2015-09-18 | 주식회사 네패스 | Process for the preparation of ligand of blue phosphorescence |
| US9997712B2 (en) | 2013-03-27 | 2018-06-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10135002B2 (en) | 2013-03-29 | 2018-11-20 | Konica Minolta, Inc. | Organic electroluminescent element, and lighting device and display device which are provided with same |
| US9537106B2 (en) | 2013-05-09 | 2017-01-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9735373B2 (en) | 2013-06-10 | 2017-08-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9673401B2 (en) | 2013-06-28 | 2017-06-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10199581B2 (en) | 2013-07-01 | 2019-02-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20160027087A (en) | 2013-07-02 | 2016-03-09 | 바스프 에스이 | Monosubstituted diazabenzimidazole carbene metal complexes for use in organic light emitting diodes |
| US10121975B2 (en) | 2013-07-03 | 2018-11-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9761807B2 (en) | 2013-07-15 | 2017-09-12 | Universal Display Corporation | Organic light emitting diode materials |
| US9324949B2 (en) | 2013-07-16 | 2016-04-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9553274B2 (en) | 2013-07-16 | 2017-01-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9224958B2 (en) | 2013-07-19 | 2015-12-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20150028290A1 (en) | 2013-07-25 | 2015-01-29 | Universal Display Corporation | Heteroleptic osmium complex and method of making the same |
| JP2016525781A (en) | 2013-07-29 | 2016-08-25 | メルク、パテント、ゲゼルシャフト、ミット、ベシュレンクテル、ハフツングMerck Patent GmbH | Electro-optic element and use thereof |
| CN105409021B (en) | 2013-07-29 | 2018-07-13 | 默克专利有限公司 | Electroluminescent device |
| EP3647393A1 (en) | 2013-07-30 | 2020-05-06 | Merck Patent GmbH | Materials for electronic devices |
| WO2015014435A1 (en) | 2013-07-30 | 2015-02-05 | Merck Patent Gmbh | Materials for electronic devices |
| US10074806B2 (en) | 2013-08-20 | 2018-09-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9831437B2 (en) | 2013-08-20 | 2017-11-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9932359B2 (en) | 2013-08-30 | 2018-04-03 | University Of Southern California | Organic electroluminescent materials and devices |
| US10199582B2 (en) | 2013-09-03 | 2019-02-05 | University Of Southern California | Organic electroluminescent materials and devices |
| US9735378B2 (en) | 2013-09-09 | 2017-08-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9748503B2 (en) | 2013-09-13 | 2017-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10003034B2 (en) | 2013-09-30 | 2018-06-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102310370B1 (en) | 2013-10-02 | 2021-10-07 | 메르크 파텐트 게엠베하 | Boron-containing compounds for use in oleds |
| US9831447B2 (en) | 2013-10-08 | 2017-11-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9293712B2 (en) | 2013-10-11 | 2016-03-22 | Universal Display Corporation | Disubstituted pyrene compounds with amino group containing ortho aryl group and devices containing the same |
| US9853229B2 (en) | 2013-10-23 | 2017-12-26 | University Of Southern California | Organic electroluminescent materials and devices |
| US20150115250A1 (en) | 2013-10-29 | 2015-04-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2015063046A1 (en) | 2013-10-31 | 2015-05-07 | Basf Se | Azadibenzothiophenes for electronic applications |
| US9306179B2 (en) | 2013-11-08 | 2016-04-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9647218B2 (en) | 2013-11-14 | 2017-05-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9905784B2 (en) | 2013-11-15 | 2018-02-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10056565B2 (en) | 2013-11-20 | 2018-08-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102153039B1 (en) | 2013-11-28 | 2020-09-07 | 삼성전자주식회사 | Carbazole-based compound and organic light emitting diode including the same |
| US9231217B2 (en) | 2013-11-28 | 2016-01-05 | Semiconductor Energy Laboratory Co., Ltd. | Synthesis method of organometallic complex, synthesis method of pyrazine derivative, 5,6-diaryl-2-pyrazyl triflate, light-emitting element, light-emitting device, electronic device, and lighting device |
| US10644251B2 (en) | 2013-12-04 | 2020-05-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN105980518B (en) | 2013-12-06 | 2019-07-12 | 默克专利有限公司 | Compound and organic electronic device |
| CN105793387B (en) | 2013-12-06 | 2019-04-30 | 默克专利有限公司 | Contain the composition comprising acrylate and/or the polymeric binder of methacrylate unit |
| WO2015082046A2 (en) | 2013-12-06 | 2015-06-11 | Merck Patent Gmbh | Substituted oxepines |
| US9876173B2 (en) | 2013-12-09 | 2018-01-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN103936792B (en) * | 2013-12-12 | 2017-04-26 | 石家庄诚志永华显示材料有限公司 | Compound containing pyrazole structural unit |
| JP6644688B2 (en) | 2013-12-12 | 2020-02-12 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| US10355227B2 (en) | 2013-12-16 | 2019-07-16 | Universal Display Corporation | Metal complex for phosphorescent OLED |
| JP6542228B2 (en) | 2013-12-19 | 2019-07-10 | メルク、パテント、ゲゼルシャフト、ミット、ベシュレンクテル、ハフツングMerck Patent GmbH | Heterocyclic spiro compounds |
| US9847496B2 (en) | 2013-12-23 | 2017-12-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10135008B2 (en) | 2014-01-07 | 2018-11-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9978961B2 (en) | 2014-01-08 | 2018-05-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9755159B2 (en) | 2014-01-23 | 2017-09-05 | Universal Display Corporation | Organic materials for OLEDs |
| US9935277B2 (en) | 2014-01-30 | 2018-04-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9590194B2 (en) | 2014-02-14 | 2017-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10003033B2 (en) | 2014-02-18 | 2018-06-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9847497B2 (en) | 2014-02-18 | 2017-12-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10707423B2 (en) | 2014-02-21 | 2020-07-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9647217B2 (en) | 2014-02-24 | 2017-05-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9502656B2 (en) | 2014-02-24 | 2016-11-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10403825B2 (en) | 2014-02-27 | 2019-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9181270B2 (en) | 2014-02-28 | 2015-11-10 | Universal Display Corporation | Method of making sulfide compounds |
| US9590195B2 (en) | 2014-02-28 | 2017-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9673407B2 (en) | 2014-02-28 | 2017-06-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9190620B2 (en) | 2014-03-01 | 2015-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9397309B2 (en) | 2014-03-13 | 2016-07-19 | Universal Display Corporation | Organic electroluminescent devices |
| US10208026B2 (en) | 2014-03-18 | 2019-02-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9748504B2 (en) | 2014-03-25 | 2017-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2015150203A1 (en) | 2014-03-31 | 2015-10-08 | Basf Se | Metal complexes, comprising carbene ligands having an o-substituted non-cyclometalated aryl group and their use in organic light emitting diodes |
| US9859504B2 (en) | 2014-03-31 | 2018-01-02 | Commonwealth Scientific And Industrial Research Organisation | Diamine compounds for phosphorescent diazaborole metal complexes and electroluminescent devices |
| AU2015201628A1 (en) | 2014-03-31 | 2015-10-15 | Commonwealth Scientific And Industrial Research Organisation | Phenylenediamine compounds for phosphorescent diazaborole metal complexes |
| US9929353B2 (en) | 2014-04-02 | 2018-03-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9691993B2 (en) | 2014-04-09 | 2017-06-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10008679B2 (en) | 2014-04-14 | 2018-06-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9905785B2 (en) | 2014-04-14 | 2018-02-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9450198B2 (en) | 2014-04-15 | 2016-09-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10256427B2 (en) | 2014-04-15 | 2019-04-09 | Universal Display Corporation | Efficient organic electroluminescent devices |
| US9741941B2 (en) | 2014-04-29 | 2017-08-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN106255687B (en) | 2014-04-30 | 2020-06-05 | 默克专利有限公司 | Material for electronic devices |
| US10457699B2 (en) | 2014-05-02 | 2019-10-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3140302B1 (en) | 2014-05-05 | 2019-08-21 | Merck Patent GmbH | Materials for organic light emitting devices |
| KR102540541B1 (en) | 2014-05-08 | 2023-06-07 | 유니버셜 디스플레이 코포레이션 | Stabilized imidazophenanthridine materials |
| US10403830B2 (en) | 2014-05-08 | 2019-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10636983B2 (en) | 2014-05-08 | 2020-04-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10301338B2 (en) | 2014-05-08 | 2019-05-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP5773033B2 (en) * | 2014-05-26 | 2015-09-02 | コニカミノルタ株式会社 | Method for manufacturing organic electroluminescent element and organic electroluminescent element |
| US9997716B2 (en) | 2014-05-27 | 2018-06-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10461260B2 (en) | 2014-06-03 | 2019-10-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN104004026A (en) * | 2014-06-09 | 2014-08-27 | 江西冠能光电材料有限公司 | Electronegative phosphor material |
| DE102014008722B4 (en) | 2014-06-18 | 2024-08-22 | Merck Patent Gmbh | Compositions for electronic devices, formulation containing them, use of the composition, use of the formulation and organic electronic device containing the composition |
| US10644246B2 (en) | 2014-06-25 | 2020-05-05 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US9911931B2 (en) | 2014-06-26 | 2018-03-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10297762B2 (en) | 2014-07-09 | 2019-05-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10566546B2 (en) | 2014-07-14 | 2020-02-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3172775B1 (en) | 2014-07-21 | 2022-04-20 | Merck Patent GmbH | Materials for electronic devices |
| US9929357B2 (en) | 2014-07-22 | 2018-03-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2016016791A1 (en) | 2014-07-28 | 2016-02-04 | Idemitsu Kosan Co., Ltd (Ikc) | 2,9-functionalized benzimidazolo[1,2-a]benzimidazoles as hosts for organic light emitting diodes (oleds) |
| US10411200B2 (en) | 2014-08-07 | 2019-09-10 | Universal Display Corporation | Electroluminescent (2-phenylpyridine)iridium complexes and devices |
| EP2982676B1 (en) | 2014-08-07 | 2018-04-11 | Idemitsu Kosan Co., Ltd. | Benzimidazo[2,1-B]benzoxazoles for electronic applications |
| US11108000B2 (en) | 2014-08-07 | 2021-08-31 | Unniversal Display Corporation | Organic electroluminescent materials and devices |
| EP2993215B1 (en) | 2014-09-04 | 2019-06-19 | Idemitsu Kosan Co., Ltd. | Azabenzimidazo[2,1-a]benzimidazoles for electronic applications |
| EP3189551B1 (en) | 2014-09-05 | 2021-01-27 | Merck Patent GmbH | Formulations and method of producing an organic electroluminescent device |
| US10135007B2 (en) | 2014-09-29 | 2018-11-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10043987B2 (en) | 2014-09-29 | 2018-08-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10749113B2 (en) | 2014-09-29 | 2020-08-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10361375B2 (en) | 2014-10-06 | 2019-07-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10854826B2 (en) | 2014-10-08 | 2020-12-01 | Universal Display Corporation | Organic electroluminescent compounds, compositions and devices |
| US10950803B2 (en) | 2014-10-13 | 2021-03-16 | Universal Display Corporation | Compounds and uses in devices |
| US9484541B2 (en) | 2014-10-20 | 2016-11-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3015469B1 (en) | 2014-10-30 | 2018-12-19 | Idemitsu Kosan Co., Ltd. | 5-(benzimidazol-2-yl)benzimidazo[1,2-a]benzimidazoles for electronic applications |
| US10868261B2 (en) | 2014-11-10 | 2020-12-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2017537085A (en) | 2014-11-11 | 2017-12-14 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| US10411201B2 (en) | 2014-11-12 | 2019-09-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10038151B2 (en) | 2014-11-12 | 2018-07-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9882151B2 (en) | 2014-11-14 | 2018-01-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9871212B2 (en) | 2014-11-14 | 2018-01-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2016079667A1 (en) | 2014-11-17 | 2016-05-26 | Idemitsu Kosan Co., Ltd. | Indole derivatives for electronic applications |
| US9761814B2 (en) | 2014-11-18 | 2017-09-12 | Universal Display Corporation | Organic light-emitting materials and devices |
| US9444075B2 (en) | 2014-11-26 | 2016-09-13 | Universal Display Corporation | Emissive display with photo-switchable polarization |
| CN107406384B (en) | 2014-12-04 | 2021-07-23 | 广州华睿光电材料有限公司 | Deuterated organic compounds, mixtures, compositions and organic electronic devices comprising said compounds |
| CN107004777B (en) | 2014-12-04 | 2019-03-26 | 广州华睿光电材料有限公司 | Polymer includes its mixture, composition, organic electronic device and monomer |
| CN107004779B (en) | 2014-12-11 | 2019-03-08 | 广州华睿光电材料有限公司 | Organic compound, mixture, composition and organic electronic device comprising it |
| EP3034507A1 (en) | 2014-12-15 | 2016-06-22 | Idemitsu Kosan Co., Ltd | 1-functionalized dibenzofurans and dibenzothiophenes for organic light emitting diodes (OLEDs) |
| EP3034506A1 (en) | 2014-12-15 | 2016-06-22 | Idemitsu Kosan Co., Ltd | 4-functionalized carbazole derivatives for electronic applications |
| US9450195B2 (en) | 2014-12-17 | 2016-09-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10636978B2 (en) | 2014-12-30 | 2020-04-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10253252B2 (en) | 2014-12-30 | 2019-04-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2016107663A1 (en) | 2014-12-30 | 2016-07-07 | Merck Patent Gmbh | Formulations and electronic devices |
| US9312499B1 (en) | 2015-01-05 | 2016-04-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9406892B2 (en) | 2015-01-07 | 2016-08-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2016112761A1 (en) | 2015-01-13 | 2016-07-21 | 广州华睿光电材料有限公司 | Conjugated polymer containing ethynyl crosslinking group, mixture, composition, organic electronic device containing the same and application thereof |
| US9711730B2 (en) | 2015-01-25 | 2017-07-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10418569B2 (en) | 2015-01-25 | 2019-09-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102610947B1 (en) | 2015-01-30 | 2023-12-06 | 메르크 파텐트 게엠베하 | Formulations with a low particle content |
| KR102626977B1 (en) | 2015-01-30 | 2024-01-19 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| US10418562B2 (en) | 2015-02-06 | 2019-09-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3054498B1 (en) | 2015-02-06 | 2017-09-20 | Idemitsu Kosan Co., Ltd. | Bisimidazodiazocines |
| EP3053918B1 (en) | 2015-02-06 | 2018-04-11 | Idemitsu Kosan Co., Ltd. | 2-carbazole substituted benzimidazoles for electronic applications |
| US10644247B2 (en) | 2015-02-06 | 2020-05-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10355222B2 (en) | 2015-02-06 | 2019-07-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10177316B2 (en) | 2015-02-09 | 2019-01-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP5831654B1 (en) | 2015-02-13 | 2015-12-09 | コニカミノルタ株式会社 | Aromatic heterocycle derivative, organic electroluminescence device using the same, illumination device and display device |
| US10144867B2 (en) | 2015-02-13 | 2018-12-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10680183B2 (en) | 2015-02-15 | 2020-06-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9929361B2 (en) | 2015-02-16 | 2018-03-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3061759B1 (en) | 2015-02-24 | 2019-12-25 | Idemitsu Kosan Co., Ltd | Nitrile substituted dibenzofurans |
| US11056657B2 (en) | 2015-02-27 | 2021-07-06 | University Display Corporation | Organic electroluminescent materials and devices |
| US10600966B2 (en) | 2015-02-27 | 2020-03-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10686143B2 (en) | 2015-03-05 | 2020-06-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10270046B2 (en) | 2015-03-06 | 2019-04-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9780316B2 (en) | 2015-03-16 | 2017-10-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3070144B1 (en) | 2015-03-17 | 2018-02-28 | Idemitsu Kosan Co., Ltd. | Seven-membered ring compounds |
| US9911928B2 (en) | 2015-03-19 | 2018-03-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9871214B2 (en) | 2015-03-23 | 2018-01-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10529931B2 (en) | 2015-03-24 | 2020-01-07 | Universal Display Corporation | Organic Electroluminescent materials and devices |
| EP3072943B1 (en) | 2015-03-26 | 2018-05-02 | Idemitsu Kosan Co., Ltd. | Dibenzofuran/carbazole-substituted benzonitriles |
| US10297770B2 (en) | 2015-03-27 | 2019-05-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6800879B2 (en) | 2015-03-30 | 2020-12-16 | メルク パテント ゲーエムベーハー | Formulations of organic functional materials containing siloxane solvents |
| EP3075737B1 (en) | 2015-03-31 | 2019-12-04 | Idemitsu Kosan Co., Ltd | Benzimidazolo[1,2-a]benzimidazole carrying aryl- or heteroarylnitril groups for organic light emitting diodes |
| US11818949B2 (en) | 2015-04-06 | 2023-11-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10693082B2 (en) | 2015-04-06 | 2020-06-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11495749B2 (en) | 2015-04-06 | 2022-11-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10403826B2 (en) | 2015-05-07 | 2019-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10777749B2 (en) | 2015-05-07 | 2020-09-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9478758B1 (en) | 2015-05-08 | 2016-10-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9859510B2 (en) | 2015-05-15 | 2018-01-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2016184540A1 (en) | 2015-05-18 | 2016-11-24 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US10256411B2 (en) | 2015-05-21 | 2019-04-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10109799B2 (en) | 2015-05-21 | 2018-10-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10418568B2 (en) | 2015-06-01 | 2019-09-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10033004B2 (en) | 2015-06-01 | 2018-07-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11925102B2 (en) | 2015-06-04 | 2024-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10818853B2 (en) | 2015-06-04 | 2020-10-27 | University Of Southern California | Organic electroluminescent materials and devices |
| US11208401B2 (en) | 2015-06-10 | 2021-12-28 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP3581633B1 (en) | 2015-06-12 | 2021-01-27 | Merck Patent GmbH | Esters containing non-aromatic cycles as solvents for oled formulations |
| US10825997B2 (en) | 2015-06-25 | 2020-11-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10873036B2 (en) | 2015-07-07 | 2020-12-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102643183B1 (en) | 2015-07-15 | 2024-03-04 | 메르크 파텐트 게엠베하 | Compositions Comprising Organic Semiconducting Compounds |
| US9978956B2 (en) | 2015-07-15 | 2018-05-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102660538B1 (en) | 2015-07-22 | 2024-04-24 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| US11127905B2 (en) | 2015-07-29 | 2021-09-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2017016632A1 (en) | 2015-07-29 | 2017-02-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2017016630A1 (en) | 2015-07-30 | 2017-02-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US11018309B2 (en) | 2015-08-03 | 2021-05-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10934292B2 (en) | 2015-08-13 | 2021-03-02 | Merck Patent Gmbh | Hexamethylindanes |
| US10696664B2 (en) | 2015-08-14 | 2020-06-30 | Merck Patent Gmbh | Phenoxazine derivatives for organic electroluminescent devices |
| US11522140B2 (en) | 2015-08-17 | 2022-12-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10522769B2 (en) | 2015-08-18 | 2019-12-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10181564B2 (en) | 2015-08-26 | 2019-01-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3341448B1 (en) | 2015-08-28 | 2020-02-12 | Merck Patent GmbH | Compounds for electronic devices |
| WO2017036572A1 (en) | 2015-08-28 | 2017-03-09 | Merck Patent Gmbh | Formulation of an organic functional material comprising an epoxy group containing solvent |
| US10361381B2 (en) | 2015-09-03 | 2019-07-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11706972B2 (en) | 2015-09-08 | 2023-07-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11302872B2 (en) | 2015-09-09 | 2022-04-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10770664B2 (en) | 2015-09-21 | 2020-09-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20170092880A1 (en) | 2015-09-25 | 2017-03-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10486600B1 (en) | 2015-09-28 | 2019-11-26 | Apple Inc. | Systems for improving side-mirror functionality of a vehicle |
| EP3356369B1 (en) | 2015-10-01 | 2022-05-04 | Idemitsu Kosan Co., Ltd | Benzimidazolo[1,2-a]benzimidazole carrying triazine groups for organic light emitting diodes |
| US10593892B2 (en) | 2015-10-01 | 2020-03-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2017056053A1 (en) | 2015-10-01 | 2017-04-06 | Idemitsu Kosan Co., Ltd. | Benzimidazolo[1,2-a]benzimidazole carrying benzimidazolo[1,2-a]benzimidazolyl groups, carbazolyl groups, benzofurane groups or benzothiophene groups for organic light emitting diodes |
| US10847728B2 (en) | 2015-10-01 | 2020-11-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3150604B1 (en) | 2015-10-01 | 2021-07-14 | Idemitsu Kosan Co., Ltd. | Benzimidazolo[1,2-a]benzimidazole carrying benzimidazolo[1,2-a]benzimidazolylyl groups, carbazolyl groups, benzofurane groups or benzothiophene groups for organic light emitting diodes |
| EP3150606B1 (en) | 2015-10-01 | 2019-08-14 | Idemitsu Kosan Co., Ltd. | Benzimidazolo[1,2-a]benzimidazoles carrying benzofurane or benzothiophene groups for organic light emitting diodes |
| US10991895B2 (en) | 2015-10-06 | 2021-04-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3368520B1 (en) | 2015-10-27 | 2023-04-26 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US10177318B2 (en) | 2015-10-29 | 2019-01-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10388892B2 (en) | 2015-10-29 | 2019-08-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10388893B2 (en) | 2015-10-29 | 2019-08-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20180319813A1 (en) | 2015-11-04 | 2018-11-08 | Idemitsu Kosan Co., Ltd | Benzimidazole fused heteroaryls |
| US11555128B2 (en) | 2015-11-12 | 2023-01-17 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Printing composition, electronic device comprising same and preparation method for functional material thin film |
| US10998507B2 (en) | 2015-11-23 | 2021-05-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10476010B2 (en) | 2015-11-30 | 2019-11-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11174258B2 (en) | 2015-12-04 | 2021-11-16 | Idemitsu Kosan Co., Ltd. | Benzimidazolo[1,2-a]benzimidazole derivatives for organic light emitting diodes |
| US10968243B2 (en) | 2015-12-04 | 2021-04-06 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Organometallic complex and application thereof in electronic devices |
| WO2017097391A1 (en) | 2015-12-10 | 2017-06-15 | Merck Patent Gmbh | Formulations containing ketones comprising non-aromatic cycles |
| EP3391428B1 (en) | 2015-12-15 | 2022-06-29 | Merck Patent GmbH | Esters containing aromatic groups as solvents for organic electronic formulations |
| JP7438661B2 (en) | 2015-12-16 | 2024-02-27 | メルク パテント ゲーエムベーハー | Formulations containing solid solvents |
| EP3390550B1 (en) | 2015-12-16 | 2022-09-28 | Merck Patent GmbH | Formulations containing a mixture of at least two different solvents |
| JP6375069B2 (en) | 2015-12-21 | 2018-08-15 | 出光興産株式会社 | Phenylquinazoline bridged with heteroatoms |
| US10957861B2 (en) | 2015-12-29 | 2021-03-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11024808B2 (en) | 2015-12-29 | 2021-06-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10135006B2 (en) | 2016-01-04 | 2018-11-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6788314B2 (en) | 2016-01-06 | 2020-11-25 | コニカミノルタ株式会社 | Organic electroluminescence element, manufacturing method of organic electroluminescence element, display device and lighting device |
| JP6651168B2 (en) | 2016-01-14 | 2020-02-19 | 国立研究開発法人産業技術総合研究所 | Method for producing cyclometallated iridium complex |
| US10312459B2 (en) * | 2016-01-27 | 2019-06-04 | Nichem Fine Technology Co., Ltd. | Compound and organic electronic device using the same |
| CN108603107B (en) | 2016-02-05 | 2022-08-26 | 默克专利有限公司 | Material for electronic devices |
| US10457864B2 (en) | 2016-02-09 | 2019-10-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10707427B2 (en) | 2016-02-09 | 2020-07-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3417033B1 (en) | 2016-02-17 | 2021-02-24 | Merck Patent GmbH | Formulation of an organic functional material |
| US10600967B2 (en) | 2016-02-18 | 2020-03-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11608327B2 (en) | 2016-03-03 | 2023-03-21 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102016003104A1 (en) | 2016-03-15 | 2017-09-21 | Merck Patent Gmbh | Container comprising a formulation containing at least one organic semiconductor |
| US11094891B2 (en) | 2016-03-16 | 2021-08-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI821807B (en) | 2016-03-17 | 2023-11-11 | 德商麥克專利有限公司 | Compounds having spirobifluorene structures |
| US10276809B2 (en) | 2016-04-05 | 2019-04-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2017178311A1 (en) | 2016-04-11 | 2017-10-19 | Merck Patent Gmbh | Heterocyclic compounds comprising dibenzofuran and/or dibenzothiophene structures |
| US10236456B2 (en) | 2016-04-11 | 2019-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2017178864A1 (en) | 2016-04-12 | 2017-10-19 | Idemitsu Kosan Co., Ltd. | Seven-membered ring compounds |
| US10566552B2 (en) | 2016-04-13 | 2020-02-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11228002B2 (en) | 2016-04-22 | 2022-01-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11228003B2 (en) | 2016-04-22 | 2022-01-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11081647B2 (en) | 2016-04-22 | 2021-08-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102385482B1 (en) | 2016-04-29 | 2022-04-12 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| US20170324049A1 (en) | 2016-05-05 | 2017-11-09 | Universal Display Corporation | Organic Electroluminescent Materials and Devices |
| US10840458B2 (en) | 2016-05-25 | 2020-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10468609B2 (en) | 2016-06-02 | 2019-11-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN116283863A (en) | 2016-06-03 | 2023-06-23 | 默克专利有限公司 | Material for organic electroluminescent device |
| WO2017216129A1 (en) | 2016-06-16 | 2017-12-21 | Merck Patent Gmbh | Formulation of an organic functional material |
| WO2017216128A1 (en) | 2016-06-17 | 2017-12-21 | Merck Patent Gmbh | Formulation of an organic functional material |
| US10727423B2 (en) | 2016-06-20 | 2020-07-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10686140B2 (en) | 2016-06-20 | 2020-06-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10672997B2 (en) | 2016-06-20 | 2020-06-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11482683B2 (en) | 2016-06-20 | 2022-10-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10651403B2 (en) | 2016-06-20 | 2020-05-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10862054B2 (en) | 2016-06-20 | 2020-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11691983B2 (en) | 2016-06-22 | 2023-07-04 | Idemitsu Kosan Co., Ltd. | Specifically substituted benzofuro- and benzothienoquinolines for organic light emitting diodes |
| TW201815998A (en) | 2016-06-28 | 2018-05-01 | 德商麥克專利有限公司 | Formulation of an organic functional material |
| US10957866B2 (en) | 2016-06-30 | 2021-03-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN109311784B (en) | 2016-07-08 | 2022-03-25 | 默克专利有限公司 | Material for organic electroluminescent device |
| US9929360B2 (en) | 2016-07-08 | 2018-03-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10566547B2 (en) | 2016-07-11 | 2020-02-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10153443B2 (en) | 2016-07-19 | 2018-12-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10720587B2 (en) | 2016-07-19 | 2020-07-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN109563402B (en) | 2016-08-04 | 2022-07-15 | 默克专利有限公司 | Preparation of organic functional materials |
| US10205105B2 (en) | 2016-08-15 | 2019-02-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2018050583A1 (en) | 2016-09-14 | 2018-03-22 | Merck Patent Gmbh | Compounds with carbazole structures |
| US11447464B2 (en) | 2016-09-14 | 2022-09-20 | Merck Patent Gmbh | Compounds with spirobifluorene-structures |
| US10608186B2 (en) | 2016-09-14 | 2020-03-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10505127B2 (en) | 2016-09-19 | 2019-12-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10680187B2 (en) | 2016-09-23 | 2020-06-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI766884B (en) | 2016-09-30 | 2022-06-11 | 德商麥克專利有限公司 | Compounds having diazadibenzofuran or diazadibenzothiophene structures, process for preparing the same and use thereof |
| KR20190053948A (en) | 2016-09-30 | 2019-05-20 | 메르크 파텐트 게엠베하 | Carbazoles having a diazadibenzofuran or diazadibenzothiophene structure |
| US11189804B2 (en) | 2016-10-03 | 2021-11-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11183642B2 (en) | 2016-10-03 | 2021-11-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11081658B2 (en) | 2016-10-03 | 2021-08-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11196010B2 (en) | 2016-10-03 | 2021-12-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11127906B2 (en) | 2016-10-03 | 2021-09-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11011709B2 (en) | 2016-10-07 | 2021-05-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI764942B (en) | 2016-10-10 | 2022-05-21 | 德商麥克專利有限公司 | Electronic device |
| US11239432B2 (en) | 2016-10-14 | 2022-02-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| DE102017008794A1 (en) | 2016-10-17 | 2018-04-19 | Merck Patent Gmbh | Materials for use in electronic devices |
| US10608185B2 (en) | 2016-10-17 | 2020-03-31 | Univeral Display Corporation | Organic electroluminescent materials and devices |
| US10236458B2 (en) | 2016-10-24 | 2019-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP7013459B2 (en) | 2016-10-31 | 2022-01-31 | メルク パテント ゲーエムベーハー | Formulation of organic functional materials |
| CN109863223B (en) | 2016-10-31 | 2023-06-20 | 默克专利有限公司 | Preparation of organic functional material |
| TWI745467B (en) | 2016-11-02 | 2021-11-11 | 德商麥克專利有限公司 | Materials for electronic devices |
| KR20230121632A (en) | 2016-11-08 | 2023-08-18 | 메르크 파텐트 게엠베하 | Compounds for electronic devices |
| EP3538521A1 (en) | 2016-11-09 | 2019-09-18 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US10340464B2 (en) | 2016-11-10 | 2019-07-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10680188B2 (en) | 2016-11-11 | 2020-06-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI756292B (en) | 2016-11-14 | 2022-03-01 | 德商麥克專利有限公司 | Compounds having an acceptor group and a donor group |
| US10897016B2 (en) | 2016-11-14 | 2021-01-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10964893B2 (en) | 2016-11-17 | 2021-03-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102580980B1 (en) | 2016-11-17 | 2023-09-20 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| US10662196B2 (en) | 2016-11-17 | 2020-05-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10153445B2 (en) | 2016-11-21 | 2018-12-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10833276B2 (en) | 2016-11-21 | 2020-11-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW201833118A (en) | 2016-11-22 | 2018-09-16 | 德商麥克專利有限公司 | Materials for electronic devices |
| WO2018095395A1 (en) | 2016-11-23 | 2018-05-31 | 广州华睿光电材料有限公司 | High polymer, mixture containing same, composition, organic electronic component, and monomer for polymerization |
| US11447496B2 (en) | 2016-11-23 | 2022-09-20 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Nitrogen-containing fused heterocyclic ring compound and application thereof |
| CN109790459B (en) | 2016-11-23 | 2022-08-12 | 广州华睿光电材料有限公司 | Organic compounds |
| EP3546532B1 (en) | 2016-11-23 | 2021-06-02 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Printing ink composition, preparation method therefor, and uses thereof |
| CN109790460B (en) | 2016-11-23 | 2023-10-13 | 广州华睿光电材料有限公司 | Boron-containing organic compound, application, organic mixture and organic electronic device |
| CN109790194B (en) | 2016-11-23 | 2021-07-23 | 广州华睿光电材料有限公司 | Metal organic complex, high polymer, composition and organic electronic device |
| EP3547385B1 (en) | 2016-11-23 | 2020-11-04 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Organic mixture, composition, and organic electronic component |
| WO2018095940A1 (en) | 2016-11-25 | 2018-05-31 | Merck Patent Gmbh | Bisbenzofuran-fused indeno[1,2-b]fluorene derivatives and related compounds as materials for organic electroluminescent devices (oled) |
| US11584753B2 (en) | 2016-11-25 | 2023-02-21 | Merck Patent Gmbh | Bisbenzofuran-fused 2,8-diaminoindeno[1,2-b]fluorene derivatives and related compounds as materials for organic electroluminescent devices (OLED) |
| JP7127026B2 (en) | 2016-11-30 | 2022-08-29 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Compound having valerolactam structure |
| US11555048B2 (en) | 2016-12-01 | 2023-01-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20190086028A (en) | 2016-12-05 | 2019-07-19 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| TW201831468A (en) | 2016-12-05 | 2018-09-01 | 德商麥克專利有限公司 | Nitrogen-containing heterocycles |
| WO2018104194A1 (en) | 2016-12-05 | 2018-06-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2018104202A1 (en) | 2016-12-06 | 2018-06-14 | Merck Patent Gmbh | Preparation process for an electronic device |
| EP3553152B1 (en) | 2016-12-08 | 2021-02-17 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Mixture, composition and organic electronic device |
| WO2018108108A1 (en) | 2016-12-13 | 2018-06-21 | 广州华睿光电材料有限公司 | Conjugated polymer and use thereof in organic electronic device |
| JP7091337B2 (en) | 2016-12-13 | 2022-06-27 | メルク パテント ゲーエムベーハー | Formulation of organic functional materials |
| US10490753B2 (en) | 2016-12-15 | 2019-11-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11545636B2 (en) | 2016-12-15 | 2023-01-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11548905B2 (en) | 2016-12-15 | 2023-01-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10811618B2 (en) | 2016-12-19 | 2020-10-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2018114744A1 (en) | 2016-12-20 | 2018-06-28 | Merck Patent Gmbh | A white light emitting solid state light source |
| US11261291B2 (en) | 2016-12-22 | 2022-03-01 | Merck Patent Gmbh | Materials for electronic devices |
| US20200098996A1 (en) | 2016-12-22 | 2020-03-26 | Merck Patent Gmbh | Mixtures comprising at least two organofunctional compounds |
| CN109790136B (en) | 2016-12-22 | 2024-01-12 | 广州华睿光电材料有限公司 | Furan cross-linking group-containing polymer and application thereof |
| US11292875B2 (en) | 2016-12-22 | 2022-04-05 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Cross-linkable polymer based on Diels-Alder reaction and use thereof in organic electronic device |
| US11152579B2 (en) | 2016-12-28 | 2021-10-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN110049986A (en) | 2017-01-04 | 2019-07-23 | 默克专利有限公司 | Material for organic electroluminescence device |
| US11780865B2 (en) | 2017-01-09 | 2023-10-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11201298B2 (en) | 2017-01-09 | 2021-12-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10804475B2 (en) | 2017-01-11 | 2020-10-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11545637B2 (en) | 2017-01-13 | 2023-01-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10629820B2 (en) | 2017-01-18 | 2020-04-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10964904B2 (en) | 2017-01-20 | 2021-03-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11053268B2 (en) | 2017-01-20 | 2021-07-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11765968B2 (en) | 2017-01-23 | 2023-09-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3571264B1 (en) | 2017-01-23 | 2021-11-24 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US11050028B2 (en) | 2017-01-24 | 2021-06-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN110198936B (en) | 2017-01-25 | 2024-03-12 | 默克专利有限公司 | Carbazole derivative |
| TWI763772B (en) | 2017-01-30 | 2022-05-11 | 德商麥克專利有限公司 | Method for forming an organic element of an electronic device |
| TWI791481B (en) | 2017-01-30 | 2023-02-11 | 德商麥克專利有限公司 | Method for forming an organic electroluminescence (el) element |
| US11407766B2 (en) | 2017-01-30 | 2022-08-09 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP3577101B1 (en) | 2017-02-02 | 2021-03-03 | Merck Patent GmbH | Materials for electronic devices |
| US12089486B2 (en) | 2017-02-08 | 2024-09-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW201835075A (en) | 2017-02-14 | 2018-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US10978647B2 (en) | 2017-02-15 | 2021-04-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10822361B2 (en) | 2017-02-22 | 2020-11-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11393987B2 (en) | 2017-03-01 | 2022-07-19 | Merck Patent Gmbh | Organic electroluminescent device |
| US20200055822A1 (en) | 2017-03-02 | 2020-02-20 | Merck Patent Gmbh | Materials for organic electronic devices |
| EP3372611B1 (en) | 2017-03-08 | 2020-06-24 | Samsung Electronics Co., Ltd. | Organometallic compound, composition containing the organometallic compound, and organic light-emitting device including the organometallic compound |
| US10745431B2 (en) | 2017-03-08 | 2020-08-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10741780B2 (en) | 2017-03-10 | 2020-08-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW201843143A (en) | 2017-03-13 | 2018-12-16 | 德商麥克專利有限公司 | Compounds containing arylamine structures |
| KR102539248B1 (en) | 2017-03-15 | 2023-06-02 | 메르크 파텐트 게엠베하 | Materials for Organic Electroluminescent Devices |
| US10672998B2 (en) | 2017-03-23 | 2020-06-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10873037B2 (en) | 2017-03-28 | 2020-12-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10910577B2 (en) | 2017-03-28 | 2021-02-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10844085B2 (en) | 2017-03-29 | 2020-11-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11056658B2 (en) | 2017-03-29 | 2021-07-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11158820B2 (en) | 2017-03-29 | 2021-10-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10862046B2 (en) | 2017-03-30 | 2020-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11139443B2 (en) | 2017-03-31 | 2021-10-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11276829B2 (en) | 2017-03-31 | 2022-03-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2018178136A1 (en) | 2017-03-31 | 2018-10-04 | Merck Patent Gmbh | Printing method for an organic light emitting diode (oled) |
| KR102632027B1 (en) | 2017-04-10 | 2024-01-31 | 메르크 파텐트 게엠베하 | Formulation of organic functional materials |
| US10777754B2 (en) | 2017-04-11 | 2020-09-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11038117B2 (en) | 2017-04-11 | 2021-06-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11778907B2 (en) | 2017-04-13 | 2023-10-03 | Merck Patent Gmbh | Composition for organic electronic devices |
| US10975113B2 (en) | 2017-04-21 | 2021-04-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11101434B2 (en) | 2017-04-21 | 2021-08-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11084838B2 (en) | 2017-04-21 | 2021-08-10 | Universal Display Corporation | Organic electroluminescent materials and device |
| CN110573515B (en) | 2017-04-25 | 2023-07-25 | 默克专利有限公司 | Compounds for electronic devices |
| US11038137B2 (en) | 2017-04-28 | 2021-06-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10910570B2 (en) | 2017-04-28 | 2021-02-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11117897B2 (en) | 2017-05-01 | 2021-09-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10941170B2 (en) | 2017-05-03 | 2021-03-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP7330898B2 (en) | 2017-05-03 | 2023-08-22 | メルク パテント ゲーエムベーハー | Formulation of organic functional material |
| US11201299B2 (en) | 2017-05-04 | 2021-12-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10870668B2 (en) | 2017-05-05 | 2020-12-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10862055B2 (en) | 2017-05-05 | 2020-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10930864B2 (en) | 2017-05-10 | 2021-02-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2018206537A1 (en) | 2017-05-11 | 2018-11-15 | Merck Patent Gmbh | Carbazole-based bodipys for organic electroluminescent devices |
| US10944060B2 (en) | 2017-05-11 | 2021-03-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3621970B1 (en) | 2017-05-11 | 2021-01-13 | Merck Patent GmbH | Organoboron complexes for organic electroluminescent devices |
| US10822362B2 (en) | 2017-05-11 | 2020-11-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10790455B2 (en) | 2017-05-18 | 2020-09-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10840459B2 (en) | 2017-05-18 | 2020-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10934293B2 (en) | 2017-05-18 | 2021-03-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10944062B2 (en) | 2017-05-18 | 2021-03-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11038115B2 (en) | 2017-05-18 | 2021-06-15 | Universal Display Corporation | Organic electroluminescent materials and device |
| CN110637017A (en) | 2017-05-22 | 2019-12-31 | 默克专利有限公司 | Hexacyclo heteroaromatic compounds for electronic devices |
| US10930862B2 (en) | 2017-06-01 | 2021-02-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW201920343A (en) | 2017-06-21 | 2019-06-01 | 德商麥克專利有限公司 | Materials for electronic devices |
| US11608321B2 (en) | 2017-06-23 | 2023-03-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11802136B2 (en) | 2017-06-23 | 2023-10-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US12098157B2 (en) | 2017-06-23 | 2024-09-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11495757B2 (en) | 2017-06-23 | 2022-11-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11814403B2 (en) | 2017-06-23 | 2023-11-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11832510B2 (en) | 2017-06-23 | 2023-11-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11678565B2 (en) | 2017-06-23 | 2023-06-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11174259B2 (en) | 2017-06-23 | 2021-11-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11552261B2 (en) | 2017-06-23 | 2023-01-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10968226B2 (en) | 2017-06-23 | 2021-04-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11758804B2 (en) | 2017-06-23 | 2023-09-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN110753685A (en) | 2017-06-23 | 2020-02-04 | 默克专利有限公司 | Material for organic electroluminescent device |
| US11725022B2 (en) | 2017-06-23 | 2023-08-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20200022010A (en) | 2017-06-26 | 2020-03-02 | 메르크 파텐트 게엠베하 | Homogeneous mixture |
| KR20240059634A (en) | 2017-06-28 | 2024-05-07 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| TWI813576B (en) | 2017-07-03 | 2023-09-01 | 德商麥克專利有限公司 | Formulations with a low content of phenol type impurities |
| KR20240148441A (en) | 2017-07-05 | 2024-10-11 | 메르크 파텐트 게엠베하 | Composition for organic electronic devices |
| US11993572B2 (en) | 2017-07-05 | 2024-05-28 | Merck Patent Gmbh | Composition for organic electronic devices |
| US11469382B2 (en) | 2017-07-12 | 2022-10-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102698061B1 (en) | 2017-07-18 | 2024-08-22 | 메르크 파텐트 게엠베하 | Formulation of organic functional materials |
| US11917843B2 (en) | 2017-07-26 | 2024-02-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11228010B2 (en) | 2017-07-26 | 2022-01-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11239433B2 (en) | 2017-07-26 | 2022-02-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11765970B2 (en) | 2017-07-26 | 2023-09-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11322691B2 (en) | 2017-07-26 | 2022-05-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11968883B2 (en) | 2017-07-26 | 2024-04-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP7413252B2 (en) | 2017-07-28 | 2024-01-15 | メルク パテント ゲーエムベーハー | Spirobifluorene derivatives for use in electronic devices |
| US11744141B2 (en) | 2017-08-09 | 2023-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11508913B2 (en) | 2017-08-10 | 2022-11-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11910699B2 (en) | 2017-08-10 | 2024-02-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11349083B2 (en) | 2017-08-10 | 2022-05-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11744142B2 (en) | 2017-08-10 | 2023-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11723269B2 (en) | 2017-08-22 | 2023-08-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11462697B2 (en) | 2017-08-22 | 2022-10-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11437591B2 (en) | 2017-08-24 | 2022-09-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11605791B2 (en) | 2017-09-01 | 2023-03-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11696492B2 (en) | 2017-09-07 | 2023-07-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11444249B2 (en) | 2017-09-07 | 2022-09-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11424420B2 (en) | 2017-09-07 | 2022-08-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN118405981A (en) | 2017-09-08 | 2024-07-30 | 默克专利有限公司 | Material for electronic devices |
| US10608188B2 (en) | 2017-09-11 | 2020-03-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3681890B1 (en) | 2017-09-12 | 2021-08-18 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US11778897B2 (en) | 2017-09-20 | 2023-10-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3466954A1 (en) | 2017-10-04 | 2019-04-10 | Idemitsu Kosan Co., Ltd. | Fused phenylquinazolines bridged with a heteroatom |
| WO2019068679A1 (en) | 2017-10-06 | 2019-04-11 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN108675975A (en) | 2017-10-17 | 2018-10-19 | 默克专利有限公司 | Material for organic electroluminescence device |
| KR102653073B1 (en) | 2017-10-24 | 2024-03-29 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| US11214587B2 (en) | 2017-11-07 | 2022-01-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11910702B2 (en) | 2017-11-07 | 2024-02-20 | Universal Display Corporation | Organic electroluminescent devices |
| US11183646B2 (en) | 2017-11-07 | 2021-11-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI785142B (en) | 2017-11-14 | 2022-12-01 | 德商麥克專利有限公司 | Composition for organic electronic devices |
| US11168103B2 (en) | 2017-11-17 | 2021-11-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20200090817A (en) | 2017-11-23 | 2020-07-29 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| TWI838352B (en) | 2017-11-24 | 2024-04-11 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI820057B (en) | 2017-11-24 | 2023-11-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11825735B2 (en) | 2017-11-28 | 2023-11-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11937503B2 (en) | 2017-11-30 | 2024-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11233204B2 (en) | 2017-12-14 | 2022-01-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10971687B2 (en) | 2017-12-14 | 2021-04-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11594690B2 (en) | 2017-12-14 | 2023-02-28 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Organometallic complex, and polymer, mixture and formulation comprising same, and use thereof in electronic device |
| CN111247159A (en) | 2017-12-14 | 2020-06-05 | 广州华睿光电材料有限公司 | Transition metal complex material and application thereof in electronic device |
| US11674080B2 (en) | 2017-12-14 | 2023-06-13 | Guangzhou Chinaray Optoelectronic Materials Ltd. | Transition metal complex, polymer, mixture, formulation and use thereof |
| US12075690B2 (en) | 2017-12-14 | 2024-08-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11233205B2 (en) | 2017-12-14 | 2022-01-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP7293229B2 (en) | 2017-12-15 | 2023-06-19 | メルク パテント ゲーエムベーハー | Formulation of organic functional material |
| US20200308129A1 (en) | 2017-12-15 | 2020-10-01 | Merck Patent Gmbh | Substituted aromatic amines for use in organic electroluminescent devices |
| TW201938562A (en) | 2017-12-19 | 2019-10-01 | 德商麥克專利有限公司 | Heterocyclic compounds |
| EP3728275B1 (en) | 2017-12-20 | 2024-09-04 | Merck Patent GmbH | Heteroaromatic compounds |
| US11700765B2 (en) | 2018-01-10 | 2023-07-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11081659B2 (en) | 2018-01-10 | 2021-08-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11515493B2 (en) | 2018-01-11 | 2022-11-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11271177B2 (en) | 2018-01-11 | 2022-03-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI811290B (en) | 2018-01-25 | 2023-08-11 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11542289B2 (en) | 2018-01-26 | 2023-01-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11367840B2 (en) | 2018-01-26 | 2022-06-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11845764B2 (en) | 2018-01-26 | 2023-12-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US12029055B2 (en) | 2018-01-30 | 2024-07-02 | The University Of Southern California | OLED with hybrid emissive layer |
| US11180519B2 (en) | 2018-02-09 | 2021-11-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11342509B2 (en) | 2018-02-09 | 2022-05-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11957050B2 (en) | 2018-02-09 | 2024-04-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11239434B2 (en) | 2018-02-09 | 2022-02-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN111712551A (en) | 2018-02-26 | 2020-09-25 | 默克专利有限公司 | Preparation of organic functional material |
| TW201938761A (en) | 2018-03-06 | 2019-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI802656B (en) | 2018-03-06 | 2023-05-21 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11557733B2 (en) | 2018-03-12 | 2023-01-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11279722B2 (en) | 2018-03-12 | 2022-03-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11217757B2 (en) | 2018-03-12 | 2022-01-04 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11142538B2 (en) | 2018-03-12 | 2021-10-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11165028B2 (en) | 2018-03-12 | 2021-11-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20200132912A (en) | 2018-03-16 | 2020-11-25 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| US11390639B2 (en) | 2018-04-13 | 2022-07-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11882759B2 (en) | 2018-04-13 | 2024-01-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11616203B2 (en) | 2018-04-17 | 2023-03-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11515494B2 (en) | 2018-05-04 | 2022-11-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11342513B2 (en) | 2018-05-04 | 2022-05-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11753427B2 (en) | 2018-05-04 | 2023-09-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11793073B2 (en) | 2018-05-06 | 2023-10-17 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11459349B2 (en) | 2018-05-25 | 2022-10-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11450822B2 (en) | 2018-05-25 | 2022-09-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2019229011A1 (en) | 2018-05-30 | 2019-12-05 | Merck Patent Gmbh | Composition for organic electronic devices |
| US11716900B2 (en) | 2018-05-30 | 2023-08-01 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11296283B2 (en) | 2018-06-04 | 2022-04-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11925103B2 (en) | 2018-06-05 | 2024-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP7322075B2 (en) | 2018-06-07 | 2023-08-07 | メルク パテント ゲーエムベーハー | organic electroluminescent device |
| US11339182B2 (en) | 2018-06-07 | 2022-05-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11603479B2 (en) | 2018-06-15 | 2023-03-14 | Merck Kgaa | Formulation of an organic functional material |
| US11228004B2 (en) | 2018-06-22 | 2022-01-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11261207B2 (en) | 2018-06-25 | 2022-03-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11581497B2 (en) | 2018-07-09 | 2023-02-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US11753425B2 (en) | 2018-07-11 | 2023-09-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN112424169A (en) | 2018-07-20 | 2021-02-26 | 默克专利有限公司 | Material for organic electroluminescent device |
| EP3604477A1 (en) | 2018-07-30 | 2020-02-05 | Idemitsu Kosan Co., Ltd. | Polycyclic compound, organic electroluminescence device, and electronic device |
| EP3608319A1 (en) | 2018-08-07 | 2020-02-12 | Idemitsu Kosan Co., Ltd. | Condensed aza cycles as organic light emitting device and materials for use in same |
| CN112585242A (en) | 2018-08-28 | 2021-03-30 | 默克专利有限公司 | Material for organic electroluminescent device |
| TWI837167B (en) | 2018-08-28 | 2024-04-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI823993B (en) | 2018-08-28 | 2023-12-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11233203B2 (en) | 2018-09-06 | 2022-01-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11485706B2 (en) | 2018-09-11 | 2022-11-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI826522B (en) | 2018-09-12 | 2023-12-21 | 德商麥克專利有限公司 | Electroluminescent devices |
| JP7459065B2 (en) | 2018-09-12 | 2024-04-01 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| TW202030902A (en) | 2018-09-12 | 2020-08-16 | 德商麥克專利有限公司 | Electroluminescent devices |
| US11718634B2 (en) | 2018-09-14 | 2023-08-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20210056432A (en) | 2018-09-24 | 2021-05-18 | 메르크 파텐트 게엠베하 | Method of manufacturing granular material |
| US11903305B2 (en) | 2018-09-24 | 2024-02-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3856717A2 (en) | 2018-09-27 | 2021-08-04 | Merck Patent GmbH | Method for producing sterically hindered, nitrogen-containing heteroaromatic compounds |
| KR20210068054A (en) | 2018-09-27 | 2021-06-08 | 메르크 파텐트 게엠베하 | Compounds that can be used as active compounds in organic electronic devices |
| US11469383B2 (en) | 2018-10-08 | 2022-10-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11495752B2 (en) | 2018-10-08 | 2022-11-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11476430B2 (en) | 2018-10-15 | 2022-10-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11515482B2 (en) | 2018-10-23 | 2022-11-29 | Universal Display Corporation | Deep HOMO (highest occupied molecular orbital) emitter device structures |
| EP3873887A1 (en) | 2018-10-31 | 2021-09-08 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US11469384B2 (en) | 2018-11-02 | 2022-10-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2022506382A (en) | 2018-11-05 | 2022-01-17 | メルク パテント ゲーエムベーハー | Compounds that can be used in organic electronic devices |
| WO2020094538A1 (en) | 2018-11-06 | 2020-05-14 | Merck Patent Gmbh | Method for forming an organic element of an electronic device |
| US20220020934A1 (en) | 2018-11-06 | 2022-01-20 | Merck Patent Gmbh | 5,6-diphenyl-5,6-dihydro-dibenz[c,e][1,2]azaphosphorin and 6-phenyl-6h-dibenzo[c,e][1,2]thiazin-5,5-dioxide derivatives and similar compounds as organic electroluminescent materials for oleds |
| CN113015720A (en) | 2018-11-14 | 2021-06-22 | 默克专利有限公司 | Compounds useful in the manufacture of organic electronic devices |
| EP3880682B1 (en) | 2018-11-15 | 2023-06-14 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US11825736B2 (en) | 2018-11-19 | 2023-11-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11963441B2 (en) | 2018-11-26 | 2024-04-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11716899B2 (en) | 2018-11-28 | 2023-08-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11672176B2 (en) | 2018-11-28 | 2023-06-06 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11690285B2 (en) | 2018-11-28 | 2023-06-27 | Universal Display Corporation | Electroluminescent devices |
| US11889708B2 (en) | 2019-11-14 | 2024-01-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11706980B2 (en) | 2018-11-28 | 2023-07-18 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11672165B2 (en) | 2018-11-28 | 2023-06-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11623936B2 (en) | 2018-12-11 | 2023-04-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11834459B2 (en) | 2018-12-12 | 2023-12-05 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11737349B2 (en) | 2018-12-12 | 2023-08-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW202039493A (en) | 2018-12-19 | 2020-11-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| EP3911655A1 (en) | 2019-01-16 | 2021-11-24 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TW202035345A (en) | 2019-01-17 | 2020-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11812624B2 (en) | 2019-01-30 | 2023-11-07 | The University Of Southern California | Organic electroluminescent materials and devices |
| US11780829B2 (en) | 2019-01-30 | 2023-10-10 | The University Of Southern California | Organic electroluminescent materials and devices |
| US11325932B2 (en) | 2019-02-08 | 2022-05-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11370809B2 (en) | 2019-02-08 | 2022-06-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US12137605B2 (en) | 2019-02-08 | 2024-11-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2020169241A1 (en) | 2019-02-18 | 2020-08-27 | Merck Patent Gmbh | Composition for organic electronic devices |
| US11773320B2 (en) | 2019-02-21 | 2023-10-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11758807B2 (en) | 2019-02-22 | 2023-09-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11871653B2 (en) | 2019-02-22 | 2024-01-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220127286A1 (en) | 2019-03-04 | 2022-04-28 | Merck Patent Gmbh | Ligands for nano-sized materials |
| US11512093B2 (en) | 2019-03-04 | 2022-11-29 | Universal Display Corporation | Compound used for organic light emitting device (OLED), consumer product and formulation |
| US11739081B2 (en) | 2019-03-11 | 2023-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2020182779A1 (en) | 2019-03-12 | 2020-09-17 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US11569480B2 (en) | 2019-03-12 | 2023-01-31 | Universal Display Corporation | Plasmonic OLEDs and vertical dipole emitters |
| US11637261B2 (en) | 2019-03-12 | 2023-04-25 | Universal Display Corporation | Nanopatch antenna outcoupling structure for use in OLEDs |
| US20220177478A1 (en) | 2019-03-20 | 2022-06-09 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2020193447A1 (en) | 2019-03-25 | 2020-10-01 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US11963438B2 (en) | 2019-03-26 | 2024-04-16 | The University Of Southern California | Organic electroluminescent materials and devices |
| US12122793B2 (en) | 2019-03-27 | 2024-10-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2022527591A (en) | 2019-04-11 | 2022-06-02 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Materials for OLED devices |
| US11639363B2 (en) | 2019-04-22 | 2023-05-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US12075691B2 (en) | 2019-04-30 | 2024-08-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11613550B2 (en) | 2019-04-30 | 2023-03-28 | Universal Display Corporation | Organic electroluminescent materials and devices comprising benzimidazole-containing metal complexes |
| US11495756B2 (en) | 2019-05-07 | 2022-11-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US12103942B2 (en) | 2019-05-13 | 2024-10-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11827651B2 (en) | 2019-05-13 | 2023-11-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11634445B2 (en) | 2019-05-21 | 2023-04-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US12010859B2 (en) | 2019-05-24 | 2024-06-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11647667B2 (en) | 2019-06-14 | 2023-05-09 | Universal Display Corporation | Organic electroluminescent compounds and organic light emitting devices using the same |
| US12077550B2 (en) | 2019-07-02 | 2024-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11920070B2 (en) | 2019-07-12 | 2024-03-05 | The University Of Southern California | Luminescent janus-type, two-coordinated metal complexes |
| US11926638B2 (en) | 2019-07-22 | 2024-03-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2022542069A (en) | 2019-07-22 | 2022-09-29 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Method for preparing ortho-metalated metal compounds |
| US11685754B2 (en) | 2019-07-22 | 2023-06-27 | Universal Display Corporation | Heteroleptic organic electroluminescent materials |
| US11708355B2 (en) | 2019-08-01 | 2023-07-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11985888B2 (en) | 2019-08-12 | 2024-05-14 | The Regents Of The University Of Michigan | Organic electroluminescent device |
| US11374181B2 (en) | 2019-08-14 | 2022-06-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11930699B2 (en) | 2019-08-15 | 2024-03-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN114222738B (en) | 2019-08-26 | 2024-05-17 | 默克专利有限公司 | Material for organic electroluminescent device |
| US11925105B2 (en) | 2019-08-26 | 2024-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11937494B2 (en) | 2019-08-28 | 2024-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11600787B2 (en) | 2019-08-30 | 2023-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP4025566A1 (en) | 2019-09-02 | 2022-07-13 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TW202122558A (en) | 2019-09-03 | 2021-06-16 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11820783B2 (en) | 2019-09-06 | 2023-11-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP4031546A1 (en) | 2019-09-16 | 2022-07-27 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| WO2021052921A1 (en) | 2019-09-19 | 2021-03-25 | Merck Patent Gmbh | Mixture of two host materials, and organic electroluminescent device comprising same |
| WO2021053046A1 (en) | 2019-09-20 | 2021-03-25 | Merck Patent Gmbh | Peri-condensed heterocyclic compounds as materials for electronic devices |
| US20210098717A1 (en) | 2019-09-26 | 2021-04-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11864458B2 (en) | 2019-10-08 | 2024-01-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11950493B2 (en) | 2019-10-15 | 2024-04-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11697653B2 (en) | 2019-10-21 | 2023-07-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2021078710A1 (en) | 2019-10-22 | 2021-04-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US11919914B2 (en) | 2019-10-25 | 2024-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20220090539A (en) | 2019-10-25 | 2022-06-29 | 메르크 파텐트 게엠베하 | Compounds that can be used in organic electronic devices |
| US11765965B2 (en) | 2019-10-30 | 2023-09-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2021089450A1 (en) | 2019-11-04 | 2021-05-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TW202130783A (en) | 2019-11-04 | 2021-08-16 | 德商麥克專利有限公司 | Organic electroluminescent device |
| TW202134252A (en) | 2019-11-12 | 2021-09-16 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TW202136181A (en) | 2019-12-04 | 2021-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TW202136471A (en) | 2019-12-17 | 2021-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| WO2021122538A1 (en) | 2019-12-18 | 2021-06-24 | Merck Patent Gmbh | Aromatic compounds for organic electroluminescent devices |
| JP2023506570A (en) | 2019-12-19 | 2023-02-16 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Polycyclic compounds for organic electroluminescent devices |
| US11778895B2 (en) | 2020-01-13 | 2023-10-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11917900B2 (en) | 2020-01-28 | 2024-02-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20220133937A (en) | 2020-01-29 | 2022-10-05 | 메르크 파텐트 게엠베하 | Benzimidazole derivatives |
| US11932660B2 (en) | 2020-01-29 | 2024-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US12084465B2 (en) | 2020-02-24 | 2024-09-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN115135741A (en) | 2020-02-25 | 2022-09-30 | 默克专利有限公司 | Use of heterocyclic compounds in organic electronic devices |
| EP4115457A1 (en) | 2020-03-02 | 2023-01-11 | Merck Patent GmbH | Use of sulfone compounds in an organic electronic device |
| WO2021180625A1 (en) | 2020-03-11 | 2021-09-16 | Merck Patent Gmbh | Organic electroluminescent apparatus |
| EP4118696B1 (en) | 2020-03-11 | 2023-12-20 | Merck Patent GmbH | Organic electroluminescent apparatus |
| CN115298847A (en) | 2020-03-17 | 2022-11-04 | 默克专利有限公司 | Heterocyclic compounds for organic electroluminescent devices |
| US20230157170A1 (en) | 2020-03-17 | 2023-05-18 | Merck Patent Gmbh | Heteroaromatic compounds for organic electroluminescent devices |
| US12018035B2 (en) | 2020-03-23 | 2024-06-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2021191058A1 (en) | 2020-03-23 | 2021-09-30 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP4126868A1 (en) | 2020-03-24 | 2023-02-08 | Merck Patent GmbH | Materials for electronic devices |
| KR20220158771A (en) | 2020-03-26 | 2022-12-01 | 메르크 파텐트 게엠베하 | Cyclic compounds for organic electroluminescent devices |
| US20230151026A1 (en) | 2020-04-02 | 2023-05-18 | Merck Patent Gmbh | Multi-layer body for diffuse transillumination |
| JP2023520710A (en) | 2020-04-06 | 2023-05-18 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Polycyclic compounds for organic electroluminescent devices |
| US12129269B2 (en) | 2020-04-13 | 2024-10-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW202214791A (en) | 2020-04-21 | 2022-04-16 | 德商麥克專利有限公司 | Formulation of an organic functional material |
| EP4139971A1 (en) | 2020-04-21 | 2023-03-01 | Merck Patent GmbH | Emulsions comprising organic functional materials |
| US11970508B2 (en) | 2020-04-22 | 2024-04-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US12035613B2 (en) | 2020-05-26 | 2024-07-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2021239772A1 (en) | 2020-05-29 | 2021-12-02 | Merck Patent Gmbh | Organic electroluminescent apparatus |
| WO2021254984A1 (en) | 2020-06-18 | 2021-12-23 | Merck Patent Gmbh | Indenoazanaphthalenes |
| EP4169082A1 (en) | 2020-06-23 | 2023-04-26 | Merck Patent GmbH | Method for producing a mixture |
| JP2023531470A (en) | 2020-06-29 | 2023-07-24 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Heteroaromatic compounds for organic electroluminescent devices |
| JP2023530915A (en) | 2020-06-29 | 2023-07-20 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Heterocyclic compounds for organic electroluminescent devices |
| EP4185574A1 (en) | 2020-07-22 | 2023-05-31 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| WO2021170886A2 (en) | 2020-08-06 | 2021-09-02 | Merck Patent Gmbh | Electronic device |
| CN116157402A (en) | 2020-08-06 | 2023-05-23 | 默克专利有限公司 | Material for organic electroluminescent device |
| US20230271989A1 (en) | 2020-08-13 | 2023-08-31 | Merck Patent Gmbh | Metal complexes |
| WO2022038065A1 (en) | 2020-08-18 | 2022-02-24 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| JP2023539825A (en) | 2020-08-19 | 2023-09-20 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| US12065451B2 (en) | 2020-08-19 | 2024-08-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW202222748A (en) | 2020-09-30 | 2022-06-16 | 德商麥克專利有限公司 | Compounds usable for structuring of functional layers of organic electroluminescent devices |
| TW202229215A (en) | 2020-09-30 | 2022-08-01 | 德商麥克專利有限公司 | Compounds for structuring of functional layers of organic electroluminescent devices |
| WO2022079068A1 (en) | 2020-10-16 | 2022-04-21 | Merck Patent Gmbh | Heterocyclic compounds for organic electroluminescent devices |
| EP4229145A1 (en) | 2020-10-16 | 2023-08-23 | Merck Patent GmbH | Compounds comprising heteroatoms for organic electroluminescent devices |
| US12137606B2 (en) | 2020-10-20 | 2024-11-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2022101171A1 (en) | 2020-11-10 | 2022-05-19 | Merck Patent Gmbh | Sulfurous compounds for organic electroluminescent devices |
| US20230416264A1 (en) | 2020-12-02 | 2023-12-28 | Merck Patent Gmbh | Heterocyclic compounds for organic electroluminescent devices |
| JP2023552761A (en) | 2020-12-08 | 2023-12-19 | メルク パテント ゲーエムベーハー | Methods for ink-based and inkjet printing |
| US20240057479A1 (en) | 2020-12-10 | 2024-02-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP4263746A1 (en) | 2020-12-18 | 2023-10-25 | Merck Patent GmbH | Nitrogenous heteroaromatic compounds for organic electroluminescent devices |
| EP4263543A1 (en) | 2020-12-18 | 2023-10-25 | Merck Patent GmbH | Nitrogenous compounds for organic electroluminescent devices |
| US20240114782A1 (en) | 2020-12-18 | 2024-04-04 | Merck Patent Gmbh | Indolo[3.2.1-jk]carbazole-6-carbonitrile derivatives as blue fluorescent emitters for use in oleds |
| JP2024502093A (en) | 2021-01-05 | 2024-01-17 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| WO2022157343A1 (en) | 2021-01-25 | 2022-07-28 | Merck Patent Gmbh | Nitrogenous compounds for organic electroluminescent devices |
| WO2022184601A1 (en) | 2021-03-02 | 2022-09-09 | Merck Patent Gmbh | Compounds for organic electroluminescent devices |
| US20240092783A1 (en) | 2021-03-18 | 2024-03-21 | Merck Patent Gmbh | Heteroaromatic compounds for organic electroluminescent devices |
| KR20240000559A (en) | 2021-04-23 | 2024-01-02 | 메르크 파텐트 게엠베하 | Formulation of organic functional materials |
| CN113201022A (en) * | 2021-04-29 | 2021-08-03 | 南京邮电大学 | Small conjugated phosphorescent metal iridium (III) complex with isomer and preparation method and application thereof |
| KR20240005806A (en) | 2021-04-29 | 2024-01-12 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| EP4330239A1 (en) | 2021-04-29 | 2024-03-06 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| WO2022229234A1 (en) | 2021-04-30 | 2022-11-03 | Merck Patent Gmbh | Nitrogenous heterocyclic compounds for organic electroluminescent devices |
| KR20240012506A (en) | 2021-05-21 | 2024-01-29 | 메르크 파텐트 게엠베하 | Method for continuous purification of at least one functional substance and device for continuous purification of at least one functional substance |
| WO2022200638A1 (en) | 2021-07-06 | 2022-09-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR20240045247A (en) | 2021-08-02 | 2024-04-05 | 메르크 파텐트 게엠베하 | Printing method by combining inks |
| WO2023036976A1 (en) | 2021-09-13 | 2023-03-16 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2023041454A1 (en) | 2021-09-14 | 2023-03-23 | Merck Patent Gmbh | Boronic heterocyclic compounds for organic electroluminescent devices |
| KR20240075872A (en) | 2021-09-28 | 2024-05-29 | 메르크 파텐트 게엠베하 | Materials for Electronic Devices |
| WO2023052314A1 (en) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materials for electronic devices |
| EP4410071A1 (en) | 2021-09-28 | 2024-08-07 | Merck Patent GmbH | Materials for electronic devices |
| EP4410074A1 (en) | 2021-09-28 | 2024-08-07 | Merck Patent GmbH | Materials for electronic devices |
| EP4423209A1 (en) | 2021-10-27 | 2024-09-04 | Merck Patent GmbH | Boronic and nitrogenous heterocyclic compounds for organic electroluminescent devices |
| WO2023094412A1 (en) | 2021-11-25 | 2023-06-01 | Merck Patent Gmbh | Materials for electronic devices |
| CN117343078A (en) | 2021-11-25 | 2024-01-05 | 北京夏禾科技有限公司 | Organic electroluminescent material and device |
| WO2023099543A1 (en) | 2021-11-30 | 2023-06-08 | Merck Patent Gmbh | Compounds having fluorene structures |
| WO2023110742A1 (en) | 2021-12-13 | 2023-06-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN118355092A (en) | 2021-12-21 | 2024-07-16 | 默克专利有限公司 | Electronic device |
| KR20240123834A (en) | 2021-12-21 | 2024-08-14 | 메르크 파텐트 게엠베하 | Electronic devices |
| CN118354991A (en) | 2021-12-21 | 2024-07-16 | 默克专利有限公司 | Method for preparing deuterated organic compounds |
| KR20240146680A (en) | 2022-02-09 | 2024-10-08 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| KR20240150795A (en) | 2022-02-14 | 2024-10-16 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| WO2023161168A1 (en) | 2022-02-23 | 2023-08-31 | Merck Patent Gmbh | Aromatic hetreocycles for organic electroluminescent devices |
| KR20240153376A (en) | 2022-02-23 | 2024-10-22 | 메르크 파텐트 게엠베하 | Nitrogen-containing heterocycles for organic electroluminescent devices |
| WO2023213837A1 (en) | 2022-05-06 | 2023-11-09 | Merck Patent Gmbh | Cyclic compounds for organic electroluminescent devices |
| WO2023222559A1 (en) | 2022-05-18 | 2023-11-23 | Merck Patent Gmbh | Process for preparing deuterated organic compounds |
| TW202411366A (en) | 2022-06-07 | 2024-03-16 | 德商麥克專利有限公司 | Method of printing a functional layer of an electronic device by combining inks |
| WO2023247662A1 (en) | 2022-06-24 | 2023-12-28 | Merck Patent Gmbh | Composition for organic electronic devices |
| CN118235543A (en) | 2022-06-24 | 2024-06-21 | 默克专利有限公司 | Composition for organic electronic devices |
| WO2024013004A1 (en) | 2022-07-11 | 2024-01-18 | Merck Patent Gmbh | Materials for electronic devices |
| WO2024033282A1 (en) | 2022-08-09 | 2024-02-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024061948A1 (en) | 2022-09-22 | 2024-03-28 | Merck Patent Gmbh | Nitrogen-containing hetreocycles for organic electroluminescent devices |
| WO2024061942A1 (en) | 2022-09-22 | 2024-03-28 | Merck Patent Gmbh | Nitrogen-containing compounds for organic electroluminescent devices |
| WO2024094592A2 (en) | 2022-11-01 | 2024-05-10 | Merck Patent Gmbh | Nitrogenous heterocycles for organic electroluminescent devices |
| WO2024105066A1 (en) | 2022-11-17 | 2024-05-23 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024126635A1 (en) | 2022-12-16 | 2024-06-20 | Merck Patent Gmbh | Formulation of an organic functional material |
| WO2024132892A1 (en) | 2022-12-19 | 2024-06-27 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024132993A1 (en) | 2022-12-19 | 2024-06-27 | Merck Patent Gmbh | Materials for electronic devices |
| WO2024133048A1 (en) | 2022-12-20 | 2024-06-27 | Merck Patent Gmbh | Method for preparing deuterated aromatic compounds |
| WO2024149694A1 (en) | 2023-01-10 | 2024-07-18 | Merck Patent Gmbh | Nitrogenous heterocycles for organic electroluminescent devices |
| WO2024153568A1 (en) | 2023-01-17 | 2024-07-25 | Merck Patent Gmbh | Heterocycles for organic electroluminescent devices |
| WO2024170605A1 (en) | 2023-02-17 | 2024-08-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024184050A1 (en) | 2023-03-07 | 2024-09-12 | Merck Patent Gmbh | Cyclic nitrogen compounds for organic electroluminescent devices |
| WO2024194264A1 (en) | 2023-03-20 | 2024-09-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024218109A1 (en) | 2023-04-20 | 2024-10-24 | Merck Patent Gmbh | Materials for electronic devices |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3718488A (en) * | 1971-03-02 | 1973-02-27 | Du Pont | Precious metal decorating compositions containing bis-chelate derivatives of palladium |
| US20020064681A1 (en) * | 2000-09-26 | 2002-05-30 | Takao Takiguchi | Luminescence device, display apparatus and metal coordination compound |
Family Cites Families (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3655679A (en) * | 1969-06-25 | 1972-04-11 | Merck & Co Inc | Certain aryl pyridine carboxylic acid derivatives |
| US3747300A (en) * | 1971-10-14 | 1973-07-24 | Mc Graw Edison Co | Portable electrostatic air cleaner |
| US3886167A (en) * | 1974-03-06 | 1975-05-27 | Us Army | 2-Aryl-6-trifluoromethyl-4-pyridylcarbinolamine antimalarials |
| US4210429A (en) * | 1977-04-04 | 1980-07-01 | Alpine Roomaire Systems, Inc. | Air purifier |
| US4244712A (en) * | 1979-03-05 | 1981-01-13 | Tongret Stewart R | Cleansing system using treated recirculating air |
| US4680299A (en) * | 1984-04-30 | 1987-07-14 | E.I. Du Pont De Nemours And Company | 2-phenyl-4-quinolinecarboxylic acids and pharmaceutical compositions thereof |
| NZ212525A (en) * | 1985-06-24 | 1989-02-24 | Dfc New Zealand Ltd Formerly D | Nitrogen-containing heterocyclic compounds, and pharmaceutical compositions containing such |
| US4847381A (en) * | 1987-08-31 | 1989-07-11 | American Cyanamid Company | 2-Phenyl-4-quinoline carboxylic acids |
| GB8909011D0 (en) | 1989-04-20 | 1989-06-07 | Friend Richard H | Electroluminescent devices |
| EP0443861B2 (en) | 1990-02-23 | 2008-05-28 | Sumitomo Chemical Company, Limited | Organic electroluminescence device |
| US5322473A (en) * | 1990-05-17 | 1994-06-21 | Quality Air Systems, Inc. | Modular wall apparatus and method for its use |
| US5160517A (en) * | 1990-11-21 | 1992-11-03 | Hicks Richard E | System for purifying air in a room |
| US5408109A (en) | 1991-02-27 | 1995-04-18 | The Regents Of The University Of California | Visible light emitting diodes fabricated from soluble semiconducting polymers |
| US5192342A (en) * | 1992-04-15 | 1993-03-09 | Baron Robert A | Apparatus for enhancing the environmental quality of work spaces |
| US5240478A (en) * | 1992-06-26 | 1993-08-31 | Messina Gary D | Self-contained, portable room air treatment apparatus and method therefore |
| DE4323916A1 (en) * | 1993-07-16 | 1995-01-19 | Basf Ag | Substituted 2-phenylpyridines |
| US5360469A (en) * | 1993-09-09 | 1994-11-01 | Baron Robert A | Apparatus for air filtration and sound masking |
| CN1075817C (en) * | 1994-07-25 | 2001-12-05 | 罗切诊断学有限公司 | Hydrophilic metal complexes |
| US5552678A (en) * | 1994-09-23 | 1996-09-03 | Eastman Kodak Company | AC drive scheme for organic led |
| US5616172A (en) * | 1996-02-27 | 1997-04-01 | Nature's Quarters, Inc. | Air treatment system |
| US5997619A (en) * | 1997-09-04 | 1999-12-07 | Nq Environmental, Inc. | Air purification system |
| US6303238B1 (en) | 1997-12-01 | 2001-10-16 | The Trustees Of Princeton University | OLEDs doped with phosphorescent compounds |
| US6099607A (en) * | 1998-07-22 | 2000-08-08 | Haslebacher; William J. | Rollably positioned, adjustably directable clean air delivery supply assembly, for use in weather protected environments to provide localized clean air, where activities require clean air quality per strict specifications |
| US6830828B2 (en) * | 1998-09-14 | 2004-12-14 | The Trustees Of Princeton University | Organometallic complexes as phosphorescent emitters in organic LEDs |
| KR20100042665A (en) | 1999-03-23 | 2010-04-26 | 유니버시티 오브 서던 캘리포니아 | Cyclometallated metal complexes as phosphorescent dopants in organic leds |
| KR100934420B1 (en) | 1999-05-13 | 2009-12-29 | 더 트러스티즈 오브 프린스턴 유니버시티 | Very high efficiency organic light emitting devices based on electrophosphorescence |
| CN1107098C (en) * | 1999-09-05 | 2003-04-30 | 吉林大学 | Phenolic group-pyridine or metal coordination compound of its derivative and their application as electroluminescence material |
| KR100794975B1 (en) | 1999-12-01 | 2008-01-16 | 더 트러스티즈 오브 프린스턴 유니버시티 | Complexes of form l2mx as phosphorescent dopants for organic leds |
| US6821645B2 (en) * | 1999-12-27 | 2004-11-23 | Fuji Photo Film Co., Ltd. | Light-emitting material comprising orthometalated iridium complex, light-emitting device, high efficiency red light-emitting device, and novel iridium complex |
| JP3929690B2 (en) * | 1999-12-27 | 2007-06-13 | 富士フイルム株式会社 | Light emitting device material, light emitting device and novel iridium complex comprising orthometalated iridium complex |
| JP4048521B2 (en) * | 2000-05-02 | 2008-02-20 | 富士フイルム株式会社 | Light emitting element |
| US20020121638A1 (en) | 2000-06-30 | 2002-09-05 | Vladimir Grushin | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US6670645B2 (en) | 2000-06-30 | 2003-12-30 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
| US6962755B2 (en) | 2000-07-17 | 2005-11-08 | Fuji Photo Film Co., Ltd. | Light emitting element and azole compound |
| JP5241053B2 (en) | 2000-08-11 | 2013-07-17 | ザ、トラスティーズ オブ プリンストン ユニバーシティ | Organometallic compounds and radiation-transfer organic electrophosphors |
| US6939624B2 (en) * | 2000-08-11 | 2005-09-06 | Universal Display Corporation | Organometallic compounds and emission-shifting organic electrophosphorescence |
| JP4154140B2 (en) | 2000-09-26 | 2008-09-24 | キヤノン株式会社 | Metal coordination compounds |
| US6464760B1 (en) * | 2000-09-27 | 2002-10-15 | John C. K. Sham | Ultraviolet air purifier |
| IL154960A0 (en) * | 2000-10-10 | 2003-10-31 | Du Pont | Polymers having attached luminescent metal complexes and devices made with sych polymers |
| SE0004054D0 (en) * | 2000-11-06 | 2000-11-06 | Astrazeneca Ab | N-type calcium channel antagonists for the treatment of pain |
| KR100798561B1 (en) * | 2000-11-30 | 2008-01-28 | 캐논 가부시끼가이샤 | Luminescent Element and Display |
| EP1349435B8 (en) * | 2000-11-30 | 2018-09-19 | Canon Kabushiki Kaisha | Luminescent element and display |
| US6835469B2 (en) * | 2001-10-17 | 2004-12-28 | The University Of Southern California | Phosphorescent compounds and devices comprising the same |
| US7250512B2 (en) * | 2001-11-07 | 2007-07-31 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds having red-orange or red emission and devices made with such compounds |
| US7166368B2 (en) * | 2001-11-07 | 2007-01-23 | E. I. Du Pont De Nemours And Company | Electroluminescent platinum compounds and devices made with such compounds |
-
2001
- 2001-06-12 US US09/879,014 patent/US20020121638A1/en not_active Abandoned
- 2001-06-27 EP EP04004542A patent/EP1431288A3/en not_active Withdrawn
- 2001-06-27 AU AU2001271550A patent/AU2001271550B2/en not_active Ceased
- 2001-06-27 CA CA002411624A patent/CA2411624A1/en not_active Abandoned
- 2001-06-27 AU AU7155001A patent/AU7155001A/en active Pending
- 2001-06-27 AT AT01950576T patent/ATE335386T1/en not_active IP Right Cessation
- 2001-06-27 JP JP2002507959A patent/JP5174310B2/en not_active Expired - Lifetime
- 2001-06-27 KR KR1020027017946A patent/KR100838010B1/en active IP Right Grant
- 2001-06-27 WO PCT/US2001/020539 patent/WO2002002714A2/en active IP Right Grant
- 2001-06-27 EP EP01950576A patent/EP1295514B1/en not_active Expired - Lifetime
- 2001-06-27 DE DE60121950T patent/DE60121950T2/en not_active Expired - Lifetime
- 2001-06-27 EP EP04004543A patent/EP1431289B1/en not_active Expired - Lifetime
- 2001-06-27 CN CNB018148611A patent/CN100438123C/en not_active Expired - Lifetime
- 2001-06-27 IL IL15331901A patent/IL153319A0/en unknown
- 2001-06-27 EP EP20040004541 patent/EP1424382A3/en not_active Withdrawn
- 2001-06-29 TW TW090115959A patent/TW593623B/en not_active IP Right Cessation
-
2003
- 2003-02-13 US US10/366,295 patent/US20030197183A1/en not_active Abandoned
- 2003-06-12 US US10/459,946 patent/US6979739B2/en not_active Expired - Lifetime
- 2003-11-24 US US10/720,954 patent/US7161172B2/en not_active Expired - Lifetime
-
2004
- 2004-11-05 US US10/983,119 patent/US20050095457A1/en not_active Abandoned
-
2012
- 2012-06-07 JP JP2012130171A patent/JP5536830B2/en not_active Expired - Lifetime
- 2012-06-07 JP JP2012130173A patent/JP5576431B2/en not_active Expired - Lifetime
- 2012-06-07 JP JP2012130172A patent/JP5615322B2/en not_active Expired - Fee Related
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3718488A (en) * | 1971-03-02 | 1973-02-27 | Du Pont | Precious metal decorating compositions containing bis-chelate derivatives of palladium |
| US20020064681A1 (en) * | 2000-09-26 | 2002-05-30 | Takao Takiguchi | Luminescence device, display apparatus and metal coordination compound |
Cited By (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050037233A1 (en) * | 2000-06-30 | 2005-02-17 | Dobbs Kerwin D. | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| US7476452B2 (en) | 2000-06-30 | 2009-01-13 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| US20070292713A9 (en) * | 2000-06-30 | 2007-12-20 | Dobbs Kerwin D | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| US20060057425A1 (en) * | 2002-02-14 | 2006-03-16 | Vladimir Grushin | Electroluminescent iridium compounds with phosphinoalkoxides and phenylpyridines or phenylpyrimidines and devices made with such compounds |
| US20030173896A1 (en) * | 2002-02-14 | 2003-09-18 | Vladimir Grushin | Electroluminescent iridium compounds with phosphinoalkoxides and phenylpyridines or phenylpyrimidines and devices made with such compounds |
| US6919139B2 (en) * | 2002-02-14 | 2005-07-19 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds with phosphinoalkoxides and phenylpyridines or phenylpyrimidines and devices made with such compounds |
| US20050186447A1 (en) * | 2002-02-14 | 2005-08-25 | Vladimir Grushin | Electroluminescent iridium compounds with phosphinoalkoxides and phenylpyridines or phenylpyrimidines and devices made with such compounds |
| US7227041B2 (en) | 2002-02-14 | 2007-06-05 | E. I. Du Pont De Nemours And Company | Process for preparing a phosphinoalkanol |
| US7164045B2 (en) | 2002-02-14 | 2007-01-16 | E.I. Du Pont De Nemours And Company | Electroluminescent iridium compounds with phosphinoalkoxides and phenylpyridines or phenylpyrimidines and devices made with such compounds |
| US20030189216A1 (en) * | 2002-03-08 | 2003-10-09 | Canon Kabushiki Kaisha | Organic light emitting device |
| US6812497B2 (en) * | 2002-03-08 | 2004-11-02 | Canon Kabushiki Kaisha | Organic light emitting device |
| US11968893B2 (en) | 2002-10-30 | 2024-04-23 | Udc Ireland Limited | Electroluminescent device |
| US8012602B2 (en) | 2002-10-30 | 2011-09-06 | Basf Se | Electroluminescent device |
| US20060041126A1 (en) * | 2002-10-30 | 2006-02-23 | Ciba Specialty Chemicals Holding Inc. | Electroluminescent device |
| US9923148B2 (en) | 2002-10-30 | 2018-03-20 | Udc Ireland Limited | Electroluminescent device |
| US20100240892A1 (en) * | 2002-10-30 | 2010-09-23 | Schaefer Thomas | Electroluminescent device |
| US11289658B2 (en) | 2002-10-30 | 2022-03-29 | Udc Ireland Limited | Electroluminescent device |
| US7816016B1 (en) | 2003-02-13 | 2010-10-19 | E. I. Du Pont De Nemours And Company | Electroluminescent iridium compounds and devices made therefrom |
| US7521130B2 (en) | 2003-11-25 | 2009-04-21 | Samsung Mobile Display Co., Ltd. | Organic electroluminescent display device having superior characteristics at high temperature |
| KR100560790B1 (en) * | 2003-11-25 | 2006-03-13 | 삼성에스디아이 주식회사 | Electroluminescent display device having a good performance at high temperature |
| US20050112401A1 (en) * | 2003-11-25 | 2005-05-26 | Samsung Sdi Co., Ltd. | Organic electroluminescent display device having superior characteristics at high temperature |
| US20090195153A1 (en) * | 2003-11-25 | 2009-08-06 | Samsung Mobile Display | Organic electroluminescent display device having superior characteristics at high temperature |
| KR101219751B1 (en) | 2003-12-02 | 2013-01-21 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | An organometallic complex |
| US9219236B2 (en) | 2003-12-02 | 2015-12-22 | Semiconductor Energy Laboratory Co., Ltd. | Organometal complex and light-emitting element using the same |
| US20110163665A1 (en) * | 2003-12-02 | 2011-07-07 | Semiconductor Energy Laboratory Co., Ltd. | Organometal complex and light-emitting element using the same |
| US8569486B2 (en) | 2003-12-02 | 2013-10-29 | Semiconductor Energy Laboratory Co., Ltd. | Organometal complex and light-emitting element using the same |
| US8278444B2 (en) | 2003-12-02 | 2012-10-02 | Semiconductor Energy Laboratory Co., Ltd. | Organometal complex and light-emitting element using the same |
| WO2005075597A3 (en) * | 2004-01-30 | 2005-12-01 | Du Pont | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| WO2005075597A2 (en) * | 2004-01-30 | 2005-08-18 | E.I. Dupont De Nemours And Company | Electroluminescent iridium compounds with fluorinated phenylpyridine ligands, and devices made with such compounds |
| US20090010305A1 (en) * | 2004-10-15 | 2009-01-08 | Koninklijke Philips Electronics, N.V. | Temperature Indicator |
| US20080192802A1 (en) * | 2004-10-15 | 2008-08-14 | Koninklijke Philips Electronics, N.V. | Colour Switching Temperature Indicator |
| US20120238047A1 (en) * | 2005-02-16 | 2012-09-20 | Bawendi Moungi G | Light emitting device including semiconductor nanocrystals |
| US7781075B2 (en) | 2005-09-07 | 2010-08-24 | Au Optronics Corporation | Organic light-emitting material and organic light-emitting device |
| JP2017171671A (en) * | 2011-10-06 | 2017-09-28 | 株式会社半導体エネルギー研究所 | Iridium complex |
| US9978960B2 (en) | 2013-06-14 | 2018-05-22 | Semiconductor Energy Laboratory Co., Ltd. | Organometallic iridium complex, light-emitting element, light-emitting device, and lighting device |
| US10431752B2 (en) | 2013-06-14 | 2019-10-01 | Semiconductor Energy Laboratory Co., Ltd. | Organometallic iridium complex, light-emitting element, light-emitting device, and lighting device |
| CN115216291A (en) * | 2014-10-08 | 2022-10-21 | 环球展览公司 | Organic electroluminescent material and device |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6979739B2 (en) | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds | |
| US7129518B2 (en) | Electroluminescent iridium compounds with fluorinated phenylpryidines, phenylpyrimidines, and phenylquinolines and devices made with such compounds | |
| US6670645B2 (en) | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds | |
| JP4299144B2 (en) | Electroluminescent iridium compounds comprising fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines, and devices made using such compounds | |
| AU2001271550A1 (en) | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds | |
| US7132681B2 (en) | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds | |
| AU2002231155A1 (en) | Electroluminescent iridium compounds with fluorinated phenylpyridines, phenylpyrimidines, and phenylquinolines and devices made with such compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |