US11482720B2 - Membrane-electrode assembly with improved durability and proton conductivity and method for manufacturing the same - Google Patents
Membrane-electrode assembly with improved durability and proton conductivity and method for manufacturing the same Download PDFInfo
- Publication number
- US11482720B2 US11482720B2 US17/065,140 US202017065140A US11482720B2 US 11482720 B2 US11482720 B2 US 11482720B2 US 202017065140 A US202017065140 A US 202017065140A US 11482720 B2 US11482720 B2 US 11482720B2
- Authority
- US
- United States
- Prior art keywords
- cerium
- conductive polymer
- ion conductive
- membrane
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/86—Inert electrodes with catalytic activity, e.g. for fuel cells
- H01M4/88—Processes of manufacture
- H01M4/8875—Methods for shaping the electrode into free-standing bodies, like sheets, films or grids, e.g. moulding, hot-pressing, casting without support, extrusion without support
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1041—Polymer electrolyte composites, mixtures or blends
- H01M8/1046—Mixtures of at least one polymer and at least one additive
- H01M8/1048—Ion-conducting additives, e.g. ion-conducting particles, heteropolyacids, metal phosphate or polybenzimidazole with phosphoric acid
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/86—Inert electrodes with catalytic activity, e.g. for fuel cells
- H01M4/8663—Selection of inactive substances as ingredients for catalytic active masses, e.g. binders, fillers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1004—Fuel cells with solid electrolytes characterised by membrane-electrode assemblies [MEA]
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/102—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer
- H01M8/1027—Polymeric electrolyte materials characterised by the chemical structure of the main chain of the ion-conducting polymer having carbon, oxygen and other atoms, e.g. sulfonated polyethersulfones [S-PES]
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1041—Polymer electrolyte composites, mixtures or blends
- H01M8/1053—Polymer electrolyte composites, mixtures or blends consisting of layers of polymers with at least one layer being ionically conductive
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1069—Polymeric electrolyte materials characterised by the manufacturing processes
- H01M8/1081—Polymeric electrolyte materials characterised by the manufacturing processes starting from solutions, dispersions or slurries exclusively of polymers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M2008/1095—Fuel cells with polymeric electrolytes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
Definitions
- the present disclosure relates to a membrane-electrode assembly and a method for manufacturing the same. More particularly, it relates to a membrane-electrode assembly with durability and proton conductivity which are greatly improved by employing an ion conductive polymer having excellent chemical durability and proton conductivity.
- a polymer electrolyte membrane fuel cell for vehicles is an electricity generator which produces electricity using electrochemical reaction between hydrogen and oxygen in air, and is known as an eco-friendly next-generation energy source which has high electricity generation efficiency and does not produce any effluent other than water. Further, the polymer electrolyte membrane fuel cell generally operates at a temperature of 95° C. or less, and may obtain high power density. Such reaction for generating electricity of the fuel cell occurs in a membrane-electrode assembly (MEA) including a perfluorinated sulfonic acid (PFSA) ionomer-based electrolyte membrane and electrodes, i.e., an anode and a cathode.
- MEA membrane-electrode assembly
- PFSA perfluorinated sulfonic acid
- Hydrogen supplied to an oxidation electrode, i.e., the anode, of the fuel cell is separated into protons and electrons.
- the protons move to a reduction electrode, i.e., the cathode, through the membrane, and the electrons moves to the cathode through an external circuit.
- oxygen molecules, the protons and the electrons react with each other, and thus produce electricity and heat and simultaneously produce water (H 2 O) as a reaction by-product.
- PFSA perfluorinated sulfonic acid
- antioxidants such as cerium (III) nitride hexahydrate
- cerium ions are combined with the terminals of sulfonic acid groups of a perfluorinated sulfonic acid ionomer, and block a path along which protons (H + ) may move.
- protons H +
- the present disclosure has been made in an effort to solve the above-described problems associated with the prior art and it is an object of the present disclosure to provide a membrane-electrode assembly having greater chemical durability and proton conductivity than an electrolyte membrane based on a perfluorinated sulfonic acid (PFSA) ionomer, such as NafionTM.
- PFSA perfluorinated sulfonic acid
- the present disclosure provides a membrane-electrode assembly including an electrolyte membrane, and a pair of electrodes formed on both surfaces of the electrolyte membrane, wherein at least one of the electrolyte membrane or the pair of electrodes includes an ion conductive polymer having proton conductive groups, wherein a compound expressed in the chemical formula MA x is combined with all or some of the proton conductive groups, where M is a lanthanoid, A is a hydrophilic functional group, and X is a number necessary to maintain a charge balance between A and M.
- the proton conductive group may include a sulfonic acid group.
- the ion conductive polymer may include one selected from the group consisting of perfluorosulfonic acid, sulfonated poly(aryl ether ketone), sulfonated poly(arylene ether sulfone) and combinations thereof.
- M may be cerium, and the hydrophilic functional group may include one selected from the group consisting of a hydroxyl group, a carboxyl group and a combination thereof.
- the compound expressed in the chemical formula MA x may include cerium hydroxide (Ce(OH) 3 ).
- the ion conductive polymer may include a carbon skeleton and a side chain expressed in the structural formula:
- the ion conductive polymer may include a porous reinforcing layer configured to be impregnated with the ion conductive polymer, and an ion transport layer provided to at least one surface of the reinforcing layer and including the ion conductive polymer.
- the present disclosure provides a method for manufacturing a membrane-electrode assembly, the method including preparing a dispersion liquid including a cerium precursor, preparing a mixture including the dispersion liquid and an ion conductive polymer including proton conductive groups, adding an acid solution to the mixture and reacting the mixture with the acid solution so as to combine cerium hydroxide (Ce(OH) 3 ) with all or some of the proton conductive groups, manufacturing an electrolyte membrane as a product of the reaction, and forming electrodes on both surfaces of the electrolyte membrane.
- Ce(OH) 3 cerium hydroxide
- the cerium precursor may include one selected from the group consisting of cerium isopropoxide (Ce(OC 3 H 7 ) 4 , cerium (III) acetate hydrate (Ce(CH 3 CO 2 ) 3 .xH 2 O), cerium (III) acetylacetonate hydrate (Ce(C 5 H 7 O 2 ) 3 .xH 2 O), cerium (III) oxalate hydrate (Ce 2 (C 2 O 4 ) 3 .xH 2 O), cerium trifluoromethanesulfonate (Ce n (CF 3 SO 3 ) x .H 2 O) and combinations thereof.
- cerium isopropoxide Ce(OC 3 H 7 ) 4
- cerium (III) acetate hydrate Ce(CH 3 CO 2 ) 3 .xH 2 O
- cerium (III) acetylacetonate hydrate Ce(C 5 H 7 O 2 ) 3 .xH 2 O
- the dispersion liquid may be prepared by dispersing the cerium precursor in a polar solvent including one selected from the group consisting of isopropanol, dimethylformamide and a combination thereof.
- the dispersion liquid may be prepared by putting the cerium precursor into a solvent and then agitating an obtained mixture for 10 to 600 minutes.
- a content of the cerium precursor included in the mixture may be 0.01 to 20 wt % with respect to a total content of the cerium precursor and the ion conductive polymer.
- the mixture may be prepared by mixing the dispersion liquid and the ion conductive polymer including the proton conductive groups and then agitating the mixed dispersion liquid and ion conductive polymer for 10 to 300 minutes.
- the acid solution may be added to the mixture and the mixture may be reacted with the acid solution at a temperature of 50° C. to 150° C. for 1 to 45 hours.
- FIG. 1 is a cross-sectional view briefly illustrating a membrane-electrode assembly in accordance with the present disclosure
- FIG. 2 is a view schematically illustrating a proton-conducting channel in an electrolyte membrane in accordance with the present disclosure
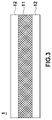
- FIG. 3 is a cross-sectional view schematically illustrating an electrolyte membrane in accordance with another embodiment of the present disclosure
- FIG. 4 is a flowchart representing a method for manufacturing a membrane-electrode assembly in accordance with the present disclosure
- FIG. 5 is a graph illustrating measurement results of fluoride emission quantities of electrolyte membranes in accordance with an example and a comparative example.
- FIG. 6 is a graph illustrating measurement results of proton conductivities of the electrolyte membranes in accordance with the example and the comparative example.
- FIG. 1 is a cross-sectional view briefly illustrating a membrane-electrode assembly in accordance with the present disclosure.
- the membrane-electrode assembly includes an electrolyte membrane 1 and a pair of electrodes 2 provided on both surfaces of the electrolyte membrane 1 .
- a pair of electrodes means an anode and a cathode, which are located opposite each other with the electrolyte membrane 1 interposed therebetween.
- FIG. 2 is a view schematically illustrating a proton-conducting channel A in the electrolyte membrane 1 in accordance with the present disclosure.
- the proton-conducting channel A is composed of main chains B forming an ion conductive polymer and side chains B′ continuously arranged along the main chains B.
- the electrolyte membrane 1 is impregnated with moisture (H 2 O) through functional groups of the side chains B′, and thus the proton-conducting channel A is formed.
- FIG. 2 illustrates one example of the ion conductive polymer, and the chemical structure of the ion conductive polymer is not limited thereto.
- the ion conductive polymer may be a polymer having proton conductive groups.
- having the proton conductive groups may mean that the main chains of the ion conductive polymer are provided with the proton conductive groups as functional groups.
- the proton conductive group may include a sulfonic acid group without being limited thereto.
- the ion conductive polymer having the proton conductive groups may include one selected from the group consisting of perfluorosulfonic acid, sulfonated poly(aryl ether ketone), sulfonated poly(arylene ether sulfone) and combinations thereof, without being limited thereto.
- the ion conductive polymer in accordance with the present disclosure is characterized in that all or some of the proton conductive groups are combined with a compound expressed as the following chemical formula: MA x
- M may be a lanthanoid, and particularly, cerium (Ce).
- A may be a hydrophilic functional group, and particularly, one selected from the group consisting of a hydroxyl group, a carboxyl group and a combination thereof.
- X may be a number which is necessary to maintain a charge balance between A and M.
- the compound expressed in chemical formula 1 may be cerium hydroxide (Ce(OH) 3 ), as shown in FIG. 2 .
- the ion conductive polymer may include a carbon skeleton and a side chain expressed in the following structural formula:
- * means an element of the carbon skeleton or an element of the side chain connected to sulfur (S). That is, the structural formula above may be a structural formula expressing a part of the side chain, or a structural formula expressing the entirety of the side chain.
- the proton-conducting channel A is expanded. Thereby, moisture more easily passes through the ion-conducting channel A, and proton conductivity of the electrolyte membrane 1 is greatly improved.
- cerium hydroxide (Ce(OH) 3 ) is used as the compound expressed in MA x , cerium hydroxide (Ce(OH) 3 ) suppresses chemical degradation of the polymer electrolyte membrane 1 caused by a hydroxyl or hydroperoxyl radical, and thus, chemical durability of the electrolyte membrane 1 is greatly improved.
- FIG. 3 is a cross-sectional view schematically illustrating an electrolyte membrane 1 in accordance with another embodiment of the present disclosure.
- the electrolyte membrane 1 may include a porous reinforcing layer 11 , and ion transport layers 12 provided to at least one surface of the reinforcing layer 11 .
- the reinforcing layer 11 serves to increase mechanical stiffness of the electrolyte membrane 1 .
- the reinforcing layer 11 may be formed of one selected from the group consisting of polytetrafluoroethylene (PTFE), expanded polytetrafluoroethylene (e-PTFE), polyethylene (PE), polypropylene (PP), poly(phenylene oxide) (PPO), polybenzimidazole (PBI), polyimide (PI), polyvinylidene fluoride (PVdF), polyvinyl chloride (PVC) and combinations thereof, and be a porous membrane having a great number of pores.
- PTFE polytetrafluoroethylene
- e-PTFE expanded polytetrafluoroethylene
- PE polyethylene
- PP polypropylene
- PPO poly(phenylene oxide)
- PBI polybenzimidazole
- PI polyimide
- PVdF polyvinylidene fluoride
- PVC polyvinyl chloride
- the reinforcing layer 11 may be a layer which is impregnated with the above-described ion conductive polymer. Further, the ion transport layer 12 may include the ion conductive polymer.
- the electrodes 2 include an anode 2 ′ which is reacted with hydrogen gas, and a cathode 2 ′′ which is reacted with oxygen gas in air.
- the anode 2 ′ splits hydrogen into protons and electrons through hydrogen oxidation reaction (HOR).
- the protons move to the cathode 2 ′′ through the electrolyte membrane 1 contacting the anode 2 ′.
- the electrons move to the cathode 2 ′′ through an external line (not shown).
- the electrodes 2 may include catalyst particles, such as carbon-supported platinum (Pt) or the like. Further, the electrodes 2 may include the above-described ion conductive polymer so as to conduct protons in the electrodes 2 . However, the electrodes 2 may include a different kind of ionomer from the ion conductive polymer.
- FIG. 4 is a flowchart representing a method for manufacturing a membrane-electrode assembly in accordance with the present disclosure.
- the method includes preparing a dispersion liquid including a cerium precursor at S 10 , preparing a mixture including the dispersion liquid and an ion conductive polymer including proton conductive groups at S 20 , adding an acid solution to the mixture and reacting the mixture with the acid solution at S 30 , manufacturing an electrolyte membrane as a product of the reaction at S 40 and forming electrodes on both surfaces of the electrolyte membrane at S 50 .
- the dispersion liquid may be prepared by dispersing the cerium precursor into a polar solvent at S 10 .
- the cerium precursor may include one selected from the group consisting of cerium isopropoxide (Ce(OC 3 H 7 ) 4 , cerium (III) acetate hydrate (Ce(CH 3 CO 2 ) 3 .xH 2 O), cerium (III) acetylacetonate hydrate (Ce(C 5 H 7 O 2 ) 3 .xH 2 O), cerium (III) oxalate hydrate (Ce 2 (C 2 O 4 ) 3 .xH 2 O), cerium trifluoromethanesulfonate (Ce n (CF 3 SO 3 ) x .H 2 O) and combinations thereof.
- the polar solvent may include one selected from the group consisting of isopropanol, dimethylformamide and a combination thereof, and particularly, a mixed solvent of isopropanol and dimethylformamide may be used.
- the dispersion liquid may be prepared by putting the cerium precursor into the solvent and agitating an obtained mixture for 10 to 600 minutes, particularly, 30 to 300 minutes.
- a dispersion method is not limited to a specific method, and, for example, the dispersion liquid may be agitated at a regular speed using a magnetic bar.
- the agitating time is excessively short, the cerium precursor may not be sufficiently dispersed, and when the agitating time is excessively long, processability may be lowered, and a mixing composition which is originally intended may be changed due to evaporation of isopropanol which volatile alcohol.
- the mixture may be prepared by mixing and agitating the dispersion liquid and the ion conductive polymer including the proton conductive groups at S 20 .
- the ion conductive polymer including the proton conductive groups has been described above, and a detailed description thereof will thus be omitted.
- the mixture may be prepared by weighing respective components such that the content of the cerium precursor is 0.01 to 20 wt %, particularly 0.1 to 10 wt %, with respect to the total content of the cerium precursor and the ion conductive polymer.
- the content of the ion conductive polymer may mean the content of the ion conductive polymer in a solid phase.
- the mixture may be prepared by mixing the dispersion liquid and the ion conductive polymer and then agitating the same for 10 to 300 minutes, particularly 30 to 120 minutes.
- the agitating time is too short, the respective components may not be uniformly dispersed, and when the agitating time is too long, processability may be lowered and the cerium precursor may be decomposed.
- cerium hydroxide caused by the cerium precursor may be combined with all or some of the proton conductive groups of the ion conductive polymer by adding the acid solution to the mixture and reacting the mixture with the acid solution at S 30 .
- the temperature of the mixture is raised to a specific temperature and then the mixture is reacted for a designated time, the cerium precursor is converted into the form of cerium hydroxide.
- Cerium hydroxide is reacted and combined with all or some of the proton conductive groups of the ion conductive polymer.
- the acid solution may include one selected from the group consisting of hydrochloric acid (HCl), sulfuric acid (H 2 SO 4 ), nitric acid (HNO 3 ), phosphoric acid (H 3 PO 4 ), hydrogen iodide (HI) and combinations thereof.
- HCl hydrochloric acid
- SO 4 sulfuric acid
- NO 3 nitric acid
- H 3 PO 4 phosphoric acid
- HI hydrogen iodide
- Such reaction may be performed at a temperature of 50° C. to 150° C., particularly 80° C. to 120° C.
- a reaction speed is too low, and thus, reaction efficiency may be greatly lowered.
- the reaction temperature is excessively high, the ion conductive polymer may be pyrolyzed.
- reaction may be performed for 1 to 24 hours, particularly 2 to 12 hours.
- reaction time is excessively short, the reaction is not sufficiently performed, and thus, reaction efficiency may be greatly lowered.
- reaction time is excessively long, processability may be reduced.
- the ion conductive polymer included in a product obtained through the above reaction may include a carbon skeleton and a side chain expressed in the following structural formula:
- * means an element of the carbon skeleton or an element of the side chain connected to sulfur (S).
- the electrolyte membrane may be manufactured using the product of the above reaction at S 40 .
- Such an electrolyte membrane manufacturing method is not limited to a specific method, and a conventional method may be employed.
- the membrane-electrode assembly may be acquired by attaching a pair of electrodes to both surfaces of the electrolyte membrane at S 50 .
- Such an electrode attachment method is not limited to a specific method, and a conventional method may be employed.
- a dispersion liquid was prepared by putting cerium isopropoxide (Ce(OC 3 H 7 ) 4 as a cerium precursor into a mixed solution of isopropanol and dimethylformamide.
- the dispersion liquid and a perfluorinated sulfonic acid dispersion liquid were mixed, such that the content of cerium isopropoxide was 1.0 wt % with respect to the total content of cerium isopropoxide and perfluorinated sulfonic acid in a solid phase.
- An obtained mixture was agitated at a low temperature (of about 25° C.) for about 2 hours.
- Deionized water and hydrochloric acid solution were put into the mixture, and then the mixture was sufficiently reacted at a temperature of about 120° C. for about 12 hours.
- the perfluorinated sulfonic acid dispersion liquid was applied to a base material and dried, thereby producing an electrolyte membrane.
- Fluoride emissions of the electrolyte membranes according to the example and the comparative example were measured. Measurement results are shown in FIG. 5 .
- the fluoride emission of the electrolyte membrane according to the example is remarkably lower than the fluoride emission of the electrolyte membrane according to the comparative example. This means that chemical durability of the electrolyte membrane according to the example is far above chemical durability of the electrolyte membrane according to the comparative example.
- Proton conductivities of the electrolyte membranes according to the example and the comparative example were measured. Measurement of the proton conductivities was performed at a relative humidity of 100% in temperature sections of 40° C. to 90° C. Measurement results are shown in FIG. 6 .
- the electrolyte membrane according to the example exhibits higher proton conductivity values than the electrolyte membrane according to the comparative example in all of the evaluation temperature sections. Particularly, it may be confirmed that the proton conductivity value of the electrolyte membrane according to the example is much higher than the proton conductivity value of the electrolyte membrane according to the comparative example at a low temperature (of 40° C.).
- the present disclosure may provide an electrolyte membrane having excellent chemical durability and proton conductivity, thus greatly improving durability and performance of a membrane-electrode assembly including the electrolyte membrane.
- the electrolyte membrane according to the present disclosure expands the size of a proton-conducting channel due to cerium hydroxide combined with terminals of side chains of an ion conductive polymer, thereby greatly improving proton conductivity of the membrane-electrode assembly.
- the electrolyte membrane according to the present disclosure suppresses chemical degradation of the polymer electrolyte membrane caused by a hydroxyl or hydroperoxyl radical, thereby greatly improving chemical durability of the membrane-electrode assembly.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Sustainable Development (AREA)
- Sustainable Energy (AREA)
- Composite Materials (AREA)
- Dispersion Chemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Conductive Materials (AREA)
- Fuel Cell (AREA)
Abstract
Description
where * may mean an element of the carbon skeleton or an element of the side chain connected to sulfur (S).
MAx
Claims (14)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020190138258A KR20210052820A (en) | 2019-11-01 | 2019-11-01 | A membrane electrode assembly with excellent durability and proton conductivity and method of manufacturing thereof |
| KR10-2019-0138258 | 2019-11-01 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20210135264A1 US20210135264A1 (en) | 2021-05-06 |
| US11482720B2 true US11482720B2 (en) | 2022-10-25 |
Family
ID=75485458
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/065,140 Active US11482720B2 (en) | 2019-11-01 | 2020-10-07 | Membrane-electrode assembly with improved durability and proton conductivity and method for manufacturing the same |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US11482720B2 (en) |
| KR (1) | KR20210052820A (en) |
| CN (1) | CN112786935A (en) |
| DE (1) | DE102020213449A1 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114276573B (en) * | 2021-12-30 | 2023-05-30 | 上海应用技术大学 | High-durability organic antioxidant chelated cerium ion composite proton exchange membrane, and preparation method and application thereof |
| CN117334975A (en) * | 2022-06-24 | 2024-01-02 | 北京清驰科技有限公司 | Proton exchange membrane and preparation method and application thereof |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140093808A1 (en) * | 2005-10-28 | 2014-04-03 | 3M Innovative Properties Company | High Durability Fuel Cell Components with Cerium Salt Additives |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005015607A (en) * | 2003-06-25 | 2005-01-20 | Toyobo Co Ltd | Sulfonated polymer, composition containing the sulfonated polymer, molding, proton exchange membrane for fuel cell, and method for producing the sulfonated polymer |
| CN100456544C (en) * | 2006-08-18 | 2009-01-28 | 中国科学院上海硅酸盐研究所 | Anode supporting solid electrolyte compound film for solid oxide fuel battery and its preparing method |
| CN101728550B (en) * | 2009-12-10 | 2011-05-04 | 山东东岳神舟新材料有限公司 | Fiber-reinforced stably-doped proton exchange membrane |
| EP3282509B1 (en) * | 2015-04-06 | 2020-09-02 | AGC Inc. | Respective methods for producing liquid composition, solid polymer electrolyte membrane, catalyst layer, and membrane electrode assembly |
| KR20200077014A (en) * | 2018-12-20 | 2020-06-30 | 현대자동차주식회사 | Chemically improved durable electrolyte membrane of membrane electrode assembly and manufacturing method thereof |
-
2019
- 2019-11-01 KR KR1020190138258A patent/KR20210052820A/en active Search and Examination
-
2020
- 2020-10-07 US US17/065,140 patent/US11482720B2/en active Active
- 2020-10-26 DE DE102020213449.9A patent/DE102020213449A1/en active Pending
- 2020-10-28 CN CN202011173651.2A patent/CN112786935A/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140093808A1 (en) * | 2005-10-28 | 2014-04-03 | 3M Innovative Properties Company | High Durability Fuel Cell Components with Cerium Salt Additives |
Non-Patent Citations (6)
| Title |
|---|
| Curtin, D.E., et al., "Advanced materials for improved PEMFC performance and life," Journal of Power Sources, vol. 131, pp. 41-48 (2004). |
| Trogadas, P. et al., "Degradation Mitigation in Polymer Electrolyte Membranes Using Cerium Oxide as a Regenerative Free-Radical Scavenger," Electrochemical and Solid-State Letters, vol. 11, pp. B113-B116 (2008). |
| Uegaki, R., et al., "Radical-induced degradation mechanism of perfluorinated polymer electrolyte membrane," Journal of Power Sources, vol. 196, pp. 9856-9986 (2011). |
| Yamato et al. (J. Chem. Research (S), 2002, 400-402). * |
| Young, A.P., et al., "Ionomer Degradation in Polymer Electrolyte Membrane Fuel Cells," Journal of The Electrochemical Society, vol. 157, pp. B425-B436 (2010). |
| Zhao, D., et al., "Cesium substituted 12-tungstophosphoric (CsxH3-xPW12O40) loaded on ceria-degradation mitigation in polymer electrolyte membranes," Journal of Power Sources, vol. 190, pp. 301-306 (2009). |
Also Published As
| Publication number | Publication date |
|---|---|
| DE102020213449A1 (en) | 2021-05-06 |
| CN112786935A (en) | 2021-05-11 |
| KR20210052820A (en) | 2021-05-11 |
| US20210135264A1 (en) | 2021-05-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100971640B1 (en) | Electrolyte membrane for solid polymer fuel cell, method for producing same and membrane electrode assembly for solid polymer fuel cell | |
| US20100081028A1 (en) | Binder for a fuel cell catalyst composition, a membrane electrode assembly for a fuel cell using the binder and a method for preparing a membrane electrode assembly | |
| US8257825B2 (en) | Polymer electrode membrane for fuel, and membrane-electrode assembly and fuel cell system comprising the same | |
| US20080118808A1 (en) | Electrolyte membrane for polymer electrolyte fuel cell, process for its production and membrane-electrode assembly for polymer electrolyte fuel cell | |
| US7179560B2 (en) | Composite electrolyte membrane and fuel cell containing the same | |
| US20090209668A1 (en) | Reinforced composite membrane for polymer electrolyte fuel cell | |
| US11482720B2 (en) | Membrane-electrode assembly with improved durability and proton conductivity and method for manufacturing the same | |
| US9711817B2 (en) | Polymer electrolyte membrane and membrane-electrode assembly for polymer electrolyte fuel cell | |
| JP5557430B2 (en) | PROTON CONDUCTIVE POLYMER ELECTROLYTE MEMBRANE, PROCESS FOR PRODUCING THE SAME, MEMBRANE-ELECTRODE ASSEMBLY USING THE SAME, AND POLYMER ELECTROLYTE FUEL CELL | |
| JP5556081B2 (en) | Ion conductive composite electrolyte membrane and fuel cell using the same | |
| US7488549B2 (en) | Proton conducting polymer, polymer membrane comprising the same, method of manufacturing the polymer membrane, and fuel cell using the polymer membrane | |
| JP5233065B2 (en) | Polymer having ionic group, polymer electrolyte material, polymer electrolyte component, membrane electrode composite, and polymer electrolyte fuel cell | |
| US20110165497A1 (en) | Method for Mitigating Fuel Cell Chemical Degradation | |
| KR20070023706A (en) | Liquid composition, method for producing same, and method for producing membrane electrode assembly for solid polymer fuel cell | |
| KR101334088B1 (en) | Composition for electrode coating of high temperature polymer electrolyte membrane fuel cell and preparation method of electrode for high temperature polymer electrolyte membrane fuel cell | |
| US20220367893A1 (en) | Durable membrane-electrode assembly with high ionic conductivity and method of manufacturing same | |
| US20040096731A1 (en) | Electrode structure for polymer electrolyte fuel cell and method for manufacturing the same | |
| US7758986B2 (en) | Proton conductor, polymer electrolyte comprising the same and fuel cell employing the polymer electrolyte | |
| US10461351B2 (en) | Polymer electrolyte membrane for fuel cell, membrane-electrode assembly for fuel cell including same, and fuel cell including same | |
| JP4742664B2 (en) | Polymer having ionic group, polymer electrolyte material, and polymer electrolyte fuel cell | |
| US7862922B2 (en) | Polymer electrolyte membrane for fuel cell and fuel cell system comprising same | |
| JP2014234445A (en) | Polymer electrolyte composition, as well as polymer electrolyte membrane, electrode catalyst layer, membrane electrode assembly, and solid polymer fuel cell using the same | |
| KR102136167B1 (en) | Polymer Electrolyte Membrane Applicable to Low Humidity Conditions and a Manufacturing Method Thereof | |
| JP4992184B2 (en) | Polymer having ionic group, polymer electrolyte material, polymer electrolyte component, membrane electrode composite, and polymer electrolyte fuel cell | |
| JP2007039536A (en) | Ionic group-containing polymer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: INDUSTRY FOUNDATION OF CHONNAM NATIONAL UNIVERSITY, KOREA, REPUBLIC OF Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:PARK, IN YU;HONG, BO KI;HONG, JAE WOON;AND OTHERS;REEL/FRAME:054001/0026 Effective date: 20200924 Owner name: KIA MOTORS CORPORATION, KOREA, REPUBLIC OF Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:PARK, IN YU;HONG, BO KI;HONG, JAE WOON;AND OTHERS;REEL/FRAME:054001/0026 Effective date: 20200924 Owner name: HYUNDAI MOTOR COMPANY, KOREA, REPUBLIC OF Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:PARK, IN YU;HONG, BO KI;HONG, JAE WOON;AND OTHERS;REEL/FRAME:054001/0026 Effective date: 20200924 |
|
| FEPP | Fee payment procedure |
Free format text: ENTITY STATUS SET TO UNDISCOUNTED (ORIGINAL EVENT CODE: BIG.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: APPLICATION DISPATCHED FROM PREEXAM, NOT YET DOCKETED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: DOCKETED NEW CASE - READY FOR EXAMINATION |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NON FINAL ACTION MAILED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: FINAL REJECTION MAILED |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: RESPONSE AFTER FINAL ACTION FORWARDED TO EXAMINER |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: NOTICE OF ALLOWANCE MAILED -- APPLICATION RECEIVED IN OFFICE OF PUBLICATIONS |
|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: PUBLICATIONS -- ISSUE FEE PAYMENT VERIFIED |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |