TW202146385A - Substituted pyrrolidine compound and use thereof in medicine - Google Patents
Substituted pyrrolidine compound and use thereof in medicine Download PDFInfo
- Publication number
- TW202146385A TW202146385A TW110114976A TW110114976A TW202146385A TW 202146385 A TW202146385 A TW 202146385A TW 110114976 A TW110114976 A TW 110114976A TW 110114976 A TW110114976 A TW 110114976A TW 202146385 A TW202146385 A TW 202146385A
- Authority
- TW
- Taiwan
- Prior art keywords
- alkyl
- methyl
- atoms
- ethyl
- propyl
- Prior art date
Links
- -1 pyrrolidine compound Chemical class 0.000 title claims abstract description 300
- 239000003814 drug Substances 0.000 title claims abstract description 39
- RWRDLPDLKQPQOW-UHFFFAOYSA-N tetrahydropyrrole Natural products C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 title abstract 6
- 150000001875 compounds Chemical class 0.000 claims abstract description 365
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 claims abstract description 46
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 43
- 150000003839 salts Chemical class 0.000 claims abstract description 39
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 38
- 201000010099 disease Diseases 0.000 claims abstract description 30
- 229940002612 prodrug Drugs 0.000 claims abstract description 28
- 239000000651 prodrug Substances 0.000 claims abstract description 28
- 201000008937 atopic dermatitis Diseases 0.000 claims abstract description 24
- 239000012453 solvate Substances 0.000 claims abstract description 24
- 206010012438 Dermatitis atopic Diseases 0.000 claims abstract description 21
- 239000002207 metabolite Substances 0.000 claims abstract description 20
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 claims abstract description 16
- 238000002360 preparation method Methods 0.000 claims abstract description 15
- 125000000217 alkyl group Chemical group 0.000 claims description 161
- 125000004429 atom Chemical group 0.000 claims description 143
- 229910052805 deuterium Inorganic materials 0.000 claims description 102
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims description 98
- 239000000460 chlorine Substances 0.000 claims description 78
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 77
- 125000000623 heterocyclic group Chemical group 0.000 claims description 75
- 229910052801 chlorine Inorganic materials 0.000 claims description 63
- 229910052731 fluorine Inorganic materials 0.000 claims description 63
- 229910052794 bromium Inorganic materials 0.000 claims description 62
- 229910052739 hydrogen Inorganic materials 0.000 claims description 62
- 239000001257 hydrogen Substances 0.000 claims description 62
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 60
- 125000001424 substituent group Chemical group 0.000 claims description 59
- 229910052740 iodine Inorganic materials 0.000 claims description 58
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 claims description 56
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 53
- 150000002431 hydrogen Chemical class 0.000 claims description 51
- 125000003545 alkoxy group Chemical group 0.000 claims description 49
- 125000001072 heteroaryl group Chemical group 0.000 claims description 48
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 46
- 125000003118 aryl group Chemical group 0.000 claims description 38
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 37
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 35
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 34
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 34
- 125000000719 pyrrolidinyl group Chemical group 0.000 claims description 33
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 claims description 32
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 claims description 31
- 125000004193 piperazinyl group Chemical group 0.000 claims description 30
- 125000003386 piperidinyl group Chemical group 0.000 claims description 30
- 125000002757 morpholinyl group Chemical group 0.000 claims description 28
- 125000004568 thiomorpholinyl group Chemical group 0.000 claims description 28
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 26
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 claims description 26
- 125000003226 pyrazolyl group Chemical group 0.000 claims description 25
- 125000004076 pyridyl group Chemical group 0.000 claims description 25
- 125000001412 tetrahydropyranyl group Chemical group 0.000 claims description 25
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 claims description 24
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 claims description 24
- 125000002883 imidazolyl group Chemical group 0.000 claims description 24
- 125000000168 pyrrolyl group Chemical group 0.000 claims description 24
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 claims description 23
- 125000000335 thiazolyl group Chemical group 0.000 claims description 23
- 125000001544 thienyl group Chemical group 0.000 claims description 23
- 125000002541 furyl group Chemical group 0.000 claims description 22
- 125000002098 pyridazinyl group Chemical group 0.000 claims description 22
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 22
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 21
- 125000002971 oxazolyl group Chemical group 0.000 claims description 21
- WAEDMQMDOHQPFL-UHFFFAOYSA-N n,n-bis(2-chloroethyl)propan-2-amine Chemical compound ClCCN(C(C)C)CCCl WAEDMQMDOHQPFL-UHFFFAOYSA-N 0.000 claims description 20
- 125000003373 pyrazinyl group Chemical group 0.000 claims description 19
- UQZPGHOJMQTOHB-UHFFFAOYSA-N 2-chloro-n-(2-chloroethyl)-n-ethylethanamine Chemical compound ClCCN(CC)CCCl UQZPGHOJMQTOHB-UHFFFAOYSA-N 0.000 claims description 18
- 125000003342 alkenyl group Chemical group 0.000 claims description 18
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 claims description 17
- 206010006451 bronchitis Diseases 0.000 claims description 16
- 125000004850 cyclobutylmethyl group Chemical group C1(CCC1)C* 0.000 claims description 16
- 125000004210 cyclohexylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 claims description 16
- 125000004851 cyclopentylmethyl group Chemical group C1(CCCC1)C* 0.000 claims description 16
- 125000000304 alkynyl group Chemical group 0.000 claims description 15
- 125000004122 cyclic group Chemical group 0.000 claims description 14
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 claims description 13
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 claims description 13
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 claims description 13
- 125000004186 cyclopropylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C1([H])[H] 0.000 claims description 13
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 claims description 13
- 230000004054 inflammatory process Effects 0.000 claims description 13
- 238000011282 treatment Methods 0.000 claims description 13
- 125000006017 1-propenyl group Chemical group 0.000 claims description 12
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 claims description 12
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 claims description 12
- 102000004861 Phosphoric Diester Hydrolases Human genes 0.000 claims description 12
- 108090001050 Phosphoric Diester Hydrolases Proteins 0.000 claims description 12
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 12
- 229940124597 therapeutic agent Drugs 0.000 claims description 12
- 125000003282 alkyl amino group Chemical group 0.000 claims description 11
- 239000003937 drug carrier Substances 0.000 claims description 11
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 10
- 206010061218 Inflammation Diseases 0.000 claims description 10
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 8
- 125000006621 (C3-C8) cycloalkyl-(C1-C6) alkyl group Chemical group 0.000 claims description 8
- 125000005842 heteroatom Chemical group 0.000 claims description 8
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 claims description 8
- 208000023504 respiratory system disease Diseases 0.000 claims description 8
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 claims description 8
- 239000002671 adjuvant Substances 0.000 claims description 7
- 125000001624 naphthyl group Chemical group 0.000 claims description 7
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 claims description 7
- 229920002554 vinyl polymer Polymers 0.000 claims description 7
- ABADUMLIAZCWJD-UHFFFAOYSA-N 1,3-dioxole Chemical compound C1OC=CO1 ABADUMLIAZCWJD-UHFFFAOYSA-N 0.000 claims description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 6
- 206010001052 Acute respiratory distress syndrome Diseases 0.000 claims description 6
- 206010009900 Colitis ulcerative Diseases 0.000 claims description 6
- 208000013616 Respiratory Distress Syndrome Diseases 0.000 claims description 6
- 201000006704 Ulcerative Colitis Diseases 0.000 claims description 6
- 201000000028 adult respiratory distress syndrome Diseases 0.000 claims description 6
- 208000006673 asthma Diseases 0.000 claims description 6
- 125000002393 azetidinyl group Chemical group 0.000 claims description 6
- 125000004043 oxo group Chemical group O=* 0.000 claims description 6
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 6
- 229960002586 roflumilast Drugs 0.000 claims description 6
- MNDBXUUTURYVHR-UHFFFAOYSA-N roflumilast Chemical compound FC(F)OC1=CC=C(C(=O)NC=2C(=CN=CC=2Cl)Cl)C=C1OCC1CC1 MNDBXUUTURYVHR-UHFFFAOYSA-N 0.000 claims description 6
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 claims description 6
- 239000003981 vehicle Substances 0.000 claims description 6
- 206010020751 Hypersensitivity Diseases 0.000 claims description 5
- 201000001263 Psoriatic Arthritis Diseases 0.000 claims description 5
- 208000036824 Psoriatic arthropathy Diseases 0.000 claims description 5
- 206010039085 Rhinitis allergic Diseases 0.000 claims description 5
- 208000026935 allergic disease Diseases 0.000 claims description 5
- 201000010105 allergic rhinitis Diseases 0.000 claims description 5
- 230000007815 allergy Effects 0.000 claims description 5
- IMOZEMNVLZVGJZ-QGZVFWFLSA-N apremilast Chemical compound C1=C(OC)C(OCC)=CC([C@@H](CS(C)(=O)=O)N2C(C3=C(NC(C)=O)C=CC=C3C2=O)=O)=C1 IMOZEMNVLZVGJZ-QGZVFWFLSA-N 0.000 claims description 5
- 229960001164 apremilast Drugs 0.000 claims description 5
- 206010003246 arthritis Diseases 0.000 claims description 5
- 208000015114 central nervous system disease Diseases 0.000 claims description 5
- 230000001684 chronic effect Effects 0.000 claims description 5
- HJORMJIFDVBMOB-UHFFFAOYSA-N rolipram Chemical compound COC1=CC=C(C2CC(=O)NC2)C=C1OC1CCCC1 HJORMJIFDVBMOB-UHFFFAOYSA-N 0.000 claims description 5
- 229950005741 rolipram Drugs 0.000 claims description 5
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 claims description 4
- HOKIHKLKOZWCRY-UHFFFAOYSA-N 2-[6-[2-(3,5-dichloropyridin-4-yl)acetyl]-2,3-dimethoxyphenoxy]-n-propylacetamide Chemical compound C1=CC(OC)=C(OC)C(OCC(=O)NCCC)=C1C(=O)CC1=C(Cl)C=NC=C1Cl HOKIHKLKOZWCRY-UHFFFAOYSA-N 0.000 claims description 4
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 claims description 4
- ZISJNXNHJRQYJO-CMDGGOBGSA-N 5-[(e)-2-phenylethenyl]-2-propan-2-ylbenzene-1,3-diol Chemical compound C1=C(O)C(C(C)C)=C(O)C=C1\C=C\C1=CC=CC=C1 ZISJNXNHJRQYJO-CMDGGOBGSA-N 0.000 claims description 4
- 206010052613 Allergic bronchitis Diseases 0.000 claims description 4
- 206010002556 Ankylosing Spondylitis Diseases 0.000 claims description 4
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 claims description 4
- 206010006458 Bronchitis chronic Diseases 0.000 claims description 4
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 claims description 4
- LERNTVKEWCAPOY-VOGVJGKGSA-N C[N+]1(C)[C@H]2C[C@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 Chemical compound C[N+]1(C)[C@H]2C[C@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 LERNTVKEWCAPOY-VOGVJGKGSA-N 0.000 claims description 4
- LUKZNWIVRBCLON-GXOBDPJESA-N Ciclesonide Chemical compound C1([C@H]2O[C@@]3([C@H](O2)C[C@@H]2[C@@]3(C[C@H](O)[C@@H]3[C@@]4(C)C=CC(=O)C=C4CC[C@H]32)C)C(=O)COC(=O)C(C)C)CCCCC1 LUKZNWIVRBCLON-GXOBDPJESA-N 0.000 claims description 4
- 206010010744 Conjunctivitis allergic Diseases 0.000 claims description 4
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 claims description 4
- 108010036949 Cyclosporine Proteins 0.000 claims description 4
- 201000003883 Cystic fibrosis Diseases 0.000 claims description 4
- 206010014561 Emphysema Diseases 0.000 claims description 4
- 108010008165 Etanercept Proteins 0.000 claims description 4
- 208000005615 Interstitial Cystitis Diseases 0.000 claims description 4
- 229940125772 JTE-052 Drugs 0.000 claims description 4
- 206010029888 Obliterative bronchiolitis Diseases 0.000 claims description 4
- 206010035664 Pneumonia Diseases 0.000 claims description 4
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 claims description 4
- 239000004012 Tofacitinib Substances 0.000 claims description 4
- VGXACJMXDYPFDB-SXMMONRFSA-N [(3r)-1-azabicyclo[2.2.2]octan-3-yl] (2s)-2-(hydroxymethyl)-4-[(r)-methylsulfinyl]-2-phenylbutanoate Chemical compound C1([C@@](CO)(C(=O)O[C@@H]2C3CCN(CC3)C2)CC[S@](=O)C)=CC=CC=C1 VGXACJMXDYPFDB-SXMMONRFSA-N 0.000 claims description 4
- 208000038016 acute inflammation Diseases 0.000 claims description 4
- 229960002964 adalimumab Drugs 0.000 claims description 4
- ISBHYKVAFKTATD-SNVBAGLBSA-N adriforant Chemical compound C1[C@H](NC)CCN1C1=CC(NCC2CC2)=NC(N)=N1 ISBHYKVAFKTATD-SNVBAGLBSA-N 0.000 claims description 4
- 208000002205 allergic conjunctivitis Diseases 0.000 claims description 4
- ATALOFNDEOCMKK-OITMNORJSA-N aprepitant Chemical compound O([C@@H]([C@@H]1C=2C=CC(F)=CC=2)O[C@H](C)C=2C=C(C=C(C=2)C(F)(F)F)C(F)(F)F)CCN1CC1=NNC(=O)N1 ATALOFNDEOCMKK-OITMNORJSA-N 0.000 claims description 4
- 229960001372 aprepitant Drugs 0.000 claims description 4
- 208000024998 atopic conjunctivitis Diseases 0.000 claims description 4
- 229950000971 baricitinib Drugs 0.000 claims description 4
- XUZMWHLSFXCVMG-UHFFFAOYSA-N baricitinib Chemical compound C1N(S(=O)(=O)CC)CC1(CC#N)N1N=CC(C=2C=3C=CNC=3N=CN=2)=C1 XUZMWHLSFXCVMG-UHFFFAOYSA-N 0.000 claims description 4
- 229950000210 beclometasone dipropionate Drugs 0.000 claims description 4
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 4
- UREBDLICKHMUKA-DVTGEIKXSA-N betamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-DVTGEIKXSA-N 0.000 claims description 4
- 229960002537 betamethasone Drugs 0.000 claims description 4
- 201000009267 bronchiectasis Diseases 0.000 claims description 4
- 201000003848 bronchiolitis obliterans Diseases 0.000 claims description 4
- 208000023367 bronchiolitis obliterans with obstructive pulmonary disease Diseases 0.000 claims description 4
- 229960004436 budesonide Drugs 0.000 claims description 4
- LWQQLNNNIPYSNX-UROSTWAQSA-N calcipotriol Chemical compound C1([C@H](O)/C=C/[C@@H](C)[C@@H]2[C@]3(CCCC(/[C@@H]3CC2)=C\C=C\2C([C@@H](O)C[C@H](O)C/2)=C)C)CC1 LWQQLNNNIPYSNX-UROSTWAQSA-N 0.000 claims description 4
- 229960002882 calcipotriol Drugs 0.000 claims description 4
- GMRQFYUYWCNGIN-NKMMMXOESA-N calcitriol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C GMRQFYUYWCNGIN-NKMMMXOESA-N 0.000 claims description 4
- 229960005084 calcitriol Drugs 0.000 claims description 4
- 235000020964 calcitriol Nutrition 0.000 claims description 4
- 239000011612 calcitriol Substances 0.000 claims description 4
- CFBUZOUXXHZCFB-OYOVHJISSA-N chembl511115 Chemical compound COC1=CC=C([C@@]2(CC[C@H](CC2)C(O)=O)C#N)C=C1OC1CCCC1 CFBUZOUXXHZCFB-OYOVHJISSA-N 0.000 claims description 4
- 208000007451 chronic bronchitis Diseases 0.000 claims description 4
- 229960003728 ciclesonide Drugs 0.000 claims description 4
- 229960001265 ciclosporin Drugs 0.000 claims description 4
- 229950001653 cilomilast Drugs 0.000 claims description 4
- 229930182912 cyclosporin Natural products 0.000 claims description 4
- LOWWYYZBZNSPDT-ZBEGNZNMSA-N delgocitinib Chemical compound C[C@H]1CN(C(=O)CC#N)[C@@]11CN(C=2C=3C=CNC=3N=CN=2)CC1 LOWWYYZBZNSPDT-ZBEGNZNMSA-N 0.000 claims description 4
- WBGKWQHBNHJJPZ-LECWWXJVSA-N desonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O WBGKWQHBNHJJPZ-LECWWXJVSA-N 0.000 claims description 4
- 229960003662 desonide Drugs 0.000 claims description 4
- 206010062952 diffuse panbronchiolitis Diseases 0.000 claims description 4
- 229960000403 etanercept Drugs 0.000 claims description 4
- 229960000676 flunisolide Drugs 0.000 claims description 4
- FEBLZLNTKCEFIT-VSXGLTOVSA-N fluocinolone acetonide Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O FEBLZLNTKCEFIT-VSXGLTOVSA-N 0.000 claims description 4
- 229960000289 fluticasone propionate Drugs 0.000 claims description 4
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 claims description 4
- 229960002848 formoterol Drugs 0.000 claims description 4
- BPZSYCZIITTYBL-UHFFFAOYSA-N formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1 BPZSYCZIITTYBL-UHFFFAOYSA-N 0.000 claims description 4
- 229960002462 glycopyrronium bromide Drugs 0.000 claims description 4
- 150000004677 hydrates Chemical class 0.000 claims description 4
- 229960000890 hydrocortisone Drugs 0.000 claims description 4
- 125000001041 indolyl group Chemical group 0.000 claims description 4
- 229960000598 infliximab Drugs 0.000 claims description 4
- 229960001361 ipratropium bromide Drugs 0.000 claims description 4
- KEWHKYJURDBRMN-ZEODDXGYSA-M ipratropium bromide hydrate Chemical compound O.[Br-].O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 KEWHKYJURDBRMN-ZEODDXGYSA-M 0.000 claims description 4
- BBTFKAOFCSOZMB-UHFFFAOYSA-N methyl 4-[[3-[6,7-dimethoxy-2-(methylamino)quinazolin-4-yl]phenyl]carbamoyl]benzoate Chemical compound C=12C=C(OC)C(OC)=CC2=NC(NC)=NC=1C(C=1)=CC=CC=1NC(=O)C1=CC=C(C(=O)OC)C=C1 BBTFKAOFCSOZMB-UHFFFAOYSA-N 0.000 claims description 4
- 229960001664 mometasone Drugs 0.000 claims description 4
- QLIIKPVHVRXHRI-CXSFZGCWSA-N mometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)C[C@@H]2O QLIIKPVHVRXHRI-CXSFZGCWSA-N 0.000 claims description 4
- 229960002744 mometasone furoate Drugs 0.000 claims description 4
- WOFMFGQZHJDGCX-ZULDAHANSA-N mometasone furoate Chemical compound O([C@]1([C@@]2(C)C[C@H](O)[C@]3(Cl)[C@@]4(C)C=CC(=O)C=C4CC[C@H]3[C@@H]2C[C@H]1C)C(=O)CCl)C(=O)C1=CC=CO1 WOFMFGQZHJDGCX-ZULDAHANSA-N 0.000 claims description 4
- LZLBRISQTJVZNP-UHFFFAOYSA-N n-(4-ethylphenyl)-3-(hydroxymethyl)-n-(2-methylpropyl)-4-(oxan-4-ylmethoxy)benzenesulfonamide Chemical compound C1=CC(CC)=CC=C1N(CC(C)C)S(=O)(=O)C(C=C1CO)=CC=C1OCC1CCOCC1 LZLBRISQTJVZNP-UHFFFAOYSA-N 0.000 claims description 4
- VFBILHPIHUPBPZ-UHFFFAOYSA-N n-[[2-[4-(difluoromethoxy)-3-propan-2-yloxyphenyl]-1,3-oxazol-4-yl]methyl]-2-ethoxybenzamide Chemical compound CCOC1=CC=CC=C1C(=O)NCC1=COC(C=2C=C(OC(C)C)C(OC(F)F)=CC=2)=N1 VFBILHPIHUPBPZ-UHFFFAOYSA-N 0.000 claims description 4
- KASDHRXLYQOAKZ-ZPSXYTITSA-N pimecrolimus Chemical compound C/C([C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@@H](OC)C[C@@H](C)C/C(C)=C/[C@H](C(C[C@H](O)[C@H]1C)=O)CC)=C\[C@@H]1CC[C@@H](Cl)[C@H](OC)C1 KASDHRXLYQOAKZ-ZPSXYTITSA-N 0.000 claims description 4
- 229960005330 pimecrolimus Drugs 0.000 claims description 4
- 206010035653 pneumoconiosis Diseases 0.000 claims description 4
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 claims description 4
- 229950000915 revatropate Drugs 0.000 claims description 4
- 229960004017 salmeterol Drugs 0.000 claims description 4
- 229960000278 theophylline Drugs 0.000 claims description 4
- 229960000257 tiotropium bromide Drugs 0.000 claims description 4
- 229960001350 tofacitinib Drugs 0.000 claims description 4
- UJLAWZDWDVHWOW-YPMHNXCESA-N tofacitinib Chemical compound C[C@@H]1CCN(C(=O)CC#N)C[C@@H]1N(C)C1=NC=NC2=C1C=CN2 UJLAWZDWDVHWOW-YPMHNXCESA-N 0.000 claims description 4
- 229950000835 tralokinumab Drugs 0.000 claims description 4
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 claims description 4
- 229960005294 triamcinolone Drugs 0.000 claims description 4
- XUYMIRYNRKXKOR-JBWFSBJDSA-N (7R)-N-[(5-ethylsulfonylpyridin-2-yl)methyl]-7-propan-2-yl-6-[[4-(trifluoromethyl)cyclohexyl]methyl]-5,7-dihydropyrrolo[3,4-b]pyridine-3-carboxamide Chemical compound CCS(=O)(=O)c1ccc(CNC(=O)c2cnc3[C@@H](C(C)C)N(CC4CCC(CC4)C(F)(F)F)Cc3c2)nc1 XUYMIRYNRKXKOR-JBWFSBJDSA-N 0.000 claims description 3
- 125000004767 (C1-C4) haloalkoxy group Chemical group 0.000 claims description 3
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 claims description 3
- 125000004890 (C1-C6) alkylamino group Chemical group 0.000 claims description 3
- 125000006719 (C6-C10) aryl (C1-C6) alkyl group Chemical group 0.000 claims description 3
- CBRJPFGIXUFMTM-WDEREUQCSA-N 1-[(2S,5R)-2-methyl-5-(7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)piperidin-1-yl]prop-2-en-1-one Chemical compound N1=CN=C(C2=C1NC=C2)N[C@@H]2CC[C@@H](N(C2)C(C=C)=O)C CBRJPFGIXUFMTM-WDEREUQCSA-N 0.000 claims description 3
- ICMFYVOUDGRBLG-NJMNTPMDSA-N 2-[3-[(5R)-5-[(7-fluoro-1,1-dimethyl-2,3-dihydroinden-5-yl)carbamoyl]-2-methoxy-7,8-dihydro-5H-1,6-naphthyridine-6-carbonyl]cyclobutyl]acetic acid Chemical compound COc1ccc2[C@@H](N(CCc2n1)C(=O)C1CC(CC(O)=O)C1)C(=O)Nc1cc2CCC(C)(C)c2c(F)c1 ICMFYVOUDGRBLG-NJMNTPMDSA-N 0.000 claims description 3
- XDBHURGONHZNJF-UHFFFAOYSA-N 6-[2-(3,4-diethoxyphenyl)-1,3-thiazol-4-yl]pyridine-2-carboxylic acid Chemical compound C1=C(OCC)C(OCC)=CC=C1C1=NC(C=2N=C(C=CC=2)C(O)=O)=CS1 XDBHURGONHZNJF-UHFFFAOYSA-N 0.000 claims description 3
- JKDGKIBAOAFRPJ-ZBINZKHDSA-N C(C)[C@H]1[C@H](NC([C@H]1F)=O)COC1=NC=CC2=CC(=C(C=C12)OC)C(=O)N Chemical compound C(C)[C@H]1[C@H](NC([C@H]1F)=O)COC1=NC=CC2=CC(=C(C=C12)OC)C(=O)N JKDGKIBAOAFRPJ-ZBINZKHDSA-N 0.000 claims description 3
- 206010012434 Dermatitis allergic Diseases 0.000 claims description 3
- BUWBRTXGQRBBHG-MJBXVCDLSA-N FC1([C@@H](C1)C(=O)N1[C@H]2CN(C[C@@H]1CC2)C1=NC(=NC=C1)NC=1C=NN(C=1)C)F Chemical compound FC1([C@@H](C1)C(=O)N1[C@H]2CN(C[C@@H]1CC2)C1=NC(=NC=C1)NC=1C=NN(C=1)C)F BUWBRTXGQRBBHG-MJBXVCDLSA-N 0.000 claims description 3
- 206010016654 Fibrosis Diseases 0.000 claims description 3
- VPNYRYCIDCJBOM-UHFFFAOYSA-M Glycopyrronium bromide Chemical compound [Br-].C1[N+](C)(C)CCC1OC(=O)C(O)(C=1C=CC=CC=1)C1CCCC1 VPNYRYCIDCJBOM-UHFFFAOYSA-M 0.000 claims description 3
- ZJVFLBOZORBYFE-UHFFFAOYSA-N Ibudilast Chemical compound C1=CC=CC2=C(C(=O)C(C)C)C(C(C)C)=NN21 ZJVFLBOZORBYFE-UHFFFAOYSA-N 0.000 claims description 3
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 claims description 3
- OGQICQVSFDPSEI-UHFFFAOYSA-N Zorac Chemical compound N1=CC(C(=O)OCC)=CC=C1C#CC1=CC=C(SCCC2(C)C)C2=C1 OGQICQVSFDPSEI-UHFFFAOYSA-N 0.000 claims description 3
- IUEWXNHSKRWHDY-PHIMTYICSA-N abrocitinib Chemical compound C1[C@@H](NS(=O)(=O)CCC)C[C@H]1N(C)C1=NC=NC2=C1C=CN2 IUEWXNHSKRWHDY-PHIMTYICSA-N 0.000 claims description 3
- 208000010668 atopic eczema Diseases 0.000 claims description 3
- 229950000321 benralizumab Drugs 0.000 claims description 3
- 229960002842 clobetasol Drugs 0.000 claims description 3
- CBGUOGMQLZIXBE-XGQKBEPLSA-N clobetasol propionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CBGUOGMQLZIXBE-XGQKBEPLSA-N 0.000 claims description 3
- HWXIGFIVGWUZAO-UHFFFAOYSA-N doxofylline Chemical compound C1=2C(=O)N(C)C(=O)N(C)C=2N=CN1CC1OCCO1 HWXIGFIVGWUZAO-UHFFFAOYSA-N 0.000 claims description 3
- 229960004483 doxofylline Drugs 0.000 claims description 3
- 230000004761 fibrosis Effects 0.000 claims description 3
- 229940043075 fluocinolone Drugs 0.000 claims description 3
- 229960001743 golimumab Drugs 0.000 claims description 3
- 229940115747 halobetasol Drugs 0.000 claims description 3
- 229960002491 ibudilast Drugs 0.000 claims description 3
- 229960004078 indacaterol Drugs 0.000 claims description 3
- QZZUEBNBZAPZLX-QFIPXVFZSA-N indacaterol Chemical compound N1C(=O)C=CC2=C1C(O)=CC=C2[C@@H](O)CNC1CC(C=C(C(=C2)CC)CC)=C2C1 QZZUEBNBZAPZLX-QFIPXVFZSA-N 0.000 claims description 3
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 claims description 3
- NVOYVOBDTVTBDX-PMEUIYRNSA-N oxitropium Chemical compound CC[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)C1=CC=CC=C1 NVOYVOBDTVTBDX-PMEUIYRNSA-N 0.000 claims description 3
- 229960000797 oxitropium Drugs 0.000 claims description 3
- 230000002265 prevention Effects 0.000 claims description 3
- 125000005344 pyridylmethyl group Chemical group [H]C1=C([H])C([H])=C([H])C(=N1)C([H])([H])* 0.000 claims description 3
- 229940054269 sodium pyruvate Drugs 0.000 claims description 3
- 229960000565 tazarotene Drugs 0.000 claims description 3
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 3
- 229950002896 tetomilast Drugs 0.000 claims description 3
- 229960003989 tocilizumab Drugs 0.000 claims description 3
- 229960002117 triamcinolone acetonide Drugs 0.000 claims description 3
- YNDXUCZADRHECN-JNQJZLCISA-N triamcinolone acetonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O YNDXUCZADRHECN-JNQJZLCISA-N 0.000 claims description 3
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 3
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims description 3
- LEHFPXVYPMWYQD-XHIJKXOTSA-N ulobetasol Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)CCl)(O)[C@@]2(C)C[C@@H]1O LEHFPXVYPMWYQD-XHIJKXOTSA-N 0.000 claims description 3
- 229960004026 vilanterol Drugs 0.000 claims description 3
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 claims description 2
- 229960005012 aclidinium bromide Drugs 0.000 claims description 2
- 150000001335 aliphatic alkanes Chemical group 0.000 claims description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 2
- DHHVAGZRUROJKS-UHFFFAOYSA-N phentermine Chemical compound CC(C)(N)CC1=CC=CC=C1 DHHVAGZRUROJKS-UHFFFAOYSA-N 0.000 claims description 2
- 208000008128 pulmonary tuberculosis Diseases 0.000 claims description 2
- 210000002345 respiratory system Anatomy 0.000 claims description 2
- 125000006223 tetrahydrofuranylmethyl group Chemical group 0.000 claims description 2
- 125000006173 tetrahydropyranylmethyl group Chemical group 0.000 claims description 2
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 claims 1
- YTZLYOJTMMZKGM-UHFFFAOYSA-N O1CN=COC=C1 Chemical compound O1CN=COC=C1 YTZLYOJTMMZKGM-UHFFFAOYSA-N 0.000 claims 1
- 241001125046 Sardina pilchardus Species 0.000 claims 1
- XLAKJQPTOJHYDR-QTQXQZBYSA-M aclidinium bromide Chemical compound [Br-].C([C@@H](C(CC1)CC2)OC(=O)C(O)(C=3SC=CC=3)C=3SC=CC=3)[N+]21CCCOC1=CC=CC=C1 XLAKJQPTOJHYDR-QTQXQZBYSA-M 0.000 claims 1
- HONIICLYMWZJFZ-UHFFFAOYSA-N azetidine Chemical compound C1CNC1 HONIICLYMWZJFZ-UHFFFAOYSA-N 0.000 claims 1
- 229960002897 heparin Drugs 0.000 claims 1
- 229920000669 heparin Polymers 0.000 claims 1
- 235000019512 sardine Nutrition 0.000 claims 1
- 125000005302 thiazolylmethyl group Chemical group [H]C1=C([H])N=C(S1)C([H])([H])* 0.000 claims 1
- 125000005301 thienylmethyl group Chemical group [H]C1=C([H])C([H])=C(S1)C([H])([H])* 0.000 claims 1
- 229960004541 umeclidinium bromide Drugs 0.000 claims 1
- PEJHHXHHNGORMP-AVADPIKZSA-M umeclidinium bromide Chemical compound [Br-].C=1C=CC=CC=1C([C@@]12CC[N@@+](CCOCC=3C=CC=CC=3)(CC1)CC2)(O)C1=CC=CC=C1 PEJHHXHHNGORMP-AVADPIKZSA-M 0.000 claims 1
- DAFYYTQWSAWIGS-DEOSSOPVSA-N vilanterol Chemical compound C1=C(O)C(CO)=CC([C@@H](O)CNCCCCCCOCCOCC=2C(=CC=CC=2Cl)Cl)=C1 DAFYYTQWSAWIGS-DEOSSOPVSA-N 0.000 claims 1
- 229940079593 drug Drugs 0.000 abstract description 18
- 101100296720 Dictyostelium discoideum Pde4 gene Proteins 0.000 abstract 1
- 101100082610 Plasmodium falciparum (isolate 3D7) PDEdelta gene Proteins 0.000 abstract 1
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 274
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 270
- 239000000243 solution Substances 0.000 description 106
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 95
- 235000019439 ethyl acetate Nutrition 0.000 description 92
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 90
- 238000005481 NMR spectroscopy Methods 0.000 description 90
- 238000004949 mass spectrometry Methods 0.000 description 89
- 230000015572 biosynthetic process Effects 0.000 description 88
- 150000002500 ions Chemical class 0.000 description 88
- 238000003786 synthesis reaction Methods 0.000 description 86
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 86
- 239000002904 solvent Substances 0.000 description 78
- 239000012074 organic phase Substances 0.000 description 74
- 238000006243 chemical reaction Methods 0.000 description 70
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 66
- 238000010898 silica gel chromatography Methods 0.000 description 62
- 239000003480 eluent Substances 0.000 description 60
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 57
- 239000007787 solid Substances 0.000 description 55
- 230000002829 reductive effect Effects 0.000 description 49
- 239000003208 petroleum Substances 0.000 description 46
- 239000000543 intermediate Substances 0.000 description 41
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 39
- 239000007788 liquid Substances 0.000 description 39
- 235000002639 sodium chloride Nutrition 0.000 description 38
- 239000000203 mixture Substances 0.000 description 36
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 34
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 33
- 125000004432 carbon atom Chemical group C* 0.000 description 32
- 229910052757 nitrogen Inorganic materials 0.000 description 32
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 30
- 238000000034 method Methods 0.000 description 28
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 21
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 21
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Chemical compound OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 20
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 20
- 239000000047 product Substances 0.000 description 20
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 19
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Substances CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 18
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 18
- 239000002585 base Substances 0.000 description 16
- FPIRBHDGWMWJEP-UHFFFAOYSA-N 1-hydroxy-7-azabenzotriazole Chemical compound C1=CN=C2N(O)N=NC2=C1 FPIRBHDGWMWJEP-UHFFFAOYSA-N 0.000 description 15
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 15
- 239000012141 concentrate Substances 0.000 description 14
- 235000008504 concentrate Nutrition 0.000 description 14
- 239000011734 sodium Substances 0.000 description 14
- 229940123932 Phosphodiesterase 4 inhibitor Drugs 0.000 description 13
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 13
- 239000003795 chemical substances by application Substances 0.000 description 13
- 239000002587 phosphodiesterase IV inhibitor Substances 0.000 description 13
- 238000003756 stirring Methods 0.000 description 13
- 239000000126 substance Substances 0.000 description 13
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 12
- 230000000694 effects Effects 0.000 description 12
- DYHSDKLCOJIUFX-UHFFFAOYSA-N tert-butoxycarbonyl anhydride Chemical compound CC(C)(C)OC(=O)OC(=O)OC(C)(C)C DYHSDKLCOJIUFX-UHFFFAOYSA-N 0.000 description 12
- 229910052799 carbon Inorganic materials 0.000 description 11
- 239000003153 chemical reaction reagent Substances 0.000 description 11
- 239000002552 dosage form Substances 0.000 description 11
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 10
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 10
- 239000000706 filtrate Substances 0.000 description 10
- 239000004094 surface-active agent Substances 0.000 description 10
- 230000001225 therapeutic effect Effects 0.000 description 10
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 9
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 9
- 239000004480 active ingredient Substances 0.000 description 9
- 125000000753 cycloalkyl group Chemical group 0.000 description 9
- 238000009472 formulation Methods 0.000 description 9
- 239000004615 ingredient Substances 0.000 description 9
- 230000002401 inhibitory effect Effects 0.000 description 9
- 239000000843 powder Substances 0.000 description 9
- 125000006413 ring segment Chemical group 0.000 description 9
- 229910052717 sulfur Inorganic materials 0.000 description 9
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 8
- 125000002947 alkylene group Chemical group 0.000 description 8
- 208000035475 disorder Diseases 0.000 description 8
- PQVSTLUFSYVLTO-UHFFFAOYSA-N ethyl n-ethoxycarbonylcarbamate Chemical compound CCOC(=O)NC(=O)OCC PQVSTLUFSYVLTO-UHFFFAOYSA-N 0.000 description 8
- 229940040692 lithium hydroxide monohydrate Drugs 0.000 description 8
- GLXDVVHUTZTUQK-UHFFFAOYSA-M lithium hydroxide monohydrate Substances [Li+].O.[OH-] GLXDVVHUTZTUQK-UHFFFAOYSA-M 0.000 description 8
- 239000012046 mixed solvent Substances 0.000 description 8
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 8
- 229910019142 PO4 Inorganic materials 0.000 description 7
- 201000004681 Psoriasis Diseases 0.000 description 7
- 238000012377 drug delivery Methods 0.000 description 7
- 150000002632 lipids Chemical class 0.000 description 7
- 239000003921 oil Substances 0.000 description 7
- 235000019198 oils Nutrition 0.000 description 7
- 235000021317 phosphate Nutrition 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 6
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 6
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 125000003710 aryl alkyl group Chemical group 0.000 description 6
- 125000002619 bicyclic group Chemical group 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 6
- 238000010348 incorporation Methods 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 239000012071 phase Substances 0.000 description 6
- 230000004224 protection Effects 0.000 description 6
- 229940044551 receptor antagonist Drugs 0.000 description 6
- 239000002464 receptor antagonist Substances 0.000 description 6
- 238000000926 separation method Methods 0.000 description 6
- 230000000699 topical effect Effects 0.000 description 6
- WJKHJLXJJJATHN-UHFFFAOYSA-N triflic anhydride Chemical compound FC(F)(F)S(=O)(=O)OS(=O)(=O)C(F)(F)F WJKHJLXJJJATHN-UHFFFAOYSA-N 0.000 description 6
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 6
- IIQXLNYQWQMHRH-UHFFFAOYSA-N 2-(cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene Chemical compound C1CC1COC2=C(C=CC(=C2)I)OC(F)F IIQXLNYQWQMHRH-UHFFFAOYSA-N 0.000 description 5
- IDDGDRWXVZBZIW-UHFFFAOYSA-N 6-[[(2-methylpropan-2-yl)oxycarbonylamino]methyl]pyridine-2-carboxylic acid Chemical compound CC(C)(C)OC(=O)NCC1=CC=CC(C(O)=O)=N1 IDDGDRWXVZBZIW-UHFFFAOYSA-N 0.000 description 5
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 5
- JGLMVXWAHNTPRF-CMDGGOBGSA-N CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O Chemical compound CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O JGLMVXWAHNTPRF-CMDGGOBGSA-N 0.000 description 5
- IVOMOUWHDPKRLL-KQYNXXCUSA-N Cyclic adenosine monophosphate Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=CN=C2N)=C2N=C1 IVOMOUWHDPKRLL-KQYNXXCUSA-N 0.000 description 5
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 5
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 239000000556 agonist Substances 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 5
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 5
- 239000011230 binding agent Substances 0.000 description 5
- 230000037396 body weight Effects 0.000 description 5
- ZOOGRGPOEVQQDX-KHLHZJAASA-N cyclic guanosine monophosphate Chemical compound C([C@H]1O2)O[P@](O)(=O)O[C@@H]1[C@H](O)[C@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-KHLHZJAASA-N 0.000 description 5
- 238000000605 extraction Methods 0.000 description 5
- 239000008101 lactose Substances 0.000 description 5
- 125000005647 linker group Chemical group 0.000 description 5
- 239000000314 lubricant Substances 0.000 description 5
- 125000002950 monocyclic group Chemical group 0.000 description 5
- 229910052760 oxygen Inorganic materials 0.000 description 5
- 229920001223 polyethylene glycol Polymers 0.000 description 5
- VLJNHYLEOZPXFW-UHFFFAOYSA-N pyrrolidine-2-carboxamide Chemical compound NC(=O)C1CCCN1 VLJNHYLEOZPXFW-UHFFFAOYSA-N 0.000 description 5
- 239000000741 silica gel Substances 0.000 description 5
- 229910002027 silica gel Inorganic materials 0.000 description 5
- 239000012312 sodium hydride Substances 0.000 description 5
- 229910000104 sodium hydride Inorganic materials 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 238000003419 tautomerization reaction Methods 0.000 description 5
- LFKDJXLFVYVEFG-UHFFFAOYSA-N tert-butyl carbamate Chemical compound CC(C)(C)OC(N)=O LFKDJXLFVYVEFG-UHFFFAOYSA-N 0.000 description 5
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 5
- AXYMXGSRCWCVIK-UHFFFAOYSA-N 2,3-dihydro-1,4,2-dioxazine Chemical compound C1NOC=CO1 AXYMXGSRCWCVIK-UHFFFAOYSA-N 0.000 description 4
- VWIOMFMPIVMLIR-UHFFFAOYSA-N 6-methoxycarbonylpyridine-2-carboxylic acid Chemical compound COC(=O)C1=CC=CC(C(O)=O)=N1 VWIOMFMPIVMLIR-UHFFFAOYSA-N 0.000 description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 4
- 239000012448 Lithium borohydride Substances 0.000 description 4
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 4
- 229930195725 Mannitol Natural products 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 4
- 239000000443 aerosol Substances 0.000 description 4
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 4
- 239000005557 antagonist Substances 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 4
- 239000003054 catalyst Substances 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 150000001975 deuterium Chemical group 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- FRQITOIRVNNQFR-UHFFFAOYSA-N ethyl 6-[[(2-methylpropan-2-yl)oxycarbonylamino]methyl]pyridine-2-carboxylate Chemical compound CCOC(=O)C1=CC=CC(CNC(=O)OC(C)(C)C)=N1 FRQITOIRVNNQFR-UHFFFAOYSA-N 0.000 description 4
- 125000005843 halogen group Chemical group 0.000 description 4
- 238000004128 high performance liquid chromatography Methods 0.000 description 4
- 150000002430 hydrocarbons Chemical group 0.000 description 4
- 230000007062 hydrolysis Effects 0.000 description 4
- 238000006460 hydrolysis reaction Methods 0.000 description 4
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 4
- 230000000155 isotopic effect Effects 0.000 description 4
- 102000004311 liver X receptors Human genes 0.000 description 4
- 108090000865 liver X receptors Proteins 0.000 description 4
- 239000000594 mannitol Substances 0.000 description 4
- 235000010355 mannitol Nutrition 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 4
- 239000004530 micro-emulsion Substances 0.000 description 4
- 201000001119 neuropathy Diseases 0.000 description 4
- 230000007823 neuropathy Effects 0.000 description 4
- 235000001968 nicotinic acid Nutrition 0.000 description 4
- 239000011664 nicotinic acid Substances 0.000 description 4
- 231100000252 nontoxic Toxicity 0.000 description 4
- 230000003000 nontoxic effect Effects 0.000 description 4
- 239000002674 ointment Substances 0.000 description 4
- 208000033808 peripheral neuropathy Diseases 0.000 description 4
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 4
- 229910052698 phosphorus Inorganic materials 0.000 description 4
- IVDFJHOHABJVEH-UHFFFAOYSA-N pinacol Chemical compound CC(C)(O)C(C)(C)O IVDFJHOHABJVEH-UHFFFAOYSA-N 0.000 description 4
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Substances [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 4
- 238000002953 preparative HPLC Methods 0.000 description 4
- 239000003380 propellant Substances 0.000 description 4
- 229920006395 saturated elastomer Polymers 0.000 description 4
- 229910052708 sodium Inorganic materials 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- 239000008107 starch Substances 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- QDZZDVQGBKTLHV-UHFFFAOYSA-N (2,4-difluorophenyl)methanamine Chemical compound NCC1=CC=C(F)C=C1F QDZZDVQGBKTLHV-UHFFFAOYSA-N 0.000 description 3
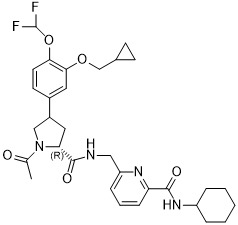
- GMPXGCDMNDJXST-KZUDCZAMSA-N (2S)-1-acetyl-4-[3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl]pyrrolidine-2-carboxylic acid Chemical compound CC(N(CC(C1)C(C=C2)=CC(OCC3CC3)=C2OC(F)F)[C@@H]1C(O)=O)=O GMPXGCDMNDJXST-KZUDCZAMSA-N 0.000 description 3
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 3
- FXODUECSYZFTGI-UHFFFAOYSA-N 1-(difluoromethoxy)-4-iodo-2-phenylmethoxybenzene Chemical compound C1=CC=C(C=C1)COC2=C(C=CC(=C2)I)OC(F)F FXODUECSYZFTGI-UHFFFAOYSA-N 0.000 description 3
- VMYQBGIPUYBFLP-UHFFFAOYSA-N 5-(azidomethyl)-2-methyl-3H-isoindol-1-one Chemical compound CN1CC2=C(C1=O)C=CC(=C2)CN=[N+]=[N-] VMYQBGIPUYBFLP-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 3
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 3
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical class OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 3
- 239000005909 Kieselgur Substances 0.000 description 3
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 3
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- 229910002651 NO3 Inorganic materials 0.000 description 3
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 208000002193 Pain Diseases 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 3
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 3
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 3
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 3
- 239000012346 acetyl chloride Substances 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- 239000003463 adsorbent Substances 0.000 description 3
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 3
- AEMOLEFTQBMNLQ-BKBMJHBISA-N alpha-D-galacturonic acid Chemical compound O[C@H]1O[C@H](C(O)=O)[C@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-BKBMJHBISA-N 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 235000006708 antioxidants Nutrition 0.000 description 3
- 229940009098 aspartate Drugs 0.000 description 3
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 239000003086 colorant Substances 0.000 description 3
- 238000006482 condensation reaction Methods 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- NXQGGXCHGDYOHB-UHFFFAOYSA-L cyclopenta-1,4-dien-1-yl(diphenyl)phosphane;dichloropalladium;iron(2+) Chemical compound [Fe+2].Cl[Pd]Cl.[CH-]1C=CC(P(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1.[CH-]1C=CC(P(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1 NXQGGXCHGDYOHB-UHFFFAOYSA-L 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 239000000796 flavoring agent Substances 0.000 description 3
- 239000011737 fluorine Substances 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 229940014259 gelatin Drugs 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 208000027866 inflammatory disease Diseases 0.000 description 3
- 239000003112 inhibitor Substances 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 3
- 239000006210 lotion Substances 0.000 description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 3
- QARBMVPHQWIHKH-UHFFFAOYSA-N methanesulfonyl chloride Chemical compound CS(Cl)(=O)=O QARBMVPHQWIHKH-UHFFFAOYSA-N 0.000 description 3
- 239000000693 micelle Substances 0.000 description 3
- 230000003551 muscarinic effect Effects 0.000 description 3
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 239000012299 nitrogen atmosphere Substances 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 239000006072 paste Substances 0.000 description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 229910000160 potassium phosphate Inorganic materials 0.000 description 3
- 235000011009 potassium phosphates Nutrition 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 3
- 229960002052 salbutamol Drugs 0.000 description 3
- 239000012279 sodium borohydride Substances 0.000 description 3
- 229910000033 sodium borohydride Inorganic materials 0.000 description 3
- 229910052938 sodium sulfate Inorganic materials 0.000 description 3
- 235000011152 sodium sulphate Nutrition 0.000 description 3
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 3
- 238000000967 suction filtration Methods 0.000 description 3
- 239000011593 sulfur Substances 0.000 description 3
- 238000001308 synthesis method Methods 0.000 description 3
- 238000010189 synthetic method Methods 0.000 description 3
- 229960001967 tacrolimus Drugs 0.000 description 3
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 3
- 229940095064 tartrate Drugs 0.000 description 3
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- LAUPTNYHVCVPFH-UHFFFAOYSA-N (2-ethoxyphenyl)methanamine Chemical compound CCOC1=CC=CC=C1CN LAUPTNYHVCVPFH-UHFFFAOYSA-N 0.000 description 2
- 125000005988 1,1-dioxo-thiomorpholinyl group Chemical group 0.000 description 2
- QJNFNEIZAPCULS-UHFFFAOYSA-N 2-(difluoromethoxy)-5-iodophenol Chemical compound OC(C=C(C=C1)I)=C1OC(F)F QJNFNEIZAPCULS-UHFFFAOYSA-N 0.000 description 2
- WOXFMYVTSLAQMO-UHFFFAOYSA-N 2-Pyridinemethanamine Chemical compound NCC1=CC=CC=N1 WOXFMYVTSLAQMO-UHFFFAOYSA-N 0.000 description 2
- 125000004398 2-methyl-2-butyl group Chemical group CC(C)(CC)* 0.000 description 2
- 125000004918 2-methyl-2-pentyl group Chemical group CC(C)(CCC)* 0.000 description 2
- 125000004922 2-methyl-3-pentyl group Chemical group CC(C)C(CC)* 0.000 description 2
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 2
- MSYGAHOHLUJIKV-UHFFFAOYSA-N 3,5-dimethyl-1-(3-nitrophenyl)-1h-pyrazole-4-carboxylic acid ethyl ester Chemical compound CC1=C(C(=O)OCC)C(C)=NN1C1=CC=CC([N+]([O-])=O)=C1 MSYGAHOHLUJIKV-UHFFFAOYSA-N 0.000 description 2
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 2
- 125000004917 3-methyl-2-butyl group Chemical group CC(C(C)*)C 0.000 description 2
- 125000004919 3-methyl-2-pentyl group Chemical group CC(C(C)*)CC 0.000 description 2
- 125000004921 3-methyl-3-pentyl group Chemical group CC(CC)(CC)* 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- OGNULTMJFVFEMD-UHFFFAOYSA-N 4-bromo-1-(difluoromethoxy)-2-nitrobenzene Chemical compound [O-][N+](=O)C1=CC(Br)=CC=C1OC(F)F OGNULTMJFVFEMD-UHFFFAOYSA-N 0.000 description 2
- GCNTZFIIOFTKIY-UHFFFAOYSA-N 4-hydroxypyridine Chemical compound OC1=CC=NC=C1 GCNTZFIIOFTKIY-UHFFFAOYSA-N 0.000 description 2
- 125000004920 4-methyl-2-pentyl group Chemical group CC(CC(C)*)C 0.000 description 2
- WQSSLICETDQKIO-UHFFFAOYSA-N 5-(aminomethyl)-2-methyl-3h-isoindol-1-one Chemical compound NCC1=CC=C2C(=O)N(C)CC2=C1 WQSSLICETDQKIO-UHFFFAOYSA-N 0.000 description 2
- AZWJQOPYJHSPRA-UHFFFAOYSA-N 5-(hydroxymethyl)-2-methyl-3h-isoindol-1-one Chemical compound OCC1=CC=C2C(=O)N(C)CC2=C1 AZWJQOPYJHSPRA-UHFFFAOYSA-N 0.000 description 2
- AYNLUTRSEXXTOK-UHFFFAOYSA-N 6-(aminomethyl)-2-methyl-3H-isoindol-1-one Chemical compound C1=C(CN)C=C2C(=O)N(C)CC2=C1 AYNLUTRSEXXTOK-UHFFFAOYSA-N 0.000 description 2
- UVMHUDDWANGYED-UHFFFAOYSA-N 6-(azidomethyl)-2-methyl-3H-isoindol-1-one Chemical compound CN(CC1=CC=C(CN=[N+]=[N-])C=C11)C1=O UVMHUDDWANGYED-UHFFFAOYSA-N 0.000 description 2
- QUVHAQPYKISQLP-UHFFFAOYSA-N 6-(hydroxymethyl)-2-methyl-3H-isoindol-1-one Chemical compound C1=C(CO)C=C2C(=O)N(C)CC2=C1 QUVHAQPYKISQLP-UHFFFAOYSA-N 0.000 description 2
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 2
- 229920001450 Alpha-Cyclodextrin Polymers 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- 208000019901 Anxiety disease Diseases 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 2
- RWWHQTRUWOHRJX-UHFFFAOYSA-N COC(=O)c1cccc(n1)C(=O)N(C)c1ccc(O)cc1 Chemical compound COC(=O)c1cccc(n1)C(=O)N(C)c1ccc(O)cc1 RWWHQTRUWOHRJX-UHFFFAOYSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 229920002261 Corn starch Polymers 0.000 description 2
- 208000011231 Crohn disease Diseases 0.000 description 2
- 102000008130 Cyclic AMP-Dependent Protein Kinases Human genes 0.000 description 2
- 108010049894 Cyclic AMP-Dependent Protein Kinases Proteins 0.000 description 2
- 102000004654 Cyclic GMP-Dependent Protein Kinases Human genes 0.000 description 2
- 108010003591 Cyclic GMP-Dependent Protein Kinases Proteins 0.000 description 2
- 229920000858 Cyclodextrin Polymers 0.000 description 2
- AEMOLEFTQBMNLQ-AQKNRBDQSA-N D-glucopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-AQKNRBDQSA-N 0.000 description 2
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 206010065390 Inflammatory pain Diseases 0.000 description 2
- 102000004388 Interleukin-4 Human genes 0.000 description 2
- 108090000978 Interleukin-4 Proteins 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- 102000042838 JAK family Human genes 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 2
- 240000007472 Leucaena leucocephala Species 0.000 description 2
- NMMIHXMBOZYNET-UHFFFAOYSA-N Methyl picolinate Chemical compound COC(=O)C1=CC=CC=N1 NMMIHXMBOZYNET-UHFFFAOYSA-N 0.000 description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 2
- UTWYMUKPGRTKJV-UHFFFAOYSA-N N-[5-bromo-2-(difluoromethoxy)phenyl]hydroxylamine Chemical compound ONc1cc(Br)ccc1OC(F)F UTWYMUKPGRTKJV-UHFFFAOYSA-N 0.000 description 2
- 108010040718 Neurokinin-1 Receptors Proteins 0.000 description 2
- 208000018737 Parkinson disease Diseases 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- 241000288906 Primates Species 0.000 description 2
- 108050000258 Prostaglandin D receptors Proteins 0.000 description 2
- 102100024218 Prostaglandin D2 receptor 2 Human genes 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 2
- 206010040070 Septic Shock Diseases 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 102100037346 Substance-P receptor Human genes 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 102000011017 Type 4 Cyclic Nucleotide Phosphodiesterases Human genes 0.000 description 2
- 108010037584 Type 4 Cyclic Nucleotide Phosphodiesterases Proteins 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 229930003316 Vitamin D Natural products 0.000 description 2
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 2
- DSFVQIUBODXRLL-UHFFFAOYSA-N acetamide;dihydrochloride Chemical compound Cl.Cl.CC(N)=O DSFVQIUBODXRLL-UHFFFAOYSA-N 0.000 description 2
- TVWAEQRFKRTYIG-JIDHJSLPSA-N acetic acid;4-[(1r)-2-[6-[2-[(2,6-dichlorophenyl)methoxy]ethoxy]hexylamino]-1-hydroxyethyl]-2-(hydroxymethyl)phenol Chemical compound CC(O)=O.C1=C(O)C(CO)=CC([C@@H](O)CNCCCCCCOCCOCC=2C(=CC=CC=2Cl)Cl)=C1 TVWAEQRFKRTYIG-JIDHJSLPSA-N 0.000 description 2
- CSCPPACGZOOCGX-WFGJKAKNSA-N acetone d6 Chemical compound [2H]C([2H])([2H])C(=O)C([2H])([2H])[2H] CSCPPACGZOOCGX-WFGJKAKNSA-N 0.000 description 2
- YRKCREAYFQTBPV-UHFFFAOYSA-N acetylacetone Chemical compound CC(=O)CC(C)=O YRKCREAYFQTBPV-UHFFFAOYSA-N 0.000 description 2
- ASMXXROZKSBQIH-VITNCHFBSA-N aclidinium Chemical compound C([C@@H](C(CC1)CC2)OC(=O)C(O)(C=3SC=CC=3)C=3SC=CC=3)[N+]21CCCOC1=CC=CC=C1 ASMXXROZKSBQIH-VITNCHFBSA-N 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 238000013019 agitation Methods 0.000 description 2
- FJXOGVLKCZQRDN-PHCHRAKRSA-N alclometasone Chemical compound C([C@H]1Cl)C2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O FJXOGVLKCZQRDN-PHCHRAKRSA-N 0.000 description 2
- 230000001476 alcoholic effect Effects 0.000 description 2
- 229940072056 alginate Drugs 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229910052783 alkali metal Inorganic materials 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- HFHDHCJBZVLPGP-RWMJIURBSA-N alpha-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO HFHDHCJBZVLPGP-RWMJIURBSA-N 0.000 description 2
- 229940043377 alpha-cyclodextrin Drugs 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 230000000844 anti-bacterial effect Effects 0.000 description 2
- 229940121375 antifungal agent Drugs 0.000 description 2
- 230000036506 anxiety Effects 0.000 description 2
- 238000011914 asymmetric synthesis Methods 0.000 description 2
- 230000001363 autoimmune Effects 0.000 description 2
- 229940077388 benzenesulfonate Drugs 0.000 description 2
- 229940050390 benzoate Drugs 0.000 description 2
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 2
- 239000012964 benzotriazole Substances 0.000 description 2
- 235000019445 benzyl alcohol Nutrition 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 229910052796 boron Inorganic materials 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- CREMABGTGYGIQB-UHFFFAOYSA-N carbon carbon Chemical compound C.C CREMABGTGYGIQB-UHFFFAOYSA-N 0.000 description 2
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 2
- 150000001733 carboxylic acid esters Chemical class 0.000 description 2
- 239000012876 carrier material Substances 0.000 description 2
- 238000009903 catalytic hydrogenation reaction Methods 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- NEHMKBQYUWJMIP-UHFFFAOYSA-N chloromethane Chemical compound ClC NEHMKBQYUWJMIP-UHFFFAOYSA-N 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 208000037976 chronic inflammation Diseases 0.000 description 2
- 230000006020 chronic inflammation Effects 0.000 description 2
- 239000003541 chymotrypsin inhibitor Substances 0.000 description 2
- 229940114081 cinnamate Drugs 0.000 description 2
- 229940001468 citrate Drugs 0.000 description 2
- 208000010877 cognitive disease Diseases 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 239000008139 complexing agent Substances 0.000 description 2
- 239000012050 conventional carrier Substances 0.000 description 2
- 239000008120 corn starch Substances 0.000 description 2
- 229940099112 cornstarch Drugs 0.000 description 2
- 239000006184 cosolvent Substances 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 230000008025 crystallization Effects 0.000 description 2
- 125000001316 cycloalkyl alkyl group Chemical group 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 125000004431 deuterium atom Chemical group 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 125000004786 difluoromethoxy group Chemical group [H]C(F)(F)O* 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- VAYGXNSJCAHWJZ-UHFFFAOYSA-N dimethyl sulfate Chemical compound COS(=O)(=O)OC VAYGXNSJCAHWJZ-UHFFFAOYSA-N 0.000 description 2
- XHFGWHUWQXTGAT-UHFFFAOYSA-N dimethylamine hydrochloride Natural products CNC(C)C XHFGWHUWQXTGAT-UHFFFAOYSA-N 0.000 description 2
- IQDGSYLLQPDQDV-UHFFFAOYSA-N dimethylazanium;chloride Chemical compound Cl.CNC IQDGSYLLQPDQDV-UHFFFAOYSA-N 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- UZUODNWWWUQRIR-UHFFFAOYSA-L disodium;3-aminonaphthalene-1,5-disulfonate Chemical compound [Na+].[Na+].C1=CC=C(S([O-])(=O)=O)C2=CC(N)=CC(S([O-])(=O)=O)=C21 UZUODNWWWUQRIR-UHFFFAOYSA-L 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000002612 dispersion medium Substances 0.000 description 2
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 2
- 229940043264 dodecyl sulfate Drugs 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 238000010931 ester hydrolysis Methods 0.000 description 2
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 2
- SAHOZFWRWQZFCH-UHFFFAOYSA-N ethyl 6-(aminomethyl)pyridine-2-carboxylate Chemical compound CCOC(=O)C1=CC=CC(CN)=N1 SAHOZFWRWQZFCH-UHFFFAOYSA-N 0.000 description 2
- FQYYIPZPELSLDK-UHFFFAOYSA-N ethyl pyridine-2-carboxylate Chemical compound CCOC(=O)C1=CC=CC=N1 FQYYIPZPELSLDK-UHFFFAOYSA-N 0.000 description 2
- KTWOOEGAPBSYNW-UHFFFAOYSA-N ferrocene Chemical compound [Fe+2].C=1C=C[CH-]C=1.C=1C=C[CH-]C=1 KTWOOEGAPBSYNW-UHFFFAOYSA-N 0.000 description 2
- 230000003176 fibrotic effect Effects 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 125000004175 fluorobenzyl group Chemical group 0.000 description 2
- 235000013355 food flavoring agent Nutrition 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 229930195712 glutamate Natural products 0.000 description 2
- 229940049906 glutamate Drugs 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 125000004438 haloalkoxy group Chemical group 0.000 description 2
- 125000001188 haloalkyl group Chemical group 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 125000004446 heteroarylalkyl group Chemical group 0.000 description 2
- 125000004415 heterocyclylalkyl group Chemical group 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000003840 hydrochlorides Chemical group 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 210000004969 inflammatory cell Anatomy 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 238000006317 isomerization reaction Methods 0.000 description 2
- 239000007951 isotonicity adjuster Substances 0.000 description 2
- 229940043355 kinase inhibitor Drugs 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 239000007937 lozenge Substances 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 229940049920 malate Drugs 0.000 description 2
- BJEPYKJPYRNKOW-UHFFFAOYSA-L malate(2-) Chemical compound [O-]C(=O)C(O)CC([O-])=O BJEPYKJPYRNKOW-UHFFFAOYSA-L 0.000 description 2
- IWYDHOAUDWTVEP-UHFFFAOYSA-M mandelate Chemical compound [O-]C(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-M 0.000 description 2
- 238000002483 medication Methods 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- UPFFKPZXJRBTDT-UHFFFAOYSA-N methyl 1-oxo-2,3-dihydroisoindole-5-carboxylate Chemical compound COC(=O)C1=CC=C2C(=O)NCC2=C1 UPFFKPZXJRBTDT-UHFFFAOYSA-N 0.000 description 2
- DTLPMSDEGCTRGW-UHFFFAOYSA-N methyl 2-methyl-1-oxo-3h-isoindole-5-carboxylate Chemical compound COC(=O)C1=CC=C2C(=O)N(C)CC2=C1 DTLPMSDEGCTRGW-UHFFFAOYSA-N 0.000 description 2
- MPCIUMPOHOGTTB-UHFFFAOYSA-N methyl 2-methyl-3-oxo-1H-isoindole-5-carboxylate Chemical compound C1=2CN(C(=O)C1=CC(C(=O)OC)=CC=2)C MPCIUMPOHOGTTB-UHFFFAOYSA-N 0.000 description 2
- FOUXXPLZXKYJRB-UHFFFAOYSA-N methyl 3-oxo-1,2-dihydroisoindole-5-carboxylate Chemical compound COC(=O)C1=CC=C2CNC(=O)C2=C1 FOUXXPLZXKYJRB-UHFFFAOYSA-N 0.000 description 2
- 229940095102 methyl benzoate Drugs 0.000 description 2
- 150000004702 methyl esters Chemical class 0.000 description 2
- YNBADRVTZLEFNH-UHFFFAOYSA-N methyl nicotinate Chemical compound COC(=O)C1=CC=CN=C1 YNBADRVTZLEFNH-UHFFFAOYSA-N 0.000 description 2
- BLWYXBNNBYXPPL-UHFFFAOYSA-N methyl pyrrolidine-2-carboxylate Chemical compound COC(=O)C1CCCN1 BLWYXBNNBYXPPL-UHFFFAOYSA-N 0.000 description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 2
- 239000008108 microcrystalline cellulose Substances 0.000 description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 description 2
- 208000027061 mild cognitive impairment Diseases 0.000 description 2
- 239000003595 mist Substances 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 2
- 229960003512 nicotinic acid Drugs 0.000 description 2
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 2
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 2
- 229960000470 omalizumab Drugs 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- RZXMPPFPUUCRFN-UHFFFAOYSA-N p-toluidine Chemical compound CC1=CC=C(N)C=C1 RZXMPPFPUUCRFN-UHFFFAOYSA-N 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 2
- 229940124531 pharmaceutical excipient Drugs 0.000 description 2
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 2
- 230000001766 physiological effect Effects 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 238000002600 positron emission tomography Methods 0.000 description 2
- 235000011056 potassium acetate Nutrition 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-M prolinate Chemical compound [O-]C(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-M 0.000 description 2
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- 150000003235 pyrrolidines Chemical class 0.000 description 2
- 229940076788 pyruvate Drugs 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 229960001860 salicylate Drugs 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 2
- 229960004540 secukinumab Drugs 0.000 description 2
- 238000002603 single-photon emission computed tomography Methods 0.000 description 2
- BEOOHQFXGBMRKU-UHFFFAOYSA-N sodium cyanoborohydride Chemical compound [Na+].[B-]C#N BEOOHQFXGBMRKU-UHFFFAOYSA-N 0.000 description 2
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000008174 sterile solution Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 125000004434 sulfur atom Chemical group 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 229940033134 talc Drugs 0.000 description 2
- 235000012222 talc Nutrition 0.000 description 2
- AKSYIQYJDJXCBX-UHFFFAOYSA-N tert-butyl 6-(hydroxymethyl)pyridine-2-carboxylate Chemical compound CC(C)(C)OC(=O)C1=CC=CC(CO)=N1 AKSYIQYJDJXCBX-UHFFFAOYSA-N 0.000 description 2
- LRHNGJREDPGGTB-UHFFFAOYSA-N tert-butyl N-[(2,4-difluorophenyl)methyl]-N-methylcarbamate Chemical compound CC(C)(C)OC(=O)N(C)CC1=CC=C(F)C=C1F LRHNGJREDPGGTB-UHFFFAOYSA-N 0.000 description 2
- MGRHKDSHJJWMHW-UHFFFAOYSA-N tert-butyl n-(cyclopropylmethyl)-n-methylcarbamate Chemical compound CC(C)(C)OC(=O)N(C)CC1CC1 MGRHKDSHJJWMHW-UHFFFAOYSA-N 0.000 description 2
- RKVWMMAKYQABGS-UHFFFAOYSA-N tert-butyl n-(pyridin-2-ylmethyl)carbamate Chemical compound CC(C)(C)OC(=O)NCC1=CC=CC=N1 RKVWMMAKYQABGS-UHFFFAOYSA-N 0.000 description 2
- XOMSOPZJGFQUAC-UHFFFAOYSA-N tert-butyl n-methyl-n-(pyridin-2-ylmethyl)carbamate Chemical compound CC(C)(C)OC(=O)N(C)CC1=CC=CC=N1 XOMSOPZJGFQUAC-UHFFFAOYSA-N 0.000 description 2
- 150000003512 tertiary amines Chemical class 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- WBYWAXJHAXSJNI-VOTSOKGWSA-M trans-cinnamate Chemical compound [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 2
- ODLHGICHYURWBS-LKONHMLTSA-N trappsol cyclo Chemical compound CC(O)COC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)COCC(O)C)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1COCC(C)O ODLHGICHYURWBS-LKONHMLTSA-N 0.000 description 2
- 235000019166 vitamin D Nutrition 0.000 description 2
- 239000011710 vitamin D Substances 0.000 description 2
- 150000003710 vitamin D derivatives Chemical class 0.000 description 2
- 229940046008 vitamin d Drugs 0.000 description 2
- LSPHULWDVZXLIL-UHFFFAOYSA-N (+/-)-Camphoric acid Chemical compound CC1(C)C(C(O)=O)CCC1(C)C(O)=O LSPHULWDVZXLIL-UHFFFAOYSA-N 0.000 description 1
- RYGOBSYXIIUFOR-UHFFFAOYSA-N (1-methylpyrazol-4-yl)boronic acid Chemical compound CN1C=C(B(O)O)C=N1 RYGOBSYXIIUFOR-UHFFFAOYSA-N 0.000 description 1
- OJZQOQNSUZLSMV-UHFFFAOYSA-N (3-aminophenyl)methanol Chemical compound NC1=CC=CC(CO)=C1 OJZQOQNSUZLSMV-UHFFFAOYSA-N 0.000 description 1
- WYQFJHHDOKWSHR-MNOVXSKESA-N (3S,4R)-3-ethyl-4-(1,5,7,10-tetrazatricyclo[7.3.0.02,6]dodeca-2(6),3,7,9,11-pentaen-12-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide Chemical compound CC[C@@H]1CN(C(=O)NCC(F)(F)F)C[C@@H]1C1=CN=C2N1C(C=CN1)=C1N=C2 WYQFJHHDOKWSHR-MNOVXSKESA-N 0.000 description 1
- QYJPFTAKVBDDPD-UHFFFAOYSA-N (4,4-difluorocyclohexyl)azanium;chloride Chemical compound Cl.NC1CCC(F)(F)CC1 QYJPFTAKVBDDPD-UHFFFAOYSA-N 0.000 description 1
- OALKYGZLLCDVEN-UHFFFAOYSA-N (5-fluoropyridin-2-yl)methanamine Chemical compound NCC1=CC=C(F)C=N1 OALKYGZLLCDVEN-UHFFFAOYSA-N 0.000 description 1
- WNUXZPQIGBMHSI-UHFFFAOYSA-N (6-bromopyridin-2-yl)methanamine Chemical compound NCC1=CC=CC(Br)=N1 WNUXZPQIGBMHSI-UHFFFAOYSA-N 0.000 description 1
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- TVQVJTVYCHMEDG-UHFFFAOYSA-N 1-[2-(aminomethyl)pyridin-4-yl]ethanol Chemical compound CC(O)C1=CC=NC(CN)=C1 TVQVJTVYCHMEDG-UHFFFAOYSA-N 0.000 description 1
- ORQVLOOOUXHZEJ-UHFFFAOYSA-N 1-cyclopropyl-n-methylmethanamine;hydrochloride Chemical compound Cl.CNCC1CC1 ORQVLOOOUXHZEJ-UHFFFAOYSA-N 0.000 description 1
- UPBHYYJZVWZCOZ-MRVPVSSYSA-N 1-o-tert-butyl 2-o-methyl (2r)-4-oxopyrrolidine-1,2-dicarboxylate Chemical compound COC(=O)[C@H]1CC(=O)CN1C(=O)OC(C)(C)C UPBHYYJZVWZCOZ-MRVPVSSYSA-N 0.000 description 1
- OLKKGIPWCIURHH-UHFFFAOYSA-N 1-oxo-2,3-dihydroisoindole-5-carboxylic acid Chemical compound OC(=O)C1=CC=C2C(=O)NCC2=C1 OLKKGIPWCIURHH-UHFFFAOYSA-N 0.000 description 1
- 125000005987 1-oxo-thiomorpholinyl group Chemical group 0.000 description 1
- 101150000874 11 gene Proteins 0.000 description 1
- GWNVDXQDILPJIG-CCHJCNDSSA-N 11-trans-Leukotriene C4 Chemical compound CCCCC\C=C/C\C=C\C=C\C=C\[C@H]([C@@H](O)CCCC(O)=O)SC[C@@H](C(=O)NCC(O)=O)NC(=O)CC[C@H](N)C(O)=O GWNVDXQDILPJIG-CCHJCNDSSA-N 0.000 description 1
- OISVCGZHLKNMSJ-UHFFFAOYSA-N 2,6-Lutidine Substances CC1=CC=CC(C)=N1 OISVCGZHLKNMSJ-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- GFPPXZDRVCSVNR-UHFFFAOYSA-N 2-[2-methyl-1-[[4-methylsulfonyl-2-(trifluoromethyl)phenyl]methyl]pyrrolo[2,3-b]pyridin-3-yl]acetic acid Chemical compound CC1=C(CC(O)=O)C2=CC=CN=C2N1CC1=CC=C(S(C)(=O)=O)C=C1C(F)(F)F GFPPXZDRVCSVNR-UHFFFAOYSA-N 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000004493 2-methylbut-1-yl group Chemical group CC(C*)CC 0.000 description 1
- BSKHPKMHTQYZBB-UHFFFAOYSA-N 2-methylpyridine Chemical compound CC1=CC=CC=N1 BSKHPKMHTQYZBB-UHFFFAOYSA-N 0.000 description 1
- 229940080296 2-naphthalenesulfonate Drugs 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000389 2-pyrrolyl group Chemical group [H]N1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- WNHPMKYMPWMAIT-UHFFFAOYSA-N 3,4,5,6-tetrahydro-2h-azepine Chemical compound C1CCC=NCC1 WNHPMKYMPWMAIT-UHFFFAOYSA-N 0.000 description 1
- KKWRTAYYCVXDIO-UHFFFAOYSA-N 3-(cyclopropylmethoxy)-4-(difluoromethoxy)aniline Chemical compound NC1=CC=C(OC(F)F)C(OCC2CC2)=C1 KKWRTAYYCVXDIO-UHFFFAOYSA-N 0.000 description 1
- IGFDIFLMMLWKKY-UHFFFAOYSA-N 3-(cyclopropylmethoxy)-4-(difluoromethoxy)benzoic acid Chemical compound OC(=O)C1=CC=C(OC(F)F)C(OCC2CC2)=C1 IGFDIFLMMLWKKY-UHFFFAOYSA-N 0.000 description 1
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- VJAXCBJUBWJIRY-UHFFFAOYSA-N 3-oxo-1,2-dihydroisoindole-5-carboxylic acid Chemical compound OC(=O)C1=CC=C2CNC(=O)C2=C1 VJAXCBJUBWJIRY-UHFFFAOYSA-N 0.000 description 1
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000001397 3-pyrrolyl group Chemical group [H]N1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- UDXHAUKLGAPSJI-UHFFFAOYSA-N 4,4-difluoro-N-methylcyclohexan-1-amine hydrochloride Chemical compound Cl.FC1(CCC(CC1)NC)F UDXHAUKLGAPSJI-UHFFFAOYSA-N 0.000 description 1
- CSDQQAQKBAQLLE-UHFFFAOYSA-N 4-(4-chlorophenyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Chemical compound C1=CC(Cl)=CC=C1C1C(C=CS2)=C2CCN1 CSDQQAQKBAQLLE-UHFFFAOYSA-N 0.000 description 1
- CUTFAPGINUFNQM-UHFFFAOYSA-N 4-bromo-2-nitrophenol Chemical compound OC1=CC=C(Br)C=C1[N+]([O-])=O CUTFAPGINUFNQM-UHFFFAOYSA-N 0.000 description 1
- VLWRKVBQUANIGI-UHFFFAOYSA-N 4-fluoro-n-methylaniline Chemical compound CNC1=CC=C(F)C=C1 VLWRKVBQUANIGI-UHFFFAOYSA-N 0.000 description 1
- 125000001255 4-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1F 0.000 description 1
- POILWHVDKZOXJZ-UHFFFAOYSA-N 4-hydroxypent-3-en-2-one Chemical compound CC(O)=CC(C)=O POILWHVDKZOXJZ-UHFFFAOYSA-N 0.000 description 1
- 125000004203 4-hydroxyphenyl group Chemical group [H]OC1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- ZFIQGRISGKSVAG-UHFFFAOYSA-N 4-methylaminophenol Chemical compound CNC1=CC=C(O)C=C1 ZFIQGRISGKSVAG-UHFFFAOYSA-N 0.000 description 1
- 125000000339 4-pyridyl group Chemical group N1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 description 1
- KDDQRKBRJSGMQE-UHFFFAOYSA-N 4-thiazolyl Chemical group [C]1=CSC=N1 KDDQRKBRJSGMQE-UHFFFAOYSA-N 0.000 description 1
- CWDWFSXUQODZGW-UHFFFAOYSA-N 5-thiazolyl Chemical group [C]1=CN=CS1 CWDWFSXUQODZGW-UHFFFAOYSA-N 0.000 description 1
- MJZJYWCQPMNPRM-UHFFFAOYSA-N 6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,6-dihydro-1,3,5-triazine-2,4-diamine Chemical compound CC1(C)N=C(N)N=C(N)N1OCCCOC1=CC(Cl)=C(Cl)C=C1Cl MJZJYWCQPMNPRM-UHFFFAOYSA-N 0.000 description 1
- OEQUBNFISYFEEG-UHFFFAOYSA-N 6-O-tert-butyl 2-O-methyl pyridine-2,6-dicarboxylate Chemical compound COC(=O)C1=CC=CC(C(=O)OC(C)(C)C)=N1 OEQUBNFISYFEEG-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 208000022309 Alcoholic Liver disease Diseases 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 208000023328 Basedow disease Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 208000019838 Blood disease Diseases 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- VHIBOFWCGOAFJE-UHFFFAOYSA-N C1=CC=C[C-]1P(C1=CC=CC=C1)C1=CC=CC=C1.C1=CC=C[C-]1P(C1=CC=CC=C1)C1=CC=CC=C1.Cl.Cl.[Fe+2] Chemical compound C1=CC=C[C-]1P(C1=CC=CC=C1)C1=CC=CC=C1.C1=CC=C[C-]1P(C1=CC=CC=C1)C1=CC=CC=C1.Cl.Cl.[Fe+2] VHIBOFWCGOAFJE-UHFFFAOYSA-N 0.000 description 1
- YGMVEEWYECLUAJ-ZGTCLIOFSA-N CC(C)OC(C=C(C(C[C@@H]1C(O)=O)CN1C(C)=O)C=C1)=C1OC(F)F Chemical compound CC(C)OC(C=C(C(C[C@@H]1C(O)=O)CN1C(C)=O)C=C1)=C1OC(F)F YGMVEEWYECLUAJ-ZGTCLIOFSA-N 0.000 description 1
- GMPXGCDMNDJXST-ARLHGKGLSA-N CC(N(CC(C1)C(C=C2)=CC(OCC3CC3)=C2OC(F)F)[C@H]1C(O)=O)=O Chemical compound CC(N(CC(C1)C(C=C2)=CC(OCC3CC3)=C2OC(F)F)[C@H]1C(O)=O)=O GMPXGCDMNDJXST-ARLHGKGLSA-N 0.000 description 1
- QWOJMRHUQHTCJG-UHFFFAOYSA-N CC([CH2-])=O Chemical compound CC([CH2-])=O QWOJMRHUQHTCJG-UHFFFAOYSA-N 0.000 description 1
- KXFOUVDJLTZHFJ-UHFFFAOYSA-N CNC(=O)C1=CC=CC(=N1)C(=O)OC Chemical compound CNC(=O)C1=CC=CC(=N1)C(=O)OC KXFOUVDJLTZHFJ-UHFFFAOYSA-N 0.000 description 1
- 102000004631 Calcineurin Human genes 0.000 description 1
- 108010042955 Calcineurin Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- PTHCMJGKKRQCBF-UHFFFAOYSA-N Cellulose, microcrystalline Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC)C(CO)O1 PTHCMJGKKRQCBF-UHFFFAOYSA-N 0.000 description 1
- 208000000668 Chronic Pancreatitis Diseases 0.000 description 1
- 206010009137 Chronic sinusitis Diseases 0.000 description 1
- ZWAUVWPEINYFIC-UHFFFAOYSA-N Cl.CNCC1=CC=C(F)C=C1F Chemical compound Cl.CNCC1=CC=C(F)C=C1F ZWAUVWPEINYFIC-UHFFFAOYSA-N 0.000 description 1
- 101710095468 Cyclase Proteins 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- 201000004624 Dermatitis Diseases 0.000 description 1
- 206010012442 Dermatitis contact Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 101100135868 Dictyostelium discoideum pde3 gene Proteins 0.000 description 1
- 101100407335 Dictyostelium discoideum pde7 gene Proteins 0.000 description 1
- 101100189582 Dictyostelium discoideum pdeD gene Proteins 0.000 description 1
- 101100351286 Dictyostelium discoideum pdeE gene Proteins 0.000 description 1
- 101100135859 Dictyostelium discoideum regA gene Proteins 0.000 description 1
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 1
- 101001117089 Drosophila melanogaster Calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1 Proteins 0.000 description 1
- 101001072031 Drosophila melanogaster Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11 Proteins 0.000 description 1
- 101100407340 Drosophila melanogaster Pde8 gene Proteins 0.000 description 1
- 101100407341 Drosophila melanogaster Pde9 gene Proteins 0.000 description 1
- 206010014824 Endotoxic shock Diseases 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 206010015150 Erythema Diseases 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 208000007882 Gastritis Diseases 0.000 description 1
- 206010018364 Glomerulonephritis Diseases 0.000 description 1
- 208000024869 Goodpasture syndrome Diseases 0.000 description 1
- 201000005569 Gout Diseases 0.000 description 1
- 208000015023 Graves' disease Diseases 0.000 description 1
- 239000007821 HATU Substances 0.000 description 1
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 1
- 206010019728 Hepatitis alcoholic Diseases 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 208000029523 Interstitial Lung disease Diseases 0.000 description 1
- 108010076876 Keratins Proteins 0.000 description 1
- 102000011782 Keratins Human genes 0.000 description 1
- 229930194542 Keto Natural products 0.000 description 1
- 239000000867 Lipoxygenase Inhibitor Substances 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 101100407337 Mus musculus Pde8a gene Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 208000009525 Myocarditis Diseases 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- AFBPFSWMIHJQDM-UHFFFAOYSA-N N-methyl-N-phenylamine Natural products CNC1=CC=CC=C1 AFBPFSWMIHJQDM-UHFFFAOYSA-N 0.000 description 1
- VRWMAVHZMUVSDV-UHFFFAOYSA-N N=1C=CC(CC(C=1)=O)=O Chemical compound N=1C=CC(CC(C=1)=O)=O VRWMAVHZMUVSDV-UHFFFAOYSA-N 0.000 description 1
- 206010051606 Necrotising colitis Diseases 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 206010065673 Nephritic syndrome Diseases 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- 208000025966 Neurological disease Diseases 0.000 description 1
- GWVIDQCUUXWKLI-UHFFFAOYSA-N O=C(NC(C=C1)=CC(OCC2CC2)=C1OC(F)F)OCC1=CC=CC=C1 Chemical compound O=C(NC(C=C1)=CC(OCC2CC2)=C1OC(F)F)OCC1=CC=CC=C1 GWVIDQCUUXWKLI-UHFFFAOYSA-N 0.000 description 1
- NEBPWXRIAZAGOT-UHFFFAOYSA-N ON(CC1CC1)C(C=C(C=C1)Br)=C1OC(F)F Chemical compound ON(CC1CC1)C(C=C(C=C1)Br)=C1OC(F)F NEBPWXRIAZAGOT-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 101150098694 PDE5A gene Proteins 0.000 description 1
- 206010033645 Pancreatitis Diseases 0.000 description 1
- 206010033647 Pancreatitis acute Diseases 0.000 description 1
- 206010033649 Pancreatitis chronic Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229920002230 Pectic acid Polymers 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical class OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 101100082606 Plasmodium falciparum (isolate 3D7) PDEbeta gene Proteins 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920002565 Polyethylene Glycol 400 Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 208000003251 Pruritus Diseases 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 208000033464 Reiter syndrome Diseases 0.000 description 1
- 208000019417 Respiration disease Diseases 0.000 description 1
- 101100135860 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) PDE2 gene Proteins 0.000 description 1
- 206010039793 Seborrhoeic dermatitis Diseases 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- 208000021386 Sjogren Syndrome Diseases 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 238000006069 Suzuki reaction reaction Methods 0.000 description 1
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 1
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical group C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 241000009298 Trigla lyra Species 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- ANGKOCUUWGHLCE-HKUYNNGSSA-N [(3s)-1,1-dimethylpyrrolidin-1-ium-3-yl] (2r)-2-cyclopentyl-2-hydroxy-2-phenylacetate Chemical compound C1[N+](C)(C)CC[C@@H]1OC(=O)[C@](O)(C=1C=CC=CC=1)C1CCCC1 ANGKOCUUWGHLCE-HKUYNNGSSA-N 0.000 description 1
- SZPWXAOBLNYOHY-UHFFFAOYSA-N [C]1=CC=NC2=CC=CC=C12 Chemical group [C]1=CC=NC2=CC=CC=C12 SZPWXAOBLNYOHY-UHFFFAOYSA-N 0.000 description 1
- SORGEQQSQGNZFI-UHFFFAOYSA-N [azido(phenoxy)phosphoryl]oxybenzene Chemical compound C=1C=CC=CC=1OP(=O)(N=[N+]=[N-])OC1=CC=CC=C1 SORGEQQSQGNZFI-UHFFFAOYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 229940022663 acetate Drugs 0.000 description 1
- PWUBONDMIMDOQY-UHFFFAOYSA-N acetonitrile;hydrochloride Chemical compound Cl.CC#N PWUBONDMIMDOQY-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 229940019903 aclidinium Drugs 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000006022 acute inflammation Effects 0.000 description 1
- 208000005298 acute pain Diseases 0.000 description 1
- 201000003229 acute pancreatitis Diseases 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-L adipate(2-) Chemical compound [O-]C(=O)CCCCC([O-])=O WNLRTRBMVRJNCN-UHFFFAOYSA-L 0.000 description 1
- 208000037883 airway inflammation Diseases 0.000 description 1
- 208000002353 alcoholic hepatitis Diseases 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical class OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 102000015007 alpha-adrenergic receptor activity proteins Human genes 0.000 description 1
- 108040006816 alpha-adrenergic receptor activity proteins Proteins 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 229940024606 amino acid Drugs 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 239000002280 amphoteric surfactant Substances 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 125000002178 anthracenyl group Chemical group C1(=CC=CC2=CC3=CC=CC=C3C=C12)* 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000181 anti-adherent effect Effects 0.000 description 1
- 230000001078 anti-cholinergic effect Effects 0.000 description 1
- 230000000843 anti-fungal effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 230000001022 anti-muscarinic effect Effects 0.000 description 1
- 239000003911 antiadherent Substances 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000012062 aqueous buffer Substances 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 125000005418 aryl aryl group Chemical group 0.000 description 1
- 125000000732 arylene group Chemical group 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 201000004339 autoimmune neuropathy Diseases 0.000 description 1
- 230000003385 bacteriostatic effect Effects 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical compound BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 1
- 229940124748 beta 2 agonist Drugs 0.000 description 1
- WHGYBXFWUBPSRW-FOUAGVGXSA-N beta-cyclodextrin Chemical class OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO WHGYBXFWUBPSRW-FOUAGVGXSA-N 0.000 description 1
- 235000011175 beta-cyclodextrine Nutrition 0.000 description 1
- 102000014974 beta2-adrenergic receptor activity proteins Human genes 0.000 description 1
- 108040006828 beta2-adrenergic receptor activity proteins Proteins 0.000 description 1
- 239000003613 bile acid Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000037086 body physiology Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 229940124630 bronchodilator Drugs 0.000 description 1
- 239000000168 bronchodilator agent Substances 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 102100029175 cGMP-specific 3',5'-cyclic phosphodiesterase Human genes 0.000 description 1
- 229910052792 caesium Inorganic materials 0.000 description 1
- TVFDJXOCXUVLDH-UHFFFAOYSA-N caesium atom Chemical compound [Cs] TVFDJXOCXUVLDH-UHFFFAOYSA-N 0.000 description 1
- 229940046731 calcineurin inhibitors Drugs 0.000 description 1
- 235000001465 calcium Nutrition 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- FATUQANACHZLRT-KMRXSBRUSA-L calcium glucoheptonate Chemical compound [Ca+2].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)C([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)C([O-])=O FATUQANACHZLRT-KMRXSBRUSA-L 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 229960001714 calcium phosphate Drugs 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- 229960003340 calcium silicate Drugs 0.000 description 1
- 235000012241 calcium silicate Nutrition 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical compound C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 239000007894 caplet Substances 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001722 carbon compounds Chemical class 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 125000004181 carboxyalkyl group Chemical group 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 150000003943 catecholamines Chemical class 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 239000003093 cationic surfactant Substances 0.000 description 1
- 108010015046 cell aggregation factors Proteins 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 208000025997 central nervous system neoplasm Diseases 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 229910052729 chemical element Inorganic materials 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 239000012069 chiral reagent Substances 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 208000027157 chronic rhinosinusitis Diseases 0.000 description 1
- 208000019425 cirrhosis of liver Diseases 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 239000013066 combination product Substances 0.000 description 1
- 229940127555 combination product Drugs 0.000 description 1
- 208000010247 contact dermatitis Diseases 0.000 description 1
- 230000009989 contractile response Effects 0.000 description 1
- 229920001531 copovidone Polymers 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 150000001924 cycloalkanes Chemical class 0.000 description 1
- 229940097362 cyclodextrins Drugs 0.000 description 1
- MRKZAZMYXYSBDG-UHFFFAOYSA-N cyclopentyl propanoate Chemical compound CCC(=O)OC1CCCC1 MRKZAZMYXYSBDG-UHFFFAOYSA-N 0.000 description 1
- IGSKHXTUVXSOMB-UHFFFAOYSA-N cyclopropylmethanamine Chemical compound NCC1CC1 IGSKHXTUVXSOMB-UHFFFAOYSA-N 0.000 description 1
- DEZRYPDIMOWBDS-UHFFFAOYSA-N dcm dichloromethane Chemical compound ClCCl.ClCCl DEZRYPDIMOWBDS-UHFFFAOYSA-N 0.000 description 1
- 230000006240 deamidation Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 230000015961 delipidation Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 229960002593 desoximetasone Drugs 0.000 description 1
- VWVSBHGCDBMOOT-IIEHVVJPSA-N desoximetasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@H](C(=O)CO)[C@@]1(C)C[C@@H]2O VWVSBHGCDBMOOT-IIEHVVJPSA-N 0.000 description 1
- 238000006193 diazotization reaction Methods 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 150000004683 dihydrates Chemical class 0.000 description 1
- 125000004852 dihydrofuranyl group Chemical group O1C(CC=C1)* 0.000 description 1
- 125000005043 dihydropyranyl group Chemical group O1C(CCC=C1)* 0.000 description 1
- 125000005057 dihydrothienyl group Chemical group S1C(CC=C1)* 0.000 description 1
- FSBVERYRVPGNGG-UHFFFAOYSA-N dimagnesium dioxido-bis[[oxido(oxo)silyl]oxy]silane hydrate Chemical compound O.[Mg+2].[Mg+2].[O-][Si](=O)O[Si]([O-])([O-])O[Si]([O-])=O FSBVERYRVPGNGG-UHFFFAOYSA-N 0.000 description 1
- UXGNZZKBCMGWAZ-UHFFFAOYSA-N dimethylformamide dmf Chemical compound CN(C)C=O.CN(C)C=O UXGNZZKBCMGWAZ-UHFFFAOYSA-N 0.000 description 1
- 125000000532 dioxanyl group Chemical group 0.000 description 1
- FVTWTVQXNAJTQP-UHFFFAOYSA-N diphenyl-[1-(2-phenylmethoxyethyl)-1-azoniabicyclo[2.2.2]octan-4-yl]methanol Chemical compound C=1C=CC=CC=1C(C12CC[N+](CCOCC=3C=CC=CC=3)(CC1)CC2)(O)C1=CC=CC=C1 FVTWTVQXNAJTQP-UHFFFAOYSA-N 0.000 description 1
- 125000005982 diphenylmethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- MKRTXPORKIRPDG-UHFFFAOYSA-N diphenylphosphoryl azide Chemical compound C=1C=CC=CC=1P(=O)(N=[N+]=[N-])C1=CC=CC=C1 MKRTXPORKIRPDG-UHFFFAOYSA-N 0.000 description 1
- 230000006806 disease prevention Effects 0.000 description 1
- 125000005883 dithianyl group Chemical group 0.000 description 1
- POULHZVOKOAJMA-UHFFFAOYSA-M dodecanoate Chemical compound CCCCCCCCCCCC([O-])=O POULHZVOKOAJMA-UHFFFAOYSA-M 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 229950003468 dupilumab Drugs 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 238000003821 enantio-separation Methods 0.000 description 1
- 230000037149 energy metabolism Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 231100000321 erythema Toxicity 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-N ethanesulfonic acid Chemical class CCS(O)(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-N 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- JHGXKXYTBMCRDX-UHFFFAOYSA-N ethyl 6-(aminomethyl)pyridine-2-carboxylate;hydrochloride Chemical compound Cl.CCOC(=O)C1=CC=CC(CN)=N1 JHGXKXYTBMCRDX-UHFFFAOYSA-N 0.000 description 1
- 238000003810 ethyl acetate extraction Methods 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 238000004299 exfoliation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 229950003562 fevipiprant Drugs 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 239000008394 flocculating agent Substances 0.000 description 1
- 229960001347 fluocinolone acetonide Drugs 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 238000001640 fractional crystallisation Methods 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 230000001408 fungistatic effect Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 1
- 230000030135 gastric motility Effects 0.000 description 1
- 208000007565 gingivitis Diseases 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 229940097042 glucuronate Drugs 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- RQFCJASXJCIDSX-UUOKFMHZSA-N guanosine 5'-monophosphate Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O RQFCJASXJCIDSX-UUOKFMHZSA-N 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 208000018706 hematopoietic system disease Diseases 0.000 description 1
- 230000002949 hemolytic effect Effects 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-M hexanoate Chemical compound CCCCCC([O-])=O FUZZWVXGSFPDMH-UHFFFAOYSA-M 0.000 description 1
- 238000000589 high-performance liquid chromatography-mass spectrometry Methods 0.000 description 1
- 239000000938 histamine H1 antagonist Substances 0.000 description 1
- 239000003395 histamine H3 receptor antagonist Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 150000005828 hydrofluoroalkanes Chemical class 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 239000003230 hygroscopic agent Substances 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 125000002632 imidazolidinyl group Chemical group 0.000 description 1
- 125000002636 imidazolinyl group Chemical group 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 125000006302 indol-3-yl methyl group Chemical group [H]N1C([H])=C(C2=C([H])C([H])=C([H])C([H])=C12)C([H])([H])* 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000012442 inert solvent Substances 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 229940100601 interleukin-6 Drugs 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 1
- FMKOJHQHASLBPH-UHFFFAOYSA-N isopropyl iodide Chemical compound CC(C)I FMKOJHQHASLBPH-UHFFFAOYSA-N 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 208000018937 joint inflammation Diseases 0.000 description 1
- 229940001447 lactate Drugs 0.000 description 1
- 229940070765 laurate Drugs 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- VNYSSYRCGWBHLG-AMOLWHMGSA-N leukotriene B4 Chemical compound CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O VNYSSYRCGWBHLG-AMOLWHMGSA-N 0.000 description 1
- 239000003199 leukotriene receptor blocking agent Substances 0.000 description 1
- 201000011486 lichen planus Diseases 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000000865 liniment Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 206010025135 lupus erythematosus Diseases 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000000391 magnesium silicate Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 229940099273 magnesium trisilicate Drugs 0.000 description 1
- 229910000386 magnesium trisilicate Inorganic materials 0.000 description 1
- 235000019793 magnesium trisilicate Nutrition 0.000 description 1
- 150000004701 malic acid derivatives Chemical class 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 230000015654 memory Effects 0.000 description 1
- COTNUBDHGSIOTA-UHFFFAOYSA-N meoh methanol Chemical compound OC.OC COTNUBDHGSIOTA-UHFFFAOYSA-N 0.000 description 1
- 230000007102 metabolic function Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- OWBKDJSKHXGOJY-UHFFFAOYSA-N methyl 3-(aminomethyl)benzoate Chemical compound COC(=O)C1=CC=CC(CN)=C1 OWBKDJSKHXGOJY-UHFFFAOYSA-N 0.000 description 1
- WGPAOTBWPMCFMM-UHFFFAOYSA-N methyl 6-(aminomethyl)pyridine-3-carboxylate Chemical compound COC(=O)C1=CC=C(CN)N=C1 WGPAOTBWPMCFMM-UHFFFAOYSA-N 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 229940050176 methyl chloride Drugs 0.000 description 1
- XMJHPCRAQCTCFT-UHFFFAOYSA-N methyl chloroformate Chemical compound COC(Cl)=O XMJHPCRAQCTCFT-UHFFFAOYSA-N 0.000 description 1
- NQMRYBIKMRVZLB-UHFFFAOYSA-N methylamine hydrochloride Chemical compound [Cl-].[NH3+]C NQMRYBIKMRVZLB-UHFFFAOYSA-N 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- 229960001238 methylnicotinate Drugs 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 150000004682 monohydrates Chemical class 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- MNWYVVFHNHMOQJ-UHFFFAOYSA-N n-(cyclopropylmethyl)hydroxylamine Chemical compound ONCC1CC1 MNWYVVFHNHMOQJ-UHFFFAOYSA-N 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- WOOWBQQQJXZGIE-UHFFFAOYSA-N n-ethyl-n-propan-2-ylpropan-2-amine Chemical compound CCN(C(C)C)C(C)C.CCN(C(C)C)C(C)C WOOWBQQQJXZGIE-UHFFFAOYSA-N 0.000 description 1
- RIVIDPPYRINTTH-UHFFFAOYSA-N n-ethylpropan-2-amine Chemical compound CCNC(C)C RIVIDPPYRINTTH-UHFFFAOYSA-N 0.000 description 1
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- UWUAILSWRYUAMP-UHFFFAOYSA-N n-methyl-1-pyridin-2-ylmethanamine;dihydrochloride Chemical compound Cl.Cl.CNCC1=CC=CC=N1 UWUAILSWRYUAMP-UHFFFAOYSA-N 0.000 description 1
- 125000003506 n-propoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-M naphthalene-2-sulfonate Chemical compound C1=CC=CC2=CC(S(=O)(=O)[O-])=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-M 0.000 description 1
- 239000006199 nebulizer Substances 0.000 description 1
- 208000004995 necrotizing enterocolitis Diseases 0.000 description 1
- 229950010012 nemolizumab Drugs 0.000 description 1
- 208000004296 neuralgia Diseases 0.000 description 1
- 208000021722 neuropathic pain Diseases 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 208000037916 non-allergic rhinitis Diseases 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- 229940049964 oleate Drugs 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-M oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC([O-])=O ZQPPMHVWECSIRJ-KTKRTIGZSA-M 0.000 description 1
- 239000006186 oral dosage form Substances 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 1
- 125000003551 oxepanyl group Chemical group 0.000 description 1
- 125000003566 oxetanyl group Chemical group 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 125000000466 oxiranyl group Chemical group 0.000 description 1
- 125000005476 oxopyrrolidinyl group Chemical group 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 229940112824 paste Drugs 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 101150037969 pde-6 gene Proteins 0.000 description 1
- 239000003961 penetration enhancing agent Substances 0.000 description 1
- JLFNLZLINWHATN-UHFFFAOYSA-N pentaethylene glycol Chemical compound OCCOCCOCCOCCOCCO JLFNLZLINWHATN-UHFFFAOYSA-N 0.000 description 1
- 125000003538 pentan-3-yl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- VLTRZXGMWDSKGL-UHFFFAOYSA-M perchlorate Inorganic materials [O-]Cl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-M 0.000 description 1
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 1
- 201000006195 perinatal necrotizing enterocolitis Diseases 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 201000001245 periodontitis Diseases 0.000 description 1
- 150000004965 peroxy acids Chemical class 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L peroxydisulfate Chemical compound [O-]S(=O)(=O)OOS([O-])(=O)=O JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 125000003884 phenylalkyl group Chemical group 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229950005184 piclamilast Drugs 0.000 description 1
- RRRUXBQSQLKHEL-UHFFFAOYSA-N piclamilast Chemical compound COC1=CC=C(C(=O)NC=2C(=CN=CC=2Cl)Cl)C=C1OC1CCCC1 RRRUXBQSQLKHEL-UHFFFAOYSA-N 0.000 description 1
- SIOXPEMLGUPBBT-UHFFFAOYSA-M picolinate Chemical compound [O-]C(=O)C1=CC=CC=N1 SIOXPEMLGUPBBT-UHFFFAOYSA-M 0.000 description 1
- 229940075930 picrate Drugs 0.000 description 1
- OXNIZHLAWKMVMX-UHFFFAOYSA-M picrate anion Chemical compound [O-]C1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1[N+]([O-])=O OXNIZHLAWKMVMX-UHFFFAOYSA-M 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-M pivalate Chemical compound CC(C)(C)C([O-])=O IUGYQRQAERSCNH-UHFFFAOYSA-M 0.000 description 1
- 229950010765 pivalate Drugs 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000151 polyglycol Polymers 0.000 description 1
- 239000010695 polyglycol Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 1
- 230000004952 protein activity Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 208000002815 pulmonary hypertension Diseases 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000003072 pyrazolidinyl group Chemical group 0.000 description 1
- 125000002206 pyridazin-3-yl group Chemical group [H]C1=C([H])C([H])=C(*)N=N1 0.000 description 1
- UBQKCCHYAOITMY-UHFFFAOYSA-N pyridin-2-ol Chemical compound OC1=CC=CC=N1 UBQKCCHYAOITMY-UHFFFAOYSA-N 0.000 description 1
- 125000006514 pyridin-2-ylmethyl group Chemical group [H]C1=C([H])C([H])=C([H])C(=N1)C([H])([H])* 0.000 description 1
- HFAFXVOPGDBAOK-UHFFFAOYSA-N pyridine-2-carbonyloxidanium;chloride Chemical compound Cl.OC(=O)C1=CC=CC=N1 HFAFXVOPGDBAOK-UHFFFAOYSA-N 0.000 description 1
- XQGIACFEVNAZRL-UHFFFAOYSA-N pyridine-2-carboxamide;dihydrochloride Chemical compound Cl.Cl.NC(=O)C1=CC=CC=N1 XQGIACFEVNAZRL-UHFFFAOYSA-N 0.000 description 1
- 125000000246 pyrimidin-2-yl group Chemical group [H]C1=NC(*)=NC([H])=C1[H] 0.000 description 1
- 125000004527 pyrimidin-4-yl group Chemical group N1=CN=C(C=C1)* 0.000 description 1
- 125000004528 pyrimidin-5-yl group Chemical group N1=CN=CC(=C1)* 0.000 description 1
- 238000005956 quaternization reaction Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 208000002574 reactive arthritis Diseases 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000006462 rearrangement reaction Methods 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000006722 reduction reaction Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 208000008742 seborrheic dermatitis Diseases 0.000 description 1
- 125000005920 sec-butoxy group Chemical group 0.000 description 1
- DCKVNWZUADLDEH-UHFFFAOYSA-N sec-butyl acetate Chemical compound CCC(C)OC(C)=O DCKVNWZUADLDEH-UHFFFAOYSA-N 0.000 description 1
- 230000036303 septic shock Effects 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 1
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 235000010288 sodium nitrite Nutrition 0.000 description 1
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical class [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 1
- MRTAVLDNYYEJHK-UHFFFAOYSA-M sodium;2-chloro-2,2-difluoroacetate Chemical compound [Na+].[O-]C(=O)C(F)(F)Cl MRTAVLDNYYEJHK-UHFFFAOYSA-M 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000011877 solvent mixture Substances 0.000 description 1
- 239000001593 sorbitan monooleate Substances 0.000 description 1
- 235000011069 sorbitan monooleate Nutrition 0.000 description 1
- 229940035049 sorbitan monooleate Drugs 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 150000005846 sugar alcohols Chemical class 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- HKARRILFPHKLQO-UHFFFAOYSA-N tert-butyl N-[[6-(dimethylcarbamoyl)pyridin-2-yl]methyl]carbamate Chemical compound CC(C)(C)OC(NCC1=NC(C(N(C)C)=O)=CC=C1)=O HKARRILFPHKLQO-UHFFFAOYSA-N 0.000 description 1
- ZPOIEEISPMGDRW-UHFFFAOYSA-N tert-butyl n-(cyclopropylmethyl)carbamate Chemical compound CC(C)(C)OC(=O)NCC1CC1 ZPOIEEISPMGDRW-UHFFFAOYSA-N 0.000 description 1
- JETMBGCMJDJPOC-UHFFFAOYSA-N tert-butyl n-[(2,4-difluorophenyl)methyl]carbamate Chemical compound CC(C)(C)OC(=O)NCC1=CC=C(F)C=C1F JETMBGCMJDJPOC-UHFFFAOYSA-N 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000005942 tetrahydropyridyl group Chemical group 0.000 description 1
- 125000005958 tetrahydrothienyl group Chemical group 0.000 description 1
- 125000004632 tetrahydrothiopyranyl group Chemical group S1C(CCCC1)* 0.000 description 1
- 150000003536 tetrazoles Chemical class 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000002053 thietanyl group Chemical group 0.000 description 1
- 125000005503 thioxanyl group Chemical group 0.000 description 1
- LERNTVKEWCAPOY-DZZGSBJMSA-N tiotropium Chemical compound O([C@H]1C[C@@H]2[N+]([C@H](C1)[C@@H]1[C@H]2O1)(C)C)C(=O)C(O)(C=1SC=CC=1)C1=CC=CS1 LERNTVKEWCAPOY-DZZGSBJMSA-N 0.000 description 1
- 229940110309 tiotropium Drugs 0.000 description 1
- 239000006208 topical dosage form Substances 0.000 description 1
- 231100000583 toxicological profile Toxicity 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 150000003852 triazoles Chemical class 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 125000002827 triflate group Chemical class FC(S(=O)(=O)O*)(F)F 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-N triflic acid Chemical class OS(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-N 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 238000000825 ultraviolet detection Methods 0.000 description 1
- 229960004258 umeclidinium Drugs 0.000 description 1
- ZDPHROOEEOARMN-UHFFFAOYSA-N undecanoic acid Chemical compound CCCCCCCCCCC(O)=O ZDPHROOEEOARMN-UHFFFAOYSA-N 0.000 description 1
- 229950000088 upadacitinib Drugs 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 229960003824 ustekinumab Drugs 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 229940070710 valerate Drugs 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 206010047470 viral myocarditis Diseases 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/401—Proline; Derivatives thereof, e.g. captopril
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/4035—Isoindoles, e.g. phthalimide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4523—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems
- A61K31/4545—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring hetero atom, e.g. pipamperone, anabasine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5377—1,4-Oxazines, e.g. morpholine not condensed and containing further heterocyclic rings, e.g. timolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/69—Boron compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/06—Anti-spasmodics, e.g. drugs for colics, esophagic dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/08—Bronchodilators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B59/00—Introduction of isotopes of elements into organic compounds ; Labelled organic compounds per se
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B59/00—Introduction of isotopes of elements into organic compounds ; Labelled organic compounds per se
- C07B59/002—Heterocyclic compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/10—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/12—Oxygen or sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/10—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/16—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/30—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members
- C07D207/34—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/36—Oxygen or sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
- C07F5/025—Boronic and borinic acid compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/05—Isotopically modified compounds, e.g. labelled
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Pulmonology (AREA)
- Rheumatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Immunology (AREA)
- Dermatology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Urology & Nephrology (AREA)
- Pain & Pain Management (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
本發明屬於藥物領域,具體涉及一類取代的吡咯烷類化合物、包含所述化合物的藥物組合物及其用途和使用方法。特別地,本發明所述的化合物是PDE4抑制劑,用於治療PDE4相關的疾病,例如特應性皮炎(AD)、銀屑病或慢性阻塞性肺病(COPD)。The present invention belongs to the field of medicine, and particularly relates to a class of substituted pyrrolidine compounds, pharmaceutical compositions comprising the compounds, and uses and methods of use thereof. In particular, the compounds described in the present invention are PDE4 inhibitors for the treatment of PDE4-related diseases such as atopic dermatitis (AD), psoriasis or chronic obstructive pulmonary disease (COPD).
環磷酸腺苷(cAMP)和環磷酸鳥苷(cGMP)是細胞內兩種重要的第二信使,主要通過啟動蛋白激酶A(PKA)和蛋白激酶G(PKG)途徑參與能量代謝、記憶、免疫反應、視覺及嗅覺形成等生理活動,其細胞內濃度的調節主要由腺(鳥)苷酸環化酶的合成和磷酸二酯酶(PDEs)的水解作用之間的平衡決定。PDEs能特異性地以3,5-環核苷酸為底物,催化細胞內的cGMP和cAMP水解生成相應的無活性的5-核苷酸,從而影響生物體的各種代謝功能。因此,抑制PDEs對引起許多細胞活性是一種很有效的途徑,能影響炎症細胞和免疫細胞活化及平滑肌細胞收縮反應。Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are two important second messengers in cells, mainly involved in energy metabolism, memory, immunity by initiating protein kinase A (PKA) and protein kinase G (PKG) pathways. The regulation of intracellular concentration of physiological activities such as reaction, vision and smell formation is mainly determined by the balance between the synthesis of adenosine (guanylate) cyclase and the hydrolysis of phosphodiesterases (PDEs). PDEs can specifically use 3,5-cyclic nucleotides as substrates to catalyze the hydrolysis of cGMP and cAMP in cells to generate corresponding inactive 5-nucleotides, thereby affecting various metabolic functions of organisms. Therefore, inhibition of PDEs is a very effective way to induce many cellular activities, which can affect the activation of inflammatory cells and immune cells and the contractile response of smooth muscle cells.
磷酸二酯酶(PDEs)迄今已報導有11個基因家族,每個家族又包括多個亞家族。PDEs分佈於多個組織中,其抑制劑具有廣泛的生理作用,其中,PDE4、PDE7和PDE8主要特異性水解cAMP,PDE5、PDE6和PDE9特異性水解cGMP,而PDE1、PDE2、PDE3、PDE10和PDE11則對cAMP和cGMP都起作用。其中,PDE4主要分佈於各種炎性細胞內,其組織分佈說明它與中樞神經系統和免疫系統息息相關,其抑制劑可用於治療各種疾病,包括過敏性和炎性疾病、糖尿病、中樞神經系統疾病和疼痛。Phosphodiesterases (PDEs) have been reported to date with 11 gene families, each of which includes multiple subfamilies. PDEs are distributed in multiple tissues, and their inhibitors have a wide range of physiological effects. Among them, PDE4, PDE7 and PDE8 mainly specifically hydrolyze cAMP, PDE5, PDE6 and PDE9 specifically hydrolyze cGMP, while PDE1, PDE2, PDE3, PDE10 and PDE11 It works on both cAMP and cGMP. Among them, PDE4 is mainly distributed in various inflammatory cells, and its tissue distribution shows that it is closely related to the central nervous system and immune system, and its inhibitors can be used to treat various diseases, including allergic and inflammatory diseases, diabetes, central nervous system diseases and pain.
目前,對PDE4的研究主要集中在免疫及炎症相關疾病中,世界上許多著名的製藥公司都把PDE4作為慢性炎症相關疾病的靶點。PDE4抑制劑發揮抗炎作用主要通過以下幾種途徑:(1)抑制多種炎症介質的活性;(2)抑制細胞黏附因子的上調和表達;(3)抑制血白細胞的活化;(4)誘導細胞凋亡;(5)誘導具有抑制活性的細胞因子的生成(如白細胞介素-6);(6)誘導兒茶酚胺類物質和內源性激素的釋放。第一代PDE4抑制劑主要有茶鹼、咯利普蘭(Rolipram)和吡拉米司特(Piclamilast)等,咯利普蘭對神經系統疾病,如帕金森病、抑鬱症和焦慮等都具有一定的治療作用。但第一代PDE4抑制劑由於嚴重的噁心、嘔吐等副作用,在臨床上的應用受到了限制;第二代PDE4抑制劑有羅氟司特(Roflumilast)和西洛司特(Cilomilast)等,其中羅氟司特用於COPD的治療,對其他炎症性疾病也有一定的治療效果,如潰瘍性結腸炎和克羅恩病。第三代PDE4抑制劑阿普斯特(Apremilast)已經用於自身免疫性疾病如銀屑病的治療,且副作用更小,病人更易耐受。WO/2000/064260披露了PDE4抑制劑Ro 20-1724 1%霜劑治療銀屑病有效。WO 2000/009504披露了另一個PDE4抑制劑CP-80633(0.5%軟膏),其明顯的改善了特應性皮炎的臨床計分(紅斑、硬結和表皮脫落)。但是,臨床上仍需要更多的可以有效治療特應性皮炎的PDE4抑制劑。At present, the research on PDE4 mainly focuses on immune and inflammation-related diseases, and many famous pharmaceutical companies in the world have used PDE4 as the target of chronic inflammation-related diseases. The anti-inflammatory effect of PDE4 inhibitors is mainly through the following ways: (1) inhibiting the activity of various inflammatory mediators; (2) inhibiting the up-regulation and expression of cell adhesion factors; (3) inhibiting the activation of blood leukocytes; (4) inducing cells apoptosis; (5) induce the production of cytokines with inhibitory activity (such as interleukin-6); (6) induce the release of catecholamines and endogenous hormones. The first-generation PDE4 inhibitors mainly include theophylline, rolipram and Piclamilast, etc. Rolipram has certain effects on neurological diseases such as Parkinson's disease, depression and anxiety. Therapeutic effect. However, the clinical application of the first-generation PDE4 inhibitors has been limited due to serious side effects such as nausea and vomiting; the second-generation PDE4 inhibitors include Roflumilast and Cilomilast, among which Roflumilast is used in the treatment of COPD and also has a certain therapeutic effect on other inflammatory diseases, such as ulcerative colitis and Crohn's disease. Apremilast, a third-generation PDE4 inhibitor, has been used in the treatment of autoimmune diseases such as psoriasis with fewer side effects and better tolerance by patients. WO/2000/064260 discloses that the PDE4 inhibitor Ro 20-1724 1% cream is effective in the treatment of psoriasis. WO 2000/009504 discloses another PDE4 inhibitor, CP-80633 (0.5% ointment), which significantly improved the clinical scores (erythema, induration and exfoliation) of atopic dermatitis. However, there is still a need for more PDE4 inhibitors that can effectively treat atopic dermatitis.
以下僅概括說明本發明的一些方面,並不局限於此。這些方面和其他部分在後面有更完整的說明。本說明書中的所有參考文獻通過整體引用於此。當本說明書的公開內容與引用文獻有差異時,以本說明書的公開內容為準。The following only outlines some aspects of the present invention, and is not limited thereto. These and other sections are described more fully later. All references in this specification are hereby incorporated by reference in their entirety. When there is a discrepancy between the disclosure content of this specification and the cited documents, the disclosure content of this specification shall prevail.
本發明提供了一類具有4型磷酸二酯酶 (Phosphodiesterase-4, PDE4) 抑制活性的化合物,用於製備預防、治療或減輕與PDE4有關的呼吸疾病、過敏、炎症、中樞神經系統疾病或非胰島素依賴糖尿病的藥物,比如慢性阻塞性肺疾病、肺氣腫、哮喘、慢性肺炎、塵肺病、支氣管炎、支氣管擴張症、肺結核纖維化病變、肺囊性纖維化、急性呼吸窘迫綜合症或呼吸道炎症;其中,支氣管炎為急性支氣管炎、慢性支氣管炎、變應性支氣管炎、彌漫性泛細支氣管炎、閉塞性細支氣管炎、過敏性結膜炎、特應性皮炎、過敏性皮炎、類風濕性關節炎、間質性膀胱炎、變應性鼻炎、潰瘍性結腸炎、強直性脊柱炎、風濕性關節炎或銀屑病關節炎等;本發明化合物能夠很好地抑制PDE4,同時具有優良的理化性質以及藥代動力學性質。The present invention provides a class of compounds with phosphodiesterase-4 (Phosphodiesterase-4, PDE4) inhibitory activity, which are used to prepare, prevent, treat or alleviate PDE4-related respiratory diseases, allergies, inflammations, central nervous system diseases or non-insulin Medications dependent on diabetes, such as chronic obstructive pulmonary disease, emphysema, asthma, chronic pneumonia, pneumoconiosis, bronchitis, bronchiectasis, tuberculosis fibrosis, cystic fibrosis, acute respiratory distress syndrome, or airway inflammation ; Among them, bronchitis is acute bronchitis, chronic bronchitis, allergic bronchitis, diffuse panbronchiolitis, obliterative bronchiolitis, allergic conjunctivitis, atopic dermatitis, allergic dermatitis, rheumatoid joint inflammation, interstitial cystitis, allergic rhinitis, ulcerative colitis, ankylosing spondylitis, rheumatoid arthritis or psoriatic arthritis, etc.; the compounds of the present invention can well inhibit PDE4 and have excellent physicochemical properties properties and pharmacokinetic properties.
本發明也提供了這些化合物的製備方法和包含這些化合物的藥物組合物以及使用這些化合物或組合物治療哺乳動物,尤其是人類的上述疾病的方法。The present invention also provides methods for the preparation of these compounds and pharmaceutical compositions comprising these compounds and methods of using these compounds or compositions to treat the above-mentioned diseases in mammals, especially humans.
具體地說:Specifically:
一方面,本發明涉及一種如式 (I) 所示的化合物或式 (I) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(I);In one aspect, the present invention relates to a compound of formula (I) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite, pharmaceutically acceptable compound of formula (I) Acceptable salts or their prodrugs: (I);
其中:in:
X為CH或N;X is CH or N;
Y為-(CH2 )m -C(=O)-NH-(CRm Rn )p -或-(CH2 )m -C(=O)-O-(CRm Rn )p -;Y is -(CH 2 ) m -C(=O)-NH-(CR m R n ) p - or -(CH 2 ) m -C(=O)-O-(CR m R n ) p -;
A環為C6-10 芳基或5-10個原子組成的雜芳基;Ring A is a C 6-10 aryl group or a heteroaryl group consisting of 5-10 atoms;
R1 為氫、氘、-ORa 或-NRc Rd ;R 1 is hydrogen, deuterium, -OR a or -NR c R d ;
R2 為C1-4 烷基、鹵代C1-4 烷基、C3-6 環烷基、C3-6 環烷基-C1-4 烷基、5-7個原子組成的雜環基或(5-7個原子組成的雜環基)-C1-4 烷基;或R 2 is C 1-4 alkyl, halogenated C 1-4 alkyl, C 3-6 cycloalkyl, C 3-6 cycloalkyl-C 1-4 alkyl, a hetero group consisting of 5-7 atoms Cyclic or (heterocyclyl consisting of 5-7 atoms)-C 1-4 alkyl; or
R2 與Ra 和其相連的原子共同形成5-7個原子組成的雜環基,所述的5-7個原子組成的雜環基任選地被選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、C1-4 烷基、C1-4 烷氧基或鹵代C1-4 烷基的取代基所取代;R 2 and R a and the connected atoms together form a heterocyclic group composed of 5-7 atoms, and the heterocyclic group composed of 5-7 atoms is optionally selected from deuterium, F, Cl, Br, Substituted by substituents of I, -OH, -CN, -NH 2 , C 1-4 alkyl, C 1-4 alkoxy or halogenated C 1-4 alkyl;
R3 為氫、氘、C1-6 烷基、C1-6 烷氧基、C2-6 烯基、C2-6 炔基、C1-6 烷基-C(=O)-、HC(=O)-、C1-6 烷基-O-C(=O)-、C1-6 烷基-S(=O)2 -或C1-6 烷基-C(=NH)-,其中,所述的C1-6 烷基、C1-6 烷氧基、C2-6 烯基、C2-6 炔基、C1-6 烷基-C(=O)-、C1-6 烷基-O-C(=O)-、C1-6 烷基-S(=O)2 -和C1-6 烷基-C(=NH)-各自獨立任選地被1、2或3個選自氘、F、Cl、Br、I、-OH、-CN或-NH2 的取代基所取代;R 3 is hydrogen, deuterium, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, C 1-6 alkyl-C(=O)-, HC(=O)-, C 1-6 alkyl-OC(=O)-, C 1-6 alkyl-S(=O) 2 - or C 1-6 alkyl-C(=NH)-, wherein said C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, C 1-6 alkyl -C (= O) -, C 1 -6Alkyl -OC(=O)-, C1-6Alkyl -S(=O) 2- and C1-6Alkyl -C(=NH)- are each independently optionally replaced by 1, 2 or 3 substituents selected from deuterium, F, Cl, Br, I, -OH, -CN or -NH 2 are substituted;
R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 和R9 各自獨立地為氫、氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-4 烷基、C1-4 烷氧基、鹵代C1-4 烷基、C1-4 烷基-C(=O)-或C1-4 烷基-S(=O)2 -; R 4, R 5a, R 5b , R 6, R 7a, R 7b, R 8 and R 9 are each independently hydrogen, deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, - NO 2 , -COOH, C 1-4 alkyl, C 1-4 alkoxy, halogenated C 1-4 alkyl, C 1-4 alkyl-C(=O)- or C 1-4 alkyl -S(=O) 2- ;
Ra 為氫、氘、C1-6 烷基、C2-6 烯基、C2-6 炔基、C3-8 環烷基、5-10個原子組成的雜環基、C6-10 芳基或5-10個原子組成的雜芳基,其中,所述的C1-6 烷基、C2-6 烯基、C2-6 炔基、C3-8 環烷基、5-10個原子組成的雜環基、C6-10 芳基和5-10個原子組成的雜芳基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-6 烷基、C1-6 烷氧基、C3-8 環烷基或5-10個原子組成的雜環基的取代基所取代;R a is hydrogen, deuterium, C 1-6 alkyl, C 2-6 alkenyl, C 2-6 alkynyl, C 3-8 cycloalkyl, heterocyclic group consisting of 5-10 atoms, C 6- 10 aryl groups or heteroaryl groups composed of 5-10 atoms, wherein the C 1-6 alkyl group, C 2-6 alkenyl group, C 2-6 alkynyl group, C 3-8 cycloalkyl group, 5 - Heterocyclyl of 10 atoms, C 6-10 aryl and heteroaryl of 5-10 atoms are each independently optionally substituted by 1, 2, 3 or 4 atoms selected from deuterium, F, Cl, Br , I, -OH, -CN, -NH 2 , -NO 2 , -COOH, C 1-6 alkyl, C 1-6 alkoxy, C 3-8 cycloalkyl or composed of 5-10 atoms Substituted by substituents of heterocyclyl;
各Rb 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、氧代、C1-6 烷基、C1-6 烷氧基、C1-6 烷基-OC(=O)-、C1-6 烷基-C(=O)-、C1-6 烷基-S(=O)2 -、C3-8 環烷基-C1-6 烷基、C3-8 環烷基、5-10個原子組成的雜環基、C6-10 芳基、5-10個原子組成的雜芳基、-NRe C(=O)C1-6 烷基、-NRe Rf 、-S(=O)2 -NRe Rf 、-C(=O)-NRe Rf 或-C1-6 烷基-NRe Rf ,其中,所述的C1-6 烷基、C1-6 烷氧基、C3-8 環烷基-C1-6 烷基、C3-8 環烷基、5-10個原子組成的雜環基、C6-10 芳基和5-10個原子組成的雜芳基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-6 烷基、C1-6 烷氨基或C1-6 烷氧基的取代基所取代;Each R b is independently deuterium, F, Cl, Br, I, -OH, -CN, -NH 2 , -NO 2 , -COOH, oxo, C 1-6 alkyl, C 1-6 alkoxy , C 1-6 alkyl-OC(=O)-, C 1-6 alkyl-C(=O)-, C 1-6 alkyl-S(=O) 2- , C 3-8 cycloalkane Base-C 1-6 alkyl, C 3-8 cycloalkyl, heterocyclic group composed of 5-10 atoms, C 6-10 aryl group, heteroaryl group composed of 5-10 atoms, -NR e C (=O)C 1-6 alkyl, -NR e R f , -S(=O) 2 -NR e R f , -C(=O)-NR e R f or -C 1-6 alkyl- NR e R f , wherein the C 1-6 alkyl, C 1-6 alkoxy, C 3-8 cycloalkyl-C 1-6 alkyl, C 3-8 cycloalkyl, 5- Heterocyclyl of 10 atoms, C 6-10 aryl and heteroaryl of 5-10 atoms are each independently optionally substituted by 1, 2, 3 or 4 atoms selected from deuterium, F, Cl, Br, I, -OH, -CN, -NH 2 , -NO 2 , -COOH, C 1-6 alkyl, C 1-6 alkylamino or C 1-6 alkoxy substituent;
Rc 和Rd 各自獨立地為氫、-OH、C1-4 烷基、鹵代C1-4 烷基、C3-6 環烷基-C1-4 烷基、C3-6 環烷基、5-7個原子組成的雜環基、(5-7個原子組成的雜環基)-C1-4 烷基、C1-4 烷基-C(=O)-或C1-4 烷基-S(=O)2 -;R c and R d are each independently hydrogen, -OH, C 1-4 alkyl, halogenated C 1-4 alkyl, C 3-6 cycloalkyl-C 1-4 alkyl, C 3-6 cyclo Alkyl, heterocyclyl of 5-7 atoms, (heterocyclyl of 5-7 atoms)-C 1-4 alkyl, C 1-4 alkyl-C(=O)- or C 1 -4 alkyl-S(=O) 2 -;
Re 和Rf 各自獨立地為氫、C1-6 烷基、C1-6 烷基-OC(=O)-、C1-6 烷基-C(=O)-、C1-6 烷基-S(=O)2 -、C3-8 環烷基、C6-10 芳基、5-10個原子組成的雜環基、5-10個原子組成的雜芳基、11-15個原子組成的雜芳基、C3-8 環烷基-C1-6 烷基、C6-10 芳基-C1-6 烷基、(5-10個原子組成的雜環基)-C1-6 烷基、(5-10個原子組成的雜芳基)-C1-6 烷基或-C1-6 烷基-NRg Rj ;或者Re 和Rf 與它們相連的N原子一起形成4-7個原子組成的雜環基;其中,所述的Re 、Rf 和4-7個原子組成的雜環基各自獨立任選地被1、2、3或4個Rh 所取代;R e and R f are each independently hydrogen, C 1-6 alkyl, C 1-6 alkyl-OC(=O)-, C 1-6 alkyl-C(=O)-, C 1-6 alkyl Alkyl-S(=O) 2 -, C 3-8 cycloalkyl, C 6-10 aryl, 5-10 atoms of heterocyclyl, 5-10 atoms of heteroaryl, 11- Heteroaryl consisting of 15 atoms, C 3-8 cycloalkyl-C 1-6 alkyl, C 6-10 aryl-C 1-6 alkyl, (heterocyclic group consisting of 5-10 atoms) -C 1-6 alkyl, (heteroaryl consisting of 5-10 atoms)-C 1-6 alkyl or -C 1-6 alkyl-NR g R j ; or R e and R f are attached to them The N atoms together form a heterocyclic group consisting of 4-7 atoms; wherein, the R e , R f and the heterocyclic group consisting of 4-7 atoms are each independently optionally replaced by 1, 2, 3 or 4 replaced by R h;
各Rh 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、C1-4 烷基、C1-4鹵代 烷基、C1-4鹵代烷氧 基或C1-4 烷氧基;Each R h is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, C 1-4 alkyl, C 1-4 haloalkyl, C 1-4 haloalkoxy or C 1-4 alkoxy;
Rg 和Rj 各自獨立地為氫、C1-4 烷基、C1-4 烷基-OC(=O)-、C1-4 烷基-C(=O)-、C1-4 烷基-S(=O)2 -、C3-6 環烷基、C6-10 芳基、5-6個原子組成的雜環基或5-6個原子組成的雜芳基;R g and R j are each independently hydrogen, C 1-4 alkyl, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-C(=O)-, C 1-4 Alkyl-S(=O) 2 -, C 3-6 cycloalkyl, C 6-10 aryl, heterocyclic group consisting of 5-6 atoms or heteroaryl group consisting of 5-6 atoms;
Rm 和Rn 各自獨立地為氫、氘、F、Cl、Br、I、-OH、-CN、-NH2 、C1-4 烷基、鹵代C1-4 烷基或-C(=O)NH2 ;R m and R n are each independently hydrogen, deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, C 1-4 alkyl, halo C 1-4 alkyl or -C ( =O)NH 2 ;
各n獨立地為0、1、2、3或4;each n is independently 0, 1, 2, 3, or 4;
m和p各自獨立地為0、1、2或3。m and p are each independently 0, 1, 2 or 3.
在一些實施方案中,A環為苯基、萘基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基、噁唑基、吲哚基、異吲哚基、、、、、或。In some embodiments, Ring A is phenyl, naphthyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, oxazole base, indolyl, isoindolyl, , , , , or .
在一些實施方案中,R3 為氫、氘、C1-4 烷基、C1-4 烷氧基、C2-4 烯基、C2-4 炔基、C1-4 烷基-C(=O)-、HC(=O)-、C1-4 烷基-O-C(=O)-、C1-4 烷基-S(=O)2 -或C1-4 烷基-C(=NH)-,其中,所述的C1-4 烷基、C1-4 烷氧基、C2-4 烯基、C2-4 炔基、C1-4 烷基-C(=O)-、C1-4 烷基-O-C(=O)-、C1-4 烷基-S(=O)2 -和C1-4 烷基-C(=NH)-各自獨立任選地被1、2或3個選自氘、F、Cl、Br、I、-OH、-CN或-NH2 的取代基所取代。In some embodiments, R 3 is hydrogen, deuterium, C 1-4 alkyl, C 1-4 alkoxy, C 2-4 alkenyl, C 2-4 alkynyl, C 1-4 alkyl-C (=O)-, HC(=O)-, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-S(=O) 2 - or C 1-4 alkyl-C (=NH)-, wherein, the C 1-4 alkyl, C 1-4 alkoxy, C 2-4 alkenyl, C 2-4 alkynyl, C 1-4 alkyl-C (= O)-, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-S(=O) 2 - and C 1-4 alkyl-C(=NH)- are each independently optional 1, 2 or 3 substituents selected from deuterium, F, Cl, Br, I , -OH, -CN , or -NH 2 to substituents.
在一些實施方案中,Ra 為氫、氘、C1-4 烷基、C2-4 烯基、C2-4 炔基、C3-6 環烷基、5-6個原子組成的雜環基、苯基或5-6個原子組成的雜芳基,其中,所述的C1-4 烷基、C2-4 烯基、C2-4 炔基、C3-6 環烷基、5-6個原子組成的雜環基、苯基和5-6個原子組成的雜芳基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-4 烷基、C1-4 烷氧基、C3-6 環烷基或5-6個原子組成的雜環基的取代基所取代。In some embodiments, R a is hydrogen, deuterium, C 1-4 alkyl, C 2-4 alkenyl, C 2-4 alkynyl, C 3-6 cycloalkyl, heterocyclic of 5-6 atoms Cyclic group, phenyl group or heteroaryl group consisting of 5-6 atoms, wherein said C 1-4 alkyl group, C 2-4 alkenyl group, C 2-4 alkynyl group, C 3-6 cycloalkyl group , 5-6 atoms of heterocyclyl, phenyl and 5-6 atoms of heteroaryl are each independently optionally replaced by 1, 2, 3 or 4 selected from deuterium, F, Cl, Br, I , -OH, -CN, -NH 2 , -NO 2 , -COOH, C 1-4 alkyl, C 1-4 alkoxy, C 3-6 cycloalkyl or a heterocycle composed of 5-6 atoms substituted by the substituents of the base.
在一些實施方案中,各Rb 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、氧代、C1-4 烷基、C1-4 烷氧基、C1-4 烷基-OC(=O)-、C1-4 烷基-C(=O)-、C1-4 烷基-S(=O)2 -、C3-6 環烷基-C1-4 烷基、C3-6 環烷基、5-6個原子組成的雜環基、苯基、5-6個原子組成的雜芳基、-NRe C(=O)C1-4 烷基、-NRe Rf 、-S(=O)2 -NRe Rf 、-C(=O)-NRe Rf 或-C1-4 烷基-NRe Rf ,其中,所述的C1-4 烷基、C1-4 烷氧基、C3-6 環烷基-C1-4 烷基、C3-6 環烷基、苯基、5-6個原子組成的雜環基和5-6個原子組成的雜芳基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-4 烷基、C1-4 烷氨基或C1-4 烷氧基的取代基所取代;其中,各Re 和Rf 具有如本發明所述的含義。In some embodiments, each R b is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, -NO 2, -COOH, oxo, C 1-4 alkyl, C 1-4 alkoxy, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-C(=O)-, C 1-4 alkyl-S(=O) 2- , C 3-6 cycloalkyl-C 1-4 alkyl, C 3-6 cycloalkyl, 5-6 atoms of heterocyclyl, phenyl, 5-6 atoms of heteroaryl, -NR e C(=O)C 1-4 alkyl, -NR e R f , -S(=O) 2 -NR e R f , -C(=O)-NR e R f or -C 1-4 alkane base-NR e R f , wherein the C 1-4 alkyl, C 1-4 alkoxy, C 3-6 cycloalkyl-C 1-4 alkyl, C 3-6 cycloalkyl, Phenyl, heterocyclyl of 5-6 atoms and heteroaryl of 5-6 atoms are each independently optionally substituted by 1, 2, 3 or 4 atoms selected from deuterium, F, Cl, Br, I, -OH, -CN, -NH 2, -NO 2, -COOH, C 1-4 alkyl, substituted C 1-4 alkylamino or C 1-4 alkoxy substituents; wherein each of R e and R f has the meaning as described in the present invention.
在一些實施方案中,Re 和Rf 各自獨立地為氫、C1-4 烷基、C1-4 烷基-OC(=O)-、C1-4 烷基-C(=O)-、C1-4 烷基-S(=O)2 -、C3-6 環烷基、C6-10 芳基、5-7個原子組成的雜環基、5-7個原子組成的雜芳基、14-15個原子組成的雜芳基、C3-6 環烷基-C1-4 烷基、C6-10 芳基-C1-4 烷基、(5-7個原子組成的雜環基)-C1-4 烷基、(5-7個原子組成的雜芳基)-C1-4 烷基或-C1-4 烷基-NRg Rj ;或者Re 和Rf 與它們相連的N原子一起形成4-6個原子組成的雜環基;其中,所述的Re 、Rf 和4-6個原子組成的雜環基各自獨立任選地被1、2、3或4個Rh 所取代;其中,各Rh 具有如本發明所述的含義。In some embodiments, R e and R f are each independently hydrogen, C 1-4 alkyl, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-C(=O) -, C 1-4 alkyl-S(=O) 2 -, C 3-6 cycloalkyl, C 6-10 aryl, 5-7 atoms heterocyclic group, 5-7 atoms Heteroaryl, heteroaryl consisting of 14-15 atoms, C 3-6 cycloalkyl-C 1-4 alkyl, C 6-10 aryl-C 1-4 alkyl, (5-7 atoms composed of heterocyclyl)-C 1-4 alkyl, (heteroaryl composed of 5-7 atoms)-C 1-4 alkyl or -C 1-4 alkyl-NR g R j ; or R e together with R f and their connected N atoms to form a heterocyclic group composed of 4-6 atoms; wherein, the heterocyclic groups composed of R e , R f and 4-6 atoms are each independently optionally replaced by 1 , 2, 3 or 4 R h ; wherein, each R h has the meaning as described in the present invention.
在一些實施方案中,R2 為甲基、乙基、正丙基、異丙基、-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 CH2 CH2 F、-CH2 CH2 CHF2 、-CH2 CH2 CF3 、-CF2 CH2 CH3 、-CH2 Cl、-CHCl2 、環丙基、環丁基、環戊基、環己基、環丙基甲基、環丁基甲基、環戊基甲基、環己基甲基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、四氫吡喃基甲基、四氫呋喃基甲基或吡咯烷基甲基;或R2 與Ra 和其相連的原子共同形成1,3-二氧雜環戊烯、1,3-二氧雜環己烯、2,3-二氫-1,4,2-二噁嗪烯或1,5,3-二氧氮雜環庚烯,所述的1,3-二氧雜環戊烯、1,3-二氧雜環己烯、2,3-二氫-1,4,2-二噁嗪烯或1,5,3-二氧氮雜環庚烯獨立任選地被選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、-CHF2 、-CF3 、-CH2 CHF2 、-CH2 CF3 或-CF2 CH3 的取代基所取代。In some embodiments, R 2 is methyl, ethyl, n-propyl, isopropyl, -CH 2 F, -CHF 2 , -CF 3 , -CH 2 CH 2 F, -CH 2 CHF 2 , - CH 2 CF 3, -CF 2 CH 3, -CH 2 CH 2 CH 2 F, -CH 2 CH 2 CHF 2, -CH 2 CH 2 CF 3, -CF 2 CH 2 CH 3, -CH 2 Cl, - CHCl 2 , cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl, piperazinyl, piperidinyl, morpholinyl , thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, tetrahydropyranyl group, tetrahydrofuranyl group or pyrrolidinyl group; or R 2 with R a and the atom thereof together form 1,3-dioxole, 1,3-dioxene, 2,3-dihydro-1,4,2-dioxazine or 1,5,3-dioxene Azacycloheptene, said 1,3-dioxole, 1,3-dioxene, 2,3-dihydro-1,4,2-dioxazine or 1 , 5,3- dioxo-azepine, optionally substituted independently selected from deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, methyl, ethyl, n-propyl, iso Propyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy, n-propoxy, iso-propoxy, -CHF 2 , -CF 3 , -CH 2 CHF 2 , -CH 2 CF 3 or -CF 2 CH 3 substituents.
在一些實施方案中,R3 為氫、氘、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、乙烯基、1-丙烯基、2-丙烯基、乙炔基、1-丙炔基、3-丙炔基、HC(=O)-、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-O-C(=O)-、乙基-O-C(=O)-、正丙基-O-C(=O)-、異丙基-O-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、甲基-C(=NH)-、乙基-C(=NH)-、正丙基-C(=NH)-或異丙基-C(=NH)-;其中,所述的甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、乙烯基、1-丙烯基、2-丙烯基、乙炔基、1-丙炔基、3-丙炔基、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-O-C(=O)-、乙基-O-C(=O)-、正丙基-O-C(=O)-、異丙基-O-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、甲基-C(=NH)-、乙基-C(=NH)-、正丙基-C(=NH)-和異丙基-C(=NH)-各自獨立任選地被1、2或3個選自氘、F、Cl、Br、I、-OH、-CN或-NH2 的取代基所取代。In some embodiments, R 3 is hydrogen, deuterium, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy , n-propoxy, isopropoxy, vinyl, 1-propenyl, 2-propenyl, ethynyl, 1-propynyl, 3-propynyl, HC(=O)-, methyl-C (=O)-, ethyl-C(=O)-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-OC(=O)-, ethyl -OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2 -, n-propyl-S(=O) 2 -, isopropyl-S(=O) 2 -, methyl-C(=NH)-, ethyl-C(=NH)-, n-propyl- C(=NH)- or isopropyl-C(=NH)-; wherein, the methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tertiary Butyl, methoxy, ethoxy, n-propoxy, isopropoxy, vinyl, 1-propenyl, 2-propenyl, ethynyl, 1-propynyl, 3-propynyl, methyl Base-C(=O)-, ethyl-C(=O)-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-OC(=O)- , ethyl-OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-S(=O) 2 -, ethyl-S(= O) 2 -, n-propyl-S(=O) 2 -, isopropyl-S(=O) 2 -, methyl-C(=NH)-, ethyl-C(=NH)-, normal Propyl-C(=NH)- and isopropyl-C(=NH)- are each independently optionally replaced by 1, 2 or 3 selected from deuterium, F, Cl, Br, I, -OH, -CN or -NH 2 substituent.
在一些實施方案中,R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 和R9 各自獨立地為氫、氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、甲基、乙基、正丙基、異丙基、甲氧基、乙氧基、正丙氧基、異丙氧基、-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 CH2 CH2 F、-CH2 CH2 CHF2 、-CH2 CH2 CF3 、-CF2 CH2 CH3 、-CH2 Cl、-CHCl2 、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -或異丙基-S(=O)2 -。In some embodiments, R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , and R 9 are each independently hydrogen, deuterium, F, Cl, Br, I, -OH, -CN , -NH 2 , -NO 2 , -COOH, methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy, n-propoxy, iso-propoxy, -CH 2 F, - CHF 2 , -CF 3 , -CH 2 CH 2 F, -CH 2 CHF 2 , -CH 2 CF 3 , -CF 2 CH 3 , -CH 2 CH 2 CH 2 F, -CH 2 CH 2 CHF 2 , - CH 2 CH 2 CF 3, -CF 2 CH 2 CH 3, -CH 2 Cl, -CHCl 2, methyl -C (= O) -, ethyl -C (= O) -, n-propyl -C ( =O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2 -, n-propyl-S(=O) 2 - or Isopropyl-S(=O) 2- .
在一些實施方案中,Ra 為氫、氘、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、乙烯基、1-丙烯基、2-丙烯基、乙炔基、1-丙炔基、3-丙炔基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、苯基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基或噁唑基;其中,所述的甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、乙烯基、1-丙烯基、2-丙烯基、乙炔基、1-丙炔基、3-丙炔基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、苯基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基和噁唑基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、甲基、乙基、正丙基、異丙基、甲氧基、乙氧基、正丙氧基、異丙氧基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基或吡咯烷基的取代基所取代。In some embodiments, R a is hydrogen, deuterium, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, vinyl, 1-propenyl , 2-propenyl, ethynyl, 1-propynyl, 3-propynyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, thio morpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, phenyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, Thiazolyl or oxazolyl; wherein, the methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, vinyl, 1-propenyl, 2-propenyl, ethynyl, 1-propynyl, 3-propynyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, thiophanyl Linyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, phenyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazole and oxazolyl are independently optionally substituted with 1,2, 3 or 4 substituents selected from deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, -NO 2, -COOH, methyl , ethyl, n-propyl, isopropyl, methoxy, ethoxy, n-propoxy, iso-propoxy, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperazinyl, piper Substituents of pyridyl, morpholinyl, thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl or pyrrolidinyl.
在一些實施方案中,各Rb 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、氧代、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、甲基-OC(=O)-、乙基-OC(=O)-、正丙基-OC(=O)-、異丙基-OC(=O)-、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、環丙基甲基、環丙基乙基、環丙基正丙基、環丙基異丙基、環丁基甲基、環戊基甲基、環己基甲基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、苯基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基、噁唑基、-NRe C(=O)甲基、-NRe C(=O)乙基、-NRe C(=O)正丙基、-NRe C(=O)異丙基、-NRe Rf 、-S(=O)2 -NRe Rf 、-C(=O)-NRe Rf 、-甲基-NRe Rf 、-乙基-NRe Rf 、-正丙基-NRe Rf 或-異丙基-NRe Rf ;其中,各Re 和Rf 具有如本發明所述的含義。In some embodiments, each R b is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, -NO 2, -COOH, oxo, methyl, ethyl, n-propyl base, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy, n-propoxy, iso-propoxy, methyl-OC(=O)-, Ethyl-OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-C(=O)-, ethyl-C(=O) -, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2 -, n-propyl- S(=O) 2 -, isopropyl-S(=O) 2 -, cyclopropyl methyl, cyclopropyl ethyl, cyclopropyl n-propyl, cyclopropyl isopropyl, cyclobutyl methyl, Cyclopentylmethyl, cyclohexylmethyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, phenyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, -NR e C(=O) methyl, -NR e C(=O) ethyl, -NR e C(=O) n-propyl, -NR e C(=O) isopropyl, -NR e R f , - S(=O) 2 -NR e R f , -C(=O)-NR e R f , -methyl-NR e R f , -ethyl-NR e R f , -n-propyl-NR e R f or -isopropyl-NR e R f ; wherein each R e and R f has the meaning as described in the present invention.
其中,所述的甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、環丙基甲基、環丙基乙基、環丙基正丙基、環丙基異丙基、環丁基甲基、環戊基甲基、環己基甲基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、苯基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基和噁唑基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、甲基、乙基、正丙基、異丙基、甲氨基、二甲氨基、甲氧基、乙氧基、正丙氧基或異丙氧基的取代基所取代。Among them, the methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropyl Oxy, cyclopropylmethyl, cyclopropylethyl, cyclopropyl-n-propyl, cyclopropylisopropyl, cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl, cyclopropyl, cyclobutyl base, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, phenyl, pyridyl, pyrimidinyl, pyridyl Azinyl, pyridazinyl, furyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl and oxazolyl are each independently optionally substituted by 1, 2, 3 or 4 selected from deuterium, F, Cl , Br, I, -OH, -CN, -NH 2 , -NO 2 , -COOH, methyl, ethyl, n-propyl, isopropyl, methylamino, dimethylamino, methoxy, ethoxy , n-propoxy or isopropoxy substituent.
在一些實施方案中,Rc 和Rd 各自獨立地為氫、-OH、甲基、乙基、正丙基、異丙基、-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 CH2 CH2 F、-CH2 CH2 CHF2 、-CH2 CH2 CF3 、-CF2 CH2 CH3 、-CH2 Cl、-CHCl2 、環丙基甲基、環丙基乙基、環丁基甲基、環戊基甲基、環己基甲基、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -或異丙基-S(=O)2 -。In some embodiments, R c and R d are each independently hydrogen, -OH, methyl, ethyl, n-propyl, isopropyl, -CH 2 F, -CHF 2, -CF 3, -CH 2 CH 2 F, -CH 2 CHF 2 , -CH 2 CF 3, -CF 2 CH 3, -CH 2 CH 2 CH 2 F, -CH 2 CH 2 CHF 2, -CH 2 CH 2 CF 3, -CF 2 CH 2 CH 3, -CH 2 Cl , -CHCl 2, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl, methyl -C (= O) - , ethyl-C(=O)-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(= O) 2 -, n-propyl-S(=O) 2 - or isopropyl-S(=O) 2 -.
在一些實施方案中,Re 和Rf 各自獨立地為氫、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲基-OC(=O)-、乙基-OC(=O)-、正丙基-OC(=O)-、異丙基-OC(=O)-、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、環丙基、環丁基、環戊基、環己基、苯基、萘基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基、噁唑基、環丙基甲基、環丙基乙基、環丙基正丙基、環丙基異丙基、環丁基甲基、環戊基甲基、環己基甲基、苯甲基、苯乙基、5-7個原子組成的雜環基-C1-4 烷基、吡啶基甲基、嘧啶基甲基、呋喃基甲基、噻吩基甲基、吡咯基甲基、吡唑基甲基、咪唑基甲基、噻唑基甲基、噁唑基甲基、-甲基-NRg Rj 、-乙基-NRg Rj 、-正丙基-NRg Rj 、-異丙基-NRg Rj 或;In some embodiments, R e and R f are each independently hydrogen, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methyl - OC(=O)-, ethyl-OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-C(=O)-, ethyl Base-C(=O)-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2- , n-propyl-S(=O) 2- , isopropyl-S(=O) 2- , cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl, naphthyl, piperazine base, piperidinyl, morpholinyl, thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furyl, thienyl, pyrrole base, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, cyclopropylmethyl, cyclopropylethyl, cyclopropyln-propyl, cyclopropylisopropyl, cyclobutylmethyl, cyclopentylmethyl base, cyclohexylmethyl, benzyl, phenethyl, heterocyclyl-C 1-4 alkyl consisting of 5-7 atoms, pyridylmethyl, pyrimidinylmethyl, furylmethyl, thienyl group, a pyrrolyl group, a pyrazolyl group, imidazolyl group, thiazolyl group, oxazolyl group, - methyl -NR g R j, - ethyl -NR g R j, - n propyl-NR g R j , -isopropyl-NR g R j or ;
或者Re 和Rf 與它們相連的N原子一起形成氮雜環丁基、吡咯烷基、哌啶基、嗎啉基、硫代嗎啉基或哌嗪基;or R e and R f taken together with the N atom to which they are attached form azetidinyl, pyrrolidinyl, piperidinyl, morpholinyl, thiomorpholinyl or piperazinyl;
其中,所述的Re 、Rf 、氮雜環丁基、吡咯烷基、哌啶基、嗎啉基、硫代嗎啉基和哌嗪基各自獨立任選地被1、2、3或4個Rh 所取代;其中,各Rh 具有如本發明所述的含義。Wherein said R e, R f, azetidinyl, pyrrolidinyl, piperidinyl, morpholinyl, thiomorpholinyl and piperazinyl each independently optionally substituted with 1,2, 3 or 4 R h is substituted; wherein, each R h has the meaning as described in the present invention.
在一些實施方案中,各Rh 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、三氟甲基、三氟甲氧基、甲氧基、乙氧基、正丙氧基、異丙氧基、正丁氧基、異丁氧基、仲丁氧基或叔丁氧基;In some embodiments, each R h is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, trifluoromethyl, trifluoromethoxy, methoxy, ethoxy, n-propoxy, iso-propoxy, n-butoxy, iso-butoxy , sec-butoxy or tert-butoxy;
Rg 和Rj 各自獨立地為氫、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲基-OC(=O)-、乙基-OC(=O)-、正丙基-OC(=O)-、異丙基-OC(=O)-、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、環丙基、環丁基、環戊基、環己基、苯基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基或噁唑基;R g and R j are each independently hydrogen, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methyl-OC(=O)- , ethyl-OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-C(=O)-, ethyl-C(=O )-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2 -, n-propyl -S(=O) 2 -, isopropyl-S(=O) 2 -, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl, piperazinyl, piperidinyl, morpholinyl , thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl or oxazolyl;
Rm 和Rn 各自獨立地為氫、氘、F、Cl、Br、I、-OH、-CN、-NH2 、甲基、乙基、正丙基、異丙基、-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 Cl、-CHCl2 或-C(=O)NH2 。R m and R n are each independently hydrogen, deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, methyl, ethyl, n-propyl, isopropyl, -CH 2 F, -CHF 2, -CF 3, -CH 2 CH 2 F, -CH 2 CHF 2, -CH 2 CF 3, -CF 2 CH 3, -CH 2 Cl, -CHCl 2 , or -C (= O) NH 2 .
另一方面,本發明涉及一種化合物,其為式 (II) 所示的化合物或式 (II) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(II);In another aspect, the present invention relates to a compound, which is a compound represented by formula (II) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (II) Products, pharmaceutically acceptable salts or their prodrugs: (II);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Ra 、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R a , R b and n has meaning in the present invention.
另一方面,本發明涉及一種化合物,其為式 (III) 所示的化合物或式 (III) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(III);In another aspect, the present invention relates to a compound, which is a compound represented by formula (III) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (III) Products, pharmaceutically acceptable salts or their prodrugs: (III);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Ra 、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R a , R b and n has meaning in the present invention.
在一些實施方案中,藥學上可接受的鹽是鹽酸鹽、氫溴酸鹽、硫酸鹽、硝酸鹽、磷酸鹽、乙酸鹽、馬來酸鹽、琥珀酸鹽、扁桃酸鹽、富馬酸鹽、丙二酸鹽、蘋果酸鹽、2-羥基丙酸鹽、丙酮酸鹽、草酸鹽、羥乙酸鹽、水楊酸鹽、葡萄糖醛酸鹽、半乳糖醛酸鹽、枸櫞酸鹽、酒石酸鹽、門冬氨酸鹽、谷氨酸鹽、苯甲酸鹽、肉桂酸鹽、對甲苯磺酸鹽、苯磺酸鹽、甲磺酸鹽、乙磺酸鹽、三氟甲磺酸鹽或它們的組合。In some embodiments, the pharmaceutically acceptable salt is hydrochloride, hydrobromide, sulfate, nitrate, phosphate, acetate, maleate, succinate, mandelate, fumaric acid Salt, Malonate, Malate, 2-Hydroxypropionate, Pyruvate, Oxalate, Glycolate, Salicylate, Glucuronate, Galacturonate, Citrate , tartrate, aspartate, glutamate, benzoate, cinnamate, p-toluenesulfonate, benzenesulfonate, mesylate, ethanesulfonate, trifluoromethanesulfonate salt or a combination thereof.
另一方面,本發明涉及一種藥物組合物,其包含本發明式 (I)、(II) 或 (III) 所示化合物或其立體異構體、幾何異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥,及藥學上可接受的賦形劑、載體、附加劑、輔劑、媒介物中的至少一種或它們的組合;In another aspect, the present invention relates to a pharmaceutical composition comprising the compound represented by formula (I), (II) or (III) of the present invention or its stereoisomer, geometric isomer, tautomer, nitrogen oxide Compounds, hydrates, solvates, metabolites, pharmaceutically acceptable salts or their prodrugs, and at least one of pharmaceutically acceptable excipients, carriers, adjuvants, adjuvants, vehicles or their combination;
所述的藥物組合物進一步包含其他附加治療劑,其中,所述的附加治療劑是:丙酮酸鈉、多索茶鹼、替托司特、泰魯司特、茶鹼、福莫特羅、沙美特羅、氟替卡松丙酸酯、咯利普蘭、吡拉米斯特、西洛司特、茚達特羅、奧達特羅、米地司坦、齊流通、沙丁醇胺、卡莫昔羅、布地奈德、二丙酸倍氯米松、氟尼縮松、羅氟奈德、環索奈德、異丙托溴銨、氧托溴銨、噻托溴銨、格隆溴銨、蕪地溴銨、維蘭特羅、阿地溴銨、Benralizumab、Tralokinumab、瑞伐托酯、克瑞沙硼、氟輕鬆醋酸酯、去羥米松、莫美他松、曲安西龍、倍他米松、阿氯米松、曲安奈德、地索奈德、氫化可的松、氯倍他索、鹵貝他索、糠酸莫米松、二氟拉松、美普克萊、他克莫司、吡美莫司、他紮羅汀、環孢素、阿普斯特、E-6005、OPA-15406、LEO-29102、DRM02、羅氟司特、異丁司特、托法替尼、JTE-052、巴瑞替尼、烏帕替尼、WBI-1001、MRX-6、GSK2981278、杜魯單抗、來金珠單抗、尼莫利珠單抗、曲洛吉努單抗、依那西普、阿達木單抗、英夫利昔單抗、尤特可單抗、塞庫吉努、奧馬珠、CIM-331、戈利木單抗和聚乙二醇化賽、妥珠單抗、卡泊三醇、骨化三醇、阿利維A酸、VTP-38543、ZPL-389、阿瑞匹坦、曲地匹坦、弗維普蘭特、OC-459、SUN 13834、SB-011、VTP-43742、ARN6039、TAK-828、JTE-451、PF-04965842、PF-06651600、PF-06700841、PF-06650833、GR-MD-02或它們的組合。The pharmaceutical composition further comprises other additional therapeutic agents, wherein the additional therapeutic agents are: sodium pyruvate, doxofylline, tetomilast, telukast, theophylline, formoterol, salmeterol, fluticasone propionate, rolipram, piramister, cilomilast, indacaterol, odaterol, midestane, qifluenza, salbutamol, carbamaze Luo, budesonide, beclomethasone dipropionate, flunisolide, roflunomide, ciclesonide, ipratropium bromide, oxytropium bromide, tiotropium bromide, glycopyrronium bromide, Destromium bromide, vilanterol, aclidinium bromide, Benralizumab, Tralokinumab, revatropate, cresabor, fluocinolone acetate, didometasone, mometasone, triamcinolone, betamethasone, aclomethasone, triamcinolone acetonide, desonide acetonide, hydrocortisone, clobetasol, halobetasol, mometasone furoate, diflurasone, meproclax, tacrolimus, pimecrolimus Limus, Tazarotene, Cyclosporine, Apremilast, E-6005, OPA-15406, LEO-29102, DRM02, Roflumilast, Ibudilast, Tofacitinib, JTE-052, Baricitinib, upadacitinib, WBI-1001, MRX-6, GSK2981278, durumumab, lekinizumab, nimolizumab, tralotinib, etanercept, Adalimumab, Infliximab, Utecalumab, Sekuginu, Omalizumab, CIM-331, Golimumab and Pegylated, Tocilizumab, Calcipotriol, Calcitriol, Aliretinoin, VTP-38543, ZPL-389, Aprepitant, Tridipitant, Faviprant, OC-459, SUN 13834, SB-011, VTP-43742, ARN6039, TAK-828, JTE-451, PF-04965842, PF-06651600, PF-06700841, PF-06650833, GR-MD-02 or combinations thereof.
另一方面,本發明涉及式 (I)、(II) 或 (III) 所示化合物或其藥物組合物在製備藥物中的用途,所述藥物用於預防、治療或減輕與4型磷酸二酯酶有關的疾病。On the other hand, the present invention relates to the use of a compound represented by formula (I), (II) or (III) or a pharmaceutical composition thereof in the preparation of a medicament for preventing, treating or alleviating phosphodiester-type 4 Enzyme-related diseases.
在一些實施方案中,本發明涉及式 (I)、(II) 或 (III) 所示化合物或其藥物組合物在製備藥物中的用途,所述與4型磷酸二酯酶有關的疾病為呼吸疾病、過敏、炎症、中樞神經系統疾病或非胰島素依賴糖尿病。In some embodiments, the present invention relates to the use of a compound represented by formula (I), (II) or (III) or a pharmaceutical composition thereof in the preparation of a medicament, wherein the disease related to phosphodiesterase type 4 is respiration Disease, allergy, inflammation, central nervous system disease or non-insulin dependent diabetes.
在一些實施方案中,所述的呼吸疾病為:慢性阻塞性肺疾病、肺氣腫、哮喘、慢性肺炎、塵肺病、支氣管炎、支氣管擴張症、肺結核纖維化病變、肺囊性纖維化、急性呼吸窘迫綜合症或呼吸道炎症;其中,支氣管炎為急性支氣管炎、慢性支氣管炎、變應性支氣管炎、彌漫性泛細支氣管炎或閉塞性細支氣管炎。In some embodiments, the respiratory disease is: chronic obstructive pulmonary disease, emphysema, asthma, chronic pneumonia, pneumoconiosis, bronchitis, bronchiectasis, pulmonary fibrotic lesions, cystic fibrosis, acute Respiratory distress syndrome or inflammation of the respiratory tract; among them, bronchitis is acute bronchitis, chronic bronchitis, allergic bronchitis, diffuse panbronchiolitis or obliterative bronchiolitis.
在一些實施方案中,所述的炎症為:過敏性結膜炎、特應性皮炎、過敏性皮炎、類風濕性關節炎、間質性膀胱炎、變應性鼻炎、潰瘍性結腸炎、強直性脊柱炎、風濕性關節炎或銀屑病關節炎。In some embodiments, the inflammation is: allergic conjunctivitis, atopic dermatitis, atopic dermatitis, rheumatoid arthritis, interstitial cystitis, allergic rhinitis, ulcerative colitis, ankylosing spine inflammation, rheumatoid arthritis or psoriatic arthritis.
另一方面,本發明涉及式 (I)、(II) 或 (III) 所示化合物的製備、分離和純化的方法。In another aspect, the present invention relates to methods for the preparation, isolation and purification of compounds of formula (I), (II) or (III).
生物試驗結果表明,本發明提供的化合物對PDE4具有較好的抑制活性,同時具有良好的藥代動力學特徵。The biological test results show that the compounds provided by the present invention have good inhibitory activity to PDE4 and have good pharmacokinetic characteristics.
本發明的任一方面的任一實施方案,可以與其他實施方案進行組合,只要它們不會出現矛盾。此外,在本發明任一方面的任一實施方案中,任一技術特徵可以適用於其他實施方案中的該技術特徵,只要它們不會出現矛盾。Any embodiment of any aspect of the invention may be combined with other embodiments so long as they do not appear to be inconsistent. Furthermore, in any embodiment of any aspect of the present invention, any technical feature may be applicable to that technical feature in other embodiments, as long as they do not contradict.
以上所述內容只概述了本發明的些許方面,但並不限於這些方面。這些方面及其他的方面的內容將在以下作更加具體完整的描述。The foregoing has outlined but not limited aspects of the invention. These and other aspects are described in more detail below.
定義和一般術語Definitions and General Terms
現在詳細描述本發明的些許實施方案,其實施例由隨附的結構式和化學式說明。本發明意圖涵蓋所有的替代、修改和等同技術方案,它們均包括在如請求項定義的本發明範圍內。所屬技術領域中具有通常知識者應認識到,許多與本發明所述類似或等同的方法和材料能夠用於實踐本發明。本發明絕不限於本發明所述的方法和材料。在所結合的文獻、專利和類似材料的一篇或多篇與本發明不同或相矛盾的情況下(包括但不限於所定義的術語、術語應用、所描述的技術,等等),以本發明為準。Certain embodiments of the present invention will now be described in detail, examples of which are illustrated by the accompanying structural and chemical formulae. The present invention is intended to cover all alternatives, modifications and equivalents, which are included within the scope of the present invention as defined by the claims. One of ordinary skill in the art would recognize that many methods and materials similar or equivalent to those described in the present invention could be used in the practice of the present invention. The present invention is in no way limited to the methods and materials described herein. In the event that one or more of the incorporated literature, patents, and similar materials differs from or contradicts the present invention (including, but not limited to, terms defined, uses of terms, techniques described, etc.), this Invention shall prevail.
應進一步認識到,本發明的些許特徵,為清楚可見,在多個獨立的實施方案中進行了描述,但也可以在單個實施例中以組合形式提供。反之,本發明的各種特徵,為簡潔起見,在單個實施方案中進行了描述,但也可以單獨或以任意適合的子組合提供。It should further be appreciated that certain features of the invention, which are, for clarity, set forth in multiple separate embodiments, can also be provided in combination in a single embodiment. Conversely, various features of the invention, which are, for brevity, described in the context of a single embodiment, can also be provided separately or in any suitable subcombination.
除非另外說明,本發明所使用的所有科技術語具有與本發明所屬技術領域中具有通常知識者的通常理解相同的含義。本發明涉及的所有專利和公開出版物通過引用方式整體併入本發明。Unless otherwise defined, all technical and scientific terms used in the present invention have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. All patents and publications referred to herein are incorporated by reference in their entirety.
除非另外說明,應當應用本發明所使用的下列定義。出於本發明的目的,化學元素與元素週期表CAS版,和《化學和物理手冊》,第75版,1994一致。此外,有機化學一般原理可參考 "Organic Chemistry", Thomas Sorrell, University Science Books, Sausalito: 1999,和 "March's Advanced Organic Chemistry" by Michael B. Smith and Jerry March, John Wiley & Sons, New York: 2007中的描述,其全部內容通過引用併入本發明。Unless otherwise stated, the following definitions used herein shall apply. For the purposes of the present invention, chemical elements are in accordance with the Periodic Table of the Elements, CAS Edition, and Handbook of Chemistry and Physics, 75th Edition, 1994. In addition, general principles of organic chemistry can be found in "Organic Chemistry", Thomas Sorrell, University Science Books, Sausalito: 1999, and "March's Advanced Organic Chemistry" by Michael B. Smith and Jerry March, John Wiley & Sons, New York: 2007 description, the entire contents of which are incorporated herein by reference.
除非另有說明或者上下文中有明顯的衝突,本發明所使用的冠詞“一”、“一個(種)”和“所述”旨在包括“至少一個”或“一個或多個”。因此,本發明所使用的這些冠詞是指一個或多於一個(即至少一個)賓語的冠詞。例如,“一組分”指一個或多個組分,即可能有多於一個的組分被考慮在所述實施方案的實施方式中採用或使用。As used herein, the articles "a", "an" and "the" are intended to include "at least one" or "one or more" unless stated otherwise or otherwise clearly contradicted by context. Accordingly, these articles, as used herein, refer to articles of one or more than one (ie, at least one) object. For example, "a component" refers to one or more components, ie, there may be more than one component contemplated for use or use in the implementation of the described embodiments.
本發明所使用的術語“受試對象”是指動物。典型地所述動物是哺乳動物。受試對象,例如也指靈長類動物(例如人類,男性或女性)、牛、綿羊、山羊、馬、犬、貓、兔、大鼠、小鼠、魚、鳥等。在些許實施方案中,所述受試對象是靈長類動物。在其他實施方案中,所述受試對象是人。The term "subject" as used herein refers to an animal. Typically the animal is a mammal. A subject, for example, also refers to primates (eg, humans, male or female), cows, sheep, goats, horses, dogs, cats, rabbits, rats, mice, fish, birds, and the like. In some embodiments, the subject is a primate. In other embodiments, the subject is a human.
本發明所使用的術語“患者”是指人(包括成人和兒童)或者其他動物。在一些實施方案中,“患者”是指人。The term "patient" as used herein refers to humans (including adults and children) or other animals. In some embodiments, "patient" refers to a human.
術語“包含”為開放式表達,即包括本發明所指明的內容,但並不排除其他方面的內容。The term "comprising" is an open-ended expression, that is, it includes the contents specified in the present invention, but does not exclude other aspects.
術語“立體異構體”是指具有相同化學構造,但原子或基團在空間上排列方式不同的化合物。立體異構體包括對映異構體、非對映異構體、構象異構體(旋轉異構體)、幾何(順/反)異構體、阻轉異構體,等等。The term "stereoisomers" refers to compounds that have the same chemical structure, but differ in the arrangement of atoms or groups in space. Stereoisomers include enantiomers, diastereomers, conformational isomers (rotamers), geometric (cis/trans) isomers, atropisomers, and the like.
術語“手性”是具有與其鏡像不能重疊性質的分子;而“非手性”是指與其鏡像可以重疊的分子。The term "chirality" refers to a molecule that has the property of being non-superimposable with its mirror image; whereas "achiral" refers to a molecule that is superimposable with its mirror image.
術語“對映異構體”是指一個化合物的兩個不能重疊但互成鏡像關係的異構體。The term "enantiomer" refers to two nonsuperimposable, but mirror-image isomers of a compound.
術語“非對映異構體”是指有兩個或多個手性中心並且其分子不互為鏡像的立體異構體。非對映異構體具有不同的物理性質,如熔點、沸點、光譜性質和反應性。非對映異構體混合物可通過高分辨分析操作如電泳和色譜,例如HPLC來分離。The term "diastereomer" refers to a stereoisomer having two or more chiral centers and whose molecules are not mirror images of each other. Diastereomers have different physical properties such as melting point, boiling point, spectral properties and reactivity. Diastereomeric mixtures can be separated by high resolution analytical procedures such as electrophoresis and chromatography, eg HPLC.
本發明所使用的立體化學定義和規則一般遵循S.P.Parker, Ed., McGraw-Hill Dictionary of Chemical Terms (1984) McGraw-Hill Book Company, New York; and Eliel, E.and Wilen, S., "Stereochemistry of Organic Compounds", John Wiley & Sons, Inc., New York, 1994。Stereochemical definitions and rules used in the present invention generally follow SP Parker, Ed., McGraw-Hill Dictionary of Chemical Terms (1984) McGraw-Hill Book Company, New York; and Eliel, E. and Wilen, S., "Stereochemistry of Organic Compounds", John Wiley & Sons, Inc., New York, 1994.
許多有機化合物以光學活性形式存在,即它們具有使平面偏振光的平面發生旋轉的能力。在描述光學活性化合物時,使用首碼D和L或R和S來表示分子關於其一個或多個手性中心的絕對構型。首碼 d 和l 或 (+) 和 (-) 是用於指定化合物所致平面偏振光旋轉的符號,其中, (-) 或 l 表示化合物是左旋的,首碼為 (+) 或 d 的化合物是右旋的。一種具體的立體異構體是對映異構體,這種異構體的混合物稱作對映異構體混合物。對映異構體的50:50混合物稱為外消旋混合物或外消旋體,當在化學反應或過程中沒有立體選擇性或立體特異性時,可出現這種情況。Many organic compounds exist in optically active forms, that is, they have the ability to rotate the plane of plane-polarized light. In describing optically active compounds, the prefixes D and L or R and S are used to denote the absolute configuration of the molecule about one or more of its chiral centers. The prefixes d and l or (+) and (-) are symbols used to designate the rotation of plane-polarized light by a compound, where (-) or l indicates that the compound is levorotatory, and the prefix is (+) or d. is right-handed. A specific stereoisomer is an enantiomer, and a mixture of such isomers is called an enantiomeric mixture. A 50:50 mixture of enantiomers is called a racemic mixture or racemate, which can occur when there is no stereoselectivity or stereospecificity in a chemical reaction or process.
本發明公開化合物的任何不對稱原子(例如,碳等)都可以以外消旋或對映體富集的形式存在,例如 (R)-、(S)- 或 (R, S)- 構型形式存在。在些許實施方案中,各不對稱原子在 (R)- 或 (S)- 構型方面具有至少50%對映體過量,至少60%對映體過量,至少70%對映體過量,至少80%對映體過量,至少90%對映體過量,至少95%對映體過量,或至少99%對映體過量。Any asymmetric atom (eg, carbon, etc.) of the compounds disclosed herein may exist in racemic or enantiomerically enriched forms, such as (R)-, (S)- or (R, S)- configurational forms exist. In some embodiments, each asymmetric atom has at least 50% enantiomeric excess, at least 60% enantiomeric excess, at least 70% enantiomeric excess, at least 80% enantiomeric excess in the (R)- or (S)- configuration % enantiomeric excess, at least 90% enantiomeric excess, at least 95% enantiomeric excess, or at least 99% enantiomeric excess.
依據起始物料和方法的選擇,本發明化合物可以以可能的異構體中的一個或它們的混合物,例如外消旋體和非對應異構體混合物(這取決於不對稱碳原子的數量)的形式存在。光學活性的 (R)- 或 (S)- 異構體可使用手性合成子或手性試劑製備,或使用常規技術拆分。如果化合物含有一個雙鍵,取代基可能為E 或Z 構型;如果化合物中含有二取代的環烷基,環烷基的取代基可能有順式或反式構型。Depending on the choice of starting materials and methods, the compounds of the present invention may be present as one of the possible isomers or as mixtures thereof, such as racemates and mixtures of diastereomers (depending on the number of asymmetric carbon atoms) form exists. Optically active (R)- or (S)- isomers can be prepared using chiral synthons or chiral reagents, or resolved using conventional techniques. If the compound contains a double bond, the substituent may be in the E or Z configuration; if the compound contains a disubstituted cycloalkyl group, the cycloalkyl substituent may have the cis or trans configuration.
所得的任何立體異構體的混合物可以依據組分物理化學性質上的差異被分離成純的或基本純的幾何異構體、對映異構體、非對映異構體,例如,通過色譜法和/或分步結晶法。The resulting mixtures of any stereoisomers may be separated into pure or substantially pure geometric isomers, enantiomers, diastereomers on the basis of differences in the physicochemical properties of the components, for example, by chromatography and/or fractional crystallization.
可以用已知的方法將任何所得終產物或中間體的外消旋體通過所屬技術領域中具有通常知識者熟悉的方法拆分成光學對映體,如,通過對獲得的其非對映異構的鹽進行分離。外消旋的產物也可以通過手性色譜來分離,如,使用手性吸附劑的高效液相色譜 (HPLC)。特別地,對映異構體可以通過不對稱合成製備,例如,可參考Jacques, et al., Enantiomers, Racemates and Resolutions (Wiley Interscience, New York, 1981);Principles of Asymmetric Synthesis (2nd Ed. Robert E. Gawley, Jeffrey Aubé, Elsevier, Oxford, UK, 2012); Eliel, E.L. Stereochemistry of Carbon Compounds (McGraw-Hill, NY, 1962);Wilen, S.H. Tables of Resolving Agents and Optical Resolutions p. 268 (E.L. Eliel, Ed., Univ. of Notre Dame Press, Notre Dame, IN 1972);Chiral Separation Techniques: A Practical Approach (Subramanian, G. Ed., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2007)。Any resulting racemate of the final product or intermediate can be resolved into the optical antipodes by methods familiar to those of ordinary skill in the art, e.g., by analysing its diastereomers obtained by known methods. The formed salt is separated. Racemic products can also be separated by chiral chromatography, eg, high performance liquid chromatography (HPLC) using chiral adsorbents. In particular, enantiomers can be prepared by asymmetric synthesis, for example, see Jacques, et al., Enantiomers, Racemates and Resolutions (Wiley Interscience, New York, 1981); Principles of Asymmetric Synthesis (2 nd Ed. Robert E. Gawley, Jeffrey Aubé, Elsevier, Oxford, UK, 2012); Eliel, EL Stereochemistry of Carbon Compounds (McGraw-Hill, NY, 1962); Wilen, SH Tables of Resolving Agents and Optical Resolutions p. 268 (EL Eliel, Ed., Univ. of Notre Dame Press, Notre Dame, IN 1972); Chiral Separation Techniques: A Practical Approach (Subramanian, G. Ed., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2007).
術語“互變異構體”或“互變異構形式”是指具有不同能量的可通過低能壘 (low energy barrier) 互相轉化的結構異構體。若互變異構是可能的(如在溶液中),則可以達到互變異構體的化學平衡。例如,質子互變異構體 (proton tautomer) (也稱為質子轉移互變異構體 (prototropic tautomer))包括通過質子遷移來進行的互相轉化,如酮-烯醇異構化和亞胺-烯胺異構化。價鍵互變異構體 (valence tautomer) 包括通過一些成鍵電子的重組來進行的互相轉化。酮-烯醇互變異構的具體實例是戊烷-2,4-二酮和4-羥基戊-3-烯-2-酮互變異構體的互變。互變異構的另一個實例是酚-酮互變異構。酚-酮互變異構的一個具體實例是吡啶-4-醇和吡啶-4(1H)-酮互變異構體的互變。除非另外指出,本發明化合物的所有互變異構體形式都在本發明的範圍之內。The term "tautomer" or "tautomeric form" refers to structural isomers having different energies that are interconvertible through a low energy barrier. A chemical equilibrium of tautomers can be achieved if tautomerism is possible (eg, in solution). For example, proton tautomers (also known as prototropic tautomers) include interconversions by migration of protons, such as keto-enol isomerization and imine-enamine isomerisation. Valence tautomers include interconversions by recombination of some of the bonding electrons. A specific example of keto-enol tautomerism is the interconversion of pentane-2,4-dione and 4-hydroxypent-3-en-2-one tautomers. Another example of tautomerism is phenol-ketone tautomerism. A specific example of phenol-ketone tautomerism is the interconversion of pyridin-4-ol and pyridin-4(lH)-one tautomers. Unless otherwise indicated, all tautomeric forms of the compounds of the present invention are within the scope of the present invention.
像本發明所描述的,本發明的化合物可以任選地被一個或多個取代基所取代,如上面的通式化合物,或者像實施例裡面特殊的例子,子類,和本發明所包含的一類化合物。As described herein, the compounds of the present invention may be optionally substituted with one or more substituents, such as the compounds of the general formula above, or as in the Examples, subclasses, and subclasses encompassed by the present invention. a class of compounds.
一般而言,術語“取代的”表示所給結構中的一個或多個氫原子被具體取代基所取代。除非其他方面表明,一個取代的基團可以有一個取代基在基團各個可取代的位置進行取代。當所給出的結構式中不只一個位置能被選自具體基團的一個或多個取代基所取代,那麼取代基可以相同或不同地在各個位置取代。In general, the term "substituted" means that one or more hydrogen atoms in a given structure have been replaced with a specified substituent. Unless otherwise indicated, a substituted group may have a substituent at each substitutable position of the group. When more than one position in a given formula can be substituted with one or more substituents selected from a particular group, the substituents can be substituted identically or differently at each position.
術語“任選地被……所取代”表示所述結構是未取代的或者被一個或多個本發明所述的取代基取代。本發明所述的取代基包括,但不限於,氘、氟、氯、溴、碘、氰基、羥基、氨基、硝基、羧基、芳基、雜芳基、烷氧基、烷基、烯基、炔基、雜環基、環烷基、環烷基烷基、雜環基烷基、芳基烷基、雜芳基烷基、氧代、鹵代烷基、鹵代烷氧基、烷基-C(=O)-、烷基-O-C(=O)-、烷基-S(=O)-、烷基-S(=O)2 -、烷基氨基、NH2 -C(=O)-、NH2 -S(=O)2 -,等等。The term "optionally substituted with" means that the structure is unsubstituted or substituted with one or more substituents described herein. The substituents described in the present invention include, but are not limited to, deuterium, fluorine, chlorine, bromine, iodine, cyano, hydroxyl, amino, nitro, carboxyl, aryl, heteroaryl, alkoxy, alkyl, alkene alkynyl, alkynyl, heterocyclyl, cycloalkyl, cycloalkylalkyl, heterocyclylalkyl, arylalkyl, heteroarylalkyl, oxo, haloalkyl, haloalkoxy, alkyl-C (=O)-, alkyl-OC(=O)-, alkyl-S(=O)-, alkyl-S(=O) 2 -, alkylamino, NH 2 -C(=O)- , NH 2 -S(=O) 2 -, and so on.
另外,需要說明的是,除非以其他方式明確指出,在本發明中所採用的描述方式“各…獨立地為”與“…各自獨立地為”和“…獨立地為”可以互換,均應做廣義理解,其既可以是指在不同基團中,相同符號之間所表達的具體選項之間互相不影響,也可以表示在相同的基團中,相同符號之間所表達的具體選項之間互相不影響。例如,結構式“-C(=O)-N(Ra Rb )”和結構式“-C1-6 亞烷基-N(Ra Rb )”兩者之間Ra 的具體選項互相之間不受影響。In addition, it should be noted that, unless clearly stated otherwise, the description modes "each independently" and "...independently" and "...independently" used in the present invention can be interchanged, and should be In a broad sense, it can either mean that in different groups, the specific options expressed between the same symbols do not affect each other, or it can mean that in the same group, the specific options expressed between the same symbols do not affect each other. For example, specific options for R a between the structural formula "-C(=O)-N(R a R b )" and the structural formula "-C 1-6 alkylene-N(R a R b )" are not affected by each other.
在本說明書的各部分,本發明公開化合物的取代基按照基團種類或範圍公開。特別指出,本發明包括這些基團種類和範圍的各個成員的每一個獨立的次級組合。例如,術語“C1 -C6 烷基”或“C1-6 烷基”特別指獨立公開的甲基、乙基、C3 烷基、C4 烷基、C5 烷基和C6 烷基。In various sections of this specification, substituents of the compounds disclosed herein are disclosed in terms of group type or scope. Specifically, the present invention includes each and every independent subcombination of each member of these group species and ranges. For example, the term "C 1 -C 6 alkyl" or "C 1-6 alkyl" refers particularly to the disclosure independently methyl, ethyl, C 3 alkyl, C 4 alkyl, C 5 alkyl, and C 6 alkyl base.
在本發明的各部分,描述了連接取代基。當該結構清楚地需要連接基團時,針對該基團所列舉的馬庫西變量應理解為連接基團。例如,如果該結構需要連接基團並且針對該變量的馬庫西基團定義列舉了如“烷基”或“芳基”,則應該理解,該“烷基”或“芳基”分別代表連接的亞烷基基團或亞芳基基團。In various parts of the present invention, linking substituents are described. When the structure clearly requires a linking group, the Markussy variables recited for that group should be understood to be the linking group. For example, if the structure requires a linking group and the definition of a Marcussy group for that variable recites, for example, "alkyl" or "aryl", it should be understood that the "alkyl" or "aryl" respectively represents the linking an alkylene group or an arylene group.
本發明使用的術語“烷基”或“烷基基團”,表示含有1至20個碳原子,飽和的直鏈或支鏈一價烴基基團,其中,所述烷基基團可以任選地被一個或多個本發明描述的取代基所取代。除非另外詳細說明,烷基基團含有1-20個碳原子。在一實施方案中,烷基基團含有1-12個碳原子;在另一實施方案中,烷基基團含有1-6個碳原子;在又一實施方案中,烷基基團含有1-4個碳原子;還在一實施方案中,烷基基團含有1-3個碳原子。The term "alkyl" or "alkyl group" used in the present invention refers to a saturated linear or branched monovalent hydrocarbon group containing 1 to 20 carbon atoms, wherein the alkyl group may optionally be is substituted with one or more substituents described herein. Unless otherwise specified, alkyl groups contain 1-20 carbon atoms. In one embodiment, the alkyl group contains 1-12 carbon atoms; in another embodiment, the alkyl group contains 1-6 carbon atoms; in yet another embodiment, the alkyl group contains 1 -4 carbon atoms; in yet another embodiment, the alkyl group contains 1-3 carbon atoms.
烷基基團的實例包含,但並不限於,甲基 (Me、-CH3 ),乙基 (Et、-CH2 CH3 ),正丙基 (n -Pr、-CH2 CH2 CH3 ),異丙基 (i -Pr、-CH(CH3 )2 ),正丁基 (n -Bu、-CH2 CH2 CH2 CH3 ),異丁基 (i -Bu、-CH2 CH(CH3 )2 ),仲丁基 (s -Bu、-CH(CH3 )CH2 CH3 ),叔丁基 (t -Bu、-C(CH3 )3 ),正戊基 (-CH2 CH2 CH2 CH2 CH3 ),2-戊基 (-CH(CH3 )CH2 CH2 CH3 ),3-戊基 (-CH(CH2 CH3 )2 ),2-甲基-2-丁基 (-C(CH3 )2 CH2 CH3 ),3-甲基-2-丁基 (-CH(CH3 )CH(CH3 )2 ),3-甲基-1-丁基 (-CH2 CH2 CH(CH3 )2 ),2-甲基-1-丁基 (-CH2 CH(CH3 )CH2 CH3 ),正己基 (-CH2 CH2 CH2 CH2 CH2 CH3 ),2-己基 (-CH(CH3 )CH2 CH2 CH2 CH3 ),3-己基 (-CH(CH2 CH3 )(CH2 CH2 CH3 )),2-甲基-2-戊基 (-C(CH3 )2 CH2 CH2 CH3 ),3-甲基-2-戊基 (-CH(CH3 )CH(CH3 )CH2 CH3 ),4-甲基-2-戊基 (-CH(CH3 )CH2 CH(CH3 )2 ),3-甲基-3-戊基 (-C(CH3 )(CH2 CH3 )2 ),2-甲基-3-戊基 (-CH(CH2 CH3 )CH(CH3 )2 ),2,3-二甲基-2-丁基 (-C(CH3 )2 CH(CH3 )2 ),3,3-二甲基-2-丁基 (-CH(CH3 )C(CH3 )3 ),正庚基,正辛基,等等。Examples of alkyl groups include, but are not limited to, methyl (Me, -CH 3), ethyl (Et, -CH 2 CH 3) , n-propyl (n -Pr, -CH 2 CH 2 CH 3 ), isopropyl (i -Pr, -CH (CH 3 ) 2), n-butyl (n -Bu, -CH 2 CH 2 CH 2 CH 3), isobutyl (i -Bu, -CH 2 CH (CH 3 ) 2 ), sec-butyl ( s- Bu, -CH(CH 3 )CH 2 CH 3 ), tert-butyl ( t -Bu, -C(CH 3 ) 3 ), n-pentyl (-CH 2 CH 2 CH 2 CH 2 CH 3), 2- pentyl (-CH (CH 3) CH 2 CH 2 CH 3), 3- pentyl (-CH (CH 2 CH 3) 2), 2- methyl 2-butyl (-C (CH 3) 2 CH 2 CH 3), 3- methyl-2-butyl (-CH (CH 3) CH ( CH 3) 2), 3- methyl-1- butyl (-CH 2 CH 2 CH (CH 3) 2), 2- methyl-1-butyl (-CH 2 CH (CH 3) CH 2 CH 3), n-hexyl (-CH 2 CH 2 CH 2 CH 2 CH 2 CH 3), 2- hexyl (-CH (CH 3) CH 2 CH 2 CH 2 CH 3), 3- hexyl (-CH (CH 2 CH 3) (CH 2 CH 2 CH 3)), 2-methyl-2-pentyl (-C (CH 3) 2 CH 2 CH 2 CH 3), 3- methyl-2-pentyl (-CH (CH 3) CH ( CH 3) CH 2 CH 3 ), 4-methyl-2-pentyl (-CH(CH 3 )CH 2 CH(CH 3 ) 2 ), 3-methyl-3-pentyl (-C(CH 3 )(CH 2 CH 3 ) 2 ), 2-methyl-3-pentyl (-CH(CH 2 CH 3 )CH(CH 3 ) 2 ), 2,3-dimethyl-2-butyl (-C(CH 3 ) 2 CH (CH 3 ) 2 ), 3,3-dimethyl-2-butyl (-CH(CH 3 )C(CH 3 ) 3 ), n-heptyl, n-octyl, and the like.
術語“亞烷基”表示從飽和的直鏈或支鏈烴中去掉兩個氫原子所得到的飽和的二價烴基基團。除非另外詳細說明,亞烷基基團含有1-12個碳原子。在一實施方案中,亞烷基基團含有1-6個碳原子;在另一實施方案中,亞烷基基團含有1-4個碳原子;在又一實施方案中,亞烷基基團含有1-3個碳原子;還在一實施方案中,亞烷基基團含有1-2個碳原子。這樣的實例包括亞甲基 (-CH2 -),亞乙基 (-CH2 CH2 -),亞丙基 (-CH2 CH2 CH2 -),亞異丙基 (-CH(CH3 )CH2 -) 等等。The term "alkylene" refers to a saturated divalent hydrocarbon radical obtained by removing two hydrogen atoms from a saturated straight or branched chain hydrocarbon. Unless otherwise specified, alkylene groups contain 1-12 carbon atoms. In one embodiment, the alkylene group contains 1-6 carbon atoms; in another embodiment, the alkylene group contains 1-4 carbon atoms; in yet another embodiment, the alkylene group A group contains 1-3 carbon atoms; in yet another embodiment, an alkylene group contains 1-2 carbon atoms. Examples of such include methylene (-CH 2 -), ethylene (-CH 2 CH 2 -), propylene (-CH 2 CH 2 CH 2 - ), isopropylene (-CH (CH 3 )CH 2 -) and so on.
術語“烯基”表示含有2-12個碳原子的直鏈或支鏈一價烴基,其中至少有一個不飽和位點,即有一個碳-碳sp2 雙鍵,其中,所述烯基基團可以任選地被一個或多個本發明所描述的取代基所取代,其包括 "cis" 和 "tans" 的定位,或者 "E" 和 "Z" 的定位。在一實施方案中,烯基基團包含2-8個碳原子;在另一實施方案中,烯基基團包含2-6個碳原子;在又一實施方案中,烯基基團包含2-4個碳原子。烯基基團的實例包括,但並不限於,乙烯基 (-CH=CH2 )、烯丙基 (-CH2 CH=CH2 ) 等等。The term "alkenyl" refers to a linear or branched monovalent hydrocarbon group containing 2 to 12 carbon atoms, wherein there is at least one site of unsaturation, i.e., a carbon-carbon sp 2 double bond, wherein the alkenyl group A group may be optionally substituted with one or more substituents described herein, including the "cis" and "tans" positions, or the "E" and "Z" positions. In one embodiment, the alkenyl group contains 2-8 carbon atoms; in another embodiment, the alkenyl group contains 2-6 carbon atoms; in yet another embodiment, the alkenyl group contains 2 -4 carbon atoms. Examples of alkenyl groups include, but are not limited to, vinyl (-CH = CH 2), allyl (-CH 2 CH = CH 2) and the like.
術語“炔基”表示含有2-12個碳原子的直鏈或支鏈一價烴基,其中至少有一個不飽和位點,即有一個碳-碳sp三鍵,其中,所述炔基基團可以任選地被一個或多個本發明所描述的取代基所取代。在一實施方案中,炔基基團包含2-8個碳原子;在另一實施方案中,炔基基團包含2-6個碳原子;在又一實施方案中,炔基基團包含2-4個碳原子。炔基基團的實例包括,但並不限於,乙炔基 (-C≡CH)、炔丙基 (-CH2 C≡CH)、1-丙炔基 (-C≡C-CH3 ) 等等。The term "alkynyl" denotes a linear or branched monovalent hydrocarbon group containing 2-12 carbon atoms, wherein there is at least one site of unsaturation, i.e., a carbon-carbon sp triple bond, wherein the alkynyl group Can be optionally substituted with one or more of the substituents described herein. In one embodiment, the alkynyl group contains 2-8 carbon atoms; in another embodiment, the alkynyl group contains 2-6 carbon atoms; in yet another embodiment, the alkynyl group contains 2 -4 carbon atoms. Examples of alkynyl groups include, but are not limited to, ethynyl (-C≡CH), propargyl (-CH 2 C≡CH), 1- propynyl (-C≡C-CH 3), etc. .
術語“羧基”,無論是單獨使用還是和其他術語連用,如“羧烷基”,表示-CO2 H或-COOH。The term "carboxyl", whether used alone or with other terms, such as "carboxyalkyl", denotes -CO 2 H or -COOH.
術語“氘”表示單個氘原子。例如,一個氘原子取代甲基中的一個氫原子,形成單-氘代甲基(-CDH2 ),兩個氘原子取代甲基中的兩個氫原子,形成雙-氘代甲基(-CD2 H),以及三個氘原子取代甲基中的三個氫原子,形成三-氘代甲基(-CD3 )。The term "deuterium" refers to a single deuterium atom. For example, one deuterium atom replaces one hydrogen atom in methyl to form mono-deuteromethyl (-CDH 2 ), and two deuterium atoms replace two hydrogen atoms in methyl to form bis-deuteromethyl (- CD 2 H), and three deuterium atoms replace the three hydrogen atoms in the methyl group to form tri-deuteromethyl (-CD 3 ).
在本發明中所使用的術語“不飽和的”表示基團中含有一個或多個不飽和度。The term "unsaturated" as used herein means that the group contains one or more degrees of unsaturation.
術語“雜原子”是指O、S、N、P和Si,包括N、S和P任何氧化態的形式;伯、仲、叔胺和季銨鹽的形式;或者雜環中氮原子上的氫被取代的形式,例如,N(像3,4-二氫-2H -吡咯基中的N),NH(像吡咯烷基中的NH)或NR(像N-取代的吡咯烷基中的NR)。The term "heteroatom" refers to O, S, N, P, and Si, including N, S, and P in any oxidation state; in the form of primary, secondary, tertiary amines, and quaternary ammonium salts; or on a nitrogen atom in a heterocyclic ring. Hydrogen substituted form, for example, N (as in 3,4-dihydro- 2H -pyrrolidinyl), NH (as in pyrrolidinyl) or NR (as in N-substituted pyrrolidinyl) NR).
術語“鹵素”是指氟(F)、氯(Cl)、溴(Br)或碘(I)。The term "halogen" refers to fluorine (F), chlorine (Cl), bromine (Br) or iodine (I).
術語“烷氧基”表示烷基基團通過氧原子與分子其餘部分相連,其中,烷基基團具有如本發明所述的含義。除非另外詳細說明,所述烷氧基基團含有1-12個碳原子。在一實施方案中,烷氧基基團含有1-6個碳原子;在另一實施方案中,烷氧基基團含有1-4個碳原子;在又一實施方案中,烷氧基基團含有1-3個碳原子。所述烷氧基基團可以任選地被一個或多個本發明描述的取代基所取代。The term "alkoxy" means that an alkyl group is attached to the remainder of the molecule through an oxygen atom, wherein the alkyl group has the meaning as described herein. Unless otherwise specified, the alkoxy groups contain 1-12 carbon atoms. In one embodiment, the alkoxy group contains 1-6 carbon atoms; in another embodiment, the alkoxy group contains 1-4 carbon atoms; in yet another embodiment, the alkoxy group The group contains 1-3 carbon atoms. The alkoxy groups may be optionally substituted with one or more substituents described herein.
烷氧基基團的實例包括,但並不限於,甲氧基 (MeO、-OCH3 ),乙氧基 (EtO、-OCH2 CH3 ),1-丙氧基 (n -PrO、n-丙氧基、-OCH2 CH2 CH3 ),2-丙氧基 (i -PrO、i -丙氧基、-OCH(CH3 )2 ),1-丁氧基 (n-BuO、n-丁氧基、-OCH2 CH2 CH2 CH3 ),2-甲基-l-丙氧基 (i -BuO、i -丁氧基、-OCH2 CH(CH3 )2 ),2-丁氧基 (s-BuO、s-丁氧基、-OCH(CH3 )CH2 CH3 ),2-甲基-2-丙氧基 (t -BuO、t -丁氧基、-OC(CH3 )3 ),1-戊氧基 (n-戊氧基、-OCH2 CH2 CH2 CH2 CH3 ),2-戊氧基 (-OCH(CH3 )CH2 CH2 CH3 ),3-戊氧基 (-OCH(CH2 CH3 )2 ),2-甲基-2-丁氧基 (-OC(CH3 )2 CH2 CH3 ),3-甲基-2-丁氧基 (-OCH(CH3 )CH(CH3 )2 ),3-甲基-l-丁氧基 (-OCH2 CH2 CH(CH3 )2 ),2-甲基-l-丁氧基 (-OCH2 CH(CH3 )CH2 CH3 ),等等。Examples of alkoxy groups include, but are not limited to, methoxy (MeO, -OCH 3), ethoxy (EtO, -OCH 2 CH 3) , 1- propoxy (n -PrO, n- Propoxy, -OCH 2 CH 2 CH 3 ), 2-propoxy ( i- PrO, i -propoxy, -OCH(CH 3 ) 2 ), 1-butoxy (n-BuO, n- Butoxy, -OCH 2 CH 2 CH 2 CH 3 ), 2-methyl-1-propoxy ( i- BuO, i -butoxy, -OCH 2 CH(CH 3 ) 2 ), 2-butanyl Oxy (s-BuO, s-butoxy, -OCH(CH 3 )CH 2 CH 3 ), 2-methyl-2-propoxy ( t- BuO, t -butoxy, -OC(CH 3) 3), 1-pentyl group (N- pentyloxy group, -OCH 2 CH 2 CH 2 CH 2 CH 3), 2- pentyloxy group (-OCH (CH 3) CH 2 CH 2 CH 3), 3-pentyloxy (-OCH(CH 2 CH 3 ) 2 ), 2-methyl-2-butoxy (-OC(CH 3 ) 2 CH 2 CH 3 ), 3-methyl-2-butoxy group (-OCH(CH 3 )CH(CH 3 ) 2 ), 3-methyl-1-butoxy (-OCH 2 CH 2 CH(CH 3 ) 2 ), 2-methyl-1-butoxy (-OCH 2 CH(CH 3 )CH 2 CH 3 ), etc.
術語“鹵代烷基”或“鹵代烷氧基”表示烷基或烷氧基基團被一個或多個鹵素原子所取代,這樣的實例包含,但並不限於,-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 CH2 CH2 F、-CH2 CH2 CHF2 、-CH2 CH2 CF3 、-CF2 CH2 CH3 、-CH2 Cl、-CHCl2 、-OCHF2 、-OCF3 等。The term "haloalkyl" or "haloalkoxy" means an alkyl or alkoxy group is substituted with one or more halogen atoms, such examples include, but are not limited to, -CH 2 F, -CHF 2, - CF 3, -CH 2 CH 2 F , -CH 2 CHF 2, -CH 2 CF 3, -CF 2 CH 3, -CH 2 CH 2 CH 2 F, -CH 2 CH 2 CHF 2, -CH 2 CH 2 CF 3 , -CF 2 CH 2 CH 3 , -CH 2 Cl, -CHCl 2 , -OCHF 2 , -OCF 3 and the like.
術語“烷氨基”表示-NH2 基團被一個或兩個烷基所取代,其中,烷基基團具有如本發明所述的含義。除非另外詳細說明,所述烷氨基基團含有1-12個碳原子。在一實施方案中,烷氨基基團含有1-6個碳原子;在另一實施方案中,烷氨基基團含有1-4個碳原子;在又一實施方案中,烷氨基基團含有1-3個碳原子。所述烷氨基的實例包括但不限於:甲基氨基、二甲基氨基、乙基氨基、二乙基氨基、甲基乙基氨基等。所述烷氨基基團可以任選地被一個或多個本發明描述的取代基所取代。The term "alkylamino" represents a -NH 2 group substituted by one or two alkyl groups, wherein the alkyl group is as defined as the present invention. Unless otherwise specified, the alkylamino group contains 1-12 carbon atoms. In one embodiment, the alkylamino group contains 1-6 carbon atoms; in another embodiment, the alkylamino group contains 1-4 carbon atoms; in yet another embodiment, the alkylamino group contains 1 -3 carbon atoms. Examples of the alkylamino group include, but are not limited to, methylamino, dimethylamino, ethylamino, diethylamino, methylethylamino, and the like. The alkylamino group may be optionally substituted with one or more substituents described herein.
術語“j-k個原子組成的”,其中各j和k獨立地為任意非零的自然數,且k>j;所述“j-k”包括j、k和兩者之間的任意自然數。典型地描述分子中成環原子的數目,在所述分子中成環原子的數目是j-k,所述的原子包括碳原子和/或O、N、S、P等雜原子。The term "consisting of j-k atoms", wherein each of j and k is independently any non-zero natural number, and k>j; said "j-k" includes j, k and any natural number in between. The number of ring-forming atoms in a molecule is typically described, where the number of ring-forming atoms in the molecule is j-k, and the atoms include carbon atoms and/or heteroatoms such as O, N, S, P, etc.
術語“環烷基”表示含有3-12個碳原子的,單價或多價的飽和單環,雙環或三環體系。在一實施方案中,環烷基包含3-12個碳原子;在另一實施方案中,環烷基包含3-8個碳原子;在又一實施方案中,環烷基包含3-6個碳原子。所述環烷基基團可以獨立任選地被一個或多個本發明所描述的取代基所取代。The term "cycloalkyl" denotes a monovalent or polyvalent saturated monocyclic, bicyclic or tricyclic ring system containing 3 to 12 carbon atoms. In one embodiment, the cycloalkyl group contains 3-12 carbon atoms; in another embodiment, the cycloalkyl group contains 3-8 carbon atoms; in yet another embodiment, the cycloalkyl group contains 3-6 carbon atoms carbon atom. The cycloalkyl groups can be independently optionally substituted with one or more substituents described herein.
術語“環烷基-烷基”或“環烷基-亞烷基”可以交換使用,都是指烷基基團被一個或多個環烷基基團所取代,其中烷基基團和環烷基基團具有如本發明所述的含義,這樣的實例包括,但並不限於環丙基甲基、環丙基乙基、環丁基甲基、環丁基乙基、環戊基甲基、環戊基乙基、環己基甲基、環己基乙基等。The terms "cycloalkyl-alkyl" or "cycloalkyl-alkylene" are used interchangeably and both refer to the substitution of an alkyl group by one or more cycloalkyl groups, wherein the alkyl group and the ring Alkyl groups have meanings as described herein, examples of which include, but are not limited to, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, cyclobutylethyl, cyclopentylmethyl, Cyclopentylethyl, cyclohexylmethyl, cyclohexylethyl, etc.
術語“雜環基”和“雜環”在此處可交換使用,都是指包含3-12個環原子的飽和或部分不飽和的單環、雙環或三環,其中,單環、雙環或三環中不包含芳香環,且至少一個環原子選自氮、硫和氧原子。除非另外說明,雜環基可以是碳基或氮基,且-CH2 -基團可以任選地被-C(=O)-替代。環的硫原子可以任選地被氧化成S-氧化物。環的氮原子可以任選地被氧化成N -氧化合物。雜環基的實例包括,但不限於:環氧乙烷基,氮雜環丁基,氧雜環丁基,硫雜環丁基,吡咯烷基,吡咯基,吡唑烷基,咪唑啉基,咪唑烷基,四氫呋喃基,二氫呋喃基,四氫噻吩基,二氫噻吩基,1,3-二氧雜環戊烷基,1,3-二氧雜環戊烯基,二硫環戊基,四氫吡喃基,二氫吡喃基,四氫噻喃基,哌啶基,四氫吡啶基,嗎啉基,硫代嗎啉基,1-氧代-硫代嗎啉基,1,1-二氧代-硫代嗎啉基,哌嗪基,二噁烷基,二噻烷基,噻噁烷基,高哌嗪基,高哌啶基,氧雜環庚烷基。雜環基中-CH2 -基團被-C(=O)-取代的實例包括,但不限於,2-氧代吡咯烷基、氧代-1,3-噻唑烷基、2-哌啶酮基和3,5-二氧代哌啶基。雜環基中硫原子被氧化的實例包括,但不限於,環丁碸基、1,1-二氧代硫代嗎啉基。所述的雜環基基團可以任選地被一個或多個本發明所描述的取代基所取代。The terms "heterocyclyl" and "heterocycle" are used interchangeably herein and both refer to a saturated or partially unsaturated monocyclic, bicyclic or tricyclic ring containing 3 to 12 ring atoms, wherein the monocyclic, bicyclic or No aromatic rings are included in the tricyclic ring, and at least one ring atom is selected from nitrogen, sulfur and oxygen atoms. Unless otherwise indicated, heterocyclyl groups may be carbon or nitrogen-based groups, and -CH 2 - groups may be optionally substituted with -C (= O) - instead. Ring sulfur atoms can optionally be oxidized to S-oxides. The nitrogen atoms of the rings can optionally be oxidized to N -oxygen compounds. Examples of heterocyclyl groups include, but are not limited to: oxiranyl, azetidinyl, oxetanyl, thietanyl, pyrrolidinyl, pyrrolyl, pyrazolidinyl, imidazolinyl , imidazolidinyl, tetrahydrofuranyl, dihydrofuranyl, tetrahydrothienyl, dihydrothienyl, 1,3-dioxolane, 1,3-dioxolyl, disulfide Amyl, tetrahydropyranyl, dihydropyranyl, tetrahydrothiopyranyl, piperidinyl, tetrahydropyridyl, morpholinyl, thiomorpholinyl, 1-oxo-thiomorpholinyl , 1,1-dioxo-thiomorpholinyl, piperazinyl, dioxanyl, dithianyl, thioxanyl, homopiperazinyl, homopiperidinyl, oxepanyl . Heterocyclyl group -CH 2 - group is -C (= O) - Examples of substituents include, but are not limited to, 2-oxo-pyrrolidinyl, oxo-1,3-thiazolidinyl, 2-piperidinyl Keto and 3,5-dioxopiperidinyl. Examples of the oxidized sulfur atom in the heterocyclyl group include, but are not limited to, cyclobutanyl, 1,1-dioxothiomorpholinyl. The heterocyclyl group may be optionally substituted with one or more substituents described herein.
術語“雜環基-烷基”或“雜環基-亞烷基”可以交換使用,都是指雜環基取代的烷基;其中雜環基和烷基基團具有如本發明所述的含義。這樣的實例包括,但並不限於硫代嗎啉-4-基甲基,四氫呋喃-3-基甲基,四氫吡喃-4基甲基,氧雜環丁烷-3-基甲基,吡咯烷-2-基甲基,嗎啉-4-基甲基等。The terms "heterocyclyl-alkyl" or "heterocyclyl-alkylene" are used interchangeably, and both refer to a heterocyclyl-substituted alkyl group; meaning. Such examples include, but are not limited to, thiomorpholin-4-ylmethyl, tetrahydrofuran-3-ylmethyl, tetrahydropyran-4-ylmethyl, oxetan-3-ylmethyl, Pyrrolidin-2-ylmethyl, morpholin-4-ylmethyl, etc.
術語“芳基”表示含有6-14個環原子,或6-12個環原子,或6-10個環原子的單環、雙環和三環的碳環體系,其中,至少一個環體系是芳香族的,其中每一個環體系包含3-7個原子組成的環,且有一個或多個附著點與分子的其餘部分相連。術語“芳基”可以和術語“芳香環”交換使用。芳基基團的實例可以包括苯基、萘基和蒽基。所述芳基基團可以獨立任選地被一個或多個本發明所描述的取代基所取代。The term "aryl" refers to monocyclic, bicyclic and tricyclic carbocyclic ring systems containing 6-14 ring atoms, or 6-12 ring atoms, or 6-10 ring atoms, wherein at least one ring system is aromatic A family of rings in which each ring system contains a ring of 3-7 atoms with one or more points of attachment to the rest of the molecule. The term "aryl" is used interchangeably with the term "aromatic ring". Examples of aryl groups may include phenyl, naphthyl, and anthracenyl. The aryl groups can be independently optionally substituted with one or more substituents described herein.
術語“芳基-烷基”或“芳基-亞烷基”可以交換使用,都是指一個或多個芳基取代的烷基基團,其中,所述芳基和烷基具有本發明所述的含義。其中一些實施方案是,芳基烷基基團是指“較低級的芳基烷基”基團,即芳基基團連接到C1-6 的烷基或亞烷基基團上。另外一些實施方案是,芳基烷基基團是指含C1-4 烷基的“苯烷基”。其中具體實例包括二苯基甲基,苯甲基、苯乙基。芳基-烷基或芳基-亞烷基上的芳基可以進一步被本發明所述的取代基所取代。The terms "aryl-alkyl" or "aryl-alkylene" are used interchangeably, and both refer to one or more aryl-substituted alkyl groups, wherein the aryl and alkyl groups have the characteristics of the present invention. stated meaning. In some embodiments, an arylalkyl group refers to a "lower arylalkyl" group, ie, an aryl group attached to a C1-6 alkyl or alkylene group. In other embodiments, an arylalkyl group refers to a "phenylalkyl" containing a C1-4 alkyl group. Specific examples thereof include diphenylmethyl, benzyl, and phenethyl. The aryl group on the aryl-alkyl or aryl-alkylene group may be further substituted with the substituents described herein.
術語“雜芳基”表示含有5-15個環原子、5-12個環原子,或5-10個環原子,或5-7個環原子的單環、雙環和三環體系,其中至少一個環體系是芳香族的,且至少一個環體系包含一個或多個雜原子,其中每一個環體系包含5-7個原子組成的環,且有一個或多個附著點與分子其餘部分相連。術語“雜芳基”可以與術語“雜芳環”,“芳雜環”或“雜芳族化合物”交換使用。所述雜芳基基團任選地被一個或多個本發明所描述的取代基所取代。在其中一些實施方案中,5-10個原子組成的雜芳基包含1,2,3或4個獨立選自O,S,N或B的雜原子。在其中一些實施方案中,11-15個原子組成的雜芳基包含1,2,3或4個獨立選自O,S,N或B的雜原子。The term "heteroaryl" refers to monocyclic, bicyclic, and tricyclic ring systems containing 5-15 ring atoms, 5-12 ring atoms, or 5-10 ring atoms, or 5-7 ring atoms, of which at least one The ring systems are aromatic and at least one ring system contains one or more heteroatoms, wherein each ring system contains a ring of 5-7 atoms with one or more points of attachment to the rest of the molecule. The term "heteroaryl" may be used interchangeably with the terms "heteroaromatic", "aromatic heterocycle" or "heteroaromatic". The heteroaryl group is optionally substituted with one or more substituents described herein. In some of these embodiments, a heteroaryl group of 5-10 atoms contains 1, 2, 3 or 4 heteroatoms independently selected from O, S, N or B. In some of these embodiments, a heteroaryl group of 11-15 atoms contains 1, 2, 3 or 4 heteroatoms independently selected from O, S, N or B.
雜芳基基團的實例包括,但並不限於,呋喃基(如 2-呋喃基、3-呋喃基)、咪唑基(如N -咪唑基、2-咪唑基、4-咪唑基、5-咪唑基)、異噁唑基(如 3-異噁唑基、4-異噁唑基、5-異噁唑基)、噁唑基(如 2-噁唑基、4-噁唑基、5-噁唑基)、吡咯基(如N -吡咯基、2-吡咯基、3-吡咯基)、吡啶基(如 2-吡啶基、3-吡啶基、4-吡啶基)、嘧啶基(如 2-嘧啶基、4-嘧啶基、5-嘧啶基)、噠嗪基(如3-噠嗪基)、噻唑基(如 2-噻唑基、4-噻唑基、5-噻唑基)、四唑基(如 5-四唑基)、三唑基(如2-三唑基和5-三唑基)、噻吩基(如 2-噻吩基、3-噻吩基)、吡唑基(如 2-吡唑基)、異噻唑基、嘧啶酮基、吡啶酮基;也包括以下的雙環,但絕不限於這些雙環:苯並咪唑基、苯並呋喃基、苯並噻吩基、吲哚基(如 2-吲哚基)、嘌呤基、喹啉基(如 2-喹啉基,3-喹啉基,4-喹啉基)、、、、、、或等等。Examples of heteroaryl groups include, but are not limited to, furyl (eg, 2-furyl, 3-furyl), imidazolyl (eg, N -imidazolyl, 2-imidazolyl, 4-imidazolyl, 5- imidazolyl), isoxazolyl (such as 3-isoxazolyl, 4-isoxazolyl, 5-isoxazolyl), oxazolyl (such as 2-oxazolyl, 4-oxazolyl, 5-isoxazolyl) -oxazolyl), pyrrolyl (eg N -pyrrolyl, 2-pyrrolyl, 3-pyrrolyl), pyridyl (eg 2-pyridyl, 3-pyridyl, 4-pyridyl), pyrimidinyl (eg 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl), pyridazinyl (such as 3-pyridazinyl), thiazolyl (such as 2-thiazolyl, 4-thiazolyl, 5-thiazolyl), tetrazole base (such as 5-tetrazolyl), triazolyl (such as 2-triazolyl and 5-triazolyl), thienyl (such as 2-thienyl, 3-thienyl), pyrazolyl (such as 2-thienyl) pyrazolyl), isothiazolyl, pyrimidinonyl, pyridinone; also including, but in no way limited to, the following bicyclic rings: benzimidazolyl, benzofuranyl, benzothienyl, indolyl (e.g. 2-indolyl), purinyl, quinolinyl (such as 2-quinolinyl, 3-quinolinyl, 4-quinolinyl), , , , , , or etc.
術語“雜芳基-烷基”或“雜芳基-亞烷基”可以交換使用,都是指烷基基團被一個或多個雜芳基所取代,其中,雜芳基和烷基基團具有本發明所述的含義,這樣的實例包括,但並不限於吡啶-2基甲基,吡啶-3基甲基,吡啶-4基甲基,嘧啶2-基甲基,吡唑-5基甲基,吡唑-4基甲基,咪唑-2-基甲基,呋喃-2-基乙基,吲哚-3-基甲基等。The terms "heteroaryl-alkyl" or "heteroaryl-alkylene" are used interchangeably and both refer to an alkyl group substituted with one or more heteroaryl groups, wherein the heteroaryl and alkyl groups are group has the meaning set forth herein, such examples include, but are not limited to, pyridin-2ylmethyl, pyridin-3ylmethyl, pyridin-4ylmethyl, pyrimidin-2-ylmethyl, pyrazole-5 ylmethyl, pyrazol-4-ylmethyl, imidazol-2-ylmethyl, furan-2-ylethyl, indol-3-ylmethyl and the like.
像本發明所描述的,取代基畫一個鍵連接到中心的環上形成的環體系(如式c所示)代表取代基在該環上任何可取代的位置都可以取代。例如,式c代表取代基R可在C環上任何可能被取代的位置上單取代或多取代,如式c1~式c19所示。 As described in the present invention, the substituent group is attached to the central ring by a bond to form a ring system (as shown in formula c), which means that the substituent group can be substituted at any substitutable position on the ring. For example, formula c represents that the substituent R can be mono- or poly-substituted at any possible substituted position on the C ring, as shown in formula c1 to formula c19.
像本發明所描述的,一個連接鍵連接到環體系上 (如式d所示) 代表連接鍵可以在環體系上任何可連接的位置與分子其餘部分相連。式d代表環上任何可能連接的位置均可與分子其餘部分相連,如式d1~式d5所示。 As described herein, a linker attached to a ring system (as shown in formula d) means that the linker can be attached to the rest of the molecule at any linkable position on the ring system. Formula d represents that any possible linking position on the ring can be connected to the rest of the molecule, as shown in formula d1 to formula d5.
本發明所使用的術語“前藥”,代表一個化合物在體內轉化為式 (I) 或式 (II) 所示的化合物。這樣的轉化受前體藥物在血液中水解或在血液或組織中經酶轉化為母體結構的影響。本發明前體藥物類化合物可以是酯,在現有的發明中酯可以作為前體藥物的有苯酯類、脂肪族(C1-24 )酯類、醯氧基甲基酯類、碳酸酯、氨基甲酸酯類和氨基酸酯類。例如本發明裡的一個化合物包含羥基,即可以將其醯化得到前體藥物形式的化合物。其他的前體藥物形式包括磷酸酯,如這些磷酸酯類化合物是經母體上的羥基磷酸化得到的。關於前體藥物完整的討論可以參考以下文獻:T. Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, Vol. 14 of the A.C.S. Symposium Series, Edward B. Roche, ed., Bioreversible Carriers in Drug Design, American Pharmaceutical Association and Pergamon Press, 1987, J. Rautio et al, Prodrugs: Design and Clinical Applications,Nature Review Drug Discovery , 2008, 7, 255-270, and S. J. Hecker et al, Prodrugs of Phosphates and Phosphonates,Journal of Medicinal Chemistry , 2008, 51, 2328-2345。As used in the present invention, the term "prodrug" refers to the conversion of a compound into a compound of formula (I) or formula (II) in vivo. Such conversion is effected by hydrolysis of the prodrug in blood or enzymatic conversion to the parent structure in blood or tissue. The prodrug compounds of the present invention can be esters. In the existing invention, esters that can be used as prodrugs include phenyl esters, aliphatic (C 1-24 ) esters, hydroxymethyl esters, carbonates, Carbamates and amino acid esters. For example, a compound of the present invention contains a hydroxyl group, which can be acylated to give the compound in the form of a prodrug. Other prodrug forms include phosphates, such as these phosphates, which are obtained by phosphorylation of the parent hydroxyl group. A complete discussion of prodrugs can be found in: T. Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, Vol. 14 of the ACS Symposium Series, Edward B. Roche, ed., Bioreversible Carriers in Drug Design , American Pharmaceutical Association and Pergamon Press, 1987, J. Rautio et al, Prodrugs: Design and Clinical Applications, Nature Review Drug Discovery , 2008, 7, 255-270, and SJ Hecker et al, Prodrugs of Phosphates and Phosphonates, Journal of Medicinal Chemistry , 2008, 51, 2328-2345.
“代謝產物”是指具體的化合物或其鹽在體內通過代謝作用所得到的產物。一個化合物的代謝產物可以通過所屬技術領域中的通常技術來進行鑒定,其活性可以通過如本發明所描述的那樣採用試驗的方法進行表徵。這樣的產物可以是通過給藥化合物經過氧化、還原、水解、醯胺化、脫醯胺作用、酯化、脫脂作用、酶裂解等等方法得到。相應地,本發明包括化合物的代謝產物,包括將本發明的化合物與哺乳動物充分接觸一段時間所產生的代謝產物。"Metabolite" refers to a product obtained by metabolism of a specific compound or salt thereof in vivo. Metabolites of a compound can be identified by conventional techniques in the art, and their activity can be characterized by experimental methods as described herein. Such products may be obtained by subjecting the administered compound to oxidation, reduction, hydrolysis, amidation, deamidation, esterification, delipidation, enzymatic cleavage, and the like. Accordingly, the present invention includes metabolites of compounds, including metabolites produced by contacting a compound of the present invention with a mammal for a sufficient period of time.
本發明所使用的“藥學上可接受的鹽”是指本發明的化合物的有機鹽和無機鹽。藥學上可接受的鹽在所屬技術領域中為通常知識者所熟知的,如文獻:S.M. Berge et al., J. Pharmaceutical Sciences, 66: 1-19, 1977所記載的。藥學上可接受的無毒的酸形成的鹽包括,但並不限於,與氨基基團反應形成的無機酸鹽有鹽酸鹽,氫溴酸鹽,磷酸鹽,硫酸鹽,高氯酸鹽,和有機酸鹽如乙酸鹽,草酸鹽,馬來酸鹽,酒石酸鹽,檸檬酸鹽,琥珀酸鹽,丙二酸鹽,或通過書籍文獻上所記載的其他方法如離子交換法來得到這些鹽。其他藥學上可接受的鹽包括己二酸鹽,藻酸鹽,抗壞血酸鹽,天冬氨酸鹽,苯磺酸鹽,苯甲酸鹽,重硫酸鹽,硼酸鹽,丁酸鹽,樟腦酸鹽,樟腦磺酸鹽,環戊基丙酸鹽,二葡萄糖酸鹽,十二烷基硫酸鹽,乙磺酸鹽,甲酸鹽,反丁烯二酸鹽,葡庚糖酸鹽,甘油磷酸鹽,葡萄糖酸鹽,半硫酸鹽,庚酸鹽,己酸鹽,氫碘酸鹽,2-羥基-乙磺酸鹽,乳糖醛酸鹽,乳酸鹽,月桂酸鹽,月桂基硫酸鹽,蘋果酸鹽,丙二酸鹽,甲磺酸鹽,2-萘磺酸鹽,煙酸鹽,硝酸鹽,油酸鹽,棕櫚酸鹽,撲酸鹽,果膠酸鹽,過硫酸鹽,3-苯基丙酸鹽,苦味酸鹽,特戊酸鹽,丙酸鹽,硬脂酸鹽,硫氰酸鹽,對甲苯磺酸鹽,十一酸鹽,戊酸鹽,等等。通過適當的鹼得到的鹽包括鹼金屬,鹼土金屬,銨和N+ (C1-4 烷基)4 的鹽。本發明也擬構思了任何所包含N的基團的化合物所形成的季銨鹽。水溶性或油溶性或分散產物可以通過季銨化作用得到。鹼金屬或鹼土金屬鹽包括鈉,鋰,鉀,鈣,鎂,等等。藥學上可接受的鹽進一步包括適當的、無毒的銨、季銨鹽和抗平衡離子形成的胺陽離子,如鹵化物,氫氧化物,羧化物,硫酸化物,磷酸化物,硝酸化物,C1-8 磺酸化物和芳香磺酸化物。As used herein, "pharmaceutically acceptable salts" refer to organic and inorganic salts of the compounds of the present invention. Pharmaceutically acceptable salts are well known to those of ordinary skill in the art, as described in SM Berge et al., J. Pharmaceutical Sciences, 66: 1-19, 1977. Pharmaceutically acceptable nontoxic acid salts include, but are not limited to, inorganic acid salts formed by reaction with amino groups including hydrochloride, hydrobromide, phosphate, sulfate, perchlorate, and Organic acid salts such as acetate, oxalate, maleate, tartrate, citrate, succinate, malonate, or obtained by other methods described in books such as ion exchange . Other pharmaceutically acceptable salts include adipate, alginate, ascorbate, aspartate, besylate, benzoate, bisulfate, borate, butyrate, camphorate , camphorsulfonate, cyclopentylpropionate, digluconate, lauryl sulfate, ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate , Gluconate, Hemisulfate, Heptanoate, Caproate, Hydroiodide, 2-Hydroxy-ethanesulfonate, Lacturonate, Lactate, Laurate, Lauryl Sulfate, Malic Acid salt, malonate, mesylate, 2-naphthalenesulfonate, nicotinate, nitrate, oleate, palmitate, pamoate, pectate, persulfate, 3-benzene propionate, picrate, pivalate, propionate, stearate, thiocyanate, p-toluenesulfonate, undecanoate, valerate, etc. Salts obtained with appropriate bases include alkali metal, alkaline earth metal, ammonium and N + (C 1-4 alkyl) 4 salts. The present invention also contemplates the quaternary ammonium salts formed by any compound containing an N group. Water- or oil-soluble or dispersible products can be obtained by quaternization. Alkali metal or alkaline earth metal salts include sodium, lithium, potassium, calcium, magnesium, and the like. Pharmaceutically acceptable salts further include appropriate, non-toxic ammonium, quaternary ammonium salts and amine cations that resist counterion formation, such as halides, hydroxides, carboxylates, sulfates, phosphates, nitrates, C 1- 8 Sulfonates and aromatic sulfonates.
本發明的“溶劑化物”是指一個或多個溶劑分子與本發明的化合物所形成的締合物。形成溶劑化物的溶劑包括,但並不限於,水,異丙醇,乙醇,甲醇,二甲亞碸,乙酸乙酯,乙酸,氨基乙醇。A "solvate" of the present invention refers to an association of one or more solvent molecules with a compound of the present invention. Solvate-forming solvents include, but are not limited to, water, isopropanol, ethanol, methanol, dimethylsulfoxide, ethyl acetate, acetic acid, aminoethanol.
本發明的“水合物”是指溶劑分子是水所形成的締合物。在一些實施例中,一個本發明化合物分子可以與一個水分子相結合,比如一水合物;在另外一些實施例中,一個本發明化合物分子可以與多於一個的水分子相結合,比如二水合物,還有一些實施例中,一個本發明化合物分子可以與少於一個的水分子相結合,比如半水合物。應注意,本發明所述的水合物保留有非水合形式的所述化合物的生物有效性。"Hydrate" in the present invention refers to an association compound in which the solvent molecule is water. In some embodiments, one molecule of a compound of the present invention may be associated with one molecule of water, such as a monohydrate; in other embodiments, a molecule of a compound of the present invention may be associated with more than one molecule of water, such as a dihydrate In yet other embodiments, one molecule of the compound of the present invention may be associated with less than one molecule of water, such as a hemihydrate. It should be noted that the hydrates of the present invention retain the bioavailability of the non-hydrated form of the compounds.
術語“氮氧化物”是指當化合物含幾個胺官能團時,可將1個或大於1個的氮原子氧化形成N -氧化物。N -氧化物的特殊實例是叔胺的N -氧化物或含氮雜環氮原子的N -氧化物。可用氧化劑例如過氧化氫或過酸(例如過氧羧酸)處理相應的胺形成N -氧化物 (參見Advanced Organic Chemistry, Wiley Interscience, 第4版, Jerry March, pages)。尤其是,N -氧化物可用L.W.Deady 的方法製備 (Syn.Comm.1977, 7, 509-514),其中例如在惰性溶劑,例如二氯甲烷中,使胺化合物與間-氯過苯甲酸 (MCPBA) 反應。The term "nitroxide" means that when the compound contains several amine functional groups, one or more nitrogen atoms can be oxidized to form N -oxides. N - Specific examples of oxides are tertiary amine N - oxide or a nitrogen-containing heterocyclic nitrogen atom of N - oxide. The corresponding amines can be treated with oxidizing agents such as hydrogen peroxide or peracids (eg, peroxycarboxylic acids) to form N -oxides (see Advanced Organic Chemistry, Wiley Interscience, 4th edition, Jerry March, pages). In particular, N -oxides can be prepared by the method of LWDeady (Syn. Comm. 1977, 7, 509-514) in which, for example, an amine compound is mixed with m-chloroperbenzoic acid (MCPBA) in an inert solvent such as dichloromethane. ) reaction.
術語“載體”包括任何溶劑,分散介質,包衣衣料,表面活性劑,抗氧化劑,防腐劑 (例如抗細菌劑、抗真菌劑),等滲劑,鹽,藥物穩定劑,黏合劑,賦形劑,分散劑,潤滑劑,甜味劑,調味劑,著色劑,或其組合物,這些載體都是所屬技術領域中具有通常知識者已知的 (如Remington's Pharmaceutical Sciences, 18th Ed. Mack Printing Company, 1990, pp. 1289-1329所述)。除了任意常規載體與活性成分不相容的情況外,涵蓋其在治療或藥物組合物中的用途。The term "carrier" includes any solvents, dispersion media, coatings, surfactants, antioxidants, preservatives (eg, antibacterial, antifungal), isotonic agents, salts, pharmaceutical stabilizers, binders, excipients Agents, dispersing agents, lubricants, sweetening agents, flavoring agents, coloring agents, or combinations thereof, such carriers are known to those of ordinary skill in the art (eg Remington's Pharmaceutical Sciences, 18th Ed. Mack Printing Company , 1990, pp. 1289-1329). Except in the event that any conventional carrier is incompatible with the active ingredient, its use in therapeutic or pharmaceutical compositions is contemplated.
如本發明所使用的術語“治療”任何疾病或病症,在其中一些實施方案中指改善疾病或病症(即減緩或阻止或減輕疾病或其至少一種臨床症狀的發展)。在另一些實施方案中,“治療”指緩和或改善至少一種身體參數,包括可能不為患者所察覺的身體參數。在另一些實施方案中,“治療”指從身體上(例如穩定可察覺的症狀)或生理學上(例如穩定身體的參數)或上述兩方面調節疾病或病症。在另一些實施方案中,“治療”指預防或延遲疾病或病症的發作、發生或惡化。The term "treating" any disease or disorder, as used herein, in some embodiments refers to ameliorating the disease or disorder (ie, slowing or arresting or alleviating the development of the disease or at least one clinical symptom thereof). In other embodiments, "treating" refers to alleviating or improving at least one physical parameter, including a physical parameter that may not be perceived by a patient. In other embodiments, "treating" refers to modulating a disease or disorder, physically (eg, stabilizing an observable symptom) or physiologically (eg, stabilizing a physical parameter), or both. In other embodiments, "treating" refers to preventing or delaying the onset, occurrence or worsening of a disease or disorder.
如本發明所使用的術語“治療有效量”或“治療有效劑量”是指能夠引發個體的生物學或醫學回應 (例如降低或抑制酶或蛋白質活性,或改善症狀、緩解病症、減緩或延遲疾病發展,或預防疾病等) 的本發明化合物的量。在一項非限定性的實施方案中,術語“治療有效量”是指當向個體施用本發明化合物時,對以下情況有效的量:(1) 至少部分地緩解、抑制、預防和/或改善 (i) 與PDE4有關,或者 (ii) 與PDE4活性相關,或者 (iii) 與PDE4的異常活性表徵的病症或疾病;或者 (2) 降低或抑制PDE4的活性;或者 (3) 降低或抑制PDE4的表達。在另一實施方案中,術語“治療有效量”是指當向細胞、或器官、或非細胞生物物質、或介質施用時,能至少部分地降低或抑制PDE4活性;或者至少部分地降低或抑制PDE4表達的有效的本發明化合物的量。The term "therapeutically effective amount" or "therapeutically effective dose" as used herein refers to a biological or medical response in an individual (eg, reducing or inhibiting enzyme or protein activity, or ameliorating symptoms, alleviating a disorder, slowing or delaying a disease) development, or prevention of disease, etc.) of the compound of the present invention. In one non-limiting embodiment, the term "therapeutically effective amount" refers to an amount effective to: (1) at least partially alleviate, inhibit, prevent and/or ameliorate when a compound of the present invention is administered to an individual (i) associated with PDE4, or (ii) associated with PDE4 activity, or (iii) a disorder or disease characterized by abnormal activity of PDE4; or (2) decreased or inhibited the activity of PDE4; or (3) decreased or inhibited PDE4 expression. In another embodiment, the term "therapeutically effective amount" refers to at least partially reducing or inhibiting PDE4 activity; or at least partially reducing or inhibiting PDE4 activity when administered to cells, or organs, or non-cellular biological substances, or mediators The amount of effective compound of the invention expressed by PDE4.
如本發明所使用的術語化合物“給予”和“給藥”化合物應當理解為向需要其的個體提供本發明的化合物或本發明化合物的前藥。應當認識到所屬技術領域中具有通常知識者通過使用有效量的本發明化合物治療目前患有此障礙的患者或者預防性地治療患有此障礙的患者。The terms "administering" and "administering" a compound as used herein are to be understood as providing a compound of the present invention or a prodrug of a compound of the present invention to an individual in need thereof. It will be appreciated that one of ordinary skill in the art can treat patients currently suffering from the disorder or prophylactically treat patients suffering from the disorder by administering an effective amount of a compound of the present invention.
如本發明所使用的術語“組合物”是指包含規定量的規定成分的產物,以及規定量的規定成分的組合所直接或間接地產生的任何產物。與藥物組合物相關的這種術語的含義包括包含活性成分 (單個或者多個) 和組成載體的惰性成分 (單個或者多個) 的產物,以及由任何兩種或多種成分混合、複合或聚集,或者由一種或多種成分分解,或者由一種或多種成分的其他類型的反應或相互作用而直接或間接產生的任何產物。因此,本發明藥物組合物包括通過將本發明化合物與可藥用載體混合而製備的任何組合物。The term "composition" as used herein refers to a product comprising the specified ingredients in the specified amounts, as well as any product that results, directly or indirectly, from combination of the specified ingredients in the specified amounts. The meaning of this term in relation to a pharmaceutical composition includes a product comprising the active ingredient (single or multiple) and inert ingredient (single or multiple) constituting the carrier, as well as being mixed, compounded or aggregated from any two or more ingredients, or any product that results directly or indirectly from the decomposition of one or more components, or from other types of reactions or interactions of one or more components. Accordingly, the pharmaceutical compositions of the present invention include any composition prepared by admixing a compound of the present invention and a pharmaceutically acceptable carrier.
另外,本發明公開的化合物、包括它們的鹽,也可以以它們的水合物形式或包含其溶劑(例如乙醇、DMSO,等等)的形式得到,用於它們的結晶。本發明公開化合物可以與藥學上可接受的溶劑(包括水)固有地或通過設計形成溶劑化物;因此,本發明旨在包括溶劑化的和未溶劑化的形式。Additionally, the compounds disclosed herein, including their salts, may also be obtained in their hydrate form or in a form containing a solvent (eg, ethanol, DMSO, etc.) for their crystallization. Compounds of the present disclosure may form solvates, inherently or by design, with pharmaceutically acceptable solvents, including water; thus, the present invention is intended to encompass both solvated and unsolvated forms.
本發明給出的任何結構式也意欲表示這些化合物未被同位素富集的形式以及同位素富集的形式。同位素富集的化合物具有本發明給出的通式描繪的結構,除了一個或多個原子被具有所選擇原子量或質量數的原子替換。可引入本發明化合物中的示例性同位素包括氫、碳、氮、氧、磷、硫、氟和氯的同位素,如2 H,3 H,11 C,13 C,14 C,15 N,17 O,18 O,18 F,31 P,32 P,35 S,36 Cl和125 I。Any structural formula given herein is also intended to represent both isotopically unenriched as well as isotopically enriched forms of these compounds. Isotopically enriched compounds have the structures depicted by the general formulae given herein, except that one or more atoms are replaced by atoms having a selected atomic weight or mass number. It can be incorporated into compounds of the invention Exemplary isotopes include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, fluorine and chlorine, such as 2 H, 3 H, 11 C , 13 C, 14 C, 15 N, 17 O , 18 O, 18 F, 31 P, 32 P, 35 S, 36 Cl and 125 I.
另一方面,本發明所述化合物包括同位素富集的本發明所定義的化合物,例如,其中存在放射性同位素,如3 H、14 C和18 F 的那些化合物,或者其中存在非放射性同位素,如2 H和13 C。該類同位素富集的化合物可用於代謝研究(使用14 C)、反應動力學研究(使用例如2 H或3 H)、檢測或成像技術,如正電子發射斷層掃描術 (PET) 或包括藥物或底物組織分佈測定的單光子發射電腦斷層成像術 (SPECT),或可用於患者的放療中。18 F富集的化合物對PET或SPECT研究而言是特別理想的。同位素富集的式 (I) 或式 (II) 所示化合物可以通過所屬技術領域中具有通常知識者熟悉的常規技術或本發明中的實施例和製備過程所描述使用合適的同位素標記試劑替代原來使用過的未標記試劑來製備。In another aspect, the compounds of the present invention include isotopically enriched compounds as defined herein, eg those in which radioactive isotopes such as 3 H, 14 C and 18 F are present, or in which non-radioactive isotopes such as 2 H and 13 C. Such isotopically enriched compounds can be used in metabolic studies (using 14 C), reaction kinetic studies (using eg 2 H or 3 H), detection or imaging techniques such as positron emission tomography (PET) or including drugs or Single-photon emission computed tomography (SPECT) for substrate tissue distribution measurement, or may be used in radiation therapy of patients. 18 F-enriched compounds are particularly ideal for PET or SPECT studies. Isotopically enriched compounds of formula (I) or formula (II) can be replaced by suitable isotope-labeled reagents by conventional techniques familiar to those of ordinary skill in the art or as described in the examples and preparation procedures of the present invention Prepared from used unlabeled reagents.
此外,較重同位素特別是氘(即,2 H或D)的取代可提供些許治療優點,這些優點是由代謝穩定性更高帶來的。例如,體內半衰期增加或劑量需求降低或治療指數得到改善帶來的。應當理解,本發明中的氘被看做式 (I) 或式 (II) 所示化合物的取代基。可以用同位素富集因子來定義該類較重同位素特別是氘的濃度。本發明所使用的術語“同位素富集因子”是指所指定同位素的同位素豐度和天然豐度之間的比例。如果本發明化合物的取代基被指定為氘,該化合物對各指定的氘原子而言具有至少3500 (各指定氘原子處52.5%的氘摻入)、至少4000 (60%的氘摻入)、至少4500 (67.5%的氘摻入),至少5000 (75%的氘摻入),至少5500(82.5%的氘摻入)、至少6000 (90%的氘摻入)、至少6333.3 (95%的氘摻入)、至少6466.7 (97%的氘摻入)、至少6600 (99%的氘摻入) 或至少6633.3(99.5%的氘摻入) 的同位素富集因子。本發明可藥用的溶劑化物包括其中結晶溶劑可以是同位素取代的例如D2 O、丙酮-d6 、DMSO-d6 的那些溶劑化物。In addition, substitution with heavier isotopes, particularly deuterium (ie, 2 H or D), may offer some therapeutic advantages that result from greater metabolic stability. For example, increased in vivo half-life or reduced dosage requirements or improved therapeutic index. It should be understood that deuterium in the present invention is regarded as a substituent of the compound represented by formula (I) or formula (II). The isotopic enrichment factor can be used to define the concentration of such heavier isotopes, especially deuterium. The term "isotopic enrichment factor" as used herein refers to the ratio between the isotopic abundance and the natural abundance of a given isotope. If a substituent of a compound of the invention is designated as deuterium, the compound has for each designated deuterium atom at least 3500 (52.5% deuterium incorporation at each designated deuterium atom), at least 4000 (60% deuterium incorporation), at least 4500 (67.5% deuterium incorporated), at least 5000 (75% deuterium incorporated), at least 5500 (82.5% deuterium incorporated), at least 6000 (90% deuterium incorporated), at least 6333.3 (95% deuterium incorporated) Deuterium incorporation), an isotopic enrichment factor of at least 6466.7 (97% deuterium incorporation), at least 6600 (99% deuterium incorporation), or at least 6633.3 (99.5% deuterium incorporation). The present invention can include pharmaceutically acceptable solvates wherein the solvent of crystallization may be isotopically substituted, for example D 2 O, acetone - d 6, DMSO- d 6 solvate of those.
本發明的化合物的描述Description of Compounds of the Invention
本發明涉及新的吡咯烷類化合物和治療特應性皮炎或慢性阻塞性肺病的方法。本發明化合物或包含所述化合物的藥物組合物作為PDE4抑制劑,對特應性皮炎或慢性阻塞性肺病有較好的治療效果。The present invention relates to novel pyrrolidine compounds and methods of treating atopic dermatitis or chronic obstructive pulmonary disease. As a PDE4 inhibitor, the compound of the present invention or the pharmaceutical composition comprising the compound has a good therapeutic effect on atopic dermatitis or chronic obstructive pulmonary disease.
一方面,本發明涉及一種化合物,其為式 (I) 所示的化合物或式 (I) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(I);In one aspect, the present invention relates to a compound, which is a compound represented by formula (I) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (I) , pharmaceutically acceptable salts or their prodrugs: (I);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R b and n has the stated meaning.
在一些實施方案中,X為CH或N。In some embodiments, X is CH or N.
在一些實施方案中,Y為-(CH2 )m -C(=O)-NH-(CRm Rn )p -或-(CH2 )m -C(=O)-O-(CRm Rn )p -;其中,Rm 、Rn 、m和p具有本發明所述的含義。In some embodiments, Y is - (CH 2) m -C ( = O) -NH- (CR m R n) p - or - (CH 2) m -C ( = O) -O- (CR m R n ) p -; wherein R m , R n , m and p have the meanings described in the present invention.
在一些實施方案中,A環為C6-10 芳基或5-10個原子組成的雜芳基。In some embodiments, Ring A is a C 6-10 aryl group or a heteroaryl group consisting of 5-10 atoms.
在一些實施方案中,R1 為氫、氘、-ORa 或-NRc Rd ;其中,Ra 、Rc 和Rd 具有本發明所述的含義。In some embodiments, R 1 is hydrogen, deuterium, -OR a or -NR c R d ; wherein Ra , R c and R d have the meanings described herein.
在一些實施方案中,R2 為C1-4 烷基、鹵代C1-4 烷基、C3-6 環烷基、C3-6 環烷基-C1-4 烷基、5-7個原子組成的雜環基或 (5-7個原子組成的雜環基)-C1-4 烷基;或In some embodiments, R 2 is C 1-4 alkyl, haloC 1-4 alkyl, C 3-6 cycloalkyl, C 3-6 cycloalkyl-C 1-4 alkyl, 5- Heterocyclyl consisting of 7 atoms or (heterocyclyl consisting of 5-7 atoms)-C 1-4 alkyl; or
R2 與Ra 和其相連的原子共同形成5-7個原子組成的雜環基,所述的5-7個原子組成的雜環基任選地被選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、C1-4 烷基、C1-4 烷氧基或鹵代C1-4 烷基的取代基所取代。R 2 and R a and the connected atoms together form a heterocyclic group composed of 5-7 atoms, and the heterocyclic group composed of 5-7 atoms is optionally selected from deuterium, F, Cl, Br, Substituents of I, -OH, -CN, -NH 2 , C 1-4 alkyl, C 1-4 alkoxy or halogenated C 1-4 alkyl.
在一些實施方案中,R3 為氫、氘、C1-6 烷基、C1-6 烷氧基、C2-6 烯基、C2-6 炔基、HC(=O)-、C1-6 烷基-C(=O)-、C1-6 烷基-O-C(=O)-、C1-6 烷基-S(=O)2 -或C1-6 烷基-C(=NH)-,其中所述的C1-6 烷基、C1-6 烷氧基、C2-6 烯基、C2-6 炔基、C1-6 烷基-C(=O)-、C1-6 烷基-S(=O)2 -和C1-6 烷基-C(=NH)-各自獨立任選地被1、2或3個選氘、F、Cl、Br、I、-OH、-CN或-NH2 的取代基所取代。In some embodiments, R 3 is hydrogen, deuterium, C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, HC(=O)-, C 1-6 alkyl-C(=O)-, C 1-6 alkyl-OC(=O)-, C 1-6 alkyl-S(=O) 2 - or C 1-6 alkyl-C (=NH)-, wherein said C 1-6 alkyl, C 1-6 alkoxy, C 2-6 alkenyl, C 2-6 alkynyl, C 1-6 alkyl-C(=O )-, C 1-6 alkyl-S(=O) 2 - and C 1-6 alkyl-C(=NH)- each independently optionally selected from 1, 2 or 3 deuterium, F, Cl, Substituents of Br, I, -OH, -CN or -NH 2 are substituted.
在一些實施方案中,R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 和R9 各自獨立地為氫、氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-4 烷基、C1-4 烷氧基、鹵代C1-4 烷基、C1-4 烷基-C(=O)-或C1-4 烷基-S(=O)2 -。In some embodiments, R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , and R 9 are each independently hydrogen, deuterium, F, Cl, Br, I, -OH, -CN , -NH 2 , -NO 2 , -COOH, C 1-4 alkyl, C 1-4 alkoxy, halogenated C 1-4 alkyl, C 1-4 alkyl -C(=O)- or C 1-4 alkyl-S(=O) 2 -.
在一些實施方案中,Ra 為氫、氘、C1-6 烷基、C2-6 烯基、C2-6 炔基、C3-8 環烷基、5-10個原子組成的雜環基、C6-10 芳基或5-10個原子組成的雜芳基,其中所述的C1-6 烷基、C2-6 烯基、C2-6 炔基、C3-8 環烷基、5-10個原子組成的雜環基、C6-10 芳基和5-10個原子組成的雜芳基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-6 烷基、C1-6 烷氧基、C3-8 環烷基或5-10個原子組成的雜環基的取代基所取代。In some embodiments, R a is hydrogen, deuterium, C 1-6 alkyl, C 2-6 alkenyl, C 2-6 alkynyl, C 3-8 cycloalkyl, heterocyclic consisting of 5-10 atoms Cyclic group, C 6-10 aryl group or heteroaryl group consisting of 5-10 atoms, wherein said C 1-6 alkyl group, C 2-6 alkenyl group, C 2-6 alkynyl group, C 3-8 Cycloalkyl, heterocyclyl of 5-10 atoms, C 6-10 aryl, and heteroaryl of 5-10 atoms are each independently optionally substituted by 1, 2, 3 or 4 atoms selected from deuterium, F, Cl, Br, I, -OH, -CN, -NH 2 , -NO 2 , -COOH, C 1-6 alkyl, C 1-6 alkoxy, C 3-8 cycloalkyl or 5- Substituted by the substituent of a heterocyclic group consisting of 10 atoms.
在一些實施方案中,各Rb 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、氧代、C1-6 烷基、C1-6 烷氧基、C1-6 烷基-OC(=O)-、C1-6 烷基-C(=O)-、C1-6 烷基-S(=O)2 -、C3-8 環烷基-C1-6 烷基、C3-8 環烷基、5-10個原子組成的雜環基、C6-10 芳基、5-10個原子組成的雜芳基、-NRe C(=O)C1-6 烷基、-NRe Rf 、-S(=O)2 -NRe Rf 、-C(=O)-NRe Rf 或-C1-6 烷基-NRe Rf ,其中所述的C1-6 烷基、C1-6 烷氧基、C3-8 環烷基-C1-6 烷基、C3-8 環烷基、5-10個原子組成的雜環基、C6-10 芳基和5-10個原子組成的雜芳基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-6 烷基、C1-6 烷氨基或C1-6 烷氧基的取代基所取代;其中,各Re 和Rf 具有本發明所述的含義。In some embodiments, each R b is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, -NO 2, -COOH, oxo, C 1-6 alkyl, C 1-6 alkoxy, C 1-6 alkyl-OC(=O)-, C 1-6 alkyl-C(=O)-, C 1-6 alkyl-S(=O) 2- , C 3-8 cycloalkyl-C 1-6 alkyl, C 3-8 cycloalkyl, heterocyclic group consisting of 5-10 atoms, C 6-10 aryl group, heteroaryl consisting of 5-10 atoms base, -NR e C(=O)C 1-6 alkyl, -NR e R f , -S(=O) 2 -NR e R f , -C(=O)-NR e R f or -C 1-6 alkyl-NR e R f , wherein said C 1-6 alkyl, C 1-6 alkoxy, C 3-8 cycloalkyl-C 1-6 alkyl, C 3-8 ring Alkyl, heterocyclyl of 5-10 atoms, C 6-10 aryl and heteroaryl of 5-10 atoms are each independently optionally substituted by 1, 2, 3 or 4 atoms selected from deuterium, F , Cl, Br, I, -OH, -CN, -NH 2 , -NO 2 , -COOH, C 1-6 alkyl, C 1-6 alkylamino or C 1-6 alkoxy substituents ; wherein, R e and R f are each have the meaning of the present invention.
在一些實施方案中,Rc 和Rd 各自獨立地為氫、-OH、C1-4 烷基、鹵代C1-4 烷基、C3-6 環烷基-C1-4 烷基、C3-6 環烷基、5-7個原子組成的雜環基、(5-7個原子組成的雜環基)-C1-4 烷基、C1-4 烷基-C(=O)-或C1-4 烷基-S(=O)2 -。In some embodiments, R c and R d are each independently hydrogen, -OH, C 1-4 alkyl, haloC 1-4 alkyl, C 3-6 cycloalkyl-C 1-4 alkyl , C 3-6 cycloalkyl, heterocyclyl composed of 5-7 atoms, (heterocyclyl composed of 5-7 atoms)-C 1-4 alkyl, C 1-4 alkyl-C (= O)- or C 1-4 alkyl-S(=O) 2 -.
在一些實施方案中,Re 和Rf 各自獨立地為氫、C1-6 烷基、C1-6 烷基-OC(=O)-、C1-6 烷基-C(=O)-、C1-6 烷基-S(=O)2 -、C3-8 環烷基、C6-10 芳基、5-10個原子組成的雜環基、5-10個原子組成的雜芳基、11-15個原子組成的雜芳基、C3-8 環烷基-C1-6 烷基、C6-10 芳基-C1-6 烷基、(5-10個原子組成的雜環基)-C1-6 烷基、(5-10個原子組成的雜芳基)-C1-6 烷基或-C1-6 烷基-NRg Rj ;或者Re 和Rf 與它們相連的N原子一起形成4-7個原子組成的雜環基;其中,所述的Re 、Rf 和4-7個原子組成的雜環基各自獨立任選地被1、2、3或4個Rh 所取代;In some embodiments, R e and R f are each independently hydrogen, C 1-6 alkyl, C 1-6 alkyl-OC(=O)-, C 1-6 alkyl-C(=O) -, C 1-6 alkyl-S(=O) 2 -, C 3-8 cycloalkyl, C 6-10 aryl, 5-10 atoms heterocyclic group, 5-10 atoms Heteroaryl, heteroaryl consisting of 11-15 atoms, C 3-8 cycloalkyl-C 1-6 alkyl, C 6-10 aryl-C 1-6 alkyl, (5-10 atoms composed of heterocyclyl)-C 1-6 alkyl, (heteroaryl composed of 5-10 atoms)-C 1-6 alkyl or -C 1-6 alkyl-NR g R j ; or R e together with R f and their connected N atoms to form a heterocyclic group composed of 4-7 atoms; wherein, the heterocyclic groups composed of R e , R f and 4-7 atoms are each independently optionally replaced by 1 , 2, 3 or 4 R h ;
其中,各Rg 、Rj 和Rh 具有本發明所述的含義。Wherein, each of R g , R j and R h has the meaning described in the present invention.
在一些實施方案中,各Rh 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、C1-4 烷基、C1-4鹵代 烷基、C1-4鹵代烷氧 基或C1-4 烷氧基。In some embodiments, each R h is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, C 1-4 alkyl, C 1-4 haloalkyl -alkyl, C 1 -4 haloalkoxy or C 1-4 alkoxy.
Rg 和Rj 各自獨立地為氫、C1-4 烷基、C1-4 烷基-OC(=O)-、C1-4 烷基-C(=O)-、C1-4 烷基-S(=O)2 -、C3-6 環烷基、C6-10 芳基、5-6個原子組成的雜環基或5-6個原子組成的雜芳基。R g and R j are each independently hydrogen, C 1-4 alkyl, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-C(=O)-, C 1-4 Alkyl-S(=O) 2 -, C 3-6 cycloalkyl, C 6-10 aryl, heterocyclic group consisting of 5-6 atoms or heteroaryl group consisting of 5-6 atoms.
在一些實施方案中,Rm 和Rn 各自獨立地為氫、氘、F、Cl、Br、I、-OH、-CN、-NH2 、C1-4 烷基、鹵代C1-4 烷基或-C(=O)NH2 。In some embodiments, R m and R n are each independently hydrogen, deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, C 1-4 alkyl, halo C 1-4 alkyl or -C (= O) NH 2.
在一些實施方案中,各n獨立地為0、1、2、3或4。In some embodiments, each n is independently 0, 1, 2, 3, or 4.
在一些實施方案中,m和p各自獨立地為0、1、2或3。In some embodiments, m and p are each independently 0, 1, 2, or 3.
在另一些實施方案中,A環為苯基、萘基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基、噁唑基、吲哚基、異吲哚基、、、、或。In other embodiments, Ring A is phenyl, naphthyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, oxa azolyl, indolyl, isoindolyl, , , , or .
在另一些實施方案中,R3 為氫、氘、C1-4 烷基、C1-4 烷氧基、C2-4 烯基、C2-4 炔基、C1-4 烷基-C(=O)-、C1-4 烷基-O-C(=O)-、HC(=O)-、C1-4 烷基-S(=O)2 -或C1-4 烷基-C(=NH)-,其中所述的C1-4 烷基、C1-4 烷氧基、C2-4 烯基、C2-4 炔基、C1-4 烷基-C(=O)-、C1-4 烷基-O-C(=O)-、C1-4 烷基-S(=O)2 -和C1-4 烷基-C(=NH)-各自獨立任選地被1、2或3個選自氘、F、Cl、Br、I、-OH、-CN或-NH2 的取代基所取代。In other embodiments, R 3 is hydrogen, deuterium, C 1-4 alkyl, C 1-4 alkoxy, C 2-4 alkenyl, C 2-4 alkynyl, C 1-4 alkyl- C(=O)-, C 1-4 alkyl-OC(=O)-, HC(=O)-, C 1-4 alkyl-S(=O) 2 - or C 1-4 alkyl- C(=NH)-, wherein said C 1-4 alkyl, C 1-4 alkoxy, C 2-4 alkenyl, C 2-4 alkynyl, C 1-4 alkyl-C(= O)-, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-S(=O) 2 - and C 1-4 alkyl-C(=NH)- are each independently optional 1, 2 or 3 substituents selected from deuterium, F, Cl, Br, I , -OH, -CN , or -NH 2 to substituents.
在另一些實施方案中,Ra 為氫、氘、C1-4 烷基、C2-4 烯基、C2-4 炔基、C3-6 環烷基、5-6個原子組成的雜環基、苯基或5-6個原子組成的雜芳基,其中,所述的C1-4 烷基、C2-4 烯基、C2-4 炔基、C3-6 環烷基、5-6個原子組成的雜環基、苯基和5-6個原子組成的雜芳基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-4 烷基、C1-4 烷氧基、C3-6 環烷基或5-6個原子組成的雜環基的取代基所取代。In other embodiments, R a is hydrogen, deuterium, C 1-4 alkyl, C 2-4 alkenyl, C 2-4 alkynyl, C 3-6 cycloalkyl, consisting of 5-6 atoms Heterocyclic group, phenyl group or heteroaryl group consisting of 5-6 atoms, wherein the C 1-4 alkyl group, C 2-4 alkenyl group, C 2-4 alkynyl group, C 3-6 cycloalkane group base, 5-6 atoms heterocyclyl, phenyl and 5-6 atoms heteroaryl are each independently optionally substituted by 1, 2, 3 or 4 atoms selected from deuterium, F, Cl, Br, I, -OH, -CN, -NH 2 , -NO 2 , -COOH, C 1-4 alkyl, C 1-4 alkoxy, C 3-6 cycloalkyl or a hetero group consisting of 5-6 atoms substituted by the substituent of the cyclic group.
在另一些實施方案中,各Rb 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、氧代、C1-4 烷基、C1-4 烷氧基、C1-4 烷基-OC(=O)-、C1-4 烷基-C(=O)-、C1-4 烷基-S(=O)2 -、C3-6 環烷基-C1-4 烷基、C3-6 環烷基、5-6個原子組成的雜環基、苯基、5-6個原子組成的雜芳基、-NRe C(=O)C1-4 烷基、-NRe Rf 、-S(=O)2 -NRe Rf 、-C(=O)-NRe Rf 或-C1-4 烷基-NRe Rf ,其中所述的C1-4 烷基、C1-4 烷氧基、C3-6 環烷基-C1-4 烷基、C3-6 環烷基、苯基、5-6個原子組成的雜環基和5-6個原子組成的雜芳基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、C1-4 烷基、C1-4 烷氨基或C1-4 烷氧基的取代基所取代;其中,各Re 和Rf 具有本發明所述的含義。In other embodiments, each R b is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, -NO 2, -COOH, oxo, C 1-4 alkyl, C 1-4 alkoxy, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-C(=O)-, C 1-4 alkyl-S(=O) 2- , C 3-6 cycloalkyl-C 1-4 alkyl, C 3-6 cycloalkyl, heterocyclic group consisting of 5-6 atoms, phenyl, heteroaryl group consisting of 5-6 atoms, - NR e C(=O)C 1-4 alkyl, -NR e R f , -S(=O) 2 -NR e R f , -C(=O)-NR e R f or -C 1-4 Alkyl-NR e R f , wherein said C 1-4 alkyl, C 1-4 alkoxy, C 3-6 cycloalkyl-C 1-4 alkyl, C 3-6 cycloalkyl, Phenyl, heterocyclyl of 5-6 atoms and heteroaryl of 5-6 atoms are each independently optionally substituted by 1, 2, 3 or 4 atoms selected from deuterium, F, Cl, Br, I, -OH, -CN, -NH 2, -NO 2, -COOH, C 1-4 alkyl, substituted C 1-4 alkylamino or C 1-4 alkoxy substituents; wherein each of R e and R f has the meaning described in the present invention.
在另一些實施方案中,Re 和Rf 各自獨立地為氫、C1-4 烷基、C1-4 烷基-OC(=O)-、C1-4 烷基-C(=O)-、C1-4 烷基-S(=O)2 -、C3-6 環烷基、C6-10 芳基、5-7個原子組成的雜環基、5-7個原子組成的雜芳基、14-15個原子組成的雜芳基、C3-6 環烷基-C1-4 烷基、C6-10 芳基-C1-4 烷基、(5-7個原子組成的雜環基)-C1-4 烷基、(5-7個原子組成的雜芳基)-C1-4 烷基或-C1-4 烷基-NRg Rj ;或者Re 和Rf 與它們相連的N原子一起形成4-6個原子組成的雜環基;其中所述的Re 、Rf 和4-6個原子組成的雜環基各自獨立任選地被1、2、3或4個Rh 所取代;其中,各Rh 、Rg 和Rj 具有本發明所述的含義。In other embodiments, R e and R f are each independently hydrogen, C 1-4 alkyl, C 1-4 alkyl-OC(=O)-, C 1-4 alkyl-C(=O )-, C 1-4 alkyl-S(=O) 2 -, C 3-6 cycloalkyl, C 6-10 aryl, heterocyclic group composed of 5-7 atoms, composed of 5-7 atoms Heteroaryl, heteroaryl consisting of 14-15 atoms, C 3-6 cycloalkyl-C 1-4 alkyl, C 6-10 aryl-C 1-4 alkyl, (5-7 atomic heterocyclyl)-C 1-4 alkyl, (5-7 atom heteroaryl)-C 1-4 alkyl or -C 1-4 alkyl-NR g R j ; or R e and R f together with the N atom to which they are attached form a heterocyclic group consisting of 4-6 atoms; wherein said R e , R f and the heterocyclic group consisting of 4-6 atoms are each independently optionally replaced by 1 , 2, 3 or 4 R h ; wherein, each R h , R g and R j has the meaning described in the present invention.
在又一些實施方案中,R2 為甲基、乙基、正丙基、異丙基、-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 CH2 CH2 F、-CH2 CH2 CHF2 、-CH2 CH2 CF3 、-CF2 CH2 CH3 、-CH2 Cl、-CHCl2 、環丙基、環丁基、環戊基、環己基、環丙基甲基、環丁基甲基、環戊基甲基、環己基甲基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、四氫吡喃基甲基、四氫呋喃基甲基或吡咯烷基甲基;或In still other embodiments, R 2 is methyl, ethyl, n-propyl, isopropyl, -CH 2 F, -CHF 2, -CF 3, -CH 2 CH 2 F, -CH 2 CHF 2, -CH 2 CF 3, -CF 2 CH 3, -CH 2 CH 2 CH 2 F, -CH 2 CH 2 CHF 2, -CH 2 CH 2 CF 3, -CF 2 CH 2 CH 3, -CH 2 Cl, -CHCl 2 , cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl, piperazinyl, piperidinyl, morpholine thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, tetrahydropyranylmethyl, tetrahydrofuranylmethyl or pyrrolidinylmethyl; or
R2 與Ra 和其相連的原子共同形成1,3-二氧雜環戊烯()、1,3-二氧雜環己烯、2,3-二氫-1,4,2-二噁嗪烯或1,5,3-二氧氮雜環庚烯,所述的1,3-二氧雜環戊烯、1,3-二氧雜環己烯、2,3-二氫-1,4,2-二噁嗪烯或1,5,3-二氧氮雜環庚烯獨立任選地被選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、-CHF2 、-CF3 、-CH2 CHF2 、-CH2 CF3 或-CF2 CH3 的取代基所取代。And R a and R 2 thereof atom together form 1,3-dioxole ( ), 1,3-dioxene, 2,3-dihydro-1,4,2-dioxazine or 1,5,3-dioxane, said 1, 3-dioxole, 1,3-dioxene, 2,3-dihydro-1,4,2-dioxazine or 1,5,3-dioxazepan alkenyl optionally substituted with independently selected from deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropoxy, -CHF 2 , -CF 3 , -CH 2 CHF 2 , -CH 2 CF 3 or -CF 2 CH 3 of substituents.
在又一些實施方案中,R3 為氫、氘、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、乙烯基、1-丙烯基、2-丙烯基、乙炔基、1-丙炔基、3-丙炔基、HC(=O)-、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-O-C(=O)-、乙基-O-C(=O)-、正丙基-O-C(=O)-、異丙基-O-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、甲基-C(=NH)-、乙基-C(=NH)-、正丙基-C(=NH)-或異丙基-C(=NH)-;In still other embodiments, R 3 is hydrogen, deuterium, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy group, n-propoxy, isopropoxy, vinyl, 1-propenyl, 2-propenyl, ethynyl, 1-propynyl, 3-propynyl, HC(=O)-, methyl- C(=O)-, ethyl-C(=O)-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-OC(=O)-, ethyl Base-OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2- , n-propyl-S(=O) 2- , isopropyl-S(=O) 2- , methyl-C(=NH)-, ethyl-C(=NH)-, n-propyl -C(=NH)- or isopropyl-C(=NH)-;
其中,所述的甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、乙烯基、1-丙烯基、2-丙烯基、乙炔基、1-丙炔基、3-丙炔基、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-O-C(=O)-、乙基-O-C(=O)-、正丙基-O-C(=O)-、異丙基-O-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、甲基-C(=NH)-、乙基-C(=NH)-、正丙基-C(=NH)-和異丙基-C(=NH)-各自獨立任選地被1、2或3個選自氘、F、Cl、Br、I、-OH、-CN或-NH2 的取代基所取代。Among them, the methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropyl Oxy, vinyl, 1-propenyl, 2-propenyl, ethynyl, 1-propynyl, 3-propynyl, methyl-C(=O)-, ethyl-C(=O)- , n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-OC(=O)-, ethyl-OC(=O)-, n-propyl-OC(= O)-, isopropyl-OC(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2 -, n-propyl-S(=O) 2 -, isopropyl Propyl-S(=O) 2 -, methyl-C(=NH)-, ethyl-C(=NH)-, n-propyl-C(=NH)- and isopropyl-C(=NH)- ) - are each independently optionally substituted with 1, 2 or 3 substituents selected from deuterium, F, Cl, Br, I , -OH, -CN , or -NH 2 to a substituent group.
在又一些實施方案中,R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 和R9 各自獨立地為氫、氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、甲基、乙基、正丙基、異丙基、甲氧基、乙氧基、正丙氧基、異丙氧基、-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 CH2 CH2 F、-CH2 CH2 CHF2 、-CH2 CH2 CF3 、-CF2 CH2 CH3 、-CH2 Cl、-CHCl2 、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -或異丙基-S(=O)2 -。In yet other embodiments, R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , and R 9 are each independently hydrogen, deuterium, F, Cl, Br, I, -OH, - CN, -NH 2 , -NO 2 , -COOH, methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy, n-propoxy, iso-propoxy, -CH 2 F, -CHF 2 , -CF 3 , -CH 2 CH 2 F, -CH 2 CHF 2 , -CH 2 CF 3 , -CF 2 CH 3 , -CH 2 CH 2 CH 2 F, -CH 2 CH 2 CHF 2 , -CH 2 CH 2 CF 3, -CF 2 CH 2 CH 3, -CH 2 Cl, -CHCl 2, methyl -C (= O) -, ethyl -C (= O) -, n-propyl -C (=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2 -, n-propyl-S(=O) 2 - or isopropyl-S(=O) 2 -.
在又一些實施方案中,Ra 為氫、氘、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、乙烯基、1-丙烯基、2-丙烯基、乙炔基、1-丙炔基、3-丙炔基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、苯基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基或噁唑基;In still other embodiments, R a is hydrogen, deuterium, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, vinyl, 1-propenyl base, 2-propenyl, ethynyl, 1-propynyl, 3-propynyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, sulfur morpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, phenyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl , thiazolyl or oxazolyl;
其中,所述的甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、乙烯基、1-丙烯基、2-丙烯基、乙炔基、1-丙炔基、3-丙炔基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、苯基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基和噁唑基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、甲基、乙基、正丙基、異丙基、甲氧基、乙氧基、正丙氧基、異丙氧基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基或吡咯烷基的取代基所取代。Among them, the methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, vinyl, 1-propenyl, 2-propenyl, ethynyl , 1-propynyl, 3-propynyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, thiomorpholinyl, tetrahydropyran , tetrahydrofuranyl, pyrrolidinyl, phenyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, and oxazolyl are each independently optionally substituted with 1,2, 3 or 4 substituents selected from deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, -NO 2, -COOH, methyl, ethyl, n-propyl , isopropyl, methoxy, ethoxy, n-propoxy, isopropoxy, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, Substituents of thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl or pyrrolidinyl.
在又一些實施方案中,各Rb 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、氧代、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、甲基-OC(=O)-、乙基-OC(=O)-、正丙基-OC(=O)-、異丙基-OC(=O)-、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、環丙基甲基、環丙基乙基、環丙基正丙基、環丙基異丙基、環丁基甲基、環戊基甲基、環己基甲基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、苯基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基、噁唑基、-NRe C(=O)甲基、-NRe C(=O)乙基、-NRe C(=O)正丙基、-NRe C(=O)異丙基、-NRe Rf 、-S(=O)2 -NRe Rf 、-C(=O)-NRe Rf 、-甲基-NRe Rf 、-乙基-NRe Rf 、-正丙基-NRe Rf 或-異丙基-NRe Rf ;In still other embodiments, each R b is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, -NO 2, -COOH, oxo, methyl, ethyl, n Propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy, n-propoxy, iso-propoxy, methyl-OC(=O)- , ethyl-OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-C(=O)-, ethyl-C(=O )-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(=O) 2 -, n-propyl -S(=O) 2 -, isopropyl-S(=O) 2 -, cyclopropylmethyl, cyclopropylethyl, cyclopropyl-n-propyl, cyclopropylisopropyl, cyclobutylmethyl , cyclopentylmethyl, cyclohexylmethyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, thiomorpholinyl, tetrahydropyranyl , tetrahydrofuranyl, pyrrolidinyl, phenyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, -NR e C(=O) methyl, -NR e C(=O) ethyl, -NR e C(=O) n-propyl, -NR e C(=O) isopropyl, -NR e R f , -S(=O) 2 -NR e R f , -C(=O)-NR e R f , -methyl-NR e R f , -ethyl-NR e R f , -n-propyl-NR e R f or -isopropyl-NR e R f ;
其中,所述的甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲氧基、乙氧基、正丙氧基、異丙氧基、環丙基甲基、環丙基乙基、環丙基正丙基、環丙基異丙基、環丁基甲基、環戊基甲基、環己基甲基、環丙基、環丁基、環戊基、環己基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、苯基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基和噁唑基各自獨立任選地被1、2、3或4個選自氘、F、Cl、Br、I、-OH、-CN、-NH2 、-NO2 、-COOH、甲基、乙基、正丙基、異丙基、甲氨基、二甲氨基、甲氧基、乙氧基、正丙氧基或異丙氧基的取代基所取代;Among them, the methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropyl Oxy, cyclopropylmethyl, cyclopropylethyl, cyclopropyl-n-propyl, cyclopropylisopropyl, cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl, cyclopropyl, cyclobutyl base, cyclopentyl, cyclohexyl, piperazinyl, piperidinyl, morpholinyl, thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, phenyl, pyridyl, pyrimidinyl, pyridyl Azinyl, pyridazinyl, furyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl and oxazolyl are each independently optionally substituted by 1, 2, 3 or 4 selected from deuterium, F, Cl , Br, I, -OH, -CN, -NH 2 , -NO 2 , -COOH, methyl, ethyl, n-propyl, isopropyl, methylamino, dimethylamino, methoxy, ethoxy , n-propoxy or isopropoxy substituent;
其中,各Re 和Rf 具有本發明所述的含義。Wherein, R e and R f are each have the meaning of the present invention.
在又一些實施方案中,Rc 和Rd 各自獨立地為氫、-OH、甲基、乙基、正丙基、異丙基、-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 CH2 CH2 F、-CH2 CH2 CHF2 、-CH2 CH2 CF3 、-CF2 CH2 CH3 、-CH2 Cl、-CHCl2 、環丙基甲基、環丙基乙基、環丁基甲基、環戊基甲基、環己基甲基、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -或異丙基-S(=O)2 -。In still other embodiments, R c and R d are each independently hydrogen, -OH, methyl, ethyl, n-propyl, isopropyl, -CH 2 F, -CHF 2, -CF 3, -CH 2 CH 2 F, -CH 2 CHF 2, -CH 2 CF 3, -CF 2 CH 3, -CH 2 CH 2 CH 2 F, -CH 2 CH 2 CHF 2, -CH 2 CH 2 CF 3, -CF 2 CH 2 CH 3, -CH 2 Cl, -CHCl 2, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl, methyl -C (= O) -, ethyl-C(=O)-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S( =O) 2 -, n-propyl-S(=O) 2 - or isopropyl-S(=O) 2 -.
在又一些實施方案中,Re 和Rf 各自獨立地為氫、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲基-OC(=O)-、乙基-OC(=O)-、正丙基-OC(=O)-、異丙基-OC(=O)-、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、環丙基、環丁基、環戊基、環己基、苯基、萘基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基、噁唑基、環丙基甲基、環丙基乙基、環丙基正丙基、環丙基異丙基、環丁基甲基、環戊基甲基、環己基甲基、苯甲基、苯乙基、5-7個原子組成的雜環基-C1-4 烷基、吡啶基甲基、嘧啶基甲基、呋喃基甲基、噻吩基甲基、吡咯基甲基、吡唑基甲基、咪唑基甲基、噻唑基甲基、噁唑基甲基、-甲基-NRg Rj 、-乙基-NRg Rj 、-正丙基-NRg Rj 、-異丙基-NRg Rj 或;In still other embodiments, R e and R f are each independently hydrogen, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methyl -OC(=O)-, ethyl-OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-C(=O)-, Ethyl-C(=O)-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(=O ) 2 -, n-propyl-S(=O) 2 -, isopropyl-S(=O) 2 -, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl, naphthyl, piper Azinyl, piperidinyl, morpholinyl, thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, Pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, cyclopropylmethyl, cyclopropylethyl, cyclopropyl-n-propyl, cyclopropylisopropyl, cyclobutylmethyl, cyclopentyl Methyl, cyclohexylmethyl, benzyl, phenethyl, heterocyclyl-C 1-4 alkyl of 5-7 atoms, pyridylmethyl, pyrimidinylmethyl, furylmethyl, thiophene group, a pyrrolyl group, a pyrazolyl group, imidazolyl group, thiazolyl group, oxazolyl group, - methyl -NR g R j, - ethyl -NR g R j, - n-propyl-NR g R j , -isopropyl-NR g R j or ;
或者Re 和Rf 與它們相連的N原子一起形成氮雜環丁基、吡咯烷基、哌啶基、嗎啉基、硫代嗎啉基或哌嗪基;or R e and R f taken together with the N atom to which they are attached form azetidinyl, pyrrolidinyl, piperidinyl, morpholinyl, thiomorpholinyl or piperazinyl;
其中,所述的Re 、Rf 、氮雜環丁基、吡咯烷基、哌啶基、嗎啉基、硫代嗎啉基和哌嗪基各自獨立任選地被1、2、3或4個Rh 所取代;Wherein said R e, R f, azetidinyl, pyrrolidinyl, piperidinyl, morpholinyl, thiomorpholinyl and piperazinyl each independently optionally substituted with 1,2, 3 or Replaced by 4 R h;
其中,各Rh 、Rg 和Rj 具有本發明所述的含義。Wherein, each of R h , R g and R j has the meaning described in the present invention.
在又一些實施方案中,各Rh 獨立地為氘、F、Cl、Br、I、-OH、-CN、-NH2 、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、三氟甲基、三氟甲氧基、甲氧基、乙氧基、正丙氧基、異丙氧基、正丁氧基、異丁氧基、仲丁氧基或叔丁氧基。In still other embodiments, each R h is independently deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, methyl, ethyl, n-propyl, isopropyl, n-butyl , isobutyl, sec-butyl, tert-butyl, trifluoromethyl, trifluoromethoxy, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy group, sec-butoxy or tert-butoxy.
在又一些實施方案中,Rg 和Rj 各自獨立地為氫、甲基、乙基、正丙基、異丙基、正丁基、異丁基、仲丁基、叔丁基、甲基-OC(=O)-、乙基-OC(=O)-、正丙基-OC(=O)-、異丙基-OC(=O)-、甲基-C(=O)-、乙基-C(=O)-、正丙基-C(=O)-、異丙基-C(=O)-、甲基-S(=O)2 -、乙基-S(=O)2 -、正丙基-S(=O)2 -、異丙基-S(=O)2 -、環丙基、環丁基、環戊基、環己基、苯基、哌嗪基、哌啶基、嗎啉基、硫代嗎啉基、四氫吡喃基、四氫呋喃基、吡咯烷基、吡啶基、嘧啶基、吡嗪基、噠嗪基、呋喃基、噻吩基、吡咯基、吡唑基、咪唑基、噻唑基或噁唑基。In yet other embodiments, R g and R j are each independently hydrogen, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, methyl -OC(=O)-, ethyl-OC(=O)-, n-propyl-OC(=O)-, isopropyl-OC(=O)-, methyl-C(=O)-, Ethyl-C(=O)-, n-propyl-C(=O)-, isopropyl-C(=O)-, methyl-S(=O) 2 -, ethyl-S(=O ) 2 -, n-propyl-S(=O) 2 -, isopropyl-S(=O) 2 -, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl, piperazinyl, piperidinyl, morpholinyl, thiomorpholinyl, tetrahydropyranyl, tetrahydrofuranyl, pyrrolidinyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, pyrrolyl, Pyrazolyl, imidazolyl, thiazolyl or oxazolyl.
在又一些實施方案中,Rm 和Rn 各自獨立地為氫、氘、F、Cl、Br、I、-OH、-CN、-NH2 、甲基、乙基、正丙基、異丙基、-CH2 F、-CHF2 、-CF3 、-CH2 CH2 F、-CH2 CHF2 、-CH2 CF3 、-CF2 CH3 、-CH2 Cl、-CHCl2 或-C(=O)NH2 。In still other embodiments, R m and R n are each independently hydrogen, deuterium, F, Cl, Br, I , -OH, -CN, -NH 2, methyl, ethyl, n-propyl, isopropyl, group, -CH 2 F, -CHF 2, -CF 3, -CH 2 CH 2 F, -CH 2 CHF 2, -CH 2 CF 3, -CF 2 CH 3, -CH 2 Cl, -CHCl 2 , or - C (= O) NH 2.
另一方面,本發明涉及一種化合物,其為式 (II) 所示的化合物或式 (II) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(II);In another aspect, the present invention relates to a compound, which is a compound represented by formula (II) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (II) Products, pharmaceutically acceptable salts or their prodrugs: (II);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Ra 、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R a , R b and n has meaning in the present invention.
另一方面,本發明涉及一種化合物,其為式 (III) 所示的化合物或式 (III) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(III);In another aspect, the present invention relates to a compound, which is a compound represented by formula (III) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (III) Products, pharmaceutically acceptable salts or their prodrugs: (III);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Ra 、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R a , R b and n has meaning in the present invention.
另一方面,本發明涉及一種化合物,其為式 (IV) 所示的化合物或式 (IV) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(IV);In another aspect, the present invention relates to a compound which is a compound represented by formula (IV) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (IV) Products, pharmaceutically acceptable salts or their prodrugs: (IV);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Ra 、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R a , R b and n has meaning in the present invention.
另一方面,本發明涉及一種化合物,其為式 (V) 所示的化合物或式 (V) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(V);In another aspect, the present invention relates to a compound which is a compound represented by formula (V) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (V) Products, pharmaceutically acceptable salts or their prodrugs: (V);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Ra 、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R a , R b and n has meaning in the present invention.
另一方面,本發明涉及一種化合物,其為式 (VI) 所示的化合物或式 (VI) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(VI);In another aspect, the present invention relates to a compound which is a compound represented by formula (VI) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (VI) Products, pharmaceutically acceptable salts or their prodrugs: (VI);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Ra 、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R a , R b and n has meaning in the present invention.
另一方面,本發明涉及一種化合物,其為式 (VII) 所示的化合物或式 (VII) 所示化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(VII);In another aspect, the present invention relates to a compound, which is a compound represented by formula (VII) or a stereoisomer, tautomer, nitrogen oxide, hydrate, solvate, metabolite of the compound represented by formula (VII) Products, pharmaceutically acceptable salts or their prodrugs: (VII);
其中,各X、Y、R1 、R2 、R3 、R4 、R5a 、R5b 、R6 、R7a 、R7b 、R8 、R9 、A、Ra 、Rb 和n具有本發明所述的含義。wherein each of X, Y, R 1 , R 2 , R 3 , R 4 , R 5a , R 5b , R 6 , R 7a , R 7b , R 8 , R 9 , A, R a , R b and n has meaning in the present invention.
在一些實施方案中,本發明包含但絕不限於具有下列之一結構的化合物或具有下列之一結構化合物的立體異構體、互變異構體、氮氧化物、水合物、溶劑化物、代謝產物、藥學上可接受的鹽或它們的前藥:(1)、(2)、(3)、(4)、(5)、(6)、(7)、(8)、(9)、(10)、(11)、(12)、(13)、(14)、(15)、(16)、(17)、(18)、(19)、(20)、(21)、(22)、(23)、(24)、(25)、(26)、(27)、(28)、(29)、(30)、(31)、(32)、(33)、(34)、(35)、(36)、(37)、(38)、(39)、(40)、(41)、(42)、(43)、(44)、(45)、(46)、(47)、(48)、(49)、(50)、(51)、(52)、(53)、(54)、(55)、(56)、(57)、(58)、(59)、(60)、(61)、(62)、(63)、(64)、(65)、(66)、(67)、(68)、(69)、(70)、(71)、(72)、(73)、(74)、(75)、(76)、(77)、(78)、(79)、(80)、(81)、(82)、(83)、(84)、(85)、(86)、(87)、(88)、(89)、(90)、(91)、(92)、(93)、(94)、(95)、(96)、(97)、(98)、(99)、(100)、(101)、(102)、(103)、(104)、(105)、(106)、(107)、(108)、(109)、(110)、(111)、(112)、(113)、(114)、(115)、(116)、(117)、(118)、(119)、(120)、(121)、(122)、(123)、(124)、(125)、(126)、(127)、(128)、(129)、(130)、(131)、(132)、(133)、(134)、(135)、(136)、(137)、(138)、(139)、(140)、(141)、(142)、(143)、(144)、(145)、(146)、(147)、(148)、(149)、(150)、(151)、(152)、(153)、(154)、(155)、(156)、(157)、(158)、(159)、(160)、(161)、(162)、(163)、(164)、(165)、(166)、(167)、(168)、(169)、(170)、(171)、(172)、(173)、(174)、(175)、(176)、(177)、(178)、(179)、(180)、(181)、(182)、(183)、(184)、(185)、(186)、(187)、(188)、(189)、(190)、(191)、(192)、(193)、(194)、(195)、(196)、(197)、(198)、(199)、(200)、(201)、(202)、(203)、(204)、(205)、(206)、(207)、(208)、(209)、(210)、(211)、(212)、(213)、(214)、(215)、(216)、(217)、(218)、(219)、(220)、(221)、(222)、(223)、(224)、(225)、(226)、(227)、(228)、(229)、(230)、(231)、(232)、(233)、(234)、(235)、(236)、(237)、(238)、(239)、(240)、(241) 或(242)。In some embodiments, the present invention includes, but is in no way limited to, compounds having one of the following structures or stereoisomers, tautomers, nitrogen oxides, hydrates, solvates, metabolites of compounds having one of the following structures , pharmaceutically acceptable salts or their prodrugs: (1), (2), (3), (4), (5), (6), (7), (8), (9), (10), (11), (12), (13), (14), (15), (16), (17), (18), (19), (20), (twenty one), (twenty two), (twenty three), (twenty four), (25), (26), (27), (28), (29), (30), (31), (32), (33), (34), (35), (36), (37), (38), (39), (40), (41), (42), (43), (44), (45), (46), (47), (48), (49), (50), (51), (52), (53), (54), (55), (56), (57), (58), (59), (60), (61), (62), (63), (64), (65), (66), (67), (68), (69), (70), (71), (72), (73), (74), (75), (76), (77), (78), (79), (80), (81), (82), (83), (84), (85), (86), (87), (88), (89), (90), (91), (92), (93), (94), (95), (96), (97), (98), (99), (100), (101), (102), (103), (104), (105), (106), (107), (108), (109), (110), (111), (112), (113), (114), (115), (116), (117), (118), (119), (120), (121), (122), (123), (124), (125), (126), (127), (128), (129), (130), (131), (132), (133), (134), (135), (136), (137), (138), (139), (140), (141), (142), (143), (144), (145), (146), (147), (148), (149), (150), (151), (152), (153), (154), (155), (156), (157), (158), (159), (160), (161), (162), (163), (164), (165), (166), (167), (168), (169), (170), (171), (172), (173), (174), (175), (176), (177), (178), (179), (180), (181), (182), (183), (184), (185), (186), (187), (188), (189), (190), (191), (192), (193), (194), (195), (196), (197), (198), (199), (200), (201), (202), (203), (204), (205), (206), (207), (208), (209), (210), (211), (212), (213), (214), (215), (216), (217), (218), (219), (220), (221), (222), (223), (224), (225), (226), (227), (228), (229), (230), (231), (232), (233), (234), (235), (236), (237), (238), (239), (240), (241) or (242).
在一些實施方案中,本發明式 (I) 所示的化合物,其藥學上可接受的鹽是鹽酸鹽、氫溴酸鹽、硫酸鹽、硝酸鹽、磷酸鹽、乙酸鹽、馬來酸鹽、琥珀酸鹽、扁桃酸鹽、富馬酸鹽、丙二酸鹽、蘋果酸鹽、2-羥基丙酸鹽、丙酮酸鹽、草酸鹽、羥乙酸鹽、水楊酸鹽、葡萄糖醛酸鹽、半乳糖醛酸鹽、枸櫞酸鹽、酒石酸鹽、門冬氨酸鹽、谷氨酸鹽、苯甲酸鹽、肉桂酸鹽、對甲苯磺酸鹽、苯磺酸鹽、甲磺酸鹽、乙磺酸鹽、三氟甲磺酸鹽或它們的組合。In some embodiments, the compound represented by formula (I) of the present invention, its pharmaceutically acceptable salt is hydrochloride, hydrobromide, sulfate, nitrate, phosphate, acetate, maleate , succinate, mandelate, fumarate, malonate, malate, 2-hydroxypropionate, pyruvate, oxalate, glycolate, salicylate, glucuronic acid Salt, galacturonate, citrate, tartrate, aspartate, glutamate, benzoate, cinnamate, p-toluenesulfonate, benzenesulfonate, methanesulfonate salts, ethanesulfonates, triflates, or combinations thereof.
一方面,本發明涉及一種藥物組合物,所述藥物組合物包含本發明公開的式 (I)、(II)、(III)、(IV)、(V) 或 (VI) 所述的化合物。In one aspect, the present invention relates to a pharmaceutical composition comprising a compound of formula (I), (II), (III), (IV), (V) or (VI) disclosed herein.
在一些實施方案中,本發明所述的藥物組合物,其進一步地包含藥學上可接受的載體、賦形劑、附加劑、輔劑、媒介物或它們的任意組合。In some embodiments, the pharmaceutical composition of the present invention further comprises a pharmaceutically acceptable carrier, excipient, adjuvant, adjuvant, vehicle or any combination thereof.
在另一些實施方案中,本發明所述的藥物組合物,進一步地包含附加治療劑,其中所述的附加治療劑是:丙酮酸鈉、多索茶鹼、替托司特、泰魯司特、茶鹼、福莫特羅、沙美特羅、氟替卡松丙酸酯、咯利普蘭、吡拉米斯特、西洛司特、茚達特羅、奧達特羅、米地司坦、齊流通、沙丁醇胺、卡莫昔羅、布地奈德、二丙酸倍氯米松、曲安奈德、氟尼縮松、糠酸莫米松、羅氟奈德、環索奈德、異丙托溴銨、氧托溴銨、噻托溴銨、格隆溴銨、蕪地溴銨、維蘭特羅、阿地溴銨、Benralizumab、Tralokinumab、瑞伐托酯、克瑞沙硼、氟輕鬆醋酸酯、去羥米松、莫美他松、曲安西龍、倍他米松、阿氯米松、地索奈德、氫化可的松、氯倍他索(clobatsol)、鹵貝他索、二氟拉松、美普克萊、他克莫司、吡美莫司、他紮羅汀、環孢素、阿普斯特、E-6005、OPA-15406、LEO-29102、DRM02、羅氟司特、異丁司特、托法替尼、JTE-052、巴瑞替尼、烏帕替尼、WBI-1001、MRX-6、GSK2981278、杜魯單抗、來金珠單抗、尼莫利珠單抗、曲洛吉努單抗、依那西普、阿達木單抗、英夫利昔單抗、尤特可單抗、塞庫吉努、奧馬珠、CIM-331、戈利木單抗和聚乙二醇化賽、妥珠單抗、卡泊三醇、骨化三醇、阿利維A酸、VTP-38543、ZPL-389、阿瑞匹坦、曲地匹坦、弗維普蘭特、、OC-459、SUN 13834、SB-011、VTP-43742、ARN6039、TAK-828、JTE-451、PF-04965842、PF-06651600、PF-06700841、PF-06650833、GR-MD-02或它們的任意組合。In other embodiments, the pharmaceutical composition of the present invention further comprises an additional therapeutic agent, wherein the additional therapeutic agent is: sodium pyruvate, doxofylline, tetomilast, telukast , theophylline, formoterol, salmeterol, fluticasone propionate, rolipram, piramister, cilomilast, indacaterol, odaterol, midestane, qifluu , salbutamol, camoxirol, budesonide, beclomethasone dipropionate, triamcinolone acetonide, flunisolide, mometasone furoate, roflunomide, ciclesonide, ipratropium bromide Ammonium, oxtropium, tiotropium, glycopyrronium, umeclidinium, vilanterol, aclidinium, benralizumab, Tralokinumab, revatropate, cresabor, fluocinolone acetate , Desoxymetasone, Mometasone, Triamcinolone, Betamethasone, Aclometasone, Desonide, Hydrocortisone, Clobetasol (clobatsol), Halobetasol, Diflurasone, Meptocline, Tacrolimus, Pimecrolimus, Tazarotene, Cyclosporine, Apremilast, E-6005, OPA-15406, LEO-29102, DRM02, Roflumilast, Ibuprofen Sturt, tofacitinib, JTE-052, baricitinib, upatinib, WBI-1001, MRX-6, GSK2981278, durumumab, lekinizumab, nimolizumab, Traloginumab, etanercept, adalimumab, infliximab, ustekinumab, secukinumab, omalizumab, CIM-331, golimumab, and pegylated acepirin, tocilizumab, calcipotriol, calcitriol, alicitretin, VTP-38543, ZPL-389, aprepitant, tripitant, faviprant, OC-459, SUN 13834, SB-011, VTP-43742, ARN6039, TAK-828, JTE-451, PF-04965842, PF-06651600, PF-06700841, PF-06650833, GR-MD-02 or any combination thereof.
另一方面,本發明涉及本發明公開的式 (I)、(II)、(III)、(IV)、(V) 或 (VI) 所示化合物或其藥物組合物在製備藥物中的用途,其中,所述藥物用於預防、治療或減輕與4型磷酸二酯酶(PDE4)有關的疾病。On the other hand, the present invention relates to the use of the compounds of formula (I), (II), (III), (IV), (V) or (VI) disclosed in the present invention or their pharmaceutical compositions in the preparation of medicines, Wherein, the medicine is used for preventing, treating or alleviating diseases related to phosphodiesterase type 4 (PDE4).
在一些實施方案中,所述與4型磷酸二酯酶有關的疾病為呼吸疾病、過敏、炎症、中樞神經系統疾病或非胰島素依賴糖尿病。In some embodiments, the disease associated with phosphodiesterase type 4 is respiratory disease, allergy, inflammation, central nervous system disease, or non-insulin dependent diabetes mellitus.
在另一些實施方案中,所述的呼吸疾病為:慢性阻塞性肺疾病、肺氣腫、哮喘、慢性肺炎、塵肺病、支氣管炎、支氣管擴張症、肺結核纖維化病變、肺囊性纖維化、急性呼吸窘迫綜合症或呼吸道炎症;其中支氣管炎包括急性支氣管炎、慢性支氣管炎、變應性支氣管炎、彌漫性泛細支氣管炎或閉塞性細支氣管炎;In other embodiments, the respiratory disease is: chronic obstructive pulmonary disease, emphysema, asthma, chronic pneumonia, pneumoconiosis, bronchitis, bronchiectasis, pulmonary tuberculosis fibrosis, cystic fibrosis, Acute respiratory distress syndrome or inflammation of the airways; where bronchitis includes acute bronchitis, chronic bronchitis, allergic bronchitis, diffuse panbronchiolitis, or bronchiolitis obliterans;
其中,所述的炎症為:過敏性結膜炎、特應性皮炎、過敏性皮炎、類風濕性關節炎、間質性膀胱炎、變應性鼻炎、潰瘍性結腸炎、強直性脊柱炎、風濕性關節炎或銀屑病關節炎。Wherein, the inflammation is: allergic conjunctivitis, atopic dermatitis, allergic dermatitis, rheumatoid arthritis, interstitial cystitis, allergic rhinitis, ulcerative colitis, ankylosing spondylitis, rheumatism Arthritis or psoriatic arthritis.
本發明另一方面涉及式 (I)、(II)、(III)、(IV)、(V) 或 (VI) 所示的化合物的製備、分離和純化的方法。Another aspect of the present invention relates to methods for the preparation, isolation and purification of compounds of formula (I), (II), (III), (IV), (V) or (VI).
另一方面,本發明涉及製備式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII) 所示化合物的中間體。In another aspect, the present invention relates to intermediates for the preparation of compounds of formula (I), (II), (III), (IV), (V), (VI) or (VII).
本發明公開化合物可含有不對稱或手性中心,因此可以不同的立體異構體形式存在。本發明旨在使式 (I) 所示化合物的所有立體異構體形式,包括但不限於非對映異構體、對映異構體、阻轉異構體和幾何 (或構象) 異構體,以及它們的混合物如外消旋混合物,成為本發明的組成部分。Compounds of the present disclosure may contain asymmetric or chiral centers and, therefore, may exist in different stereoisomeric forms. The present invention is intended to make all stereoisomeric forms of the compounds of formula (I), including but not limited to diastereomers, enantiomers, atropisomers and geometric (or conformational) isomers Compounds, as well as mixtures thereof such as racemic mixtures, form part of the present invention.
在本發明公開的結構中,當任意特定的手性原子的立體化學未指明時,則該結構的所有立體異構體都考慮在本發明之內,並且作為本發明公開化合物包括在本發明中。當立體化學被表示特定構型的實楔形線 (solid wedge) 或虛線指明時,則該結構的立體異構體就此明確和定義。In a structure disclosed herein, when the stereochemistry of any particular chiral atom is not specified, then all stereoisomers of that structure are contemplated within the present invention and are included in the present invention as compounds disclosed herein . When stereochemistry is indicated by a solid wedge or dashed line representing a particular configuration, then the stereoisomers of that structure are identified and defined.
式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII)所示化合物可以以不同的互變異構體形式存在,並且所有這些互變異構體,都包括在本發明範圍內。Compounds of formula (I), (II), (III), (IV), (V), (VI) or (VII) may exist in different tautomeric forms, and all these tautomers, All are included in the scope of the present invention.
本發明化合物的藥物組合物、製劑和給藥PHARMACEUTICAL COMPOSITIONS, FORMULATIONS AND ADMINISTRATION OF THE COMPOUNDS OF THE INVENTION
本發明提供一種藥物組合物,包括式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII) 所示化合物或其單獨的立體異構體,異構體的外消旋或非外消旋混合物或其藥學上可接受的鹽或溶劑化物。在本發明的一個實施方式中,所述藥物組合物進一步包含至少一種藥學上可接受的載體、賦形劑或吸附劑,以及任選地,其他的治療和/或預防成分。The present invention provides a pharmaceutical composition comprising a compound represented by formula (I), (II), (III), (IV), (V), (VI) or (VII) or its individual stereoisomer, isomeric Racemic or non-racemic mixtures of isomers or pharmaceutically acceptable salts or solvates thereof. In one embodiment of the present invention, the pharmaceutical composition further comprises at least one pharmaceutically acceptable carrier, excipient or adsorbent, and optionally, other therapeutic and/or prophylactic ingredients.
合適的載體、賦形劑或吸附劑對於所屬技術領域中具有通常知識者是熟知的並且詳細描述於例如Ansel H. C. et al., Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (2004) Lippincott, Williams & Wilkins, Philadelphia;Gennaro A. R. et al., Remington:The Science and Practice of Pharmacy (2000) Lippincott, Williams & Wilkins, Philadelphia;和Rowe R. C., Handbook of Pharmaceutical Excipients (2005) Pharmaceutical Press, Chicago中。Suitable carriers, excipients or adsorbents are well known to those of ordinary skill in the art and are described in detail in, for example, Ansel HC et al., Ansel's Pharmaceutical Dosage Forms and Drug Delivery Systems (2004) Lippincott, Williams & Wilkins, Philadelphia; Gennaro AR et al., Remington: The Science and Practice of Pharmacy (2000) Lippincott, Williams & Wilkins, Philadelphia; and Rowe RC, Handbook of Pharmaceutical Excipients (2005) Pharmaceutical Press, Chicago.
本發明所用“藥學上可接受的賦形劑”意指與給藥劑型或藥物組合物一致性相關的藥學上可接受的材料、混合物或溶媒。每種賦形劑在混合時必須與藥物組合物的其他成分相容,以避免對患者給藥時會大大降低本發明公開化合物的功效的相互作用和會導致不是藥學上可接受的藥物組合物的相互作用。此外,每種賦形劑必須是藥學上可接受的,例如,具有足夠高的純度。"Pharmaceutically acceptable excipient" as used in the present invention means a pharmaceutically acceptable material, mixture or vehicle that is relevant to the consistency of the dosage form or pharmaceutical composition for administration. Each excipient must, when mixed, be compatible with the other ingredients of the pharmaceutical composition to avoid interactions that would greatly reduce the efficacy of the disclosed compounds when administered to a patient and would result in a pharmaceutical composition that is not pharmaceutically acceptable Interaction. In addition, each excipient must be pharmaceutically acceptable, eg, of sufficiently high purity.
合適的藥學上可接受的賦形劑會依所選具體劑型而不同。此外,可根據它們在組合物中的特定功能來選擇藥學上可接受的賦形劑。例如,可選擇能有助於生產均一劑型的些許藥學上可接受的賦形劑。可選擇能有助於生產穩定劑型的些許藥學上可接受的賦形劑。可選擇對患者給藥時有助於攜帶或運輸本發明化合物從身體的一個器官或部分到身體的另一個器官或部分的些許藥學上可接受的賦形劑。可選擇增強患者依從性的些許藥學上可接受的賦形劑。Suitable pharmaceutically acceptable excipients will vary depending on the particular dosage form chosen. Furthermore, pharmaceutically acceptable excipients can be selected based on their particular function in the composition. For example, a number of pharmaceutically acceptable excipients can be selected to aid in the production of a uniform dosage form. A number of pharmaceutically acceptable excipients may be selected to aid in the production of stable dosage forms. A number of pharmaceutically acceptable excipients may be selected which aid in carrying or transporting the compounds of the present invention from one organ or part of the body to another organ or part of the body when administered to a patient. Several pharmaceutically acceptable excipients may be selected to enhance patient compliance.
一些合適的賦型劑實例包括乳糖、葡萄糖、蔗糖、山梨糖醇、甘露糖醇、澱粉、阿拉伯膠、磷酸鈣、藻酸鹽、西黃蓍膠、明膠、矽酸鈣、微晶纖維素、聚乙烯吡咯烷酮、纖維素、水、糖漿和甲基纖維素。合適的藥學上可接受的賦形劑還包括以下類型的賦形劑:溶媒、拋射劑、增溶劑、助溶劑、乳化劑、著色劑、黏合劑、崩解劑、填充劑、潤滑劑、潤濕劑、滲透壓調節劑、穩定劑、助流劑、矯味劑、防腐劑、助懸劑、包衣材料、芳香劑、抗黏著劑、抗氧劑、螯合劑、滲透促進劑、pH 調節劑、增塑劑、表面活性劑、發泡劑、消泡劑、增稠劑、包合劑、保濕劑、吸收劑、稀釋劑、絮凝劑與反絮凝劑和助濾劑。具有通常知識者可認識到,些許藥學上可接受的賦形劑可提供不只一種功能,並提供可供選擇的功能,這取決於製劑中存在多少該賦形劑和製劑中存在哪些其他賦形劑。可以採用所屬技術領域的已知方法來配製本發明化合物,以便對患者給藥後能快速、持續或延緩釋放出活性組份。Some examples of suitable excipients include lactose, glucose, sucrose, sorbitol, mannitol, starch, acacia, calcium phosphate, alginate, tragacanth, gelatin, calcium silicate, microcrystalline cellulose, Polyvinylpyrrolidone, cellulose, water, syrup and methylcellulose. Suitable pharmaceutically acceptable excipients also include the following types of excipients: vehicles, propellants, solubilizers, solubilizers, emulsifiers, colorants, binders, disintegrants, fillers, lubricants, lubricants, etc. Wetting agent, osmotic pressure regulator, stabilizer, glidant, flavoring agent, preservative, suspending agent, coating material, fragrance, anti-adherent, antioxidant, chelating agent, penetration enhancer, pH adjuster , plasticizers, surfactants, foaming agents, defoaming agents, thickeners, inclusion agents, humectants, absorbents, diluents, flocculants and deflocculants and filter aids. Those of ordinary skill will recognize that some pharmaceutically acceptable excipients may serve more than one function, and may provide alternative functions, depending on how much of that excipient is present in the formulation and what other excipients are present in the formulation agent. The compounds of the present invention may be formulated using methods known in the art to provide rapid, sustained or delayed release of the active ingredient after administration to a patient.
技術人員掌握所屬技術領域的知識和技能,以使他們能選擇用於本發明的適當量的合適的藥學上可接受的賦形劑。此外,存在大量技術人員可獲得的資源,他們描述藥學上可接受的賦形劑,並用於選擇合適的藥學上可接受的賦形劑。實例包括Remington's Pharmaceutical Sciences (Mack Publishing Company), The Handbook of Pharmaceutical Additives (Gower Publishing Limited), and The Handbook of Pharmaceutical Excipients (the American Pharmaceutical Association and the Pharmaceutical Press)。Skilled artisans possess the knowledge and skills in the art to enable them to select appropriate amounts of suitable pharmaceutically acceptable excipients for use in the present invention. In addition, there are numerous resources available to the skilled artisan describing pharmaceutically acceptable excipients and for use in selecting suitable pharmaceutically acceptable excipients. Examples include Remington's Pharmaceutical Sciences (Mack Publishing Company), The Handbook of Pharmaceutical Additives (Gower Publishing Limited), and The Handbook of Pharmaceutical Excipients (the American Pharmaceutical Association and the Pharmaceutical Press).
在Remington: The Science and Practice of Pharmacy, 21st edition, 2005, ed. D.B. Troy, Lippincott Williams & Wilkins, Philadelphia, and Encyclopedia of Pharmaceutical Technology, eds. J. Swarbrick and J. C. Boylan, 1988-1999, Marcel Dekker, New York中披露了用於配置藥學上可接受的組合物的各種載體,和用於其製備的通常技術,這些文獻各自的內容通過引用併入本發明。除任何諸如因產生任何不期望的生物作用,或以有害方式與藥學上可接受組合物中的任何其他成分發生相互作用而與本發明化合物不相容的任何常用載體外,關注其應用屬於本發明的範圍。In Remington: The Science and Practice of Pharmacy, 21st edition, 2005, ed. DB Troy, Lippincott Williams & Wilkins, Philadelphia, and Encyclopedia of Pharmaceutical Technology, eds. J. Swarbrick and JC Boylan, 1988-1999, Marcel Dekker, New Various carriers for formulating pharmaceutically acceptable compositions, and general techniques for their preparation, are disclosed in York, the contents of each of which are incorporated herein by reference. Except for any conventional carrier that is incompatible with the compounds of the present invention, such as by producing any undesired biological effect, or interacting in a deleterious manner with any other ingredient of the pharmaceutically acceptable composition, concerns for its use are within the scope of this scope of invention.
合適的藥學上可接受的載體取決於藥物形式並且是所屬技術領域中具有通常知識者所知的。Suitable pharmaceutically acceptable carriers depend on the pharmaceutical form and are known to those of ordinary skill in the art.
如本發明中使用的,“藥學上可接受的載體”包括任何和全部的溶劑和溶劑混合物、塗層、絡合劑、固體載體、分散體介質、表面活性賦形劑、抗細菌和抗真菌藥,用於藥物活性物質的等滲和吸收延遲劑,和其混合物,這些同樣是所屬技術領域中已知的。As used herein, "pharmaceutically acceptable carrier" includes any and all solvents and solvent mixtures, coatings, complexing agents, solid carriers, dispersion media, surface active excipients, antibacterial and antifungal agents , isotonic and absorption delaying agents for pharmaceutically active substances, and mixtures thereof, which are likewise known in the art.
用於藥學上可接受的載體的非限制性實例包括具有選自如下組分的那些:乳糖,明膠,糖醇 (例如澱粉,甘露醇,玉米澱粉等),植物油,滑石,硬脂酸鎂,膠體二氧化矽,羧甲基纖維素,微晶纖維素,十二烷硫酸鈉,緩衝水溶液,共聚維酮,聚山梨酸酯,乙醇,丙二醇,聚二醇 (優選地聚乙二醇,例如PEG400),Tween®80 (即PEG (20),山梨糖醇一油酸酯),DMSO,水和助溶劑的混合物,例如包括醇如乙醇和/或聚二醇如聚乙二醇的水溶液,多元醇如甘油和/或聚乙二醇與脂肪酸的酯,表面活性劑如陰離子、陽離子、非離子和兩性表面活性劑,絡合劑如環糊精,例如α-環糊精 (α-CD) 或者羥丙基-β-環糊精 (HP-β-CD),膽汁酸或者脂質,例如動物或者植物磷脂的鹽,成膠束劑,和油如玉米油,或前面提及的兩種或更多種組分的混合物。Non-limiting examples for pharmaceutically acceptable carriers include those having components selected from the group consisting of lactose, gelatin, sugar alcohols (eg, starch, mannitol, cornstarch, etc.), vegetable oils, talc, magnesium stearate, Colloidal silica, carboxymethyl cellulose, microcrystalline cellulose, sodium lauryl sulfate, aqueous buffer solutions, copovidone, polysorbate, ethanol, propylene glycol, polyethylene glycol (preferably polyethylene glycol, such as PEG400), Tween® 80 (i.e. PEG(20), sorbitan monooleate), DMSO, mixtures of water and cosolvents, for example aqueous solutions including alcohols such as ethanol and/or polyglycols such as polyethylene glycol, Polyols such as glycerol and/or polyethylene glycol esters with fatty acids, surfactants such as anionic, cationic, nonionic and amphoteric surfactants, complexing agents such as cyclodextrins, for example alpha-cyclodextrin (alpha-CD) or hydroxypropyl-beta-cyclodextrin (HP-beta-CD), bile acids or lipids, such as salts of animal or vegetable phospholipids, micelle-forming agents, and oils such as corn oil, or both or Mixtures of more components.
以下提及可用於本發明的藥物組合物的進一步的合適的藥學上可接受的載體以及合適的添加劑的非限制性實例。Non-limiting examples of further suitable pharmaceutically acceptable carriers and suitable additives that can be used in the pharmaceutical compositions of the present invention are mentioned below.
在一個實施方案中,本發明涉及本發明的藥物組合物,其在水介質中形成基於脂質的藥物輸送系統(DDS)。所述藥物組合物,除式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII) 所示的化合物中的至少一種化合物或其鹽以外,還包括至少一種表面活性劑。合適的表面活性劑的非限制性實例是如上所述的。在各種實施方案中,基於脂質的藥物輸送系統形成以下結構:(1)脂質體 (即水中層狀相的分散閉合的雙層組裝體);(2)非層狀相 (例如立方體、六角形、海綿狀物) 的奈米顆粒;或(3)膠束、乳狀液、微乳狀液 (即脂質和表面活性劑的簡單自組裝結構)。In one embodiment, the present invention relates to a pharmaceutical composition of the present invention that forms a lipid-based drug delivery system (DDS) in an aqueous medium. The pharmaceutical composition, in addition to at least one compound or a salt thereof among the compounds represented by formula (I), (II), (III), (IV), (V), (VI) or (VII), also At least one surfactant is included. Non-limiting examples of suitable surfactants are described above. In various embodiments, lipid-based drug delivery systems form the following structures: (1) liposomes (ie, dispersed closed bilayer assemblies of lamellar phases in water); (2) non-lamellar phases (eg, cubic, hexagonal) , sponge) nanoparticles; or (3) micelles, emulsions, microemulsions (ie simple self-assembled structures of lipids and surfactants).
在一些實施方案中,形成膠束、乳狀液或者微乳狀液的基於脂質的藥物輸送系統是優選的。用於形成膠束、乳狀液或微乳狀液的合適的表面活性劑或者表面活性劑混合物的親水親油平衡值 (HLB-值) 一般為約8-18,約10-18,或約12- 16。基於脂質的藥物輸送系統形成自乳化藥物輸送系統 (SEDDS) 或者自微乳化藥物輸送系統 (SMEDDS)。SEDDS和SMEDDS是油 (即脂質,例如式 (I) 的化合物或者其鹽)、至少一種表面活性劑、任選地至少一種助溶劑和任選地至少一種助表面活性劑的混合物,理想地各向同性的,在溫和的攪拌下當被引入水相時,其自發地乳化而形成水包油乳化劑。溫和的攪拌可以例如由胃的活動性提供。In some embodiments, lipid-based drug delivery systems that form micelles, emulsions, or microemulsions are preferred. Suitable surfactants or surfactant mixtures for forming micelles, emulsions, or microemulsions generally have a hydrophilic-lipophilic balance (HLB-value) of about 8-18, about 10-18, or about 12-16. Lipid-based drug delivery systems form self-emulsifying drug delivery systems (SEDDS) or self-microemulsifying drug delivery systems (SMEDDS). SEDDS and SMEDDS are mixtures of oils (ie lipids, such as compounds of formula (I) or salts thereof), at least one surfactant, optionally at least one co-solvent and optionally at least one co-surfactant, ideally each Isotropic, it spontaneously emulsifies to form oil-in-water emulsifiers when introduced into the aqueous phase with gentle agitation. Gentle agitation may be provided, for example, by gastric motility.
本發明公開的藥物組合物使用所屬技術領域中具有通常知識者已知的技術和方法來製備。所屬技術領域一些常用方法的描述可參見Remington's Pharmaceutical Sciences (Mack Publishing Company)。The pharmaceutical compositions disclosed herein are prepared using techniques and methods known to those of ordinary skill in the art. A description of some commonly used methods in the art can be found in Remington's Pharmaceutical Sciences (Mack Publishing Company).
因此,另一方面,本發明涉及製備藥物組合物的工藝,所述藥物組合物包含本發明公開化合物和藥學上可接受的賦形劑、載體、輔劑、溶媒或它們的組合,該工藝包括混合各種成分。包含本發明公開化合物的藥物組合物,可以在例如環境溫度和大氣壓下混合來製備。Accordingly, in another aspect, the present invention relates to a process for preparing a pharmaceutical composition comprising a compound disclosed herein and a pharmaceutically acceptable excipient, carrier, adjuvant, vehicle or combination thereof, the process comprising Mix various ingredients. Pharmaceutical compositions containing compounds of the present disclosure can be prepared by mixing, for example, at ambient temperature and atmospheric pressure.
本發明公開的化合物通常被配製成適合於通過所需途徑對患者給藥的劑型。例如,所述劑型包括那些適合於以下給藥途徑的劑型:(1)口服給藥,例如片劑、膠囊劑、囊片劑、丸劑、含片劑、粉劑、糖漿劑、酏劑、混懸劑、溶液劑、乳劑、香包劑和扁囊劑;(2)胃腸外給藥,例如無菌溶液劑、混懸劑和複溶粉末;(3)透皮給藥,例如透皮貼片劑;(4)直腸給藥,例如栓劑;(5)吸入,例如氣霧劑、溶液劑和乾粉劑;和(6)局部給藥,例如乳膏劑、油膏劑、洗劑、溶液劑、糊劑、噴霧劑、泡沫劑和凝膠劑。The compounds disclosed herein are generally formulated in a dosage form suitable for administration to a patient by the desired route. For example, the dosage forms include those suitable for the following routes of administration: (1) oral administration, such as tablets, capsules, caplets, pills, troches, powders, syrups, elixirs, suspensions (2) parenteral administration, such as sterile solutions, suspensions and reconstituted powders; (3) transdermal administration, such as transdermal patches (4) rectal administration, such as suppositories; (5) inhalation, such as aerosols, solutions, and dry powders; and (6) topical administration, such as creams, ointments, lotions, solutions, pastes , sprays, foams and gels.
將各種固體口服劑型用於本發明化合物的給藥,例如片劑、膠囊、顆粒、錠劑和散裝粉末的固體劑型。可以將本發明化合物單獨給藥或與所屬技術領域已知的各種藥學上可接受的載體和賦形劑(例如,蔗糖、甘露醇、乳糖、澱粉)組合給藥,包括但不限於助懸劑、增溶劑、緩衝劑、黏合劑、崩解劑、防腐劑、著色劑、調味劑、潤滑劑等。定時釋放膠囊、片劑和凝膠劑對於本發明化合物的給藥也是有利的。Various solid oral dosage forms are used for the administration of the compounds of the present invention, such as solid dosage forms of tablets, capsules, granules, lozenges and bulk powders. The compounds of the present invention can be administered alone or in combination with various pharmaceutically acceptable carriers and excipients known in the art (eg, sucrose, mannitol, lactose, starch), including but not limited to suspending agents , solubilizers, buffers, binders, disintegrants, preservatives, colorants, flavors, lubricants, etc. Timed release capsules, tablets and gels are also advantageous for the administration of the compounds of the present invention.
可以將各種外用劑型用於本發明化合物的給藥,例如洗劑、軟膏劑、酊劑、擦劑、醑劑、粉劑、霜劑、油劑、糊劑、硬膏劑、塗膜劑和氣霧劑。局部給藥還可以包括通過例如透皮貼片的方式進行的透皮給藥。可以將本發明化合物單獨給藥或與所屬技術領域已知的各種藥學上可接受的載體、稀釋劑和賦形劑組合給藥,包括但不限於溶劑、油性溶劑、稀釋劑、安定劑、吸收延遲劑、崩散劑、乳化劑、抗氧化劑、黏合劑、結合劑、增黏劑、增溶劑、分散劑、懸乳化劑、潤滑劑、吸濕劑、脂質體、微乳和β-環糊精等。Various topical dosage forms can be used to administer the compounds of this invention, such as lotions, ointments, tinctures, liniments, elixirs, powders, creams, oils, pastes, plasters, films and aerosols. Topical administration may also include transdermal administration by means of, for example, transdermal patches. The compounds of the present invention can be administered alone or in combination with various pharmaceutically acceptable carriers, diluents and excipients known in the art, including but not limited to solvents, oily Delaying agents, disintegrating agents, emulsifiers, antioxidants, binders, binders, tackifiers, solubilizers, dispersants, suspoemulsifiers, lubricants, hygroscopic agents, liposomes, microemulsions and beta-cyclodextrins Wait.
對於呼吸道疾病的治療,優選本發明的化合物通過吸入給藥。For the treatment of respiratory diseases, the compounds of the present invention are preferably administered by inhalation.
可吸入製備物包括可吸入的粉劑、含推進劑的計量氣霧劑或不含推進劑的可吸入製劑。為此,可以以粉劑(最好為微粒化形式)直接給藥,或通過含有它們的噴霧溶液劑或混懸液給藥。Inhalable preparations include inhalable powders, propellant-containing metered-dose aerosols or propellant-free inhalable formulations. For this purpose, they can be administered directly as powders, preferably in micronized form, or by spray solutions or suspensions containing them.
可以向本發明的粉末化合物加入賦形劑或載體,所述賦形劑或載體通常是無毒的並且對於本發明的化合物為化學惰性的,例如乳糖或適合於改善可呼吸部分的任何其他添加劑。An excipient or carrier, which is generally non-toxic and chemically inert to the compound of the present invention, such as lactose or any other additive suitable for improving the respirable moiety, may be added to the powder compound of the present invention.
包含氣體推進劑例如氫氟烷烴的吸入氣霧劑可以包含溶液或分散型形式的本發明化合物。推進劑驅動的製劑還可以包含其他成分,例如共溶劑、穩定劑和任選的其他賦形劑。Inhalation aerosols containing a propellant gas such as a hydrofluoroalkane may contain a compound of the present invention in solution or dispersed form. The propellant-driven formulation may also contain other ingredients such as co-solvents, stabilizers, and optionally other excipients.
含本發明化合物的不含推進劑的可吸入製劑可以是在含水介質、醇類介質或含水酒精介質中的溶液或懸浮液形式,並且它們可以通過現有技術已知的噴射霧化器或超聲霧化器遞送,或者通過細霧霧化器(soft-mist nebulizers)例如Respimat® 遞送。The propellant-free inhalable formulations containing the compounds of the present invention may be in the form of solutions or suspensions in aqueous, alcoholic or aqueous alcoholic media, and they may be produced by spray nebulizers or ultrasonic mists as known in the art. gasifier delivery, by a fine mist or nebulizer (soft-mist nebulizers) e.g. Respimat ® delivery.
本發明所使用的術語“治療有效量”是指足以顯示出有益的治療效果的各活性組分的總量。例如,給藥或使體內達到平衡的足以治療、治癒或減輕疾病的症狀的量。特殊的治療方案所需的有效量依賴於多種因素,包括治療的疾病,疾病的嚴重程度,使用的特定藥物的活性,給藥方式,特定藥物的清除率,治療持續時間,聯合用藥,年齡,體重,性別,飲食和病人的健康等。所屬技術領域關於“治療有效量”需要考慮的其他因素的描述可參見Gilman et al., eds., Goodman And Gilman’s: The Pharmacological Bases of Therapeutics, 8th ed., Pergamon Press, 1990; Remington's Pharmaceutical Sciences, 17th ed., Mack Publishing Company, Easton, Pa., 1990。The term "therapeutically effective amount" as used herein refers to the total amount of each active ingredient sufficient to exhibit a beneficial therapeutic effect. For example, an amount sufficient to treat, cure or reduce symptoms of a disease is administered or brought into balance in the body. The effective amount required for a particular treatment regimen will depend on a variety of factors, including the disease being treated, the severity of the disease, the activity of the particular drug used, the mode of administration, the clearance of the particular drug, the duration of treatment, concomitant medications, age, Weight, gender, diet and patient's health, etc. Those skilled in the description of other factors need to be considered a "therapeutically effective amount" can be found in Gilman et al, eds, Goodman And Gilman's: The Pharmacological Bases of Therapeutics, 8 th ed, Pergamon Press, 1990; Remington's Pharmaceutical Sciences,... 17 th ed., Mack Publishing Company , Easton, Pa., 1990.
本發明的化合物的劑量取決於多種因素,包括要治療的具體疾病、症狀的嚴重程度、給藥途徑、劑量間隔頻率、所用的具體化合物、化合物的效力、毒理學特徵和藥代動力學的特徵。The dosage of a compound of the present invention will depend on a variety of factors, including the specific disease to be treated, the severity of the symptoms, the route of administration, the frequency of dosage intervals, the specific compound used, the potency of the compound, its toxicological profile and its pharmacokinetics. feature.
可與載體材料相組合從而產生單劑量形式的活性成分的量將取決於被治療的宿主和特定的施用方式而變化。例如,意欲塗抹施用給人的製劑可以方便地含有約5 mg至約250 mg/千克體重/天的活性劑,其與合適且方便的量的載體材料(可佔總組合物的大約5%至大約95%)相複合。單位劑量形式一般將包含大約1 mg至大約500 mg的活性成分。The amount of active ingredient that can be combined with a carrier material to produce a single dosage form will vary depending upon the host being treated and the particular mode of administration. For example, formulations intended for smearing to humans may conveniently contain from about 5 mg to about 250 mg/kg body weight/day of the active agent in combination with a suitable and convenient amount of carrier material (which may range from about 5% to about 5% of the total composition to about 250 mg/kg body weight/day). about 95%) are compounded. A unit dosage form will generally contain from about 1 mg to about 500 mg of active ingredient.
有利地,它們以5-250 mg/千克體重/天,優選地25-150 mg/千克體重/天劑量給藥。Advantageously, they are administered at a dose of 5-250 mg/kg body weight/day, preferably 25-150 mg/kg body weight/day.
術語“給藥”指給個體提供治療有效量的藥物,給藥方式包括口服,舌下,靜脈,皮下,經皮,肌內,皮內,鞘內,硬膜上,眼內,顱內,吸入,直腸,陰道等。給藥劑型包括膏劑,洗劑,片劑,膠囊劑,丸劑,飛散性粉末劑,顆粒劑,栓劑,丹劑,錠劑,注射劑,無菌溶液或非水溶液劑,懸浮劑,乳劑,貼片劑等。活性組分與無毒的藥學上可接受的載體(如葡萄糖,乳糖,阿拉伯樹膠,明膠,甘露醇,澱粉糊,三矽酸鎂,滑石粉,玉米澱粉,角蛋白,矽膠,土豆澱粉,尿素,右旋糖酐等)複合。The term "administration" refers to the provision of a therapeutically effective amount of a drug to an individual by means of oral, sublingual, intravenous, subcutaneous, transdermal, intramuscular, intradermal, intrathecal, epidural, intraocular, intracranial, Inhalation, rectal, vaginal, etc. Dosage forms include ointments, lotions, tablets, capsules, pills, powders, granules, suppositories, pills, lozenges, injections, sterile solutions or non-aqueous solutions, suspensions, emulsions, patches Wait. The active ingredient is combined with a non-toxic pharmaceutically acceptable carrier (such as glucose, lactose, acacia, gelatin, mannitol, starch paste, magnesium trisilicate, talc, corn starch, keratin, silica gel, potato starch, urea, Dextran, etc.) complex.
優選的,給藥途徑會隨著臨床特徵而變化,劑量的變化必須依賴於正在治療的病人的情況,醫生會根據個體患者來確定合適的劑量。每單位劑量的治療有效量取決於體重,生理機能和選擇的接種方案。每單位劑量的化合物是指每次給藥時化合物的重量,不包括載體的重量(藥物裡含有載體)。Preferably, the route of administration will vary with clinical characteristics, the dosage will depend on the condition of the patient being treated, and the physician will determine the appropriate dosage on an individual patient basis. The therapeutically effective amount per unit dose depends on body weight, physiology and the chosen vaccination regimen. Compound per unit dose refers to the weight of the compound per administration, excluding the weight of the carrier (which is contained in the drug).
本發明提供的藥物組合物可以配製成單劑量或多劑量給藥。所述單劑量製劑被包裝在安瓿劑、小瓶或注射器中。所述多劑量腸胃外製劑必須包含抑菌或抑真菌濃度的抗微生物劑。所有的腸胃外製劑都必須是無菌的,如所屬技術領域已知和實踐的。The pharmaceutical compositions provided by the present invention can be formulated for single-dose or multiple-dose administration. The single-dose formulation is packaged in ampoules, vials or syringes. The multiple-dose parenteral formulation must contain a bacteriostatic or fungistatic concentration of the antimicrobial agent. All parenteral formulations must be sterile, as known and practiced in the art.
本發明提供的藥物組合物可以與不會損害預期的治療作用的其他活性成分共同配製,或者與補充預期的作用的物質共同配製。The pharmaceutical compositions provided by the present invention can be co-formulated with other active ingredients that do not impair the intended therapeutic effect, or with substances that supplement the intended effect.
在一實施方案中,本發明的治療方法包括對有需要的患者給予安全有效量的本發明化合物或包含本發明化合物的藥物組合物。本發明各實施方案包括通過對有需要的患者給予安全有效量的本發明化合物或包含本發明化合物的藥物組合物,來治療本發明提及的疾病。In one embodiment, the methods of treatment of the present invention comprise administering to a patient in need thereof a safe and effective amount of a compound of the present invention or a pharmaceutical composition comprising a compound of the present invention. Various embodiments of the present invention encompass the treatment of diseases referred to herein by administering to a patient in need thereof a safe and effective amount of a compound of the present invention or a pharmaceutical composition comprising a compound of the present invention.
在一實施方案中,本發明化合物或包含本發明化合物的藥物組合物可以一次性給藥,或者根據給藥方案,在指定時間段內,在不同的時間間隔給藥若干次。例如,每天給藥一次、兩次、三次或四次。在一實施方案中,每天給藥一次。在又一實施方案中,每天給藥兩次。可以給藥直至達到想要的治療效果或無限期地維持想要的治療效果。本發明化合物或包含本發明化合物的藥物組合物的合適給藥方案取決於該化合物的藥代動力學性質,例如吸收、分佈和半衰期,這些可以由技術人員測定。此外,本發明化合物或包含本發明化合物的藥物組合物的合適給藥方案,包括實施該方案的持續時間,取決於被治療的疾病、被治療疾病的嚴重程度、被治療患者的年齡和身體狀況、被治療患者的醫療史、同時療法的性質、想要的治療效果等在技術人員知識和經驗範圍內的因素。這樣的技術人員還應該理解,對於個體患者對給藥方案的反應,或隨著時間推移個體患者需要變化時,可要求調整適宜的給藥方案。In one embodiment, a compound of the present invention or a pharmaceutical composition comprising a compound of the present invention may be administered once, or several times at different time intervals over a specified period of time, depending on the dosing regimen. For example, it is administered once, twice, three times or four times a day. In one embodiment, the administration is once a day. In yet another embodiment, the administration is twice daily. Administration may be performed until the desired therapeutic effect is achieved or maintained indefinitely. A suitable dosing regimen for a compound of the present invention or a pharmaceutical composition comprising a compound of the present invention depends on the pharmacokinetic properties of the compound, such as absorption, distribution and half-life, which can be determined by the skilled artisan. In addition, a suitable dosing regimen for a compound of the present invention or a pharmaceutical composition comprising a compound of the present invention, including the duration for which the regimen is carried out, depends on the disease being treated, the severity of the disease being treated, the age and physical condition of the patient being treated , the medical history of the patient being treated, the nature of the concurrent therapy, the desired therapeutic effect, etc., factors within the knowledge and experience of the skilled person. Such skilled artisans will also appreciate that adjustments to appropriate dosing regimens may be required as individual patient responses to dosing regimens change, or as individual patient needs change over time.
本發明化合物可以與一種或多種其他治療劑同時,或在其之前或之後給藥。本發明化合物可以與其他治療劑通過相同或不同給藥途徑分別給藥,或與之以同一藥物組合物形式給藥。這由所屬技術領域中具有通常知識者根據患者的健康、年齡、體重等身體的實際情況選擇。如果配製為固定劑量,這種聯用產品使用本發明的化合物(在本發明所描述的劑量範圍之內)和其他藥學活性劑(在其劑量範圍之內)。The compounds of the present invention can be administered concurrently with, before or after one or more other therapeutic agents. The compounds of the present invention can be administered separately with other therapeutic agents by the same or different route of administration, or in the same pharmaceutical composition. This is selected by those with ordinary knowledge in the technical field according to the actual physical conditions of the patient, such as health, age, and weight. If formulated as a fixed dose, such a combination product employs a compound of the invention (within the dosage range described herein) and the other pharmaceutically active agent (within its dosage range).
相應地,在一個方面,本發明包括聯合用藥,其包括一定數量的至少一種本發明的化合物或其可藥用鹽、溶劑化物、酯或前體藥物和有效量的一種或多種上述附加治療劑。Accordingly, in one aspect, the present invention includes combinations comprising an amount of at least one compound of the present invention, or a pharmaceutically acceptable salt, solvate, ester or prodrug thereof, and an effective amount of one or more of the aforementioned additional therapeutic agents .
式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII)所示的化合物可以與用於預防、治療或減輕式 (I)、 (II)、(III)、(IV)、(V)、(VI) 或 (VII) 所示的化合物適用的疾病或症狀的其他藥物聯用。這些其他藥物可通過其常用的途徑和量與式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII)所示的化合物同時或相繼給藥。當式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII)所示的化合物與一種或多種其他藥物同時使用時,含有這類其他藥物以及式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII)所示的化合物的藥物單位劑型是優選的。The compound represented by formula (I), (II), (III), (IV), (V), (VI) or (VII) can be used for prevention, treatment or alleviation of formula (I), (II), The compound of (III), (IV), (V), (VI) or (VII) is suitable for use in combination with other drugs for diseases or symptoms. These other drugs can be administered simultaneously or sequentially with the compounds of formula (I), (II), (III), (IV), (V), (VI) or (VII) by their usual routes and amounts. When the compound represented by formula (I), (II), (III), (IV), (V), (VI) or (VII) is used concomitantly with one or more other drugs, such other drugs and formula Pharmaceutical unit dosage forms of the compounds of (I), (II), (III), (IV), (V), (VI) or (VII) are preferred.
在各種實施方案中,本發明中所述的化合物與其他藥物結合來提供用於慢性阻塞性肺病 (COPD)、特應性皮炎 (AD) 、銀屑病或其他狀況的聯合治療。本發明的藥物組合物包括本發明中所述的PDE4抑制劑中的至少一種和附加治療劑,附加治療劑的實例包括但不限於:In various embodiments, the compounds described in this invention are combined with other drugs to provide combination therapy for chronic obstructive pulmonary disease (COPD), atopic dermatitis (AD), psoriasis or other conditions. The pharmaceutical composition of the present invention includes at least one of the PDE4 inhibitors described in the present invention and an additional therapeutic agent. Examples of the additional therapeutic agent include, but are not limited to:
(1) β2-激動劑,例如沙丁醇胺、福莫特羅、沙美特羅和卡莫昔羅;(1) β2-agonists, such as salbutamol, formoterol, salmeterol and camoxirol;
(2) 皮質類固醇類,例如布地奈德、二丙酸倍氯米松、丙酸氟替卡松、氟尼縮松、糠酸莫米松、羅氟奈德、環索奈德、氟輕鬆醋酸酯、去羥米松、莫美他松、曲安西龍、倍他米松、阿氯米松、地索奈德、氫化可的松、美普克萊;(2) Corticosteroids, such as budesonide, beclomethasone dipropionate, fluticasone propionate, flunisolide, mometasone furoate, roflunomide, ciclesonide, fluocinolone acetonide, didanoate Methasone, Mometasone, Triamcinolone, Betamethasone, Aclometasone, Desonide, Hydrocortisone, Meproclair;
(3) 抗膽鹼能藥或抗毒蕈鹼藥,例如異丙托溴銨、氧托溴銨、噻托溴銨、格隆溴銨、瑞伐托酯;(3) Anticholinergic or antimuscarinic drugs, such as ipratropium bromide, oxytropium bromide, tiotropium bromide, glycopyrronium bromide, revatropate;
(4) 局部鈣調磷酸酶抑制劑,例如他克莫司、吡美莫司、環孢素;(4) Topical calcineurin inhibitors, such as tacrolimus, pimecrolimus, cyclosporine;
(5) PDE4抑制劑的局部製劑,例如阿普斯特、異丁司特、E-6005、OPA-15406、LEO-29102、DRM02、羅氟司特、克瑞沙硼;(5) Topical preparations of PDE4 inhibitors, such as Apremilast, Ibudilast, E-6005, OPA-15406, LEO-29102, DRM02, Roflumilast, Cresaborone;
(6) JAK激酶抑制劑的局部製劑,例如托法替尼、JTE-052、巴瑞替尼、烏帕替尼;(6) Topical preparations of JAK kinase inhibitors, such as tofacitinib, JTE-052, baricitinib, upatinib;
(7) 局部非甾體抗炎藥物,例如WBI-1001、MRX-6;(7) Topical non-steroidal anti-inflammatory drugs, such as WBI-1001, MRX-6;
(8) 局部ROR藥劑,例如GSK2981278;(8) Topical ROR agents, such as GSK2981278;
(9) 可注射抗IL4、IL-31、IL-22、IL-33、IL-12、IL-23、IL-17、IgE、IL-4治療藥物,例如杜魯單抗 (Dupilumab)、來金珠單抗、尼莫利珠單抗 (Nemolizumab)、曲洛吉努單抗、依那西普、阿達木單抗、英夫利昔單抗、尤特可單抗、塞庫吉努 (Secukinumab)、奧馬珠 (Omazumilab)、CIM-331;(9) Injectable anti-IL4, IL-31, IL-22, IL-33, IL-12, IL-23, IL-17, IgE, IL-4 therapeutic drugs, such as Dupilumab, Akinizumab, Nemolizumab, Tralokinumab, Etanercept, Adalimumab, Infliximab, Utecalumab, Secukinumab , Omazumilab, CIM-331;
(10) 維生素D類似物,例如卡泊三醇、骨化三醇;(10) Vitamin D analogs, such as calcipotriol, calcitriol;
(11) 口服視黃酸衍生物,例如阿利維A酸;(11) Oral retinoic acid derivatives, such as alivet A acid;
(12) 口服肝X受體(LXR)選擇性激動劑,例如VTP-38543;(12) Oral liver X receptor (LXR) selective agonists, such as VTP-38543;
(13) 口服H4受體拮抗劑,例如ZPL-389;(13) Oral H4 receptor antagonists, such as ZPL-389;
(14) 口服NK1受體拮抗劑,例如阿瑞匹坦、曲地匹坦;(14) Oral NK1 receptor antagonists, such as aprepitant and tripipitant;
(15) 口服CRTH2受體拮抗劑,例如弗維普蘭特(Fevipiprant)、和OC-459;(15) Oral CRTH2 receptor antagonists, such as Fevipiprant, and OC-459;
(16) 口服糜蛋白酶抑制劑,例如SUN 13834。(16) Oral chymotrypsin inhibitors such as SUN 13834.
優選地,給與單獨的或與其他活性成分組合的式 (I) 或式 (II) 所示的化合物用於預防和/或治療呼吸疾病或皮膚炎症疾病,例如慢性阻塞性肺病 (COPD)、特應性皮炎 (AD) 或銀屑病。Preferably, the compound of formula (I) or formula (II) is administered alone or in combination with other active ingredients for the prevention and/or treatment of respiratory diseases or skin inflammatory diseases, such as chronic obstructive pulmonary disease (COPD), Atopic dermatitis (AD) or psoriasis.
包含本發明化合物或藥物組合物給藥的治療方法,進一步包括對患者進行其他抗慢性阻塞性肺病 (COPD) 或特應性皮炎藥物(聯合治療)的給藥,其中其他抗慢性阻塞性肺病 (COPD) 或特應性皮炎的藥物為上述附加治療劑中的藥物或它們的組合物。A method of treatment comprising the administration of a compound or pharmaceutical composition of the present invention, further comprising administering to the patient other anti-chronic obstructive pulmonary disease (COPD) or atopic dermatitis drugs (combination therapy), wherein the other anti-chronic obstructive pulmonary disease (COPD) The drug for COPD) or atopic dermatitis is one of the above-mentioned additional therapeutic agents or a combination thereof.
本發明提供在需要這種治療的患者中治療肺病(例如,COPD、氣喘或纖維囊腫)或炎症(例如,特應性皮炎或銀屑病)的方法,該方法包括聯合給予所述患者治療有效量的至少一種式 (I) 或式 (II) 所示化合物,或其藥學上可接受的鹽或溶劑合物,以及至少一種選自下列的化合物:類固醇(如糖皮質激素)、鈣調磷酸酶抑制劑、PDE4抑制劑、JAK激酶抑制劑、半胱氨醯基白三烯拮抗劑、非甾體抗炎藥物、局部ROR藥劑、抗IL4抗體、IL-31抗體、IL-22抗體、IL-33抗體、IL-12抗體、IL-23抗體、IL-17抗體、IgE抗體、IL-4抗體、維生素D類似物、肝X受體(LXR)選擇性激動劑、組胺H1拮抗劑、組胺H3拮抗劑、H4受體拮抗劑、NK1受體拮抗劑、CRTH2受體拮抗劑、糜蛋白酶抑制劑、5-脂氧合酶抑制劑、β-2腎上腺素受體 (adrenoceptor) 激動劑、α-腎上腺素受體激動劑、蕈毒鹼M1拮抗劑、蕈毒鹼M3拮抗劑、蕈毒鹼M2激動劑、NK3拮抗劑、LTB4拮抗劑、支氣管擴張劑、PDE抑制劑。The present invention provides methods of treating pulmonary disease (eg, COPD, asthma or fibrocysts) or inflammation (eg, atopic dermatitis or psoriasis) in a patient in need of such treatment comprising administering to said patient in combination a therapeutically effective Amount of at least one compound represented by formula (I) or formula (II), or a pharmaceutically acceptable salt or solvate thereof, and at least one compound selected from the group consisting of steroids (such as glucocorticoids), calcineurin Enzyme Inhibitors, PDE4 Inhibitors, JAK Kinase Inhibitors, Cysteinyl Leukotriene Antagonists, Non-Steroidal Anti-Inflammatory Drugs, Topical ROR Agents, Anti-IL4 Antibodies, IL-31 Antibodies, IL-22 Antibodies, IL -33 antibody, IL-12 antibody, IL-23 antibody, IL-17 antibody, IgE antibody, IL-4 antibody, vitamin D analog, liver X receptor (LXR) selective agonist, histamine H1 antagonist, Histamine H3 antagonists, H4 receptor antagonists, NK1 receptor antagonists, CRTH2 receptor antagonists, chymotrypsin inhibitors, 5-lipoxygenase inhibitors, β-2 adrenoceptor agonists , α-adrenoceptor agonists, muscarinic M1 antagonists, muscarinic M3 antagonists, muscarinic M2 agonists, NK3 antagonists, LTB4 antagonists, bronchodilators, PDE inhibitors.
本發明化合物和藥物組合物的用途Use of the Compounds and Pharmaceutical Compositions of the Invention
本發明化合物或本發明的組合物中化合物的量可以有效地可探測地拮抗PDE4以治療以下疾病:疼痛(例如,急性疼痛、急性炎性疼痛、慢性炎性疼痛和神經病性疼痛)、急性炎症、慢性炎症、銀屑病關節炎、類風濕性關節炎、牛皮癬、增生性和炎性皮膚病(例如特應性皮炎、脂溢性皮炎、接觸性皮炎)、哮喘、慢性阻塞性肺病 (COPD)、關節炎、炎性腸道疾病、節段性回腸炎、潰瘍性結腸炎、敗血性休克、內毒素性休克、格蘭氏陰性菌敗血症、腎小球性腎炎、帕金森氏病、阿爾茨海默氏病、輕度認知損害 (MCI)、抑鬱症、焦慮症、急性呼吸道窘迫綜合征、骨關節炎、強直性脊柱炎、多發性硬化、牙齦炎、牙周炎、搔癢症、皰疹、CNS腫瘤、間質性肺炎、過敏、結晶誘發的關節炎、急性胰腺炎、慢性胰腺炎、急性酒精性肝炎、壞死性小腸結腸炎、慢性鼻竇炎、急性呼吸窘迫綜合症、肺高血壓、痛風、酒精性肝病、狼瘡、癌症、過敏性鼻炎、非過敏性鼻炎、自身免疫性溶血綜合征、自身免疫性肝炎、自身免疫性神經病變、肝硬化、纖維化疾病、胃炎、Goodpasture氏綜合征、格雷夫斯氏病、Gullain-Barre病、橋本氏甲狀腺炎、HIV-相關的自身免疫性綜合征和血液疾病、扁平苔癬、心肌炎(包括病毒性心肌炎)、神經病變(包括例如,IgA神經病變、細胞膜神經病變和特發性神經病變)、腎炎綜合征、萊特爾氏綜合征、斯耶葛籣氏綜合征、系統性紅斑狼瘡,該方法包括施於該病患有效量的至少一種式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII) 所示化合物或其藥學上可接受的鹽或溶劑合物。The amount of the compound of the invention or the compound in the composition of the invention is effective to detectably antagonize PDE4 to treat the following conditions: pain (eg, acute pain, acute inflammatory pain, chronic inflammatory pain, and neuropathic pain), acute inflammation , chronic inflammation, psoriatic arthritis, rheumatoid arthritis, psoriasis, proliferative and inflammatory skin diseases (eg, atopic dermatitis, seborrheic dermatitis, contact dermatitis), asthma, chronic obstructive pulmonary disease (COPD) ), arthritis, inflammatory bowel disease, Crohn's disease, ulcerative colitis, septic shock, endotoxic shock, gram-negative sepsis, glomerulonephritis, Parkinson's disease, Alzheimer's disease Zheimer's Disease, Mild Cognitive Impairment (MCI), Depression, Anxiety, Acute Respiratory Distress Syndrome, Osteoarthritis, Ankylosing Spondylitis, Multiple Sclerosis, Gingivitis, Periodontitis, Pruritus, Blisters Rash, CNS tumor, interstitial pneumonia, allergy, crystal-induced arthritis, acute pancreatitis, chronic pancreatitis, acute alcoholic hepatitis, necrotizing enterocolitis, chronic sinusitis, acute respiratory distress syndrome, pulmonary hypertension , gout, alcoholic liver disease, lupus, cancer, allergic rhinitis, non-allergic rhinitis, autoimmune hemolytic syndrome, autoimmune hepatitis, autoimmune neuropathy, liver cirrhosis, fibrotic diseases, gastritis, Goodpasture's syndrome symptoms, Graves' disease, Gullin-Barre disease, Hashimoto's thyroiditis, HIV-related autoimmune syndromes and blood disorders, lichen planus, myocarditis (including viral myocarditis), neuropathy (including, for example, IgA neuropathy, cell membrane neuropathy and idiopathic neuropathy), nephritic syndrome, Reiter's syndrome, Sjogren's syndrome, systemic lupus erythematosus, the method comprising administering to the patient an effective amount of at least one of A compound represented by formula (I), (II), (III), (IV), (V), (VI) or (VII) or a pharmaceutically acceptable salt or solvate thereof.
一般合成步驟General synthetic steps
一般地,本發明的化合物可以通過本發明所描述的方法製備得到,除非有進一步的說明,其中取代基的定義如式 (I)、(II)、(III)、(IV)、(V)、(VI) 或 (VII)所示。以下的反應方案和實施例用於進一步舉例說明本發明的內容。In general, the compounds of the present invention can be prepared by the methods described herein, unless otherwise specified, wherein the substituents are as defined in formula (I), (II), (III), (IV), (V) , (VI) or (VII). The following reaction schemes and examples are used to further illustrate the content of the present invention.
所屬技術領域中具有通常知識者將認識到:本發明所描述的化學反應可以用來合適地製備許多本發明的其他化合物,且用於製備本發明的化合物的其他方法都被認為是在本發明的範圍之內。例如,根據本發明那些非例證的化合物的合成可以成功地被所屬技術領域的通常知識者通過修飾方法完成,如適當的保護干擾基團,通過利用其他已知的試劑除了本發明所描述的,或將反應條件做一些常規的修改。另外,本發明所公開的反應或已知的反應條件也公認地適用於本發明其他化合物的製備。One of ordinary skill in the art will recognize that the chemical reactions described in this invention can be used to suitably prepare many other compounds of this invention, and that other methods for preparing compounds of this invention are considered within the scope of this invention. within the range. For example, the synthesis of those non-exemplified compounds according to the present invention can be successfully accomplished by one of ordinary skill in the art by modifying methods, such as appropriate protection of interfering groups, by utilizing other known reagents in addition to those described herein, Or make some routine modifications to the reaction conditions. In addition, the reactions disclosed herein or known reaction conditions are also acknowledged to be applicable to the preparation of other compounds of the present invention.
以下所描述的實施例,除非其他方面表明所有的溫度定為攝氏度。試劑購買於商品供應商如Aldrich Chemical Company, Arco Chemical Company and Alfa Chemical Company,使用時都沒有經過進一步純化,除非其他方面表明。一般的試劑從汕頭西隴化工廠、廣東光華化學試劑廠、廣州化學試劑廠、天津好寓宇化學品有限公司、青島騰龍化學試劑有限公司和青島海洋化工廠購買得到。In the examples described below, unless otherwise indicated all temperatures are in degrees Celsius. Reagents were purchased from commercial suppliers such as Aldrich Chemical Company, Arco Chemical Company and Alfa Chemical Company and were used without further purification unless otherwise indicated. General reagents were purchased from Shantou Xilong Chemical Reagent Factory, Guangdong Guanghua Chemical Reagent Factory, Guangzhou Chemical Reagent Factory, Tianjin Haoyuyu Chemical Co., Ltd., Qingdao Tenglong Chemical Reagent Co., Ltd. and Qingdao Ocean Chemical Factory.
無水四氫呋喃、二氧六環、甲苯、乙醚是經過金屬鈉回流乾燥得到。無水二氯甲烷和氯仿是經過氫化鈣回流乾燥得到。乙酸乙酯、石油醚、正己烷、N ,N -二甲基乙醯胺和N ,N -二甲基甲醯胺是經無水硫酸鈉事先乾燥使用。Anhydrous tetrahydrofuran, dioxane, toluene and diethyl ether are obtained by refluxing and drying with metallic sodium. Anhydrous dichloromethane and chloroform were obtained by refluxing with calcium hydride. Ethyl acetate, petroleum ether, n-hexane, N , N -dimethylacetamide and N , N -dimethylformamide were dried over anhydrous sodium sulfate before use.
以下反應一般是在氮氣或氬氣正壓下或在無水溶劑上套一乾燥管(除非其他方面表明),反應瓶都塞上合適的橡皮塞,底物通過注射器打入。玻璃器皿都是乾燥過的。The following reactions are generally carried out under positive nitrogen or argon pressure or a drying tube is set over anhydrous solvent (unless otherwise indicated), the reaction flasks are plugged with suitable rubber stoppers, and the substrate is injected through a syringe. Glassware is dry.
色譜柱是使用矽膠柱。矽膠(300-400目)購於青島海洋化工廠。核磁共振氫譜的測試條件是:室溫條件下,布魯克 (Bruker) 400 MHz或600 MHz的核磁儀,以CDC13 、DMSO-d6 、CD3 OD或丙酮-d6 為溶劑(報導以ppm為單位),用TMS (0 ppm) 或氯仿 (7.26 ppm) 作為參照標準。當出現多重峰的時候,將使用以下的縮寫:s (singlet,單峰),d (doublet,雙峰),t (triplet,三重峰),q (quartet,四重峰),m (multiplet,多重峰),br (broadened,寬峰),dd (doublet of doublets,雙二重峰),dt (doublet of triplets,雙三重峰)。偶合常數,用赫茲 (Hz) 表示。The chromatographic column is a silica gel column. Silica gel (300-400 mesh) was purchased from Qingdao Ocean Chemical Factory. The test conditions for NMR spectroscopy are: Bruker 400 MHz or 600 MHz NMR instrument at room temperature, with CDC1 3 , DMSO- d 6 , CD 3 OD or acetone- d 6 as solvent (reported in ppm units), with TMS (0 ppm) or chloroform (7.26 ppm) as reference standards. When multiplets are present, the following abbreviations are used: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet, multiplet), br (broadened), dd (doublet of doublets), dt (doublet of triplets). Coupling constant, expressed in Hertz (Hz).
低解析度質譜 (MS) 資料測定的條件是:Agilent 6120 Quadrupole HPLC-MS (管柱型號:Zorbax SB-C18, 2.1 x 30 mm, 3.5 μm, 6 min, 流速為0.6 mL/min,流動相:5%-95% (含0.1%甲酸的CH3 CN) 在 (含0.1%甲酸的H2 O)中的比例)),在210/254 nm用UV檢測,用電噴霧電離模式 (ESI)。Low-resolution mass spectrometry (MS) data were determined under the following conditions: Agilent 6120 Quadrupole HPLC-MS (column model: Zorbax SB-C18, 2.1 x 30 mm, 3.5 μm, 6 min, flow rate 0.6 mL/min, mobile phase: ratio of 5% -95% (containing 0.1% formic acid in CH 3 CN) in (H containing 0.1% formic acid 2 O) in)), with UV detection at 210/254 nm, electrospray ionization mode (ESI).
化合物純度的表徵方式為:Agilent 1260製備型高效液相色譜 (Pre-HPLC) 或Calesep Pump 250製備型高效液相色譜 (Pre-HPLC) (管柱型號:NOVASEP, 50/80 mm, DAC),在210 nm/254 nm用UV檢測。The purity of the compounds was characterized by: Agilent 1260 Preparative High Performance Liquid Chromatography (Pre-HPLC) or Calesep Pump 250 Preparative High Performance Liquid Chromatography (Pre-HPLC) (column type: NOVASEP, 50/80 mm, DAC), Detection with UV at 210 nm/254 nm.
本發明所述的各手性化合物的立體構型使用以下其中之一的分析方法進行拆分:The stereoconfiguration of each chiral compound described in the present invention is resolved by one of the following analytical methods:
分析方法:Analytical method:
1. 使用AS-H柱(廠家:大賽璐,規格:4.6 × 250 mm,5 μm),流動相條件:柱溫30℃,流速1 mL/min,30%乙醇(0.1%三氟乙酸),70%正己烷;1. Use an AS-H column (manufacturer: Daicel, size: 4.6 × 250 mm, 5 μm), mobile phase conditions: column temperature 30 °C, flow rate 1 mL/min, 30% ethanol (0.1% trifluoroacetic acid), 70% n-hexane;
2. 使用OD-H柱(廠家:大賽璐,規格:4.6 × 250 mm,5 μm)流動相條件:柱溫30℃,流速1 mL/min,30%乙醇,70%正己烷;2. Use OD-H column (manufacturer: Daicel, size: 4.6 × 250 mm, 5 μm) mobile phase conditions: column temperature 30 °C, flow rate 1 mL/min, 30% ethanol, 70% n-hexane;
3. 使用IC柱(廠家:大賽璐,規格:4.6 × 250 mm,5 μm)流動相條件:柱溫30℃,流速0.8 mL/min,50%乙醇,50%正己烷。3. Use an IC column (manufacturer: Daicel, size: 4.6 × 250 mm, 5 μm) mobile phase conditions: column temperature 30°C, flow rate 0.8 mL/min, 50% ethanol, 50% n-hexane.
以下簡寫詞的使用貫穿本發明:
中間體的合成方法: Synthesis of intermediates:
重要中間體 M-4 可以通過中間體的合成方法製備得到,其中,除非另外說明,R2 和Ra 具有如本發明所述的含義。起始物料 M-1 與物料BnOH在弱鹼和疊氮磷酸二苯酯條件下發生重排反應,形成氨基保護基得到中間體 M-2,中間體 M-2 經過催化氫化還原得到中間體 M-3,中間體 M-3 經過重氮化反應,再與KI發生取代反應得到中間體 M-4。Important intermediates M-4 can be prepared by synthetic methods of intermediates wherein, unless otherwise stated, R 2 and R a have the meanings as described in the present invention. The starting material M-1 and the material BnOH undergo rearrangement reaction under the condition of weak base and diphenylphosphoric azide to form an amino protecting group to obtain intermediate M-2, and intermediate M-2 undergoes catalytic hydrogenation reduction to obtain intermediate M -3, the intermediate M-3 undergoes a diazotization reaction, and then undergoes a substitution reaction with KI to obtain the intermediate M-4.
目標產物合成方法:Target product synthesis method:
合成方法一: Synthesis method one:
目標化合物 S-8 可以通過合成方法一製備得到,其中,X代表鹵素原子,Rx 為-NH2 或-OH,Rxo 為-NH-或-O-,除非另外說明,A環、R2 、Ra 、Rb 、Rm 、Rn 、p和n具有如本發明所述的含義。化合物 S-1 在鹼性(例如DIPEA或TEA等)條件下經烯醇化反應後再經過取代反應得到化合物 S-2,化合物 S-2 與化合物 S'-2 在鹼性條件下通過硼基化反應得到化合物 S-3,化合物 S-3與中間體 M-4 在偶聯試劑條件下發生Suzuki偶聯反應得到化合物 S-4,化合物 S-4 經催化氫化得到化合物 S-5,化合物 S-5 在酸性條件下脫去Boc保護基,再經醯化反應得到化合物 S-6,化合物 S-6 在鹼性條件下經酯水解反應得到化合物 S-7,化合物 S-7 與化合物 S'-7 在合適的縮合劑作用下經縮合反應得到目標化合物 S-8。The target compound S-8 can be prepared by synthetic method 1, wherein, X represents a halogen atom, R x is -NH 2 or -OH, R xo is -NH- or -O-, unless otherwise specified, A ring, R 2 , R a , R b , R m , R n , p and n have the meanings as described in the present invention. Compound S-1 is subjected to enolation reaction under basic conditions (such as DIPEA or TEA, etc.) and then undergoes substitution reaction to obtain compound S-2. Compound S-2 and compound S'-2 undergo boronylation under basic conditions Reaction to obtain compound S-3, compound S-3 and intermediate M-4 undergo Suzuki coupling reaction under coupling reagent conditions to obtain compound S-4, compound S-4 is subjected to catalytic hydrogenation to obtain compound S-5, compound S- 5 Under acidic conditions, the Boc protecting group was removed, and then compound S-6 was obtained by acylation reaction, and compound S-6 was obtained by ester hydrolysis reaction under basic conditions to obtain compound S-7. Compound S-7 and compound S'- 7 The target compound S-8 is obtained by condensation reaction under the action of a suitable condensing agent.
合成方法二: Synthesis method two:
目標化合物 S-11 可以通過合成方法二製備得到,其中,Rx 為-NH2 或-OH,Rxo 為-NH-或-O-,除非另外說明,A環、R2 、Ra 、Re 、Rf 、Rm 、Rn 和p具有如本發明所述的含義。化合物 S-7與化合物 S''-7 在合適的縮合劑作用下經縮合反應得到化合物 S-9,化合物 S-9在鹼性條件下經酯水解反應得到化合物 S-10,化合物 S-10 與化合物 S'-10 在合適的縮合劑作用下經縮合反應得到目標化合物 S-11。The target compound S-11 can be prepared by synthetic method 2, wherein, R x is -NH 2 or -OH, R xo is -NH- or -O-, unless otherwise specified, A ring, R 2 , R a , R e, R f, R m, R n and p have the meanings as described in the present invention. Compound S-7 and compound S''-7 undergo condensation reaction under the action of a suitable condensing agent to obtain compound S-9, compound S-9 undergoes ester hydrolysis under basic conditions to obtain compound S-10, compound S-10 The target compound S-11 is obtained by condensation reaction with compound S'-10 under the action of a suitable condensing agent.
中間體的合成Synthesis of Intermediates
中間體1:2-(環丙基甲氧基)-1-(二氟甲氧基)-4-碘苯 Intermediate 1: 2-(Cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene
步驟1:化合物 (3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)氨基甲酸苯甲酯的合成Step 1: Synthesis of compound (3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)carbamate benzyl
將3-(環丙甲氧基)-4-(二氟甲氧基)苯甲酸 (5.0 g, 19.4 mmol)溶於甲苯 (25 mL) 中,加入疊氮磷酸二苯酯 (5.0 mL, 23.0 mmol),三乙胺 (4.3 mL, 31.0 mmol),室溫下反應1h,加入苯甲醇 (3.0 mL, 29.0 mmol),轉移至90℃加熱反應3 h。減壓濃縮除去溶劑,剩餘物加入水 (100 ml),用乙酸乙酯萃取 (25mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到白色固體5.01 g,收率71%。3-(Cyclopropylmethoxy)-4-(difluoromethoxy)benzoic acid (5.0 g, 19.4 mmol) was dissolved in toluene (25 mL) and diphenylphosphoryl azide (5.0 mL, 23.0 mL) was added. mmol), triethylamine (4.3 mL, 31.0 mmol), react at room temperature for 1 h, add benzyl alcohol (3.0 mL, 29.0 mmol), transfer to 90 °C and heat for 3 h. Concentrated under reduced pressure to remove the solvent, the residue was added with water (100 ml), extracted with ethyl acetate (25 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) to obtain 5.01 g of a white solid with a yield of 71%.
1 H NMR (600 MHz, CDCl3 ) δ (ppm): δ 7.35-7.43 (m, 5H), 7.09 (d,J = 8.6 Hz, 1H), 6.65-6.69 (m, 1H), 6.58 (t,J F-H = 75.8 Hz, 1H), 5.22 (s, 3H), 3.88 (s, 3H), 1.27-1.34 (m, 1H), 0.58-0.73 (m, 2H), 0.29-0.44 ( m, 2H). 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): δ 7.35-7.43 (m, 5H), 7.09 (d, J = 8.6 Hz, 1H), 6.65-6.69 (m, 1H), 6.58 (t, J FH = 75.8 Hz, 1H), 5.22 (s, 3H), 3.88 (s, 3H), 1.27-1.34 (m, 1H), 0.58-0.73 (m, 2H), 0.29-0.44 (m, 2H).
MS (ESI, pos.ion) m/z: 364.10 [M+H]+ .MS (ESI, pos.ion) m/z: 364.10 [M+H] + .
步驟2:化合物3-(環丙基甲氧基)-4-(二氟甲氧基)苯胺的合成Step 2: Synthesis of compound 3-(cyclopropylmethoxy)-4-(difluoromethoxy)aniline
將 (3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)氨基甲酸苯甲酯 (145 mg, 0.45 mmol),溶於無水甲醇 (6 mL) 中,加入鈀炭 (50 mg),排除空氣,通入氫氣室溫反應2 h。用矽藻土抽濾除去催化劑,濾液濃縮得到淡紅色液體102 mg,收率98%。Benzyl (3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)carbamate (145 mg, 0.45 mmol) was dissolved in anhydrous methanol (6 mL) and palladium was added Carbon (50 mg) was removed, and hydrogen was passed through to react at room temperature for 2 h. The catalyst was removed by suction filtration with diatomaceous earth, and the filtrate was concentrated to obtain 102 mg of a light red liquid with a yield of 98%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 6.94 (d,J = 8.4 Hz, 1H), 6.47 (t,J F-H = 76.3 Hz, 1H), 6.26 (d,J = 2.2 Hz, 1H), 6.20 (dd,J = 8.4, 2.4 Hz, 1H), 3.80 (d,J = 6.8 Hz, 2H), 1.25-1.31 (m, 1H), 0.58-0.65 (m, 2H), 0.30-0.35 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 6.94 (d, J = 8.4 Hz, 1H), 6.47 (t, J FH = 76.3 Hz, 1H), 6.26 (d, J = 2.2 Hz, 1H) ), 6.20 (dd, J = 8.4, 2.4 Hz, 1H), 3.80 (d, J = 6.8 Hz, 2H), 1.25-1.31 (m, 1H), 0.58-0.65 (m, 2H), 0.30-0.35 ( m, 2H).
MS (ESI, pos.ion) m/z: 230.10 [M+H]+ .MS (ESI, pos.ion) m/z: 230.10 [M+H] + .
步驟3:化合物2-(環丙基甲氧基)-1-(二氟甲氧基)-4-碘苯的合成Step 3: Synthesis of compound 2-(cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene
將化合物 3-(環丙基甲氧基)-4-(二氟甲氧基)苯胺鹽酸鹽 (17 g, 64.0 mmol),溶於1,4-二氧六環(85 mL) 和水 (30 mL) 中,0℃以下加入濃鹽酸 (17 mL),降溫到-15℃,逐滴加入亞硝酸鈉 (5.3 g, 77.0 mmol) 和水 (15 mL) 的溶液,控制溫度在-15℃至-5℃之間,0℃下繼續反應40 min,加入碘化鉀 (13.8 g, 83.1 mmol) 和水 (15 mL) 的溶液,控制溫度在-15℃至-5℃之間,滴加完畢後,在0℃下繼續反應2 h,加入水 (200 mL) 停止反應,用乙酸乙酯萃取 (200mL × 2),有機相用飽和亞硫酸鈉洗一次,無水硫酸鈉乾燥,減壓濃縮,得到淺棕色液體21 g,收率96%。Compound 3-(cyclopropylmethoxy)-4-(difluoromethoxy)aniline hydrochloride (17 g, 64.0 mmol) was dissolved in 1,4-dioxane (85 mL) and water (30 mL), add concentrated hydrochloric acid (17 mL) below 0 °C, cool down to -15 °C, add a solution of sodium nitrite (5.3 g, 77.0 mmol) and water (15 mL) dropwise, and control the temperature at -15 °C. Between ℃ and -5℃, continue the reaction for 40 min at 0℃, add a solution of potassium iodide (13.8 g, 83.1 mmol) and water (15 mL), control the temperature between -15℃ and -5℃, and complete the dropwise addition. After that, the reaction was continued at 0 °C for 2 h, water (200 mL) was added to stop the reaction, extracted with ethyl acetate (200 mL × 2), the organic phase was washed once with saturated sodium sulfite, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a pale Brown liquid 21 g, yield 96%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.21-7.27 (m, 2H), 6.89 (d,J = 8.1 Hz, 1H), 6.59 (t,J F-H = 75.2 Hz, 1H), 3.84 (d,J = 6.9 Hz, 2H), 1.23-1.29 (m, 1H), 0.61-0.69 (m, 2H), 0.32-0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.21-7.27 (m, 2H), 6.89 (d, J = 8.1 Hz, 1H), 6.59 (t, J FH = 75.2 Hz, 1H), 3.84 (d, J = 6.9 Hz, 2H), 1.23-1.29 (m, 1H), 0.61-0.69 (m, 2H), 0.32-0.40 (m, 2H).
GC-MS: m/z 340.0.GC-MS: m/z 340.0.
中間體2:中間體2-(苄氧基)-1-(二氟甲氧基)-4-碘苯 Intermediate 2: Intermediate 2-(benzyloxy)-1-(difluoromethoxy)-4-iodobenzene
步驟1:化合物2-(二氟甲氧基)-5-碘苯酚的合成Step 1: Synthesis of compound 2-(difluoromethoxy)-5-iodophenol
將化合物2-(環丙基甲氧基)-1-(二氟甲氧基)-4-碘苯 (4.9 g, 14.0 mmol) 溶解在乙腈 (8 mL) 中,加入水(10 mL) 和濃鹽酸 (10 mL),80℃反應4 h,加入氫氧化鈉溶液調節pH=5,用乙酸乙酯萃取 (5 mL × 3),有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 5/1),得到淡黃色液體1.8 g,產率44%。Compound 2-(cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene (4.9 g, 14.0 mmol) was dissolved in acetonitrile (8 mL), water (10 mL) and Concentrated hydrochloric acid (10 mL), react at 80 °C for 4 h, add sodium hydroxide solution to adjust pH=5, extract with ethyl acetate (5 mL × 3), dry the organic phase with anhydrous sodium sulfate, remove the solvent, and concentrate the solution for silica gel Column chromatography separation (eluent: petroleum ether/ethyl acetate (v/v) = 5/1) gave 1.8 g of pale yellow liquid with a yield of 44%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 10.30 (s, 1H), 7.28 (d,J = 2.0 Hz, 1H)), 7.15 (dd,J = 8.4, 2.0 Hz, 1H), 7.02 (t,J F-H = 74.7 Hz, 1H), 6.90 (d,J = 8.4 Hz, 1H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 10.30 (s, 1H), 7.28 (d, J = 2.0 Hz, 1H)), 7.15 (dd, J = 8.4, 2.0 Hz, 1H) , 7.02 (t, J FH = 74.7 Hz, 1H), 6.90 (d, J = 8.4 Hz, 1H).
MS (ESI, pos.ion) m/z: 286.00 [M].MS (ESI, pos.ion) m/z: 286.00 [M].
步驟2:化合物2-(苄氧基)-1-(二氟甲氧基)-4-碘苯的合成Step 2: Synthesis of compound 2-(benzyloxy)-1-(difluoromethoxy)-4-iodobenzene
將化合物2-(二氟甲氧基)-5-碘苯酚 (3.8 g, 13.0 mmol) 和碳酸鉀 (5.8 g, 42.0 mmol) 於N ,N -二甲基甲醯胺 (30 mL) 中混合均勻,加入苄溴 (2.5 mL, 21.0 mmol),在100℃反應16 h,過濾除去固體,濾液濃縮,加入水 (50 mL),用乙酸乙酯 (25 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到淡黃色液體4.8 g,產率96%。Compound 2-(difluoromethoxy)-5-iodophenol (3.8 g, 13.0 mmol) and potassium carbonate (5.8 g, 42.0 mmol ) were mixed in N , N -dimethylformamide (30 mL) Homogeneous, added benzyl bromide (2.5 mL, 21.0 mmol), reacted at 100 °C for 16 h, filtered to remove the solid, concentrated the filtrate, added water (50 mL), extracted with ethyl acetate (25 mL × 3), the organic phase was combined with anhydrous After drying over sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) to obtain 4.8 g of pale yellow liquid with a yield of 96%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.57 (d,J = 1.9 Hz, 1H), 7.45-7.47 (m, 2H), 7.41 (s, 1H), 7.39 (d,J = 3.4 Hz, 1H), 7.33-7.37 (m, 2H), 7.09 (t,J F-H = 74.2 Hz, 1H), 7.00 (d,J = 8.4 Hz, 1H), 5.18 (s, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.57 (d, J = 1.9 Hz, 1H), 7.45-7.47 (m, 2H), 7.41 (s, 1H), 7.39 (d, J = 3.4 Hz, 1H), 7.33-7.37 (m, 2H), 7.09 (t, J FH = 74.2 Hz, 1H), 7.00 (d, J = 8.4 Hz, 1H), 5.18 (s, 2H).
中間體3:中間體6-(氨基甲基)-N,N -二甲基吡啶醯胺二鹽酸鹽 Intermediate 3: Intermediate 6-(aminomethyl) -N,N -lutidine pyridinamide dihydrochloride
步驟1:化合物6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸乙酯的合成Step 1: Synthesis of compound 6-(((tert-butoxycarbonyl)amino)methyl)picolinate ethyl ester
將6-(氨基甲基)吡啶甲酸乙酯 (2.0 g, 9.2 mmol) 溶於無水DMF (15 mL)中,0℃下加入N ,N -二異丙基乙胺 (4.1 mL, 23 mmol) 和二碳酸二叔丁酯 (2.8 mL, 12 mmol),室溫反應1.5 h,加水 (20 mL),加二氯甲烷萃取 (10 mL × 3),有機相合併後用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/1),得到黃色液體1.98 g,產率77%。Ethyl 6-(aminomethyl)picolinate (2.0 g, 9.2 mmol) was dissolved in dry DMF (15 mL), and N , N -diisopropylethylamine (4.1 mL, 23 mmol) was added at 0°C and di-tert-butyl dicarbonate (2.8 mL, 12 mmol), react at room temperature for 1.5 h, add water (20 mL), add dichloromethane for extraction (10 mL × 3), combine the organic phases, dry with anhydrous sodium sulfate, remove The solvent and the concentrated solution were separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 3/1) to obtain 1.98 g of a yellow liquid with a yield of 77%.
1 H NMR (600 MHz, CDCl3 ) δ (ppm): 8.01 (t,J = 8.1 Hz, 1H), 7.79 – 7.83 (m, 1H), 7.50 (t,J = 7.0 Hz, 1H), 4.50 – 4.55 (m, 2H), 4.45 – 4.50 (m, 2H), 1.42 – 1.47 (m, 12H). 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 8.01 (t, J = 8.1 Hz, 1H), 7.79 – 7.83 (m, 1H), 7.50 (t, J = 7.0 Hz, 1H), 4.50 – 4.55 (m, 2H), 4.45 – 4.50 (m, 2H), 1.42 – 1.47 (m, 12H).
MS (ESI, pos.ion) m/z: 281.25 [M+H]+ .MS (ESI, pos.ion) m/z: 281.25 [M+H] + .
步驟2:6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸的合成Step 2: Synthesis of 6-(((tert-butoxycarbonyl)amino)methyl)picolinic acid
將6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸乙酯 (700 mg, 2.5 mmol) 和一水合氫氧化鋰 (527 mg, 12.6 mmol) 加入至四氫呋喃 (5 mL) 和水 (5 mL) 混合溶劑中,50℃反應1.5 h後停止,加稀鹽酸調節pH=6,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,得到淺黃色油狀物606 mg,收率96%。Ethyl 6-(((tert-butoxycarbonyl)amino)methyl)picolinate (700 mg, 2.5 mmol) and lithium hydroxide monohydrate (527 mg, 12.6 mmol) were added to tetrahydrofuran (5 mL) and water ( 5 mL) mixed solvent, the reaction was stopped after 1.5 h at 50 °C, diluted hydrochloric acid was added to adjust pH=6, extracted with ethyl acetate (10 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain a pale yellow oil The substance was 606 mg, and the yield was 96%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.06 (d,J = 7.6 Hz, 1H), 7.99 (t,J = 7.7 Hz, 1H), 7.60 (d,J = 7.6 Hz, 1H), 4.44 (s, 2H), 1.48 (s, 9H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.06 (d, J = 7.6 Hz, 1H), 7.99 (t, J = 7.7 Hz, 1H), 7.60 (d, J = 7.6 Hz, 1H) ), 4.44 (s, 2H), 1.48 (s, 9H).
MS (ESI, pos.ion) m/z: 253.10 [M+H]+ .MS (ESI, pos.ion) m/z: 253.10 [M+H] + .
步驟3:((6-(二甲基氨基甲醯基)吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 3: Synthesis of tert-butyl ((6-(dimethylcarbamoyl)pyridin-2-yl)methyl)carbamate
將6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸 (587-1-2) (600 mg, 2.38 mmol),二甲胺鹽酸鹽 (971 mg, 11.9 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (2.3 g, 12 mmol) 和N -羥基-7-氮雜苯並三氮唑 (488 mg, 3.59 mmol) 溶於二氯甲烷 (10 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (2.4 mL, 15 mmol),室溫攪拌19 h,加水 (15 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到淺黃色黏稠固體554 mg,收率83%。6-(((tert-butoxycarbonyl)amino)methyl)picolinic acid (587-1-2) (600 mg, 2.38 mmol), dimethylamine hydrochloride (971 mg, 11.9 mmol), 1-ethyl Alkyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (2.3 g, 12 mmol) and N -hydroxy-7-azabenzotriazole (488 mg, 3.59 mmol) were dissolved in In dichloromethane (10 mL), N , N -diisopropylethylamine (2.4 mL, 15 mmol) was added dropwise to this solution at 0 °C, stirred at room temperature for 19 h, added with water (15 mL), followed by Dichloromethane extraction (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) , 554 mg of light yellow viscous solid was obtained with a yield of 83%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.78 (d,J = 7.7 Hz, 1H), 7.50 (d,J = 7.6 Hz, 1H), 7.33 (d,J = 7.8 Hz, 1H), 4.47 (d,J = 5.2 Hz, 2H), 3.16 (s, 3H), 3.07 (s, 3H), 1.48 (s, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.78 (d, J = 7.7 Hz, 1H), 7.50 (d, J = 7.6 Hz, 1H), 7.33 (d, J = 7.8 Hz, 1H) , 4.47 (d, J = 5.2 Hz, 2H), 3.16 (s, 3H), 3.07 (s, 3H), 1.48 (s, 9H).
MS (ESI, pos.ion) m/z: 280.30 [M+H]+ .MS (ESI, pos.ion) m/z: 280.30 [M+H] + .
步驟4:6-(氨基甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽的合成Step 4: Synthesis of 6-(aminomethyl) -N , N -lutidylpyridinium dihydrochloride
將化合物 ((6-(二甲基氨基甲醯基)吡啶-2-基)甲基)氨基甲酸叔丁酯 (587-1-1) (550 mg, 1.97 mmol) 溶解於甲醇 (5 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (5 mL),室溫攪拌1 h,除去溶劑,得到白色固體486 mg,收率97%。Compound ((6-(dimethylaminocarbamoyl)pyridin-2-yl)methyl)carbamate tert-butyl ester (587-1-1) (550 mg, 1.97 mmol) was dissolved in methanol (5 mL) To the solution, 4 mol/L HCl in ethyl acetate solution (5 mL) was added, stirred at room temperature for 1 h, and the solvent was removed to obtain 486 mg of white solid with a yield of 97%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.02 (t,J = 7.8 Hz, 1H), 7.59 (t,J = 8.1 Hz, 2H), 4.37 (s, 2H), 3.16 (s, 3H), 3.07 (s, 3H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.02 (t, J = 7.8 Hz, 1H), 7.59 (t, J = 8.1 Hz, 2H), 4.37 (s, 2H), 3.16 (s , 3H), 3.07 (s, 3H).
MS (ESI, pos.ion) m/z: 180.15 [M+H-2HCl]+ .MS (ESI, pos.ion) m/z: 180.15 [M+H-2HCl] + .
中間體4:中間體 (3-(氨甲基)苯基)氨基甲酸甲酯鹽酸鹽 Intermediate 4: Intermediate (3-(aminomethyl)phenyl)carbamate hydrochloride
步驟1:化合物N -(3-((叔丁氧羰基氨基)甲基)苯基)氨基甲酸甲酯的合成Step 1: Synthesis of compound N- (3-((tert-butoxycarbonylamino)methyl)phenyl)carbamate
將化合物3-氨基苄基氨基甲酸叔丁酯 (503 mg, 2.26 mmol) 溶解在二氯甲烷 (6 mL) 中,0℃條件下加入氯甲酸甲酯 (0.35 mL, 4.5 mmol) 和N ,N -二異丙基乙胺 (1.2 mL, 7.3 mmol),室溫攪拌20 h後停止反應,加水溶液洗有機相 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到白色固體511 mg,產率90%。The compound tert-butyl 3-aminobenzylcarbamate (503 mg, 2.26 mmol) was dissolved in dichloromethane (6 mL), methyl chloroformate (0.35 mL, 4.5 mmol) and N , N were added at 0°C -Diisopropylethylamine (1.2 mL, 7.3 mmol), the reaction was stopped after stirring at room temperature for 20 h, the organic phase was washed with an aqueous solution (10 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the concentrated solution was subjected to a silica gel column layer Separation (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) gave 511 mg of white solid with a yield of 90%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.26-7.32 (m, 3H), 7.01 (d,J = 6.9 Hz, 1H), 6.71 (br.s, 1H), 4.89 (br.s, 1H), 4.30 (d,J = 5.2 Hz, 2H), 3.79 (s, 3H), 1.48 (s, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.26-7.32 (m, 3H), 7.01 (d, J = 6.9 Hz, 1H), 6.71 (br.s, 1H), 4.89 (br.s , 1H), 4.30 (d, J = 5.2 Hz, 2H), 3.79 (s, 3H), 1.48 (s, 9H).
MS (ESI, pos.ion) m/z: 303.10[M+Na]+ .MS (ESI, pos.ion) m/z: 303.10[M+Na] + .
步驟2:化合物 (3-(氨甲基)苯基)氨基甲酸甲酯鹽酸鹽的合成Step 2: Synthesis of Compound (3-(aminomethyl)phenyl)carbamic acid methyl ester hydrochloride
將化合物N -(3-((叔丁氧羰基氨基)甲基)苯基)氨基甲酸甲酯 (511 mg, 1.8 mmol) 溶解於二氯甲烷 (2 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (3 mL),室溫攪拌1 h,除去溶劑,得到白色固體316 mg,收率96%。Compound N- (3-((tert-butoxycarbonylamino)methyl)phenyl)carbamate (511 mg, 1.8 mmol) was dissolved in dichloromethane (2 mL) solution, and 4 mol/L HCl was added The ethyl acetate solution (3 mL) was stirred at room temperature for 1 h, and the solvent was removed to obtain 316 mg of a white solid with a yield of 96%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 9.79 (s, 1H), 8.48 (br.s, 2H), 7.56 (s, 1H), 7.43 (d,J = 7.8 Hz, 1H), 7.32 (t,J = 7.8 Hz, 1H), 7.16 (d,J = 7.2 Hz, 1H), 3.94 (s, 2H), 3.67 (s, 3H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 9.79 (s, 1H), 8.48 (br.s, 2H), 7.56 (s, 1H), 7.43 (d, J = 7.8 Hz, 1H ), 7.32 (t, J = 7.8 Hz, 1H), 7.16 (d, J = 7.2 Hz, 1H), 3.94 (s, 2H), 3.67 (s, 3H).
MS (ESI, pos.ion) m/z: 181.10 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 181.10 [M+H-HCl] + .
中間體5:中間體 (6-(1-甲基-1H -吡唑-4-基)吡啶-2-基)甲胺二鹽酸鹽 Intermediate 5: Intermediate (6-(1-methyl- 1H -pyrazol-4-yl)pyridin-2-yl)methanamine dihydrochloride
步驟1:化合物 ((6-溴吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 1: Synthesis of compound ((6-bromopyridin-2-yl)methyl)carbamate tert-butyl ester
將化合物 (6-溴吡啶-2-基)甲胺 (1.0 g, 5.4 mmol) 溶解於二氯甲烷 (15 mL) 溶液中,加入N ,N -二異丙基乙胺 (3.0 mL, 18.0 mmol),在0℃下加入二碳酸二叔丁酯 (1.5 mL, 6.5 mmol),攪拌20 min轉移至室溫反應5 h,加水洗 (50 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到白色固體1.42 g,收率92%。The compound (6-bromopyridin-2-yl)methanamine (1.0 g, 5.4 mmol) was dissolved in a solution of dichloromethane (15 mL), and N , N -diisopropylethylamine (3.0 mL, 18.0 mmol) was added. ), di-tert-butyl dicarbonate (1.5 mL, 6.5 mmol) was added at 0°C, stirred for 20 min, transferred to room temperature for 5 h, washed with water (50 mL), and then extracted with dichloromethane (5 mL × 3) , the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) to obtain a white solid 1.42 g, yield 92%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.73 (t,J = 7.7 Hz, 1H), 7.50 (d,J = 7.9 Hz, 1H), 7.48 (br.s, 1H), 7.30 (d,J = 7.5 Hz, 1H), 4.19 (d,J = 6.0 Hz, 2H), 1.40 (s, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.73 (t, J = 7.7 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.48 (br.s, 1H), 7.30 (d, J = 7.5 Hz, 1H), 4.19 (d, J = 6.0 Hz, 2H), 1.40 (s, 9H).
MS (ESI, pos.ion) m/z: 233.10 [M-55]+ .MS (ESI, pos.ion) m/z: 233.10 [M-55] + .
步驟2:化合物 ((6-(1-甲基-1H -吡唑-4-基)吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 2: Synthesis of compound ((6-(1-methyl- 1H -pyrazol-4-yl)pyridin-2-yl)methyl)carbamate tert-butyl ester
將化合物 ((6-溴吡啶-2-基)甲基)氨基甲酸叔丁酯(298 mg, 1.0 mmol),(1-甲基-1H -吡唑-4-基)硼酸 (153 mg, 1.2 mmol),碳酸鈉 (332 mg, 3.1 mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (42 mg, 0.06 mmol) 溶解於無水1,4-二氧六環 (6 mL) 和水 (2 mL) 中,將反應瓶中空氣抽走,通入氮氣,在100℃下反應21 h,加水洗 (50 mL),然後乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到196 mg淡黃色液體,收率66%。Compound ((6-bromopyridin-2-yl)methyl)carbamic acid tert-butyl ester (298 mg, 1.0 mmol), (1-methyl- 1H -pyrazol-4-yl)boronic acid (153 mg, 1.2 mmol), sodium carbonate (332 mg, 3.1 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (42 mg, 0.06 mmol) were dissolved in anhydrous 1,4- Dioxane (6 mL) and water (2 mL), the air in the reaction flask was evacuated, nitrogen was introduced, the reaction was carried out at 100 ° C for 21 h, washed with water (50 mL), and then extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) to obtain 196 mg of pale yellow liquid, The yield is 66%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.25 (s, 1H), 7.97 (s, 1H), 7.72 (t,J = 7.7 Hz, 1H), 7.49 (d,J = 7.8 Hz, 1H), 7.41 (br.s, 1H), 7.04 (d,J = 7.6 Hz, 1H), 4.22 (d,J = 5.9 Hz, 2H), 3.88 (s, 3H), 1.42 (s, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.25 (s, 1H), 7.97 (s, 1H), 7.72 (t, J = 7.7 Hz, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.41 (br.s, 1H), 7.04 (d, J = 7.6 Hz, 1H), 4.22 (d, J = 5.9 Hz, 2H), 3.88 (s, 3H), 1.42 (s, 9H) ).
MS (ESI, pos.ion) m/z: 289.25 [M+H]+ .MS (ESI, pos.ion) m/z: 289.25 [M+H] + .
步驟3:化合物 (6-(1-甲基-1H -吡唑-4-基)吡啶-2-基)甲胺二鹽酸鹽的合成Step 3: Synthesis of compound (6-(1-methyl- 1H -pyrazol-4-yl)pyridin-2-yl)methanamine dihydrochloride
將化合物 ((6-(1-甲基-1H -吡唑-4-基)吡啶-2-基)甲基)氨基甲酸叔丁酯 (186 mg, 0.64 mmol) 溶解於二氯甲烷 (2 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (3 mL),室溫攪拌40 min,除去溶劑,得到淡黃色固體116 mg,收率95%。Compound ((6-(1-methyl- 1H -pyrazol-4-yl)pyridin-2-yl)methyl)carbamate (186 mg, 0.64 mmol) was dissolved in dichloromethane (2 mL) solution, 4 mol/L HCl in ethyl acetate solution (3 mL) was added, stirred at room temperature for 40 min, and the solvent was removed to obtain 116 mg of pale yellow solid with a yield of 95%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.52 (br.s, 2H), 8.47 (s, 1H), 8.20 (s, 1H), 7.87 (t,J = 7.8 Hz, 1H), 7.67 (d,J = 7.8 Hz, 1H), 7.31 (d,J = 7.5 Hz, 1H), 4.14-4.26 (m, 2H), 3.90 (s, 3H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.52 (br.s, 2H), 8.47 (s, 1H), 8.20 (s, 1H), 7.87 (t, J = 7.8 Hz, 1H ), 7.67 (d, J = 7.8 Hz, 1H), 7.31 (d, J = 7.5 Hz, 1H), 4.14-4.26 (m, 2H), 3.90 (s, 3H).
MS (ESI, pos.ion) m/z: 189.20 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 189.20 [M+H-HCl] + .
中間體6:中間體6-(氨基甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺二鹽酸鹽 Intermediate 6: Intermediate 6- (aminomethyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methyl acyl pyridine dihydrochloride
步驟1:化合物6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸乙酯的合成Step 1: Synthesis of compound 6-(((tert-butoxycarbonyl)amino)methyl)picolinate ethyl ester
將6-(氨基甲基)吡啶甲酸乙酯 (2.0 g, 9.2 mmol) 溶於無水N ,N -二甲基甲醯胺 (15 mL) 中,0℃下加入N ,N -二異丙基乙胺 (4.1 mL, 23 mmol) 和二碳酸二叔丁酯 (2.8 mL, 12 mmol),室溫反應1.5 h,加水 (20 mL),加二氯甲烷萃取 (10 mL × 3),有機相合併後用無水硫酸鈉乾燥,除去溶劑,濃縮液進行柱分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/1),得到黃色液體1.98 g,收率77%。Ethyl 6-(aminomethyl)picolinate (2.0 g, 9.2 mmol) was dissolved in anhydrous N , N -dimethylformamide (15 mL), and N , N -diisopropyl was added at 0°C Ethylamine (4.1 mL, 23 mmol) and di-tert-butyl dicarbonate (2.8 mL, 12 mmol), react at room temperature for 1.5 h, add water (20 mL), add dichloromethane for extraction (10 mL × 3), the organic phase After the combination, it was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was subjected to column separation (eluent: petroleum ether/ethyl acetate (v/v) = 3/1) to obtain 1.98 g of a yellow liquid with a yield of 77%.
1 H NMR (600 MHz, CDCl3 ) δ(ppm): 8.01 (t,J = 8.1 Hz, 1H), 7.79 – 7.83 (m, 1H), 7.50 (t,J = 7.0 Hz, 1H), 4.50 – 4.55 (m, 2H), 4.45 – 4.50 (m, 2H), 1.42 – 1.47 (m, 12H). 1 H NMR (600 MHz, CDCl 3 ) δ(ppm): 8.01 (t, J = 8.1 Hz, 1H), 7.79 – 7.83 (m, 1H), 7.50 (t, J = 7.0 Hz, 1H), 4.50 – 4.55 (m, 2H), 4.45 – 4.50 (m, 2H), 1.42 – 1.47 (m, 12H).
MS (ESI, pos.ion) m/z: 281.25 [M+H]+ .MS (ESI, pos.ion) m/z: 281.25 [M+H] + .
步驟2:化合物6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸的合成Step 2: Synthesis of compound 6-(((tert-butoxycarbonyl)amino)methyl)picolinic acid
將6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸乙酯(700 mg, 2.5 mmol) 和一水合氫氧化鋰 (527 mg, 12.6 mmol) 加入至四氫呋喃 (5 mL) 和水 (5 mL) 混合溶劑中,50℃反應1.5 h後停止,加稀鹽酸調節pH=6,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,得到淺黃色油狀物606 mg,手率96%。Ethyl 6-(((tert-butoxycarbonyl)amino)methyl)picolinate (700 mg, 2.5 mmol) and lithium hydroxide monohydrate (527 mg, 12.6 mmol) were added to tetrahydrofuran (5 mL) and water ( 5 mL) mixed solvent, the reaction was stopped after 1.5 h at 50 °C, diluted hydrochloric acid was added to adjust pH=6, extracted with ethyl acetate (10 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain a pale yellow oil 606 mg of substance, with a hand rate of 96%.
1 H NMR (400 MHz, CD3 OD) δ(ppm): 8.06 (d,J = 7.6 Hz, 1H), 7.99 (t,J = 7.7 Hz, 1H), 7.60 (d,J = 7.6 Hz, 1H), 4.44 (s, 2H), 1.48 (s, 9H). 1 H NMR (400 MHz, CD 3 OD) δ(ppm): 8.06 (d, J = 7.6 Hz, 1H), 7.99 (t, J = 7.7 Hz, 1H), 7.60 (d, J = 7.6 Hz, 1H) ), 4.44 (s, 2H), 1.48 (s, 9H).
MS (ESI, pos.ion) m/z: 253.10 [M+H]+ .MS (ESI, pos.ion) m/z: 253.10 [M+H] + .
步驟3:化合物 ((6-((4,4-二氟環己基)(甲基)甲醯氨基)吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 3: Synthesis of compound ((6-((4,4-difluorocyclohexyl)(methyl)carbamoylamino)pyridin-2-yl)methyl)carbamic acid tert-butyl ester
將6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸(347 mg, 1.38 mmol),4,4-二氟-N -甲基環己胺鹽酸鹽(387 mg, 2.08 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (1.4 g, 7.3 mmol) 和N -羥基-7-氮雜苯並三氮唑 (382 mg, 2.81 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (1.8 mL, 11.0 mmol),室溫攪拌5 h,加水 (35 mL),用二氯甲烷萃取 (15 mL × 3),有機相用無水硫酸鈉乾燥,濃縮,矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到白色固體486 mg,收率92%。6-(((tert-butoxycarbonyl)amino)methyl)picolinic acid (347 mg, 1.38 mmol), 4,4-difluoro- N -methylcyclohexylamine hydrochloride (387 mg, 2.08 mmol) , 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.4 g, 7.3 mmol) and N -hydroxy-7-azabenzotriazole (382 mg, 2.81 mmol) was dissolved in dichloromethane (5 mL), cooled to 0 °C, added N , N -diisopropylethylamine (1.8 mL, 11.0 mmol), stirred at room temperature for 5 h, added water (35 mL), Extracted with dichloromethane (15 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/1) to obtain 486 mg of white solid, yield 92%.
1 H NMR (400 MHz, CDCl3 ) δ(ppm): 7.76 – 7.83 (m, 1H), 7.56 (d,J = 7.7 Hz, 0.5H), 7.46 (d,J = 7.7 Hz, 0.5H), 7.32 – 7.37 (m, 1H), 4.67 – 4.75 (m, 0.5H), 4.47 (d,J = 5.0 Hz, 2H), 3.81 – 3.90 (m, 0.5H), 3.04 (s, 1.5H), 2.89 (s, 1.5H), 2.11 – 2.28 (m, 3H), 1.86 – 2.07 (m, 5H), 1.48 (s, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ(ppm): 7.76 – 7.83 (m, 1H), 7.56 (d, J = 7.7 Hz, 0.5H), 7.46 (d, J = 7.7 Hz, 0.5H), 7.32 – 7.37 (m, 1H), 4.67 – 4.75 (m, 0.5H), 4.47 (d, J = 5.0 Hz, 2H), 3.81 – 3.90 (m, 0.5H), 3.04 (s, 1.5H), 2.89 (s, 1.5H), 2.11 – 2.28 (m, 3H), 1.86 – 2.07 (m, 5H), 1.48 (s, 9H).
MS (ESI, pos.ion) m/z: 384.60 [M+H]+ .MS (ESI, pos.ion) m/z: 384.60 [M+H] + .
步驟4:化合物6-(氨基甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺二鹽酸鹽的合成Step 4: Compound 6- (aminomethyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine dihydrochloride Amides
將化合物 ((6-((4,4-二氟環己基)(甲基)甲醯氨基)吡啶-2-基)甲基)氨基甲酸叔丁酯(486 mg, 1.27 mmol) 溶解於甲醇 (5 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (10 mL),室溫攪拌1.5 h,減壓濃縮,得白色固體423 mg,收率93%。Compound (tert-butyl ((6-((4,4-difluorocyclohexyl)(methyl)carbamoylamino)pyridin-2-yl)methyl)carbamate (486 mg, 1.27 mmol) was dissolved in methanol ( 5 mL) solution, 4 mol/L HCl in ethyl acetate solution (10 mL) was added, stirred at room temperature for 1.5 h, and concentrated under reduced pressure to obtain 423 mg of white solid with a yield of 93%.
1 H NMR (400 MHz, CD3 OD) δ(ppm): 8.00 – 8.05 (m, 1H), 7.56 – 7.61 (m, 2H), 4.57 – 4.62 (m, 0.5H), 4.36 (s, 2H), 3.59 – 3.67 (m, 0.5H), 3.03 (s, 1H), 2.91 (s, 2H), 2.04 – 2.24 (m, 4H), 1.90 – 1.98 (m, 4H). 1 H NMR (400 MHz, CD 3 OD) δ(ppm): 8.00 – 8.05 (m, 1H), 7.56 – 7.61 (m, 2H), 4.57 – 4.62 (m, 0.5H), 4.36 (s, 2H) , 3.59 – 3.67 (m, 0.5H), 3.03 (s, 1H), 2.91 (s, 2H), 2.04 – 2.24 (m, 4H), 1.90 – 1.98 (m, 4H).
MS (ESI, pos.ion) m/z: 284.10 [M+H-2HCl]+ .MS (ESI, pos.ion) m/z: 284.10 [M+H-2HCl] + .
中間體7:中間體6-(氨基甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺二鹽酸鹽 Intermediate 7: Intermediate 6- (aminomethyl) - N - (4- fluorophenyl) - N - methyl acyl pyridine dihydrochloride
步驟1:化合物 ((6-((4-氟苯基)(甲基)甲醯氨基)吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 1: Synthesis of compound ((6-((4-fluorophenyl)(methyl)carbamoylamino)pyridin-2-yl)methyl)carbamate tert-butyl ester
將6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸(236 mg, 0.94 mmol),4-氟-N -甲基苯胺 (182 mg, 1.45 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (913 mg, 4.76 mmol) 和N -羥基-7-氮雜苯並三氮唑(262 mg, 1.92 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.96 mL, 5.80 mmol),室溫攪拌5 h,加水 (15 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮,矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到淺黃色油狀物287 mg,收率85%。6-(((tert-butoxycarbonyl)amino)methyl)picolinic acid (236 mg, 0.94 mmol), 4-fluoro- N -methylaniline (182 mg, 1.45 mmol), 1-ethyl-(3 -Dimethylaminopropyl)carbodiimide hydrochloride (913 mg, 4.76 mmol) and N -hydroxy-7-azabenzotriazole (262 mg, 1.92 mmol) were dissolved in dichloromethane ( 10 mL), cooled to 0 °C, added N , N -diisopropylethylamine (0.96 mL, 5.80 mmol), stirred at room temperature for 5 h, added water (15 mL), and extracted with dichloromethane (10 mL). × 3), the organic phase was dried with anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/1) to obtain 287 mg of pale yellow oil, Yield 85%.
1 H NMR (400 MHz, CDCl3 ) δ(ppm): 7.64 – 7.67 (m, 1H), 7.57 – 7.58 (m, 1H), 7.06 – 7.13 (m, 3H), 6.93 – 6.98 (m, 2H), 4.21 (s, 2H), 3.52 (s, 3H), 1.51 (s, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ(ppm): 7.64 – 7.67 (m, 1H), 7.57 – 7.58 (m, 1H), 7.06 – 7.13 (m, 3H), 6.93 – 6.98 (m, 2H) , 4.21 (s, 2H), 3.52 (s, 3H), 1.51 (s, 9H).
MS (ESI, pos.ion) m/z: 360.10 [M+H]+ .MS (ESI, pos.ion) m/z: 360.10 [M+H] + .
步驟2:化合物6-(氨基甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺二鹽酸鹽的合成Step 2: Compound 6- (aminomethyl) - N - (4- fluorophenyl) - N - methylpyridine dihydrochloride Amides
將化合物 ((6-((4-氟苯基)(甲基)甲醯氨基)吡啶-2-基)甲基)氨基甲酸叔丁酯(260 mg, 1.97 mmol) 溶解於甲醇 (5 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (10 mL),室溫攪拌1.5 h,減壓濃縮,得淺黃色黏稠固體184 mg,收率93%。Compound (tert-butyl ((6-((4-fluorophenyl)(methyl)carbamoylamino)pyridin-2-yl)methyl)carbamate (260 mg, 1.97 mmol) was dissolved in methanol (5 mL) To the solution, 4 mol/L HCl in ethyl acetate solution (10 mL) was added, stirred at room temperature for 1.5 h, and concentrated under reduced pressure to obtain 184 mg of a light yellow viscous solid with a yield of 93%.
1 H NMR (400 MHz, CD3 OD) δ(ppm): 7.73 – 7.78 (m, 1H), 7.38 – 7.40 (m, 1H), 7.30 – 7.32 (m, 1H), 7.22 – 7.29 (m, 2H), 7.00 – 7.04 (m, 2H), 4.21 (s, 2H), 3.50 (s, 3H). 1 H NMR (400 MHz, CD 3 OD) δ(ppm): 7.73 – 7.78 (m, 1H), 7.38 – 7.40 (m, 1H), 7.30 – 7.32 (m, 1H), 7.22 – 7.29 (m, 2H) ), 7.00 – 7.04 (m, 2H), 4.21 (s, 2H), 3.50 (s, 3H).
MS (ESI, pos.ion) m/z: 260.10 [M+H-2HCl]+ .MS (ESI, pos.ion) m/z: 260.10 [M+H-2HCl] + .
中間體8:中間體N -(5-溴-2-(二氟甲氧基)-4-(甲磺醯基)苯基)-N -(環丙基甲基)羥胺 Intermediate 8: Intermediate N - (5- bromo-2- (difluoromethoxy) -4- (acyl methanesulfonamide) phenyl) - N - (cyclopropylmethyl) hydroxylamine
步驟1:化合物4-溴-1-(二氟甲氧基)-2-硝基苯的合成Step 1: Synthesis of compound 4-bromo-1-(difluoromethoxy)-2-nitrobenzene
將化合物4-溴-2-硝基苯酚 (3.0 g, 14 mmol) 溶於N ,N -二甲基甲醯胺 (15 mL),加入二氟氯乙酸鈉 (3.8 g, 25 mmol) 和碳酸銫 (8.1 g, 25 mmol),120℃反應2 h後停止,加水 (25 mL),用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 (v/v) = 5/1),得到淺紅色液體1.6 g,產率43%。The compound 4-bromo-2-nitrophenol (3.0 g, 14 mmol) was dissolved in N , N -dimethylformamide (15 mL), sodium difluorochloroacetate (3.8 g, 25 mmol) and carbonic acid were added. Cesium (8.1 g, 25 mmol), the reaction was stopped at 120 °C for 2 h, water (25 mL) was added, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and subjected to silica gel column Chromatographic separation (eluent: petroleum ether/ethyl acetate (v/v) = 5/1) gave 1.6 g of a light red liquid with a yield of 43%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.09 (d,J = 2.3 Hz, 1H), 7.76 (dd,J = 8.8, 2.4 Hz, 1H), 7.32 (d,J = 8.8 Hz, 1H), 6.63 (t,J F-H = 72.5 Hz, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.09 (d, J = 2.3 Hz, 1H), 7.76 (dd, J = 8.8, 2.4 Hz, 1H), 7.32 (d, J = 8.8 Hz, 1H), 6.63 (t, J FH = 72.5 Hz, 1H).
MS (ESI, pos.ion) m/z: 267.00 [M].MS (ESI, pos.ion) m/z: 267.00 [M].
步驟2:化合物N -(5-溴-2-(二氟甲氧基)苯基)羥胺的合成Step 2: Synthesis of compound N- (5-bromo-2-(difluoromethoxy)phenyl)hydroxylamine
將化合物4-溴-1-(二氟甲氧基)-2-硝基苯(1.6 g, 6 mmol),氯化銨 (961 mg, 18 mmol) 溶於1,4-二氧六環 (20 mL) 和水 (10 mL) 的混合溶劑中,冰浴中加入鋅粉 (1.2 g, 18 mmol),室溫反應4 h,過濾除去固體,濾液濃縮,用乙酸乙酯萃取 (50 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 (v/v) = 15/1),得到淺黃色液體648 mg,產率46%。Compound 4-bromo-1-(difluoromethoxy)-2-nitrobenzene (1.6 g, 6 mmol), ammonium chloride (961 mg, 18 mmol) was dissolved in 1,4-dioxane ( 20 mL) and water (10 mL), zinc powder (1.2 g, 18 mmol) was added to the ice bath, the reaction was carried out at room temperature for 4 h, the solid was removed by filtration, the filtrate was concentrated, and extracted with ethyl acetate (50 mL × 3), the organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 15/1) to obtain 648 mg of pale yellow liquid, Yield 46%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.49 (d,J = 2.2 Hz, 1H), 7.14 (br.s, 1H), 7.06 (dd,J = 8.6, 2.3 Hz, 1H), 6.94 (d,J = 8.6 Hz, 1H), 5.34 (d,J = 1.8 Hz, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.49 (d, J = 2.2 Hz, 1H), 7.14 (br.s, 1H), 7.06 (dd, J = 8.6, 2.3 Hz, 1H), 6.94 (d, J = 8.6 Hz, 1H), 5.34 (d, J = 1.8 Hz, 1H).
MS (ESI, pos.ion) m/z: 254.10 [M+H]+ .MS (ESI, pos.ion) m/z: 254.10 [M+H] + .
步驟3:化合物N -(5-溴-2-(二氟甲氧基)苯基)-N -(環丙基甲基)羥胺的合成Step 3: Compound N - (5- bromo-2- (difluoromethoxy) phenyl) - N - Synthesis of (cyclopropylmethyl) hydroxylamine
將化合物N -(5-溴-2-(二氟甲氧基)苯基)羥胺 (284 mg, 1.12 mmol),環丙基甲醛 (250 mg, 3.57 mmol) 溶於乙醇 (5 mL) 和醋酸 (358 mg, 5.97 mmol) 的混合溶劑中,加入氰基硼氫化鈉 (374 mg, 3.95 mmol),室溫反應12 h,濃縮,加水 (10 mL),用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 20/1),得到黃褐色固體243 mg,產率69%。Compound N- (5-bromo-2-(difluoromethoxy)phenyl)hydroxylamine (284 mg, 1.12 mmol), cyclopropylcarbaldehyde (250 mg, 3.57 mmol) was dissolved in ethanol (5 mL) and acetic acid (358 mg, 5.97 mmol) in a mixed solvent, sodium cyanoborohydride (374 mg, 3.95 mmol) was added, reacted at room temperature for 12 h, concentrated, added with water (10 mL), and extracted with ethyl acetate (5 mL × 3 ), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 20/1) to obtain 243 mg of a yellow-brown solid, the product rate of 69%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.74 (d,J = 2.2 Hz, 1H), 7.18 (dd,J = 8.5, 2.3 Hz, 1H), 6.95 (d,J = 8.7 Hz, 1H), 6.51 (t,J F-H = 74.7 Hz, 1H), 5.87 (s, 1H), 3.03 (d,J = 6.9 Hz, 1H), 1.12 – 1.22 (m, 1H), 0.51 – 0.56 (m, 2H), 0.24 – 0.28 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.74 (d, J = 2.2 Hz, 1H), 7.18 (dd, J = 8.5, 2.3 Hz, 1H), 6.95 (d, J = 8.7 Hz, 1H), 6.51 (t, J FH = 74.7 Hz, 1H), 5.87 (s, 1H), 3.03 (d, J = 6.9 Hz, 1H), 1.12 – 1.22 (m, 1H), 0.51 – 0.56 (m, 2H), 0.24 – 0.28 (m, 2H).
MS (ESI, pos.ion) m/z: 308.05 [M+H]+ .MS (ESI, pos.ion) m/z: 308.05 [M+H] + .
步驟4:化合物N -(5-溴-2-(二氟甲氧基)-4-(甲磺醯基)苯基)-N -(環丙基甲基)羥胺的合成Step 4: The compound N - (5- bromo-2- (difluoromethoxy) -4- (acyl methanesulfonamide) phenyl) - N - (cyclopropylmethyl) hydroxylamine Synthesis of
將化合物N -(5-溴-2-(二氟甲氧基)苯基)-N -(環丙基甲基)羥胺(300 mg, 1.06 mmol) 溶解在二氯甲烷 (6 mL) 中,加入N ,N -二異丙基乙胺 (1.0 mL, 6.10 mmol),0℃條件下滴加甲磺醯氯 (354 mg, 3.09 mmol),室溫攪拌3 h後停止,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 8/1),得到淺褐色液體121 mg,產率31%。The compound N - (5- bromo-2- (difluoromethoxy) phenyl) - N - (cyclopropylmethyl) hydroxylamine (300 mg, 1.06 mmol) was dissolved in dichloromethane (6 mL), N , N -diisopropylethylamine (1.0 mL, 6.10 mmol) was added, methanesulfonic acid chloride (354 mg, 3.09 mmol) was added dropwise at 0 °C, the mixture was stirred at room temperature for 3 h, and then stopped, and water (15 mL) was added. , extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 8/ 1), 121 mg of light brown liquid was obtained, and the yield was 31%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.16 (s, 1H), 6.82 (s, 1H), 6.51 (t,J F-H = 73.3 Hz, 1H), 4.41 (br.s, 1H), 3.23 (s, 1H), 2.96 – 2.99 (m, 1H), 1.10 – 1.16 (m, 1H), 0.61 – 0.65 (m, 2H), 0.27 – 0.31 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.16 (s, 1H), 6.82 (s, 1H), 6.51 (t, J FH = 73.3 Hz, 1H), 4.41 (br.s, 1H) , 3.23 (s, 1H), 2.96 – 2.99 (m, 1H), 1.10 – 1.16 (m, 1H), 0.61 – 0.65 (m, 2H), 0.27 – 0.31 (m, 2H).
MS (ESI, pos.ion) m/z: 386.10 [M+H]+ .MS (ESI, pos.ion) m/z: 386.10 [M+H] + .
中間體9:中間體1-環丙基-N -甲基甲胺鹽酸鹽 Intermediate 9: Intermediate 1-Cyclopropyl- N -methylmethanamine hydrochloride
步驟1:化合物環丙基甲基氨基甲酸叔丁酯的合成Step 1: Synthesis of compound tert-butyl cyclopropylmethylcarbamate
將化合物環丙基甲胺 (0.51 g, 7.11 mmol) 溶解於二氯甲烷 (15 mL) 溶液中,加入N ,N -二異丙基乙胺(3.5 mL, 21 mmol),在-10℃下加入二碳酸二叔丁酯 (1.9 mL, 8.3 mmol),攪拌15 min轉移至室溫反應3 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/1),得到無色液體810 mg,收率67%。The compound cyclopropylmethylamine (0.51 g, 7.11 mmol) was dissolved in dichloromethane (15 mL) solution, N , N -diisopropylethylamine (3.5 mL, 21 mmol) was added, at -10 °C Di-tert-butyl dicarbonate (1.9 mL, 8.3 mmol) was added, stirred for 15 min, transferred to room temperature for 3 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sulfuric acid The solution was dried over sodium, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 3/1) to obtain 810 mg of a colorless liquid with a yield of 67%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 6.84 (br.s, 1H), 2.80 (t,J = 6.1 Hz, 2H), 1.38 (s, 9H), 0.82-0.92(m, 1H), 0.34-0.38 (m, 2H), 0.09-0.14 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 6.84 (br.s, 1H), 2.80 (t, J = 6.1 Hz, 2H), 1.38 (s, 9H), 0.82-0.92(m , 1H), 0.34-0.38 (m, 2H), 0.09-0.14 (m, 2H).
MS (ESI, pos.ion) m/z: 116.25 [M-55]+ .MS (ESI, pos.ion) m/z: 116.25 [M-55] + .
步驟2:化合物(環丙基甲基)(甲基)氨基甲酸叔丁酯的合成Step 2: Synthesis of compound (cyclopropylmethyl)(methyl)carbamate tert-butyl ester
將氫化鈉 (0.47 g, 11.9 mmol) 溶解於N ,N -二甲基甲醯胺 (15 mL) 溶液中,在冰浴中加入環丙基甲基氨基甲酸叔丁酯(0.81 g, 4.7 mmol),5 min後加入碘甲烷 (0. 5 mL, 8.0 mmol),冰浴中繼續反應20 min,轉移至室溫反應7 h,加水 (100 mL),用乙酸乙酯萃取 (25 mL × 3),有機相用無水硫酸鈉乾燥,濃縮得到淡黃色液體790 mg,收率90%。Sodium hydride (0.47 g, 11.9 mmol) was dissolved in N , N -dimethylformamide (15 mL) solution, and tert-butyl cyclopropylmethylcarbamate (0.81 g, 4.7 mmol) was added in an ice bath. ), iodomethane (0.5 mL, 8.0 mmol) was added after 5 min, the reaction was continued in an ice bath for 20 min, transferred to room temperature for 7 h, water (100 mL) was added, and extracted with ethyl acetate (25 mL × 3 ), the organic phase was dried over anhydrous sodium sulfate and concentrated to obtain 790 mg of pale yellow liquid with a yield of 90%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 3.04 (d,J = 6.9 Hz, 1H), 2.82 (s, 1H), 1.39 (s, 9H), 0.89-0.97 (m, 1H), 0.41-0.45 (m, 2H), 0.16-0.20 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 3.04 (d, J = 6.9 Hz, 1H), 2.82 (s, 1H), 1.39 (s, 9H), 0.89-0.97 (m, 1H) ), 0.41-0.45 (m, 2H), 0.16-0.20 (m, 2H).
MS (ESI, pos.ion) m/z: 130.20 [M-55]+ .MS (ESI, pos.ion) m/z: 130.20 [M-55] + .
步驟3:化合物1-(環丙基)-N -甲基甲胺鹽酸鹽的合成Step 3: Synthesis of compound 1-(cyclopropyl) -N -methylmethanamine hydrochloride
將化合物(環丙基甲基)(甲基)氨基甲酸叔丁酯(0.79 g, 4.3 mmol) 溶解於二氯甲烷 (6 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (8 mL),室溫攪拌1.5 h,除去溶劑,得到淡黃色液體350 mg,收率97%。The compound (cyclopropylmethyl)(methyl)carbamate tert-butyl ester (0.79 g, 4.3 mmol) was dissolved in dichloromethane (6 mL) solution, and 4 mol/L HCl in ethyl acetate solution (8 mL), stirred at room temperature for 1.5 h, and removed the solvent to obtain 350 mg of pale yellow liquid with a yield of 97%.
1 H NMR (400 MHz, DMSO-d6 ): δ (ppm) 2.74 (d,J = 7.4 Hz, 2H), 1.91 (s, 3H), 1.00-1.11 (m, 1H), 0.53-0.57 (m, 2H), 0.33-0.38 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ): δ (ppm) 2.74 (d, J = 7.4 Hz, 2H), 1.91 (s, 3H), 1.00-1.11 (m, 1H), 0.53-0.57 (m , 2H), 0.33-0.38 (m, 2H).
中間體10:中間體1-(2,4-二氟苯基)-N -甲基甲胺鹽酸鹽 Intermediate 10: Intermediate 1-(2,4-Difluorophenyl) -N -methylmethanamine hydrochloride
步驟1:化合物2,4-二氟苄基氨基甲酸叔丁酯的合成Step 1: Synthesis of compound tert-butyl 2,4-difluorobenzylcarbamate
將化合物2,4-二氟苯基甲胺 (1.01 g, 7.1 mmol) 溶解於二氯甲烷 (15 mL) 溶液中,加入N ,N -二異丙基乙胺 (3.5 mL, 21 mmol),在-10℃下加入二碳酸二叔丁酯 (2.0 mL, 8.7 mmol),攪拌15 min轉移至室溫反應5 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到無色液體1.4 g,收率82%。Compound 2,4-difluorophenylmethylamine (1.01 g, 7.1 mmol) was dissolved in dichloromethane (15 mL) solution, N , N -diisopropylethylamine (3.5 mL, 21 mmol) was added, Di-tert-butyl dicarbonate (2.0 mL, 8.7 mmol) was added at -10°C, stirred for 15 min, transferred to room temperature and reacted for 5 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), The organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) to obtain 1.4 g of a colorless liquid, yield 82 %.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.31-7.39 (m, 2H), 7.15-7.20 (m, 1H), 7.06 (t,J = 8.0 Hz, 1H), 4.14 (d,J = 5.4 Hz, 2H), 1.39 (s, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.31-7.39 (m, 2H), 7.15-7.20 (m, 1H), 7.06 (t, J = 8.0 Hz, 1H), 4.14 (d , J = 5.4 Hz, 2H), 1.39 (s, 9H).
MS (ESI, pos.ion) m/z: 266.15 [M+Na]+ .MS (ESI, pos.ion) m/z: 266.15 [M+Na] + .
步驟2:化合物 (2,4-二氟苄基)(甲基)氨基甲酸叔丁酯的合成Step 2: Synthesis of compound (2,4-difluorobenzyl)(methyl)carbamate tert-butyl ester
將氫化鈉 (0.63 g, 15.8 mmol) 溶解於N ,N -二甲基甲醯胺 (15 mL) 溶液中,在0℃下加入2,4-二氟苄基氨基甲酸叔丁酯(1.4 g, 5.8 mmol),5 min後再加入碘甲烷 (0.65 mL, 10.0 mmol),0℃下反應20 min,轉移至室溫反應12h,加水 (100 mL),用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到淡黃色液體1.3 g,收率88%。Sodium hydride (0.63 g, 15.8 mmol) was dissolved in N , N -dimethylformamide (15 mL) solution, and tert-butyl 2,4-difluorobenzylcarbamate (1.4 g) was added at 0°C , 5.8 mmol), then add iodomethane (0.65 mL, 10.0 mmol) for 5 min, react at 0 °C for 20 min, transfer to room temperature for 12 h, add water (100 mL), extract with ethyl acetate (5 mL × 3 ), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) to obtain 1.3 g of pale yellow liquid, The yield is 88%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.27-7.33 (m, 1H), 7.20-7.26 (m, 1H), 7.09 (t,J = 7.9 Hz, 1H), 4.39 (s, 2H), 2.78 (s, 3H), 1.38 (s, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.27-7.33 (m, 1H), 7.20-7.26 (m, 1H), 7.09 (t, J = 7.9 Hz, 1H), 4.39 (s , 2H), 2.78 (s, 3H), 1.38 (s, 9H).
MS (ESI, pos.ion) m/z: 280.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 280.20 [M+Na] + .
步驟3:化合物1-(2,4-二氟苯基)-N -甲基甲胺鹽酸鹽的合成Step 3: Synthesis of compound 1-(2,4-difluorophenyl) -N -methylmethanamine hydrochloride
將化合物 (2,4-二氟苄基)(甲基)氨基甲酸叔丁酯(1.3 g, 5.1 mmol) 溶解於二氯甲烷 (6 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (10 mL),室溫攪拌1 h,除去溶劑,得到淡黃色固體763 mg,收率96%。The compound (2,4-difluorobenzyl)(methyl)carbamate tert-butyl ester (1.3 g, 5.1 mmol) was dissolved in dichloromethane (6 mL) solution, and 4 mol/L HCl in ethyl acetate was added The solution (10 mL) was stirred at room temperature for 1 h, and the solvent was removed to obtain 763 mg of a pale yellow solid with a yield of 96%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 9.47-9.61 (m, 1H), 7.74-7.81 (m, 1H), 7.34-7.39 (m, 1H), 7.21 (t,J = 8.5 Hz, 1H), 4.13 (s, 2H), 2.54 (s, 2H), 2.51 (s, 1H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 9.47-9.61 (m, 1H), 7.74-7.81 (m, 1H), 7.34-7.39 (m, 1H), 7.21 (t, J = 8.5 Hz, 1H), 4.13 (s, 2H), 2.54 (s, 2H), 2.51 (s, 1H).
MS (ESI, pos.ion) m/z: 158.20 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 158.20 [M+H-HCl] + .
中間體11:中間體N -甲基-1-(吡啶-2-基)甲胺二鹽酸鹽 Intermediate 11: Intermediate N -methyl-1-(pyridin-2-yl)methanamine dihydrochloride
步驟1:化合物 (吡啶-2-基甲基)氨基甲酸叔丁酯的合成Step 1: Synthesis of compound (pyridin-2-ylmethyl)carbamate tert-butyl ester
將化合物2-吡啶甲胺 (510 mg, 4.70 mmol) 溶解於二氯甲烷 (15 mL) 溶液中,加入N ,N -二異丙基乙胺 (2.3 mL, 14 mmol),在-10℃下加入二碳酸二叔丁酯 (1.3 mL, 5.7 mmol),攪拌15 min轉移至室溫反應8 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到無色液體620 mg,收率64%。The compound 2-pyridylmethylamine (510 mg, 4.70 mmol) was dissolved in dichloromethane (15 mL) solution, N , N -diisopropylethylamine (2.3 mL, 14 mmol) was added, at -10 °C Di-tert-butyl dicarbonate (1.3 mL, 5.7 mmol) was added, stirred for 15 min, transferred to room temperature for 8 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sulfuric acid The solution was dried over sodium, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/1) to obtain 620 mg of a colorless liquid with a yield of 64%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.48 (d,J = 4.1 Hz, 1H), 7.76 (t,J = 7.3 Hz, 1H), 7.41 (br.s, 1H), 7.21-7.29 (m, 2H), 4.22 (d,J = 5.9 Hz, 2H), 1.41 (s, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.48 (d, J = 4.1 Hz, 1H), 7.76 (t, J = 7.3 Hz, 1H), 7.41 (br.s, 1H), 7.21-7.29 (m, 2H), 4.22 (d, J = 5.9 Hz, 2H), 1.41 (s, 9H).
MS (ESI, pos.ion) m/z: 209.10 [M+H]+ .MS (ESI, pos.ion) m/z: 209.10 [M+H] + .
步驟2:化合物N -甲基-(吡啶-2-基甲基)氨基甲酸叔丁酯的合成Step 2: Synthesis of compound N -methyl-(pyridin-2-ylmethyl)carbamate tert-butyl ester
將氫化鈉 (310 mg, 7.6 mmol) 溶解於N ,N -二甲基甲醯胺 (10 mL) 溶液中,在0℃下加入(吡啶-2-基甲基)氨基甲酸叔丁酯(620 mg, 2.98 mmol),5 min後再加入碘甲烷 (0.3 mL, 5.0 mmol),0℃下反應20 min,轉移至室溫反應18 h,加水洗 (100 mL),用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到淡黃色液體580 mg,收率88%。Sodium hydride (310 mg, 7.6 mmol) was dissolved in N , N -dimethylformamide (10 mL) solution, and tert-butyl (pyridin-2-ylmethyl)carbamate (620 °C) was added at 0 °C. mg, 2.98 mmol), then add iodomethane (0.3 mL, 5.0 mmol) for 5 min, react at 0 °C for 20 min, transfer to room temperature and react for 18 h, add water (100 mL), and extract with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/1) to obtain 580 mg of pale yellow liquid, The yield is 88%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.52 (d,J = 4.3 Hz, 1H), 7.78 (t,J = 7.4 Hz, 1H), 7.26 – 7.29 (m, 1H), 7.19 (d,J = 7.6 Hz, 1H), 4.45 (s, 2H), 2.86 (s, 3H), 1.43 (s, 4H), 1.30 (s, 5H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.52 (d, J = 4.3 Hz, 1H), 7.78 (t, J = 7.4 Hz, 1H), 7.26 – 7.29 (m, 1H), 7.19 (d, J = 7.6 Hz, 1H), 4.45 (s, 2H), 2.86 (s, 3H), 1.43 (s, 4H), 1.30 (s, 5H).
MS (ESI, pos.ion) m/z: 223.25 [M+H]+ .MS (ESI, pos.ion) m/z: 223.25 [M+H] + .
步驟3:化合物N -甲基-1-(吡啶-2-基)甲胺二鹽酸鹽的合成Step 3: Synthesis of compound N -methyl-1-(pyridin-2-yl)methanamine dihydrochloride
將化合物甲基 (吡啶-2-基甲基)氨基甲酸叔丁酯(580 mg, 2.6 mmol) 溶解於二氯甲烷 (6 mL)溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (8 mL),室溫攪拌1 h,除去溶劑,得到灰色固體310 mg,收率98%。The compound methyl(pyridin-2-ylmethyl)carbamate tert-butyl ester (580 mg, 2.6 mmol) was dissolved in dichloromethane (6 mL) solution, and 4 mol/L HCl in ethyl acetate solution (8 mL), stirred at room temperature for 1 h, and removed the solvent to obtain 310 mg of a gray solid with a yield of 98%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.69 (d,J = 4.4 Hz, 1H), 8.02 (t,J = 6.8 Hz, 1H), 7.68 – 7.70 (m, 1H), 7.52 – 7.55 (m, 1H), 4.33 (t,J = 5.5 Hz, 2H), 2.60 (t,J = 4.8 Hz, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.69 (d, J = 4.4 Hz, 1H), 8.02 (t, J = 6.8 Hz, 1H), 7.68 – 7.70 (m, 1H), 7.52 – 7.55 (m, 1H), 4.33 (t, J = 5.5 Hz, 2H), 2.60 (t, J = 4.8 Hz, 2H).
MS (ESI, pos.ion) m/z: 123.30 [M+H]+ .MS (ESI, pos.ion) m/z: 123.30 [M+H] + .
中間體12:中間體N -((6-(氨基甲基)吡啶-2-基)甲基)-N -(4,4-二氟環己基)乙醯胺二鹽酸鹽 Intermediate 12: Intermediate N - ((6- (aminomethyl) pyridin-2-yl) methyl) - N - (4,4- difluoro-cyclohexyl) as acetamide dihydrochloride
步驟1:化合物 ((6-(羥甲基)吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 1: Synthesis of compound ((6-(hydroxymethyl)pyridin-2-yl)methyl)carbamate tert-butyl ester
將化合物6-(((叔丁氧羰基)氨基)甲基)吡啶甲酸乙酯 (747 mg, 2.67 mmol) 溶於無水四氫呋喃 (10 mL),冰浴下加入硼氫化鋰 (304 mg, 14.0 mmol),室溫反應2 h後停止,加入碎冰,用乙酸乙酯萃取 (10 mL × 3),合併有機相後,用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到白色固體515 mg,收率81%。The compound 6-(((tert-butoxycarbonyl)amino)methyl)picolinate (747 mg, 2.67 mmol) was dissolved in anhydrous tetrahydrofuran (10 mL), and lithium borohydride (304 mg, 14.0 mmol) was added under ice bath. ), the reaction was stopped after 2 h at room temperature, crushed ice was added, extracted with ethyl acetate (10 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (washed Removal agent: petroleum ether/ethyl acetate (v/v) = 1/2) to obtain 515 mg of white solid with a yield of 81%.
MS (ESI, pos.ion) m/z: 239.30 [M+H]+ .MS (ESI, pos.ion) m/z: 239.30 [M+H] + .
步驟2:化合物 ((6-甲醯基吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 2: Synthesis of compound ((6-carboxypyridin-2-yl)methyl)carbamic acid tert-butyl ester
將二甲基亞碸 (669 mg, 8.56 mmol) 溶於二氯甲烷 (2 mL),-78℃下加入草醯氯 (1.1 g, 8.70 mmol) 的二氯甲烷溶液 (3 mL),在此溫度下反應30 min後加入化合物 ((6-(羥甲基)吡啶-2-基)甲基)氨基甲酸叔丁酯(510 mg, 2.14 mmol) 的二氯甲烷溶液 (10 mL),繼續反應30 min,-78℃下加入三乙胺 (1.5 mL, 11 mmol),緩慢升高到室溫,水洗有機相 (10 mL ×3 ),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/1),得到白色固體459 mg,收率90%。Dimethyl sulfoxide (669 mg, 8.56 mmol) was dissolved in dichloromethane (2 mL), and a solution of oxalic chloride (1.1 g, 8.70 mmol) in dichloromethane (3 mL) was added at -78°C, where After reacting for 30 min at the temperature, a dichloromethane solution (10 mL) of the compound ((6-(hydroxymethyl)pyridin-2-yl)methyl)carbamate (510 mg, 2.14 mmol) in dichloromethane (10 mL) was added, and the reaction was continued. For 30 min, triethylamine (1.5 mL, 11 mmol) was added at -78°C, the temperature was slowly raised to room temperature, the organic phase was washed with water (10 mL × 3 ), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the solution was concentrated. Separation by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 3/1) gave 459 mg of white solid with a yield of 90%.
MS (ESI, pos.ion) m/z: 237.10 [M+H]+ .MS (ESI, pos.ion) m/z: 237.10 [M+H] + .
步驟3:化合物 ((6-(((4,4-二氟環己基)氨基)甲基)吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 3: Synthesis of compound ((6-(((4,4-difluorocyclohexyl)amino)methyl)pyridin-2-yl)methyl)carbamic acid tert-butyl ester
將化合物 ((6-甲醯基吡啶-2-基)甲基)氨基甲酸叔丁酯(233 mg, 0.99 mmol) 和4,4-二氟環己胺鹽酸鹽 (217 mg, 1.26 mmol) 溶於乙醇 (5 mL) 中,加入乙酸 (59 mg, 0.98 mmol) 和氰基硼氫化鈉 (187 mg, 2.98 mmol),50℃反應6 h,減壓除去乙醇,加水洗 (10 mL),乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/5),得到無色液體323 mg,收率92%。Compound ((6-carbamoylpyridin-2-yl)methyl)carbamate tert-butyl ester (233 mg, 0.99 mmol) and 4,4-difluorocyclohexylamine hydrochloride (217 mg, 1.26 mmol) Dissolve in ethanol (5 mL), add acetic acid (59 mg, 0.98 mmol) and sodium cyanoborohydride (187 mg, 2.98 mmol), react at 50 °C for 6 h, remove ethanol under reduced pressure, wash with water (10 mL), Ethyl acetate extraction (10 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/5 ) to obtain 323 mg of a colorless liquid with a yield of 92%.
MS (ESI, pos.ion) m/z: 356.25 [M+H]+ .MS (ESI, pos.ion) m/z: 356.25 [M+H] + .
步驟4:化合物 ((6-((N -(4,4-二氟環己基)乙醯氨基)甲基)吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 4: Synthesis of compound ((6-(( N- (4,4-difluorocyclohexyl)acetamido)methyl)pyridin-2-yl)methyl)carbamate tert-butyl ester
將化合物 ((6-(((4,4-二氟環己基)氨基)甲基)吡啶-2-基)甲基)氨基甲酸叔丁酯(322 mg, 0.91 mmol) 溶解在二氯甲烷 (8 mL)中,加入N ,N -二異丙基乙胺 (0.75 mL, 4.5 mmol),0℃條件下滴加乙醯氯 (0.2 mL, 3.0 mmol),室溫攪拌5 h後停止反應,加水 (20 mL),二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯( v/v ) = 1/4),得到白色固體212 mg,產率59%。Compound ((6-(((4,4-difluorocyclohexyl)amino)methyl)pyridin-2-yl)methyl)carbamate (322 mg, 0.91 mmol) was dissolved in dichloromethane ( 8 mL), N , N -diisopropylethylamine (0.75 mL, 4.5 mmol) was added, acetyl chloride (0.2 mL, 3.0 mmol) was added dropwise at 0 °C, the reaction was stopped after stirring at room temperature for 5 h, Water (20 mL) was added, extracted with dichloromethane (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/4) to obtain 212 mg of white solid with a yield of 59%.
MS (ESI, pos.ion) m/z: 398.30 [M+H]+ .MS (ESI, pos.ion) m/z: 398.30 [M+H] + .
步驟5:化合物N -((6-(氨基甲基)吡啶-2-基)甲基)-N -(4,4-二氟環己基)乙醯胺二鹽酸鹽的合成Step 5: The compound N - ((6- (aminomethyl) pyridin-2-yl) methyl) - N - Synthesis of (4,4-difluoro-cyclohexyl) as acetamide dihydrochloride
將化合物 ((6-((N -(4,4-二氟環己基)乙醯氨基)甲基)吡啶-2-基)甲基)氨基甲酸叔丁酯(200 mg, 0.5 mmol) 溶解於二氯甲烷 (4 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (6 mL),室溫攪拌30 min,除去溶劑,得到182 mg白色固體,收率97%。Compound ((6-(( N- (4,4-difluorocyclohexyl)acetamido)methyl)pyridin-2-yl)methyl)carbamic acid tert-butyl ester (200 mg, 0.5 mmol) was dissolved in Dichloromethane (4 mL) solution was added with 4 mol/L HCl in ethyl acetate solution (6 mL), stirred at room temperature for 30 min, and the solvent was removed to obtain 182 mg of white solid with a yield of 97%.
MS (ESI, pos.ion) m/z: 298.30 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 298.30 [M+H-HCl] + .
中間體13:6-(氨基甲基)-2-甲基異吲哚啉-1-酮 Intermediate 13: 6-(Aminomethyl)-2-methylisoindolin-1-one
步驟1:3-氧代異吲哚啉-5-甲酸甲酯的合成Step 1: Synthesis of methyl 3-oxoisoindoline-5-carboxylate
將化合物3-氧代異吲哚啉-5-甲酸 (1.00 g, 5.60 mmol),N ,N’ -羰基二咪唑 (960 mg, 5.92 mmol) 溶於N ,N’ -二甲基甲醯胺 (20 mL) 中,60℃下攪拌30 min,依次加入N ,N -二異丙基乙胺 (1.90 mL,11.0 mmol) 與甲醇 (0.50 mL,11.0 mmol),60℃下攪拌3.5 h。減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1),得到淺黃色固體 (900 mg, 產率83%)。The compound 3-oxoisoindoline-5-carboxylic acid (1.00 g, 5.60 mmol), N , N' -carbonyldiimidazole (960 mg, 5.92 mmol) was dissolved in N , N' -dimethylformamide (20 mL), stirred at 60 °C for 30 min, then added N , N -diisopropylethylamine (1.90 mL, 11.0 mmol) and methanol (0.50 mL, 11.0 mmol), and stirred at 60 °C for 3.5 h. The solvent was removed by concentration under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain a pale yellow solid (900 mg, yield 83%).
1 H NMR (400 MHz, DMSO-d6 ): δ (ppm) 8.15 – 8.18 (m, 1H), 7.95 (s, 2H), 4.47 (s, 2H), 3.89 (s, 3H). 1 H NMR (400 MHz, DMSO- d 6 ): δ (ppm) 8.15 – 8.18 (m, 1H), 7.95 (s, 2H), 4.47 (s, 2H), 3.89 (s, 3H).
MS (ESI, pos.ion) m/z: 192.10 [M+H]+ .MS (ESI, pos.ion) m/z: 192.10 [M+H] + .
步驟2:2-甲基-3-氧代異吲哚啉-5-甲酸甲酯的合成Step 2: Synthesis of methyl 2-methyl-3-oxoisoindoline-5-carboxylate
將化合物3-氧代異吲哚啉-5-甲酸甲酯 (1.00 g, 5.23 mmol),氫化鈉 (271 mg, 6.78 mmol) 溶於N ,N’ -二甲基甲醯胺 (40 mL) 中,在氮氣氛圍下50℃下攪拌20 min,加入硫酸二甲酯 (866 mg, 6.79 mmol),升溫至100℃攪拌反應2 h。停止反應,減壓濃縮除去溶劑,用乙酸乙酯 (40 mL) 溶解,用水 (20 mL × 2) 洗滌,無水硫酸鈉乾燥有機相,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 石油醚/乙酸乙酯 (v/v) = 1/4),得到褐色固體 (450 mg, 產率42%)。Compound 3-oxoisoindoline-5-carboxylic acid methyl ester (1.00 g, 5.23 mmol), sodium hydride (271 mg, 6.78 mmol) was dissolved in N , N' -dimethylformamide (40 mL) was stirred at 50 °C for 20 min under nitrogen atmosphere, dimethyl sulfate (866 mg, 6.79 mmol) was added, the temperature was raised to 100 °C and the reaction was stirred for 2 h. The reaction was stopped, concentrated under reduced pressure to remove the solvent, dissolved in ethyl acetate (40 mL), washed with water (20 mL × 2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent, and the concentrate was separated by silica gel column chromatography ( Eluent: petroleum ether/ethyl acetate (v/v) = 1/4) to give a brown solid (450 mg, 42% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 8.50 (s, 1H), 8.20 – 8.26 (m, 1H), 7.51 (d,J = 7.9 Hz, 1H), 4.43 (s, 2H), 3.95 (s, 3H), 3.22 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 8.50 (s, 1H), 8.20 – 8.26 (m, 1H), 7.51 (d, J = 7.9 Hz, 1H), 4.43 (s, 2H), 3.95 (s, 3H), 3.22 (s, 3H).
MS (ESI, pos.ion) m/z: 206.15 [M+H]+ .MS (ESI, pos.ion) m/z: 206.15 [M+H] + .
步驟3:6-(羥甲基)-2-甲基異吲哚啉-1-酮的合成Step 3: Synthesis of 6-(hydroxymethyl)-2-methylisoindolin-1-one
將化合物2-甲基-3-氧代異吲哚啉-5-羧酸甲酯(460 mg, 2.24 mmol),溶於四氫呋喃 (25 mL) 中,加入硼氫化鋰 (195 mg, 8.95 mmol),50℃下攪拌6 h,停止反應,加水 (10 mL) 攪拌5 min,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 10/1),得到白色固體 (370 mg, 產率93%)。The compound 2-methyl-3-oxoisoindoline-5-carboxylic acid methyl ester (460 mg, 2.24 mmol) was dissolved in tetrahydrofuran (25 mL), and lithium borohydride (195 mg, 8.95 mmol) was added , stirred at 50°C for 6 h, stopped the reaction, added water (10 mL), stirred for 5 min, concentrated under reduced pressure to remove the solvent, and separated the concentrate by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 10/1) to give a white solid (370 mg, 93% yield).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.76 (s, 1H), 7.57 – 7.64 (m, 1H), 7.51 – 7.56 (m, 1H), 4.71 (s, 2H), 4.49 (s, 2H), 3.21 (s, 3H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.76 (s, 1H), 7.57 – 7.64 (m, 1H), 7.51 – 7.56 (m, 1H), 4.71 (s, 2H), 4.49 ( s, 2H), 3.21 (s, 3H).
MS (ESI, pos.ion) m/z: 178.20 [M+H]+ .MS (ESI, pos.ion) m/z: 178.20 [M+H] + .
步驟4:(2-甲基-3-氧代異吲哚啉-5-基)甲基 甲磺酸酯的合成Step 4: Synthesis of (2-methyl-3-oxoisoindolin-5-yl)methyl mesylate
將化合物6-(羥甲基)-2-甲基異吲哚-1-酮(252 mg, 1.42 mmol) 溶於二氯甲烷 (10 mL) 中,加入N ,N -二異丙基乙胺 (737 mg,0.94 mmol),冰浴下攪拌5 min後,加入甲磺醯氯 (325 mg,2.84 mmol),室溫下攪拌1 h,停止反應,加水 (10 mL) 攪拌5 min,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 石油醚/乙酸乙酯 (v/v) = 1/4),得到淺黃色固體 (240 mg, 產率66%)。Compound 6-(hydroxymethyl)-2-methylisoindol-1-one (252 mg, 1.42 mmol) was dissolved in dichloromethane (10 mL), and N , N -diisopropylethylamine was added (737 mg, 0.94 mmol), stirred under ice bath for 5 min, added methanesulfonic acid chloride (325 mg, 2.84 mmol), stirred at room temperature for 1 h, stopped the reaction, added water (10 mL), stirred for 5 min, reduced pressure The solvent was removed by concentration, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/4) to obtain a pale yellow solid (240 mg, yield 66%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.87 (s, 1H), 7.57 – 7.63 (m, 1H), 7.46 – 7.52 (m, 1H), 5.31 (s, 2H), 4.40 (s, 2H), 3.21 (s, 3H), 2.98 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.87 (s, 1H), 7.57 – 7.63 (m, 1H), 7.46 – 7.52 (m, 1H), 5.31 (s, 2H), 4.40 (s , 2H), 3.21 (s, 3H), 2.98 (s, 3H).
MS (ESI, pos.ion) m/z: 256.15 [M+H]+ .MS (ESI, pos.ion) m/z: 256.15 [M+H] + .
步驟5:6-(疊氮甲基)-2-甲基異吲哚啉-1-酮的合成Step 5: Synthesis of 6-(azidomethyl)-2-methylisoindolin-1-one
將化合物(2-甲基-3-氧代異吲哚啉-5-基)甲基 甲磺酸酯(240 mg, 0.94 mmol) 與疊氮化鈉 (305 mg, 4.69 mmol) 溶於N ,N’ -二甲基甲醯胺 (10 mL) 中,80℃下攪拌2.5 h,減壓濃縮除去溶劑,乙酸乙酯 (20 mL) 溶解,用水 (10 mL × 2) 洗滌,有機相用無水硫酸鈉乾燥,減壓濃縮得到淺黃色固體 (181 mg, 產率95%)。Compound (2-methyl-3-oxoisoindolin-5-yl)methylmethanesulfonate (240 mg, 0.94 mmol) and sodium azide (305 mg, 4.69 mmol) were dissolved in N , N' -dimethylformamide (10 mL), stirred at 80 °C for 2.5 h, concentrated under reduced pressure to remove the solvent, dissolved in ethyl acetate (20 mL), washed with water (10 mL × 2), and the organic phase was washed with anhydrous Dry over sodium sulfate and concentrate under reduced pressure to give a pale yellow solid (181 mg, 95% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.79 (s, 1H), 7.43 – 7.53 (m, 2H), 4.43 (s, 2H), 4.39 (s, 2H), 3.21 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.79 (s, 1H), 7.43 – 7.53 (m, 2H), 4.43 (s, 2H), 4.39 (s, 2H), 3.21 (s, 3H) ).
MS (ESI, pos.ion) m/z: 203.15 [M+H]+ .MS (ESI, pos.ion) m/z: 203.15 [M+H] + .
步驟6:6-(氨基甲基)-2-甲基異吲哚啉-1-酮的合成Step 6: Synthesis of 6-(aminomethyl)-2-methylisoindolin-1-one
將化合物6-(疊氮甲基)-2-甲基異吲哚啉-1-酮(180 mg, 0.89 mmol) 溶於甲醇 (10 mL)中,加入Pd/C (20 mg, 0.10 g/g),通入氫氣,室溫下攪拌30 min,通過矽藻土抽濾除去催化劑,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 10/1),得到白色固體 (67 mg, 42%)。Compound 6-(azidomethyl)-2-methylisoindolin-1-one (180 mg, 0.89 mmol) was dissolved in methanol (10 mL), Pd/C (20 mg, 0.10 g/ g), pass into hydrogen, stir for 30 min at room temperature, remove the catalyst through diatomaceous earth suction filtration, concentrate under reduced pressure to remove the solvent, and separate the concentrate by silica gel column chromatography (eluent: dichloromethane/methanol (v/ v) = 10/1) to give a white solid (67 mg, 42%).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.73 (s, 1H), 7.27 – 7.63 (m, 1H), 7.50 – 7.56 (m, 1H), 4.48 (s, 2H), 3.93 (s, 2H), 3.19 (s, 3H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.73 (s, 1H), 7.27 – 7.63 (m, 1H), 7.50 – 7.56 (m, 1H), 4.48 (s, 2H), 3.93 ( s, 2H), 3.19 (s, 3H).
MS (ESI, pos.ion) m/z: 177.20 [M+H]+ .MS (ESI, pos.ion) m/z: 177.20 [M+H] + .
中間體14:5-(氨基甲基)-2-甲基異吲哚啉-1-酮 Intermediate 14: 5-(Aminomethyl)-2-methylisoindolin-1-one
步驟1:1-氧代異吲哚啉-5-甲酸甲酯的合成Step 1: Synthesis of methyl 1-oxoisoindoline-5-carboxylate
將化合物1-氧代異吲哚啉-5-甲酸 (2.00 g, 11.0 mmol),N ,N’ -羰基二咪唑 (2.00 g, 12.0 mmol) 溶於N ,N’ -二甲基甲醯胺 (40 mL) 中,60℃下攪拌30 min,依次加入N ,N -二異丙基乙胺 (7.50 mL,45.0 mmol) 與甲醇 (1.80 mL,45.0 mmol),60℃下攪拌3.5 h。減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1),得到淺黃色固體 (1.00 g, 產率46%)。Compound 1-oxoisoindoline-5-carboxylic acid (2.00 g, 11.0 mmol), N , N' -carbonyldiimidazole (2.00 g, 12.0 mmol) was dissolved in N , N' -dimethylformamide (40 mL), stirred at 60 °C for 30 min, added N , N -diisopropylethylamine (7.50 mL, 45.0 mmol) and methanol (1.80 mL, 45.0 mmol) successively, and stirred at 60 °C for 3.5 h. The solvent was removed by concentration under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain a pale yellow solid (1.00 g, yield 46%).
1 H NMR (600 MHz, DMSO-d6 ): δ (ppm) 8.81 (s, 1H), 8.16 (s, 1H), 8.05 (d,J = 7.9 Hz, 1H), 7.79 (d,J = 7.9 Hz, 1H), 4.44 (s, 2H), 3.89 (s, 3H). 1 H NMR (600 MHz, DMSO- d 6 ): δ (ppm) 8.81 (s, 1H), 8.16 (s, 1H), 8.05 (d, J = 7.9 Hz, 1H), 7.79 (d, J = 7.9 Hz, 1H), 4.44 (s, 2H), 3.89 (s, 3H).
MS (ESI, pos.ion) m/z: 192.20 [M+H]+ .MS (ESI, pos.ion) m/z: 192.20 [M+H] + .
步驟2:2-甲基-1-氧代異吲哚啉-5-甲酸甲酯的合成Step 2: Synthesis of methyl 2-methyl-1-oxoisoindoline-5-carboxylate
將化合物1-氧代異吲哚啉-5-甲酸甲酯 (945 mg, 4.94 mmol),氫化鈉 (300 mg, 7.50 mmol) 溶於N ,N’ -二甲基甲醯胺 (30 mL) 中,在氮氣氛圍下50℃下攪拌20 min,加入硫酸二甲酯 (944 mg, 7.41 mmol) ,升溫至100℃攪拌反應2 h。停止反應,減壓濃縮除去溶劑,用乙酸乙酯 (20 mL) 溶解,用水 (10 mL) 洗滌,無水硫酸鈉乾燥有機相,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 石油醚/乙酸乙酯 (v/v) = 1/4),得到褐色固體 (600 mg, 產率59%)。Compound 1-oxoisoindoline-5-carboxylic acid methyl ester (945 mg, 4.94 mmol), sodium hydride (300 mg, 7.50 mmol) was dissolved in N , N' -dimethylformamide (30 mL) was stirred at 50 °C for 20 min under nitrogen atmosphere, dimethyl sulfate (944 mg, 7.41 mmol) was added, the temperature was raised to 100 °C and the reaction was stirred for 2 h. The reaction was stopped, concentrated under reduced pressure to remove the solvent, dissolved in ethyl acetate (20 mL), washed with water (10 mL), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent, and the concentrate was separated by silica gel column chromatography (eluting Reagent: petroleum ether/ethyl acetate (v/v) = 1/4) to give a brown solid (600 mg, 59% yield).
1 H NMR (600 MHz, CDCl3): δ (ppm) 8.14 (d, J = 8.0 Hz, 1H), 8.12 (s, 1H), 7.89 (d, J = 7.9 Hz, 1H), 4.43 (s, 2H), 3.95 (s, 3H), 3.22 (s, 3H). 1 H NMR (600 MHz, CDCl3): δ (ppm) 8.14 (d, J = 8.0 Hz, 1H), 8.12 (s, 1H), 7.89 (d, J = 7.9 Hz, 1H), 4.43 (s, 2H) ), 3.95 (s, 3H), 3.22 (s, 3H).
MS (ESI, pos.ion) m/z: 206.20 [M+H]+ .MS (ESI, pos.ion) m/z: 206.20 [M+H] + .
步驟3:5-(羥甲基)-2-甲基異吲哚-1-酮的合成Step 3: Synthesis of 5-(hydroxymethyl)-2-methylisoindol-1-one
將化合物2-甲基-1-氧代異吲哚啉-5-甲酸甲酯(600 mg, 2.92 mmol),溶於四氫呋喃 (25 mL) 中,加入硼氫化鋰 (254 mg, 11.7 mmol),50℃下攪拌5 h,停止反應,加水 (10 mL) 攪拌5 min,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1),得到白色固體 (437 mg, 84%)。The compound 2-methyl-1-oxoisoindoline-5-carboxylic acid methyl ester (600 mg, 2.92 mmol) was dissolved in tetrahydrofuran (25 mL), lithium borohydride (254 mg, 11.7 mmol) was added, Stir at 50°C for 5 h, stop the reaction, add water (10 mL), stir for 5 min, concentrate under reduced pressure to remove the solvent, and separate the concentrate by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20 /1) to give a white solid (437 mg, 84%).
1 H NMR (600 MHz, CDCl3 ): δ (ppm) 7.76 (d,J = 7.8 Hz, 1H), 7.46 (s, 1H), 7.40 (d,J = 7.7 Hz, 1H), 4.76 – 4.83 (m, 2H), 4.34 (s, 2H), 3.18 (s, 3H). 1 H NMR (600 MHz, CDCl 3 ): δ (ppm) 7.76 (d, J = 7.8 Hz, 1H), 7.46 (s, 1H), 7.40 (d, J = 7.7 Hz, 1H), 4.76 – 4.83 ( m, 2H), 4.34 (s, 2H), 3.18 (s, 3H).
MS (ESI, pos.ion) m/z: 178.20 [M+H]+ .MS (ESI, pos.ion) m/z: 178.20 [M+H] + .
步驟4:(2-甲基-1-氧代異吲哚啉-5-基)甲基 甲磺酸酯的合成Step 4: Synthesis of (2-methyl-1-oxoisoindolin-5-yl)methylmethanesulfonate
將化合物5-(羥甲基)-2-甲基異吲哚-1-酮(250 mg, 1.41 mmol) 溶於二氯甲烷 (10 mL) 中,加入N ,N -二異丙烯基乙醯胺 (732 mg,5.64 mmol),冰浴下攪拌5 min後,加入甲磺醯氯 (326 mg,2.84 mmol),室溫下攪拌1 h,停止反應,加水 (10 mL) 攪拌5 min,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 石油醚/乙酸乙酯 (v/v) = 1/4),得到白色固體 (250 mg, 產率69%)。The compound 5-(hydroxymethyl)-2-methylisoindol-1-one (250 mg, 1.41 mmol) was dissolved in dichloromethane (10 mL), and N , N -diisopropenylacetone was added Amine (732 mg, 5.64 mmol), stirred under ice bath for 5 min, added methanesulfonic acid chloride (326 mg, 2.84 mmol), stirred at room temperature for 1 h, stopped the reaction, added water (10 mL), stirred for 5 min, reduced The solvent was removed by pressure concentration, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/4) to obtain a white solid (250 mg, yield 69%).
化合物206-4:1 H NMR (600 MHz, CDCl3 ): δ (ppm) 7.86 (d,J = 7.8 Hz, 1H), 7.51 (s, 1H), 7.49 (d,J = 7.8 Hz, 1H), 5.31 (s, 2H), 4.40 (s, 2H), 3.21 (s, 3H), 2.99 (s, 3H).Compound 206-4: 1 H NMR (600 MHz, CDCl 3 ): δ (ppm) 7.86 (d, J = 7.8 Hz, 1H), 7.51 (s, 1H), 7.49 (d, J = 7.8 Hz, 1H) , 5.31 (s, 2H), 4.40 (s, 2H), 3.21 (s, 3H), 2.99 (s, 3H).
MS (ESI, pos.ion) m/z: 256.15 [M+H]+ .MS (ESI, pos.ion) m/z: 256.15 [M+H] + .
步驟5:5-(疊氮甲基)-2-甲基異吲哚啉-1-酮的合成Step 5: Synthesis of 5-(azidomethyl)-2-methylisoindolin-1-one
將化合物(2-甲基-1-氧代異吲哚啉-5-基)甲基 甲磺酸酯(250 mg, 0.98 mmol) 與疊氮化鈉 (318 mg, 4.89 mmol) 溶於N ,N’ -二甲基甲醯胺 (10 mL) 中,80℃下攪拌3 h,減壓濃縮除去溶劑,乙酸乙酯 (20 mL) 溶解,用水 (10 mL × 2) 洗滌,有機相用無水硫酸鈉乾燥,減壓濃縮除去溶劑得到淺黃色固體 (198 mg, 產率100%)。Compound (2-methyl-1-oxoisoindolin-5-yl)methylmethanesulfonate (250 mg, 0.98 mmol) and sodium azide (318 mg, 4.89 mmol) were dissolved in N , N' -dimethylformamide (10 mL), stirred at 80 °C for 3 h, concentrated under reduced pressure to remove the solvent, dissolved in ethyl acetate (20 mL), washed with water (10 mL × 2), and the organic phase was washed with anhydrous It was dried over sodium sulfate and concentrated under reduced pressure to remove the solvent to give a pale yellow solid (198 mg, 100% yield).
1 H NMR (600 MHz, CDCl3 ): δ (ppm) 7.84 (d,J = 7.7 Hz, 1H), 7.37 – 7.43 (m, 2H), 4.45 (s, 2H), 4.39 (s, 2H), 3.20 (s, 3H). 1 H NMR (600 MHz, CDCl 3 ): δ (ppm) 7.84 (d, J = 7.7 Hz, 1H), 7.37 – 7.43 (m, 2H), 4.45 (s, 2H), 4.39 (s, 2H), 3.20 (s, 3H).
MS (ESI, pos.ion) m/z: 203.20 [M+H]+ .MS (ESI, pos.ion) m/z: 203.20 [M+H] + .
步驟6:5-(氨基甲基)-2-甲基異吲哚啉-1-酮的合成Step 6: Synthesis of 5-(aminomethyl)-2-methylisoindolin-1-one
將化合物5-(疊氮甲基)-2-甲基異吲哚啉-1-酮 (206-5) (198 mg, 0.98 mmol) 溶於甲醇 (10 ml)中,加入Pd/C (30 mg, 0.10 g/g),通入氫氣,室溫下攪拌30 min,通過矽藻土抽濾除去催化劑,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 10/1),得到白色固體 (84 mg, 49%)。Compound 5-(azidomethyl)-2-methylisoindolin-1-one (206-5) (198 mg, 0.98 mmol) was dissolved in methanol (10 ml), Pd/C (30 mg, 0.10 g/g), pass through hydrogen, stir at room temperature for 30 min, remove the catalyst through diatomaceous earth suction filtration, concentrate under reduced pressure to remove the solvent, and separate the concentrate by silica gel column chromatography (eluent: dichloromethane) /methanol (v/v) = 10/1) to give a white solid (84 mg, 49%).
1 H NMR (600 MHz, CD3 OD): δ (ppm) 7.71 (d,J = 7.8 Hz, 1H), 7.54 (s, 1H), 7.47 (d,J = 7.8 Hz, 1H), 4.48 (s, 2H), 3.92 (s, 2H), 3.19 (s, 3H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 7.71 (d, J = 7.8 Hz, 1H), 7.54 (s, 1H), 7.47 (d, J = 7.8 Hz, 1H), 4.48 (s , 2H), 3.92 (s, 2H), 3.19 (s, 3H).
MS (ESI, pos.ion) m/z: 177.20 [M+H]+ .MS (ESI, pos.ion) m/z: 177.20 [M+H] + .
中間體15:6-(羥基甲基)-N -(對甲苯基)吡啶醯胺 Intermediate 15: 6- (hydroxymethyl) - N - (p-tolyl) pyridine Amides
步驟1:化合物6-(對甲苯甲氨甲醯基)吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-(p-toluylcarbamoyl) picolinate methyl ester
將化合物6-(甲氧羰基)吡啶甲酸 (2.00 g, 11 mol) ,對甲苯胺 (1.42 g, 13.3 mmol) 溶解在二氯甲烷 (50 mL) 溶液中,加入1-羥基-7-偶氮苯並三氮唑 (HOAT) (2.30 g, 16.9 mmol ),在0 ℃下冷卻,再加入N ,N -二異丙基乙胺 (DIPEA) (4.3 g, 33.3 mmol),1- (3-二甲基氨基丙基) -3-乙基碳二亞胺 (EDCI) (4.2g, 21.9 mmol),轉移到室溫下攪拌反應17 h。加水停止反應,用二氯甲烷 (100 mL × 3) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/乙酸乙酯(v/v) =4/1),得到淡黃色固體 (2.37 g, 79.40 %)。Compound 6-(methoxycarbonyl)picolinic acid (2.00 g, 11 mol), p-toluidine (1.42 g, 13.3 mmol) were dissolved in dichloromethane (50 mL) solution, 1-hydroxy-7-azo was added Benzotriazole (HOAT) (2.30 g, 16.9 mmol), cooled at 0 °C, then added N , N -diisopropylethylamine (DIPEA) (4.3 g, 33.3 mmol), 1-(3- Dimethylaminopropyl)-3-ethylcarbodiimide (EDCI) (4.2 g, 21.9 mmol), transferred to room temperature and stirred for 17 h. The reaction was stopped by adding water, extracted with dichloromethane (100 mL × 3), the organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/ v) = 4/1) to give a pale yellow solid (2.37 g, 79.40 %).
1 H NMR (400 MHz, CDCl3 ) δ 9.97 (s, 1H), 8.49 (dd,J = 7.8, 1.1 Hz, 1H), 8.26 (dd,J = 7.7, 1.1 Hz, 1H), 8.06 (t,J = 7.8 Hz, 1H), 7.69 (d,J = 8.4 Hz, 2H), 7.20 (d,J =8.2Hz, 2H), 4.05 (s, 3H), 2.35 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ 9.97 (s, 1H), 8.49 (dd, J = 7.8, 1.1 Hz, 1H), 8.26 (dd, J = 7.7, 1.1 Hz, 1H), 8.06 (t, J = 7.8 Hz, 1H), 7.69 (d, J = 8.4 Hz, 2H), 7.20 (d, J =8.2Hz, 2H), 4.05 (s, 3H), 2.35 (s, 3H).
MS (ESI, pos.ion) m/z: 271.10 [M+H]+ .MS (ESI, pos.ion) m/z: 271.10 [M+H] + .
步驟2:化合物6-(羥基甲基)-N -(對甲苯基)吡啶醯胺的合成Step 2: Compound 6- (hydroxymethyl) - N - Synthesis of (p-tolyl) pyridine Amides of
將化合物6-(對甲苯甲氨甲醯基) 吡啶甲酸甲酯 (1.68 g, 6.22 mmol) 溶解在乾燥的四氫呋喃溶液 (15 mL) 中,再加入硼氫化鈉 (400 mg, 10.58 mmol),在室溫下攪拌反應24 h。加飽和食鹽水 (15 mL) 及(20 mL) 水,停止反應,用乙酸乙酯 (20 mL × 2) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/ EtOAc(v/v) =3/2) ,得到白色固體 (1.26 g, 84.70 %)。Compound 6-(p-toluylcarbamoyl)methyl picolinate (1.68 g, 6.22 mmol) was dissolved in dry tetrahydrofuran solution (15 mL), sodium borohydride (400 mg, 10.58 mmol) was added, and The reaction was stirred at room temperature for 24 h. Saturated brine (15 mL) and (20 mL) water were added to stop the reaction, extracted with ethyl acetate (20 mL × 2), the organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: petroleum ether/EtOAc (v/v) = 3/2) to give a white solid (1.26 g, 84.70 %).
1 H NMR (400 MHz, CDCl3 ) δ 9.77 (s, 1H), 8.21 (d,J = 7.6 Hz, 1H), 7.91 (t,J = 7.7 Hz, 1H), 7.65 (d,J = 8.3 Hz, 2H), 7.54 (d,J = 7.7 Hz, 1H), 7.19 (d,J = 8.1 Hz, 2H), 4.88 (s, 2H), 2.83 (br.s, 1H), 2.35 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ 9.77 (s, 1H), 8.21 (d, J = 7.6 Hz, 1H), 7.91 (t, J = 7.7 Hz, 1H), 7.65 (d, J = 8.3 Hz , 2H), 7.54 (d, J = 7.7 Hz, 1H), 7.19 (d, J = 8.1 Hz, 2H), 4.88 (s, 2H), 2.83 (br.s, 1H), 2.35 (s, 3H) .
MS (ESI, pos.ion) m/z: 243.10 [M+H]+ .MS (ESI, pos.ion) m/z: 243.10 [M+H] + .
中間體16:6-(羥甲基)-N -甲基吡啶甲醯胺 Intermediate 16: 6-(Hydroxymethyl) -N -picolinamide
步驟1:6-(甲基氨基甲醯基)吡啶甲酸甲酯的合成Step 1: Synthesis of methyl 6-(methylcarbamoyl)picolinate
將化合物6-(甲氧羰基)吡啶甲酸 (2.00 g, 11 mol),鹽酸甲胺 (2.3 g, 34.1 mmol) 溶解在二氯甲烷 (50 mL) 溶液中,加入1-羥基-7-偶氮苯並三氮唑 (HOAT) (2.30 g, 16.9 mmol ),在0 ℃下冷卻,再加入N,N-二異丙基乙胺 (DIPEA) (5.7 g, 44.1 mmol),1- (3-二甲基氨基丙基) -3-乙基碳二亞胺 (EDCI) (4.2g, 21.9 mmol),轉移到室溫下攪拌反應9 h。加水停止反應,用二氯甲烷 (100 mL × 2) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/EtOAc =1 : 2 ),得到白色固體 (1.63 g, 產率76.00 %)。Compound 6-(methoxycarbonyl)picolinic acid (2.00 g, 11 mol), methylamine hydrochloride (2.3 g, 34.1 mmol) were dissolved in dichloromethane (50 mL) solution, 1-hydroxy-7-azo was added Benzotriazole (HOAT) (2.30 g, 16.9 mmol), cooled at 0 °C, then added N,N-diisopropylethylamine (DIPEA) (5.7 g, 44.1 mmol), 1-(3- Dimethylaminopropyl)-3-ethylcarbodiimide (EDCI) (4.2 g, 21.9 mmol), transferred to room temperature and stirred for 9 h. The reaction was stopped by adding water, extracted with dichloromethane (100 mL × 2), the organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: petroleum ether/EtOAc = 1 : 2 ) , a white solid (1.63 g, 76.00 % yield) was obtained.
1 H NMR (400 MHz, CDCl3 ) δ 8.39 (dd,J = 7.8, 0.9 Hz, 1H), 8.22 (dd,J = 7.8, 1.0 Hz, 1H), 8.11 (br.s, 1H), 8.01 (t,J = 7.8 Hz, 1H), 4.02 (s, 3H), 3.06 (d,J = 5.1 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ 8.39 (dd, J = 7.8, 0.9 Hz, 1H), 8.22 (dd, J = 7.8, 1.0 Hz, 1H), 8.11 (br.s, 1H), 8.01 ( t, J = 7.8 Hz, 1H), 4.02 (s, 3H), 3.06 (d, J = 5.1 Hz, 3H).
MS (ESI, pos.ion) m/z: 195.10 [M+H]+ .MS (ESI, pos.ion) m/z: 195.10 [M+H] + .
步驟2:6-(羥甲基)-N -甲基吡啶甲醯胺的合成Step 2: Synthesis of 6-(hydroxymethyl) -N-picolinamide
將化合物6-(甲基氨基甲醯基)吡啶甲酸甲酯 (800 mg, 4.12 mmol) 溶於乾燥的四氫呋喃(15 mL) 溶液中,在0 ℃下冷卻,加入硼氫化鋰 (230 mg, 10.56 mmol),再轉移到室溫下攪拌反應1 h。加入飽和食鹽水 (15 mL) 攪拌,停止反應,用乙酸乙酯 (20 mL×3) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/EtOAc =3:7),得到白色固體 (610 mg, 產率89.10 %)。Compound 6-(methylcarbamoyl)picolinate (800 mg, 4.12 mmol) was dissolved in dry tetrahydrofuran (15 mL) solution, cooled at 0 °C, and lithium borohydride (230 mg, 10.56 mmol) was added. mmol), and then transferred to room temperature and stirred for 1 h. Saturated brine (15 mL) was added and stirred to stop the reaction, extracted with ethyl acetate (20 mL×3), the organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: Petroleum ether/EtOAc = 3:7) to give a white solid (610 mg, 89.10% yield).
1 H NMR (400 MHz, CDCl3 ) δ 8.11 (d,J = 7.7 Hz, 1H), 7.96 (br.s, 1H), 7.85 (t,J = 7.7 Hz, 1H), 7.46 (d,J = 7.8 Hz, 1H), 4.81 (s, 2H), 3.12 (br.s, 1H), 3.04 (d,J = 5.1 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ 8.11 (d, J = 7.7 Hz, 1H), 7.96 (br.s, 1H), 7.85 (t, J = 7.7 Hz, 1H), 7.46 (d, J = 7.8 Hz, 1H), 4.81 (s, 2H), 3.12 (br.s, 1H), 3.04 (d, J = 5.1 Hz, 3H).
MS (ESI, pos.ion) m/z: 167.20 [M+H]+ .MS (ESI, pos.ion) m/z: 167.20 [M+H] + .
中間體17:6-(羥甲基)吡啶甲酸叔丁酯 Intermediate 17: tert-Butyl 6-(hydroxymethyl)picolinate
步驟1:2-叔丁基 6-甲基 吡啶-2, 6-二甲酸酯的合成Step 1: Synthesis of 2-tert-butyl 6-methylpyridine-2,6-dicarboxylate
將化合物6-(甲氧羰基)吡啶甲酸 (500 mg, 2.76 mmol),溶解在四氫呋喃 (10 mL) 溶劑中,氮氣置換三次後,氮氣氛圍下加入N ,N' -二異丙基氨基甲酸叔丁酯 (600 mg, 2.99 mmol),原料加入完之後,轉入到60 °C溫度下攪拌反應3 h。減壓濃縮除去四氫呋喃溶劑,再加入二氯甲烷 (10 mL) 溶劑溶解,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/乙酸乙酯(v/v)= 4/1),得到白色固體產物 (190 mg, 產率29.01 %)。Compound 6-(methoxycarbonyl)picolinic acid (500 mg, 2.76 mmol) was dissolved in tetrahydrofuran (10 mL) solvent, nitrogen was replaced three times, and tertiary N , N' -diisopropylcarbamate was added under nitrogen atmosphere Butyl ester (600 mg, 2.99 mmol), after the raw material was added, was transferred to 60 °C and stirred for 3 h. Concentrate under reduced pressure to remove the tetrahydrofuran solvent, add dichloromethane (10 mL) to dissolve the solvent, concentrate under reduced pressure, and purify by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) , the product was obtained as a white solid (190 mg, 29.01 % yield).
1 H NMR (400 MHz, CDCl3 ) δ 8.27 (d,J = 7.8 Hz, 1H), 8.21 (d,J = 7.8 Hz, 1H), 7.97 (t,J = 7.8 Hz, 1H), 4.01 (s, 3H), 1.65 (s, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ 8.27 (d, J = 7.8 Hz, 1H), 8.21 (d, J = 7.8 Hz, 1H), 7.97 (t, J = 7.8 Hz, 1H), 4.01 (s , 3H), 1.65 (s, 9H).
MS (ESI, pos.ion) m/z: 182.15 [M+H]+ .MS (ESI, pos.ion) m/z: 182.15 [M+H] + .
步驟2:6-(羥甲基)吡啶甲酸叔丁酯的合成Step 2: Synthesis of tert-butyl 6-(hydroxymethyl)picolinate
將化合物2-叔丁基 6-甲基 吡啶-2, 6-二甲酸酯 (190 mg, 0.80 mmol) 溶解在乾燥四氫呋喃 (10 mL) 溶劑中,再加入硼氫化鈉 (99 mg, 2.62 mmol),在室溫下攪拌反應2.5 h。加入飽和食鹽水 (20 mL) 攪拌,停止反應,用乙酸乙酯 (20 mL × 2) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/乙酸乙酯(v/v)=3/7),得到白色固體 (60 mg, 35.81 %)。Compound 2-tert-butyl 6-methylpyridine-2,6-dicarboxylate (190 mg, 0.80 mmol) was dissolved in dry tetrahydrofuran (10 mL) solvent, and sodium borohydride (99 mg, 2.62 mmol) was added. ), and the reaction was stirred at room temperature for 2.5 h. Saturated brine (20 mL) was added and stirred to stop the reaction, extracted with ethyl acetate (20 mL × 2), the organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: Petroleum ether/ethyl acetate (v/v)=3/7) to give a white solid (60 mg, 35.81 %).
1 H NMR (400 MHz, CDCl3 ) δ 7.94 (d,J = 7.7 Hz, 1H), 7.80 (t,J = 7.7 Hz, 1H), 7.44 (d,J = 7.8 Hz, 1H), 4.84 (s, 2H), 3.92 (br.s, 1H), 1.63 (s, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ 7.94 (d, J = 7.7 Hz, 1H), 7.80 (t, J = 7.7 Hz, 1H), 7.44 (d, J = 7.8 Hz, 1H), 4.84 (s , 2H), 3.92 (br.s, 1H), 1.63 (s, 9H).
MS (ESI, pos.ion) m/z: 210.15 [M+H]+ .MS (ESI, pos.ion) m/z: 210.15 [M+H] + .
中間體18:6-(羥甲基)-N - (4-羥苯基)-N -甲基吡啶醯胺 Intermediate 18: 6- (hydroxymethyl) - N - (4- hydroxyphenyl) - N - methylpyridine Amides
步驟1:化合物6-((4-羥苯基)(甲基)氨基甲醯基)吡啶羧酸甲酯的合成Step 1: Synthesis of compound 6-((4-hydroxyphenyl)(methyl)carbamoyl)picolinate methyl ester
將化合物6-(甲氧羰基)吡啶甲酸(1.00 g, 5.52 mmol) ,對甲氨基酚 (820 mg, 6.62 mmol) 溶解在DMF (15 ml) 溶液中,加入2-(7-偶氮苯並三氮唑)-N ,N ,N' ,N' -四甲基脲六氟磷酸酯(HATU) (3.15 g, 8.28 mmol ),在0 ℃下冷卻,再加入N ,N -二異丙基乙胺 (DIPEA) (1.28 g, 9.94 mmol),轉移到室溫下攪拌反應1 h。加水停止反應,用乙酸乙酯 (10 mL × 4) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (PE/ EtOAc (v/v)=1/4),得到淡褐色液體 (1.2g, 產率76 %)。Compound 6-(methoxycarbonyl)picolinic acid (1.00 g, 5.52 mmol), p-methylaminophenol (820 mg, 6.62 mmol) were dissolved in DMF (15 ml) solution, 2-(7-azobenzoic acid was added) triazole) -N , N , N' , N' -tetramethylurea hexafluorophosphate (HATU) (3.15 g, 8.28 mmol), cooled at 0 °C, and added N , N -diisopropyl Ethylamine (DIPEA) (1.28 g, 9.94 mmol), transferred to room temperature and stirred for 1 h. The reaction was stopped by adding water, extracted with ethyl acetate (10 mL × 4), the organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (PE/EtOAc (v/v)=1/4) , a light brown liquid (1.2 g, 76 % yield) was obtained.
1 H NMR (400 MHz, DMSO-d6 ) 9.49 (br.s, 1H), 7.90 – 7.83 (m, 2H), 7.54 – 7.48 (m, 1H), 6.95 (d,J = 8.5 Hz, 2H), 6.55 (d,J = 8.5 Hz, 2H), 3.84 (s, 3H), 3.34 (s, 3H). 1 H NMR (400 MHz, DMSO- d 6 ) 9.49 (br.s, 1H), 7.90 – 7.83 (m, 2H), 7.54 – 7.48 (m, 1H), 6.95 (d, J = 8.5 Hz, 2H) , 6.55 (d, J = 8.5 Hz, 2H), 3.84 (s, 3H), 3.34 (s, 3H).
MS (ESI, pos.ion) m/z: 287.10 [M+H]+ .MS (ESI, pos.ion) m/z: 287.10 [M+H] + .
步驟2:化合物6-(羥甲基)-N - (4-羥苯基)-N -甲基吡啶醯胺的合成Step 2: Compound 6- (hydroxymethyl) - N - (4- hydroxyphenyl) - N - Synthesis of Amides methylpyridine
將化合物6-((4-羥苯基)(甲基)氨基甲醯基)吡啶羧酸甲酯 (1.20 g, 4.19 mmol) 溶解在乾燥的四氫呋喃溶液 (15 mL) 中,再加入硼氫化鈉 (480 mg, 12.57 mmol),在室溫下攪拌反應13 h停止,加稀鹽酸調節pH=6,減壓濃縮,通過矽膠柱層析純化 (MeOH/ DCM(v/v) =1:25) ,得到淺黃色固體 (604 mg, 56 %)。The compound, methyl 6-((4-hydroxyphenyl)(methyl)carbamoyl)picolinate (1.20 g, 4.19 mmol) was dissolved in dry tetrahydrofuran solution (15 mL) and sodium borohydride was added (480 mg, 12.57 mmol), the reaction was stopped by stirring at room temperature for 13 h, dilute hydrochloric acid was added to adjust pH=6, concentrated under reduced pressure, and purified by silica gel column chromatography (MeOH/DCM(v/v)=1:25) , a pale yellow solid (604 mg, 56%) was obtained.
1 H NMR (400 MHz, DMSO-d6 ) 9.44 (s, 1H), 7.67 (d,J = 7.6 Hz, 1H), 7.28 (d,J = 7.5 Hz, 1H), 7.17 (d,J = 7.3 Hz, 1H), 6.91 (d,J = 8.0 Hz, 2H), 6.55 (d,J = 7.9 Hz, 2H), 5.33 (s, 1H), 4.33 (d,J = 4.9 Hz, 2H), 3.30 (s, 3H). 1 H NMR (400 MHz, DMSO- d 6 ) 9.44 (s, 1H), 7.67 (d, J = 7.6 Hz, 1H), 7.28 (d, J = 7.5 Hz, 1H), 7.17 (d, J = 7.3 Hz, 1H), 6.91 (d, J = 8.0 Hz, 2H), 6.55 (d, J = 7.9 Hz, 2H), 5.33 (s, 1H), 4.33 (d, J = 4.9 Hz, 2H), 3.30 ( s, 3H).
MS (ESI, pos.ion) m/z: 259.20 [M+H]+ .MS (ESI, pos.ion) m/z: 259.20 [M+H] + .
實施例Example
實施例1:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(2,4-二氟苄基)吡咯烷-2-甲醯胺 Example 1: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (2,4- Difluorobenzyl)pyrrolidine-2-carboxamide
步驟1:化合物 (R )-1-叔丁基 2-甲基 4-(((三氟甲基)磺醯基)氧基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 1: Compound (R) -1- tert-butyl 2-methyl 4 - (((trifluoromethyl) sulfonyl acyl) oxy) -1 H - pyrrole -1,2 (2 H, 5 H) - Synthesis of dicarboxylates
將化合物Boc-4-氧代-D-脯氨酸甲酯 (0.98 g, 4.0 mmol) 溶解在二氯甲烷 (15 mL) 溶液中,-15℃下加入N ,N -二異丙基乙胺 (3.4 mL, 20.5 mmol),三氟甲磺酸酐 (1.3 mL, 7.7 mmol),-15℃下攪拌10 min後,轉移至室溫反應18 h,停止反應,加入水 (50 mL),用二氯甲烷萃取 (5 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 6/1),得黃色油狀物1.3 g,產率86%。Compound Boc-4-oxo-D-proline methyl ester (0.98 g, 4.0 mmol) was dissolved in dichloromethane (15 mL) solution, and N , N -diisopropylethylamine was added at -15°C (3.4 mL, 20.5 mmol), trifluoromethanesulfonic anhydride (1.3 mL, 7.7 mmol), stirred at -15 °C for 10 min, transferred to room temperature and reacted for 18 h, stopped the reaction, added water (50 mL), mixed with 2 Extracted with methyl chloride (5 mL × 3), dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 6/1) to obtain yellow oil 1.3 g, 86% yield.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 5.71 (dd,J = 18.8, 1.6 Hz, 1H), 4.99-5.06 (m, 1H), 4.24-4.41 (m, 2H), 3.76 (s, 3H), 1.42 – 1.47 (m, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 5.71 (dd, J = 18.8, 1.6 Hz, 1H), 4.99-5.06 (m, 1H), 4.24-4.41 (m, 2H), 3.76 (s , 3H), 1.42 – 1.47 (m, 9H).
MS (ESI, pos.ion) m/z: 398.10 [M+Na]+ .MS (ESI, pos.ion) m/z: 398.10 [M+Na] + .
步驟2:化合物 (R )-1-叔丁基 2-甲基 4-(4,4,5,5-四甲基-1,3,2-二氧雜硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 2: Compound ( R )-1-tert-butyl 2-methyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl)-1 Synthesis of H -pyrrole-1,2( 2H , 5H)-dicarboxylate
將化合物 (R )-1-叔丁基 2-甲基 4-(((三氟甲基)磺醯基)氧基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (1.3 g, 3.5 mmol),聯硼酸頻那醇酯 (1.3 g, 5.1 mmol),醋酸鉀(1.1 g, 11.2 mmol) 和 [1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (130 mg, 0.2 mmol) 混合在乾燥的1,4-二氧六環 (30mL) 溶液中,氮氣保護下100℃反應13 h,冷卻至室溫,將反應液抽濾,濾液中加入50 mL水,用乙酸乙酯 (5 mL × 3) 萃取,合併有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 6/1),得淡紅色液體1.05 g,產率85%。The compound (R) -1- tert-butyl 2-methyl 4 - (((trifluoromethyl) sulfonyl acyl) oxy) -1 H - pyrrole -1,2 (2 H, 5 H) - two Carboxylic acid ester (1.3 g, 3.5 mmol), pinacol diboronate (1.3 g, 5.1 mmol), potassium acetate (1.1 g, 11.2 mmol) and [1,1'-bis(diphenylphosphino)di Ferrocene] palladium dichloride (130 mg, 0.2 mmol) was mixed in dry 1,4-dioxane (30 mL) solution, reacted at 100 °C for 13 h under nitrogen protection, cooled to room temperature, and the reaction solution was pumped up filter, add 50 mL of water to the filtrate, extract with ethyl acetate (5 mL × 3), combine the organic phases and dry with anhydrous sodium sulfate, remove the solvent, and separate the concentrated solution by silica gel column chromatography (eluent: petroleum ether/acetic acid). Ethyl ester (v/v) = 6/1) to obtain light red liquid 1.05 g, yield 85%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 6.33 (dd,J = 23.4, 1.9 Hz, 1H), 5.01-5.12 (m, 1H), 4.26-4.40 (m, 2H), 3.71 – 3.73 (m, 3H), 1.42 – 1.47 (m, 9H), 1.26 (s, 12H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 6.33 (dd, J = 23.4, 1.9 Hz, 1H), 5.01-5.12 (m, 1H), 4.26-4.40 (m, 2H), 3.71 – 3.73 (m, 3H), 1.42 – 1.47 (m, 9H), 1.26 (s, 12H).
MS (ESI, pos.ion) m/z: 376.15 [M+Na]+ .MS (ESI, pos.ion) m/z: 376.15 [M+Na] + .
步驟3:化合物 (R )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 3: Compound (R) -1- tert-butyl 2-methyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) -1 H - pyrrole -1 Synthesis of ,2( 2H , 5H)-dicarboxylate
將化合物 (R )-1-叔丁基 2-甲基 4-(4,4,5,5-四甲基-1,3,2-二氧雜硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (1.15 g, 3.3 mmol),2-(環丙基甲氧基)-1-(二氟甲氧基)-4-碘苯 (1.1 g, 3.2 mmol),磷酸鉀 (2.1 g, 9.9 mmol) 和 [1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (120 mg, 0.16 mmol) 混合在乾燥的1,4-二氧六環 (15mL) 溶液中,氮氣保護下100℃反應3 h,將反應液抽濾,濾液中加入50 mL水,再用乙酸乙酯 (5 mL × 3) 萃取,合併有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 8/1)得淡紅色液體1.13 g,產率80%。The compound (R) -1- tert-butyl 2-methyl-4- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -1 H - Pyrrole-1,2( 2H , 5H )-dicarboxylate (1.15 g, 3.3 mmol), 2-(cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene (1.1 g, 3.2 mmol), potassium phosphate (2.1 g, 9.9 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium (120 mg, 0.16 mmol) were mixed on dry In a solution of 1,4-dioxane (15 mL), the reaction was carried out at 100 °C for 3 h under nitrogen protection, the reaction solution was suction filtered, 50 mL of water was added to the filtrate, and then extracted with ethyl acetate (5 mL × 3). The combined organic phases were dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 8/1) to obtain 1.13 g of a light red liquid, with a yield of 1.13 g. 80%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.14 (d,J = 8.2 Hz, 1H), 6.90-6.97 (m, 2H), 6.63 (t,J F-H = 75.4 Hz, 1H), 6.00-6.03 (m, 1H), 5.11-5.19 (m, 1H), 4.47-4.66 (m, 2H), 3.86-3.90 (m, 2H), 3.76 (d,J = 4.0 Hz, 3H), 1.46 – 1.52 (m, 9H), 1.26-1.31 (m, 1H), 0.61-0.70 (m, 2H), 0.33-0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.14 (d, J = 8.2 Hz, 1H), 6.90-6.97 (m, 2H), 6.63 (t, J FH = 75.4 Hz, 1H), 6.00 -6.03 (m, 1H), 5.11-5.19 (m, 1H), 4.47-4.66 (m, 2H), 3.86-3.90 (m, 2H), 3.76 (d, J = 4.0 Hz, 3H), 1.46 – 1.52 (m, 9H), 1.26-1.31 (m, 1H), 0.61-0.70 (m, 2H), 0.33-0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 462.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 462.20 [M+Na] + .
步驟4:化合物 (2R )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯的合成Step 4: Compound ( 2R )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2 - Synthesis of dicarboxylates
將化合物 (R )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (1.1 g, 2.5 mmol) 溶於甲醇 (15 mL),加入Pd/C (260 mg, 10%),通入氫氣室溫反應7 h,然後將反應液抽濾,濾液濃縮得到990 mg黃色液體,收率90%。The compound (R) -1- tert-butyl 2-methyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) -1 H - pyrrole-1,2 ( 2H , 5H )-dicarboxylate (1.1 g, 2.5 mmol) was dissolved in methanol (15 mL), Pd/C (260 mg, 10%) was added, and hydrogen was passed through to react at room temperature for 7 h, and then the The reaction solution was suction filtered, and the filtrate was concentrated to obtain 990 mg of yellow liquid with a yield of 90%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.10 (d,J = 8.0 Hz, 1H), 6.77-6.81 (m, 2H), 6.60 (t,J F-H = 74.2 Hz, 1H), 4.30-4.40 (m, 1H), 3.91-3.95 (m, 0.5H), 4.02-4.06 (m, 0.5H), 3.84-3.87 (m, 2H), 3.76 (d,J = 6.7 Hz, 3H), 3.30-3.45 (m, 2H), 2.62-2.68 (m, 1H), 1.99-2.07 (m, 1H), 1.43 – 1.47 (m, 9H), 1.28-1.31 (m, 1H), 0.62-0.67 (m, 2H), 0.33-0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.10 (d, J = 8.0 Hz, 1H), 6.77-6.81 (m, 2H), 6.60 (t, J FH = 74.2 Hz, 1H), 4.30 -4.40 (m, 1H), 3.91-3.95 (m, 0.5H), 4.02-4.06 (m, 0.5H), 3.84-3.87 (m, 2H), 3.76 (d, J = 6.7 Hz, 3H), 3.30 -3.45 (m, 2H), 2.62-2.68 (m, 1H), 1.99-2.07 (m, 1H), 1.43 – 1.47 (m, 9H), 1.28-1.31 (m, 1H), 0.62-0.67 (m, 2H), 0.33-0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 464.25 [M+Na]+ .MS (ESI, pos.ion) m/z: 464.25 [M+Na] + .
步驟5:化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽的合成Step 5: Synthesis of compound (2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride
將化合物 (2R )-1-叔丁基-2-甲基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯 (990 mg, 2.2 mmol) 溶解於二氯甲烷 (6 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (8 mL),室溫攪拌50 min,除去溶劑,得白色固體751 mg,收率98%。The compound ( 2R )-1-tert-butyl-2-methyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2 -Dicarboxylate (990 mg, 2.2 mmol) was dissolved in dichloromethane (6 mL) solution, 4 mol/L HCl in ethyl acetate solution (8 mL) was added, stirred at room temperature for 50 min, and the solvent was removed to obtain White solid 751 mg, yield 98%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.14 (d,J = 8.2 Hz, 1H), 7.07 (s, 1H), 6.91-6.94 (m, 1H), 6.76 (t,J F-H = 75.5 Hz, 2H), 4.60-4.64 (m, 1H), 3.94 (d,J = 6.9 Hz, 2H), 3.90 (s, 3H), 3.78-3.83 (m, 1H), 3.65-3.72 (m, 1H), 3.33-3.39 (m, 1H), 2.82-2.89 (m, 1H), 2.20-2.29 (m, 1H), 1.28-1.36 (m, 1H), 0.63-0.66 (m, 2H), 0.37-0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.14 (d, J = 8.2 Hz, 1H), 7.07 (s, 1H), 6.91-6.94 (m, 1H), 6.76 (t, J FH = 75.5 Hz, 2H), 4.60-4.64 (m, 1H), 3.94 (d, J = 6.9 Hz, 2H), 3.90 (s, 3H), 3.78-3.83 (m, 1H), 3.65-3.72 (m, 1H), 3.33-3.39 (m, 1H), 2.82-2.89 (m, 1H), 2.20-2.29 (m, 1H), 1.28-1.36 (m, 1H), 0.63-0.66 (m, 2H), 0.37- 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 342.20 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 342.20 [M+H-HCl] + .
步驟6:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯的合成Step 6: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester Synthesis
將化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (203 mg, 0.6 mmol) 溶解在二氯甲烷 (5 mL) 中,0℃條件下滴加N ,N -二異丙基乙胺 (0.2 mL, 1.0 mmol) 和乙醯氯 (0.1 mL, 1.0 mmol),室溫攪拌5 h後停止反應,加水溶液洗有機相 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得淺黃色液體186 mg,產率82%。Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride (203 mg, 0.6 mmol) was dissolved in dichloromethane (5 mL), N , N -diisopropylethylamine (0.2 mL, 1.0 mmol) and acetyl chloride (0.1 mL, 1.0 mmol) were added dropwise at 0 °C, room temperature The reaction was stopped after stirring for 5 h, the organic phase was washed with aqueous solution (10 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v ) = 1/1), 186 mg of light yellow liquid was obtained, and the yield was 82%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.11 (d,J = 8.2 Hz, 1H), 7.04 (s, 1H), 6.90-6.94 (m, 1H), 6.74 (t,J F-H = 75.6 Hz, 1H), 4.45-4.49 (m, 1H), 4.08-4.12 (m, 1H), 3.93 (d,J = 6.8 Hz, 2H), 3.75 (s, 3H), 3.50-3.64 (m, 2H), 2.67-2.74 (m, 1H), 2.13 (s, 3H), 1.99-2.06 (m, 1H), 1.27-1.33 (m, 1H), 0.62-0.67 (m, 2H), 0.36-0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.11 (d, J = 8.2 Hz, 1H), 7.04 (s, 1H), 6.90-6.94 (m, 1H), 6.74 (t, J FH = 75.6 Hz, 1H), 4.45-4.49 (m, 1H), 4.08-4.12 (m, 1H), 3.93 (d, J = 6.8 Hz, 2H), 3.75 (s, 3H), 3.50-3.64 (m, 2H), 2.67-2.74 (m, 1H), 2.13 (s, 3H), 1.99-2.06 (m, 1H), 1.27-1.33 (m, 1H), 0.62-0.67 (m, 2H), 0.36-0.40 ( m, 2H).
MS (ESI, pos.ion) m/z: 384.20 [M+H]+ .MS (ESI, pos.ion) m/z: 384.20 [M+H] + .
步驟7:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸的合成Step 7: Synthesis of compound (2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯 (426 mg, 1.1 mmol) 溶於四氫呋喃 (6 mL) 和水 (4 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (203 mg,4.8 mmol),50℃反應3 h後停止,加鹽酸調節溶液pH=1,再用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得無色液體388 mg,產率95%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester (426 mg, 1.1 mmol) was dissolved in a mixed solvent of tetrahydrofuran (6 mL) and water (4 mL), then lithium hydroxide monohydrate (203 mg, 4.8 mmol) was added, the reaction was stopped after 3 h at 50 °C, and hydrochloric acid was added to adjust the solution pH=1, then extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 388 mg of a colorless liquid with a yield of 95%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.12 (d,J = 8.1 Hz, 1H), 7.05 (d,J = 1.5 Hz, 1H), 6.878-6.93 (m, 1H), 6.74 (t,J F-H = 74.9 Hz, 1H), 4.43 – 4.47 (m, 1H), 4.08 – 4.15 (m, 1H), 3.94 (d,J = 6.8 Hz, 2H), 3.50 – 3.64 (m, 2H), 2.72 – 2.78 (m, 1H), 2.09 (s, 3H), 2.00 – 2.08 (m, 1H), 1.27-1.36 (m, 1H), 0.63-0.67 (m, 2H), 0.37-0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.12 (d, J = 8.1 Hz, 1H), 7.05 (d, J = 1.5 Hz, 1H), 6.878-6.93 (m, 1H), 6.74 (t, J FH = 74.9 Hz, 1H), 4.43 – 4.47 (m, 1H), 4.08 – 4.15 (m, 1H), 3.94 (d, J = 6.8 Hz, 2H), 3.50 – 3.64 (m, 2H) , 2.72 – 2.78 (m, 1H), 2.09 (s, 3H), 2.00 – 2.08 (m, 1H), 1.27-1.36 (m, 1H), 0.63-0.67 (m, 2H), 0.37-0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 370.25 [M+H]+ .MS (ESI, pos.ion) m/z: 370.25 [M+H] + .
步驟8:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(2,4-二氟苄基)吡咯烷-2-甲醯胺的合成Step 8: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (2,4-di Synthesis of Fluorobenzyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (123 mg, 0.33 mmol),(2,4-二氟苯基)甲胺 (96 mg, 0.67 mmol),1-乙基-3-(3-二甲胺基丙基)碳二亞胺鹽酸鹽 (160 mg, 0.83 mmol)和N -羥基-7-氮雜苯並三氮唑 (82 mg, 0.60 mmol) 溶於二氯甲烷 (6 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌21 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得白色固體113 mg,收率68%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (123 mg, 0.33 mmol), (2,4-difluorophenyl)methanamine (96 mg, 0.67 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (160 mg, 0.83 mmol) and N -hydroxy-7-azabenzotriazole (82 mg, 0.60 mmol) were dissolved in dichloromethane (6 mL), to this solution was added dropwise N , N at 0 °C -Diisopropylethylamine (0.4 mL, 2.0 mmol), stir at room temperature for 21 h, add water (15 mL), extract with dichloromethane (5 mL × 3), combine the organic phases, dry with anhydrous sodium sulfate, reduce It was concentrated under pressure, and the residue was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/2) to obtain 113 mg of white solid with a yield of 68%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.46-7.52 (m, 1H), 7.08-7.12 (m, 2H), 6.93-6.97 (m, 3H), 6.74 (t,J F-H = 75.7 Hz, 1H), 4.44-4.49 (m, 3H), 4.05-4.11 (m, 1H), 3.93 (d,J = 6.8 Hz, 2H), 3.61-3.66 (m, 1H), 3.44-3.53 (m, 1H), 2.61-2.68 (m, 1H), 2.14 (s, 3H), 2.00-2.09 (m, 1H), 1.27-1.35 (m, 1H), 0.62-0.67 (m, 2H), 0.37-0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.46-7.52 (m, 1H), 7.08-7.12 (m, 2H), 6.93-6.97 (m, 3H), 6.74 (t, J FH = 75.7 Hz, 1H), 4.44-4.49 (m, 3H), 4.05-4.11 (m, 1H), 3.93 (d, J = 6.8 Hz, 2H), 3.61-3.66 (m, 1H), 3.44-3.53 (m , 1H), 2.61-2.68 (m, 1H), 2.14 (s, 3H), 2.00-2.09 (m, 1H), 1.27-1.35 (m, 1H), 0.62-0.67 (m, 2H), 0.37-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 495.25 [M+H]+ .MS (ESI, pos.ion) m/z: 495.25 [M+H] + .
實施例2:化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(2,4-二氟苄基)吡咯烷-2-甲醯胺 Example 2: Compound ( 2S )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (2,4- Difluorobenzyl)pyrrolidine-2-carboxamide
步驟1:化合物 (S )-1-叔丁基 2-甲基 4-(((三氟甲基)磺醯基)氧基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 1: The compound (S) -1- tert-butyl 2-methyl 4 - (((trifluoromethyl) sulfonyl acyl) oxy) -1 H - pyrrole -1,2 (2 H, 5 H) - Synthesis of dicarboxylates
將化合物Boc-4-氧代-L-脯氨酸甲酯 (5.0 g, 20.6 mmol) 溶解在二氯甲烷 (100 mL) 溶液中,冷卻至-10℃,加入N,N -二異丙基乙胺 (17.0 mL, 103 mmol),緩慢滴加三氟甲磺酸酐 (9.9 g, 35 mmol),轉移至室溫反應16 h,加入水 (100 mL) 淬滅反應,攪拌10min後分離有機相,無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 10/1),得淺黃色油狀物7.1 g,產率92%。Compound Boc-4-oxo-L-proline methyl ester (5.0 g, 20.6 mmol) was dissolved in dichloromethane (100 mL) solution, cooled to -10 °C, and N,N -diisopropyl was added Ethylamine (17.0 mL, 103 mmol) was slowly added dropwise with trifluoromethanesulfonic anhydride (9.9 g, 35 mmol), transferred to room temperature for 16 h, water (100 mL) was added to quench the reaction, and the organic phase was separated after stirring for 10 min , dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 10/1) to obtain 7.1 g of a light yellow oily product. rate of 92%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 5.70 – 5.74 (m, 1H), 5.00 – 5.02 (m, 1H), 4.28 – 4.36 (m, 2H), 3.75 – 3.77 (m, 3H),1.43 – 1.48 (m, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 5.70 – 5.74 (m, 1H), 5.00 – 5.02 (m, 1H), 4.28 – 4.36 (m, 2H), 3.75 – 3.77 (m, 3H) ,1.43 – 1.48 (m, 9H).
MS (ESI, pos.ion) m/z: 320.90 [M-55]+ .MS (ESI, pos.ion) m/z: 320.90 [M-55] + .
步驟2:化合物 (S )-1-叔丁基 2-甲基 4-(4,4,5,5-四甲基-1,3,2-二氧雜硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 2: Compound ( S )-1-tert-butyl 2-methyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl)-1 Synthesis of H -pyrrole-1,2( 2H , 5H)-dicarboxylate
將化合物 (S )-1-叔丁基 2-甲基 4-(((三氟甲基)磺醯基)氧基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯(2.9 g, 7.7 mmol),聯硼酸頻那醇酯 (2.9 g, 11 mmol),醋酸鉀 (2.3 g, 23 mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (50 mg, 0.068 mmol) 混合在乾燥的1,4-二氧六環 (40mL) 溶液中,氮氣保護下100℃反應5 h,冷卻至室溫,過濾,濾液濃縮,剩餘物加水溶液 (30 mL) 和乙酸乙酯 (50 mL),攪拌均勻後分離出有機相,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/1),得到淺褐色液體1.44 g,產率51%。The compound (S) -1- tert-butyl 2-methyl 4 - (((trifluoromethyl) sulfonyl acyl) oxy) -1 H - pyrrole -1,2 (2 H, 5 H) - two Carboxylic acid ester (2.9 g, 7.7 mmol), pinacol diboronate (2.9 g, 11 mmol), potassium acetate (2.3 g, 23 mmol) and [1,1'-bis(diphenylphosphino)bis Ferrocene] palladium dichloride (50 mg, 0.068 mmol) was mixed in dry 1,4-dioxane (40 mL) solution, reacted at 100 °C for 5 h under nitrogen protection, cooled to room temperature, filtered, and the filtrate was concentrated , the residue was added with aqueous solution (30 mL) and ethyl acetate (50 mL), and the organic phase was separated after stirring evenly. The organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: Petroleum ether/ethyl acetate (v/v) = 3/1) to obtain light brown liquid 1.44 g, yield 51%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 6.23 – 6.32 (m, 1H), 5.03 – 5.09 (m, 1H), 4.23 – 4.29 (m, 2H), 3.77 (s, 3H),1.44 (s, 6H), 1.26 – 1.30 (m, 9H), 1.22 (s, 3H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 6.23 – 6.32 (m, 1H), 5.03 – 5.09 (m, 1H), 4.23 – 4.29 (m, 2H), 3.77 (s, 3H), 1.44 (s, 6H), 1.26 – 1.30 (m, 9H), 1.22 (s, 3H).
步驟3:化合物 (S )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 3: Compound (S) -1- tert-butyl 2-methyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) -1 H - pyrrole -1 Synthesis of ,2( 2H , 5H)-dicarboxylate
將化合物 (S )-1-叔丁基 2-甲基 4-(4,4,5,5-四甲基-1,3,2-二氧雜硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (886 mg, 3.2 mmol),2-(環丙基甲氧基)-1-(二氟甲氧基)-4-碘苯 (1.1 g, 3.2 mmol),磷酸鉀 (2.7 g, 13 mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (110 mg, 0.15 mmol) 混合在乾燥的1,4-二氧六環 (25mL) 溶液中,氮氣保護下100℃反應5 h,冷卻至室溫,濃縮,剩餘物加水溶液 (20 mL) 和乙酸乙酯 (30 mL),攪拌均勻後分離出有機相,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/1),得到淺褐色液體576 mg,產率41%。The compound (S) -1- tert-butyl 2-methyl-4- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -1 H - Pyrrole-1,2( 2H , 5H )-dicarboxylate (886 mg, 3.2 mmol), 2-(cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene (1.1 g, 3.2 mmol), potassium phosphate (2.7 g, 13 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]dichloride palladium (110 mg, 0.15 mmol) were mixed on dry In a solution of 1,4-dioxane (25 mL), reacted at 100 °C for 5 h under nitrogen protection, cooled to room temperature, concentrated, the residue was added with aqueous solution (20 mL) and ethyl acetate (30 mL), stirred well After separating the organic phase, the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 3/1) to obtain light brown Liquid 576 mg, yield 41%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.12 – 7.15 (m, 1H), 6.93 – 6.96 (m, 2H), 6.63 (t,J F-H = 75.4 Hz, 1H), 5.99 – 6.02 (m, 1H), 5.10 – 5.19 (m, 1H), 4.47 – 4.65 (m, 2H), 3.85 – 5.90 (m, 2H), 3.75 (s, 3H), 1.45 – 1.52 (m, 9H), 1.23-1.33 (m, 1H), 0.63 – 0.67 (m, 2H), 0.33 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.12 – 7.15 (m, 1H), 6.93 – 6.96 (m, 2H), 6.63 (t, J FH = 75.4 Hz, 1H), 5.99 – 6.02 ( m, 1H), 5.10 – 5.19 (m, 1H), 4.47 – 4.65 (m, 2H), 3.85 – 5.90 (m, 2H), 3.75 (s, 3H), 1.45 – 1.52 (m, 9H), 1.23- 1.33 (m, 1H), 0.63 – 0.67 (m, 2H), 0.33 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 462.10 [M+Na]+ .MS (ESI, pos.ion) m/z: 462.10 [M+Na] + .
步驟4:化合物 (2S )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯的合成Step 4: Compound ( 2S )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2 - Synthesis of dicarboxylates
將化合物 (S )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (263 mg, 0.6 mmol),加入甲醇 (8 mL) 溶解,加入Pd/C (54 mg,),常溫常壓下氫氣反應6 h,抽濾,濾液濃縮得到無色液體258 mg,收率97%。The compound (S) -1- tert-butyl 2-methyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) -1 H - pyrrole-1,2 ( 2H , 5H )-dicarboxylate (263 mg, 0.6 mmol) was added with methanol (8 mL) to dissolve, added with Pd/C (54 mg,), reacted with hydrogen at room temperature and pressure for 6 h, suction filtered, The filtrate was concentrated to obtain 258 mg of a colorless liquid with a yield of 97%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.10 (d,J = 7.9 Hz, 1H), 6.77 – 6.81 (m, 2H), 6.59 (t,J F-H = 75.6 Hz, 1H), 4.30 – 4.40 (m, 1H), 4.02 – 4.06 (m, 0.6H), 3.91 – 3.95 (m, 0.4H), 3.85 (t,J = 6.7 Hz, 2H), 3.75 – 3.77 (m, 3H), 3.38 – 3.44 (m, 1H), 3.27 – 3.36 (m, 1H), 2.60 – 2.68 (m, 1H), 1.96 – 2.07 (m, 1H), 1.43 – 1.46 (m, 9H), 1.23 – 1.36 (m, 1H), 0.63 – 0.66 (m, 2H), 0.33 – 0.36 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.10 (d, J = 7.9 Hz, 1H), 6.77 – 6.81 (m, 2H), 6.59 (t, J FH = 75.6 Hz, 1H), 4.30 – 4.40 (m, 1H), 4.02 – 4.06 (m, 0.6H), 3.91 – 3.95 (m, 0.4H), 3.85 (t, J = 6.7 Hz, 2H), 3.75 – 3.77 (m, 3H), 3.38 – 3.44 (m, 1H), 3.27 – 3.36 (m, 1H), 2.60 – 2.68 (m, 1H), 1.96 – 2.07 (m, 1H), 1.43 – 1.46 (m, 9H), 1.23 – 1.36 (m, 1H), 0.63 – 0.66 (m, 2H), 0.33 – 0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 464.10 [M+Na]+ .MS (ESI, pos.ion) m/z: 464.10 [M+Na] + .
步驟5:化合物 (2S )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽的合成Step 5: Synthesis of Compound (2S )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物 (2S )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯(252 mg, 0.57 mmol) 溶解於二氯甲烷 (5 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (5 mL),室溫攪拌30 min,減壓濃縮,得到無色液體202 mg,收率94%。The compound ( 2S )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-di Carboxylic acid ester (252 mg, 0.57 mmol) was dissolved in dichloromethane (5 mL) solution, 4 mol/L HCl in ethyl acetate solution (5 mL) was added, stirred at room temperature for 30 min, and concentrated under reduced pressure to obtain colorless Liquid 202 mg, yield 94%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.14 (d,J = 8.4 Hz, 1H), 7.08 (d,J = 1.6 Hz, 1H), 6.92 (dd,J = 8.3, 1.6 Hz, 1H), 6.76 (t,J F-H = 75.5 Hz, 1H), 4.60 – 4.65 (m, 1H), 3.94 (d,J = 6.9 Hz, 2H), 3.90 (s, 3H), 3.78 – 3.83 (m, 1H), 3.63 – 3.73 (m, 1H), 3.33 – 3.40 (m, 1H), 2.82 – 2.89 (m, 1H), 2.20 – 2.29 (m, 1H), 1.27 – 1.35 (m, 1H), 0.63 – 0.68 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.14 (d, J = 8.4 Hz, 1H), 7.08 (d, J = 1.6 Hz, 1H), 6.92 (dd, J = 8.3, 1.6 Hz , 1H), 6.76 (t, J FH = 75.5 Hz, 1H), 4.60 – 4.65 (m, 1H), 3.94 (d, J = 6.9 Hz, 2H), 3.90 (s, 3H), 3.78 – 3.83 (m , 1H), 3.63 – 3.73 (m, 1H), 3.33 – 3.40 (m, 1H), 2.82 – 2.89 (m, 1H), 2.20 – 2.29 (m, 1H), 1.27 – 1.35 (m, 1H), 0.63 – 0.68 (m, 2H), 0.37 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 342.40 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 342.40 [M+H-HCl] + .
步驟6:化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯的合成Step 6: Compound ( 2S )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester Synthesis
將化合物 (2S )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽(198 mg, 0.53 mmol) 溶解在二氯甲烷 (10 mL) 中,加入N,N -二異丙基乙胺 (0.2 mL, 1.0 mmol),冰浴中滴加乙醯氯 (70 mg, 0.8 mmol),室溫攪拌5.5 h後停止反應,加水 (10 mL),二氯甲烷萃取 (10 mL × 3),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到淺褐色液體176 mg,產率87%。Compound ( 2S )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride (198 mg, 0.53 mmol) was dissolved in dichloromethane (10 mL), N,N -diisopropylethylamine (0.2 mL, 1.0 mmol) was added, acetonitrile chloride (70 mg, 0.8 mmol) was added dropwise in an ice bath, room temperature After stirring for 5.5 h, the reaction was stopped, water (10 mL) was added, and dichloromethane was extracted (10 mL × 3). The organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent). : petroleum ether/ethyl acetate (v/v) = 2/1) to obtain a light brown liquid 176 mg, a yield of 87%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.12 (d,J = 8.6 Hz, 1H), 6.82 (s, 1H), 6.78-6.82 (m, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 4.45 – 4.49 (m, 1H), 3.91 – 3.95 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.77 (s, 3H), 3.60 (t,J = 10.4 Hz, 1H), 3.36 – 3.45 (m, 1H), 2.62 – 2.68 (m, 1H), 2.11 (s, 3H), 2.02 – 2.09 (m, 1H), 1.27-1.33 (m, 1H), 0.63-0.68 (m, 2H), 0.34-0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.12 (d, J = 8.6 Hz, 1H), 6.82 (s, 1H), 6.78-6.82 (m, 1H), 6.60 (t, J FH = 75.5 Hz, 1H), 4.45 – 4.49 (m, 1H), 3.91 – 3.95 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.77 (s, 3H), 3.60 (t, J = 10.4 Hz, 1H), 3.36 – 3.45 (m, 1H), 2.62 – 2.68 (m, 1H), 2.11 (s, 3H), 2.02 – 2.09 (m, 1H), 1.27-1.33 (m, 1H), 0.63- 0.68 (m, 2H), 0.34-0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 384.15 [M+H]+ .MS (ESI, pos.ion) m/z: 384.15 [M+H] + .
步驟7:化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸的合成Step 7: Synthesis of compound (2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid
將化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯 (92 mg, 0.24 mmol) 溶解在四氫呋喃 (5 mL) 中,加入一水合氫氧化鋰(51 mg, 1.22 mmol) 和水 (3 mL),45℃反應50 min,加稀鹽酸調節pH=1,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,得白色固體84 mg,產率94%。The compound ( 2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester (92 mg, 0.24 mmol) was dissolved in tetrahydrofuran (5 mL), added lithium hydroxide monohydrate (51 mg, 1.22 mmol) and water (3 mL), reacted at 45 °C for 50 min, added dilute hydrochloric acid to adjust pH=1, and acetic acid Ethyl ester extraction (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 84 mg of a white solid with a yield of 94%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.09 – 7.13 (m, 1H), 7.05 (d,J = 1.5 Hz, 1H), 6.88 – 6.93 (m, 1H), 6.74 (t,J F-H = 74.9 Hz, 1H), 4.43 – 4.47 (m, 1H), 4.08 – 4.12 (m, 1H), 3.94 (d,J = 6.8 Hz, 2H), 3.58 – 3.64 (m, 1H), 3.50 – 3.57 (m, 1H), 2.72 – 2.78 (m, 1H), 2.14 (s, 3H), 2.00 – 2.05 (m, 1H), 1.27-1.36 (m, 1H), 0.63-0.67 (m, 2H), 0.37-0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.09 – 7.13 (m, 1H), 7.05 (d, J = 1.5 Hz, 1H), 6.88 – 6.93 (m, 1H), 6.74 (t, J FH = 74.9 Hz, 1H), 4.43 – 4.47 (m, 1H), 4.08 – 4.12 (m, 1H), 3.94 (d, J = 6.8 Hz, 2H), 3.58 – 3.64 (m, 1H), 3.50 – 3.57 (m, 1H), 2.72 – 2.78 (m, 1H), 2.14 (s, 3H), 2.00 – 2.05 (m, 1H), 1.27-1.36 (m, 1H), 0.63-0.67 (m, 2H), 0.37-0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 370.10 [M+H]+ .MS (ESI, pos.ion) m/z: 370.10 [M+H] + .
步驟8:化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(2,4-二氟苄基)吡咯烷-2-甲醯胺的合成Step 8: Compound ( 2S )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (2,4-di Synthesis of Fluorobenzyl)pyrrolidine-2-carboxamide
將化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (107 mg, 0.29 mmol),2,4-二氟苄胺 (48 mg, 0.34 mmol) 和N -羥基-7-氮雜苯並三氮唑 (59 mg, 0.43 mmol) 溶於二氯甲烷 (5 mL)中,冷卻至0℃,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (254 mg, 1.33 mmol) 和N,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌4 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:EA ( v ) = 100%),得到白色固體101 mg,收率70%。The compound ( 2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (107 mg, 0.29 mmol), 2,4-difluorobenzylamine (48 mg, 0.34 mmol) and N -hydroxy-7-azabenzotriazole (59 mg, 0.43 mmol) were dissolved in dichloromethane (5 mL) , cooled to 0°C, added 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (254 mg, 1.33 mmol) and N,N -diisopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 4 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography ( Eluent: EA (v) = 100%) to give a white solid 101 mg in 70% yield.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.34 – 7.40 (m, 1H), 7.22 (br.s, 1H), 7.13 (d,J = 8.2 Hz, 1H), 6.91 (d,J = 1.6 Hz, 1H), 6.83 – 6.89 (m, 2H), 6.63 (t,J F-H = 75.6 Hz, 1H), 4.55 – 4.59 (m, 1H), 4.44 – 4.52 (m, 2H), 3.90 – 3.96 (m, 1H), 3.89 (d,J = 6.9 Hz, 2H), 3.48 – 3.54 (m, 1H), 3.27 – 3.36 (m, 1H), 2.47 – 2.62 (m, 2H), 2.14 (s, 3H), 1.28-1.37 (m, 1H), 0.65-0.69 (m, 2H), 0.36-0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.34 – 7.40 (m, 1H), 7.22 (br.s, 1H), 7.13 (d, J = 8.2 Hz, 1H), 6.91 (d, J = 1.6 Hz, 1H), 6.83 – 6.89 (m, 2H), 6.63 (t, J FH = 75.6 Hz, 1H), 4.55 – 4.59 (m, 1H), 4.44 – 4.52 (m, 2H), 3.90 – 3.96 (m, 1H), 3.89 (d, J = 6.9 Hz, 2H), 3.48 – 3.54 (m, 1H), 3.27 – 3.36 (m, 1H), 2.47 – 2.62 (m, 2H), 2.14 (s, 3H) ), 1.28-1.37 (m, 1H), 0.65-0.69 (m, 2H), 0.36-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 495.20 [M+H]+ .MS (ESI, pos.ion) m/z: 495.20 [M+H] + .
實施例3:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(吡啶-2-基甲基)吡咯烷-2-甲醯胺 Example 3: Compound (2 R) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - (pyridin-2 ylmethyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (123 mg, 0.33 mmol),2-吡啶甲胺 (96 mg, 0.89 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (160 mg, 0.83 mmol) 和N -羥基-7-氮雜苯並三氮唑 (82 mg, 0.60 mmol) 溶於二氯甲烷 (6 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌20 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到淡黃色液體53 mg,收率34%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (123 mg, 0.33 mmol), 2-pyridylmethylamine (96 mg, 0.89 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (160 mg, 0.83 mmol) and N- Hydroxy-7-azabenzotriazole (82 mg, 0.60 mmol) was dissolved in dichloromethane (6 mL), to this solution was added dropwise N , N -diisopropylethylamine ( 0.4 mL, 2.0 mmol), stirred at room temperature for 20 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1), 53 mg of pale yellow liquid was obtained, and the yield was 34%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.47 (d,J = 4.6 Hz, 1H), 7.84 (t,J = 7.7 Hz, 1H), 7.57 (d,J = 7.9 Hz, 1H), 7.30-7.33 (m, 1H), 7.09-7.10 (m, 2H), 6.93-6.94 (m, 1H), 6.75 (t,J F-H = 75.8 Hz, 1H), 4.45-4.55 (m, 3H), 4.09-4.13 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.63-3.69 (m, 1H), 3.48-3.56 (m, 1H), 2.66-2.73 (m, 1H), 2.16 (s, 3H), 2.05-2.12 (m, 1H), 1.28-1.34 (m, 1H), 0.62-0.67 (m, 2H), 0.37-0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.47 (d, J = 4.6 Hz, 1H), 7.84 (t, J = 7.7 Hz, 1H), 7.57 (d, J = 7.9 Hz, 1H) ), 7.30-7.33 (m, 1H), 7.09-7.10 (m, 2H), 6.93-6.94 (m, 1H), 6.75 (t, J FH = 75.8 Hz, 1H), 4.45-4.55 (m, 3H) , 4.09-4.13 (m, 1H), 3.93 (d, J = 6.9 Hz, 2H), 3.63-3.69 (m, 1H), 3.48-3.56 (m, 1H), 2.66-2.73 (m, 1H), 2.16 (s, 3H), 2.05-2.12 (m, 1H), 1.28-1.34 (m, 1H), 0.62-0.67 (m, 2H), 0.37-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 460.30 [M+H]+ .MS (ESI, pos.ion) m/z: 460.30 [M+H] + .
實施例4:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((5-氟吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 4: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((5-fluoro Pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (121 mg, 0.33 mmol),(5-氟吡啶-2-基)甲胺 (91 mg, 0.72 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (143 mg, 0.75 mmol) 和N -羥基-7-氮雜苯並三氮唑 (81 mg, 0.60 mmol) 溶於二氯甲烷 (6 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌18 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到無色液體61 mg,收率39%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (121 mg, 0.33 mmol), (5-fluoropyridin-2-yl)methanamine (91 mg, 0.72 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (143 mg , 0.75 mmol) and N -hydroxy-7-azabenzotriazole (81 mg, 0.60 mmol) were dissolved in dichloromethane (6 mL), to this solution was added dropwise N , N - Diisopropylethylamine (0.4 mL, 2.0 mmol) was stirred at room temperature for 18 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, The concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 61 mg of a colorless liquid with a yield of 39%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.38 (s, 1H), 7.59-7.62 (m, 2H), 7.09-7.12 (m, 2H), 6.93-6.94 (m, 1H), 6.75 (t,J F-H = 75.4 Hz, 1H), 4.60 (s, 2H), 4.48-4.55 (m, 1H), 4.08-4.13 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.63-3.68 (m, 1H), 3.47-3.56 (m, 1H), 2.65-2.72 (m, 1H), 2.16 (s, 3H), 1.98-2.11 (m, 1H), 1.27-1.33 (m, 1H), 0.62-0.65 (m, 2H), 0.37-0.40 (m, 2H); 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.38 (s, 1H), 7.59-7.62 (m, 2H), 7.09-7.12 (m, 2H), 6.93-6.94 (m, 1H), 6.75 (t, J FH = 75.4 Hz, 1H), 4.60 (s, 2H), 4.48-4.55 (m, 1H), 4.08-4.13 (m, 1H), 3.93 (d, J = 6.9 Hz, 2H), 3.63-3.68 (m, 1H), 3.47-3.56 (m, 1H), 2.65-2.72 (m, 1H), 2.16 (s, 3H), 1.98-2.11 (m, 1H), 1.27-1.33 (m, 1H) ), 0.62-0.65 (m, 2H), 0.37-0.40 (m, 2H);
MS (ESI, pos.ion) m/z: 478.30 [M+H]+ .MS (ESI, pos.ion) m/z: 478.30 [M+H] + .
實施例5:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-甲醯氨基)甲基)吡啶羧酸乙酯 Example 5: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carbamoylamino ) methyl) ethyl picolinate
步驟1:化合物(R )-1-叔丁基 2-甲基 4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 1: Compound (R) -1- tert-butyl 2-methyl-4- (3- (benzyloxy) -4- (difluoromethoxy) phenyl) -1 H - pyrrole-1,2 ( Synthesis of 2H , 5H)-dicarboxylate
將化合物 (R )-1-叔丁基 2-甲基 4-(4,4,5,5-四甲基-1,3,2-二氧雜戊硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯(2.1 g, 5.9 mmol),2-(苄氧基)-1-(二氟甲氧基)-4-碘苯 (861-1-2) (5.8 g, 2.2 mmol),磷酸鉀 (5.0 g, 24.0 mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (88 mg, 0.12 mmol) 混合在乾燥的1,4-二氧六環 (20 mL) 溶液中,氮氣保護下100℃反應5 h,將反應液抽濾,濾液中加入20 mL水,用乙酸乙酯 (15 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到黃褐色液體2.1g,產率74%。The compound (R) -1- tert-butyl 2-methyl-4- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -1 H -pyrrole-1,2( 2H , 5H )-dicarboxylate (2.1 g, 5.9 mmol), 2-(benzyloxy)-1-(difluoromethoxy)-4-iodobenzene (861 -1-2) (5.8 g, 2.2 mmol), potassium phosphate (5.0 g, 24.0 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (88 mg, 0.12 mmol) in dry 1,4-dioxane (20 mL) solution, react at 100 °C for 5 h under nitrogen protection, filter the reaction solution with suction, add 20 mL of water to the filtrate, wash with ethyl acetate (15 mL) × 3) Extraction, the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, concentrated, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) to obtain a yellow-brown liquid 2.1 g, 74% yield.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.37 – 7.44 (m, 5H), 7.18 – 7.20 (m, 1H), 7.04 (s, 1H), 6.95 – 7.01 (m, 1H), 6.60 (t,J F-H = 75.0 Hz, 1H), 6.00 – 6.03 (m, 1H), 5.13 – 5.21 (m, 3H), 4.54 – 4.67 (m, 2H), 3.78 – 3.79 (m, 3H), 1.48 – 1.55 (m, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.37 – 7.44 (m, 5H), 7.18 – 7.20 (m, 1H), 7.04 (s, 1H), 6.95 – 7.01 (m, 1H), 6.60 (t, J FH = 75.0 Hz, 1H), 6.00 – 6.03 (m, 1H), 5.13 – 5.21 (m, 3H), 4.54 – 4.67 (m, 2H), 3.78 – 3.79 (m, 3H), 1.48 – 1.55 (m, 9H).
MS (ESI, pos.ion) m/z: 498.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 498.20 [M+Na] + .
步驟2:化合物 (R )-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-羧酸甲酯鹽酸鹽的合成Step 2: Compound ( R )-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro- 1H -pyrrole-2-carboxylate methyl ester Synthesis of hydrochloride
將化合物 (R )-1-叔丁基 2-甲基 4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯(2.3 g, 4.8 mmol) 溶解於二氯甲烷 (5 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (15 mL),室溫反應1 h,除去溶劑,得到淺黃色液體2.02 g,收率100%。The compound (R) -1- tert-butyl 2-methyl-4- (3- (benzyloxy) -4- (difluoromethoxy) phenyl) -1 H - pyrrole -1,2 (2 H , 5H )-dicarboxylate (2.3 g, 4.8 mmol) was dissolved in dichloromethane (5 mL) solution, 4 mol/L HCl in ethyl acetate solution (15 mL) was added, and the reaction was carried out at room temperature for 1 h, The solvent was removed to obtain 2.02 g of a pale yellow liquid with a yield of 100%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.48 – 7.50 (m, 2H), 7.33 – 7.43 (m, 4H), 7.23 (d,J = 8.3 Hz, 1H), 7.12 (dd,J = 8.3, 1.9 Hz, 1H), 6.81 (t,J F-H = 74.8 Hz, 1H), 6.45 – 6.46 (m, 1H), 5.40 – 5.43 (m, 1H), 5.24 (s, 2H), 4.53 – 4.62 (m, 2H), 3.93 (s, 3H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.48 – 7.50 (m, 2H), 7.33 – 7.43 (m, 4H), 7.23 (d, J = 8.3 Hz, 1H), 7.12 (dd, J = 8.3, 1.9 Hz, 1H), 6.81 (t, J FH = 74.8 Hz, 1H), 6.45 – 6.46 (m, 1H), 5.40 – 5.43 (m, 1H), 5.24 (s, 2H), 4.53 – 4.62 (m, 2H), 3.93 (s, 3H).
MS (ESI, pos.ion) m/z: 376.05 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 376.05 [M+H-HCl] + .
步驟3:化合物 (R )-1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-羧酸甲酯的合成Step 3: Compound ( R )-1-Acetyl-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro- 1H -pyrrole- Synthesis of 2-carboxylate methyl ester
將化合物 (R )-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-羧酸甲酯鹽酸鹽 (2.0 g, 4.9 mmol) 溶解在二氯甲烷 (10 mL) 中,加入N ,N -二異丙基乙胺 (4.0 mL, 24 mmol),冷卻至0℃後加入乙醯氯 (1.1 g, 14 mmol),室溫攪拌3 h後停止反應,加水 (20 mL × 3) 攪拌,二氯甲烷萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到淺黃色液體1.8 g,產率89%。The compound ( R )-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro- 1H -pyrrole-2-carboxylate methyl ester hydrochloride The salt (2.0 g, 4.9 mmol) was dissolved in dichloromethane (10 mL), N , N -diisopropylethylamine (4.0 mL, 24 mmol) was added, and acetyl chloride (1.1 g) was added after cooling to 0 °C , 14 mmol), stirred at room temperature for 3 h, then stopped the reaction, added water (20 mL × 3), stirred, extracted with dichloromethane (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/2) to obtain 1.8 g of a pale yellow liquid with a yield of 89%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.36 – 7.47 (m, 5H), 7.20 (d,J = 8.2 Hz, 1H), 7.05 – 7.06 (m, 1H), 6.95 (dd,J = 8.3, 1.9 Hz, 1H), 6.61 (t,J F-H = 74.8 Hz, 1H), 6.07 – 6.10 (m, 1H), 5.27 – 5.35 (m, 1H), 5.17 (s, 2H), 5.16 (s, 1H), 4.73 – 4.80 (m, 1H), 4.58 – 4.66 (m, 1H), 3.83 (s, 1H), 3.79 (s, 2H), 2.22 (s, 2H), 2.11 (s, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.36 – 7.47 (m, 5H), 7.20 (d, J = 8.2 Hz, 1H), 7.05 – 7.06 (m, 1H), 6.95 (dd, J = 8.3, 1.9 Hz, 1H), 6.61 (t, J FH = 74.8 Hz, 1H), 6.07 – 6.10 (m, 1H), 5.27 – 5.35 (m, 1H), 5.17 (s, 2H), 5.16 (s , 1H), 4.73 – 4.80 (m, 1H), 4.58 – 4.66 (m, 1H), 3.83 (s, 1H), 3.79 (s, 2H), 2.22 (s, 2H), 2.11 (s, 1H).
MS (ESI, pos.ion) m/z: 418.60 [M+H]+ .MS (ESI, pos.ion) m/z: 418.60 [M+H] + .
步驟4:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-羧酸甲酯 的合成Step 4: Synthesis of compound (2R )-1-acetoxy-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carboxylate methyl ester
將化合物 (R )-1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-羧酸甲酯 (4.5 g, 11 mmol) 溶於甲醇 (30 mL),加入Pd/C (450 mg, 10%),通入氫氣,室溫反應5 h,過濾除去催化劑,濾液濃縮得到淺褐色液體3.1 g,產率87%。The compound ( R )-1-acetyl-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro- 1H -pyrrole-2- Methyl carboxylate (4.5 g, 11 mmol) was dissolved in methanol (30 mL), Pd/C (450 mg, 10%) was added, hydrogen was passed through, the reaction was carried out at room temperature for 5 h, the catalyst was removed by filtration, and the filtrate was concentrated to obtain light brown Liquid 3.1 g, yield 87%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.10 (d,J = 8.3 Hz, 1H), 6.96 (d,J = 1.9 Hz, 1H), 6.79 (dd,J = 8.3, 2.0 Hz, 1H), 6.56 (t,J F-H = 73.7 Hz, 1H), 4.48 – 4.53 (m, 1H), 3.94 – 3.98 (m, 1H), 3.78 (s, 3H), 3.63 (t,J = 10.5 Hz, 1H), 3.38 – 3.47 (m, 1H), 2.65 – 2.71 (m, 1H), 2.14 (s, 3H), 2.05 – 2.11 (m, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.10 (d, J = 8.3 Hz, 1H), 6.96 (d, J = 1.9 Hz, 1H), 6.79 (dd, J = 8.3, 2.0 Hz, 1H), 6.56 (t, J FH = 73.7 Hz, 1H), 4.48 – 4.53 (m, 1H), 3.94 – 3.98 (m, 1H), 3.78 (s, 3H), 3.63 (t, J = 10.5 Hz, 1H), 3.38 – 3.47 (m, 1H), 2.65 – 2.71 (m, 1H), 2.14 (s, 3H), 2.05 – 2.11 (m, 1H).
MS (ESI, pos.ion) m/z: 330.00 [M+H]+ .MS (ESI, pos.ion) m/z: 330.00 [M+H] + .
步驟5:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-羧酸甲酯 的合成Step 5: Synthesis of compound (2R )-1-acetoxy-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carboxylate methyl ester
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-羧酸甲酯 (1.3 g, 3.9mmol) 溶於無水N ,N -二甲基甲醯胺 (10 mL),加入碳酸鉀 (1.8 g, 13 mmol) 和2-碘丙烷 (1.5 g, 8.8 mmol),80℃加熱反應4 h,減壓除去溶劑,剩餘物加水 (50 mL),乙酸乙酯萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1),得到淺褐色液體1.5 g,產率100%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carboxylic acid methyl ester (1.3 g, 3.9 mmol) was dissolved in Anhydrous N , N -dimethylformamide (10 mL), potassium carbonate (1.8 g, 13 mmol) and 2-iodopropane (1.5 g, 8.8 mmol) were added, the reaction was heated at 80 °C for 4 h, and the solvent was removed under reduced pressure , the residue was added with water (50 mL), extracted with ethyl acetate (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v /v ) = 50/1) to obtain 1.5 g of a light brown liquid with a yield of 100%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.14 (d,J = 8.1 Hz, 1H), 6.87 (s, 1H), 6.83 (dd,J = 8.0, 1.9 Hz, 1H), 6.56 (t,J F-H = 75.5 Hz, 1H), 4.56 – 4.61 (m, 1H), 4.48 – 4.55 (m, 1H), 3.94 – 3.98 (m, 1H), 3.79 (s, 3H), 3.63 (t,J = 10.5 Hz, 1H), 3.40 – 3.48 (m, 1H), 2.65 – 2.72 (m, 1H), 2.14 (s, 3H), 2.02 – 2.11 (m, 1H), 1.37 (d,J = 6.0 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.14 (d, J = 8.1 Hz, 1H), 6.87 (s, 1H), 6.83 (dd, J = 8.0, 1.9 Hz, 1H), 6.56 ( t, J FH = 75.5 Hz, 1H), 4.56 – 4.61 (m, 1H), 4.48 – 4.55 (m, 1H), 3.94 – 3.98 (m, 1H), 3.79 (s, 3H), 3.63 (t, J = 10.5 Hz, 1H), 3.40 – 3.48 (m, 1H), 2.65 – 2.72 (m, 1H), 2.14 (s, 3H), 2.02 – 2.11 (m, 1H), 1.37 (d, J = 6.0 Hz, 6H).
MS (ESI, pos.ion) m/z: 372.30 [M+H]+ .MS (ESI, pos.ion) m/z: 372.30 [M+H] + .
步驟6:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-羧酸 的合成Step 6: Synthesis of compound (2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-羧酸甲酯 (750 mg, 2.02 mmol) 溶於四氫呋喃 (10 mL) 和水 (5 mL) 的混合溶劑中,加入一水合氫氧化鋰 (297 mg, 7.08 mmol),50℃反應1 h後停止,加稀鹽酸調節溶液pH=1,減壓除去四氫呋喃,剩餘物用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淺褐色液體665 mg,產率92%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carboxylate methyl ester (750 mg, 2.02 mmol ) was dissolved in a mixed solvent of tetrahydrofuran (10 mL) and water (5 mL), lithium hydroxide monohydrate (297 mg, 7.08 mmol) was added, the reaction was stopped after 1 h at 50 °C, and diluted hydrochloric acid was added to adjust the pH of the solution to 1. The tetrahydrofuran was removed under reduced pressure, the residue was extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 665 mg of light brown liquid with a yield of 92%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.12 (d,J = 8.2 Hz, 1H), 7.04 – 7.08 (m, 1H), 6.93 (dd,J = 8.3, 1.8 Hz, 1H), 6.69 (t,J F-H = 75.6 Hz, 1H), 4.63 – 4.71 (m, 1H), 4.44 – 4.48 (m, 1H), 4.09 – 4.13 (m, 1H), 3.57 – 3.63 (m, 1H), 3.50 – 3.56 (m, 1H), 2.72 – 2.79 (m, 1H), 2.14 (s, 3H), 1.99 – 2.08 (m, 1H), 1.35 (d,J = 6.0 Hz, 6H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.12 (d, J = 8.2 Hz, 1H), 7.04 – 7.08 (m, 1H), 6.93 (dd, J = 8.3, 1.8 Hz, 1H) , 6.69 (t, J FH = 75.6 Hz, 1H), 4.63 – 4.71 (m, 1H), 4.44 – 4.48 (m, 1H), 4.09 – 4.13 (m, 1H), 3.57 – 3.63 (m, 1H), 3.50 – 3.56 (m, 1H), 2.72 – 2.79 (m, 1H), 2.14 (s, 3H), 1.99 – 2.08 (m, 1H), 1.35 (d, J = 6.0 Hz, 6H).
MS (ESI, pos.ion) m/z: 358.30 [M+H]+ .MS (ESI, pos.ion) m/z: 358.30 [M+H] + .
步驟7:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-甲醯氨基)甲基)吡啶羧酸乙酯 的合成Step 7: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carbamoylamino) Synthesis of ethyl methyl)picolinate
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-羧酸 (110 mg, 0.31 mmol),6-氨基甲基吡啶-2-羧酸乙酯鹽酸鹽 (132 mg, 0.52 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (295 mg, 1.54 mmol) 和N -羥基-7-氮雜苯並三氮唑 (83 mg, 0.61 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (358 mg, 2.77 mmol),室溫反應6 h,加水洗 (10 mL × 3) 攪拌,分離有機相,有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1),得到淺黃色固體107 mg,產率66%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carboxylic acid (110 mg, 0.31 mmol), 6-Aminomethylpyridine-2-carboxylate ethyl ester hydrochloride (132 mg, 0.52 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (295 mg , 1.54 mmol) and N -hydroxy-7-azabenzotriazole (83 mg, 0.61 mmol) were dissolved in dichloromethane (10 mL), cooled to 0 °C, and N , N -diisopropyl was added Ethylamine (358 mg, 2.77 mmol) was reacted at room temperature for 6 h, washed with water (10 mL × 3), stirred, the organic phase was separated, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography ( Eluent: dichloromethane/methanol (v/v) = 50/1) to give a pale yellow solid 107 mg with a yield of 66%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.02 (d,J = 7.6 Hz, 1H), 7.84 (t,J = 7.8 Hz, 1H), 7.57 (d,J = 7.8 Hz, 1H), 7.48 (br.s, 1H), 7.11 (d,J = 8.2 Hz, 1H), 6.93 (s, 1H), 6.87 (d,J = 7.3 Hz, 1H), 6.55 (t,J F-H = 75.6 Hz, 1H), 4.66 – 4.78 (m, 2H), 4.53 – 4.64 (m, 2H), 4.12 (q,J = 7.0 Hz, 2H), 3.96 – 4.01 (m, 1H), 3.61 (t,J = 10.7 Hz, 1H), 3.30 – 3.42 (m, 1H), 2.59 – 2.66 (m, 1H), 2.40 – 2.49 (m, 1H), 2.17 (s, 3H), 1.44 (t,J = 7.1 Hz, 3H), 1.37 (d,J = 6.1 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.02 (d, J = 7.6 Hz, 1H), 7.84 (t, J = 7.8 Hz, 1H), 7.57 (d, J = 7.8 Hz, 1H) , 7.48 (br.s, 1H), 7.11 (d, J = 8.2 Hz, 1H), 6.93 (s, 1H), 6.87 (d, J = 7.3 Hz, 1H), 6.55 (t, J FH = 75.6 Hz , 1H), 4.66 – 4.78 (m, 2H), 4.53 – 4.64 (m, 2H), 4.12 (q, J = 7.0 Hz, 2H), 3.96 – 4.01 (m, 1H), 3.61 (t, J = 10.7 Hz, 1H), 3.30 – 3.42 (m, 1H), 2.59 – 2.66 (m, 1H), 2.40 – 2.49 (m, 1H), 2.17 (s, 3H), 1.44 (t, J = 7.1 Hz, 3H) , 1.37 (d, J = 6.1 Hz, 6H).
MS (ESI, pos.ion) m/z: 520.10 [M+H]+ .MS (ESI, pos.ion) m/z: 520.10 [M+H] + .
實施例6:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-甲醯氨基)甲基)吡啶羧酸鹽酸鹽 Example 6: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carbamoylamino ) methyl) pyridine carboxylic acid hydrochloride
將化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-甲醯氨基)甲基)吡啶羧酸乙酯 (55 mg, 0.11 mmol) 溶於四氫呋喃 (6 mL) 和水 (3 mL) 的混合溶劑中,加入一水合氫氧化鋰 (22 mg, 0.52 mmol),50℃反應1.5 h後停止,加稀鹽酸調節溶液pH=1,減壓除去溶劑,剩餘物用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,除去溶劑得到55 mg淺黃色固體,產率98%。The compound 6-((( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carbamoylamino)methyl ) ethyl pyridinecarboxylate (55 mg, 0.11 mmol) was dissolved in a mixed solvent of tetrahydrofuran (6 mL) and water (3 mL), lithium hydroxide monohydrate (22 mg, 0.52 mmol) was added, and the reaction was carried out at 50 °C for 1.5 h After stopping, dilute hydrochloric acid was added to adjust the pH of the solution to 1, the solvent was removed under reduced pressure, the residue was extracted with ethyl acetate (10 mL × 2), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 55 mg of pale yellow solids, yield 98%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.06 (d,J = 7.0 Hz, 1H), 7.99 (t,J = 7.5 Hz, 1H), 7.76 (d,J = 7.4 Hz, 1H), 7.06 – 7.15 (m, 2H), 6.94 (d,J = 7.8 Hz, 1H), 6.70 (t,J F-H = 75.6 Hz, 1H), 4.64 – 4.74 (m, 2H), 4.51 – 4.58 (m, 2H), 4.10 – 4.14 (m, 1H), 3.66 (t,J = 10.4 Hz, 1H), 3.47 – 3.56 (m, 1H), 2.67 – 2.75 (m, 1H), 2.16 (s, 3H), 2.04 – 2.13 (m, 1H), 1.35 (d,J = 5.7 Hz, 6H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.06 (d, J = 7.0 Hz, 1H), 7.99 (t, J = 7.5 Hz, 1H), 7.76 (d, J = 7.4 Hz, 1H) ), 7.06 – 7.15 (m, 2H), 6.94 (d, J = 7.8 Hz, 1H), 6.70 (t, J FH = 75.6 Hz, 1H), 4.64 – 4.74 (m, 2H), 4.51 – 4.58 (m , 2H), 4.10 – 4.14 (m, 1H), 3.66 (t, J = 10.4 Hz, 1H), 3.47 – 3.56 (m, 1H), 2.67 – 2.75 (m, 1H), 2.16 (s, 3H), 2.04 – 2.13 (m, 1H), 1.35 (d, J = 5.7 Hz, 6H).
MS (ESI, pos.ion) m/z: 492.10 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 492.10 [M+H-HCl] + .
實施例7:化合物3-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-甲醯氨基)甲基)苯甲酸甲酯 Example 7: Compound 3-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carbamoylamino ) methyl) methyl benzoate
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-羧酸 (110 mg, 0.31 mmol),3-氨基甲基苯甲酸甲酯 (86 mg, 0.52 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (297 mg, 1.55 mmol) 和N -羥基-7-氮雜苯並三氮唑 (81 mg, 0.60 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (278 mg, 2.15 mmol),室溫反應3 h,加水洗 (10 mL × 3) 攪拌,分離有機相,有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1),得到無色液體112 mg,產率72%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carboxylic acid (110 mg, 0.31 mmol), Methyl 3-aminomethylbenzoate (86 mg, 0.52 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (297 mg, 1.55 mmol) and N- Hydroxy-7-azabenzotriazole (81 mg, 0.60 mmol) was dissolved in dichloromethane (10 mL), and after cooling to 0 °C, N , N -diisopropylethylamine (278 mg, 2.15 mmol), react at room temperature for 3 h, add water and wash (10 mL × 3), stir, separate the organic phase, dry the organic phase with anhydrous sodium sulfate, concentrate under reduced pressure, and conduct silica gel column chromatography (eluent: dichloromethane). /methanol (v/v) = 50/1) to obtain 112 mg of a colorless liquid with a yield of 72%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.95 – 7.96 (m, 2H), 7.53 (d,J = 7.7 Hz, 1H), 7.43 (d,J = 7.9 Hz, 1H), 7.13 (d,J = 8.2 Hz, 1H), 6.97 (s, 1H), 6.88 (dd,J = 8.2, 1.7 Hz, 1H), 6.57 (t,J F-H = 75.6 Hz, 1H), 4.57 – 4.68 (m, 2H), 4.49 – 4.58 (m, 2H), 3.94 – 3.99 (m, 1H), 3.92 (s, 3H), 3.52 (t,J = 10.8 Hz, 1H), 3.29 – 3.35 (m, 1H), 2.63 – 2.71 (m, 1H), 2.50 – 2.57 (m, 1H), 2.17 (s, 3H), 1.36 – 1.38 (m, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.95 – 7.96 (m, 2H), 7.53 (d, J = 7.7 Hz, 1H), 7.43 (d, J = 7.9 Hz, 1H), 7.13 ( d, J = 8.2 Hz, 1H), 6.97 (s, 1H), 6.88 (dd, J = 8.2, 1.7 Hz, 1H), 6.57 (t, J FH = 75.6 Hz, 1H), 4.57 – 4.68 (m, 2H), 4.49 – 4.58 (m, 2H), 3.94 – 3.99 (m, 1H), 3.92 (s, 3H), 3.52 (t, J = 10.8 Hz, 1H), 3.29 – 3.35 (m, 1H), 2.63 – 2.71 (m, 1H), 2.50 – 2.57 (m, 1H), 2.17 (s, 3H), 1.36 – 1.38 (m, 6H).
MS (ESI, pos.ion) m/z: 505.10 [M+H]+ .MS (ESI, pos.ion) m/z: 505.10 [M+H] + .
實施例8:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-甲醯氨基)甲基)苯甲酸 Example 8: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carbamoylamino )methyl)benzoic acid
將化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-甲醯氨基)甲基)苯甲酸甲酯 (63 mg, 0.12 mmol) 溶於四氫呋喃 (6 mL) 和水 (3 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (26 mg, 0.62 mmol),50℃反應1.5 h後停止,加稀鹽酸調節溶液pH=1,減壓除去四氫呋喃,剩餘物用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體53 mg,產率86%。The compound 6-((( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carbamoylamino)methyl ) methyl benzoate (63 mg, 0.12 mmol) was dissolved in a mixed solvent of tetrahydrofuran (6 mL) and water (3 mL), then lithium hydroxide monohydrate (26 mg, 0.62 mmol) was added, and the reaction was carried out at 50 °C for 1.5 h After stopping, dilute hydrochloric acid was added to adjust the pH of the solution to 1, the tetrahydrofuran was removed under reduced pressure, the residue was extracted with ethyl acetate (10 mL × 2), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 53 mg of white solid with a yield of 86 mg %.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.99 (s, 1H), 7.93 (d,J = 7.7 Hz, 1H), 7.62 (d,J = 7.2 Hz, 1H), 7.46 (d,J = 7.6 Hz, 1H), 7.06 – 7.12 (m, 2H), 6.93 (d,J = 8.5 Hz, 1H), 6.69 (t,J F-H = 75.6 Hz, 1H), 4.63 – 4.70 (m, 1H), 4.50 (s, 2H), 4.08 – 4.12 (m, 1H), 3.52 (t,J = 10.5 Hz, 1H), 3.44 – 3.55 (m, 1H), 3.31 – 3.38 (m, 1H), 2.64 – 2.72 (m, 1H), 2.15 (s, 3H), 2.01 – 2.12 (m, 1H), 1.35 (d,J = 5.8 Hz, 6H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.99 (s, 1H), 7.93 (d, J = 7.7 Hz, 1H), 7.62 (d, J = 7.2 Hz, 1H), 7.46 (d , J = 7.6 Hz, 1H), 7.06 – 7.12 (m, 2H), 6.93 (d, J = 8.5 Hz, 1H), 6.69 (t, J FH = 75.6 Hz, 1H), 4.63 – 4.70 (m, 1H) ), 4.50 (s, 2H), 4.08 – 4.12 (m, 1H), 3.52 (t, J = 10.5 Hz, 1H), 3.44 – 3.55 (m, 1H), 3.31 – 3.38 (m, 1H), 2.64 – 2.72 (m, 1H), 2.15 (s, 3H), 2.01 – 2.12 (m, 1H), 1.35 (d, J = 5.8 Hz, 6H).
MS (ESI, pos.ion) m/z: 491.40 [M+H]+ .MS (ESI, pos.ion) m/z: 491.40 [M+H] + .
實施例9:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)-N -(3-(羥基甲基)苯基)吡咯烷-2-甲醯胺 Example 9: Compound (2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-isopropoxyphenyl) - N - (3- (hydroxymethyl) benzene yl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-羧酸 (60 mg, 0.17 mmol),3-氨基苯甲醇 (62 mg, 0.50 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (163 mg, 0.85 mmol) 和N -羥基-7-氮雜苯並三氮唑 (46 mg, 0.34 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (153 mg, 1.18 mmol),室溫反應12 h,加水洗 (10 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 35/1),得到淺褐色固體53 mg,產率68%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carboxylic acid (60 mg, 0.17 mmol), 3-Aminobenzyl alcohol (62 mg, 0.50 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (163 mg, 0.85 mmol) and N -hydroxy-7- Azabenzotriazole (46 mg, 0.34 mmol) was dissolved in dichloromethane (10 mL), and after cooling to 0 °C, N , N -diisopropylethylamine (153 mg, 1.18 mmol) was added, The reaction was carried out at room temperature for 12 h, washed with water (10 mL), extracted with dichloromethane (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: Dichloromethane/methanol (v/v) = 35/1), 53 mg of light brown solid was obtained in 68% yield.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.68 (s, 1H), 7.42 (d,J = 7.8 Hz, 1H), 7.37 (s, 1H), 7.12 – 7.15 (m, 2H), 6.96 (s, 1H), 6.93 (d,J = 7.5 Hz, 1H), 6.87 (d,J = 8.0 Hz, 1H), 6.56 (t,J F-H = 75.5 Hz, 1H), 4.74 – 4.78 (m, 1H), 4.52 – 4.64 (m, 3H), 3.97 – 4.01 (m, 1H), 3.62 – 3.67 (m, 1H), 3.34 – 3.45 (m, 1H), 2.58 – 2.66 (m, 1H), 2.46 – 2.54 (m, 1H), 2.20 (s, 3H), 1.37 (t,J = 5.9 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.68 (s, 1H), 7.42 (d, J = 7.8 Hz, 1H), 7.37 (s, 1H), 7.12 – 7.15 (m, 2H), 6.96 (s, 1H), 6.93 (d, J = 7.5 Hz, 1H), 6.87 (d, J = 8.0 Hz, 1H), 6.56 (t, J FH = 75.5 Hz, 1H), 4.74 – 4.78 (m, 1H), 4.52 – 4.64 (m, 3H), 3.97 – 4.01 (m, 1H), 3.62 – 3.67 (m, 1H), 3.34 – 3.45 (m, 1H), 2.58 – 2.66 (m, 1H), 2.46 – 2.54 (m, 1H), 2.20 (s, 3H), 1.37 (t, J = 5.9 Hz, 6H).
MS (ESI, pos.ion) m/z: 463.30 [M+H]+ .MS (ESI, pos.ion) m/z: 463.30 [M+H] + .
實施例10:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧苯基)-N -(2-乙氧基苄基)吡咯烷-2-甲醯胺 Example 10: Compound (2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-isopropoxyphenyl) - N - (2- ethoxy-benzyl) pyrrolidin Alkyl-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-羧酸 (60 mg, 0.17 mmol),2-乙氧基苄胺 (52 mg, 0.34 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (163 mg, 0.85 mmol) 和N -羥基-7-氮雜苯並三氮唑 (46 mg, 0.34 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (153 mg, 1.18 mmol),室溫反應12 h,加水洗 (10 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 35/1),得到白色黏稠固體79 mg,產率96%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carboxylic acid (60 mg, 0.17 mmol), 2-Ethoxybenzylamine (52 mg, 0.34 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (163 mg, 0.85 mmol) and N -hydroxy- 7-Azabenzotriazole (46 mg, 0.34 mmol) was dissolved in dichloromethane (10 mL), cooled to 0 °C, and N , N -diisopropylethylamine (153 mg, 1.18 mmol) was added. ), reacted at room temperature for 12 h, washed with water (10 mL), extracted with dichloromethane (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluting Reagent: dichloromethane/methanol (v/v) = 35/1) to obtain 79 mg of white viscous solid with a yield of 96%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.23 – 7.29 (m, 2H), 7.08 – 7.14 (m, 2H), 6.96 (s, 1H), 6.84 – 6.92 (m, 2H), 6.56 (t,J F-H = 75.6 Hz, 1H), 4.51 – 4.62 (m, 3H), 4.37 – 4.47 (m, 1H), 4.10 (q,J = 6.9 Hz, 2H), 3.92 – 3.96 (m, 1H), 3.51 (t,J = 10.8 Hz, 1H), 3.23 – 3.32 (m, 1H), 2.47 – 2.63 (m, 2H), 2.14 (s, 3H), 1.47 (t,J = 6.9 Hz, 3H), 1.37 (d,J = 5.7 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.23 – 7.29 (m, 2H), 7.08 – 7.14 (m, 2H), 6.96 (s, 1H), 6.84 – 6.92 (m, 2H), 6.56 (t, J FH = 75.6 Hz, 1H), 4.51 – 4.62 (m, 3H), 4.37 – 4.47 (m, 1H), 4.10 (q, J = 6.9 Hz, 2H), 3.92 – 3.96 (m, 1H) , 3.51 (t, J = 10.8 Hz, 1H), 3.23 – 3.32 (m, 1H), 2.47 – 2.63 (m, 2H), 2.14 (s, 3H), 1.47 (t, J = 6.9 Hz, 3H), 1.37 (d, J = 5.7 Hz, 6H).
MS (ESI, pos.ion) m/z: 491.25 [M+H]+ .MS (ESI, pos.ion) m/z: 491.25 [M+H] + .
實施例11:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(2-乙氧基苄基)吡咯烷-2-甲醯胺 Example 11: Compound (2 R) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - (2- ethoxyethyl benzyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (50 mg, 0.14 mmol),(2-乙氧基苯基)甲胺 (41 mg, 0.27 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (132 mg, 0.69 mmol) 和N -羥基-7-氮雜苯並三氮唑 (37 mg, 0.27 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (107 mg, 0.83 mmol),室溫反應11 h,加入二氯甲烷 (15 mL),加水洗有機相 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到白色黏稠固體39 mg,產率57%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (50 mg, 0.14 mmol), (2-ethoxyphenyl)methylamine (41 mg, 0.27 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (132 mg, 0.69 mmol) and N -hydroxy-7-azabenzotriazole (37 mg, 0.27 mmol) were dissolved in dichloromethane (10 mL), cooled to 0 °C, and N , N -diisopropylethyl acetate was added. Amine (107 mg, 0.83 mmol) was reacted at room temperature for 11 h, dichloromethane (15 mL) was added, the organic phase was washed with water (20 mL × 2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was subjected to Separation by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/2) gave 39 mg of a white viscous solid with a yield of 57%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.23 – 7.28 (m, 1H), 7.09 – 7.12 (m, 2H), 6.85 – 6.94 (m, 4H), 6.62 (t,J F-H = 75.5 Hz, 1H), 4.53 – 4.59 (m, 2H), 4.39 – 4.47 (m, 1H), 4.09 (q,J = 7.0 Hz, 2H), 3.84 – 3.95 (m, 3H), 3.51 (t,J = 10.7 Hz, 1H), 3.24 – 3.34 (m, 1H), 2.89 – 2.95 (m, 0.3H), 2.48 – 2.61 (m, 1.7H), 2.14 (s, 2.4H), 1.90 (s, 0.6H), 1.45 (t,J = 7.0 Hz, 3H), 1.28 – 1.36 (m, 1H), 0.69 – 0.70 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.23 – 7.28 (m, 1H), 7.09 – 7.12 (m, 2H), 6.85 – 6.94 (m, 4H), 6.62 (t, J FH = 75.5 Hz, 1H), 4.53 – 4.59 (m, 2H), 4.39 – 4.47 (m, 1H), 4.09 (q, J = 7.0 Hz, 2H), 3.84 – 3.95 (m, 3H), 3.51 (t, J = 10.7 Hz, 1H), 3.24 – 3.34 (m, 1H), 2.89 – 2.95 (m, 0.3H), 2.48 – 2.61 (m, 1.7H), 2.14 (s, 2.4H), 1.90 (s, 0.6H) , 1.45 (t, J = 7.0 Hz, 3H), 1.28 – 1.36 (m, 1H), 0.69 – 0.70 (m, 2H), 0.36 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 503.10 [M+H]+ .MS (ESI, pos.ion) m/z: 503.10 [M+H] + .
實施例12:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N,N -二甲基煙醯胺 Example 12: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -formamido)methyl) -N,N -dimethylnicotinamide
步驟1:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)煙酸甲酯 的合成Step 1: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of methyl formamido)methyl)nicotinate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (300 mg, 0.81 mmol),6-(氨基甲基)煙酸甲酯 (206 mg, 1.24 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (780 mg, 4.07 mmol) 和N -羥基-7-氮雜苯並三氮唑 (166 mg, 1.22 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.6 mL, 4.0 mmol),室溫反應17 h,加水洗 (15 mL),然後二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到淺黃色黏稠固體213 mg,收率51%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (300 mg, 0.81 mmol), methyl 6-(aminomethyl)nicotinate (206 mg, 1.24 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (780 mg, 4.07 mmol) and N -hydroxy-7-azabenzotriazole (166 mg, 1.22 mmol) were dissolved in dichloromethane (5 mL), to this solution was added dropwise N , N -dichloromethane at 0°C Isopropylethylamine (0.6 mL, 4.0 mmol) was reacted at room temperature for 17 h, washed with water (15 mL), and then extracted with dichloromethane (10 mL × 3). The organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the solution was concentrated. The liquid was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 213 mg of a light yellow viscous solid with a yield of 51%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.15 (s, 1H), 8.28 (dd,J = 8.1, 2.0 Hz, 1H), 7.43 (d,J = 8.1 Hz, 1H), 7.12 (d,J = 8.2 Hz, 1H), 6.91 (s, 1H), 6.87 (d,J = 8.2 Hz, 1H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.61 – 4.69 (m, 3H), 3.94 – 3.99 (m, 1H), 3.97 (s, 3H), 3.88 (d,J = 6.9 Hz, 2H), 3.57 (t,J = 10.7 Hz, 1H), 3.31 – 3.41 (m, 1H), 2.46 – 2.63 (m, 2H), 2.16 (s, 3H), 1.27 – 1.35 (m, 1H), 0.65 – 0.69 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.15 (s, 1H), 8.28 (dd, J = 8.1, 2.0 Hz, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.12 ( d, J = 8.2 Hz, 1H), 6.91 (s, 1H), 6.87 (d, J = 8.2 Hz, 1H), 6.62 (t, J FH = 75.6 Hz, 1H), 4.61 – 4.69 (m, 3H) , 3.94 – 3.99 (m, 1H), 3.97 (s, 3H), 3.88 (d, J = 6.9 Hz, 2H), 3.57 (t, J = 10.7 Hz, 1H), 3.31 – 3.41 (m, 1H), 2.46 – 2.63 (m, 2H), 2.16 (s, 3H), 1.27 – 1.35 (m, 1H), 0.65 – 0.69 (m, 2H), 0.36 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 518.10 [M+H]+ .MS (ESI, pos.ion) m/z: 518.10 [M+H] + .
步驟2:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)煙酸的合成Step 2: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of formamido)methyl)nicotinic acid
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)煙酸甲酯 (154 mg, 0.3 mmol) 溶於四氫呋喃 (10 mL) 和水 (5 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (86 mg, 2.05 mmol),50℃反應3 h後停止,加稀鹽酸調節溶液pH=1,再用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體137 mg,收率91%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)methyl nicotinate (154 mg, 0.3 mmol) was dissolved in a mixed solvent of tetrahydrofuran (10 mL) and water (5 mL), and then lithium hydroxide monohydrate (86 mg, 2.05 mmol) was added, 50 The reaction was stopped after 3 h at ℃, diluted hydrochloric acid was added to adjust the pH of the solution to 1, and then extracted with ethyl acetate (10 mL × 3). The organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 137 mg of white solid with a yield of 91%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 9.07 (s, 1H), 8.42 (dd,J = 8.2, 1.9 Hz, 1H), 7.71 (d,J = 8.2 Hz, 1H), 7.12 (d,J = 8.2 Hz, 1H), 7.09 (s, 1H), 6.93 – 6.96 (m, 1H), 6.75 (t,J F-H = 75.7 Hz, 1H), 4.51 – 4.73 (m, 2H), 4.10 – 4.14 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.66 (t,J = 10.6 Hz, 1H), 3.51 – 3.56 (m, 1H), 2.68 – 2.74 (m, 1H), 2.17 (s, 3H), 2.01 – 2.13 (m, 2H), 1.27 – 1.35 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 9.07 (s, 1H), 8.42 (dd, J = 8.2, 1.9 Hz, 1H), 7.71 (d, J = 8.2 Hz, 1H), 7.12 (d, J = 8.2 Hz, 1H), 7.09 (s, 1H), 6.93 – 6.96 (m, 1H), 6.75 (t, J FH = 75.7 Hz, 1H), 4.51 – 4.73 (m, 2H), 4.10 – 4.14 (m, 1H), 3.93 (d, J = 6.9 Hz, 2H), 3.66 (t, J = 10.6 Hz, 1H), 3.51 – 3.56 (m, 1H), 2.68 – 2.74 (m, 1H), 2.17 (s, 3H), 2.01 – 2.13 (m, 2H), 1.27 – 1.35 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 504.10 [M+H]+ .MS (ESI, pos.ion) m/z: 504.10 [M+H] + .
步驟3:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N,N -二甲基煙醯胺 的合成Step 3: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of formamido)methyl) -N,N -dimethylnicotinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)煙酸 (130 mg, 0.26 mmol),二甲胺鹽酸鹽 (103 mg, 1.26 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (249 mg, 1.3 mmol) 和N -羥基-7-氮雜苯並三氮唑 (58 mg, 0.43 mmol) 溶於二氯甲烷 (5 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.28 mL, 1.7 mmol),室溫反應19 h,加水洗 (15 mL),然後二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體103 mg,收率75%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)nicotinic acid (130 mg, 0.26 mmol), dimethylamine hydrochloride (103 mg, 1.26 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide The acid salt (249 mg, 1.3 mmol) and N -hydroxy-7-azabenzotriazole (58 mg, 0.43 mmol) were dissolved in dichloromethane (5 mL), and the solution was added dropwise at 0°C N , N -diisopropylethylamine (0.28 mL, 1.7 mmol) was added, the reaction was carried out at room temperature for 19 h, washed with water (15 mL), and then extracted with dichloromethane (10 mL × 3). The organic phase was washed with anhydrous sodium sulfate. After drying, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 103 mg of white solid with a yield of 75%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.60 (s, 1H), 7.74 (d,J = 7.5 Hz, 1H), 7.61 (br.s, 1H), 7.39 (d,J = 7.9 Hz, 1H), 7.11 (d,J = 8.1 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 8.0 Hz, 1H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.49-4.68 (m, 3H), 3.91-3.98 (m, 1H), 3.88 (d,J = 6.8 Hz, 2H), 3.54-3.60 (m, 1H), 3.26-3.41 (m, 1H), 3.13 (s, 3H), 3.01 (s, 3H), 2.41-2.62 (m, 2H), 2.14 (s, 3H), 1.24-1.35 (m, 1H), 0.62-0.67 (m, 2H), 0.34-0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.60 (s, 1H), 7.74 (d, J = 7.5 Hz, 1H), 7.61 (br.s, 1H), 7.39 (d, J = 7.9 Hz, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.90 (s, 1H), 6.86 (d, J = 8.0 Hz, 1H), 6.62 (t, J FH = 75.6 Hz, 1H), 4.49 -4.68 (m, 3H), 3.91-3.98 (m, 1H), 3.88 (d, J = 6.8 Hz, 2H), 3.54-3.60 (m, 1H), 3.26-3.41 (m, 1H), 3.13 (s , 3H), 3.01 (s, 3H), 2.41-2.62 (m, 2H), 2.14 (s, 3H), 1.24-1.35 (m, 1H), 0.62-0.67 (m, 2H), 0.34-0.39 (m , 2H).
MS (ESI, pos.ion) m/z: 531.15 [M+H]+ .MS (ESI, pos.ion) m/z: 531.15 [M+H] + .
實施例13:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((4-(1-羥乙基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 13: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((4-( 1-Hydroxyethyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (748-1) (92 mg, 0.25 mmol),化合物1-(2-(氨甲基)吡啶-4-基)乙醇 (101 mg, 0.54mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (143 mg, 0.75 mmol) 和N -羥基-7-氮雜苯並三氮唑 (72 mg, 0.53 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌17 h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體52 mg,收率41%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (748-1 ) (92 mg, 0.25 mmol), compound 1-(2-(aminomethyl)pyridin-4-yl)ethanol (101 mg, 0.54 mmol), 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (143 mg, 0.75 mmol) and N -hydroxy-7-azabenzotriazole (72 mg, 0.53 mmol) were dissolved in dichloromethane (6 mL) at room temperature To this solution was added dropwise N , N -diisopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 17 h, added water (50 mL), extracted with dichloromethane (5 mL × 3), the organic phase was It was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 52 mg of white solid, with a yield of 41%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.81-8.84 (m, 0.3H), 8.53-8.58 (m, 0.7H), 8.39-8.41 (m, 1H), 7.36 (s, 0.7H), 7.28 (s, 0.3H), 7.22 (s, 1H), 7.10-7.14 (m, 1H), 7.05-7.06 (m, 1H), 7.03 (t,J F-H = 78.2 Hz, 1H), 6.89 (d,J = 8.5 Hz, 1H), 5.33-5.39 (m, 1H), 4.64-4.73 (m, 1H), 4.35-4.42 (m, 3H), 4.19-4.23 (m, 0.3H), 4.05-4.09 (m, 0.7H), 3.90 (d,J = 6.9 Hz, 2H), 3.34-3.47 (m, 2H), 2.51-2.64 (m, 1H), 2.02 (s, 3H), 1.89-1.98 (m, 1H), 1.31 (d,J = 6.5 Hz, 3H), 1.24-1.27 (m, 1H), 0.54-0.60 (m, 2H), 0.31-0.38 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.81-8.84 (m, 0.3H), 8.53-8.58 (m, 0.7H), 8.39-8.41 (m, 1H), 7.36 (s, 0.7H), 7.28 (s, 0.3H), 7.22 (s, 1H), 7.10-7.14 (m, 1H), 7.05-7.06 (m, 1H), 7.03 (t, J FH = 78.2 Hz, 1H), 6.89 (d, J = 8.5 Hz, 1H), 5.33-5.39 (m, 1H), 4.64-4.73 (m, 1H), 4.35-4.42 (m, 3H), 4.19-4.23 (m, 0.3H), 4.05 -4.09 (m, 0.7H), 3.90 (d, J = 6.9 Hz, 2H), 3.34-3.47 (m, 2H), 2.51-2.64 (m, 1H), 2.02 (s, 3H), 1.89-1.98 ( m, 1H), 1.31 (d, J = 6.5 Hz, 3H), 1.24-1.27 (m, 1H), 0.54-0.60 (m, 2H), 0.31-0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 504.20 [M+H]+ .MS (ESI, pos.ion) m/z: 504.20 [M+H] + .
實施例14:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(1-羥乙基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 14: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( 1-Hydroxyethyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (82 mg, 0.22 mmol),化合物1-(6-(氨甲基)吡啶-2-基)乙醇 (73 mg, 0.48 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (123 mg, 0.64 mmol) 和N -羥基-7-氮雜苯並三氮唑 (74 mg, 0.54 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌17 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體43 mg,收率38%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (82 mg, 0.22 mmol), compound 1-(6-(aminomethyl)pyridin-2-yl)ethanol (73 mg, 0.48 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide The hydrochloride salt (123 mg, 0.64 mmol) and N -hydroxy-7-azabenzotriazole (74 mg, 0.54 mmol) were dissolved in dichloromethane (6 mL) and added dropwise to this solution at room temperature N , N -diisopropylethylamine (0.4 mL, 2.0 mmol) was added, stirred at room temperature for 17 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sodium sulfate After drying, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 43 mg of a white solid with a yield of 38%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.75-8.83 (m, 0.2H), 8.50-8.62 (m, 0.8H), 7.70-7.79 (m, 1H), 7.32-7.39 (m, 1H), 7.24-7.26 (m, 1H), 7.06-7.14 (m, 2H), 7.03 (t,J F-H = 74.6 Hz, 1H), 6.86-6.90 (m, 1H), 5.26-5.43 (m, 1H), 4.64-4.75 (m, 1H), 4.28-4.44 (m, 3H), 4.02-4.12 (m, 1H), 3.90 (d,J = 6.1 Hz, 2H), 3.43-3.57 (m, 2H), 2.56-2.67 (m, 1H), 2.03 (s, 3H), 1.86-1.98 (m, 1H), 1.30-1.36 (m, 3H), 1.23-1.26 (m, 1H), 0.51-0.62 (m, 2H), 0.28-0.38 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.75-8.83 (m, 0.2H), 8.50-8.62 (m, 0.8H), 7.70-7.79 (m, 1H), 7.32-7.39 ( m, 1H), 7.24-7.26 (m, 1H), 7.06-7.14 (m, 2H), 7.03 (t, J FH = 74.6 Hz, 1H), 6.86-6.90 (m, 1H), 5.26-5.43 (m , 1H), 4.64-4.75 (m, 1H), 4.28-4.44 (m, 3H), 4.02-4.12 (m, 1H), 3.90 (d, J = 6.1 Hz, 2H), 3.43-3.57 (m, 2H) ), 2.56-2.67 (m, 1H), 2.03 (s, 3H), 1.86-1.98 (m, 1H), 1.30-1.36 (m, 3H), 1.23-1.26 (m, 1H), 0.51-0.62 (m , 2H), 0.28-0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 504.20 [M+H]+ .MS (ESI, pos.ion) m/z: 504.20 [M+H] + .
實施例15:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(羥甲基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 15: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( Hydroxymethyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (85 mg, 0.23 mmol),化合物 (6-(氨甲基)吡啶-2-基)甲醇 (61 mg, 0.35mmol),1-乙基-3-(3-二甲胺基丙基)碳二亞胺鹽酸鹽 (143 mg, 0.75 mmol) 和N -羥基-7-氮雜苯並三氮唑 (72 mg, 0.53 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌20 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體64 mg,收率56%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (85 mg, 0.23 mmol), compound (6-(aminomethyl)pyridin-2-yl)methanol (61 mg, 0.35mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide salt The acid salt (143 mg, 0.75 mmol) and N -hydroxy-7-azabenzotriazole (72 mg, 0.53 mmol) were dissolved in dichloromethane (6 mL), and to this solution was added dropwise at room temperature N , N -diisopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 20 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was dried over anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 64 mg of white solid with a yield of 56%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.52-8.55 (m, 1H), 7.71-7.75 (m, 1H), 7.25-7.35 (m, 2H), 7.12 (d,J = 7.7 Hz, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.8 Hz, 1H), 6.89 (d,J = 8.4 Hz, 1H), 5.36-5.41 (m, 1H), 4.52 (d,J = 4.8 Hz, 2H), 4.27-4.42 (m, 3H), 4.05-4.09 (m, 1H), 3.90 (d,J = 6.5 Hz, 2H), 3.40-3.51 (m, 2H), 2.55-2.64 (m, 1H), 2.03 (s, 3H), 1.88-1.98 (m, 1H), 1.23-1.35 (m, 1H), 0.53-0.59 (m, 2H), 0.31-0.37 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.52-8.55 (m, 1H), 7.71-7.75 (m, 1H), 7.25-7.35 (m, 2H), 7.12 (d, J = 7.7 Hz, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.8 Hz, 1H), 6.89 (d, J = 8.4 Hz, 1H), 5.36-5.41 (m, 1H), 4.52 (d , J = 4.8 Hz, 2H), 4.27-4.42 (m, 3H), 4.05-4.09 (m, 1H), 3.90 (d, J = 6.5 Hz, 2H), 3.40-3.51 (m, 2H), 2.55- 2.64 (m, 1H), 2.03 (s, 3H), 1.88-1.98 (m, 1H), 1.23-1.35 (m, 1H), 0.53-0.59 (m, 2H), 0.31-0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 490.20 [M+H]+ .MS (ESI, pos.ion) m/z: 490.20 [M+H] + .
實施例16:化合物 (3-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)苯基)氨基甲酸甲酯 Example 16: Compound (3-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine- 2-Methylamino)methyl)phenyl)carbamate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (748-1) (85 mg, 0.23 mmol),化合物 (3-(氨甲基)苯基)氨基甲酸甲酯鹽酸鹽 (803-2) (96 mg, 0.53 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (148 mg, 0.77 mmol) 和N -羥基-7-氮雜苯並三氮唑 (74 mg, 0.54 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌14 h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體46mg,收率38%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (748-1 ) (85 mg, 0.23 mmol), compound (3-(aminomethyl)phenyl)carbamate methyl ester hydrochloride (803-2) (96 mg, 0.53 mmol), 1-ethyl-(3-di Methylaminopropyl)carbodiimide hydrochloride (148 mg, 0.77 mmol) and N -hydroxy-7-azabenzotriazole (74 mg, 0.54 mmol) were dissolved in dichloromethane (6 mL) ), N , N -diisopropylethylamine (0.4 mL, 2.0 mmol) was added dropwise to this solution at room temperature, stirred at room temperature for 14 h, added water (50 mL), and extracted with dichloromethane (5 mL). × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 46 mg of white solid, which was collected rate 38%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 9.60- 9.64 (m, 1H), 8.72-8.75 (m, 0.3H), 8.41-8.43 (m, 0.7H), 7.37-7.42 (m, 1H), 7.28-7.32 (m, 1H), 7.20-7.23 (m, 1H), 7.09-7.13 (m, 1H), 7.04-7.05 (m, 1H), 7.02 (t,J F-H = 69.5 Hz, 1H), 6.88-6.94 (m, 2H), 4.18-4.34 (m, 3H), 4.03-4.07 (m, 1H), 3.89 (d,J = 6.8 Hz, 2H), 3.63 (s, 3H), 3.39-3.49 (m, 2H), 2.56-2.61 (m, 1H), 2.01 (s, 3H), 1.87-1.96 (m, 1H), 1.22-1.33 (m, 1H), 0.54-0.60 (m, 2H), 0.31-0.35 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 9.60-9.64 (m, 1H), 8.72-8.75 (m, 0.3H), 8.41-8.43 (m, 0.7H), 7.37-7.42 ( m, 1H), 7.28-7.32 (m, 1H), 7.20-7.23 (m, 1H), 7.09-7.13 (m, 1H), 7.04-7.05 (m, 1H), 7.02 (t, J FH = 69.5 Hz , 1H), 6.88-6.94 (m, 2H), 4.18-4.34 (m, 3H), 4.03-4.07 (m, 1H), 3.89 (d, J = 6.8 Hz, 2H), 3.63 (s, 3H), 3.39-3.49 (m, 2H), 2.56-2.61 (m, 1H), 2.01 (s, 3H), 1.87-1.96 (m, 1H), 1.22-1.33 (m, 1H), 0.54-0.60 (m, 2H) ), 0.31-0.35 (m, 2H).
MS (ESI, pos.ion) m/z: 532.20 [M+H]+ .MS (ESI, pos.ion) m/z: 532.20 [M+H] + .
實施例17:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((嘧啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 17: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((pyrimidine-2 -yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (92 mg, 0.25 mmol),化合物嘧啶-2-甲胺 (103 mg, 0.71mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (150 mg, 0.78 mmol) 和N -羥基-7-氮雜苯並三氮唑 (71 mg, 0.52 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下加入N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌15 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體49 mg,收率42%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (92 mg, 0.25 mmol), compound pyrimidine-2-methylamine (103 mg, 0.71 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (150 mg, 0.78 mmol) and N -Hydroxy-7-azabenzotriazole (71 mg, 0.52 mmol) was dissolved in dichloromethane (6 mL), and N , N -diisopropylethylamine (0.4 mL, 2.0 mL) was added at room temperature mmol), stirred at room temperature for 15 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (elution Reagent: dichloromethane/methanol (v/v) = 15/1) to obtain 49 mg of white solid with a yield of 42%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.75 (d,J = 4.7 Hz, 2H), 7.39 (t,J = 4.4 Hz, 1H), 7.12 (t,J = 8.0 Hz, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.86-6.90 (m, 1H), 4.38-4.56 (m, 3H), 4.19-4.24 (m, 0.5H), 4.03-4.07 (m, 0.5H), 3.87-3.90 (m, 2H), 3.39-3.48 (m, 2H), 2.74-2.80 (m, 0.5H), 2.74-2.80 (m, 0.5H), 1.98-2.07 (m, 1H), 2.01 (s, 3H), 1.23-1.32 (m, 1H), 0.53-0.61 (m, 2H), 0.29-0.36 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.75 (d, J = 4.7 Hz, 2H), 7.39 (t, J = 4.4 Hz, 1H), 7.12 (t, J = 8.0 Hz, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.86-6.90 (m, 1H), 4.38-4.56 (m, 3H), 4.19-4.24 (m, 0.5H) , 4.03-4.07 (m, 0.5H), 3.87-3.90 (m, 2H), 3.39-3.48 (m, 2H), 2.74-2.80 (m, 0.5H), 2.74-2.80 (m, 0.5H), 1.98 -2.07 (m, 1H), 2.01 (s, 3H), 1.23-1.32 (m, 1H), 0.53-0.61 (m, 2H), 0.29-0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 461.25 [M+H]+ .MS (ESI, pos.ion) m/z: 461.25 [M+H] + .
實施例18:化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(1-羥基乙基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 18: Compound ( 2S )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( 1-Hydroxyethyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
步驟1:(2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸的合成Step 1: Synthesis of (2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid
將化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯(410 mg, 1.07 mmol) 溶於四氫呋喃 (5 mL) 和水 (5 mL) 的混合溶劑中,加入一水合氫氧化鋰 (230 mg, 5.48mmol),50℃反應4 h後停止。除去溶劑,加稀鹽酸調節溶液pH=1,用乙酸乙酯萃取 (10 mL × 3),合併有機相,無水硫酸鈉乾燥,減壓濃縮得白色固體365 mg,產率92%。The compound ( 2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester (410 mg, 1.07 mmol) was dissolved in a mixed solvent of tetrahydrofuran (5 mL) and water (5 mL), lithium hydroxide monohydrate (230 mg, 5.48 mmol) was added, and the reaction was stopped after 4 h at 50 °C. The solvent was removed, diluted hydrochloric acid was added to adjust the pH of the solution to 1, extracted with ethyl acetate (10 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 365 mg of white solid with a yield of 92%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.13 (d,J = 8.0 Hz, 1H), 6.83 (s, 1H), 6.80-6.81 (m, 1H), 6.61 (t,J F-H = 75.5 Hz, 1H), 4.56-4.60 (m, 1H), 3.94-3.98 (m, 1H), 3.87 (d,J = 6.9 Hz, 2H), 3.51-3.57 (m, 1H), 3.34-3.43 (m, 1H), 2.62 – 2.69 (m, 1H), 2.33-2.41 (m, 1H), 2.18 (s, 3H), 1.25 – 1.34 (m, 1H), 0.63-0.68 (m, 2H), 0.34-0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.13 (d, J = 8.0 Hz, 1H), 6.83 (s, 1H), 6.80-6.81 (m, 1H), 6.61 (t, J FH = 75.5 Hz, 1H), 4.56-4.60 (m, 1H), 3.94-3.98 (m, 1H), 3.87 (d, J = 6.9 Hz, 2H), 3.51-3.57 (m, 1H), 3.34-3.43 (m , 1H), 2.62 – 2.69 (m, 1H), 2.33-2.41 (m, 1H), 2.18 (s, 3H), 1.25 – 1.34 (m, 1H), 0.63-0.68 (m, 2H), 0.34-0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 370.20 [M+H]+ .MS (ESI, pos.ion) m/z: 370.20 [M+H] + .
步驟2:(2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(1-羥基乙基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺的合成Step 2: ( 2S )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-(1- Synthesis of Hydroxyethyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (97 mg, 0.26 mmol),1-(6-(氨基甲基)吡啶-2-基)乙醇二鹽酸鹽 (84 mg, 0.55 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (103 mg, 0.54 mmol) 和N -羥基-7-氮雜苯並三氮唑 (69 mg, 0.51 mmol) 溶於二氯甲烷 (4 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.22 mL, 1.3 mmol),室溫攪拌7 h,反應液水洗 (5 mL × 3),無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇( v/v ) = 20/1),得淡黃色固體68 mg,產率51%。The compound ( 2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (97 mg, 0.26 mmol), 1-(6-(aminomethyl)pyridin-2-yl)ethanol dihydrochloride (84 mg, 0.55 mmol), 1-ethyl-(3-dimethylaminopropyl)carboline Diimine hydrochloride (103 mg, 0.54 mmol) and N -hydroxy-7-azabenzotriazole (69 mg, 0.51 mmol) were dissolved in dichloromethane (4 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.22 mL, 1.3 mmol) was added, stirred at room temperature for 7 h, the reaction solution was washed with water (5 mL × 3), dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography Separation (eluent: dichloromethane/methanol (v/v) = 20/1) gave a pale yellow solid 68 mg, yield 51%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.55 (t,J = 5.9 Hz, 1H), 7.72 (t,J = 7.8 Hz, 1H), 7.34-7.40 (m, 1H), 7.26 (d,J = 7.7 Hz, 1H), 7.10-7.14 (m, 1H), 7.05-7.07 (m, 1H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.89 (d,J = 9.5 Hz, 1H), 5.33-5.35 (m, 1H), 4.64-4.72 (m, 1H), 4.32-4.41 (m, 3H), 4.06-4.10 (m, 1H), 3.90 (d,J = 6.9 Hz, 2H), 3.37-3.52 (m, 2H), 2.58-2.65 (m, 1H), 2.03 (s, 3H), 1.84-1.93 (m, 1H), 1.33-1.36 (m, 3H), 1.24-1.27 (m, 1H), 0.55-0.60 (m, 2H), 0.31-0.38 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.55 (t, J = 5.9 Hz, 1H), 7.72 (t, J = 7.8 Hz, 1H), 7.34-7.40 (m, 1H), 7.26 (d, J = 7.7 Hz, 1H), 7.10-7.14 (m, 1H), 7.05-7.07 (m, 1H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.89 (d, J = 9.5 Hz, 1H), 5.33-5.35 (m, 1H), 4.64-4.72 (m, 1H), 4.32-4.41 (m, 3H), 4.06-4.10 (m, 1H), 3.90 (d, J = 6.9 Hz, 2H), 3.37-3.52 (m, 2H), 2.58-2.65 (m, 1H), 2.03 (s, 3H), 1.84-1.93 (m, 1H), 1.33-1.36 (m, 3H), 1.24-1.27 ( m, 1H), 0.55-0.60 (m, 2H), 0.31-0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 504.30 [M+H]+ .MS (ESI, pos.ion) m/z: 504.30 [M+H] + .
實施例19:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((4-(羥基甲基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 19: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((4-( Hydroxymethyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 2-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)異煙酸甲酯 (79 mg, 0.15 mmol) 溶於四氫呋喃 (6 mL) 中,在冰浴中加入硼氫化鋰 (48 mg,2.20 mmol),室溫反應4 h後停止,加水 (50 mL),用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體36 mg,收率48%。The compound 2-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)isonicotinic acid methyl ester (79 mg, 0.15 mmol) was dissolved in tetrahydrofuran (6 mL), lithium borohydride (48 mg, 2.20 mmol) was added in an ice bath, and the reaction was stopped after 4 h at room temperature. Water (50 mL) was added, extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/ v) = 20/1) to obtain 36 mg of white solid, yield 48%.
MS (ESI, pos.ion) m/z: 490.20 [M+H]+ .MS (ESI, pos.ion) m/z: 490.20 [M+H] + .
實施例20:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((4-(2-羥丙基-2-基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 20: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((4-( 2-Hydroxypropyl-2-yl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (203 mg, 0.55 mmol),2-(2-(氨甲基)吡啶-4-基)異丙醇二鹽酸鹽 (186 mg, 1.12 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (223 mg,1.16 mmol) 和N -羥基-7-氮雜苯並三氮唑 (115 mg, 0.85 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.5 mL, 3.0 mmol),室溫攪拌21 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),合併有機相,用無水硫酸鈉乾燥,除去溶劑,剩餘物進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得白色固體221 mg,收率77%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (203 mg, 0.55 mmol), 2-(2-(aminomethyl)pyridin-4-yl)isopropanol dihydrochloride (186 mg, 1.12 mmol), 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (223 mg, 1.16 mmol) and N -hydroxy-7-azabenzotriazole (115 mg, 0.85 mmol) were dissolved in dichloromethane (6 mL) and cooled to 0 ℃, add N , N -diisopropylethylamine (0.5 mL, 3.0 mmol), stir at room temperature for 21 h, add water (15 mL), extract with dichloromethane (5 mL × 3), combine the organic phases, It was dried with anhydrous sodium sulfate, the solvent was removed, and the residue was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/2) to obtain 221 mg of white solid with a yield of 77%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.41 (d,J = 5.2 Hz, 1H), 7.64 (s, 1H), 7.43 (d,J = 3.9 Hz, 1H), 7.08-7.12 (m, 2H), 6.90-6.94 (m, 1H), 6.75 (t,J F-H = 75.7 Hz, 1H), 4.50-4.65(m, 1H), 4.50-4.60(m, 2H), 4.09-4.13 (m, 1H), 3.93 (d,J = 6.8 Hz, 2H), 3.65 (d,J = 10.6 Hz, 1H), 3.47-3.55 (m, 1H), 2.67-2.74 (m, 1H), 2.15 (s, 3H), 2.08-2.15 (m, 1H), 1.54 (d,J = 3.4 Hz, 6H), 1.27-1.34 (m, 1H), 0.62-0.67 (m, 2H), 0.36-0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.41 (d, J = 5.2 Hz, 1H), 7.64 (s, 1H), 7.43 (d, J = 3.9 Hz, 1H), 7.08-7.12 (m, 2H), 6.90-6.94 (m, 1H), 6.75 (t, J FH = 75.7 Hz, 1H), 4.50-4.65(m, 1H), 4.50-4.60(m, 2H), 4.09-4.13 ( m, 1H), 3.93 (d, J = 6.8 Hz, 2H), 3.65 (d, J = 10.6 Hz, 1H), 3.47-3.55 (m, 1H), 2.67-2.74 (m, 1H), 2.15 (s , 3H), 2.08-2.15 (m, 1H), 1.54 (d, J = 3.4 Hz, 6H), 1.27-1.34 (m, 1H), 0.62-0.67 (m, 2H), 0.36-0.41 (m, 2H) ).
MS (ESI, pos.ion) m/z: 518.20 [M+H]+ .MS (ESI, pos.ion) m/z: 518.20 [M+H] + .
實施例21:(2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((4-(2-羥基丙烷-2-基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 21: ( 2S )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((4-(2 -Hydroxypropan-2-yl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (100 mg, 0.27 mmol),2-(2-(氨基甲基)吡啶-4-基)異丙醇二鹽酸鹽 (77 mg, 0.33 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (408 mg, 2.13 mmol) 和N -羥基-7-氮雜苯並三氮唑 (87 mg, 0.64 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下冷卻,滴加N ,N -二異丙基乙胺 (0.45 mL, 2.6 mmol),室溫攪拌12 h,加水 (20 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體91 mg,收率64%。The compound ( 2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (100 mg, 0.27 mmol), 2-(2-(aminomethyl)pyridin-4-yl)isopropanol dihydrochloride (77 mg, 0.33 mmol), 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (408 mg, 2.13 mmol) and N -hydroxy-7-azabenzotriazole (87 mg, 0.64 mmol) were dissolved in dichloromethane (5 mL) at 0°C Under cooling, N , N -diisopropylethylamine (0.45 mL, 2.6 mmol) was added dropwise, stirred at room temperature for 12 h, added water (20 mL), extracted with dichloromethane (10 mL × 3), the organic phase was It was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 91 mg of white solid with a yield of 64%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.45 (d,J = 5.1 Hz, 1H), 7.67 (s, 1H), 7.19 (d,J = 4.4 Hz, 1H), 7.13 (d,J = 8.1 Hz, 1H), 6.91 (s, 1H), 6.86 (d,J = 8.3 Hz, 1H), 6.63 (t,J F-H = 75.6 Hz, 1H), 4.78 – 4.84 (m, 1H), 4.51 – 4.55 (m, 1H), 4.41 – 4.46 (m, 1H), 3.93 – 3.97 (m, 1H), 3.88 (d,J = 6.9 Hz, 2H), 3.60 – 3.65 (m, 1H), 3.32 – 3.43 (m, 1H), 2.57 – 2.64 (m, 1H), 2.37 – 2.45 (m, 1H), 2.14 (s, 3H), 1.55 (s, 3H), 1.57 (s, 3H), 1.25-1.37 (m, 1H), 0.65-0.69 (m, 2H), 0.36-0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.45 (d, J = 5.1 Hz, 1H), 7.67 (s, 1H), 7.19 (d, J = 4.4 Hz, 1H), 7.13 (d, J = 8.1 Hz, 1H), 6.91 (s, 1H), 6.86 (d, J = 8.3 Hz, 1H), 6.63 (t, J FH = 75.6 Hz, 1H), 4.78 – 4.84 (m, 1H), 4.51 – 4.55 (m, 1H), 4.41 – 4.46 (m, 1H), 3.93 – 3.97 (m, 1H), 3.88 (d, J = 6.9 Hz, 2H), 3.60 – 3.65 (m, 1H), 3.32 – 3.43 (m, 1H), 2.57 – 2.64 (m, 1H), 2.37 – 2.45 (m, 1H), 2.14 (s, 3H), 1.55 (s, 3H), 1.57 (s, 3H), 1.25-1.37 (m , 1H), 0.65-0.69 (m, 2H), 0.36-0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 518.15 [M+H]+ .MS (ESI, pos.ion) m/z: 518.15 [M+H] + .
實施例22:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(2-羥基丙基-2-基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 22: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( 2-Hydroxypropyl-2-yl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (67 mg, 0.18 mmol),2-(6-(氨基甲基)吡啶-2-基)丙烷-2-羥基 (93 mg, 0.56 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (103 mg, 0.54 mmol) 和N -羥基-7-氮雜苯並三氮唑 (65 mg, 0.48 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌18 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體32 mg,收率34%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (67 mg, 0.18 mmol), 2-(6-(aminomethyl)pyridin-2-yl)propane-2-hydroxy (93 mg, 0.56 mmol), 1-ethyl-(3-dimethylaminopropyl)carboline Diimine hydrochloride (103 mg, 0.54 mmol) and N -hydroxy-7-azabenzotriazole (65 mg, 0.48 mmol) were dissolved in dichloromethane (6 mL), cooled to 0 °C, Add N , N -diisopropylethylamine (0.3 mL, 2.0 mmol), stir at room temperature for 18 h, add water (15 mL), extract with dichloromethane (5 mL × 3), and dry the organic phase with anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 32 mg of white solid with a yield of 34%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.76 (t,J = 7.8 Hz, 1H), 7.49 (d,J = 7.8 Hz, 1H), 7.34 (d,J = 7.7 Hz, 1H), 7.09-7.12 (m, 2H), 6.92-6.94 (m, 1H), 6.74 (t,J F-H = 75.7 Hz, 1H), 4.50-4.60 (m, 3H),4.09-4.13 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.63-3.68 (m, 1H), 3.48-3.55 (m, 1H), 2.66-2.73 (m, 1H), 2.16 (s, 3H), 2.03-2.12 (m, 1H), 1.54 (s, 6H), 1.27-1.35 (m, 1H), 0.62-0.67 (m, 2H), 0.37-0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.76 (t, J = 7.8 Hz, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 7.7 Hz, 1H) ), 7.09-7.12 (m, 2H), 6.92-6.94 (m, 1H), 6.74 (t, J FH = 75.7 Hz, 1H), 4.50-4.60 (m, 3H), 4.09-4.13 (m, 1H) , 3.93 (d, J = 6.9 Hz, 2H), 3.63-3.68 (m, 1H), 3.48-3.55 (m, 1H), 2.66-2.73 (m, 1H), 2.16 (s, 3H), 2.03-2.12 (m, 1H), 1.54 (s, 6H), 1.27-1.35 (m, 1H), 0.62-0.67 (m, 2H), 0.37-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 518.20 [M+H]+ .MS (ESI, pos.ion) m/z: 518.20 [M+H] + .
實施例23:(2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((4-(2-羥基丙烷-2-基)吡啶-2-基)甲基)-1-(甲磺醯基)吡咯烷-2-甲醯胺 Example 23: ( 2R )-4-(3-(Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((4-(2-hydroxypropane-2- yl)pyridin-2-yl)methyl)-1-(methylsulfonyl)pyrrolidine-2-carbamide
步驟1:化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-(甲磺醯基)吡咯烷-2-羧酸甲酯 的合成Step 1: Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-(methylsulfonyl)pyrrolidine-2-carboxy Synthesis of Methyl Acid
將化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (418 mg, 1.1 mmol) 溶解在二氯甲烷 (5 mL) 中,0℃條件下加入N ,N -二異丙基乙胺 (0.36 mL, 2.2 mmol) 和甲磺醯氯 (0.13 mL, 1.7 mmol),室溫攪拌4 h後停止反應,加水 (15mL),二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:EtOAc ( v ) = 100%),得到白色固體154 mg,產率33%。Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride (418 mg, 1.1 mmol) was dissolved in dichloromethane (5 mL), N , N -diisopropylethylamine (0.36 mL, 2.2 mmol) and methanesulfonyl chloride (0.13 mL, 1.7 mmol) were added at 0°C, and the mixture was heated to room temperature. The reaction was stopped after stirring for 4 h, water (15 mL) was added, and dichloromethane was extracted (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: EtOAc (v) = 100 %) to obtain 154 mg of white solid with a yield of 33%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.11 (d,J = 7.9 Hz, 1H), 6.77-6.79 (m, 2H), 6.59 (t,J F-H = 71.0 Hz, 1H), 4.63 – 4.67 (m, 1H), 4.05 – 4.08 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.78 (s, 3H), 3.38 – 3.46 (m, 1H), 3.30 – 3.37 (m, 1H), 3.08 (s, 3H), 2.74 – 2.81 (m, 1H), 2.04 – 2.13 (m, 1H), 1.24 – 1.33 (m, 1H), 0.63 – 0.68 (m, 2H), 0.34 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.11 (d, J = 7.9 Hz, 1H), 6.77-6.79 (m, 2H), 6.59 (t, J FH = 71.0 Hz, 1H), 4.63 – 4.67 (m, 1H), 4.05 – 4.08 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.78 (s, 3H), 3.38 – 3.46 (m, 1H), 3.30 – 3.37 (m , 1H), 3.08 (s, 3H), 2.74 – 2.81 (m, 1H), 2.04 – 2.13 (m, 1H), 1.24 – 1.33 (m, 1H), 0.63 – 0.68 (m, 2H), 0.34 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 420.20 [M+H]+ .MS (ESI, pos.ion) m/z: 420.20 [M+H] + .
步驟2:化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-(甲磺醯基)吡咯烷-2-羧酸 的合成Step 2: Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-(methylsulfonyl)pyrrolidine-2-carboxy acid synthesis
將化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-(甲磺醯基)吡咯烷-2-羧酸甲酯 (151 mg, 0.36 mmol) 溶解在四氫呋喃 (5 mL) 中,加入一水合氫氧化鋰 (75 mg, 1.79 mmol) 和水 (5 mL),45℃反應1 h,加稀鹽酸調節pH=1,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,得白色固體143 mg,產率98%。The compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-(methylsulfonyl)pyrrolidine-2-carboxylate methyl The ester (151 mg, 0.36 mmol) was dissolved in tetrahydrofuran (5 mL), lithium hydroxide monohydrate (75 mg, 1.79 mmol) and water (5 mL) were added, and the reaction was carried out at 45 °C for 1 h, and dilute hydrochloric acid was added to adjust pH=1 , extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 143 mg of white solid with a yield of 98%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.10 (d,J = 8.2 Hz, 1H), 7.05 (d,J = 1.7 Hz, 1H), 6.90 (dd,J = 8.2, 1.8 Hz, 1H), 6.73 (t,J F-H = 74.9 Hz, 1H), 4.49 – 4.53 (m, 1H), 3.96 – 4.00 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.41 – 3.50 (m, 2H), 3.08 (s, 3H), 2.80 – 2.84 (m, 1H), 2.09 – 2.17 (m, 1H), 1.28-1.36 (m, 1H), 0.62-0.67 (m, 2H), 0.37-0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.10 (d, J = 8.2 Hz, 1H), 7.05 (d, J = 1.7 Hz, 1H), 6.90 (dd, J = 8.2, 1.8 Hz , 1H), 6.73 (t, J FH = 74.9 Hz, 1H), 4.49 – 4.53 (m, 1H), 3.96 – 4.00 (m, 1H), 3.93 (d, J = 6.9 Hz, 2H), 3.41 – 3.50 (m, 2H), 3.08 (s, 3H), 2.80 – 2.84 (m, 1H), 2.09 – 2.17 (m, 1H), 1.28-1.36 (m, 1H), 0.62-0.67 (m, 2H), 0.37 -0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 406.20 [M+H]+ .MS (ESI, pos.ion) m/z: 406.20 [M+H] + .
步驟3:化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((4-(2-羥基丙烷-2-基)吡啶-2-基)甲基)-1-(甲磺醯基)吡咯烷-2-甲醯胺 的合成Step 3: Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((4-(2-hydroxypropane-2- Synthesis of yl)pyridin-2-yl)methyl)-1-(methylsulfonyl)pyrrolidine-2-carboxamide
將化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-(甲磺醯基)吡咯烷-2-羧酸 (66 mg, 0.16 mmol),2-(2-(氨基甲基)吡啶-4-基)異丙醇二鹽酸鹽 (58 mg, 0.24 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (156 mg, 0.81 mmol) 和N -羥基-7-氮雜苯並三氮唑 (33 mg, 0.24 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.2 mL, 1.0 mmol),室溫攪拌5 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到淺黃色固體87 mg,收率96%。The compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-(methylsulfonyl)pyrrolidine-2-carboxylic acid ( 66 mg, 0.16 mmol), 2-(2-(aminomethyl)pyridin-4-yl)isopropanol dihydrochloride (58 mg, 0.24 mmol), 1-ethyl-(3-dimethylamino) propyl)carbodiimide hydrochloride (156 mg, 0.81 mmol) and N -hydroxy-7-azabenzotriazole (33 mg, 0.24 mmol) were dissolved in dichloromethane (5 mL), N , N -diisopropylethylamine (0.2 mL, 1.0 mmol) was added dropwise to this solution at 0°C, stirred at room temperature for 5 h, added with water (15 mL), and extracted with dichloromethane (5 mL × 3 ), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 87 mg of a pale yellow solid, which was collected rate of 96%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.44 (d,J = 5.2 Hz, 1H), 7.54 (br.s, 1H), 7.43 (s, 1H), 7.25 (d,J = 5.9 Hz, 1H), 7.08 (d,J = 8.0 Hz, 1H), 6.80 (s, 1H), 6.79 (d,J = 10.3 Hz, 1H), 6.59 (t,J F-H = 75.6 Hz, 1H), 4.47 – 4.65 (m, 3H), 3.99 – 4.03 (m, 1H), 3.85 (d,J = 6.8 Hz, 2H), 3.33 – 3.47 (m, 2H), 3.02 (s, 3H), 2.76 – 2.82 (m, 1H), 2.25 – 2.33 (m, 1H), 1.54 (s, 6H), 1.25-1.32 (m, 1H), 0.61-0.66 (m, 2H), 0.32-0.36 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.44 (d, J = 5.2 Hz, 1H), 7.54 (br.s, 1H), 7.43 (s, 1H), 7.25 (d, J = 5.9 Hz, 1H), 7.08 (d, J = 8.0 Hz, 1H), 6.80 (s, 1H), 6.79 (d, J = 10.3 Hz, 1H), 6.59 (t, J FH = 75.6 Hz, 1H), 4.47 – 4.65 (m, 3H), 3.99 – 4.03 (m, 1H), 3.85 (d, J = 6.8 Hz, 2H), 3.33 – 3.47 (m, 2H), 3.02 (s, 3H), 2.76 – 2.82 (m , 1H), 2.25 – 2.33 (m, 1H), 1.54 (s, 6H), 1.25-1.32 (m, 1H), 0.61-0.66 (m, 2H), 0.32-0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 554.30 [M+H]+ .MS (ESI, pos.ion) m/z: 554.30 [M+H] + .
實施例24:化合物 (2R )-1-乙醯基-N -(2-氨基-1-(2,4-二氟苯基)-2-氧代乙基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯胺 Example 24: Compound ( 2R )-1-Acetyl- N- (2-amino-1-(2,4-difluorophenyl)-2-oxoethyl)-4-(3-( Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxamide
步驟1:化合物2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)-2-(2,4-二氟苯基)乙酸乙酯 的合成Step 1: Compound 2-(( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-methyl Synthesis of Acylamino)-2-(2,4-difluorophenyl)acetate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸) (178 mg, 0.48 mmol),2-氨基-2-(2,4-二氟苯基)乙酸乙酯鹽酸鹽 (149 mg, 0.59 mmol) 和N -羥基-7-氮雜苯並三氮唑(101 mg, 0.74 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (467 mg, 2.44 mmol) 和N ,N -二異丙基乙胺 (0.5 mL, 3.0 mmol),室溫反應16 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/3),得到白色固體147 mg,收率54%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid) (178 mg , 0.48 mmol), 2-amino-2-(2,4-difluorophenyl)ethyl acetate hydrochloride (149 mg, 0.59 mmol) and N -hydroxy-7-azabenzotriazole (101 mg, 0.74 mmol) was dissolved in dichloromethane (5 mL), cooled to 0 °C, added 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (467 mg, 2.44 mmol) and N , N -diisopropylethylamine (0.5 mL, 3.0 mmol), reacted at room temperature for 16 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was washed with anhydrous After drying over sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/3) to obtain 147 mg of white solid, with a yield of 54%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.33-7.44 (m, 1H), 7.09-7.12 (m, 1H), 6.82-6.91 (m, 4H), 6.62 (t,J F-H = 75.6 Hz, 1H), 5.71 – 5.74 (m, 1H), 4.62 – 4.68 (m, 1H), 4.17 – 4.27 (m, 2H), 3.90 – 3.98 (m, 1H), 3.88 (d,J = 6.8 Hz, 2H), 3.44 – 3.52 (m, 1H), 3.28 – 3.38 (m, 1H), 2.46 – 2.57 (m, 2H), 2.10 – 2.19 (m, 3H), 1.27-1.35 (m, 1H), 1.20 – 1.23 (m, 3H), 0.64-0.69 (m, 2H), 0.37-0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.33-7.44 (m, 1H), 7.09-7.12 (m, 1H), 6.82-6.91 (m, 4H), 6.62 (t, J FH = 75.6 Hz, 1H), 5.71 – 5.74 (m, 1H), 4.62 – 4.68 (m, 1H), 4.17 – 4.27 (m, 2H), 3.90 – 3.98 (m, 1H), 3.88 (d, J = 6.8 Hz, 2H), 3.44 – 3.52 (m, 1H), 3.28 – 3.38 (m, 1H), 2.46 – 2.57 (m, 2H), 2.10 – 2.19 (m, 3H), 1.27-1.35 (m, 1H), 1.20 – 1.23 (m, 3H), 0.64-0.69 (m, 2H), 0.37-0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 567.30 [M+H]+ .MS (ESI, pos.ion) m/z: 567.30 [M+H] + .
步驟2:化合物2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)-2-(2,4-二氟苯基)乙酸 的合成Step 2: Compound 2-(( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-methyl Synthesis of acylamino)-2-(2,4-difluorophenyl)acetic acid
將化合物2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)-2-(2,4-二氟苯基)乙酸乙酯 (140 mg, 0.25 mmol) 溶於四氫呋喃 (5 mL) 和 水(5 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (54 mg,1.29 mmol),50℃反應3 h後停止,加濃鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體131 mg,收率98%。The compound 2-(( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino )-2-(2,4-difluorophenyl)ethyl acetate (140 mg, 0.25 mmol) was dissolved in a mixed solvent of tetrahydrofuran (5 mL) and water (5 mL), and then lithium hydroxide monohydrate ( 54 mg, 1.29 mmol), the reaction was stopped after 3 h at 50 °C, concentrated hydrochloric acid was added to adjust the pH of the solution to 1, extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain a white solid 131 mg, the yield is 98%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.43 – 7.53 (m, 1H), 6.91 – 7.12 (m, 5H), 6.53 – 6.94 (m, 1H), 5.74 – 5.78 (m, 1H), 4.54 – 4.68 (m, 1H), 4.05 – 4.15 (m, 1H), 3.89 – 3.95 (m, 2H), 3.56 – 3.63 (m, 1H), 3.44 – 3.52 (m, 1H), 2.57 – 2.76 (m, 1H), 2.03 – 2.13 (m, 4H), 1.27-1.36 (m, 1H), 0.61-0.67 (m, 2H), 0.37-0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.43 – 7.53 (m, 1H), 6.91 – 7.12 (m, 5H), 6.53 – 6.94 (m, 1H), 5.74 – 5.78 (m, 1H) ), 4.54 – 4.68 (m, 1H), 4.05 – 4.15 (m, 1H), 3.89 – 3.95 (m, 2H), 3.56 – 3.63 (m, 1H), 3.44 – 3.52 (m, 1H), 2.57 – 2.76 (m, 1H), 2.03 – 2.13 (m, 4H), 1.27-1.36 (m, 1H), 0.61-0.67 (m, 2H), 0.37-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 539.20 [M+H]+ .MS (ESI, pos.ion) m/z: 539.20 [M+H] + .
步驟3:化合物 (2R )-1-乙醯基-N -(2-氨基-1-(2,4-二氟苯基)-2-氧代乙基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯胺 的合成Step 3: Compound ( 2R )-1-Acetyl- N- (2-amino-1-(2,4-difluorophenyl)-2-oxoethyl)-4-(3-(cyclo) Synthesis of propylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxamide
將化合物2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)-2-(2,4-二氟苯基)乙酸 (130 mg, 0.24 mmol),氯化銨 (132 mg, 2.4 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (242 mg, 1.26 mmol) 和N -羥基-7-氮雜苯並三氮唑 (51 mg, 0.37 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫反應16 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/3),得到白色固體37 mg,收率29%。The compound 2-(( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino )-2-(2,4-difluorophenyl)acetic acid (130 mg, 0.24 mmol), ammonium chloride (132 mg, 2.4 mmol), 1-ethyl-(3-dimethylaminopropyl)carbon Diimide hydrochloride (242 mg, 1.26 mmol) and N -hydroxy-7-azabenzotriazole (51 mg, 0.37 mmol) were dissolved in dichloromethane (5 mL) at 0°C N , N -diisopropylethylamine (0.4 mL, 2.0 mmol) was added dropwise to this solution, the reaction was carried out at room temperature for 16 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic The phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/3) to obtain 37 mg of white solid, yield 29% .
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.41-7.50 (m, 1H), 7.05-7.14 (m, 2H), 6.96-7.05 (m, 2H), 6.88-6.95 (m, 1H), 6.73 (t,J F-H = 75.6 Hz, 1H), 5.72 (s, 1H), 4.44 – 4.52 (m, 1H), 4.03 – 4.12 (m, 1H), 3.92 (d,J = 5.9 Hz, 1H), 3.61 – 3.67 (m, 1H), 3.43 – 3.55 (m, 1H), 2.50 – 2.57 (m, 3H), 3.13 (m, 3H), 2.03 – 2.09 (m, 1H), 1.31-1.40 (m, 1H), 0.60-0.66 (m, 2H), 0.35-0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.41-7.50 (m, 1H), 7.05-7.14 (m, 2H), 6.96-7.05 (m, 2H), 6.88-6.95 (m, 1H) ), 6.73 (t, J FH = 75.6 Hz, 1H), 5.72 (s, 1H), 4.44 – 4.52 (m, 1H), 4.03 – 4.12 (m, 1H), 3.92 (d, J = 5.9 Hz, 1H) ), 3.61 – 3.67 (m, 1H), 3.43 – 3.55 (m, 1H), 2.50 – 2.57 (m, 3H), 3.13 (m, 3H), 2.03 – 2.09 (m, 1H), 1.31-1.40 (m , 1H), 0.60-0.66 (m, 2H), 0.35-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 538.15 [M+H]+ .MS (ESI, pos.ion) m/z: 538.15 [M+H] + .
實施例25:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 Example 25: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N , N -lutidyl pyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (172 mg, 0.34 mmol),二甲胺鹽酸鹽 (142 mg, 1.74 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (321 mg, 1.67 mmol) 和N -羥基-7-氮雜苯並三氮唑 (72 mg, 0.53 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體94 mg,收率52%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (172 mg, 0.34 mmol), dimethylamine hydrochloride (142 mg, 1.74 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide The acid salt (321 mg, 1.67 mmol) and N -hydroxy-7-azabenzotriazole (72 mg, 0.53 mmol) were dissolved in dichloromethane (5 mL), and the solution was added dropwise at 0°C N , N -diisopropylethylamine (0.4 mL, 2.0 mmol) was added, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sodium sulfate After drying, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 94 mg of white solid, with a yield of 52%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.92 (t,J = 7.9 Hz, 1H), 7.60 (d,J = 7.9 Hz, 1H), 7.45 (d,J = 7.7 Hz, 1H), 7.12 (d,J = 8.2 Hz, 1H), 7.09 (s, 1H), 6.92 – 6.94 (m, 1H), 6.75 (t,J F-H = 75.7 Hz, 1H), 4.48 – 4.65 (m, 3H), 4.09 – 4.13 (m, 1H), 3.93 (d,J = 6.8 Hz, 2H), 3.66 (t,J = 10.6 Hz, 1H), 3.49 – 3.56 (m, 1H), 3.10 (s, 3H), 3.01 (s, 3H), 2.66 – 2.73 (m, 1H), 2.19 (s, 3H), 2.06 – 2.16 (m, 1H), 1.27 – 1.33 (m, 1H), 0.62 – 0.67 (m, 2H), 0.36 – 0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.92 (t, J = 7.9 Hz, 1H), 7.60 (d, J = 7.9 Hz, 1H), 7.45 (d, J = 7.7 Hz, 1H) ), 7.12 (d, J = 8.2 Hz, 1H), 7.09 (s, 1H), 6.92 – 6.94 (m, 1H), 6.75 (t, J FH = 75.7 Hz, 1H), 4.48 – 4.65 (m, 3H) ), 4.09 – 4.13 (m, 1H), 3.93 (d, J = 6.8 Hz, 2H), 3.66 (t, J = 10.6 Hz, 1H), 3.49 – 3.56 (m, 1H), 3.10 (s, 3H) , 3.01 (s, 3H), 2.66 – 2.73 (m, 1H), 2.19 (s, 3H), 2.06 – 2.16 (m, 1H), 1.27 – 1.33 (m, 1H), 0.62 – 0.67 (m, 2H) , 0.36 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 531.10 [M+H]+ .MS (ESI, pos.ion) m/z: 531.10 [M+H] + .
實施例26:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 Example 26: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -formamido)methyl)picolinic acid
步驟1:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸乙酯 的合成Step 1: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of ethyl formamido)methyl)picolinate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (201 mg, 0.54 mmol),6-(氨甲基)吡啶甲酸乙酯鹽酸鹽 (142 mg, 0.66 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (516 mg, 2.69 mmol) 和N -羥基-7-氮雜苯並三氮唑 (111 mg, 0.82 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.6 mL, 4.0 mmol),室溫反應16 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色黏稠固體216 mg,收率74%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (201 mg, 0.54 mmol), 6-(aminomethyl)picolinate ethyl ester hydrochloride (142 mg, 0.66 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 516 mg, 2.69 mmol) and N -hydroxy-7-azabenzotriazole (111 mg, 0.82 mmol) were dissolved in dichloromethane (5 mL), and N was added dropwise to this solution at 0 °C, N -diisopropylethylamine (0.6 mL, 4.0 mmol) was reacted at room temperature for 16 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, removed The solvent and the concentrated solution were separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 216 mg of white viscous solid with a yield of 74%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.02 (d,J = 7.5 Hz, 1H), 7.83 (t,J = 7.7 Hz, 1H), 7.56 (d,J = 7.7 Hz, 1H), 7.11 (d,J = 8.1 Hz, 1H), 6.89 (s, 1H), 6.86 (d,J = 8.2 Hz, 1H), 6.62 (t,J F-H = 75.7 Hz, 1H), 4.59 – 4.77 (m, 3H), 4.47 (q,J = 7.1 Hz, 2H), 3.95 – 4.00 (m, 1H), 3.88 (d,J = 6.9 Hz, 2H), 3.61 (t,J = 10.6 Hz, 1H), 3.32 – 3.39 (m, 1H), 2.58 – 2.65 (m, 1H), 2.40 – 2.48 (m, 1H), 2.17 (s, 3H), 1.44 (t,J = 7.2 Hz, 3H), 1.27 – 1.33 (m, 1H), 0.64 – 0.69 (m, 2H), 0.35 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.02 (d, J = 7.5 Hz, 1H), 7.83 (t, J = 7.7 Hz, 1H), 7.56 (d, J = 7.7 Hz, 1H) , 7.11 (d, J = 8.1 Hz, 1H), 6.89 (s, 1H), 6.86 (d, J = 8.2 Hz, 1H), 6.62 (t, J FH = 75.7 Hz, 1H), 4.59 – 4.77 (m , 3H), 4.47 (q, J = 7.1 Hz, 2H), 3.95 – 4.00 (m, 1H), 3.88 (d, J = 6.9 Hz, 2H), 3.61 (t, J = 10.6 Hz, 1H), 3.32 – 3.39 (m, 1H), 2.58 – 2.65 (m, 1H), 2.40 – 2.48 (m, 1H), 2.17 (s, 3H), 1.44 (t, J = 7.2 Hz, 3H), 1.27 – 1.33 (m , 1H), 0.64 – 0.69 (m, 2H), 0.35 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 532.30 [M+H]+ .MS (ESI, pos.ion) m/z: 532.30 [M+H] + .
步驟2:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 的合成Step 2: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of carboxylamino)methyl)picolinic acid
將化合物 6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸乙酯 (232 mg, 0.44 mmol) 溶於四氫呋喃 (6 mL) 和水 (4 mL) 的混合溶劑中,加入一水合氫氧化鋰(72 mg, 1.72 mmol),50℃反應3 h後停止,加鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體213 mg,收率97%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)ethyl picolinate (232 mg, 0.44 mmol) was dissolved in a mixed solvent of tetrahydrofuran (6 mL) and water (4 mL), and lithium hydroxide monohydrate (72 mg, 1.72 mmol) was added, at 50°C The reaction was stopped after 3 h, hydrochloric acid was added to adjust the pH of the solution to 1, extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 213 mg of a white solid with a yield of 97%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.06 (d,J = 7.5 Hz, 1H), 7.99 (t,J = 7.7 Hz, 1H), 7.75 (d,J = 7.7 Hz, 1H), 7.11 (d,J = 8.2 Hz, 1H), 7.09 (s, 1H), 6.92 – 6.94 (m, 1H), 6.74 (t,J F-H = 75.7 Hz, 1H), 4.51 – 4.73 (m, 3H), 4.09 – 4.14 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.66 (t,J = 10.6 Hz, 1H), 3.49 – 3.55 (m, 1H), 2.67 – 2.73 (m, 1H), 2.16 (s, 3H), 2.07 – 2.12 (m, 1H), 1.27 – 1.35 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.06 (d, J = 7.5 Hz, 1H), 7.99 (t, J = 7.7 Hz, 1H), 7.75 (d, J = 7.7 Hz, 1H) ), 7.11 (d, J = 8.2 Hz, 1H), 7.09 (s, 1H), 6.92 – 6.94 (m, 1H), 6.74 (t, J FH = 75.7 Hz, 1H), 4.51 – 4.73 (m, 3H) ), 4.09 – 4.14 (m, 1H), 3.93 (d, J = 6.9 Hz, 2H), 3.66 (t, J = 10.6 Hz, 1H), 3.49 – 3.55 (m, 1H), 2.67 – 2.73 (m, 1H), 2.16 (s, 3H), 2.07 – 2.12 (m, 1H), 1.27 – 1.35 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 504.30 [M+H]+ .MS (ESI, pos.ion) m/z: 504.30 [M+H] + .
實施例27:化合物 6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶醯胺 Example 27: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl)pyridylamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (101 mg, 0.20 mmol),氯化銨 (112 mg, 2.09 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (96 mg, 0.50 mmol) 和N -羥基-7-氮雜苯並三氮唑 (67 mg, 0.49 mmol) 溶於二氯甲烷 (6 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.2 mL, 1.0 mmol),室溫攪拌6 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體73 mg,收率72%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (101 mg, 0.20 mmol), ammonium chloride (112 mg, 2.09 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 96 mg, 0.50 mmol) and N -hydroxy-7-azabenzotriazole (67 mg, 0.49 mmol) were dissolved in dichloromethane (6 mL), and N was added dropwise to this solution at 0°C, N -diisopropylethylamine (0.2 mL, 1.0 mmol), stirred at room temperature for 6 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed , the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 73 mg of white solid with a yield of 72%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.01 (d,J = 7.8 Hz, 1H), 7.95 (t,J = 7.6 Hz, 1H), 7.65 (d,J = 7.6 Hz, 1H), 7.08-7.12 (m, 2H), 6.91-6.95 (m, 1H), 6.74 (t,J F-H = 75.7 Hz, 1H), 4.54-4.71 (m, 3H), 4.09-4.13 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.63-3.68 (m, 1H), 3.47-3.56 (m, 1H), 2.66-2.73 (m, 1H), 2.16 (s, 3H), 2.03-2.13 (m, 1H), 1.27-1.35 (m, 1H), 0.62-0.67 (m, 2H), 0.34-0.40 (m, 2H); 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.01 (d, J = 7.8 Hz, 1H), 7.95 (t, J = 7.6 Hz, 1H), 7.65 (d, J = 7.6 Hz, 1H) ), 7.08-7.12 (m, 2H), 6.91-6.95 (m, 1H), 6.74 (t, J FH = 75.7 Hz, 1H), 4.54-4.71 (m, 3H), 4.09-4.13 (m, 1H) , 3.93 (d, J = 6.9 Hz, 2H), 3.63-3.68 (m, 1H), 3.47-3.56 (m, 1H), 2.66-2.73 (m, 1H), 2.16 (s, 3H), 2.03-2.13 (m, 1H), 1.27-1.35 (m, 1H), 0.62-0.67 (m, 2H), 0.34-0.40 (m, 2H);
MS (ESI, pos.ion) m/z: 503.30 [M+H]+ .MS (ESI, pos.ion) m/z: 503.30 [M+H] + .
實施例28:化合物 6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基吡啶醯胺 Example 28: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -picolinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (97 mg, 0.19 mmol),甲胺鹽酸鹽 (81 mg, 1.20 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (86 mg, 0.45 mmol) 和N -羥基-7-氮雜苯並三氮唑 (56 mg, 0.41 mmol) 溶於二氯甲烷 (6 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.2 mL, 1.0 mmol),室溫攪拌20 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體58 mg,收率58%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (97 mg, 0.19 mmol), methylamine hydrochloride (81 mg, 1.20 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride Salt (86 mg, 0.45 mmol) and N -hydroxy-7-azabenzotriazole (56 mg, 0.41 mmol) were dissolved in dichloromethane (6 mL) and added dropwise to this solution at 0°C N , N -diisopropylethylamine (0.2 mL, 1.0 mmol), stirred at room temperature for 20 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, The solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 58 mg of a white solid with a yield of 58%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.91-7.98 (m, 2H), 7.60 (d,J = 7.3 Hz, 1H), 7.08-7.12 (m, 2H), 6.92-6.94 (m, 1H), 6.74 (t,J F-H = 75.7 Hz, 1H), 4.51-4.61 (m, 3H), 4.07-4.17 (m, 1H), 3.92 (d,J = 6.6 Hz, 2H), 3.61-3.72 (m, 1H), 3.46-3.57 (m, 1H), 2.97 (s, 3H), 2.65-2.76 (m, 1H), 2.16 (s, 3H), 2.06-2.14 (m, 1H), 1.27-1.34 (m, 1H), 0.62-0.66 (m, 2H), 0.36-0.40 (m, 2H); 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.91-7.98 (m, 2H), 7.60 (d, J = 7.3 Hz, 1H), 7.08-7.12 (m, 2H), 6.92-6.94 ( m, 1H), 6.74 (t, J FH = 75.7 Hz, 1H), 4.51-4.61 (m, 3H), 4.07-4.17 (m, 1H), 3.92 (d, J = 6.6 Hz, 2H), 3.61- 3.72 (m, 1H), 3.46-3.57 (m, 1H), 2.97 (s, 3H), 2.65-2.76 (m, 1H), 2.16 (s, 3H), 2.06-2.14 (m, 1H), 1.27- 1.34 (m, 1H), 0.62-0.66 (m, 2H), 0.36-0.40 (m, 2H);
MS (ESI, pos.ion) m/z: 517.30 [M+H]+ .MS (ESI, pos.ion) m/z: 517.30 [M+H] + .
實施例29:化合物2-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基異煙醯胺 Example 29: Compound 2-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -formamido)methyl) -N , N -dimethylisonicotinamide
步驟1:化合物2-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)異煙酸甲酯的合成Step 1: Compound 2-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of methyl formamido)methyl)isonicotinate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (103 mg, 0.28 mmol),2-(氨基甲基)異煙酸甲酯 (102 mg, 0.61 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽(113 mg, 0.59 mmol) 和N -羥基-7-氮雜苯並三氮唑 (65 mg, 0.48 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌17 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到淡黃色液體103 mg,收率71%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (103 mg, 0.28 mmol), methyl 2-(aminomethyl)isonicotinate (102 mg, 0.61 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (113 mg , 0.59 mmol) and N -hydroxy-7-azabenzotriazole (65 mg, 0.48 mmol) were dissolved in dichloromethane (6 mL), cooled to 0 °C, and N , N -diisopropyl was added Ethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 17 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was subjected to silica gel column Chromatographic separation (eluent: dichloromethane/methanol (v/v) = 15/1) gave 103 mg of pale yellow liquid with a yield of 71%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.67-8.70 (m, 1H), 7.96 (s, 1H), 7.80-7.81 (m, 1H), 7.09-7.12 (m, 2H), 6.93-6.95 (m, 1H), 6.74 (t,J F-H = 75.7 Hz, 1H), 4.52-4.66 (m, 3H), 4.09-4.14 (m, 1H), 3.93 (s, 3H), 3.93 (d,J = 4.6 Hz, 2H), 3.63-3.68 (m, 1H), 3.47-3.57 (m, 1H), 2.69-2.76 (m, 1H), 2.15 (s, 3H), 2.07-2.15 (m, 1H), 1.27-1.33 (m, 1H), 0.62-0.66 (m, 2H), 0.32-0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.67-8.70 (m, 1H), 7.96 (s, 1H), 7.80-7.81 (m, 1H), 7.09-7.12 (m, 2H), 6.93-6.95 (m, 1H), 6.74 (t, J FH = 75.7 Hz, 1H), 4.52-4.66 (m, 3H), 4.09-4.14 (m, 1H), 3.93 (s, 3H), 3.93 (d , J = 4.6 Hz, 2H), 3.63-3.68 (m, 1H), 3.47-3.57 (m, 1H), 2.69-2.76 (m, 1H), 2.15 (s, 3H), 2.07-2.15 (m, 1H) ), 1.27-1.33 (m, 1H), 0.62-0.66 (m, 2H), 0.32-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 518.30 [M+H]+ .MS (ESI, pos.ion) m/z: 518.30 [M+H] + .
步驟2:化合物2-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)異煙酸的合成Step 2: Compound 2-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of formamido)methyl)isonicotinic acid
將化合物 2-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)異煙酸甲酯 (97 mg, 0.19 mmol) 溶於四氫呋喃 (6 mL) 和水 (4 mL) 的混合溶劑中,加入一水合氫氧化鋰 (43 mg, 1.03 mmol),50℃反應2 h後停止,加鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體92 mg,收率97%。The compound 2-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)isonicotinate (97 mg, 0.19 mmol) was dissolved in a mixed solvent of tetrahydrofuran (6 mL) and water (4 mL), lithium hydroxide monohydrate (43 mg, 1.03 mmol) was added, 50 The reaction was stopped after 2 h at ℃, hydrochloric acid was added to adjust the pH of the solution to 1, extracted with ethyl acetate (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 92 mg of a white solid with a yield of 97%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.66-8.69 (m, 1H), 7.96 (s, 1H), 7.82 (d,J = 4.8 Hz, 1H), 7.05-7.12 (m, 2H), 6.91-6.95 (m, 1H), 6.74 (t,J F-H = 75.7 Hz, 1H), 4.52-4.69 (m, 3H), 4.09-4.13 (m, 1H), 3.92 (d,J = 6.8 Hz, 2H), 3.61-3.67 (m, 1H), 3.47-3.56 (m, 1H), 2.69-2.75 (m, 1H), 2.13-2.23 (m, 1H), 2.15 (s, 3H), 1.24-1.35 (m, 1H), 0.61-0.66 (m, 2H), 0.35-0.39 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.66-8.69 (m, 1H), 7.96 (s, 1H), 7.82 (d, J = 4.8 Hz, 1H), 7.05-7.12 (m, 2H), 6.91-6.95 (m, 1H), 6.74 (t, J FH = 75.7 Hz, 1H), 4.52-4.69 (m, 3H), 4.09-4.13 (m, 1H), 3.92 (d, J = 6.8 Hz, 2H), 3.61-3.67 (m, 1H), 3.47-3.56 (m, 1H), 2.69-2.75 (m, 1H), 2.13-2.23 (m, 1H), 2.15 (s, 3H), 1.24- 1.35 (m, 1H), 0.61-0.66 (m, 2H), 0.35-0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 504.10 [M+H]+ .MS (ESI, pos.ion) m/z: 504.10 [M+H] + .
步驟3:化合物2-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基異煙醯胺的合成Step 3: Compound 2-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of Carboxylamido)methyl) -N , N -Dimethylisonicotinamide
將化合物2-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)異煙酸 (92 mg, 0.18 mmol),二甲胺鹽酸鹽 (71 mg, 0.87 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (76 mg, 0.40 mmol) 和N -羥基-7-氮雜苯並三氮唑 (46 mg, 0.34 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.2 mL, 1.0 mmol),室溫攪拌18 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體71 mg,收率73%。The compound 2-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)isonicotinic acid (92 mg, 0.18 mmol), dimethylamine hydrochloride (71 mg, 0.87 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide The hydrochloride (76 mg, 0.40 mmol) and N -hydroxy-7-azabenzotriazole (46 mg, 0.34 mmol) were dissolved in dichloromethane (6 mL), cooled to 0 °C, added with N , N -diisopropylethylamine (0.2 mL, 1.0 mmol), stirred at room temperature for 18 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed , and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 71 mg of white solid with a yield of 73%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.59 (d,J = 5.0 Hz, 1H), 7.55 (s, 1H), 7.32 (d,J = 5.0 Hz, 1H), 7.05-7.12 (m, 2H), 6.92-6.94 (m, 1H), 6.75 (t,J F-H = 75.7 Hz, 1H), 4.49-4.61 (m, 3H), 4.09-4.13 (m, 1H), 3.94 (d,J = 6.8 Hz, 2H), 3.63-3.68 (m, 1H), 3.48-3.54 (m, 1H), 3.12 (s, 3H), 2.96 (s, 3H), 2.66-2.73 (m, 1H), 2.15 (s, 3H), 2.05-2.13 (m, 1H), 1.26-1.33 (m, 1H), 0.63-0.66 (m, 2H), 0.37-0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.59 (d, J = 5.0 Hz, 1H), 7.55 (s, 1H), 7.32 (d, J = 5.0 Hz, 1H), 7.05-7.12 (m, 2H), 6.92-6.94 (m, 1H), 6.75 (t, J FH = 75.7 Hz, 1H), 4.49-4.61 (m, 3H), 4.09-4.13 (m, 1H), 3.94 (d, J = 6.8 Hz, 2H), 3.63-3.68 (m, 1H), 3.48-3.54 (m, 1H), 3.12 (s, 3H), 2.96 (s, 3H), 2.66-2.73 (m, 1H), 2.15 (s, 3H), 2.05-2.13 (m, 1H), 1.26-1.33 (m, 1H), 0.63-0.66 (m, 2H), 0.37-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 531.25 [M+H]+ .MS (ESI, pos.ion) m/z: 531.25 [M+H] + .
實施例30:化合物6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 Example 30: Compound 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methane yl) -N , N -lutidine pyridylamine dihydrochloride
步驟1:化合物 (2R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 的合成Step 1: Compound ( 2R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxy acid synthesis
將化合物 (2R )-1-叔丁基-2-甲基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯 (362 mg, 0.82 mmol) 溶於四氫呋喃 (3 mL) 和水 (5 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (176 mg, 4.2 mmol),50℃反應2.5 h後停止,加鹽酸調節溶液pH=4,再用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體338 mg,收率96%。The compound ( 2R )-1-tert-butyl-2-methyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2 -Dicarboxylate (362 mg, 0.82 mmol) was dissolved in a mixed solvent of tetrahydrofuran (3 mL) and water (5 mL), then lithium hydroxide monohydrate (176 mg, 4.2 mmol) was added, and the reaction was carried out at 50 °C for 2.5 h After stopping, adding hydrochloric acid to adjust the pH of the solution to 4, and then extracting with ethyl acetate (10 mL × 3). The organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 338 mg of white solid with a yield of 96%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.10 (d,J = 8.2 Hz, 1H), 7.03 (s, 1H), 6.88-6.90 (m, 1H), 6.73 (t,J F-H = 75.7 Hz, 1H), 4.31 – 4.37 (m, 1H), 3.92 – 3.99 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.35 – 3.45 (m, 2H), 2.69 – 2.74 (m, 1H), 1.87 – 1.96 (m, 1H), 1.44 – 1.46 (m, 9H), 1.29-1.36 (m, 1H), 0.62-0.66 (m, 2H), 0.37-0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.10 (d, J = 8.2 Hz, 1H), 7.03 (s, 1H), 6.88-6.90 (m, 1H), 6.73 (t, J FH = 75.7 Hz, 1H), 4.31 – 4.37 (m, 1H), 3.92 – 3.99 (m, 1H), 3.93 (d, J = 6.9 Hz, 2H), 3.35 – 3.45 (m, 2H), 2.69 – 2.74 ( m, 1H), 1.87 – 1.96 (m, 1H), 1.44 – 1.46 (m, 9H), 1.29-1.36 (m, 1H), 0.62-0.66 (m, 2H), 0.37-0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 450.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 450.20 [M+Na] + .
步驟2:化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2-(((6-(二甲基氨基甲醯基)吡啶-2-基)甲基)氨基甲醯基)吡咯烷-1-羧酸叔丁酯 的合成Step 2: Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-(((6-(dimethylaminocarboxy) Synthesis of tert-butyl)pyridin-2-yl)methyl)carbamoyl)pyrrolidine-1-carboxylate
將化合物 (2R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (334 mg, 0.78 mmol),6-(氨基甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 (238 mg, 0.94 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (746 mg, 3.89 mmol) 和N -羥基-7-氮雜苯並三氮唑 (162 mg, 1.19 mmol) 溶於二氯甲烷 (10 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.8 mL, 5.0 mmol),室溫反應12 h,加水洗 (15 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到淺黃色黏稠固體356 mg,收率77%。The compound ( 2R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid ( 334 mg, 0.78 mmol), 6-(aminomethyl) -N , N -lutidine dihydrochloride (238 mg, 0.94 mmol), 1-ethyl-(3-dimethylaminopropyl) yl)carbodiimide hydrochloride (746 mg, 3.89 mmol) and N -hydroxy-7-azabenzotriazole (162 mg, 1.19 mmol) were dissolved in dichloromethane (10 mL), 0 N , N -diisopropylethylamine (0.8 mL, 5.0 mmol) was added dropwise to this solution at ℃, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and then extracted with dichloromethane (5 mL × 3 ), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 356 mg of a light yellow viscous solid, Yield 77%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.75 – 7.81 (m, 1H), 7.49 – 7.57 (m, 1H), 7.33 – 7.40 (m, 1H), 7.11 (d,J = 8.1 Hz, 1H), 6.80 – 6.86 (m, 2H), 6.61 (t,J F-H = 75.7 Hz, 1H), 4.60 – 4.70 (m, 2H), 4.34 – 4.49 (m, 1H), 4.02 – 4.24 (m, 1H), 3.87 (d,J = 6.8 Hz, 2H), 3.37 – 3.36 (m, 2H), 3.15 (s, 3H), 3.06 (s, 3H), 2.61 – 2.75 (m, 1H), 2.15 – 2.39 (m, 1H), 1.38 – 1.49 (m, 9H), 1.27 – 1.36 (m, 1H), 0.64 – 0.68 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.75 – 7.81 (m, 1H), 7.49 – 7.57 (m, 1H), 7.33 – 7.40 (m, 1H), 7.11 (d, J = 8.1 Hz , 1H), 6.80 – 6.86 (m, 2H), 6.61 (t, J FH = 75.7 Hz, 1H), 4.60 – 4.70 (m, 2H), 4.34 – 4.49 (m, 1H), 4.02 – 4.24 (m, 1H), 3.87 (d, J = 6.8 Hz, 2H), 3.37 – 3.36 (m, 2H), 3.15 (s, 3H), 3.06 (s, 3H), 2.61 – 2.75 (m, 1H), 2.15 – 2.39 (m, 1H), 1.38 – 1.49 (m, 9H), 1.27 – 1.36 (m, 1H), 0.64 – 0.68 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 589.20 [M+H]+ .MS (ESI, pos.ion) m/z: 589.20 [M+H] + .
步驟3:化合物6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 的合成Step 3: Compound 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl Synthesis of ) -N , N -lutidine pyridylamine dihydrochloride
將化合物 (2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2-(((6-(二甲基氨基甲醯基)吡啶-2-基)甲基)氨基甲醯基)吡咯烷-1-羧酸叔丁酯 (350 mg, 0.59 mmol) 溶解於甲醇 (5 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (5 mL),室溫攪拌2 h,除去溶劑,得到白色固體332 mg,收率97%。The compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-(((6-(dimethylcarbamoyl) Pyridin-2-yl)methyl)carbamoyl)pyrrolidine-1-carboxylate tert-butyl ester (350 mg, 0.59 mmol) was dissolved in methanol (5 mL) solution, 4 mol/L HCl in ethyl acetate was added The ester solution (5 mL) was stirred at room temperature for 2 h, and the solvent was removed to obtain 332 mg of a white solid with a yield of 97%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.08 (t,J = 7.8 Hz, 1H), 7.61 (d,J = 7.6 Hz, 2H), 7.14 (d,J = 8.2 Hz, 1H), 7.08 (s, 1H), 6.93 (d,J = 9.9 Hz, 1H), 6.76 (t,J F-H = 75.5 Hz, 1H), 4.64-4.73 (m, 2H), 4.55-4.60 (m, 1H), 3.94 (d,J = 6.9 Hz, 2H), 3.78-3.83 (m, 1H), 3.66-3.76 (m, 1H), 3.34-3.42 (m, 1H), 3.10 (s, 3H), 3.02 (s, 3H), 2.89-2.97 (m, 1H), 2.12-2.21 (m, 1H), 1.28-1.37 (m, 1H), 0.63-0.67 (m, 2H), 0.37-0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.08 (t, J = 7.8 Hz, 1H), 7.61 (d, J = 7.6 Hz, 2H), 7.14 (d, J = 8.2 Hz, 1H) ), 7.08 (s, 1H), 6.93 (d, J = 9.9 Hz, 1H), 6.76 (t, J FH = 75.5 Hz, 1H), 4.64-4.73 (m, 2H), 4.55-4.60 (m, 1H) ), 3.94 (d, J = 6.9 Hz, 2H), 3.78-3.83 (m, 1H), 3.66-3.76 (m, 1H), 3.34-3.42 (m, 1H), 3.10 (s, 3H), 3.02 ( s, 3H), 2.89-2.97 (m, 1H), 2.12-2.21 (m, 1H), 1.28-1.37 (m, 1H), 0.63-0.67 (m, 2H), 0.37-0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 489.30 [M+H-2HCl]+ .MS (ESI, pos.ion) m/z: 489.30 [M+H-2HCl] + .
實施例31:6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-乙基吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺;以及Example 31: 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-ethylpyrrolidine-2-methyl (acylamino)methyl) -N , N -lutidamide; and
實施例32:6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-乙烯基吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 實施例31:和實施例32: Example 32: 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-vinylpyrrolidine-2-methyl Acrylamido)methyl) -N , N -lutidamide Example 31: and Example 32:
將化合物6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 (74 mg, 0.13 mmol) 和對甲苯磺酸 (4 mg, 0.023 mmol) 溶解於乙醇 (3 mL) 溶液中,加入40%乙醛水溶液 (0.15 mL),50℃加熱反應50 min,冷卻後加入硼氫化鈉室繼續反應2 h,除去溶劑,加入水 (3 mL),乙酸乙酯萃取 (5 mL × 3),無水硫酸鈉乾燥,濃縮後經矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-乙基吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 (實施例31),淺黃色黏稠固體26 mg,收率38%,和6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-乙烯基吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 (實施例32),淺黃色黏稠固體12 mg,收率17%。Compound 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)- N , N -lutidyl pyridamide dihydrochloride (74 mg, 0.13 mmol) and p-toluenesulfonic acid (4 mg, 0.023 mmol) were dissolved in ethanol (3 mL) solution, and 40% acetaldehyde in water was added ( 0.15 mL), heated at 50 °C for 50 min, cooled and added to sodium borohydride to continue the reaction for 2 h, removed the solvent, added water (3 mL), extracted with ethyl acetate (5 mL × 3), dried over anhydrous sodium sulfate, concentrated After separation by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1), 6-((( 2R )-4-(3-(cyclopropylmethoxy) was obtained )-4-(difluoromethoxy)phenyl)-1-ethylpyrrolidine-2-carbamoylamino)methyl) -N , N -lutidyl pyridamide (Example 31), pale yellow Sticky solid 26 mg, 38% yield, and 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-ethene acyl pyrrolidine-2-yl) methyl) - N, N - dimethyl pyridine Amides (Example 32), as a pale yellow viscous solid was 12 mg, 17% yield.
實施例31:Example 31:
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 (t,J = 7.8 Hz, 1H), 7.52 (d,J = 7.6 Hz, 1H), 7.23 (d,J = 7.8 Hz, 1H), 7.05 (d,J = 8.6 Hz, 1H), 6.84 (d,J = 6.3 Hz, 1H), 6.83 (s, 1H), 6.60 (t,J F-H = 75.7 Hz, 1H), 4.51-4.68 (m, 2H), 3.84 (d,J = 6.9 Hz, 2H), 3.32-3.40 (m, 2H), 3.24-3.30 (m, 1H), 3.13 (s, 3H), 3.03 (s, 3H), 2.88-2.97 (m, 1H), 2.71-2.81 (m, 2H), 2.48-2.59 (m, 1H), 2.22-2.25 (m, 1H), 1.28-1.37 (m, 1H), 1.13 (t,J = 7.1 Hz, 3H), 0.62-0.66 (m, 2H), 0.32-0.36 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 (t, J = 7.8 Hz, 1H), 7.52 (d, J = 7.6 Hz, 1H), 7.23 (d, J = 7.8 Hz, 1H) , 7.05 (d, J = 8.6 Hz, 1H), 6.84 (d, J = 6.3 Hz, 1H), 6.83 (s, 1H), 6.60 (t, J FH = 75.7 Hz, 1H), 4.51-4.68 (m , 2H), 3.84 (d, J = 6.9 Hz, 2H), 3.32-3.40 (m, 2H), 3.24-3.30 (m, 1H), 3.13 (s, 3H), 3.03 (s, 3H), 2.88- 2.97 (m, 1H), 2.71-2.81 (m, 2H), 2.48-2.59 (m, 1H), 2.22-2.25 (m, 1H), 1.28-1.37 (m, 1H), 1.13 (t, J = 7.1 Hz, 3H), 0.62-0.66 (m, 2H), 0.32-0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 517.20 [M+H]+ .MS (ESI, pos.ion) m/z: 517.20 [M+H] + .
實施例32:Example 32:
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.78 (t,J = 7.8 Hz, 1H), 7.55 (d,J = 7.6 Hz, 1H), 7.32 (d,J = 7.8 Hz, 1H), 7.11 (d,J = 8.5 Hz, 1H), 6.82 (s, 1H), 6.81 (d,J = 6.3 Hz, 1H), 6.62 (t,J F-H = 75.7 Hz, 1H), 5.30-5.45 (m, 1H), 4.76-4.88 (m, 2H), 4.42 (d,J = 15.8 Hz, 1H), 3.93-3.97 (m, 1H), 3.88 (d,J = 6.8 Hz, 2H), 3.36-3.45 (m, 1H), 3.13-3.21 (m, 1H), 3.13 (s, 3H), 3.05 (s, 3H), 2.58-2.68 (m, 2H), 2.19-2.25 (m, 1H), 2.00-2.05 (m, 1H), 1.28-1.37 (m, 1H), 0.64-0.69 (m, 2H), 0.36-0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.78 (t, J = 7.8 Hz, 1H), 7.55 (d, J = 7.6 Hz, 1H), 7.32 (d, J = 7.8 Hz, 1H) , 7.11 (d, J = 8.5 Hz, 1H), 6.82 (s, 1H), 6.81 (d, J = 6.3 Hz, 1H), 6.62 (t, J FH = 75.7 Hz, 1H), 5.30-5.45 (m , 1H), 4.76-4.88 (m, 2H), 4.42 (d, J = 15.8 Hz, 1H), 3.93-3.97 (m, 1H), 3.88 (d, J = 6.8 Hz, 2H), 3.36-3.45 ( m, 1H), 3.13-3.21 (m, 1H), 3.13 (s, 3H), 3.05 (s, 3H), 2.58-2.68 (m, 2H), 2.19-2.25 (m, 1H), 2.00-2.05 ( m, 1H), 1.28-1.37 (m, 1H), 0.64-0.69 (m, 2H), 0.36-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 515.35 [M+H]+ .MS (ESI, pos.ion) m/z: 515.35 [M+H] + .
實施例33:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -乙基吡啶醯胺 Example 33: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -ethylpyridamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.2 mmol),乙胺鹽酸鹽(163 mg, 2.0 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.0 mmol) 和N -羥基-7-氮雜苯並三氮唑 (41 mg, 0.3 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體58 mg,收率55%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.2 mmol), ethylamine hydrochloride (163 mg, 2.0 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride Salt (192 mg, 1.0 mmol) and N -hydroxy-7-azabenzotriazole (41 mg, 0.3 mmol) were dissolved in dichloromethane (5 mL) and added dropwise to this solution at 0°C N , N -diisopropylethylamine (0.4 mL, 2.0 mmol), reacted at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was dried over anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 58 mg of white solid with a yield of 55%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.00 (br.s, 1H), 8.65 (s, 1H), 8.12 (d,J = 7.1 Hz, 1H), 7.82 (br.s, 1H), 7.38 (d,J = 7.1 Hz, 1H), 7.13 (d,J = 7.6 Hz, 1H), 6.87 – 6.93 (m, 2H), 6.62 (t,J F-H = 75.7 Hz, 1H), 4.81 – 4.86 (m, 1H), 4.58 – 4.71 (m, 2H), 3.91 – 4.00 (m, 1H), 3.89 (d,J = 7.3 Hz, 2H), 3.49 – 3.67 (m, 2H), 3.44 – 3.50 (m, 1H), 3.29 – 3.38 (m, 1H), 2.77 – 2.86 (m, 1H), 2.47 – 2.55 (m, 1H), 2.18 (s, 3H), 1.26 – 1.35 (m, 4H), 0.61 – 0.70 (m, 2H), 0.32 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.00 (br.s, 1H), 8.65 (s, 1H), 8.12 (d, J = 7.1 Hz, 1H), 7.82 (br.s, 1H) ), 7.38 (d, J = 7.1 Hz, 1H), 7.13 (d, J = 7.6 Hz, 1H), 6.87 – 6.93 (m, 2H), 6.62 (t, J FH = 75.7 Hz, 1H), 4.81 – 4.86 (m, 1H), 4.58 – 4.71 (m, 2H), 3.91 – 4.00 (m, 1H), 3.89 (d, J = 7.3 Hz, 2H), 3.49 – 3.67 (m, 2H), 3.44 – 3.50 ( m, 1H), 3.29 – 3.38 (m, 1H), 2.77 – 2.86 (m, 1H), 2.47 – 2.55 (m, 1H), 2.18 (s, 3H), 1.26 – 1.35 (m, 4H), 0.61 – 0.70 (m, 2H), 0.32 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 531.30 [M+H]+ .MS (ESI, pos.ion) m/z: 531.30 [M+H] + .
實施例34:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二乙基吡啶醯胺 Example 34: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N , N -diethylpyridamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.2 mmol),二乙胺(73 mg, 1.0 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.0 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.3 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.0 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體63 mg,收率56%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.2 mmol), diethylamine (73 mg, 1.0 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 192 mg, 1.0 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.3 mmol) were dissolved in dichloromethane (5 mL), and N was added dropwise to this solution at 0°C, N -diisopropylethylamine (0.17 mL, 1.0 mmol), reacted at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, removed The solvent and the concentrated solution were separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 63 mg of white solid with a yield of 56%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 (t,J = 7.6 Hz, 1H), 7.48 (br.s, 1H), 7.42 (d,J = 7.4 Hz, 1H), 7.37 (d,J = 7.6 Hz, 1H), 7.12 (d,J = 7.9 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 8.1 Hz, 1H), 6.62 (t,J F-H = 75.5 Hz, 1H), 4.55 – 4.61 (m, 3H), 3.93 – 3.97 (m, 1H), 3.88 (d,J = 6.8 Hz, 2H), 3.56 – 3.62 (m, 3H), 3.27 – 3.38 (m, 3H), 3.55 – 3.62 (m, 1H), 2.39 – 2.49 (m, 1H), 2.14 (s, 3H), 1.26 – 1.35 (m, 1H), 1.28 (t,J = 6.7 Hz, 3H), 1.18 (t,J = 6.7 Hz, 3H), 0.64 – 0.69 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 (t, J = 7.6 Hz, 1H), 7.48 (br.s, 1H), 7.42 (d, J = 7.4 Hz, 1H), 7.37 ( d, J = 7.6 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H), 6.90 (s, 1H), 6.86 (d, J = 8.1 Hz, 1H), 6.62 (t, J FH = 75.5 Hz , 1H), 4.55 – 4.61 (m, 3H), 3.93 – 3.97 (m, 1H), 3.88 (d, J = 6.8 Hz, 2H), 3.56 – 3.62 (m, 3H), 3.27 – 3.38 (m, 3H) ), 3.55 – 3.62 (m, 1H), 2.39 – 2.49 (m, 1H), 2.14 (s, 3H), 1.26 – 1.35 (m, 1H), 1.28 (t, J = 6.7 Hz, 3H), 1.18 ( t, J = 6.7 Hz, 3H), 0.64 – 0.69 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 559.30 [M+H]+ .MS (ESI, pos.ion) m/z: 559.30 [M+H] + .
實施例35:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯胺基)甲基)-N ,N -二丙基吡啶醯胺 Example 35: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxamido)methyl) -N , N -dipropylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.2 mmol),二正丙胺 (64 mg, 0.63 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.0 mmol) 和N -羥基-7-氮雜苯並三氮唑 (42 mg, 0.3 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.0 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體76 mg,收率65%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.2 mmol), di-n-propylamine (64 mg, 0.63 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 192 mg, 1.0 mmol) and N -hydroxy-7-azabenzotriazole (42 mg, 0.3 mmol) were dissolved in dichloromethane (5 mL), and N was added dropwise to this solution at 0°C, N -diisopropylethylamine (0.17 mL, 1.0 mmol), reacted at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, removed The solvent and the concentrated solution were separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 76 mg of white solid with a yield of 65%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 (t,J = 7.7 Hz, 1H), 7.43 (br.s, 1H), 7.40 (d,J = 7.8 Hz, 1H), 7.37 (d,J = 7.8 Hz, 1H), 7.12 (d,J = 8.0 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 8.1 Hz, 1H), 6.62 (t,J F-H = 75.5 Hz, 1H), 4.50 – 4.62 (m, 3H), 3.93 – 3.98 (m, 1H), 3.88 (d,J = 6.8 Hz, 2H), 3.55 – 3.61 (m, 1H), 3.45 – 3.49 (m, 2H), 3.32 – 3.39 (m, 1H), 3.19 – 3.25 (m, 2H), 2.55 – 2.62 (m, 1H), 2.40 – 2.48 (m, 1H), 2.15 (s, 3H), 1.58 – 1.62 (m, 2H), 1.26 – 1.35 (m, 1H), 1.00 (t,J = 7.3 Hz, 3H), 0.85 – 0.91 (m, 2H), 0.77 (t,J = 7.3 Hz, 3H), 0.64 – 0.68 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 (t, J = 7.7 Hz, 1H), 7.43 (br.s, 1H), 7.40 (d, J = 7.8 Hz, 1H), 7.37 ( d, J = 7.8 Hz, 1H), 7.12 (d, J = 8.0 Hz, 1H), 6.90 (s, 1H), 6.86 (d, J = 8.1 Hz, 1H), 6.62 (t, J FH = 75.5 Hz , 1H), 4.50 – 4.62 (m, 3H), 3.93 – 3.98 (m, 1H), 3.88 (d, J = 6.8 Hz, 2H), 3.55 – 3.61 (m, 1H), 3.45 – 3.49 (m, 2H) ), 3.32 – 3.39 (m, 1H), 3.19 – 3.25 (m, 2H), 2.55 – 2.62 (m, 1H), 2.40 – 2.48 (m, 1H), 2.15 (s, 3H), 1.58 – 1.62 (m , 2H), 1.26 – 1.35 (m, 1H), 1.00 (t, J = 7.3 Hz, 3H), 0.85 – 0.91 (m, 2H), 0.77 (t, J = 7.3 Hz, 3H), 0.64 – 0.68 ( m, 2H), 0.36 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 587.45 [M+H]+ .MS (ESI, pos.ion) m/z: 587.45 [M+H] + .
實施例36:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基-N -正丙基吡啶醯胺 Example 36: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -methyl- N -n-propylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (102 mg, 0.2 mmol),N -甲基正丙胺 (43 mg, 0.6 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.0 mmol) 和N -羥基-7-氮雜苯並三氮唑 (45 mg, 0.33 mmol) 溶於二氯甲烷 (5 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.15 mL, 0.9 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體59 mg,收率52%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (102 mg, 0.2 mmol), N -methyl-n-propylamine (43 mg, 0.6 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide salt The acid salt (192 mg, 1.0 mmol) and N -hydroxy-7-azabenzotriazole (45 mg, 0.33 mmol) were dissolved in dichloromethane (5 mL), and the solution was added dropwise at 0°C N , N -diisopropylethylamine (0.15 mL, 0.9 mmol) was added, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sodium sulfate After drying, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 59 mg of a white solid with a yield of 52%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 (t,J = 7.1 Hz, 1H), 7.36 – 7.49 (m, 2H), 7.12 (d,J = 7.6 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 7.8 Hz, 1H), 6.62 (t,J F-H = 75.4 Hz, 1H), 4.47 – 4.67 (m, 3H), 3.90 – 3.98 (m, 1H), 3.88 (d,J = 6.5 Hz, 2H), 3.52 – 3.60 (m, 2H), 3.25 – 3.39 (m, 2H), 3.10 (s, 1.5H), 3.00 (s, 1.5H), 2.52 – 2.63 (m, 1H), 2.38 – 2.50 (m, 1H), 2.14 (s, 3H), 1.58 – 1.74 (m, 2H), 1.26 – 1.35 (m, 1H), 0.94 – 1.04 (m, 1.5H), 0.72 – 0.81 (m, 1.5H), 0.62 – 0.70 (m, 2H), 0.33 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 (t, J = 7.1 Hz, 1H), 7.36 – 7.49 (m, 2H), 7.12 (d, J = 7.6 Hz, 1H), 6.90 ( s, 1H), 6.86 (d, J = 7.8 Hz, 1H), 6.62 (t, J FH = 75.4 Hz, 1H), 4.47 – 4.67 (m, 3H), 3.90 – 3.98 (m, 1H), 3.88 ( d, J = 6.5 Hz, 2H), 3.52 – 3.60 (m, 2H), 3.25 – 3.39 (m, 2H), 3.10 (s, 1.5H), 3.00 (s, 1.5H), 2.52 – 2.63 (m, 1H), 2.38 – 2.50 (m, 1H), 2.14 (s, 3H), 1.58 – 1.74 (m, 2H), 1.26 – 1.35 (m, 1H), 0.94 – 1.04 (m, 1.5H), 0.72 – 0.81 (m, 1.5H), 0.62 – 0.70 (m, 2H), 0.33 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 559.20 [M+H]+ .MS (ESI, pos.ion) m/z: 559.20 [M+H] + .
實施例37:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(3-(二甲基甲醯胺)苄基)吡咯烷-2-甲醯胺 Example 37: Compound (2 R) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - (3- (two Methylformamide)benzyl)pyrrolidine-2-formamide
步驟1:化合物 3-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)苯甲酸甲酯 的合成Step 1: Compound 3-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of methyl formamido)methyl)benzoate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (83 mg, 0.22 mmol),3-(氨甲基)苯甲酸甲酯 (97 mg, 0.48 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (93 mg, 0.48 mmol) 和N -羥基-7-氮雜苯並三氮唑 (47 mg, 0.34 mmol) 溶於二氯甲烷 (6 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌5 h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到無色液體81 mg,收率70%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (83 mg, 0.22 mmol), methyl 3-(aminomethyl)benzoate (97 mg, 0.48 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (93 mg, 0.48 mmol) and N -hydroxy-7-azabenzotriazole (47 mg, 0.34 mmol) were dissolved in dichloromethane (6 mL), to this solution was added dropwise N , N -dichloromethane at 0°C Isopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 5 h, added water (50 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution Silica gel column chromatography was performed (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 81 mg of a colorless liquid with a yield of 70%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.98 (s, 1H), 7.91 (d,J = 7.8 Hz, 1H), 7.62 (d,J = 7.6 Hz, 1H), 7.47 (t,J = 7.7 Hz, 1H), 7.11 (d,J = 8.2 Hz, 1H), 7.08 (s, 1H), 6.92-6.93 (m, 1H), 6.74 (t,J F-H = 73.6 Hz, 1H), 4.57-4.64 (m, 1H), 4.49 (s, 2H), 4.08-4.12 (m, 1H), 3.92 (d,J = 6.9 Hz, 2H), 3.89 (s, 3H), 3.62-3.67 (m, 1H), 3.47-3.51 (m, 1H), 2.65-2.72 (m, 1h), 2.15 (s, 3H), 2.01-2.12 (m, 1H), 1.32-1.36 (m, 1H), 0.62-0.65 (m, 2H), 0.35-0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.98 (s, 1H), 7.91 (d, J = 7.8 Hz, 1H), 7.62 (d, J = 7.6 Hz, 1H), 7.47 (t , J = 7.7 Hz, 1H), 7.11 (d, J = 8.2 Hz, 1H), 7.08 (s, 1H), 6.92-6.93 (m, 1H), 6.74 (t, J FH = 73.6 Hz, 1H), 4.57-4.64 (m, 1H), 4.49 (s, 2H), 4.08-4.12 (m, 1H), 3.92 (d, J = 6.9 Hz, 2H), 3.89 (s, 3H), 3.62-3.67 (m, 1H), 3.47-3.51 (m, 1H), 2.65-2.72 (m, 1h), 2.15 (s, 3H), 2.01-2.12 (m, 1H), 1.32-1.36 (m, 1H), 0.62-0.65 ( m, 2H), 0.35-0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 517.20 [M+H]+ .MS (ESI, pos.ion) m/z: 517.20 [M+H] + .
步驟2:化合物3-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)苯甲酸的合成Step 2: Compound 3-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of formamido)methyl)benzoic acid
將化合物 3-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)苯甲酸甲酯 (78 mg, 0.15 mmol) 溶於四氫呋喃 (4 mL) 和水 (2 mL) 的混合溶劑中,加入一水合氫氧化鋰 (32 mg, 0.76 mmol),50℃反應2 h後停止,加鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體70 mg,收率94%。The compound 3-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)methyl benzoate (78 mg, 0.15 mmol) was dissolved in a mixed solvent of tetrahydrofuran (4 mL) and water (2 mL), added lithium hydroxide monohydrate (32 mg, 0.76 mmol), 50 ° C The reaction was stopped after 2 h, hydrochloric acid was added to adjust the pH of the solution to 1, extracted with ethyl acetate (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 70 mg of a white solid with a yield of 94%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.51 (t,J = 5.8 Hz, 1H), 7.79-7.88 (m, 2H), 7.50-7.56 (m, 1H), 7.42-7.47 (m, 1H), 7.09-7.13 (m, 1H), 7.05 (s, 1H), 7.02 (t,J F-H = 74.9 Hz, 1H), 6.88 (d,J = 9.6 Hz, 1H), 4.30-4.38 (m, 3H), 4.04-4.08 (m, 1H), 3.89 (d,J = 6.9 Hz, 2H), 3.40-3.50 (m, 2H), 2.56-2.62 (m, 1H), 2.02 (s, 3H), 1.84-1.92 (m, 1H), 1.26-1.32 (m, 1H), 0.54-0.59 (m, 2H), 0.32-0.35 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.51 (t, J = 5.8 Hz, 1H), 7.79-7.88 (m, 2H), 7.50-7.56 (m, 1H), 7.42-7.47 (m, 1H), 7.09-7.13 (m, 1H), 7.05 (s, 1H), 7.02 (t, J FH = 74.9 Hz, 1H), 6.88 (d, J = 9.6 Hz, 1H), 4.30-4.38 (m, 3H), 4.04-4.08 (m, 1H), 3.89 (d, J = 6.9 Hz, 2H), 3.40-3.50 (m, 2H), 2.56-2.62 (m, 1H), 2.02 (s, 3H) ), 1.84-1.92 (m, 1H), 1.26-1.32 (m, 1H), 0.54-0.59 (m, 2H), 0.32-0.35 (m, 2H).
MS (ESI, pos.ion) m/z: 503.10 [M+H]+ .MS (ESI, pos.ion) m/z: 503.10 [M+H] + .
步驟3:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(3-(二甲基甲醯胺)苄基)吡咯烷-2-甲醯胺的合成Step 3: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (3-(dimethyl) Synthesis of carboxamide)benzyl)pyrrolidine-2-carboxamide
將化合物3-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)苯甲酸 (71 mg, 0.14 mmol),二甲胺鹽酸鹽 (67 mg, 0.82 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (76 mg, 0.40 mmol) 和N -羥基-7-氮雜苯並三氮唑 (42 mg, 0.31 mmol) 溶於二氯甲烷 (6 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺(0.2 mL, 1.0 mmol),室溫攪拌15 h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體54 mg,收率72%。The compound 3-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)benzoic acid (71 mg, 0.14 mmol), dimethylamine hydrochloride (67 mg, 0.82 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide The acid salt (76 mg, 0.40 mmol) and N -hydroxy-7-azabenzotriazole (42 mg, 0.31 mmol) were dissolved in dichloromethane (6 mL), and the solution was added dropwise at 0°C Add N , N -diisopropylethylamine (0.2 mL, 1.0 mmol), stir at room temperature for 15 h, add water (50 mL), extract with dichloromethane (5 mL × 3), and dry the organic phase with anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 54 mg of white solid with a yield of 72%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.49 (t,J = 5.6 Hz, 1H), 7.33-7.38 (m, 2H), 7.28-7.32 (m, 1H), 7.23-7.27 (m, 1H), 7.12 (d,J = 8.0 Hz, 1H), 7.05 (s, 1H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.88 (d,J = 8.8 Hz, 1H), 4.31-4.37 (m, 2H), 4.04-4.08 (m, 1H), 3.90 (d,J = 6.8 Hz, 2H), 3.41-3.51 (m, 2H), 2.96 (s, 3H), 2.88 (s, 3H), 2.55-2.62 (m, 1H), 2.02 (s, 3H), 1.82-1.91 (m, 1H), 1.23-1.35 (m, 1H), 0.54-0.60 (m, 2H), 0.31-0.35 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.49 (t, J = 5.6 Hz, 1H), 7.33-7.38 (m, 2H), 7.28-7.32 (m, 1H), 7.23-7.27 (m, 1H), 7.12 (d, J = 8.0 Hz, 1H), 7.05 (s, 1H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.88 (d, J = 8.8 Hz, 1H), 4.31-4.37 (m, 2H), 4.04-4.08 (m, 1H), 3.90 (d, J = 6.8 Hz, 2H), 3.41-3.51 (m, 2H), 2.96 (s, 3H), 2.88 (s, 3H), 2.55-2.62 (m, 1H), 2.02 (s, 3H), 1.82-1.91 (m, 1H), 1.23-1.35 (m, 1H), 0.54-0.60 (m, 2H), 0.31-0.35 ( m, 2H).
MS (ESI, pos.ion) m/z: 530.30 [M+H]+ .MS (ESI, pos.ion) m/z: 530.30 [M+H] + .
實施例38:化合物 6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二異丙基吡啶醯胺 Example 38: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N , N -diisopropylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (92 mg, 0.18 mmol),N ,N -二異丙基胺 (89 mg, 0.88mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (143 mg, 0.75 mmol) 和N -羥基-7-氮雜苯並三氮唑 (81 mg, 0.60 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌5 h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體51 mg,收率47%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (92 mg, 0.18 mmol), N , N -diisopropylamine (89 mg, 0.88 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Imine hydrochloride (143 mg, 0.75 mmol) and N -hydroxy-7-azabenzotriazole (81 mg, 0.60 mmol) were dissolved in dichloromethane (6 mL) and added to this solution at room temperature N , N -diisopropylethylamine (0.4 mL, 2.0 mmol) was added dropwise to it, stirred at room temperature for 5 h, added with water (50 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sulfuric acid The solution was dried over sodium, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 51 mg of white solid with a yield of 47%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.60 (t,J = 5.8 Hz, 1H), 7.82 (t,J = 7.6 Hz, 1H), 7.43 (d,J = 7.8 Hz, 1H), 7.24-7.30 (m, 1H), 7.12 (d,J = 8.3 Hz, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.8 Hz, 1H),6.89 (d,J = 8.9 Hz, 1H), 4.33-4.42 (m, 3H), 4.05-4.09 (m, 1H), 3.90 (d,J = 6.8 Hz, 2H), 3.55-3.65 (m, 2H), 3.39-3.52 (m, 2H), 2.56-2.65 (m, 1H), 2.03 (s, 3H), 1.89-1.99 (m, 1H), 1.43 (d,J = 5.7 Hz, 6H), 1.24-1.27 (m, 1H), 1.09 (d,J = 6.1 Hz, 6H), 0.54-0.60 (m, 2H), 0.30-0.36 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.60 (t, J = 5.8 Hz, 1H), 7.82 (t, J = 7.6 Hz, 1H), 7.43 (d, J = 7.8 Hz, 1H), 7.24-7.30 (m, 1H), 7.12 (d, J = 8.3 Hz, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.8 Hz, 1H), 6.89 (d, J = 8.9 Hz, 1H), 4.33-4.42 (m, 3H), 4.05-4.09 (m, 1H), 3.90 (d, J = 6.8 Hz, 2H), 3.55-3.65 (m, 2H), 3.39-3.52 (m , 2H), 2.56-2.65 (m, 1H), 2.03 (s, 3H), 1.89-1.99 (m, 1H), 1.43 (d, J = 5.7 Hz, 6H), 1.24-1.27 (m, 1H), 1.09 (d, J = 6.1 Hz, 6H), 0.54-0.60 (m, 2H), 0.30-0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 587.45 [M+H]+ .MS (ESI, pos.ion) m/z: 587.45 [M+H] + .
實施例39:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -乙基-N -甲基吡啶醯胺 Example 39: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -ethyl- N -picolinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (102 mg, 0.20 mmol),N -乙基甲基胺 (59 mg, 1.00 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (198 mg, 1.03 mmol) 和N -羥基-7-氮雜苯並三氮唑 (46 mg, 0.34 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到淺黃色固體28 mg,收率25%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (102 mg, 0.20 mmol), N -ethylmethylamine (59 mg, 1.00 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide The hydrochloride salt (198 mg, 1.03 mmol) and N -hydroxy-7-azabenzotriazole (46 mg, 0.34 mmol) were dissolved in dichloromethane (5 mL) and added to this solution at 0°C N ,N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added dropwise, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sulfuric acid The solution was dried over sodium, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 28 mg of a pale yellow solid with a yield of 25%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.77 (t,J = 7.6 Hz, 1H), 7.36 – 7.46 (m, 2H), 7.12 (d,J = 8.0 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 7.2 Hz, 1H), 6.62 (t,J F-H = 75.4 Hz, 1H), 4.49 – 4.67 (m, 3H), 3.91 – 3.97 (m, 1H), 3.88 (d,J = 6.9 Hz, 2H), 3.55 – 3.63 (m, 2H), 3.30 – 3.39 (m, 2H), 3.11 (s, 2H), 3.01 (s, 1H), 2.54 – 2.62 (m, 1H), 2.41 – 2.49 (m, 1H), 2.14 (s, 3H), 1.26 – 1.35 (m, 1H), 1.20 (t,J = 7.0 Hz, 3H), 0.63 – 0.70 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.77 (t, J = 7.6 Hz, 1H), 7.36 – 7.46 (m, 2H), 7.12 (d, J = 8.0 Hz, 1H), 6.90 ( s, 1H), 6.86 (d, J = 7.2 Hz, 1H), 6.62 (t, J FH = 75.4 Hz, 1H), 4.49 – 4.67 (m, 3H), 3.91 – 3.97 (m, 1H), 3.88 ( d, J = 6.9 Hz, 2H), 3.55 – 3.63 (m, 2H), 3.30 – 3.39 (m, 2H), 3.11 (s, 2H), 3.01 (s, 1H), 2.54 – 2.62 (m, 1H) , 2.41 – 2.49 (m, 1H), 2.14 (s, 3H), 1.26 – 1.35 (m, 1H), 1.20 (t, J = 7.0 Hz, 3H), 0.63 – 0.70 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 545.30 [M+H]+ .MS (ESI, pos.ion) m/z: 545.30 [M+H] + .
實施例40:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二正丁基吡啶醯胺 Example 40: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N , N -di-n-butylpyridinamide
將化合物6-(((2R)-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (97 mg, 0.19 mmol),二正丁基胺 (57 mg, 0.44 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (41 mg, 0.30 mmol) 溶於二氯甲烷 (5 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.16 mL, 0.97 mmol),室溫反應12 h,加水洗 (15 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體41 mg,收率35%。Compound 6-(((2R)-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino )methyl)picolinic acid (97 mg, 0.19 mmol), di-n-butylamine (57 mg, 0.44 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (192 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (41 mg, 0.30 mmol) were dissolved in dichloromethane (5 mL), to this solution was added dropwise N at 0°C , N -diisopropylethylamine (0.16 mL, 0.97 mmol), reacted at room temperature for 12 h, washed with water (15 mL), then extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, The solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 41 mg of white solid with a yield of 35%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 (t,J = 7.7 Hz, 1H), 7.40 (d,J = 7.1 Hz, 1H), 7.37 (d,J = 7.9 Hz, 1H),7.13 (d,J = 8.0 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 8.5 Hz, 1H), 6.62 (t,J F-H = 75.5 Hz, 1H), 4.54 – 4.67 (m, 3H), 3.93 – 3.99 (m, 1H), 3.89 (d,J = 6.7 Hz, 2H), 3.55 – 3.61 (m, 1H), 3.49 – 3.52 (m, 2H), 3.31 – 3.39 (m, 1H), 3.25 – 3.29 (m, 2H), 2.53 – 2.63 (m, 1H), 2.41 – 2.49 (m, 1H), 2.15 (s, 3H), 1.63 – 1.73 (m, 2H), 1.51 – 1.59 (m, 2H), 1.39 – 1.47 (m, 2H), 1.26 – 1.34 (m, 1H), 1.13 – 1.19 (m, 2H), 0.99 (t,J = 7.2 Hz, 3H), 0.80 (t,J = 7.3 Hz, 3H), 0.64 – 0.70 (m, 2H), 0.35 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 (t, J = 7.7 Hz, 1H), 7.40 (d, J = 7.1 Hz, 1H), 7.37 (d, J = 7.9 Hz, 1H) ,7.13 (d, J = 8.0 Hz, 1H), 6.90 (s, 1H), 6.86 (d, J = 8.5 Hz, 1H), 6.62 (t, J FH = 75.5 Hz, 1H), 4.54 – 4.67 (m , 3H), 3.93 – 3.99 (m, 1H), 3.89 (d, J = 6.7 Hz, 2H), 3.55 – 3.61 (m, 1H), 3.49 – 3.52 (m, 2H), 3.31 – 3.39 (m, 1H) ), 3.25 – 3.29 (m, 2H), 2.53 – 2.63 (m, 1H), 2.41 – 2.49 (m, 1H), 2.15 (s, 3H), 1.63 – 1.73 (m, 2H), 1.51 – 1.59 (m , 2H), 1.39 – 1.47 (m, 2H), 1.26 – 1.34 (m, 1H), 1.13 – 1.19 (m, 2H), 0.99 (t, J = 7.2 Hz, 3H), 0.80 (t, J = 7.3 Hz, 3H), 0.64 – 0.70 (m, 2H), 0.35 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 615.35 [M+H]+ .MS (ESI, pos.ion) m/z: 615.35 [M+H] + .
實施例41:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(2-(二甲氨基)乙基)-N -甲基吡啶醯胺 Example 41: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (2- (dimethylamino) ethyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N 1 ,N 1 ,N 2 -三甲基乙二胺 (60 mg, 0.59 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (191 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (56 mg, 0.41 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (152 mg, 1.18 mmol),室溫反應4.5 h,加水 (30 mL) 攪拌5 min,分離有機相,水相用二氯甲烷萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 24/1),得到白色固體55 mg,收率47%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N 1 , N 1 , N 2 -trimethylethylenediamine (60 mg, 0.59 mmol), 1-ethyl-(3-dimethylamino) propyl)carbodiimide hydrochloride (191 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (56 mg, 0.41 mmol) were dissolved in dichloromethane (5 mL), Cool to 0°C, add N , N -diisopropylethylamine (152 mg, 1.18 mmol), react at room temperature for 4.5 h, add water (30 mL) and stir for 5 min, separate the organic phase, and extract the aqueous phase with dichloromethane (10 mL × 2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 24/1) to obtain a white Solid 55 mg, yield 47%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.71 – 7.79 (m, 1H), 7.44 – 7.53 (m, 1H), 7.37 – 7.40 (m, 1H), 7.10 (d,J = 8.1 Hz, 1H), 6.87 (s, 1H), 6.83 (d,J = 8.2, Hz, 1H), 6.60 (t,J F-H = 75.6 Hz, 1H), 4.46 – 4.63 (m, 2H), 3.90 – 3.94 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.67 – 3.70 (m, 1H), 3.57 (d,J = 10.6 Hz, 1H), 3.48 – 3.51 (m, 1H), 3.20 – 3.37 (m, 2H), 3.12 (s, 1.5H), 3.03 (s, 1.5H), 2.52 – 2.64 (m, 3H), 2.37 – 2.42 (m, 1H), 2.32 (s, 3H), 2.13 (s, 3H), 2.11 (s, 3H), 1.21 – 1.28 (m, 1H), 0.62 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.71 – 7.79 (m, 1H), 7.44 – 7.53 (m, 1H), 7.37 – 7.40 (m, 1H), 7.10 (d, J = 8.1 Hz , 1H), 6.87 (s, 1H), 6.83 (d, J = 8.2, Hz, 1H), 6.60 (t, J FH = 75.6 Hz, 1H), 4.46 – 4.63 (m, 2H), 3.90 – 3.94 ( m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.67 – 3.70 (m, 1H), 3.57 (d, J = 10.6 Hz, 1H), 3.48 – 3.51 (m, 1H), 3.20 – 3.37 (m, 2H), 3.12 (s, 1.5H), 3.03 (s, 1.5H), 2.52 – 2.64 (m, 3H), 2.37 – 2.42 (m, 1H), 2.32 (s, 3H), 2.13 (s , 3H), 2.11 (s, 3H), 1.21 – 1.28 (m, 1H), 0.62 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 588.30 [M+H]+ .MS (ESI, pos.ion) m/z: 588.30 [M+H] + .
實施例42:化合物6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-丙醯基吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 Example 42: Compound 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-propionylpyrrolidine-2 -Carboxylamido)methyl) -N , N -lutidyl pyridinamide
將丙酸 (33 mg, 0.45 mmol),6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 (70 mg, 0.12 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (121 mg, 0.63 mmol) 和N -羥基-7-氮雜苯並三氮唑 (32 mg, 0.24 mmol) 溶於二氯甲烷 (8 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.1 mL, 0.6 mmol),室溫攪拌19 h,加水 (15 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體41 mg,收率60%。Propionic acid (33 mg, 0.45 mmol), 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N , N -lutidine dihydrochloride (70 mg, 0.12 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Amine hydrochloride (121 mg, 0.63 mmol) and N -hydroxy-7-azabenzotriazole (32 mg, 0.24 mmol) were dissolved in dichloromethane (8 mL) and added to this solution at 0°C N , N -diisopropylethylamine (0.1 mL, 0.6 mmol) was added dropwise to it, stirred at room temperature for 19 h, water (15 mL) was added, extracted with dichloromethane (10 mL × 3), the organic phase was washed with anhydrous sulfuric acid The solution was dried over sodium, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 41 mg of white solid with a yield of 60%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.77 (t,J = 7.3 Hz, 1H), 7.52 (br.s, 1H), 7.47 (d,J = 7.3 Hz, 1H), 7.37 (d,J = 7.3 Hz, 1H), 7.12 (d,J = 7.8 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 7.8 Hz, 1H), 6.62 (t,J F-H = 75.4 Hz, 1H), 4.53 – 4.65 (m, 3H), 3.95 – 3.99 (m, 1H), 3.88 (d,J = 6.6 Hz, 2H), 3.52 (t,J = 10.4 Hz, 1H), 3.27 – 3.38 (m, 1H), 3.14 (s, 3H), 3.06 (s, 3H), 2.52 – 2.64 (m, 1H), 2.32 – 2.49 (m, 3H), 1.27 – 1.33 (m, 1H), 1.17 (t,J = 7.0 Hz, 3H), 0.64 – 0.68 (m, 2H), 0.34 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.77 (t, J = 7.3 Hz, 1H), 7.52 (br.s, 1H), 7.47 (d, J = 7.3 Hz, 1H), 7.37 ( d, J = 7.3 Hz, 1H), 7.12 (d, J = 7.8 Hz, 1H), 6.90 (s, 1H), 6.86 (d, J = 7.8 Hz, 1H), 6.62 (t, J FH = 75.4 Hz , 1H), 4.53 – 4.65 (m, 3H), 3.95 – 3.99 (m, 1H), 3.88 (d, J = 6.6 Hz, 2H), 3.52 (t, J = 10.4 Hz, 1H), 3.27 – 3.38 ( m, 1H), 3.14 (s, 3H), 3.06 (s, 3H), 2.52 – 2.64 (m, 1H), 2.32 – 2.49 (m, 3H), 1.27 – 1.33 (m, 1H), 1.17 (t, J = 7.0 Hz, 3H), 0.64 – 0.68 (m, 2H), 0.34 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 545.20 [M+H]+ .MS (ESI, pos.ion) m/z: 545.20 [M+H] + .
實施例43:化合物6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-(2-氟乙醯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 Example 43: Compound 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-(2-fluoroacetyl ) pyrrolidine-2-carbamoylamino)methyl) -N , N -lutidyl pyridylamine
將2-氟乙酸 (37 mg, 0.47 mmol),6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 (90 mg, 0.16 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (152 mg, 0.79 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.14 mL, 0.85 mmol),室溫攪拌7 h,加水 (15 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體73 mg,收率83%。2-Fluoroacetic acid (37 mg, 0.47 mmol), 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine -2-Carboxylamido)methyl) -N , N -lutidine dihydrochloride (90 mg, 0.16 mmol), 1-ethyl-(3-dimethylaminopropyl)carboamide Diimine hydrochloride (152 mg, 0.79 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL), and the mixture was heated to 0 °C. N , N -diisopropylethylamine (0.14 mL, 0.85 mmol) was added dropwise to this solution, stirred at room temperature for 7 h, added with water (15 mL), extracted with dichloromethane (10 mL × 3), and the organic phase was After drying over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 73 mg of a white solid with a yield of 83%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.77 (t,J = 7.3 Hz, 1H), 7.57 (br.s, 1H), 7.35 – 7.45 (m, 2H), 7.13 (d,J = 7.8 Hz, 1H), 6.89 (s, 1H), 6.81 – 6.89 (m, 1H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.91 – 5.05 (m, 2H), 4.55 – 4.68 (m, 3H), 3.93 – 4.02 (m, 1H), 3.88 (d,J = 5.5 Hz, 2H), 3.53 – 3.58 (m, 1H), 3.29 – 3.40 (m, 1H), 3.14 (s, 3H), 3.04 (s, 3H), 2.56 – 2.66 (m, 1H), 2.33 – 2.43 (m, 1H), 1.24 – 1.36 (m, 1H), 0.60 – 0.70 (m, 2H), 0.33 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.77 (t, J = 7.3 Hz, 1H), 7.57 (br.s, 1H), 7.35 – 7.45 (m, 2H), 7.13 (d, J = 7.8 Hz, 1H), 6.89 (s, 1H), 6.81 – 6.89 (m, 1H), 6.62 (t, J FH = 75.6 Hz, 1H), 4.91 – 5.05 (m, 2H), 4.55 – 4.68 (m , 3H), 3.93 – 4.02 (m, 1H), 3.88 (d, J = 5.5 Hz, 2H), 3.53 – 3.58 (m, 1H), 3.29 – 3.40 (m, 1H), 3.14 (s, 3H), 3.04 (s, 3H), 2.56 – 2.66 (m, 1H), 2.33 – 2.43 (m, 1H), 1.24 – 1.36 (m, 1H), 0.60 – 0.70 (m, 2H), 0.33 – 0.40 (m, 2H) ).
MS (ESI, pos.ion) m/z: 549.20 [M+H]+ .MS (ESI, pos.ion) m/z: 549.20 [M+H] + .
實施例44:化合物6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-(2,2-二氟乙醯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 Example 44: Compound 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-1-(2,2-difluoro Acetyl)pyrrolidine-2-carbamoylamino)methyl) -N , N -lutidyl pyridinamide
將2,2-二氟乙酸 (36 mg, 0.38 mmol),6-(((2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 (70 mg, 0.12 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (118 mg, 0.62 mmol) 和N -羥基-7-氮雜苯並三氮唑 (32 mg, 0.24 mmol) 溶於二氯甲烷 (8 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.1 mL, 0.6 mmol),室溫攪拌12 h,加水 (15 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體46 mg,收率65%。2,2-Difluoroacetic acid (36 mg, 0.38 mmol), 6-((( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl )pyrrolidine-2-carbamoylamino)methyl) -N , N -lutidine dihydrochloride (70 mg, 0.12 mmol), 1-ethyl-(3-dimethylaminopropyl) ) carbodiimide hydrochloride (118 mg, 0.62 mmol) and N -hydroxy-7-azabenzotriazole (32 mg, 0.24 mmol) were dissolved in dichloromethane (8 mL) at 0°C N , N -diisopropylethylamine (0.1 mL, 0.6 mmol) was added dropwise to this solution under conditions, stirred at room temperature for 12 h, added with water (15 mL), extracted with dichloromethane (10 mL × 3), The organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 46 mg of white solid, yield 65% .
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.77 (t,J = 7.6 Hz, 1H), 7.46 (d,J = 6.8 Hz, 1H), 7.34 (d,J = 7.7 Hz, 1H), 7.14 (d,J = 7.9 Hz, 1H), 6.89 (s, 1H), 6.84 (d,J = 7.8 Hz, 1H), 6.62 (t,J F-H = 75.6 Hz, 1H), 6.08 (t,J F-H = 53.4 Hz, 1H), 4.55 – 4.68 (m, 3H), 4.24 – 4.32 (m, 1H), 3.89 (d,J = 6.7 Hz, 2H), 3.63 (t,J = 11.1 Hz, 1H), 3.32 – 3.44 (m, 1H), 3.15 (s, 3H), 3.04 (s, 3H), 2.60 – 2.69 (m, 1H), 2.35 – 2.43 (m, 1H), 1.27 – 1.39 (m, 1H), 0.64 – 0.69 (m, 2H), 0.36 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.77 (t, J = 7.6 Hz, 1H), 7.46 (d, J = 6.8 Hz, 1H), 7.34 (d, J = 7.7 Hz, 1H) , 7.14 (d, J = 7.9 Hz, 1H), 6.89 (s, 1H), 6.84 (d, J = 7.8 Hz, 1H), 6.62 (t, J FH = 75.6 Hz, 1H), 6.08 (t, J FH = 53.4 Hz, 1H), 4.55 – 4.68 (m, 3H), 4.24 – 4.32 (m, 1H), 3.89 (d, J = 6.7 Hz, 2H), 3.63 (t, J = 11.1 Hz, 1H), 3.32 – 3.44 (m, 1H), 3.15 (s, 3H), 3.04 (s, 3H), 2.60 – 2.69 (m, 1H), 2.35 – 2.43 (m, 1H), 1.27 – 1.39 (m, 1H), 0.64 – 0.69 (m, 2H), 0.36 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 567.30 [M+H]+ .MS (ESI, pos.ion) m/z: 567.30 [M+H] + .
實施例45:化合物 6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 Example 45: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carbamoylamino)methyl ) -N , N -lutidine pyridamide
步驟1:化合物(R )-1-叔丁基 2-甲基 4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 的合成Step 1: Compound (R) -1- tert-butyl 2-methyl-4- (3- (benzyloxy) -4- (difluoromethoxy) phenyl) -1 H - pyrrole-1,2 ( Synthesis of 2H , 5H)-dicarboxylate
將化合物 (R )-1-叔丁基 2-甲基 4-(4,4,5,5-四甲基-1,3,2-二氧雜戊硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (5.6 g, 16.0 mmol),2-(苄氧基)-1-(二氟甲氧基)-4-碘苯 (861-1-2) (4.79 g, 12.7 mmol),磷酸鉀 (8.1 g, 38.0 mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (478 mg, 0.65 mmol) 混合在乾燥的1,4-二氧六環 (60 mL) 溶液中,氮氣保護下100℃反應4 h,將反應液抽濾,濾液中加入水 (50mL),用乙酸乙酯 (25 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到淡紅色液體3.6 g,收率59%。The compound (R) -1- tert-butyl 2-methyl-4- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -1 H -pyrrole-1,2( 2H , 5H )-dicarboxylate (5.6 g, 16.0 mmol), 2-(benzyloxy)-1-(difluoromethoxy)-4-iodobenzene (861 -1-2) (4.79 g, 12.7 mmol), potassium phosphate (8.1 g, 38.0 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (478 mg, 0.65 mmol) were mixed in dry 1,4-dioxane (60 mL) solution, reacted at 100 °C for 4 h under nitrogen protection, the reaction solution was suction filtered, water (50 mL) was added to the filtrate, and ethyl acetate (25 mL) was added to the filtrate. mL × 3) extraction, the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) to obtain light red Liquid 3.6 g, yield 59%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm):7.37 – 7.44 (m, 5H), 7.18 – 7.20 (m, 1H), 7.04 (s, 1H), 6.95 – 7.01 (m, 1H), 6.60 (t,J F-H = 75.0 Hz, 1H), 6.00 – 6.03 (m, 1H), 5.12 – 5.22 (m, 1H), 5.16 (d,J = 9.1 Hz, 2H), 4.54 – 4.67 (m, 2H), 3.78 (s, 3H), 1.48 – 1.55 (m, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.37 – 7.44 (m, 5H), 7.18 – 7.20 (m, 1H), 7.04 (s, 1H), 6.95 – 7.01 (m, 1H), 6.60 (t, J FH = 75.0 Hz, 1H), 6.00 – 6.03 (m, 1H), 5.12 – 5.22 (m, 1H), 5.16 (d, J = 9.1 Hz, 2H), 4.54 – 4.67 (m, 2H) , 3.78 (s, 3H), 1.48 – 1.55 (m, 9H).
MS (ESI, pos.ion) m/z: 498.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 498.20 [M+Na] + .
步驟2:化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-(羥基)苯基)吡咯烷-1,2-二羧酸酯 的合成Step 2: Compound ( 2R )-1-tert-butyl 2-methyl 4-(4-(difluoromethoxy)-3-(hydroxy)phenyl)pyrrolidine-1,2-dicarboxylate Synthesis
將化合物 (R )-1-叔丁基 2-甲基 4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (1.48 g, 3.1 mmol) 溶於甲醇 (30 mL),加入Pd/C (1.0 g, 10%),通入氫氣室溫反應24 h,過濾除去催化劑,濾液濃縮得到黃色液體860 mg,收率71%。The compound (R) -1- tert-butyl 2-methyl-4- (3- (benzyloxy) -4- (difluoromethoxy) phenyl) -1 H - pyrrole -1,2 (2 H , 5H )-dicarboxylate (1.48 g, 3.1 mmol) was dissolved in methanol (30 mL), Pd/C (1.0 g, 10%) was added, and hydrogen was passed through to react at room temperature for 24 h, the catalyst was removed by filtration, and the filtrate was Concentrated to obtain 860 mg of yellow liquid with a yield of 71%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 9.93 (s, 1H), 7.04 (d,J = 8.2 Hz, 1H), 6.99 (t,J F-H = 75.2 Hz, 1H), 6.85 (d,J = 1.8 Hz, 1H), 6.72 (d,J = 8.3 Hz, 1H), 4.26 - 4.30 (m, 1H), 3.84 - 3.88 (m, 1H), 3.69 (s, 2H), 3.66 (s, 1H), 3.11 - 3.19 (m, 2H), 2.58 - 2.65 (m, 1H), 1.77 - 1.88 (m, 1H), 1.49 (m, 3H), 1.39 (m, 6H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 9.93 (s, 1H), 7.04 (d, J = 8.2 Hz, 1H), 6.99 (t, J FH = 75.2 Hz, 1H), 6.85 (d, J = 1.8 Hz, 1H), 6.72 (d, J = 8.3 Hz, 1H), 4.26 - 4.30 (m, 1H), 3.84 - 3.88 (m, 1H), 3.69 (s, 2H), 3.66 ( s, 1H), 3.11 - 3.19 (m, 2H), 2.58 - 2.65 (m, 1H), 1.77 - 1.88 (m, 1H), 1.49 (m, 3H), 1.39 (m, 6H).
MS (ESI, pos.ion) m/z: 410.15 [M+Na]+ .MS (ESI, pos.ion) m/z: 410.15 [M+Na] + .
步驟3:化合物 (2R )-4-(4-(二氟甲氧基)-3-(羥基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 的合成Step 3: Synthesis of compound (2R )-4-(4-(difluoromethoxy)-3-(hydroxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-(羥基)苯基)吡咯烷-1,2-二羧酸酯 (860 mg, 2.2 mmol) 溶解於二氯甲烷 (3 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (3 mL),室溫攪拌1 h,除去溶劑,得到白色固體623 mg,收率97%。The compound ( 2R )-1-tert-butyl 2-methyl 4-(4-(difluoromethoxy)-3-(hydroxy)phenyl)pyrrolidine-1,2-dicarboxylate (860 mg, 2.2 mmol) was dissolved in dichloromethane (3 mL) solution, 4 mol/L HCl in ethyl acetate solution (3 mL) was added, stirred at room temperature for 1 h, and the solvent was removed to obtain a white solid 623 mg, yield 97%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 10.02 (s, 1H), 7.08 (d,J = 8.2 Hz, 1H), 7.00 (t,J F-H = 75.1 Hz, 1H), 6.92 (d,J = 1.8 Hz, 1H), 6.79 (dd,J = 8.3, 1.9 Hz, 1H), 4.49 - 4.54 (m, 1H), 3.79 (s, 3H), 3.61 - 3.66 (m, 1H), 3.42 - 3.51 (m, 1H), 3.11 - 3.17 (m, 1H), 2.64 - 2.70 (m, 1H), 1.99-2.10 (m, 1H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 10.02 (s, 1H), 7.08 (d, J = 8.2 Hz, 1H), 7.00 (t, J FH = 75.1 Hz, 1H), 6.92 (d, J = 1.8 Hz, 1H), 6.79 (dd, J = 8.3, 1.9 Hz, 1H), 4.49 - 4.54 (m, 1H), 3.79 (s, 3H), 3.61 - 3.66 (m, 1H), 3.42 - 3.51 (m, 1H), 3.11 - 3.17 (m, 1H), 2.64 - 2.70 (m, 1H), 1.99-2.10 (m, 1H).
MS (ESI, pos.ion) m/z: 288.10 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 288.10 [M+H-HCl] + .
步驟4:化合物 (2R )-1-乙醯基-4-(3-(乙醯氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸甲酯 的合成Step 4: Synthesis of compound (2R )-1-acetyl-4-(3-(acetyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester
將化合物 (2R )-4-(4-(二氟甲氧基)-3-(羥基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (269 mg, 0.93 mmol) 溶解在二氯甲烷 (6 mL) 中,加入N ,N -二異丙基乙胺 (0.6 mL, 4.0 mmol),冰浴中加入乙醯氯 (0.3 mL, 4.0 mmol),室溫攪拌19 h後停止反應,加入水 (15 mL),用二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到淺黃色液體263 mg,收率75%。Compound ( 2R )-4-(4-(difluoromethoxy)-3-(hydroxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride (269 mg, 0.93 mmol) was dissolved in In methyl chloride (6 mL), N , N -diisopropylethylamine (0.6 mL, 4.0 mmol) was added, acetyl chloride (0.3 mL, 4.0 mmol) was added in an ice bath, and the reaction was stopped after stirring at room temperature for 19 h , water (15 mL) was added, extracted with dichloromethane (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/ v) = 1/2) to obtain 263 mg of pale yellow liquid with a yield of 75%.
MS (ESI, pos.ion) m/z: 372.20 [M+H]+ .MS (ESI, pos.ion) m/z: 372.20 [M+H] + .
步驟5:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-(羥基)苯基)吡咯烷-2-甲酸的合成Step 5: Synthesis of compound (2R )-1-acetyl-4-(4-(difluoromethoxy)-3-(hydroxy)phenyl)pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(3-(乙醯氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸甲酯 (263 mg, 0.71 mmol) 溶於四氫呋喃 (5 mL) 和水 (4 mL) 的混合溶劑中,加入一水合氫氧化鋰 (172 mg, 3.1 mmol),50℃反應2 h後停止,加濃鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淡黃色固體213 mg,收率95%。Compound ( 2R )-1-acetoxy-4-(3-(acetoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester (263 mg, 0.71 mmol) was dissolved in a mixed solvent of tetrahydrofuran (5 mL) and water (4 mL), lithium hydroxide monohydrate (172 mg, 3.1 mmol) was added, the reaction was stopped after 2 h at 50 °C, and concentrated hydrochloric acid was added to adjust the pH of the solution to 1 , extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 213 mg of a pale yellow solid with a yield of 95%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 9.89 (s, 1H), 7.06 (d,J = 8.2 Hz, 1H), 6.99 (t,J F-H = 75.1 Hz, 1H), 6.85 - 6.88 (m, 1H), 6.74 - 6.76 (m, 1H), 4.19 - 4.24 (m, 1H), 4.01 - 4.06 (m, 1H), 3.27 - 3.42 (m, 2H), 2.59 - 2.67 (m, 1H), 2.00 (s, 3H), 1.75 - 1.83 (m, 1H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 9.89 (s, 1H), 7.06 (d, J = 8.2 Hz, 1H), 6.99 (t, J FH = 75.1 Hz, 1H), 6.85 - 6.88 (m, 1H), 6.74 - 6.76 (m, 1H), 4.19 - 4.24 (m, 1H), 4.01 - 4.06 (m, 1H), 3.27 - 3.42 (m, 2H), 2.59 - 2.67 (m, 1H), 2.00 (s, 3H), 1.75 - 1.83 (m, 1H).
MS (ESI, pos.ion) m/z: 316.10 [M+H]+ .MS (ESI, pos.ion) m/z: 316.10 [M+H] + .
步驟6:化合物 6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺的合成Step 6: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carbamoylamino)methyl) - Synthesis of N , N -lutidyl pyridamide
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲酸 (72 mg, 0.23 mmol),6-(氨基甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 (94 mg, 0.52 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (88 mg, 0.46 mmol) 和N -羥基-7-氮雜苯並三氮唑 (61 mg, 0.45 mmol) 溶於二氯甲烷 (6 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌4 h,加水洗 (50 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體23 mg,收率21%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carboxylic acid (72 mg, 0.23 mmol), 6-(amino Methyl) -N , N -lutidine dihydrochloride (94 mg, 0.52 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 88 mg, 0.46 mmol) and N -hydroxy-7-azabenzotriazole (61 mg, 0.45 mmol) were dissolved in dichloromethane (6 mL), and N was added dropwise to this solution at 0°C, N -diisopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 4 h, washed with water (50 mL), and then extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, removed The solvent and the concentrated solution were separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 23 mg of white solid with a yield of 21%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 9.88 (s, 1H), 8.61 (t,J = 5.9 Hz, 1H), 7.86 (t,J = 7.7 Hz, 1H), 7.46 (d,J = 7.9 Hz, 1H), 7.32 - 7.40 (m, 1H), 7.03 - 7.07 (m, 1H), 6.99 (t,J F-H = 75.1 Hz, 1H), 6.86 - 6.89 (m, 1H), 6.72 - 6.77 (m, 1H), 4.32 - 4.50 (m, 3H), 4.02 - 4.05 (m, 1H), 3.38 - 3.46 (m, 2H), 2.96 - 2.98 (m, 3H), 2.88 - 2.89 (m, 3H), 2.51 - 2.62 (m, 1H), 2.02 (s, 3H), 1.82 - 1.87 (m, 1H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 9.88 (s, 1H), 8.61 (t, J = 5.9 Hz, 1H), 7.86 (t, J = 7.7 Hz, 1H), 7.46 ( d, J = 7.9 Hz, 1H), 7.32 - 7.40 (m, 1H), 7.03 - 7.07 (m, 1H), 6.99 (t, J FH = 75.1 Hz, 1H), 6.86 - 6.89 (m, 1H), 6.72 - 6.77 (m, 1H), 4.32 - 4.50 (m, 3H), 4.02 - 4.05 (m, 1H), 3.38 - 3.46 (m, 2H), 2.96 - 2.98 (m, 3H), 2.88 - 2.89 (m , 3H), 2.51 - 2.62 (m, 1H), 2.02 (s, 3H), 1.82 - 1.87 (m, 1H).
MS (ESI, pos.ion) m/z: 477.25 [M+H]+ .MS (ESI, pos.ion) m/z: 477.25 [M+H] + .
實施例46:化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 Example 46: Compound 6-((( 2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl) - N , N -lutidine pyridamide
步驟1:化合物2-(二氟甲氧基)-5-碘苯酚的合成Step 1: Synthesis of compound 2-(difluoromethoxy)-5-iodophenol
將化合物2-(環丙基甲氧基)-1-(二氟甲氧基)-4-碘苯 (5.0 g, 14.71 mmol) 溶於乙腈 (25 mL) 和濃鹽酸 (10 mL) 的混合溶劑中,80℃反應6 h後停止,減壓濃縮,加水 (10 mL),用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,經矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 10/1),得到淺黃色液體3.23 g,產率76%。Compound 2-(cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene (5.0 g, 14.71 mmol) was dissolved in a mixture of acetonitrile (25 mL) and concentrated hydrochloric acid (10 mL) In the solvent, the reaction was stopped at 80 °C for 6 h, concentrated under reduced pressure, added water (10 mL), extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and subjected to silica gel column chromatography Separation (eluent: petroleum ether/ethyl acetate (v/v) = 10/1) gave a pale yellow liquid 3.23 g in 76% yield.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.39 (d,J = 2.0 Hz, 1H), 7.23 (dd,J = 8.5, 2.0 Hz, 1H), 6.87 (d,J = 8.5 Hz, 1H), 6.53 (t,J F-H = 73.2 Hz, 1H), 5.72 (br.s, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.39 (d, J = 2.0 Hz, 1H), 7.23 (dd, J = 8.5, 2.0 Hz, 1H), 6.87 (d, J = 8.5 Hz, 1H), 6.53 (t, J FH = 73.2 Hz, 1H), 5.72 (br.s, 1H).
MS (ESI, pos.ion) m/z: 285.90 [M].MS (ESI, pos.ion) m/z: 285.90 [M].
步驟2:化合物1,2-雙(二氟甲氧基)-4-碘苯的合成Step 2: Synthesis of compound 1,2-bis(difluoromethoxy)-4-iodobenzene
將化合物2-(二氟甲氧基)-5-碘苯酚 (770 mg, 2.69 mmol) 溶於N ,N -二甲基甲醯胺 (10 mL),加入二氟氯乙酸鈉 (733 mg, 4.81 mmol) 和碳酸銫 (1.6 g, 4.90 mmol),120℃反應4 h後停止,加水 (25 mL),用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,濃縮得到淺黃色液體831 mg,產率91%。Compound 2-(difluoromethoxy)-5-iodophenol (770 mg, 2.69 mmol) was dissolved in N , N -dimethylformamide (10 mL), and sodium difluorochloroacetate (733 mg, 10 mL) was added. 4.81 mmol) and cesium carbonate (1.6 g, 4.90 mmol), the reaction was stopped after 4 h at 120 °C, water (25 mL) was added, extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and concentrated to obtain Light yellow liquid 831 mg, yield 91%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.61 (s, 1H), 7.58 (dd,J = 8.5, 2.0 Hz, 1H), 7.03 (d,J = 8.5 Hz, 1H), 6.53 (t,J F-H = 73.1 Hz, 1H), 6.52 (t,J F-H = 73.1 Hz, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.61 (s, 1H), 7.58 (dd, J = 8.5, 2.0 Hz, 1H), 7.03 (d, J = 8.5 Hz, 1H), 6.53 ( t, J FH = 73.1 Hz, 1H), 6.52 (t, J FH = 73.1 Hz, 1H).
MS (ESI, pos.ion) m/z: 335.90 [M].MS (ESI, pos.ion) m/z: 335.90 [M].
步驟3:化合物 (R )-1-叔丁基 2-甲基 4-(3,4-雙(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 3: Compound (R) -1- tert-butyl 2-methyl-4- (3,4-bis (difluoromethoxy) phenyl) -1 H - pyrrole -1,2 (2 H, 5 H )-Dicarboxylate Synthesis
將(R )-1-叔丁基 2-甲基 4-(4,4,5,5-四甲基-1,3,2-二氧雜戊硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (800 mg, 2.27 mmol),1,2-雙(二氟甲氧基)-4-碘苯 (868-1) (790 mg, 2.35 mmol),磷酸鉀 (2.0 g, 9.4 mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (35 mg, 0.05 mmol) 混合在乾燥的1,4-二氧六環 (10mL) 溶液中,氮氣保護下100℃反應2.5h,將反應液抽濾,濾液中加入水 (20 mL),用乙酸乙酯 (10 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 6/1),得到765 mg褐色液體,產率77%。The (R) -1- tert-butyl 2-methyl-4- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -1 H - Pyrrole-1,2( 2H , 5H )-dicarboxylate (800 mg, 2.27 mmol), 1,2-bis(difluoromethoxy)-4-iodobenzene (868-1) (790 mg , 2.35 mmol), potassium phosphate (2.0 g, 9.4 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]dichloride palladium (35 mg, 0.05 mmol) were mixed in dry 1, 4-dioxane (10 mL) solution was reacted at 100 °C for 2.5 h under nitrogen protection, the reaction solution was suction filtered, water (20 mL) was added to the filtrate, extracted with ethyl acetate (10 mL × 3), and the organic phases were combined. It was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 6/1) to obtain 765 mg of brown liquid with a yield of 77%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.25 – 7.31 (m, 3H), 6.56 (t,J F-H = 73.4 Hz, 1H), 6.55 (t,J F-H = 73.4 Hz, 1H), 6.07-6.12 (m, 1H), 5.14-5.23 (m, 1H), 4.49-4.68 (m, 2H), 3.78-3.79 (m, 3H), 1.48 – 1.54 (m, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.25 – 7.31 (m, 3H), 6.56 (t, J FH = 73.4 Hz, 1H), 6.55 (t, J FH = 73.4 Hz, 1H), 6.07-6.12 (m, 1H), 5.14-5.23 (m, 1H), 4.49-4.68 (m, 2H), 3.78-3.79 (m, 3H), 1.48 – 1.54 (m, 9H).
MS (ESI, pos.ion) m/z: 458.05 [M+Na]+ .MS (ESI, pos.ion) m/z: 458.05 [M+Na] + .
步驟4:化合物 (2R )-1-叔丁基 2-甲基 4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯的合成Step 4: Synthesis of compound (2R )-1-tert-butyl 2-methyl 4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物 (R )-1-叔丁基 2-甲基 4-(3,4-雙(二氟甲氧基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (755 mg, 1.74 mmol) 溶於甲醇 (6 mL),加入Pd/C (76 mg, 10%),通入氫氣室溫反應19 h,將反應液抽濾,濾液濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到無色液體653 mg,產率86%。The compound (R) -1- tert-butyl 2-methyl-4- (3,4-bis (difluoromethoxy) phenyl) -1 H - pyrrole -1,2 (2 H, 5 H) - Dicarboxylate (755 mg, 1.74 mmol) was dissolved in methanol (6 mL), Pd/C (76 mg, 10%) was added, hydrogen was passed through it, and the reaction was carried out at room temperature for 19 h. The reaction solution was suction filtered, and the filtrate was concentrated. Silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) gave 653 mg of a colorless liquid with a yield of 86%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.24 (d,J = 8.3 Hz, 1H), 7.12-7.16 (m, 2H), 6.54 (t,J F-H = 73.5 Hz, 1H), 6.52 (t,J F-H = 73.5 Hz, 1H), 4.34-4.44 (m, 1H), 3.96-4.10 (m, 1H), 3.78 – 3.79 (m, 3H), 3.34-3.46 (m, 2H), 2.67-2.73 (m, 1H), 2.01-2.09 (m, 1H), 1.45 – 1.49 (m, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.24 (d, J = 8.3 Hz, 1H), 7.12-7.16 (m, 2H), 6.54 (t, J FH = 73.5 Hz, 1H), 6.52 (t, J FH = 73.5 Hz, 1H), 4.34-4.44 (m, 1H), 3.96-4.10 (m, 1H), 3.78 – 3.79 (m, 3H), 3.34-3.46 (m, 2H), 2.67- 2.73 (m, 1H), 2.01-2.09 (m, 1H), 1.45 – 1.49 (m, 9H).
MS (ESI, pos.ion) m/z: 460.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 460.20 [M+Na] + .
步驟5:化合物 (2R )-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽的合成Step 5: Synthesis of Compound (2R )-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物 (2R )-1-叔丁基 2-甲基 4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯 (650 mg, 1.49 mmol) 溶解於二氯甲烷 (4 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (6 mL),室溫攪拌1.5 h,除去溶劑,得到淺黃色液體552 mg,收率98%。Compound ( 2R )-1-tert-butyl 2-methyl 4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (650 mg, 1.49 mmol) was dissolved in dichloromethane (4 mL) solution, 4 mol/L HCl in ethyl acetate solution (6 mL) was added, stirred at room temperature for 1.5 h, and the solvent was removed to obtain 552 mg of pale yellow liquid, yield 98% .
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.28-7.34 (m, 3H), 6.86 (t,J F-H = 73.6 Hz, 1H), 4.62 – 4.64 (m, 1H), 3.90 (s, 3H), 3.81-3.87 (m, 1H), 3.69-3.77 (m, 1H), 3.37 (d,J = 11.0 Hz, 1H), 2.85-2.92 (m, 1H), 2.20-2.29 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.28-7.34 (m, 3H), 6.86 (t, J FH = 73.6 Hz, 1H), 4.62 – 4.64 (m, 1H), 3.90 (s , 3H), 3.81-3.87 (m, 1H), 3.69-3.77 (m, 1H), 3.37 (d, J = 11.0 Hz, 1H), 2.85-2.92 (m, 1H), 2.20-2.29 (m, 1H) ).
MS (ESI, pos.ion) m/z: 338.15 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 338.15 [M+H-HCl] + .
步驟6:化合物 (2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯的合成Step 6: Synthesis of compound (2R )-1-acetoxy-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester
將化合物 (2R )-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (550 mg, 1.48 mmol) 溶解在二氯甲烷 (8 mL) 中,加入N ,N -二異丙基乙胺 (0.95 mL, 5.70 mmol),0℃條件下加入乙醯氯 (0.35 mL, 4.90 mmol),室溫攪拌4 h後停止反應,加水 (20 mL) 攪拌,二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/4),得到淺黃色液體586 mg,產率95%。Compound ( 2R )-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride (550 mg, 1.48 mmol) was dissolved in dichloromethane ( 8 mL), N , N -diisopropylethylamine (0.95 mL, 5.70 mmol) was added, acetyl chloride (0.35 mL, 4.90 mmol) was added at 0 °C, the reaction was stopped after stirring at room temperature for 4 h, and water was added. (20 mL) stirred, extracted with dichloromethane (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/4) to obtain 586 mg of pale yellow liquid with a yield of 95%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.24-7.314 (m, 3H), 6.85 (t,J F-H = 73.7 Hz, 1H), 6.81 (t,J F-H = 73.7 Hz, 1H), 4.46 – 4.51 (m, 1H), 4.09-4.15 (m, 1H), 3.76 (s, 3H), 3.62 (t,J = 8.2 Hz, 1H), 3.57-3.61 (m, 1H), 2.72-2.77 (m, 1H), 2.14 (s, 3H), 2.01-2.07 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.24-7.314 (m, 3H), 6.85 (t, J FH = 73.7 Hz, 1H), 6.81 (t, J FH = 73.7 Hz, 1H) , 4.46 – 4.51 (m, 1H), 4.09-4.15 (m, 1H), 3.76 (s, 3H), 3.62 (t, J = 8.2 Hz, 1H), 3.57-3.61 (m, 1H), 2.72-2.77 (m, 1H), 2.14 (s, 3H), 2.01-2.07 (m, 1H).
MS (ESI, pos.ion) m/z: 380.10 [M+H]+ .MS (ESI, pos.ion) m/z: 380.10 [M+H] + .
步驟7:化合物 (2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-羧酸的合成Step 7: Synthesis of Compound (2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯 (530 mg, 1.40 mmol) 溶於四氫呋喃 (8 mL) 和水 (5 mL) 的混合溶劑中,加入一水合氫氧化鋰 (203 mg, 4.8 mmol),50℃反應2 h後停止,加鹽酸調節溶液pH=6,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體552 mg,收率92%。Compound ( 2R )-1-acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester (530 mg, 1.40 mmol) was dissolved in tetrahydrofuran (8 mL) and water (5 mL) in a mixed solvent, lithium hydroxide monohydrate (203 mg, 4.8 mmol) was added, the reaction was stopped after 2 h at 50 °C, and the pH of the solution was adjusted to 6 by adding hydrochloric acid, and extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 552 mg of a white solid with a yield of 92%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.25 – 7.31 (m, 3H), 6.85 (t,J F-H = 73.7 Hz, 1H), 6.81 (t,J F-H = 73.7 Hz, 1H), 4.44 – 4.49 (m, 1H), 4.11-4.15 (m, 1H), 3.54 – 3.64 (m, 2H), 3.54-3.60 (m, 1H), 2.74-2.81 (m, 1H), 2.14 (s, 3H), 2.00-2.08 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.25 – 7.31 (m, 3H), 6.85 (t, J FH = 73.7 Hz, 1H), 6.81 (t, J FH = 73.7 Hz, 1H) , 4.44 – 4.49 (m, 1H), 4.11-4.15 (m, 1H), 3.54 – 3.64 (m, 2H), 3.54-3.60 (m, 1H), 2.74-2.81 (m, 1H), 2.14 (s, 3H), 2.00-2.08 (m, 1H).
MS (ESI, pos.ion) m/z: 366.15 [M+H]+ .MS (ESI, pos.ion) m/z: 366.15 [M+H] + .
步驟8:化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸乙酯的合成Step 8: Compound 6-((( 2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)pyridine Synthesis of Ethyl Formate
將化合物 (2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-羧酸 (500 mg, 1.37 mmol),6-(氨基甲基)吡啶甲酸乙酯鹽酸鹽 (415 mg, 1.64 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (1.3 g, 6.80 mmol) 和N -羥基-7-氮雜苯並三氮唑 (280 mg, 2.06 mmol) 溶於二氯甲烷 (15 mL) 中,0℃條件下滴加N ,N -二異丙基乙胺 (1.4 mL, 8.5 mmol),室溫反應16 h,加水洗 (10 mL × 3) 有機相,有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到淺黃色固體482 mg,收率66%。Compound ( 2R )-1-acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (500 mg, 1.37 mmol), 6-(amino Methyl)picolinate hydrochloride (415 mg, 1.64 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.3 g, 6.80 mmol) and N -Hydroxy-7-azabenzotriazole (280 mg, 2.06 mmol) was dissolved in dichloromethane (15 mL), and N , N -diisopropylethylamine (1.4 mL, 8.5 mmol), react at room temperature for 16 h, add water to wash (10 mL × 3) the organic phase, dry the organic phase with anhydrous sodium sulfate, remove the solvent, and separate the concentrated solution by silica gel column chromatography (eluent: dichloromethane/methanol). (v/v)=40/1), 482 mg of light yellow solid was obtained, and the yield was 66%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.02 (d,J = 7.6 Hz, 1H), 7.83 (t,J = 7.8 Hz, 1H), 7.55 (d,J = 7.8 Hz, 1H), 7.48 (br.s, 1H), 7.14 – 7.24 (m, 3H), 6.55 (t,J F-H = 73.5 Hz, 1H), 6.52 (t,J F-H = 73.5 Hz, 1H), 4.61 – 4.76 (m, 3H), 4.47 (q= 7.1 Hz, 2H), 3.99 – 4.04 (m, 1H), 3.62 (t,J = 10.6 Hz, 1H), 3.38 – 3.45 (m, 1H), 2.61 – 2.68 (m, 1H), 2.42 – 2.50 (m, 1H), 2.18 (s, 3H), 1.44 (t,J = 7.1 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.02 (d, J = 7.6 Hz, 1H), 7.83 (t, J = 7.8 Hz, 1H), 7.55 (d, J = 7.8 Hz, 1H) , 7.48 (br.s, 1H), 7.14 – 7.24 (m, 3H), 6.55 (t, J FH = 73.5 Hz, 1H), 6.52 (t, J FH = 73.5 Hz, 1H), 4.61 – 4.76 (m , 3H), 4.47 (q= 7.1 Hz, 2H), 3.99 – 4.04 (m, 1H), 3.62 (t, J = 10.6 Hz, 1H), 3.38 – 3.45 (m, 1H), 2.61 – 2.68 (m, 1H), 2.42 – 2.50 (m, 1H), 2.18 (s, 3H), 1.44 (t, J = 7.1 Hz, 3H).
MS (ESI, pos.ion) m/z: 528.15 [M+H]+ .MS (ESI, pos.ion) m/z: 528.15 [M+H] + .
步驟9:化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 的合成Step 9: Compound 6-((( 2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)pyridine Synthesis of Formic Acid
將化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸乙酯 (470 mg, 0.39 mmol) 溶於四氫呋喃 (8 mL) 和水 (5 mL) 的混合溶劑中,加入一水合氫氧化鋰 (183 mg, 4.36 mmol),50℃反應2 h後停止,加稀鹽酸調節溶液pH=6,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體426 mg,收率95%。The compound ethyl 6-((( 2R )-1-acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinate The ester (470 mg, 0.39 mmol) was dissolved in a mixed solvent of tetrahydrofuran (8 mL) and water (5 mL), lithium hydroxide monohydrate (183 mg, 4.36 mmol) was added, the reaction was stopped after 2 h at 50 °C, and the dilution was added. The pH of the solution was adjusted to 6 with hydrochloric acid, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 426 mg of a white solid with a yield of 95%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.06 (d,J = 7.5 Hz, 1H), 7.99 (t,J = 7.7 Hz, 1H), 7.75 (d,J = 7.7 Hz, 1H), 7.26 – 7.32 (m, 3H), 6.85 (t,J F-H = 73.8 Hz, 1H), 6.81 (t,J F-H = 73.8 Hz, 1H), 4.53 – 4.73 (m, 3H), 4.11 – 4.17 (m, 1H), 3.66 (t,J = 10.9 Hz, 1H), 3.55 – 3.60 (m, 1H), 2.70 – 2.76 (m, 1H), 2.16 (s, 3H), 2.06 – 2.15 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.06 (d, J = 7.5 Hz, 1H), 7.99 (t, J = 7.7 Hz, 1H), 7.75 (d, J = 7.7 Hz, 1H) ), 7.26 – 7.32 (m, 3H), 6.85 (t, J FH = 73.8 Hz, 1H), 6.81 (t, J FH = 73.8 Hz, 1H), 4.53 – 4.73 (m, 3H), 4.11 – 4.17 ( m, 1H), 3.66 (t, J = 10.9 Hz, 1H), 3.55 – 3.60 (m, 1H), 2.70 – 2.76 (m, 1H), 2.16 (s, 3H), 2.06 – 2.15 (m, 1H) .
MS (ESI, pos.ion) m/z: 500.10 [M+H]+ .MS (ESI, pos.ion) m/z: 500.10 [M+H] + .
步驟10:化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 的合成Step 10: Compound 6-((( 2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)- Synthesis of N , N -lutidyl pyridamide
將化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (80 mg, 0.16 mmol),二甲胺鹽酸鹽 (66 mg, 0.81 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (151 mg, 0.79 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.27 mL, 1.60 mmol),室溫反應10 h,加水洗 (15 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體52 mg,收率61%。The compound 6-((( 2R )-1-acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinic acid ( 80 mg, 0.16 mmol), dimethylamine hydrochloride (66 mg, 0.81 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (151 mg, 0.79 mmol) ) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL), and N , N -diisopropyl was added dropwise to this solution at 0°C Ethylamine (0.27 mL, 1.60 mmol), reacted at room temperature for 10 h, washed with water (15 mL), then extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was carried out Separation by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) gave 52 mg of white solid with a yield of 61%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.77 (t,J = 7.7 Hz, 1H), 7.56 (br.s, 1H), 7.47 (d,J = 7.5 Hz, 1H), 7.38 (d,J = 7.8 Hz, 1H), 7.13 – 7.25 (m, 3H), 6.56 (t,J F-H = 73.5 Hz, 1H), 6.53 (t,J F-H = 73.5 Hz, 1H), 4.51 – 4.67 (m, 3H), 3.99 (dd,J = 9.9, 7.6 Hz, 1H), 3.58 (t,J = 10.6 Hz, 1H), 3.34 – 3.43 (m, 1H), 3.14 (s, 3H), 3.06 (s, 3H), 2.58 – 2.65 (m, 1H), 2.42 – 2.50 (m, 1H), 2.15 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.77 (t, J = 7.7 Hz, 1H), 7.56 (br.s, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.38 ( d, J = 7.8 Hz, 1H), 7.13 – 7.25 (m, 3H), 6.56 (t, J FH = 73.5 Hz, 1H), 6.53 (t, J FH = 73.5 Hz, 1H), 4.51 – 4.67 (m , 3H), 3.99 (dd, J = 9.9, 7.6 Hz, 1H), 3.58 (t, J = 10.6 Hz, 1H), 3.34 – 3.43 (m, 1H), 3.14 (s, 3H), 3.06 (s, 3H), 2.58 – 2.65 (m, 1H), 2.42 – 2.50 (m, 1H), 2.15 (s, 3H).
13 C NMR (100 MHz, CDCl3 ) δ (ppm):171.3, 169.8, 168.8, 156.0, 153.9, 142.4, 141.3, 138.51, 137.7, 125.3, 122.6, 122.2, 121.6, 118.3, 115.7, 113.1, 59.9, 54.7, 44.7, 43.3, 39.0, 35.6, 35.5, 22.6. 13 C NMR (100 MHz, CDCl 3 ) δ (ppm): 171.3, 169.8, 168.8, 156.0, 153.9, 142.4, 141.3, 138.51, 137.7, 125.3, 122.6, 122.2, 121.6, 118.3, 115. , 44.7, 43.3, 39.0, 35.6, 35.5, 22.6.
MS (ESI, pos.ion) m/z: 527.15 [M+H]+ .MS (ESI, pos.ion) m/z: 527.15 [M+H] + .
實施例47:化合物 6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 Example 47: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-methyl Acrylamido)methyl) -N , N -lutidyl pyridamide
步驟1:化合物 (2R )-1-叔丁基 2-甲基 4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯的合成Step 1: Compound ( 2R )-1-tert-butyl 2-methyl 4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-di Synthesis of Carboxylic Acid Esters
將化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-1,2-二羧酸酯 (487 mg, 1.25 mmol),碳酸鉀 (364 mg, 2.63 mmol) 溶於N ,N -二甲基甲醯胺 (10 mL) 中,室溫下向此溶液中加入環戊基溴 (303 mg, 2.03 mmol),70℃下反應5 h,加水洗 (50 mL),用乙酸乙酯萃取 (15 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到淡黃色液體273 mg,收率47%。Compound ( 2R )-1-tert-butyl 2-methyl 4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-1,2-dicarboxylate (487 mg, 1.25 mmol), potassium carbonate (364 mg, 2.63 mmol) was dissolved in N , N -dimethylformamide (10 mL), to this solution was added cyclopentyl bromide (303 mg, 2.03 mmol) at room temperature , reacted at 70 °C for 5 h, washed with water (50 mL), extracted with ethyl acetate (15 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent). : petroleum ether/ethyl acetate (v/v) = 2/1) to obtain 273 mg of pale yellow liquid with a yield of 47%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.09 (d,J = 8.2 Hz, 1H), 7.04 (s, 1H), 6.94 (t,J F-H = 74.8 Hz, 1H), 6.84 (d,J = 8.3 Hz, 1H), 4.91-4.94 (m, 1H), 4.27-4.31 (m, 1H), 3.86-3.93 (m, 1H), 3.69 (s, 2H), 3.66 (s, 1H), 3.37-3.45 (m, 1H), 3.18-3.267 (m, 1H), 2.60-2.68 (m, 1H), 1.85-1.93 (m, 3H), 1.66-1.76 (m, 4H), 1.55-1.61 (m, 2H), 1.35-1.40 (m, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.09 (d, J = 8.2 Hz, 1H), 7.04 (s, 1H), 6.94 (t, J FH = 74.8 Hz, 1H), 6.84 (d, J = 8.3 Hz, 1H), 4.91-4.94 (m, 1H), 4.27-4.31 (m, 1H), 3.86-3.93 (m, 1H), 3.69 (s, 2H), 3.66 (s, 1H) ), 3.37-3.45 (m, 1H), 3.18-3.267 (m, 1H), 2.60-2.68 (m, 1H), 1.85-1.93 (m, 3H), 1.66-1.76 (m, 4H), 1.55-1.61 (m, 2H), 1.35-1.40 (m, 9H).
MS (ESI, pos.ion) m/z: 478.30 [M+Na]+ .MS (ESI, pos.ion) m/z: 478.30 [M+Na] + .
步驟2:化合物 (2R )-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 的合成Step 2: Synthesis of Compound (2R )-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物 (2R )-1-叔丁基 2-甲基 4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯 (412 mg, 0.88 mmol) 溶解於二氯甲烷 (4 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (3 mL),室溫攪拌1 h,除去溶劑,得到無色液體269 mg,收率76%。The compound ( 2R )-1-tert-butyl 2-methyl 4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylic acid The ester (412 mg, 0.88 mmol) was dissolved in dichloromethane (4 mL) solution, 4 mol/L HCl in ethyl acetate solution (3 mL) was added, stirred at room temperature for 1 h, and the solvent was removed to obtain 269 mg of a colorless liquid , the yield is 76%.
MS (ESI, pos.ion) m/z: 356.20 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 356.20 [M+H-HCl] + .
步驟3:化合物 (2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯的合成Step 3: Synthesis of compound (2R )-1-acetoxy-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester
將化合物 (2R )-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (269 mg, 0.76 mmol) 溶解在二氯甲烷 (6 mL) 中,加入N ,N -二異丙基乙胺 (0.5 mL, 3.0 mmol),0℃條件下滴加乙醯氯 (0.2 mL, 3.0 mmol),室溫攪拌4 h後停止反應,加入50 mL水洗,用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到淺黃色液體213 mg,收率70%。Compound ( 2R )-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride (269 mg, 0.76 mmol) It was dissolved in dichloromethane (6 mL), N , N -diisopropylethylamine (0.5 mL, 3.0 mmol) was added, acetyl chloride (0.2 mL, 3.0 mmol) was added dropwise at 0°C, and the mixture was stirred at room temperature The reaction was stopped after 4 h, washed with 50 mL of water, extracted with dichloromethane (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate). (v/v) = 1/2), 213 mg of light yellow liquid was obtained, and the yield was 70%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.11 (d,J = 8.3 Hz, 1H), 7.04 - 7.08 (m, 1H), 6.94 (t,J F-H = 74.8 Hz, 1H), 6.87 (d,J = 8.3, 1.7 Hz, 1H), 4.91 - 4.94 (m, 1H), 4.29 - 4.34 (m, 1H), 4.06 - 4.09 (m, 1H), 3.63 (s, 3H), 3.41 - 3.49 (m, 2H), 2.61 - 2.68 (m, 1H), 2.01 (s, 3H), 1.83 - 1.91 (m, 3H), 1.68 - 1.77 (m, 4H), 1.54 - 1.62 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.11 (d, J = 8.3 Hz, 1H), 7.04 - 7.08 (m, 1H), 6.94 (t, J FH = 74.8 Hz, 1H) , 6.87 (d, J = 8.3, 1.7 Hz, 1H), 4.91 - 4.94 (m, 1H), 4.29 - 4.34 (m, 1H), 4.06 - 4.09 (m, 1H), 3.63 (s, 3H), 3.41 - 3.49 (m, 2H), 2.61 - 2.68 (m, 1H), 2.01 (s, 3H), 1.83 - 1.91 (m, 3H), 1.68 - 1.77 (m, 4H), 1.54 - 1.62 (m, 2H) .
MS (ESI, pos.ion) m/z: 398.25 [M+H]+ .MS (ESI, pos.ion) m/z: 398.25 [M+H] + .
步驟4:化合物 (2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸的合成Step 4: Synthesis of Compound (2R )-1-Acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯 (213 mg, 0.54 mmol) 溶於四氫呋喃 (6 mL) 和水 (3 mL) 的混合溶劑中,加入一水合氫氧化鋰 (113 mg, 2.69 mmol),50℃反應2 h後停止,加鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淡黃色液體197 mg,收率96%。The compound ( 2R )-1-acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate methyl ester (213 mg, 0.54 mmol) was dissolved in a mixed solvent of tetrahydrofuran (6 mL) and water (3 mL), lithium hydroxide monohydrate (113 mg, 2.69 mmol) was added, the reaction was stopped at 50 °C for 2 h, and hydrochloric acid was added to adjust the pH of the solution to 1 , extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 197 mg of pale yellow liquid, with a yield of 96%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.11 (d,J = 8.3 Hz, 1H), 7.04 - 7.08 (m, 1H), 6.95 (t,J F-H = 74.8 Hz, 1H), 6.87 (d,J = 8.3, 1.7 Hz, 1H), 4.91 - 4.94 (m, 1H), 4.21 - 4.25 (m, 1H), 4.03 - 4.10 (m, 1H), 3.40 - 3.49 (m, 2H), 2.63 - 2.69 (m, 1H), 2.01 (s, 3H), 1.82 - 1.95 (m, 3H), 1.67 - 1.78 (m, 4H), 1.53 - 1.63 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.11 (d, J = 8.3 Hz, 1H), 7.04 - 7.08 (m, 1H), 6.95 (t, J FH = 74.8 Hz, 1H) , 6.87 (d, J = 8.3, 1.7 Hz, 1H), 4.91 - 4.94 (m, 1H), 4.21 - 4.25 (m, 1H), 4.03 - 4.10 (m, 1H), 3.40 - 3.49 (m, 2H) , 2.63 - 2.69 (m, 1H), 2.01 (s, 3H), 1.82 - 1.95 (m, 3H), 1.67 - 1.78 (m, 4H), 1.53 - 1.63 (m, 2H).
MS (ESI, pos.ion) m/z: 384.10 [M+H]+ .MS (ESI, pos.ion) m/z: 384.10 [M+H] + .
步驟5:化合物6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸乙酯 的合成Step 5: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate The synthesis of amino) methyl) picolinate ethyl ester
將化合物 (2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (197 mg, 0.51 mmol),6-(氨基甲基)吡啶甲酸乙酯鹽酸鹽 (164 mg, 0.76 mmol),1-乙基-(3-二甲氨基丙基)碳醯二亞胺鹽酸鹽 (213 mg, 1.11 mmol) 和N -羥基-7-氮雜苯並三氮唑 (106 mg, 0.78 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.6 mL, 4.0 mmol),室溫攪拌7 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到淡黃色固體231 mg,收率82%。Compound ( 2R )-1-acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (197 mg, 0.51 mmol) ), ethyl 6-(aminomethyl)picolinate hydrochloride (164 mg, 0.76 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (213 mg, 1.11 mmol) and N -hydroxy-7-azabenzotriazole (106 mg, 0.78 mmol) were dissolved in dichloromethane (6 mL), to this solution was added dropwise N , N -diiso Propylethylamine (0.6 mL, 4.0 mmol), stirred at room temperature for 7 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution Silica gel column chromatography was performed (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 231 mg of a pale yellow solid with a yield of 82%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.65-8.68 (m, 1H), 7.90 - 7.98 (m, 2H), 7.67 (d,J = 7.3 Hz, 1H), 7.11 (d,J = 8.3 Hz, 1H), 7.04 - 7.06 (m, 1H), 6.95 (t,J F-H = 74.8 Hz, 1H), 6.87 - 6.89 (m, 1H), 4.85 - 4.90 (m, 1H), 4.29 - 4.52 (m, 5H), 4.04 - 4.10 (m, 1H), 3.44 - 3.56 (m, 2H), 2.60 - 2.67 (m, 1H), 2.04 (s, 3H), 1.85 - 1.94 (m, 3H), 1.66 -1.78 (m, 4H), 1.52 - 1.62 (m, 2H), 1.31 (t,J = 7.1 Hz, 3H) 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.65-8.68 (m, 1H), 7.90 - 7.98 (m, 2H), 7.67 (d, J = 7.3 Hz, 1H), 7.11 (d , J = 8.3 Hz, 1H), 7.04 - 7.06 (m, 1H), 6.95 (t, J FH = 74.8 Hz, 1H), 6.87 - 6.89 (m, 1H), 4.85 - 4.90 (m, 1H), 4.29 - 4.52 (m, 5H), 4.04 - 4.10 (m, 1H), 3.44 - 3.56 (m, 2H), 2.60 - 2.67 (m, 1H), 2.04 (s, 3H), 1.85 - 1.94 (m, 3H) , 1.66 -1.78 (m, 4H), 1.52 - 1.62 (m, 2H), 1.31 (t, J = 7.1 Hz, 3H)
MS (ESI, pos.ion) m/z: 546.30 [M+H]+ .MS (ESI, pos.ion) m/z: 546.30 [M+H] + .
步驟6:化合物6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 的合成Step 6: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Synthesis of Amino)methyl)picolinic acid
將化合物 6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸乙酯 (231 mg, 0.42 mmol) 溶於四氫呋喃 (6 mL) 和水 (4 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (96 mg, 1.71 mmol),50℃反應2 h後停止,加鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淡黃色固體215 mg,收率98%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino) Methyl)picolinate (231 mg, 0.42 mmol) was dissolved in a mixed solvent of tetrahydrofuran (6 mL) and water (4 mL), and then lithium hydroxide monohydrate (96 mg, 1.71 mmol) was added, and the reaction was carried out at 50 °C After 2 h, the solution was stopped, and hydrochloric acid was added to adjust the pH of the solution to 1. The solution was extracted with ethyl acetate (5 mL × 3). The organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 215 mg of a pale yellow solid with a yield of 98%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.67 (t,J = 6.0 Hz, 1H), 7.90 - 7.96 (m, 2H), 7.65 (d,J = 7.3 Hz, 1H), 7.12 (d,J = 8.4 Hz, 1H), 7.08 - 7.24 (m, 1H), 6.95 (t,J F-H = 74.8 Hz, 1H), 6.87-6.90 (m, 1H), 4.88 - 4.92 (m, 1H), 4.35 - 4.53 (m, 3H), 4.08 - 4.10 (m, 1H), 3.43 - 3.52 (m, 2H), 2.60 - 2.67 (m, 1H), 2.04 (s, 3H), 1.85 - 1.94 (m, 3H), 1.67 - 1.77 (m, 4H), 1.54 - 1.63 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.67 (t, J = 6.0 Hz, 1H), 7.90 - 7.96 (m, 2H), 7.65 (d, J = 7.3 Hz, 1H), 7.12 (d, J = 8.4 Hz, 1H), 7.08 - 7.24 (m, 1H), 6.95 (t, J FH = 74.8 Hz, 1H), 6.87-6.90 (m, 1H), 4.88 - 4.92 (m, 1H) ), 4.35 - 4.53 (m, 3H), 4.08 - 4.10 (m, 1H), 3.43 - 3.52 (m, 2H), 2.60 - 2.67 (m, 1H), 2.04 (s, 3H), 1.85 - 1.94 (m , 3H), 1.67 - 1.77 (m, 4H), 1.54 - 1.63 (m, 2H).
MS (ESI, pos.ion) m/z: 518.25 [M+H]+ .MS (ESI, pos.ion) m/z: 518.25 [M+H] + .
步驟7:化合物 6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺的合成Step 7: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Synthesis of Amino)methyl) -N , N -lutidyl pyridamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (76 mg, 0.15 mmol),二甲胺鹽酸鹽 (45 mg, 1.0 mmol),1-乙基-(3-二甲氨基丙基)碳醯二亞胺鹽酸鹽 (86 mg, 0.45 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.31 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌20 h,加水洗 (50 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行柱分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體37 mg,收率46%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino) Methyl)picolinic acid (76 mg, 0.15 mmol), dimethylamine hydrochloride (45 mg, 1.0 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 86 mg, 0.45 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.31 mmol) were dissolved in dichloromethane (6 mL), to this solution was added dropwise N , N at room temperature -Diisopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 20 h, washed with water (50 mL), then extracted with dichloromethane (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed , and the concentrated solution was subjected to column separation (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 37 mg of white solid with a yield of 46%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.56 - 8.86 (m, 1H), 7.80 - 7.92 (m, 1H), 7.47 (d,J = 7.5 Hz, 1H), 7.32 - 7.41 (m, 1H), 7.01 - 7.15 (m, 2H), 6.95 (t,J F-H = 74.8 Hz, 1H), 6.82 - 6.91 (m, 1H), 4.84 - 4.97 (m, 1H), 4.30 - 4.52 (m, 3H), 4.02 - 4.26 (m, 1H), 3.39 - 3.57 (m, 2H), 2.98 (s, 3H), 2.91 (s, 3H), 2.56 - 2.71 (m, 1H), 2.04 (s, 3H), 1.83 - 1.94 (m, 3H), 1.67 - 1.77 (m, 4H), 1.54 - 1.63 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.56 - 8.86 (m, 1H), 7.80 - 7.92 (m, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.32 - 7.41 (m, 1H), 7.01 - 7.15 (m, 2H), 6.95 (t, J FH = 74.8 Hz, 1H), 6.82 - 6.91 (m, 1H), 4.84 - 4.97 (m, 1H), 4.30 - 4.52 ( m, 3H), 4.02 - 4.26 (m, 1H), 3.39 - 3.57 (m, 2H), 2.98 (s, 3H), 2.91 (s, 3H), 2.56 - 2.71 (m, 1H), 2.04 (s, 3H), 1.83 - 1.94 (m, 3H), 1.67 - 1.77 (m, 4H), 1.54 - 1.63 (m, 2H).
MS (ESI, pos.ion) m/z: 545.30 [M+H]+ .MS (ESI, pos.ion) m/z: 545.30 [M+H] + .
實施例48:化合物 6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-(乙氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 Example 48: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-(ethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl) -N , N -lutidine
步驟1:化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-1,2-二羧酸酯 的合成Step 1: Compound ( 2R )-1-tert-butyl 2-methyl 4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-1,2-dicarboxylate Synthesis
將化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-羥基苯基)吡咯-1,2-二羧酸酯 (489 mg, 1.26 mmol),碳酸鉀 (364 mg, 2.63 mmol) 溶於N ,N -二甲基甲醯胺 (10 mL) 中,室溫下向此溶液中加入碘乙烷 (0.2 mL, 3.0 mmol),70℃下反應3 h,加水洗 (50 mL),然後乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到淡黃色液體323 mg,收率62%。Compound ( 2R )-1-tert-butyl 2-methyl 4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrole-1,2-dicarboxylate (489 mg, 1.26 mmol), potassium carbonate (364 mg, 2.63 mmol) was dissolved in N , N -dimethylformamide (10 mL), to this solution was added iodoethane (0.2 mL, 3.0 mmol) at room temperature, 70 The reaction was carried out at ℃ for 3 h, washed with water (50 mL), and then extracted with ethyl acetate (5 mL × 3). The organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) to obtain 323 mg of pale yellow liquid with a yield of 62%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.10 (d,J = 8.2 Hz, 1H), 7.06 (s, 1H), 7.01 (t,J F-H = 74.9 Hz, 1H), 6.86 (dd,J = 8.3, 1.7 Hz, 1H), 4.26 - 4.32 (m, 1H), 4.10 (q,J = 7.0 Hz, 2H), 3.85 - 3.95 (m, 1H), 3.65 - 3.72 (m, 3H), 3.39 - 3.47 (m, 1H), 3.16 - 3.26 (m, 1H), 2.58 - 2.69 (m, 1H), 1.88 - 2.00 (m, 1H), 1.39 - 1.40 (m, 3H), 1.33 - 1.35 (m, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.10 (d, J = 8.2 Hz, 1H), 7.06 (s, 1H), 7.01 (t, J FH = 74.9 Hz, 1H), 6.86 (dd, J = 8.3, 1.7 Hz, 1H), 4.26 - 4.32 (m, 1H), 4.10 (q, J = 7.0 Hz, 2H), 3.85 - 3.95 (m, 1H), 3.65 - 3.72 (m, 3H) ), 3.39 - 3.47 (m, 1H), 3.16 - 3.26 (m, 1H), 2.58 - 2.69 (m, 1H), 1.88 - 2.00 (m, 1H), 1.39 - 1.40 (m, 3H), 1.33 - 1.35 (m, 9H).
MS (ESI, pos.ion) m/z: 438.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 438.20 [M+Na] + .
步驟2:化合物 (2R )-4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 的合成Step 2: Synthesis of compound (2R )-4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride
將化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-1,2-二羧酸酯 (323 mg, 0.58 mmol) 溶解於二氯甲烷 (4 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (3 mL),室溫攪拌1 h,除去溶劑,得到淡黃色液體175 mg,收率94%。The compound ( 2R )-1-tert-butyl 2-methyl 4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-1,2-dicarboxylate (323 mg, 0.58 mmol) was dissolved in dichloromethane (4 mL) solution, 4 mol/L HCl in ethyl acetate solution (3 mL) was added, stirred at room temperature for 1 h, and the solvent was removed to obtain 175 mg of pale yellow liquid, which was collected rate of 94%.
MS (ESI, pos.ion) m/z: 316.10 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 316.10 [M+H-HCl] + .
步驟3:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 的合成Step 3: Synthesis of Compound (2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride
將化合物 (2R )-4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (175 mg, 0.56 mmol) 溶解在二氯甲烷 (6 mL) 中,加入N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),0℃條件下滴加乙醯氯 (0.1 mL, 1.0 mmol),室溫攪拌16 h後停止反應,加入水 (50 mL) 有機相,水相用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到淺黃色液體166 mg,收率84%。Compound ( 2R )-4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride (175 mg, 0.56 mmol) was dissolved in two In methyl chloride (6 mL), N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) was added, and acetyl chloride (0.1 mL, 1.0 mmol) was added dropwise at 0 °C, and the mixture was stirred at room temperature for 16 h. The reaction was stopped, water (50 mL) was added to the organic phase, the aqueous phase was extracted with dichloromethane (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ Ethyl acetate (v/v) = 1/2) to obtain light yellow liquid 166 mg, yield 84%.
MS (ESI, pos.ion) m/z: 358.10 [M+H]+ .MS (ESI, pos.ion) m/z: 358.10 [M+H] + .
步驟4:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-2-羧酸 的合成Step 4: Synthesis of compound (2R )-1-acetyl-4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (166 mg, 0.46 mmol) 溶於四氫呋喃 (6 mL) 和水 (3 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (113 mg, 2.69 mmol),50℃反應2 h後停止,加鹽酸調節溶液pH=1,再用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淡黃色液體149 mg,收率93%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride (166 mg, 0.46 mmol) was dissolved in a mixed solvent of tetrahydrofuran (6 mL) and water (3 mL), then lithium hydroxide monohydrate (113 mg, 2.69 mmol) was added, the reaction was stopped after 2 h at 50 °C, and hydrochloric acid was added to adjust the pH of the solution = 1, and then extracted with ethyl acetate (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 149 mg of light yellow liquid, the yield was 93%.
MS (ESI, pos.ion) m/z: 344.20 [M+H]+ .MS (ESI, pos.ion) m/z: 344.20 [M+H] + .
步驟5:化合物 6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-(乙氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基吡啶醯胺 的合成Step 5: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-(ethoxy)phenyl)pyrrolidine-2-carbamoylamino )methyl) -N , N -lutidyl pyridamide synthesis
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-(乙氧基)苯基)吡咯烷-2-羧酸 (67 mg, 0.20 mmol),6-(氨基甲基)-N ,N -二甲基吡啶醯胺二鹽酸鹽 (96 mg, 0.54 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (88 mg, 0.46 mmol) 和N -羥基-7-氮雜苯並三氮唑 (56 mg, 0.41 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌16 h,加水洗 (50 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行柱分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體56 mg,收率57%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-(ethoxy)phenyl)pyrrolidine-2-carboxylic acid (67 mg, 0.20 mmol) , 6-(aminomethyl) -N , N -lutidylpyridamide dihydrochloride (96 mg, 0.54 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Amine hydrochloride (88 mg, 0.46 mmol) and N -hydroxy-7-azabenzotriazole (56 mg, 0.41 mmol) were dissolved in dichloromethane (6 mL) and added to this solution at room temperature N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) was added dropwise, stirred at room temperature for 16 h, washed with water (50 mL), then extracted with dichloromethane (5 mL × 3), the organic phase was washed with anhydrous sulfuric acid After drying over sodium, the solvent was removed, and the concentrated solution was subjected to column separation (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 56 mg of a white solid with a yield of 57%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.61 - 8.83 (m, 1H), 7.81 - 7.92 (m, 1H), 7.31 - 7.52 (m, 2H), 7.04 - 7.16 (m, 2H), 7.01 (t,J F-H = 74.8 Hz, 1H), 6.89 (d,J = 7.9 Hz, 1H), 4.29 - 4.56 (m, 3H), 3.99 - 4.21 (m, 3H), 3.39 - 3.56 (m, 2H), 2.97 (s, 3H), 2.90 (s, 3H), 2.54-2.69 (m, 1H), 2.03 (s, 3H), 1.81 - 1.90 (m, 1H), 1.28 - 1.38 (m, 3H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.61 - 8.83 (m, 1H), 7.81 - 7.92 (m, 1H), 7.31 - 7.52 (m, 2H), 7.04 - 7.16 (m, 1H) 2H), 7.01 (t, J FH = 74.8 Hz, 1H), 6.89 (d, J = 7.9 Hz, 1H), 4.29 - 4.56 (m, 3H), 3.99 - 4.21 (m, 3H), 3.39 - 3.56 ( m, 2H), 2.97 (s, 3H), 2.90 (s, 3H), 2.54-2.69 (m, 1H), 2.03 (s, 3H), 1.81 - 1.90 (m, 1H), 1.28 - 1.38 (m, 3H).
MS (ESI, pos.ion) m/z: 505.20 [M+H]+ .MS (ESI, pos.ion) m/z: 505.20 [M+H] + .
實施例49:化合物6-((2R -1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基-d6 -吡啶醯胺 Example 49: Compound 6-(( 2R- 1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-methyl acyl amino) methyl) - N, N - dimethyl - d 6 - pyridin Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (90 mg, 0.18 mmol),N ,N -二甲基-d6 -胺鹽酸鹽 (77 mg, 0.88 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (173 mg, 0.9 mmol) 和N -羥基-7-氮雜苯並三氮唑 (48 mg, 0.35 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),室溫反應12 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 25/1),得到白色固體68 mg,收率70%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate amino) methyl) pyridine-carboxylic acid (90 mg, 0.18 mmol), N, N - dimethyl - d 6 - amine hydrochloride (77 mg, 0.88 mmol), 1- ethyl - (3-dimethylaminopropyl propyl)carbodiimide hydrochloride (173 mg, 0.9 mmol) and N -hydroxy-7-azabenzotriazole (48 mg, 0.35 mmol) were dissolved in dichloromethane (5 mL), N , N -diisopropylethylamine (0.15 mL, 0.91 mmol) was added dropwise to this solution at 0°C, the reaction was carried out at room temperature for 12 h, water (15 mL) was added, and the mixture was extracted with dichloromethane (5 mL × 3 ), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 25/1) to obtain a white solid 68 mg, yield 70%.
1 H NMR (600 MHz, CD3 OD) δ (ppm): 7.93 (t,J = 7.7 Hz, 1H), 7.61 (d,J = 7.6 Hz, 1H), 7.46 (d,J = 7.3 Hz, 1H), 7.12 (d,J = 7.9 Hz, 1H), 7.09 (s, 1H), 6.94 (d,J = 7.9 Hz, 1H), 6.75 (t,J F-H = 75.7 Hz, 1H), 4.49 – 4.65 (m, 3H), 4.10 – 4.13 (m, 1H), 3.94 (d,J = 6.7 Hz, 2H), 3.66 (t,J = 10.5 Hz, 1H), 3.48 – 3.56 (m, 1H), 2.68 – 2.72 (m, 1H), 2.16 (s, 3H), 2.05 – 2.21 (m, 1H), 1.32 – 1.39 (m, 1H), 0.63 – 0.67 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (600 MHz, CD 3 OD) δ (ppm): 7.93 (t, J = 7.7 Hz, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.46 (d, J = 7.3 Hz, 1H) ), 7.12 (d, J = 7.9 Hz, 1H), 7.09 (s, 1H), 6.94 (d, J = 7.9 Hz, 1H), 6.75 (t, J FH = 75.7 Hz, 1H), 4.49 – 4.65 ( m, 3H), 4.10 – 4.13 (m, 1H), 3.94 (d, J = 6.7 Hz, 2H), 3.66 (t, J = 10.5 Hz, 1H), 3.48 – 3.56 (m, 1H), 2.68 – 2.72 (m, 1H), 2.16 (s, 3H), 2.05 – 2.21 (m, 1H), 1.32 – 1.39 (m, 1H), 0.63 – 0.67 (m, 2H), 0.37 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 537.25 [M+H]+ .MS (ESI, pos.ion) m/z: 537.25 [M+H] + .
實施例50:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二甲基-d6 -吡啶醯胺 Example 50: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethane oxy) phenyl) pyrrolidine-2-acyl-amino) methyl) - N, N - dimethyl - d 6 - pyridin Amides
步驟1:化合物 (2R )-1-叔丁基 2-甲基 4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯的合成Step 1: Compound ( 2R )-1-tert-Butyl 2-methyl 4-(3-(cyclopropyl-(1,1-duteuteto)methoxy)-4-(difluoromethoxy) )Phenyl)pyrrolidine-1,2-dicarboxylate synthesis
將 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-1,2-二羧酸酯 (630 mg, 1.63mmol),1,1-二氘代環丙基甲醇 (247 mg, 3.33mmol),三苯基膦 (1.08g, 14.12mmol) 溶解在乾燥的四氫呋喃 (10mL) 溶液中,冰浴條件下緩慢加入偶氮二甲酸二異丙酯 (247 mg, 3.33mmol),室溫反應4 h,加入水 (20mL),再用乙酸乙酯 (5 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,除去溶劑,減壓濃縮,經矽膠柱層析色譜分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到無色液體653 mg,收率90%。( 2R )-1-tert-butyl 2-methyl 4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-1,2-dicarboxylate (630 mg, 1.63 mmol), 1,1-dideuterated cyclopropylmethanol (247 mg, 3.33 mmol), triphenylphosphine (1.08 g, 14.12 mmol) were dissolved in dry tetrahydrofuran (10 mL) solution, slowly added under ice bath conditions Diisopropyl azodicarboxylate (247 mg, 3.33 mmol) was reacted at room temperature for 4 h, water (20 mL) was added, and then extracted with ethyl acetate (5 mL × 3). The organic phase was dried over anhydrous sodium sulfate and removed. The solvent was concentrated under reduced pressure and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) to obtain 653 mg of a colorless liquid with a yield of 90%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm):7.12 (d,J = 7.9 Hz, 1H), 6.80 – 6.83 (m, 2H), 6.61 (t,J F-H = 73.6 Hz, 1H), 4.94 – 5.00 (m, 1H), 4.32 – 4.42 (m, 1H), 3.94 – 4.08 (m, 1H), 3.78 – 3.79 (m, 3H), 3.40 – 3.47 (m, 1H), 3.29 – 3.38 (m, 1H), 2.62 – 2.70 (m, 1H), 1.98 – 2.09 (m, 1H), 1.45 – 1.48 (m, 9H), 0.64 – 0.68 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.12 (d, J = 7.9 Hz, 1H), 6.80 – 6.83 (m, 2H), 6.61 (t, J FH = 73.6 Hz, 1H), 4.94 – 5.00 (m, 1H), 4.32 – 4.42 (m, 1H), 3.94 – 4.08 (m, 1H), 3.78 – 3.79 (m, 3H), 3.40 – 3.47 (m, 1H), 3.29 – 3.38 (m, 1H), 2.62 – 2.70 (m, 1H), 1.98 – 2.09 (m, 1H), 1.45 – 1.48 (m, 9H), 0.64 – 0.68 (m, 2H), 0.36 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 466.10 [M+Na]+ .MS (ESI, pos.ion) m/z: 466.10 [M+Na] + .
步驟2:化合物 (2R )-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽的合成Step 2: Compound ( 2R )-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of Carboxylic Acid Methyl Hydrochloride
將化合物 (2R )-1-叔丁基 2-甲基 4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯 (400 mg, 0.9 mmol) 溶解於二氯甲烷 (5 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (10 mL),室溫反應3 h,除去溶劑,得到淺黃色黏稠固體314 mg,收率92%。The compound (2 R )-1-tert-butyl 2-methyl 4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy)benzene yl)pyrrolidine-1,2-dicarboxylate (400 mg, 0.9 mmol) was dissolved in dichloromethane (5 mL) solution, added 4 mol/L HCl in ethyl acetate solution (10 mL), room temperature The reaction was carried out for 3 h, and the solvent was removed to obtain 314 mg of a light yellow viscous solid with a yield of 92%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.14 (d,J = 8.2 Hz, 1H), 7.07 (d,J = 2.0 Hz, 1H), 6.92 (dd,J = 8.3, 1.9 Hz, 1H), 6.76 (t,J F-H = 75.5 Hz, 1H), 4.60 – 4.64 (m, 1H), 3.90 (s, 3H), 3.76 – 3.83 (m, 1H), 3.63 – 3.71 (m, 1H), 3.32 – 3.39 (m, 1H), 2.82 – 2.89 (m, 1H), 2.20 – 2.29 (m, 1H), 1.26 – 1.34 (m, 1H), 0.62 – 0.68 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.14 (d, J = 8.2 Hz, 1H), 7.07 (d, J = 2.0 Hz, 1H), 6.92 (dd, J = 8.3, 1.9 Hz , 1H), 6.76 (t, J FH = 75.5 Hz, 1H), 4.60 – 4.64 (m, 1H), 3.90 (s, 3H), 3.76 – 3.83 (m, 1H), 3.63 – 3.71 (m, 1H) , 3.32 – 3.39 (m, 1H), 2.82 – 2.89 (m, 1H), 2.20 – 2.29 (m, 1H), 1.26 – 1.34 (m, 1H), 0.62 – 0.68 (m, 2H), 0.37 – 0.41 ( m, 2H).
MS (ESI, pos.ion) m/z: 344.10 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 344.10 [M+H-HCl] + .
步驟3:化合物 (2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯的合成Step 3: Compound ( 2R )-1-Acetyl-4-(3-(Cyclopropyl-(1,1-Dideutero)methoxy)-4-(difluoromethoxy)phenyl ) Synthesis of methyl pyrrolidine-2-carboxylate
將化合物 (2R )-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽(343 mg, 0.91 mmol) 溶解在二氯甲烷 (10 mL) 中,加入N ,N -二異丙基乙胺 (1.0 mL, 6.1 mmol),0℃條件下加入乙醯氯 (0.3 mL, 4.0 mmol),室溫攪拌16 h後停止反應,加水溶液洗有機相 (10 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到無色液體222 mg,產率63%。The compound ( 2R )-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid Methyl ester hydrochloride (343 mg, 0.91 mmol) was dissolved in dichloromethane (10 mL), N , N -diisopropylethylamine (1.0 mL, 6.1 mmol) was added, and acetyl chloride was added at 0°C (0.3 mL, 4.0 mmol), the reaction was stopped after stirring at room temperature for 16 h, an aqueous solution was added to wash the organic phase (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluting Agent: petroleum ether/ethyl acetate (v/v) = 1/1) to obtain 222 mg of a colorless liquid with a yield of 63%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.15 (d,J = 8.7 Hz, 1H), 6.80 – 6.84 (m, 2H), 6.63 (t,J F-H = 73.6 Hz, 1H), 4.48 – 4.52 (m, 1H), 3.93 – 3.98 (m, 1H), 3.79 (s, 3H), 3.63 (t,J = 10.5 Hz, 1H), 3.38 – 3.47 (m, 2H), 2.64 – 2.71 (m, 1H), 2.13 (s, 3H), 2.02 – 2.07 (m, 1H), 1.27 – 1.33 (m, 1H), 0.65 – 0.70 (m, 2H), 0.36 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.15 (d, J = 8.7 Hz, 1H), 6.80 – 6.84 (m, 2H), 6.63 (t, J FH = 73.6 Hz, 1H), 4.48 – 4.52 (m, 1H), 3.93 – 3.98 (m, 1H), 3.79 (s, 3H), 3.63 (t, J = 10.5 Hz, 1H), 3.38 – 3.47 (m, 2H), 2.64 – 2.71 (m , 1H), 2.13 (s, 3H), 2.02 – 2.07 (m, 1H), 1.27 – 1.33 (m, 1H), 0.65 – 0.70 (m, 2H), 0.36 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 386.50 [M+H]+ .MS (ESI, pos.ion) m/z: 386.50 [M+H] + .
步驟4:化合物 (2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸的合成Step 4: Compound ( 2R )-1-Acetyl-4-(3-(Cyclopropyl-(1,1-Dideutero)methoxy)-4-(difluoromethoxy)phenyl ) Synthesis of pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯 (217 mg, 0.56 mmol) 溶於四氫呋喃 (5 mL) 和水 (5 mL) 的混合溶劑中,加入一水合氫氧化鋰 (121 mg, 2.88 mmol),50℃反應1 h後停止,加稀鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淺黃色液體208 mg,收率99%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy)phenyl)pyrrole Methyl alkane-2-carboxylate (217 mg, 0.56 mmol) was dissolved in a mixed solvent of tetrahydrofuran (5 mL) and water (5 mL), lithium hydroxide monohydrate (121 mg, 2.88 mmol) was added, and the reaction was carried out at 50 °C After 1 h, the solution was stopped, diluted hydrochloric acid was added to adjust the pH of the solution to 1, extracted with ethyl acetate (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 208 mg of a light yellow liquid with a yield of 99%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.11 (d,J = 8.2 Hz, 1H), 7.05 (d,J = 1.9 Hz, 1H), 6.90 – 6.93 (m, 1H), 6.74 (t,J F-H = 74.9 Hz, 1H), 4.43 – 4.47 (m, 1H), 4.08 – 4.15 (m, 1H), 3.49 – 3.56 (m, 1H), 3.56 – 3.64 (m, 1H), 2.72 – 2.78 (m, 1H), 2.14 (s, 3H), 2.00 – 2.08 (m, 1H), 1.28 – 1.346 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.11 (d, J = 8.2 Hz, 1H), 7.05 (d, J = 1.9 Hz, 1H), 6.90 – 6.93 (m, 1H), 6.74 (t, J FH = 74.9 Hz, 1H), 4.43 – 4.47 (m, 1H), 4.08 – 4.15 (m, 1H), 3.49 – 3.56 (m, 1H), 3.56 – 3.64 (m, 1H), 2.72 – 2.78 (m, 1H), 2.14 (s, 3H), 2.00 – 2.08 (m, 1H), 1.28 – 1.346 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 372.10 [M+H]+ .MS (ESI, pos.ion) m/z: 372.10 [M+H] + .
步驟5:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸乙酯的合成Step 5: Compound 6-((( 2R )-1-Acetyl-4-(3-(Cyclopropyl-(1,1-Dideutero)methoxy)-4-(difluoromethoxy) Synthesis of Ethyl)phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (205 mg, 0.55 mmol),6-(氨基甲基)吡啶甲酸乙酯鹽酸鹽 (267 mg, 1.09 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (523 mg, 2.73 mmol) 和N -羥基-7-氮雜苯並三氮唑 (158 mg, 1.16 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.7 mL, 4.0 mmol),室溫反應17 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色黏稠固體267 mg,產率90%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy)phenyl)pyrrole Alkane-2-carboxylic acid (205 mg, 0.55 mmol), 6-(aminomethyl)picolinate ethyl ester hydrochloride (267 mg, 1.09 mmol), 1-ethyl-(3-dimethylaminopropyl) ) carbodiimide hydrochloride (523 mg, 2.73 mmol) and N -hydroxy-7-azabenzotriazole (158 mg, 1.16 mmol) were dissolved in dichloromethane (10 mL) and cooled to After 0 °C, N , N -diisopropylethylamine (0.7 mL, 4.0 mmol) was added, the reaction was carried out at room temperature for 17 h, water (15 mL) was added, and the mixture was extracted with dichloromethane (5 mL × 3). Dry over anhydrous sodium sulfate, remove the solvent, concentrate, and conduct silica gel column chromatography separation (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 267 mg of a white viscous solid with a yield of 90%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.02 (d,J = 7.5 Hz, 1H), 7.84 (t,J = 7.8 Hz, 1H), 7.56 (d,J = 7.8 Hz, 1H), 7.11 (d,J = 8.1 Hz, 1H), 6.89 (s, 1H), 6.86 (dd,J = 8.2, 1.8 Hz, 1H), 6.62 (t,J F-H = 75.7 Hz, 1H), 4.59 – 4.77 (m, 3H), 4.47 (q,J = 7.1 Hz, 2H), 3.95 – 3.99 (m, 1H), 3.62 (t,J = 10.6 Hz, 1H), 3.29 – 3.41 (m, 1H), 2.58 – 2.65 (m, 1H), 2.39 – 2.48 (m, 1H), 2.16 (s, 3H), 1.44 (t,J = 7.2 Hz, 3H), 1.25 – 1.31 (m, 1H), 0.64 – 0.68 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.02 (d, J = 7.5 Hz, 1H), 7.84 (t, J = 7.8 Hz, 1H), 7.56 (d, J = 7.8 Hz, 1H) , 7.11 (d, J = 8.1 Hz, 1H), 6.89 (s, 1H), 6.86 (dd, J = 8.2, 1.8 Hz, 1H), 6.62 (t, J FH = 75.7 Hz, 1H), 4.59 – 4.77 (m, 3H), 4.47 (q, J = 7.1 Hz, 2H), 3.95 – 3.99 (m, 1H), 3.62 (t, J = 10.6 Hz, 1H), 3.29 – 3.41 (m, 1H), 2.58 – 2.65 (m, 1H), 2.39 – 2.48 (m, 1H), 2.16 (s, 3H), 1.44 (t, J = 7.2 Hz, 3H), 1.25 – 1.31 (m, 1H), 0.64 – 0.68 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 534.70 [M+H]+ .MS (ESI, pos.ion) m/z: 534.70 [M+H] + .
步驟6:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸的合成Step 6: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy) )Phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinic acid synthesis
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸乙酯 (267 mg, 0.5 mmol) 溶於四氫呋喃 (5 mL) 和水 (5 mL) 的混合溶劑中,加入一水合氫氧化鋰 (101 mg, 2.5 mmol),50℃反應1 h後停止,加稀鹽酸調節溶液pH=6,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到242 mg淺黃色固體,產率95%。Compound 6-(((2 R )-1-acetoxy-4-(3-(cyclopropyl-(1,1-duteuteto)methoxy)-4-(difluoromethoxy) Phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinate (267 mg, 0.5 mmol) was dissolved in a mixed solvent of tetrahydrofuran (5 mL) and water (5 mL), and lithium hydroxide monohydrate was added (101 mg, 2.5 mmol), the reaction was stopped after 1 h at 50 °C, diluted hydrochloric acid was added to adjust the pH of the solution to 6, extracted with ethyl acetate (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 242 mg Pale yellow solid, 95% yield.
MS (ESI, pos.ion) m/z: 506.70 [M+H]+ .MS (ESI, pos.ion) m/z: 506.70 [M+H] + .
步驟7:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯胺基)甲基)-N ,N -二甲基-d6 -吡啶醯胺 的合成Step 7: Compound 6-((( 2R )-1-Acetyl-4-(3-(Cyclopropyl-(1,1-Dideutero)methoxy)-4-(difluoromethoxy) yl) phenyl) pyrrolidine-2-acyl) methyl) - N, N - dimethyl - d 6 - synthesis of Amides pyridine
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (94 mg, 0.18 mmol),N ,N -二甲基-d6 -胺鹽酸鹽(77 mg, 0.88 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (173 mg, 0.91 mmol) 和N -羥基-7-氮雜苯並三氮唑 (48 mg, 0.35 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),室溫反應18 h,加水 (15 mL),二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 25/1),得到白色固體42 mg,產率42%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy)benzene yl) pyrrolidine-2-acyl-amino) methyl) pyridine-carboxylic acid (94 mg, 0.18 mmol), N, N - dimethyl - d 6 - amine hydrochloride (77 mg, 0.88 mmol), 1- ethyl Alkyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (173 mg, 0.91 mmol) and N -hydroxy-7-azabenzotriazole (48 mg, 0.35 mmol) were dissolved in In dichloromethane (5 mL), after cooling to 0 °C, N , N -diisopropylethylamine (0.15 mL, 0.91 mmol) was added, the reaction was carried out at room temperature for 18 h, water (15 mL) was added, and dichloromethane was extracted. (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, concentrated, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 25/1) to obtain a white solid 42 mg, 42% yield.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.90 – 7.95 (m, 1H), 7.60 (d,J = 7.5 Hz, 1H), 7.45 (d,J = 7.1 Hz, 1H), 7.05 – 7.12 (m, 2H), 6.90 (s, 1H), 6.75 (t,J F-H = 75.6 Hz, 1H), 4.48 – 4.65 (m, 3H), 4.07 – 4.15 (m, 1H), 3.63 – 3.69 (m, 1H), 3.47 – 3.57 (m, 1H), 2.65 – 2.74 (m, 1H), 2.16 (s, 3H), 2.01 – 2.13 (m, 1H), 1.24 – 1.37 (m, 1H), 0.60 – 0.68 (m, 2H), 0.34 – 0.42 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.90 – 7.95 (m, 1H), 7.60 (d, J = 7.5 Hz, 1H), 7.45 (d, J = 7.1 Hz, 1H), 7.05 – 7.12 (m, 2H), 6.90 (s, 1H), 6.75 (t, J FH = 75.6 Hz, 1H), 4.48 – 4.65 (m, 3H), 4.07 – 4.15 (m, 1H), 3.63 – 3.69 ( m, 1H), 3.47 – 3.57 (m, 1H), 2.65 – 2.74 (m, 1H), 2.16 (s, 3H), 2.01 – 2.13 (m, 1H), 1.24 – 1.37 (m, 1H), 0.60 – 0.68 (m, 2H), 0.34 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 539.30 [M+H]+ .MS (ESI, pos.ion) m/z: 539.30 [M+H] + .
實施例51:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -環丙基吡啶醯胺 Example 51: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -cyclopropylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (102 mg, 0.2 mmol),環丙胺 (43 mg, 0.6 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.0 mmol) 和N -羥基-7-氮雜苯並三氮唑 (45 mg, 0.33 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.15 mL, 0.9 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體59 mg,收率52%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (102 mg, 0.2 mmol), cyclopropylamine (43 mg, 0.6 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (192 mg, 1.0 mmol) and N -hydroxy-7-azabenzotriazole (45 mg, 0.33 mmol) were dissolved in dichloromethane (5 mL), to this solution was added dropwise N , N at 0°C -Diisopropylethylamine (0.15 mL, 0.9 mmol), reacted at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed , the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 59 mg of white solid with a yield of 52%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.96 (br.s, 1H), 8.77 (br.s, 1H), 8.14 (d,J = 7.5 Hz, 1H), 7.83 (t,J = 7.7 Hz, 1H), 7.37 (d,J = 7.6 Hz, 1H), 7.14 (d,J = 8.0 Hz, 1H), 6.94 (s, 1H), 6.89 (d,J = 8.0 Hz, 1H), 6.63 (t,J F-H = 75.6 Hz, 1H), 4.85 (t,J = 8.0 Hz, 1H), 4.56 – 4.71 (m, 2H), 3.94 – 4.00 (m, 1H), 3.90 (d,J = 6.7 Hz, 2H), 3.42 – 3.47 (m, 1H), 3.29 – 3.38 (m, 1H), 2.99 – 3.06 (m, 1H), 2.82 – 2.90 (m, 1H), 2.47 – 2.54 (m, 1H), 2.21 (s, 3H), 1.23 – 1.37 (m, 3H), 0.80 – 0.92 (m, 2H), 0.62 – 0.70 (m, 2H), 0.33 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.96 (br.s, 1H), 8.77 (br.s, 1H), 8.14 (d, J = 7.5 Hz, 1H), 7.83 (t, J = 7.7 Hz, 1H), 7.37 (d, J = 7.6 Hz, 1H), 7.14 (d, J = 8.0 Hz, 1H), 6.94 (s, 1H), 6.89 (d, J = 8.0 Hz, 1H), 6.63 (t, J FH = 75.6 Hz, 1H), 4.85 (t, J = 8.0 Hz, 1H), 4.56 – 4.71 (m, 2H), 3.94 – 4.00 (m, 1H), 3.90 (d, J = 6.7 Hz, 2H), 3.42 – 3.47 (m, 1H), 3.29 – 3.38 (m, 1H), 2.99 – 3.06 (m, 1H), 2.82 – 2.90 (m, 1H), 2.47 – 2.54 (m, 1H), 2.21 (s, 3H), 1.23 – 1.37 (m, 3H), 0.80 – 0.92 (m, 2H), 0.62 – 0.70 (m, 2H), 0.33 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 543.20 [M+H]+ .MS (ESI, pos.ion) m/z: 543.20 [M+H] + .
實施例52:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -環丙基-N -甲基吡啶醯胺 Example 52: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -cyclopropyl- N -methylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (80 mg, 0.16 mmol),N -甲基環丙胺 (35 mg, 0.33 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (154 mg, 0.8 mmol) 和N -羥基-7-氮雜苯並三氮唑 (33 mg, 0.24 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),室溫反應5 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體71 mg,收率63%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (80 mg, 0.16 mmol), N -methylcyclopropylamine (35 mg, 0.33 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide salt The acid salt (154 mg, 0.8 mmol) and N -hydroxy-7-azabenzotriazole (33 mg, 0.24 mmol) were dissolved in dichloromethane (5 mL), and the solution was added dropwise at 0°C N , N -diisopropylethylamine (0.15 mL, 0.91 mmol) was added, reacted at room temperature for 5 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sodium sulfate After drying, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 71 mg of white solid, with a yield of 63%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.77 (t,J = 7.4 Hz, 1H), 7.54 (br.s, 1H), 7.42 – 7.44 (m, 1H), 7.36 (d,J = 7.6 Hz, 1H), 7.12 (d,J = 7.9 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 7.6 Hz, 1H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.49 – 4.67 (m, 3H), 3.93 – 3.97 (m, 1H), 3.88 (d,J = 6.8 Hz, 2H), 3.56 (t,J = 10.6 Hz, 1H), 3.27 – 3.38 (m, 1H), 3.15 (s, 3H), 2.97 – 3.07 (m, 1H), 2.43 – 2.62 (m, 2H), 2.14 (s, 3H), 1.24 – 1.37 (m, 1H), 0.62 – 0.71 (m, 2H), 0.47 – 0.55 (m, 2H), 0.31 – 0.42 (m, 4H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.77 (t, J = 7.4 Hz, 1H), 7.54 (br.s, 1H), 7.42 – 7.44 (m, 1H), 7.36 (d, J = 7.6 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H), 6.90 (s, 1H), 6.86 (d, J = 7.6 Hz, 1H), 6.62 (t, J FH = 75.6 Hz, 1H) , 4.49 – 4.67 (m, 3H), 3.93 – 3.97 (m, 1H), 3.88 (d, J = 6.8 Hz, 2H), 3.56 (t, J = 10.6 Hz, 1H), 3.27 – 3.38 (m, 1H) ), 3.15 (s, 3H), 2.97 – 3.07 (m, 1H), 2.43 – 2.62 (m, 2H), 2.14 (s, 3H), 1.24 – 1.37 (m, 1H), 0.62 – 0.71 (m, 2H) ), 0.47 – 0.55 (m, 2H), 0.31 – 0.42 (m, 4H).
MS (ESI, pos.ion) m/z: 557.20 [M+H]+ .MS (ESI, pos.ion) m/z: 557.20 [M+H] + .
實施例53:化合物6-(((2R )-1-乙醯基-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -環己基-N -甲基吡啶醯胺 Example 53: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Carboxylamido)methyl) -N -cyclohexyl- N -methylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (102 mg, 0.20 mmol),N -甲基環己胺 (46 mg, 0.41 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (196 mg, 1.02 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體39 mg,收率32%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (102 mg, 0.20 mmol), N -methylcyclohexylamine (46 mg, 0.41 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide The hydrochloride salt (196 mg, 1.02 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL) and added to this solution at 0°C N ,N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added dropwise, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sulfuric acid The solution was dried over sodium, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 39 mg of white solid with a yield of 32%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.73 – 7.79 (m, 1H), 7.35 – 7.44 (m, 2H), 7.12 (d,J = 8.4 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 7.0 Hz, 1H), 6.62 (t,J F-H = 75.5 Hz, 1H), 4.48 – 4.67 (m, 3H), 3.90 – 3.98 (m, 1H), 3.88 (d,J = 6.2 Hz, 2H), 3.53 – 3.61 (m, 1H), 3.35 – 3.45 (m, 2H), 3.01 (s, 2H), 2.83(s, 1H), 2.51 – 2.62 (m, 1H), 2.39 – 2.50 (m, 1H), 2.14 (s, 3H), 1.69 – 1.83 (m, 4H), 1.44 – 1.60 (m, 4H), 1.26 – 1.34 (m, 1H), 1.02 – 1.19 (m, 2H), 0.63 – 0.70 (m, 2H), 0.34 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.73 – 7.79 (m, 1H), 7.35 – 7.44 (m, 2H), 7.12 (d, J = 8.4 Hz, 1H), 6.90 (s, 1H) ), 6.86 (d, J = 7.0 Hz, 1H), 6.62 (t, J FH = 75.5 Hz, 1H), 4.48 – 4.67 (m, 3H), 3.90 – 3.98 (m, 1H), 3.88 (d, J = 6.2 Hz, 2H), 3.53 – 3.61 (m, 1H), 3.35 – 3.45 (m, 2H), 3.01 (s, 2H), 2.83(s, 1H), 2.51 – 2.62 (m, 1H), 2.39 – 2.50 (m, 1H), 2.14 (s, 3H), 1.69 – 1.83 (m, 4H), 1.44 – 1.60 (m, 4H), 1.26 – 1.34 (m, 1H), 1.02 – 1.19 (m, 2H), 0.63 – 0.70 (m, 2H), 0.34 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 599.20 [M+H]+ .MS (ESI, pos.ion) m/z: 599.20 [M+H] + .
實施例54:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -((5-氟吡啶-2-基)甲基)-N -甲基吡啶醯胺 Example 54: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -((5-fluoropyridin-2-yl)methyl) -N -methylpyridamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (102 mg, 0.21 mmol),1-(5-氟吡啶-2-基)-N -甲基甲胺 (57 mg, 0.41 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (40 mg, 0.29 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.0 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體47 mg,收率37%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (102 mg, 0.21 mmol), 1-(5-fluoropyridin-2-yl) -N -methylmethanamine (57 mg, 0.41 mmol), 1-ethyl-(3- Dimethylaminopropyl)carbodiimide hydrochloride (192 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (40 mg, 0.29 mmol) were dissolved in dichloromethane (5 mL), N , N -diisopropylethylamine (0.17 mL, 1.0 mmol) was added dropwise to this solution at 0 °C, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and extracted with dichloromethane. (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain a white solid 47 mg, yield 37%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.38 – 8.42 (m, 1H), 7.72 – 7.80 (m, 1H), 7.34 – 7.54 (m, 4H), 7.10 (d,J = 7.7 Hz, 1H), 6.88 (s, 1H), 6.84 (d,J = 7.5 Hz, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 4.77 – 4.85 (m, 2H), 4.47 – 4.61 (m, 3H), 3.84 – 3.94 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.46 – 3.56 (m, 1H), 3.26 – 3.36 (m, 1H), 3.10 (s, 3H), 2.40 – 2.56 (m, 2H), 2.11 (s, 3H), 1.24 – 1.34 (m, 1H), 0.61 – 0.66 (m, 2H), 0.31 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.38 – 8.42 (m, 1H), 7.72 – 7.80 (m, 1H), 7.34 – 7.54 (m, 4H), 7.10 (d, J = 7.7 Hz , 1H), 6.88 (s, 1H), 6.84 (d, J = 7.5 Hz, 1H), 6.60 (t, J FH = 75.5 Hz, 1H), 4.77 – 4.85 (m, 2H), 4.47 – 4.61 (m , 3H), 3.84 – 3.94 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.46 – 3.56 (m, 1H), 3.26 – 3.36 (m, 1H), 3.10 (s, 3H), 2.40 – 2.56 (m, 2H), 2.11 (s, 3H), 1.24 – 1.34 (m, 1H), 0.61 – 0.66 (m, 2H), 0.31 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 626.20 [M+H]+ .MS (ESI, pos.ion) m/z: 626.20 [M+H] + .
實施例55:化合物 (2R )-1-乙醯基-N -(3-(環己基(甲基)氨甲醯基)苯甲基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯胺 Example 55: Compound ( 2R )-1-Acetyl- N- (3-(cyclohexyl(methyl)carbamoyl)benzyl)-4-(3-(cyclopropylmethoxy) )-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxamide
將化合物3-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)苯甲酸 (97 mg, 0.19 mmol),N -甲基環己基胺 (76 mg, 0.67 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (103 mg, 0.53 mmol) 和N -羥基-7-氮雜苯並三氮唑 (69 mg, 0.50 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌17 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體63 mg,收率54%。The compound 3-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)benzoic acid (97 mg, 0.19 mmol), N -methylcyclohexylamine (76 mg, 0.67 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide The hydrochloride salt (103 mg, 0.53 mmol) and N -hydroxy-7-azabenzotriazole (69 mg, 0.50 mmol) were dissolved in dichloromethane (6 mL), cooled to 0 °C, added with N , N -diisopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 17 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, removed The solvent and the concentrated solution were separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 63 mg of white solid with a yield of 54%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.31-7.38 (m, 2H), 7.16-7.26 (m, 2H), 7.09-7.13 (m, 1H), 7.05 (d,J = 1.6 Hz, 1H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.87 (d,J = 9.8 Hz, 1H), 4.30-4.35 (m, 3H), 4.04-4.08 (m, 1H), 3.90 (d,J = 6.9 Hz, 2H), 3.37-3.51 (m, 2H), 3.15-3.28 (m, 1H), 2.70-2.82 (m, 3H), 2.55-2.62 (m, 1H), 2.01 (s, 3H), 1.84-1.97 (m, 1H), 1.40-1.61 (m, 6H), 1.22-1.28 (m, 3H), 0.80-0.87 (m, 2H), 0.52-0.62 (m, 2H), 0.30-0.37 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.31-7.38 (m, 2H), 7.16-7.26 (m, 2H), 7.09-7.13 (m, 1H), 7.05 (d, J = 1.6 Hz, 1H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.87 (d, J = 9.8 Hz, 1H), 4.30-4.35 (m, 3H), 4.04-4.08 (m, 1H), 3.90 (d, J = 6.9 Hz, 2H), 3.37-3.51 (m, 2H), 3.15-3.28 (m, 1H), 2.70-2.82 (m, 3H), 2.55-2.62 (m, 1H), 2.01 (s , 3H), 1.84-1.97 (m, 1H), 1.40-1.61 (m, 6H), 1.22-1.28 (m, 3H), 0.80-0.87 (m, 2H), 0.52-0.62 (m, 2H), 0.30 -0.37 (m, 2H).
實施例56:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基-N -(四氫-2H -吡喃-4-基)吡啶醯胺 Example 56: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -methyl- N- (tetrahydro- 2H -pyran-4-yl)pyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -甲基四氫-2H -吡喃-4-胺 (93 mg, 0.81 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (54 mg, 0.40 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到74 mg白色固體,產率62%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N -methyltetrahydro- 2H -pyran-4-amine (93 mg, 0.81 mmol), 1-ethyl-(3-dimethyl Aminopropyl)carbodiimide hydrochloride (192 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (54 mg, 0.40 mmol) were dissolved in dichloromethane (5 mL) , cooled to 0°C, added N , N -diisopropylethylamine (0.17 mL, 1.00 mmol), reacted at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), The organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 74 mg of white solid with a yield of 62% .
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.91 – 7.94 (m, 1H), 7.57 – 7.63 (m, 1H), 7.41 – 7.45 (m, 1H), 7.12 (d,J = 8.2 Hz, 1H), 7.09 (s, 1H), 6.92 – 6.94 (m, 1H), 6.56 – 6.92 (m, 1H), 4.45 – 4.59 (m, 3H), 4.09 – 4.13 (m, 1H), 3.98 – 4.05 (m, 1H), 3.93 (d,J = 6.7 Hz, 2H), 3.69 – 3.79 (m, 1H), 3.66 (t,J = 10.5 Hz, 1H), 3.48 – 3.56 (m, 2H), 3.20 – 3.26 (m, 1H), 3.01 (s, 1.5H), 2.84 (s, 1.5H), 2.65 – 2.74 (m, 1H), 2.15 (s, 3H), 2.05 – 2.13 (m, 1H), 1.82 – 2.01 (m, 3H), 1.60 – 1.76 (m, 2H), 1.26 – 1.34 (m, 1H), 0.62 – 0.66 (m, 2H), 0.37 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.91 – 7.94 (m, 1H), 7.57 – 7.63 (m, 1H), 7.41 – 7.45 (m, 1H), 7.12 (d, J = 8.2 Hz , 1H), 7.09 (s, 1H), 6.92 – 6.94 (m, 1H), 6.56 – 6.92 (m, 1H), 4.45 – 4.59 (m, 3H), 4.09 – 4.13 (m, 1H), 3.98 – 4.05 (m, 1H), 3.93 (d, J = 6.7 Hz, 2H), 3.69 – 3.79 (m, 1H), 3.66 (t, J = 10.5 Hz, 1H), 3.48 – 3.56 (m, 2H), 3.20 – 3.26 (m, 1H), 3.01 (s, 1.5H), 2.84 (s, 1.5H), 2.65 – 2.74 (m, 1H), 2.15 (s, 3H), 2.05 – 2.13 (m, 1H), 1.82 – 2.01 (m, 3H), 1.60 – 1.76 (m, 2H), 1.26 – 1.34 (m, 1H), 0.62 – 0.66 (m, 2H), 0.37 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 601.30 [M+H]+ .MS (ESI, pos.ion) m/z: 601.30 [M+H] + .
實施例57:化合物 (2R )-1-乙醯基-N -((6-(氮雜環丁烷-1-羰基)吡啶-2-基)甲基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-吡咯烷-2-甲醯胺 Example 57: Compound ( 2R )-1-Acetyl- N -((6-(azetidine-1-carbonyl)pyridin-2-yl)methyl)-4-(3-(cyclo) Propylmethoxy)-4-(difluoromethoxy)phenyl)-pyrrolidine-2-carboxamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (102 mg, 0.20 mmol),氮雜環丁烷 (96 mg, 1.68 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (156 mg, 0.81 mmol) 和N -羥基-7-氮雜苯並三氮唑 (87 mg, 0.64 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌18 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到淡黃色固體5 mg,收率5%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (102 mg, 0.20 mmol), azetidine (96 mg, 1.68 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride The salt (156 mg, 0.81 mmol) and N -hydroxy-7-azabenzotriazole (87 mg, 0.64 mmol) were dissolved in dichloromethane (6 mL), cooled to 0 °C, and N , N - Diisopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 18 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and concentrated The liquid was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 5 mg of pale yellow solid with a yield of 5%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.89 (t,J = 7.7 Hz, 1H), 7.75-7.80 (m, 1H), 7.52 (d,J = 7.7 Hz, 1H), 7.13 (d,J = 8.1 Hz, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.88 (d,J = 9.2 Hz, 1H), 4.57-4.61 (m, 2H), 4.35-4.45 (m, 3H), 4.03-4.07 (m, 3H), 3.90 (d,J = 6.8 Hz, 2H), 3.43-3.52 (m, 2H), 2.21-2.28 (m, 2H), 2.03 (s, 3H), 1.91-1.99 (m, 2H), 1.28-1.31 (m, 1H), 0.53-0.59 (m, 2H), 0.30-0.34 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.89 (t, J = 7.7 Hz, 1H), 7.75-7.80 (m, 1H), 7.52 (d, J = 7.7 Hz, 1H), 7.13 (d, J = 8.1 Hz, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.88 (d, J = 9.2 Hz, 1H), 4.57-4.61 (m, 2H), 4.35-4.45 (m, 3H), 4.03-4.07 (m, 3H), 3.90 (d, J = 6.8 Hz, 2H), 3.43-3.52 (m, 2H), 2.21-2.28 (m, 2H) , 2.03 (s, 3H), 1.91-1.99 (m, 2H), 1.28-1.31 (m, 1H), 0.53-0.59 (m, 2H), 0.30-0.34 (m, 2H).
MS (ESI, pos.ion) m/z: 543.20 [M+H]+ .MS (ESI, pos.ion) m/z: 543.20 [M+H] + .
實施例58:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(吡咯烷-1-羰基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 58: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( Pyrrolidine-1-carbonyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (94 mg, 0.18 mmol),四氫吡咯 (69 mg, 0.97 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (86 mg, 0.45 mmol) 和N -羥基-7-氮雜苯並三氮唑 (52 mg, 0.38 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.2 mL, 1.0 mmol),室溫攪拌7 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體48 mg,收率46%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (94 mg, 0.18 mmol), tetrahydropyrrole (69 mg, 0.97 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 86 mg, 0.45 mmol) and N -hydroxy-7-azabenzotriazole (52 mg, 0.38 mmol) were dissolved in dichloromethane (6 mL), cooled to 0 °C, added with N , N -diiso Propylethylamine (0.2 mL, 1.0 mmol), stirred at room temperature for 7 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was carried out Separation by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) gave 48 mg of white solid with a yield of 46%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.59 (t,J = 6.0 Hz, 1H), 7.84-7.90 (m, 1H), 7.48-7.55 (m, 2H), 7.12-7.14 (m, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.88 (d,J = 9.5 Hz, 1H), 4.41-4.53 (m, 1H), 4.33-4.39 (m, 2H), 4.06-4.09 (m, 1H), 3.90 (d,J = 6.9 Hz, 2H), 3.51-3.59 (m, 2H), 3.39-3.49 (m, 4H), 2.58-2.63 (m, 1H), 2.03 (s, 3H), 1.89-1.94 (m, 1H), 1.80-1.83 (m, 4H), 1.23-1.26 (m, 1H), 0.54-0.59 (m, 2H), 0.33-0.36 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.59 (t, J = 6.0 Hz, 1H), 7.84-7.90 (m, 1H), 7.48-7.55 (m, 2H), 7.12-7.14 (m, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.88 (d, J = 9.5 Hz, 1H), 4.41-4.53 (m, 1H), 4.33-4.39 (m, 2H), 4.06-4.09 (m, 1H), 3.90 (d, J = 6.9 Hz, 2H), 3.51-3.59 (m, 2H), 3.39-3.49 (m, 4H), 2.58-2.63 (m , 1H), 2.03 (s, 3H), 1.89-1.94 (m, 1H), 1.80-1.83 (m, 4H), 1.23-1.26 (m, 1H), 0.54-0.59 (m, 2H), 0.33-0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 557.40 [M+H]+ .MS (ESI, pos.ion) m/z: 557.40 [M+H] + .
實施例59:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(哌啶-1-羰基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 59: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( Piperidine-1-carbonyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (103 mg, 0.20 mmol),哌啶 (68 mg, 0.80 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (154 mg, 0.80 mmol) 和N -羥基-7-氮雜苯並三氮唑 (86 mg, 0.63 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌20 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體75 mg,收率64%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (103 mg, 0.20 mmol), piperidine (68 mg, 0.80 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (154 mg, 0.80 mmol) and N -hydroxy-7-azabenzotriazole (86 mg, 0.63 mmol) were dissolved in dichloromethane (6 mL), cooled to 0 °C, and N , N -diisopropyl was added Ethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 20 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was washed with silica gel Column chromatography separation (eluent: dichloromethane/methanol (v/v) = 15/1) gave 75 mg of white solid, yield 64%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.59 (t,J = 5.7 Hz, 1H), 7.85 (t,J = 7.5 Hz, 1H), 7.46 (d,J = 7.8 Hz, 1H), 7.32-7.38 (m, 1H), 7.10-7.14 (m, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.8 Hz, 1H), 6.88 (d,J = 8.9 Hz, 1H), 4.33-4.43 (m, 3H), 4.05-4.09 (m, 1H), 3.90 (d,J = 6.8 Hz, 2H), 3.53-3.59 (m, 2H), 3.39-3.52 (m, 2H), 3.21-3.27 (m, 2H), 2.56-2.64 (m, 1H), 2.03 (s, 3H), 1.87-1.97 (m, 1H), 1.53-1.58 (m, 4H), 1.40-1.43 (m, 2H), 1.25-1.34 (m, 1H), 0.53-0.59 (m, 2H), 0.30-0.35 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.59 (t, J = 5.7 Hz, 1H), 7.85 (t, J = 7.5 Hz, 1H), 7.46 (d, J = 7.8 Hz, 1H) ), 7.32-7.38 (m, 1H), 7.10-7.14 (m, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.8 Hz, 1H), 6.88 (d, J = 8.9 Hz, 1H ), 4.33-4.43 (m, 3H), 4.05-4.09 (m, 1H), 3.90 (d, J = 6.8 Hz, 2H), 3.53-3.59 (m, 2H), 3.39-3.52 (m, 2H), 3.21-3.27 (m, 2H), 2.56-2.64 (m, 1H), 2.03 (s, 3H), 1.87-1.97 (m, 1H), 1.53-1.58 (m, 4H), 1.40-1.43 (m, 2H) ), 1.25-1.34 (m, 1H), 0.53-0.59 (m, 2H), 0.30-0.35 (m, 2H).
MS (ESI, pos.ion) m/z: 571.40 [M+H]+ .MS (ESI, pos.ion) m/z: 571.40 [M+H] + .
實施例60:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(嗎啉-4-羰基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 60: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( Morpholine-4-carbonyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (103 mg, 0.20 mmol),嗎啉 (75 mg, 0.86mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (165 mg, 0.86 mmol) 和N -羥基-7-氮雜苯並三氮唑 (86 mg, 0.62 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌14 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體72 mg,收率61%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (103 mg, 0.20 mmol), morpholine (75 mg, 0.86 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (165 mg, 0.86 mmol) and N -hydroxy-7-azabenzotriazole (86 mg, 0.62 mmol) were dissolved in dichloromethane (6 mL), cooled to 0 °C, and N , N -diisopropyl was added Ethylethylamine (0.4 mL, 2.0 mmol), stirred at room temperature for 14 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was washed with silica gel Column chromatography separation (eluent: dichloromethane/methanol (v/v) = 15/1) gave 72 mg of a white solid with a yield of 61%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.60 (t,J = 5.8 Hz, 1H), 7.88 (t,J = 7.6 Hz, 1H), 7.49 (d,J = 7.9 Hz, 1H), 7.43 (d,J = 7.8 Hz, 1H), 7.13 (d,J = 8.1 Hz, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.89 (d,J = 9.1 Hz, 1H), 4.33-4.44 (m, 3H), 4.05-4.09 (m, 1H), 3.90 (d,J = 6.9 Hz, 2H), 3.60-3.66 (m, 4H), 3.50-3.55 (m, 2H), 3.40-3.48 (m, 4H), 2.55-2.64 (m, 1H), 2.03 (s, 3H), 1.87-1.98 (m, 1H), 1.25-1.29 (m, 1H), 0.54-0.60 (m, 2H), 0.33-0.37 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.60 (t, J = 5.8 Hz, 1H), 7.88 (t, J = 7.6 Hz, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.43 (d, J = 7.8 Hz, 1H), 7.13 (d, J = 8.1 Hz, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.89 (d , J = 9.1 Hz, 1H), 4.33-4.44 (m, 3H), 4.05-4.09 (m, 1H), 3.90 (d, J = 6.9 Hz, 2H), 3.60-3.66 (m, 4H), 3.50- 3.55 (m, 2H), 3.40-3.48 (m, 4H), 2.55-2.64 (m, 1H), 2.03 (s, 3H), 1.87-1.98 (m, 1H), 1.25-1.29 (m, 1H), 0.54-0.60 (m, 2H), 0.33-0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 573.25 [M+H]+ .MS (ESI, pos.ion) m/z: 573.25 [M+H] + .
實施例61:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(4-甲基哌嗪-1-羰基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 61: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( 4-Methylpiperazine-1-carbonyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (92 mg, 0.18 mmol),1-甲基哌嗪 (89 mg, 0.89mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (163 mg, 0.85 mmol) 和N -羥基-7-氮雜苯並三氮唑 (89 mg, 0.65 mmol) 溶於二氯甲烷 (6 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌14 h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到淡黃色固體67 mg,收率63%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (92 mg, 0.18 mmol), 1-methylpiperazine (89 mg, 0.89 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide salt The acid salt (163 mg, 0.85 mmol) and N -hydroxy-7-azabenzotriazole (89 mg, 0.65 mmol) were dissolved in dichloromethane (6 mL), and the solution was added dropwise at 0°C Add N , N -diisopropylethylamine (0.4 mL, 2.0 mmol), stir at room temperature for 14 h, add water (50 mL), extract with dichloromethane (5 mL × 3), and dry the organic phase with anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 67 mg of pale yellow solid with a yield of 63%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.61 (t,J = 5.8 Hz, 1H), 7.87 (t,J = 7.6 Hz, 1H), 7.48 (d,J = 7.8 Hz, 1H), 7.40 (d,J = 7.8 Hz, 1H), 7.13 (d,J = 8.1 Hz, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.8 Hz, 1H), 6.88 (d,J = 8.8 Hz, 1H), 4.42-4.53 (m, 1H), 4.33-4.39 (m, 2H), 4.05-4.09 (m, 1H), 3.90 (d,J = 6.8 Hz, 2H), 3.57-3.69 (m, 2H), 3.37-3.51 (m, 4H), 2.58-2.64 (m, 1H), 2.40-2.451 (m, 2H), 2.25-2.40 (m, 2H), 2.24 (s, 3H), 2.03 (s, 3H), 1.89-1.99 (m, 1H), 1.25-1.34 (m, 1H), 0.54-0.60 (m, 2H), 0.31-0.36 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.61 (t, J = 5.8 Hz, 1H), 7.87 (t, J = 7.6 Hz, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 7.8 Hz, 1H), 7.13 (d, J = 8.1 Hz, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.8 Hz, 1H), 6.88 (d , J = 8.8 Hz, 1H), 4.42-4.53 (m, 1H), 4.33-4.39 (m, 2H), 4.05-4.09 (m, 1H), 3.90 (d, J = 6.8 Hz, 2H), 3.57- 3.69 (m, 2H), 3.37-3.51 (m, 4H), 2.58-2.64 (m, 1H), 2.40-2.451 (m, 2H), 2.25-2.40 (m, 2H), 2.24 (s, 3H), 2.03 (s, 3H), 1.89-1.99 (m, 1H), 1.25-1.34 (m, 1H), 0.54-0.60 (m, 2H), 0.31-0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 586.20 [M+H]+ .MS (ESI, pos.ion) m/z: 586.20 [M+H] + .
實施例62:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(1-甲基-1H -吡唑-5-基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 62: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( 1-Methyl-1 H -pyrazol-5-yl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
步驟1:化合物 ((6-溴吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 1: Synthesis of compound ((6-bromopyridin-2-yl)methyl)carbamate tert-butyl ester
將化合物 (6-溴吡啶-2-基)甲胺 (1.0 g, 5.4 mmol) 溶解於二氯甲烷 (15 mL) 溶液中,加入N ,N -二異丙基乙胺 (3.0 mL, 18.0 mmol),在0℃下加入二叔丁基二碳酸酯 (1.5 mL, 6.5 mmol),攪拌20 min轉移至室溫反應5 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到白色固體1.42 g,收率92%。The compound (6-bromopyridin-2-yl)methanamine (1.0 g, 5.4 mmol) was dissolved in a solution of dichloromethane (15 mL), and N , N -diisopropylethylamine (3.0 mL, 18.0 mmol) was added. ), di-tert-butyl dicarbonate (1.5 mL, 6.5 mmol) was added at 0 °C, stirred for 20 min, transferred to room temperature for 5 h, washed with water (50 mL), and extracted with dichloromethane (5 mL × 3 ), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) to obtain 1.42 g of white solid, which was collected rate of 92%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.73 (t,J = 7.7 Hz, 1H), 7.50 (d,J = 7.9 Hz, 1H), 7.48 (br.s, 1H), 7.30 (d,J = 7.5 Hz, 1H), 4.19 (d,J = 6.0 Hz, 2H), 1.40 (s, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.73 (t, J = 7.7 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.48 (br.s, 1H), 7.30 (d, J = 7.5 Hz, 1H), 4.19 (d, J = 6.0 Hz, 2H), 1.40 (s, 9H).
MS (ESI, pos.ion) m/z: 233.10 [M-55]+ .MS (ESI, pos.ion) m/z: 233.10 [M-55] + .
步驟2:化合物 ((6-(1-甲基-1H -吡唑-5-基)吡啶-2-基)甲基)氨基甲酸叔丁酯的合成Step 2: Synthesis of compound ((6-(1-methyl- 1H -pyrazol-5-yl)pyridin-2-yl)methyl)carbamate tert-butyl ester
將化合物 ((6-溴吡啶-2-基)甲基)氨基甲酸叔丁酯 (198 mg, 0.69 mmol),1-甲基-5-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)-1H -吡唑 (153 mg, 0.74 mmol)、碳酸鈉 (232 mg, 2.19 mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (29 mg, 0.04 mmol) 溶於無水1,4-二氧六環 (6 mL) 中,排除空氣,通入氮氣,在100℃下反應18 h,加水 (50 mL),用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到淡黃色液體143 mg,收率72%。Compound ((6-bromopyridin-2-yl)methyl)carbamic acid tert-butyl ester (198 mg, 0.69 mmol), 1-methyl-5-(4,4,5,5-tetramethyl-1 , 3,2-dioxaborolan-2-yl) -1 H - pyrazole (153 mg, 0.74 mmol), sodium carbonate (232 mg, 2.19 mmol) and [1,1'-bis (diphenylphosphino The phosphino)ferrocene]palladium dichloride (29 mg, 0.04 mmol) was dissolved in anhydrous 1,4-dioxane (6 mL), the air was removed, nitrogen was passed through, and the reaction was carried out at 100 °C for 18 h, Water (50 mL) was added, extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) to obtain 143 mg of pale yellow liquid with a yield of 72%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.86 (t,J = 7.8 Hz, 1H), 7.65 (d,J = 7.8 Hz, 1H), 7.45-7.48 (m, 2H), 7.24 (d,J = 7.6 Hz, 1H), 6.78 (d,J = 1.4 Hz, 1H), 4.30 (d,J = 6.0 Hz, 2H), 4.16 (s, 3H), 1.41 (s, 9H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.86 (t, J = 7.8 Hz, 1H), 7.65 (d, J = 7.8 Hz, 1H), 7.45-7.48 (m, 2H), 7.24 (d, J = 7.6 Hz, 1H), 6.78 (d, J = 1.4 Hz, 1H), 4.30 (d, J = 6.0 Hz, 2H), 4.16 (s, 3H), 1.41 (s, 9H).
MS (ESI, pos.ion) m/z: 289.15 [M+H]+ .MS (ESI, pos.ion) m/z: 289.15 [M+H] + .
步驟3:化合物 (6-(1-甲基-1H -吡唑-5-基)吡啶-2-基)甲胺二鹽酸鹽的合成Step 3: Synthesis of compound (6-(1-methyl- 1H -pyrazol-5-yl)pyridin-2-yl)methanamine dihydrochloride
將化合物 ((6-(1-甲基-1H -吡唑-5-基)吡啶-2-基)甲基)氨基甲酸叔丁酯 (112 mg, 0.39 mmol) 溶解於二氯甲烷 (2 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (3 mL),室溫攪拌1 h,除去溶劑,得到淡黃色固體71 mg,收率97%。Compound ((6-(1-methyl- 1H -pyrazol-5-yl)pyridin-2-yl)methyl)carbamate (112 mg, 0.39 mmol) was dissolved in dichloromethane (2 mL) solution, 4 mol/L HCl in ethyl acetate solution (3 mL) was added, stirred at room temperature for 1 h, and the solvent was removed to obtain 71 mg of pale yellow solid with a yield of 97%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.48-8.63 (m, 2H), 7.96 (t,J = 7.8 Hz, 1H), 7.78 (d,J = 7.8 Hz, 1H), 7.47-7.50 (m, 2H), 6.84 (d,J = 1.7 Hz, 1H), 4.27 (d,J = 4.9 Hz, 2H), 4.19 (s, 3H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.48-8.63 (m, 2H), 7.96 (t, J = 7.8 Hz, 1H), 7.78 (d, J = 7.8 Hz, 1H), 7.47-7.50 (m, 2H), 6.84 (d, J = 1.7 Hz, 1H), 4.27 (d, J = 4.9 Hz, 2H), 4.19 (s, 3H).
MS (ESI, pos.ion) m/z: 189.30 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 189.30 [M+H-HCl] + .
步驟4:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(1-甲基-1H -吡唑-5-基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺的合成Step 4: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-(1 Synthesis of -methyl- 1H -pyrazol-5-yl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (92 mg, 0.25 mmol),化合物 (6-(1-甲基-1H -吡唑-5-基)吡啶-2-基)甲胺二鹽酸鹽 (811-3) (103 mg, 0.55 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (150 mg, 0.78 mmol) 和N -羥基-7-氮雜苯並三氮唑 (71 mg, 0.52 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌6 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體67 mg,收率50%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (92 mg, 0.25 mmol), compound (6-(1-methyl- 1H -pyrazol-5-yl)pyridin-2-yl)methanamine dihydrochloride (811-3) (103 mg, 0.55 mmol), 1 -Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (150 mg, 0.78 mmol) and N -hydroxy-7-azabenzotriazole (71 mg, 0.52 mmol) It was dissolved in dichloromethane (6 mL), and N , N -diisopropylethylamine (0.4 mL, 2.0 mmol) was added dropwise to this solution at room temperature, stirred at room temperature for 6 h, and washed with water (50 mL) , extracted with dichloromethane (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/ 1), 67 mg of white solid was obtained, and the yield was 50%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.62 (s, 1H), 7.84-7.90 (m, 1H), 7.64-7.67 (m, 1H), 7.48 (s, 1H), 7.39-7.40 (m, 1H), 7.10-7.16 (m, 1H), 7.06 (s, 2H), 7.04 (t,J F-H = 75.0 Hz, 1H), 6.88-6.91 (m, 1H), 6.78 (s, 1H), 4.38-4.53 (m, 3H), 4.15 (s, 3H), 4.05-4.10 (m, 1H), 3.90 (d,J = 4.7 Hz, 2H), 3.43-3.50 (m, 2H), 2.57-2.67 (m, 1H), 2.04 (s, 3H), 1.88-1.96 (m, 1H), 1.23-1.26 (m, 1H), 0.50-0.61 (m, 2H), 0.28-0.38 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.62 (s, 1H), 7.84-7.90 (m, 1H), 7.64-7.67 (m, 1H), 7.48 (s, 1H), 7.39 -7.40 (m, 1H), 7.10-7.16 (m, 1H), 7.06 (s, 2H), 7.04 (t, J FH = 75.0 Hz, 1H), 6.88-6.91 (m, 1H), 6.78 (s, 1H), 4.38-4.53 (m, 3H), 4.15 (s, 3H), 4.05-4.10 (m, 1H), 3.90 (d, J = 4.7 Hz, 2H), 3.43-3.50 (m, 2H), 2.57 -2.67 (m, 1H), 2.04 (s, 3H), 1.88-1.96 (m, 1H), 1.23-1.26 (m, 1H), 0.50-0.61 (m, 2H), 0.28-0.38 (m, 2H) .
MS (ESI, pos.ion) m/z: 540.15 [M+H]+ .MS (ESI, pos.ion) m/z: 540.15 [M+H] + .
實施例63:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-(1-甲基-1H -吡唑-4-基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 63: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( 1-Methyl-1 H -pyrazol-4-yl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (91 mg, 0.25 mmol),化合物 (6-(1-甲基-1H -吡唑-4-基)吡啶-2-基)甲胺二鹽酸鹽 (96 mg, 0.51 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (153 mg, 0.79 mmol) 和N -羥基-7-氮雜苯並三氮唑 (71 mg, 0.52 mmol)溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌6 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體53 mg,收率39%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (91 mg, 0.25 mmol), compound (6-(1-methyl- 1H -pyrazol-4-yl)pyridin-2-yl)methanamine dihydrochloride (96 mg, 0.51 mmol), 1-ethyl-( 3-Dimethylaminopropyl)carbodiimide hydrochloride (153 mg, 0.79 mmol) and N -hydroxy-7-azabenzotriazole (71 mg, 0.52 mmol) in dichloromethane (6 mL), N , N -diisopropylethylamine (0.4 mL, 2.0 mmol) was added dropwise to this solution at room temperature, stirred at room temperature for 6 h, washed with water (50 mL), and washed with dichloromethane. Extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain a white Solid 53 mg, yield 39%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.55 (br.s, 1H), 8.26 (s, 1H), 7.99 (s, 1H), 7.68-7.72 (m, 1H), 7.48-7.52 (m, 1H), 7.18-7.21 (m, 1H), 7.09-7.13 (m, 1H), 7.05-7.08 (m, 2H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.89 (d,J = 8.3 Hz, 1H), 4.31-4.48 (m, 3H), 4.00-4.20 (m, 1H), 3.84-3.93 (m, 5H), 3.40-3.57 (m, 2H), 2.56-2.74 (m, 1H), 2.04 (s, 3H), 1.91-1.96 (m, 1H), 1.22-1.29 (m, 1H), 0.49-0.65 (m, 2H), 0.27-0.40 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.55 (br.s, 1H), 8.26 (s, 1H), 7.99 (s, 1H), 7.68-7.72 (m, 1H), 7.48 -7.52 (m, 1H), 7.18-7.21 (m, 1H), 7.09-7.13 (m, 1H), 7.05-7.08 (m, 2H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.89 ( d, J = 8.3 Hz, 1H), 4.31-4.48 (m, 3H), 4.00-4.20 (m, 1H), 3.84-3.93 (m, 5H), 3.40-3.57 (m, 2H), 2.56-2.74 ( m, 1H), 2.04 (s, 3H), 1.91-1.96 (m, 1H), 1.22-1.29 (m, 1H), 0.49-0.65 (m, 2H), 0.27-0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 540.20 [M+H]+ .MS (ESI, pos.ion) m/z: 540.20 [M+H] + .
實施例64:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基-N -苯基吡啶醯胺 Example 64: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -methyl- N -phenylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.19 mmol),N -甲基苯胺 (578 mg, 0.53 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (1925 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (40 mg, 0.29 mmol) 溶於二氯甲烷 (5 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.13 mL, 0.79 mmol),室溫反應18 h,加水洗 (15 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體41 mg,收率36%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.19 mmol), N -methylaniline (578 mg, 0.53 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride Salt (1925 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (40 mg, 0.29 mmol) were dissolved in dichloromethane (5 mL) and added dropwise to this solution at 0°C N , N -diisopropylethylamine (0.13 mL, 0.79 mmol), reacted at room temperature for 18 h, washed with water (15 mL), then extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 41 mg of white solid with a yield of 36%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.48 – 7.61 (m, 1H), 7.09 – 7.26 (m, 8H), 6.88 (s, 1H), 6.84 (d,J = 7.7 Hz, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 4.430 – 4.55 (m, 1H), 4.31 – 4.45 (m, 2H), 3.87 – 3.96 (m, 1H), 3.86 (d,J = 6.7 Hz, 2H), 3.50 – 3.61 (m, 1H), 3.51 (s, 3H), 3.26 – 3.41 (m, 1H), 2.50 – 2.61 (m, 1H), 2.28 – 2.39 (m, 1H), 2.11 (s, 3H), 1.24 – 1.37 (m, 1H), 0.60 – 0.66 (m, 2H), 0.30 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.48 – 7.61 (m, 1H), 7.09 – 7.26 (m, 8H), 6.88 (s, 1H), 6.84 (d, J = 7.7 Hz, 1H) ), 6.60 (t, J FH = 75.5 Hz, 1H), 4.430 – 4.55 (m, 1H), 4.31 – 4.45 (m, 2H), 3.87 – 3.96 (m, 1H), 3.86 (d, J = 6.7 Hz , 2H), 3.50 – 3.61 (m, 1H), 3.51 (s, 3H), 3.26 – 3.41 (m, 1H), 2.50 – 2.61 (m, 1H), 2.28 – 2.39 (m, 1H), 2.11 (s , 3H), 1.24 – 1.37 (m, 1H), 0.60 – 0.66 (m, 2H), 0.30 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 593.25 [M+H]+ .MS (ESI, pos.ion) m/z: 593.25 [M+H] + .
實施例65:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基-N -(4-甲基苯基)吡啶醯胺 Example 65: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -methyl- N- (4-methylphenyl)pyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -甲基-4-甲基苯胺 (62 mg, 0.51 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (251 mg, 1.31 mmol) 和N -羥基-7-氮雜苯並三氮唑 (54 mg, 0.40 mmol) 溶於二氯甲烷 (5 mL)中,冷卻至0℃,加入N ,N -二異丙基乙胺 (183 mg, 1.42 mmol),室溫反應22 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體84 mg,產率69%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N -methyl-4-methylaniline (62 mg, 0.51 mmol), 1-ethyl-(3-dimethylaminopropyl)carbanide Diimine hydrochloride (251 mg, 1.31 mmol) and N -hydroxy-7-azabenzotriazole (54 mg, 0.40 mmol) were dissolved in dichloromethane (5 mL), cooled to 0 °C, N , N -diisopropylethylamine (183 mg, 1.42 mmol) was added, the reaction was carried out at room temperature for 22 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sodium sulfate After drying, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 84 mg of a white solid with a yield of 69%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.52 (br.s, 1H), 7.10 - 7.27 (m, 4H), 6.86 - 7.00 (m, 5H), 6.61 (t,J = 75.5 Hz, 1H), 4.43 - 4.50 (m, 3H), 3.87 - 3.94 (m, 3H), 3.53 - 3.65 (m, 1H), 3.45 (s, 3H), 3.25 - 3.42 (m, 1H), 2.50 - 2.64 (m, 1H), 2.26 - 2.40 (m, 1H), 2.26 (s, 3H), 2.13 (s, 3H), 1.26 - 1.35 (m, 1H), 0.60 - 0.72 (m, 2H), 0.29 - 0.45 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.52 (br.s, 1H), 7.10 - 7.27 (m, 4H), 6.86 - 7.00 (m, 5H), 6.61 (t, J = 75.5 Hz , 1H), 4.43 - 4.50 (m, 3H), 3.87 - 3.94 (m, 3H), 3.53 - 3.65 (m, 1H), 3.45 (s, 3H), 3.25 - 3.42 (m, 1H), 2.50 - 2.64 (m, 1H), 2.26 - 2.40 (m, 1H), 2.26 (s, 3H), 2.13 (s, 3H), 1.26 - 1.35 (m, 1H), 0.60 - 0.72 (m, 2H), 0.29 - 0.45 (m, 2H).
MS (ESI, pos.ion) m/z: 607.40 [M+H]+ .MS (ESI, pos.ion) m/z: 607.40 [M+H] + .
實施例66:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 Example 66: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (4- fluorophenyl) - N - methylpyridine Amides
將化合物6-(((2R)-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (102 mg, 0.20 mmol),4-氟-N -甲基苯胺 (50 mg, 0.40 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (40 mg, 0.29 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到49 mg白色固體,收率39%。Compound 6-(((2R)-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino ) methyl)picolinic acid (102 mg, 0.20 mmol), 4-fluoro- N -methylaniline (50 mg, 0.40 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Amine hydrochloride (192 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (40 mg, 0.29 mmol) were dissolved in dichloromethane (5 mL) and added to this solution at 0°C N ,N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added dropwise to it, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous It was dried over sodium sulfate, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 49 mg of white solid with a yield of 39%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.55 – 7.68 (m, 1H), 7.28 – 7.43 (m, 1H), 7.04 – 7.17 (m, 4H), 6.84 – 6.98 (m, 4H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.40 – 4.51 (m, 3H), 3.86 – 3.98 (m, 1H), 3.88 (d,J = 5.8 Hz, 2H), 3.49 – 3.59 (m, 1H), 3.50 (s, 3H), 3.28 – 3.43 (m, 1H), 2.49 – 2.60 (m, 1H), 2.36 – 2.48 (m, 1H), 2.15 (s, 3H), 1.26 – 1.36 (m, 1H), 0.66 – 0.70 (m, 2H), 0.33 – 0.342 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.55 – 7.68 (m, 1H), 7.28 – 7.43 (m, 1H), 7.04 – 7.17 (m, 4H), 6.84 – 6.98 (m, 4H) , 6.62 (t, J FH = 75.6 Hz, 1H), 4.40 – 4.51 (m, 3H), 3.86 – 3.98 (m, 1H), 3.88 (d, J = 5.8 Hz, 2H), 3.49 – 3.59 (m, 1H), 3.50 (s, 3H), 3.28 – 3.43 (m, 1H), 2.49 – 2.60 (m, 1H), 2.36 – 2.48 (m, 1H), 2.15 (s, 3H), 1.26 – 1.36 (m, 1H), 0.66 – 0.70 (m, 2H), 0.33 – 0.342 (m, 2H).
MS (ESI, pos.ion) m/z: 611.30 [M+H]+ .MS (ESI, pos.ion) m/z: 611.30 [M+H] + .
實施例67:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(2,4-二氟苯基)-N -甲基吡啶醯胺 Example 67: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (2,4-difluorophenyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (86 mg, 0.17 mmol),2,4-二氟-N -甲基苯胺 (41 mg, 0.29 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (173 mg, 0.90 mmol) 和N -羥基-7-氮雜苯並三氮唑 (48 mg, 0.35 mmol) 溶於二氯甲烷 (5 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),室溫反應12 h,加水洗 (15 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到33 mg白色固體,收率30%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (86 mg, 0.17 mmol), 2,4-difluoro- N -methylaniline (41 mg, 0.29 mmol), 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (173 mg, 0.90 mmol) and N -hydroxy-7-azabenzotriazole (48 mg, 0.35 mmol) were dissolved in dichloromethane (5 mL) at 0°C N , N -diisopropylethylamine (0.15 mL, 0.91 mmol) was added dropwise to this solution, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and then extracted with dichloromethane (5 mL × 3), The organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 33 mg of white solid, yield 30% .
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.60 – 7.66 (m, 1H), 7.44 – 7.52 (m, 1H), 7.18 (d,J = 7.5Hz, 1H), 7.06 – 7.13 (m, 3H), 6.89 (s, 1H), 6.84 (d,J = 8.0Hz, 1H), 6.69 – 6.76 (m, 1H), 6.60 (t,J F-H = 75.6 Hz, 1H), 4.45 – 4.58 (m, 1H), 4.25 – 4.38 (m, 2H), 3.88 – 3.98 (m, 1H), 3.86 (d,J = 6.6 Hz, 2H), 3.56 (d,J = 10.4Hz, 1H), 3.42 (s, 3H), 3.25 – 3.41 (m, 1H), 2.48 – 2.62 (m, 1H), 2.34 – 2.44 (m, 1H), 2.13 (s, 3H), 1.24 – 1.36 (m, 1H), 0.62 – 0.66 (m, 2H), 0.32 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.60 – 7.66 (m, 1H), 7.44 – 7.52 (m, 1H), 7.18 (d, J = 7.5Hz, 1H), 7.06 – 7.13 (m , 3H), 6.89 (s, 1H), 6.84 (d, J = 8.0Hz, 1H), 6.69 – 6.76 (m, 1H), 6.60 (t, J FH = 75.6 Hz, 1H), 4.45 – 4.58 (m , 1H), 4.25 – 4.38 (m, 2H), 3.88 – 3.98 (m, 1H), 3.86 (d, J = 6.6 Hz, 2H), 3.56 (d, J = 10.4Hz, 1H), 3.42 (s, 3H), 3.25 – 3.41 (m, 1H), 2.48 – 2.62 (m, 1H), 2.34 – 2.44 (m, 1H), 2.13 (s, 3H), 1.24 – 1.36 (m, 1H), 0.62 – 0.66 ( m, 2H), 0.32 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 629.30 [M+H]+ .MS (ESI, pos.ion) m/z: 629.30 [M+H] + .
實施例68:化合物6-(((2R )-1-乙醯基-4-(3-((2-環丙基丙烷-2-基)氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 Example 68: Compound 6-((( 2R )-1-acetyl-4-(3-((2-cyclopropylpropan-2-yl)oxy)-4-(difluoromethoxy) ) phenyl) pyrrolidine-2-acyl-amino) methyl) - N - (4- fluorophenyl) - N - methylpyridine Amides
步驟1:化合物 (R )-1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-羧酸 的合成Step 1: Compound ( R )-1-Acetyl-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro- 1H -pyrrole- Synthesis of 2-Carboxylic Acid
將化合物 (R )-1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-羧酸甲酯 (1.8 g, 0.56 mmol) 溶於四氫呋喃 (15 mL) 和水 (8 mL) 的混合溶劑中,加入一水合氫氧化鋰 (964 mg,23 mmol),50℃反應2 h後停止,加稀鹽酸調節溶液pH=1,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淺黃色固體1.6 g,收率94%。The compound ( R )-1-acetyl-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro- 1H -pyrrole-2- Methyl carboxylate (1.8 g, 0.56 mmol) was dissolved in a mixed solvent of tetrahydrofuran (15 mL) and water (8 mL), lithium hydroxide monohydrate (964 mg, 23 mmol) was added, and the reaction was stopped after 2 h at 50 °C , dilute hydrochloric acid was added to adjust the pH of the solution to 1, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 1.6 g of a pale yellow solid with a yield of 94%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.49 – 7.51 (m, 2H), 7.38 – 7.42 (m, 2H), 7.29 – 7.32 (m, 2H), 7.19 (d,J = 8.3 Hz, 1H), 7.08 – 7.11 (m, 1H), 6.78 (t,J F-H = 74.8 Hz, 1H), 6.31 – 6.34 (m, 1H), 5.39 – 5.42 (m, 0.3H), 5.22 – 5.25 (m, 0.7H), 5.22 (s, 2H), 4.75 – 4.82 (m, 2H), 2.22 (s, 2H), 2.07 (s, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.49 – 7.51 (m, 2H), 7.38 – 7.42 (m, 2H), 7.29 – 7.32 (m, 2H), 7.19 (d, J = 8.3 Hz, 1H), 7.08 – 7.11 (m, 1H), 6.78 (t, J FH = 74.8 Hz, 1H), 6.31 – 6.34 (m, 1H), 5.39 – 5.42 (m, 0.3H), 5.22 – 5.25 ( m, 0.7H), 5.22 (s, 2H), 4.75 – 4.82 (m, 2H), 2.22 (s, 2H), 2.07 (s, 1H).
MS (ESI, pos.ion) m/z: 404.05 [M+H]+ .MS (ESI, pos.ion) m/z: 404.05 [M+H] + .
步驟2:化合物 (R )-6-((1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺的合成Step 2: Compound ( R )-6-((1-Acetyl-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro-1 H - pyrrole-2-acyl-amino) methyl) - N - (4- fluorophenyl) - N - synthesis of Amides methylpyridine
將化合物 (R )-1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-羧酸 (200 mg, 0.50 mmol),6-(氨基甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺二鹽酸鹽 (889-1) (181 mg, 0.54 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (476 mg, 2.48 mmol) 和N -羥基-7-氮雜苯並三氮唑 (136 mg, 1.00 mmol)溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.6 mL, 4.0 mmol),室溫反應12 h,加水洗 (20 mL) 攪拌,分離有機相,有機相用無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到301 mg白色固體,收率94%。The compound ( R )-1-acetyl-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro- 1H -pyrrole-2- carboxylic acid (200 mg, 0.50 mmol), 6- ( aminomethyl) - N - (4- fluorophenyl) - N - methyl acyl pyridine dihydrochloride (889-1) (181 mg, 0.54 mmol ), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (476 mg, 2.48 mmol) and N -hydroxy-7-azabenzotriazole (136 mg, 1.00 mmol) was dissolved in dichloromethane (10 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.6 mL, 4.0 mmol) was added, the reaction was carried out at room temperature for 12 h, and washed with water (20 mL). ) was stirred, the organic phase was separated, the organic phase was dried with anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 301 mg of white solid, Yield 94%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.37 – 7.47 (m, 6H), 7.16 – 7.22 (m, 2H), 7.00 – 7.12 (m, 4H), 6.86 – 6.98 (m, 3H), 6.60 (t,J F-H = 74.9 Hz, 1H), 6.17 – 6.20 (m, 1H), 5.30 – 5.36 (m, 0.7H), 5.19 – 5.23 (m, 0.3H), 5.17 (s, 2H), 4.61 – 4.78 (m, 2H), 4.29 – 4.49 (m, 2H), 3.50 (s, 3H), 2.22 (s, 2H), 2.07 (s, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.37 – 7.47 (m, 6H), 7.16 – 7.22 (m, 2H), 7.00 – 7.12 (m, 4H), 6.86 – 6.98 (m, 3H) , 6.60 (t, J FH = 74.9 Hz, 1H), 6.17 – 6.20 (m, 1H), 5.30 – 5.36 (m, 0.7H), 5.19 – 5.23 (m, 0.3H), 5.17 (s, 2H), 4.61 – 4.78 (m, 2H), 4.29 – 4.49 (m, 2H), 3.50 (s, 3H), 2.22 (s, 2H), 2.07 (s, 1H).
MS (ESI, pos.ion) m/z: 645.10 [M+H]+ .MS (ESI, pos.ion) m/z: 645.10 [M+H] + .
步驟3:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 的合成Step 3: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carbamoylamino)methyl) - Synthesis of N- (4-fluorophenyl) -N-picolinamide
將化合物 (R )-6-((1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 (301 mg, 0.47mmol) 溶於甲醇 (10 mL),加入Pd/C (33 mg, 10%),通入氫氣室溫反應8 h,過濾除去催化劑,濾液濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 25/1),得到白色固體227 mg,收率87%。Compound (R) -6 - ((1- acetyl-4- (3- (benzyloxy) -4- (difluoromethoxy) phenyl) -2,5-dihydro -1 H - acyl pyrrole-2-ylamino) methyl) - N - (4- fluorophenyl) - N - Amides methylpyridine (301 mg, 0.47mmol) was dissolved in methanol (10 mL), was added Pd / C (33 mg, 10%), passed hydrogen into the reaction at room temperature for 8 h, filtered to remove the catalyst, concentrated the filtrate, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 25/1) to obtain White solid 227 mg, yield 87%.
1 H NMR (600 MHz, CDCl3 ) δ (ppm): 7.54 – 7.58 (m, 1H), 7.17 – 7.24 (m, 2H), 7.00 – 7.14 (m, 4H), 6.92 – 6.98 (m, 2H), 6.78 (d,J = 8.0 Hz, 1H), 6.55 (t,J F-H = 74.4 Hz, 1H), 4.55 – 4.63 (m, 0.4H), 4.43 – 4.53 (m, 2H), 4.28 – 4.33 (m, 0.6H), 4.13 – 4.16 (m, 0.4H), 3.92 – 3.96 (m, 0.7H), 3.61 – 3.65 (m, 1H), 3.52 – 3.54 (m, 3H), 3.29 – 3.40 (m, 1H), 2.81 – 2.86 (m, 0.3H), 2.55 – 2.61 (m, 0.7H), 2.32 – 2.39 (m, 1H), 2.17 (s, 2H), 2.08 (s, 1H). 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 7.54 – 7.58 (m, 1H), 7.17 – 7.24 (m, 2H), 7.00 – 7.14 (m, 4H), 6.92 – 6.98 (m, 2H) , 6.78 (d, J = 8.0 Hz, 1H), 6.55 (t, J FH = 74.4 Hz, 1H), 4.55 – 4.63 (m, 0.4H), 4.43 – 4.53 (m, 2H), 4.28 – 4.33 (m , 0.6H), 4.13 – 4.16 (m, 0.4H), 3.92 – 3.96 (m, 0.7H), 3.61 – 3.65 (m, 1H), 3.52 – 3.54 (m, 3H), 3.29 – 3.40 (m, 1H) ), 2.81 – 2.86 (m, 0.3H), 2.55 – 2.61 (m, 0.7H), 2.32 – 2.39 (m, 1H), 2.17 (s, 2H), 2.08 (s, 1H).
MS (ESI, pos.ion) m/z: 557.15 [M+H]+ .MS (ESI, pos.ion) m/z: 557.15 [M+H] + .
步驟4:化合物6-(((2R )-1-乙醯基-4-(3-((2-環丙基丙烷-2-基)氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺的合成Step 4: Compound 6-((( 2R )-1-acetyl-4-(3-((2-cyclopropylpropan-2-yl)oxy)-4-(difluoromethoxy) phenyl) pyrrolidine-2-acyl-amino) methyl) - N - (4- fluorophenyl) - N - synthesis of Amides methylpyridine
將6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 (112 mg, 0.20mmol),1-環丙基-1-甲基乙醇 (60 mg, 0.60mmol),三苯基膦 (101 mg, 0.50mmol)溶解在乾燥的四氫呋喃 (10mL) 溶液中,冰浴條件下緩慢加入偶氮二甲醯胺 (100 mg, 0.58mmol),30℃反應12 h,加入水 (10mL),用乙酸乙酯 (5 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體56 mg,收率43%。The 6 - (((2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-hydroxyphenyl) pyrrolidine-2-acyl-amino) methyl) - N - (4-Fluorophenyl) -N -picolinamide (112 mg, 0.20 mmol), 1-cyclopropyl-1-methylethanol (60 mg, 0.60 mmol), triphenylphosphine (101 mg, 0.50 mmol) was dissolved in dry tetrahydrofuran (10 mL) solution, azodimethylamide (100 mg, 0.58 mmol) was slowly added under ice bath conditions, reacted at 30 °C for 12 h, added water (10 mL), and washed with ethyl acetate. (5 mL × 3) extracted, the organic phase was dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 56 mg of white solid , the yield is 43%.
1 H NMR (600 MHz, CDCl3 ) δ (ppm): 7.57 – 7.65 (m, 1H), 7.31 – 7.38 (m, 1H), 7.17 – 7.20 (m, 1H), 7.12 (d,J = 8.2 Hz, 1H), 7.05 – 7.09 (m, 3H), 7.00 (d,J = 8.2 Hz, 1H), 6.90 – 6.96 (m, 2H), 6.58 (t,J F-H = 75.7 Hz, 1H), 4.50 – 4.54 (m, 1H), 4.36 – 4.44 (m, 2H), 3.95 – 3.98 (m, 1H), 3.57 (t,J = 10.7 Hz, 1H), 3.51 (s, 3H), 3.31 – 3.39 (m, 1H), 3.54 – 3.59 (m, 1H), 3.38 – 3.46 (m, 1H), 2.16 (s, 3H), 1.26 (s, 6H), 1.16 – 1.23 (m, 1H), 0.48 – 0.51 (m, 2H), 0.38 – 0.40 (m, 2H). 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 7.57 – 7.65 (m, 1H), 7.31 – 7.38 (m, 1H), 7.17 – 7.20 (m, 1H), 7.12 (d, J = 8.2 Hz , 1H), 7.05 – 7.09 (m, 3H), 7.00 (d, J = 8.2 Hz, 1H), 6.90 – 6.96 (m, 2H), 6.58 (t, J FH = 75.7 Hz, 1H), 4.50 – 4.54 (m, 1H), 4.36 – 4.44 (m, 2H), 3.95 – 3.98 (m, 1H), 3.57 (t, J = 10.7 Hz, 1H), 3.51 (s, 3H), 3.31 – 3.39 (m, 1H) ), 3.54 – 3.59 (m, 1H), 3.38 – 3.46 (m, 1H), 2.16 (s, 3H), 1.26 (s, 6H), 1.16 – 1.23 (m, 1H), 0.48 – 0.51 (m, 2H) ), 0.38 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 661.15 [M+Na]+ .MS (ESI, pos.ion) m/z: 661.15 [M+Na] + .
實施例69:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 Example 69: Compound 6 - (((2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2 methyl acyl amino) methyl) - N - (4- fluorophenyl) - N - methylpyridine Amides
步驟1:化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-1,2-二羧酸酯合成Step 1: Compound (2 R) -1- tert-butyl 2-methyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) -pyrrolidine-l, Synthesis of Dicarboxylates
將化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-1,2-二羧酸酯 (510 mg, 1.32 mmol) 溶於乾燥的四氫呋喃 (10 mL) 中,冷卻至0℃後,加入60%氫化鈉 (71 mg, 1.78 mmol),室溫反應30 min,冰浴中加入碘甲烷-d3 (382 mg, 2.64 mmol),室溫反應7 h後停止,緩慢加冰水 (10 mL),乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,減壓濃縮,進行矽膠柱層析色分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到無色液體127 mg,產率24%。Compound ( 2R )-1-tert-butyl 2-methyl 4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-1,2-dicarboxylate (510 mg, 1.32 mmol) was dissolved in dry tetrahydrofuran (10 mL), cooled to 0 °C, 60% sodium hydride (71 mg, 1.78 mmol) was added, the reaction was carried out at room temperature for 30 min, and iodomethane- d 3 (382 mmol) was added to the ice bath mg, 2.64 mmol), the reaction was stopped after 7 h at room temperature, ice water (10 mL) was slowly added, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and concentrated under reduced pressure to carry out Silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) gave 127 mg of a colorless liquid with a yield of 24%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.13 (d,J = 8.1 Hz, 1H), 6.81 – 6.85 (m, 2H), 6.54 (t,J F-H = 75.2 Hz, 1H), 4.34 – 4.44 (m, 1H), 4.06 – 4.10 (m, 0.6H), 3.95 – 3.99 (m, 0.4H), 3.79 (d,J = 6.9 Hz, 1H), 3.43 – 3.49 (m, 1H), 3.33 – 3.39 (m, 1H), 2.64 – 2.72 (m, 1H), 2.01 – 2.12 (m, 1H), 1.46 – 1.49 (m, 9H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.13 (d, J = 8.1 Hz, 1H), 6.81 – 6.85 (m, 2H), 6.54 (t, J FH = 75.2 Hz, 1H), 4.34 – 4.44 (m, 1H), 4.06 – 4.10 (m, 0.6H), 3.95 – 3.99 (m, 0.4H), 3.79 (d, J = 6.9 Hz, 1H), 3.43 – 3.49 (m, 1H), 3.33 – 3.39 (m, 1H), 2.64 – 2.72 (m, 1H), 2.01 – 2.12 (m, 1H), 1.46 – 1.49 (m, 9H).
MS (ESI, pos.ion) m/z: 349.20 [M-55]+ .MS (ESI, pos.ion) m/z: 349.20 [M-55] + .
步驟2:化合物 (2R )-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-羧酸甲酯鹽酸鹽的合成Step 2: Compound (2 R) -4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物 (2R )-1-叔丁基 2-甲基 4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-1,2-二羧酸酯 (123 mg, 0.30 mmol) 溶解於二氯甲烷 (6 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (5 mL),室溫反應1 h,除去溶劑,得到無色液體102 mg,產率98%。Compound (2 R) -1- tert-butyl 2-methyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-1,2-dicarboximide The acid ester (123 mg, 0.30 mmol) was dissolved in dichloromethane (6 mL) solution, 4 mol/L HCl in ethyl acetate solution (5 mL) was added, the reaction was carried out at room temperature for 1 h, and the solvent was removed to obtain a colorless liquid 102 mg, 98% yield.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.14 (d,J = 8.2 Hz, 1H), 7.10 (d,J = 1.9 Hz, 1H), 6.92 (dd,J = 8.2, 1.9 Hz, 1H), 6.71 (t,J F-H = 75.4 Hz, 1H), 4.61 – 4.65 (m, 1H), 3.90 (s, 3H), 3.80 – 3.84 (m, 1H), 3.68 – 3.75 (m, 1H), 3.36 – 3.41 (m, 1H), 2.84 – 2.90 (m, 1H), 2.22 – 2.31 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.14 (d, J = 8.2 Hz, 1H), 7.10 (d, J = 1.9 Hz, 1H), 6.92 (dd, J = 8.2, 1.9 Hz , 1H), 6.71 (t, J FH = 75.4 Hz, 1H), 4.61 – 4.65 (m, 1H), 3.90 (s, 3H), 3.80 – 3.84 (m, 1H), 3.68 – 3.75 (m, 1H) , 3.36 – 3.41 (m, 1H), 2.84 – 2.90 (m, 1H), 2.22 – 2.31 (m, 1H).
MS (ESI, pos.ion) m/z: 305.15 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 305.15 [M+H-HCl] + .
步驟3:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基) 吡咯烷-2-羧酸甲酯的合成Step 3: Compound (2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2-carboxylate synthesis
將化合物 (2R )-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (100 mg, 0.29 mmol) 溶解在二氯甲烷 (3 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),乙醯氯 (69 mg, 0.88 mmol),室溫反應4 h後停止,加水 (15 mL),二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析色譜分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到淺黃色液體93 mg,產率91%。Compound (2 R) -4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2-carboxylic acid methyl ester hydrochloride (100 mg, 0.29 mmol ) was dissolved in dichloromethane (3 mL), cooled to 0 °C, added N , N -diisopropylethylamine (0.15 mL, 0.91 mmol), acetyl chloride (69 mg, 0.88 mmol), room temperature The reaction was stopped after 4 h, water (15 mL) was added, and dichloromethane was extracted (10 mL × 3). The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ Ethyl acetate (v/v) = 1/1) to obtain a pale yellow liquid 93 mg with a yield of 91%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.14 (d,J = 7.9 Hz, 1H), 6.85 (s, 1H), 6.79 – 6.83 (m, 1H), 6.55 (t,J F-H = 75.1 Hz, 1H), 4.49 – 4.53 (m, 1H), 3.95 – 3.99 (m, 1H), 3.79 (s, 3H), 3.65 (t,J = 10.4 Hz, 1H), 3.41 – 3.48 (m, 1H), 2.66 – 2.73 (m, 1H), 2.14 (s, 3H), 2.04 – 2.10 (m, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.14 (d, J = 7.9 Hz, 1H), 6.85 (s, 1H), 6.79 – 6.83 (m, 1H), 6.55 (t, J FH = 75.1 Hz, 1H), 4.49 – 4.53 (m, 1H), 3.95 – 3.99 (m, 1H), 3.79 (s, 3H), 3.65 (t, J = 10.4 Hz, 1H), 3.41 – 3.48 (m, 1H) ), 2.66 – 2.73 (m, 1H), 2.14 (s, 3H), 2.04 – 2.10 (m, 1H).
MS (ESI, pos.ion) m/z: 347.10 [M+H]+ .MS (ESI, pos.ion) m/z: 347.10 [M+H] + .
步驟4:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-羧酸 的合成Step 4: Compound (2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-羧酸甲酯 (92 mg, 0.27 mmol) 溶於四氫呋喃 (3 mL) 和水 (3 mL) 的混合溶劑中,加入一水合氫氧化鋰 (56 mg, 1.34 mmol),50℃反應30 min後停止,加稀鹽酸調節溶液pH=1,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淺黃色液體87 mg,收率98%。Compound (2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2-carboxylate (92 mg , 0.27 mmol) was dissolved in a mixed solvent of tetrahydrofuran (3 mL) and water (3 mL), lithium hydroxide monohydrate (56 mg, 1.34 mmol) was added, the reaction was stopped at 50 °C for 30 min, and diluted hydrochloric acid was added to adjust the pH of the solution = 1, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 87 mg of light yellow liquid, the yield was 98%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.12 (d,J = 8.2 Hz, 1H), 7.08 (d,J = 1.8 Hz, 1H), 6.93 (dd,J = 8.3, 2.0 Hz, 1H), 6.69 (t,J F-H = 75.5 Hz, 1H), 4.44 – 4.49 (m, 1H), 4.09 – 4.15 (m, 1H), 3.49 – 3.56 (m, 1H), 3.58 – 3.65 (m, 1H), 2.73 – 2.80 (m, 1H), 2.14 (s, 3H), 2.02 – 2.10 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.12 (d, J = 8.2 Hz, 1H), 7.08 (d, J = 1.8 Hz, 1H), 6.93 (dd, J = 8.3, 2.0 Hz , 1H), 6.69 (t, J FH = 75.5 Hz, 1H), 4.44 – 4.49 (m, 1H), 4.09 – 4.15 (m, 1H), 3.49 – 3.56 (m, 1H), 3.58 – 3.65 (m, 1H), 2.73 – 2.80 (m, 1H), 2.14 (s, 3H), 2.02 – 2.10 (m, 1H).
MS (ESI, pos.ion) m/z: 333.10 [M+H]+ .MS (ESI, pos.ion) m/z: 333.10 [M+H] + .
步驟5:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺的合成Step 5: Compound 6 - (((2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2- acyl amino) methyl) - N - (4- fluorophenyl) - N - acyl amine-methylpyridine
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-羧酸 (45 mg, 0.14 mmol),6-(氨基甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺二鹽酸鹽 (889-1) (61 mg, 0.18 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (132 mg, 0.69 mmol) 和N -羥基-7-氮雜苯並三氮唑 (36 mg, 0.26 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.16 mL, 0.97 mmol),室溫反應11 h,加水 (20 mL) 攪拌5 min,用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體37 mg,產率47%。Compound (2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2-carboxylic acid (45 mg, 0.14 mmol), 6- (aminomethyl) - N - (4- fluorophenyl) - N - methyl acyl pyridine dihydrochloride (889-1) (61 mg, 0.18 mmol), 1- ethyl - (3-Dimethylaminopropyl)carbodiimide hydrochloride (132 mg, 0.69 mmol) and N -hydroxy-7-azabenzotriazole (36 mg, 0.26 mmol) in dichloro In methane (10 mL), after cooling to 0 °C, N , N -diisopropylethylamine (0.16 mL, 0.97 mmol) was added, the reaction was carried out at room temperature for 11 h, water (20 mL) was added, stirred for 5 min, and dichloromethane was added. Methane extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) , 37 mg of white solid was obtained, and the yield was 47%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.52 – 7.67 (m, 1H), 7.34 (br.s, 1H), 7.00 – 7.20 (m, 5H), 6.83 – 6.99 (m, 4H), 6.55 (t,J F-H = 75.2 Hz, 1H), 4.47 – 4.57 (m, 1H), 4.32 – 4.47 (m, 2H), 3.95 – 3.99 (m, 1H), 3.60 (t,J = 10.5 Hz, 1H), 3.49 (s, 3H), 3.30 – 3.45 (m, 1H), 2.52 – 2.62 (m, 1H), 2.39 – 2.48 (m, 1H), 2.15 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.52 – 7.67 (m, 1H), 7.34 (br.s, 1H), 7.00 – 7.20 (m, 5H), 6.83 – 6.99 (m, 4H) , 6.55 (t, J FH = 75.2 Hz, 1H), 4.47 – 4.57 (m, 1H), 4.32 – 4.47 (m, 2H), 3.95 – 3.99 (m, 1H), 3.60 (t, J = 10.5 Hz, 1H), 3.49 (s, 3H), 3.30 – 3.45 (m, 1H), 2.52 – 2.62 (m, 1H), 2.39 – 2.48 (m, 1H), 2.15 (s, 3H).
MS (ESI, pos.ion) m/z: 574.35 [M+H]+ .MS (ESI, pos.ion) m/z: 574.35 [M+H] + .
實施例70:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘代)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 Example 70: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethyl oxy) phenyl) pyrrolidine-2-acyl-amino) methyl) - N - (4- fluorophenyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (90 mg, 0.18 mmol),4-氟-N -甲基苯 (44 mg, 0.35 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (172 mg, 0.9 mmol) 和N -羥基-7-氮雜苯並三氮唑 (49 mg, 0.36 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.21 mL, 1.3 mmol),室溫反應18 h,加水 (15 mL),二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體46 mg,產率43%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy)benzene yl)pyrrolidine-2-carbamoylamino)methyl)picolinic acid (90 mg, 0.18 mmol), 4-fluoro- N -methylbenzene (44 mg, 0.35 mmol), 1-ethyl-(3-di- Methylaminopropyl)carbodiimide hydrochloride (172 mg, 0.9 mmol) and N -hydroxy-7-azabenzotriazole (49 mg, 0.36 mmol) were dissolved in dichloromethane (5 mL) ), cooled to 0 °C, added N , N -diisopropylethylamine (0.21 mL, 1.3 mmol), reacted at room temperature for 18 h, added water (15 mL), and extracted with dichloromethane (5 mL × 3) , the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, concentrated, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 46 mg of white solid, yield 43 %.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.63 – 7.77 (m, 1H), 7.34 – 7.48 (m, 1H), 7.08 – 7.28 (m, 5H), 6.90 – 7.02 (m, 3H), 6.74 (t,J F-H = 75.2 Hz, 1H), 4.38 – 4.53 (m, 2H), 4.21 – 4.32 (m, 1H), 4.06 – 4.15 (m, 1H), 3.62 – 3.67 (m, 1H), 3.37 – 3.54 (m, 1H), 3.46 (s, 3H), 2.61 – 2.75 (m, 1H), 2.14 (s, 3H), 1.99 – 2.13 (m, 1H), 1.26 – 1.40 (m, 1H), 0.60 – 0.68 (m, 2H), 0.32 – 0.44 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.63 – 7.77 (m, 1H), 7.34 – 7.48 (m, 1H), 7.08 – 7.28 (m, 5H), 6.90 – 7.02 (m, 3H) ), 6.74 (t, J FH = 75.2 Hz, 1H), 4.38 – 4.53 (m, 2H), 4.21 – 4.32 (m, 1H), 4.06 – 4.15 (m, 1H), 3.62 – 3.67 (m, 1H) , 3.37 – 3.54 (m, 1H), 3.46 (s, 3H), 2.61 – 2.75 (m, 1H), 2.14 (s, 3H), 1.99 – 2.13 (m, 1H), 1.26 – 1.40 (m, 1H) , 0.60 – 0.68 (m, 2H), 0.32 – 0.44 (m, 2H).
MS (ESI, pos.ion) m/z: 613.80 [M+H]+ .MS (ESI, pos.ion) m/z: 613.80 [M+H] + .
實施例71:化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 Example 71: Compound 6-((( 2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl) - N- (4-Fluorophenyl) -N -picolinamide
將化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (80 mg, 0.16 mmol),4-氟-N -甲基苯胺 (26 mg, 0.21 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (151 mg, 0.79 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.13 mL, 0.79 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體81 mg,收率83%。The compound 6-((( 2R )-1-acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinic acid ( 80 mg, 0.16 mmol), 4-fluoro- N -methylaniline (26 mg, 0.21 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (151 mg , 0.79 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL), to this solution was added dropwise N , N - Diisopropylethylamine (0.13 mL, 0.79 mmol) was reacted at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed, The concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 81 mg of white solid with a yield of 83%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.56 – 7.65 (m, 1H), 7.31 – 7.38 (m, 1H), 7.17 – 7.25 (m, 3H), 7.05 – 7.14 (m, 3H), 6.91 – 6.98 (m, 2H), 6.56 (t,J F-H = 73.6 Hz, 1H), 6.53 (t,J F-H = 73.6 Hz, 1H), 4.48 – 4.60 (m, 1H), 4.34 – 4.48 (m, 2H), 3.97 – 4.01 (m, 1H), 3.58 (t,J = 10.6 Hz, 1H), 3.50 (s, 3H), 3.30 – 3.45 (m, 1H), 2.55 – 2.60 (m, 1H), 2.38 – 2.40 (m, 1H), 2.16 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.56 – 7.65 (m, 1H), 7.31 – 7.38 (m, 1H), 7.17 – 7.25 (m, 3H), 7.05 – 7.14 (m, 3H) , 6.91 – 6.98 (m, 2H), 6.56 (t, J FH = 73.6 Hz, 1H), 6.53 (t, J FH = 73.6 Hz, 1H), 4.48 – 4.60 (m, 1H), 4.34 – 4.48 (m , 2H), 3.97 – 4.01 (m, 1H), 3.58 (t, J = 10.6 Hz, 1H), 3.50 (s, 3H), 3.30 – 3.45 (m, 1H), 2.55 – 2.60 (m, 1H), 2.38 – 2.40 (m, 1H), 2.16 (s, 3H).
MS (ESI, pos.ion) m/z: 607.20 [M+H]+ .MS (ESI, pos.ion) m/z: 607.20 [M+H] + .
實施例72:化合物6-(((2R )-1-乙醯基-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 Example 72: Compound 6-((( 2R )-1-Acetyl-4-(2,2-difluorobenzo[ d ][1,3]dioxocen-5-yl)pyrrolidine- 2-acyl-amino) methyl) - N - (4- fluorophenyl) - N - methylpyridine Amides
步驟1:化合物 (R )-1-叔丁基 2-甲基 4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 1: Compound (R) -1- tert-butyl 2-methyl-4- (2,2-difluorobenzo [d] [1,3] dioxol-5-yl) -1 H - pyrrole - Synthesis of 1,2( 2H , 5H)-dicarboxylate
將 (R )-1-叔丁基-2-甲基-4-(4,4,5,5-四甲基-1,3,2-二氧雜戊硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (320 mg, 0.9mmol),5-溴-2,2-二氟苯並[d ][1,3]二噁茂 (475 mg, 2.0mmol),磷酸鉀 (1.1g, 5.2mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (26 mg) 混合在乾燥的1,4-二氧六環 (8 mL) 溶液中,氮氣保護下100℃反應12 h,冷卻至室溫,減壓濃縮,剩餘物加入水 (20mL),用乙酸乙酯 (15 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到淺褐色液體324 mg,產率93%。( R )-1-tert-butyl-2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-1 H -pyrrole-1,2( 2H , 5H )-dicarboxylate (320 mg, 0.9 mmol), 5-bromo-2,2-difluorobenzo[ d ][1,3]dioxin (475 mg, 2.0 mmol), potassium phosphate (1.1 g, 5.2 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]dichloride palladium (26 mg) were mixed in dry 1, 4-dioxane (8 mL) solution was reacted at 100 °C for 12 h under nitrogen protection, cooled to room temperature, concentrated under reduced pressure, the residue was added with water (20 mL), and extracted with ethyl acetate (15 mL × 3) , the organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1) to obtain a light brown liquid 324 mg, yield 93%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm):7.14 (s, 1H), 7.05 – 7.11 (m, 2H), 6.05 – 6.07 (m, 0.4H), 6.00 – 6.02 (m, 0.6H), 5.20 – 5.23 (m, 0.4H), 5.12 – 5.15 (m, 0.6H), 4.50 – 4.67 (m, 2H), 3.79 (s, 1H), 3.78 (s, 2H), 1.54 (s, 3.4H), 1.48 (s, 5.6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.14 (s, 1H), 7.05 – 7.11 (m, 2H), 6.05 – 6.07 (m, 0.4H), 6.00 – 6.02 (m, 0.6H) , 5.20 – 5.23 (m, 0.4H), 5.12 – 5.15 (m, 0.6H), 4.50 – 4.67 (m, 2H), 3.79 (s, 1H), 3.78 (s, 2H), 1.54 (s, 3.4H) ), 1.48 (s, 5.6H).
MS (ESI, pos.ion) m/z: 406.00 [M+Na]+ .MS (ESI, pos.ion) m/z: 406.00 [M+Na] + .
步驟2:化合物 (2R )-1-叔丁基 2-甲基 4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-1,2-二羧酸酯的合成Step 2: Compound ( 2R )-1-tert-butyl 2-methyl 4-(2,2-difluorobenzo[ d ][1,3]dioxocen-5-yl)pyrrolidine-1, Synthesis of 2-dicarboxylate
將化合物 (R )-1-叔丁基 2-甲基 4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (320 mg, 0.83mmol) 溶於甲醇 (8mL),加入Pd/C (32 mg),通入氫氣室溫反應5 h,過濾除去催化劑,濾液濃縮,得到無色液體306 mg,產率95%。The compound (R) -1- tert-butyl 2-methyl-4- (2,2-difluorobenzo [d] [1,3] dioxol-5-yl) -1 H - pyrrole-1, 2( 2H , 5H )-dicarboxylate (320 mg, 0.83 mmol) was dissolved in methanol (8 mL), Pd/C (32 mg) was added, hydrogen was passed through it and reacted at room temperature for 5 h, the catalyst was removed by filtration, and the filtrate was Concentration gave 306 mg of a colorless liquid with a yield of 95%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 6.95 – 7.03 (m, 3H), 4.33 – 4.43 (m, 1H), 4.04 – 4.10 (m, 0.6H), 3.94 – 4.00 (m, 0.4H), 3.79 (s, 1H), 3.78 (s, 2H), 3.42 (t,J = 10.1 Hz, 1H), 3.32 – 3.41 (m, 1H), 2.64 – 2.71 (m, 1H), 2.00 – 2.09 (m, 1H), 1.49 (s, 3H), 1.45 (s, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 6.95 – 7.03 (m, 3H), 4.33 – 4.43 (m, 1H), 4.04 – 4.10 (m, 0.6H), 3.94 – 4.00 (m, 0.4 H), 3.79 (s, 1H), 3.78 (s, 2H), 3.42 (t, J = 10.1 Hz, 1H), 3.32 – 3.41 (m, 1H), 2.64 – 2.71 (m, 1H), 2.00 – 2.09 (m, 1H), 1.49 (s, 3H), 1.45 (s, 6H).
MS (ESI, pos.ion) m/z: 408.10 [M+Na]+ .MS (ESI, pos.ion) m/z: 408.10 [M+Na] + .
步驟3:化合物 (2R )-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-羧酸甲酯鹽酸鹽的合成Step 3: Synthesis of Compound (2R )-4-(2,2-difluorobenzo[ d ][1,3]dioxoc-5-yl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物 (2R )-1-叔丁基 2-甲基 4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-1,2-二羧酸酯 (300 mg, 0.78 mmol) 溶解於二氯甲烷 (5 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (5 mL),室溫反應1 h,除去溶劑,得到白色固體248 mg,產率99%。The compound (2 R )-1-tert-butyl 2-methyl 4-(2,2-difluorobenzo[ d ][1,3]dioxol-5-yl)pyrrolidine-1,2- Dicarboxylate (300 mg, 0.78 mmol) was dissolved in dichloromethane (5 mL) solution, 4 mol/L HCl in ethyl acetate solution (5 mL) was added, the reaction was carried out at room temperature for 1 h, and the solvent was removed to obtain a white Solid 248 mg, 99% yield.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.29 (s, 1H), 7.20 – 7.22(m, 1H), 7.16 – 7.18 (m, 1H), 4.61 – 4.66 (m, 1H), 3.90 (s, 3H), 3.69 – 3.85 (m, 2H), 3.34 – 3.38 (m, 1H), 2.84 – 2.91 (m, 1H), 2.20 – 2.28 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.29 (s, 1H), 7.20 – 7.22 (m, 1H), 7.16 – 7.18 (m, 1H), 4.61 – 4.66 (m, 1H), 3.90 (s, 3H), 3.69 – 3.85 (m, 2H), 3.34 – 3.38 (m, 1H), 2.84 – 2.91 (m, 1H), 2.20 – 2.28 (m, 1H).
MS (ESI, pos.ion) m/z: 286.00 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 286.00 [M+H-HCl] + .
步驟4:化合物 (2R )-1-乙醯基-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-羧酸甲酯 的合成Step 4: Compound ( 2R )-1-Acetyl-4-(2,2-difluorobenzo[ d ][1,3]dioxol-5-yl)pyrrolidine-2-carboxylic acid methyl Synthesis of Esters
將化合物 (2R )-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-羧酸甲酯鹽酸鹽 (245 mg, 0.76 mmol) 溶解在二氯甲烷 (6 mL)中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.63 mL, 3.8 mmol) 和乙醯氯 (181 mg, 2.3 mmol),室溫攪拌3 h後停止,加水 (15 mL),分離有機相,有機相用無水硫酸鈉乾燥,濃縮,進行矽膠柱層析色譜分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到淺黃色液體221 mg,產率88%。The compound ( 2R )-4-(2,2-difluorobenzo[ d ][1,3]dioxol-5-yl)pyrrolidine-2-carboxylate methyl ester hydrochloride (245 mg, 0.76 mmol) was dissolved in dichloromethane (6 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.63 mL, 3.8 mmol) and acetyl chloride (181 mg, 2.3 mmol) were added, and the After 3 h of warm stirring, water (15 mL) was added, and the organic phase was separated. The organic phase was dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/2) to obtain 221 mg of pale yellow liquid with a yield of 88%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 6.95 – 7.05 (m, 3H), 6.55 (t,J F-H = 75.1 Hz, 1H), 4.49 – 4.53 (m, 1H), 3.95 – 3.99 (m, 1H), 3.79 (s, 3H), 3.62 (t,J = 10.4 Hz, 1H), 3.42 – 3.51 (m, 1H), 2.66 – 2.73 (m, 1H), 2.13 (s, 3H), 2.00 – 2.10 (m, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 6.95 – 7.05 (m, 3H), 6.55 (t, J FH = 75.1 Hz, 1H), 4.49 – 4.53 (m, 1H), 3.95 – 3.99 ( m, 1H), 3.79 (s, 3H), 3.62 (t, J = 10.4 Hz, 1H), 3.42 – 3.51 (m, 1H), 2.66 – 2.73 (m, 1H), 2.13 (s, 3H), 2.00 – 2.10 (m, 1H).
MS (ESI, pos.ion) m/z: 328.20 [M+H]+ .MS (ESI, pos.ion) m/z: 328.20 [M+H] + .
步驟5:化合物 (2R )-1-乙醯基-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-羧酸的合成Step 5: Compound ( 2R )-1-Acetyl-4-(2,2-difluorobenzo[ d ][1,3]dioxol-5-yl)pyrrolidine-2-carboxylic acid synthesis
將化合物 (2R )-1-乙醯基-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-羧酸甲酯 (220 mg, 0.67 mmol) 溶於四氫呋喃 (3 mL) 和水 (3 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (56 mg, 1.34 mmol),50℃反應30 min後停止,加稀鹽酸調節溶液pH=1,再用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淺黃色液體207 mg,收率98%。The compound ( 2R )-1-acetyl-4-(2,2-difluorobenzo[ d ][1,3]dioxol-5-yl)pyrrolidine-2-carboxylate methyl ester ( 220 mg, 0.67 mmol) was dissolved in a mixed solvent of tetrahydrofuran (3 mL) and water (3 mL), and then lithium hydroxide monohydrate (56 mg, 1.34 mmol) was added, the reaction was stopped at 50 °C for 30 min, and diluted hydrochloric acid was added. The pH of the solution was adjusted to 1, and then extracted with ethyl acetate (10 mL × 3). The organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain 207 mg of a light yellow liquid with a yield of 98%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.26 (s, 1H), 7.14 – 7.19(m, 2H), 4.44 – 4.48 (m, 1H), 4.09 – 4.15 (m, 1H), 3.56 – 3.63 (m, 2H), 2.74 – 2.80 (m, 1H), 2.14 (s, 3H), 2.00 – 2.08 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.26 (s, 1H), 7.14 – 7.19 (m, 2H), 4.44 – 4.48 (m, 1H), 4.09 – 4.15 (m, 1H), 3.56 – 3.63 (m, 2H), 2.74 – 2.80 (m, 1H), 2.14 (s, 3H), 2.00 – 2.08 (m, 1H).
MS (ESI, pos.ion) m/z: 314.20 [M+H]+ .MS (ESI, pos.ion) m/z: 314.20 [M+H] + .
步驟6:化合物6-(((2R )-1-乙醯基-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺的合成Step 6: Compound 6-((( 2R )-1-Acetyl-4-(2,2-difluorobenzo[ d ][1,3]dioxocen-5-yl)pyrrolidine-2 - a XI) methyl) - N - (4- fluorophenyl) - N - acyl amine-methylpyridine
將化合物 (2R )-1-乙醯基-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-羧酸 (60 mg, 0.19 mmol),6-(氨基甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺二鹽酸鹽 (889-1) (77 mg, 0.23 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (182 mg, 0.95 mmol) 和N -羥基-7-氮雜苯並三氮唑 (52 mg, 0.38 mmol) 溶於二氯甲烷 (6 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.22 mL, 1.3 mmol),室溫反應11 h,加水 (20 mL) 攪拌5 min,分離有機相,有機相用無水硫酸鈉乾燥,濃縮,進行矽膠柱層析色譜分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體64 mg,再經過高效液相色譜製備分離得到白色固體39 mg,收率36%。The compound ( 2R )-1-acetyl-4-(2,2-difluorobenzo[ d ][1,3]dioxoc-5-yl)pyrrolidine-2-carboxylic acid (60 mg , 0.19 mmol), 6- (aminomethyl) - N - (4- fluorophenyl) - N - methyl acyl pyridine dihydrochloride (889-1) (77 mg, 0.23 mmol), 1- ethyl Alkyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (182 mg, 0.95 mmol) and N -hydroxy-7-azabenzotriazole (52 mg, 0.38 mmol) were dissolved in In dichloromethane (6 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.22 mL, 1.3 mmol) was added, the reaction was carried out at room temperature for 11 h, water (20 mL) was added, stirred for 5 min, and separated. The organic phase was dried with anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 64 mg of white solid, which was then subjected to high-efficiency Preparative separation by liquid chromatography gave 39 mg of white solid with a yield of 36%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.50 – 7.63 (m, 2H), 7.19 – 7.28 (m, 1H), 7.13 – 6.92 (m, 7H), 4.53 – 4.57 (m, 1H), 4.34 – 4.47 (m, 2H), 3.93 – 3.98 (m, 1H), 3.57 (t,J = 10.6 Hz, 1H), 3.50 (s, 3H), 3.35 – 3.49 (m, 1H), 2.55 – 2.62 (m, 1H), 2.33 – 2.47 (m, 1H), 2.13 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.50 – 7.63 (m, 2H), 7.19 – 7.28 (m, 1H), 7.13 – 6.92 (m, 7H), 4.53 – 4.57 (m, 1H) , 4.34 – 4.47 (m, 2H), 3.93 – 3.98 (m, 1H), 3.57 (t, J = 10.6 Hz, 1H), 3.50 (s, 3H), 3.35 – 3.49 (m, 1H), 2.55 – 2.62 (m, 1H), 2.33 – 2.47 (m, 1H), 2.13 (s, 3H).
MS (ESI, pos.ion) m/z: 555.85 [M+H]+ .MS (ESI, pos.ion) m/z: 555.85 [M+H] + .
實施例73:化合物6-(((2R )-1-乙醯基-4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 Example 73: Compound 6-((( 2R )-1-Acetyl-4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2 - (methanesulfonamide acyl) phenyl) pyrrolidine-2-acyl-amino) methyl) - N - (4- fluorophenyl) - N - methylpyridine Amides
步驟1:化合物 (R )-1-叔丁基 2-甲基 4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯的合成Step 1: Compound ( R )-1-tert-butyl 2-methyl 4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methyl) sulfonic yl) phenyl) -1 H - pyrrole -1,2 (2 H, 5 H) - synthesis of esters of dicarboxylic acids
將化合物 (R )-1-叔丁基 2-甲基 4-(4,4,5,5-四甲基-1,3,2-二氧雜戊硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (300 mg, 0.85mmol),N -(5-溴-2-(二氟甲氧基)-4-(甲磺醯基)苯基)-N -(環丙基甲基)羥胺 (334 mg, 0.86mmol),磷酸鉀 (721 mg, 3.40mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (24 mg) 混合在乾燥的1,4-二氧六環 (8 mL) 溶液中,氮氣保護下100℃反應3 h,冷卻至室溫,減壓濃縮,剩餘物加入水 (20mL),用乙酸乙酯 (15 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 5/1),得到淺黃色液體381 mg,產率84%。The compound (R) -1- tert-butyl 2-methyl-4- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -1 H -pyrrole-1,2( 2H , 5H )-dicarboxylate (300 mg, 0.85 mmol), N- (5-bromo-2-(difluoromethoxy)-4-(methylsulfonyl) ) phenyl) - N - (cyclopropylmethyl) hydroxylamine (334 mg, 0.86mmol), potassium phosphate (721 mg, 3.40mmol) and [1,1'-bis (diphenylphosphino) ferrocene ] Palladium dichloride (24 mg) was mixed in dry 1,4-dioxane (8 mL) solution, reacted at 100 °C for 3 h under nitrogen protection, cooled to room temperature, concentrated under reduced pressure, and the residue was added with water (20 mL), extracted with ethyl acetate (15 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 5/1) to obtain 381 mg of pale yellow liquid with a yield of 84%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm):7.14 – 7.15 (m, 1H), 6.53 (t,JF-H = 73.3 Hz, 1H), 6.49 (s, 1H), 6.19 – 6.21 (m, 0.7H), 6.10 – 6.12 (m, 0.3H), 5.19 – 5.23 (m, 0.3H), 5.14 – 5.17 (m, 0.7H), 4.58 – 4.67 (m, 2H), 3.79 (s, 3H), 3.03 – 3.05 (m, 3H), 2.95 – 2.98 (m, 2H), 1.45 – 1.53 (m, 9H), 1.08 – 1.16 (m, 1H), 0.60 – 0.65 (m, 2H), 0.26 – 0.30 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.14 – 7.15 (m, 1H), 6.53 (t, J FH = 73.3 Hz, 1H), 6.49 (s, 1H), 6.19 – 6.21 (m, 0.7H), 6.10 – 6.12 (m, 0.3H), 5.19 – 5.23 (m, 0.3H), 5.14 – 5.17 (m, 0.7H), 4.58 – 4.67 (m, 2H), 3.79 (s, 3H), 3.03 – 3.05 (m, 3H), 2.95 – 2.98 (m, 2H), 1.45 – 1.53 (m, 9H), 1.08 – 1.16 (m, 1H), 0.60 – 0.65 (m, 2H), 0.26 – 0.30 (m , 2H).
MS (ESI, pos.ion) m/z: 533.90 [M+H]+ .MS (ESI, pos.ion) m/z: 533.90 [M+H] + .
步驟2:化合物 (2R )-1-叔丁基 2-甲基 4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-1,2-二羧酸酯的合成Step 2: Compound ( 2R )-1-tert-butyl 2-methyl 4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-( Synthesis of Methylsulfonyl)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物 (R )-1-叔丁基 2-甲基 4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯 (380 mg, 0.71mmol) 溶於甲醇 (8mL),加入Pd/C (32 mg, 10%),通入氫氣室溫反應24 h,過濾除去催化劑,濾液濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/1),得到無色液體226 mg,產率59%。The compound ( R )-1-tert-butyl-2-methyl 4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methanesulfonyl) yl) phenyl) -1 H - pyrrole -1,2 (2 H, 5 H) - dicarboxylate (380 mg, 0.71mmol) was dissolved in methanol (8mL), was added Pd / C (32 mg, 10 % ), passed into hydrogen and reacted at room temperature for 24 h, filtered to remove the catalyst, the filtrate was concentrated, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v)=3/1) to obtain a colorless liquid 226 mg, 59% yield.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.01 (s, 1H), 6.65 (s, 1H), 6.48 (t,JF-H = 73.6 Hz, 1H), 4.33 – 4.42 (m, 2H), 3.92 – 4.04 (m, 1H), 3.80 (s, 1H), 3.64 – 3.73 (m, 1H), 3.43 – 3.50 (m, 1H), 3.24 (s, 3H), 2.96 – 3.00 (m, 2H), 2.67 – 2.75 (m, 1H), 1.96 – 2.04 (m, 1H), 1.48 (s, 3.4H), 1.45 (s, 5.6H), 1.07 – 1.14 (m, 1H), 0.60 – 0.64 (m, 2H), 0.27 – 0.31 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.01 (s, 1H), 6.65 (s, 1H), 6.48 (t, J FH = 73.6 Hz, 1H), 4.33 – 4.42 (m, 2H) , 3.92 – 4.04 (m, 1H), 3.80 (s, 1H), 3.64 – 3.73 (m, 1H), 3.43 – 3.50 (m, 1H), 3.24 (s, 3H), 2.96 – 3.00 (m, 2H) , 2.67 – 2.75 (m, 1H), 1.96 – 2.04 (m, 1H), 1.48 (s, 3.4H), 1.45 (s, 5.6H), 1.07 – 1.14 (m, 1H), 0.60 – 0.64 (m, 2H), 0.27 – 0.31 (m, 2H).
MS (ESI, pos.ion) m/z: 535.10 [M+H]+ .MS (ESI, pos.ion) m/z: 535.10 [M+H] + .
步驟3:化合物 (2R )-4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽的合成Step 3: Compound ( 2R )-4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methylsulfonyl)phenyl)pyrrole Synthesis of Alkane-2-Carboxylic Acid Methyl Hydrochloride
將化合物 (2R )-1-叔丁基 2-甲基 4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-1,2-二羧酸酯 (221 mg, 0.41 mmol) 溶解於二氯甲烷 (3 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (6 mL),室溫反應1 h,除去溶劑,得到白色固體191 mg,產率98%。The compound (2 R )-1-tert-butyl 2-methyl 4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methanesulfonic acid) Acyl)phenyl)pyrrolidine-1,2-dicarboxylate (221 mg, 0.41 mmol) was dissolved in dichloromethane (3 mL) solution, 4 mol/L HCl in ethyl acetate solution (6 mL) was added ), reacted at room temperature for 1 h, and removed the solvent to obtain 191 mg of white solid with a yield of 98%.
1 H NMR (600 MHz, CD3 OD) δ (ppm): 7.30 (s, 1H), 7.29 (s, 1H), 6.99 (t,JF-H = 72.8 Hz, 1H), 4.65 – 4.68 (m, 1H), 4.05 – 4.08 (m, 1H), 3.92 (s, 3H), 3.82 – 3.86 (m, 1H), 3.46 (s, 3H), 3.37 – 3.41 (m, 1H), 3.21 (d,J = 7.0 Hz, 2H), 2.84 – 2.88 (m, 1H), 2.29 – 2.35 (m, 1H), 1.15 – 1.20 (m, 1H), 0.64 – 0.67 (m, 2H), 0.35 – 0.37 (m, 2H). 1 H NMR (600 MHz, CD 3 OD) δ (ppm): 7.30 (s, 1H), 7.29 (s, 1H), 6.99 (t, J FH = 72.8 Hz, 1H), 4.65 – 4.68 (m, 1H) ), 4.05 – 4.08 (m, 1H), 3.92 (s, 3H), 3.82 – 3.86 (m, 1H), 3.46 (s, 3H), 3.37 – 3.41 (m, 1H), 3.21 (d, J = 7.0 Hz, 2H), 2.84 – 2.88 (m, 1H), 2.29 – 2.35 (m, 1H), 1.15 – 1.20 (m, 1H), 0.64 – 0.67 (m, 2H), 0.35 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 435.05 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 435.05 [M+H-HCl] + .
步驟4:化合物 (2R )-1-乙醯基-4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-羧酸甲酯的合成Step 4: Compound ( 2R )-1-Acetyl-4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methylsulfonyl Synthesis of methyl)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (190 mg, 0.41 mmol) 溶解在二氯甲烷 (6 mL) 中,在冰浴中加入N ,N -二異丙基乙胺 (0.41 mL, 2.5 mmol) 和乙醯氯 (96 mg, 1.22 mmol),室溫攪拌40min後停止,加水 (15 mL),用二氯甲烷 (15 mL × 3) 萃取,有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到無色液體162 mg,產率84%。Compound ( 2R )-4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methylsulfonyl)phenyl)pyrrolidine- Methyl 2-carboxylate hydrochloride (190 mg, 0.41 mmol) was dissolved in dichloromethane (6 mL), N , N -diisopropylethylamine (0.41 mL, 2.5 mmol) and Acetyl chloride (96 mg, 1.22 mmol), stirred at room temperature for 40 min, then stopped, added water (15 mL), extracted with dichloromethane (15 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and silica gel Column chromatography separation (eluent: petroleum ether/ethyl acetate (v/v) = 1/2) gave 162 mg of a colorless liquid with a yield of 84%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.00 (s, 1H), 6.56 (s, 1H), 6.50 (t,JF-H = 73.4 Hz, 1H), 4.48 – 4.52 (m, 1H), 4.33 – 4.40 (m, 1H), 3.98 – 4.02 (m, 1H), 3.80 (s, 3H), 3.74 – 3.80 (m, 1H), 3.57 – 3.62 (m, 1H), 3.26 (s, 3H), 3.37 – 3.41 (m, 1H), 2.98 – 3.02 (m, 2H), 2.64 – 2.71 (m, 1H), 2.12 (s, 3H), 2.01 – 2.09 (m, 1H), 1.07 – 1.16 (m, 1H), 0.61 – 0.66 (m, 2H), 0.29 – 0.33 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.00 (s, 1H), 6.56 (s, 1H), 6.50 (t, J FH = 73.4 Hz, 1H), 4.48 – 4.52 (m, 1H) , 4.33 – 4.40 (m, 1H), 3.98 – 4.02 (m, 1H), 3.80 (s, 3H), 3.74 – 3.80 (m, 1H), 3.57 – 3.62 (m, 1H), 3.26 (s, 3H) , 3.37 – 3.41 (m, 1H), 2.98 – 3.02 (m, 2H), 2.64 – 2.71 (m, 1H), 2.12 (s, 3H), 2.01 – 2.09 (m, 1H), 1.07 – 1.16 (m, 1H), 0.61 – 0.66 (m, 2H), 0.29 – 0.33 (m, 2H).
MS (ESI, pos.ion) m/z: 477.90 [M+H]+ .MS (ESI, pos.ion) m/z: 477.90 [M+H] + .
步驟5:化合物 (2R )-1-乙醯基-4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-羧酸的合成Step 5: Compound ( 2R )-1-Acetyl-4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methylsulfonyl Synthesis of yl)phenyl)pyrrolidine-2-carboxylic acid
將化合物 (2R )-1-乙醯基-4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-羧酸甲酯 (153 mg, 0.32 mmol) 溶於四氫呋喃 (4 mL) 和水 (4 mL) 的混合溶劑中,加入一水合氫氧化鋰 (67 mg, 1.60 mmol),50℃反應1 h後停止,加稀鹽酸調節溶液pH=1,用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到淺褐色固體133 mg,收率89%。The compound ( 2R )-1-acetyl-4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methylsulfonyl) Phenyl)pyrrolidine-2-carboxylate methyl ester (153 mg, 0.32 mmol) was dissolved in a mixed solvent of tetrahydrofuran (4 mL) and water (4 mL), and lithium hydroxide monohydrate (67 mg, 1.60 mmol) was added , the reaction was stopped after 1 h at 50 °C, diluted hydrochloric acid was added to adjust the pH of the solution to 1, extracted with ethyl acetate (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain 133 mg of a light brown solid with a yield of 89 %.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.10 (s, 1H), 6.79 (t,JF-H = 73.8 Hz, 1H), 7.14 – 7.19(m, 2H), 6.77 (s, 1H), 4.45 – 4.49 (m, 1H), 4.05 – 4.13 (m, 1H), 3.80 – 3.89 (m, 1H), 3.61 – 3.73 (m, 1H), 3.35 (s, 3H), 3.05 – 3.08 (m, 2H), 2.71 – 2.78 (m, 1H),2.12 (s, 2H), 2.01 (s, 1H), 2.01 – 2.10 (m, 1H), 1.10 – 1.14 (m, 1H), 0.55 – 0.60 (m, 2H), 0.28 – 0.31 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.10 (s, 1H), 6.79 (t, J FH = 73.8 Hz, 1H), 7.14 – 7.19 (m, 2H), 6.77 (s, 1H) ), 4.45 – 4.49 (m, 1H), 4.05 – 4.13 (m, 1H), 3.80 – 3.89 (m, 1H), 3.61 – 3.73 (m, 1H), 3.35 (s, 3H), 3.05 – 3.08 (m , 2H), 2.71 – 2.78 (m, 1H), 2.12 (s, 2H), 2.01 (s, 1H), 2.01 – 2.10 (m, 1H), 1.10 – 1.14 (m, 1H), 0.55 – 0.60 (m , 2H), 0.28 – 0.31 (m, 2H).
MS (ESI, pos.ion) m/z: 463.90 [M+H]+ .MS (ESI, pos.ion) m/z: 463.90 [M+H] + .
步驟6:化合物6-(((2R )-1-乙醯基-4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺的合成Step 6: Compound 6-((( 2R )-1-Acetyl-4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2- (acyl methanesulfonamide) phenyl) pyrrolidine-2-acyl-amino) methyl) - N - (4- fluorophenyl) - N - acyl amine-methylpyridine
將化合物 (2R )-1-乙醯基-4-(5-((環丙基甲基)(羥基)氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-羧酸 (60 mg, 0.13 mmol),6-(氨基甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺二鹽酸鹽 (51 mg, 0.15 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (124 mg, 0.65 mmol) 和N -羥基-7-氮雜苯並三氮唑(36 mg, 0.26 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),室溫反應12 h,加水 (20 mL) 攪拌5 min,分離有機相,有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體53 mg,產率58%。The compound ( 2R )-1-acetyl-4-(5-((cyclopropylmethyl)(hydroxy)amino)-4-(difluoromethoxy)-2-(methylsulfonyl) phenyl) pyrrolidine-2-carboxylic acid (60 mg, 0.13 mmol), 6- ( aminomethyl) - N - (4- fluorophenyl) - N - methyl acyl pyridine dihydrochloride (51 mg , 0.15 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (124 mg, 0.65 mmol) and N -hydroxy-7-azabenzotriazole ( 36 mg, 0.26 mmol) was dissolved in dichloromethane (5 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.15 mL, 0.91 mmol) was added, the reaction was carried out at room temperature for 12 h, and water ( 20 mL) was stirred for 5 min, the organic phase was separated, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1), 53 mg of white solid was obtained in 58% yield.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.61 (br.s, 1H), 7.33 – 7.46 (m, 1H), 7.16 – 7.24 (m, 1H), 6.98 – 7.09 (m, 5H), 6.68 (s, 1H), 6.50 (t,J F-H = 74.0 Hz, 1H), 4.35 – 4.83 (m, 4H), 3.97 – 4.07 (m, 1H), 3.63 – 3.74 (m, 1H), 3.50 (s, 3H), 3.65 (s, 3H), 2.93 – 3.04 (m, 2H), 2.48 – 2.59 (m, 1H), 2.36 – 2.48 (m, 1H), 2.13(s, 3H), 1.05 – 1.14 (m, 1H), 0.56 – 0.64 (m, 2H), 0.25 – 0.32 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.61 (br.s, 1H), 7.33 – 7.46 (m, 1H), 7.16 – 7.24 (m, 1H), 6.98 – 7.09 (m, 5H) , 6.68 (s, 1H), 6.50 (t, J FH = 74.0 Hz, 1H), 4.35 – 4.83 (m, 4H), 3.97 – 4.07 (m, 1H), 3.63 – 3.74 (m, 1H), 3.50 ( s, 3H), 3.65 (s, 3H), 2.93 – 3.04 (m, 2H), 2.48 – 2.59 (m, 1H), 2.36 – 2.48 (m, 1H), 2.13(s, 3H), 1.05 – 1.14 ( m, 1H), 0.56 – 0.64 (m, 2H), 0.25 – 0.32 (m, 2H).
MS (ESI, pos.ion) m/z: 704.40 [M+H]+ .MS (ESI, pos.ion) m/z: 704.40 [M+H] + .
實施例74:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基-N -(4-甲氧基苯基)吡啶醯胺 Example 74: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxyamino)methyl) -N -methyl- N- (4-methoxyphenyl)pyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -甲基-4-甲氧基苯胺 (68 mg, 0.50 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (251 mg, 1.31 mmol) 和N -羥基-7-氮雜苯並三氮唑 (54 mg, 0.40 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (180 mg, 1.39 mmol),室溫反應22 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到灰色固體73 mg,收率59%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N -methyl-4-methoxyaniline (68 mg, 0.50 mmol), 1-ethyl-(3-dimethylaminopropyl) carbon Diimide hydrochloride (251 mg, 1.31 mmol) and N -hydroxy-7-azabenzotriazole (54 mg, 0.40 mmol) were dissolved in dichloromethane (5 mL) and cooled to 0°C , N , N -diisopropylethylamine (180 mg, 1.39 mmol) was added, the reaction was carried out at room temperature for 22 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sulfuric acid The solution was dried over sodium, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 73 mg of a gray solid with a yield of 59%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.63 (br.s, 1H), 7.19 - 7.31 (m, 2H), 7.05 - 7.12 (m, 3H), 6.77 - 6.92 (m, 4H), 6.62 (t, J = 75.5 Hz, 1H), 4.38 - 4.62 (m, 3H), 3.81 - 3.99 (m, 1H), 3.89 (s, 2H), 3.64 - 3.80 (m, 1H), 3.74 (s, 3H), 3.49 (s, 3H), 3.33 - 3.57 (m, 1H), 2.50 - 2.78 (m, 1H), 2.17 (s, 3H), 1.26 - 1.35 (m, 1H), 0.60 - 0.71 (m, 2H), 0.35 - 0.43 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.63 (br.s, 1H), 7.19 - 7.31 (m, 2H), 7.05 - 7.12 (m, 3H), 6.77 - 6.92 (m, 4H) , 6.62 (t, J = 75.5 Hz, 1H), 4.38 - 4.62 (m, 3H), 3.81 - 3.99 (m, 1H), 3.89 (s, 2H), 3.64 - 3.80 (m, 1H), 3.74 (s , 3H), 3.49 (s, 3H), 3.33 - 3.57 (m, 1H), 2.50 - 2.78 (m, 1H), 2.17 (s, 3H), 1.26 - 1.35 (m, 1H), 0.60 - 0.71 (m , 2H), 0.35 - 0.43 (m, 2H).
MS (ESI, pos.ion) m/z: 623.1 0 [M+H]+ .MS (ESI, pos.ion) m/z: 623.1 0 [M+H] + .
實施例75:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(2-環丙基丙烷-2-基)吡啶醯胺 Example 75: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (2- cyclopropyl-2-yl) pyridin Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),1-環丙基-1-甲基乙胺鹽酸鹽 (59 mg, 0.43 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (191 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應16 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體59 mg,產率50%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), 1-cyclopropyl-1-methylethylamine hydrochloride (59 mg, 0.43 mmol), 1-ethyl-(3-dimethylamino) propyl)carbodiimide hydrochloride (191 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL), Cool to 0°C, add N , N -diisopropylethylamine (0.17 mL, 1.00 mmol), react at room temperature for 16 h, add water (15 mL), extract with dichloromethane (5 mL × 3), the organic phase It was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 59 mg of white solid with a yield of 50%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.92 – 7.99 (m, 2H), 7.66 (d,J = 5.4 Hz, 1H), 7.11 (d,J = 8.2 Hz, 1H), 7.07 (s, 1H), 6.93 (s, 1H), 6.73 (t,J F-H = 71.4 Hz, 1H), 4.51 – 4.65 (m, 3H), 4.08 – 4.13 (m, 1H), 3.91 (d,J = 6.8 Hz, 2H), 3.66 (d,J = 10.5 Hz, 1H), 3.47 – 3.56 (m, 1H), 2.68 – 2.74 (m, 1H), 2.15 (s, 3H), 2.03 – 2.14 (m, 1H), 3.16 (s, 3H), 3.15 (s, 3H), 1.24 – 1.36 (m, 2H), 0.60 – 0.65 (m, 2H), 0.40 – 0.46 (m, 4H), 0.31 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.92 – 7.99 (m, 2H), 7.66 (d, J = 5.4 Hz, 1H), 7.11 (d, J = 8.2 Hz, 1H), 7.07 (s, 1H), 6.93 (s, 1H), 6.73 (t, J FH = 71.4 Hz, 1H), 4.51 – 4.65 (m, 3H), 4.08 – 4.13 (m, 1H), 3.91 (d, J = 6.8 Hz, 2H), 3.66 (d, J = 10.5 Hz, 1H), 3.47 – 3.56 (m, 1H), 2.68 – 2.74 (m, 1H), 2.15 (s, 3H), 2.03 – 2.14 (m, 1H) ), 3.16 (s, 3H), 3.15 (s, 3H), 1.24 – 1.36 (m, 2H), 0.60 – 0.65 (m, 2H), 0.40 – 0.46 (m, 4H), 0.31 – 0.41 (m, 2H) ).
MS (ESI, pos.ion) m/z: 585.20 [M+H]+ .MS (ESI, pos.ion) m/z: 585.20 [M+H] + .
實施例76:化合物 6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N- (環丙基甲基)-N -甲基吡啶醯胺 Example 76: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N- (cyclopropylmethyl) -N -methylpyridamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (98 mg, 0.19 mmol),1-環丙基-N -甲基甲胺鹽酸鹽 (,合成方法參見中間體9) (63 mg, 0.52 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (127 mg, 0.66 mmol) 和N -羥基-7-氮雜苯並三氮唑 (89 mg, 0.65 mmol) 溶於二氯甲烷 (6 mL)中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌6h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體46 mg,收率41%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (98 mg, 0.19 mmol), 1-cyclopropyl- N -methylmethanamine hydrochloride (see Intermediate 9 for synthesis method) (63 mg, 0.52 mmol), 1-ethyl Alkyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (127 mg, 0.66 mmol) and N -hydroxy-7-azabenzotriazole (89 mg, 0.65 mmol) were dissolved in In dichloromethane (6 mL), N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) was added dropwise to this solution at room temperature, stirred at room temperature for 6 h, water (50 mL) was added, and dichloromethane was added. Methane extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain White solid 46 mg, yield 41%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.64 (br.s, 1H), 7.86 (t,J = 7.7 Hz, 1H), 7.47 (d,J = 7.8 Hz, 1H), 7.32-7.40 (m, 1H), 7.13 (d,J = 8.1 Hz, 1H), 7.07 (s, 1H), 7.03 (t,J F-H = 75.0 Hz, 1H), 6.89 (d,J = 7.1 Hz, 1H), 4.30-4.56 (m, 3H), 4.05-4.10 (m, 1H), 3.91 (d,J = 6.4 Hz, 2H), 3.38-3.52 (m, 2H), 3.10-3.12 (m, 1H), 2.92-3.03 (m, 3H), 2.55-2.66 (m, 1H), 2.03 (s, 3H), 1.85-1.96 (m, 1H),1.23-1.29 (m, 1H), 0.95-1.06 (m, 1H), 0.53-0.60 (m, 2H), 0.37-0.51 (m, 2H), 0.30-0.36 (m, 2H), 0.22-0.29 (m, 1H), 0.02-0.09 (m, 1H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.64 (br.s, 1H), 7.86 (t, J = 7.7 Hz, 1H), 7.47 (d, J = 7.8 Hz, 1H), 7.32-7.40 (m, 1H), 7.13 (d, J = 8.1 Hz, 1H), 7.07 (s, 1H), 7.03 (t, J FH = 75.0 Hz, 1H), 6.89 (d, J = 7.1 Hz, 1H), 4.30-4.56 (m, 3H), 4.05-4.10 (m, 1H), 3.91 (d, J = 6.4 Hz, 2H), 3.38-3.52 (m, 2H), 3.10-3.12 (m, 1H) , 2.92-3.03 (m, 3H), 2.55-2.66 (m, 1H), 2.03 (s, 3H), 1.85-1.96 (m, 1H), 1.23-1.29 (m, 1H), 0.95-1.06 (m, 1H), 0.53-0.60 (m, 2H), 0.37-0.51 (m, 2H), 0.30-0.36 (m, 2H), 0.22-0.29 (m, 1H), 0.02-0.09 (m, 1H).
MS (ESI, pos.ion) m/z: 571.20 [M+H]+ .MS (ESI, pos.ion) m/z: 571.20 [M+H] + .
實施例77:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(環己基甲基)-N -甲基吡啶醯胺 Example 77: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (cyclohexylmethyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -甲基-環己基甲胺 (51 mg, 0.40 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (196 mg, 1.02 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到淺黃色固體39 mg,收率32%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N -methyl-cyclohexylmethylamine (51 mg, 0.40 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Imine hydrochloride (196 mg, 1.02 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL) and added to this at 0°C N , N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added dropwise to the solution, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and extracted with dichloromethane (5 mL × 3). Dry over anhydrous sodium sulfate, remove the solvent, and separate the concentrated solution by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 39 mg of a pale yellow solid with a yield of 32%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.72 – 7.76 (m, 1H), 7.32 – 7.42 (m, 2H), 7.10 (d,J = 8.1 Hz, 1H), 6.88 (s, 1H), 6.84 (d,J = 8.0 Hz, 1H), 6.60 (t,J F-H = 75.6 Hz, 1H), 4.48 – 4.65 (m, 3H), 3.91 – 3.95 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.53 – 3.58 (m, 1H), 3.36 – 3.41 (m, 1H), 3.27 – 3.36 (m, 1H), 3.18 – 3.22 (m, 1H), 3.08 (s, 1.5H), 2.98(s, 1.5H), 2.52 – 2.60 (m, 1H), 2.39 – 2.47 (m, 1H), 2.12 (s, 3H), 1.71 – 1.78 (m, 3H), 1.54 – 1.66 (m, 6H), 1.26 – 1.34 (m, 1H), 1.02 – 1.17 (m, 2H), 0.62 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.72 – 7.76 (m, 1H), 7.32 – 7.42 (m, 2H), 7.10 (d, J = 8.1 Hz, 1H), 6.88 (s, 1H) ), 6.84 (d, J = 8.0 Hz, 1H), 6.60 (t, J FH = 75.6 Hz, 1H), 4.48 – 4.65 (m, 3H), 3.91 – 3.95 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.53 – 3.58 (m, 1H), 3.36 – 3.41 (m, 1H), 3.27 – 3.36 (m, 1H), 3.18 – 3.22 (m, 1H), 3.08 (s, 1.5H) , 2.98(s, 1.5H), 2.52 – 2.60 (m, 1H), 2.39 – 2.47 (m, 1H), 2.12 (s, 3H), 1.71 – 1.78 (m, 3H), 1.54 – 1.66 (m, 6H) ), 1.26 – 1.34 (m, 1H), 1.02 – 1.17 (m, 2H), 0.62 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 613.30 [M+H]+ .MS (ESI, pos.ion) m/z: 613.30 [M+H] + .
實施例78:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -苄基-N -甲基吡啶醯胺 Example 78: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -benzyl- N -methylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (80 mg, 0.16 mmol),N -甲基苄胺 (38 mg, 0.24 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (155 mg, 0.81 mmol) 和N -羥基-7-氮雜苯並三氮唑 (32 mg, 0.24 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體81 mg,收率84%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (80 mg, 0.16 mmol), N -methylbenzylamine (38 mg, 0.24 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide salt The acid salt (155 mg, 0.81 mmol) and N -hydroxy-7-azabenzotriazole (32 mg, 0.24 mmol) were dissolved in dichloromethane (5 mL) and added dropwise to the solution at 0°C N , N -diisopropylethylamine (0.15 mL, 0.91 mmol) was added, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous sodium sulfate After drying, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 81 mg of white solid with a yield of 84%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 – 7.80 (m, 1H), 7.51 – 7.61 (m, 1H), 7.28 – 7.38 (m, 6H), 7.12 (d,J = 7.2 Hz, 1H), 6.79 – 6.90 (m, 2H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.50 – 4.83 (m, 4H), 4.08 – 4.14 (m, 1H), 3.84 – 3.96 (m, 1H), 3.87 (d,J = 6.3 Hz, 2H), 3.50 – 3.57 (m, 1H), 3.22 – 3.36 (m, 1H), 3.04 (s, 2H), 2.96 (s, 1H), 2.42 – 2.61 (m, 1H), 2.25 – 2.39 (m, 1H), 2.10 (s, 3H), 1.24 – 1.37 (m, 1H), 0.64 – 0.68 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 – 7.80 (m, 1H), 7.51 – 7.61 (m, 1H), 7.28 – 7.38 (m, 6H), 7.12 (d, J = 7.2 Hz , 1H), 6.79 – 6.90 (m, 2H), 6.62 (t, J FH = 75.6 Hz, 1H), 4.50 – 4.83 (m, 4H), 4.08 – 4.14 (m, 1H), 3.84 – 3.96 (m, 1H), 3.87 (d, J = 6.3 Hz, 2H), 3.50 – 3.57 (m, 1H), 3.22 – 3.36 (m, 1H), 3.04 (s, 2H), 2.96 (s, 1H), 2.42 – 2.61 (m, 1H), 2.25 – 2.39 (m, 1H), 2.10 (s, 3H), 1.24 – 1.37 (m, 1H), 0.64 – 0.68 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 607.40 [M+H]+ .MS (ESI, pos.ion) m/z: 607.40 [M+H] + .
實施例79:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(嘧啶-2-基甲基)吡啶醯胺 Example 79: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (pyrimidin-2-ylmethyl) pyridin Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (80 mg, 0.16 mmol),2-氨基甲基嘧啶鹽酸鹽 (36 mg, 0.25 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (153 mg, 0.8 mmol) 和N -羥基-7-氮雜苯並三氮唑 (33 mg, 0.24 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體71 mg,收率74%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (80 mg, 0.16 mmol), 2-aminomethylpyrimidine hydrochloride (36 mg, 0.25 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Imine hydrochloride (153 mg, 0.8 mmol) and N -hydroxy-7-azabenzotriazole (33 mg, 0.24 mmol) were dissolved in dichloromethane (5 mL) and added to this at 0°C N , N -diisopropylethylamine (0.15 mL, 0.91 mmol) was added dropwise to the solution, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and extracted with dichloromethane (5 mL × 3). It was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 71 mg of white solid with a yield of 74%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.64 (br.s, 1H), 8.71 (s, 2H), 8.42 (br.s, 1H), 8.09 – 8.16 (m, 1H), 7.77 – 7.87 (m, 1H), 7.36 – 7.44 (m, 1H), 7.09 – 7.18 (m, 2H), 6.81 – 6.91 (m, 2H), 6.60 (t,J F-H = 75.6 Hz, 1H), 4.88 – 5.05 (m, 2H), 4.56 – 4.79 (m, 3H), 3.78 – 3.96 (m, 3H), 3.40 – 3.49 (m, 1H), 3.24 – 3.32 (m, 1H), 2.65 – 2.75 (m, 1H), 2.46 – 2.55 (m, 1H), 2.05 (s, 3H), 1.23 – 1.37 (m, 1H), 0.62 – 0.69 (m, 2H), 0.34 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.64 (br.s, 1H), 8.71 (s, 2H), 8.42 (br.s, 1H), 8.09 – 8.16 (m, 1H), 7.77 – 7.87 (m, 1H), 7.36 – 7.44 (m, 1H), 7.09 – 7.18 (m, 2H), 6.81 – 6.91 (m, 2H), 6.60 (t, J FH = 75.6 Hz, 1H), 4.88 – 5.05 (m, 2H), 4.56 – 4.79 (m, 3H), 3.78 – 3.96 (m, 3H), 3.40 – 3.49 (m, 1H), 3.24 – 3.32 (m, 1H), 2.65 – 2.75 (m, 1H) ), 2.46 – 2.55 (m, 1H), 2.05 (s, 3H), 1.23 – 1.37 (m, 1H), 0.62 – 0.69 (m, 2H), 0.34 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 595.40 [M+H]+ .MS (ESI, pos.ion) m/z: 595.40 [M+H] + .
實施例80:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -苄基吡啶醯胺 Example 80: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -benzylpyridamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (98 mg, 0.19 mmol),苄胺 (43 mg, 0.4 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.0 mmol) 和N -羥基-7-氮雜苯並三氮唑 (40 mg, 0.3 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.16 mL, 0.97 mmol),室溫反應12 h,加水洗 (15 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體82 mg,收率71%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (98 mg, 0.19 mmol), benzylamine (43 mg, 0.4 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (192 mg, 1.0 mmol) and N -hydroxy-7-azabenzotriazole (40 mg, 0.3 mmol) were dissolved in dichloromethane (5 mL), to this solution was added dropwise N , N at 0°C -Diisopropylethylamine (0.16 mL, 0.97 mmol), reacted at room temperature for 12 h, washed with water (15 mL), then extracted with dichloromethane (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed , the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 82 mg of white solid with a yield of 71%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.50 (br.s, 1H), 8.68 (br.s, 1H), 8.17 (d,J = 7.5 Hz, 1H), 7.85 (t,J = 7.6 Hz, 1H), 7.24 – 7.44 (m, 6H), 7.13 (d,J = 8.1 Hz, 1H), 6.92 (s, 1H), 6.87 (d,J = 7.9 Hz, 1H), 6.63 (t,J F-H = 75.6 Hz, 1H), 4.78 – 4.89 (m, 2H), 4.58 – 4.71 (m, 3H), 3.84 – 3.93 (m, 1H), 3.89 (d,J = 6.8 Hz, 2H), 3.38 – 3.43 (m, 1H), 3.25 – 3.33 (m, 1H), 2.77 – 2.85 (m, 1H), 2.45 – 2.52 (m, 1H), 1.93 (s, 3H), 1.23 – 1.37 (m, 1H), 0.65 – 0.69 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.50 (br.s, 1H), 8.68 (br.s, 1H), 8.17 (d, J = 7.5 Hz, 1H), 7.85 (t, J = 7.6 Hz, 1H), 7.24 – 7.44 (m, 6H), 7.13 (d, J = 8.1 Hz, 1H), 6.92 (s, 1H), 6.87 (d, J = 7.9 Hz, 1H), 6.63 (t , J FH = 75.6 Hz, 1H), 4.78 – 4.89 (m, 2H), 4.58 – 4.71 (m, 3H), 3.84 – 3.93 (m, 1H), 3.89 (d, J = 6.8 Hz, 2H), 3.38 – 3.43 (m, 1H), 3.25 – 3.33 (m, 1H), 2.77 – 2.85 (m, 1H), 2.45 – 2.52 (m, 1H), 1.93 (s, 3H), 1.23 – 1.37 (m, 1H) , 0.65 – 0.69 (m, 2H), 0.36 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 593.20 [M+H]+ .MS (ESI, pos.ion) m/z: 593.20 [M+H] + .
實施例81:化合物 6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N- (2,4-二氟苯基)-N -甲基吡啶醯胺 Example 81: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N- (2,4-difluorophenyl) -N -methylpyridylamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (96 mg, 0.19 mmol),1-(2,4-二氟苯基)-N -甲基甲胺 (73 mg, 0.46mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (131 mg, 0.68 mmol) 和N -羥基-7-氮雜苯並三氮唑 (85 mg, 0.62 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下加入N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌13 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體52 mg,收率42%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (96 mg, 0.19 mmol), 1-(2,4-difluorophenyl) -N -methylmethanamine (73 mg, 0.46 mmol), 1-ethyl-(3- Dimethylaminopropyl)carbodiimide hydrochloride (131 mg, 0.68 mmol) and N -hydroxy-7-azabenzotriazole (85 mg, 0.62 mmol) were dissolved in dichloromethane (6 mL), N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) was added at room temperature, stirred at room temperature for 13 h, washed with water (50 mL), and extracted with dichloromethane (5 mL × 3) , the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 52 mg of white solid, yield 42 %.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.62 (br.s, 1H), 7.85-7.92 (m, 1H), 7.38-7.51 (m, 3H), 7.24-7.36 (m, 1H), 7.09-7.13 (m, 2H), 7.05 (s, 1H), 7.03 (t,J F-H = 75.0 Hz, 1H), 6.87 (d,J = 8.8 Hz, 1H), 4.60-4.71 (m, 2H), 4.31-4.52 (m, 3H), 4.05-4.08 (m, 1H), 3.90 (d,J = 6.5 Hz, 2H), 3.39-3.50 (m, 2H), 2.90 (s, 3H), 2.55-2.65 (m, 1H), 2.02 (s, 3H), 1.84-1.97 (m, 1H), 1.24-1.31 (m, 1H), 0.53-0.59 (m, 2H), 0.31-0.36 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.62 (br.s, 1H), 7.85-7.92 (m, 1H), 7.38-7.51 (m, 3H), 7.24-7.36 (m, 1H), 7.09-7.13 (m, 2H), 7.05 (s, 1H), 7.03 (t, J FH = 75.0 Hz, 1H), 6.87 (d, J = 8.8 Hz, 1H), 4.60-4.71 (m, 2H), 4.31-4.52 (m, 3H), 4.05-4.08 (m, 1H), 3.90 (d, J = 6.5 Hz, 2H), 3.39-3.50 (m, 2H), 2.90 (s, 3H), 2.55 -2.65 (m, 1H), 2.02 (s, 3H), 1.84-1.97 (m, 1H), 1.24-1.31 (m, 1H), 0.53-0.59 (m, 2H), 0.31-0.36 (m, 2H) .
MS (ESI, pos.ion) m/z: 643.20 [M+H]+ .MS (ESI, pos.ion) m/z: 643.20 [M+H] + .
實施例82:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4-氟苄基)-N -甲基吡啶醯胺 Example 82: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (4- fluorobenzyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -甲基-4-氟苄胺(55 mg, 0.40 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (196 mg, 1.02 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體37 mg,收率30%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N -methyl-4-fluorobenzylamine (55 mg, 0.40 mmol), 1-ethyl-(3-dimethylaminopropyl)carbanium Diimine hydrochloride (196 mg, 1.02 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL) and added to the mixture at 0°C. N , N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added dropwise to this solution, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and extracted with dichloromethane (5 mL × 3). It was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 37 mg of white solid, yield 30%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.75 – 7.86 (m, 1H), 7.53 – 7.61 (m, 1H), 7.25 – 7.39 (m, 3H), 7.05 – 7.13 (m, 3H), 6.82 – 6.96 (m, 2H), 6.62 (t,J F-H = 75.7 Hz, 1H), 4.48 – 4.73 (m, 4H), 4.11 – 4.19 (m, 0.5H), 3.85 – 3.95 (m, 0.5H), 3.88 ( d,J = 5.8 Hz, 2H), 3.51 – 3.57 (m, 1H), 3.25 – 3.38 (m, 1H), 3.02 (s, 2H), 2.96 (s, 1H), 2.27 – 2.60 (m, 2H), 2.11 (s, 3H), 2.06 – 1.94 (m, 1H), 1.29 – 1.36 (m, 1H), 0.62 – 0.70 (m, 2H), 0.33 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.75 – 7.86 (m, 1H), 7.53 – 7.61 (m, 1H), 7.25 – 7.39 (m, 3H), 7.05 – 7.13 (m, 3H) , 6.82 – 6.96 (m, 2H), 6.62 (t, J FH = 75.7 Hz, 1H), 4.48 – 4.73 (m, 4H), 4.11 – 4.19 (m, 0.5H), 3.85 – 3.95 (m, 0.5H) ), 3.88 ( d, J = 5.8 Hz, 2H), 3.51 – 3.57 (m, 1H), 3.25 – 3.38 (m, 1H), 3.02 (s, 2H), 2.96 (s, 1H), 2.27 – 2.60 ( m, 2H), 2.11 (s, 3H), 2.06 – 1.94 (m, 1H), 1.29 – 1.36 (m, 1H), 0.62 – 0.70 (m, 2H), 0.33 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 625.20 [M+H]+ .MS (ESI, pos.ion) m/z: 625.20 [M+H] + .
實施例83:化合物 6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N- 甲基-N- ((吡啶-2-基)甲基)吡啶醯胺 Example 83: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N- methyl -N- ((pyridin-2-yl)methyl)pyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (93 mg, 0.18 mmol),化合物N -甲基-1-(吡啶-2-基)甲胺二鹽酸鹽 (63 mg, 0.52mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (121 mg, 0.63 mmol) 和N -羥基-7-氮雜苯並三氮唑 (87 mg, 0.64 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌5 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體52 mg,收率46%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (93 mg, 0.18 mmol), compound N -methyl-1-(pyridin-2-yl)methanamine dihydrochloride (63 mg, 0.52 mmol), 1-ethyl-( 3-Dimethylaminopropyl)carbodiimide hydrochloride (121 mg, 0.63 mmol) and N -hydroxy-7-azabenzotriazole (87 mg, 0.64 mmol) in dichloromethane (6 mL), N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) was added dropwise to this solution at room temperature, stirred at room temperature for 5 h, washed with water (50 mL), and washed with dichloromethane. Extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain a white Solid 52 mg, yield 46%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.48-8.63 (m, 2H), 7.73-7.91 (m, 2H), 7.40-7.45 (m, 1H), 7.25-7.38 (m, 2H), 7.10-7.13 (m, 1H), 7.06 (s, 1H), 7.03 (t,J F-H = 74.7 Hz, 1H), 6.88 (d,J = 8.5 Hz, 1H), 4.67-4.74 (m, 2H), 4.26-4.50 (m, 3H), 4.03-4.10 (m, 1H), 3.90 (d,J = 6.4 Hz, 2H), 3.42-3.52 (m, 2H), 2.94-2.98 (m, 3H), 2.55-2.65 (m, 1H), 2.03 (d,J = 3.8 Hz, 3H), 1.86-1.95 (m, 1H), 1.22-1.26 (m, 1H), 0.51-0.60 (m, 2H), 0.30-0.38 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.48-8.63 (m, 2H), 7.73-7.91 (m, 2H), 7.40-7.45 (m, 1H), 7.25-7.38 (m, 2H), 7.10-7.13 (m, 1H), 7.06 (s, 1H), 7.03 (t, J FH = 74.7 Hz, 1H), 6.88 (d, J = 8.5 Hz, 1H), 4.67-4.74 (m, 2H), 4.26-4.50 (m, 3H), 4.03-4.10 (m, 1H), 3.90 (d, J = 6.4 Hz, 2H), 3.42-3.52 (m, 2H), 2.94-2.98 (m, 3H) , 2.55-2.65 (m, 1H), 2.03 (d, J = 3.8 Hz, 3H), 1.86-1.95 (m, 1H), 1.22-1.26 (m, 1H), 0.51-0.60 (m, 2H), 0.30 -0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 608.25 [M+H]+ .MS (ESI, pos.ion) m/z: 608.25 [M+H] + .
實施例84:化合物 6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N ,N -二環己基吡啶醯胺 Example 84: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N , N -dicyclohexylpyridamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (86 mg, 0.17 mmol),二環己基胺 (96 mg, 0.53mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (96 mg, 0.50 mmol) 和N -羥基-7-氮雜苯並三氮唑 (46 mg, 0.34 mmol) 溶於二氯甲烷 (6 mL)中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌13h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體53 mg,收率46%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (86 mg, 0.17 mmol), dicyclohexylamine (96 mg, 0.53 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (96 mg, 0.50 mmol) and N -hydroxy-7-azabenzotriazole (46 mg, 0.34 mmol) were dissolved in dichloromethane (6 mL), to this solution was added dropwise N at room temperature, N -diisopropylethylamine (0.3 mL, 2.0 mmol), stirred at room temperature for 13 h, added water (50 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, The concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 53 mg of white solid with a yield of 46%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.51-8.74 (m, 1H), 7.76-7.88 (m, 1H), 7.39-7.41 (m, 1H), 7.20-7.31 (m, 1H), 7.04-7.12 (m, 2H), 7.03 (t,J F-H = 75.0 Hz, 1H), 6.89 (d,J = 7.3 Hz, 1H), 4.30-4.52 (m, 3H), 4.02-4.13 (m, 1H), 3.84-3.96 (m, 2H), 3.40-3.53 (m, 1H), 3.05-3.22 (m, 2H), 2.52-2.67 (m, 1H), 2.37-2.49 (m, 1H), 2.04 (s, 3H), 1.84-1.99 (m, 1H), 1.60-1.79 (m, 6H), 1.42-1.58 (m, 6H), 1.11-1.32 (m, 5H), 0.84-1.02 (m, 4H), 0.51-0.61 (m, 2H), 0.28-0.39 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.51-8.74 (m, 1H), 7.76-7.88 (m, 1H), 7.39-7.41 (m, 1H), 7.20-7.31 (m, 1H), 7.04-7.12 (m, 2H), 7.03 (t, J FH = 75.0 Hz, 1H), 6.89 (d, J = 7.3 Hz, 1H), 4.30-4.52 (m, 3H), 4.02-4.13 ( m, 1H), 3.84-3.96 (m, 2H), 3.40-3.53 (m, 1H), 3.05-3.22 (m, 2H), 2.52-2.67 (m, 1H), 2.37-2.49 (m, 1H), 2.04 (s, 3H), 1.84-1.99 (m, 1H), 1.60-1.79 (m, 6H), 1.42-1.58 (m, 6H), 1.11-1.32 (m, 5H), 0.84-1.02 (m, 4H) ), 0.51-0.61 (m, 2H), 0.28-0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 667.30 [M+H]+ .MS (ESI, pos.ion) m/z: 667.30 [M+H] + .
實施例85:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -環己基-N -(環丙基甲基)吡啶醯胺 Example 85: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -cyclohexyl- N- (cyclopropylmethyl)pyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -(環丙基甲基)環己胺 (60 mg, 0.39 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (40 mg, 0.29 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體59 mg,收率46%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N- (cyclopropylmethyl)cyclohexylamine (60 mg, 0.39 mmol), 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (192 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (40 mg, 0.29 mmol) were dissolved in dichloromethane (5 mL) at 0°C N , N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added dropwise to this solution, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), The organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 59 mg of white solid, yield 46% .
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.66 – 7.76 (m, 1H), 7.53 (br.s, 1H), 7.26 – 7.37 (m, 2H), 7.08 (d,J = 6.4 Hz, 1H), 6.78 – 6.88 (m, 2H), 6.59 (t,J F-H = 75.5 Hz, 1H), 4.45 – 4.61 (m, 3H), 3.85 – 3.96 (m, 3H), 3.50 – 3.60 (m, 1H), 3.34 – 3.45 (m, 1H), 3.22 – 3.35 (m, 2H), 3.07 – 3.21 (m, 1H), 2.50 – 2.64 (m, 1H), 2.30 – 2.44 (m, 1H), 2.11 (s, 3H), 1.84 – 1.98 (m, 6H), 1.34 – 1.54 (m, 4H), 1.14 – 1.33 (m, 2H), 0.58 – 0.66 (m, 2H), 0.47 – 0.56 (m, 2H), 0.29 – 0.39 (m, 4H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.66 – 7.76 (m, 1H), 7.53 (br.s, 1H), 7.26 – 7.37 (m, 2H), 7.08 (d, J = 6.4 Hz , 1H), 6.78 – 6.88 (m, 2H), 6.59 (t, J FH = 75.5 Hz, 1H), 4.45 – 4.61 (m, 3H), 3.85 – 3.96 (m, 3H), 3.50 – 3.60 (m, 1H), 3.34 – 3.45 (m, 1H), 3.22 – 3.35 (m, 2H), 3.07 – 3.21 (m, 1H), 2.50 – 2.64 (m, 1H), 2.30 – 2.44 (m, 1H), 2.11 ( s, 3H), 1.84 – 1.98 (m, 6H), 1.34 – 1.54 (m, 4H), 1.14 – 1.33 (m, 2H), 0.58 – 0.66 (m, 2H), 0.47 – 0.56 (m, 2H), 0.29 – 0.39 (m, 4H).
MS (ESI, pos.ion) m/z: 639.35 [M+H]+ .MS (ESI, pos.ion) m/z: 639.35 [M+H] + .
實施例86:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 86: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (102 mg, 0.20 mmol),4,4-二氟-N -甲基環己胺鹽酸鹽 (56 mg, 0.30 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (192 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (55 mg, 0.40 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到色固體82 mg白,收率63%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (102 mg, 0.20 mmol), 4,4-difluoro- N -methylcyclohexylamine hydrochloride (56 mg, 0.30 mmol), 1-ethyl-(3-dimethyl aminopropyl)carbodiimide hydrochloride (192 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (55 mg, 0.40 mmol) in dichloromethane (5 mL) N , N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added dropwise to this solution at 0°C, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 82 mg of a colored solid White, yield 63%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.71 – 7.82 (m, 1H), 7.48 (br.s, 1H), 7.35 – 7.45 (m, 2H), 7.10 (d,J = 6.4 Hz, 1H), 6.88 (s, 1H), 6.84 (d,J = 6.9 Hz, 1H), 6.60 (t,J F-H = 75.6 Hz, 1H), 4.49 – 4.68 (m, 3H), 3.87 – 3.96 (m, 1H), 3.86 (d,J = 5.5 Hz, 2H), 3.67 – 3.80 (m, 1H), 3.48 – 3.56 (m, 1H), 3.25 – 3.38 (m, 1H), 2.99 (s, 1.5H), 2.86 (s, 1.5H), 2.42 – 2.51 (m, 2H), 2.13 – 2.27 (m, 2H), 2.11 (s, 3H), 1.84 – 1.96 (m, 6H), 1.24 – 1.35 (m, 1H), 0.59 – 0.68 (m, 2H), 0.31 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.71 – 7.82 (m, 1H), 7.48 (br.s, 1H), 7.35 – 7.45 (m, 2H), 7.10 (d, J = 6.4 Hz , 1H), 6.88 (s, 1H), 6.84 (d, J = 6.9 Hz, 1H), 6.60 (t, J FH = 75.6 Hz, 1H), 4.49 – 4.68 (m, 3H), 3.87 – 3.96 (m , 1H), 3.86 (d, J = 5.5 Hz, 2H), 3.67 – 3.80 (m, 1H), 3.48 – 3.56 (m, 1H), 3.25 – 3.38 (m, 1H), 2.99 (s, 1.5H) , 2.86 (s, 1.5H), 2.42 – 2.51 (m, 2H), 2.13 – 2.27 (m, 2H), 2.11 (s, 3H), 1.84 – 1.96 (m, 6H), 1.24 – 1.35 (m, 1H) ), 0.59 – 0.68 (m, 2H), 0.31 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 635.30 [M+H]+ .MS (ESI, pos.ion) m/z: 635.30 [M+H] + .
實施例87:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(環丙基甲基)-N -(4,4-二氟環己基)吡啶醯胺 Example 87: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (cyclopropylmethyl) - N - (4,4- difluoro-cyclohexyl) pyridin Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -(環丙基甲基)-4,4-二氟環己胺鹽酸鹽 (68 mg, 0.30 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (193 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (54 mg, 0.40 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.2 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到淺黃色固體101 mg,收率75%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N- (cyclopropylmethyl)-4,4-difluorocyclohexylamine hydrochloride (68 mg, 0.30 mmol), 1-ethyl- (3-Dimethylaminopropyl)carbodiimide hydrochloride (193 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (54 mg, 0.40 mmol) in dichloro In methane (5 mL), N , N -diisopropylethylamine (0.2 mL, 1.00 mmol) was added dropwise to this solution at 0°C, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and washed with two Chloromethane extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1), 101 mg of light yellow solid was obtained, and the yield was 75%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.71 – 7.81 (m, 1H), 7.57 (br.s, 1H), 7.40 – 7.50 (m, 2H), 7.12 (d,J = 5.4 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 6.9 Hz, 1H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.46 – 4.65 (m, 3H), 3.88 – 3.96 (m, 1H), 3.88 (d,J = 4.1 Hz, 2H), 3.67 – 3.82 (m, 1H), 3.53 – 3.58 (m, 1H), 3.26 – 3.37 (m, 2H), 3.16 – 3.27 (m, 1H), 2.46 – 2.55 (m, 2H), 2.13 – 2.23 (m, 2H), 2.13 (s, 3H), 1.94 – 2.05 (m, 6H), 1.55 – 1.73 (m, 1H), 1.26 – 1.34 (m, 1H), 0.81 – 0.94 (m, 1H), 0.62 – 0.70 (m, 2H), 0.52 – 0.62 (m, 1H), 0.35 – 0.44 (m, 4H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.71 – 7.81 (m, 1H), 7.57 (br.s, 1H), 7.40 – 7.50 (m, 2H), 7.12 (d, J = 5.4 Hz , 1H), 6.90 (s, 1H), 6.86 (d, J = 6.9 Hz, 1H), 6.62 (t, J FH = 75.6 Hz, 1H), 4.46 – 4.65 (m, 3H), 3.88 – 3.96 (m , 1H), 3.88 (d, J = 4.1 Hz, 2H), 3.67 – 3.82 (m, 1H), 3.53 – 3.58 (m, 1H), 3.26 – 3.37 (m, 2H), 3.16 – 3.27 (m, 1H) ), 2.46 – 2.55 (m, 2H), 2.13 – 2.23 (m, 2H), 2.13 (s, 3H), 1.94 – 2.05 (m, 6H), 1.55 – 1.73 (m, 1H), 1.26 – 1.34 (m , 1H), 0.81 – 0.94 (m, 1H), 0.62 – 0.70 (m, 2H), 0.52 – 0.62 (m, 1H), 0.35 – 0.44 (m, 4H).
MS (ESI, pos.ion) m/z: 675.30 [M+H]+ .MS (ESI, pos.ion) m/z: 675.30 [M+H] + .
實施例88:化合物 6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 88: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carbamoylamino)methyl ) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲酸 (67 mg, 0.21 mmol),6-(氨基甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺二鹽酸鹽 (94 mg, 0.33 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (88 mg, 0.46 mmol) 和N -羥基-7-氮雜苯並三氮唑 (54 mg, 0.39 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌17 h,加水洗 (50 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體65 mg,收率52%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carboxylic acid (67 mg, 0.21 mmol), 6-(amino methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methyl acyl pyridine dihydrochloride (94 mg, 0.33 mmol), 1- ethyl - (3-dimethylaminopropyl ) carbodiimide hydrochloride (88 mg, 0.46 mmol) and N -hydroxy-7-azabenzotriazole (54 mg, 0.39 mmol) were dissolved in dichloromethane (6 mL) at room temperature N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) was added dropwise to this solution, stirred at room temperature for 17 h, washed with water (50 mL), and then extracted with dichloromethane (5 mL × 3), The organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 65 mg of white solid, yield 52% .
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 9.87 (s, 1H), 8.60 (t,J = 5.9 Hz, 1H), 7.82 - 7.93 (m, 1H), 7.34 - 7.50 (m, 2H), 7.04 - 7.07 (m, 1H), 6.99 (t,J F-H = 75.1 Hz, 1H), 6.86 - 6.89 (m, 1H), 6.75 (d,J = 9.8 Hz, 1H), 4.30 - 4.53 (m, 3H), 3.99 - 4.07 (m, 1H), 3.61 - 3.69 (m, 1H), 3.36 - 3.45 (m, 1H), 2.71 – 2.78 (m, 3H), 2.53 - 2.62 (m, 1H), 1.91 - 2.15 (m, 3H), 2.02 (s, 3H), 1.65 - 1.90 (m, 7H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 9.87 (s, 1H), 8.60 (t, J = 5.9 Hz, 1H), 7.82 - 7.93 (m, 1H), 7.34 - 7.50 (m , 2H), 7.04 - 7.07 (m, 1H), 6.99 (t, J FH = 75.1 Hz, 1H), 6.86 - 6.89 (m, 1H), 6.75 (d, J = 9.8 Hz, 1H), 4.30 - 4.53 (m, 3H), 3.99 - 4.07 (m, 1H), 3.61 - 3.69 (m, 1H), 3.36 - 3.45 (m, 1H), 2.71 - 2.78 (m, 3H), 2.53 - 2.62 (m, 1H) , 1.91 - 2.15 (m, 3H), 2.02 (s, 3H), 1.65 - 1.90 (m, 7H).
MS (ESI, pos.ion) m/z: 581.30 [M+H]+ .MS (ESI, pos.ion) m/z: 581.30 [M+H] + .
實施例89:化合物 6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-2-甲醯氨基)甲基)-N -環己基-N -甲基吡啶醯胺 Example 89: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-2-carbamoylamino) Methyl) -N -cyclohexyl- N -picoline pyridamide
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-2-羧酸 (67 mg, 0.20 mmol),6-(氨基甲基)-N -環己基-N -甲基吡啶醯胺二鹽酸鹽 (94 mg, 0.38 mmol,合成方法參考中間體6),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (88 mg, 0.46 mmol) 和N -羥基-7-氮雜苯並三氮唑 (54 mg, 0.40 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌16 h,加水洗 (50 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體96 mg,收率85%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-2-carboxylic acid (67 mg, 0.20 mmol), 6 -(Aminomethyl) -N -cyclohexyl- N -picolinamide dihydrochloride (94 mg, 0.38 mmol, refer to Intermediate 6 for the synthesis method), 1-ethyl-(3-dimethylamino) propyl)carbodiimide hydrochloride (88 mg, 0.46 mmol) and N -hydroxy-7-azabenzotriazole (54 mg, 0.40 mmol) were dissolved in dichloromethane (6 mL), N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) was added dropwise to this solution at room temperature, stirred at room temperature for 16 h, washed with water (50 mL), and then extracted with dichloromethane (5 mL × 3 ), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain a white solid 96 mg, yield 85%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.55 - 8.67 (m, 1H), 7.82 - 7.88 (m, 1H), 7.44 - 7.49 (m, 1H), 7.31 - 7.36 (m, 1H), 7.13 (d,J = 8.2 Hz, 1H), 7.07 (t,J = 8.3 Hz, 1H), 7.02 (t,J F-H = 74.8 Hz, 1H), 6.85 - 6.93 (m, 1H), 4.26 - 4.45 (m, 3H), 4.01 - 4.15 (m, 3H), 3.40 - 3.52 (m, 2H), 2.86 (s, 1.6H), 2.70 (s, 1.4H), 2.57 - 2.66 (m, 1H), 2.04 (d,J = 4.2 Hz, 3H), 1.86 - 1.96 (m, 1H), 1.40 - 1.81 (m, 8H), 1.31 - 1.37 (m, 3H), 1.21 - 1.29 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.55 - 8.67 (m, 1H), 7.82 - 7.88 (m, 1H), 7.44 - 7.49 (m, 1H), 7.31 - 7.36 (m, 1H) 1H), 7.13 (d, J = 8.2 Hz, 1H), 7.07 (t, J = 8.3 Hz, 1H), 7.02 (t, J FH = 74.8 Hz, 1H), 6.85 - 6.93 (m, 1H), 4.26 - 4.45 (m, 3H), 4.01 - 4.15 (m, 3H), 3.40 - 3.52 (m, 2H), 2.86 (s, 1.6H), 2.70 (s, 1.4H), 2.57 - 2.66 (m, 1H) , 2.04 (d, J = 4.2 Hz, 3H), 1.86 - 1.96 (m, 1H), 1.40 - 1.81 (m, 8H), 1.31 - 1.37 (m, 3H), 1.21 - 1.29 (m, 2H).
MS (ESI, pos.ion) m/z: 573.35 [M+H]+ .MS (ESI, pos.ion) m/z: 573.35 [M+H] + .
實施例90:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-((N -(4,4-二氟環己基)乙醯氨基)甲基)吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 90: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-( ( N- (4,4-Difluorocyclohexyl)acetamido)methyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (150 mg, 0.41 mmol),N -((6-(氨基甲基)吡啶-2-基)甲基)-N -(4,4-二氟環己基)乙醯胺二鹽酸鹽 (166 mg, 0.45 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (391 mg, 2.04 mmol) 和N -羥基-7-氮雜苯並三氮唑 (112 mg, 0.82 mmol) 溶於二氯甲烷 (15 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫反應3 h,加水洗 (15 mL),用二氯甲烷萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體117 mg,收率44%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (150 mg, 0.41 mmol), N - (( 6- ( aminomethyl) pyridin-2-yl) methyl) - N - (4,4- difluoro-cyclohexyl) as acetamide dihydrochloride (166 mg, 0.45 mmol ), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (391 mg, 2.04 mmol) and N -hydroxy-7-azabenzotriazole (112 mg, 0.82 mmol) was dissolved in dichloromethane (15 mL), N , N -diisopropylethylamine (0.4 mL, 2.0 mmol) was added dropwise to this solution at 0 °C, the reaction was carried out at room temperature for 3 h, and washed with water. (15 mL), extracted with dichloromethane (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v ) = 30/1) to obtain 117 mg of white solid, yield 44%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.71 – 7.81 (m, 1H), 7.36 – 7.42 (m, 1H), 7.22 – 7.26 (m, 1H), 7.10 – 7.13 (m, 2H), 6.93 – 6.94 (m, 1H), 6.74 (t,J F-H = 75.7 Hz, 1H), 4.61 – 4.66 (m, 2H), 4.44 – 4.56 (m, 3H), 4.09 – 4.13 (m, 1H), 4.00 – 4.11 (m, 1H), 3.93 – 3.95 (m, 2H), 3.67 (t,J = 10.6 Hz, 1H), 3.50 – 3.56 (m, 1H), 2.67 – 2.73 (m, 1H), 2.31 (s, 1H), 2.16 (s, 3H), 2.13 (s, 2H), 2.06 – 2.10 (m, 1H), 1.99 – 2.03 (m, 4H), 1.63 – 1.81 (m, 4H), 1.27 – 1.35 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.71 – 7.81 (m, 1H), 7.36 – 7.42 (m, 1H), 7.22 – 7.26 (m, 1H), 7.10 – 7.13 (m, 2H) ), 6.93 – 6.94 (m, 1H), 6.74 (t, J FH = 75.7 Hz, 1H), 4.61 – 4.66 (m, 2H), 4.44 – 4.56 (m, 3H), 4.09 – 4.13 (m, 1H) , 4.00 – 4.11 (m, 1H), 3.93 – 3.95 (m, 2H), 3.67 (t, J = 10.6 Hz, 1H), 3.50 – 3.56 (m, 1H), 2.67 – 2.73 (m, 1H), 2.31 (s, 1H), 2.16 (s, 3H), 2.13 (s, 2H), 2.06 – 2.10 (m, 1H), 1.99 – 2.03 (m, 4H), 1.63 – 1.81 (m, 4H), 1.27 – 1.35 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 649.30 [M+H]+ .MS (ESI, pos.ion) m/z: 649.30 [M+H] + .
實施例91:化合物 6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 91: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-methyl acyl amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (76 mg, 0.15 mmol),4,4-二氟-N -甲基環己基胺鹽酸鹽 (96 mg, 0.64 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (96 mg, 0.50 mmol) 和N -羥基-7-氮雜苯並三氮唑 (46 mg, 0.34 mmol) 溶於二氯甲烷 (6 mL)中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌17 h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體46 mg,收率48%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino) Methyl)picolinic acid (76 mg, 0.15 mmol), 4,4-difluoro- N -methylcyclohexylamine hydrochloride (96 mg, 0.64 mmol), 1-ethyl-(3-dimethylamino) propyl)carbodiimide hydrochloride (96 mg, 0.50 mmol) and N -hydroxy-7-azabenzotriazole (46 mg, 0.34 mmol) were dissolved in dichloromethane (6 mL), N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) was added dropwise to this solution at room temperature, stirred at room temperature for 17 h, added with water (50 mL), and extracted with dichloromethane (5 mL × 3) , the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 46 mg of white solid, yield 48 %.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.61 (t,J = 6.0 Hz, 1H), 7.84-7.89 (m, 1H), 7.34-7.51 (m, 2H), 7.12 (d,J = 8.2 Hz, 1H), 7.04-7.07 (m, 1H), 6.95 (t,J F-H = 74.8 Hz, 1H), 6.84-6.89 (m, 1H), 4.87-4.93 (m, 1H), 4.34-4.52 (m, 3H), 4.01-4.12 (m, 1H), 3.41-3.52 (m, 2H), 2.71-2.87 (m, 3H), 2.58-2.67 (m, 1H), 2.04 (m, 3H), 1.99-2.13 (m, 4H), 1.83-1.93 (m, 4H), 1.53-1.74 (m, 10H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.61 (t, J = 6.0 Hz, 1H), 7.84-7.89 (m, 1H), 7.34-7.51 (m, 2H), 7.12 (d , J = 8.2 Hz, 1H), 7.04-7.07 (m, 1H), 6.95 (t, J FH = 74.8 Hz, 1H), 6.84-6.89 (m, 1H), 4.87-4.93 (m, 1H), 4.34 -4.52 (m, 3H), 4.01-4.12 (m, 1H), 3.41-3.52 (m, 2H), 2.71-2.87 (m, 3H), 2.58-2.67 (m, 1H), 2.04 (m, 3H) , 1.99-2.13 (m, 4H), 1.83-1.93 (m, 4H), 1.53-1.74 (m, 10H).
MS (ESI, pos.ion) m/z: 649.20 [M+H]+ .MS (ESI, pos.ion) m/z: 649.20 [M+H] + .
實施例92:化合物6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(環丙基甲基)-N -(4,4-二氟環己基)吡啶醯胺 Example 92: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-methyl acyl amino) methyl) - N - (cyclopropylmethyl) - N - (4,4- difluoro-cyclohexyl) pyridin Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環戊氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (76 mg, 0.15 mmol),N -(環丙基甲基)-4,4-二氟環己基胺鹽酸鹽 (96 mg, 0.51 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (96 mg, 0.50 mmol) 和N -羥基-7-氮雜苯並三氮唑 (46 mg, 0.34 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下加入N ,N -二異丙基乙胺 (0.3 mL, 2.0 mmol),室溫攪拌17 h,加水 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行柱分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體66 mg,收率65%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopentyloxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino) Methyl)picolinic acid (76 mg, 0.15 mmol), N- (cyclopropylmethyl)-4,4-difluorocyclohexylamine hydrochloride (96 mg, 0.51 mmol), 1-ethyl-(3 -Dimethylaminopropyl)carbodiimide hydrochloride (96 mg, 0.50 mmol) and N -hydroxy-7-azabenzotriazole (46 mg, 0.34 mmol) were dissolved in dichloromethane ( 6 mL), add N , N -diisopropylethylamine (0.3 mL, 2.0 mmol) at room temperature, stir at room temperature for 17 h, add water (50 mL), extract with dichloromethane (5 mL × 3) , the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was subjected to column separation (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 66 mg of white solid with a yield of 65%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.61 (t,J = 6.0 Hz, 1H), 7.83-7.87 (m, 1H), 7.31-7.51 (m, 2H), 7.12 (d,J = 8.4 Hz, 1H), 7.04-7.08 (m, 1H), 6.95 (t,J F-H = 74.8 Hz, 1H), 6.84-6.91 (m, 1H), 4.88-4.93 (m, 1H), 4.34-4.50 (m, 3H), 4.05-4.12 (m, 1H), 3.40-3.52 (m, 2H), 3.18-3.25 (m, 1H), 3.08-3.12 (m, 1H), 2.58-2.65 (m, 1H), 2.39 (d,J = 6.7 Hz, 2H), 2.04 (s, 3H), 1.96-2.10 (m, 4H), 1.84-1.92 (m, 4H), 1.74-1.80 (m, 8H), 1.32-1.39 (m, 1H), 0.37-0.40 (m, 2H), 0.09-0.11 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.61 (t, J = 6.0 Hz, 1H), 7.83-7.87 (m, 1H), 7.31-7.51 (m, 2H), 7.12 (d , J = 8.4 Hz, 1H), 7.04-7.08 (m, 1H), 6.95 (t, J FH = 74.8 Hz, 1H), 6.84-6.91 (m, 1H), 4.88-4.93 (m, 1H), 4.34 -4.50 (m, 3H), 4.05-4.12 (m, 1H), 3.40-3.52 (m, 2H), 3.18-3.25 (m, 1H), 3.08-3.12 (m, 1H), 2.58-2.65 (m, 1H), 2.39 (d, J = 6.7 Hz, 2H), 2.04 (s, 3H), 1.96-2.10 (m, 4H), 1.84-1.92 (m, 4H), 1.74-1.80 (m, 8H), 1.32 -1.39 (m, 1H), 0.37-0.40 (m, 2H), 0.09-0.11 (m, 2H).
MS (ESI, pos.ion) m/z: 689.35 [M+H]+ .MS (ESI, pos.ion) m/z: 689.35 [M+H] + .
實施例93:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-乙氧基苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 93: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-ethoxyphenyl)pyrrolidine-2-carbamoylamino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 (60 mg, 0.10 mmol),碳酸鉀 (36 mg, 0.26 mmol) 溶於N ,N -二甲基甲醯胺 (3 mL)中,室溫加入碘乙烷 (0.1 mL, 1.0 mmol),70℃下反應17 h,加水 (50 mL),用乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體33 mg,收率52%。The compound 6-((( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carbamoylamino)methyl) -N -(4,4-Difluorocyclohexyl) -N -picolinamide (60 mg, 0.10 mmol), potassium carbonate (36 mg, 0.26 mmol) in N , N -dimethylformamide (3 mL), add iodoethane (0.1 mL, 1.0 mmol) at room temperature, react at 70 °C for 17 h, add water (50 mL), extract with ethyl acetate (5 mL × 3), and dry the organic phase with anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 33 mg of white solid with a yield of 52%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.60 (t,J = 5.9 Hz, 1H), 7.84-7.90 (m, 1H), 7.34-7.51 (m, 2H), 7.05-7.14 (m, 2H), 7.02 (t,J F-H = 74.9 Hz, 1H), 6.89 (d,J = 8.5 Hz, 1H), 4.33-4.51 (m, 3H), 4.08-4.12 (m, 3H), 3.41-3.52 (m, 2H), 2.71-2.87 (m, 3H), 2.58-2.64 (m, 1H), 2.03 (s, 3H), 1.63-2.12 (m, 10H), 1.34 (t,J = 6.7 Hz, 3H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.60 (t, J = 5.9 Hz, 1H), 7.84-7.90 (m, 1H), 7.34-7.51 (m, 2H), 7.05-7.14 (m, 2H), 7.02 (t, J FH = 74.9 Hz, 1H), 6.89 (d, J = 8.5 Hz, 1H), 4.33-4.51 (m, 3H), 4.08-4.12 (m, 3H), 3.41 -3.52 (m, 2H), 2.71-2.87 (m, 3H), 2.58-2.64 (m, 1H), 2.03 (s, 3H), 1.63-2.12 (m, 10H), 1.34 (t, J = 6.7 Hz , 3H).
MS (ESI, pos.ion) m/z: 609.30 [M+H]+ .MS (ESI, pos.ion) m/z: 609.30 [M+H] + .
實施例94:化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -環己基-N -甲基吡啶醯胺 Example 94: Compound 6-((( 2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl) - N -Cyclohexyl- N -picolinamide
將化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (80 mg, 0.16 mmol),N -甲基環己胺 (36 mg, 0.32 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (156 mg, 0.81 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下滴加N ,N -二異丙基乙胺 (0.13 mL, 0.79 mmol),室溫反應22 h,加水洗 (15 mL),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體42 mg,收率43%。The compound 6-((( 2R )-1-acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinic acid ( 80 mg, 0.16 mmol), N -methylcyclohexylamine (36 mg, 0.32 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (156 mg, 0.81 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL), and N , N -diisopropylethylamine was added dropwise at 0°C (0.13 mL, 0.79 mmol), reacted at room temperature for 22 h, washed with water (15 mL), then extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was subjected to silica gel column layer 42 mg of white solid was obtained, and the yield was 43%.
1 H NMR (600 MHz, CDCl3 ) δ (ppm): 7.76 (s, 1H), 7.51 – 7.54 (m, 1H), 7.35 – 7.38 (m, 2H), 7.12 – 7.28 (m, 3H), 6.56 (t,J F-H = 73.5 Hz, 1H), 6.53 (t,J F-H = 73.5 Hz, 1H), 4.50 – 4.63 (m, 3H), 3.99 (dd,J = 9.9, 7.6 Hz, 1H), 3.58 (t,J = 10.6 Hz, 1H), 3.30 – 3.46 (m, 2H), 3.00 (s, 2H), 2.84 (s, 1H), 2.58 – 2.64 (m, 1H), 2.40 – 2.47 (m, 1H), 2.15 (s, 3H), 1.70 – 1.85 (m, 6H), 1.48 – 1.56 (m, 4H). 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 7.76 (s, 1H), 7.51 – 7.54 (m, 1H), 7.35 – 7.38 (m, 2H), 7.12 – 7.28 (m, 3H), 6.56 (t, J FH = 73.5 Hz, 1H), 6.53 (t, J FH = 73.5 Hz, 1H), 4.50 – 4.63 (m, 3H), 3.99 (dd, J = 9.9, 7.6 Hz, 1H), 3.58 ( t, J = 10.6 Hz, 1H), 3.30 – 3.46 (m, 2H), 3.00 (s, 2H), 2.84 (s, 1H), 2.58 – 2.64 (m, 1H), 2.40 – 2.47 (m, 1H) , 2.15 (s, 3H), 1.70 – 1.85 (m, 6H), 1.48 – 1.56 (m, 4H).
MS (ESI, pos.ion) m/z: 595.30 [M+H]+ .MS (ESI, pos.ion) m/z: 595.30 [M+H] + .
實施例95:化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 95: Compound 6-((( 2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl) - N- (4,4-Difluorocyclohexyl) -N -picoline pyridamide
將化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (80 mg, 0.16 mmol),4,4-二氟-N -甲基環己胺鹽酸鹽 (36 mg, 0.19 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (151 mg, 0.79 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.13 mL, 0.79 mmol),室溫反應15 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體23mg,收率22%。The compound 6-((( 2R )-1-acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinic acid ( 80 mg, 0.16 mmol), 4,4-difluoro- N -methylcyclohexylamine hydrochloride (36 mg, 0.19 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Imine hydrochloride (151 mg, 0.79 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL) and added to this at 0°C N , N -diisopropylethylamine (0.13 mL, 0.79 mmol) was added dropwise to the solution, the reaction was carried out at room temperature for 15 h, washed with water (15 mL), and extracted with dichloromethane (5 mL × 3). It was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 23 mg of white solid with a yield of 22%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.79 (br.s, 1H), 7.36 – 7.50 (m, 3H), 7.13 – 7.26 (m, 3H), 6.56 (t,J F-H = 73.6 Hz, 1H), 6.53 (t,J F-H = 73.6 Hz, 1H), 4.51 – 4.67 (m, 4H), 3.93 – 4.03 (m, 1H), 3.50 – 3.62 (m, 1H), 3.30 – 3.46 (m, 1H), 3.02 (s, 1H), 2.90 (s, 2H), 2.45 – 2.64 (m, 2H), 2.05 – 2.29 (m, 2H), 2.16 (s, 3H), 1.91 – 2.04 (m, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.79 (br.s, 1H), 7.36 – 7.50 (m, 3H), 7.13 – 7.26 (m, 3H), 6.56 (t, J FH = 73.6 Hz, 1H), 6.53 (t, J FH = 73.6 Hz, 1H), 4.51 – 4.67 (m, 4H), 3.93 – 4.03 (m, 1H), 3.50 – 3.62 (m, 1H), 3.30 – 3.46 (m , 1H), 3.02 (s, 1H), 2.90 (s, 2H), 2.45 – 2.64 (m, 2H), 2.05 – 2.29 (m, 2H), 2.16 (s, 3H), 1.91 – 2.04 (m, 6H) ).
MS (ESI, pos.ion) m/z: 631.30 [M+H]+ .MS (ESI, pos.ion) m/z: 631.30 [M+H] + .
實施例96:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -環戊基-N -甲基吡啶醯胺 Example 96: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -cyclopentyl- N -methylpyridylamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -甲基環戊胺鹽酸鹽 (55 mg, 0.410 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (191 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體59 mg,收率50%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N -methylcyclopentylamine hydrochloride (55 mg, 0.410 mmol), 1-ethyl-(3-dimethylaminopropyl)carbohydride Diimine hydrochloride (191 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL) and added to the solution at 0 °C. N , N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added dropwise to this solution, the reaction was carried out at room temperature for 12 h, washed with water (15 mL), and extracted with dichloromethane (5 mL × 3). It was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 59 mg of white solid, with a yield of 50%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 (s, 1H), 7.50 (br.s, 1H), 7.35 – 7.41 (m, 2H), 7.12 (d,J = 7.1 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 7.1 Hz, 1H), 6.62 (t,J F-H = 75.6 Hz, 1H), 5.02 – 5.10 (m, 0.4H), 4.48 – 4.65 (m, 3H), 3.99 – 4.09 (m, 0.6H), 3.91 – 3.97 (m, 1H), 3.88 (d,J = 5.8 Hz, 2H), 3.55 – 3.61 (m, 1H), 3.29 – 3.38 (m, 1H), 3.00 (s, 2H), 2.85 (s, 1H), 2.56 – 2.61 (m, 1H), 2.40 – 2.47 (m, 1H), 2.14 (s, 3H), 1.68 – 1.84 (m, 8H), 1.26 – 1.34 (m, 1H), 0.65 – 0.68 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 (s, 1H), 7.50 (br.s, 1H), 7.35 – 7.41 (m, 2H), 7.12 (d, J = 7.1 Hz, 1H ), 6.90 (s, 1H), 6.86 (d, J = 7.1 Hz, 1H), 6.62 (t, J FH = 75.6 Hz, 1H), 5.02 – 5.10 (m, 0.4H), 4.48 – 4.65 (m, 3H), 3.99 – 4.09 (m, 0.6H), 3.91 – 3.97 (m, 1H), 3.88 (d, J = 5.8 Hz, 2H), 3.55 – 3.61 (m, 1H), 3.29 – 3.38 (m, 1H) ), 3.00 (s, 2H), 2.85 (s, 1H), 2.56 – 2.61 (m, 1H), 2.40 – 2.47 (m, 1H), 2.14 (s, 3H), 1.68 – 1.84 (m, 8H), 1.26 – 1.34 (m, 1H), 0.65 – 0.68 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 585.30 [M+H]+ .MS (ESI, pos.ion) m/z: 585.30 [M+H] + .
實施例97:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -環丁基-N -甲基吡啶醯胺 Example 97: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxylamido)methyl) -N -cyclobutyl- N -methylpyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (114 mg, 0.23 mmol),N -甲基環丁胺鹽酸鹽 (48 mg, 0.40 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (191 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應12 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體82 mg,收率63%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (114 mg, 0.23 mmol), N -methylcyclobutylamine hydrochloride (48 mg, 0.40 mmol), 1-ethyl-(3-dimethylaminopropyl)carbohydride Diimine hydrochloride (191 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) were dissolved in dichloromethane (5 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.17 mL, 1.00 mmol) was added, the reaction was carried out at room temperature for 12 h, water (15 mL) was added, extracted with dichloromethane (5 mL × 3), and the organic phase was dried over anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 82 mg of white solid with a yield of 63%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.74 (s, 1H), 7.58 (br.s, 1H), 7.29 – 7.39 (m, 2H), 7.12 (d,J = 5.8 Hz, 1H), 6.89 (s, 1H), 6.85 (d,J = 6.6 Hz, 1H), 6.61 (t,J F-H = 75.6 Hz, 1H), 4.97 – 5.12 (m, 0.4H), 4.48 – 4.66 (m, 3H), 4.15 – 4.28 (m, 0.6H), 3.78 – 3.96 (m, 3H), 3.51 – 3.63 (m, 1H), 3.25 – 3.39 (m, 1H), 3.09 (s, 2H), 2.94 (s, 1H), 2.52 – 2.63 (m, 1H), 2.35 – 2.45 (m, 1H), 2.12 (s, 3H), 1.94 – 2.04 (m, 4H), 1.62 – 1.74 (m, 2H), 1.26 – 1.34 (m, 1H), 0.58 – 0.68 (m, 2H), 0.31 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.74 (s, 1H), 7.58 (br.s, 1H), 7.29 – 7.39 (m, 2H), 7.12 (d, J = 5.8 Hz, 1H ), 6.89 (s, 1H), 6.85 (d, J = 6.6 Hz, 1H), 6.61 (t, J FH = 75.6 Hz, 1H), 4.97 – 5.12 (m, 0.4H), 4.48 – 4.66 (m, 3H), 4.15 – 4.28 (m, 0.6H), 3.78 – 3.96 (m, 3H), 3.51 – 3.63 (m, 1H), 3.25 – 3.39 (m, 1H), 3.09 (s, 2H), 2.94 (s , 1H), 2.52 – 2.63 (m, 1H), 2.35 – 2.45 (m, 1H), 2.12 (s, 3H), 1.94 – 2.04 (m, 4H), 1.62 – 1.74 (m, 2H), 1.26 – 1.34 (m, 1H), 0.58 – 0.68 (m, 2H), 0.31 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 571.30 [M+H]+ .MS (ESI, pos.ion) m/z: 571.30 [M+H] + .
實施例98:化合物6-(((2R )-1-乙醯基-4-(3-(2,2-二氟乙氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 98: Compound 6-((( 2R )-1-Acetyl-4-(3-(2,2-difluoroethoxy)-4-(difluoromethoxy)phenyl)pyrrole alkyl-2-acyl-amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物 6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 (53 mg, 0.09 mmol),碳酸鉀 (36 mg, 0.26 mmol) 溶於N ,N -二甲基甲醯胺 (3 mL) 中,室溫下向此溶液中加入2-溴-1,1-二氟乙烷 (0.1 mL, 1.0 mmol),70℃下反應12 h,加水洗 (50 mL),然後乙酸乙酯萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體31 mg,收率52%。The compound 6-((( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carbamoylamino)methyl) -N -(4,4-Difluorocyclohexyl) -N -picolinamide (53 mg, 0.09 mmol), potassium carbonate (36 mg, 0.26 mmol) in N , N -dimethylformamide (3 mL), 2-bromo-1,1-difluoroethane (0.1 mL, 1.0 mmol) was added to this solution at room temperature, reacted at 70 °C for 12 h, washed with water (50 mL), and then ethyl acetate Extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain a white Solid 31 mg, yield 52%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.60 - 8.63 (m, 1H), 7.81 - 7.95 (m, 1H), 7.31 - 7.51 (m, 2H), 7.12 - 7.24 (m, 2H), 7.04 (t,J F-H = 74.9 Hz, 1H), 6.91 - 7.01 (m, 1H), 6.40 (t,J F-H = 54.6 Hz, 1H), 4.30 - 4.57 (m, 5H), 4.09 - 4.23 (m, 1H), 3.41 - 3.74 (m, 3H), 2.86 (s, 1.5H), 2.73 (s, 1.5H), 2.55 - 2.67 (m, 1H), 1.95 - 2.15 (m, 6H), 1.62 - 1.88 (m, 6H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.60 - 8.63 (m, 1H), 7.81 - 7.95 (m, 1H), 7.31 - 7.51 (m, 2H), 7.12 - 7.24 (m, 2H), 7.04 (t, J FH = 74.9 Hz, 1H), 6.91 - 7.01 (m, 1H), 6.40 (t, J FH = 54.6 Hz, 1H), 4.30 - 4.57 (m, 5H), 4.09 - 4.23 (m, 1H), 3.41 - 3.74 (m, 3H), 2.86 (s, 1.5H), 2.73 (s, 1.5H), 2.55 - 2.67 (m, 1H), 1.95 - 2.15 (m, 6H), 1.62 - 1.88 (m, 6H).
MS (ESI, pos.ion) m/z: 645.30 [M+H]+ .MS (ESI, pos.ion) m/z: 645.30 [M+H] + .
實施例99:化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氘環己基)-N -甲基吡啶醯胺 Example 99: Compound 6-((( 2R )-1-Acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl) - N- (4,4-dideuterocyclohexyl) -N -methylpyridamide
將化合物6-(((2R )-1-乙醯基-4-(3,4-雙(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),4,4-二氘-N -甲基環己胺鹽酸鹽 (67 mg, 0.44 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (193 mg, 1.01 mmol) 和N -羥基-7-氮雜苯並三氮唑 (53 mg, 0.39 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.26 mL, 1.60 mmol),室溫反應12 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體66 mg,收率55%。The compound 6-((( 2R )-1-acetyl-4-(3,4-bis(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino)methyl)picolinic acid ( 100 mg, 0.20 mmol), 4,4-dideutero- N -methylcyclohexylamine hydrochloride (67 mg, 0.44 mmol), 1-ethyl-(3-dimethylaminopropyl)carbohydride Imine hydrochloride (193 mg, 1.01 mmol) and N -hydroxy-7-azabenzotriazole (53 mg, 0.39 mmol) were dissolved in dichloromethane (5 mL) and added to this at 0°C N , N -diisopropylethylamine (0.26 mL, 1.60 mmol) was added dropwise to the solution, the reaction was carried out at room temperature for 12 h, water (15 mL) was added, extracted with dichloromethane (5 mL × 3), and the organic phase was washed with anhydrous It was dried over sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 66 mg of white solid with a yield of 55%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.92 (br.s, 1H), 7.58 – 7.63 (m, 1H), 7.40 (d,J = 6.7 Hz, 1H), 7.25 – 7.34 (m, 3H), 6.86 (t,J F-H = 73.6 Hz, 1H), 6.82 (t,J F-H = 73.6 Hz, 1H), 4.48 – 4.65 (m, 3H), 4.10 – 4.20 (m, 1H), 3.61 – 3.69 (m, 1H), 3.51 – 3.60 (m, 1H), 3.36 – 3.46 (m, 1H), 3.00 (s, 1.6H), 2.82 (s, 1.4H), 2.68 – 2.79 (m, 1H), 2.17 (s, 3H), 2.01 – 2.12 (m, 1H), 1.71 – 1.88 (m, 4H), 1.53 – 1.66 (m, 2H), 1.37 – 1.49 (m, 1H), 1.04 – 1.12 (m, 1H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.92 (br.s, 1H), 7.58 – 7.63 (m, 1H), 7.40 (d, J = 6.7 Hz, 1H), 7.25 – 7.34 ( m, 3H), 6.86 (t, J FH = 73.6 Hz, 1H), 6.82 (t, J FH = 73.6 Hz, 1H), 4.48 – 4.65 (m, 3H), 4.10 – 4.20 (m, 1H), 3.61 – 3.69 (m, 1H), 3.51 – 3.60 (m, 1H), 3.36 – 3.46 (m, 1H), 3.00 (s, 1.6H), 2.82 (s, 1.4H), 2.68 – 2.79 (m, 1H) , 2.17 (s, 3H), 2.01 – 2.12 (m, 1H), 1.71 – 1.88 (m, 4H), 1.53 – 1.66 (m, 2H), 1.37 – 1.49 (m, 1H), 1.04 – 1.12 (m, 1H).
MS (ESI, pos.ion) m/z: 597.70 [M+H]+ .MS (ESI, pos.ion) m/z: 597.70 [M+H] + .
實施例100:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氘環己基)-N -甲基吡啶醯胺 Example 100: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (cyclohexyl of 4,4-dideutero-yl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (90 mg, 0.18 mmol),4,4-二氘-N -甲基環己胺鹽酸鹽 (42 mg, 0.28 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (173 mg, 0.9 mmol) 和N -羥基-7-氮雜苯並三氮唑 (49 mg, 0.36 mmol) 溶於二氯甲烷 (5 mL) 中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.21 mL, 1.3 mmol),室溫反應10 h,加水 (25 mL),二氯甲烷萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體46 mg,收率42%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (90 mg, 0.18 mmol), 4,4-dideutero- N -methylcyclohexylamine hydrochloride (42 mg, 0.28 mmol), 1-ethyl-(3-dimethyl aminopropyl)carbodiimide hydrochloride (173 mg, 0.9 mmol) and N -hydroxy-7-azabenzotriazole (49 mg, 0.36 mmol) in dichloromethane (5 mL) N , N -diisopropylethylamine (0.21 mL, 1.3 mmol) was added dropwise to this solution at 0°C, reacted at room temperature for 10 h, added water (25 mL), extracted with dichloromethane (10 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 46 mg of white solid, which was collected rate 42%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.92 (s, 1H), 7.57 – 7.64 (m, 1H), 7.40 (d,J = 7.3 Hz, 1H), 7.12 (d,J = 8.3 Hz, 1H), 7.10 (s, 1H), 6.92 – 6.95 (m, 1H), 6.75 (t,J F-H = 75.6 Hz, 1H), 4.51 – 4.64 (m, 3H), 4.08 – 4.15 (m, 1H), 3.94 (d,J = 5.7 Hz, 2H), 3.67 (t,J = 10.2 Hz, 1H), 3.46 – 3.56 (m, 1H), 3.36 – 3.45 (m, 1H), 3.00 (s, 1.7H), 2.81 (s, 1.3H), 2.64 – 2.75 (m, 1H), 2.17 (s, 3H), 2.01 – 2.12 (m, 1H), 1.71 – 1.88 (m, 4H), 1.53 – 1.65 (m, 2H), 1.35 – 1.47 (m, 2H), 1.03 – 1.11 (m, 1H), 0.62 – 0.68 (m, 2H), 0.38 – 0.42 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.92 (s, 1H), 7.57 – 7.64 (m, 1H), 7.40 (d, J = 7.3 Hz, 1H), 7.12 (d, J = 8.3 Hz, 1H), 7.10 (s, 1H), 6.92 – 6.95 (m, 1H), 6.75 (t, J FH = 75.6 Hz, 1H), 4.51 – 4.64 (m, 3H), 4.08 – 4.15 (m, 1H), 3.94 (d, J = 5.7 Hz, 2H), 3.67 (t, J = 10.2 Hz, 1H), 3.46 – 3.56 (m, 1H), 3.36 – 3.45 (m, 1H), 3.00 (s, 1.7 H), 2.81 (s, 1.3H), 2.64 – 2.75 (m, 1H), 2.17 (s, 3H), 2.01 – 2.12 (m, 1H), 1.71 – 1.88 (m, 4H), 1.53 – 1.65 (m , 2H), 1.35 – 1.47 (m, 2H), 1.03 – 1.11 (m, 1H), 0.62 – 0.68 (m, 2H), 0.38 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 601.30 [M+H]+ .MS (ESI, pos.ion) m/z: 601.30 [M+H] + .
實施例101:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氘環己基)-N -甲基吡啶醯胺 Example 101: Compound 6-((( 2R )-1-acetoxy-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy) yl) phenyl) pyrrolidine-2-acyl-amino) methyl) - N - (cyclohexyl of 4,4-dideutero-yl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基(1,1-二氘)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (90 mg, 0.18 mmol),4,4-二氘-N -甲基環己胺鹽酸鹽 (40 mg, 0.26 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (175 mg, 0.91 mmol) 和N -羥基-7-氮雜苯並三氮唑 (53 mg, 0.39 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.26 mL, 1.6 mmol),室溫反應15 h,加水 (15 mL),二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體46 mg,收率43%。Compound 6-(((2 R )-1-acetyl-4-(3-(cyclopropyl(1,1-dideutero)methoxy)-4-(difluoromethoxy)phenyl )pyrrolidine-2-carbamoylamino)methyl)picolinic acid (90 mg, 0.18 mmol), 4,4-dideutero- N -methylcyclohexylamine hydrochloride (40 mg, 0.26 mmol), 1- Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (175 mg, 0.91 mmol) and N -hydroxy-7-azabenzotriazole (53 mg, 0.39 mmol) were dissolved In dichloromethane (5 mL), after cooling to 0 ℃, N , N -diisopropylethylamine (0.26 mL, 1.6 mmol) was added, the reaction was carried out at room temperature for 15 h, water (15 mL) was added, dichloromethane Extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, concentrated, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain a white Solid 46 mg, yield 43%.
1 H NMR (600 MHz, CD3 OD) δ (ppm): 7.90 – 7.94 (m, 1H), 7.57 – 7.63 (m, 1H), 7.40 (d,J = 7.5 Hz, 1H), 7.12 (d,J = 7.9 Hz, 1H), 7.09 (s, 1H), 6.93 (d,J = 7.7 Hz, 1H), 6.75 (t,J F-H = 75.8 Hz, 1H), 4.47 – 4.64 (m, 3H), 4.09 – 4.14 (m, 1H), 3.66 (t,J = 10.4 Hz, 1H), 3.47 – 3.55 (m, 1H), 3.37 – 3.44 (m, 1H), 3.00 (s, 1.5H), 2.81 (s, 1.5H), 2.67 – 2.72 (m, 1H), 2.17 (s, 3H), 2.07 – 2.12 (m, 1H), 1.73 – 1.87 (m, 4H), 1.51 – 1.66 (m, 2H), 1.38 – 1.45 (m, 1H), 1.28 – 1.36 (m, 1H), 1.05 – 1.10 (m, 1H), 0.63 – 0.66 (m, 2H), 0.38 – 0.40 (m, 2H). 1 H NMR (600 MHz, CD 3 OD) δ (ppm): 7.90 – 7.94 (m, 1H), 7.57 – 7.63 (m, 1H), 7.40 (d, J = 7.5 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H), 7.09 (s, 1H), 6.93 (d, J = 7.7 Hz, 1H), 6.75 (t, J FH = 75.8 Hz, 1H), 4.47 – 4.64 (m, 3H), 4.09 – 4.14 (m, 1H), 3.66 (t, J = 10.4 Hz, 1H), 3.47 – 3.55 (m, 1H), 3.37 – 3.44 (m, 1H), 3.00 (s, 1.5H), 2.81 (s, 1.5H), 2.67 – 2.72 (m, 1H), 2.17 (s, 3H), 2.07 – 2.12 (m, 1H), 1.73 – 1.87 (m, 4H), 1.51 – 1.66 (m, 2H), 1.38 – 1.45 (m, 1H), 1.28 – 1.36 (m, 1H), 1.05 – 1.10 (m, 1H), 0.63 – 0.66 (m, 2H), 0.38 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 603.35 [M+H]+ .MS (ESI, pos.ion) m/z: 603.35 [M+H] + .
實施例102:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 102: Compound 6-(((2 R )-1-acetoxy-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy) yl) phenyl) pyrrolidine-2-acyl-amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基-(1,1-二氘)甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (90 mg, 0.18 mmol),4,4-二氟-N -甲基環己胺鹽酸鹽 (49 mg, 0.27 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (172 mg, 0.9 mmol) 和N -羥基-7-氮雜苯並三氮唑 (49 mg, 0.36 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.21 mL, 1.3 mmol),室溫反應18 h,加水 (15 mL),二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體77 mg,收率68%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropyl-(1,1-dideutero)methoxy)-4-(difluoromethoxy)benzene yl)pyrrolidine-2-carbamoylamino)methyl)picolinic acid (90 mg, 0.18 mmol), 4,4-difluoro- N -methylcyclohexylamine hydrochloride (49 mg, 0.27 mmol), 1 -Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (172 mg, 0.9 mmol) and N -hydroxy-7-azabenzotriazole (49 mg, 0.36 mmol) It was dissolved in dichloromethane (5 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.21 mL, 1.3 mmol) was added, the reaction was carried out at room temperature for 18 h, water (15 mL) was added, and dichloromethane was added. Methane extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, concentrated, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain White solid 77 mg, yield 68%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.90 – 7.95 (m, 1H), 7.58 – 7.63 (m, 1H), 7.42 – 7.47 (m, 1H), 7.12 (d,J = 7.9 Hz, 1H), 7.09 (s, 1H), 6.91 – 6.95 (m, 1H), 6.75 (t,J F-H = 75.6 Hz, 1H), 4.45 – 4.64 (m, 3H), 4.07 – 4.14 (m, 1H), 3.66 – 3.78 (m, 0.5H), 3.66 (t,J = 10.5 Hz, 1H), 3.46 – 3.57 (m, 1H), 3.33 – 3.42 (m, 0.5H), 3.00 (s, 1.5H), 2.84 (s, 1.5H), 2.64 – 2.74 (m, 1H), 2.16 (s, 3H), 2.01 – 2.12 (m, 1H), 1.80 – 2.10 (m, 8H), 1.29 – 1.38 (m, 1H), 0.61 – 0.67 (m, 2H), 0.35 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.90 – 7.95 (m, 1H), 7.58 – 7.63 (m, 1H), 7.42 – 7.47 (m, 1H), 7.12 (d, J = 7.9 Hz, 1H), 7.09 (s, 1H), 6.91 – 6.95 (m, 1H), 6.75 (t, J FH = 75.6 Hz, 1H), 4.45 – 4.64 (m, 3H), 4.07 – 4.14 (m, 1H) ), 3.66 – 3.78 (m, 0.5H), 3.66 (t, J = 10.5 Hz, 1H), 3.46 – 3.57 (m, 1H), 3.33 – 3.42 (m, 0.5H), 3.00 (s, 1.5H) , 2.84 (s, 1.5H), 2.64 – 2.74 (m, 1H), 2.16 (s, 3H), 2.01 – 2.12 (m, 1H), 1.80 – 2.10 (m, 8H), 1.29 – 1.38 (m, 1H) ), 0.61 – 0.67 (m, 2H), 0.35 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 637.80 [M+H]+ .MS (ESI, pos.ion) m/z: 637.80 [M+H] + .
實施例103:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 103: Compound 6 - (((2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2 methyl acyl amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-甲氧基-d3 -苯基)吡咯烷-2-羧酸 (45 mg, 0.14 mmol),6-(氨基甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺二鹽酸鹽 (62 mg, 0.17 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (132 mg, 0.69 mmol) 和N -羥基-7-氮雜苯並三氮唑 (39 mg, 0.29 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.16 mL, 0.97 mmol),室溫反應11 h,加水 (20 mL) 攪拌5 min,用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,減壓濃縮,進行矽膠柱層析分離( 洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體32 mg,收率39%。Compound (2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-methoxy - d 3 - phenyl) pyrrolidine-2-carboxylic acid (45 mg, 0.14 mmol), 6- (aminomethyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methyl acyl pyridine dihydrochloride (62 mg, 0.17 mmol), 1- ethyl - (3 -Dimethylaminopropyl)carbodiimide hydrochloride (132 mg, 0.69 mmol) and N -hydroxy-7-azabenzotriazole (39 mg, 0.29 mmol) were dissolved in dichloromethane ( 10 mL), cooled to 0 °C, added N , N -diisopropylethylamine (0.16 mL, 0.97 mmol), reacted at room temperature for 11 h, added water (20 mL), stirred for 5 min, and extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain White solid 32 mg, yield 39%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 – 7.82 (m, 1H), 7.37 – 7.49 (m, 3H), 7.13 (d,J = 8.0 Hz, 1H), 6.93 (s, 1H), 6.87 (d,J = 7.9 Hz, 1H), 6.55 (t,J F-H = 75.2 Hz, 1H), 4.64 – 4.72 (m, 0.6H), 4.54 – 4.64 (m, 3H), 3.95 – 3.99 (m, 1H), 3.72 – 3861 (m, 0.4H), 3.55 – 3.61 (m, 1H), 3.30 – 3.41 (m, 1H), 3.02 (s, 1.3H), 2.89 (s, 1.7H), 2.50 – 2.61 (m, 2H), 2.17 – 2.27 (m, 2H), 2.15 (s, 3H), 1.85 – 2.03 (m, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 – 7.82 (m, 1H), 7.37 – 7.49 (m, 3H), 7.13 (d, J = 8.0 Hz, 1H), 6.93 (s, 1H) ), 6.87 (d, J = 7.9 Hz, 1H), 6.55 (t, J FH = 75.2 Hz, 1H), 4.64 – 4.72 (m, 0.6H), 4.54 – 4.64 (m, 3H), 3.95 – 3.99 ( m, 1H), 3.72 – 3861 (m, 0.4H), 3.55 – 3.61 (m, 1H), 3.30 – 3.41 (m, 1H), 3.02 (s, 1.3H), 2.89 (s, 1.7H), 2.50 – 2.61 (m, 2H), 2.17 – 2.27 (m, 2H), 2.15 (s, 3H), 1.85 – 2.03 (m, 6H).
MS (ESI, pos.ion) m/z: 598.40 [M+H]+ .MS (ESI, pos.ion) m/z: 598.40 [M+H] + .
實施例104:化合物6-(((2R )-1-乙醯基-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 104: Compound 6-((( 2R )-1-Acetyl-4-(2,2-difluorobenzo[ d ][1,3]dioxocen-5-yl)pyrrolidine- 2-acyl-amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物 (2R )-1-乙醯基-4-(2,2-二氟苯並[d ][1,3]二噁茂-5-基)吡咯烷-2-羧酸 (60 mg, 0.19 mmol),6-(氨基甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺二鹽酸鹽 (82 mg, 0.23 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (182 mg, 0.95 mmol) 和N -羥基-7-氮雜苯並三氮唑 (53 mg, 0.39 mmol) 溶於二氯甲烷 (6 mL)中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.23 mL, 1.4 mmol),室溫反應11 h,加水 (20 mL) 攪拌5 min,用二氯甲烷萃取 (15 mL × 3) 萃取,合併有機相,用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體72 mg,收率65%。The compound ( 2R )-1-acetyl-4-(2,2-difluorobenzo[ d ][1,3]dioxoc-5-yl)pyrrolidine-2-carboxylic acid (60 mg , 0.19 mmol), 6- (aminomethyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methyl acyl pyridine dihydrochloride (82 mg, 0.23 mmol), 1- ethyl - (3-Dimethylaminopropyl)carbodiimide hydrochloride (182 mg, 0.95 mmol) and N -hydroxy-7-azabenzotriazole (53 mg, 0.39 mmol) in dichloro In methane (6 mL), after cooling to 0 °C, N , N -diisopropylethylamine (0.23 mL, 1.4 mmol) was added, the reaction was carried out at room temperature for 11 h, water (20 mL) was added and stirred for 5 min. Extraction with methane (15 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) , 72 mg of white solid was obtained, and the yield was 65%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.72 – 7.83 (m, 1H), 7.57 (br.s, 1H), 7.34 – 7.48 (m, 2H), 6.94 – 7.07 (m, 3H), 4.47 – 4.72 (m, 3.6H), 3.90 – 4.02 (m, 1H), 3.70 – 3.80 (m, 0.4H), 3.52 – 3.57 (m, 1H), 3.29 – 3.43 (m, 1H), 3.01 (s, 1.3H), 2.88 (s, 1.7H), 2.46 – 2.56 (m, 2H), 2.12 – 2.25 (m, 2H), 2.13 (s, 3H) , 1.90 – 1.99 (m, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.72 – 7.83 (m, 1H), 7.57 (br.s, 1H), 7.34 – 7.48 (m, 2H), 6.94 – 7.07 (m, 3H) , 4.47 – 4.72 (m, 3.6H), 3.90 – 4.02 (m, 1H), 3.70 – 3.80 (m, 0.4H), 3.52 – 3.57 (m, 1H), 3.29 – 3.43 (m, 1H), 3.01 ( s, 1.3H), 2.88 (s, 1.7H), 2.46 – 2.56 (m, 2H), 2.12 – 2.25 (m, 2H), 2.13 (s, 3H) , 1.90 – 1.99 (m, 6H).
MS (ESI, pos.ion) m/z: 579.10 [M+H]+ .MS (ESI, pos.ion) m/z: 579.10 [M+H] + .
實施例105:化合物6-(((2R )-1-乙醯基-4-(5-((環丙基甲基)-羥基氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 105: Compound 6-((( 2R )-1-Acetyl-4-(5-((cyclopropylmethyl)-hydroxyamino)-4-(difluoromethoxy)-2- (acyl methanesulfonamide) phenyl) pyrrolidine-2-acyl-amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
將化合物 (2R )-1-乙醯基-4-(5-((環丙基甲基)-羥基氨基)-4-(二氟甲氧基)-2-(甲磺醯基)苯基)吡咯烷-2-羧酸 (60 mg, 0.13 mmol),6-(氨基甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺二鹽酸鹽 (55 mg, 0.15 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (164 mg, 0.65 mmol) 和N -羥基-7-氮雜苯並三氮唑 (35 mg, 0.26 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.15 mL, 0.91 mmol),室溫反應12 h,加水 (20 mL) 攪拌5 min,分離有機相,水相用二氯甲烷萃取 (5 mL × 3),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體63 mg,收率67%。The compound ( 2R )-1-acetyl-4-(5-((cyclopropylmethyl)-hydroxyamino)-4-(difluoromethoxy)-2-(methylsulfonyl)benzene yl) pyrrolidine-2-carboxylic acid (60 mg, 0.13 mmol), 6- ( aminomethyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methyl acyl pyridine dihydrochloride ( 55 mg, 0.15 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (164 mg, 0.65 mmol) and N -hydroxy-7-azabenzotriazepine azole (35 mg, 0.26 mmol) was dissolved in dichloromethane (5 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.15 mL, 0.91 mmol) was added, and the reaction was carried out at room temperature for 12 h, Water (20 mL) was added, stirred for 5 min, the organic phase was separated, the aqueous phase was extracted with dichloromethane (5 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluting) Reagent: dichloromethane/methanol (v/v) = 30/1) to obtain 63 mg of white solid with a yield of 67%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.74 – 7.87 (m, 1H), 7.52 – 7.63 (m, 1H), 7.37 – 7.49 (m, 2H), 6.97 (s, 1H), 6.69 (s, 1H), 6.50 (t,J F-H = 73.6 Hz, 1H), 4.54 – 4.74 (m, 4H), 4.30 – 4.39 (m, 1H), 3.99 – 4.08 (m, 1H), 3.64 – 3.81 (m, 1H), 3.46 – 3.53 (m, 1H), 3.25 (s, 3H), 2.89 – 3.02 (m, 5H), 2.45 – 2.60 (m, 2H), 2.12 – 2.27 (m, 2H), 2.12 (s, 3H), 1.83 – 2.04 (m, 6H) , 1.05 – 1.13 (m, 1H), 0.55 – 0.65 (m, 2H), 0.22 – 0.34 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.74 – 7.87 (m, 1H), 7.52 – 7.63 (m, 1H), 7.37 – 7.49 (m, 2H), 6.97 (s, 1H), 6.69 (s, 1H), 6.50 (t, J FH = 73.6 Hz, 1H), 4.54 – 4.74 (m, 4H), 4.30 – 4.39 (m, 1H), 3.99 – 4.08 (m, 1H), 3.64 – 3.81 ( m, 1H), 3.46 – 3.53 (m, 1H), 3.25 (s, 3H), 2.89 – 3.02 (m, 5H), 2.45 – 2.60 (m, 2H), 2.12 – 2.27 (m, 2H), 2.12 ( s, 3H), 1.83 – 2.04 (m, 6H) , 1.05 – 1.13 (m, 1H), 0.55 – 0.65 (m, 2H), 0.22 – 0.34 (m, 2H).
MS (ESI, pos.ion) m/z: 728.80 [M+H]+ .MS (ESI, pos.ion) m/z: 728.80 [M+H] + .
實施例106:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基-N -((1r ,4R )-4-甲基環己基)吡啶醯胺 Example 106: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - methyl - N - ((1 r, 4 R) -4- methylcyclohexyl) pyridin Amides
步驟1:化合物叔丁基 ((1r ,4r )-4-甲基環己基)氨基甲酸酯 的合成Step 1: Synthesis of compound tert-butyl(( 1r , 4r )-4-methylcyclohexyl)carbamate
將 (1r ,4r )-4-甲基環己胺 (800 mg, 7.06 mmol) 和N ,N -二異丙基乙胺 (1.4 mL, 8.50 mmol) 溶於二氯甲烷 (8 mL) 中,冰浴下加入二碳酸二叔丁酯 (1.7 g, 7.80 mmol),室溫反應9 h,加水 (20 mL) 攪拌5 min,分離有機相,有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 20/1),得到白色固體612 mg,收率40%。( 1r , 4r )-4-methylcyclohexylamine (800 mg, 7.06 mmol) and N , N -diisopropylethylamine (1.4 mL, 8.50 mmol) were dissolved in dichloromethane (8 mL) , add di-tert-butyl dicarbonate (1.7 g, 7.80 mmol) under ice bath, react at room temperature for 9 h, add water (20 mL) and stir for 5 min, separate the organic phase, dry the organic phase with anhydrous sodium sulfate, remove the solvent, The concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 20/1) to obtain 612 mg of white solid with a yield of 40%.
1 H NMR (600 MHz, CDCl3 ) δ (ppm): 4.36 (brs, 1H), 3.31 – 3.41 (m, 1H), 1.97 – 2.00 (m, 2H), 1.69 – 1.72 (m, 2H), 1.46 (s, 9H), 1.27 – 1.37 (m, 1H), 1.01 – 1.14 (m, 4H), 0.90 (d,J = 6.5 Hz, 3H). 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 4.36 (brs, 1H), 3.31 – 3.41 (m, 1H), 1.97 – 2.00 (m, 2H), 1.69 – 1.72 (m, 2H), 1.46 (s, 9H), 1.27 – 1.37 (m, 1H), 1.01 – 1.14 (m, 4H), 0.90 (d, J = 6.5 Hz, 3H).
MS (ESI, pos.ion) m/z: 158.25 [M-55]+ .MS (ESI, pos.ion) m/z: 158.25 [M-55] + .
步驟2:化合物叔丁基 甲基 ((1r ,4r )-4-甲基環己基)氨基甲酸酯的合成Step 2: Synthesis of compound tert-butylmethyl(( 1r , 4r )-4-methylcyclohexyl)carbamate
將叔丁基 ((1r ,4r )-4-甲基環己基)氨基甲酸酯 (300 mg, 1.41 mmol) 溶於無水N ,N -二甲基甲醯胺 (8 mL) 中,冰浴下加入60%氫化鈉 (84 mg, 2.10 mmol),室溫反應30 min,冰浴下加入碘甲烷 (260 mg, 1.83 mmol),室溫反應9 h,加水 (25 mL),用乙酸乙酯萃取 (10 mL × 3),分離有機相,有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 25/1),得到無色液體129 m g,收率40%。tert-Butyl(( 1r , 4r )-4-methylcyclohexyl)carbamate (300 mg, 1.41 mmol) was dissolved in anhydrous N , N -dimethylformamide (8 mL), Add 60% sodium hydride (84 mg, 2.10 mmol) under ice bath, react at room temperature for 30 min, add iodomethane (260 mg, 1.83 mmol) under ice bath, react at room temperature for 9 h, add water (25 mL), add acetic acid Ethyl ester extraction (10 mL × 3), the organic phase was separated, the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 25/1) to obtain 129 mg of a colorless liquid with a yield of 40%.
1 H NMR (600 MHz, CDCl3 ) δ (ppm): 3.75 – 4.04 (m, 1H), 2.72 (s, 3H), 1.73 – 1.76 (m, 2H), 1.64 – 1.67 (m, 2H), 1.39 – 1.51 (m, 2H), 1.47 (s, 9H), 1.25 – 1.34 (m, 1H), 1.01 – 1.10 (m, 2H), 0.90 (d,J = 6.5 Hz, 3H). 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 3.75 – 4.04 (m, 1H), 2.72 (s, 3H), 1.73 – 1.76 (m, 2H), 1.64 – 1.67 (m, 2H), 1.39 – 1.51 (m, 2H), 1.47 (s, 9H), 1.25 – 1.34 (m, 1H), 1.01 – 1.10 (m, 2H), 0.90 (d, J = 6.5 Hz, 3H).
MS (ESI, pos.ion) m/z: 172.25 [M-55]+ .MS (ESI, pos.ion) m/z: 172.25 [M-55] + .
步驟3:化合物 (1r ,4r )-N ,4-二甲基環己胺鹽酸鹽的合成Step 3: Synthesis of compound (1r , 4r ) -N ,4-dimethylcyclohexylamine hydrochloride
將化合物叔丁基 甲基 ((1r ,4r )-4-甲基環己基)氨基甲酸酯 (120 mg, 0.53 mmol) 溶解於二氯甲烷 (3 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (6 mL),室溫攪拌1 h,減壓濃縮,得到白色固體83 mg,收率96%。Compound tert-butylmethyl(( 1r , 4r )-4-methylcyclohexyl)carbamate (120 mg, 0.53 mmol) was dissolved in dichloromethane (3 mL) solution, and 4 mol/L HCl was added The ethyl acetate solution (6 mL) was stirred at room temperature for 1 h, and concentrated under reduced pressure to obtain 83 mg of a white solid with a yield of 96%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 2.94 – 3.00 (m, 1H), 2.69 (s, 3H), 2.11 – 2.14 (m, 2H), 1.85 – 1.88 (m, 2H), 1.32 – 1.43 (m, 3H), 1.02 – 1.12 (m, 2H), 0.95 (d,J = 6.5 Hz, 3H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 2.94 – 3.00 (m, 1H), 2.69 (s, 3H), 2.11 – 2.14 (m, 2H), 1.85 – 1.88 (m, 2H), 1.32 – 1.43 (m, 3H), 1.02 – 1.12 (m, 2H), 0.95 (d, J = 6.5 Hz, 3H).
MS (ESI, pos.ion) m/z: 128.30 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 128.30 [M+H-HCl] + .
步驟4:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基-N -((1r ,4R )-4-甲基環己基)吡啶醯胺 的合成Step 4: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Synthesis of Carboxylamido)methyl) -N -methyl- N -((1 r ,4 R )-4-methylcyclohexyl)pyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),(1r ,4r )-N ,4-二甲基環己胺鹽酸鹽 (59 mg, 0.36 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (191 mg, 1.00 mmol) 和N -羥基-7-氮雜苯並三氮唑 (43 mg, 0.32 mmol)溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (0.17 mL, 1.00 mmol),室溫反應6 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體41 mg,收率33%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), ( 1r , 4r ) -N ,4-dimethylcyclohexylamine hydrochloride (59 mg, 0.36 mmol), 1-ethyl-( 3-Dimethylaminopropyl)carbodiimide hydrochloride (191 mg, 1.00 mmol) and N -hydroxy-7-azabenzotriazole (43 mg, 0.32 mmol) in dichloromethane (5 mL), cooled to 0 °C, added N , N -diisopropylethylamine (0.17 mL, 1.00 mmol), reacted at room temperature for 6 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 41 mg of white solid , the yield is 33%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.72 – 7.80 (m, 1H), 7.34 – 7.50 (m, 3H), 7.12 (d,J = 7.6 Hz, 1H), 6.90 (s, 1H), 6.86 (d,J = 7.7 Hz, 1H), 6.62 (t,J F-H = 75.5 Hz, 1H), 4.47 – 4.65 (m, 3.6H), 3.90 – 3.99 (m, 1H), 3.88 (d,J = 6.6 Hz, 2H), 3.55 – 3.62 (m, 1H), 3.29 – 3.45 (m, 1.4H), 3.00 (s, 1.7H), 2.83 (s, 1.3H), 2.53 – 2.63 (m, 1H), 2.38 – 2.48 (m, 1H), 2.14 (s, 3H), 1.52 – 1.72 (m, 5H), 1.14 – 1.37 (m, 5H), 0.94 (d,J = 5.5 Hz, 1.5H), 0.83 (d,J = 5.5 Hz, 1.5H), 0.63 – 0.70 (m, 2H), 0.33 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.72 – 7.80 (m, 1H), 7.34 – 7.50 (m, 3H), 7.12 (d, J = 7.6 Hz, 1H), 6.90 (s, 1H) ), 6.86 (d, J = 7.7 Hz, 1H), 6.62 (t, J FH = 75.5 Hz, 1H), 4.47 – 4.65 (m, 3.6H), 3.90 – 3.99 (m, 1H), 3.88 (d, J = 6.6 Hz, 2H), 3.55 – 3.62 (m, 1H), 3.29 – 3.45 (m, 1.4H), 3.00 (s, 1.7H), 2.83 (s, 1.3H), 2.53 – 2.63 (m, 1H) ), 2.38 – 2.48 (m, 1H), 2.14 (s, 3H), 1.52 – 1.72 (m, 5H), 1.14 – 1.37 (m, 5H), 0.94 (d, J = 5.5 Hz, 1.5H), 0.83 (d, J = 5.5 Hz, 1.5H), 0.63 – 0.70 (m, 2H), 0.33 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 613.45 [M+H]+ .MS (ESI, pos.ion) m/z: 613.45 [M+H] + .
實施例107:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -甲基-N -(4-氯苯基)吡啶醯胺 Example 107: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Carboxyamino)methyl) -N -methyl- N- (4-chlorophenyl)pyridinamide
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),N -甲基-4-氯苯胺 (70 mg, 0.50 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (251 mg, 1.31 mmol) 和N -羥基-7-氮雜苯並三氮唑 (54 mg, 0.40 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (183 mg, 1.42 mmol),室溫反應22 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到白色固體82 mg,收率65%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), N -methyl-4-chloroaniline (70 mg, 0.50 mmol), 1-ethyl-(3-dimethylaminopropyl)carboamide Imine hydrochloride (251 mg, 1.31 mmol) and N -hydroxy-7-azabenzotriazole (54 mg, 0.40 mmol) were dissolved in dichloromethane (5 mL), cooled to 0 °C, added N , N -diisopropylethylamine (183 mg, 1.42 mmol), reacted at room temperature for 22 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was dried over anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 82 mg of white solid with a yield of 65%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.64 (br.s, 1H), 7.35 - 7.48 (m, 1H), 7.18 - 7.28 (m, 2H), 6.97 - 7.16 (m, 4H), 6.83 - 6.95 (m, 2H), 6.63 (t,J F-H = 75.5 Hz, 1H), 4.42 - 4.51 (m, 3H),3.88 - 4.00 (m, 3H), 3.51 - 3.65 (m, 1H), 3.51 (s, 3H), 3.29 - 3.45 (m, 1H), 2.51 - 2.64 (m, 1H), 2.35 - 2.63 (m, 1H), 2.15 (s, 3H), 1.26 - 1.35 (m, 1H), 0.64 - 0.71 (m, 2H), 0.36 - 0.43 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.64 (br.s, 1H), 7.35 - 7.48 (m, 1H), 7.18 - 7.28 (m, 2H), 6.97 - 7.16 (m, 4H) , 6.83 - 6.95 (m, 2H), 6.63 (t, J FH = 75.5 Hz, 1H), 4.42 - 4.51 (m, 3H), 3.88 - 4.00 (m, 3H), 3.51 - 3.65 (m, 1H), 3.51 (s, 3H), 3.29 - 3.45 (m, 1H), 2.51 - 2.64 (m, 1H), 2.35 - 2.63 (m, 1H), 2.15 (s, 3H), 1.26 - 1.35 (m, 1H), 0.64 - 0.71 (m, 2H), 0.36 - 0.43 (m, 2H).
MS (ESI, pos.ion) m/z: 627.10 [M+H]+ .MS (ESI, pos.ion) m/z: 627.10 [M+H] + .
實施例108:化合物6-(((2R )-1-乙醯基-4-(3-(異丙氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 Example 108: Compound 6-((( 2R )-1-Acetyl-4-(3-(isopropoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-methyl acyl amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methylpyridine Amides
步驟1:化合物 (R )-6-((1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 的合成Step 1: Compound ( R )-6-((1-Acetyl-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro-1 H - pyrrole-2-acyl-amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - synthesis of Amides methylpyridine
將化合物 (R )-1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-羧酸 (300 mg, 0.74 mmol),6-(氨基甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺二鹽酸鹽 (301 mg, 0.85 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (718 mg, 3.75 mmol) 和N -羥基-7-氮雜苯並三氮唑 (203 mg, 1.49 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (0.9 mL, 5.0 mmol),室溫反應7 h,加水洗 (20 mL) 攪拌,分離有機相,有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析色譜分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體387 mg,收率77%。The compound ( R )-1-acetyl-4-(3-(benzyloxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro- 1H -pyrrole-2- carboxylic acid (300 mg, 0.74 mmol), 6- ( aminomethyl) - N - (of 4,4-difluoro-cyclohexyl) - N - methyl acyl pyridine dihydrochloride (301 mg, 0.85 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (718 mg, 3.75 mmol) and N -hydroxy-7-azabenzotriazole (203 mg, 1.49 mmol) ) was dissolved in dichloromethane (10 mL), cooled to 0 °C, N , N -diisopropylethylamine (0.9 mL, 5.0 mmol) was added, the reaction was carried out at room temperature for 7 h, washed with water (20 mL) and stirred , the organic phase was separated, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 387 mg of white solid , the yield is 77%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.75 – 7.81 (m, 1H), 7.41 – 7.49 (m, 5H), 7.34 – 7.40 (m, 2H), 7.18 – 7.20 (m, 1H), 7.07 – 7.10 (m, 1H), 6.93 – 7.01 (m, 1H), 6.61 (t,J F-H = 74.9 Hz, 1H), 6.18 – 6.23 (m, 1H), 5.37 – 5.41 (m, 0.7H), 5.23 – 5.27 (m, 0.3H), 5.17 (s, 2H), 4.66 – 4.74 (m, 2H), 4.55 – 4.65 (m, 3H), 2.87 (s, 2H), 2.78 (s, 1H), 2.24 (s, 3H), 2.07 (s, 1H), 2.06 – 2.16 (m, 2H), 1.82 – 2.04 (m, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.75 – 7.81 (m, 1H), 7.41 – 7.49 (m, 5H), 7.34 – 7.40 (m, 2H), 7.18 – 7.20 (m, 1H) , 7.07 – 7.10 (m, 1H), 6.93 – 7.01 (m, 1H), 6.61 (t, J FH = 74.9 Hz, 1H), 6.18 – 6.23 (m, 1H), 5.37 – 5.41 (m, 0.7H) , 5.23 – 5.27 (m, 0.3H), 5.17 (s, 2H), 4.66 – 4.74 (m, 2H), 4.55 – 4.65 (m, 3H), 2.87 (s, 2H), 2.78 (s, 1H), 2.24 (s, 3H), 2.07 (s, 1H), 2.06 – 2.16 (m, 2H), 1.82 – 2.04 (m, 6H).
MS (ESI, pos.ion) m/z: 669.15 [M+H]+ .MS (ESI, pos.ion) m/z: 669.15 [M+H] + .
步驟2:化合物6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 的合成Step 2: Compound 6-((( 2R )-1-Acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carbamoylamino)methyl) - Synthesis of N- (4,4-difluorocyclohexyl) -N-picolinamide
將化合物(R )-6-((1-乙醯基-4-(3-(苄氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 (380 mg, 0.57mmol) 溶於甲醇 (10 mL),加入Pd/C (53 mg),通入氫氣室溫反應12 h,過濾除去催化劑,濾液濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 25/1),得到白色固體316 mg,收率95%。Compound (R) -6 - ((1- acetyl-4- (3- (benzyloxy) -4- (difluoromethoxy) phenyl) -2,5-dihydro -1 H - acyl pyrrole-2-ylamino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - Amides methylpyridine (380 mg, 0.57mmol) was dissolved in methanol (10 mL), was added Pd / C (53 mg) was passed into hydrogen and reacted at room temperature for 12 h, the catalyst was removed by filtration, the filtrate was concentrated, and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 25/1) to obtain 316 mg of white solid, yield 95%.
MS (ESI, pos.ion) m/z: 581.10 [M+H]+ .MS (ESI, pos.ion) m/z: 581.10 [M+H] + .
步驟3:化合物6-(((2R )-1-乙醯基-4-(3-(異丙氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺的合成Step 3: Compound 6-((( 2R )-1-Acetyl-4-(3-(isopropoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate amino) methyl) - N - (of 4,4-difluoro-cyclohexyl) - N - acyl amine-methylpyridine
將6-(((2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲醯氨基)甲基)-N -(4,4-二氟環己基)-N -甲基吡啶醯胺 (70 mg, 0.12mmol),2-碘丙烷 (61 mg, 0.36mmol),碳酸鉀 (83 mg, 0.60mmol) 溶解在乾燥的N ,N -二甲基甲醯胺 (3mL) 溶液中,80℃反應2 h,加入水 (30mL),用乙酸乙酯 (10 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到白色固體63 mg,收率83%。The 6 - (((2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-hydroxyphenyl) pyrrolidine-2-acyl-amino) methyl) - N - (4,4-Difluorocyclohexyl) -N -picolinamide (70 mg, 0.12 mmol), 2-iodopropane (61 mg, 0.36 mmol), potassium carbonate (83 mg, 0.60 mmol) were dissolved in dry In a solution of N , N -dimethylformamide (3mL), react at 80°C for 2 h, add water (30mL), extract with ethyl acetate (10mL × 3), dry the organic phase with anhydrous sodium sulfate, concentrate , and separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 63 mg of white solid with a yield of 83%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.91 – 7.95 (m, 1H), 7.59 – 7.64 (m, 1H), 7.42 – 7.48 (m, 1H), 7.12 – 7.13 (m, 2H), 6.94 (d,J = 8.0 Hz, 1H), 6.70 (t,J F-H = 75.7 Hz, 1H), 4.61 – 4.70 (m, 2H), 4.45 – 4.55 (m, 2H), 4.10 – 4.15 (m, 1H), 3.70 – 3.77 (m, 1H), 3.52 – 3.69 (m, 1H), 3.47 – 3.58 (m, 1H), 3.30 (s, 1.5H), 2.84 (s, 1.5H), 2.66 – 2.74 (m, 1H), 2.16 (s, 3H), 2.01 – 2.08 (m, 1H), 1.76 – 2.00 (m, 8H), 1.35 (d,J = 5.7 Hz, 6H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.91 – 7.95 (m, 1H), 7.59 – 7.64 (m, 1H), 7.42 – 7.48 (m, 1H), 7.12 – 7.13 (m, 2H) ), 6.94 (d, J = 8.0 Hz, 1H), 6.70 (t, J FH = 75.7 Hz, 1H), 4.61 – 4.70 (m, 2H), 4.45 – 4.55 (m, 2H), 4.10 – 4.15 (m , 1H), 3.70 – 3.77 (m, 1H), 3.52 – 3.69 (m, 1H), 3.47 – 3.58 (m, 1H), 3.30 (s, 1.5H), 2.84 (s, 1.5H), 2.66 – 2.74 (m, 1H), 2.16 (s, 3H), 2.01 – 2.08 (m, 1H), 1.76 – 2.00 (m, 8H), 1.35 (d, J = 5.7 Hz, 6H).
MS (ESI, pos.ion) m/z: 623.20 [M+H]+ .MS (ESI, pos.ion) m/z: 623.20 [M+H] + .
實施例109:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(9-氨基-5,7-二氫二苯並[c ,e ]氧雜卓-3-基)吡啶醯胺 Example 109: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 - A XI) methyl) - N - (9- amino-5,7-dihydro-dibenzo [c, e] Zhuo oxa-3-yl) pyridin Amides
步驟1:化合物4,4'-二硝基-[1,1'-聯苯]-2,2'-二甲醛的合成Step 1: Synthesis of compound 4,4'-dinitro-[1,1'-biphenyl]-2,2'-dicarbaldehyde
將2-溴-5-硝基苯甲醛 (1.0 g, 4.3 mmol),聯硼酸頻那醇酯 (1.3 g, 5.1 mmol),醋酸鉀 (1.3 g, 13 mmol)和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀 (50 mg, 0.07 mmol) 混合在乾燥的1,4-二氧六環 (15mL) 溶液中,氮氣保護下100℃反應4 h,冷卻至室溫,將反應液抽濾,濾液濃縮後加入水 (30mL),用乙酸乙酯 (10 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 5/1),得到淺黃色固體752 mg,收率58%。Combine 2-bromo-5-nitrobenzaldehyde (1.0 g, 4.3 mmol), pinacol diboronate (1.3 g, 5.1 mmol), potassium acetate (1.3 g, 13 mmol) and [1,1'-bis (diphenylphosphino)ferrocene]palladium dichloride (50 mg, 0.07 mmol) was mixed with dry 1,4-dioxane (15 mL) solution, reacted at 100 °C for 4 h under nitrogen protection, cooled After reaching room temperature, the reaction solution was filtered with suction. After the filtrate was concentrated, water (30 mL) was added, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 5/1) to obtain 752 mg of a pale yellow solid with a yield of 58%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.91 (s, 2H), 8.87 (d,J = 2.3 Hz, 2H), 8.56 (dd,J = 8.3, 2.3 Hz, 2H), 7.53 (d,J = 8.3 Hz, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.91 (s, 2H), 8.87 (d, J = 2.3 Hz, 2H), 8.56 (dd, J = 8.3, 2.3 Hz, 2H), 7.53 ( d, J = 8.3 Hz, 2H).
MS (ESI, pos.ion) m/z: 301.20 [M+H]+ .MS (ESI, pos.ion) m/z: 301.20 [M+H] + .
步驟2:化合物4,4'-二硝基-[1,1'-聯苯]-2,2'-二甲醇的合成Step 2: Synthesis of compound 4,4'-dinitro-[1,1'-biphenyl]-2,2'-dimethanol
將化合物4,4'-二硝基-[1,1'-聯苯]-2,2'-二甲醛 (740 mg, 2.47 mmol) 溶解在甲醇 (10 mL) 中,冷卻至0℃,加入硼氫化鈉 (203 mg, 5.37 mmol),室溫攪拌30 min後停止反應,加水 (25 mL),乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到淺黃色固體255 mg,收率34%。Compound 4,4'-dinitro-[1,1'-biphenyl]-2,2'-dicarbaldehyde (740 mg, 2.47 mmol) was dissolved in methanol (10 mL), cooled to 0 °C, added Sodium borohydride (203 mg, 5.37 mmol) was stirred at room temperature for 30 min to stop the reaction, water (25 mL) was added, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the concentrated solution was subjected to silica gel column Chromatographic separation (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) gave 255 mg of pale yellow solid, yield 34%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.50 (s, 2H), 8.26 (dd,J = 8.3, 2.3 Hz, 2H), 7.35 (d,J = 8.3 Hz, 2H), 4.46 (dd,J = 37.0, 12.7 Hz, 4H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.50 (s, 2H), 8.26 (dd, J = 8.3, 2.3 Hz, 2H), 7.35 (d, J = 8.3 Hz, 2H), 4.46 ( dd, J = 37.0, 12.7 Hz, 4H).
MS (ESI, pos.ion) m/z: 327.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 327.20 [M+Na] + .
步驟3:化合物3,9-二硝基-5,7-二氫二苯並[c ,e ]氧雜卓的合成Step 3: Synthesis of compound 3,9-dinitro-5,7-dihydrodibenzo[ c , e]oxazepine
將4,4'-二硝基-[1,1'-聯苯]-2,2'-二甲醇 (152 mg, 0.50mmol),三丁基膦 (231 mg, 1.14mmol) 溶解在乾燥的四氫呋喃 (5 mL) 溶液中,冰浴條件下緩慢加入偶氮二甲醯胺 (196 mg, 1.14mmol),室溫反應3 h,加入水 (30mL),用乙酸乙酯 (15 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析色譜分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/1),得到淺黃色固體106 mg,收率74%。4,4'-Dinitro-[1,1'-biphenyl]-2,2'-dimethanol (152 mg, 0.50 mmol), tributylphosphine (231 mg, 1.14 mmol) were dissolved in dry To the solution of tetrahydrofuran (5 mL), azodimethylamide (196 mg, 1.14 mmol) was slowly added under ice bath conditions, and the reaction was carried out at room temperature for 3 h, water (30 mL) was added, and ethyl acetate (15 mL × 3) was added. Extracted, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 3/1) to obtain 106 mg of pale yellow solid, Yield 74%.
1 H NMR (400 MHz,d6 -DMSO) δ (ppm): 8.51 (d,J = 2.2 Hz, 2H), 8.44 (dd,J = 8.5, 2.3 Hz, 2H), 7.99 (d,J = 8.5 Hz, 2H), 4.43 (s, 4H). 1 H NMR (400 MHz, d 6 -DMSO) δ (ppm): 8.51 (d, J = 2.2 Hz, 2H), 8.44 (dd, J = 8.5, 2.3 Hz, 2H), 7.99 (d, J = 8.5 Hz, 2H), 4.43 (s, 4H).
步驟4:化合物5,7-二氫二苯並[c ,e ]氧雜卓-3,9-二胺的合成Step 4: Synthesis of compound 5,7-dihydrodibenzo[ c , e ]oxazepine-3,9-diamine
將化合物3,9-二硝基-5,7-二氫二苯並[c ,e ]氧雜卓 (100 mg, 0.35mmol) 溶於甲醇 (10 mL),加入Pd/C (20 mg),通入氫氣室溫反應5 h,過濾除去催化劑,濾液濃縮,得淺黃色固體64 mg,收率81%。Compound 3,9-dinitro-5,7-dihydrodibenzo[ c , e ]oxazepine (100 mg, 0.35 mmol) was dissolved in methanol (10 mL), Pd/C (20 mg) was added , hydrogen was introduced into the reaction at room temperature for 5 h, the catalyst was removed by filtration, and the filtrate was concentrated to obtain 64 mg of a pale yellow solid with a yield of 81%.
1 H NMR (400 MHz,d6 -DMSO) δ (ppm): 7.11 (d,J = 8.1 Hz, 2H), 6.65 (dd,J = 8.1, 2.2 Hz, 2H), 6.60 (d,J = 2.1 Hz, 2H), 5.11 (s, 4H), 4.08 (s, 4H). 1 H NMR (400 MHz, d 6 -DMSO) δ (ppm): 7.11 (d, J = 8.1 Hz, 2H), 6.65 (dd, J = 8.1, 2.2 Hz, 2H), 6.60 (d, J = 2.1 Hz, 2H), 5.11 (s, 4H), 4.08 (s, 4H).
MS (ESI, pos.ion) m/z: 227.20 [M+H]+ .MS (ESI, pos.ion) m/z: 227.20 [M+H] + .
步驟5:化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)-N -(9-氨基-5,7-二氫二苯並[c ,e ]氧雜卓-3-基)吡啶醯胺的合成Step 5: Compound 6-((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- methyl acyl amino) methyl) - N - (9- amino-5,7-dihydro-dibenzo synthesis [c, e] Zhuo oxa-3-yl) pyridin-acyl amine
將化合物6-(((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)甲基)吡啶甲酸 (100 mg, 0.20 mmol),5,7-二氫二苯並[c ,e ]氧雜卓-3,9-二胺 (73 mg, 0.32 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (251 mg, 1.31 mmol) 和N -羥基-7-氮雜苯並三氮唑 (54 mg, 0.40 mmol)溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (183 mg, 1.42 mmol),室溫反應3 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到淺褐色固體44 mg,收率31%。The compound 6-((( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Amino)methyl)picolinic acid (100 mg, 0.20 mmol), 5,7-dihydrodibenzo[ c , e ]oxazepine-3,9-diamine (73 mg, 0.32 mmol), 1-ethyl Alkyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (251 mg, 1.31 mmol) and N -hydroxy-7-azabenzotriazole (54 mg, 0.40 mmol) were dissolved in In dichloromethane (5 mL), cooled to 0 °C, N , N -diisopropylethylamine (183 mg, 1.42 mmol) was added, the reaction was carried out at room temperature for 3 h, washed with water (15 mL), and washed with dichloromethane. Extraction (5 mL × 3), the organic phase was dried with anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain a pale Brown solid 44 mg, yield 31%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 10.74 (s, 1H), 8.98 (s, 1H), 8.24 (d,J = 7.4 Hz, 1H), 8.19 (d,J = 7.9 Hz, 1H), 8.04 (s, 1H), 7.86 - 7.90 (m, 1H), 7.49 (d,J = 7.2 Hz, 1H), 7.43 (d,J = 7.5 Hz, 1H), 7.36 (d,J = 8.0 Hz, 1H), 7.11 (d,J = 8.1 Hz, 1H), 6.92 (s, 1H), 6.87 (d,J = 8.1 Hz, 1H), 6.81 (d,J = 7.8 Hz, 1H), 6.76 (s, 1H), 6.61 (t,J F-H = 75.6 Hz, 1H), 4.86 - 4.90 (m, 1H), 4.62 - 4.80 (m, 2H), 4.39 (s, 2H), 4.30 (s, 2H), 3.93 - 3.97 (m, 1H), 3.88 (d,J = 6.7 Hz, 2H), 3.34 - 3.45 (m, 2H), 2.82 - 2.90 (m, 1H), 2.47 - 2.54 (m, 1H), 2.10 (s, 3H), 1.68 - 1.79 (m, 1H), 0.63 - 0.66 (m, 2H), 0.34 - 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 10.74 (s, 1H), 8.98 (s, 1H), 8.24 (d, J = 7.4 Hz, 1H), 8.19 (d, J = 7.9 Hz, 1H), 8.04 (s, 1H), 7.86 - 7.90 (m, 1H), 7.49 (d, J = 7.2 Hz, 1H), 7.43 (d, J = 7.5 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.92 (s, 1H), 6.87 (d, J = 8.1 Hz, 1H), 6.81 (d, J = 7.8 Hz, 1H), 6.76 ( s, 1H), 6.61 (t, J FH = 75.6 Hz, 1H), 4.86 - 4.90 (m, 1H), 4.62 - 4.80 (m, 2H), 4.39 (s, 2H), 4.30 (s, 2H), 3.93 - 3.97 (m, 1H), 3.88 (d, J = 6.7 Hz, 2H), 3.34 - 3.45 (m, 2H), 2.82 - 2.90 (m, 1H), 2.47 - 2.54 (m, 1H), 2.10 ( s, 3H), 1.68 - 1.79 (m, 1H), 0.63 - 0.66 (m, 2H), 0.34 - 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 712.50 [M+H]+ .MS (ESI, pos.ion) m/z: 712.50 [M+H] + .
實施例110:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((3-氧代異吲哚-5-基)甲基)吡咯烷-2-甲醯胺 Example 110: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((3-oxygen isoindol-5-yl)methyl)pyrrolidin-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (120 mg, 0.32 mmol) 和6-(氨基甲基)異吲哚-1-酮 (57 mg, 0.35 mmol) 溶於二氯甲烷 (8 mL) 和N ,N -二甲基甲醯胺 (1 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (88 mg, 0.65 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (186 mg, 0.97 mmol) 和N ,N -二異丙基乙胺 (167 mg, 1.29 mmol),室溫反應5 h,減壓濃縮除去溶劑,加水 (40 mL),用乙酸乙酯萃取 (25 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體97 mg,收率58%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (120 mg, 0.32 mmol) and 6-(aminomethyl)isoindol-1-one (57 mg, 0.35 mmol) in dichloromethane (8 mL) and N , N -dimethylformamide (1 mL) , was added N -hydroxy-7-azabenzotriazole (88 mg, 0.65 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (186 mg, 0.97 mmol) and N , N -diisopropylethylamine (167 mg, 1.29 mmol), reacted at room temperature for 5 h, concentrated under reduced pressure to remove the solvent, added water (40 mL), extracted with ethyl acetate (25 mL × 2 ), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 97 mg of white solid, which was collected rate 58%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.42 (br.s, 1H), 7.84 (br.s, 1H), 7.43 – 7.60 (m, 2H), 7.25 – 7.33 (m, 1H), 7.12 (d,J = 7.4 Hz, 1H), 6.83 – 6.95 (m, 2H), 6.61 (t,J F-H = 75.7 Hz, 1H), 4.91 – 5.08 (m, 1H), 4.62 – 4.78 (m, 1H), 4.10 – 4.26 (m, 1H), 3.90 – 4.03 (m, 2H), 3.88 (d,J = 4.7 Hz, 2H), 3.61 – 3.76 (m, 1H), 3.26 – 3.46 (m, 1H), 2.55 – 2.73 (m, 1H), 2.29 – 2.44 (m, 1H), 2.16 (s, 3H), 1.21 – 1.36 (m, 1H), 0.59 – 0.69 (m, 2H), 0.31 – 0.42 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.42 (br.s, 1H), 7.84 (br.s, 1H), 7.43 – 7.60 (m, 2H), 7.25 – 7.33 (m, 1H) , 7.12 (d, J = 7.4 Hz, 1H), 6.83 – 6.95 (m, 2H), 6.61 (t, J FH = 75.7 Hz, 1H), 4.91 – 5.08 (m, 1H), 4.62 – 4.78 (m, 1H), 4.10 – 4.26 (m, 1H), 3.90 – 4.03 (m, 2H), 3.88 (d, J = 4.7 Hz, 2H), 3.61 – 3.76 (m, 1H), 3.26 – 3.46 (m, 1H) , 2.55 – 2.73 (m, 1H), 2.29 – 2.44 (m, 1H), 2.16 (s, 3H), 1.21 – 1.36 (m, 1H), 0.59 – 0.69 (m, 2H), 0.31 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 514.15 [M+H]+ .MS (ESI, pos.ion) m/z: 514.15 [M+H] + .
實施例111:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((1-氧代異吲哚-5-基)甲基)吡咯烷-2-甲醯胺 Example 111: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((1-oxygen isoindol-5-yl)methyl)pyrrolidin-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (100 mg, 0.27 mmol) 和5-(氨基甲基)異吲哚-1-酮 (43 mg, 0.27 mmol) 溶於二氯甲烷 (4 mL) 和N ,N -二甲基甲醯胺 (4 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (73 mg, 0.54 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (156 mg, 0.81 mmol) 和N ,N -二異丙基乙胺 (141 mg, 1.09 mmol),室溫反應15 h,減壓濃縮除去溶劑,加水 (40 mL),用乙酸乙酯萃取 (25 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到白色固體57 mg,收率41%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (100 mg, 0.27 mmol) and 5-(aminomethyl)isoindol-1-one (43 mg, 0.27 mmol) in dichloromethane (4 mL) and N , N -dimethylformamide (4 mL) , was added N -hydroxy-7-azabenzotriazole (73 mg, 0.54 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (156 mg, 0.81 mmol) and N , N -diisopropylethylamine (141 mg, 1.09 mmol), reacted at room temperature for 15 h, concentrated under reduced pressure to remove the solvent, added water (40 mL), extracted with ethyl acetate (25 mL × 2 ), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 57 mg of white solid, which was collected rate 41%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.01 (brs, 1H), 7.63 (br.s, 1H), 7.46 (d,J = 7.1 Hz, 1H), 7.34 (s, 1H), 7.30 (d,J = 7.3 Hz, 1H), 7.12 (d,J = 7.9 Hz, 1H), 6.91 (s, 1H), 6.86 (d,J = 7.5 Hz, 1H),6.61 (t,J F-H = 75.6 Hz, 1H), 4.65 – 4.80 (m, 2H), 4.16 – 4.33 (m, 3H), 3.87 – 3.98 (m, 1H), 3.87 (d,J = 6.5 Hz, 2H), 3.66 (d,J = 10.5 Hz, 1H), 3.28 – 3.43 (m, 1H), 2.56 – 2.69 (m, 1H), 2.34 – 2.48 (m, 1H), 2.15 (s, 3H), 1.21 – 1.36 (m, 1H), 0.59 – 0.69 (m, 2H), 0.31 – 0.42 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.01 (brs, 1H), 7.63 (br.s, 1H), 7.46 (d, J = 7.1 Hz, 1H), 7.34 (s, 1H), 7.30 (d, J = 7.3 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H), 6.91 (s, 1H), 6.86 (d, J = 7.5 Hz, 1H), 6.61 (t, J FH = 75.6 Hz, 1H), 4.65 – 4.80 (m, 2H), 4.16 – 4.33 (m, 3H), 3.87 – 3.98 (m, 1H), 3.87 (d, J = 6.5 Hz, 2H), 3.66 (d, J = 10.5 Hz, 1H), 3.28 – 3.43 (m, 1H), 2.56 – 2.69 (m, 1H), 2.34 – 2.48 (m, 1H), 2.15 (s, 3H), 1.21 – 1.36 (m, 1H), 0.59 – 0.69 (m, 2H), 0.31 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 514.25 [M+H]+ .MS (ESI, pos.ion) m/z: 514.25 [M+H] + .
實施例112:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(3,5-二氯吡啶-4-基)吡咯烷-2-甲醯胺 Example 112: Compound (2 R) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - (3,5- Dichloropyridin-4-yl)pyrrolidine-2-carboxamide
第一步反應:將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (200 mg, 0.54 mmol) 溶於二氯甲烷 (5 mL) 中,-20℃條件下加入三乙胺 (0.3 mL, 2.0 mmol) 和三聚氟氰(0.14 mL, 1.7 mmol),-20℃反應1 h,加冰水洗 (20 mL × 3),然後二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮。The first reaction: compound (2 R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Acid (200 mg, 0.54 mmol) was dissolved in dichloromethane (5 mL), triethylamine (0.3 mL, 2.0 mmol) and cyanuric fluoride (0.14 mL, 1.7 mmol) were added at -20°C, -20 The reaction was carried out at ℃ for 1 h, washed with ice and water (20 mL × 3), and then extracted with dichloromethane (5 mL × 3). The organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure.
第二步反應:將3,5-二氯-4-氨基吡啶 (108 mg, 0.66 mmol) 溶於乾燥的N ,N -二甲基甲醯胺 (7 mL) 中,冰浴下加入60%氫化鈉 (34 mg, 0.85 mmol),室溫反應1h,在冰浴下加入第一步反應的濃縮液,室溫反應2h後停止。減壓除去N ,N -二甲基甲醯胺,剩餘物加水 (10 mL),用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (洗脫劑:乙酸乙酯 ( v ) = 100%),得到白色固體183 mg,收率65%。The second reaction step: 3,5-dichloro-4-aminopyridine (108 mg, 0.66 mmol) was dissolved in dry N , N -dimethylformamide (7 mL), and 60% of the solution was added under ice bath. Sodium hydride (34 mg, 0.85 mmol) was reacted at room temperature for 1 h, the concentrated solution of the first reaction was added under an ice bath, and the reaction was stopped after 2 h at room temperature. N , N -dimethylformamide was removed under reduced pressure, water (10 mL) was added to the residue, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: ethyl acetate (v) = 100%) to obtain 183 mg of a white solid with a yield of 65%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.48 (br.s, 1H), 8.54 (s, 2H), 7.15 (d,J = 8.1 Hz, 1H), 6.93 (s, 1H), 6.90 (d,J = 8.2 Hz, 1H), 6.73 (t,J F-H = 75.5 Hz, 1H), 4.97 (t,J = 8.1 Hz, 1H), 4.01 – 4.06 (m, 1H), 3.90 (d,J = 6.9 Hz, 2H), 3.50 (t,J = 10.6 Hz, 1H), 3.36 – 3.45 (m, 1H), 2.81 – 2.89 (m, 1H), 2.58 – 2.65 (m, 1H), 2.24 (s, 3H),1.27 – 1.36 (m, 1H), 0.65 – 0.69 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.48 (br.s, 1H), 8.54 (s, 2H), 7.15 (d, J = 8.1 Hz, 1H), 6.93 (s, 1H), 6.90 (d, J = 8.2 Hz, 1H), 6.73 (t, J FH = 75.5 Hz, 1H), 4.97 (t, J = 8.1 Hz, 1H), 4.01 – 4.06 (m, 1H), 3.90 (d, J = 6.9 Hz, 2H), 3.50 (t, J = 10.6 Hz, 1H), 3.36 – 3.45 (m, 1H), 2.81 – 2.89 (m, 1H), 2.58 – 2.65 (m, 1H), 2.24 (s , 3H), 1.27 – 1.36 (m, 1H), 0.65 – 0.69 (m, 2H), 0.36 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 514.20 [M+H]+ .MS (ESI, pos.ion) m/z: 514.20 [M+H] + .
實施例113:化合物 (2R )-1-乙醯基-N -(3-(氨基甲基)苯基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯胺鹽酸鹽 Example 113: Compound ( 2R )-1-Acetyl- N- (3-(aminomethyl)phenyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethyl) Oxy)phenyl)pyrrolidine-2-carboxamide hydrochloride
步驟1:化合物3-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)苄基氨基甲酸叔丁酯的合成Step 1: Compound 3-(( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-methyl Synthesis of tert-butyl acylamino)benzylcarbamate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸(82 mg, 0.22 mmol),化合物3-氨基苄基氨基甲酸叔丁酯 (96 mg, 0.43 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (143 mg, 0.74 mmol) 和N -羥基-7-氮雜苯並三氮唑 (72 mg, 0.53 mmol) 溶於二氯甲烷 (6 mL) 中,室溫下向此溶液中滴加N ,N -二異丙基乙胺 (0.4 mL, 2.0 mmol),室溫攪拌21 h,加水洗 (50 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體112 mg,收率88%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (82 mg, 0.22 mmol), compound tert-butyl 3-aminobenzylcarbamate (96 mg, 0.43 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (143 mg, 0.74 mmol) and N -hydroxy-7-azabenzotriazole (72 mg, 0.53 mmol) were dissolved in dichloromethane (6 mL), to this solution was added dropwise N , N -diiso Propylethylamine (0.4 mL, 2.0 mmol), stirred at room temperature for 21 h, washed with water (50 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution Silica gel column chromatography was performed (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 112 mg of a white solid with a yield of 88%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 7.44-7.52 (m, 3H), 7.33-7.39 (m, 1H), 7.20-7.24 (m, 1H), 7.10-7.14 (m, 1H), 7.05-7.07 (m, 1H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.88-6.93 (m, 2H), 4.42-4.47 (m, 1H), 4.05-4.12 (m, 3H), 3.91 (d,J = 6.9 Hz, 2H), 3.44-3.52 (m, 2H), 2.60-2.65 (m, 1H), 2.02 (s, 3H), 1.95-2.00 (m, 1H), 1.40 (s, 9H), 1.31-1.35 (m, 1H), 0.55-0.59 (m, 2H), 0.30-0.37 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 7.44-7.52 (m, 3H), 7.33-7.39 (m, 1H), 7.20-7.24 (m, 1H), 7.10-7.14 (m, 1H), 7.05-7.07 (m, 1H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.88-6.93 (m, 2H), 4.42-4.47 (m, 1H), 4.05-4.12 (m, 3H) ), 3.91 (d, J = 6.9 Hz, 2H), 3.44-3.52 (m, 2H), 2.60-2.65 (m, 1H), 2.02 (s, 3H), 1.95-2.00 (m, 1H), 1.40 ( s, 9H), 1.31-1.35 (m, 1H), 0.55-0.59 (m, 2H), 0.30-0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 596.25 [M+Na]+ .MS (ESI, pos.ion) m/z: 596.25 [M+Na] + .
步驟2:化合物 (2R )-1-乙醯基-N -(3-(氨基甲基)苯基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯胺鹽酸鹽的合成Step 2: Compound ( 2R )-1-Acetyl- N- (3-(aminomethyl)phenyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy) Synthesis of phenyl) phenyl) pyrrolidine-2-carboxamide hydrochloride
將化合物3-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲醯氨基)苄基氨基甲酸叔丁酯 (112 mg, 0.2 mmol) 溶解於二氯甲烷 (2 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (3 mL),室溫攪拌30 min,除去溶劑,得到白色固體53 mg,收率96%。The compound 3-(( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbamoylamino ) tert-butyl benzylcarbamate (112 mg, 0.2 mmol) was dissolved in dichloromethane (2 mL) solution, 4 mol/L HCl in ethyl acetate solution (3 mL) was added, stirred at room temperature for 30 min, removed solvent to obtain 53 mg of white solid with a yield of 96%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 10.36 (s, 1H), 7.77- 7.79 (m, 1H), 7.59-7.64 (m, 1H), 7.33 (t,J = 8.0 Hz, 1H), 7.19-7.23 (m, 1H), 7.09-7.14 (m, 2H), 7.03 (t,J F-H = 74.9 Hz, 1H), 6.87-6.91 (m, 1H), 4.49-4.53 (m, 1H), 4.08-4.11 (m, 1H), 3.2 (s, 2H), 3.91 (d,J = 6.9 Hz, 2H), 3.45-3.54 (m, 2H), 2.60-2.70 (m, 1H), 2.02 (s, 3H), 1.87-1.98 (m, 1H), 1.23-1.27 (m, 1H), 0.51-0.61 (m, 2H), 0.29-0.36 (m, 2H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 10.36 (s, 1H), 7.77-7.79 (m, 1H), 7.59-7.64 (m, 1H), 7.33 (t, J = 8.0 Hz , 1H), 7.19-7.23 (m, 1H), 7.09-7.14 (m, 2H), 7.03 (t, J FH = 74.9 Hz, 1H), 6.87-6.91 (m, 1H), 4.49-4.53 (m, 1H), 4.08-4.11 (m, 1H), 3.2 (s, 2H), 3.91 (d, J = 6.9 Hz, 2H), 3.45-3.54 (m, 2H), 2.60-2.70 (m, 1H), 2.02 (s, 3H), 1.87-1.98 (m, 1H), 1.23-1.27 (m, 1H), 0.51-0.61 (m, 2H), 0.29-0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 474.15 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 474.15 [M+H-HCl] + .
實施例114:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(3-(羥甲基)苯基)吡咯烷-2-甲醯胺 Example 114: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (3-(hydroxyl) Methyl)phenyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (100 mg, 0.27 mmol),(3-氨基苯基)甲醇 (52 mg, 0.42 mmol) 溶於乾燥的N ,N -二甲基甲醯胺 (5 mL)中,加入N -羥基-7-氮雜苯並三氮唑 (73 mg, 0.54 mmol),在冰浴中加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (259 mg, 1.35 mmol) 和N ,N -二異丙基乙胺 (174 mg, 1.35 mmol),室溫反應12 h,加水 (20 mL) 攪拌5 min,乙酸乙酯萃取 (10 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 54/1),得到淺紅色固體85 mg,收率62%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (100 mg, 0.27 mmol), (3-aminophenyl)methanol (52 mg, 0.42 mmol) was dissolved in dry N , N -dimethylformamide (5 mL), N -hydroxy-7-azabenzone was added Triazole (73 mg, 0.54 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (259 mg, 1.35 mmol) and N , N were added in an ice bath -Diisopropylethylamine (174 mg, 1.35 mmol), react at room temperature for 12 h, add water (20 mL), stir for 5 min, extract with ethyl acetate (10 mL × 2), dry the organic phase with anhydrous sodium sulfate, reduce It was concentrated under pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 54/1) to obtain 85 mg of a light red solid with a yield of 62%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.64 (s, 1H), 7.39 (d,J = 8.1 Hz, 1H), 7.36 (s, 1H), 7.13 (d,J = 8.1 Hz, 1H), 7.11 (d,J = 8.2 Hz, 1H), 6.92 (d,J =9.3 Hz, 1H), 6.91 (s, 1H), 6.85 (d,J =8.1 Hz, 1H), 6.61 (t,J F-H = 75.6 Hz, 1H), 4.75 (t,J = 8.1 Hz, 1H), 4.50 – 4.57 (m, 2H), 3.94 – 3.98 (m, 1H), 3.87 (d,J = 6.9 Hz, 2H), 3.61 (t,J = 10.7 Hz, 1H), 3.31 – 3.40 (m, 1H), 2.87 (br.s, 1H), 2.46 – 2.63 (m, 2H), 2.17 (s, 3H), 1.25 – 1.36 (m, 1H), 0.62 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.64 (s, 1H), 7.39 (d, J = 8.1 Hz, 1H), 7.36 (s, 1H), 7.13 (d, J = 8.1 Hz, 1H), 7.11 (d, J = 8.2 Hz, 1H), 6.92 (d, J =9.3 Hz, 1H), 6.91 (s, 1H), 6.85 (d, J =8.1 Hz, 1H), 6.61 (t, J FH = 75.6 Hz, 1H), 4.75 (t, J = 8.1 Hz, 1H), 4.50 – 4.57 (m, 2H), 3.94 – 3.98 (m, 1H), 3.87 (d, J = 6.9 Hz, 2H) , 3.61 (t, J = 10.7 Hz, 1H), 3.31 – 3.40 (m, 1H), 2.87 (br.s, 1H), 2.46 – 2.63 (m, 2H), 2.17 (s, 3H), 1.25 – 1.36 (m, 1H), 0.62 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 475.15 [M+H]+ .MS (ESI, pos.ion) m/z: 475.15 [M+H] + .
實施例115:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-羥基-1,3-二氫苯並[c][1,2]噁硼烷-5-基)吡咯烷-2-甲醯胺 Example 115: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (1-hydroxy- 1,3-Dihydrobenzo[c][1,2]oxaboran-5-yl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (100 mg, 0.27 mmol),2-羥甲基-5-氨基苯硼酸半酯 (48 mg, 0.32 mmol) 溶於乾燥的N ,N -二甲基甲醯胺 (5 mL)中,加入N -羥基-7-氮雜苯並三氮唑 (73 mg, 0.54 mmol),在冰浴中加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (259 mg, 1.35 mmol) 和N ,N -二異丙基乙胺 (174 mg, 1.35 mmol),室溫反應5 h,加水 (20 mL) 攪拌5 min,乙酸乙酯萃取 (10 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 54/1),得到白色固體35 mg,收率25%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (100 mg, 0.27 mmol), 2-hydroxymethyl-5-aminophenylboronic acid half ester (48 mg, 0.32 mmol) was dissolved in dry N , N -dimethylformamide (5 mL) and N -hydroxy-7 - Azabenzotriazole (73 mg, 0.54 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (259 mg, 1.35 mmol) was added in an ice bath ) and N , N -diisopropylethylamine (174 mg, 1.35 mmol), react at room temperature for 5 h, add water (20 mL), stir for 5 min, extract with ethyl acetate (10 mL × 2), use anhydrous sodium sulfate The organic phase was dried, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 54/1) to obtain 35 mg of white solid, yield 25%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.78 (s, 1H), 7.62 (d,J = 7.8 Hz, 1H), 7.47 (d,J = 7.7 Hz, 1H), 7.06 – 7.12 (m, 2H), 6.94 (d,J = 10.2 Hz, 1H), 6.74 (t,J F-H = 75.0 Hz, 1H), 5.06 (s, 2H), 4.56 – 4.62 (m, 1H), 4.10 – 4.15 (m, 1H), 3.93 (d,J = 6.9 Hz, 2H), 3.68 (t,J = 10.5 Hz, 1H), 3.49 – 3.59 (m, 1H), 2.68 – 2.75 (m, 1H), 2.05 – 2.20 (m, 1H), 2.16 (s, 3H), 1.25 – 1.37 (m, 1H), 0.61 – 0.66 (m, 2H), 0.35 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.78 (s, 1H), 7.62 (d, J = 7.8 Hz, 1H), 7.47 (d, J = 7.7 Hz, 1H), 7.06 – 7.12 ( m, 2H), 6.94 (d, J = 10.2 Hz, 1H), 6.74 (t, J FH = 75.0 Hz, 1H), 5.06 (s, 2H), 4.56 – 4.62 (m, 1H), 4.10 – 4.15 ( m, 1H), 3.93 (d, J = 6.9 Hz, 2H), 3.68 (t, J = 10.5 Hz, 1H), 3.49 – 3.59 (m, 1H), 2.68 – 2.75 (m, 1H), 2.05 – 2.20 (m, 1H), 2.16 (s, 3H), 1.25 – 1.37 (m, 1H), 0.61 – 0.66 (m, 2H), 0.35 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 501.10 [M+H]+ .MS (ESI, pos.ion) m/z: 501.10 [M+H] + .
實施例116:化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)-N -(2-乙氧基苯基)吡咯烷-2-甲醯胺 Example 116: Compound (2 R) -1- acetyl-4- (4- (difluoromethoxy) -3-isopropoxyphenyl) - N - (2- ethoxyphenyl) Pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(4-(二氟甲氧基)-3-異丙氧基苯基)吡咯烷-2-羧酸 (60 mg, 0.17 mmol),2-乙氧基苯胺 (69 mg, 0.50 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (160 mg, 0.83 mmol) 和N -羥基-7-氮雜苯並三氮唑 (45 mg, 0.33 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃後,加入N ,N -二異丙基乙胺 (155 mg, 1.20 mmol),室溫反應3 h,加水洗 (10 mL × 3) 攪拌,分離有機相,有機相用無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1),得到淺褐色固體71 mg,收率88%。Compound ( 2R )-1-acetyl-4-(4-(difluoromethoxy)-3-isopropoxyphenyl)pyrrolidine-2-carboxylic acid (60 mg, 0.17 mmol), 2-Ethoxyaniline (69 mg, 0.50 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (160 mg, 0.83 mmol) and N -hydroxy-7 -Azabenzotriazole (45 mg, 0.33 mmol) was dissolved in dichloromethane (10 mL), and after cooling to 0 °C, N , N -diisopropylethylamine (155 mg, 1.20 mmol) was added , reacted at room temperature for 3 h, washed with water (10 mL × 3), stirred, separated the organic phase, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: dichloromethane/methanol ( v/v ) = 50/1) to obtain 71 mg of a light brown solid with a yield of 88%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.98 (s, 1H), 8.38 (d,J = 7.8 Hz, 1H), 7.13 (d,J = 8.2 Hz, 1H), 7.03 (d,J = 7.7 Hz, 1H), 6.94 – 6.99 (m, 2H), 6.85 – 6.91 (m, 2H), 6.55 (t,J F-H = 75.6 Hz, 1H), 4.79 (t,J = 8.1 Hz, 1H), 4.52 – 4.64 (m, 1H), 4.12 (q,J = 7.0 Hz, 2H), 3.99 – 4.03 (m, 1H), 3.56 (t,J = 10.6 Hz, 1H), 3.34 – 3.43 (m, 1H), 2.64 (t,J = 8.7 Hz, 2H), 2.20 (s, 3H), 1.51 (t,J = 6.9 Hz, 3H), 1.37 (d,J = 6.0 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.98 (s, 1H), 8.38 (d, J = 7.8 Hz, 1H), 7.13 (d, J = 8.2 Hz, 1H), 7.03 (d, J = 7.7 Hz, 1H), 6.94 – 6.99 (m, 2H), 6.85 – 6.91 (m, 2H), 6.55 (t, J FH = 75.6 Hz, 1H), 4.79 (t, J = 8.1 Hz, 1H) , 4.52 – 4.64 (m, 1H), 4.12 (q, J = 7.0 Hz, 2H), 3.99 – 4.03 (m, 1H), 3.56 (t, J = 10.6 Hz, 1H), 3.34 – 3.43 (m, 1H) ), 2.64 (t, J = 8.7 Hz, 2H), 2.20 (s, 3H), 1.51 (t, J = 6.9 Hz, 3H), 1.37 (d, J = 6.0 Hz, 6H).
MS (ESI, pos.ion) m/z: 477.15 [M+H]+ .MS (ESI, pos.ion) m/z: 477.15 [M+H] + .
實施例117:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-氧代異吲哚-5-基)吡咯烷-2-甲醯胺 Example 117: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (1-oxo Isoindol-5-yl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (150 mg, 0.41 mmol),5-氨基異吲哚-1-酮 (72 mg, 0.49 mmol) 和N -羥基-7-氮雜苯並三氮唑 (110 mg, 0.81 mmol) 溶於二氯甲烷 (12 mL) 和N ,N -二甲基甲醯胺 (4 mL) 中,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (233 mg, 1.22 mmol) 和N ,N -二異丙基乙胺 (210 mg, 1.62 mmol),室溫反應5 h,減壓濃縮除去溶劑,加水 (20 mL),用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 25/1),得到淺褐色固體126 mg,收率62%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (150 mg, 0.41 mmol), 5-aminoisoindol-1-one (72 mg, 0.49 mmol) and N -hydroxy-7-azabenzotriazole (110 mg, 0.81 mmol) were dissolved in dichloromethane (12 mL) ) and N , N -dimethylformamide (4 mL), add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (233 mg, 1.22 mmol) and N , N -diisopropylethylamine (210 mg, 1.62 mmol), reacted at room temperature for 5 h, concentrated under reduced pressure to remove the solvent, added water (20 mL), extracted with ethyl acetate (10 mL × 2), the organic phase was It was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 25/1) to obtain 126 mg of light brown solid, yield 62% .
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 10.22 (s, 1H), 8.61 (s, 1H), 8.10 (s, 1H), 7.39 – 7.59 (m, 1H), 7.04 – 7.17 (m, 1H), 6.85 – 6.97 (m, 3H), 6.61 (t,J = 75.3 Hz, 1H), 4.68 – 4.87 (m, 1H), 4.15 – 4.36 (m, 2H), 3.95 – 4.09 (m, 1H), 3.78 – 3.93 (m, 2H), 3.62 – 3.77 (m, 1H), 3.32 – 3.56 (m, 1H), 2.56 – 2.78 (m, 1H), 2.29 – 2.46 (m, 1H), 2.24 (s, 3H), 1.26 – 1.37 (m, 1H), 0.57 – 0.71 (m, 2H), 0.27 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 10.22 (s, 1H), 8.61 (s, 1H), 8.10 (s, 1H), 7.39 – 7.59 (m, 1H), 7.04 – 7.17 (m , 1H), 6.85 – 6.97 (m, 3H), 6.61 (t, J = 75.3 Hz, 1H), 4.68 – 4.87 (m, 1H), 4.15 – 4.36 (m, 2H), 3.95 – 4.09 (m, 1H) ), 3.78 – 3.93 (m, 2H), 3.62 – 3.77 (m, 1H), 3.32 – 3.56 (m, 1H), 2.56 – 2.78 (m, 1H), 2.29 – 2.46 (m, 1H), 2.24 (s , 3H), 1.26 – 1.37 (m, 1H), 0.57 – 0.71 (m, 2H), 0.27 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 500.10 [M+H]+ .MS (ESI, pos.ion) m/z: 500.10 [M+H] + .
實施例118:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(3-氧代異吲哚-5-基)吡咯烷-2-甲醯胺 Example 118: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (3-oxo Isoindol-5-yl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (203 mg, 0.55 mmol),6-氨基異吲哚-1-酮 (98 mg, 0.66 mmol) 和N -羥基-7-氮雜苯並三氮唑 (149 mg, 1.09 mmol) 溶於無水N ,N -二甲基甲醯胺 (8 mL) 中,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (310 mg, 1.62 mmol) 和N ,N -二異丙基乙胺 (283 mg, 2.19 mmol),室溫反應10 h,減壓濃縮除去溶劑,加水 (30 mL),用乙酸乙酯萃取 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到白色固體203 mg,收率74%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (203 mg, 0.55 mmol), 6-aminoisoindol-1-one (98 mg, 0.66 mmol) and N -hydroxy-7-azabenzotriazole (149 mg, 1.09 mmol) in anhydrous N , N -diazole Methylformamide (8 mL) was added 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (310 mg, 1.62 mmol) and N , N -diisopropyl Ethylethylamine (283 mg, 2.19 mmol), reacted at room temperature for 10 h, concentrated under reduced pressure to remove the solvent, added water (30 mL), extracted with ethyl acetate (20 mL × 2), the organic phase was dried over anhydrous sodium sulfate, reduced It was concentrated under pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 203 mg of white solid with a yield of 74%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 10.18 (br.s, 1H), 8.10 (s, 1H), 7.90 (s, 1H), 7.05 – 7.19 (m, 2H), 6.80 – 6.93 (m, 2H), 6.59 (t,J F-H = 75.8 Hz, 1H), 4.87 – 5.05 (m, 1H), 4.11 – 4.35 (m, 2H), 3.90 – 4.03 (m, 1H), 3.77 – 3.90 (m, 2H), 3.56 – 3.70 (m, 1H), 3.31 – 3.50 (m, 1H), 2.70 – 2.87 (m, 1H), 2.26 – 2.43 (m, 1H), 2.11 – 2.20 (s, 3H), 1.23 – 1.32 (m, 1H), 0.55 – 0.68 (m, 2H), 0.26 – 0.33 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 10.18 (br.s, 1H), 8.10 (s, 1H), 7.90 (s, 1H), 7.05 – 7.19 (m, 2H), 6.80 – 6.93 (m, 2H), 6.59 (t, J FH = 75.8 Hz, 1H), 4.87 – 5.05 (m, 1H), 4.11 – 4.35 (m, 2H), 3.90 – 4.03 (m, 1H), 3.77 – 3.90 ( m, 2H), 3.56 – 3.70 (m, 1H), 3.31 – 3.50 (m, 1H), 2.70 – 2.87 (m, 1H), 2.26 – 2.43 (m, 1H), 2.11 – 2.20 (s, 3H), 1.23 – 1.32 (m, 1H), 0.55 – 0.68 (m, 2H), 0.26 – 0.33 (m, 2H).
MS (ESI, pos.ion) m/z: 500.45 [M+H]+ .MS (ESI, pos.ion) m/z: 500.45 [M+H] + .
實施例119:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-羥基-1,3-二氫苯並[c ][1,2]氧雜環戊硼烷-6-基)吡咯烷-2-甲醯胺 Example 119: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (1-hydroxy- 1,3-Dihydrobenzo[ c ][1,2]oxaborolane-6-yl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (200 mg, 0.54 mmol),2-羥甲基-6-氨基苯硼酸半酯 (96 mg, 0.65 mmol) 和N -羥基-7-氮雜苯並三氮唑 (147 mg, 1.08 mmol) 溶於無水N ,N -二甲基甲醯胺 (5 mL) 中,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (312 mg, 1.63 mmol) 和N ,N -二異丙基乙胺 (279 mg, 2.16 mmol),室溫反應10 h,減壓濃縮除去溶劑,加水 (30 mL),用乙酸乙酯萃取 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 25/1),得到白色固體166 mg,收率61%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (200 mg, 0.54 mmol), 2-hydroxymethyl-6-aminophenylboronic acid half ester (96 mg, 0.65 mmol) and N -hydroxy-7-azabenzotriazole (147 mg, 1.08 mmol) were dissolved in anhydrous N , N -dimethylformamide (5 mL) was added 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (312 mg, 1.63 mmol) and N , N - Diisopropylethylamine (279 mg, 2.16 mmol) was reacted at room temperature for 10 h, concentrated under reduced pressure to remove the solvent, added water (30 mL), extracted with ethyl acetate (20 mL × 2), and the organic phase was washed with anhydrous sodium sulfate It was dried and concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 25/1) to obtain 166 mg of white solid with a yield of 61%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.45 (br.s, 1H), 7.70 (s, 2H), 7.03 – 7.16 (m, 2H), 6.94 (s, 1H), 6.75 – 6.87 (m, 1H), 6.61 (t,J F-H = 75.4 Hz, 1H), 4.77 – 5.00 (m, 1H), 4.88 (s, 2H), 3.85 – 4.00 (m, 1H), 3.83 – 3.90 (m, 2H), 3.61 – 3.76 (m, 1H), 3.30 – 3.50 (m, 1H), 2.56 – 2.74 (m, 1H), 2.38 – 2.52 (m, 1H), 2.13 (s, 3H), 1.23 – 1.32 (m, 1H), 0.55 – 0.68 (m, 2H), 0.27 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.45 (br.s, 1H), 7.70 (s, 2H), 7.03 – 7.16 (m, 2H), 6.94 (s, 1H), 6.75 – 6.87 (m, 1H), 6.61 (t, J FH = 75.4 Hz, 1H), 4.77 – 5.00 (m, 1H), 4.88 (s, 2H), 3.85 – 4.00 (m, 1H), 3.83 – 3.90 (m, 2H), 3.61 – 3.76 (m, 1H), 3.30 – 3.50 (m, 1H), 2.56 – 2.74 (m, 1H), 2.38 – 2.52 (m, 1H), 2.13 (s, 3H), 1.23 – 1.32 ( m, 1H), 0.55 – 0.68 (m, 2H), 0.27 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 501.40 [M+H]+ .MS (ESI, pos.ion) m/z: 501.40 [M+H] + .
實施例120:化合物6-((((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)氧基)甲基)煙酸甲酯 Example 120: Compound 6-(((( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine- 2-Carbonyl)oxy)methyl)nicotinic acid methyl ester
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (60 mg, 0.16 mmol),6-羥甲基煙酸甲酯 (32 mg, 0.19 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (155 mg, 0.81 mmol) 和N -羥基-7-氮雜苯並三氮唑 (44 mg, 0.32 mmol) 溶於二氯甲烷 (20 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (126 mg, 0.97 mmol),室溫反應20 h,加水洗有機相 (10 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到淺黃色黏稠固體54 mg,收率64%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (60 mg, 0.16 mmol), methyl 6-hydroxymethylnicotinate (32 mg, 0.19 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (155 mg, 0.81 mmol) ) and N -hydroxy-7-azabenzotriazole (44 mg, 0.32 mmol) were dissolved in dichloromethane (20 mL), cooled to 0 °C, added with N , N -diisopropylethylamine ( 126 mg, 0.97 mmol), react at room temperature for 20 h, add water to wash the organic phase (10 mL × 3), dry the organic phase with anhydrous sodium sulfate, remove the solvent, and separate the concentrated solution by silica gel column chromatography (eluent: dichloride Methane/methanol (v/v) = 30/1) to obtain 54 mg of light yellow viscous solid, yield 64%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 9.16 (s, 1H), 8.35 (dd,J = 8.2, 2.0 Hz, 1H), 7.61 (d,J = 8.2 Hz, 1H), 7.15 (d,J = 8.7 Hz, 1H), 6.85 (s, 1H), 6.82 – 6.85 (m, 1H), 6.63 (t,J F-H = 75.5 Hz, 1H), 5.30 – 5.52 (m, 2H), 4.61 – 4.66 (m, 1H), 3.97 – 4.03 (m, 1H), 3.97 (s, 3H), 3.88 (d,J = 6.9 Hz, 2H), 3.63 – 3.68 (m, 1H), 3.43 – 3.52 (m, 1H), 2.72 – 2.79 (m, 1H), 2.09 – 2.22 (m, 1H), 2.15 (s, 3H), 1.27 – 1.36 (m, 1H), 0.65 – 0.70 (m, 2H), 0.36 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.16 (s, 1H), 8.35 (dd, J = 8.2, 2.0 Hz, 1H), 7.61 (d, J = 8.2 Hz, 1H), 7.15 ( d, J = 8.7 Hz, 1H), 6.85 (s, 1H), 6.82 – 6.85 (m, 1H), 6.63 (t, J FH = 75.5 Hz, 1H), 5.30 – 5.52 (m, 2H), 4.61 – 4.66 (m, 1H), 3.97 – 4.03 (m, 1H), 3.97 (s, 3H), 3.88 (d, J = 6.9 Hz, 2H), 3.63 – 3.68 (m, 1H), 3.43 – 3.52 (m, 1H), 2.72 – 2.79 (m, 1H), 2.09 – 2.22 (m, 1H), 2.15 (s, 3H), 1.27 – 1.36 (m, 1H), 0.65 – 0.70 (m, 2H), 0.36 – 0.40 ( m, 2H).
MS (ESI, pos.ion) m/z: 519.25 [M+H]+ .MS (ESI, pos.ion) m/z: 519.25 [M+H] + .
實施例121:化合物 (2R )-2-乙氧基苄基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 121: Compound ( 2R )-2-ethoxybenzyl-1-acetoxy-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrole Alkane-2-carboxylate
將化合物 (2R )-1-乙醯基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (50 mg, 0.14 mmol),2-乙氧基苄醇 (61 mg, 0.40 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (130 mg, 0.68 mmol) 和N -羥基-7-氮雜苯並三氮唑 (36 mg, 0.26 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (105 mg, 0.81 mmol),室溫反應44 h,加水洗有機相 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到白色黏稠固體33 mg,收率48%。The compound ( 2R )-1-acetyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (50 mg, 0.14 mmol), 2-ethoxybenzyl alcohol (61 mg, 0.40 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (130 mg, 0.68 mmol) and N -Hydroxy-7-azabenzotriazole (36 mg, 0.26 mmol) was dissolved in dichloromethane (10 mL), cooled to 0 °C, added with N , N -diisopropylethylamine (105 mg, 0.81 mmol), react at room temperature for 44 h, add water to wash the organic phase (20 mL × 2), dry the organic phase with anhydrous sodium sulfate, concentrate under reduced pressure, and separate the residue by silica gel column chromatography (eluent: petroleum ether/acetic acid) Ethyl ester (v/v) = 1/1) to obtain 33 mg of white viscous solid, yield 48%.
1 H NMR (600 MHz, CDCl3 ) δ (ppm): 7.37 (d,J = 7.4 Hz, 1H), 7.28 – 7.32 (m, 1H), 7.10 – 7.14 (m, 1H), 6.94 – 6.97 (m, 1H), 6.87 – 6.91 (m, 1H), 6.83 (s, 1H), 6.67 – 6.81 (m, 1H), 6.62 (t,J F-H = 75.5 Hz, 1H), 5.23 – 5.37 (m, 2H), 4.51 – 4.60 (m, 1H), 4.06 (q,J = 6.9 Hz, 2H), 3.94 – 3.97 (m, 1H), 3.87 (d,J = 6.9 Hz, 2H), 3.62 – 3.65 (m, 1H), 3.38 – 3.45 (m, 1H), 2.84 – 2.89 (m, 0.3H), 2.69 – 2.73 (m, 0.7 H), 2.14 (s, 2.3H), 1.98 (s, 0.7H), 2.02 – 2.11 (m, 1H), 1.41 (t,J = 6.9 Hz, 3H), 1.29 – 1.36 (m, 1H), 0.66 – 0.69 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 7.37 (d, J = 7.4 Hz, 1H), 7.28 – 7.32 (m, 1H), 7.10 – 7.14 (m, 1H), 6.94 – 6.97 (m , 1H), 6.87 – 6.91 (m, 1H), 6.83 (s, 1H), 6.67 – 6.81 (m, 1H), 6.62 (t, J FH = 75.5 Hz, 1H), 5.23 – 5.37 (m, 2H) , 4.51 – 4.60 (m, 1H), 4.06 (q, J = 6.9 Hz, 2H), 3.94 – 3.97 (m, 1H), 3.87 (d, J = 6.9 Hz, 2H), 3.62 – 3.65 (m, 1H) ), 3.38 – 3.45 (m, 1H), 2.84 – 2.89 (m, 0.3H), 2.69 – 2.73 (m, 0.7 H), 2.14 (s, 2.3H), 1.98 (s, 0.7H), 2.02 – 2.11 (m, 1H), 1.41 (t, J = 6.9 Hz, 3H), 1.29 – 1.36 (m, 1H), 0.66 – 0.69 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 504.20 [M+H]+ .MS (ESI, pos.ion) m/z: 504.20 [M+H] + .
實施例122:化合物 (2R )-(2-乙氧基-3-氟苄基) -1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 122: Compound ( 2R )-(2-ethoxy-3-fluorobenzyl)-1-acetoxy-4-(3-(cyclopropylmethoxy)-4-(difluoromethyl) Oxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (50 mg, 0.14 mmol),2-乙氧基-3-氟苄醇 (46 mg, 0.27 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (130 mg, 0.68 mmol) 和N -羥基-7-氮雜苯並三氮唑 (36 mg, 0.26 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (105 mg, 0.81 mmol),室溫反應11 h,加水洗有機相 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到白色黏稠固體17 mg,收率24%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (50 mg, 0.14 mmol), 2-ethoxy-3-fluorobenzyl alcohol (46 mg, 0.27 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (130 mg, 0.68 mmol) and N -hydroxy-7-azabenzotriazole (36 mg, 0.26 mmol) were dissolved in dichloromethane (10 mL), cooled to 0 °C, and N , N -diisopropylethyl acetate was added. Amine (105 mg, 0.81 mmol), react at room temperature for 11 h, add water to wash the organic phase (20 mL × 2), dry the organic phase with anhydrous sodium sulfate, concentrate under reduced pressure, and separate the residue by silica gel column chromatography (eluent). : petroleum ether/ethyl acetate (v/v) = 1/1) to obtain 17 mg of white viscous solid, yield 24%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.01 – 7.18 (m, 4H), 6.83 (s, 1H), 6.82 (d,J = 7.1 Hz, 1H), 6.62 (t,J F-H = 75.5 Hz, 1H), 5.25 – 5.35 (m, 2H), 4.52 – 4.58 (m, 1H), 4.19 (q,J = 7.0 Hz, 2H), 3.94 – 3.98 (m, 1H), 3.87 (d,J = 7.0 Hz, 2H), 3.63 (t,J = 10.4 Hz, 1H), 3.37 – 3.47 (m, 1H), 2.65 – 2.72 (m, 1H), 2.14 (s, 2.4H), 1.97 (s, 0.6H), 2.02 – 2.11 (m, 1H), 1.40 (t,J = 7.0 Hz, 3H), 1.27 – 1.36 (m, 1H), 0.66 – 0.70 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.01 – 7.18 (m, 4H), 6.83 (s, 1H), 6.82 (d, J = 7.1 Hz, 1H), 6.62 (t, J FH = 75.5 Hz, 1H), 5.25 – 5.35 (m, 2H), 4.52 – 4.58 (m, 1H), 4.19 (q, J = 7.0 Hz, 2H), 3.94 – 3.98 (m, 1H), 3.87 (d, J = 7.0 Hz, 2H), 3.63 (t, J = 10.4 Hz, 1H), 3.37 – 3.47 (m, 1H), 2.65 – 2.72 (m, 1H), 2.14 (s, 2.4H), 1.97 (s, 0.6 H), 2.02 – 2.11 (m, 1H), 1.40 (t, J = 7.0 Hz, 3H), 1.27 – 1.36 (m, 1H), 0.66 – 0.70 (m, 2H), 0.36 – 0.39 (m, 2H) .
MS (ESI, pos.ion) m/z: 522.15 [M+H]+ .MS (ESI, pos.ion) m/z: 522.15 [M+H] + .
實施例123:化合物 (2R )-(6-(異丙基(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 123: Compound ( 2R )-(6-(isopropyl(methyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethyl) oxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-(異丙基(甲基)氨基甲醯基)吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-(isopropyl(methyl)carbamoyl)methyl picolinate
將化合物2,6-吡啶二羧酸單甲酯 (500 mg, 2.76 mmol),N -異丙基甲胺 (264 mg, 2.64 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (1.30 g, 6.80 mmol) 和N -羥基-7-氮雜苯並三氮唑 (751 mg, 5.52 mmol) 溶於二氯甲烷 (20 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (1.10 g, 8.51 mmol),室溫反應1 h,加水洗 (20 mL × 2) 有機相,分離有機相,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到白色固體622 mg,收率95%。Compound 2,6-pyridinedicarboxylate monomethyl ester (500 mg, 2.76 mmol), N -isopropylmethylamine (264 mg, 2.64 mmol), 1-ethyl-(3-dimethylaminopropyl) ) carbodiimide hydrochloride (1.30 g, 6.80 mmol) and N -hydroxy-7-azabenzotriazole (751 mg, 5.52 mmol) were dissolved in dichloromethane (20 mL) and cooled to 0 °C, add N , N -diisopropylethylamine (1.10 g, 8.51 mmol), react at room temperature for 1 h, add water to wash (20 mL × 2) the organic phase, separate the organic phase, dry with anhydrous sodium sulfate, reduce It was concentrated under pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/1) to obtain 622 mg of white solid with a yield of 95%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.17 (d,J = 7.6 Hz, 1H), 7.96 (t,J = 7.8 Hz, 1H), 7.78 (t,J = 7.8 Hz, 1H), 4.93 – 5.00 (m, 0.4H), 4.01 – 4.09 (m, 0.6H), 4.01 (s, 3H), 3.00 (s, 2H), 2.92 (s, 1H), 1.24 – 1.77 (m, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.17 (d, J = 7.6 Hz, 1H), 7.96 (t, J = 7.8 Hz, 1H), 7.78 (t, J = 7.8 Hz, 1H) , 4.93 – 5.00 (m, 0.4H), 4.01 – 4.09 (m, 0.6H), 4.01 (s, 3H), 3.00 (s, 2H), 2.92 (s, 1H), 1.24 – 1.77 (m, 6H) .
MS (ESI, pos.ion) m/z: 237.25 [M+H]+ .MS (ESI, pos.ion) m/z: 237.25 [M+H] + .
步驟2:化合物6-(羥甲基)-N -異丙基-N -甲基吡啶醯胺的合成Step 2: Synthesis of compound 6-(hydroxymethyl) -N -isopropyl- N-picolinamide
將化合物6-(異丙基(甲基)氨基甲醯基)吡啶甲酸甲酯 (680 mg, 2.39 mmol) 溶於乾燥四氫呋喃 (5 mL) 中,冰浴中加入硼氫化鋰 (96 mg, 4.51 mmol),室溫反應30 min後停止,加入飽和氯化鈉水溶液 (10 mL),乙酸乙酯萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 100/1),得到白色固體423 mg,收率78%。The compound 6-(isopropyl(methyl)carbamoyl)picolinate (680 mg, 2.39 mmol) was dissolved in dry tetrahydrofuran (5 mL), and lithium borohydride (96 mg, 4.51 mg) was added to the ice bath. mmol), the reaction was stopped after 30 min at room temperature, saturated aqueous sodium chloride solution (10 mL) was added, extracted with ethyl acetate (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was subjected to silica gel column Chromatographic separation (eluent: dichloromethane/methanol (v/v) = 100/1) gave 423 mg of a white solid with a yield of 78%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.17 (t,J = 7.7 Hz, 1H), 7.447.35 – 7.24 (m, 1H), 4.94 – 5.01 (m, 0.4H), 4.78 (s, 3H), 2.99 (s, 1.8H), 2.83 (s, 1.2H), 1.20 – 1.26 (m, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.17 (t, J = 7.7 Hz, 1H), 7.447.35 – 7.24 (m, 1H), 4.94 – 5.01 (m, 0.4H), 4.78 ( s, 3H), 2.99 (s, 1.8H), 2.83 (s, 1.2H), 1.20 – 1.26 (m, 6H).
MS (ESI, pos.ion) m/z: 209.20 [M+H]+ .MS (ESI, pos.ion) m/z: 209.20 [M+H] + .
步驟3:化合物 (2R )-(6-(異丙基(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 3: Compound ( 2R )-(6-(isopropyl(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethoxy) Synthesis of yl)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (80 mg, 0.22 mmol),6-(羥甲基)-N -異丙基-N -甲基吡啶醯胺 (56 mg, 0.27 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (207 mg, 1.08 mmol) 和N -羥基-7-氮雜苯並三氮唑 (58 mg, 0.43 mmol) 溶於二氯甲烷(10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (141 mg, 1.09 mmol),室溫反應6 h,加水 (15 mL),有機相水洗 (10 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 54/1),得到淺褐色黏稠固體56 mg,收率46%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (80 mg, 0.22 mmol), 6-(hydroxymethyl) -N -isopropyl- N -picoline amide (56 mg, 0.27 mmol), 1-ethyl-(3-dimethylaminopropyl)carboamide Diimine hydrochloride (207 mg, 1.08 mmol) and N -hydroxy-7-azabenzotriazole (58 mg, 0.43 mmol) were dissolved in dichloromethane (10 mL), cooled to 0 °C, N , N -diisopropylethylamine (141 mg, 1.09 mmol) was added, the reaction was carried out at room temperature for 6 h, water (15 mL) was added, the organic phase was washed with water (10 mL × 2), the organic phase was dried with anhydrous sodium sulfate, and the mixture was reduced It was concentrated under pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 54/1) to obtain 56 mg of light brown viscous solid with a yield of 46%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.80 (t,J = 7.7 Hz, 1H), 7.41 – 7.52 (m, 2H), 7.12 (d,J = 8.3 Hz, 1H), 6.82 (s, 1H), 6.77 – 6.81 (m, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 5.23 – 5.44 (m, 2H), 4.89 – 4.96 (m, 0.4H), 4.57 – 4.61 (m, 1H), 3.92 – 4.00 (m, 0.6H), 3.92 – 3.97 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.63 (t,J = 10.4 Hz, 1H), 3.38 – 3.50 (m, 1H), 2.94 (s, 1.8H), 2.80 (s, 1.2H), 2.68 – 2.74 (m, 1H), 2.04 – 2.16 (m, 1H), 2.12 (s, 3H), 1.25 – 1.32 (m, 1H), 1.15 – 1.21 (m, 6H), 0.63– 0.67(m, 2H), 0.34 – 0.37(m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.80 (t, J = 7.7 Hz, 1H), 7.41 – 7.52 (m, 2H), 7.12 (d, J = 8.3 Hz, 1H), 6.82 ( s, 1H), 6.77 – 6.81 (m, 1H), 6.60 (t, J FH = 75.5 Hz, 1H), 5.23 – 5.44 (m, 2H), 4.89 – 4.96 (m, 0.4H), 4.57 – 4.61 ( m, 1H), 3.92 – 4.00 (m, 0.6H), 3.92 – 3.97 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.63 (t, J = 10.4 Hz, 1H), 3.38 – 3.50 (m, 1H), 2.94 (s, 1.8H), 2.80 (s, 1.2H), 2.68 – 2.74 (m, 1H), 2.04 – 2.16 (m, 1H), 2.12 (s, 3H), 1.25 – 1.32 (m, 1H), 1.15 – 1.21 (m, 6H), 0.63 – 0.67(m, 2H), 0.34 – 0.37(m, 2H).
MS (ESI, pos.ion) m/z: 560.20 [M+H]+ .MS (ESI, pos.ion) m/z: 560.20 [M+H] + .
實施例124:化合物 (2R )-(6-((2-(二甲氨基)乙基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 124: Compound ( 2R )-(6-((2-(dimethylamino)ethyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4- (3-(Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-((2-(二甲氨基)乙基)(甲基)氨基甲醯基)吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-((2-(dimethylamino)ethyl)(methyl)carbamoyl)picolinate methyl ester
將化合物2,6-吡啶二羧酸單甲酯 (500 mg, 2.76 mmol),N1 ,N1 ,N2 -三甲基乙二胺 (368 mg, 3.60 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (1.30 g, 6.80 mmol) 和N -羥基-7-氮雜苯並三氮唑 (753 mg, 5.53 mmol) 溶於二氯甲烷 (20 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (1.10 g, 8.51 mmol),室溫反應8.5 h,加水 (20 mL × 2) 洗有機相,分離有機相,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到淺褐色液體667 mg,收率91%。The compound 2,6-pyridinedicarboxylate monomethyl ester (500 mg, 2.76 mmol), N 1 , N 1 , N 2 -trimethylethylenediamine (368 mg, 3.60 mmol), 1-ethyl-( 3-Dimethylaminopropyl)carbodiimide hydrochloride (1.30 g, 6.80 mmol) and N -hydroxy-7-azabenzotriazole (753 mg, 5.53 mmol) in dichloromethane (20 mL), cooled to 0 °C, added N , N -diisopropylethylamine (1.10 g, 8.51 mmol), reacted at room temperature for 8.5 h, added water (20 mL × 2) to wash the organic phase, and separated the organic phase , dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrated solution was subjected to silica gel column separation (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 667 mg of light brown liquid with a yield of 91%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.17 (d,J = 7.7 Hz, 1H), 7.84 – 7.89 (m, 1H), 4.01 (s, 3H), 2.33 (s, 2H), 2.24 (s, 1H), 2.10 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.17 (d, J = 7.7 Hz, 1H), 7.84 – 7.89 (m, 1H), 4.01 (s, 3H), 2.33 (s, 2H), 2.24 (s, 1H), 2.10 (s, 3H).
MS (ESI, pos.ion) m/z: 266.10 [M+H]+ .MS (ESI, pos.ion) m/z: 266.10 [M+H] + .
步驟2:化合物N -(2-(二甲氨基)乙基)-6-(羥甲基)-N -甲基吡啶醯胺的合成Step 2: Synthesis of compound N- (2-(dimethylamino)ethyl)-6-(hydroxymethyl) -N -methylpyridamide
將化合物6-((2-(二甲氨基)乙基)(甲基)氨基甲醯基)吡啶甲酸甲酯 (360 mg, 1.36 mmol) 溶於甲醇 (5 mL) 中,冰浴下加入硼氫化鈉 (410 mg,10.8 mmol),室溫反應3 h後停止,減壓濃縮,濃縮液進行矽膠柱分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 10/1),得到無色液體214 mg,產率66%。Compound 6-((2-(dimethylamino)ethyl)(methyl)carbamoyl)picolinate (360 mg, 1.36 mmol) was dissolved in methanol (5 mL), and boron was added under ice bath Sodium hydride (410 mg, 10.8 mmol), the reaction was stopped after 3 h at room temperature, concentrated under reduced pressure, and the concentrated solution was subjected to silica gel column separation (eluent: dichloromethane/methanol (v/v) = 10/1) to obtain Colorless liquid 214 mg, 66% yield.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.75 – 7.79 (m, 1H), 7.69 (d,J = 7.7, Hz, 0.6H), 7.50 (d,J = 7.6, Hz, 0.4H), 7.31 (d,J = 7.9, Hz, 0.4H), 7.27 (d,J = 7.8, Hz, 0.6H), 4.47 (s, 0.8H), 4.72 (s, 1.2H), 3.69 (t,J = 6.8, Hz, 0.7H), 3.59 (t,J = 6.8, Hz, 1.3H), 3.14 (s, 2.2H), 3.05 (s, 0.8H), 2.73 (t,J = 6.8, Hz, 1.3H), 2.62 (t,J = 6.8, Hz, 0.7H), 2.33 (s, 2H), 2.19 (s, 4H) 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.75 – 7.79 (m, 1H), 7.69 (d, J = 7.7, Hz, 0.6H), 7.50 (d, J = 7.6, Hz, 0.4H ), 7.31 (d, J = 7.9, Hz, 0.4H), 7.27 (d, J = 7.8, Hz, 0.6H), 4.47 (s, 0.8H), 4.72 (s, 1.2H), 3.69 (t, J = 6.8, Hz, 0.7H), 3.59 (t, J = 6.8, Hz, 1.3H), 3.14 (s, 2.2H), 3.05 (s, 0.8H), 2.73 (t, J = 6.8, Hz, 1.3H), 2.62 (t, J = 6.8, Hz, 0.7H), 2.33 (s, 2H), 2.19 (s, 4H)
MS (ESI, pos.ion) m/z: 238.20 [M+H]+ .MS (ESI, pos.ion) m/z: 238.20 [M+H] + .
步驟3:化合物 (2R )-(6-((2-(二甲氨基)乙基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 的合成Step 3: Compound ( 2R )-(6-((2-(dimethylamino)ethyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-( Synthesis of 3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (60 mg, 0.16 mmol),N -(2-(二甲氨基)乙基)-6-(羥甲基)-N -甲基吡啶醯胺 (45 mg, 0.19 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (207 mg, 1.08 mmol) 和N -羥基-7-氮雜苯並三氮唑 (44 mg, 0.32 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (83 mg, 0.64 mmol),室溫反應9 h,加二氯甲烷 (15 mL),有機相水洗 (20 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇( v/v ) = 24/1),得到白色黏稠固體47 mg,收率49%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (60 mg, 0.16 mmol), N- (2-(dimethylamino)ethyl)-6-(hydroxymethyl) -N -picolinamide (45 mg, 0.19 mmol), 1-ethyl-(3-diamino) Methylaminopropyl)carbodiimide hydrochloride (207 mg, 1.08 mmol) and N -hydroxy-7-azabenzotriazole (44 mg, 0.32 mmol) were dissolved in dichloromethane (10 mL) ), cooled to 0°C, added N , N -diisopropylethylamine (83 mg, 0.64 mmol), reacted at room temperature for 9 h, added dichloromethane (15 mL), washed the organic phase with water (20 mL × 2 ), the organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 24/1) to obtain 47 mg of white viscous solid, Yield 49%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.78 – 7.83 (m, 1H), 7.50 – 7.57 (m, 2H), 7.12 (d,J = 8.7 Hz, 1H), 6.82 (s, 1H), 6.78 – 6.2 (m, 1H), 6.61 (t,J F-H = 75.5 Hz, 1H), 5.23 – 5.45 (m, 2H), 4.56 – 4.60 (m, 1H), 3.93 – 3.98 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.60 – 3.70 (m, 2H), 3.41 – 3.51 (m, 2H), 3.11 (s, 1.5H), 3.06 (s, 1.5H), 2.67 – 2.72 (m, 2H), 2.55 – 2.61 (m, 1H), 2.39 (s, 3H), 2.18 – 2.26 (m, 1H), 2.14 (s, 3H), 2.12 (s, 3H), 1.25 – 1.36 (m, 1H), 0.63 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.78 – 7.83 (m, 1H), 7.50 – 7.57 (m, 2H), 7.12 (d, J = 8.7 Hz, 1H), 6.82 (s, 1H) ), 6.78 – 6.2 (m, 1H), 6.61 (t, J FH = 75.5 Hz, 1H), 5.23 – 5.45 (m, 2H), 4.56 – 4.60 (m, 1H), 3.93 – 3.98 (m, 1H) , 3.86 (d, J = 6.9 Hz, 2H), 3.60 – 3.70 (m, 2H), 3.41 – 3.51 (m, 2H), 3.11 (s, 1.5H), 3.06 (s, 1.5H), 2.67 – 2.72 (m, 2H), 2.55 – 2.61 (m, 1H), 2.39 (s, 3H), 2.18 – 2.26 (m, 1H), 2.14 (s, 3H), 2.12 (s, 3H), 1.25 – 1.36 (m , 1H), 0.63 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 589.30 [M+H]+ .MS (ESI, pos.ion) m/z: 589.30 [M+H] + .
實施例125:化合物 (2R )-(6-(甲基(丙基)氨甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 125: Compound ( 2R )-(6-(methyl(propyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethoxy) yl)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-(甲基(丙基)氨基甲醯基)-2-吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-(methyl(propyl)carbamoyl)-2-picolinate methyl ester
取化合物吡啶二羧酸單甲酯 (1.00 g , 5.5 mmol) 於50 mL單口瓶,加入35 mL二氯甲烷,攪拌,溶解,再室溫加入N ,N -甲基丙基胺 (0.70 g, 9.4 mmol) 以及N -羥基-7-氮雜苯並三氮唑 (1.50 g, 11 mmol),之後冰浴下依次加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (3.23 g, 16.7 mmol) 和N ,N -二異丙基乙胺(5.73 g , 44 mmol),最後室溫攪拌7 h,停止反應,水洗3次 (30 mL × 3),無水Na2 SO4 乾燥,濃縮,進行矽膠柱層析分離 (梯度洗脫, 洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3:1, 2:1),得到淡黃色油狀物1.21 g,收率92.8%。The compound pyridinedicarboxylate monomethyl ester (1.00 g, 5.5 mmol) was taken in a 50 mL single-neck flask, 35 mL of dichloromethane was added, stirred and dissolved, and N , N -methylpropylamine (0.70 g, 9.4 mmol) and N -hydroxy-7-azabenzotriazole (1.50 g, 11 mmol), followed by 1-ethyl-(3-dimethylaminopropyl)carbodiimide under ice bath Amine hydrochloride (3.23 g, 16.7 mmol) and N , N -diisopropylethylamine (5.73 g, 44 mmol), and finally stirred at room temperature for 7 h to stop the reaction, washed with water 3 times (30 mL × 3), It was dried over anhydrous Na 2 SO 4 , concentrated, and separated by silica gel column chromatography (gradient elution, eluent: petroleum ether/ethyl acetate (v/v) = 3:1, 2:1) to obtain pale yellow oil The compound was 1.21 g, and the yield was 92.8%.
1 H-NMR (400 MHz, CDCl3 ) δ (ppm): 8.17 (t,J = 8.1 Hz, 1H), 7.96 (t,J = 8.0 Hz, 1H), 7.85 (t,J = 8.1 Hz, 1H), 4.03 (s, 3H), 3.53 (t,J = 8.1 Hz ,1H), 3.41 (t,J = 8.1 Hz, 1H), 3.12 (d,J = 8.1 Hz, 3H), 1.80 – 1.70 (m, 2H),1.01 (t,J = 8.1 Hz, 1H), 0.81 (t,J = 8.1 Hz ,2H). 1 H-NMR (400 MHz, CDCl 3 ) δ (ppm): 8.17 (t, J = 8.1 Hz, 1H), 7.96 (t, J = 8.0 Hz, 1H), 7.85 (t, J = 8.1 Hz, 1H) ), 4.03 (s, 3H), 3.53 (t, J = 8.1 Hz, 1H), 3.41 (t, J = 8.1 Hz, 1H), 3.12 (d, J = 8.1 Hz, 3H), 1.80 – 1.70 (m , 2H), 1.01 (t, J = 8.1 Hz, 1H), 0.81 (t, J = 8.1 Hz, 2H).
MS (ESI, pos.ion) m/z: 237.20 [M+H]+ .MS (ESI, pos.ion) m/z: 237.20 [M+H] + .
步驟2:化合物6-羥甲基-N ,N -甲基丙基-2-吡啶甲醯胺的合成Step 2: Synthesis of compound 6-hydroxymethyl- N , N -methylpropyl-2-picolinamide
取化合物6-(甲基(丙基)氨基甲醯基)-2-吡啶甲酸甲酯 (0.60 g, 2.5 mmol) 於100 mL單口瓶,加入10 mL無水四氫呋喃,之後冰浴下加入硼氫化鋰 (0.15 g, 6.9 mmol),室溫攪拌5 h,停止反應,冰浴下加入20 mL飽和氯化鈉溶液,乙酸乙酯萃取 (15 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離(梯度洗脫, 洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1:1, 1:2),得到淺青色油狀物290 mg,收率55%。Take the compound 6-(methyl(propyl)carbamoyl)-2-picolinate methyl ester (0.60 g, 2.5 mmol) in a 100 mL single-neck flask, add 10 mL of anhydrous tetrahydrofuran, and then add lithium borohydride under an ice bath (0.15 g, 6.9 mmol), stirred at room temperature for 5 h, stopped the reaction, added 20 mL of saturated sodium chloride solution under ice bath, extracted with ethyl acetate (15 mL × 3), dried over anhydrous sodium sulfate, concentrated, and carried out a silica gel column Chromatographic separation (gradient elution, eluent: petroleum ether/ethyl acetate (v/v) = 1:1, 1:2) gave 290 mg of light cyan oil with a yield of 55%.
1 H-NMR (400 MHz, CDCl3 ) δ (ppm): 7.79 (t,J = 8.1 Hz, 1H), 7.49 (t,J = 8.0 Hz, 1H), 7.32 (t,J = 8.1 Hz, 1H), 4.79 (s, 2H), 3.54 (t,J = 8.1 Hz ,1H), 3.28 (t,J = 8.1 Hz, 1H), 3.12 (s, 2H), 3.01 (s, 1H),1.8-1.6 (m, 2H), 1.01 (t,J = 8.1 Hz ,1H), 0.80 (t,J = 8.1 Hz ,2H). 1 H-NMR (400 MHz, CDCl 3 ) δ (ppm): 7.79 (t, J = 8.1 Hz, 1H), 7.49 (t, J = 8.0 Hz, 1H), 7.32 (t, J = 8.1 Hz, 1H) ), 4.79 (s, 2H), 3.54 (t, J = 8.1 Hz, 1H), 3.28 (t, J = 8.1 Hz, 1H), 3.12 (s, 2H), 3.01 (s, 1H), 1.8-1.6 (m, 2H), 1.01 (t, J = 8.1 Hz, 1H), 0.80 (t, J = 8.1 Hz, 2H).
MS (ESI, pos.ion) m/z: 209.20 [M+H]+ .MS (ESI, pos.ion) m/z: 209.20 [M+H] + .
步驟3:化合物 (2R )-(6-(甲基(丙基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 3: Compound ( 2R )-(6-(methyl(propyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethoxy) )-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
取化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (120 mg , 0.32 mmol) 於50 mL單口瓶,加入10 mL二氯甲烷,攪拌,溶解,在室溫加入6-羥甲基-N ,N -甲基丙基-2-吡啶甲醯胺 (80 mg,0.40 mmol) 以及N -羥基-7-氮雜苯並三氮唑 (80 mg, 0.60 mmol),之後冰浴下依次加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (210 mg,1.10 mmol) 和N ,N -二異丙基乙胺 (300 mg , 2.30 mmol),最後室溫攪拌5.5 h,停止反應,水洗3次 (30 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (梯度洗脫, 洗脫劑:二氯甲烷/甲醇 ( v/v ) = 7:1,6:1),得淡黃色油狀物147.6 mg,收率81%。Take compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (120 mg, 0.32 mmol) in a 50 mL single-neck flask, add 10 mL of dichloromethane, stir, dissolve, and add 6-hydroxymethyl- N , N -methylpropyl-2-picolinamide (80 mg, 0.40 mmol) at room temperature mmol) and N -hydroxy-7-azabenzotriazole (80 mg, 0.60 mmol), followed by the addition of 1-ethyl-(3-dimethylaminopropyl)carbodiimide under ice bath Hydrochloride (210 mg, 1.10 mmol) and N , N -diisopropylethylamine (300 mg, 2.30 mmol), finally stirred at room temperature for 5.5 h to stop the reaction, washed with water 3 times (30 mL × 3), anhydrous dried over sodium sulfate, concentrated, and separated by silica gel column chromatography (gradient elution, eluent: dichloromethane/methanol (v/v) = 7:1, 6:1) to obtain 147.6 mg of pale yellow oil, Yield 81%.
1 H-NMR (400 MHz, CDCl3 ) δ (ppm): 7.87-7.76 (m , 1H), 7.58-7.50 (m , 2H), 7.18-7.10 (m, 1H), 6.88-6.83 (m, 2H), 6.67 (t,J F-H = 75.6 Hz, 1H), 5.47-5.42 (m, 1H), 5.34-5.28 (m, 2H), 4.65-4.56 (m, 1H), 3.98 (t,J = 8.0 Hz, 1H), 3.92-3.85 (m, 2H), 3.65(t,J = 8.1 Hz ,1H), 3.55-3.40 (m, 2H), 3.33-3.25 (m, 1H), 3.15-3.06 (m, 2H), 3.02-2.97 (m, 1H), 2.78-2.68 (m, 1H), 2.18-2.12 (m, 3H), 1.36-1.24 (m, 3H), 0.99 (t,J = 8.0 Hz, 1H), 0.79 (t,J = 8.0 Hz, 2H), 0.71-0.64 (m, 2H), 0.41-0.33 (m, 2H). 1 H-NMR (400 MHz, CDCl 3 ) δ (ppm): 7.87-7.76 (m , 1H), 7.58-7.50 (m , 2H), 7.18-7.10 (m, 1H), 6.88-6.83 (m, 2H) ), 6.67 (t, J FH = 75.6 Hz, 1H), 5.47-5.42 (m, 1H), 5.34-5.28 (m, 2H), 4.65-4.56 (m, 1H), 3.98 (t, J = 8.0 Hz , 1H), 3.92-3.85 (m, 2H), 3.65(t, J = 8.1 Hz ,1H), 3.55-3.40 (m, 2H), 3.33-3.25 (m, 1H), 3.15-3.06 (m, 2H) ), 3.02-2.97 (m, 1H), 2.78-2.68 (m, 1H), 2.18-2.12 (m, 3H), 1.36-1.24 (m, 3H), 0.99 (t, J = 8.0 Hz, 1H), 0.79 (t, J = 8.0 Hz, 2H), 0.71-0.64 (m, 2H), 0.41-0.33 (m, 2H).
MS (ESI, pos.ion) m/z: 560.20 [M+H]+ .MS (ESI, pos.ion) m/z: 560.20 [M+H] + .
實施例126:化合物(2R )-(6-二甲基氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 126: Compound ( 2R )-(6-dimethylaminocarbamoyl)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy)-4 -(Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-(二甲基氨基甲醯基)-2-吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-(dimethylaminocarboxy)-2-picolinate methyl ester
取化合物吡啶二羧酸單甲酯 (1.02 g , 5.63 mmol) 於100 mL單口瓶,加入40 mL二氯甲烷,攪拌,溶解,再室溫加入鹽酸二甲胺 (2.49 g, 9.4 mmol) 以及N -羥基-7-氮雜苯並三氮唑 (1.50 g, 11 mmol),之後冰浴下依次加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (3.23 g, 16.7 mmol) 和N ,N -二異丙基乙胺(5.73 g, 44 mmol),最後室溫攪拌5 h,停止反應,水洗3次 (30 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (梯度洗脫, 洗脫劑:石油醚/乙酸乙酯 ( v/ v) =2:1, 1:1),得到淡黃色油狀物1.05 g,收率89.6%。Take the compound pyridinedicarboxylate monomethyl ester (1.02 g, 5.63 mmol) in a 100 mL single-neck flask, add 40 mL of dichloromethane, stir, dissolve, and then add dimethylamine hydrochloride (2.49 g, 9.4 mmol) and N at room temperature. -Hydroxy-7-azabenzotriazole (1.50 g, 11 mmol), followed by the addition of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 3.23 g, 16.7 mmol) and N , N -diisopropylethylamine (5.73 g, 44 mmol), and finally stirred at room temperature for 5 h to stop the reaction, washed with water 3 times (30 mL × 3), dried over anhydrous sodium sulfate, Concentrated and separated by silica gel column chromatography (gradient elution, eluent: petroleum ether/ethyl acetate (v/v) = 2:1, 1:1) to obtain 1.05 g of pale yellow oil, yield 89.6 %.
1 H-NMR (400 MHz, CDCl3 ) δ (ppm): 8.22-8.17 (m, 1H), 7.98 (t,J = 8.0 Hz, 1H), 7.90-7.85 (m, 1H), 4.03 (s, 3H), 3.19-3.13 (m, 5H), 3.02-2.88 (m, 1H). 1 H-NMR (400 MHz, CDCl 3 ) δ (ppm): 8.22-8.17 (m, 1H), 7.98 (t, J = 8.0 Hz, 1H), 7.90-7.85 (m, 1H), 4.03 (s, 3H), 3.19-3.13 (m, 5H), 3.02-2.88 (m, 1H).
MS (ESI, pos.ion) m/z: 209.10 [M+H]+ .MS (ESI, pos.ion) m/z: 209.10 [M+H] + .
步驟2:化合物6-羥甲基-N ,N -二甲基-2-吡啶甲醯胺的合成Step 2: Synthesis of compound 6-hydroxymethyl- N , N -dimethyl-2-picolinamide
取化合物6-(二甲基氨基甲醯基)-2-吡啶甲酸甲酯 (0.52 g, 2.5 mmol) 於25 mL單口瓶,加入6 mL無水四氫呋喃,之後冰浴下加入硼氫化鋰 (0.11 g, 5.0 mmol),室溫攪拌3 h,停止反應,冰浴下加入20 mL飽和氯化鈉溶液,乙酸乙酯萃取 (15 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (梯度洗脫, 洗脫劑:DCM/MeOH (v/v) = 39:1, 6:1),得到白色固體260 mg,收率58%。Take compound 6-(dimethylaminocarboxy)-2-picolinate methyl ester (0.52 g, 2.5 mmol) in a 25 mL single-neck flask, add 6 mL of anhydrous tetrahydrofuran, and then add lithium borohydride (0.11 g under ice bath) , 5.0 mmol), stirred at room temperature for 3 h, stopped the reaction, added 20 mL of saturated sodium chloride solution under ice bath, extracted with ethyl acetate (15 mL × 3), dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (gradient elution, eluent: DCM/MeOH (v/v) = 39:1, 6:1) to give a white solid 260 mg, yield 58%.
1 H-NMR (400 MHz, CDCl3 ) δ (ppm): 7.81 (t,J = 8.1 Hz, 1H), 7.55-7.50 (m, 1H), 7.37-7.30 (m, 1H), 4.80 (s, 2H), 3.17 (s, 3H), 3.07 (s, 1H). 1 H-NMR (400 MHz, CDCl 3 ) δ (ppm): 7.81 (t, J = 8.1 Hz, 1H), 7.55-7.50 (m, 1H), 7.37-7.30 (m, 1H), 4.80 (s, 2H), 3.17 (s, 3H), 3.07 (s, 1H).
MS (ESI, pos.ion) m/z: 181.25 [M+H]+ .MS (ESI, pos.ion) m/z: 181.25 [M+H] + .
步驟3:化合物 (2R )-(6-(二甲基)氨甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 3: Compound ( 2R )-(6-(dimethyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethoxy)- Synthesis of 4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
取化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (120 mg, 0.32 mmol) 於25 mL單口瓶,加入11 mL二氯甲烷,攪拌,溶解,再室溫加入6-羥甲基-N ,N -二甲基-2-吡啶甲醯胺 (60 mg, 0.33 mmol) 以及N -羥基-7-氮雜苯並三氮唑 (90 mg, 0.65 mmol),之後冰浴下依次加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (200 mg, 1.03 mmol) 和N ,N -二異丙基乙胺 (400 mg, 3.03 mmol),最後室溫攪拌17 h,停止反應,水洗3次 (30 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (梯度洗脫,洗脫劑:二氯甲烷/甲醇 ( v/v ) = 11:1,10:1,9:1),得到淡黃色油狀物78.9mg,收率44.6%。Take compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (120 mg, 0.32 mmol) in a 25 mL single-neck flask, add 11 mL of dichloromethane, stir, dissolve, and then add 6-hydroxymethyl- N , N -dimethyl-2-picolinamide (60 mg, 0.33 mmol at room temperature) ) and N -hydroxy-7-azabenzotriazole (90 mg, 0.65 mmol), followed by the addition of 1-ethyl-(3-dimethylaminopropyl)carbodiimide salt under ice bath acid (200 mg, 1.03 mmol) and N , N -diisopropylethylamine (400 mg, 3.03 mmol), and finally stirred at room temperature for 17 h to stop the reaction, washed with water 3 times (30 mL × 3), anhydrous sulfuric acid Dry over sodium, concentrate, and separate by silica gel column chromatography (gradient elution, eluent: dichloromethane/methanol (v/v) = 11:1, 10:1, 9:1) to give a pale yellow oil 78.9 mg, yield 44.6%.
1 H-NMR (400 MHz, CDCl3 ) δ (ppm): 7.84 (t,J = 8.1 Hz, 1H), 7.61-7.52 (m, 2H), 7.18-7.12 (m, 1H), 6.88-6.83 (m, 2H), 6.63 (t,J F-H = 75.6 Hz, 1H), 5.47-5.42 (m, 1H), 5.34-5.28 (m, 2H), 4.65-4.56 (m, 1H), 4.02-3.96 (m, 1H), 3.92-3.85 (m, 2H), 3.68-3.62(m, 1H), 3.13 (s, 3H), 3.06 (s, 3H), 2.18-2.12 (m, 3H), 2.04-2.00 (m, 1H), 0.94-0.85 (m, 2H), 0.71-0.64 (m, 2H), 0.41-0.33 (m, 2H). 1 H-NMR (400 MHz, CDCl 3 ) δ (ppm): 7.84 (t, J = 8.1 Hz, 1H), 7.61-7.52 (m, 2H), 7.18-7.12 (m, 1H), 6.88-6.83 ( m, 2H), 6.63 (t, J FH = 75.6 Hz, 1H), 5.47-5.42 (m, 1H), 5.34-5.28 (m, 2H), 4.65-4.56 (m, 1H), 4.02-3.96 (m , 1H), 3.92-3.85 (m, 2H), 3.68-3.62(m, 1H), 3.13 (s, 3H), 3.06 (s, 3H), 2.18-2.12 (m, 3H), 2.04-2.00 (m , 1H), 0.94-0.85 (m, 2H), 0.71-0.64 (m, 2H), 0.41-0.33 (m, 2H).
MS (ESI, pos.ion) m/z: 532.20 [M+H]+ .MS (ESI, pos.ion) m/z: 532.20 [M+H] + .
實施例127:化合物 (2R )-(6-((氰甲基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 127: Compound ( 2R )-(6-((cyanomethyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropyl) methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物2-(甲基氨基)乙腈鹽酸鹽的合成Step 1: Synthesis of compound 2-(methylamino)acetonitrile hydrochloride
將化合物叔丁基(氰甲基)(甲基)氨基甲酸酯 (300 mg, 0.066 mmol) 溶解在二氯甲烷 (5 mL) 溶液中,加入HCl的乙酸乙酯溶液 (4 M, 8 mL),室溫反應30 min,減壓濃縮,得到淺褐色固體176 mg,收率93%。Compound tert-butyl(cyanomethyl)(methyl)carbamate (300 mg, 0.066 mmol) was dissolved in dichloromethane (5 mL) solution, HCl in ethyl acetate (4 M, 8 mL) was added ), reacted at room temperature for 30 min, and concentrated under reduced pressure to obtain 176 mg of a light brown solid with a yield of 93%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 4.34 (s, 1H), 3.84 (s, 1H), 2.87 (s, 1.5H), 2.75 (s, 1.5H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 4.34 (s, 1H), 3.84 (s, 1H), 2.87 (s, 1.5H), 2.75 (s, 1.5H).
MS (ESI, pos.ion) m/z: 70.10 [M-HCl+H]+ .MS (ESI, pos.ion) m/z: 70.10 [M-HCl+H] + .
步驟2:化合物6-((氰甲基)(甲基)氨基甲醯基)吡啶甲酸甲酯的合成Step 2: Synthesis of compound 6-((cyanomethyl)(methyl)carbamoyl)picolinate methyl ester
將化合物2,6-吡啶二羧酸單甲酯 (300 mg, 1.66 mmol),2-(甲基氨基)乙腈鹽酸鹽(172 mg, 1.61 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (634 mg, 3.31 mmol) 和N -羥基-7-氮雜苯並三氮唑 (333 mg, 2.45 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (624 mg, 4.83 mmol),室溫反應6 h,加水 (20 mL × 2) 洗,分離有機相,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到淺褐色液體121 mg,收率31%。Compound 2,6-pyridinedicarboxylate monomethyl ester (300 mg, 1.66 mmol), 2-(methylamino)acetonitrile hydrochloride (172 mg, 1.61 mmol), 1-ethyl-(3-dimethyl aminopropyl)carbodiimide hydrochloride (634 mg, 3.31 mmol) and N -hydroxy-7-azabenzotriazole (333 mg, 2.45 mmol) in dichloromethane (10 mL) , cooled to 0°C, added N , N -diisopropylethylamine (624 mg, 4.83 mmol), reacted at room temperature for 6 h, added water (20 mL × 2) to wash, separated the organic phase, and dried over anhydrous sodium sulfate , concentrated under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 121 mg of light brown liquid with a yield of 31%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.24 – 8.27 (m, 1H), 8.16 – 8.18 (m, 0.5H), 7.99 – 8.07 (m, 1.5H), 4.95 (s, 0.8H), 4.52 (s, 1.2H), 4.03 (s, 3H), 3.38 (s, 1.8H), 3.29 (s, 1.2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.24 – 8.27 (m, 1H), 8.16 – 8.18 (m, 0.5H), 7.99 – 8.07 (m, 1.5H), 4.95 (s, 0.8H) ), 4.52 (s, 1.2H), 4.03 (s, 3H), 3.38 (s, 1.8H), 3.29 (s, 1.2H).
MS (ESI, pos.ion) m/z: 234.05 [M+H]+ .MS (ESI, pos.ion) m/z: 234.05 [M+H] + .
步驟3:化合物N -(氰甲基)-6-(羥甲基)-N -甲基吡啶醯胺的合成Step 3: Synthesis of Compound N- (cyanomethyl)-6-(hydroxymethyl) -N -picolinamide
將化合物6-((氰甲基)(甲基)氨基甲醯基)吡啶甲酸甲酯(115 mg, 0.49 mmol) 溶於甲醇 (5 mL)中,冰浴下加入硼氫化鈉 (149 mg, 3.94 mmol),室溫反應2.5 h後停止,減壓濃縮,濃縮液進行矽膠柱分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 15/1),得到無色液體29 mg,收率28%。Compound 6-((cyanomethyl)(methyl)carbamoyl)picolinate methyl ester (115 mg, 0.49 mmol) was dissolved in methanol (5 mL), and sodium borohydride (149 mg, 3.94 mmol), the reaction was stopped after 2.5 h at room temperature, concentrated under reduced pressure, and the concentrated solution was subjected to silica gel column separation (eluent: dichloromethane/methanol (v/v) = 15/1) to obtain 29 mg of a colorless liquid, which was collected rate of 28%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.86 – 7.91 (m, 1H), 7.81 (d,J = 7.5, Hz, 0.4H), 7.67 (d,J = 7.6, Hz, 0.6H), 7.44 (t,J = 8.6, Hz, 1H), 4.85 (d,J = 13.0, Hz, 2H), 4.55 (d,J = 8.5, Hz, 2H), 3.32 (br.s, 1H), 3.28 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.86 – 7.91 (m, 1H), 7.81 (d, J = 7.5, Hz, 0.4H), 7.67 (d, J = 7.6, Hz, 0.6H) ), 7.44 (t, J = 8.6, Hz, 1H), 4.85 (d, J = 13.0, Hz, 2H), 4.55 (d, J = 8.5, Hz, 2H), 3.32 (br.s, 1H), 3.28 (s, 3H).
MS (ESI, pos.ion) m/z: 206.20 [M+H]+ .MS (ESI, pos.ion) m/z: 206.20 [M+H] + .
步驟4:化合物 (2R )-(6-((氰甲基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 4: Compound ( 2R )-(6-((cyanomethyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropyl) Synthesis of Methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (50 mg, 0.14 mmol),N -(氰甲基)-6-(羥甲基)-N -甲基吡啶醯胺 (23 mg, 0.11 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (107 mg, 0.56 mmol) 和N -羥基-7-氮雜苯並三氮唑 (32 mg, 0.24 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (78 mg, 0.60 mmol),室溫反應14 h,加水 (15 mL) 攪拌5 min,二氯甲烷萃取 (10 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 54/1),得到淺褐色黏稠固體16 mg,收率25%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (50 mg, 0.14 mmol), N- (cyanomethyl)-6-(hydroxymethyl) -N -picolinamide (23 mg, 0.11 mmol), 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (107 mg, 0.56 mmol) and N -hydroxy-7-azabenzotriazole (32 mg, 0.24 mmol) were dissolved in dichloromethane (10 mL) and cooled to 0 ℃, N , N -diisopropylethylamine (78 mg, 0.60 mmol) was added, the reaction was carried out at room temperature for 14 h, water (15 mL) was added, stirred for 5 min, extracted with dichloromethane (10 mL × 2), and the mixture was washed with anhydrous sulfuric acid. The organic phase was dried over sodium, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 54/1) to obtain 16 mg of light brown viscous solid, yield 25% .
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.82 – 7.94 (m, 1.5H), 7.58 – 7.70 (m, 1.5H), 7.12 (d,J = 7.6 Hz, 1H), 6.82 (s, 2H), 6.61 (t,J F-H = 75.5 Hz, 1H), 5.43 – 5.51 (m, 1H), 5.22 – 5.30 (m, 1H), 4.56 – 4.76 (m, 2H), 4.43 – 4.51 (m, 1H), 3.90 – 3.99 (m, 1H), 3.86 (d,J = 6.0 Hz, 2H), 3.60 – 3.65 (m, 1H), 3.37 – 3.51 (m, 1H), 3.24 (d,J = 11.4 Hz, 3H), 2.66 – 2.77 (m, 1H), 2.11 (s, 3H), 1.97 – 2.05 (m, 1H), 1.25 – 1.35 (m, 1H), 0.57 – 0.69 (m, 2H), 0.30 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.82 – 7.94 (m, 1.5H), 7.58 – 7.70 (m, 1.5H), 7.12 (d, J = 7.6 Hz, 1H), 6.82 (s , 2H), 6.61 (t, J FH = 75.5 Hz, 1H), 5.43 – 5.51 (m, 1H), 5.22 – 5.30 (m, 1H), 4.56 – 4.76 (m, 2H), 4.43 – 4.51 (m, 1H), 3.90 – 3.99 (m, 1H), 3.86 (d, J = 6.0 Hz, 2H), 3.60 – 3.65 (m, 1H), 3.37 – 3.51 (m, 1H), 3.24 (d, J = 11.4 Hz , 3H), 2.66 – 2.77 (m, 1H), 2.11 (s, 3H), 1.97 – 2.05 (m, 1H), 1.25 – 1.35 (m, 1H), 0.57 – 0.69 (m, 2H), 0.30 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 557.20 [M+H]+ .MS (ESI, pos.ion) m/z: 557.20 [M+H] + .
實施例128:化合物 (2R )-(6-((2-甲氧基乙基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 128: Compound ( 2R )-(6-((2-methoxyethyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetoxy-4-(3 -(Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-((2-甲氧基乙基)氨基甲醯基)吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-((2-methoxyethyl)carbamoyl)picolinate methyl ester
將化合物2,6-吡啶二羧酸單甲酯 (500 mg, 2.76 mmol),2-甲氧基乙胺 (402 mg, 5.35 mmol) 溶於二氯甲烷 (20 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (563 mg, 4.14 mmol),在冰浴中加加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (1.1 g, 5.70 mmol) 和N ,N -二異丙基乙胺 (890 mg, 6.89 mmol),室溫反應5 h,有機相用水洗 (20 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,剩餘物用矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到白色固體598 mg,收率91%。The compound 2,6-pyridinedicarboxylate monomethyl ester (500 mg, 2.76 mmol), 2-methoxyethylamine (402 mg, 5.35 mmol) was dissolved in dichloromethane (20 mL), N -hydroxyl was added -7-Azabenzotriazole (563 mg, 4.14 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.1 g) was added in an ice bath , 5.70 mmol) and N , N -diisopropylethylamine (890 mg, 6.89 mmol), reacted at room temperature for 5 h, the organic phase was washed with water (20 mL × 2), the organic phase was dried with anhydrous sodium sulfate, and the pressure was reduced. Concentrated, and the residue was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/1) to obtain 598 mg of white solid with a yield of 91%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.42 (br.s, 1H), 8.39 (d,J = 7.8 Hz, 1H), 8.23 (d,J = 7.8 Hz, 1H), 8.01 (t,J = 7.8 Hz, 1H), 4.02 (s, 3H), 3.69 – 3.73 (m, 2H), 3.61 (t,J = 5.2 Hz, 2H), 3.41 (s, 3H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.42 (br.s, 1H), 8.39 (d, J = 7.8 Hz, 1H), 8.23 (d, J = 7.8 Hz, 1H), 8.01 (t, J = 7.8 Hz, 1H), 4.02 (s, 3H), 3.69 – 3.73 (m, 2H), 3.61 (t, J = 5.2 Hz, 2H), 3.41 (s, 3H).
MS (ESI, pos.ion) m/z: 239.10 [M-HCl+H]+ .MS (ESI, pos.ion) m/z: 239.10 [M-HCl+H] + .
步驟2:化合物6-((2-甲氧基乙基)(甲基)氨基甲醯基)吡啶甲酸甲酯的合成Step 2: Synthesis of compound 6-((2-methoxyethyl)(methyl)carbamoyl)picolinate methyl ester
將化合物6-((2-甲氧基乙基)氨基甲醯基)吡啶甲酸甲酯 (585 mg, 2.46 mmol) 溶於無水N ,N -二甲基甲醯胺 (8 mL) 中,在冰浴中加入60%氫化鈉 (128 mg, 3.20 mmol),室溫反應1 h後加入碘甲烷 (1.1 g, 7.70 mmol),60℃反應8 h後停止反應,減壓除去溶劑,加水 (20 mL),水相用乙酸乙酯萃取 (10 mL × 3),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到淺褐色固體199 mg,收率32%。The compound 6-((2-methoxyethyl)carbamoyl)picolinate (585 mg, 2.46 mmol) was dissolved in anhydrous N , N -dimethylformamide (8 mL), in Add 60% sodium hydride (128 mg, 3.20 mmol) to an ice bath, react at room temperature for 1 h, add iodomethane (1.1 g, 7.70 mmol), react at 60 °C for 8 h, stop the reaction, remove the solvent under reduced pressure, add water (20 mL), the aqueous phase was extracted with ethyl acetate (10 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate) (v/v) = 2/1) to obtain 199 mg of a light brown solid with a yield of 32%.
MS (ESI, pos.ion) m/z: 253.10 [M+H]+ .MS (ESI, pos.ion) m/z: 253.10 [M+H] + .
步驟3:化合物6-(羥甲基)-N -(2-甲氧乙基)-N -甲基吡啶醯胺的合成Step 3: Compound 6- (hydroxymethyl) - N - (2- methoxyethyl) - N - Synthesis of Amides methylpyridine
將化合物6-((2-甲氧基乙基)(甲基)氨基甲醯基)吡啶甲酸甲酯 (195 mg, 0.77 mmol) 溶於甲醇 (5 mL) 中,冰浴中加入硼氫化鈉 (233 mg,6.16 mmol),室溫反應3.5 h後停止,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1),得到無色液體162 mg,收率93%。The compound 6-((2-methoxyethyl)(methyl)carbamoyl)picolinate (195 mg, 0.77 mmol) was dissolved in methanol (5 mL), and sodium borohydride was added to the ice bath (233 mg, 6.16 mmol), the reaction was stopped after 3.5 h at room temperature, concentrated under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 50/1) to obtain Colorless liquid 162 mg, yield 93%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.76 – 7.80 (m, 1H), 7.56 (d,J = 7.6 Hz, 0.6H), 7.50 (d,J = 7.6 Hz, 0.4H), 7.28 – 7.31 (m, 1H), 4.77 (s, 2H), 3.68 – 3.76 (m, 2H), 3.57 – 3.61 (m, 2H), 3.40 (s, 1.4H), 3.30 (s, 1.6H), 3.16 (s, 1.4H), 3.10 (s, 1.6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.76 – 7.80 (m, 1H), 7.56 (d, J = 7.6 Hz, 0.6H), 7.50 (d, J = 7.6 Hz, 0.4H), 7.28 – 7.31 (m, 1H), 4.77 (s, 2H), 3.68 – 3.76 (m, 2H), 3.57 – 3.61 (m, 2H), 3.40 (s, 1.4H), 3.30 (s, 1.6H), 3.16 (s, 1.4H), 3.10 (s, 1.6H).
MS (ESI, pos.ion) m/z: 225.20 [M+H]+ .MS (ESI, pos.ion) m/z: 225.20 [M+H] + .
步驟4:化合物 (2R )-(6-((2-甲氧基乙基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 4: Compound ( 2R )-(6-((2-methoxyethyl)(methyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3- Synthesis of (cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (107 mg, 0.29 mmol),6-(羥甲基)-N -(2-甲氧基乙基)-N -甲基吡啶醯胺 (11029-1) (50 mg, 0.22 mmol) 溶於二氯甲烷 (10 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (61 mg, 0.45 mmol),在冰浴中加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (214 mg, 1.12 mmol) 和N ,N -二異丙基乙胺 (144 mg, 1.11 mmol),室溫反應6 h,加水 (20 mL) 攪拌5 min,二氯甲烷萃取 (5 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 54/1),得到淺褐色黏稠固體78 mg,收率60%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (107 mg, 0.29 mmol), 6- (hydroxymethyl) - N - (2- methoxyethyl) - N - methylpyridine Amides (11029-1) (50 mg, 0.22 mmol) was dissolved in dichloromethane (10 mL), N -hydroxy-7-azabenzotriazole (61 mg, 0.45 mmol) was added, and 1-ethyl-(3-dimethylaminopropyl)carbodiimide was added in an ice bath Amine hydrochloride (214 mg, 1.12 mmol) and N , N -diisopropylethylamine (144 mg, 1.11 mmol), react at room temperature for 6 h, add water (20 mL), stir for 5 min, and extract with dichloromethane ( 5 mL × 2), the organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 54/1) to obtain light brown The viscous solid was 78 mg, the yield was 60%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.81 (t,J = 7.6 Hz, 1H), 7.49 – 7.56 (m, 2H), 7.12 (d,J = 8.3 Hz, 1H), 6.82 (s, 1H), 6.78 – 6.81 (m, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 5.24 – 5.44 (m, 2H), 4.56 – 4.61 (m, 1H), 3.93 – 3.98 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.65 – 3.72 (m, 2H), 3.62 (t,J = 10.4 Hz, 1H), 3.50 – 3.58 (m, 2H), 3.39 – 3.49 (m, 1H), 3.37 (s, 1.5H), 3.25 (s, 1.5H), 3.13 (s, 1.5H), 3.08 (s, 1.5H), 2.67 – 2.74 (m, 1H), 2.06 – 2.14 (m, 1H), 2.12 (s, 3H), 1.25 – 1.36 (m, 1H), 0.62 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.81 (t, J = 7.6 Hz, 1H), 7.49 – 7.56 (m, 2H), 7.12 (d, J = 8.3 Hz, 1H), 6.82 ( s, 1H), 6.78 – 6.81 (m, 1H), 6.60 (t, J FH = 75.5 Hz, 1H), 5.24 – 5.44 (m, 2H), 4.56 – 4.61 (m, 1H), 3.93 – 3.98 (m , 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.65 – 3.72 (m, 2H), 3.62 (t, J = 10.4 Hz, 1H), 3.50 – 3.58 (m, 2H), 3.39 – 3.49 ( m, 1H), 3.37 (s, 1.5H), 3.25 (s, 1.5H), 3.13 (s, 1.5H), 3.08 (s, 1.5H), 2.67 – 2.74 (m, 1H), 2.06 – 2.14 ( m, 1H), 2.12 (s, 3H), 1.25 – 1.36 (m, 1H), 0.62 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 576.20 [M+H]+ .MS (ESI, pos.ion) m/z: 576.20 [M+H] + .
實施例129:化合物 (2R )-(6-(甲基(2,2,2-三氟乙基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 129: Compound ( 2R )-(6-(methyl(2,2,2-trifluoroethyl)carbamoyl)pyridin-2-yl)methyl 1-acetoxyl-4-( 3-(Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-(甲基(2,2,2-三氟乙基)氨基甲醯基)吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-(methyl(2,2,2-trifluoroethyl)carbamoyl)picolinate methyl ester
將化合物2,6-吡啶二羧酸單甲酯 (400 mg, 2.21 mmol),N -甲基-2,2,2-三氟乙胺鹽酸鹽 (330 mg, 2.21 mmol) 溶於二氯甲烷 (15 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (450 mg, 3.31 mmol),在冰浴中加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (846 mg, 4.41 mmol) 和N ,N -二異丙基乙胺 (999 mg, 7.73 mmol),室溫反應3.5 h,有機相用水洗 (20 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,剩餘物用矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/1),得到無色液體436 mg,收率71%。The compound 2,6-pyridinedicarboxylate monomethyl ester (400 mg, 2.21 mmol), N -methyl-2,2,2-trifluoroethylamine hydrochloride (330 mg, 2.21 mmol) was dissolved in dichloro In methane (15 mL), N -hydroxy-7-azabenzotriazole (450 mg, 3.31 mmol) was added, and 1-ethyl-(3-dimethylaminopropyl)carbon was added in an ice bath Diimide hydrochloride (846 mg, 4.41 mmol) and N , N -diisopropylethylamine (999 mg, 7.73 mmol) were reacted at room temperature for 3.5 h, the organic phase was washed with water (20 mL × 2), The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/1) to obtain a colorless liquid 436 mg, yield 71%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 8.19 – 8.21 (m, 1H), 7.89 – 8.01 (m, 2H), 4.62 (q,J = 7.5, Hz, 1.2H), 4.21 (q,J = 9.0, Hz, 0.8H), 4.01 (s, 3H), 3.30 (s, 1.3H), 3.25 (s, 1.7H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 8.19 – 8.21 (m, 1H), 7.89 – 8.01 (m, 2H), 4.62 (q, J = 7.5, Hz, 1.2H), 4.21 ( q, J = 9.0, Hz, 0.8H), 4.01 (s, 3H), 3.30 (s, 1.3H), 3.25 (s, 1.7H).
MS (ESI, pos.ion) m/z: 277.20 [M +H]+ .MS (ESI, pos.ion) m/z: 277.20 [M +H] + .
步驟2:化合物6-(羥甲基)-N -甲基N -(2,2,2-三氟乙基)-吡啶醯胺的合成Step 2: Synthesis of compound 6-(hydroxymethyl) -N -methyl N- (2,2,2-trifluoroethyl)-pyridinamide
將化合物6-(甲基(2,2,2-三氟乙基)氨基甲醯基)吡啶甲酸甲酯 (430 mg, 1.56 mmol) 溶於甲醇 (5 mL) 中,冰浴中加入硼氫化鈉 (294 mg,7.78 mmol),室溫反應1.5 h後停止,減壓濃縮,濃縮液進行矽膠柱分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 66/1),得到無色液體310 mg,收率80%。The compound 6-(methyl(2,2,2-trifluoroethyl)carbamoyl)picolinate (430 mg, 1.56 mmol) was dissolved in methanol (5 mL), and hydroboration was added to the ice bath Sodium (294 mg, 7.78 mmol), the reaction was stopped after 1.5 h at room temperature, concentrated under reduced pressure, and the concentrated solution was subjected to silica gel column separation (eluent: dichloromethane/methanol (v/v) = 66/1) to obtain colorless Liquid 310 mg, yield 80%.
H NMR (400 MHz, CDCl3 ) δ (ppm): 7.80 – 7.85 (m, 1H), 7.55 – 7.64 (m, 1H), 7.37 (d,J = 8.8, Hz, 1H), 4.79 (d,J = 4.3, Hz, 2H), 4.32 (q,J = 8.6, Hz, 1H), 4.22 (q,J = 8.9, Hz, 1H), 3.24 (s, 1.5H), 3.19 (s, 1.5H).H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.80 – 7.85 (m, 1H), 7.55 – 7.64 (m, 1H), 7.37 (d, J = 8.8, Hz, 1H), 4.79 (d, J = 4.3, Hz, 2H), 4.32 (q, J = 8.6, Hz, 1H), 4.22 (q, J = 8.9, Hz, 1H), 3.24 (s, 1.5H), 3.19 (s, 1.5H).
MS (ESI, pos.ion) m/z: 249.20 [M+H]+ .MS (ESI, pos.ion) m/z: 249.20 [M+H] + .
步驟3:化合物 (2R )-(6-(甲基(2,2,2-三氟乙基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 3: Compound ( 2R )-(6-(methyl(2,2,2-trifluoroethyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3 Synthesis of -(Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (97 mg, 0.26 mmol),6-(羥甲基)-N -甲基-N -(2,2,2-三氟乙基)-吡啶醯胺 (50 mg, 0.20 mmol) 溶於二氯甲烷 (10 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (61 mg, 0.45 mmol),在冰浴中加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (214 mg, 1.12 mmol) 和N ,N -二異丙基乙胺 (144 mg, 1.11 mmol),室溫反應11 h,加水 (20 mL) 攪拌5 min,二氯甲烷萃取 (5 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 54/1),得到淺褐色黏稠固體68 mg,收率56%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (97 mg, 0.26 mmol), 6-(hydroxymethyl) -N -methyl- N- (2,2,2-trifluoroethyl)-pyridinamide (50 mg, 0.20 mmol) in dichloromethane (10 mL) ), N -hydroxy-7-azabenzotriazole (61 mg, 0.45 mmol) was added, and 1-ethyl-(3-dimethylaminopropyl)carbodiimide was added in an ice bath Hydrochloride (214 mg, 1.12 mmol) and N , N -diisopropylethylamine (144 mg, 1.11 mmol), react at room temperature for 11 h, add water (20 mL), stir for 5 min, and extract with dichloromethane (5 mL × 2), the organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 54/1) to obtain a light brown viscous Solid 68 mg, yield 56%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.83 – 7.88 (m, 1H), 7.69 – 7.71 (m, 0.5H), 7.55 – 7.59 (m, 1.5H), 7.12 (d,J = 8.5 Hz, 1H), 6.82 (s, 1H), 6.75 – 6.81 (m, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 5.38 – 5.46 (m, 1H), 5.22 – 5.29 (m, 15H), 4.56 – 4.60 (m, 1H), 4.42 – 4.48 (m, 1H), 4.15 – 4.22 (m, 1H), 3.93 – 3.98 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.63 (t,J = 10.4 Hz, 1H), 3.39 – 3.49 (m, 1H), 3.19 – 3.21 (m, 3H), 2.66 – 2.74 (m, 1H), 2.05 – 2.14 (m, 1H), 2.12 (s, 3H), 1.25 – 1.36 (m, 1H), 0.63 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.83 – 7.88 (m, 1H), 7.69 – 7.71 (m, 0.5H), 7.55 – 7.59 (m, 1.5H), 7.12 (d, J = 8.5 Hz, 1H), 6.82 (s, 1H), 6.75 – 6.81 (m, 1H), 6.60 (t, J FH = 75.5 Hz, 1H), 5.38 – 5.46 (m, 1H), 5.22 – 5.29 (m, 15H), 4.56 – 4.60 (m, 1H), 4.42 – 4.48 (m, 1H), 4.15 – 4.22 (m, 1H), 3.93 – 3.98 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H) , 3.63 (t, J = 10.4 Hz, 1H), 3.39 – 3.49 (m, 1H), 3.19 – 3.21 (m, 3H), 2.66 – 2.74 (m, 1H), 2.05 – 2.14 (m, 1H), 2.12 (s, 3H), 1.25 – 1.36 (m, 1H), 0.63 – 0.67 (m, 2H), 0.33 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 600.20 [M+H]+ .MS (ESI, pos.ion) m/z: 600.20 [M+H] + .
實施例130:化合物 (2R )-(6-(嗎啉-4-羰基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 130: Compound ( 2R )-(6-(morpholine-4-carbonyl)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy)-4 -(Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-(嗎啉-4-羰基)-2-吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-(morpholine-4-carbonyl)-2-picolinate methyl ester
取化合物吡啶二羧酸單甲酯 (1.04 g, 5.74 mmol) 於50 mL單口瓶,加入32 mL二氯甲烷,攪拌,溶解,在室溫加入嗎啉 (0.82 g, 9.4 mmol) 以及N -羥基-7-氮雜苯並三氮唑 (1.50 g, 10.8 mmol),之後冰浴下依次加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (3.21 g, 16.6 mmol) 和N ,N -二異丙基乙胺 (5.78 g, 44.6 mmol),最後室溫攪拌3 h,停止反應,水洗 (30 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (梯度洗脫, 洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2:1, 1:1),得白色固體1.30 g,收率90.4%。Take the compound pyridinedicarboxylate monomethyl ester (1.04 g, 5.74 mmol) in a 50 mL single-necked flask, add 32 mL of dichloromethane, stir, dissolve, add morpholine (0.82 g, 9.4 mmol) and N -hydroxyl at room temperature -7-Azabenzotriazole (1.50 g, 10.8 mmol), followed by the addition of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (3.21 g) in an ice bath , 16.6 mmol) and N , N -diisopropylethylamine (5.78 g, 44.6 mmol), and finally stirred at room temperature for 3 h to stop the reaction, washed with water (30 mL × 3), dried over anhydrous sodium sulfate, concentrated, and subjected to silica gel Column chromatography separation (gradient elution, eluent: petroleum ether/ethyl acetate (v/v) = 2:1, 1:1) gave 1.30 g of a white solid with a yield of 90.4%.
1 H-NMR (400 MHz, CDCl3 ) δ (ppm): 8.23-8.18(m, 1H), 8.00 (t,J = 8.0 Hz, 1H), 7.96-7.91 (m, 1H), 4.03 (s, 3H), 3.85 (s, 4H), 3.75 (s, 4H). 1 H-NMR (400 MHz, CDCl 3 ) δ (ppm): 8.23-8.18 (m, 1H), 8.00 (t, J = 8.0 Hz, 1H), 7.96-7.91 (m, 1H), 4.03 (s, 3H), 3.85 (s, 4H), 3.75 (s, 4H).
MS (ESI, pos.ion) m/z: 251.10 [M+H]+ .MS (ESI, pos.ion) m/z: 251.10 [M+H] + .
步驟2:化合物 (6-(羥甲基)吡啶基-2-基)(嗎啉基)甲酮的合成Step 2: Synthesis of Compound (6-(hydroxymethyl)pyridin-2-yl)(morpholinyl)methanone
取化合物6-(嗎啉-4-羰基)-2-吡啶甲酸甲酯 (0.71 g, 2.8 mmol) 於100 mL單口瓶,加入6 mL無水四氫呋喃,之後冰浴下加入硼氫化鋰 (0.14 g, 6.4 mmol),室溫攪拌5 h,停止反應,冰浴下加入20 mL飽和NaCl溶液,乙酸乙酯萃取 (15 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (梯度洗脫, 洗脫劑:二氯甲烷/甲醇 ( v/v ) = 12:1, 11:1),得白色固體209 mg,收率33%。The compound 6-(morpholine-4-carbonyl)-2-picolinate methyl ester (0.71 g, 2.8 mmol) was taken into a 100 mL single-neck flask, 6 mL of anhydrous tetrahydrofuran was added, and then lithium borohydride (0.14 g, 6.4 mmol), stirred at room temperature for 5 h, stopped the reaction, added 20 mL of saturated NaCl solution in an ice bath, extracted with ethyl acetate (15 mL × 3), dried over anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (gradient washing). For removal, eluent: dichloromethane/methanol (v/v) = 12:1, 11:1) to obtain 209 mg of white solid, yield 33%.
MS (ESI, pos.ion) m/z: 223.10 [M+H]+ .MS (ESI, pos.ion) m/z: 223.10 [M+H] + .
步驟3:化合物 (2R )-(6-(嗎啉-4-羰基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 3: Compound ( 2R )-(6-(morpholine-4-carbonyl)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy)-4- Synthesis of (difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
取化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (96.9 mg, 0.26 mmol) 於25 mL單口瓶,加入10 mL二氯甲烷,攪拌,溶解,再室溫加入6-羥甲基-N ,N -甲基丙基-2-吡啶甲醯胺 (69.6 mg, 0.31 mmol) 以及N -羥基-7-氮雜苯並三氮唑 (77.1 mg, 0.56 mmol),之後冰浴下依次加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (159.0 mg, 0.82 mmol) 和N ,N -二異丙基乙胺 (285.0 mg , 2.20 mmol),最後室溫攪拌6 h,停止反應,水洗 (30 mL × 3),無水硫酸鈉乾燥,濃縮,進行矽膠柱層析分離 (梯度洗脫, 洗脫劑:二氯甲烷/甲醇 ( v/v ) = 7:1, 6:1),得淡黃色油狀物78.1 mg,收率51.9%。Take compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (96.9 mg, 0.26 mmol) in a 25 mL single-neck flask, add 10 mL of dichloromethane, stir, dissolve, and then add 6-hydroxymethyl- N , N -methylpropyl-2-picolinamide (69.6 mg, 0.31 mmol) at room temperature mmol) and N -hydroxy-7-azabenzotriazole (77.1 mg, 0.56 mmol), followed by the addition of 1-ethyl-(3-dimethylaminopropyl)carbodiimide under ice bath Hydrochloride (159.0 mg, 0.82 mmol) and N , N -diisopropylethylamine (285.0 mg, 2.20 mmol), and finally stirred at room temperature for 6 h to stop the reaction, washed with water (30 mL × 3), anhydrous sodium sulfate dried, concentrated, and separated by silica gel column chromatography (gradient elution, eluent: dichloromethane/methanol (v/v) = 7:1, 6:1) to obtain pale yellow oil 78.1 mg, yield 51.9%.
1 H-NMR (400 MHz, CDCl3 ) δ (ppm): 7.89-7.82 (m , 1H), 7.62-7.58 (m , 1H),7.57-7.54 (m, 1H), 7.18-7.14 (m, 1H), 6.88-6.83 (m, 2H), 6.64 (t,J F-H = 75.6 Hz, 1H), 5.47-5.42 (m, 1H), 5.28-5.24 (m, 1H), 4.64-4.58 (m, 1H), 4.02-3.97 (m, 1H), 3.92-3.86 (m, 2H), 3.84-3.76(m, 4H), 3.72-3.67 (m, 2H), 3.65-3.60 (m, 2H), 2.76-2.71(m, 1H), 2.27-2.23 (m, 1H), 2.15 (s, 2H), 2.07-2.01 (m, 2H), 0.93-0.87 (m, 2H), 0.71-0.67 (m , 2H), 0.40-0.35 (m, 2H). 1 H-NMR (400 MHz, CDCl 3 ) δ (ppm): 7.89-7.82 (m, 1H), 7.62-7.58 (m, 1H), 7.57-7.54 (m, 1H), 7.18-7.14 (m, 1H) ), 6.88-6.83 (m, 2H), 6.64 (t, J FH = 75.6 Hz, 1H), 5.47-5.42 (m, 1H), 5.28-5.24 (m, 1H), 4.64-4.58 (m, 1H) , 4.02-3.97 (m, 1H), 3.92-3.86 (m, 2H), 3.84-3.76(m, 4H), 3.72-3.67 (m, 2H), 3.65-3.60 (m, 2H), 2.76-2.71( m, 1H), 2.27-2.23 (m, 1H), 2.15 (s, 2H), 2.07-2.01 (m, 2H), 0.93-0.87 (m, 2H), 0.71-0.67 (m , 2H), 0.40- 0.35 (m, 2H).
MS (ESI, pos.ion) m/z: 574.60 [M+H]+ .MS (ESI, pos.ion) m/z: 574.60 [M+H] + .
實施例131:化合物 (2R )-(6-(環己基(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 131: Compound ( 2R )-(6-(cyclohexyl(methyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethoxy) yl)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-(環己基(甲基)氨基甲醯基)吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-(cyclohexyl(methyl)carbamoyl)methyl picolinate
將化合物2,6-吡啶二羧酸單甲酯 (500 mg, 2.76 mmol),N -甲基環己胺 (374 mg, 3.30 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (1.30 g, 6.80 mmol) 和N -羥基- 7-氮雜苯並三氮唑 (753 mg, 5.53 mmol)溶於二氯甲烷 (20 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (1.5 mL, 8.10 mmol),室溫反應17.5 h,加水洗 (20 mL × 2) 有機相,分離有機相,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離(洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/2),得到淺褐色液體754 mg,收率98%。Compound 2,6-pyridinedicarboxylate monomethyl ester (500 mg, 2.76 mmol), N -methylcyclohexylamine (374 mg, 3.30 mmol), 1-ethyl-(3-dimethylaminopropyl) ) carbodiimide hydrochloride (1.30 g, 6.80 mmol) and N -hydroxy-7-azabenzotriazole (753 mg, 5.53 mmol) were dissolved in dichloromethane (20 mL) and cooled to 0 °C, N , N -diisopropylethylamine (1.5 mL, 8.10 mmol) was added, the reaction was carried out at room temperature for 17.5 h, the organic phase was washed with water (20 mL × 2), the organic phase was separated, dried over anhydrous sodium sulfate, and then reduced It was concentrated under pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 3/2) to obtain 754 mg of light brown liquid with a yield of 98%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.17 (t,J = 8.8 Hz, 1H), 7.93 – 7.98 (m, 1H), 7.78 – 7.85 (m, 1H), 4.50 – 4.58 (m, 0.4H), 4.02 (s, 1.2H), 4.01 (s, 1.8H), 3.63 – 3.70 (m, 0.6H), 3.03 (s, 1.8H), 2.93 (s, 1.2H), 1.78 – 1.97 (m, 4H), 1.46 – 1.49 (m, 4H), 1.09 – 1.17 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.17 (t, J = 8.8 Hz, 1H), 7.93 – 7.98 (m, 1H), 7.78 – 7.85 (m, 1H), 4.50 – 4.58 (m , 0.4H), 4.02 (s, 1.2H), 4.01 (s, 1.8H), 3.63 – 3.70 (m, 0.6H), 3.03 (s, 1.8H), 2.93 (s, 1.2H), 1.78 – 1.97 (m, 4H), 1.46 – 1.49 (m, 4H), 1.09 – 1.17 (m, 2H).
MS (ESI, pos.ion) m/z: 277.20 [M+H]+ .MS (ESI, pos.ion) m/z: 277.20 [M+H] + .
步驟2:化合物N -環己基-6-(羥甲基)-N -甲基吡啶醯胺的合成Step 2: Synthesis of compound N -cyclohexyl-6-(hydroxymethyl) -N-picolinamide
將化合物6-(環己基(甲基)氨基甲醯基)吡啶甲酸甲酯 (750 mg, 2.71 mmol) 溶於四氫呋喃(10 mL) 中,冰浴下加入硼氫化鋰 (117 mg, 5.50 mmol),室溫反應2.5 h後停止,加入飽和氯化鈉水溶液 (30 mL),乙酸乙酯萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,得到白色固體632 mg,收率93%。The compound 6-(cyclohexyl(methyl)carbamoyl)picolinate (750 mg, 2.71 mmol) was dissolved in tetrahydrofuran (10 mL), and lithium borohydride (117 mg, 5.50 mmol) was added under ice bath. , the reaction was stopped after 2.5 h at room temperature, saturated aqueous sodium chloride solution (30 mL) was added, extracted with ethyl acetate (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 632 mg of white solid, which was collected rate 93%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.97 (t,J = 7.8 Hz, 1H), 7.62 – 7.66 (m, 1H), 7.41 (d,J = 7.6 Hz, 1H), 4.72 – 4.73 (m, 2H), 3.36 – 3.42 (m, 0.6H), 3.01 (s, 1.8H), 2.82 (s, 1.2H), 2.58 – 2.65 (m, 0.4H), 1.76 – 1.93 (m, 4H), 1.42 – 1.67 (m, 4H), 1.06 – 1.21 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.97 (t, J = 7.8 Hz, 1H), 7.62 – 7.66 (m, 1H), 7.41 (d, J = 7.6 Hz, 1H), 4.72 – 4.73 (m, 2H), 3.36 – 3.42 (m, 0.6H), 3.01 (s, 1.8H), 2.82 (s, 1.2H), 2.58 – 2.65 (m, 0.4H), 1.76 – 1.93 (m, 4H), 1.42 – 1.67 (m, 4H), 1.06 – 1.21 (m, 2H).
MS (ESI, pos.ion) m/z: 249.30 [M+H]+ .MS (ESI, pos.ion) m/z: 249.30 [M+H] + .
步驟3:化合物 (2R )-(6-(環己基(甲基)氨基甲醯基)吡啶-2-基)甲基-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 3: Compound ( 2R )-(6-(cyclohexyl(methyl)carbamoyl)pyridin-2-yl)methyl-1-acetyl-4-(3-(cyclopropylmethoxy) Synthesis of yl)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (80 mg, 0.22 mmol),N -環己基-6-(羥甲基)-N -甲基吡啶醯胺 (53 mg, 0.21 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (207 mg, 1.08 mmol) 和N -羥基-7-氮雜苯並三氮唑 (60 mg, 0.44 mmol) 溶於二氯甲烷 (8 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (167 mg, 1.29 mmol),室溫反應13 h,加二氯甲烷 (15 mL),有機相水洗 (20 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1),得到白色黏稠固體83 mg,收率64%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (80 mg, 0.22 mmol), N -cyclohexyl-6-(hydroxymethyl) -N -picoline amide (53 mg, 0.21 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Imine hydrochloride (207 mg, 1.08 mmol) and N -hydroxy-7-azabenzotriazole (60 mg, 0.44 mmol) were dissolved in dichloromethane (8 mL), cooled to 0 °C, added N , N -diisopropylethylamine (167 mg, 1.29 mmol), react at room temperature for 13 h, add dichloromethane (15 mL), wash the organic phase with water (20 mL × 2), and dry the organic phase with anhydrous sodium sulfate , concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 50/1) to obtain 83 mg of white viscous solid with a yield of 64%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.80 – 7.85 (m, 1H), 7.45 – 7.55 (m, 2H), 7.15 (d,J = 8.4 Hz, 1H), 6.85 (s, 1H), 6.80 – 6.84 (m, 1H), 6.63 (t,J F-H = 75.5 Hz, 1H), 5.25 – 5.46 (m, 2H), 4.59 – 4.64 (m, 1H), 3.95 – 4.01 (m, 1H), 3.89 (d,J = 6.7 Hz, 2H), 3.63 – 3.68 (m, 1H), 3.40 – 3.54 (m, 2H), 3.00 (s, 1.8H), 2.83 (s, 1.2H), .70 – 2.77 (m, 1H), 2.10 – 2.17 (m, 1H), 2.15 (s, 3H), 1.73 – 1.87 (m, 4H), 1.46 – 1.58 (m, 4H), 1.28 – 1.31 (m, 1H), 1.05 – 1.16 (m, 2H), 0.65 – 0.69 (m, 2H), 0.36 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.80 – 7.85 (m, 1H), 7.45 – 7.55 (m, 2H), 7.15 (d, J = 8.4 Hz, 1H), 6.85 (s, 1H) ), 6.80 – 6.84 (m, 1H), 6.63 (t, J FH = 75.5 Hz, 1H), 5.25 – 5.46 (m, 2H), 4.59 – 4.64 (m, 1H), 3.95 – 4.01 (m, 1H) , 3.89 (d, J = 6.7 Hz, 2H), 3.63 – 3.68 (m, 1H), 3.40 – 3.54 (m, 2H), 3.00 (s, 1.8H), 2.83 (s, 1.2H), .70 – 2.77 (m, 1H), 2.10 – 2.17 (m, 1H), 2.15 (s, 3H), 1.73 – 1.87 (m, 4H), 1.46 – 1.58 (m, 4H), 1.28 – 1.31 (m, 1H), 1.05 – 1.16 (m, 2H), 0.65 – 0.69 (m, 2H), 0.36 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 600.30 [M+H]+ .MS (ESI, pos.ion) m/z: 600.30 [M+H] + .
實施例132:化合物 (2R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 132: Compound ( 2R )-(6-(methyl(p-tolyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethyl) oxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-(甲基(對甲苯基)氨基甲醯基)吡啶甲酸甲酯的合成Step 1: Synthesis of compound 6-(methyl(p-tolyl)carbamoyl)picolinate methyl ester
將化合物2,6-吡啶二羧酸單甲酯 (500 mg, 2.76 mmol),N -甲基-4-甲基苯胺 (402 mg, 3.32 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (1.30 g, 6.80 mmol) 和N -羥基-7-氮雜苯並三氮唑 (753 mg, 5.53 mmol) 溶於二氯甲烷 (20 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (1.10 g, 8.51 mmol),室溫反應17 h,加水洗 (20 mL × 2) 有機相,分離有機相,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 3/2),得到淺褐色液體761 mg,收率97%。Compound 2,6-pyridinedicarboxylate monomethyl ester (500 mg, 2.76 mmol), N -methyl-4-methylaniline (402 mg, 3.32 mmol), 1-ethyl-(3-dimethylaniline) Aminopropyl)carbodiimide hydrochloride (1.30 g, 6.80 mmol) and N -hydroxy-7-azabenzotriazole (753 mg, 5.53 mmol) were dissolved in dichloromethane (20 mL) , cooled to 0 °C, N , N -diisopropylethylamine (1.10 g, 8.51 mmol) was added, the reaction was carried out at room temperature for 17 h, and the organic phase was washed with water (20 mL × 2), and the organic phase was separated and washed with anhydrous sodium sulfate. It was dried, concentrated under reduced pressure, and the concentrated solution was subjected to silica gel column separation (eluent: petroleum ether/ethyl acetate (v/v) = 3/2) to obtain 761 mg of light brown liquid with a yield of 97%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.97 (d,J = 7.5 Hz, 1H), 7.73 (t,J = 8.8 Hz, 1H), 7.57 (d,J = 7.4 Hz, 1H), 6.96 – 7.01 (m, 4H), 3.92 (s, 3H), 3.51 (s, 3H), 2.26 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.97 (d, J = 7.5 Hz, 1H), 7.73 (t, J = 8.8 Hz, 1H), 7.57 (d, J = 7.4 Hz, 1H) , 6.96 – 7.01 (m, 4H), 3.92 (s, 3H), 3.51 (s, 3H), 2.26 (s, 3H).
MS (ESI, pos.ion) m/z: 285.20 [M+H]+ .MS (ESI, pos.ion) m/z: 285.20 [M+H] + .
步驟2:化合物6-(羥甲基)-N -甲基-N -(對甲苯基)吡啶醯胺的合成Step 2: Synthesis of compound 6-(hydroxymethyl) -N -methyl- N- (p-tolyl)pyridinamide
將化合物6-(甲基(對甲苯基)氨基甲醯基)吡啶甲酸甲酯 (680 mg, 2.39 mmol) 溶於甲醇 (8 mL) 中,冰浴中加入硼氫化鈉 (271 mg,7.16 mmol),室溫反應3 h後停止,加入飽和氯化鈉水溶液 (30 mL),乙酸乙酯萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/4),得到白色固體171 mg,收率27%。The compound 6-(methyl(p-tolyl)carbamoyl)picolinate (680 mg, 2.39 mmol) was dissolved in methanol (8 mL), and sodium borohydride (271 mg, 7.16 mmol) was added to the ice bath. ), the reaction was stopped after 3 h at room temperature, saturated aqueous sodium chloride solution (30 mL) was added, extracted with ethyl acetate (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was subjected to a silica gel column layer. Separation (eluent: petroleum ether/ethyl acetate (v/v) = 1/4) to obtain 171 mg of white solid, yield 27%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.58 – 7.67 (m, 2H), 6.97 – 7.07 (m, 5H), 4.49 (s, 2H), 3.52 (s, 3H), 2.85 (br.s, 1H), 2.30 (s, 3H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.58 – 7.67 (m, 2H), 6.97 – 7.07 (m, 5H), 4.49 (s, 2H), 3.52 (s, 3H), 2.85 ( br.s, 1H), 2.30 (s, 3H).
MS (ESI, pos.ion) m/z: 257.20 [M+H]+ .MS (ESI, pos.ion) m/z: 257.20 [M+H] + .
步驟3:化合物 (2R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 3: Compound ( 2R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethoxy) Synthesis of yl)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (80 mg, 0.22 mmol),6-(羥甲基)-N -甲基-N -(對甲苯基)吡啶醯胺 (52 mg, 0.22 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (207 mg, 1.08 mmol) 和N -羥基-7-氮雜苯並三氮唑 (58 mg, 0.43 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (141 mg, 1.09 mmol),室溫反應9 h,加二氯甲烷 (15 mL),有機相水洗 (20 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/2),得到白色黏稠固體87 mg,收率65%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (80 mg, 0.22 mmol), 6-(hydroxymethyl) -N -methyl- N- (p-tolyl)pyridinamide (52 mg, 0.22 mmol), 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (207 mg, 1.08 mmol) and N -hydroxy-7-azabenzotriazole (58 mg, 0.43 mmol) were dissolved in dichloromethane (10 mL) and cooled to 0 ℃, add N , N -diisopropylethylamine (141 mg, 1.09 mmol), react at room temperature for 9 h, add dichloromethane (15 mL), wash the organic phase with water (20 mL × 2), wash with anhydrous sodium sulfate The organic phase was dried, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/2) to obtain 87 mg of white viscous solid with a yield of 65%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.54 – 7.66 (m, 1H), 7.31 – 7.40 (m, 1H), 7.22 – 7.28 (m, 1H), 7.14 (d,J = 8.3 Hz, 1H), 6.93 – 6.99 (m, 4H), 6.84 (s, 1H), 6.82 (d,J = 3.9 Hz, 1H), 6.62 (t,J F-H = 75.5 Hz, 1H), 5.04 – 5.24 (m, 2H), 4.55 – 4.59 (m, 1H), 3.95 – 3.99 (m, 1H), 3.88 (d,J = 6.9 Hz, 2H), 3.63 (t,J = 10.5 Hz, 1H), 3.39 – 3.52 (m, 1H), 3.48 (s, 3H), 2.68 – 2.74 (m, 1H), 2.27 (s, 3H), 2.13 (s, 3H), 2.03 – 2.11 (m, 1H), 1.27 – 1.35 (m, 1H), 0.65– 0.69 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.54 – 7.66 (m, 1H), 7.31 – 7.40 (m, 1H), 7.22 – 7.28 (m, 1H), 7.14 (d, J = 8.3 Hz , 1H), 6.93 – 6.99 (m, 4H), 6.84 (s, 1H), 6.82 (d, J = 3.9 Hz, 1H), 6.62 (t, J FH = 75.5 Hz, 1H), 5.04 – 5.24 (m , 2H), 4.55 – 4.59 (m, 1H), 3.95 – 3.99 (m, 1H), 3.88 (d, J = 6.9 Hz, 2H), 3.63 (t, J = 10.5 Hz, 1H), 3.39 – 3.52 ( m, 1H), 3.48 (s, 3H), 2.68 – 2.74 (m, 1H), 2.27 (s, 3H), 2.13 (s, 3H), 2.03 – 2.11 (m, 1H), 1.27 – 1.35 (m, 1H), 0.65 – 0.69 (m, 2H), 0.36 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 608.50 [M+H]+ .MS (ESI, pos.ion) m/z: 608.50 [M+H] + .
實施例133:化合物 (2R )-(6-((4-氟苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 133: Compound ( 2R )-(6-((4-fluorophenyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-( Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-羥甲基吡啶-2-羧酸甲酯的合成Step 1: Synthesis of compound 6-hydroxymethylpyridine-2-carboxylate methyl ester
將化合物2,6-吡啶二羧酸單甲酯 (1.0 g, 5.5 mmol) 溶解在無水四氫呋喃 (10 mL) 溶液中,加入N ,N -羰基二咪唑 (1.1 g, 6.8 mmol),50℃加熱反應1 h後停止,冷卻至室溫,加入硼氫化鈉 (320 mg, 8.4 mmol),室溫反應2.5 h,加入水 (25 mL) 淬滅反應,用乙酸乙酯萃取 (30 mL × 6),無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 1/4),得無色液體704 mg,收率76%。Compound 2,6-pyridinedicarboxylate monomethyl ester (1.0 g, 5.5 mmol) was dissolved in anhydrous tetrahydrofuran (10 mL) solution, added N , N -carbonyldiimidazole (1.1 g, 6.8 mmol), heated at 50 °C The reaction was stopped after 1 h, cooled to room temperature, sodium borohydride (320 mg, 8.4 mmol) was added, the reaction was carried out at room temperature for 2.5 h, water (25 mL) was added to quench the reaction, and extracted with ethyl acetate (30 mL × 6) , dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/4) to obtain 704 mg of a colorless liquid with a yield of 76%.
1 H NMR (400 MHz, DMSO-d6 ) δ (ppm): 8.00 (t,J = 7.7 Hz, 1H), 7.94 (t,J = 8.3 Hz, 1H), 7.72 (d,J = 7.7 Hz, 1H), 4.62 (s, 2H), 3.88 (s, 3H). 1 H NMR (400 MHz, DMSO- d 6 ) δ (ppm): 8.00 (t, J = 7.7 Hz, 1H), 7.94 (t, J = 8.3 Hz, 1H), 7.72 (d, J = 7.7 Hz, 1H), 4.62 (s, 2H), 3.88 (s, 3H).
MS (ESI, pos.ion) m/z: 168.20 [M+H]+ .MS (ESI, pos.ion) m/z: 168.20 [M+H] + .
步驟2:化合物6-(((叔丁基二甲基矽基)氧基)甲基)吡啶甲酸甲酯的合成Step 2: Synthesis of compound 6-(((tert-butyldimethylsilyl)oxy)methyl)picolinate methyl ester
將化合物6-羥甲基吡啶-2-羧酸甲酯 (700 mg, 4.19 mmol) 溶解在二氯甲烷 (10 mL) 溶液中,加入三乙胺 (639 mg, 6.31 mmol),冰浴中加入叔丁基二甲矽基三氟甲磺酸酯 (1.3 g, 4.90 mmol),室溫反應3 h,停止反應,水洗有機相 (20 mL × 2),無水硫酸鈉乾燥,減壓濃縮,進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 10/1),得無色液體744 mg,收率63%。The compound 6-hydroxymethylpyridine-2-carboxylic acid methyl ester (700 mg, 4.19 mmol) was dissolved in dichloromethane (10 mL) solution, triethylamine (639 mg, 6.31 mmol) was added, and the ice bath was added tert-Butyldimethylsilyl trifluoromethanesulfonate (1.3 g, 4.90 mmol) was reacted at room temperature for 3 h, the reaction was stopped, the organic phase was washed with water (20 mL × 2), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and carried out Silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 10/1) gave 744 mg of a colorless liquid with a yield of 63%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.02 (d,J = 7.7 Hz, 1H), 7.88 (t,J = 7.8 Hz, 1H), 7.76 (d,J = 7.8 Hz, 1H), 4.96 (s, 2H), 4.02 (s, 3H), 0.98 (s, 9H), 0.14 (s, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.02 (d, J = 7.7 Hz, 1H), 7.88 (t, J = 7.8 Hz, 1H), 7.76 (d, J = 7.8 Hz, 1H) , 4.96 (s, 2H), 4.02 (s, 3H), 0.98 (s, 9H), 0.14 (s, 6H).
MS (ESI, pos.ion) m/z: 282.20 [M+H]+ .MS (ESI, pos.ion) m/z: 282.20 [M+H] + .
步驟3:化合物6-(((叔丁基二甲基矽基)氧基)甲基)吡啶甲酸的合成Step 3: Synthesis of compound 6-(((tert-butyldimethylsilyl)oxy)methyl)picolinic acid
將化合物6-(((叔丁基二甲基矽基)氧基)甲基)吡啶甲酸甲酯 (740 mg, 2.63 mmol) 溶於四氫呋喃 (5 mL) 和水 (5 mL) 的混合溶劑中,加入一水合氫氧化鋰 (220 mg, 5.24mmol),60℃反應45 min後停止,加稀鹽酸調節溶液pH=6,用乙酸乙酯萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體232 mg,收率33%。The compound 6-(((tert-butyldimethylsilyl)oxy)methyl)picolinate (740 mg, 2.63 mmol) was dissolved in a mixed solvent of tetrahydrofuran (5 mL) and water (5 mL) , Lithium hydroxide monohydrate (220 mg, 5.24 mmol) was added, the reaction was stopped after 45 min at 60 °C, diluted hydrochloric acid was added to adjust the pH of the solution to 6, extracted with ethyl acetate (20 mL × 3), and the organic phase was washed with anhydrous sodium sulfate. After drying, the solvent was removed to obtain 232 mg of a white solid with a yield of 33%.
MS (ESI, pos.ion) m/z: 268.20 [M+H]+ .MS (ESI, pos.ion) m/z: 268.20 [M+H] + .
步驟4:化合物6-(((叔丁基二甲基矽基)氧基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺的合成Step 4: Compound 6 - (((tert-butyldimethyl silicon based) oxy) methyl) - N - (4- fluorophenyl) - N - acyl amine-methylpyridine
將化合物6-(((叔丁基二甲基矽基)氧基)甲基)吡啶甲酸 (200 mg, 0.75 mmol),4-氟-N -甲基苯胺 (134 mg, 0.99 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (716mg, 3.74mmol) 和N -羥基-7-氮雜苯並三氮唑 (203 mg, 1.49 mmol) 溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (280 mg, 2.17 mmol),室溫反應10 h,加入二氯甲烷 (15 mL),加水洗有機相 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 4/1),得到淺褐色液體113 mg,收率44%。Compound 6-(((tert-butyldimethylsilyl)oxy)methyl)picolinic acid (200 mg, 0.75 mmol), 4-fluoro- N -methylaniline (134 mg, 0.99 mmol), 1 -Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (716 mg, 3.74 mmol) and N -hydroxy-7-azabenzotriazole (203 mg, 1.49 mmol) were dissolved In dichloromethane (10 mL), cooled to 0 °C, N , N -diisopropylethylamine (280 mg, 2.17 mmol) was added, the reaction was carried out at room temperature for 10 h, dichloromethane (15 mL) was added, The organic phase was washed with water (20 mL × 2), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 4/1 ) to obtain 113 mg of light brown liquid with a yield of 44%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.60 – 7.69 (m, 1H), 7.34 – 7.40 (m, 2H), 6.99 – 7.12 (m, 2H), 6.86 – 6.95(m, 2H), 4.55 (s, 2H), 3.50 (s, 3H), 0.94 (s, 9H), 0.07 (s, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.60 – 7.69 (m, 1H), 7.34 – 7.40 (m, 2H), 6.99 – 7.12 (m, 2H), 6.86 – 6.95 (m, 2H) , 4.55 (s, 2H), 3.50 (s, 3H), 0.94 (s, 9H), 0.07 (s, 6H).
MS (ESI, pos.ion) m/z: 375.20 [M+H]+ .MS (ESI, pos.ion) m/z: 375.20 [M+H] + .
步驟5:化合物N -(4-氟苯基)-6-(羥甲基)-N -甲基吡啶醯胺的合成Step 5: Synthesis of compound N- (4-fluorophenyl)-6-(hydroxymethyl) -N -picoline pyridinamide
將化合物6-(((叔丁基二甲基矽基)氧基)甲基)-N -(4-氟苯基)-N -甲基吡啶醯胺 (107 mg, 0.29 mmol) 溶於四氫呋喃 (5 mL) 中,加入3 mol/L四丁基氟化銨的四氫呋喃溶液 (1.5 mL),室溫反應2 h,加入飽和氯化鈉 (5 mL),乙酸乙酯萃取 (15 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:乙酸乙酯 ( v ) = 100%),得到白色固體68 mg,收率91%。Compound 6 - (((tert-butyldimethyl silicon based) oxy) methyl) - N - (4- fluorophenyl) - N - Amides methylpyridine (107 mg, 0.29 mmol) was dissolved in tetrahydrofuran (5 mL), add 3 mol/L tetrabutylammonium fluoride solution in tetrahydrofuran (1.5 mL), react at room temperature for 2 h, add saturated sodium chloride (5 mL), extract with ethyl acetate (15 mL × 2 ), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: ethyl acetate (v) = 100%) to obtain 68 mg of white solid with a yield of 91%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.67 – 7.71 (m, 1H), 7.59 – 7.61 (m, 1H), 7.04 – 7.13 (m, 3H), 6.94 – 6.98 (m, 2H), 4.52 (s, 2H), 3.52 (s, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.67 – 7.71 (m, 1H), 7.59 – 7.61 (m, 1H), 7.04 – 7.13 (m, 3H), 6.94 – 6.98 (m, 2H) , 4.52 (s, 2H), 3.52 (s, 3H).
MS (ESI, pos.ion) m/z: 261.20 [M+H]+ .MS (ESI, pos.ion) m/z: 261.20 [M+H] + .
步驟6:化合物 (2R )-(6-((4-氟苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 6: Compound ( 2R )-(6-((4-fluorophenyl)(methyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclo Synthesis of Propylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (51 mg, 0.14 mmol),N -(4-氟苯基)-6-(羥甲基)-N -甲基吡啶醯胺 (35 mg, 0.14 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (260mg, 1.36 mmol) 和N -羥基-7-氮雜苯並三氮唑 (55 mg, 0.40 mmol)溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (174 mg, 1.35 mmol),室溫反應10 h,加入二氯甲烷 (15 mL),加水洗有機相 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到59 mg白色黏稠固體,收率70%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (51 mg, 0.14 mmol), N- (4-fluorophenyl)-6-(hydroxymethyl) -N -picolinamide (35 mg, 0.14 mmol), 1-ethyl-(3-dimethylaminopropyl) yl)carbodiimide hydrochloride (260 mg, 1.36 mmol) and N -hydroxy-7-azabenzotriazole (55 mg, 0.40 mmol) were dissolved in dichloromethane (10 mL) and cooled to 0 °C, N , N -diisopropylethylamine (174 mg, 1.35 mmol) was added, the reaction was carried out at room temperature for 10 h, dichloromethane (15 mL) was added, and the organic phase was washed with water (20 mL × 2). It was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 30/1) to obtain 59 mg of white viscous solid, yield 70% .
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.59 – 7.68 (m, 1H), 7.31 – 7.43 (m, 2H), 7.12 (d,J = 8.0 Hz, 1H), 6.97 – 7.05 (m, 2H), 6.84 – 6.93 (m, 2H), 6.82 (s, 1H), 6.77 – 6.81 (m, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 5.10 – 5.21 (m, 1H), 4.90 – 5.02 (m, 1H), 4.51 – 4.55 (m, 1H), 3.92 – 3.97 (m, 1H), 3.86 (d,J = 6.8 Hz, 2H), 3.61 (t,J = 10.4 Hz, 1H), 3.46 (s, 3H), 3.39 – 3.50 (m, 1H), 2.64 – 2.71 (m, 1H), 2.11 (s, 3H), 2.01 – 2.11 (m, 1H), 1.25 – 1.34 (m, 1H), 0.62– 0.67(m, 2H), 0.34 – 0.38(m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.59 – 7.68 (m, 1H), 7.31 – 7.43 (m, 2H), 7.12 (d, J = 8.0 Hz, 1H), 6.97 – 7.05 (m , 2H), 6.84 – 6.93 (m, 2H), 6.82 (s, 1H), 6.77 – 6.81 (m, 1H), 6.60 (t, J FH = 75.5 Hz, 1H), 5.10 – 5.21 (m, 1H) , 4.90 – 5.02 (m, 1H), 4.51 – 4.55 (m, 1H), 3.92 – 3.97 (m, 1H), 3.86 (d, J = 6.8 Hz, 2H), 3.61 (t, J = 10.4 Hz, 1H) ), 3.46 (s, 3H), 3.39 – 3.50 (m, 1H), 2.64 – 2.71 (m, 1H), 2.11 (s, 3H), 2.01 – 2.11 (m, 1H), 1.25 – 1.34 (m, 1H) ), 0.62 – 0.67(m, 2H), 0.34 – 0.38(m, 2H).
MS (ESI, pos.ion) m/z: 612.20 [M+H]+ .MS (ESI, pos.ion) m/z: 612.20 [M+H] + .
實施例134:化合物 (2R )-(3-(氨基甲醯基)苄基) 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 134: Compound ( 2R )-(3-(aminocarboxy)benzyl)1-acetoxy-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy) )Phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (150 mg, 0.41 mmol),3-(羥甲基)苯甲醯胺 (74 mg, 0.49 mmol) 和N -羥基-7-氮雜苯並三氮唑 (111 mg, 0.82 mmol) 溶於二氯甲烷 (8 mL) 和N ,N -二甲基甲醯胺 (1 mL) 中,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (234 mg, 1.22 mmol) 和N ,N -二異丙基乙胺 (210 mg, 1.62 mmol),室溫反應5 h,減壓濃縮除去溶劑,加水 (30 mL),用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 35/1),得到白色固體137 mg,收率67%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (150 mg, 0.41 mmol), 3-(hydroxymethyl)benzamide (74 mg, 0.49 mmol) and N -hydroxy-7-azabenzotriazole (111 mg, 0.82 mmol) were dissolved in dichloromethane (8 mL) and N , N -dimethylformamide (1 mL), add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (234 mg, 1.22 mmol) and N , N -diisopropylethylamine (210 mg, 1.62 mmol), reacted at room temperature for 5 h, concentrated under reduced pressure to remove the solvent, added water (30 mL), extracted with ethyl acetate (10 mL × 2), organic The phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 35/1) to obtain 137 mg of white solid, yield 67% .
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.98 (s, 1H), 7.89 – 7.95 (m, 1H), 7.35 – 7.47 (m, 2H), 7.12 (d,J = 7.3 Hz, 1H), 6.78 – 6.89 (m, 2H), 6.60 (t,J F-H = 75.4 Hz, 1H), 5.55 (d,J = 13.0 Hz, 1H), 5.12 (d,J = 13.1 Hz, 1H), 4.54 – 4.63 (m, 1H), 3.92 – 3.99 (m, 1H), 3.86 (d,J = 6.0 Hz, 2H), 3.60 – 3.66 (m, 1H), 3.39 – 3.50 (m, 1H), 2.65 – 2.76 (m, 1H), 2.05 – 2.17 (m, 1H), 2.10 (s, 3H), 1.21 – 1.32 (m, 1H), 0.56– 0.69 (m, 2H), 0.26 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.98 (s, 1H), 7.89 – 7.95 (m, 1H), 7.35 – 7.47 (m, 2H), 7.12 (d, J = 7.3 Hz, 1H ), 6.78 – 6.89 (m, 2H), 6.60 (t, J FH = 75.4 Hz, 1H), 5.55 (d, J = 13.0 Hz, 1H), 5.12 (d, J = 13.1 Hz, 1H), 4.54 – 4.63 (m, 1H), 3.92 – 3.99 (m, 1H), 3.86 (d, J = 6.0 Hz, 2H), 3.60 – 3.66 (m, 1H), 3.39 – 3.50 (m, 1H), 2.65 – 2.76 ( m, 1H), 2.05 – 2.17 (m, 1H), 2.10 (s, 3H), 1.21 – 1.32 (m, 1H), 0.56 – 0.69 (m, 2H), 0.26 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 503.15 [M+H]+ .MS (ESI, pos.ion) m/z: 503.15 [M+H] + .
實施例135:化合物 (2R )-(6-(氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 135: Compound ( 2R )-(6-(aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethoxy)-4-( Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (150 mg, 0.41 mmol),6-(羥甲基)吡啶醯胺 (74 mg, 0.49 mmol) 和N -羥基-7-氮雜苯並三氮唑 (110 mg, 0.81 mmol) 溶於二氯甲烷 (8 mL) 和N ,N -二甲基甲醯胺 (1 mL) 中,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (233 mg, 1.22 mmol) 和N ,N -二異丙基乙胺 (209 mg, 1.62 mmol),室溫反應5 h,減壓濃縮除去溶劑,加水 (30 mL),用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 35/1),得到白色固體112 mg,收率55%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (150 mg, 0.41 mmol), 6-(hydroxymethyl)pyridamide (74 mg, 0.49 mmol) and N -hydroxy-7-azabenzotriazole (110 mg, 0.81 mmol) were dissolved in dichloromethane (8 mL) ) and N , N -dimethylformamide (1 mL), add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (233 mg, 1.22 mmol) and N , N -diisopropylethylamine (209 mg, 1.62 mmol), reacted at room temperature for 5 h, concentrated under reduced pressure to remove the solvent, added water (30 mL), extracted with ethyl acetate (10 mL × 2), the organic phase was It was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 35/1) to obtain 112 mg of white solid with a yield of 55%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.11 (d,J = 7.3 Hz, 1H), 8.07 (br.s, 1H), 7.85 – 7.89 (m, 1H), 7.56 (d,J = 7.4 Hz, 1H), 7.11 (d,J = 7.9 Hz, 1H), 6.76 – 6.88 (m, 2H), 6.60 (t,J F-H = 75.5 Hz, 1H), 5.48 (d,J = 13.7 Hz, 1H), 5.22 (d,J = 13.7 Hz, 1H), 4.59 – 4.63 (m, 1H), 3.95 – 3.99 (m, 1H), 3.85 (d,J = 6.6 Hz, 2H), 3.61 – 3.66 (m, 1H), 3.39 – 3.50 (m, 1H), 2.67 – 2.77 (m, 1H), 2.05 – 2.17 (m, 1H), 2.12 (s, 3H), 1.21 – 1.32 (m, 1H), 0.59– 0.68 (m, 2H), 0.28 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.11 (d, J = 7.3 Hz, 1H), 8.07 (br.s, 1H), 7.85 – 7.89 (m, 1H), 7.56 (d, J = 7.4 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H), 6.76 – 6.88 (m, 2H), 6.60 (t, J FH = 75.5 Hz, 1H), 5.48 (d, J = 13.7 Hz, 1H), 5.22 (d, J = 13.7 Hz, 1H), 4.59 – 4.63 (m, 1H), 3.95 – 3.99 (m, 1H), 3.85 (d, J = 6.6 Hz, 2H), 3.61 – 3.66 (m , 1H), 3.39 – 3.50 (m, 1H), 2.67 – 2.77 (m, 1H), 2.05 – 2.17 (m, 1H), 2.12 (s, 3H), 1.21 – 1.32 (m, 1H), 0.59 – 0.68 (m, 2H), 0.28 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 504.10 [M+H]+ .MS (ESI, pos.ion) m/z: 504.10 [M+H] + .
實施例136:化合物 (2R )-(3-氧代異吲哚-5-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 136: Compound ( 2R )-(3-oxoisoindol-5-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy)-4-(difluoro) Methoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (150 mg, 0.41 mmol),6-(羥甲基)異吲哚-1-酮 (99 mg, 0.61 mmol) 和N -羥基-7-氮雜苯並三氮唑 (110 mg, 0.81 mmol) 溶於二氯甲烷 (8 mL) 和N ,N -二甲基甲醯胺 (1 mL) 中,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (233 mg, 1.22 mmol) 和N ,N -二異丙基乙胺 (209 mg, 1.62 mmol),室溫反應6 h,減壓濃縮除去溶劑,加水 (30 mL),用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 35/1),得到白色固體19 mg,收率9%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (150 mg, 0.41 mmol), 6-(hydroxymethyl)isoindol-1-one (99 mg, 0.61 mmol) and N -hydroxy-7-azabenzotriazole (110 mg, 0.81 mmol) in dichloro To methane (8 mL) and N , N -dimethylformamide (1 mL), add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (233 mg, 1.22 mmol) and N , N -diisopropylethylamine (209 mg, 1.62 mmol), reacted at room temperature for 6 h, concentrated under reduced pressure to remove the solvent, added water (30 mL), extracted with ethyl acetate (10 mL × 2 ), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 35/1) to obtain 19 mg of white solid, which was collected rate of 9%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.85 (s, 1H), 7.59 (d,J = 7.9 Hz, 1H), 7.47 (d,J = 7.8 Hz, 1H), 7.25 – 7.21 (m, 1H), 7.10 (d,J = 8.0 Hz, 1H), 6.80 (s, 1H), 6.79 (d,J = 7.7 Hz, 1H), 6.59 (t,J F-H = 75.5 Hz, 1H), 5.29 (s, 2H), 4.52 – 4.56 (m, 1H), 4.45 (s, 2H), 3.92 – 3.96 (m, 1H), 3.85 (d,J = 6.9 Hz, 2H), 3.59 – 3.65 (m, 1H), 3.37 – 3.46 (m, 1H), 2.64 – 2.71 (m, 1H), 2.12 (s, 3H), 1.99 – 2.08 (m, 1H), 1.25 – 1.33 (m, 1H), 0.61– 0.66 (m, 2H), 0.32 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.85 (s, 1H), 7.59 (d, J = 7.9 Hz, 1H), 7.47 (d, J = 7.8 Hz, 1H), 7.25 – 7.21 ( m, 1H), 7.10 (d, J = 8.0 Hz, 1H), 6.80 (s, 1H), 6.79 (d, J = 7.7 Hz, 1H), 6.59 (t, J FH = 75.5 Hz, 1H), 5.29 (s, 2H), 4.52 – 4.56 (m, 1H), 4.45 (s, 2H), 3.92 – 3.96 (m, 1H), 3.85 (d, J = 6.9 Hz, 2H), 3.59 – 3.65 (m, 1H) ), 3.37 – 3.46 (m, 1H), 2.64 – 2.71 (m, 1H), 2.12 (s, 3H), 1.99 – 2.08 (m, 1H), 1.25 – 1.33 (m, 1H), 0.61 – 0.66 (m , 2H), 0.32 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 515.20 [M+H]+ .MS (ESI, pos.ion) m/z: 515.20 [M+H] + .
實施例137:化合物 (2R )-(1-氧代異吲哚-5-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 137: Compound ( 2R )-(1-oxoisoindol-5-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy)-4-(difluoro) Methoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (200 mg, 0.54 mmol) 和5-(羥甲基)異吲哚-1-酮 (220 mg, 1.35 mmol) 溶於N ,N -二甲基甲醯胺 (5 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (147 mg, 1.08 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (312 mg, 1.63 mmol) 和N ,N -二異丙基乙胺 (201 mg, 1.56 mmol),室溫反應11 h,減壓濃縮除去溶劑,加水 (30 mL),用乙酸乙酯萃取 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇( v/v ) = 25/1),得到白色固體129 mg,收率46%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (200 mg, 0.54 mmol) and 5-(hydroxymethyl)isoindol-1-one (220 mg, 1.35 mmol) were dissolved in N , N -dimethylformamide (5 mL) and N -hydroxy-7- Azabenzotriazole (147 mg, 1.08 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (312 mg, 1.63 mmol) and N , N- Diisopropylethylamine (201 mg, 1.56 mmol) was reacted at room temperature for 11 h, concentrated under reduced pressure to remove the solvent, added water (30 mL), extracted with ethyl acetate (20 mL × 2), and the organic phase was washed with anhydrous sodium sulfate It was dried, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 25/1) to obtain 129 mg of white solid, yield 46%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.86 (d,J = 6.8 Hz, 1H), 7.52 (s, 1H), 7.43 – 7.47 (m, 1H), 7.11 (d,J = 7.7 Hz, 1H), 6.81 (s, 1H), 6.80 (d,J = 7.5 Hz, 1H), 6.60 (t,J F-H = 75.6 Hz, 1H), 5.24 – 5.36 (m, 2H), 4.50 – 4.59 (m, 1H), 4.47 (s, 2H), 3.90 – 3.99 (m, 1H), 3.85 (d,J = 6.5 Hz, 2H), 3.57 – 3.66 (m, 1H), 3.37 – 3.50 (m, 1H), 2.63 – 2.74 (m, 1H), 2.13 (s, 3H), 2.00 – 2.08 (m, 1H), 1.25 – 1.33 (m, 1H), 0.60– 0.69 (m, 2H), 0.30 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.86 (d, J = 6.8 Hz, 1H), 7.52 (s, 1H), 7.43 – 7.47 (m, 1H), 7.11 (d, J = 7.7 Hz, 1H), 6.81 (s, 1H), 6.80 (d, J = 7.5 Hz, 1H), 6.60 (t, J FH = 75.6 Hz, 1H), 5.24 – 5.36 (m, 2H), 4.50 – 4.59 ( m, 1H), 4.47 (s, 2H), 3.90 – 3.99 (m, 1H), 3.85 (d, J = 6.5 Hz, 2H), 3.57 – 3.66 (m, 1H), 3.37 – 3.50 (m, 1H) , 2.63 – 2.74 (m, 1H), 2.13 (s, 3H), 2.00 – 2.08 (m, 1H), 1.25 – 1.33 (m, 1H), 0.60 – 0.69 (m, 2H), 0.30 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 515.10 [M+H]+ .MS (ESI, pos.ion) m/z: 515.10 [M+H] + .
實施例138:化合物 (2R )-(1-羥基-1,3-二氫苯並[c ][1,2]噁硼烷-5-基) 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 138: Compound ( 2R )-(1-hydroxy-1,3-dihydrobenzo[ c ][1,2]oxaboran-5-yl)1-acetyl-4-(3- (Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (100 mg, 0.27 mmol),2-羥基甲基-5-羥基苯硼酸半酯 (48 mg, 0.32 mmol) 溶於乾燥的N ,N -二甲基甲醯胺 (5 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (73 mg, 0.54 mmol),在冰浴中加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (259 mg, 1.35 mmol) 和N ,N -二異丙基乙胺 (174 mg, 1.35 mmol),室溫反應5 h,加水 (20 mL) 攪拌5 min,乙酸乙酯萃取 (10 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 54/1),得到白色固體42 mg,收率31%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (100 mg, 0.27 mmol), 2-hydroxymethyl-5-hydroxyphenylboronic acid half ester (48 mg, 0.32 mmol) was dissolved in dry N , N -dimethylformamide (5 mL) and N -hydroxy-7 - Azabenzotriazole (73 mg, 0.54 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (259 mg, 1.35 mmol) was added in an ice bath ) and N , N -diisopropylethylamine (174 mg, 1.35 mmol), react at room temperature for 5 h, add water (20 mL), stir for 5 min, extract with ethyl acetate (10 mL × 2), use anhydrous sodium sulfate The organic phase was dried, concentrated under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 54/1) to obtain 42 mg of white solid with a yield of 31%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.70 (s, 1H), 7.18 (s, 1H), 7.14 (d,J = 8.1 Hz, 1H), 7.11 (d,J = 7.8 Hz, 1H), 7.09 (s, 1H), 6.97 (d,J = 7.9 Hz, 1H), 6.76 (t,J F-H = 75.6 Hz, 1H), 5.08 (s, 2H), 4.68 – 4.71 (m, 1H), 4.15 – 4.18 (m, 1H), 3.94 (d,J = 6.7 Hz, 2H), 3.71 (t,J = 10.2 Hz, 1H), 3.61 – 3.68 (m, 1H), 2.86 – 2.90 (m, 1H), 2.22 – 2.28 (m, 1H), 2.19 (s, 3H), 1.31 – 1.38 (m, 1H), 0.61 – 0.66 (m, 2H), 0.37 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.70 (s, 1H), 7.18 (s, 1H), 7.14 (d, J = 8.1 Hz, 1H), 7.11 (d, J = 7.8 Hz, 1H), 7.09 (s, 1H), 6.97 (d, J = 7.9 Hz, 1H), 6.76 (t, J FH = 75.6 Hz, 1H), 5.08 (s, 2H), 4.68 – 4.71 (m, 1H) , 4.15 – 4.18 (m, 1H), 3.94 (d, J = 6.7 Hz, 2H), 3.71 (t, J = 10.2 Hz, 1H), 3.61 – 3.68 (m, 1H), 2.86 – 2.90 (m, 1H) ), 2.22 – 2.28 (m, 1H), 2.19 (s, 3H), 1.31 – 1.38 (m, 1H), 0.61 – 0.66 (m, 2H), 0.37 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 502.10 [M+H]+ .MS (ESI, pos.ion) m/z: 502.10 [M+H] + .
實施例139:化合物 (2R )-(3-氧代異吲哚-5-基) 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 139: Compound ( 2R )-(3-oxoisoindol-5-yl)1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy) yl)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (200 mg, 0.54 mmol),5-羥基異吲哚-1-酮 (96 mg, 0.64 mmol) 和N -羥基-7-氮雜苯並三氮唑 (149 mg, 1.09 mmol) 溶於二氯甲烷 (12 mL) 和N ,N -二甲基甲醯胺 (4 mL) 中,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (313 mg, 1.63 mmol) 和N ,N -二異丙基乙胺 (282 mg, 2.18 mmol),室溫反應5 h,減壓濃縮除去溶劑,加水 (40 mL),用乙酸乙酯萃取 (15 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 35/1),得到白色固體163 mg,收率60%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (200 mg, 0.54 mmol), 5-hydroxyisoindol-1-one (96 mg, 0.64 mmol) and N -hydroxy-7-azabenzotriazole (149 mg, 1.09 mmol) were dissolved in dichloromethane (12 mL) ) and N , N -dimethylformamide (4 mL), add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (313 mg, 1.63 mmol) and N , N -diisopropylethylamine (282 mg, 2.18 mmol), reacted at room temperature for 5 h, concentrated under reduced pressure to remove the solvent, added water (40 mL), extracted with ethyl acetate (15 mL × 2), the organic phase was It was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 35/1) to obtain 163 mg of white solid with a yield of 60%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.60 (s, 1H), 7.43 – 7.48 (m, 2H), 7.24 – 7.34 (m, 1H), 7.16 (d,J = 6.0 Hz, 1H), 6.80 – 6.94 (m, 2H), 6.64 (t,J = 75.3 Hz, 1H), 4.64 – 4.80 (m, 1H), 4.46 (s, 2H), 3.96 – 4.09 (m, 1H), 3.89 (d,J = 4.6 Hz, 2H), 3.63 – 3.74 (m, 1H), 3.46 – 3.60 (m, 1H), 2.77 – 2.92 (m, 1H), 2.17 – 2.32 (m, 1H), 2.16 (s, 3H), 1.26 – 1.38 (m, 1H), 0.59 – 0.71 (m, 2H), 0.31 – 0.44 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.60 (s, 1H), 7.43 – 7.48 (m, 2H), 7.24 – 7.34 (m, 1H), 7.16 (d, J = 6.0 Hz, 1H) ), 6.80 – 6.94 (m, 2H), 6.64 (t, J = 75.3 Hz, 1H), 4.64 – 4.80 (m, 1H), 4.46 (s, 2H), 3.96 – 4.09 (m, 1H), 3.89 ( d, J = 4.6 Hz, 2H), 3.63 – 3.74 (m, 1H), 3.46 – 3.60 (m, 1H), 2.77 – 2.92 (m, 1H), 2.17 – 2.32 (m, 1H), 2.16 (s, 3H), 1.26 – 1.38 (m, 1H), 0.59 – 0.71 (m, 2H), 0.31 – 0.44 (m, 2H).
MS (ESI, pos.ion) m/z: 501.10 [M+H]+ .MS (ESI, pos.ion) m/z: 501.10 [M+H] + .
實施例140:化合物 (2R )-(2-甲基-1-氧代異吲哚-5-基) 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯;以及Example 140: Compound ( 2R )-(2-methyl-1-oxoisoindol-5-yl)1-acetyl-4-(3-(cyclopropylmethoxy)-4- (difluoromethoxy)phenyl)pyrrolidine-2-carboxylate; and
實施例141:化合物 (2R )-(2,3-二甲基-1-氧代異吲哚-5-基) 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 實施例140:和實施例141: Example 141: Compound ( 2R )-(2,3-Dimethyl-1-oxoisoindol-5-yl)1-acetyl-4-(3-(cyclopropylmethoxy) -4-(Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate Example 140: and Example 141:
步驟1:化合物5-(苄氧基)異吲哚-1-酮 的合成Step 1: Synthesis of compound 5-(benzyloxy)isoindol-1-one
將5-羥基異吲哚-1-酮 (400 mg, 2.68 mmol) 和碳酸鉀 (741 mg, 5.36 mmol) 混合,加入無水N ,N -二甲基甲醯胺 (5 mL) 溶液和苄溴 (688 mg, 4.02 mmol),80℃反應4 h,冷卻至室溫,加入水 (60 mL) 析出白色固體,抽濾,濾餅乾燥,得到白色固體536 mg,收率84%。5-Hydroxyisoindol-1-one (400 mg, 2.68 mmol) and potassium carbonate (741 mg, 5.36 mmol) were mixed, anhydrous N , N -dimethylformamide (5 mL) solution and benzyl bromide were added (688 mg, 4.02 mmol), reacted at 80 °C for 4 h, cooled to room temperature, added water (60 mL) to precipitate a white solid, suction filtered, and the filter cake was dried to obtain 536 mg of a white solid with a yield of 84%.
1 H NMR (400 MHz,d6 -DMSO) δ (ppm): 8.32 (br.s, 1H), 7.58 (d,J = 8.4 Hz, 1H), 7.46 – 7.47 (m, 2H), 7.38 – 7.42 (m, 2H), 7.32 – 7.36 (m, 1H), 7.21 (s, 1H), 7.09 (dd,J = 8.4, 2.0 Hz, 1H), 5.18 (s, 2H), 4.31 (s, 2H). 1 H NMR (400 MHz, d 6 -DMSO) δ (ppm): 8.32 (br.s, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.46 – 7.47 (m, 2H), 7.38 – 7.42 (m, 2H), 7.32 – 7.36 (m, 1H), 7.21 (s, 1H), 7.09 (dd, J = 8.4, 2.0 Hz, 1H), 5.18 (s, 2H), 4.31 (s, 2H).
MS (ESI, pos.ion) m/z: 240.10 [M+H]+ .MS (ESI, pos.ion) m/z: 240.10 [M+H] + .
步驟2:化合物5-(苄氧基)-2-甲基異吲哚-1-酮 和5-(苄氧基)-2,3-二甲基異吲哚-1-酮 的合成Step 2: Synthesis of Compounds 5-(benzyloxy)-2-methylisoindol-1-one and 5-(benzyloxy)-2,3-dimethylisoindol-1-one
將5-(苄氧基)異吲哚-1-酮 (400 mg, 1.67 mmol) 溶解在無水二甲基亞風 (5 mL) 溶液中, 在冰浴中冷卻,加入60%氫化鈉 (133 mg, 3.33 mmol) 反應30 min,再加入碘甲烷 (475 mg, 3.33 mmol) 反應4 h,加入水 (60mL) 淬滅反應,用二氯甲烷 (10 mL × 2) 萃取,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 40/1),得到混合物淺黃色液體414 mg,總收率95%。5-(benzyloxy)isoindol-1-one (400 mg, 1.67 mmol) was dissolved in anhydrous dimethyl sulfoxide (5 mL) solution, cooled in an ice bath, and 60% sodium hydride (133 mL) was added. mg, 3.33 mmol) for 30 min, then iodomethane (475 mg, 3.33 mmol) was added to react for 4 h, water (60 mL) was added to quench the reaction, extracted with dichloromethane (10 mL × 2), and the organic phase was combined with anhydrous sulfuric acid After drying over sodium, the solvent was removed, and the concentrated solution was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 40/1) to obtain 414 mg of the mixture as a pale yellow liquid, with a total yield of 95%.
步驟3:化合物5-羥基-2-甲基異吲哚-1-酮和5-羥基-2,3-二甲基異吲哚-1-酮的合成Step 3: Synthesis of Compounds 5-hydroxy-2-methylisoindol-1-one and 5-hydroxy-2,3-dimethylisoindol-1-one
將化合物5-(苄氧基)-2-甲基異吲哚-1-酮 (11136-1-2) 和5-(苄氧基)-2,3-二甲基異吲哚-1-酮 (11154-1-2) 的混合物 (675 mg) 溶於甲醇 (8 mL),加入Pd/C (200 mg, 0.3 g/g),通入氫氣室溫反應1h,然後將反應液抽濾,濾液濃縮,經過高效液相色譜分離得到5-羥基-2-甲基異吲哚-1-酮 (11136-1) 白色固體153 mg和5-羥基-2,3-二甲基異吲哚-1-酮 (11154-1) 白色固體72 mg。The compounds 5-(benzyloxy)-2-methylisoindole-1-one (11136-1-2) and 5-(benzyloxy)-2,3-dimethylisoindole-1- A mixture of ketones (11154-1-2) (675 mg) was dissolved in methanol (8 mL), Pd/C (200 mg, 0.3 g/g) was added, hydrogen was passed through for reaction at room temperature for 1 h, and the reaction solution was suction filtered , the filtrate was concentrated and separated by high performance liquid chromatography to obtain 153 mg of 5-hydroxy-2-methylisoindol-1-one (11136-1) as a white solid and 5-hydroxy-2,3-dimethylisoindole -1-One (11154-1) White solid 72 mg.
化合物5-羥基-2-甲基異吲哚-1-酮:1 H NMR (400 MHz,d6 -DMSO) δ (ppm): 10.07 (br.s, 1H), 7.46 (d,J = 8.2 Hz, 1H), 6.90 (s, 1H), 6.84 (d,J = 8.2 Hz, 1H), 4.33 (s, 2H), 3.01 (s, 3H).Compound 5-hydroxy-2-methylisoindol-1-one: 1 H NMR (400 MHz, d 6 -DMSO) δ (ppm): 10.07 (br.s, 1H), 7.46 (d, J = 8.2 Hz, 1H), 6.90 (s, 1H), 6.84 (d, J = 8.2 Hz, 1H), 4.33 (s, 2H), 3.01 (s, 3H).
MS (ESI, pos.ion) m/z: 164.10 [M+H]+ .MS (ESI, pos.ion) m/z: 164.10 [M+H] + .
化合物5-羥基-2,3-二甲基異吲哚-1-酮:1 H NMR (400 MHz,d6 -DMSO) δ (ppm): 10.09 (br.s, 1H), 7.45 (d,J = 8.2 Hz, 1H), 6.91 (s, 1H), 6.84 (d,J = 8.2 Hz, 1H), 4.42 (q,J = 6.6 Hz, 1H), 2.95 (s, 3H), 1.37 (d,J = 6.7 Hz, 3H).Compound 5-hydroxy-2,3-dimethylisoindol-1-one: 1 H NMR (400 MHz, d 6 -DMSO) δ (ppm): 10.09 (br.s, 1H), 7.45 (d, J = 8.2 Hz, 1H), 6.91 (s, 1H), 6.84 (d, J = 8.2 Hz, 1H), 4.42 (q, J = 6.6 Hz, 1H), 2.95 (s, 3H), 1.37 (d, J = 6.7 Hz, 3H).
MS (ESI, pos.ion) m/z: 178.10 [M+H]+ .MS (ESI, pos.ion) m/z: 178.10 [M+H] + .
步驟4:化合物 (2R )-(2-甲基-1-氧代異吲哚-5-基) 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 (實施例140) 的合成Step 4: Compound ( 2R )-(2-methyl-1-oxoisoindol-5-yl)1-acetyl-4-(3-(cyclopropylmethoxy)-4-( Synthesis of difluoromethoxy)phenyl)pyrrolidine-2-carboxylate (Example 140)
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (120 mg, 0.32 mmol) 和5-羥基-2-甲基異吲哚-1-酮 (58 mg, 0.36 mmol) 溶於二氯甲烷 (4 mL) 和N ,N -二甲基甲醯胺 (2 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (89 mg, 0.65 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (187 mg, 0.98 mmol) 和N ,N -二異丙基乙胺 (169 mg, 1.31 mmol),室溫反應11 h,減壓濃縮除去溶劑,加水 (20 mL),用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 35/1),得到白色固體132 mg,收率79%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (120 mg, 0.32 mmol) and 5-hydroxy-2-methylisoindol-1-one (58 mg, 0.36 mmol) in dichloromethane (4 mL) and N , N -dimethylformamide (2 mL) , was added N -hydroxy-7-azabenzotriazole (89 mg, 0.65 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (187 mg) , 0.98 mmol) and N , N -diisopropylethylamine (169 mg, 1.31 mmol), reacted at room temperature for 11 h, concentrated under reduced pressure to remove the solvent, added water (20 mL), extracted with ethyl acetate (10 mL × 2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 35/1) to obtain 132 mg of white solid, Yield 79%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.83 (d,J = 6.9 Hz, 1H), 7.29 (s, 1H), 7.09 – 7.22 (m, 2H), 6.78 – 6.90 (m, 2H), 6.61 (t,J F-H = 75.6 Hz, 1H), 4.61 – 4.72 (m, 1H), 4.35 (s, 2H), 3.95 – 4.05 (m, 1H), 3.87 (d,J = 5.7 Hz, 2H), 3.62 – 3.75 (m, 1H), 3.46 – 3.59 (m, 1H), 3.18 (s, 3H), 2.74 – 2.87 (m, 1H), 2.16 – 2.28 (m, 1H), 2.15 (s, 3H), 1.21 – 1.32 (m, 1H), 0.56 – 0.69 (m, 2H), 0.27 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.83 (d, J = 6.9 Hz, 1H), 7.29 (s, 1H), 7.09 – 7.22 (m, 2H), 6.78 – 6.90 (m, 2H) ), 6.61 (t, J FH = 75.6 Hz, 1H), 4.61 – 4.72 (m, 1H), 4.35 (s, 2H), 3.95 – 4.05 (m, 1H), 3.87 (d, J = 5.7 Hz, 2H ), 3.62 – 3.75 (m, 1H), 3.46 – 3.59 (m, 1H), 3.18 (s, 3H), 2.74 – 2.87 (m, 1H), 2.16 – 2.28 (m, 1H), 2.15 (s, 3H) ), 1.21 – 1.32 (m, 1H), 0.56 – 0.69 (m, 2H), 0.27 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 515.25 [M+H]+ .MS (ESI, pos.ion) m/z: 515.25 [M+H] + .
步驟5:化合物 (2R )-(2,3-二甲基-1-氧代異吲哚-5-基) 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 (實施例141) 的合成Step 5: Compound ( 2R )-(2,3-Dimethyl-1-oxoisoindol-5-yl)1-acetyl-4-(3-(cyclopropylmethoxy)- Synthesis of 4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate (Example 141)
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (120 mg, 0.32 mmol) 和5-羥基-2,3-二甲基異吲哚-1-酮 (63 mg, 0.36 mmol) 溶於二氯甲烷 (4 mL) 和N ,N -二甲基甲醯胺 (2 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (89 mg, 0.65 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (187 mg, 0.98 mmol) 和N ,N -二異丙基乙胺 (169 mg, 1.31 mmol),室溫反應11 h,減壓濃縮除去溶劑,加水 (20 mL),用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 35/1),得到白色固體137 mg,收率80%。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (120 mg, 0.32 mmol) and 5-hydroxy-2,3-dimethylisoindol-1-one (63 mg, 0.36 mmol) in dichloromethane (4 mL) and N , N -dimethylformamide ( 2 mL), add N -hydroxy-7-azabenzotriazole (89 mg, 0.65 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (187 mg, 0.98 mmol) and N , N -diisopropylethylamine (169 mg, 1.31 mmol), reacted at room temperature for 11 h, concentrated under reduced pressure to remove the solvent, added water (20 mL), extracted with ethyl acetate ( 10 mL × 2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 35/1) to obtain a white solid 137 mg, yield 80%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.82 (d,J = 7.7 Hz, 1H), 7.26 (s, 1H), 7.16 – 7.21 (m, 1H), 7.15 (d,J = 9.0 Hz, 1H), 6.80 – 6.90 (m, 2H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.66 – 4.69 (m, 1H), 4.37 – 4.47 (m, 1H), 3.96 – 4.05 (m, 1H), 3.87 (d,J = 6.4 Hz, 2H), 3.65 – 3.75 (m, 1H), 3.48 – 3.59 (m, 1H), 3.11 (s, 3H), 2.77 – 2.88 (m, 1H), 2.16 – 2.28 (m, 1H), 2.16 (s, 3H), 1.47 (d,J = 5.9 Hz, 3H), 1.21 – 1.32 (m, 1H), 0.60 – 0.69 (m, 2H), 0.30 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.82 (d, J = 7.7 Hz, 1H), 7.26 (s, 1H), 7.16 – 7.21 (m, 1H), 7.15 (d, J = 9.0 Hz, 1H), 6.80 – 6.90 (m, 2H), 6.62 (t, J FH = 75.6 Hz, 1H), 4.66 – 4.69 (m, 1H), 4.37 – 4.47 (m, 1H), 3.96 – 4.05 (m , 1H), 3.87 (d, J = 6.4 Hz, 2H), 3.65 – 3.75 (m, 1H), 3.48 – 3.59 (m, 1H), 3.11 (s, 3H), 2.77 – 2.88 (m, 1H), 2.16 – 2.28 (m, 1H), 2.16 (s, 3H), 1.47 (d, J = 5.9 Hz, 3H), 1.21 – 1.32 (m, 1H), 0.60 – 0.69 (m, 2H), 0.30 – 0.41 ( m, 2H).
MS (ESI, pos.ion) m/z: 529.30 [M+H]+ .MS (ESI, pos.ion) m/z: 529.30 [M+H] + .
實施例142:化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((6-氟吡啶-2-基)甲基)吡咯烷-2-甲醯胺 Example 142: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((6-fluoro Pyridin-2-yl)methyl)pyrrolidine-2-carboxamide
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (203 mg, 0.55 mmol),(6-氟吡啶-2-基)甲胺二鹽酸鹽 (162 mg, 0.81 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (521 mg, 2.72 mmol) 和N -羥基-7-氮雜苯並三氮唑 (149 mg, 1.09 mmol) 溶於二氯甲烷 (5 mL)中,0℃條件下向此溶液中滴加N ,N -二異丙基乙胺 (0.7 mL, 4.0 mmol),室溫反應16 h,加水洗 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1),得到淺黃色固體163 mg,收率62%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (203 mg, 0.55 mmol), (6-fluoropyridin-2-yl)methanamine dihydrochloride (162 mg, 0.81 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride Salt (521 mg, 2.72 mmol) and N -hydroxy-7-azabenzotriazole (149 mg, 1.09 mmol) were dissolved in dichloromethane (5 mL) and added dropwise to this solution at 0°C N , N -diisopropylethylamine (0.7 mL, 4.0 mmol), reacted at room temperature for 16 h, washed with water (15 mL), extracted with dichloromethane (5 mL × 3), and the organic phase was dried over anhydrous sodium sulfate , the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain 163 mg of a pale yellow solid with a yield of 62%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.75 – 7.81 (m, 1H), 7.24 (d,J = 5.4 Hz, 1H), 7.12 (d,J = 8.1 Hz, 1H), 6.90 (s, 1H), 6.81 – 6.87 (m, 2H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.54 – 4.63 (m, 3H), 3.94 – 3.98 (m, 1H), 3.88 (d,J = 6.9 Hz, 2H), 3.57 (t,J = 10.7 Hz, 1H), 3.30 – 3.42 (m, 1H), 2.54 – 2.62 (m, 1H), 2.42 – 2.51 (m, 1H), 2.16 (s, 3H), 1.24 – 1.33 (m, 1H), 0.64 – 0.69 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.75 – 7.81 (m, 1H), 7.24 (d, J = 5.4 Hz, 1H), 7.12 (d, J = 8.1 Hz, 1H), 6.90 ( s, 1H), 6.81 – 6.87 (m, 2H), 6.62 (t, J FH = 75.6 Hz, 1H), 4.54 – 4.63 (m, 3H), 3.94 – 3.98 (m, 1H), 3.88 (d, J = 6.9 Hz, 2H), 3.57 (t, J = 10.7 Hz, 1H), 3.30 – 3.42 (m, 1H), 2.54 – 2.62 (m, 1H), 2.42 – 2.51 (m, 1H), 2.16 (s, 3H), 1.24 – 1.33 (m, 1H), 0.64 – 0.69 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 478.30 [M+H]+ .MS (ESI, pos.ion) m/z: 478.30 [M+H] + .
實施例143:化合物 (6-(乙基(甲基)氨基甲醯基)吡啶-2-基)甲基 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 143: Compound (6-(ethyl(methyl)carbamoyl)pyridin-2-yl)methyl( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy) yl)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物6-(乙基(甲基)氨基甲醯基)吡啶甲酸甲酯 的合成Step 1: Synthesis of compound 6-(ethyl(methyl)carbamoyl)methyl picolinate
將化合物2,6-吡啶二羧酸單甲酯 (500 mg, 2.76 mmol),N -乙基甲基胺 (327 mg, 5.53 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (2.65 g, 13.8 mmol) 和N -羥基-7-氮雜苯並三氮唑 (750 mg, 5.51 mmol)溶於二氯甲烷 (10 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (2.1 g, 16.0 mmol),室溫反應5 h,加水 (20 mL),用二氯甲烷萃取 (15 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:石油醚/乙酸乙酯 ( v/v ) = 2/1),得到淺褐色液體592 mg,收率96%。Compound 2,6-pyridinedicarboxylate monomethyl ester (500 mg, 2.76 mmol), N -ethylmethylamine (327 mg, 5.53 mmol), 1-ethyl-(3-dimethylaminopropyl) ) carbodiimide hydrochloride (2.65 g, 13.8 mmol) and N -hydroxy-7-azabenzotriazole (750 mg, 5.51 mmol) were dissolved in dichloromethane (10 mL) and cooled to 0 °C, add N , N -diisopropylethylamine (2.1 g, 16.0 mmol), react at room temperature for 5 h, add water (20 mL), extract with dichloromethane (15 mL × 3), the organic phase is washed with anhydrous It was dried over sodium sulfate, concentrated under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 2/1) to obtain 592 mg of light brown liquid with a yield of 96%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 8.16 – 8.19 (m, 1H), 7.96 (t,J = 7.8 Hz, 1H), 7.85 (t,J = 6.3 Hz, 1H), 4.02 (s, 3H), 3.25 – 3.30 (m, 2H), 3.11 – 3.12 (m, 3H), 1.24 – 1.28 (m, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.16 – 8.19 (m, 1H), 7.96 (t, J = 7.8 Hz, 1H), 7.85 (t, J = 6.3 Hz, 1H), 4.02 ( s, 3H), 3.25 – 3.30 (m, 2H), 3.11 – 3.12 (m, 3H), 1.24 – 1.28 (m, 3H).
MS (ESI, pos.ion) m/z: 223.20 [M+H]+ .MS (ESI, pos.ion) m/z: 223.20 [M+H] + .
步驟2:化合物N -乙基-6-(羥甲基)-N -甲基吡啶醯胺的合成Step 2: Synthesis of compound N -ethyl-6-(hydroxymethyl) -N-picolinamide
將化合物6-(乙基(甲基)氨基甲醯基)吡啶甲酸甲酯 (370 mg, 1.66 mmol) 溶於四氫呋喃 (6 mL) 中,冰浴下加入硼氫化鋰 (354 mg, 16.6 mmol),室溫反應1 h後停止,加入飽和氯化鈉水溶液 (10 mL),乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 30/1),得到淺黃色液體116 mg,收率36%。The compound 6-(ethyl(methyl)carbamoyl)picolinate methyl ester (370 mg, 1.66 mmol) was dissolved in tetrahydrofuran (6 mL), and lithium borohydride (354 mg, 16.6 mmol) was added under ice bath. , the reaction was stopped after 1 h at room temperature, saturated aqueous sodium chloride solution (10 mL) was added, extracted with ethyl acetate (10 mL × 2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was subjected to silica gel column chromatography Separation (eluent: dichloromethane/methanol (v/v) = 30/1) gave 116 mg of pale yellow liquid, yield 36%.
1 H NMR (400 MHz,d6 -DMSO) δ (ppm): 7.90 (d,J = 7.7 Hz, 1H), 7.53 (d,J = 7.7 Hz, 1H), 7.36 (d,J = 7.6 Hz, 1H), 5.49 (t,J = 5.9 Hz, 1H), 4.56 – 4.58 (m, 2H), 3.47 (q,J = 0.8 Hz, 1H), 3.22 (q,J = 1.2 Hz, 1H), 2.96 (s, 1.7H), 2.88 (s, 1.3H), 1.14 (t,J = 7.1 Hz, 1.2H), 1.08 (t,J = 7.0 Hz, 1.8H). 1 H NMR (400 MHz, d 6 -DMSO) δ (ppm): 7.90 (d, J = 7.7 Hz, 1H), 7.53 (d, J = 7.7 Hz, 1H), 7.36 (d, J = 7.6 Hz, 1H), 5.49 (t, J = 5.9 Hz, 1H), 4.56 – 4.58 (m, 2H), 3.47 (q, J = 0.8 Hz, 1H), 3.22 (q, J = 1.2 Hz, 1H), 2.96 ( s, 1.7H), 2.88 (s, 1.3H), 1.14 (t, J = 7.1 Hz, 1.2H), 1.08 (t, J = 7.0 Hz, 1.8H).
MS (ESI, pos.ion) m/z: 195.20 [M+H]+ .MS (ESI, pos.ion) m/z: 195.20 [M+H] + .
步驟3:化合物 (6-(乙基(甲基)氨基甲醯基)吡啶-2-基)甲基 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 3: Compound (6-(ethyl(methyl)carbamoyl)pyridin-2-yl)methyl( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy) )-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (70 mg, 0.19 mmol),N -乙基-6-(羥甲基)-N -甲基吡啶醯胺 (52 mg, 0.27 mmol),1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (181 mg, 0.94 mmol) 和N -羥基-7-氮雜苯並三氮唑 (51 mg, 0.37 mmol) 溶於二氯甲烷 (5 mL) 中,冷卻至0℃,加入N ,N -二異丙基乙胺 (146 mg, 0.13 mmol),室溫反應12 h,加水 (15 mL),用二氯甲烷萃取 (5 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 25/1),得到淺黃色黏稠固體216 mg,收率49%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (70 mg, 0.19 mmol), N -ethyl-6-(hydroxymethyl) -N -picoline amide (52 mg, 0.27 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide Imine hydrochloride (181 mg, 0.94 mmol) and N -hydroxy-7-azabenzotriazole (51 mg, 0.37 mmol) were dissolved in dichloromethane (5 mL), cooled to 0 °C, added N , N -diisopropylethylamine (146 mg, 0.13 mmol), reacted at room temperature for 12 h, added water (15 mL), extracted with dichloromethane (5 mL × 3), the organic phase was dried over anhydrous sodium sulfate, The solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 25/1) to obtain 216 mg of a light yellow viscous solid with a yield of 49%.
1 H NMR (400 MHz, CDCl3 ) δ (ppm): 7.84 (t,J = 7.7 Hz, 1H), 7.51 – 7.55 (m, 2H), 7.15 (d,J = 8.6 Hz, 1H), 6.84 (s, 1H), 6.80 – 6.84 (m, 1H), 6.63 (t,J F-H = 71.5 Hz, 1H), 5.26 – 5.47 (m, 2H), 4.59 – 4.64 (m, 1H), 3.96 – 4.01 (m, 1H), 3.88 (d,J = 6.9 Hz, 2H), 3.56 – 3.68 (m, 2H), 3.41 – 3.52 (m, 1H), 3.33 – 3.40 (m, 1H), 3.09 (s, 1.8H), 3.01 (s, 1.2H), 2.70 – 2.77 (m, 1H), 2.15 (s, 3H), 2.06 – 1.15 (m, 1H), 1.25 – 1.34 (m, 1H), 1.17 – 1.26 (m, 3H), 0.65 – 0.69 (m, 2H), 0.35 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.84 (t, J = 7.7 Hz, 1H), 7.51 – 7.55 (m, 2H), 7.15 (d, J = 8.6 Hz, 1H), 6.84 ( s, 1H), 6.80 – 6.84 (m, 1H), 6.63 (t, J FH = 71.5 Hz, 1H), 5.26 – 5.47 (m, 2H), 4.59 – 4.64 (m, 1H), 3.96 – 4.01 (m , 1H), 3.88 (d, J = 6.9 Hz, 2H), 3.56 – 3.68 (m, 2H), 3.41 – 3.52 (m, 1H), 3.33 – 3.40 (m, 1H), 3.09 (s, 1.8H) , 3.01 (s, 1.2H), 2.70 – 2.77 (m, 1H), 2.15 (s, 3H), 2.06 – 1.15 (m, 1H), 1.25 – 1.34 (m, 1H), 1.17 – 1.26 (m, 3H) ), 0.65 – 0.69 (m, 2H), 0.35 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 546.30 [M+H]+ .MS (ESI, pos.ion) m/z: 546.30 [M+H] + .
實施例144:化合物 (2R )-1-異吲哚酮-5-基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯 Example 144: Compound ( 2R )-1-isoindolinone-5-yl 1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)benzene yl)pyrrolidine-2-carboxylate
將化合物 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (128 mg, 0.35 mmol),5-羥基異吲哚-1-酮 (61 mg, 0.41 mmol) 和N -羥基-7-氮雜苯並三氮唑 (91 mg, 0.67 mmol) 溶於N ,N -二甲基甲醯胺 (15 mL) 中,冷卻至0℃,加入1-乙基-(3-二甲基氨基丙基)碳醯二亞胺鹽酸鹽 (186 mg, 0.97 mmol) 和N ,N -二異丙基乙胺 (127 mg, 1.29 mmol),室溫反應7 h,減壓濃縮除去溶劑,加水 (20 mL),用乙酸乙酯萃取 (10 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:DCM/MeOH ( v/v ) = 35/1),得到淺褐色固體59 mg,收率34%。The compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (128 mg, 0.35 mmol), 5-hydroxyisoindol-1-one (61 mg, 0.41 mmol) and N -hydroxy-7-azabenzotriazole (91 mg, 0.67 mmol) in N , N -dimethyl carbodiimide (15 mL), cooled to 0°C, added 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (186 mg, 0.97 mmol) and N , N -Diisopropylethylamine (127 mg, 1.29 mmol), reacted at room temperature for 7 h, concentrated under reduced pressure to remove the solvent, added water (20 mL), extracted with ethyl acetate (10 mL × 2), the organic phase was washed with anhydrous sulfuric acid It was dried over sodium, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: DCM/MeOH (v/v) = 35/1) to obtain 59 mg of a light brown solid with a yield of 34%.
1 H NMR (400 MHz, CD3 OD) δ (ppm): 7.83 (d,J = 8.3 Hz, 1H), 7.33 (s, 1H), 7.23 (d,J = 8.3 Hz, 1H), 7.15 (d,J = 8.2 Hz, 1H), 7.07 (s, 1H), 6.96 – 6.99 (m, 1H), 6.76 (t,J F-H = 74.9 Hz, 1H), 4.50 (s, 2H), 4.14 – 4.18 (m, 1H), 3.90 (d,J = 7.0 Hz, 2H), 3.62 – 3.77 (m, 3H), 2.85 – 2.93 (m, 1H), 2.19 – 2.28 (m, 1H), 2.20 (s, 3H), 1.21 – 1.28 (m, 1H), 0.59– 0.63 (m, 2H), 0.30 – 0.34 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ (ppm): 7.83 (d, J = 8.3 Hz, 1H), 7.33 (s, 1H), 7.23 (d, J = 8.3 Hz, 1H), 7.15 (d , J = 8.2 Hz, 1H), 7.07 (s, 1H), 6.96 – 6.99 (m, 1H), 6.76 (t, J FH = 74.9 Hz, 1H), 4.50 (s, 2H), 4.14 – 4.18 (m , 1H), 3.90 (d, J = 7.0 Hz, 2H), 3.62 – 3.77 (m, 3H), 2.85 – 2.93 (m, 1H), 2.19 – 2.28 (m, 1H), 2.20 (s, 3H), 1.21 – 1.28 (m, 1H), 0.59 – 0.63 (m, 2H), 0.30 – 0.34 (m, 2H).
MS-ESI: m/z 501.05 [M+H]+ .MS-ESI: m/z 501.05 [M+H] + .
實施例145:1-羥基-1,3-二氫苯並[c ][1,2]氧雜環戊硼烷-5-基 (2R )-1-乙醯基-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸酯 Example 145: 1-Hydroxy-1,3-dihydrobenzo[ c ][1,2]oxaborol-5-yl( 2R )-1-acetoxy-4-(6- Ethoxypyridin-3-yl)pyrrolidine-2-carboxylate
步驟1: 化合物2-乙氧基-5-碘吡啶的合成Step 1: Synthesis of compound 2-ethoxy-5-iodopyridine
往封管中加入2-羥基-5-碘吡啶 (1.50 g,4.00 mmol),無水碳酸鉀 (1.90 g, 14.0 mmol),DMF (12 ml),在室溫下攪拌30 min後,加入碘乙烷 (3 g, 19.2 mmol), 80℃下攪拌反應12 h。加入乙酸乙酯 (100 mL) 稀釋,用飽和氯化銨溶液洗滌有機相 (40 mL),用無水硫酸鈉乾燥有機相,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 石油醚/乙酸乙酯 (v/v) = 4/1) 得到黃色固體 (1.00 g, 產率59%)。2-Hydroxy-5-iodopyridine (1.50 g, 4.00 mmol), anhydrous potassium carbonate (1.90 g, 14.0 mmol), DMF (12 ml) were added to the sealed tube, and after stirring at room temperature for 30 min, ethyl iodide was added. alkane (3 g, 19.2 mmol), and the reaction was stirred at 80 °C for 12 h. Ethyl acetate (100 mL) was added to dilute, the organic phase (40 mL) was washed with saturated ammonium chloride solution, the organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent, and the concentrate was separated by silica gel column chromatography (eluent). : petroleum ether/ethyl acetate (v/v) = 4/1) to give a yellow solid (1.00 g, 59% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.50 (d,J = 2.3 Hz, 1H), 7.41 (dd,J = 9.5, 2.5 Hz, 1H), 6.39 (d,J = 9.5 Hz, 1H), 3.95 (q,J = 7.2 Hz, 2H), 1.35 (t,J = 7.2 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.50 (d, J = 2.3 Hz, 1H), 7.41 (dd, J = 9.5, 2.5 Hz, 1H), 6.39 (d, J = 9.5 Hz, 1H), 3.95 (q, J = 7.2 Hz, 2H), 1.35 (t, J = 7.2 Hz, 3H).
MS (ESI, pos.ion) m/z: 250.00 [M+H]+ .MS (ESI, pos.ion) m/z: 250.00 [M+H] + .
步驟2: 化合物1-叔丁基 2-甲基 (R )-4-(6-乙氧基吡啶-3-基)-1H -吡咯-1,2-(2H, 5H )-二甲酸酯的合成Step 2: compound 1-tert-butyl 2-methyl (R) -4- (6- ethoxy-pyridin-3-yl) -1 H - pyrrole -1,2- (2 H, 5 H) - two Synthesis of Formate
將(R )-1-叔丁基-2-甲基-4-(4,4,5,5-四甲基-1,3,2-二氧雜環戊硼烷-2-基)-1H -吡咯-1,2(2H ,5H )-二羧酸酯(2.00 g, 4.40 mmol),2-乙氧基-5-碘吡啶 (1.00 g, 4.00 mmol),磷酸鉀 (3.40 g, 16.1 mmol) 和[1,1'-雙(二苯基膦基)二茂鐵]二氯化鈀(Pd(dppf)Cl2 ) (147 mg, 0.20 mmol) 混合在乾燥的1,4-二氧六環 (10 mL) 溶液中,氮氣保護下100℃反應22 h,冷卻至室溫後抽濾,濾液中加入水 (50 mL),用乙酸乙酯 (15 mL × 3) 萃取,有機相合併後用無水硫酸鈉乾燥,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 石油醚/乙酸乙酯 ( v/v ) = 1/4) 得到黃色液體 (0.80 g, 產率57%)。( R )-1-tert-butyl-2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)- 1H -pyrrole-1,2( 2H , 5H )-dicarboxylate (2.00 g, 4.40 mmol), 2-ethoxy-5-iodopyridine (1.00 g, 4.00 mmol), potassium phosphate (3.40 g, 16.1 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (Pd(dppf)Cl 2 ) (147 mg, 0.20 mmol) were mixed in dry 1,4 - Dioxane (10 mL) solution, reacted at 100 °C for 22 h under nitrogen protection, cooled to room temperature, filtered with suction, added water (50 mL) to the filtrate, extracted with ethyl acetate (15 mL × 3), The organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent, and the concentrated solution was separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/4) to obtain a yellow liquid (0.80 g, 57% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.46 (dd,J = 9.5, 2.6 Hz, 1H), 7.14 – 7.20 (m, 1H), 6.58 (d,J = 9.5 Hz, 1H), 5.79 – 5.88 (m, 1H), 5.04 – 5.16 (m, 1H), 4.35 – 4.56 (m, 2H), 3.94 – 4.03 (m, 2H), 3.75 (d,J = 3.9 Hz, 3H), 1.52 (s, 3H), 1.45 (s, 6H), 1.32 – 1.39 (m, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.46 (dd, J = 9.5, 2.6 Hz, 1H), 7.14 – 7.20 (m, 1H), 6.58 (d, J = 9.5 Hz, 1H), 5.79 – 5.88 (m, 1H), 5.04 – 5.16 (m, 1H), 4.35 – 4.56 (m, 2H), 3.94 – 4.03 (m, 2H), 3.75 (d, J = 3.9 Hz, 3H), 1.52 ( s, 3H), 1.45 (s, 6H), 1.32 – 1.39 (m, 3H).
MS (ESI, pos.ion) m/z: 349.65 [M+H]+ .MS (ESI, pos.ion) m/z: 349.65 [M+H] + .
步驟3: 化合物1-(叔丁基) 2-甲基 (2R )-4-(6-乙氧基吡啶-3-基)吡咯烷-1,2-二甲酸酯的合成Step 3: Synthesis of compound 1-(tert-butyl)2-methyl( 2R )-4-(6-ethoxypyridin-3-yl)pyrrolidine-1,2-dicarboxylate
將化合物 1-叔丁基 2-甲基 (R )-4-(6-乙氧基吡啶-3-基)-1H -吡咯-1,2-(2H, 5H )-二甲酸酯 (0.8 g, 2.3 mmol) 溶於甲醇 (15 mL),加入Pd/C (80 mg, 0.10 g/g),通入氫氣,室溫反應23 h,過濾除去催化劑,濾液濃縮得到黃色油狀物 (639 mg, 產率79%)。The compound 1-tert-butyl 2-methyl (R) -4- (6- ethoxy-pyridin-3-yl) -1 H - pyrrole -1,2- (2 H, 5 H) - dicarboxylic acid The ester (0.8 g, 2.3 mmol) was dissolved in methanol (15 mL), Pd/C (80 mg, 0.10 g/g) was added, hydrogen was passed through, the reaction was carried out at room temperature for 23 h, the catalyst was removed by filtration, and the filtrate was concentrated to obtain a yellow oil compound (639 mg, 79% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.19 – 7.26 (m, 1H), 7.08 – 7.12 (m, 1H), 6.56 (d,J = 9.4 Hz, 1H), 4.27 – 4.40 (m, 1H), 3.91 – 3.99 (m, 0.5H), 3.95 (q,J = 7.2 Hz, 2H), 3.82 – 3.89 (m, 0.5H), 3.75 (d,J = 6.3 Hz, 3H), 3.27 – 3.38 (m, 1H), 3.05 – 3.17 (m, 1H), 2.51 – 2.63 (m, 1H), 1.84 – 1.98 (m, 1H), 1.38 – 1.49 (m, 9H), 1.34 (t,J = 7.2 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.19 – 7.26 (m, 1H), 7.08 – 7.12 (m, 1H), 6.56 (d, J = 9.4 Hz, 1H), 4.27 – 4.40 (m , 1H), 3.91 – 3.99 (m, 0.5H), 3.95 (q, J = 7.2 Hz, 2H), 3.82 – 3.89 (m, 0.5H), 3.75 (d, J = 6.3 Hz, 3H), 3.27 – 3.38 (m, 1H), 3.05 – 3.17 (m, 1H), 2.51 – 2.63 (m, 1H), 1.84 – 1.98 (m, 1H), 1.38 – 1.49 (m, 9H), 1.34 (t, J = 7.2 Hz, 3H).
MS (ESI, pos.ion) m/z: 351.20 [M+H]+ .MS (ESI, pos.ion) m/z: 351.20 [M+H] + .
步驟4: 化合物(2R )-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸甲酯鹽酸鹽的合成Step 4: Synthesis of Compound (2R )-4-(6-ethoxypyridin-3-yl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物1-(叔丁基) 2-甲基 (2R ) -4-(6-乙氧基吡啶-3-基)吡咯烷-1,2-二甲酸酯 (0.64 g, 1.80 mmol) 溶解於二氯甲烷 (6 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (8 mL),室溫攪拌50 min,減壓濃縮除去溶劑,得到淺黃色固體 (585 mg, 產率100%)。Compound 1-(tert-butyl)2-methyl( 2R )-4-(6-ethoxypyridin-3-yl)pyrrolidine-1,2-dicarboxylate (0.64 g, 1.80 mmol) It was dissolved in dichloromethane (6 mL) solution, 4 mol/L HCl in ethyl acetate solution (8 mL) was added, stirred at room temperature for 50 min, concentrated under reduced pressure to remove the solvent to obtain a pale yellow solid (585 mg, yield 100%).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.83 – 7.89 (m, 1H), 7.70 – 7.76 (m, 1H), 6.74 (d,J = 9.2 Hz, 1H), 4.61 (dd,J = 10.5, 7.5 Hz, 1H), 4.11 (q,J = 7.1 Hz, 2H), 3.88 (s, 3H), 3.72 – 3.80 (m, 1H), 3.53 – 3.67 (m, 1H), 3.27 – 3.34 (m, 1H), 2.75 – 2.86 (m, 1H), 2.20 (q,J = 12.0 Hz, 1H), 1.37 (t,J = 7.2 Hz, 3H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.83 – 7.89 (m, 1H), 7.70 – 7.76 (m, 1H), 6.74 (d, J = 9.2 Hz, 1H), 4.61 (dd, J = 10.5, 7.5 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.88 (s, 3H), 3.72 – 3.80 (m, 1H), 3.53 – 3.67 (m, 1H), 3.27 – 3.34 (m, 1H), 2.75 – 2.86 (m, 1H), 2.20 (q, J = 12.0 Hz, 1H), 1.37 (t, J = 7.2 Hz, 3H).
MS (ESI, pos.ion) m/z: 251.20 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 251.20 [M+H-HCl] + .
步驟5: 化合物(2R )-1-乙醯基-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸甲酯的合成Step 5: Synthesis of Compound (2R )-1-Acetyl-4-(6-ethoxypyridin-3-yl)pyrrolidine-2-carboxylic acid methyl ester
將化合物(2R )-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸甲酯鹽酸鹽 (520 mg, 1.80 mmol) 溶解在二氯甲烷 (8 mL) 中,加入N ,N -二異丙基乙胺 (1.89 mL, 10.9 mmol),冷卻至0 ℃,加入乙醯氯 (0.39 mL, 5.4 mmol),室溫攪拌3 h後停止反應,加水 (15 mL),二氯甲烷萃取 (10 mL × 3),合併有機相用無水硫酸鈉乾燥,減壓濃縮除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1) 得到褐色液體 (378 mg, 71%)。Compound ( 2R )-4-(6-ethoxypyridin-3-yl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride (520 mg, 1.80 mmol) was dissolved in dichloromethane (8 mL), N , N -diisopropylethylamine (1.89 mL, 10.9 mmol) was added, cooled to 0 °C, acetyl chloride (0.39 mL, 5.4 mmol) was added, the reaction was stopped after stirring at room temperature for 3 h, and water (15 mL) was added. , extracted with dichloromethane (10 mL × 3), the combined organic phases were dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to give a brown liquid (378 mg, 71%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.28 (d,J = 2.6 Hz, 1H), 7.12 – 7.17 (m, 1H), 6.58 (d,J = 9.3 Hz, 1H), 4.42 – 4.51 (m, 1H), 3.91 – 4.02 (m, 2H), 3.83 – 3.91 (m, 1H), 3.73 – 3.79 (m, 3H), 3.52 (t,J = 10.4 Hz, 1H), 3.15 – 3.27 (m, 1H), 2.53 – 2.63 (m, 1H), 2.11 (s, 3H), 1.89 – 1.97 (m, 1H), 1.33 – 1.38 (m, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.28 (d, J = 2.6 Hz, 1H), 7.12 – 7.17 (m, 1H), 6.58 (d, J = 9.3 Hz, 1H), 4.42 – 4.51 (m, 1H), 3.91 – 4.02 (m, 2H), 3.83 – 3.91 (m, 1H), 3.73 – 3.79 (m, 3H), 3.52 (t, J = 10.4 Hz, 1H), 3.15 – 3.27 ( m, 1H), 2.53 – 2.63 (m, 1H), 2.11 (s, 3H), 1.89 – 1.97 (m, 1H), 1.33 – 1.38 (m, 3H).
MS (ESI, pos.ion) m/z: 293.15 [M+H]+ .MS (ESI, pos.ion) m/z: 293.15 [M+H] + .
步驟6: (2R )-1-乙醯基-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸的合成Step 6: Synthesis of (2R )-1-Acetyl-4-(6-ethoxypyridin-3-yl)pyrrolidine-2-carboxylic acid
將化合物(2R )-1-乙醯基-4-(6-乙氧基吡啶-3-基)吡咯烷-2-羧酸甲酯 (350 mg, 1.20 mmol) 溶於四氫呋喃 (6 mL) 和水 (3 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (100 mg, 2.40 mmol),50℃反應1.5 h後停止,加稀鹽酸調節溶液Ph = 1,減壓濃縮除去溶劑,進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 8/1) 得到白色固體 (375 mg, 99%)。Compound ( 2R )-methyl 1-acetyl-4-(6-ethoxypyridin-3-yl)pyrrolidine-2-carboxylate (350 mg, 1.20 mmol) was dissolved in tetrahydrofuran (6 mL) In a mixed solvent of water (3 mL), lithium hydroxide monohydrate (100 mg, 2.40 mmol) was added, and the reaction was stopped after 1.5 h at 50 °C. Diluted hydrochloric acid was added to adjust the pH of the solution to 1. Silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 8/1) gave a white solid (375 mg, 99%).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.60 – 7.64 (m, 2H), 6.55 (d,J = 9.0 Hz, 1H), 4.32 (dd,J = 16.9, 8.0 Hz, 1H), 4.10 – 4.16 (m, 0.6H), 4.03 (q,J = 7.1 Hz, 2H), 3.93 – 3.98 (m, 0.4H), 3.51 – 3.58 (m, 0.4H), 3.25 – 3.33 (m, 0.6H), 3.09 – 3.24 (m, 1H), 2.57 – 2.79 (m, 1H), 2.10 (s, 1H), 2.01 (s, 2H), 1.83 – 1.98 (m, 1H), 1.33 (t,J = 7.1 Hz, 3H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.60 – 7.64 (m, 2H), 6.55 (d, J = 9.0 Hz, 1H), 4.32 (dd, J = 16.9, 8.0 Hz, 1H) , 4.10 – 4.16 (m, 0.6H), 4.03 (q, J = 7.1 Hz, 2H), 3.93 – 3.98 (m, 0.4H), 3.51 – 3.58 (m, 0.4H), 3.25 – 3.33 (m, 0.6 H), 3.09 – 3.24 (m, 1H), 2.57 – 2.79 (m, 1H), 2.10 (s, 1H), 2.01 (s, 2H), 1.83 – 1.98 (m, 1H), 1.33 (t, J = 7.1 Hz, 3H).
MS (ESI, pos.ion) m/z: 279.25 [M+H]+ .MS (ESI, pos.ion) m/z: 279.25 [M+H] + .
步驟7: 1-羥基-1,3-二氫苯並[c ][1,2]氧雜環戊硼烷-5-基 (2R )-1-乙醯基-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸酯的合成Step 7: 1-Hydroxy-1,3-dihydrobenzo[ c ][1,2]oxaborolane-5-yl( 2R )-1-acetoxy-4-(6-ethyl) Synthesis of Oxypyridin-3-yl)pyrrolidine-2-carboxylate
將化合物(2R )-1-乙醯基-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸 (200 mg, 0.64 mmol),2-羥基甲基-5-羥基苯硼酸半酯 (114 mg, 0.76 mmol) 溶於N ,N -二甲基甲醯胺 (10 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (265 mg, 1.91 mmol),在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (365 mg, 1.91 mmol) 和N ,N -二異丙基乙胺 (494 mg, 3.81 mmol),室溫反應23 h,加水 (20 mL) 攪拌5 min,二氯甲烷萃取 (5 mL × 2),用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 15/1),得到白色固體 (100 mg, 產率56%)。Compound ( 2R )-1-acetyl-4-(6-ethoxypyridin-3-yl)pyrrolidine-2-carboxylic acid (200 mg, 0.64 mmol), 2-hydroxymethyl-5-hydroxy Phenylboronic acid half ester (114 mg, 0.76 mmol) was dissolved in N , N -dimethylformamide (10 mL), and N -hydroxy-7-azabenzotriazole (265 mg, 1.91 mmol) was added , 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (365 mg, 1.91 mmol) and N , N -diisopropylethylamine (494 mg) were added in an ice bath , 3.81 mmol), reacted at room temperature for 23 h, added water (20 mL), stirred for 5 min, extracted with dichloromethane (5 mL × 2), dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was subjected to silica gel column chromatography Isolation (eluent: dichloromethane/methanol (v/v) = 15/1) gave a white solid (100 mg, 56% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.75 (d,J = 8.0 Hz, 1H), 7.32 (dd,J = 9.4, 2.5 Hz, 1H), 7.18 – 7.23 (m, 1H), 7.11 (s, 1H), 7.06 (d,J = 7.9 Hz, 1H), 6.63 (d,J = 9.4 Hz, 1H), 5.03 (s, 2H), 4.63 – 4.72 (m, 1H), 3.90 – 4.03 (m, 3H), 3.60 (t,J = 10.4 Hz, 1H), 3.25 – 3.39 (m, 1H), 2.68 – 2.82 (m, 1H), 2.16 (s, 3H), 2.09 – 2.13 (m, 1H), 1.33 (t,J = 7.2 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.75 (d, J = 8.0 Hz, 1H), 7.32 (dd, J = 9.4, 2.5 Hz, 1H), 7.18 – 7.23 (m, 1H), 7.11 (s, 1H), 7.06 (d, J = 7.9 Hz, 1H), 6.63 (d, J = 9.4 Hz, 1H), 5.03 (s, 2H), 4.63 – 4.72 (m, 1H), 3.90 – 4.03 (m, 3H), 3.60 (t, J = 10.4 Hz, 1H), 3.25 – 3.39 (m, 1H), 2.68 – 2.82 (m, 1H), 2.16 (s, 3H), 2.09 – 2.13 (m, 1H) ), 1.33 (t, J = 7.2 Hz, 3H).
MS (ESI, pos.ion) m/z: m/z: 411.10 [M+H]+ .MS (ESI, pos.ion) m/z: m/z: 411.10 [M+H] + .
實施例146:(6-(乙基(甲基)氨基甲醯基)吡啶-2-基)甲基 (2R )-1-乙醯基-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸酯 Example 146: (6-(Ethyl(methyl)carbamoyl)pyridin-2-yl)methyl( 2R )-1-acetyl-4-(6-ethoxypyridine-3- yl)pyrrolidine-2-carboxylate
將化合物(2R )-1-乙醯基-4-(6-乙氧基吡啶-3-基)吡咯烷-2-甲酸 (100 mg, 0.36 mmol,實施例145步驟6),N -乙基-6-(羥基甲基)-N -甲基吡啶醯胺 (74 mg, 0.38 mmol) 溶於N ,N -二甲基甲醯胺 (5 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (132 mg, 0.95 mmol),在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (184 mg, 0.95 mmol) 和N ,N -二異丙基乙胺 (247 mg, 1.90 mmol),室溫反應23 h,減壓濃縮除去溶劑,加水 (10 mL) 攪拌5 min,二氯甲烷萃取 (20 mL × 2),合併有機相用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1),得到白色固體 (60 mg, 產率:42%)。Compound ( 2R )-1-acetyl-4-(6-ethoxypyridin-3-yl)pyrrolidine-2-carboxylic acid (100 mg, 0.36 mmol, Example 145 step 6), N -ethyl yl-6-(hydroxymethyl) -N -picolinamide (74 mg, 0.38 mmol) was dissolved in N , N -dimethylformamide (5 mL) and N -hydroxy-7-nitrogen was added Heterobenzotriazole (132 mg, 0.95 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (184 mg, 0.95 mmol) and N , N -diisopropylethylamine (247 mg, 1.90 mmol), reacted at room temperature for 23 h, concentrated under reduced pressure to remove the solvent, added water (10 mL), stirred for 5 min, extracted with dichloromethane (20 mL × 2), The combined organic phases were dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain a white solid (60 mg, product rate: 42%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.77 – 7.85 (m, 1H), 7.45 – 7.54 (m, 2H), 7.23 – 7.26 (m, 1H), 7.15 – 7.18 (m, 1H), 6.58 (d,J = 9.4 Hz, 1H), 5.36 – 5.44 (m, 1H), 5.21 – 5.31 (m, 1H), 4.52 – 4.62 (m, 1H), 3.93 – 4.04 (m, 2H), 3.83 – 3.92 (m, 1H), 3.48 – 3.62 (m, 2H), 3.29 – 3.37 (m, 1H), 3.19 – 3.28 (m, 1H), 3.07 (s, 2H), 2.98 (s, 1H), 2.56 – 2.69 (m, 1H), 2.11 (s, 3H), 2.00 – 2.05 (m, 1H), 1.34 (t,J = 7.1 Hz, 3H), 1.20 – 1.23 (m, 1H), 1.12 – 1.19 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.77 – 7.85 (m, 1H), 7.45 – 7.54 (m, 2H), 7.23 – 7.26 (m, 1H), 7.15 – 7.18 (m, 1H) , 6.58 (d, J = 9.4 Hz, 1H), 5.36 – 5.44 (m, 1H), 5.21 – 5.31 (m, 1H), 4.52 – 4.62 (m, 1H), 3.93 – 4.04 (m, 2H), 3.83 – 3.92 (m, 1H), 3.48 – 3.62 (m, 2H), 3.29 – 3.37 (m, 1H), 3.19 – 3.28 (m, 1H), 3.07 (s, 2H), 2.98 (s, 1H), 2.56 – 2.69 (m, 1H), 2.11 (s, 3H), 2.00 – 2.05 (m, 1H), 1.34 (t, J = 7.1 Hz, 3H), 1.20 – 1.23 (m, 1H), 1.12 – 1.19 (m , 2H).
MS (ESI, pos.ion) m/z: 455.20[M+H]+ .MS (ESI, pos.ion) m/z: 455.20[M+H] + .
實施例147:(2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-氧代異吲哚啉-5-基)吡咯烷-2-甲醯胺 Example 147: ( 2S )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (1-oxoiso Indolin-5-yl)pyrrolidine-2-carboxamide
將化合物(2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸(151 mg, 0.41 mmol,實施例18步驟1),5-氨基異吲哚啉-1-酮 (89 mg, 0.60 mmol) 和N -羥基-7-氮雜苯並三氮唑 (110 mg, 0.81 mmol) 溶於二氯甲烷/無水N ,N -二甲基甲醯胺 (8 mL, ( v/v ) = 3/1) 中,在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (243 mg, 1.26 mmol) 和N ,N -二異丙基乙胺 (268 uL, 1.62 mmol),室溫攪拌3 h。加飽和食鹽水 (20 mL),水相用二氯甲烷萃取 (50 mL × 3),合併有機相,用無水硫酸鈉乾燥,減壓除去溶劑,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1) 得到黃白色固體 (161 mg, 79%, HPLC 98.44%)。Compound ( 2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (151 mg, 0.41 mmol, Example 18 step 1), 5-aminoisoindolin-1-one (89 mg, 0.60 mmol) and N -hydroxy-7-azabenzotriazole (110 mg, 0.81 mmol) were dissolved in Dichloromethane/anhydrous N , N -dimethylformamide (8 mL, (v/v) = 3/1), add 1-ethyl-3-(3-dimethylaminopropane) in an ice bath base) carbodiimide hydrochloride (243 mg, 1.26 mmol) and N , N -diisopropylethylamine (268 uL, 1.62 mmol), and stirred at room temperature for 3 h. Saturated brine (20 mL) was added, the aqueous phase was extracted with dichloromethane (50 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography (eluent). : dichloromethane/methanol (v/v) = 50/1) to give an off-white solid (161 mg, 79%, HPLC 98.44%).
1 H NMR (600 MHz, CDCl3 ): δ (ppm) 10.25 (s, 1H), 8.93 (s, 1H), 8.11 (s, 1H),7.43 (d,J = 8.1 Hz, 1H), 7.11 (d,J = 8.1 Hz, 1H), 6.92 (d,J = 8.0 Hz, 1H), 6.89 (s, 1H), 6.84 (d,J = 8.2 Hz, 1H), 6.60 (t,J = 75.6 Hz, 1H), 4.76 (t,J = 7.4 Hz, 1H), 4.30 – 4.16 (m, 2H), 4.07 – 3.96 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.72 (t,J = 9.9 Hz, 1H), 3.48 – 3.36 (m, 1H), 2.73 – 2.61 (m, 1H), 2.37 – 2.27 (m, 1H), 2.24 (s, 3H), 1.30 – 1.21 (m, 1H), 0.67 – 0.60 (m, 2H), 0.38 – 0.30 (m, 2H). 1 H NMR (600 MHz, CDCl 3 ): δ (ppm) 10.25 (s, 1H), 8.93 (s, 1H), 8.11 (s, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.11 ( d, J = 8.1 Hz, 1H), 6.92 (d, J = 8.0 Hz, 1H), 6.89 (s, 1H), 6.84 (d, J = 8.2 Hz, 1H), 6.60 (t, J = 75.6 Hz, 1H), 4.76 (t, J = 7.4 Hz, 1H), 4.30 – 4.16 (m, 2H), 4.07 – 3.96 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.72 (t, J = 9.9 Hz, 1H), 3.48 – 3.36 (m, 1H), 2.73 – 2.61 (m, 1H), 2.37 – 2.27 (m, 1H), 2.24 (s, 3H), 1.30 – 1.21 (m, 1H), 0.67 – 0.60 (m, 2H), 0.38 – 0.30 (m, 2H).
MS (ESI, pos.ion) m/z: 500.20 [M+H]+ .MS (ESI, pos.ion) m/z: 500.20 [M+H] + .
實施例148:(6-(甲基(p -甲苯基)氨基甲醯基)吡啶-2-基)甲基 (2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 148: (6-(Methyl( p -tolyl)carbamoyl)pyridin-2-yl)methyl( 2S )-1-acetyl-4-(3-(cyclopropylmethyl) oxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸 (107 mg, 0.29 mmol,實施例18步驟1),6-羥甲基-N -甲基-N -對甲苯基-吡啶甲醯胺 (90 mg, 0.35 mmol) 和N -羥基-7-氮雜苯並三氮唑 (74 mg, 0.54 mmol) 溶於二氯甲烷 (10 mL) 中,在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (157 mg, 81 mmol) 和N ,N -二異丙基乙胺 (179 uL, 1.08 mmol),室溫攪拌3 h。加飽和食鹽水 (20 mL),水相用二氯甲烷萃取 (50 mL × 3),合併有機相,用無水硫酸鈉乾燥,減壓除去溶劑,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1) 得到白色固體 (80 mg, 產率79%)。The compound ( 2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (107 mg, 0.29 mmol, Example 18 step 1), 6-hydroxymethyl- N -methyl- N -p-tolyl-picolinamide (90 mg, 0.35 mmol) and N -hydroxy-7-azabenzotri Azole (74 mg, 0.54 mmol) was dissolved in dichloromethane (10 mL), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (157 mg, 81 mmol) and N , N -diisopropylethylamine (179 uL, 1.08 mmol), and stirred at room temperature for 3 h. Saturated brine (20 mL) was added, the aqueous phase was extracted with dichloromethane (50 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography (eluent). : dichloromethane/methanol (v/v) = 50/1) to give a white solid (80 mg, 79% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.57 (s, 1H), 7.38 – 7.18 (m, 2H), 7.11 (d,J = 8.1 Hz, 1H), 7.03 – 6.86 (m, 4H), 6.84 – 6.76 (m, 2H), 6.60 (t,J = 75.5 Hz, 1H), 5.25 – 4.98 (m, 2H), 4.59 – 1.49 (m, 1H), 3.94 (t,J = 8.0 Hz, 1H), 3.85 (d,J = 6.8 Hz, 2H), 3.61 (t,J = 10.1 Hz, 1H), 3.51 – 3.34 (m, 4H), 2.74 – 2.64 (m, 1H), 2.25 (s, 3H), 2.11 (s, 3H), 2.16 – 2.02 (m, 1H), 1.33 – 1.21 (m, 1H), 0.69 – 0.58 (m, 2H), 0.41 – 0.28 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.57 (s, 1H), 7.38 – 7.18 (m, 2H), 7.11 (d, J = 8.1 Hz, 1H), 7.03 – 6.86 (m, 4H) ), 6.84 – 6.76 (m, 2H), 6.60 (t, J = 75.5 Hz, 1H), 5.25 – 4.98 (m, 2H), 4.59 – 1.49 (m, 1H), 3.94 (t, J = 8.0 Hz, 1H), 3.85 (d, J = 6.8 Hz, 2H), 3.61 (t, J = 10.1 Hz, 1H), 3.51 – 3.34 (m, 4H), 2.74 – 2.64 (m, 1H), 2.25 (s, 3H) ), 2.11 (s, 3H), 2.16 – 2.02 (m, 1H), 1.33 – 1.21 (m, 1H), 0.69 – 0.58 (m, 2H), 0.41 – 0.28 (m, 2H).
MS (ESI, pos.ion) m/z: 608.20 [M+H]+ .MS (ESI, pos.ion) m/z: 608.20 [M+H] + .
實施例149:(2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((3-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺 Example 149: ( 2S )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((3-oxo Isoindolin-5-yl)methyl)pyrrolidine-2-carboxamide
將化合物(2S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基) 吡咯烷-2-羧酸 (86 mg, 0.23 mmol),6-氨甲基異吲哚啉-1-酮 (53 mg, 0.33 mmol) 和N -羥基-7-氮雜苯並三氮唑 (73 mg, 0.54 mmol) 溶於於二氯甲烷/無水N ,N -二甲基甲醯胺 (8 mL, ( v/v ) = 1/1) 中,在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (157 mg, 81 mmol) 和N ,N -二異丙基乙胺 (179 uL, 1.08 mmol),室溫攪拌3 h。加飽和食鹽水 (20 mL),水相用二氯甲烷萃取 (50 mL × 3),合併有機相,用無水硫酸鈉乾燥,減壓除去溶劑,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 20/1) 得到白色固體 (66 mg, 產率55%)。The compound ( 2S )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (86 mg, 0.23 mmol), 6-aminomethylisoindolin-1-one (53 mg, 0.33 mmol) and N -hydroxy-7-azabenzotriazole (73 mg, 0.54 mmol) in dichloro Methane/anhydrous N , N -dimethylformamide (8 mL, (v/v) = 1/1), add 1-ethyl-3-(3-dimethylaminopropyl) in an ice bath Carbodiimide hydrochloride (157 mg, 81 mmol) and N , N -diisopropylethylamine (179 uL, 1.08 mmol) were stirred at room temperature for 3 h. Saturated brine (20 mL) was added, the aqueous phase was extracted with dichloromethane (50 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography (eluent). : dichloromethane/methanol (v/v) = 20/1) to give a white solid (66 mg, 55% yield).
1 H NMR (600 MHz, CDCl3 ): δ (ppm) 8.51 (d,J = 7.2 Hz, 1H), 7.98 (s, 1H), 7.50 – 7.47 (m, 2H), 7.27 (d,J = 8.3 Hz, 1H), 7.11 (d,J = 8.1 Hz, 1H), 6.90 (d,J = 1.7 Hz, 1H), 6.85 (dd,J = 8.2, 1.8 Hz, 1H), 6.61 (t,J = 75.6 Hz, 1H), 5.05 – 4.98 (m, 1H), 4.68 (t,J = 8.4 Hz, 1H), 4.13 (d,J = 17.3 Hz, 1H), 3.99 – 3.93 (m, 1H), 3.92 (d,J = 7.7 Hz, 1H), 3.87 (d,J = 6.9 Hz, 2H), 3.86 – 3.83 (m, 1H), 3.70 (t,J = 10.7 Hz, 1H), 3.39 – 3.31 (m, 1H), 2.67 – 2.62 (m, 1H), 2.37 – 2.30 (m, 1H), 2.15 (s, 3H), 1.30 – 1.24 (m, 1H), 0.66 – 0.62 (m, 2H), 0.37 – 0.33 (m, 2H). 1 H NMR (600 MHz, CDCl 3 ): δ (ppm) 8.51 (d, J = 7.2 Hz, 1H), 7.98 (s, 1H), 7.50 – 7.47 (m, 2H), 7.27 (d, J = 8.3 Hz, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.90 (d, J = 1.7 Hz, 1H), 6.85 (dd, J = 8.2, 1.8 Hz, 1H), 6.61 (t, J = 75.6 Hz, 1H), 5.05 – 4.98 (m, 1H), 4.68 (t, J = 8.4 Hz, 1H), 4.13 (d, J = 17.3 Hz, 1H), 3.99 – 3.93 (m, 1H), 3.92 (d , J = 7.7 Hz, 1H), 3.87 (d, J = 6.9 Hz, 2H), 3.86 – 3.83 (m, 1H), 3.70 (t, J = 10.7 Hz, 1H), 3.39 – 3.31 (m, 1H) , 2.67 – 2.62 (m, 1H), 2.37 – 2.30 (m, 1H), 2.15 (s, 3H), 1.30 – 1.24 (m, 1H), 0.66 – 0.62 (m, 2H), 0.37 – 0.33 (m, 2H).
MS (ESI, pos.ion) m/z: 514.20 [M+H]+ .MS (ESI, pos.ion) m/z: 514.20 [M+H] + .
實施例150: (2R ,4R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-氧代異吲哚啉-5-基)吡咯烷-2-甲醯胺 Example 150: (2 R, 4 R ) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - (1- oxoisoindolin-5-yl)pyrrolidine-2-carboxamide
步驟1:化合物 (2R ,4S )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4-羥基吡咯烷-1,2-二甲酸酯的合成Step 1: Compound ( 2R , 4S )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4- Synthesis of Hydroxypyrrolidine-1,2-Dicarboxylate
將化合物2-(環丙基甲氧基)-1-(二氟甲氧基)-4-碘苯 (5.0 g, 14.7 mmol) 溶解在無水四氫呋喃 (40 mL) 溶液中,氮氣保護下冷卻至-78℃,加入1.3 mol/L異丙基氯化鎂-氯化鋰的四氫呋喃溶液 (16 mL, 20.8 mmol),反應1.5 h,緩慢加入N -Boc -4-氧代-D -脯氨酸甲酯 (3.5 g, 14.0 mmol) 的無水四氫呋喃 (20 mL) 溶液,-78℃下繼續反應4 h後停止,依次加入飽和氯化銨水溶液 (70 mL) 和 水(30 mL),用乙酸乙酯萃取 (35 mL × 2),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:PE/EtOAc ( v/v ) = 3/1) 得淺褐色油狀物 (2.9 g, 產率43%)。合成參考:Synthesis of 4-cis -Phenyl-L-proline via HydrogenolysisJ. Org. Chem. 2001, 66, 3593-3596 。Compound 2-(cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene (5.0 g, 14.7 mmol) was dissolved in anhydrous tetrahydrofuran (40 mL) solution, cooled under nitrogen protection to -78°C, add 1.3 mol/L isopropylmagnesium chloride-lithium chloride solution in tetrahydrofuran (16 mL, 20.8 mmol), react for 1.5 h, slowly add N - Boc- 4-oxo- D -proline methyl ester (3.5 g, 14.0 mmol) in anhydrous tetrahydrofuran (20 mL), the reaction was continued at -78 °C for 4 h and then stopped, and saturated aqueous ammonium chloride solution (70 mL) and water (30 mL) were added successively, and extracted with ethyl acetate (35 mL × 2), the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: PE/EtOAc (v/v) = 3/1) to obtain light brown Oil (2.9 g, 43% yield). Synthesis reference: Synthesis of 4- cis- Phenyl-L-proline via Hydrogenolysis J. Org. Chem. 2001, 66, 3593-3596 .
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 6.84 – 7.23 (m, 1H), 7.19 – 7.22 (m, 1H), 7.12 (d,J = 8.3 Hz, 1H), 7.02 – 7.07 (m, 1H), 5.56 (d,J = 8.7 Hz, 1H), 4.38 – 4.45 (m, 1H), 3.89 (d,J = 6.9 Hz, 2H), 3.64 – 3.66 (m, 3H), 3.55 – 3.60 (m, 2H), 2.62 – 2.69 (m, 1H), 2.18 – 2.25 (m, 1H), 1.37 – 1.41 (m, 9H), 1.19 – 1.28 (m, 1H), 0.55 – 0.59 (m, 2H), 0.32 – 0.36 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 6.84 – 7.23 (m, 1H), 7.19 – 7.22 (m, 1H), 7.12 (d, J = 8.3 Hz, 1H), 7.02 – 7.07 (m , 1H), 5.56 (d, J = 8.7 Hz, 1H), 4.38 – 4.45 (m, 1H), 3.89 (d, J = 6.9 Hz, 2H), 3.64 – 3.66 (m, 3H), 3.55 – 3.60 ( m, 2H), 2.62 – 2.69 (m, 1H), 2.18 – 2.25 (m, 1H), 1.37 – 1.41 (m, 9H), 1.19 – 1.28 (m, 1H), 0.55 – 0.59 (m, 2H), 0.32 – 0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 384.15 [M-H2 O-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 384.15 [MH 2 O- t -Bu+2H] + .
步驟2:化合物 (2R ,4S )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4-羥基吡咯烷-2-甲酸的合成Step 2: Compound ( 2R , 4S )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4- Synthesis of Hydroxypyrrolidine-2-carboxylic Acid
將化合物(2R ,4S )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4-羥基吡咯烷-1,2-二甲酸酯 (770 mg, 1.68 mmol) 溶於四氫呋喃 (10 mL) 和水 (10 mL) 的混合溶劑中,加入一水合氫氧化鋰 (285 mg,6.79 mmol),室溫反應1.5 h後停止,加鹽酸調節溶液pH=4,再用乙酸乙酯萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥, 減壓濃縮,得到白色固體 (741 mg, 產率99%)。Compound (2 R ,4 S )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4-hydroxypyrrole Alkane-1,2-dicarboxylate (770 mg, 1.68 mmol) was dissolved in a mixed solvent of tetrahydrofuran (10 mL) and water (10 mL), lithium hydroxide monohydrate (285 mg, 6.79 mmol) was added, and the The warm reaction was stopped after 1.5 h, and hydrochloric acid was added to adjust the pH of the solution to 4, followed by extraction with ethyl acetate (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a white solid (741 mg, yield 99 %).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.23 (s, 1H), 7.06 – 7.14 (m, 2H), 6.76 (t,J F-H = 75.6 Hz, 1H), 4.45 – 4.53 (m, 1H), 3.94 (d,J = 6.6 Hz, 2H), 3.67 – 3.76 (m, 2H), 2.73 – 2.79 (m, 1H), 2.42 – 2.45 (m, 1H), 1.48 – 1.50 (m, 9H), 1.24 – 1.35 (m, 1H), 0.60 – 0.68 (m, 2H), 0.34 – 0.42 (m, 2H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.23 (s, 1H), 7.06 – 7.14 (m, 2H), 6.76 (t, J FH = 75.6 Hz, 1H), 4.45 – 4.53 (m , 1H), 3.94 (d, J = 6.6 Hz, 2H), 3.67 – 3.76 (m, 2H), 2.73 – 2.79 (m, 1H), 2.42 – 2.45 (m, 1H), 1.48 – 1.50 (m, 9H) ), 1.24 – 1.35 (m, 1H), 0.60 – 0.68 (m, 2H), 0.34 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 466.10 [M+Na]+ .MS (ESI, pos.ion) m/z: 466.10 [M+Na] + .
步驟3:化合物 (1S ,4R )-叔丁基 1-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-3-氧代-2-氧雜-5-氮雜雙環[2.2.1]庚烷-5-甲酸酯的合成Step 3: Compound ( 1S , 4R )-tert-butyl 1-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-3-oxo-2-oxo Synthesis of Hetero-5-azabicyclo[2.2.1]heptane-5-carboxylate
將(2R ,4S )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4-羥基吡咯烷-2-甲酸 (817 mg, 1.84 mmol) 溶解在二氯甲烷 (25 mL) 溶液中,加入N ,N -二異丙基乙胺 (717 mg, 5.55 mmol),在冰浴中冷卻,加入甲磺醯氯 (317 mg, 2.77 mmol),室溫反應3 h,加入水 (30 mL) 洗有機相,分離有機相,水相用二氯甲烷萃取 (5 mL × 2),合併有機相合,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:PE/EtOAc ( v/v ) = 6/1) 得到淺黃色液體 (681 mg, 產率87%)。( 2R , 4S )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4-hydroxypyrrolidine -2-carboxylic acid (817 mg, 1.84 mmol) was dissolved in dichloromethane (25 mL) solution, N , N -diisopropylethylamine (717 mg, 5.55 mmol) was added, cooled in an ice bath, methyl methane was added Sulfonyl chloride (317 mg, 2.77 mmol), react at room temperature for 3 h, add water (30 mL) to wash the organic phase, separate the organic phase, extract the aqueous phase with dichloromethane (5 mL × 2), combine the organic phases, use It was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: PE/EtOAc (v/v) = 6/1) to obtain a pale yellow liquid (681 mg, yield 87%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.24 (d,J = 8.3 Hz, 1H), 7.10 (d,J = 1.9 Hz, 1H), 6.96 (dd,J = 8.3, 2.0 Hz, 1H), 6.67 (t,J F-H = 75.2 Hz, 1H), 4.66 – 4.82 (m, 1H), 3.91 (d,J = 6.9 Hz, 2H), 3.62 – 3.75 (m, 2H), 2.43 – 2.46 (m, 1H), 2.29 – 2.32 (m, 1H), 1.51 (s, 9H), 1.26 – 1.37 (m, 1H), 0.66 – 0.71 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.24 (d, J = 8.3 Hz, 1H), 7.10 (d, J = 1.9 Hz, 1H), 6.96 (dd, J = 8.3, 2.0 Hz, 1H), 6.67 (t, J FH = 75.2 Hz, 1H), 4.66 – 4.82 (m, 1H), 3.91 (d, J = 6.9 Hz, 2H), 3.62 – 3.75 (m, 2H), 2.43 – 2.46 ( m, 1H), 2.29 – 2.32 (m, 1H), 1.51 (s, 9H), 1.26 – 1.37 (m, 1H), 0.66 – 0.71 (m, 2H), 0.37 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 448.10 [M+Na]+ .MS (ESI, pos.ion) m/z: 448.10 [M+Na] + .
步驟4:化合物 (R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲酸的合成Step 4: Compound ( R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro Synthesis of -1 H -pyrrole-2-carboxylic acid
將化合物(1S ,4R )-叔丁基 1-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-3-氧代-2-氧雜-5-氮雜雙環[2.2.1]庚烷-5-甲酸酯(1.4 g, 3.29 mmol) 溶於二氯甲烷 (50 mL),加入三氟乙酸 (TFA) (824 mg, 7.23 mmol),室溫反應2 h,加入水 (40 mL) 洗有機相,分離有機相,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:PE/EtOAc ( v/v ) = 1/2) 得到淺褐色固體 (1.2 g, 產率86%)。The compound (1 S ,4 R )-tert-butyl 1-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-3-oxo-2-oxa- 5-Azabicyclo[2.2.1]heptane-5-carboxylate (1.4 g, 3.29 mmol) was dissolved in dichloromethane (50 mL), trifluoroacetic acid (TFA) (824 mg, 7.23 mmol) was added, The reaction was carried out at room temperature for 2 h, water (40 mL) was added to wash the organic phase, the organic phase was separated, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: PE/EtOAc (v/v ) = 1/2) to give a light brown solid (1.2 g, 86% yield).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.16 – 7.20 (m, 1H), 7.14 (d,J = 8.3 Hz, 1H), 7.01 – 7.06 (m, 1H), 6.79 (t,J F-H = 75.4 Hz, 1H), 6.24 – 6.29 (m, 1H), 5.06 – 5.12 (m, 1H), 4.51 – 4.62 (m, 2H), 3.96 (d,J = 6.8 Hz, 2H), 1.49 – 1.54 (m, 9H), 1.24 – 1.37 (m, 1H), 0.62 – 0.67 (m, 2H), 0.38 – 0.42 (m, 2H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.16 – 7.20 (m, 1H), 7.14 (d, J = 8.3 Hz, 1H), 7.01 – 7.06 (m, 1H), 6.79 (t, J FH = 75.4 Hz, 1H), 6.24 – 6.29 (m, 1H), 5.06 – 5.12 (m, 1H), 4.51 – 4.62 (m, 2H), 3.96 (d, J = 6.8 Hz, 2H), 1.49 – 1.54 (m, 9H), 1.24 – 1.37 (m, 1H), 0.62 – 0.67 (m, 2H), 0.38 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 370.10 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 370.10 [M- t- Bu+2H] + .
步驟5:化合物 (2R ,4R )-1-(叔丁氧羰基)-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸的合成Step 5: Compound ( 2R , 4R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Synthesis of formic acid
將化合物(R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲酸(1.6 g, 3.80 mmol) 溶於乙醇 (27 mL) 和無水四氫呋喃 (3 mL),加入三苯基膦氯化銠 (452 mg, 0.49 mmol) 和三乙胺 (572 mg, 5.65 mmol),通入氫氣0.5 MPa壓力下室溫反應3天,然後將反應液濃縮,加水 (27 mL),加稀鹽酸調節pH =1,用乙酸乙酯萃取 (30 mL × 3),合併有機相合,用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑:PE/EtOAc ( v/v ) = 1/1) 得到淺黃色液體 (1.2 g, 產率75%, 4R:4S=4:1),經過手性柱拆分得到(2R ,4R )-1-(叔丁氧羰基)-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸。合成參考:Carboxylate-Directed Highly Stereoselective Homogeneous Hydrogenation of Cyclic Olefins with Wilkinson’s Catalyst.Organic Letters. 2003 Vol. 5, No. 9 1587 - 1589 。Compound ( R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro-1 H -pyrrole-2-carboxylic acid (1.6 g, 3.80 mmol) was dissolved in ethanol (27 mL) and anhydrous tetrahydrofuran (3 mL), triphenylphosphine rhodium chloride (452 mg, 0.49 mmol) and triethylamine (572 mmol) were added. mg, 5.65 mmol), passed into hydrogen and reacted at room temperature under 0.5 MPa pressure for 3 days, then concentrated the reaction solution, added water (27 mL), added dilute hydrochloric acid to adjust pH=1, extracted with ethyl acetate (30 mL × 3) , the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: PE/EtOAc (v/v) = 1/1) to obtain a pale yellow liquid (1.2 g, yield rate of 75%, 4R: 4S = 4 : 1), resolution via chiral column to give (2 R, 4 R) -1- ( tert-butoxycarbonyl) -4- (3- (cyclopropylmethoxy) - 4-(Difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid. Synthetic reference: Carboxylate-Directed Highly Stereoselective Homogeneous Hydrogenation of Cyclic Olefins with Wilkinson's Catalyst. Organic Letters. 2003 Vol. 5, No. 9 1587 - 1589 .
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.10 (d,J = 8.2 Hz, 1H), 7.00 – 7.03 (m, 1H), 6.86 – 6.90 (m, 1H), 6.73 (t,J F-H = 75.7 Hz, 1H), 4.38 – 4.46 (m, 1H), 3.89 – 3.97 (m, 1H), 3.92 (d,J = 6.9 Hz, 2H), 3.49 – 3.57 (m, 1H), 3.35 – 3.41 (m, 1H), 2.43 – 2.51 (m, 1H), 2.32 – 2.39 (m, 1H), 1.46 – 1.49 (m, 9H), 1.26 – 1.35 (m, 1H), 0.61 – 0.66 (m, 2H), 0.36 – 0.40 (m, 2H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.10 (d, J = 8.2 Hz, 1H), 7.00 – 7.03 (m, 1H), 6.86 – 6.90 (m, 1H), 6.73 (t, J FH = 75.7 Hz, 1H), 4.38 – 4.46 (m, 1H), 3.89 – 3.97 (m, 1H), 3.92 (d, J = 6.9 Hz, 2H), 3.49 – 3.57 (m, 1H), 3.35 – 3.41 (m, 1H), 2.43 – 2.51 (m, 1H), 2.32 – 2.39 (m, 1H), 1.46 – 1.49 (m, 9H), 1.26 – 1.35 (m, 1H), 0.61 – 0.66 (m, 2H) ), 0.36 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 372.10 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 372.10 [M- t- Bu+2H] + .
步驟6:化合物 (2R ,4R )-叔丁基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2-((1-氧代異吲哚啉-5-基)氨基甲醯基)吡咯烷-1-甲酸酯的合成Step 6: Compound ( 2R , 4R )-tert-butyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-((1-oxo Synthesis of isoindolin-5-yl)carbamoyl)pyrrolidine-1-carboxylate
將化合物(2R ,4R )-1-(叔丁氧羰基)-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸(120 mg, 0.28 mmol),5-氨基異吲哚-1-酮 (50 mg, 0.34 mmol) 和N -羥基-7-氮雜苯並三氮唑 (76 mg, 0.56 mmol) 溶於二氯甲烷 (12 mL) 和無水DMF (4 mL) 中,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (167 mg, 0.87 mmol) 和N ,N -二異丙基乙胺 (169 mg, 1.31 mmol),室溫反應10 h,減壓濃縮,剩餘物加水 (20 mL),用乙酸乙酯萃取 (20 mL × 2),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:DCM/ MeOH ( v/v ) = 23/1) 得到白色固體 (83 mg, 產率53%)。The compound ( 2R , 4R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (120 mg, 0.28 mmol), 5-aminoisoindol-1-one (50 mg, 0.34 mmol) and N -hydroxy-7-azabenzotriazole (76 mg, 0.56 mmol) in dichloro Methane (12 mL) and dry DMF (4 mL) were added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (167 mg, 0.87 mmol) and N , N - Diisopropylethylamine (169 mg, 1.31 mmol) was reacted at room temperature for 10 h, concentrated under reduced pressure, the residue was added with water (20 mL), extracted with ethyl acetate (20 mL × 2), the organic phases were combined and washed with anhydrous It was dried over sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: DCM/MeOH (v/v) = 23/1) to obtain a white solid (83 mg, yield 53%).
化合物11178-3: MS (ESI, pos.ion) m/z: 558.20 [M+H]+ .Compound 11178-3: MS (ESI, pos.ion) m/z: 558.20 [M+H] + .
步驟7:化合物 (2R ,4R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-氧代異吲哚啉-5-基) 吡咯烷-2-甲醯胺鹽酸鹽的合成Step 7: Compound ( 2R , 4R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (1-oxoisoindoline Synthesis of -5-yl)pyrrolidine-2-carboxamide hydrochloride
將化合物(2R ,4R )-叔丁基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2-((1-氧代異吲哚啉-5-基)氨基甲醯基)吡咯烷-1-甲酸酯 (11178-3) (83 mg, 0.15 mmol) 溶解於二氯甲烷 (4 mL) 溶液中,加入4 mol/L HCl的1,4-二氧六環溶液 (1.5 mL),室溫攪拌2 h,除去溶劑,得到白色固體 (74 mg, 產率100%)。Compound (2 R ,4 R )-tert-butyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-((1-oxoisoindium) Indolin-5-yl)carbamoyl)pyrrolidine-1-carboxylate (11178-3) (83 mg, 0.15 mmol) was dissolved in dichloromethane (4 mL) solution, and 4 mol/L HCl was added A solution of 1,4-dioxane (1.5 mL) was stirred at room temperature for 2 h, and the solvent was removed to give a white solid (74 mg, 100% yield).
步驟8:化合物 (2R ,4R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-氧代異吲哚啉-5-基)吡咯烷-2-甲醯胺的合成Step 8: Compound (2 R, 4 R) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - (1- Synthesis of oxoisoindolin-5-yl)pyrrolidine-2-carboxamide
將化合物(2R ,4R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-氧代異吲哚啉-5-基) 吡咯烷-2-甲醯胺鹽酸鹽(73 mg, 0.15 mmol) 溶解在二氯甲烷 (8 mL) 中,在冰浴中冷卻,加入N ,N -二異丙基乙胺 (191 mg, 1.48 mmol) 和乙醯氯 (59 mg, 0.75 mmol),室溫攪拌1 h後停止反應,加水 (20 mL) 攪拌 2 min,分離有機相,水相用二氯甲烷萃取 (10 mL × 2),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:DCM/ MeOH ( v/v ) = 35/1) 得到褐色固體 (27 mg, 產率36%)。Compound ( 2R , 4R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N- (1-oxoisoindoline-5 -yl)pyrrolidine-2-carboxamide hydrochloride (73 mg, 0.15 mmol) was dissolved in dichloromethane (8 mL), cooled in an ice bath, added with N , N -diisopropylethylamine ( 191 mg, 1.48 mmol) and acetyl chloride (59 mg, 0.75 mmol), the reaction was stopped after stirring at room temperature for 1 h, water (20 mL) was added and stirred for 2 min, the organic phase was separated, and the aqueous phase was extracted with dichloromethane (10 mL). × 2), the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: DCM/MeOH (v/v) = 35/1) to obtain a brown solid (27 mg) , yield 36%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 10.18 (br.s, 1H), 8.08 (s, 1H), 7.46 – 7.58 (m, 1H), 7.14 (d,J = 7.4 Hz, 1H), 7.01 – 7.10 (m, 1H), 6.86 – 6.91 (m, 2H), 6.62 (t,J F-H = 75.6 Hz, 1H), 4.72 – 4.89 (m, 1H), 4.20 – 4.35(m, 2H), 3.96 – 4.10 (m, 1H), 3.88 (d,J = 5.5 Hz, 2H), 3.61 – 3.74 (m, 1H), 3.32 – 3.53 (m, 1H), 2.59 – 2.74 (m, 1H), 2.37 – 2.53 (m, 1H), 2.26 (s, 3H), 1.23 – 1.34 (m, 1H), 0.60 – 0.71 (m, 2H), 0.31 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 10.18 (br.s, 1H), 8.08 (s, 1H), 7.46 – 7.58 (m, 1H), 7.14 (d, J = 7.4 Hz, 1H ), 7.01 – 7.10 (m, 1H), 6.86 – 6.91 (m, 2H), 6.62 (t, J FH = 75.6 Hz, 1H), 4.72 – 4.89 (m, 1H), 4.20 – 4.35(m, 2H) , 3.96 – 4.10 (m, 1H), 3.88 (d, J = 5.5 Hz, 2H), 3.61 – 3.74 (m, 1H), 3.32 – 3.53 (m, 1H), 2.59 – 2.74 (m, 1H), 2.37 – 2.53 (m, 1H), 2.26 (s, 3H), 1.23 – 1.34 (m, 1H), 0.60 – 0.71 (m, 2H), 0.31 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 500.05 [M+H]+ .MS (ESI, pos.ion) m/z: 500.05 [M+H] + .
實施例151:(2R, 4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-氧代異吲哚啉-5-基)吡咯烷-2-甲醯胺 Example 151: (2 R, 4 S ) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - (1- oxoisoindolin-5-yl)pyrrolidine-2-carboxamide
步驟1:化合物 (2R ,4S )-1-(叔丁氧羰基)-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸的合成Step 1: Compound ( 2R , 4S )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 -Synthesis of formic acid
將化合物(R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲酸(500 mg, 1.18 mmol,實施例150步驟4) 溶於乙醇 (27 mL),加入Pd/C (400 mg) 和三乙胺 (178 mg, 1.76 mmol),通入氫氣室溫反應12 h,然後將反應液抽濾,濾液濃縮得到淺褐色液體 (483 mg, 產率96%)。合成參考:Discovery of Potent and Specific Dihydroisoxazole Inhibitors of Human Transglutaminase 2.J .Med. Chem. 2014, 57, 9042 - 9064 。Compound ( R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro-1 H -pyrrole-2-carboxylic acid (500 mg, 1.18 mmol, Example 150, step 4) was dissolved in ethanol (27 mL), added with Pd/C (400 mg) and triethylamine (178 mg, 1.76 mmol), passed through Hydrogen was reacted at room temperature for 12 h, then the reaction solution was suction filtered, and the filtrate was concentrated to obtain a light brown liquid (483 mg, yield 96%). Synthetic reference: Discovery of Potent and Specific Dihydroisoxazole Inhibitors of Human Transglutaminase 2. J. Med. Chem. 2014, 57, 9042 - 9064 .
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.07 – 7.09 (m, 2H), 6.89 – 6.92 (m, 1H), 6.73 (t,J F-H = 75.8 Hz, 1H), 4.19 – 4.25 (m, 1H), 3.89 – 3.97 (m, 1H), 3.98 (d,J = 6.9 Hz, 2H), 3.44 (d,J = 10.6 Hz, 1H), 3.32 – 3.38 (m, 1H), 2.61 – 2.68 (m, 1H), 1.98 – 2.07 (m, 1H), 1.48 (s, 9H), 1.28 – 1.34 (m, 1H), 0.61 – 0.66 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.07 – 7.09 (m, 2H), 6.89 – 6.92 (m, 1H), 6.73 (t, J FH = 75.8 Hz, 1H), 4.19 – 4.25 (m, 1H), 3.89 – 3.97 (m, 1H), 3.98 (d, J = 6.9 Hz, 2H), 3.44 (d, J = 10.6 Hz, 1H), 3.32 – 3.38 (m, 1H), 2.61 – 2.68 (m, 1H), 1.98 – 2.07 (m, 1H), 1.48 (s, 9H), 1.28 – 1.34 (m, 1H), 0.61 – 0.66 (m, 2H), 0.37 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 372.10 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 372.10 [M- t- Bu+2H] + .
步驟2:化合物 (2R ,4S )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯的合成Step 2: Compound ( 2R , 4S )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine- Synthesis of 1,2-Dicarboxylate
將化合物(2R ,4S )-1-(叔丁氧羰基)-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸(280 mg, 0.66 mmol),甲醇 (106 mg, 3.31 mmol) 和N -羥基-7-氮雜苯並三氮唑 (178 mg, 1.31 mmol) 溶於二氯甲烷 (8 mL) 中,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (503 mg, 2.62 mmol) 和N ,N -二異丙基乙胺 (339 mg, 2.62 mmol),室溫反應8 h,加水 (20 mL) 停止反應,用二氯甲烷萃取 (5 mL × 2),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:PE/ EtOAc ( v/v ) = 5/1) 得到無色液體 (165 mg, 產率57%)。Compound ( 2R , 4S )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (280 mg, 0.66 mmol), methanol (106 mg, 3.31 mmol) and N -hydroxy-7-azabenzotriazole (178 mg, 1.31 mmol) were dissolved in dichloromethane (8 mL) and 1 -Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (503 mg, 2.62 mmol) and N , N -diisopropylethylamine (339 mg, 2.62 mmol), room temperature After 8 h of reaction, water (20 mL) was added to stop the reaction, extracted with dichloromethane (5 mL × 2), the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent). :PE/EtOAc (v/v) = 5/1) to give a colorless liquid (165 mg, 57% yield).
1 H NMR (400 MHz, CDCl3 ): δ ppm 7.10 (d,J = 7.9 Hz, 1H), 6.77 – 6.81 (m, 2H), 6.59 (t,J F-H = 75.6 Hz, 1H), 4.30 – 4.40 (m, 1H), 4.02 – 4.06 (m, 0.6H), 3.91 – 3.95 (m, 0.4H), 3.84 – 3.86 (m, 2H), 3.76 (d,J = 7.1 Hz, 3H), 3.38 – 3.44 (m, 1H), 3.26 – 3.36 (m, 1H), 2.61 – 2.68 (m, 1H), 1.96 – 2.07 (m, 1H), 1.43 – 1.46 (m, 9H), 1.25 – 1.33 (m, 1H), 0.62 – 0.67 (m, 2H), 0.33 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ ppm 7.10 (d, J = 7.9 Hz, 1H), 6.77 – 6.81 (m, 2H), 6.59 (t, J FH = 75.6 Hz, 1H), 4.30 – 4.40 (m, 1H), 4.02 – 4.06 (m, 0.6H), 3.91 – 3.95 (m, 0.4H), 3.84 – 3.86 (m, 2H), 3.76 (d, J = 7.1 Hz, 3H), 3.38 – 3.44 (m, 1H), 3.26 – 3.36 (m, 1H), 2.61 – 2.68 (m, 1H), 1.96 – 2.07 (m, 1H), 1.43 – 1.46 (m, 9H), 1.25 – 1.33 (m, 1H) , 0.62 – 0.67 (m, 2H), 0.33 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 386.40 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 386.40 [M- t- Bu+2H] + .
步驟3:化合物(2R ,4S )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸甲酯鹽酸鹽的合成Step 3: Compound ( 2R , 4S )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride synthesis
將化合物(2R ,4S )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯 (11179-3) (4.03 g, 9.13 mmol) 溶解於二氯甲烷 (50 mL) 溶液中,加入3 mol/L HCl的1,4-二氧六環溶液 (20 mL),室溫攪拌4.5 h,除去溶劑,得到淺黃色固體 (3.5 g, 100%)。The compound ( 2R , 4S )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1, 2-Dicarboxylate (11179-3) (4.03 g, 9.13 mmol) was dissolved in dichloromethane (50 mL) solution, and 3 mol/L HCl in 1,4-dioxane solution (20 mL) was added was stirred at room temperature for 4.5 h, and the solvent was removed to give a pale yellow solid (3.5 g, 100%).
1 H NMR (400 MHz, CD3 OD) δ: (ppm) 7.14 (d,J = 8.2 Hz, 1H), 7.09 (d,J = 1.8 Hz, 1H), 6.93 (dd,J = 8.3, 1.8 Hz, 1H), 6.76 (t,J F-H = 75.5 Hz, 1H), 4.61 – 4.65 (m, 1H), 3.94 (d,J = 6.9 Hz, 2H), 3.90 (s, 3H), 3.79 – 3.83 (m, 1H), 3.64 – 3.74 (m, 1H), 3.37 (t,J = 11.1 Hz, 1H), 2.82 – 2.89 (m, 1H), 2.20 – 2.29 (m, 1H), 1.27 – 1.35 (m, 1H), 0.63 – 0.68 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ: (ppm) 7.14 (d, J = 8.2 Hz, 1H), 7.09 (d, J = 1.8 Hz, 1H), 6.93 (dd, J = 8.3, 1.8 Hz , 1H), 6.76 (t, J FH = 75.5 Hz, 1H), 4.61 – 4.65 (m, 1H), 3.94 (d, J = 6.9 Hz, 2H), 3.90 (s, 3H), 3.79 – 3.83 (m , 1H), 3.64 – 3.74 (m, 1H), 3.37 (t, J = 11.1 Hz, 1H), 2.82 – 2.89 (m, 1H), 2.20 – 2.29 (m, 1H), 1.27 – 1.35 (m, 1H) ), 0.63 – 0.68 (m, 2H), 0.37 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 342.15 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 342.15 [M+H-HCl] + .
步驟4:化合物 (2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸甲酯的合成Step 4: Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid Synthesis of methyl esters
將化合物(2R ,4S )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸甲酯鹽酸鹽 (3.5 g, 9.30 mmol) 溶解在二氯甲烷 (50 mL) 中,在冰浴中冷卻,加入N ,N -二異丙基乙胺 (6.0 g, 46.0 mmol) 和乙醯氯 (2.2 g, 28.0 mmol),室溫攪拌2.5 h後停止反應,加水 (50 mL) 攪拌 2 min,分離有機相,水相用二氯甲烷萃取 (20 mL × 2),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:PE/EtOAc ( v/v ) = 1/3) 得到褐色液體 (3.4 g, 96%)。Compound ( 2R , 4S )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride (3.5 g , 9.30 mmol) was dissolved in dichloromethane (50 mL), cooled in an ice bath, N , N -diisopropylethylamine (6.0 g, 46.0 mmol) and acetyl chloride (2.2 g, 28.0 mmol) were added , the reaction was stopped after stirring at room temperature for 2.5 h, water (50 mL) was added and stirred for 2 min, the organic phase was separated, the aqueous phase was extracted with dichloromethane (20 mL × 2), the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure , and the residue was subjected to silica gel column chromatography (eluent: PE/EtOAc (v/v) = 1/3) to obtain a brown liquid (3.4 g, 96%).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.09 – 7.13 (m, 1H), 6.82 (s, 1H), 6.78 – 6.81 (m, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 4.45 – 4.49 (m, 1H), 3.91 – 3.95 (m, 1H), 3.84 – 3.87 (m, 2H), 3.77 (s, 3H), 3.60 (t,J = 10.4 Hz, 1H), 3.36 – 3.45 (m, 1H), 2.62 – 2.68 (m, 1H), 2.11 (s, 2.5H), 2.02 – 2.06 (m, 1H), 1.98 (s, 0.5H), 1.24 – 1.33 (m, 1H), 0.63 – 0.68 (m, 2H), 0.34 – 0.37 (m, 2H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.09 – 7.13 (m, 1H), 6.82 (s, 1H), 6.78 – 6.81 (m, 1H), 6.60 (t, J FH = 75.5 Hz , 1H), 4.45 – 4.49 (m, 1H), 3.91 – 3.95 (m, 1H), 3.84 – 3.87 (m, 2H), 3.77 (s, 3H), 3.60 (t, J = 10.4 Hz, 1H), 3.36 – 3.45 (m, 1H), 2.62 – 2.68 (m, 1H), 2.11 (s, 2.5H), 2.02 – 2.06 (m, 1H), 1.98 (s, 0.5H), 1.24 – 1.33 (m, 1H) ), 0.63 – 0.68 (m, 2H), 0.34 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 384.10 [M+H]+ .MS (ESI, pos.ion) m/z: 384.10 [M+H] + .
步驟5:化合物(2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸的合成Step 5: Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid Synthesis
將化合物(2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸甲酯(11179-5) (3.4 g, 8.90 mmol) 溶於四氫呋喃 (40 mL) 和水 (20 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (1.1 mg,26.0 mmol),50℃反應1 h後停止,冰浴中冷卻,加入稀鹽酸調節溶液pH=1,減壓除去四氫呋喃,再用乙酸乙酯萃取 (25 mL×3),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,得到淺黃色固體 (3.3 g, 100%)。Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid Ester (11179-5) (3.4 g, 8.90 mmol) was dissolved in a mixed solvent of tetrahydrofuran (40 mL) and water (20 mL), then lithium hydroxide monohydrate (1.1 mg, 26.0 mmol) was added, and the reaction was carried out at 50 °C for 1 After h, it was stopped, cooled in an ice bath, diluted hydrochloric acid was added to adjust the pH of the solution to 1, tetrahydrofuran was removed under reduced pressure, extracted with ethyl acetate (25 mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. Obtained as a pale yellow solid (3.3 g, 100%).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.09 – 7.12 (m, 1H), 7.01 – 7.05 (m, 1H), 6.87 – 6.93 (m, 1H), 6.54 – 6.90 (m, 1H), 4.64 – 4.68 (m, 0.2H), 4.43 – 4.47 (m, 0.8H), 4.22 – 4.26 (m, 0.2H), 4.08 – 4.12 (m, 0.8H), 3.91 – 3.94 (m, 2H), 3.57 – 3.63 (m, 1H), 3.47 – 3.55 (m, 1H), 2.88 – 2.95 (m, 0.2H), 2.71 – 2.78 (m, 0.8H), 2.14 (s, 2.5H), 2.00 – 2.08 (m, 1H), 2.02 (s, 0.5H), 1.26 – 1.33 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.09 – 7.12 (m, 1H), 7.01 – 7.05 (m, 1H), 6.87 – 6.93 (m, 1H), 6.54 – 6.90 (m, 1H) ), 4.64 – 4.68 (m, 0.2H), 4.43 – 4.47 (m, 0.8H), 4.22 – 4.26 (m, 0.2H), 4.08 – 4.12 (m, 0.8H), 3.91 – 3.94 (m, 2H) , 3.57 – 3.63 (m, 1H), 3.47 – 3.55 (m, 1H), 2.88 – 2.95 (m, 0.2H), 2.71 – 2.78 (m, 0.8H), 2.14 (s, 2.5H), 2.00 – 2.08 (m, 1H), 2.02 (s, 0.5H), 1.26 – 1.33 (m, 1H), 0.62 – 0.67 (m, 2H), 0.37 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 370.20 [M+H]+ .MS (ESI, pos.ion) m/z: 370.20 [M+H] + .
步驟6:化合物 (2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(1-氧代異吲哚啉-5-基)吡咯烷-2-甲醯胺的合成Step 6: Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - (1- Synthesis of oxoisoindolin-5-yl)pyrrolidine-2-carboxamide
將化合物(2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (120 mg, 0.32 mmol),5-氨基異吲哚-1-酮 (57 mg, 0.38 mmol) 和N -羥基-7-氮雜苯並三氮唑 (88 mg, 0.65 mmol) 溶於DCM (12 mL) 和DMF (4 mL) 中,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (187 mg, 0.98 mmol) 和N ,N -二異丙基乙胺 (169 mg, 1.31 mmol),室溫反應8 h,減壓濃縮除去溶劑,加水 (20 mL),用乙酸乙酯萃取 (20 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑: DCM/MeOH ( v/v ) = 17/1) 得到淺黃色固體 (122 mg, 產率75%)。Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid (120 mg, 0.32 mmol), 5-aminoisoindol-1-one (57 mg, 0.38 mmol) and N -hydroxy-7-azabenzotriazole (88 mg, 0.65 mmol) in DCM (12 mL) ) and DMF (4 mL), add 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (187 mg, 0.98 mmol) and N , N -diisopropylethyl acetate Amine (169 mg, 1.31 mmol), reacted at room temperature for 8 h, concentrated under reduced pressure to remove the solvent, added water (20 mL), extracted with ethyl acetate (20 mL × 2), the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure , the residue was separated by silica gel column chromatography (eluent: DCM/MeOH (v/v) = 17/1) to obtain a pale yellow solid (122 mg, yield 75%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 10.20 (br.s, 1H), 8.35 (br.s, 1H), 8.08 (s, 1H), 7.44 – 7.58 (m, 1H), 7.12 (d,J = 7.5 Hz, 1H), 6.97 – 7.06 (m, 1H), 6.90 (s, 1H), 6.85 (d,J = 7.4 Hz, 1H), 6.61 (t,J F-H = 75.6 Hz, 1H), 4.70 – 4.87 (m, 1H), 4.18 – 4.35(m, 2H), 3.96 – 4.09 (m, 1H), 3.87 (d,J = 6.6 Hz, 2H), 3.63 – 3.76 (m, 1H), 3.36 – 3.53 (m, 1H), 2.59 – 2.76 (m, 1H), 2.32 – 2.47 (m, 1H), 2.24 (s, 3H), 1.23 – 1.34 (m, 1H), 0.57 – 0.71 (m, 2H), 0.28 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 10.20 (br.s, 1H), 8.35 (br.s, 1H), 8.08 (s, 1H), 7.44 – 7.58 (m, 1H), 7.12 (d, J = 7.5 Hz, 1H), 6.97 – 7.06 (m, 1H), 6.90 (s, 1H), 6.85 (d, J = 7.4 Hz, 1H), 6.61 (t, J FH = 75.6 Hz, 1H ), 4.70 – 4.87 (m, 1H), 4.18 – 4.35(m, 2H), 3.96 – 4.09 (m, 1H), 3.87 (d, J = 6.6 Hz, 2H), 3.63 – 3.76 (m, 1H), 3.36 – 3.53 (m, 1H), 2.59 – 2.76 (m, 1H), 2.32 – 2.47 (m, 1H), 2.24 (s, 3H), 1.23 – 1.34 (m, 1H), 0.57 – 0.71 (m, 2H) ), 0.28 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 498.50 [M-H]- .MS (ESI, pos.ion) m/z: 498.50 [MH] - .
實施例152:(2R ,4S )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 152: ( 2R , 4S )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropyl) methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物 (2R ,4S )-1-叔丁基 2-((6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯的合成Step 1: Compound ( 2R , 4S )-1-tert-butyl 2-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl)4-(3- Synthesis of (cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物(2R ,4S )-1-(叔丁氧羰基)-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (298 mg, 0.70 mmol,實施151步驟1),6-(羥甲基)-N -甲基-N -(對甲基苯基)吡啶醯胺 (179 mg, 0.70 mmol)和1-羥基苯並三唑 (142 mg, 1.05 mmol) 溶於乾燥的DMF (5 mL) 中,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (201 mg, 1.05 mmol) 和N -甲基嗎啡啉 (142 mg, 1.40 mmol),室溫反應17 h,加水 (30 mL) 停止反應,用乙酸乙酯萃取 (15 mL × 2),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:PE/ EtOAc ( v/v ) = 1/1) 得到白色黏稠固體 (285 mg, 產率61%)。Compound ( 2R , 4S )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (298 mg, 0.70 mmol, carried out step 1 of 151), 6-(hydroxymethyl) -N -methyl- N- (p-methylphenyl)pyridinamide (179 mg, 0.70 mmol) and 1-hydroxybenzene Natriazole (142 mg, 1.05 mmol) was dissolved in dry DMF (5 mL) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (201 mg, 1.05 mmol) and N -methylmorpholine (142 mg, 1.40 mmol), reacted at room temperature for 17 h, added water (30 mL) to stop the reaction, extracted with ethyl acetate (15 mL × 2), combined the organic phases, washed with anhydrous sulfuric acid It was dried over sodium, concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: PE/EtOAc (v/v) = 1/1) to obtain a white viscous solid (285 mg, yield 61%).
1 H NMR (400 MHz, CDCl3 ): δ ppm 7.49 – 7.62 (m, 1H), 7.22 – 7.33 (m, 1H), 7.13 – 7.19 (m, 1H), 7.09 (d,J = 7.9 Hz, 1H), 6.86 – 7.03 (m, 4H), 6.78 – 6.83 (m, 2H), 6.59 (t,J F-H = 75.6 Hz, 1H), 5.19 – 4.97 (m, 2H), 4.37 – 4.48 (m, 1H), 4.05 – 4.09 (m, 0.5H), 3.92 – 3.96 (m, 0.5H), 3.82 – 3.86 (m, 2H), 3.46 (s, 3H), 3.26 – 3.44 (m, 2H), 2.62 – 2.74 (m, 1H), 2.25 (s, 3H), 1.97 – 2.10 (m, 1H), 1.37 (s, 4.5H), 1.45 (s, 4.5H), 1.24 – 1.31 (m, 1H), 0.60 – 0.68 (m, 2H), 0.31 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ ppm 7.49 – 7.62 (m, 1H), 7.22 – 7.33 (m, 1H), 7.13 – 7.19 (m, 1H), 7.09 (d, J = 7.9 Hz, 1H ), 6.86 – 7.03 (m, 4H), 6.78 – 6.83 (m, 2H), 6.59 (t, J FH = 75.6 Hz, 1H), 5.19 – 4.97 (m, 2H), 4.37 – 4.48 (m, 1H) , 4.05 – 4.09 (m, 0.5H), 3.92 – 3.96 (m, 0.5H), 3.82 – 3.86 (m, 2H), 3.46 (s, 3H), 3.26 – 3.44 (m, 2H), 2.62 – 2.74 ( m, 1H), 2.25 (s, 3H), 1.97 – 2.10 (m, 1H), 1.37 (s, 4.5H), 1.45 (s, 4.5H), 1.24 – 1.31 (m, 1H), 0.60 – 0.68 ( m, 2H), 0.31 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 666.30 [M+H]+ .MS (ESI, pos.ion) m/z: 666.30 [M+H] + .
步驟2:化合物 (2R ,4S )-(6-(甲基(對甲苯基)氨基甲醯基) 吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽的合成Step 2: Compound ( 2R , 4S )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)- Synthesis of 4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride
將化合物(2R ,4S )-1-叔丁基 2-((6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯 (1184-6) (280 mg, 0.42 mmol) 溶解於二氯甲烷 (12 mL) 溶液中,加入4 mol/L HCl的1,4-二氧六環溶液 (5 mL),室溫攪拌3 h,減壓濃縮除去溶劑,得到淺黃色固體 (297 mg, 100%,含有溶劑)。The compound ( 2R , 4S )-1-tert-butyl 2-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl)4-(3-(cyclo) Propylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (1184-6) (280 mg, 0.42 mmol) was dissolved in dichloromethane (12 mL) ) solution, added 4 mol/L HCl in 1,4-dioxane solution (5 mL), stirred at room temperature for 3 h, concentrated under reduced pressure to remove the solvent to obtain a pale yellow solid (297 mg, 100%, containing solvent ).
1 H NMR (400 MHz, CD3 OD) δ: (ppm) 7.76 – 7.85 (m, 1H), 7.46 – 7.52 (m, 1H), 7.29 – 7.36 (m, 1H), 7.09 – 7.16 (m, 2H), 7.00 – 7.08 (m, 4H), 6.94 – 6.97 (m, 1H), 6.75 (t,J F-H = 75.6 Hz, 1H), 5.29 – 5.39 (m, 2H), 4.73 – 4.78 (m, 1H), 3.95 (d,J = 6.8 Hz, 2H), 3.82 – 3.87 (m, 1H), 3.71 – 3.78 (m, 1H), 3.36 – 3.47 (m, 1H), 3.45 (s, 3H), 2.87 – 2.95 (m, 1H), 2.30 – 2.43 (m, 1H), 2.24 (s, 3H), 1.26 – 1.38 (m, 1H), 0.61 – 0.68 (m, 2H), 0.35 – 0.42 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ: (ppm) 7.76 – 7.85 (m, 1H), 7.46 – 7.52 (m, 1H), 7.29 – 7.36 (m, 1H), 7.09 – 7.16 (m, 2H) ), 7.00 – 7.08 (m, 4H), 6.94 – 6.97 (m, 1H), 6.75 (t, J FH = 75.6 Hz, 1H), 5.29 – 5.39 (m, 2H), 4.73 – 4.78 (m, 1H) , 3.95 (d, J = 6.8 Hz, 2H), 3.82 – 3.87 (m, 1H), 3.71 – 3.78 (m, 1H), 3.36 – 3.47 (m, 1H), 3.45 (s, 3H), 2.87 – 2.95 (m, 1H), 2.30 – 2.43 (m, 1H), 2.24 (s, 3H), 1.26 – 1.38 (m, 1H), 0.61 – 0.68 (m, 2H), 0.35 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 566.20 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 566.20 [M+H-HCl] + .
步驟3:化合物 (2R ,4S )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 3: Compound ( 2R , 4S )-(6-(methyl(p-tolyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropyl) Synthesis of methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2R ,4S )-(6-(甲基(對甲苯基)氨基甲醯基) 吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽(253 mg, 0.42 mmol) 溶解在二氯甲烷 (8 mL) 中,在冰浴中冷卻,加入N ,N -二異丙基乙胺 (269 mg, 2.08 mmol) 和乙醯氯 (82 mg, 1.05 mmol),室溫攪拌4 h後停止反應,加水 (30 mL) 攪拌 2 min,分離有機相,水相用二氯甲烷萃取 (5 mL × 2),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:DCM/MeOH ( v/v ) = 23/1) 得到白色固體 (184 mg, 產率72%)。Compound ( 2R , 4S )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4- (Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride (253 mg, 0.42 mmol) was dissolved in dichloromethane (8 mL), cooled in an ice bath, N , N - Diisopropylethylamine (269 mg, 2.08 mmol) and acetyl chloride (82 mg, 1.05 mmol) were stirred at room temperature for 4 h to stop the reaction, water (30 mL) was added and stirred for 2 min, the organic phase was separated, and the aqueous phase was Dichloromethane extraction (5 mL × 2), combined organic phases, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: DCM/MeOH (v/v) = 23/1 ) to give a white solid (184 mg, 72% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.50 – 7.64 (m, 1H), 7.27 – 7.38 (m, 1H), 7.20 – 7.27 (m, 1H), 7.08 – 7.13 (m, 1H), 6.86 – 7.05 (m, 4H), 6.82 (s, 1H), 6.80 (d,J = 3.9 Hz, 1H), 6.60 (t,J F-H = 75.5 Hz, 1H), 5.01 – 5.22 (m, 2H), 4.53 – 4.57 (m, 1H), 3.92 – 3.97 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.61 (t,J = 10.4 Hz, 1H), 3.46 (s, 3H), 3.37 – 3.45 (m, 1H), 2.66 – 2.72 (m, 1H), 2.25 (s, 3H), 2.11 (s, 3H), 2.04 – 2.09 (m, 1H), 1.24 – 1.34 (m, 1H), 0.63 – 0.67 (m, 2H), 0.34 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.50 – 7.64 (m, 1H), 7.27 – 7.38 (m, 1H), 7.20 – 7.27 (m, 1H), 7.08 – 7.13 (m, 1H) , 6.86 – 7.05 (m, 4H), 6.82 (s, 1H), 6.80 (d, J = 3.9 Hz, 1H), 6.60 (t, J FH = 75.5 Hz, 1H), 5.01 – 5.22 (m, 2H) , 4.53 – 4.57 (m, 1H), 3.92 – 3.97 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.61 (t, J = 10.4 Hz, 1H), 3.46 (s, 3H), 3.37 – 3.45 (m, 1H), 2.66 – 2.72 (m, 1H), 2.25 (s, 3H), 2.11 (s, 3H), 2.04 – 2.09 (m, 1H), 1.24 – 1.34 (m, 1H), 0.63 – 0.67 (m, 2H), 0.34 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 608.30 [M+H]+ .MS (ESI, pos.ion) m/z: 608.30 [M+H] + .
實施例153:(3-異吲哚啉-5-基)甲基 (2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 153: (3-indol-5-yl) methyl (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (di Fluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (200 mg, 0.54 mmol,實施例151步驟5),6-(羥甲基)異吲哚-1-酮 (221 mg, 1.35 mmol) 和N -羥基-7-氮雜苯並三氮唑 (146 mg, 1.08 mmol) 溶於無水DMF (8 mL) 中,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (311 mg, 1.62 mmol),加入N ,N -二異丙基乙胺 (279 mg, 2.16 mmol),室溫反應11 h,減壓濃縮除去溶劑,加水 (40 mL),用乙酸乙酯萃取 (25 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑: DCM/MeOH ( v/v ) = 17/1) 得到白色固體 (176 mg, 產率63%)。Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid (200 mg, 0.54 mmol, Example 151, step 5), 6-(hydroxymethyl)isoindol-1-one (221 mg, 1.35 mmol) and N -hydroxy-7-azabenzotriazole (146 mg , 1.08 mmol) was dissolved in dry DMF (8 mL), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (311 mg, 1.62 mmol) was added, N , N -Diisopropylethylamine (279 mg, 2.16 mmol), reacted at room temperature for 11 h, concentrated under reduced pressure to remove the solvent, added water (40 mL), extracted with ethyl acetate (25 mL × 2), the organic phase was washed with anhydrous sulfuric acid It was dried over sodium, concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: DCM/MeOH (v/v) = 17/1) to obtain a white solid (176 mg, yield 63%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.85 (s, 1H), 7.59 (d,J = 6.3 Hz, 1H), 7.47 (d,J = 5.7 Hz, 1H), 7.14 (br.s, 1H), 7.10 (d,J = 6.7 Hz, 1H), 6.80 (s, 1H), 6.72 – 6.80 (m, 1H), 6.59 (t,J F-H = 75.7 Hz, 1H), 5.29 (s, 2H), 4.49 – 4.59 (m, 1H), 4.45 (s, 2H), 3.89 – 3.99 (m, 1H), 3.85 (d,J = 4.3 Hz, 2H), 3.55 – 3.66 (m, 1H), 3.35 – 3.48 (m, 1H), 2.60 – 2.74 (m, 1H), 2.12 (s, 3H), 1.99 – 2.08 (m, 1H), 1.25 – 1.33 (m, 1H), 0.58– 0.69 (m, 2H), 0.27 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.85 (s, 1H), 7.59 (d, J = 6.3 Hz, 1H), 7.47 (d, J = 5.7 Hz, 1H), 7.14 (br. s, 1H), 7.10 (d, J = 6.7 Hz, 1H), 6.80 (s, 1H), 6.72 – 6.80 (m, 1H), 6.59 (t, J FH = 75.7 Hz, 1H), 5.29 (s, 2H), 4.49 – 4.59 (m, 1H), 4.45 (s, 2H), 3.89 – 3.99 (m, 1H), 3.85 (d, J = 4.3 Hz, 2H), 3.55 – 3.66 (m, 1H), 3.35 – 3.48 (m, 1H), 2.60 – 2.74 (m, 1H), 2.12 (s, 3H), 1.99 – 2.08 (m, 1H), 1.25 – 1.33 (m, 1H), 0.58– 0.69 (m, 2H) , 0.27 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 515.20 [M+H]+ .MS (ESI, pos.ion) m/z: 515.20 [M+H] + .
實施例154:(2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((3-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺 Embodiment 154 cases of: (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - ((3 -oxoisoindolin-5-yl)methyl)pyrrolidine-2-carboxamide
將化合物(2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (120 mg, 0.32 mmol,實施例151步驟5),6-(氨甲基)異吲哚-1-酮 (58 mg, 0.36 mmol) 和N -羥基-7-氮雜苯並三氮唑 (89 mg, 0.65 mmol) 溶於無水DMF (4 mL) 和二氯甲烷 (2 mL) 中,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (188 mg, 0.98 mmol),加入N ,N -二異丙基乙胺 (167 mg, 1.29 mmol),室溫反應14 h,減壓濃縮除去溶劑,加水 (20 mL),用乙酸乙酯萃取 (15 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑: DCM/MeOH ( v/v ) = 15/1) 得到白色固體 (113 mg, 產率68%)。Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid (120 mg, 0.32 mmol, Example 151 step 5), 6-(aminomethyl)isoindol-1-one (58 mg, 0.36 mmol) and N -hydroxy-7-azabenzotriazole (89 mg , 0.65 mmol) was dissolved in dry DMF (4 mL) and dichloromethane (2 mL), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (188 mg, 0.98 mmol), N , N -diisopropylethylamine (167 mg, 1.29 mmol) was added, the reaction was carried out at room temperature for 14 h, concentrated under reduced pressure to remove the solvent, added water (20 mL), and extracted with ethyl acetate (15 mL × 2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: DCM/MeOH (v/v) = 15/1) to obtain a white solid (113 mg, yield 68%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 8.42 (br.s, 1H), 7.84 (br.s, 1H), 7.45 – 7.56 (m, 2H), 7.25 – 7.34 (m, 1H), 7.12 (d,J = 8.0 Hz, 1H), 6.90 (s, 1H), 6.85 (d,J = 7.9 Hz, 1H), 6.61 (t,J F-H = 75.7 Hz, 1H), 4.93 – 4.99 (m, 1H), 4.62 – 4.74 (m, 1H), 4.10 – 4.20 (m, 1H), 3.99 – 4.07 (m, 1H), 3.89 – 3.97 (m, 2H), 3.88 (d,J = 6.8 Hz, 2H), 3.62 – 3.74 (m, 1H), 3.28 – 3.41 (m, 1H), 2.59 – 2.69 (m, 1H), 2.29 – 2.44 (m, 1H), 2.15 (s, 3H), 1.21 – 1.36 (m, 1H), 0.59 – 0.69 (m, 2H), 0.31 – 0.42 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 8.42 (br.s, 1H), 7.84 (br.s, 1H), 7.45 – 7.56 (m, 2H), 7.25 – 7.34 (m, 1H) , 7.12 (d, J = 8.0 Hz, 1H), 6.90 (s, 1H), 6.85 (d, J = 7.9 Hz, 1H), 6.61 (t, J FH = 75.7 Hz, 1H), 4.93 – 4.99 (m , 1H), 4.62 – 4.74 (m, 1H), 4.10 – 4.20 (m, 1H), 3.99 – 4.07 (m, 1H), 3.89 – 3.97 (m, 2H), 3.88 (d, J = 6.8 Hz, 2H ), 3.62 – 3.74 (m, 1H), 3.28 – 3.41 (m, 1H), 2.59 – 2.69 (m, 1H), 2.29 – 2.44 (m, 1H), 2.15 (s, 3H), 1.21 – 1.36 (m , 1H), 0.59 – 0.69 (m, 2H), 0.31 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 514.20 [M+H]+ .MS (ESI, pos.ion) m/z: 514.20 [M+H] + .
實施例155:(2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((1-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺 Example 155: (2 R, 4 S ) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) - N - ((1 -oxoisoindolin-5-yl)methyl)pyrrolidine-2-carboxamide
將化合物(2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (120 mg, 0.32 mmol,實施例151步驟5),5-(氨甲基)異吲哚-1-酮 (52 mg, 0.32 mmol) 和N -羥基-7-氮雜苯並三氮唑 (89 mg, 0.65 mmol) 溶於無水DMF (8 mL) 中,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (188 mg, 0.98 mmol),加入N ,N -二異丙基乙胺 (167 mg, 1.29 mmol),室溫反應18 h,減壓濃縮除去溶劑,加水 (20 mL),用乙酸乙酯萃取 (15 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑: DCM/MeOH ( v/v ) = 15/1) 得到白色固體 (70 mg, 產率49%)。Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid (120 mg, 0.32 mmol, Example 151, step 5), 5-(aminomethyl)isoindol-1-one (52 mg, 0.32 mmol) and N -hydroxy-7-azabenzotriazole (89 mg , 0.65 mmol) was dissolved in dry DMF (8 mL), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (188 mg, 0.98 mmol) was added, N , N -Diisopropylethylamine (167 mg, 1.29 mmol), reacted at room temperature for 18 h, concentrated under reduced pressure to remove the solvent, added water (20 mL), extracted with ethyl acetate (15 mL × 2), the organic phase was washed with anhydrous sulfuric acid It was dried over sodium, concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: DCM/MeOH (v/v) = 15/1) to obtain a white solid (70 mg, yield 49%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 8.10 (br.s, 1H), 7.78 (br.s, 1H), 7.38 – 7.44 (m, 1H), 7.33 (s, 1H), 7.29 (d,J = 7.2 Hz, 1H), 7.11 (d,J = 8.0 Hz, 1H), 6.91 (s, 1H), 6.86 (d,J = 7.9 Hz, 1H), 6.61 (t,J F-H = 75.6 Hz, 1H), 4.70 – 4.88 (m, 2H), 4.08 – 4.23 (m, 1H), 4.19 (s, 2H), 3.90 – 3.97 (m, 1H), 3.87 (d,J = 6.8 Hz, 2H), 3.65 – 3.73 (m, 1H), 3.27 – 3.42 (m, 1H), 2.56 – 2.68 (m, 1H), 2.34 – 2.45 (m, 1H), 2.15 (s, 3H), 1.21 – 1.36 (m, 1H), 0.59 – 0.69 (m, 2H), 0.29 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 8.10 (br.s, 1H), 7.78 (br.s, 1H), 7.38 – 7.44 (m, 1H), 7.33 (s, 1H), 7.29 (d, J = 7.2 Hz, 1H), 7.11 (d, J = 8.0 Hz, 1H), 6.91 (s, 1H), 6.86 (d, J = 7.9 Hz, 1H), 6.61 (t, J FH = 75.6 Hz, 1H), 4.70 – 4.88 (m, 2H), 4.08 – 4.23 (m, 1H), 4.19 (s, 2H), 3.90 – 3.97 (m, 1H), 3.87 (d, J = 6.8 Hz, 2H) , 3.65 – 3.73 (m, 1H), 3.27 – 3.42 (m, 1H), 2.56 – 2.68 (m, 1H), 2.34 – 2.45 (m, 1H), 2.15 (s, 3H), 1.21 – 1.36 (m, 1H), 0.59 – 0.69 (m, 2H), 0.29 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 514.20 [M+H]+ .MS (ESI, pos.ion) m/z: 514.20 [M+H] + .
實施例156:(2R ,4R )- (6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 156: ( 2R , 4R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropyl) methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物 (2R ,4R )-1-叔丁基 2-((6-(甲基(對甲苯基) 氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯的合成Step 1: Compound ( 2R , 4R )-1-tert-butyl 2-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl)4-(3- Synthesis of (cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物(2R ,4R )-1-(叔丁氧羰基)-4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (900 mg, 2.11 mmol,實施例150步驟5),6-(羥甲基)-N -甲基-N -(對甲基苯基)吡啶醯胺 (50 mg, 0.34 mmol) 和1-羥基苯並三唑 (426 mg, 3.15 mmol) 溶於乾燥的DMF (8 mL) 中,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (605 mg, 3.16 mmol),冰浴中加入N –甲基嗎啡啉 (426 mg, 4.21 mmol),室溫反應10 h,加水 (130 mL),用乙酸乙酯萃取 (25 mL × 3),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:DCM/ EtOAc ( v/v ) = 10/1) 得到淺褐色固體 (983 mg, 產率70%)。The compound ( 2R , 4R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (900 mg, 2.11 mmol, Example 150, step 5), 6-(hydroxymethyl) -N -methyl- N- (p-methylphenyl)pyridinamide (50 mg, 0.34 mmol) and 1-hydroxyl Benzotriazole (426 mg, 3.15 mmol) was dissolved in dry DMF (8 mL) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (605 mg, 3.16 mmol), N -methylmorpholine (426 mg, 4.21 mmol) was added to the ice bath, the reaction was carried out at room temperature for 10 h, water (130 mL) was added, extracted with ethyl acetate (25 mL × 3), the organic phases were combined, It was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: DCM/EtOAc (v/v) = 10/1) to obtain a light brown solid (983 mg, yield 70%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.49 – 7.61 (m, 1H), 7.13 – 7.32 (m, 2H), 7.09 (d,J = 7.8 Hz, 1H), 6.86 – 7.01 (m, 4H), 6.76 – 6.81 (m, 2H), 6.59 (t,J F-H = 75.6 Hz, 1H), 4.99 – 5.18 (m, 2H), 4.37 – 4.48 (m, 1H), 4.05 – 4.09 (m, 0.5H), 3.92 – 3.96 (m, 0.5H), 3.82 – 3.86 (m, 2H), 3.46 (s, 3H), 3.28 – 3.44 (m, 2H), 2.64 – 2.73 (m, 1H), 2.28 – 2.41 (m, 0.5H), 2.24 (s, 3H), 1.99 – 2.11 (m, 0.5H), 1.37 – 1.45 (m, 9H), 1.22 – 1.33 (m, 1H), 0.61 – 0.67 (m, 2H), 0.31 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.49 – 7.61 (m, 1H), 7.13 – 7.32 (m, 2H), 7.09 (d, J = 7.8 Hz, 1H), 6.86 – 7.01 (m , 4H), 6.76 – 6.81 (m, 2H), 6.59 (t, J FH = 75.6 Hz, 1H), 4.99 – 5.18 (m, 2H), 4.37 – 4.48 (m, 1H), 4.05 – 4.09 (m, 0.5H), 3.92 – 3.96 (m, 0.5H), 3.82 – 3.86 (m, 2H), 3.46 (s, 3H), 3.28 – 3.44 (m, 2H), 2.64 – 2.73 (m, 1H), 2.28 – 2.41 (m, 0.5H), 2.24 (s, 3H), 1.99 – 2.11 (m, 0.5H), 1.37 – 1.45 (m, 9H), 1.22 – 1.33 (m, 1H), 0.61 – 0.67 (m, 2H) ), 0.31 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 666.30 [M+H]+ .MS (ESI, pos.ion) m/z: 666.30 [M+H] + .
步驟2:化合物 (2R ,4R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽的合成Step 2: Compound ( 2R , 4R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)- Synthesis of 4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride
將化合物(2R ,4R )-1-叔丁基 2-((6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯 (880 mg, 1.32 mmol) 溶解於二氯甲烷 (12 mL) 溶液中,加入4 mol/L HCl的1,4-二氧六環溶液 (5 mL),室溫攪拌1 h,除去溶劑,得到淺黃色固體 (817 mg, 產率100%)。The compound ( 2R , 4R )-1-tert-butyl 2-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl)4-(3-(cyclo) Propylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (880 mg, 1.32 mmol) was dissolved in dichloromethane (12 mL) and added A solution of 4 mol/L HCl in 1,4-dioxane (5 mL) was stirred at room temperature for 1 h, and the solvent was removed to obtain a pale yellow solid (817 mg, yield 100%).
步驟3:化合物 (2R ,4R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 3: Compound ( 2R , 4R )-(6-(methyl(p-tolyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropyl) Synthesis of methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2R ,4R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽(795 mg, 1.32 mmol) 溶解在二氯甲烷 (15 mL) 中,在冰浴中冷卻,加入N ,N -二異丙基乙胺 (682 mg, 5.28 mmol) 和乙醯氯 (259 mg, 3.30 mmol),室溫攪拌3 h後停止反應,加水 (20 mL) 攪拌 2 min,分離有機相,水相用二氯甲烷萃取 (10 mL × 2),合併有機相,用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑:DCM/ MeOH ( v/v ) = 23/1) 和製備純化得到白色固體 (614 mg, 產率77%,手性HPLC純度2R4R:23.43%, 2R4S:75.35%)。再進行手性製備得到白色固體(83 mg)。Compound (2 R ,4 R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4- (Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride (795 mg, 1.32 mmol) was dissolved in dichloromethane (15 mL), cooled in an ice bath, and N , N- Diisopropylethylamine (682 mg, 5.28 mmol) and acetyl chloride (259 mg, 3.30 mmol) were stirred at room temperature for 3 h to stop the reaction, water (20 mL) was added and stirred for 2 min, the organic phase was separated, and the aqueous phase was Dichloromethane extraction (10 mL × 2), combined organic phases, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: DCM/MeOH (v/v) = 23/1 ) and preparative purification gave a white solid (614 mg, 77% yield, chiral HPLC purity 2R4R: 23.43%, 2R4S: 75.35%). An additional chiral preparation gave a white solid (83 mg).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.55 – 7.65 (m, 1H), 7.22 – 7.35 (m, 2H), 7.12 – 7.16 (m, 1H), 6.87 – 7.05 (m, 4H), 6.79 – 6.83 (m, 2H), 6.63 (t,J F-H = 75.6 Hz, 1H), 5.02 – 5.25 (m, 2H), 4.79 (d,J = 8.1 Hz, 0.8H), 4.61 (d,J = 8.4 Hz, 0.2H), 4.05 – 4.09 (m, 1H), 3.86 – 3.90 (m, 2H), 3.63 – 3.72 (m, 1H), 3.49 – 3.53 (m, 1H), 3.47 (s, 3H), 2.34 – 2.47 (m, 2H), 2.27 (s, 3H), 2.13 (s, 2.5H), 2.04 (s, 0.5H), 1.22 – 1.35 (m, 1H), 0.65 – 0.70 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.55 – 7.65 (m, 1H), 7.22 – 7.35 (m, 2H), 7.12 – 7.16 (m, 1H), 6.87 – 7.05 (m, 4H) , 6.79 – 6.83 (m, 2H), 6.63 (t, J FH = 75.6 Hz, 1H), 5.02 – 5.25 (m, 2H), 4.79 (d, J = 8.1 Hz, 0.8H), 4.61 (d, J = 8.4 Hz, 0.2H), 4.05 – 4.09 (m, 1H), 3.86 – 3.90 (m, 2H), 3.63 – 3.72 (m, 1H), 3.49 – 3.53 (m, 1H), 3.47 (s, 3H) , 2.34 – 2.47 (m, 2H), 2.27 (s, 3H), 2.13 (s, 2.5H), 2.04 (s, 0.5H), 1.22 – 1.35 (m, 1H), 0.65 – 0.70 (m, 2H) , 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 608.25 [M+H]+ .MS (ESI, pos.ion) m/z: 608.25 [M+H] + .
實施例157:(2S ,4S )- (6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 157: ( 2S , 4S )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropyl) methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物(2S ,4R )-1-叔丁基 2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4-羥基吡咯烷-1,2-二甲酸酯的合成Step 1: Compound ( 2S , 4R )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4-hydroxy Synthesis of Pyrrolidine-1,2-Dicarboxylate
將化合物2-(環丙基甲氧基)-1-(二氟甲氧基)-4-碘苯 (10.410 g, 26.73 mmol) 溶於無水四氫呋喃 (62 mL), 冷至-20℃, 滴入異丙基氯化鎂-氯化鋰 (22 mL, 29.00 mmol, 1.3 mol/L 的四氫呋喃溶液),室溫攪拌2 h, 置於-78℃攪拌0.5 h, 滴入(2S )-1-叔丁基氧羰基-4-氧代脯氨酸甲酯(5.00 g, 20.60 mmol) 的無水四氫呋喃 (20 mL), 置於-70℃攪拌2 h。加入飽和氯化銨溶液(5 mL)淬滅反應, 加入乙酸乙酯 (100 mL) 稀釋,有機相用飽和食鹽水洗滌 (100 mL), 水相用乙酸乙酯萃取 (50 mL × 3) ,合併有機相,有機相用無水無水硫酸鈉乾燥,濃縮得黃色液體, 矽膠柱層析 (洗脫劑:PE/AcOEt ( v/v ) = 4/1) 純化得黃色透明液體 (5.21 g, 產率55.40%)。合成參考:Synthesis of 4-cis -Phenyl-L-proline via HydrogenolysisJ. Org. Chem. 2001, 66, 3593-3596 。Compound 2-(cyclopropylmethoxy)-1-(difluoromethoxy)-4-iodobenzene (10.410 g, 26.73 mmol) was dissolved in anhydrous tetrahydrofuran (62 mL), cooled to -20°C, dropwise Add isopropylmagnesium chloride-lithium chloride (22 mL, 29.00 mmol, 1.3 mol/L tetrahydrofuran solution), stir at room temperature for 2 h, place at -78 °C and stir for 0.5 h, add dropwise ( 2S )-1-tert. Butyloxycarbonyl-4-oxoproline methyl ester (5.00 g, 20.60 mmol) in dry tetrahydrofuran (20 mL) was stirred at -70 °C for 2 h. Saturated ammonium chloride solution (5 mL) was added to quench the reaction, ethyl acetate (100 mL) was added to dilute, the organic phase was washed with saturated brine (100 mL), and the aqueous phase was extracted with ethyl acetate (50 mL × 3), The organic phases were combined, dried over anhydrous anhydrous sodium sulfate, concentrated to obtain a yellow liquid, and purified by silica gel column chromatography (eluent: PE/AcOEt (v/v) = 4/1) to obtain a yellow transparent liquid (5.21 g, yield rate 55.40%). Synthesis reference: Synthesis of 4- cis- Phenyl-L-proline via Hydrogenolysis J. Org. Chem. 2001, 66, 3593-3596 .
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.19 (d,J = 14.9 Hz, 1H), 7.13 (d,J = 8.3 Hz, 1H), 6.95 (d,J = 8.3 Hz, 1H), 6.62 (t,J = 75.6 Hz, 1H), 4.57 – 4.42 (m, 1H), 3.95 – 3.84 (m, 3H), 3.82 (d,J = 6.0 Hz, 3H), 3.76 – 3.62 (m, 1H), 2.68 – 2.58 (m, 1H), 2.33 – 2.21 (m, 1H), 1.45 (d,J = 8.5 Hz, 9H), 1.28 – 1.24 (m, 1H), 0.69 – 0.60 (m, 2H), 0.38 – 0.29 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.19 (d, J = 14.9 Hz, 1H), 7.13 (d, J = 8.3 Hz, 1H), 6.95 (d, J = 8.3 Hz, 1H) , 6.62 (t, J = 75.6 Hz, 1H), 4.57 – 4.42 (m, 1H), 3.95 – 3.84 (m, 3H), 3.82 (d, J = 6.0 Hz, 3H), 3.76 – 3.62 (m, 1H) ), 2.68 – 2.58 (m, 1H), 2.33 – 2.21 (m, 1H), 1.45 (d, J = 8.5 Hz, 9H), 1.28 – 1.24 (m, 1H), 0.69 – 0.60 (m, 2H), 0.38 – 0.29 (m, 2H).
MS (ESI, pos.ion) m/z: 340.10 [M-H2 O-Boc+2H]+ .MS (ESI, pos.ion) m/z: 340.10 [MH 2 O-Boc+2H] + .
步驟2:化合物(2S ,4R )-1-叔丁氧羰基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4-羥基吡咯烷-2-甲酸的合成Step 2: Compound ( 2S , 4R )-1-tert-butoxycarbonyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4-hydroxypyrrolidine Synthesis of -2-carboxylic acid
將化合物(2S ,4R )-1-叔丁基2-甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4-羥基吡咯烷-1,2-二甲酸酯(5.21 g, 11.38 mmol),氫氧化鋰一水合物 (560 mg, 22.91 mmol) 溶於四氫呋喃/水 (13 mL, ( v/v ) = 5/1) 中,室溫攪拌4 h。置於冰浴冷卻10 min, 滴入稀鹽酸 (4 mol/L) 調節pH = 4, 加入飽和食鹽水 (50 mL),用乙酸乙酯 (100 mL × 3)萃取,合併有機相,用無水硫酸鈉乾燥,減壓濃縮,得淺褐色固體 (5.15 g, 產率100% )。The compound ( 2S , 4R )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4-hydroxypyrrolidine -1,2-Dicarboxylate (5.21 g, 11.38 mmol), lithium hydroxide monohydrate (560 mg, 22.91 mmol) in tetrahydrofuran/water (13 mL, (v/v) = 5/1) , and stirred at room temperature for 4 h. Place in an ice bath to cool for 10 min, add dilute hydrochloric acid (4 mol/L) dropwise to adjust pH = 4, add saturated brine (50 mL), extract with ethyl acetate (100 mL × 3), combine the organic phases, wash with anhydrous Dry over sodium sulfate and concentrate under reduced pressure to give a light brown solid (5.15 g, yield 100%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.14 – 7.05 (m, 2H), 6.93 (d,J = 8.2 Hz, 1H), 6.61 (t,J = 75.6 Hz, 1H), 4.58 – 4.46 (m, 2H), 3.91 – 3.82 (m, 2H), 3.82 – 3.70 (m, 1H), 3.67 – 3.50 (m, 1H), 2.64 – 2.52 (m, 1H), 2.47 (d,J = 13.6 Hz, 1H), 1.41 (s, 9H), 1.27 – 1.22 (m, 1H), 0.66 – 0.54 (m, 2H), 0.39 – 0.25 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.14 – 7.05 (m, 2H), 6.93 (d, J = 8.2 Hz, 1H), 6.61 (t, J = 75.6 Hz, 1H), 4.58 – 4.46 (m, 2H), 3.91 – 3.82 (m, 2H), 3.82 – 3.70 (m, 1H), 3.67 – 3.50 (m, 1H), 2.64 – 2.52 (m, 1H), 2.47 (d, J = 13.6 Hz, 1H), 1.41 (s, 9H), 1.27 – 1.22 (m, 1H), 0.66 – 0.54 (m, 2H), 0.39 – 0.25 (m, 2H).
MS (ESI, pos.ion) m/z: 370.10 [M-H2 O-tBu+2H]+ .MS (ESI, pos.ion) m/z: 370.10 [MH 2 O-tBu+2H] + .
步驟3:化合物(1R ,4S )-叔丁基 1-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-3-氧代-2-氧雜-5-氮雜雙環[2.2.1]庚烷-5-甲酸酯的合成Step 3: Compound ( 1R , 4S )-tert-butyl 1-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-3-oxo-2-oxa -Synthesis of 5-azabicyclo[2.2.1]heptane-5-carboxylate
將化合物(2S ,4R )-1-叔丁氧羰基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4-羥基吡咯烷-2-甲酸 (5.10 g, 11.50 mmol) 和N ,N -二異丙基乙胺 (5.6 mL, 34.00 mmol) 溶於二氯甲烷 (45 mL) 中,在0℃下緩慢滴入甲磺醯氯 (1.3 mL, 17.00 mmol),室溫攪拌2 h。加入二氯甲烷 (100 mL) 稀釋,有機相用水 (50 mL × 3)洗滌, 用無水硫酸鈉乾燥,濃縮得黃色液體, 矽膠柱層析 (洗脫劑:PE/AcOEt ( v/v ) = 5/1) 純化得黃色液體 (3.19 g, 產率65.20% )。The compound ( 2S , 4R )-1-tert-butoxycarbonyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4-hydroxypyrrolidine-2 - Formic acid (5.10 g, 11.50 mmol) and N , N -diisopropylethylamine (5.6 mL, 34.00 mmol) were dissolved in dichloromethane (45 mL), and methanesulfonyl chloride was slowly added dropwise at 0 °C ( 1.3 mL, 17.00 mmol), stirred at room temperature for 2 h. Dichloromethane (100 mL) was added to dilute, and the organic phase was washed with water (50 mL × 3), dried over anhydrous sodium sulfate, and concentrated to obtain a yellow liquid. Silica gel column chromatography (eluent: PE/AcOEt (v/v) = 5/1) was purified to give a yellow liquid (3.19 g, yield 65.20%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.19 (d,J = 8.3 Hz, 1H), 7.07 (d,J = 1.9 Hz, 1H), 6.93 (dd,J = 8.3, 2.0 Hz, 1H), 6.64 (t,J = 75.2 Hz, 1H), 4.69 (br.s, 1H), 3.88 (d,J = 6.9 Hz, 2H), 3.70 – 3.58 (m, 2H), 2.42 (d,J = 11.9 Hz, 1H), 2.27 (d,J = 10.6 Hz, 1H), 1.47 (s, 9H), 1.29 – 1.24 (m, 1H), 0.67 – 0.61 (m, 2H), 0.38 – 0.32 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.19 (d, J = 8.3 Hz, 1H), 7.07 (d, J = 1.9 Hz, 1H), 6.93 (dd, J = 8.3, 2.0 Hz, 1H), 6.64 (t, J = 75.2 Hz, 1H), 4.69 (br.s, 1H), 3.88 (d, J = 6.9 Hz, 2H), 3.70 – 3.58 (m, 2H), 2.42 (d, J = 11.9 Hz, 1H), 2.27 (d, J = 10.6 Hz, 1H), 1.47 (s, 9H), 1.29 – 1.24 (m, 1H), 0.67 – 0.61 (m, 2H), 0.38 – 0.32 (m, 2H).
MS (ESI, pos.ion) m/z: 370.10 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 370.10 [M- t- Bu+2H] + .
步驟4:化合物(2S )-1-(叔丁基氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲酸的合成Step 4: Compound ( 2S )-1-(tert-butyloxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2,5- Synthesis of Dihydro- 1H -pyrrole-2-carboxylic acid
將化合物(1R ,4S )-叔丁基 1-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-3-氧代-2-氧雜-5-氮雜雙環[2.2.1]庚烷-5-甲酸酯 (3.15 g, 7.40 mmol) 溶於二氯甲烷 (85 mL) 中, 滴入三氟乙酸 (5.6 mL, 34.00 mmol) 的二氯甲烷溶液 (20 mL), 室溫攪拌2.5 h。有機相用水 (80 mL × 3) 洗滌, 用無水硫酸鈉乾燥,濃縮得黃色液體, 矽膠柱層析 (洗脫劑:PE/AcOEt ( v/v ) = 5/1) 純化得黃色固體 (1.53 g, 產率48.50% )。The compound ( 1R , 4S )-tert-butyl 1-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-3-oxo-2-oxa-5 - Azabicyclo[2.2.1]heptane-5-carboxylate (3.15 g, 7.40 mmol) was dissolved in dichloromethane (85 mL) and added dropwise to trifluoroacetic acid (5.6 mL, 34.00 mmol) in dichloromethane Methane solution (20 mL), stirred at room temperature for 2.5 h. The organic phase was washed with water (80 mL × 3), dried over anhydrous sodium sulfate, and concentrated to obtain a yellow liquid, which was purified by silica gel column chromatography (eluent: PE/AcOEt (v/v) = 5/1) to obtain a yellow solid (1.53 g, yield 48.50%).
MS (ESI, pos.ion) m/z: 370.10 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 370.10 [M- t- Bu+2H] + .
步驟5:化合物(2S )-1-叔丁基氧羰基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-吡咯烷-2-甲酸的合成Step 5: Compound ( 2S )-1-tert-butyloxycarbonyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-pyrrolidine-2-carboxylic acid synthesis
將化合物(2S )-1-叔丁基氧羰基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲酸(1.04 g, 2.45 mmol),三(三苯基膦)氯化銠 (295 mg, 0.32 mmol),三乙胺 (490 uL, 3.52 mmol),無水四氫呋喃 (2 mL)溶於無水乙醇 (18 mL) 中,排除空氣, 在氫氣 (0.8 MPa) 氛圍下,室溫攪拌4天。濃縮溶劑,向剩餘物加入乙酸乙酯 (50 mL) 稀釋,用稀鹽酸(1 M/L)調節pH = 1,分離有機相,水相用乙酸乙酯萃取 (30 mL × 3),合併有機相,用無水硫酸鈉乾燥,濃縮得黃色液體,矽膠柱層析純化 (洗脫劑:PE/AcOEt ( v/v ) = 5/1) 得白色黏稠固體(843 mg, 80.45%)。合成參考:Carboxylate-Directed Highly Stereoselective Homogeneous Hydrogenation of Cyclic Olefins with Wilkinson’s Catalyst.Organic Letters. 2003 Vol. 5, No. 9 1587 - 1589 。The compound ( 2S )-1-tert-butyloxycarbonyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro-1 H -pyrrole-2-carboxylic acid (1.04 g, 2.45 mmol), tris(triphenylphosphine)rhodium chloride (295 mg, 0.32 mmol), triethylamine (490 uL, 3.52 mmol), anhydrous tetrahydrofuran (2 mL) Dissolve in absolute ethanol (18 mL), remove air, and stir at room temperature for 4 days under hydrogen (0.8 MPa) atmosphere. The solvent was concentrated, ethyl acetate (50 mL) was added to the residue to dilute, the pH was adjusted to 1 with dilute hydrochloric acid (1 M/L), the organic phase was separated, the aqueous phase was extracted with ethyl acetate (30 mL × 3), and the organic The phase was dried over anhydrous sodium sulfate and concentrated to obtain a yellow liquid, which was purified by silica gel column chromatography (eluent: PE/AcOEt (v/v) = 5/1) to obtain a white viscous solid (843 mg, 80.45%). Synthetic reference: Carboxylate-Directed Highly Stereoselective Homogeneous Hydrogenation of Cyclic Olefins with Wilkinson's Catalyst. Organic Letters. 2003 Vol. 5, No. 9 1587 - 1589 .
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.15 – 7.06 (m, 1H), 6.85 – 6.73 (m, 2H), 6.59 (t,J = 75.6 Hz, 1H), 4.56 – 4.30 (m, 1H), 4.09 – 3.92 (m, 1H), 3.92 – 3.79 (m, 2H), 3.56 – 3.38 (m, 1H), 3.39 – 3.23 (m, 1H), 2.77 – 2.54 (m, 1H), 2.50 – 2.14 (m, 1H), 1.49 – 1.44 (m, 9H), 1.33 – 1.18 (m, 1H), 0.68 – 0.55 (m, 2H), 0.41 – 0.27 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.15 – 7.06 (m, 1H), 6.85 – 6.73 (m, 2H), 6.59 (t, J = 75.6 Hz, 1H), 4.56 – 4.30 (m , 1H), 4.09 – 3.92 (m, 1H), 3.92 – 3.79 (m, 2H), 3.56 – 3.38 (m, 1H), 3.39 – 3.23 (m, 1H), 2.77 – 2.54 (m, 1H), 2.50 – 2.14 (m, 1H), 1.49 – 1.44 (m, 9H), 1.33 – 1.18 (m, 1H), 0.68 – 0.55 (m, 2H), 0.41 – 0.27 (m, 2H).
MS (ESI, pos.ion) m/z: 372.05 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 372.05 [M- t- Bu+2H] + .
步驟6:化合物(2S )-1-叔丁基 2-((6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯的合成Step 6: Compound ( 2S )-1-tert-butyl 2-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropyl) Synthesis of Methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物(2S )-1-叔丁基氧羰基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-吡咯烷-2-甲酸 (840 mg, 1.97 mmol),6-羥甲基-N -甲基-N -對甲苯基-吡啶甲醯胺 (554 mg, 2.16 mmol) 和1-羥基苯並三唑 (406 mg, 2.94 mmol) 溶於N ,N -二甲基甲醯胺 (15 mL) 中,在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (568 mg, 2.95 mmol) 和N -甲基嗎啉 (440 uL, 3.90 mmol),室溫攪拌18 h。加水 (100 mL)稀釋,有機相用二氯甲烷 (50 mL × 3)萃取,用無水硫酸鈉乾燥,減壓除去溶劑,剩餘物進行矽膠柱層析分離 (洗脫劑:60-90石油醚/乙酸乙酯 ( v/v ) = 3/2) 得到白色固體 (940 mg, 71.85 %)。The compound ( 2S )-1-tert-butyloxycarbonyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-pyrrolidine-2-carboxylic acid (840 mg , 1.97 mmol), 6-hydroxymethyl- N -methyl- N -p-tolyl-picolinamide (554 mg, 2.16 mmol) and 1-hydroxybenzotriazole (406 mg, 2.94 mmol) were dissolved in N , N -dimethylformamide (15 mL), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (568 mg, 2.95 mmol) was added in an ice bath ) and N -methylmorpholine (440 uL, 3.90 mmol) and stirred at room temperature for 18 h. Water (100 mL) was added to dilute, the organic phase was extracted with dichloromethane (50 mL × 3), dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: 60-90 petroleum ether). /ethyl acetate (v/v) = 3/2) to give a white solid (940 mg, 71.85%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.60 – 7.48 (m, 1H), 7.30 – 7.12 (m, 2H), 7.07 (d,J = 8.0 Hz, 1H), 7.00 – 6.83 (m, 4H), 6.80 – 6.74 (m, 2H), 6.56 (t,J = 75.7 Hz, 1H), 5.18 – 4.96 (m, 2H), 4.60 – 4.33 (m, 1H), 4.02 – 3.88 (m, 1H), 3.86 – 3.78 (m, 2H), 3.53 – 3.45 (m, 1H), 3.42 (s, 3H), 3.40 – 3.29 (m, 1H), 2.42 – 2.26 (m, 2H), 2.22 (s, 3H), 1.43 (s, 5H), 1.36 (s, 4H), 1.27 – 1.23 (m, 1H), 0.65 – 0.58 (m, 2H), 0.35 – 0.28 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.60 – 7.48 (m, 1H), 7.30 – 7.12 (m, 2H), 7.07 (d, J = 8.0 Hz, 1H), 7.00 – 6.83 (m , 4H), 6.80 – 6.74 (m, 2H), 6.56 (t, J = 75.7 Hz, 1H), 5.18 – 4.96 (m, 2H), 4.60 – 4.33 (m, 1H), 4.02 – 3.88 (m, 1H) ), 3.86 – 3.78 (m, 2H), 3.53 – 3.45 (m, 1H), 3.42 (s, 3H), 3.40 – 3.29 (m, 1H), 2.42 – 2.26 (m, 2H), 2.22 (s, 3H) ), 1.43 (s, 5H), 1.36 (s, 4H), 1.27 – 1.23 (m, 1H), 0.65 – 0.58 (m, 2H), 0.35 – 0.28 (m, 2H).
MS (ESI, pos.ion) m/z: 666.10 [M+H]+ .MS (ESI, pos.ion) m/z: 666.10 [M+H] + .
步驟7:化合物(2S )-(6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽的合成Step 7: Compound ( 2S )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4-( Synthesis of difluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride
將化合物(2S )-1-叔丁基 2-((6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯 (940 mg, 1.41 mmol) 溶於二氯甲烷 (14 mL) 中,注入氯化氫的二氧六環溶液 (3.5 mL, 14 mmol, 4 mol/L)溶液,室溫攪拌3 h。減壓除去溶劑,得到黃色泡沫狀固體 (850 mg, 99.88% )。Compound ( 2S )-1-tert-butyl 2-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy) yl)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (940 mg, 1.41 mmol) was dissolved in dichloromethane (14 mL) and injected with hydrogen chloride in dioxane The solution of the ring solution (3.5 mL, 14 mmol, 4 mol/L) was stirred at room temperature for 3 h. The solvent was removed under reduced pressure to give a yellow foamy solid (850 mg, 99.88%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 8.28 – 8.05 (m, 1H), 7.86 – 7.69 (m, 2H), 7.25 – 7.11 (m, 2H), 7.11 – 6.95 (m, 4H), 6.93 – 6.78 (m, 1H), 6.56 (t,J = 75.6 Hz, 1H), 5.90 – 5.56 (m, 1H), 5.19 – 4.82 (m, 1H), 4.09 – 3.93 (m, 1H), 3.93 – 3.80 (m, 2H), 3.80 – 3.70 (m, 1H), 3.66 – 3.62 (m, 1H), 3.62 – 3.56 (m, 1H), 3.44 (s, 3H), 2.85 – 2.67 (m, 1H), 2.53 – 2.34 (m, 1H), 2.30 – 2.12 (m, 3H), 1.28 – 1.17 (m, 1H), 0.64 – 0.49 (m, 2H), 0.38 – 0.25 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 8.28 – 8.05 (m, 1H), 7.86 – 7.69 (m, 2H), 7.25 – 7.11 (m, 2H), 7.11 – 6.95 (m, 4H) , 6.93 – 6.78 (m, 1H), 6.56 (t, J = 75.6 Hz, 1H), 5.90 – 5.56 (m, 1H), 5.19 – 4.82 (m, 1H), 4.09 – 3.93 (m, 1H), 3.93 – 3.80 (m, 2H), 3.80 – 3.70 (m, 1H), 3.66 – 3.62 (m, 1H), 3.62 – 3.56 (m, 1H), 3.44 (s, 3H), 2.85 – 2.67 (m, 1H) , 2.53 – 2.34 (m, 1H), 2.30 – 2.12 (m, 3H), 1.28 – 1.17 (m, 1H), 0.64 – 0.49 (m, 2H), 0.38 – 0.25 (m, 2H).
MS (ESI, pos.ion) m/z: 566.10 [M-HCl+H]+ .MS (ESI, pos.ion) m/z: 566.10 [M-HCl+H] + .
步驟8:化合物(2S ,4S )-(6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯的合成Step 8: Compound ( 2S , 4S )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropyl) Synthesis of methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2S )-((6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羧酸酯鹽酸鹽 (850 mg, 1.41 mmol),三乙胺 (596 uL, 4.23 mmol) 溶於二氯甲烷 (14 mL) 中,在0℃下緩慢滴入乙醯氯 (151 uL, 2.12 mmol), 室溫攪拌2 h。加入二氯甲烷 (50 mL),有機相用飽和食鹽水 (30 mL × 3)洗滌,用無水硫酸鈉乾燥,減壓除去溶劑,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50:1 ) 得到黃色黏稠液體 (690 mg, 80.44%, (2S,4S):(2S,4R) = 63:23), 手性製備拆分得白色固體 (144 mg, 16.78%, (2S,4S) = 100%)。Compound ( 2S )-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4-(di Fluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride (850 mg, 1.41 mmol), triethylamine (596 uL, 4.23 mmol) was dissolved in dichloromethane (14 mL) at 0 Acetyl chloride (151 uL, 2.12 mmol) was slowly added dropwise at ℃, stirred at room temperature for 2 h. Dichloromethane (50 mL) was added, the organic phase was washed with saturated brine (30 mL × 3), and dried over anhydrous sodium sulfate , the solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 50:1) to obtain a yellow viscous liquid (690 mg, 80.44%, (2S,4S) :(2S,4R) = 63:23), resolved by chiral preparation to give a white solid (144 mg, 16.78%, (2S,4S) = 100%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.56 (br.s, 1H), 7.31 – 7.17 (m, 2H), 7.11 (d,J = 8.2 Hz, 1H), 7.02 – 6.85 (m, 4H), 6.83 – 6.74 (m, 2H), 6.59 (t,J = 75.6 Hz, 1H), 5.23 – 5.00 (m, 2H), 4.75 (d,J = 8.2 Hz, 1H), 4.10 – 3.99 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.70 – 3.58 (m, 1H), 3.52 – 3.44 (m, 1H), 3.43 (s, 3H), 2.45 – 2.37 (m, 1H), 2.37 – 2.27 (m, 1H), 2.24 (s, 3H), 2.10 (s, 2.5H), 2.00 (s, 0.5H), 1.28 – 1.24 (m, 1H), 0.68 – 0.59 (m, 2H), 0.38 – 0.30 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.56 (br.s, 1H), 7.31 – 7.17 (m, 2H), 7.11 (d, J = 8.2 Hz, 1H), 7.02 – 6.85 (m , 4H), 6.83 – 6.74 (m, 2H), 6.59 (t, J = 75.6 Hz, 1H), 5.23 – 5.00 (m, 2H), 4.75 (d, J = 8.2 Hz, 1H), 4.10 – 3.99 ( m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.70 – 3.58 (m, 1H), 3.52 – 3.44 (m, 1H), 3.43 (s, 3H), 2.45 – 2.37 (m, 1H) , 2.37 – 2.27 (m, 1H), 2.24 (s, 3H), 2.10 (s, 2.5H), 2.00 (s, 0.5H), 1.28 – 1.24 (m, 1H), 0.68 – 0.59 (m, 2H) , 0.38 – 0.30 (m, 2H).
MS (ESI, pos.ion) m/z: 608.20 [M+H]+ .MS (ESI, pos.ion) m/z: 608.20 [M+H] + .
實施例158:(2S ,4R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 158: ( 2S , 4R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropyl) methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物(2S ,4R )-1-叔丁基氧羰基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-吡咯烷-2-甲酸的合成Step 1: Compound ( 2S , 4R )-1-tert-butyloxycarbonyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-pyrrolidine-2 -Synthesis of formic acid
將化合物(2S )-1-(叔丁基氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2,5-二氫-1H -吡咯-2-甲酸(640 mg, 1.50 mmol,實施例157步驟4) 溶於乙醇 (31 mL),加入Pd/C (520 mg, 10%Pd, 55% H2 O) 和三乙胺 (319 uL, 2.29 mmol),排除空氣,氫氣氛圍下,室溫攪拌17 h。把反應液通過矽藻土濾去固體,濾液減壓除去溶劑得到無色透明液體 (645 mg, 產率100%)。合成參考:Discovery of Potent and Specific Dihydroisoxazole Inhibitors of Human Transglutaminase 2.J .Med. Chem. 2014, 57, 9042 - 9064 。Compound ( 2S )-1-(tert-butyloxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2,5-dihydro -1 H - pyrrole-2-carboxylic acid (640 mg, 1.50 mmol, Example 157, step 4) was dissolved in ethanol (31 mL), was added Pd / C (520 mg, 10 % Pd, 55% H 2 O) and tris Ethylamine (319 uL, 2.29 mmol) was removed from the air and stirred at room temperature for 17 h under a hydrogen atmosphere. The reaction solution was filtered through celite to remove the solid, and the filtrate was removed from the solvent under reduced pressure to obtain a colorless transparent liquid (645 mg, yield 100%). Synthetic reference: Discovery of Potent and Specific Dihydroisoxazole Inhibitors of Human Transglutaminase 2. J. Med. Chem. 2014, 57, 9042 - 9064 .
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.03 (d,J = 8.1 Hz, 1H), 6.82 (d,J = 9.1 Hz, 1H), 6.77 (d,J = 7.4 Hz, 1H), 6.55 (t,J = 75.8 Hz, 1H), 4.25 – 4.19 (m, 1H), 4.04 – 3.84 (m, 1H), 3.88 – 3.80 (m, 2H), 3.38 (t,J = 10.7 Hz, 1H), 3.27 – 3.15 (m, 1H), 2.72 – 2.55 (m, 1H), 2.07 – 1.92 (m, 1H), 1.41 (s, 9H), 1.29 – 1.18 (m, 1H), 0.63 – 0.56 (m, 2H), 0.34 – 0.28 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.03 (d, J = 8.1 Hz, 1H), 6.82 (d, J = 9.1 Hz, 1H), 6.77 (d, J = 7.4 Hz, 1H) , 6.55 (t, J = 75.8 Hz, 1H), 4.25 – 4.19 (m, 1H), 4.04 – 3.84 (m, 1H), 3.88 – 3.80 (m, 2H), 3.38 (t, J = 10.7 Hz, 1H) ), 3.27 – 3.15 (m, 1H), 2.72 – 2.55 (m, 1H), 2.07 – 1.92 (m, 1H), 1.41 (s, 9H), 1.29 – 1.18 (m, 1H), 0.63 – 0.56 (m , 2H), 0.34 – 0.28 (m, 2H).
MS (ESI, pos.ion) m/z: 372.05 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 372.05 [M- t- Bu+2H] + .
步驟2:化合物(2S ,4R )-1-叔丁基 2-((6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯的合成Step 2: Compound ( 2S , 4R )-1-tert-butyl 2-(((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-( Synthesis of Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物(2S ,4R )-1-叔丁基氧羰基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-吡咯烷-2-甲酸(600 mg, 1.40 mmol),6-羥甲基-N -甲基-N -對甲苯基-吡啶甲醯胺 (396 mg, 1.55 mmol) 和1-羥基苯並三唑 (290 mg, 2.10 mmol) 溶於N ,N -二甲基甲醯胺 (10 mL) 中,在冰浴中冷卻,加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (405 mg, 2.10 mmol) 和N -甲基嗎啉 (310 uL, 2.80 mmol),室溫攪拌21 h。加水 (50 mL)稀釋,有機相用二氯甲烷(50 mL × 3)萃取,用無水硫酸鈉乾燥,減壓除去溶劑,剩餘物進行矽膠柱層析分離 (洗脫劑:60-90石油醚/乙酸乙酯 ( v/v ) = 3/2) 得到白色固體 (650.0 mg, 0.98 mmol, 產率 69.55%)。The compound ( 2S , 4R )-1-tert-butyloxycarbonyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-pyrrolidine-2-carboxylic acid (600 mg, 1.40 mmol), 6-hydroxymethyl- N -methyl- N -p-tolyl-picolinamide (396 mg, 1.55 mmol) and 1-hydroxybenzotriazole (290 mg, 2.10 mmol) ) was dissolved in N , N -dimethylformamide (10 mL), cooled in an ice bath, and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride was added ( 405 mg, 2.10 mmol) and N -methylmorpholine (310 uL, 2.80 mmol), stirred at room temperature for 21 h. Water (50 mL) was added to dilute, the organic phase was extracted with dichloromethane (50 mL × 3), dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: 60-90 petroleum ether). /ethyl acetate (v/v) = 3/2) to give a white solid (650.0 mg, 0.98 mmol, 69.55% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) ) 7.62 – 7.47 (m, 1H), 7.32 – 7.22 (m, 1H), 7.19 – 7.06 (m, 1H), 7.08 (d,J = 8.0 Hz, 1H), 7.02 – 6.85 (m, 4H), 6.81 – 6.77 (m, 2H), 6.58 (t,J = 75.6 Hz, 1H), 5.19 – 4.97 (m, 2H), 4.49 – 4.33 (m, 1H), 4.08 – 3.89 (m, 1H), 3.85 – 3.82 (m, 2H), 3.45 (s, 3H), 3.42 – 3.35 (m, 1H), 3.35 – 3.27 (m, 1H), 2.73 – 2.62 (m, 1H), 2.10 – 1.97 (m, 1H), 2.03 (s, 3H), 1.45 (s, 4H), 1.36 (s, 5H), 1.25 – 1.23 (m, 1H), 0.66 – 0.60 (m, 2H), 0.37 – 0.31 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) ) 7.62 – 7.47 (m, 1H), 7.32 – 7.22 (m, 1H), 7.19 – 7.06 (m, 1H), 7.08 (d, J = 8.0 Hz, 1H), 7.02 – 6.85 (m, 4H), 6.81 – 6.77 (m, 2H), 6.58 (t, J = 75.6 Hz, 1H), 5.19 – 4.97 (m, 2H), 4.49 – 4.33 (m, 1H), 4.08 – 3.89 (m, 1H), 3.85 – 3.82 (m, 2H), 3.45 (s, 3H), 3.42 – 3.35 (m, 1H), 3.35 – 3.27 (m, 1H), 2.73 – 2.62 ( m, 1H), 2.10 – 1.97 (m, 1H), 2.03 (s, 3H), 1.45 (s, 4H), 1.36 (s, 5H), 1.25 – 1.23 (m, 1H), 0.66 – 0.60 (m, 2H), 0.37 – 0.31 (m, 2H).
MS (ESI, pos.ion) m/z: 666.30 [M+H]+ .MS (ESI, pos.ion) m/z: 666.30 [M+H] + .
步驟3:化合物(2S ,4R )-(6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽的合成Step 3: Compound ( 2S , 4R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)- Synthesis of 4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride
將化合物(2S ,4R )-1-叔丁基 2-((6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯 (11195-2) (600 mg, 0.90 mmol) 溶於二氯甲烷 (10 mL) 中,注入氯化氫的二氧六環溶液 (2.5 mL, 10.0 mmol, 4 mol/L)溶液,室溫攪拌2 h。減壓除去溶劑,得到褐色黏稠液體 (542 mg, 99.88% )。Compound ( 2S , 4R )-1-tert-butyl 2-(((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropyl) ylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (11195-2) (600 mg, 0.90 mmol) in dichloromethane (10 mL) Into the solution, a solution of hydrogen chloride in dioxane (2.5 mL, 10.0 mmol, 4 mol/L) was injected, and the mixture was stirred at room temperature for 2 h. The solvent was removed under reduced pressure to obtain a brown viscous liquid (542 mg, 99.88%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 8.02 – 7.82 (m, 1H), 7.27 – 7.15 (m, 1H), 7.10 – 6.95 (m, 5H), 6.92 – 6.79 (m, 3H), 6.57 (t,J = 75.7 Hz, 1H), 5.62 – 5.49 (m, 2H), 4.95 – 4.75 (m, 1H), 3.90 – 3.85 (m, 2H), 3.85 – 3.79 (m, 1H), 3.74 – 3.68 (m, 1H), 3.64 – 3.56 (m, 1H), 3.46 – 3.36 (m, 3H), 2.82 – 2.66 (m, 1H), 2.51 – 2.35 (m, 1H), 2.19 (s, 3H), 1.26 – 1.20 (m, 1H), 0.61 – 0.53 (m, 2H), 0.35 – 0.25 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 8.02 – 7.82 (m, 1H), 7.27 – 7.15 (m, 1H), 7.10 – 6.95 (m, 5H), 6.92 – 6.79 (m, 3H) , 6.57 (t, J = 75.7 Hz, 1H), 5.62 – 5.49 (m, 2H), 4.95 – 4.75 (m, 1H), 3.90 – 3.85 (m, 2H), 3.85 – 3.79 (m, 1H), 3.74 – 3.68 (m, 1H), 3.64 – 3.56 (m, 1H), 3.46 – 3.36 (m, 3H), 2.82 – 2.66 (m, 1H), 2.51 – 2.35 (m, 1H), 2.19 (s, 3H) , 1.26 – 1.20 (m, 1H), 0.61 – 0.53 (m, 2H), 0.35 – 0.25 (m, 2H).
MS (ESI, pos.ion) m/z: 566.15 [M-HCl+H]+ .MS (ESI, pos.ion) m/z: 566.15 [M-HCl+H] + .
步驟4:化合物(2S ,4R )-(6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 4: Compound ( 2S , 4R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropyl) Synthesis of methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2S ,4R )-((6-(甲基(對甲苯基)氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽(500 mg, 0.83 mmol),三乙胺 (351 uL, 2.49 mmol) 溶於二氯甲烷 (8 mL) 中,在0℃下緩慢滴入乙醯氯 (89 uL, 1.25 mmol), 室溫攪拌2 h。加入二氯甲烷 (50 mL),有機相用飽和食鹽水 (30 mL × 3)洗滌,用無水硫酸鈉乾燥,減壓除去溶劑,剩餘物進行矽膠柱層析分離 (洗脫劑:二氯甲烷/甲醇 ( v/v ) = 50/1 ) 得到黃色黏稠液體 (333 mg, 66.00%)。Compound ( 2S , 4R )-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4 -(Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride (500 mg, 0.83 mmol), triethylamine (351 uL, 2.49 mmol) in dichloromethane (8 mL) , Acetyl chloride (89 uL, 1.25 mmol) was slowly added dropwise at 0°C, and stirred at room temperature for 2 h. Dichloromethane (50 mL) was added, and the organic phase was washed with saturated brine (30 mL × 3), washed with anhydrous After drying over sodium sulfate, the solvent was removed under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 50/1) to obtain a yellow viscous liquid (333 mg, 66.00%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.68 – 7.47 (m, 1H), 7.36 – 7.28 (m, 1H), 7.25 – 7.18 (m, 1H), 7.14 – 7.04 (m, 1H), 7.03 – 6.84 (m, 4H), 6.81 (s, 1H), 6.79 – 6.75 (m, 1H), 6.58 (t,J = 75.5 Hz, 1H), 5.20 – 5.00 (m, 2H), 4.53 (t,J = 8.5 Hz, 1H), 3.97 – 3.88 (m, 1H), 3.84 (d,J = 6.9 Hz, 2H), 3.60 (t,J = 10.5 Hz, 1H), 3.44 (s, 3H), 3.41 – 3.29 (m, 1H), 2.72 – 2.61 (m, 1H), 2.23 (s, 3H), 2.09 (s, 3H), 2.07 – 2.03 (m, 1H), 1.27 – 1.22 (m, 1H), 0.65 – 0.61 (m, 2H), 0.35 – 0.32 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.68 – 7.47 (m, 1H), 7.36 – 7.28 (m, 1H), 7.25 – 7.18 (m, 1H), 7.14 – 7.04 (m, 1H) , 7.03 – 6.84 (m, 4H), 6.81 (s, 1H), 6.79 – 6.75 (m, 1H), 6.58 (t, J = 75.5 Hz, 1H), 5.20 – 5.00 (m, 2H), 4.53 (t , J = 8.5 Hz, 1H), 3.97 – 3.88 (m, 1H), 3.84 (d, J = 6.9 Hz, 2H), 3.60 (t, J = 10.5 Hz, 1H), 3.44 (s, 3H), 3.41 – 3.29 (m, 1H), 2.72 – 2.61 (m, 1H), 2.23 (s, 3H), 2.09 (s, 3H), 2.07 – 2.03 (m, 1H), 1.27 – 1.22 (m, 1H), 0.65 – 0.61 (m, 2H), 0.35 – 0.32 (m, 2H).
MS (ESI, pos.ion) m/z: 608.20 [M+H]+ .MS (ESI, pos.ion) m/z: 608.20 [M+H] + .
實施例159:(2-甲基-3-氧代異吲哚啉-5-基)甲基 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 159: (2-Methyl-3-oxoisoindolin-5-yl)methyl( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)- 4-(Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酸 (100 mg, 0.27 mmol,實施例1步驟7),6-(羥甲基)-2-甲基異吲哚-1-酮 (50 mg, 0.28 mmol) 溶於二氯甲烷 (10 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (113 mg, 0.81 mmol),在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (157 mg, 0.81 mmol) 和N ,N -二異丙基乙胺 (141 mg, 1.09 mmol),室溫反應22 h,加水 (10 mL) 攪拌5 min,二氯甲烷萃取 (20 mL × 2),合併有機相後用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 1/1),得到白色固體 (40 mg, 產率28%)。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (100 mg, 0.27 mmol, Example 1, step 7), 6-(hydroxymethyl)-2-methylisoindol-1-one (50 mg, 0.28 mmol) was dissolved in dichloromethane (10 mL), N- Hydroxy-7-azabenzotriazole (113 mg, 0.81 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (157 mg) was added in an ice bath , 0.81 mmol) and N , N -diisopropylethylamine (141 mg, 1.09 mmol), react at room temperature for 22 h, add water (10 mL), stir for 5 min, extract with dichloromethane (20 mL × 2), combine The organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 1/1) to obtain a white solid (40 mg , yield 28%).
1 H NMR (400 MHz, CDCl3 ) :δ (ppm) 7.82 (s, 1H), 7.51 – 7.58 (m, 1H), 7.39 – 7.48 (m, 1H), 7.10 (d,J = 7.9 Hz, 1H), 6.75 – 6.84 (m, 2H), 6.59 (t,J = 75.6 Hz, 1H), 5.23 – 5.34 (m, 2H), 4.47 – 4.60 (m, 1H), 4.30 – 4.42 (m, 2H), 3.90 – 4.00 (m, 1H), 3.79 – 3.89 (m, 2H), 3.61 (t,J = 10.1 Hz, 1H), 3.34 – 3.51 (m, 1H), 3.15 – 3.29 (m, 3H), 2.59 – 2.74 (m, 1H), 2.12 (s, 2.6H), 1.95 – 2.06 (m, 1H), 1.92 (s, 0.4H), 1.22 – 1.26 (m, 1H), 0.59 – 0.71 (m, 2H), 0.29 – 0.41 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.82 (s, 1H), 7.51 – 7.58 (m, 1H), 7.39 – 7.48 (m, 1H), 7.10 (d, J = 7.9 Hz, 1H ), 6.75 – 6.84 (m, 2H), 6.59 (t, J = 75.6 Hz, 1H), 5.23 – 5.34 (m, 2H), 4.47 – 4.60 (m, 1H), 4.30 – 4.42 (m, 2H), 3.90 – 4.00 (m, 1H), 3.79 – 3.89 (m, 2H), 3.61 (t, J = 10.1 Hz, 1H), 3.34 – 3.51 (m, 1H), 3.15 – 3.29 (m, 3H), 2.59 – 2.74 (m, 1H), 2.12 (s, 2.6H), 1.95 – 2.06 (m, 1H), 1.92 (s, 0.4H), 1.22 – 1.26 (m, 1H), 0.59 – 0.71 (m, 2H), 0.29 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 529.20 [M+H]+ .MS (ESI, pos.ion) m/z: 529.20 [M+H] + .
實施例160:(1,2-二甲基-3-氧代異吲哚啉-5-基)甲基 (2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 160: (1,2-Dimethyl-3-oxoisoindolin-5-yl)methyl( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy) yl)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (100 mg, 0.27 mmol,實施例1步驟7),6-(羥甲基)-2,3-二甲基異吲哚-1-酮 (50 mg, 0.28 mmol) 溶於二氯甲烷 (10 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (113 mg, 0.81 mmol),在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (157 mg, 0.81 mmol) 和N ,N -二異丙基乙胺 (141 mg, 1.09 mmol),室溫反應20 h,加水 (10 mL) 攪拌5 min,二氯甲烷萃取 (20 mL × 2),合併有機相後用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 1/1),得到白色固體 (40 mg, 27%, HPLC純度84.31%)。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (100 mg, 0.27 mmol, Example 1, step 7), 6-(hydroxymethyl)-2,3-dimethylisoindol-1-one (50 mg, 0.28 mmol) was dissolved in dichloromethane (10 mL), added N -Hydroxy-7-azabenzotriazole (113 mg, 0.81 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride ( 157 mg, 0.81 mmol) and N , N -diisopropylethylamine (141 mg, 1.09 mmol), react at room temperature for 20 h, add water (10 mL), stir for 5 min, and extract with dichloromethane (20 mL × 2) , after combining the organic phases, the organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrated solution was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v)=1/1) to obtain a white solid ( 40 mg, 27%, HPLC purity 84.31%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.80 (s, 1H), 7.52 – 7.59 (m, 1H), 7.41 (d,J = 7.7 Hz, 1H), 7.10 (d,J = 7.9 Hz, 1H), 6.73 – 6.83 (m, 2H), 6.59 (t,J = 75.5 Hz, 1H), 5.22 – 5.30 (m, 2H), 4.49 – 4.58 (m, 1H), 4.39 – 4.48 (m, 1H), 3.89 – 3.97 (m, 1H), 3.80 – 3.89 (m, 2H), 3.57 – 3.65 (m, 1H), 3.35 – 3.46 (m, 1H), 3.11 (s, 2.6H), 3.02 (s, 0.4H), 2.62 – 2.72 (m, 1H), 2.12 (s, 2.6H), 1.97 – 2.08 (m, 1H), 1.92 (s, 0.4H), 1.43 – 1.50 (m, 3H), 1.22 – 1.25 (m, 1H), 0.60 – 0.68 (m, 2H), 0.31 – 0.38 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.80 (s, 1H), 7.52 – 7.59 (m, 1H), 7.41 (d, J = 7.7 Hz, 1H), 7.10 (d, J = 7.9 Hz, 1H), 6.73 – 6.83 (m, 2H), 6.59 (t, J = 75.5 Hz, 1H), 5.22 – 5.30 (m, 2H), 4.49 – 4.58 (m, 1H), 4.39 – 4.48 (m, 1H), 3.89 – 3.97 (m, 1H), 3.80 – 3.89 (m, 2H), 3.57 – 3.65 (m, 1H), 3.35 – 3.46 (m, 1H), 3.11 (s, 2.6H), 3.02 (s , 0.4H), 2.62 – 2.72 (m, 1H), 2.12 (s, 2.6H), 1.97 – 2.08 (m, 1H), 1.92 (s, 0.4H), 1.43 – 1.50 (m, 3H), 1.22 – 1.25 (m, 1H), 0.60 – 0.68 (m, 2H), 0.31 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 543.20 [M+H]+ .MS (ESI, pos.ion) m/z: 543.20 [M+H] + .
實施例161:(2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((2-甲基-3-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺 Example 161: ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((2-methyl) -3-oxoisoindolin-5-yl)methyl)pyrrolidine-2-carboxamide
步驟1:化合物(2R )-叔丁基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2-((((2-甲基-3-氧代異吲哚啉-5-基)甲基)氨基甲醯基)吡咯烷-1-羧酸酯的合成Step 1: Compound ( 2R )-tert-butyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-((((2-methyl- Synthesis of 3-oxoisoindolin-5-yl)methyl)carbamoyl)pyrrolidine-1-carboxylate
將化合物(2R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (150 mg, 0.35 mmol),6-(氨基甲基)-2-甲基異吲哚-1-酮(64 mg, 0.36 mmol,中間體13) 溶於N ,N ’-二甲基甲醯胺 (6 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (146 mg, 1.05 mmol),在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (203 mg, 1.05 mmol) 和N ,N -二異丙基乙胺 (182 mg, 1.41 mmol),室溫反應6 h,加水 (10 mL) 攪拌5 min,二氯甲烷萃取 (20 mL × 2),合併有機相後用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1),得到白色固體 (157 mg, 產率76%)。The compound ( 2R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (150 mg, 0.35 mmol), 6-(aminomethyl)-2-methylisoindol-1-one (64 mg, 0.36 mmol, intermediate 13) in N , N' -dimethylformamide ( 6 mL), add N -hydroxy-7-azabenzotriazole (146 mg, 1.05 mmol), add 1-ethyl-3-(3-dimethylaminopropyl) carbodiazepine in an ice bath Imine hydrochloride (203 mg, 1.05 mmol) and N , N -diisopropylethylamine (182 mg, 1.41 mmol), react at room temperature for 6 h, add water (10 mL), stir for 5 min, and extract with dichloromethane (20 mL × 2), combine the organic phases, dry the organic phases with anhydrous sodium sulfate, concentrate under reduced pressure, and separate the concentrate by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1 ) to give a white solid (157 mg, 76% yield).
1 H NMR (600 MHz, CD3 OD) : δ (ppm) 7.67 – 7.73 (m, 1H), 7.55 – 7.63 (m, 1H), 7.47 – 7.54 (m, 1H), 7.05 – 7.10 (m, 1H), 7.03 (s, 1H), 6.85 – 6.90 (m, 1H), 6.71 (t,J = 75.8 Hz, 1H), 4.55 – 4.62 (m, 1H), 4.47 (s, 2H), 4.38 – 4.45 (m, 1H), 4.26 – 4.35 (m, 1H), 3.94 – 3.99 (m, 1H), 3.90 (d,J = 6.6 Hz, 2H), 3.34 – 3.47 (m, 2H), 3.18 (s, 3H), 2.59 – 2.69 (m, 1H), 2.03 – 2.06 (m, 1H), 1.47 (s, 3H), 1.34 (s, 6H), 1.22 – 1.28 (m, 1H), 0.58 – 0.65 (m, 2H), 0.33 – 0.40 (m, 2H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 7.67 – 7.73 (m, 1H), 7.55 – 7.63 (m, 1H), 7.47 – 7.54 (m, 1H), 7.05 – 7.10 (m, 1H) ), 7.03 (s, 1H), 6.85 – 6.90 (m, 1H), 6.71 (t, J = 75.8 Hz, 1H), 4.55 – 4.62 (m, 1H), 4.47 (s, 2H), 4.38 – 4.45 ( m, 1H), 4.26 – 4.35 (m, 1H), 3.94 – 3.99 (m, 1H), 3.90 (d, J = 6.6 Hz, 2H), 3.34 – 3.47 (m, 2H), 3.18 (s, 3H) , 2.59 – 2.69 (m, 1H), 2.03 – 2.06 (m, 1H), 1.47 (s, 3H), 1.34 (s, 6H), 1.22 – 1.28 (m, 1H), 0.58 – 0.65 (m, 2H) , 0.33 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 486.25 [M-Boc +2H]+ .MS (ESI, pos.ion) m/z: 486.25 [M- Boc +2H] + .
步驟2:化合物(2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(((2-甲基-3-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺鹽酸鹽的合成Step 2: Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -(((2-methyl-3-oxo Synthesis of isoindolin-5-yl)methyl)pyrrolidine-2-carboxamide hydrochloride
將化合物(2R )-叔丁基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2-((((2-甲基-3-氧代異吲哚啉-5-基)甲基)氨基甲醯基)吡咯烷-1-羧酸酯 (11204-1) (150 mg, 0.26 mmol) 溶解於二氯甲烷 (10 mL) 溶液中,加入4 mol/L HCl的二氧六環溶液 (5 mL),室溫攪拌50 min,減壓濃縮除去溶劑,得到白色固體 (134 mg, 100%)。The compound ( 2R )-tert-butyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-(((((2-methyl-3- Oxoisoindolin-5-yl)methyl)carbamoyl)pyrrolidine-1-carboxylate (11204-1) (150 mg, 0.26 mmol) dissolved in dichloromethane (10 mL) solution , 4 mol/L HCl in dioxane solution (5 mL) was added, stirred at room temperature for 50 min, and concentrated under reduced pressure to remove the solvent to obtain a white solid (134 mg, 100%).
1 H NMR (600 MHz, CD3 OD) : δ (ppm) 7.69 (s, 1H), 7.51 – 7.58 (m, 2H), 7.10 (d,J = 8.2 Hz, 1H), 7.05 (d,J = 1.5 Hz, 1H), 6.87 – 6.92 (m, 1H), 6.73 (t,J = 75.5 Hz, 1H), 4.57 (d,J = 3.6 Hz, 2H), 4.48 (s, 2H), 4.43 – 4.47 (m, 1H), 3.90 (d,J = 6.8 Hz, 2H), 3.75 – 3.80 (m, 1H), 3.56 – 3.60 (m, 1H), 3.34 – 3.40 (m, 1H), 3.19 (s, 3H), 2.83 – 2.90 (m, 1H), 2.09 – 2.17 (m, 1H), 1.24 – 1.29 (m, 1H), 0.60 – 0.66 (m, 2H), 0.34 – 0.39 (m, 2H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 7.69 (s, 1H), 7.51 – 7.58 (m, 2H), 7.10 (d, J = 8.2 Hz, 1H), 7.05 (d, J = 1.5 Hz, 1H), 6.87 – 6.92 (m, 1H), 6.73 (t, J = 75.5 Hz, 1H), 4.57 (d, J = 3.6 Hz, 2H), 4.48 (s, 2H), 4.43 – 4.47 ( m, 1H), 3.90 (d, J = 6.8 Hz, 2H), 3.75 – 3.80 (m, 1H), 3.56 – 3.60 (m, 1H), 3.34 – 3.40 (m, 1H), 3.19 (s, 3H) , 2.83 – 2.90 (m, 1H), 2.09 – 2.17 (m, 1H), 1.24 – 1.29 (m, 1H), 0.60 – 0.66 (m, 2H), 0.34 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 486.75 [M-HCl+H]+ .MS (ESI, pos.ion) m/z: 486.75 [M-HCl+H] + .
步驟3:化合物(2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(((2-甲基-3-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺的合成Step 3: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -(((2-methyl Synthesis of yl-3-oxoisoindolin-5-yl)methyl)pyrrolidine-2-carboxamide
將化合物(2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -(((2-甲基-3-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺鹽酸鹽(134 mg, 0.26 mmol) 溶解在二氯甲烷 (10 mL) 中,加入N ,N -二異丙基乙胺 (167 mg, 1.28 mmol),冷卻至0℃,加入乙醯氯 (59 mg, 0.76 mmol),室溫攪拌1 h後停止反應,加水(10 mL)淬滅反應,二氯甲烷萃取 (40 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1) 得到白色固體 (100 mg, 產率74%)。Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -(((2-methyl-3-oxoisoindium Doolin-5-yl)methyl)pyrrolidine-2-carboxamide hydrochloride (134 mg, 0.26 mmol) was dissolved in dichloromethane (10 mL) and N , N -diisopropylethylamine was added (167 mg, 1.28 mmol), cooled to 0 °C, added with acetyl chloride (59 mg, 0.76 mmol), stirred at room temperature for 1 h, then stopped the reaction, added water (10 mL) to quench the reaction, and extracted with dichloromethane (40 mL × 2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain a white solid (100 mg) , yield 74%).
1 H NMR (600 MHz, CD3 OD) : δ (ppm) 7.66 – 7.70 (m, 1H), 7.61 (d,J = 7.6 Hz, 1H), 7.50 – 7.55 (m, 1H), 7.03 – 7.12 (m, 2H), 6.88 – 6.94 (m, 1H), 6.72 (t,J = 75.7 Hz, 1H), 4.54 – 4.60 (m, 1H), 4.51 (s, 2H), 4.47 (s, 2H), 4.04 – 4.10 (m, 1H), 3.86 – 3.95 (m, 2H), 3.58 – 3.67 (m, 1H), 3.43 – 3.53 (m, 1H), 3.19 (s, 3H), 2.60 – 2.71 (m, 1H), 2.13 (s, 2.4H), 2.00 – 2.09 (m, 1H), 1.91 (s, 0.6H), 1.23 – 1.28 (m, 1H), 0.58 – 0.66 (m, 2H), 0.32 – 0.40 (m, 2H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 7.66 – 7.70 (m, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.50 – 7.55 (m, 1H), 7.03 – 7.12 ( m, 2H), 6.88 – 6.94 (m, 1H), 6.72 (t, J = 75.7 Hz, 1H), 4.54 – 4.60 (m, 1H), 4.51 (s, 2H), 4.47 (s, 2H), 4.04 – 4.10 (m, 1H), 3.86 – 3.95 (m, 2H), 3.58 – 3.67 (m, 1H), 3.43 – 3.53 (m, 1H), 3.19 (s, 3H), 2.60 – 2.71 (m, 1H) , 2.13 (s, 2.4H), 2.00 – 2.09 (m, 1H), 1.91 (s, 0.6H), 1.23 – 1.28 (m, 1H), 0.58 – 0.66 (m, 2H), 0.32 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 528.20 [M +H]+ .MS (ESI, pos.ion) m/z: 528.20 [M +H] + .
實施例162:(2R )-(2-甲基-1-氧代異吲哚啉-5-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 162: ( 2R )-(2-Methyl-1-oxoisoindolin-5-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy)- 4-(Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:化合物(2R )-1-叔丁基 2-(((2-甲基-1-氧代異吲哚啉-5-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯的合成Step 1: Compound ( 2R )-1-tert-butyl 2-(((2-methyl-1-oxoisoindolin-5-yl)methyl)4-(3-(cyclopropylmethyl) Synthesis of oxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物(2R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (150 mg, 0.35 mmol,實施例1步驟4),5-(羥甲基)-2-甲基異吲哚-1-酮 (64 mg, 0.36 mmol) 溶於N ,N’ -二甲基甲醯胺 (6 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (146 mg, 1.05 mmol),在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (203 mg, 1.05 mmol) 和N ,N -二異丙基乙胺 (182 mg, 1.41 mmol),室溫反應6 h,加水 (10 mL) 攪拌5 min,二氯甲烷萃取 (20 mL × 2),合併有機相後用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 石油醚/乙酸乙酯 (v/v) = 1/1),得到無色液體 (130 mg, 產率63%)。The compound ( 2R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (150 mg, 0.35 mmol, Example 1, step 4), 5-(hydroxymethyl)-2-methylisoindol-1-one (64 mg, 0.36 mmol) in N , N' -dimethylformamide amine (6 mL), N -hydroxy-7-azabenzotriazole (146 mg, 1.05 mmol), and 1-ethyl-3-(3-dimethylaminopropyl) in an ice bath Carbodiimide hydrochloride (203 mg, 1.05 mmol) and N , N -diisopropylethylamine (182 mg, 1.41 mmol), react at room temperature for 6 h, add water (10 mL) and stir for 5 min, dichloride Extracted with methane (20 mL × 2), combined the organic phases, dried the organic phases with anhydrous sodium sulfate, concentrated under reduced pressure, and separated the concentrate by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v) = 1/1) to give a colorless liquid (130 mg, 63% yield).
1 H NMR (600 MHz, CDCl3 ) : δ (ppm) 7.78 – 7.85 (m, 1H), 7.41 – 7.47 (m, 2H), 7.08 (d,J = 8.1 Hz, 1H), 6.73 – 6.82 (m, 2H), 6.59 (t,J = 75.6 Hz, 1H), 5.32 – 5.38 (m, 1H), 5.19 – 5.24 (m, 1H), 4.42 – 4.49 (m, 1H), 4.34 – 4.38 (m, 2H), 3.90 – 3.97 (m, 1H), 3.83 (t,J = 6.8 Hz, 2H), 3.39 – 3.47 (m, 1H), 3.29 – 3.38 (m, 1H), 3.16 – 3.24 (m, 3H), 2.63 – 2.72 (m, 1H), 1.97 – 2.05 (m, 1H), 1.46 (s, 4H), 1.35 (s, 5H), 1.23 – 1.25 (m, 1H), 0.61 – 0.67 (m, 2H), 0.31 – 0.38 (m, 2H). 1 H NMR (600 MHz, CDCl 3 ): δ (ppm) 7.78 – 7.85 (m, 1H), 7.41 – 7.47 (m, 2H), 7.08 (d, J = 8.1 Hz, 1H), 6.73 – 6.82 (m , 2H), 6.59 (t, J = 75.6 Hz, 1H), 5.32 – 5.38 (m, 1H), 5.19 – 5.24 (m, 1H), 4.42 – 4.49 (m, 1H), 4.34 – 4.38 (m, 2H) ), 3.90 – 3.97 (m, 1H), 3.83 (t, J = 6.8 Hz, 2H), 3.39 – 3.47 (m, 1H), 3.29 – 3.38 (m, 1H), 3.16 – 3.24 (m, 3H), 2.63 – 2.72 (m, 1H), 1.97 – 2.05 (m, 1H), 1.46 (s, 4H), 1.35 (s, 5H), 1.23 – 1.25 (m, 1H), 0.61 – 0.67 (m, 2H), 0.31 – 0.38 (m, 2H).
MS (ESI, pos.ion) m/z: 531.40 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 531.40 [M- t- Bu+2H] + .
步驟2:化合物(2R )-(2-甲基-1-氧代異吲哚啉-5-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽的合成Step 2: Compound ( 2R )-(2-methyl-1-oxoisoindolin-5-yl)methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethyl) Synthesis of oxy)phenyl)pyrrolidine-2-carboxylate hydrochloride
將化合物(2R )-1-叔丁基2-(((2-甲基-1-氧代異吲哚啉-5-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯 (120 mg, 0.20 mmol) 溶解於二氯甲烷 (10 mL) 溶液中,加入4 mol/L HCl的二氧六環溶液 (5 mL),室溫攪拌50 min,減壓濃縮除去溶劑,得到白色固體 (107 mg, 100%)。Compound ( 2R )-1-tert-butyl 2-(((2-methyl-1-oxoisoindolin-5-yl)methyl)4-(3-(cyclopropylmethoxy) )-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (120 mg, 0.20 mmol) was dissolved in dichloromethane (10 mL) solution, and 4 mol/L HCl was added The solution in dioxane (5 mL) was stirred at room temperature for 50 min, and concentrated under reduced pressure to remove the solvent to obtain a white solid (107 mg, 100%).
1 H NMR (600 MHz, CD3 OD) : δ (ppm) 7.76 (d,J = 7.7 Hz, 1H), 7.62 (s, 1H), 7.56 (d,J = 7.8 Hz, 1H), 7.10 (d,J = 8.1 Hz, 1H), 7.02 (s, 1H), 6.84 – 6.88 (m, 1H), 6.73 (t,J = 75.6 Hz, 1H), 5.49 (s, 0.5H), 5.43 (s, 1.5H), 4.65 – 4.70 (m, 1H), 4.50 (s, 2H), 3.90 (d,J = 6.8 Hz, 2H), 3.76 – 3.82 (m, 1H), 3.59 (d,J = 4.6 Hz, 1H), 3.37 (t,J = 11.1 Hz, 1H), 3.20 (s, 3H), 2.84 – 2.90 (m, 1H), 2.18 – 2.26 (m, 1H), 1.27 – 1.30 (m, 1H), 0.59 – 0.66 (m, 2H), 0.31 – 0.40 (m, 2H). 1 H NMR (600 MHz, CD 3 OD) : δ (ppm) 7.76 (d, J = 7.7 Hz, 1H), 7.62 (s, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.10 (d , J = 8.1 Hz, 1H), 7.02 (s, 1H), 6.84 – 6.88 (m, 1H), 6.73 (t, J = 75.6 Hz, 1H), 5.49 (s, 0.5H), 5.43 (s, 1.5 H), 4.65 – 4.70 (m, 1H), 4.50 (s, 2H), 3.90 (d, J = 6.8 Hz, 2H), 3.76 – 3.82 (m, 1H), 3.59 (d, J = 4.6 Hz, 1H) ), 3.37 (t, J = 11.1 Hz, 1H), 3.20 (s, 3H), 2.84 – 2.90 (m, 1H), 2.18 – 2.26 (m, 1H), 1.27 – 1.30 (m, 1H), 0.59 – 0.66 (m, 2H), 0.31 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 487.20 [M-HCl+H]+ .MS (ESI, pos.ion) m/z: 487.20 [M-HCl+H] + .
步驟3:化合物(2R )-(2-甲基-1-氧代異吲哚啉-5-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 3: Compound ( 2R )-(2-methyl-1-oxoisoindolin-5-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy)- Synthesis of 4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物(2R )-(2-甲基-1-氧代異吲哚啉-5-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽(107 mg, 0.20 mmol) 溶解在二氯甲烷 (10 mL) 中,加入N ,N -二異丙基乙胺 (133 mg, 1.03 mmol),冷卻至0℃,加入乙醯氯 (48 mg, 0.60 mmol),室溫攪拌1 h後停止反應,加水(10 mL),二氯甲烷萃取 (40 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1) 得到白色固體 (70 mg, 產率65%)。Compound ( 2R )-(2-methyl-1-oxoisoindolin-5-yl)methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy) )phenyl)pyrrolidine-2-carboxylate hydrochloride (107 mg, 0.20 mmol) was dissolved in dichloromethane (10 mL), N , N -diisopropylethylamine (133 mg, 1.03 mmol) was added ), cooled to 0 °C, acetyl chloride (48 mg, 0.60 mmol) was added, the reaction was stopped after stirring at room temperature for 1 h, water (10 mL) was added, extracted with dichloromethane (40 mL × 2), the organic phase was washed with anhydrous sulfuric acid It was dried over sodium, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain a white solid (70 mg, yield 65%).
1 H NMR (600 MHz, CD3 OD) : δ (ppm) 7.74 (d,J = 7.8 Hz, 1H), 7.58 (s, 1H), 7.50 (d,J = 7.9 Hz, 1H), 7.08 (d,J = 8.2 Hz, 1H), 7.01 (s, 1H), 6.87 (d,J = 8.1 Hz, 1H), 6.72 (t,J = 75.6 Hz, 1H), 5.22 – 5.37 (m, 2H), 4.52 – 4.57 (m, 1H), 4.49 (s, 2H), 4.07 – 4.13 (m, 1H), 3.89 (d,J = 6.9 Hz, 2H), 3.61 (t,J = 10.4 Hz, 1H), 3.49 – 3.58 (m, 1H), 3.19 (s, 3H), 2.69 – 2.77 (m, 1H), 2.13 (s, 2.5H), 1.97 – 2.06 (m, 1H), 1.94 (s, 0.5H), 1.22 – 1.28 (m, 1H), 0.58 – 0.65 (m, 2H), 0.32 – 0.39 (m, 2H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 7.74 (d, J = 7.8 Hz, 1H), 7.58 (s, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.08 (d , J = 8.2 Hz, 1H), 7.01 (s, 1H), 6.87 (d, J = 8.1 Hz, 1H), 6.72 (t, J = 75.6 Hz, 1H), 5.22 – 5.37 (m, 2H), 4.52 – 4.57 (m, 1H), 4.49 (s, 2H), 4.07 – 4.13 (m, 1H), 3.89 (d, J = 6.9 Hz, 2H), 3.61 (t, J = 10.4 Hz, 1H), 3.49 – 3.58 (m, 1H), 3.19 (s, 3H), 2.69 – 2.77 (m, 1H), 2.13 (s, 2.5H), 1.97 – 2.06 (m, 1H), 1.94 (s, 0.5H), 1.22 – 1.28 (m, 1H), 0.58 – 0.65 (m, 2H), 0.32 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 529.20 [M +H]+ .MS (ESI, pos.ion) m/z: 529.20 [M +H] + .
實施例163:(2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((2-甲基-1-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺 Example 163: ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((2-methyl) -1-oxoisoindolin-5-yl)methyl)pyrrolidine-2-carboxamide
步驟1:化合物(2R )-叔丁基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2-(((2-甲基-1-氧代異吲哚啉-5-基)甲基)氨基甲醯基)吡咯烷-1-甲酸酯的合成Step 1: Compound ( 2R )-tert-butyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-(((2-methyl-1 -Synthesis of oxoisoindolin-5-yl)methyl)carbamoyl)pyrrolidine-1-carboxylate
將化合物(2R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (150 mg, 0.35 mmol,實施例1步驟4),5-(氨基甲基)-2-甲基異吲哚-1-酮 (64 mg, 0.36 mmol,中間體14) 溶於N ,N ’-二甲基甲醯胺 (6 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (146 mg, 1.05 mmol),在冰浴中加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (203 mg, 1.05 mmol) 和N ,N -二異丙基乙胺 (182 mg, 1.41 mmol),室溫反應6 h,加水 (10 mL) 攪拌5 min,二氯甲烷萃取 (20 mL × 2),合併有機相後用無水硫酸鈉乾燥有機相,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 石油醚/乙酸乙酯 (v/v) = 1/4),得到白色固體 (100 mg, 產率49%)。The compound ( 2R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (150 mg, 0.35 mmol, Example 1, step 4), 5-(aminomethyl)-2-methylisoindol-1-one (64 mg, 0.36 mmol, intermediate 14) in N , N' -di In methylformamide (6 mL), N -hydroxy-7-azabenzotriazole (146 mg, 1.05 mmol) was added, and 1-ethyl-3-(3-dimethylene) was added in an ice bath Aminopropyl)carbodiimide hydrochloride (203 mg, 1.05 mmol) and N , N -diisopropylethylamine (182 mg, 1.41 mmol), react at room temperature for 6 h, add water (10 mL) and stir for 5 min, extracted with dichloromethane (20 mL × 2), combined the organic phases, dried the organic phases with anhydrous sodium sulfate, concentrated under reduced pressure, and separated the concentrate by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v /v) = 1/4) to give a white solid (100 mg, 49% yield).
1 H NMR (600 MHz, CD3 OD) : δ (ppm) 7.66 – 7.73 (m, 1H), 7.50 – 7.59 (m, 1H), 7.47 (d,J = 7.8 Hz, 1H), 7.07 (d,J = 8.2 Hz, 1H), 7.03 (s, 1H), 6.86 – 6.92 (m, 1H), 6.71 (t,J = 75.7 Hz, 1H), 4.54 – 4.61 (m, 2H), 4.47 (s, 2H), 4.28 – 4.38 (m, 1H), 3.94 – 4.01 (m, 1H), 3.90 (d,J = 6.8 Hz, 2H), 3.35 – 3.49 (m, 2H), 3.18 (s, 3H), 2.59 – 2.69 (m, 1H), 1.99 – 2.07 (m, 1H), 1.49 (s, 3H), 1.34 (s, 6H), 1.23 – 1.28 (m, 1H), 0.57 – 0.67 (m, 2H), 0.31 – 0.41 (m, 2H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 7.66 – 7.73 (m, 1H), 7.50 – 7.59 (m, 1H), 7.47 (d, J = 7.8 Hz, 1H), 7.07 (d, J = 8.2 Hz, 1H), 7.03 (s, 1H), 6.86 – 6.92 (m, 1H), 6.71 (t, J = 75.7 Hz, 1H), 4.54 – 4.61 (m, 2H), 4.47 (s, 2H) ), 4.28 – 4.38 (m, 1H), 3.94 – 4.01 (m, 1H), 3.90 (d, J = 6.8 Hz, 2H), 3.35 – 3.49 (m, 2H), 3.18 (s, 3H), 2.59 – 2.69 (m, 1H), 1.99 – 2.07 (m, 1H), 1.49 (s, 3H), 1.34 (s, 6H), 1.23 – 1.28 (m, 1H), 0.57 – 0.67 (m, 2H), 0.31 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 586.25 [M+H]+ .MS (ESI, pos.ion) m/z: 586.25 [M+H] + .
步驟2:化合物(2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((2-甲基-1-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺鹽酸鹽的合成Step 2: Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((2-methyl-1-oxoiso Synthesis of indolin-5-yl)methyl)pyrrolidine-2-carboxamide hydrochloride
將化合物(2R )-叔丁基4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-2-(((2-甲基-1-氧代異吲哚啉-5-基)甲基)氨基甲醯基)吡咯烷-1-甲酸酯(100 mg, 0.17 mmol) 溶解於二氯甲烷 (10 mL) 溶液中,加入4 mol/L HCl的二氧六環溶液 (5 mL),室溫攪拌50 min,減壓濃縮除去溶劑,得到白色固體 (90 mg, 100%)。The compound ( 2R )-tert-butyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-(((2-methyl-1-oxo Isoindolin-5-yl)methyl)carbamoyl)pyrrolidine-1-carboxylate (100 mg, 0.17 mmol) was dissolved in dichloromethane (10 mL) solution, added 4 mol/L A solution of HCl in dioxane (5 mL) was stirred at room temperature for 50 min, and concentrated under reduced pressure to remove the solvent to obtain a white solid (90 mg, 100%).
1 H NMR (600 MHz, CD3 OD) : δ (ppm) 7.74 (d,J = 7.8 Hz, 1H), 7.53 (s, 1H), 7.47 (d,J = 7.8 Hz, 1H), 7.13 (d,J = 8.2 Hz, 1H), 7.06 (d,J = 1.8 Hz, 1H), 6.89 – 6.93 (m, 1H), 6.76 (t,J = 75.5 Hz, 1H), 4.57 – 4.62 (m, 2H), 4.50 (s, 2H), 4.44 – 4.48 (m, 1H), 3.90 – 3.94 (m, 2H), 3.75 – 3.78 (m, 1H), 3.58 – 3.62 (m, 1H), 3.36 – 3.43 (m, 1H), 3.21 (s, 3H), 2.85 – 2.91 (m, 1H), 2.09 – 2.16 (m, 1H), 1.26 – 1.29 (m, 1H), 0.61 – 0.69 (m, 2H), 0.34 – 0.41 (m, 2H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 7.74 (d, J = 7.8 Hz, 1H), 7.53 (s, 1H), 7.47 (d, J = 7.8 Hz, 1H), 7.13 (d , J = 8.2 Hz, 1H), 7.06 (d, J = 1.8 Hz, 1H), 6.89 – 6.93 (m, 1H), 6.76 (t, J = 75.5 Hz, 1H), 4.57 – 4.62 (m, 2H) , 4.50 (s, 2H), 4.44 – 4.48 (m, 1H), 3.90 – 3.94 (m, 2H), 3.75 – 3.78 (m, 1H), 3.58 – 3.62 (m, 1H), 3.36 – 3.43 (m, 1H), 3.21 (s, 3H), 2.85 – 2.91 (m, 1H), 2.09 – 2.16 (m, 1H), 1.26 – 1.29 (m, 1H), 0.61 – 0.69 (m, 2H), 0.34 – 0.41 ( m, 2H).
MS (ESI, pos.ion) m/z: 486.35 [M-HCl+H]+ .MS (ESI, pos.ion) m/z: 486.35 [M-HCl+H] + .
步驟3:化合物(2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((2-甲基-1-氧代異吲哚啉-5-基)甲基)吡咯烷-2-甲醯胺的合成Step 3: Compound ( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((2-methyl) Synthesis of -1-oxoisoindolin-5-yl)methyl)pyrrolidine-2-carboxamide
將化合物(2R )-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-N -((2-甲基-1-氧代異吲哚-5-基)甲基)吡咯烷-2-甲醯胺鹽酸鹽(90 mg, 0.17 mmol) 溶解在二氯甲烷 (10 mL) 中,加入N ,N -二異丙基乙胺 (113 mg, 0.87 mmol),冷卻至0℃,加入乙醯氯 (44 mg, 0.56 mmol),室溫攪拌1 h後停止反應,加水(10 mL),二氯甲烷萃取 (40 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,濃縮液進行矽膠柱層析分離 (洗脫劑: 二氯甲烷/甲醇 (v/v) = 20/1) 得到白色固體 (75 mg, 產率82%)。Compound ( 2R )-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl) -N -((2-methyl-1-oxoisoindole) -5-yl)methyl)pyrrolidine-2-carboxamide hydrochloride (90 mg, 0.17 mmol) was dissolved in dichloromethane (10 mL) and N , N -diisopropylethylamine (113 mg, 0.87 mmol), cooled to 0 °C, added acetyl chloride (44 mg, 0.56 mmol), stirred at room temperature for 1 h, then stopped the reaction, added water (10 mL), extracted with dichloromethane (40 mL × 2), organic The phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by silica gel column chromatography (eluent: dichloromethane/methanol (v/v) = 20/1) to obtain a white solid (75 mg, yield 82%) ).
1 H NMR (600 MHz, CD3 OD) : δ (ppm) 7.68 – 7.73 (m, 1H), 7.55 (s, 1H), 7.44 – 7.49 (m, 1H), 7.04 – 7.12 (m, 2H), 6.89 – 6.93 (m, 1H), 6.73 (t,J = 75.7 Hz, 1H), 4.57 – 4.65 (m, 1H), 4.48 (s, 2H), 4.47 (s, 1H), 4.43 – 4.46 (m, 1H), 4.06 – 4.12 (m, 1H), 3.85 – 3.94 (m, 2H), 3.63 (t,J = 10.6 Hz, 1H), 3.44 – 3.53 (m, 1H), 3.18 (s, 3H), 2.62 – 2.70 (m, 1H), 2.14 (s, 3H), 2.02 – 2.09 (m, 1H), 1.25 – 1.29 (m, 1H), 0.59 – 0.66 (m, 2H), 0.33 – 0.39 (m, 2H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 7.68 – 7.73 (m, 1H), 7.55 (s, 1H), 7.44 – 7.49 (m, 1H), 7.04 – 7.12 (m, 2H), 6.89 – 6.93 (m, 1H), 6.73 (t, J = 75.7 Hz, 1H), 4.57 – 4.65 (m, 1H), 4.48 (s, 2H), 4.47 (s, 1H), 4.43 – 4.46 (m, 1H), 4.06 – 4.12 (m, 1H), 3.85 – 3.94 (m, 2H), 3.63 (t, J = 10.6 Hz, 1H), 3.44 – 3.53 (m, 1H), 3.18 (s, 3H), 2.62 – 2.70 (m, 1H), 2.14 (s, 3H), 2.02 – 2.09 (m, 1H), 1.25 – 1.29 (m, 1H), 0.59 – 0.66 (m, 2H), 0.33 – 0.39 (m, 2H) .
MS (ESI, pos.ion) m/z: 528.40 [M +H]+ .MS (ESI, pos.ion) m/z: 528.40 [M +H] + .
實施例164:(2R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-甲醯基吡咯烷-2-甲酸酯 Example 164: ( 2R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4-( Difluoromethoxy)phenyl)-1-carboxypyrrolidine-2-carboxylate
步驟1:化合物 (2R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸的合成Step 1: Compound ( 2R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid Synthesis
將化合物(2R )-1-叔丁基 2-甲基 4-(3-(環丙甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二羧酸酯(2.4 g, 5.4 mmol,實施例1步驟4) 溶於四氫呋喃 (20 mL) 和水 (10 mL) 的混合溶劑中,再加入一水合氫氧化鋰 (688 mg,16.4 mmol),室溫反應5 h後停止,加鹽酸調節溶液pH=4,減壓除去四氫呋喃,用乙酸乙酯萃取 (30 mL×3),有機相用無水硫酸鈉乾燥,除去溶劑得到無色黏稠固體 (2.3 g, 產率99%)。The compound ( 2R )-1-tert-butyl 2-methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate The acid ester (2.4 g, 5.4 mmol, step 4 of Example 1) was dissolved in a mixed solvent of tetrahydrofuran (20 mL) and water (10 mL), and then lithium hydroxide monohydrate (688 mg, 16.4 mmol) was added, and the mixture was dissolved at room temperature. The reaction was stopped after 5 h, hydrochloric acid was added to adjust the pH of the solution to 4, tetrahydrofuran was removed under reduced pressure, extracted with ethyl acetate (30 mL×3), the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed to obtain a colorless viscous solid (2.3 g, yielded rate 99%).
1 H NMR (400 MHz, CD3 OD): δ (ppm) 7.10 (d,J = 8.2 Hz, 1H), 7.03 (s, 1H), 6.89 (d,J = 8.1 Hz, 1H), 6.73 (t,J F-H = 75.5 Hz, 1H), 4.31 – 4.37 (m, 1H), 3.92 – 3.99 (m, 1H), 3.92 (d,J = 6.9 Hz, 2H), 3.35 – 3.47 (m, 2H), 2.69 – 2.76 (m, 1H), 1.97 – 2.09 (m, 1H), 1.46 – 1.49 (m, 9H), 1.24 – 1.34 (m, 1H), 0.62 – 0.66 (m, 2H), 0.37 – 0.41 (m, 2H). 1 H NMR (400 MHz, CD 3 OD): δ (ppm) 7.10 (d, J = 8.2 Hz, 1H), 7.03 (s, 1H), 6.89 (d, J = 8.1 Hz, 1H), 6.73 (t , J FH = 75.5 Hz, 1H), 4.31 – 4.37 (m, 1H), 3.92 – 3.99 (m, 1H), 3.92 (d, J = 6.9 Hz, 2H), 3.35 – 3.47 (m, 2H), 2.69 – 2.76 (m, 1H), 1.97 – 2.09 (m, 1H), 1.46 – 1.49 (m, 9H), 1.24 – 1.34 (m, 1H), 0.62 – 0.66 (m, 2H), 0.37 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 450.20 [M+Na]+ .MS (ESI, pos.ion) m/z: 450.20 [M+Na] + .
步驟2:化合物 (2R )-1-叔丁基 2-((6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯的合成Step 2: Compound ( 2R )-1-tert-butyl 2-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl)4-(3-(cyclopropyl) Synthesis of methoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物(2R )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸(1.10 g, 2.60 mmol) 和6-(羥甲基)-N -甲基-N -(對甲基苯基)吡啶醯胺 (690 mg, 0.70 mmol) 溶於二氯甲烷 (30 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (700 mg, 5.14 mmol)和1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (1.50 g, 7.80 mmol),冰浴下加入N ,N -二異丙基乙胺 (1.3 g, 10.0 mmol),室溫反應12 h,加水 (30 mL),用二氯甲烷萃取 (10 mL×2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑: PE/EtOAc ( v/v ) =2/1) 得到白色黏稠固體 (1.1 g, 62%)。The compound ( 2R )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (1.10 g, 2.60 mmol) and 6-(hydroxymethyl) -N -methyl- N- (p-methylphenyl)pyridinamide (690 mg, 0.70 mmol) were dissolved in dichloromethane (30 mL) and added N -Hydroxy-7-azabenzotriazole (700 mg, 5.14 mmol) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.50 g, 7.80 mmol) ), N , N -diisopropylethylamine (1.3 g, 10.0 mmol) was added under ice bath, the reaction was carried out at room temperature for 12 h, water (30 mL) was added, extracted with dichloromethane (10 mL×2), the organic phase was It was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: PE/EtOAc (v/v) = 2/1) to obtain a white viscous solid (1.1 g, 62%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.49 – 7.61 (m, 1H), 7.13 – 7.32 (m, 2H), 7.09 (d,J = 7.9 Hz, 1H), 6.86 – 7.03 (m, 4H), 6.76 – 6.81 (m, 2H), 6.59 (t,J F-H = 75.6 Hz, 1H), 4.99 – 5.18 (m, 2H), 4.37 – 4.48 (m, 1H), 4.05 – 4.09 (m, 0.5H), 3.92 – 3.96 (m, 0.5H), 3.82 – 3.86 (m, 2H), 3.46 (s, 3H), 3.28 – 3.44 (m, 2H), 2.63 – 2.75 (m, 1H), 2.25 (s, 3H), 1.99 – 2.10 (m, 1H), 1.37 – 1.45 (m, 9H), 1.22 – 1.33 (m, 1H), 0.61 – 0.67 (m, 2H), 0.32 – 0.37 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.49 – 7.61 (m, 1H), 7.13 – 7.32 (m, 2H), 7.09 (d, J = 7.9 Hz, 1H), 6.86 – 7.03 (m , 4H), 6.76 – 6.81 (m, 2H), 6.59 (t, J FH = 75.6 Hz, 1H), 4.99 – 5.18 (m, 2H), 4.37 – 4.48 (m, 1H), 4.05 – 4.09 (m, 0.5H), 3.92 – 3.96 (m, 0.5H), 3.82 – 3.86 (m, 2H), 3.46 (s, 3H), 3.28 – 3.44 (m, 2H), 2.63 – 2.75 (m, 1H), 2.25 ( s, 3H), 1.99 – 2.10 (m, 1H), 1.37 – 1.45 (m, 9H), 1.22 – 1.33 (m, 1H), 0.61 – 0.67 (m, 2H), 0.32 – 0.37 (m, 2H).
MS (ESI, pos.ion) m/z: 666.30 [M+H]+ .MS (ESI, pos.ion) m/z: 666.30 [M+H] + .
步驟3:化合物 (2R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽的合成Step 3: Compound ( 2R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4-( Synthesis of difluoromethoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride
將化合物(2R )-1-叔丁基 2-((6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1,2-二甲酸酯(1.1 g, 2.70 mmol) 溶解於二氯甲烷 (15 mL) 溶液中,加入4 mol/L HCl的乙酸乙酯溶液 (5 mL),室溫攪拌1.5 h,減壓除去溶劑,得到淺黃色固體 (1.0 g, 100%)。Compound ( 2R )-1-tert-butyl 2-((6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl)4-(3-(cyclopropylmethyl) oxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (1.1 g, 2.70 mmol) was dissolved in dichloromethane (15 mL) solution, and 4 mol/ L HCl in ethyl acetate (5 mL) was stirred at room temperature for 1.5 h, and the solvent was removed under reduced pressure to give a pale yellow solid (1.0 g, 100%).
1 H NMR (400 MHz, CD3 OD) δ: (ppm) 7.76 – 7.85 (m, 1H), 7.46 – 7.52 (m, 1H), 7.29 – 7.36 (m, 1H), 7.09 – 7.16 (m, 2H), 7.00 – 7.08 (m, 4H), 6.94 – 6.97 (m, 1H), 6.75 (t,J F-H = 75.6 Hz, 1H), 5.29 – 5.39 (m, 2H), 4.73 – 4.78 (m, 1H), 3.95 (d,J = 6.8 Hz, 2H), 3.82 – 3.87 (m, 1H), 3.71 – 3.78 (m, 1H), 3.36 – 3.47 (m, 1H), 3.45 (s, 3H), 2.87 – 2.95 (m, 1H), 2.30 – 2.43 (m, 1H), 2.24 (s, 3H), 1.26 – 1.38 (m, 1H), 0.61 – 0.68 (m, 2H), 0.35 – 0.42 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ: (ppm) 7.76 – 7.85 (m, 1H), 7.46 – 7.52 (m, 1H), 7.29 – 7.36 (m, 1H), 7.09 – 7.16 (m, 2H) ), 7.00 – 7.08 (m, 4H), 6.94 – 6.97 (m, 1H), 6.75 (t, J FH = 75.6 Hz, 1H), 5.29 – 5.39 (m, 2H), 4.73 – 4.78 (m, 1H) , 3.95 (d, J = 6.8 Hz, 2H), 3.82 – 3.87 (m, 1H), 3.71 – 3.78 (m, 1H), 3.36 – 3.47 (m, 1H), 3.45 (s, 3H), 2.87 – 2.95 (m, 1H), 2.30 – 2.43 (m, 1H), 2.24 (s, 3H), 1.26 – 1.38 (m, 1H), 0.61 – 0.68 (m, 2H), 0.35 – 0.42 (m, 2H).
MS (ESI, pos.ion) m/z: 566.20 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 566.20 [M+H-HCl] + .
步驟4:化合物 (2R )-(6-(甲基(對甲基苯基)氨基甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-1-甲醯基吡咯烷-2-甲酸酯的合成Step 4: Compound ( 2R )-(6-(methyl(p-methylphenyl)carbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4 Synthesis of -(difluoromethoxy)phenyl)-1-carboxypyrrolidine-2-carboxylate
將化合物(2R )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽(100 mg, 0.17 mmol) 和N -羥基-7-氮雜苯並三氮唑 (45 mg, 0.33 mmol) 溶於二氯甲烷 (8 mL) 中,加入甲酸 (45 mg, 0.98 mmol),冰浴下加入1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (127 mg, 0.66 mmol) 和N ,N -二異丙基乙胺 (306 mg, 2.37 mmol),室溫反應2 h,加水 (20 mL) 攪拌2min,用二氯甲烷萃取 (15 mL × 2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑: DCM/MeOH ( v/v ) =15/1) 得到白色黏稠固體 (73 mg, 產率74%)。Compound ( 2R )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl4-(3-(cyclopropylmethoxy)-4-(difluoro) Methoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride (100 mg, 0.17 mmol) and N -hydroxy-7-azabenzotriazole (45 mg, 0.33 mmol) in dichloro In methane (8 mL), formic acid (45 mg, 0.98 mmol) was added, and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (127 mg, 0.66 mmol) was added under ice bath ) and N , N -diisopropylethylamine (306 mg, 2.37 mmol), reacted at room temperature for 2 h, added water (20 mL), stirred for 2 min, extracted with dichloromethane (15 mL × 2), the organic phase was washed with anhydrous It was dried over sodium sulfate, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: DCM/MeOH (v/v) = 15/1) to obtain a white viscous solid (73 mg, yield 74%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 8.35 (s, 1H), 7.55 – 7.63 (m, 1H), 7.23 – 7.33 (m, 1H), 7.12 – 7.16 (m, 1H), 6.89 – 7.04 (m, 4H), 6.79 – 6.84 (m, 2H), 6.49 – 6.75 (m, 1H), 5.07 – 5.22 (m, 2H), 4.57 – 4.65 (m, 1H), 4.26 – 4.31 (m, 0.2H), 4.06 – 4.09 (m, 0.8H), 3.86 – 3.89 (m, 2H), 3.60 – 3.64 (m, 1H), 3.49 (s, 3H), 3.36 – 3.41 (m, 1H), 2.77 – 2.86 (m, 1H), 2.27 (s, 3H), 2.11 – 2.17 (m, 1H), 1.28 – 1.36 (m, 1H), 0.66 – 0.69 (m, 2H), 0.36 – 0.40 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 8.35 (s, 1H), 7.55 – 7.63 (m, 1H), 7.23 – 7.33 (m, 1H), 7.12 – 7.16 (m, 1H), 6.89 – 7.04 (m, 4H), 6.79 – 6.84 (m, 2H), 6.49 – 6.75 (m, 1H), 5.07 – 5.22 (m, 2H), 4.57 – 4.65 (m, 1H), 4.26 – 4.31 (m, 0.2H), 4.06 – 4.09 (m, 0.8H), 3.86 – 3.89 (m, 2H), 3.60 – 3.64 (m, 1H), 3.49 (s, 3H), 3.36 – 3.41 (m, 1H), 2.77 – 2.86 (m, 1H), 2.27 (s, 3H), 2.11 – 2.17 (m, 1H), 1.28 – 1.36 (m, 1H), 0.66 – 0.69 (m, 2H), 0.36 – 0.40 (m, 2H).
MS (ESI, pos.ion) m/z: 594.20 [M+H]+ .MS (ESI, pos.ion) m/z: 594.20 [M+H] + .
實施例165:2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)異吲哚啉-5-甲醯胺 Example 165: 2-(( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbonyl ) isoindoline-5-carboxamide
步驟1:化合物 2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)異吲哚-5-甲酸甲酯的合成Step 1: Compound 2-(( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbonyl ) Synthesis of methyl isoindole-5-carboxylate
將化合物(2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (300 mg, 0.81 mmol,實施例1步驟7) 和異吲哚-5-羧酸甲酯 (220 mg, 0.89 mmol) 溶於DMF (8 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (221 mg, 1.62 mmol)和1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (467 mg, 2.44 mmol),冰浴下加入N ,N -二異丙基乙胺 (419 mg, 3.24 mmol),室溫反應5 h,加水 (20 mL),用乙酸乙酯萃取 (5 mL×2),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑: DCM/MeOH ( v/v ) = 35/1) 得到淺褐色固體 (397 mg, 產率93%)。Compound ( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylic acid (300 mg, 0.81 mmol, Example 1, step 7) and methyl isoindole-5-carboxylate (220 mg, 0.89 mmol) were dissolved in DMF (8 mL) and N -hydroxy-7-azabenzotriazole ( 221 mg, 1.62 mmol) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (467 mg, 2.44 mmol), N , N -diisopropyl was added under ice bath Ethylamine (419 mg, 3.24 mmol), reacted at room temperature for 5 h, added water (20 mL), extracted with ethyl acetate (5 mL×2), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was subjected to silica gel Column chromatography (eluent: DCM/MeOH (v/v) = 35/1) gave a light brown solid (397 mg, 93% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.95 – 8.02 (m, 2H), 7.32 – 7.39 (m, 1H), 7.14 (d,J = 8.1 Hz, 1H), 6.94 (s, 1H), 6.87 – 6.90 (m, 1H), 6.63 (t,J F-H = 75.6 Hz, 1H), 5.41 (t,J = 13.8 Hz, 1H), 4.75 – 5.01 (m, 4H), 3.93 – 3.99 (m, 1H), 3.95 (s, 3H), 3.89 (d,J = 6.9 Hz, 2H), 3.76 (t,J = 10.7 Hz, 1H), 3.38 – 3.50 (m, 1H), 2.58 – 2.67 (m, 1H), 2.22 – 2.35 (m, 1H), 2.14 (s, 3H), 1.28 – 1.35 (m, 1H), 0.64– 0.69 (m, 2H), 0.35 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.95 – 8.02 (m, 2H), 7.32 – 7.39 (m, 1H), 7.14 (d, J = 8.1 Hz, 1H), 6.94 (s, 1H) ), 6.87 – 6.90 (m, 1H), 6.63 (t, J FH = 75.6 Hz, 1H), 5.41 (t, J = 13.8 Hz, 1H), 4.75 – 5.01 (m, 4H), 3.93 – 3.99 (m , 1H), 3.95 (s, 3H), 3.89 (d, J = 6.9 Hz, 2H), 3.76 (t, J = 10.7 Hz, 1H), 3.38 – 3.50 (m, 1H), 2.58 – 2.67 (m, 1H), 2.22 – 2.35 (m, 1H), 2.14 (s, 3H), 1.28 – 1.35 (m, 1H), 0.64 – 0.69 (m, 2H), 0.35 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 529.15 [M+H]+ .MS (ESI, pos.ion) m/z: 529.15 [M+H] + .
步驟2:化合物2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)異吲哚啉-5-甲酸的合成Step 2: Compound 2-(( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbonyl ) Synthesis of isoindoline-5-carboxylic acid
將化合物2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)異吲哚-5-羧酸甲酯 (11208-1) (340 mg, 0.64 mmol) 溶於四氫呋喃 (10 mL) 和水 (5 mL) 的混合溶劑中,加入一水合氫氧化鋰 (135 mg,3.22 mmol),50℃反應30 min後停止,加鹽酸調節溶液pH=2,再用乙酸乙酯萃取 (10 mL×3),有機相用無水硫酸鈉乾燥,除去溶劑得到白色固體 (309 mg, 93%)。The compound 2-(( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbonyl)iso Methyl indole-5-carboxylate (11208-1) (340 mg, 0.64 mmol) was dissolved in a mixed solvent of tetrahydrofuran (10 mL) and water (5 mL), and lithium hydroxide monohydrate (135 mg, 3.22 mL) was added. mmol), the reaction was stopped after 30 min at 50 °C, hydrochloric acid was added to adjust the pH of the solution to 2, and then extracted with ethyl acetate (10 mL × 3), the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed to obtain a white solid (309 mg, 93 %).
1 H NMR (600 MHz, CD3 OD): δ (ppm) 8.03 (s, 1H), 8.02 (d,J = 7.9 Hz, 1H), 7.47 (t,J = 7.5 Hz, 1H), 7.13 (s, 1H), 7.12 (d,J = 8.4 Hz, 1H), 6.95 – 6.96 (m, 1H), 6.75 (t,J F-H = 75.7 Hz, 1H), 5.27 – 5.31 (m, 1H), 5.02 – 5.06 (m, 1H), 4.90 – 4.94 (m, 1H), 4.80 – 4.85 (m, 2H), 4.10 – 4.14 (m, 1H), 3.92 – 3.95 (m, 2H), 3.69 (t,J = 10.7 Hz, 1H), 3.53 – 3.60 (m, 1H), 2.75 – 2.80 (m, 1H), 2.14 (s, 3H), 2.05 – 2.13 (m, 1H), 1.29 – 1.36 (m, 1H), 0.64 – 0.67 (m, 2H), 0.38 – 0.41 (m, 2H). 1 H NMR (600 MHz, CD 3 OD): δ (ppm) 8.03 (s, 1H), 8.02 (d, J = 7.9 Hz, 1H), 7.47 (t, J = 7.5 Hz, 1H), 7.13 (s , 1H), 7.12 (d, J = 8.4 Hz, 1H), 6.95 – 6.96 (m, 1H), 6.75 (t, J FH = 75.7 Hz, 1H), 5.27 – 5.31 (m, 1H), 5.02 – 5.06 (m, 1H), 4.90 – 4.94 (m, 1H), 4.80 – 4.85 (m, 2H), 4.10 – 4.14 (m, 1H), 3.92 – 3.95 (m, 2H), 3.69 (t, J = 10.7 Hz , 1H), 3.53 – 3.60 (m, 1H), 2.75 – 2.80 (m, 1H), 2.14 (s, 3H), 2.05 – 2.13 (m, 1H), 1.29 – 1.36 (m, 1H), 0.64 – 0.67 (m, 2H), 0.38 – 0.41 (m, 2H).
MS (ESI, pos.ion) m/z: 515.15 [M+H]+ .MS (ESI, pos.ion) m/z: 515.15 [M+H] + .
步驟3:化合物 2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)異吲哚啉-5-甲醯胺的合成Step 3: Compound 2-(( 2R )-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbonyl ) Synthesis of isoindoline-5-carboxamide
將化合物2-((2R )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)異吲哚-5-羧酸 (90 mg, 0.17 mmol) 和氯化銨 (930 mg, 1.74 mmol) 溶於無水DMF (5 mL) 中,加入N -羥基-7-氮雜苯並三氮唑 (35 mg, 0.26 mmol)和1-乙基-3-(3-二甲胺丙基)碳二亞胺鹽酸鹽 (467 mg, 2.44 mmol),冰浴下加入N ,N -二異丙基乙胺 (134 mg, 0.70 mmol),室溫反應17 h,減壓濃縮,加水 (40 mL) 攪拌2min,用乙酸乙酯萃取 (10 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,剩餘物進行矽膠柱層析分離 (洗脫劑: DCM/MeOH ( v/v ) = 8/1) 得到白色固體 (78 mg, 87%, HPLC 純度98.12%)。The compound 2-(( 2R )-1-acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carbonyl)iso Indole-5-carboxylic acid (90 mg, 0.17 mmol) and ammonium chloride (930 mg, 1.74 mmol) were dissolved in dry DMF (5 mL) and N -hydroxy-7-azabenzotriazole ( 35 mg, 0.26 mmol) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (467 mg, 2.44 mmol), N , N -diisopropyl was added under ice bath Ethylamine (134 mg, 0.70 mmol) was reacted at room temperature for 17 h, concentrated under reduced pressure, added water (40 mL), stirred for 2 min, extracted with ethyl acetate (10 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and the mixture was dried under reduced pressure. After concentration, the residue was subjected to silica gel column chromatography (eluent: DCM/MeOH (v/v) = 8/1) to obtain a white solid (78 mg, 87%, HPLC purity 98.12%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.21 – 7.79 (m, 2H), 7.30 – 7.34 (m, 1H), 7.13 (d,J = 8.1 Hz, 1H), 6.92 (s, 1H), 6.88 (d,J = 8.1 Hz, 1H), 6.63 (t,J F-H = 75.6 Hz, 1H), 5.37 (t,J = 15.2 Hz, 1H), 4.91 – 4.96 (m, 1H), 4.83 – 4.88 (m, 1H), 4.74 – 4.79 (m, 2H), 3.96 – 3.99 (m, 1H), 3.89 (d,J = 6.9 Hz, 2H), 3.73 (t,J = 10.6 Hz, 1H), 3.40 – 3.48 (m, 1H), 2.59 – 2.66 (m, 1H), 2.22 – 2.30 (m, 1H), 2.13 (s, 3H), 1.28 – 1.32 (m, 1H), 0.65– 0.68 (m, 2H), 0.36 – 0.39 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.21 – 7.79 (m, 2H), 7.30 – 7.34 (m, 1H), 7.13 (d, J = 8.1 Hz, 1H), 6.92 (s, 1H) ), 6.88 (d, J = 8.1 Hz, 1H), 6.63 (t, J FH = 75.6 Hz, 1H), 5.37 (t, J = 15.2 Hz, 1H), 4.91 – 4.96 (m, 1H), 4.83 – 4.88 (m, 1H), 4.74 – 4.79 (m, 2H), 3.96 – 3.99 (m, 1H), 3.89 (d, J = 6.9 Hz, 2H), 3.73 (t, J = 10.6 Hz, 1H), 3.40 – 3.48 (m, 1H), 2.59 – 2.66 (m, 1H), 2.22 – 2.30 (m, 1H), 2.13 (s, 3H), 1.28 – 1.32 (m, 1H), 0.65– 0.68 (m, 2H) , 0.36 – 0.39 (m, 2H).
MS (ESI, pos.ion) m/z: 514.20 [M+H]+ .MS (ESI, pos.ion) m/z: 514.20 [M+H] + .
實施例166:(2R ,4S )-(6-(對甲苯基氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 166: ( 2R , 4S )-(6-(p-tolylaminocarboxy)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy) )-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:(2R , 4S )-1-叔丁基 2-(( 6-(對甲苯基氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1, 2-二甲酸酯的合成Step 1: ( 2R , 4S )-1-tert-butyl 2-((6-(p-tolylcarbamoyl)pyridin-2-yl)methyl)4-(3-(cyclopropylmethyl) Synthesis of oxy)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物 (2R , 4S )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (400 mg, 0.94 mmol,實施例151步驟1),6-(羥甲基)-N -(對甲苯基)吡啶甲酸醯胺 (280 mg, 1.2 mmol) 及1-羥基苯並三唑 (189 mg, 1.4 mmol) 溶解在N ,N -二甲基甲醯胺 (10 mL) 溶液中,在0 ℃下冷卻後,再加入1- (3-二甲氨基丙基) -3-乙基碳二亞胺 (EDCI) (360 mg, 1.9 mmol),N-甲基嗎啡啉(NMM) (190 mg, 1.9 mmol),轉移到室溫下攪拌反應18 h。加水 (50 mL) 停止反應,用乙酸乙酯 (20 mL × 3) 萃取有機相後,用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/EtOAc (v/v)= 4 / 1),得到淺褐色油狀產物 (320 mg, 52.47 %)。Compound (2 R , 4 S )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- carboxylic acid (400 mg, 0.94 mmol, Example 151, step 1), 6- (hydroxymethyl) - N - (p-tolyl) Amides picolinic acid (280 mg, 1.2 mmol) and 1-hydroxybenzotriazole ( 189 mg, 1.4 mmol) was dissolved in N , N -dimethylformamide (10 mL) solution, cooled at 0 °C, and then added 1-(3-dimethylaminopropyl)-3-ethyl Carbodiimide (EDCI) (360 mg, 1.9 mmol), N-methylmorpholine (NMM) (190 mg, 1.9 mmol), transferred to room temperature and stirred for 18 h. Water (50 mL) was added to stop the reaction, the organic phase was extracted with ethyl acetate (20 mL × 3), dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: petroleum ether/EtOAc (v/v)=4/1) to give the product as a light brown oil (320 mg, 52.47 %).
1 H NMR (400 MHz, CDCl3 ) δ 9.85(d,J =24.1Hz, 1H), 8.24 (dd,J = 14.0, 7.7 Hz, 1H), 8.02 – 7.87 (m, 1H), 7.69 – 7.51 (m, 3H), 7.18 (dd,J = 8.6, 3.1 Hz, 2H), 7.07 (dd,J = 8.0, 5.5 Hz, 1H), 6.83 – 6.76 (m, 2H), 6.76 – 6.37 (m, 1H), 5.52 – 5.27 (m, 2H), 4.60 – 4.43 (m, 1H), 4.13 (m, 1H), 3.82 (t,J = 7.2 Hz, 2H), 3.51 – 3.30 (m, 2H), 2.81 – 2.68 (m, 1H), 2.35 (s, 3H), 2.18 – 2.00 (m, 1H), 1.43 (d,J = 22.5Hz, 9H), 1.30-1.22 (m, 1H), 0.67-0.61 (m, 2H), 0.36-0.32 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ 9.85 (d, J =24.1 Hz, 1H), 8.24 (dd, J = 14.0, 7.7 Hz, 1H), 8.02 – 7.87 (m, 1H), 7.69 – 7.51 ( m, 3H), 7.18 (dd, J = 8.6, 3.1 Hz, 2H), 7.07 (dd, J = 8.0, 5.5 Hz, 1H), 6.83 – 6.76 (m, 2H), 6.76 – 6.37 (m, 1H) , 5.52 – 5.27 (m, 2H), 4.60 – 4.43 (m, 1H), 4.13 (m, 1H), 3.82 (t, J = 7.2 Hz, 2H), 3.51 – 3.30 (m, 2H), 2.81 – 2.68 (m, 1H), 2.35 (s, 3H), 2.18 – 2.00 (m, 1H), 1.43 (d, J = 22.5Hz, 9H), 1.30-1.22 (m, 1H), 0.67-0.61 (m, 2H) ), 0.36-0.32 (m, 2H).
MS (ESI, pos.ion) m/z: 652.30 [M+H]+ .MS (ESI, pos.ion) m/z: 652.30 [M+H] + .
步驟2:(2R , 4S )-(6-(對甲苯基氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽的合成Step 2: ( 2R , 4S )-(6-(p-tolylcarbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4-(difluoro) Synthesis of Methoxy)phenyl)pyrrolidine-2-carboxylate hydrochloride
將化合物 (2R , 4S )-1-叔丁基 2-((6-(對甲苯基氨甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1, 2-二羧酸酯 (320 mg, 0.49 mmol) 溶解在二氯甲烷 (5 mL) 溶液中,再加入1, 4-二氧六環鹽酸溶液 (1.5 mL, 6.0 mmol),在室溫下攪拌反應1 h。停止反應,減壓濃縮一次後,再加入二氯甲烷溶液 (20 mL) 溶解,再次減壓濃縮,得到淺褐色油狀粗產物 (340 mg, 100.00 %) 。Compound (2 R , 4 S )-1-tert-butyl 2-((6-(p-tolylcarbamoyl)pyridin-2-yl)methyl)4-(3-(cyclopropylmethoxy) yl)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (320 mg, 0.49 mmol) was dissolved in dichloromethane (5 mL), followed by the addition of 1,4 - Dioxane hydrochloric acid solution (1.5 mL, 6.0 mmol), and the reaction was stirred at room temperature for 1 h. The reaction was stopped, after concentration under reduced pressure once, dichloromethane solution (20 mL) was added to dissolve, and concentrated under reduced pressure again to obtain a light brown oily crude product (340 mg, 100.00%).
1 H NMR (400 MHz, CD3 OD) δ 8.18 (d,J = 7.7 Hz, 1H), 8.09 (t,J = 7.8 Hz, 1H), 8.00 (s, 1H), 7.73 (d,J = 7.8 Hz, 1H), 7.59 (d,J = 8.4 Hz, 2H), 7.17 (d,J = 8.3 Hz, 2H), 7.06 (d,J = 8.2 Hz, 1H), 7.02 (d,J = 2.1 Hz, 1H), 6.86 (dd,J = 8.2, 2.1 Hz, 1H), 6.72 (t,J = 75.5 Hz, 1H), 5.66 – 5.44 (m, 2H), 3.90 – 3.84 (m, 2H), 3.82 – 3.71 (m, 2H), 3.64 – 3.58 (m, 1H), 3.39 (t,J = 11.0 Hz, 1H), 3.05-2.96 (m, 1H), 2.88 (s, 3H), 2.37-2.29 (m, 1H), 1.32-1.22 (m, 1H), 0.69 – 0.59 (m, 2H), 0.38-0.34 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ 8.18 (d, J = 7.7 Hz, 1H), 8.09 (t, J = 7.8 Hz, 1H), 8.00 (s, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.59 (d, J = 8.4 Hz, 2H), 7.17 (d, J = 8.3 Hz, 2H), 7.06 (d, J = 8.2 Hz, 1H), 7.02 (d, J = 2.1 Hz, 1H), 6.86 (dd, J = 8.2, 2.1 Hz, 1H), 6.72 (t, J = 75.5 Hz, 1H), 5.66 – 5.44 (m, 2H), 3.90 – 3.84 (m, 2H), 3.82 – 3.71 (m, 2H), 3.64 – 3.58 (m, 1H), 3.39 (t, J = 11.0 Hz, 1H), 3.05-2.96 (m, 1H), 2.88 (s, 3H), 2.37-2.29 (m, 1H) ), 1.32-1.22 (m, 1H), 0.69 – 0.59 (m, 2H), 0.38-0.34 (m, 2H).
MS (ESI, pos.ion) m/z: 552.25 [M+H]+ .MS (ESI, pos.ion) m/z: 552.25 [M+H] + .
步驟3:(2R , 4S )-(6-(對甲苯甲氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 3: ( 2R , 4S )-(6-(p-tolylaminocarboxy)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy) Synthesis of -4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R , 4S )-(6-(對甲苯基氨甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯鹽酸鹽 (320 mg, 0.58 mmol) 溶解在二氯甲烷 (8.0 mL) 溶液中,在0 ℃下冷卻,加入N ,N -二異丙基乙胺 ( DIPEA ) (340 mL, 2.6 mmol ) 和乙醯氯 (114 mg, 1.45 mmol ),轉移到室溫下攪拌反應3 h。加水 (25 mL) 停止反應,分離有機相,水相用二氯甲烷 (25 mL × 2) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/EtOAc(v/v) = 3/7),得到白色黏稠固體 (150 mg, 產率41.40 %)。Compound (2 R , 4 S )-(6-(p-tolylcarbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethyl) Oxy)phenyl)pyrrolidine-2-carboxylate hydrochloride (320 mg, 0.58 mmol) was dissolved in dichloromethane (8.0 mL) solution, cooled at 0 °C, added with N , N -diisopropyl Ethylethylamine (DIPEA) (340 mL, 2.6 mmol) and acetyl chloride (114 mg, 1.45 mmol) were transferred to room temperature and stirred for 3 h. Water (25 mL) was added to stop the reaction, the organic phase was separated, the aqueous phase was extracted with dichloromethane (25 mL × 2), the organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent). : petroleum ether/EtOAc (v/v) = 3/7) to give a white viscous solid (150 mg, 41.40 % yield).
1 H NMR (400 MHz, CDCl3 ) δ 9.97 (s, 1H), 8.23 (d,J = 7.7 Hz, 1H), 7.93 (t,J = 7.8 Hz, 1H), 7.67 (d,J = 8.4 Hz, 2H), 7.62 (d,J = 7.8 Hz, 1H), 7.17 (d,J = 8.1 Hz, 2H), 7.10 (d,J = 8.1 Hz, 1H), 6.84 – 6.78 (m, 2H), 6.60 (t,J = 75.5 Hz, 1H), 5.50 (d,J = 13.7 Hz, 1H), 5.33 (d,J = 13.6 Hz, 1H), 4.66 – 4.59 (m, 1H), 4.01 – 3.93 (m, 1H), 3.84 (d,J = 6.9 Hz, 2H), 3.64 (t,J = 10.4 Hz, 1H), 3.52 – 3.40 (m, 1H), 2.78 – 2.69 (m, 1H), 2.34 (s, 3H), 2.17 – 2.07 (m, 1H), 2.12 (s, 3H), 1.32 – 1.22 (m, 1H), 0.69 – 0.61 (m, 2H), 0.41-0.33 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ 9.97 (s, 1H), 8.23 (d, J = 7.7 Hz, 1H), 7.93 (t, J = 7.8 Hz, 1H), 7.67 (d, J = 8.4 Hz , 2H), 7.62 (d, J = 7.8 Hz, 1H), 7.17 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 8.1 Hz, 1H), 6.84 – 6.78 (m, 2H), 6.60 (t, J = 75.5 Hz, 1H), 5.50 (d, J = 13.7 Hz, 1H), 5.33 (d, J = 13.6 Hz, 1H), 4.66 – 4.59 (m, 1H), 4.01 – 3.93 (m, 1H), 3.84 (d, J = 6.9 Hz, 2H), 3.64 (t, J = 10.4 Hz, 1H), 3.52 – 3.40 (m, 1H), 2.78 – 2.69 (m, 1H), 2.34 (s, 3H) ), 2.17 – 2.07 (m, 1H), 2.12 (s, 3H), 1.32 – 1.22 (m, 1H), 0.69 – 0.61 (m, 2H), 0.41-0.33 (m, 2H).
MS (ESI, pos.ion) m/z: 593.05 [M]+ .MS (ESI, pos.ion) m/z: 593.05 [M] + .
實施例167: (2R ,4S )-(6-(甲基氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 167: ( 2R , 4S )-(6-(methylaminocarboxy)pyridin-2-yl)methyl 1-acetoxy-4-(3-(cyclopropylmethoxy) -4-(Difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
步驟1:(2R , 4S )-1-叔丁基 2-((6-(甲基氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1, 2-二甲酸酯的合成Step 1: ( 2R , 4S )-1-tert-butyl 2-((6-(methylaminocarboxy)pyridin-2-yl)methyl)4-(3-(cyclopropylmethoxy) Synthesis of yl)-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate
將化合物 (2R , 4S )-1-(叔丁氧羰基)-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (400 mg, 0.94 mmol,實施例151步驟1) ,6-(羥甲基)-N -甲基吡啶甲醯胺 (188 mg, 1.1 mmol,中間體16 ),1-羥基苯並三唑 (190 mg, 1.4 mmol) 溶解在N ,N -二甲基甲醯胺 (10 mL) 溶液中,在0 ℃下冷卻,再加入1- (3-二甲氨基丙基)-3-乙基碳二亞胺 (EDCI) (358 mg, 1.9 mmol),N -甲基嗎啡啉(NMM) (190 mg, 1.9 mmol),轉移到室溫下攪拌反應14 h。加水 (50 mL) 停止,用乙酸乙酯 (20 mL×3) 萃取有機相後,用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/EtOAc (v/v)=4/1),得到淺褐色油狀物 (530 mg, 產率98.39 %)。Compound (2 R , 4 S )-1-(tert-butoxycarbonyl)-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2- Formic acid (400 mg, 0.94 mmol, Example 151, step 1), 6-(hydroxymethyl) -N -picolinamide (188 mg, 1.1 mmol, intermediate 16), 1-hydroxybenzotriazole (190 mg, 1.4 mmol) was dissolved in N , N -dimethylformamide (10 mL) solution, cooled at 0 °C, and 1-(3-dimethylaminopropyl)-3-ethyl was added Carbodiimide (EDCI) (358 mg, 1.9 mmol), N -methylmorpholine (NMM) (190 mg, 1.9 mmol), transferred to room temperature and stirred for 14 h. After adding water (50 mL), the organic phase was extracted with ethyl acetate (20 mL×3), dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: petroleum ether/EtOAc ( v/v)=4/1) to give a light brown oil (530 mg, 98.39 % yield).
1 H NMR (400 MHz, CDCl3 ) δ 8.39 (m, 1H), 7.92 – 7.82 (m, 1H), 7.55 – 7.48 (m, 1H), 7.11 (d,J = 8.1 Hz, 1H), 6.81 (d,J = 10.4 Hz, 2H), 6.61 (t,J = 75.6 Hz, 1H), 5.43 – 5.27 (m, 1H), 4.60 – 4.42 (m, 1H), 4.12 – 3.95 (m, 1H), 3.85 (t,J = 7.8 Hz, 2H), 3.51 – 3.28 (m, 2H), 3.05 – 3.00 (m, 3H), 2.82 – 2.70 (m, 1H), 2.15 – 1.99 (m, 2H), 1.52 – 1.44 (s, 6H), 1.39 (s, 3H), 1.27 – 1.21 (s, 1H), 0.66 – 0.61 (m, 2H), 0.36 – 0.31 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ 8.39 (m, 1H), 7.92 – 7.82 (m, 1H), 7.55 – 7.48 (m, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.81 ( d, J = 10.4 Hz, 2H), 6.61 (t, J = 75.6 Hz, 1H), 5.43 – 5.27 (m, 1H), 4.60 – 4.42 (m, 1H), 4.12 – 3.95 (m, 1H), 3.85 (t, J = 7.8 Hz, 2H), 3.51 – 3.28 (m, 2H), 3.05 – 3.00 (m, 3H), 2.82 – 2.70 (m, 1H), 2.15 – 1.99 (m, 2H), 1.52 – 1.44 (s, 6H), 1.39 (s, 3H), 1.27 – 1.21 (s, 1H), 0.66 – 0.61 (m, 2H), 0.36 – 0.31 (m, 2H).
MS (ESI, pos.ion) m/z: 576.20 [M+H]+ .MS (ESI, pos.ion) m/z: 576.20 [M+H] + .
步驟2:(2R, 4S)-(6-(甲基氨基甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸鹽酸鹽的合成Step 2: (2R, 4S)-(6-(Methylaminocarbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy) )Phenyl)pyrrolidine-2-carboxylate hydrochloride synthesis
將化合物 (2R , 4S )-1-叔丁基 2-((6-(甲基氨基甲醯基)吡啶-2-基)甲基) 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-1, 2- 二甲酸酯 (530 mg, 0.92 mmol) 溶解在二氯甲烷 (5 mL) 溶液中,再加入1, 4-二氧六環鹽酸溶液 (2.5 mL, 10.0 mmol),在室溫下攪拌反應1 h。減壓濃縮一次後,再加入二氯甲烷溶液 (20 mL) 溶解,再次減壓濃縮,得到淺褐色油狀粗產物 (435 mg, 產率92.27 %)Compound (2 R , 4 S )-1-tert-butyl 2-((6-(methylaminocarbamoyl)pyridin-2-yl)methyl)4-(3-(cyclopropylmethoxy) )-4-(difluoromethoxy)phenyl)pyrrolidine-1,2-dicarboxylate (530 mg, 0.92 mmol) was dissolved in dichloromethane (5 mL) and 1,4- Dioxane hydrochloric acid solution (2.5 mL, 10.0 mmol) was stirred at room temperature for 1 h. After concentration under reduced pressure once, dichloromethane solution (20 mL) was added to dissolve, and concentrated under reduced pressure again to obtain a light brown oily crude product (435 mg, yield 92.27%)
1 H NMR (400 MHz, CD3 OD) δ 7.97 – 7.90 (m, 1H), 7.90 – 7.84 (m, 2H), 7.06 – 6.99 (m, 1H), 6.95 (s, 1H), 6.83 – 6.77 (m, 1H), 6.64 (t,J = 75.6 Hz, 1H), 5.38 (d,J = 6.6 Hz, 2H), 3.85 – 3.77 (m, 2H), 3.74 – 3.53 (m, 4H), 3.32 – 3.20 (m, 1H), 2.83 (s, 3H), 2.24 – 2.05 (m, 1H), 1.14 – 1.25 (m, 1H), 0.55 – 0.51 (m, 2H), 0.30 – 0.24 (m, 2H). 1 H NMR (400 MHz, CD 3 OD) δ 7.97 – 7.90 (m, 1H), 7.90 – 7.84 (m, 2H), 7.06 – 6.99 (m, 1H), 6.95 (s, 1H), 6.83 – 6.77 ( m, 1H), 6.64 (t, J = 75.6 Hz, 1H), 5.38 (d, J = 6.6 Hz, 2H), 3.85 – 3.77 (m, 2H), 3.74 – 3.53 (m, 4H), 3.32 – 3.20 (m, 1H), 2.83 (s, 3H), 2.24 – 2.05 (m, 1H), 1.14 – 1.25 (m, 1H), 0.55 – 0.51 (m, 2H), 0.30 – 0.24 (m, 2H).
MS (ESI, pos.ion) m/z: 476.20 [M+H]+ .MS (ESI, pos.ion) m/z: 476.20 [M+H] + .
步驟3:(2R , 4S )-(6-(甲基氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯的合成Step 3: ( 2R , 4S )-(6-(methylaminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3-(cyclopropylmethoxy)- Synthesis of 4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R , 4S )-(6-(甲基氨基甲醯基)吡啶-2-基)甲基 4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸鹽酸鹽 (435 mg, 0.91 mmol) 溶解在二氯甲 (8 mL) 烷溶液中,在0 ℃下冷卻,加入N, N-二異丙基乙胺 ( DIPEA ) (550 mL, 4.3 mmol ) 及乙醯氯 (200 mg, 2.3 mmol ),轉移到室溫下攪拌反應2 h。加水 (25 mL) 停止反應,分離有機相,水相用二氯甲烷 (25 mL × 2) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:二氯甲烷/甲醇(v/v)= 50/1),得到淺褐色油狀物 (133 mg, 產率27.73 %)。Compound (2 R , 4 S )-(6-(methylcarbamoyl)pyridin-2-yl)methyl 4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy) yl)phenyl)pyrrolidine-2-carboxylate hydrochloride (435 mg, 0.91 mmol) was dissolved in dichloromethane (8 mL) alkane solution, cooled at 0 °C, and N,N-diisopropyl was added Ethylamine (DIPEA) (550 mL, 4.3 mmol) and acetyl chloride (200 mg, 2.3 mmol) were transferred to room temperature and stirred for 2 h. Water (25 mL) was added to stop the reaction, the organic phase was separated, the aqueous phase was extracted with dichloromethane (25 mL × 2), the organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent). : dichloromethane/methanol (v/v) = 50/1) to give a light brown oil (133 mg, yield 27.73 %).
1 H NMR (400 MHz, CDCl3 ) δ 8.34 (br.s, 1H), 8.13 (d,J = 7.7 Hz, 1H), 7.86 (t,J = 7.8 Hz, 1H), 7.51 (d,J = 7.8 Hz, 1H), 7.13 (d,J = 8.7 Hz, 1H), 6.87 – 6.79 (m, 2H), 6.61 (t,J = 75.5 Hz, 1H), 5.52 (d,J = 13.8 Hz, 1H), 5.20 (d,J = 13.8 Hz, 1H), 4.65 – 4.61 (m, 1H), 400 – 3.96 (m, 1H), 3.86 (d,J = 6.9 Hz, 2H), 3.65 (t,J = 10.4 Hz, 1H), 3.52 – 3.39 (m, 1H), 3.03 (d,J = 5.0 Hz, 3H), 2.79 – 2.69 (m, 1H), 2.14 (s, 3H), 2.11 – 1.96 (m, 1H), 1.33 – 1.22 (m, 1H), 0.69 – 0.63 (m, 2H), 0.36 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ 8.34 (br.s, 1H), 8.13 (d, J = 7.7 Hz, 1H), 7.86 (t, J = 7.8 Hz, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.13 (d, J = 8.7 Hz, 1H), 6.87 – 6.79 (m, 2H), 6.61 (t, J = 75.5 Hz, 1H), 5.52 (d, J = 13.8 Hz, 1H) , 5.20 (d, J = 13.8 Hz, 1H), 4.65 – 4.61 (m, 1H), 400 – 3.96 (m, 1H), 3.86 (d, J = 6.9 Hz, 2H), 3.65 (t, J = 10.4 Hz, 1H), 3.52 – 3.39 (m, 1H), 3.03 (d, J = 5.0 Hz, 3H), 2.79 – 2.69 (m, 1H), 2.14 (s, 3H), 2.11 – 1.96 (m, 1H) , 1.33 – 1.22 (m, 1H), 0.69 – 0.63 (m, 2H), 0.36 (m, 2H).
MS (ESI, pos.ion) m/z: 518.20 [M+H]+ .MS (ESI, pos.ion) m/z: 518.20 [M+H] + .
實施例168:6-((((2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)氧基)甲基)吡啶甲酸 Example 168: 6 - (((( 2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrole Alkyl-2-carbonyl)oxy)methyl)picolinic acid
步驟1:叔丁基 6-((((2R , 4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)氧基)甲基)吡啶甲酸酯的合成Step 1: tert-Butyl 6 - ((((2 R , 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl ) Synthesis of pyrrolidine-2-carbonyl)oxy)methyl)picolinate
將化合物 (2R , 4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (540 mg, 1.46 mmol,實施例151步驟5) 溶於N ,N -二甲醯胺 (15 mL) 溶劑中,再加入6-(羥甲基)吡啶甲酸叔丁酯 (374 mg,1.79 mmol,中間體17),1-羥基苯並三唑 (HOBT) (300 mg, 2.22 mmol),轉移到0 ℃下,再加入1-(3-二甲基氨基丙基)-3-乙基碳二亞胺 (EDCI) (637 mg, 3.32 mmol),N -甲基嗎啡啉(NMM) (370 mg, 3.66 mmol),加入原料完之後,轉移到室溫下攪拌反應17 h。加水 (50 mL)停止反應,再用乙酸乙酯 (20 mL×3) 萃取有機相後,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:石油醚/乙酸乙酯(v/v)=1/4 ),得到淺褐色油狀物 (590 mg, 產率71.99 %)。Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid (540 mg, 1.46 mmol, Example 151, step 5) was dissolved in N , N -dimethylamide (15 mL) solvent, followed by the addition of tert-butyl 6-(hydroxymethyl)picolinate (374 mg, 1.79 mmol, intermediate Compound 17), 1-hydroxybenzotriazole (HOBT) (300 mg, 2.22 mmol), transferred to 0 °C, followed by addition of 1-(3-dimethylaminopropyl)-3-ethylcarbodiidene Amine (EDCI) (637 mg, 3.32 mmol), N -methylmorpholine (NMM) (370 mg, 3.66 mmol), after adding the starting materials, transfer to room temperature and stir the reaction for 17 h. Water (50 mL) was added to stop the reaction, and the organic phase was extracted with ethyl acetate (20 mL×3). The organic phase was dried over anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v)=1/4) to give a light brown oil (590 mg, 71.99% yield).
1H NMR (400 MHz, CDCl3 ) δ 7.94 (d,J = 7.8 Hz, 1H), 7.84 (t,J = 7.8 Hz, 1H), 7.66 (d,J = 7.7 Hz, 1H), 7.12 (d,J = 8.7 Hz, 1H), 6.85 – 6.80 (m, 2H), 6.61 (d,J = 75.5 Hz, 1H), 5.56 – 5.33 (m, 2H), 4.64 – 4.57 (m, 1H), 4.01 – 3.91 (m, 1H), 3.88 – 3.82 (m, 2H), 3.63 (t,J = 10.5 Hz, 1H), 3.51 – 3.42 (m, 1H), 2.79 – 2.68 (m, 1H), 2.19 – 2.10 (m, 1H), 2.13 (s, 3H), 1.61 (s, 9H), 1.34 – 1.24 (m, 1H), 0.68 – 0.63 (m, 2H), 0.38 – 0.34 (m, 2H).1H NMR (400 MHz, CDCl 3 ) δ 7.94 (d, J = 7.8 Hz, 1H), 7.84 (t, J = 7.8 Hz, 1H), 7.66 (d, J = 7.7 Hz, 1H), 7.12 (d, J = 8.7 Hz, 1H), 6.85 – 6.80 (m, 2H), 6.61 (d, J = 75.5 Hz, 1H), 5.56 – 5.33 (m, 2H), 4.64 – 4.57 (m, 1H), 4.01 – 3.91 (m, 1H), 3.88 – 3.82 (m, 2H), 3.63 (t, J = 10.5 Hz, 1H), 3.51 – 3.42 (m, 1H), 2.79 – 2.68 (m, 1H), 2.19 – 2.10 (m , 1H), 2.13 (s, 3H), 1.61 (s, 9H), 1.34 – 1.24 (m, 1H), 0.68 – 0.63 (m, 2H), 0.38 – 0.34 (m, 2H).
MS (ESI, pos.ion) m/z: 561.30 [M+H]+ .MS (ESI, pos.ion) m/z: 561.30 [M+H] + .
步驟2:6-((((2R, 4S)-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)氧基)甲基)吡啶甲酸的合成Step 2: 6-((((2R, 4S)-1-Acetyl-4-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2 Synthesis of -carbonyl)oxy)methyl)picolinic acid
將化合物叔丁基 6-((((2R , 4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基氧基)甲基]吡啶甲酸酯 (620 mg, 1.11 mmol) 溶解在二氯甲烷 (20 mL) 溶劑中,再滴加三氟乙酸 (4.59 g, 40.62 mmol),在室溫下攪拌反應11 h。停止反應,通過HPLC製備純化,得到白色黏稠固體 (210 mg, 產率34.84 %)。The compound tert-butyl 6 - ((((2 R , 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) Pyrrolidine-2-carbonyloxy)methyl]picolinate (620 mg, 1.11 mmol) was dissolved in dichloromethane (20 mL) solvent, and trifluoroacetic acid (4.59 g, 40.62 mmol) was added dropwise to the solution. The reaction was stirred at room temperature for 11 h. The reaction was stopped and purified by HPLC prep to give a white viscous solid (210 mg, 34.84% yield).
1 H NMR(400 MHz, CDCl3 ) δ 8.16 (d, J = 7.6 Hz, 1H), 7.96 (t, J = 7.8 Hz, 1H), 7.65 (d, J = 7.8 Hz, 1H), 7.14 (d, J = 8.7 Hz, 1H), 6.84 (s, 1H), 6.83 (d, J = 6.4 Hz, 1H), 6.62 (t, J = 75.5 Hz, 1H), 5.57 (d, J = 14.1 Hz, 1H), 5.29 (d, J = 8.7 Hz, 1H), 4.66 – 4.59 (m, 1H), 3.99 (t, J = 8.6 Hz, 1H), 3.87 (d, J = 6.9 Hz, 2H), 3.65 (t, J = 10.5 Hz, 1H), 3.54 – 3.43 (m, 1H), 2.79 – 2.69 (m, 1H), 2.19 – 2.09 (m, 1H), 2.16 (s, 3H), 1.35 – 1.21 (m, 1H), 0.69 – 0.64 (m, 2H), 0.38 – 0.34 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ) δ 8.16 (d, J = 7.6 Hz, 1H), 7.96 (t, J = 7.8 Hz, 1H), 7.65 (d, J = 7.8 Hz, 1H), 7.14 (d , J = 8.7 Hz, 1H), 6.84 (s, 1H), 6.83 (d, J = 6.4 Hz, 1H), 6.62 (t, J = 75.5 Hz, 1H), 5.57 (d, J = 14.1 Hz, 1H) ), 5.29 (d, J = 8.7 Hz, 1H), 4.66 – 4.59 (m, 1H), 3.99 (t, J = 8.6 Hz, 1H), 3.87 (d, J = 6.9 Hz, 2H), 3.65 (t , J = 10.5 Hz, 1H), 3.54 – 3.43 (m, 1H), 2.79 – 2.69 (m, 1H), 2.19 – 2.09 (m, 1H), 2.16 (s, 3H), 1.35 – 1.21 (m, 1H) ), 0.69 – 0.64 (m, 2H), 0.38 – 0.34 (m, 2H).
MS (ESI, pos.ion) m/z: 505.10 [M+H]+ .MS (ESI, pos.ion) m/z: 505.10 [M+H] + .
實施例169:(2R ,4S )-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲酸酯 Example 169: ( 2R , 4S )-(6-(methyl(p-tolyl)carbamoyl)pyridin-2-yl)methyl 1-acetoxy-4-(4-(difluoro) Methoxy)-3-hydroxyphenyl)pyrrolidine-2-carboxylate
步驟1:6-((((2R , 4S )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-羰基)氧基)甲基)吡啶甲酸 的合成Step 1: 6 - (((( 2 R, 4 S) -1- acetyl-4- (4- (difluoromethoxy) -3-hydroxyphenyl) pyrrolidine-2-carbonyl) oxy ) methyl) picolinic acid synthesis
將化合物叔丁基6-((((2R , 4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-羰基)氧基)甲基)吡啶甲酸酯 (185 mg, 0.33 mmol,實施例168步驟1) 溶於二氯甲烷 (5.5 mL) 溶劑中,再加入溴化鋅 (74 mg, 0.33 mmol),在室溫下攪拌反應23.5 h。加入水 (20 mL),攪拌2分鐘,分離有機相,水相再加入二氯甲烷溶劑 (20 mL × 2) 萃取,合併有機相,並用無水硫酸鈉乾燥30 min,通過矽膠柱層析純化 (洗脫劑:二氯甲烷/甲醇(v/v)=10/1),得到白色固體 (22 mg, 產率14.80 %)The compound tert-butyl 6 - ((((2 R , 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) Pyrrolidine-2-carbonyl)oxy)methyl)picolinate (185 mg, 0.33 mmol, Example 168, step 1) was dissolved in dichloromethane (5.5 mL) solvent and zinc bromide (74 mg) was added , 0.33 mmol), and the reaction was stirred at room temperature for 23.5 h. Water (20 mL) was added, stirred for 2 minutes, the organic phase was separated, the aqueous phase was then extracted with dichloromethane solvent (20 mL × 2), the organic phases were combined, dried over anhydrous sodium sulfate for 30 min, and purified by silica gel column chromatography ( Eluent: dichloromethane/methanol (v/v)=10/1) to give a white solid (22 mg, yield 14.80 %)
MS (ESI, pos.ion) m/z: 451.20 [M+H]+ .MS (ESI, pos.ion) m/z: 451.20 [M+H] + .
步驟2:(2R , 4S)-(6-(甲基(對甲苯基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-甲酸酯的合成Step 2: ( 2R ,4S)-(6-(methyl(p-tolyl)aminocarbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(4-(difluoromethoxy) Synthesis of phenyl)-3-hydroxyphenyl)pyrrolidine-2-carboxylate
將化合物6-((((2R , 4S )-1-乙醯基-4-(4-(二氟甲氧基)-3-羥基苯基)吡咯烷-2-羰基)氧基)甲基)吡啶甲酸 (100 mg, 0.22 mmol),N -甲基-對甲基苯胺 (40 mg, 0.33 mmol),溶於二氯甲烷溶劑 (5 mL) 中,再加入1-羥基苯並三唑 (HOBT) (148 mg, 1.1 mmol),在0 ℃下冷卻,加入1-(3-二甲基氨基丙基)-3-乙基碳二亞胺 (EDCI) ( 126 mg, 0.66 mmol),N ,N -二異丙基乙胺 (DIPEA) ( 142 mg, 1.1 mmol )。原料加入完之後,轉移到室溫下攪拌反應7 h。加水停止反應,用二氯甲烷 (100 mL×2) 萃取,有機相用無水硫酸鈉乾燥30 min,減壓濃縮,通過矽膠柱層析純化 (洗脫劑:二氯甲烷/甲醇(v/v)=16/1),得到白色固體產物 (40 mg, 產率28.50 %)Compound 6-((((2 R , 4 S )-1-acetyl-4-(4-(difluoromethoxy)-3-hydroxyphenyl)pyrrolidine-2-carbonyl)oxy) Methyl)picolinic acid (100 mg, 0.22 mmol), N -methyl-p-methylaniline (40 mg, 0.33 mmol), dissolved in dichloromethane solvent (5 mL), and 1-hydroxybenzotriazine was added oxazole (HOBT) (148 mg, 1.1 mmol), cooled at 0 °C, added 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDCI) (126 mg, 0.66 mmol) , N , N -diisopropylethylamine (DIPEA) (142 mg, 1.1 mmol). After the addition of the raw materials, the reaction was stirred at room temperature for 7 h. The reaction was stopped by adding water, extracted with dichloromethane (100 mL×2), the organic phase was dried with anhydrous sodium sulfate for 30 min, concentrated under reduced pressure, and purified by silica gel column chromatography (eluent: dichloromethane/methanol (v/v )=16/1) to obtain a white solid product (40 mg, yield 28.50%)
1 H NMR (400 MHz, CDCl3 ) δ 7.51 (s, 1H), 7.13 – 7.02 (m, 3H), 7.01 – 6.96 (m, 2H), 6.94 – 6.86 (m, 2H), 6.83 – 6.64 (m, 2H), 6.61 (t,J = 74.7 Hz, 1H), 5.33 (d,J = 13.8 Hz, 1H), 4.97 (d,J = 13.2 Hz, 1H), 4.61 – 4.52 (m, 1H), 3.91 (t,J = 8.8 Hz, 1H), 3.55 – 3.48 (m, 3H), 3.44 – 3.33 (m, 1H), 2.69 – 2.57 (m, 1H), 2.25 (s, 3H), 2.17 – 2.084 (m, 1H), 2.10 (s, 3H), 1.37 – 1.267 (m, 1H). 1 H NMR (400 MHz, CDCl 3 ) δ 7.51 (s, 1H), 7.13 – 7.02 (m, 3H), 7.01 – 6.96 (m, 2H), 6.94 – 6.86 (m, 2H), 6.83 – 6.64 (m , 2H), 6.61 (t, J = 74.7 Hz, 1H), 5.33 (d, J = 13.8 Hz, 1H), 4.97 (d, J = 13.2 Hz, 1H), 4.61 – 4.52 (m, 1H), 3.91 (t, J = 8.8 Hz, 1H), 3.55 – 3.48 (m, 3H), 3.44 – 3.33 (m, 1H), 2.69 – 2.57 (m, 1H), 2.25 (s, 3H), 2.17 – 2.084 (m , 1H), 2.10 (s, 3H), 1.37 – 1.267 (m, 1H).
MS (ESI, pos.ion) m/z: 554.20 [M+H]+ .MS (ESI, pos.ion) m/z: 554.20 [M+H] + .
實施例170:(6-((4-羥基苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 (2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸酯 Example 170: (6 - ((4-hydroxyphenyl) (methyl) carbamoyl acyl) pyridin-2-yl) methyl (2 R, 4 S) -1- acetyl-4- (3 -(Cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)pyrrolidine-2-carboxylate
將化合物 (2R ,4S )-1-乙醯基-4-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)吡咯烷-2-甲酸 (300 mg, 0.81 mmol,實施例151步驟5),6-(羥甲基)-N -(4-羥苯基)-N -甲基吡啶醯胺 (250 mg, 0.97 mmol,中間體18) 和1-羥基苯並三唑 (130 mg, 0.97 mmol) 溶解在乾燥的N ,N -二甲基甲醯胺 (5 ml) 溶液中,加入1- (3-二甲氨基丙基) -3-乙基碳二亞胺 (EDCI) (190 mg, 0.97 mmol)和N -甲基嗎啡啉 (NMM) (120 mg, 1.22 mmol),轉移到室溫下攪拌反應16 h。加水 (30 mL) 停止反應,用乙酸乙酯 (10 ml × 3) 萃取有機相後,用無水硫酸鈉乾燥,減壓濃縮,通過矽膠柱層析純化 (MeOH/DCM ( v/v )=1/50),得到白色固體 (36 mg, 產率6.98 %)。Compound (2 R, 4 S) -1- acetyl-4- (3- (cyclopropylmethoxy) -4- (difluoromethoxy) phenyl) pyrrolidine-2-carboxylic acid (300 mg, 0.81 mmol, 151 Example 5 step), 6- (hydroxymethyl) - N - (4- hydroxyphenyl) - N - Amides methylpyridine (250 mg, 0.97 mmol, intermediate 18) and 1 -Hydroxybenzotriazole (130 mg, 0.97 mmol) was dissolved in dry N , N -dimethylformamide (5 ml) solution, 1-(3-dimethylaminopropyl)-3-ethyl was added carbodiimide (EDCI) (190 mg, 0.97 mmol) and N -methylmorpholine (NMM) (120 mg, 1.22 mmol), transferred to room temperature and stirred for 16 h. Water (30 mL) was added to stop the reaction, the organic phase was extracted with ethyl acetate (10 ml × 3), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by silica gel column chromatography (MeOH/DCM (v/v)=1 /50) to give a white solid (36 mg, 6.98% yield).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 9.46 (s, 1H), 7.69 (t,J = 7.2 Hz, 1H), 7.28 – 7.21 (m, 2H), 7.12 (d,J = 8.1 Hz, 1H), 7.07 (s, 1H), 7.02 (t,J F-H = 74.9 Hz, 1H), 6.94 – 6.84 (m, 3H), 6.60 – 6.51 (m, 2H), 5.01 (s, 2H), 4.47 – 4.36 (m, 1H), 4.14 – 4.05 (m, 1H), 3.90 (d,J = 6.3 Hz, 2H), 3.58 – 3.40 (m, 2H), 3.28 (s, 3H), 2.75 – 2.65 (m, 1H), 2.03 (s, 3H), 2.02 – 1.91 (m, 1H), 1.29 – 1.17 (m, 1H), 0.62 – 0.51 (m, 2H), 0.38 – 0.26 (m, 2H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 9.46 (s, 1H), 7.69 (t, J = 7.2 Hz, 1H), 7.28 – 7.21 (m, 2H), 7.12 (d, J = 8.1 Hz, 1H), 7.07 (s, 1H), 7.02 (t, J FH = 74.9 Hz, 1H), 6.94 – 6.84 (m, 3H), 6.60 – 6.51 (m, 2H), 5.01 (s, 2H), 4.47 – 4.36 (m, 1H), 4.14 – 4.05 (m, 1H), 3.90 (d, J = 6.3 Hz, 2H), 3.58 – 3.40 (m, 2H), 3.28 (s, 3H), 2.75 – 2.65 ( m, 1H), 2.03 (s, 3H), 2.02 – 1.91 (m, 1H), 1.29 – 1.17 (m, 1H), 0.62 – 0.51 (m, 2H), 0.38 – 0.26 (m, 2H).
MS (ESI, pos.ion) m/z: 610.15 [M+H]+ ..MS (ESI, pos.ion) m/z: 610.15 [M+H] + ..
實施例171:(2R ,4S )-(6-((4-羥基苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸酯 Example 171 : ( 2R , 4S )-(6-((4-hydroxyphenyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3 -Ethoxy-4-methoxyphenyl)pyrrolidine-2-carboxylate
步驟1:化合物3-乙氧基-4-甲氧基苯甲酸甲酯的合成Step 1: Synthesis of compound 3-ethoxy-4-methoxybenzoic acid methyl ester
將化合物 3-羥基-4-甲氧基苯甲酸甲酯(5.00 g, 27.45 mmol), 溴乙烷 (4.49 g, 41.17 mmol)和碳酸鉀 (11.83 g, 82.35 mmol) 混合在丙酮 (25 mL) 中, 置於60℃下反應10 h,加入二氯甲烷 (100 mL)稀釋,有機相用飽和食鹽水 (100 mL × 3)洗滌,用無水硫酸鈉乾燥,減壓旋蒸濃縮得到黃色固體 (5.23 g, 產率90.63%).Compound 3-hydroxy-4-methoxybenzoic acid methyl ester (5.00 g, 27.45 mmol), bromoethane (4.49 g, 41.17 mmol) and potassium carbonate (11.83 g, 82.35 mmol) were mixed in acetone (25 mL) was placed at 60 °C for reaction for 10 h, diluted with dichloromethane (100 mL), the organic phase was washed with saturated brine (100 mL × 3), dried over anhydrous sodium sulfate, and concentrated by rotary evaporation under reduced pressure to obtain a yellow solid ( 5.23 g, yield 90.63%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.66 (dd,J = 8.4, 1.7 Hz, 1H), 7.54 (d,J = 1.6 Hz, 1H), 6.88 (d,J = 8.4 Hz, 1H), 4.15 (q,J = 7.0 Hz, 2H), 3.92 (s, 3H), 3.88 (s, 3H), 1.48 (t,J = 7.0 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.66 (dd, J = 8.4, 1.7 Hz, 1H), 7.54 (d, J = 1.6 Hz, 1H), 6.88 (d, J = 8.4 Hz, 1H), 4.15 (q, J = 7.0 Hz, 2H), 3.92 (s, 3H), 3.88 (s, 3H), 1.48 (t, J = 7.0 Hz, 3H).
MS (ESI, pos.ion) m/z: 211.10 [M+H]+ .MS (ESI, pos.ion) m/z: 211.10 [M+H] + .
步驟2:化合物3-乙氧基-4-甲氧基苯甲酸的合成Step 2: Synthesis of compound 3-ethoxy-4-methoxybenzoic acid
將化合物3-乙氧基-4-甲氧基苯甲酸甲酯(10.46 g, 49.76 mmol), 氫氧化鈉 (3.98 g, 99.52 mmol,乙醇 (30 mL),水 (10 mL) 混合均勻,置於50℃攪拌2 h。滴入稀鹽酸 (4 M/L) 調節pH=1,加入水(100 mL),攪拌(析出大量固體),過濾,所得濾餅用水(50 mL × 3)洗滌,真空60℃乾燥12 h,得白色固體 (9.76 g, 產率97%)。The compound 3-ethoxy-4-methoxybenzoic acid methyl ester (10.46 g, 49.76 mmol), sodium hydroxide (3.98 g, 99.52 mmol, ethanol (30 mL), water (10 mL) were mixed uniformly, and set aside. Stir for 2 h at 50 ° C. Dilute hydrochloric acid (4 M/L) was added dropwise to adjust pH=1, water (100 mL) was added, stirred (a large amount of solid was precipitated), filtered, and the obtained filter cake was washed with water (50 mL × 3), Dry under vacuum at 60 °C for 12 h to obtain a white solid (9.76 g, yield 97%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.77 (dd,J = 8.4, 1.9 Hz, 1H), 7.60 (d,J = 1.8 Hz, 1H), 6.92 (d,J = 8.5 Hz, 1H), 4.17 (q,J = 7.0 Hz, 2H), 3.95 (s, 3H), 1.50 (t,J = 7.0 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.77 (dd, J = 8.4, 1.9 Hz, 1H), 7.60 (d, J = 1.8 Hz, 1H), 6.92 (d, J = 8.5 Hz, 1H), 4.17 (q, J = 7.0 Hz, 2H), 3.95 (s, 3H), 1.50 (t, J = 7.0 Hz, 3H).
MS (ESI, pos.ion) m/z: 197.15 [M+H]+ .MS (ESI, pos.ion) m/z: 197.15 [M+H] + .
步驟3:化合物苄基 (3-乙氧基-4-甲氧基苯基)氨基甲酸酯的合成Step 3: Synthesis of compound benzyl(3-ethoxy-4-methoxyphenyl)carbamate
步驟一:室溫下,向兩口瓶(100 mL)(A瓶)加入3-乙氧基-4-甲氧基苯甲酸 (9.40 g, 47.91 mmol ),三乙胺 (6.30 g, 62.28 mmol ),甲苯 (50 mL),置於冰浴中攪拌,緩慢滴加入疊氮磷酸二苯酯 (14.50 g, 52.70 mmol ),室溫中攪拌2 h。Step 1: At room temperature, add 3-ethoxy-4-methoxybenzoic acid (9.40 g, 47.91 mmol), triethylamine (6.30 g, 62.28 mmol) to a two-necked flask (100 mL) (A bottle) , toluene (50 mL), stirred in an ice bath, slowly added dropwise diphenylphosphoryl azide (14.50 g, 52.70 mmol), and stirred at room temperature for 2 h.
步驟二:向另一個單口瓶(250 mL)(B瓶)加入甲苯 (50 mL),苯甲醇 (5.70 g, 52.70 mmol),加熱至110℃,把A瓶物料滴入B瓶中,滴畢保溫攪拌2 h。停止加熱攪拌,冷至室溫, 反應液用水洗滌(100 mL),再用5% 氫氧化鈉水溶液洗滌(100 mL × 3),用無水Na2 SO4 乾燥,減壓旋蒸濃縮得黃色固體。向粗品加入石油醚/乙酸乙酯 (50 mL, v/v=10/1) 攪拌3 h, 過濾得白色固體 (11.55 g, 產率80.00%).Step 2: Add toluene (50 mL) and benzyl alcohol (5.70 g, 52.70 mmol) to another single-necked bottle (250 mL) (B bottle), heat to 110°C, drop the material from A bottle into B bottle, and drip it. Keep stirring for 2 h. The heating and stirring were stopped, cooled to room temperature, the reaction solution was washed with water (100 mL), then washed with 5% aqueous sodium hydroxide solution (100 mL × 3), dried over anhydrous Na 2 SO 4 , and concentrated by rotary evaporation under reduced pressure to obtain a yellow solid . Petroleum ether/ethyl acetate (50 mL, v/v=10/1) was added to the crude product, stirred for 3 h, and filtered to obtain a white solid (11.55 g, yield 80.00%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.44 – 7.31 (m, 5H), 6.85 – 6.72 (m, 2H), 6.59 (s, 1H), 5.19 (s, 2H), 4.08 (d,J = 6.3 Hz, 2H), 3.84 (s, 3H), 1.45 (t,J = 7.0 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.44 – 7.31 (m, 5H), 6.85 – 6.72 (m, 2H), 6.59 (s, 1H), 5.19 (s, 2H), 4.08 (d , J = 6.3 Hz, 2H), 3.84 (s, 3H), 1.45 (t, J = 7.0 Hz, 3H).
MS (ESI, pos.ion) m/z: 302.10 [M+H]+ .MS (ESI, pos.ion) m/z: 302.10 [M+H] + .
步驟4:化合物3-乙氧基-4-甲氧基苯胺的合成Step 4: Synthesis of compound 3-ethoxy-4-methoxyaniline
向高壓釜(1 L)中加入苄基 (3-乙氧基-4-甲氧基苯基)氨基甲酸酯(11.50 g, 38.16 mmol ), 10% 鈀碳 (0.61 g, 0.57 mmol ), 甲醇 (40 mL),排除空氣,通入氫氣(0.5 MPa),室溫攪拌2 h。反應液通過矽藻土過濾,濾液經減壓濃縮得褐色固體 (6.38 g, 產率100% )To the autoclave (1 L) was added benzyl(3-ethoxy-4-methoxyphenyl)carbamate (11.50 g, 38.16 mmol), 10% palladium on carbon (0.61 g, 0.57 mmol), Methanol (40 mL), remove air, pass hydrogen (0.5 MPa), and stir at room temperature for 2 h. The reaction solution was filtered through celite, and the filtrate was concentrated under reduced pressure to obtain a brown solid (6.38 g, yield 100%)
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 6.71 (d,J = 8.4 Hz, 1H), 6.31 (d,J = 2.5 Hz, 1H), 6.23 (dd,J = 8.4, 2.6 Hz, 1H), 4.04 (q,J = 7.0 Hz, 2H), 3.79 (s, 3H), 1.44 (t,J = 7.0 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 6.71 (d, J = 8.4 Hz, 1H), 6.31 (d, J = 2.5 Hz, 1H), 6.23 (dd, J = 8.4, 2.6 Hz, 1H), 4.04 (q, J = 7.0 Hz, 2H), 3.79 (s, 3H), 1.44 (t, J = 7.0 Hz, 3H).
MS (ESI, pos.ion) m/z: 168.20 [M+H]+ .MS (ESI, pos.ion) m/z: 168.20 [M+H] + .
步驟5:化合物3-乙氧基-4-甲氧基碘苯的合成Step 5: Synthesis of compound 3-ethoxy-4-methoxyiodobenzene
將化合物3-乙氧基-4-甲氧基苯胺 (6.40 g, 38.28 mmol),溶於1,4-二氧六環(30 mL)和水(11 mL)中,冷卻至0℃,加入濃鹽酸(9.7 mL, 36% aq),攪拌均勻,冷卻至-15℃,逐滴加入亞硝酸鈉(2.91 g, 42.11 mmol)和水(7 mL)的溶液,控制滴加溫度在-15℃至-5℃之間,滴畢回溫至-5℃攪拌30 min後,加入碘化鉀 (8.26 g, 49.76 mmol) 和水 (12 mL) 的溶液,控制滴加溫度在-15℃至-5℃之間,滴畢回溫至0℃下攪拌2 h. 加入亞硫酸氫鈉(1.99 g, 19.14 mmol) 攪拌1 h淬滅反應,向反應液加入石油醚(200 mL),有機相用水洗滌(100 mL ×3),用無水Na2 SO4乾燥,減壓濃縮得黃色黏稠液體。矽膠柱層析(石油醚/乙酸乙酯(v/v)= 10/1)純化得白色固體 (6.24 g , 產率58.62% )Compound 3-ethoxy-4-methoxyaniline (6.40 g, 38.28 mmol) was dissolved in 1,4-dioxane (30 mL) and water (11 mL), cooled to 0 °C, added Concentrated hydrochloric acid (9.7 mL, 36% aq), stirred evenly, cooled to -15 °C, and a solution of sodium nitrite (2.91 g, 42.11 mmol) and water (7 mL) was added dropwise, and the dropwise temperature was controlled at -15 °C After dropping to -5°C, return to -5°C and stir for 30 min, then add a solution of potassium iodide (8.26 g, 49.76 mmol) and water (12 mL), and control the dropwise temperature at -15°C to -5°C. After dropping, the temperature was returned to 0 °C and stirred for 2 h. Sodium bisulfite (1.99 g, 19.14 mmol) was added and stirred for 1 h to quench the reaction. Petroleum ether (200 mL) was added to the reaction solution, and the organic phase was washed with water ( 100 mL × 3), dried over anhydrous Na 2 SO 4 , and concentrated under reduced pressure to obtain a yellow viscous liquid. Silica gel column chromatography (petroleum ether/ethyl acetate (v/v) = 10/1) was purified to give a white solid (6.24 g, yield 58.62%)
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.21 (dd,J = 8.4, 1.9 Hz, 1H), 7.12 (d,J = 1.8 Hz, 1H), 6.62 (d,J = 8.4 Hz, 1H), 4.06 (q,J = 7.0 Hz, 2H), 3.84 (s, 3H), 1.46 (t,J = 7.0 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.21 (dd, J = 8.4, 1.9 Hz, 1H), 7.12 (d, J = 1.8 Hz, 1H), 6.62 (d, J = 8.4 Hz, 1H), 4.06 (q, J = 7.0 Hz, 2H), 3.84 (s, 3H), 1.46 (t, J = 7.0 Hz, 3H).
GC-MS: m/z 278.00 [M]+ .GC-MS: m/z 278.00 [M] + .
步驟6:化合物2-(3-乙氧基-4-甲氧基苯基)-4,4,5,5-四甲基-1,3,2-二惡唑烷的合成Step 6: Synthesis of compound 2-(3-ethoxy-4-methoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxazolidine
將3-乙氧基-4-甲氧基碘苯 (1277-6) (6.24 g, 22.44 mmol),聯硼酸頻那醇酯 (5.70 g, 22.44 mmol),醋酸鉀 (3.30 g, 33.66 mmol), 醋酸鈀 (0.25 g, 1.12 mmol) , 2-二環己基磷-2’-甲基聯苯(Mephos) (0.82 g, 2.24 mmol) 混合在N ,N -二甲基甲醯胺 (36 mL) 中,氮氣保護下80℃攪拌6 h。冷卻至室溫,反應液中加入水 (100 mL),用石油醚 (100 mL × 3)萃取反應液,合併有機相,無水硫酸鈉乾燥有機相,減壓旋蒸濃縮得到褐色液體,矽膠柱層析 (石油醚/乙酸乙酯(v/v)=10/1) 純化得黃色固體 (4.85 g, 產率:77.70%)3-Ethoxy-4-methoxyiodobenzene (1277-6) (6.24 g, 22.44 mmol), pinacol diboronate (5.70 g, 22.44 mmol), potassium acetate (3.30 g, 33.66 mmol) , palladium acetate (0.25 g, 1.12 mmol), 2-dicyclohexylphosphorus-2'-methylbiphenyl (Mephos) (0.82 g, 2.24 mmol) were mixed in N , N -dimethylformamide (36 mL) ), stirred at 80 °C for 6 h under nitrogen protection. Cooled to room temperature, water (100 mL) was added to the reaction solution, the reaction solution was extracted with petroleum ether (100 mL × 3), the organic phases were combined, the organic phases were dried over anhydrous sodium sulfate, and concentrated by rotary evaporation under reduced pressure to obtain a brown liquid. Chromatography (petroleum ether/ethyl acetate (v/v)=10/1) was purified to give a yellow solid (4.85 g, yield: 77.70%)
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.41 (d,J = 8.0 Hz, 1H), 7.29 (s, 1H), 6.88 (d,J = 8.0 Hz, 1H), 4.15 (q,J = 7.0 Hz, 2H), 3.89 (s, 3H), 1.47 (t,J = 7.0 Hz, 3H), 1.33 (s, 12H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.41 (d, J = 8.0 Hz, 1H), 7.29 (s, 1H), 6.88 (d, J = 8.0 Hz, 1H), 4.15 (q, J = 7.0 Hz, 2H), 3.89 (s, 3H), 1.47 (t, J = 7.0 Hz, 3H), 1.33 (s, 12H).
MS (ESI, pos.ion) m/z: 279.35 [M+H]+ .MS (ESI, pos.ion) m/z: 279.35 [M+H] + .
步驟7:化合物 (R )-1-叔丁基 2-甲基 4-(3-乙氧基-4-甲氧基苯基)-1H -吡咯-1,2(2H , 5H )-二甲酸酯的合成Step 7: Compound (R) -1- tert-butyl 2-methyl-4- (3-ethoxy-4-methoxyphenyl) -1 H - pyrrole -1,2 (2 H, 5 H) - Synthesis of Diformate
將化合物2-(3-乙氧基-4-甲氧基苯基)-4,4,5,5-四甲基-1,3,2-二氧雜硼烷 (2.0 g, 7.19 mmol),(R )-1-叔丁基 2-甲基 4-(((三氟甲基)磺醯基)氧基)1H -吡咯-1, 2(2H , 5H )-二羧酸酯 (2.7 g, 7.19 mmol),N -甲基嗎啡啉 (1.6 g, 15.82 mmol),二環己基-[2-(2-甲基苯基)苯基]膦 (0.13 g, 0.36 mmol) 和醋酸鈀 (0.04 g, 0.18 mmol) 混合甲苯 (10 mL) 和水 (5 mL) 混合溶液中,氮氣保護下80℃反應1 h,停止反應,冷卻至室溫。向反應液加入水 (50 mL),用乙酸乙酯 (30 mL × 3) 萃取,有機相合用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱層析分離 (洗脫劑:PE/EtOAc ( v/v ) = 5/1) 得到黃色液體 (1.1 g, 產率44%)。Compound 2-(3-ethoxy-4-methoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane (2.0 g, 7.19 mmol) , ( R )-1-tert-butyl 2-methyl 4-(((trifluoromethyl)sulfonyl)oxy) 1H -pyrrole- 1,2(2H , 5H )-dicarboxylic acid ester (2.7 g, 7.19 mmol), N -methylmorpholine (1.6 g, 15.82 mmol), dicyclohexyl-[2-(2-methylphenyl)phenyl]phosphine (0.13 g, 0.36 mmol) and Palladium acetate (0.04 g, 0.18 mmol) was mixed with a mixed solution of toluene (10 mL) and water (5 mL), and reacted at 80 °C for 1 h under nitrogen protection. The reaction was stopped and cooled to room temperature. Water (50 mL) was added to the reaction solution, extracted with ethyl acetate (30 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was separated by silica gel column chromatography (eluent: PE/EtOAc ( v/v ) = 5/1) to give a yellow liquid (1.1 g, 44% yield).
MS (ESI, pos.ion) m/z: 278.18 [M-Boc +2H]+ .MS (ESI, pos.ion) m/z: 278.18 [M- Boc +2H] + .
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.93 – 6.88 (m, 2H), 6.85 – 6.81 (m, 1H), 5.94 – 5.89 (m, 1H), 5.18 – 5.08 (m, 1H), 4.66 – 4.47 (m, 2H), 4.14 – 4.07 (m, 2H), 3.88 (s, 3H), 3.75 (d,J = 4.8 Hz, 3H), 1.52 (s, 3H), 1.50 – 1.43 (m, 9H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.93 – 6.88 (m, 2H), 6.85 – 6.81 (m, 1H), 5.94 – 5.89 (m, 1H), 5.18 – 5.08 (m, 1H), 4.66 – 4.47 (m, 2H), 4.14 – 4.07 (m, 2H), 3.88 (s, 3H), 3.75 (d, J = 4.8 Hz, 3H), 1.52 (s, 3H), 1.50 – 1.43 (m, 9H).
步驟8:化合物 (2R , 4S )-1-叔丁基 2-甲基 4-(3-乙氧基-4-甲氧基苯基)吡咯烷-1,2-二甲酸酯的合成Step 8: Compound ( 2R , 4S )-1-tert-butyl 2-methyl 4-(3-ethoxy-4-methoxyphenyl)pyrrolidine-1,2-dicarboxylate synthesis
將化合物 (R )-1-叔丁基 2-甲基 4-(3-乙氧基-4-甲氧基苯基)-1H -吡咯-1,2(2H , 5H )-二甲酸酯 (2.0 g, 5.30 mmol),鈀碳 (0.45 g, 5.30 mmol) 溶於35 mL甲醇中,在氫氣氛圍下,室溫反應12 h,原料反應完全,停止反應。用矽藻土過濾鈀碳,有機相減壓濃縮得到黃色液體 (1.26 g, 產率63%)。The compound (R) -1- tert-butyl 2-methyl-4- (3-ethoxy-4-methoxyphenyl) -1 H - pyrrole -1,2 (2 H, 5 H) - two Formate (2.0 g, 5.30 mmol) and palladium on carbon (0.45 g, 5.30 mmol) were dissolved in 35 mL of methanol, and reacted at room temperature for 12 h under a hydrogen atmosphere. The reaction of the raw materials was complete and the reaction was stopped. The palladium carbon was filtered through celite, and the organic phase was concentrated under reduced pressure to obtain a yellow liquid (1.26 g, yield 63%).
MS (ESI, pos.ion) m/z: 280.20 [M-Boc +2H]+ .MS (ESI, pos.ion) m/z: 280.20 [M- Boc +2H] + .
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.82 – 6.74 (m, 3H), 4.39 – 4.29 (m, 1H), 4.11 – 4.05 (m, 2H), 4.02 – 4.00 (m, 0.5H), 3.95 – 3.89 (m, 0.5H), 3.85 (s, 3H), 3.76 (d,J = 7.0 Hz, 3H), 3.44 – 3.37 (m, 1H), 3.33 – 3.24 (m, 1H), 2.66 – 2.57 (m, 1H), 2.07 – 1.96 (m, 1H), 1.50 – 1.44 (m, 6H), 1.43 (s, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.82 – 6.74 (m, 3H), 4.39 – 4.29 (m, 1H), 4.11 – 4.05 (m, 2H), 4.02 – 4.00 (m, 0.5H) , 3.95 – 3.89 (m, 0.5H), 3.85 (s, 3H), 3.76 (d, J = 7.0 Hz, 3H), 3.44 – 3.37 (m, 1H), 3.33 – 3.24 (m, 1H), 2.66 – 2.57 (m, 1H), 2.07 – 1.96 (m, 1H), 1.50 – 1.44 (m, 6H), 1.43 (s, 6H).
步驟9:化合物 (2R , 4S )-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸甲酯鹽酸鹽的合成Step 9: Synthesis of Compound (2R , 4S )-4-(3-ethoxy-4-methoxyphenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物(2R , 4S )-1-叔丁基 2-甲基 4-(3-乙氧基-4-甲氧基苯基)吡咯烷-1,2-二甲酸酯 (1.2 g, 3.16 mmol) 溶於10 mL 二氯甲烷溶液中,再加入鹽酸1, 4-二氧六環溶液 (3.94 mL, 4.01 mol/L),室溫反應1.5 h,原料反應完全,停止反應。減壓蒸餾,除去溶劑,濃縮液得到黃色液體 (1.02 g, 產率100%)。Compound (2 R , 4 S )-1-tert-butyl 2-methyl 4-(3-ethoxy-4-methoxyphenyl)pyrrolidine-1,2-dicarboxylate (1.2 g , 3.16 mmol) was dissolved in 10 mL of dichloromethane solution, and then hydrochloric acid 1,4-dioxane solution (3.94 mL, 4.01 mol/L) was added, and the reaction was carried out at room temperature for 1.5 h. The reaction of the raw materials was completed and the reaction was stopped. The solvent was removed by distillation under reduced pressure, and the concentrated solution gave a yellow liquid (1.02 g, yield 100%).
MS (ESI, pos.ion) m/z: 280.30 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 280.30 [M+H-HCl] + .
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.86 (s, 1H), 6.79 (s, 2H), 4.67 – 4.57 (m, 1H), 4.09 (q,J = 6.9 Hz, 2H), 3.90 – 3.78 (m, 1H), 3.83 (s, 6H), 3.64 – 3.52 (m, 2H), 2.82 – 2.70 (m, 1H), 2.28 – 2.17 (m, 1H), 1.44 (t,J = 6.9 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.86 (s, 1H), 6.79 (s, 2H), 4.67 – 4.57 (m, 1H), 4.09 (q, J = 6.9 Hz, 2H), 3.90 – 3.78 (m, 1H), 3.83 (s, 6H), 3.64 – 3.52 (m, 2H), 2.82 – 2.70 (m, 1H), 2.28 – 2.17 (m, 1H), 1.44 (t, J = 6.9 Hz , 3H).
MS (ESI, pos.ion) m/z: 280.30 [M+H-HCl]+ .MS (ESI, pos.ion) m/z: 280.30 [M+H-HCl] + .
步驟10:化合物 (2R , 4S )-1-乙醯基-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸甲酯的合成Step 10: Compound (2 R, 4 S) -1- acetyl-4- (3-ethoxy-4-methoxyphenyl) pyrrolidine-2-carboxylic acid methyl ester Synthesis of
將化合物 (2R , 4S )-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸甲酯鹽酸鹽 (1.0 g, 3.17 mmol) 溶於20 mL二氯甲烷溶液中,在冰浴條件下,加入N ,N -二異丙基乙胺 (1.64 g, 12.68 mmol),乙醯氯 (0.50 g, 6.34 mmol),室溫反應1 h,原料反應完全,停止反應。加入飽和食鹽水淬滅反應,用二氯甲烷萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,除去溶劑,濃縮液進行矽膠柱分離 (洗脫劑:DCM/MeOH ( v/v ) = 20/1) 得到黃色液體 (0.95 g, 產率93%)。Compound ( 2R , 4S )-4-(3-ethoxy-4-methoxyphenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride (1.0 g, 3.17 mmol) was dissolved in 20 mL of In the methyl chloride solution, under ice bath conditions, N , N -diisopropylethylamine (1.64 g, 12.68 mmol), acetyl chloride (0.50 g, 6.34 mmol) were added, and the reaction was carried out at room temperature for 1 h, and the reaction of the raw materials was complete. , stop the reaction. Saturated brine was added to quench the reaction, extracted with dichloromethane (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, the solvent was removed, and the concentrated solution was subjected to silica gel column separation (eluent: DCM/MeOH (v/v) = 20/1) to give a yellow liquid (0.95 g, 93% yield).
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.84 – 6.80 (m, 1H), 6.79 – 6.77 (m, 1H), 6.76 – 6.74 (m, 1H), 4.46 (dd,J = 9.7, 7.5 Hz, 1H), 4.08 (q,J = 6.9 Hz, 2H), 3.93 – 3.89 (m, 1H), 3.85 (s, 3H), 3.76 (s, 3H), 3.59 (t,J = 10.5 Hz, 1H), 3.42 – 3.33 (m, 1H), 2.66 – 2.60 (m, 1H), 2.10 (s, 3H), 2.05 – 2.00 (m, 1H), 1.46 (t,J = 7.0 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.84 – 6.80 (m, 1H), 6.79 – 6.77 (m, 1H), 6.76 – 6.74 (m, 1H), 4.46 (dd, J = 9.7, 7.5 Hz, 1H), 4.08 (q, J = 6.9 Hz, 2H), 3.93 – 3.89 (m, 1H), 3.85 (s, 3H), 3.76 (s, 3H), 3.59 (t, J = 10.5 Hz, 1H) ), 3.42 – 3.33 (m, 1H), 2.66 – 2.60 (m, 1H), 2.10 (s, 3H), 2.05 – 2.00 (m, 1H), 1.46 (t, J = 7.0 Hz, 3H).
MS (ESI, pos.ion) m/z: 322.20 [M+H]+ .MS (ESI, pos.ion) m/z: 322.20 [M+H] + .
步驟11:化合物 (2R , 4S )-1-乙醯基-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸的合成Step 11: Compound (2 R, 4 S) -1- acetyl-4- (3-ethoxy-4-methoxyphenyl) pyrrolidine-2-carboxylic acid Synthesis of
將化合物 (2R , 4S )-1-乙醯基-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸甲酯溶於四氫呋喃 (8 mL) 和水 (4 mL) 的混合溶劑中,加入氫氧化鋰 (59 mg, 2.48 mmol),置於50℃加熱條件下反應1.5 h。原料反應完全,停止反應,加入飽和食鹽水 (30 mL),用稀鹽酸調節pH=3,用乙酸乙酯萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮,得黃色固體 (320 mg, 產率84%)。Compound (2 R, 4 S) -1- acetyl-4- (3-ethoxy-4-methoxyphenyl) pyrrolidine-2-carboxylate was dissolved in tetrahydrofuran (8 mL) and water (4 mL) of mixed solvent, lithium hydroxide (59 mg, 2.48 mmol) was added, and the reaction was carried out under heating at 50 °C for 1.5 h. The reaction of the raw materials was completed, the reaction was stopped, saturated brine (30 mL) was added, pH=3 was adjusted with dilute hydrochloric acid, extracted with ethyl acetate (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a yellow Solid (320 mg, 84% yield).
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.85 – 6.81 (m, 1H), 6.80 (s, 1H), 6.78 – 6.76 (m, 1H), 4.58 (t,J = 8.6 Hz, 1H), 4.13 – 4.07 (m, 2H), 3.96 – 3.92 (m, 1H), 3.86 (s, 3H), 3.54 (t,J = 10.6 Hz, 1H), 3.39 – 3.31 (m, 1H), 2.69 – 2.62 (m, 1H), 2.39 – 2.30 (m, 1H), 2.18 (s, 3H), 1.47 (t,J = 7.0 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.85 – 6.81 (m, 1H), 6.80 (s, 1H), 6.78 – 6.76 (m, 1H), 4.58 (t, J = 8.6 Hz, 1H) , 4.13 – 4.07 (m, 2H), 3.96 – 3.92 (m, 1H), 3.86 (s, 3H), 3.54 (t, J = 10.6 Hz, 1H), 3.39 – 3.31 (m, 1H), 2.69 – 2.62 (m, 1H), 2.39 – 2.30 (m, 1H), 2.18 (s, 3H), 1.47 (t, J = 7.0 Hz, 3H).
MS (ESI, pos.ion) m/z: 308.15 [M+H]+ .MS (ESI, pos.ion) m/z: 308.15 [M+H] + .
步驟12:化合物 (2R , 4S )-(6-((4-((叔丁基二甲基矽烷基)氧基)苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸酯的合成Step 12: Compound ( 2R , 4S )-(6-((4-((tert-butyldimethylsilyl)oxy)phenyl)(methyl)carbamoyl)pyridin-2-yl ) Synthesis of methyl 1-acetyl-4-(3-ethoxy-4-methoxyphenyl) pyrrolidine-2-carboxylate
將化合物 (2R , 4S )-1-乙醯基-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸 (140 mg, 0.44 mmol) 、N -(4-((叔丁基二甲基矽烷基)氧基)苯基)-6-(羥甲基)-N -甲基吡啶甲醯胺 (150 mg, 0.40 mmol) 和1-羥基-7-氮雜苯並三唑 (110 mg, 0.80 mmol) 溶於二氯甲烷 (20 mL)溶液中,冰浴條件下,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亞胺鹽酸鹽 (150 mg, 0. 80 mmol) 和N ,N -二異丙基乙胺 (160 mg, 1.20 mmol),室溫下反應18 h,原料反應完全,停止反應,加入飽和食鹽水 (50 mL),用二氯甲烷萃取 (30 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮矽膠柱層析 (洗脫劑:PE/EtOAc ( v/v ) = 1/1) 分離提純,得淡黃色液體 (200 mg, 產率76%)。Compound (2 R, 4 S) -1- acetyl-4- (3-ethoxy-4-methoxyphenyl) pyrrolidine-2-carboxylic acid (140 mg, 0.44 mmol), N - ( 4-((tert-Butyldimethylsilyl)oxy)phenyl)-6-(hydroxymethyl) -N -picolinamide (150 mg, 0.40 mmol) and 1-hydroxy-7- Azabenzotriazole (110 mg, 0.80 mmol) was dissolved in dichloromethane (20 mL) solution, and 1-(3-dimethylaminopropyl)-3-ethylcarbodie Imine hydrochloride (150 mg, 0.80 mmol) and N , N -diisopropylethylamine (160 mg, 1.20 mmol) were reacted at room temperature for 18 h, the reaction of the raw materials was complete, the reaction was stopped, and saturated common salt was added Water (50 mL), extracted with dichloromethane (30 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure for silica gel column chromatography (eluent: PE/EtOAc (v/v) = 1/1 ) was separated and purified to obtain pale yellow liquid (200 mg, yield 76%).
1 H NMR (400 MHz, CDCl3 ) δ (ppm)7.58 – 7.55 (m, 1H), 7.33 (d,J = 8.2 Hz, 1H), 7.22 (d,J = 6.7 Hz, 1H), 6.91 – 6.89 (m, 2H), 6.84 – 6.79 (m, 2H), 6.77 – 6.76 (m, 1H), 6.63 (d,J = 7.9 Hz, 2H), 5.26 – 5.23 (m, 1H), 5.07 – 5.03 (m, 1H), 4.56 – 4.52 (m, 1H), 4.14 – 4.05 (m, 3H), 3.95 – 3.90 (m, 1H), 3.86 (s, 3H), 3.60 (t,J = 10.5 Hz, 1H), 3.46 (s, 3H), 3.40 – 3.37 (m, 1H), 2.70 – 2.64 (m, 1H), 2.11 (s, 3H), 1.46 (t,J = 7.0 Hz, 3H), 0.93 (s, 9H), 0.13 (s, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 7.58 – 7.55 (m, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.22 (d, J = 6.7 Hz, 1H), 6.91 – 6.89 (m, 2H), 6.84 – 6.79 (m, 2H), 6.77 – 6.76 (m, 1H), 6.63 (d, J = 7.9 Hz, 2H), 5.26 – 5.23 (m, 1H), 5.07 – 5.03 (m , 1H), 4.56 – 4.52 (m, 1H), 4.14 – 4.05 (m, 3H), 3.95 – 3.90 (m, 1H), 3.86 (s, 3H), 3.60 (t, J = 10.5 Hz, 1H), 3.46 (s, 3H), 3.40 – 3.37 (m, 1H), 2.70 – 2.64 (m, 1H), 2.11 (s, 3H), 1.46 (t, J = 7.0 Hz, 3H), 0.93 (s, 9H) , 0.13 (s, 6H).
MS (ESI, pos.ion) m/z: 662.20 [M+H]+ .MS (ESI, pos.ion) m/z: 662.20 [M+H] + .
步驟13: (2R , 4S )-(6-((4-羥基苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-乙氧基-4-甲氧基苯基)吡咯烷-2-甲酸酯的合成Step 13: ( 2R , 4S )-(6-((4-hydroxyphenyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3- Synthesis of Ethoxy-4-methoxyphenyl)pyrrolidine-2-carboxylate
將化合物 (2R , 4S )-(6-((4-((叔丁基二甲基矽烷基)氧基)苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-乙氧基-4-甲氧基苯基) 吡咯烷-2-甲酸酯 (200 mg, 0.30 mmol) 溶於四氫呋喃 (10 mL) 中,加入四丁基氟化銨溶液 (0.60 mL, 1.0 mmol/L),室溫反應1 h。原料反應完全,停止反應,加入飽和食鹽水 (30 mL),用乙酸乙酯萃取 (20 mL × 3),有機相用無水硫酸鈉乾燥,減壓濃縮矽膠柱層析 (洗脫劑:DCM/MeOH (v/v) = 20/1) 分離提純,得白色固體 (136 mg, 產率82%)。Compound (2 R , 4 S )-(6-((4-((tert-butyldimethylsilyl)oxy)phenyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-Acetyl-4-(3-ethoxy-4-methoxyphenyl)pyrrolidine-2-carboxylate (200 mg, 0.30 mmol) was dissolved in tetrahydrofuran (10 mL) and added with tetrahydrofuran Butylammonium fluoride solution (0.60 mL, 1.0 mmol/L) was reacted at room temperature for 1 h. The reaction of the raw materials was completed, the reaction was stopped, saturated brine (30 mL) was added, extracted with ethyl acetate (20 mL × 3), the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure for silica gel column chromatography (eluent: DCM/ MeOH (v/v) = 20/1) was isolated and purified to obtain a white solid (136 mg, 82% yield).
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 7.54 (t,J = 7.7 Hz, 1H), 7.36 (d,J = 7.7 Hz, 1H), 7.22 (d,J = 7.6 Hz, 1H), 6.88 – 6.84 (m, 2H), 6.82 – 6.77 (m, 2H), 6.76 – 6.74 (m, 1H), 6.72 – 6.66 (m, 2H), 5.11 – 5.06 (m, 1H), 4.96 – 4.93 (m, 1H), 4.55 (t,J = 8.6 Hz, 1H), 4.08 (q,J = 7.0 Hz, 2H), 3.96 – 3.91 (m, 1H), 3.85 (s, 3H), 3.62 (t,J = 10.5 Hz, 1H), 3.46 (s, 3H), 3.41 – 3.37 (m, 1H), 2.72 – 2.66 (m, 1H), 2.14 (s, 3H), 2.08 – 2.01 (m, 1H), 1.46 (t,J = 7.0 Hz, 3H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 7.54 (t, J = 7.7 Hz, 1H), 7.36 (d, J = 7.7 Hz, 1H), 7.22 (d, J = 7.6 Hz, 1H), 6.88 – 6.84 (m, 2H), 6.82 – 6.77 (m, 2H), 6.76 – 6.74 (m, 1H), 6.72 – 6.66 (m, 2H), 5.11 – 5.06 (m, 1H), 4.96 – 4.93 (m , 1H), 4.55 (t, J = 8.6 Hz, 1H), 4.08 (q, J = 7.0 Hz, 2H), 3.96 – 3.91 (m, 1H), 3.85 (s, 3H), 3.62 (t, J = 10.5 Hz, 1H), 3.46 (s, 3H), 3.41 – 3.37 (m, 1H), 2.72 – 2.66 (m, 1H), 2.14 (s, 3H), 2.08 – 2.01 (m, 1H), 1.46 (t , J = 7.0 Hz, 3H).
MS (ESI, pos.ion) m/z: 548.70 [M+H]+ .MS (ESI, pos.ion) m/z: 548.70 [M+H] + .
實施例172:(2R ,4S )- (6-((4-羥基苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-異丙氧基-4-甲氧基苯基)吡咯烷-2-甲酸酯 Example 172: ( 2R , 4S )-(6-((4-hydroxyphenyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3 -Isopropoxy-4-methoxyphenyl)pyrrolidine-2-carboxylate
步驟1:化合物3-異丙氧基-4-甲氧基苯甲酸甲酯的合成Step 1: Synthesis of compound 3-isopropoxy-4-methoxybenzoic acid methyl ester
將化合物 3-羥基-4-甲氧基苯甲酸甲酯 (5.00 g, 27.45 mmol),異丙基碘 (7.00 g, 41.17 mmol) 和碳酸鉀 (11.83 g, 82.35 mmol) 混合在N ,N -二甲基甲醯胺 (25 mL) 中,置於80 ℃下攪拌10 h。加入乙酸乙酯 (100 mL) 稀釋,有機相用飽和食鹽水 (100 mL × 3) 洗滌,再用無水硫酸鈉乾燥,減壓濃縮得到黃色液體 (5.48 g, 產率89.02 %).The compound 3-hydroxy-4-methoxybenzoic acid methyl ester (5.00 g, 27.45 mmol), isopropyl iodide (7.00 g, 41.17 mmol) and potassium carbonate (11.83 g, 82.35 mmol) were mixed in N , N - dimethylformamide (25 mL), and stirred at 80 °C for 10 h. Ethyl acetate (100 mL) was added for dilution, the organic phase was washed with saturated brine (100 mL × 3), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a yellow liquid (5.48 g, yield 89.02 %).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.62 (dd,J = 8.5, 1.8 Hz, 1H), 7.53 (d,J = 1.7 Hz, 1H), 6.85 (d,J = 8.5 Hz, 1H), 4.60 – 4.54 (m, 1H), 3.86 (s, 3H), 3.84 (s, 3H), 1.35 (d,J = 6.1 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.62 (dd, J = 8.5, 1.8 Hz, 1H), 7.53 (d, J = 1.7 Hz, 1H), 6.85 (d, J = 8.5 Hz, 1H), 4.60 – 4.54 (m, 1H), 3.86 (s, 3H), 3.84 (s, 3H), 1.35 (d, J = 6.1 Hz, 6H).
MS (ESI, pos.ion) m/z: 225.20 [M+H]+ .MS (ESI, pos.ion) m/z: 225.20 [M+H] + .
步驟2:化合物3-異丙氧基-4-甲氧基苯甲酸的合成Step 2: Synthesis of compound 3-isopropoxy-4-methoxybenzoic acid
將化合物3-異丙氧基-4-甲氧基苯甲酸甲酯 (11.05 g, 49.28 mmol),氫氧化鈉 (3.94 g, 98.56 mmol),乙醇 (30 mL),水 (10 mL) 混合均勻,置於50 ℃攪拌2 h。滴入稀鹽酸 (4 M/L) 調節pH=1,加入水 (100 mL),攪拌 (析出大量固體),過濾,所得濾餅用水 (50 mL×3) 洗滌,真空60 ℃乾燥12 h,得白色固體 (10.36 g, 產率100 %)。Compound 3-isopropoxy-4-methoxybenzoic acid methyl ester (11.05 g, 49.28 mmol), sodium hydroxide (3.94 g, 98.56 mmol), ethanol (30 mL), water (10 mL) were mixed uniformly , and stirred at 50 °C for 2 h. Dilute hydrochloric acid (4 M/L) was added dropwise to adjust pH=1, water (100 mL) was added, stirred (a large amount of solid was precipitated), filtered, and the obtained filter cake was washed with water (50 mL×3), dried in vacuum at 60 °C for 12 h, A white solid (10.36 g, 100 % yield) was obtained.
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.76 (dd,J = 8.5, 1.9 Hz, 1H), 7.62 (d,J = 1.8 Hz, 1H), 6.92 (d,J = 8.5 Hz, 1H), 4.66 – 4.60 (m, 1H), 3.93 (s, 3H), 1.40 (d,J = 6.1 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.76 (dd, J = 8.5, 1.9 Hz, 1H), 7.62 (d, J = 1.8 Hz, 1H), 6.92 (d, J = 8.5 Hz, 1H), 4.66 – 4.60 (m, 1H), 3.93 (s, 3H), 1.40 (d, J = 6.1 Hz, 6H).
MS (ESI, pos.ion) m/z: 211.15 [M+H]+ .MS (ESI, pos.ion) m/z: 211.15 [M+H] + .
步驟3:化合物苄基 (3-異丙氧基-4-甲氧基苯基)氨基甲酸酯的合成Step 3: Synthesis of compound benzyl(3-isopropoxy-4-methoxyphenyl)carbamate
步驟一:室溫下,向兩口瓶 (A瓶) 中加入3-異丙氧基-4-甲氧基苯甲酸 (10.30 g, 48.99 mmol ),三乙胺 (6.44 g, 663.69 mmol ),甲苯 (50 ml) ,置於冰浴中攪拌,緩慢滴加疊氮磷酸二苯酯 (DPPA) (14.83 g, 53.89 mmol ),室溫中攪拌2 h。Step 1: at room temperature, add 3-isopropoxy-4-methoxybenzoic acid (10.30 g, 48.99 mmol), triethylamine (6.44 g, 663.69 mmol), toluene (50 ml), placed in an ice bath and stirred, slowly added dropwise diphenylphosphoryl azide (DPPA) (14.83 g, 53.89 mmol), and stirred at room temperature for 2 h.
步驟二:向另一個三口瓶 (B瓶) 加入甲苯 (50 ml) ,苯甲醇 (5.83 g, 53.89 mmol ),加熱至110 ℃,把A瓶物料滴入B瓶中,滴畢恆溫攪拌2 h。停止加熱攪拌,冷至室溫,反應液用水洗滌 (100 mL),再用5 %氫氧化鈉水溶液洗滌 (100 mL×3),用無水Na2 SO4 乾燥,減壓濃縮得黃色固體。向粗品加入石油醚/乙酸乙酯 (50 mL, v/v = 10:1) 攪拌3 h, 過濾得白色固體 (11.34 g, 產率73.40 %).Step 2: Add toluene (50 ml) and benzyl alcohol (5.83 g, 53.89 mmol) to another three-necked bottle (B bottle), heat to 110 °C, drop the material from bottle A into bottle B, and stir at constant temperature for 2 h after dropping . The heating and stirring were stopped, cooled to room temperature, the reaction solution was washed with water (100 mL), then washed with 5% aqueous sodium hydroxide solution (100 mL×3), dried over anhydrous Na 2 SO 4 , and concentrated under reduced pressure to obtain a yellow solid. Petroleum ether/ethyl acetate (50 mL, v/v = 10:1) was added to the crude product, stirred for 3 h, and filtered to obtain a white solid (11.34 g, yield 73.40 %).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.47 – 7.30 (m, 5H), 7.20 – 7.11 (m, 1H), 6.86 – 6.72 (m, 2H), 6.56 (s, 1H), 5.19 (s, 2H), 3.82 (s, 3H), 1.36 (d,J = 5.9 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.47 – 7.30 (m, 5H), 7.20 – 7.11 (m, 1H), 6.86 – 6.72 (m, 2H), 6.56 (s, 1H), 5.19 (s, 2H), 3.82 (s, 3H), 1.36 (d, J = 5.9 Hz, 6H).
MS (ESI, pos.ion) m/z: 316.20 [M+H]+ .MS (ESI, pos.ion) m/z: 316.20 [M+H] + .
步驟4:化合物3-異丙氧基-4-甲氧基苯胺的合成Step 4: Synthesis of compound 3-isopropoxy-4-methoxyaniline
向高壓釜中加入苄基 (3-異丙氧基-4-甲氧基苯基)氨基甲酸酯 (11.34 g, 35.96 mmol ),10 %鈀碳 (0.51 g, 0.54 mmol ),甲醇 (40 mL),置換氫氣 (0.5 MPa),室溫攪拌2 h。反應液通過矽藻土過濾,收集濾液經減壓濃縮得褐色固體 (6.52 g , 產率100 % )。To the autoclave was added benzyl(3-isopropoxy-4-methoxyphenyl)carbamate (11.34 g, 35.96 mmol), 10% palladium on carbon (0.51 g, 0.54 mmol), methanol (40 mL), replaced with hydrogen (0.5 MPa), and stirred at room temperature for 2 h. The reaction solution was filtered through celite, and the filtrate was collected and concentrated under reduced pressure to obtain a brown solid (6.52 g, yield 100%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 6.71 (d,J = 8.4 Hz, 1H), 6.33 (d,J = 2.4 Hz, 1H), 6.24 (dd,J = 8.4, 2.5 Hz, 1H), 4.49 – 4.43 (p,J = 6.1 Hz, 1H), 3.77 (s, 3H), 1.34 (d,J = 6.1 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 6.71 (d, J = 8.4 Hz, 1H), 6.33 (d, J = 2.4 Hz, 1H), 6.24 (dd, J = 8.4, 2.5 Hz, 1H), 4.49 – 4.43 (p, J = 6.1 Hz, 1H), 3.77 (s, 3H), 1.34 (d, J = 6.1 Hz, 6H).
MS (ESI, pos.ion) m/z: 182.20 [M+H]+ .MS (ESI, pos.ion) m/z: 182.20 [M+H] + .
步驟5:化合物3-異丙氧基-4-甲氧基碘苯的合成Step 5: Synthesis of compound 3-isopropoxy-4-methoxyiodobenzene
將化合物3-異丙氧基-4-甲氧基苯胺 (6.90 g, 38.07 mmol),溶於1, 4-二氧六環 (30 mL)和水 (11 mL) 中,冷卻至0 ℃,加入濃鹽酸 (9.6 mL, 36% aq),攪拌均勻,冷卻至-15 ℃,逐滴加入亞硝酸鈉 (2.89 g, 41.88 mmol) 和水 (7 mL) 的溶液,控制滴加溫度在-15 ℃至-5 ℃之間,滴畢回溫至-5 ℃攪拌30 min後,加入碘化鉀 (8.22 g, 49.49 mmol) 和水 (12 mL) 的溶液,控制滴加溫度在-15 ℃至-5 ℃之間,滴畢回溫至0 ℃下攪拌2 h. 加入亞硫酸氫鈉 (1.98 g, 19.04 mmol) 攪拌1 h淬滅反應,向反應液加入石油醚 (200 mL),有機相用水洗滌(100 mL × 3),用無水Na2 SO4 乾燥,減壓濃縮得黃色黏稠液體。矽膠柱層析 (洗脫劑:石油醚/乙酸乙酯(v/v)=10/1) 純化得白色固體 (7.83 g, 產率70.41 % )Compound 3-isopropoxy-4-methoxyaniline (6.90 g, 38.07 mmol) was dissolved in 1,4-dioxane (30 mL) and water (11 mL), cooled to 0 °C, Add concentrated hydrochloric acid (9.6 mL, 36% aq), stir well, cool to -15 °C, add a solution of sodium nitrite (2.89 g, 41.88 mmol) and water (7 mL) dropwise, control the dropwise temperature at -15 Between ℃ and -5 ℃, return to -5 ℃ and stir for 30 min after dropping, add a solution of potassium iodide (8.22 g, 49.49 mmol) and water (12 mL), and control the dropwise temperature at -15 ℃ to -5 ℃. After dropping, the temperature was returned to 0 °C and stirred for 2 h. Sodium bisulfite (1.98 g, 19.04 mmol) was added and stirred for 1 h to quench the reaction. Petroleum ether (200 mL) was added to the reaction solution, and the organic phase was washed with water (100 mL × 3), dried over anhydrous Na 2 SO 4 and concentrated under reduced pressure to obtain a yellow viscous liquid. Silica gel column chromatography (eluent: petroleum ether/ethyl acetate (v/v)=10/1) was purified to obtain a white solid (7.83 g, yield 70.41 %)
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 7.21 (dd,J = 8.5, 1.9 Hz, 1H), 7.16 (d,J = 1.9 Hz, 1H), 6.62 (d,J = 8.5 Hz, 1H), 4.53 – 4.44 (m, 1H), 3.82 (s, 3H), 1.36 (d,J = 6.1 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.21 (dd, J = 8.5, 1.9 Hz, 1H), 7.16 (d, J = 1.9 Hz, 1H), 6.62 (d, J = 8.5 Hz, 1H), 4.53 – 4.44 (m, 1H), 3.82 (s, 3H), 1.36 (d, J = 6.1 Hz, 6H).
GC-MS: m/z 292.0 [M]+ .GC-MS: m/z 292.0 [M] + .
步驟6:化合物2-(3-異丙氧基-4-甲氧基苯基)-4, 4, 5, 5-四甲基-1, 3, 2-二氧雜環戊硼烷的合成Step 6: Synthesis of compound 2-(3-isopropoxy-4-methoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane
將3-異丙氧基-4-甲氧基碘苯 (8.64 g, 26.63 mmol),聯硼酸頻那醇酯 (6.76 g, 26.63 mmol),醋酸鉀 (39.2 g, 39.95 mmol),醋酸鈀 (0.30 g, 1.33 mmol),2-二環己基磷-2’-甲基聯苯 (Mephos) (0.97 g, 2.66 mmol) 混合在N ,N -二甲基甲醯胺 (48 mL) 中,氮氣保護下80 ℃攪拌6 h。冷卻至室溫,反應液中加入水 (100 mL),用石油醚 (100 mL×3) 萃取反應液,合併有機相,無水硫酸鈉乾燥有機相,減壓濃縮得到褐色液體,通過矽膠柱層析 (洗脫劑:石油醚/乙酸乙酯(v/v)=10/1) 純化得黃色固體 (5.04 g, 產率64.78 %)。3-Isopropoxy-4-methoxyiodobenzene (8.64 g, 26.63 mmol), pinacol diboronate (6.76 g, 26.63 mmol), potassium acetate (39.2 g, 39.95 mmol), palladium acetate ( 0.30 g, 1.33 mmol), 2-dicyclohexylphosphorus-2'-methylbiphenyl (Mephos) (0.97 g, 2.66 mmol) was mixed in N , N -dimethylformamide (48 mL) under nitrogen Stir at 80 °C for 6 h under the protection. Cool to room temperature, add water (100 mL) to the reaction solution, extract the reaction solution with petroleum ether (100 mL×3), combine the organic phases, dry the organic phases with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a brown liquid, which is passed through a silica gel column layer. It was purified by analysis (eluent: petroleum ether/ethyl acetate (v/v)=10/1) to obtain a yellow solid (5.04 g, yield 64.78%).
1 H NMR (400 MHz, CDCl3 ): δ (ppm) 77.41 (d,J = 8.0 Hz, 1H), 7.33 (s, 1H), 6.88 (d,J = 8.0 Hz, 1H), 4.67 – 4.56 (m, 1H), 3.87 (s, 3H), 1.37 (d,J = 6.1 Hz, 6H), 1.33 (s, 12H). 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 77.41 (d, J = 8.0 Hz, 1H), 7.33 (s, 1H), 6.88 (d, J = 8.0 Hz, 1H), 4.67 – 4.56 ( m, 1H), 3.87 (s, 3H), 1.37 (d, J = 6.1 Hz, 6H), 1.33 (s, 12H).
MS (ESI, pos.ion) m/z: 293.25 [M+H]+ .MS (ESI, pos.ion) m/z: 293.25 [M+H] + .
步驟7:化合物 (R ) -1-叔丁基 2-甲基 4-(3-異丙氧基-4-甲氧基苯基)-1H -吡咯-1, 2 (2H , 5H )-二甲酸酯的合成Step 7: Compound (R) -1- tert-butyl 2-methyl-4- (3-isopropoxy-4-methoxy-phenyl) -1 H - pyrrole -1, 2 (2 H, 5 H )-Diformate Synthesis
將化合物 (R )-1-叔丁基 2-甲基 4-((((三氟甲基)磺醯基)甲氧基)-1H -吡咯-1, 2 (2H , 5H )-二甲酸酯 (5.50 g, 14.65 mmol,中間體M2 ),2-(3-(環丙基甲氧基)-4-(二氟甲氧基)苯基)-4, 4, 5, 5-四甲基-1, 3, 2-二氧雜環戊硼烷 (4.49 g, 15.38 mmol),醋酸鈀 (82.23 mg, 0.37 mmol),二環己基-[2-(2-甲基苯基)苯基] 膦 (267.00 mg, 0.73 mmol),溶解於甲苯 (30 mL) 溶劑中,再加入4-甲基嗎啉 (3.25 g, 32.08 mmol),水 (15 mL),在氮氣氛圍下,轉入到80 ℃攪拌反應2 h,冷卻至室溫,加入水 (100 mL),用乙酸乙酯 (50 mL × 3) 萃取,有機相用無水硫酸鈉乾燥30 min,通過矽膠柱層純化 (洗脫劑: 乙酸乙酯/石油醚(v/v)=3/20),得到褐色黏稠狀 (5 g, 產率87.19 %)。The compound (R) -1- tert-butyl 2-methyl 4 - ((((trifluoromethyl) sulfonyl acyl) methoxy) -1 H - pyrrole -1, 2 (2 H, 5 H) - Dicarboxylate (5.50 g, 14.65 mmol, Intermediate M 2 ), 2-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-4,4,5 , 5-tetramethyl-1,3,2-dioxaborolane (4.49 g, 15.38 mmol), palladium acetate (82.23 mg, 0.37 mmol), dicyclohexyl-[2-(2-methyl) phenyl) phenyl] phosphine (267.00 mg, 0.73 mmol), dissolved in toluene (30 mL) solvent, and then added 4-methylmorpholine (3.25 g, 32.08 mmol), water (15 mL), under nitrogen atmosphere Then, it was transferred to 80 °C and stirred for 2 h, cooled to room temperature, added with water (100 mL), extracted with ethyl acetate (50 mL × 3), the organic phase was dried with anhydrous sodium sulfate for 30 min, and passed through a silica gel column layer. Purification (eluent: ethyl acetate/petroleum ether (v/v) = 3/20) gave a brown sticky (5 g, 87.19 % yield).
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.96 – 6.90 (m, 2H), 6.85 – 6.82 (m, 1H), 5.93 – 5.87 (m, 1H), 5.17 – 5.08 (m, 1H), 4.66 – 4.46 (m, 3H), 3.85 (s, 3H), 3.74 (d,J = 5.0 Hz, 3H), 1.52 (s, 3H), 1.45 (s, 6H), 1.36 (d,J = 6.1 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.96 – 6.90 (m, 2H), 6.85 – 6.82 (m, 1H), 5.93 – 5.87 (m, 1H), 5.17 – 5.08 (m, 1H), 4.66 – 4.46 (m, 3H), 3.85 (s, 3H), 3.74 (d, J = 5.0 Hz, 3H), 1.52 (s, 3H), 1.45 (s, 6H), 1.36 (d, J = 6.1 Hz , 6H).
MS (ESI, pos.ion) m/z: 336.05 [M-t -Bu+2H]+ .MS (ESI, pos.ion) m/z: 336.05 [M- t- Bu+2H] + .
步驟8:化合物 (2R , 4S )-1-叔丁基 2-甲基 4-(3-異丙氧基-4-甲氧基苯基)吡咯烷-1, 2-二甲酸酯的合成Step 8: Compound ( 2R , 4S )-1-tert-butyl 2-methyl 4-(3-isopropoxy-4-methoxyphenyl)pyrrolidine-1,2-dicarboxylate Synthesis
將化合物 (R )-1-叔丁基 2-甲基 4-(3-異丙氧基-4-甲氧基苯基)-1H -吡咯-1, 2 (2H , 5H )-二羧酸酯 (5.55 g, 14.18 mmol) 溶解於的甲醇 (90 mL) 溶劑中,再加入鈀碳 (0.56 mg, 1.42 mmol),在氫氣氛圍下,室溫攪拌反應4 h。停止反應,用矽藻土過濾,減壓濃縮,得到無色黏稠狀 (4.45 g, 產率79.76 %)。The compound (R) -1- tert-butyl 2-methyl-4- (3-isopropoxy-4-methoxy-phenyl) -1 H - pyrrole -1, 2 (2 H, 5 H) - Dicarboxylate (5.55 g, 14.18 mmol) was dissolved in methanol (90 mL) solvent, then palladium carbon (0.56 mg, 1.42 mmol) was added, and the reaction was stirred at room temperature for 4 h under a hydrogen atmosphere. The reaction was stopped, filtered through celite, and concentrated under reduced pressure to obtain a colorless viscous substance (4.45 g, yield 79.76%).
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.83 – 6.76 (m, 3H), 4.53 – 4.46 (m, 1H), 4.39 – 4.29 (m, 1H), 4.04 – 3.89 (m, 1H), 3.82 (s, 3H), 3.75 (s, 3H), 3.42 – 3.35 (m, 1H), 3.32 – 3.23 (m, 1H), 2.65 – 2.57 (m, 1H), 2.06 – 1.95 (m, 1H), 1.44 (s, 9H), 1.34 (d, J= 6.0 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.83 – 6.76 (m, 3H), 4.53 – 4.46 (m, 1H), 4.39 – 4.29 (m, 1H), 4.04 – 3.89 (m, 1H), 3.82 (s, 3H), 3.75 (s, 3H), 3.42 – 3.35 (m, 1H), 3.32 – 3.23 (m, 1H), 2.65 – 2.57 (m, 1H), 2.06 – 1.95 (m, 1H), 1.44 (s, 9H), 1.34 (d, J= 6.0 Hz, 6H).
MS (ESI, pos.ion) m/z: 337.40 [M-t -Bu+H]+ .MS (ESI, pos.ion) m/z: 337.40 [M- t- Bu+H] + .
步驟9:化合物 (2R ,4S )-4-(3-異丙氧基-4-甲氧基苯基)吡咯烷-2-甲酸甲酯鹽酸鹽的合成Step 9: Synthesis of Compound (2R , 4S )-4-(3-isopropoxy-4-methoxyphenyl)pyrrolidine-2-carboxylic acid methyl ester hydrochloride
將化合物(2R , 4S )-1-叔丁基 2-甲基 4-(4-甲氧基-3-(丙氧基)苯基)吡咯烷-1, 2-二羧酸酯(1.70g, 4.32mmol) 溶解於二氯甲烷 (3 mL) 溶劑中,再加入氯化氫/1, 4-二氧六環 (9 mL) 溶液, 加入原料完之後,在室溫下攪拌反應2 h。停止反應,減壓濃縮一次後,再加入二氯甲烷 (20 mL) 溶解,再次減壓濃縮,得到淺黃色黏稠狀物 (1.27 g, 產率100 %)The compound ( 2R , 4S )-1-tert-butyl 2-methyl 4-(4-methoxy-3-(propoxy)phenyl)pyrrolidine-1,2-dicarboxylate ( 1.70g, 4.32mmol) was dissolved in dichloromethane (3 mL) solvent, and then hydrogen chloride/1,4-dioxane (9 mL) solution was added. After adding the raw materials, the reaction was stirred at room temperature for 2 h. The reaction was stopped and concentrated under reduced pressure once, then dichloromethane (20 mL) was added to dissolve, and concentrated under reduced pressure again to obtain a light yellow viscous substance (1.27 g, yield 100%)
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.84 (s, 1H), 6.80 (s, 2H), 4.66 – 4.58 (m, 1H), 4.56 – 4.50 (m, 1H), 3.83 (s, 3H), 3.81 (s, 3H), 3.64 – 3.61 (m, 1H), 3.55 – 3.47 (m, 1H), 2.82 – 2.73 (m, 1H), 2.25 – 2.10 (m, 2H), 1.34 (d,J = 4.9 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.84 (s, 1H), 6.80 (s, 2H), 4.66 – 4.58 (m, 1H), 4.56 – 4.50 (m, 1H), 3.83 (s, 3H), 3.81 (s, 3H), 3.64 – 3.61 (m, 1H), 3.55 – 3.47 (m, 1H), 2.82 – 2.73 (m, 1H), 2.25 – 2.10 (m, 2H), 1.34 (d, J = 4.9 Hz, 6H).
MS (ESI, pos.ion) m/z: 294.30[M+H-HCl]+ .MS (ESI, pos.ion) m/z: 294.30[M+H-HCl] + .
步驟10:化合物 (2R , 4S )-1-乙醯基-4-(3-異丙氧基-4-甲氧基苯基)吡咯烷-2-甲酸甲酯的合成Step 10: Compound (2 R, 4 S) -1- acetyl-4- (3-isopropoxy-4-methoxyphenyl) pyrrolidine-2-carboxylic acid methyl ester Synthesis of
將化合物 (2R ,4S )-4-(3-異丙氧基-4-甲氧基苯基)吡咯烷-2-羧酸甲酯鹽酸鹽 (1.7 g, 5.79 mmol) 溶解於二氯甲烷 (10 mL) 溶劑中,在冰浴中冷卻,緩慢依次加入乙基二異丙胺 (DIPEA) (2.99 g, 23.16 mmol) 及乙醯氯 (0.91 g , 11.58 mmol ),轉入到室溫下攪拌反應2 h。停止反應,反應液水洗 (50 mL×1),有機相用二氯甲烷 (20 mL) 萃取一次,合併有機相,再用飽和食鹽水 (50mL) 洗一次,分離有機相,有機相加無水硫酸鈉乾燥30 min,減壓濃縮,矽膠柱層析分離 (洗脫劑:乙酸乙酯/石油醚=80 %),得到淺褐色黏稠狀 (1.386 g, 產率71.37 %)Compound ( 2R , 4S )-4-(3-isopropoxy-4-methoxyphenyl)pyrrolidine-2-carboxylate methyl ester hydrochloride (1.7 g, 5.79 mmol) was dissolved in two In a solvent of methyl chloride (10 mL), cooled in an ice bath, ethyldiisopropylamine (DIPEA) (2.99 g, 23.16 mmol) and acetyl chloride (0.91 g, 11.58 mmol) were slowly added sequentially, and the temperature was brought to room temperature. The reaction was stirred for 2 h. The reaction was stopped, the reaction solution was washed with water (50 mL×1), the organic phase was extracted once with dichloromethane (20 mL), the organic phases were combined, washed once with saturated brine (50 mL), the organic phase was separated, and anhydrous sulfuric acid was added to the organic phase. It was dried over sodium for 30 min, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: ethyl acetate/petroleum ether=80%) to obtain a light brown viscous (1.386 g, yield 71.37%)
1 H NMR (400 MHz, CDCl3 ) δ (ppm) 6.84 – 6.75 (m, 3H), 4.54 – 4.43 (m, 2H), 3.93 – 3.88 (m, 1H), 3.83 (s, 3H), 3.76 (s, 3H), 3.58 (t,J = 10.5 Hz, 1H), 3.41 – 3.31 (m, 1H), 2.66 – 2.59 (m, 1H), 2.10 (s, 3H), 2.07 – 2.00 (m, 1H), 1.34 (d,J = 6.1 Hz, 6H). 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 6.84 – 6.75 (m, 3H), 4.54 – 4.43 (m, 2H), 3.93 – 3.88 (m, 1H), 3.83 (s, 3H), 3.76 ( s, 3H), 3.58 (t, J = 10.5 Hz, 1H), 3.41 – 3.31 (m, 1H), 2.66 – 2.59 (m, 1H), 2.10 (s, 3H), 2.07 – 2.00 (m, 1H) , 1.34 (d, J = 6.1 Hz, 6H).
MS (ESI, pos.ion) m/z: 336.35 [M+H]+ .MS (ESI, pos.ion) m/z: 336.35 [M+H] + .
步驟11:化合物 (2R , 4S )-1-乙醯基-4-(3-異丙氧基-4-甲氧基苯基)吡咯烷-2-甲酸的合成Step 11: Compound (2 R, 4 S) -1- acetyl-4- (3-isopropoxy-4-methoxyphenyl) pyrrolidine-2-carboxylic acid
將化合物 (2R , 4S )-1-乙醯基-4-(3-異丙氧基-4-甲氧基苯基)吡咯烷-2-甲酸甲酯 (0.62 g, 1.86 mmol),水合氫氧化鋰 (0.16 g, 3.72 mmol) 溶於四氫呋喃 (5 mL) 及水 (2.5 mL),在50 ℃下攪拌反3 h。停止反應,在-5 ℃中冷卻,滴加稀鹽酸 (0.5 M/L),調節pH=2,加入飽和食鹽水 (50 mL×2) 洗滌,分離有機相,無水硫酸鈉乾燥30 min,減壓濃縮,得到淺黃色固體 (561 mg, 產率93.85 %)Compound (2 R, 4 S) -1- acetyl-4- (3-isopropoxy-4-methoxyphenyl) pyrrolidine-2-carboxylic acid methyl ester (0.62 g, 1.86 mmol), Hydrate lithium hydroxide (0.16 g, 3.72 mmol) was dissolved in tetrahydrofuran (5 mL) and water (2.5 mL), and stirred at 50 °C for 3 h. Stop the reaction, cool at -5 °C, add dilute hydrochloric acid (0.5 M/L) dropwise, adjust pH=2, add saturated brine (50 mL×2) to wash, separate the organic phase, dry over anhydrous sodium sulfate for 30 min, reduce Concentrated under pressure to give a pale yellow solid (561 mg, 93.85% yield)
1 H NMR (400 MHz, DMSO-d 6 ) δ 6.91 – 6.85 (m, 2H), 6.84 – 6.78 (m, 1H), 4.59 – 4.50 (m, 2H), 4.23 – 4.19 (m, 1H), 4.06 – 4.00 (m, 1H), 3.72 (s, 3H), 3.30 – 3.18 (m, 1H), 2.67 – 2.58 (m, 1H), 2.00 (s, 3H), 1.91 – 1.78 (m, 2H), 1.24 (d,J = 6.0 Hz, 6H). 1 H NMR (400 MHz, DMSO- d 6 ) δ 6.91 – 6.85 (m, 2H), 6.84 – 6.78 (m, 1H), 4.59 – 4.50 (m, 2H), 4.23 – 4.19 (m, 1H), 4.06 – 4.00 (m, 1H), 3.72 (s, 3H), 3.30 – 3.18 (m, 1H), 2.67 – 2.58 (m, 1H), 2.00 (s, 3H), 1.91 – 1.78 (m, 2H), 1.24 (d, J = 6.0 Hz, 6H).
MS (ESI, pos.ion) m/z: 322.20 [M+H]+ .MS (ESI, pos.ion) m/z: 322.20 [M+H] + .
步驟12:化合物 (2R ,4S )-(6-((4-羥基苯基)(甲基)氨基甲醯基)吡啶-2-基)甲基 1-乙醯基-4-(3-異丙氧基-4-甲氧基苯基)吡咯烷-2-甲酸酯的合成Step 12: Compound ( 2R , 4S )-(6-((4-hydroxyphenyl)(methyl)carbamoyl)pyridin-2-yl)methyl 1-acetyl-4-(3 -Synthesis of isopropoxy-4-methoxyphenyl)pyrrolidine-2-carboxylate
將化合物 (2R , 4S )-1-乙醯基-4-(4-甲氧基-3-(丙-2-基氧基)苯基)吡咯烷-2-甲酸 (151.04 mg, 0.47 mmol),6-(羥甲基)-N -(4-羥苯基)-N -甲基吡啶-2-羧醯胺 (145.66 mg, 0.56 mmol),N -(3-二甲基氨基丙基)-N' -乙基碳二亞胺鹽酸鹽 (135.15 mg, 0.7 mmol),1-羥基苯並三唑(76.21 mg, 0.56 mmol) ,4-甲基嗎啉 (76.06 mg, 0.75 mmol),溶解在二氯甲烷 (5 mL) 溶劑中,在室溫下攪拌反應5 h。停止反應,加入水 (20 mL),用乙酸乙酯 (10 mL×2) 萃取,有機相再用飽和食鹽水洗 (20 mL) 一次,有機相用無水硫酸鈉乾燥,減壓濃縮,矽膠柱層析分離 (洗脫劑:甲醇/二氯甲烷(v/v)=5%),得到白色固體 (30 mg, 產率13.06 %)Compound (2 R, 4 S) -1- acetyl-4- (4-methoxy-3- (prop-2-yloxy) phenyl) pyrrolidine-2-carboxylic acid (151.04 mg, 0.47 mmol), 6- (hydroxymethyl) - N - (4- hydroxyphenyl) - N - methylpyridine-2carboxamide (145.66 mg, 0.56 mmol), N - (3- dimethylaminopropyl yl) - N '- ethylcarbodiimide hydrochloride (135.15 mg, 0.7 mmol), 1- hydroxybenzotriazole (76.21 mg, 0.56 mmol), 4- methylmorpholine (76.06 mg, 0.75 mmol ), dissolved in dichloromethane (5 mL) solvent, and the reaction was stirred at room temperature for 5 h. The reaction was stopped, water (20 mL) was added, extracted with ethyl acetate (10 mL×2), the organic phase was washed with saturated brine (20 mL) once, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and a silica gel column layer was used. Separation (eluent: methanol/dichloromethane (v/v)=5%) gave a white solid (30 mg, yield 13.06%)
1 H NMR (400 MHz, DMSO-d 6 ) δ 9.46 (s, 1H), 7.69 (t,J = 8.0 Hz, 1H), 7.27 – 7.21 (m, 2H), 6.94 – 6.86 (m, 4H), 6.85 – 6.79 (m, 1H), 6.55 (d,J = 8.2 Hz, 2H), 5.09 – 4.95 (m, 2H), 4.57 – 4.49 (m, 1H), 4.43 – 4.35 (m, 1H), 4.09 – 4.02 (m, 1H), 3.72 (s, 3H), 3.29 (s, 3H), 3.50 – 3.40 (m, 2H), 2.03 (s, 3H), 1.99 – 1.85 (m, 2H), 1.22 (d,J = 5.9 Hz, 6H). 1 H NMR (400 MHz, DMSO- d 6 ) δ 9.46 (s, 1H), 7.69 (t, J = 8.0 Hz, 1H), 7.27 – 7.21 (m, 2H), 6.94 – 6.86 (m, 4H), 6.85 – 6.79 (m, 1H), 6.55 (d, J = 8.2 Hz, 2H), 5.09 – 4.95 (m, 2H), 4.57 – 4.49 (m, 1H), 4.43 – 4.35 (m, 1H), 4.09 – 4.02 (m, 1H), 3.72 (s, 3H), 3.29 (s, 3H), 3.50 – 3.40 (m, 2H), 2.03 (s, 3H), 1.99 – 1.85 (m, 2H), 1.22 (d, J = 5.9 Hz, 6H).
MS (ESI, pos.ion) m/z: 562.50 [M+H]+ .MS (ESI, pos.ion) m/z: 562.50 [M+H] + .
生物試驗biological test
生物實施例1:本發明化合物對PDE4B2酶抑制作用的測定Biological Example 1: Determination of the Inhibitory Effect of the Compounds of the Invention on PDE4B2 Enzyme
1. 實驗方法1. Experimental method
本發明採用以下方法對本發明化合物進行生物試驗:(1)採用BPS生產試劑盒(BPS,Cat. No.60343),按照製造商提供的說明書,採用螢光偏振方法檢測化合物對PDE4B2酶抑制作用。(2)將PDE4B2酶濃度配製為83.33 pg/μL,終濃度為27.78 pg/μL;底物FAM-Cyclic-3’,5’-AMP濃度配製為300 nM,反應終濃度為100 nM,酶及底物稀釋液均使用試劑盒自帶緩衝液PDE Assay buffer;Binding Agent利用試劑盒自帶Binding Agent Diluent進行100倍稀釋,備用。反應體系如表1所示。
[表1] 化合物對PDE4B2酶 IC50
檢測體系
採用384孔板進行檢測,實驗設置受試樣品孔、陽性對照孔、陰性對照孔及空白孔,每個樣品利用雙複孔檢測10個濃度下對PDE4B2酶濃度的抑制作用,利用PDE4B2酶及FAM-Cyclic-3’,5’-AMP底物反應孔作為陽性對照,FAM-Cyclic-3’,5’-AMP底物孔作為陰性對照,緩衝液孔作為空白對照。各孔按表1順序加入相應樣品、酶、底物及緩衝液後,25℃恆溫箱孵育1 h,然後每孔加入已配置好的Binding Agent 15 μL,並於25℃恆溫振盪器振搖1 h後,利用PHER Astar FS多功能酶標儀(BMG)在FP485/525波長處進行檢測。利用Graph Pad Prism 5軟體對化合物不同濃度下對PDE4B2酶抑制作用進行作圖,計算IC50 。A 384-well plate was used for detection. The experiment was set up with test sample wells, positive control wells, negative control wells and blank wells. Each sample was tested for the inhibitory effect on the PDE4B2 enzyme concentration at 10 concentrations by double wells. PDE4B2 enzyme and FAM were used for the detection The -Cyclic-3',5'-AMP substrate reaction well was used as a positive control, the FAM-Cyclic-3',5'-AMP substrate well was used as a negative control, and the buffer well was used as a blank control. After adding the corresponding samples, enzymes, substrates and buffers to each well in the order of Table 1, incubate at 25°C for 1 h, then add 15 μL of the configured Binding Agent to each well, and shake at 25°C for 1 hour. After h, detection was performed at the wavelength of FP485/525 using a PHER Astar FS multifunctional microplate reader (BMG). Graph Pad Prism 5 software was used to plot the inhibitory effects of compounds on PDE4B2 at different concentrations, and IC 50 was calculated.
2. 實驗結果2. Experimental results
按照上述方法測定本發明實施例提供的化合物對PDE4B2酶的抑制作用,發現本發明化合物對PDE4B2酶具有抑制作用,其IC50
值小於1 μM;進一步發現,本發明部分實施例化合物的對PDE4B2酶的抑制作用的IC50
值小於500 nM;更進一步發現,本發明部分實施例化合物的對PDE4B2酶的抑制作用的IC50
值小於200 nM。具體地,本發明部分實施例化合物對PDE4B2酶抑制作用的測定結果參見表2。
[表2] 本發明部分化合物對PDE4B2酶抑制作用的測定結果
3. 實驗結論3. Experimental conclusion
實驗證明,本發明化合物在體外對PDE4B2酶普遍具有較高的抑制作用;特別是表2中的化合物,它們在對PDE4B2酶的體外抑制篩選實驗中表現出顯著的抑制活性。Experiments show that the compounds of the present invention generally have a high inhibitory effect on PDE4B2 enzyme in vitro; especially the compounds in Table 2, they show significant inhibitory activity in the in vitro inhibition screening experiment on PDE4B2 enzyme.
生物實施例2:本發明化合物對PMA誘導急性特應性皮炎小鼠模型Biological Example 2: PMA-induced acute atopic dermatitis mouse model by the compounds of the present invention
1. 試驗方法1. Test method
選取雌性ICR小鼠,體重26-28 g,動物適應性飼養7天后,隨機分為正常組、模型組、和各化合物組,正常組4隻,其餘每組10隻。除正常組小鼠外,其餘小鼠用20 μL濃度為0.25 mg/mL的PMA (Phorbol 12-myristate 13-acetate,佛波酯) 溶液 (溶於無水乙醇) 塗抹於右耳造模,正常組塗抹相應溶媒。各化合物組在造模前30 min進行第一次給藥和造模後15 min進行第二次給藥,各組動物耳部塗抹20 μL濃度為15 mg/mL受試藥物溶液 [乙醇:丙酮=1:1(v/v)],正常組、模型組動物塗抹相應溶媒。在第二次給藥6小時後處死動物,每隻動物剪下右耳用8 mm打耳器在固定位置獲取耳片並進行秤重,隨後液氮保存耳片,然後加入500 μL生理鹽水用勻漿機勻漿,離心後取上清液,檢測上清液中的IL-1β和IL-6濃度並用歸一化法計算蛋白濃度。Select female ICR mice, weighing 26-28 g, and after 7 days of adaptive feeding, they were randomly divided into normal group, model group, and each compound group, with 4 mice in normal group and 10 mice in each other group. Except for the mice in the normal group, the other mice were smeared with 20 μL of PMA (Phorbol 12-myristate 13-acetate, phorbol ester) solution (dissolved in absolute ethanol) with a concentration of 0.25 mg/mL on the right ear model. The normal group Apply the corresponding solvent. Each compound group was given the first administration 30 minutes before modeling and the second administration 15 minutes after modeling. Animals in each group were smeared with 20 μL of the test drug solution with a concentration of 15 mg/mL [ethanol:acetone]. =1:1(v/v)], the animals in the normal group and model group were smeared with the corresponding vehicle. The animals were sacrificed 6 hours after the second administration, the right ear of each animal was cut off and the ear piece was obtained at a fixed position with an 8 mm ear punch and weighed. Homogenize in a homogenizer, take the supernatant after centrifugation, detect the concentrations of IL-1β and IL-6 in the supernatant, and calculate the protein concentration by the normalization method.
2. 實驗結果2. Experimental results
表3~9為本發明化合物對PMA誘導急性特應性皮炎小鼠耳厚度、耳重量以及耳部炎症因子分泌的影響 (Mean ± SEM,動物數量N = 4 or N = 10,與模型組相比,*表示P
< 0.05,**表示P
< 0.01)。
[表3] 化合物對小鼠耳厚度以及耳重量的影響 (Mean ± Sem)
3. 實驗結論3. Experimental conclusion
由上述實驗結果可知,與模型組比較,本發明的化合物均能顯著地降低PMA誘導的急性特應性皮炎小鼠耳厚度和耳重量以及耳部炎症因子IL-1β和IL-6的分泌 (P < 0.05 )。As can be seen from the above experimental results, compared with the model group, the compounds of the present invention can significantly reduce the ear thickness and ear weight and the secretion of ear inflammatory factors IL-1β and IL-6 in mice with acute atopic dermatitis induced by PMA ( P < 0.05 ).
生物實施例3:本發明化合物對OXA誘導慢性特應性皮炎小鼠模型Biological Example 3: OXA-induced chronic atopic dermatitis mouse model by the compounds of the present invention
1. 實驗方法1. Experimental method
選取雄性Balb/c小鼠,體重24-26 g,動物適應性飼養7天后,除正常組外,其餘動物在第0天和第一天用40 μL 1%的OXA溶液 (溶於丙酮) 塗於小鼠雙耳致敏,在第7天和第8天用40 μL 0.5%的OXA溶液塗於小鼠雙耳重複致敏,正常組塗抹相應溶媒。從第12天開始,除正常組外,其餘動物用20 μl 0.5%的OXA溶液塗於小鼠右耳進行激發,每週兩次,正常組塗抹相應溶媒,每次激發24 h後測量右耳厚度。除正常組外,其餘動物在第13天根據耳厚度的結果隨機分為模型組和各化合物組,正常組5隻,其餘每組10隻。在第14天開始給藥,每天兩次,各給藥組動物耳部塗抹20 μL濃度為15 mg/mL受試藥物溶液 [乙醇:丙酮=1:1 (v/v)],正常組、模型組動物塗抹相應溶媒。第29天測量耳厚度後處死,每隻動物剪下右耳用8 mm打耳器在固定位置獲取耳片並進行秤重,隨後液氮保存耳片,然後加入500 μL生理鹽水用勻漿機勻漿,離心後取上清,檢測上清中的IL-1β、IL-4、IL-5、IL-6和TNF-α濃度並用歸一化法計算蛋白濃度。Male Balb/c mice with a body weight of 24-26 g were selected. After 7 days of adaptive feeding, except for the normal group, the rest of the animals were coated with 40 μL of 1% OXA solution (dissolved in acetone) on the 0th and the first day. Mice were sensitized on both ears, and 40 μL of 0.5% OXA solution was applied to both ears of mice on the 7th and 8th days to repeat the sensitization, and the normal group was smeared with the corresponding vehicle. From the 12th day, except for the normal group, the other animals were challenged with 20 μl of 0.5% OXA solution applied to the right ear of the mice, twice a week, the normal group was applied with the corresponding vehicle, and the right ear was measured 24 hours after each challenge thickness. Except for the normal group, the other animals were randomly divided into the model group and each compound group on the 13th day according to the results of ear thickness, with 5 animals in the normal group and 10 animals in each other group. The administration started on the 14th day, twice a day, 20 μL of the test drug solution [ethanol:acetone=1:1 (v/v)] with a concentration of 15 mg/mL was applied to the ears of the animals in each administration group. Animals in the model group were smeared with the corresponding vehicle. On the 29th day, the ear thickness was measured and then sacrificed. The right ear of each animal was cut off and the ear piece was obtained at a fixed position with an 8 mm ear punch and weighed. Then, the ear piece was stored in liquid nitrogen, and then 500 μL of normal saline was added with a homogenizer. After homogenization, the supernatant was taken after centrifugation, and the concentrations of IL-1β, IL-4, IL-5, IL-6 and TNF-α in the supernatant were detected and the protein concentration was calculated by the normalization method.
2. 實驗結果2. Experimental results
由結果可知,與模型組比較,本發明的化合物從第20天開始到給藥結束均能顯著的降低OXA誘導慢性特應性皮炎小鼠耳厚度和終點耳重量以及耳部炎症因子IL-1β、IL-4、IL-5、IL-6和TNF-α的分泌 (P < 0.01 )。It can be seen from the results that, compared with the model group, the compounds of the present invention can significantly reduce the ear thickness and end-point ear weight of OXA-induced chronic atopic dermatitis mice and the ear inflammatory factor IL-1β from the 20th day to the end of administration. , IL-4, IL-5, IL-6 and TNF-α secretion ( P < 0.01 ).
對於所屬技術領域中具有通常知識者顯而易見的是,本發明內容並不限於前述說明性實施例,而且可以體現在其他具體形式中而又不偏離其實質特性。因此,預期各實施例在所有方面都被視作說明性的且非限制性的,應參照所附申請專利範圍,而不是前述實施例,因此,在所附申請專利範圍等同內容的含義和範圍內的所有變化都包括在本發明中。It will be apparent to those of ordinary skill in the art that this disclosure is not limited to the foregoing illustrative embodiments, but may be embodied in other specific forms without departing from essential characteristics thereof. Accordingly, it is intended that the various embodiments be regarded in all respects as illustrative and non-restrictive, and reference should be made to the appended claims, rather than to the foregoing embodiments, and, accordingly, to the meaning and scope of equivalents in the appended claims All changes within are included in the present invention.
在本說明書的描述中,參考術語“一個實施例”、“一些實施例”、“示例”、“具體示例”或“一些示例”等的描述意指結合該實施例或示例描述的具體特徵、結構、材料或者特點包含於本發明的至少一個實施例或示例中。在本說明書中,對上述術語的示意性表述不一定指的是相同的實施例或示例。而且,描述的具體特徵、結構、材料或者特點可以在任何的一個或多個實施例或示例中以合適的方式結合。In the description of this specification, reference to the terms "one embodiment," "some embodiments," "example," "specific example," or "some examples", etc., means a specific feature described in connection with the embodiment or example, A structure, material, or feature is included in at least one embodiment or example of the present invention. In this specification, schematic representations of the above terms do not necessarily refer to the same embodiment or example. Furthermore, the particular features, structures, materials or characteristics described may be combined in any suitable manner in any one or more embodiments or examples.
最後,需要注意的是,還有其他方式用來實施本發明。相應地,本發明的實施例是將作為例證進行說明,但並不限於本發明所描述的內容,還可能是在本發明範圍內所作的修改或在請求項中所添加的等同內容。本發明所引用的所有出版物或專利都將作為本發明的參考文獻。Finally, it should be noted that there are other ways of implementing the invention. Accordingly, the embodiments of the present invention are described as examples, but are not limited to what is described in the present invention, and may also be modifications within the scope of the present invention or equivalents added in the claims. All publications or patents cited herein are incorporated herein by reference.
Claims (20)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010354590 | 2020-04-29 | ||
| CN202010354590.3 | 2020-04-29 | ||
| CN202010523655 | 2020-06-10 | ||
| CN202010523655.2 | 2020-06-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| TW202146385A true TW202146385A (en) | 2021-12-16 |
Family
ID=78161349
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW110114976A TW202146385A (en) | 2020-04-29 | 2021-04-26 | Substituted pyrrolidine compound and use thereof in medicine |
Country Status (3)
| Country | Link |
|---|---|
| CN (1) | CN113563245B (en) |
| TW (1) | TW202146385A (en) |
| WO (1) | WO2021218992A1 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FI3448391T3 (en) | 2016-04-27 | 2024-06-19 | Abbvie Mfg Management Unlimited Company | Methods of treatment of diseases in which il-13 activity is detrimental using anti-il-13 antibodies |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6348602B1 (en) * | 1999-12-23 | 2002-02-19 | Icos Corporation | Cyclic AMP-specific phosphodiesterase inhibitors |
| US6258833B1 (en) * | 1999-12-23 | 2001-07-10 | Icos Corporation | Cyclic AMP-specific phosphodiesterase inhibitors |
| IL161317A0 (en) * | 2001-10-16 | 2004-09-27 | Memory Pharm Corp | 4-(4-alkoxy-3-hydroxyphenyl)-2-pyrrolidone derivatives as pde-4 inhibitors for the treatment of neurological syndromes |
| US7585882B2 (en) * | 2004-10-20 | 2009-09-08 | Memory Pharmaceuticals Corporation | Phosphodiesterase 4 inhibitors |
| WO2010075086A2 (en) * | 2008-12-15 | 2010-07-01 | Auspex Pharmaceuticals, Inc. | Pyrrolidinone inhibitors of pde-4 |
-
2021
- 2021-04-26 TW TW110114976A patent/TW202146385A/en unknown
- 2021-04-28 WO PCT/CN2021/090489 patent/WO2021218992A1/en active Application Filing
- 2021-04-28 CN CN202110468583.0A patent/CN113563245B/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN113563245B (en) | 2024-10-15 |
| WO2021218992A1 (en) | 2021-11-04 |
| CN113563245A (en) | 2021-10-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6388991B2 (en) | Aminoheteroarylbenzamides as kinase inhibitors | |
| TWI780562B (en) | 1,3-thiazol-2-yl substituted benzamides | |
| CN112204009A (en) | Modulators of integrated stress pathways | |
| JP2023071767A (en) | Brm targeting compound and related methods of use | |
| JP2024038329A (en) | Irak decomposing agent and use of the same | |
| AU2022228150A1 (en) | Polycyclic compounds and methods for the targeted degradation of rapidly accelerated fibrosarcoma polypeptides | |
| JP2021503458A (en) | New dihydroisoxazole compounds and their use for the treatment of hepatitis B | |
| CN105566321B (en) | Heteroaromatic compounds and their use in medicine | |
| JP2015521645A (en) | 5-azaindazole compounds and methods of use | |
| EP2976338B1 (en) | N-(2-cyano heterocyclyl)pyrazolo pyridones as janus kinase inhibitors | |
| CN112513021B (en) | ROR gamma antagonist and application thereof in medicines | |
| EP4089080A1 (en) | RORyT INHIBITOR, PREPARATION METHOD THEREFOR AND USE THEREOF | |
| TW202146384A (en) | Substituted five-membered aza-ring compound and use thereof in drug | |
| TWI689497B (en) | Heteroaromatic derivatives and parmaceutical applications thereof | |
| CN113563245B (en) | Substituted pyrrolidines and their use in medicine | |
| JP2014501772A (en) | Novel sulfamide piperazine derivatives as protein tyrosine kinase inhibitors and their pharmaceutical use | |
| EP4089079A1 (en) | Ror gamma t inhibitor, preparation method therefor and use thereof | |
| RU2797922C2 (en) | Bicyclic ketones and methods of their use | |
| CN113563246A (en) | Substituted aza five-membered ring derivative and application thereof in medicine | |
| CN116354862A (en) | Substituted azacyclic compounds and application thereof in medicines | |
| US20230365531A1 (en) | Inhibitors of human respiratory syncytial virus and metapneumo virus | |
| CN112707873A (en) | Substituted oxazole derivative and application thereof in medicine | |
| JP2022151801A (en) | heterocyclic compound | |
| CN116354992A (en) | PDE4 inhibitor and application thereof in medicines | |
| CN117412959A (en) | Substituted 1-aryl-1 '-heteroaryl compounds, substituted 1,1' -biaryl compounds, and methods of use thereof |