KR20220111620A - A pharmaceutical composition for use of prevention or treatment of COVID-19 infection - Google Patents
A pharmaceutical composition for use of prevention or treatment of COVID-19 infection Download PDFInfo
- Publication number
- KR20220111620A KR20220111620A KR1020210042181A KR20210042181A KR20220111620A KR 20220111620 A KR20220111620 A KR 20220111620A KR 1020210042181 A KR1020210042181 A KR 1020210042181A KR 20210042181 A KR20210042181 A KR 20210042181A KR 20220111620 A KR20220111620 A KR 20220111620A
- Authority
- KR
- South Korea
- Prior art keywords
- pharmaceutical composition
- day
- covid
- infection
- virus
- Prior art date
Links
- 208000025721 COVID-19 Diseases 0.000 title claims abstract description 41
- 239000008194 pharmaceutical composition Substances 0.000 title claims abstract description 31
- 230000002265 prevention Effects 0.000 title claims description 7
- 208000015181 infectious disease Diseases 0.000 claims abstract description 20
- 208000024891 symptom Diseases 0.000 claims abstract description 13
- 239000003937 drug carrier Substances 0.000 claims abstract description 5
- 241000700605 Viruses Species 0.000 claims description 16
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 claims description 12
- 238000000034 method Methods 0.000 claims description 11
- 239000002775 capsule Substances 0.000 claims description 7
- 239000003826 tablet Substances 0.000 claims description 7
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 6
- 235000019359 magnesium stearate Nutrition 0.000 claims description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 6
- 239000003814 drug Substances 0.000 claims description 5
- 239000000203 mixture Substances 0.000 claims description 5
- 229920002472 Starch Polymers 0.000 claims description 4
- 239000001913 cellulose Substances 0.000 claims description 4
- 235000010980 cellulose Nutrition 0.000 claims description 4
- 229920002678 cellulose Polymers 0.000 claims description 4
- ZCGNOVWYSGBHAU-UHFFFAOYSA-N favipiravir Chemical compound NC(=O)C1=NC(F)=CNC1=O ZCGNOVWYSGBHAU-UHFFFAOYSA-N 0.000 claims description 4
- 229950008454 favipiravir Drugs 0.000 claims description 4
- 239000008107 starch Substances 0.000 claims description 4
- 235000019698 starch Nutrition 0.000 claims description 4
- 229940124597 therapeutic agent Drugs 0.000 claims description 4
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 claims description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 claims description 3
- 201000010099 disease Diseases 0.000 claims description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 3
- 235000011187 glycerol Nutrition 0.000 claims description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N lactose group Chemical group OC1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@@H](O)[C@H](O2)CO)[C@H](O1)CO GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 claims description 3
- 239000007788 liquid Substances 0.000 claims description 3
- WHTVZRBIWZFKQO-AWEZNQCLSA-N (S)-chloroquine Chemical compound ClC1=CC=C2C(N[C@@H](C)CCCN(CC)CC)=CC=NC2=C1 WHTVZRBIWZFKQO-AWEZNQCLSA-N 0.000 claims description 2
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 claims description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims description 2
- 108010010803 Gelatin Proteins 0.000 claims description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 2
- 229920000084 Gum arabic Polymers 0.000 claims description 2
- KJHKTHWMRKYKJE-SUGCFTRWSA-N Kaletra Chemical compound N1([C@@H](C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](CC=2C=CC=CC=2)NC(=O)COC=2C(=CC=CC=2C)C)CC=2C=CC=CC=2)CCCNC1=O KJHKTHWMRKYKJE-SUGCFTRWSA-N 0.000 claims description 2
- 229930195725 Mannitol Natural products 0.000 claims description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 claims description 2
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 claims description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 2
- 229930006000 Sucrose Natural products 0.000 claims description 2
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 claims description 2
- 239000000205 acacia gum Substances 0.000 claims description 2
- 235000010489 acacia gum Nutrition 0.000 claims description 2
- 229940072056 alginate Drugs 0.000 claims description 2
- 235000010443 alginic acid Nutrition 0.000 claims description 2
- 229920000615 alginic acid Polymers 0.000 claims description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 claims description 2
- 239000001506 calcium phosphate Substances 0.000 claims description 2
- 229910000389 calcium phosphate Inorganic materials 0.000 claims description 2
- 235000011010 calcium phosphates Nutrition 0.000 claims description 2
- 239000000378 calcium silicate Substances 0.000 claims description 2
- 229910052918 calcium silicate Inorganic materials 0.000 claims description 2
- 235000012241 calcium silicate Nutrition 0.000 claims description 2
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 claims description 2
- 229960003677 chloroquine Drugs 0.000 claims description 2
- WHTVZRBIWZFKQO-UHFFFAOYSA-N chloroquine Natural products ClC1=CC=C2C(NC(C)CCCN(CC)CC)=CC=NC2=C1 WHTVZRBIWZFKQO-UHFFFAOYSA-N 0.000 claims description 2
- CJBJHOAVZSMMDJ-HEXNFIEUSA-N darunavir Chemical compound C([C@@H]([C@H](O)CN(CC(C)C)S(=O)(=O)C=1C=CC(N)=CC=1)NC(=O)O[C@@H]1[C@@H]2CCO[C@@H]2OC1)C1=CC=CC=C1 CJBJHOAVZSMMDJ-HEXNFIEUSA-N 0.000 claims description 2
- 229960005107 darunavir Drugs 0.000 claims description 2
- 239000008121 dextrose Substances 0.000 claims description 2
- 229920000159 gelatin Polymers 0.000 claims description 2
- 239000008273 gelatin Substances 0.000 claims description 2
- 235000019322 gelatine Nutrition 0.000 claims description 2
- 235000011852 gelatine desserts Nutrition 0.000 claims description 2
- 229940112586 kaletra Drugs 0.000 claims description 2
- 239000008101 lactose Substances 0.000 claims description 2
- 239000000845 maltitol Substances 0.000 claims description 2
- 235000010449 maltitol Nutrition 0.000 claims description 2
- VQHSOMBJVWLPSR-WUJBLJFYSA-N maltitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-WUJBLJFYSA-N 0.000 claims description 2
- 229940035436 maltitol Drugs 0.000 claims description 2
- 239000000594 mannitol Substances 0.000 claims description 2
- 235000010355 mannitol Nutrition 0.000 claims description 2
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 claims description 2
- 229920000609 methyl cellulose Polymers 0.000 claims description 2
- 239000001923 methylcellulose Substances 0.000 claims description 2
- 235000010981 methylcellulose Nutrition 0.000 claims description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 claims description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 claims description 2
- 239000008108 microcrystalline cellulose Substances 0.000 claims description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 claims description 2
- 239000002480 mineral oil Substances 0.000 claims description 2
- 235000010446 mineral oil Nutrition 0.000 claims description 2
- 238000002625 monoclonal antibody therapy Methods 0.000 claims description 2
- MQQNFDZXWVTQEH-UHFFFAOYSA-N nafamostat Chemical compound C1=CC(N=C(N)N)=CC=C1C(=O)OC1=CC=C(C=C(C=C2)C(N)=N)C2=C1 MQQNFDZXWVTQEH-UHFFFAOYSA-N 0.000 claims description 2
- 229950009865 nafamostat Drugs 0.000 claims description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 claims description 2
- 229960003415 propylparaben Drugs 0.000 claims description 2
- RWWYLEGWBNMMLJ-MEUHYHILSA-N remdesivir Drugs C([C@@H]1[C@H]([C@@H](O)[C@@](C#N)(O1)C=1N2N=CN=C(N)C2=CC=1)O)OP(=O)(N[C@@H](C)C(=O)OCC(CC)CC)OC1=CC=CC=C1 RWWYLEGWBNMMLJ-MEUHYHILSA-N 0.000 claims description 2
- RWWYLEGWBNMMLJ-YSOARWBDSA-N remdesivir Chemical compound NC1=NC=NN2C1=CC=C2[C@]1([C@@H]([C@@H]([C@H](O1)CO[P@](=O)(OC1=CC=CC=C1)N[C@H](C(=O)OCC(CC)CC)C)O)O)C#N RWWYLEGWBNMMLJ-YSOARWBDSA-N 0.000 claims description 2
- 229960000329 ribavirin Drugs 0.000 claims description 2
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 claims description 2
- 239000000600 sorbitol Substances 0.000 claims description 2
- 235000010356 sorbitol Nutrition 0.000 claims description 2
- 239000005720 sucrose Substances 0.000 claims description 2
- 239000000454 talc Substances 0.000 claims description 2
- 229910052623 talc Inorganic materials 0.000 claims description 2
- 235000012222 talc Nutrition 0.000 claims description 2
- 229940021747 therapeutic vaccine Drugs 0.000 claims description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 claims description 2
- KCFYEAOKVJSACF-UHFFFAOYSA-N umifenovir Chemical compound CN1C2=CC(Br)=C(O)C(CN(C)C)=C2C(C(=O)OCC)=C1CSC1=CC=CC=C1 KCFYEAOKVJSACF-UHFFFAOYSA-N 0.000 claims description 2
- 229960004626 umifenovir Drugs 0.000 claims description 2
- 230000005727 virus proliferation Effects 0.000 claims description 2
- 235000010447 xylitol Nutrition 0.000 claims description 2
- 239000000811 xylitol Substances 0.000 claims description 2
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 claims description 2
- 229960002675 xylitol Drugs 0.000 claims description 2
- -1 nitacosanide Chemical compound 0.000 claims 2
- JNTOCHDNEULJHD-UHFFFAOYSA-N Penciclovir Chemical compound N1C(N)=NC(=O)C2=C1N(CCC(CO)CO)C=N2 JNTOCHDNEULJHD-UHFFFAOYSA-N 0.000 claims 1
- 229960001179 penciclovir Drugs 0.000 claims 1
- 238000009832 plasma treatment Methods 0.000 claims 1
- 229940027755 thymomodulin Drugs 0.000 abstract description 3
- 239000005723 virus inoculator Substances 0.000 description 19
- 210000004072 lung Anatomy 0.000 description 14
- 108090000623 proteins and genes Proteins 0.000 description 14
- 241001678559 COVID-19 virus Species 0.000 description 10
- 210000001519 tissue Anatomy 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 8
- 230000037396 body weight Effects 0.000 description 7
- 230000008595 infiltration Effects 0.000 description 7
- 238000001764 infiltration Methods 0.000 description 7
- 210000004969 inflammatory cell Anatomy 0.000 description 7
- 238000011081 inoculation Methods 0.000 description 7
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 6
- 241000699670 Mus sp. Species 0.000 description 6
- 230000002354 daily effect Effects 0.000 description 6
- 230000014509 gene expression Effects 0.000 description 6
- 230000003902 lesion Effects 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 208000032843 Hemorrhage Diseases 0.000 description 5
- 238000011888 autopsy Methods 0.000 description 5
- 210000001541 thymus gland Anatomy 0.000 description 5
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000000314 lubricant Substances 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 238000003753 real-time PCR Methods 0.000 description 4
- 241000283690 Bos taurus Species 0.000 description 3
- 101150013191 E gene Proteins 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 206010060891 General symptom Diseases 0.000 description 3
- 208000024777 Prion disease Diseases 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 208000034158 bleeding Diseases 0.000 description 3
- 230000000740 bleeding effect Effects 0.000 description 3
- 108091027963 non-coding RNA Proteins 0.000 description 3
- 102000042567 non-coding RNA Human genes 0.000 description 3
- 239000002831 pharmacologic agent Substances 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 239000008213 purified water Substances 0.000 description 3
- 208000023504 respiratory system disease Diseases 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 230000004580 weight loss Effects 0.000 description 3
- 241000766026 Coregonus nasus Species 0.000 description 2
- 206010011224 Cough Diseases 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- 208000000059 Dyspnea Diseases 0.000 description 2
- 206010013975 Dyspnoeas Diseases 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 206010068319 Oropharyngeal pain Diseases 0.000 description 2
- 201000007100 Pharyngitis Diseases 0.000 description 2
- 206010035664 Pneumonia Diseases 0.000 description 2
- 108010078233 Thymalfasin Proteins 0.000 description 2
- 102400000800 Thymosin alpha-1 Human genes 0.000 description 2
- UGPMCIBIHRSCBV-XNBOLLIBSA-N Thymosin beta 4 Chemical compound N([C@@H](CC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O)C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(C)=O UGPMCIBIHRSCBV-XNBOLLIBSA-N 0.000 description 2
- 102100035000 Thymosin beta-4 Human genes 0.000 description 2
- 230000005856 abnormality Effects 0.000 description 2
- 239000011149 active material Substances 0.000 description 2
- 238000009098 adjuvant therapy Methods 0.000 description 2
- 238000003149 assay kit Methods 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- HUSUHZRVLBSGBO-UHFFFAOYSA-L calcium;dihydrogen phosphate;hydroxide Chemical compound O.[Ca+2].OP([O-])([O-])=O HUSUHZRVLBSGBO-UHFFFAOYSA-L 0.000 description 2
- 244000309466 calf Species 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 231100000517 death Toxicity 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 230000003203 everyday effect Effects 0.000 description 2
- 238000007490 hematoxylin and eosin (H&E) staining Methods 0.000 description 2
- 208000006454 hepatitis Diseases 0.000 description 2
- 231100000283 hepatitis Toxicity 0.000 description 2
- 238000005286 illumination Methods 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000011812 mixed powder Substances 0.000 description 2
- 230000001575 pathological effect Effects 0.000 description 2
- 238000010149 post-hoc-test Methods 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 210000002345 respiratory system Anatomy 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 235000012239 silicon dioxide Nutrition 0.000 description 2
- 229960002920 sorbitol Drugs 0.000 description 2
- NZVYCXVTEHPMHE-ZSUJOUNUSA-N thymalfasin Chemical compound CC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O NZVYCXVTEHPMHE-ZSUJOUNUSA-N 0.000 description 2
- 229960004231 thymalfasin Drugs 0.000 description 2
- LCJVIYPJPCBWKS-NXPQJCNCSA-N thymosin Chemical group SC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@H](C(C)C)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](C(C)C)C(=O)N[C@H](CO)C(=O)N[C@H](CO)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H]([C@@H](C)CC)C(=O)N[C@H]([C@H](C)O)C(=O)N[C@H](C(C)C)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](C(C)C)C(=O)N[C@H](C(C)C)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@H](CCC(O)=O)C(O)=O LCJVIYPJPCBWKS-NXPQJCNCSA-N 0.000 description 2
- 108010079996 thymosin beta(4) Proteins 0.000 description 2
- 238000009423 ventilation Methods 0.000 description 2
- 230000009385 viral infection Effects 0.000 description 2
- 238000005303 weighing Methods 0.000 description 2
- AMFDITJFBUXZQN-KUBHLMPHSA-N (2s,3s,4r,5r)-2-(4-amino-5h-pyrrolo[3,2-d]pyrimidin-7-yl)-5-(hydroxymethyl)pyrrolidine-3,4-diol Chemical compound C=1NC=2C(N)=NC=NC=2C=1[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O AMFDITJFBUXZQN-KUBHLMPHSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- YASYEJJMZJALEJ-UHFFFAOYSA-N Citric acid monohydrate Chemical compound O.OC(=O)CC(O)(C(O)=O)CC(O)=O YASYEJJMZJALEJ-UHFFFAOYSA-N 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 241000711573 Coronaviridae Species 0.000 description 1
- 208000001528 Coronaviridae Infections Diseases 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 238000000585 Mann–Whitney U test Methods 0.000 description 1
- 241000699673 Mesocricetus auratus Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000551546 Minerva Species 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- 101150025571 SELENOF gene Proteins 0.000 description 1
- 241000181331 Saron Species 0.000 description 1
- 108010046075 Thymosin Proteins 0.000 description 1
- 102000007501 Thymosin Human genes 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 235000001465 calcium Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 229940084030 carboxymethylcellulose calcium Drugs 0.000 description 1
- 230000004709 cell invasion Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 208000027744 congestion Diseases 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 230000035622 drinking Effects 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 229950002031 galidesivir Drugs 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 208000021760 high fever Diseases 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 206010025482 malaise Diseases 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 210000003928 nasal cavity Anatomy 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 229940100688 oral solution Drugs 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 239000012264 purified product Substances 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 208000037816 tissue injury Diseases 0.000 description 1
- 230000006648 viral gene expression Effects 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 208000016261 weight loss Diseases 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/01—Hydrolysed proteins; Derivatives thereof
- A61K38/012—Hydrolysed proteins; Derivatives thereof from animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Immunology (AREA)
- Oncology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Zoology (AREA)
- Communicable Diseases (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
본 발명은 코비드-19 감염증을 예방하거나 치료할 수 있는 약제학적 조성물에 대한 것이다.The present invention relates to a pharmaceutical composition capable of preventing or treating Covid-19 infection.
신종 코로나 바이러스(2019 novel Corona virus; severe acute respiratory syndrome coronavirus 2; SARS-CoV-2)는 2019년 12월에 중국 우한에서 발생한 신종 코로나 바이러스 감염증의 원인 바이러스(2019-nCoV)이다. 이 신종 바이러스는 2019년 말 처음 인체 감염이 확인되었다는 의미에서 '코로나-19' 또는 '코비드-19'로 명명된다. 신종 코로나바이러스에 의한 유행성 질환은 호흡기를 통해 감염되며, 감염 초기에 전염성이 강한 특징을 보인다. 감염 후에는 인후통, 고열, 기침, 호흡곤란 등의 증상을 거쳐 폐렴으로 발전하여 사망까지도 발생할 수 있는 중대 호흡기 질환으로, 승인되거나 정립된 치료제가 없이 증상에 따른 약제나 위중 환자의 경우 산소 마스크 또는 인공호흡기 등의 보존적 치료에 의지하고 있다.The 2019 novel Corona virus (2019 novel Corona virus; severe acute
한편, 치모모둘린(Thymomodulin)은 간염, 호흡기 질환 등 세균 및 바이러스성 감염질환의 보조치료제로 시판되고 있으며, 송아지 흉선에서 추출한 단백질의 분해 정제물이다. 분자량은 1kD 내지 10kD이며, 치모신(Thymosin) 알파1과 치모신 베타4를 포함하는 것으로 알려져 있다. On the other hand, thymomodulin is marketed as an adjuvant treatment for bacterial and viral infectious diseases such as hepatitis and respiratory diseases, and is a purified product of protein extracted from calf thymus. The molecular weight is 1 kD to 10 kD, and is known to include
이에, 본 발명의 하나의 목적은, 안전하고 유효하게 코비드-19를 예방하거나 치료할 수 있는 약제학적 조성물 및 그 제조 방법을 제공하는 것에 있다.Accordingly, one object of the present invention is to provide a pharmaceutical composition that can safely and effectively prevent or treat Covid-19 and a method for preparing the same.
본 발명의 다른 목적은, 치모모둘린의 코비드-19 치료제 또는 예방제로서의 신규 용도를 제공하는 데 있다.Another object of the present invention is to provide a novel use of chymomodulin as a therapeutic or prophylactic agent for Covid-19.
본 발명의 또 다른 목적은 코비드-19 바이러스의 증식을 억제할 수 있는 약제학적 조성물을 제공하는 데 있다.Another object of the present invention is to provide a pharmaceutical composition capable of inhibiting the proliferation of the Covid-19 virus.
본 발명의 일 양태는, 치모모둘린 및 약제학적으로 허용되는 담체를 포함하는 코로나 19 바이러스 (COVID-19, 코비드-19) 감염증의 예방 또는 치료 용도의, 약제학적 조성물에 관한 것이다. One aspect of the present invention relates to a pharmaceutical composition for use in the prevention or treatment of Corona 19 virus (COVID-19, Covid-19) infection comprising chimomodulin and a pharmaceutically acceptable carrier.
활성 물질인 치모모둘린(thymomodulin)은 송아지 흉선에서 추출한 단백질의 산 또는 효소 분해 정제물로, 치모신 단편 5에 해당하는 여러 펩타이드의 혼합물이다. 치모모둘린의 분자량은 1kD 내지 10kD이며, 이의 CAS 넘버는 90803-92-2이다. 치모신(Thymosin) 알파1, 치모신 베타4 및 치모신 감마 등을 포함하며 현재 간염, 호흡기 질환 등 세균 및 바이러스성 감염질환의 보조치료제로 시판되고 있다. 본 발명의 발명자들은 치모모둘린이 코비드-19 증식을 억제할 뿐만 아니라, 코비드-19 감염으로 인한 체중 감소를 예방 또는 완화하고, 염증, 울혈, 출혈, 조직 상해 등의 제반 병리학적 결과를 경감 내지 완화시킬 수 있음을 밝혀내었다.The active substance, thymomodulin, is an acid or enzymatic digest of a protein extracted from calf thymus, and is a mixture of several peptides corresponding to
치모모둘린은 부작용이 거의 없는 것으로 알려져 있으므로 본 발명에서 제공하는 치모모둘린의 코비드-19 감염증 치료 또는 예방의 신규 용도는, 코비드-19를 안전하고 유효하게 치료 또는 예방할 수 있음을 의미한다.Since chimomodulin is known to have few side effects, the novel use of chimomodulin for the treatment or prevention of Covid-19 infection provided by the present invention means that it can safely and effectively treat or prevent Covid-19. .
약제학적 조성물의 총 중량을 기준으로, 치모모둘린은 5 내지 35 중량%, 구체적으로는 10 내지 30 중량%, 보다 구체적으로는 20 내지 30 중량%의 양으로 포함될 수 있다. Based on the total weight of the pharmaceutical composition, chymomodulin may be included in an amount of 5 to 35% by weight, specifically 10 to 30% by weight, more specifically 20 to 30% by weight.
코비드-19 바이러스 감염증의 예방 또는 치료를 위한 치모모둘린의 투여량은 환자의 나이, 성별, 체중, 및 감염증의 중등도에 따라 달라질 수 있으나, 1일 40mg 내지 800mg의 범위이다. 보다 구체적으로 치모모둘린의 1일 투여량은 80mg, 160mg 또는 240mg이다. 코비드-19 예방 또는 치료를 위한 약제학적 조성물의 투여 방법은 1일 1회 또는 1일 2회, 치모모둘린의 하루 투여량 80mg 내지 800mg을 1주 내지 24주 동안 투여하는 것이다. 구체적으로는 1일 1회 또는 2회 치모모둘린의 하루 투여량 80mg 내지 240mg을 4주 내지 20주 동안 투여하는 것이다. 보다 구체적으로, 1일 1회 또는 2회 치모모둘린의 하루 투여량 80mg 내지 160mg을 4주 내지 12주 동안 투여하는 것이다. 특히, 코로나 19 바이러스(코비드-19) 감염증의 예방을 위해서 건강한 사람, 고령자, 또는 기저질환자에게 치모모둘린의 하루 투여량 80mg 내지 800mg을, 1일 1회 또는 1일 2회, 4주 내지 24주 동안 투여할 수 있고, 구체적으로는 1일 1회 또는 2회 치모모둘린의 하루 투여량 80mg 내지 240mg을 4주 내지 20주 동안 투여할 수 있다. 특히 코로나 19 바이러스(코비드-19) 감염증의 치료를 위해 코로나 19 바이러스 감염자에게 치모모둘린의 하루 투여량 80mg 내지 800mg을, 1일 1회 또는 1일 2회, 1주 내지 24주 동안 투여할 수 있고, 구체적으로는 1일 1회 또는 2회 치모모둘린의 하루 투여량 80mg 내지 240mg을 2주 내지 20주 동안 투여할 수 있다.The dosage of chymomodulin for the prevention or treatment of Covid-19 virus infection may vary depending on the age, sex, weight, and severity of the infection of the patient, but ranges from 40 mg to 800 mg per day. More specifically, the daily dose of chymomodulin is 80 mg, 160 mg or 240 mg. The administration method of the pharmaceutical composition for the prevention or treatment of Covid-19 is to administer 80 mg to 800 mg of chimomodulin once a day or twice a day, for 1 week to 24 weeks. Specifically, a daily dose of 80 mg to 240 mg of chimomodulin once or twice a day is administered for 4 to 20 weeks. More specifically, a daily dose of 80 mg to 160 mg of chimomodulin once or twice a day is administered for 4 to 12 weeks. In particular, for the prevention of Corona 19 virus (COVID-19) infection, a daily dose of 80 mg to 800 mg of chimomodulin to a healthy person, the elderly, or an underlying disease, once a day or twice a day, 4 weeks to It may be administered for 24 weeks, and specifically, a daily dose of 80 mg to 240 mg of chimomodulin once or twice a day may be administered for 4 to 20 weeks. In particular, for the treatment of Corona 19 virus (COVID-19) infection, a daily dose of 80 mg to 800 mg of zymomodulin is administered to a person infected with Corona 19 virus, once a day or twice a day, for 1 week to 24 weeks. and specifically 80 mg to 240 mg of chimomodulin once or twice a day may be administered for 2 to 20 weeks.
상기 약제학적 조성물은 경구 투여용일 수 있다. 이에 약제학적 조성물은 환제, 산제, 액제, 정제 또는 캡슐제 형태로 제형화될 수 있으며, 구체예에서 액제, 캡슐제 또는 정제로 제형화될 수 있다. The pharmaceutical composition may be for oral administration. Accordingly, the pharmaceutical composition may be formulated in the form of pills, powders, liquids, tablets or capsules, and in embodiments may be formulated as liquids, capsules or tablets.
상기 약제학적으로 허용되는 담체로는 예를 들어, 락토즈, 덱스트로스, 슈크로스, 솔비톨, 만니톨, 자일리톨, 말티톨, 전분, 글리세린, 전분, 아카시아 고무, 알지네이트, 젤라틴, 칼슘포스페이트, 칼슘실리케이트, 셀룰로즈, 메틸셀룰로즈, 미정질셀룰로즈, 폴리비닐피롤리돈, 물, 메틸히드록시벤조에이트, 프로필 히드록시벤조에이트, 탈크, 마그네슘스테아레이트 또는 광물유를 들 수 있다. Examples of the pharmaceutically acceptable carrier include lactose, dextrose, sucrose, sorbitol, mannitol, xylitol, maltitol, starch, glycerin, starch, acacia gum, alginate, gelatin, calcium phosphate, calcium silicate, cellulose , methylcellulose, microcrystalline cellulose, polyvinylpyrrolidone, water, methylhydroxybenzoate, propylhydroxybenzoate, talc, magnesium stearate or mineral oil.
상기 약제학적 조성물은 추가로 충진제, 증량제, 결합제, 습윤제, 붕해제 등을 추가로 포함할 수 있다. The pharmaceutical composition may further include a filler, an extender, a binder, a wetting agent, a disintegrant, and the like.
상기 약제학적 조성물은 코비드-19의 증식 억제 또는 이의 감염증의 예방 또는 치료에 효과적이다. 코비드-19 감염 증상은, 발열, 권태감, 기침, 호흡곤란, 폐렴, 인후통, 두통, 설사, 오심, 체중 감소 등이며, 본 발명의 약제학적 조성물을 반복 투여시, 상기 하나 이상의 증상이 경감되거나 없어질 수 있다.The pharmaceutical composition is effective for inhibiting the proliferation of Covid-19 or preventing or treating an infection thereof. Symptoms of Covid-19 infection are fever, malaise, cough, dyspnea, pneumonia, sore throat, headache, diarrhea, nausea, weight loss, etc., and when the pharmaceutical composition of the present invention is repeatedly administered, one or more symptoms are relieved or can disappear
상기 약제학적 조성물은 코비드-19 예방, 치료 또는 증상 완화에 효과가 있는 다른 치료제, 백신 또는 감염 증상 완화제와 함께 사용될 수 있다. 이러한 치료제 또는 감염 증상 완화제로는, 바이러스에 감염된 환자의 혈장을 이용한 혈장치료, 단클론 항체 치료제, 리바비린, 펜시클로비르, 니타코사니드, 나파모스태트, 렘데시비르, 파비피라비르, 클로로퀸, 칼레트라, 아비간, 아르비돌, 갈리데시비르, 다루나비르 등을 들 수 있다.The pharmaceutical composition may be used in combination with other therapeutic agents, vaccines, or alleviation agents for symptoms of infection that are effective in preventing, treating, or relieving symptoms of Covid-19. Such therapeutic agents or alleviating symptoms of infection include plasma therapy using plasma from a patient infected with the virus, monoclonal antibody therapy, ribavirin, fenciclovir, nitacosanide, nafamostat, remdesivir, favipiravir, chloroquine, kaletra, Avigan, Arbidol, Galidesivir, Darunavir and the like are mentioned.
본 발명의 약제학적 조성물은 안전하고 유효하게 코비드-19 감염증 또는 감염에 따른 증상을 완화, 예방하거나 치료할 수 있다.The pharmaceutical composition of the present invention can safely and effectively alleviate, prevent or treat Covid-19 infection or symptoms caused by infection.
도 1은 코비드-19 접종 후 3 일째부터 7 일째까지 G1(정상군: 미처리 대조군), G2(감염군: 코비드-19 감염 및 치료제 미투여), G3(치모모둘린 투여군: 코비드-19 감염 및 치모모둘린 투여)의 육안상 출혈 및 손상 수준을 비교하기 위한, hamster의 폐 사진이다.
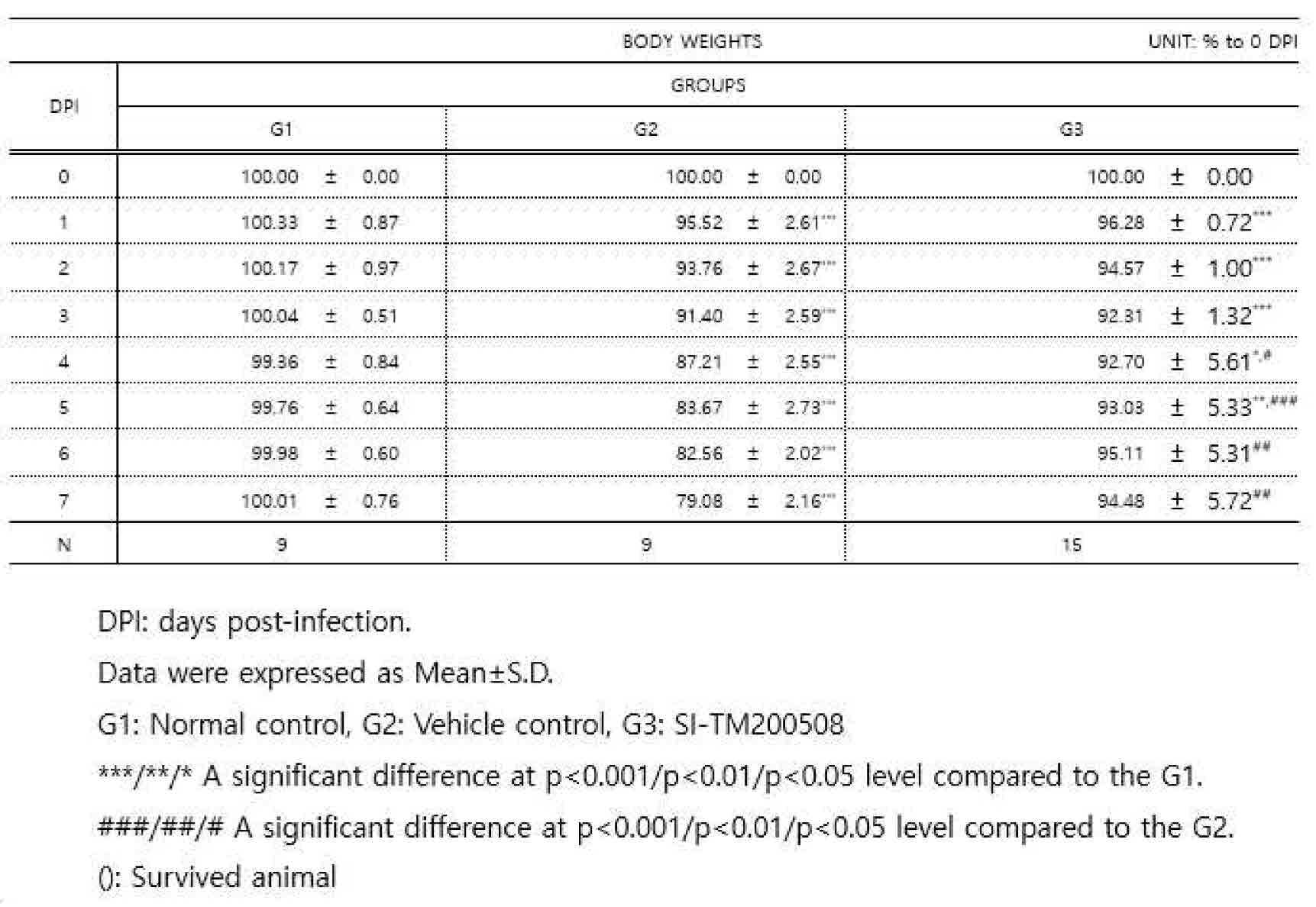
도 2는 코비드-19 접종 후 4일째, 5일째, 6일째, 및 7일째의 G1, G2, G3의 바이러스 접종 전 체중 기준 변화율을 도시한 그래프이다.
도 3은 코비드-19 접종 후 3일째, 5일째, 및 7일째의 G1, G2, G3의 폐포의 염증세포 침윤 수준을 나타내는 그래프이다.
도 4는 코비드-19 접종 후 3일째, 5일째, 및 7일째의 G1, G2, G3의 간질의 염증세포 침윤 수준을 나타내는 그래프이다.
도 5는 코비드-19 접종 후 3일째, 5일째, 및 7일째의 G2 및 G3의 부검한 폐조직에서 분리한 FAM(E gene)의 RNA 유전자 상대 발현율을 나타내는 그래프이다.
도 6는 코비드-19 접종 후 3일째, 5일째, 및 7일째의 G2 및 G3의 부검한 폐조직에서 분리한 RED(RdRP gene)의 RNA 유전자 상대 발현율을 나타내는 그래프이다.
도 7은 코비드-19 접종 후 3일째, 5일째, 및 7일째의 G2 및 G3의 부검한 폐조직에서 분리한 Cy5(N gene)의 RNA 유전자 상대 발현율을 나타내는 그래프이다.1 shows from the 3rd to the 7th day after the Covid-19 inoculation, G1 (normal group: untreated control), G2 (infected group: Covid-19 infection and no treatment), G3 (chimomodulin administered group: Covid- 19 Infection and administration of chymomodulin) are photographs of the lungs of hamsters for comparison of gross bleeding and damage levels.
Figure 2 is a graph showing the rate of change based on the body weight before the virus inoculation of G1, G2, G3 on the 4th, 5th, 6th, and 7th days after the Covid-19 inoculation.
3 is a graph showing the level of inflammatory cell infiltration in the alveoli of G1, G2, and G3 on the 3rd, 5th, and 7th days after covid-19 inoculation.
4 is a graph showing the level of inflammatory cell infiltration in the stroma of G1, G2, and G3 on the 3rd, 5th, and 7th days after covid-19 inoculation.
5 is a graph showing the relative expression rate of the RNA gene of FAM (E gene) isolated from the autopsied lung tissue of G2 and G3 on the 3rd, 5th, and 7th days after covid-19 inoculation.
6 is a graph showing the relative expression rate of the RNA gene of RED (RdRP gene) isolated from the autopsied lung tissue of G2 and G3 on the 3rd, 5th, and 7th days after Covid-19 inoculation.
7 is a graph showing the relative expression rate of the RNA gene of Cy5 (N gene) isolated from the autopsied lung tissue of G2 and G3 on the 3rd, 5th, and 7th days after Covid-19 inoculation.
이하, 실시예를 통하여 본 발명을 보다 상세히 설명하고자 한다. 그러나, 이들 실시 예는 본 발명을 예시적으로 설명하기 위한 것으로 본 발명의 범위가 이들 실시 예에 국한되는 것은 아니다.Hereinafter, the present invention will be described in more detail through examples. However, these examples are for illustrative purposes only, and the scope of the present invention is not limited to these examples.
실험예 1 Experimental Example 1
Syrian hamster(생산자 및 공급원: JANVIRER LABS (프랑스)/새론바이오)의 4주령의 수컷 33마리(체중 80-100g)를 온도 23±3 ℃, 상대습도 55±15 %, 환기횟수 10~20회/hr, 조명시간 12 시간 (오전 8 시 점등 ~ 오후 8 시 소등) 및 조도 150~300 Lux로 설정한 주식회사 노터스 설치류 사육구역에서 사육하였다. 사육기간 중 동물실의 온/습도, 환기횟수 및 조도 등의 환경조건은 정기적으로 측정하였다. 환경측정 결과, 시험의 결과에 나쁜 영향을 끼칠만한 이상은 관찰되지 않았다. 사료는 자유롭게 섭취하도록 하였으며, 물은 정수된 물을 폴리카보네이트제 음수병을 이용하여 자유롭게 섭취하도록 하였다. 33 4-week-old males (weight 80-100 g) of Syrian hamster (producer and supplier: JANVIRER LABS (France)/Saron Bio) were treated with a temperature of 23±3 ℃, a relative humidity of 55±15 %, and the number of ventilation 10 to 20 times/ hr, illumination time of 12 hours (lights from 8 am to 8 pm), and illumination was set at 150-300 Lux. During the breeding period, environmental conditions such as temperature/humidity, ventilation frequency, and illuminance of the animal room were measured regularly. As a result of environmental measurement, no abnormalities that could adversely affect the results of the test were observed. Feed was freely ingested, and purified water was freely ingested using a polycarbonate drinking bottle.
상기 햄스터를 아래 표와 같이 G1(정상군: 미처리 대조군), G2(감염군: 코비드-19 감염 및 치료제 미투여), G3(치모모둘린 투여군: 코비드-19 감염 및 치모모둘린 투여)으로 무작위로 나누었다:G1 (normal group: untreated control group), G2 (infected group: covid-19 infection and no treatment), G3 (chimomodulin administered group: covid-19 infection and chymomodulin administration) as shown in the table below. were randomly divided into:
[표 1][Table 1]
시험군의 구성Composition of the test group
G3군에 투여되는 치모모둘린은 다음 방법으로 제조되었다: 치모모둘린 분말 가루 5 g(공급처: 삼익제약㈜)을 칭량한 후 증류수 100 mL을 넣고 10 분간 교반하여 제조하였다.Chimomodulin administered to group G3 was prepared by the following method: After weighing 5 g of chimomodulin powder (supplier: Samik Pharmaceutical Co., Ltd.), 100 mL of distilled water was added, followed by stirring for 10 minutes.
바이러스 감염은 공시 바이러스인 SARS-CoV-2 (NCCP43326)를 계대 배양하여 104 TCID50/mL의 바이러스를 PBS 용액에 준비하여 사용하였으며, 이 바이러스 용액 200 μL를 햄스터의 좌측 비강으로 투여하여 감염시켰다(G2군 및 G3군).Virus infection was performed by subculturing the test virus SARS-CoV-2 (NCCP43326) and using a virus of 10 4 TCID50/mL in PBS solution, and 200 μL of this virus solution was administered to the left nasal cavity of the hamster to infect ( G2 and G3 groups).
G3군은 총 8주간 치모모둘린을 상기 용량으로 1일 1회 경구 투여하였고, 코비드-19로 감염시킨 후 동일 용량으로 추가 1주간 투여를 지속하여 총 9주간 치모모둘린을 투여하였다.Group G3 was orally administered chimomodulin at the above dose once a day for a total of 8 weeks, and after infection with Covid-19, administration was continued for an additional 1 week at the same dose, and chimomodulin was administered for a total of 9 weeks.
G2군은 상기 G3군과 동일한 시기에 동일한 방법으로 코비드-19로 감염시켰다.The G2 group was infected with Covid-19 at the same time and in the same manner as the G3 group.
실험예 2 : 관찰항목 및 시험방법 및 그 결과 Experimental Example 2 : Observation items and test methods and results
1. 일반증상 관찰1. Observation of general symptoms
바이러스 접종 직후부터 7 일 동안 매일 일반증상으로서 생존율과 체중 변화를 관찰하였다. 이상이 있을 경우에만 임상증상 관찰기록지에 이상증상, 정도 및 관찰시간 등을 기록하였다. 일반증상 관찰 결과, 전 실험기간 중 사망동물은 관찰되지 않았으며, 시험물질 투여에 의한 특이증상은 관찰되지 않았다. Survival rate and weight change were observed as general symptoms every day for 7 days immediately after virus inoculation. Only in the case of abnormalities, abnormal symptoms, severity, and observation time were recorded on the clinical symptom observation record sheet. As a result of the observation of general symptoms, no deaths were observed during the entire experimental period, and no specific symptoms were observed due to the administration of the test substance.
2. 체중측정2. Weighing
바이러스 접종 당일부터 7 일 동안 매일 전자저울을 이용하여 개체별 체중을 측정하여 기록하고, DPI 0 대비 체중의 변화율을 아래 표 2에 나타내었다:Each individual body weight was measured and recorded using an electronic scale every day for 7 days from the day of virus inoculation, and the rate of change of body weight compared to
[표 2][Table 2]
체중 측정 결과, 바이러스 접종 후 1 일째부터 5 일째까지 감염군 (G2) 및 SI-TM200508 (치모모둘린) 투여군 (G3)의 체중 수준은 정상군 (G1)에 비하여 유의하게 낮았으며 (p<0.001, p<0.01 또는 p<0.05), 바이러스 접종 후 6 일째부터 7 일째까지 G2의 체중 수준은 G1에 비하여 유의하게 낮았다 (p<0.05). 바이러스 접종 후 4 일째부터 7 일째까지 G3의 체중 수준은 G2에 비하여 유의하게 높았다 (p<0.001, p<0.01 또는 p<0.05)(도 2 참조).As a result of body weight measurement, the body weight levels of the infected group (G2) and the SI-TM200508 (chimomodulin) administered group (G3) were significantly lower than those of the normal group (G1) from
상기 결과는 코비드-19 감염으로 인한 체중 감소를 치모모둘린 투여로 유의하게 완화시킬 수 있음을 의미한다.The above results mean that weight loss due to Covid-19 infection can be significantly alleviated by administration of chymomodulin.
3. 부검 및 조직병리3. Autopsy and histopathology
바이러스 접종 직후부터 지정된 일별로 부검하였다. 바이러스 접종 후 3 일째 G1 군과 G2 군에서 각 군당 3 마리, 5 일째에 3 마리, 7 일째에 3 마리 부검하여 폐조직에서 보이는 병변 부위 일부를 NBF에 고정하여 H&E 염색 후 검경하였다. G3 군의 경우 바이러스 접종 후 3 일째 5 마리, 5 일째에 5 마리, 7 일째에 5 마리를 부검하여 폐조직에서 보이는 병변 부위 일부를 NBF에 고정하여 H&E 염색 후 검경하였다. 상기 폐포 및 간질의 염증성 세포 침윤 정도를 관찰하고 그 결과를 아래 표 3 내지 표 4에 나타내었다(도 3 내지 도 4 참조) :Autopsies were performed on designated days immediately after virus inoculation. On the 3rd day after virus inoculation, 3 mice in each group in the G1 and G2 groups, 3 mice on the 5th day, and 3 mice on the 7th day were necropsied. Part of the lesion visible in the lung tissue was fixed in NBF, followed by H&E staining and microscopic examination. In the case of the G3 group, 5 mice on the 3rd day, 5 mice on the 5th day, and 5 mice on the 7th day after virus inoculation were autopsied. Some of the lesions visible in the lung tissue were fixed in NBF, followed by H&E staining and microscopic examination. The degree of inflammatory cell infiltration of the alveoli and interstitium was observed, and the results are shown in Tables 3 to 4 below (see FIGS. 3 to 4):
[표 3][Table 3]
[표 4][Table 4]
G1은 전체 부검 기간 동안 조직병리 결과에서 이상병변이 관찰되지 않았으며, 바이러스 접종 후 7 일째에 G2의 폐포의 염증세포 침윤 수준은 G1에 비하여 유의하게 높았으며 (p<0.01), G3의 폐포 염증세포 침윤 수준은 G2에 비하여 유의하게 낮았다 (p<0.05)[표 3 참조]. 바이러스 접종 후 7일째의 G2의 간질 염증세포 침윤 수준은 G1에 비하여 유의하게 높았으며 (p<0.05), G3의 간질 염증세포 침윤 수준은 G2에 비하여 유의하게 낮았다 (p<0.05)[표 4 참조]. 또한 G3는 G2에 비하여 폐조직의 울혈 및 출혈 수준이 낮았다.In G1, no abnormal lesions were observed in histopathology results during the entire autopsy period, and on the 7th day after virus inoculation, the level of inflammatory cell infiltration in the alveoli of G2 was significantly higher than that of G1 (p<0.01), and alveolar inflammation of G3 The level of cell invasion was significantly lower than that of G2 (p<0.05) [see Table 3]. On the 7th day after virus inoculation, the level of G2 interstitial inflammatory cell infiltration was significantly higher than that of G1 (p<0.05), and the level of G3 interstitial inflammatory cell infiltration was significantly lower than that of G2 (p<0.05) [see Table 4]. ]. In addition, G3 had lower levels of congestion and bleeding in lung tissue compared to G2.
상기 결과는 코비드-19 감염으로 야기되는 폐포 및 간질의 염증세포 침윤, 울혈 및 출혈 등의 병리학적인 결과들이 치모모둘린 처리에 의해 감소되거나 완화될 수 있음을 의미한다.These results mean that pathological consequences such as alveolar and interstitial inflammatory cell infiltration, congestion and hemorrhage caused by Covid-19 infection can be reduced or alleviated by chymomodulin treatment.
추가로 바이러스 접종 후 3 일째, 5 일째 및 7 일째에서 부검한 폐 조직의 육안 소견을 평가하였다. G1은 전체 부검 기간 동안 육안 소견에서 이상병변이 관찰되지 않았다. 바이러스 접종 후 3 일째부터 7 일째까지 G3의 육안상 출혈 및 손상 수준은 G2 대비 적게 관찰되었음을 도 1a 내지 도 1c의 폐 사진을 통해 확인할 수 있다. 도 1a는 바이러스 접종 후 3 일째의 G1, G2, G3군의 폐 사진이고, 도 1b는 바이러스 접종 후 5일째의 G1, G2, G3군의 폐 사진이며 도 1c는 바이러스 접종 후 7일째의 G1, G2, G3군의 폐 사진이다.Additionally, macroscopic findings of autopsied lung tissues were evaluated on the 3rd, 5th, and 7th days after virus inoculation. In G1, no abnormal lesions were observed in gross findings during the entire autopsy period. From the 3rd to the 7th day after virus inoculation, it can be seen from the lung photographs of FIGS. 1A to 1C that the level of gross hemorrhage and damage of G3 was less than that of G2. Figure 1a is a photograph of the lungs of the G1, G2, and G3 groups on the 3rd day after virus inoculation, Figure 1b is a photograph of the lungs of the G1, G2, and G3 groups on the 5th day after the virus inoculation, and Figure 1c is the G1, These are pictures of the lungs of groups G2 and G3.
4. Real time RT-PCR4. Real-time RT-PCR
부검시 채취한 폐 조직에서 보이는 병변 부위 일부를 RNA extraction 하여 Seegene Allplex 2019-nCoV assay kit (Cat no. RP10243X)를 통해 바이러스 잔존량을 평가하였다. 평가 방법은 다음과 같다:Part of the lesion seen in the lung tissue collected at the time of autopsy was RNA extracted and the remaining amount of virus was evaluated using the Seegene Allplex 2019-nCoV assay kit (Cat no. RP10243X). The evaluation method is as follows:
병변 부위 일부 (약 1 g)를 EP-TUBE에 넣고, RNA prep 하였다. GeneAll 사의 키트를 사용하였으며 방법은 제조사의 방법을 따라 진행하였다 (GeneAll hybrid-R RNA purification kit; GeneAll, 3033522).A part of the lesion site (about 1 g) was placed in EP-TUBE and RNA prep was performed. GeneAll's kit was used, and the method was performed according to the manufacturer's method (GeneAll hybrid-R RNA purification kit; GeneAll, 3033522).
분리한 RNA를 nanodrop (Take3 Multi-Volume plate, BioTeK, Instruments, VT, USA)을 이용하여 정량한 뒤, 각 실험군별로 동일한 농도 (100 ng/μL)로 희석하였다. The isolated RNA was quantified using nanodrop (Take3 Multi-Volume plate, BioTeK, Instruments, VT, USA), and then diluted to the same concentration (100 ng/μL) for each experimental group.
동량으로 희석된 RNA template를 Seegene Allplex 2019-nCoV assay kit (Cat no. RP10243X)를 사용하여 FAM, HEX, RED, Cy5 파장에서 각각의 유전자 발현을 확인하였다. Real time RT-PCR 조건은 키트에 동봉된 제조사의 방법을 따라 진행하였다. 이중 바이러스 관련된 유전자 확인을 위하여 FAM (E gene), RED (RdRP gene) 및 Cy5 (N gene) 이용하였고, HEX를 이용한 Internal control gene의 발현 유무를 확인함으로써 Real time PCR 수행의 신뢰성을 확보하였다. 또한 이후 결과 확인 부분에서 internal control gene의 ct value 값을 이용하여, 해당 virus gene ct value 값들을 normalization 시켜서 유전자 발현 정도를 확인하여 비교하였다. Using the same amount of diluted RNA template, Seegene Allplex 2019-nCoV assay kit (Cat no. RP10243X) was used to confirm the expression of each gene at FAM, HEX, RED, and Cy5 wavelengths. Real time RT-PCR conditions were performed according to the manufacturer's method enclosed in the kit. FAM (E gene), RED (RdRP gene) and Cy5 (N gene) were used to confirm the double virus-related gene, and the reliability of real-time PCR was secured by checking the expression of the internal control gene using HEX. In addition, using the ct value of the internal control gene in the subsequent result confirmation section, the ct value of the virus gene was normalized to confirm and compare the gene expression level.
상기 FAM, RED, 및 Cy5는 코비드-19를 구성하는 단백질 유전자로 코비드-19 감염 여부를 확인하는 진단 검사에 이용되고 있으며, 이들 각각 또는 모두의 존재를 확인하면 감염 양성으로 진단하고 있다.The FAM, RED, and Cy5 are protein genes constituting Covid-19 and are used in a diagnostic test to confirm whether or not Covid-19 infection is present. If the presence of each or all of them is confirmed, the infection is diagnosed as positive.
바이러스 접종 후 3 일째, 5 일째 및 7 일째에서 부검한 폐조직에서 분리한 RNA의 바이러스 유전자 발현량을 확인하고 (FAM: E gene, RED: RdRP gene, Cy5: N gene), 그 결과를 표 5 내지 표 7에 나타내었다(도 5 내지 도 7 결과 참조).Check the viral gene expression level of RNA isolated from the lung tissue autopsied on the 3rd, 5th and 7th day after virus inoculation (FAM: E gene, RED: RdRP gene, Cy5: N gene), and the results are shown in Table 5 to Table 7 (see the results of Figures 5 to 7).
[표 5][Table 5]
[표 6][Table 6]
[표 7][Table 7]
Real time RT-PCR 결과, 바이러스 접종 후 5 일째 및 7 일째 G3의 FAM 수준은 G2에 비하여 유의하게 낮았다. (p<0.01/p<0.05). 그리고 바이러스 접종 후 3 일째, 5 일째 및 7 일째 G3의 Cy5의 수준은 G2에 비하여 유의하게 낮았다 (p<0.001/p<0.01/p<0.05). 또한 바이러스 접종 후 3 일째 및 7 일째 G3의 RED의 수준은 G2에 비하여 유의하게 낮았다(p<0.001/p<0.01). 상기 결과로부터 코비드-19 바이러스 증식이 치모모둘린 투여에 의해 효과적으로 억제되고 있음을 알 수 있다.As a result of real time RT-PCR, the FAM level of G3 was significantly lower than that of G2 on the 5th and 7th days after virus inoculation. (p<0.01/p<0.05). And on the 3rd, 5th, and 7th days after virus inoculation, the level of Cy5 of G3 was significantly lower than that of G2 (p<0.001/p<0.01/p<0.05). In addition, the level of RED in G3 on the 3rd and 7th days after virus inoculation was significantly lower than that of G2 (p<0.001/p<0.01). From the above results, it can be seen that the Covid-19 virus proliferation is effectively inhibited by chymomodulin administration.
상기 결과들의 통계 처리는, 자료의 정규성을 가정하였고, 모수적인 다중비교 (parametric multiple comparison procedures) 또는 비모수적인 다중비교 (non-parametric multiple comparison procedures)를 이용하여 분석하였다. 모수적 일원분산분석 (One-way ANOVA) 결과가 유의하였을 경우, Dunnett's multiple comparison test를 이용하여 사후검정을 실시하였고, 비모수적 Kruskal-Wallis'H-test 분석 결과가 유의하였을 경우, Mann Whitney U test를 이용하여 사후검정을 실시하였다.Statistical processing of the results assumed normality of the data, and was analyzed using parametric multiple comparison procedures or non-parametric multiple comparison procedures. If the parametric one-way ANOVA result was significant, a post hoc test was performed using Dunnett's multiple comparison test. If the nonparametric Kruskal-Wallis'H-test analysis result was significant, the Mann Whitney U test A post-hoc test was performed using
제조실시예 1 :치모모둘린 함유 캡슐제의 제조Preparation Example 1: Preparation of chymomodulin-containing capsules
본 실시예에 따른 정제의 제조는 유효물질과 부형제를 1차적으로 혼합한 후, 활택제인 스테아르산마그네슘으로 2차 혼합을 진행하여 캡슐 충진하는 것을 기본으로 하였다.The preparation of the tablet according to this embodiment was based on the primary mixing of the active material and the excipient, followed by secondary mixing with magnesium stearate, a lubricant, to fill the capsule.
약리학적 활성성분인 치모모둘린 (전염성 해면상뇌증감염을 방지하기 위하여 대한민국산, 뉴질랜드산, 중국산, 브라질산 등의 건강한 소에서 흉선을 채취하여 처리공정 후 제조) 80mg에 부형제로써 이산화규소 5mg, 미결정셀룰로오스 30mg, 카르복시메틸셀룰로오스칼슘 120mg, 인산수소칼슘수화물 10mg을 드럼형 또는 브이형 혼합기에 투입하여 25±1분 동안 균질하게 혼합하였다. 이후 활택제로서 스테아르산마그네슘 5mg 을 첨가하여 5±1분 동안 균질하게 2차 혼합하였다.Silicon dioxide 5mg as an excipient to 80mg of chymomodulin, a pharmacologically active ingredient (manufactured after processing by collecting thymus from healthy cattle from Korea, New Zealand, China, Brazil, etc. to prevent contagious spongiform encephalopathy infection) Cellulose 30 mg,
상기 혼합된 혼합분말을 캡슐제에 충전하여 치모모둘린 함유 캡슐제를 제조하였다. The mixed powder was filled in capsules to prepare chymomodulin-containing capsules.
제조실시예 2 :치모모둘린 함유 정제의 제조Preparation Example 2: Preparation of chymomodulin-containing tablets
본 실시예에 따른 정제의 제조는 유효물질과 부형제를 1차적으로 혼합한 후, 활택제인 스테아르산마그네슘으로 2차 혼합을 진행하여 직접 타정하는 것을 기본으로 하였다.The preparation of the tablet according to this example was based on the primary mixing of the active material and the excipient, followed by secondary mixing with magnesium stearate, a lubricant, and direct tableting.
약리학적 활성성분인 치모모둘린 (전염성 해면상뇌증감염을 방지하기 위하여 대한민국산, 뉴질랜드산, 중국산, 브라질산 등의 건강한 소에서 흉선을 채취하여 처리공정 후 제조) 80mg에 부형제로써 이산화규소 5mg, 미결정셀룰로오스 52mg, 카르복시메틸셀룰로오스칼슘 120mg, 인산수소칼슘수화물 10mg을 드럼형 또는 브이형 혼합기에 투입하여 25±1분 동안 균질하게 혼합하였다. 이후 활택제로서 스테아르산마그네슘 3mg 을 첨가하여 5±1분 동안 균질하게 혼합하였다. Silicon dioxide 5mg as an excipient to 80mg of chymomodulin, a pharmacologically active ingredient (manufactured after processing by collecting thymus from healthy cattle from Korea, New Zealand, China, Brazil, etc. to prevent contagious spongiform encephalopathy infection) 52 mg of cellulose, 120 mg of calcium carboxymethylcellulose, and 10 mg of calcium hydrogen phosphate hydrate were put into a drum-type or V-type mixer and homogeneously mixed for 25±1 minutes. Then, 3 mg of magnesium stearate as a lubricant was added and homogeneously mixed for 5±1 minutes.
상기 혼합된 혼합분말을 로터리타정기를 이용하여 총 중량 270밀리그램, 평균 경도 10±5 Kp으로 타정하여 정제를 제조하였다.Tablets were prepared by tableting the mixed powder to a total weight of 270 mg and an average hardness of 10±5 Kp using a rotary tableting machine.
제조실시예 3 :치모모둘린 함유 내용 액제의 제조Preparation Example 3: Preparation of an oral solution containing chymomodulin
약리학적 활성성분인 치모모둘린 (전염성 해면상뇌증감염을 방지하기 위하여 대한민국산, 뉴질랜드산, 중국산, 브라질산 등의 건강한 소에서 흉선을 채취하여 처리공정 후 제조) 400mg에 정제수 80mL, 농글리세린 10mL 및 에탄올 적량을 가하여 약 1시간 교반하여 녹이고, 70% D-소르비톨액, 시트르산수화물을 적량씩 첨가하고, 미생물번식 방지를 위하여 보존제로 벤조산나트륨 또는 파라옥시벤조산메틸 또는 파라옥시벤조산프로틸을 적량씩 첨가 후 약 30분 교반 후 정제수로 100mL로 맞추어 액제를 제조하였다.Pharmacologically active ingredient chymomodulin (to prevent infectious spongiform encephalopathy infection, thymus from healthy cattle from Korea, New Zealand, China, Brazil, etc. is collected and processed after processing) 400 mg of purified
Claims (11)
11. The method of claim 10, wherein the therapeutic agent is plasma treatment using plasma from a patient infected with a virus, monoclonal antibody therapy, ribavirin, penciclovir, nitacosanide, nafamostat, remdesivir, favipiravir, chloroquine, kaletra, avigan, arbidol, gallidesivir, or darunavir, a pharmaceutical composition.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR20210014470 | 2021-02-02 | ||
| KR1020210014470 | 2021-02-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20220111620A true KR20220111620A (en) | 2022-08-09 |
Family
ID=82844945
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020210042181A KR20220111620A (en) | 2021-02-02 | 2021-03-31 | A pharmaceutical composition for use of prevention or treatment of COVID-19 infection |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR20220111620A (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20070072919A (en) | 2004-10-26 | 2007-07-06 | 코닌클리케 필립스 일렉트로닉스 엔.브이. | Compensating gain of an optical recording apparatus |
| KR20200063275A (en) | 2020-05-02 | 2020-06-04 | 최기은 | Corona virus prevention and treatment drugs |
-
2021
- 2021-03-31 KR KR1020210042181A patent/KR20220111620A/en active Search and Examination
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20070072919A (en) | 2004-10-26 | 2007-07-06 | 코닌클리케 필립스 일렉트로닉스 엔.브이. | Compensating gain of an optical recording apparatus |
| KR20200063275A (en) | 2020-05-02 | 2020-06-04 | 최기은 | Corona virus prevention and treatment drugs |
Non-Patent Citations (4)
| Title |
|---|
| Drugs Exp Clin Res, 1985, 11(9), 665-9 |
| Minerva Med. 1987 Sep15: 78(17): 1281-9 |
| Pediatr Med Chir. 1988; 10(6): 603-7 |
| Pediatr Med Chir. 1990; 12(3): 229-32 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2799071B1 (en) | Levocetirizine and montelukast for the treatment of influenza and common cold | |
| JP2018514589A5 (en) | ||
| CN113244212B (en) | Application of baicalein in preparing medicament for preventing and/or treating novel coronavirus infection diseases | |
| WO2021207325A1 (en) | Natural extract and their components for use in mitigating acute respiratory distress syndrome | |
| JPH0635382B2 (en) | Uses of fluoxetine as an anxiolytic | |
| Blair | Remdesivir: a review in COVID-19 | |
| Naik et al. | Therapeutic Strategies in the Management of COVID-19 | |
| CN114504578A (en) | Medicine for treating and preventing related diseases caused by virus infection and application thereof | |
| JP2004525940A (en) | Duloxetine for the treatment of hot flashes | |
| EP4129290A1 (en) | Pharmaceutical composition for preventing or treating epidemic rna viral infectious disease | |
| US20050038018A1 (en) | Meloxicam compositions | |
| TWI581794B (en) | Methods and formulation for improving oral availability of cpt-11 while reducing cpt-11 induced gastronintestinal toxicity in cancer therapy | |
| KR20220111620A (en) | A pharmaceutical composition for use of prevention or treatment of COVID-19 infection | |
| KR101887561B1 (en) | Pharmaceutical composition for improving hepatitis virus-induced liver fibrosis | |
| CN114903910A (en) | Application of apigenin-7-O-beta-D-glucoside in preparation of medicine for treating inflammatory bowel disease | |
| CN102716128A (en) | Pharmaceutical composition for treating asthma | |
| WO2020194042A1 (en) | Treating influenza using substituted polycyclic pyridone derivatives and prodrugs thereof in a subject having influenza and a severe influenza condition | |
| US7820690B2 (en) | Method of treating or inhibiting a non-digestive tract derived abdominal disorder associated with pain using a 5-HT, receptor antagonist | |
| JP2002265354A (en) | Anti-helicobacter pylori agent composition | |
| RU2819722C2 (en) | Use of 1-(2-(1n-imidazol-4-yl)ethyl)piperidine-2,6-dione for treating cough caused by viral infections | |
| NZ533421A (en) | The use of darifenacin for the reduction of urgency in patients suffering from overactive bladder | |
| US20230123135A1 (en) | Method of treatment using meta-arsenite | |
| JP2009108042A (en) | Pharmaceutical composition containing azelastine and ambroxol | |
| JP2009007332A (en) | Pharmaceutical composition containing azelastines and ephedorines | |
| JP6644917B2 (en) | Compositions and drug combination methods for treating enterovirus infection |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination |