KR20130088807A - Novel ctla-4igg (cytotoxic t lymphocyte antigen 4-immunoglobulin g) fusion protein - Google Patents
Novel ctla-4igg (cytotoxic t lymphocyte antigen 4-immunoglobulin g) fusion protein Download PDFInfo
- Publication number
- KR20130088807A KR20130088807A KR1020130011443A KR20130011443A KR20130088807A KR 20130088807 A KR20130088807 A KR 20130088807A KR 1020130011443 A KR1020130011443 A KR 1020130011443A KR 20130011443 A KR20130011443 A KR 20130011443A KR 20130088807 A KR20130088807 A KR 20130088807A
- Authority
- KR
- South Korea
- Prior art keywords
- ctla
- 4igg
- fusion protein
- igg
- liposomes
- Prior art date
Links
- 102000037865 fusion proteins Human genes 0.000 title claims abstract description 41
- 108020001507 fusion proteins Proteins 0.000 title claims abstract description 41
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 title claims abstract description 8
- 102100031547 HLA class II histocompatibility antigen, DO alpha chain Human genes 0.000 title claims abstract description 7
- 101000866278 Homo sapiens HLA class II histocompatibility antigen, DO alpha chain Proteins 0.000 title claims abstract description 7
- 229940027941 immunoglobulin g Drugs 0.000 title claims abstract description 7
- 210000001744 T-lymphocyte Anatomy 0.000 claims abstract description 33
- 150000002632 lipids Chemical class 0.000 claims abstract description 29
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 19
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 claims abstract description 15
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Chemical group NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims abstract description 14
- 150000001413 amino acids Chemical group 0.000 claims abstract description 14
- 235000018417 cysteine Nutrition 0.000 claims abstract description 14
- 235000001014 amino acid Nutrition 0.000 claims abstract description 13
- 239000004472 Lysine Chemical group 0.000 claims abstract description 12
- 108060003951 Immunoglobulin Proteins 0.000 claims abstract description 8
- 102000018358 immunoglobulin Human genes 0.000 claims abstract description 8
- 208000023275 Autoimmune disease Diseases 0.000 claims abstract description 7
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 claims abstract description 5
- 239000002502 liposome Substances 0.000 claims description 67
- 239000003981 vehicle Substances 0.000 claims description 18
- 230000000694 effects Effects 0.000 claims description 17
- 238000000034 method Methods 0.000 claims description 13
- 230000002401 inhibitory effect Effects 0.000 claims description 8
- 230000001506 immunosuppresive effect Effects 0.000 claims description 7
- 206010062016 Immunosuppression Diseases 0.000 claims description 5
- 239000004480 active ingredient Substances 0.000 claims description 5
- 206010012601 diabetes mellitus Diseases 0.000 claims description 3
- 239000000693 micelle Substances 0.000 claims description 3
- 210000000056 organ Anatomy 0.000 claims description 3
- 238000002054 transplantation Methods 0.000 claims description 3
- 206010012438 Dermatitis atopic Diseases 0.000 claims description 2
- 208000006673 asthma Diseases 0.000 claims description 2
- 201000008937 atopic dermatitis Diseases 0.000 claims description 2
- 230000001079 digestive effect Effects 0.000 claims description 2
- 208000024711 extrinsic asthma Diseases 0.000 claims description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 claims description 2
- 208000011231 Crohn disease Diseases 0.000 claims 1
- 125000003473 lipid group Chemical group 0.000 claims 1
- 210000004899 c-terminal region Anatomy 0.000 abstract description 5
- 230000036039 immunity Effects 0.000 abstract description 3
- 239000003795 chemical substances by application Substances 0.000 abstract description 2
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 abstract 1
- 210000004027 cell Anatomy 0.000 description 50
- 230000027455 binding Effects 0.000 description 32
- 108090000623 proteins and genes Proteins 0.000 description 27
- 102000004169 proteins and genes Human genes 0.000 description 24
- 235000018102 proteins Nutrition 0.000 description 21
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 13
- 102000013816 Cytotoxic T-lymphocyte antigen 4 Human genes 0.000 description 13
- 108020004414 DNA Proteins 0.000 description 12
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 12
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 12
- 239000000427 antigen Substances 0.000 description 11
- 102000036639 antigens Human genes 0.000 description 11
- 108091007433 antigens Proteins 0.000 description 11
- 210000000612 antigen-presenting cell Anatomy 0.000 description 10
- 239000003814 drug Substances 0.000 description 9
- 239000000203 mixture Substances 0.000 description 9
- 230000028993 immune response Effects 0.000 description 8
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 8
- 229920002684 Sepharose Polymers 0.000 description 7
- 230000000735 allogeneic effect Effects 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 7
- 238000013519 translation Methods 0.000 description 7
- 241000699666 Mus <mouse, genus> Species 0.000 description 6
- 241000699670 Mus sp. Species 0.000 description 6
- 239000011324 bead Substances 0.000 description 6
- UQLDLKMNUJERMK-UHFFFAOYSA-L di(octadecanoyloxy)lead Chemical compound [Pb+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O UQLDLKMNUJERMK-UHFFFAOYSA-L 0.000 description 6
- 238000010586 diagram Methods 0.000 description 6
- 239000012528 membrane Substances 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 230000000638 stimulation Effects 0.000 description 6
- 239000004698 Polyethylene Substances 0.000 description 5
- 108091008874 T cell receptors Proteins 0.000 description 5
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 5
- 238000001514 detection method Methods 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 210000000265 leukocyte Anatomy 0.000 description 5
- 230000035772 mutation Effects 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 238000013518 transcription Methods 0.000 description 5
- 230000035897 transcription Effects 0.000 description 5
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 4
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 4
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 206010052779 Transplant rejections Diseases 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 239000000872 buffer Substances 0.000 description 4
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 4
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 4
- 230000001965 increasing effect Effects 0.000 description 4
- 210000004698 lymphocyte Anatomy 0.000 description 4
- -1 lysine amino acid Chemical class 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 229940124597 therapeutic agent Drugs 0.000 description 4
- 238000001262 western blot Methods 0.000 description 4
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 3
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 3
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 239000002033 PVDF binder Substances 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 230000000692 anti-sense effect Effects 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 3
- 239000012228 culture supernatant Substances 0.000 description 3
- 238000004520 electroporation Methods 0.000 description 3
- 230000002708 enhancing effect Effects 0.000 description 3
- 239000008103 glucose Substances 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000007799 mixed lymphocyte reaction assay Methods 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- 235000020183 skimmed milk Nutrition 0.000 description 3
- 230000001360 synchronised effect Effects 0.000 description 3
- 229940104230 thymidine Drugs 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- 108010084313 CD58 Antigens Proteins 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 241000699802 Cricetulus griseus Species 0.000 description 2
- 238000001712 DNA sequencing Methods 0.000 description 2
- 206010011968 Decreased immune responsiveness Diseases 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 108090000288 Glycoproteins Proteins 0.000 description 2
- 102000003886 Glycoproteins Human genes 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- 102100022339 Integrin alpha-L Human genes 0.000 description 2
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 108010064548 Lymphocyte Function-Associated Antigen-1 Proteins 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 2
- 101710160107 Outer membrane protein A Proteins 0.000 description 2
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 2
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 2
- 108010047620 Phytohemagglutinins Proteins 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- OVLIFGQSBSNGHY-KKHAAJSZSA-N Val-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C(C)C)N)O OVLIFGQSBSNGHY-KKHAAJSZSA-N 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 239000011543 agarose gel Substances 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 229940098773 bovine serum albumin Drugs 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 230000024245 cell differentiation Effects 0.000 description 2
- 230000032823 cell division Effects 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 230000002860 competitive effect Effects 0.000 description 2
- 230000006196 deacetylation Effects 0.000 description 2
- 238000003381 deacetylation reaction Methods 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000001125 extrusion Methods 0.000 description 2
- 239000012091 fetal bovine serum Substances 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- BRZYSWJRSDMWLG-CAXSIQPQSA-N geneticin Chemical compound O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](C(C)O)O2)N)[C@@H](N)C[C@H]1N BRZYSWJRSDMWLG-CAXSIQPQSA-N 0.000 description 2
- 108010049041 glutamylalanine Proteins 0.000 description 2
- 239000000710 homodimer Substances 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 210000004153 islets of langerhan Anatomy 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 238000002703 mutagenesis Methods 0.000 description 2
- 231100000350 mutagenesis Toxicity 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 230000001885 phytohemagglutinin Effects 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 210000004988 splenocyte Anatomy 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 2
- PHIQHXFUZVPYII-ZCFIWIBFSA-O (R)-carnitinium Chemical compound C[N+](C)(C)C[C@H](O)CC(O)=O PHIQHXFUZVPYII-ZCFIWIBFSA-O 0.000 description 1
- SLKDGVPOSSLUAI-PGUFJCEWSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine zwitterion Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OCCN)OC(=O)CCCCCCCCCCCCCCC SLKDGVPOSSLUAI-PGUFJCEWSA-N 0.000 description 1
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- BLGHHPHXVJWCNK-GUBZILKMSA-N Ala-Gln-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O BLGHHPHXVJWCNK-GUBZILKMSA-N 0.000 description 1
- ZDYNWWQXFRUOEO-XDTLVQLUSA-N Ala-Gln-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZDYNWWQXFRUOEO-XDTLVQLUSA-N 0.000 description 1
- DPNZTBKGAUAZQU-DLOVCJGASA-N Ala-Leu-His Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N DPNZTBKGAUAZQU-DLOVCJGASA-N 0.000 description 1
- QPBSRMDNJOTFAL-AICCOOGYSA-N Ala-Leu-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QPBSRMDNJOTFAL-AICCOOGYSA-N 0.000 description 1
- OPZJWMJPCNNZNT-DCAQKATOSA-N Ala-Leu-Met Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)O)N OPZJWMJPCNNZNT-DCAQKATOSA-N 0.000 description 1
- SFPRJVVDZNLUTG-OWLDWWDNSA-N Ala-Trp-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SFPRJVVDZNLUTG-OWLDWWDNSA-N 0.000 description 1
- GXCSUJQOECMKPV-CIUDSAMLSA-N Arg-Ala-Gln Chemical compound C[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O GXCSUJQOECMKPV-CIUDSAMLSA-N 0.000 description 1
- NONSEUUPKITYQT-BQBZGAKWSA-N Arg-Asn-Gly Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)NCC(=O)O)N)CN=C(N)N NONSEUUPKITYQT-BQBZGAKWSA-N 0.000 description 1
- MZRBYBIQTIKERR-GUBZILKMSA-N Arg-Glu-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MZRBYBIQTIKERR-GUBZILKMSA-N 0.000 description 1
- GNYUVVJYGJFKHN-RVMXOQNASA-N Arg-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N GNYUVVJYGJFKHN-RVMXOQNASA-N 0.000 description 1
- NMTANZXPDAHUKU-ULQDDVLXSA-N Arg-Tyr-Lys Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(O)=O)CC1=CC=C(O)C=C1 NMTANZXPDAHUKU-ULQDDVLXSA-N 0.000 description 1
- RAQMSGVCGSJKCL-FOHZUACHSA-N Asn-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(N)=O RAQMSGVCGSJKCL-FOHZUACHSA-N 0.000 description 1
- GQRDIVQPSMPQME-ZPFDUUQYSA-N Asn-Ile-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O GQRDIVQPSMPQME-ZPFDUUQYSA-N 0.000 description 1
- FHETWELNCBMRMG-HJGDQZAQSA-N Asn-Leu-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O FHETWELNCBMRMG-HJGDQZAQSA-N 0.000 description 1
- FBODFHMLALOPHP-GUBZILKMSA-N Asn-Lys-Glu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O FBODFHMLALOPHP-GUBZILKMSA-N 0.000 description 1
- FTNRWCPWDWRPAV-BZSNNMDCSA-N Asn-Phe-Phe Chemical compound C([C@H](NC(=O)[C@H](CC(N)=O)N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 FTNRWCPWDWRPAV-BZSNNMDCSA-N 0.000 description 1
- HNXWVVHIGTZTBO-LKXGYXEUSA-N Asn-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O HNXWVVHIGTZTBO-LKXGYXEUSA-N 0.000 description 1
- HPASIOLTWSNMFB-OLHMAJIHSA-N Asn-Thr-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O HPASIOLTWSNMFB-OLHMAJIHSA-N 0.000 description 1
- WUQXMTITJLFXAU-JIOCBJNQSA-N Asn-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)N)N)O WUQXMTITJLFXAU-JIOCBJNQSA-N 0.000 description 1
- BCADFFUQHIMQAA-KKHAAJSZSA-N Asn-Thr-Val Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BCADFFUQHIMQAA-KKHAAJSZSA-N 0.000 description 1
- LKIYSIYBKYLKPU-BIIVOSGPSA-N Asp-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)O)N)C(=O)O LKIYSIYBKYLKPU-BIIVOSGPSA-N 0.000 description 1
- UZFHNLYQWMGUHU-DCAQKATOSA-N Asp-Lys-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O UZFHNLYQWMGUHU-DCAQKATOSA-N 0.000 description 1
- CUQDCPXNZPDYFQ-ZLUOBGJFSA-N Asp-Ser-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O CUQDCPXNZPDYFQ-ZLUOBGJFSA-N 0.000 description 1
- OZBXOELNJBSJOA-UBHSHLNASA-N Asp-Ser-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N OZBXOELNJBSJOA-UBHSHLNASA-N 0.000 description 1
- BJDHEININLSZOT-KKUMJFAQSA-N Asp-Tyr-Lys Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(O)=O BJDHEININLSZOT-KKUMJFAQSA-N 0.000 description 1
- XQFLFQWOBXPMHW-NHCYSSNCSA-N Asp-Val-His Chemical compound N[C@@H](CC(=O)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)O XQFLFQWOBXPMHW-NHCYSSNCSA-N 0.000 description 1
- 108010045634 B7 Antigens Proteins 0.000 description 1
- 102000005738 B7 Antigens Human genes 0.000 description 1
- 102000038504 B7-1 Antigen Human genes 0.000 description 1
- 108010035053 B7-1 Antigen Proteins 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-N Betaine Natural products C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 1
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 1
- 108010029697 CD40 Ligand Proteins 0.000 description 1
- 101150013553 CD40 gene Proteins 0.000 description 1
- 102100032937 CD40 ligand Human genes 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- GHOKWGTUZJEAQD-UHFFFAOYSA-N Chick antidermatitis factor Natural products OCC(C)(C)C(O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-UHFFFAOYSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 241000699679 Cricetulus migratorius Species 0.000 description 1
- AOZBJZBKFHOYHL-AVGNSLFASA-N Cys-Glu-Tyr Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O AOZBJZBKFHOYHL-AVGNSLFASA-N 0.000 description 1
- OHLLDUNVMPPUMD-DCAQKATOSA-N Cys-Leu-Val Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N OHLLDUNVMPPUMD-DCAQKATOSA-N 0.000 description 1
- ZXCAQANTQWBICD-DCAQKATOSA-N Cys-Lys-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CS)N ZXCAQANTQWBICD-DCAQKATOSA-N 0.000 description 1
- KJJASVYBTKRYSN-FXQIFTODSA-N Cys-Pro-Asp Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CS)N)C(=O)N[C@@H](CC(=O)O)C(=O)O KJJASVYBTKRYSN-FXQIFTODSA-N 0.000 description 1
- NXQCSPVUPLUTJH-WHFBIAKZSA-N Cys-Ser-Gly Chemical compound SC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O NXQCSPVUPLUTJH-WHFBIAKZSA-N 0.000 description 1
- NDNZRWUDUMTITL-FXQIFTODSA-N Cys-Ser-Val Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NDNZRWUDUMTITL-FXQIFTODSA-N 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 241000620209 Escherichia coli DH5[alpha] Species 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- MFJAPSYJQJCQDN-BQBZGAKWSA-N Gln-Gly-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O MFJAPSYJQJCQDN-BQBZGAKWSA-N 0.000 description 1
- JKGHMESJHRTHIC-SIUGBPQLSA-N Gln-Ile-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N JKGHMESJHRTHIC-SIUGBPQLSA-N 0.000 description 1
- ZBKUIQNCRIYVGH-SDDRHHMPSA-N Gln-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N ZBKUIQNCRIYVGH-SDDRHHMPSA-N 0.000 description 1
- JNENSVNAUWONEZ-GUBZILKMSA-N Gln-Lys-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O JNENSVNAUWONEZ-GUBZILKMSA-N 0.000 description 1
- VNTGPISAOMAXRK-CIUDSAMLSA-N Gln-Pro-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O VNTGPISAOMAXRK-CIUDSAMLSA-N 0.000 description 1
- HNAUFGBKJLTWQE-IFFSRLJSSA-N Gln-Val-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CCC(=O)N)N)O HNAUFGBKJLTWQE-IFFSRLJSSA-N 0.000 description 1
- 102000006395 Globulins Human genes 0.000 description 1
- 108010044091 Globulins Proteins 0.000 description 1
- RLZBLVSJDFHDBL-KBIXCLLPSA-N Glu-Ala-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O RLZBLVSJDFHDBL-KBIXCLLPSA-N 0.000 description 1
- DWBBKNPKDHXIAC-SRVKXCTJSA-N Glu-Leu-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCC(O)=O DWBBKNPKDHXIAC-SRVKXCTJSA-N 0.000 description 1
- WIKMTDVSCUJIPJ-CIUDSAMLSA-N Glu-Ser-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCN=C(N)N WIKMTDVSCUJIPJ-CIUDSAMLSA-N 0.000 description 1
- BPCLDCNZBUYGOD-BPUTZDHNSA-N Glu-Trp-Glu Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=CNC2=C1 BPCLDCNZBUYGOD-BPUTZDHNSA-N 0.000 description 1
- MLILEEIVMRUYBX-NHCYSSNCSA-N Glu-Val-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O MLILEEIVMRUYBX-NHCYSSNCSA-N 0.000 description 1
- BUEFQXUHTUZXHR-LURJTMIESA-N Gly-Gly-Pro zwitterion Chemical compound NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O BUEFQXUHTUZXHR-LURJTMIESA-N 0.000 description 1
- AFWYPMDMDYCKMD-KBPBESRZSA-N Gly-Leu-Tyr Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 AFWYPMDMDYCKMD-KBPBESRZSA-N 0.000 description 1
- GMTXWRIDLGTVFC-IUCAKERBSA-N Gly-Lys-Glu Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O GMTXWRIDLGTVFC-IUCAKERBSA-N 0.000 description 1
- GWCJMBNBFYBQCV-XPUUQOCRSA-N Gly-Val-Ala Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O GWCJMBNBFYBQCV-XPUUQOCRSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- FYVHHKMHFPMBBG-GUBZILKMSA-N His-Gln-Asp Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N FYVHHKMHFPMBBG-GUBZILKMSA-N 0.000 description 1
- CNHSMSFYVARZLI-YJRXYDGGSA-N His-His-Thr Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CNHSMSFYVARZLI-YJRXYDGGSA-N 0.000 description 1
- 108010027412 Histocompatibility Antigens Class II Proteins 0.000 description 1
- 102000018713 Histocompatibility Antigens Class II Human genes 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000994375 Homo sapiens Integrin alpha-4 Proteins 0.000 description 1
- 101000599852 Homo sapiens Intercellular adhesion molecule 1 Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- CYHJCEKUMCNDFG-LAEOZQHASA-N Ile-Gln-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)NCC(=O)O)N CYHJCEKUMCNDFG-LAEOZQHASA-N 0.000 description 1
- JDAWAWXGAUZPNJ-ZPFDUUQYSA-N Ile-Glu-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N JDAWAWXGAUZPNJ-ZPFDUUQYSA-N 0.000 description 1
- XLCZWMJPVGRWHJ-KQXIARHKSA-N Ile-Glu-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N XLCZWMJPVGRWHJ-KQXIARHKSA-N 0.000 description 1
- VEPIBPGLTLPBDW-URLPEUOOSA-N Ile-Phe-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N VEPIBPGLTLPBDW-URLPEUOOSA-N 0.000 description 1
- FQYQMFCIJNWDQZ-CYDGBPFRSA-N Ile-Pro-Pro Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 FQYQMFCIJNWDQZ-CYDGBPFRSA-N 0.000 description 1
- PELCGFMHLZXWBQ-BJDJZHNGSA-N Ile-Ser-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)O)N PELCGFMHLZXWBQ-BJDJZHNGSA-N 0.000 description 1
- HXIDVIFHRYRXLZ-NAKRPEOUSA-N Ile-Ser-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)O)N HXIDVIFHRYRXLZ-NAKRPEOUSA-N 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 102100032818 Integrin alpha-4 Human genes 0.000 description 1
- 108010064593 Intercellular Adhesion Molecule-1 Proteins 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- 241000880493 Leptailurus serval Species 0.000 description 1
- GRZSCTXVCDUIPO-SRVKXCTJSA-N Leu-Arg-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O GRZSCTXVCDUIPO-SRVKXCTJSA-N 0.000 description 1
- KVMULWOHPPMHHE-DCAQKATOSA-N Leu-Glu-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O KVMULWOHPPMHHE-DCAQKATOSA-N 0.000 description 1
- HYIFFZAQXPUEAU-QWRGUYRKSA-N Leu-Gly-Leu Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(C)C HYIFFZAQXPUEAU-QWRGUYRKSA-N 0.000 description 1
- UBZGNBKMIJHOHL-BZSNNMDCSA-N Leu-Leu-Phe Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C([O-])=O)CC1=CC=CC=C1 UBZGNBKMIJHOHL-BZSNNMDCSA-N 0.000 description 1
- IDGZVZJLYFTXSL-DCAQKATOSA-N Leu-Ser-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IDGZVZJLYFTXSL-DCAQKATOSA-N 0.000 description 1
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 1
- 239000000232 Lipid Bilayer Substances 0.000 description 1
- KCXUCYYZNZFGLL-SRVKXCTJSA-N Lys-Ala-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O KCXUCYYZNZFGLL-SRVKXCTJSA-N 0.000 description 1
- OIQSIMFSVLLWBX-VOAKCMCISA-N Lys-Leu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OIQSIMFSVLLWBX-VOAKCMCISA-N 0.000 description 1
- QQPSCXKFDSORFT-IHRRRGAJSA-N Lys-Lys-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCCN QQPSCXKFDSORFT-IHRRRGAJSA-N 0.000 description 1
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 1
- YTJFXEDRUOQGSP-DCAQKATOSA-N Lys-Pro-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YTJFXEDRUOQGSP-DCAQKATOSA-N 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 102000043131 MHC class II family Human genes 0.000 description 1
- 108091054438 MHC class II family Proteins 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- QRHWTCJBCLGYRB-FXQIFTODSA-N Met-Ala-Cys Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CS)C(O)=O QRHWTCJBCLGYRB-FXQIFTODSA-N 0.000 description 1
- DSZFTPCSFVWMKP-DCAQKATOSA-N Met-Ser-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCCN DSZFTPCSFVWMKP-DCAQKATOSA-N 0.000 description 1
- FXBKQTOGURNXSL-HJGDQZAQSA-N Met-Thr-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(O)=O FXBKQTOGURNXSL-HJGDQZAQSA-N 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-O N,N,N-trimethylglycinium Chemical compound C[N+](C)(C)CC(O)=O KWIUHFFTVRNATP-UHFFFAOYSA-O 0.000 description 1
- PESQCPHRXOFIPX-UHFFFAOYSA-N N-L-methionyl-L-tyrosine Natural products CSCCC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 PESQCPHRXOFIPX-UHFFFAOYSA-N 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 1
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 1
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- QACUPNAKIPYZAW-RMQWDSPGSA-N O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O QACUPNAKIPYZAW-RMQWDSPGSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- LJUUGSWZPQOJKD-JYJNAYRXSA-N Phe-Arg-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccccc1)C(O)=O LJUUGSWZPQOJKD-JYJNAYRXSA-N 0.000 description 1
- YKUGPVXSDOOANW-KKUMJFAQSA-N Phe-Leu-Asp Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O YKUGPVXSDOOANW-KKUMJFAQSA-N 0.000 description 1
- CJAHQEZWDZNSJO-KKUMJFAQSA-N Phe-Lys-Cys Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CS)C(O)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 CJAHQEZWDZNSJO-KKUMJFAQSA-N 0.000 description 1
- ZJPGOXWRFNKIQL-JYJNAYRXSA-N Phe-Pro-Pro Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=CC=C1 ZJPGOXWRFNKIQL-JYJNAYRXSA-N 0.000 description 1
- JSGWNFKWZNPDAV-YDHLFZDLSA-N Phe-Val-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 JSGWNFKWZNPDAV-YDHLFZDLSA-N 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 1
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 1
- LGSANCBHSMDFDY-GARJFASQSA-N Pro-Glu-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCC(=O)O)C(=O)N2CCC[C@@H]2C(=O)O LGSANCBHSMDFDY-GARJFASQSA-N 0.000 description 1
- BWCZJGJKOFUUCN-ZPFDUUQYSA-N Pro-Ile-Gln Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(O)=O BWCZJGJKOFUUCN-ZPFDUUQYSA-N 0.000 description 1
- VZKBJNBZMZHKRC-XUXIUFHCSA-N Pro-Ile-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O VZKBJNBZMZHKRC-XUXIUFHCSA-N 0.000 description 1
- ABSSTGUCBCDKMU-UWVGGRQHSA-N Pro-Lys-Gly Chemical compound NCCCC[C@@H](C(=O)NCC(O)=O)NC(=O)[C@@H]1CCCN1 ABSSTGUCBCDKMU-UWVGGRQHSA-N 0.000 description 1
- LEIKGVHQTKHOLM-IUCAKERBSA-N Pro-Pro-Gly Chemical compound OC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 LEIKGVHQTKHOLM-IUCAKERBSA-N 0.000 description 1
- DYJTXTCEXMCPBF-UFYCRDLUSA-N Pro-Tyr-Phe Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CC3=CC=CC=C3)C(=O)O DYJTXTCEXMCPBF-UFYCRDLUSA-N 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 229920005654 Sephadex Polymers 0.000 description 1
- 239000012507 Sephadex™ Substances 0.000 description 1
- OYEDZGNMSBZCIM-XGEHTFHBSA-N Ser-Arg-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OYEDZGNMSBZCIM-XGEHTFHBSA-N 0.000 description 1
- SMIDBHKWSYUBRZ-ACZMJKKPSA-N Ser-Glu-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O SMIDBHKWSYUBRZ-ACZMJKKPSA-N 0.000 description 1
- MUJQWSAWLLRJCE-KATARQTJSA-N Ser-Leu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MUJQWSAWLLRJCE-KATARQTJSA-N 0.000 description 1
- RWDVVSKYZBNDCO-MELADBBJSA-N Ser-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CO)N)C(=O)O RWDVVSKYZBNDCO-MELADBBJSA-N 0.000 description 1
- RHAPJNVNWDBFQI-BQBZGAKWSA-N Ser-Pro-Gly Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O RHAPJNVNWDBFQI-BQBZGAKWSA-N 0.000 description 1
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 1
- VFWQQZMRKFOGLE-ZLUOBGJFSA-N Ser-Ser-Cys Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N)O VFWQQZMRKFOGLE-ZLUOBGJFSA-N 0.000 description 1
- NVNPWELENFJOHH-CIUDSAMLSA-N Ser-Ser-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)N NVNPWELENFJOHH-CIUDSAMLSA-N 0.000 description 1
- HNDMFDBQXYZSRM-IHRRRGAJSA-N Ser-Val-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HNDMFDBQXYZSRM-IHRRRGAJSA-N 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 description 1
- 239000006180 TBST buffer Substances 0.000 description 1
- YLXAMFZYJTZXFH-OLHMAJIHSA-N Thr-Asn-Asp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N)O YLXAMFZYJTZXFH-OLHMAJIHSA-N 0.000 description 1
- HJOSVGCWOTYJFG-WDCWCFNPSA-N Thr-Glu-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N)O HJOSVGCWOTYJFG-WDCWCFNPSA-N 0.000 description 1
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 1
- KZURUCDWKDEAFZ-XVSYOHENSA-N Thr-Phe-Asn Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)O KZURUCDWKDEAFZ-XVSYOHENSA-N 0.000 description 1
- LKJCABTUFGTPPY-HJGDQZAQSA-N Thr-Pro-Gln Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O LKJCABTUFGTPPY-HJGDQZAQSA-N 0.000 description 1
- GFRIEEKFXOVPIR-RHYQMDGZSA-N Thr-Pro-Lys Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O GFRIEEKFXOVPIR-RHYQMDGZSA-N 0.000 description 1
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 1
- QJIODPFLAASXJC-JHYOHUSXSA-N Thr-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O QJIODPFLAASXJC-JHYOHUSXSA-N 0.000 description 1
- XVHAUVJXBFGUPC-RPTUDFQQSA-N Thr-Tyr-Phe Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O XVHAUVJXBFGUPC-RPTUDFQQSA-N 0.000 description 1
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- SNWIAPVRCNYFNI-SZMVWBNQSA-N Trp-Met-Arg Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N SNWIAPVRCNYFNI-SZMVWBNQSA-N 0.000 description 1
- GFUOTIPYXKAPAH-BVSLBCMMSA-N Trp-Pro-Phe Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O GFUOTIPYXKAPAH-BVSLBCMMSA-N 0.000 description 1
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 1
- RCMWNNJFKNDKQR-UFYCRDLUSA-N Tyr-Pro-Phe Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 RCMWNNJFKNDKQR-UFYCRDLUSA-N 0.000 description 1
- VXFXIBCCVLJCJT-JYJNAYRXSA-N Tyr-Pro-Pro Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O VXFXIBCCVLJCJT-JYJNAYRXSA-N 0.000 description 1
- BYOHPUZJVXWHAE-BYULHYEWSA-N Val-Asn-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N BYOHPUZJVXWHAE-BYULHYEWSA-N 0.000 description 1
- CWSIBTLMMQLPPZ-FXQIFTODSA-N Val-Cys-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](C(C)C)N CWSIBTLMMQLPPZ-FXQIFTODSA-N 0.000 description 1
- FXVDGDZRYLFQKY-WPRPVWTQSA-N Val-Gly-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)C(C)C FXVDGDZRYLFQKY-WPRPVWTQSA-N 0.000 description 1
- RHYOAUJXSRWVJT-GVXVVHGQSA-N Val-His-Glu Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N RHYOAUJXSRWVJT-GVXVVHGQSA-N 0.000 description 1
- ZIGZPYJXIWLQFC-QTKMDUPCSA-N Val-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](C(C)C)N)O ZIGZPYJXIWLQFC-QTKMDUPCSA-N 0.000 description 1
- LKUDRJSNRWVGMS-QSFUFRPTSA-N Val-Ile-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N LKUDRJSNRWVGMS-QSFUFRPTSA-N 0.000 description 1
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 1
- DEGUERSKQBRZMZ-FXQIFTODSA-N Val-Ser-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O DEGUERSKQBRZMZ-FXQIFTODSA-N 0.000 description 1
- VIKZGAUAKQZDOF-NRPADANISA-N Val-Ser-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O VIKZGAUAKQZDOF-NRPADANISA-N 0.000 description 1
- SDHZOOIGIUEPDY-JYJNAYRXSA-N Val-Ser-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 SDHZOOIGIUEPDY-JYJNAYRXSA-N 0.000 description 1
- BZDGLJPROOOUOZ-XGEHTFHBSA-N Val-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N)O BZDGLJPROOOUOZ-XGEHTFHBSA-N 0.000 description 1
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 1
- BGTDGENDNWGMDQ-KJEVXHAQSA-N Val-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](C(C)C)N)O BGTDGENDNWGMDQ-KJEVXHAQSA-N 0.000 description 1
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- NFXWJYUDIOHFAW-UHFFFAOYSA-N acetic acid;tetradecanoic acid Chemical compound CC(O)=O.CCCCCCCCCCCCCC(O)=O NFXWJYUDIOHFAW-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 230000003698 anagen phase Effects 0.000 description 1
- 238000003975 animal breeding Methods 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 108010093581 aspartyl-proline Proteins 0.000 description 1
- 108010038633 aspartylglutamate Proteins 0.000 description 1
- 108010047857 aspartylglycine Proteins 0.000 description 1
- 108010068265 aspartyltyrosine Proteins 0.000 description 1
- 238000003149 assay kit Methods 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229960003237 betaine Drugs 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 102000023732 binding proteins Human genes 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000012888 bovine serum Substances 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 229960004203 carnitine Drugs 0.000 description 1
- 230000021164 cell adhesion Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 230000019771 cognition Effects 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 description 1
- 108010060199 cysteinylproline Proteins 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- SQNZJJAZBFDUTD-UHFFFAOYSA-N durene Chemical compound CC1=CC(C)=C(C)C=C1C SQNZJJAZBFDUTD-UHFFFAOYSA-N 0.000 description 1
- 239000012149 elution buffer Substances 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 235000013861 fat-free Nutrition 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 230000005251 gamma ray Effects 0.000 description 1
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 108010089804 glycyl-threonine Proteins 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 230000008629 immune suppression Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 108010031424 isoleucyl-prolyl-proline Proteins 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 1
- 108010057821 leucylproline Proteins 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 108010003700 lysyl aspartic acid Proteins 0.000 description 1
- 108010054155 lysyllysine Proteins 0.000 description 1
- 125000005439 maleimidyl group Chemical group C1(C=CC(N1*)=O)=O 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 108010005942 methionylglycine Proteins 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 229960003512 nicotinic acid Drugs 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 239000006259 organic additive Substances 0.000 description 1
- 229940055726 pantothenic acid Drugs 0.000 description 1
- 235000019161 pantothenic acid Nutrition 0.000 description 1
- 239000011713 pantothenic acid Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 108010084572 phenylalanyl-valine Proteins 0.000 description 1
- 108010073101 phenylalanylleucine Proteins 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 238000012257 pre-denaturation Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 108010077112 prolyl-proline Proteins 0.000 description 1
- 108010087846 prolyl-prolyl-glycine Proteins 0.000 description 1
- 108010020432 prolyl-prolylisoleucine Proteins 0.000 description 1
- 108010031719 prolyl-serine Proteins 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 239000011535 reaction buffer Substances 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- BBCGXZULVWBCGP-UHFFFAOYSA-N s-acetyl 2-(2,5-dioxopyrrolidin-1-yl)ethanethioate Chemical compound CC(=O)SC(=O)CN1C(=O)CCC1=O BBCGXZULVWBCGP-UHFFFAOYSA-N 0.000 description 1
- 238000003118 sandwich ELISA Methods 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 108010048818 seryl-histidine Proteins 0.000 description 1
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 1
- RYMZZMVNJRMUDD-HGQWONQESA-N simvastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 RYMZZMVNJRMUDD-HGQWONQESA-N 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 238000006177 thiolation reaction Methods 0.000 description 1
- 108010061238 threonyl-glycine Proteins 0.000 description 1
- 230000036962 time dependent Effects 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 108010080629 tryptophan-leucine Proteins 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 235000010374 vitamin B1 Nutrition 0.000 description 1
- 239000011691 vitamin B1 Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 235000016804 zinc Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K19/00—Hybrid peptides, i.e. peptides covalently bound to nucleic acids, or non-covalently bound protein-protein complexes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Gastroenterology & Hepatology (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Zoology (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
본 발명은 새로운 CTLA-4IgG 융합 단백질에 관한 것이다. 보다 구체적으로 본 발명은 면역 글로불린 CH3 도메인 잔기의 C 말단의 아미노산인 라이신이 시스테인으로 치환된 CTLA-4IgG 융합 단백질, 상기 CTLA-4IgG 융합 단백질을 유효성분으로 포함하는 면역 억제용 약학적 조성물, 및 CTLA-4IgG 융합 단백질의 C 말단의 아미노산인 라이신을 시스테인으로 치환하는 단계;를 포함하는 CTLA-4IgG 융합 단백질과 지질 비히클과의 결합력을 높이는 방법에 관한 것이다.
The present invention relates to a novel CTLA-4IgG fusion protein. More specifically, the present invention provides a CTLA-4IgG fusion protein in which lysine, an amino acid at the C terminus of an immunoglobulin CH3 domain residue, is substituted with cysteine, an immunosuppressive pharmaceutical composition comprising the CTLA-4IgG fusion protein as an active ingredient, and CTLA. And replacing the lysine, an amino acid at the C terminus of the -4IgG fusion protein, with cysteine; and a method for increasing the binding force between the CTLA-4IgG fusion protein and the lipid vehicle.
세포성 면역 반응은 T 림프구의 아구변형, 세포분열 및 세포 분화 등의 순차적인 과정을 통한 T 림프구 활성화를 통해 일어난다. T 림프구의 활성화는 세포 부착, 자극 또는 인지 그리고 동조자극의 순서로 일어나며, T 림프구와 항원제시세포가 갖는 세포 표면 항원 단백들 간의 상호 작용에 의해 형성되는 신호 전달체계에 의해 이루어진다.The cellular immune response occurs through the activation of T lymphocytes through sequential processes, such as submersion, cell division, and cell differentiation of T lymphocytes. Activation of T lymphocytes occurs in the order of cell adhesion, stimulation or recognition, and co-stimulation, and is achieved by signal transduction systems formed by the interaction between T lymphocytes and the cell surface antigenic proteins present in antigen presenting cells.
세포 부착(접촉)은 T 림프구와 항원제시세포가 서로 자극을 주고받기 위하여 만나는 단계를 말하며, 각 세포의 표면 단백질인 백혈구 항원에 의해 중재 된다. 이때 T 림프구에서는 ICAM (CD54), LFA-1(CD11a/18), VLA (CD49d/29) CD2 등이, 항원제시세포에서는 LFA-1(CD11a/18), ICAM-1 (CD45), VCAM (CD106), LFA-3 등이 백혈구 항원으로 관여하는 것으로 알려져 있다. Cell attachment (contact) refers to the stage where T lymphocytes and antigen-presenting cells meet to stimulate each other and are mediated by leukocyte antigens, which are surface proteins of each cell. In T lymphocytes, ICAM (CD54), LFA-1 (CD11a / 18), VLA (CD49d / 29) CD2, and the like, LFA-1 (CD11a / 18), ICAM-1 (CD45), VCAM ( CD106), LFA-3 and the like are known to be involved as leukocyte antigens.
자극은 인지라고도 하며 T 림프구가 클론 특이적 항원을 인식하여 항원 특이성을 나타내는 단계이다. 이 단계에 관여하는 백혈구 항원으로 T 세포에는 T 세포 수용체 (T cell receptor, TCR)와 CD3, CD4, CD8 등이 있으며, 항원제시세포에는 MHC class Ⅰ, Ⅱ 등이 있다. Stimulation, also known as cognition, is the stage at which T lymphocytes recognize clone specific antigens to express antigen specificity. Leukocyte antigens involved in this stage include T cell receptors (TCR), CD3, CD4, and CD8, and antigen presenting cells include MHC class I and II.
동조자극은 항원을 인식한 T세포가 아구변형과 세포분열 및 세포분화를 개시하도록 하는 단계로서, 이 단계에 관여하는 대표적인 백혈구 항원으로는 T 림프구의 CD2, CD40L, CD28, CTLA-4 등과 항원제시세포의 LFA-3, CD40, B7-1, B7-2 등이 있다.Synchronous stimulation causes antigen-recognized T cells to initiate apoptosis, cell division, and cell differentiation. Representative leukocyte antigens involved in this step include antigens such as CD2, CD40L, CD28, CTLA-4, etc. of T lymphocytes. Cell LFA-3, CD40, B7-1, B7-2 and the like.
T 세포의 활성화는 항원제시세포 표면의 항원 펩타이드 함유 MHC Ⅱ 분자가 T 세포의 TCR/CD3 분자 복합체에 결합함으로써 시작된다. 그러나 TCR/CD3에 의한 항원특이적 신호체계만으로는 완전한 면역 반응을 일으키기에 불충분하며 후속되는 이차 신호체계 즉 동조자극이 없는 상태에서 TCR/CD3에 항원이 재차 결합하게 되면 오히려 면역무력증(anergy) 혹은 클론 불활성화를 일으키게 된다. 동조자극은 주로 항원제시세포 표면 항원인 B7과 T 세포 표면 항원인 CD28의 상호결합에 의한 이차신호체계에 의해 유도되며, 이때 CTLA-4는 CD28과 경쟁적으로 B7에 결합함으로써 면역 반응 억제 기능을 하는 것으로 보고되고 있다. 이와 같이 후속되는 동조자극에 관여하는 수용체군을 부속분자라 부르며, CD28과 CTLA-4가 핵심적인 역할을 하는 것으로 알려져 있다.Activation of T cells is initiated by binding of antigenic peptide-containing MHC II molecules on the surface of antigen presenting cells to the TCR / CD3 molecule complex of T cells. However, antigen-specific signaling by TCR / CD3 alone is not sufficient to elicit a complete immune response. When antigens recombine to TCR / CD3 in the absence of subsequent secondary signaling, i.e., synchronous stimulation, it is rather anergy or clone. Inactivation will occur. Synchronous stimulation is mainly induced by secondary signaling system by the interaction of antigen-presenting cell surface antigen B7 and T cell surface antigen CD28, where CTLA-4 competes with CD28 to inhibit immune response by binding to B7. It is reported. This group of receptors involved in subsequent co-stimulation is called accessory molecules, and CD28 and CTLA-4 are known to play key roles.
CD28은 T 림프구의 표면에 존재하는 호모다이머 당단백질 (homodimer glycoprotein)로 T 림프구의 활성화 후에 증가하며, 단독으로 T 림프구를 활성화하지는 못하나, PHA(phytohemagglutinin)나 PMA(phorbor myristate acetate)의 자극이 있을 때, 또는 CD3과 CD28에 대한 항체 자극이 있을 때 T 림프구 활성을 현저히 강화시키는 것으로 알려져 있다.CD28 is a homodimer glycoprotein on the surface of T lymphocytes, which increases after activation of T lymphocytes and does not activate T lymphocytes alone, but may be stimulated by phytohemagglutinin (PHA) or phorbor myristate acetate (PMA). It is known to significantly enhance T lymphocyte activity when or when there is antibody stimulation against CD3 and CD28.
한편, CTLA-4는 휴지기의 T 림프구에서는 발현하지 않고 활성화된 T 림프구 표면에 CD28의 2~3% 정도의 수준에서 발현되어 있다고 알려져 있으며, CD28과 마찬가지로, 호모다이머 당단백질 (homodimer glycoprotein)로 존재하고, 단백질을 코딩하는 유전자 구성에 있어서 CD28과 67%의 동일성을 가지는 것으로 알려져 있다.CTLA-4 is not expressed in resting T lymphocytes but is expressed on the surface of activated T lymphocytes at the level of 2-3% of CD28. Like CD28, CTLA-4 is present as a homodimer glycoprotein. In addition, it is known to have 67% identity with CD28 in the gene encoding the protein.
백혈구 항원의 신호 전달에 의해 일어나는 면역반응은 이식거부반응과 자가면역질환 등을 유발하게 하는 원인이 된다. 특히, 세포독성 (cytotoxic) T 림프구가 최종적으로 목적세포를 사멸시킨다는 점에서 이의 활성을 저하시킴으로써 면역 반응을 억제하고자 하는 연구가 많이 이루어지고 있다.Immune responses caused by signal transduction of leukocyte antigens cause graft rejection and autoimmune diseases. In particular, many studies have been made to suppress the immune response by decreasing its activity in that cytotoxic T lymphocytes finally kill target cells.
본 발명자들은 리포좀에의 효과적인 방향성을 부여하고, 높은 T 세포 억제 효과를 갖는 신규한 CTLA-4IgG 융합 단백질을 개발하기 위하여 예의 노력한 결과, 면역 글로불린 CH3 도메인 잔기의 C 말단의 아미노산인 라이신을 시스테인으로 치환시킨 돌연변이체가 리포좀에의 결합력을 높이며, 높은 T 세포 억제능을 나타냄을 확인함으로써 본 발명을 완성하였다.
The present inventors have made intensive efforts to develop a novel CTLA-4IgG fusion protein that gives effective directivity to liposomes and has high T cell inhibitory effect. The present invention was completed by confirming that the mutants increase binding ability to liposomes and exhibit high T cell inhibitory ability.
본 발명의 목적은 신규한 CTLA-4IgG 융합 단백질을 제공함에 있다.It is an object of the present invention to provide novel CTLA-4IgG fusion proteins.
또한 본 발명의 목적은 상기 CTLA-4IgG 융합 단백질을 유효성분으로 포함하는 면역 억제용 약학적 조성물을 제공함에 있다.It is also an object of the present invention to provide a pharmaceutical composition for immunosuppression comprising the CTLA-4IgG fusion protein as an active ingredient.
또한 본 발명의 목적은 CTLA-4IgG 융합 단백질의 C 말단의 아미노산인 라이신을 시스테인으로 치환하는 단계;를 포함하는 CTLA-4IgG 융합 단백질과 지질 비히클과의 결합력을 높이는 방법을 제공함에 있다.
It is also an object of the present invention to provide a method for enhancing the binding force between the CTLA-4IgG fusion protein and the lipid vehicle, comprising the step of: replacing the lysine amino acid of the C-terminal of the CTLA-4IgG fusion protein with cysteine.
상기와 같은 목적을 달성하기 위하여, 본 발명은 면역 글로불린 CH3 도메인 잔기의 C 말단의 아미노산인 라이신이 시스테인으로 치환된 CTLA-4IgG (cytotoxic T lymphocyte antigen 4-Immunoglobulin G) 융합 단백질을 제공한다. In order to achieve the above object, the present invention provides a cytotoxic T lymphocyte antigen 4-Immunoglobulin G (CTLA-4IgG) fusion protein in which lysine, an amino acid at the C terminus of an immunoglobulin CH3 domain residue, is substituted with cysteine.
또한, 본 발명은 상기 CTLA-4IgG 융합 단백질을 유효성분으로 포함하는 면역 억제용 약학적 조성물을 제공한다.In addition, the present invention provides a pharmaceutical composition for immunosuppression comprising the CTLA-4IgG fusion protein as an active ingredient.
또한, 본 발명은 CTLA-4IgG 융합 단백질의 C 말단의 아미노산인 라이신을 시스테인으로 치환하는 단계;를 포함하는 CTLA-4IgG 융합 단백질과 지질 비히클과의 결합력을 높이는 방법을 제공한다.
In another aspect, the present invention provides a method for enhancing the binding force between the CTLA-4IgG fusion protein and the lipid vehicle, comprising the step of: replacing the lysine amino acid of the C-terminal of the CTLA-4IgG fusion protein with cysteine.
본 발명에 따른 CTLA-4IgG 융합 단백질은 면역 글로불린 CH3 도메인 잔기의 C 말단의 아미노산인 라이신을 시스테인으로 치환함으로써, 지질 비히클에 효과적으로 결합하며, T 세포를 높은 활성으로 억제하는바, 효과적인 자가면역 질환 치료제 또는 장기이식반응 억제제 등 면역 억제용 약학적 조성물로 이용될 수 있다.
The CTLA-4IgG fusion protein according to the present invention effectively binds to a lipid vehicle and inhibits T cells with high activity by replacing lysine, an amino acid at the C terminus of an immunoglobulin CH3 domain residue with cysteine, an effective autoimmune disease therapeutic agent. Or it may be used as a pharmaceutical composition for suppressing immune such as organ transplant inhibitors.
도 1은 CTLA-4IgG의 면역 반응에서의 다양한 역할을 나타낸 도이다.
도 2는 본 발명에 따른 CTLA-4IgG3의 돌연변이에 의한 효과를 나타낸 도이다.
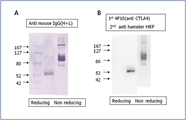
도 3은 본 발명에 따른 신규한 CTLA-4IgG3(K397C)를 확인한 결과를 나타낸 도이다. ((A) CTLA-4IgG3 아미노산 서열의 구성도임 (빨간색, 파란색, 초록색 및 짙은 빨간색은 각각 CTLA-4IgG의 세포 외 도메인, 경첩 영역 (hinge region), CH2 및 CH3을 나타냄) (B) PCR 산물의 1% 아가로오스 겔 전기영동 결과, (C) CTLA-4IgG3의 야생형 및 돌연변이형의 in vitro 전사 및 번역 결과)
도 4는 본 발명에 따른 신규한 CTLA-4IgG3(K397C)의 웨스턴 블로팅 에세이 결과를 나타낸 도이다. ((A) K397C의 IgG3 부분은 항마우스-IgG와 결합된 알칼리 포스파타아제 (alkaline phosphatase, AP)에 의하여 확인됨, (B) K397C의 CTLA-4 세포 외 도메인은 4F10과 HRP conjugated anti-hamster IgG (H+L)를 이용하여 확인됨)
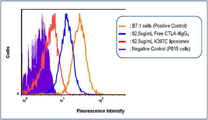
도 5는 야생형 CTLA-4IgG3 또는 K397C의 P815B7-1 결합 에세이 결과를 나타낸 도이다. ((A) 5x105의 B7-1가 표면에 발현되는 P815 (P815B7-1) 세포를 항 마우스 CD80-FITC 및 isotype control로 염색함, (B) 및 (C) 50, 1,000, 및 20,000 ng/mL의 야생형 CTLA-4IgG3 (B) 및 K397C (C)와 함께 배양함.)
도 6은 야생형 CTLA-4IgG3 또는 K397C의 P815B7-1 세포와의 결합을 나타낸 도이다.
도 7은 야생형 CTLA-4IgG3 또는 K397C의 혼합 림프구 반응 결과를 나타낸 도이다.
도 8은 야생형 CTLA-4IgG3 또는 K397C가 리포좀 분자에 결합된 분자 수 계산 결과를 나타낸 도이다.
도 9는 자유 CTLA-4IgG3 또는 K397C 리포좀의 경쟁적 결합 에세이 결과를 나타낸 도이다.
도 10은 자유 CTLA-4IgG3 또는 K397C 리포좀의 P815B7-1 세포와의 결합을 나타낸 도이다.
도 11은 자유 CTLA-4IgG3 , CTLA-4IgG3 리포좀 또는 K397C 리포좀의 혼합림프구반응 억제 효과를 나타낸 결과를 나타낸 도이다.
도 12는 K397C 또는 K397C 리포좀의 동종 췌도 이식 모델에서의 이식 거부 반응을 확인한 결과를 나타낸 도이다. 1 shows various roles of CTLA-4IgG in the immune response.
Figure 2 is a diagram showing the effect of the mutation of CTLA-4IgG 3 according to the present invention.
Figure 3 is a view showing the results confirmed the novel CTLA-4IgG 3 (K397C) according to the present invention. ((A) CTLA-4IgG 3 Schematic diagram of the amino acid sequence (red, blue, green and dark red represent the extracellular domain, hinge region, CH2 and CH3 of CTLA-4IgG, respectively) (B) 1% agarose gel of PCR product Electrophoresis results (C) in vitro transcription and translation of wild and mutant forms of CTLA-4IgG 3 )
4 is a diagram showing the results of Western blotting assay of novel CTLA-4IgG 3 (K397C) according to the present invention. ((A) IgG 3 portion of K397C was identified by alkaline phosphatase (AP) coupled with anti-mouse-IgG. (B) CTLA-4 extracellular domain of K397C was 4F10 and HRP conjugated anti- confirmed using hamster IgG (H + L)
Figure 5 shows the results of P815B7-1 binding assay of wild type CTLA-4IgG 3 or K397C. ((A) P815 (P815B7-1) cells expressing B7-1 at 5 × 10 5 surface were stained with anti mouse CD80-FITC and isotype control, (B) and (C) 50, 1,000, and 20,000 ng / Incubate with mL of wild type CTLA-4IgG 3 (B) and K397C (C).)
6 wild type CTLA-4IgG 3 Or K397C shows the binding to P815B7-1 cells.
Figure 7 is a diagram showing the results of mixed lymphocyte response of wild type CTLA-4IgG 3 or K397C.
8 is a diagram showing the result of calculating the number of molecules in which wild type CTLA-4IgG 3 or K397C is bound to liposome molecules.
Figure 9 shows the results of a competitive binding assay of free CTLA-4IgG 3 or K397C liposomes.
10 shows binding of free CTLA-4IgG 3 or K397C liposomes with P815B7-1 cells.
11 shows free CTLA-4IgG 3 , CTLA-4IgG 3 It is a figure showing the result showing the inhibitory effect of mixed lymphocyte reaction of liposomes or K397C liposomes.
12 is a diagram showing the results of confirming the transplant rejection response in a homogeneous islet transplantation model of K397C or K397C liposomes.
본 발명은 서열번호 3의 아미노산 서열로 표시되는, 면역 글로불린 CH3 도메인 잔기의 C 말단의 아미노산인 라이신이 시스테인으로 치환된 CTLA-4IgG (cytotoxic T lymphocyte antigen 4-Immunoglobulin G) 융합 단백질을 제공한다.The present invention provides a cytotoxic T lymphocyte antigen 4-Immunoglobulin G (CTLA-4IgG) fusion protein in which lysine, an amino acid at the C terminus of an immunoglobulin CH3 domain residue, is represented by the amino acid sequence of SEQ ID NO: 3, substituted with cysteine.
상기 CTLA-4IgG 융합 단백질은 야생형인 CTLA-4IgG 융합 단백질에 비해 지질 비히클에의 방향성이 높으며, 지질 비히클과 결합하여 T 세포의 활성을 효과적으로 억제하는 작용을 수행함으로써 효과적인 면역 억제가 가능하다. The CTLA-4IgG fusion protein has a higher directivity to the lipid vehicle than the wild type CTLA-4IgG fusion protein, and effectively binds to the lipid vehicle to effectively inhibit T cell activity, thereby effectively suppressing immunity.
본 발명에 있어서 용어 “CTLA-4 (cytotoxic T lymphocyte antigen-4)”는 CD28과 유사한 구조를 갖는 단백질로, T 림프구가 활성화되었을 때 발현하는 T 세포 활성 항원의 일종이다. CTLA-4는 항원 제시세포 (APC) 표면의 B7-1 (CD80), B7-2 (CD86)와의 결합력이 CD28의 20배 이상이며, B7과 결합한 후 T 림프구의 활성을 억제하는 신호를 전달하는 역할을 담당한다. CTLA-4의 면역 반응에서의 다양한 역할을 도 1에 나타내었다.In the present invention, the term “CTLA-4 (cytotoxic T lymphocyte antigen-4)” is a protein having a structure similar to that of CD28, which is a kind of T cell active antigen expressed when T lymphocytes are activated. CTLA-4 has a binding capacity of B7-1 (CD80) and B7-2 (CD86) on the surface of antigen presenting cells (APC) at least 20 times that of CD28, and transmits a signal that inhibits T lymphocyte activity after binding to B7. Play a role. Various roles of CTLA-4 in the immune response are shown in FIG. 1.
본 발명의 CTLA-4는 CTLA-4가 존재할 수 있는 모든 개체로부터 유래할 수 있으며, 바람직하게는 인간, 영장류, 설치류 등의 포유류일 수 있다.CTLA-4 of the present invention may be derived from any individual in which CTLA-4 may be present, and may preferably be mammals such as humans, primates, rodents and the like.
본 발명에 있어서 용어 “CTLA-4IgG”는 CTLA-4와 면역 글로불린의 Fc 부위를 유전자 재조합을 통해 융합시킨 구조이다. 본 발명의 “CTLA-4IgG”은 생체 투여시 항원 제시세포 (APC) 표면에 존재하는 B7 (CD80 및 CD86)에 결합하여 APC로부터 T 림프구의 표면에 존재하는 CD28에 가해지는 공조 자극(Costimulattion signal)을 차단하고 면역무력상태(anergy)를 유도함으로써 면역반응을 억제하는 효과를 발생시킨다.In the present invention, the term "CTLA-4IgG" is a structure in which the Fc region of CTLA-4 and immunoglobulin is fused through gene recombination. The "CTLA-4IgG" of the present invention binds to B7 (CD80 and CD86) present on the surface of antigen presenting cells (APC) upon in vivo administration and is applied to CD28 present on the surface of T lymphocytes from APC. Block and induce an immune force (anergy) to produce an effect of suppressing the immune response.
본 발명자는 CTLA-4IgG를 지질 비히클에 결합하여 B7 분자와 결합능을 증가시키고 T세포의 활성을 억제하는 방법에 있어서, 지질 비히클 바람직하게는 리포좀과의 결합능력을 높이기 위하여 CTLA-4IgG 융합 단백질의 면역 글로불린 CH3의 C 말단 (C-terminal)의 아미노산이 시스테인 (cystein)에 해당하면 지질 비히클에의 높은 결합효율을 보인다는 것을 최초로 밝혔다. 돌연변이가 발생한 재조합 CTLA-4IgG 융합 단백질의 지질비히클과의 결합을 도식한 결과는 도 2와 같다.In a method of binding CTLA-4IgG to a lipid vehicle to increase binding capacity with B7 molecules and inhibiting T cell activity, the present inventors have suggested that immunity of CTLA-4IgG fusion protein to enhance binding ability with a lipid vehicle, preferably liposomes. For the first time, the amino acid at the C-terminal of globulin CH3 corresponds to cystein, which shows high binding efficiency to lipid vehicles. Figure 2 shows the result of binding the mutant recombinant CTLA-4IgG fusion protein with the lipid vehicle.
본 발명에 있어서, 상기 면역 글로불린 IgG의 종류는 제한되지 않으며, IgG1, IgG2, IgG3 , IgG4 일 수 있고, 본 발명에서는 일례로 IgG3 을 이용하였다. In the present invention, the type of the immunoglobulin IgG is not limited, IgG 1 , IgG 2 , IgG 3 , IgG 4 In the present invention, for example IgG 3 Was used.
본 발명에서 CTLA-4IgG 융합 단백질은 지질 비히클(vehicle)과 결합한 것일 수 있다. 상기 지질 비히클은 지질을 포함하고 있으며, CTLA-4IgG를 표적세포에 전달할 수 있는 모든 비히클을 포함하며, 바람직하게는 리포좀 (liposome) 또는 마이셀 (Micelle)일 수 있으며, 보다 바람직하게는 리포좀일 수 있다.
In the present invention, the CTLA-4IgG fusion protein may be bound to a lipid vehicle. The lipid vehicle includes lipids, and includes all vehicles capable of delivering CTLA-4IgG to target cells, preferably liposomes or micelles, more preferably liposomes. .
또한, 본 발명은 상기 신규한 CTLA-4IgG (cytotoxic T lymphocyte antigen 4-Immunoglobulin G) 융합 단백질을 유효성분으로 포함하는 면역 억제용 약학적 조성물을 제공한다. The present invention also provides a pharmaceutical composition for immunosuppression comprising the novel CTLA-4IgG (cytotoxic T lymphocyte antigen 4-Immunoglobulin G) fusion protein as an active ingredient.
상기 면역 억제는 장기이식반응 억제용 또는 자가면역질환의 예방 또는 치료용 일 수 있으며, 상기 자가면역 질환은 이에 제한되지는 않으나, 류마티스 관절염, 전신 홍반성 낭창, 소화기 당뇨병, 아토피 피부염, 천식 또는 크론병 일 수 있다.The immune suppression may be for inhibiting organ transplantation or for preventing or treating autoimmune diseases, and the autoimmune disease is not limited thereto, but rheumatoid arthritis, systemic lupus erythematosus, digestive diabetes, atopic dermatitis, asthma or crohn It can be a bottle.
상기 융합 단백질은 전체 약학적 조성물에서 0.001 내지 50중량%, 바람직하게는 0.01 내지 20중량% 포함될 수 있다. 또한, 상기 융합 단백질은 전체 약학적 조성물에서 상기 약학적 조성물이 투여되는 개체의 1kg당 적정량이 투여될 수 있도록 함량을 조절할 수 있다. The fusion protein may be included in 0.001 to 50% by weight, preferably 0.01 to 20% by weight in the total pharmaceutical composition. In addition, the fusion protein may be adjusted in a total pharmaceutical composition so that an appropriate amount may be administered per kg of the individual to which the pharmaceutical composition is administered.
본 발명의 약학적 조성물은 약효를 증가시키지는 않으나 약학적 조성물에 통상 사용되어 냄새, 맛, 시각 등을 향상시킬 수 있는 성분을 추가로 포함할 수 있다. 또한, 상기 조성물은 비타민 B1, B2, B6, C, E, 니아신, 카르니친, 베타인, 엽산 판토텐산, 비오틴, 아연, 철, 칼슘, 크롬, 마그네슘 및 이들의 혼합물 등의 무기 또는 유기 첨가물들을 추가로 포함할 수 있다. The pharmaceutical composition of the present invention does not increase the efficacy, but may further include ingredients that are commonly used in the pharmaceutical composition to improve the smell, taste, time and the like. The composition may further comprise inorganic or organic additives such as vitamins B1, B2, B6, C, E, niacin, carnitine, betaine, folic acid pantothenic acid, biotin, zinc, iron, calcium, chromium, magnesium, As shown in FIG.
본 발명의 상기 약학적 조성물은 약학적으로 허용 가능한 담체를 포함하고 경구 또는 비경구용의 인체 또는 수의용으로 제형화될 수 있다. 본 발명의 조성물을 제제화하는 경우 충진제, 증량제, 결합제, 습윤제, 붕해제 및 계면활성제 등의 희석제 또는 부형제를 사용할 수 있다. 경구투여를 위한 고형제제에는 정제, 환제, 산제, 과립제 및 캡슐제 등이 포함되며, 이러한 고형제제는 본 발명의 조성물에 적어도 하나 이상의 부형제 예를 들면, 전분, 칼슘카보네이트(Calcium carbonate), 수크로오스(Sucrose), 락토오스(Lactose) 또는 젤라틴 등을 섞어 조제한다. 또한 단순한 부형제 이외에 마그네슘, 스티레이트, 탈크 같은 윤활제를 사용할 수 있다. 경구를 위한 액상제제로는 현탁제, 내용액제, 유제 및 시럽제 등이 해당되는데 흔히 사용되는 단순희석제인 물 및 리퀴드 파라핀 이외에 여러 가지 부형제, 예를 들면 습윤제, 감미제, 방향제 및 보존제 등이 포함될 수 있다. 비경구투여를 위한 제제에는 멸균된 수용액, 비수성용제, 현탁제, 유제, 동결건조제제 및 좌제가 포함된다. 비수성용제 및 현탁용제로는 프로필렌글리콜(Propylene glycol), 폴리에틸렌 글리콜 및 올리브 오일과 같은 식물성 기름, 에틸올레이트와 같은 주사 가능한 에스테르 등이 사용될 수 있다.The pharmaceutical composition of the present invention comprises a pharmaceutically acceptable carrier and can be formulated for human or veterinary use for oral or parenteral use. When the composition of the present invention is formulated, diluents or excipients such as fillers, extenders, binders, wetting agents, disintegrating agents and surfactants can be used. Solid preparations for oral administration include tablets, pills, powders, granules, capsules, and the like, and such solid preparations include at least one excipient in the composition of the present invention, for example, starch, calcium carbonate, sucrose ( Sucrose), lactose (Lactose) or gelatin and mix. In addition to simple excipients, lubricants such as magnesium, styrene, and talc may be used. Examples of the liquid preparation for oral use include suspensions, solutions, emulsions and syrups, and various excipients such as wetting agents, sweetening agents, fragrances and preservatives in addition to commonly used simple diluents such as water and liquid paraffin . Formulations for parenteral administration include sterilized aqueous solutions, non-aqueous solutions, suspensions, emulsions, freeze-dried preparations and suppositories. As the non-aqueous solvent and suspending solvent, vegetable oils such as propylene glycol, polyethylene glycol and olive oil, injectable esters such as ethyl oleate and the like can be used.
본 발명의 상기 약학적 조성물은 개체에 투여하여 면역작용을 억제할 수 있다. The pharmaceutical composition of the present invention can be administered to a subject to inhibit immune action.
본 발명에서 사용된 용어, “투여”는 어떠한 적절한 방법으로 개체에 본 발명의 약학적 조성물을 도입하는 것을 의미한다. 투여 경로는 목적 조직에 도달할 수 있는 한 어떠한 일반적인 경로를 통하여 경구 또는 비경구 투여될 수 있다. 또한, 본 발명의 약학적 조성물이 표적 세포로 이동할 수 있도록 하는 임의의 장치에 의해 투여될 수 있다.As used herein, the term “administration” means introducing a pharmaceutical composition of the invention to a subject in any suitable manner. The route of administration may be oral or parenteral via any general route so long as it can reach the desired tissue. In addition, the pharmaceutical composition of the present invention may be administered by any device that allows migration to target cells.
본 발명의 조성물은 약학적으로 유효한 양으로 투여한다. 본 발명에서 사용된 용어, “약학적으로 유효한 양”은 의학적 치료에 적용 가능한 합리적인 수혜/위험 비율로 질환을 치료하기에 충분한 양을 의미하며, 유효 용량 수준은 개체의 성별, 연령, 중증도, 약물의 활성, 약물에 대한 민감도, 투여 시간, 투여 경로 및 배출 비율, 치료 기간, 동시 사용되는 약물을 포함한 요소 및 기타 의학 분야에 잘 알려진 요소에 따라 결정될 수 있다. 본 발명의 조성물은 개별 치료제로 투여하거나 다른 치료제와 병용하여 투여될 수 있고 종래의 치료제와는 순차적 또는 동시에 투여될 수 있으며, 단일 또는 다중 투여될 수 있다. 상기 요소를 모두 고려하여 부작용 없이 최소한의 양으로 최대 효과를 얻을 수 있는 양을 투여하는 것이 중요하며, 당업자에 의해 용이하게 결정될 수 있다. The composition of the present invention is administered in a pharmaceutically effective amount. As used herein, the term “pharmaceutically effective amount” means an amount sufficient to treat a disease at a reasonable benefit / risk ratio applicable to medical treatment, and the effective dose level is the sex, age, severity, drug of the individual. Can be determined according to the activity of the drug, the sensitivity to the drug, the time of administration, the route of administration and the rate of release, the duration of treatment, factors including the drug used concurrently and other factors well known in the medical field. The composition of the present invention can be administered as an individual therapeutic agent or in combination with other therapeutic agents, and can be administered sequentially or simultaneously with conventional therapeutic agents, and can be administered singly or in multiple doses. It is important to take into account all of the above factors and to administer the amount in which the maximum effect can be obtained in a minimal amount without adverse effect, and can be easily determined by those skilled in the art.
본 발명의 조성물은 면역억제를 위하여 단독으로, 또는 수술, 방사선 치료, 호르몬 치료, 화학 치료 및 생물학적 반응 조절제를 사용하는 방법들과 병용하여 사용할 수 있다.
The composition of the present invention may be used alone or in combination with methods using surgery, radiation therapy, hormone therapy, chemotherapy and biological response modulators for immunosuppression.
또한, 본 발명은 CTLA-4IgG 융합 단백질의 C 말단의 아미노산인 라이신을 시스테인으로 치환하는 단계;를 포함하는 CTLA-4IgG 융합 단백질과 지질 비히클과의 결합력을 높이는 방법을 제공한다. 상기 지질 비히클의 종류는 제한되지 않으나, 바람직하게는 리포좀 또는 마이셀일 수 있으며, 보다 바람직하게는 리포좀이다.
In another aspect, the present invention provides a method for enhancing the binding force between the CTLA-4IgG fusion protein and the lipid vehicle, comprising the step of: replacing the lysine amino acid of the C-terminal of the CTLA-4IgG fusion protein with cysteine. The type of the lipid vehicle is not limited, but may preferably be liposomes or micelles, more preferably liposomes.
이하, 본 발명을 실시예에 의거하여 보다 구체적으로 설명한다. 이는 오로지 본 발명을 보다 구체적으로 설명하기 위한 것으로, 본 발명의 요지에 따라 발명의 범위가 이들 실시예에 의해 제한되지 않는다는 것은 당업계에서 통상의 지식을 가진 자에 있어서 자명할 것이다.
Hereinafter, the present invention will be described more specifically based on examples. This is only to explain the present invention in more detail, it will be apparent to those skilled in the art that the scope of the invention is not limited by these examples in accordance with the gist of the present invention.
실시예Example 1. 시료 및 방법 1. Samples and Methods
1-1. 실험동물1-1. Experimental animal
BALB/c (NIH, Bethesda, Maryland, USA)와 C57BL/6 (NIH, Bethesda, Maryland, USA)마우스는 Koatech (Kyunggi-do, KOREA)에서 구매하였으며, 서울대학교 의과대학 무병 동물 사육시설에서 보관하였다.
BALB / c (NIH, Bethesda, Maryland, USA) and C57BL / 6 (NIH, Bethesda, Maryland, USA) mice were purchased from Koatech (Kyunggi-do, KOREA) and stored in disease-free animal breeding facilities at Seoul National University College of Medicine. .
1-2. 세포배양1-2. Cell culture
항-마우스 CTLA4 항체를 분비하는 하이브리도마 세포주인 4F10 (HB304, American Type Culture Ccollection, Manassas, VA, USA) 및 형질감염된 마우스 비만세포인 P815B7.1 세포주 (TIB-64, American Type Culture Collection, Manassas, VA, USA)는 고 글루코오스 함유 DMEM (Dulbecco’s Modified Eagles Medium, Gibco/BRL, Grand Island, NY, USA)에서 배양 및 유지하였으며, 상기 배지는 10 mM의 HEPES (Sigma-Aldrich Inc., St. Louis, MO, USA), 100M의 불필수 아미노산, 55M의 2-머캅토에탄올(β-ME) 및 50 g/mL의 겐타마이신 (Gibco/BRL, Grand Island, NY, USA), 및 열불활성화 (heat-inactivated) 소태아혈청 (fetal bovine serum, FBS, Gibco/BRL, Grand Island, NY, USA)을 포함한다. 4F10 (HB304, American Type Culture Ccollection, Manassas, VA, USA), a hybridoma cell line secreting anti-mouse CTLA4 antibody, and P815B7.1 cell line (TIB-64, American Type Culture Collection, Manassas, transfected mouse mast cells) , VA, USA) were incubated and maintained in high glucose containing DMEM (Dulbecco's Modified Eagles Medium, Gibco / BRL, Grand Island, NY, USA) and the medium was 10 mM HEPES (Sigma-Aldrich Inc., St. Louis). , MO, USA), 100 M of essential amino acids, 55 M 2-mercaptoethanol (β-ME) and 50 g / mL gentamicin (Gibco / BRL, Grand Island, NY, USA), and heat inactivation (heat -inactivated fetal bovine serum (Ftal bovine serum, FBS, Gibco / BRL, Grand Island, NY, USA).
중국 햄스터 난소 (Chinese hamster ovary, CHO) K-1 세포 (SNUMC의 Dr. Cho가 제공)는 DMEM 배지에서 생장하였으며, 단백질 준비를 위하여 완전 무혈청배지인 Opti-CHO (Gibco/BRL, Grand Island, NY, USA)에서 대량배양하였다. Chinese hamster ovary (CHO) K-1 cells (provided by Dr. Cho of SNUMC) were grown in DMEM medium and used for complete protein preparation, Opti-CHO (Gibco / BRL, Grand Island, Mass culture in NY, USA).
모든 세포는 37 ℃에서 7% CO2의 조건에서 생장하였다.
All cells were grown at 37 ° C. under 7% CO 2 .
1-3. 1-3. DNADNA 구조체 및 형질감염 Constructs and Transfection
마우스 CTLA-4IgG3의 DNA구조체는 Dr. Jeffery A. Bluestone의 실험실에서 얻었다. 모든 플라스미드 DNA는 DNA preparation kit (Genenmed, Seoul, Korea)를 이용하여 준비하였다.The DNA construct of mouse CTLA-4IgG 3 was determined by Dr. Got from Jeffery A. Bluestone's lab. All plasmid DNA was prepared using a DNA preparation kit (Genenmed, Seoul, Korea).
C-말단 잔기의 AAA(lysine)를 ACA(cysteine)로 돌연변이 시키는 IgG3의 말단의 점돌연변이는 공지된 PCR 방법에 의하여 제작하였다. 보다 구체적으로, PCR 반응은 시스테인 (하기 안티센스 프라이머 서열 중 굵게 표시)을 포함하는 두 프라이머와 Han-pfu 중합효소 (Genenmed, Seoul, Korea)에 의하여 수행되었다 (Sense: 5’-GTG GTA CCT TTA ATG AAA-3’(18 mer) (서열번호 1), antisense: 5’-GCT CTA GAG CTG TTC TCA ACA ACC AGG GGA GCG A-3’(34 mer) (서열번호 2)). 1 ng의 CTLA-4IgG3 DNA, 0.2 uM의 센스 및 안티센스 프라이머, 5μl의 10 x 반응 완충액 (Han-pfu 폴리머라아제가 보충됨), 10 mM의 dNTP, 1μl의 Han-pfu 중합효소를 최종 부피 50μl이 되게 혼합한 후, 94℃에서 3 분간 전-변성 (pre-denaturation) 후, 94 ℃에서 1분간 변성, 58℃에서 1분간 어닐링, 72℃에서 1분간 신장 단계를 35 사이클 반복한 후, 72℃에서 10분간 후-신장 (post elongation)을 수행하였다.Point mutations of the ends of IgG 3 to a mutation AAA (lysine) in the C- terminal residue with ACA (cysteine) was prepared by the known PCR method. More specifically, the PCR reaction was carried out by Han-pfu polymerase (Genenmed, Seoul, Korea) and two primers containing cysteine (in bold in the antisense primer sequence below) (Sense: 5'-GTG GTA CCT TTA ATG AAA-3 '(18 mer) (SEQ ID NO: 1), antisense: 5'-GCT CTA GAG CTG TTC TCA ACA ACC AGG GGA GCG A-3' (34 mer) (SEQ ID NO: 2)). Final volume of 1 ng CTLA-4IgG 3 DNA, 0.2 uM sense and antisense primer, 5
PCT 결과물은 KpnI 및 XbaI에 의하여 절단하였으며, pcDNA3.1 (Invitrogen Life Technology, Carlsbad, CA, USA)에 라이게이션하였다. PCT results were cut by KpnI and XbaI and ligated to pcDNA3.1 (Invitrogen Life Technology, Carlsbad, Calif., USA).
이 후, 준비된 플라스미드 DNA를 E. coli DH5 알파에 형질전환하였다. 돌연변이 자리는 전체 길이의 DNA 시퀀싱 분석을 이용하여 확인하였다. CHO 세포는 대수성장기에서 수집하였으며, 전기천공법을 아래와 같은 조건으로 수행하였다. 10μg의 pcDNA3.1-CTLA4 야생형 또는 본 발명의 pcDNA3.1-CTLA-4IgG3 Cys (이하 "K397C"라고 표기)를 각각 전기천공 크벳 (cuvette)의 5x106 세포와 혼합한 뒤, 25F와 무한저항 (infinite resistance)에서 300 volt로 Gene pulser X cell system (Bio-RAD Laboratories, Hercules, California, USA)을 이용하여 전기천공하였으며, 100 mm의 배양 디쉬에 플레이팅 하였다.
Thereafter, the prepared plasmid DNA was transformed into E. coli DH5 alpha. Mutagenesis was identified using full length DNA sequencing analysis. CHO cells were collected in logarithmic growth phase, and electroporation was performed under the following conditions. 10 μg of pcDNA3.1-CTLA4 wild type or pcDNA3.1-CTLA-4IgG 3 Cys of the present invention (hereinafter referred to as "K397C") were respectively mixed with 5x10 6 cells of electroporation cuvette, followed by 25F and infinite resistance. (infinite resistance) was electroporated using a Gene pulser X cell system (Bio-RAD Laboratories, Hercules, California, USA) at 300 volt and plated on a 100 mm culture dish.
1-4. 1-4. inin vitrovitro 전사 및 번역 Transcription and Translation
in vitro 전사 및 번역에는 TNT Quick Coupled Transcription/Translation System (Promega)을 사용하였으며, 번역 산물을 검출하기 위해 TranscendTM Non-Radioactive Translation Detection System (Promega)을 사용하였다. TNT Quick Coupled Transcription / Translation System (Promega) was used for in vitro transcription and translation, and TranscendTM Non-Radioactive Translation Detection System (Promega) was used to detect translation products.
보다 구체적으로, 1 μg의 pcDNA3.1-CTLA4IgG3 및 pcDNA3.1-CTLA4IgCys는 50μl의 반응 혼합물 (40μl TNT T7 quick master mix, 1 mM 메티오닌 및 2μl TranscendTM Biotin-Lysyl-tRNA를 포함)에서 30℃, 90분간 전사 및 번역되었다. 반응 산물은 SDS-PAGE로 분리하였으며, PVDF 막 (membrane)으로 옮겼다. 구체적으로 막 이동 (Membrane Transfer)은 193 mM의 글리신 (glycine), 25 mM의 Trisand, 20%의 메탄올에서 2시간 동안 100V 및 4℃ 조건에서 수행하였다. 이후, 막을 5%의 무지방 분유 및 0.1%의 Tween-20을 포함한 20 mM의 Tris/HCl(pH 7.3)/137 mM NaCl(TBST) 완충액에 담근 후, 실온에서 3시간 동안 방치하였다. 웨스턴 블랏을 위해 1:10,000으로 희석된 검출 항체인 streptavidin-HRP conjugate를 첨가한 후, 2시간 동안 실온에서 배양하였다. 검출은 Supersignal West Pico Chemiluminescent Substrate (Pierce, Thermo scientific, Rockford, IL, USA)를 이용하였다.
More specifically, 1 μg of pcDNA3.1-CTLA4IgG3 and pcDNA3.1-CTLA4IgCys were reacted at 50 ° C. in a reaction mixture of 50 μl (including 40 μl TNT T7 quick master mix, 1 mM methionine and 2 μl TranscendTM Biotin-Lysyl-tRNA). Transcribed and translated for a minute. The reaction product was separated by SDS-PAGE and transferred to PVDF membrane. Specifically, membrane transfer was performed at 193 mM glycine, 25 mM Trisand, 20% methanol at 100V and 4 ° C. for 2 hours. The membrane was then immersed in 20 mM Tris / HCl (pH 7.3) / 137 mM NaCl (TBST) buffer containing 5% nonfat dry milk and 0.1% Tween-20 and then left at room temperature for 3 hours. For Western blot, the streptavidin-HRP conjugate, a detection antibody diluted 1: 10,000, was added and then incubated at room temperature for 2 hours. Detection was performed using Supersignal West Pico Chemiluminescent Substrate (Pierce, Thermo scientific, Rockford, IL, USA).
1-5. 분비된 단백질 측정 및 정량1-5. Measuring and Quantifying Secreted Proteins
선발 및 대량 배양 중에서의 CTLA-4IgG량을 정량하기 위하여, 샌드위치-ELISA가 아래의 항체들을 이용하여 수행하였다. 포획 항체 (Capturing antibody), 검출항체 (detecting antibody)로 항-CTLA4 단클론성 항체 (4F10), HRP conjugated 항-마우스 IgG3 항체)를 이용하였다. 2μg/mL의 PBS 에 희석된 항-CTLA4 모노크로날 항체는 96-well MaxiSorpTM ELISA plate (Nunc, Rochester, NY, USA)에 코팅하였으며, 4℃에서 하룻밤 배양하였으며, 0.05%의 Tween-20 (Sigma-Aldrich Inc., St. Louis, MO, USA)을 포함하는 PBST로 3 차례 세척하였다. PBST에 포함된 5%의 탈지분유 (Difco, Spark, MD, USA)를 이용하여 각 웰을 블로킹한 뒤, 희석된 샘플 (1:100~1:100,000)을 각각의 웰에 첨가한 후 상온에서 2시간 동안 반응하였으며, PBST를 이용하여 5차례 세척하였다. 100μl의 TMB 기질 용액 (eBioscience, San Diego, CA, USA)이 각 웰에 5분간 더해졌으며, 동일한 부피의 0.18M의 H2SO4가 발색반응을 멈추기 위하여 사용되었다. 플레이트는 450nm의 흡광도에서 판독되었다.
In order to quantify the amount of CTLA-4IgG in selection and mass culture, sandwich-ELISA was performed using the following antibodies. Capturing antibody, anti-CTLA4 monoclonal antibody (4F10), HRP conjugated anti-mouse IgG 3 antibody) were used as a detecting antibody. Anti-CTLA4 monoclonal antibody diluted in 2 μg / mL PBS was coated on a 96-well MaxiSorpTM ELISA plate (Nunc, Rochester, NY, USA), incubated overnight at 4 ° C, and 0.05% Tween-20 (Sigma) 3 washes with PBST, including Aldrich Inc., St. Louis, MO, USA). Each well was blocked using 5% skim milk powder (Difco, Spark, MD, USA) contained in PBST, and then diluted samples (1: 100-1: 100,000) were added to each well at room temperature. The reaction was carried out for 2 hours, and washed five times using PBST. 100 μl of TMB substrate solution (eBioscience, San Diego, CA, USA) was added to each well for 5 minutes and an equal volume of 0.18 M H 2 SO 4 was used to stop the color reaction. The plate was read at absorbance of 450 nm.
1-6. 1-6. CTLACTLA -4-4 IgGIgG 33 의 준비Preparation of
총 배양 상층액을 수득하고 Avanti J-E high speed centrifuge (Beckman Coulters, Fullerton, CA, USA)를 이용하여 12000 x g로 4℃에서 원심분리하였다. 원심 분리 후, 상층액을 수확하여 steriletop 필터 (Millipore, Billerica, MA, USA)로 여과하였다. 4F10-sepharose 컬럼을 3 bed volume의 PBS와 평형시킨 뒤, 여과된 샘플을 컬럼에 적용시키고 겔 속으로 완전히 흐르도록 하였다. 몇 번의 회전 뒤, 30 mL의 Immunopure® IgG 용출 완충액 (Pierce Biotechnology, Rockford, IL, USA)을 컬럼에 더하였으며, pH 8.0인 100 μl의 1M Tris를 포함하는 1.5 mL 튜브에 1.0 mL의 분획물을 수집하였다. 수집된 분획물은 NanoDrop, ND-1000 (Thermo Scientific, Wilmington, DE, USA)을 이용하여 정량하였으며, Amicon Ultra centrifugal filter (Millipore, Billerica, CA, USA)로 농축하였다. 농축된 분획물은 BCA assay kit (Pierce Biotechnology, Rockford, IL, USA)로 정량하였다.
Total culture supernatants were obtained and centrifuged at 4 ° C. at 12000 × g using Avanti JE high speed centrifuge (Beckman Coulters, Fullerton, Calif., USA). After centrifugation, the supernatant was harvested and filtered with a steriletop filter (Millipore, Billerica, Mass., USA). After equilibrating the 4F10-sepharose column with 3 bed volume of PBS, the filtered sample was applied to the column and allowed to flow completely into the gel. After several turns, 30 mL of Immunopure ® IgG elution buffer (Pierce Biotechnology, Rockford, IL, USA) was added to the column and 1.0 mL fractions were collected in a 1.5 mL tube containing 100 μl of 1M Tris at pH 8.0. It was. Collected fractions were quantified using NanoDrop, ND-1000 (Thermo Scientific, Wilmington, DE, USA) and concentrated with Amicon Ultra centrifugal filter (Millipore, Billerica, Calif., USA). The concentrated fractions were quantified by BCA assay kit (Pierce Biotechnology, Rockford, IL, USA).
1-7. 1-7. 웨스턴Western 블롯팅Blotting
5μg~10μg의 정제된 CTLA-4IgG3 단백질은 환원 (reducing)과 비-환원(non-reducing)의 2가지 조건에서 SDS-PAGE로 로딩하였으며, PVDF 막으로 옮겼다. 그 뒤, PVDF 막을 5%의 탈지분유를 포함하는 PBST에서 2시간 동안 상온에서 배양하였으며, 15mL의 2%의 탈지분유를 포함하는 PBST에서 10μg의 항-마우스 CTLA4 항체인 4F10로 프로브화하였다. 이를 상온에서 2시간 또는 4℃에서 밤새도록 가벼운 쉐이킹 상태로 배양한 뒤, PBST로 3차례 세척하였으며, 검출 항체 (detection antibody)로서 HRP-conjugated anti Armenian hamster IgG (Santa Cruz Biotechnology, Delaware, CA, USA)를 첨가한 후, 2시간 동안 상온에서 배양하였다. 2mL의 Enhanced Chemiluminescent (ECL) 시약 (Pierce Biotechnology, Rockford, IL, USA)을 이용하여 검출하였다.
5 μg-10 μg of purified CTLA-4IgG 3 protein was loaded by SDS-PAGE under two conditions, reducing and non-reducing, and transferred to PVDF membrane. PVDF membranes were then incubated for 2 hours at room temperature in PBST containing 5% skim milk powder and probed with 4F10, 10 μg anti-mouse CTLA4 antibody in PBST containing 15 mL of 2% skim milk powder. The cells were incubated in a light shaking state at room temperature for 2 hours or 4 ° C. overnight, and then washed three times with PBST. HRP-conjugated anti Armenian hamster IgG (Santa Cruz Biotechnology, Delaware, CA, USA) ) Was added and incubated at room temperature for 2 hours. Detection was performed using 2 mL of Enhanced Chemiluminescent (ECL) reagent (Pierce Biotechnology, Rockford, IL, USA).
1-8. 결합 에세이1-8. Combined essay
각기 다른 농도의 CTLA-4IgG3 단백질을 5x105의 마우스 B7.1 발현 세포주인 P815 B7.1에 부가하였으며, 4℃에서 1시간 동안 배양하였다. 상기 세포를 소혈청알부민 (bovine serum albumin) (0.5%, Sigma-Aldrich Inc., St. Louis, MO, USA)과 아지드화나트륨 (sodium azide) (0.05%, Sigma-Aldrich Inc., St. Louis, MO, USA)을 포함하는 FACS 완충액을 이용하여 2차례 세척하였으며, FITC-conjugated 항 마우스 IgG 다클론 항체 (SC-2010, Santa Cruz Biotechnology, Delaware, CA, USA)와 함께 1시간 동안 다시 배양하였다. FITC 양성 세포를 FACS Canto II (Becton Dickinson, Mountain View, CA, USA)를 이용하여 검출하였고, FACS Diva software(Becton Dickinson, Mountain View, CA, USA)를 이용하여 분석하였다.
Different concentrations of CTLA-4IgG 3 protein were added to P815 B7.1, a 5 × 10 5 mouse B7.1 expressing cell line, and incubated at 4 ° C. for 1 hour. The cells were treated with bovine serum albumin (0.5%, Sigma-Aldrich Inc., St. Louis, MO, USA) and sodium azide (0.05%, Sigma-Aldrich Inc., St. Washed twice with FACS buffer containing Louis, MO, USA) and re-incubated with FITC-conjugated anti mouse IgG polyclonal antibody (SC-2010, Santa Cruz Biotechnology, Delaware, CA, USA) for 1 hour. It was. FITC positive cells were detected using FACS Canto II (Becton Dickinson, Mountain View, CA, USA) and analyzed using FACS Diva software (Becton Dickinson, Mountain View, CA, USA).
1-9. 동종 혼합 림프구 반응 (1-9. Homologous mixed lymphocyte response ( AllogeneicAllogeneic MixedMixed LymphocyteLymphocyte ReactionReaction , allogeneic , allogeneic MLRMLR ))
정상 BALB/c mice에서 5x105의 비장세포를 수확하였으며, 이를 동종 혼합 림프구 반응에서 자극자 세포로 이용하였다. 이 자극자는 20 Gy로 γ-선 조사하여 T 세포를 제거하였다. 동일한 수의 반응자 세포는 C57BL/6 mice의 비장세포로부터 준비하였다. 자극자 및 반응자 세포 모두 96-웰 둥근 바닥 플레이트 (Falcon, Becton Dickinson, CA, USA)에 총 부피가 0.2mL이 되도록 동일한 세포 수 (5x105)로 혼합하였다. 다양한 농도의 CTLA-4IgG3 (야생형), CTLA-4IgG3 리포좀, K397C (돌연변이형) 및 K397C 리포좀이 웰에 분리되어 더해졌다. 혼합된 세포들은 24, 48, 72시간 동안 37℃에서 배양하였으며, 18시간 동안 1μ Ci의 [3H]-thymidine (Amersham Bioscience, Bucks, UK)로 펄스 (pulse)되었다. T 세포의 증식에 따른 3H-thymidine의 흡수량은 Wallac MicroBeta ® TriLux (PerkinElmer, Waltham, MA, USA)를 이용한 신텔레이션 계수 (scintillation counting)로 측정하였다.
5x10 5 splenocytes were harvested from normal BALB / c mice and used as stimulator cells in allogeneic mixed lymphocyte response. This stimulator was irradiated with γ-ray at 20 Gy to remove T cells. The same number of responder cells were prepared from splenocytes of C57BL / 6 mice. Both stimulator and responder cells were mixed in 96-well round bottom plates (Falcon, Becton Dickinson, Calif., USA) with the same cell count ( 5 × 10 5 ) to a total volume of 0.2 mL. Various concentrations of CTLA-4IgG 3 (wild type), CTLA-4IgG 3 liposomes, K397C (mutant) and K397C liposomes were added to the wells separately. Mixed cells were incubated at 37 ° C. for 24, 48 and 72 hours and pulsed with 1 μ Ci of [ 3 H] -thymidine (Amersham Bioscience, Bucks, UK) for 18 hours. 3 H-thymidine uptake by T cell proliferation was measured by scintillation counting using Wallac MicroBeta ® TriLux (PerkinElmer, Waltham, MA, USA).
1-10. 경쟁적 결합 에세이1-10. Competitive Combination Essay
배양한 P815 세포와 세포 표면에 B7-1을 표현하는 P815 세포 (B7.1 cells)를 수확하여 카운트하였으며, FACS 완충액으로 (0.5%의 소혈청알부민과 0.05%의 아지드화 나트륨을 포함하는 PBS) 2차례 세척한 뒤, 5x106 cells/mL로 희석하여 튜브에 분배하였다. 20mL의 각각의 다른 농도의 CTLA-4IgG3 리포좀, K397C 리포좀 또는 자유 CTLA-4IgG3가 세포에 더해졌으며, 30분간 4℃에서 배양하였다. 그리고 10 mL의 0.5 mg/mL CTLA-4IgG3-FITC (WT의 CTLA-4IgG3과 함께 형성)가 각 샘플에 더해졌으며 4℃에서 45분간 배양하였다. 반응을 끝낸 세포들은 모두 FACS 완충액으로 3차례 세척하였으며, 형광은 488/530 nm에서 FACS Canto II (Becton Dickinson, Mountain View, CA, USA)로 측정하고, FACS Diva software (Becton Dickinson, Mountain View, CA, USA)로 측정하였다.
Cultured P815 cells and P815 cells expressing B7-1 (B7.1 cells) were harvested and counted on the cell surface, and were counted with FACS buffer (PBS containing 0.5% bovine serum albumin and 0.05% sodium azide). After washing twice, the solution was diluted to 5 × 10 6 cells / mL and dispensed into the tube. 20 mL of each different concentration of CTLA-4IgG 3 liposomes, K397C liposomes or free CTLA-4IgG 3 was added to the cells and incubated at 4 ° C. for 30 minutes. And 10 mL of 0.5 mg / mL CTLA-4IgG 3 -FITC (formed with CTT-4IgG 3 of WT) was added to each sample and incubated at 4 ° C. for 45 minutes. After completion of the reaction, all cells were washed three times with FACS buffer, the fluorescence was measured by FACS Canto II (Becton Dickinson, Mountain View, CA, USA) at 488/530 nm, and FACS Diva software (Becton Dickinson, Mountain View, CA). , USA).
1-11. 1-11. 리포좀의Liposomal 준비 Ready
리포좀은 말레이미드 작용기를 갖는 PE의 유도체인 2 mole %의 MPB-PE(maleimideparabenzoic-PE)와 2:1의 몰랄비의 포스파티딜콜린 (phosphatidycholine) (PC, Avanti Lipid Inc., Alabaster, AL, USA)과 콜레스테롤 (Calbiochem, La Jolla, CA, USA)로 구성하였다. 이를 혼합한 뒤, 용매를 증발시키고 (Rotovapor R-200, Buchi), 다층상 지질 필름을 HBSE (10 mM HEPES, 140 mM NaCl, 1 mM EDTA, pH 7.0)로 수화시켰다. 지질 용액은 4차례의 동결-융해 반복을 받았으며, 0.1mm 크기의 폴리카보네이트 필터 (polycarbonate filters) (Nucleopore Corning, Acton, MA, USA)를 이용하여 4차례의 막 압출 (Lipex, Vancouver, BC, Canada)을 수행하였다. 압출 후, 리포좀은 사용될 때까지 4℃에서 아르곤 가스 하에 보관하였다.
Liposomes are 2 mole% MPB-PE (maleimideparabenzoic-PE), a derivative of PE with maleimide functional groups, and 2: 1 molalbi phosphatidycholine (PC, Avanti Lipid Inc., Alabaster, AL, USA) Cholesterol (Calbiochem, La Jolla, CA, USA). After mixing this, the solvent was evaporated (Rotovapor R-200, Buchi) and the multilayered lipid film was hydrated with HBSE (10 mM HEPES, 140 mM NaCl, 1 mM EDTA, pH 7.0). The lipid solution was subjected to four freeze-thaw cycles and four membrane extrusions (Lipex, Vancouver, BC, Canada) using 0.1 mm polycarbonate filters (Nucleopore Corning, Acton, MA, USA). ) Was performed. After extrusion, liposomes were stored under argon gas at 4 ° C. until used.
1-12. 1-12. CTLACTLA -4-4 IgGIgG 33 의 of 리포좀에의Liposomes 결합 Combination
SATASATA (( SuccinimidylSuccinimidyl -S--S- AcetylthioAcetylthio -- acetateacetate )를 이용한 ) CTLACTLA -4-4 IgGIgG 33 의 변형Variation of
2 mg/mL 농도로 PBS에 담겨진 CTLA-4IgG3는 25 ℃에서 25분동안 메틸 포말레이드에 용해될 수 있는 SATA와 함께 배양하였다. CTLA-4IgG3와 SATA의 몰랄 비 및 부피비는 각각 1:20 및 1:100이다. 반응하지 않은 SATA는 4 ℃에서 투석을 통하여 밤새 제거하였다. 탈아세틸화는 리포좀과의 혼합 바로 뒤에 25℃에서 2시간 동안 탈아세틸화 용액 (0.5 M hydroxylamine, 50 mM sodium phosph ate, 25 mM EDTA, pH 7.0)에서 수행하였다.
CTLA-4IgG 3 in PBS at a concentration of 2 mg / mL was incubated with SATA, which can be dissolved in methyl pomale for 25 minutes at 25 ° C. The molar ratio and volume ratio of CTLA-4IgG 3 and SATA are 1:20 and 1: 100, respectively. Unreacted SATA was removed overnight via dialysis at 4 ° C. Deacetylation was performed in deacetylation solution (0.5 M hydroxylamine, 50 mM sodium phosphate, 25 mM EDTA, pH 7.0) for 2 hours at 25 ° C. immediately after mixing with liposomes.
디티오트레이톨(dithiothreitol, DTT)을Dithiothreitol (DTT) 이용한 Used K397CK397C 의 변형 Variation of
K397C 단백질은 25℃에서 15분간 2 mM의 DTT로 환원하였다. 반응이 끝난 뒤, 환원된 단백질은 세파덱스 G-25 컬럼(GE Healthcare, San Jose, CA, USA)을 이용하여 DTT로부터 정제하였다. 용출액을 농축하였으며 단백질 농도는 UV 흡광도를 통하여 결정하였다.
K397C protein was reduced with 2 mM DTT at 25 ° C. for 15 minutes. After the reaction, the reduced protein was purified from DTT using a Sephadex G-25 column (GE Healthcare, San Jose, Calif., USA). The eluate was concentrated and protein concentration was determined via UV absorbance.
maleimidemaleimide -- PEPE -포함 -include 리포좀에의Liposomes 결합 Combination
Maleimide-포함 리포좀을 지질과 CTLA-4IgG3이 290:1의 몰랄비를 갖도록 탈아세틸화된 CTLA-4IgG3-linked SATA 용액 (야생형) 및 환원된 K397C (돌연변이형)에 첨가하였으며, 4℃에서 아르곤 가스 존재 하에서 가벼운 교반으로 밤새 배양하였다. 결합 후, 결합되지 않은 자유 CTLA-4IgG3 단백질은 겔 여과 (1 x 20 cm sepharose CL-4B column) (GE Healthcare, San Jose, CA, USA)를 통하여 제거하였으며, 용출액은 수집하였다. 결합된 CTLA-4IgG3와 컬럼에서의 리포좀 분획에의 인지질의 양은 각각 양적 SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis)와 포스파타아제 어세이를 이용하여 검사하였다. 결합된 리포좀 당 CTLA-4IgG3 분자는 100 nm의 리포좀 크기에 기초하여 계산하였다.
Maleimide-containing liposomes were added to a deacetylated CTLA-4IgG 3 -linked SATA solution (wild type) and reduced K397C (mutant) such that the lipid and CTLA-4IgG 3 had a 290: 1 molar ratio. Incubate overnight with light agitation in the presence of argon gas. After binding, unbound free CTLA-4IgG 3 Protein was removed via gel filtration (1 × 20 cm sepharose CL-4B column) (GE Healthcare, San Jose, CA, USA) and eluate was collected. The amount of bound CTLA-4IgG 3 and the phospholipids in the liposome fraction in the column were examined using quantitative SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and phosphatase assay, respectively. CTLA-4IgG 3 molecules per bound liposome were calculated based on liposome size of 100 nm.
실시예Example 2. 결과 2. Results
2-1. 2-1. CTLACTLA -4 -4 IgGIgG 33 과 돌연변이의 Of mutations DNADNA 구조체 Structure
IgG3의 CH3 도메인의 C 말단의 아미노산은 본래 라이신이지만, 이를 PCR을 이용한 돌연변이 생성을 통해 시스테인으로 변경하였다. 최종 생성된 돌연변이 CTLA-IgG3 융합 단백질의 아미노산 서열은 서열번호3 및 도 3A에 표시하였다. The amino acid at the C terminus of the CH3 domain of IgG 3 was originally lysine, but this was changed to cysteine through mutagenesis using PCR. The amino acid sequence of the resulting mutated CTLA-IgG 3 fusion protein is shown in SEQ ID NO: 3 and FIG. 3A.
돌연변이를 갖는 PCR 산물은 1 % 아가오로스 겔에서의 전기영동을 통해 확인하였다. 그 결과를 도 3B에 나타내었다. PCR products with mutations were identified by electrophoresis on 1% agarose gels. The results are shown in Figure 3B.
도 3B에 나타낸 바와 같이, 1.2 kb 크기임을 확인하였다. As shown in Figure 3B, it was confirmed that the size is 1.2 kb.
또한, 상기 DNA 구조체는 진균세포 발현 벡터인 pcDNA3.1으로 서브클론하였으며, PCR 산물의 총 DNA 서열은 DNA 시퀀싱 분석(Macrogen corp. Seoul)에 의해 확인하였다. pcDNA3.1-CTLA-4IgG3 단백질 발현은 TNT® Quick Coupled Transcription/Translation System (Promega, Madison, MI, USA), SDS-PAGE 및 웨스턴 블랏을 통하여 확인하였다. 그 결과를 도 3C에 나타내었다. In addition, the DNA construct was subcloned with pcDNA3.1, a fungal cell expression vector, and the total DNA sequence of the PCR product was confirmed by DNA sequencing analysis (Macrogen corp. Seoul). pcDNA3.1-CTLA-4IgG 3 protein expression was confirmed by TNT ® Quick Coupled Transcription / Translation System (Promega, Madison, MI, USA), SDS-PAGE and Western blot. The results are shown in Figure 3C.
도 3C에 나타낸 바와 같이, 야생형과 돌연변이형 CTLA-4IgG3 모두 45 KDa의 단백질 밴드를 나타내었다.
As shown in FIG. 3C, both wild type and mutant CTLA-4IgG 3 exhibited a protein band of 45 KDa.
2-2. 2-2. DNADNA 의 형질주입 및 세포주의 정제 및 안정화And Stabilization of Transfection and Cell Lines in Cells
pcDNA3.1-CTLA-4IgG3와 pcDNA3.1-CTLA-4IgG3 Cys의 두 가지 형태의 구조체를 endotoxin free plasmid maxi prep kit (MACHEREY-NAGEL GmbH & Co. KG, Neumann-Neander-Straße, Duren, Germany)를 이용하여 정제하였다. 정제된 DNA 구조체를 중국 햄스터 난소 (CHO)세포 안으로 전기천공법을 이용하여 형질주입하였다. 2 mg/mL의 Geneticin® (G-418) 존재하에서 형질주입된 세포를 선발한 뒤, 안정화된 세포주의 확보를 위하여 콜로니를 선발하였으며, 높은 수준의 CTLA-4IgG3 단백질을 분비하는 세포를 선택하기 위하여, ELISA를 이용하여 분비된 단백질을 측정 및 정량하였다.
Two types of constructs, pcDNA3.1-CTLA-4IgG 3 and pcDNA3.1-CTLA-4IgG 3 Cys, were prepared using endotoxin free plasmid maxi prep kit (MACHEREY-NAGEL GmbH & Co. KG, Neumann-Neander-Straße, Duren, Germany). Purified using). Purified DNA constructs were transfected into Chinese hamster ovary (CHO) cells using electroporation. After transfected cells were selected in the presence of 2 mg / mL of Geneticin ® (G-418), colonies were selected to secure a stable cell line, and cells were selected to secrete high levels of CTLA-4IgG 3 protein. For this purpose, the secreted protein was measured and quantified using ELISA.
2-3. 대량 배양 및 정제2-3. Mass Culture and Purification
안정화된 세포주의 설계 이후, 세포 대량배양을 위하여 무혈청 CHO 세포 배지에 접종하였다. 총 6L의 배양 상층액을 수득하였으며, 본 발명의 CTLA-4IgG3의 돌연변이형 (K397C)은 protein G 세파로오스 비드 (sepharose bead)에 대한 낮은 결합력 때문에, 세파로오스 비드 (sepharose bead)에 결합된 항-CTLA4 mAb인 4F10에 의하여 정제하였다. 정제된 4F10 항체는 생산자가 제공한 프로토콜에 따라 CNBr-활성화된 세파로오스 비드와 교차연결하였다. 4F10-세파로오스 비드 (sepharose bead)와 CTLA4와의 결합력은 1mL의 비드 슬러리 당 1mg이다. 총 2L의 배양 상층액이 단백질 정제를 위하여 1차례의 반응 당 3 mL의 4F10 세파로오스 비드를 사용하였다. 정제된 단백질은 SDS-PAGE 수행 결과 환원 조건에서는 55 KDa, 비-환원 조건에서는 110 KDa로 확인되었다 (도 4). 이러한 단백질 크기의 상승은 CHO세포에서 타겟 단백질의 글루코시레이션 때문일 것으로 판단된다.
After the design of the stabilized cell lines, they were seeded in serum-free CHO cell medium for cell mass culture. A total of 6 L of culture supernatant was obtained, and the mutant (K397C) of CTLA-4IgG 3 of the present invention bound to Sepharose beads because of their low binding capacity to protein G sepharose beads. Purified by 4F10, an anti-CTLA4 mAb. Purified 4F10 antibody was crosslinked with CNBr-activated Sepharose beads according to the protocol provided by the producer. The binding force between 4F10-sepharose beads and CTLA4 is 1 mg per 1 mL bead slurry. A total of 2 L of culture supernatant used 3 mL of 4F10 Sepharose beads per reaction for protein purification. Purified protein was found to be 55 KDa under reducing conditions and 110 KDa under non-reducing conditions as a result of SDS-PAGE (FIG. 4). This increase in protein size is believed to be due to glucosination of the target protein in CHO cells.
2-4. 2-4. K397CK397C 의 B7-1과의 결합 에세이Combination Essay with B7-1
정제된 K397C 단백질이 그의 타겟 분자인 B7-1(CD80)에 결합하는지를 확인하기 위하여, 다양한 함량의 정제된 단백질이 B7-1이 발현되는 P815 세포주인 P815B7-1에 첨가하였다. 유동세포계수 (flow cytometry)를 이용하여 검출하기 위하여 0.5mg의 FITC-conjugated anti-mouse IgG 항체와 함께 배양하였다. 그 결과를 도 5 및 6에 나타내었다. To confirm that the purified K397C protein binds to its target molecule, B7-1 (CD80), various amounts of purified protein were added to P815B7-1, a P815 cell line expressing B7-1. Cultures were incubated with 0.5 mg of FITC-conjugated anti-mouse IgG antibody for detection using flow cytometry. The results are shown in FIGS. 5 and 6.
도 5 및 도 6에 나타낸 바와 같이, 평균 형광감도가 농도비례적으로 증가하였다. 특히, K397C는 야생형인 CTLA-4IgG3와 비교하여 유사한 결합효력을 보였다.
As shown in Fig. 5 and 6, the average fluorescence sensitivity was increased proportionally. In particular, K397C showed a similar binding effect compared to the wild type CTLA-4IgG 3 .
2-5. 2-5. K397CK397C 의 of MLRMLR 반응 억제 효과 Inhibitory effect
면역반응에서의 K397C의 역할을 확인하기 위하여, K397C 또는 야생형인 CTLA-4IgG3를 MHC-disparate allogeneic (BALB/c 및 C57BL/6) MLR 조건에 첨가하였다. 그 결과를 도 7에 나타내었다. To confirm the role of K397C in the immune response, K397C or wild type CTLA-4IgG 3 was added to MHC-disparate allogeneic (BALB / c and C57BL / 6) MLR conditions. The results are shown in Fig.
도 7에 나타낸 바와 같이, 음성대조군에서는 시간 의존적으로 CPM 값 (count per minute, proliferation index)이 증가하였으나, K397C (0.5 ~6.0 mg) 처리시, 생장 반응이 농도의존적으로 현저하게 감소하였다. 이러한 결과를 통해 K397C가 T 세포 증식 억제 효과를 가지고 있음을 확인하였다.
As shown in FIG. 7, in the negative control group, the CPM value (count per minute, proliferation index) increased in a time-dependent manner, but when K397C (0.5-6.0 mg) treatment, growth response was significantly decreased in a concentration-dependent manner. These results confirm that K397C has a T cell proliferation inhibitory effect.
2-6. 2-6. K397CK397C 의 of 리포좀과의With liposomes 결합 효율 Coupling efficiency
단백질이 지질 비히클 (예, 리포좀)에 공유결합하는 몇 가지 방법이 종래 개시된 바가 있다. 단백질에 리포좀을 결합하는 방법 중 하나는 헤테로이기능시약 (heterobifunctional reagent)인 SATA(succinimidy-S-actylthioacetate) 및 4MPB-PE(4-(p-phenylbutyryl)phophatidyl ethanolamine)을 이용한 티올화 (thiolation)이다. 상기 방법을 이용하여 K397C 또는 야생형인 CTLA-4IgG3(1.3 mg) 을 리포좀에 결합하였다. 리포좀과의 결합 후, 결합효율은 양적 SDS-PAGE를 이용하여 측정하였다. 그 결과를 도 8 및 표 1에 나타내었다. Several methods of covalently binding proteins to lipid vehicles (eg liposomes) have been previously disclosed. One method of binding liposomes to proteins is thiolation using a heterobifunctional reagent, SATA (succinimidy-S-actylthioacetate) and 4MPB-PE (4- (p-phenylbutyryl) phophatidyl ethanolamine). Using this method K397C or wild type CTLA-4IgG 3 (1.3 mg) was bound to liposomes. After binding to liposomes, binding efficiency was measured using quantitative SDS-PAGE. The results are shown in Figure 8 and Table 1.
아래는 CTLA-4IgG3에 사용된 기본 방정식이다. Below is the basic equation used for CTLA-4IgG 3 .
y=7×106x-437870y = 7 × 10 6 x-437870
y는 단백질의 강도, x는 단백질의 질량.
y is the strength of the protein, x is the mass of the protein.
또한, CTLA-4IgG3 리포좀 및 K397C 리포좀에서의 CTLA-4IgG3의 질량을 계산하기 위하여, 아래의 식을 이용하였다. Further, the following equation was used to CTLA-4IgG 3 to calculate the mass of the CTLA-4IgG 3 K397C in a liposome and the liposome.
x=y/7×106+437870
x = y / 7 × 10 6 +437870
또한, CTLA-4IgG3 분자는 아래의 식을 이용하여 계산하였다.In addition, CTLA-4IgG 3 molecules were calculated using the following formula.
CTLA-4IgG3 의 분자=(CTLA-4IG3×10-4의 질량/분자량)×6.02×1023 CTLA-4IgG of 3 molecules = × 6.02 × 10 23 (CTLA -4IG 3 × 10 -4 by weight / molecular weight)
도 8 및 표 1에 나타낸 바와 같이, 리포좀 샘플의 인산염 (또는 지질)의 양은 포스파타아제 에세이 (phosphatase assay)를 통하여 결정된 알려진 양을 가지고 있었다. 리포좀의 크기는 100 nm이며, 1 지질 머리기(lipid head group)의 부분은 50Å2이며, 지질 이중층의 두께는 5 nm이며, 하나의 리포좀에서 지질의 수는 113,725 lipid 정도였다. 이에 따라, 리포좀의 수를 계산하기 위하여 이 샘플의 지질의 몰량에 따른 아보가드로수 및 지질:리포좀의 비율이 사용되었다. 결론적으로, CTLA-4IgG3 리포좀 및 K397C 리포좀에서, 하나의 리포좀 당 CTLA-4IgG3 분자는 각각 183 및 156 분자로 확인되었다. 특히, K397C 리포좀의 CTLA-4IgG3 총질량은 1.267 mg였다. K397C 1.3mg의 K397C 단백질이 결합에 사용된 것을 보면, 대부분의 모든 K397C이 리포좀과 결합됨을 알 수 있다. 이러한 결과는 K397C가 리포좀의 말레이미드 (maleimide)와 직접적으로 결합함에 따라 야생형인 CTLA-4IgG3보다 높은 결합 효율을 나타냄을 확인하였다.
As shown in Figure 8 and Table 1, the amount of phosphate (or lipid) of the liposome sample had a known amount determined through a phosphatase assay. The size of the liposome was 100 nm, 1 part of the lipid head group was 50 mm 2 , the thickness of the lipid bilayer was 5 nm, and the number of lipids in one liposome was about 113,725 lipids. Accordingly, in order to calculate the number of liposomes, the ratio of avogadro and lipid: liposomes according to the molar amount of lipids of this sample was used. In conclusion, in CTLA-4IgG 3 liposomes and K397C liposomes, CTLA-4IgG 3 per liposome The molecule was identified as 183 and 156 molecules, respectively. In particular, the total mass of CTLA-4IgG 3 of K397C liposome was 1.267 mg. K397C 1.3 mg of K397C protein was used for binding, indicating that most of all K397C are bound to liposomes. These results confirmed that K397C showed higher binding efficiency than the wild type CTLA-4IgG 3 as it directly binds to the maleimide of liposomes.
2-7. 2-7. K397CK397C 리포좀의Liposomal P815B7P815B7 -1과의 결합 확인Binding to -1
P815 B7-1 세포를 62.5μg/mL 농도의 자유 CTLA-4IgG3 또는 K397C 리포좀과 선배양한 뒤, 4℃에서 CTLA-4IgG3 \-FITC과 후배양하였다. 이것은 높은 농도의 CTLA-4IgG3-FITC와 경쟁될 P815B7-1 세포의 블록되지 않고 남은 B7-1 리간드 혹은 선결합된 B7 조각을 모니터링하기 위한 것이다. 그 결과를 도 9에 나타내었다. P815 B7-1 cells were precultured with free CTLA-4IgG 3 or K397C liposomes at a concentration of 62.5 μg / mL and then incubated with CTLA-4IgG 3 \ -FITC at 4 ° C. This is to monitor the unblocked B7-1 ligand or prebound B7 fragments of P815B7-1 cells to compete with high concentrations of CTLA-4IgG 3 -FITC. The results are shown in Fig.
도 9에 나타난 바와 같이, P815B7-1 세포에 결합한 CTLA-4IgG3-FITC는 하나의 피크를 나타내고 있다 (도 9의 오랜지색). CTLA-4IgG3-FITC는 B7-1을 발현하지 않는 P815 세포에는 결합하지 않았다. 이것은 CTLA-4IgG3-FITC가 B7-1에 특이적인 방법으로 결합함을 확인한 결과이다. 또한, 62.5 mg/mL의 K397C 리포좀과 선배양한 P815B7.1는 추가적인 CTLA-4IgG3-FITC의 B7-1에의 결합이 블록됨에 반하여 (도 9의 빨간색 피크), 62.5 mg/mL의 자유 CTLA-4IgG3와 함께 선배양한 경우 B-7에의 결합이 부분적으로 블록되었다(도 9의 파란색). 이러한 결과를 통하여, K397C 리포좀이 야생형인 자유 CTLA-4IgG3보다 B-7에 높은 결합효율을 가지고 있음을 확인하였다. As shown in FIG. 9, CTLA-4IgG 3 -FITC bound to P815B7-1 cells showed one peak (orange in FIG. 9). CTLA-4IgG 3 -FITC did not bind to P815 cells that did not express B7-1. This confirms that CTLA-4IgG 3 -FITC binds to B7-1 in a specific way. In addition, P815B7.1 pre-cultured with 62.5 mg / mL K397C liposome blocked additional CTLA-4IgG 3 -FITC binding to B7-1 (red peak in FIG. 9), while 62.5 mg / mL free CTLA- When precultured with 4IgG 3 , binding to B-7 was partially blocked (blue in FIG. 9). Through these results, it was confirmed that K397C liposomes had higher binding efficiency to B-7 than wild type free CTLA-4IgG 3 .
또한, 농도의존성 및 자유 CTLA-4IgG3와 비교한 결합활성을 확인하기 위하여, P815 B7-1 세포를 다양한 농도의 자유 CTLA-4IgG3, CTLA-4IgG3 리포좀 또는 K397C 리포좀과 선배양한 후, CTLA-4IgG3-FITC로 염색하였다. 그 결과를 도 10에 나타내었다. In addition, to confirm the concentration-dependent and binding activity compared to free CTLA-4IgG 3 , P815 B7-1 cells were preincubated with various concentrations of free CTLA-4IgG 3 , CTLA-4IgG 3 liposomes or K397C liposomes, followed by CTLA Stained with -4IgG 3 -FITC. The results are shown in Fig.
도 10에 나타낸 바와 같이, P815 B7-1 세포에 10 mL의 CTLA-4IgG3-FITC의 부과는 유세포분석에서 100%에 가까운 FITC 염색을 나타내었다. 염색 컨트롤의 %는 P815 B7-1세포의 B7-1에 CTLA-4IgG3-FITC의 결합 경쟁 수준을 나타낸 것이다. CTLA-4IgG3-FITC로 모니터링 하였을 때(도 10에서 검은색의 채워진 사각형), 약 190 mg/mL 농도에서 자유 CTLA-4IgG3는 세포 표면의 B7-1 분자의 50%를 블록하였다. 각각 50 mg/mL (도 10에서 파란색 채워진 삼각형)과 2.5mg/mL (도 10에서 빨간색 채워진 삼각형)에서 CTLA-4IgG3 리포좀과 K397C 리포좀은 50%의 경쟁을 나타내었다. 이러한 결과를 통하여, K397C 리포좀이 CTLA-4IgG3 또는 CTLA-4IgG3 리포좀에 비하여 현격한 블로킹 효과를 보임을 확인하였다. 특히, K397C 리포좀은 자유 CTLA-4IgG3에 비하여 100배의 높은 결합 효율을 가지고 있었다. 이는 리포좀 표면의 IgG의 방향성에의 다가 리간드 효과 (multivalent ligand effect)를 통한 것으로 예상된다.
As shown in FIG. 10, imposition of 10 mL of CTLA-4IgG 3 -FITC on P815 B7-1 cells showed near 100% FITC staining in flow cytometry. % Of staining controls show the competitive level of CTLA-4IgG 3 -FITC binding to B7-1 in P815 B7-1 cells. When monitored with CTLA-4IgG 3 -FITC (black filled squares in FIG. 10), at a concentration of about 190 mg / mL free CTLA-4IgG 3 blocked 50% of the B7-1 molecules on the cell surface. CTLA-4IgG 3 liposomes and K397C liposomes showed 50% competition at 50 mg / mL (blue filled triangle in FIG. 10) and 2.5 mg / mL (red filled triangle in FIG. 10), respectively. Through these results, it was confirmed that K397C liposomes showed a marked blocking effect compared to CTLA-4IgG 3 or CTLA-4IgG 3 liposomes. In particular, K397C liposomes had a 100-fold higher binding efficiency than free CTLA-4IgG 3 . This is expected through the multivalent ligand effect on the orientation of IgG on the liposome surface.
2-8. 2-8. K397CK397C 리포좀으로With liposomes 인한 Due to 동종이계Homogeneous (( allogeneicallogeneic ) ) MLRMLR 의 봉쇄 효과Containment effect
APC의 B7에 대한 K397C 리포좀의 블로킹 효과 및 그에 의한 T 세포 활성 및 생장 저해를 확인하였다. 이를 위하여 동종이계 혼합림프구반응 (allogeneic mixed lymphocyte reaction, allogeneic MLR)이 수행하였다. 다양한 농도의 자유 CTLA-4IgG3, CTLA-4IgG3 리포좀, 또는 K397C 리포좀을 BALB/c 마우스의 방사선 조사된 비장 세포와 함께 혼합하였으며, T 세포의 3H-티미딘 (thymidine)의 C57BL/6 비장세포에의 융합을 모니터링하였다. 그 결과를 도 11에 나타내었다. The blocking effect of K397C liposomes on B7 of APC and thereby T cell activity and growth inhibition were confirmed. To this end, an allogeneic mixed lymphocyte reaction (allogeneic MLR) was performed. Various concentrations of free CTLA-4IgG 3 , CTLA-4IgG 3 liposomes, or K397C liposomes were mixed with irradiated spleen cells of BALB / c mice, and C57BL / 6 spleen of 3 H-thymidine in T cells. Fusion to the cells was monitored. The results are shown in Fig.
도 11에 나타난 것과 같이, 72시간의 혼합 림프구 배양에서, 3H-티미딘은 K397C 리포좀에 의하여 효과적으로 흡수가 억제되었으며, K397C 리포좀의 농도에 의존적으로 증가하였다. 특히, K397C 리포좀은 자유 CTLA-4IgG3와 CTLA-4IgG3 리포좀보다 T세포의 생장 억제에 높은 효과를 나타내었다.
As shown in FIG. 11, in 72 hours of mixed lymphocyte culture, 3 H-thymidine was effectively inhibited by K397C liposomes and increased depending on the concentration of K397C liposomes. In particular, K397C liposomes showed higher effects on inhibition of T cell growth than free CTLA-4IgG 3 and CTLA-4IgG 3 liposomes.
2.9. 동종 2.9. the same kind 췌도Islet 이식모델에서의 이식 거부반응의 비교. Comparison of transplant rejection in the transplant model.
Balb/c 마우스의 췌도에서 인슐린의 분비하는 췌도(islet)를 분리하고, STZ를 처리하여 당뇨를 유발시킨 C57BL/6 마우스의 신장 캡슐에 이식한 후, K397C와 K397C 리포좀을 투여하였다. K397C와 K397C 리포좀은 이식 당일(day 0)부터 2주 간 격일로 투여하였다 (0,2,4,6,8,10,12,14 일). 이식받은 마우스의 꼬리 끝에서 혈액을 소량 얻어 혈당을 측정하고, 혈당이 250mg/dl을 2일 연속으로 넘어가면 거부반응이 왔다고 판정하였다. 그 결과를 도 12에 나타내었다. Insulin-releasing pancreatic islets were isolated from the pancreatic islets of Balb / c mice and transplanted into kidney capsules of C57BL / 6 mice, which were treated with STZ to induce diabetes, and then administered K397C and K397C liposomes. K397C and K397C liposomes were administered every other day for two weeks from day 0 (
도 12에 나타낸 바와 같이, K397C 리포좀은 K397C에 비해 이식 거부반응이 나타나지 않음을 확인하였다.
As shown in FIG. 12, K397C liposomes were confirmed to have no transplant rejection compared to K397C.
<110> SNU R&DB FOUNDATION <120> Novel CTLA-4IgG (cytotoxic T lymphocyte antigen 4-Immunoglobulin G) fusion protein <160> 3 <170> KopatentIn 1.71 <210> 1 <211> 18 <212> DNA <213> Artificial Sequence <220> <223> Primer-Sense <400> 1 gtggtacctt taatgaaa 18 <210> 2 <211> 34 <212> DNA <213> Artificial Sequence <220> <223> Primer-Antisense <400> 2 gctctagagc tgttctcaac aaccagggga gcga 34 <210> 3 <211> 397 <212> PRT <213> Artificial Sequence <220> <223> CTLA-IgG <400> 3 Met Ala Cys Leu Gly Leu Arg Arg Tyr Lys Ala Gln Leu Gln Leu Pro 1 5 10 15 Ser Arg Thr Trp Pro Phe Val Ala Leu Leu Thr Leu Leu Phe Ile Pro 20 25 30 Val Phe Ser Glu Ala Ile Gln Val Thr Gln Pro Ser Val Val Leu Ala 35 40 45 Ser Ser His Gly Val Ala Ser Phe Pro Cys Glu Tyr Ser Pro Ser His 50 55 60 Asn Thr Asp Glu Val Arg Val Thr Val Leu Arg Gln Thr Asn Asp Gln 65 70 75 80 Met Thr Glu Val Cys Ala Thr Thr Phe Thr Glu Lys Asn Thr Val Gly 85 90 95 Phe Leu Asp Tyr Pro Phe Cys Ser Gly Thr Phe Asn Glu Ser Arg Val 100 105 110 Asn Leu Thr Ile Gln Gly Leu Arg Ala Val Asp Thr Gly Leu Tyr Leu 115 120 125 Cys Lys Val Glu Leu Met Tyr Pro Pro Pro Tyr Phe Val Gly Met Gly 130 135 140 Asn Gly Thr Gln Ile Tyr Val Ile Asp Pro Glu Pro Cys Pro Asp Ser 145 150 155 160 Asp Lys Arg Ile Glu Pro Arg Ile Pro Lys Pro Ser Thr Pro Pro Gly 165 170 175 Ser Ser Cys Pro Pro Gly Asn Ile Leu Gly Gly Pro Ser Val Phe Ile 180 185 190 Phe Pro Pro Lys Pro Lys Asp Ala Leu Met Ile Ser Leu Thr Pro Lys 195 200 205 Val Thr Cys Val Val Val Asp Val Ser Glu Asp Asp Pro Asp Val His 210 215 220 Val Ser Trp Phe Val Asp Asn Lys Glu Val His Thr Ala Trp Thr Gln 225 230 235 240 Pro Arg Glu Ala Gln Tyr Asn Ser Thr Phe Arg Val Val Ser Ala Leu 245 250 255 Pro Ile Gln His Gln Asp Trp Met Arg Gly Lys Glu Phe Lys Cys Lys 260 265 270 Val Asn Asn Lys Ala Leu Pro Ala Pro Ile Glu Arg Thr Ile Ser Lys 275 280 285 Pro Lys Gly Arg Ala Gln Thr Pro Gln Val Tyr Thr Ile Pro Pro Pro 290 295 300 Arg Glu Gln Met Ser Lys Lys Lys Val Ser Leu Thr Cys Leu Val Thr 305 310 315 320 Asn Phe Phe Ser Glu Ala Ile Ser Val Glu Trp Glu Arg Asn Gly Glu 325 330 335 Leu Glu Gln Asp Tyr Lys Asn Thr Pro Pro Ile Leu Asp Ser Asp Gly 340 345 350 Thr Tyr Phe Leu Tyr Ser Lys Leu Thr Val Asp Thr Asp Ser Trp Leu 355 360 365 Gln Gly Glu Ile Phe Thr Cys Ser Val Val His Glu Ala Leu His Asn 370 375 380 His His Thr Gln Lys Asn Leu Ser Arg Ser Pro Gly Cys 385 390 395 <110> SNU R & DB FOUNDATION <120> Novel CTLA-4IgG (cytotoxic T lymphocyte antigen 4-Immunoglobulin G) fusion protein <160> 3 <170> Kopatentin 1.71 <210> 1 <211> 18 <212> DNA <213> Artificial Sequence <220> <223> Primer-Sense <400> 1 gtggtacctt taatgaaa 18 <210> 2 <211> 34 <212> DNA <213> Artificial Sequence <220> Primer-Antisense <400> 2 gctctagagc tgttctcaac aaccagggga gcga 34 <210> 3 <211> 397 <212> PRT <213> Artificial Sequence <220> <223> CTLA-IgG <400> 3 Met Ala Cys Leu Gly Leu Arg Arg Tyr Lys Ala Gln Leu Gln Leu Pro 1 5 10 15 Ser Arg Thr Trp Pro Phe Val Ala Leu Leu Thr Leu Leu Phe Ile Pro 20 25 30 Val Phe Ser Glu Ala Ile Gln Val Thr Gln Pro Ser Val Val Leu Ala 35 40 45 Ser Ser His Gly Val Ala Ser Phe Pro Cys Glu Tyr Ser Pro Ser His 50 55 60 Asn Thr Asp Glu Val Arg Val Thr Val Leu Arg Gln Thr Asn Asp Gln 65 70 75 80 Met Thr Glu Val Cys Ala Thr Thr Phe Thr Glu Lys Asn Thr Val Gly 85 90 95 Phe Leu Asp Tyr Pro Phe Cys Ser Gly Thr Phe Asn Glu Ser Arg Val 100 105 110 Asn Leu Thr Ile Gln Gly Leu Arg Ala Val Asp Thr Gly Leu Tyr Leu 115 120 125 Cys Lys Val Glu Leu Met Tyr Pro Pro Pro Tyr Phe Val Gly Met Gly 130 135 140 Asn Gly Thr Gln Ile Tyr Val Ile Asp Pro Glu Pro Cys Pro Asp Ser 145 150 155 160 Asp Lys Arg Ile Glu Pro Arg Ile Pro Lys Pro Ser Thr Pro Pro Gly 165 170 175 Ser Ser Cys Pro Pro Gly Asn Ile Leu Gly Gly Pro Ser Val Phe Ile 180 185 190 Phe Pro Pro Lys Pro Lys Asp Ala Leu Met Ile Ser Leu Thr Pro Lys 195 200 205 Val Thr Cys Val Val Val Asp Val Ser Glu Asp Asp Pro Asp Val His 210 215 220 Val Ser Trp Phe Val Asp Asn Lys Glu Val His Thr Ala Trp Thr Gln 225 230 235 240 Pro Arg Glu Ala Gln Tyr Asn Ser Thr Phe Arg Val Val Ser Ala Leu 245 250 255 Pro Ile Gln His Gln Asp Trp Met Arg Gly Lys Glu Phe Lys Cys Lys 260 265 270 Val Asn Asn Lys Ala Leu Pro Ala Pro Ile Glu Arg Thr Ile Ser Lys 275 280 285 Pro Lys Gly Arg Ala Gln Thr Pro Gln Val Tyr Thr Ile Pro Pro Pro 290 295 300 Arg Glu Gln Met Ser Lys Lys Lys Val Ser Leu Thr Cys Leu Val Thr 305 310 315 320 Asn Phe Phe Ser Glu Ala Ile Ser Val Glu Trp Glu Arg Asn Gly Glu 325 330 335 Leu Glu Gln Asp Tyr Lys Asn Thr Pro Pro Ile Leu Asp Ser Asp Gly 340 345 350 Thr Tyr Phe Leu Tyr Ser Lys Leu Thr Val Asp Thr Asp Ser Trp Leu 355 360 365 Gln Gly Glu Ile Phe Thr Cys Ser Val Val His Glu Ala Leu His Asn 370 375 380 His His Thr Gln Lys Asn Leu Ser Arg Ser Pro Gly Cys 385 390 395
Claims (9)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020120009982 | 2012-01-31 | ||
| KR20120009982 | 2012-01-31 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20130088807A true KR20130088807A (en) | 2013-08-08 |
| KR101536151B1 KR101536151B1 (en) | 2015-07-14 |
Family
ID=49215003
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020130011443A KR101536151B1 (en) | 2012-01-31 | 2013-01-31 | Novel CTLA-4IgG (cytotoxic T lymphocyte antigen 4-Immunoglobulin G) fusion protein |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR101536151B1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015182953A1 (en) * | 2014-05-27 | 2015-12-03 | Samsung Electronics Co., Ltd. | Composition and kit for prevention or treatment of arthritis, and method using the same |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7829064B2 (en) * | 1999-05-10 | 2010-11-09 | Immunomedics, Inc. | Anti-CD74 immunoconjugates and methods |
| BRPI0514259A (en) * | 2004-08-11 | 2008-06-03 | Trubion Pharmaceuticals Inc | binding domain fusion protein |
| LT2536745T (en) * | 2010-02-19 | 2016-09-26 | Xencor, Inc. | Novel ctla4-ig immunoadhesins |
-
2013
- 2013-01-31 KR KR1020130011443A patent/KR101536151B1/en active IP Right Grant
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015182953A1 (en) * | 2014-05-27 | 2015-12-03 | Samsung Electronics Co., Ltd. | Composition and kit for prevention or treatment of arthritis, and method using the same |
Also Published As
| Publication number | Publication date |
|---|---|
| KR101536151B1 (en) | 2015-07-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7221338B2 (en) | Immune effector cells genetically engineered with CS1-specific chimeric antigen receptors | |
| US11578130B2 (en) | Regulatable chimeric antigen receptor | |
| KR100496307B1 (en) | Monkey monoclonal antibodies specific to human b7.1 and/or b7.2 primatized forms, pharmaceutical compositions | |
| JP6647868B2 (en) | Treatment of cancer with humanized anti-EGFRvIII chimeric antigen receptor | |
| JP4837880B2 (en) | Method for protecting allogeneic island grafts using soluble CTLA4 mutant molecules | |
| US9828425B2 (en) | Anti-ILT5 antibodies and ILT5-binding antibody fragments | |
| US8846397B2 (en) | Immunoregulation by anti-ILT5 antibodies and ILT5-binding antibody fragments | |
| JP2019106995A (en) | Treatment of cancer using humanized anti-CD19 chimeric antigen receptor | |
| JP2019206538A (en) | Treatment of cancer using chimeric antigen receptor | |
| JP7004761B2 (en) | Improved Cell Compositions and Methods for Cancer Treatment | |
| TW201843173A (en) | Anti-icos agonist antibodies and uses thereof | |
| CN110225927A (en) | Chimeric antigen receptor for treating cancer | |
| KR20100126811A (en) | A monoclonal antibody and a method thereof | |
| JP2017528433A (en) | Low immunoenhancing dose of mTOR inhibitor and CAR combination | |
| KR20000053137A (en) | Identification of unique binding interactions between certain antibodies and the human b7.1 and b7.2 co-stimulatory antigens | |
| RU2727900C2 (en) | Pharmaceutical composition for preventing or treating diseases mediated by regulatory t-cells | |
| CN110177568A (en) | Combination treatment for treating cancer | |
| CA3177488A1 (en) | Compositions and methods for tcr reprogramming using cd70 specific fusion proteins | |
| KR101536151B1 (en) | Novel CTLA-4IgG (cytotoxic T lymphocyte antigen 4-Immunoglobulin G) fusion protein | |
| JP2021505659A (en) | TRPV6 Inhibitors and Combination Therapies for Cancer Treatment | |
| WO2024060140A1 (en) | Egfrviii chimeric antigen receptor and use thereof | |
| CN118369332A (en) | CD 33-targeting antigen recognizing receptor and use thereof | |
| Kim et al. | Functional characteristics of C-terminal lysine to cysteine mutant Form of CTLA-4Ig | |
| CA3142487A1 (en) | Oligodendrocyte-derived extracellular vesicles for therapy of multiple sclerosis | |
| Pearson et al. | Development of a Chimeric Anti-CD40 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| E701 | Decision to grant or registration of patent right | ||
| GRNT | Written decision to grant | ||
| FPAY | Annual fee payment |
Payment date: 20180620 Year of fee payment: 4 |
|
| FPAY | Annual fee payment |
Payment date: 20190625 Year of fee payment: 5 |