KR20120138265A - Sterilzation treatment apparatus for water of aquarium for living fish - Google Patents
Sterilzation treatment apparatus for water of aquarium for living fish Download PDFInfo
- Publication number
- KR20120138265A KR20120138265A KR1020110057607A KR20110057607A KR20120138265A KR 20120138265 A KR20120138265 A KR 20120138265A KR 1020110057607 A KR1020110057607 A KR 1020110057607A KR 20110057607 A KR20110057607 A KR 20110057607A KR 20120138265 A KR20120138265 A KR 20120138265A
- Authority
- KR
- South Korea
- Prior art keywords
- seawater
- alloy
- live fish
- metal
- sterilization
- Prior art date
Links
- 241000251468 Actinopterygii Species 0.000 title claims abstract description 51
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 title claims abstract description 26
- 230000001954 sterilising effect Effects 0.000 claims abstract description 59
- 239000013535 sea water Substances 0.000 claims abstract description 57
- 238000004659 sterilization and disinfection Methods 0.000 claims abstract description 53
- 229910045601 alloy Inorganic materials 0.000 claims abstract description 31
- 239000000956 alloy Substances 0.000 claims abstract description 31
- 229910001092 metal group alloy Inorganic materials 0.000 claims abstract description 28
- 229910021645 metal ion Inorganic materials 0.000 claims abstract description 28
- 238000006479 redox reaction Methods 0.000 claims abstract description 12
- 239000010949 copper Substances 0.000 claims description 22
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 16
- 229910052802 copper Inorganic materials 0.000 claims description 14
- 238000000034 method Methods 0.000 claims description 14
- 239000002184 metal Substances 0.000 claims description 10
- 229910052751 metal Inorganic materials 0.000 claims description 10
- 230000009467 reduction Effects 0.000 claims description 6
- JBQYATWDVHIOAR-UHFFFAOYSA-N tellanylidenegermanium Chemical compound [Te]=[Ge] JBQYATWDVHIOAR-UHFFFAOYSA-N 0.000 claims description 3
- 150000002739 metals Chemical class 0.000 claims description 2
- 229920001429 chelating resin Polymers 0.000 claims 1
- 230000000694 effects Effects 0.000 abstract description 17
- 241000894006 Bacteria Species 0.000 abstract description 13
- 241000233866 Fungi Species 0.000 abstract description 5
- 230000002265 prevention Effects 0.000 abstract description 3
- 239000011701 zinc Substances 0.000 description 10
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 8
- 230000008878 coupling Effects 0.000 description 8
- 238000010168 coupling process Methods 0.000 description 8
- 238000005859 coupling reaction Methods 0.000 description 8
- 229910052725 zinc Inorganic materials 0.000 description 7
- 238000001914 filtration Methods 0.000 description 6
- 241000195493 Cryptophyta Species 0.000 description 5
- 230000003647 oxidation Effects 0.000 description 5
- 238000007254 oxidation reaction Methods 0.000 description 5
- 229910001297 Zn alloy Inorganic materials 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 229910000881 Cu alloy Inorganic materials 0.000 description 3
- 241000588724 Escherichia coli Species 0.000 description 3
- PTFCDOFLOPIGGS-UHFFFAOYSA-N Zinc dication Chemical compound [Zn+2] PTFCDOFLOPIGGS-UHFFFAOYSA-N 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 230000029142 excretion Effects 0.000 description 3
- 239000003456 ion exchange resin Substances 0.000 description 3
- 229920003303 ion-exchange polymer Polymers 0.000 description 3
- 238000012423 maintenance Methods 0.000 description 3
- 244000005700 microbiome Species 0.000 description 3
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 2
- 230000000844 anti-bacterial effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- 239000013522 chelant Substances 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000008439 repair process Effects 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 235000014102 seafood Nutrition 0.000 description 2
- 206010004022 Bacterial food poisoning Diseases 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- 206010008631 Cholera Diseases 0.000 description 1
- 206010016952 Food poisoning Diseases 0.000 description 1
- 208000019331 Foodborne disease Diseases 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 239000010828 animal waste Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000000721 bacterilogical effect Effects 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000005660 chlorination reaction Methods 0.000 description 1
- 239000011362 coarse particle Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- TVZPLCNGKSPOJA-UHFFFAOYSA-N copper zinc Chemical compound [Cu].[Zn] TVZPLCNGKSPOJA-UHFFFAOYSA-N 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- ALKZAGKDWUSJED-UHFFFAOYSA-N dinuclear copper ion Chemical compound [Cu].[Cu] ALKZAGKDWUSJED-UHFFFAOYSA-N 0.000 description 1
- 238000007599 discharging Methods 0.000 description 1
- QNDQILQPPKQROV-UHFFFAOYSA-N dizinc Chemical compound [Zn]=[Zn] QNDQILQPPKQROV-UHFFFAOYSA-N 0.000 description 1
- 239000003651 drinking water Substances 0.000 description 1
- 235000020188 drinking water Nutrition 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 239000003344 environmental pollutant Substances 0.000 description 1
- 235000020774 essential nutrients Nutrition 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 230000000855 fungicidal effect Effects 0.000 description 1
- 239000000417 fungicide Substances 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 235000019645 odor Nutrition 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 239000002574 poison Substances 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 231100000719 pollutant Toxicity 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 239000002351 wastewater Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K63/00—Receptacles for live fish, e.g. aquaria; Terraria
- A01K63/04—Arrangements for treating water specially adapted to receptacles for live fish
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K63/00—Receptacles for live fish, e.g. aquaria; Terraria
- A01K63/003—Aquaria; Terraria
- A01K63/006—Accessories for aquaria or terraria
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/42—Treatment of water, waste water, or sewage by ion-exchange
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Environmental Sciences (AREA)
- Marine Sciences & Fisheries (AREA)
- Animal Husbandry (AREA)
- Biodiversity & Conservation Biology (AREA)
- Farming Of Fish And Shellfish (AREA)
Abstract
Description
The present invention relates to an apparatus for sterilizing a live fish tank, and more particularly, to an apparatus for sterilizing a live fish tank using a redox principle to remove various algae, bacteria, and bacteria that may occur in the live fish tank. It is about.
In general, seafood is rich in nutrients such as DHA and EPA, but since seafood and sea water contain bacteria, microorganisms, some fish species and pathogens such as cholera, it is identified as one of the main causes of marine ecosystem destruction and food poisoning. Therefore, bacterial food poisoning accidents are frequently generated due to unsanitary treatment in fish sashimi using coastal seawater.
There are various sterilization methods in consideration of the use and condition of water. Among them, sterilization method by the chemicals used at home is generally widely used due to low cost and certainty and rapid effect, but serious side effects occur due to the harmfulness of the drug itself There is a problem.
Another method is known as ozone sterilization, ultraviolet sterilization and chlorine sterilization, but these methods have serious problems in terms of installation cost, disinfection effect and side effects.
In other words, the ozone sterilization method and the ultraviolet sterilization method do not leave any residual seawater with sterilization effect, so there is no safety against contamination after sterilization and the treatment cost is high. In addition, chlorination sterilizes the taste and smell, and chemical reactions between the organism and chlorine in water produce trihalomethane, which is harmful to the human body.
In addition, in this reality, the alternative method is to lower the water temperature in the aquarium to prevent bacterial growth or to frequently change the aquarium's seawater, but even in the summer when the temperature is high, it is not enough precautions. Seawater can also be contaminated, so sterilization of aquariums, tanks, etc. is essential.
That is, in tanks, it is used while continuously circulating a certain amount of seawater, so it must be sterilized for circulating seawater. If sterilization is not done for circulating seawater, moss, algae, fungus, etc. are generated, odors are generated, and dissolved oxygen There is also a problem in that the environment is reduced to the inhabitants.
The present invention is to solve the above-mentioned problems of the prior art, by applying a sterilization system using the redox principle of the metal to the water tank sterilization effect of various fungi, sterilization effect of algae, moss and mold, prevention of scale adhesion It is an object of the present invention to provide a sterilization apparatus of a live fish tank that provides an effect.
Another object of the present invention is to apply a sterilization system using the redox principle of the metal to the tank to increase the replacement cycle of seawater in the tank can reduce the cost and labor associated with the replacement of live fish tanks The purpose is to provide.
Still another object of the present invention is to provide a sterilization apparatus for a live fish tank by ionization according to oxidation of a metal alloy made of copper (Cu) and zinc (Zn) in using the redox principle of the metal alloy. The purpose is.
According to the present invention, the object and the tank for storing the seawater therein; A pump for sucking the sea water so that the sea water in the water tank circulates; A circulation pipe having one side coupled to the pump and the other side connected to one side of the tank to circulate the seawater sucked by the pump; And a metal alloy part composed of a plurality of metals having different standard reduction potentials, and disposing the metal alloy part to contact the sea water transported through the circulation pipe to sterilize the sea water by redox reaction of the metal alloy part. An alloy filter unit; It is achieved by a sterilization treatment apparatus of a live fish tank comprising a metal ion filter unit for removing the metal ions generated in the alloy filter unit.
Here, the metal alloy portion is characterized in that the hollow is formed to widen the contact area with the water transported through the circulation pipe.
In addition, the metal ion filter unit is characterized in that consisting of a chelate resin.
The metal alloy portion is formed of an alloy containing copper (Cu) and zinc (Zn).
Sterilization apparatus of a live fish tank according to the present invention having the configuration as described above sterilizes various fungi, algae, moss and mold of the tank, aquarium, etc., using the redox principle, and prevents adhesion of scale in the circulation pipe do.
In other words, by using the sterilization apparatus of the live fish tank according to the present invention in the coastal sashimi and inland fat sashimi using live fish tank can reconsider the sanitary credibility of sashimi can increase the income of fishermen and improve the public health.
In addition, it is possible to increase the replacement cycle of seawater in the tank, thereby reducing the cost and labor associated with seawater replacement.
In other words, it is possible to reduce the economic burden due to the burden of seawater purchase and the reduction of logistics costs for transporting supplemental water in the case of inland water, and labor for water quality maintenance.
1 is a conceptual diagram of a sterilization apparatus of a live fish tank according to the present invention,
Figure 2 is a view showing the alloy filter portion of the live fish tank sterilization treatment apparatus according to the present invention,
Figure 3 is a view showing a second embodiment of the alloy filter portion of the live fish tank sterilization apparatus according to the present invention,
4 is a view showing another form of the metal alloy portion according to the second embodiment of the alloy filter portion of FIG.
5 is a view showing the reaction of the metal ions discharged from the alloy filter unit of the sterilization apparatus of a live fish tank according to the present invention.
Hereinafter, with reference to the accompanying drawings will be described in detail embodiments according to the present invention. Here, in describing the embodiments according to the present invention, the same reference numerals are used for the same components, and description thereof may be omitted as necessary.
Referring to Figure 1, the live fish tank sterilization apparatus according to the present invention includes a
Here, the
The
The
First Embodiment
Referring to FIG. 2, the
Here, the
As shown in FIG. 2, the
Here, the
In other words, Redox is a compound word of reduction and oxidation and it uses the redox principle that occurs naturally between metal alloy and water.
In particular, the
Here, copper (Cu) is an essential nutrient for mammals including humans, but is mostly ingested from food, but in other organisms such as microorganisms, the demand is very low at 1 to 10 levels. Will be removed.
Therefore, when the redox reaction energy is increased, the sterilization effect of various fungi, the sterilization effect of algae, moss and mold, and the effect of preventing the adhesion of scales will be described in detail with reference to FIG. 5.
The principle of sterilization by redox reaction is as follows.
Cu / Zn 0- > Cu +2 / Zn +2 + 2e - Reduction
H 2 O + 2e - -> OH - + .H oxidation
H 2 O + .H-> .OH + H 2
Therefore, the
That is, in the process of redox reaction, the metal emits electrons (e-) through oxidation. First, the electrons act on cells of bacteria such as bacteria to hemolyze the cell wall to kill the bacteria. Secondly, the released electrons interact with seawater to produce hydrogen radicals (.H), and hydrogen radicals react with seawater to produce hydroxyl radicals (.OH). It reaches about 800 times (Cl). Third, a hydroxyl group (OH-) is produced in the reaction process, and the pH increases due to the hydroxyl group. When the pH is 9.5 or more, it becomes an environmental condition that various bacteria are difficult to inhabit, so that effective sterilization is possible because it makes strong sterilization and the culture environment of bacteria difficult during the redox reaction.
In addition, one
In addition, the
On the other hand, the
2 (HCO3 -) + Ca 2 + -> CaCO 3 + H 2 O + CO 2
The metal ion is Ca + 2 or Mg + 2 and compete with consequently interfere with the generation and crystal growth of CaCO 3 or MgCO 3. So, to remove the scale-forming material, such as Mg 2 +, Na 2 +, SiO 2 2 +.
The anti-scaling effect is one of the great effects of the redox reaction, and in general, the scale is charged by the
However, in the process of redox reaction, charge (e-), which is the nucleus of scale formation, is provided in the solution so that the scale to be attached to the circulating
Hereinafter, the correlation between the sterilizing power and the metal ion concentration according to the metal content will be described.
Copper zinc alloys (copper: zinc ratio = 10: 0, 9: 1, 8: 2, 7: 3, 6: 4) prepared to test the sterilization power and metal ion concentration according to the metal content are as wide as possible 5 g of the copper / zinc alloy sample thus processed was placed in 1 L of seawater and tested for pH, general bacteria, copper and zinc ion concentration at 1, 2, 4, and 8 days with stirring. .
The experimental results are shown in Table 1 below.
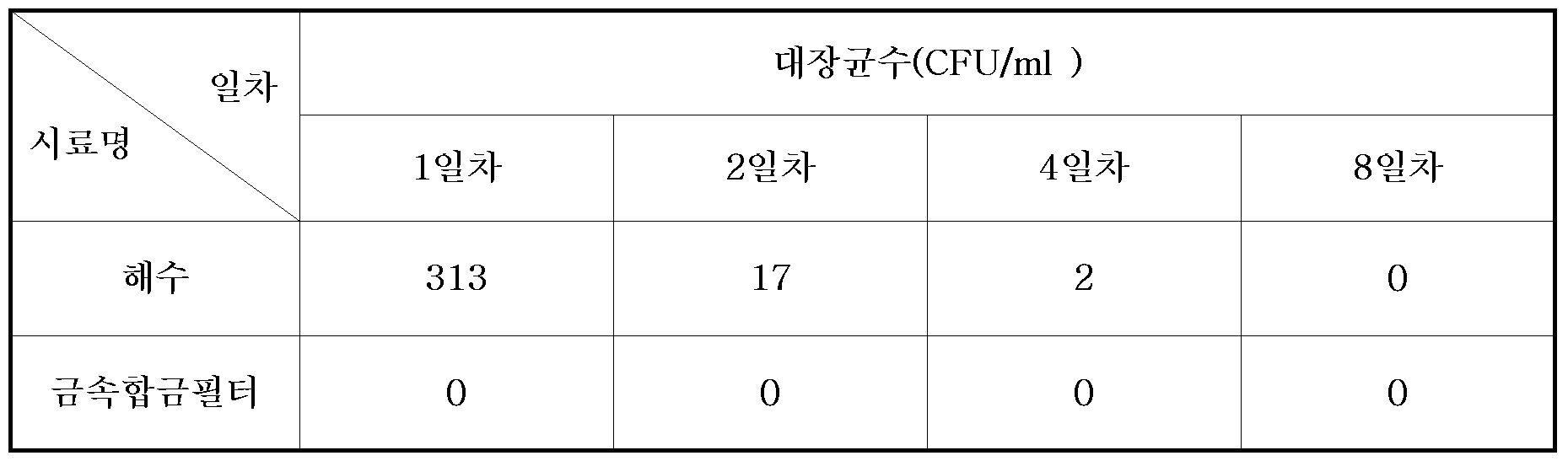
TABLE 1 Changes in pH, general bacterial counts, and copper and zinc ion concentrations over different periods of treatment of seawater with copper / zinc alloys.
According to this result, the bactericidal power of the bacteria was the most excellent bactericidal power of the alloy with a weight ratio of copper and zinc 7: 3. That is, according to the experimental results shown in Table 1 below, the
However, the concentration of metal ions may exceed 1 mg / L on a drinking water basis. In this case, metal ions should be removed.
At this time, in order to remove the copper and zinc ions eluted from the copper / zinc metal alloy remaining in the sea water, the
That is, since the metal ions in the sea water may act as a poison, the metal ions can be safely removed using the metal
Here, the ion exchange resin can be used as the metal
In this case, the ion exchange resin used may catch salts of seawater when using general ion exchange resins, and may reduce salinity of seawater. Therefore, chelate resins that selectively hold copper and zinc and pass salts of seawater are used. It is preferable.
Therefore, ions remaining as stable residual fungicide in water are filtered by the metal
In order to comparatively measure the sterilizing power of the
<Table 2> Bacterial Determination of Metal Alloy Sterilization System and General Seawater for Seawater E. Coli.
Referring to Table 2, E. coli was not detected due to the sterilizing action of the
On the contrary, when only the sea water was circulated, the number of bacteria was gradually reduced, but it was judged that it was killed by lack of nutrients.
Therefore, it can be seen that the
Second Embodiment
Hereinafter, a second embodiment of the alloy filter part 40a of the present invention will be described in detail with reference to the accompanying drawings. In addition, in describing the second embodiment of the alloy filter part 40a of the present invention, the same reference numerals are used for the same components as those of the first embodiment, and the description thereof may be omitted.
Figure 3 is a view showing a second embodiment of the alloy filter portion 40a of the sterilization apparatus of a live fish tank according to the present invention, Figure 4 is a metal according to a second embodiment of the alloy filter portion 40a of FIG. Another figure of the
In the second embodiment, a hollow is formed inside the
3 to 4, the
Here, although the hollow is formed inside the
In addition, as shown in FIGS. 3 to 4, a thread is formed on the outer circumferential surface of the
Here, the
In more detail, the
Live fish tanks can be operated for a long time without discharging waste water or replenishing new water, unlike fish farms in fish farms where untreated animal waste and fish excretion seawater are dissolved, and live fish tanks do not feed live fish. Pollution load is smaller than in fish farms. Therefore, in the case of live fish tanks, a small and
However, as described above, since the
While the invention has been shown and described with reference to certain preferred embodiments thereof, it is to be understood that the invention is not limited to the disclosed embodiments. Those skilled in the art will appreciate that various modifications, additions and substitutions are possible, without departing from the scope of the appended claims, The genius will be so self-evident. Accordingly, the true scope of the present invention should be determined by the technical idea of the appended claims.
1: Sterilization apparatus of live fish tank 10: Tank
20: pump 30: circulation pipe
40: alloy filter portion 50: metal ion filter portion
60: filtration system
Claims (4)
A pump for sucking the sea water so that the sea water in the water tank circulates;
A circulation pipe having one side coupled to the pump and the other side connected to one side of the tank to circulate the seawater sucked by the pump;
And a metal alloy part composed of a plurality of metals having different standard reduction potentials, and disposing the metal alloy part to contact the sea water transported through the circulation pipe to sterilize the sea water by redox reaction of the metal alloy part. An alloy filter unit;
Sterilization treatment apparatus of a live fish tank comprising a metal ion filter to remove the metal ions produced by the alloy filter.
The metal alloy portion is a sterilization treatment apparatus for a live fish tank, characterized in that the hollow is formed to widen the contact area with the water transported through the circulation pipe.
The metal ion filter unit of the live fish tank sterilization treatment, characterized in that consisting of a chelating resin.
The metal alloy portion sterilization apparatus of a live fish tank, characterized in that formed of an alloy containing copper (Cu) and zinc (Zn).
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020110057607A KR20120138265A (en) | 2011-06-14 | 2011-06-14 | Sterilzation treatment apparatus for water of aquarium for living fish |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020110057607A KR20120138265A (en) | 2011-06-14 | 2011-06-14 | Sterilzation treatment apparatus for water of aquarium for living fish |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20120138265A true KR20120138265A (en) | 2012-12-26 |
Family
ID=47905086
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020110057607A KR20120138265A (en) | 2011-06-14 | 2011-06-14 | Sterilzation treatment apparatus for water of aquarium for living fish |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR20120138265A (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101458177B1 (en) * | 2013-06-25 | 2014-11-04 | 아산텍 주식회사 | Water-treatment system usning alloy catalyst reactor system |
| CN104686411A (en) * | 2015-01-07 | 2015-06-10 | 中国水产科学研究院东海水产研究所 | Ground-based copper alloy culture pond and usage method |
| KR20160043238A (en) * | 2014-10-10 | 2016-04-21 | 주식회사 대창 | Direct-coupled seawater purify filter coupled to the seawater supply pipe of inland fish farm water tank |
| CN109631858A (en) * | 2019-02-22 | 2019-04-16 | 中国人民解放军海军大连舰艇学院 | A kind of portable float-type automatic tide gauge |
-
2011
- 2011-06-14 KR KR1020110057607A patent/KR20120138265A/en not_active Application Discontinuation
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101458177B1 (en) * | 2013-06-25 | 2014-11-04 | 아산텍 주식회사 | Water-treatment system usning alloy catalyst reactor system |
| KR20160043238A (en) * | 2014-10-10 | 2016-04-21 | 주식회사 대창 | Direct-coupled seawater purify filter coupled to the seawater supply pipe of inland fish farm water tank |
| CN104686411A (en) * | 2015-01-07 | 2015-06-10 | 中国水产科学研究院东海水产研究所 | Ground-based copper alloy culture pond and usage method |
| CN109631858A (en) * | 2019-02-22 | 2019-04-16 | 中国人民解放军海军大连舰艇学院 | A kind of portable float-type automatic tide gauge |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Lacasa et al. | Electrochemical disinfection of simulated ballast water on conductive diamond electrodes | |
| JP6131342B2 (en) | Method for producing sterilized cultured water and method for culturing flowing water-sterilized water fish using the same | |
| US8454837B2 (en) | Systems and methods for generation of low zeta potential mineral crystals to enhance quality of liquid solutions | |
| US10414677B2 (en) | Ozone-assisted fluid treatment apparatus and method | |
| KR101498990B1 (en) | Continuous Flow Sterile Water Fish Aquaculture System | |
| Ahile et al. | Are iron chelates suitable to perform photo-Fenton at neutral pH for secondary effluent treatment? | |
| US20060175254A1 (en) | Systems and methods for treatment of liquid solutions for use with livestock operations | |
| KR101547566B1 (en) | A Method for Preparing Sterile Water Using the Electrolyzing and Continuous Flow Sterile Water Fish Aquaculture System Using It | |
| JP2012239938A (en) | Water-quality improvement apparatus, water-quality improving method and metal ion water generator | |
| KR20120138265A (en) | Sterilzation treatment apparatus for water of aquarium for living fish | |
| Samocha et al. | System treatment and preparation | |
| KR20150093293A (en) | Method for Preparing Sterilized Water using Seawater and Sterilization System for Marine Products | |
| KR101715822B1 (en) | A disinfectant aquaculture method for fish and shellfish by using eletrolytic mixed oxidant | |
| JP2004132592A (en) | Electrochemical water treatment method and water treatment system | |
| JP2010036082A (en) | Composite circulation antibacterial bathtub system, composite circulation antibacterial warm water supply system, composite circulation antibacterial cooling tower system, composite circulation antibacterial pool system, composite circulation antibacterial water and sewerage system and composite circulation antibacterial water system for agriculture, fishing and fisheries | |
| JP3918133B2 (en) | Water purification method and purification device | |
| Allen et al. | Water quality and water delivery systems | |
| JPH08164390A (en) | Electrochemical treatment of water to be treated | |
| Arkush | Water quality | |
| Paneva et al. | Methods of cleaning and disinfection of drinking water in livestock farms. | |
| US20230039534A1 (en) | Water remediation system | |
| KR20190005554A (en) | Sterilization and deodorization device by dilute hydrogen peroxide solution with zinc electrode | |
| RU2377192C1 (en) | Method for biological decontamination of treated waste waters | |
| JP3145670U (en) | Compound circulation antibacterial bathtub system, compound circulation antibacterial hot water system, compound circulation antibacterial cooling tower system, compound circulation antibacterial pool system, compound circulation antibacterial water and sewage system and compound circulation antibacterial agriculture fishery water industry water system | |
| Sun et al. | Inactivation of Chironomid larvae with ozone |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| E601 | Decision to refuse application |