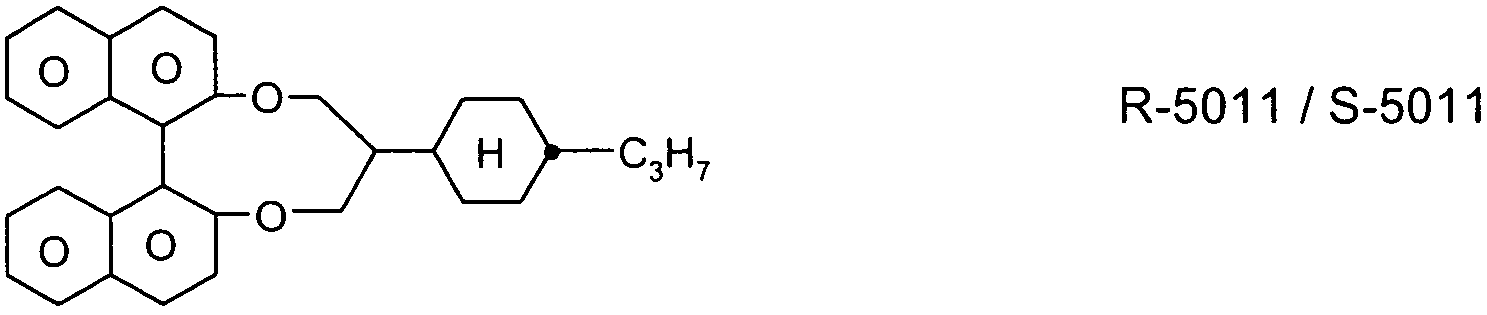KR101770833B1 - Liquid crystalline medium and liquid crystal display - Google Patents
Liquid crystalline medium and liquid crystal display Download PDFInfo
- Publication number
- KR101770833B1 KR101770833B1 KR1020117007227A KR20117007227A KR101770833B1 KR 101770833 B1 KR101770833 B1 KR 101770833B1 KR 1020117007227 A KR1020117007227 A KR 1020117007227A KR 20117007227 A KR20117007227 A KR 20117007227A KR 101770833 B1 KR101770833 B1 KR 101770833B1
- Authority
- KR
- South Korea
- Prior art keywords
- formula
- liquid crystal
- compound
- crystal medium
- compounds
- Prior art date
Links
- 0 *c(cc1)ccc1-c1ccc(-c2cc(F)c(*Oc(cc3F)cc(F)c3F)c(F)c2)c(F)c1 Chemical compound *c(cc1)ccc1-c1ccc(-c2cc(F)c(*Oc(cc3F)cc(F)c3F)c(F)c2)c(F)c1 0.000 description 5
- DYSJQUQJVBYIOT-UHFFFAOYSA-N Cc(ccc(C)c1F)c1F Chemical compound Cc(ccc(C)c1F)c1F DYSJQUQJVBYIOT-UHFFFAOYSA-N 0.000 description 1
- HBXFIXSFKULBOG-UHFFFAOYSA-N Cc1cc(F)c(C)c(F)c1 Chemical compound Cc1cc(F)c(C)c(F)c1 HBXFIXSFKULBOG-UHFFFAOYSA-N 0.000 description 1
- OKSMWLUJGZQUQH-UHFFFAOYSA-N Cc1cc([F]c(cc2C)c(C)cc2F)c(C)c(F)c1 Chemical compound Cc1cc([F]c(cc2C)c(C)cc2F)c(C)c(F)c1 OKSMWLUJGZQUQH-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/42—Mixtures of liquid crystal compounds covered by two or more of the preceding groups C09K19/06 - C09K19/40
- C09K19/46—Mixtures of liquid crystal compounds covered by two or more of the preceding groups C09K19/06 - C09K19/40 containing esters
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/52—Liquid crystal materials characterised by components which are not liquid crystals, e.g. additives with special physical aspect: solvents, solid particles
- C09K19/58—Dopants or charge transfer agents
- C09K19/586—Optically active dopants; chiral dopants
- C09K19/588—Heterocyclic compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K2019/0444—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit characterized by a linking chain between rings or ring systems, a bridging chain between extensive mesogenic moieties or an end chain group
- C09K2019/0448—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit characterized by a linking chain between rings or ring systems, a bridging chain between extensive mesogenic moieties or an end chain group the end chain group being a polymerizable end group, e.g. -Sp-P or acrylate
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K2019/0444—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit characterized by a linking chain between rings or ring systems, a bridging chain between extensive mesogenic moieties or an end chain group
- C09K2019/0466—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit characterized by a linking chain between rings or ring systems, a bridging chain between extensive mesogenic moieties or an end chain group the linking chain being a -CF2O- chain
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/10—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing at least two benzene rings
- C09K19/20—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing at least two benzene rings linked by a chain containing carbon and oxygen atoms as chain links, e.g. esters or ethers
- C09K19/2007—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing at least two benzene rings linked by a chain containing carbon and oxygen atoms as chain links, e.g. esters or ethers the chain containing -COO- or -OCO- groups
- C09K2019/2035—Ph-COO-Ph
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/30—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing saturated or unsaturated non-aromatic rings, e.g. cyclohexane rings
- C09K19/3001—Cyclohexane rings
- C09K19/3066—Cyclohexane rings in which the rings are linked by a chain containing carbon and oxygen atoms, e.g. esters or ethers
- C09K19/3068—Cyclohexane rings in which the rings are linked by a chain containing carbon and oxygen atoms, e.g. esters or ethers chain containing -COO- or -OCO- groups
- C09K2019/3075—Cy-COO-Ph
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/34—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring
- C09K19/3402—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having oxygen as hetero atom
- C09K2019/3422—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having oxygen as hetero atom the heterocyclic ring being a six-membered ring
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/34—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring
- C09K19/3402—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having oxygen as hetero atom
- C09K2019/343—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having oxygen as hetero atom the heterocyclic ring being a seven-membered ring
- C09K2019/3433—Seven-membered ring with oxygen(s) in fused, bridged or spiro ring systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/34—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring
- C09K19/3402—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having oxygen as hetero atom
- C09K2019/3438—Crown ethers
Landscapes
- Chemical & Material Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Liquid Crystal Substances (AREA)
Abstract
The present invention also provides a pharmaceutical composition comprising one or more compounds selected from the group consisting of 1, 2 or 3 compounds of formula (I) and compounds selected from the group of compounds of formula (II) and (III) And a liquid crystal display comprising said medium, in particular a TN-display, in particular an active matrix display, which comprises at least one chiral dopant and at least one chiral dopant, .
Description
The present invention relates to a liquid crystalline medium, preferably a genetically positively nematic medium comprising at least one genetically positive compound and at least one genetic neutral compound, preferably comprising at least one chiral dopant and preferably encapsulated in a polymer matrix, and To a liquid crystal display comprising said medium, in particular a display which is operated in a reflective mode and is preferably addressed by an active matrix.
Liquid crystal displays (LCDs) are widely used to display information. LCDs are used in direct view and projection displays. The electro-optic mode used in most displays is still a twisted nematic (TN) mode and various variations thereof. In addition to the above schemes, a super twisted nematic (STN) scheme and more recently, an optical compensated bend (OCB) scheme and an electrically controlled birefringence (ECB) scheme and various variations thereof, such as vertically aligned nematic Oriented ITO vertically aligned nematic (PVA) and polymer stabilized vertical aligned nematic (PSVA) and multi-domain vertically aligned nematic (MVA) methods and other methods are increasingly being used. All of these methods use an electric field that is substantially perpendicular to the substrate or liquid crystal layer. In addition to these schemes, it is also possible to provide a method of fabricating a substrate or liquid crystal layer substantially as in a in-plane switching (abbreviated as IPS) scheme (e.g., as disclosed in DE 40 00 451 and
The liquid crystal (LC) according to the present invention is preferably used in an improved LCD using cholesteric liquid crystals, also known as chiral nematic liquid crystals, which have a short helical pitch and a high dielectric anisotropy especially for advanced products . These are particularly useful for operating in a reflective manner because cholesteric liquid crystals with appropriate cholesteric pitches selectively color light as they are reflected and avoid using color filters in the LCD.
The cholesteric liquid crystal may be encapsulated in a polymer matrix, for example as PDLC or NCAP.
In these products, there is a need for a novel liquid crystal medium with improved properties. Therefore, there is a need for a liquid crystal medium with improved behavior. Their rotational viscosity should be as low as possible. In addition to the above variables, the medium should exhibit a suitably wide range of nematic phases, suitable birefringence (? N) (preferably in the range of 0.100 to 0.300) and moderately high dielectric anisotropy (??). It should be high enough to allow a significantly lower operating voltage. Preferably, it should be 10 or more so as to allow a driver that is easily accessible with a suitably low operating voltage. However, since? Is particularly detrimental to at least some high specific resistance, which is another requirement especially in the case of active matrix addressing, ?? should preferably be 40 or less, and especially 35 or less. Most preferably, the DELTA epsilon should be in the range of 20 to 30.
The display according to the invention is preferably an active matrix LCD (abbreviated AMD), preferably addressed by a matrix of thin film transistors (TFT). However, the liquid crystal according to the present invention can also be advantageously used in displays having other known addressing means.
Liquid crystal compositions suitable for LCDs, especially TN-displays, are already well known. However, the composition has significant weaknesses. Most of these, among other defects, result in disadvantageously long response times and / or too low a contrast ratio in many products. They also have insufficient reliability and stability, most generally for exposure to heat, moisture, or light (especially UV) radiation, especially when these one or more stressors are combined together.
Therefore, it is possible to obtain a high nematic phase range, an appropriate optical anisotropy (? N), a high?, A low viscosity, in particular a low rotational viscosity (? 1 ), a high contrast ratio in the display, There is a great demand for a liquid crystal medium having improved properties suitable for practical products such as reliability.
Surprisingly, it has recently been found that a liquid phase medium having a suitable phase range, suitably high? E and? N, and suitably low viscosity can be realized, while exhibiting no or only at least substantially reduced weaknesses of the prior art materials.
Such an improved liquid crystal medium according to the present invention comprises at least the following components:
- one or more compounds of formula (I)
[In the above formula,
R 1 is alkyl, alkoxy, fluorinated alkyl or fluorinated alkoxy, alkenyl, alkenyloxy, alkoxyalkyl, fluorinated alkenyl or fluorinated alkenyloxy, preferably alkyl or alkoxyalkyl, most preferably n- Alkyl,
The or ego,
L 11 and L 12 independently of one another are H or F, preferably F,
X < 1 > is CN or NCS, preferably CN,
i is 0 or 1, preferably 0,
- at least one genetically positive compound, preferably selected from the group consisting of compounds of the formulas II and III, preferably comprising at least one of each of these compounds, preferably having a dielectric anisotropy greater than 3:
[In the above formula,
R 2 and R 3 independently of one another are alkyl, alkoxy, fluorinated alkyl or fluorinated alkoxy having 1 to 7 carbon atoms, alkenyl having 2 to 7 carbon atoms, alkenyloxy, alkoxyalkyl or fluorinated alk Preferably R < 2 > and R < 3 > are alkyl or alkenyl,
To Independently of one another,
or , And preferably
or ego,
L 21 , L 22 , L 31 and L 32 independently of one another are H or F, L 21 and / or L 31 are preferably F,
X 2 and X 3 independently of one another are halogen, halogenated alkyl or alkoxy having 1 to 3 carbon atoms, or halogenated alkenyl or alkenyloxy having 2 or 3 carbon atoms, preferably F, Cl, -OCF 3 or -CF 3 , most preferably F, Cl or -OCF 3 ,
Z 3 is -CH 2 CH 2 -, -CF 2 CF 2 -, -COO-, trans -CH = CH-, trans -CF = CF-, -CH 2 O- or a single bond, preferably -CH 2 CH 2 -, -COO-, trans-CH = CH- or a single bond, most preferably -COO-, trans-CH = CH- or a single bond,
l, m, n and o are independently of each other 0 or 1, and
Optionally at least one genetic neutral compound selected from the group consisting of compounds of formula IV and V, preferably comprising at least one of each of these compounds:
[In the above formula,
R 41 to R 52 independently of one another have the significance given for R 2 in formula II, preferably R 41 is alkyl and R 42 is alkyl or alkoxy, or R 41 is alkenyl and R 42 is alkyl , Preferably R 51 is alkyl and R 52 is alkyl or alkenyl, or R 51 is alkenyl and R 52 is alkyl or alkenyl, preferably alkyl,
And Independently of one another, and Lt; / RTI > are present independently from each other,
or
, And preferablyAnd One or more of ego,
And Independently of one another, and Lt; / RTI > are present independently from each other,
or
, And preferablyAnd One or more of or ego,
Z 41 to Z 52 are independently of each other and, when Z 41 and / or Z 51 are present two times, they also independently of one another are -CH 2 CH 2 -, -COO-, trans-CH = -CF = CF-, -CH 2 O-, -CF 2 O-, -C≡C- or a single bond, preferably, Z 41 and Z 42, and at least one of Z 51 and Z 52 is a single one or more of Lt; / RTI &
p and q are independently of each other 0, 1 or 2,
p is preferably 0 or 1.
Wherein the medium is a compound of formula III wherein n and o are all 1 and Z 3 is preferably a single bond and all the rings are 1,4-phenylene optionally substituted one or two times independently of one another. ) And a compound of formula V, wherein q is 2 and Z 51 and Z 52 are preferably all single bonds.
Alternatively or additionally to the compounds of formula (II) and / or (III), the medium according to the invention may comprise one or more genetically-positive compounds of formula (VI)
[In the above formula,
R 6 is an alkenyl, alkenyloxy City, alkenyl, alkoxy alkyl, or fluoride having an alkyl, alkoxy, fluorinated alkyl or fluorinated alkoxy, 2 to 7 carbon atoms having 7 carbon atoms, preferably Alkyl or alkenyl,
To Independently of one another,
or ego,
L 61 and L 62 independently of one another are H or F, preferably L 61 is F,
X 6 is halogen, halogenated alkyl or alkoxy having 1 to 3 carbon atoms, or halogenated alkenyl or alkenyloxy having 2 or 3 carbon atoms, preferably F, Cl, -OCF 3 or -CF 3 , most preferably F, Cl or -OCF 3 ,
Z 6 represents -CH 2 CH 2 -, -CF 2 CF 2 -, -COO-, trans-CH═CH-, trans-CF═CF- or -CH 2 O-, preferably -CH 2 CH 2 - a, -, -COO- or trans -CH = CH-, most preferably -COO- or -CH 2 CH 2
r is 0 or 1;
In a preferred embodiment of the present invention, the liquid crystal medium according to the present invention comprises at least one polymerizable compound. The polymerizable compound is a non-mesogenic compound such as the known EHA, 2EHA, or a mesogenic compound. These polymerizable mesogenic compounds are referred to herein as "reactive mesogens" (abbreviated as RM). These polymerizable compounds can be either one-reactive or multi-reactive, preferably both reactive, whether mesogenic or non-mesogenic. Preferably, the medium comprises both one or more mono-reactive compounds and one or more multi-reactive, preferably e-reactive compounds. Most preferably, the medium comprises one or more RMs, but a non-mesogenic compound may additionally be present.
The RM can be chiral or non-chiral and can comprise an acrylate / methacrylate group or another polymerizable group. In a particularly preferred embodiment, RM is a chiral compound (since it is possible to simply adjust the selective reflection wavelength by polymerizing a predetermined amount of the chiral RM), and therefore no further twist of the liquid crystal material is required, so that a cholesteric pitch And as a result can form selective reflection at longer wavelengths. The generated cholesteric pitch can be stabilized advantageously for further changes, for example, by the use of a suitable filter (e.g. UV filter) to protect the liquid crystal from ambient radiation.
When a chiral reactive mesogen is used in the liquid crystal medium according to the present invention, it is also preferable to use a photoinitiator in the medium in many cases when exposed to UV radiation. The use of photoinitiators results in a significant reduction in the required UV radiation dose.
Chiral reactive mesogens can be used in the liquid crystal media according to the present invention, since only chiral compounds are present in the medium. However, in a preferred embodiment of the present invention, chiral reactive mesogens are used with conventional (non-reactive) chiral dopants. In this preferred embodiment, a preferred starting value of the cholesteric pitch, or alternatively a value somewhat closer to the already preferred value, can be fixed by one or more conventional chiral dopant (s). The further use of one or more chiral reactive mesogens then allows the cholesteric pitch to be further adjusted by depleting the chiral reactive mesogens after exposing the medium to UV radiation.
In the latter of these, in many cases it is preferred to use conventional chiral dopants and chiral reactive mesogens having the same designation of HTP for each other. In this embodiment, only a small total concentration is required to achieve a short cholesteric pitch, and the overall physical properties of the mesogenic host material change only to a relatively small degree. This preferred embodiment results in relatively low requirements for the protection of the medium against further wavelength changes of selective reflection after the desired value is achieved by UV radiation.
However, in some cases, it is advantageous to use one or more conventional chiral dopants and one or more chiral reactive mesogens, wherein the conventional chiral dopants have different HTPs than the chiral reactive mesogens. In this case, the center wavelength of the selective reflection can be fixed by a conventional chiral dopant. This central wavelength can then be shifted to longer wavelengths by use of the chiral reactive mesogens. This effect can then be reversed by exposure of the medium to UV radiation. In particular, this latter preferred embodiment results in the lowest requirement for protection of the medium against further wavelength changes of selective reflection after the desired value is achieved by UV radiation.
As a first approximation, it should be noted that the mixture of chiral compounds, i.e. the HTP of the conventional chiral dopant and the chiral reactive mesogen, approximates the respective HTP values weighed by their respective concentrations in said medium .
The RM may be one-reactive or bi- or multi-reactive. Particularly preferably, a liquid crystal having a functional group at each end or a substance containing at least one azo-reactive compound (cross-linking agent) which is at least mesogenic is usable, for example, based on a diacrylate-based RM.
Where the medium comprises one or more polymerizable compounds, it preferably further comprises one or more polymerization initiators, such as photoinitiators and / or thermal initiators.
The liquid crystal medium according to the present invention may be stabilized by polymerization of each of the polymer precursors composed of said one or more polymerizable compounds and optionally said one or more initiators, or stabilized in a preferred embodiment. Preferably, the stabilizing polymer has the form of a polymer network. That is, the non-polymerizable liquid crystal material / mesogenic material is present in a somewhat continuous form in which the somewhat smooth strands of the polymeric material are dispersed. Polymer networks Stabilized liquid crystals are described, for example, in Dierking, I., Adv. Mater. 12, No. 3, pp. 167-181 (2000). In a preferred embodiment of the present invention, the liquid crystal medium according to the present invention comprises at least one polymerizable compound, preferably RM.
The host mixture contains a liquid crystal compound having a low molecular weight and preferably one or more chiral dopants in an amount sufficient to effect selective reflection of the electromagnetic spectrum in the visible range. These cholesteric phases having a relatively short cholesteric pitch are preferably stabilized by the polymer. Stabilization of the (cholesteric) phase is accomplished by adding to the chiral liquid crystal host mixture a mixture comprising at least one polymerizable compound, preferably a RM, preferably a one-reactive and a re-reactive RM, and a suitable photo- For example by exposing the polymerizable compound to UV radiation for a short period of time. Preferably, the polymerization is carried out in an electro-optic cell maintained at a predetermined temperature on the cholesteric phase of the chiral liquid crystal host mixture.
The mesogenic mono-reactive compounds according to the present invention preferably comprise one or more ring elements linked together by a direct bond or via a linking group, wherein two of these ring members are optionally either directly or the same or different Can be connected to one another via connectors. The ring element is preferably selected from the group consisting of 4, 5, 6 or 7 membered rings, preferably 5 or 6 membered rings.
The RMs used according to the invention are preferably selected from the group of compounds of the formulas VIIA and VIIB:
In this formula,
R 71 is H, F, Cl, Br, I, CN, NO 2 , NCS, SF 5 , SO 2 CF 3 ; Or linear or branched, preferably having 1 to 20 C-atoms, unsubstituted or monosubstituted or polysubstituted by F, Cl, Br, I or CN, optionally substituted with one or more nonadjacent CH 2 Wherein each of the groups is independently selected from the group consisting of -O-, -S-, -NH-, -NR 01- , -SiR 01 R 02 -, -CO- , -COO-, -OCO-, -OCO-O-, -S-CO-, -CO-S-, -CY 01 = CY 02- or -C≡C, preferably H, halogen; N-alkyl having 1 to 7, preferably 2 to 5 C-atoms, n-alkoxy; Alkenyl, alkenyloxy or alkoxyalkyl having 2 to 7, preferably 2 to 5 C-atoms; Or CN, NCS; Halogen, preferably F, Cl; Halogenated alkyl, alkenyl or alkoxy, preferably mono-, di- or oligo-fluoroalkyl, alkenyl or alkoxy, particularly preferably CF 3 , OCF 2 H or OCF 3 ,
R 01 and R 02 are independently of each other H or alkyl having 1 to 12 C-atoms,
or ego,Is preferably a mesogenic residue comprising at least one ring, most preferably a < RTI ID = 0.0 > Lt; / RTI > is a divalent radical of formula &
To Are independently of one another an aromatic and / or cycloaliphatic ring, or a group containing two or more fused aromatic or cycloaliphatic rings, optionally containing one or more heteroatoms selected from N, O and / or S Optionally mono- or poly-substituted by R 72 ,
Z 71 to Z 74 independently represent -O-, -S-, -CO-, -CO-O-, -O-CO-, -S-CO-, -CO- O-, -CO-NR 01 -, -NR 01 -CO-, -OCH 2 -, -CH 2 O-, -SCH 2 -, -CH 2 S-, -CF 2 O-, -OCF 2 - -CF 2 S-, -SCF-, -CH 2 CH 2 -, -CF 2 CH 2 -, -CH 2 CF 2 -, -CF 2 CF 2 -, -CH═N-, -N═CH-, -N = N-, -CH = CR 01 -, -CY 01 = CY 02 -, -C≡C-, - (CH 2) 4 -, -CH = CH-CO-O-, -O-CO- CH = CH- or a single bond,
Y 01 and Y 02 are independently of each other F, Cl or CN, alternatively one of them may be H,
R 72 is H or alkyl, preferably H, or alkyl having 1 to 10 C-atoms,
PG 71 is a polymerizable group or a reactive group,
SP 71 is a spacer group or a single bond,
X 71 has one of the meanings given for Z 71 and is preferably selected from the group consisting of -O-, -CO-O-, -O-CO-, -CF 2 O-, -OCF 2 -, -CH 2 O- , -OCH 2 - or a single bond;
Lt; RTI ID = 0.0 > ≪ / RTI > have the meanings given for &
PG 72 and PG 73 independently of each other have one of the meanings given for PG 71 ,
SP 72 and SP 73 independently of each other have one of the meanings given for SP 11 ,
X 72 and X 73 independently of one another have one of the meanings given for X 71 above.
In a preferred embodiment of the present invention, the precursor of the polymer comprises, in addition to the compound (s) of formula (VIIA), preferably at least one e-reactive mesogenic monomer of formula (VIIB).
The compounds of formulas VIIA and VIIB according to the invention may be chiral compounds.
Particularly preferred is a polymer precursor comprising at least one compound of formula (VIIA) and / or (VIIB)
Z 71 and / or Z 74 are independently selected from the group consisting of -O-, -CO-O-, -OCO-, -O-CO-O-, -CH 2 -O-, -O-CH 2 -, -CF 2 -O -, -O-CF 2 -, -C≡C-, -CH = CH- or a single bond, most preferably -CO-O-, -O-CO- or -O-, and / or;
Z 71 is not a single bond;
Ring A < 71 > is phenylene optionally substituted with one or more groups R < 1 >;
R 71 is alkyl or alkoxy having 1 to 12, preferably 1 to 8 C-atoms, or alkenyl, alkenyloxy having 2 to 12, preferably 2 to 7 C-atoms, / RTI >
- SP 71 is optionally mono- or polysubstituted by F, and one or more non-adjacent CH 2 in each case independently of one another can be replaced by -O-, -CH = CH- or -C≡C-, Quot; A 71 "through a group selected from -O-, -CO-O-, -O-CO-, -O-CO-O- and a single bond, An alkylene having an atom and / or;
- SP 71 is a single bond.
Preferred for MG 72 for X 73 is the same as for MG 71 for X 71 .
In a preferred embodiment, the rings A 71 to A 73 independently of one another are an aromatic or alicyclic ring, preferably a 5, 6 or 7 membered ring, or two or more, preferably two or three fused aromatic or alicyclic rings is a group containing, where these rings are optionally N, O and / or contain one or more heteroatoms selected from S, and optionally is monosubstituted or polysubstituted by L 7, L 7 is F, Cl, Br, CN , OH, NO 2 , and / or an alkyl, alkoxy, alkylcarbonyl or alkoxycarbonyl group having 1 to 12 C atoms, wherein one or more H atoms are optionally substituted with F or Cl.
L 7 is preferably F, Cl, CN, OH, NO 2 , CH 3 , C 2 H 5 , OCH 3 , OC 2 H 5 , COCH 3 , COC 2 H 5 , COOCH 3 , COOC 2 H 5 , CF 3 , OCF 3 , OCHF 2 or OC 2 F 5 , especially F, Cl, CN, CH 3 , C 2 H 5 , OCH 3 , COCH 3 or OCF 3 , most preferably F, Cl, CH 3 , OCH 3 or a COCH 3.
Preferred rings A 71 to A 73 are, for example, furan, pyrrole, thiophene, oxazole, thiazole, thiadiazole, imidazole, phenylene, cyclohexylene, cyclohexeneylene, pyridine, pyrimidine, pyrazine, Indene, naphthalene, tetrahydronaphthalene, decahydronaphthalene, tetrahydropyran, anthracene, phenanthrene and fluorene.
Particularly preferably, at least one of these rings A 71 to A 73 is furan-2,5-diyl, thiophene-2, or furan-2,5-diyl in which one or two non-adjacent CH 2 groups are optionally replaced by O and / 2,5-diyl, thienothiophen-2,5-diyl, dithienothiophene-2,6-diyl, pyrrole-2,5-diyl, 1,4-phenylene, azulene- Diyl, pyrimidine-2,5-diyl, naphthalene-2,6-diyl, 1,2,3,4-tetrahydro-naphthalene-2,6-di Yl, indene-2,5-diyl or 1,4-cyclohexylene, wherein these groups are unsubstituted or monosubstituted or polysubstituted by L as defined above.
Preferably,
To Lt; / RTI > Or a mirror image thereof,R is alkyl having 1 to 12, preferably 1 to 7 C-atoms, or alkenyl or alkynyl having 2 to 12, preferably 2 to 7 C-atoms, One or more non-adjacent -CH 2 - groups not adjacent to the phenyl ring may be replaced by -O- and / or -CH = CH- and / or one or more H-atoms may be replaced by halogen, preferably F And / or;
Preferably,
The or to be.In a preferred embodiment of the present invention,
Contain only single cyclic rings A 71 to A 73 . Very preferably, it is a group having one or two 5 and / or 6-membered rings.Preferred sub-formulas of this group are listed below. For simplicity, Phe in these groups is 1,4-phenylene, PheL is a 1,4-phenylene group substituted with 1 to 4 L groups as defined above, and Cyc is 1,4-cyclohexylene , Pyd is pyridine-2,5-diyl, and Pyr is pyrimidine-2,5-diyl. The following list of preferred groups includes sub-formulas VII-1 to VII-20 and their enantiomers:
In these preferred groups, Z has the meaning of Z 71 described in formula VIIA. Preferably, Z is -COO-, -OCO-, -CH 2 CH 2 -, -C≡C- or a single bond.
Most preferably, the group
Are selected from the following formulas (VIIa) to (VIIj) and their enantiomers:
In this formula,
L is an alkyl, alkoxy, alkylcarbonyl or alkoxycarbonyl group having F, Cl, Br, CN, OH, NO 2 and / or 1 to 12 C atoms wherein one or more H atoms are optionally F or Cl And,
r is 0, 1, 2, 3 or 4, preferably 0, 1 or 2.
In these preferred formulas
Is very preferably or , As well as , Wherein each L independently has one of the meanings given above.Particularly preferred compounds of formula I are those wherein r is 1 or 2,
.More preferred compounds of formula I are those wherein r is 1
/ RTI > and / or < RTI ID = 0.0 > .Are very preferably the following compounds:
In this formula,
The 1,4-phenylene ring may optionally be substituted by R, preferably alkyl, preferably methyl, and / or alkoxy, and / or halogen, preferably F.
More preferably,
Are the following compounds or enantiomers thereof: < RTI ID = 0.0 >
In this formula,
R is not adjacent to the have the above-described means, preferably at least one -CH 2 - group is optionally -O- and / or -CH = CH- may be substituted by, and / or one or more H atom is a halogen, preferably Alkyl, preferably n-alkyl, having from one to six C-atoms which may be replaced by F.
In a preferred embodiment of the invention, the liquid crystal medium according to the invention comprises the compounds of the sub-formulas I-1 to I-5, preferably the sub-formulas I-2, I-4 and I-5, Comprises one or more compounds of formula (I) selected from compounds of sub-formula (I-2):
In this formula,
R < 1 > has each of the meanings given for formula (I) above, preferably alkyl, most preferably n-alkyl and X < 1 >
In a preferred embodiment of the invention, the liquid crystal medium according to the invention comprises at least one compound selected from the group of compounds of the formulas II-1 and II-2, preferably the compound of the formula II-2:
In this formula,
The variables have the respective meanings given above for formula II, and X 2 is preferably F or -OCF 3 .
Preferably, the medium is selected from the group of compounds of formulas II-1 and II-2, wherein L 21 and L 22 are all F.
Preferably, the medium comprises at least one compound of formula II-1, preferably a compound selected from compounds of the formulas II-1a to II-1c, preferably compounds of the formula II-1c:
In this formula,
L 21 and L 22 are both F and L 23 and L 24 are both H, or L 21 , L 22 , L 23 and L 24 are both F.
In a preferred embodiment, the medium comprises at least one compound of formula (II-1c) wherein L 21 , L 22 , L 23 and L 24 are both F.
Preferably, the medium comprises at least one compound selected from the group of compounds of formulas II-2a to II-2c, preferably compounds of formula II-2c:
In this formula,
L 23 to L 27 are H or F independently of each other and other variables, and L 21 and L 22 are preferably all F, and two or three of L 23 to L 27 , Most preferably L 23 to L 25 are F and others of L 21 to L 27 are H or F, preferably H, and X 2 is preferably F or -OCF 3 , most preferably F .
Particularly preferred compounds of formula II-2 are those of formula II-2c-1:
Wherein R < 2 > has the meaning described above.
In another preferred embodiment of the present invention, the medium comprises at least one compound selected from the group of compounds of the formulas III-1 and III-2:
In the above formula, the variables have the respective meanings described above for the formula (III).
The medium is preferably a compound selected from the group of compounds of the formula III-1, preferably those of the formulas III-1a to III-1f, preferably those of the formulas III-1a, III-1c and III- And most preferably one or more compounds of each of formulas (III-1a) and / or (III-1c) and / or (III-1d)
L < 33 > to L < 37 > independently of each other and other variables are H or F, L < 31 > and L < 32 > are preferably all F, and L 33 to L 37 Two or three, most preferably L 33 to L 35 are F, and others of L 31 to L 37 are H or F, preferably H, and X 3 is preferably F or -OCF 3 .
Most preferred compounds of formula III-1 are selected from the group consisting of compounds of formula III-1a-1, III-1c-1 and III-1d-1:
Wherein R < 3 > has the meaning described above.
The compound of formula (IV) is preferably selected from the group consisting of compounds of formula IV-1 to IV-7, more preferably compounds of formula IV-6 and / or IV-7:
In this formula,
R 41 and R 42 are in having the respective meanings set forth in Formula IV, in particular the general formula IV-1 and IV-5, R 41 is preferably an alkenyl is the alkenyl alkyl or alkenyl, preferably, R 42 is preferably R 41 and R 42 are preferably both alkyl, and R 41 in formula IV-4 is preferably alkyl or alkenyl, preferably alkyl, in formula IV-2, , Preferably alkyl, and R < 42 > is preferably alkyl or alkoxy, preferably alkoxy.
Preferably the medium comprises one or more compounds selected from the group consisting of compounds of formulas IV-6 and IV-7; Most preferably at least one compound of each of formulas IV-6 and IV-7.
A preferred compound of formula IV-6 is a compound of formula CPTP-n-m and CPTP-n-Om, more preferably a compound of CPTP-n-Om. Preferred compounds of formula IV-7 are compounds of formula CPGP-n-m. The definitions of these abbreviations (acronyms) are set forth in the following Tables A to C and are illustrated in Table D below.
In a preferred embodiment, the liquid crystal medium according to the invention comprises at least one compound of the formula V selected from the group consisting of compounds of the formulas V-1 to V-6:
In this formula,
R 51 and R 52 each have the meanings given above in formula V, R 51 is preferably alkyl, more preferably n-alkyl, R 52 in formula V-1 is preferably alkenyl, Is preferably 3-alkenyl and most preferably - (CH 2 ) 2 -CH = CH-CH 3 , in formula V-3 R 52 is preferably alkyl or alkenyl, preferably n- 3-alkenyl, most preferably - (CH 2 ) 2 -CH = CH 2 , R 52 in formula V-3 is preferably alkyl, and "F 0/1 " in formula It is F.
Preferred compounds of the formula V-1 are the compounds of the formulas PP-n-2V and PP-n-2Vm, more preferably the compounds of the formula PP-1-2V1. Preferred compounds of formula V-2 are the compounds of formula PTP-n-Om, particularly preferably PTP-1-O2, PTP-2-O1 and PTP-3-O1. Preferred compounds of formula V-3 are those of formula PGP-nm, PGP-n-2V and PGP-n-2Vm, more preferably compounds of formula PGP-2-m, PGP- / RTI > Preferred compounds of the formula V-4 are the compounds of the formula PPTUI-n-m, particularly preferably the compounds of PPTUI-3-2, PPTUI-3-3, PPTUI-3-4 and PPTUI-4-4. A preferred compound of formula V-5 is a compound of formula PGGIP-n-m. A preferred compound of formula V-6 is a compound of formula PGIGP-n-m. The definitions of these abbreviations (acronyms) are illustrated in the following Tables A to C and illustrated in Table D below.
The compound of formula (VI) is preferably a compound selected from the group consisting of compounds of formula (VI-1) and (VI-2), preferably compounds of formula (VI-1)
In this formula,
The variables have the respective meanings given above and the variables L 63 and L 64 independently of each other and other variables are H or F, preferably Z 6 is -CH 2 -CH 2 -, preferably X 6 Is F.
Preferably, the liquid crystal medium according to the invention comprises a compound selected from the group consisting of compounds of formulas I to VI and VIIa and VIIb, more preferably compounds of formulas I to V and VIIa and / or VIIb, Preferably consists essentially of, or more preferably consists essentially of, or most preferably consists of, these compounds.
"Containing" in connection with a composition, unless otherwise explicitly defined, means that a relevant entity, e.g., a medium or component, is present in the composition or components or compounds or compounds described, preferably at least 10% Means that it contains 20% or more.
As used herein, "predominantly ", unless the context clearly dictates otherwise, means that the associated entity contains at least 55%, preferably at least 60%, and most preferably at least 70% of the listed components or components or compounds or compounds .
As used herein, "consisting essentially of" means that, unless otherwise expressly defined, the associated entity is intended to include at least 80%, preferably at least 90%, and most preferably at least 95% of the listed components or components or compounds or compounds .
As used herein, "totally occurring" means that, unless expressly defined otherwise, the related entity contains at least 98%, preferably at least 99%, and most preferably 100.0% of the listed components or components or compounds or compounds it means.
In addition, other mesogenic compounds not explicitly mentioned above may optionally and advantageously be used in the medium according to the invention. Such compounds are known to those skilled in the art.
The liquid crystal medium according to the present invention has a brightening point of 85 DEG C or higher, preferably 90 DEG C or higher.
The Δn at 589 nm (Na D ) and 20 ° C. of the liquid crystal medium according to the present invention is preferably in the range of 0.150 to 0.350, more preferably in the range of 0.170 to 0.250, and most preferably in the range of 0.180 to 0.220 Range.
The Δε at 1 kHz and 20 ° C. of the liquid crystal medium according to the present invention is preferably 10 or more, preferably 15 or more, more preferably 20 or more, most preferably 25 or more, preferably 40 or less Preferably 35 or less, more preferably 10 or more and 40 or less, and most preferably 20 to 30.
The nematic phase of the medium according to the present invention free of chiral dopants is preferably at least 0 ° C and at least 80 ° C, more preferably at least -20 ° C and at most 85 ° C, most preferably at least -20 ° C and not less than 90 ° C , In particular at least -30 캜 or lower and 95 캜 or higher.
The liquid crystal medium is preferably characterized for comparison purposes in a TN display operating at a second transition minimum according to Gooch and Tarry with an optical retardation (d 占 n) in the range of 1.0 占 퐉 to 1.1 占 퐉 . However, they are preferably used as cholesteric liquid crystals, also referred to as chiral nematic liquid crystals having a rather short cholesteric pitch, and preferably their cholesteric pitches are selected such that their selective reflection wavelength is within the visible range of the electromagnetic spectrum , I.e. within the range of 400 to 800 nm.
The liquid crystal medium preferably has a spiral twist in the range of not less than 20 μm -1 , preferably not less than 40 μm -1 , more preferably not less than 60 μm -1 , and most preferably not less than 80 μm -1 and not more than 260 μm -1 And at least one chiral dopant having an absolute value of helical twisting power (HTP).
The liquid crystal medium preferably contains a total of 50 to 100%, more preferably 70 to 100%, more preferably 80 to 100%, especially 90 to 100% of the compounds of formulas I, II, III, IV, V and VI Compounds, preferably compounds of formula (I), (II), (III), (IV) and (V).
The liquid crystal medium more preferably comprises or more preferably consists of the compounds of the formulas I, II, III, IV, V and VI, preferably the compounds of the formulas I, II, III, IV and V Or more preferably consist essentially of, or most preferably consist entirely of these.
The compound of formula (I) is preferably used in the medium at a total concentration of from 1 to 35%, more preferably from 2 to 30%, even more preferably from 3 to 20% and most preferably from 5 to 15% do.
The compounds of Formulas II and III are used together in the medium at a total concentration of 40 to 80%, more preferably 45 to 75%, even more preferably 50 to 70%, and most preferably 55 to 65% of the total mixture do.
The compound of formula II is preferably used in the medium at a total concentration of 15 to 35%, more preferably 20 to 30%, most preferably 22 to 28% of the total mixture.
The compound of formula III is preferably used in the medium at a total concentration of 20 to 45%, more preferably 25 to 40%, most preferably 30 to 35% of the total mixture.
The compound of formula IV is preferably used in the medium at a total concentration of from 10 to 35%, more preferably from 15 to 30%, and most preferably from 20 to 25% of the total mixture.
The compound of formula V is preferably used in the medium at a total concentration of 10 to 30%, more preferably 12 to 28%, most preferably 16 to 23% of the total mixture.
The compound of formula (VI) is preferably used in the medium at a total concentration of 0 to 30%, more preferably 0 to 15%, most preferably 1 to 10% of the total mixture.
The polymerizable compounds, preferably the compounds of formulas VIIA and / or VIIB, are preferably present in a total concentration of from 0 to 10%, more preferably from 0 to 7%, most preferably from 0.5 to 5% .
Preferably, at least one polymerization initiator, preferably at least one photoinitiator, is used. The concentration of the initiator is 0.1-10%, more preferably 0.2-5%, most preferably 0.5-2% of the total concentration of the polymerizable compounds.
Preferably the media according to the invention further comprise one or more chiral compounds as chiral dopants for adjusting their cholesteric pitch. Their total concentration in the medium according to the invention is preferably in the range 0.1 to 15%, more preferably 1 to 10%, most preferably 2 to 6%.
Optionally, the media according to the present invention may comprise additional liquid crystal compounds to adjust the physical properties. Such compounds are known to those skilled in the art. Their concentration in the medium according to the invention is preferably 0 to 30%, more preferably 0.1 to 20%, most preferably 1 to 15%.
The medium according to the invention preferably comprises at least one compound of the formula:
- Formula I-2; And / or
- Formula II-1c, preferably a compound of the formula PUQU-n-F; And / or
- a compound of the formula II-2c, preferably a compound of the formula II-2c-1; And / or
- formula III-1a, preferably formula III-1a-1; And / or
delete
- a compound of the formula III-1c, preferably a compound of the formula III-1c-1; And / or
- formula (III-1d), preferably formula (III-1d-1); And / or
- formula IV-6, preferably formula CPTP-n-Om and / or CPTP-n-m; And / or
- formula IV-7, preferably formula CPGP-n-Om and / or CPGP-n-m; And / or
- formula V-1, preferably the formula PP-n-mV and / or PP-n-mVI; And / or
- formula V-2, preferably formula PTP-n-Om; And / or
- a compound of the formula V-3, preferably a compound of the formula PGP-n-m and / or PGP-n-mV; And / or
- the formula V-4, preferably the formula PPTUI-n-m; And / or
- R-5011 or S-5011; And / or
At least one reactive polymerizable compound; And / or
At least one polymerization initiator.
The term " dielectrically positive "is used herein to refer to a compound or component having a DELTA epsilon over 3.0, and the expression" dielectrically neutral " , And the expression "dielectrically negative" is used for a compound or an ingredient having a [Delta] [epsilon] less than -1.5. [Delta] is determined at a frequency of 1 kHz and at 20 [deg.] C. The dielectrically anisotropy of each compound is determined from the results of a 10% solution of each individual compound in the nematic host mixture. If the solubility of each compound in the host mixture is less than 10%, the concentration is reduced to a factor of 2 until the product mixture is stable enough to at least determine its properties. Preferably, however, the concentration is maintained at least 5% to maintain the significance of the result as high as possible. The capacity of the test mixture is determined in both cells having a homeotropic orientation and cells having a homogeneous orientation. The cell gap in both types of cells is about 20 탆. The applied voltage is a rectangular wave with a frequency of 1 kHz and is typically a mean square root of 0.5 to 1.0 V (rms), but this is always selected to be less than the threshold of the capacity of each test mixture.
Δε is defined as (ε ∥ -ε ⊥), ε av is defined as / 3 (ε ∥ + 2ε ⊥ ). For genetically positive compounds, the mixture ZLI-4792 is used as the host mixture, not only for the genetic neutrality but also for the genetic negative compound, the mixture ZLI-3086 is the product of the German Merck KGaA . The permittivity of the compound is determined from the change in the respective value of the host mixture when the compound of interest is added. The value is extrapolated to a 100% concentration of the compound of interest.
The components having a nematic phase at a measurement temperature of 20 DEG C are measured as such and the others are handled like compounds.
Unless expressly stated otherwise, the term "threshold voltage" is used herein to refer to the optical threshold and is presented as 10% relative contrast (V 10 ), the term "saturation voltage" Contrast (V 90 ). Also, the capacitance threshold voltage (V 0 ), also referred to as the Freedericks-threshold (V Fr ), is used only when explicitly mentioned.
Unless expressly stated otherwise, the ranges of variables described herein all include limits.
Unless specifically stated otherwise, all concentrations throughout the disclosure are presented in mass% and are for each individual mixture, all temperatures are given in degrees Celsius, and all temperature differences are noted in degrees Celsius. All physical properties are described in "Merck Liquid Crystals, Physical Properties of Liquid Crystals ", Status Nov. 1997, Merck KGaA, Germany] and is described for a temperature of 20 ° C, unless explicitly stated otherwise. The optical anisotropy (? N) is determined at a wavelength of 589.3 nm. The dielectric anisotropy (DELTA epsilon) is determined at a frequency of 1 kHz. All other electro-optical properties as well as the threshold voltage were measured using a test cell manufactured by Merck KGaA in Germany. The test cell for measuring [Delta] [epsilon] had a cell gap of about 20 [mu] m. The electrode was a circular ITO electrode with an area of 1.13 cm 2 and a guard ring. The orientation layer was homeotropic alignment was for (ε ⊥) lecithin, horizontal orientation (ε ∥) polyimide AL-1054 of Japanese Shin tetik rubber (Synthetic Rubber) for. The capacitance was determined with Solatron 1260, a frequency response analyzer using a sine wave with a voltage of 0.3 V rms . The test cell used had a cell gap selected to have a suitable optical delay below the first transmission minimum according to the molar and terry, typically about 0.45 mu m <" 1 & gt ;. The light used for the electrooptical measurement was white light. We used equipment available from Autronic-Melchers, Karlsruhe, Germany. The characteristic voltage was determined under vertical observation. The threshold voltage V 10 , the mid-gray voltage V 50 and the saturation voltage V 90 were determined for 10%, 50% and 90% relative contrast, respectively.
Response time will increase time (τ on) for a change from 0 to the time of 90% relative contrast (t 90 -t 0) (including any that is, the delay time (t 10 -t 0)), respectively, again from 100% Decay time τ off with respect to the change time (t 100 -t 10 ) of the relative contrast which becomes 10%, and the total response time (τ total = τ on + τ off ).
The liquid crystal medium according to the present invention may contain further additives in usual concentrations. The total concentration of these additional components ranges from 0 to 10%, preferably from 0.1 to 6%, based on the total mixture. The concentration of each of the individual compounds used is preferably in the range of 0.1 to 3%. When reference is made herein to the concentration values and ranges of the liquid crystal components and compounds of the liquid crystal medium, the concentrations of these components and similar additives are not taken into account. This is also effective with the concentration of dichroic dye used in the mixture which is not calculated when the concentration of the compounds and components of the host mixture is not specified. The concentration of each of the above additives is always described for the final doped mixture.
The liquid crystal medium according to the present invention comprises several compounds, preferably 3 to 30, more preferably 4 to 20, most preferably 4 to 16 compounds. These compounds are mixed in a conventional manner. In general, compounds used in smaller amounts are more soluble in compounds used. It is particularly easy to observe that the dissolution process is completed when the temperature is higher than the luminescent point of the compound used at a higher concentration. However, it is also possible to use a so-called pre-mixture, which may be a homogeneous or eutectic mixture of the compound, or a so-called multi-container system (multi -bottle-systems) may be used to prepare the media.
The liquid crystal medium according to the present invention, which preferably comprises one or more chiral dopants, selectively reflects radiation in the visible range of the electromagnetic spectrum, i.e. in the range of 400 to 800 nm. Preferably, the bands of selective reflection thereof extend into the range of the wavelengths, more preferably the center wavelengths of these reflection bands are within this range, and most preferably these fully reflected bands are within the range Lt; / RTI > Preferably they have a selective reflection of half full width (1/2 FWHM) in the range of 15 to 60 nm, preferably 20 to 55 nm, most preferably 25 to 50 nm. In particular, the relative half-life width, that is, the ratio of the half-value width to the center wavelength of the reflection band is preferably 1 to 20%, more preferably 2 to 16%, further preferably 4 to 10% 8%.
The center wavelength of the selective reflection generated at the stated temperatures can be calculated from the actual center of the chiral dopant in the host used through the estimation of the polynomial series (I).
In this formula,
alpha, beta and gamma are material constants specific to the combination of given chiral dopants in a given host mixture.
c (dop.) is the concentration of the chiral dopant in the host mixture.
In many practical cases, the first term ("α · [c (dop.)] -1 '') provides sufficiently accurate results, even if only the first term is taken into account. The variable "a" is similar to the reciprocal of HTP (i.e., HTP- 1 ). Here, in determining the selective reflection wavelength of the cholesteric LC similar to the "Bragg" reflection, the effective refractive index of the mixture should be further considered for more accurate numerical calculation.
Typically, the variables alpha, beta and gamma are more strongly dependent on the type of chiral dopant than the particular liquid crystal mixture used.
Obviously, they depend on the enantiomeric excess of each chiral dopant. They have their maximum absolute values for pure enantiomers and 0 for racemates. Herein, these values are for pure enantiomers with an enantiomeric excess of 98% or more.
Preferably, the absolute value of the variable alpha of each chiral dopant in each liquid crystal medium according to the present invention is in the range of 5 to 25 nm, more preferably 10 to 20 nm, most preferably 12 to 16 nm.
These media may comprise more than one chiral dopant. When they comprise two or more chiral dopants, they can be advantageously chosen in one of the known ways of compensating for, for example, the cholesteric pitch, and thus the temperature dependence of the selective reflection wavelength. Here, in one host mixture, the chiral dopant having the same symbol of the variable a according to the characteristics of the higher-order term of the above-mentioned equation (I) Can be used.
More preferably, as an embodiment of the present invention using a single chiral dopant, it shows that it has a low temperature dependence of the chiral pitch induced in each host mixture, i. The liquid crystal medium according to the present invention by the addition of suitable additives can be obtained by using a liquid crystal medium such as TN, TN-AMD, ECB-AMD, VAN-AMD, IPS and OCB LCD or by using PDLC, NCAP, PN LCD, Can be modified to be used in all known types of liquid crystal displays using a complex system such as a PA LCD.
The transition T (S, N) from the melting point T (C, N), the smectic phase S to the nematic phase N and the nominal point T (N, I) of the liquid crystal are described in degrees Celsius.
In the present specification, particularly in the following examples, the structure of the liquid crystal compound is represented by abbreviations also called "acronyms ". The abbreviations of the abbreviations into their corresponding formulas are simplified according to the following three Tables A to C.
C n H 2n + 1 , C m H 2m + 1 and C l H 2l + 1 groups are straight chain alkyl groups having n, m and 1 carbon atoms respectively and C n H 2n , C m H 2m and C l H 2l group all are each preferably is (CH 2) and n, l (CH 2) m and (CH 2), -CH = CH- is preferably a trans-or E - it is vinylene.
Table A lists the codes used for the ring elements, Table B lists the codes for the linkers, and Table C lists the codes for the left and right extremities of the molecule.
Table D lists the exemplary structures of the molecules and their respective codes together.
Table A: Ring elements
Table B: Connectors
Table C:
In the above, n and m are integers, respectively, and "... " represents the spacing distance from other abbreviations in the table.
The liquid crystal medium according to the present invention preferably contains, in addition to the compounds of formula (I), one or more compounds selected from the group consisting of compounds of the following table.
Table D
Table E lists the chiral dopants preferably used in the liquid crystal media according to the invention.
Table E
In a preferred embodiment of the invention, the medium according to the invention comprises at least one compound selected from the group consisting of the compounds of Table E,
Table F lists the stabilizers preferably used in the liquid crystal media according to the invention.
Table F
In the above table, "n" means an integer of 1 to 12.
In a preferred embodiment of the invention, the medium according to the invention comprises at least one compound selected from the group consisting of the compounds of Table F.
The liquid crystal medium according to the invention preferably comprises at least 4, preferably at least 6 compounds selected from the group consisting of the compounds of Table D, preferably those selected from the group consisting of the compounds of Table D More preferably 7 or more, preferably 8 or more, preferably 3 or more different.
Figure 1 shows UV reflection peaks of cholesteric liquid crystal (CLC) mixtures A-1 and A-2 according to an embodiment of the present invention.
Figure 2 shows UV reflection peaks of cholesteric liquid crystal mixtures B-1 and B-3 according to an embodiment of the present invention.
Example
The examples provided below illustrate the invention without limiting the invention in any way.
However, the physical properties and composition show to the person skilled in the art which properties can be obtained and the extent to which they can be varied. In particular, combinations of various properties that can be advantageously obtained are well-defined for those skilled in the art.
A liquid crystal mixture is implemented with the composition and characteristics given in the following table. Investigate its optical performance. Especially its reflection spectrum.
Example One
Example 1.1 and 1.2
3.2% or 2.3% of the chiral dopant R-5011, obtained from Merck KGaA of Darmstadt, Germany, was added to 96.8% and 97.7% of the mixture AO from Example 1, respectively, to give each A -1 < / RTI > and A-2, respectively. The transition temperature from the cholesteric liquid crystal phase to the isotropic phase (i.e., clearing point) was 83.5 ° C for the mixture A-1 having 3.2% R-5011 and 86.5 ° C for the mixture having 2.3% R-5011 .
The mixture A-1 exhibits reflection in the blue spectral range from 377 to 435 nm (FWHM) as shown in Fig. 1 and mixture A-2 also shows the green spectrum in the range from 506 to 576 nm (FWHM) Region. ≪ / RTI > The "R" in the drawing - the comma on the axis represents the decimal point.
Example 1.3
Similar to Example 1.1, 1.6% chiral dopant R-5011 was added to 98.4% mixture A-0 from Example 1 to produce cholesteric mixture A-3. This mixture had selective reflection in the red visible spectrum region.
In the same way, it is easy to implement a mixture that reflects in any desired spectral region using the respective chiral dopant concentration.
The results of all the examples are shown in Table 2 below. The variable "?" Was also calculated for each individual example. And, as expected, it was almost constant for the combination of the chiral dopant R-5011 and the host mixture A-0 and was (12.7 ± 0.3) nm for the concentration used herein.
Example 2
Example 2.1 to 2.4
Example 2.1
3.2% chiral dopant R-5011 was added to 96.8% of the mixture B-0 from Example 2 to produce each cholesteric mixture B-1 with a relatively short cholesteric pitch. The transition temperature (i.e., luminescent point) of the mixture B-1 from the cholesteric liquid crystal phase to the isotropic phase was 74.5 占 폚. Mixture B-1 showed a reflection in the blue spectral range from 418 to 488 nm (FWHM).
Example 2.2
2.6% of the chiral dopant R-5011 was added to 97.4% of the mixture B-0 from Example 2 to give each cholesteric mixture having a short cholesteric pitch, somewhat longer than the mixture B-1 of Example 2.1 B-2. The conspicuous point of mixture B-2 was 76.5 ° C. Mixture B-2 showed a reflection in the green spectral range from 512 to 590 nm (FWHM). The center wavelength of the selective reflection was 551 nm.
Example 2.3
2.2% of the chiral dopant R-5011 was added to 97.8% of the mixture B-0 from Example 2 to give the respective cholesteric mixture B-0 having a cholesteric pitch much longer than the mixture B- 3. The conspicuous point of the mixture B-3 was 77.5 ° C. Mixture B-3 showed reflections in the red spectral range of 600 to 693 nm (FWHM).
The production spectra of the selective reflections for the mixtures B-1 and B-3 of Examples 2.1 and 2.3 are all shown in Fig. Drawing "R" - The comma on the axis represents the decimal point. In this figure, the results for Mixture B-2 of Example 2.2 were not included to avoid any confusion due to overlapping spectra. The band of selective reflection of mixture B-2 almost completely fills the gap in the visible spectrum between the two mixtures B-1 and B-2.
Example 2.4
2.7% of the chiral dopant R-5011 was added to 97.3% of the mixture B-0 from Example 2 to give each cholesteric mixture having a short cholesteric pitch, which is somewhat shorter than the mixture B-2 of Example 2.2 B-4 was prepared. The conspicuous point of the mixture B-4 was 76.0 占 폚. The mixture B-4 showed a reflection in the green spectral range. The center wavelength of the selective reflection was about 530 nm.
The results for the mixtures B-2 and B-4 show that they have a selective reflection width with a slightly smaller bandwidth than the mixture B-2 and a near coincident central wavelength between the two mixtures B-2 and B-4 Lt; RTI ID = 0.0 > A-2 < / RTI >
Example 2.5
2.43% of the chiral dopant R-5011 was added to 97.57% of the mixture B-0 from Example 2 to give a mixture of each cholesteric mixture having a short cholesteric pitch slightly longer than the mixture B- Mixture B-5 was prepared. The conspicuous point of the mixture B-5 was 77.0 占 폚. The mixture B-5 showed a reflection in the yellow spectrum region. The center wavelength of the selective reflection was about 584 nm, and the range was 541 to 626 nm (FWHM).
Example 2.6
2.13% of the chiral dopant R-5011 was added to 97.87% of the mixture B-0 from Example 2 to obtain a mixture of the respective cholesteric Mixture B-6 was prepared. The conspicuous point of the mixture B-6 was 78 占 폚. Mixture B-6 showed a reflection in the red spectrum region. The center wavelength of the selective reflection was about 670 nm, and the range was 619 to 720 nm (FWHM).
The variable "a" for the combination of chiral dopant R-5011 and host mixture B-0 was (14.3 0.2) nm for the concentration used herein.
Example 3
In this example, Mixture B-2 of Example 2.2, consisting of 2.6% R-5011 and 97.4% Mixture B-0 and having a reflection in the green spectral region, was used as the starting mixture, . To this end, RM-1 (bis-methacrylate), a reactive mesogen of the following formula, is added to mixture B-2:
Photoinitiator Irgacure (R) 651 (IRG-651 (registered trademark) (available from Ciba, Switzerland)):
≪ / RTI >
The concentration of RM used is 1%, and the concentration of photoinitiator is set to 1% of RM concentration.
The mixture is homogenized on a hot plate for 20 minutes, once heated to 60 DEG C (Series A) and its properties are investigated. This is then heated to the same temperature for 20 minutes on the second hot plate (Series B) and again illuminated. The mixture (B2M1-1) is examined as described above. This is further filled into the test cell and then the reactive mesogens are polymerized by exposure to UV radiation.
Example 4
Example 4.1 to 4.3
Similar to Example 3, the mixture B-2 of Example 2.2 is stabilized with the polymer formed in the material. The concentration of RM is again changed from 1% to 3% to a mixture of 5%, and the concentration of photoinitiator is set to RM of 1% in each case. However, here different RMs, e-reactive mesogens, RM2 (bis-acrylate) of the following formula are used:
These mixtures (B2M2-1 to B2M2-3) are heated on a hot plate at a temperature of 60 DEG C for 20 minutes to homogenize them and irradiate them as described above. These are further filled into the test cell and then the reactive mesogens are polymerized by exposure to UV radiation.
Example 5
Example 5.1 to 5.3
Similar to Examples 3 and 4, a basic mixture is stabilized with the polymer formed in the material. The concentration of RM is again changed from 1% to 3% to a mixture of 5%, and the concentration of photoinitiator is set to RM of 1% in each case. Here, similarly to Example 4, the re-reactive mesogen RM2 is used.
However, here the mixture B-6 of Example 2.6 is used.
These respective mixtures (B6M2-1 to B6M2-3) are heated on a hot plate for 20 minutes at a temperature of 60 DEG C to homogenize them and irradiate them as described above. These are further filled into the test cell and then the reactive mesogens are polymerized by exposure to UV radiation.
Example 6
A cholesteric mixture C-1 with a short cholesteric pitch was prepared by adding 2.8% of the chiral dopant R-5011 to 97.2% of the mixture C-0. The set point of the mixture C-1 was 84.0 占 폚. The mixture C-1 showed a reflection in the green spectral range. The center wavelength of the selective reflection was about 526 nm. The band of selective reflection is in the range of 484 to 567 nm (FWHM), so DELTA lambda / 2 is 42 nm.
Example 7
Example 7.1 to 7.4
Alternatively, 2.7, 3.1, 3.2 and 3.3% of the chiral dopant R-5011, respectively, were added to the respective amounts of the mixture D-0 to give cholesteric mixtures D-4 to D-1 having relatively short cholesteric pitch . The conspicuous points of these mixtures were 78.5, 77.0, 77.0 and 76.5 ° C, respectively. Mixture D-4 showed reflection in the green spectral region, and all three mixtures D-1 to D-3 showed reflection in the blue spectral region. Changes in the concentration of the chiral dopant in these three mixtures make it possible to select a suitable blue color. The center wavelength of the selective reflection of the mixture D-4 is about 536 nm, and then the mixtures continue to move towards shorter wavelengths and the concentration of the chiral dopant increases.
Example 8
Example 8.1 to 8.3
3.28%, 2.82% or 2.27% of the chiral dopant R-5011 obtained from Merck KGaA of Darmstadt, Germany was added to 96.72%, 97.18% and 97.73% of the mixture E-0 from Example 8, respectively, Each cholesteric mixture, designated E-1 to E-3, respectively, having a cholesteric pitch was prepared. The transition temperature (i.e., clearing point) from the cholesteric liquid crystal phase to the isotropic phase is 73.5 ° C in the case of the mixture E-1 having 3.28% R-5011 and in the mixture E-2 having 2.82% R- And 75.0 占 폚 for the mixture E-3 having 2.27% R-5011 and 76.0 占 폚 for the mixture E-3 having 2.27% R-5011.
Mixture E-1 exhibits reflections in the blue spectral range from 438 to 510 nm (FWHM), mixture E-2 has selective reflection in the green spectral range from 490 to 567 nm (FWHM) And has selective reflection in the red spectral region ranging from 595 to 681 nm (FWHM).
The results of all examples are shown in Table 13 below. The variable "?" Was also calculated for each individual example. And, as expected, it was almost constant for the combination of the chiral dopant R-5011 and the host mixture E-0 and was (15.0 ± 0.5) nm for the concentration used herein.
Example 9
To a 97.61% mixture F-0 was added 2.39% chiral dopant R-5011. The product mixture F-1 has a center wavelength of 86.0 DEG C and a selective reflection at 558 nm. The band of selective reflection is in the range of 524 to 592 nm (FWHM), so DELTA lambda / 2 is 34 nm.
Example 10 to 13
To each of these mixtures (G-0 to J-0), a predetermined amount of chiral dopant R-5011 was provided. The respective concentrations were 2.73%, 2.22%, 3.02% and 2.93% for the respective mixtures G-1, H-1, I-1 and J-1.
Example 14
To the liquid crystal host mixture B-0 of Example 2 was added a chiral reactive mesogen (RM3 *) of the formula:
Was added to mixture B-2. This reactive mesogen RM3 * had a chiral structure and the compound used had an enantiomeric excess. To this host mixture B-0 was added 4.75% RM3 *. The three cells were then filled with the product mixture. The cell for LC was made of AF glass and had an orientation layer of AL3046 (available from JSR of Japan). The primary cells of these cells were irradiated as they were. The variable α was here 21.2 nm. The secondary and tertiary cells of these cells were each exposed to UV radiation with different doses. A high pressure mercury lamp EXECURE-w 3000 (available from Hoya Schott, Japan) was used. The intensity of the UV radiation was 100 mW / cm. A blocking filter with a blocking wavelength of 320 nm was placed between the lamp and the LC cell. The secondary cell was exposed to UV radiation with an energy of 22 J, and the tertiary cell was exposed to 71 J.
Obviously, the selective reflection wavelength can be changed to any desired color simply by changing the intensity / energy of the UV radiation.
Example 15
In this example, 1.19% chiral dopant R-5011 and 3.00% chiral reactive mesogen RM3 * were added to the liquid crystal host mixture B-0 of Example 2. R-5011 and RM3 * both had positive HTP values. Similar to in Example 14, several cells were filled with the product mixture and processed and examined as described in Example 14. [ Here, four cells were fabricated. The primary cell was irradiated as is. Each of the secondary cells was exposed to UV radiation having an energy of 60 J, the tertiary cell was exposed to 120 J, and the quaternary cell was exposed to 300 J.
Obviously, also here the selective reflection wavelength can be changed to any desired color by a change in the intensity / energy of the UV radiation.
Example 16
1.19% chiral dopant R-5011 and 3.00% chiral reactive mesogen RM3 * were mixed with 0.030% photoinitiator Irgacure 651 (abbreviation IRG-651 (registered trademark), Shibaura, Switzerland) Liquid crystal host mixture B-0. Similar to in Example 15, four cells were also prepared, treated and examined. Primary cells were also examined as is. Each of the secondary cells was exposed to UV radiation having an energy of 30 J, the tertiary cell was exposed to 60 J, and the quaternary cell was exposed to 120 J.
Obviously, also here the selective reflection wavelength can be changed to any desired color by a change in the intensity / energy of the UV radiation. The energy of the UV radiation required to achieve a given wavelength shift of selective reflection is significantly lower when a photoinitiator is used, as in this embodiment, as compared to the situation under the absence of photoinitiator as in Example 15 above. Starting with one identical wavelength of selective reflection (i.e., 452 +/- 1 nm) in both cases, the longer wavelength value of a given selective reflection (e.g., 598 +/- 3 nm) is significantly lower Of the energy dose. This results in reduced exposure of the medium to UV radiation and increased reliability.
These results are summarized and compared in the following table.
Claims (27)
[In the above formula,
R 1 is alkyl, alkoxy, fluorinated alkyl or fluorinated alkoxy, alkenyl, alkenyloxy, alkoxyalkyl, fluorinated alkenyl or fluorinated alkenyloxy,
The or ego,
L 11 and L 12 independently of one another are H or F,
X < 1 > is CN or NCS,
i is 0 or 1,
- at least one compound of formula II:
[In the above formula,
R 2 is alkyl, alkoxy, fluorinated alkyl or fluorinated alkoxy having 1 to 7 carbon atoms or alkenyl, alkenyloxy, alkoxyalkyl or fluorinated alkenyl having 2 to 7 carbon atoms,
To Independently of one another,
or ego,
L 21 and L 22 independently of one another are H or F,
X 2 is halogen, halogenated alkyl or alkoxy having 1 to 3 carbon atoms, or halogenated alkenyl or alkenyloxy having 2 or 3 carbon atoms,
l and m are independently of each other 0 or 1,
- at least one compound of the formula V:
[In the above formula,
R 51 and R 52 independently of one another have the meanings given for R 2 in the above formula (II)
And Independently of one another, and Lt; / RTI > are present independently from each other,
or ego,
Z 51 and Z 52 independently of one another and, when Z 51 is present two times, also independently of one another, -CH 2 CH 2 -, -COO-, trans-CH = CH-, trans- , -CH 2 O-, -CF 2 O-, -C≡C- or a single bond,
q is 0, 1 or 2, and
- one or more chiral compounds
And a liquid crystal layer,
Said liquid crystal medium having a cholesteric pitch suitable for selectively reflecting light so that the center wavelength of the selective reflection is in the visible range of the spectrum,
589 nm (Na D ) and birefringence (? N) at 20 占 폚 of 0.1999 or more and 0.350 or less.
Wherein the total concentration of the compound of formula (I) in the medium is in the range of 1 mass% to 35 mass%.
Wherein the liquid crystal medium comprises at least one compound of formula < RTI ID = 0.0 > (I-2)
In this formula,
R 1 and X 1 have the meanings described above for Formula I in Claim 1, respectively.
Wherein the liquid crystal medium comprises at least one compound of the formula < RTI ID = 0.0 > (II-2c-1)
In this formula,
And R < 2 > have the meanings described in claim 1.
Wherein the liquid crystal medium comprises at least one compound of the formula < RTI ID = 0.0 > (III-1d-1)
In this formula,
R 3 is alkyl, alkoxy, fluorinated alkyl or fluorinated alkoxy having 1 to 7 carbon atoms, or alkenyl, alkenyloxy, alkoxyalkyl or fluorinated alkenyl having 2 to 7 carbon atoms.
Wherein the liquid crystal medium comprises at least one compound of the following formula R-5011 or S-5011:
.
Lt; RTI ID = 0.0 > 1, < / RTI > wherein the liquid crystal medium comprises at least one polymerizable compound.
Wherein the liquid crystal medium comprises a compound of formula V-1, a compound of V-2, or both:
In this formula,
R 51 and R 52 each have the meanings given in claim 1.
Characterized in that the liquid crystal medium is the liquid crystal medium according to claim 1.
Wherein the liquid crystal medium comprises at least one non-reactive chiral compound (i.e., chiral dopant).
Characterized in that the liquid crystal medium further comprises a photoinitiator.
Characterized in that the liquid crystal medium has a selective reflection wavelength in the near ultraviolet or visible range of more than 250 nm of the electromagnetic spectrum.
Wherein the medium selectively reflects light having a color selected from red, green and blue.
Applications Claiming Priority (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP08015344 | 2008-08-29 | ||
| EP08015344.8 | 2008-08-29 | ||
| EP08019804.7 | 2008-11-13 | ||
| EP08019804 | 2008-11-13 | ||
| EP09000778.2 | 2009-01-21 | ||
| EP09000778 | 2009-01-21 | ||
| EP09007720.7 | 2009-06-10 | ||
| EP09007720 | 2009-06-10 | ||
| PCT/EP2009/006045 WO2010022891A1 (en) | 2008-08-29 | 2009-08-20 | Liquid crystalline medium and liquid crystal display |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167026948A Division KR20160118375A (en) | 2008-08-29 | 2009-08-20 | Liquid crystalline medium and liquid crystal display |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20110058860A KR20110058860A (en) | 2011-06-01 |
| KR101770833B1 true KR101770833B1 (en) | 2017-08-24 |
Family
ID=41217728
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117007227A KR101770833B1 (en) | 2008-08-29 | 2009-08-20 | Liquid crystalline medium and liquid crystal display |
| KR1020167026948A KR20160118375A (en) | 2008-08-29 | 2009-08-20 | Liquid crystalline medium and liquid crystal display |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167026948A KR20160118375A (en) | 2008-08-29 | 2009-08-20 | Liquid crystalline medium and liquid crystal display |
Country Status (2)
| Country | Link |
|---|---|
| KR (2) | KR101770833B1 (en) |
| WO (1) | WO2010022891A1 (en) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6280366B2 (en) * | 2010-05-06 | 2018-02-14 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツングMerck Patent Gesellschaft mit beschraenkter Haftung | Liquid crystal medium and liquid crystal display |
| US20140217325A1 (en) * | 2011-09-05 | 2014-08-07 | Merck Patent Gmbh | Liquid-crystalline medium and high-frequency components comprising same |
| EP2760971B1 (en) * | 2011-09-27 | 2015-11-25 | Merck Patent GmbH | Liquid crystal medium and high-frequency components comprising same |
| KR102128242B1 (en) * | 2012-03-06 | 2020-06-30 | 메르크 파텐트 게엠베하 | Cholesteric liquid crystal medium and liquid crystal display |
| JPWO2015162950A1 (en) * | 2014-04-25 | 2017-04-13 | Jnc株式会社 | Liquid crystal composition and liquid crystal display element |
| CN106459763A (en) * | 2014-05-09 | 2017-02-22 | 默克专利股份有限公司 | Liquid-crystalline medium and high-frequency components comprising same |
| WO2016066242A1 (en) * | 2014-10-31 | 2016-05-06 | Merck Patent Gmbh | Liquid-crystalline medium and high-frequency components comprising same |
| KR20190042636A (en) * | 2016-08-24 | 2019-04-24 | 메르크 파텐트 게엠베하 | Liquid crystal medium and liquid crystal display |
| KR102537813B1 (en) * | 2016-11-11 | 2023-05-31 | 메르크 파텐트 게엠베하 | Liquid crystalline medium and liquid crystal display |
| CN107418597A (en) * | 2017-07-21 | 2017-12-01 | 扬州高捷电子科技有限公司 | Low temperature liquid crystal material and its application |
| US11760931B2 (en) | 2017-12-06 | 2023-09-19 | Merck Patent Gmbh | Liquid-crystalline medium for use in a switching element |
| JP7420717B2 (en) * | 2017-12-06 | 2024-01-23 | メルク・パテント・ゲゼルシヤフト・ミツト・ベシユレンクテル・ハフツング | Liquid crystal media for use in switching elements |
| CN109929564B (en) * | 2017-12-19 | 2024-03-12 | 默克专利股份有限公司 | Liquid crystal medium and liquid crystal display |
| KR20190076882A (en) * | 2017-12-22 | 2019-07-02 | 메르크 파텐트 게엠베하 | Liquid crystalline medium and liquid crystal display |
| CN108707464A (en) * | 2018-06-05 | 2018-10-26 | 晶美晟光电材料(南京)有限公司 | A kind of liquid-crystal composition and its application with high optics anisotropic |
| TWI775904B (en) | 2018-07-24 | 2022-09-01 | 德商馬克專利公司 | Liquid-crystal lens |
| US11952526B2 (en) * | 2019-08-30 | 2024-04-09 | Merck Patent Gmbh | LC medium |
| JP2023508301A (en) * | 2019-12-24 | 2023-03-02 | 北京八億時空液晶科技股▲ふん▼有限公司 | Applications in liquid crystal compositions and devices adaptable to relatively wide operating temperatures |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040089843A1 (en) * | 2002-08-03 | 2004-05-13 | Merck Patent Gmbh | Liquid-crystalline medium and liquid-crystal display having high twist |
| US20040222404A1 (en) * | 2003-02-14 | 2004-11-11 | Harald Hirschmann | Liquid-crystalline medium |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TW523621B (en) * | 1997-06-11 | 2003-03-11 | Koninkl Philips Electronics Nv | Multi-domain liquid-crystal display device |
| JP4058481B2 (en) * | 2001-04-12 | 2008-03-12 | 日東電工株式会社 | Polymerizable liquid crystal compound and optical film |
| KR100920912B1 (en) * | 2001-05-21 | 2009-10-12 | 메르크 파텐트 게엠베하 | Chiral compounds |
| EP1273585B1 (en) * | 2001-07-02 | 2005-02-16 | MERCK PATENT GmbH | Chiral compounds |
| DE10204790A1 (en) * | 2002-02-06 | 2003-08-14 | Merck Patent Gmbh | Liquid crystalline medium |
| EP1813662B1 (en) * | 2006-01-27 | 2009-10-21 | MERCK PATENT GmbH | Liquid crystalline medium and liquid crystal display |
| US7879256B2 (en) * | 2006-03-31 | 2011-02-01 | E. I. Du Pont De Nemours And Company | Liquid crystal compositions, polymer networks derived therefrom and process for making the same |
| DE102007007143A1 (en) * | 2006-10-04 | 2008-04-10 | Merck Patent Gmbh | Liquid crystalline medium |
| DE602007004922D1 (en) * | 2006-11-27 | 2010-04-08 | Merck Patent Gmbh | Liquid-crystalline medium and liquid-crystal display |
-
2009
- 2009-08-20 KR KR1020117007227A patent/KR101770833B1/en active IP Right Grant
- 2009-08-20 KR KR1020167026948A patent/KR20160118375A/en not_active Application Discontinuation
- 2009-08-20 WO PCT/EP2009/006045 patent/WO2010022891A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040089843A1 (en) * | 2002-08-03 | 2004-05-13 | Merck Patent Gmbh | Liquid-crystalline medium and liquid-crystal display having high twist |
| US20040222404A1 (en) * | 2003-02-14 | 2004-11-11 | Harald Hirschmann | Liquid-crystalline medium |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20160118375A (en) | 2016-10-11 |
| KR20110058860A (en) | 2011-06-01 |
| WO2010022891A1 (en) | 2010-03-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101770833B1 (en) | Liquid crystalline medium and liquid crystal display | |
| JP6280366B2 (en) | Liquid crystal medium and liquid crystal display | |
| JP6526745B2 (en) | Liquid crystal medium and liquid crystal display | |
| KR101725562B1 (en) | Liquid crystalline medium and liquid crystal display | |
| JP5627857B2 (en) | Liquid crystal medium and liquid crystal display | |
| KR20160137393A (en) | Liquid-crystalline medium and liquid-crystal display comprising the same | |
| KR20190013577A (en) | Liquid-crystalline medium and liquid-crystal display comprising the same | |
| KR20160122074A (en) | Liquid-crystalline medium and liquid-crystal display comprising the same | |
| JP6961485B2 (en) | Liquid crystal medium and high frequency elements including it | |
| KR20180105719A (en) | Liquid crystal media and high frequency components containing them | |
| JP2019163236A (en) | Compounds for homeotropic alignment of liquid-crystalline media | |
| KR20160105484A (en) | Liquid crystal medium and liquid crystal display | |
| EP3212734A1 (en) | Liquid-crystalline medium and high-frequency components comprising same | |
| WO2009115226A1 (en) | Liquid crystalline medium and liquid crystal display | |
| KR101863377B1 (en) | Liquid crystalline medium and liquid crystalline display | |
| KR20150002847A (en) | Liquid crystal medium and liquid crystal display | |
| JP2017524046A (en) | Liquid crystal medium and high frequency component including the same | |
| KR20170038058A (en) | Polymerisable mesogenic compound, liquid crystal medium and liquid crystal display | |
| EP3240859A1 (en) | Bimesogenic compounds and mesogenic media | |
| JP6752795B2 (en) | Liquid crystal medium and high frequency elements including it | |
| KR102128242B1 (en) | Cholesteric liquid crystal medium and liquid crystal display | |
| CN109929564B (en) | Liquid crystal medium and liquid crystal display | |
| EP1213337B1 (en) | Liquid crystalline medium and liquid crystal display | |
| KR101647510B1 (en) | Liquid crystalline medium and liquid crystal display | |
| TW201716557A (en) | Liquid crystal medium and liquid crystal display |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| AMND | Amendment | ||
| E902 | Notification of reason for refusal | ||
| AMND | Amendment | ||
| E601 | Decision to refuse application | ||
| AMND | Amendment | ||
| E902 | Notification of reason for refusal | ||
| AMND | Amendment | ||
| X701 | Decision to grant (after re-examination) | ||
| GRNT | Written decision to grant |