JP4847988B2 - Spring wire with excellent corrosion fatigue characteristics - Google Patents
Spring wire with excellent corrosion fatigue characteristics Download PDFInfo
- Publication number
- JP4847988B2 JP4847988B2 JP2008184734A JP2008184734A JP4847988B2 JP 4847988 B2 JP4847988 B2 JP 4847988B2 JP 2008184734 A JP2008184734 A JP 2008184734A JP 2008184734 A JP2008184734 A JP 2008184734A JP 4847988 B2 JP4847988 B2 JP 4847988B2
- Authority
- JP
- Japan
- Prior art keywords
- less
- corrosion
- temperature
- steel
- test piece
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Landscapes
- Heat Treatment Of Steel (AREA)
- Heat Treatment Of Articles (AREA)
Description
本発明は、調質(焼入れ・焼戻し)した状態で使用されるコイルばねの素材として有用なばね用鋼(ばね鋼)に関するものであり、より詳細には腐食疲労特性に優れたばね用鋼に関するものである。 TECHNICAL FIELD The present invention relates to a spring steel (spring steel) useful as a material for a coil spring used in a tempered (quenched / tempered) state, and more particularly to a spring steel having excellent corrosion fatigue characteristics. It is.
自動車等に用いられるばねには、排ガス低減や燃費向上のため軽量化が求められ、その一環として高強度化が指向されている。高強度化されたばね(焼入れ・焼戻し後の引張強度が、例えば、1900MPa以上であるばね)では、一般に、水素脆性や腐食疲労による早期折損が問題となる。 Spring used in automobiles and the like is required to be light in weight for reducing exhaust gas and improving fuel efficiency, and as a part of this, high strength is aimed at. In the case of a highly strengthened spring (a spring having a tensile strength after quenching / tempering of, for example, 1900 MPa or more), in general, premature breakage due to hydrogen embrittlement or corrosion fatigue becomes a problem.
そのような問題を解決するため、従来、様々な技術が提案されている。例えばCrは一般に耐食性向上元素として知られているが、特許文献1は、塩水噴霧サイクル試験後の低歪速度での引張試験を行った場合にはCr添加では却って耐食性が低下する場合があること、このような場合の耐食性を向上させるにはCuやNiが効果的であり、CuとNiの合計量をCrの2倍以上にすることを提案している。
特許文献2は、Cが腐食疲労強度低下原因であるとしてCを低減すること、そしてCの低減によって懸念される耐へたり性の低下を、Si、Cu、Niなどの添加で防止すること、CuやNiは耐食性を高めるためにも有効であることを教示している。
しかしこれら特許文献1〜2では、技術レベルが十分に高いとは言えず、腐食疲労強度のさらなる向上余地がある。例えば、特許文献1〜2では、Niについては単に耐食性に優れるとの認識に過ぎず、その詳細な作用メカニズムや功罪両面の詳細検討が不足している。また同様のことは、Ni以外の元素についてもいえる。
Patent Document 2 describes that C is reduced because C is a cause of a decrease in corrosion fatigue strength, and a decrease in sag resistance that is a concern due to the reduction of C is prevented by addition of Si, Cu, Ni, and the like. It teaches that Cu and Ni are also effective for enhancing corrosion resistance.
However, in these
本発明の目的は、腐食疲労強度(特に調質後の腐食疲労強度)をより高いレベルで改善できるばね用鋼を提供することにある。 An object of the present invention is to provide a spring steel that can improve corrosion fatigue strength (particularly, corrosion fatigue strength after tempering) at a higher level.
本発明者らは、前記課題を解決するために鋭意検討を重ねた結果、腐食疲労強度を向上するには、フェライト脱炭を防止した上で、鋼の強度(硬さ)、腐食ピット形状、耐水素脆性の3点を改善する必要があることを見出し、しかもこの3点に及ぼす種々の元素の複雑な影響を解明し、本発明を完成した。 As a result of intensive studies in order to solve the above problems, the present inventors have improved the corrosion fatigue strength in order to prevent ferrite decarburization, and then the steel strength (hardness), corrosion pit shape, The present inventors have found that it is necessary to improve the three points of hydrogen embrittlement resistance, and have clarified the complex influence of various elements on these three points, thereby completing the present invention.
従って前記目的を達成し得た本発明のばね用鋼とは、C:0.38〜0.47%(質量%の意味。以下同じ)、Si:1.9〜2.5%、Mn:0.6〜1.3%、Cr:0.4%以下(0%を含む)、Cu:0.7%以下(0%を含む)、Ni:0.7%以下(0%を含む)、Ti:0.05〜0.15%、及びAl:0.003〜0.1%を含有し、残部が鉄及び不可避不純物であって、フェライト脱炭層深さが0.01mm以下であり、さらに下記式(1)で示されるCeq1が0.580以上、下記式(2)で示されるCeq2が0.49以下、下記式(3)で示されるCeq3が0.570以下である点にその特徴がある。
Ceq1=[C]+0.11[Si]−0.07[Mn]−0.05[Ni]+0.02[Cr] … (1)
Ceq2=[C]+0.30[Cr]−0.15[Ni]−0.70[Cu] … (2)
Ceq3=[C]−0.04[Si]+0.24[Mn]+0.10[Ni]+0.20[Cr]−0.89[Ti]−1.92[Nb] … (3)
(上記式中、[ ]は鋼中の各元素の含有量(質量%)を表す。なお鋼が、上記式に記載された元素(例えばCr)を含有しない場合、その対応する項目(例えば[Cr])にはゼロを代入して、Ceq1〜3を計算すればよい。)
Therefore, the spring steel of the present invention that can achieve the above-mentioned object is: C: 0.38 to 0.47% (meaning mass%; the same applies hereinafter), Si: 1.9 to 2.5%, Mn: 0.6 to 1.3%, Cr: 0.4% or less (including 0%), Cu: 0.7% or less (including 0%), Ni: 0.7% or less (including 0%) Ti: 0.05-0.15%, and Al: 0.003-0.1%, the balance is iron and inevitable impurities, and the ferrite decarburized layer depth is 0.01 mm or less, Further, Ceq1 represented by the following formula (1) is 0.580 or more, Ceq2 represented by the following formula (2) is 0.49 or less, and Ceq3 represented by the following formula (3) is 0.570 or less. There are features.
Ceq1 = [C] +0.11 [Si] −0.07 [Mn] −0.05 [Ni] +0.02 [Cr] (1)
Ceq2 = [C] +0.30 [Cr] −0.15 [Ni] −0.70 [Cu] (2)
Ceq3 = [C] −0.04 [Si] +0.24 [Mn] +0.10 [Ni] +0.20 [Cr] −0.89 [Ti] −1.92 [Nb] (3)
(In the above formula, [] represents the content (% by mass) of each element in the steel. When the steel does not contain the element (for example, Cr) described in the above formula, the corresponding item (for example, [ Cr]) is substituted with zero and Ceq1 to 3 are calculated.)
本発明のばね用鋼として、下記に示す腐食試験を行った後に、試験片表面に観察される腐食ピットの中から、深さが大きいものから順に5個以上の腐食ピットを選択し、それら腐食ピットの下記式(4)で示されるアスペクト比の平均値が0.9以下であるものが好ましい。
アスペクト比=(腐食ピットの深さ×2)/(腐食ピットの幅) … (4)
腐食試験:
ばね用鋼を温度925℃で10分加熱した後、温度70℃の油で冷却して油焼入れし、次いで温度400℃で60分加熱して焼戻しを行った後、表面を800番のエメリー紙で研磨して腐食試験用の試験片を作製する。
この試験片に、5質量%のNaCl水溶液を、JIS Z 2371に従い35℃で8時間噴霧し、その後、試験片を湿度60%及び温度35℃の湿潤環境に16時間保持することを1サイクルとして、これを合計14サイクル行う。
その後、錆を除去してから、試験片表面の腐食ピットをレーザー顕微鏡にて観察する。
As the steel for springs of the present invention, after performing the corrosion test shown below, five or more corrosion pits are selected from the corrosion pits observed on the surface of the test piece in descending order, and these corrosions are selected. The average value of the aspect ratio represented by the following formula (4) of the pit is preferably 0.9 or less.
Aspect ratio = (depth of corrosion pit × 2) / (width of corrosion pit) (4)
Corrosion test:
After heating the spring steel at a temperature of 925 ° C. for 10 minutes, cooling it with oil at a temperature of 70 ° C. and quenching it, followed by heating at a temperature of 400 ° C. for 60 minutes and tempering the surface, the surface is
The test piece was sprayed with 5% by mass NaCl aqueous solution at 35 ° C. for 8 hours in accordance with JIS Z 2371, and then the test piece was kept in a humid environment of 60% humidity and 35 ° C. for 16 hours as one cycle. This is done for a total of 14 cycles.
Then, after removing rust, the corrosion pits on the surface of the test piece are observed with a laser microscope.
本発明のばね用鋼は、必要に応じて、さらにNb:0.1%以下(0%を含まない)、B:0.005%以下(0%を含まない)などを単独で又は組み合わせて含有していても良い。また本発明のばね用鋼中で、Pが0.02%以下(0%を含まない)、Sが0.02%以下(0%を含まない)、Nが0.007%以下(0%を含まない)、Oが0.0015%以下(0%を含まない)になっていることが望ましい。 In the spring steel of the present invention, Nb: 0.1% or less (not including 0%), B: 0.005% or less (not including 0%), etc., alone or in combination, as necessary. You may contain. In the spring steel of the present invention, P is 0.02% or less (excluding 0%), S is 0.02% or less (not including 0%), and N is 0.007% or less (0% It is desirable that O is 0.0015% or less (excluding 0%).
本発明によれば、フェライト脱炭を防止した上で、種々の合金元素が適切に制御されているため、調質(焼入れ・焼戻し)後の鋼の硬さを向上でき、腐食ピットの形状を平坦化でき、且つ水素脆化に対する耐性を向上させることができ、その結果、優れた腐食疲労強度を実現することができる。さらに本発明のばね用鋼は、合金元素が節約されており、経済性にも優れている。 According to the present invention, since various alloying elements are appropriately controlled after preventing ferrite decarburization, the hardness of the steel after tempering (quenching / tempering) can be improved, and the shape of the corrosion pit can be reduced. Planarization and resistance to hydrogen embrittlement can be improved. As a result, excellent corrosion fatigue strength can be realized. Furthermore, the spring steel of the present invention saves alloy elements and is excellent in economy.
本発明鋼は、フェライト脱炭が防止されており、しかも調質後の硬さが高く、腐食により生じるピットが平らであり、かつ耐水素脆性が向上している点に特徴がある。このような鋼は、腐食疲労強度が優れている。前記フェライト脱炭の防止は、製造条件を工夫することによって達成できる。調質後の硬さ、腐食ピット形状、耐水素脆性は、フェライト脱炭を防止した上で、合金元素を適切に制御することによって(すなわち上記Ceq1〜3を適切にすることによって)達成できる。以下、順に説明する。
本発明では、製造方法を工夫することによってフェライト脱炭を防止している。フェライト脱炭は、合金元素を制御することによっても低減できるが、その場合には合金元素の添加量が増大して経済性が低下する虞があり、またCeq1〜3の制御との両立が難しくなるため、製造方法の工夫によってフェライト脱炭を防止することとした。
The steel of the present invention is characterized in that ferrite decarburization is prevented, hardness after tempering is high, pits generated by corrosion are flat, and hydrogen brittleness resistance is improved. Such steel has excellent corrosion fatigue strength. Prevention of the ferrite decarburization can be achieved by devising manufacturing conditions. The hardness after tempering, corrosion pit shape, and hydrogen embrittlement resistance can be achieved by preventing ferrite decarburization and appropriately controlling the alloy elements (that is, by appropriately adjusting the above Ceq1 to Ceq3). Hereinafter, it demonstrates in order.
In the present invention, ferrite decarburization is prevented by devising a manufacturing method. Ferritic decarburization can also be reduced by controlling the alloy element, but in that case, there is a risk that the added amount of the alloy element will increase and the economic efficiency will be lowered, and it is difficult to achieve compatibility with the control of Ceq1 to Ceq3. Therefore, it was decided to prevent ferrite decarburization by devising the manufacturing method.
通常、ばね用鋼(線材)は、鋼材を加熱及び熱間圧延し、コイリング後、冷却床で冷却することによって製造されており、本発明ではこの製造条件を適正に調整することによって、フェライト脱炭を防止した。具体的には、鋼材の化学成分の平衡状態図(C量だけを変化させたときの平衡状態図。Thermo−Calcを利用して作図できる)でC=0質量%としたときのA1変態点、A3変態点、及びA4変態点をそれぞれ、A1(C=0)変態点、A3(C=0)変態点、及びA4(C=0)変態点と称したとき、熱間圧延の仕上げ圧延中の鋼の最高到達温度を、A3(C=0)変態点以上、A4(C=0)変態点以下にすることによってフェライト脱炭を防止した。 Usually, spring steel (wire material) is manufactured by heating and hot rolling the steel material, coiling, and then cooling in a cooling bed. In the present invention, by appropriately adjusting the manufacturing conditions, ferrite removal is achieved. Prevented charcoal. Specifically, in the equilibrium diagram of the chemical composition of steel (equilibrium diagram when only the amount of C is changed; drawing can be made using Thermo-Calc), the A 1 transformation when C = 0 mass% Point, A 3 transformation point, and A 4 transformation point are referred to as A 1 (C = 0) transformation point, A 3 (C = 0) transformation point, and A 4 (C = 0) transformation point, respectively. Ferrite decarburization was prevented by setting the maximum temperature of the steel during the hot rolling finish rolling to the A 3 (C = 0) transformation point or more and the A 4 (C = 0) transformation point or less.
上記製造条件において注目すべきは、C=0質量%の状態を想定して圧延条件(特に仕上げ圧延の最高到達温度)を設定している点である。この利点は、鋼材の全成分量から計算したA3変態点以上の温度に保持して、相変態による炭素拡散を防止しつつ鋼材を圧延した場合と対比すると明瞭になる。全成分量から計算したA3変態点以上の温度に保持しても、圧延中、鋼表面の炭素濃度は徐々に低下していく。そして亜共析鋼では、C量が減少すると、A3変態点が上昇する。一方、圧延温度(鋼の温度)は、特に粗圧延及び中間圧延段階で徐々に低下していく。徐々に低下していく圧延温度が、徐々に上昇していく鋼表面のA3変態点に達すると、鋼表面では相変態が生じ、炭素拡散によるフェライト脱炭が急速に進行する。これに対して、仕上げ圧延でA3(C=0)変態点以上になるようにすると、たとえ仕上げ圧延前に(例えば、粗圧延後の中間圧延段階で)A3(C=0)変態点以下になってフェライト脱炭が生じていても、Cの逆拡散によってフェライト脱炭層を消失させることができる。なお仕上げ圧延の最高到達温度の上限をA4(C=0)変態点以下にしたのは、この温度を超えると、鋼表面にδフェライトが生成し、逆にフェライト脱炭が進行してしまうためである。またA4(C=0)変態点以上になると、極めて高温になるため、トータル脱炭(全脱炭)も進行してしまう。 What should be noted in the above production conditions is that the rolling conditions (particularly the highest temperature reached in finish rolling) are set assuming the state of C = 0% by mass. This advantage is retained in A 3 transformation point or above the temperature calculated from the total components of the steel product, become apparent when compared with the case of rolled steel while preventing carbon diffusion by phase transformation. Be held in A 3 transformation point or above the temperature calculated from the total component amount, during the rolling, the carbon concentration of the steel surface is gradually reduced. And in hypoeutectoid steel, when the C content decreases, the A 3 transformation point increases. On the other hand, the rolling temperature (steel temperature) gradually decreases particularly in the rough rolling and intermediate rolling stages. Rolling temperature that gradually decreases and gradually reaches the A 3 transformation point rises and goes steel surfaces, the steel surface occurs phase transformation, ferrite decarburization proceeds rapidly by carbon diffusion. On the other hand, if the A3 (C = 0) transformation point or higher is set in the finish rolling, the A3 (C = 0) transformation point before the finish rolling (for example, in the intermediate rolling stage after the rough rolling ). Even if ferrite decarburization occurs in the following manner, the ferrite decarburized layer can be eliminated by reverse diffusion of C. In addition, the upper limit of the maximum ultimate temperature of finish rolling was made below the A 4 (C = 0) transformation point. If this temperature is exceeded, δ ferrite is generated on the steel surface, and on the contrary, ferrite decarburization proceeds. Because. Moreover, since it will become very high when it becomes more than an A4 (C = 0) transformation point, total decarburization (total decarburization) will also advance.
仕上げ圧延の好ましい温度範囲は、1000℃以上(特に1050℃以上)、1250℃以下(特に1200℃以下)である。仕上げ圧延直前の圧延温度(例えば中間圧延の最終温度)は、特に限定されないが、通常850℃以上(好ましくは860℃)以上である。
本発明の仕上げ圧延温度は、通常の仕上げ圧延温度に比べて高い。仕上げ圧延温度を前記範囲にするためには、例えば、仕上げ圧延前に通常行っている水冷を省略(水冷を弱くすることを含む)し、仕上げ圧延時の加工発熱を利用して、鋼材温度を高めればよい。
The preferable temperature range of finish rolling is 1000 ° C. or higher (particularly 1050 ° C. or higher) and 1250 ° C. or lower (particularly 1200 ° C. or lower). The rolling temperature immediately before finish rolling (for example, the final temperature of intermediate rolling) is not particularly limited, but is usually 850 ° C. or higher (preferably 860 ° C.) or higher.
The finish rolling temperature of the present invention is higher than the normal finish rolling temperature. In order to set the finish rolling temperature within the above range, for example, the water cooling that is normally performed before finish rolling is omitted (including weakening the water cooling), and the steel material temperature is set using the processing heat generated during finish rolling. You only have to increase it.
なおフェライト脱炭を確実に防止するには、仕上げ圧延の温度制御に加え、圧延前の鋼の加熱温度、熱間圧延してコイリングした後の冷却床への載置温度、コイル(リング)の冷却条件なども制御する必要がある。 In order to prevent ferrite decarburization without fail, in addition to controlling the temperature of finish rolling, the heating temperature of the steel before rolling, the mounting temperature on the cooling bed after hot rolling and coiling, and the coil (ring) It is necessary to control the cooling conditions.
熱間圧延前の鋼の加熱温度は、900℃以上(好ましくはA3(C=0)変態点以上)、A4(C=0)変態点以下(好ましくは1250℃以下)である。加熱温度が低すぎると、フェライト−オーステナイト域での滞留時間が長くなる。また熱間圧延の生産性が低下する。一方、加熱温度がA4(C=0)変態点を超えると、δ−フェライト変態に起因するフェライト脱炭、及び高温加熱に起因するトータル脱炭(全脱炭)が進行する。 The heating temperature of the steel before hot rolling is 900 ° C. or higher (preferably A 3 (C = 0) transformation point or higher) and A 4 (C = 0) transformation point or lower (preferably 1250 ° C. or lower). When the heating temperature is too low, the residence time in the ferrite-austenite region becomes long. Moreover, the productivity of hot rolling is reduced. On the other hand, when the heating temperature exceeds the A 4 (C = 0) transformation point, ferrite decarburization due to the δ-ferrite transformation and total decarburization (total decarburization) due to high-temperature heating proceed.
冷却床への載置温度は、熱間圧延からコイリングまでの冷却条件を反映したものである。載置温度は、900℃以上、好ましくは940℃以上である。載置温度が低すぎると、フェライト単相域での滞留時間が長くなってフェライト脱炭が生じ易くなる。なお載置温度の上限はフェライト脱炭とは無関係であるが、A1(C=0)変態点+50℃以下(好ましくは1000℃以下、特に975℃以下)にすることが推奨される。載置温度が高すぎると、冷却時に過冷組織(ベイナイトやマルテンサイト)が発生し易くなり、ばね用鋼の加工性が劣化する。
なお仕上げ圧延後、コイリングまでの条件は、コイリング後の線材(ばね用鋼)を、そのまま所定の載置温度で冷却床に供給可能なように設計される。通常は、仕上げ圧延後、水冷又は風冷(好ましくは水冷)によって所定の載置温度近くまで急冷してから、コイリングする。急冷によって、冷却床での冷却開始までにフェライト脱炭が開始するのを防止することもできる。
The mounting temperature on the cooling floor reflects the cooling conditions from hot rolling to coiling. The mounting temperature is 900 ° C. or higher, preferably 940 ° C. or higher. If the mounting temperature is too low, the residence time in the ferrite single-phase region becomes long and ferrite decarburization is likely to occur. The upper limit of the mounting temperature is irrelevant to ferrite decarburization, but it is recommended to set the A 1 (C = 0) transformation point + 50 ° C. or lower (preferably 1000 ° C. or lower, particularly 975 ° C. or lower). If the mounting temperature is too high, a supercooled structure (bainite or martensite) is likely to occur during cooling, and the workability of the spring steel deteriorates.
The conditions from finish rolling to coiling are designed so that the coiled wire (spring steel) can be supplied as it is to the cooling bed at a predetermined mounting temperature. Usually, after finish rolling, coiling is performed after quenching to near a predetermined mounting temperature by water cooling or air cooling (preferably water cooling). By rapid cooling, it is possible to prevent the start of ferrite decarburization before the start of cooling in the cooling bed.
冷却床では、コイルの密部(冷却コンベアの幅方向両端)とコイルの疎部(冷却コンベアの幅方向中央)に分けて、600〜750℃の温度範囲の冷却速度を制御することが重要である。コイルの密部は、疎部に比べて冷却速度が遅くなり易く、この冷却速度が過度に遅くなると脱炭(特にフェライト脱炭)が生ずる。従ってコイル密部の冷却速度は、1.0℃/秒以上(好ましくは1.2℃/秒以上)にする。一方、フェライト脱炭防止とは無関係であるが、コイルの疎部は、密部に比べて冷却速度が速くなり易く、この冷却速度が過度に速くなると過冷組織が発生し易くなり、加工性に悪影響が出ることがある。従って過冷組織を防止する場合には、コイルの疎部の冷却速度は、8℃/秒以下(7℃/秒以下)にすることが推奨される。コイル密部及び疎部の冷却速度は、例えば、それぞれの場所にあたる風量を調節することによって別々に調整できる。 In the cooling floor, it is important to control the cooling rate in the temperature range of 600 to 750 ° C. by dividing the coil into dense portions (both ends in the width direction of the cooling conveyor) and sparse portions (center in the width direction of the cooling conveyor). is there. The dense portion of the coil tends to have a lower cooling rate than the sparse portion, and decarburization (particularly, ferrite decarburization) occurs when the cooling rate is excessively slow. Therefore, the cooling rate of the coil dense portion is set to 1.0 ° C./second or more (preferably 1.2 ° C./second or more). On the other hand, although it has nothing to do with the prevention of ferrite decarburization, the sparse part of the coil tends to have a faster cooling rate than the dense part. May be adversely affected. Therefore, in order to prevent an overcooled structure, it is recommended that the cooling rate of the sparse part of the coil be 8 ° C./second or less (7 ° C./second or less). The cooling rate of the coil dense part and the sparse part can be adjusted separately, for example, by adjusting the air volume corresponding to each location.
上記のようにしてばね用鋼(線材)のフェライト脱炭層を低減できる。本発明のばね用鋼では、フェライト脱炭層は、0.01mm以下(好ましくは、0.00mm)である。なお全脱炭層深さも浅いほど好ましく、例えば、0.25mm以下(好ましくは0.05〜0.20mm程度)である。 As described above, the ferrite decarburized layer of the spring steel (wire) can be reduced. In the spring steel of the present invention, the ferrite decarburized layer is 0.01 mm or less (preferably 0.00 mm). The total decarburized layer depth is preferably as shallow as possible, for example, 0.25 mm or less (preferably about 0.05 to 0.20 mm).
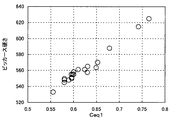
本発明のばね用鋼は、フェライト脱炭層が低減されているだけでなく、1)調質(焼入れ・焼戻し)後の硬さが高く、2)腐食により生じるピットが平らであり、かつ3)耐水素脆性が向上している点に特徴がある。フェライト脱炭の防止に加え、これら3つの特徴を兼ね備えることによって、腐食疲労強度を高めることができる。そして本発明の最大の特徴は、1)硬さ、2)ピット形状、3)耐水素脆性に与える合金元素の複雑な関係を明瞭に解明し、それぞれ、Ceq1、Ceq2、Ceq3と極めて高い相関関係があることを突き止めた点にある(図1〜3参照)。Ceq1が大きいほど硬くなり、Ceq2が小さいほどピット形状が平らになり、Ceq3が小さいほど耐水素脆性が向上し、それぞれ腐食疲労強度を高めるのに有利に作用する。 The spring steel of the present invention has not only a reduced ferrite decarburized layer, but also 1) high hardness after tempering (quenching / tempering), 2) flat pits caused by corrosion, and 3) It is characterized by improved hydrogen embrittlement resistance. In addition to preventing ferrite decarburization, the corrosion fatigue strength can be increased by combining these three characteristics. The most important features of the present invention are 1) hardness, 2) pit shape, and 3) the complex relationship of alloying elements that give resistance to hydrogen embrittlement, which is clearly elucidated, and extremely high correlation with Ceq1, Ceq2, and Ceq3, respectively. It is in the point where it was determined that there is (see FIGS. The larger Ceq1 is, the harder it is, and the smaller Ceq2 is, the flatter the pit shape is. The smaller Ceq3 is, the better the hydrogen embrittlement resistance is.
Ceq1=[C]+0.11[Si]−0.07[Mn]−0.05[Ni]+0.02[Cr] … (1)
Ceq2=[C]+0.30[Cr]−0.15[Ni]−0.70[Cu] … (2)
Ceq3=[C]−0.04[Si]+0.24[Mn]+0.10[Ni]+0.20[Cr]−0.89[Ti]−1.92[Nb] … (3)
Ceq1 = [C] +0.11 [Si] −0.07 [Mn] −0.05 [Ni] +0.02 [Cr] (1)
Ceq2 = [C] +0.30 [Cr] −0.15 [Ni] −0.70 [Cu] (2)
Ceq3 = [C] −0.04 [Si] +0.24 [Mn] +0.10 [Ni] +0.20 [Cr] −0.89 [Ti] −1.92 [Nb] (3)
上記式から把握されるように例えば、Niは、Ceq1(硬さ)とCeq3(耐水素脆性)の観点からは腐食疲労強度にとって不利に作用し、Ceq2(ピット形状)の観点からは腐食疲労強度にとって有利に作用する。他の合金元素も、同様に、複雑な関与の仕方をする。本発明によれば、各元素を個別に制御するのでなく、Ceq1〜3の視点にたって総合的に制御することによって、腐食疲労強度を確実に高めることができる。 As can be seen from the above formula, for example, Ni acts against corrosion fatigue strength from the viewpoint of Ceq1 (hardness) and Ceq3 (hydrogen embrittlement resistance), and corrosion fatigue strength from the viewpoint of Ceq2 (pit shape). It works for you. Other alloying elements have a complex involvement as well. According to the present invention, the corrosion fatigue strength can be reliably increased by comprehensively controlling each element from the viewpoint of Ceq1 to Ceq3, instead of controlling each element individually.
Ceq1の範囲は、0.580以上、好ましくは0.59以上、より好ましくは0.60以上である。Ceq2は、0.49以下、好ましくは0.47以下、より好ましくは0.45以下、特に0.43以下である。Ceq3は、0.570以下、好ましくは0.54以下、より好ましくは0.52以下である。 The range of Ceq1 is 0.580 or more, preferably 0.59 or more, more preferably 0.60 or more. Ceq2 is 0.49 or less, preferably 0.47 or less, more preferably 0.45 or less, and particularly 0.43 or less. Ceq3 is 0.570 or less, preferably 0.54 or less, more preferably 0.52 or less.
本発明のばね用鋼の硬さは、例えば、540HV以上(例えば、540〜580HV程度)である。なお前記硬さは、ロックウェルC硬さや引張強さに換算すると、52〜54HRC程度、1900〜2000MPa程度に相当する。 The hardness of the spring steel of the present invention is, for example, 540 HV or higher (for example, about 540 to 580 HV). The hardness corresponds to about 52 to 54 HRC and about 1900 to 2000 MPa when converted to Rockwell C hardness and tensile strength.
また本発明のばね用鋼によって達成されるピット形状は、下記腐食試験を実施することによって求まるアスペクト比によって特定でき、そのアスペクト比は、例えば、0.9以下程度(例えば、0.3〜0.85程度)である。
腐食試験:
(a)ばね用鋼を温度925℃で10分加熱した後、温度70℃の油で冷却して油焼入れし、次いで温度400℃で60分加熱して焼戻しを行った後(その後、必要なら表面を削って縮径した後(例えば、直径を0.25mm程度短くした後))、次いで表面を800番のエメリー紙で研磨して腐食試験用の試験片を作成する。
(b)この試験片に、5質量%のNaCl水溶液を、JIS Z 2371に従い35℃で8時間噴霧し、その後、試験片を湿度60%及び温度35℃の湿潤環境に16時間保持することを1サイクルとして、これを合計14サイクル行う。
(c)クエン酸アンモニウム(98.7%)を蒸留水で10質量%に希釈した液に常温で試験片を浸し、塩水噴霧で発生した錆びを除去する。次いで試験片表面の腐食ピットをレーザー顕微鏡にて観察し、試験片表面に観察される腐食ピットの中から、深さが大きいものから順に5個以上の腐食ピットを選択し、それら腐食ピットのアスペクト比を下記式(4)に従って算出する。
アスペクト比=(腐食ピットの深さ×2)/(腐食ピットの幅) … (4)
Moreover, the pit shape achieved by the spring steel of the present invention can be specified by an aspect ratio obtained by performing the following corrosion test, and the aspect ratio is, for example, about 0.9 or less (for example, 0.3 to 0). .85 or so).
Corrosion test:
(A) After heating the spring steel at a temperature of 925 ° C. for 10 minutes, cooling with oil at a temperature of 70 ° C. and quenching, then heating at a temperature of 400 ° C. for 60 minutes and tempering (after that, if necessary After cutting the surface to reduce the diameter (for example, after reducing the diameter by about 0.25 mm), the surface is then polished with No. 800 emery paper to prepare a test piece for a corrosion test.
(B) A 5 mass% NaCl aqueous solution is sprayed on this test piece at 35 ° C. for 8 hours in accordance with JIS Z 2371, and then the test piece is kept in a humid environment of 60% humidity and 35 ° C. for 16 hours. A total of 14 cycles are performed as one cycle.
(C) A test piece is immersed at room temperature in a solution obtained by diluting ammonium citrate (98.7%) to 10% by mass with distilled water, and rust generated by salt spray is removed. Next, the corrosion pits on the surface of the test piece are observed with a laser microscope, and from the corrosion pits observed on the surface of the test piece, five or more corrosion pits are selected in descending order, and the aspect of these corrosion pits is selected. The ratio is calculated according to the following formula (4).
Aspect ratio = (depth of corrosion pit × 2) / (width of corrosion pit) (4)
本発明のばね用鋼の水素割れ寿命は、例えば、720秒以上(例えば、800〜1200秒程度)である。水素割れ寿命は、以下のようにして求めることができる。
ばね用鋼を温度925℃で10分加熱した後、温度70℃の油で冷却して油焼入れし、次いで温度400℃で60分加熱して焼戻しを行い、試験片にする。4点曲げによって1400MPaの応力を作用させながら、試験片を硫酸(0.5mol/L)及びチオシアン酸カリウム(0.01mmol/L)の混合水溶液に浸漬する。ポテンションスタットを用いてSCE電極よりも卑である−700mVの電圧をかけ、割れが発生するまでの時間を測定する。
The hydrogen cracking life of the spring steel of the present invention is, for example, 720 seconds or longer (for example, about 800 to 1200 seconds). The hydrogen cracking life can be obtained as follows.
The spring steel is heated at a temperature of 925 ° C. for 10 minutes, then cooled with oil at a temperature of 70 ° C. and oil-quenched, and then heated at a temperature of 400 ° C. for 60 minutes for tempering to obtain a test piece. The test piece is immersed in a mixed aqueous solution of sulfuric acid (0.5 mol / L) and potassium thiocyanate (0.01 mmol / L) while applying a stress of 1400 MPa by 4-point bending. A voltage of −700 mV, which is lower than the SCE electrode, is applied using a potentiostat, and the time until cracking occurs is measured.
本発明のばね用鋼の腐食疲労強度は、例えば、290MPa以上(好ましくは300〜400MPa程度)である。腐食疲労強度は、例えば、以下のようにして求めることができる。 The corrosion fatigue strength of the spring steel of the present invention is, for example, 290 MPa or more (preferably about 300 to 400 MPa). The corrosion fatigue strength can be determined as follows, for example.
腐食疲労強度:
(a)ばね用鋼を温度925℃で10分加熱した後、温度70℃の油で冷却して油焼入れし、次いで温度400℃で60分加熱して焼戻しを行った後、JIS試験片(疲労試験片)に加工する。
(b)この疲労試験片の平行部を800番のエメリー紙で研磨した。試験片の掴み部が腐食しないように被膜で保護した後、この試験片に、5質量%のNaCl水溶液を、JIS Z 2371規格に従い35℃で8時間噴霧し、その後、試験片を湿度60%及び温度35℃の湿潤環境に16時間保持することを1サイクルとして、これを合計14サイクル行った後、小野式回転曲げ疲労試験で疲労試験を実施する。10MPa間隔で負荷応力を増大させながら、各負荷応力につき5本の試験片を用いて疲労試験を実施し、5本の試験片全てで1000万回まで折損しなかった応力を、腐食疲労強度とする。
Corrosion fatigue strength:
(A) After the spring steel was heated at a temperature of 925 ° C. for 10 minutes, cooled with oil at a temperature of 70 ° C. and oil-quenched, then heated at a temperature of 400 ° C. for 60 minutes and tempered, then a JIS test piece ( Fatigue test piece).
(B) The parallel part of this fatigue test piece was polished with # 800 emery paper. After protecting the grip of the test piece with a coating so that it does not corrode, this test piece was sprayed with 5% by weight NaCl aqueous solution at 35 ° C. for 8 hours in accordance with JIS Z 2371 standard. Further, holding for 16 hours in a humid environment at a temperature of 35 ° C. is one cycle, and after performing this for a total of 14 cycles, a fatigue test is performed by an Ono-type rotary bending fatigue test. While increasing the load stress at 10 MPa intervals, a fatigue test was performed using five test pieces for each load stress, and the stress that was not broken up to 10 million times by all five test pieces was determined as the corrosion fatigue strength. To do.
本発明を適用できるばね用鋼の成分組成は、以下の通りである。
C:0.38〜0.47%、
Si:1.9〜2.5%、
Mn:0.6〜1.3%、
Cr:0.4%以下(0%を含む)、
Cu:0.7%以下(0%を含む)、
Ni:0.7%以下(0%を含む)、
Ti:0.05〜0.15%、及び
Al:0.003〜0.1%、
残部:鉄及び不可避不純物
合金元素量(成分)の限定理由について詳述する。
The composition of the spring steel to which the present invention can be applied is as follows.
C: 0.38 to 0.47%,
Si: 1.9 to 2.5%,
Mn: 0.6 to 1.3%
Cr: 0.4% or less (including 0%),
Cu: 0.7% or less (including 0%),
Ni: 0.7% or less (including 0%),
Ti: 0.05-0.15%, and Al: 0.003-0.1%,
The remainder: iron and inevitable impurities The reason for limiting the amount (component) of alloying elements will be described in detail.
C:0.38〜0.47%
Cは、鋼中に必須的に含まれ、焼入れ・焼戻し後の強度(硬さ)の向上に寄与する。しかしC量が多すぎると、腐食ピットのアスペクト比が増大することで腐食ピットへの応力集中が増大し、また鋼中マトリックスの靱性が劣化することで耐水素脆性も劣化する。その結果、C量が過剰であると腐食疲労特性が劣化する。そこでC量を、0.38%以上(好ましくは0.39%以上)、0.47%以下(好ましくは0.45%以下、より好ましくは0.43%以下)と定めた。
C: 0.38 to 0.47%
C is essential in steel and contributes to improvement in strength (hardness) after quenching and tempering. However, if the amount of C is too large, the aspect ratio of the corrosion pits increases, the stress concentration on the corrosion pits increases, and the toughness of the matrix in steel deteriorates, so that the hydrogen embrittlement resistance also deteriorates. As a result, if the amount of C is excessive, the corrosion fatigue characteristics deteriorate. Accordingly, the C content is determined to be 0.38% or more (preferably 0.39% or more) and 0.47% or less (preferably 0.45% or less, more preferably 0.43% or less).
Si:1.9〜2.5%
Siは、固溶強化元素として強度向上に寄与し、耐力も向上させる。そのためSi量が少なすぎると、マトリックス強度が不足する。さらにSiは、焼戻し時の炭化物析出温度を高温側にシフトさせて、焼戻し脆性域を高温側にシフトさせることによって、耐水素脆性を向上させる作用も有する。しかしSi量が過剰であると、調質加熱時の炭化物の溶け込みを阻害し、強度が低下する。そこでSi量を、1.9%以上(好ましくは1.95%以上)、2.5%以下(好ましくは2.3%以下、より好ましくは2.2%以下)と定めた。
Si: 1.9 to 2.5%
Si contributes to strength improvement as a solid solution strengthening element and also improves proof stress. Therefore, if the amount of Si is too small, the matrix strength is insufficient. Furthermore, Si also has the effect of improving hydrogen embrittlement resistance by shifting the carbide precipitation temperature during tempering to the high temperature side and shifting the temper embrittlement region to the high temperature side. However, if the amount of Si is excessive, the penetration of carbides during temper heating is hindered and the strength is reduced. Therefore, the Si amount is determined to be 1.9% or more (preferably 1.95% or more) and 2.5% or less (preferably 2.3% or less, more preferably 2.2% or less).
Mn:0.6〜1.3%
Mnは、平衡状態図におけるオーステナイト領域を広げる元素(オーステナイトフォーマー元素)であり、安定してフェライト脱炭を抑制するのに有効である。しかしMn量が過剰であると、鋼中マトリックスの靱性が低下して耐水素脆性が劣化し、その結果、腐食疲労特性が劣化する。そこでMn量を、0.6%以上(好ましくは0.65%以上、より好ましくは0.7%以上)、1.3%以下(好ましくは1.1%以下、より好ましくは0.9%以下)と定めた。
Mn: 0.6 to 1.3%
Mn is an element (austenite former element) that expands the austenite region in the equilibrium diagram, and is effective in stably suppressing ferrite decarburization. However, if the amount of Mn is excessive, the toughness of the matrix in the steel is lowered and the hydrogen embrittlement resistance is deteriorated. As a result, the corrosion fatigue characteristics are deteriorated. Therefore, the amount of Mn is 0.6% or more (preferably 0.65% or more, more preferably 0.7% or more), 1.3% or less (preferably 1.1% or less, more preferably 0.9%). The following:
Cr:0.4%以下(0%を含む)
Crは、固溶強化により鋼のマトリックスを強化し、また焼入性を向上させる作用を有する。こうした作用を発揮させるために鋼中にCrを、好ましくは0.1%以上(より好ましくは0.15%以上、さらに好ましくは0.20%以上)の量で含有させることが推奨される。しかしCrは、腐食ピット底部のpH値を下げて、腐食ピットのアスペクト比を増大させる(鋭利にする)という作用を有し、これは腐食疲労特性に悪影響を及ぼす。従って本発明では、Cr量の上限を、0.4%以下(好ましくは0.3%以下、より好ましくは0.25%以下)と定めた。
Cr: 0.4% or less (including 0%)
Cr strengthens the steel matrix by solid solution strengthening, and has the effect of improving hardenability. In order to exert such an effect, it is recommended that Cr is contained in the steel in an amount of preferably 0.1% or more (more preferably 0.15% or more, and further preferably 0.20% or more). However, Cr has the effect of lowering the pH value at the bottom of the corrosion pit and increasing (sharpening) the aspect ratio of the corrosion pit, which adversely affects the corrosion fatigue properties. Therefore, in the present invention, the upper limit of the Cr amount is set to 0.4% or less (preferably 0.3% or less, more preferably 0.25% or less).
Cu:0.7%以下(0%を含む)
Cuは、電気化学的に鉄よりも貴な元素であり、鋼の耐食性を高める作用を有する。さらにCuは、腐食中に生じる錆のアモルファス組成を増大させて、腐食原因の1つであるCl元素が腐食ピット底部に濃化することを抑制する作用を有する。この作用によって、腐食ピットのアスペクト比が制限され、応力集中が緩和され、腐食疲労特性が向上する。こうした作用を発揮させるために鋼中にCuを、好ましくは0.1%以上(より好ましくは0.15%以上、さらに好ましくは0.22%以上)の量で含有させることが推奨される。しかしCu添加によって、熱間圧延割れが生ずることがある。従って本発明では、Cu量の上限を0.7%以下(好ましくは0.5%以下、より好ましくは0.4%以下、特に0.35%以下)と定めた。
Cu: 0.7% or less (including 0%)
Cu is an element that is electrochemically nobler than iron and has the effect of enhancing the corrosion resistance of steel. Furthermore, Cu has an action of increasing the amorphous composition of rust generated during corrosion and suppressing concentration of Cl element, which is one of the causes of corrosion, at the bottom of the corrosion pit. By this action, the aspect ratio of the corrosion pit is limited, the stress concentration is relaxed, and the corrosion fatigue characteristics are improved. In order to exert such an effect, it is recommended that Cu be contained in the steel in an amount of preferably 0.1% or more (more preferably 0.15% or more, further preferably 0.22% or more). However, hot rolling cracks may occur due to the addition of Cu. Therefore, in the present invention, the upper limit of the amount of Cu is set to 0.7% or less (preferably 0.5% or less, more preferably 0.4% or less, particularly 0.35% or less).
Ni:0.7%以下(0%を含む)
Niは、Cuと同様に、耐食性を高める作用、及び錆のアモルファス組成を増大させて、腐食ピットのアスペクト比を低減させる作用を有する。こうした作用を発揮させるために鋼中にNiを、好ましくは0.1%以上(より好ましくは0.15%以上、さらに好ましくは0.20%以上)の量で含有させることが推奨される。しかしNiは、調質(焼入れ・焼戻し)後のマトリックス中の残留オーステナイト量を増大させる作用を有し、その結果、調質後の硬さ(引張強さ)を低減させる。さらに耐水素脆性も低下させる。従って本発明では、Ni量の上限を、0.7%以下、好ましくは0.5%以下、さらに好ましくは0.4%以下、特に0.35%以下と定めた。
Ni: 0.7% or less (including 0%)
Ni, like Cu, has the effect of increasing the corrosion resistance and the effect of increasing the amorphous composition of rust and reducing the aspect ratio of the corrosion pits. In order to exert such an effect, it is recommended that Ni is contained in the steel in an amount of preferably 0.1% or more (more preferably 0.15% or more, and further preferably 0.20% or more). However, Ni has the effect of increasing the amount of retained austenite in the matrix after tempering (quenching / tempering), and as a result, reduces the hardness (tensile strength) after tempering. Furthermore, hydrogen brittleness resistance is also reduced. Therefore, in the present invention, the upper limit of the amount of Ni is set to 0.7% or less, preferably 0.5% or less, more preferably 0.4% or less, and particularly 0.35% or less.
本発明のばね用鋼は、上記Cr、Cu及びNiのいずれも含まなくても良いが、好ましくはCr、Cu及びNiの中から少なくとも1種、より好ましくはCr及びNiのいずれか1種を、上述した量で含有することが好ましい。 The spring steel of the present invention may not contain any of the above Cr, Cu and Ni, but preferably contains at least one of Cr, Cu and Ni, more preferably any one of Cr and Ni. It is preferable to contain in the above-mentioned amount.
Ti:0.05〜0.15%
Tiは、焼入れ・焼戻し後の旧オーステナイト結晶粒を微細化し、大気耐久性及び耐水素脆性の向上に有効である。しかしTi量が過剰であると、粗大なTi窒化物が析出し、疲労特性が劣化する。そこでTi量を、0.05%以上(好ましくは0.06%以上、より好ましくは0.07%以上)、0.15%以下(好ましくは0.1%以下、より好ましくは0.09%以下、特に0.085%以下)と定めた。
Ti: 0.05 to 0.15%
Ti refines the prior austenite crystal grains after quenching and tempering, and is effective in improving air durability and hydrogen embrittlement resistance. However, if the amount of Ti is excessive, coarse Ti nitride precipitates and the fatigue characteristics deteriorate. Therefore, the Ti amount is 0.05% or more (preferably 0.06% or more, more preferably 0.07% or more), 0.15% or less (preferably 0.1% or less, more preferably 0.09%). Hereinafter, it was determined as 0.085% or less in particular.
Al:0.003〜0.1%
Alは、溶鋼処理時の脱酸剤として作用する元素である。またAlは、微細なAl窒化物を形成し、そのピニング効果によって結晶粒を微細化する作用を有する。しかしAl量が過剰であると、粗大なAl酸化物を形成し、疲労特性に悪影響を及ぼす。そこでAl量を、0.003%以上(好ましくは0.005%以上)、0.1%以下(好ましくは0.05%以下、より好ましくは0.03%以下)と定めた。
Al: 0.003-0.1%
Al is an element that acts as a deoxidizer during the treatment of molten steel. Moreover, Al has the effect | action which forms fine Al nitride and refines | miniaturizes a crystal grain by the pinning effect. However, if the amount of Al is excessive, a coarse Al oxide is formed and the fatigue characteristics are adversely affected. Therefore, the Al content is set to 0.003% or more (preferably 0.005% or more) and 0.1% or less (preferably 0.05% or less, more preferably 0.03% or less).
本発明で用いるばね用鋼の残部は、実質的に鉄である。但し鉄原料(スクラップを含む)、副原料などの資材、製造設備等の状況によって持ち込まれる不可避不純物が鋼中に含まれることは、当然に許容される。この不可避不純物を厳密に制御してもよく、例えばP、S、O、Nなどを以下の範囲に制御してもよい。 The balance of the spring steel used in the present invention is substantially iron. However, it is naturally allowed that steel contains unavoidable impurities brought in depending on the situation of materials such as iron materials (including scrap), auxiliary materials, and manufacturing equipment. This inevitable impurity may be strictly controlled. For example, P, S, O, N, etc. may be controlled within the following range.
P:0.02%以下(0%を含まない)
Pは、旧オーステナイト粒界に偏析して粒界を脆化させ、疲労特性を低下させる元素である。そこでP量は、できるだけ低いほど好ましく、例えば0.02%以下(好ましくは0.01%以下)に制御してもよい。
P: 0.02% or less (excluding 0%)
P is an element that segregates at the prior austenite grain boundaries, embrittles the grain boundaries, and lowers fatigue characteristics. Therefore, the P amount is preferably as low as possible, and may be controlled to, for example, 0.02% or less (preferably 0.01% or less).
S:0.02%以下(0%を含まない)
Sは、旧オーステナイト粒界に偏析して粒界を脆化させ、疲労特性を低下させる元素である。そこでS量は、できるだけ低いほど好ましく、例えば0.02%以下(好ましくは0.01%以下)に制御してもよい。
S: 0.02% or less (excluding 0%)
S is an element that segregates at the prior austenite grain boundaries, embrittles the grain boundaries, and lowers fatigue characteristics. Therefore, the S amount is preferably as low as possible, and may be controlled to, for example, 0.02% or less (preferably 0.01% or less).
N:0.007%以下(0%を含まない)
N量が多くなるほど、TiやAlと共に粗大な窒化物を形成し、疲労特性に悪影響を及ぼす。そこでN量はできるだけ少ないほど好ましく、例えば0.007%以下(好ましくは0.005%以下)に制御してもよい。一方、N量を低減しすぎると、生産性が著しく低下する。またNはAlと共に窒化物を形成して、結晶粒の微細化に貢献する。この観点からすればN量を、0.001%以上(好ましくは0.002%以上)に設定することが好ましい。
N: 0.007% or less (excluding 0%)
As the amount of N increases, coarse nitrides are formed together with Ti and Al, which adversely affects fatigue characteristics. Therefore, the N content is preferably as small as possible, and may be controlled to be, for example, 0.007% or less (preferably 0.005% or less). On the other hand, if the amount of N is reduced too much, productivity is significantly reduced. N forms a nitride with Al and contributes to refinement of crystal grains. From this viewpoint, it is preferable to set the N amount to 0.001% or more (preferably 0.002% or more).
O:0.0015%以下(0%を含まない)
O量が過剰になると、粗大な酸化物系介在物(Al2O3など)が形成され、疲労特性に悪影響を及ぼす。そこでO量の上限を、0.0015%以下(好ましくは0.0010%以下)と定めた。一方、O量の下限は、工業生産上、一般に0.0002%以上(好ましくは0.0004%以上)である。
O: 0.0015% or less (excluding 0%)
When the amount of O becomes excessive, coarse oxide inclusions (Al 2 O 3 and the like) are formed, which adversely affects fatigue characteristics. Therefore, the upper limit of the O amount is set to 0.0015% or less (preferably 0.0010% or less). On the other hand, the lower limit of the amount of O is generally 0.0002% or more (preferably 0.0004% or more) in industrial production.
さらに本発明のばね用鋼は、必要に応じて、以下の選択元素を含有していても良い。 Furthermore, the spring steel of the present invention may contain the following selective elements as necessary.
Nb:0.1%以下(0%を含まない)
Nbは、微細な化合物(Nb炭化物、窒化物、硫化物やこれらの複合化合物)を形成して、耐水素脆性を向上させる作用を有する元素である。さらにNbは、結晶粒微細化効果を発揮して、靱性や耐力を高める作用も有する。そこで必要に応じてNbを、好ましくは0.01%以上(より好ましくは0.02%以上)の量で含有させることが推奨される。しかしNb量が過剰であると、焼入れ加熱時にオーステナイト中に固溶されない炭化物の量が増大し、充分な強度が得られなくなる。またNb量が過剰であると、粗大なNb窒化物を形成して、疲労折損が生じ易くなる。そこで含有させる場合のNb量を、0.1%以下(好ましくは0.05%以下)と定めた。
Nb: 0.1% or less (excluding 0%)
Nb is an element that has a function of improving the resistance to hydrogen embrittlement by forming fine compounds (Nb carbides, nitrides, sulfides, and complex compounds thereof). Further, Nb has an effect of increasing the toughness and proof stress by exerting a crystal grain refining effect. Therefore, it is recommended that Nb be contained in an amount of 0.01% or more (more preferably 0.02% or more) as necessary. However, if the amount of Nb is excessive, the amount of carbide that is not dissolved in austenite during quenching heating increases, and sufficient strength cannot be obtained. On the other hand, if the amount of Nb is excessive, coarse Nb nitride is formed and fatigue breakage is likely to occur. Therefore, the amount of Nb in the case of inclusion is determined to be 0.1% or less (preferably 0.05% or less).
B:0.005%以下(0%を含まない)
Bは、Pの粒界偏析を防止して粒界を清浄化し、耐水素脆性や靱延性を向上させるために有効な元素である。また少量のBを添加するだけで、多量の合金元素を添加しなくとも焼入性を増大させることができるので、圧延後の徐冷中に生じる線材表層のフェライト析出を抑えると共に、ばね製造時の焼入時の硬さを深くまで確保できる。そこで必要に応じてBを、好ましくは0.0003%以上(より好ましくは0.0005%以上)の量で含有させることが推奨される。しかしB量が過剰になると、Fe23(CB)6等のB化合物が形成されて、フリーのBが減少するため、Pの粒界偏析を防止する効果が飽和する。さらにこのB化合物は粗大であることが多いため、疲労折損の起点となって疲労特性が低下し得る。そこで含有させる場合のB量を、0.005%以下(好ましくは0.004%以下)と定めた。
B: 0.005% or less (excluding 0%)
B is an element that is effective for preventing grain boundary segregation of P, purifying the grain boundaries, and improving hydrogen embrittlement resistance and toughness. Also, by adding a small amount of B, the hardenability can be increased without adding a large amount of alloy elements, so that ferrite precipitation on the wire surface layer that occurs during slow cooling after rolling can be suppressed, and at the time of spring production The hardness when entering can be secured deeply. Therefore, it is recommended to contain B in an amount of preferably 0.0003% or more (more preferably 0.0005% or more) as required. However, when the amount of B is excessive, B compounds such as Fe 23 (CB) 6 are formed and free B is reduced, so that the effect of preventing P grain boundary segregation is saturated. Furthermore, since this B compound is often coarse, it can become a starting point of fatigue breakage and fatigue characteristics can be lowered. Therefore, the B content in the case of inclusion is set to 0.005% or less (preferably 0.004% or less).
本発明のばね用鋼の線径は、例えば、9〜25mm(好ましくは10〜20mm)である。 The wire diameter of the spring steel of the present invention is, for example, 9 to 25 mm (preferably 10 to 20 mm).
以下、実施例を挙げて本発明をより具体的に説明するが、本発明は以下の実施例によって制限を受けるものではなく、上記・下記の趣旨に適合し得る範囲で適当に変更を加えて実施することも勿論可能であり、それらはいずれも本発明の技術的範囲に包含される。 EXAMPLES Hereinafter, the present invention will be described in more detail with reference to examples. However, the present invention is not limited by the following examples, and appropriate modifications are made within a range that can meet the above and the following purposes. Of course, it is possible to implement them, and they are all included in the technical scope of the present invention.
参考例
表1に示す化学成分組成の鋼(鋼種SA〜SG)を80トンの転炉にて溶製し、連続鋳造で400mm角のブルームを作製し、さらに分塊圧延して155mm角のビレットにした。このビレットを加熱した後、熱間圧延し、載置温度近くまで水冷した後、コイリングし、ステルモア冷却設備の冷却床(コンベア)に載置し、コイル密部とコイル疎部に供給する風量を調節しながら衝風冷却することによって、直径14.3mmのばね用線材を2トン作製した。詳細な製造条件は、表2に示した通りである。また表2中、冷却速度は、温度750℃〜600℃の間の速度である。
Reference Example Steel having the chemical composition shown in Table 1 (steel grades SA to SG) was melted in an 80-ton converter, a 400 mm square bloom was produced by continuous casting, and further rolled into pieces to form a 155 mm square billet. I made it. After heating this billet, it is hot-rolled, water-cooled to near the placement temperature, coiled, placed on the cooling floor (conveyor) of the Stealmore cooling equipment, and the air volume supplied to the coil dense part and coil sparse part Two tons of a wire rod for a spring having a diameter of 14.3 mm was produced by blast cooling while adjusting. Detailed manufacturing conditions are as shown in Table 2. In Table 2, the cooling rate is a temperature between 750 ° C. and 600 ° C.
得られた線材の脱炭層深さを以下のようにして調べた。
線材コイルのトップ部(圧延始め)及びボトム部(圧延終わり)からそれぞれ5巻き目を寸断した。トップ側及びボトム側の1巻きをそれぞれ8等分に分割し、合計16本の線材片を作製し、各線材片から10mm程度のサンプルを切断した。このサンプルを、切断面(横断面)が表面に出るようにしながら樹脂に埋込み、エメリー紙及びダイヤモンド粒子を用いて湿式研磨し、次いでピクラール液でエッチングして、合計16個の脱炭層深さ測定用試験片を作製した。これら試験片を光学顕微鏡にて観察倍率200倍で観察し、表層の全脱炭層深さ及びフェライト脱炭層深さを測定した。この測定法は、JIS G 0558の顕微鏡による測定法に従った。16個のサンプルの中で、全脱炭層深さ及びフェライト脱炭層深さの最大値を、各線材コイルにおける「全脱炭層深さ」及び「フェライト脱炭層深さ」とした。
The decarburized layer depth of the obtained wire was examined as follows.
The 5th roll was cut off from the top part (rolling start) and the bottom part (rolling end) of the wire coil. Each turn on the top side and bottom side was divided into 8 equal parts to produce a total of 16 wire pieces, and a sample of about 10 mm was cut from each wire piece. This sample was embedded in a resin with the cut surface (cross section) coming out to the surface, wet-polished with emery paper and diamond particles, and then etched with Picral liquid to measure a total of 16 decarburized layer depths. A test piece was prepared. These test pieces were observed with an optical microscope at an observation magnification of 200 times, and the total decarburized layer depth and the ferrite decarburized layer depth of the surface layer were measured. This measuring method followed the measuring method by the microscope of JIS G 0558. Among the 16 samples, the maximum values of the total decarburization layer depth and the ferrite decarburization layer depth were defined as “total decarburization layer depth” and “ferrite decarburization layer depth” in each wire coil.
測定結果を表2に示す。また表2には、化学成分組成(但しC=0%)からThermo−Calcで計算したA1変態点、A3変態点、及びA4変態点(すなわちA1(C=0)変態
点、A3(C=0)変態点、A4(C=0)変態点)も記載した。なお鋼種SGでは、C=0%付近でA3及びA4線が結合してしまい、A3(C=0)変態点及びA4(C=0)変態点が消失した。
The measurement results are shown in Table 2. Table 2 also shows the A 1 transformation point, A 3 transformation point, and A 4 transformation point (that is, A 1 (C = 0) transformation point ) calculated by Thermo-Calc from the chemical composition (where C = 0%), A 3 (C = 0) transformation point, A 4 (C = 0) transformation point) are also described. In steel grade SG, the A 3 and A 4 wires were combined around C = 0%, and the A 3 (C = 0) transformation point and the A 4 (C = 0) transformation point disappeared.
製造条件が適切である線材SA−1、SB−1、SC−1、SD−1、SE−1、及びSF−1は、フェライト脱炭層深さが0.00mmであった。 As for the wire rods SA-1, SB-1, SC-1, SD-1, SE-1, and SF-1 whose manufacturing conditions are appropriate, the ferrite decarburized layer depth was 0.00 mm.
一方、加熱温度が高い線材SA−3及びSE−2、仕上げ圧延中の最高到達温度が低い線材SA−2及びSC−2、仕上げ圧延中の最高到達温度が高い線材SB−2及びSF−2、載置温度が低い線材SD−2、コイル密部の冷却速度が小さい線材SB−3及びSE−3、並びにA3(C=0)変態点やA4(C=0)変態点が消失してしまった線材SG−1はフェライト脱炭が生じている。 On the other hand, the wire rods SA-3 and SE-2 having high heating temperatures, the wire rods SA-2 and SC-2 having low maximum ultimate temperatures during finish rolling, and the wire rods SB-2 and SF-2 having high maximum ultimate temperatures during finish rolling. The wire SD-2 with a low mounting temperature, the wires SB-3 and SE-3 with a low cooling rate of the coil dense portion, and the A 3 (C = 0) transformation point and the A 4 (C = 0) transformation point disappear. The wire SG-1 that has been subjected to ferrite decarburization has occurred.
実験例
表3に示す化学成分組成の鋼を150kgの小型真空溶解炉にて溶製し、熱間鍛造して155mm角のビレットを作製した。化学成分組成から計算されるCeq1〜3を、表5に示す。前記ビレットを加熱した後、熱間圧延し、載置温度近くまで水冷したあと、コイリングし、ステルモア冷却設備の冷却床(コンベア)に載置し、コイル密部とコイル疎部に供給する風量を調節しながら衝風冷却することによって、直径13.5mmのばね用鋼(線材)を作製した。詳細な製造条件は、表4に示した通りである。また表4中、冷却速度は600〜750℃の間の速度である。なお表4には、化学成分組成(但しC=0%)からThermo−Calcで計算したA1(C=0)変態点、A3(C=0)変態点及びA4(C=0)変態点を記載した。
Experimental Example Steel having a chemical composition shown in Table 3 was melted in a 150 kg small vacuum melting furnace and hot forged to produce a 155 mm square billet. Table 5 shows Ceq1 to 3 calculated from the chemical composition. After heating the billet, it is hot-rolled, water-cooled to near the placement temperature, coiled, placed on the cooling floor (conveyor) of the Stealmore cooling equipment, and the air volume supplied to the coil dense part and coil sparse part By blast cooling while adjusting, a spring steel (wire) having a diameter of 13.5 mm was produced. Detailed manufacturing conditions are as shown in Table 4. In Table 4, the cooling rate is between 600 and 750 ° C. Table 4 shows the A 1 (C = 0) transformation point, A 3 (C = 0) transformation point and A 4 (C = 0) calculated by Thermo-Calc from the chemical composition (provided that C = 0% ). The transformation point is described.
得られたばね用鋼の脱炭層強さ、調質(焼入れ・焼戻し)後の疲労強度、ビッカース硬さ、腐食ピットのアスペクト比、及び水素脆化割れ寿命を以下のようにして調べた。 The obtained spring steel was examined for decarburized layer strength, fatigue strength after tempering (quenching / tempering), Vickers hardness, aspect ratio of corrosion pits, and hydrogen embrittlement crack life as follows.
(1)脱炭層深さ
線材コイルのボトム側(圧延終わり)から3、4及び5巻き目を寸断し、1巻きをそれぞれ8等分に分割し、合計24本の線材片を作製した。線材片から、それぞれ10mm程度切断して、サンプルを取得した。このサンプルを、切断面(横断面)が表面にでるようにしながら樹脂に埋込み、エメリー紙及びダイヤモンド粒子を用いて湿式研磨し、次いでピクラール液でエッチングして、合計24個の脱炭層深さ測定用試験片を作製した。これら試験片を光学顕微鏡にて観察倍率200倍で観察し、参考例の場合と同様にして、全脱炭層深さ(DmT)、フェライト脱炭層深さ(DmF)を求めた。
(1) Decarburized layer depth The third, fourth, and fifth turns were cut from the bottom side (rolling end) of the wire coil, and each turn was divided into eight equal parts to produce a total of 24 wire pieces. Samples were obtained by cutting about 10 mm each from the wire pieces. This sample was embedded in resin with the cut surface (cross section) appearing on the surface, wet-polished with emery paper and diamond particles, and then etched with Picral liquid to measure a total of 24 decarburized layer depths. A test piece was prepared. These test pieces were observed with an optical microscope at an observation magnification of 200 times, and the total decarburized layer depth (DmT) and ferrite decarburized layer depth (DmF) were obtained in the same manner as in the reference example.
(2)疲労強度
前記線材片を引き抜き加工(摩棒加工)及び切断して、直径12.5mm×長さ70mmのサンプルを作製した。このサンプルを、温度925℃で10分加熱した後、温度70℃の油で冷却して油焼入れし、次いで温度400℃で60分加熱して焼戻しを行った。焼入れ・焼戻しを行った鋼を、掴み部径12mm、平行部径8mmnJIS Z2274の1号試験片(疲労試験片)に加工した。
(2) Fatigue strength The wire piece was drawn (braded) and cut to prepare a sample having a diameter of 12.5 mm and a length of 70 mm. The sample was heated at a temperature of 925 ° C. for 10 minutes, then cooled with oil at a temperature of 70 ° C. and oil-quenched, and then heated at a temperature of 400 ° C. for 60 minutes for tempering. The quenched and tempered steel was processed into a No. 1 test piece (fatigue test piece) of grip part diameter 12 mm and parallel part diameter 8 mm n JIS Z2274.
この疲労試験片の平行部を800番のエメリー紙で研磨した。試験片の掴み部が腐食しないようにエナメル被膜で保護した後、この試験片に、5質量%のNaCl水溶液を、JIS Z 2371に従い35℃で8時間噴霧し、その後、試験片を湿度60%及び温度35℃の湿潤環境に16時間保持することを1サイクルとして、これを合計14サイクル行った。この腐食試験を行った試験片は、小野式回転曲げ疲労試験に供するまで、真空デシケータに保管した。10MPa間隔で負荷応力を増大させながら、各負荷応力につき5本の試験片を用いて小野式回転曲げ疲労試験を実施し、5本の試験片全てで1000万回まで折損しなかった応力を、腐食疲労強度とした。結果を表5に示す。 The parallel portion of this fatigue test piece was polished with # 800 emery paper. After protecting the gripping portion of the test piece with an enamel coating, the test piece was sprayed with 5 mass% NaCl aqueous solution at 35 ° C. for 8 hours in accordance with JIS Z 2371, and then the test piece was subjected to a humidity of 60%. In addition, holding for 16 hours in a humid environment at a temperature of 35 ° C. was regarded as one cycle, and this was performed for a total of 14 cycles. The specimen subjected to this corrosion test was stored in a vacuum desiccator until it was subjected to an Ono-type rotary bending fatigue test. While increasing the load stress at 10 MPa intervals, the Ono-type rotary bending fatigue test was performed using 5 test pieces for each load stress, and the stress that did not break up to 10 million times in all 5 test pieces, Corrosion fatigue strength. The results are shown in Table 5.
(3)ビッカース硬さ
前記線材片を摩棒加工及び切断して、直径12.5mm×長さ60mmのサンプルを作製した。このサンプルを、疲労試験と同じ条件にて焼入れ・焼戻しして、ビッカース硬さ測定用の試験片を作製した。この試験片を横断面が露出するようにして樹脂に埋込み、研磨・鏡面仕上げした後、表層から深さ0.1mmの位置を10kgの荷重でビッカース硬さ試験を行い、ビッカース硬さを測定した。結果を表5に示す。図1に、ビッカース硬さとCeq1との関係を示すグラフを記載する。また表5には、ビッカース硬さから換算した引張強度を記載する(表5中で「換算TS」と記載)。この換算には、下記式(5)を使用した:
TS=58.33×(−9.751+0.16491×HV
−9.4457×10-5×HV2)−1135.7 … (5)
〔上記式中、TSは引張強さ(MPa)を表し、HVはビッカース硬さを表す。〕
(3) Vickers hardness The wire piece was processed and cut to prepare a sample having a diameter of 12.5 mm and a length of 60 mm. This sample was quenched and tempered under the same conditions as the fatigue test to prepare a test piece for measuring the Vickers hardness. After embedding this test piece in a resin so that the cross section was exposed, polishing and mirror finishing, a Vickers hardness test was performed at a position of 0.1 mm depth from the surface layer with a load of 10 kg, and the Vickers hardness was measured. . The results are shown in Table 5. FIG. 1 shows a graph showing the relationship between Vickers hardness and Ceq1. Table 5 describes the tensile strength converted from Vickers hardness (described as “converted TS” in Table 5). The following formula (5) was used for this conversion:
TS = 58.33 × (−9.751 + 0.16491 × HV
−9.4457 × 10 −5 × HV 2 ) −1135.7 (5)
[In the above formula, TS represents tensile strength (MPa), and HV represents Vickers hardness. ]
(4)腐食ピットのアスペクト比
前記の線材片を摩棒加工及び切断して、直径12.5mm×長さ120mmのサンプルを作製した。このサンプルを、疲労試験と同じ条件にて焼入れ・焼戻しした後、直径10mm×長さ100mmの形状に機械加工して、アスペクト比測定用の試験片を作製した。試験片の表面を800番のエメリー紙で研磨した。腐食しないように、この試験片の両端10mmをエナメル被覆で保護し、この試験片に、5質量%のNaCl水溶液を、JIS Z 2371に従い35℃で8時間噴霧し、その後、試験片を湿度60%及び温度35℃の湿潤環境に16時間保持することを1サイクルとして、これを合計14サイクル行った。その後、クエン酸アンモニウム(98.7%)を蒸留水で10質量%に希釈した液に常温で試験片を浸し、塩水噴霧で発生した錆びを除去し、試験片表面の腐食ピットをレーザー顕微鏡(レーザーテック社製「1LM21W」、倍率:100〜200倍)にて観察した。鋼種ごとに5本の試験片を使用した。5本の試験片表面に観察される腐食ピットの中から、深さが大きいものから順に10個の腐食ピットを選択し、各腐食ピットの深さ及び幅を上記式(4)に代入してアスペクト比を求め、これらの平均値を求めた。結果を表5に示す。図2に、腐食ピットのアスペクト比(平均値)とCeq2との関係を示すグラフを記載する。
(4) Aspect ratio of corrosion pit The above-mentioned wire piece was processed and cut into a sample having a diameter of 12.5 mm and a length of 120 mm. The sample was quenched and tempered under the same conditions as in the fatigue test, and then machined into a shape having a diameter of 10 mm and a length of 100 mm to prepare a test piece for measuring the aspect ratio. The surface of the test piece was polished with # 800 emery paper. In order not to corrode, 10 mm of both ends of the test piece were protected with enamel coating, and 5% by mass of NaCl aqueous solution was sprayed on the test piece at 35 ° C. for 8 hours in accordance with JIS Z 2371. This was performed for a total of 14 cycles, with one cycle being held in a humid environment with a% and a temperature of 35 ° C. for 16 hours. Thereafter, the test piece is immersed in a solution obtained by diluting ammonium citrate (98.7%) to 10% by mass with distilled water at room temperature to remove rust generated by spraying with salt water. Observation was performed with “1LM21W” manufactured by Lasertec Corporation, magnification: 100 to 200 times. Five test pieces were used for each steel type. From the five corrosion pits observed on the surface of the test piece, ten corrosion pits are selected in descending order of depth, and the depth and width of each corrosion pit are substituted into the above equation (4). The aspect ratio was determined, and the average value of these was determined. The results are shown in Table 5. FIG. 2 shows a graph showing the relationship between the aspect ratio (average value) of corrosion pits and Ceq2.
(5)水素脆化割れ寿命
前記の線材片を摩棒加工及び切断して、直径12.5mm×長さ70mmのサンプルを作製した。このサンプルを、疲労試験と同じ条件にて焼入れ・焼戻ししてから、幅10mm×厚さ1.5mm×長さ65mmの試験片を切り出した。この試験片に対して、4点曲げによって1400MPaの応力を作用させながら、試験片を硫酸(0.5mol/L)及びチオシアン酸カリウム(0.01mmol/L)の混合水溶液に浸漬した。ポテンションスタットを用いてSCE電極よりも卑である−700mVの電圧をかけ、割れが発生するまでの時間を測定した。結果を表5に示す。図3に、水素脆化割れ寿命とCeq3との関係を示すグラフを記載する。
(5) Hydrogen embrittlement crack life The above-mentioned wire rod piece was processed with a wand and cut to prepare a sample having a diameter of 12.5 mm and a length of 70 mm. This sample was quenched and tempered under the same conditions as in the fatigue test, and then a test piece having a width of 10 mm, a thickness of 1.5 mm, and a length of 65 mm was cut out. The test piece was immersed in a mixed aqueous solution of sulfuric acid (0.5 mol / L) and potassium thiocyanate (0.01 mmol / L) while applying a stress of 1400 MPa to the test piece by four-point bending. Using a potentiostat, a voltage of -700 mV, which is lower than the SCE electrode, was applied, and the time until cracking occurred was measured. The results are shown in Table 5. FIG. 3 shows a graph showing the relationship between the hydrogen embrittlement crack life and Ceq3.
表3〜5の結果から、フェライト脱炭しておらず、かつ本発明のCeq1〜3の要件を満たす鋼種A〜Lは、良好な腐食疲労強度(例えば、290MPa以上)を示す。一方、Ceq1が0.580未満である鋼種Mは、ビッカース硬さが低く、そのため疲労強度が低くなっている。Ceq2が0.49を超える鋼種O〜Qは、腐食ピットのアスペクト比が大きく、そのため疲労強度が低くなっている。Ceq3が0.570を超える鋼種N〜Uは、水素脆化割れ寿命が短く、そのため疲労強度が低くなっている。
From the results of Tables 3 to 5, steel types A to L that are not decarburized by ferrite and satisfy the requirements of
また表3〜5の結果から示されるように、フェライト脱炭を防止した鋼において、そのビッカース硬さ、腐食ピットのアスペクト比及び水素脆化割れ寿命は、腐食疲労強度に影響する。そして図1〜3のグラフから示されるように、これらビッカース硬さ、腐食ピットのアスペクト比及び水素脆化割れ寿命は、それぞれCeq1〜3と極めて高い相関関係がある。従って鋼の化学成分組成を、本発明のCeq1〜3の要件を満たすように調整することで、ビッカース硬さ、腐食ピットのアスペクト比及び水素脆化割れ寿命を制御でき、良好な腐食疲労強度を達成できる。
Moreover, as shown from the results of Tables 3 to 5, the Vickers hardness, the aspect ratio of the corrosion pits, and the hydrogen embrittlement crack life affect the corrosion fatigue strength in the steel in which the ferrite decarburization is prevented. As shown in the graphs of FIGS. 1 to 3, the Vickers hardness, the aspect ratio of the corrosion pits, and the hydrogen embrittlement crack life have a very high correlation with
Claims (5)
Si:1.9〜2.5%、
Mn:0.6〜1.3%、
Cr:0.4%以下(0%を含む)、
Cu:0.7%以下(0%を含む)、
Ni:0.7%以下(0%を含む)、
Ti:0.05〜0.15%、及び
Al:0.003〜0.1%
を含有し、残部が鉄及び不可避不純物からなり、
フェライト脱炭層深さが0.00mmであり、
下記式(1)で示されるCeq1が0.580以上であり、
下記式(2)で示されるCeq2が0.49以下であり、
下記式(3)で示されるCeq3が0.54以下であることを特徴とするばね用線材。
Ceq1=[C]+0.11[Si]−0.07[Mn]−0.05[Ni]+0.02[Cr] … (1)
Ceq2=[C]+0.30[Cr]−0.15[Ni]−0.70[Cu] … (2)
Ceq3=[C]−0.04[Si]+0.24[Mn]+0.10[Ni]+0.20[Cr]−0.89[Ti]−1.92[Nb] … (3)
〔上記式中、[ ]は鋼中の各元素の含有量(質量%)を表す。〕 C: 0.38 to 0.47% (meaning mass%, the same shall apply hereinafter)
Si: 1.9 to 2.5%,
Mn: 0.6 to 1.3%
Cr: 0.4% or less (including 0%),
Cu: 0.7% or less (including 0%),
Ni: 0.7% or less (including 0%),
Ti: 0.05-0.15%, and Al: 0.003-0.1%
And the balance consists of iron and inevitable impurities,
The ferrite decarburized layer depth is 0.00 mm,
Ceq1 represented by the following formula (1) is 0.580 or more,
Ceq2 represented by the following formula (2) is 0.49 or less,
Ceq3 shown by following formula (3) is 0.54 or less , The wire for springs characterized by the above-mentioned.
Ceq1 = [C] +0.11 [Si] −0.07 [Mn] −0.05 [Ni] +0.02 [Cr] (1)
Ceq2 = [C] +0.30 [Cr] −0.15 [Ni] −0.70 [Cu] (2)
Ceq3 = [C] −0.04 [Si] +0.24 [Mn] +0.10 [Ni] +0.20 [Cr] −0.89 [Ti] −1.92 [Nb] (3)
[In the above formula, [] represents the content (% by mass) of each element in the steel. ]
アスペクト比=(腐食ピットの深さ×2)/(腐食ピットの幅) … (4)
腐食試験:
ばね用線材を温度925℃で10分加熱した後、温度70℃の油で冷却して油焼入れし、次いで温度400℃で60分加熱して焼戻しを行った後、表面を800番のエメリー紙
で研磨して腐食試験用の試験片を作製する。
この試験片に、5質量%のNaCl水溶液を、JIS Z 2371に従い35℃で8時間噴霧し、その後、試験片を湿度60%及び温度35℃の湿潤環境に16時間保持することを1サイクルとして、これを合計14サイクル行う。
その後、錆を除去してから、試験片表面の腐食ピットをレーザー顕微鏡にて観察する。 After conducting the corrosion test shown below, from the corrosion pits observed on the surface of the specimen, select five or more corrosion pits in descending order of depth, and use the following equation (4) for those corrosion pits. The spring wire according to claim 1, wherein the average aspect ratio shown is 0.9 or less.
Aspect ratio = (depth of corrosion pit × 2) / (width of corrosion pit) (4)
Corrosion test:
The spring wire is heated at a temperature of 925 ° C. for 10 minutes, cooled with oil at a temperature of 70 ° C. and then oil-quenched, then heated at a temperature of 400 ° C. for 60 minutes and tempered, and then the surface is number 800 emery paper A specimen for corrosion test is prepared by polishing with
The test piece was sprayed with 5% by mass NaCl aqueous solution at 35 ° C. for 8 hours in accordance with JIS Z 2371, and then the test piece was kept in a humid environment of 60% humidity and 35 ° C. for 16 hours as one cycle. This is done for a total of 14 cycles.
Then, after removing rust, the corrosion pits on the surface of the test piece are observed with a laser microscope.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008184734A JP4847988B2 (en) | 2007-07-20 | 2008-07-16 | Spring wire with excellent corrosion fatigue characteristics |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007190001 | 2007-07-20 | ||
| JP2007190001 | 2007-07-20 | ||
| JP2008184734A JP4847988B2 (en) | 2007-07-20 | 2008-07-16 | Spring wire with excellent corrosion fatigue characteristics |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009046764A JP2009046764A (en) | 2009-03-05 |
| JP4847988B2 true JP4847988B2 (en) | 2011-12-28 |
Family
ID=40499241
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008184734A Expired - Fee Related JP4847988B2 (en) | 2007-07-20 | 2008-07-16 | Spring wire with excellent corrosion fatigue characteristics |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4847988B2 (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101115761B1 (en) * | 2008-12-26 | 2012-06-12 | 주식회사 포스코 | Steel restrained from surface decarborization and manufacturing method for the same |
| JP6027302B2 (en) * | 2009-12-22 | 2016-11-16 | 株式会社神戸製鋼所 | High strength tempered spring steel |
| JP4900516B2 (en) | 2010-03-29 | 2012-03-21 | Jfeスチール株式会社 | Spring steel and manufacturing method thereof |
| JP5250609B2 (en) | 2010-11-11 | 2013-07-31 | 日本発條株式会社 | Steel for high strength spring, method for producing high strength spring, and high strength spring |
| JP6036396B2 (en) * | 2013-02-25 | 2016-11-30 | 新日鐵住金株式会社 | Spring steel and spring steel with excellent corrosion resistance |
| JP6452454B2 (en) * | 2014-02-28 | 2019-01-16 | 株式会社神戸製鋼所 | Rolled material for high strength spring and wire for high strength spring |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3429258B2 (en) * | 2000-07-31 | 2003-07-22 | 株式会社神戸製鋼所 | Spring steel with excellent environmental resistance |
| JP3896902B2 (en) * | 2002-06-06 | 2007-03-22 | 大同特殊鋼株式会社 | High-strength spring steel with excellent corrosion fatigue strength |
-
2008
- 2008-07-16 JP JP2008184734A patent/JP4847988B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009046764A (en) | 2009-03-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101031679B1 (en) | Method of producing steel wire material for spring | |
| JP5070149B2 (en) | Spring wire and method for manufacturing the same | |
| JP4476846B2 (en) | High strength spring steel with excellent cold workability and quality stability | |
| JP5064060B2 (en) | Steel wire for high-strength spring, high-strength spring, and manufacturing method thereof | |
| JP5079788B2 (en) | Non-tempered steel for martensitic hot forging and hot-forged non-tempered steel parts | |
| KR100823780B1 (en) | Steel wire rod for steel spring excellent in pickling and descaling property | |
| JP6027302B2 (en) | High strength tempered spring steel | |
| US20170058376A1 (en) | Rolled material for high strength spring, and wire for high strength spring | |
| JP6816738B2 (en) | Steel wire manufacturing method | |
| KR101750668B1 (en) | Steel for spring, and method for producing spring | |
| KR100891764B1 (en) | Spring steel wire rod excellent in pickling performance | |
| KR102507715B1 (en) | High-strength steel sheet and manufacturing method thereof | |
| US10030282B2 (en) | Ferrite-based stainless steel plate having excellent resistance against scale peeling, and method for manufacturing same | |
| JP5796782B2 (en) | High strength spring steel wire rod and high strength spring with excellent skin machinability | |
| JP4847988B2 (en) | Spring wire with excellent corrosion fatigue characteristics | |
| KR102512610B1 (en) | High-strength steel sheet and its manufacturing method | |
| JP5679455B2 (en) | Spring steel, spring steel wire and spring | |
| WO2017191792A1 (en) | Steel wire for spring having exceptional spring coiling properties, and method for producing same | |
| JP6338012B2 (en) | Suspension spring steel and manufacturing method thereof | |
| JP2005350736A (en) | High-strength steel having superior corrosion resistance and fatigue characteristics for spring, and manufacturing method therefor | |
| JP4900251B2 (en) | Manufacturing method of nitrided parts | |
| KR0146799B1 (en) | Method for manufacturing stabilizer | |
| JPH06299296A (en) | Steel for high strength spring excellent in decarburizing resistance |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20101228 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110125 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110328 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20110531 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110830 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20110907 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20111011 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20111014 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20141021 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4847988 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |