JP4620215B2 - Diaphragm sensor - Google Patents
Diaphragm sensor Download PDFInfo
- Publication number
- JP4620215B2 JP4620215B2 JP2000132990A JP2000132990A JP4620215B2 JP 4620215 B2 JP4620215 B2 JP 4620215B2 JP 2000132990 A JP2000132990 A JP 2000132990A JP 2000132990 A JP2000132990 A JP 2000132990A JP 4620215 B2 JP4620215 B2 JP 4620215B2
- Authority
- JP
- Japan
- Prior art keywords
- gas
- membrane
- detection electrode
- liquid separation
- separation membrane
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Landscapes
- Measuring Oxygen Concentration In Cells (AREA)
Description
【0001】
【発明の属する技術分野】
本発明は、センサ本体と気液分離膜で、内部に検知極、対極、電解液(内部液)などを保持した構造の隔膜式センサに関する。さらに詳しくは、塩素の漏洩ガス等、低濃度ガスの検知に適し、かつ微小に製作が可能な隔膜式センサに関する。
【0002】
【従来の技術】
塩素ガス等の毒性ガス検知用センサとしては、従来からセンチメートルサイズの電量式及び定電位電解式センサが実用化されている。漏洩ガス検知警報器などに広く用いられており、許容濃度(ACGIH又は日本産業衛生学会の勧告値)付近の低濃度ガスに対しても迅速に応答する性能を有したセンサとなっている。
【0003】
これらの電量式及び定電位電解式塩素ガスセンサは、気液分離膜(隔膜)とセンサ本体等の内部に、作用極(検知極)・対極などとともに内部液(電解液)などを保持した構造となっている。両方式は、作用極一対極間に適当な電圧を印加しながらセンサ出力としての電流を測定するなど類似点も多く、最近では測定原理上の区分にかかわりなく、他の類似した測定方式をも含めてアンペロメトリックセンサや電流測定式センサ、或いは電気化学センサやEC(Electrochemical)センサと呼ばれるケースが多くなってきている。
【0004】
図9に上記従来技術に係るECセンサの一例を示す。図9のECセンサは、筒状のセンサ本体51と、中央に多孔質の隔膜52を保持した隔膜保持体53とを備えている。そして、ナット54をセンサ本体51と螺合することにより、隔膜保持体53がOリング55を介してセンサ本体51の先端に装着されている。また、電極本体51の中央空間に、センサ上部(図示せず)から電極支持体56が垂下されており、電極支持体56の先端には検知電極57が、中間の凹部には対極58が各々支持されている。そして、これら各部材の間に形成される空間に電解液59が充填されている。このECセンサ全体の大きさは、例えば、26mmΦ×58mmである。
【0005】
一方、微小ガスセンサの研究開発はこれまでにも多くの研究者によって行われてきており、報告も多数となっている。しかし、微小ECセンサに関する報告の殆どは、酸素センサ又はバイオセンサに関する内容である。このことは、これらの微小センサのニーズが医療などの分野に存在していることや、また、測定対象物質となる酸素が高濃度(%オーダー)であるためにポアサイズや気孔率が小さい気液分離膜で使用可能であり、ふっ素化合物やシリコーンなどのフィルムも使用できるために比較的簡単に気液分離構造を構築できること、及び酸素センサの内部液はほぼ塩化カリウム溶液に近い組成であるために周囲のセンサ部材等を侵食したりすることも殆どないこと、などが大きく関与し、研究報告も多数に及んでいると考えられる。
【0006】
図10に、上記微小センサの一例として、特公平6−1254号公報に記載された酸素電極を示す。図10において、シリコン基板71は、異方性エッチングにより作成された溝を有すると共に、その全面に絶縁膜72を被着せしめられている。シリコン基板71の溝には、2本の金電極73A及び73Bが対をなして接着せしめられている。金電極73A及び73Bは、それぞれの先端が溝の外側まで延在している。また、シリコン基板71の溝には、電解液を含ませたゲル74が満たされている。さらに、溝の上部には、シリコン基板71の上部の全面を覆う形で、液体状の材料を塗布することにより形成されたガス透過性膜75が被覆されている。このセンサの大きさは、幅4mm×長さ15mm×厚さ350μmである。
【0007】
【発明が解決しようとする課題】
図9に示す従来のECセンサにおいても、発生源となり得る箇所の近傍の狭い場所への設置を可能としたり、低コスト化を促進する観点から微小化が望まれている。また、省エネルギーや、電解液をはじめとする廃棄物の少量化という環境上の配慮からも微小化が望まれている。
【0008】
しかし、これをそのままの構造で微小化して製作することはできない。すなわち、電解液59を確実に内部に保持するために使用されているOリング55やナット54等は、サイズを小さくしようとしても限界がある。また、検知電極57と隔膜52との位置関係は、隔膜52を透過した被測定ガスが溶解する電解液59の層の厚さや、被測定ガスが検知電極57に到達するまでの距離等のセンサの感度に影響を及ぼす事項に直接かかわるので、厳密な管理が必要である。しかしながら、たとえOリング55等を微小化できたとしても、ナットの締め付けという方法で検知電極57と隔膜52との位置関係を微小サイズにおいて厳密に管理することは困難である。
【0009】
一方、図10に示した酸素電極のような構成は、毒性ガス測定用の微小ECセンサには応用されていない。なぜなら、上記構成では、以下の理由により測定対象ガスがppb〜ppmオーダーと低濃度ガスであり、しかも迅速な応答や長期安定性などが要求されるという仕様を達成することが困難だからである。
【0010】
すなわち、低濃度測定の場合には、十分なガス透過性が確保されつつも、内部液を確実に保持できる気液分離膜を用いなければならない。しかしながら、上記酸素電極における液体状の材料の塗布により形成された膜は非多孔質膜である。この場合、気体は連続膜を構成する分子間を通って透過することになるので、膜に接した気体の極一部しか透過できない。酸素のように、20%前後のガスを測定するのであればこれで構わないが、ppb〜ppmオーダーの低濃度ガスを対象とする場合には、充分な透過量を確保できない。
【0011】
また、図10の酸素電極では、電解液を非液体としてある程度の機械的強度を持たせ、これにより気液分離膜を形成する液体状材料の塗布を可能としている。そのため、気液分離膜と検知電極との位置関係を一定にしにくく、低濃度測定の場合、精度上問題が生じる。
【0012】
本発明は、上記事情に鑑みてなされたもので、微小化した場合にも、低濃度ガスの測定にも対応できる充分なガス透過性を確保できると共に、内部液も確実に保持でき、しかも、検知電極と気液分離膜の位置関係を一定する等の精密な加工が可能なことにより良好な測定精度が得られる隔膜式センサを提供することを課題とする。
【0013】
【課題を解決するための手段】
本発明者らは上記課題を検討した結果、請求項1の発明として、センサ本体と、気液分離膜と、センサ本体と気液分離膜との間に充填された電解液と、電解液に接触する検知電極及び対極とを備える隔膜式センサにおいて、気液分離膜が、シリコンウエハーに孔あけ加工した膜材又はアルミナを陽極酸化して多孔質化した膜材により構成され、センサ本体が、同一平面に設けられて気液分離膜が接合される一以上の膜接合面と、膜接合面に囲まれる位置に設けられた電解液収容溝と、電解液収容溝底面から膜接合面と離間して突設され先端が膜接合面と同一平面上の近傍にまで伸張する検知電極台とを有すると共に、このセンサ本体の検知電極台の先端には検知電極が、電解液収容溝の底面には対極が各々設けられ、検知電極は、膜接合面と同一平面上よりも電解液収容溝の底面方向に位置し、気液分離膜と検知電極との位置関係が膜接合面と検知電極台の位置関係によって安定した関係に保持されるように構成されていることを特徴とする隔膜式センサを提供する。
【0014】
本発明における気液分離膜は一般に隔膜とも称される。また、電解液は内部液とも称される。また、検知電極及び対極は、各々、電流測定式センサにおいて作用極及び対極とも称されるものである。また両極は、電子授受の方向性を考慮して、何れかをアノード、何れかをカソードと称される場合もある。さらに、電圧測定式センサにおいて、検知極及び対極は、各々感応電極(膜)及び参照電極とも称されるものである。本発明における隔膜式センサは、これら種々の測定原理や代替の名称の有無を問わず、検知極と対極との間において、気液分離膜を通過して電解液に入る測定対象ガスに対応して得られる電気信号を何らかの方式で検知する隔膜式センサを総て対象とするものである。
【0015】
本発明において、電解液としては、液体状の他、加粘性物質を加えたゾル状や、ゲル形成物質や樹脂等にしみ込ませた半固体状や固体状等、非液体状のものも採用できる。膜接合面は、ドーナツ状や四角枠等、内部に電解液収容溝を囲む単一の面として形成しても良いし、2以上の膜接合面を、電解液収容溝を囲むように、同一平面上に配置しても良い。
【0016】
本発明においては、センサ本体が簡単な構造のため、微小なサイズ・形状が精度良く加工できる。そして、その膜接合面に気液分離膜を接合するので、充分なガス透過性を確保できる気液分離膜を選択して装着可能である。気液分離膜と検知電極とは、膜接合面と検知電極台によって安定した位置関係を保持できる。従って、微小サイズであっても、精度良い測定が可能となる。また、内部液も液体、非液体にかかわらず確実に保持できる。
【0017】
請求項1の発明は、センサ本体の形状をより特定することにより、さらに加工が容易となる。すなわち、請求項2に記載した発明の如く、センサ本体と、気液分離膜と、センサ本体と気液分離膜との間に充填された電解液と、電解液に接触する検知電極及び対極とを備える隔膜式センサにおいて、気液分離膜が、シリコンウエハーに孔あけ加工した膜材又はアルミナを陽極酸化して多孔質化した膜材により構成され、センサ本体が、同一平面に平行して設けられて気液分離膜が接合される一対の膜接合面と、膜接合面に挟まれる位置に設けられた底面が長方形の電解液収容溝と、電解液収容溝底面から突設され先端が膜接合面と同一平面上の近傍にまで伸張する先端面が膜接合面と平行する長方形の検知電極台とを有すると共に、このセンサ本体の検知電極台の先端面には検知電極が、電解液収容溝の底面には対極が各々設けられ、検知電極は、膜接合面と同一平面上よりも電解液収容溝の底面方向に位置し、気液分離膜と検知電極との位置関係が膜接合面と検知電極台の位置関係によって安定した関係に保持されるように構成されていることを特徴とする隔膜式センサとすることができる。
【0018】
本発明によれば、電解液収容溝や検知電極台を、直線加工により設けることができるので、製作が容易である。なお、センサ本体全体の形状は特に限定されず、例えば円柱等としてもよいが、直方体の如く直線で囲まれた形状にすれば、より製作が容易となる。
【0019】
センサ本体の材質に特に限定はないが、請求項3に記載した如く、石英等のガラス製とすることにより、安価な材料で、精度良い加工を迅速に行うことができる。また、電解液による腐食を受けにくく、種々の測定原理の隔膜電極に対応できる。センサ本体の材質としては、この他、樹脂材等を適宜使用できる。
【0020】
気液分離膜は、請求項4に記載の如く、電解液に面する内面が親水性で、試料ガスに面する外面が撥水性の多孔質膜とすることができる。本発明によれば、外面を撥水性としたので、外部に電解液が漏出しにくい。そのため、充分なガス透過を確保できる気孔率の高い多孔質膜を採用できる。また、外部からの汚れの影響も受けにくくなる。さらに、電解液に接する内面を親水性としたので、電解液とのなじみが良い。そのため、検知電極と気液分離膜との距離を極限まで狭くしても検知電極が電解液に接することができる。したがって、センサ全体をより小さく形成することができる。
【0021】
本発明は、請求項5に記載の如く、検知電極と対極との間に電圧を印加し、気液分離膜を透過した測定対象ガスによって、両極間の分極が解除された際に流れる電流を検知する方式、すなわち電量式とすることができる。電量式の場合、化学的浸食性のほとんどない中性付近の電解液を用いることができる。そのため、ガラス等に限らず、種々の材質を用いることができるので、半導体加工技術を用いてのセンサの微小化にも適応できる。
【0022】
電量式ガスセンサは、請求項6に記載の如く、例えば塩素ガスセンサとして構成できる。この場合、電解液としては臭化物イオン溶液を用いる。なお、検知電極及び対極の材質としては、白金、銀、金、プラセオジム、パラジウム、イリジウム、ロジウム等種々の貴金属が選択できる。
【0023】
【発明の実施の形態】
以下、図に沿って本発明の実施形態を説明する。図1は本発明に係る隔膜式センサの1実施形態を示す分解斜視図である。図1の隔膜式センサは、センサ本体1と、センサ本体1に設けられた検知電極2及び対極3と、気液分離膜4とから構成されている。
【0024】
図1の隔膜式センサについて、図2〜図7を適宜参照しながらさらに詳しく説明する。なお、図2は平面図、図3は気液分離膜4を除いた平面図、図3〜図7は、各々図2におけるIV〜VII断面図である。
【0025】
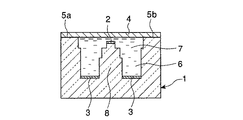
図3及び図4に示すように、センサ本体1は膜接合面5a,5bとを備えている。膜接合面5a,5bは長方形で、同一平面上に互いに平行して設けられている。また、膜接合面5a,5bに挟まれる位置に底面が長方形の電解液収容溝6が設けられており、電解液7が収容されるようになっている。また、電解液収容溝6の中央部分に、検知電極台8が突設されている。この検知電極台8の先端は、膜接合面5a,5bと同一平面上の近傍にまで伸張しており、その先端面は、膜接合面5a,5bと平行する長方形となっている。
【0026】
検知電極2は、検知電極台8の先端面に設けられている。また、対極3は、電解液収容溝6の検知電極台8が設けられていない残りの底面部分に、検知電極台8を挟むように設けられている。検知電極2からは、図5にも示すようにリード線9が導出されている。また、対極3からは図6にも示すようにリード線10が導出されている。なお、図1においては、これらリード線9,10の図示を省略してある。
【0027】
気液分離膜4は、図7に示すように膜接合面5b(及び5a)に接合され、図2に示すようにリード線9,10の導出を妨げない位置までセンサ本体1を覆っている。また、センサ本体1自身にも、リード線9,10を導出しやすくするために気液分離膜4で覆われていない部分に切り込みが設けられている。また、センサ本体1には、検知極2と対極3との間の電気的絶縁を図ることを目的として、薄い切り込みが各部分に適宜設けられている。
【0028】
本実施形態において、センサ本体1は石英ガラス製で、精密ダイシングソーにより、所望の寸法形状に、精密に加工されている。なお、センサ本体の加工方法としては、この他、レーザー加工等を用いてもよく、エッチング等、半導体加工技術を適宜採用してもよい。検知電極及び対極は、蒸着、スバッタ、イオン蒸着(I−BAD)等公知の方法により、センサ本体上に膜状に形成されている。両極からのリード線9,10には、極細の金線等が用いられる。
【0029】
気液分離膜4としては、図9の従来技術に係る電極ではテトラフルオロエチレン等のフッ素化合物製の多孔質メンプレン等が用いられているが、緩みやすいため、微小電極として作成するには適していない。検討の結果、シリコンウエハーをDeepRIE(反応性イオンエッチング)により孔あけ加工した膜材や、アルミナを陽極酸化して多孔質化した膜材等が好適に使用できた。例えば、厚さ160μmのシリコンウェハーに、DeepRIEにより、ポアサイズがφ30μmからφ100μm程度の貫通孔をあけることができる。また、厚さ10μmのアルミナに陽極酸化法によって、ポアサイズφ0.08μm程度の貫通口をあけることができる。
【0030】
また、気液分離膜4の撥水化には、シラン化処理やプラズマCVD(化学的気相堆積)法が好適に使用できる。撥水化の処理は、膜の両面に行ってもよいが、試料ガスに接する外側片面のみとする方が、気液分離膜4と検知電極2との間を極限まで近接させることができるので、より微細な電極とすることが可能となる。
【0031】
気液分離膜4のセンサ本体1への接合方法は、接着、陽極接合、ふっ化酸接合、水ガラス結合等の接合方法が適宜採用できる。なお、気液分離膜4と検知電極2との位置関係をより安定させるために、検知電極台8の先端面に適当なスペーサを設けて、このスペーサと膜接合面5a,5bで気液分離膜4を支えるようにしても差し支えない。
【0032】
電解液7は、気液分離膜4をセンサ本体1に接合した後に、細いシリンジやチューブ等を介して電解液収容溝6内に注入される。なお、電解液7は、センサ本体を小さく形成することにより、そのままでも毛細管現象により、気液分離膜4に接する位置まで充分に保持されるが、電解液7の露出部分に接着剤等をコーティングすることにより、蒸発や漏出が的確に防止でき、扱いがより容易となる。
【0033】
本実施形態によれば、センサ本体1の加工が容易であり、全体を5mm×5mm×3mm程度のミリメートルサイズで精度良く製作できる。また、このセンサ本体1の膜接合面5a,5bに気液分離膜を接合することにより、気液分離膜4と検知電極2の位置関係を良好に安定させることができる。さらに、気液分離膜4は、低濃度ガスの測定にも対応できる充分なガス透過性を確保できると共に、内部液も確実に保持できる。すなわち、本実施形態によれば、毒性ガス等の低濃度ガスの測定が可能な微小電極を提供できるものである。
【0034】
【実施例】
本発明の一実施例として、実施形態として図1〜図7に示した構造で、塩素センサを作成した。本実施例において、センサ本体1は、精密ダイシングソーにより、5mm×5mm×3mmの石英ガラスブロックを加工することにより製作した。具体的には、両サイドに膜接合面5a,5bを略1mm幅ずつ残し、電解液収容溝6を、石英ガラスブロックの中央部に幅3mmで、さらにその中央に検知電極台8の基台部分を幅1mmで設けた。検知電極台8の先端面と、膜接合面5a,5bが存在する平面との距離は、約0.1mmとした。
【0035】
検知電極2及び対極3は白金製で、電解液7としては、濃厚な臭化物イオン溶液を用いた。検知電極2及び対極3は、膜接合面5a,5bを予めレジストしておいてから、ECRスパッタにより設けた。具体的には、レジストは、石英ガラスブロックに密着強化剤をスピンコートした上に、ポジタイプのレジストをスピンコートすることにより行った。また、ECRスパッタは、エリオニクス社製EIS−200ER装置を用いて、チタンをバインダーとして行った。この後、エタノールを使用して超音波洗浄を行い、レジストを除去し、膜接合面4にスパッタされたチタン及び白金を除去した。これにより、チタン層が12nm程度、白金層が120nm程度の電極が成膜できた。検知電極2は、表面積が0.1mm×3.3mmとなるよう、検知電極台8ごと、両端をダイシングソーによりカットした。対極3は、電解液収容溝6の底面の検知電極台8を挟む位置に、1mm×5mmの表面積のものが各々形成された。両極には、リード線9,10として、φ25μmの金線をワイヤボンディングした。
【0036】
気液分離膜4としては、厚さ160μmのシリコンウエハーにDeepRIEによりポアサイズがφ30μmの貫通孔をあけたものを、シラン化処理により撥水化して用いた。シラン化処理は、処理剤として1,1,1,3,3,3−ヘキサメチルジシラザンの入ったデシケータ中にDeepRIE加工後の膜を24時間放置することにより行った。気液分離膜4のセンサ本体1への接合は、シリコーン接着剤を薄く塗布した後に貼り合わせ、24時間以上放置して接着硬化させることにより行った。
【0037】
以上のように製作したセンサの両電極間に電圧(250〜300mV)を印加すると、対極3で次反応が起こり分極し、一旦電流は停止する。
2H++2e−→H2 (1)
そこに、電解液7に気液分離膜4を透過してきた塩素ガスが入ると、ハロゲン交換反応が起こり、検知電極2で臭素ガスが発生する。
2Br−+Cl2→Br2+2Cl− (2)
そして、これらの臭素と水素が反応して分極が解除(復極)し、電流が流れる。
Br2+H2→2Br−+2H+ (3)
この電流を検知することにより、試料ガス中の塩素濃度を求められるものである。
【0038】
図8に、上記実施例の塩素センサにより得られたデータを示す。図8に示すように、得られる電流Iと塩素濃度Cとの関係は、I=−15.35C−1.033(nA)、相関係数=0.999となり、良好な直線性が得られた。繰り返し性・応答速度などでも図9に示す従来センサーと同等の良好な特性が得られ、毒性ガスとしての許容濃度付近の低濃度測定にも対応可能な性能を有していることが確認された。
【0039】
なお、電流値は、検知する塩素ガス濃度に比例するので、本実施例のセンサによって得られる電流はnAレベルで、図9の従来センサによって得られるμAレベルよりも著しく小さい。このことにより、アンプの消費電流が低減されるので、装置全体の消費電力を著しく減少させることができる。すなわち、センサの微小化は、単に小さいサイズによる取扱いの便利さだけでなく、消費電力低減という効果をもたらし、装置全体の電池駆動等も可能とするものである。
【0040】
【発明の効果】
本発明によれば、微小化した場合にも、低濃度ガスの測定にも対応できる充分なガス透過性を確保できると共に、内部液も確実に保持でき、しかも、検知電極と気液分離膜の位置関係を一定する等の精密な加工が可能なことにより良好な測定精度が得られる隔膜式センサを提供することができる。
【図面の簡単な説明】
【図1】本発明の一実施形態に係る隔膜式センサの分解斜視図である。
【図2】本発明の一実施形態に係る隔膜式センサの平面図である。
【図3】図2の平面図から、気液分離膜4を除いた平面図である。
【図4】図2の平面図のIV−IV断面図である。
【図5】図2の平面図のV−V断面図である。
【図6】図2の平面図のVI一VI断面図である。
【図7】図2の平面図のVII−VII断面図である。
【図8】本発明の一実施例に係る塩素センサにより得られたデータである。
【図9】従来技術に係るECセンサの一例である。
【図10】従来技術に係る微小酸素電極である。
【符号の説明】
1 センサ本体
2 検知電極
3 対極
4 気液分離膜
5a,5b 膜接合面
6 電解液収容溝
7 電解液
8 検知電極台
9,10 リード線[0001]
BACKGROUND OF THE INVENTION
The present invention relates to a diaphragm type sensor having a structure in which a detection electrode, a counter electrode, an electrolytic solution (internal liquid) and the like are held in a sensor body and a gas-liquid separation membrane. More specifically, the present invention relates to a diaphragm type sensor that is suitable for detection of low-concentration gas such as chlorine leakage gas and can be manufactured minutely.
[0002]
[Prior art]
As sensors for detecting toxic gases such as chlorine gas, centimeter-sized coulometric and constant potential electrolytic sensors have been put into practical use. The sensor is widely used for leaked gas detection alarms and the like, and is a sensor having the capability of quickly responding to a low-concentration gas near an allowable concentration (ACGIH or recommended value of the Japan Society for Occupational Health).
[0003]
These coulometric and constant-potential electrolytic chlorine gas sensors have a structure in which an internal liquid (electrolyte) is held together with a working electrode (detection electrode), a counter electrode, etc. inside a gas-liquid separation membrane (diaphragm) and the sensor body It has become. Both methods have many similarities, such as measuring the current as the sensor output while applying an appropriate voltage between the working electrode, and recently, other similar measurement methods can be used regardless of the measurement principle. In addition, cases called amperometric sensors, amperometric sensors, electrochemical sensors, and EC (Electrochemical) sensors are increasing.
[0004]
FIG. 9 shows an example of an EC sensor according to the above prior art. The EC sensor shown in FIG. 9 includes a cylindrical sensor
[0005]
On the other hand, research and development of micro gas sensors have been performed by many researchers so far, and many reports have been made. However, most of the reports on micro EC sensors are about oxygen sensors or biosensors. This is because the need for these microsensors exists in the medical field, and because of the high concentration (% order) of oxygen as the measurement target substance, the gas-liquid with a small pore size and porosity. Because it can be used as a separation membrane, and a film such as a fluorine compound or silicone can be used, a gas-liquid separation structure can be constructed relatively easily, and the internal solution of the oxygen sensor has a composition close to that of a potassium chloride solution. The fact that the surrounding sensor members and the like are hardly eroded is greatly involved, and it is considered that many research reports have been made.
[0006]
FIG. 10 shows an oxygen electrode described in Japanese Patent Publication No. 6-1254 as an example of the minute sensor. In FIG. 10, a
[0007]
[Problems to be solved by the invention]
Also in the conventional EC sensor shown in FIG. 9, miniaturization is desired from the viewpoint of enabling installation in a narrow place near a place that can be a generation source and promoting cost reduction. In addition, miniaturization is also desired for energy saving and environmental considerations such as reducing the amount of waste including electrolytes.
[0008]
However, it cannot be manufactured by miniaturizing the structure as it is. That is, the O-
[0009]
On the other hand, the configuration like the oxygen electrode shown in FIG. 10 is not applied to a micro EC sensor for measuring toxic gas. This is because, in the above configuration, it is difficult to achieve the specification that the gas to be measured is a low-concentration gas of the order of ppb to ppm and that quick response and long-term stability are required for the following reasons.
[0010]
That is, in the case of low concentration measurement, it is necessary to use a gas-liquid separation membrane that can reliably retain the internal liquid while ensuring sufficient gas permeability. However, the film formed by applying the liquid material on the oxygen electrode is a non-porous film. In this case, since the gas permeates through the molecules constituting the continuous film, only a very small part of the gas in contact with the film can permeate. This can be used as long as a gas of around 20% is measured, such as oxygen. However, when a low-concentration gas of the order of ppb to ppm is targeted, a sufficient amount of permeation cannot be ensured.
[0011]
Further, in the oxygen electrode of FIG. 10, the electrolytic solution is made non-liquid and has some mechanical strength, thereby enabling application of a liquid material that forms a gas-liquid separation membrane. Therefore, it is difficult to make the positional relationship between the gas-liquid separation membrane and the detection electrode constant, and in the case of low concentration measurement, there is a problem in accuracy.
[0012]
The present invention has been made in view of the above circumstances, and even when it is miniaturized, it can ensure sufficient gas permeability that can accommodate measurement of low-concentration gas, and can also reliably hold internal liquid, It is an object of the present invention to provide a diaphragm type sensor that can obtain good measurement accuracy by performing precise processing such as making the positional relationship between a detection electrode and a gas-liquid separation membrane constant.
[0013]
[Means for Solving the Problems]
As a result of studying the above problems, the inventors of the present invention have, as the invention of
[0014]
The gas-liquid separation membrane in the present invention is generally also referred to as a diaphragm. The electrolytic solution is also referred to as an internal solution. Further, the detection electrode and the counter electrode are also referred to as a working electrode and a counter electrode in the current measurement type sensor, respectively. In addition, in consideration of the direction of electron exchange, either of the electrodes may be referred to as an anode and either as a cathode. Further, in the voltage measurement type sensor, the detection electrode and the counter electrode are also referred to as a sensitive electrode (membrane) and a reference electrode, respectively. The diaphragm type sensor according to the present invention corresponds to the measurement target gas that passes through the gas-liquid separation membrane and enters the electrolytic solution between the detection electrode and the counter electrode regardless of the presence of these various measurement principles and alternative names. The present invention is intended for all diaphragm type sensors that detect electrical signals obtained by any method.
[0015]
In the present invention, as the electrolytic solution, in addition to a liquid form, a non-liquid form such as a sol form added with a viscous substance, a semi-solid form or a solid form soaked in a gel-forming substance or a resin, etc. can be adopted. . The membrane bonding surface may be formed as a single surface surrounding the electrolyte accommodating groove, such as a donut shape or a square frame, and two or more membrane bonding surfaces are the same so as to surround the electrolyte accommodating groove You may arrange | position on a plane.
[0016]
In the present invention, since the sensor body has a simple structure, a minute size and shape can be processed with high accuracy. Since the gas-liquid separation membrane is bonded to the membrane bonding surface, a gas-liquid separation membrane that can ensure sufficient gas permeability can be selected and mounted. The gas-liquid separation membrane and the detection electrode can maintain a stable positional relationship by the membrane bonding surface and the detection electrode base. Therefore, even if it is a very small size, it is possible to measure with high accuracy. In addition, the internal liquid can be reliably held regardless of whether it is liquid or non-liquid.
[0017]
According to the first aspect of the present invention, the processing is further facilitated by specifying the shape of the sensor body more. That is, as in the invention described in
[0018]
According to the present invention, since the electrolytic solution containing groove and the detection electrode base can be provided by linear processing, the manufacturing is easy. The shape of the entire sensor body is not particularly limited, and may be, for example, a cylinder or the like. However, if the sensor body is surrounded by a straight line such as a rectangular parallelepiped, the manufacturing becomes easier.
[0019]
Although there is no particular limitation on the material of the sensor body, as described in
[0020]
As described in
[0021]
According to the present invention, the current flowing when the polarization between the electrodes is released by the measurement target gas that has passed through the gas-liquid separation membrane by applying a voltage between the detection electrode and the counter electrode. A detection method, that is, a coulometric method can be used. In the case of the coulometric method, a neutral electrolyte solution having almost no chemical erosion property can be used. Therefore, not only glass and the like, but various materials can be used, so that it can be applied to miniaturization of sensors using semiconductor processing technology.
[0022]
The coulometric gas sensor can be configured as a chlorine gas sensor, for example, as described in
[0023]
DETAILED DESCRIPTION OF THE INVENTION
Hereinafter, embodiments of the present invention will be described with reference to the drawings. FIG. 1 is an exploded perspective view showing one embodiment of a diaphragm type sensor according to the present invention. The diaphragm type sensor shown in FIG. 1 includes a sensor
[0024]
The diaphragm type sensor in FIG. 1 will be described in more detail with reference to FIGS. 2 is a plan view, FIG. 3 is a plan view excluding the gas-
[0025]
As shown in FIGS. 3 and 4, the
[0026]
The
[0027]
The gas-
[0028]
In this embodiment, the sensor
[0029]
As the gas-
[0030]
In addition, silanization or plasma CVD (chemical vapor deposition) can be suitably used to make the gas-
[0031]
As a method for bonding the gas-
[0032]
The
[0033]
According to this embodiment, the processing of the
[0034]
【Example】
As an example of the present invention, a chlorine sensor was created with the structure shown in FIGS. 1 to 7 as an embodiment. In this example, the
[0035]
The
[0036]
As the gas-
[0037]
When a voltage (250 to 300 mV) is applied between both electrodes of the sensor manufactured as described above, the next reaction takes place at the
2H + + 2e − → H 2 (1)
When the chlorine gas that has permeated the gas-
2Br − + Cl 2 → Br 2 + 2Cl − (2)
These bromine and hydrogen react to depolarize (depolarize) and a current flows.
Br 2 + H 2 → 2Br − + 2H + (3)
By detecting this current, the chlorine concentration in the sample gas can be obtained.
[0038]
FIG. 8 shows data obtained by the chlorine sensor of the above example. As shown in FIG. 8, the relationship between the obtained current I and the chlorine concentration C is I = -15.35C-1.033 (nA), the correlation coefficient = 0.999, and good linearity is obtained. It was. Good characteristics equivalent to the conventional sensor shown in Fig. 9 were obtained in terms of repeatability and response speed, and it was confirmed that it has the capability to handle low concentration measurements near the permissible concentration as a toxic gas. .
[0039]
Since the current value is proportional to the chlorine gas concentration to be detected, the current obtained by the sensor of this embodiment is nA level, which is significantly smaller than the μA level obtained by the conventional sensor of FIG. As a result, the current consumption of the amplifier is reduced, so that the power consumption of the entire apparatus can be significantly reduced. That is, the miniaturization of the sensor not only provides convenience of handling due to a small size but also brings about an effect of reducing power consumption, and enables battery driving of the entire apparatus.
[0040]
【The invention's effect】
According to the present invention, sufficient gas permeability that can cope with measurement of low concentration gas even when miniaturized can be secured, the internal liquid can be securely held, and the detection electrode and the gas-liquid separation membrane can be maintained. It is possible to provide a diaphragm type sensor that can obtain good measurement accuracy by performing precise processing such as fixing the positional relationship.
[Brief description of the drawings]
FIG. 1 is an exploded perspective view of a diaphragm type sensor according to an embodiment of the present invention.
FIG. 2 is a plan view of a diaphragm type sensor according to an embodiment of the present invention.
3 is a plan view obtained by removing the gas-
4 is a cross-sectional view taken along the line IV-IV of the plan view of FIG.
5 is a VV cross-sectional view of the plan view of FIG. 2. FIG.
6 is a VI-VI cross-sectional view of the plan view of FIG. 2. FIG.
7 is a cross-sectional view taken along the line VII-VII of the plan view of FIG.
FIG. 8 is data obtained by a chlorine sensor according to an example of the present invention.
FIG. 9 is an example of an EC sensor according to the prior art.
FIG. 10 is a micro oxygen electrode according to the prior art.
[Explanation of symbols]
DESCRIPTION OF
Claims (6)
気液分離膜が、シリコンウエハーに孔あけ加工した膜材又はアルミナを陽極酸化して多孔質化した膜材により構成され、
センサ本体が、同一平面に設けられて気液分離膜が接合される一以上の膜接合面と、膜接合面に囲まれる位置に設けられた電解液収容溝と、電解液収容溝底面から膜接合面と離間して突設され先端が膜接合面と同一平面上の近傍にまで伸張する検知電極台とを有すると共に、このセンサ本体の検知電極台の先端には検知電極が、電解液収容溝の底面には対極が各々設けられ、検知電極は、膜接合面と同一平面上よりも電解液収容溝の底面方向に位置し、気液分離膜と検知電極との位置関係が膜接合面と検知電極台の位置関係によって安定した関係に保持されるように構成されていることを特徴とする隔膜式センサ。In a diaphragm type sensor comprising a sensor body, a gas-liquid separation membrane, an electrolyte filled between the sensor body and the gas-liquid separation membrane, and a detection electrode and a counter electrode in contact with the electrolyte,
The gas-liquid separation membrane is composed of a membrane material that has been perforated in a silicon wafer or a membrane material that has been made porous by anodizing alumina.
The sensor main body is provided on the same plane and has one or more membrane bonding surfaces to which the gas-liquid separation membrane is bonded; an electrolyte containing groove provided at a position surrounded by the membrane bonding surface; and a membrane from the bottom of the electrolytic solution containing groove A detection electrode base that protrudes away from the bonding surface and extends to the vicinity of the same plane as the membrane bonding surface, and the detection electrode is disposed at the tip of the detection electrode base of the sensor body. A counter electrode is provided on the bottom surface of the groove, and the detection electrode is positioned in the bottom surface direction of the electrolyte solution storage groove rather than on the same plane as the membrane bonding surface, and the positional relationship between the gas-liquid separation membrane and the detection electrode is The diaphragm type sensor is configured to be held in a stable relationship by the positional relationship between the detection electrode base and the detection electrode base.
気液分離膜が、シリコンウエハーに孔あけ加工した膜材又はアルミナを陽極酸化して多孔質化した膜材により構成され、
センサ本体が、同一平面に平行して設けられて気液分離膜が接合される一対の膜接合面と、膜接合面に挟まれる位置に設けられた底面が長方形の電解液収容溝と、電解液収容溝底面から突設され先端が膜接合面と同一平面上の近傍にまで伸張する先端面が膜接合面と平行する長方形の検知電極台とを有すると共に、このセンサ本体の検知電極台の先端面には検知電極が、電解液収容溝の底面には対極が各々設けられ、検知電極は、膜接合面と同一平面上よりも電解液収容溝の底面方向に位置し、気液分離膜と検知電極との位置関係が膜接合面と検知電極台の位置関係によって安定した関係に保持されるように構成されていることを特徴とする隔膜式センサ。In a diaphragm type sensor comprising a sensor body, a gas-liquid separation membrane, an electrolyte filled between the sensor body and the gas-liquid separation membrane, and a detection electrode and a counter electrode in contact with the electrolyte,
The gas-liquid separation membrane is composed of a membrane material that has been perforated in a silicon wafer or a membrane material that has been made porous by anodizing alumina.
A sensor body is provided in parallel to the same plane and a gas-liquid separation membrane is joined to a pair of membrane joining surfaces, an electrolyte solution containing groove having a rectangular bottom surface provided between the membrane joining surfaces, A leading end surface protruding from the bottom surface of the liquid storage groove and extending to the vicinity of the same plane as the membrane junction surface, and a rectangular detection electrode base parallel to the membrane junction surface. A sensing electrode is provided on the front end surface, and a counter electrode is provided on the bottom surface of the electrolytic solution housing groove, and the sensing electrode is located on the bottom surface side of the electrolytic solution housing groove rather than on the same plane as the membrane bonding surface. A diaphragm type sensor characterized in that the positional relationship between the electrode and the detection electrode is maintained in a stable relationship by the positional relationship between the membrane bonding surface and the detection electrode base.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2000132990A JP4620215B2 (en) | 2000-03-28 | 2000-03-28 | Diaphragm sensor |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2000132990A JP4620215B2 (en) | 2000-03-28 | 2000-03-28 | Diaphragm sensor |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2001281204A JP2001281204A (en) | 2001-10-10 |
| JP4620215B2 true JP4620215B2 (en) | 2011-01-26 |
Family
ID=18641576
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2000132990A Expired - Fee Related JP4620215B2 (en) | 2000-03-28 | 2000-03-28 | Diaphragm sensor |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4620215B2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106645295A (en) * | 2016-12-27 | 2017-05-10 | 北京七星华创电子股份有限公司 | Gas leakage detection device and gas leakage position detection method |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5629094B2 (en) * | 2007-01-19 | 2014-11-19 | アエロクライン・アクチボラゲットAerocrine Ab | Analysis equipment |
| EP2131187A4 (en) | 2007-03-29 | 2015-07-01 | Nec Corp | Measurement device |
| JP5843754B2 (en) * | 2010-03-09 | 2016-01-13 | アークレイ株式会社 | Electrochemical sensor |
| JP5392918B2 (en) * | 2010-06-18 | 2014-01-22 | 理研計器株式会社 | Constant potential electrolytic acid gas detector |
| CN108732162B (en) * | 2018-05-29 | 2024-07-26 | 四川轻化工大学 | Rapid detection device and detection method for arsenic concentration in water |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS54150196A (en) * | 1978-05-17 | 1979-11-26 | Osaka Soda Co Ltd | Method of detecting gas |
| JPH04346065A (en) * | 1991-05-24 | 1992-12-01 | Hitachi Ltd | Oxygen sensor |
| JPH05223774A (en) * | 1991-01-21 | 1993-08-31 | Hitachi Ltd | Oxygen sensor, its manufacture, and flow cell utilizing it |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01265881A (en) * | 1988-02-02 | 1989-10-23 | Choichi Furuya | Biocatalyst membrane with hydrophobic porous layer |
| JPH03183943A (en) * | 1989-12-14 | 1991-08-09 | Hitachi Ltd | Oxygen sensor |
-
2000
- 2000-03-28 JP JP2000132990A patent/JP4620215B2/en not_active Expired - Fee Related
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS54150196A (en) * | 1978-05-17 | 1979-11-26 | Osaka Soda Co Ltd | Method of detecting gas |
| JPH05223774A (en) * | 1991-01-21 | 1993-08-31 | Hitachi Ltd | Oxygen sensor, its manufacture, and flow cell utilizing it |
| JPH04346065A (en) * | 1991-05-24 | 1992-12-01 | Hitachi Ltd | Oxygen sensor |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106645295A (en) * | 2016-12-27 | 2017-05-10 | 北京七星华创电子股份有限公司 | Gas leakage detection device and gas leakage position detection method |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2001281204A (en) | 2001-10-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU689625B2 (en) | Method of measuring gas concentrations and microfabricated sensing device for practicing same | |
| JPH02232554A (en) | Sensor construction | |
| EP0064337B1 (en) | Carbon dioxide measurement | |
| US20170160228A1 (en) | Potentiometric sensor | |
| JP2000512745A (en) | Membrane electrode for measuring glucose concentration in liquid | |
| JP2512843B2 (en) | Carbon dioxide sensor | |
| US10139362B2 (en) | Sensor head, electrochemical sensor, and method for using electrochemical sensor | |
| JPH01112149A (en) | Electrochemical electrode construction | |
| JPH06508432A (en) | Electrical analysis of liquids and detection elements used for it | |
| JP4620215B2 (en) | Diaphragm sensor | |
| KR101488438B1 (en) | Electrochemical gas sensor | |
| JPH0351B2 (en) | ||
| JP3318405B2 (en) | Reference electrode | |
| JPH09170998A (en) | Reference electrode assembly | |
| JP2006171000A (en) | Steam sensor | |
| Jin et al. | Determination of clozapine by capillary zone electrophoresis following end‐column amperometric detection with simplified capillary/electrode alignment | |
| US20070227908A1 (en) | Electrochemical cell sensor | |
| CN217156396U (en) | Tectorial membrane multi-chamber water quality analysis sensor | |
| JP4216846B2 (en) | Electrodes for electrochemical measurements and electrochemical measurement methods | |
| JP3530627B2 (en) | Method and apparatus for measuring oxygen content in gas | |
| Nei | Some milestones in the 50-year history of electrochemical oxygen sensor development | |
| US20080116083A1 (en) | Electrochemical gas sensor with at least one punctiform measuring electrode | |
| JP4758752B2 (en) | pH electrode | |
| JPH06148124A (en) | Disposable ion sensor unit | |
| KR0177196B1 (en) | Sensor for dissolved oxygen using ph-isfet |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20061121 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A712 Effective date: 20061218 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20090527 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090609 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090806 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100302 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100426 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20101019 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20101028 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131105 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4620215 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313117 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R371 | Transfer withdrawn |
Free format text: JAPANESE INTERMEDIATE CODE: R371 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313117 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |