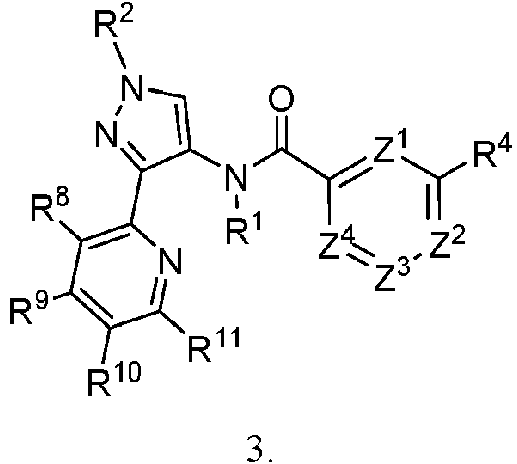
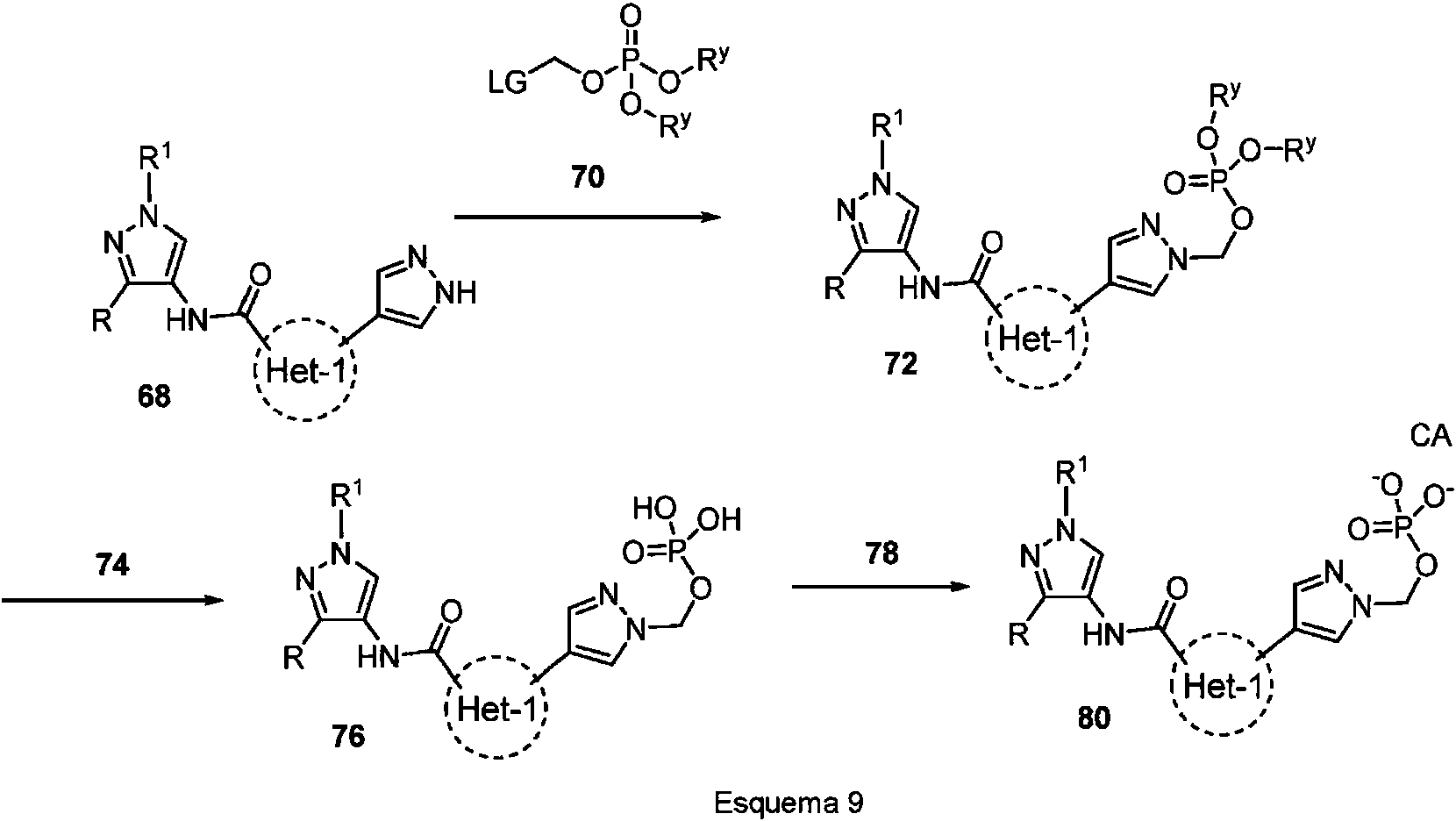
ES2946001T3 - Pyrazole amide compounds as IRAK inhibitors - Google Patents
Pyrazole amide compounds as IRAK inhibitors Download PDFInfo
- Publication number
- ES2946001T3 ES2946001T3 ES17794624T ES17794624T ES2946001T3 ES 2946001 T3 ES2946001 T3 ES 2946001T3 ES 17794624 T ES17794624 T ES 17794624T ES 17794624 T ES17794624 T ES 17794624T ES 2946001 T3 ES2946001 T3 ES 2946001T3
- Authority
- ES
- Spain
- Prior art keywords
- pyrazol
- pyridin
- methyl
- carboxamide
- bipyridine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000003112 inhibitor Substances 0.000 title claims abstract description 21
- -1 Pyrazole amide compounds Chemical class 0.000 title claims description 295
- 150000001875 compounds Chemical class 0.000 claims abstract description 239
- 239000000203 mixture Substances 0.000 claims abstract description 87
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 62
- 238000000034 method Methods 0.000 claims abstract description 62
- 201000010099 disease Diseases 0.000 claims abstract description 36
- 125000000217 alkyl group Chemical group 0.000 claims description 112
- 125000003545 alkoxy group Chemical group 0.000 claims description 78
- 125000003118 aryl group Chemical group 0.000 claims description 51
- 229910052736 halogen Inorganic materials 0.000 claims description 49
- 150000002367 halogens Chemical group 0.000 claims description 49
- 125000001931 aliphatic group Chemical group 0.000 claims description 45
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 42
- IBBMAWULFFBRKK-UHFFFAOYSA-N picolinamide Chemical compound NC(=O)C1=CC=CC=N1 IBBMAWULFFBRKK-UHFFFAOYSA-N 0.000 claims description 41
- 125000004076 pyridyl group Chemical group 0.000 claims description 40
- 125000001072 heteroaryl group Chemical group 0.000 claims description 36
- 150000003839 salts Chemical class 0.000 claims description 34
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 claims description 33
- 229910052739 hydrogen Inorganic materials 0.000 claims description 33
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 31
- 229910052799 carbon Inorganic materials 0.000 claims description 30
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 claims description 29
- 125000003226 pyrazolyl group Chemical group 0.000 claims description 27
- 125000004093 cyano group Chemical class *C#N 0.000 claims description 26
- 208000035475 disorder Diseases 0.000 claims description 25
- 125000000623 heterocyclic group Chemical group 0.000 claims description 25
- 125000001041 indolyl group Chemical group 0.000 claims description 23
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 22
- 125000001188 haloalkyl group Chemical class 0.000 claims description 21
- 125000004853 tetrahydropyridinyl group Chemical group N1(CCCC=C1)* 0.000 claims description 21
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 claims description 18
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 18
- 229940124530 sulfonamide Drugs 0.000 claims description 18
- 150000003456 sulfonamides Chemical class 0.000 claims description 18
- 150000001408 amides Chemical class 0.000 claims description 17
- 125000003566 oxetanyl group Chemical group 0.000 claims description 16
- 125000003373 pyrazinyl group Chemical group 0.000 claims description 16
- 125000001412 tetrahydropyranyl group Chemical group 0.000 claims description 16
- 125000000753 cycloalkyl group Chemical class 0.000 claims description 15
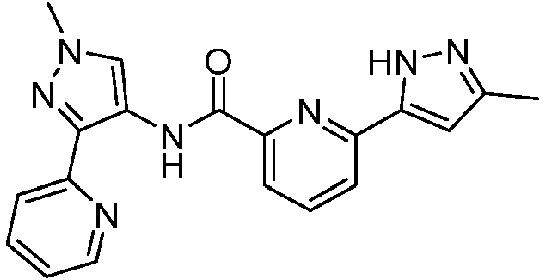
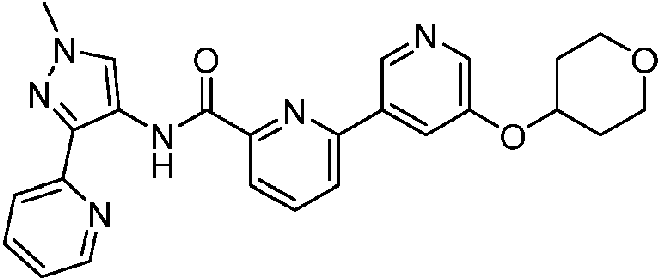
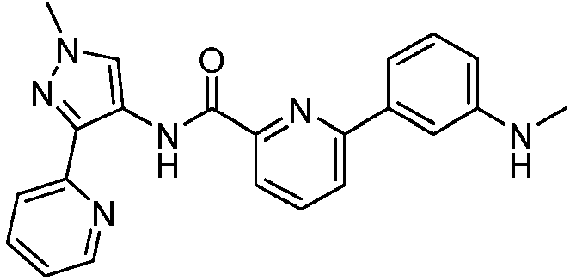
- XSLDPQKBOPPWOQ-UHFFFAOYSA-N 6-(5-fluoropyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound FC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 XSLDPQKBOPPWOQ-UHFFFAOYSA-N 0.000 claims description 14
- 125000002541 furyl group Chemical group 0.000 claims description 14
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 14
- 125000000168 pyrrolyl group Chemical group 0.000 claims description 14
- 125000003386 piperidinyl group Chemical group 0.000 claims description 13
- 239000011734 sodium Substances 0.000 claims description 13
- 206010028980 Neoplasm Diseases 0.000 claims description 12
- 239000002253 acid Substances 0.000 claims description 11
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 11
- 208000010668 atopic eczema Diseases 0.000 claims description 10
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 10
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 claims description 10
- 102100036342 Interleukin-1 receptor-associated kinase 1 Human genes 0.000 claims description 8
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 8
- 238000002054 transplantation Methods 0.000 claims description 8
- YFMFQTLBMUWLEA-UHFFFAOYSA-N 6-bromo-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound BrC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 YFMFQTLBMUWLEA-UHFFFAOYSA-N 0.000 claims description 7
- 208000035143 Bacterial infection Diseases 0.000 claims description 7
- 208000022362 bacterial infectious disease Diseases 0.000 claims description 7
- 201000011510 cancer Diseases 0.000 claims description 7
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 7
- 208000023275 Autoimmune disease Diseases 0.000 claims description 6
- WNEWQZJKZDJENB-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrazol-5-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=CC=NN1)=O)C1=NC=CC=C1 WNEWQZJKZDJENB-UHFFFAOYSA-N 0.000 claims description 6
- 230000000172 allergic effect Effects 0.000 claims description 6
- 230000000302 ischemic effect Effects 0.000 claims description 6
- 208000023504 respiratory system disease Diseases 0.000 claims description 6
- 125000001313 C5-C10 heteroaryl group Chemical group 0.000 claims description 5
- 206010052779 Transplant rejections Diseases 0.000 claims description 5
- 208000036142 Viral infection Diseases 0.000 claims description 5
- 230000007812 deficiency Effects 0.000 claims description 5
- 230000002401 inhibitory effect Effects 0.000 claims description 5
- 229910052708 sodium Inorganic materials 0.000 claims description 5
- 230000009385 viral infection Effects 0.000 claims description 5
- YTWFFDQDKDWZSS-UHFFFAOYSA-N 6-(1-methylpyrazol-4-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=CC(=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 YTWFFDQDKDWZSS-UHFFFAOYSA-N 0.000 claims description 4
- MPPIKNIKMWPIMW-UHFFFAOYSA-N 6-(1H-indazol-5-ylamino)-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound N1N=CC2=CC(=CC=C12)NC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 MPPIKNIKMWPIMW-UHFFFAOYSA-N 0.000 claims description 4
- XSVKYJNEULVCJF-UHFFFAOYSA-N 6-(1H-indol-5-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound N1C=CC2=CC(=CC=C12)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 XSVKYJNEULVCJF-UHFFFAOYSA-N 0.000 claims description 4
- QISHWQBGAHZIBL-UHFFFAOYSA-N 6-(1H-indol-6-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound N1C=CC2=CC=C(C=C12)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 QISHWQBGAHZIBL-UHFFFAOYSA-N 0.000 claims description 4
- AQKKKMXHUZDXQZ-UHFFFAOYSA-N 6-(2-carbamoylpyridin-4-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC(=NC=C1)C(=O)N)C1=NC=CC=C1 AQKKKMXHUZDXQZ-UHFFFAOYSA-N 0.000 claims description 4
- PYDKXYPIVUBFFI-UHFFFAOYSA-N 6-(2-methylpyridin-4-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CC1=NC=CC(=C1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 PYDKXYPIVUBFFI-UHFFFAOYSA-N 0.000 claims description 4
- ARIHXSDAAHHPAO-UHFFFAOYSA-N 6-(3-aminophenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound NC=1C=C(C=CC=1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 ARIHXSDAAHHPAO-UHFFFAOYSA-N 0.000 claims description 4
- MIWTVMNDBKPSKV-UHFFFAOYSA-N 6-(3-cyanophenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C(#N)C=1C=C(C=CC=1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 MIWTVMNDBKPSKV-UHFFFAOYSA-N 0.000 claims description 4
- TYEXPVYMHODEBM-UHFFFAOYSA-N 6-(3-methoxyphenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound COC=1C=C(C=CC=1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 TYEXPVYMHODEBM-UHFFFAOYSA-N 0.000 claims description 4
- WIZBNHIVWBGYGZ-UHFFFAOYSA-N 6-(3-methylphenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=C(C=CC=1)C)=O)C1=NC=CC=C1 WIZBNHIVWBGYGZ-UHFFFAOYSA-N 0.000 claims description 4
- USLRNBUNKZGXDM-UHFFFAOYSA-N 6-(4-fluorophenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound FC1=CC=C(C=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 USLRNBUNKZGXDM-UHFFFAOYSA-N 0.000 claims description 4
- QHCSVMAQBCJRAQ-UHFFFAOYSA-N 6-(4-methoxyphenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound COC1=CC=C(C=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 QHCSVMAQBCJRAQ-UHFFFAOYSA-N 0.000 claims description 4
- QESDZBJLKSOLCP-UHFFFAOYSA-N 6-(4-methylphenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=CC=C(C=C1)C)=O)C1=NC=CC=C1 QESDZBJLKSOLCP-UHFFFAOYSA-N 0.000 claims description 4
- XEHNDXZRJSEGMY-UHFFFAOYSA-N 6-(5-aminopyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound NC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 XEHNDXZRJSEGMY-UHFFFAOYSA-N 0.000 claims description 4
- ZTKBSDIZABEXSU-UHFFFAOYSA-N 6-(5-carbamoylpyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C(=O)N)C1=NC=CC=C1 ZTKBSDIZABEXSU-UHFFFAOYSA-N 0.000 claims description 4
- XOYAYZMHQUHOBA-UHFFFAOYSA-N 6-(5-cyclopropylpyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C1(CC1)C=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 XOYAYZMHQUHOBA-UHFFFAOYSA-N 0.000 claims description 4
- YEGGGGKTCMGEDA-UHFFFAOYSA-N 6-(5-methyl-1H-pyrazol-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CC1=NNC(=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 YEGGGGKTCMGEDA-UHFFFAOYSA-N 0.000 claims description 4
- DHVXVRPZOLWLOQ-UHFFFAOYSA-N 6-(5-methyl-1H-pyrazol-4-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CC1=NNC=C1C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 DHVXVRPZOLWLOQ-UHFFFAOYSA-N 0.000 claims description 4
- VWSQZAUEFLVYHM-UHFFFAOYSA-N 6-(5-methylpyridin-3-yl)-N-(1-propan-2-yl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C(C)(C)N1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C)C1=NC=CC=C1 VWSQZAUEFLVYHM-UHFFFAOYSA-N 0.000 claims description 4
- DFHQGQLEUPPPOZ-UHFFFAOYSA-N 6-(6-aminopyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound NC1=CC=C(C=N1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 DFHQGQLEUPPPOZ-UHFFFAOYSA-N 0.000 claims description 4
- MWHQKWCDDQOMFJ-UHFFFAOYSA-N 6-(6-methoxypyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound COC1=CC=C(C=N1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 MWHQKWCDDQOMFJ-UHFFFAOYSA-N 0.000 claims description 4
- WGZGEHTYEYFSMZ-UHFFFAOYSA-N 6-(6-methylpyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CC1=CC=C(C=N1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 WGZGEHTYEYFSMZ-UHFFFAOYSA-N 0.000 claims description 4
- CLVCFSJYIBLYIE-UHFFFAOYSA-N 6-(furan-2-yl)-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound O1C(=CC=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 CLVCFSJYIBLYIE-UHFFFAOYSA-N 0.000 claims description 4
- LJTVHTHEADQLJN-UHFFFAOYSA-N 6-[3-(dimethylamino)phenyl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN(C=1C=C(C=CC=1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1)C LJTVHTHEADQLJN-UHFFFAOYSA-N 0.000 claims description 4
- UNQLQIURCLHBCW-UHFFFAOYSA-N 6-[3-(methanesulfonamido)phenyl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=CC(=CC=C1)NS(=O)(=O)C)=O)C1=NC=CC=C1 UNQLQIURCLHBCW-UHFFFAOYSA-N 0.000 claims description 4
- PIZJQJVCPSXEDW-UHFFFAOYSA-N 6-[3-(methylamino)phenyl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=CC(=CC=C1)NC)=O)C1=NC=CC=C1 PIZJQJVCPSXEDW-UHFFFAOYSA-N 0.000 claims description 4
- XXLDLSQLGWAJCH-UHFFFAOYSA-N 6-[5-(2-hydroxypropan-2-yl)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound OC(C)(C)C=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 XXLDLSQLGWAJCH-UHFFFAOYSA-N 0.000 claims description 4
- CLURMQRDVHKAOY-UHFFFAOYSA-N 6-[5-(2-methoxyethylamino)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound COCCNC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 CLURMQRDVHKAOY-UHFFFAOYSA-N 0.000 claims description 4
- NGGCNCSANXVJMK-UHFFFAOYSA-N 6-[5-(4-methylpiperazin-1-yl)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)N1CCN(CC1)C)C1=NC=CC=C1 NGGCNCSANXVJMK-UHFFFAOYSA-N 0.000 claims description 4
- OILCSRYERXEJDA-UHFFFAOYSA-N 6-[5-(cyclopropylamino)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C1(CC1)NC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 OILCSRYERXEJDA-UHFFFAOYSA-N 0.000 claims description 4
- JFLPUZLWLHRFPJ-UHFFFAOYSA-N 6-bromo-N-(1-propan-2-yl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound BrC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C(C)C)C1=NC=CC=C1 JFLPUZLWLHRFPJ-UHFFFAOYSA-N 0.000 claims description 4
- HMRSCDSRPFRSBU-UHFFFAOYSA-N 6-bromo-N-[1-(2-morpholin-4-ylethyl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound BrC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCN1CCOCC1)C1=NC=CC=C1 HMRSCDSRPFRSBU-UHFFFAOYSA-N 0.000 claims description 4
- UKPPOPPQQBENOU-UHFFFAOYSA-N 6-bromo-N-[1-[2-(4-methylpiperazin-1-yl)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound BrC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCN1CCN(CC1)C)C1=NC=CC=C1 UKPPOPPQQBENOU-UHFFFAOYSA-N 0.000 claims description 4
- RQFDXVWIASFERM-UHFFFAOYSA-N 6-bromo-N-[3-(5-methoxypyridin-2-yl)-1-methylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound BrC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=C(C=C1)OC RQFDXVWIASFERM-UHFFFAOYSA-N 0.000 claims description 4
- 208000024172 Cardiovascular disease Diseases 0.000 claims description 4
- 208000034486 Multi-organ failure Diseases 0.000 claims description 4
- MHUWGNVVRLOSPS-UHFFFAOYSA-N N-(1-methyl-3-pyrazin-2-ylpyrazol-4-yl)-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CN=C1 MHUWGNVVRLOSPS-UHFFFAOYSA-N 0.000 claims description 4
- CFDHPFXYWBVRLU-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1,2,3,6-tetrahydropyridin-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1CCNCC=1)C1=NC=CC=C1 CFDHPFXYWBVRLU-UHFFFAOYSA-N 0.000 claims description 4
- YXOPVLBBHKEGFU-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1,2,3,6-tetrahydropyridin-5-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1CNCCC=1)C1=NC=CC=C1 YXOPVLBBHKEGFU-UHFFFAOYSA-N 0.000 claims description 4
- CDUFDFWBJIXZOV-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=CC=C1 CDUFDFWBJIXZOV-UHFFFAOYSA-N 0.000 claims description 4
- BGAHUBMKTIMLKQ-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(2-oxo-1H-pyridin-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC(NC=C1)=O)C1=NC=CC=C1 BGAHUBMKTIMLKQ-UHFFFAOYSA-N 0.000 claims description 4
- WMFRKNLZJADXLW-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(5-morpholin-4-ylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)N1CCOCC1)C1=NC=CC=C1 WMFRKNLZJADXLW-UHFFFAOYSA-N 0.000 claims description 4
- GXWWBENTRFKXOS-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(5-pyrrolidin-1-ylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)N1CCCC1)C1=NC=CC=C1 GXWWBENTRFKXOS-UHFFFAOYSA-N 0.000 claims description 4
- DGRGTRRSRUZYRX-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-[5-(2,2,2-trifluoroethoxy)pyridin-3-yl]pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)OCC(F)(F)F)C1=NC=CC=C1 DGRGTRRSRUZYRX-UHFFFAOYSA-N 0.000 claims description 4
- ZIHZRMOLAJFLSX-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-[5-(2,2,2-trifluoroethylamino)pyridin-3-yl]pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)NCC(F)(F)F)C1=NC=CC=C1 ZIHZRMOLAJFLSX-UHFFFAOYSA-N 0.000 claims description 4
- KRRSTIHOJJTDKU-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-[5-(oxan-4-ylamino)pyridin-3-yl]pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)NC1CCOCC1)C1=NC=CC=C1 KRRSTIHOJJTDKU-UHFFFAOYSA-N 0.000 claims description 4
- ZVDUZAXLVFBSHL-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-[5-(oxan-4-yloxy)pyridin-3-yl]pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)OC1CCOCC1)C1=NC=CC=C1 ZVDUZAXLVFBSHL-UHFFFAOYSA-N 0.000 claims description 4
- BVBJYALXDVUQHA-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-[5-(propan-2-ylamino)pyridin-3-yl]pyridine-2-carboxamide Chemical compound C(C)(C)NC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 BVBJYALXDVUQHA-UHFFFAOYSA-N 0.000 claims description 4
- ILCNCQAQXDIAKW-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-phenylpyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=CC=CC=C1)=O)C1=NC=CC=C1 ILCNCQAQXDIAKW-UHFFFAOYSA-N 0.000 claims description 4
- JRSQSDAMAOLHLG-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-piperidin-4-ylpyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1CCNCC1)=O)C1=NC=CC=C1 JRSQSDAMAOLHLG-UHFFFAOYSA-N 0.000 claims description 4
- NHUYDUPUQWCSDI-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-pyridin-3-ylpyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=CC=1)C1=NC=CC=C1 NHUYDUPUQWCSDI-UHFFFAOYSA-N 0.000 claims description 4
- JHCWNSSQHCMTOF-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-pyrimidin-5-ylpyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NC=NC=1)=O)C1=NC=CC=C1 JHCWNSSQHCMTOF-UHFFFAOYSA-N 0.000 claims description 4
- SSVWXXYELFPARR-UHFFFAOYSA-N N-(1-propan-2-yl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound C(C)(C)N1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=CC=C1 SSVWXXYELFPARR-UHFFFAOYSA-N 0.000 claims description 4
- SJLWTKYOGKDEKK-UHFFFAOYSA-N N-[1-(2-morpholin-4-ylethyl)-3-pyridin-2-ylpyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound O1CCN(CC1)CCN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=CC=C1 SJLWTKYOGKDEKK-UHFFFAOYSA-N 0.000 claims description 4
- HRIXYHGQUSEYGX-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-[[6-(4-methylpiperazin-1-yl)-5-(trifluoromethyl)pyridin-3-yl]amino]pyridine-2-carboxamide Chemical compound COCCOCCN1N=C(C(=C1)NC(C1=NC(=CC=C1)NC=1C=NC(=C(C=1)C(F)(F)F)N1CCN(CC1)C)=O)C1=NC=CC=C1 HRIXYHGQUSEYGX-UHFFFAOYSA-N 0.000 claims description 4
- WNMNUJCKTZQBRI-UHFFFAOYSA-N N-[1-[2-(4-methylpiperazin-1-yl)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)CCN1CCN(CC1)C)C1=NC=CC=C1 WNMNUJCKTZQBRI-UHFFFAOYSA-N 0.000 claims description 4
- USSHPGMPPJPPMG-UHFFFAOYSA-N N-[3-(5-methoxypyridin-2-yl)-1-methylpyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound COC=1C=CC(=NC=1)C1=NN(C=C1NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C USSHPGMPPJPPMG-UHFFFAOYSA-N 0.000 claims description 4
- XTJATVFXVUWXHM-UHFFFAOYSA-N N-[3-(5-methoxypyridin-2-yl)-1-methylpyrazol-4-yl]-6-(1H-pyrazol-5-yl)pyridine-2-carboxamide Chemical compound COC=1C=CC(=NC=1)C1=NN(C=C1NC(C1=NC(=CC=C1)C1=CC=NN1)=O)C XTJATVFXVUWXHM-UHFFFAOYSA-N 0.000 claims description 4
- 210000003743 erythrocyte Anatomy 0.000 claims description 4
- 230000003832 immune regulation Effects 0.000 claims description 4
- 208000027866 inflammatory disease Diseases 0.000 claims description 4
- 208000017169 kidney disease Diseases 0.000 claims description 4
- 208000029744 multiple organ dysfunction syndrome Diseases 0.000 claims description 4
- 208000015122 neurodegenerative disease Diseases 0.000 claims description 4
- 230000019100 sperm motility Effects 0.000 claims description 4
- 208000010110 spontaneous platelet aggregation Diseases 0.000 claims description 4
- FEXNXLHQERMJCY-UHFFFAOYSA-N 6-(2-methoxypyridin-4-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound COC1=NC=CC(=C1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 FEXNXLHQERMJCY-UHFFFAOYSA-N 0.000 claims description 3
- VNANXNCMWFAFIP-UHFFFAOYSA-N 6-(3-fluorophenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound FC=1C=C(C=CC=1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 VNANXNCMWFAFIP-UHFFFAOYSA-N 0.000 claims description 3
- CTGOYKGXHHKJGU-UHFFFAOYSA-N 6-(3-fluoropyridin-4-yl)-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound FC=1C=NC=CC=1C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 CTGOYKGXHHKJGU-UHFFFAOYSA-N 0.000 claims description 3
- LUFQILDTORCFBE-UHFFFAOYSA-N 6-(4-cyanophenyl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C(#N)C1=CC=C(C=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 LUFQILDTORCFBE-UHFFFAOYSA-N 0.000 claims description 3
- MAGPVJYVFDLJOB-UHFFFAOYSA-N 6-(5-methoxypyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound COC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 MAGPVJYVFDLJOB-UHFFFAOYSA-N 0.000 claims description 3
- APUJOSAJKMFDLV-UHFFFAOYSA-N 6-(5-methylpyridin-3-yl)-N-[1-(2-morpholin-4-ylethyl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)CCN1CCOCC1)C1=NC=CC=C1 APUJOSAJKMFDLV-UHFFFAOYSA-N 0.000 claims description 3
- IOKFOAHIJOZAJT-UHFFFAOYSA-N 6-(6-acetamidopyridin-3-yl)-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound C(C)(=O)NC1=CC=C(C=N1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 IOKFOAHIJOZAJT-UHFFFAOYSA-N 0.000 claims description 3
- AHEMVKIGMLDUTR-UHFFFAOYSA-N 6-(6-aminopyridin-3-yl)-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound NC1=CC=C(C=N1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 AHEMVKIGMLDUTR-UHFFFAOYSA-N 0.000 claims description 3
- CYSXLQNEOGZKEW-UHFFFAOYSA-N 6-[1-(3-chlorophenyl)pyrazol-4-yl]-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound ClC=1C=C(C=CC=1)N1N=CC(=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 CYSXLQNEOGZKEW-UHFFFAOYSA-N 0.000 claims description 3
- VRZKKXDSQFOSPF-UHFFFAOYSA-N 6-[5-(cyclopropylmethylamino)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C1(CC1)CNC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 VRZKKXDSQFOSPF-UHFFFAOYSA-N 0.000 claims description 3
- QSHZGNXMEVFJQT-UHFFFAOYSA-N 6-[5-(dimethylamino)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN(C=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1)C QSHZGNXMEVFJQT-UHFFFAOYSA-N 0.000 claims description 3
- FWXAXFVJPJGLFG-UHFFFAOYSA-N 6-[5-(methanesulfonamido)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)NS(=O)(=O)C)C1=NC=CC=C1 FWXAXFVJPJGLFG-UHFFFAOYSA-N 0.000 claims description 3
- HHJXVVMBYBZUEV-UHFFFAOYSA-N 6-[5-(methylamino)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)NC)C1=NC=CC=C1 HHJXVVMBYBZUEV-UHFFFAOYSA-N 0.000 claims description 3
- QIXDTJIVVDJWOJ-UHFFFAOYSA-N 6-[5-(tert-butylamino)pyridin-3-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C(C)(C)(C)NC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 QIXDTJIVVDJWOJ-UHFFFAOYSA-N 0.000 claims description 3
- 208000004852 Lung Injury Diseases 0.000 claims description 3
- FDAPCQUJJXNQPB-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrrolo[2,3-b]pyridin-5-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=C2C(=NC=1)NC=C2)=O)C1=NC=CC=C1 FDAPCQUJJXNQPB-UHFFFAOYSA-N 0.000 claims description 3
- GKCZAXMLCIERKT-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrrolo[3,2-b]pyridin-6-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=C2C(=NC=1)C=CN2)=O)C1=NC=CC=C1 GKCZAXMLCIERKT-UHFFFAOYSA-N 0.000 claims description 3
- KQOMLDVKMOWBDP-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(5-methylsulfonylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)S(=O)(=O)C)C1=NC=CC=C1 KQOMLDVKMOWBDP-UHFFFAOYSA-N 0.000 claims description 3
- UPRRTHKTIIJXMQ-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(5-propan-2-yloxypyridin-3-yl)pyridine-2-carboxamide Chemical compound C(C)(C)OC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 UPRRTHKTIIJXMQ-UHFFFAOYSA-N 0.000 claims description 3
- HFNKGFWFCBGWMH-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(6-oxo-1H-pyridin-3-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CNC(C=C1)=O)C1=NC=CC=C1 HFNKGFWFCBGWMH-UHFFFAOYSA-N 0.000 claims description 3
- IDWDKTFTGKZLIO-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-[2-(2,2,2-trifluoroethylamino)pyridin-4-yl]pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC(=NC=C1)NCC(F)(F)F)C1=NC=CC=C1 IDWDKTFTGKZLIO-UHFFFAOYSA-N 0.000 claims description 3
- HOCZSWHZJFOILY-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-[2-(trifluoromethyl)pyridin-4-yl]pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC(=NC=C1)C(F)(F)F)C1=NC=CC=C1 HOCZSWHZJFOILY-UHFFFAOYSA-N 0.000 claims description 3
- FGWMUEVZWWWDOC-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-[5-(trifluoromethyl)pyridin-3-yl]pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C(F)(F)F)C1=NC=CC=C1 FGWMUEVZWWWDOC-UHFFFAOYSA-N 0.000 claims description 3
- PUPPPIPMWLLAKT-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-(2-methylpyridin-4-yl)pyridine-2-carboxamide Chemical compound COCCOCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC(=NC=C1)C)C1=NC=CC=C1 PUPPPIPMWLLAKT-UHFFFAOYSA-N 0.000 claims description 3
- HRLCWWYONSERCF-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-(3-methylpyridin-4-yl)pyridine-2-carboxamide Chemical compound COCCOCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=C(C=NC=C1)C)C1=NC=CC=C1 HRLCWWYONSERCF-UHFFFAOYSA-N 0.000 claims description 3
- OXDCUKPMHVQPPG-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound COCCOCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C)C1=NC=CC=C1 OXDCUKPMHVQPPG-UHFFFAOYSA-N 0.000 claims description 3
- AWUCYIHTUQYADB-UHFFFAOYSA-N N-[3-(5-methoxypyridin-2-yl)-1-methylpyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound COC=1C=CC(=NC=1)C1=NN(C=C1NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C)C AWUCYIHTUQYADB-UHFFFAOYSA-N 0.000 claims description 3
- VPVRRABFOQYLLW-UHFFFAOYSA-N N-[3-(5-methoxypyridin-2-yl)-1-methylpyrazol-4-yl]-6-(5-propan-2-yloxypyridin-3-yl)pyridine-2-carboxamide Chemical compound C(C)(C)OC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=C(C=C1)OC VPVRRABFOQYLLW-UHFFFAOYSA-N 0.000 claims description 3
- CRYBNBNPSYBZHG-UHFFFAOYSA-N N-[3-(5-methoxypyridin-2-yl)-1-methylpyrazol-4-yl]-6-[5-(propan-2-ylamino)pyridin-3-yl]pyridine-2-carboxamide Chemical compound C(C)(C)NC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=C(C=C1)OC CRYBNBNPSYBZHG-UHFFFAOYSA-N 0.000 claims description 3
- 206010069363 Traumatic lung injury Diseases 0.000 claims description 3
- 150000003857 carboxamides Chemical class 0.000 claims description 3
- 231100000515 lung injury Toxicity 0.000 claims description 3
- PZERTJJBSGHBDT-UHFFFAOYSA-N 4-(5-methylpyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyrimidine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=NC=CC(=N1)C=1C=NC=C(C=1)C)C1=NC=CC=C1 PZERTJJBSGHBDT-UHFFFAOYSA-N 0.000 claims description 2
- IJXZPRZXGWHTOW-UHFFFAOYSA-N 5-[1-(cyclopropylmethyl)pyrazol-4-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-3-carboxamide Chemical compound C1(CC1)CN1N=CC(=C1)C=1C=NC=C(C(=O)NC=2C(=NN(C=2)C)C2=NC=CC=C2)C=1 IJXZPRZXGWHTOW-UHFFFAOYSA-N 0.000 claims description 2
- VMWANCHMURDXEZ-UHFFFAOYSA-N 6-(2-aminopyridin-4-yl)oxy-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound NC1=NC=CC(=C1)OC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 VMWANCHMURDXEZ-UHFFFAOYSA-N 0.000 claims description 2
- SYDDUVPHHRKPDS-UHFFFAOYSA-N 6-(2-cyanopyridin-4-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C(#N)C1=NC=CC(=C1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 SYDDUVPHHRKPDS-UHFFFAOYSA-N 0.000 claims description 2
- KNKKUSAYDDZVCV-UHFFFAOYSA-N 6-(2-methoxypyridin-4-yl)-N-(1-methyl-3-pyrazin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound COC1=NC=CC(=C1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CN=C1 KNKKUSAYDDZVCV-UHFFFAOYSA-N 0.000 claims description 2
- INCSVMCMRPKSKB-UHFFFAOYSA-N 6-(5-carbamoylpyridin-3-yl)-N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound O1CC(C1)N1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C(=O)N)C1=NC=CC=C1 INCSVMCMRPKSKB-UHFFFAOYSA-N 0.000 claims description 2
- FJSYAJRSQGIJQE-UHFFFAOYSA-N 6-(5-cyanopyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C(#N)C=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 FJSYAJRSQGIJQE-UHFFFAOYSA-N 0.000 claims description 2
- RKLYDUQONRJJOO-UHFFFAOYSA-N 6-(5-fluoropyridin-3-yl)-N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound FC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C1COC1)C1=NC=CC=C1 RKLYDUQONRJJOO-UHFFFAOYSA-N 0.000 claims description 2
- JONCVOGCNFSUSX-UHFFFAOYSA-N 6-(5-methylpyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyrazine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=NC(=CN=C1)C=1C=NC=C(C=1)C)C1=NC=CC=C1 JONCVOGCNFSUSX-UHFFFAOYSA-N 0.000 claims description 2
- WBGXDYKTCIQOCS-UHFFFAOYSA-N 6-(5-methylpyridin-3-yl)-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 WBGXDYKTCIQOCS-UHFFFAOYSA-N 0.000 claims description 2
- HYAOKGQVLFOURI-UHFFFAOYSA-N 6-(5-methylpyridin-3-yl)-N-[1-(oxan-4-yl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C1CCOCC1)C1=NC=CC=C1 HYAOKGQVLFOURI-UHFFFAOYSA-N 0.000 claims description 2
- FTBVODWLCFBWJG-UHFFFAOYSA-N 6-(5-methylpyridin-3-yl)-N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C1COC1)C1=NC=CC=C1 FTBVODWLCFBWJG-UHFFFAOYSA-N 0.000 claims description 2
- UTFIYNDXTDJFSS-UHFFFAOYSA-N 6-(6-acetamidopyridin-3-yl)-N-(1-methyl-3-pyrazin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C(C)(=O)NC1=CC=C(C=N1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CN=C1 UTFIYNDXTDJFSS-UHFFFAOYSA-N 0.000 claims description 2
- IGCHUSNDZRCYRI-UHFFFAOYSA-N 6-(furan-3-yl)-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound O1C=C(C=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 IGCHUSNDZRCYRI-UHFFFAOYSA-N 0.000 claims description 2
- GUGVGJRMVSPOQY-UHFFFAOYSA-N 6-[(2,5-dimethylpyridin-4-yl)amino]-N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound CC1=NC=C(C(=C1)NC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1)C GUGVGJRMVSPOQY-UHFFFAOYSA-N 0.000 claims description 2
- DVXJDONJRNPQFV-UHFFFAOYSA-N 6-[2-(cyclopropylmethylamino)pyridin-4-yl]-N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound C1(CC1)CNC1=NC=CC(=C1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 DVXJDONJRNPQFV-UHFFFAOYSA-N 0.000 claims description 2
- KGKPLIRQEVCSOW-UHFFFAOYSA-N 6-[5-(methanesulfonamido)pyridin-3-yl]-N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound CS(=O)(=O)NC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C1COC1)C1=NC=CC=C1 KGKPLIRQEVCSOW-UHFFFAOYSA-N 0.000 claims description 2
- ZYWAAFNAJJOPHJ-UHFFFAOYSA-N 6-bromo-N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound BrC1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C1COC1)C1=NC=CC=C1 ZYWAAFNAJJOPHJ-UHFFFAOYSA-N 0.000 claims description 2
- XMEVRHDZSJABSC-UHFFFAOYSA-N 6-pyridin-4-yl-N-(5-pyridin-2-yl-1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound N1=C(C=CC=C1)C1=NNC=C1NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1 XMEVRHDZSJABSC-UHFFFAOYSA-N 0.000 claims description 2
- XPMAFQKCWLYVBM-UHFFFAOYSA-N C(=O)O.COC1CC(C1)N1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC1)=O)C1=NC=CC=C1 Chemical compound C(=O)O.COC1CC(C1)N1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC1)=O)C1=NC=CC=C1 XPMAFQKCWLYVBM-UHFFFAOYSA-N 0.000 claims description 2
- WQQDCXIRWZWRFQ-UHFFFAOYSA-N C(=O)O.COCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 Chemical compound C(=O)O.COCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 WQQDCXIRWZWRFQ-UHFFFAOYSA-N 0.000 claims description 2
- VBEZZALLHDTQPY-UHFFFAOYSA-N C(=O)O.N1=C(C=CC=C1)C1=NNC=C1NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1 Chemical compound C(=O)O.N1=C(C=CC=C1)C1=NNC=C1NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1 VBEZZALLHDTQPY-UHFFFAOYSA-N 0.000 claims description 2
- DHCKHPSJTFRTSS-UHFFFAOYSA-N C(=O)O.OCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 Chemical compound C(=O)O.OCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 DHCKHPSJTFRTSS-UHFFFAOYSA-N 0.000 claims description 2
- UAJQPPLYZNGPHW-UHFFFAOYSA-N FC(C(=O)O)(F)F.CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 Chemical compound FC(C(=O)O)(F)F.CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 UAJQPPLYZNGPHW-UHFFFAOYSA-N 0.000 claims description 2
- 208000010718 Multiple Organ Failure Diseases 0.000 claims description 2
- AUUNLBJDFMBHLY-UHFFFAOYSA-N N-(1-methyl-3-pyrazin-2-ylpyrazol-4-yl)-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=CN=C1 AUUNLBJDFMBHLY-UHFFFAOYSA-N 0.000 claims description 2
- SUJSKUINUBXEPX-UHFFFAOYSA-N N-(1-methyl-3-pyrazin-2-ylpyrazol-4-yl)-6-(2-phenylmethoxypyridin-4-yl)pyridine-2-carboxamide Chemical compound C(C1=CC=CC=C1)OC1=NC=CC(=C1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CN=C1 SUJSKUINUBXEPX-UHFFFAOYSA-N 0.000 claims description 2
- KBDHZSTYCGWVDX-UHFFFAOYSA-N N-(1-methyl-3-pyrazin-2-ylpyrazol-4-yl)-6-pyridin-3-ylpyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=CC=1)C1=NC=CN=C1 KBDHZSTYCGWVDX-UHFFFAOYSA-N 0.000 claims description 2
- SDNHMPJDNHXGJS-UHFFFAOYSA-N N-(1-methyl-3-pyrazin-2-ylpyrazol-4-yl)-6-pyridin-4-ylpyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CN=C1 SDNHMPJDNHXGJS-UHFFFAOYSA-N 0.000 claims description 2
- CUXOCWJQNNLPBW-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-2-(1H-pyrazol-4-yl)pyrimidine-4-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=NC(=NC=C1)C=1C=NNC=1)C1=NC=CC=C1 CUXOCWJQNNLPBW-UHFFFAOYSA-N 0.000 claims description 2
- QLXFNWSHNIKICE-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-2-(1H-pyrazol-5-yl)pyrimidine-4-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=NC(=NC=C1)C1=NNC=C1)C1=NC=CC=C1 QLXFNWSHNIKICE-UHFFFAOYSA-N 0.000 claims description 2
- CZLAYOUZKKJWEM-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-4-(1H-pyrazol-5-ylamino)pyrimidine-2-carboxamide Chemical compound N1N=C(C=C1)NC1=NC(=NC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=CC=C1 CZLAYOUZKKJWEM-UHFFFAOYSA-N 0.000 claims description 2
- NVDXRJSTBVRZEH-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-4-[(1-methyl-3-pyridin-2-ylpyrazol-4-yl)amino]pyrimidine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=NC=CC(=N1)NC=1C(=NN(C=1)C)C1=NC=CC=C1)C1=NC=CC=C1 NVDXRJSTBVRZEH-UHFFFAOYSA-N 0.000 claims description 2
- NGISUHLZZCSBNH-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-5-pyridin-3-ylpyridine-3-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C=1C=C(C=NC=1)C=1C=NC=CC=1)C1=NC=CC=C1 NGISUHLZZCSBNH-UHFFFAOYSA-N 0.000 claims description 2
- VKEQHWSTCUTOMC-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-5-pyridin-4-ylpyridine-3-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C=1C=C(C=NC=1)C1=CC=NC=C1)C1=NC=CC=C1 VKEQHWSTCUTOMC-UHFFFAOYSA-N 0.000 claims description 2
- KLFINWWQKBJLBL-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrazol-4-yl)pyrazine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=NC(=CN=C1)C=1C=NNC=1)C1=NC=CC=C1 KLFINWWQKBJLBL-UHFFFAOYSA-N 0.000 claims description 2
- QBFMYJJUPUMKPO-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-pyridin-4-ylpyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 QBFMYJJUPUMKPO-UHFFFAOYSA-N 0.000 claims description 2
- DEOBVQTYUKPGBQ-UHFFFAOYSA-N N-[1-(1,3-dihydroxypropan-2-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound OCC(CO)N1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=CC=C1 DEOBVQTYUKPGBQ-UHFFFAOYSA-N 0.000 claims description 2
- CIBVHEMUCJEOHD-UHFFFAOYSA-N N-[1-(1,3-dihydroxypropan-2-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound OCC(CO)N1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C)C1=NC=CC=C1 CIBVHEMUCJEOHD-UHFFFAOYSA-N 0.000 claims description 2
- JQOLZWMZPQNPDV-UHFFFAOYSA-N N-[1-(1,3-dihydroxypropan-2-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-pyridin-3-ylpyridine-2-carboxamide Chemical compound OCC(CO)N1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=CC=1)C1=NC=CC=C1 JQOLZWMZPQNPDV-UHFFFAOYSA-N 0.000 claims description 2
- JATYFSUURANRGU-UHFFFAOYSA-N N-[1-(2-hydroxyethyl)-3-pyridin-2-ylpyrazol-4-yl]-6-pyridin-4-ylpyridine-2-carboxamide Chemical compound OCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 JATYFSUURANRGU-UHFFFAOYSA-N 0.000 claims description 2
- PMTKMCYZRJKWRW-UHFFFAOYSA-N N-[1-(2-methoxyethyl)-3-pyridin-2-ylpyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound COCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C)C1=NC=CC=C1 PMTKMCYZRJKWRW-UHFFFAOYSA-N 0.000 claims description 2
- ZQBWZEWRBKGYPX-UHFFFAOYSA-N N-[1-(2-methoxyethyl)-3-pyridin-2-ylpyrazol-4-yl]-6-pyridin-4-ylpyridine-2-carboxamide Chemical compound COCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 ZQBWZEWRBKGYPX-UHFFFAOYSA-N 0.000 claims description 2
- NWWNOUYPLRRQOM-UHFFFAOYSA-N N-[1-(3-methoxycyclobutyl)-3-pyridin-2-ylpyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound COC1CC(C1)N1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=CC=C1 NWWNOUYPLRRQOM-UHFFFAOYSA-N 0.000 claims description 2
- KKRQEHOOKMNSKE-UHFFFAOYSA-N N-[1-(3-methoxycyclobutyl)-3-pyridin-2-ylpyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound COC1CC(C1)N1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C)C1=NC=CC=C1 KKRQEHOOKMNSKE-UHFFFAOYSA-N 0.000 claims description 2
- PJBHQFFWMDZEQB-UHFFFAOYSA-N N-[1-(3-methoxypropyl)-3-pyridin-2-ylpyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound COCCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C)C1=NC=CC=C1 PJBHQFFWMDZEQB-UHFFFAOYSA-N 0.000 claims description 2
- CTERVWNHIIUHHX-UHFFFAOYSA-N N-[1-(oxan-4-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-pyridin-4-ylpyridine-2-carboxamide Chemical compound N1=C(C=CC=C1)C1=NN(C=C1NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1CCOCC1 CTERVWNHIIUHHX-UHFFFAOYSA-N 0.000 claims description 2
- YPZLKMTXXWBDKG-UHFFFAOYSA-N N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound O1CC(C1)N1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=CC=C1 YPZLKMTXXWBDKG-UHFFFAOYSA-N 0.000 claims description 2
- IIDSJDWCARFIHF-UHFFFAOYSA-N N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-(1H-pyrazol-5-yl)pyridine-2-carboxamide Chemical compound O1CC(C1)N1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=NNC=C1)=O)C1=NC=CC=C1 IIDSJDWCARFIHF-UHFFFAOYSA-N 0.000 claims description 2
- OZCZXEHAMAZRCZ-UHFFFAOYSA-N N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-(5-propan-2-yloxypyridin-3-yl)pyridine-2-carboxamide Chemical compound C(C)(C)OC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C1COC1)C1=NC=CC=C1 OZCZXEHAMAZRCZ-UHFFFAOYSA-N 0.000 claims description 2
- RUEBCRLIQPJMHA-UHFFFAOYSA-N N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-[5-(trifluoromethyl)pyridin-3-yl]pyridine-2-carboxamide Chemical compound O1CC(C1)N1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C(F)(F)F)C1=NC=CC=C1 RUEBCRLIQPJMHA-UHFFFAOYSA-N 0.000 claims description 2
- QIVWPDQIUOITEN-UHFFFAOYSA-N N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]-6-pyridin-3-ylpyridine-2-carboxamide Chemical compound O1CC(C1)N1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=CC=1)C1=NC=CC=C1 QIVWPDQIUOITEN-UHFFFAOYSA-N 0.000 claims description 2
- WUIVZVMEHJPHPB-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-(1H-pyrazol-5-yl)pyridine-2-carboxamide Chemical compound COCCOCCN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=NNC=C1)=O)C1=NC=CC=C1 WUIVZVMEHJPHPB-UHFFFAOYSA-N 0.000 claims description 2
- LKNSSFXQHNJHKA-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-(2-methoxypyridin-4-yl)pyridine-2-carboxamide Chemical compound COC1=NC=CC(=C1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 LKNSSFXQHNJHKA-UHFFFAOYSA-N 0.000 claims description 2
- NRTKCYMKHIXADV-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-(6-methoxy-1H-indol-2-yl)pyridine-2-carboxamide Chemical compound COC1=CC=C2C=C(NC2=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)CCOCCOC)C1=NC=CC=C1 NRTKCYMKHIXADV-UHFFFAOYSA-N 0.000 claims description 2
- YXTCYPXNBZGFOT-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-pyridin-4-yloxypyridine-2-carboxamide Chemical compound COCCOCCN1N=C(C(=C1)NC(C1=NC(=CC=C1)OC1=CC=NC=C1)=O)C1=NC=CC=C1 YXTCYPXNBZGFOT-UHFFFAOYSA-N 0.000 claims description 2
- RQWIEKGLBXIJNP-UHFFFAOYSA-N N-[1-[2-(2-methoxyethoxy)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-pyridin-4-ylpyridine-2-carboxamide Chemical compound COCCOCCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1 RQWIEKGLBXIJNP-UHFFFAOYSA-N 0.000 claims description 2
- LETCYIQUOFVQDF-UHFFFAOYSA-N N-[1-[2-(4-methylpiperazin-1-yl)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1CCN(CC1)CCN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=CC=C1 LETCYIQUOFVQDF-UHFFFAOYSA-N 0.000 claims description 2
- DGLFAPYNKMSWNP-UHFFFAOYSA-N N-[1-[2-(diethylamino)ethyl]-3-pyridin-2-ylpyrazol-4-yl]-6-pyridin-4-ylpyridine-2-carboxamide Chemical compound C(C)N(CCN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C1=CC=NC=C1)C1=NC=CC=C1)CC DGLFAPYNKMSWNP-UHFFFAOYSA-N 0.000 claims description 2
- YHERGZIQGARUMZ-UHFFFAOYSA-N N-[1-methyl-3-(5-morpholin-4-ylpyridin-2-yl)pyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=C(C=C1)N1CCOCC1 YHERGZIQGARUMZ-UHFFFAOYSA-N 0.000 claims description 2
- AAVVDPLIXQKZJV-UHFFFAOYSA-N N-[1-methyl-3-(5-morpholin-4-ylpyridin-2-yl)pyrazol-4-yl]-6-(1H-pyrazol-5-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=NNC=C1)=O)C1=NC=C(C=C1)N1CCOCC1 AAVVDPLIXQKZJV-UHFFFAOYSA-N 0.000 claims description 2
- GEIWKWHHXMOABF-UHFFFAOYSA-N N-[1-methyl-3-(5-morpholin-4-ylpyridin-2-yl)pyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=C(C=C1)N1CCOCC1 GEIWKWHHXMOABF-UHFFFAOYSA-N 0.000 claims description 2
- QBCQBRJSPPFQGU-UHFFFAOYSA-N N-[1-methyl-3-[5-(4-methylpiperazin-1-yl)pyridin-2-yl]pyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=C(C=C1)N1CCN(CC1)C QBCQBRJSPPFQGU-UHFFFAOYSA-N 0.000 claims description 2
- RZBGMCDBMBLVDA-UHFFFAOYSA-N N-[1-methyl-3-[5-(4-methylpiperazin-1-yl)pyridin-2-yl]pyrazol-4-yl]-6-(1H-pyrazol-5-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=NNC=C1)=O)C1=NC=C(C=C1)N1CCN(CC1)C RZBGMCDBMBLVDA-UHFFFAOYSA-N 0.000 claims description 2
- LOPFFNPXPYOFLA-UHFFFAOYSA-N N-[1-methyl-3-[5-(4-methylpiperazin-1-yl)pyridin-2-yl]pyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=C(C=C1)N1CCN(CC1)C LOPFFNPXPYOFLA-UHFFFAOYSA-N 0.000 claims description 2
- PNWGNXDYGATMTJ-UHFFFAOYSA-N N-[1-methyl-3-[5-(oxetan-3-yloxy)pyridin-2-yl]pyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)C1=NC=C(C=C1)OC1COC1 PNWGNXDYGATMTJ-UHFFFAOYSA-N 0.000 claims description 2
- DWCZNRSYDLCZFT-UHFFFAOYSA-N N-[3-[5-(2-hydroxy-2-methylpropoxy)pyridin-2-yl]-1-methylpyrazol-4-yl]-6-(1H-pyrazol-5-yl)pyridine-2-carboxamide Chemical compound OC(COC=1C=CC(=NC=1)C1=NN(C=C1NC(C1=NC(=CC=C1)C1=NNC=C1)=O)C)(C)C DWCZNRSYDLCZFT-UHFFFAOYSA-N 0.000 claims description 2
- GEMNJLQVOOGTOU-UHFFFAOYSA-N N-[3-[5-(2-hydroxy-2-methylpropoxy)pyridin-2-yl]-1-methylpyrazol-4-yl]-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound OC(COC=1C=CC(=NC=1)C1=NN(C=C1NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)C)C)(C)C GEMNJLQVOOGTOU-UHFFFAOYSA-N 0.000 claims description 2
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 claims description 2
- FUXJMHXHGDAHPD-UHFFFAOYSA-N pyrimidine-2-carboxamide Chemical compound NC(=O)C1=NC=CC=N1 FUXJMHXHGDAHPD-UHFFFAOYSA-N 0.000 claims description 2
- 101710199015 Interleukin-1 receptor-associated kinase 1 Proteins 0.000 claims 3
- MAPIAZXXQVPWLA-UHFFFAOYSA-N C(=O)O.C(N)(=O)C1=NC=CC=C1 Chemical compound C(=O)O.C(N)(=O)C1=NC=CC=C1 MAPIAZXXQVPWLA-UHFFFAOYSA-N 0.000 claims 1
- OECLKIBSCKPXER-UHFFFAOYSA-N N-(1-methyl-3-pyrazin-2-ylpyrazol-4-yl)-6-[5-(2,2,2-trifluoroethoxy)pyridin-3-yl]pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=CC=CC(=N1)C=1C=NC=C(C=1)OCC(F)(F)F)C1=NC=CN=C1 OECLKIBSCKPXER-UHFFFAOYSA-N 0.000 claims 1
- PLDQTSUDAYSAJH-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-4-(1H-pyrazol-4-yl)pyrimidine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=NC=CC(=N1)C=1C=NNC=1)C1=NC=CC=C1 PLDQTSUDAYSAJH-UHFFFAOYSA-N 0.000 claims 1
- VBHOYVGKKKACQX-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrazol-5-yl)pyrazine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(=O)C1=NC(=CN=C1)C1=NNC=C1)C1=NC=CC=C1 VBHOYVGKKKACQX-UHFFFAOYSA-N 0.000 claims 1
- PJPAZQZYSCBCII-UHFFFAOYSA-N N-[1-methyl-3-[5-(oxetan-3-yloxy)pyridin-2-yl]pyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C1=NC=C(C=C1)OC1COC1 PJPAZQZYSCBCII-UHFFFAOYSA-N 0.000 claims 1
- USNJNLBTPOYYAD-UHFFFAOYSA-N N-[1-methyl-3-[5-(oxetan-3-yloxy)pyridin-2-yl]pyrazol-4-yl]-6-(1H-pyrazol-5-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=NNC=C1)=O)C1=NC=C(C=C1)OC1COC1 USNJNLBTPOYYAD-UHFFFAOYSA-N 0.000 claims 1
- DGBILAKHPMOPTD-UHFFFAOYSA-N N-[3-[5-(2-hydroxy-2-methylpropoxy)pyridin-2-yl]-1-methylpyrazol-4-yl]-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound OC(COC=1C=CC(=NC=1)C1=NN(C=C1NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)C)(C)C DGBILAKHPMOPTD-UHFFFAOYSA-N 0.000 claims 1
- 108091000080 Phosphotransferase Proteins 0.000 abstract description 4
- 102000020233 phosphotransferase Human genes 0.000 abstract description 4
- 102000002467 interleukin receptors Human genes 0.000 abstract description 2
- 108010093036 interleukin receptors Proteins 0.000 abstract description 2
- 238000005160 1H NMR spectroscopy Methods 0.000 description 98
- 238000006243 chemical reaction Methods 0.000 description 98
- 239000002904 solvent Substances 0.000 description 80
- 239000000243 solution Substances 0.000 description 53
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 52
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 41
- 208000006673 asthma Diseases 0.000 description 39
- 125000001424 substituent group Chemical group 0.000 description 34
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 33
- 239000007787 solid Substances 0.000 description 32
- 229910001868 water Inorganic materials 0.000 description 32
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 30
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 29
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 28
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 27
- 239000002585 base Substances 0.000 description 27
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 25
- 239000000047 product Substances 0.000 description 25
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 24
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 23
- 239000003814 drug Substances 0.000 description 23
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 22
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 22
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 21
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 21
- 239000003153 chemical reaction reagent Substances 0.000 description 20
- 238000004587 chromatography analysis Methods 0.000 description 20
- 125000004122 cyclic group Chemical group 0.000 description 20
- 239000011541 reaction mixture Substances 0.000 description 20
- 125000004432 carbon atom Chemical group C* 0.000 description 19
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 18
- 238000011282 treatment Methods 0.000 description 18
- 210000004027 cell Anatomy 0.000 description 17
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 16
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 16
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 15
- 239000003054 catalyst Substances 0.000 description 15
- 239000003638 chemical reducing agent Substances 0.000 description 15
- 206010039073 rheumatoid arthritis Diseases 0.000 description 15
- 239000000741 silica gel Substances 0.000 description 15
- 229910002027 silica gel Inorganic materials 0.000 description 15
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 14
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 14
- 239000003795 chemical substances by application Substances 0.000 description 14
- 235000019439 ethyl acetate Nutrition 0.000 description 14
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 14
- 108090000623 proteins and genes Proteins 0.000 description 14
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 13
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 13
- 238000005481 NMR spectroscopy Methods 0.000 description 13
- 150000002148 esters Chemical class 0.000 description 13
- 235000019441 ethanol Nutrition 0.000 description 13
- 238000009472 formulation Methods 0.000 description 13
- 235000018102 proteins Nutrition 0.000 description 13
- 102000004169 proteins and genes Human genes 0.000 description 13
- 238000001665 trituration Methods 0.000 description 13
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 12
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 12
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 12
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 12
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 12
- 229910052757 nitrogen Inorganic materials 0.000 description 12
- 229910052702 rhenium Inorganic materials 0.000 description 12
- 238000002965 ELISA Methods 0.000 description 11
- 206010020751 Hypersensitivity Diseases 0.000 description 11
- 239000002671 adjuvant Substances 0.000 description 11
- 208000026935 allergic disease Diseases 0.000 description 11
- 229940098773 bovine serum albumin Drugs 0.000 description 11
- 239000008194 pharmaceutical composition Substances 0.000 description 11
- 239000012453 solvate Substances 0.000 description 11
- 230000001225 therapeutic effect Effects 0.000 description 11
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 10
- 150000001204 N-oxides Chemical class 0.000 description 10
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 10
- 230000008901 benefit Effects 0.000 description 10
- 238000005859 coupling reaction Methods 0.000 description 10
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 10
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 10
- 238000002360 preparation method Methods 0.000 description 10
- 229940124597 therapeutic agent Drugs 0.000 description 10
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 10
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 9
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 9
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 9
- 230000007815 allergy Effects 0.000 description 9
- 238000009835 boiling Methods 0.000 description 9
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 125000005842 heteroatom Chemical group 0.000 description 9
- 239000001257 hydrogen Substances 0.000 description 9
- 230000006872 improvement Effects 0.000 description 9
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 9
- 229910000104 sodium hydride Inorganic materials 0.000 description 9
- 238000004809 thin layer chromatography Methods 0.000 description 9
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 9
- STBLNCCBQMHSRC-BATDWUPUSA-N (2s)-n-[(3s,4s)-5-acetyl-7-cyano-4-methyl-1-[(2-methylnaphthalen-1-yl)methyl]-2-oxo-3,4-dihydro-1,5-benzodiazepin-3-yl]-2-(methylamino)propanamide Chemical compound O=C1[C@@H](NC(=O)[C@H](C)NC)[C@H](C)N(C(C)=O)C2=CC(C#N)=CC=C2N1CC1=C(C)C=CC2=CC=CC=C12 STBLNCCBQMHSRC-BATDWUPUSA-N 0.000 description 8
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 8
- 201000004624 Dermatitis Diseases 0.000 description 8
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 8
- 239000000010 aprotic solvent Substances 0.000 description 8
- 125000003710 aryl alkyl group Chemical group 0.000 description 8
- 125000001246 bromo group Chemical group Br* 0.000 description 8
- 230000001684 chronic effect Effects 0.000 description 8
- 229940125878 compound 36 Drugs 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 125000002346 iodo group Chemical group I* 0.000 description 8
- 150000002500 ions Chemical class 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 230000000802 nitrating effect Effects 0.000 description 8
- 229910052705 radium Inorganic materials 0.000 description 8
- 229910052701 rubidium Inorganic materials 0.000 description 8
- 239000012312 sodium hydride Substances 0.000 description 8
- 239000006228 supernatant Substances 0.000 description 8
- 208000024891 symptom Diseases 0.000 description 8
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 8
- 239000003981 vehicle Substances 0.000 description 8
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 description 7
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 7
- 239000012979 RPMI medium Substances 0.000 description 7
- 238000004458 analytical method Methods 0.000 description 7
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 7
- 229910000024 caesium carbonate Inorganic materials 0.000 description 7
- 238000001514 detection method Methods 0.000 description 7
- 239000002158 endotoxin Substances 0.000 description 7
- 239000012091 fetal bovine serum Substances 0.000 description 7
- 239000003921 oil Substances 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- 229910000027 potassium carbonate Inorganic materials 0.000 description 7
- 239000003755 preservative agent Substances 0.000 description 7
- 229910000029 sodium carbonate Inorganic materials 0.000 description 7
- HUWSZNZAROKDRZ-RRLWZMAJSA-N (3r,4r)-3-azaniumyl-5-[[(2s,3r)-1-[(2s)-2,3-dicarboxypyrrolidin-1-yl]-3-methyl-1-oxopentan-2-yl]amino]-5-oxo-4-sulfanylpentane-1-sulfonate Chemical compound OS(=O)(=O)CC[C@@H](N)[C@@H](S)C(=O)N[C@@H]([C@H](C)CC)C(=O)N1CCC(C(O)=O)[C@H]1C(O)=O HUWSZNZAROKDRZ-RRLWZMAJSA-N 0.000 description 6
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 6
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 6
- 206010018364 Glomerulonephritis Diseases 0.000 description 6
- 206010019663 Hepatic failure Diseases 0.000 description 6
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 6
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 6
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 6
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- 201000004681 Psoriasis Diseases 0.000 description 6
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 6
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 6
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 6
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 6
- 239000006180 TBST buffer Substances 0.000 description 6
- 206010046851 Uveitis Diseases 0.000 description 6
- 230000001154 acute effect Effects 0.000 description 6
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 125000001309 chloro group Chemical group Cl* 0.000 description 6
- 229940125807 compound 37 Drugs 0.000 description 6
- 238000001816 cooling Methods 0.000 description 6
- 230000008878 coupling Effects 0.000 description 6
- 238000010168 coupling process Methods 0.000 description 6
- 229910052805 deuterium Inorganic materials 0.000 description 6
- UQLDLKMNUJERMK-UHFFFAOYSA-L di(octadecanoyloxy)lead Chemical compound [Pb+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O UQLDLKMNUJERMK-UHFFFAOYSA-L 0.000 description 6
- 239000008101 lactose Substances 0.000 description 6
- 208000007903 liver failure Diseases 0.000 description 6
- 231100000835 liver failure Toxicity 0.000 description 6
- 210000004072 lung Anatomy 0.000 description 6
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 6
- 235000019198 oils Nutrition 0.000 description 6
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 6
- 229940002612 prodrug Drugs 0.000 description 6
- 239000000651 prodrug Substances 0.000 description 6
- 230000000069 prophylactic effect Effects 0.000 description 6
- 201000000306 sarcoidosis Diseases 0.000 description 6
- 229920006395 saturated elastomer Polymers 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000006467 substitution reaction Methods 0.000 description 6
- 239000000725 suspension Substances 0.000 description 6
- IUSARDYWEPUTPN-OZBXUNDUSA-N (2r)-n-[(2s,3r)-4-[[(4s)-6-(2,2-dimethylpropyl)spiro[3,4-dihydropyrano[2,3-b]pyridine-2,1'-cyclobutane]-4-yl]amino]-3-hydroxy-1-[3-(1,3-thiazol-2-yl)phenyl]butan-2-yl]-2-methoxypropanamide Chemical compound C([C@H](NC(=O)[C@@H](C)OC)[C@H](O)CN[C@@H]1C2=CC(CC(C)(C)C)=CN=C2OC2(CCC2)C1)C(C=1)=CC=CC=1C1=NC=CS1 IUSARDYWEPUTPN-OZBXUNDUSA-N 0.000 description 5
- YJLIKUSWRSEPSM-WGQQHEPDSA-N (2r,3r,4s,5r)-2-[6-amino-8-[(4-phenylphenyl)methylamino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C=1C=C(C=2C=CC=CC=2)C=CC=1CNC1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O YJLIKUSWRSEPSM-WGQQHEPDSA-N 0.000 description 5
- PYRKKGOKRMZEIT-UHFFFAOYSA-N 2-[6-(2-cyclopropylethoxy)-9-(2-hydroxy-2-methylpropyl)-1h-phenanthro[9,10-d]imidazol-2-yl]-5-fluorobenzene-1,3-dicarbonitrile Chemical compound C1=C2C3=CC(CC(C)(O)C)=CC=C3C=3NC(C=4C(=CC(F)=CC=4C#N)C#N)=NC=3C2=CC=C1OCCC1CC1 PYRKKGOKRMZEIT-UHFFFAOYSA-N 0.000 description 5
- 206010009900 Colitis ulcerative Diseases 0.000 description 5
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 5
- 101000852483 Homo sapiens Interleukin-1 receptor-associated kinase 1 Proteins 0.000 description 5
- 101000977771 Homo sapiens Interleukin-1 receptor-associated kinase 4 Proteins 0.000 description 5
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 5
- 108010072621 Interleukin-1 Receptor-Associated Kinases Proteins 0.000 description 5
- 102000006940 Interleukin-1 Receptor-Associated Kinases Human genes 0.000 description 5
- 102100023533 Interleukin-1 receptor-associated kinase 4 Human genes 0.000 description 5
- 239000002177 L01XE27 - Ibrutinib Substances 0.000 description 5
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 5
- 229920002472 Starch Polymers 0.000 description 5
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 5
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 5
- 201000006704 Ulcerative Colitis Diseases 0.000 description 5
- 239000000654 additive Substances 0.000 description 5
- 150000001412 amines Chemical class 0.000 description 5
- 125000004429 atom Chemical group 0.000 description 5
- 210000000988 bone and bone Anatomy 0.000 description 5
- 239000000872 buffer Substances 0.000 description 5
- 150000001732 carboxylic acid derivatives Chemical group 0.000 description 5
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 5
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 229960001507 ibrutinib Drugs 0.000 description 5
- XYFPWWZEPKGCCK-GOSISDBHSA-N ibrutinib Chemical compound C1=2C(N)=NC=NC=2N([C@H]2CN(CCC2)C(=O)C=C)N=C1C(C=C1)=CC=C1OC1=CC=CC=C1 XYFPWWZEPKGCCK-GOSISDBHSA-N 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- 201000010659 intrinsic asthma Diseases 0.000 description 5
- 206010023332 keratitis Diseases 0.000 description 5
- 235000019359 magnesium stearate Nutrition 0.000 description 5
- 201000006417 multiple sclerosis Diseases 0.000 description 5
- 229910017604 nitric acid Inorganic materials 0.000 description 5
- 238000012587 nuclear overhauser effect experiment Methods 0.000 description 5
- 239000011591 potassium Substances 0.000 description 5
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 5
- 125000004307 pyrazin-2-yl group Chemical group [H]C1=C([H])N=C(*)C([H])=N1 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 229910052938 sodium sulfate Inorganic materials 0.000 description 5
- 235000011152 sodium sulphate Nutrition 0.000 description 5
- 235000019698 starch Nutrition 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 5
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 4
- YQOLEILXOBUDMU-KRWDZBQOSA-N (4R)-5-[(6-bromo-3-methyl-2-pyrrolidin-1-ylquinoline-4-carbonyl)amino]-4-(2-chlorophenyl)pentanoic acid Chemical compound CC1=C(C2=C(C=CC(=C2)Br)N=C1N3CCCC3)C(=O)NC[C@H](CCC(=O)O)C4=CC=CC=C4Cl YQOLEILXOBUDMU-KRWDZBQOSA-N 0.000 description 4
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 4
- 201000004384 Alopecia Diseases 0.000 description 4
- 208000032467 Aplastic anaemia Diseases 0.000 description 4
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 4
- 208000009137 Behcet syndrome Diseases 0.000 description 4
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 description 4
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 4
- 201000009030 Carcinoma Diseases 0.000 description 4
- 101150065749 Churc1 gene Proteins 0.000 description 4
- 208000011231 Crohn disease Diseases 0.000 description 4
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 4
- 206010011831 Cytomegalovirus infection Diseases 0.000 description 4
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 4
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 4
- 206010060742 Endocrine ophthalmopathy Diseases 0.000 description 4
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 4
- 108010010803 Gelatin Proteins 0.000 description 4
- 102000006395 Globulins Human genes 0.000 description 4
- 108010044091 Globulins Proteins 0.000 description 4
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 4
- 239000007821 HATU Substances 0.000 description 4
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 4
- 101000977768 Homo sapiens Interleukin-1 receptor-associated kinase 3 Proteins 0.000 description 4
- 102100023530 Interleukin-1 receptor-associated kinase 3 Human genes 0.000 description 4
- 108010065637 Interleukin-23 Proteins 0.000 description 4
- 102000013264 Interleukin-23 Human genes 0.000 description 4
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 4
- 206010029240 Neuritis Diseases 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- 206010033645 Pancreatitis Diseases 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- 229930006000 Sucrose Natural products 0.000 description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 4
- PSLUFJFHTBIXMW-WYEYVKMPSA-N [(3r,4ar,5s,6s,6as,10s,10ar,10bs)-3-ethenyl-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-6-(2-pyridin-2-ylethylcarbamoyloxy)-5,6,6a,8,9,10-hexahydro-2h-benzo[f]chromen-5-yl] acetate Chemical compound O([C@@H]1[C@@H]([C@]2(O[C@](C)(CC(=O)[C@]2(O)[C@@]2(C)[C@@H](O)CCC(C)(C)[C@@H]21)C=C)C)OC(=O)C)C(=O)NCCC1=CC=CC=N1 PSLUFJFHTBIXMW-WYEYVKMPSA-N 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 229940100198 alkylating agent Drugs 0.000 description 4
- 239000002168 alkylating agent Substances 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 4
- 238000010171 animal model Methods 0.000 description 4
- 239000002246 antineoplastic agent Substances 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 229960002170 azathioprine Drugs 0.000 description 4
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 4
- 230000000903 blocking effect Effects 0.000 description 4
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical compound OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 description 4
- 239000012267 brine Substances 0.000 description 4
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 4
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 4
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 238000012054 celltiter-glo Methods 0.000 description 4
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 229940125773 compound 10 Drugs 0.000 description 4
- 229940126543 compound 14 Drugs 0.000 description 4
- 229940125844 compound 46 Drugs 0.000 description 4
- 210000004087 cornea Anatomy 0.000 description 4
- 229940127089 cytotoxic agent Drugs 0.000 description 4
- 238000010790 dilution Methods 0.000 description 4
- 239000012895 dilution Substances 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- GWNFQAKCJYEJEW-UHFFFAOYSA-N ethyl 3-[8-[[4-methyl-5-[(3-methyl-4-oxophthalazin-1-yl)methyl]-1,2,4-triazol-3-yl]sulfanyl]octanoylamino]benzoate Chemical compound CCOC(=O)C1=CC(NC(=O)CCCCCCCSC2=NN=C(CC3=NN(C)C(=O)C4=CC=CC=C34)N2C)=CC=C1 GWNFQAKCJYEJEW-UHFFFAOYSA-N 0.000 description 4
- 208000024711 extrinsic asthma Diseases 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 239000000796 flavoring agent Substances 0.000 description 4
- 235000019634 flavors Nutrition 0.000 description 4
- 239000008273 gelatin Substances 0.000 description 4
- 229920000159 gelatin Polymers 0.000 description 4
- 229940014259 gelatin Drugs 0.000 description 4
- 235000019322 gelatine Nutrition 0.000 description 4
- 235000011852 gelatine desserts Nutrition 0.000 description 4
- 150000004678 hydrides Chemical class 0.000 description 4
- 206010021198 ichthyosis Diseases 0.000 description 4
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 4
- 208000030603 inherited susceptibility to asthma Diseases 0.000 description 4
- 208000023589 ischemic disease Diseases 0.000 description 4
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 4
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical group S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 4
- 229960001428 mercaptopurine Drugs 0.000 description 4
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 4
- 201000005962 mycosis fungoides Diseases 0.000 description 4
- 125000004433 nitrogen atom Chemical group N* 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- 239000012044 organic layer Substances 0.000 description 4
- 239000003960 organic solvent Substances 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 229910052763 palladium Inorganic materials 0.000 description 4
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 4
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 4
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 4
- WVYADZUPLLSGPU-UHFFFAOYSA-N salsalate Chemical compound OC(=O)C1=CC=CC=C1OC(=O)C1=CC=CC=C1O WVYADZUPLLSGPU-UHFFFAOYSA-N 0.000 description 4
- 239000000377 silicon dioxide Substances 0.000 description 4
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 4
- 239000012279 sodium borohydride Substances 0.000 description 4
- 229910000033 sodium borohydride Inorganic materials 0.000 description 4
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 4
- 235000010356 sorbitol Nutrition 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- 239000005720 sucrose Substances 0.000 description 4
- 229960004793 sucrose Drugs 0.000 description 4
- 229910052717 sulfur Inorganic materials 0.000 description 4
- 208000011580 syndromic disease Diseases 0.000 description 4
- 230000009885 systemic effect Effects 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 4
- MPDDTAJMJCESGV-CTUHWIOQSA-M (3r,5r)-7-[2-(4-fluorophenyl)-5-[methyl-[(1r)-1-phenylethyl]carbamoyl]-4-propan-2-ylpyrazol-3-yl]-3,5-dihydroxyheptanoate Chemical compound C1([C@@H](C)N(C)C(=O)C2=NN(C(CC[C@@H](O)C[C@@H](O)CC([O-])=O)=C2C(C)C)C=2C=CC(F)=CC=2)=CC=CC=C1 MPDDTAJMJCESGV-CTUHWIOQSA-M 0.000 description 3
- STPKWKPURVSAJF-LJEWAXOPSA-N (4r,5r)-5-[4-[[4-(1-aza-4-azoniabicyclo[2.2.2]octan-4-ylmethyl)phenyl]methoxy]phenyl]-3,3-dibutyl-7-(dimethylamino)-1,1-dioxo-4,5-dihydro-2h-1$l^{6}-benzothiepin-4-ol Chemical compound O[C@H]1C(CCCC)(CCCC)CS(=O)(=O)C2=CC=C(N(C)C)C=C2[C@H]1C(C=C1)=CC=C1OCC(C=C1)=CC=C1C[N+]1(CC2)CCN2CC1 STPKWKPURVSAJF-LJEWAXOPSA-N 0.000 description 3
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 3
- DEVSOMFAQLZNKR-RJRFIUFISA-N (z)-3-[3-[3,5-bis(trifluoromethyl)phenyl]-1,2,4-triazol-1-yl]-n'-pyrazin-2-ylprop-2-enehydrazide Chemical compound FC(F)(F)C1=CC(C(F)(F)F)=CC(C2=NN(\C=C/C(=O)NNC=3N=CC=NC=3)C=N2)=C1 DEVSOMFAQLZNKR-RJRFIUFISA-N 0.000 description 3
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 3
- NPRYCHLHHVWLQZ-TURQNECASA-N 2-amino-9-[(2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-ynylpurin-8-one Chemical compound NC1=NC=C2N(C(N(C2=N1)[C@@H]1O[C@@H]([C@H]([C@H]1O)F)CO)=O)CC#C NPRYCHLHHVWLQZ-TURQNECASA-N 0.000 description 3
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 description 3
- LVEFFFUKMDNCBN-UHFFFAOYSA-N 4-ethoxycyclohexan-1-one Chemical compound CCOC1CCC(=O)CC1 LVEFFFUKMDNCBN-UHFFFAOYSA-N 0.000 description 3
- XFJBGINZIMNZBW-CRAIPNDOSA-N 5-chloro-2-[4-[(1r,2s)-2-[2-(5-methylsulfonylpyridin-2-yl)oxyethyl]cyclopropyl]piperidin-1-yl]pyrimidine Chemical compound N1=CC(S(=O)(=O)C)=CC=C1OCC[C@H]1[C@@H](C2CCN(CC2)C=2N=CC(Cl)=CN=2)C1 XFJBGINZIMNZBW-CRAIPNDOSA-N 0.000 description 3
- XURXQNUIGWHWHU-UHFFFAOYSA-N 6-bromopyridine-2-carboxylic acid Chemical compound OC(=O)C1=CC=CC(Br)=N1 XURXQNUIGWHWHU-UHFFFAOYSA-N 0.000 description 3
- ZJZLKONLVWLQRK-UHFFFAOYSA-N 8-ethoxy-1,4-dioxaspiro[4.5]decane Chemical compound C1CC(OCC)CCC21OCCO2 ZJZLKONLVWLQRK-UHFFFAOYSA-N 0.000 description 3
- 208000024827 Alzheimer disease Diseases 0.000 description 3
- 239000004475 Arginine Substances 0.000 description 3
- 201000001320 Atherosclerosis Diseases 0.000 description 3
- PKMUHQIDVVOXHQ-HXUWFJFHSA-N C[C@H](C1=CC(C2=CC=C(CNC3CCCC3)S2)=CC=C1)NC(C1=C(C)C=CC(NC2CNC2)=C1)=O Chemical compound C[C@H](C1=CC(C2=CC=C(CNC3CCCC3)S2)=CC=C1)NC(C1=C(C)C=CC(NC2CNC2)=C1)=O PKMUHQIDVVOXHQ-HXUWFJFHSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- 208000015943 Coeliac disease Diseases 0.000 description 3
- 108010036949 Cyclosporine Proteins 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 3
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 3
- 206010014561 Emphysema Diseases 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 206010019799 Hepatitis viral Diseases 0.000 description 3
- 101000746373 Homo sapiens Granulocyte-macrophage colony-stimulating factor Proteins 0.000 description 3
- 102000019223 Interleukin-1 receptor Human genes 0.000 description 3
- 108050006617 Interleukin-1 receptor Proteins 0.000 description 3
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 3
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 3
- 239000004472 Lysine Substances 0.000 description 3
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 3
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 3
- 208000001132 Osteoporosis Diseases 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 208000002193 Pain Diseases 0.000 description 3
- 208000018737 Parkinson disease Diseases 0.000 description 3
- 206010034277 Pemphigoid Diseases 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 206010040047 Sepsis Diseases 0.000 description 3
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 3
- 108010090804 Streptavidin Proteins 0.000 description 3
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 3
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 3
- SPXSEZMVRJLHQG-XMMPIXPASA-N [(2R)-1-[[4-[(3-phenylmethoxyphenoxy)methyl]phenyl]methyl]pyrrolidin-2-yl]methanol Chemical compound C(C1=CC=CC=C1)OC=1C=C(OCC2=CC=C(CN3[C@H](CCC3)CO)C=C2)C=CC=1 SPXSEZMVRJLHQG-XMMPIXPASA-N 0.000 description 3
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 125000002015 acyclic group Chemical group 0.000 description 3
- 125000002252 acyl group Chemical group 0.000 description 3
- 229910001413 alkali metal ion Inorganic materials 0.000 description 3
- 125000003342 alkenyl group Chemical group 0.000 description 3
- 239000013566 allergen Substances 0.000 description 3
- 201000009961 allergic asthma Diseases 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 230000001093 anti-cancer Effects 0.000 description 3
- 229940121363 anti-inflammatory agent Drugs 0.000 description 3
- 239000002260 anti-inflammatory agent Substances 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 235000006708 antioxidants Nutrition 0.000 description 3
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 3
- 230000001363 autoimmune Effects 0.000 description 3
- 125000002619 bicyclic group Chemical group 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 206010006451 bronchitis Diseases 0.000 description 3
- 208000000594 bullous pemphigoid Diseases 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 150000001721 carbon Chemical group 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 235000010980 cellulose Nutrition 0.000 description 3
- 229920002678 cellulose Polymers 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 235000015165 citric acid Nutrition 0.000 description 3
- 238000004440 column chromatography Methods 0.000 description 3
- 229940126142 compound 16 Drugs 0.000 description 3
- 229940127573 compound 38 Drugs 0.000 description 3
- 229940127271 compound 49 Drugs 0.000 description 3
- 229940126179 compound 72 Drugs 0.000 description 3
- 125000000392 cycloalkenyl group Chemical group 0.000 description 3
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 3
- 239000007884 disintegrant Substances 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 239000002702 enteric coating Substances 0.000 description 3
- 238000009505 enteric coating Methods 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 235000003599 food sweetener Nutrition 0.000 description 3
- 150000004676 glycans Chemical class 0.000 description 3
- 125000005843 halogen group Chemical group 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 239000005556 hormone Chemical class 0.000 description 3
- 150000004677 hydrates Chemical class 0.000 description 3
- 150000002431 hydrogen Chemical class 0.000 description 3
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 3
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 3
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 3
- 229960003444 immunosuppressant agent Drugs 0.000 description 3
- 239000003018 immunosuppressive agent Substances 0.000 description 3
- 230000002757 inflammatory effect Effects 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 3
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 3
- 239000010410 layer Substances 0.000 description 3
- 230000003902 lesion Effects 0.000 description 3
- 239000012280 lithium aluminium hydride Substances 0.000 description 3
- 239000000314 lubricant Substances 0.000 description 3
- 230000036210 malignancy Effects 0.000 description 3
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 3
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 3
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 3
- 239000008108 microcrystalline cellulose Substances 0.000 description 3
- 229940016286 microcrystalline cellulose Drugs 0.000 description 3
- 239000002480 mineral oil Substances 0.000 description 3
- 235000010446 mineral oil Nutrition 0.000 description 3
- 125000002950 monocyclic group Chemical group 0.000 description 3
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 3
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 3
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 3
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 3
- PIDFDZJZLOTZTM-KHVQSSSXSA-N ombitasvir Chemical compound COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NC1=CC=C([C@H]2N([C@@H](CC2)C=2C=CC(NC(=O)[C@H]3N(CCC3)C(=O)[C@@H](NC(=O)OC)C(C)C)=CC=2)C=2C=CC(=CC=2)C(C)(C)C)C=C1 PIDFDZJZLOTZTM-KHVQSSSXSA-N 0.000 description 3
- 230000003287 optical effect Effects 0.000 description 3
- 150000007530 organic bases Chemical class 0.000 description 3
- 239000012074 organic phase Substances 0.000 description 3
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 229960002621 pembrolizumab Drugs 0.000 description 3
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 3
- 229910052698 phosphorus Inorganic materials 0.000 description 3
- 229920001282 polysaccharide Polymers 0.000 description 3
- 239000005017 polysaccharide Substances 0.000 description 3
- 229920000136 polysorbate Polymers 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 229960004618 prednisone Drugs 0.000 description 3
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 3
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 3
- 230000002685 pulmonary effect Effects 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 3
- 238000010992 reflux Methods 0.000 description 3
- 125000006413 ring segment Chemical group 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- 238000010898 silica gel chromatography Methods 0.000 description 3
- 229910052710 silicon Inorganic materials 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- 229960002930 sirolimus Drugs 0.000 description 3
- 239000008109 sodium starch glycolate Substances 0.000 description 3
- 229920003109 sodium starch glycolate Polymers 0.000 description 3
- 229940079832 sodium starch glycolate Drugs 0.000 description 3
- 239000011877 solvent mixture Substances 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 239000003765 sweetening agent Substances 0.000 description 3
- 239000000454 talc Substances 0.000 description 3
- 229910052623 talc Inorganic materials 0.000 description 3
- 229940033134 talc Drugs 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- 239000003039 volatile agent Substances 0.000 description 3
- 229910052725 zinc Inorganic materials 0.000 description 3
- 239000011701 zinc Substances 0.000 description 3
- NWZSZGALRFJKBT-KNIFDHDWSA-N (2s)-2,6-diaminohexanoic acid;(2s)-2-hydroxybutanedioic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O.NCCCC[C@H](N)C(O)=O NWZSZGALRFJKBT-KNIFDHDWSA-N 0.000 description 2
- RDJGLLICXDHJDY-NSHDSACASA-N (2s)-2-(3-phenoxyphenyl)propanoic acid Chemical compound OC(=O)[C@@H](C)C1=CC=CC(OC=2C=CC=CC=2)=C1 RDJGLLICXDHJDY-NSHDSACASA-N 0.000 description 2
- WWTBZEKOSBFBEM-SPWPXUSOSA-N (2s)-2-[[2-benzyl-3-[hydroxy-[(1r)-2-phenyl-1-(phenylmethoxycarbonylamino)ethyl]phosphoryl]propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)O)C(=O)C(CP(O)(=O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1C=CC=CC=1)CC1=CC=CC=C1 WWTBZEKOSBFBEM-SPWPXUSOSA-N 0.000 description 2
- XDBHWPLGGBLUHH-UHFFFAOYSA-N (3-cyanophenyl)boronic acid Chemical compound OB(O)C1=CC=CC(C#N)=C1 XDBHWPLGGBLUHH-UHFFFAOYSA-N 0.000 description 2
- FVEDGBRHTGXPOK-UHFFFAOYSA-N (5-fluoropyridin-3-yl)boronic acid Chemical compound OB(O)C1=CN=CC(F)=C1 FVEDGBRHTGXPOK-UHFFFAOYSA-N 0.000 description 2
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 2
- LBUJPTNKIBCYBY-UHFFFAOYSA-N 1,2,3,4-tetrahydroquinoline Chemical compound C1=CC=C2CCCNC2=C1 LBUJPTNKIBCYBY-UHFFFAOYSA-N 0.000 description 2
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 2
- KKHFRAFPESRGGD-UHFFFAOYSA-N 1,3-dimethyl-7-[3-(n-methylanilino)propyl]purine-2,6-dione Chemical compound C1=NC=2N(C)C(=O)N(C)C(=O)C=2N1CCCN(C)C1=CC=CC=C1 KKHFRAFPESRGGD-UHFFFAOYSA-N 0.000 description 2
- HKQTYQDNCKMNHZ-UHFFFAOYSA-N 1,4-dioxaspiro[4.5]decan-8-ol Chemical compound C1CC(O)CCC21OCCO2 HKQTYQDNCKMNHZ-UHFFFAOYSA-N 0.000 description 2
- WZZBNLYBHUDSHF-DHLKQENFSA-N 1-[(3s,4s)-4-[8-(2-chloro-4-pyrimidin-2-yloxyphenyl)-7-fluoro-2-methylimidazo[4,5-c]quinolin-1-yl]-3-fluoropiperidin-1-yl]-2-hydroxyethanone Chemical compound CC1=NC2=CN=C3C=C(F)C(C=4C(=CC(OC=5N=CC=CN=5)=CC=4)Cl)=CC3=C2N1[C@H]1CCN(C(=O)CO)C[C@@H]1F WZZBNLYBHUDSHF-DHLKQENFSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- FPIPGXGPPPQFEQ-UHFFFAOYSA-N 13-cis retinol Natural products OCC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-UHFFFAOYSA-N 0.000 description 2
- WGFNXGPBPIJYLI-UHFFFAOYSA-N 2,6-difluoro-3-[(3-fluorophenyl)sulfonylamino]-n-(3-methoxy-1h-pyrazolo[3,4-b]pyridin-5-yl)benzamide Chemical compound C1=C2C(OC)=NNC2=NC=C1NC(=O)C(C=1F)=C(F)C=CC=1NS(=O)(=O)C1=CC=CC(F)=C1 WGFNXGPBPIJYLI-UHFFFAOYSA-N 0.000 description 2
- UKFTXWKNVSVVCJ-UHFFFAOYSA-N 2-[(6-hydrazinylpyridazin-3-yl)-(2-hydroxyethyl)amino]ethanol;hydron;dichloride Chemical class Cl.Cl.NNC1=CC=C(N(CCO)CCO)N=N1 UKFTXWKNVSVVCJ-UHFFFAOYSA-N 0.000 description 2
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 2
- NBGAYCYFNGPNPV-UHFFFAOYSA-N 2-aminooxybenzoic acid Chemical group NOC1=CC=CC=C1C(O)=O NBGAYCYFNGPNPV-UHFFFAOYSA-N 0.000 description 2
- WRMNZCZEMHIOCP-UHFFFAOYSA-N 2-phenylethanol Chemical compound OCCC1=CC=CC=C1 WRMNZCZEMHIOCP-UHFFFAOYSA-N 0.000 description 2
- VEKIYFGCEAJDDT-UHFFFAOYSA-N 2-pyridin-3-ylpyridine Chemical compound N1=CC=CC=C1C1=CC=CN=C1 VEKIYFGCEAJDDT-UHFFFAOYSA-N 0.000 description 2
- LXRDCGVUPNWEGX-UHFFFAOYSA-N 4-ethoxycyclohexan-1-ol Chemical compound CCOC1CCC(O)CC1 LXRDCGVUPNWEGX-UHFFFAOYSA-N 0.000 description 2
- DQAZPZIYEOGZAF-UHFFFAOYSA-N 4-ethyl-n-[4-(3-ethynylanilino)-7-methoxyquinazolin-6-yl]piperazine-1-carboxamide Chemical compound C1CN(CC)CCN1C(=O)NC(C(=CC1=NC=N2)OC)=CC1=C2NC1=CC=CC(C#C)=C1 DQAZPZIYEOGZAF-UHFFFAOYSA-N 0.000 description 2
- KGKUBINPASMHDZ-UHFFFAOYSA-N 5-methoxy-2-(1-methyl-4-nitropyrazol-3-yl)pyridine Chemical compound COC=1C=CC(=NC=1)C1=NN(C=C1[N+](=O)[O-])C KGKUBINPASMHDZ-UHFFFAOYSA-N 0.000 description 2
- USAGMMSAFJZDCV-UHFFFAOYSA-N 5-methoxy-2-(1-methylpyrazol-3-yl)pyridine Chemical compound COC=1C=CC(=NC=1)C1=NN(C=C1)C USAGMMSAFJZDCV-UHFFFAOYSA-N 0.000 description 2
- JQEPZWIMBARBKN-UHFFFAOYSA-N 5-methoxy-2-(1h-pyrazol-5-yl)pyridine Chemical compound N1=CC(OC)=CC=C1C1=CC=NN1 JQEPZWIMBARBKN-UHFFFAOYSA-N 0.000 description 2
- VNUBTOULBWHIRO-UHFFFAOYSA-N 6-(5-methylpyridin-3-yl)-N-(1-methyl-3-pyrrolidin-1-ylpyrazol-4-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)N1CCCC1 VNUBTOULBWHIRO-UHFFFAOYSA-N 0.000 description 2
- 208000030507 AIDS Diseases 0.000 description 2
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 2
- 208000002874 Acne Vulgaris Diseases 0.000 description 2
- 208000009304 Acute Kidney Injury Diseases 0.000 description 2
- 208000007788 Acute Liver Failure Diseases 0.000 description 2
- 208000026872 Addison Disease Diseases 0.000 description 2
- 201000010000 Agranulocytosis Diseases 0.000 description 2
- 208000035285 Allergic Seasonal Rhinitis Diseases 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 206010002065 Anaemia megaloblastic Diseases 0.000 description 2
- 208000028185 Angioedema Diseases 0.000 description 2
- 206010002660 Anoxia Diseases 0.000 description 2
- 241000976983 Anoxia Species 0.000 description 2
- 206010003559 Asthma late onset Diseases 0.000 description 2
- 102100034605 Atrial natriuretic peptide receptor 3 Human genes 0.000 description 2
- 208000032116 Autoimmune Experimental Encephalomyelitis Diseases 0.000 description 2
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 2
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 2
- 208000023328 Basedow disease Diseases 0.000 description 2
- 206010004659 Biliary cirrhosis Diseases 0.000 description 2
- 208000033222 Biliary cirrhosis primary Diseases 0.000 description 2
- 206010006474 Bronchopulmonary aspergillosis allergic Diseases 0.000 description 2
- 208000011691 Burkitt lymphomas Diseases 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 208000005623 Carcinogenesis Diseases 0.000 description 2
- 208000031229 Cardiomyopathies Diseases 0.000 description 2
- 208000002177 Cataract Diseases 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 2
- 206010008609 Cholangitis sclerosing Diseases 0.000 description 2
- 208000002691 Choroiditis Diseases 0.000 description 2
- 206010009208 Cirrhosis alcoholic Diseases 0.000 description 2
- LYAUBLPHKYSCSK-UHFFFAOYSA-N Cl.Cl.CN1N=C(C(=C1)N)C1=NC=CC=C1 Chemical compound Cl.Cl.CN1N=C(C(=C1)N)C1=NC=CC=C1 LYAUBLPHKYSCSK-UHFFFAOYSA-N 0.000 description 2
- 206010009657 Clostridium difficile colitis Diseases 0.000 description 2
- 206010066968 Corneal leukoma Diseases 0.000 description 2
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 206010012438 Dermatitis atopic Diseases 0.000 description 2
- 206010012441 Dermatitis bullous Diseases 0.000 description 2
- 206010012442 Dermatitis contact Diseases 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- 208000007342 Diabetic Nephropathies Diseases 0.000 description 2
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 2
- 206010014950 Eosinophilia Diseases 0.000 description 2
- 206010014954 Eosinophilic fasciitis Diseases 0.000 description 2
- 206010015150 Erythema Diseases 0.000 description 2
- 206010015218 Erythema multiforme Diseases 0.000 description 2
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- DCXXMTOCNZCJGO-UHFFFAOYSA-N Glycerol trioctadecanoate Natural products CCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC DCXXMTOCNZCJGO-UHFFFAOYSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 208000024869 Goodpasture syndrome Diseases 0.000 description 2
- 201000005569 Gout Diseases 0.000 description 2
- 206010018691 Granuloma Diseases 0.000 description 2
- 206010072579 Granulomatosis with polyangiitis Diseases 0.000 description 2
- 208000035895 Guillain-Barré syndrome Diseases 0.000 description 2
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 2
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 2
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 2
- 208000032759 Hemolytic-Uremic Syndrome Diseases 0.000 description 2
- 101000924488 Homo sapiens Atrial natriuretic peptide receptor 3 Proteins 0.000 description 2
- 101000852980 Homo sapiens Interleukin-23 subunit alpha Proteins 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- 206010020850 Hyperthyroidism Diseases 0.000 description 2
- 206010021074 Hypoplastic anaemia Diseases 0.000 description 2
- 206010021143 Hypoxia Diseases 0.000 description 2
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 2
- 201000003838 Idiopathic interstitial pneumonia Diseases 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- UETNIIAIRMUTSM-UHFFFAOYSA-N Jacareubin Natural products CC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)O UETNIIAIRMUTSM-UHFFFAOYSA-N 0.000 description 2
- 229940122245 Janus kinase inhibitor Drugs 0.000 description 2
- 208000007766 Kaposi sarcoma Diseases 0.000 description 2
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 2
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- 206010024380 Leukoderma Diseases 0.000 description 2
- GWNVDXQDILPJIG-SHSCPDMUSA-N Leukotriene C4 Natural products CCCCCC=C/CC=C/C=C/C=C/C(SCC(NC(=O)CCC(N)C(=O)O)C(=O)NCC(=O)O)C(O)CCCC(=O)O GWNVDXQDILPJIG-SHSCPDMUSA-N 0.000 description 2
- 208000012309 Linear IgA disease Diseases 0.000 description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- 208000000682 Megaloblastic Anemia Diseases 0.000 description 2
- ZRVUJXDFFKFLMG-UHFFFAOYSA-N Meloxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC1=NC=C(C)S1 ZRVUJXDFFKFLMG-UHFFFAOYSA-N 0.000 description 2
- 208000027530 Meniere disease Diseases 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 2
- 241001139947 Mida Species 0.000 description 2
- 208000019695 Migraine disease Diseases 0.000 description 2
- 206010049567 Miller Fisher syndrome Diseases 0.000 description 2
- 206010027910 Mononeuritis Diseases 0.000 description 2
- 208000034578 Multiple myelomas Diseases 0.000 description 2
- 201000002481 Myositis Diseases 0.000 description 2
- ZSXGLVDWWRXATF-UHFFFAOYSA-N N,N-dimethylformamide dimethyl acetal Chemical compound COC(OC)N(C)C ZSXGLVDWWRXATF-UHFFFAOYSA-N 0.000 description 2
- NXSGKGMHGZEMJY-UHFFFAOYSA-N N-(1-methyl-3-morpholin-4-ylpyrazol-4-yl)-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)N1CCOCC1 NXSGKGMHGZEMJY-UHFFFAOYSA-N 0.000 description 2
- PUIFTQINEBWPRC-UHFFFAOYSA-N N-(1-methyl-3-morpholin-4-ylpyrazol-4-yl)-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)N1CCOCC1 PUIFTQINEBWPRC-UHFFFAOYSA-N 0.000 description 2
- VSAXMBWPYGNZRO-UHFFFAOYSA-N N-(1-methyl-3-piperidin-1-ylpyrazol-4-yl)-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)N1CCCCC1 VSAXMBWPYGNZRO-UHFFFAOYSA-N 0.000 description 2
- QGNKFRNKJBNDMF-UHFFFAOYSA-N N-(1-methyl-3-piperidin-1-ylpyrazol-4-yl)-6-(5-methylpyridin-3-yl)pyridine-2-carboxamide Chemical compound CC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)NC=1C(=NN(C=1)C)N1CCCCC1 QGNKFRNKJBNDMF-UHFFFAOYSA-N 0.000 description 2
- VXYVOJKFBSRWMD-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrrol-2-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1NC=CC=1)=O)C1=NC=CC=C1 VXYVOJKFBSRWMD-UHFFFAOYSA-N 0.000 description 2
- GKYARCRAVRHLAN-UHFFFAOYSA-N N-(1-methyl-3-pyridin-2-ylpyrazol-4-yl)-6-(1H-pyrrol-3-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C1=CNC=C1)=O)C1=NC=CC=C1 GKYARCRAVRHLAN-UHFFFAOYSA-N 0.000 description 2
- ZXSGVPOVTCUYII-UHFFFAOYSA-N N-(1-methyl-3-pyrrolidin-1-ylpyrazol-4-yl)-6-(1H-pyrazol-4-yl)pyridine-2-carboxamide Chemical compound CN1N=C(C(=C1)NC(C1=NC(=CC=C1)C=1C=NNC=1)=O)N1CCCC1 ZXSGVPOVTCUYII-UHFFFAOYSA-N 0.000 description 2
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 2
- 206010051606 Necrotising colitis Diseases 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 208000008589 Obesity Diseases 0.000 description 2
- 101150003085 Pdcl gene Proteins 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 208000031845 Pernicious anaemia Diseases 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 206010036105 Polyneuropathy Diseases 0.000 description 2
- 208000003971 Posterior uveitis Diseases 0.000 description 2
- 208000012654 Primary biliary cholangitis Diseases 0.000 description 2
- 206010036774 Proctitis Diseases 0.000 description 2
- ZTHYODDOHIVTJV-UHFFFAOYSA-N Propyl gallate Chemical compound CCCOC(=O)C1=CC(O)=C(O)C(O)=C1 ZTHYODDOHIVTJV-UHFFFAOYSA-N 0.000 description 2
- 229940079156 Proteasome inhibitor Drugs 0.000 description 2
- 208000003100 Pseudomembranous Enterocolitis Diseases 0.000 description 2
- 206010037128 Pseudomembranous colitis Diseases 0.000 description 2
- 208000003670 Pure Red-Cell Aplasia Diseases 0.000 description 2
- 208000006311 Pyoderma Diseases 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 206010037779 Radiculopathy Diseases 0.000 description 2
- 208000033626 Renal failure acute Diseases 0.000 description 2
- 206010063837 Reperfusion injury Diseases 0.000 description 2
- 208000002200 Respiratory Hypersensitivity Diseases 0.000 description 2
- 208000007014 Retinitis pigmentosa Diseases 0.000 description 2
- VYGQUTWHTHXGQB-FFHKNEKCSA-N Retinol Palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C VYGQUTWHTHXGQB-FFHKNEKCSA-N 0.000 description 2
- 206010039085 Rhinitis allergic Diseases 0.000 description 2
- 206010039094 Rhinitis perennial Diseases 0.000 description 2
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 2
- 206010039705 Scleritis Diseases 0.000 description 2
- 206010039710 Scleroderma Diseases 0.000 description 2
- 206010048908 Seasonal allergy Diseases 0.000 description 2
- 206010039793 Seborrhoeic dermatitis Diseases 0.000 description 2
- 206010039966 Senile dementia Diseases 0.000 description 2
- 206010040070 Septic Shock Diseases 0.000 description 2
- 208000009359 Sezary Syndrome Diseases 0.000 description 2
- 208000021388 Sezary disease Diseases 0.000 description 2
- 208000021386 Sjogren Syndrome Diseases 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- 208000007107 Stomach Ulcer Diseases 0.000 description 2
- 201000009594 Systemic Scleroderma Diseases 0.000 description 2
- 206010042953 Systemic sclerosis Diseases 0.000 description 2
- 208000031673 T-Cell Cutaneous Lymphoma Diseases 0.000 description 2
- 208000001106 Takayasu Arteritis Diseases 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- 239000004098 Tetracycline Substances 0.000 description 2
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 2
- 206010053615 Thermal burn Diseases 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- AOBORMOPSGHCAX-UHFFFAOYSA-N Tocophersolan Chemical compound OCCOC(=O)CCC(=O)OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C AOBORMOPSGHCAX-UHFFFAOYSA-N 0.000 description 2
- 102000002689 Toll-like receptor Human genes 0.000 description 2
- 108020000411 Toll-like receptor Proteins 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 208000024780 Urticaria Diseases 0.000 description 2
- 208000001445 Uveomeningoencephalitic Syndrome Diseases 0.000 description 2
- 206010047115 Vasculitis Diseases 0.000 description 2
- 241000863480 Vinca Species 0.000 description 2
- FPIPGXGPPPQFEQ-BOOMUCAASA-N Vitamin A Natural products OC/C=C(/C)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-BOOMUCAASA-N 0.000 description 2
- 229930003268 Vitamin C Natural products 0.000 description 2
- 229930003427 Vitamin E Natural products 0.000 description 2
- 208000034705 Vogt-Koyanagi-Harada syndrome Diseases 0.000 description 2
- 206010000496 acne Diseases 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 201000011040 acute kidney failure Diseases 0.000 description 2
- 208000012998 acute renal failure Diseases 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 206010064930 age-related macular degeneration Diseases 0.000 description 2
- 230000032683 aging Effects 0.000 description 2
- 230000010085 airway hyperresponsiveness Effects 0.000 description 2
- 208000010002 alcoholic liver cirrhosis Diseases 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 150000007824 aliphatic compounds Chemical class 0.000 description 2
- 125000005907 alkyl ester group Chemical group 0.000 description 2
- 125000000304 alkynyl group Chemical group 0.000 description 2
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 2
- 208000006778 allergic bronchopulmonary aspergillosis Diseases 0.000 description 2
- 201000010105 allergic rhinitis Diseases 0.000 description 2
- 231100000360 alopecia Toxicity 0.000 description 2
- 208000004631 alopecia areata Diseases 0.000 description 2
- 230000007953 anoxia Effects 0.000 description 2
- 230000000781 anti-lymphocytic effect Effects 0.000 description 2
- 230000000340 anti-metabolite Effects 0.000 description 2
- 230000001494 anti-thymocyte effect Effects 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 239000003146 anticoagulant agent Substances 0.000 description 2
- 229940127219 anticoagulant drug Drugs 0.000 description 2
- 229940100197 antimetabolite Drugs 0.000 description 2
- 239000002256 antimetabolite Substances 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 239000007900 aqueous suspension Substances 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 201000008937 atopic dermatitis Diseases 0.000 description 2
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 229960000686 benzalkonium chloride Drugs 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 2
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 229960001948 caffeine Drugs 0.000 description 2
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 2
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 2
- 230000036952 cancer formation Effects 0.000 description 2
- 208000035269 cancer or benign tumor Diseases 0.000 description 2
- 150000001718 carbodiimides Chemical class 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 231100000504 carcinogenesis Toxicity 0.000 description 2
- 239000005018 casein Substances 0.000 description 2
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 2
- 235000021240 caseins Nutrition 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 229920003086 cellulose ether Polymers 0.000 description 2
- JQXXHWHPUNPDRT-BQVAUQFYSA-N chembl1523493 Chemical compound O([C@](C1=O)(C)O\C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)/C=C\C=C(C)/C(=O)NC=2C(O)=C3C(O)=C4C)C)OC)C4=C1C3=C(O)C=2C=NN1CCN(C)CC1 JQXXHWHPUNPDRT-BQVAUQFYSA-N 0.000 description 2
- 230000000973 chemotherapeutic effect Effects 0.000 description 2
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 2
- 229960001231 choline Drugs 0.000 description 2
- 208000020832 chronic kidney disease Diseases 0.000 description 2
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 2
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 2
- 201000010002 cicatricial pemphigoid Diseases 0.000 description 2
- 229960001265 ciclosporin Drugs 0.000 description 2
- MYSWGUAQZAJSOK-UHFFFAOYSA-N ciprofloxacin Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)O)=CN1C1CC1 MYSWGUAQZAJSOK-UHFFFAOYSA-N 0.000 description 2
- 230000007882 cirrhosis Effects 0.000 description 2
- 208000019425 cirrhosis of liver Diseases 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- OROGSEYTTFOCAN-DNJOTXNNSA-N codeine Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC OROGSEYTTFOCAN-DNJOTXNNSA-N 0.000 description 2
- 206010009887 colitis Diseases 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 229940126208 compound 22 Drugs 0.000 description 2
- 208000010247 contact dermatitis Diseases 0.000 description 2
- 239000003246 corticosteroid Substances 0.000 description 2
- 239000012043 crude product Substances 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 230000008025 crystallization Effects 0.000 description 2
- 239000012228 culture supernatant Substances 0.000 description 2
- 201000007241 cutaneous T cell lymphoma Diseases 0.000 description 2
- 229960004397 cyclophosphamide Drugs 0.000 description 2
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 2
- 229930182912 cyclosporin Natural products 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 201000001981 dermatomyositis Diseases 0.000 description 2
- 229960003957 dexamethasone Drugs 0.000 description 2
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 2
- 239000008121 dextrose Substances 0.000 description 2
- 208000033679 diabetic kidney disease Diseases 0.000 description 2
- 235000019700 dicalcium phosphate Nutrition 0.000 description 2
- 229960001259 diclofenac Drugs 0.000 description 2
- DCOPUUMXTXDBNB-UHFFFAOYSA-N diclofenac Chemical compound OC(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl DCOPUUMXTXDBNB-UHFFFAOYSA-N 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 150000002016 disaccharides Chemical class 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 229950009791 durvalumab Drugs 0.000 description 2
- 239000000428 dust Substances 0.000 description 2
- 238000003912 environmental pollution Methods 0.000 description 2
- 201000001564 eosinophilic gastroenteritis Diseases 0.000 description 2
- 230000008029 eradication Effects 0.000 description 2
- 231100000321 erythema Toxicity 0.000 description 2
- AEUTYOVWOVBAKS-UWVGGRQHSA-N ethambutol Chemical compound CC[C@@H](CO)NCCN[C@@H](CC)CO AEUTYOVWOVBAKS-UWVGGRQHSA-N 0.000 description 2
- 229960005293 etodolac Drugs 0.000 description 2
- XFBVBWWRPKNWHW-UHFFFAOYSA-N etodolac Chemical compound C1COC(CC)(CC(O)=O)C2=N[C]3C(CC)=CC=CC3=C21 XFBVBWWRPKNWHW-UHFFFAOYSA-N 0.000 description 2
- XUFQPHANEAPEMJ-UHFFFAOYSA-N famotidine Chemical compound NC(N)=NC1=NC(CSCCC(N)=NS(N)(=O)=O)=CS1 XUFQPHANEAPEMJ-UHFFFAOYSA-N 0.000 description 2
- 229960001596 famotidine Drugs 0.000 description 2
- 229960001419 fenoprofen Drugs 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 125000001153 fluoro group Chemical group F* 0.000 description 2
- 229960002390 flurbiprofen Drugs 0.000 description 2
- SYTBZMRGLBWNTM-UHFFFAOYSA-N flurbiprofen Chemical compound FC1=CC(C(C(O)=O)C)=CC=C1C1=CC=CC=C1 SYTBZMRGLBWNTM-UHFFFAOYSA-N 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 230000035784 germination Effects 0.000 description 2
- 208000007565 gingivitis Diseases 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 206010019692 hepatic necrosis Diseases 0.000 description 2
- 208000006454 hepatitis Diseases 0.000 description 2
- 231100000283 hepatitis Toxicity 0.000 description 2
- 229960001340 histamine Drugs 0.000 description 2
- IKDUDTNKRLTJSI-UHFFFAOYSA-N hydrazine monohydrate Substances O.NN IKDUDTNKRLTJSI-UHFFFAOYSA-N 0.000 description 2
- 125000001183 hydrocarbyl group Chemical group 0.000 description 2
- OROGSEYTTFOCAN-UHFFFAOYSA-N hydrocodone Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OC OROGSEYTTFOCAN-UHFFFAOYSA-N 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 230000003463 hyperproliferative effect Effects 0.000 description 2
- 229960001680 ibuprofen Drugs 0.000 description 2
- 201000002597 ichthyosis vulgaris Diseases 0.000 description 2
- 230000001861 immunosuppressant effect Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 229960000905 indomethacin Drugs 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 229960000598 infliximab Drugs 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 150000007529 inorganic bases Chemical class 0.000 description 2
- 201000006334 interstitial nephritis Diseases 0.000 description 2
- 230000000968 intestinal effect Effects 0.000 description 2
- 230000003903 intestinal lesions Effects 0.000 description 2
- 208000012947 ischemia reperfusion injury Diseases 0.000 description 2
- 208000023569 ischemic bowel disease Diseases 0.000 description 2
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 2
- 201000010666 keratoconjunctivitis Diseases 0.000 description 2
- DKYWVDODHFEZIM-UHFFFAOYSA-N ketoprofen Chemical compound OC(=O)C(C)C1=CC=CC(C(=O)C=2C=CC=CC=2)=C1 DKYWVDODHFEZIM-UHFFFAOYSA-N 0.000 description 2
- 229960000991 ketoprofen Drugs 0.000 description 2
- 229960004752 ketorolac Drugs 0.000 description 2
- OZWKMVRBQXNZKK-UHFFFAOYSA-N ketorolac Chemical compound OC(=O)C1CCN2C1=CC=C2C(=O)C1=CC=CC=C1 OZWKMVRBQXNZKK-UHFFFAOYSA-N 0.000 description 2
- 229940043355 kinase inhibitor Drugs 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 201000010260 leiomyoma Diseases 0.000 description 2
- GWNVDXQDILPJIG-NXOLIXFESA-N leukotriene C4 Chemical compound CCCCC\C=C/C\C=C/C=C/C=C/[C@H]([C@@H](O)CCCC(O)=O)SC[C@@H](C(=O)NCC(O)=O)NC(=O)CC[C@H](N)C(O)=O GWNVDXQDILPJIG-NXOLIXFESA-N 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- KXGCNMMJRFDFNR-WDRJZQOASA-N linaclotide Chemical compound C([C@H](NC(=O)[C@@H]1CSSC[C@H]2C(=O)N[C@H]3CSSC[C@H](N)C(=O)N[C@H](C(N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N2)=O)CSSC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(N)=O)NC3=O)C(=O)N[C@H](C(NCC(=O)N1)=O)[C@H](O)C)C(O)=O)C1=CC=C(O)C=C1 KXGCNMMJRFDFNR-WDRJZQOASA-N 0.000 description 2
- 108010024409 linaclotide Proteins 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 201000005202 lung cancer Diseases 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- 206010025135 lupus erythematosus Diseases 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 239000000845 maltitol Substances 0.000 description 2
- 235000010449 maltitol Nutrition 0.000 description 2
- VQHSOMBJVWLPSR-WUJBLJFYSA-N maltitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-WUJBLJFYSA-N 0.000 description 2
- 229940035436 maltitol Drugs 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 229960001855 mannitol Drugs 0.000 description 2
- 208000008585 mastocytosis Diseases 0.000 description 2
- 229940013798 meclofenamate Drugs 0.000 description 2
- SBDNJUWAMKYJOX-UHFFFAOYSA-M meclofenamic acid(1-) Chemical compound CC1=CC=C(Cl)C(NC=2C(=CC=CC=2)C([O-])=O)=C1Cl SBDNJUWAMKYJOX-UHFFFAOYSA-M 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 229960003464 mefenamic acid Drugs 0.000 description 2
- HYYBABOKPJLUIN-UHFFFAOYSA-N mefenamic acid Chemical compound CC1=CC=CC(NC=2C(=CC=CC=2)C(O)=O)=C1C HYYBABOKPJLUIN-UHFFFAOYSA-N 0.000 description 2
- 231100001016 megaloblastic anemia Toxicity 0.000 description 2
- 229960001929 meloxicam Drugs 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- PJCHRFYQNWYOHE-UHFFFAOYSA-N methyl 6-(5-bromopyridin-3-yl)pyridine-2-carboxylate Chemical compound BrC=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)OC PJCHRFYQNWYOHE-UHFFFAOYSA-N 0.000 description 2
- OXKHNVMIALFNJO-UHFFFAOYSA-N methyl 6-(5-cyclopropylpyridin-3-yl)pyridine-2-carboxylate Chemical compound C1(CC1)C=1C=C(C=NC=1)C1=NC(=CC=C1)C(=O)OC OXKHNVMIALFNJO-UHFFFAOYSA-N 0.000 description 2
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 2
- 229960002216 methylparaben Drugs 0.000 description 2
- 229960004584 methylprednisolone Drugs 0.000 description 2
- 206010027599 migraine Diseases 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 208000013734 mononeuritis simplex Diseases 0.000 description 2
- 201000005518 mononeuropathy Diseases 0.000 description 2
- 201000006938 muscular dystrophy Diseases 0.000 description 2
- 206010028417 myasthenia gravis Diseases 0.000 description 2
- RTGDFNSFWBGLEC-SYZQJQIISA-N mycophenolate mofetil Chemical compound COC1=C(C)C=2COC(=O)C=2C(O)=C1C\C=C(/C)CCC(=O)OCCN1CCOCC1 RTGDFNSFWBGLEC-SYZQJQIISA-N 0.000 description 2
- 229960004866 mycophenolate mofetil Drugs 0.000 description 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 229960002009 naproxen Drugs 0.000 description 2
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 2
- 230000017074 necrotic cell death Effects 0.000 description 2
- 208000004995 necrotizing enterocolitis Diseases 0.000 description 2
- 208000004296 neuralgia Diseases 0.000 description 2
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 2
- 229960003301 nivolumab Drugs 0.000 description 2
- 208000008338 non-alcoholic fatty liver disease Diseases 0.000 description 2
- 206010053219 non-alcoholic steatohepatitis Diseases 0.000 description 2
- 230000000414 obstructive effect Effects 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 229960002739 oxaprozin Drugs 0.000 description 2
- OFPXSFXSNFPTHF-UHFFFAOYSA-N oxaprozin Chemical compound O1C(CCC(=O)O)=NC(C=2C=CC=CC=2)=C1C1=CC=CC=C1 OFPXSFXSNFPTHF-UHFFFAOYSA-N 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 201000006195 perinatal necrotizing enterocolitis Diseases 0.000 description 2
- 201000001245 periodontitis Diseases 0.000 description 2
- 210000004261 periodontium Anatomy 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical compound OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 2
- 239000011574 phosphorus Substances 0.000 description 2
- 239000003757 phosphotransferase inhibitor Chemical class 0.000 description 2
- 229960002702 piroxicam Drugs 0.000 description 2
- QYSPLQLAKJAUJT-UHFFFAOYSA-N piroxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC1=CC=CC=N1 QYSPLQLAKJAUJT-UHFFFAOYSA-N 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 201000004338 pollen allergy Diseases 0.000 description 2
- 201000006292 polyarteritis nodosa Diseases 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 208000005987 polymyositis Diseases 0.000 description 2
- 208000019629 polyneuritis Diseases 0.000 description 2
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 2
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 2
- 229940068968 polysorbate 80 Drugs 0.000 description 2
- 229920000053 polysorbate 80 Polymers 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 229960005205 prednisolone Drugs 0.000 description 2
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 2
- 238000004321 preservation Methods 0.000 description 2
- 208000025638 primary cutaneous T-cell non-Hodgkin lymphoma Diseases 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 2
- 229960003415 propylparaben Drugs 0.000 description 2
- 239000002599 prostaglandin synthase inhibitor Substances 0.000 description 2
- 239000003207 proteasome inhibitor Substances 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- 150000003212 purines Chemical class 0.000 description 2
- ZDYVRSLAEXCVBX-UHFFFAOYSA-N pyridinium p-toluenesulfonate Chemical compound C1=CC=[NH+]C=C1.CC1=CC=C(S([O-])(=O)=O)C=C1 ZDYVRSLAEXCVBX-UHFFFAOYSA-N 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 238000002271 resection Methods 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 201000003068 rheumatic fever Diseases 0.000 description 2
- 206010039083 rhinitis Diseases 0.000 description 2
- 229960001225 rifampicin Drugs 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 229960000953 salsalate Drugs 0.000 description 2
- 208000010157 sclerosing cholangitis Diseases 0.000 description 2
- 208000008742 seborrheic dermatitis Diseases 0.000 description 2
- 230000000580 secretagogue effect Effects 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 230000035939 shock Effects 0.000 description 2
- 208000004003 siderosis Diseases 0.000 description 2
- 201000009890 sinusitis Diseases 0.000 description 2
- 208000017520 skin disease Diseases 0.000 description 2
- 239000001509 sodium citrate Substances 0.000 description 2
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 2
- 229960002920 sorbitol Drugs 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000005846 sugar alcohols Chemical class 0.000 description 2
- NCEXYHBECQHGNR-QZQOTICOSA-N sulfasalazine Chemical compound C1=C(O)C(C(=O)O)=CC(\N=N\C=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-QZQOTICOSA-N 0.000 description 2
- 229960001940 sulfasalazine Drugs 0.000 description 2
- NCEXYHBECQHGNR-UHFFFAOYSA-N sulfasalazine Natural products C1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-UHFFFAOYSA-N 0.000 description 2
- 125000004434 sulfur atom Chemical group 0.000 description 2
- 229960000894 sulindac Drugs 0.000 description 2
- MLKXDPUZXIRXEP-MFOYZWKCSA-N sulindac Chemical compound CC1=C(CC(O)=O)C2=CC(F)=CC=C2\C1=C/C1=CC=C(S(C)=O)C=C1 MLKXDPUZXIRXEP-MFOYZWKCSA-N 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 210000001258 synovial membrane Anatomy 0.000 description 2
- 229920001059 synthetic polymer Polymers 0.000 description 2
- 229960001967 tacrolimus Drugs 0.000 description 2
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 2
- 235000019364 tetracycline Nutrition 0.000 description 2
- 150000003522 tetracyclines Chemical class 0.000 description 2
- YAPQBXQYLJRXSA-UHFFFAOYSA-N theobromine Chemical compound CN1C(=O)NC(=O)C2=C1N=CN2C YAPQBXQYLJRXSA-UHFFFAOYSA-N 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 230000036575 thermal burns Effects 0.000 description 2
- 229960004659 ticarcillin Drugs 0.000 description 2
- OHKOGUYZJXTSFX-KZFFXBSXSA-N ticarcillin Chemical compound C=1([C@@H](C(O)=O)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)C=CSC=1 OHKOGUYZJXTSFX-KZFFXBSXSA-N 0.000 description 2
- 229960001017 tolmetin Drugs 0.000 description 2
- UPSPUYADGBWSHF-UHFFFAOYSA-N tolmetin Chemical compound C1=CC(C)=CC=C1C(=O)C1=CC=C(CC(O)=O)N1C UPSPUYADGBWSHF-UHFFFAOYSA-N 0.000 description 2
- 230000008733 trauma Effects 0.000 description 2
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 229950005972 urelumab Drugs 0.000 description 2
- 230000003966 vascular damage Effects 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 235000013311 vegetables Nutrition 0.000 description 2
- 201000001862 viral hepatitis Diseases 0.000 description 2
- 235000019155 vitamin A Nutrition 0.000 description 2
- 239000011719 vitamin A Substances 0.000 description 2
- 235000019154 vitamin C Nutrition 0.000 description 2
- 239000011718 vitamin C Substances 0.000 description 2
- 229940046009 vitamin E Drugs 0.000 description 2
- 235000019165 vitamin E Nutrition 0.000 description 2
- 239000011709 vitamin E Substances 0.000 description 2
- 229940045997 vitamin a Drugs 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- VCGRFBXVSFAGGA-UHFFFAOYSA-N (1,1-dioxo-1,4-thiazinan-4-yl)-[6-[[3-(4-fluorophenyl)-5-methyl-1,2-oxazol-4-yl]methoxy]pyridin-3-yl]methanone Chemical compound CC=1ON=C(C=2C=CC(F)=CC=2)C=1COC(N=C1)=CC=C1C(=O)N1CCS(=O)(=O)CC1 VCGRFBXVSFAGGA-UHFFFAOYSA-N 0.000 description 1
- RYGOBSYXIIUFOR-UHFFFAOYSA-N (1-methylpyrazol-4-yl)boronic acid Chemical compound CN1C=C(B(O)O)C=N1 RYGOBSYXIIUFOR-UHFFFAOYSA-N 0.000 description 1
- UAOUIVVJBYDFKD-XKCDOFEDSA-N (1R,9R,10S,11R,12R,15S,18S,21R)-10,11,21-trihydroxy-8,8-dimethyl-14-methylidene-4-(prop-2-enylamino)-20-oxa-5-thia-3-azahexacyclo[9.7.2.112,15.01,9.02,6.012,18]henicosa-2(6),3-dien-13-one Chemical compound C([C@@H]1[C@@H](O)[C@@]23C(C1=C)=O)C[C@H]2[C@]12C(N=C(NCC=C)S4)=C4CC(C)(C)[C@H]1[C@H](O)[C@]3(O)OC2 UAOUIVVJBYDFKD-XKCDOFEDSA-N 0.000 description 1
- DHQMUJSACXTPEA-UHFFFAOYSA-N (2-methoxypyridin-4-yl)boronic acid Chemical compound COC1=CC(B(O)O)=CC=N1 DHQMUJSACXTPEA-UHFFFAOYSA-N 0.000 description 1
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- CIDUJQMULVCIBT-MQDUPKMGSA-N (2r,3r,4r,5r)-2-[(1s,2s,3r,4s,6r)-4-amino-3-[[(2s,3r)-3-amino-6-(aminomethyl)-3,4-dihydro-2h-pyran-2-yl]oxy]-6-(ethylamino)-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol Chemical compound O([C@@H]1[C@@H](N)C[C@H]([C@@H]([C@H]1O)O[C@@H]1[C@@H]([C@@H](NC)[C@@](C)(O)CO1)O)NCC)[C@H]1OC(CN)=CC[C@H]1N CIDUJQMULVCIBT-MQDUPKMGSA-N 0.000 description 1
- LITBAYYWXZOHAW-XDZRHBBOSA-N (2s,5r,6r)-6-[[(2r)-2-[(4-ethyl-2,3-dioxopiperazine-1-carbonyl)amino]-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;(2s,3s,5r)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4$l^{6}-thia-1-azabicyclo[3.2.0]hept Chemical compound C([C@]1(C)S([C@H]2N(C(C2)=O)[C@H]1C(O)=O)(=O)=O)N1C=CN=N1.O=C1C(=O)N(CC)CCN1C(=O)N[C@H](C=1C=CC=CC=1)C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 LITBAYYWXZOHAW-XDZRHBBOSA-N 0.000 description 1
- KNXQDJCZSVHEIW-UHFFFAOYSA-N (3-fluorophenyl)boronic acid Chemical compound OB(O)C1=CC=CC(F)=C1 KNXQDJCZSVHEIW-UHFFFAOYSA-N 0.000 description 1
- NLLGFYPSWCMUIV-UHFFFAOYSA-N (3-methoxyphenyl)boronic acid Chemical compound COC1=CC=CC(B(O)O)=C1 NLLGFYPSWCMUIV-UHFFFAOYSA-N 0.000 description 1
- BJQCPCFFYBKRLM-UHFFFAOYSA-N (3-methylphenyl)boronic acid Chemical compound CC1=CC=CC(B(O)O)=C1 BJQCPCFFYBKRLM-UHFFFAOYSA-N 0.000 description 1
- VCOPTHOUUNAYKQ-WBTCAYNUSA-N (3s)-3,6-diamino-n-[[(2s,5s,8e,11s,15s)-15-amino-11-[(6r)-2-amino-1,4,5,6-tetrahydropyrimidin-6-yl]-8-[(carbamoylamino)methylidene]-2-(hydroxymethyl)-3,6,9,12,16-pentaoxo-1,4,7,10,13-pentazacyclohexadec-5-yl]methyl]hexanamide;(3s)-3,6-diamino-n-[[(2s,5s,8 Chemical compound N1C(=O)\C(=C/NC(N)=O)NC(=O)[C@H](CNC(=O)C[C@@H](N)CCCN)NC(=O)[C@H](C)NC(=O)[C@@H](N)CNC(=O)[C@@H]1[C@@H]1NC(N)=NCC1.N1C(=O)\C(=C/NC(N)=O)NC(=O)[C@H](CNC(=O)C[C@@H](N)CCCN)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CNC(=O)[C@@H]1[C@@H]1NC(N)=NCC1 VCOPTHOUUNAYKQ-WBTCAYNUSA-N 0.000 description 1
- ODNCSIZLKBQURO-UHFFFAOYSA-N (4-ethoxycyclohexyl) 4-nitrobenzenesulfonate Chemical compound [N+](=O)([O-])C1=CC=C(C=C1)S(=O)(=O)OC1CCC(CC1)OCC ODNCSIZLKBQURO-UHFFFAOYSA-N 0.000 description 1
- NQMRYYAAICMHPE-UHFFFAOYSA-N (4-methoxyphenyl)boron Chemical compound [B]C1=CC=C(OC)C=C1 NQMRYYAAICMHPE-UHFFFAOYSA-N 0.000 description 1
- BIWQNIMLAISTBV-UHFFFAOYSA-N (4-methylphenyl)boronic acid Chemical compound CC1=CC=C(B(O)O)C=C1 BIWQNIMLAISTBV-UHFFFAOYSA-N 0.000 description 1
- SGKRLCUYIXIAHR-AKNGSSGZSA-N (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1=CC=C2[C@H](C)[C@@H]([C@H](O)[C@@H]3[C@](C(O)=C(C(N)=O)C(=O)[C@H]3N(C)C)(O)C3=O)C3=C(O)C2=C1O SGKRLCUYIXIAHR-AKNGSSGZSA-N 0.000 description 1
- FFTVPQUHLQBXQZ-KVUCHLLUSA-N (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O FFTVPQUHLQBXQZ-KVUCHLLUSA-N 0.000 description 1
- SOVUOXKZCCAWOJ-HJYUBDRYSA-N (4s,4as,5ar,12ar)-9-[[2-(tert-butylamino)acetyl]amino]-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=C(NC(=O)CNC(C)(C)C)C(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O SOVUOXKZCCAWOJ-HJYUBDRYSA-N 0.000 description 1
- GUXHBMASAHGULD-SEYHBJAFSA-N (4s,4as,5as,6s,12ar)-7-chloro-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1([C@H]2O)=C(Cl)C=CC(O)=C1C(O)=C1[C@@H]2C[C@H]2[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]2(O)C1=O GUXHBMASAHGULD-SEYHBJAFSA-N 0.000 description 1
- ICCGFOKNFZWCTJ-UHFFFAOYSA-N (5-bromopyridin-3-yl)boronic acid Chemical compound OB(O)C1=CN=CC(Br)=C1 ICCGFOKNFZWCTJ-UHFFFAOYSA-N 0.000 description 1
- ISDFOFZTZUILPE-UHFFFAOYSA-N (5-methoxypyridin-3-yl)boronic acid Chemical compound COC1=CN=CC(B(O)O)=C1 ISDFOFZTZUILPE-UHFFFAOYSA-N 0.000 description 1
- ZYXMOCKFGABUGK-UHFFFAOYSA-N (5-methyl-1h-pyrazol-4-yl)boronic acid Chemical compound CC1=NNC=C1B(O)O ZYXMOCKFGABUGK-UHFFFAOYSA-N 0.000 description 1
- REONQWGHSQHTAC-UHFFFAOYSA-N (5-methylpyridin-3-yl)boronic acid Chemical compound CC1=CN=CC(B(O)O)=C1 REONQWGHSQHTAC-UHFFFAOYSA-N 0.000 description 1
- YGXNPQDXWDCWET-UHFFFAOYSA-N (5-methylsulfonylpyridin-3-yl)boronic acid Chemical compound CS(=O)(=O)C1=CN=CC(B(O)O)=C1 YGXNPQDXWDCWET-UHFFFAOYSA-N 0.000 description 1
- XQHUPBAXLPBXOT-UHFFFAOYSA-N (5-propan-2-yloxypyridin-3-yl)boronic acid Chemical compound CC(C)OC1=CN=CC(B(O)O)=C1 XQHUPBAXLPBXOT-UHFFFAOYSA-N 0.000 description 1
- NPJBPJIQMCHZLJ-UHFFFAOYSA-N (6-aminopyridin-3-yl)boronic acid Chemical compound NC1=CC=C(B(O)O)C=N1 NPJBPJIQMCHZLJ-UHFFFAOYSA-N 0.000 description 1
- DHADXDMPEUWEAS-UHFFFAOYSA-N (6-methoxypyridin-3-yl)boronic acid Chemical compound COC1=CC=C(B(O)O)C=N1 DHADXDMPEUWEAS-UHFFFAOYSA-N 0.000 description 1
- MZUSCPDSQJSBSY-UHFFFAOYSA-N (6-methylpyridin-3-yl)boronic acid Chemical compound CC1=CC=C(B(O)O)C=N1 MZUSCPDSQJSBSY-UHFFFAOYSA-N 0.000 description 1
- YYGNTYWPHWGJRM-UHFFFAOYSA-N (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene Chemical compound CC(C)=CCCC(C)=CCCC(C)=CCCC=C(C)CCC=C(C)CCC=C(C)C YYGNTYWPHWGJRM-UHFFFAOYSA-N 0.000 description 1
- WDLWHQDACQUCJR-ZAMMOSSLSA-N (6r,7r)-7-[[(2r)-2-azaniumyl-2-(4-hydroxyphenyl)acetyl]amino]-8-oxo-3-[(e)-prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)/C=C/C)C(O)=O)=CC=C(O)C=C1 WDLWHQDACQUCJR-ZAMMOSSLSA-N 0.000 description 1
- IPYWNMVPZOAFOQ-NABDTECSSA-N (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(carboxymethoxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;trihydrate Chemical compound O.O.O.S1C(N)=NC(C(=N\OCC(O)=O)\C(=O)N[C@@H]2C(N3C(=C(C=C)CS[C@@H]32)C(O)=O)=O)=C1 IPYWNMVPZOAFOQ-NABDTECSSA-N 0.000 description 1
- MMRINLZOZVAPDZ-LSGRDSQZSA-N (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(1-methylpyrrolidin-1-ium-1-yl)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;chloride Chemical compound Cl.S([C@@H]1[C@@H](C(N1C=1C([O-])=O)=O)NC(=O)\C(=N/OC)C=2N=C(N)SC=2)CC=1C[N+]1(C)CCCC1 MMRINLZOZVAPDZ-LSGRDSQZSA-N 0.000 description 1
- HMLGSIZOMSVISS-ONJSNURVSA-N (7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(2,2-dimethylpropanoyloxymethoxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Chemical compound N([C@@H]1C(N2C(=C(C=C)CSC21)C(O)=O)=O)C(=O)\C(=N/OCOC(=O)C(C)(C)C)C1=CSC(N)=N1 HMLGSIZOMSVISS-ONJSNURVSA-N 0.000 description 1
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 description 1
- MINDHVHHQZYEEK-UHFFFAOYSA-N (E)-(2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-(beta)-methyl-2H-pyran-2-crotonic acid ester with 9-hydroxynonanoic acid Natural products CC(O)C(C)C1OC1CC1C(O)C(O)C(CC(C)=CC(=O)OCCCCCCCCC(O)=O)OC1 MINDHVHHQZYEEK-UHFFFAOYSA-N 0.000 description 1
- HFXWYVXONBPYRW-VOTSOKGWSA-N (E)-3-(dimethylamino)-1-(5-methoxypyridin-2-yl)prop-2-en-1-one Chemical compound CN(/C=C/C(=O)C1=NC=C(C=C1)OC)C HFXWYVXONBPYRW-VOTSOKGWSA-N 0.000 description 1
- RXZBMPWDPOLZGW-XMRMVWPWSA-N (E)-roxithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=N/OCOCCOC)/[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 RXZBMPWDPOLZGW-XMRMVWPWSA-N 0.000 description 1
- QKDHBVNJCZBTMR-LLVKDONJSA-N (R)-temafloxacin Chemical compound C1CN[C@H](C)CN1C(C(=C1)F)=CC2=C1C(=O)C(C(O)=O)=CN2C1=CC=C(F)C=C1F QKDHBVNJCZBTMR-LLVKDONJSA-N 0.000 description 1
- TVYLLZQTGLZFBW-ZBFHGGJFSA-N (R,R)-tramadol Chemical compound COC1=CC=CC([C@]2(O)[C@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-ZBFHGGJFSA-N 0.000 description 1
- XUBOMFCQGDBHNK-JTQLQIEISA-N (S)-gatifloxacin Chemical compound FC1=CC(C(C(C(O)=O)=CN2C3CC3)=O)=C2C(OC)=C1N1CCN[C@@H](C)C1 XUBOMFCQGDBHNK-JTQLQIEISA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- LRANPJDWHYRCER-UHFFFAOYSA-N 1,2-diazepine Chemical compound N1C=CC=CC=N1 LRANPJDWHYRCER-UHFFFAOYSA-N 0.000 description 1
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- FTNJQNQLEGKTGD-UHFFFAOYSA-N 1,3-benzodioxole Chemical compound C1=CC=C2OCOC2=C1 FTNJQNQLEGKTGD-UHFFFAOYSA-N 0.000 description 1
- WORJRXHJTUTINR-UHFFFAOYSA-N 1,4-dioxane;hydron;chloride Chemical compound Cl.C1COCCO1 WORJRXHJTUTINR-UHFFFAOYSA-N 0.000 description 1
- RCHPXFFBQVRUPD-UHFFFAOYSA-N 1-(1-methyl-4-nitropyrazol-3-yl)piperidine Chemical compound CN1N=C(C(=C1)[N+](=O)[O-])N1CCCCC1 RCHPXFFBQVRUPD-UHFFFAOYSA-N 0.000 description 1
- AYBJWQOPVRJOSD-UHFFFAOYSA-N 1-(2-methyl-4-nitropyrazol-3-yl)piperidine Chemical compound CN1N=CC([N+]([O-])=O)=C1N1CCCCC1 AYBJWQOPVRJOSD-UHFFFAOYSA-N 0.000 description 1
- JCFAFZULKPYMQV-UHFFFAOYSA-N 1-(5-methoxypyridin-2-yl)ethanone Chemical compound COC1=CC=C(C(C)=O)N=C1 JCFAFZULKPYMQV-UHFFFAOYSA-N 0.000 description 1
- PVOAHINGSUIXLS-UHFFFAOYSA-N 1-Methylpiperazine Chemical compound CN1CCNCC1 PVOAHINGSUIXLS-UHFFFAOYSA-N 0.000 description 1
- 102100025573 1-alkyl-2-acetylglycerophosphocholine esterase Human genes 0.000 description 1
- LLSRLLPHUVVLQJ-UHFFFAOYSA-N 1-cycloheptyldiazepane Chemical compound C1CCCCCC1N1NCCCCC1 LLSRLLPHUVVLQJ-UHFFFAOYSA-N 0.000 description 1
- SJJCQDRGABAVBB-UHFFFAOYSA-N 1-hydroxy-2-naphthoic acid Chemical compound C1=CC=CC2=C(O)C(C(=O)O)=CC=C21 SJJCQDRGABAVBB-UHFFFAOYSA-N 0.000 description 1
- REFZGDUPQPZPIP-UHFFFAOYSA-N 1-methyl-3-morpholin-4-ylpyrazol-4-amine Chemical compound CN1C=C(N)C(N2CCOCC2)=N1 REFZGDUPQPZPIP-UHFFFAOYSA-N 0.000 description 1
- AGSABRJWZQWJIL-UHFFFAOYSA-N 1-methyl-3-piperidin-1-ylpyrazol-4-amine Chemical compound CN1C=C(N)C(N2CCCCC2)=N1 AGSABRJWZQWJIL-UHFFFAOYSA-N 0.000 description 1
- GYPVTYIWNZLODS-UHFFFAOYSA-N 1-methyl-3-pyrrolidin-1-ylpyrazol-4-amine Chemical compound CN1C=C(N)C(N2CCCC2)=N1 GYPVTYIWNZLODS-UHFFFAOYSA-N 0.000 description 1
- WJOHMRCBYOJCHT-UHFFFAOYSA-N 1-methyl-4-[2-(4-nitro-3-pyridin-2-ylpyrazol-1-yl)ethyl]piperazine Chemical compound CN1CCN(CC1)CCN1N=C(C(=C1)[N+](=O)[O-])C1=NC=CC=C1 WJOHMRCBYOJCHT-UHFFFAOYSA-N 0.000 description 1
- LTPUJLYMKBQHBX-UHFFFAOYSA-N 1-methyl-4-nitro-3-pyrrolidin-1-ylpyrazole Chemical compound CN1N=C(C(=C1)[N+](=O)[O-])N1CCCC1 LTPUJLYMKBQHBX-UHFFFAOYSA-N 0.000 description 1
- VNCFDMNAMVXUCT-UHFFFAOYSA-N 1-propan-2-yl-3-pyridin-2-ylpyrazol-4-amine Chemical compound CC(C)N1C=C(N)C(C=2N=CC=CC=2)=N1 VNCFDMNAMVXUCT-UHFFFAOYSA-N 0.000 description 1
- SPXOTSHWBDUUMT-UHFFFAOYSA-N 138-42-1 Chemical compound OS(=O)(=O)C1=CC=C([N+]([O-])=O)C=C1 SPXOTSHWBDUUMT-UHFFFAOYSA-N 0.000 description 1
- VHADYSUJZAPXOW-UHFFFAOYSA-N 1h-indol-5-ylboronic acid Chemical compound OB(O)C1=CC=C2NC=CC2=C1 VHADYSUJZAPXOW-UHFFFAOYSA-N 0.000 description 1
- ZVMHOIWRCCZGPZ-UHFFFAOYSA-N 1h-indol-6-ylboronic acid Chemical compound OB(O)C1=CC=C2C=CNC2=C1 ZVMHOIWRCCZGPZ-UHFFFAOYSA-N 0.000 description 1
- KEZNMOUMHOZFRA-UHFFFAOYSA-N 1h-pyrazol-4-ylboronic acid Chemical compound OB(O)C=1C=NNC=1 KEZNMOUMHOZFRA-UHFFFAOYSA-N 0.000 description 1
- NEUWPDLMDVINSN-UHFFFAOYSA-N 1h-pyrazol-5-ylboronic acid Chemical compound OB(O)C=1C=CNN=1 NEUWPDLMDVINSN-UHFFFAOYSA-N 0.000 description 1
- WADSQZHEAXPENM-UHFFFAOYSA-N 1h-pyrrol-2-ylboronic acid Chemical compound OB(O)C1=CC=CN1 WADSQZHEAXPENM-UHFFFAOYSA-N 0.000 description 1
- ZITITTQOWAPHDT-UHFFFAOYSA-N 1h-pyrrol-3-ylboronic acid Chemical compound OB(O)C=1C=CNC=1 ZITITTQOWAPHDT-UHFFFAOYSA-N 0.000 description 1
- KCHKRCSVKZQYCA-UHFFFAOYSA-N 1h-pyrrolo[2,3-b]pyridin-5-ylboronic acid Chemical compound OB(O)C1=CN=C2NC=CC2=C1 KCHKRCSVKZQYCA-UHFFFAOYSA-N 0.000 description 1
- UWKQJZCTQGMHKD-UHFFFAOYSA-N 2,6-di-tert-butylpyridine Chemical compound CC(C)(C)C1=CC=CC(C(C)(C)C)=N1 UWKQJZCTQGMHKD-UHFFFAOYSA-N 0.000 description 1
- FURIZZHQBLGJPP-UHFFFAOYSA-N 2-(1-propan-2-ylpyrazol-3-yl)pyridine Chemical compound CC(C)N1C=CC(C=2N=CC=CC=2)=N1 FURIZZHQBLGJPP-UHFFFAOYSA-N 0.000 description 1
- ZAACCUDXIQXAFN-UHFFFAOYSA-N 2-(4-nitro-1-propan-2-ylpyrazol-3-yl)pyridine Chemical compound CC(C)N1C=C(C(=N1)C1=CC=CC=N1)[N+]([O-])=O ZAACCUDXIQXAFN-UHFFFAOYSA-N 0.000 description 1
- NBLNFSHWRISQGX-UHFFFAOYSA-N 2-[(5-chloro-4-nitropyrazol-1-yl)methoxy]ethyl-trimethylsilane Chemical compound C[Si](C)(C)CCOCN1N=CC([N+]([O-])=O)=C1Cl NBLNFSHWRISQGX-UHFFFAOYSA-N 0.000 description 1
- WZPBZJONDBGPKJ-VEHQQRBSSA-L 2-[(z)-[1-(2-amino-1,3-thiazol-4-yl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfonatoazetidin-3-yl]amino]-2-oxoethylidene]amino]oxy-2-methylpropanoate Chemical compound O=C1N(S([O-])(=O)=O)[C@@H](C)[C@@H]1NC(=O)C(=N/OC(C)(C)C([O-])=O)\C1=CSC(N)=N1 WZPBZJONDBGPKJ-VEHQQRBSSA-L 0.000 description 1
- FIWGJYZBBBBXPY-UHFFFAOYSA-N 2-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-yl]propan-2-ol Chemical compound CC(C)(O)C1=CN=CC(B2OC(C)(C)C(C)(C)O2)=C1 FIWGJYZBBBBXPY-UHFFFAOYSA-N 0.000 description 1
- MSWZFWKMSRAUBD-IVMDWMLBSA-N 2-amino-2-deoxy-D-glucopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-IVMDWMLBSA-N 0.000 description 1
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 description 1
- YSUIQYOGTINQIN-UZFYAQMZSA-N 2-amino-9-[(1S,6R,8R,9S,10R,15R,17R,18R)-8-(6-aminopurin-9-yl)-9,18-difluoro-3,12-dihydroxy-3,12-bis(sulfanylidene)-2,4,7,11,13,16-hexaoxa-3lambda5,12lambda5-diphosphatricyclo[13.2.1.06,10]octadecan-17-yl]-1H-purin-6-one Chemical compound NC1=NC2=C(N=CN2[C@@H]2O[C@@H]3COP(S)(=O)O[C@@H]4[C@@H](COP(S)(=O)O[C@@H]2[C@@H]3F)O[C@H]([C@H]4F)N2C=NC3=C2N=CN=C3N)C(=O)N1 YSUIQYOGTINQIN-UZFYAQMZSA-N 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- 229940013085 2-diethylaminoethanol Drugs 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- ASUDFOJKTJLAIK-UHFFFAOYSA-N 2-methoxyethanamine Chemical compound COCCN ASUDFOJKTJLAIK-UHFFFAOYSA-N 0.000 description 1
- 125000004200 2-methoxyethyl group Chemical group [H]C([H])([H])OC([H])([H])C([H])([H])* 0.000 description 1
- CTRPRMNBTVRDFH-UHFFFAOYSA-N 2-n-methyl-1,3,5-triazine-2,4,6-triamine Chemical class CNC1=NC(N)=NC(N)=N1 CTRPRMNBTVRDFH-UHFFFAOYSA-N 0.000 description 1
- FUBFWTUFPGFHOJ-UHFFFAOYSA-N 2-nitrofuran Chemical class [O-][N+](=O)C1=CC=CO1 FUBFWTUFPGFHOJ-UHFFFAOYSA-N 0.000 description 1
- 125000006088 2-oxoazepinyl group Chemical group 0.000 description 1
- 125000004638 2-oxopiperazinyl group Chemical group O=C1N(CCNC1)* 0.000 description 1
- 125000004637 2-oxopiperidinyl group Chemical group O=C1N(CCCC1)* 0.000 description 1
- 125000000389 2-pyrrolyl group Chemical group [H]N1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- LJGHYPLBDBRCRZ-UHFFFAOYSA-N 3-(3-aminophenyl)sulfonylaniline Chemical group NC1=CC=CC(S(=O)(=O)C=2C=C(N)C=CC=2)=C1 LJGHYPLBDBRCRZ-UHFFFAOYSA-N 0.000 description 1
- WEVYNIUIFUYDGI-UHFFFAOYSA-N 3-[6-[4-(trifluoromethoxy)anilino]-4-pyrimidinyl]benzamide Chemical compound NC(=O)C1=CC=CC(C=2N=CN=C(NC=3C=CC(OC(F)(F)F)=CC=3)C=2)=C1 WEVYNIUIFUYDGI-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- 125000004180 3-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C(F)=C1[H] 0.000 description 1
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- 125000004207 3-methoxyphenyl group Chemical group [H]C1=C([H])C(*)=C([H])C(OC([H])([H])[H])=C1[H] 0.000 description 1
- YZASJUALPAEXBC-UHFFFAOYSA-N 4-(1-methyl-4-nitropyrazol-3-yl)morpholine Chemical compound CN1N=C(C(=C1)[N+](=O)[O-])N1CCOCC1 YZASJUALPAEXBC-UHFFFAOYSA-N 0.000 description 1
- UGHOJWLZPSTTKB-UHFFFAOYSA-N 4-(2-bromoethyl)morpholine;hydrobromide Chemical compound Br.BrCCN1CCOCC1 UGHOJWLZPSTTKB-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- XMOHTWBLUXNLLR-UHFFFAOYSA-N 4-(4-nitro-1h-pyrazol-5-yl)morpholine Chemical compound [O-][N+](=O)C1=CNN=C1N1CCOCC1 XMOHTWBLUXNLLR-UHFFFAOYSA-N 0.000 description 1
- CGOUSLMICDEEGL-UHFFFAOYSA-N 4-[2-(3-pyridin-2-ylpyrazol-1-yl)ethyl]morpholine Chemical compound N1=C(C=CC=C1)C1=NN(C=C1)CCN1CCOCC1 CGOUSLMICDEEGL-UHFFFAOYSA-N 0.000 description 1
- NIDCIXMXEYGXCL-UHFFFAOYSA-N 4-[2-(4-nitro-3-pyridin-2-ylpyrazol-1-yl)ethyl]morpholine Chemical compound [N+](=O)([O-])C=1C(=NN(C=1)CCN1CCOCC1)C1=NC=CC=C1 NIDCIXMXEYGXCL-UHFFFAOYSA-N 0.000 description 1
- WZRJTRPJURQBRM-UHFFFAOYSA-N 4-amino-n-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide;5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine Chemical compound O1C(C)=CC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1.COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 WZRJTRPJURQBRM-UHFFFAOYSA-N 0.000 description 1
- KVCQTKNUUQOELD-UHFFFAOYSA-N 4-amino-n-[1-(3-chloro-2-fluoroanilino)-6-methylisoquinolin-5-yl]thieno[3,2-d]pyrimidine-7-carboxamide Chemical compound N=1C=CC2=C(NC(=O)C=3C4=NC=NC(N)=C4SC=3)C(C)=CC=C2C=1NC1=CC=CC(Cl)=C1F KVCQTKNUUQOELD-UHFFFAOYSA-N 0.000 description 1
- 125000004801 4-cyanophenyl group Chemical group [H]C1=C([H])C(C#N)=C([H])C([H])=C1* 0.000 description 1
- 125000001255 4-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1F 0.000 description 1
- LBUNNMJLXWQQBY-UHFFFAOYSA-N 4-fluorophenylboronic acid Chemical compound OB(O)C1=CC=C(F)C=C1 LBUNNMJLXWQQBY-UHFFFAOYSA-N 0.000 description 1
- 125000004172 4-methoxyphenyl group Chemical group [H]C1=C([H])C(OC([H])([H])[H])=C([H])C([H])=C1* 0.000 description 1
- MRNATRCYVZJFLC-UHFFFAOYSA-N 4-nitro-5-pyrrolidin-1-yl-1H-pyrazole Chemical compound [N+](=O)([O-])C=1C(=NNC=1)N1CCCC1 MRNATRCYVZJFLC-UHFFFAOYSA-N 0.000 description 1
- JXRGUPLJCCDGKG-UHFFFAOYSA-N 4-nitrobenzenesulfonyl chloride Chemical compound [O-][N+](=O)C1=CC=C(S(Cl)(=O)=O)C=C1 JXRGUPLJCCDGKG-UHFFFAOYSA-N 0.000 description 1
- 125000000339 4-pyridyl group Chemical group N1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 description 1
- DAISWHFZWZZBBD-UHFFFAOYSA-N 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-amine Chemical compound O1C(C)(C)C(C)(C)OB1C1=CN=CC(N)=C1 DAISWHFZWZZBBD-UHFFFAOYSA-N 0.000 description 1
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- 125000006163 5-membered heteroaryl group Chemical group 0.000 description 1
- NTLHRXLEEUZSGN-UHFFFAOYSA-N 5-methoxy-2-(2-methyl-4-nitropyrazol-3-yl)pyridine Chemical compound COC1=CC=C(N=C1)C1=C(C=NN1C)[N+]([O-])=O NTLHRXLEEUZSGN-UHFFFAOYSA-N 0.000 description 1
- GPIPOBMZCUQXEF-UHFFFAOYSA-N 6-(1-methylpyrazol-4-yl)-N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound CN1N=CC(=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C1COC1)C1=NC=CC=C1 GPIPOBMZCUQXEF-UHFFFAOYSA-N 0.000 description 1
- LVVACKKTPDWXJM-UHFFFAOYSA-N 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1h-pyrrolo[3,2-b]pyridine Chemical compound O1C(C)(C)C(C)(C)OB1C1=CN=C(C=CN2)C2=C1 LVVACKKTPDWXJM-UHFFFAOYSA-N 0.000 description 1
- SQZYUUWZPGSAER-UHFFFAOYSA-N 6-(5-methyl-1H-pyrazol-3-yl)-N-[1-(oxetan-3-yl)-3-pyridin-2-ylpyrazol-4-yl]pyridine-2-carboxamide Chemical compound CC1=NNC(=C1)C1=CC=CC(=N1)C(=O)NC=1C(=NN(C=1)C1COC1)C1=NC=CC=C1 SQZYUUWZPGSAER-UHFFFAOYSA-N 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- XZIIFPSPUDAGJM-UHFFFAOYSA-N 6-chloro-2-n,2-n-diethylpyrimidine-2,4-diamine Chemical compound CCN(CC)C1=NC(N)=CC(Cl)=N1 XZIIFPSPUDAGJM-UHFFFAOYSA-N 0.000 description 1
- ZXFHUDPUFXOJLA-UHFFFAOYSA-N 6-pyridin-3-ylpyridine-2-carboxamide Chemical compound N1=C(C=CC=C1C(=O)N)C=1C=NC=CC=1 ZXFHUDPUFXOJLA-UHFFFAOYSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 1
- CYJRNFFLTBEQSQ-UHFFFAOYSA-N 8-(3-methyl-1-benzothiophen-5-yl)-N-(4-methylsulfonylpyridin-3-yl)quinoxalin-6-amine Chemical compound CS(=O)(=O)C1=C(C=NC=C1)NC=1C=C2N=CC=NC2=C(C=1)C=1C=CC2=C(C(=CS2)C)C=1 CYJRNFFLTBEQSQ-UHFFFAOYSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- GSDSWSVVBLHKDQ-UHFFFAOYSA-N 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid Chemical compound FC1=CC(C(C(C(O)=O)=C2)=O)=C3N2C(C)COC3=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 208000007815 Acquired Hyperostosis Syndrome Diseases 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 206010001076 Acute sinusitis Diseases 0.000 description 1
- 102000011767 Acute-Phase Proteins Human genes 0.000 description 1
- 108010062271 Acute-Phase Proteins Proteins 0.000 description 1
- 208000003200 Adenoma Diseases 0.000 description 1
- 206010001233 Adenoma benign Diseases 0.000 description 1
- 208000026326 Adult-onset Still disease Diseases 0.000 description 1
- 208000022309 Alcoholic Liver disease Diseases 0.000 description 1
- 235000019489 Almond oil Nutrition 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- 244000144730 Amygdalus persica Species 0.000 description 1
- 208000008884 Aneurysmal Bone Cysts Diseases 0.000 description 1
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 1
- WZPBZJONDBGPKJ-UHFFFAOYSA-N Antibiotic SQ 26917 Natural products O=C1N(S(O)(=O)=O)C(C)C1NC(=O)C(=NOC(C)(C)C(O)=O)C1=CSC(N)=N1 WZPBZJONDBGPKJ-UHFFFAOYSA-N 0.000 description 1
- QNZCBYKSOIHPEH-UHFFFAOYSA-N Apixaban Chemical compound C1=CC(OC)=CC=C1N1C(C(=O)N(CC2)C=3C=CC(=CC=3)N3C(CCCC3)=O)=C2C(C(N)=O)=N1 QNZCBYKSOIHPEH-UHFFFAOYSA-N 0.000 description 1
- 229930091051 Arenine Natural products 0.000 description 1
- 206010003210 Arteriosclerosis Diseases 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- 108010011485 Aspartame Proteins 0.000 description 1
- 206010003557 Asthma exercise induced Diseases 0.000 description 1
- 206010003645 Atopy Diseases 0.000 description 1
- 208000032568 B-cell prolymphocytic leukaemia Diseases 0.000 description 1
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 1
- 239000012664 BCL-2-inhibitor Substances 0.000 description 1
- 229940125565 BMS-986016 Drugs 0.000 description 1
- 229940124291 BTK inhibitor Drugs 0.000 description 1
- 108010001478 Bacitracin Proteins 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 108010027612 Batroxobin Proteins 0.000 description 1
- 229940123711 Bcl2 inhibitor Drugs 0.000 description 1
- 206010004446 Benign prostatic hyperplasia Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 206010051728 Bone erosion Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- 241000167854 Bourreria succulenta Species 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 206010006448 Bronchiolitis Diseases 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- 208000023611 Burkitt leukaemia Diseases 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- JQUCWIWWWKZNCS-LESHARBVSA-N C(C1=CC=CC=C1)(=O)NC=1SC[C@H]2[C@@](N1)(CO[C@H](C2)C)C=2SC=C(N2)NC(=O)C2=NC=C(C=C2)OC(F)F Chemical compound C(C1=CC=CC=C1)(=O)NC=1SC[C@H]2[C@@](N1)(CO[C@H](C2)C)C=2SC=C(N2)NC(=O)C2=NC=C(C=C2)OC(F)F JQUCWIWWWKZNCS-LESHARBVSA-N 0.000 description 1
- FTGOEGANYLGKMK-UHFFFAOYSA-N C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)C1=CC([N+]([O-])=O)=CC=C1S(Cl)(=O)=O Chemical compound C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)C1=CC([N+]([O-])=O)=CC=C1S(Cl)(=O)=O FTGOEGANYLGKMK-UHFFFAOYSA-N 0.000 description 1
- 125000000041 C6-C10 aryl group Chemical group 0.000 description 1
- HGYRTEROZSENSA-UHFFFAOYSA-N CCCCCCCCCCCCCCCC(O)=O.CCCCCCCCCCCCCCCC(O)=O.[SeH2] Chemical compound CCCCCCCCCCCCCCCC(O)=O.CCCCCCCCCCCCCCCC(O)=O.[SeH2] HGYRTEROZSENSA-UHFFFAOYSA-N 0.000 description 1
- JGLMVXWAHNTPRF-CMDGGOBGSA-N CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O Chemical compound CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O JGLMVXWAHNTPRF-CMDGGOBGSA-N 0.000 description 1
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 229940122739 Calcineurin inhibitor Drugs 0.000 description 1
- 101710192106 Calcineurin-binding protein cabin-1 Proteins 0.000 description 1
- 102100024123 Calcineurin-binding protein cabin-1 Human genes 0.000 description 1
- 108010065839 Capreomycin Proteins 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 208000005024 Castleman disease Diseases 0.000 description 1
- GNWUOVJNSFPWDD-XMZRARIVSA-M Cefoxitin sodium Chemical compound [Na+].N([C@]1(OC)C(N2C(=C(COC(N)=O)CS[C@@H]21)C([O-])=O)=O)C(=O)CC1=CC=CS1 GNWUOVJNSFPWDD-XMZRARIVSA-M 0.000 description 1
- PTHCMJGKKRQCBF-UHFFFAOYSA-N Cellulose, microcrystalline Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC)C(CO)O1 PTHCMJGKKRQCBF-UHFFFAOYSA-N 0.000 description 1
- 229930186147 Cephalosporin Natural products 0.000 description 1
- JZUFKLXOESDKRF-UHFFFAOYSA-N Chlorothiazide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NCNS2(=O)=O JZUFKLXOESDKRF-UHFFFAOYSA-N 0.000 description 1
- 206010008690 Chondrocalcinosis pyrophosphate Diseases 0.000 description 1
- 206010009137 Chronic sinusitis Diseases 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- HZZVJAQRINQKSD-UHFFFAOYSA-N Clavulanic acid Natural products OC(=O)C1C(=CCO)OC2CC(=O)N21 HZZVJAQRINQKSD-UHFFFAOYSA-N 0.000 description 1
- 108010078777 Colistin Proteins 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000032170 Congenital Abnormalities Diseases 0.000 description 1
- 206010010741 Conjunctivitis Diseases 0.000 description 1
- 206010010744 Conjunctivitis allergic Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 206010011224 Cough Diseases 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- HTJDQJBWANPRPF-UHFFFAOYSA-N Cyclopropylamine Chemical compound NC1CC1 HTJDQJBWANPRPF-UHFFFAOYSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- DYDCUQKUCUHJBH-UWTATZPHSA-N D-Cycloserine Chemical compound N[C@@H]1CONC1=O DYDCUQKUCUHJBH-UWTATZPHSA-N 0.000 description 1
- DYDCUQKUCUHJBH-UHFFFAOYSA-N D-Cycloserine Natural products NC1CONC1=O DYDCUQKUCUHJBH-UHFFFAOYSA-N 0.000 description 1
- 102100036279 DNA (cytosine-5)-methyltransferase 1 Human genes 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- MQJKPEGWNLWLTK-UHFFFAOYSA-N Dapsone Chemical compound C1=CC(N)=CC=C1S(=O)(=O)C1=CC=C(N)C=C1 MQJKPEGWNLWLTK-UHFFFAOYSA-N 0.000 description 1
- 108010013198 Daptomycin Proteins 0.000 description 1
- ZBNZXTGUTAYRHI-UHFFFAOYSA-N Dasatinib Chemical compound C=1C(N2CCN(CCO)CC2)=NC(C)=NC=1NC(S1)=NC=C1C(=O)NC1=C(C)C=CC=C1Cl ZBNZXTGUTAYRHI-UHFFFAOYSA-N 0.000 description 1
- 101710088194 Dehydrogenase Proteins 0.000 description 1
- FMTDIUIBLCQGJB-UHFFFAOYSA-N Demethylchlortetracyclin Natural products C1C2C(O)C3=C(Cl)C=CC(O)=C3C(=O)C2=C(O)C2(O)C1C(N(C)C)C(O)=C(C(N)=O)C2=O FMTDIUIBLCQGJB-UHFFFAOYSA-N 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 206010053177 Epidermolysis Diseases 0.000 description 1
- 206010014989 Epidermolysis bullosa Diseases 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 208000004657 Exercise-Induced Asthma Diseases 0.000 description 1
- UIOFUWFRIANQPC-JKIFEVAISA-N Floxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=C(F)C=CC=C1Cl UIOFUWFRIANQPC-JKIFEVAISA-N 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- IECPWNUMDGFDKC-UHFFFAOYSA-N Fusicsaeure Natural products C12C(O)CC3C(=C(CCC=C(C)C)C(O)=O)C(OC(C)=O)CC3(C)C1(C)CCC1C2(C)CCC(O)C1C IECPWNUMDGFDKC-UHFFFAOYSA-N 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- 108010015899 Glycopeptides Proteins 0.000 description 1
- 102000002068 Glycopeptides Human genes 0.000 description 1
- 240000004670 Glycyrrhiza echinata Species 0.000 description 1
- 235000001453 Glycyrrhiza echinata Nutrition 0.000 description 1
- 235000006200 Glycyrrhiza glabra Nutrition 0.000 description 1
- 235000017382 Glycyrrhiza lepidota Nutrition 0.000 description 1
- 208000009329 Graft vs Host Disease Diseases 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- AIJTTZAVMXIJGM-UHFFFAOYSA-N Grepafloxacin Chemical compound C1CNC(C)CN1C(C(=C1C)F)=CC2=C1C(=O)C(C(O)=O)=CN2C1CC1 AIJTTZAVMXIJGM-UHFFFAOYSA-N 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 241000700721 Hepatitis B virus Species 0.000 description 1
- 208000005100 Herpetic Keratitis Diseases 0.000 description 1
- 108010007267 Hirudins Proteins 0.000 description 1
- 102000007625 Hirudins Human genes 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000931098 Homo sapiens DNA (cytosine-5)-methyltransferase 1 Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 1
- GRSZFWQUAKGDAV-KQYNXXCUSA-N IMP Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H]1N1C(NC=NC2=O)=C2N=C1 GRSZFWQUAKGDAV-KQYNXXCUSA-N 0.000 description 1
- 206010021245 Idiopathic thrombocytopenic purpura Diseases 0.000 description 1
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 1
- 241000764773 Inna Species 0.000 description 1
- 229940121740 Inosine monophosphate dehydrogenase inhibitor Drugs 0.000 description 1
- 102000051628 Interleukin-1 receptor antagonist Human genes 0.000 description 1
- 108700021006 Interleukin-1 receptor antagonist Proteins 0.000 description 1
- 102100026018 Interleukin-1 receptor antagonist protein Human genes 0.000 description 1
- 101710144554 Interleukin-1 receptor antagonist protein Proteins 0.000 description 1
- 102000003814 Interleukin-10 Human genes 0.000 description 1
- 108090000174 Interleukin-10 Proteins 0.000 description 1
- 102000000588 Interleukin-2 Human genes 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 108010076561 Interleukin-23 Subunit p19 Proteins 0.000 description 1
- 102100036705 Interleukin-23 subunit alpha Human genes 0.000 description 1
- JUZNIMUFDBIJCM-ANEDZVCMSA-N Invanz Chemical compound O=C([C@H]1NC[C@H](C1)SC=1[C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)NC1=CC=CC(C(O)=O)=C1 JUZNIMUFDBIJCM-ANEDZVCMSA-N 0.000 description 1
- 206010023232 Joint swelling Diseases 0.000 description 1
- 208000003456 Juvenile Arthritis Diseases 0.000 description 1
- 206010059176 Juvenile idiopathic arthritis Diseases 0.000 description 1
- 206010023347 Keratoacanthoma Diseases 0.000 description 1
- 201000002287 Keratoconus Diseases 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 1
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 1
- 239000002067 L01XE06 - Dasatinib Substances 0.000 description 1
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 1
- 239000005536 L01XE08 - Nilotinib Substances 0.000 description 1
- 239000003798 L01XE11 - Pazopanib Substances 0.000 description 1
- 239000002145 L01XE14 - Bosutinib Substances 0.000 description 1
- 239000002144 L01XE18 - Ruxolitinib Substances 0.000 description 1
- 239000004166 Lanolin Substances 0.000 description 1
- XNRVGTHNYCNCFF-UHFFFAOYSA-N Lapatinib ditosylate monohydrate Chemical compound O.CC1=CC=C(S(O)(=O)=O)C=C1.CC1=CC=C(S(O)(=O)=O)C=C1.O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 XNRVGTHNYCNCFF-UHFFFAOYSA-N 0.000 description 1
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- GSDSWSVVBLHKDQ-JTQLQIEISA-N Levofloxacin Chemical compound C([C@@H](N1C2=C(C(C(C(O)=O)=C1)=O)C=C1F)C)OC2=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-JTQLQIEISA-N 0.000 description 1
- 229910010084 LiAlH4 Inorganic materials 0.000 description 1
- 108010028921 Lipopeptides Proteins 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 208000005777 Lupus Nephritis Diseases 0.000 description 1
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 1
- 206010052178 Lymphocytic lymphoma Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 241000701076 Macacine alphaherpesvirus 1 Species 0.000 description 1
- TYMRLRRVMHJFTF-UHFFFAOYSA-N Mafenide Chemical compound NCC1=CC=C(S(N)(=O)=O)C=C1 TYMRLRRVMHJFTF-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 235000006679 Mentha X verticillata Nutrition 0.000 description 1
- 235000002899 Mentha suaveolens Nutrition 0.000 description 1
- 235000001636 Mentha x rotundifolia Nutrition 0.000 description 1
- XADCESSVHJOZHK-UHFFFAOYSA-N Meperidine Chemical compound C=1C=CC=CC=1C1(C(=O)OCC)CCN(C)CC1 XADCESSVHJOZHK-UHFFFAOYSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- 208000024599 Mooren ulcer Diseases 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- 102000010168 Myeloid Differentiation Factor 88 Human genes 0.000 description 1
- 108010077432 Myeloid Differentiation Factor 88 Proteins 0.000 description 1
- 240000009023 Myrrhis odorata Species 0.000 description 1
- 235000007265 Myrrhis odorata Nutrition 0.000 description 1
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- AYCPARAPKDAOEN-LJQANCHMSA-N N-[(1S)-2-(dimethylamino)-1-phenylethyl]-6,6-dimethyl-3-[(2-methyl-4-thieno[3,2-d]pyrimidinyl)amino]-1,4-dihydropyrrolo[3,4-c]pyrazole-5-carboxamide Chemical compound C1([C@H](NC(=O)N2C(C=3NN=C(NC=4C=5SC=CC=5N=C(C)N=4)C=3C2)(C)C)CN(C)C)=CC=CC=C1 AYCPARAPKDAOEN-LJQANCHMSA-N 0.000 description 1
- JUUFBMODXQKSTD-UHFFFAOYSA-N N-[2-amino-6-[(4-fluorophenyl)methylamino]-3-pyridinyl]carbamic acid ethyl ester Chemical compound N1=C(N)C(NC(=O)OCC)=CC=C1NCC1=CC=C(F)C=C1 JUUFBMODXQKSTD-UHFFFAOYSA-N 0.000 description 1
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- GADIHNNHORVFIG-UHFFFAOYSA-N N-tert-butyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-amine Chemical compound C(C)(C)(C)NC=1C=NC=C(C=1)B1OC(C(O1)(C)C)(C)C GADIHNNHORVFIG-UHFFFAOYSA-N 0.000 description 1
- 239000007832 Na2SO4 Substances 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 206010029098 Neoplasm skin Diseases 0.000 description 1
- 206010029216 Nervousness Diseases 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- LUZWXMGSPXIQHB-UHFFFAOYSA-N OC(=O)CC(C1=CC=C(C=C1)[N+]([O-])=O)C1=C(O)C2=C(OC1=O)C=CC=C2 Chemical compound OC(=O)CC(C1=CC=C(C=C1)[N+]([O-])=O)C1=C(O)C2=C(OC1=O)C=CC=C2 LUZWXMGSPXIQHB-UHFFFAOYSA-N 0.000 description 1
- 206010073938 Ophthalmic herpes simplex Diseases 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- BRUQQQPBMZOVGD-XFKAJCMBSA-N Oxycodone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(OC)C2=C5[C@@]13CCN4C BRUQQQPBMZOVGD-XFKAJCMBSA-N 0.000 description 1
- 239000004100 Oxytetracycline Substances 0.000 description 1
- 239000012270 PD-1 inhibitor Substances 0.000 description 1
- 239000012668 PD-1-inhibitor Substances 0.000 description 1
- 239000012271 PD-L1 inhibitor Substances 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 101100272976 Panax ginseng CYP716A53v2 gene Proteins 0.000 description 1
- 239000005662 Paraffin oil Substances 0.000 description 1
- 241000721454 Pemphigus Species 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- 229930195708 Penicillin V Natural products 0.000 description 1
- 235000012550 Pimpinella anisum Nutrition 0.000 description 1
- 208000007452 Plasmacytoma Diseases 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 108010093965 Polymyxin B Proteins 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 206010065857 Primary Effusion Lymphoma Diseases 0.000 description 1
- 208000028561 Primary cutaneous T-cell lymphoma Diseases 0.000 description 1
- 208000035416 Prolymphocytic B-Cell Leukemia Diseases 0.000 description 1
- 208000033759 Prolymphocytic T-Cell Leukemia Diseases 0.000 description 1
- 208000004403 Prostatic Hyperplasia Diseases 0.000 description 1
- 244000018633 Prunus armeniaca Species 0.000 description 1
- 235000009827 Prunus armeniaca Nutrition 0.000 description 1
- 235000006040 Prunus persica var persica Nutrition 0.000 description 1
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 1
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 1
- 206010037575 Pustular psoriasis Diseases 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- MUPFEKGTMRGPLJ-RMMQSMQOSA-N Raffinose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 MUPFEKGTMRGPLJ-RMMQSMQOSA-N 0.000 description 1
- 208000037656 Respiratory Sounds Diseases 0.000 description 1
- 206010057190 Respiratory tract infections Diseases 0.000 description 1
- 240000007651 Rubus glaucus Species 0.000 description 1
- 235000011034 Rubus glaucus Nutrition 0.000 description 1
- 235000009122 Rubus idaeus Nutrition 0.000 description 1
- 201000004854 SAPHO syndrome Diseases 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 201000010848 Schnitzler Syndrome Diseases 0.000 description 1
- 208000034189 Sclerosis Diseases 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- 201000010208 Seminoma Diseases 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 208000004346 Smoldering Multiple Myeloma Diseases 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- NHUHCSRWZMLRLA-UHFFFAOYSA-N Sulfisoxazole Chemical compound CC1=NOC(NS(=O)(=O)C=2C=CC(N)=CC=2)=C1C NHUHCSRWZMLRLA-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 206010042970 T-cell chronic lymphocytic leukaemia Diseases 0.000 description 1
- 208000026651 T-cell prolymphocytic leukemia Diseases 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- BHEOSNUKNHRBNM-UHFFFAOYSA-N Tetramethylsqualene Natural products CC(=C)C(C)CCC(=C)C(C)CCC(C)=CCCC=C(C)CCC(C)C(=C)CCC(C)C(C)=C BHEOSNUKNHRBNM-UHFFFAOYSA-N 0.000 description 1
- WKDDRNSBRWANNC-UHFFFAOYSA-N Thienamycin Natural products C1C(SCCN)=C(C(O)=O)N2C(=O)C(C(O)C)C21 WKDDRNSBRWANNC-UHFFFAOYSA-N 0.000 description 1
- 208000031981 Thrombocytopenic Idiopathic Purpura Diseases 0.000 description 1
- 206010043561 Thrombocytopenic purpura Diseases 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 231100000579 Toxinosis Toxicity 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- MUPFEKGTMRGPLJ-UHFFFAOYSA-N UNPD196149 Natural products OC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1 MUPFEKGTMRGPLJ-UHFFFAOYSA-N 0.000 description 1
- 208000025865 Ulcer Diseases 0.000 description 1
- 206010064996 Ulcerative keratitis Diseases 0.000 description 1
- 206010070488 Upper-airway cough syndrome Diseases 0.000 description 1
- 108091008605 VEGF receptors Proteins 0.000 description 1
- 108010059993 Vancomycin Proteins 0.000 description 1
- 235000009499 Vanilla fragrans Nutrition 0.000 description 1
- 244000263375 Vanilla tahitensis Species 0.000 description 1
- 235000012036 Vanilla tahitensis Nutrition 0.000 description 1
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 208000016025 Waldenstroem macroglobulinemia Diseases 0.000 description 1
- 206010047924 Wheezing Diseases 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- ZWBTYMGEBZUQTK-PVLSIAFMSA-N [(7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,32-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-1'-(2-methylpropyl)-6,23-dioxospiro[8,33-dioxa-24,27,29-triazapentacyclo[23.6.1.14,7.05,31.026,30]tritriaconta-1(32),2,4,9,19,21,24,26,30-nonaene-28,4'-piperidine]-13-yl] acetate Chemical compound CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C2=O)c2c4NC5(CCN(CC(C)C)CC5)N=c4c(=NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)c(O)c2c(O)c3C ZWBTYMGEBZUQTK-PVLSIAFMSA-N 0.000 description 1
- PHLLTVDZCKUNJN-UHFFFAOYSA-N [2-(trifluoromethyl)pyridin-4-yl]boronic acid Chemical compound OB(O)C1=CC=NC(C(F)(F)F)=C1 PHLLTVDZCKUNJN-UHFFFAOYSA-N 0.000 description 1
- YZQQHZXHCXAJAV-UHFFFAOYSA-N [3-(dimethylamino)phenyl]boronic acid Chemical compound CN(C)C1=CC=CC(B(O)O)=C1 YZQQHZXHCXAJAV-UHFFFAOYSA-N 0.000 description 1
- XUIQQIRLFMCWLN-UHFFFAOYSA-N [3-(methanesulfonamido)phenyl]boronic acid Chemical compound CS(=O)(=O)NC1=CC=CC(B(O)O)=C1 XUIQQIRLFMCWLN-UHFFFAOYSA-N 0.000 description 1
- QCHZDFUJSFGGJV-UHFFFAOYSA-N [5-(2,2,2-trifluoroethoxy)pyridin-3-yl]boronic acid Chemical compound OB(O)C1=CN=CC(OCC(F)(F)F)=C1 QCHZDFUJSFGGJV-UHFFFAOYSA-N 0.000 description 1
- LPIQAYCWHTUVJQ-UHFFFAOYSA-N [5-(oxan-4-yloxy)pyridin-3-yl]boronic acid Chemical compound OB(O)C1=CN=CC(OC2CCOCC2)=C1 LPIQAYCWHTUVJQ-UHFFFAOYSA-N 0.000 description 1
- SFBQNNGMEKUJAN-UHFFFAOYSA-N [5-(trifluoromethyl)pyridin-3-yl]boronic acid Chemical compound OB(O)C1=CN=CC(C(F)(F)F)=C1 SFBQNNGMEKUJAN-UHFFFAOYSA-N 0.000 description 1
- BOGSOFADOWIECK-UHFFFAOYSA-N [N].C=1C=NNC=1 Chemical compound [N].C=1C=NNC=1 BOGSOFADOWIECK-UHFFFAOYSA-N 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 229960002054 acenocoumarol Drugs 0.000 description 1
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 1
- 150000001241 acetals Chemical class 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 239000012445 acidic reagent Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 208000017733 acquired polycythemia vera Diseases 0.000 description 1
- 125000000641 acridinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3C=C12)* 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 229960002964 adalimumab Drugs 0.000 description 1
- 208000009956 adenocarcinoma Diseases 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 210000004100 adrenal gland Anatomy 0.000 description 1
- 238000012387 aerosolization Methods 0.000 description 1
- ULXXDDBFHOBEHA-CWDCEQMOSA-N afatinib Chemical compound N1=CN=C2C=C(O[C@@H]3COCC3)C(NC(=O)/C=C/CN(C)C)=CC2=C1NC1=CC=C(F)C(Cl)=C1 ULXXDDBFHOBEHA-CWDCEQMOSA-N 0.000 description 1
- 229960001686 afatinib Drugs 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 125000003158 alcohol group Chemical group 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910001420 alkaline earth metal ion Inorganic materials 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 150000001345 alkine derivatives Chemical class 0.000 description 1
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 125000005360 alkyl sulfoxide group Chemical group 0.000 description 1
- 208000002205 allergic conjunctivitis Diseases 0.000 description 1
- 239000008168 almond oil Substances 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 229940037003 alum Drugs 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 230000001668 ameliorated effect Effects 0.000 description 1
- 229960004821 amikacin Drugs 0.000 description 1
- LKCWBDHBTVXHDL-RMDFUYIESA-N amikacin Chemical compound O([C@@H]1[C@@H](N)C[C@H]([C@@H]([C@H]1O)O[C@@H]1[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O1)O)NC(=O)[C@@H](O)CCN)[C@H]1O[C@H](CN)[C@@H](O)[C@H](O)[C@H]1O LKCWBDHBTVXHDL-RMDFUYIESA-N 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 229960003022 amoxicillin Drugs 0.000 description 1
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 1
- 229940038195 amoxicillin / clavulanate Drugs 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 229940043312 ampicillin / sulbactam Drugs 0.000 description 1
- 229960004238 anakinra Drugs 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000000843 anti-fungal effect Effects 0.000 description 1
- 230000001355 anti-mycobacterial effect Effects 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 229940045686 antimetabolites antineoplastic purine analogs Drugs 0.000 description 1
- 239000003926 antimycobacterial agent Substances 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- 229940045713 antineoplastic alkylating drug ethylene imines Drugs 0.000 description 1
- 229960003886 apixaban Drugs 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 239000011260 aqueous acid Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 229960003121 arginine Drugs 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 229940046844 aromatase inhibitors Drugs 0.000 description 1
- 229940003446 arsphenamine Drugs 0.000 description 1
- VLAXZGHHBIJLAD-UHFFFAOYSA-N arsphenamine Chemical compound [Cl-].[Cl-].C1=C(O)C([NH3+])=CC([As]=[As]C=2C=C([NH3+])C(O)=CC=2)=C1 VLAXZGHHBIJLAD-UHFFFAOYSA-N 0.000 description 1
- 208000011775 arteriosclerosis disease Diseases 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 description 1
- 239000000605 aspartame Substances 0.000 description 1
- 235000010357 aspartame Nutrition 0.000 description 1
- 229960003438 aspartame Drugs 0.000 description 1
- FKQQKMGWCJGUCS-UHFFFAOYSA-N atromentin Chemical compound O=C1C(O)=C(C=2C=CC(O)=CC=2)C(=O)C(O)=C1C1=CC=C(O)C=C1 FKQQKMGWCJGUCS-UHFFFAOYSA-N 0.000 description 1
- AAEDGQBSNHENEM-UHFFFAOYSA-N atromentin Natural products OCC1(O)C2=C(C(=O)C(=C(C2=O)c3ccc(O)cc3)O)c4ccc(O)cc14 AAEDGQBSNHENEM-UHFFFAOYSA-N 0.000 description 1
- 201000003710 autoimmune thrombocytopenic purpura Diseases 0.000 description 1
- 229960003005 axitinib Drugs 0.000 description 1
- RITAVMQDGBJQJZ-FMIVXFBMSA-N axitinib Chemical compound CNC(=O)C1=CC=CC=C1SC1=CC=C(C(\C=C\C=2N=CC=CC=2)=NN2)C2=C1 RITAVMQDGBJQJZ-FMIVXFBMSA-N 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 229940022777 azasan Drugs 0.000 description 1
- 125000002785 azepinyl group Chemical group 0.000 description 1
- 125000002393 azetidinyl group Chemical group 0.000 description 1
- 229960004099 azithromycin Drugs 0.000 description 1
- MQTOSJVFKKJCRP-BICOPXKESA-N azithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)N(C)C[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 MQTOSJVFKKJCRP-BICOPXKESA-N 0.000 description 1
- JTWOMNBEOCYFNV-NFFDBFGFSA-N azlocillin Chemical compound N([C@@H](C(=O)N[C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C=1C=CC=CC=1)C(=O)N1CCNC1=O JTWOMNBEOCYFNV-NFFDBFGFSA-N 0.000 description 1
- 229960003623 azlocillin Drugs 0.000 description 1
- 229960003644 aztreonam Drugs 0.000 description 1
- 229960003071 bacitracin Drugs 0.000 description 1
- 229930184125 bacitracin Natural products 0.000 description 1
- CLKOFPXJLQSYAH-ABRJDSQDSA-N bacitracin A Chemical compound C1SC([C@@H](N)[C@@H](C)CC)=N[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]1C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2N=CNC=2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC1 CLKOFPXJLQSYAH-ABRJDSQDSA-N 0.000 description 1
- 239000003855 balanced salt solution Substances 0.000 description 1
- IPOKCKJONYRRHP-FMQUCBEESA-N balsalazide Chemical compound C1=CC(C(=O)NCCC(=O)O)=CC=C1\N=N\C1=CC=C(O)C(C(O)=O)=C1 IPOKCKJONYRRHP-FMQUCBEESA-N 0.000 description 1
- 229960004168 balsalazide Drugs 0.000 description 1
- 210000000678 band cell Anatomy 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 229960004669 basiliximab Drugs 0.000 description 1
- 229960002210 batroxobin Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000002047 benzodioxolyl group Chemical group O1OC(C2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 125000004619 benzopyranyl group Chemical group O1C(C=CC2=C1C=CC=C2)* 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 1
- 229960003237 betaine Drugs 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- OIRCOABEOLEUMC-GEJPAHFPSA-N bivalirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 OIRCOABEOLEUMC-GEJPAHFPSA-N 0.000 description 1
- 108010055460 bivalirudin Proteins 0.000 description 1
- 229960001500 bivalirudin Drugs 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- UWTDFICHZKXYAC-UHFFFAOYSA-N boron;oxolane Chemical compound [B].C1CCOC1 UWTDFICHZKXYAC-UHFFFAOYSA-N 0.000 description 1
- 125000005620 boronic acid group Chemical class 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- UBPYILGKFZZVDX-UHFFFAOYSA-N bosutinib Chemical compound C1=C(Cl)C(OC)=CC(NC=2C3=CC(OC)=C(OCCCN4CCN(C)CC4)C=C3N=CC=2C#N)=C1Cl UBPYILGKFZZVDX-UHFFFAOYSA-N 0.000 description 1
- 229960003736 bosutinib Drugs 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 201000008275 breast carcinoma Diseases 0.000 description 1
- 229960003735 brodalumab Drugs 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 239000000337 buffer salt Substances 0.000 description 1
- RMRJXGBAOAMLHD-IHFGGWKQSA-N buprenorphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]11CC[C@]3([C@H](C1)[C@](C)(O)C(C)(C)C)OC)CN2CC1CC1 RMRJXGBAOAMLHD-IHFGGWKQSA-N 0.000 description 1
- 229960001736 buprenorphine Drugs 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229940046731 calcineurin inhibitors Drugs 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 229960004602 capreomycin Drugs 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- 229940041011 carbapenems Drugs 0.000 description 1
- 229960003669 carbenicillin Drugs 0.000 description 1
- FPPNZSSZRUTDAP-UWFZAAFLSA-N carbenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)C(C(O)=O)C1=CC=CC=C1 FPPNZSSZRUTDAP-UWFZAAFLSA-N 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- BLMPQMFVWMYDKT-NZTKNTHTSA-N carfilzomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)[C@]1(C)OC1)NC(=O)CN1CCOCC1)CC1=CC=CC=C1 BLMPQMFVWMYDKT-NZTKNTHTSA-N 0.000 description 1
- 229960002438 carfilzomib Drugs 0.000 description 1
- 108010021331 carfilzomib Proteins 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- QYIYFLOTGYLRGG-GPCCPHFNSA-N cefaclor Chemical compound C1([C@H](C(=O)N[C@@H]2C(N3C(=C(Cl)CS[C@@H]32)C(O)=O)=O)N)=CC=CC=C1 QYIYFLOTGYLRGG-GPCCPHFNSA-N 0.000 description 1
- 229960005361 cefaclor Drugs 0.000 description 1
- 229960004841 cefadroxil Drugs 0.000 description 1
- NBFNMSULHIODTC-CYJZLJNKSA-N cefadroxil monohydrate Chemical compound O.C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C)C(O)=O)=CC=C(O)C=C1 NBFNMSULHIODTC-CYJZLJNKSA-N 0.000 description 1
- 229960000603 cefalotin Drugs 0.000 description 1
- OLVCFLKTBJRLHI-AXAPSJFSSA-N cefamandole Chemical compound CN1N=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)[C@H](O)C=3C=CC=CC=3)[C@H]2SC1 OLVCFLKTBJRLHI-AXAPSJFSSA-N 0.000 description 1
- 229960003012 cefamandole Drugs 0.000 description 1
- 229960001139 cefazolin Drugs 0.000 description 1
- MLYYVTUWGNIJIB-BXKDBHETSA-N cefazolin Chemical compound S1C(C)=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)CN3N=NN=C3)[C@H]2SC1 MLYYVTUWGNIJIB-BXKDBHETSA-N 0.000 description 1
- 229960003719 cefdinir Drugs 0.000 description 1
- RTXOFQZKPXMALH-GHXIOONMSA-N cefdinir Chemical compound S1C(N)=NC(C(=N\O)\C(=O)N[C@@H]2C(N3C(=C(C=C)CS[C@@H]32)C(O)=O)=O)=C1 RTXOFQZKPXMALH-GHXIOONMSA-N 0.000 description 1
- 229960004069 cefditoren Drugs 0.000 description 1
- KMIPKYQIOVAHOP-YLGJWRNMSA-N cefditoren Chemical compound S([C@@H]1[C@@H](C(N1C=1C(O)=O)=O)NC(=O)\C(=N/OC)C=2N=C(N)SC=2)CC=1\C=C/C=1SC=NC=1C KMIPKYQIOVAHOP-YLGJWRNMSA-N 0.000 description 1
- 229960002100 cefepime Drugs 0.000 description 1
- 229960002129 cefixime Drugs 0.000 description 1
- 229960004682 cefoperazone Drugs 0.000 description 1
- GCFBRXLSHGKWDP-XCGNWRKASA-N cefoperazone Chemical compound O=C1C(=O)N(CC)CCN1C(=O)N[C@H](C=1C=CC(O)=CC=1)C(=O)N[C@@H]1C(=O)N2C(C(O)=O)=C(CSC=3N(N=NN=3)C)CS[C@@H]21 GCFBRXLSHGKWDP-XCGNWRKASA-N 0.000 description 1
- 229960004261 cefotaxime Drugs 0.000 description 1
- AZZMGZXNTDTSME-JUZDKLSSSA-M cefotaxime sodium Chemical compound [Na+].N([C@@H]1C(N2C(=C(COC(C)=O)CS[C@@H]21)C([O-])=O)=O)C(=O)\C(=N/OC)C1=CSC(N)=N1 AZZMGZXNTDTSME-JUZDKLSSSA-M 0.000 description 1
- 229960002682 cefoxitin Drugs 0.000 description 1
- 229960005090 cefpodoxime Drugs 0.000 description 1
- WYUSVOMTXWRGEK-HBWVYFAYSA-N cefpodoxime Chemical compound N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC)C(O)=O)C(=O)C(=N/OC)\C1=CSC(N)=N1 WYUSVOMTXWRGEK-HBWVYFAYSA-N 0.000 description 1
- 229960002580 cefprozil Drugs 0.000 description 1
- 229960000484 ceftazidime Drugs 0.000 description 1
- NMVPEQXCMGEDNH-TZVUEUGBSA-N ceftazidime pentahydrate Chemical compound O.O.O.O.O.S([C@@H]1[C@@H](C(N1C=1C([O-])=O)=O)NC(=O)\C(=N/OC(C)(C)C(O)=O)C=2N=C(N)SC=2)CC=1C[N+]1=CC=CC=C1 NMVPEQXCMGEDNH-TZVUEUGBSA-N 0.000 description 1
- 229960004086 ceftibuten Drugs 0.000 description 1
- SSWTVBYDDFPFAF-DKOGRLLHSA-N ceftibuten dihydrate Chemical compound O.O.S1C(N)=NC(C(=C\CC(O)=O)\C(=O)N[C@@H]2C(N3C(=CCS[C@@H]32)C(O)=O)=O)=C1 SSWTVBYDDFPFAF-DKOGRLLHSA-N 0.000 description 1
- 229960001991 ceftizoxime Drugs 0.000 description 1
- NNULBSISHYWZJU-LLKWHZGFSA-N ceftizoxime Chemical compound N([C@@H]1C(N2C(=CCS[C@@H]21)C(O)=O)=O)C(=O)\C(=N/OC)C1=CSC(N)=N1 NNULBSISHYWZJU-LLKWHZGFSA-N 0.000 description 1
- 229960004755 ceftriaxone Drugs 0.000 description 1
- VAAUVRVFOQPIGI-SPQHTLEESA-N ceftriaxone Chemical compound S([C@@H]1[C@@H](C(N1C=1C(O)=O)=O)NC(=O)\C(=N/OC)C=2N=C(N)SC=2)CC=1CSC1=NC(=O)C(=O)NN1C VAAUVRVFOQPIGI-SPQHTLEESA-N 0.000 description 1
- 229960001668 cefuroxime Drugs 0.000 description 1
- JFPVXVDWJQMJEE-IZRZKJBUSA-N cefuroxime Chemical compound N([C@@H]1C(N2C(=C(COC(N)=O)CS[C@@H]21)C(O)=O)=O)C(=O)\C(=N/OC)C1=CC=CO1 JFPVXVDWJQMJEE-IZRZKJBUSA-N 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 229940107810 cellcept Drugs 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 229940106164 cephalexin Drugs 0.000 description 1
- ZAIPMKNFIOOWCQ-UEKVPHQBSA-N cephalexin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C)C(O)=O)=CC=CC=C1 ZAIPMKNFIOOWCQ-UEKVPHQBSA-N 0.000 description 1
- 229940124587 cephalosporin Drugs 0.000 description 1
- 150000001780 cephalosporins Chemical class 0.000 description 1
- VUFGUVLLDPOSBC-XRZFDKQNSA-M cephalothin sodium Chemical compound [Na+].N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC(=O)C)C([O-])=O)C(=O)CC1=CC=CS1 VUFGUVLLDPOSBC-XRZFDKQNSA-M 0.000 description 1
- 229960003115 certolizumab pegol Drugs 0.000 description 1
- 210000003679 cervix uteri Anatomy 0.000 description 1
- 235000019693 cherries Nutrition 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 208000002849 chondrocalcinosis Diseases 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 230000006020 chronic inflammation Effects 0.000 description 1
- 208000027157 chronic rhinosinusitis Diseases 0.000 description 1
- DHSUYTOATWAVLW-WFVMDLQDSA-N cilastatin Chemical compound CC1(C)C[C@@H]1C(=O)N\C(=C/CCCCSC[C@H](N)C(O)=O)C(O)=O DHSUYTOATWAVLW-WFVMDLQDSA-N 0.000 description 1
- 229960004912 cilastatin Drugs 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 1
- 229960003405 ciprofloxacin Drugs 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 229960002626 clarithromycin Drugs 0.000 description 1
- AGOYDEPGAOXOCK-KCBOHYOISA-N clarithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@](C)([C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)OC)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 AGOYDEPGAOXOCK-KCBOHYOISA-N 0.000 description 1
- 229940090805 clavulanate Drugs 0.000 description 1
- HZZVJAQRINQKSD-PBFISZAISA-N clavulanic acid Chemical compound OC(=O)[C@H]1C(=C/CO)/O[C@@H]2CC(=O)N21 HZZVJAQRINQKSD-PBFISZAISA-N 0.000 description 1
- 229950002334 clenoliximab Drugs 0.000 description 1
- 229960002227 clindamycin Drugs 0.000 description 1
- KDLRVYVGXIQJDK-AWPVFWJPSA-N clindamycin Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@H](C)Cl)[C@@H]1[C@H](O)[C@H](O)[C@@H](O)[C@@H](SC)O1 KDLRVYVGXIQJDK-AWPVFWJPSA-N 0.000 description 1
- 229960004287 clofazimine Drugs 0.000 description 1
- WDQPAMHFFCXSNU-BGABXYSRSA-N clofazimine Chemical compound C12=CC=CC=C2N=C2C=C(NC=3C=CC(Cl)=CC=3)C(=N/C(C)C)/C=C2N1C1=CC=C(Cl)C=C1 WDQPAMHFFCXSNU-BGABXYSRSA-N 0.000 description 1
- 229960003326 cloxacillin Drugs 0.000 description 1
- LQOLIRLGBULYKD-JKIFEVAISA-N cloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=CC=CC=C1Cl LQOLIRLGBULYKD-JKIFEVAISA-N 0.000 description 1
- 229940047766 co-trimoxazole Drugs 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 229960004126 codeine Drugs 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- 229960003346 colistin Drugs 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 201000002758 colorectal adenoma Diseases 0.000 description 1
- 229940105050 combination of penicillins Drugs 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 229940125782 compound 2 Drugs 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- 239000001767 crosslinked sodium carboxy methyl cellulose Substances 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 208000022993 cryopyrin-associated periodic syndrome Diseases 0.000 description 1
- 229940043378 cyclin-dependent kinase inhibitor Drugs 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 1
- WLVKDFJTYKELLQ-UHFFFAOYSA-N cyclopropylboronic acid Chemical compound OB(O)C1CC1 WLVKDFJTYKELLQ-UHFFFAOYSA-N 0.000 description 1
- IGSKHXTUVXSOMB-UHFFFAOYSA-N cyclopropylmethanamine Chemical compound NCC1CC1 IGSKHXTUVXSOMB-UHFFFAOYSA-N 0.000 description 1
- 229960003077 cycloserine Drugs 0.000 description 1
- 230000010250 cytokine signaling pathway Effects 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 229960003850 dabigatran Drugs 0.000 description 1
- YBSJFWOBGCMAKL-UHFFFAOYSA-N dabigatran Chemical compound N=1C2=CC(C(=O)N(CCC(O)=O)C=3N=CC=CC=3)=CC=C2N(C)C=1CNC1=CC=C(C(N)=N)C=C1 YBSJFWOBGCMAKL-UHFFFAOYSA-N 0.000 description 1
- 229960002465 dabrafenib Drugs 0.000 description 1
- BFSMGDJOXZAERB-UHFFFAOYSA-N dabrafenib Chemical compound S1C(C(C)(C)C)=NC(C=2C(=C(NS(=O)(=O)C=3C(=CC=CC=3F)F)C=CC=2)F)=C1C1=CC=NC(N)=N1 BFSMGDJOXZAERB-UHFFFAOYSA-N 0.000 description 1
- 229960002806 daclizumab Drugs 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- SUYRLXYYZQTJHF-VMBLUXKRSA-N dalfopristin Chemical compound O=C([C@@H]1N(C2=O)CC[C@H]1S(=O)(=O)CCN(CC)CC)O[C@H](C(C)C)[C@H](C)\C=C\C(=O)NC\C=C\C(\C)=C\[C@@H](O)CC(=O)CC1=NC2=CO1 SUYRLXYYZQTJHF-VMBLUXKRSA-N 0.000 description 1
- 229960002615 dalfopristin Drugs 0.000 description 1
- 108700028430 dalfopristin Proteins 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 229960000860 dapsone Drugs 0.000 description 1
- DOAKLVKFURWEDJ-QCMAZARJSA-N daptomycin Chemical compound C([C@H]1C(=O)O[C@H](C)[C@@H](C(NCC(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@H](CO)C(=O)N[C@H](C(=O)N1)[C@H](C)CC(O)=O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)CCCCCCCCC)C(=O)C1=CC=CC=C1N DOAKLVKFURWEDJ-QCMAZARJSA-N 0.000 description 1
- 229960005484 daptomycin Drugs 0.000 description 1
- 229960002448 dasatinib Drugs 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 125000005507 decahydroisoquinolyl group Chemical group 0.000 description 1
- 229960003603 decitabine Drugs 0.000 description 1
- 229960002398 demeclocycline Drugs 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- INUNXTSAACVKJS-OAQYLSRUSA-N dextromoramide Chemical compound C([C@@H](C)C(C(=O)N1CCCC1)(C=1C=CC=CC=1)C=1C=CC=CC=1)N1CCOCC1 INUNXTSAACVKJS-OAQYLSRUSA-N 0.000 description 1
- 229960003701 dextromoramide Drugs 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 229940095079 dicalcium phosphate anhydrous Drugs 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940042935 dichlorodifluoromethane Drugs 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- YFAGHNZHGGCZAX-JKIFEVAISA-N dicloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=C(Cl)C=CC=C1Cl YFAGHNZHGGCZAX-JKIFEVAISA-N 0.000 description 1
- 229960001585 dicloxacillin Drugs 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- XYYVYLMBEZUESM-UHFFFAOYSA-N dihydrocodeine Natural products C1C(N(CCC234)C)C2C=CC(=O)C3OC2=C4C1=CC=C2OC XYYVYLMBEZUESM-UHFFFAOYSA-N 0.000 description 1
- 125000004655 dihydropyridinyl group Chemical group N1(CC=CC=C1)* 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000013024 dilution buffer Substances 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- XHFGWHUWQXTGAT-UHFFFAOYSA-N dimethylamine hydrochloride Natural products CNC(C)C XHFGWHUWQXTGAT-UHFFFAOYSA-N 0.000 description 1
- IQDGSYLLQPDQDV-UHFFFAOYSA-N dimethylazanium;chloride Chemical compound Cl.CNC IQDGSYLLQPDQDV-UHFFFAOYSA-N 0.000 description 1
- 125000005879 dioxolanyl group Chemical group 0.000 description 1
- SVDHSZFEQYXRDC-UHFFFAOYSA-N dipipanone Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)CC)CC(C)N1CCCCC1 SVDHSZFEQYXRDC-UHFFFAOYSA-N 0.000 description 1
- 229960002500 dipipanone Drugs 0.000 description 1
- WLOHNSSYAXHWNR-NXPDYKKBSA-N dirithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H]2O[C@H](COCCOC)N[C@H]([C@@H]2C)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 WLOHNSSYAXHWNR-NXPDYKKBSA-N 0.000 description 1
- 229960004100 dirithromycin Drugs 0.000 description 1
- 231100000676 disease causative agent Toxicity 0.000 description 1
- NWOYIVRVSJDTLK-YSDBFZIDSA-L disodium;(2s,5r,6r)-6-[[(2r)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate;(1r,4s)-3,3-dimethyl-2,2,6-trioxo-2$l^{6}-thiabicyclo[3.2.0]heptane-4-carboxylate Chemical compound [Na+].[Na+].O=S1(=O)C(C)(C)[C@H](C([O-])=O)C2C(=O)C[C@H]21.C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C([O-])=O)(C)C)=CC=CC=C1 NWOYIVRVSJDTLK-YSDBFZIDSA-L 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N dodecahydrosqualene Natural products CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 description 1
- AVAACINZEOAHHE-VFZPANTDSA-N doripenem Chemical compound C=1([C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)S[C@@H]1CN[C@H](CNS(N)(=O)=O)C1 AVAACINZEOAHHE-VFZPANTDSA-N 0.000 description 1
- 229960000895 doripenem Drugs 0.000 description 1
- 229960003722 doxycycline Drugs 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 229940121647 egfr inhibitor Drugs 0.000 description 1
- 229960004137 elotuzumab Drugs 0.000 description 1
- 230000008451 emotion Effects 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940079360 enema for constipation Drugs 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 150000002085 enols Chemical group 0.000 description 1
- 229960002549 enoxacin Drugs 0.000 description 1
- IDYZIJYBMGIQMJ-UHFFFAOYSA-N enoxacin Chemical compound N1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 IDYZIJYBMGIQMJ-UHFFFAOYSA-N 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 229960001433 erlotinib Drugs 0.000 description 1
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 1
- 229960002770 ertapenem Drugs 0.000 description 1
- 229960003276 erythromycin Drugs 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- 229960000285 ethambutol Drugs 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- LJQKCYFTNDAAPC-UHFFFAOYSA-N ethanol;ethyl acetate Chemical compound CCO.CCOC(C)=O LJQKCYFTNDAAPC-UHFFFAOYSA-N 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- AEOCXXJPGCBFJA-UHFFFAOYSA-N ethionamide Chemical compound CCC1=CC(C(N)=S)=CC=N1 AEOCXXJPGCBFJA-UHFFFAOYSA-N 0.000 description 1
- 229960002001 ethionamide Drugs 0.000 description 1
- 201000009320 ethmoid sinusitis Diseases 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 229940012017 ethylenediamine Drugs 0.000 description 1
- 208000024695 exercise-induced bronchoconstriction Diseases 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 229960002428 fentanyl Drugs 0.000 description 1
- IVLVTNPOHDFFCJ-UHFFFAOYSA-N fentanyl citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 IVLVTNPOHDFFCJ-UHFFFAOYSA-N 0.000 description 1
- 229950010512 fezakinumab Drugs 0.000 description 1
- 229950010043 fletikumab Drugs 0.000 description 1
- 229960004273 floxacillin Drugs 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 229960003667 flupirtine Drugs 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- KANJSNBRCNMZMV-ABRZTLGGSA-N fondaparinux Chemical compound O[C@@H]1[C@@H](NS(O)(=O)=O)[C@@H](OC)O[C@H](COS(O)(=O)=O)[C@H]1O[C@H]1[C@H](OS(O)(=O)=O)[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](OS(O)(=O)=O)[C@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O[C@@H]4[C@@H]([C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O4)NS(O)(=O)=O)[C@H](O3)C(O)=O)O)[C@@H](COS(O)(=O)=O)O2)NS(O)(=O)=O)[C@H](C(O)=O)O1 KANJSNBRCNMZMV-ABRZTLGGSA-N 0.000 description 1
- 229960001318 fondaparinux Drugs 0.000 description 1
- 229960004421 formestane Drugs 0.000 description 1
- OSVMTWJCGUFAOD-KZQROQTASA-N formestane Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1O OSVMTWJCGUFAOD-KZQROQTASA-N 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 229960000308 fosfomycin Drugs 0.000 description 1
- YMDXZJFXQJVXBF-STHAYSLISA-N fosfomycin Chemical compound C[C@@H]1O[C@@H]1P(O)(O)=O YMDXZJFXQJVXBF-STHAYSLISA-N 0.000 description 1
- 229950005309 fostamatinib Drugs 0.000 description 1
- GKDRMWXFWHEQQT-UHFFFAOYSA-N fostamatinib Chemical compound COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3N=C4N(COP(O)(O)=O)C(=O)C(C)(C)OC4=CC=3)C(F)=CN=2)=C1 GKDRMWXFWHEQQT-UHFFFAOYSA-N 0.000 description 1
- 201000006916 frontal sinusitis Diseases 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 229960001625 furazolidone Drugs 0.000 description 1
- PLHJDBGFXBMTGZ-WEVVVXLNSA-N furazolidone Chemical compound O1C([N+](=O)[O-])=CC=C1\C=N\N1C(=O)OCC1 PLHJDBGFXBMTGZ-WEVVVXLNSA-N 0.000 description 1
- 229960004675 fusidic acid Drugs 0.000 description 1
- IECPWNUMDGFDKC-MZJAQBGESA-N fusidic acid Chemical compound O[C@@H]([C@@H]12)C[C@H]3\C(=C(/CCC=C(C)C)C(O)=O)[C@@H](OC(C)=O)C[C@]3(C)[C@@]2(C)CC[C@@H]2[C@]1(C)CC[C@@H](O)[C@H]2C IECPWNUMDGFDKC-MZJAQBGESA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 229960003923 gatifloxacin Drugs 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 229940062737 gengraf Drugs 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 229960002442 glucosamine Drugs 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- 229960001743 golimumab Drugs 0.000 description 1
- 239000003979 granulating agent Substances 0.000 description 1
- 229960000642 grepafloxacin Drugs 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 125000004438 haloalkoxy group Chemical group 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 108010005808 hementin Proteins 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- 230000007866 hepatic necrosis Effects 0.000 description 1
- 201000010884 herpes simplex virus keratitis Diseases 0.000 description 1
- 231100000171 higher toxicity Toxicity 0.000 description 1
- WQPDUTSPKFMPDP-OUMQNGNKSA-N hirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(OS(O)(=O)=O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]2CSSC[C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@H](C(NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N2)=O)CSSC1)C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)CSSC1)C(C)C)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 WQPDUTSPKFMPDP-OUMQNGNKSA-N 0.000 description 1
- 229940006607 hirudin Drugs 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 229960002885 histidine Drugs 0.000 description 1
- 239000003667 hormone antagonist Chemical class 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- XGIHQYAWBCFNPY-AZOCGYLKSA-N hydrabamine Chemical compound C([C@@H]12)CC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC[C@@]1(C)CNCCNC[C@@]1(C)[C@@H]2CCC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC1 XGIHQYAWBCFNPY-AZOCGYLKSA-N 0.000 description 1
- BHEPBYXIRTUNPN-UHFFFAOYSA-N hydridophosphorus(.) (triplet) Chemical compound [PH] BHEPBYXIRTUNPN-UHFFFAOYSA-N 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 150000003840 hydrochlorides Chemical class 0.000 description 1
- LLPOLZWFYMWNKH-CMKMFDCUSA-N hydrocodone Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)CC(=O)[C@@H]1OC1=C2C3=CC=C1OC LLPOLZWFYMWNKH-CMKMFDCUSA-N 0.000 description 1
- 229960000240 hydrocodone Drugs 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- WVLOADHCBXTIJK-YNHQPCIGSA-N hydromorphone Chemical compound O([C@H]1C(CC[C@H]23)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O WVLOADHCBXTIJK-YNHQPCIGSA-N 0.000 description 1
- 229960001410 hydromorphone Drugs 0.000 description 1
- 229950000801 hydroxyprogesterone caproate Drugs 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 229940075628 hypomethylating agent Drugs 0.000 description 1
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 1
- 229960001101 ifosfamide Drugs 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- 125000002632 imidazolidinyl group Chemical group 0.000 description 1
- 125000002636 imidazolinyl group Chemical group 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 229960002182 imipenem Drugs 0.000 description 1
- GSOSVVULSKVSLQ-JJVRHELESA-N imipenem hydrate Chemical compound O.C1C(SCCNC=N)=C(C(O)=O)N2C(=O)[C@H]([C@H](O)C)[C@H]21 GSOSVVULSKVSLQ-JJVRHELESA-N 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 1
- 229940045207 immuno-oncology agent Drugs 0.000 description 1
- 239000000677 immunologic agent Substances 0.000 description 1
- 229940124541 immunological agent Drugs 0.000 description 1
- 239000002584 immunological anticancer agent Substances 0.000 description 1
- 230000002134 immunopathologic effect Effects 0.000 description 1
- 229940073062 imuran Drugs 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000004531 indol-5-yl group Chemical group [H]N1C([H])=C([H])C2=C([H])C(*)=C([H])C([H])=C12 0.000 description 1
- 208000015266 indolent plasma cell myeloma Diseases 0.000 description 1
- 125000003387 indolinyl group Chemical group N1(CCC2=CC=CC=C12)* 0.000 description 1
- 125000003406 indolizinyl group Chemical group C=1(C=CN2C=CC=CC12)* 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229910001867 inorganic solvent Inorganic materials 0.000 description 1
- 239000003049 inorganic solvent Substances 0.000 description 1
- 239000002348 inosinate dehydrogenase inhibitor Substances 0.000 description 1
- 235000013902 inosinic acid Nutrition 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 208000026876 intravascular large B-cell lymphoma Diseases 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- HVTICUPFWKNHNG-UHFFFAOYSA-N iodoethane Chemical compound CCI HVTICUPFWKNHNG-UHFFFAOYSA-N 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 229960005386 ipilimumab Drugs 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 229940045996 isethionic acid Drugs 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000004594 isoindolinyl group Chemical group C1(NCC2=CC=CC=C12)* 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- 229960003350 isoniazid Drugs 0.000 description 1
- QRXWMOHMRWLFEY-UHFFFAOYSA-N isoniazide Chemical compound NNC(=O)C1=CC=NC=C1 QRXWMOHMRWLFEY-UHFFFAOYSA-N 0.000 description 1
- FMKOJHQHASLBPH-UHFFFAOYSA-N isopropyl iodide Chemical compound CC(C)I FMKOJHQHASLBPH-UHFFFAOYSA-N 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 125000005956 isoquinolyl group Chemical group 0.000 description 1
- 125000004628 isothiazolidinyl group Chemical group S1N(CCC1)* 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 125000003965 isoxazolidinyl group Chemical group 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 229960005435 ixekizumab Drugs 0.000 description 1
- 201000002215 juvenile rheumatoid arthritis Diseases 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000000832 lactitol Substances 0.000 description 1
- VQHSOMBJVWLPSR-JVCRWLNRSA-N lactitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-JVCRWLNRSA-N 0.000 description 1
- 235000010448 lactitol Nutrition 0.000 description 1
- 229960003451 lactitol Drugs 0.000 description 1
- 235000019388 lanolin Nutrition 0.000 description 1
- 229940039717 lanolin Drugs 0.000 description 1
- 229960004891 lapatinib Drugs 0.000 description 1
- 208000003849 large cell carcinoma Diseases 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- 210000000867 larynx Anatomy 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 229960003784 lenvatinib Drugs 0.000 description 1
- WOSKHXYHFSIKNG-UHFFFAOYSA-N lenvatinib Chemical compound C=12C=C(C(N)=O)C(OC)=CC2=NC=CC=1OC(C=C1Cl)=CC=C1NC(=O)NC1CC1 WOSKHXYHFSIKNG-UHFFFAOYSA-N 0.000 description 1
- OTQCKZUSUGYWBD-BRHMIFOHSA-N lepirudin Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)C(C)C)[C@@H](C)O)[C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(C)C)[C@@H](C)O)C1=CC=C(O)C=C1 OTQCKZUSUGYWBD-BRHMIFOHSA-N 0.000 description 1
- 229960004408 lepirudin Drugs 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 229960003376 levofloxacin Drugs 0.000 description 1
- 201000011486 lichen planus Diseases 0.000 description 1
- 229940010454 licorice Drugs 0.000 description 1
- 229960000812 linaclotide Drugs 0.000 description 1
- 229940041028 lincosamides Drugs 0.000 description 1
- 229960003907 linezolid Drugs 0.000 description 1
- TYZROVQLWOKYKF-ZDUSSCGKSA-N linezolid Chemical compound O=C1O[C@@H](CNC(=O)C)CN1C(C=C1F)=CC=C1N1CCOCC1 TYZROVQLWOKYKF-ZDUSSCGKSA-N 0.000 description 1
- 229940084408 linzess Drugs 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 229950011263 lirilumab Drugs 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 231100000149 liver necrosis Toxicity 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 229960002422 lomefloxacin Drugs 0.000 description 1
- ZEKZLJVOYLTDKK-UHFFFAOYSA-N lomefloxacin Chemical compound FC1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNC(C)C1 ZEKZLJVOYLTDKK-UHFFFAOYSA-N 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 201000007919 lymphoplasmacytic lymphoma Diseases 0.000 description 1
- 229960003646 lysine Drugs 0.000 description 1
- JMZFEHDNIAQMNB-UHFFFAOYSA-N m-aminophenylboronic acid Chemical compound NC1=CC=CC(B(O)O)=C1 JMZFEHDNIAQMNB-UHFFFAOYSA-N 0.000 description 1
- 125000000040 m-tolyl group Chemical group [H]C1=C([H])C(*)=C([H])C(=C1[H])C([H])([H])[H] 0.000 description 1
- 201000000564 macroglobulinemia Diseases 0.000 description 1
- 239000003120 macrolide antibiotic agent Substances 0.000 description 1
- 229940041033 macrolides Drugs 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 208000002780 macular degeneration Diseases 0.000 description 1
- 229960003640 mafenide Drugs 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 229960001708 magnesium carbonate Drugs 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 229950002736 marizomib Drugs 0.000 description 1
- 229950007254 mavrilimumab Drugs 0.000 description 1
- 201000008836 maxillary sinusitis Diseases 0.000 description 1
- 238000010907 mechanical stirring Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- FRQMUZJSZHZSGN-HBNHAYAOSA-N medroxyprogesterone Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](O)(C(C)=O)CC[C@H]21 FRQMUZJSZHZSGN-HBNHAYAOSA-N 0.000 description 1
- 229960004616 medroxyprogesterone Drugs 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 229960002260 meropenem Drugs 0.000 description 1
- DMJNNHOOLUXYBV-PQTSNVLCSA-N meropenem Chemical compound C=1([C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)S[C@@H]1CN[C@H](C(=O)N(C)C)C1 DMJNNHOOLUXYBV-PQTSNVLCSA-N 0.000 description 1
- KBOPZPXVLCULAV-UHFFFAOYSA-N mesalamine Chemical compound NC1=CC=C(O)C(C(O)=O)=C1 KBOPZPXVLCULAV-UHFFFAOYSA-N 0.000 description 1
- 229960004963 mesalazine Drugs 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 125000001434 methanylylidene group Chemical group [H]C#[*] 0.000 description 1
- PMRYVIKBURPHAH-UHFFFAOYSA-N methimazole Chemical compound CN1C=CNC1=S PMRYVIKBURPHAH-UHFFFAOYSA-N 0.000 description 1
- 235000006109 methionine Nutrition 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- SGNCOKUHMXLGAH-UHFFFAOYSA-N methyl 6-bromopyridine-2-carboxylate Chemical compound COC(=O)C1=CC=CC(Br)=N1 SGNCOKUHMXLGAH-UHFFFAOYSA-N 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- NQMRYBIKMRVZLB-UHFFFAOYSA-N methylamine hydrochloride Chemical compound [Cl-].[NH3+]C NQMRYBIKMRVZLB-UHFFFAOYSA-N 0.000 description 1
- SNVLJLYUUXKWOJ-UHFFFAOYSA-N methylidenecarbene Chemical compound C=[C] SNVLJLYUUXKWOJ-UHFFFAOYSA-N 0.000 description 1
- 229960003085 meticillin Drugs 0.000 description 1
- 229960000282 metronidazole Drugs 0.000 description 1
- VAOCPAMSLUNLGC-UHFFFAOYSA-N metronidazole Chemical compound CC1=NC=C([N+]([O-])=O)N1CCO VAOCPAMSLUNLGC-UHFFFAOYSA-N 0.000 description 1
- 206010072221 mevalonate kinase deficiency Diseases 0.000 description 1
- YPBATNHYBCGSSN-VWPFQQQWSA-N mezlocillin Chemical compound N([C@@H](C(=O)N[C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C=1C=CC=CC=1)C(=O)N1CCN(S(C)(=O)=O)C1=O YPBATNHYBCGSSN-VWPFQQQWSA-N 0.000 description 1
- 229960000198 mezlocillin Drugs 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 229960004023 minocycline Drugs 0.000 description 1
- 239000002829 mitogen activated protein kinase inhibitor Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 235000019426 modified starch Nutrition 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 229940041009 monobactams Drugs 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Natural products O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 229960003702 moxifloxacin Drugs 0.000 description 1
- FABPRXSRWADJSP-MEDUHNTESA-N moxifloxacin Chemical compound COC1=C(N2C[C@H]3NCCC[C@H]3C2)C(F)=CC(C(C(C(O)=O)=C2)=O)=C1N2C1CC1 FABPRXSRWADJSP-MEDUHNTESA-N 0.000 description 1
- 229960003128 mupirocin Drugs 0.000 description 1
- 229930187697 mupirocin Natural products 0.000 description 1
- DDHVILIIHBIMQU-YJGQQKNPSA-L mupirocin calcium hydrate Chemical compound O.O.[Ca+2].C[C@H](O)[C@H](C)[C@@H]1O[C@H]1C[C@@H]1[C@@H](O)[C@@H](O)[C@H](C\C(C)=C\C(=O)OCCCCCCCCC([O-])=O)OC1.C[C@H](O)[C@H](C)[C@@H]1O[C@H]1C[C@@H]1[C@@H](O)[C@@H](O)[C@H](C\C(C)=C\C(=O)OCCCCCCCCC([O-])=O)OC1 DDHVILIIHBIMQU-YJGQQKNPSA-L 0.000 description 1
- 229940014456 mycophenolate Drugs 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- 206010028537 myelofibrosis Diseases 0.000 description 1
- 229940083410 myfortic Drugs 0.000 description 1
- JORAUNFTUVJTNG-BSTBCYLQSA-N n-[(2s)-4-amino-1-[[(2s,3r)-1-[[(2s)-4-amino-1-oxo-1-[[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-bis(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]amino]-3-h Chemical compound CC(C)CCCCC(=O)N[C@@H](CCN)C(=O)N[C@H]([C@@H](C)O)CN[C@@H](CCN)C(=O)N[C@H]1CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCN)NC1=O.CCC(C)CCCCC(=O)N[C@@H](CCN)C(=O)N[C@H]([C@@H](C)O)CN[C@@H](CCN)C(=O)N[C@H]1CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCN)NC1=O JORAUNFTUVJTNG-BSTBCYLQSA-N 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- HFQQLRKRUDQYED-UHFFFAOYSA-N n-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-yl]methanesulfonamide Chemical compound O1C(C)(C)C(C)(C)OB1C1=CN=CC(NS(C)(=O)=O)=C1 HFQQLRKRUDQYED-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- GPXLMGHLHQJAGZ-JTDSTZFVSA-N nafcillin Chemical compound C1=CC=CC2=C(C(=O)N[C@@H]3C(N4[C@H](C(C)(C)S[C@@H]43)C(O)=O)=O)C(OCC)=CC=C21 GPXLMGHLHQJAGZ-JTDSTZFVSA-N 0.000 description 1
- 229960000515 nafcillin Drugs 0.000 description 1
- MHWLWQUZZRMNGJ-UHFFFAOYSA-N nalidixic acid Chemical compound C1=C(C)N=C2N(CC)C=C(C(O)=O)C(=O)C2=C1 MHWLWQUZZRMNGJ-UHFFFAOYSA-N 0.000 description 1
- 229960000210 nalidixic acid Drugs 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 125000004593 naphthyridinyl group Chemical group N1=C(C=CC2=CC=CN=C12)* 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 239000006199 nebulizer Substances 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 208000025402 neoplasm of esophagus Diseases 0.000 description 1
- 208000025440 neoplasm of neck Diseases 0.000 description 1
- 229960000808 netilmicin Drugs 0.000 description 1
- 208000021722 neuropathic pain Diseases 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229960001346 nilotinib Drugs 0.000 description 1
- HHZIURLSWUIHRB-UHFFFAOYSA-N nilotinib Chemical compound C1=NC(C)=CN1C1=CC(NC(=O)C=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)=CC(C(F)(F)F)=C1 HHZIURLSWUIHRB-UHFFFAOYSA-N 0.000 description 1
- 229960004378 nintedanib Drugs 0.000 description 1
- XZXHXSATPCNXJR-ZIADKAODSA-N nintedanib Chemical compound O=C1NC2=CC(C(=O)OC)=CC=C2\C1=C(C=1C=CC=CC=1)\NC(C=C1)=CC=C1N(C)C(=O)CN1CCN(C)CC1 XZXHXSATPCNXJR-ZIADKAODSA-N 0.000 description 1
- 229960000564 nitrofurantoin Drugs 0.000 description 1
- NXFQHRVNIOXGAQ-YCRREMRBSA-N nitrofurantoin Chemical compound O1C([N+](=O)[O-])=CC=C1\C=N\N1C(=O)NC(=O)C1 NXFQHRVNIOXGAQ-YCRREMRBSA-N 0.000 description 1
- 125000006574 non-aromatic ring group Chemical group 0.000 description 1
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 1
- 239000002687 nonaqueous vehicle Substances 0.000 description 1
- MVPQUSQUURLQKF-MCPDASDXSA-E nonasodium;(2s,3s,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3s,4s,5r,6r)-2-carboxylato-4,5-dimethoxy-6-[(2r,3r,4s,5r,6s)-6-methoxy-4,5-disulfonatooxy-2-(sulfonatooxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-4,5-disulfonatooxy-2-(sulfonatooxymethyl)oxan-3-yl]oxy-4,5-di Chemical compound [Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[O-]S(=O)(=O)O[C@@H]1[C@@H](OS([O-])(=O)=O)[C@@H](OC)O[C@H](COS([O-])(=O)=O)[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@@H]2[C@@H]([C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3[C@@H]([C@@H](OC)[C@H](O[C@@H]4[C@@H]([C@@H](OC)[C@H](OC)[C@@H](COS([O-])(=O)=O)O4)OC)[C@H](O3)C([O-])=O)OC)[C@@H](COS([O-])(=O)=O)O2)OS([O-])(=O)=O)[C@H](C([O-])=O)O1 MVPQUSQUURLQKF-MCPDASDXSA-E 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 229960001180 norfloxacin Drugs 0.000 description 1
- OGJPXUAPXNRGGI-UHFFFAOYSA-N norfloxacin Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 OGJPXUAPXNRGGI-UHFFFAOYSA-N 0.000 description 1
- 125000001736 nosyl group Chemical group S(=O)(=O)(C1=CC=C([N+](=O)[O-])C=C1)* 0.000 description 1
- 208000007892 occupational asthma Diseases 0.000 description 1
- 229950005751 ocrelizumab Drugs 0.000 description 1
- 125000005060 octahydroindolyl group Chemical group N1(CCC2CCCCC12)* 0.000 description 1
- 125000005061 octahydroisoindolyl group Chemical group C1(NCC2CCCCC12)* 0.000 description 1
- 229960002450 ofatumumab Drugs 0.000 description 1
- 229960001699 ofloxacin Drugs 0.000 description 1
- QQBDLJCYGRGAKP-FOCLMDBBSA-N olsalazine Chemical compound C1=C(O)C(C(=O)O)=CC(\N=N\C=2C=C(C(O)=CC=2)C(O)=O)=C1 QQBDLJCYGRGAKP-FOCLMDBBSA-N 0.000 description 1
- 229960004110 olsalazine Drugs 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 229960003278 osimertinib Drugs 0.000 description 1
- DUYJMQONPNNFPI-UHFFFAOYSA-N osimertinib Chemical compound COC1=CC(N(C)CCN(C)C)=C(NC(=O)C=C)C=C1NC1=NC=CC(C=2C3=CC=CC=C3N(C)C=2)=N1 DUYJMQONPNNFPI-UHFFFAOYSA-N 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- 229940127084 other anti-cancer agent Drugs 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- UWYHMGVUTGAWSP-JKIFEVAISA-N oxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=CC=CC=C1 UWYHMGVUTGAWSP-JKIFEVAISA-N 0.000 description 1
- 229960001019 oxacillin Drugs 0.000 description 1
- 125000001715 oxadiazolyl group Chemical group 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- AHVQYHFYQWKUKB-UHFFFAOYSA-N oxan-4-amine Chemical compound NC1CCOCC1 AHVQYHFYQWKUKB-UHFFFAOYSA-N 0.000 description 1
- 125000000160 oxazolidinyl group Chemical group 0.000 description 1
- 125000005968 oxazolinyl group Chemical group 0.000 description 1
- 125000006299 oxetan-3-yl group Chemical group [H]C1([H])OC([H])([H])C1([H])* 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 125000005476 oxopyrrolidinyl group Chemical group 0.000 description 1
- 229960002085 oxycodone Drugs 0.000 description 1
- 229960000625 oxytetracycline Drugs 0.000 description 1
- IWVCMVBTMGNXQD-PXOLEDIWSA-N oxytetracycline Chemical compound C1=CC=C2[C@](O)(C)[C@H]3[C@H](O)[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-PXOLEDIWSA-N 0.000 description 1
- 235000019366 oxytetracycline Nutrition 0.000 description 1
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 1
- 125000001037 p-tolyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])[H] 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 229960004390 palbociclib Drugs 0.000 description 1
- AHJRHEGDXFFMBM-UHFFFAOYSA-N palbociclib Chemical compound N1=C2N(C3CCCC3)C(=O)C(C(=O)C)=C(C)C2=CN=C1NC(N=C1)=CC=C1N1CCNCC1 AHJRHEGDXFFMBM-UHFFFAOYSA-N 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 201000010198 papillary carcinoma Diseases 0.000 description 1
- 229960005489 paracetamol Drugs 0.000 description 1
- 230000004796 pathophysiological change Effects 0.000 description 1
- 229960000639 pazopanib Drugs 0.000 description 1
- CUIHSIWYWATEQL-UHFFFAOYSA-N pazopanib Chemical compound C1=CC2=C(C)N(C)N=C2C=C1N(C)C(N=1)=CC=NC=1NC1=CC=C(C)C(S(N)(=O)=O)=C1 CUIHSIWYWATEQL-UHFFFAOYSA-N 0.000 description 1
- 229940121655 pd-1 inhibitor Drugs 0.000 description 1
- 229940121656 pd-l1 inhibitor Drugs 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 229940056360 penicillin g Drugs 0.000 description 1
- 229940056367 penicillin v Drugs 0.000 description 1
- 150000002960 penicillins Chemical class 0.000 description 1
- VOKSWYLNZZRQPF-GDIGMMSISA-N pentazocine Chemical compound C1C2=CC=C(O)C=C2[C@@]2(C)[C@@H](C)[C@@H]1N(CC=C(C)C)CC2 VOKSWYLNZZRQPF-GDIGMMSISA-N 0.000 description 1
- 229960005301 pentazocine Drugs 0.000 description 1
- 208000022719 perennial allergic rhinitis Diseases 0.000 description 1
- 229960000482 pethidine Drugs 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- 239000002831 pharmacologic agent Substances 0.000 description 1
- 125000001791 phenazinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3N=C12)* 0.000 description 1
- NFBAXHOPROOJAW-UHFFFAOYSA-N phenindione Chemical compound O=C1C2=CC=CC=C2C(=O)C1C1=CC=CC=C1 NFBAXHOPROOJAW-UHFFFAOYSA-N 0.000 description 1
- 229960000280 phenindione Drugs 0.000 description 1
- 125000001484 phenothiazinyl group Chemical group C1(=CC=CC=2SC3=CC=CC=C3NC12)* 0.000 description 1
- 125000001644 phenoxazinyl group Chemical group C1(=CC=CC=2OC3=CC=CC=C3NC12)* 0.000 description 1
- BPLBGHOLXOTWMN-MBNYWOFBSA-N phenoxymethylpenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)COC1=CC=CC=C1 BPLBGHOLXOTWMN-MBNYWOFBSA-N 0.000 description 1
- DQDAYGNAKTZFIW-UHFFFAOYSA-N phenprocoumon Chemical compound OC=1C2=CC=CC=C2OC(=O)C=1C(CC)C1=CC=CC=C1 DQDAYGNAKTZFIW-UHFFFAOYSA-N 0.000 description 1
- 229960004923 phenprocoumon Drugs 0.000 description 1
- 125000000286 phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 1
- 150000003014 phosphoric acid esters Chemical class 0.000 description 1
- 125000004592 phthalazinyl group Chemical group C1(=NN=CC2=CC=CC=C12)* 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229950010773 pidilizumab Drugs 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 229960002292 piperacillin Drugs 0.000 description 1
- 229940104641 piperacillin / tazobactam Drugs 0.000 description 1
- WCMIIGXFCMNQDS-IDYPWDAWSA-M piperacillin sodium Chemical compound [Na+].O=C1C(=O)N(CC)CCN1C(=O)N[C@H](C=1C=CC=CC=1)C(=O)N[C@@H]1C(=O)N2[C@@H](C([O-])=O)C(C)(C)S[C@@H]21 WCMIIGXFCMNQDS-IDYPWDAWSA-M 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 125000004482 piperidin-4-yl group Chemical group N1CCC(CC1)* 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920001515 polyalkylene glycol Polymers 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 208000037244 polycythemia vera Diseases 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229940113116 polyethylene glycol 1000 Drugs 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920000024 polymyxin B Polymers 0.000 description 1
- XDJYMJULXQKGMM-UHFFFAOYSA-N polymyxin E1 Natural products CCC(C)CCCCC(=O)NC(CCN)C(=O)NC(C(C)O)C(=O)NC(CCN)C(=O)NC1CCNC(=O)C(C(C)O)NC(=O)C(CCN)NC(=O)C(CCN)NC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(CCN)NC1=O XDJYMJULXQKGMM-UHFFFAOYSA-N 0.000 description 1
- KNIWPHSUTGNZST-UHFFFAOYSA-N polymyxin E2 Natural products CC(C)CCCCC(=O)NC(CCN)C(=O)NC(C(C)O)C(=O)NC(CCN)C(=O)NC1CCNC(=O)C(C(C)O)NC(=O)C(CCN)NC(=O)C(CCN)NC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(CCN)NC1=O KNIWPHSUTGNZST-UHFFFAOYSA-N 0.000 description 1
- 229960005266 polymyxin b Drugs 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 229910000160 potassium phosphate Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 150000003140 primary amides Chemical class 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 229940072288 prograf Drugs 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- ABBQGOCHXSPKHJ-WUKNDPDISA-N prontosil Chemical compound NC1=CC(N)=CC=C1\N=N\C1=CC=C(S(N)(=O)=O)C=C1 ABBQGOCHXSPKHJ-WUKNDPDISA-N 0.000 description 1
- LVOICKNPHXSSQM-UHFFFAOYSA-N prop-2-en-1-one Chemical compound C=C[C]=O LVOICKNPHXSSQM-UHFFFAOYSA-N 0.000 description 1
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 239000000473 propyl gallate Substances 0.000 description 1
- 235000010388 propyl gallate Nutrition 0.000 description 1
- 229940075579 propyl gallate Drugs 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 208000028172 protozoa infectious disease Diseases 0.000 description 1
- 125000001042 pteridinyl group Chemical group N1=C(N=CC2=NC=CN=C12)* 0.000 description 1
- 229940117820 purinethol Drugs 0.000 description 1
- 229960005206 pyrazinamide Drugs 0.000 description 1
- IPEHBUMCGVEMRF-UHFFFAOYSA-N pyrazinecarboxamide Chemical compound NC(=O)C1=CN=CC=N1 IPEHBUMCGVEMRF-UHFFFAOYSA-N 0.000 description 1
- 125000003072 pyrazolidinyl group Chemical group 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- 125000004528 pyrimidin-5-yl group Chemical group N1=CN=CC(=C1)* 0.000 description 1
- HZFPPBMKGYINDF-UHFFFAOYSA-N pyrimidin-5-ylboronic acid Chemical compound OB(O)C1=CN=CN=C1 HZFPPBMKGYINDF-UHFFFAOYSA-N 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 150000007660 quinolones Chemical class 0.000 description 1
- 125000005493 quinolyl group Chemical group 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 125000004621 quinuclidinyl group Chemical group N12C(CC(CC1)CC2)* 0.000 description 1
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-N raffinose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1 MUPFEKGTMRGPLJ-ZQSKZDJDSA-N 0.000 description 1
- 229940099538 rapamune Drugs 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 125000000946 retinyl group Chemical group [H]C([*])([H])/C([H])=C(C([H])([H])[H])/C([H])=C([H])/C([H])=C(C([H])([H])[H])/C([H])=C([H])/C1=C(C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])C1(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 235000019172 retinyl palmitate Nutrition 0.000 description 1
- 229940108325 retinyl palmitate Drugs 0.000 description 1
- 239000011769 retinyl palmitate Substances 0.000 description 1
- 229960000885 rifabutin Drugs 0.000 description 1
- WDZCUPBHRAEYDL-GZAUEHORSA-N rifapentine Chemical compound O([C@](C1=O)(C)O/C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)\C=C\C=C(C)/C(=O)NC=2C(O)=C3C(O)=C4C)C)OC)C4=C1C3=C(O)C=2\C=N\N(CC1)CCN1C1CCCC1 WDZCUPBHRAEYDL-GZAUEHORSA-N 0.000 description 1
- 229960003040 rifaximin Drugs 0.000 description 1
- NZCRJKRKKOLAOJ-XRCRFVBUSA-N rifaximin Chemical compound OC1=C(C(O)=C2C)C3=C4N=C5C=C(C)C=CN5C4=C1NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@H](C)[C@@H](OC)\C=C\O[C@@]1(C)OC2=C3C1=O NZCRJKRKKOLAOJ-XRCRFVBUSA-N 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- KGFYHTZWPPHNLQ-AWEZNQCLSA-N rivaroxaban Chemical compound S1C(Cl)=CC=C1C(=O)NC[C@@H]1OC(=O)N(C=2C=CC(=CC=2)N2C(COCC2)=O)C1 KGFYHTZWPPHNLQ-AWEZNQCLSA-N 0.000 description 1
- 229960001148 rivaroxaban Drugs 0.000 description 1
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 description 1
- 229960000371 rofecoxib Drugs 0.000 description 1
- 238000002390 rotary evaporation Methods 0.000 description 1
- 229960005224 roxithromycin Drugs 0.000 description 1
- HFNKQEVNSGCOJV-OAHLLOKOSA-N ruxolitinib Chemical compound C1([C@@H](CC#N)N2N=CC(=C2)C=2C=3C=CNC=3N=CN=2)CCCC1 HFNKQEVNSGCOJV-OAHLLOKOSA-N 0.000 description 1
- 229960000215 ruxolitinib Drugs 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- NGWSFRIPKNWYAO-SHTIJGAHSA-N salinosporamide A Chemical compound C([C@@H]1[C@H](O)[C@]23C(=O)O[C@]2([C@H](C(=O)N3)CCCl)C)CCC=C1 NGWSFRIPKNWYAO-SHTIJGAHSA-N 0.000 description 1
- NGWSFRIPKNWYAO-UHFFFAOYSA-N salinosporamide A Natural products N1C(=O)C(CCCl)C2(C)OC(=O)C21C(O)C1CCCC=C1 NGWSFRIPKNWYAO-UHFFFAOYSA-N 0.000 description 1
- 229940063122 sandimmune Drugs 0.000 description 1
- 238000003118 sandwich ELISA Methods 0.000 description 1
- 229950006348 sarilumab Drugs 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 229960004540 secukinumab Drugs 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 235000011649 selenium Nutrition 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 235000019615 sensations Nutrition 0.000 description 1
- 208000013223 septicemia Diseases 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 229960003600 silver sulfadiazine Drugs 0.000 description 1
- QRUBYZBWAOOHSV-UHFFFAOYSA-M silver trifluoromethanesulfonate Chemical compound [Ag+].[O-]S(=O)(=O)C(F)(F)F QRUBYZBWAOOHSV-UHFFFAOYSA-M 0.000 description 1
- UEJSSZHHYBHCEL-UHFFFAOYSA-N silver(1+) sulfadiazinate Chemical compound [Ag+].C1=CC(N)=CC=C1S(=O)(=O)[N-]C1=NC=CC=N1 UEJSSZHHYBHCEL-UHFFFAOYSA-N 0.000 description 1
- 229940115586 simulect Drugs 0.000 description 1
- XGVXKJKTISMIOW-ZDUSSCGKSA-N simurosertib Chemical compound N1N=CC(C=2SC=3C(=O)NC(=NC=3C=2)[C@H]2N3CCC(CC3)C2)=C1C XGVXKJKTISMIOW-ZDUSSCGKSA-N 0.000 description 1
- 229950006094 sirukumab Drugs 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 208000010721 smoldering plasma cell myeloma Diseases 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 235000011083 sodium citrates Nutrition 0.000 description 1
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 229940001584 sodium metabisulfite Drugs 0.000 description 1
- 235000010262 sodium metabisulphite Nutrition 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 230000007928 solubilization Effects 0.000 description 1
- 238000005063 solubilization Methods 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 229960003787 sorafenib Drugs 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 229940035044 sorbitan monolaurate Drugs 0.000 description 1
- DZZWHBIBMUVIIW-DTORHVGOSA-N sparfloxacin Chemical compound C1[C@@H](C)N[C@@H](C)CN1C1=C(F)C(N)=C2C(=O)C(C(O)=O)=CN(C3CC3)C2=C1F DZZWHBIBMUVIIW-DTORHVGOSA-N 0.000 description 1
- 229960004954 sparfloxacin Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 229960000268 spectinomycin Drugs 0.000 description 1
- UNFWWIHTNXNPBV-WXKVUWSESA-N spectinomycin Chemical compound O([C@@H]1[C@@H](NC)[C@@H](O)[C@H]([C@@H]([C@H]1O1)O)NC)[C@]2(O)[C@H]1O[C@H](C)CC2=O UNFWWIHTNXNPBV-WXKVUWSESA-N 0.000 description 1
- 201000006923 sphenoid sinusitis Diseases 0.000 description 1
- 206010062113 splenic marginal zone lymphoma Diseases 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 229940031439 squalene Drugs 0.000 description 1
- TUHBEKDERLKLEC-UHFFFAOYSA-N squalene Natural products CC(=CCCC(=CCCC(=CCCC=C(/C)CCC=C(/C)CC=C(C)C)C)C)C TUHBEKDERLKLEC-UHFFFAOYSA-N 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 125000005415 substituted alkoxy group Chemical group 0.000 description 1
- 125000000547 substituted alkyl group Chemical group 0.000 description 1
- 125000005346 substituted cycloalkyl group Chemical group 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- SKIVFJLNDNKQPD-UHFFFAOYSA-N sulfacetamide Chemical compound CC(=O)NS(=O)(=O)C1=CC=C(N)C=C1 SKIVFJLNDNKQPD-UHFFFAOYSA-N 0.000 description 1
- 229960002673 sulfacetamide Drugs 0.000 description 1
- 229960000654 sulfafurazole Drugs 0.000 description 1
- 229960005404 sulfamethoxazole Drugs 0.000 description 1
- 229950008188 sulfamidochrysoidine Drugs 0.000 description 1
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- JLKIGFTWXXRPMT-UHFFFAOYSA-N sulphamethoxazole Chemical compound O1C(C)=CC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1 JLKIGFTWXXRPMT-UHFFFAOYSA-N 0.000 description 1
- 235000011149 sulphuric acid Nutrition 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000002511 suppository base Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- UJMBCXLDXJUMFB-GLCFPVLVSA-K tartrazine Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)C1=NN(C=2C=CC(=CC=2)S([O-])(=O)=O)C(=O)C1\N=N\C1=CC=C(S([O-])(=O)=O)C=C1 UJMBCXLDXJUMFB-GLCFPVLVSA-K 0.000 description 1
- 229960000943 tartrazine Drugs 0.000 description 1
- 239000004149 tartrazine Substances 0.000 description 1
- 235000012756 tartrazine Nutrition 0.000 description 1
- 238000003419 tautomerization reaction Methods 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- ONUMZHGUFYIKPM-MXNFEBESSA-N telavancin Chemical compound O1[C@@H](C)[C@@H](O)[C@](NCCNCCCCCCCCCC)(C)C[C@@H]1O[C@H]1[C@H](OC=2C3=CC=4[C@H](C(N[C@H]5C(=O)N[C@H](C(N[C@@H](C6=CC(O)=C(CNCP(O)(O)=O)C(O)=C6C=6C(O)=CC=C5C=6)C(O)=O)=O)[C@H](O)C5=CC=C(C(=C5)Cl)O3)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](NC(=O)[C@@H](CC(C)C)NC)[C@H](O)C3=CC=C(C(=C3)Cl)OC=2C=4)O[C@H](CO)[C@@H](O)[C@@H]1O ONUMZHGUFYIKPM-MXNFEBESSA-N 0.000 description 1
- 229960005240 telavancin Drugs 0.000 description 1
- 108010089019 telavancin Proteins 0.000 description 1
- LJVAJPDWBABPEJ-PNUFFHFMSA-N telithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)[C@@H](C)C(=O)O[C@@H]([C@]2(OC(=O)N(CCCCN3C=C(N=C3)C=3C=NC=CC=3)[C@@H]2[C@@H](C)C(=O)[C@H](C)C[C@@]1(C)OC)C)CC)[C@@H]1O[C@H](C)C[C@H](N(C)C)[C@H]1O LJVAJPDWBABPEJ-PNUFFHFMSA-N 0.000 description 1
- 229960003250 telithromycin Drugs 0.000 description 1
- 229960004576 temafloxacin Drugs 0.000 description 1
- 229960001114 temocillin Drugs 0.000 description 1
- BVCKFLJARNKCSS-DWPRYXJFSA-N temocillin Chemical compound N([C@]1(OC)C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C(C(O)=O)C=1C=CSC=1 BVCKFLJARNKCSS-DWPRYXJFSA-N 0.000 description 1
- IWVCMVBTMGNXQD-UHFFFAOYSA-N terramycin dehydrate Natural products C1=CC=C2C(O)(C)C3C(O)C4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-UHFFFAOYSA-N 0.000 description 1
- VVDCRJGWILREQH-UHFFFAOYSA-N tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,6-dihydro-2h-pyridine-1-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCC(B2OC(C)(C)C(C)(C)O2)=C1 VVDCRJGWILREQH-UHFFFAOYSA-N 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- 125000005207 tetraalkylammonium group Chemical group 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 229940040944 tetracyclines Drugs 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 229960004559 theobromine Drugs 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 229960002178 thiamazole Drugs 0.000 description 1
- 125000006090 thiamorpholinyl sulfone group Chemical group 0.000 description 1
- 125000006089 thiamorpholinyl sulfoxide group Chemical group 0.000 description 1
- 229960003053 thiamphenicol Drugs 0.000 description 1
- OTVAEFIXJLOWRX-NXEZZACHSA-N thiamphenicol Chemical compound CS(=O)(=O)C1=CC=C([C@@H](O)[C@@H](CO)NC(=O)C(Cl)Cl)C=C1 OTVAEFIXJLOWRX-NXEZZACHSA-N 0.000 description 1
- 125000001984 thiazolidinyl group Chemical group 0.000 description 1
- 125000002769 thiazolinyl group Chemical group 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 125000004568 thiomorpholinyl group Chemical group 0.000 description 1
- 208000030901 thyroid gland follicular carcinoma Diseases 0.000 description 1
- 208000013076 thyroid tumor Diseases 0.000 description 1
- 229960004089 tigecycline Drugs 0.000 description 1
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 229960000707 tobramycin Drugs 0.000 description 1
- NLVFBUXFDBBNBW-PBSUHMDJSA-N tobramycin Chemical compound N[C@@H]1C[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N NLVFBUXFDBBNBW-PBSUHMDJSA-N 0.000 description 1
- 229960003989 tocilizumab Drugs 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 235000010384 tocopherol Nutrition 0.000 description 1
- 229960001295 tocopherol Drugs 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 229950001802 toralizumab Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 229960004380 tramadol Drugs 0.000 description 1
- TVYLLZQTGLZFBW-GOEBONIOSA-N tramadol Natural products COC1=CC=CC([C@@]2(O)[C@@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-GOEBONIOSA-N 0.000 description 1
- 229960004066 trametinib Drugs 0.000 description 1
- LIRYPHYGHXZJBZ-UHFFFAOYSA-N trametinib Chemical compound CC(=O)NC1=CC=CC(N2C(N(C3CC3)C(=O)C3=C(NC=4C(=CC(I)=CC=4)F)N(C)C(=O)C(C)=C32)=O)=C1 LIRYPHYGHXZJBZ-UHFFFAOYSA-N 0.000 description 1
- LLPOLZWFYMWNKH-UHFFFAOYSA-N trans-dihydrocodeinone Natural products C1C(N(CCC234)C)C2CCC(=O)C3OC2=C4C1=CC=C2OC LLPOLZWFYMWNKH-UHFFFAOYSA-N 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 229940074410 trehalose Drugs 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- WLPUWLXVBWGYMZ-UHFFFAOYSA-N tricyclohexylphosphine Chemical compound C1CCCCC1P(C1CCCCC1)C1CCCCC1 WLPUWLXVBWGYMZ-UHFFFAOYSA-N 0.000 description 1
- 229960001082 trimethoprim Drugs 0.000 description 1
- IEDVJHCEMCRBQM-UHFFFAOYSA-N trimethoprim Chemical compound COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 IEDVJHCEMCRBQM-UHFFFAOYSA-N 0.000 description 1
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- 229960005041 troleandomycin Drugs 0.000 description 1
- LQCLVBQBTUVCEQ-QTFUVMRISA-N troleandomycin Chemical compound O1[C@@H](C)[C@H](OC(C)=O)[C@@H](OC)C[C@@H]1O[C@@H]1[C@@H](C)C(=O)O[C@H](C)[C@H](C)[C@H](OC(C)=O)[C@@H](C)C(=O)[C@@]2(OC2)C[C@H](C)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)OC(C)=O)[C@H]1C LQCLVBQBTUVCEQ-QTFUVMRISA-N 0.000 description 1
- 229960000497 trovafloxacin Drugs 0.000 description 1
- WVPSKSLAZQPAKQ-CDMJZVDBSA-N trovafloxacin Chemical compound C([C@H]1[C@@H]([C@H]1C1)N)N1C(C(=CC=1C(=O)C(C(O)=O)=C2)F)=NC=1N2C1=CC=C(F)C=C1F WVPSKSLAZQPAKQ-CDMJZVDBSA-N 0.000 description 1
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 231100000397 ulcer Toxicity 0.000 description 1
- 208000010576 undifferentiated carcinoma Diseases 0.000 description 1
- 150000003672 ureas Chemical class 0.000 description 1
- 210000003932 urinary bladder Anatomy 0.000 description 1
- 210000001215 vagina Anatomy 0.000 description 1
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 1
- 229960003165 vancomycin Drugs 0.000 description 1
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 description 1
- UHTHHESEBZOYNR-UHFFFAOYSA-N vandetanib Chemical compound COC1=CC(C(/N=CN2)=N/C=3C(=CC(Br)=CC=3)F)=C2C=C1OCC1CCN(C)CC1 UHTHHESEBZOYNR-UHFFFAOYSA-N 0.000 description 1
- 208000001319 vasomotor rhinitis Diseases 0.000 description 1
- 235000019871 vegetable fat Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 229960003862 vemurafenib Drugs 0.000 description 1
- GPXBXXGIAQBQNI-UHFFFAOYSA-N vemurafenib Chemical compound CCCS(=O)(=O)NC1=CC=C(F)C(C(=O)C=2C3=CC(=CN=C3NC=2)C=2C=CC(Cl)=CC=2)=C1F GPXBXXGIAQBQNI-UHFFFAOYSA-N 0.000 description 1
- PNVNVHUZROJLTJ-UHFFFAOYSA-N venlafaxine Chemical compound C1=CC(OC)=CC=C1C(CN(C)C)C1(O)CCCCC1 PNVNVHUZROJLTJ-UHFFFAOYSA-N 0.000 description 1
- 229960004688 venlafaxine Drugs 0.000 description 1
- 201000005539 vernal conjunctivitis Diseases 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 229960005080 warfarin Drugs 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- ZXIBCJHYVWYIKI-PZJWPPBQSA-N ximelagatran Chemical compound C1([C@@H](NCC(=O)OCC)C(=O)N2[C@@H](CC2)C(=O)NCC=2C=CC(=CC=2)C(\N)=N\O)CCCCC1 ZXIBCJHYVWYIKI-PZJWPPBQSA-N 0.000 description 1
- 229960001522 ximelagatran Drugs 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
- 229940055760 yervoy Drugs 0.000 description 1
- 229950009002 zanolimumab Drugs 0.000 description 1
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4965—Non-condensed pyrazines
- A61K31/497—Non-condensed pyrazines containing further heterocyclic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/444—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring heteroatom, e.g. amrinone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4523—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems
- A61K31/4545—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring hetero atom, e.g. pipamperone, anabasine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5377—1,4-Oxazines, e.g. morpholine not condensed and containing further heterocyclic rings, e.g. timolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Pulmonology (AREA)
- Rheumatology (AREA)
- Biomedical Technology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Neurology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Reproductive Health (AREA)
- Endocrinology (AREA)
- Urology & Nephrology (AREA)
- Virology (AREA)
- Obesity (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Abstract
Las realizaciones descritas se refieren a nuevos inhibidores de quinasas asociadas al receptor de interleucina (IRAK) de fórmula 1 y composiciones que comprenden dichos inhibidores. También se describen métodos para preparar y usar los compuestos y composiciones. Los compuestos y/o composiciones divulgados pueden usarse para tratar o prevenir una enfermedad o afección asociada con IRAK. (Traducción automática con Google Translate, sin valor legal)The described embodiments relate to new interleukin receptor associated kinase (IRAK) inhibitors of formula 1 and compositions comprising said inhibitors. Methods for preparing and using the compounds and compositions are also described. The disclosed compounds and/or compositions may be used to treat or prevent a disease or condition associated with IRAQ. (Automatic translation with Google Translate, without legal value)
Description
DESCRIPCIÓNDESCRIPTION
Compuestos de pirazol amida como inhibidores de IRAKPyrazole amide compounds as IRAK inhibitors
CAMPOFIELD
Esta descripción se refiere a compuestos de amida, y a realizaciones de un método para obtener y usar los compuestos, tal como para inhibir la quinasa asociada al receptor de interleuquina (IRAK), y para tratar enfermedades y afecciones relacionadas con IRAK.This disclosure relates to amide compounds, and to embodiments of a method of making and using the compounds, such as for inhibiting interleukin receptor-associated kinase (IRAK), and for treating IRAK-related diseases and conditions.
ANTECEDENTESBACKGROUND
Las quinasas asociadas al receptor de interleuquina-1 (IRAKs) son mediadores importantes de los procesos de señalización, tales como los receptores tipo toll (TLR, por sus siglas en inglés) y los procesos de señalización del receptor de interleuquina-1 (IL-1R). Las IRAKs se han implicado en la modulación de las redes de señalización que controlan la inflamación, la apoptosis y la diferenciación celular. Se han identificado cuatro genes IRAK en el genoma humano (IRAK1, IRAK2, IRAK3 e IRAK4), y estudios han revelado papeles biológicos distintos y no redundantes. Se ha demostrado que IRAK1 e IRAK4 exhiben actividad quinasa. El documento WO2015/068856 describe inhibidores de IRAK.Interleukin-1 receptor-associated kinases (IRAKs) are important mediators of signaling processes, such as toll-like receptors (TLRs) and interleukin-1 receptor (IL-1) signaling processes. 1R). IRAKs have been implicated in the modulation of signaling networks that control inflammation, apoptosis, and cell differentiation. Four IRAK genes have been identified in the human genome (IRAK1, IRAK 2 , IRAK3 and IRAK4), and studies have revealed distinct and non-redundant biological roles. IRAK1 and IRAK4 have been shown to exhibit kinase activity. WO2015/068856 describes IRAK inhibitors.
SUMARIOSUMMARY
La invención es como se define en las reivindicaciones. Por consiguiente, la invención proporciona un compuesto que tiene una fórmula 1The invention is as defined in the claims. Accordingly, the invention provides a compound having a formula 1
o una sal del mismo, en la que:or a salt thereof, wherein:
el anillo A es heteroarilo;ring A is heteroaryl;
R1 es H o alquilo C1-6 ;R1 is H or C 1-6 alkyl;
R2 es H, alifático, heteroalifático, o heterociclilo;R2 is H, aliphatic, heteroaliphatic, or heterocyclyl;
cada Z1, Z2, Z3, y Z4, es independientemente N o CR3, en la que al menos uno de Z1, Z2, Z3, y Z4 es N; cada R3 es independientemente H, alifático, o heteroalifático; yeach Z1, Z2, Z3, and Z4, is independently N or CR3, wherein at least one of Z1, Z2, Z3, and Z4 is N; each R3 is independently H, aliphatic, or heteroaliphatic; and
R4 es halógeno, heteroarilo, heterocicloalifático, arilo, -NH-heteroarilo, u -O-heteroarilo.R4 is halogen, heteroaryl, heterocycloaliphatic, aryl, -NH-heteroaryl, or -O-heteroaryl.
La invención también proporciona una composición que comprende un compuesto como se define anteriormente, y un excipiente farmacéuticamente aceptable.The invention also provides a composition comprising a compound as defined above, and a pharmaceutically acceptable excipient.
Ciertas realizaciones descritas se refieren a compuestos que tienen una fórmula 1Certain described embodiments refer to compounds having a formula 1
y/o una sal del mismo. Un experto en la técnica apreciará que los compuestos dentro de la fórmula 1 también pueden ser solvatos, incluidos hidratos, N-óxidos de los mismos. Con referencia a la fórmula 1, el anillo A es heteroarilo, tal como piridinilo, o pirazinilo. En realizaciones particulares, el anillo A es piridin-2-ilo. En otros ejemplos, el anillo A esand/or a salt thereof. One skilled in the art will appreciate that compounds within formula 1 may also be solvates, including hydrates, N-oxides thereof. Referring to formula 1, ring A is heteroaryl, such as pyridinyl, or pyrazinyl. In particular embodiments, ring A is pyridin-2-yl. In other examples, ring A is
en la que cada Q es independientemente CH, CRm o N, Rm es Rb, y x es 0, 1, o 2; al menos un Q es N. Rb es independientemente en cada aparición -OH, -CF3 , -CN, -Orc, -SO2 Rc, - NRdRd, -N(H)SO2 Rc, -C(O)OH, -N(H)C(O)Rc, -C(O)ORc, -C(O)NRdRd, =O, o halógeno. Rc es independientemente, para cada aparición, alquilo de C1-6, cicloalquilo de C3-6, heteroaliciclilo de C3-6, aralquilo, alquilo de C1-6 sustituido con 1,2 o 3 Re, aromático de C5-10, aromático de C5-10 sustituido con 1,2 o 3 Re. Rd es independientemente, para cada aparición, H, alquilo de C1-6 opcionalmente sustituido con 1 ,2 o 3 Re, cicloalquilo de C3-6 opcionalmente sustituido con 1, 2 o 3 Re, heteroaliciclilo de C3-6 opcionalmente sustituido con 1,2 o 3 Re, aromático de C5-10 opcionalmente sustituido con 1,2 o 3 Ra o Rb, o dos grupos Rd, junto con el nitrógeno unido a ella, forman un resto heteroaliciclilo de C3-6 opcionalmente sustituido con alquilo de C1-6 y opcionalmente interrumpido con uno o dos -O- o -N(R8), en el que R9 es R70. Ra es independientemente, para cada aparición, H, D, alquilo de C1-6, cicloalquilo de C3-6, aromático de C5-10, o heterocicloalifático de C3-6. Y Re es independientemente, para cada aparición, halógeno, alquilo de C1-6, cicloalquilo de C3-6, u -ORa.wherein each Q is independently CH, CRm, or N, Rm is Rb, and x is 0, 1, or 2; at least one Q is N. Rb is independently at each occurrence -OH, -CF3 , -CN, -O rc , -SO2 Rc, -NRdRd, -N(H)SO2 Rc, -C(O)OH, -N (H)C(O)Rc, -C(O)ORc, -C(O)NRdRd, =O, or halogen. Rc is independently, for each occurrence, C 1-6 alkyl, C 3-6 cycloalkyl, C 3-6 heteroalicyclyl, aralkyl, C 1-6 alkyl substituted with 1,2 or 3 Re, C 5 aromatic -10 , C 5-10 aromatic substituted with 1,2 or 3 Re. Rd is independently, for each occurrence, H, C 1-6 alkyl optionally substituted with 1,2 or 3 Re, C 3-6 cycloalkyl optionally substituted with 1, 2 or 3 Re, C 3-6 heteroalicyclyl optionally substituted with 1,2 or 3 Re, C 5-10 aromatic optionally substituted with 1,2 or 3 Ra or Rb, or two Rd groups, together with nitrogen attached thereto, form a C 3-6 heteroalicyclyl moiety optionally substituted with C 1-6 alkyl and optionally interrupted with one or two -O- or -N(R 8 ), where R 9 is R 7 0. Ra is independently, for each occurrence, H, D, C 1-6 alkyl, C 3-6 cycloalkyl, C 5-10 aromatic, or C 3-6 heterocycloaliphatic. Y Re is independently, for each occurrence, halogen, C 1-6 alkyl, C 3-6 cycloalkyl, or -ORa.
R1 es H o alquilo de C1-6; R2 es H, alifático, heteroalifático, o heterociclilo, y puede ser H, heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático. En algunas realizaciones, R2 es Ra, Ra sustituido con Rb, Ra sustituido con 1 o 2 Rc, o Ra sustituido con Rd. Y en realizaciones particulares, R2 es H, CH3,R1 is H or C 1-6 alkyl; R2 is H, aliphatic, heteroaliphatic, or heterocyclyl, and can be H, 3-10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, alkyl unsubstituted C 1-6 alkyl, or C 1-6 alkyl substituted with -OH, amino, alkoxy, or heterocycloaliphatic. In some embodiments, R2 is Ra, Ra substituted with Rb, Ra substituted with 1 or 2 Rc, or Ra substituted with Rd. And in particular embodiments, R2 is H, CH 3 ,
En ciertas realizaciones de fórmula 1, el anillo A es piridinilo o pirazinilo; R1 es H; y/o R2 es H, heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático.In certain embodiments of Formula 1, Ring A is pyridinyl or pyrazinyl; R1 is H; and/or R2 is H, 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C 1-6 substituted with -OH, amino, alkoxy, or heterocycloaliphatic.
También con respecto a la fórmula 1, cada Z1, Z2, Z3, y Z4 es independientemente N o CR3, en la que al menos uno de Z1, Z2, Z3, y Z4 es N, y cada R3 es independientemente H, alifático, o heteroalifático. R4 puede ser halógeno, heteroarilo, heterocicloalifático, arilo, -NH-heteroarilo, u -O-heteroarilo.Also with respect to formula 1, each Z1, Z2, Z3, and Z4 is independently N or CR3, wherein at least one of Z1, Z2, Z3, and Z4 is N, and each R3 is independently H, aliphatic, or heteroaliphatic. R4 can be halogen, heteroaryl, heterocycloaliphatic, aryl, -NH-heteroaryl, or -O-heteroaryl.
En algunas realizaciones, el restoIn some embodiments, the rest
es piridinilo, pirimidinilo, o pirazinilo. En algunos ejemplos, Z1 es N.En otros ejemplos, Z1 es N, y Z2, Z3, y Z4 son CR3; Z1 y Z2 son N, y Z3 y Z4 son CR3; Z1 y Z3 son N, y Z2 y Z4 son CR3; Z1 y Z4 son N, y Z2 y Z3 son CR3; o Z3 es N, y Z1, Z2, y Z4 son CR3. En cualquiera de las realizaciones, R1 puede ser H.is pyridinyl, pyrimidinyl, or pyrazinyl. In some examples, Z1 is N. In other examples, Z1 is N, and Z2, Z3, and Z4 are CR3; Z1 and Z2 are N, and Z3 and Z4 are CR3; Z1 and Z3 are N, and Z2 and Z4 are CR3; Z1 and Z4 are N, and Z2 and Z3 are CR3; or Z3 is N, and Z1, Z2, and Z4 are CR3. In any of the embodiments, R1 can be H.
R4 puede ser halógeno, heterocicloalifático, arilo, heteroarilo, -NH-heteroarilo, u -O-heteroarilo, tal como Br, heteroarilo de 5 a 10 miembros, heterocicloalifático de 3 a 6 miembros, arilo de 6 a 10 miembros, -NH-(heteroarilo de 5 a 10 miembros), u -O-(heteroarilo de 5 a 10 miembros). En algunas realizaciones, R4 es piridinilo, pirimidinilo, pirazolilo, NH-pirazolilo, pirrolilo, -O-piridinilo -NH-piridinilo, indolilo, furanilo, -NH-benzopirazolilo, pirrolopiridinilo, fenilo, tetrahidropiridinilo, piperidinilo, o 2-oxo-1,2-dihidropiridinilo. En otros ejemplos, R4 es Br,R4 can be halogen, heterocycloaliphatic, aryl, heteroaryl, -NH-heteroaryl, or -O-heteroaryl, such as Br, 5-10 membered heteroaryl, 3-6 membered heterocycloaliphatic, 6-10 membered aryl, -NH- (5 to 10 membered heteroaryl), or -O-(5 to 10 membered heteroaryl). In some embodiments, R4 is pyridinyl, pyrimidinyl, pyrazolyl, NH-pyrazolyl, pyrrolyl, -O-pyridinyl -NH-pyridinyl, indolyl, furanyl, -NH-benzopyrazolyl, pyrrolopyridinyl, phenyl, tetrahydropyridinyl, piperidinyl, or 2-oxo-1,2-dihydropyridinyl. In other examples, R4 is Br,
en las que y es 0, 1 o 2, y cada Rp es independientemente Ra, Rb, Ra sustituido con Rb, o o R Ra sustituid Rp puede ser independientemente -CH3 , -OCH3 , - NH2 , -CF3 , F, -CN,wherein y is 0, 1, or 2, and each Rp is independently Ra, Rb, Ra substituted with Rb, oo R Ra substituted Rp can independently be -CH 3 , -OCH 3 , -NH 2 , -CF 3 , F , -CN,
En realizaciones particulares, R4 es Br; piridinilo no sustituido; piridinilo sustituido con alquilo de C1-6 , haloalquilo, amino, heterocicloalifático, cicloalquilo, -CN, alcoxi, -O-heterocicloalifático, -NH-heterocicloalifático, halógeno, sulfonamida, -O-bencilo, carboxilo, sulfonilo, -NH-cicloalquilo, o amida; pirimidinilo no sustituido; pirazolilo no sustituido; pirazolilo sustituido con alquilo de C1-6 ; -NH-pirazolilo no sustituido; -NH-pirazolilo sustituido con alquilo de C1-6 , o heteroarilo; pirrolilo; -O-piridinilo no sustituido; -O-piridinilo sustituido con amino; -NH-piridinilo sustituido con alquilo de C1-6, haloalquilo, o heterocicloalifático; indolilo no sustituido; indolilo sustituido con alcoxi; furanilo; -NH-benzopirazolilo; pirrolopiridinilo; fenilo no sustituido; fenilo sustituido con halógeno, alquilo de C1-6 , alcoxi, -CN, amino, o sulfonamida; tetrahidropiridinilo no sustituido; tetrahidropiridinilo sustituido con terc-butoxicarbonilo; piperidinilo; o 2-oxo-1,2-dihidropiridinilo.In particular embodiments, R4 is Br; unsubstituted pyridinyl; C 1-6 alkyl-substituted pyridinyl, haloalkyl, amino, heterocycloaliphatic, cycloalkyl, -CN, alkoxy, -O-heterocycloaliphatic, -NH-heterocycloaliphatic, halogen, sulfonamide, -O-benzyl, carboxyl, sulfonyl, -NH-cycloalkyl , or amide; unsubstituted pyrimidinyl; unsubstituted pyrazolyl; pyrazolyl substituted with C 1-6 alkyl; -NH-pyrazolyl unsubstituted; -NH-pyrazolyl substituted with C 1-6 alkyl, or heteroaryl; pyrrolyl; -O-pyridinyl unsubstituted; -O-pyridinyl substituted with amino; -NH-pyridinyl substituted with C 1-6 alkyl, haloalkyl, or heterocycloaliphatic; unsubstituted indolyl; alkoxy substituted indolyl; furanyl; -NH-benzopyrazolyl; pyrrolopyridinyl; unsubstituted phenyl; phenyl substituted with halogen, C 1-6 alkyl, alkoxy, -CN, amino, or sulfonamide; unsubstituted tetrahydropyridinyl; tetrahydropyridinyl substituted with tert-butoxycarbonyl; piperidinyl; or 2-oxo-1,2-dihydropyridinyl.
Los compuestos descritos también pueden tener una fórmula 2The described compounds can also have a formula 2
y/o una sal, solvato, N-óxido, de los mismos, en la que los sustituyentes comunes son como se establece para la fórmula 1, y cada uno de R5, R6, y R7 es independientemente H o alquilo, tal como alquilo de C1-6. En algunos ejemplos, cada uno de R5, R6, y R' es H. En algunas realizaciones de la fórmula 2, el anillo A es piridin-2-ilo, o pirazin-2-ilo. and/or a salt, solvate, N-oxide, thereof, wherein the common substituents are as set forth for Formula 1, and R5, R6, and R7 are each independently H or alkyl, such as C 1-6 alkyl. In some examples, R5, R6, and R' are each H. In some embodiments of formula 2, ring A is pyridin-2-yl, or pyrazin-2-yl.
También se describen compuestos que tienen la fórmula 3Also described are compounds having the formula 3
y/o una sal, solvato, N-óxido, de los mismos, en la que los sustituyentes comunes son como se establece para las fórmulas 1 y 2, y cada uno de R8, R9, R10 y R11 es independientemente H, alifático, halógeno, heterocicloalifático, alcoxi, u -O-heterocicloalifático, tal como H, halógeno, heterocicloalifático de 3 a 6 miembros, alcoxi, u -O-(heterocicloalifático de 3 a 6 miembros). En ciertas realizaciones, R8 es H o halógeno; R9 y R11 son H; y/o R10 es H, F, morfolino, N-metilpiperidin-1-ilo, metoxi, 2-hidroxi-2-metilpropoxi, u -O-oxetanilo. Y en realizaciones particulares, cada uno de R8, R9, y R11 es H; R9 y R11 son H, y R8 y R10 son F; o cada uno de R8, R9, R10 y R11 es H.and/or a salt, solvate, N-oxide, thereof, wherein the common substituents are as set forth for formulas 1 and 2, and R8 , R9, R10, and R11 are each independently H, aliphatic , halogen, heterocycloaliphatic, alkoxy, or -O-heterocycloaliphatic, such as H, halogen, 3- to 6-membered heterocycloaliphatic, alkoxy, or -O-(3- to 6-membered heterocycloaliphatic). In certain embodiments, R 8 is H or halogen; R9 and R11 are H; and/or R10 is H, F, morpholino, N-methylpiperidin-1-yl, methoxy, 2-hydroxy-2-methylpropoxy, or -O-oxetanyl. And in particular embodiments, each of R 8 , R 9 , and R 11 is H; R9 and R11 are H, and R8 and R10 are F; or R8 , R9, R10, and R11 are each H.
Los compuestos descritos también pueden tener una fórmula 8The described compounds can also have a formula 8
y/o una sal, solvato, N-óxido, de los mismos, en la que los sustituyentes son como se establece para las fórmulas 1 3. En algunas realizaciones, R1 es H; R2 es H, heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático; R4 es Br; piridinilo no sustituido; piridinilo sustituido con alquilo de C1-6 , haloalquilo, amino, heterocicloalifático, cicloalquilo, -CN, alcoxi, -O-heterocicloalifático, -NH-heterocicloalifático, halógeno, sulfonamida, -O-bencilo, carboxilo, sulfonilo, -NH-cicloalquilo, o amida; pirimidinilo no sustituido; pirazolilo no sustituido; pirazolilo sustituido con alquilo de C1-6 ; -NH-pirazolilo no sustituido; -NH-pirazolilo sustituido con alquilo de C1-6 , o heteroarilo; pirrolilo; -O-piridinilo no sustituido; -O-piridinilo sustituido con amino; -NH-piridinilo sustituido con alquilo de C1-6 , haloalquilo, o heterocicloalifático; indolilo no sustituido; indolilo sustituido con alcoxi; furanilo; -NH-benzopirazolilo; pirrolopiridinilo; fenilo no sustituido; fenilo sustituido con halógeno, alquilo de C1-6 , alcoxi, -CN, amino, o sulfonamida; tetrahidropiridinilo no sustituido; tetrahidropiridinilo sustituido con terc-butoxicarbonilo; piperidinilo; o 2-oxo-1,2-dihidropiridinilo; R8 es H o F; R9 y R11 son H; y R10 es H, F, morfolino, N-metilpiperidinilo, metoxi, 2-hidroxi-2-metilpropoxi, u -O-oxetanilo. Y en realizaciones particulares, cada uno de R8, R9, R10 y R11 es H; R8, R9, y R11 es H y R10 es morfolino de 3 a 6 miembros o N-metilpiperidinilo, metoxi, 2-hidroxi-2-metilpropoxi, u -O-oxetanilo; o R8 y R10 son F, y R9 y R11 son H.and/or a salt, solvate, N-oxide, thereof, in which the substituents are as set forth for Formulas 1-3. In some embodiments, R1 is H; R2 is H, 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C alkyl 1-6 substituted with -OH, amino, alkoxy, or heterocycloaliphatic; R4 is Br; unsubstituted pyridinyl; C 1-6 alkyl-substituted pyridinyl, haloalkyl, amino, heterocycloaliphatic, cycloalkyl, -CN, alkoxy, -O-heterocycloaliphatic, -NH-heterocycloaliphatic, halogen, sulfonamide, -O-benzyl, carboxyl, sulfonyl, -NH-cycloalkyl , or amide; unsubstituted pyrimidinyl; unsubstituted pyrazolyl; pyrazolyl substituted with C 1-6 alkyl; -NH-pyrazolyl unsubstituted; -NH-pyrazolyl substituted with C 1-6 alkyl, or heteroaryl; pyrrolyl; -O-pyridinyl unsubstituted; -O-pyridinyl substituted with amino; -NH-pyridinyl substituted with C 1-6 alkyl, haloalkyl, or heterocycloaliphatic; unsubstituted indolyl; alkoxy substituted indolyl; furanyl; -NH-benzopyrazolyl; pyrrolopyridinyl; unsubstituted phenyl; phenyl substituted with halogen, C 1-6 alkyl, alkoxy, -CN, amino, or sulfonamide; unsubstituted tetrahydropyridinyl; tetrahydropyridinyl substituted with tert-butoxycarbonyl; piperidinyl; or 2-oxo-1,2-dihydropyridinyl; R 8 is H or F; R9 and R11 are H; and R10 is H, F, morpholino, N-methylpiperidinyl, methoxy, 2-hydroxy-2-methylpropoxy, or -O-oxetanyl. And in particular embodiments, each of R 8 , R 9 , R 10 and R 11 is H; R 8 , R 9 , and R 11 is H and R 10 is 3- to 6-membered morpholino or N-methylpiperidinyl, methoxy, 2-hydroxy-2-methylpropoxy, or -O-oxetanyl; or R 8 and R10 are F, and R9 and R11 are H.
Compuestos de acuerdo con la presente divulgación también se pueden formular como composiciones que comprenden uno o más compuestos de acuerdo con cualquiera de las fórmulas 1-9 y un excipiente farmacéuticamente aceptable. Composiciones de este tipo también pueden incluir un agente terapéutico adicional.Compounds according to the present disclosure may also be formulated as compositions comprising one or more compounds according to any of formulas 1-9 and a pharmaceutically acceptable excipient. Compositions of this type can also include an additional therapeutic agent.
También se describen métodos para obtener y usar dichos compuestos y composiciones (debe señalarse, para evitar dudas, que cualesquiera referencias descritas a métodos de tratamiento médico que usan los compuestos y composiciones no es parte de la invención, sino en el contexto de la invención debe interpretarse, en cambio, como los compuestos y composiciones para uso en los métodos relevantes de tratamiento médico). Por ejemplo, un método descrito para usar compuestos dentro de las fórmulas 1-9 comprende administrar a un sujeto que lo necesita una cantidad efectiva de un compuesto, dos o más compuestos, o una composición que comprende al menos un compuesto, de acuerdo con cualquiera o todas las fórmulas 1-9. El método puede ser particularmente adecuado para tratar una enfermedad o afección para la que está indicado un inhibidor de IRAK, incluido un inhibidor de IRAK1, IRAK2, IRAK3 y/o IRAK4. La enfermedad puede ser una enfermedad autoinmune, un trastorno inflamatorio, una enfermedad cardiovascular, un trastorno neurodegenerativo, un trastorno alérgico, una insuficiencia multi-orgánica, una enfermedad renal, una enfermedad de agregación plaquetaria, cáncer, trasplante, motilidad espermática, deficiencia de eritrocitos, rechazo del injerto, lesión pulmonar, enfermedad respiratoria, afección isquémica, infección bacteriana, infección vírica, trastorno de la regulación inmunitaria o una combinación de los mismos.Methods of making and using said compounds and compositions are also described (it should be noted, for the avoidance of doubt, that any references disclosed to methods of medical treatment using the compounds and compositions are not part of the invention, but rather in the context of the invention should be rather be construed as the compounds and compositions for use in the relevant methods of medical treatment). For example, a described method of using compounds within formulas 1-9 comprises administering to a subject in need thereof an effective amount of one compound, two or more compounds, or a composition comprising at least one compound, according to any or all formulas 1-9. The method may be particularly suitable for treating a disease or condition for which an IRAK inhibitor is indicated, including an IRAK1 inhibitor, IRAK 2 , IRAK3 and/or IRAK4. The disease may be autoimmune disease, inflammatory disorder, cardiovascular disease, neurodegenerative disorder, allergic disorder, multi-organ failure, kidney disease, platelet aggregation disease, cancer, transplantation, sperm motility, erythrocyte deficiency , graft rejection, lung injury, respiratory disease, ischemic condition, bacterial infection, viral infection, immune regulation disorder, or a combination thereof.
Los métodos descritos para usar compuestos de acuerdo con las fórmulas 1-9 también incluyen inhibir una proteína IRAK poniendo en contacto la proteína IRAK con una cantidad efectiva de un compuesto o compuestos, o una composición que comprende un compuesto o compuestos, de acuerdo con cualquiera o todas las fórmulas 1-9, en los que el compuesto tiene una CE50 mayor que 0 a 5 μM, típicamente de 0 a 1 μM, y con muchos compuestos descritos que tienen una CE50 sustancialmente menor que 1 μM. La proteína IRAK puede estar en un sujeto, o el método puede comprender poner en contacto la proteína IRAK in vitro. The described methods for using compounds according to formulas 1-9 also include inhibiting an IRAK protein by contacting the IRAK protein with an effective amount of a compound or compounds, or a composition comprising a compound or compounds, according to either or all of formulas 1-9, where the compound has an EC 50 greater than 0 to 5 µM, typically 0 to 1 µM, and with many compounds disclosed having an EC 50 substantially less than 1 µM. The IRAK protein may be in a subject, or the method may comprise contacting the IRAK protein in vitro.
Los anteriores y otros objetos, características y ventajas de la invención se harán más evidentes a partir de la siguiente descripción detallada.The foregoing and other objects, features, and advantages of the invention will become more apparent from the following detailed description.
DESCRIPCIÓN DETALLADADETAILED DESCRIPTION
I. DefinicionesI. Definitions
Las siguientes explicaciones de términos, expresiones y métodos se proporcionan para describir mejor la presente divulgación y para guiar a los expertos ordinarios en la técnica en la práctica de la presente divulgación. Las formas singulares "un", "una" y "el", "la" se refieren a uno o más de uno, a menos que el contexto indique claramente lo contrario. El término "o" se refiere a un solo elemento de elementos alternativos establecidos o una combinación de dos o más elementos, a menos que el contexto indique claramente lo contrario. Tal como se utiliza en esta memoria, "comprende" significa "incluye". Por lo tanto, "que comprende A o B" significa "que incluye A, B o A y B", sin excluir elementos adicionales.The following explanations of terms, expressions, and methods are provided to better describe the present disclosure and to guide those of ordinary skill in the art in the practice of the present disclosure. The singular forms "un", "una" and "el", "la" refer to one or more than one, unless the context clearly indicates otherwise. The term "or" refers to a single item of set alternative items or a combination of two or more items, unless the context clearly indicates otherwise. As used herein, "comprises" means "includes". Therefore, "comprising A or B" means "including A, B or A and B", without excluding additional elements.
A menos que se indique lo contrario, todos los números que expresan cantidades de componentes, pesos moleculares, porcentajes, temperaturas, tiempos, etcétera, tal como se utilizan en la memoria descriptiva o las reivindicaciones, deben entenderse modificados por el término "aproximadamente". Por consiguiente, a menos que se indique lo contrario, implícita o explícitamente, los parámetros numéricos recogidos son aproximaciones que pueden depender de las propiedades deseadas que se buscan y/o los límites de detección en condiciones/métodos de ensayo estándares. Cuando se distinguen directa y explícitamente las realizaciones de la técnica anterior comentada, los números de realización no son aproximados, a menos que se mencione expresamente la palabra "aproximadamente". Unless otherwise indicated, all numbers expressing amounts of components, molecular weights, percentages, temperatures, times, etc., as used in the specification or claims, are to be understood as modified by the term "about". Therefore, unless explicitly or implicitly stated otherwise, the reported numerical parameters are approximations that may depend on the desired properties being sought and/or detection limits under standard conditions/test methods. When directly and explicitly distinguishing the discussed prior art embodiments, the embodiment numbers are not approximate, unless the word "approximately" is expressly mentioned.
A menos que se explique lo contrario, todas las expresiones y términos técnicos y científicos utilizados en esta memoria tienen el mismo significado que comúnmente entiende un experto ordinario en la técnica a la que pertenece esta divulgación. Aunque se pueden utilizar métodos y materiales similares o equivalentes a los descritos en esta memoria en la práctica o ensayo de la presente divulgación, más adelante se describen métodos y materiales adecuados. Los materiales, métodos y ejemplos son solo ilustrativos y no pretenden ser limitativos.Unless otherwise explained, all technical and scientific expressions and terms used in this specification have the same meaning as is commonly understood by an ordinary person skilled in the art to which this disclosure pertains. Although methods and materials similar or equivalent to those described herein may be used in the practice or testing of the present disclosure, suitable methods and materials are described below. The materials, methods, and examples are illustrative only and are not intended to be limiting.
Cuando se representan o describen estructuras químicas, a menos que se indique explícitamente lo contrario, se supone que todos los carbonos incluyen hidrógeno, de modo que cada uno de los carbonos se ajusta a una valencia de cuatro. Por ejemplo, en la estructura del lado izquierdo del siguiente esquema hay nueve átomos de hidrógeno implicados. Los nueve átomos de hidrógeno se representan en la estructura de la derecha.When depicting or describing chemical structures, unless explicitly stated otherwise, all carbons are assumed to include hydrogen, so each carbon fits a valence of four. For example, in the structure on the left side of the following diagram there are nine hydrogen atoms involved. The nine hydrogen atoms are represented in the structure on the right.
A veces, un átomo particular en una estructura se describe en una fórmula textual como si tuviera hidrógeno o átomos de hidrógeno, por ejemplo -CH2CH2-. Una persona experta ordinaria en la técnica entenderá que las técnicas descriptivas antes mencionadas son comunes en las técnicas químicas para proporcionar brevedad y simplicidad a la descripción de estructuras orgánicas.Sometimes a particular atom in a structure is described in a textual formula as having hydrogen or hydrogen atoms, for example -CH 2 CH 2 -. A person of ordinary skill in the art will understand that the aforementioned descriptive techniques are common in the chemical arts to provide brevity and simplicity in the description of organic structures.
Si un grupo R se representa como "flotante" en un sistema de anillo tal como, por ejemplo, en el grupo:If a group R is represented as "floating" in a ring system such as, for example, in the group:
entonces, a menos que se defina de otra manera, un sustituyente R puede residir en cualquier átomo del sistema de anillos bicíclicos condensados, excluyendo el átomo que lleva el enlace con el símbolo "then, unless otherwise defined, a substituent R may reside on any atom in the fused bicyclic ring system, excluding the atom bearing the bond with the symbol "
J V U VJ V U V
", siempre que se forme una estructura estable. En el ejemplo representado, el grupo R puede residir en un átomo en el anillo de 5 miembros o el de 6 miembros del sistema de anillo de indolilo.", provided a stable structure is formed. In the depicted example, the R group may reside on either a 5-membered or 6-membered ring atom of the indolyl ring system.
Cuando hay más de uno de estos grupos "flotantes" representados tal como, por ejemplo, en las fórmulas:When there is more than one of these "floating" groups represented such as, for example, in the formulas:
en que hay dos grupos, a saber, el R y el enlace que indica unión a una estructura principal; entonces, a menos que se defina de otra manera, los grupos "flotantes" pueden residir en cualquier átomo del sistema de anillos, suponiendo nuevamente que cada uno reemplaza un hidrógeno representado, implícito o expresamente definido en el sistema de anillos y que una disposición de este tipo formaría un compuesto químicamente estable.in that there are two groups, namely the R and the bond indicating attachment to a parent structure; then, unless otherwise defined, "floating" groups may reside on any atom in the ring system, again assuming that each replaces a depicted, implicitly, or expressly defined hydrogen in the ring system and that an arrangement of this type would form a chemically stable compound.
Cuando un grupo R se representa como existente en un sistema de anillos tal como, por ejemplo, en la fórmula:When a group R is represented as existing in a ring system such as, for example, in the formula:
en que, en este ejemplo, y puede ser más de uno, asumiendo que cada uno reemplaza a un hidrógeno actualmente representado, implícito o definido expresamente en el anillo; entonces, a menos que se defina de otro modo, dos Rs pueden residir en el mismo carbono. Un ejemplo simple es cuando R es un grupo metilo. La estructura representada puede existir como un dimetilo geminal en un carbono del anillo representado (un carbono "anular"). En otro ejemplo, dos Rs en el mismo carbono, incluyendo ese mismo carbono, pueden formar un anillo, creando así una estructura de anillo espirocíclico (un grupo "espirociclilo"). Por ejemplo, como se muestra a continuación, dos Rs pueden formar un anillo de piperidina en una disposición espirocíclica con el ciclohexano, tal comowhere, in this example, y can be more than one, assuming each replaces a currently represented, implied, or expressly defined hydrogen on the ring; then, unless otherwise defined, two Rs may reside on the same carbon. A simple example is when R is a methyl group. The depicted structure can exist as a geminal dimethyl on a depicted ring carbon (a "ring" carbon). In another example, two Rs on the same carbon, including that same carbon, can form a ring, thus creating a spirocyclic ring structure (a "spirocyclyl" group). For example, as shown below, two Rs can form a piperidine ring in a spirocyclic arrangement with cyclohexane, such as
Como se usa aquí, el término "sustituido" se refiere a todos los modificadores posteriores en un término; por ejemplo, en el término "arilalquilo de C1-8 sustituido", la sustitución puede ocurrir en la "porción alquílica de C1-8", la porción "arílica", o en ambas porciones del grupo arilalquilo de C1-8.As used herein, the term "substituted" refers to all subsequent modifiers in a term; for example, in the term "substituted C 1-8 arylalkyl", substitution can occur on the "C 1-8 alkyl portion", the "aryl" portion, or on both portions of the C 1-8 arylalkyl group .
"Sustituido", cuando se utiliza para modificar un grupo o resto específicado, significa que al menos uno, y quizás dos o más átomos de hidrógeno del grupo o resto especificado está reemplazado independientemente con los mismos o diferentes grupos sustituyentes como se define más adelante. En una realización particular, un grupo, resto o sustituyente puede estar sustituido o no sustituido, a menos que se defina expresamente como "no sustituido" o "sustituido". Por consiguiente, cualquiera de los grupos especificados en esta memoria puede estar sustituido o no sustituido. En realizaciones particulares, el sustituyente puede definirse expresamente o no como sustituido, pero todavía se contempla que esté opcionalmente sustituido. Por ejemplo, un resto "alquilo" o "pirazolilo" puede estar no sustituido o sustituido, pero un "alquilo no sustituido" o un "pirazolilo no sustituido" no está sustituido."Substituted", when used to modify a specified group or moiety, means that at least one, and perhaps two or more, hydrogen atoms of the specified group or moiety are independently replaced with the same or different substituent groups as defined below. In a particular embodiment, a group, moiety, or substituent may be substituted or unsubstituted, unless expressly defined as "unsubstituted" or "substituted." Accordingly, any of the groups specified herein may be substituted or unsubstituted. In particular embodiments, the substituent may or may not be expressly defined as substituted, but is still contemplated to be optionally substituted. For example, an "alkyl" or "pyrazolyl" moiety may be unsubstituted or substituted, but an "unsubstituted alkyl" or "unsubstituted pyrazolyl" is unsubstituted.
Los "sustituyentes" o "grupos sustituyentes" para sustituir uno o más átomos de hidrógeno en átomos de carbono saturados en el grupo o resto especificado son, a menos que se especifique lo contrario, -R60, halo, =O, -OR70, -SR70, -N(R80)2 , haloalquilo, perhaloalquilo, -CN, -NOz, =N2 , -N3 , -SO2R70, -SO3-M+, -SO3R70, -OSO2 R70, -OSO3-M+ -OSO3 R70, -P(O)(O-)2(M+)2 , -P(O)(O-)2M2+, -P(O)(OR70)O-M+, -P(O)(OR70)2 , -C(O)R70, -C(S)R70, -C(NR70)R70, -CO2-M+, -CO2 R70, -C(S)OR70, -C(O)N(R80)2 , -C(NR70)(R80)2 , -OC(O)R70, -OC(S)R70, -OCO2-M+, -OCO2R70, -OC(S)OR70, -NR70C(O)R70, -NR70C(S)R70, -NR70CO2-M+, -NR70CO2 R70, -NR70C(S)OR70, -NR70C(O)N(R80)2 , -NR70C(NR70)R70 y -NR70C(NR70)N(R80)2 , en los que R60 es alifático, heteroalifático o cicloalifático de C1-10, típicamente, alifático de C1-6, más típicamente alquilo de C1-6, en los que R60 opcionalmente puede estar sustituido; cada R70 es independientemente, para cada aparición, hidrógeno o R60; cada R80 es independientemente, para cada aparición, R70, o alternativamente, dos grupos R80, tomados junto con el átomo de nitrógeno al que están unidos, forman un heterocicloalifático de 3 a 7 miembros, que incluye opcionalmente de 1 a 4 de los mismos o diferentes heteroátomos adicionales seleccionados de O, N y S, de los cuales N opcionalmente tiene sustitución R70, tal como H o sustitución con alquilo de C1-C3 ; y cada M+ es un contraión con una sola carga neta positiva. Cada uno de los M+ es independientemente para cada aparición, por ejemplo, un ion de metal alcalino, tal como K+, Na+, Li+; un ion amonio, tal como N(R60)4; un ion de aminoácido protonado, tal como un ion lisina o un ion arginina; o un ion de metal alcalinotérreo, tal como [Ca2+]0.5, [Mg2+]0.5 o [Ba2+]0.5 (un subíndice "0,5" significa, por ejemplo, que uno de los contraiones para iones alcalinotérreos divalentes pueden ser una forma ionizada de un compuesto de la invención y el otro un contraión típico tal como cloruro, o dos compuestos ionizados pueden servir como contraiones para iones alcalinotérreos divalentes de este tipo, o un compuesto doblemente ionizado puede servir como el contraión para iones alcalinotérreos divalentes de este tipo). Como ejemplos específicos, -N(R80)2 incluye -NH2 , -NH-alquilo, -NH-pirrolidin-3-ilo, N-pirrolidinilo, N-piperazinilo, 4N-metil-piperazin-1-ilo, N-morfolinilo, y similares. Cualesquier dos átomos de hidrógeno en un solo carbono también pueden estar reemplazados, por ejemplo, con =O, =NR70, =N-OR70, =N2 o =S."Substituents" or "substituent groups" for substituting one or more hydrogen atoms on saturated carbon atoms in the specified group or moiety are, unless otherwise specified, -R60, halo, =O, -OR70, - SR70, -N(R80) 2 , haloalkyl, perhaloalkyl, -CN, -NOz, =N 2 , -N 3 , -SO 2 R 70 , -SO 3 -M+, -SO 3 R 70 , -OSO 2 R 70 , -OSO 3 -M+ -OSO 3 R 70 , -P(O)(O-) 2 (M+) 2 , -P(O)(O-) 2 M2+, -P(O)(OR70)O-M+ , -P(O)(OR70) 2 , -C(O)R70, -C(S)R70, -C(NR70)R70, -CO 2 -M+, -CO 2 R 70 , -C(S)OR70 , -C(O)N(R80) 2 , -C(NR70)(R80) 2 , -OC(O)R70, -OC(S)R70, -OCO 2 -M+, -OCO 2 R 70 , -OC (S)OR70, -NR70C(O)R70, -NR70C(S)R70, -NR70CO 2 -M+, -NR70CO 2 R 7 0, -NR70C(S)OR70, -NR70C(O)N(R80) 2 , -NR70C(NR70)R70 and -NR70C(NR70)N(R80) 2 , where R60 is C 1-10 aliphatic, heteroaliphatic or cycloaliphatic, typically C 1-6 aliphatic, more typically C 1- alkyl 6 , where R60 may optionally be substituted; each R 7 0 is independently, for each occurrence, hydrogen or R60; each R 8 0 is independently, for each occurrence, R 7 0, or alternatively, two R 8 0 groups, taken together with the nitrogen atom to which they are attached, form a 3 to 7-membered heterocycloaliphatic, optionally including 1 to 4 of the same or different additional heteroatoms selected from O, N and S, of which N optionally has R 7 0 substitution, such as H or C 1 -C 3 alkyl substitution; and each M+ is a counterion with a single net positive charge. Each of the M+ is independently for each occurrence, for example, an alkali metal ion, such as K+, Na+, Li+; an ammonium ion, such as N(R60)4; an amino acid ion protonated, such as a lysine ion or an arginine ion; or an alkaline earth metal ion, such as [Ca2+] 0.5 , [Mg2+] 0.5 or [Ba2+]0.5 (a subscript "0.5" means, for example, that one of the counterions for divalent alkaline earth ions may be an ionized form of one compound of the invention and the other a typical counterion such as chloride, or two ionized compounds can serve as counterions for divalent alkaline earth ions of this type, or one doubly ionized compound can serve as the counterion for divalent alkaline earth ions of this type) . As specific examples, -N(R80) 2 includes -NH 2 , -NH-alkyl, -NH-pyrrolidin-3-yl, N-pyrrolidinyl, N-piperazinyl, 4N-methyl-piperazin-1-yl, N-morpholinyl , and the like. Any two hydrogen atoms on a single carbon may also be replaced, for example, with =O, =NR70, =N-OR70, =N 2 or =S.
Los grupos sustituyentes para reemplazar átomos de hidrógeno en átomos de carbono insaturados en grupos que contienen carbonos insaturados son, a menos que se especifique lo contrario, -R60, halo, -O-M+, -OR70, -SR70, -S-M+, Substituent groups for replacing hydrogen atoms on unsaturated carbon atoms in groups containing unsaturated carbons are, unless otherwise specified, -R60, halo, -O-M+, -OR70, -SR70, -S-M+,
-N(R80)2 , haloalquilo, perhaloalquilo, -CN, -OCN, -SCN, -NO, -NOz, -N3 , -SO2 R70, -SO3-M+, -SO3 R70, -OSO2R70,-N(R80) 2 , haloalkyl, perhaloalkyl, -CN, -OCN, -SCN, -NO, -NOz, -N 3 , -SO 2 R 70 , -SO 3 -M+, -SO 3 R 70 , -OSO 2 R70,
-OSO3-M+ -OSO3 R70, -PO3-2(M+)2, -PO3-2M2+, -P(O)(OR70)O-M+, -P(O)(OR70)2 , -C(O)R70,-OSO 3 -M+ -OSO 3 R 70 , -PO 3 -2(M+) 2 , -PO 3 -2M2+, -P(O)(OR70)O-M+, -P(O)(OR70) 2 , - C(O)R70,
-C(S)R70, -C(NR70)R70, -CO2-M+, -CO2R70, -C(S)OR70, -C(O)NR80R80, -C(NR70)N(R80)2,-C(S)R70, -C(NR70)R70, -CO 2 -M+, -CO 2 R70, -C(S)OR70, -C(O)NR80R80, -C(NR70)N(R80) 2 ,
-OC(O)R70, -OC(S)R70, -OCO2-M+, -OCO2R70, -OC(S)OR70, -NR70C(O)R70,-OC(O)R70, -OC(S)R70, -OCO 2 -M+, -OCO 2 R70, -OC(S)OR70, -NR70C(O)R70,
-NR70C(S)R70, -NR70CO2-M+, -NR70CO2R70, -NR70C(S)OR70, -NR70C(O)N(R80)2,-NR70C(S)R70, -NR70CO 2 -M+, -NR70CO 2 R70, -NR70C(S)OR70, -NR70C(O)N(R80) 2 ,
-NR70C(NR70)R70 y -NR70C(NR70)N(R80)2 , en los que R60, R70, R80 y M+ son como se han definido anteriormente, con la condición de que, en el caso de alqueno o alquino sustituido, los sustituyentes no sean -O-M+, -OR70, -SR70, o -S-M+.-NR70C(NR70)R70 and -NR70C(NR70)N(R80) 2 , where R60, R 7 0, R 8 0 and M+ are as defined above, provided that, in the case of alkene or substituted alkyne, the substituents are not -O-M+, -OR70, -SR70, or -S-M+.
Los grupos sustituyentes para reemplazar átomos de hidrógeno en átomos de nitrógeno en grupos que contienen tales átomos de nitrógeno son, a menos que se especifique lo contrario, -R60, -O-M+, -OR70, -SR70, -S-M+, -N(R80)2 , haloalquilo, perhaloalquilo, -CN, -NO, -NOz,Substituent groups for replacing hydrogen atoms on nitrogen atoms in groups containing such nitrogen atoms are, unless otherwise specified, -R60, -O-M+, -OR70, -SR70, -S-M+, - N(R80) 2 , haloalkyl, perhaloalkyl, -CN, -NO, -NOz,
-S(O)2 R70, -SO3-M+, -SO3 R70, -OS(O)2 R70, -OSO3-M+ -OSO3 R70, -PO32-(M+)2,-S(O) 2 R 7 0, -SO 3 -M+, -SO 3 R 70 , -OS(O) 2 R 7 0, -OSO 3 -M+ -OSO 3 R 70 , -PO 3 2-(M+ ) 2 ,
-PO32-M2+, -P(O)(OR70)O-M+, -P(O)(OR70)(OR70), -C(O)R70, -C(S)R70, -C(NR70)R70,-PO 3 2-M2+, -P(O)(OR70)O-M+, -P(O)(OR70)(OR70), -C(O)R70, -C(S)R70, -C(NR70) R70,
-CO2R70, -C(S)OR70, -C(O)NR80R80, -C(NR70)NR80R80, -OC(O)R70, -OC(S)R70, -OCO2R70,-CO 2 R70, -C(S)OR70, -C(O)NR80R80, -C(NR70)NR80R80, -OC(O)R70, -OC(S)R70, -OCO 2 R70,
-OC(S)OR70, -NR70C(O)R70, -NR70C(S)R70, -NR70CO2R70, -NR70C(S)OR70, -NR70C(O)N(R80)2,-OC(S)OR70, -NR70C(O)R70, -NR70C(S)R70, -NR70CO 2 R70, -NR70C(S)OR70, -NR70C(O)N(R80) 2 ,
-NR70C(NR70)R70 y -NR70C(NR70)N(R80)2 , en los que R60, R70, R80 y M+ son como se definen anteriormente. -NR70C(NR70)R70 and -NR70C(NR70)N(R80) 2 , where R60, R 7 0, R 8 0 and M+ are as defined above.
En una realización, un grupo que está sustituido tiene al menos un sustituyente hasta el número de sustituyentes posibles para un resto particular, tal como 1 sustituyente, 2 sustituyentes, 3 sustituyentes o 4 sustituyentes.In one embodiment, a group that is substituted has at least one substituent up to the number of substituents possible for a particular moiety, such as 1 substituent, 2 substituents, 3 substituents, or 4 substituents.
Adicionalmente, en realizaciones en las que un grupo o resto está sustituido con un sustituyente sustituido, el anidamiento de sustituyentes sustituidos de este tipo se limita a tres, evitando así la formación de polímeros. Por lo tanto, en un grupo o resto que comprende un primer grupo que es un sustituyente en un segundo grupo que es a su vez un sustituyente en un tercer grupo, que está unido a la estructura original, el primer grupo (más externo) solo puede estar sustituido con sustituyentes no sustituidos. Por ejemplo, en un grupo que comprende -(aril-1 )-(aril-2)-(aril-3), el aril-3 solo puede estar sustituido con sustituyentes que no estén sustituidos.Additionally, in embodiments where a group or moiety is substituted with a substituted substituent, the nesting of such substituted substituents is limited to three, thus avoiding polymer formation. Therefore, in a group or moiety that comprises a first group that is a substituent in a second group that is in turn a substituent in a third group, which is attached to the original structure, the first (outermost) group only it may be substituted with unsubstituted substituents. For example, in a group comprising -(aryl-1)-(aryl-2)-(aryl-3), the aryl-3 can only be substituted with substituents that are unsubstituted.
Cualquier grupo o resto definido en esta memoria se puede conectar a cualquier otra parte de una estructura divulgada, tal como una estructura principal o central, como entendería una persona ordinaria en la técnica, por ejemplo, al considerar las reglas de valencia, la comparación con especies ejemplares y/o considerando la funcionalidad, a menos que la conectividad del grupo o resto con la otra parte de la estructura se establezca expresamente o esté implícita en el contexto.Any group or moiety defined herein can be connected to any other part of a disclosed structure, such as a parent or core structure, as would be understood by one of ordinary skill in the art, for example, when considering valence rules, comparison with exemplary species and/or considering functionality, unless the connectivity of the group or moiety with the other part of the structure is expressly stated or implied by the context.
"Acilo" se refiere al grupo -C(O)R, en el que R es H, alifático, heteroalifático, heterocíclico, o aromático. Los restos acilo ejemplares incluyen, pero no se limitan a, -C(O)H, -C(O)alquilo, -C(O)alquilo de C1-C6 , - C(O)haloalquilo de C1-C6, -C(O)cicloalquilo, -C(O)alquenilo, -C(O)cicloalquenilo, -C(O)arilo, -C(O)heteroarilo o -C(O)heterociclilo. Ejemplos específicos incluyen -C(O)H, -C(O)Me, -C(O)Et o -C(O)ciclopropilo."Acyl" refers to the group -C(O)R, where R is H, aliphatic, heteroaliphatic, heterocyclic, or aromatic. Exemplary acyl moieties include, but are not limited to, -C(O)H, -C(O)alkyl, -C(O) C1 - C6alkyl , -C(O)C1 - C6haloalkyl , -C(O)cycloalkyl, -C(O)alkenyl, -C(O)cycloalkenyl, -C(O)aryl, -C(O)heteroaryl or -C(O)heterocyclyl. Specific examples include -C(O)H, -C(O)Me, -C(O)Et or -C(O)cyclopropyl.
"Alifático" se refiere a un grupo o resto sustancialmente basado en hidrocarburo. Un grupo o resto alifático puede ser acíclico, que incluye grupos alquilo, alquenilo o alquinilo, versiones cíclicas de los mismos, tales como grupos o restos cicloalifáticos que incluyen cicloalquilo, cicloalquenilo o cicloalquinilo, y que además incluyen disposiciones de cadena lineal y ramificada, e igualmente todos los estereoisómeros e isómeros de posición. A menos que se indique expresamente lo contrario, un grupo alifático contiene de uno a veinticinco átomos de carbono (C1-25); por ejemplo, de uno a quince (C1-15), de uno a diez (C1-10), de uno a seis (C1-6), o de uno a cuatro átomos de carbono (C1-4) para un grupo o resto alifático acíclico, o de tres a quince (C3-15) de tres a diez (C3-10), de tres a seis (C3-6), o de tres a cuatro (C3-4) átomos de carbono para un grupo o resto cicloalifático. Un grupo alifático puede estar sustituido o no sustituido, a menos que se le aluda expresamente como un "alifático no sustituido" o un "alifático sustituido". Un grupo alifático puede estar sustituido con uno o más sustituyentes (hasta dos sustituyentes por cada carbono de metileno en una cadena alifática, o hasta un sustituyente por cada carbono de un doble enlace -C=C- en una cadena alifática, o hasta un sustituyente para un carbono de un grupo metino terminal)."Aliphatic" refers to a substantially hydrocarbon-based group or moiety. An aliphatic group or moiety can be acyclic, including alkyl, alkenyl or alkynyl groups, cyclic versions thereof, such as cycloaliphatic groups or moieties including cycloalkyl, cycloalkenyl or cycloalkynyl, and further including straight and branched chain arrangements, and likewise all stereoisomers and positional isomers. Unless stated expressly to the contrary, an aliphatic group contains from one to twenty-five carbon atoms (C 1-25 ); for example, from one to fifteen (C 1-15 ), from one to ten (C 1-10 ), from one to six (C 1-6 ), or from one to four carbon atoms (C 1-4 ) for an acyclic aliphatic group or residue, or from three to fifteen (C 3-15 ), from three to ten (C 3-10 ), from three to six (C 3-6 ), or from three to four (C 3- 4 ) carbon atoms for a cycloaliphatic group or moiety. An aliphatic group may be substituted or unsubstituted, unless expressly referred to as an "unsubstituted aliphatic" or a "substituted aliphatic". An aliphatic group may be substituted with one or more substituents (up to two substituents for each methylene carbon in an aliphatic chain, or up to one substituent for each carbon of a -C=C- double bond in an aliphatic chain, or up to one substituent for a carbon of a terminal methine group).
"Alifático inferior" se refiere a un grupo alifático que contiene de uno a diez átomos de carbono (C1-10), tal como de uno a seis (C1-6), o de uno a cuatro (C1-4) átomos de carbono; o de tres a diez (C3-10), tal como de tres a seis (C3-6) átomos de carbono para un grupo cicloalifático inferior."Lower aliphatic" refers to an aliphatic group containing one to ten carbon atoms (C 1-10 ), such as one to six (C 1-6 ), or one to four (C 1-4 ). carbon atoms; or three to ten (C 3-10 ), such as three to six (C 3-6 ) carbon atoms for a lower cycloaliphatic group.
"Alcoxi" se refiere al grupo -OR, en el que R es un grupo alquilo sustituido o no sustituido, o cicloalquilo sustituido o no sustituido. En determinados ejemplos, R es un grupo alquilo C1-6 o un grupo cicloalquilo C3-6. Metoxi (-OCH3) y etoxi (-OCH2CH3) son grupos alcoxi ejemplares. En un alcoxi sustituido, R es alquilo sustituido o cicloalquilo sustituido, ejemplos de los cuales en los compuestos descritos en esta memoria incluyen grupos haloalcoxi, tales como -OCF2H. "Alkoxy" refers to the group -OR, where R is a substituted or unsubstituted alkyl, or substituted or unsubstituted cycloalkyl group. In certain examples, R is a C 1-6 alkyl group or a C 3-6 cycloalkyl group. Methoxy (-OCH 3 ) and ethoxy (-OCH 2 CH 3 ) are exemplary alkoxy groups. In a substituted alkoxy, R is substituted alkyl or substituted cycloalkyl, examples of which in the compounds described herein include haloalkoxy groups, such as -OCF 2 H.
"Alcoxialquilo" se refiere al grupo -alquilo-OR, en el que R es un grupo alquilo sustituido o no sustituido, o cicloalquilo sustituido o no sustituido."Alkoxyalkyl" refers to the group -alkyl-OR, where R is a substituted or unsubstituted alkyl, or substituted or unsubstituted cycloalkyl group.
"Alquilo" se refiere a un grupo hidrocarbilo alifático saturado que tiene de 1 a 25 (C1-25) átomos de carbono, normalmente de 1 a 10 (C1-10) átomos de carbono, tal como 1 a 6 (C1-6) átomos de carbono. Un resto alquilo puede estar sustituido o no sustituido. Este término incluye, a modo de ejemplo, grupos hidrocarbilo lineales y ramificados tales como metilo (CH3), etilo (-CH2CH3), n-propilo (-CH2CH2CH3), isopropilo (-Ch (CH3)2), n-butilo (-CH2-CH2CH2CH3), isobutilo (-CH2CH2 (CH3)2), sec-butilo (-CH(CH3)(CH2CH3), t-butilo (-C(CH3)3), n-pentilo (-CH2CH2CH2CH2CH3), y neopentilo (-CH2C(CH3)3)."Alkyl" refers to a saturated aliphatic hydrocarbyl group having 1 to 25 (C 1-25 ) carbon atoms, typically 1 to 10 (C 1-10 ) carbon atoms, such as 1 to 6 (C 1 -6 ) carbon atoms. An alkyl moiety can be substituted or unsubstituted. This term includes, by way of example, linear and branched hydrocarbyl groups such as methyl (CH 3 ), ethyl (-CH 2 CH 3 ), n-propyl (-CH 2 CH 2 CH 3 ), isopropyl (-C h ( CH 3 ) 2 ), n-butyl (-CH 2 -CH 2 CH 2 CH 3 ), isobutyl (-CH 2 CH 2 (CH 3 ) 2 ), sec-butyl (-CH(CH 3 )(CH 2 CH 3 ), t-butyl (-C(CH 3 ) 3 ), n-pentyl (-CH 2 CH 2 CH 2 CH 2 CH 3 ), and neopentyl (-CH 2 C(CH 3 ) 3 ).
"Amino" se refiere al grupo -NH2 , -NHR o -NRR, en el que cada R se selecciona independientemente de H, alifático, heteroalifático, aromático, incluyendo tanto arilo como heteroarilo, o heterocicloalifático, o dos grupos R, junto con el nitrógeno unido a ellos, forman un anillo heterocíclico. Ejemplos de anillos heterocíclicos de este tipo incluyen aquellos en los que dos grupos R, junto con el nitrógeno al que están unidos, forman un anillo - (CH2)2-5-, opcionalmente interrumpido por uno o dos grupos heteroátomo, tales como -O- o -N(R8) como en los grupos"Amino" refers to the group -NH 2 , -NHR or -NRR, where each R is independently selected from H, aliphatic, heteroaliphatic, aromatic, including both aryl and heteroaryl, or heterocycloaliphatic, or two R groups, together with the nitrogen attached to them form a heterocyclic ring. Examples of such heterocyclic rings include those in which two R groups, together with the nitrogen to which they are attached, form a -(CH 2 ) 2 -5- ring, optionally interrupted by one or two heteroatom groups, such as - O- or -N(R 8 ) as in the groups
en donde Rg es R70, -C(O)R70, -C(O)OR60 o -C(O)N(R8Vwhere Rg is R 7 0, -C(O)R70, -C(O)OR60, or -C(O)N(R8V
"Am ida" o "carboxamida" se refiere al grupo -N(R)acilo, o -C(O)amino, en el que R es hidrógeno, heteroalifático o alifático, tal como alquilo, particularmente alquilo de C1-6."Amide" or "carboxamide" refers to the group -N(R)acyl, or -C(O)amino, where R is hydrogen, heteroaliphatic or aliphatic, such as alkyl, particularly C 1-6 alkyl.
"A rom ático" se refiere a un grupo o resto cíclico conjugado de, a menos que se especifique lo contrario, 5 a 15 átomos anulares, que tiene un solo anillo (por ejemplo, fenilo, piridinilo o pirazolilo) o múltiples anillos condensados en los que al menos un anillo es aromático (por ejemplo, naftilo, indolilo o pirazolopiridinilo), es decir, al menos un anillo, y opcionalmente múltiples anillos condensados, tienen un sistema de electrones n deslocalizado continuo. Típicamente, el número de electrones n fuera del plano corresponde a la regla de Hückel (4n 2). El punto de unión a la estructura principal es típicamente a través de una porción aromática del sistema de anillos condensados. Por ejemplo,"Romantic A" refers to a conjugated cyclic group or moiety of, unless otherwise specified, 5 to 15 ring atoms, having a single ring (for example, phenyl, pyridinyl, or pyrazolyl) or multiple fused rings in those in which at least one ring is aromatic (eg, naphthyl, indolyl, or pyrazolopyridinyl), ie, at least one ring, and optionally multiple fused rings, have a continuous n delocalized electron system. Typically, the number of out-of-plane electrons n corresponds to Hückel's rule (4n 2). The point of attachment to the backbone is typically via an aromatic portion of the fused ring system. For example,
Sin embargo, en determinados ejemplos, el contexto o la divulgación expresa pueden indicar que el punto de unión es a través de una parte no aromática del sistema de anillos condensados. Por ejemplo,However, in certain examples, the context or the express disclosure may indicate that the point of attachment is through a non-aromatic portion of the fused ring system. For example,
. Un grupo o resto aromático puede comprender solo átomos de carbono en el anillo tal como en un grupo o resto arilo, o puede comprender uno o más átomos de carbono en el anillo y uno o más heteroátomos en el anillo que comprenden un solo par de electrones (p. ej., S, O, N, P o Si) tal como en un grupo o resto heteroarilo. A menos que se indique lo contrario, un grupo aromático puede estar sustituido o no sustituido.. An aromatic group or moiety may comprise only ring carbon atoms such as in an aryl group or moiety, or may comprise one or more ring carbon atoms and one or more ring heteroatoms comprising a single pair of electrons (eg, S, O, N, P, or Si) such as in a heteroaryl group or moiety. Unless otherwise indicated, an aromatic group may be substituted or unsubstituted.
"A rilo " se refiere a un grupo carbocíclico aromático de, a menos que se especifique lo contrario, 6 a 15 átomos de carbono, que tiene un solo anillo (por ejemplo, fenilo) o múltiples anillos condensados, en los que al menos un anillo es aromático (por ejemplo, 1,2,3,4-tetrahidroquinolina, benzodioxol y similares). Si alguna parte del anillo aromático contiene un heteroátomo, el grupo es heteroarilo y no arilo. Los grupos arilo pueden ser, por ejemplo, monocíclicos, bicíclicos, tricíclicos o tetracíclicos. A menos que se indique lo contrario, un grupo arilo puede estar sustituido o no sustituido."Aryl" refers to an aromatic carbocyclic group of, unless otherwise specified, 6 to 15 carbon atoms, having a single ring (for example, phenyl) or multiple fused rings, in which at least one ring is aromatic (for example, 1,2,3,4-tetrahydroquinoline, benzodioxole, and the like). If any part of the aromatic ring contains a heteroatom, the group is heteroaryl and not aryl. Aryl groups can be, for example, monocyclic, bicyclic, tricyclic or tetracyclic. Unless otherwise indicated, an aryl group may be substituted or unsubstituted.
"A ra lifático" se refiere a un grupo arilo unido al parental a través de un resto alifático. Aralifático incluye grupos aralquilo o arilalquilo tales como bencilo y feniletilo."Liphatic A ra" refers to an aryl group attached to the parent through an aliphatic moiety. Araliphatic includes aralkyl or arylalkyl groups such as benzyl and phenylethyl.
"Carboxilo" o "carboxila to" se refiere a -COzH, -C(O)O-, o sales de los mismos."Carboxyl" or "carboxyl tho" refers to -COzH, -C(O)O-, or salts thereof.
"Éster carboxílico" o "carboxi éster" se refiere al grupo -C(O)OR, en el que R es alifático, heteroalifático, cíclico, heterocíclico, y aromático, incluyendo tanto arilo como heteroarilo."Carboxylic ester" or "carboxy ester" refers to the group -C(O)OR, where R is aliphatic, heteroaliphatic, cyclic, heterocyclic, and aromatic, including both aryl and heteroaryl.
°C iano" se refiere al grupo -CN.°C iano" refers to the group -CN.
°C icloalifá tico" se refiere a un grupo alifático cíclico que tiene un solo anillo (por ejemplo, ciclohexilo), o múltiples anillos, tal como en un sistema condensado, puenteado o espirocíclico, al menos uno de los cuales es alifático. Típicamente, el punto de unión a la estructura principal es a través de una parte alifática del sistema de múltiples anillos. Cicloalifático incluye sistemas saturados e insaturados, incluyendo cicloalquilo, cicloalquenilo y cicloalquinilo. Un grupo cicloalifático puede contener de tres a veinticinco átomos de carbono; por ejemplo, de tres a quince, de tres a diez, o de tres a seis átomos de carbono. A menos que se indique lo contrario, un grupo cicloalifático puede estar sustituido o no sustituido. Grupos cicloalifáticos ejemplares incluyen, pero no se limitan a ciclopropilo, ciclobutilo, ciclopentilo, ciclohexilo, cicloheptilo, ciclopentenilo o ciclohexenilo.°C "cycloaliphatic" refers to a cyclic aliphatic group having a single ring (eg, cyclohexyl), or multiple rings, such as in a fused, bridged, or spirocyclic system, at least one of which is aliphatic. Typically, the point of attachment to the backbone is through an aliphatic portion of the multiple ring system.Cycloaliphatic includes saturated and unsaturated systems, including cycloalkyl, cycloalkenyl, and cycloalkynyl.A cycloaliphatic group can contain from three to twenty-five carbon atoms; for example , three to fifteen, three to ten, or three to six carbon atoms. Unless otherwise indicated, a cycloaliphatic group may be substituted or unsubstituted. Exemplary cycloaliphatic groups include, but are not limited to cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclopentenyl or cyclohexenyl.
"Halo," "haluro" o "halógeno" se refiere a fluoro, cloro, bromo o yodo."Halo," "halide" or "halogen" refers to fluoro, chloro, bromo, or iodo.
"Haloalquilo" se refiere a un resto alquilo sustituido con uno o más halógenos. Restos haloalquilo ejemplares incluyen -CH2 F, -CHF2 y -CF3."Haloalkyl" refers to an alkyl moiety substituted with one or more halogens. Exemplary haloalkyl moieties include -CH 2 F, -CHF 2 and -CF 3 .
"Heteroalifático" se refiere a un compuesto o grupo alifático que tiene al menos un heteroátomo y al menos un átomo de carbono, es decir, uno o más átomos de carbono de un compuesto o grupo alifático que comprende al menos dos átomos de carbono se han reemplazado por un átomo que tiene al menos un par de electrones solitario, normalmente nitrógeno, oxígeno, fósforo, silicio, o azufre. Compuestos o grupos heteroalifáticos pueden estar sustituidos o no sustituidos, ramificados o no ramificados, quirales o aquirales y/o acíclicos o cíclicos tal como un grupo heterocicloalifático."Heteroaliphatic" refers to an aliphatic compound or group having at least one heteroatom and at least one carbon atom, that is, one or more carbon atoms of an aliphatic compound or group comprising at least two carbon atoms have been replaced by an atom that has at least one lone pair of electrons, usually nitrogen, oxygen, phosphorus, silicon, or sulfur. Heteroaliphatic compounds or groups may be substituted or unsubstituted, branched or unbranched, chiral or achiral, and/or acyclic or cyclic such as a heterocycloaliphatic group.
"Heteroarilo" se refiere a un grupo o resto aromático de, a menos que se especifique lo contrario, 5 a 15 átomos anulares, que comprende al menos un átomo de carbono y al menos un heteroátomo, tal como N, S, O, P o Si. Un grupo o resto heteroarilo puede comprender un solo anillo (p. ej., piridinilo, pirimidinilo o pirazolilo) o múltiples anillos condensados (p. ej., indolilo, benzopirazolilo o pirazolopiridinilo). Grupos o restos heteroarilo pueden ser, por ejemplo, monocíclicos, bicíclicos, tricíclicos o tetracíclicos. A menos que se indique lo contrario, un grupo o resto heteroarilo puede estar sustituido o no sustituido."Heteroaryl" refers to an aromatic group or moiety of, unless otherwise specified, 5 to 15 ring atoms, comprising at least one carbon atom and at least one heteroatom, such as N, S, O, P or if. A heteroaryl group or moiety can comprise a single ring (eg, pyridinyl, pyrimidinyl, or pyrazolyl) or multiple fused rings (eg, indolyl, benzopyrazolyl, or pyrazolopyridinyl). Heteroaryl groups or moieties can be, for example, monocyclic, bicyclic, tricyclic or tetracyclic. Unless otherwise indicated, a heteroaryl group or moiety may be substituted or unsubstituted.
"H eterocic lilo", "heterociclo" y "heterociclo" se refieren a sistemas anulares tanto aromáticos como no aromáticos, y más específicamente se refieren a un resto anular estable de tres a quince miembros que comprende al menos un átomo de carbono, y típicamente varios átomos de carbono, y al menos uno, tal como de uno a cinco, heteroátomos. El o los heteroátomos pueden ser átomos de nitrógeno, fósforo, oxígeno, silicio o azufre. El resto heterociclilo puede ser un resto monocíclico o puede comprender múltiples anillos tal como en un sistema de anillo bicíclico o tricíclico, con la condición de que al menos uno de los anillos contenga un heteroátomo. Un resto de múltiples anillos puede incluir sistemas de anillos condensados o puenteados, así como sistemas espirocíclicos; y cualesquiera átomos de nitrógeno, fósforo, carbono, silicio o azufre en el resto heterociclilo puede oxidarse opcionalmente a varios estados de oxidación. Por conveniencia, los nitrógenos, en particular, pero no exclusivamente, los definidos como nitrógenos aromáticos anulares, incluyen su forma de N-óxido correspondiente, aunque no se definen explícitamente como tales en un ejemplo particular. Por lo tanto, para un compuesto que tiene, por ejemplo, un anillo piridinilo, el piridinil-N-óxido correspondiente se incluye como otro compuesto de la invención, a menos que esté expresamente excluido o excluido por el contexto. Además, los átomos de nitrógeno anulares pueden estar opcionalmente cuaternizados. Heterociclo incluye restos heteroarílicos, y restos heteroaliciclílicos o heterocicloalifáticos , que son anillos de heterociclilo que están parcial o totalmente saturados. Ejemplos de grupos heterociclilo incluyen, pero no se limitan a azetidinilo, oxetanilo, acridinilo, benzodioxolilo, benzodioxanilo, benzofuranilo, carbazoilo, cinnolinilo, dioxolanilo, indolizinilo, naftiridinilo, perhidroazepinilo, fenazinilo, fenotiazinilo, fenoxazinilo, ftalazinilo, pteridinilo, purinilo, quinazolinilo, quinoxalinilo, quinolinilo, isoquinolinilo, tetrazoilo, tetrahidroisoquinolilo, piperidinilo, piperazinilo, 2-oxopiperazinilo, 2oxopiperidinilo, 2-oxopirrolidinilo, 2-oxoazepinilo, azepinilo, pirrolilo, 4-piperidonilo, pirrolidinilo, pirazolilo, pirazolidinilo, imidazolilo, imidazolinilo, imidazolidinilo, dihidropiridinilo, tetrahidropiridinilo, piridinilo, pirazinilo, pirimidinilo, piridazinilo, oxazolilo, oxazolinilo, oxazolidinilo, triazolilo, isoxazolilo, isoxazolidinilo, morfolinilo, tiazolilo, tiazolinilo, tiazolidinilo, isotiazolilo, quinuclidinilo, isotiazolidinilo, indolilo, isoindolilo, indolinilo, isoindolinilo, octahidroindolilo, octahidroisoindolilo, quinolilo, isoquinolilo, decahidroisoquinolilo, bencimidazolilo, tiadiazolilo, benzopiranilo, benzotiazolilo, benzoxazolilo, furilo, diazabicicloheptano, diazapano, diazepina, tetrahidrofurilo, tetrahidropiranilo, tienilo, benzotienilo, tiamorfolinilo, tiamorfolinil sulfóxido, tiamorfolinil sulfona, dioxafosfolanilo y oxadiazolilo."Heterocyclyl", "heterocycle" and "heterocycle" refer to both aromatic and non-aromatic ring systems, and more specifically refer to a three to fifteen membered stable ring moiety comprising at least one carbon atom, and typically several carbon atoms, and at least one, such as one to five, heteroatoms. The heteroatom(s) may be nitrogen, phosphorus, oxygen, silicon or sulfur atoms. The heterocyclyl moiety may be a monocyclic moiety or may comprise multiple rings such as in a bicyclic or tricyclic ring system, provided that at least one of the rings contains a heteroatom. A multi-ring moiety can include fused or bridged ring systems, as well as spirocyclic systems; and any nitrogen, phosphorous, carbon, silicon, or sulfur atoms in the heterocyclyl moiety may optionally be oxidized to various oxidation states. For convenience, nitrogens, particularly, but not exclusively, those defined as ring aromatic nitrogens, include their corresponding N-oxide form, although they are not explicitly defined as such in a particular example. Thus, for a compound having, for example, a pyridinyl ring, the corresponding pyridinyl-N-oxide is included as another compound of the invention, unless expressly excluded or excluded by context. In addition, the ring nitrogen atoms may optionally be quaternized. Heterocycle includes heteroaryl moieties, and "heteroalicyclylic" or "heterocycloaliphatic" moieties, which are heterocyclyl rings that are partially or fully saturated. Examples of heterocyclyl groups include, but are not limited to, azetidinyl, oxetanyl, acridinyl, benzodioxolyl, benzodioxanyl, benzofuranyl, carbazoyl, cinnolinyl, dioxolanyl, indolizinyl, naphthyridinyl, perhydroazepinyl, phenazinyl, phenothiazinyl, phenoxazinyl, phthalazinyl, pteridinyl, purin yl, quinazolinyl, quinoxalinyl , quinolinyl, isoquinolinyl, tetrazoyl, tetrahydroisoquinolyl, piperidinyl, piperazinyl, 2-oxopiperazinyl, 2oxopiperidinyl, 2-oxopyrrolidinyl, 2-oxoazepinyl, azepinyl, pyrrolyl, 4-piperidinyl, pyrrolidinyl, pyrazolyl, pyrazolidinyl, imidazolyl, imidazolinyl, imidazolidinyl, dihydropyridinyl, tetrahydropyridinyl, pyridinyl, pyrazinyl, pyrimidinyl, pyridazinyl, oxazole yl, oxazolinyl, oxazolidinyl, triazolyl, isoxazolyl, isoxazolidinyl, morpholinyl, thiazolyl, thiazolinyl, thiazolidinyl, isothiazolyl, quinuclidinyl, isothiazolidinyl, indolyl, isoindolyl, indolinyl, isoindolinyl, octahydroindolyl, octahydroisoindolyl, quinolyl, isoquinolyl, decahydroisoquinolyl, benzimidazolyl, thiadiazolyl, benzopyranyl, benzothiazolyl, benzoxazolyl, furyl, diazabicycloheptane, diazapane, diazepine, tetrahydrofuryl, tetrahydropyranyl, thienyl, benzothienyl, thiamorpholinyl, thiamorpholinyl sulfoxide, thiamorpholinyl sulfone, dioxaphospholanyl, and oxadiazolyl.
"H idroxilo" se refiere al grupo -OH."Hydroxy" refers to the group -OH.
"N itro" se refiere al grupo -NOz."Nitro" refers to the group -NOz.
"Fosfato" se refiere al grupo -O-P(O)(OR')2 , en el que cada -OR' es independientemente -OH; -O-alifático, tales como -O-alquilo u -O-cicloalquilo; -O-aromático, incluyendo tanto -O-arilo como -O-heteroarilo; -O-aralquilo; u -OR' es -O-M+, en el que cada M+ es un contraión cargado positivamente. A modo de ejemplo, M+ puede ser un ion de metal alcalino, tal como K+, Na+, Li+; un ion amonio, tal como N(R")4, en el que R" es H, alifático, heterociclilo o arilo; o un ion alcalinotérreo, tal como [Ca2+]0,5, [Mg2+]0,5, o [Ba21]0,5. Fosfono-oxialquilo se refiere al grupo -alquil-fosfato, tal como, por ejemplo, -CH2OP(O)(OH)2 , o una sal del mismo, tal como -CH2OP(O)(O-Na+)2 , y (((dialcoxifosforil)oxi)alquilo) se refiere al éster dialquílico de un grupo fosfono-oxialquilo tal como, por ejemplo, -CH2OP(O)(O-terc.-butilo)2."Phosphate" refers to the group -OP(O)(OR') 2 , where each -OR' is independently -OH; -O-aliphatic, such as -O-alkyl or -O-cycloalkyl; -O-aromatic, including both -O-aryl and -O-heteroaryl; -O-aralkyl; u -OR' is -O-M+, where each M+ is a positively charged counterion. By way of example, M+ can be an alkali metal ion, such as K+, Na+, Li+; an ammonium ion, such as N(R") 4 , where R" is H, aliphatic, heterocyclyl, or aryl; or an alkaline earth ion, such as [Ca2+]0.5, [Mg2+]0.5, or [Ba21]0.5. Phosphono-oxyalkyl refers to the group -alkyl-phosphate, such as, for example, -CH 2 OP(O)(OH) 2 , or a salt thereof, such as -CH 2 OP(O)(O-Na+) 2 , and (((dialkoxyphosphoryl)oxy)alkyl) refers to the dialkyl ester of a phosphono-oxyalkyl group such as, for example, -CH 2 OP(O)(O-tert-butyl) 2 .
"Fosfonato" se refiere al grupo -P(O)(OR')2 , en el que cada -OR' es independientemente -OH; -O-alifático, tales como -O-alquilo u -O-cicloalquilo; -O-aromático, incluyendo tanto -O-arilo como -O-heteroarilo; u -O-aralquilo; u -OR' es -O-M+, en el que cada M+ es un contraión cargado positivamente. A modo de ejemplo, M+ puede ser un ion de metal alcalino, tal como K+, Na+, Li+; un ion amonio, tal como N(R")4, en el que R" es H, alifático, heterociclilo o arilo; o un ion alcalinotérreo, tal como [Ca21]0,5, [Mg2+]0,5, o [Ba21]0,5. Fosfonoalquilo se refiere al grupo -alquil-fosfonato, tal como, por ejemplo, -CH2 P(O)(OH)2 , o CH2 P(O)(O-Na+)2 y (((dialcoxifosforil)oxi)alquilo) se refiere al éster dialquílico de un grupo fosfonoalquilo tal como, por ejemplo, -CH2 P(O)(O-terc.-butilo)2."Phosphonate" refers to the group -P(O)(OR') 2 , where each -OR' is independently -OH; -O-aliphatic, such as -O-alkyl or -O-cycloalkyl; -O-aromatic, including both -O-aryl and -O-heteroaryl; u -O-aralkyl; u -OR' is -O-M+, where each M+ is a positively charged counterion. By way of example, M+ can be an alkali metal ion, such as K+, Na+, Li+; an ammonium ion, such as N(R")4, where R" is H, aliphatic, heterocyclyl, or aryl; or an alkaline earth ion, such as [Ca21]0.5, [Mg2+]0.5, or [Ba21]0.5. "Phosphonoalkyl" refers to the group -alkyl-phosphonate, such as, for example, -CH 2 P(O)(OH) 2 , or CH 2 P(O)(O-Na+) 2 and (((dialkoxyphosphoryl)oxy)alkyl ) refers to the dialkyl ester of a phosphonoalkyl group such as, for example, -CH 2 P(O)(O-tert.-butyl) 2 .
"Paciente" o "Sujeto" se refiere a mamíferos y otros animales, particularmente seres humanos. Por lo tanto, los métodos descritos son aplicables tanto a la terapia humana como a aplicaciones veterinarias"Patient" or "Subject" refers to mammals and other animals, particularly humans. Therefore, the described methods are applicable to both human therapy and veterinary applications.
"Excipiente farmacéuticamente aceptable" se refiere a una sustancia, distinta del ingrediente activo, que se incluye en una formulación del ingrediente activo. Tal como se utiliza en esta memoria, un excipiente puede incorporarse en partículas de una composición farmacéutica, o puede mezclarse físicamente con partículas de una composición farmacéutica. Un excipiente se puede utilizar, por ejemplo, para diluir un agente activo y/o para modificar propiedades de una composición farmacéutica. Excipientes pueden incluir, pero no se limitan a antiadherentes, aglutinantes, recubrimientos, recubrimientos entéricos, desintegrantes, aromatizantes, edulcorantes, colorantes, lubricantes, deslizantes, absorbentes, conservantes, adyuvantes, soportes o vehículos. Los excipientes pueden ser almidones y almidones modificados, celulosa y derivados de celulosa, sacáridos y sus derivados, tales como disacáridos, polisacáridos y alcoholes de azúcar, proteínas, polímeros sintéticos, polímeros reticulados, antioxidantes, aminoácidos o conservantes. Excipientes ejemplares incluyen, pero no se limitan a estearato de magnesio, ácido esteárico, estearina vegetal, sacarosa, lactosa, almidones, hidroxipropil celulosa, hidroxipropil metilcelulosa, xilitol, sorbitol, maltitol, gelatina, polivinilpirrolidona (PVP), polietilenglicol (PEG), tocoferol succinato de polietilenglicol 1000 (también conocido como vitamina E TPGS o TPGS), carboximetil celulosa, dipalmitoil fosfatidil colina (DPPC), vitamina A, vitamina E, vitamina C, palmitato de retinilo, selenio, cisteína, metionina, ácido cítrico, citrato de sodio, metilparabeno, propilparabeno, azúcar, sílice, talco, carbonato de magnesio, glicolato de almidón de sodio, tartrazina, aspartamo, cloruro de benzalconio, aceite de sésamo, galato de propilo, metabisulfito de sodio o lanolina."Pharmaceutically acceptable carrier" refers to a substance, other than the active ingredient, that is included in a formulation of the active ingredient. As used herein, an excipient can be incorporated into particles of a pharmaceutical composition, or it can be physically mixed with particles of a pharmaceutical composition. An excipient can be used, for example, to dilute an active agent and/or to modify properties of a pharmaceutical composition. Excipients may include, but are not limited to release agents, binders, coatings, enteric coatings, disintegrants, flavors, sweeteners, colorants, lubricants, glidants, absorbents, preservatives, adjuvants, carriers, or vehicles. The excipients can be starches and modified starches, cellulose and cellulose derivatives, saccharides and their derivatives, such as disaccharides, polysaccharides and sugar alcohols, proteins, synthetic polymers, cross-linked polymers, antioxidants, amino acids or preservatives. Exemplary excipients include, but are not limited to magnesium stearate, stearic acid, vegetable stearin, sucrose, lactose, starches, hydroxypropyl cellulose, hydroxypropyl methylcellulose, xylitol, sorbitol, maltitol, gelatin, polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), tocopherol. polyethylene glycol 1000 succinate (also known as vitamin E TPGS or TPGS), carboxymethyl cellulose, dipalmitoyl phosphatidyl choline (DPPC), vitamin A, vitamin E, vitamin C, retinyl palmitate, selenium, cysteine, methionine, citric acid, sodium citrate , methylparaben, propylparaben, sugar, silica, talc, magnesium carbonate, sodium starch glycolate, tartrazine, aspartame, benzalkonium chloride, sesame oil, propyl gallate, sodium metabisulfite, or lanolin.
Un "adyuvante" es un excipiente que modifica el efecto de otros agentes, típicamente el ingrediente activo. Los adyuvantes son a menudo agentes farmacológicos y/o inmunológicos. Un adyuvante puede modificar el efecto de un ingrediente activo aumentando una respuesta inmune. Un adyuvante también puede actuar como agente estabilizador para una formulación. Adyuvantes ejemplares incluyen, pero no se limitan a hidróxido de aluminio, alumbre, fosfato de aluminio, bacterias muertas, escualeno, detergentes, citoquinas, aceite de parafina y adyuvantes combinados tal como el adyuvante completo de Freund o el adyuvante incompleto de Freund.An "adjuvant" is an excipient that modifies the effect of other agents, typically the active ingredient. Adjuvants are often pharmacological and/or immunological agents. An adjuvant can modify the effect of an active ingredient by increasing an immune response. An adjuvant can also act as a stabilizing agent for a formulation. Exemplary adjuvants include, but are not limited to aluminum hydroxide, alum, aluminum phosphate, killed bacteria, squalene, detergents, cytokines, paraffin oil, and combined adjuvants such as Freund's complete adjuvant or Freund's incomplete adjuvant.
"Soporte farmacéuticamente aceptable" se refiere a un excipiente que es un soporte o vehículo, tal como un auxiliar de suspensión, un auxiliar de solubilización o un auxiliar de aerosolización. Remington: The Science and Practice of Pharmacy, The University of the Sciences in Philadelphia, Editor, Lippincott, Williams, & Wilkins, Philadelphia, PA, 21a Edición (2005), describe composiciones y formulaciones ejemplares adecuadas para la administración farmacéutica de una o más composiciones y agentes farmacéuticos adicionales."Pharmaceutically acceptable carrier" refers to an excipient that is a carrier or vehicle, such as a suspending aid, solubilization aid, or aerosolization aid. Remington: The Science and Practice of Pharmacy, The University of the Sciences in Philadelphia, Publisher, Lippincott, Williams, & Wilkins, Philadelphia, PA, 21st Edition (2005), describes exemplary compositions and formulations suitable for pharmaceutical administration of one or more additional pharmaceutical compositions and agents.
En general, la naturaleza del soporte dependerá del modo particular de administración que se emplee. Por ejemplo, las formulaciones parenterales comprenden habitualmente fluidos inyectables que incluyen fluidos farmacéutica y fisiológicamente aceptables, tales como agua, solución salina fisiológica, soluciones salinas equilibradas, dextrosa acuosa, glicerol o similares como vehículo. En algunos ejemplos, el soporte farmacéuticamente aceptable puede ser estéril para ser adecuado para la administración a un sujeto (por ejemplo, mediante inyección parenteral, intramuscular o subcutánea). Además de los soportes biológicamente neutros, las composiciones farmacéuticas a administrar pueden contener cantidades menores de sustancias auxiliares no tóxicas, tales como agentes humectantes o emulsionantes, conservantes y agentes tamponadores del pH y similares, por ejemplo, acetato de sodio o monolaurato de sorbitán.In general, the nature of the carrier will depend on the particular mode of administration that is employed. For example, parenteral formulations usually comprise injectable fluids including pharmaceutically and physiologically acceptable fluids such as water, physiological saline, balanced salt solutions, aqueous dextrose, glycerol, or the like as a vehicle. In some examples, the pharmaceutically acceptable carrier may be sterile to be suitable for administration to a subject (eg, by parenteral, intramuscular injection or subcutaneous). In addition to biologically neutral carriers, the pharmaceutical compositions to be administered may contain minor amounts of non-toxic auxiliary substances, such as wetting or emulsifying agents, preservatives and pH buffering agents and the like, for example sodium acetate or sorbitan monolaurate.
"Sal farmacéuticamente aceptable" se refiere a sales farmacéuticamente aceptables de un compuesto que se derivan de una diversidad de contraiones orgánicos e inorgánicos como sabrá una persona de experiencia ordinaria en la técnica e incluyen, a modo de ejemplo únicamente, sodio, potasio, calcio, magnesio, amonio, tetraalquilamonio y similares; y cuando la molécula contiene una funcionalidad básica, sales de ácidos orgánicos o inorgánicos, tales como hidrocloruro, hidrobromuro, tartrato, mesilato, acetato, maleato, oxalato y similares. Las "sales por adición de ácidos farmacéuticamente aceptables" son un subconjunto de "sales farmacéuticamente aceptables" que conservan la eficacia biológica de las bases libres mientras se forman por participantes ácidos. En particular, los compuestos descritos forman sales con una variedad de ácidos farmacéuticamente aceptables, incluidos, sin limitación, ácidos inorgánicos tales como ácido clorhídrico, ácido bromhídrico, ácido sulfúrico, ácido nítrico, ácido fosfórico, y similares, así como ácidos orgánicos tales como ácido fórmico, ácido acético, ácido trifluoroacético, ácido propiónico, ácido glicólico, ácido pirúvico, ácido oxálico, ácido maleico, ácido malónico, ácido succínico, ácido fumárico, ácido tartárico, ácido cítrico, ácido benzoico, ácido cinámico, ácido mandélico, ácido bencenosulfónico, ácido isetiónico, ácido metanosulfónico, ácido etanosulfónico, ácido p-toluenosulfónico, ácido salicílico, ácido xinafoico, y similares. Las "sales por adición de bases farmacéuticamente aceptables" son un subconjunto de "sales farmacéuticamente aceptables" que se derivan de bases inorgánicas, tales como sales de sodio, potasio, litio, amonio, calcio, magnesio, hierro, zinc, cobre, manganeso, aluminio y similares. Sales ejemplares son las sales de amonio, potasio, sodio, calcio y magnesio. Las sales derivadas de bases orgánicas farmacéuticamente aceptables incluyen, pero no se limitan a, sales de aminas primarias, secundarias y terciarias, aminas sustituidas, incluyendo aminas sustituidas naturales, aminas cíclicas, y resinas de intercambio iónico básicas, tales como isopropilamina, trimetilamina, dietilamina, trietilamina, tripropilamina, tris(hidroximetil)aminometano (Tris), etanolamina, 2-dimetilaminoetanol, 2-dietilaminoetanol, diciclohexilamina, lisina, arginina, histidina, cafeína, procaína, hidrabamina, colina, betaína, etilendiamina, glucosamina, metilglucamina, teobromina, purinas, piperazina, piperidina, N-etilpiperidina, resinas de poliaminas, y similares. Bases orgánicas ejemplares son isopropilamina, dietilamina, tris(hidroximetil)aminometano (Tris), etanolamina, trimetilamina, diciclohexilamina, colina y cafeína. (Véase, por ejemplo, S. M. Berge, et al., "Pharmaceutical Salts," J. Pharm. Sci., 1977; 66: 1-19.) En realizaciones descritas particulares, los compuestos de amida pueden ser formiato, trifluoroactato, hidrocloruro, o sal de sodio."Pharmaceutically acceptable salt" refers to pharmaceutically acceptable salts of a compound that are derived from a variety of organic and inorganic counterions as will be known to one of ordinary skill in the art and include, by way of example only, sodium, potassium, calcium, magnesium, ammonium, tetraalkylammonium and the like; and when the molecule contains basic functionality, salts of organic or inorganic acids, such as hydrochloride, hydrobromide, tartrate, mesylate, acetate, maleate, oxalate, and the like. "Pharmaceutically acceptable acid addition salts" are a subset of "pharmaceutically acceptable salts" that retain the biological efficacy of free bases while being formed by acidic participants. In particular, the disclosed compounds form salts with a variety of pharmaceutically acceptable acids, including, without limitation, inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the like, as well as organic acids such as formic acid, acetic acid, trifluoroacetic acid, propionic acid, glycolic acid, pyruvic acid, oxalic acid, maleic acid, malonic acid, succinic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, cinnamic acid, mandelic acid, benzenesulfonic acid, isethionic acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid, xinafoic acid, and the like. "Pharmaceutically acceptable basic addition salts" are a subset of "pharmaceutically acceptable salts" that are derived from inorganic bases, such as sodium, potassium, lithium, ammonium, calcium, magnesium, iron, zinc, copper, manganese, aluminum and the like. Exemplary salts are the ammonium, potassium, sodium, calcium and magnesium salts. Salts derived from pharmaceutically acceptable organic bases include, but are not limited to, salts of primary, secondary, and tertiary amines, substituted amines, including natural substituted amines, cyclic amines, and basic ion exchange resins, such as isopropylamine, trimethylamine, diethylamine. , triethylamine, tripropylamine, tris(hydroxymethyl)aminomethane (Tris), ethanolamine, 2-dimethylaminoethanol, 2-diethylaminoethanol, dicyclohexylamine, lysine, arginine, histidine, caffeine, procaine, hydrabamine, choline, betaine, ethylenediamine, glucosamine, methylglucamine, theobromine, purines, piperazine, piperidine, N-ethylpiperidine, polyamine resins, and the like. Exemplary organic bases are isopropylamine, diethylamine, tris(hydroxymethyl)aminomethane (Tris), ethanolamine, trimethylamine, dicyclohexylamine, choline, and caffeine. (See, for example, S. M. Berge, et al., "Pharmaceutical Salts," J. Pharm. Sci., 1977; 66: 1-19.) In particular disclosed embodiments, the amide compounds can be formate, trifluoroactate, hydrochloride , or sodium salt.
"Cantidad efectiva", con respecto a un compuesto o composición, se refiere a una cantidad del compuesto o composición suficiente para lograr un resultado deseado particular, tal como inhibir una proteína o enzima, particularmente una quinasa IRAK; para provocar una respuesta biológica o médica deseada en un tejido, sistema, sujeto o paciente; para tratar un trastorno o enfermedad específica; para mejorar o erradicar uno o más de sus síntomas; y/o para prevenir la aparición de la enfermedad o trastorno. La cantidad de un compuesto que constituye una "cantidad eficaz" puede variar dependiendo del compuesto, del resultado deseado, del estado de la enfermedad y de su gravedad, la edad del paciente a tratar y similares."Effective amount", with respect to a compound or composition, refers to an amount of the compound or composition sufficient to achieve a particular desired result, such as inhibiting a protein or enzyme, particularly an IRAK kinase; to elicit a desired biological or medical response in a tissue, system, subject, or patient; to treat a specific disease or disorder; to ameliorate or eradicate one or more of your symptoms; and/or to prevent the onset of the disease or disorder. The amount of a compound constituting an "effective amount" may vary depending on the compound, the desired result, the state of the disease and its severity, the age of the patient to be treated, and the like.
"Profármaco" se refiere a compuestos que se transforman in vivo para producir un compuesto biológicamente activo, particularmente el compuesto parental, por ejemplo, por hidrólisis en el intestino o conversión enzimática. Ejemplos comunes de restos de profármacos incluyen, pero no se limitan a formas de éster y amida de un compuesto que tiene una forma activa que porta un resto de ácido carboxílico. Ejemplos de ésteres farmacéuticamente aceptables de los compuestos de esta invención incluyen, pero no se limitan a ésteres de grupos fosfato y ácidos carboxílicos, tales como ésteres alifáticos, particularmente ésteres alquílicos (por ejemplo, ésteres alquílicos C1-6). Otros restos de profármaco incluyen ésteres de fosfato tal como -CH2-O-P(O)(OR ')2 o una de sus sales, en donde R' es H o alquilo C1-6. Ésteres aceptables también incluyen ésteres de cicloalquilo y ésteres de arilalquilo tales como, pero no limitados a bencilo. Los ejemplos de amidas farmacéuticamente aceptables de los compuestos descritos aquí incluyen, pero no se limitan a, amidas primarias, y amidas de alquilo secundarias y terciarias (por ejemplo, con entre alrededor de uno y alrededor de seis carbonos). Las amidas y los ésteres de realizaciones ejemplares descritas de los compuestos de acuerdo con la presente invención se pueden preparar de acuerdo con métodos convencionales. Una discusión detallada de los profármacos.se proporciona en T. Higuchi y V. Stella, "Pro-drugs as Novel Delivery Systems", Vol. 14 de la Serie de Simposios de A.C.S. y en Bioreversible Carriers in Drug Design, ed., Edward B. Roche, American Pharmaceutical Association y Pergamon Press, 1987. "Prodrug" refers to compounds that are transformed in vivo to produce a biologically active compound, particularly the parent compound, for example, by hydrolysis in the intestine or enzymatic conversion. Common examples of prodrug moieties include, but are not limited to, ester and amide forms of a compound having an active form bearing a carboxylic acid moiety. Examples of pharmaceutically acceptable esters of the compounds of this invention include, but are not limited to, esters of phosphate groups and carboxylic acids, such as aliphatic esters, particularly alkyl esters (eg, C 1-6 alkyl esters). Other prodrug moieties include phosphate esters such as -CH 2 -OP(O)(OR ')2 or a salt thereof, where R' is H or C 1 -6 alkyl. Acceptable esters also include cycloalkyl esters and arylalkyl esters such as, but not limited to, benzyl. Examples of pharmaceutically acceptable amides of the compounds described herein include, but are not limited to, primary amides, and secondary and tertiary alkyl amides (eg, with between about one and about six carbons). Amides and esters of the described exemplary embodiments of the compounds according to the present invention may be prepared according to conventional methods. A detailed discussion of prodrugs is provided in T. Higuchi and V. Stella, "Pro-drugs as Novel Delivery Systems," Vol. 14 of the ACS Symposium Series and in Bioreversible Carriers in Drug Design, ed., Edward B. Roche, American Pharmaceutical Association and Pergamon Press, 1987.
"Solvato" se refiere a un complejo formado por la combinación de moléculas de disolvente con moléculas o iones de un soluto. El disolvente puede ser un disolvente orgánico, un disolvente inorgánico o una mezcla de ambos. Los disolventes ejemplares incluyen, pero no se limitan a, alcoholes, tales como metanol, etanol, propanol; amidas, tales como amidas N,N-dialifaticas, tales como N,N-dimetilformamida; tetrahidrofurano; alquilsulfóxidos, tales como dimetilsulfóxido; agua; y combinaciones de los mismos. Los compuestos descritos en esta memoria pueden existir en formas no solvatadas, así como solvatadas cuando se combinan con disolventes, farmacéuticamente aceptables o no, tales como agua, etanol y similares. Formas solvatadas de los compuestos descritos en esta memoria están dentro del alcance de las realizaciones descritas en esta memoria. "Solvate" refers to a complex formed by the combination of solvent molecules with molecules or ions of a solute. The solvent can be an organic solvent, an inorganic solvent, or a mixture of both. Exemplary solvents include, but are not limited to, alcohols, such as methanol, ethanol, propanol; amides, such as N,N-dialiphatic amides, such as N,N-dimethylformamide; tetrahydrofuran; alkyl sulfoxides, such as dimethyl sulfoxide; water; and combinations thereof. The compounds described herein can exist in unsolvated as well as solvated forms when combined with solvents, pharmaceutically acceptable or not, such as water, ethanol, and the like. Solvated forms of the compounds described herein are within the scope of the embodiments described herein.
"Sulfonam ida" se refiere al grupo o resto -SO2amino, o -N(R)sulfonilo, en el que R es H, alifático, heteroalifático, cíclico, heterocíclico, incluyendo aromático, tanto arilo como heteroarilo."Sulfonamide" refers to the group or moiety -SO 2 amino, or -N(R)sulfonyl, where R is H, aliphatic, heteroaliphatic, cyclic, heterocyclic, including aromatic, both aryl and heteroaryl.
"Sulfanilo" se refiere al grupo o -SH, -S-alifático, -S-heteroalifático, -S-cíclico, -S-heterociclilo, incluyendo -S-aromático, tanto -S-arilo como -S-heteroarilo."Sulfanyl" refers to the group o -SH, -S-aliphatic, -S-heteroaliphatic, -S-cyclic, -S-heterocyclyl, including -S-aromatic, both -S-aryl and -S-heteroaryl.
"S ulfin ilo " se refiere al grupo o resto -S(O)H, -S(O)alifático, -S(O)heteroalifático, -S(O)cíclico, -S(O)heterociclilo, incluyendo aromático, tanto -S(O)arilo como -S(O)heteroarilo."Sulfinyl" refers to the group or moiety -S(O)H, -S(O)aliphatic, -S(O)heteroaliphatic, -S(O)cyclic, -S(O)heterocyclyl, including aromatic, both -S(O)aryl as -S(O)heteroaryl.
"Sulfonilo" se refiere al grupo: -SOzH, -SO2alifático, -SO2heteroalifático, -SO2cíclico, - SO2 heterociclilo, incluyendo sulfonilos aromáticos, incluyendo tanto -SO2arilo como -SO2heteroarilo."Sulfonyl" refers to the group: -SOzH, -SO 2 aliphatic, -SO 2 heteroaliphatic, -SO 2 cyclic, -SO 2 heterocyclyl, including aromatic sulfonyls, including both -SO 2 aryl and -SO 2 heteroaryl.
"Tratar" o "tra tam iento", tal como se utiliza en esta memoria, se refiere al tratamiento de una enfermedad o afección de interés en un paciente o sujeto, en particular un ser humano que padece la enfermedad o afección de interés, e incluye, a modo de ejemplo, y sin limitación:"Treating" or "treatment" as used herein refers to the treatment of a disease or condition of interest in a patient or subject, in particular a human suffering from the disease or condition of interest, and includes, by way of example, and without limitation:
(i) prevenir que la enfermedad o afección se produzca en un paciente o sujeto, en particular, cuando dicho paciente o sujeto está predispuesto a la afección pero aún no se le ha diagnosticado que la tenga;(i) prevent the disease or condition from occurring in a patient or subject, in particular, where said patient or subject is predisposed to the condition but has not yet been diagnosed as having it;
(ii) inhibir la enfermedad o afección, por ejemplo, deteniendo o ralentizando su desarrollo;(ii) inhibit the disease or condition, for example by stopping or slowing its development;
(iii) aliviar la enfermedad o afección, por ejemplo, provocando la regresión de la enfermedad o afección o un síntoma de la misma; o(iii) alleviate the disease or condition, for example, by causing regression of the disease or condition or a symptom thereof; either
(iv) estabilizar la enfermedad o afección.(iv) stabilize the disease or condition.
Tal como se utiliza en esta memoria, los términos "enfermedad" y "afección" se pueden utilizar indistintamente o pueden ser diferentes en el sentido de que la enfermedad o afección particular puede no tener un agente causante conocido (por lo que aún no se ha determinado la etiología) y, por lo tanto, todavía no se reconoce como una enfermedad, sino solo como una afección o un síndrome indeseable, en que los médicos han identificado un conjunto más o menos específico de síntomas.As used herein, the terms "disease" and "condition" may be used interchangeably or may be different in the sense that the particular disease or condition may not have a known causative agent (so it has not yet been determined). determined the etiology) and, therefore, it is not yet recognized as a disease, but only as an undesirable condition or syndrome, in which doctors have identified a more or less specific set of symptoms.
Las definiciones anteriores y las siguientes fórmulas generales no pretenden incluir patrones de sustitución inadmisibles (p. ej., metilo sustituido con 5 grupos fluoro). Patrones de sustitución inadmisibles de este tipo son fácilmente reconocidos por una persona con experiencia ordinaria en la técnica.The above definitions and the following general formulas are not intended to include impermissible substitution patterns (eg, methyl substituted with 5 fluoro groups). Impermissible substitution patterns of this type are readily recognized by one of ordinary skill in the art.
Cualquiera de los grupos a los que se hace referencia en esta memoria puede estar opcionalmente sustituido con al menos uno, posiblemente dos o más sustituyentes como se define en esta memoria. Es decir, un grupo sustituido tiene al menos uno, posiblemente dos o más, hidrógenos sustituibles reemplazados por un sustituyente o sustituyentes como se define en esta memoria, a menos que el contexto indique lo contrario o una fórmula estructural particular impida la sustitución.Any of the groups referred to herein may be optionally substituted with at least one, possibly two or more substituents as defined herein. That is, a substituted group has at least one, possibly two or more, substitutable hydrogens replaced by a substituent(s) as defined herein, unless the context otherwise indicates or a particular structural formula precludes substitution.
Un experto ordinario en la técnica apreciará que los compuestos pueden exhibir los fenómenos de tautomerismo, isomería conformacional, isomería geométrica y/o isomería óptica. Por ejemplo, determinados compuestos descritos pueden incluir uno o más centros quirales y/o dobles enlaces y, como consecuencia, pueden existir como estereoisómeros, como isómeros de doble enlace (es decir, isómeros geométricos), enantiómeros, diastereómeros y mezclas de los mismos tales como mezclas racémicas. Como otro ejemplo, determinados compuestos descritos pueden existir en varias formas tautoméricas, incluyendo la forma enol, la forma ceto y mezclas de las mismas. Como los diversos nombres de compuestos, fórmulas y dibujos de compuestos dentro de la memoria descriptiva y las reivindicaciones pueden representar solo una de las posibles formas tautoméricas, isoméricas conformacionales, isoméricas ópticas o isoméricas geométricas, una persona experta ordinaria en la técnica apreciará que los compuestos descritos abarcan cualquier forma tautomérica, isomérica conformacional, isomérica óptica y/o isomérica geométrica de los compuestos descritos en esta memoria, así como mezclas de estas diversas formas isoméricas diferentes. En casos de rotación limitada, p. ej., alrededor del enlace amida o entre dos anillos unidos directamente, como los anillos de pirazolilo y piridinilo, también son posibles los atropisómeros y también se incluyen específicamente en los compuestos de la invención.One of ordinary skill in the art will appreciate that the compounds may exhibit the phenomena of tautomerism, conformational isomerism, geometric isomerism, and/or optical isomerism. For example, certain described compounds may include one or more chiral centers and/or double bonds and, as a consequence, may exist as stereoisomers, as double bond isomers (i.e., geometric isomers), enantiomers, diastereomers, and mixtures thereof such as racemic mixtures. As another example, certain disclosed compounds can exist in various tautomeric forms, including the enol form, the keto form, and mixtures thereof. As the various compound names, formulas, and drawings of compounds within the specification and claims may represent only one of the possible tautomeric, conformational isomeric, optical isomeric, or geometric isomeric forms, one of ordinary skill in the art will appreciate that compounds described encompass any tautomeric, conformational isomeric, optical isomeric, and/or geometric isomeric forms of the compounds described herein, as well as mixtures of these various different isomeric forms. In cases of limited rotation, e.g. eg around the amide bond or between two directly attached rings, such as pyrazolyl and pyridinyl rings, atropisomers are also possible and are also specifically included in the compounds of the invention.
En cualquier realización, cualquiera o todos los hidrógenos presentes en el compuesto, o en un grupo o resto particular dentro del compuesto, pueden ser reemplazados por un deuterio o un tritio. Así, una enumeración de alquilo incluye alquilo deuterado, en que desde uno hasta el número máximo de hidrógenos presentes pueden ser reemplazados por deuterio. Por ejemplo, etilo puede ser C2H5 o C2H5 , en que de 1 a 5 hidrógenos están reemplazados por deuterio tal como en C2 DxHs-x.In any embodiment, any or all of the hydrogens present in the compound, or in a particular group or moiety within the compound, may be replaced by a deuterium or a tritium. Thus, a listing of alkyl includes deuterated alkyl, where from one to the maximum number of hydrogens present can be replaced by deuterium. For example, ethyl can be C 2 H 5 or C 2 H 5 , where 1 to 5 hydrogens are replaced by deuterium such as in C 2 DxHs-x.
II. Compuestos con actividad IRAK y Composiciones que Comprenden Compuestos con actividad IRAK A. Compuestos de amidaII. Compounds with IRAK Activity and Compositions Comprising Compounds with IRAK Activity A. Amide compounds
Se describen aquí compuestos de amida, métodos para preparar los compuestos, y métodos para usar los compuestos. En una realización, los compuestos descritos son inhibidores de tirosina quinasa. En una realización particular, los compuestos son útiles para bloquear una o más rutas de señalización de citoquinas, tal como la ruta de señalización de IL-17. Para ciertas realizaciones, los compuestos de amida son útiles para tratar afecciones en las que la inhibición de una ruta de quinasa asociada al receptor de interleuquina-1 (IRAK) es terapéuticamente útil. En algunas realizaciones, los compuestos inhiben directamente una proteína IRAK, tal como IRAK1, IRAK2, IRAK3, IRAK4 o una combinación de las mismas.Amide compounds, methods of preparing the compounds, and methods of using the compounds are described herein. In one embodiment, the disclosed compounds are tyrosine kinase inhibitors. In a particular embodiment, the compounds are useful for blocking one or more cytokine signaling pathways, such as the IL-17 signaling pathway. For certain embodiments, the amide compounds are useful for treating conditions in which inhibition of an interleukin-1 receptor-associated kinase (IRAK) pathway is therapeutically useful. In some embodiments, the compounds directly inhibit an IRAK protein, such as IRAK1, IRAK 2 , IRAK3, IRAK4, or a combination thereof.
Los compuestos de amida ejemplares dentro del alcance de la presente invención tienen una fórmula general 1Exemplary amide compounds within the scope of the present invention have a general formula 1
Un experto en la técnica apreciará que también se pueden formar sales, N-óxidos y/o solvatos, incluyendo hidratos, de tales compuestos, y en consecuencia, se entiende que las sales, N-óxidos y/o solvatos están incluidos dentro del alcance de las fórmulas generales descritas 1 -9.One skilled in the art will appreciate that salts, N-oxides and/or solvates, including hydrates, of such compounds may also be formed, and accordingly, it is understood that salts, N-oxides and/or solvates are included within the scope of the general formulas described 1 -9.
Con referencia a la fórmula 1, el anillo A es heteroarilo. En algunas realizaciones, el anillo A es piridinilo o pirazinilo. En algunas realizaciones, el anillo A esReferring to formula 1, ring A is heteroaryl. In some embodiments, ring A is pyridinyl or pyrazinyl. In some embodiments, ring A is
en el que cada Q es independientemente CH, CRm o N, Rm es Rb, y x es 0, 1, o 2; y al menos uno de Q es N.wherein each Q is independently CH, CRm, or N, Rm is Rb, and x is 0, 1, or 2; and at least one of Q is N.
Ra es independientemente, para cada aparición, H, D, alquilo de C1-6, cicloalquilo de C3-6, aromático de C5-10, o heterocicloalifático de C3-6;Ra is independently, for each occurrence, H, D, C 1-6 alkyl, C 3-6 cycloalkyl, C 5-10 aromatic, or C 3-6 heterocycloaliphatic;
Rb es independientemente, para cada aparición, -OH, -CF3 , -CN, -ORc, -SO2Rc, -NRdRd, -N(H)SO2Rc, -C(O)OH, -N(H)C(O)Rc, -C(O)ORc, -C(O)NRdRd, =O, o halógeno;Rb is independently, for each occurrence, -OH, -CF 3 , -CN, -ORc, -SO 2 Rc, -NRdRd, -N(H)SO 2 Rc, -C(O)OH, -N(H) C(O)Rc, -C(O)ORc, -C(O)NRdRd, =O, or halogen;
Rc es independientemente, para cada aparición, alquilo de C1-6, cicloalquilo de C3-6, heteroaliciclilo de C3-6, aralquilo, alquilo de C1-6 sustituido con 1,2 o 3 Re, aromático de C5-10, aromático de C5-10 sustituido con 1,2 o 3 Re;Rc is independently, for each occurrence, C 1-6 alkyl, C 3-6 cycloalkyl, C 3-6 heteroalicyclyl, aralkyl, C 1-6 alkyl substituted with 1,2 or 3 Re, C 5 aromatic -10 , C 5-10 aromatic substituted with 1,2 or 3 Re;
Rd es independientemente, para cada aparición, H, alquilo de C1-6 opcionalmente sustituido con 1 ,2 o 3 Re, cicloalquilo de C3-6 opcionalmente sustituido con 1,2 o 3 Re, heteroaliciclilo de C3-6 opcionalmente sustituido con 1,2 o 3 Re, aromático de C5-10 opcionalmente sustituido con 1,2 o 3 Ra o Rb, o dos grupos Rd, junto con el nitrógeno unido a ella, forman un resto heteroaliciclilo de C3-6 opcionalmente sustituido con alquilo de C1-6 y opcionalmente interrumpido con uno o dos -O- o -N(R8), en el que R9 es R70; yRd is independently, for each occurrence, H, C 1-6 alkyl optionally substituted with 1, 2 or 3 Re, C 3-6 cycloalkyl optionally substituted with 1, 2 or 3 Re, C 3-6 heteroalicyclyl optionally substituted with 1,2 or 3 Re, C 5-10 aromatic optionally substituted with 1,2 or 3 Ra or Rb, or two Rd groups, together with the nitrogen attached to it, form an optionally substituted C 3-6 heteroalicyclyl moiety with C 1-6 alkyl and optionally interrupted with one or two -O- or -N(R 8 ), where R 9 is R 7 0; and
Re es independientemente, para cada aparición, halógeno, alquilo de C1-6, cicloalquilo de C3-6, u -ORa. En algunas realizaciones, el anillo A esRe is independently, for each occurrence, halogen, C 1-6 alkyl, C 3-6 cycloalkyl, or -ORa. In some embodiments, ring A is
R1 es H, o Ci-6. En realizaciones particulares, R1 es H.R1 is H, or Ci-6. In particular embodiments, R1 is H.
R2 es H; alifático, incluyendo alquilo, alquenilo, alquinilo, y cicloalifático, tal como cicloalquilo; heteroalifático; o heterociclilo. En algunas realizaciones, R2 es H, cicloalquilo de C3-6, heterocicloalifático, tal como heterocicloalifático de 3 a 6 miembros, o heteroalifático, tal como heteroalifático de 3 a 10 miembros.R2 is H; aliphatic, including alkyl, alkenyl, alkynyl, and cycloaliphatic, such as cycloalkyl; heteroaliphatic; or heterocyclyl. In some embodiments, R 2 is H, C 3-6 cycloalkyl, heterocycloaliphatic, such as 3-6 membered heterocycloaliphatic, or heteroaliphatic, such as 3-10 membered heteroaliphatic.
En algunas realizaciones, R2 es H, alquilo de C1-6, heteroalifático, tal como heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático.In some embodiments, R 2 is H, C 1-6 alkyl, heteroaliphatic, such as 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy substituted cyclohexyl and/or hydroxy, unsubstituted C 1-6 alkyl, or C 1-6 alkyl substituted with -OH, amino, alkoxy, or heterocycloaliphatic.
En algunas realizaciones, R2 es Ra, Ra sustituido con Rb, Ra sustituido con 1 o 2 Rc, o Ra sustituido con Rd. R2 puede ser H, CH3,In some embodiments, R2 is Ra, Ra substituted with Rb, Ra substituted with 1 or 2 Rc, or Ra substituted with Rd. R2 can be H, CH 3 ,
En realizaciones particulares, el anillo A es piridinilo o pirazinilo; R1 es H; y R2 es H, heteroalifático, tal como heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático.In particular embodiments, ring A is pyridinyl or pyrazinyl; R1 is H; and R2 is H, heteroaliphatic, such as 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C 1-6 alkyl substituted with -OH, amino, alkoxy, or heterocycloaliphatic.
Con respecto a la fórmula 1, el anillo A puede ser: 1B) heteroarilo; 1E) piridinilo; 1F) pirazinilo; 1J) piridin-2-ilo; 1K) pirazin-2-ilo; 1L) 5-(2-hidroxi-2-metilpropoxi)piridin-2-ilo; 1M) 5-oxetan-3-iloxipiridin-2-ilo; 1N) 5-morfolinopiridin-2-ilo; 1O) 5-(4-metilpiperazin-1-il)piridin-2-ilo; 1P) 3,5-difluoropiridin-2-ilo; o 1Q) 5-metoxipiridin-2-ilo.With respect to formula 1, ring A can be: 1B) heteroaryl; 1E) pyridinyl; 1F) pyrazinyl; 1J) pyridin-2-yl; 1K) pyrazin-2-yl; 1L) 5-(2-hydroxy-2-methylpropoxy)pyridin-2-yl; 1M) 5-oxetan-3-yloxypyridin-2-yl; 1N) 5-morpholinopyridin-2-yl; 1O) 5-(4-methylpiperazin-1-yl)pyridin-2-yl; 1P) 3,5-difluoropyridin-2-yl; or 1Q) 5-methoxypyridin-2-yl.
Con respecto al anillo A de las realizaciones 1B a 1Q, R2 puede ser, en cualquier combinación con 1B a 1Q: 2A) H; 2B) alifático; 2C) heteroalifático; 2D) heterocicloalifático; 2E) alquilo de C1-6; 2F) metilo; 2G) isopropilo; 2H) 2-morfolinoetilo; 2I) 2-(4-metilpiperazin-1-il)etilo; 2J) 2-hidroxietilo; 2K) 2-(2-metoxietoxi)etilo; 2L) 2-metoxiciclobut-1-ilo; 2M) 4-tetrahidropiran; 2N) 2-metoxietilo; 2O) 3-metoxipropilo; 2P) (1r,4r)-4-etoxiciclohex-1-ilo; 2Q) oxetan-3-ilo; 3R) 1,3-dihidroxipropan-2-ilo; o 2S) 2-(N,N-dietilamino)etilo.With respect to ring A of embodiments 1B to 1Q, R2 may be, in any combination with 1B to 1Q: 2A) H; 2B) aliphatic; 2C) heteroaliphatic; 2D) heterocycloaliphatic; 2E) C 1-6 alkyl; 2F) methyl; 2G) isopropyl; 2H) 2-morpholinoethyl; 2I) 2-(4-methylpiperazin-1-yl)ethyl; 2J) 2-hydroxyethyl; 2K) 2-(2-methoxyethoxy)ethyl; 2L) 2-methoxycyclobut-1-yl; 2M) 4-tetrahydropyran; 2N) 2-methoxyethyl; 2O ) 3-methoxypropyl; 2P) (1r,4r)-4-ethoxycyclohex-1-yl; 2Q) oxetan-3-yl; 3R) 1,3-dihydroxypropan-2-yl; or 2S) 2-(N,N-diethylamino)ethyl.
Una persona de experiencia ordinaria en la técnica comprenderá que cualquiera de 2A a 2S puede combinarse con cualquiera de 1B a 1Q para formar cualquiera y todas las combinaciones entre estos sustituyentesOne of ordinary skill in the art will understand that any of 2A to 2S can be combined with any of 1B to 1Q to form any and all combinations between these substituents.
Cada Z1, Z2, Z3, y Z4, es independientemente N o CR3, en la que al menos uno de Z1, Z2, Z3, y Z4 es N. Cada R3 es independientemente H, alifático, o heteroalifático, y puede ser H o alquilo, tal como H o alquilo de C1-6. En algunas realizaciones, cada R3 es H. En algunas realizaciones, uno de Z1, Z2, Z3, y Z4 es N, y los restantes son CR3. En otras realizaciones, dos de Z1, Z2, Z3, y Z4 son N, y los restantes son CR3. En algunas realizaciones, el restoEach Z1, Z2, Z3, and Z4, is independently N or CR3, wherein at least one of Z1, Z2, Z3, and Z4 is N. Each R3 is independently H, aliphatic, or heteroaliphatic, and can be H or alkyl, such as H or C 1-6 alkyl. In some embodiments, each R3 is H. In some embodiments, one of Z1, Z2, Z3, and Z4 is N, and the remainder are CR3. In other embodiments, two of Z1, Z2, Z3, and Z4 are N, and the remainder are CR3. In some embodiments, the rest
es piridinilo, pirimidinilo, o pirazinilo. En realizaciones particulares, Z1 es N, y Z2, Z3, y Z4 son CR3; tanto Z1 como Z2 son N, y Z3 y Z4 son CR3; tanto Z1 como Z3 son N, y Z2 y Z4 son CR3; tanto Z1 como Z4 son N, y Z2 y Z3 son CR3; o Z3 es N, y Z1, Z2, y Z4 son CR3. En cualquiera de estas realizaciones, el anillo A es piridinilo o pirazinilo; R1 es H; y R2 es H, heteroalifático, tal como heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático.is pyridinyl, pyrimidinyl, or pyrazinyl. In particular embodiments, Z1 is N, and Z2, Z3, and Z4 are CR3; both Z1 and Z2 are N, and Z3 and Z4 are CR3; both Z1 and Z3 are N, and Z2 and Z4 are CR3; both Z1 and Z4 are N, and Z2 and Z3 are CR3; or Z3 is N, and Z1, Z2, and Z4 are CR3. In any of these embodiments, ring A is pyridinyl or pyrazinyl; R1 is H; and R2 is H, heteroaliphatic, such as 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl , or C 1-6 alkyl substituted with -OH, amino, alkoxy, or heterocycloaliphatic.
En realizaciones particulares, Z1 es N, y Z2, Z3, y Z4 son CR3; tanto Z1 como Z2 son N, y Z3 y Z4 son CR3; tanto Z1 como Z3 son N, y Z2 y Z4 son CR3; tanto Z1 como Z4 son N, y Z2 y Z3 son CR3; o Z3 es N, y Z1, Z2, y Z4 son CR3. En cualquiera de estas realizaciones, el anillo A es piridinilo o pirazinilo; R1 es H; R2 es H, heteroalifático, tal como heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático; y cada R3 es H.In particular embodiments, Z1 is N, and Z2, Z3, and Z4 are CR3; both Z1 and Z2 are N, and Z3 and Z4 are CR3; both Z1 and Z3 are N, and Z2 and Z4 are CR3; both Z1 and Z4 are N, and Z2 and Z3 are CR3; or Z3 is N, and Z1, Z2, and Z4 are CR3. In any of these embodiments, ring A is pyridinyl or pyrazinyl; R1 is H; R2 is H, heteroaliphatic, such as 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C 1-6 alkyl substituted with -OH, amino, alkoxy, or heterocycloaliphatic; and each R3 is H.
En cualquiera de las realizaciones de fórmula 1, R4 puede ser halógeno, heterocicloalifático, arilo, heteroarilo, -NH-heteroarilo, u -O-heteroarilo. R4 puede ser halógeno, tal como Br; heteroarilo de 5 a 10 miembros, heterocicloalifático de 3 a 6 miembros, arilo de 6 a 10 miembros, -NH-(heteroarilo de 5 a 10 miembros), u -O-(heteroarilo de 5 a 10 miembros). Y en algunas realizaciones, R4 es halógeno, piridinilo pirimidinilo, pirazolilo, - NH-pirazolilo, pirrolilo, -O-piridinilo -NH-piridinilo indolilo, furanilo, -NH-benzopirazolilo, pirrolopiridinilo fenilo, tetrahidropiridinilo piperidinilo, o 2-oxo-1,2-dihidropiridinilo.In any of the embodiments of formula 1, R4 can be halogen, heterocycloaliphatic, aryl, heteroaryl, -NH-heteroaryl, or -O-heteroaryl. R4 can be halogen, such as Br; 5 to 10 membered heteroaryl, 3 to 6 membered heterocycloaliphatic, 6 to 10 membered aryl, -NH-(5 to 10 membered heteroaryl), or -O-(5 to 10 membered heteroaryl). And in some embodiments, R4 is halogen, pyridinyl pyrimidinyl, pyrazolyl, -NH-pyrazolyl, pyrrolyl, -O-pyridinyl, -NH-pyridinyl indolyl, furanyl, -NH-benzopyrazolyl, pyrrolopyridinyl phenyl, tetrahydropyridinyl piperidinyl, or 2-oxo-1 ,2-dihydropyridinyl.
R4 puede ser Br,R4 can be Br,
en las que y es 0, 1 o 2, y cada Rp es independientemente Ra, Rb, Ra sustituido con Rb, o Ra sustituido con Rc. En ciertas realizaciones, cada Rp es independientemente -CH3 , -OCH3 , -NH2 , - CF3 , F, -CN,wherein y is 0, 1, or 2, and each Rp is independently Ra, Rb, Ra substituted with Rb, or Ra substituted with Rc. In certain embodiments, each Rp is independently -CH 3 , -OCH 3 , -NH 2 , -CF 3 , F, -CN,
En realizaciones particulares, Z1 es N, y Z2, Z3, y Z4 son CR3; tanto Z1 como Z2 son N, y Z3 y Z4 son CR3; tanto Z1 como Z3 son N, y Z2 y Z4 son CR3; tanto Z1 como Z4 son N, y Z2 y Z3 son CR3; o Z3 es N, y Z1, Z2, y Z4 son CR3. En cualquiera de estas realizaciones, el anillo A es piridinilo o pirazinilo; R1 es H; R2 es H, heteroalifático, tal como heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático; cada R3 es H; y R4 es Br; piridinilo no sustituido; piridinilo sustituido con alquilo de C1-6 , haloalquilo, amino, heterocicloalifático, cicloalquilo, -CN, alcoxi, -O-heterocicloalifático, -NH-heterocicloalifático, halógeno, sulfonamida, -O-bencilo, carboxilo, sulfonilo, -NH-cicloalquilo, o amida; pirimidinilo no sustituido; pirazolilo no sustituido; pirazolilo sustituido con alquilo de Ci-e; -NH-pirazolilo no sustituido; -NH-pirazolilo sustituido con alquilo de Ci-6, o heteroarilo; pirrolilo; -O-piridinilo no sustituido; -O-piridinilo sustituido con amino; -NH-piridinilo sustituido con alquilo de Ci-6, haloalquilo, o heterocicloalifático; indolilo no sustituido; indolilo sustituido con alcoxi; furanilo; -NH-benzopirazolilo; pirrolopiridinilo; fenilo no sustituido; fenilo sustituido con halógeno, alquilo de Ci-6, alcoxi, -CN, amino, 0 sulfonamida; tetrahidropiridinilo no sustituido; tetrahidropiridinilo sustituido con terc-butoxicarbonilo; piperidinilo; o 2-oxo-1,2-dihidropiridinilo.In particular embodiments, Z1 is N, and Z2, Z3, and Z4 are CR3; both Z1 and Z2 are N, and Z3 and Z4 are CR3; both Z1 and Z3 are N, and Z2 and Z4 are CR3; both Z1 and Z4 are N, and Z2 and Z3 are CR3; or Z3 is N, and Z1, Z2, and Z4 are CR3. In any of these embodiments, ring A is pyridinyl or pyrazinyl; R1 is H; R2 is H, heteroaliphatic, such as 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C 1-6 alkyl substituted with -OH, amino, alkoxy, or heterocycloaliphatic; each R3 is H; and R4 is Br; unsubstituted pyridinyl; C 1-6 alkyl-substituted pyridinyl, haloalkyl, amino, heterocycloaliphatic, cycloalkyl, -CN, alkoxy, -O-heterocycloaliphatic, -NH-heterocycloaliphatic, halogen, sulfonamide, -O-benzyl, carboxyl, sulfonyl, -NH-cycloalkyl , or amide; unsubstituted pyrimidinyl; pyrazolyl not substituted; Ci-e alkyl substituted pyrazolyl; -NH-pyrazolyl unsubstituted; -NH-pyrazolyl substituted with C1-6 alkyl, or heteroaryl; pyrrolyl; -O-pyridinyl unsubstituted; -O-pyridinyl substituted with amino; -NH-pyridinyl substituted with Ci-6 alkyl, haloalkyl, or heterocycloaliphatic; unsubstituted indolyl; alkoxy substituted indolyl; furanyl; -NH-benzopyrazolyl; pyrrolopyridinyl; unsubstituted phenyl; phenyl substituted with halogen, C1-6 alkyl, alkoxy, -CN, amino, or sulfonamide; unsubstituted tetrahydropyridinyl; tetrahydropyridinyl substituted with tert-butoxycarbonyl; piperidinyl; or 2-oxo-1,2-dihydropyridinyl.
Con respecto a la fórmula 1, R4 puede ser, en cualquier combinación con las realizaciones 1B a 1Q del anillo A, las realizaciones 2A a 2S de R2, y siendo R1 H: 3A) halógeno; 3B) heterociclilo; 3C) arilo; 3D) heteroarilo; 3E) heterocicloalifático; 3F) heteroarilo de 5 miembros; 3G) heteroarilo de 6 miembros; 3H) piridinilo; 3I) pirimidinilo; 3J) pirazolilo; 3K) pirrolilo; 3L) indolilo; 3M) pirazolopiridinilo; 3N) furanilo; 3O) -NH-heteroarilo; 3P) -O-heteroarilo; 3Q) tetrahidropiridinilo; 3R) bromo; 3S) piridin-3-ilo; 3T) 3-fluoropiridin-3-ilo; 3U) 5-cianopiridin-3-ilo; 3V) pirimidin-5-ilo; 3W) 1 H-pirazol-5-ilo; 3X) 3-metil-1 H-pirazol-5-ilo; 3Y) 1 -metil-1 H-pirazol-4-ilo; 3Z) 1 H-pirazol-4-ilo; 3AA) 1 H-pirrol-2-ilo; 3aB) 1 H-pirrol-3-ilo; 3aC) 5-metilpiridin-3-ilo; 3AD) 5-ciclopropilpiridin-3-ilo; 3aE) 5-trifluorometilpiridin-3-ilo; 3AF) 2-trifluorometilpiridin-4-ilo; 3AG) 5-metoxipiridin-3-ilo; 3AH) 2-metoxipiridin-5-ilo; 3AI) 2-metoxipiridin-4-ilo; 3AJ) 5-isopropoxipiridin-3-ilo; 3AK) 5-((tetrahidro-2H-piran)oxi)piridin-3-ilo; 3AL) 5-(2,2,2-trifluoroetoxi)piridin-3-ilo; 3AM) 5-aminopiridin-3-ilo; 3AN) 5-(metilamino)piridin-3-ilo; 3AO) 5-(ciclopropilamino)piridin-3-ilo; 3AP) 5-(isopropilamino)piridin-3-ilo; 3AQ) 5-(terc-butiloamino)piridin-3-ilo; 3AR) 5-((tetrahidro-2H-piran)amino)piridin-3-ilo; 3AS) 5-((2-metoxietil)amino)piridin-3-ilo; 3AT) 5-((ciclopropilmetil)amino)piridin-3-ilo; 3AU) 5-((2,2,2-trifluoroetil)amino)piridin-3-ilo; 3AV) 5-(metilsulfonamido)piridin-3-ilo; 3AW) 5-(dimetilamino)piridin-3-ilo; 3Ax) 5-(pirrolidin-1 -il)piridin-3-ilo; 3AY) 5-(morfolino)piridin-3-ilo; 3AZ) 5-(4-metilpiperazin-1-il)piridin-3-ilo; 3BA) 5-metilsulfonilpiridin-3-ilo; 3BB) 5-carbamoilpiridin-3-ilo; 3BC) 2-carbamoilpiridin-4-ilo; 3BD) 2-cianopiridin-4-ilo; 3BE) 6-aminopiridin-3-ilo; 3BF) 1H-pirrolo[2,3-b]piridin-5-ilo; 3BG) 1H-pirrolo[3,2-b]piridin-6-ilo; 3bH) 1 H-indol-5-ilo; 3BI) 1h-indol-6-ilo; 3BJ) fenilo; 3BK) m-tolilo; 3bL) p-tolilo; 3BM) 3-metoxifenilo; 3bN) 4-metoxifenilo; 3BO) 3-cianofenilo; 3BP) 4-cianofenilo; 3BQ) 3-fluorofenilo; 3BR) 4-fluorofenilo; 3BS) 3-aminofenilo; 3BT) 3-(metilamino)fenilo; 3BU) 3-(dimetilamino)fenilo; 3BV) 3-(metilsulfonamido)fenilo; 3BW) 1'-(terc-butoxicarbonil)-1',2',3',6'-tetrahidropiridin-4-ilo; 3BX) 1',2',3',6'-tetrahidropiridin-4-ilo; 3BY) piperazin-4-ilo; 3BZ) 1'-(terc-butoxicarbonil)-1',2',3',6'- tetrahidropiridin-3-ilo; 3CA) 1',2',3',6'-tetrahidropiridin-3-ilo; 3CB) 3-metil-1 H-pirazol-4-ilo; 6CC) piridin-4-ilo; 3CD) piridin-4-iloxi; 3CE) 2-oxo-1,2-dihidropiridin-4-ilo; 3CF) 6-oxo-1,6-dihidropiridin-3-ilo; 3CG) 6-acetamidopiridin-3-ilo; 3CH) 1-(ciclopropilmetil)-1H-pirazol-4-ilo; 3CI) 1 H-pirazol-3-ilo; 3CJ) (1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)amino; 3CK) (1 H-pirazol-3-il)amino; 3CL) (3-metil-1H-pirazol-4-il)amino; 3CM) 5-carboxipiridin-3-ilo; 3Cn) 3-fluoropiridin-4-ilo; 3CO) 3-metilpiridin-4-ilo; 3CP) 2-metilpiridin-4-ilo; 3CQ) 6-metoxi-1H-indol-2-ilo; 3CR) furan-3-ilo; 3CS) furan-2-ilo; 3CT) (1H-indazol-5-il)amino; 3Cu) (2H-indazol-5-il)amino; 3CV) 5-(2-hidroxipropan-2-il)piridin-3-ilo; 3CW) 2-metilpiridin-4-ilo; 3CX) 2-metilpiridin-5-ilo; 3CY) (2-aminopiridin-4-il)oxi; 3CZ) (2,5-dimetilpiridin-4-il)amino; 3DA) (6-(4-metilpiperazin-1 -il)-5-(trifluorometil)piridin-3-il)amino; 3DB) 2-((ciclopropilmetil)amino)piridin-4-ilo; o 3DC) 2-((2,2,2-trifluoroetil)amino)piridin-4-ilo.With respect to formula 1, R4 can be, in any combination with ring A embodiments 1B to 1Q, R2 embodiments 2A to 2S, and R1 being H: 3A) halogen; 3B) heterocyclyl; 3C) aryl; 3D) heteroaryl; 3E) heterocycloaliphatic; 3F) 5-membered heteroaryl; 3G) 6-membered heteroaryl; 3H) pyridinyl; 3I) pyrimidinyl; 3J) pyrazolyl; 3K) pyrrolyl; 3L) indolyl; 3M) pyrazolopyridinyl; 3N) furanyl; 3O) -NH-heteroaryl; 3P) -O-heteroaryl; 3Q) tetrahydropyridinyl; 3R) bromine; 3S) pyridin-3-yl; 3T) 3-fluoropyridin-3-yl; 3U) 5-cyanopyridin-3-yl; 3V) pyrimidin-5-yl; 3W) 1H-pyrazol-5-yl; 3X) 3-methyl-1H-pyrazol-5-yl; 3Y) 1 -methyl-1 H -pyrazol-4-yl; 3Z) 1H-pyrazol-4-yl; 3AA) 1H-pyrrol-2-yl; 3 to B) 1H-pyrrol-3-yl; 3 to C) 5-methylpyridin-3-yl; 3AD) 5-cyclopropylpyridin-3-yl; 3 to E) 5-trifluoromethylpyridin-3-yl; 3AF) 2- trifluoromethylpyridin-4-yl; 3AG) 5-methoxypyridin-3-yl; 3AH) 2-methoxypyridin-5-yl; 3AI) 2-methoxypyridin-4-yl; 3AJ) 5-isopropoxypyridin-3-yl; 3AK) 5-((tetrahydro-2H-pyran)oxy)pyridin-3-yl; 3AL) 5-(2,2,2-trifluoroethoxy)pyridin-3-yl; 3AM) 5-aminopyridin-3-yl; 3AN) 5-(methylamino)pyridin-3-yl; 3AO) 5-(cyclopropylamino)pyridin-3-yl; 3AP) 5-(isopropylamino)pyridin-3-yl; 3AQ) 5-(tert-butylamino)pyridin-3-yl; 3AR) 5-((tetrahydro-2H-pyran)amino)pyridin-3-yl; 3AS) 5-((2-methoxyethyl)amino)pyridin-3-yl; 3AT) 5-((cyclopropylmethyl)amino)pyridin-3-yl; 3AU) 5-((2,2,2- trifluoroethyl)amino)pyridin-3-yl; 3AV) 5-(methylsulfonamido)pyridin-3-yl; 3AW) 5-(dimethylamino)pyridin-3-yl; 3A x ) 5- (pyrrolidin-1-yl)pyridin-3-yl; 3AY) 5-(morpholino)pyridin-3-yl; 3AZ) 5-(4-methylpiperazin-1-yl)pyridin-3-yl; 3BA) 5-methylsulfonylpyridin-3-yl; 3BB) 5-carbamoylpyridin-3-yl; 3BC) 2-carbamoylpyridin-4-yl; 3BD) 2-cyanopyridin-4-yl; 3BE) 6- aminopyridin-3-yl; 3BF) 1H-pyrrolo[2,3-b]pyridin-5-yl; 3BG) 1H-pyrrolo[3,2-b]pyridin-6-yl; 3b H) 1H-indol-5-yl; 3BI) 1 h -indol-6-yl; 3BJ) phenyl; 3BK) m-tolyl; 3b L) p-tolyl; 3BM) 3-methoxyphenyl; 3b N) 4-methoxyphenyl; 3BO) 3-cyanophenyl; 3BP) 4-cyanophenyl; 3BQ) 3-fluorophenyl; 3BR) 4-fluorophenyl; 3BS) 3-aminophenyl; 3BT) 3-(methylamino)phenyl; 3BU) 3-(dimethylamino)phenyl; 3BV) 3-(methylsulfonamido)phenyl; 3BW) 1'-(tert-butoxycarbonyl)-1',2',3',6'-tetrahydropyridin-4-yl; 3BX) 1',2',3',6'-tetrahydropyridin-4-yl; 3BY) piperazin-4-yl; 3BZ) 1'-(tert-butoxycarbonyl)-1',2',3',6'-tetrahydropyridin-3-yl; 3CA) 1',2',3',6'-tetrahydropyridin-3-yl; 3CB) 3-methyl-1H-pyrazol-4-yl; 6CC) pyridin-4-yl; 3CD) pyridin-4-yloxy; 3CE) 2-oxo-1,2-dihydropyridin-4-yl; 3CF) 6-oxo-1,6-dihydropyridin-3-yl; 3CG) 6-acetamidopyridin-3-yl; 3CH) 1-(cyclopropylmethyl)-1H-pyrazol-4-yl; 3CI) 1H-pyrazol-3-yl; 3CJ) (1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)amino; 3CK) (1 H-pyrazol-3-yl)amino; 3CL) (3-methyl-1H-pyrazol-4-yl)amino; 3CM) 5-carboxypyridin-3-yl; 3C n ) 3-fluoropyridin-4-yl; 3CO) 3-methylpyridin-4-yl; 3CP) 2-methylpyridin-4-yl; 3CQ) 6-methoxy-1H-indol-2-yl; 3CR) furan-3-yl; 3CS) furan-2-yl; 3CT) (1H-indazol-5-yl)amino; 3C u ) (2H-indazol-5-yl)amino; 3CV) 5-(2-hydroxypropan-2-yl)pyridin-3-yl; 3CW) 2-methylpyridin-4-yl; 3CX) 2-methylpyridin-5-yl; 3CY) (2-aminopyridin-4-yl)oxy; 3CZ) (2,5-dimethylpyridin-4-yl)amino; 3DA) (6-(4-methylpiperazin-1-yl)-5-(trifluoromethyl)pyridin-3-yl)amino; 3DB) 2-((cyclopropylmethyl)amino)pyridin-4-yl; or 3DC) 2-((2,2,2-trifluoroethyl)amino)pyridin-4-yl.
Un experto en la técnica comprenderá que cualquiera de 3A a 3DC puede combinarse con cualquiera de 1B a 1Q, cualquiera de 2A a 2FS, y siendo R1 H, para formar cualquiera y todas las combinaciones entre tales sustituyentes. Con respecto a las realizaciones 1B a 1Q del anillo A, las realizaciones 2A a 2S de R2, las realizaciones 3A a 3DC de R4, y siendo R1 hidrógeno, Z1, Z2, Z3 y Z4 pueden ser, en cualquier combinación con 1B a 1Q, 2A a 2S, y 3A a 3DC: 4A) Z1 es N y Z2, Z3 y Z4 son CH; 4B) Z1 y Z2 son N y Z3 y Z4 son CH; 4C) Z1 y Z3 son N y Z2 y Z4 son CH; 4D) Z1 y Z4 son N y Z2 y Z3 son CH; o 4E) Z3 es N y Z1, Z2 y Z4 son CH.One skilled in the art will understand that any of 3A to 3DC can be combined with any of 1B to 1Q, any of 2A to 2FS, and R1 being H, to form any and all combinations between such substituents. With respect to ring A embodiments 1B to 1Q, R2 embodiments 2A to 2S, R4 embodiments 3A to 3DC, and R1 being hydrogen, Z1, Z2, Z3 and Z4 may be, in any combination with 1B to 1Q , 2A to 2S, and 3A to 3DC: 4A) Z1 is N and Z2, Z3 and Z4 are CH; 4B) Z1 and Z2 are N and Z3 and Z4 are CH; 4C) Z1 and Z3 are N and Z2 and Z4 are CH; 4D) Z1 and Z4 are N and Z2 and Z3 are CH; or 4E) Z3 is N and Z1, Z2 and Z4 are CH.
Un experto en la técnica comprenderá que cualquiera de 4A a 4E puede combinarse con cualquiera de 1B a 1Q, cualquiera de 2A a 2S, cualquiera de 3A a 3DC, y siendo R1 H, para formar cualquiera y todas las combinaciones entre tales sustituyentes.One skilled in the art will understand that any of 4A to 4E can be combined with any of 1B to 1Q, any of 2A to 2S, any of 3A to 3DC, and R1 being H, to form any and all combinations between such substituents.
En algunas realizaciones of fórmula 1, el compuesto tiene la fórmula 2In some embodiments of formula 1, the compound has formula 2
Con referencia a la fórmula 2, R1, R2, R4 y el anillo A son tal como se definen para la fórmula 1. Cada uno de R5, R6, y R7 es independientemente H o alquilo, tal como alquilo de C1-6 , y en ciertas realizaciones, cada uno de R5, R6, y R7 es H.With reference to formula 2, R1, R2, R4 and ring A are as defined for formula 1. R5, R6, and R7 are each independently H or alkyl, such as C 1-6 alkyl, and in certain embodiments, each of R5, R6, and R7 is H.
En realizaciones particulares de fórmula 2, el anillo A es piridin-2-ilo o pirazin-2-ilo; R1 es H; R2 es H, heteroalifático, tal como heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de Ci-6 no sustituido, o alquilo de Ci-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático; cada R3 es H; y R4 es Br; piridinilo no sustituido; piridinilo sustituido con alquilo de C1-6 , haloalquilo, amino, heterocicloalifático, cicloalquilo, -CN, alcoxi, -O-heterocicloalifático, -NH-heterocicloalifático, halógeno, sulfonamida, -O-bencilo, carboxilo, sulfonilo, -NH-cicloalquilo, o amida; pirimidinilo no sustituido; pirazolilo no sustituido; pirazolilo sustituido con alquilo de C1-6 ; -NH-pirazolilo no sustituido; -NH-pirazolilo sustituido con alquilo de C1-6 , o heteroarilo; pirrolilo; -O-piridinilo no sustituido; -O-piridinilo sustituido con amino; -NH-piridinilo sustituido con alquilo de C1-6 , haloalquilo, o heterocicloalifático; indolilo no sustituido; indolilo sustituido con alcoxi; furanilo; -NH-benzopirazolilo; pirrolopiridinilo; fenilo no sustituido; fenilo sustituido con halógeno, alquilo de C1-6, alcoxi, -CN, amino, o sulfonamida; tetrahidropiridinilo no sustituido; tetrahidropiridinilo sustituido con tercbutoxicarbonilo; piperidinilo; o 2-oxo-1,2-dihidropiridinilo.In particular embodiments of formula 2, ring A is pyridin-2-yl or pyrazin-2-yl; R1 is H; R2 is H, heteroaliphatic, such as 3-10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy-substituted cyclobutyl and/or hydroxy, cyclohexyl, alkoxy-substituted cyclohexyl and/or hydroxy, unsubstituted Ci-6 alkyl, or -OH, amino, alkoxy, or heterocycloaliphatic substituted Ci-6 alkyl; each R3 is H; and R4 is Br; unsubstituted pyridinyl; C 1-6 alkyl-substituted pyridinyl, haloalkyl, amino, heterocycloaliphatic, cycloalkyl, -CN, alkoxy, -O-heterocycloaliphatic, -NH-heterocycloaliphatic, halogen, sulfonamide, -O-benzyl, carboxyl, sulfonyl, -NH-cycloalkyl , or amide; unsubstituted pyrimidinyl; unsubstituted pyrazolyl; pyrazolyl substituted with C 1-6 alkyl; -NH-pyrazolyl unsubstituted; -NH-pyrazolyl substituted with C 1-6 alkyl, or heteroaryl; pyrrolyl; -O-pyridinyl unsubstituted; -O-pyridinyl substituted with amino; -NH-pyridinyl substituted with C 1-6 alkyl, haloalkyl, or heterocycloaliphatic; unsubstituted indolyl; alkoxy substituted indolyl; furanyl; -NH-benzopyrazolyl; pyrrolopyridinyl; unsubstituted phenyl; phenyl substituted with halogen, C 1 -6 alkyl, alkoxy, -CN, amino, or sulfonamide; unsubstituted tetrahydropyridinyl; tetrahydropyridinyl substituted with tert-butoxycarbonyl; piperidinyl; or 2-oxo-1,2-dihydropyridinyl.
Con respecto a la fórmula 2, un experto en la técnica comprenderá que cuando R1, R5, R6, y R7 son H, cualquiera de las realizaciones 1B a 1Q del anillo A, se puede combinar con cualquiera de las realizaciones 2A a 2S de R2, y cualquiera de las realizaciones 3A a 3DC de R4 para formar todas y cada una de las combinaciones entre tales sustituyentes.With respect to formula 2, one skilled in the art will understand that when R1, R5, R6, and R7 are H, any of the embodiments 1B to 1Q of ring A, can be combined with any of the embodiments 2A to 2S of R2, and any of the embodiments 3A to 3DC of R4 to form any and all combinations between such substituents.
En algunas realizaciones de las fórmulas 1 y 2, el anillo A es piridinilo, tal como piridin-2-ilo; o pirazinilo, tal como pirazin-2-ilo. En realizaciones particulares, tales compuestos, o sales, solvatos, hidrates, tienen una fórmula 3In some embodiments of formulas 1 and 2, ring A is pyridinyl, such as pyridin-2-yl; or pyrazinyl, such as pyrazin-2-yl. In particular embodiments, such compounds, or salts, solvates, hydrates, have a formula 3
Con respecto a la fórmula 3, R1, R2, R3, R4, Z1, Z2, Z3, y Z4 son como se definen para las fórmulas 1 y 2. Cada uno de R8, R9, R10 y R11 es independientemente H, alifático, halógeno, heterocicloalifático, alcoxi, u -O-heterocicloalifático. Cada uno de R8, R9, R10 y R11 puede ser independientemente H, halógeno, heterocicloalifático de 3 a 6 miembros, alcoxi, u -O-(heterocicloalifático de 3 a 6 miembros). En algunas realizaciones, R9 y R11 son H, R8 es H o halógeno, tal como F, y R10 es halógeno, tal como F, heterocicloalifático de 3 a 6 miembros, tal como morfolino o N-metilpiperidinilo, alcoxi, tal como metoxi o 2-hidroxi-2-metilpropoxi, u -O-oxetanilo. En algunas realizaciones, cada uno de R8, R9, R10 y R11 es H. En otras realizaciones, R8, R9, y R11 son H, y R10 es heterocicloalifático de 3 a 6 miembros, tal como morfolino o N-metilpiperidin-1-ilo; alcoxi, tal como metoxi o 2-hidroxi-2-metilpropoxi; u -O-oxetanilo. En otras ciertas realizaciones, R9 y R11 son H, y R8 y R10 son F.With respect to formula 3, R1, R2, R3, R4, Z1, Z2, Z3, and Z4 are as defined for formulas 1 and 2. R8, R9, R10, and R11 are each independently H, aliphatic, halogen, heterocycloaliphatic, alkoxy, or -O-heterocycloaliphatic. Each of R 8 , R 9 , R 10 and R 11 can independently be H, halogen, 3 to 6 membered heterocycloaliphatic, alkoxy, or -O-(3 to 6 membered heterocycloaliphatic). In some embodiments, R9 and R11 are H, R8 is H or halogen, such as F, and R10 is halogen, such as F, 3- to 6-membered heterocycloaliphatic, such as morpholino or N-methylpiperidinyl, alkoxy, such as methoxy. or 2-hydroxy-2-methylpropoxy, or -O-oxetanyl. In some embodiments, R8 , R9, R10, and R11 are each H. In other embodiments, R8 , R9, and R11 are H, and R10 is 3- to 6-membered heterocycloaliphatic, such as morpholino or N-methylpiperidine- 1-yl; alkoxy, such as methoxy or 2-hydroxy-2-methylpropoxy; u -O-oxetanyl. In certain other embodiments, R9 and R11 are H, and R8 and R10 are F.
En realizaciones particulares, Z1 es N, y Z2, Z3, y Z4 son CR3; tanto Z1 como Z2 son N, y Z3 y Z4 son CR3; tanto Z1 como Z3 son N, y Z2 y Z4 son CR3; tanto Z1 como Z4 son N, y Z2 y Z3 son CR3; o Z3 es N, y Z1, Z2, y Z4 son CR3. En cualquiera de estas realizaciones, R1 es H; R2 es H, heteroalifático, tal como heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático; cada R3 es H; R8 es H o F; R9 es H; R10 es H, F, heterocicloalifático de 3 a 6 miembros, tal como morfolino o N-metilpiperidinilo, alcoxi, tal como metoxi o 2-hidroxi-2-metilpropoxi, u -O-oxetanilo; y R11 es H.In particular embodiments, Z1 is N, and Z2, Z3, and Z4 are CR3; both Z1 and Z2 are N, and Z3 and Z4 are CR3; both Z1 and Z3 are N, and Z2 and Z4 are CR3; both Z1 and Z4 are N, and Z2 and Z3 are CR3; or Z3 is N, and Z1, Z2, and Z4 are CR3. In any of these embodiments, R1 is H; R2 is H, heteroaliphatic, such as 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C 1-6 alkyl substituted with -OH, amino, alkoxy, or heterocycloaliphatic; each R3 is H; R 8 is H or F; R9 is H; R10 is H, F, 3- to 6-membered heterocycloaliphatic, such as morpholino or N-methylpiperidinyl, alkoxy, such as methoxy or 2-hydroxy-2-methylpropoxy, or -O-oxetanyl; and R11 is H.
En algunas realizaciones of fórmula 3, el compuesto tiene la fórmula 4In some embodiments of formula 3, the compound has formula 4
Con respecto a la fórmula 4, R1, R2, R3, R4, R8, R9, R10 y R11 son como se definen para fórmula 3, y Z5 y Z6 son independientemente N o CR3, con la condición de que al menos uno de Z5 y Z6 sea CR3. En algunas realizaciones, tanto Z5 como Z6 son CR3. En otras realizaciones, Z5 es N y Z6 es CR3, y en realizaciones alternativas, Z5 es CR3 y Z6 es N. En algunas realizaciones, el compuesto tiene una fórmula seleccionada deWith respect to Formula 4, R1, R2, R3, R4, R8 , R9, R10, and R11 are as defined for Formula 3, and Z5 and Z6 are independently N or CR3, provided that at least one of Z5 and Z6 are CR3. In some embodiments, both Z5 and Z6 are CR3. In other embodiments, Z5 is N and Z6 is CR3, and in alternate embodiments, Z5 is CR3 and Z6 is N. In some embodiments, the compound has a formula selected from
Con respecto a las fórmulas 5, 6 y 7, R1, R2, R4, R8, R9, R10 y R11 son como se definen para la fórmula 4.With respect to Formulas 5, 6, and 7, R1, R2, R4, R8 , R9, R10, and R11 are as defined for Formula 4.
En ciertas realizaciones of fórmula 3, el compuesto tiene la fórmula 8In certain embodiments of formula 3, the compound has formula 8
Con referencia a la fórmula 8, R1, R2, R4, R8, R9, R10, y R11 son como se definen para la fórmula 3, y cada uno de R5, R6, y R7 es independientemente H o alquilo, tal como alquilo de C1-6. En ciertas realizaciones, cada uno de R5, R6, y R7 es H, conduciendo a compuestos que tienen la fórmula 9Referring to Formula 8, R1, R2, R4, R8 , R9, R10, and R11 are as defined for Formula 3, and R5, R6, and R7 are each independently H or alkyl, such as C 1-6 alkyl. In certain embodiments, each of R5, R6, and R7 is H, leading to compounds having formula 9
En realizaciones particulares de fórmulas 8 y 9, R1 es H; R2 es H, heteroalifático, tal como heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático; R4 es Br; piridinilo no sustituido; piridinilo sustituido con alquilo de C1-6 , haloalquilo, amino, heterocicloalifático, cicloalquilo, -CN, alcoxi, -O-heterocicloalifático, -NH-heterocicloalifático, halógeno, sulfonamida, -O-bencilo, carboxilo, sulfonilo, -NH-cicloalquilo, o amida; pirimidinilo no sustituido; pirazolilo no sustituido; pirazolilo sustituido con alquilo de C1-6 ; -NH-pirazolilo no sustituido; -NH-pirazolilo sustituido con alquilo de C1-6 , o heteroarilo; pirrolilo; -O-piridinilo no sustituido; -O-piridinilo sustituido con amino; -NH-piridinilo sustituido con alquilo de C1-6, haloalquilo, o heterocicloalifático; indolilo no sustituido; indolilo sustituido con alcoxi; furanilo; -NH-benzopirazolilo; pirrolopiridinilo; fenilo no sustituido; fenilo sustituido con halógeno, alquilo de C1-6 , alcoxi, -CN, amino, o sulfonamida; tetrahidropiridinilo no sustituido; tetrahidropiridinilo sustituido con terc-butoxicarbonilo; piperidinilo; o 2-oxo-1,2-dihidropiridinilo; R8 es H o F; R9 y R11 son H; y R10 es H; F; heterocicloalifático de 3 a 6 miembros, tal como morfolino o N-metilpiperidinilo; alcoxi, tal como metoxi o 2-hidroxi-2-metilpropoxi; u -O-oxetanilo. Y en ciertas realizaciones, cada uno de R8, R9, R10 y R11 es H; R8, R9, y R11 son H y R10 es heterocicloalifático de 3 a 6 miembros, tal como morfolino o N-metilpiperidinilo, alcoxi, tal como metoxi o 2-hidroxi-2-metilpropoxi, u -O-oxetanilo, R8 y R10 son F, y R9 y R11 son H.In particular embodiments of formulas 8 and 9, R1 is H; R2 is H, heteroaliphatic, such as 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C 1-6 alkyl substituted with -OH, amino, alkoxy, or heterocycloaliphatic; R4 is Br; unsubstituted pyridinyl; C 1-6 alkyl-substituted pyridinyl, haloalkyl, amino, heterocycloaliphatic, cycloalkyl, -CN, alkoxy, -O-heterocycloaliphatic, -NH-heterocycloaliphatic, halogen, sulfonamide, -O-benzyl, carboxyl, sulfonyl, -NH-cycloalkyl , or amide; unsubstituted pyrimidinyl; unsubstituted pyrazolyl; pyrazolyl substituted with C 1-6 alkyl; -NH-pyrazolyl unsubstituted; -NH-pyrazolyl substituted with C 1-6 alkyl, or heteroaryl; pyrrolyl; -O-pyridinyl unsubstituted; -O-pyridinyl substituted with amino; -NH-pyridinyl substituted with C 1-6 alkyl, haloalkyl, or heterocycloaliphatic; unsubstituted indolyl; alkoxy substituted indolyl; furanyl; -NH-benzopyrazolyl; pyrrolopyridinyl; unsubstituted phenyl; phenyl substituted with halogen, C 1-6 alkyl, alkoxy, -CN, amino, or sulfonamide; unsubstituted tetrahydropyridinyl; tetrahydropyridinyl substituted with tert-butoxycarbonyl; piperidinyl; or 2-oxo-1,2-dihydropyridinyl; R 8 is H or F; R9 and R11 are H; and R10 is H; F; 3 to 6 membered heterocycloaliphatic, such as morpholino or N-methylpiperidinyl; alkoxy, such as methoxy or 2-hydroxy-2-methylpropoxy; u -O-oxetanyl. And in certain embodiments, each of R 8 , R 9 , R 10 and R 11 is H; R 8 , R 9 , and R 11 are H and R 10 is 3- to 6-membered heterocycloaliphatic, such as morpholino or N-methylpiperidinyl, alkoxy, such as methoxy or 2-hydroxy-2-methylpropoxy, or -O-oxetanyl, R 8 and R10 are F, and R9 and R11 are H.
Ciertos compuestos descritos ejemplares dentro del alcance de una o más de las fórmulas generales 1-9 incluyen: Certain exemplary described compounds within the scope of one or more of General Formulas 1-9 include:
Los compuestos descritos ejemplares dentro del alcance de una o más de las fórmulas generales 1 -9 incluyen:Exemplary compounds described within the scope of one or more of General Formulas 1-9 include:
I-1: 6-bromo-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-1: 6-bromo-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide;
I-2: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-2: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-3: 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-3: 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-4: 5'-ciano-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-4: 5'-cyano-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-5: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(pirimidin-5-il)picolinamida;I-5: N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(pyrimidin-5-yl)picolinamide;
I-6: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-5-il)picolinamida;I-6: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-5-yl)picolinamide;
I-7: 6-(3-metil-1 H-pirazol-5-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-7: 6-(3-methyl-1H-pyrazol-5-yl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide;
I-8: 6-(1 -metil-1 H-pirazol-4-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-8: 6-(1-methyl-1H-pyrazol-4-yl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide;
1 -9: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;1-9: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide;
I-10: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-2-il)picolinamida;I-10: N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(1 H-pyrazol-2-yl)picolinamide;
I-11: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-3-il)picolinamida;I-11: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-3-yl)picolinamide;
I-12: 5'-metil-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-12: 5'-methyl-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-13: 5'-ciclopropil-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-13: 5'-cyclopropyl-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-14: 5'-(2-hidroxipropan-2-il)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; I-15: 2'-metil-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;I-14: 5'-(2-hydroxypropan-2-yl)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'- bipyridine]-6-carboxamide; I-15: 2'-methyl-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
I-16: 6'-metil-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-16: 6'-methyl-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-17: N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(trifluorometil)-[2,3'-bipiridin]-6-carboxamida;I-17: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(trifluoromethyl)-[2,3'-bipyridine]-6-carboxamide ;
I-18: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-2'-(trifluorometil)-[2,4'-bipiridin]-6-carboxamida;I-18: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-2'-(trifluoromethyl)-[2,4'-bipyridine]-6-carboxamide ;
I-19: 5'-metoxiN-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-19: 5'-methoxy N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-20: 6'-metoxi-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-20: 6'-methoxy-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I - 21 : 2 '-m e to x i-N -(1 -m e til-3 -(p ir id in -2 - il) -1 H -p ira z o l-4 - i l) -[2 ,4 '-b ip ir id in ]-6 -c a rb o x a m id a ; I - 21 : 2 '-me to x iN -(1 -me til-3 -(p ir id in -2 - il) -1 H -p ira zo l-4 - il) -[2 ,4 '- b ip ir id in ]-6 -ca rb oxam id a ;
I-22 : 5 '- is o p ro p o x i-N -(1 -m e ti l-3 -(p ir id in -2 - i l) -1 H -p ira z o l-4 - i l) -[2 ,3 '-b ip ir id in ]-6 -c a rb o x a m id a ;I-22 : 5 '- is o prop o x i-N -(1 -m e ti l-3 -(p ir id in -2 - i l) -1 H -p ira z o l-4 - i l) -[2 ,3 '-b ip ir id in ]-6 -c a rb o x a m id a ;
I-23: N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-((tetrahidro-2H-piran-4-il)oxi)-[2,3'-bipiridin]-6-carboxamida; I-24: N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-5'-(2,2,2-trifluoroetoxi)-[2,3'-bipiridin]-6-carboxamida;I-23: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-((tetrahydro-2H-pyran-4-yl)oxy)-[ 2,3'-bipyridine]-6-carboxamide; I-24: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-5'-(2,2,2-trifluoroethoxy)-[2,3'-bipyridine ]-6-carboxamide;
I-25: 5'-amino-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-25: 5'-amino-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-26: N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(metilamino)-[2,3'-bipiridin]-6-carboxamida;I-26: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(methylamino)-[2,3'-bipyridine]-6-carboxamide ;
I-27: 5'-(ciclopropilamino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-27: 5'-(cyclopropylamino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide ;
I-28: 5'-(isopropilamino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-28: 5'-(isopropylamino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide ;
I-29: 5'-(terc-butiloamino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-29: 5'-(tert-butylamino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]-6 -carboxamide;
I-30: N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-5'-((tetrahidro-2H-piran-4-il)amino)-[2,3'-bipiridin]-6-carboxamida;I-30: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-5'-((tetrahydro-2H-pyran-4-yl)amino)-[2 ,3'-bipyridine]-6-carboxamide;
I-31: 5'-((2-metoxietil)amino)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-31: 5'-((2-methoxyethyl)amino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin ]-6-carboxamide;
I-32: 5'-((ciclopropilmetil)amino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; I-33: N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-((2,2,2-trifluoroetil)amino)-[2,3'-bipiridin]-6-carboxamida; I-34: N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(metilsulfonamido)-[2,3'-bipiridin]-6-carboxamida;I-32: 5'-((cyclopropylmethyl)amino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide; I-33: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-((2,2,2-trifluoroethyl)amino)-[2, 3'-bipyridine]-6-carboxamide; I-34: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(methylsulfonamido)-[2,3'-bipyridine]-6-carboxamide ;
I-35: 5'-(dimetilamino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-35: 5'-(dimethylamino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide ;
I-36: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(pirrolidin-1 -il)-[2,3'-bipiridin]-6-carboxamida;I-36: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(pyrrolidin-1-yl)-[2,3'-bipyridin] -6-carboxamide;
I-37: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-morfolino-[2,3'-bipiridin]-6-carboxamida;I-37: N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-5'-morpholino-[2,3'-bipyridine]-6-carboxamide;
I-38: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(4-metilpiperazin-1 -il)-[2,3'-bipiridin]-6-carboxamida;I-38: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(4-methylpiperazin-1-yl)-[2,3'- bipyridine]-6-carboxamide;
I-39: N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(metilsulfonil)-[2,3'-bipiridin]-6-carboxamida;I-39: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(methylsulfonyl)-[2,3'-bipyridine]-6-carboxamide ;
I-40: N6-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-5',6-dicarboxamida;I-40: N6-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-5',6-dicarboxamide;
I-41: N6-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-2',6-dicarboxamida;I-41: N6-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-2',6-dicarboxamide;
I-42: 2'-ciano-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;I-42: 2'-cyano-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
I-43: 6'-amino-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-43: 6'-amino-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
I-44: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirrolo[2,3-b]piridin-5-il)picolinamida;I-44: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrrolo[2,3-b]pyridin-5-yl )picolinamide;
I-45: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirrolo[3,2-b]piridin-6-il)picolinamida;I-45: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrrolo[3,2-b]pyridin-6-yl )picolinamide;
I-46: 6-(1 H-indol-5-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-46: 6-(1H-indol-5-yl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide;
I-47: 6-(1 H-indol-6-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-47: 6-(1H-indol-6-yl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide;
I-48: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-fenilpicolinamida;I-48: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-phenylpicolinamide;
I-49: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(m-tolil)picolinamida;I-49: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(m-tolyl)picolinamide;
I-50: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(p-tolil)picolinamida;I-50: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(p-tolyl)picolinamide;
I-51: 6-(3-metoxifenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-51: 6-(3-methoxyphenyl)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
I-52: 6-(4-metoxifenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-52: 6-(4-methoxyphenyl)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
I-53: 6-(3-cianofenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-53: 6-(3-cyanophenyl)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
I-54 : 6 -(4 -c ia n o fe n il) -N -(1 -m e til-3 -(p ir id in -2 - il) -1 H -p ira z o l-4 - il)p ic o lin a m id a ; I-54 : 6 -(4 -c ia no fen il) -N -(1 -methyl-3 -(p ir id in -2 - il) -1 H -p ira zo l-4 - il) pico linamida ;
-55 : 6 -(3 - flu o ro fe n il) -N -(1 -m e t il-3 -(p ir id in -2 - il) -1 H -p ira z o l-4 - i l)p ic o lin a m id a ;-55 : 6 -(3 - fluoro phenyl) -N -(1 -m e til-3 -(p ir id in -2 - il) -1 H -p ira z o l-4 - i l)p ic o lin a m id a ;
-56: 6-(4-fluorofenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-56: 6-(4-fluorophenyl)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
-57: 6-(3-aminofenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-57: 6-(3-aminophenyl)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
-58: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(3-(metilamino)fenil)picolinamida;-58: N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(3-(methylamino)phenyl)picolinamide;
-59: 6-(3-(dimetilamino)fenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-59: 6-(3-(dimethylamino)phenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide;
-60: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(3-(metilsulfonamido)fenil)picolinamida;-60: N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(3-(methylsulfonamido)phenyl)picolinamide;
-61 -((1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)carbamoil)-3',6'-dihidro-[2,4'-bipiridin]-1 '(2'H)-carboxilato de tercbutilo;-61 -((1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)carbamoyl)-3',6'-dihydro-[2,4'-bipyridin]-1' tert-butyl (2'H)-carboxylate;
-62: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-1 ',2',3',6'-tetrahidro-[2,4'-bipiridin]-6-carboxamida;-62: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-1',2',3',6'-tetrahydro-[2,4'- bipyridine]-6-carboxamide;
-63: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(piperidin-4-il)picolinamida;-63: N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(piperidin-4-yl)picolinamide;
-64-((1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)carbamoil)-5',6'-dihidro-[2,3'-bipiridin]-1 '(2'H)-carboxilato de tercbutilo;-64-((1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)carbamoyl)-5',6'-dihydro-[2,3'-bipyridin]-1' tert-butyl (2'H)-carboxylate;
-65: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-1 ',2',5',6'-tetrahidro-[2,3'-bipiridin]-6-carboxamida;-65: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-1',2',5',6'-tetrahydro-[2,3'- bipyridine]-6-carboxamide;
-66: 6-bromo-N-(3-(5-metoxipiridin-2-il)-1 -metil-1 H-pirazol-4-il)picolinamida;-66: 6-bromo-N-(3-(5-methoxypyridin-2-yl)-1-methyl-1 H-pyrazol-4-yl)picolinamide;
-67: N-(3-(5-metoxipiridin-2-il)-1 -metil-1 H-pirazol-4-il)-6-(1 H-pirazol-5-il)picolinamida;-67: N-(3-(5-methoxypyridin-2-yl)-1-methyl-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-5-yl)picolinamide;
-68: N-(3-(5-metoxipiridin-2-il)-1 -metil-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;-68: N-(3-(5-methoxypyridin-2-yl)-1-methyl-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide;
-69: N-(3-(5-metoxipiridin-2-il)-1 -metil-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida;-69: N-(3-(5-methoxypyridin-2-yl)-1-methyl-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridine]-6-carboxamide;
-70: 5'-isopropoxi-N-(3-(5-metoxipiridin-2-il)-1-metil-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-70: 5'-isopropoxy-N-(3-(5-methoxypyridin-2-yl)-1-methyl-1 H-pyrazol-4-yl)-[2,3'-bipyridin]-6-carboxamide;
-71: 5'-(isopropilamino)-N-(3-(5-metoxipiridin-2-il)-1 -metil-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; -78: 6-(3-metil-1 H-pirazol-4-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-71: 5'-(isopropylamino)-N-(3-(5-methoxypyridin-2-yl)-1-methyl-1 H-pyrazol-4-yl)-[2,3'-bipyridin]-6- carboxamide; -78: 6-(3-methyl-1 H -pyrazol-4-yl)-N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
-79: 6-bromo-N-(1 -isopropil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-79: 6-bromo-N-(1-isopropyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
-80: N-(1 -isopropil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;-80: N-(1-isopropyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide;
-81: N-(1 -isopropil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida;-81: N-(1-isopropyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridine]-6-carboxamide;
-82: 6-bromo-N-(1 -(2-morfolinoetil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-82: 6-bromo-N-(1 -(2-morpholinoethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
-83: N-(1 -(2-morfolinoetil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;-83: N-(1 -(2-morpholinoethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide;
-84: 5'-metil-N-(1-(2-morfolinoetil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-84: 5'-methyl-N-(1-(2-morpholinoethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]-6- carboxamide;
-85: 6-bromo-N-(1 -(2-(4-metilpiperazin-1 -il)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-85: 6-bromo-N-(1 -(2-(4-methylpiperazin-1-yl)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide;
-86: N-(1 -(2-(4-metilpiperazin-1 -il)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;-86: N-(1 -(2-(4-methylpiperazin-1 -yl)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazole -4-yl)picolinamide;
-87: 5'-metil-N-(1 -(2-(4-metilpiperazin-1 -il)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; -88: N-(1 -(2-hidroxietil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-87: 5'-methyl-N-(1 -(2-(4-methylpiperazin-1 -yl)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2 ,3'-bipyridine]-6-carboxamide; -88: N-(1 -(2-hydroxyethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
-89: N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(piridin-4-iloxi)picolinamida;-89: N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(pyridin-4-yloxy)picolinamide;
-90: N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-90: N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridin]-6- carboxamide;
-91: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-2'-oxo-1 ',2'-dihidro-[2,4'-bipiridin]-6-carboxamida;-91: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-2'-oxo-1',2'-dihydro-[2,4'-bipyridine ]-6-carboxamide;
-92 : N -(1 -m e t il-3 -(p ir id in -2 - il) -1 H -p ira z o l-4 - il) -6 '-o x o -1 ',6 '- d ih id ro -[2 ,3 '-b ip ir id in ] -6 -c a rb o x a m id a ; -92 : N -(1 -met il-3 -(p ir id in -2 - il) -1 H -p ira zo l-4 - il) -6 '-oxo -1 ',6 '- d ih id ro -[2,3 '-b ip ir id in ] -6 -ca rb oxam id a ;
-93 : N -(1 -m e til-3 -(p ira z in -2 - il) -1 H -p ira z o l-4 - il) -5 '-(2 ,2 ,2 - t r i f lu o ro e to x i) -[2 ,3 '-b ip ir id in ] -6 -c a rb o x a m id a ;-93 : N -(1 -m e til-3 -(p ira z in -2 - yl) -1 H -p ira z o l-4 - yl) -5 '-(2 ,2 ,2 - t r i fluo ro e to x i) -[2,3 '-b ip ir id in ] -6 -c a rb o x a m id a ;
-94: N-(3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-94: N-(3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
-95: N-(1 -(2-metoxietil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-95: N-(1 -(2-methoxyethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
-96: 2'-metoxi-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida; -97: 6'-amino-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; -98: 6'-acetamido-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; -99: 2'-metoxi-N-(1 -metil-3-(pirazin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-96: 2'-methoxy-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'- bipyridine]-6-carboxamide; -97: 6'-amino-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'- bipyridine]-6-carboxamide; -98: 6'-acetamido-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'- bipyridine]-6-carboxamide; -99: 2'-methoxy-N-(1-methyl-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
-100: N-(1 -metil-3-(pirazin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-100: N-(1-methyl-3-(pyrazin-2-yl)-1 H -pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
-101: N-(1 -metil-3-(pirazin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-101: N-(1-methyl-3-(pyrazin-2-yl)-1 H -pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
-102: 6'-acetamido-N-(1 -metil-3-(pirazin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-102: 6'-acetamido-N-(1-methyl-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
-103: 2'-(benciloxi)-N-(1 -metil-3-(pirazin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-103: 2'-(benzyloxy)-N-(1-methyl-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
-104: N-(1 -(2-(dietilamino)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-104: N-(1 -(2-(diethylamino)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
-105: formiato de N-(1 -metil-3-(pirazin-2-il)-1 H-pirazol-4-il)-5'-(2,2,2-trifluoroetoxi)-[2,3'-bipiridin]-6-carboxamida;-105: N-(1-methyl-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-5'-(2,2,2-trifluoroethoxy)-[2,3' formate -bipyridine]-6-carboxamide;
-106: formiato de N-(3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-106: N-(3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide formate;
-107: formiato de N-(1 -(2-metoxietil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-107: N-(1 -(2-methoxyethyl)-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide formate;
-108: formiato de N-(1 -(2-hidroxietil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-108: N-(1 -(2-hydroxyethyl)-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide formate;
-109: 5-(1 -(ciclopropilmetil)-1 H-pirazol-4-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)nicotinamida;-109: 5-(1 -(cyclopropylmethyl)-1H-pyrazol-4-yl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)nicotinamide;
-110: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[3,4'-bipiridin]-5-carboxamida;-110: N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-[3,4'-bipyridine]-5-carboxamide;
-111: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[3,3'-bipiridin]-5-carboxamida;-111: N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-[3,3'-bipyridine]-5-carboxamide;
-112: N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-112: N-(1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
-113: 6-bromo-N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-113: 6-bromo-N-(1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide;
-114: 5'-metil-N-(1-(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-114: 5'-methyl-N-(1-(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide;
-115: 5'-(metilsulfonamido)-N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; -116: N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;-115: 5'-(methylsulfonamido)-N-(1-(oxetan-3-yl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridin ]-6-carboxamide; -116: N-(1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide;
-117: N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-3-il)picolinamida;-117: N-(1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-3-yl)picolinamide;
-118: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-4-((1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)amino)pirimidin-2-carboxamida;-118: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-4-((1-methyl-3-(pyridin-2-yl)-1H -pyrazol-4-yl)amino)pyrimidine-2-carboxamide;
-119: (metilsulfonil)(6-((1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)carbamoil)-[2,3'-bipiridin]-5'-il)amida sódica;-119: (methylsulfonyl)(6-((1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)carbamoyl)-[2,3'-bipyridine sodium ]-5'-yl)amide;
-120: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-4-(5-metilpiridin-3-il)pirimidin-2-carboxam ida;-120: N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-4-(5-methylpyridin-3-yl)pyrimidine-2-carboxamide;
-121: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(5-metilpiridin-3-il)pirazin-2-carboxamida;-121: N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(5-methylpyridin-3-yl)pyrazine-2-carboxamide;
-122: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)pirazin-2-carboxamida;-122: N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)pyrazine-2-carboxamide;
-123 : N -(1 -m e til-3 -(p ir id in -2 - il) -1 H -p ira z o l-4 - il) -6 -(1 H -p ira z o l-3 - i l)p ira z in -2 -c a rb o x a m id a ; -123 : N -(1 -me til-3 -(p ir id in -2 - il) -1 H -p ira zo l-4 - il) -6 -(1 H -p ira zo l-3 - il) p ira z in -2 -ca rb oxam id a ;
I-124 : N -(1 -m e t il-3 -(p ir id in -2 - il) -1 H -p ira z o l-4 - il) -4 -(1 H -p ira z o l-4 - i l)p ir im id in -2 -c a rb o x a m id a ;I-124 : N -(1 -m e til-3 -(p ir id in -2 - il) -1 H -p ira z o l-4 - il) -4 -(1 H -p ira z o l-4 -i l)p ir im id in -2 -c a rb o x a m id a ;
I-125: N-(1 -(1,3-dihidroxipropan-2-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-125: N-(1 -(1,3-dihydroxypropan-2-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide;
I-126: N-(1 -(1,3-dihidroxipropan-2-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida; I-127: N-(1-(1,3-dihidroxipropan-2-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;I-126: N-(1 -(1,3-dihydroxypropan-2-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3 '-bipyridine]-6-carboxamide; I-127: N-(1-(1,3-dihydroxypropan-2-yl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4 -yl)picolinamide;
I-128: 4-((1 H-pirazol-3-il)amino)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)pirimidin-2-carboxamida;I-128: 4-((1 H-pyrazol-3-yl)amino)-N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)pyrimidin-2- carboxamide;
I-129: 4-((3-metil-1 H-pirazol-4-il)amino)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)pirimidin-2-carboxamida; I-130: 5'-isopropoxi-N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-129: 4-((3-methyl-1H-pyrazol-4-yl)amino)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl) pyrimidine-2-carboxamide; I-130: 5'-isopropoxy-N-(1-(oxetan-3-yl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridin] -6-carboxamide;
I-131: 5'-fluoro-N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-131: 5'-fluoro-N-(1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin] -6-carboxamide;
I-132: N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(trifluorometil)-[2,3'-bipiridin]-6-carboxamida;I-132: N-(1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(trifluoromethyl)-[2,3'- bipyridine]-6-carboxamide;
I-133: N6-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-5',6-dicarboxamida;I-133: N6-(1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]-5',6 -dicarboxamide;
I-134: 6-(1 -metil-1 H-pirazol-4-il)-N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-134: 6-(1-methyl-1H-pyrazol-4-yl)-N-(1-(oxetan-3-yl)-3-(pyridin-2-yl)-1H-pyrazol-4 -yl)picolinamide;
I-135: ácido 6-((1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)carbamoil)-[2,3'-bipiridin]-5'-carboxílico;I-135: 6-((1 -(oxetan-3-yl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)carbamoyl)-[2,3'-bipyridin]- acid 5'-carboxylic;
I-136: 6-(3-metil-1 H-pirazol-5-il)-N-(1 -(oxetan-3-il)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-136: 6-(3-methyl-1H-pyrazol-5-yl)-N-(1-(oxetan-3-yl)-3-(pyridin-2-yl)-1H-pyrazol-4 -yl)picolinamide;
I-137: N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-2-(1 H-pirazol-4-il)pirimidin-4-carboxamida;I-137: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-2-(1H-pyrazol-4-yl)pyrimidine-4-carboxamide;
I-138: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-2-(1 H-pirazol-3-il)pirimidin-4-carboxamida;I-138: N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-2-(1 H-pyrazol-3-yl)pyrimidine-4-carboxamide;
I-139: formiato de N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(piridin-4-iloxi)picolinam ida; I-140: formiato de N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida; I-141: formiato de 2'-metoxi-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;I-139: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(pyridin-4-yloxy) formate )picolinam ide; I-140: N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,4'-bipyridin] formate -6-carboxamide; I-141: 2'-methoxy-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,4 formate '-bipyridine]-6-carboxamide;
I-142: formiato de 6'-amino-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-142: 6'-amino-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2, 3'-bipyridine]-6-carboxamide;
I-143: formiato de 6'-acetamido-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;I-143: 6'-acetamido-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2, 3'-bipyridine]-6-carboxamide;
I-144: formiato de 3'-fluoro-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;I-144: 3'-fluoro-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2, 4'-bipyridine]-6-carboxamide;
I-145: formiato de N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-3'-metil-[2,4'-bipiridin]-6-carboxamida;I-145: N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-3'-methyl-[2, 4'-bipyridine]-6-carboxamide;
I-146: formiato de N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-2'-metil-[2,4'-bipiridin]-6-carboxamida;I-146: N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-2'-methyl-[2, 4'-bipyridine]-6-carboxamide;
I-147: formiato de 6-(6-metoxi-1 H-indol-2-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-147: 6-(6-methoxy-1H-indol-2-yl)-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 formate H-pyrazol-4-yl)picolinamide;
I-148: formiato de 6-(1 -(3-clorofenil)-1 H-pirazol-4-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-148: 6-(1 -(3-chlorophenyl)-1H-pyrazol-4-yl)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2- yl)-1 H-pyrazol-4-yl)picolinamide;
I-149: formiato de N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida;I-149: N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2, 3'-bipyridine]-6-carboxamide;
I-150: formiato de N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-3-il)picolinamida; I-151: 2,2,2-trifluoroacetato de 6-(furan-3-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida; I-150: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol- 3-yl)picolinamide; I-151: 6-(furan-3-yl)-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-2,2,2-trifluoroacetate -pyrazol-4-yl)picolinamide;
-152: 2,2,2-trifluoroacetato de 6-(furan-2-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-l)picolinamida;-152: 6-(furan-2-yl)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H- 2,2,2-trifluoroacetate pyrazol-4-l)picolinamide;
-153: formiato de N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida; -154: formiato de 6-((1 H-indazol-5-il)amino)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-153: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-formate -yl)picolinamide; -154: 6-((1 H-indazol-5-yl)amino)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H- formate pyrazol-4-yl)picolinamide;
-155: formiato de 6-((2H-indazol-5-il)amino)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-155: 6-((2H-indazol-5-yl)amino)-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazole formate -4-yl)picolinamide;
-156: formiato de 6-((2-aminopiridin-4-il)oxi)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-156: 6-((2-aminopyridin-4-yl)oxy)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazole formate -4-yl)picolinamide;
-157: formiato de 6-((2,5-dimetilpiridin-4-il)amino)-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;-157: 6-((2,5-dimethylpyridin-4-yl)amino)-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H formate -pyrazol-4-yl)picolinamide;
-158: formiato de N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-((6-(4-metilpiperazin-1 -il)-5-(trifluorometil)piridin-3-il)amino)picolinamida;-158: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-((6-(4- methylpiperazin-1-yl)-5-(trifluoromethyl)pyridin-3-yl)amino)picolinamide;
-159: formiato de 2'-((ciclopropilmetil)amino)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-159: 2'-((cyclopropylmethyl)amino)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,4'-bipyridin] formate -6-carboxamide;
-160: 2,2,2-trifluoroacetato de N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-160: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide 2,2,2-trifluoroacetate ;
-161: formiato de N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-2'-((2,2,2-trifluoroetil)amino)-[2,4'-bipiridin]-6-carboxamida;-161: N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-2'-((2,2,2-trifluoroethyl)amino)-[2, 4'-bipyridine]-6-carboxamide;
-162: formiato de N-(1 -(3-metoxiciclobutil)-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida;-162: N-(1 -(3-methoxycyclobutyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridin]- formate 6-carboxamide;
-163: formiato de N-(1 -(3-metoxiciclobutil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida; -164: 5'-metil-N-(3-(piridin-2-il)-1 -(tetrahidro-2H-piran-4-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; -165: 6-(1 H-pirazol-4-il)-N-(3-(piridin-2-il)-1 -(tetrahidro-2H-piran-4-il)-1 H-pirazol-4-il)picolinamida;-163: N-(1-(3-methoxycyclobutyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide formate; -164: 5'-methyl-N-(3-(pyridin-2-yl)-1 -(tetrahydro-2H-pyran-4-yl)-1 H-pyrazol-4-yl)-[2,3' -bipyridine]-6-carboxamide; -165: 6-(1H-pyrazol-4-yl)-N-(3-(pyridin-2-yl)-1 -(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4- il)picolinamide;
-166: 6-(1 H-pirazol-3-il)-N-(3-(piridin-2-il)-1 -(tetrahidro-2H-piran-4-il)-1 H-pirazol-4-il)picolinamida;-166: 6-(1H-pyrazol-3-yl)-N-(3-(pyridin-2-yl)-1 -(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4- il)picolinamide;
-167: N-(3-(piridin-2-il)-1-(tetrahidro-2H-piran-4-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;-167: N-(3-(pyridin-2-yl)-1-(tetrahydro-2H-pyran-4-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridin]-6 -carboxamide;
-168: N-(1 -(2-metoxietil)-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida;-168: N-(1 -(2-methoxyethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridin]-6- carboxamide;
-169: N-(1 -(3-metoxipropil)-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida;-169: N-(1 -(3-methoxypropyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridin]-6- carboxamide;
-170: 5'-metil-N-(1 -metil-3-(5-morfolinopiridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-170: 5'-methyl-N-(1-methyl-3-(5-morpholinopyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide;
-171: N-(1 -metil-3-(5-morfolinopiridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;-171: N-(1 -methyl-3-(5-morpholinopyridin-2-yl)-1 H -pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide;
-172: N-(1 -metil-3-(5-morfolinopiridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-3-il)picolinamida;-172: N-(1 -methyl-3-(5-morpholinopyridin-2-yl)-1 H -pyrazol-4-yl)-6-(1 H-pyrazol-3-yl)picolinamide;
-173: 5'-metil-N-(1 -metil-3-(5-(4-metilpiperazin-1 -il)piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; -174: N-(1 -metil-3-(5-(4-metilpiperazin-1 -il)piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;-173: 5'-methyl-N-(1-methyl-3-(5-(4-methylpiperazin-1-yl)pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3 '-bipyridine]-6-carboxamide; -174: N-(1-methyl-3-(5-(4-methylpiperazin-1-yl)pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4 -yl)picolinamide;
-175: N-(1 -metil-3-(5-(4-metilpiperazin-1 -il)piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-3-il)picolinamida;-175: N-(1-methyl-3-(5-(4-methylpiperazin-1-yl)pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-3 -yl)picolinamide;
-176: dihidrocloruro de 5'-metil-N-(1 -metil-3-(5-morfolinopiridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-176: 5'-methyl-N-(1-methyl-3-(5-morpholinopyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridin]-6- dihydrochloride carboxamide;
-177: dihidrocloruro de 5'-metil-N-(1 -metil-3-(5-(4-metilpiperazin-1 -il)piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida;-177: 5'-methyl-N-(1-methyl-3-(5-(4-methylpiperazin-1-yl)pyridin-2-yl)-1H-pyrazol-4-yl)-[2 dihydrochloride ,3'-bipyridine]-6-carboxamide;
-178: N-(3-(5-(2-hidroxi-2-metilpropoxi)piridin-2-il)-1 -metil-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida; -178: N-(3-(5-(2-hydroxy-2-methylpropoxy)pyridin-2-yl)-1-methyl-1 H-pyrazol-4-yl)-5'-methyl-[2,3 '-bipyridine]-6-carboxamide;
I-179 : N -(3 -(5 -(2 -h id ro x i-2 -m e t ilp ro p o x i)p ir id in -2 - il) -1 -m e til-1 H -p ira z o l-4 - il) -6 -(1 H -p ira z o l-4 - il)p ic o lin a m id a ; I-180: N-(3-(5-(2-hidroxi-2-metilpropoxi)piridin-2-il)-1 -metil-1 H-pirazol-4-il)-6-(1 H-pirazol-3-il)picolinamida; I-181: 5'-metil-N-(1 -metil-3-(5-(oxetan-3-iloxi)piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida; I-182: N-(1 -metil-3-(5-(oxetan-3-iloxi)piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;I-179 : N -(3 -(5 -(2 -h id ro x i-2 -m e tilp ro p o xi)p ir id in -2 - il) -1 -m e til-1 H -p ira z o l -4-il) -6 -(1H-pyrazol-4-il)picolinamide; I-180: N-(3-(5-(2-hydroxy-2-methylpropoxy)pyridin-2-yl)-1 -methyl-1 H-pyrazol-4-yl)-6-(1 H-pyrazol- 3-yl)picolinamide; I-181: 5'-methyl-N-(1-methyl-3-(5-(oxetan-3-yloxy)pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3' -bipyridine]-6-carboxamide; I-182: N-(1 -methyl-3-(5-(oxetan-3-yloxy)pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4- il)picolinamide;
I-183: N-(1 -metil-3-(5-(oxetan-3-iloxi)piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-3-il)picolinamida;I-183: N-(1 -methyl-3-(5-(oxetan-3-yloxy)pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-3- il)picolinamide;
I-184: N-(3-(3,5-difluoropiridin-2-il)-1 -((1 r,4r)-4-etoxiciclohexil)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida; I-185: 3'-fluoro-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida; I-186: N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-3'-metil-[2,4'-bipiridin]-6-carboxamida;I-184: N-(3-(3,5-difluoropyridin-2-yl)-1 -((1 r,4r)-4-ethoxycyclohexyl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide; I-185: 3'-fluoro-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,4' -bipyridine]-6-carboxamide; I-186: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-3'-methyl-[2,4' -bipyridine]-6-carboxamide;
I-187: N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-2'-metil-[2,4'-bipiridin]-6-carboxamida;I-187: N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-2'-methyl-[2,4' -bipyridine]-6-carboxamide;
I-188: 6-(6-metoxi-1 H-indol-2-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida; I-189: 6-(1 -(3-clorofenil)-1 H-pirazol-4-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-188: 6-(6-methoxy-1 H-indol-2-yl)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H- pyrazol-4-yl)picolinamide; I-189: 6-(1 -(3-chlorophenyl)-1 H-pyrazol-4-yl)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl) -1 H-pyrazol-4-yl)picolinamide;
I-190: N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida;I-190: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3' -bipyridine]-6-carboxamide;
I-191: N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-3-il)picolinamida;I-191: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-3- il)picolinamide;
I-192: 6-(furan-3-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-192: 6-(furan-3-yl)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide ;
I-193: 6-(furan-2-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-193: 6-(furan-2-yl)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide ;
I-193: 6-(furan-2-il)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida;I-193: 6-(furan-2-yl)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide ;
I-194: N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;I-194: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4- il)picolinamide;
1-195: 6-((1 H-indazol-5-il)amino)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida; I-196: 6-((2H-indazol-5-il)amino)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida; I-197: 6-((2-aminopiridin-4-il)oxi)-N-(1-(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida; I-198: 6-((2,5-dimetilpiridin-4-il)amino)-N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida; I-199: N-(1 -(2-(2-metoxietoxi)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-((6-(4-metilpiperazin-1 -il)-5-(trifluorometil)piridin-3-il)amino)picolinamida;1-195: 6-((1 H-indazol-5-yl)amino)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazole -4-yl)picolinamide; I-196: 6-((2H-indazol-5-yl)amino)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol- 4-yl)picolinamide; I-197: 6-((2-aminopyridin-4-yl)oxy)-N-(1-(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1H-pyrazol- 4-yl)picolinamide; I-198: 6-((2,5-dimethylpyridin-4-yl)amino)-N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H- pyrazol-4-yl)picolinamide; I-199: N-(1 -(2-(2-methoxyethoxy)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-((6-(4-methylpiperazine -1-yl)-5-(trifluoromethyl)pyridin-3-yl)amino)picolinamide;
I-200: 2'-((ciclopropilmetil)amino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida; I-201: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida;I-200: 2'-((cyclopropylmethyl)amino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridin]- 6-carboxamide; I-201: N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide;
I-202: N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-2'-((2,2,2 trifluoroetil)amino)-[2,4'-bipiridin]-6-carboxamida; I-203: N-(1-(3-metoxiciclobutil)-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida;I-202: N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-2'-((2,2,2 trifluoroethyl)amino)-[2,4 '-bipyridine]-6-carboxamide; I-203: N-(1-(3-methoxycyclobutyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridin]-6 -carboxamide;
I-204: N-(1 -(3-metoxiciclobutil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;I-204: N-(1 -(3-methoxycyclobutyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide;
I-205: N-(1 -((1 r,4r)-4-etoxiciclohexil)-3-(pirazin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida;I-205: N-(1 -((1 r,4r)-4-ethoxycyclohexyl)-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol- 4-yl)picolinamide;
I-206: N-(1 -((1 r,4r)-4-etoxiciclohexil)-3-(pirazin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida; I-207: N-(1 -metil-3-(pirazin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida; oI-206: N-(1 -((1 r,4r)-4-ethoxycyclohexyl)-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2, 3'-bipyridine]-6-carboxamide; I-207: N-(1 -methyl-3-(pyrazin-2-yl)-1 H -pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide; either
I-208: 5'-metil-N-(1 -metil-3-(pirazin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida. I-208: 5'-methyl-N-(1-methyl-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide.
B. SíntesisB. Summary
Los compuestos de amida descritos se pueden preparar como se ejemplifica a continuación, y como entenderá un experto en la técnica de la síntesis orgánica. Una síntesis ejemplar puede incluir el siguiente 1a etapa de reacción de acuerdo con el Esquema 1.The described amide compounds can be prepared as exemplified below, and as will be understood by one skilled in the art of organic synthesis. An exemplary synthesis may include the following 1st reaction step according to Scheme 1.
Compuesto de acetilo 2 se hace reaccionar con dimetilformamida dimetilacetal 4 a una temperatura de reacción adecuada, tal como de aproximadamente 85 °C a aproximadamente 130 °C, para formar el compuesto intermedio 6. El compuesto intermedio 6 se hace reaccionar luego con hidrato de hidrazina 8 para formar compuesto de pirazol 10. La reacción se lleva a cabo en un disolvente adecuado, por ejemplo un alcohol, tal como etanol, metanol o isopropanol, y normalmente se calienta, tal como a reflujo.Acetyl compound 2 is reacted with dimethylformamide dimethyl acetal 4 at a suitable reaction temperature, such as from about 85°C to about 130°C, to form intermediate 6. Intermediate 6 is then reacted with hydrazine hydrate 8 to form pyrazole compound 10. The reaction is carried out in a suitable solvent, for example an alcohol, such as ethanol, methanol or isopropanol, and is usually heated, such as under reflux.
A continuación se proporciona una 2° etapa de reacción en la síntesis ejemplar de acuerdo con el Esquema 2.A 2nd reaction step in the exemplary synthesis according to Scheme 2 is provided below.
Compuesto 10 se nitra utilizando un reactivo de nitración adecuado o una mezcla de reactivos 12 para formar compuesto 14. Condiciones de nitración adecuadas incluyen hacer reaccionar el compuesto 10 con ácido nítrico, tal como ácido nítrico fumante, opcionalmente en presencia de ácido sulfúrico. Típicamente, el compuesto 10 y el ácido nítrico se añaden lentamente, uno al otro. Se puede utilizar enfriamiento, por ejemplo utilizando un baño de hielo, para mantener la temperatura de reacción dentro de un intervalo adecuado tal como de aproximadamente 0 °C hasta menos de 50 °C, de 0 °C hasta 20 °C o de 0 °C hasta 10 °C. Una vez completada la adición, se deja que la reacción prosiga hasta que la reacción se complete sustancialmente, y se puede dejar calentar a temperatura ambiente para facilitar la reacción. Opcionalmente, se puede añadir reactivo de nitración adicional, o una mezcla de reactivos de nitración, para facilitar que la reacción prosiga hasta su compleción. A continuación, la reacción se extingue tal como mediante la adición de agua y/o hielo, y el producto se separa o extrae del acuoso y se purifica si es necesario. Técnicas de purificación adecuadas para purificar un producto de cualquier reacción descrita en esta memoria incluyen, pero no se limitan a cristalización, destilación y/o cromatografía.Compound 10 is nitrated using a suitable nitrating reagent or reagent mixture 12 to form compound 14. Suitable nitrating conditions include reacting compound 10 with nitric acid, such as fuming nitric acid, optionally in the presence of sulfuric acid. Typically, compound 10 and nitric acid are slowly added to each other. Cooling, for example using an ice bath, may be used to maintain the reaction temperature within a suitable range such as about 0°C to less than 50°C, 0°C to 20°C, or 0°C. C up to 10°C. After the addition is complete, the reaction is allowed to proceed until substantially complete, and may be allowed to warm to room temperature to facilitate the reaction. Optionally, additional nitrating reagent, or a mixture of nitrating reagents, can be added to facilitate the reaction to proceed to completion. The reaction is then quenched such as by addition of water and/or ice, and the product is separated or extracted from aqueous and purified if necessary. Purification techniques suitable for purifying a product of any reaction described herein include, but are not limited to, crystallization, distillation, and/or chromatography.
Continuando con la referencia al Esquema 2, compuesto 14 se hace reaccionar luego con compuesto 16 para formar compuesto 18. Compuesto 16 comprende un resto R1 deseado y un grupo saliente, LG, adecuado. Los grupos salientes adecuados incluyen cualquier grupo que actúe como grupo saliente para facilitar la adición del resto R1 al compuesto 14. Grupos salientes adecuados incluyen, pero no se limitan a halógenos, típicamente bromo, cloro o yodo, y grupos tosilato o mesilato. Compuesto 14 se hace reaccionar con compuesto 16 en un disolvente adecuado y típicamente en presencia de una base. Disolventes adecuados incluyen cualquier disolvente que facilite la reacción tales como disolventes apróticos. Disolventes adecuados incluyen, pero no se limitan a DMF (dimetilformamida), THF (tetrahidrofurano), DMSO (dimetilsulfóxido), acetonitrilo, disolventes clorados, tales como diclorometano y cloroformo, DMA (dimetilacetamida), dioxano, N-metilpirrolidona o combinaciones de los mismos. Bases adecuadas incluyen cualquier base que facilite las reacciones tal, como un hidruro, típicamente hidruro de sodio, o un carbonato, tal como carbonato de potasio, carbonato de sodio o carbonato de cesio. La reacción puede proseguir a temperatura ambiente, o la mezcla de reacción puede calentarse tal como a 50 °C, 100 °C o más, según se requiera. A continuación, compuesto 18 se aísla de la mezcla de reacción y se purifica si es necesario.Continuing with reference to Scheme 2, compound 14 is then reacted with compound 16 to form compound 18. Compound 16 comprises a desired R1 moiety and a suitable leaving group, LG. Suitable leaving groups include any group that acts as a leaving group to facilitate the addition of the R 1 moiety to compound 14. Suitable leaving groups include, but are not limited to halogens, typically bromo, chloro or iodo, and tosylate or mesylate groups. Compound 14 is reacted with compound 16 in a suitable solvent and typically in the presence of a base. Suitable solvents include any solvent that facilitates the reaction such as aprotic solvents. Suitable solvents include, but are not limited to, DMF (dimethylformamide), THF (tetrahydrofuran), DMSO (dimethylsulfoxide), acetonitrile, chlorinated solvents such as dichloromethane and chloroform, DMA (dimethylacetamide), dioxane, N-methylpyrrolidone, or combinations thereof. . Suitable bases include any base that facilitates the reactions such as a hydride, typically sodium hydride, or a carbonate, such as potassium carbonate, sodium carbonate or cesium carbonate. The reaction can proceed at room temperature, or the reaction mixture can be heated such as 50°C, 100°C or higher as required. Compound 18 is then isolated from the reaction mixture and purified if necessary.
Compuesto 18 se hace reaccionar con un agente reductor 20 adecuado para reducir el resto nitro a una amina. Agentes reductores adecuados incluyen, pero no se limitan a: hidrógeno gaseoso en presencia de un catalizador tal como un catalizador de paladio; un borohidruro tal como borohidruro de sodio, opcionalmente en presencia de un catalizador tal como un catalizador de níquel; zinc metálico en ácido acético; o polvo de hierro en agua o agua y ácido. En determinadas realizaciones, se utiliza gas hidrógeno en combinación con un catalizador de paladio sobre carbono y en un disolvente adecuado tal como acetato de etilo o metanol. En algunas realizaciones, se utiliza una combinación de agentes y/o técnicas reductoras. Por ejemplo, la reducción puede realizarse inicialmente utilizando un primer método que comprende un primer agente y/o técnica reductora, pero da como resultado una mezcla de productos. El primer método puede repetirse y/o puede realizarse un segundo método, que comprende un segundo agente y/o técnica reductora. Una vez que se completa la reacción, según se indica por una técnica analítica tal como LC-MS, TLC o HPLC, el compuesto producto 22 se aísla y se purifica si es necesario.Compound 18 is reacted with a suitable reducing agent 20 to reduce the nitro moiety to an amine. Suitable reducing agents include, but are not limited to: gaseous hydrogen in the presence of a catalyst such as as a palladium catalyst; a borohydride such as sodium borohydride, optionally in the presence of a catalyst such as a nickel catalyst; metallic zinc in acetic acid; or iron powder in water or water and acid. In certain embodiments, hydrogen gas is used in combination with a palladium on carbon catalyst and in a suitable solvent such as ethyl acetate or methanol. In some embodiments, a combination of reducing agents and/or techniques is used. For example, the reduction may initially be carried out using a first method that comprises a first reducing agent and/or technique, but results in a mixture of products. The first method can be repeated and/or a second method can be performed, comprising a second reducing agent and/or technique. Once the reaction is complete, as indicated by an analytical technique such as LC-MS, TLC, or HPLC, product compound 22 is isolated and purified if necessary.
A continuación se proporciona una 3a etapa de la secuencia de reacción ejemplar de acuerdo con el Esquema 3.An exemplary 3rd step reaction sequence according to Scheme 3 is provided below.
El Compuesto 22 se hace reaccionar con un ácido carboxílico 24 para formar amida 26. El ácido carboxílico 24 puede activarse por cualquier método adecuado, y después se puede hacer reaccionar con la amina 22. Métodos de activación adecuados incluyen, pero no se limitan a formar el cloruro de ácido tal como por tratamiento con cloruro de tionilo; tratamiento con hexafluorofosfato de 1-[bis(dimetilamino)metileno]-1H-1,2,3-triazolo[4,5-b]piridinio 3-óxido (HATU) y una base tal como diisopropiletilamina (DIPEA); tratamiento con carbonildiimidazol (CDI); o tratamiento con una carbodiimida tal como diciclohexilcarbodiimida (DCC) o 1 -etil-3-(3-dimetilaminopropil)carbodiimida (EDC).Compound 22 is reacted with carboxylic acid 24 to form amide 26. Carboxylic acid 24 can be activated by any suitable method, and can then be reacted with amine 22. Suitable activation methods include, but are not limited to forming the acid chloride such as by treatment with thionyl chloride; treatment with 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) and a base such as diisopropylethylamine (DIPEA); treatment with carbonyldiimidazole (CDI); or treatment with a carbodiimide such as dicyclohexylcarbodiimide (DCC) or 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC).
La amida 26 se acopla entonces con el compuesto 28 para formar compuesto 30 usando cualquier reacción de acoplamiento adecuada para formar un enlace entre dos anillos. En el ejemplo del Esquema 3, se muestra un acoplamiento de ácido borónico utilizando ácido borónico 28, en que el grupo saliente Lg en compuesto 26 es típicamente bromo o yodo. Otros grupos funcionales de acoplamiento adecuados incluyen trialquil estaño o ésteres borónicos. La reacción de acoplamiento transcurre típicamente en presencia de un catalizador adecuado. Para un acoplamiento de ácido borónico, el catalizador es típicamente un catalizador de paladio, tal como PdCl2(dppf)2 , Pd[P(Ph)a]2Cl2 , acetato de paladio y trifenilfosfina, o tetrakis(trifenilfosfina)paladio(0) . La reacción se realiza en presencia de una base, tal como carbonato de sodio, potasio o cesio, y se realiza en un disolvente o mezcla de disolventes adecuado, tal como dioxano, dioxano/agua o DME (dimetoxietano)/etanol/agua. La reacción se puede calentar a una temperatura adecuada tal como a una temperatura dentro del intervalo mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como a una temperatura de 50 °C a 125 °C, típicamente de aproximadamente 100 °C, y/o se agita durante un período de tiempo adecuado, tal como de 1 hora a 3 días, de 6 horas a 24 horas o de 12 horas a 18 horas, para facilitar que la reacción prosiga hasta su compleción. A continuación, el compuesto 30 se aísla de la mezcla de reacción y se purifica mediante una técnica adecuada. Amide 26 is then coupled with compound 28 to form compound 30 using any suitable coupling reaction to form a bond between two rings. In the example of Scheme 3, a boronic acid coupling using boronic acid 28 is shown, where the leaving group L g in compound 26 is typically bromo or iodo. Other suitable coupling functional groups include trialkyl tin or boronic esters. The coupling reaction typically proceeds in the presence of a suitable catalyst. For a boronic acid coupling, the catalyst is typically a palladium catalyst, such as PdCl 2 (dppf) 2 , Pd[P(Ph)a] 2 Cl 2 , palladium acetate and triphenylphosphine, or tetrakis(triphenylphosphine)palladium( 0) . The reaction is carried out in the presence of a base, such as sodium, potassium, or cesium carbonate, and is carried out in a suitable solvent or solvent mixture, such as dioxane, dioxane/water, or DME (dimethoxyethane)/ethanol/water. The reaction can be heated to a suitable temperature such as a temperature within the range greater than room temperature up to the boiling point of the selected solvent, such as 50°C to 125°C, typically about 100°C. C, and/or stirred for a suitable period of time, such as 1 hour to 3 days, 6 hours to 24 hours, or 12 hours to 18 hours, to allow the reaction to proceed to completion. Next, compound 30 is isolated from the reaction mixture and purified by a suitable technique.
Una síntesis ejemplar alternativa puede incluir la siguiente 1a etapa de reacción de acuerdo con el Esquema 4. An alternative exemplary synthesis may include the following 1st reaction step according to Scheme 4.
El compuesto 32 se nitra usando un reactivo de nitración adecuado, o una mezcla de reactivos 34, para formar el compuesto 36. Condiciones de nitración adecuadas incluyen hacer reaccionar el compuesto 32 con ácido nítrico, tal como ácido nítrico fumante, opcionalmente en presencia de ácido sulfúrico. Típicamente, el compuesto 32 y el ácido nítrico se añaden lentamente, uno al otro. Se puede utilizar enfriamiento, por ejemplo utilizando un baño de hielo, para mantener la temperatura de reacción dentro de un intervalo adecuado tal como de aproximadamente 0 °C hasta menos de 50 °C, de 0 °C hasta 20 °C o de 0 °C hasta 10 °C. Una vez completada la adición, se deja que la reacción prosiga hasta que la reacción se complete sustancialmente, y se puede dejar calentar a temperatura ambiente para facilitar la reacción. Opcionalmente, se puede añadir reactivo de nitración adicional, o una mezcla de reactivos de nitración, para facilitar que la reacción prosiga hasta su compleción. A continuación, la reacción se extingue tal como mediante la adición de agua y/o hielo, y el producto se separa o extrae de la fase acuosa y se purifica si se requiere. Técnicas de purificación adecuadas para purificar un producto de cualquier reacción descrita en esta memoria incluyen, pero no se limitan a cristalización, destilación y/o cromatografía.Compound 32 is nitrated using a suitable nitrating reagent, or reagent mixture 34, to form compound 36. Suitable nitrating conditions include reacting compound 32 with nitric acid, such as fuming nitric acid, optionally in the presence of acid. sulfuric. Typically, compound 32 and nitric acid are slowly added to each other. Cooling, for example using an ice bath, may be used to maintain the reaction temperature within a suitable range such as about 0°C to less than 50°C, 0°C to 20°C, or 0°C. C up to 10°C. After the addition is complete, the reaction is allowed to proceed until substantially complete, and may be allowed to warm to room temperature to facilitate the reaction. Optionally, additional nitrating reagent, or a mixture of nitrating reagents, can be added to facilitate the reaction to proceed to completion. The reaction is then quenched such as by the addition of water and/or ice, and the product is separated or extracted from the aqueous phase and purified if required. Purification techniques suitable for purifying a product of any reaction described herein include, but are not limited to, crystallization, distillation, and/or chromatography.
Continuando con la referencia al Esquema 4, el compuesto 36 se hace reaccionar entonces con el compuesto 38 para formar el compuesto 40. El compuesto 38 comprende un anillo deseado como se describe con referencia a las fórmulas generales, que incluye, a modo de ejemplo, un anillo de ciclobutilo, ciclopentilo o ciclohexilo, y un grupo saliente adecuado, LG. Los grupos salientes adecuados incluyen cualquier grupo que actúe como grupo saliente para facilitar la adición del anillo al compuesto 36. Grupos salientes adecuados incluyen, pero no se limitan a halógenos, típicamente bromo, cloro o yodo, y grupos tosilato o mesilato. Compuesto 36 se hace reaccionar con compuesto 38 en un disolvente adecuado y típicamente en presencia de una base. Disolventes adecuados incluyen cualquier disolvente que facilite la reacción tales como disolventes apróticos. Disolventes adecuados incluyen, pero no se limitan a DMF, THF, DMSO, acetonitrilo, disolventes clorados, tales como diclorometano y cloroformo, DMA, dioxano, N-metilpirrolidona o combinaciones de los mismos. Bases adecuadas incluyen cualquier base que facilite la reacción tal como un hidruro, típicamente hidruro de sodio, o un carbonato, tal como carbonato de potasio, carbonato de sodio o carbonato de cesio. La reacción puede proseguir a temperatura ambiente, o la mezcla de reacción puede calentarse tal como a una temperatura dentro del intervalo de temperaturas mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como a 50 °C, 100 °C o mayor, según se requiera. A continuación, compuesto 40 se aísla de la mezcla de reacción y se purifica si es necesario.Continuing with reference to Scheme 4, compound 36 is then reacted with compound 38 to form compound 40. Compound 38 comprises a desired ring as described with reference to the general formulas, including, by way of example, a cyclobutyl, cyclopentyl or cyclohexyl ring, and a suitable leaving group, LG. Suitable leaving groups include any group that acts as a leaving group to facilitate ring addition to compound 36. Suitable leaving groups include, but are not limited to halogens, typically bromo, chloro or iodo, and tosylate or mesylate groups. Compound 36 is reacted with compound 38 in a suitable solvent and typically in the presence of a base. Suitable solvents include any solvent that facilitates the reaction such as aprotic solvents. Suitable solvents include, but are not limited to, DMF, THF, DMSO, acetonitrile, chlorinated solvents, such as dichloromethane and chloroform, DMA, dioxane, N-methylpyrrolidone, or combinations thereof. Suitable bases include any base that facilitates the reaction such as a hydride, typically sodium hydride, or a carbonate, such as potassium carbonate, sodium carbonate or cesium carbonate. The reaction can proceed at room temperature, or the reaction mixture can be heated such as at a temperature within the range of temperatures greater than room temperature up to the boiling point of the selected solvent, such as at 50°C, 100°C or higher, as required. Compound 40 is then isolated from the reaction mixture and purified if necessary.
Compuesto 40 se hace reaccionar con un agente reductor 42 adecuado para reducir el resto carbonilo a un grupo hidroxilo. Agentes reductores adecuados incluyen, pero no se limitan a borohidruro de sodio, hidruro de diisobutilaluminio o hidruro de litio y aluminio. La reacción se realiza en un disolvente adecuado para facilitar la reacción, tal como un alcohol, en particular metanol o etanol, THF o dietil éter. La reacción puede proseguir a temperatura ambiente, o la mezcla de reacción puede calentarse tal como a una temperatura mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como a una temperatura de 50 °C, 100 °C o mayor. Alternativamente, la mezcla de reacción se puede enfriar según se requiera, por ejemplo por debajo de 20 °C, por debajo de 10 °C o por debajo de 0 °C. Una vez que se completa la reacción, según lo indicado por una técnica analítica tal como LC-MS, TLC o HPLC, el compuesto producto 44 se aísla y purifica si es necesario, mediante una técnica adecuada tal como cromatografía en columna. Alternativa o adicionalmente se puede aislar compuesto 45 .Compound 40 is reacted with a suitable reducing agent 42 to reduce the carbonyl moiety to a hydroxyl group. Suitable reducing agents include, but are not limited to sodium borohydride, diisobutylaluminum hydride, or lithium aluminum hydride. The reaction is carried out in a suitable solvent to facilitate the reaction, such as an alcohol, in particular methanol or ethanol, THF or diethyl ether. The reaction can proceed at room temperature, or the reaction mixture can be heated such as above room temperature to the boiling point of the selected solvent, such as 50°C, 100°C or higher. Alternatively, the reaction mixture can be cooled as required, for example below 20°C, below 10°C or below 0°C. After the reaction is complete, as indicated by an analytical technique such as LC-MS, TLC or HPLC, product compound 44 is isolated and purified if necessary, by a suitable technique such as column chromatography. Alternatively or additionally compound 45 can be isolated.
Opcionalmente, el compuesto 44, y/o el compuesto 45, puede reaccionar con el compuesto 46 para formar el compuesto 48 y/o el compuesto 49. Compuesto 46 comprende un resto Rx deseado y un grupo saliente, LG, adecuado. Grupos salientes adecuados incluyen cualquier grupo que actuará como un grupo saliente para facilitar la adición del resto Rx a compuesto 44 y/o compuesto 45. Grupos salientes adecuados incluyen, pero no se limitan a halógenos, típicamente bromo, cloro o yodo, y grupos tosilato o mesilato. Compuesto 44/45 se hace reaccionar con compuesto 46 en un disolvente adecuado y típicamente en presencia de una base u otro reactivo o reactivos que facilitan la reacción. Disolventes adecuados incluyen cualquier disolvente que facilite la reacción tales como disolventes apróticos. Disolventes adecuados incluyen, pero no se limitan a DMF, THF, DMSO, acetonitrilo, disolventes clorados, tales como diclorometano y cloroformo, DMA, dioxano, N-metilpirrolidona o combinaciones de los mismos. Bases o reactivos adecuados que facilitan la reacción incluyen, pero no se limitan a triflato de plata, 2,6-di-t-butilpiridina, hidruro de sodio o combinaciones de los mismos. Típicamente, compuesto 46 se añade lentamente a la mezcla de reacción. Se puede utilizar enfriamiento, tal como mediante un baño de hielo, para mantener la temperatura de reacción dentro de un intervalo adecuado, tal como desde aproximadamente 0 °C hasta menos de 50 °C, desde 0 °C hasta 20 °C o desde 0 °C. hasta 10 °C. Una vez que se completa la adición de 46, se permite que la reacción prosiga hasta que la reacción se complete sustancialmente y se puede dejar calentar a temperatura ambiente, o la reacción se puede calentar a una temperatura dentro del intervalo mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como 50 °C, 100 °C o mayor, para facilitar la reacción. Una vez completada la reacción, como puede indicarse mediante una técnica analítica, tal como LC-MS, TLC o HPLC, el compuesto producto 48 y/o compuesto 49 se aísla y purifica si es necesario, mediante una técnica adecuada, tal como cromatografía en columna.Optionally, compound 44, and/or compound 45, can be reacted with compound 46 to form compound 48 and/or compound 49. Compound 46 comprises a desired Rx moiety and a suitable leaving group, LG. Suitable leaving groups include any group that will act as a leaving group to facilitate the addition of the Rx moiety to compound 44 and/or compound 45. Suitable leaving groups include, but are not limited to halogen, typically bromo, chloro or iodo, and tosylate or mesylate groups. Compound 44/45 is reacted with compound 46 in a suitable solvent and typically in the presence of a base or other reagent(s) that facilitate the reaction. Suitable solvents include any solvent that facilitates the reaction such as aprotic solvents. Suitable solvents include, but are not limited to, DMF, THF, DMSO, acetonitrile, chlorinated solvents, such as dichloromethane and chloroform, DMA, dioxane, N-methylpyrrolidone, or combinations thereof. Suitable bases or reagents that facilitate the reaction include, but are not limited to, silver triflate, 2,6-di-t-butylpyridine, sodium hydride, or combinations thereof. Typically, compound 46 is slowly added to the reaction mixture. Cooling, such as by using an ice bath, may be used to maintain the reaction temperature within a suitable range, such as from about 0°C to less than 50°C, from 0°C to 20°C, or from 0°C. °C up to 10°C. Once the addition of 46 is complete, the reaction is allowed to proceed until the reaction is substantially complete and can be allowed to warm to room temperature, or the reaction can be warmed to a temperature within the range greater than room temperature until the boiling point of the selected solvent, such as 50°C, 100°C or higher, to facilitate the reaction. After the reaction is complete, as can be indicated by an analytical technique, such as LC-MS, TLC or HPLC, the product compound 48 and/or compound 49 is isolated and purified if necessary, by a suitable technique, such as chromatography on column.
Otra ruta sintética ejemplar para el compuesto 48 y/o el compuesto 49 se ilustra en el Esquema 5.Another exemplary synthetic route for compound 48 and/or compound 49 is illustrated in Scheme 5.
Con referencia al Esquema 5, compuesto 36 se disuelve en un disolvente adecuado con enfriamiento y se trata con una base tal como hidruro de sodio. Disolventes adecuados incluyen, pero no se limita a disolventes apróticos, tales como 1,4-dioxano, THF, DMF, acetonitrilo, éter o una combinación de los mismos. Se añade el compuesto de ciclohexilo 37, que tiene un grupo saliente de 4-nitrobencenosulfona (nosilo, o Nos), y la reacción se calienta a una temperatura adecuada para facilitar una reacción, tal como a una temperatura dentro de un intervalo mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como de 50°C a 200°C o mayor, típicamente de 90°C a 150°C. La reacción se puede agitar, tal como agitando manualmente o agitando mecánicamente. Se puede añadir compuesto 37 adicional si es necesario, para facilitar que la reacción prosiga hasta su compleción. Referring to Scheme 5, compound 36 is dissolved in a suitable solvent with cooling and treated with a base such as sodium hydride. Suitable solvents include, but are not limited to, aprotic solvents, such as 1,4-dioxane, THF, DMF, acetonitrile, ether, or a combination thereof. Cyclohexyl compound 37, which has a 4-nitrobenzenesulfone (nosyl, or Nos) leaving group, is added, and the reaction is heated to a suitable temperature to facilitate a reaction, such as to a temperature within a range higher than the room temperature to the boiling point of the selected solvent, such as 50°C to 200°C or higher, typically 90°C to 150°C. The reaction can be stirred, such as by hand stirring or mechanical stirring. Additional compound 37 can be added if necessary to facilitate the reaction to proceed to completion.
La mezcla de reacción se extingue tal como mediante la adición de solución de bicarbonato de sodio, y los productos se extraen en un disolvente orgánico tal como acetato de etilo o cloroformo. Los compuestos 48 y 49 se pueden separar y/o purificar mediante cualquier técnica adecuada, o combinación de técnicas, tal como cromatografía o trituración.The reaction mixture is quenched such as by adding sodium bicarbonate solution, and the products are extracted into an organic solvent such as ethyl acetate or chloroform. Compounds 48 and 49 can be separated and/or purified by any suitable technique, or combination of techniques, such as chromatography or trituration.
El compuesto de 4-nitrobencensulfonato 37 se puede preparar de acuerdo con una ruta sintética ejemplar de acuerdo con el Esquema 6. The 4-nitrobenzenesulfonate compound 37 can be prepared according to an exemplary synthetic route according to Scheme 6.
Con referencia al Esquema 6, 1,4-dioxaespiro[4.5]decan-8-ol 37-1 se trata primero con una base, tal como hidruro de sodio o terc-butóxido de potasio, en un disolvente adecuado, y después con RX-LG, para formar los compuestos 37 2 , en los que LG es un grupo saliente adecuado, tal como cloruro, bromuro, yoduro, tosilato o mesilato. Disolventes adecuados incluyen, pero no se limitan a disolventes apróticos, tales como THF, DMF, acetonitrilo, dioxano, éter o una combinación de los mismos. Después de que la reacción haya proseguido sustancialmente hasta la compleción, se añade ácido acuoso tal como HCl, para extinguir la reacción y formar compuesto 37-3.Referring to Scheme 6, 1,4-dioxaspiro[4.5]decan-8-ol 37-1 is first treated with a base, such as sodium hydride or potassium tert-butoxide, in a suitable solvent, and then with RX -LG, to form compounds 37 2 , where LG is a suitable leaving group, such as chloride, bromide, iodide, tosylate or mesylate. Suitable solvents include, but are not limited to, aprotic solvents, such as THF, DMF, acetonitrile, dioxane, ether, or a combination thereof. After the reaction has gone substantially to completion, aqueous acid such as HCl is added to quench the reaction to form compound 37-3.
Compuesto 37-3 se trata luego con un agente reductor para formar compuesto 37-4.. El compuesto 37-4 puede ser sustancialmente un isómero o, alternativamente, un compuesto 37-4 puede ser una mezcla de isómeros cis y trans, y en algunas realizaciones, el compuesto 37-4 comprende una mezcla 2:1 de isómeros cis: trans. El agente reductor puede ser cualquier agente que pueda reducir el resto carbonilo a un resto alcohol. Agentes reductores adecuados incluyen, pero no se limitan a hidruro de litio y aluminio, hidruro de diisobutilaluminio, borano-THF o un reactivo de borohidruro tal como borohidruro de sodio. Disolventes adecuados para facilitar la reacción incluyen, pero no se limitan a THF, éter o una combinación de los mismos.Compound 37-3 is then treated with a reducing agent to form Compound 37-4. Compound 37-4 may be substantially one isomer or, alternatively, Compound 37-4 may be a mixture of cis and trans isomers, and in In some embodiments, compound 37-4 comprises a 2:1 mixture of cis:trans isomers. The reducing agent can be any agent that can reduce the carbonyl moiety to an alcohol moiety. Suitable reducing agents include, but are not limited to lithium aluminum hydride, diisobutylaluminum hydride, borane-THF, or a borohydride reagent such as sodium borohydride. Suitable solvents to facilitate the reaction include, but are not limited to THF, ether, or a combination thereof.
Compuesto 37-4 se trata con cloruro de 4-nitrobencenosulfonilo (nosilo) en presencia de una base, para formar compuesto 37. La base puede ser cualquier base adecuada que facilite la reacción y puede ser una base orgánica, tal como trimetilamina, 1,4-diazabiciclo[2.2.2]octano (DABCO), piridina o base de Hunig; o una base inorgánica, tal como carbonato de potasio, carbonato de sodio o carbonato de cesio. La reacción puede proseguir en un disolvente adecuado, típicamente un disolvente aprótico, tal como piridina, THF o un disolvente clorado, tal como diclorometano o cloroformo. Compuesto 37 puede ser sustancialmente un isómero o, alternativamente, compuesto 37 puede ser una mezcla de isómeros. La relación de isómeros puede modificarse mediante una técnica adecuada, tal como cromatografía o trituración. En algunas realizaciones, la relación de isómeros se modifica a alrededor de 8:1 cis a trans por trituración.Compound 37-4 is treated with 4-nitrobenzenesulfonyl (nosyl) chloride in the presence of base to form compound 37. The base can be any suitable base that facilitates the reaction and can be an organic base, such as trimethylamine, 1, 4-diazabicyclo[2.2.2]octane (DABCO), pyridine or Hunig's base; or an inorganic base, such as potassium carbonate, sodium carbonate, or cesium carbonate. The reaction can proceed in a suitable solvent, typically an aprotic solvent, such as pyridine, THF, or a chlorinated solvent, such as dichloromethane or chloroform. Compound 37 can be substantially one isomer or, alternatively, Compound 37 can be a mixture of isomers. The isomer ratio can be modified by a suitable technique, such as chromatography or trituration. In some embodiments, the isomer ratio is changed to about 8:1 cis to trans by trituration.
Alternativamente, compuesto 40 se puede preparar de acuerdo con una vía de síntesis ejemplar de acuerdo con el Esquema 7. Alternatively, compound 40 can be prepared according to an exemplary synthetic route according to Scheme 7.
Con respecto al Esquema 7, compuesto 36 se hace reaccionar con compuesto 50 para formar compuesto 52. Compuesto 50 comprende un anillo deseado, tal como un anillo de ciclobutilo, ciclopentilo o ciclohexilo, un grupo saliente LG, adecuado y un resto carbonilo protegido, tal como un acetal o un cetal. En el ejemplo anterior se muestra un resto cetal cíclico. Los grupos salientes adecuados incluyen cualquier grupo que actúe como grupo saliente para facilitar la adición del anillo al compuesto 36, e incluyen, pero no se limitan a, halógenos, típicamente bromo, cloro o yodo, y grupos tosilato o mesilato. Compuesto 36 se hace reaccionar con compuesto 50 en un disolvente adecuado y típicamente en presencia de una base. Disolventes adecuados incluyen cualquier disolvente que facilite la reacción tales como disolventes apróticos. Disolventes adecuados incluyen, pero no se limitan a DMF, THF, DMSO, acetonitrilo, disolventes clorados, tales como diclorometano y cloroformo, DMA, dioxano, N-metilpirrolidona o combinaciones de los mismos. Bases adecuadas incluyen cualquier base que facilite las reacciones tal, como un hidruro, típicamente hidruro de sodio, o un carbonato, tal como carbonato de potasio, carbonato de sodio o carbonato de cesio. La reacción puede proseguir a temperatura ambiente o, alternativamente, la mezcla de reacción puede calentarse tal como a una temperatura dentro del intervalo de temperaturas mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como a 50 °C, 100 °C o mayor, según se requiera. A continuación, compuesto 52 se aísla de la mezcla de reacción y se purifica, si se requiere, mediante una técnica adecuada tal como cromatografía en columna.Referring to Scheme 7, compound 36 is reacted with compound 50 to form compound 52. Compound 50 comprises a desired ring, such as a cyclobutyl, cyclopentyl, or cyclohexyl ring, a suitable leaving group LG, and a protected carbonyl moiety, such as as an acetal or a ketal. A cyclic ketal moiety is shown in the example above. Suitable leaving groups include any group that acts as a leaving group to facilitate ring addition to compound 36, and include, but are not limited to, halogens, typically bromo, chloro, or iodo, and tosylate or mesylate groups. Compound 36 is reacted with compound 50 in a suitable solvent and typically in the presence of base. Suitable solvents include any solvent that facilitates the reaction such as aprotic solvents. Suitable solvents include, but are not limited to, DMF, THF, DMSO, acetonitrile, chlorinated solvents, such as dichloromethane and chloroform, DMA, dioxane, N-methylpyrrolidone, or combinations thereof. Suitable bases include any base that facilitates the reactions such as a hydride, typically sodium hydride, or a carbonate, such as potassium carbonate, sodium carbonate or cesium carbonate. The reaction can proceed at room temperature or, alternatively, the reaction mixture can be heated such as at a temperature within the range of temperatures greater than room temperature to the boiling point of the selected solvent, such as at 50 °C, 100 °C C or greater, as required. Compound 52 is then isolated from the reaction mixture and purified, if required, by a suitable technique such as column chromatography.
Compuesto 52 se hace reaccionar luego con un reactivo 54 adecuado para formar compuesto 40. Reactivo 54 puede ser cualquier reactivo adecuado para eliminar el grupo protector y/o formar el resto carbonilo. En la síntesis ejemplar mostrada en el Esquema 5, el grupo protector es un cetal cíclico, y reactivos 54 adecuados incluyen, pero no se limitan a tosilato de piridinio (PPTS), ácido para-toluenosulfónico, ácido clorhídrico o ácido acético. La reacción se realiza en un disolvente o mezcla de disolventes adecuados para facilitar la reacción, tal como acetona, THF, ácido acético, agua o una combinación de los mismos. La reacción puede proseguir a temperatura ambiente o, alternativamente, la mezcla de reacción puede calentarse tal como a una temperatura dentro del intervalo de temperaturas mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como a 50 °C, 100 °C o mayor, según se requiera. A continuación, compuesto 40 se aísla de la mezcla de reacción y se purifica, si se requiere, mediante una técnica adecuada tal como cromatografía en columna.Compound 52 is then reacted with a suitable reagent 54 to form compound 40. Reagent 54 can be any suitable reagent to remove the protecting group and/or form the carbonyl moiety. In the exemplary synthesis shown in Scheme 5, the protecting group is a cyclic ketal, and suitable reagents include, but are not limited to, pyridinium tosylate (PPTS), para-toluenesulfonic acid, hydrochloric acid, or acetic acid. The reaction is carried out in a suitable solvent or solvent mixture to facilitate the reaction, such as acetone, THF, acetic acid, water, or a combination thereof. The reaction can proceed at room temperature or, alternatively, the reaction mixture can be heated such as at a temperature within the range of temperatures greater than room temperature to the boiling point of the selected solvent, such as at 50 °C, 100 °C C or greater, as required. Compound 40 is then isolated from the reaction mixture and purified, if required, by a suitable technique such as column chromatography.
A continuación, se proporciona una 2a etapa de la secuencia de reacción ejemplar de acuerdo con el Esquema 8.An exemplary 2nd step reaction sequence according to Scheme 8 is provided below.
El compuesto 48 se hace reaccionar con un agente reductor 56 adecuado, para reducir el resto nitro a una amina. En determinadas realizaciones en las que el compuesto producto deseado comprende un resto hidroxilo, se puede utilizar compuesto 44 en lugar de compuesto 48. Agentes reductores adecuados incluyen, pero no se limitan a: hidrógeno gaseoso en presencia de un catalizador tal como un catalizador de paladio; un borohidruro tal como borohidruro de sodio, opcionalmente en presencia de un catalizador tal como un catalizador de níquel; zinc metálico en ácido acético; o polvo de hierro en agua o agua y ácido. En determinadas realizaciones, se utiliza hidrógeno gaseoso, en presencia de un catalizador de paladio sobre carbono, y en un disolvente adecuado, tal como acetato de etilo o metanol. En algunas realizaciones, se utiliza una combinación de agentes y/o técnicas reductoras. Por ejemplo, la reducción puede realizarse inicialmente utilizando un primer método que comprende un primer agente y/o técnica reductora, pero da como resultado una mezcla de productos. El primer método puede repetirse y/o puede realizarse un segundo método, que comprende un segundo agente y/o técnica reductora. Una vez que se completa la reacción, según se indica por una técnica analítica tal como LC-MS, TLC o HPLC, el compuesto producto 58 se aísla y se purifica si es necesario. Compound 48 is reacted with a suitable reducing agent 56, to reduce the nitro moiety to an amine. In certain embodiments where the desired product compound comprises a hydroxyl moiety, compound 44 may be used in place of compound 48. Suitable reducing agents include, but are not limited to: hydrogen gas in the presence of a catalyst such as a palladium catalyst ; a borohydride such as sodium borohydride, optionally in the presence of a catalyst such as a nickel catalyst; metallic zinc in acetic acid; or iron powder in water or water and acid. In certain embodiments, hydrogen gas is used, in the presence of a palladium-on-carbon catalyst, and in a suitable solvent, such as ethyl acetate or methanol. In some embodiments, a combination of reducing agents and/or techniques is used. For example, the reduction can initially carried out using a first method that comprises a first reducing agent and/or technique, but results in a mixture of products. The first method can be repeated and/or a second method can be performed, comprising a second reducing agent and/or technique. Once the reaction is complete, as indicated by an analytical technique such as LC-MS, TLC, or HPLC, product compound 58 is isolated and purified if necessary.
Compuesto 58 se hace reaccionar con un ácido carboxílico 60 para formar amida 62. El ácido carboxílico 60 se activa mediante cualquier método adecuado y luego se hace reaccionar con el grupo funcional amina de compuesto 58. Los métodos de activación adecuados incluyen, pero no se limitan a: formar el cloruro de ácido por tratamiento con cloruro de tionilo; tratamiento con hexafluorofosfato de 1-[bis(dimetilamino)metilen]-1H-1,2,3-triazolo[4,5-b]piridinio 3-óxido (HATU) y una base tal como diisopropiletilamina (DIPEA); por tratamiento con carbonildiimidazol (CDI); o tratamiento con una carbodiimida, tal como diciclohexilcarbodiimida (DCC) o 1 -etil-3-(3-dimetilaminopropil)carbodiimida (EDC). Compound 58 is reacted with a carboxylic acid 60 to form amide 62. Carboxylic acid 60 is activated by any suitable method and then reacted with the amine functional group of compound 58. Suitable activation methods include, but are not limited to a: form the acid chloride by treatment with thionyl chloride; treatment with 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) and a base such as diisopropylethylamine (DIPEA); by treatment with carbonyldiimidazole (CDI); or treatment with a carbodiimide, such as dicyclohexylcarbodiimide (DCC) or 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC).
Compuesto 62 se acopla después con compuesto 64 para formar compuesto 66 utilizando cualquier reacción de acoplamiento adecuada para formar un enlace entre dos anillos. En el ejemplo anterior se muestra un acoplamiento de éster borónico, en que el grupo saliente LG en compuesto 62 es típicamente bromo o yodo. Otros grupos funcionales de acoplamiento adecuados incluyen trialquil estaño o ácidos borónicos. La reacción de acoplamiento transcurre típicamente en presencia de un catalizador adecuado. Para un acoplamiento de éster borónico o ácido borónico, el catalizador suele ser un catalizador de paladio, tal como PdCl2(dppf)2 , Pd[P(Ph)3]2Cl2, acetato de paladio y trifenilfosfina, oCompound 62 is then coupled with compound 64 to form compound 66 using any suitable coupling reaction to form a bond between two rings. A boronic ester coupling is shown in the above example, in which the leaving group LG in compound 62 is typically bromo or iodo. Other suitable coupling functional groups include trialkyl tin or boronic acids. The coupling reaction typically proceeds in the presence of a suitable catalyst. For a boronic ester or boronic acid coupling, the catalyst is usually a palladium catalyst, such as PdCl 2 (dppf) 2 , Pd[P(Ph) 3 ]2Cl2, palladium acetate and triphenylphosphine, or
tetraquis(trifenilfosfina)paladio(0). La reacción se realiza en presencia de una base, tal como carbonato de sodio, potasio o cesio, y se realiza en un disolvente o mezcla de disolventes adecuado, tal como dioxano, dioxano/agua o DME/etanol/agua. La reacción se puede calentar a una temperatura adecuada tal como de 50 °C a 125 °C, típicamente de aproximadamente 100 °C, y/o se agita durante un período de tiempo adecuado, tal como de 1 hora a 3 días, de 6 horas a 24 horas o de 12 horas a 18 horas, para facilitar que la reacción prosiga hasta su compleción. A continuación, compuesto 66 se aísla de la mezcla de reacción y se purifica mediante una técnica adecuada.tetrakis(triphenylphosphine)palladium(0). The reaction is carried out in the presence of a base, such as sodium, potassium or cesium carbonate, and is carried out in a suitable solvent or solvent mixture, such as dioxane, dioxane/water or DME/ethanol/water. The reaction can be heated to a suitable temperature such as 50°C to 125°C, typically about 100°C, and/or stirred for a suitable period of time, such as 1 hour to 3 days, 6 hours to 24 hours or from 12 hours to 18 hours, to allow the reaction to proceed to completion. Compound 66 is then isolated from the reaction mixture and purified by a suitable technique.
Determinadas realizaciones pueden comprender un resto fosfato. El Esquema 9 proporciona una síntesis ejemplar de determinadas realizaciones de este tipo.Certain embodiments may comprise a phosphate moiety. Scheme 9 provides an exemplary summary of certain such embodiments.
El compuesto 68 se hace reaccionar con el compuesto 70 para formar compuesto 72. Compuesto 70 comprende restos Ry deseados y un grupo saliente, LG, adecuado. Restos Ry típicos incluyen, pero no se limitan a alifáticos tales como alquilo, típicamente metilo, etilo, propilo, isopropilo o t-butilo; arilo; heteroalifático; o heterocíclico. Los dos restos Ry pueden ser iguales o diferentes. Grupos salientes adecuados incluyen, pero no se limitan a halógenos, típicamente bromo, cloro o yodo, y grupos tosilato o mesilato. Compuesto 68 se hace reaccionar con compuesto 70 en un disolvente adecuado y típicamente en presencia de una base. Disolventes adecuados incluyen cualquier disolvente que facilite la reacción tales como disolventes apróticos. Disolventes adecuados incluyen, pero no se limitan a DMF, THF, DMSO, acetonitrilo, disolventes clorados, tales como diclorometano y cloroformo, DMA, dioxano, N-metilpirrolidona o combinaciones de los mismos. Bases adecuadas incluyen cualquier base que facilite las reacciones tal, como un hidruro, típicamente hidruro de sodio, o un carbonato, tal como carbonato de potasio, carbonato de sodio o carbonato de cesio. La reacción puede proseguir a temperatura ambiente o, alternativamente, la mezcla de reacción puede calentarse tal como a una temperatura dentro del intervalo de temperaturas mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como a 50 °C, 100 °C o mayor, según se requiera. A continuación, compuesto 72 se aísla de la mezcla de reacción y se purifica si es necesario.Compound 68 is reacted with compound 70 to form compound 72. Compound 70 comprises the desired Ry moieties and a suitable leaving group, LG. Typical Ry moieties include, but are not limited to, aliphatics such as alkyl, typically methyl, ethyl, propyl, isopropyl, or t-butyl; aryl; heteroaliphatic; or heterocyclic. The two Ry moieties may be the same or different. Suitable leaving groups include, but are not limited to halogens, typically bromo, chloro or iodo, and tosylate or mesylate groups. Compound 68 is reacted with compound 70 in a suitable solvent and typically in the presence of base. Suitable solvents include any solvent that facilitates the reaction such as aprotic solvents. Suitable solvents include, but are not limited to, DMF, THF, DMSO, acetonitrile, chlorinated solvents, such as dichloromethane and chloroform, DMA, dioxane, N-methylpyrrolidone, or combinations thereof. Suitable bases include any base that facilitates the reactions such as a hydride, typically sodium hydride, or a carbonate, such as potassium carbonate, sodium carbonate or cesium carbonate. The reaction can proceed at room temperature or, alternatively, the reaction mixture can be heated such as to a temperature within the range of temperatures greater than room temperature. to the boiling point of the selected solvent, such as at 50°C, 100°C or higher, as required. Compound 72 is then isolated from the reaction mixture and purified if necessary.
Compuesto 72 se hace reaccionar luego con compuesto 74 para formar compuesto 76. Compuesto 74 puede ser cualquier compuesto adecuado para formar los restos ácidos en el compuesto 76. Compuesto 74 puede ser un reactivo de carácter ácido, tal como ácido trifluoroacético, ácido clorhídrico o ácido bromhídrico, o puede ser un reactivo de carácter básico, tal como como hidróxido de sodio, hidróxido de litio o hidróxido de potasio. Disolventes adecuados incluyen, pero no se limitan a disolventes clorados, tales como diclorometano y cloroformo, alcoholes, tales como metanol y etanol, agua o combinaciones de los mismos. La reacción puede proseguir a temperatura ambiente o, alternativamente, la mezcla de reacción puede calentarse tal como a una temperatura dentro del intervalo de temperaturas mayor que la temperatura ambiente hasta el punto de ebullición del disolvente seleccionado, tal como a 50 °C, 100 °C o mayor, según se requiera. La mezcla de reacción también se puede enfriar, por ejemplo, por debajo de 20 °C, por debajo de 10 °C, por debajo de 0 °C o menos. Una vez que se completa la reacción, según se indica mediante una técnica analítica tal como LC-MS, TLC o HPLC, el compuesto producto 76 se aísla y se purifica si es necesario, mediante una técnica adecuada tal como agitación o tratamiento con ultrasonidos, en un disolvente o sistema de disolventes adecuado. Disolventes o sistemas de disolventes adecuados incluyen, pero no se limitan a acetona/agua, acetona, dietil éter o alcohol/agua.Compound 72 is then reacted with compound 74 to form compound 76. Compound 74 can be any suitable compound to form the acid moieties in compound 76. Compound 74 can be an acidic reagent, such as trifluoroacetic acid, hydrochloric acid, or hydrobromic, or it can be a basic reagent, such as sodium hydroxide, lithium hydroxide or potassium hydroxide. Suitable solvents include, but are not limited to, chlorinated solvents, such as dichloromethane and chloroform, alcohols, such as methanol and ethanol, water, or combinations thereof. The reaction can proceed at room temperature or, alternatively, the reaction mixture can be heated such as at a temperature within the range of temperatures greater than room temperature to the boiling point of the selected solvent, such as at 50 °C, 100 °C C or greater, as required. The reaction mixture can also be cooled, for example, below 20°C, below 10°C, below 0°C or less. After the reaction is complete, as indicated by an analytical technique such as LC-MS, TLC or HPLC, product compound 76 is isolated and purified if necessary, by a suitable technique such as shaking or sonication, in a suitable solvent or solvent system. Suitable solvents or solvent systems include, but are not limited to, acetone/water, acetone, diethyl ether, or alcohol/water.
El compuesto 76 se hace reaccionar entonces con el compuesto 78 para formar el compuesto de sal 80. El compuesto 78 puede ser cualquier compuesto que proporcione un contraión adecuado (CI) para el compuesto de sal 80, tal como hidróxido de calcio, hidróxido de sodio, hidróxido de potasio, hidróxido de litio, amoníaco, trimetilamina, tris(hidroximetil)aminometano, o un aminoácido tal como lisina o arginina. Un experto en la técnica apreciará que si el contraión CI tiene una sola carga positiva, tal como en Na+, K+, Li+, o NH4+, entonces el compuesto 80 comprenderá dos iones CI, mientras que si el contraión CI tiene dos cargas positivas, tal como en Cl2+, el compuesto 80 comprenderá un ion CI.Compound 76 is then reacted with compound 78 to form salt compound 80. Compound 78 can be any compound that provides a suitable counterion (CI) for salt compound 80, such as calcium hydroxide, sodium hydroxide , potassium hydroxide, lithium hydroxide, ammonia, trimethylamine, tris(hydroxymethyl)aminomethane, or an amino acid such as lysine or arginine. One skilled in the art will appreciate that if the CI counterion has a single positive charge, such as in Na+, K+, Li+, or NH 4 +, then compound 80 will comprise two CI ions, whereas if the CI counterion has two positive charges , as in Cl 2 + , compound 80 will comprise a CI ion.
C. Combinaciones de Agentes TerapéuticosC. Combinations of Therapeutic Agents
Los compuestos de amida de la presente invención se pueden usar solos, en combinación entre sí, o como complemento o en combinación con otras terapias establecidas. En otro aspecto, los compuestos de la presente invención se pueden utilizar en combinación con otros agentes terapéuticos útiles para el trastorno o la afección que se esté tratando. Estos compuestos pueden administrarse simultáneamente, secuencialmente en cualquier orden, por la misma vía de administración o por una vía diferente.The amide compounds of the present invention can be used alone, in combination with each other, or as an adjunct to or in combination with other established therapies. In another aspect, the compounds of the present invention may be used in combination with other therapeutic agents useful for the disorder or condition being treated. These compounds can be administered simultaneously, sequentially in any order, by the same route of administration or by a different route.
En algunas realizaciones, el segundo agente terapéutico es un analgésico, un antibiótico, un anticoagulante, un anticuerpo, un agente antiinflamatorio, un inmunosupresor, un agonista de guanilato ciclasa-C, un secretagogo intestinal, un antiviral, anticancerígeno, antifúngico o un combinación de los mismos. El agente antiinflamatorio puede ser un esteroide o un agente antiinflamatorio no esteroide. En determinadas realizaciones, el agente antiinflamatorio no esteroide se selecciona de aminosalicilatos, inhibidores de la ciclooxigenasa, diclofenaco, etodolaco, famotidina, fenoprofeno, flurbiprofeno, ketoprofeno, ketorolaco, ibuprofeno, indometacina, meclofenamato, ácido mefenámico, meloxicam, nambumetona, naproxeno, oxaprozina, piroxicam, salsalato, sulindac, tolmetina o una combinación de los mismos. En algunas realizaciones, el inmunosupresor es mercaptopurina, un corticosteroide, un agente alquilante, un inhibidor de calcineurina, un inhibidor de inosina monofosfato deshidrogenasa, globulina antilinfocítica, globulina antitimcítica, un anticuerpo anti-células T o una combinación de los mismos. En una realización, el anticuerpo es infliximab.In some embodiments, the second therapeutic agent is an analgesic, an antibiotic, an anticoagulant, an antibody, an anti-inflammatory agent, an immunosuppressant, a guanylate cyclase-C agonist, an intestinal secretagogue, an antiviral, anticancer, antifungal, or a combination of the same. The anti-inflammatory agent can be a steroid or a non-steroidal anti-inflammatory agent. In certain embodiments, the non-steroidal anti-inflammatory agent is selected from aminosalicylates, cyclooxygenase inhibitors, diclofenac, etodolac, famotidine, fenoprofen, flurbiprofen, ketoprofen, ketorolac, ibuprofen, indomethacin, meclofenamate, mefenamic acid, meloxicam, nambumetone, naproxen, oxaprozin, piroxicam, salsalate, sulindac, tolmetin, or a combination thereof. In some embodiments, the immunosuppressant is mercaptopurine, a corticosteroid, an alkylating agent, a calcineurin inhibitor, an inosine monophosphate dehydrogenase inhibitor, antilymphocyte globulin, antithymocyte globulin, an anti-T cell antibody, or a combination thereof. In one embodiment, the antibody is infliximab.
En algunas realizaciones, los presentes compuestos pueden utilizarse con otros agentes anticancerígenos o citotóxicos. Diversas clases de compuestos anticancerígenos y antineoplásicos incluyen, pero no se limitan a agentes alquilantes, antimetabolitos, inhibidores de BCL-2, alquiloides de vinca, taxanos, antibióticos, enzimas, citoquinas, complejos de coordinación de platino, inhibidores de proteasoma, ureas sustituidas, inhibidores de quinasa, hormonas y antagonistas de hormonas, y agentes hipometilantes, por ejemplo inhibidores de DNMT, tales como azacitidina y decitabina. Ejemplos de agentes alquilantes incluyen, sin limitación, meclorotamina, ciclofosfamida, ifosfamida, melfalán, clorambucilo, etileniminas, metilmelaminas, sulfonatos de alquilo (p. ej., busulfano) y carmustina. Antimetabolitos ejemplares incluyen, a modo de ejemplo y no de limitación, metotrexato análogo del ácido fólico; fluorouracilo análogo de pirimidina, arabinósido de citosina; análogos de purina mercaptopurina, tioguanina y azatioprina. Alquiloides de vinca ejemplares incluyen, a modo de ejemplo y no de limitación, vinblastina, vincristina, paclitaxel y colchicina. Ejemplos de antibióticos incluyen, a modo de ejemplo y no de limitación, actinomicina D, daunorrubicina y bleomicina. Una enzima ejemplar eficaz como un agente antineoplásico incluye L-asparaginasa. Compuestos de coordinación ejemplares incluyen, a modo de ejemplo y no de limitación, cisplatino y carboplatino. Hormonas y compuestos relacionados con hormonas ejemplares incluyen, a modo de ejemplo y no de limitación, adrenocorticosteroides prednisona y dexametasona; inhibidores de la aromatasa amino glutetimida, formestano y anastrozol; compuestos de progestina caproato de hidroxiprogesterona, medroxiprogesterona; y el compuesto antiestrógeno tamoxifeno. In some embodiments, the present compounds can be used with other anticancer or cytotoxic agents. Various classes of anticancer and antineoplastic compounds include, but are not limited to, alkylating agents, antimetabolites, BCL-2 inhibitors, vinca alkyloids, taxanes, antibiotics, enzymes, cytokines, platinum coordination complexes, proteasome inhibitors, substituted ureas, kinase inhibitors, hormones and hormone antagonists, and hypomethylating agents, for example DNMT inhibitors, such as azacitidine and decitabine. Examples of alkylating agents include, without limitation, mechlorotamine, cyclophosphamide, ifosfamide, melphalan, chlorambucil, ethyleneimines, methylmelamines, alkyl sulfonates (eg, busulfan), and carmustine. Exemplary antimetabolites include, by way of example and not limitation, folic acid analog methotrexate; fluorouracil pyrimidine analog, cytosine arabinoside; purine analogues mercaptopurine, thioguanine and azathioprine. Exemplary vinca alkyloids include, by way of example and not limitation, vinblastine, vincristine, paclitaxel, and colchicine. Examples of antibiotics include, by way of example and not limitation, actinomycin D, daunorubicin, and bleomycin. An exemplary enzyme effective as an antineoplastic agent includes L-asparaginase. Exemplary coordination compounds include, by way of example and not limitation, cisplatin and carboplatin. Exemplary hormones and hormone-related compounds include, by way of example and not limitation, adrenocorticosteroids prednisone and dexamethasone; aromatase inhibitors amino glutethimide, formestane and anastrozole; progestin compounds hydroxyprogesterone caproate, medroxyprogesterone; and the anti-estrogen compound tamoxifen.
Estos y otros compuestos anticancerígenos útiles se describen en Merck Index, 13a Ed. (O'Neil M. J. et al., ed.) Merck Publishing Group (2001) y Goodman and Gilman's The Pharmacological Basis of Therapeutics, 12a Edición, Brunton L.L. ed., Capítulos 60-63, McGraw Hill, (2011).These and other useful anticancer compounds are described in the Merck Index, 13th Ed. (O'Neil M.J. et al., ed.) Merck Publishing Group (2001) and Goodman and Gilman's The Pharmacological Basis of Therapeutics, 12th Edition, Brunton L.L. ed., Chapters 60-63, McGraw Hill, (2011).
Entre los anticuerpos CTLA 4 que se pueden utilizar en combinación con los inhibidores actualmente descritos se encuentra ipilimumab, comercializado como YERVOY® por Bristol-Myers Squibb.Among the CTLA 4 antibodies that can be used in combination with currently described inhibitors is ipilimumab, marketed as YERVOY® by Bristol-Myers Squibb.
Otros agentes quimioterapéuticos para combinación incluyen agentes de inmunooncología tales como inhibidores de la vía del punto de control, por ejemplo, inhibidores de PD-1, tales como nivolumab y lambrolizumab, e inhibidores de PD-L1, tales como pembrolizumab, MEDI-4736 y MPDL3280NRG7446. Los inhibidores de puntos de control adicionales para la combinación con los compuestos descritos aquí incluyen agentes Anti-LAG-3, tales como BMS-986016 (MDX-1408).Other combination chemotherapeutic agents include immuno-oncology agents such as checkpoint pathway inhibitors, for example, PD-1 inhibitors, such as nivolumab and lambrolizumab, and PD-L1 inhibitors, such as pembrolizumab, MEDI-4736, and MPDL3280N RG7446. Additional checkpoint inhibitors for combination with the compounds described herein include Anti-LAG-3 agents, such as BMS-986016 (MDX-1408).
Otros agentes quimioterapéuticos para combinar con los inhibidores descritos en la presente incluyen agentes anti-SLAMF7, tal como el anticuerpo monoclonal humanizado elotuzumab (BMS-901608), agentes anti-KIR, tal como el anticuerpo monoclonal anti-KIR lirilumab (BMS-986015), y agentes anti-CD137, tal como el anticuerpo monoclonal completamente humano urelumab (BMS-663513).Other chemotherapeutic agents to combine with the inhibitors described herein include anti-SLAMF7 agents, such as the humanized monoclonal antibody elotuzumab (BMS-901608), anti-KIR agents, such as the anti-KIR monoclonal antibody lirilumab (BMS-986015) , and anti-CD137 agents, such as the fully human monoclonal antibody urelumab (BMS-663513).
Los compuestos antiproliferativos adicionales útiles en combinación con los compuestos de la presente invención incluyen, a modo de ejemplo y no de limitación, anticuerpos dirigidos contra los receptores del factor de crecimiento (por ejemplo, anti-Her2); y citoquinas tales como interferón-a e interferón-Y, interleuquina-2 y GM-CSF.Additional antiproliferative compounds useful in combination with the compounds of the present invention include, by way of example and not limitation, antibodies directed against growth factor receptors (eg, anti-Her2); and cytokines such as interferon-a and interferon-Y, interleukin-2, and GM-CSF.
Los agentes quimioterapéuticos adicionales útiles en combinación con los presentes compuestos de amida incluyen inhibidores de proteasoma, tales como bortezomib, carfilzomib, marizomib, y similares.Additional chemotherapeutic agents useful in combination with the present amide compounds include proteasome inhibitors, such as bortezomib, carfilzomib, marizomib, and the like.
Ejemplos de inhibidores de quinasa que son útiles en combinación con los compuestos descritos en esta memoria, particularmente en el tratamiento de tumores malignos, incluyen inhibidores de Btk tales como ibrutinib, inhibidores de CDK tales como palbociclib, inhibidores de EGFR, tales como afatinib, erlotinib, gefitinib, lapatinib, osimertinib y vandetinib, inhibidores de Mek, tales como trametinib, inhibidores de Raf, tales como dabrafenib, sorafenib y vemurafenib, inhibidores de VEGFR, tales como axitinib, lenvatinib, nintedanib, pazopanib, inhibidores de BCR-Abl, tales como bosutinib, dasatinib, imatinib y nilotinib, inhibidores de Syk, tales como fostamatinib, e inhibidores de JAK tal como ruxolitinib. En otras realizaciones, el segundo agente terapéutico se puede seleccionar de cualquiera de los siguientes:Examples of kinase inhibitors that are useful in combination with the compounds described herein, particularly in the treatment of malignancies, include Btk inhibitors such as ibrutinib, CDK inhibitors such as palbociclib, EGFR inhibitors such as afatinib, erlotinib , gefitinib, lapatinib, osimertinib and vandetinib, Mek inhibitors such as trametinib, Raf inhibitors such as dabrafenib, sorafenib and vemurafenib, VEGFR inhibitors such as axitinib, lenvatinib, nintedanib, pazopanib, BCR-Abl inhibitors such such as bosutinib, dasatinib, imatinib, and nilotinib, Syk inhibitors such as fostamatinib, and JAK inhibitors such as ruxolitinib. In other embodiments, the second therapeutic agent can be selected from any of the following:
Analgésicos - morfina, fentanilo, hidromorfona, oxicodona, codeína, acetaminofeno, hidrocodona, buprenorfina, tramadol, venlafaxina, flupirtina, meperidina, pentazocina, dextromoramida, dipipanona; Analgesics - morphine, fentanyl, hydromorphone, oxycodone, codeine, acetaminophen, hydrocodone, buprenorphine, tramadol, venlafaxine, flupirtine, meperidine, pentazocine, dextromoramide, dipipanone;
antibióticos-aminoglicósidos (p. ej., amikacina, gentamicina, kanamicina, neomicina, netilmicina, tobramicina y paromicina), carbapenémicos (p. ej., ertapenem, doripenem, imipenem, cilastatina y meropenem), cefalosporinas (p. ej., cefadroxilo, cefazolina, cefalotina, cefalexina, cefaclor, cefamandol, cefoxitina, cefprozil, cefuroxima, cefixima, cefdinir, cefditoren, cefoperazona, cefotaxima, cefpodoxima, ceftazidima, ceftibuten, ceftizoxima, ceftriaxona, cefepima y cefobiprol), glicopéptidos (p. ej., teicoplanina, vancomicina y telavancina), lincosamidas (p. ej., clindamicina e incomisina), lipopéptidos (p. ej., daptomicina), macrólidos (azitromicina, claritromicina, diritromicina, eritromicina, roxitromicina, troleandomicina, telitromicina y espectinomicina), monobactámicos (p. ej., aztreonam), nitrofuranos (p. ej., furazolidona y nitrofurantoína), penicilinas (p. ej., amoxicilina, ampicilina, azlocilina, carbenicilina, cloxacilina, dicloxacilina, flucloxacilina, mezlocilina, meticilina, nafcilina, oxacilina, penicilina G, penicilina V, piperacilina, temocilina y ticarcilina), combinaciones de penicilinas (p. ej., amoxicilina/clavulanato, ampicilina/sulbactam, piperacilina/tazobactam y ticarcilina/clavulanato), polipéptidos (p. ej., bacitracina, colistina y polimixina B), quinolonas (p. ej., ciprofloxacina, enoxacina, gatifloxacina, levofloxacina, lomefloxacina, moxifloxacina, ácido nalidíxico, norfloxacina, ofloxacina, trovafloxacina, grepafloxacina, esparfloxacina y temafloxacina), sulfonamidas (p. ej., mafenida, sulfonamidocrisoidina, sulfacetamida, sulfadiazina, sulfadiazina de plata, sulfametoxazol, sulfasalazina, sulfisoxazol, trimetoprim y trimetoprim-sulfametoxazol), tetraciclinas (p. ej., demeclociclina, doxiciclina, minociclina, oxitetraciclina y tetraciclina), compuestos antimicobacterianos (p. ej., clofazimina, dapsona, capreomicina, cicloserina, etambutol, etionamida, isoniazida, pirazinamida, rifampicina (rifampina), rifabutina, rifapentina y estreptomicina) y otros, tales como arsfenamina, cloranfenicol, fosfomicina, ácido fusídico, linezolid, metronidazol, mupirocina, platensimicina, quinuprisina/dalfopristina, rifaximina, tiamfenicol, tigeciclina y timidazol;antibiotics-aminoglycosides (eg, amikacin, gentamicin, kanamycin, neomycin, netilmicin, tobramycin, and paromycin), carbapenems (eg, ertapenem, doripenem, imipenem, cilastatin, and meropenem), cephalosporins (eg, cefadroxil , cefazolin, cephalothin, cephalexin, cefaclor, cefamandole, cefoxitin, cefprozil, cefuroxime, cefixime, cefdinir, cefditoren, cefoperazone, cefotaxime, cefpodoxime, ceftazidime, ceftibuten, ceftizoxime, ceftriaxone, cefepime, and cefobiprol), glycopeptides (eg, teicoplan inna , vancomycin, and telavancin), lincosamides (eg, clindamycin, and incomysin), lipopeptides (eg, daptomycin), macrolides (azithromycin, clarithromycin, dirithromycin, erythromycin, roxithromycin, troleandomycin, telithromycin, and spectinomycin), monobactams (p eg, aztreonam), nitrofurans (eg, furazolidone and nitrofurantoin), penicillins (eg, amoxicillin, ampicillin, azlocillin, carbenicillin, cloxacillin, dicloxacillin, flucloxacillin, mezlocillin, methicillin, nafcillin, oxacillin, penicillin G , penicillin V, piperacillin, temocillin and ticarcillin), combinations of penicillins (eg. g., amoxicillin/clavulanate, ampicillin/sulbactam, piperacillin/tazobactam, and ticarcillin/clavulanate), polypeptides (eg, bacitracin, colistin, and polymyxin B), quinolones (eg, ciprofloxacin, enoxacin, gatifloxacin, levofloxacin, lomefloxacin, moxifloxacin, nalidixic acid, norfloxacin, ofloxacin, trovafloxacin, grepafloxacin, sparfloxacin, and temafloxacin), sulfonamides (eg, mafenide, sulfonamidochrysoidine, sulfacetamide, sulfadiazine, silver sulfadiazine, sulfamethoxazole, sulfasalazine, sulfisoxazole, trimethoprim and trimethoprim-sulfamethoxazole ), tetracyclines (eg, demeclocycline, doxycycline, minocycline, oxytetracycline, and tetracycline), antimycobacterial compounds (eg, clofazimine, dapsone, capreomycin, cycloserine, ethambutol, ethionamide, isoniazid, pyrazinamide, rifampicin (rifampin), rifabutin , rifapentin, and streptomycin) and others, such as arsphenamine, chloramphenicol, fosfomycin, fusidic acid, linezolid, metronidazole, mupirocin, platensimicin, quinuprisin/dalfopristin, rifaximin, thiamphenicol, tigecycline, and thymidazole;
anticuerpos - anticuerpos anti-TNF-a, por ejemplo infliximab (Remicade™), adalimumab, golimumab, certolizumab; anticuerpos anti-células B, por ejemplo rituximab; anticuerpos anti-IL-6, por ejemplo tocilizumab; anticuerpos anti-IL-1, por ejemplo anakinra; anticuerpos anti-PD-1 y/o anti-PD-L1, por ejemplo nivolumab, pembrolizumab, pidilizumab, BMS-936559, MPDL3280A, AMP-224, MEDI4736; ixekizumab, brodalumab, ofatumumab, sirukumab, clenoliximab, clazakiumab, fezakinumab, fletikumab, mavrilimumab, ocrelizumab, sarilumab, secukinumab, toralizumab, zanolimumab; antibodies - anti-TNF-a antibodies, eg infliximab (Remicade™), adalimumab, golimumab, certolizumab; anti-B cell antibodies, for example rituximab; anti-IL-6 antibodies, for example tocilizumab; anti-IL-1 antibodies, for example anakinra; anti-PD-1 and/or anti-PD-L1 antibodies, for example nivolumab, pembrolizumab, pidilizumab, BMS-936559, MPDL3280A, AMP-224, MEDI4736; ixekizumab, brodalumab, ofatumumab, sirukumab, clenoliximab, clazakiumab, fezakinumab, fletikumab, mavrilimumab, ocrelizumab, sarilumab, secukinumab, toralizumab, zanolimumab;
anticoagulantes - warfarina (Coumadin™), acenocumarol, fenprocumona, atromentina, fenindiona, heparina, fondaparinux, idraparinux, rivaroxabán, apixabán, hirudina, lepirudina, bivalirudina, argatrobam, dabigatrán, ximelagatrán, batroxobina, hementinaanticoagulants - warfarin (Coumadin™), acenocoumarol, phenprocoumon, atromentin, phenindione, heparin, fondaparinux, idraparinux, rivaroxaban, apixaban, hirudin, lepirudin, bivalirudin, argatrobam, dabigatran, ximelagatran, batroxobin, hementin
agentes antiinflamatorios - esteroides, p. ej., budesonida, agentes antiinflamatorios no esteroides, p. ej., aminosalicilatos (p. ej., sulfasalazina, mesalamina, olsalazina y balsalazida), inhibidores de la ciclooxigenasa (inhibidores de la COX-2, tales como rofecoxib, celecoxib), diclofenaco, etodolaco, famotidina, fenoprofeno, flurbiprofeno, ketoprofeno, ketorolaco, ibuprofeno, indometacina, meclofenamato, ácido mefenámico, meloxicam, nambumetona, naproxeno, oxaprozina, piroxicam, salsalato, sulindaco, tolmetina; anti-inflammatory agents - steroids, e.g. eg, budesonide, non-steroidal anti-inflammatory agents, e.g. eg, aminosalicylates (eg, sulfasalazine, mesalamine, olsalazine, and balsalazide), cyclooxygenase inhibitors (COX-2 inhibitors, such as rofecoxib, celecoxib), diclofenac, etodolac, famotidine, fenoprofen, flurbiprofen, ketoprofen, ketorolac, ibuprofen, indomethacin, meclofenamate, mefenamic acid, meloxicam, nambumetone, naproxen, oxaprozin, piroxicam, salsalate, sulindac, tolmetin;
inmunosupresores - mercaptopurina, corticosteroides, tales como dexametasona, hidrocortisona, prednisona, metilprednisolona y prednisolona, agentes alquilantes tales como ciclofosfamida, inhibidores de la calcineurina, tales como ciclosporina, sirolimus y tacrolimus, inhibidores de la inosina monofosfato deshidrogenasa (IMPDH), tales como micofenolato, micofenolato mofetil y azatioprina, y agentes diseñados para suprimir la inmunidad celular al tiempo que dejan intacta la respuesta inmunológica humoral del receptor, incluyendo diversos anticuerpos (por ejemplo, globulina antilinfocítica (ALG), globulina antitimocítica (ATG), anticuerpos monoclonales anti-células T (OKT3)) e irradiación. Azatioprina está disponible actualmente de Salix Pharmaceuticals, Inc. bajo el nombre de marca Azasan; mercaptopurina está disponible actualmente de Gate Pharmaceuticals, Inc. bajo el nombre de marca Purinethol; prednisona y prednisolona están actualmente disponibles de Roxane Laboratories, Inc.; metilprednisolona está disponible actualmente de Pfizer; sirolimus (rapamicina) está actualmente disponible de Wyeth-Ayerst bajo el nombre de marca Rapamune; tacrolimus está actualmente disponible de Fujisawa bajo el nombre de marca Prograf; ciclosporina está actualmente disponible de Novartis bajo el nombre de marca Sandimmune y Abbott bajo el nombre de marca Gengraf; inhibidores de la IMPDH, tales como micofenolato de mofetilo y ácido micofenólico, están actualmente disponibles de Roche bajo el nombre de marca Cellcept y Novartis bajo el nombre de marca Myfortic; azatioprina está actualmente disponible de Glaxo Smith Kline bajo el nombre de marca Imuran; y los anticuerpos están actualmente disponibles de Ortho Biotech bajo el nombre de marca Orthoclone, Novartis bajo el nombre de marca Simulect (basiliximab) y Roche bajo el nombre de marca Zenapax (daclizumab); yimmunosuppressants - mercaptopurine, corticosteroids such as dexamethasone, hydrocortisone, prednisone, methylprednisolone, and prednisolone, alkylating agents such as cyclophosphamide, calcineurin inhibitors such as cyclosporine, sirolimus, and tacrolimus, inosine monophosphate dehydrogenase (IMPDH) inhibitors, such as mycophenolate , mycophenolate mofetil, and azathioprine, and agents designed to suppress cellular immunity while leaving the recipient's humoral immune response intact, including various antibodies (eg, antilymphocyte globulin (ALG), antithymocyte globulin (ATG), anti-cell monoclonal antibodies). T(OKT3)) and irradiation. Azathioprine is currently available from Salix Pharmaceuticals, Inc. under the brand name Azasan; Mercaptopurine is currently available from Gate Pharmaceuticals, Inc. under the brand name Purinethol; prednisone and prednisolone are currently available from Roxane Laboratories, Inc.; Methylprednisolone is currently available from Pfizer; sirolimus (rapamycin) is currently available from Wyeth-Ayerst under the brand name Rapamune; tacrolimus is currently available from Fujisawa under the brand name Prograf; Cyclosporine is currently available from Novartis under the brand name Sandimmune and Abbott under the brand name Gengraf; IMPDH inhibitors, such as mycophenolate mofetil and mycophenolic acid, are currently available from Roche under the brand name Cellcept and Novartis under the brand name Myfortic; Azathioprine is currently available from Glaxo Smith Kline under the brand name Imuran; and the antibodies are currently available from Ortho Biotech under the brand name Orthoclone, Novartis under the brand name Simulect (basiliximab) and Roche under the brand name Zenapax (daclizumab); and
agonistas del receptor de guanilato ciclasa-C o secretagogos intestinales - por ejemplo, linaclotida, vendida bajo el nombre Linzess.guanylate cyclase-C receptor agonists or intestinal secretagogues - eg linaclotide, sold under the name Linzess.
Estos diversos agentes se pueden utilizar de acuerdo con sus dosis estándares o comunes, como se especifica en la información de prescripción que acompaña a las formas comercialmente disponibles de los fármacos (véase también la información de prescripción en la edición de 2006 de The Physician's Desk Reference).These various agents can be used according to their standard or common dosages, as specified in the prescribing information accompanying the commercially available forms of the drugs (see also the prescribing information in the 2006 edition of The Physician's Desk Reference). ).
D. Composiciones que comprenden compuestos de amidaD. Compositions Comprising Amide Compounds
Los compuestos de amida descritos se pueden usar solos, en cualquier combinación, y en combinación con, o como complemento de, al menos un segundo agente terapéutico, y además los compuestos de amida y el al menos un segundo agente terapéutico se pueden usar en combinación con cualquier aditivo adecuado útil para formar composiciones para la administración a un sujeto. Los aditivos se pueden incluir en composiciones farmacéuticas para una diversidad de propósitos tales como diluir una composición para administrarla a un sujeto, facilitar el procesamiento de la formulación, proporcionar propiedades materiales ventajosas a la formulación, facilitar la dispersión desde un dispositivo de administración, estabilizar la formulación (p. ej., antioxidantes o tampones), para proporcionar un sabor o consistencia agradable o apetecible a la formulación, o similares. Aditivos típicos incluyen, a modo de ejemplo y sin limitación: excipientes farmacéuticamente aceptables; soportes farmacéuticamente aceptables; y/o adyuvantes tales como mono-, di- y poli-sacáridos, alcoholes de azúcar y otros polioles, tales como lactosa, glucosa, rafinosa, melecitosa, lactitol, maltitol, trehalosa, sacarosa, manitol, almidón o combinaciones de los mismos; tensioactivos, tales como sorbitoles, difosfatidilcolina y lecitina; agentes conferidores de consistencia; tampones, tales como tampones fosfato y citrato; antiadherentes tales como estearato de magnesio; aglutinantes tales como sacáridos (incluyendo disacáridos, tales como sacarosa y lactosa), polisacáridos (tales como almidones, celulosa, celulosa microcristalina, éteres de celulosa (como hidroxipropil celulosa), gelatina, polímeros sintéticos (tales como polivinilpirrolidona, polialquilenglicoles); revestimientos (tales como éteres de celulosa, incluyendo hidroxipropilmetilcelulosa, goma laca, zeína de proteína de maíz y gelatina); auxiliares de liberación (tales como revestimientos entéricos); desintegrantes (tales como crospovidona, carboximetilcelulosa sódica reticulada y glicolato de almidón sódico); cargas (tales como fosfato de calcio dibásico, grasas y aceites vegetales, lactosa, sacarosa, glucosa, manitol, sorbitol, carbonato de calcio y estearato de magnesio); sabores y edulcorantes (tales como menta, cereza, anís, melocotón, albaricoque o regaliz, frambuesa y vainilla ; lubricantes (tales como minerales, ejemplificados por talco o sílice, grasas, ejemplificados por estearina vegetal, estearato de magnesio o ácido esteárico); conservantes (tales como antioxidantes ejemplificados por vitamina A, vitamina E, vitamina C, palmitato de retinilo y selenio, aminoácidos, ejemplificados por cisteína y metionina, ácido cítrico y citrato de sodio, parabenos, ejemplificados por metilparabeno y propilparabeno); colorantes; auxiliares de compresión; agentes emulsionantes; agentes de encapsulación; gomas; agentes de granulación; y combinaciones de los mismos. The described amide compounds can be used alone, in any combination, and in combination with, or as an adjunct to, at least one second therapeutic agent, and furthermore the amide compounds and the at least one second therapeutic agent can be used in combination. with any suitable additive useful to form compositions for administration to a subject. Additives can be included in pharmaceutical compositions for a variety of purposes such as diluting a composition for administration to a subject, facilitating processing of the formulation, providing advantageous material properties to the formulation, facilitating dispersion from a delivery device, stabilizing the formulation (eg, antioxidants or buffers), to provide a pleasant or palatable flavor or consistency to the formulation, or the like. Typical additives include, by way of example and without limitation: pharmaceutically acceptable excipients; pharmaceutically acceptable carriers; and/or adjuvants such as mono-, di-, and polysaccharides, sugar alcohols, and other polyols, such as lactose, glucose, raffinose, melecitose, lactitol, maltitol, trehalose, sucrose, mannitol, starch, or combinations thereof; surfactants, such as sorbitols, diphosphatidylcholine, and lecithin; consistency conferring agents; buffers, such as phosphate and citrate buffers; anti-stick agents such as magnesium stearate; binders such as saccharides (including disaccharides, such as sucrose and lactose), polysaccharides (such as starches, cellulose, microcrystalline cellulose, cellulose ethers (such as hydroxypropyl cellulose), gelatin, synthetic polymers (such as polyvinylpyrrolidone, polyalkylene glycols), coatings (such as cellulose ethers, including hydroxypropylmethylcellulose, shellac, corn protein zein, and gelatin); release aids (such as enteric coatings); disintegrants (such as crospovidone, cross-linked sodium carboxymethylcellulose, and sodium starch glycolate); fillers (such as dibasic calcium phosphate, vegetable fats and oils, lactose, sucrose, glucose, mannitol, sorbitol, calcium carbonate, and magnesium stearate); flavors and sweeteners (such as mint, cherry, anise, peach, apricot or licorice, raspberry, and vanilla lubricants (such as minerals, exemplified by talc or silica, greases, exemplified by vegetable stearin, magnesium stearate or stearic acid); preservatives (such as antioxidants exemplified by vitamin A, vitamin E, vitamin C, retinyl and selenium palmitate, amino acids, exemplified by cysteine and methionine, citric acid and sodium citrate, parabens, exemplified by methylparaben and propylparaben); dyes; compression aids; emulsifying agents; encapsulation agents; gums; granulating agents; and combinations thereof.
III. Métodos de UsoIII. Methods of Use
A. Enfermedades/TrastornosA. Diseases/Disorders
Los compuestos de amida descritos, así como sus combinaciones y/o composiciones, pueden usarse para mejorar, tratar o prevenir una variedad de enfermedades y/o trastornos. En realizaciones particulares, el compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, pueden ser útiles para tratar afecciones en las que la inhibición de una ruta de quinasa asociada al receptor de interleuquina-1 (IRAK) es terapéuticamente útil. En algunas realizaciones, los compuestos inhiben directamente una proteína IRAK, tal como IRAK1, IRAK2, IRAK3, IRAK4 o una combinación de las mismas. En ciertas realizaciones, los compuestos de amida descritos son útiles para tratar, prevenir o mejorar enfermedades autoinmunes, trastornos inflamatorios, enfermedades cardiovasculares, trastornos nerviosos, trastornos neurodegenerativos, trastornos alérgicos, asma, pancreatitis, insuficiencia multiorgánica, enfermedades renales, agregación plaquetaria, cáncer, trasplante, motilidad espermática, deficiencia de eritrocitos, rechazo del injerto, lesiones pulmonares, enfermedades respiratorias, afecciones isquémicas, e infecciones bacterianas y virales.The described amide compounds, as well as their combinations and/or compositions, can be used to ameliorate, treat or prevent a variety of diseases and/or disorders. In particular embodiments, the amide compound, combinations of amide compounds, or compositions thereof, may be useful for treating conditions in which inhibition of an interleukin-1 receptor associated kinase (IRAK) pathway is therapeutically useful. . In some embodiments, the compounds directly inhibit an IRAK protein, such as IRAK1, IRAK 2 , IRAK3, IRAK4, or a combination thereof. In certain embodiments, the described amide compounds are useful for treating, preventing, or ameliorating autoimmune diseases, inflammatory disorders, cardiovascular diseases, nervous disorders, neurodegenerative disorders, allergic disorders, asthma, pancreatitis, multiple organ failure, renal diseases, platelet aggregation, cancer, transplantation, sperm motility, red blood cell deficiency, graft rejection, lung lesions, respiratory diseases, ischemic conditions, and bacterial and viral infections.
En algunas realizaciones, el compuesto de amida, combinaciones de compuestos de amida. o composiciones de los mismos. pueden usarse para tratar o prevenir enfermedades alérgicas, esclerosis lateral amiotrófica (ELA), lupus eritematoso sistémico, artritis reumatoide, diabetes mellitus tipo I, enfermedad inflamatoria intestinal, cirrosis biliar, uveítis, esclerosis múltiple, enfermedad de Crohn, colitis ulcerosa, penfigoide ampolloso, sarcoidosis, psoriasis, miositis autoinmune, granulomatosis de Wegener, ictiosis, oftalmopatía de Graves, o asma.In some embodiments, the amide compound, combinations of amide compounds. or compositions thereof. may be used to treat or prevent allergic diseases, amyotrophic lateral sclerosis (ALS), systemic lupus erythematosus, rheumatoid arthritis, type I diabetes mellitus, inflammatory bowel disease, biliary cirrhosis, uveitis, multiple sclerosis, Crohn's disease, ulcerative colitis, bullous pemphigoid, sarcoidosis, psoriasis, autoimmune myositis, Wegener's granulomatosis, ichthyosis, Graves' ophthalmopathy, or asthma.
El compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, también pueden ser útiles para mejorar, tratar o prevenir trastornos de la regulación inmunitaria relacionados con el rechazo del trasplante de médula ósea o de órganos, o la enfermedad de injerto contra hospedante. Los ejemplos de trastornos de la regulación inflamatoria e inmunitaria que pueden tratarse con los presentes compuestos incluyen, pero no se limitan a, trasplante de órganos o tejidos, enfermedades de injerto contra hospedante provocadas por el trasplante, síndromes autoinmunes que incluyen artritis reumatoide, lupus eritematoso sistémico, tiroiditis de Hashimoto, esclerosis múltiple, esclerosis sistémica, miastenia grave, diabetes tipo I, uveítis, uveítis posterior, encefalomielitis alérgica, glomerulonefritis, enfermedades autoinmunes posinfecciosas que incluyen fiebre reumática y glomerulonefritis posinfecciosa, enfermedades inflamatorias e hiperproliferativas de la piel, psoriasis, dermatitis atópica, dermatitis por contacto, dermatitis eccematosa, dermatitis seborreica, liquen plano, pénfigo, penfigoide ampolloso, epidermólisis ampollosa, urticaria, angioedemas, vasculitis, eritema, eosinofilia cutánea, lupus eritematoso, acné, alopecia areata, queratoconjuntivitis, conjuntivitis vernal, uveítis asociada a la enfermedad de Behcet, queratitis , queratitis herpética, córnea cónica, distrofia epitelial de la córnea, leucoma corneal, pénfigo ocular, úlcera de Mooren, escleritis, oftalmopatía de Graves, síndrome de Vogt-Koyanagi-Harada, sarcoidosis, alergia al polen, enfermedad obstructiva reversible de las vías respiratorias, asma bronquial, asma alérgica, asma intrínseca, asma extrínseca, asma por polvo, asma crónica o inveterada, asma tardía e hiperreactividad de las vías respiratorias, bronquitis, úlceras gástricas, daño vascular causado por enfermedades isquémicas y trombosis, enfermedades isquémicas del intestino, enfermedades inflamatorias del intestino, enterocolitis necrosante, lesiones intestinales asociadas con quemaduras térmicas, celiaquías, proctitis, gastroenteritis eosinofílica, mastocitosis, enfermedad de Crohn, colitis ulcerosa, migraña, rinitis, eccema, nefritis intersticial, síndrome de Goodpasture, síndrome urémico hemolítico, nefropatía diabética, miositis múltiple, síndrome de Guillain-Barré, enfermedad de Meniere, polineuritis, neuritis múltiple, mononeuritis, radiculopatía, hipertiroidismo, enfermedad de Basedow, aplasia pura de glóbulos rojos, anemia aplásica, anemia hipoplásica, púrpura trombocitopénica idiopática, anemia hemolítica autoinmune, agranulocitosis, anemia perniciosa, anemia megaloblástica, aneritroplasia, osteoporosis, sarcoidosis, pulmón fibroide, neumonía intersticial idiopática, dermatomiositis, leucodermia vulgar, ictiosis vulgar, sensibilidad fotoalérgica, linfoma cutáneo de células T, leucemia linfocítica crónica, arteriosclerosis, aterosclerosis, síndrome de aortitis, poliarteritis nodosa, miocardosis, esclerodermia, granuloma de Wegener, síndrome de Sjogren, adiposis, fascitis eosinofílica, lesiones de las encías, periodonto, hueso alveolar, sustancia ósea dental, glomerulonefritis, alopecia de patrón masculino o alopecia senil al prevenir la depilación o facilitar la germinación del cabello y/o promover la generación y el crecimiento del cabello, distrofia muscular, pioderma y síndrome de Sezary, enfermedad de Addison, lesión por isquemia-reperfusión de órganos que se produce tras la conservación, trasplante o enfermedad isquémica, choque por endotoxinas, colitis seudomembranosa, colitis causada por fármacos o radiación, insuficiencia renal aguda isquémica, insuficiencia renal crónica, toxinosis causada por oxígeno pulmonar o fármacos, cáncer de pulmón, enfisema, catarata, siderosis, retinitis pigmentosa, degeneración macular senil, cicatrización vítrea, quemadura alcalina de la córnea, dermatitis eritema multiforme, dermatitis ampollosa IgA lineal y dermatitis del cemento, gingivitis, periodontitis, septicemia, pancreatitis, enfermedades causadas por la contaminación ambiental, envejecimiento, carcinogénesis, metástasis de carcinoma e hipobaropatía, enfermedad causada por liberación de histaminas o leucotrieno-C4, enfermedad de Behcet, hepatitis autoinmune, cirrosis biliar primaria, colangitis esclerosante, resección hepática parcial, necrosis hepática aguda, necrosis causada por toxina, hepatitis viral, choque o anoxia, hepatitis por virus B, hepatitis no Nno B, cirrosis, hepatopatía alcohólica, incluyendo cirrosis alcohólica, esteatohepatitis no alcohólica (NASH), insuficiencia hepática, insuficiencia hepática fulminante, insuficiencia hepática de inicio tardío, insuficiencia hepática “aguda sobre crónica”, aumento del efecto quimioterapéutico, infección por citomegalovirus, infección por HCMV, SIDA, cáncer, demencia senil, enfermedad de Parkinson, trauma, o infección bacteriana crónica. The amide compound, combinations of amide compounds, or compositions thereof, may also be useful for ameliorating, treating, or preventing disorders of immune regulation related to bone marrow or organ transplant rejection, or graft disease. against host. Examples of disorders of inflammatory and immune regulation that can be treated with the present compounds include, but are not limited to, organ or tissue transplantation, graft-versus-host diseases caused by transplantation, autoimmune syndromes including rheumatoid arthritis, lupus erythematosus systemic, Hashimoto's thyroiditis, multiple sclerosis, systemic sclerosis, myasthenia gravis, type I diabetes, uveitis, posterior uveitis, allergic encephalomyelitis, glomerulonephritis, post-infectious autoimmune diseases including rheumatic fever and post-infectious glomerulonephritis, inflammatory and hyperproliferative skin diseases, psoriasis, Atopic dermatitis, contact dermatitis, eczematous dermatitis, seborrheic dermatitis, mixture flat, pénfigigo, blistering penfigoid, ampouple epidermolysis, urticaria, angioedemas, vasculitis, erythema, cutaneous eosinophilia, lupus erythematosus, acne, alopecia areata, keratoconjunctivitis, conjunctivitis vernal vernal , associated uveitis Behcet's disease, keratitis, herpetic keratitis, conical cornea, epithelial dystrophy of the cornea, corneal leukoma, ocular pemphigus, Mooren's ulcer, scleritis, Graves' ophthalmopathy, Vogt-Koyanagi-Harada syndrome, sarcoidosis, pollen allergy, reversible obstructive airway disease, bronchial asthma, allergic asthma, intrinsic asthma, extrinsic asthma, dust asthma, chronic or inveterate asthma, late-onset asthma and airway hyperresponsiveness, bronchitis, gastric ulcers, vascular damage caused by ischemic diseases and thrombosis, ischemic bowel diseases, inflammatory bowel diseases, necrotizing enterocolitis, intestinal lesions associated with thermal burns, celiac disease, proctitis, eosinophilic gastroenteritis, mastocytosis, Crohn's disease, ulcerative colitis, migraine, rhinitis, eczema, interstitial nephritis, Goodpasture syndrome , hemolytic uremic syndrome, diabetic nephropathy, multiple myositis, Guillain-Barré syndrome, Meniere's disease, polyneuritis, multiple neuritis, mononeuritis, radiculopathy, hyperthyroidism, Basedow's disease, pure red cell aplasia, aplastic anemia, hypoplastic anemia, thrombocytopenic purpura idiopathic, autoimmune hemolytic anemia, agranulocytosis, pernicious anemia, megaloblastic anemia, anerythroplasia, osteoporosis, sarcoidosis, fibroid lung, idiopathic interstitial pneumonia, dermatomyositis, leukoderma vulgaris, ichthyosis vulgaris, photoallergic sensitivity, cutaneous T-cell lymphoma, chronic lymphocytic leukemia, arteries clerosis, atherosclerosis, aortitis syndrome, polyarteritis nodosa, myocardosis, scleroderma, Wegener's granuloma, Sjogren's syndrome, adiposis, eosinophilic fasciitis, gum lesions, periodontium, alveolar bone, dental bone substance, glomerulonephritis, male pattern alopecia or senile alopecia al prevent hair removal or facilitate hair germination and/or promote hair generation and growth, muscular dystrophy, pyoderma and Sezary syndrome, Addison's disease, ischemia-reperfusion injury to organs that occurs after preservation, transplantation or Ischemic disease, endotoxin shock, pseudomembranous colitis, colitis caused by drugs or radiation, ischemic acute renal failure, chronic renal failure, pulmonary oxygen or drug toxins, lung cancer, emphysema, cataract, siderosis, retinitis pigmentosa, senile macular degeneration , vitreous healing, corneal alkali burn, dermatitis erythema multiforme, linear IgA bullous dermatitis and cementum dermatitis, gingivitis, periodontitis, septicemia, pancreatitis, diseases caused by environmental pollution, aging, carcinogenesis, carcinoma metastasis and hypobaropathy, disease caused due to histamine or leukotriene-C4 release, Behcet's disease, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, partial hepatic resection, acute hepatic necrosis, necrosis caused by toxin, viral hepatitis, shock or anoxia, B virus hepatitis, non-hepatitis N non-B, cirrhosis, alcoholic liver disease including alcoholic cirrhosis, nonalcoholic steatohepatitis (NASH), liver failure, fulminant liver failure, late-onset liver failure, “acute on chronic” liver failure, increased chemotherapeutic effect, cytomegalovirus infection, HCMV infection, AIDS, cancer, senile dementia, Parkinson's disease, trauma, or chronic bacterial infection.
En determinadas realizaciones, los presentes compuestos son útiles para tratar el dolor nervioso, incluyendo el dolor neuropático y el dolor inducido por inflamación.In certain embodiments, the present compounds are useful for treating nerve pain, including neuropathic pain and pain induced by inflammation.
En ciertas realizaciones, el compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, son útiles para tratar y/o prevenir artritis reumatoide, artritis psoriásica, osteoartritis, lupus eritematoso sistémico, nefritis lúpica, espondilitis anquilosante, osteoporosis, esclerosis sistémica, esclerosis múltiple, psoriasis, en particular psoriasis pustulosa, diabetes tipo I, diabetes tipo II, enfermedad inflamatoria intestinal (enfermedad de Cronh y colitis ulcerosa), hiperinmunoglobulinemia d y síndrome de fiebre periódica, síndromes periódicos asociados a la criopirina, síndrome de Schnitzler, artritis idiopática juvenil sistémica, enfermedad de Still de inicio en la adultez, gota, brotes de gota, seudogota, síndrome sapho, enfermedad de Castleman, septicemia, accidente cerebrovascular, aterosclerosis, celiaquía, DIRA (deficiencia del antagonista del receptor de Il-1), enfermedad de Alzheimer, enfermedad de Parkinson.In certain embodiments, the amide compound, combinations of amide compounds, or compositions thereof, are useful for treating and/or preventing rheumatoid arthritis, psoriatic arthritis, osteoarthritis, systemic lupus erythematosus, lupus nephritis, ankylosing spondylitis, osteoporosis, sclerosis systemic, multiple sclerosis, psoriasis, in particular pustular psoriasis, type I diabetes, type II diabetes, inflammatory bowel disease (Cronh's disease and ulcerative colitis), hyperimmunoglobulinemia d and periodic fever syndrome, cryopyrin-associated periodic syndromes, Schnitzler syndrome, systemic juvenile idiopathic arthritis, adult-onset Still's disease, gout, gout flare-ups, pseudogout, sapho syndrome, Castleman's disease, sepsis, stroke, atherosclerosis, celiac disease, DIRA (Il-1 receptor antagonist deficiency) , Alzheimer's disease, Parkinson's disease.
Las enfermedades proliferativas que pueden tratarse con el compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, incluyen tumores benignos o malignos, tumores sólidos, carcinoma del cerebro, riñón, hígado, glándula suprarrenal, vejiga, mama, estómago, tumores gástricos, ovarios, colon, recto, próstata, páncreas, pulmón, vagina, cuello uterino, testículos, tracto genitourinario, esófago, laringe, piel, hueso o tiroides, sarcoma, glioblastomas, neuroblastomas, mieloma múltiple, cáncer gastrointestinal, especialmente carcinoma de colon o adenoma colorrectal, un tumor del cuello y la cabeza, una hiperproliferación epidérmica, psoriasis, hiperplasia prostática, una neoplasia, una neoplasia de carácter epitelial, adenoma, adenocarcinoma, queratoacantoma, carcinoma epidermoide, carcinoma de células grandes, carcinoma de pulmón no microcítico, linfomas, Hodgkin y no Hodgkin, un carcinoma mamario, carcinoma folicular, carcinoma indiferenciado, carcinoma papilar, seminoma, melanoma, trastornos provocados por IL-1, un trastorno provocado por MyD88 (tal como el linfoma difuso de células B grandes ABC (DLBCL), macroglobulinemia de Waldenstrom, linfoma de Hodgkin, linfoma cutáneo primario de células T o leucemia linfocítica crónica), mieloma múltiple latente o indolente, o neoplasias malignas hematológicas (incluyendo leucemia, leucemia mieloide aguda (AML), DLBCL, ABC DLBCL, leucemia linfocítica crónica (CLL), linfoma linfocítico crónico, linfoma de efusión primaria, linfoma/leucemia de Burkitt, leucemia linfocítica aguda, leucemia prolinfocítica de células B, linfoma linfoplasmocitario, síndromes mielodisplásicos (MDS), mielofibrosis, policitemia vera, sarcoma de Kaposi, macroglobulinemia de Waldenstrom (WM), linfoma de la zona marginal esplénica, mieloma múltiple, plasmocitoma, linfoma intravascular de células B grandes). En particular, los compuestos descritos en esta memoria son útiles en el tratamiento de tumores malignos resistentes a fármacos, tales como aquellos resistentes a inhibidores de JAK, tumores malignos resistentes a ibrutinib, incluyendo tumores malignos hematológicos resistentes a ibrutinib, tales como CLL resistente a ibrutinib y macroglobulinemia de Waldenstrom resistente a ibrutinib. Proliferative diseases that can be treated with the amide compound, combinations of amide compounds, or compositions thereof, include benign or malignant tumors, solid tumors, carcinoma of the brain, kidney, liver, adrenal gland, bladder, breast, stomach, gastric, ovarian, colon, rectal, prostate, pancreas, lung, vagina, cervix, testicles, genitourinary tract, esophagus, larynx, skin, bone or thyroid tumors, sarcoma, glioblastomas, neuroblastomas, multiple myeloma, gastrointestinal cancer, especially carcinoma of colon or colorectal adenoma, a tumor of the neck and head, an epidermal hyperproliferation, psoriasis, prostatic hyperplasia, a neoplasm, a neoplasm of an epithelial nature, adenoma, adenocarcinoma, keratoacanthoma, squamous cell carcinoma, large cell carcinoma, non-small cell lung carcinoma , lymphomas, Hodgkin and non-Hodgkin, a breast carcinoma, follicular carcinoma, undifferentiated carcinoma, papillary carcinoma, seminoma, melanoma, disorders caused by IL-1, a disorder caused by MyD88 (such as diffuse large B-cell lymphoma ABC (DLBCL ), Waldenstrom macroglobulinemia, Hodgkin lymphoma, primary cutaneous T-cell lymphoma or chronic lymphocytic leukemia), smoldering or indolent multiple myeloma, or hematologic malignancies (including leukemia, acute myeloid leukemia (AML), DLBCL, ABC DLBCL, lymphocytic leukemia (CLL), chronic lymphocytic lymphoma, primary effusion lymphoma, Burkitt lymphoma/leukemia, acute lymphocytic leukemia, B-cell prolymphocytic leukemia, lymphoplasmacytic lymphoma, myelodysplastic syndromes (MDS), myelofibrosis, polycythemia vera, Kaposi's sarcoma, macroglobulinemia of Waldenstrom (WM), splenic marginal zone lymphoma, multiple myeloma, plasmacytoma, intravascular large B-cell lymphoma). In particular, the compounds described herein are useful in the treatment of drug-resistant malignancies, such as those resistant to JAK inhibitors, ibrutinib-resistant malignancies, including ibrutinib-resistant hematologic malignancies, such as ibrutinib-resistant CLL. and Waldenstrom's macroglobulinemia resistant to ibrutinib.
Los ejemplos de trastornos alérgicos que pueden tratarse usando el compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, incluyen, pero no se limitan a, asma (por ejemplo, asma atópica, asma alérgica, asma bronquial atópica mediada por IgE, asma no atópica, asma bronquial, asma no alérgica, asma esencial, asma verdadera, asma intrínseca causada por alteraciones fisiopatológicas, asma esencial de causa desconocida o no aparente, asma enfisematosa, asma inducida por ejercicio, asma inducida por emociones, asma extrínseca causada por factores ambientales, asma inducida por aire frío, asma ocupacional, asma infecciosa causada por o asociada con una infección bacteriana, fúngica, protozoaria o viral, asma incipiente, síndrome del lactante sibilante, bronquiolitis, variante del asma con tos, o asma inducida por fármacos), aspergilosis broncopulmonar alérgica (ABPA), rinitis alérgica, rinitis alérgica perenne, rinitis perenne, rinitis vasomotora, goteo posnasal, sinusitis purulenta o no purulenta, sinusitis aguda o crónica, y sinusitis etmoidal, frontal, maxilar o esfenoidal.Examples of allergic disorders that can be treated using the amide compound, combinations of amide compounds, or compositions thereof, include, but are not limited to, asthma (eg, atopic asthma, allergic asthma, atopic bronchial asthma mediated by IgE, non-atopic asthma, bronchial asthma, non-allergic asthma, essential asthma, true asthma, intrinsic asthma caused by pathophysiological changes, essential asthma of unknown or unknown cause, emphysematous asthma, exercise-induced asthma, emotion-induced asthma, extrinsic asthma caused by environmental factors, cold air-induced asthma, occupational asthma, infectious asthma caused by or associated with a bacterial, fungal, protozoal, or viral infection, incipient asthma, wheezing infant syndrome, bronchiolitis, variant asthma with cough, or induced asthma from drugs), allergic bronchopulmonary aspergillosis (ABPA), allergic rhinitis, perennial allergic rhinitis, perennial rhinitis, vasomotor rhinitis, postnasal drip, purulent or nonpurulent sinusitis, acute or chronic sinusitis, and ethmoid, frontal, maxillary, or sphenoid sinusitis.
Como otro ejemplo, la artritis reumatoide (RA, por sus siglas en inglés) resulta típicamente en hinchazón, dolor, pérdida de movimiento y sensibilidad en las articulaciones objetivo en todo el cuerpo. La RA se caracteriza por inflamación crónica de la membrana sinovial densamente poblada de linfocitos. La membrana sinovial, que suele tener una capa de células de espesor, se vuelve intensamente celular y adopta una forma similar al tejido linfoide, incluyendo las células dendríticas, células T, NK de banda, macrófagos y agrupaciones de células plasmáticas. Este proceso, así como una plétora de mecanismos inmunopatológicos, incluyendo la formación de complejos antígeno-inmunoglobulina, resultan finalmente en la destrucción de la integridad de la articulación, lo que resulta en deformidad, pérdida permanente de la función y/o erosión ósea en la articulación o cerca de ella. El compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, pueden usarse para tratar, mejorar o prevenir uno cualquiera, varios o todos estos síntomas de RA. Por lo tanto, en el contexto de la RA, se considera que los compuestos proporcionan un beneficio terapéutico cuando se logra una reducción o mejora de cualquiera de los síntomas comúnmente asociados con la RA, independientemente de si el tratamiento resulta en un tratamiento concomitante de la RA subyacente y/o en una reducción en la cantidad de factor reumatoide circulante ("RF", por sus siglas en inglés).As another example, rheumatoid arthritis (RA) typically results in swelling, pain, loss of motion, and tenderness in target joints throughout the body. RA is characterized by chronic inflammation of the synovial membrane densely populated with lymphocytes. The synovial membrane, which is usually one cell layer thick, becomes intensely cellular and takes on a form similar to lymphoid tissue, including dendritic cells, T cells, NK band cells, macrophages, and plasma cell clusters. This process, as well as a plethora of immunopathological mechanisms, including the formation of antigen-immunoglobulin complexes, ultimately results in the destruction of joint integrity, resulting in deformity, permanent loss of function, and/or bone erosion at the joint. joint or near it. The amide compound, combinations of amide compounds, or compositions thereof, can be used to treat, ameliorate, or prevent any, several, or all of these RA symptoms. Therefore, in the context of RA, compounds are considered to provide therapeutic benefit when a reduction or amelioration of any of the symptoms commonly associated with RA is achieved, regardless of whether the treatment results in concomitant treatment of RA. underlying RA and/or a reduction in the amount of circulating rheumatoid factor ("RF").
El American College of Rheumatology (ACR, por sus siglas en inglés) ha desarrollado criterios para definir la mejoría y la remisión clínica en la RA. Una vez alcanzado dicho parámetro, el ACR20 (criterio ACR para una mejora clínica del 20%), requiere una mejora del 20% en el recuento de articulaciones sensibles e inflamadas, así como una mejora del 20% en 3 de los siguientes 5 parámetros: evaluación global del paciente, evaluación global del médico, evaluación del paciente, evaluación del dolor por parte del paciente, grado de discapacidad y nivel de reaccionante de fase aguda. The American College of Rheumatology (ACR) has developed criteria to define clinical improvement and remission in RA. Once this parameter is reached, the ACR20 (ACR criteria for a 20% clinical improvement), requires a 20% improvement in the count of tender and swollen joints, as well as a 20% improvement in 3 of the following 5 parameters: patient global assessment, physician global assessment, patient assessment, patient assessment of pain, degree of disability, and acute phase reactant level.
Estos criterios se han ampliado para una mejora del 50% y el 70% en ACR50 y ACR70, respectivamente. Otros criterios incluyen los criterios de Paulu y la progresión radiográfica (p. ej., puntuación de Sharp).These criteria have been expanded for a 50% and 70% improvement in ACR50 and ACR70, respectively. Other criteria include the Paulu criteria and radiographic progression (eg, Sharp score).
En algunas realizaciones, el beneficio terapéutico en pacientes que padecen RA se logra cuando el paciente presenta un ACR20. En realizaciones específicas, se pueden lograr mejoras ACR de ACRC50 o incluso ACR70.In some embodiments, therapeutic benefit in patients suffering from RA is achieved when the patient has an ACR20. In specific embodiments, ACR improvements of ACRC50 or even ACR70 can be achieved.
B. Formulaciones y AdministraciónB. Formulations and Administration
Las composiciones farmacéuticas que comprenden uno o más compuestos de amida activos de la invención se pueden fabricar mediante procedimientos de mezclamiento, disolución, granulación, obtención de grageas, levigación, emulsionamiento, encapsulamiento, atrapamiento, o liofilización. Las composiciones se pueden formular utilizando uno o más excipientes, diluyentes, soportes, adyuvantes o auxiliares fisiológicamente aceptables para proporcionar preparados que se pueden utilizar farmacéuticamente.Pharmaceutical compositions comprising one or more active amide compounds of the invention may be manufactured by mixing, dissolving, granulating, drageeizing, levigating, emulsifying, encapsulating, entrapment, or freeze-drying processes. The compositions may be formulated using one or more physiologically acceptable excipients, diluents, carriers, adjuvants or auxiliaries to provide pharmaceutically usable preparations.
El o los compuestos activos se pueden formular en las composiciones farmacéuticas per se, o en forma de hidrato, solvato, N-óxido o sal farmacéuticamente aceptable. Típicamente, sales de este tipo son más solubles en soluciones acuosas que los correspondientes ácidos y bases libres, pero también se pueden formar sales que tienen una menor solubilidad que los correspondientes ácidos y bases libres. The active compound(s) can be formulated into the pharmaceutical compositions per se, or in pharmaceutically acceptable hydrate, solvate, N-oxide or salt form . Typically, salts of this type are more soluble in aqueous solutions than the corresponding free acids and bases, but salts that have a lower solubility than the corresponding free acids and bases can also be formed.
Composiciones farmacéuticas de la invención pueden adoptar una forma adecuada para virtualmente cualquier modo de administración, incluyendo, por ejemplo, tópica, ocular, oral, bucal, sistémica, nasal, inyección, tal como i.v. o i.p., transdérmica, rectal, vaginal, etc., o una forma adecuada para la administración por inhalación o insuflación.Pharmaceutical compositions of the invention may take a form suitable for virtually any mode of administration, including, for example, topical, ocular, oral, buccal, systemic, nasal, injection, such as i.v. or i.p., transdermal, rectal, vaginal, etc., or a form suitable for administration by inhalation or insufflation.
Para la administración tópica, el o los compuestos activos, hidrato, solvato, N-óxido o sal farmacéuticamente aceptable se pueden formular como disoluciones, geles, ungüentos, cremas, suspensiones, etc., como se conoce en la técnica. For topical administration, the active compound(s), hydrate, solvate, N-oxide, or pharmaceutically acceptable salt may be formulated as solutions, gels, ointments, creams, suspensions, etc., as known in the art.
Las formulaciones sistémicas incluyen aquellas diseñadas para administración por inyección, p. ej., inyección subcutánea, intravenosa, intramuscular, intratecal o intraperitoneal, así como aquellas diseñadas para administración transdérmica, transmucosal, oral o pulmonar.Systemic formulations include those designed for administration by injection, e.g. eg, subcutaneous, intravenous, intramuscular, intrathecal, or intraperitoneal injection, as well as those designed for transdermal, transmucosal, oral, or pulmonary administration.
Preparados inyectables útiles incluyen suspensiones, soluciones o emulsiones estériles del o de los compuestos activos en vehículos acuosos u oleosos. Las composiciones también pueden contener agentes de formulación, tales como agentes de suspensión, estabilización y/o dispersión. Las formulaciones para inyección pueden presentarse en forma de dosificación unitaria, p. ej., en ampollas o en recipientes multidosis, y pueden contener conservantes añadidos.Useful injectable preparations include sterile suspensions, solutions or emulsions of the active compound(s) in aqueous or oily vehicles. The compositions may also contain formulatory agents, such as suspending, stabilizing and/or dispersing agents. Formulations for injection may be presented in unit dosage form, e.g. in ampoules or multi-dose containers, and may contain added preservatives.
Alternativamente, la formulación inyectable se puede proporcionar en forma de polvo para reconstitución con un vehículo adecuado, incluyendo, pero no limitado a agua estéril apirógena, tampón, solución de dextrosa, etc., antes de su uso. Con este fin, el o los compuestos activos pueden secarse mediante cualquier técnica conocida en la técnica, tal como liofilización, y reconstituirse antes de su uso.Alternatively, the injectable formulation may be provided in powder form for reconstitution with a suitable vehicle, including, but not limited to, sterile, pyrogen-free water, buffer, dextrose solution, etc., prior to use. To this end, the active compound(s) can be dried by any technique known in the art, such as lyophilization, and reconstituted before use.
Para la administración transmucosa, se utilizan en la formulación penetrantes apropiados para la barrera a atravesar. Penetrantes de este tipo son conocidos en la técnica.For transmucosal administration, penetrants appropriate for the barrier to be traversed are used in the formulation. Penetrants of this type are known in the art.
Para la administración oral, las composiciones farmacéuticas pueden adoptar la forma de, por ejemplo, pastillas, comprimidos o cápsulas preparadas por medios convencionales con excipientes farmacéuticamente aceptables, tales como: agentes aglutinantes (p. ej., almidón de maíz pregelatinizado, polivinilpirrolidona o hidroxipropilmetilcelulosa); cargas (p. ej., lactosa, celulosa microcristalina o hidrogenofosfato de calcio); lubricantes (p. ej., estearato de magnesio, talco o sílice); desintegrantes (p. ej., almidón de patata o glicolato de almidón sódico); y/o agentes humectantes (p. ej., laurilsulfato de sodio). Los comprimidos se pueden recubrir mediante métodos bien conocidos en la técnica, por ejemplo, con azúcares, películas o recubrimientos entéricos.For oral administration, the pharmaceutical compositions may take the form of, for example, pills, tablets or capsules prepared by conventional means with pharmaceutically acceptable excipients such as: binding agents (eg pregelatinized corn starch, polyvinylpyrrolidone or hydroxypropylmethylcellulose ); fillers (eg, lactose, microcrystalline cellulose, or calcium hydrogen phosphate); lubricants (eg, magnesium stearate, talc, or silica); disintegrants (eg, potato starch or sodium starch glycolate); and/or wetting agents (eg, sodium lauryl sulfate). Tablets can be coated by methods well known in the art, for example, with sugars, films or enteric coatings.
Las preparaciones líquidas para administración oral pueden adoptar la forma de, por ejemplo, elixires, soluciones, jarabes o suspensiones, o pueden presentarse como un producto seco para su reconstitución con agua u otro vehículo adecuado antes de su uso. Preparaciones líquidas de este tipo se pueden preparar por medios convencionales con aditivos farmacéuticamente aceptables tales como: agentes de suspensión (p. ej., jarabe de sorbitol, derivados de celulosa o grasas comestibles hidrogenadas); agentes emulsionantes (p. ej., lecitina o goma arábiga); vehículos no acuosos (p. ej., aceite de almendras, ésteres aceitosos, alcohol etílico, cremophore™ o aceites vegetales fraccionados); y conservantes (p. ej., p-hidroxibenzoatos de metilo o propilo o ácido sórbico). Los preparados también pueden contener sales tampón, conservantes, aromatizantes, colorantes y edulcorantes, según convenga.Liquid preparations for oral administration may take the form of, for example, elixirs, solutions, syrups or suspensions, or they may be presented as a dry product for reconstitution with water or other suitable vehicle before use. Liquid preparations of this type can be prepared by conventional means with pharmaceutically acceptable additives such as: suspending agents (eg, sorbitol syrup, cellulose derivatives, or hydrogenated edible fats); emulsifying agents (eg, lecithin or acacia); non-aqueous vehicles (eg, almond oil, oily esters, ethyl alcohol, cremophore™, or fractionated vegetable oils); and preservatives (eg, methyl or propyl p-hydroxybenzoates or sorbic acid). The preparations may also contain buffer salts, preservatives, flavors, colors and sweeteners, as appropriate.
Preparaciones para administración oral pueden formularse adecuadamente para dar una liberación controlada del compuesto activo o profármaco, como es bien conocido.Preparations for oral administration can be suitably formulated to give a controlled release of the active compound or prodrug, as is well known.
Para la administración bucal, las composiciones pueden adoptar la forma de comprimidos o pastillas formuladas de manera convencional For buccal administration, the compositions may take the form of conventionally formulated tablets or lozenges.
Para las vías de administración rectal y vaginal, el o los compuestos activos pueden formularse como soluciones (para enemas de retención), supositorios o ungüentos que contienen bases de supositorios convencionales, tales como manteca de cacao u otros glicéridos.For rectal and vaginal routes of administration, the active compound(s) may be formulated as solutions (for retention enemas), suppositories, or ointments containing conventional suppository bases, such as cocoa butter or other glycerides.
Para la administración nasal o la administración por inhalación o insuflación, el o los compuestos activos, hidrato, solvato, N-óxido, sal farmacéuticamente aceptable se pueden administrar convenientemente en forma de pulverización en forma de aerosol a partir de paquetes presurizados o un nebulizador con el uso de un propelente adecuado, por ejemplo diclorodifluorometano, triclorofluorometano, diclorotetrafluoroetano, fluorocarbonos, dióxido de carbono u otro gas adecuado. En el caso de un aerosol presurizado, la unidad de dosificación puede determinarse proporcionando una válvula para suministrar una cantidad medida. Cápsulas y cartuchos para uso en un inhalador o insuflador (por ejemplo, cápsulas y cartuchos compuestos de gelatina) se pueden formular para que contengan una mezcla de polvo del compuesto y una base de polvo adecuada tal como lactosa o almidónFor nasal administration or administration by inhalation or insufflation, the active compound(s), hydrate, solvate, N-oxide, pharmaceutically acceptable salt may conveniently be administered as an aerosol spray from pressurized packets or a nebulizer with the use of a suitable propellant, for example dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, fluorocarbons, carbon dioxide or other suitable gas. In the case of a pressurized aerosol, the dosage unit can be determined by providing a valve to deliver a metered amount. Capsules and cartridges for use in an inhaler or insufflator (for example, gelatin composite capsules and cartridges) may be formulated to contain a powder mixture of the compound and a suitable powder base such as lactose or starch.
Un ejemplo específico de una formulación de suspensión acuosa, adecuada para administración nasal, que usa dispositivos de pulverización nasal comercialmente disponibles incluye los siguientes ingredientes: compuesto activo (0,5 20 mg/ml); cloruro de benzalconio (0,1 0,2 mg/ml); polisorbato 80 (TWEEN® 80; 0,55 mg/ml); carboximetilcelulosa sódica o celulosa microcristalina (1 15 mg/ml); feniletanol (14 mg/ml); y dextrosa (2050 mg/ml). El pH de la suspensión final se puede ajustar en el intervalo de aproximadamente pH 5 a pH 7, siendo típico un pH de aproximadamente pH 5,5.A specific example of an aqueous suspension formulation, suitable for nasal administration, using commercially available nasal spray devices includes the following ingredients: active compound (0.5-20 mg/ml); benzalkonium chloride (0.1-0.2 mg/ml); polysorbate 80 (TWEEN® 80; 0.55 mg/ml); sodium carboxymethylcellulose or microcrystalline cellulose (1-15 mg/ml); phenylethanol (14mg/ml); and dextrose (2050 mg/ml). The pH of the final suspension can be adjusted in the range of about pH 5 to pH 7, with a pH of about pH 5.5 being typical.
Otro ejemplo específico de una suspensión acuosa, adecuada para la administración de los compuestos por inhalación, contiene 20 mg/ml del compuesto o compuestos de amida, 1% (v/v) de polisorbato 80 (TWEEN® 80), citrato 50 mM y/o cloruro de sodio al 0,9%.Another specific example of an aqueous suspension, suitable for administration of the compounds by inhalation, contains 20 mg/ml of the amide compound(s), 1% (v/v) polysorbate 80 (TWEEN® 80), 50 mM citrate and /or 0.9% sodium chloride.
Para la administración ocular, el o los compuestos de amida activos pueden formularse como una disolución, emulsión, suspensión, etc., adecuada para la administración en el ojo. En la técnica se conocen una diversidad de vehículos adecuados para administrar compuestos al ojo. Ejemplos específicos no limitantes se describen en las Pat. de EE.UU. N°s 6.261.547; 6.197.934; 6.056.950; 5.800.807; 5.776.445; 5.698.219; 5.521.222; 5.403.841; 5.077.033; 4.882.150; y 4.738.851.For ocular administration, the active amide compound(s) may be formulated as a solution, emulsion, suspension, etc., suitable for administration to the eye. A variety of vehicles suitable for delivering compounds to the eye are known in the art. Specific, non-limiting examples are described in US Pat. US Nos. 6,261,547; 6,197,934; 6,056,950; 5,800,807; 5,776,445; 5,698,219; 5,521,222; 5,403,841; 5,077,033; 4,882,150; and 4,738,851.
Para la administración prolongada, el o los compuestos de amida activos se pueden formular como una preparación de depósito para la administración por implantación o inyección intramuscular. El ingrediente activo puede formularse con materiales poliméricos o hidrófobos adecuados (p. ej., como una emulsión en un aceite aceptable) o resinas de intercambio iónico, o como derivados escasamente solubles, p. ej., como una sal escasamente soluble. Alternativamente, se pueden utilizar sistemas de administración transdérmicos fabricados como un disco o parche adhesivo que libera lentamente el o los compuestos activos para la absorción percutánea. Con este fin, pueden utilizarse potenciadores de la permeación para facilitar la penetración transdérmica del o de los compuestos activos. Parches transdérmicos adecuados se describen, por ejemplo, en las Pat. de EE.UU. N°s 5.407.713; 5.352.456; 5.332.213; 5.336.168; 5.290.561; 5.254.346; 5.164.189; 5.163.899; 5.088.977; 5.087.240; 5.008.110 y 4.921.475. For prolonged administration, the active amide compound(s) can be formulated as a depot preparation for administration by implantation or intramuscular injection. The active ingredient may be formulated with suitable polymeric or hydrophobic materials (eg as an emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble derivatives, eg. g., as a sparingly soluble salt. Alternatively, transdermal delivery systems manufactured as an adhesive disc or patch that slowly release the active compound(s) for percutaneous absorption may be used. To this end, permeation enhancers may be used to facilitate transdermal penetration of the active compound(s). Suitable transdermal patches are described, for example, in US Pat. US Nos. 5,407,713; 5,352,456; 5,332,213; 5,336,168; 5,290,561; 5,254,346; 5,164,189; 5,163,899; 5,088,977; 5,087,240; 5,008,110 and 4,921,475.
Alternativamente, se pueden emplear otros sistemas de administración farmacéutica. Los liposomas y las emulsiones son ejemplos bien conocidos de vehículos de administración que se pueden usar para administrar el o los compuestos activos. También se pueden emplear determinados disolventes orgánicos tal como dimetilsulfóxido (DMSO), aunque habitualmente a costa de una mayor toxicidad.Alternatively, other pharmaceutical delivery systems may be employed. Liposomes and emulsions are well known examples of delivery vehicles that can be used to deliver the active compound(s). Certain organic solvents such as dimethyl sulfoxide (DMSO) can also be used, although usually at the cost of increased toxicity.
Las composiciones farmacéuticas pueden, si se desea, presentarse en un envase o dispositivo dispensador que puede contener una o más formas de dosificación unitaria que contienen el o los compuestos activos. El envase puede comprender, por ejemplo, una lámina metálica o de plástico tal como un envase blister. El envase o dispositivo dispensador puede ir acompañado de instrucciones de administración.The pharmaceutical compositions may, if desired, be presented in a container or dispensing device that may contain one or more unit dosage forms containing the active compound(s). The pack may comprise, for example, metal or plastic foil such as a blister pack. The package or dispensing device may be accompanied by instructions for administration.
C. DosificacionesC. Dosages
El compuesto de amida, o combinaciones de compuestos de amida, generalmente se usarán en una cantidad efectiva para lograr el resultado deseado, por ejemplo en una cantidad efectiva para tratar, prevenir o mejorar una afección particular. El o los compuestos de amida, o las composiciones de los mismos, pueden administrarse terapéuticamente para lograr un beneficio terapéutico, o profilácticamente para lograr un beneficio profiláctico. Beneficio terapéutico significa la erradicación o mejora del trastorno subyacente que se está tratando y/o la erradicación o mejora de uno o más de los síntomas asociados con el trastorno subyacente de manera que el paciente informa de una mejora en la sensación o afección, a pesar de que el paciente aún puede estar afectado con el trastorno subyacente. Por ejemplo, la administración de un compuesto a un paciente que padece una alergia proporciona un beneficio terapéutico no solo cuando se erradica o mejora la respuesta alérgica subyacente, sino también cuando el paciente informa de una disminución en la gravedad o duración de los síntomas asociados con la alergia después de exposición al alérgeno. Como otro ejemplo, el beneficio terapéutico en el contexto del asma incluye una mejora en la respiración después del inicio de un ataque de asma o una reducción en la frecuencia o gravedad de los episodios de asma. El beneficio terapéutico también incluye detener o ralentizar la progresión de la enfermedad, independientemente de si se logra una mejoría.The amide compound, or combinations of amide compounds, will generally be used in an amount effective to achieve the desired result, for example in an amount effective to treat, prevent, or ameliorate a particular condition. The amide compound(s), or compositions thereof, may be administered therapeutically to achieve therapeutic benefit, or prophylactically to achieve prophylactic benefit. Therapeutic benefit means the eradication or amelioration of the underlying disorder being treated and/or the eradication or amelioration of one or more of the symptoms associated with the underlying disorder such that the patient reports an improvement in sensation or condition, despite that the patient may still be affected with the underlying disorder. For example, administration of a compound to a patient suffering from an allergy provides therapeutic benefit not only when the underlying allergic response is eradicated or ameliorated, but also when the patient reports a decrease in the severity or duration of symptoms associated with the allergy. allergy after allergen exposure. As another example, therapeutic benefit in the context of asthma includes an improvement in breathing after the onset of an asthma attack or a reduction in the frequency or severity of asthma episodes. The benefit Therapeutic also includes stopping or slowing the progression of the disease, regardless of whether improvement is achieved.
Como saben los expertos en la técnica, la dosificación preferida de compuestos de amida puede depender de diversos factores, incluyendo la edad, el peso, el estado de salud general, y la gravedad del estado del paciente o sujeto que se está tratando. También puede ser necesario adaptar la dosis al sexo del individuo, y/o a la capacidad pulmonar del individuo, cuando se administra por inhalación. La dosificación también se puede adaptar a personas que padecen más de una afección o aquellas personas que tienen afecciones adicionales que afectan a la capacidad pulmonar y a la capacidad de respirar normalmente, por ejemplo, enfisema, bronquitis, neumonía e infecciones respiratorias. La dosificación y la frecuencia de administración del o de los compuestos de amida, o de sus composiciones, también dependerán de si el o los compuestos de amida se formulan para el tratamiento de episodios agudos de una afección, o para el tratamiento profiláctico de un trastorno. Una persona con conocimientos ordinarios en la técnica podrá determinar la dosis óptima para un individuo en particular.As is known to those skilled in the art, the preferred dosage of amide compounds may depend on a number of factors, including the age, weight, general health, and severity of the condition of the patient or subject being treated. It may also be necessary to tailor the dose to the sex of the individual, and/or the lung capacity of the individual, when administered by inhalation. Dosage can also be tailored for people who have more than one condition or those who have additional conditions that affect lung capacity and the ability to breathe normally, for example, emphysema, bronchitis, pneumonia, and respiratory infections. The dosage and frequency of administration of the amide compound(s) or compositions thereof will also depend on whether the amide compound(s) are formulated for the treatment of acute episodes of a condition, or for the prophylactic treatment of a disorder. . One of ordinary skill in the art will be able to determine the optimum dosage for a particular individual.
Para la administración profiláctica, el compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, se pueden administrar a un paciente o sujeto en riesgo de desarrollar una de las afecciones descritas previamente. Por ejemplo, si se desconoce si un paciente o sujeto es alérgico a un fármaco en particular, el compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, se pueden administrar antes de la administración del fármaco para evitar o mejorar una respuesta alérgica al fármaco. Alternativamente, se puede utilizar la administración profiláctica para evitar o mejorar la aparición de síntomas en un paciente diagnosticado con el trastorno subyacente. Por ejemplo, uno o más compuestos de amida, o una composición de los mismos, se puede administrar a un alérgico antes de la exposición esperada al alérgeno. Un compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, también se pueden administrar profilácticamente a individuos sanos, que se exponen repetidamente a agentes conocidos por una de las enfermedades descritas anteriormente para prevenir la aparición del trastorno. Por ejemplo, un compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, se pueden administrar a un individuo sano, que se expone repetidamente a un alérgeno que se sabe que induce alergias, tal como el látex, en un esfuerzo por evitar que el individuo desarrolle una alergia. Alternativamente, un compuesto de amida, combinaciones de compuestos de amida, o composiciones de los mismos, se pueden administrar a un paciente que padece asma antes de participar en actividades que desencadenan ataques de asma, para disminuir la gravedad de un episodio asmático o evitarlo por completo.For prophylactic administration, the amide compound, combinations of amide compounds, or compositions thereof, can be administered to a patient or subject at risk of developing one of the previously described conditions. For example, if it is not known whether a patient or subject is allergic to a particular drug, the amide compound, combinations of amide compounds, or compositions thereof, may be administered prior to drug administration to prevent or ameliorate a allergic response to the drug. Alternatively, prophylactic administration may be used to prevent or ameliorate the onset of symptoms in a patient diagnosed with the underlying disorder. For example, one or more amide compounds, or a composition thereof, can be administered to an allergy sufferer prior to the expected exposure to the allergen. An amide compound, combinations of amide compounds, or compositions thereof, may also be administered prophylactically to healthy individuals, who are repeatedly exposed to agents known for one of the diseases described above to prevent the onset of the disorder. For example, an amide compound, combinations of amide compounds, or compositions thereof, can be administered to a healthy individual, who is repeatedly exposed to an allergen known to induce allergies, such as latex, in an effort to to prevent the individual from developing an allergy. Alternatively, an amide compound, combinations of amide compounds, or compositions thereof, may be administered to a patient suffering from asthma prior to engaging in activities that trigger asthma attacks, in order to lessen the severity of an asthmatic episode or to avoid it for good. complete.
Las dosificaciones eficaces se pueden estimar inicialmente a partir de ensayos in vitro. Por ejemplo, se puede formular una dosificación inicial para uso en sujetos para lograr una concentración de compuesto activo en sangre o suero circulante que esté en o por encima de una CI50 o CE50 del compuesto particular medido en un ensayo in vitro. Las dosificaciones se pueden calcular para lograr concentraciones circulantes en sangre o suero de este tipo, teniendo en cuenta la biodisponibilidad del compuesto particular. Fingl & Woodbury, "General Principles," En: The Pharmaceutical Basis of Therapeutics de Goodman y Gilman, Capítulo 1, páginas 1 -46, Pergamon Press, y las referencias citadas allí, proporcionan una guía adicional sobre las dosificaciones eficaces. Effective dosages can be initially estimated from in vitro assays. For example, an initial dosage for use in subjects may be formulated to achieve a concentration of active compound in circulating blood or serum that is at or above an IC50 or EC50 of the particular compound as measured in an in vitro assay. Dosages can be calculated to achieve circulating blood or serum concentrations of this type, taking into account the bioavailability of the particular compound. Fingl & Woodbury, "General Principles," In: Goodman and Gilman's The Pharmaceutical Basis of Therapeutics, Chapter 1, pages 1-46, Pergamon Press, and the references cited therein, provide additional guidance on effective dosages.
En algunas realizaciones, los compuestos descritos tienen una CE50 de más de 0 a 20 μM, tal como de más de 0 a 10 μM, de más de 0 a 5 μM, de más de 0 a 1 μM, de más de 0 a 0,5 μM, de más de 0 a 0,1 μM, o de más de 0 a 0,05 μM. In some embodiments, the disclosed compounds have an EC 50 of greater than 0 to 20 µM, such as greater than 0 to 10 µM, greater than 0 to 5 µM, greater than 0 to 1 µM, greater than 0 to 0.5 μM, greater than 0 to 0.1 μM, or greater than 0 to 0.05 μM.
Las dosificaciones iniciales también se pueden estimar a partir de datos in vivo tal como modelos animales. Modelos animales útiles para testar la eficacia de compuestos para tratar o prevenir las diversas enfermedades arriba descritas son bien conocidos en la técnica. Modelos animales adecuados de hipersensibilidad o reacciones alérgicas se describen en Foster, (1995) Allergy 50(21Suμl):6-9, discusión 34-38 y Tumas et al., (2001), J. Allergy Clin. Immunol. Starting dosages can also be estimated from in vivo data such as animal models. Animal models useful for testing the efficacy of compounds in treating or preventing the various diseases described above are well known in the art. Suitable animal models of hypersensitivity or allergic reactions are described in Foster, (1995) Allergy 50(21Suµl):6-9, discussion 34-38 and Tumas et al., (2001), J. Allergy Clin. Immunol.
107(6): 1025-1033. Modelos animales adecuados de rinitis alérgica se describen en Szelenyi et al., (2000), Arzneimittelforschung 50(11): 1037-42; Kawaguchi et al., (1994), Clin. Exp. Allergy 24(3):238-244 y Sugimoto et al., (2000), Immunopharmacology 48(1): 1-7. Personas de experiencia ordinaria en la técnica pueden adaptar dicha información para determinar las dosificaciones adecuadas para la administración humana.107(6): 1025-1033. Suitable animal models of allergic rhinitis are described in Szelenyi et al., (2000), Arzneimittelforschung 50(11): 1037-42; Kawaguchi et al., (1994), Clin. Exp. Allergy 24(3):238-244 and Sugimoto et al., (2000), Immunopharmacology 48(1): 1-7. Persons of ordinary skill in the art can adapt such information to determine suitable dosages for human administration.
Las cantidades de dosificación de los compuestos de amida descritos estarán típicamente en el intervalo de alrededor de más de 0 mg/kg/día, tal como 0,0001 mg/kg/día o 0,001 mg/kg/día o 0,01 mg/kg/día, hasta al menos menos alrededor de 100 mg/kg/día. Más típicamente, la dosificación (o cantidad eficaz) puede variar de aproximadamente 0,0025 mg/kg a aproximadamente 1 mg/kg administrados al menos una vez al día, tal como de 0,01 mg/kg a aproximadamente 0,5 mg/kg o de aproximadamente 0,05 mg/kg a aproximadamente 0,15 mg/kg. La dosificación diaria total varía típicamente de aproximadamente 0,1 mg/kg a aproximadamente 5 mg/kg o a aproximadamente 20 mg/kg al día, tal como de 0,5 mg/kg a aproximadamente 10 mg/kg al día o de aproximadamente 0,7 mg/kg al día a aproximadamente 2,5 mg/kg/día. Las cantidades de dosificación pueden ser mayores o menores, dependiendo, entre otros factores, de la actividad del compuesto de amida, su biodisponibilidad, el modo de administración, y diversos factores discutidos anteriormente.Dosage amounts of the amide compounds described will typically be in the range of about greater than 0 mg/kg/day, such as 0.0001 mg/kg/day or 0.001 mg/kg/day or 0.01 mg/ kg/day, to at least less than about 100 mg/kg/day. More typically, the dosage (or effective amount) can range from about 0.0025 mg/kg to about 1 mg/kg administered at least once daily, such as from 0.01 mg/kg to about 0.5 mg/ kg or from about 0.05 mg/kg to about 0.15 mg/kg. Total daily dosage typically ranges from about 0.1 mg/kg to about 5 mg/kg or about 20 mg/kg per day, such as from 0.5 mg/kg to about 10 mg/kg per day or from about 0 0.7 mg/kg per day to approximately 2.5 mg/kg/day. Dosage amounts can be higher or lower, depending, among other factors, on the activity of the amide compound, its bioavailability, the mode of administration, and various factors discussed above.
La cantidad de dosificación y el intervalo de dosificación se pueden ajustar para que los individuos proporcionen niveles plasmáticos del compuesto de amida que sean suficientes para mantener el efecto terapéutico o profiláctico. Por ejemplo, los compuestos se pueden administrar una vez al día, múltiples veces al día, una vez a la semana, múltiples veces a la semana (p. ej., cada dos días), una vez al mes, múltiples veces al mes o una vez al año, dependiendo, entre otras cosas, del modo de administración, de la indicación específica que se esté tratando y del criterio del médico que prescribe. Las personas de una experiencia ordinaria en la técnica serán capaces de optimizar las dosificaciones locales eficaces sin una experimentación excesiva.The dosage amount and dosage interval can be adjusted for individuals to provide plasma levels of the amide compound that are sufficient to maintain therapeutic or prophylactic effect. For example, the compounds can be administered once a day, multiple times a day, once a week, multiple times a week (eg, every other day), once a month, multiple times a month, or once a year, depending, among other things, the mode of administration, the specific indication being treated and the criteria of the prescribing physician. Those of ordinary skill in the art will be able to optimize effective local dosages without undue experimentation.
Las composiciones que comprenden uno o más de los compuestos de amida descritos normalmente comprenden desde más de 0 hasta 99% del compuesto o compuestos de amida, y/u otro agente terapéutico por porcentaje en peso total. Más típicamente, las composiciones que comprenden uno o más de los compuestos de amida descritos comprenden de alrededor de 1 a alrededor de 20 por ciento en peso total del compuesto de amida y otro agente terapéutico, y de alrededor de 80 a alrededor de 99 por ciento en peso de un aditivo farmacéuticamente aceptable. Preferiblemente, el compuesto de amida, las combinaciones de los compuestos de amida, o las composiciones de los mismos, proporcionarán un beneficio terapéutico o profiláctico sin causar una toxicidad sustancial. La toxicidad del compuesto de amida se puede determinar usando procedimientos farmacéuticos estándar. La relación de dosis entre el efecto tóxico y terapéutico (o profiláctico) es el índice terapéutico. Se prefieren los compuestos de amida que exhiben altos índices terapéuticos.Compositions comprising one or more of the amide compounds typically described comprise from greater than 0 to 99% of the amide compound(s), and/or other therapeutic agent by total weight percent. More typically, compositions comprising one or more of the described amide compounds comprise from about 1 to about 20 percent by total weight of the amide compound and other therapeutic agent, and from about 80 to about 99 percent by weight of a pharmaceutically acceptable additive. Preferably, the amide compound, combinations of amide compounds, or compositions thereof will provide therapeutic or prophylactic benefit without causing substantial toxicity. The toxicity of the amide compound can be determined using standard pharmaceutical procedures. The dose relationship between the toxic and therapeutic (or prophylactic) effect is the therapeutic index. Amide compounds that exhibit high therapeutic indices are preferred.
IV. EjemplosIV. examples
La siguiente sección proporciona ejemplos y ejemplos de referencia. Cualquier compuesto que esté fuera del alcance de las reivindicaciones se incluye sólo con fines de referencia.The following section provides examples and reference examples. Any compounds that are outside the scope of the claims are included for reference purposes only.
Ejemplo 1Example 1
6-bromo-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-1)6-bromo-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-1)
Una disolución en CH2G 2 (40 ml) de ácido 6-bromopicolínico (2,02 g, 10 mmoles), dihidrocloruro de 1-metil-3-(piridin-2-il)-1H-pirazol-4-amina (2,52 g, 10,2 mmoles), HATU (4,56 g, 12 mmoles) y DIPEA (5,4 ml, 31 mmoles) se agitó a temperatura ambiente durante 15 horas. La reacción se completó según se monitorizó por LC-MS. A la mezcla de reacción, se añadió una disolución acuosa saturada de NaHCO3 (alrededor de 100 ml), se mezcló bien, se separaron dos capas; la capa acuosa se extrajo con CH2G 2 (20 ml x 2). Las capas orgánicas se combinaron, se secaron (Na2SO4), se filtraron, se eliminó el disolvente a vacío. El producto bruto se trató adicionalmente con una disolución acuosa saturada de NaHCO3 (alrededor de 80 ml), se mezcló bien, y el precipitado se recogió por filtración, lavando con H2O (alrededor de 10 ml x 5), se secó adicionalmente a vacío. Se obtuvo el compuesto 6-bromo-N-(1 -metil-3-(piridin-2-il)-1H-pirazol-4-il)picolinamida como un sólido de color beige claro: 2,70 g (75% de rendimiento); RMN 1H (300 MHz, cloroformo-d) 5 13.09 (s, 1H), 8.84 (ddd, j = 4,9, 1,8, 1,0 Hz, 1H), 8,36 (s, 1H), 8,21 (dd, j = 7,5, 1,0 Hz, 1H), 8,04 (ddd, j = 8,1, 1,1, 1,1 Hz, 1H), 7,77 (ddd, j = 8,1, 7,5, 1,8 Hz, parcialmente solapado, 1H), 7,78 - 7,73 (m, parcialmente solapado, 1H), 7,65 (dd, j = 7,9, 1,0 Hz, 1H), 7,26 - 7,22 (m, parcialmente solapado con CHCta, 1H), 3,98 (s, 3H); LRMS (M+H) m/z 358,45, 360,33. A CH 2 G 2 (40 mL) solution of 6-bromopicolinic acid (2.02 g, 10 mmol), 1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-amine dihydrochloride ( 2.52 g, 10.2 mmol), HATU (4.56 g, 12 mmol) and DIPEA (5.4 mL, 31 mmol) was stirred at room temperature for 15 hours. The reaction was complete as monitored by LC-MS. To the reaction mixture, saturated aqueous NaHCO 3 solution (ca. 100 mL) was added, mixed well, two layers were separated; the aqueous layer was extracted with CH 2 G 2 (20 ml x 2). The organic layers were combined, dried (Na 2 SO 4 ), filtered, the solvent removed in vacuo. The crude product was further treated with saturated aqueous NaHCO 3 solution (ca. 80 mL), mixed well, and the precipitate collected by filtration, washing with H 2 O (ca. 10 mL x 5), dried further. to empty. The compound 6-bromo-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide was obtained as a light beige solid: 2.70 g (75% of performance); 1 H NMR (300 MHz, chloroform-d) 13.09 (s, 1H), 8.84 (ddd, j = 4.9, 1.8, 1.0 Hz, 1H), 8.36 (s, 1H), 8.21 (dd, j = 7.5, 1.0 Hz, 1H), 8.04 (ddd, j = 8.1, 1.1, 1.1 Hz, 1H), 7.77 (ddd, j = 8.1, 7.5, 1.8 Hz, partially overlapped, 1H), 7.78 - 7.73 (m, partially overlapped, 1H), 7.65 (dd, j = 7.9, 1 .0 Hz, 1H), 7.26-7.22 (m, partially overlapped with CHCta, 1H), 3.98 (s, 3H); LRMS (M+H) m/z 358.45, 360.33.
Ejemplo 2Example 2
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin] -6-carboxamida (I-2)N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-2)
Escala de 0,1 mmol, con ácido (5-fluoropiridin-3-il)borónico, 2,6 mg, 6,8% de rendimiento. RMN (300 MHz, DMSO-ofe) 5 12,53 (s, 1H), 9,71 (d, J = 2,0 Hz, 1H), 8,82 - 8,80 (m, 2H), 8,69 (ddd, J = 8,0, 1,7, 1,7 Hz, 1 H), 8,55 (s, 1 H), 8,37 (dd, J = 7,2, 1,7 Hz, 1H), 8,25 - 8,17 (m, 2H), 8,06 (d, J = 8,0 Hz, 1H), 7,95 (ddd, J = 7,9, 7,9, 1,7 Hz, 1H), 7,72 (dd, J = 7,9, 4,8 Hz, 1H), 7,45 (ddd, J = 7,3, 5,0, 1,1 Hz, 1H), 4,00 (s, 3H); LRMS (M+H) 0.1 mmol scale, with (5-fluoropyridin-3-yl)boronic acid, 2.6 mg, 6.8% yield. NMR (300 MHz, DMSO-ofe) 12.53 (s, 1H), 9.71 (d, J =2.0 Hz, 1H), 8.82-8.80 (m, 2H), 8, 69 (ddd, J = 8.0, 1.7, 1.7 Hz, 1 H), 8.55 (s, 1 H), 8.37 (dd, J = 7.2, 1.7 Hz, 1H), 8.25 - 8.17 (m, 2H), 8.06 (d, J = 8.0 Hz, 1H), 7.95 (ddd, J = 7.9, 7.9, 1, 7 Hz, 1H), 7.72 (dd, J = 7.9, 4.8 Hz, 1H), 7.45 (ddd, J = 7.3, 5.0, 1.1 Hz, 1H), 4.00 (s, 3H); LRMS (M+H)
Ejemplo 3Example 3
5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-3)5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-3)
Una disolución en 1,4-dioxano (3 ml) de 6-bromo-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)picolinamida (71,6 mg, 0,2 mmoles), ácido (5-fluoropiridin-3-il)borónico (42,3 mg, 0,3 mmoles), Pd(PPh3)4 (23,1 mg, 0,02 mmoles), y disolución acuosa 2 M de Na2CÜ3 (0,3 ml, 0,6 mmoles) se calentó en microondas a 150°C durante 60 minutos. El disolvente se eliminó a vacío, y tras la cromatografía en gel de sílice, se obtuvo 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida como un sólido gris claro: 328.8 mg (88% de rendimiento); 1H RMN (300 MHz, DMSO-ofe) δ 12,48 (s, 1 H), 9,60 (s, 1 H), 8,83 - 8,80 (m, 2H), 8,61 (ddd, J = 9,9, 2,1,2,1 Hz, 1H), 8,56 (s, 1 H), 8,45 (dd, J = 7,1, 1,6 Hz, 1 H), 8,28 -8,21 (m, 2H), 8,08 (d, J = 7,9 Hz, 1 H), 7,96 (td, J = 7,9, 1,6 Hz, 1H), 7,46 - 7,42 (m, 1 H), 3,99 (s, 4H); LRMS (M+H) m/z375,62.A 1,4-dioxane (3 mL) solution of 6-bromo-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (71.6 mg, 0 0.2 mmol), (5-fluoropyridin-3-yl)boronic acid (42.3 mg, 0.3 mmol), Pd(PPh3)4 (23.1 mg, 0.02 mmol), and 2 M aqueous solution of Na 2 CÜ3 (0.3 mL, 0.6 mmol) was heated in the microwave at 150°C for 60 minutes. The solvent was removed in vacuo, and after chromatography on silica gel, 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)- was obtained. [2,3'-bipyridine]-6-carboxamide as a light gray solid: 328.8 mg (88% yield); 1H NMR (300 MHz, DMSO-ofe) δ 12.48 (s, 1H), 9.60 (s, 1H), 8.83-8.80 (m, 2H), 8.61 (ddd , J = 9.9, 2.1,2.1 Hz, 1H), 8.56 (s, 1H), 8.45 (dd, J = 7.1, 1.6 Hz, 1H), 8.28 -8.21 (m, 2H), 8.08 (d, J =7.9 Hz, 1H), 7.96 (td, J =7.9, 1.6 Hz, 1H), 7.46-7.42 (m, 1H), 3.99 (s, 4H); LRMS (M+H) m/z 375.62.
Ejemplo 4Example 4
5'-ciano-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida(I-4)5'-Cyano-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide(I-4)
Escala de 0,1 mmol, con ácido (5-metil-1H-pirazol-4-il)borónico, 2,6 mg, 6,8% de rendimiento. (300 MHz, DMSÜ-ofe) δ 12,54 (s, 1H), 9,97 (s, 1H), 9,28 (d, J = 1,9 Hz, 1H), 9,18 (dd, J = 2,1,2,1 Hz, 1H), 8,85 (a d, J = 5,0 Hz, 1H), 8,59 (s, 1H), 8,52 (dd, J = 7,0, 1,8 Hz, 1 H), 8,35 - 8,27 (m, 2H), 8,10 (a d, J= 7,9 Hz, 1H), 7,99 (ddd, J= 7,9, 7,9, 1,7 Hz, 1H), 7,46 (ddd, J= 7,4, 5,0, 1,3 Hz, 1H), 4,02 (s, 3H); LRMS (M+H) m/0.1 mmol scale, with (5-methyl-1H-pyrazol-4-yl)boronic acid, 2.6 mg, 6.8% yield. (300 MHz, DMSÜ-ofe) δ 12.54 (s, 1H), 9.97 (s, 1H), 9.28 (d, J = 1.9 Hz, 1H), 9.18 (dd, J = 2,1,2.1 Hz, 1H), 8.85 (ad, J = 5.0 Hz, 1H), 8.59 (s, 1H), 8.52 (dd, J = 7.0, 1.8 Hz, 1H), 8.35 - 8.27 (m, 2H), 8.10 (ad, J= 7.9 Hz, 1H), 7.99 (ddd, J= 7.9, 7.9, 1.7 Hz, 1H), 7.46 (ddd, J= 7.4, 5.0, 1.3 Hz, 1H), 4.02 (s, 3H); LRMS (M+H) m/
Ejemplo 5Example 5
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(pirimidin-5-il)picolinamida (I-5)N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(pyrimidin-5-yl)picolinamide (I-5)
Escala de 0,1 mmol, con ácido pirimidin-5-ilborónico, 5,8 mg, 16% de rendimiento. ofe) δ 12,52 (s, 1H), 9,80 (s, 2H), 9,43 (s, 1 H), 8,81 (d, J = 5,7 Hz, 1H), 8,56 (s, 1H), 8,49 (dd, J = 7,4, 1,3 Hz, 1H), 8,32 - 8,23 (m, 2H), 8,08 (d, J = 8,9 Hz, 1 H), 7,97 (ddd, J = 8,1,8,1, 1,7 Hz, 1H), 7,47 (ddd, J = 7,4, 5,0, 1,1 Hz, 1H), 4,01 (s, 4H); LRMS (M+H) m/z 8._ 0.1 mmol scale, with pyrimidin-5-ylboronic acid, 5.8 mg, 16% yield. ofe) δ 12.52 (s, 1H), 9.80 (s, 2H), 9.43 (s, 1H), 8.81 (d, J = 5.7 Hz, 1H), 8.56 (s, 1H), 8.49 (dd, J = 7.4, 1.3 Hz, 1H), 8.32 - 8.23 (m, 2H), 8.08 (d, J = 8.9 Hz, 1H), 7.97 (ddd, J = 8,1,8,1, 1.7 Hz, 1H), 7.47 (ddd, J = 7.4, 5.0, 1.1 Hz , 1H), 4.01 (s, 4H); LRMS (M+H) m/z 8._
Ejemplo 6Example 6
N-(1 -metil-3 -(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-5-il)picolinamida (I-6)N-(1 -methyl-3 -(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-5-yl)picolinamide (I-6)
Escala de 0,1 mmol, con ácido (1H-pirazol-5-il)borónico, 12,2 mg, 35% de rendimiento. ofe) 5 13,28 (s, 1H), 12,48 (s, 1H), 8,83 (s, 1 H), 8,58 (s, 1H), 8,23 (dd, J = 6,9, 2,2 Hz, 1H), 8,17 - 8,04 (m, 4H), 7,97 (ddd, J = 7,8, 7,8, 1,7 Hz, 1H), 7,45 (ddd, J = 7,3, 5,0, 1,0 Hz, 1H), 7,20 (d, J = 2,2 Hz, 1H), 4,01 (s, 3H); LRMS (M+H) m/z6,57._ 0.1 mmol scale, with (1H-pyrazol-5-yl)boronic acid, 12.2 mg, 35% yield. ofe) 5 13.28 (s, 1H), 12.48 (s, 1H), 8.83 (s, 1H), 8.58 (s, 1H), 8.23 (dd, J = 6, 9, 2.2 Hz, 1H), 8.17 - 8.04 (m, 4H), 7.97 (ddd, J = 7.8, 7.8, 1.7 Hz, 1H), 7.45 (ddd, J = 7.3, 5.0, 1.0 Hz, 1H), 7.20 (d, J = 2.2 Hz, 1H), 4.01 (s, 3H); LRMS (M+H) m/z6.57._
Ejemplo 7Example 7
6-(3-metil-1 H-pirazol-5-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-7)6-(3-methyl-1 H-pyrazol-5-yl)-N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide (I-7)
Ejemplo 8Example 8
6-(1 -metil-1 H-pirazol-4-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-8)6-(1 -methyl-1 H-pyrazol-4-yl)-N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide (I-8)
Escala de 0,1 mmol, con ácido (1-metil-1H-pirazol-4-il)borónico, 5,6 mg, 16% de rendimiento.1H RMN (300 MHz, DMSO-ofe) δ 12,38 (s, 1H), 8,75 (a d, J= 4,9 Hz, 1H), 8,57 (s, 1H), 8,48 (s, 1H), 8,29 (s, 1H), 8,10 - 8,05 (m, 2H), 8,01 - 7,96 (m, 2H), 7,93 (dd, J = 7,7, 1,2 Hz, 1H), 7,51 - 7,46 (m, 1H), 4,03 (s, 3H), 4,01 (s, 3H); LRMS (M+H) m/z360,65. Ejemplo 90.1 mmol scale, with (1-methyl-1H-pyrazol-4-yl)boronic acid, 5.6 mg, 16% yield. 1 H NMR (300 MHz, DMSO-ofe) δ 12.38 (s, 1H), 8.75 (ad, J= 4.9 Hz, 1H), 8.57 (s, 1H), 8.48 ( s, 1H), 8.29 (s, 1H), 8.10 - 8.05 (m, 2H), 8.01 - 7.96 (m, 2H), 7.93 (dd, J = 7, 7, 1.2 Hz, 1H), 7.51-7.46 (m, 1H), 4.03 (s, 3H), 4.01 (s, 3H); LRMS (M+H) m/z 360.65. Example 9
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida (I-9)N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide (I-9)
Una disolución en dioxano (4,5 ml) de 6-bromo-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)picolinamida (107,5 mg, 0,3 mmoles), ácido (1H-pirazol-4-il)borónico (50,4 mg, 0,45 mmoles), Pd(PPh3)4 (34,7 mg, 0,03 mmoles), y disolución acuosa 2 M de Na2¿03 (0,45 ml, 0,9 mmoles) se calentó en microondas a 150°C durante 45 minutos. El sólido se eliminó por filtración a través de una almohadilla de celite, lavando con MeOH. El filtrado se recolectó, el disolvente se eliminó a vacío, tras la cromatografía en gel de sílice, se obtuvo N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida como un sólido amarillo pálido: 93.4 mg (90% de rendimineto): 1H Rm N (300 MHz, DMSO-ofe) 513,31 (s, 1H), 12,38 (s, 1H), 8,76 (ddd, J = 5,0, 1,7, 1,0 Hz, 1H), 8,58 (s, 1H), 8,56 (d, J = 1,3 Hz, 1H), 8,36 (d, J = 1,7 Hz, 1H), 8,12 - 8,05 (m, 2H), 8,02 - 7,96 (m, 3H), 7,47 (ddd, J = 7,4, 5,0, 1,3 Hz, 1 H), 4,01 (s, 3H); LRMS (M+H) m/z 346,59. Ejemplo 10A dioxane (4.5 mL) solution of 6-bromo-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (107.5 mg, 0.3 mmol), (1H-pyrazol-4-yl)boronic acid (50.4 mg, 0.45 mmol), Pd(PPh3)4 (34.7 mg, 0.03 mmol), and 2 M aqueous Na solution 2 03 (0.45 mL, 0.9 mmol) was microwaved at 150°C for 45 minutes. The solid was removed by filtration through a pad of celite, washing with MeOH. The filtrate was collected, the solvent was removed in vacuo, after chromatography on silica gel, N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6 was obtained. -(1H-pyrazol-4-yl)picolinamide as a pale yellow solid: 93.4 mg (90% yield): 1 H Rm N (300 MHz, DMSO-ofe) 513.31 (s, 1H), 12, 38 (s, 1H), 8.76 (ddd, J = 5.0, 1.7, 1.0 Hz, 1H), 8.58 (s, 1H), 8.56 (d, J = 1, 3 Hz, 1H), 8.36 (d, J = 1.7 Hz, 1H), 8.12 - 8.05 (m, 2H), 8.02 - 7.96 (m, 3H), 7, 47 (ddd, J =7.4, 5.0, 1.3Hz, 1H), 4.01 (s, 3H); LRMS (M+H) m/z 346.59. Example 10
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirrol-2-il)picolinamida (I-10)N-(1 -methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrrol-2-yl)picolinamide (I-10)
Escala de 0,08 mmoles, con ácido (1H-pirrol-2-il)borónico, 19,5 mg, 71% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) δ 12,42 (s, 1H), 11,56 (s, 1H), 8,74 (ddd, J= 5,0, 1,7, 0,9 Hz, 1H), 8,56 (s, 1H), 8,09 - 8,03 (m, 2H), 8,00 - 7,90 (m, 3H), 7,46 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 7,15 - 7,12 (m, 2H), 6,37 (ddd, J = 3,3, 2,4, 2,4 Hz, 1H), 4,01 (s, 3H); LRMS (M+H) m/z 345,56. 0.08 mmol scale, with (1H-pyrrol-2-yl)boronic acid, 19.5 mg, 71% yield. 1 H NMR (300 MHz, DMSO-ofe) δ 12.42 (s, 1H), 11.56 (s, 1H), 8.74 (ddd, J= 5.0, 1.7, 0.9 Hz , 1H), 8.56 (s, 1H), 8.09 - 8.03 (m, 2H), 8.00 - 7.90 (m, 3H), 7.46 (ddd, J = 7.4 , 5.0, 1.3 Hz, 1H), 7.15-7.12 (m, 2H), 6.37 (ddd, J = 3.3, 2.4, 2.4 Hz, 1H), 4.01 (s, 3H); LRMS (M+H) m/z 345.56.
Ejemplo 11Example 11
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1H-pirrol-3-il)picolinamida (I-11)N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrrol-3-yl)picolinamide (I-11)
Escala de 0,08 mmoles, con ácido (1H-pirrol-3-il)borónico, 9,4 mg, 34% de rendimiento. 1H RMN (300 MHz, DMSO-de) δ 12,37 (s, 1H), 11,33 (s, 1H), 8,82 (ddd, J = 5,0, 1,7, 1,0 Hz, 1H), 8,58 (s, 1 H), 8,09 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 8,01 -7,95 (m, 2H), 7,90 - 7,87 (m, 2H), 7,74 (ddd, J = 2,8, 1,8, 1,8 Hz, 1H), 7,45 (ddd, J = 7,4, 5,0, 1,3 Hz, 1 H), 7,02 (dd, J = 2,5, 2,5 Hz, 1 H), 6,95 (ddd, J = 2,6, 1,6, 1,6 Hz, 1 H), 4,01 (s, 3H); LRMS (M+H) m/z345,60.0.08 mmol scale, with (1H-pyrrol-3-yl)boronic acid, 9.4 mg, 34% yield. 1 H NMR (300 MHz, DMSO- de) δ 12.37 (s, 1H), 11.33 (s, 1H), 8.82 (ddd, J = 5.0, 1.7, 1.0 Hz , 1H), 8.58 (s, 1H), 8.09 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 8.01 -7.95 (m, 2H) , 7.90 - 7.87 (m, 2H), 7.74 (ddd, J = 2.8, 1.8, 1.8 Hz, 1H), 7.45 (ddd, J = 7.4, 5.0, 1.3 Hz, 1 H), 7.02 (dd, J = 2.5, 2.5 Hz, 1 H), 6.95 (ddd, J = 2.6, 1.6, 1.6Hz, 1H), 4.01 (s, 3H); LRMS (M+H) m/z 345.60.
Ejemplo 12Example 12
5'-metil-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida(I-12)5'-methyl-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide(I-12)
Escala de 0,3 mmoles, con ácido (5-metilpiridin-3-il)borónico, 95,7 mg, 86% de rendimiento. 1H RMN (300 MHz, DMSO-d6) δ 12,62 (s, 1 H), 9,55 (s, 1H), 8,81 (ddd, J = 4,9, 1,6, 1,0 Hz, 1H), 8,66 (d, J = 1,9 Hz, 1H), 8,58 (s, 1H), 8,48 (dd, J = 1,6, 1,6 Hz, 1H), 8,38 (dd, J = 7,1, 1,8 Hz, 1H), 8,27 - 8,19 (m, 2H), 8,09 (ddd, J = 8,1, 1,0, 1,0 Hz, 1H), 7,98 (ddd, J = 7,7, 7,7, 1,8 Hz, 1H), 7,47 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 4,02 (s, 3H), 2,51 (s, 3H); LRMS (M+H) m/z 371,64. 0.3 mmol scale, with (5-methylpyridin-3-yl)boronic acid, 95.7 mg, 86% yield. 1H NMR (300 MHz, DMSO-d6) δ 12.62 (s, 1H), 9.55 (s, 1H), 8.81 (ddd, J = 4.9, 1.6, 1.0 Hz, 1H), 8.66 (d, J = 1.9 Hz, 1H), 8.58 (s, 1H), 8.48 (dd, J = 1.6, 1.6 Hz, 1H), 8.38 (dd, J = 7.1, 1.8 Hz, 1H), 8.27 - 8.19 (m, 2H), 8.09 (ddd, J = 8.1, 1.0, 1 .0 Hz, 1H), 7.98 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.47 (ddd, J = 7.4, 5.0, 1.3 Hz, 1H), 4.02 (s, 3H), 2.51 (s, 3H); LRMS (M+H) m/z 371.64.
Ejemplo 13Example 13
5'-bromo-[2,3'-bipiridin]-6-carboxilato de metiloMethyl 5'-bromo-[2,3'-bipyridine]-6-carboxylate
Una disolución en dioxano/H2O (15/1,5 ml) de 6-bromopicolinato de metilo (648,1 mg, 3 mmoles), ácido (5-bromopiridin-3-il)borónico (726,5 mg, 3,6 mmoles), Pd(PPh)3)4 (173,2 mg, 0,15 mmoles), y disolución acuosa 2 M de Na2CO3 (0,795 ml, 7,5 mmoles) se calentó en microondas a 150°C durante 60 minutos. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice, las fracciones con el producto deseado se trituraron adicionalmente en hexanos-EtOAc (alrededor de 9:0,5). El compuesto del título se obtuvo como un sólido blanquecino: 582.3 mg (66% de rendimiento): 1H RMN (300 MHz, Cloroformo-d) 59,12 (d, J = 1,9 Hz, 1H), 8,75 (d, J = 2,2 Hz, 1H), 8,59 (dd, J = 2,1,2,1 Hz, 1H), 8,15 (dd, J = 7,4, 1,4 Hz, 1H), 7,98 (dd, J = 7,9, 7,4 Hz, 1H), 7,92 (dd, J = 7,9, 1,4 Hz, 1H), 4,05 (s, 3H); LRMS (M+H) m/z295,28.A dioxane/H2O (15/1.5 mL) solution of methyl 6-bromopicolinate (648.1 mg, 3 mmol), (5-bromopyridin-3-yl)boronic acid (726.5 mg, 3.6 mmol), Pd(PPh) 3 )4 (173.2 mg, 0.15 mmol), and 2 M aqueous Na 2 CO 3 solution (0.795 mL, 7.5 mmol) was microwaved at 150°C for 60 minutes. The solvent was removed in vacuo, after chromatography on silica gel, the fractions with the desired product were triturated further in hexanes-EtOAc (ca. 9:0.5). The title compound was obtained as an off-white solid: 582.3 mg (66% yield): 1H NMR (300 MHz, Chloroform-d) 59.12 (d, J = 1.9 Hz, 1H), 8.75 (d, J = 2.2 Hz, 1H), 8.59 (dd, J = 2,1,2.1 Hz, 1H), 8.15 (dd, J = 7.4, 1.4 Hz, 1H), 7.98 (dd, J = 7.9, 7.4 Hz, 1H), 7.92 (dd, J = 7.9, 1.4 Hz, 1H), 4.05 (s, 3H ); LRMS (M+H) m/z 295.28.
Ejemplo 14Example 14
5'-ciclopropil-[2,3'-bipiridin]-6-carboxilato de metiloMethyl 5'-cyclopropyl-[2,3'-bipyridine]-6-carboxylate
Una disolución en tolueno/H2O (3/0,3 ml) de 5'-bromo-[2,3'-bipiridin]-6-carboxilato de metilo y metilo (35,2 mg, 0,12 mmoles), éster MIDA de ácido ciclopropilborónico (35,5 mg, 0,18 mmoles), Pd(OAc)2 (1,3 mg, 0,006 mmoles), triciclohexilfosfina (3,4 mg, 0,012 mmoles) y fosfato de potasio (89,2 mg, 0,42 mmoles) se calentó en microondas a 150°C durante 30 minutos, seguido de otras 39 horas a 100°C, tras añadir éster MIDA adicional y catalizadores, hasta que la reacción se completó según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice, se obtuvo el compuesto del título como un sólido pegajoso amarillo claro: 1H RMN (300 MHz, Cloroformo-o) 5 8,96 (d, J = 2,1 Hz, 1 H), 8,47 (d, J = 2,2 Hz, 1 H), 8,11 (dd, J = 7,1, 1,7 Hz, 1 H), 8,04 (ddd, J= 2,2, 2,2, 0,5 Hz, 1H), 7,95 (dd, J = 7,9, 7,1 Hz, 1H), 7,90 (dd, J = 7,9, 1,7 Hz, 1H), 4,04 (s, 3H), 2,06 - 1,97 (m, 1H), 1,11- 1,05 (m, 2H), 0,87 - 0,82 (m, 2H); LRMS (M+H) m/z 255,36. A toluene/H2O (3/0.3 mL) solution of methyl 5'-bromo-[2,3'-bipyridine]-6-carboxylate (35.2 mg, 0.12 mmol), MIDA ester cyclopropylboronic acid (35.5 mg, 0.18 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), tricyclohexylphosphine (3.4 mg, 0.012 mmol), and potassium phosphate (89.2 mg, 0.42 mmol) was microwaved at 150°C for 30 minutes, followed by another 39 hours at 100°C, after adding additional MIDA ester and catalysts, until that the reaction was complete as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel the title compound was obtained as a light yellow sticky solid: 1H NMR (300 MHz, Chloroform-o) 5 8.96 (d, J = 2, 1 Hz, 1 H), 8.47 (d, J = 2.2 Hz, 1 H), 8.11 (dd, J = 7.1, 1.7 Hz, 1 H), 8.04 (ddd , J= 2.2, 2.2, 0.5 Hz, 1H), 7.95 (dd, J = 7.9, 7.1 Hz, 1H), 7.90 (dd, J = 7.9 , 1.7 Hz, 1H), 4.04 (s, 3H), 2.06 - 1.97 (m, 1H), 1.11- 1.05 (m, 2H), 0.87 - 0, 82(m, 2H); LRMS (M+H) m/z 255.36.
Ejemplo 15Example 15
5'-ciclopropil-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-13)5'-cyclopropyl-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-13)
Una disolución en MeOH (0,5 ml) de 5'-ciclopropil-[2,3'-bipiridin]-6-carboxilato de metilo (~0,12 mmoles) y una disolución acuosa de NaOH (1 N, 126 μl) se agitó a 50°C durante 1 hora. La reacción se completó según lo monitorizado por LC-MS, los volátiles se eliminaron a vacío, y el producto bruto se usó directamente en la siguiente reacción sin purificación adicional. Una disolución en CH2G 2 (2 ml) del ácido, 1 -metil-3-(piridin-2-il)-1 H-pirazol-4-amina di-ácido clorhídrico (29,7 mg, 0,12 mmoles), HATU (55 mg, 0,14 mmoles) y DIPEA (63 μl, 0,36 mmoles) se agitó a temperatura ambiente durante 1 hora, la reacción se completó según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice, el producto se trituró adicionalmente en hexanos-EtOAc. El compuesto del título se obtuvo como un sólido blanco: 7,9 mg (17% de rendimiento en 3 etapas); 1H RMN (300 MHz, DMSO-de) 5 12,61 (s, 1H), 9,55 (s, 1H), 8,84 (d, J = 4,9 Hz, 1H), 8,61 (d, J = 2,1 Hz, 1H), 8,57 (s, 1H), 8,40 (dd, J = 7.0, 1,9 Hz, 1H), 8,26 - 8,18 (m, 3H), 8,08 (d, J = 8,0 Hz, 1H), 7,97 (ddd, J = 7,8, 7,8, 1,7 Hz, 1 H), 7,45 (ddd, J = 7,3, 5.0, 1,2 Hz, 1 H), 4,01 (s, 3H), 2,18 - 2,09 (m, 1H), 1,15 - 1,09 (m, 2H), 0,98 - 0,95 (m, 2H); LRMS (M+H) m/z 397,59. Ejemplo 16A MeOH (0.5 mL) solution of methyl 5'-cyclopropyl-[2,3'-bipyridine]-6-carboxylate (~0.12 mmol) and aqueous NaOH solution (1 N, 126 μL) it was stirred at 50°C for 1 hour. The reaction was complete as monitored by LC-MS, the volatiles were removed in vacuo, and the crude product was used directly in the next reaction without further purification. A CH 2 G 2 (2 mL) solution of the acid, 1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-amine di-hydrochloric acid (29.7 mg, 0.12 mmol ), HATU (55 mg, 0.14 mmol) and DIPEA (63 µL, 0.36 mmol) were stirred at room temperature for 1 hour, the reaction was complete as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel the product was triturated further in hexanes-EtOAc. The title compound was obtained as a white solid: 7.9 mg (17% yield over 3 steps); 1 H NMR (300 MHz, DMSO-de) 12.61 (s, 1H), 9.55 (s, 1H), 8.84 (d, J = 4.9 Hz, 1H), 8.61 ( d, J = 2.1 Hz, 1H), 8.57 (s, 1H), 8.40 (dd, J = 7.0, 1.9 Hz, 1H), 8.26 - 8.18 (m, 3H ), 8.08 (d, J = 8.0 Hz, 1H), 7.97 (ddd, J = 7.8, 7.8, 1.7 Hz, 1H), 7.45 (ddd, J = 7.3, 5.0, 1.2 Hz, 1H), 4.01 (s, 3H), 2.18 - 2.09 (m, 1H), 1.15 - 1.09 (m, 2H) , 0.98-0.95 (m, 2H); LRMS (M+H) m/z 397.59. Example 16
5'-(2-hidroxipropan-2-il)-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6- carboxamida (I-14) Escala de 0,1 mmol, con 2-(5-(4,4,5,5-tetrametil-1,3,2-dioxaborolan-2-il)piridin-3-il)propan-2-ol, 29,8 mg, 72% de rendimiento. 1H RMN (300 MHz, DMSO-de) 5 12,62 (s, 1H), 9,64 (s, 1H), 8,93 (d, J = 2,1 Hz, 1H), 8,91 (ddd, J = 5,0, 1,7, 1,0 Hz, 1H), 8,59 - 8,58 (m, 2H), 8,39 (dd, J = 6,9, 2,1 Hz, 1 H), 8,28 - 8,21 (m, 2H), 8,08 (ddd, J = 8,1,8,1, 1,1 Hz, 1H), 7,97 (ddd, J = 7,8, 7,8, 1,8 Hz, 1H), 7,45 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 5,41 (s, 1H), 4,02 (s, 3H), 1,61 (s, 6H); LRMS (M+H) m/z 415,66. 5'-(2-hydroxypropan-2-yl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridin]-6- carboxamide (I-14) 0.1 mmol scale, with 2-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-yl)propan- 2-ol, 29.8 mg, 72% yield. 1H NMR (300 MHz, DMSO-de) 12.62 (s, 1H), 9.64 (s, 1H), 8.93 (d, J = 2.1 Hz, 1H), 8.91 (ddd , J = 5.0, 1.7, 1.0 Hz, 1H), 8.59 - 8.58 (m, 2H), 8.39 (dd, J = 6.9, 2.1 Hz, 1 H), 8.28 - 8.21 (m, 2H), 8.08 (ddd, J = 8,1,8.1, 1.1 Hz, 1H), 7.97 (ddd, J = 7, 8, 7.8, 1.8 Hz, 1H), 7.45 (ddd, J = 7.4, 5.0, 1.3 Hz, 1H), 5.41 (s, 1H), 4.02 (s, 3H), 1.61 (s, 6H); LRMS (M+H) m/z 415.66.
Ejemplo 17Example 17
2'-metil-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida (I-15)2'-methyl-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide (I-15)
Escala de 0,08 mmoles, con 2-metil-4-(4,4,5,5-tetrametil-1,3,2-dioxaborlan-2-il)piridina, 10,8 mg, 36% de rendimiento.0.08 mmol scale, with 2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborlan-2-yl)pyridine, 10.8 mg, 36% yield.
1HNMR (300 MHz, DMSO-de) 5 12,57 (s, 1H), 8,78 (d, 1HNMR (300 MHz, DMSO-de) 5 12.57 (s, 1H), 8.78 (d, J J = 5,1 Hz, 1H), 8,66 (a d, = 5.1 Hz, 1H), 8.66 (at d, J J = 4,7 Hz, 1H), 8,55 (s, 1H), 8,41 -8,36 (m, 1H), 8,25 (d, = 4.7 Hz, 1H), 8.55 (s, 1H), 8.41 -8.36 (m, 1H), 8.25 (d, J J = 0,8 Hz, 1H), 8,24 (s, 1H), 8,15 - 8,12 (m, 2H), 8,06 (d, = 0.8 Hz, 1H), 8.24 (s, 1H), 8.15 - 8.12 (m, 2H), 8.06 (d, J J = 8,0 Hz, 1H), 7,96 (ddd, = 8.0 Hz, 1H), 7.96 (ddd, J J = 7,7, 7,7, 1,7 Hz, 1 H), 7,46 (ddd, = 7.7, 7.7, 1.7 Hz, 1H), 7.46 (ddd, J J = 7,3, 5,0, 1,3 Hz, 1 H), 4,01 (s, 3H), 2,67 (s, 3H); LRMS (M+H) = 7.3, 5.0, 1.3 Hz, 1H), 4.01 (s, 3H), 2.67 (s, 3H); LRMS (M+H) m/z m/z 371,64. 371.64.
Ejemplo 18Example 18
6'-metil-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin] -6-carboxamida (I-16)6'-methyl-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-16)
Escala de 0,08 mmoles, con ácido (6-metilpiridin-3-il)borónico, 20,4 mg, 69% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 512,55 (s, 1H), 9,58 (s, 1H), 8,84 (ddd, J = 5,0, 1,7, 0,9 Hz, 1 H), 8,63 (dd, J = 8,1,2,4 Hz, 1H), 8,59 (s, 1H), 8,37 (dd, J = 7,2, 1,8 Hz, 1H), 8,25 - 8,17 (m, 2H), 8,10 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,99 (ddd, J = 7,8, 7,8, 1,8 Hz, 1H), 7,60 (d, J = 8,2 Hz, 1H), 7,49 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 4,02 (s, 3H), 2,67 (s, 3H); LRMS (M+H) m/z371,67. 0.08 mmol scale, with (6-methylpyridin-3-yl)boronic acid, 20.4 mg, 69% yield. 1 H NMR (300 MHz, DMSO-ofe) 512.55 (s, 1H), 9.58 (s, 1H), 8.84 (ddd, J = 5.0, 1.7, 0.9 Hz, 1H), 8.63 (dd, J = 8,1,2.4 Hz, 1H), 8.59 (s, 1H), 8.37 (dd, J = 7.2, 1.8 Hz, 1H), 8.25 - 8.17 (m, 2H), 8.10 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.99 (ddd, J = 7, 8, 7.8, 1.8 Hz, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.49 (ddd, J = 7.4, 5.0, 1.3 Hz , 1H), 4.02 (s, 3H), 2.67 (s, 3H); LRMS (M+H) m/z 371.67.
Ejemplo 19Example 19
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(trifluorometil)-[2,3'-bipiridin]-6-carboxamida (I-17)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(trifluoromethyl)-[2,3'-bipyridine]-6-carboxamide (I-17 )
Escala de 0,08 mmoles, con ácido (5-(trifluorometil)piridin-3-il)borónico, 24,6 mg, 72% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 512,43 (s, 1H), 9,95 (s, 1H), 9,16 (s, 1H), 8,90 (s, 1H), 8,69 (d, J = 4,8 Hz, 1 H), 8,49 - 8,47 (m, 2H), 8,24 - 8,16 (m, 2H), 8,01 (d, J = 8,0 Hz, 1H), 7,90 (ddd, J = 7,9, 7,9, 1,5 Hz, 1H), 7,36 - 7,32 (m, 1H), 3,98 (s, 3H); LRMS (M+H) m/z 425,62.0.08 mmol scale, with (5-(trifluoromethyl)pyridin-3-yl)boronic acid, 24.6 mg, 72% yield. 1H NMR (300 MHz, DMSO-ofe) 512.43 (s, 1H), 9.95 (s, 1H), 9.16 (s, 1H), 8.90 (s, 1H), 8.69 ( d, J = 4.8 Hz, 1H), 8.49 - 8.47 (m, 2H), 8.24 - 8.16 (m, 2H), 8.01 (d, J = 8.0 Hz, 1H), 7.90 (ddd, J = 7.9, 7.9, 1.5 Hz, 1H), 7.36 - 7.32 (m, 1H), 3.98 (s, 3H) ; LRMS (M+H) m/z 425.62.
Ejemplo 20Example 20
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-2'-(trifluorometil)-[2,4'-bipiridin]-6-carboxamida (I-18)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-2'-(trifluoromethyl)-[2,4'-bipyridine]-6-carboxamide (I-18 )
Escala de 0,08 mmoles, con ácido (2-(trifluorometil)piridin-4-il)borónico, 17,2 mg, 51% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 512,47 (s, 1H), 9,09 (d, J = 5,0 Hz, 1 H), 8,63 - 8,60 (m, 2H), 8,55 - 8,50 (m, 3H), 8,27 - 8,26 (m, 2H), 8,02 (d, J = 8,0 Hz, 1 H), 7,92 (ddd, J = 7,8, 7,8, 1,7 Hz, 1 H), 7,37 (ddd, J = 7,2, 5,0, 1,2 Hz, 1 H), 3,99 (s, 3H); LRMS (M+H) m/z 425,67.0.08 mmol scale, with (2-(trifluoromethyl)pyridin-4-yl)boronic acid, 17.2 mg, 51% yield. 1H NMR (300 MHz, DMSO-ofe) 512.47 (s, 1H), 9.09 (d, J = 5.0 Hz, 1H), 8.63-8.60 (m, 2H), 8 .55 - 8.50 (m, 3H), 8.27 - 8.26 (m, 2H), 8.02 (d, J = 8.0 Hz, 1H), 7.92 (ddd, J = 7.8, 7.8, 1.7 Hz, 1H), 7.37 (ddd, J = 7.2, 5.0, 1.2 Hz, 1H), 3.99 (s, 3H) ; LRMS (M+H) m/z 425.67.
Ejemplo 21Example 21
5'-metoxi-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-19)5'-methoxy-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-19)
Escala de 0,08 mmoles, con ácido (5-metoxipiridin-3-il)borónico, 21,5 mg, 70% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) δ 12,58 (s, 1H), 9,38 (d, J = 1,8 Hz, 1H), 8,88 (ddd, J = 5,0, 1,7, 1,0 Hz, 1H), 8,59 (s, 1H), 8,56 (d, J = 2,8 Hz, 1 H), 8,45 (dd, J = 7,1, 1,8 Hz, 1H), 8,28 - 8,20 (m, 3H), 8,09 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,98 (ddd, J= 8,1,7,5, 1,8 Hz, 1 H), 7,46 (ddd, J= 7,4, 5,0, 1,3 Hz, 1 H), 4,03 (s, 3H), 4,02 (s, 3H); LRMS (M+H) m/z 387,62. 0.08 mmol scale, with (5-methoxypyridin-3-yl)boronic acid, 21.5 mg, 70% yield. 1H NMR (300 MHz, DMSO-ofe) δ 12.58 (s, 1H), 9.38 (d, J = 1.8 Hz, 1H), 8.88 (ddd, J = 5.0, 1, 7, 1.0 Hz, 1H), 8.59 (s, 1H), 8.56 (d, J = 2.8 Hz, 1H), 8.45 (dd, J = 7.1, 1, 8 Hz, 1H), 8.28 - 8.20 (m, 3H), 8.09 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.98 (ddd, J = 8,1,7,5, 1.8 Hz, 1H), 7.46 (ddd, J= 7.4, 5.0, 1.3 Hz, 1H), 4.03 (s, 3H ), 4.02 (s, 3H); LRMS (M+H) m/z 387.62.
Ejemplo 22Example 22
6'-metoxi-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-20)6'-methoxy-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-20)
Escala de 0,08 mmoles con ácido (6-metoxipiridin-3-il)borónico, 20,4 mg, 66% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 512,55 (s, 1 H), 9,27 (dd, J = 2,5, 0,6 Hz, 1H), 8,80 (ddd, J = 5,0, 1,7, 1,0 Hz, 1 H), 8,69 (dd, J = 8,7, 2,5 Hz, 1H), 8,58 (s, 1H), 8,30 (dd, J = 7,6, 1,4 Hz, 1H), 8,19 (dd, J = 7,6, 7,6 Hz, 1H), 8,14 (dd, J =7,6, 1,4 Hz, 1H), 8,08 (ddd, J = 8,1, 1,1, 1,1 Hz, 1 H), 7,97 (ddd, J = 8,1,7,5, 1,8 Hz, 1H), 7,48 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 7,18 (dd, J = 8,7, 0,6 Hz, 1 H), 4,05 (s, 3H), 4,01 (s, 3H); LRMS (M+H) m/z387,61.0.08 mmol scale with (6-methoxypyridin-3-yl)boronic acid, 20.4 mg, 66% yield. 1H NMR (300 MHz, DMSO-ofe) 512.55 (s, 1H), 9.27 (dd, J = 2.5, 0.6 Hz, 1H), 8.80 (ddd, J = 5, 0, 1.7, 1.0 Hz, 1H), 8.69 (dd, J = 8.7, 2.5 Hz, 1H), 8.58 (s, 1H), 8.30 (dd, J = 7.6, 1.4 Hz, 1H), 8.19 (dd, J = 7.6, 7.6 Hz, 1H), 8.14 (dd, J = 7.6, 1.4 Hz , 1H), 8.08 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.97 (ddd, J = 8.1,7.5, 1.8 Hz, 1H), 7.48 (ddd, J = 7.4, 5.0, 1.3 Hz, 1H), 7.18 (dd, J = 8.7, 0.6 Hz, 1H), 4, 05 (s, 3H), 4.01 (s, 3H); LRMS (M+H) m/z 387.61.
Ejemplo 23Example 23
2'-metoxi-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida (I-21)2'-Methoxy-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide (I-21)
Escala de 0,08 mmoles, con ácido (2-metoxipiridin-4-il)borónico, 16 mg, 52% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 512,52 (s, 1H), 8,86 (ddd, J = 5,0, 1,7, 1,0 Hz, 1H), 8,58 (s, 1H), 8,50 (dd, J = 5,4, 0,6 Hz, 1H), 8,43 (dd, J = 5,5, 3,5 Hz, 1H), 8,27 (s, 1H), 8,26 (d, J = 2,2 Hz, 1H), 8,08 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,99 (dd, J = 7,4, 1,8 Hz, 1H), 7,94 (dd, J = 5,4, 1,5 Hz, 1H), 7,84 (dd, J = 1,4, 0,7 Hz, 1H), 7,47 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 4,05 (s, 3H), 4,01 (s, 3H); LRMS (M+H) m/z387,52.0.08 mmol scale, with (2-methoxypyridin-4-yl)boronic acid, 16 mg, 52% yield. 1H NMR (300 MHz, DMSO-ofe) 512.52 (s, 1H), 8.86 (ddd, J = 5.0, 1.7, 1.0 Hz, 1H), 8.58 (s, 1H ), 8.50 (dd, J = 5.4, 0.6 Hz, 1H), 8.43 (dd, J = 5.5, 3.5 Hz, 1H), 8.27 (s, 1H) , 8.26 (d, J = 2.2 Hz, 1H), 8.08 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.99 (dd, J = 7 .4, 1.8 Hz, 1H), 7.94 (dd, J = 5.4, 1.5 Hz, 1H), 7.84 (dd, J = 1.4, 0.7 Hz, 1H) , 7.47 (ddd, J = 7.4, 5.0, 1.3 Hz, 1H), 4.05 (s, 3H), 4.01 (s, 3H); LRMS (M+H) m/z 387.52.
Ejemplo 24Example 24
5'-isopropoxi-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-22)5'-Isopropoxy-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-22)
Escala de 0,3 mmoles, con ácido (5-isopropoxipiridin-3-il)borónico, 99,3 mg, 80% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) δ 12,54 (s, 1H), 9,31 (d, J = 1,8 Hz, 1H), 8,93 (ddd, J = 4,9, 1,6, 0,9 Hz, 1H), 8,59 (s, 1H), 8,54 (d, J = 2,7 Hz, 1H), 8,45 (dd, J = 6,6, 2,3 Hz, 1 H), 8,27 - 8,21 (m, 3H), 8,10 (ddd, J = 8,1,0,9, 0,9 Hz, 1 H), 7,98 (ddd, J = 7,7, 7,7, 1,8 Hz, 1H), 7,44 (ddd, J = 7,4, 5,0, 1,2 Hz, 1H), 4,97 (hept, J = 6,0 Hz, 1H), 4,02 (s, 3H), 1,41 (d, J = 6,0 Hz, 6H); LRMS (M+H) m/z 415,67.0.3 mmol scale, with (5-isopropoxypyridin-3-yl)boronic acid, 99.3 mg, 80% yield. 1H NMR (300 MHz, DMSO-ofe) δ 12.54 (s, 1H), 9.31 (d, J = 1.8 Hz, 1H), 8.93 (ddd, J = 4.9, 1, 6, 0.9 Hz, 1H), 8.59 (s, 1H), 8.54 (d, J = 2.7 Hz, 1H), 8.45 (dd, J = 6.6, 2.3 Hz, 1H), 8.27 - 8.21 (m, 3H), 8.10 (ddd, J = 8.1,0.9, 0.9 Hz, 1H), 7.98 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.44 (ddd, J = 7.4, 5.0, 1.2 Hz, 1H), 4.97 (hept, J = 6.0Hz, 1H), 4.02 (s, 3H), 1.41 (d, J = 6.0Hz, 6H); LRMS (M+H) m/z 415.67.
Ejemplo 25Example 25
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-((tetrahidro-2H-piran-4-il)oxi)-[2,3'- bipiridina]-6-carboxamida (I-23) N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-((tetrahydro-2H-pyran-4-yl)oxy)-[2,3' - bipyridine]-6-carboxamide (I-23)
Escala de 0,3 mmoles, con ácido (5-((tetrahidro-2H-piran-4-il)oxi)piridin-3-il)borónico, 110,4 mg, 81% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 512,55 (s, 1H), 9,34 (d, J = 1,7 Hz, 1H), 8,92 (ddd, J= 5,0, 1,6, 0,9 Hz, 1 H), 8,61 (d, J= 2,7 Hz, 1H), 8,60 (s, 1H), 8,46 (dd, J= 6,8, 2,1 Hz, 1 H), 8,31 - 8,29 (m, 1 H), 8,28 - 8,21 (m, 2H), 8,10 (a d, J= 8,0 Hz, 1 H), 7,99 (ddd, J = 7,7, 7,7, 1,7 Hz, 1 H), 7,44 (ddd, J = 7,4, 5,0, 1,2 Hz, 1H), 4,92 (tt, J = 8,5, 4,1 Hz, 1 H), 4,02 (s, 3H), 3,94 (dt, J = 11,7, 4,2 Hz, 2H), 3,55 (ddd, J = 11,7, 9,5, 2,6 Hz, 2H), 2,15 - 2,10 (m, 2H), 1,71 (dtd, J = 13,0, 9,5, 4,1 Hz, 2H); LRMS (M+H) m/z457,61.0.3 mmol scale, with (5-((tetrahydro-2H-pyran-4-yl)oxy)pyridin-3-yl)boronic acid, 110.4 mg, 81% yield. 1 H NMR (300 MHz, DMSO-ofe) 512.55 (s, 1H), 9.34 (d, J = 1.7 Hz, 1H), 8.92 (ddd, J= 5.0, 1, 6, 0.9Hz, 1H), 8.61 (d, J= 2.7 Hz, 1H), 8.60 (s, 1H), 8.46 (dd, J= 6.8, 2.1 Hz, 1H), 8.31 - 8.29 (m, 1H), 8.28 - 8.21 (m, 2H), 8.10 (ad, J= 8.0 Hz, 1H), 7.99 (ddd, J =7, 7, 7.7, 1.7 Hz, 1H), 7.44 (ddd, J = 7.4, 5.0, 1.2 Hz, 1H), 4.92 (tt, J = 8.5 , 4.1 Hz, 1H), 4.02 (s, 3H), 3.94 (sd, J = 11.7, 4.2 Hz, 2H), 3.55 (ddd, J = 11.7 , 9.5, 2.6 Hz, 2H), 2.15-2.10 (m, 2H), 1.71 (dtd, J =13.0, 9.5, 4.1 Hz, 2H); LRMS (M+H) m/z 457.61.
Ejemplo 26Example 26
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(2,2,2-trifluoroetoxi)-[2,3'-bipiridin]-6-carboxamida (I-24) N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(2,2,2-trifluoroethoxy)-[2,3'-bipyridin]-6 -carboxamide (I-24)
Escala de 0,1 mmol, con ácido (5-(2,2,2-trifluoroetoxi)piridin-3-il)borónico, 30,8 mg, 68% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) δ 12,55 (s, 1H), 9,29 (d, J = 2,4 Hz, 1 H), 8,81 - 8,77 (m, 2H), 8,58 (s, 1H), 8,35 (dd, J = 7,5, 1,3 Hz, 1H), 8,22 (dd, J = 7,6, 7,6 Hz, 1H), 8,17 (dd, J = 7,9, 1,1 Hz, 1H), 8,09 (d, J = 8,2 Hz, 1H), 7,98 (ddd, J = 7,7, 7,7, 1,6 Hz, 1H), 7,49 (ddd, J = 6,7, 4,7, 1,0 Hz, 1H), 7,38 (d, J = 8,7 Hz, 1H), 5,21 (c, J = 9,1 Hz, 2H), 4,01 (s, 3H); LRMS (M+H) m/z 455,53.0.1 mmol scale, with (5-(2,2,2-trifluoroethoxy)pyridin-3-yl)boronic acid, 30.8 mg, 68% yield. 1H NMR (300 MHz, DMSO-ofe) δ 12.55 (s, 1H), 9.29 (d, J = 2.4 Hz, 1H), 8.81-8.77 (m, 2H), 8.58 (s, 1H), 8.35 (dd, J = 7.5, 1.3 Hz, 1H), 8.22 (dd, J = 7.6, 7.6 Hz, 1H), 8 .17 (dd, J = 7.9, 1.1 Hz, 1H), 8.09 (d, J = 8.2 Hz, 1H), 7.98 (ddd, J = 7.7, 7.7 , 1.6 Hz, 1H), 7.49 (ddd, J = 6.7, 4.7, 1.0 Hz, 1H), 7.38 (d, J = 8.7 Hz, 1H), 5 .21 (c, J = 9.1 Hz, 2H), 4.01 (s, 3H); LRMS (M+H) m/z 455.53.
Ejemplo 27Example 27
5'-amino-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-25)5'-amino-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-25)
Escala de 0,08 mmoles, con 5-(4,4,5,5-tetrametil-1,3,2-dioxaborolan-2-il)piridin-3-amina, 13,3 mg, 45% de rendimiento.1H RMN (300 MHz, DMSO-ofe) δ 12,67 (s, 1H), 8,86 (d, J = 1,9 Hz, 1H), 8,81 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,58 (s, 1H), 8,24 - 8,15 (m, 4H), 8,07 (ddd, J= 8,1, 1,1, 1,1 Hz, 1H), 7,97 (ddd, J= 7,7, 7,7, 1,8 Hz, 1 H), 7,73 -7,71 (m, 1H), 7,43 (ddd, J= 7,4, 5,0, 1,3 Hz, 1H), 5,60 (s, 2H), 4,03 (s, 3H); LRMS (M+H) m/z372,64.0.08 mmol scale, with 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-amine, 13.3 mg, 45% yield.1H NMR (300 MHz, DMSO-ofe) δ 12.67 (s, 1H), 8.86 (d, J = 1.9 Hz, 1H), 8.81 (ddd, J = 5.0, 1.7 , 0.9 Hz, 1H), 8.58 (s, 1H), 8.24 - 8.15 (m, 4H), 8.07 (ddd, J= 8.1, 1.1, 1.1 Hz, 1H), 7.97 (ddd, J= 7.7, 7.7, 1.8 Hz, 1H), 7.73 -7.71 (m, 1H), 7.43 (ddd, J = 7.4, 5.0, 1.3Hz, 1H), 5.60 (s, 2H), 4.03 (s, 3H); LRMS (M+H) m/z 372.64.
Ejemplo 28Example 28
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(metilamino)-[2,3'-bipiridin]-6-carboxamida (I-26)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(methylamino)-[2,3'-bipyridine]-6-carboxamide (I-26 )
Una disolución en NMP (1 ml) de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (22,5 mg, 0,06 mmoles), hidrocloruro de metilamina (> 5 eq.) y NaHCO3 (>2 eq) se calentó a 150 - 180°C durante 20 días hasta que se formó >50% del producto deseado, según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice, el compuesto del título se obtuvo como un sólido de color beige: 14 mg (61% de rendimiento.); 1H RMN (300 MHz, DMSO-ofe) 512,66 (s, 1H), 8,97 (d, J = 1,9 Hz, 1H), 8,81 (ddd, J = 5,0, 1,7, 1,0 Hz, 1H), 8,58 (s, 1 H), 8,29 (dd, J = 7,1,2,0 Hz, 1H), 8,25 - 8,17 (m, 3H), 8,08 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,97 (ddd, J = 8,1,7,5, 1,8 Hz, 1H), 7,61 -7,60(m, 1H), 7,44 (ddd, J =7,4, 5,0, 1,3 Hz, 1H), 6,18 (c, J = 5,0 Hz, 1 H), 4,02 (s, 3H), 2,85 (d, J = 5,0 Hz, 3H); LRMS (M+H) m/z386,68. An NMP (1 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide (22.5 mg, 0.06 mmol), methylamine hydrochloride (>5 eq.) and NaHCO 3 (>2 eq.) was heated at 150-180°C for 20 days until >50% formed. of the desired product, as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel the title compound was obtained as a beige solid: 14 mg (61% yield.); 1H NMR (300 MHz, DMSO-ofe) 512.66 (s, 1H), 8.97 (d, J = 1.9 Hz, 1H), 8.81 (ddd, J = 5.0, 1.7 , 1.0 Hz, 1H), 8.58 (s, 1H), 8.29 (dd, J = 7.1,2.0 Hz, 1H), 8.25 - 8.17 (m, 3H ), 8.08 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.97 (ddd, J = 8,1,7.5, 1.8 Hz, 1H), 7.61 -7.60(m, 1H), 7.44 (ddd, J =7.4, 5.0, 1.3 Hz, 1H), 6.18 (c, J = 5.0 Hz, 1H), 4.02 (s, 3H), 2.85 (d, J =5.0 Hz, 3H); LRMS (M+H) m/z 386.68.
Ejemplo 29Example 29
5'-(ciclopropilamino)-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-27)5'-(cyclopropylamino)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-27)
Una disolución en NMP (1 ml) de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (22,5 mg, 0,06 mmoles), ciclopropanamina (> 5 eq.) y NaHCO3 (>2 eq) se calentó a 140 - 150°C durante 12 días hasta que se formó >50% del producto deseado, según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y trituración adicional con hexanos-EtOAc, el compuesto del título se obtuvo como un sólido blanquecino: 1,8 mg (7% de rendimiento.); 1H RMN (300 MHz, DMSO-afe) 512,67 (s, 1H), 9,00 (d, J = 1,9 Hz, 1H), 8,80 (ddd, J= 5,0, 1,7, 0,9 Hz, 1H), 8,58 (s, 1H), 8,30 (d, J = 2,6 Hz, 1H), 8,24 - 8,19 (m, 3H), 8,08 (ddd, J= 8,1, 1,1, 1,1 Hz, 1H), 7,97 (ddd, J = 7,7, 7,7, 1,8 Hz, 1H), 7,76 - 7,75 (m, 1 H), 7,42 (ddd, J= 7,3, 5,0, 1,2 Hz, 1H), 6,62 (d, J= 1,7 Hz, 1H), 4,02 (s, 3H), 3,35 - 3,33 (m, overlapped with HzO, 1H), 0,84 - 0,78 (m, 2H), 0,52 - 0,48 (m, 2H); LRMS (M+H) m/z 412,73.An NMP (1 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide (22.5 mg, 0.06 mmol), cyclopropanamine (>5 eq.) and NaHCO 3 (>2 eq.) was heated at 140-150°C for 12 days until >50% product was formed. desired, as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel and further trituration with hexanes-EtOAc, the title compound was obtained as an off-white solid: 1.8 mg (7% yield.); 1H NMR (300 MHz, DMSO-afe) 512.67 (s, 1H), 9.00 (d, J = 1.9 Hz, 1H), 8.80 (ddd, J= 5.0, 1.7 , 0.9 Hz, 1H), 8.58 (s, 1H), 8.30 (d, J = 2.6 Hz, 1H), 8.24-8.19 (m, 3H), 8.08 (ddd, J= 8.1, 1.1, 1.1 Hz, 1H), 7.97 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.76 - 7 .75 (m, 1H), 7.42 (ddd, J= 7.3, 5.0, 1.2 Hz, 1H), 6.62 (d, J= 1.7 Hz, 1H), 4 .02 (s, 3H), 3.35 - 3.33 (m, overlapped with HzO, 1H), 0.84 - 0.78 (m, 2H), 0.52 - 0.48 (m, 2H) ; LRMS (M+H) m/z 412.73.
Ejemplo 30Example 30
5'-(isopropilamino)-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-28)5'-(isopropylamino)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-28)
Una disolución en NMP (1 ml) de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6- carboxamida (22,5 mg, 0,06 mmoles) y propan-2-amina (> 5 eq.) se calentó a 120 - 150°C durante 24 días hasta que se formó >50% del producto deseado. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y la trituración adicional en hexanos-EtOAc, el compuesto del título se obtuvo como un sólido de color beige claro: 7,1 mg (29% de rendimiento): 1H RMN (300 MHz, DMSO-de) 512,66 (s, 1H), 8,90 (d, J= 1,9 Hz, 1H), 8,80 (ddd, J= 5,0, 1,7, 1,0 Hz, 1 H), 8,58 (s, 1H), 8,27 - 8,16 (m, 4H), 8,08 (ddd, J= 8,1, 1,1, 1,1 Hz, 1H), 7,97 (ddd, J= 7,7, 7,7, 1,8 Hz, 1H), 7,66 - 7,64 (m, 1H), 7,43 (ddd, J= 7,4, 5,0, 1,3 Hz, 1H), 5,95 (d, J = 8,3 Hz, 1 H), 4,01 (s, 3H), 3,84 - 3,73 (m, 1H), 1,22 (d, J= 6,3 Hz, 6H); LRMS (M+H) m/z 414,73.An NMP (1 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide (22.5 mg, 0.06 mmol) and propan-2-amine (>5 eq.) was heated at 120-150°C for 24 days until >50% of the desired product was formed. The solvent was removed in vacuo, after silica gel chromatography and further trituration in hexanes-EtOAc the title compound was obtained as a light beige solid: 7.1 mg (29% yield): 1H NMR (300 MHz, DMSO-de) 512.66 (s, 1H), 8.90 (d, J= 1.9 Hz, 1H), 8.80 (ddd, J= 5.0, 1.7, 1 0.0 Hz, 1H), 8.58 (s, 1H), 8.27 - 8.16 (m, 4H), 8.08 (ddd, J= 8.1, 1.1, 1.1 Hz , 1H), 7.97 (ddd, J= 7.7, 7.7, 1.8 Hz, 1H), 7.66 - 7.64 (m, 1H), 7.43 (ddd, J= 7 .4, 5.0, 1.3 Hz, 1H), 5.95 (d, J = 8.3 Hz, 1H), 4.01 (s, 3H), 3.84 - 3.73 (m , 1H), 1.22 (d, J= 6.3 Hz, 6H); LRMS (M+H) m/z 414.73.
Ejemplo 31Example 31
5'-(terc-butilamino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-29)5'-(tert-Butylamino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I -29)
Escala de 0,1 mmol, con N-(terc-butil)-5-(4,4,5,5-tetrametil-1,3,2-dioxaborolan-2-il)piridin-3-amina, 15,2 mg, 36% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) δ 12,65 (s, 1H), 8,94 (d, J = 1,6 Hz, 1H), 8,82 (d, J = 4,4 Hz, 1H), 8,58 (s, 1H), 8,32 (d, J= 2,6 Hz, 1 H), 8,22 - 8,19 (m, 3H), 8,08 (a d, J= 8,0 Hz, 1H), 7,97 (ddd, J= 7,8, 7,8, 1,7 Hz, 1H), 7,81 -7,79 (m, 1H), 7,43 (ddd, J = 7,3, 5,0, 1,0 Hz, 1H), 5,84 (s a, 1H), 4,02 (s, 3H), 1,42 (s, 9H); LRMS (M+H) m/z 428,66. 0.1 mmol scale, with N-(tert-butyl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-amine, 15.2 mg, 36% yield. 1H NMR (300 MHz, DMSO-ofe) δ 12.65 (s, 1H), 8.94 (d, J = 1.6 Hz, 1H), 8.82 (d, J = 4.4 Hz, 1H ), 8.58 (s, 1H), 8.32 (d, J= 2.6 Hz, 1H), 8.22 - 8.19 (m, 3H), 8.08 (bd, J= 8 .0 Hz, 1H), 7.97 (ddd, J= 7.8, 7.8, 1.7 Hz, 1H), 7.81 -7.79 (m, 1H), 7.43 (ddd, J = 7.3, 5.0, 1.0 Hz, 1H), 5.84 (bs, 1H), 4.02 (s, 3H), 1.42 (s, 9H); LRMS (M+H) m/z 428.66.
Ejemplo 32Example 32
N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-5'-((tetrahidro-2H-piran-4-il)amino)-[2,3'-bipiridina]-6-carboxamida (I-30)N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-5'-((tetrahydro-2H-pyran-4-yl)amino)-[2,3'- bipyridine]-6-carboxamide (I-30)
Una disolución en NMP (1,5 ml) de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (30 mg, 0,08 mmoles) y tetrahidro-2H-piran-4-amina 40 mg, 0,4 mmoles) se calentó a 150°C durante 19 días hasta que se formó >50% del producto deseado, según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y trituración adicional con hexanos-EtOAc, el compuesto del título se obtuvo como un sólido de color beige claro: 5 mg (14% de rendimiento); 1H RMN (300 MHz, DMSO-afe) 512,66 (s, 1H), 8,93 (d, J = 1,8 Hz, 1H), 8,80 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,58 (s, 1 H), 8,30 - 8,17 (m, 4H), 8,08 (ddd, J = 8,1, 1,0, 1,0 Hz, 1H), 7,97 (ddd, J = 7,7, 7,7, 1,8 Hz, 1H), 7,72 - 7,71 (m, 1H), 7,43 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 6,10 (d, J = 8,3 Hz, 1H), 4,02 (s, 3H), 3,91 (ddd, J = 11,3, 3,2, 3,2 Hz, 2H), 3,75 - 3,63 (m, 1H), 3,47 - 3,36 (m, parcialmente solapado con H2O, 2H), 2,00 - 1,95 (m, 2H), 1,63 (s, 1H), 1,53 - 1,40 (m, 2H); LRMS (M+H) m/z456,46.An NMP (1.5 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine ]-6-carboxamide (30 mg, 0.08 mmol) and tetrahydro-2H-pyran-4-amine 40 mg, 0.4 mmol) was heated at 150°C for 19 days until >50% product was formed desired, as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel and further trituration with hexanes-EtOAc, the title compound was obtained as a light beige solid: 5mg (14% yield); 1H NMR (300 MHz, DMSO-afe) 512.66 (s, 1H), 8.93 (d, J = 1.8 Hz, 1H), 8.80 (ddd, J = 5.0, 1.7 , 0.9 Hz, 1H), 8.58 (s, 1H), 8.30 - 8.17 (m, 4H), 8.08 (ddd, J = 8.1, 1.0, 1, 0 Hz, 1H), 7.97 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.72 - 7.71 (m, 1H), 7.43 (ddd, J = 7.4, 5.0, 1.3 Hz, 1H), 6.10 (d, J = 8.3 Hz, 1H), 4.02 (s, 3H), 3.91 (ddd, J = 11.3, 3.2, 3.2 Hz, 2H), 3.75 - 3.63 (m, 1H), 3.47 - 3.36 (m, partially overlapped with H2O, 2H), 2.00 - 1.95 (m, 2H), 1.63 (s, 1H), 1.53-1.40 (m, 2H); LRMS (M+H) m/z 456.46.
Ejemplo 33Example 33
5'-((2-metoxietil)amino)-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-31) Una disolución en NMP (1,2 ml) de 5'-fluoro-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (22,5 mg, 0,06 mmoles) y 2-metoxietan-1-amina (>5 eq) se calentó a 140 - 150°C durante 13 días hasta que se completó la reacción, según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y trituración adicional con hexanos-EtOAc, el compuesto del título se obtuvo como un sólido de color canela: 14 mg (54% de rendimiento)); 1H RMN (300 MHz, DMSO-ofe) δ 12,64 (s, 1H), 8,97 (d, J = 1,7 Hz, 1H), 8,82 (ddd, J= 5,0, 1,6, 0,9 Hz, 1 H), 8,57 (s, 1 H), 8,28 - 8,16 (m, 4H), 8,07 (a d, J= 8,0 Hz, 1H), 7,96 (ddd, J = 7,8, 7,8, 1,7 Hz, 1 H), 7,70 - 7,68 (m, 1 H), 7,43 (ddd, J = 7,4, 5,0, 1,2 Hz, 1 H), 6,13 (t, J = 5,7 Hz, 1 H), 4,01 (s, 3H), 3,59 (t, J= 5,5 Hz, 2H), 3,41 - 3,36 (m, parcialmente solapado con H2O, 2H), 3.34 (s, 3H); LRMS (M+H) m/z 430.72.5'-((2-methoxyethyl)amino)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-31) An NMP (1.2 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2, 3'-Bipyridine]-6-carboxamide (22.5 mg, 0.06 mmol) and 2-methoxyethane-1-amine (>5 eq) was heated at 140 - 150°C for 13 days until reaction was complete , as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel and further trituration with hexanes-EtOAc, the title compound was obtained as a tan solid: 14 mg (54% yield)); 1H NMR (300 MHz, DMSO-ofe) δ 12.64 (s, 1H), 8.97 (d, J = 1.7 Hz, 1H), 8.82 (ddd, J= 5.0, 1, 6, 0.9 Hz, 1H), 8.57 (s, 1H), 8.28 - 8.16 (m, 4H), 8.07 (bd, J= 8.0 Hz, 1H), 7.96 (ddd, J = 7.8, 7.8, 1.7 Hz, 1H), 7.70 - 7.68 (m, 1H), 7.43 (ddd, J = 7.4 , 5.0, 1.2 Hz, 1H), 6.13 (t, J =5.7 Hz, 1H), 4.01 (s, 3H), 3.59 (t, J= 5, 5 Hz, 2H), 3.41-3.36 (m, partially overlapped with H2O, 2H), 3.34 (s, 3H); LRMS (M+H) m/z 430.72.
Ejemplo 34Example 34
5'-((ciclopropilmetil)amino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-32) Una mezcla de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (22,5 mg, 0,06 mmoles) y ciclopropilmetanamina (1 ml) se calentó a 150 - 180°C durante 20 días hasta que se formó >50% del producto deseado, según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y trituración adicional con hexanos-EtOAc, el compuesto del título se obtuvo como un sólido de color beige: 8.6 mg (34% de rendimiento)); 1H RMN (300 MHz, DMSO-de) δ 12,65 (s, 1H), 8,94 (d, J = 1,7 Hz, 1H), 8,81 (a d, J = 5,0 Hz, 1H), 8,58 (s, 1H), 8,28 - 8,16 (m, 4H), 8,08 (a d, J = 8,2 Hz, 1 H), 7,96 (ddd, J = 7,5, 7,5, 1,3 Hz, 1H), 7,66 (dd, J = 2,1 Hz, 1H), 7,45 - 7,41 (m, 1 H), 6,21 (t, J= 5,4 Hz, 1H), 4,01 (s, 3H), 3,09 - 3,05 (m, 2H), 1,20 - 1,07 (m, 1H), 0,57 - 0,51 (m, 2H), 0,30 - 0,26 (m, 2H); LRMS (M+H) m/z426,75.5'-((cyclopropylmethyl)amino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide ( I-32) A mixture of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]-6- carboxamide (22.5 mg, 0.06 mmol) and cyclopropylmethanamine (1 mL) was heated at 150-180°C for 20 days until >50% of the desired product was formed, as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel and further trituration with hexanes-EtOAc, the title compound was obtained as a beige solid: 8.6 mg (34% yield)); 1H NMR (300 MHz, DMSO-de) δ 12.65 (s, 1H), 8.94 (d, J = 1.7 Hz, 1H), 8.81 (d, J = 5.0 Hz, 1H ), 8.58 (s, 1H), 8.28 - 8.16 (m, 4H), 8.08 (bd, J = 8.2 Hz, 1H), 7.96 (ddd, J = 7 .5, 7.5, 1.3 Hz, 1H), 7.66 (dd, J = 2.1 Hz, 1H), 7.45 - 7.41 (m, 1H), 6.21 (t , J= 5.4 Hz, 1H), 4.01 (s, 3H), 3.09 - 3.05 (m, 2H), 1.20 - 1.07 (m, 1H), 0.57 - 0.51 (m, 2H), 0.30-0.26 (m, 2H); LRMS (M+H) m/z 426.75.
Ejemplo 35Example 35
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-((2,2,2-trifluoroetil)amino)-[2,3'-bipiridin]-6-carboxamida (I-33) 1H RMN (300 MHz, DMSO-afe) 512,64 (s, 1H), 9,10 (d, J = 1,7 Hz, 1H), 8,84 (a d, J= 4,9 Hz, 1H), 8,58 (s, 1H), 8,36 (d, J = 2,6 Hz, 1H), 8,32 (dd, J = 7,5, 1,5 Hz, 1H), 8,25 (dd, J = 7,5, 7,5 Hz, 1 H), 8,20 (dd, J= 7,5, 1,6 Hz, 1 H), 8,08 (d, J = 8,1 Hz, 1H), 7,97 (ddd, J = 7,7, 7,7, 1,7 Hz, 1 H), 7,89 (dd, J = 2,1,2,1 Hz, 1 H), 7,43 (ddd, J= 7,3, 5,0, 1,2 Hz, 1H), 6,79 (t, J= 6,9 Hz, 1H), 4,26 - 4,14 (m, 2H), 4,02 (s, 3H); LRMS (M+H) m/z454,69.N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-5'-((2,2,2-trifluoroethyl)amino)-[2,3'-bipyridine ]-6-carboxamide (I-33) 1H NMR (300 MHz, DMSO-afe) 512.64 (s, 1H), 9.10 (d, J = 1.7 Hz, 1H), 8.84 (d, J= 4.9 Hz, 1H) , 8.58 (s, 1H), 8.36 (d, J = 2.6 Hz, 1H), 8.32 (dd, J = 7.5, 1.5 Hz, 1H), 8.25 ( dd, J = 7.5, 7.5 Hz, 1 H), 8.20 (dd, J = 7.5, 1.6 Hz, 1 H), 8.08 (d, J = 8.1 Hz , 1H), 7.97 (ddd, J = 7.7, 7.7, 1.7 Hz, 1H), 7.89 (dd, J = 2,1,2.1 Hz, 1H), 7.43 (ddd, J= 7.3, 5.0, 1.2 Hz, 1H), 6.79 (t, J= 6.9 Hz, 1H), 4.26 - 4.14 (m, 2H), 4.02 (s, 3H); LRMS (M+H) m/z 454.69.
Ejemplo 36Example 36
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(metilsulfonamido)-[2,3'-bipiridin]-6-carboxamida (I-34) N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-5'-(methylsulfonamido)-[2,3'-bipyridine]-6-carboxamide (I-34 )
Escala de 0,08 mmoles, con N-(5-(4,4,5,5tetrametil-1,3,2-dioxaborolan-2-il)piridin-3-il)metanosulfonamida, 21,3 mg, 59% de rendimiento. 1H RMN (300 MHz, DMSO-afe) δ 12,63 (s, 1H), 10,27 (s, 1H), 9,48 (d, J= 2,0 Hz, 1H), 8,83 (ddd, J= 5,0, 1,7, 1,0 Hz, 1 H), 8,69 (d, J= 2,4 Hz, 1H), 8,59 (s, 1 H), 8,36 - 8,35 (m, 1H), 8,31 - 8,23 (m, 3H), 8,08 (ddd, J= 8,1, 1,1, 1,1 Hz, 1H), 7,97 (ddd, J = 8,1,7,5, 1,8 Hz, 1H), 7,43 (ddd, J= 7,4, 5,0, 1,3 Hz, 1 H), 4,02 (s, 3H), 3,20 (s, 3H); LRMS (M+H) m/z 450,67.0.08 mmol scale, with N-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-yl)methanesulfonamide, 21.3 mg, 59% de performance. 1H NMR (300 MHz, DMSO-afe) δ 12.63 (s, 1H), 10.27 (s, 1H), 9.48 (d, J= 2.0 Hz, 1H), 8.83 (ddd , J= 5.0, 1.7, 1.0 Hz, 1H), 8.69 (d, J= 2.4 Hz, 1H), 8.59 (s, 1H), 8.36 - 8.35 (m, 1H), 8.31 - 8.23 (m, 3H), 8.08 (ddd, J= 8.1, 1.1, 1.1 Hz, 1H), 7.97 ( ddd, J = 8,1,7.5, 1.8 Hz, 1H), 7.43 (ddd, J= 7.4, 5.0, 1.3 Hz, 1H), 4.02 (s , 3H), 3.20 (s, 3H); LRMS (M+H) m/z 450.67.
Ejemplo 37Example 37
5'-(dimetilamino)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-35)5'-(dimethylamino)-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-35 )
Una disolución en NMP (1 ml) de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (22,5 mg, 0,06 mmoles), hidrocloruro de dimetilamina (> 5 eq.) y NaHCO3 (>2 eq) se calentó a 120 - 150°C durante 11 días hasta que se formó >50% del producto deseado, según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y trituración adicional con hexanos-EtOAc, el compuesto del título se obtuvo como un sólido de color canela: 4.3 mg (18% de rendimiento)); 1H RMN (300 MHz, DMSO-afe) δ 12,65 (s, 1H), 9,12 (d, J = 1,7 Hz, 1H), 8,83 (a d, J = 4,9 Hz, 1 H), 8,58 (s, 1H), 8,40 (dd, J = 7,2, 1,8 Hz, 1 H), 8,35 (d, J = 2,8 Hz, 1H), 8,26 -8,18 (m, 2H), 8,09 (d, J = 8,0 Hz, 1 H), 7,97 (ddd, J = 7,7, 7,7, 1,8 Hz, 1 H), 7,80 - 7,79 (m, 1 H), 7,44 (ddd, J= 7,4, 5,0, 1,2 Hz, 1 H), 4,02 (s, 3H), 3,08 (s, 6H); LRMS (M+H) m/z400,66.An NMP (1 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide (22.5 mg, 0.06 mmol), dimethylamine hydrochloride (>5 eq.) and NaHCO 3 (>2 eq.) was heated at 120-150°C for 11 days until >50% formed. of the desired product, as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel and further trituration with hexanes-EtOAc, the title compound was obtained as a tan solid: 4.3 mg (18% yield)); 1H NMR (300 MHz, DMSO-afe) δ 12.65 (s, 1H), 9.12 (d, J = 1.7 Hz, 1H), 8.83 (d, J = 4.9 Hz, 1 H), 8.58 (s, 1H), 8.40 (dd, J = 7.2, 1.8 Hz, 1H), 8.35 (d, J = 2.8 Hz, 1H), 8 .26 -8.18 (m, 2H), 8.09 (d, J = 8.0 Hz, 1 H), 7.97 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.80 - 7.79 (m, 1H), 7.44 (ddd, J= 7.4, 5.0, 1.2 Hz, 1H), 4.02 (s, 3H ), 3.08 (s, 6H); LRMS (M+H) m/z 400.66.
Ejemplo 38Example 38
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(pirrolidin-1-il)-[2,3'-bipiridin]-6-carboxamida (I-36)N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-5'-(pyrrolidin-1-yl)-[2,3'-bipyridine]-6-carboxamide (I-36)
Una disolución en NMP (1 ml) de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (22,5 mg, 0,06 mmoles) y pirrolidina (0,1 ml) se calentó a 120 - 150°C durante 4 días hasta que se completó la reacción, según se monitorizó mediante LC-m S. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y trituración adicional con hexanos-EtOAc, el compuesto del título se obtuvo como un sólido amarillo: 14,9 mg (58% de rendimiento): 1H RMN (300 MHz, DMSO-afe) δ 12,66 (s, 1H), 9,06 (d, J= 1,8 Hz, 1H), 8,81 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,58 (s, 1H), 8,37 (dd, J = 7,2, 1,8 Hz, 1H), 8,25 - 8,17 (m, 3H), 8,08 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,97 (ddd, J = 7,7, 7,7, 1,8 Hz, 1 H), 7,60 - 7,59 (m, 1H), 7,44 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 4,02 (s, 3H), 3,44 - 3,39 (m, 4H), 2,05 - 2,01 (m, 4H); LRMS (M+H) m/z426,69. An NMP (1 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide (22.5 mg, 0.06 mmol) and pyrrolidine (0.1 mL) was heated at 120-150°C for 4 days until reaction was complete, as monitored by LC-mS. Solvent was removed in vacuo, after chromatography on silica gel and further trituration with hexanes-EtOAc, the title compound was obtained as a yellow solid: 14.9 mg (58% yield): 1H NMR (300 MHz, DMSO -afe) δ 12.66 (s, 1H), 9.06 (d, J= 1.8 Hz, 1H), 8.81 (ddd, J = 5.0, 1.7, 0.9 Hz, 1H), 8.58 (s, 1H), 8.37 (dd, J = 7.2, 1.8 Hz, 1H), 8.25 - 8.17 (m, 3H), 8.08 (ddd , J = 8.1, 1.1, 1.1 Hz, 1H), 7.97 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.60 - 7, 59 (m, 1H), 7.44 (ddd, J = 7.4, 5.0, 1.3 Hz, 1H), 4.02 (s, 3H), 3.44 - 3.39 (m, 4H), 2.05-2.01 (m, 4H); LRMS (M+H) m/z 426.69.
Ejemplo 39Example 39
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-morfolino-[2,3'-bipiridin]-6-carboxamida (I-37)N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-5'-morpholino-[2,3'-bipyridine]-6-carboxamide (I-37)
Una disolución en NMP (1 ml) de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (22,5 mg, 0,06 mmoles) y morfolina (0,1 ml) se calentó a 120 - 150°C durante 11 días hasta que se completó la reacción, según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y trituración adicional con hexanos-EtOAc, el compuesto del título se obtuvo como un sólido blanquecino: 21,4 mg (87% de rendimiento)); 1H RMN (300 MHz, DMSO-afe) δ 12,64 (s, 1H), 9,23 (d, J = 1,7 Hz, 1H), 8,82 (a d, J = 5,0 Hz, 1H), 8,58 (s, 1 H), 8,54 (d, J = 2,7 Hz, 1 H), 8,42 (dd, J = 7,2, 1,7 Hz, 1H), 8,26 - 8,18 (m, 2H), 8,10 - 8,07 (m, 2H), 7,98 (ddd, J= 7,7, 7,7, 1,7 Hz, 1H), 7,46 (ddd, J= 7,4, 5,0, 1,2 Hz, 1H), 4,02 (s, 3H), 3,84 - 3,81 (m, 4H), 3,37 - 3,34(m, parcialmente solapado con H2O, 2H), 3.34 (s, 4H); LRMS (M+H) m/z 442.59.An NMP (1 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide (22.5 mg, 0.06 mmol) and morpholine (0.1 mL) was heated at 120-150°C for 11 days until reaction was complete, as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel and further trituration with hexanes-EtOAc, the title compound was obtained as an off-white solid: 21.4 mg (87% yield)); 1H NMR (300 MHz, DMSO-afe) δ 12.64 (s, 1H), 9.23 (d, J = 1.7 Hz, 1H), 8.82 (bd, J = 5.0 Hz, 1H ), 8.58 (s, 1H), 8.54 (d, J = 2.7 Hz, 1H), 8.42 (dd, J = 7.2, 1.7 Hz, 1H), 8 .26 - 8.18 (m, 2H), 8.10 - 8.07 (m, 2H), 7.98 (ddd, J= 7.7, 7.7, 1.7 Hz, 1H), 7 .46 (ddd, J= 7.4, 5.0, 1.2 Hz, 1H), 4.02 (s, 3H), 3.84 - 3.81 (m, 4H), 3.37 - 3 .34(m, partially overlapped with H2O, 2H), 3.34 (s, 4H); LRMS (M+H) m/z 442.59.
Ejemplo 40Example 40
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(4-metilpiperazin-1-il)-[2,3'-bipiridin]-6-carboxamida (I-38)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(4-methylpiperazin-1-yl)-[2,3'-bipyridin]-6 -carboxamide (I-38)
Una disolución en NMP (1 ml) de 5'-fluoro-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (22,5 mg, 0,06 mmoles) y 1-metilpiperazina (0,1 ml) se calentó a 120 - 150°C durante 11 días hasta que se formó >50% del producto deseado, según se monitorizó mediante LC-MS. El disolvente se eliminó a vacío, tras la cromatografía en gel de sílice y trituración adicional con hexanos-EtOAc, el compuesto del título se obtuvo como un sólido de color tostado claro: 12.4 mg (45% de rendimiento); 1H RMN (300 MHz, DMSO-afe) δ 12,64 (s, 1H), 9,19 (d, J= 1,7 Hz, 1H), 8,81 (ddd, J = 4,9, 1,6, 0,9 Hz, 1H), 8,58 (s, 1H), 8,53 (d, J = 2,7 Hz, 1H), 8,41 (dd, J= 7,0, 1,9 Hz, 1H), 8,26 - 8,18 (m, 2H), 8,10 - 8,07 (m, 2H), 7,98 (ddd, J = 7,7, 7,7, 1,8 Hz, 1H), 7,45 (ddd, J= 7,4, 5,0, 1,3 Hz, 1 H), 4,02 (s, 3H), 3,38 -3,35 (m, parcialmente solapado con H2O, 4H), 2,56 - 2,52 (m, solapado con DMSO, 4H), 2.28 (s, 3H); LRMS (M+H) m/z 455.77.An NMP (1 mL) solution of 5'-fluoro-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridin]- 6-carboxamide (22.5 mg, 0.06 mmol) and 1-methylpiperazine (0.1 mL) was heated at 120-150°C for 11 days until >50% of the desired product was formed, as monitored by LC-MS. The solvent was removed in vacuo, after chromatography on silica gel and further trituration with hexanes-EtOAc, the title compound was obtained as a light tan solid: 12.4 mg (45% yield); 1H NMR (300 MHz, DMSO-afe) δ 12.64 (s, 1H), 9.19 (d, J= 1.7 Hz, 1H), 8.81 (ddd, J = 4.9, 1, 6, 0.9 Hz, 1H), 8.58 (s, 1H), 8.53 (d, J = 2.7 Hz, 1H), 8.41 (dd, J= 7.0, 1.9 Hz, 1H), 8.26 - 8.18 (m, 2H), 8.10 - 8.07 (m, 2H), 7.98 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.45 (ddd, J= 7.4, 5.0, 1.3 Hz, 1H), 4.02 (s, 3H), 3.38 -3.35 (m, partially overlapped with H2O, 4H), 2.56-2.52 (m, overlapped with DMSO, 4H), 2.28 (s, 3H); LRMS (M+H) m/z 455.77.
Ejemplo 41Example 41
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-(metilsulfonil)-[2,3'-bipiridin]-6-carboxamida (I-39)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-(methylsulfonyl)-[2,3'-bipyridine]-6-carboxamide (I-39 )
Escala de 0,1 mmol, con ácido (5-(metilsulfonil)piridin-3-il)borónico, 26,1 mg, 45% de rendimiento. 1H RMN (300 MHz, DMSO-afe) 512,58 (s, 1 H), 10,02 (d, J = 2,1 Hz, 1H), 9,31 (d, J = 2,1 Hz, 1H), 9,05 (dd, J = 2,1,2,1 Hz, 1 H), 8,83 (ddd, J = 5,0, 1,7, 1,0 Hz, 1H), 8,59 (s, 1H), 8,54 (dd, J = 6,7, 2,2 Hz, 1 H), 8,35 - 8,28 (m, 2H), 8,08 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,97 (ddd, J = 7,8, 7,8, 1,8 Hz, 1H), 7,43 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 4,02 (s, 3H), 3,50 (s, 3H); LRMS (M+H) m/z 435,45. 0.1 mmol scale, with (5-(methylsulfonyl)pyridin-3-yl)boronic acid, 26.1 mg, 45% yield. 1H NMR (300 MHz, DMSO-afe) 512.58 (s, 1H), 10.02 (d, J = 2.1 Hz, 1H), 9.31 (d, J = 2.1 Hz, 1H ), 9.05 (dd, J = 2,1,2.1 Hz, 1H), 8.83 (ddd, J = 5.0, 1.7, 1.0 Hz, 1H), 8.59 (s, 1H), 8.54 (dd, J = 6.7, 2.2 Hz, 1H), 8.35 - 8.28 (m, 2H), 8.08 (ddd, J = 8, 1, 1.1, 1.1 Hz, 1H), 7.97 (ddd, J = 7.8, 7.8, 1.8 Hz, 1H), 7.43 (ddd, J = 7.4, 5.0, 1.3Hz, 1H), 4.02 (s, 3H), 3.50 (s, 3H); LRMS (M+H) m/z 435.45.
Ejemplo 42Example 42
N6-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-5',6-dicarboxamida (I-40)N6-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-5',6-dicarboxamide (I-40)
Ejemplo 43Example 43
N6-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,4'-bipiridin]-2',6-dicarboxamida (I-41)N6-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,4'-bipyridine]-2',6-dicarboxamide (I-41)
Ejemplo 44Example 44
2'-ciano-N-(1-metil-3-(piridin-2-il)-1H-pirazol-4-il)-[2,4'-bipiridin]-6-carboxamida(I-42)2'-Cyano-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,4'-bipyridine]-6-carboxamide(I-42)
Ejemplo 45Example 45
6'-amino-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-43)6'-amino-N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-43)
Escala de 0,1 mmol, con ácido (6-aminopiridin-3-il)borónico, 18,8 mg, 51% de rendimiento.1H RMN (300 MHz, DMSO-de) 512,49 (s, 1 H), 9,14 (d, J = 2,2 Hz, 1H), 8,84 (a d, J = 4,9 Hz, 1 H), 8,58 (s, 1 H), 8,36 (dd, J = 8,7, 2,5 Hz, 1 H), 8,14 (dd, J = 7,8, 1,6 Hz, 1H), 8,13 - 7,94 (m, 4H), 7,44 (ddd, J = 7,4, 5,0, 1,2 Hz, 1 H), 6,70 (d, J = 8,7 Hz, 1H), 6,53 (s, 2H), 4,01 (s, 3H); LRMS (M+H) m/z372,50.0.1 mmol scale, with (6-aminopyridin-3-yl)boronic acid, 18.8 mg, 51% yield.1H NMR (300 MHz, DMSO- de) 512.49 (s, 1H), 9.14 (d, J = 2.2 Hz, 1H), 8.84 (dd, J = 4.9 Hz, 1H), 8.58 (s, 1H), 8.36 (dd, J = 8.7, 2.5 Hz, 1H), 8.14 (dd, J = 7.8, 1.6 Hz, 1H), 8.13 - 7.94 (m, 4H), 7.44 (ddd, J = 7.4, 5.0, 1.2 Hz, 1H), 6.70 (d, J = 8.7 Hz, 1H), 6.53 (s, 2H), 4.01 (s, 3H); LRMS (M+H) m/z 372.50.
Ejemplo 46Example 46
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirrolo[2,3-b]piridin-5-il)picolinamida (I-44) N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrrolo[2,3-b]pyridin-5-yl)picolinamide (I -44)
Escala de 0,1 mmol, con ácido (1 H-pirrolo[2,3-b]piridin-5-il)borónico, 26,1 mg, 66% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 512,64 (s, 1H), 12,06 (s, 1H), 9,46 (d, J = 2,1 Hz, 1H), 8,95 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,92 (d, J = 2,0 Hz, 1H), 8,60 (s, 1H), 8,39 (dd, J = 7,7, 1,2 Hz, 1H), 8,20 (dd, J = 7,7, 7,7 Hz, 1H), 8,15 (d, J = 1,2 Hz, 1H), 8,11 (d, J = 8,2 Hz, 1H), 8,00 (ddd, J = 7,7, 7,7, 1,8 Hz, 1 H), 7,66 (dd, J= 3,3, 2,6 Hz, 1H), 7,46 (ddd, J = 7,4, 5,0, 1,3 Hz, 1 H), 6,67 (dd, J = 3,4, 1,8 Hz, 1 H), 4,03 (s, 3H); LRMS (M+H) m/z396,57.0.1 mmol scale, with (1H-pyrrolo[2,3-b]pyridin-5-yl)boronic acid, 26.1 mg, 66% yield. 1H NMR (300 MHz, DMSO-ofe) 512.64 (s, 1H), 12.06 (s, 1H), 9.46 (d, J = 2.1 Hz, 1H), 8.95 (ddd, J = 5.0, 1.7, 0.9 Hz, 1H), 8.92 (d, J = 2.0 Hz, 1H), 8.60 (s, 1H), 8.39 (dd, J = 7.7, 1.2 Hz, 1H), 8.20 (dd, J = 7.7, 7.7 Hz, 1H), 8.15 (d, J = 1.2 Hz, 1H), 8 .11 (d, J = 8.2 Hz, 1H), 8.00 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.66 (dd, J= 3, 3, 2.6 Hz, 1H), 7.46 (ddd, J = 7.4, 5.0, 1.3 Hz, 1H), 6.67 (dd, J = 3.4, 1.8 Hz, 1H), 4.03 (s, 3H); LRMS (M+H) m/z 396.57.
Ejemplo 47Example 47
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirrolo[3,2-b]piridin-6-il)picolinamida (I-45)N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrrolo[3,2-b]pyridin-6-yl)picolinamide (I -Four. Five)
Escala de 0,1 mmol, con 6-(4,4,5,5-tetrametil-1,3,2-dioxaborolan-2-il)-1H-pirrolo[3,2-b]piridina, 9,7 mg, 25% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) δ 12,67 (s, 1H), 11,68 (s, 1H), 9,60 (d, J= 2,0 Hz, 1H), 8,93 (ddd, J= 5,0, 1,6, 0,9 Hz, 1H), 8,60 - 8,59 (m, 2H), 8,38 (dd, J= 7,6, 1,3 Hz, 1H), 8,21 (dd, J= 7,6, 7,6 Hz, 1 H), 8,15 (dd, J= 7,6, 1,3 Hz, 1 H), 8,10 (ddd, J= 8,1, 1,1, 1,1 Hz, 1H), 7,98 (ddd, J= 7,7, 7,7, 1,8 Hz, 1H), 7,87 - 7,85 (m, 1H), 7,47 (ddd, J= 7,4, 5,0, 1,3 Hz, 1 H), 6,78 - 6,77 (m, 1H), 4,03 (s, 3H); LRMS (M+H) m/z 396,59.0.1 mmol scale, with 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrrolo[3,2-b]pyridine, 9.7 mg , 25% yield. 1H NMR (300 MHz, DMSO-ofe) δ 12.67 (s, 1H), 11.68 (s, 1H), 9.60 (d, J= 2.0 Hz, 1H), 8.93 (ddd , J= 5.0, 1.6, 0.9 Hz, 1H), 8.60 - 8.59 (m, 2H), 8.38 (dd, J= 7.6, 1.3 Hz, 1H ), 8.21 (dd, J= 7.6, 7.6 Hz, 1 H), 8.15 (dd, J= 7.6, 1.3 Hz, 1 H), 8.10 (ddd, J= 8.1, 1.1, 1.1 Hz, 1H), 7.98 (ddd, J= 7.7, 7.7, 1.8 Hz, 1H), 7.87 - 7.85 ( m, 1H), 7.47 (ddd, J= 7.4, 5.0, 1.3 Hz, 1H), 6.78 - 6.77 (m, 1H), 4.03 (s, 3H ); LRMS (M+H) m/z 396.59.
Ejemplo 48Example 48
6-(1 H-indol-5-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-46)6-(1 H-indol-5-yl)-N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide (I-46)
Escala de 0,1 mmol, con ácido (1 H-indol-5-il)borónico, 27,9 mg, 70% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 5 12,67 (s, 1 H), 11,40 (s, 1H), 8,85 (ddd, J= 5,0, 1,6, 0,9 Hz, 1H), 8,65 (d, J = 1,3 Hz, 1H), 8,60 (s, 1 H), 8,29 (dd, J= 7,7, 1,3 Hz, 1 H), 8,20 (dd, J= 8,6, 1,7 Hz, 1H), 8,16 - 8,07 (m, 3H), 8,00 (ddd, J= 7,7, 7,7, 1,8 Hz, 1 H), 7,68 (d, J = 8,6 Hz, 1H), 7,54-7,52(m, 1H), 7,46 (ddd, J= 7,4, 5,0, 1,2 Hz, 1H), 6,65 - 6,64 (m, 1 H), 4,02 (s, 3H); LRMS (M+H) m/z 395,57. Ejemplo 490.1 mmol scale, with (1H-indol-5-yl)boronic acid, 27.9 mg, 70% yield. 1H NMR (300 MHz, DMSO-ofe) 5 12.67 (s, 1H), 11.40 (s, 1H), 8.85 (ddd, J= 5.0, 1.6, 0.9 Hz , 1H), 8.65 (d, J =1.3 Hz, 1H), 8.60 (s, 1H), 8.29 (dd, J= 7.7, 1.3 Hz, 1H) , 8.20 (dd, J= 8.6, 1.7 Hz, 1H), 8.16 - 8.07 (m, 3H), 8.00 (ddd, J= 7.7, 7.7, 1.8 Hz, 1H), 7.68 (d, J = 8.6 Hz, 1H), 7.54-7.52(m, 1H), 7.46 (ddd, J= 7.4, 5.0, 1.2 Hz, 1H), 6.65-6.64 (m, 1H), 4.02 (s, 3H); LRMS (M+H) m/z 395.57. Example 49
6-(1 H-indol-6-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-47)6-(1 H-indol-6-yl)-N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide (I-47)
Escala de 0,1 mmol, con ácido (1 H-indol-6-il)borónico, 37,8 mg, 96% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 5 12,69 (s, 1H), 11,44 (s, 1 H), 8,76 (a d, J= 4,9 Hz, 1H), 8,60 (s, 1H), 8,32 (s, 1H), 8,27 (dd, J = 7,6, 1,4 Hz, 1H), 8,20 -8,07 (m, 4H), 7,98 (ddd, J= 7,7, 7,7, 1,7 Hz, 1 H), 7,87 (d, J= 8,3 Hz, 1H), 7,56 (dd, J = 2,7, 2,7 Hz, 1 H), 7,45 (ddd, J= 7,3, 5,0, 1,2 Hz, 1 H), 6,61 - 6,60 (m, 1H), 4,02 (s, 3H); LRMS (M+H) m/z 395,54. 0.1 mmol scale, with (1H-indol-6-yl)boronic acid, 37.8 mg, 96% yield. 1H NMR (300 MHz, DMSO-ofe) 5 12.69 (s, 1H), 11.44 (s, 1H), 8.76 (ad, J= 4.9 Hz, 1H), 8.60 ( s, 1H), 8.32 (s, 1H), 8.27 (dd, J = 7.6, 1.4 Hz, 1H), 8.20 -8.07 (m, 4H), 7.98 (ddd, J= 7.7, 7.7, 1.7 Hz, 1H), 7.87 (d, J= 8.3 Hz, 1H), 7.56 (dd, J =2.7, 2.7 Hz, 1H), 7.45 (ddd, J= 7.3, 5.0, 1.2 Hz, 1H), 6.61 - 6.60 (m, 1H), 4.02 (s, 3H); LRMS (M+H) m/z 395.54.
Ejemplo 50Example 50
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-fenilpicolinamida (I-48)N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-phenylpicolinamide (I-48)
Escala de 0,08 mmoles, con ácido fenilborónico, 20,5 mg, 72% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) δ 12,58 (s, 1 H), 8,75 (ddd, J= 5,0, 1,7, 1,0 Hz, 1H), 8,59 (s, 1H), 8,45 - 8,42 (m, 2H), 8,32 (dd, J= 6,9, 2,1 Hz, 1H), 8,24 - 8,17 (m, 2H), 8,09 (ddd, J= 8,0, 1,1, 1,1 Hz, 1 H), 7,98 (ddd, J= 7,7, 7,7, 1,8 Hz, 1H), 7,75 - 7,70 (m, 2H), 7,66 - 7,61 (m, 1H), 7,49 (ddd, J= 7,4, 5,0, 1,3 Hz, 1 H), 4,02 (s, 3H); LRMS (M+H) m/z356,59.0.08 mmol scale, with phenylboronic acid, 20.5 mg, 72% yield. 1H NMR (300 MHz, DMSO-ofe) δ 12.58 (s, 1H), 8.75 (ddd, J= 5.0, 1.7, 1.0 Hz, 1H), 8.59 (s , 1H), 8.45 - 8.42 (m, 2H), 8.32 (dd, J= 6.9, 2.1 Hz, 1H), 8.24 - 8.17 (m, 2H), 8.09 (ddd, J= 8.0, 1.1, 1.1 Hz, 1H), 7.98 (ddd, J= 7.7, 7.7, 1.8 Hz, 1H), 7 .75 - 7.70 (m, 2H), 7.66 - 7.61 (m, 1H), 7.49 (ddd, J= 7.4, 5.0, 1.3 Hz, 1H), 4.02 (s, 3H); LRMS (M+H) m/z 356.59.
Ejemplo 51Example 51
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(m-tolil)picolinamida (I-49)N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(m-tolyl)picolinamide (I-49)
Escala de 0,08 mmoles, con ácido m-tolilborónico, 15,8 mg, 71% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 5 12,63 (s, 1H), 8,67 (a d, J= 4,9 Hz, 1H), 8,58 (s, 1H), 8,29 - 8,17 (m, 4H), 8,12 (s, 1H), 8,07 (d, J= 8,1 Hz, 1H), 7,97 (ddd, J= 7,7, 7,7, 1,8 Hz, 1H), 7,61 (dd, J= 7,6, 7,6 Hz, 1 H), 7,48 - 7,43 (m, 2H), 4,02 (s, 3H), 2,51 (s, 3H); LRMS (M+H) m/z 370,63.0.08 mmol scale, with m-tolylboronic acid, 15.8 mg, 71% yield. 1H NMR (300 MHz, DMSO-ofe) 5 12.63 (s, 1H), 8.67 (bd, J= 4.9 Hz, 1H), 8.58 (s, 1H), 8.29 - 8 .17 (m, 4H), 8.12 (s, 1H), 8.07 (d, J= 8.1 Hz, 1H), 7.97 (ddd, J= 7.7, 7.7, 1 0.8 Hz, 1H), 7.61 (dd, J= 7.6, 7.6 Hz, 1H), 7.48 - 7.43 (m, 2H), 4.02 (s, 3H), 2.51 (s, 3H); LRMS (M+H) m/z 370.63.
Ejemplo 52Example 52
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(p-tolil)picolinamida (I-50)N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(p-tolyl)picolinamide (I-50)
Escala de 0,08 mmoles, con ácido p-tolilborónico, 22,1 mg, 75% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) 5 12,64 (s, 1 H), 8,68 (a d, J= 4,9 Hz, 1H), 8,58 (s, 1H), 8,28 (dd, J= 6,7, 2,4 Hz, 1H), 8,25 - 8,17 (m, 3H), 8,13 (s, 1H), 8,08 (d, J= 8,1 Hz, 1H), 7,98 (ddd, J= 7,7, 7,7, 1,8 Hz, 1H), 7,62 (dd, J = 7,6, 7,6 Hz, 1 H), 7,48 - 7,43 (m, 2H), 4,02 (s, 3H), 2,51 (s, 3H); LRMS (M+H) m/z370,18.0.08 mmol scale, with p-tolylboronic acid, 22.1 mg, 75% yield. 1H NMR (300 MHz, DMSO-ofe) 5 12.64 (s, 1H), 8.68 (ad, J= 4.9 Hz, 1H), 8.58 (s, 1H), 8.28 ( dd, J= 6.7, 2.4 Hz, 1H), 8.25 - 8.17 (m, 3H), 8.13 (s, 1H), 8.08 (d, J= 8.1 Hz , 1H), 7.98 (ddd, J= 7.7, 7.7, 1.8 Hz, 1H), 7.62 (dd, J = 7.6, 7.6 Hz, 1H), 7 .48-7.43 (m, 2H), 4.02 (s, 3H), 2.51 (s, 3H); LRMS (M+H) m/z 370.18.
Ejemplo 53Example 53
6-(3-metoxifenil)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-51)6-(3-methoxyphenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-51)
Escala de 0,08 mmoles, con ácido (3-metoxifenil)borónico, 2,2 mg, 7% de rendimiento. 1H RMN (300 MHz, DMSO-ofe) δ 12,58 (s, 1H), 8,82 (a d, J= 5,0 Hz, 1H), 8,59 (s, 1H), 8,33 (dd, J= 6,4, 2,7 Hz, 1H), 8,23 - 8,17 (m, 2H), 8,08 (d, J= 8,0 Hz, 1 H), 8,01 - 7,94 (m, 3H), 7,65 (dd, J= 8,0, 8,0 Hz, 1H), 7,48 - 7,44 (m, 1H), 7,23 (dd, J= 8,1,2,6 Hz, 1 H), 4,02 (s, 3H), 3,95 (s, 3H); LRMS (M+H) m/z386,58. 0.08 mmol scale, with (3-methoxyphenyl)boronic acid, 2.2 mg, 7% yield. 1H NMR (300 MHz, DMSO-ofe) δ 12.58 (s, 1H), 8.82 (ad, J= 5.0 Hz, 1H), 8.59 (s, 1H), 8.33 (dd , J= 6.4, 2.7 Hz, 1H), 8.23 - 8.17 (m, 2H), 8.08 (d, J= 8.0 Hz, 1H), 8.01 - 7 .94 (m, 3H), 7.65 (dd, J= 8.0, 8.0 Hz, 1H), 7.48 - 7.44 (m, 1H), 7.23 (dd, J= 8 ,1,2.6 Hz, 1H), 4.02 (s, 3H), 3.95 (s, 3H); LRMS (M+H) m/z 386.58.
Ejemplo 54Example 54
6-(4-metoxifenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-52)6-(4-methoxyphenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-52)
Escala de 0,08 mmoles, con ácido (4-metoxifenil)borónico, 16 mg, 52% de rendimiento.1H RMN (300 MHz, DMSO-afe) δ 12,54 (s, 1H), 8,77 - 8,75 (m, 1 H), 8,58 (s, 1H), 8,40 - 8,37 (m, 2H), 8,24 (dd, J= 7,5, 1,6 Hz, 1H), 8,17 - 8,07 (m, 3H), 7,98 (ddd, J= 7,5, 7,5, 1,7 Hz, 1 H), 7,51 (ddd, J= 7,4, 5,0, 1,3 Hz, 1 H), 7,27 - 7,24 (m, 2H), 4,01 (s, 3H), 3,94 (s, 3H);0.08 mmol scale, with (4-methoxyphenyl)boronic acid, 16 mg, 52% yield.1H NMR (300 MHz, DMSO-afe) δ 12.54 (s, 1H), 8.77 - 8, 75 (m, 1H), 8.58 (s, 1H), 8.40 - 8.37 (m, 2H), 8.24 (dd, J= 7.5, 1.6 Hz, 1H), 8.17 - 8.07 (m, 3H), 7.98 (ddd, J= 7.5, 7.5, 1.7 Hz, 1H), 7.51 (ddd, J= 7.4, 5.0, 1.3 Hz, 1H), 7.27-7.24 (m, 2H), 4.01 (s, 3H), 3.94 (s, 3H);
LRMS (M+H) m/z386,64.LRMS (M+H) m/z 386.64.
Ejemplo 55Example 55
6-(3-cianofenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-53)6-(3-cyanophenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-53)
Escala de 0,08 mmoles, con ácido (3-cianofenil)borónico, 21 mg, 69% de rendimiento. 1H RMN (300 MHz, DMSO-afe) δ 12,46 (s, 1 H), 8,73 - 8,66 (m, 3H), 8,53 (s, 1H), 8,37 (dd, J = 6,1,2,7 Hz, 1H), 8,24 - 8,17 (m, 2H), 8,07 - 8,03 (m, 2H), 7,97 - 7,86 (m, 2H), 7,41 - 7,37 (m, 1 H), 4,00 (s, 3H); LRMS (M+H) m/z381,61.0.08 mmol scale, with (3-cyanophenyl)boronic acid, 21 mg, 69% yield. 1H NMR (300 MHz, DMSO-afe) δ 12.46 (s, 1H), 8.73-8.66 (m, 3H), 8.53 (s, 1H), 8.37 (dd, J = 6.1,2.7 Hz, 1H), 8.24 - 8.17 (m, 2H), 8.07 - 8.03 (m, 2H), 7.97 - 7.86 (m, 2H ), 7.41-7.37 (m, 1H), 4.00 (s, 3H); LRMS (M+H) m/z 381.61.
Ejemplo 56Example 56
6-(4-cianofenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-54)6-(4-cyanophenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-54)
Escala de 0,08 mmoles, con ácido (3-cianofenil)borónico, 9,1 mg, 30% de rendimiento. 6) 512,50 (s, 1H), 8,72 (a d, J = 5,2 Hz, 1H), 8,60 - 8,57 (m, 2H), 8,43- 8,36 (m, 1 H), 8,27 - 8,19 (m, 3H), 8,08 (d, J = 7,9 Hz, 1H), 8,00 - 7,96 (m, 1 H), 7,69 - 7,58 (m, 2H), 7,53 - 7,49 (m, 1 H), 4,02 (s, 3H); LRMS (M+H) m/z6.0.08 mmol scale, with (3-cyanophenyl)boronic acid, 9.1 mg, 30% yield. 6) 512.50 (s, 1H), 8.72 (ad, J = 5.2 Hz, 1H), 8.60 - 8.57 (m, 2H), 8.43- 8.36 (m, 1H), 8.27 - 8.19 (m, 3H), 8.08 (d, J = 7.9 Hz, 1H), 8.00 - 7.96 (m, 1H), 7.69 - 7.58 (m, 2H), 7.53 - 7.49 (m, 1H), 4.02 (s, 3H); LRMS (M+H) m/z6.
Ejemplo 57Example 57
6-(3-fluorofenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-55)6-(3-fluorophenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-55)
Escala de 0,08 mmoles, con ácido (3-fluorofenil)borónico, 20,7 mg, 69% de rendimiento. 1H RMN (300 MHz, DMSO-afe) 512,51 (s, 1 H), 8,75 (ddd, J = 5,0, 1,7, 1,0 Hz, 1H), 8,59 (s, 1H), 8,37 (dd, J = 6,3, 2,7 Hz, 1 H), 8,27 - 8,21 (m, 4H), 8,09 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,98 (ddd, J = 8,1,7,5, 1,8 Hz, 1H), 7,79 - 7,72 (m, 1H), 7,50 - 7,43 (m, 2H), 4,02 (s, 3H); LRMS (M+H) m/z374,64. 0.08 mmol scale, with (3-fluorophenyl)boronic acid, 20.7 mg, 69% yield. 1H NMR (300 MHz, DMSO-afe) 512.51 (s, 1H), 8.75 (ddd, J = 5.0, 1.7, 1.0 Hz, 1H), 8.59 (s, 1H), 8.37 (dd, J = 6.3, 2.7 Hz, 1H), 8.27 - 8.21 (m, 4H), 8.09 (ddd, J = 8.1, 1 .1, 1.1 Hz, 1H), 7.98 (ddd, J = 8,1,7.5, 1.8 Hz, 1H), 7.79 - 7.72 (m, 1H), 7, 50 - 7.43 (m, 2H), 4.02 (s, 3H); LRMS (M+H) m/z 374.64.
Ejemplo 58Example 58
6-(4-fluorofenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-56)6-(4-fluorophenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-56)
Escala de 0,08 mmoles, con ácido (4-fluorofenil)borónico, 22 mg, 74% de rendimiento. (300 MHz, DMSO-afe) δ 12,52 (s, 1 H), 8,72 (ddd, J = 5,0, 1,6, 0,9 Hz, 1H), 8,58 (s, 1H), 8,49 - 8,44 (m, 2H), 8,30 (dd, J = 7,0, 2,0 Hz, 1H), 8,22 - 8,15 (m, 2H), 8,08 (a d, J = 8,0 Hz, 1 H), 7,98 (ddd, J = 7,7, 7,7, 1,8 Hz, 1 H), 7,60 - 7,54 (m, 2H), 7,50 (ddd, J = 7,3, 5,0, 1,3 Hz, 1 H), 4,01 (s, 3H); LRMS (M+H)4.0.08 mmol scale, with (4-fluorophenyl)boronic acid, 22 mg, 74% yield. (300 MHz, DMSO-afe) δ 12.52 (s, 1H), 8.72 (ddd, J = 5.0, 1.6, 0.9 Hz, 1H), 8.58 (s, 1H ), 8.49 - 8.44 (m, 2H), 8.30 (dd, J = 7.0, 2.0 Hz, 1H), 8.22 - 8.15 (m, 2H), 8, 08 (ad, J = 8.0 Hz, 1 H), 7.98 (ddd, J = 7.7, 7.7, 1.8 Hz, 1 H), 7.60 - 7.54 (m, 2H), 7.50 (ddd, J =7.3, 5.0, 1.3 Hz, 1H), 4.01 (s, 3H); LRMS (M+H)4.
Ejemplo 59Example 59
6-(3-aminofenil)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-57)6-(3-aminophenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-57)
Escala de 0,08 mmoles, con ácido (3-aminofenil)borónico, 18 mg, 61% de rendimiento. 1H RMN (300 MHz, DMSO-afe) δ 12,68 (s, 1 H), 8,75 (a d, J = 4,8 Hz, 1H), 8,58 (s, 1H), 8,19 - 8,05 (m, 4H), 7,96 (ddd, J = 7,7, 7,7, 1,7 Hz, 1H), 7,56 (a d, J = 7,7 Hz, 1H), 7,47 (dd, J = 1,9, 1,9 Hz, 1H), 7,42 (ddd, J = 7,4, 5,0, 1,2 Hz, 1H), 7,36 (dd, J = 7,8, 7,8 Hz, 1H), 6,83 (dd, J = 7,6, 1,7 Hz, 1H), 5,33 (s, 2H), 4,01 (s, 3H); LRMS (M+H) m/z 371,60.0.08 mmol scale, with (3-aminophenyl)boronic acid, 18 mg, 61% yield. 1H NMR (300 MHz, DMSO-afe) δ 12.68 (s, 1H), 8.75 (bd, J = 4.8 Hz, 1H), 8.58 (s, 1H), 8.19 - 8.05 (m, 4H), 7.96 (ddd, J = 7.7, 7.7, 1.7 Hz, 1H), 7.56 (bd, J = 7.7 Hz, 1H), 7 .47 (dd, J = 1.9, 1.9 Hz, 1H), 7.42 (ddd, J = 7.4, 5.0, 1.2 Hz, 1H), 7.36 (dd, J = 7.8, 7.8 Hz, 1H), 6.83 (dd, J = 7.6, 1.7 Hz, 1H), 5.33 (s, 2H), 4.01 (s, 3H) ; LRMS (M+H) m/z 371.60.
Ejemplo 60Example 60
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(3-(metilamino)fenil)picolinamida (I-58)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(3-(methylamino)phenyl)picolinamide (I-58)
1H RMN (300 MHz, DMSO-afe) δ 12,68 (s, 1H), 8,68 (a d, J = 4,9 Hz, 1H), 8,58 (s, 1H), 8,18 - 8,13 (m, 3H), 8,06 (a d, J = 8,0 Hz, 1 H), 7,96 (ddd, J = 7,7, 7,7, 1,7 Hz, 1H), 7,64 (d, J = 7,7 Hz, 1H), 7,47 - 7,41 (m, 2H), 7,36 (dd, J = 1,8, 1,8 Hz, 1H), 6,79 (dd, J = 7,7, 1,9 Hz, 1H), 5,88 - 5,86 (m, 1 H), 4,01 (s, 3H), 2,80 (d, J = 4,2 Hz, 3H); LRMS (M+H) m/z 385,68.1H NMR (300 MHz, DMSO-afe) δ 12.68 (s, 1H), 8.68 (bd, J = 4.9 Hz, 1H), 8.58 (s, 1H), 8.18-8 .13 (m, 3H), 8.06 (bd, J = 8.0 Hz, 1H), 7.96 (ddd, J = 7.7, 7.7, 1.7 Hz, 1H), 7 .64 (d, J = 7.7 Hz, 1H), 7.47 - 7.41 (m, 2H), 7.36 (dd, J = 1.8, 1.8 Hz, 1H), 6, 79 (dd, J = 7.7, 1.9 Hz, 1H), 5.88 - 5.86 (m, 1H), 4.01 (s, 3H), 2.80 (d, J = 4 .2Hz, 3H); LRMS (M+H) m/z 385.68.
Ejemplo 61Example 61
6-(3-(dimetilamino)fenil)-N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-59)6-(3-(dimethylamino)phenyl)-N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-59)
Escala de 0,08 mmoles, con ácido (3-(dimetilamino)fenil)borónico, 13,2 mg, 41% de rendimiento. 1H RMN (300 MHz, DMSO-afe) 512,66 (s, 1H), 8,65 (ddd, J = 4,9, 1,7, 0,9 Hz, 1H), 8,58 (s, 1H), 8,28 (dd, J = 6,8, 2,3 Hz, 1H), 8,20 - 8,14 (m, 2H), 8,07 (ddd, J = 8,1, 1,0, 1,0 Hz, 1 H), 7,97 (ddd, J = 7,7, 7,7, 1,8 Hz, 1 H), 7,78 (a d, J = 7,8 Hz, 1 H), 7,57 - 7,51 (m, 2H), 7,43 (ddd, J = 7,3, 5,0, 1,3 Hz, 1H), 6,98 (dd, J = 8,0, 2,2 Hz, 1 H), 4,02 (s, 3H), 3,03 (s, 6H); LRMS (M+H) m/z 399,64. 0.08 mmol scale, with (3-(dimethylamino)phenyl)boronic acid, 13.2 mg, 41% yield. 1H NMR (300 MHz, DMSO-afe) 512.66 (s, 1H), 8.65 (ddd, J = 4.9, 1.7, 0.9 Hz, 1H), 8.58 (s, 1H ), 8.28 (dd, J = 6.8, 2.3 Hz, 1H), 8.20 - 8.14 (m, 2H), 8.07 (ddd, J = 8.1, 1.0 , 1.0 Hz, 1 H), 7.97 (ddd, J = 7.7, 7.7, 1.8 Hz, 1 H), 7.78 (dd, J = 7.8 Hz, 1 H ), 7.57 - 7.51 (m, 2H), 7.43 (ddd, J = 7.3, 5.0, 1.3 Hz, 1H), 6.98 (dd, J = 8.0 , 2.2 Hz, 1H), 4.02 (s, 3H), 3.03 (s, 6H); LRMS (M+H) m/z 399.64.
Ejemplo 62Example 62
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(3-(metilsulfonamido)fenil)picolinamida (I-60)N-(1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(3-(methylsulfonamido)phenyl)picolinamide (I-60)
Escala de 0,08 mmoles, con ácido (3-(metilsulfonamido)fenil)borónico, 21,7 mg, 41% de rendimiento. 1H RMN (300 MHz, DMSO-afe) δ 12,64 (s, 1H), 9,99 (s, 1H), 8,72 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,58 (s, 1H), 8,26 - 8,21 (m, 4H), 8,07 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 8,03 (dd, J = 1,8, 1,8 Hz, 1H), 7,97 (ddd, J = 7,7, 7,7, 1,8 Hz, 1H), 7,72 (dd, J = 7,9, 7,9 Hz, 1H), 7,49 (ddd, J = 8,0, 2,1, 0,9 Hz, 1H), 7,42 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 4,02 (s, 3H), 3,11 (s, 3H); LRMS (M+H) m/z449,45.0.08 mmol scale, with (3-(methylsulfonamido)phenyl)boronic acid, 21.7 mg, 41% yield. 1H NMR (300 MHz, DMSO-afe) δ 12.64 (s, 1H), 9.99 (s, 1H), 8.72 (ddd, J = 5.0, 1.7, 0.9 Hz, 1H), 8.58 (s, 1H), 8.26-8.21 (m, 4H), 8.07 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 8 .03 (dd, J = 1.8, 1.8 Hz, 1H), 7.97 (ddd, J = 7.7, 7.7, 1.8 Hz, 1H), 7.72 (dd, J = 7.9, 7.9 Hz, 1H), 7.49 (ddd, J = 8.0, 2.1, 0.9 Hz, 1H), 7.42 (ddd, J = 7.4, 5 0, 1.3Hz, 1H), 4.02 (s, 3H), 3.11 (s, 3H); LRMS (M+H) m/z 449.45.
Ejemplo 63Example 63
6-((1-metil-3-(piridin-2-il)-1H-pirazol-4-il)carbamoil)-3',6'-dihidro-[2,4'-bipiridin]-1'(2'H)-carboxilato de tercbutilo (I-61)6-((1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)carbamoyl)-3',6'-dihydro-[2,4'-bipyridin]-1'(2 'H)-tert-butyl carboxylate (I-61)
Escala de 0,15 mmoles, con 4-(4,4,5,5-tetrametil-1,3,2-dioxaborolan-2-il)-3,6-dihidropiridin-1(2H)-carboxilato de tercbutilo, 33,1 mg, 48% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 512,40 (s, 1H), 8,60 (ddd, J = 5,0, 1,8, 0,9 Hz, 1H), 8,49 (s, 1 H), 8,15 (dd, J = 7,7, 0,9 Hz, 1H), 8,10 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,86 (dd, J = 7,8, 7,8 Hz, 1H), 7,76 (ddd, J = 8,1,7,5, 1,8 Hz, 1H), 7,58 (d, J = 7,8 Hz, 1H), 7,22 (ddd, J = 7,5, 5,0, 1,2 Hz, 1H), 6,93 (s a, 1H), 4,26 (s a, 2H), 3,99 (s, 3H), 3,78 (t, J = 5,6 Hz, 2H), 2,90 (s a, 2H), 1,53 (s, 9H); LRMS (M+H) m/z461,80.0.15 mmol scale, with tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,6-dihydropyridine-1(2H)-carboxylate, 33 0.1mg, 48% yield. 1H NMR (300 MHz, Chloroform-d) 512.40 (s, 1H), 8.60 (ddd, J = 5.0, 1.8, 0.9 Hz, 1H), 8.49 (s, 1 H), 8.15 (dd, J = 7.7, 0.9 Hz, 1H), 8.10 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.86 (dd, J = 7.8, 7.8 Hz, 1H), 7.76 (ddd, J = 8,1,7.5, 1.8 Hz, 1H), 7.58 (d, J = 7 .8 Hz, 1H), 7.22 (ddd, J = 7.5, 5.0, 1.2 Hz, 1H), 6.93 (sa, 1H), 4.26 (sa, 2H), 3 .99 (s, 3H), 3.78 (t, J =5.6 Hz, 2H), 2.90 (bs, 2H), 1.53 (s, 9H); LRMS (M+H) m/z 461.80.
Ejemplo 64Example 64
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-1 ',2',3',6'tetrahidro-[2,4'-bipiridin]-6-carboxamida (I-62)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-1',2',3',6'tetrahydro-[2,4'-bipyridin]-6 -carboxamide (I-62)
1H RMN (300 MHz, Cloroformo-a) 512,41 (s, 1H), 8,59 (ddd, J = 5,0, 1,8, 0,9 Hz, 1H), 8,48 (s, 1H), 8,12 (dd, J = 7,7, 0,9 Hz, 1H), 8,09 (ddd, J = 8,1, 1,1, 1,1 Hz, 1 H), 7,83 (dd, J = 7,8, 7,8z Hz, 1 H), 7,75 (ddd, J = 8,0, 7,6, 1,8 Hz, 1 H), 7,55 (dd, J = 8,0, 0,9 Hz, 1 H), 7,20 (ddd, J = 7,5, 5,0, 1,2 Hz, 1 H), 7,04 - 7,00 (m, 1 H), 3,98 (s, 3H), 3,73 - 3,70 (m, 2H), 3,25 (t, J = 5,7 Hz, 2H), 2,83 - 2,79 (m, 2H), 2,06 (s, 1 H); LRMS (M+H) m/z361,48.1H NMR (300 MHz, Chloroform-a) 512.41 (s, 1H), 8.59 (ddd, J = 5.0, 1.8, 0.9 Hz, 1H), 8.48 (s, 1H ), 8.12 (dd, J = 7.7, 0.9 Hz, 1H), 8.09 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.83 (dd, J = 7.8, 7.8z Hz, 1H), 7.75 (ddd, J = 8.0, 7.6, 1.8Hz, 1H), 7.55 (dd, J = 8.0, 0.9 Hz, 1 H), 7.20 (ddd, J = 7.5, 5.0, 1.2 Hz, 1 H), 7.04 - 7.00 (m, 1 H), 3.98 (s, 3H), 3.73 - 3.70 (m, 2H), 3.25 (t, J = 5.7 Hz, 2H), 2.83 - 2.79 (m , 2H), 2.06 (s, 1H); LRMS (M+H) m/z 361.48.
Ejemplo 65Example 65
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(piperidin-4-il)picolinamida (I-63) N-(1 -methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-6-(piperidin-4-yl)picolinamide (I-63)
1H RMN (300 MHz, Cloroformo-a) 512,46 (s, 1H), 8,60 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,45 (s, 1H), 8,17 (dd, J = 7,7, 0,7 Hz, 1 H), 8,08 (ddd, J = 8,1, 1,0, 1,0 Hz, 1H), 7,88 (dd, J = 7,8, 7,8 Hz, 1 H), 7,75 (ddd, J = 7,8, 7,8, 1,8 Hz, 1H), 7,40 (d, J = 7,3 Hz, 1H), 7,29 - 7,25 (m, parcialmente solapado con cloroformo, 1H), 3,98 (s, 3H), 3,70 (dt, J = 12,8, 3,0 Hz, 2H), 3,24 - 3,15 (m, 3H), 2,52 - 2,32 (m, 4H); LRMS (M+H) m/z363,63.1H NMR (300 MHz, Chloroform-a) 512.46 (s, 1H), 8.60 (ddd, J = 5.0, 1.7, 0.9 Hz, 1H), 8.45 (s, 1H ), 8.17 (dd, J = 7.7, 0.7 Hz, 1H), 8.08 (ddd, J = 8.1, 1.0, 1.0 Hz, 1H), 7.88 (dd, J = 7.8, 7.8 Hz, 1H), 7.75 (ddd, J = 7.8, 7.8, 1.8 Hz, 1H), 7.40 (d, J = 7.3 Hz, 1H), 7.29 - 7.25 (m, partially overlapped with chloroform, 1H), 3.98 (s, 3H), 3.70 (dt, J = 12.8, 3.0 Hz, 2H), 3.24-3.15 (m, 3H), 2.52-2.32 (m, 4H); LRMS (M+H) m/z 363.63.
Ejemplo 66Example 66
6-((1-metil-3-(piridin-2-il)-1H-pirazol-4-il)carbamoil)-5',6'-dihidro-[2,3'-bipiridin]-1'(2'H)-carboxilato de tercbutilo (I-64)6-((1-methyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)carbamoyl)-5',6'-dihydro-[2,3'-bipyridin]-1'(2 'H)-tert-butyl carboxylate (I-64)
Ejemplo 67Example 67
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-1 ',2',5',6'-tetrahidro-[2,3'-bipiridin]-6 -carboxamida (I-65) Ejemplo 68N-(1-methyl-3-(pyridin-2-yl)-1 H -pyrazol-4-yl)-1',2',5',6'-tetrahydro-[2,3'-bipyridin]- 6-carboxamide (I-65) Example 68
(£)-3-(dimetilamino)-1 -(5-metoxipiridin-2-il)prop-2-en-1 -ona(£)-3-(dimethylamino)-1 -(5-methoxypyridin-2-yl)prop-2-en-1-one
Una disolución en 1,1-dimetoxi-N,N-dimetilmetanamina (1,4 ml, 10 mmoles) de 1-(5-metoxipiridin-2-il)etano-1-ona (755,8 mg, 5 mmoles) se agitó a 110°C. Después de 16 horas, la reacción se completó, monitorizada mediante LC-MS. El disolvente se eliminó a vacío, el compuesto del título se obtuvo como un sólido marrón: 1H RMN (300 MHz, Cloroformo-a) 58,30 (dd, J = 2,9, 0,6 Hz, 1 H), 8,15 (dd, J = 8,7, 0,6 Hz, 1 H), 7,87 (d, J = 12,7 Hz, 1 H), 7,28 - 7,24 (m, partially overlapped with CHCh, 1H), 6,42 (d, J = 12,7 Hz, 1H), 3,90 (s, 3H), 3,16 (s a, 3H), 2,95 (s a, 3H); LRMS (M+H) m/z 207,37.A 1,1-dimethoxy-N,N-dimethylmethanamine (1.4 mL, 10 mmol) solution of 1-(5-methoxypyridin-2-yl)ethane-1-one (755.8 mg, 5 mmol) was stirred at 110°C. After 16 hours, the reaction was complete, monitored by LC-MS. The solvent was removed in vacuo, the title compound was obtained as a brown solid: 1H NMR (300 MHz, Chloroform-a) 58.30 (dd, J = 2.9, 0.6 Hz, 1H), 8 .15 (dd, J = 8.7, 0.6 Hz, 1 H), 7.87 (d, J = 12.7 Hz, 1 H), 7.28 - 7.24 (m, partially overlapped with CHCh, 1H), 6.42 (d, J =12.7 Hz, 1H), 3.90 (s, 3H), 3.16 (bs, 3H), 2.95 (bs, 3H); LRMS (M+H) m/z 207.37.
Ejemplo 69Example 69
5-metoxi-2-(1H-pirazol-3-il)piridina5-methoxy-2-(1H-pyrazol-3-yl)pyridine
Una disolución en EtOH (5 ml) de (E)-3-(dimetilamino)-1 -(5-metoxipiridin-2-il)prop-2-en-1 -ona (alrededor de 5 mmoles) e hidrato de hidrazina (0,32 ml, 6,5 mmoles) se agitó a 85°C durante 2 horas. La reacción se completó según se monitorizó por LC-MS. Después de enfriar hasta temperatura ambiente, se añadió H2O (alrededor de 10 ml), y la mayor parte del disolvente orgánico se eliminó a vacío. La mezcla se extrajo con EtOAc (15 ml x 2), las capas orgánicas se combinaron, se lavaron con H2O, se secaron (Na2SO4), se filtraron, y el disolvente se eliminó a vacío. El compuesto del título se obtuvo como un sólido marrón: 801,5 mg (91 % de rendimiento en 2 etapas):1 H RMN (300 MHz, Cloroformoa) 5 10,89 (v s a, 1H), 8,31 (dd, J = 3,0, 0,7 Hz, 1H), 7,66 (d, J = 8,7 Hz, 1 H), 7,63 (d, J = 2,0 Hz, 1H), 7,26 (dd, J = 8,7, 3,0 Hz, 1 H), 6,69 (d, J = 2,0 Hz, 1H), 3,90 (s, 3H); LRMS (M+H) m/z 176,30. An EtOH (5 mL) solution of (E)-3-(dimethylamino)-1 -(5-methoxypyridin-2-yl)prop-2-en-1-one (ca. 5 mmol) and hydrazine hydrate ( 0.32 mL, 6.5 mmol) was stirred at 85°C for 2 hours. The reaction was complete as monitored by LC-MS. After cooling to room temperature, H2O (ca. 10 mL) was added, and most of the organic solvent was removed in vacuo. The mixture was extracted with EtOAc (15 mL x 2), the organic layers combined, washed with H2O, dried ( Na2SO4 ), filtered, and the solvent removed in vacuo . The title compound was obtained as a brown solid: 801.5 mg (91% yield in 2 steps): 1 H NMR (300 MHz, Chloroformoa) 5 10.89 (vsa, 1H), 8.31 (dd, J = 3.0, 0.7 Hz, 1H), 7.66 (d, J = 8.7 Hz, 1H), 7.63 (d, J = 2.0 Hz, 1H), 7.26 (dd, J = 8.7, 3.0 Hz, 1H), 6.69 (d, J = 2.0 Hz, 1H), 3.90 (s, 3H); LRMS (M+H) m/z 176.30.
Ejemplo 70Example 70
5-metoxi-2-(1-metil-1H-pirazol-3-il)piridina5-methoxy-2-(1-methyl-1H-pyrazol-3-yl)pyridine
En un baño de hielo, a una disolución en THF (20 ml) de 5-metoxi-2-(1 H-pirazol-3-il)piridina (801 mg, 4,58 mmoles), se añadió NaH (dispersión al 60% en aceite mineral, 274,5 mg, 6,87 mmoles) en porciones. La reacción se mantuvo a 0°C durante 30 minutos, después se dejó calentar hasta temperatura ambiente durante 20 minutos. Después de volver a enfriar hasta 0°C, se añadió gota a gota Mel (0,3 ml, 4,81 mmoles). A continuación, la reacción se dejó calentar hasta temperatura ambiente, y después se calentó a 50°C durante la noche. Mediante LC-MS, la reacción se completó. La reacción se enfrió hasta temperatura ambiente, se paralizó con una disolución acuosa saturada de NH4Cl (alrededor de 50 ml). La mayor parte del THF se eliminó mediante evaporación rotatoria, y la mezcla se extrajo con EtOAc (50 ml x 2). Las capas orgánicas se combinaron, se secaron (Na2SO4), se filtraron, se eliminó el disolvente a vacío. El compuesto del título se obtuvo como un aceite marrón (contiene aceite mineral): 1H RMN (300 MHz, Cloroformo-a) 5 8,32 (dd, J = 3,0, 0,7 Hz, 1H), 7,84 (dd, J = 8,7, 0,7 Hz, 1H), 7,39 (d, J = 2,3 Hz, 1H), 7,24 (dd, J = 8,7, 3,0 Hz, 1 H), 6,77 (d, J = 2,3 Hz, 1H), 3,96 (s, 3H), 3,89 (s, 3H); LRMS (M+H) m/z 190,35.In an ice bath, to a THF (20 mL) solution of 5-methoxy-2-(1H-pyrazol-3-yl)pyridine (801 mg, 4.58 mmol), NaH (60% dispersion) was added. % in mineral oil, 274.5 mg, 6.87 mmol) portionwise. The reaction was held at 0°C for 30 minutes, then allowed to warm to room temperature over 20 minutes. After re-cooling to 0°C, Mel (0.3 mL, 4.81 mmol) was added dropwise. The reaction was then allowed to warm to room temperature, then warmed to 50°C overnight. By LC-MS, the reaction was complete. The reaction was cooled to room temperature, quenched with saturated aqueous NH4Cl (ca. 50 mL). Most of the THF was removed by rotary evaporation, and the mixture was extracted with EtOAc (50 mL x 2). The organic layers were combined, dried (Na 2 SO 4 ), filtered, the solvent removed in vacuo. The title compound was obtained as a brown oil (contains mineral oil): 1H NMR (300 MHz, Chloroform-a) 5 8.32 (dd, J = 3.0, 0.7 Hz, 1H), 7.84 (dd, J = 8.7, 0.7 Hz, 1H), 7.39 (d, J = 2.3 Hz, 1H), 7.24 (dd, J = 8.7, 3.0 Hz, 1H), 6.77 (d, J =2.3 Hz, 1H), 3.96 (s, 3H), 3.89 (s, 3H); LRMS (M+H) m/z 190.35.
Ejemplo 71Example 71
5-metoxi-2-(1 -metil-4-nitro-1 H-pirazol-3-il)piridina5-methoxy-2-(1-methyl-4-nitro-1H-pyrazol-3-yl)pyridine
Sobre baño de hielo, a una disolución en H2SO4 (5 ml) de 5-metoxi-2-(1 -metil-1 H-pirazol-3-il)piridina (alrededor de 4,58 mmoles), se añadió gota a gota HNO3 (disolución ac. al 90%, 0,252 ml). Tras la adición, la reacción se dejó calentar hasta temperatura ambiente. Después de 17 horas, la reacción se detuvo vertiéndola sobre hielo, después se basificó con NaOH sólido y Na2CO3 hasta alrededor de pH = 8. El precipitado se recogió por filtración (se eliminó el material de partida (SM) sin reaccionar y el producto deseado <alrededor de 1: 1>, que no se separan en la columna de sílice), y se purificó adicionalmente mediante cromatografía en gel de sílice para eliminar el subproducto di-nitro. El compuesto del título se obtuvo como un sólido de color canela: 663,2 mg (62% de rendimiento en 2 etapas); 1H RMN (300 MHz, Cloroformo-d) 58,43 (dd, J = 3,0, 0,7 Hz, 1H), 8,23 (d, J = 0,5 Hz, 1H), 7,75 (dd, J = 8,7, 0,6 Hz, 1H), 7,29 (dd, J = 8,7, 3,0 Hz, 1H), 4,01 (s, 3H), 3,92 (s, 3H); LRMS (M+H) m/z 235,42. La estructura se confirmó, en términos de regioselectividad, mediante el experimento 1D-NOE: se observó NOE entre el grupo metilo y el protón en el pirazol. También se aisló el otro regioisómero:On an ice bath, to a H2SO4 (5 mL) solution of 5-methoxy-2-(1-methyl-1H-pyrazol-3-yl)pyridine (ca. 4.58 mmol), was added dropwise to drop HNO 3 (90% aq. solution, 0.252 mL). After the addition, the reaction was allowed to warm to room temperature. After 17 hours, the reaction was quenched by pouring it onto ice, then made basic with solid NaOH and Na 2 CO 3 to about pH = 8. The precipitate was collected by filtration (removed unreacted starting material (SM) and desired product <about 1:1>, not separating on silica column), and further purified by silica gel chromatography to remove di-nitro by-product. The title compound was obtained as a tan solid: 663.2 mg (62% yield over 2 steps); 1H NMR (300 MHz, Chloroform-d) 58.43 (dd, J = 3.0, 0.7 Hz, 1H), 8.23 (d, J = 0.5 Hz, 1H), 7.75 ( dd, J = 8.7, 0.6 Hz, 1H), 7.29 (dd, J = 8.7, 3.0 Hz, 1H), 4.01 (s, 3H), 3.92 (s , 3H); LRMS (M+H) m/z 235.42. The structure was confirmed, in terms of regioselectivity, by the 1D-NOE experiment: NOE was observed between the methyl group and the proton in the pyrazole. The other regioisomer was also isolated:
5-metoxi-2-(1 -metil-4-nitro-1 H-pirazol-5-il)piridina5-methoxy-2-(1-methyl-4-nitro-1H-pyrazol-5-yl)pyridine
1H RMN (300 MHz, Cloroformo-d) 58,24 (d, J = 0,4 Hz, 1H), 8,01 (d, J = 8,6 Hz, 1 H), 7,61 (d, J = 8,6 Hz, 1 H), 4,04 (s, 3H), 4,03 (d, J = 0,4 Hz, 2H). 1H NMR (300 MHz, Chloroform-d) 58.24 (d, J = 0.4 Hz, 1H), 8.01 (d, J = 8.6 Hz, 1H), 7.61 (d, J = 8.6 Hz, 1H), 4.04 (s, 3H), 4.03 (d, J = 0.4 Hz, 2H).
Ejemplo 72Example 72
dih idrocloruro de 3-(5-metoxipiridin-2-il)-1 -metil-1 H-pirazol-4-amina3-(5-methoxypyridin-2-yl)-1-methyl-1H-pyrazol-4-amine dihydrochloride
Una disolución en EtOAc (20 ml) de 5-metoxi-2-(1-metil-4-nitro-1 H-pirazol-3-il)piridina (663 mg, 2,83 mmoles) y Pd-C (10% en C, 50% húmedo, 100 mg), se agitó en un matraz Parr a 30 psi de hidrógeno. Después de 21 horas, la reacción se completó según se monitorizó mediante LC-MS. El sólido se eliminó por filtración a través de una almohadilla de celite, se lavó con EtOAc y MeOH, y el filtrado se recogió en un matraz con disolución 4 M de HCl-dioxano (1,5 ml). Se formó un precipitado, y se recogió por filtración, lavando con EtOAc, y se secó adicionalmente a vacío. El compuesto del título se obtuvo como sal di-HCl: 703,8 mg (90% de rendimiento); 1H RMN (300 MHz, DMSO-afe) ó 10,06 (s a, 2H), 8,37 (dd, J = 3,0, 0,7 Hz, 1H), 8,04 (d, J = 3,7 Hz, 1H), 7,94 (dd, J = 8,8, 0,7 Hz, 1H), 7,60 (dd, J = 8,8, 3,0 Hz, 1 H), 3,96 (s, 3H), 3,93 (s, 3H), 3,64 (s a, 2H); LRMS (M+H) m/z 205,32.An EtOAc (20 mL) solution of 5-methoxy-2-(1-methyl-4-nitro-1H-pyrazol-3-yl)pyridine (663 mg, 2.83 mmol) and Pd-C (10% in C, 50% wet, 100 mg), was shaken in a Parr flask under 30 psi of hydrogen. After 21 hours, the reaction was complete as monitored by LC-MS. The solid was filtered off through a celite pad, washed with EtOAc and MeOH, and the filtrate collected in a flask with 4M HCl-dioxane solution (1.5 mL). A precipitate formed, and was collected by filtration, washing with EtOAc, and dried further in vacuo. The title compound was obtained as di-HCl salt: 703.8 mg (90% yield); 1H NMR (300 MHz, DMSO-afe) or 10.06 (bs, 2H), 8.37 (dd, J = 3.0, 0.7 Hz, 1H), 8.04 (d, J = 3, 7 Hz, 1H), 7.94 (dd, J = 8.8, 0.7 Hz, 1H), 7.60 (dd, J = 8.8, 3.0 Hz, 1H), 3.96 (s, 3H), 3.93 (s, 3H), 3.64 (bs, 2H); LRMS (M+H) m/z 205.32.
Ejemplo 73Example 73
6-bromo-N-(3-(5-metoxipiridin-2-il)-1-metil-1 H-pirazol-4-il)picolinamida (I-66)6-Bromo-N-(3-(5-methoxypyridin-2-yl)-1-methyl-1H-pyrazol-4-yl)picolinamide (I-66)
Escala de 0,8 mmoles, con ácido 6-bromopicolínico, 239,9 mg, 77% de rendimiento. 1H RMN (300 MHz, Cloroformod) δ 12,98 (s, 1H), 8,56 (dd, J = 3,0, 0,7 Hz, 1H), 8,33 (s, 1H), 8,20 (dd, J = 7,5, 1,0 Hz, 1H), 7,98 (dd, J = 8,8, 0,7 Hz, 1H), 7,75 (dd, J = 7,9, 7,5 Hz, 1 H), 7,65 (dd, J = 7,9, 1,0 Hz, 1H), 7,32 (dd, J = 8,8, 3,0 Hz, 1H), 3,96 (s, 3H), 3,94 (s, 3H); LRMS (M+H) m/z390,60.0.8 mmol scale, with 6-bromopicolinic acid, 239.9 mg, 77% yield. 1H NMR (300 MHz, Chloroformod) δ 12.98 (s, 1H), 8.56 (dd, J = 3.0, 0.7 Hz, 1H), 8.33 (s, 1H), 8.20 (dd, J = 7.5, 1.0 Hz, 1H), 7.98 (dd, J = 8.8, 0.7 Hz, 1H), 7.75 (dd, J = 7.9, 7 .5 Hz, 1H), 7.65 (dd, J = 7.9, 1.0 Hz, 1H), 7.32 (dd, J = 8.8, 3.0 Hz, 1H), 3, 96 (s, 3H), 3.94 (s, 3H); LRMS (M+H) m/z 390.60.
Ejemplo 74Example 74
N-(3-(5-metoxipiridin-2-il)-1 -metil-1 H-pirazol-4-il)-6-(1 H-pirazol-5-il)picolinamida (I-67)N-(3-(5-methoxypyridin-2-yl)-1 -methyl-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-5-yl)picolinamide (I-67)
Escala de 0,08 mmoles, 22,3 mg, 71% de rendimiento. 1H RMN (300 MHz, DMSO-afe) 5 13,29 (s, 1H), 12,40 (s, 1H), 8,52 (s, 1H), 8,46 (d, J = 2,4 Hz, 1 H), 8,23 (dd, J = 6,8, 2,0 Hz, 1H), 8,15 - 8,08 (m, 3H), 8,01 (d, J = 8,8 Hz, 1H), 7,59 (dd, J = 8,9, 2,9 Hz, 1 H), 7,20 (s a, 1H), 3,98 (s, 3H), 3,95 (s, 3H); LRMS (M+H) m/z 376,61. 0.08 mmol scale, 22.3 mg, 71% yield. 1H NMR (300 MHz, DMSO-afe) 5 13.29 (s, 1H), 12.40 (s, 1H), 8.52 (s, 1H), 8.46 (d, J = 2.4 Hz , 1H), 8.23 (dd, J = 6.8, 2.0 Hz, 1H), 8.15 - 8.08 (m, 3H), 8.01 (d, J = 8.8 Hz , 1H), 7.59 (dd, J = 8.9, 2.9 Hz, 1H), 7.20 (sa, 1H), 3.98 (s, 3H), 3.95 (s, 3H ); LRMS (M+H) m/z 376.61.
Ejemplo 75Example 75
N-(3-(5-metoxipiridin-2-il)-1 -metil-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida (I-68)N-(3-(5-methoxypyridin-2-yl)-1 -methyl-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide (I-68)
Escala de 0,08 mmoles, 11,1 mg, 37% de rendimiento.1H RMN (300 MHz, DMSO-afe) 5 13,34 (s, 1H), 12,32 (s, 1H), 8,55 (s, 1 H), 8,53 (s, 1 H), 8,43 (d, J = 2,6 Hz, 1 H), 8,37 (s, 1H), 8,19 - 7,96 (m, 4H), 7,62 (dd, J = 8,9, 3,0 Hz, 1 H), 3,98 (s, 3H), 3,97 (s, 3H); LRMS (M+H) m/z376,60.0.08 mmol scale, 11.1 mg, 37% yield.1H NMR (300 MHz, DMSO-afe) 13.34 (s, 1H), 12.32 (s, 1H), 8.55 ( s, 1H), 8.53 (s, 1H), 8.43 (d, J = 2.6 Hz, 1H), 8.37 (s, 1H), 8.19 - 7.96 ( m, 4H), 7.62 (dd, J =8.9, 3.0 Hz, 1H), 3.98 (s, 3H), 3.97 (s, 3H); LRMS (M+H) m/z 376.60.
Ejemplo 76Example 76
N-(3-(5-metoxipiridin-2-il)-1-metil-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida (I-69)N-(3-(5-methoxypyridin-2-yl)-1-methyl-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridine]-6-carboxamide (I-69 )
Escala de 0,08 mmoles, 28,2 mg, 88% de rendim iento.^ RMN (300 MHz, DMSO-afe) δ 12,48 (s, 1H), 9,53 (s, 1H), 8,59 (dd, J = 2,0, 0,7 Hz, 1H), 8,49 (a d, J = 2,9 Hz, 1H), 8,48 (s, 1H), 8,39 (ddd, J = 2,1,2,1,0,7 Hz, 1H), 8,30 (dd, J = 7,4, 1,6 Hz, 1 H), 8,21 - 8,12 (m, 2H), 7,97 (dd, J = 8,9, 0,5 Hz, 1H), 7,55 (dd, J = 8,9, 3,0 Hz, 1H), 3,93 (s, 3H), 3,91 (s, 3H), 2,44 (s, 3H); LRMS (M+H) m/z401,66.0.08 mmol scale, 28.2 mg, 88% yield.^ NMR (300 MHz, DMSO-afe) δ 12.48 (s, 1H), 9.53 (s, 1H), 8.59 (dd, J = 2.0, 0.7 Hz, 1H), 8.49 (dd, J = 2.9 Hz, 1H), 8.48 (s, 1H), 8.39 (ddd, J = 2,1,2,1,0.7 Hz, 1H), 8.30 (dd, J = 7.4, 1.6 Hz, 1H), 8.21 - 8.12 (m, 2H), 7.97 (dd, J = 8.9, 0.5 Hz, 1H), 7.55 (dd, J = 8.9, 3.0 Hz, 1H), 3.93 (s, 3H), 3 .91 (s, 3H), 2.44 (s, 3H); LRMS (M+H) m/z 401.66.
Ejemplo 77Example 77
5'-isopropoxi-N-(3-(5-metoxipiridin-2-il)-1-metil-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-70) Escala de 0,08 mmoles, 24,1 mg, 68% de rendimiento.. 1H RMN (300 MHz, DMSO-afe) 512,41 (s, 1H), 9,31 (d, J = 1,8 Hz, 1H), 8,57 (a d, J = 2,9 Hz, 1H), 8,49 - 8,48 (m, 2H), 8,37 (dd, J = 7,0, 2,0 Hz, 1H), 8,21 - 8,13 (m, 3H), 7,98 (dd, J = 8,9, 0,5 Hz, 1 H), 7,56 (dd, J = 8,9, 3,0 Hz, 1H), 4,92 (hept, J = 6,0 Hz, 1H), 3,97 (s, 3H), 3,94 (s, 3H), 1,38 (d, J = 6,0 Hz, 6H); LRMS (M+H) m/z445,73.5'-Isopropoxy-N-(3-(5-methoxypyridin-2-yl)-1-methyl-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-70 ) 0.08 mmol scale, 24.1 mg, 68% yield. 1H NMR (300 MHz, DMSO-afe) 512.41 (s, 1H), 9.31 (d, J = 1.8 Hz , 1H), 8.57 (ad, J = 2.9 Hz, 1H), 8.49 - 8.48 (m, 2H), 8.37 (dd, J = 7.0, 2.0 Hz, 1H), 8.21 - 8.13 (m, 3H), 7.98 (dd, J = 8.9, 0.5 Hz, 1H), 7.56 (dd, J = 8.9, 3 .0 Hz, 1H), 4.92 (hept, J = 6.0 Hz, 1H), 3.97 (s, 3H), 3.94 (s, 3H), 1.38 (d, J = 6 .0Hz, 6H); LRMS (M+H) m/z 445.73.
Ejemplo 78Example 78
5'-(isopropilamino)-N-(3-(5-metoxipiridin-2-il)-1-metil-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-71) 1H RMN (300 MHz, DMSO-ds) δ 12,60 (s, 1H), 8,92 (d, J = 1,8 Hz, 1H), 8,54 - 8,53 (m, 2H), 8,27 - 8,16 (m, 4H), 8,01 (d, J = 8,8 Hz, 1H), 7,63 - 7,62 (m, 1H), 7,59 (dd, J = 8,9, 3,0 Hz, 1H), 5,95 (d, J = 8,2 Hz, 1H), 3,99 (s, 3H), 3,97 (s, 3H), 3,83 - 3,72 (m, 1H), 1,21 (d, J = 6,3 Hz, 6H); LRMS (M+H) m/z444,80.5'-(isopropylamino)-N-(3-(5-methoxypyridin-2-yl)-1-methyl-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I- 71) 1 H NMR (300 MHz, DMSO-ds) δ 12.60 (s, 1H), 8.92 (d, J = 1.8 Hz, 1H), 8.54-8.53 (m, 2H), 8.27 - 8.16 (m, 4H), 8.01 (d, J = 8.8 Hz, 1H), 7.63 - 7.62 (m, 1H), 7.59 (dd, J = 8.9, 3.0 Hz, 1H), 5.95 (d, J = 8.2 Hz, 1H), 3.99 (s, 3H), 3.97 (s, 3H), 3.83 - 3.72 (m, 1H), 1.21 (d, J =6.3Hz, 6H); LRMS (M+H) m/z 444.80.
Ejemplo 79Example 79
4-nitro-3-(pirrolidin-1 -il)-1 H-pirazol4-nitro-3-(pyrrolidin-1 -yl)-1 H-pyrazole
Una disolución en BuOH (5 ml) de 5-cloro-4-nitro-1 -((2-(trimetilsilil)etoxi)metil)-1 H-pirazol (1,39 g, 5 mmoles) y pirrolidina (0,575 ml, 7 mmoles) se se agitó a 120°C durante 15 horas, después de lo cual la LC-MS indicó que la reacción se había completado. Los volátiles se eliminaron a vacío, y la mezcla de reacción bruta se trató con EtOH (10 ml) y HCl 6N (ac., 3 ml), y se agitó a 70°C durante 3 horas. Los volátiles se eliminaron a vacío. Después de la trituración con EtOH-EtOAc, el compuesto del título se obtuvo como un sólido de color naranja: 631 mg como sal de HCl; ; 1H RMN (300 MHz, DMSO-afe) 58,09 (s, 1H), 3,49 - 3,45 (m, 4H), 1,97 - 1,92 (m, 4H); LRMS (M+H) m/z 183,24. Ejemplo 80A BuOH (5 mL) solution of 5-chloro-4-nitro-1 -((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrazole (1.39 g, 5 mmol) and pyrrolidine (0.575 mL, 7 mmol) was stirred at 120°C for 15 hours, after which LC-MS indicated the reaction was complete. The volatiles were removed in vacuo, and the crude reaction mixture was treated with EtOH (10 mL) and 6N HCl (aq, 3 mL), and stirred at 70°C for 3 hours. Volatiles were removed in vacuo. After trituration with EtOH-EtOAc, the title compound was obtained as an orange solid: 631 mg as HCl salt; ; 1H NMR (300 MHz, DMSO-afe) 58.09 (s, 1H), 3.49-3.45 (m, 4H), 1.97-1.92 (m, 4H); LRMS (M+H) m/z 183.24. Example 80
1-(4-nitro-1H-pirazol-3-ilo)1-(4-nitro-1H-pyrazol-3-yl)
Escala de 5 mmoles, con piperidina, 667,1 mg. . 1H RMN (300 MHz, DMSO-ds) 5 8,40 (s, 1H), 3,25 - 3,21 (m, 4H), 1,70 - 1,60 (m, 6H); LRMS (M+H) m/z 197,33.5 mmol scale, with piperidine, 667.1 mg. . 1H NMR (300 MHz, DMSO-ds) 8.40 (s, 1H), 3.25-3.21 (m, 4H), 1.70-1.60 (m, 6H); LRMS (M+H) m/z 197.33.
Ejemplo 81Example 81
4-(4-nitro-1H-pirazol-3-il)morfolina4-(4-nitro-1H-pyrazol-3-yl)morpholine
Escala de 5 mmoles, con morfolina, 540 mg. 1H RMN (300 MHz, DMSO-ds) 58,58 (s, 1H), 3,78 - 3,75 (m, 4H), 3,27 -3,23 (m, 4H); LRMS (M+H) m/z 199,26.5 mmol scale, with morpholine, 540 mg. 1H NMR (300 MHz, DMSO-ds) 58.58 (s, 1H), 3.78-3.75 (m, 4H), 3.27-3.23 (m, 4H); LRMS (M+H) m/z 199.26.
Ejemplo 82Example 82
1-metil-4-nitro-3-(pirrolidin-1-il)-1H-pirazol1-methyl-4-nitro-3-(pyrrolidin-1-yl)-1H-pyrazole
397,2 mg, 40% de rendimiento en 3 etapas. 1H RMN (300 MHz, Cloroformo-d) 58,03 (s, 1H), 3,76 (s, 3H), 3,38 - 3,33 (m, 4H), 2,08 - 2,04 (m, 4H); LRMS (M+H) m/z 197,34. La estructura se confirmó mediante el experimento 1D-NOE: se observó NOE entre los protones del grupo metilo y el protón del pirazol. 397.2 mg, 40% yield in 3 steps. 1H NMR (300 MHz, Chloroform-d) 58.03 (s, 1H), 3.76 (s, 3H), 3.38-3.33 (m, 4H), 2.08-2.04 (m , 4H); LRMS (M+H) m/z 197.34. The structure was confirmed by 1D-NOE experiment: NOE was observed between the protons of the methyl group and the proton of the pyrazole.
Ejemplo 83Example 83
1-metil-3-(pirrolidin-1-il)-1H-pirazol-4-amina1-methyl-3-(pyrrolidin-1-yl)-1H-pyrazol-4-amine
0,34 g como sal de 2 HCl. 1H RMN (300 MHz, Cloroformo-d) 57,98 (c, J = 0,6 Hz, 1H), 3,76 (d, J = 0,5 Hz, 3H), 3,48 - 3,43 (m, 4H), 1,96 - 1,91 (m, 4H); LRMS (M+H) m/z 167,28.0.34 g as the 2-HCl salt. 1H NMR (300 MHz, Chloroform-d) 57.98 (c, J = 0.6 Hz, 1H), 3.76 (d, J = 0.5 Hz, 3H), 3.48 - 3.43 ( m, 4H), 1.96-1.91 (m, 4H); LRMS (M+H) m/z 167.28.
Ejemplo 84Example 84
1-(1-metil-4-nitro-1H-pirazol-3-il)piperidina1-(1-methyl-4-nitro-1H-pyrazol-3-yl)piperidine
460,1 mg, 44% de rendimiento en 3 etapas. 1H RMN (300 MHz, Cloroformo-a) 58,04 (d, J = 0,6 Hz, 1H), 3,78 (d, J = 0,5 Hz, 4H), 3,22 - 3,19 (m, 4H), 1,75 - 1,68 (m, 4H), 1,64 - 1,56 (m, 2H); LRMS (M+H) m/z 211,36. La estructura se confirmó mediante el experimento 1D-NOE: se observó NOE entre los protones del grupo metilo y el protón del pirazol. También se aisló y caracterizó el otro regioisómero:460.1 mg, 44% yield over 3 steps. 1H NMR (300 MHz, Chloroform-a) 58.04 (d, J = 0.6 Hz, 1H), 3.78 (d, J = 0.5 Hz, 4H), 3.22-3.19 ( m, 4H), 1.75-1.68 (m, 4H), 1.64-1.56 (m, 2H); LRMS (M+H) m/z 211.36. The structure was confirmed by 1D-NOE experiment: NOE was observed between the protons of the methyl group and the proton of the pyrazole. The other regioisomer was also isolated and characterized:
1-(1-metil-4-nitro-1H-pirazol-5-il)piperidina1-(1-methyl-4-nitro-1H-pyrazol-5-yl)piperidine
1H RMN (300 MHz, Cloroformo-d) 58,01 (s, 1 H), 3,75 (s, 3H), 3,16 - 3,13 (m, 4H), 1,73 - 1,67 (m, 6H).1H NMR (300 MHz, Chloroform-d) 58.01 (s, 1H), 3.75 (s, 3H), 3.16-3.13 (m, 4H), 1.73-1.67 ( m, 6H).
Ejemplo 85Example 85
dih idrocloruro de 1-metil-3-(piperidin-1-il)-1 H-pirazol-4-amina1-methyl-3-(piperidin-1-yl)-1H-pyrazol-4-amine dih hydrochloride
317,1 mg, como sal de 2HCl, 91% de rendimiento 1H RMN (300 MHz, DMSO-d6) 59,75 (s a, 2H), 7,74 (s, 1H), 3,73 (s, 3H), 3,66 (s, 2H), 3,02 - 2,98 (m, 4H), 1,69 - 1,61 (m, 4H), 1,58 - 1,52 (m, 2H); LRMS (M+H) m/z 181,38.317.1 mg, as 2HCl salt, 91% yield 1H NMR (300 MHz, DMSO-d6) 59.75 (bs, 2H), 7.74 (s, 1H), 3.73 (s, 3H) , 3.66 (s, 2H), 3.02-2.98 (m, 4H), 1.69-1.61 (m, 4H), 1.58-1.52 (m, 2H); LRMS (M+H) m/z 181.38.
Ejemplo 86Example 86
4-(1-metil-4-nitro-1H-pirazol-3-il)morfolina 4-(1-methyl-4-nitro-1H-pyrazol-3-yl)morpholine
337 mg, 37% de rendimiento en 3 etapas. RMN 1H (300 MHz, Cloroformo-d) 58,07 (d, J = 0,5 Hz, 1H), 3,88 - 3,85 (m, 4H), 3,81 (d, J = 0,5 Hz, 3H), 3,32 - 3,29 (m, 4H); LRMS (M+H) m/z 213,34. La estructura se confirmó mediante el experimento 1D-NOE: se observó NOE entre los protones del grupo metilo y el protón del pirazol.337 mg, 37% yield in 3 steps. 1H NMR (300 MHz, Chloroform-d) 58.07 (d, J = 0.5 Hz, 1H), 3.88 - 3.85 (m, 4H), 3.81 (d, J = 0.5 Hz, 3H), 3.32-3.29 (m, 4H); LRMS (M+H) m/z 213.34. The structure was confirmed by 1D-NOE experiment: NOE was observed between the protons of the methyl group and the proton of the pyrazole.
Ejemplo 87Example 87
1 -metil-3-morfolino-1 H-pirazol-4-amina1 -methyl-3-morpholino-1H-pyrazol-4-amine
328,7 mg, como sal de 2HCl, 81% de rendimiento 1H RMN (300 MHz, DMSO-afe) 510,00 (s a, 2H), 7,80 (d, J = 0,9 Hz, 1H), 5,31 (s a, 2H), 3,75 - 3,72 (m, 7H), 3,05 - 3,02 (m, 4H); LRMS (M+H) m/z 183,30.328.7 mg, as 2HCl salt, 81% yield 1H NMR (300 MHz, DMSO-afe) 510.00 (bs, 2H), 7.80 (d, J =0.9 Hz, 1H), 5 .31 (bs, 2H), 3.75-3.72 (m, 7H), 3.05-3.02 (m, 4H); LRMS (M+H) m/z 183.30.
Ejemplo 88Example 88
N-(1-metil-3-(pirrolidin-1-il)-1H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida (I-72)N-(1-methyl-3-(pyrrolidin-1-yl)-1H-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide (I-72)
Escala de 0,08 mmoles, 20,7 mg, 77% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 5 10,47 (s a, 1H), 9,85 (s, 1H), 8,14 (s, 2H), 8,09 (dd, J = 7,8, 1,0 Hz, 1H), 7,95 (s, 1H), 7,88 (dd, J = 7,8, 7,8 Hz, 1H), 7,65 (dd, J = 7,8, 1,0 Hz, 1H), 3,78 (s, 3H), 3,49 - 3,45 (m, 4H), 2,02 - 1,97 (m, 4H); LRMS (M+H) m/z338,60.0.08 mmol scale, 20.7 mg, 77% yield. 1H NMR (300 MHz, Chloroform-d) 10.47 (bs, 1H), 9.85 (s, 1H), 8.14 (s, 2H), 8.09 (dd, J = 7.8, 1.0 Hz, 1H), 7.95 (s, 1H), 7.88 (dd, J = 7.8, 7.8 Hz, 1H), 7.65 (dd, J = 7.8, 1 0.0 Hz, 1H), 3.78 (s, 3H), 3.49-3.45 (m, 4H), 2.02-1.97 (m, 4H); LRMS (M+H) m/z 338.60.
Ejemplo 89Example 89
N-(1-metil-3-(piperidin-1-il)-1H-pirazol-4-il)-6-(1H-pirazol-4-il)picolinamida (I-73)N-(1-methyl-3-(piperidin-1-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide (I-73)
Escala de 0,08 mmoles, 13,1 mg, 47% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 5 10,52 (s a, 1H), 9,82 (s, 1H), 8,20 (s, 2H), 8,09 (dd, J = 7,8, 1,0 Hz, 1H), 8,05 (s, 1 H), 7,88 (dd, J = 7,8, 7,8 Hz, 1H), 7,65 (dd, J = 7,8, 1,0 Hz, 1 h ), 3,81 (s, 3H), 3,12 - 3,09 (m, 4H), 1,83 - 1,75 (m, 4H), 1,66 - 1,59 (m, parcialmente solapado con H2O, 2H); LRMS (M+H) m/z 352,52.0.08 mmol scale, 13.1 mg, 47% yield. 1H NMR (300 MHz, Chloroform-d) 10.52 (bs, 1H), 9.82 (s, 1H), 8.20 (s, 2H), 8.09 (dd, J = 7.8, 1.0 Hz, 1H), 8.05 (s, 1H), 7.88 (dd, J = 7.8, 7.8 Hz, 1H), 7.65 (dd, J = 7.8, 1.0 Hz, 1h), 3.81 (s, 3H), 3.12 - 3.09 (m, 4H), 1.83 - 1.75 (m, 4H), 1.66 - 1, 59 (m, partially overlapped with H2O, 2H); LRMS (M+H) m/z 352.52.
Ejemplo 90Example 90
N-(1 -metil-3-morfolino-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida (I-74)N-(1-methyl-3-morpholino-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide (I-74)
Escala de 0,08 mmoles, 22 mg, 78% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 510,61 (s a, 1H), 9,81 (s, 1H), 8,18 (s, 2H), 8,09 (dd, J = 7,8, 1,0 Hz, 1 H), 8,04 (s, 1H), 7,89 (d, J = 7,8, 7,8 Hz, 1H), 7,67 (dd, J = 7,9, 1,0 Hz, 1H), 3,94 - 3,91 (m, 4H), 3,82 (s, 3H), 3,18 - 3,15 (m, 4H); LRMS (M+H) m/z 354,53. 0.08 mmol scale, 22 mg, 78% yield. 1H NMR (300 MHz, Chloroform-d) 510.61 (bs, 1H), 9.81 (s, 1H), 8.18 (s, 2H), 8.09 (dd, J = 7.8, 1 .0 Hz, 1H), 8.04 (s, 1H), 7.89 (d, J = 7.8, 7.8 Hz, 1H), 7.67 (dd, J = 7.9, 1 0.0 Hz, 1H), 3.94-3.91 (m, 4H), 3.82 (s, 3H), 3.18-3.15 (m, 4H); LRMS (M+H) m/z 354.53.
Ejemplo 91Example 91
5'-metil-N-(1-metil-3-(pirrolidin-1-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-75)5'-methyl-N-(1-methyl-3-(pyrrolidin-1-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-75)
Escala de 0,08 mmoles, 19,6 mg, 68% de rendimiento. 1H RMN (300 MHz, Cloroformo-a) 59,90 (s, 1H), 9,04 (d, J = 2,1 Hz, 1 H), 8,55 (dd, J = 2,1,0,7 Hz, 1H), 8,25 (dd, J = 7,6, 1,1 Hz, 1H), 8,15 - 8,13 (m, 1H), 8,03 - 7,98 (m, 2H), 7,92 (dd, J = 7,9, 1,1 Hz, 1H), 3,78 (s, 3H), 3,49 - 3,45 (m, 4H), 2,47 (s, 3H), 2,02 - 1,98 (m, 4H); LRMS (M+H) m/z363,66. 0.08 mmol scale, 19.6 mg, 68% yield. 1H NMR (300 MHz, Chloroform-a) 59.90 (s, 1H), 9.04 (d, J = 2.1 Hz, 1H), 8.55 (dd, J = 2.1.0, 7 Hz, 1H), 8.25 (dd, J = 7.6, 1.1 Hz, 1H), 8.15 - 8.13 (m, 1H), 8.03 - 7.98 (m, 2H ), 7.92 (dd, J = 7.9, 1.1 Hz, 1H), 3.78 (s, 3H), 3.49 - 3.45 (m, 4H), 2.47 (s, 3H), 2.02-1.98 (m, 4H); LRMS (M+H) m/z 363.66.
Ejemplo 92Example 92
5'-metil-N-(1-metil-3-(piperidin-1-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-76)5'-methyl-N-(1-methyl-3-(piperidin-1-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-76)
Escala de 0,08 mmoles, 14 mg, 46% de rendimiento. 1H RMN (300 MHz, Cloroformo-a) 59,87 (s, 1H), 9,07 (d, J = 1,9 Hz, 1 H), 8,57 - 8,56 (m, 1H), 8,27 - 8,24 (m, 2H), 8,05 (s, 1 H), 8,04 - 7,98 (m, 1H), 7,94 (dd, J = 7,9, 1,2 Hz, 1 H), 3,82 (s, 3H), 3,11 - 3,08 (m, 5H), 2,48 (s, 3H), 1,83 - 1,75 (m, 4H), 1,66 - 1,59 (m, parcialmente solapado con HzO, 5H); LRMS (M+H) m/z 377,71.0.08 mmol scale, 14 mg, 46% yield. 1H NMR (300 MHz, Chloroform-a) 59.87 (s, 1H), 9.07 (d, J = 1.9 Hz, 1H), 8.57-8.56 (m, 1H), 8 .27 - 8.24 (m, 2H), 8.05 (s, 1H), 8.04 - 7.98 (m, 1H), 7.94 (dd, J = 7.9, 1.2 Hz, 1H), 3.82 (s, 3H), 3.11 - 3.08 (m, 5H), 2.48 (s, 3H), 1.83 - 1.75 (m, 4H), 1.66-1.59 (m, partially overlapped with HzO, 5H); LRMS (M+H) m/z 377.71.
Ejemplo 93Example 93
5'-metil-N-(1-metil-3-morfolino-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-77)5'-methyl-N-(1-methyl-3-morpholino-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-77)
Escala de 0,08 mmoles, 19,4 mg, 64% de rendimiento. .1H RMN (300 MHz, Cloroformo-a) 59,86 (s, 1H), 9,06 (d, J = 2,1 Hz, 1 H), 8,58 (dd, J = 2,1,0,7 Hz, 1H), 8,25 (dd, J = 7,5, 1,2 Hz, 1H), 8,21 - 8,19 (m, 1H), 8,04 - 7,99 (m, 2H), 7,94 (dd, J = 7,9, 1,2 Hz, 1 H), 3,92 - 3,89 (m, 4H), 3,83 (s, 3H), 3,17 - 3,14 (m, 4H), 2,51 (s a, 3H); LRMS (M+H) m/z 379,69. 0.08 mmol scale, 19.4 mg, 64% yield. .1H NMR (300 MHz, Chloroform-a) 59.86 (s, 1H), 9.06 (d, J = 2.1 Hz, 1H), 8.58 (dd, J = 2.1.0 0.7 Hz, 1H), 8.25 (dd, J = 7.5, 1.2 Hz, 1H), 8.21 - 8.19 (m, 1H), 8.04 - 7.99 (m, 2H), 7.94 (dd, J = 7.9, 1.2 Hz, 1H), 3.92 - 3.89 (m, 4H), 3.83 (s, 3H), 3.17 - 3.14 (m, 4H), 2.51 (bs, 3H); LRMS (M+H) m/z 379.69.
Ejemplo 94Example 94
6-(3-metil-1 H-pirazol-4-il)-N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-78)6-(3-methyl-1 H-pyrazol-4-yl)-N-(1 -methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)picolinamide (I-78)
Escala de 0,1 mmol, con 3-metil-4-(4,4,5,5tetrametil-1,3,2-dioxaborlan-2-il)-1H-pirazol, 19,1 mg, 53% de rendimiento.0.1 mmol scale, with 3-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborlan-2-yl)-1H-pyrazole, 19.1 mg, 53% yield.
1H RMN (300 MHz, DMSO-afe) δ 12,98, 12,93 (s, s, dos atropisómeros, 1H), 12,25, 12,23 (s, s, dos atropisómeros 1H), 8,62 - 8,56 (m, 2H), 8,41,8,22 (s, s, dos atropisómeros, 1H), 8,10 - 8,05 (m, 2H), 8,00 - 7,93 (m, 2H), 7,87 - 7,83 (m, 1H), 7,45 - 7,40 (m, 1H), 4,01 (s, 3H), 2,66, 2,60 (s, s, dos atropisómeros, 3H); LRMS (M+H) m/z 360,68. 1H NMR (300 MHz, DMSO-afe) δ 12.98, 12.93 (s, s, two atropisomers, 1H), 12.25, 12.23 (s, s, two atropisomers 1H), 8.62 - 8.56 (m, 2H), 8.41.8.22 (s, s, two atropisomers, 1H), 8.10 - 8.05 (m, 2H), 8.00 - 7.93 (m, 2H), 7.87 - 7.83 (m, 1H), 7.45 - 7.40 (m, 1H), 4.01 (s, 3H), 2.66, 2.60 (s, s, two atropisomers, 3H); LRMS (M+H) m/z 360.68.
Ejemplo 95Example 95
2-(1 -isopropil-1 H-pirazol-3-il)piridina2-(1 -isopropyl-1H-pyrazol-3-yl)pyridine
Escala de 40 mmoles, con 2-yodopropano, se usó un aceite marrón claro directamente en la siguiente reacción. 1H RMN (300 MHz, Cloroformo-a) 58,62 (ddd, J = 4,9, 1,8, 1,0 Hz, 1H), 7,94 (ddd, J = 8,0, 1,1, 1,1 Hz, 1H), 7,73 - 7,67 (m, 1H), 7,48 (d, J = 2,4 Hz, 1H), 7,17 (ddd, J = 7,5, 4,9, 1,2 Hz, 1H), 6,87 (d, J = 2,4 Hz, 1H), 4,60 (hept, J = 6,7 Hz, 1H), 1,56 (d, J = 6,7 Hz, 6H); LRMS (M+H) m/z 188,22.40 mmol scale, with 2-iodopropane, a light brown oil was used directly in the next reaction. 1H NMR (300 MHz, Chloroform-a) 58.62 (ddd, J = 4.9, 1.8, 1.0 Hz, 1H), 7.94 (ddd, J = 8.0, 1.1, 1.1 Hz, 1H), 7.73 - 7.67 (m, 1H), 7.48 (d, J = 2.4 Hz, 1H), 7.17 (ddd, J = 7.5, 4 .9, 1.2 Hz, 1H), 6.87 (d, J = 2.4 Hz, 1H), 4.60 (hept, J = 6.7 Hz, 1H), 1.56 (d, J = 6.7Hz, 6H); LRMS (M+H) m/z 188.22.
Ejemplo 96Example 96
2-(1 -isopropil-4-nitro-1 H-pirazol-3-il)piridina2-(1 -isopropyl-4-nitro-1H-pyrazol-3-yl)pyridine
Escala de 40 mmoles, tras la cromatografía en gel de sílice, un sólido amarillo: 8,79 g, 95% de rendimiento en 2 etapas.40 mmol scale, after silica gel chromatography, a yellow solid: 8.79 g, 95% yield in 2 steps.
1H RMN (300 MHz, Cloroformo-a) 58,75 (ddd, J = 4,9, 1,7, 1,0 Hz, 1H), 8,29 (s, 1H), 7,84 - 7,72 (m, 2H), 7,36 (ddd, J = 7,4, 4,9, 1,4 Hz, 1 H), 4,62 (hept, J = 6,7 Hz, 1H), 1,61 (d, J = 6,7 Hz, 6H); LRMS (M+H) m/z233,28.1H NMR (300 MHz, Chloroform-a) 58.75 (ddd, J = 4.9, 1.7, 1.0 Hz, 1H), 8.29 (s, 1H), 7.84-7.72 (m, 2H), 7.36 (ddd, J = 7.4, 4.9, 1.4 Hz, 1H), 4.62 (hept, J = 6.7 Hz, 1H), 1.61 (d, J =6.7Hz, 6H); LRMS (M+H) m/z 233.28.
Ejemplo 97Example 97
1-isopropil-3-(piridin-2-il)-1H-pirazol-4-amina, dih idrocloruro1-isopropyl-3-(pyridin-2-yl)-1H-pyrazol-4-amine, dihydrochloride
Obtenido como sal de di-HCl, un sólido amarillo claro: 9,99 g (96 % de rendimiento); 1H RMN (300 MHz, Cloroformo-d) 58,52 (ddd, J = 5,0, 1,8, 1,0 Hz, 1H), 7,97 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,66 (ddd, J = 8,1,7,5, 1,8 Hz, 1H), 7,09 - 7,05 (m, 2H), 4,43 (hept, J = 6,7 Hz, 1 H), 4,07 (s a, 2H), 1,49 (d, J = 6,7 Hz, 6H); LRMS (M+H) m/z203,28.Obtained as di-HCl salt, a light yellow solid: 9.99 g (96% yield); 1H NMR (300 MHz, Chloroform- d) 58.52 (ddd, J = 5.0, 1.8, 1.0 Hz, 1H), 7.97 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.66 (ddd, J = 8,1,7.5, 1.8 Hz, 1H), 7.09 - 7.05 (m, 2H), 4.43 (hept , J = 6.7 Hz, 1H), 4.07 (sa, 2H), 1.49 (d, J = 6.7 Hz, 6H); LRMS (M+H) m/z 203.28.
Ejemplo 98Example 98
6-bromo-N-(1-isopropil-3-(piridin-2-il)-1H-pirazol-4-il)picolinamida (I-79)6-bromo-N-(1-isopropyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-79)
Escala de 1,0 mmoles, con ácido 6-bromopicolínico, 244,7 mg, 63% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 513,11 (s, 1H), 8,84 (ddd, J = 4,9, 1,8, 1,0 Hz, 1H), 8,41 (s, 1H), 8,20 (dd, J = 7,5, 1,0 Hz, 1H), 8,07 (ddd, J = 8,1, 1,1, 1,1 Hz, 1 H), 7,79 - 7,73 (m, 2H), 7,65 (dd, J = 7,9, 1,0 Hz, 1H), 7,22 (ddd, J = 7,5, 4,9, 1,1 Hz, 1 H), 4,56 (hept, J = 6,7 Hz, 1H), 1,58 (d, J = 6,7 Hz, 6H); LRMS (M+H) m/z386,58, 388,49.1.0 mmol scale, with 6-bromopicolinic acid, 244.7 mg, 63% yield. 1H NMR (300 MHz, Chloroform- d) 13.11 (s, 1H), 8.84 (ddd, J = 4.9, 1.8, 1.0 Hz, 1H), 8.41 (s, 1H), 8.20 (dd, J = 7.5, 1.0 Hz, 1H), 8.07 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.79 - 7.73 (m, 2H), 7.65 (dd, J = 7.9, 1.0 Hz, 1H), 7.22 ( ddd, J = 7.5, 4.9, 1.1 Hz, 1H), 4.56 (hept, J = 6.7 Hz, 1H), 1.58 (d, J = 6.7 Hz, 6H); LRMS (M+H) m/z 386.58, 388.49.
Ejemplo 99Example 99
N-(1 -isopropil-3-(piridin-2-il)-1 H-pi razol-4-i l)-6-(1 H-pirazol-4-il)picolinamida (I-80)N-(1-isopropyl-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide (I-80)
Escala de 0,1 mmoles, 32 mg, 86% de rendimiento.1H RMN (300 MHz, DMSO-de) 5 13,31 (s, 1H), 12,38 (s, 1H), 8,76 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,61 (s, 1H), 8,56 (s, 1H), 8,36 - 8,35 (m, 1H), 8,13 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 8,08 (dd, J = 8,0, 7,3 Hz, 1 H), 8,02 - 7,96 (m, 3H), 7,47 (ddd, J = 7,4, 5,0, 1,3 Hz, 1H), 4,69 (hept, J = 6,7 Hz, 1H), 1,55 (d, J = 6,7 Hz, 6H); LRMS (M+H) m/z374,69.0.1 mmol scale, 32 mg, 86% yield.1H NMR (300 MHz, DMSO-de) 13.31 (s, 1H), 12.38 (s, 1H), 8.76 (ddd, J = 5.0, 1.7, 0.9 Hz, 1H), 8.61 (s, 1H), 8.56 (s, 1H), 8.36 - 8.35 (m, 1H), 8 .13 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 8.08 (dd, J = 8.0, 7.3 Hz, 1H), 8.02 - 7, 96 (m, 3H), 7.47 (ddd, J = 7.4, 5.0, 1.3 Hz, 1H), 4.69 (hept, J = 6.7 Hz, 1H), 1.55 (d, J =6.7Hz, 6H); LRMS (M+H) m/z 374.69.
Ejemplo 100Example 100
N-(1-isopropil-3-(piridin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida (I-81)N-(1-isopropyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridine]-6-carboxamide (I-81)
Escala de 0,1 mmoles, 31,4 mg, 79% de rendimiento. 1H RMN (300 MHz, DMSO-afe) 512,63 (s, 1H), 9,55 (d, J = 2,1 Hz, 1H), 8,81 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,67 - 8,66 (m, 1H), 8,61 (s, 1 H), 8,48 (ddd, J = 2,1,2,1,0,7 Hz, 1 H), 8,38 (dd, J = 7,4, 1,6 Hz, 1H), 8,28 - 8,19 (m, 2H), 8,12 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 8,01 - 7,95 (m, 1H), 7,47 (ddd, J = 7,4, 5,0, 1,2 Hz, 1 H), 4,69 (hept, J = 6,7 Hz, 1H), 2,51 (s, 3H), 1,56 (d, J = 6,7 Hz, 6H); LRMS (M+H) m/z399,69. Ejemplo 1010.1 mmol scale, 31.4 mg, 79% yield. 1H NMR (300 MHz, DMSO-afe) 512.63 (s, 1H), 9.55 (d, J = 2.1 Hz, 1H), 8.81 (ddd, J = 5.0, 1.7 , 0.9 Hz, 1H), 8.67 - 8.66 (m, 1H), 8.61 (s, 1H), 8.48 (ddd, J = 2.1,2,1.0, 7 Hz, 1H), 8.38 (dd, J = 7.4, 1.6 Hz, 1H), 8.28 - 8.19 (m, 2H), 8.12 (ddd, J = 8, 1, 1.1, 1.1 Hz, 1H), 8.01 - 7.95 (m, 1H), 7.47 (ddd, J = 7.4, 5.0, 1.2 Hz, 1H ), 4.69 (hept, J = 6.7 Hz, 1H), 2.51 (s, 3H), 1.56 (d, J = 6.7 Hz, 6H); LRMS (M+H) m/z 399.69. Example 101
4-(2-(3-(piridin-2-il)-1 H-pirazol-1-il)etil)morfolina4-(2-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)ethyl)morpholine
Escala de 15 mmoles, con hidrobromuro de 4-(2-bromoetil)morfolina, se usó un aceite naranja-marrón claro directamente en la siguiente reacción. 1H RMN (300 MHz, Cloroformo-d) 58,63 (ddd, J = 4,9, 1,8, 1,0 Hz, 1H), 7,90 (ddd, J = 8,0, 1,1, 1,1 Hz, 1 H), 7,70 (ddd, J = 8,0, 7,5, 1,8 Hz, 1 H), 7,53 (d, J = 2,3 Hz, 1H), 7,19 (ddd, J = 7,5, 4,9, 1,2 Hz, 1H), 6,85 (d, J = 2,3 Hz, 1H), 4,32 (t, J = 6,6 Hz, 2H), 3,72 - 3,68 (m, 4H), 2,87 (t, J = 6,6 Hz, 2H), 2,51 - 2,48 (m, 4H); LRMS (M+H) m/z259,53. 15 mmol scale, with 4-(2-bromoethyl)morpholine hydrobromide, a light orange-brown oil was used directly in the next reaction. 1H NMR (300 MHz, Chloroform-d) 58.63 (ddd, J = 4.9, 1.8, 1.0 Hz, 1H), 7.90 (ddd, J = 8.0, 1.1, 1.1 Hz, 1H), 7.70 (ddd, J = 8.0, 7.5, 1.8 Hz, 1H), 7.53 (d, J = 2.3 Hz, 1H), 7.19 (ddd, J = 7.5, 4.9, 1.2 Hz, 1H), 6.85 (d, J = 2.3 Hz, 1H), 4.32 (t, J = 6, 6 Hz, 2H), 3.72-3.68 (m, 4H), 2.87 (t, J =6.6 Hz, 2H), 2.51-2.48 (m, 4H); LRMS (M+H) m/z 259.53.
Ejemplo 102Example 102
4-(2-(4-nitro-3-(piridin-2-il)-1 H-pirazol-1 -il)etil)morfolina4-(2-(4-nitro-3-(pyridin-2-yl)-1H-pyrazol-1-yl)ethyl)morpholine
Escala de 15 mmoles, 3,145 mg, 69% de rendimiento. .1H RMN (300 MHz, Cloroformo-d) 58,75 (ddd, J = 4,9, 1,7, 1,0 Hz, 1H), 8,41 (s, 1H), 7,81 (ddd, J = 7,9, 7,2, 1,7 Hz, 1H), 7,76 (ddd, J = 7,9, 1,6, 1,0 Hz, 1H), 7,37 (ddd, J = 7,2, 4,9, 1,6 Hz, 1H), 4,32 - 4,30 (m, 2H), 3,74 - 3,71 (m, 4H), 2,89 - 2,85 (m, 2H), 2,54 - 2,51 (m, 4H); LRMS (M+H) m/z304,56. Se obtuvo una fracción mixta (1,17 g) con alrededor de 26% del otro regioisómero. La estructura del compuesto del título se confirmó mediante el experimento 1D-NOE: se observó NOE entre protones de CH2 que se conectan al nitrógeno del pirazol y el protón en el pirazol.15 mmol scale, 3.145 mg, 69% yield. .1H NMR (300 MHz, Chloroform-d) 58.75 (ddd, J = 4.9, 1.7, 1.0 Hz, 1H), 8.41 (s, 1H), 7.81 (ddd, J = 7.9, 7.2, 1.7 Hz, 1H), 7.76 (ddd, J = 7.9, 1.6, 1.0 Hz, 1H), 7.37 (ddd, J = 7.2, 4.9, 1.6 Hz, 1H), 4.32 - 4.30 (m, 2H), 3.74 - 3.71 (m, 4H), 2.89 - 2.85 ( m, 2H), 2.54-2.51 (m, 4H); LRMS (M+H) m/z 304.56. A mixed fraction (1.17 g) was obtained with about 26% of the other regioisomer. The structure of the title compound was confirmed by 1D-NOE experiment: NOE was observed between CH 2 protons connecting to the pyrazole nitrogen and the proton in the pyrazole.
Ejemplo 103Example 103
trih idrocloruro de 1-(2-morfolinoetil)-3-(piridin-2-il)-1H-pirazol-4-amina1-(2-morpholinoethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-amine trihydrochloride
Escala de 10,35 mmoles, obtenida como sal de trihidrocloruro: 2,92 g (74 % de rendimiento); 1H RMN (300 MHz, DMSO-ofe) 511,55 (s, 1H), 10,26 (s a, 2H), 8,70 (ddd, J = 4,9, 1,3, 1,3 Hz, 1H), 8,28 (s, 1H), 8,04 - 7,97 (m, 2H), 7,49 (ddd, J = 5,1,5,1,3,5 Hz, 1H), 5,20 (s a, 3H), 4,80 (t, J = 6,7 Hz, 2H), 3,98 - 3,88 (m, 4H), 3,70 (t, J = 6,7 Hz, 2H), 3,49 - 3,40 (m, 2H), 3,25 - 3,17 (m, 2H); LRMS (M+H) m/z274,54.10.35 mmol scale, obtained as the trihydrochloride salt: 2.92 g (74% yield); 1H NMR (300 MHz, DMSO-ofe) 511.55 (s, 1H), 10.26 (sa, 2H), 8.70 (ddd, J = 4.9, 1.3, 1.3 Hz, 1H ), 8.28 (s, 1H), 8.04 - 7.97 (m, 2H), 7.49 (ddd, J = 5,1,5,1,3.5 Hz, 1H), 5, 20 (sa, 3H), 4.80 (t, J = 6.7 Hz, 2H), 3.98-3.88 (m, 4H), 3.70 (t, J = 6.7 Hz, 2H ), 3.49-3.40 (m, 2H), 3.25-3.17 (m, 2H); LRMS (M+H) m/z 274.54.
Ejemplo 104Example 104
6-bromo-N-(1-(2-morfolinoetil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-82)6-Bromo-N-(1-(2-morpholinoethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-82)
Escala de 1,0 mmol, 323,6 g, 71% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 5 13,05 (s, 1H), 8,86 (brd, J = 4,7 Hz, 1 H), 8,50 (s, 1H), 8,21 (d, J = 7,4 Hz, 1H), 8,03 (d, J = 8,0 Hz, 1H), 7,82 - 7,74 (m, 2H), 7,66 (d, J = 7,9 Hz, 1H), 7,30 - 7,26 (m, 1H, parcialmente solapado con CHCh), 4,77 - 4,70 (m, 2H), 4,03 - 3,94 (m, 4H), 3,54 - 3,44 (m, 2H), 3,02 - 2,84 (m, 4H); LRMS (M+H) m/z457,61,459,50. 1.0 mmol scale, 323.6 g, 71% yield. 1H NMR (300 MHz, Chloroform-d) 13.05 (s, 1H), 8.86 (brd, J = 4.7 Hz, 1H), 8.50 (s, 1H), 8.21 ( d, J = 7.4 Hz, 1H), 8.03 (d, J = 8.0 Hz, 1H), 7.82 - 7.74 (m, 2H), 7.66 (d, J = 7 .9 Hz, 1H), 7.30 - 7.26 (m, 1H, partially overlapped with CHCh), 4.77 - 4.70 (m, 2H), 4.03 - 3.94 (m, 4H) , 3.54-3.44 (m, 2H), 3.02-2.84 (m, 4H); LRMS (M+H) m/z 457,61,459.50.
Ejemplo 105Example 105
N-(1 -(2-morfolinoetil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida (I-83)N-(1 -(2-morpholinoethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide (I-83)
Escala de 0,1 mmoles, 27,8 mg, 63% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 512,56 (s, 1H), 10,35 (v s a, 1H), 8,73 (ddd, J = 5,0, 1,8, 0,9 Hz, 1H), 8,56 (s, 1H), 8,33 (s, 2H), 8,13 - 8,09 (m, 2H), 7,88 (dd, J = 7,8, 7,8 Hz, 1H), 7,76 (ddd, J = 8,1,7,5, 1,8 Hz, 1H), 7,65 (dd, J = 7,8, 1,0 Hz, 1H), 7,23 (ddd, J = 7,5, 5,0, 1,1 Hz, 1H), 4,32 (t, J = 6,8 Hz, 2H), 3,74 - 3,71 (m, 4H), 2,92 (t, J = 6,8 Hz, 2H), 2,55 - 2,52 (m, 4H); LRMS (M+H) m/z445,79.0.1 mmol scale, 27.8 mg, 63% yield. 1H NMR (300 MHz, Chloroform-d) 512.56 (s, 1H), 10.35 (vsa, 1H), 8.73 (ddd, J = 5.0, 1.8, 0.9 Hz, 1H ), 8.56 (s, 1H), 8.33 (s, 2H), 8.13 - 8.09 (m, 2H), 7.88 (dd, J = 7.8, 7.8 Hz, 1H), 7.76 (ddd, J = 8,1,7.5, 1.8 Hz, 1H), 7.65 (dd, J = 7.8, 1.0 Hz, 1H), 7.23 (ddd, J = 7.5, 5.0, 1.1 Hz, 1H), 4.32 (t, J = 6.8 Hz, 2H), 3.74 - 3.71 (m, 4H), 2.92 (t, J =6.8 Hz, 2H), 2.55-2.52 (m, 4H); LRMS (M+H) m/z 445.79.
Ejemplo 106Example 106
5'-metil-N-(1-(2-morfolinoetil)-3-(piridin-2-il)-1 H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-84)5'-methyl-N-(1-(2-morpholinoethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I -84)
Escala de 0,1 mmol, 27,9 g, 59% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 512,83 (s, 1H), 9,38 (a d, J = 2,1 Hz, 1 H), 8,74 (ddd, J = 5,0, 1,8, 1,0 Hz, 1 H), 8,61 (dd, J = 2,1,0,7 Hz, 1H), 8,55 (s, 1H), 8,29-8,26 (m, 2H), 8,10 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 8,04 - 7,99 (m, 1H), 7,93 (dd, J = 7,9, 1,2 Hz, 1H), 7,76 (ddd, J = 8,1,7,5, 1,8 Hz, 1H), 7,26 -7,22 (m, 1H, parcialmente solapado con CHCh), 4,32 (s, J = 6,8 Hz, 2H), 3,74 - 3,71 (m, 4H), 2,92 (t, J = 6,8 Hz, 2H), 2,55 - 2,52 (m, 4H), 2,51 (s a, 3H); LRMS (M+H) m/z 470,85.0.1 mmol scale, 27.9 g, 59% yield. 1H NMR (300 MHz, Chloroform-d) 512.83 (s, 1H), 9.38 (ad, J = 2.1 Hz, 1H), 8.74 (ddd, J = 5.0, 1, 8, 1.0 Hz, 1H), 8.61 (dd, J = 2.1,0.7 Hz, 1H), 8.55 (s, 1H), 8.29-8.26 (m, 2H), 8.10 (ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 8.04 - 7.99 (m, 1H), 7.93 (dd, J = 7, 9, 1.2 Hz, 1H), 7.76 (ddd, J = 8,1,7,5, 1.8 Hz, 1H), 7.26 -7.22 (m, 1H, partially overlapped with CHCh ), 4.32 (s, J = 6.8 Hz, 2H), 3.74 - 3.71 (m, 4H), 2.92 (t, J = 6.8 Hz, 2H), 2.55 - 2.52 (m, 4H), 2.51 (bs, 3H); LRMS (M+H) m/z 470.85.
Ejemplo 107Example 107
1 -metil-4-(2-(4-nitro-3-(piridin-2-il)-1 H-pirazol-1 -il)etil)piperazina1-methyl-4-(2-(4-nitro-3-(pyridin-2-yl)-1H-pyrazol-1-yl)ethyl)piperazine
Escala de 10 mmoles, 1s etapa: el bruto se usó directamente en la siguiente reacción; 2a etapa: tras la cromatografía en gel de sílice, 1,69 g, 53% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 58,75 (ddd, J = 4,9, 1,7, 1,0 Hz, 1H), 8,42 (s, 1H), 7,84 - 7,74 (m, 2H), 7,37 (ddd, J = 7,1,4,9, 1,6 Hz, 1 H), 4,33 - 4,29 (m, 2H), 2,89 - 2,85 (m, 2H), 2,60 -2,39 (m, 8H), 2,31 (s, 3H); LRMS (M+H) m/z317,63. 10 mmol scale, 1s stage: crude was used directly in the next reaction; 2nd stage: after chromatography on silica gel, 1.69 g, 53% yield. 1H NMR (300 MHz, Chloroform-d) 58.75 (ddd, J = 4.9, 1.7, 1.0 Hz, 1H), 8.42 (s, 1H), 7.84-7.74 (m, 2H), 7.37 (ddd, J = 7,1,4,9, 1.6 Hz, 1H), 4.33 - 4.29 (m, 2H), 2.89 - 2, 85 (m, 2H), 2.60 -2.39 (m, 8H), 2.31 (s, 3H); LRMS (M+H) m/z 317.63.
Ejemplo 108Example 108
trih idrocloruro de 1-(2-(4-metilpiperazin-1-il)etil)-3-(piridin-2-il)-1H-pirazol-4-amina Escala de 5,3 mmoles, obtenido como sal de trih idrocloruro: 1,67 g, 79% de rendimiento; LRMS (M+H) m/z 287,55.1-(2-(4-methylpiperazin-1-yl)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-amine trihydrochloride 5.3 mmol scale, obtained as the trihydrochloride salt : 1.67 g, 79% yield; LRMS (M+H) m/z 287.55.
Ejemplo 109Example 109
6-bromo-N-(1-(2-(4-metilpiperazin-1-il)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)picolinamida (I-85)6-Bromo-N-(1-(2-(4-methylpiperazin-1-yl)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)picolinamide (I-85)
Escala de 1,0 mmol, 342,1 g, 73% de rendimiento. 1H RMN (300 MHz, Cloroformo-d) 5 13,10 (s, 1H), 8,85 (ddd, J = 4,9, 1,7, 1,0 Hz, 1H), 8,40 (s, 1H), 8,19 (dd, J = 7,5, 0,9 Hz, 1H), 8,03 (ddd, J = 8,1, 1,0, 1,0 Hz, 1H), 7,78 (ddd, J = 7,8, 7,8, 1,8 Hz, 1H), 7,77 (dd, J = 7,7, 7,7 Hz, 1H), 7,67 (dd, J = 7,9, 0,9 Hz, 1H), 7,28 - 7,24 (m, parcialmente solapado con CHCh, 1 H), 4,27 (t, J = 5,9 Hz, 2H), 3,39 - 3,29 (m, 2H), 3,01 (t, J = 5,9 Hz, 2H), 3,03 - 2,81 (m, 6H), 2,73 (s, 3H); LRMS (M+H) m/z472,68.1.0 mmol scale, 342.1 g, 73% yield. 1H NMR (300 MHz, Chloroform-d) 13.10 (s, 1H), 8.85 (ddd, J = 4.9, 1.7, 1.0 Hz, 1H), 8.40 (s, 1H), 8.19 (dd, J = 7.5, 0.9 Hz, 1H), 8.03 (ddd, J = 8.1, 1.0, 1.0 Hz, 1H), 7.78 (ddd, J = 7.8, 7.8, 1.8 Hz, 1H), 7.77 (dd, J = 7.7, 7.7 Hz, 1H), 7.67 (dd, J = 7 .9, 0.9 Hz, 1H), 7.28 - 7.24 (m, partially overlapped with CHCh, 1H), 4.27 (t, J = 5.9 Hz, 2H), 3.39 - 3.29 (m, 2H), 3.01 (t, J =5.9 Hz, 2H), 3.03-2.81 (m, 6H), 2.73 (s, 3H); LRMS (M+H) m/z 472.68.
Ejemplo 110Example 110
N-(1 -(2-(4-metilpiperazin-1 -il)etil)-3-(piridin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il) picolinamida (I-86) Escala de 0,1 mmoles, 15,2 mg, 33% de rendimiento. 1H RMN (300 MHz, DMSO-afe) 513,35 (s a, 1H), 12,38 (s, 1H), 8,76 (a d, J = 4,9 Hz, 1 H), 8,63 (s, 1 H), 8,46 (s a, 2H), 8,12 - 8,05 (m, 2H), 8,01 - 7,96 (m, 3H), 7,47 (ddd, J = 7,4, 5,0, 1,0 Hz, 1H), 4,38 (t, J = 6,5 Hz, 2H), 3,44 (v s a, 2H), 2,85 (t, J = 6,5 Hz, 2H), 2,57 - 2,52 (m, solapado con DMSO, 6H), 2,29 (s, 3H); LRMS (M+H) m/z458,81. N-(1 -(2-(4-methylpiperazin-1 -yl)ethyl)-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4- il) picolinamide (I-86) 0.1 mmol scale, 15.2 mg, 33% yield. 1H NMR (300 MHz, DMSO-afe) 513.35 (sa, 1H), 12.38 (s, 1H), 8.76 (bd, J = 4.9 Hz, 1H), 8.63 (s , 1H), 8.46 (sa, 2H), 8.12 - 8.05 (m, 2H), 8.01 - 7.96 (m, 3H), 7.47 (ddd, J = 7, 4, 5.0, 1.0 Hz, 1H), 4.38 (t, J = 6.5 Hz, 2H), 3.44 (vsa, 2H), 2.85 (t, J = 6.5 Hz, 2H), 2.57-2.52 (m, overlapped with DMSO, 6H), 2.29 (s, 3H); LRMS (M+H) m/z 458.81.
Ejemplo 111Example 111
5'-metil-N-(1-(2-(4-metilpiperazin-1-il)etil)-3-(piridin-2-il)-1H-pirazol-4-il)-[2,3'- bipiridina]-6-carboxamida (I-87) 5'-methyl-N-(1-(2-(4-methylpiperazin-1-yl)ethyl)-3-(pyridin-2-yl)-1H-pyrazol-4-yl)-[2,3'- bipyridine]-6-carboxamide (I-87)
Escala de 0,1 mmol, 9,1 mg, 19% de rendimiento. 1H RMN (300 MHz, DMSO-afe) 512,61 (s, 1H), 9,54 (d, J = 1,8 Hz, 1H), 8,80 (a d, J = 4,5 Hz, 1H), 8,66 (d, J = 1,0 Hz, 1 H), 8,63 (s, 1 H), 8,47 (s, 1H), 8,37 (dd, J = 7,1, 1,8 Hz, 1H), 8,27 - 8,19 (m, 2H), 8,09 (a d, J = 8,0 Hz, 1H), 7,98 (ddd, J = 7,8, 7,8, 1,7 Hz, 1H), 7,47 (ddd, J = 7,4, 5,0, 1,0 Hz, 1H), 4,39 (t, J = 6,5 Hz, 2H), 3,38 (v s a, 2H), 2,84 (t, J = 6,5 Hz, 2H), 2,50 (s, 3H), 2,57 - 2,51 (m,solapado con DMSO, 2H), 2,42 (s a, 4H), 2,22 (s, 3H); LRMS (M+H) m/z483,88.0.1 mmol scale, 9.1 mg, 19% yield. 1H NMR (300 MHz, DMSO-afe) 512.61 (s, 1H), 9.54 (d, J = 1.8 Hz, 1H), 8.80 (d, J = 4.5 Hz, 1H) , 8.66 (d, J = 1.0 Hz, 1H), 8.63 (s, 1H), 8.47 (s, 1H), 8.37 (dd, J = 7.1, 1 .8 Hz, 1H), 8.27 - 8.19 (m, 2H), 8.09 (ad, J = 8.0 Hz, 1H), 7.98 (ddd, J = 7.8, 7, 8, 1.7 Hz, 1H), 7.47 (ddd, J = 7.4, 5.0, 1.0 Hz, 1H), 4.39 (t, J = 6.5 Hz, 2H), 3.38 (vsa, 2H), 2.84 (t, J =6.5 Hz, 2H), 2.50 (s, 3H), 2.57-2.51 (m,overlapped with DMSO, 2H) , 2.42 (bs, 4H), 2.22 (s, 3H); LRMS (M+H) m/z 483.88.
Ejemplo 112Example 112
N-(1-metil-3-(piridin-2-il)-1 H-pirazol-4-il)-2'-oxo-1',2'-dihidro-[2,4'-bipiridin]-6-carboxamida (I-91)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-2'-oxo-1',2'-dihydro-[2,4'-bipyridin]-6 -carboxamide (I-91)
1H RMN (300 MHz, DMSO-afe) δ 12,48 (s, 1H), 11,72 (s a, 1H), 9,00 (ddd, J = 5,0, 1,7, 0,9 Hz, 1H), 8,59 (s, 1H), 8,35 (dd, J = 7,0, 2,0 Hz, 1 H), 8,27 - 8,24 (m, 2H), 8,08 (ddd, J = 8,1, 1,1, 1,1 Hz, 1H), 7,97 (ddd, J = 7,7, 7,7, 1,7 Hz, 1H), 7,73 (d, J = 6,8 Hz, 1H), 7,14 (dd, J = 6,9, 1,8 Hz, 1H), 6,34 (ddd, J = 9,2, 1,0, 1,0 Hz, 1H), 6,18 (ddd, J = 6,5, 6,5, 1,2 Hz, 1 H), 4,01 (s, 3H); LRMS (M+H) m/z373,60.1H NMR (300 MHz, DMSO-afe) δ 12.48 (s, 1H), 11.72 (sa, 1H), 9.00 (ddd, J = 5.0, 1.7, 0.9 Hz, 1H), 8.59 (s, 1H), 8.35 (dd, J = 7.0, 2.0 Hz, 1H), 8.27 - 8.24 (m, 2H), 8.08 ( ddd, J = 8.1, 1.1, 1.1 Hz, 1H), 7.97 (ddd, J = 7.7, 7.7, 1.7 Hz, 1H), 7.73 (d, J = 6.8 Hz, 1H), 7.14 (dd, J = 6.9, 1.8 Hz, 1H), 6.34 (ddd, J = 9.2, 1.0, 1.0 Hz , 1H), 6.18 (ddd, J = 6.5, 6.5, 1.2 Hz, 1H), 4.01 (s, 3H); LRMS (M+H) m/z 373.60.
Ejemplo 113Example 113
N-(1 -metil-3-(piridin-2-il)-1 H-pirazol-4-il)-6'-oxo-1 ',6'-dihidro-[2,3'-bipiridin]-6-carboxamida (I-92)N-(1-methyl-3-(pyridin-2-yl)-1 H-pyrazol-4-yl)-6'-oxo-1',6'-dihydro-[2,3'-bipyridin]-6 -carboxamide (I-92)
1H RMN (300 MHz, DMSO-afe) δ 12,39 (s, 1H), 12,30 (s, 1H), 8,73 - 8,70 (m, 1H), 8,57 (s, 1H), 8,52 (dd, J = 9,6, 2,7 Hz, 1H), 8,41 (d, J = 2,9 Hz, 1H), 8,16 (dd, J = 7,6, 1,8 Hz, 1H), 8,12 (d, J = 7,2 Hz, 1H), 8,07 - 8,04 (m, 2H), 7,98 (ddd, J = 7,7, 7,7, 1,7 Hz, 1H), 7,48 (ddd, J = 7,4, 5,0, 1,4 Hz, 1H), 6,70 (d, J = 9,6 Hz, 1H), 4,01 (s, 3H); LRMS (M+H) m/z 373,62. 1H NMR (300 MHz, DMSO-afe) δ 12.39 (s, 1H), 12.30 (s, 1H), 8.73-8.70 (m, 1H), 8.57 (s, 1H) , 8.52 (dd, J = 9.6, 2.7 Hz, 1H), 8.41 (d, J = 2.9 Hz, 1H), 8.16 (dd, J = 7.6, 1 0.8 Hz, 1H), 8.12 (d, J = 7.2 Hz, 1H), 8.07 - 8.04 (m, 2H), 7.98 (ddd, J = 7.7, 7, 7, 1.7 Hz, 1H), 7.48 (ddd, J = 7.4, 5.0, 1.4 Hz, 1H), 6.70 (d, J = 9.6 Hz, 1H), 4.01 (s, 3H); LRMS (M+H) m/z 373.62.
Ejemplo 114Example 114
N-(3-(3,5-difluoropiridin-2-il)-1 -(írans-4-etoxiciclohexil)-1 HLpirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida (I-184) 1H RMN (300 MHz, Cloroformo-d) 512,17 (s, 1 H), 8,58 (s a, 1H), 8,47 (d, J = 2,4 Hz, 1 H), 8,26 (s a, 2H), 8,10 (dd, J = 7,7, 1,0 Hz, 1 H), 7,88 (dd, J = 7,8, 7,8 Hz, 1H), 7,65 (dd, J = 7,9, 1,1 Hz, 1H), 7,36 (ddd, J = 10,7, 8,3, 2,4 Hz, 1H), 4,28 (tt, J = 11,8, 3,8 Hz, 1H), 3,57 (c, J = 7,0 Hz, 2H), 3,38 (tt, J = 10,7, 4,1 Hz, 1H), 2,33 - 2,20 (m, 4H), 1,97 - 1,84 (m, 2H), 1,55 - 1,41 (m, 2H), 1,23 (t, J = 7,0 Hz, 3H); LRMS (M+H) m/z494,28.N-(3-(3,5-difluoropyridin-2-yl)-1 -(írans-4-ethoxycyclohexyl)-1 HLpyrazol-4-yl)-6-(1H-pyrazol-4-yl)picolinamide (I -184) 1H NMR (300 MHz, Chloroform-d) 512.17 (s, 1H), 8.58 (bs, 1H), 8.47 (d, J = 2.4 Hz, 1H), 8 .26 (sa, 2H), 8.10 (dd, J = 7.7, 1.0 Hz, 1H), 7.88 (dd, J = 7.8, 7.8 Hz, 1H), 7 .65 (dd, J = 7.9, 1.1 Hz, 1H), 7.36 (ddd, J = 10.7, 8.3, 2.4 Hz, 1H), 4.28 (tt, J = 11.8, 3.8 Hz, 1H), 3.57 (c, J = 7.0 Hz, 2H), 3.38 (tt, J = 10.7, 4.1 Hz, 1H), 2 .33 - 2.20 (m, 4H), 1.97 - 1.84 (m, 2H), 1.55 - 1.41 (m, 2H), 1.23 (t, J = 7.0 Hz , 3H); LRMS (M+H) m/z 494.28.
Ejemplo 115Example 115
N-(1 -(írans-4-etoxiciclohexil)-3-(pirazin-2-il)-1 H-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida (I-205) N- ( 1 -(írans-4-ethoxycyclohexyl)-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide (I-205 )
1H RMN (400 MHz, Cloroformo-d) δ 12,19 (s, 1H), 10,57 (s a, 1H), 9,38 (d, J = 1,5 Hz, 1H), 8,64 (dd, J = 2,7, 1,6 Hz, 1H), 8,53 (s, 1 H), 8,48 (d, J = 2,6 Hz, 1 H), 8,31 (s, 2H), 8,09 (dd, J = 7,7, 1,0 Hz, 1 H), 7,88 (dd, J = 7,8, 7,8 Hz, 1 H), 7,65 (dd, J = 7,9, 1,0 Hz, 1H), 4,22 (tt, J = 11,8, 3,9 Hz, 1H), 3,57 (c, J = 7,0 Hz, 2H), 3,38 (tt, J = 10,6, 4,1 Hz, 1H), 2,31 - 2,20 (m, 4H), 1,98 - 1,88 (m, 2H), 1,53 - 1,43 (m, 2H), 1,23 (t, J = 7,0 Hz, 3H); LRMS (M+H) m/z 459,3.1H NMR (400 MHz, Chloroform-d) δ 12.19 (s, 1H), 10.57 (sa, 1H), 9.38 (d, J = 1.5 Hz, 1H), 8.64 (dd , J = 2.7, 1.6 Hz, 1H), 8.53 (s, 1H), 8.48 (d, J = 2.6 Hz, 1H), 8.31 (s, 2H) , 8.09 (dd, J = 7.7, 1.0 Hz, 1 H), 7.88 (dd, J = 7.8, 7.8 Hz, 1 H), 7.65 (dd, J = 7.9, 1.0 Hz, 1H), 4.22 (tt, J = 11.8, 3.9 Hz, 1H), 3.57 (c, J = 7.0 Hz, 2H), 3 .38 (tt, J = 10.6, 4.1 Hz, 1H), 2.31 - 2.20 (m, 4H), 1.98 - 1.88 (m, 2H), 1.53 - 1 .43 (m, 2H), 1.23 (t, J = 7.0 Hz, 3H); LRMS (M+H) m/z 459.3.
Ejemplo 116Example 116
N-(1 -(írans-4-etoxiciclohexil)-3-(pirazin-2-il)-1 H-pirazol-4-il)-5'-metil-[2,3'-bipiridin]-6-carboxamida (I-206) 1H RMN (400 MHz, Cloroformo-d) δ 12,43 (s, 1H), 9,46 (dd, J = 2,2, 0,7 Hz, 1H), 9,37 (d, J = 1,5 Hz, 1H), 8,72 (dd, J = 2,7, 1,6 Hz, 1H), 8,60 (dd, J = 2,1,0,9 Hz, 1H), 8,52 (s, 1H), 8,51 (d, J = 2,7 Hz, 1 H), 8,25 (dd, J = 7,6, 1,0 Hz, 1 H), 8,15 (ddd, J = 2,1,2,1,0,9 Hz, 1H), 8,01 (dd, J = 7,8, 7,8 Hz, 1H), 7,92 (dd, J = 7,9, 1,0 Hz, 1H), 4,23 (tt, J = 11,8, 3,9 Hz, 1H), 3,57 (c, J = 7,0 Hz, 2H), 3,38 (tt, J = 10,6, 4,1 Hz, 1H), 2,49 (a d, J = 0,7 Hz, 3H), 2,32 - 2,21 (m, 4H), 1,98 -1,88 (m, 2H), 1,53 - 1,43 (m, 2H), 1,23 (t, J = 7,0 Hz, 3H); LRMS (M+H) m/z 484,3. N- ( 1 -(írans-4-ethoxycyclohexyl)-3-(pyrazin-2-yl)-1 H-pyrazol-4-yl)-5'-methyl-[2,3'-bipyridine]-6-carboxamide (I-206) 1H NMR (400 MHz, Chloroform-d) δ 12.43 (s, 1H), 9.46 (dd, J = 2.2, 0.7 Hz, 1H), 9.37 (d , J = 1.5 Hz, 1H), 8.72 (dd, J = 2.7, 1.6 Hz, 1H), 8.60 (dd, J = 2.1,0.9 Hz, 1H) , 8.52 (s, 1H), 8.51 (d, J = 2.7 Hz, 1 H), 8.25 (dd, J = 7.6, 1.0 Hz, 1 H), 8, 15 (ddd, J = 2,1,2,1,0.9 Hz, 1H), 8.01 (dd, J = 7.8, 7.8 Hz, 1H), 7.92 (dd, J = 7.9, 1.0 Hz, 1H), 4.23 (tt, J = 11.8, 3.9 Hz, 1H), 3.57 (c, J = 7.0 Hz, 2H), 3, 38 (tt, J = 10.6, 4.1 Hz, 1H), 2.49 (bd, J = 0.7 Hz, 3H), 2.32-2.21 (m, 4H), 1.98 -1.88 (m, 2H), 1.53-1.43 (m, 2H), 1.23 (t, J = 7.0 Hz, 3H); LRMS (M+H) m/z 484.3.
Ejemplo 117Example 117
N-(1 -metil-3-(pirazin-2-il)-1 -W-pirazol-4-il)-6-(1 H-pirazol-4-il)picolinamida (I-207) N- ( 1 -methyl-3-(pyrazin-2-yl)-1 -W-pyrazol-4-yl)-6-(1 H-pyrazol-4-yl)picolinamide (I-207)
1H RMN (400 MHz, Cloroformo-a) 512,19 (s, 1H), 9,39 (d, J = 1,6 Hz, 1H), 8,67 (dd, J = 2,7, 1,6 Hz, 1H), 8,51 (d, J = 2,7 Hz, 1 H), 8,50 (s, 1 H), 8,31 (s, 2H), 8,11 (dd, J = 7,7, 1,0 Hz, 1H), 7,90 (t, J = 7,8 Hz, 1 H), 7,66 (dd, J = 7,8, 1,0 Hz, 1H), 4,02 (s, 3H); LRMS (M+H) m/z347,3.1H NMR (400 MHz, Chloroform-a) 512.19 (s, 1H), 9.39 (d, J = 1.6 Hz, 1H), 8.67 (dd, J = 2.7, 1.6 Hz, 1H), 8.51 (d, J = 2.7 Hz, 1H), 8.50 (s, 1H), 8.31 (s, 2H), 8.11 (dd, J = 7 .7, 1.0 Hz, 1H), 7.90 (t, J = 7.8 Hz, 1H), 7.66 (dd, J = 7.8, 1.0 Hz, 1H), 4, 02(s, 3H); LRMS (M+H) m/z 347.3.
Ejemplo 118Example 118
5'-metil-N-(1-metil-3-(pirazin-2-il)-1H-pirazol-4-il)-[2,3'-bipiridin]-6-carboxamida (I-208)5'-methyl-N-(1-methyl-3-(pyrazin-2-yl)-1H-pyrazol-4-yl)-[2,3'-bipyridine]-6-carboxamide (I-208)
1H RMN (400 MHz, Cloroformo-a) 512,43 (s, 1H), 9,46 (d, J = 2,0 Hz, 1H), 9,37 (d, J = 1,5 Hz, 1H), 8,74 (dd, J = 2,7, 1,6 Hz, 1H), 8,61 (dd, J = 2,1,0,9 Hz, 1H), 8,53 (d, J = 2,6 Hz, 1H), 8,49 (s, 1H), 8,26 (dd, J = 7,6, 1,0 Hz, 1H), 8,16 (ddd, J = 2,1,2,1, 0,9 Hz, 1H), 8,01 (dd, J = 7,8, 7,8 Hz, 1H), 7,93 (dd, J = 7,9, 1,0 Hz, 1H), 4,02 (s, 3H), 2,50 (c, J = 0,7 Hz, 3H); LRMS (M+H) m/z372,3.1H NMR (400 MHz, Chloroform-a) 512.43 (s, 1H), 9.46 (d, J = 2.0 Hz, 1H), 9.37 (d, J = 1.5 Hz, 1H) , 8.74 (dd, J = 2.7, 1.6 Hz, 1H), 8.61 (dd, J = 2,1,0.9 Hz, 1H), 8.53 (d, J = 2 .6 Hz, 1H), 8.49 (s, 1H), 8.26 (dd, J = 7.6, 1.0 Hz, 1H), 8.16 (ddd, J = 2.1,2, 1, 0.9 Hz, 1H), 8.01 (dd, J = 7.8, 7.8 Hz, 1H), 7.93 (dd, J = 7.9, 1.0 Hz, 1H), 4.02 (s, 3H), 2.50 (c, J =0.7 Hz, 3H); LRMS (M+H) m/z 372.3.
Ejemplo 119Example 119
Preparación de 8-etoxi-1,4-dioxaespiro[4.5]decanoPreparation of 8-ethoxy-1,4-dioxaspiro[4.5]decane
Hidruro de sodio (dispersión al 60% en aceite mineral, 74,3 g, 1,858 mol) en un matraz de fondo redondo se lavó cuatro veces con hexanos. Se preparó una suspensión en THF (1 L) y se enfrió a 0 °C. Gradualmente, se añadió 1,4-dioxaespiro[4,5]decan-8-ol (150 g, 929 mmoles) y la reacción se dejó calentar a temperatura ambiente. Después de dos horas, la reacción se enfrió de nuevo a 0 °C y se añadió yodoetano a través de un embudo de goteo a lo largo de una hora. La reacción se dejó calentar gradualmente a temperatura ambiente, después de lo cual se instaló un condensador de reflujo. La reacción se monitorizó mediante TLC (1: 1 acetato de etilo en hexanos). Una vez completada, la reacción se extinguió con hielo, el disolvente se redujo en volumen al vacío y luego se diluyó con acetato de etilo y agua. Después de extraer dos veces con acetato de etilo, las capas orgánicas reunidas se lavaron con salmuera, se secaron sobre sulfato de sodio sólido, se filtraron y se concentraron al vacío. Después de secar a alto vacío, se obtuvieron 166,9 g de 8-etoxi-1,4-dioxaespiro[4,5]decano como un aceite amarillo.Sodium hydride (60% dispersion in mineral oil, 74.3 g, 1.858 mol) in a round bottom flask was washed four times with hexanes. A suspension was made in THF (1 L) and cooled to 0 °C. Gradually, 1,4-dioxaspiro[4,5]decan-8-ol (150 g, 929 mmol) was added and the reaction was allowed to warm to room temperature. After two hours, the reaction was cooled back to 0 °C and iodoethane was added via a dropping funnel over one hour. The reaction was allowed to gradually warm to room temperature, after which a reflux condenser was installed. The reaction was monitored by TLC (1:1 ethyl acetate in hexanes). Upon completion, the reaction was quenched with ice, the solvent reduced by volume in vacuo, and then diluted with ethyl acetate and water. After extracting twice with ethyl acetate, the combined organic layers were washed with brine, dried over solid sodium sulfate, filtered, and concentrated in vacuo. After drying under high vacuum, 166.9 g of 8-ethoxy-1,4-dioxaspiro[4,5]decane were obtained as a yellow oil.
1H RMN (300 MHz, Cloroformo-d) 53,90 (t, J = 2,2 Hz, 4H), 3,45 (c, J = 7,0 Hz, 2H), 3,40 - 3,32 (m, 1 H), 1,82 - 1,62 (m, 6H), 1,56 - 1,46 (m, 2H), 1,16 (t, J = 7,0 Hz, 3H).1H NMR (300 MHz, Chloroform-d) 53.90 (t, J = 2.2 Hz, 4H), 3.45 (c, J = 7.0 Hz, 2H), 3.40 - 3.32 ( m, 1H), 1.82-1.62 (m, 6H), 1.56-1.46 (m, 2H), 1.16 (t, J=7.0 Hz, 3H).
Ejemplo 120 Example 120
Preparación de 4-etoxiciclohexan-1-onaPreparation of 4-ethoxycyclohexan-1-one
A una disolución de 8-etoxi-1,4-dioxaespiro[4,5]decano (166,9 g, 896,1 mmoles) en THF (700 ml) se añadió una disolución acuosa 3 M de HCl (597 ml). La solución se calentó a 90 °C con un condensador de reflujo adjunto durante dos horas. La reacción se monitorizó mediante TLC (1: 1 acetato de etilo en hexanos) tomando partes alícuotas inactivadas con NaOH acuoso, y extrayéndolas en acetato de etilo. Al finalizar, el volumen de disolvente se redujo al vacío y se ajustó a pH 8 con una solución de NaOH 4M. Esto se extrajo dos veces con acetato de etilo, las fases orgánicas reunidas se lavaron con salmuera, se secaron sobre sulfato de sodio sólido, se filtraron y se concentraron al vacío. Después de secar a alto vacío, se obtuvieron 124,2 g de 4-etoxiciclohexan-1-ona deseada en forma de un aceite amarillo.To a solution of 8-ethoxy-1,4-dioxaspiro[4,5]decane (166.9 g, 896.1 mmol) in THF (700 mL) was added a 3M aqueous HCl solution (597 mL). The solution was heated at 90 °C with an attached reflux condenser for two hours. The reaction was monitored by TLC (1:1 ethyl acetate in hexanes) by taking aliquots quenched with aqueous NaOH, and extracting into ethyl acetate. On completion, the volume of solvent was reduced in vacuo and adjusted to pH 8 with 4M NaOH solution. This was extracted twice with ethyl acetate, the combined organic phases washed with brine, dried over solid sodium sulphate, filtered and concentrated in vacuo. After drying under high vacuum, 124.2 g of the desired 4-ethoxycyclohexan-1-one were obtained as a yellow oil.
1H RMN (300 MHz, Cloroformo-d) 53,74 - 3,61 (m, 1H), 3,53 (c, J = 7,0 Hz, 2H), 2,63 - 2,46 (m, 2H), 2,30 - 2,15 (m, 2H), 2,12 - 1,82 (m, 4H), 1,21 (t, J = 7,0 Hz, 3H). 1 H NMR (300 MHz, Chloroform-d) 53.74 - 3.61 (m, 1H), 3.53 (c, J = 7.0 Hz, 2H), 2.63 - 2.46 (m, 2H), 2.30-2.15 (m, 2H), 2.12-1.82 (m, 4H), 1.21 (t, J =7.0 Hz, 3H).
Ejemplo 121Example 121
Preparación de (1s,4s)-4-etoxiciclohexan-1-olPreparation of (1s,4s)-4-ethoxycyclohexan-1-ol
Una disolución de 4-etoxiciclohexan-1 -ona (121,6 g, 855 mmoles) en 750 ml de THF se enfrió hasta -78°C. Se añadió LiAlH4 sólido (95%, 51,2 g, 1,28 mol) en porciones, durante una hora, y después se calentó gradualmente hasta -10°C. Después de una hora, se tomó una alícuota, y se inactivó para el análisis por TLC en 1: 1 acetato de etilo en hexanos, que mostró que la reacción se había completado. La reacción se enfrió nuevamente a -78 °C y se extinguió con la adición gota a gota de una solución de NaOH 4 M (641 ml). La mezcla se diluyó y se lavó tres veces con acetato de etilo, separando por decantación el disolvente. Las fases orgánicas reunidas se lavaron con NaOH 1 M y salmuera, se secaron sobre sulfato de sodio sólido, se filtraron y se concentraron al vacío. Después de secar a alto vacío, se obtuvieron 117,9 g de 4-etoxiciclohexan-1 -ol (en una relación ~2:1 de isómeros 1 s,4s y 2s,4s) como un aceite amarillo.A solution of 4-ethoxycyclohexan-1 -one (121.6 g, 855 mmol) in 750 mL THF was cooled to -78°C. Solid LiAlH4 (95%, 51.2 g, 1.28 mol) was added portionwise over one hour, then gradually warmed to -10°C. After one hour, an aliquot was taken, and quenched for analysis by TLC in 1:1 ethyl acetate in hexanes, which showed the reaction to be complete. The reaction was cooled back to -78 °C and quenched with the dropwise addition of 4 M NaOH solution (641 mL). The mixture was diluted and washed three times with ethyl acetate, decanting off the solvent. The combined organic phases were washed with 1M NaOH, brine, dried over solid sodium sulfate, filtered, and concentrated in vacuo. After drying under high vacuum, 117.9 g of 4-ethoxycyclohexan-1-ol (in ~2:1 ratio of 1s,4s and 2s,4s isomers) was obtained as a yellow oil.
1H RMN (300 MHz, Cloroformo-d) 53,76 - 3,61 (m, 1 H), 3,52 - 3,38 (m, 2H), 3,34 (dp, J = 6,3, 3,2 Hz, 1H), 3,27 - 3,18 (m, ~0,5H), 2,01 - 1,92 (m, 1 H), 1,84 - 1,47 (m, 6H), 1,33 - 1,25 (m, 1H), 1,17 (td, J = 7,0, 2,5 Hz, 3H).1H NMR (300 MHz, Chloroform-d) 53.76 - 3.61 (m, 1H), 3.52 - 3.38 (m, 2H), 3.34 (dp, J = 6.3, 3 .2 Hz, 1H), 3.27 - 3.18 (m, ~0.5H), 2.01 - 1.92 (m, 1H), 1.84 - 1.47 (m, 6H), 1.33-1.25 (m, 1H), 1.17 (td, J=7.0, 2.5Hz, 3H).
Ejemplo 122Example 122
Preparación de (1r,4r)-4-nitrobencenosulfonato de 4-etoxiciclohexiloPreparation of 4-ethoxycyclohexyl (1r,4r)-4-nitrobenzenesulfonate
A una disolución de 4-etoxiciclohexan-1 -ol (en una relación ~2:1 de isómeros 1 s,4s y 2s,4s; 112,9 g, 782,9 mmoles) en CH2G 2 (800 ml), enfriada hasta 0°C, se añadió 1,4-diazabiciclo[2.2.2]octano (105,4 g, 939,5 mmoles). Se añadió en porciones cloruro de 4-nitrobencenosulfonilo a 0°C durante 1 hora y luego la reacción se calentó gradualmente a temperatura ambiente durante la noche. Cuando la reacción pareció completa por análisis TLC en acetato de etilo en hexanos 1:1, se extinguió con la adición de solución acuosa saturada de NaHCO3. Después de dilución adicional con CH2G 2 y agua, la fase orgánica se aisló y se lavó adicionalmente con solución de NaHCO3, agua y salmuera. Después de secar sobre sulfato de sodio sólido, la solución se filtró, se concentró al vacío y se secó a alto vacío. El sólido resultante se trituró en acetato de etilo y hexanos para producir 160,8 g de 4-nitrobencenosulfonato de 4-etoxiciclohexilo (como una mezcla de 1r,4r y 1s,4s) como un sólido blanquecino. To a solution of 4-ethoxycyclohexan-1 -ol (~2:1 ratio of 1s,4s and 2s,4s isomers; 112.9 g, 782.9 mmol) in CH2G 2 (800 mL), cooled to 0°C, 1,4-diazabicyclo[2.2.2]octane (105.4 g, 939.5 mmol) was added. 4-Nitrobenzenesulfonyl chloride was added portionwise at 0°C over 1 hour and then the reaction gradually warmed to room temperature overnight. When the reaction appeared complete by TLC analysis in 1:1 ethyl acetate in hexanes, it was quenched with the addition of saturated aqueous NaHCO 3 solution. After further dilution with CH 2 G 2 and water, the organic phase was isolated and further washed with NaHCO 3 solution, water and brine. After drying over solid sodium sulfate, the solution was filtered, concentrated in vacuo and dried under high vacuum. The resulting solid was triturated in ethyl acetate and hexanes to yield 160.8 g of 4-ethoxycyclohexyl 4-nitrobenzenesulfonate (as a mixture of 1r,4r and 1s,4s) as an off-white solid.
1H RMN (300 MHz, Cloroformo-d) 58,51 - 8,31 (m, 2H), 8,17 - 8,03 (m, 2H), 4,73 (dtt, J = 11,7, 8,1,4,0 Hz, 1H), 3,44 (qd, J = 7,0, 1,5 Hz, 2H), 3,34 (tt, J = 6,7, 3,1 Hz, 1H), 1,99 - 1,87 (m, 2H), 1,81 - 1,53 (m, 6H), 1,16 (td, J = 7,0, 2,8 Hz, 3H). 1 H NMR (300 MHz, Chloroform-d) 58.51 - 8.31 (m, 2H), 8.17 - 8.03 (m, 2H), 4.73 (dtt, J = 11.7, 8 ,1,4.0 Hz, 1H), 3.44 (qd, J = 7.0, 1.5 Hz, 2H), 3.34 (tt, J = 6.7, 3.1 Hz, 1H) , 1.99-1.87 (m, 2H), 1.81-1.53 (m, 6H), 1.16 (td, J =7.0, 2.8 Hz, 3H).
Ejemplo 123Example 123
Ensayo de IL23p19 inducido por LPS en células THP-1 (con IFNy cebado)LPS-induced IL23p19 assay in THP-1 cells (with IFNy primed)
Materiales y EquipoMaterials and Equipment
Células THP-1 (ATCC, n.° de cat. TIB-202), dimetilsulfóxido (DMSO) (Sigma-Aldrich, n.° de cat. D2650), RPMI 1640 (Cellgro, n.° de cat. 10-040-CM), suero bovino fetal (Sigma, n.° de cat. F4135), seroalbúmina bovina (BSA) (Sigma-Aldrich, n.° de cat. A7906), LPS (serotipo K-235, Sigma, número de producto L 2143), IFNy (Peprotech, n.° de cat.THP-1 cells (ATCC, cat. no. TIB-202), dimethyl sulfoxide (DMSO) (Sigma-Aldrich, cat. no. D2650), RPMI 1640 (Cellgro, cat. no. 10-040 -CM), fetal bovine serum (Sigma, cat. no. F4135), bovine serum albumin (BSA) (Sigma-Aldrich, cat. no. A7906), LPS (serotype K-235, Sigma, product no. L 2143), IFNy (Peprotech, cat. no.
300-02)300-02)
Anticuerpo de captura: IL-23p19 humano ELISA (e-Bioscience, Cat. n° 14-7238-85), Anticuerpo de detección: IL-12 (p40/p70) anti-humano de Ratón Primario Biotinilado (e-Bioscience, n.° de Cat. 13-7129-85), estreptavidina secundaria conjugada con HRP (R&D Systems, n.° de cat. DY998), amortiguador de lavado PBST 1x (tableta PBS-Tween) (VWR International, n.° de cat. 80058-558), amortiguador de bloqueo ELISA (PBS con 1% de BSA), amortiguador de dilución de ELISA (PBS con 1% de BSA), placas MaxiSorp Black Immuno de fondo plano de 384 pocillos (Thermo Scientific, n.° de cat. 12-565-346), placas de cultivo tisular blancas de fondo plano de 384 pocillos (Thermo Scientific, n.° de cat.Capture antibody: human IL-23p19 ELISA (e-Bioscience, Cat. no. 14-7238-85), Detection antibody: Biotinylated Primary Mouse anti-human IL-12 (p40/p70) (e-Bioscience, no. 13-7129-85), HRP-conjugated secondary streptavidin (R&D Systems, cat. no. DY998), 1x PBST wash buffer (PBS-Tween tablet) (VWR International, cat. no. 80058-558), ELISA blocking buffer (PBS with 1% BSA), ELISA dilution buffer (PBS with 1% BSA), MaxiSorp Black Immuno 384-well flat-bottom plates (Thermo Scientific, # 12-565-346), white 384-well flat-bottom tissue culture plates (Thermo Scientific, cat. no.
12-565-343), sustrato quimioluminiscente Super Signal ELISA Pico (Thermo Scientific, n.° de cat. 37070), reactivo Cell Titer Glo (Promega, n.° de cat. G7573), control positivo, inhibidor de IKK2VI (Calbiochem, n.° de cat. 401483), lavador de placas AquaMax 4000 (Molecular Devices), luminómetro, contador Wallac Victor2 1420 Multilabel.12-565-343), Super Signal ELISA Pico chemiluminescent substrate (Thermo Scientific, cat. no. 37070), Cell Titer Glo reagent (Promega, cat. no. G7573), positive control, IKK 2 VI inhibitor (Calbiochem, cat. no. 401483), AquaMax 4000 plate washer (Molecular Devices), luminometer, Wallac Victor2 1420 Multilabel counter.
MétodoMethod
Estim ulación de Células THP-1:Stim ulation of THP-1 Cells:
El día 1, se sembraron 50K/pocillo de células THP-1 y se cebaron con IFNy (50 ng/ml) en placas de 384 pocillos durante aproximadamente 18 horas en medio RPMI con FBS al 10%. El día 2, el compuesto se diluyó en serie en DMSO de 5 mM en diluciones de 3 veces, y después se diluyó 1: 125 en medio RPMI con 10% de FBS. Se añadieron 50 μl/pocillo de compuesto 2x a 50 μl/pocillo de células THP-1 (cebadas con IFNy) por duplicado en placas de cultivo tisular de 384 pocillos. Las células se preincubaron con el compuesto durante 1 hora a 37°C, COz al 5% antes de la adición de 10 μl/pocillo de 1 1x LPS para dar una concentración final de 1 μg/ml de LPS. El día 3, después de la estimulación durante 18 horas a 37 °C, 5% de CO2 , se centrifugó la placa de ensayo y se recogieron 70 μl/pocillo de sobrenadante. Se midió la proteína IL-23p19 en 70 μl/pocillo de sobrenadante mediante ELISA tipo sándwich, y se añadieron 25 μl/pocillo de reactivo Cell Titer Glo a las células restantes para medir la toxicidad del compuesto. On day 1, 50K/well THP-1 cells were seeded and primed with IFNγ (50 ng/ml) in 384-well plates for approximately 18 hours in RPMI medium with 10% FBS. On day 2, the compound was serially diluted in 5 mM DMSO in 3-fold dilutions, and then diluted 1:125 in RPMI medium with 10% FBS. 50 µl/well of 2x compound was added to 50 µl/well of THP-1 cells (primed with IFNγ) in duplicate in 384-well tissue culture plates. Cells were preincubated with compound for 1 hour at 37°C, 5% COz before the addition of 10 µl/well 1x LPS to give a final concentration of 1 µg/ml LPS. On day 3, after stimulation for 18 hours at 37°C, 5% CO 2 , the assay plate was centrifuged and 70 µl/well supernatant was collected. IL-23p19 protein in 70 µl/well of supernatant was measured by sandwich ELISA, and 25 µl/well of Cell Titer Glo reagent was added to the remaining cells to measure compound toxicity.
E L IS A tipo Sándw ich para IL-23p19 humana:E L IS A sandwich type for human IL-23p19:
Placas Maxisorp immuno ELISA se recubrieron previamente con 25 μl/pocillo de anticuerpo de captura anti-IL-23p19 (2,5 μg/ml) en PBS durante la noche a temperatura ambiente. Después de lavar con PBST 1x, las placas se bloquearon usando 100 μl/pocillo de BSA al 1 % en PBS durante 2 horas a temperatura ambiente. Las placas se lavaron tres veces con PBST 1 x, y se añadieron 70 μl/pocillo de sobrenadante. Las placas se incubaron a temperatura ambiente durante 2 horas con agitación, y se lavaron tres veces con PBST 1x. Se añadieron 25 μl/pocillo de anticuerpo de detección anti-IL-12 (p40/p70) marcado con biotina (100 ng/ml) en PBS con BSA al 1% y las placas se incubaron a temperatura ambiente durante 2 horas con agitación. Después de lavar tres veces con PBST 1x, se añadieron 25 μl/pocillo de estreptavidina-HRP (1:200) en PBS con BSA al 1%, y las placas se incubaron a temperatura ambiente durante 20 minutos con agitación. Las placas se lavaron tres veces con PBST 1x, y se añadieron 25 μl/pocillo de sustrato quimioluminiscente Super Signal ELISA Pico. Las placas se leyeron con un luminómetro y los valores de quimioluminiscencia se introdujeron en Athena (Rigel) para el ajuste de la curva, el cálculo de CE50 y el almacenamiento en la base de datos. Los resultados se muestran en la Tabla 1.Maxisorp immuno ELISA plates were precoated with 25 µl/well of anti-IL-23p19 capture antibody (2.5 µg/ml) in PBS overnight at room temperature. After washing with 1x PBST, the plates were blocked using 100 µl/well 1% BSA in PBS for 2 hours at room temperature. The plates were washed three times with 1 x PBST, and 70 µl/well of supernatant was added. The plates were incubated at room temperature for 2 hours with shaking, and washed three times with 1x PBST. 25 µl/well of biotinylated anti-IL-12 (p40/p70) detection antibody (100 ng/ml) in PBS with 1% BSA was added and the plates were incubated at room temperature for 2 hours with shaking. After washing three times with 1x PBST, 25 µl/well streptavidin-HRP (1:200) in PBS with 1% BSA was added, and the plates were incubated at room temperature for 20 min with shaking. Plates were washed three times with 1x PBST, and 25 µl/well Super Signal ELISA Pico chemiluminescent substrate was added. Plates were read with a luminometer and chemiluminescence values were entered into Athena (Rigel) for curve fitting, EC50 calculation and storage in the database. The results are shown in Table 1.
Ejemplo 124Example 124
Detección de compuestos mediante células DCDetection of compounds by DC cells
MaterialesMaterials
Células PBMC humanas (Todas las células, Cat n° PB002)Human PBMC cells (All cells, Cat # PB002)
Medio de crecimiento RPMI que contienen FBS al 10%RPMI growth medium containing 10% FBS
IFNy (Peprotech, Cat n° 300-02) IFNy (Peprotech, Cat no. 300-02)
GMCSF (Peprotech, Cat n° 300-03) e IL4 (Peprotech Cat n° 200-04)GMCSF (Peprotech, Cat #300-03) and IL4 (Peprotech Cat #200-04)
Placas blancas de fondo transparente de 96 pocillos (Fisher, Cat. n° 07-200-587, Corning n° 3903) LPS (hacer 2,5 mg/ml de material en PBS) de Sigma Aldrich (Cat n° L2018-5MG)96-well white clear bottom plates (Fisher, Cat. No. 07-200-587, Corning #3903) LPS (make 2.5 mg/ml material in PBS) from Sigma Aldrich (Cat. No. L2018-5MG )
Reactivo Cell Titer Glo (Promega, Cat n° G7573)Cell Titer Glo Reagent (Promega, Cat No. G7573)
Controles positivos, inhibidor de IKK2VI (Calbiochem, Cat n° 401483)Positive controls, IKK 2 VI inhibitor (Calbiochem, Cat no. 401483)
ProtocoloProtocol
I. Diferenciación de PBMCs a células DC:I. Differentiation of PBMCs to DC cells:
Células PBMC humanas (400 millones) obtenidas del proveedor se transfirieron a un matraz T-175 que contenía 15 ml de medio RPMI (FBS al 10%) y se incubaron durante 2 horas a 37 °C. Después de 2 horas, se aspiró cuidadosamente el medio que incluía células flotantes y se añadieron 12 ml de medio RPMI fresco (FBS al 10%) que contenía GMCSF (100 ng/ml) e IL4 (20 ng/ml), y el matraz se mantuvo en una incubadora a 37 °C durante 7 días. Después de 3 días, se añadieron al matraz GMCSF (100 ng/ml) e IL4 (20 ng/ml) frescos y se continuó con la incubación. Después de 7 días, las células completamente diferenciadas se recogieron por centrifugación (1200 rpm / 5 min) y aspirando el medio. Las células se suspendieron en medio RPMI fresco (FBS al 10%) que contenía 50 ng/ml de IFNy (1000 U/ml) y luego se sembraron en placas (50 K/pocillo en 100 μl) en una placa blanca de fondo transparente de 96 pocillos y se dejaron en una incubadora a 37 °C durante 24 horas.Human PBMC cells (400 million) obtained from the supplier were transferred to a T-175 flask containing 15 mL RPMI medium (10% FBS) and incubated for 2 hours at 37°C. After 2 hours, the medium including floating cells was carefully aspirated and 12 ml of fresh RPMI medium (10% FBS) containing GMCSF (100 ng/ml) and IL4 (20 ng/ml) was added, and the flask it was kept in an incubator at 37 °C for 7 days. After 3 days, fresh GMCSF (100 ng/ml) and IL4 (20 ng/ml) were added to the flask and incubation continued. After 7 days, fully differentiated cells were collected by centrifugation (1200 rpm/5 min) and aspirating the medium. Cells were suspended in fresh RPMI medium (10% FBS) containing 50 ng/ml IFNγ (1000 U/ml) and then plated (50 K/well in 100 µL) on a clear-bottomed white plate. of 96 wells and left in an incubator at 37 °C for 24 hours.
II. Adición de compuestos:II. Addition of compounds:
Después de 24 horas de incubación, se añadieron 100 μl de medio RPMI que contenía compuesto de ensayo concentrado 2X por pocillo al medio de cultivo celular anterior (la concentración final se convierte en 1X), y las placas se preincubaron durante 1 hora a 37°C antes de estimular con LPS.After 24 hours of incubation, 100 µL of RPMI medium containing 2X concentrated test compound per well was added to the above cell culture medium (final concentration becomes 1X), and plates were pre-incubated for 1 hour at 37°C. C before stimulating with LPS.
Después de 1 hora de preincubación del compuesto, se añadieron 10 μl por pocillo de disolución de LPS concentrada 20X en medio RPMI, para dar una concentración final de 1 μg/ml. La mezcla se agitó y las placas se incubaron a 37 °C durante 18 horas adicionalesAfter 1 hour of compound preincubation, 10 µl per well of 20X concentrated LPS solution in RPMI medium was added, to give a final concentration of 1 µg/ml. The mixture was vortexed and the plates were incubated at 37°C for an additional 18 hours.
Se recogieron cuidadosamente 155 μl del sobrenadante de cada uno de los pocillos (sin que la punta tocara el fondo del pocillo) y a los restantes 50 μl / pocillo de la placa de cultivo celular se añadieron 50 μl de reactivo Cell Titer Glo. La mezcla se incubó durante 1-2 minutos en un agitador y se leyó la intensidad de luminiscencia de la placa para determinar la citotoxicidad del compuesto. El sobrenadante del cultivo celular recogido anteriormente se usó para llevar a cabo ELISA de IL23 (65 μl - Sobrenadante) y ELISA de IL 10 (90 μl - Sobrenadante) como se describe a continuación.155 µL of the supernatant from each well was carefully collected (without the tip touching the bottom of the well) and 50 µL of Cell Titer Glo reagent was added to the remaining 50 µL/well of the cell culture plate. The mixture was incubated for 1-2 minutes on a shaker and the luminescence intensity of the plate was read to determine the cytotoxicity of the compound. The cell culture supernatant collected above was used to carry out IL23 ELISA (65 µl - Supernatant) and IL10 ELISA (90 µl - Supernatant) as described below.
Ejemplo 125Example 125
IL-23 humana (p19/p40) Protocolo ELISA (e-Biosciences)Human IL-23 (p19/p40) ELISA Protocol (e-Biosciences)
Materiales:Materials:
Placas blancas opacas de alta unión de 96 pocillos (de Pierce, Cat n° 15042);96-well high-binding opaque white plates (from Pierce, Cat #15042);
PBS 1X; amortiguador de lavado TBST 1X;1X PBS; TBST 1X washout buffer;
Solución de Bloqueo: Caseína al 0,5% en PBS (de BDH, Cat n° 440203H);Blocking Solution: 0.5% Casein in PBS (from BDH, Cat #440203H);
Solución de Dilución: BSA al 1% en PBS (BSA al 10% de Fisher, Cat n° 37525);Dilution Solution: 1% BSA in PBS (10% BSA from Fisher, Cat #37525);
Anticuerpo de captura: IL-23 anti-humano de rata (p19) (e-Biosciences, Cat. n° 14-7238-85);Capture antibody: rat anti-human IL-23 (p19) (e-Biosciences, Cat. no. 14-7238-85);
Anticuerpo de Detección: IL-12 (p40/p70) anti-humano de Ratón Primario Biotinilado (e-bioscience, Cat n° 13-7129-85);Detection Antibody: Biotinylated Primary Mouse anti-human IL-12 (p40/p70) (e-bioscience, Cat n° 13-7129-85);
Estreptavidina conjugada con HRP secundaria (R&D Systems, Cat n° DY998);Secondary HRP-conjugated streptavidin (R&D Systems, Cat #DY998);
IL-23 rHumana (e-biosciences, Cat n° 34-8239) (concentración inicial sugerida = 5 ng/ml en medio de cultivo celular RPMI); rHuman IL-23 (e-biosciences, Cat #34-8239) (suggested initial concentration = 5 ng/ml in RPMI cell culture medium);
Sobrenadante de Cultivo Celular (65 gl de células THP-1 cebadas con IFNy (50 ng/ml - 1000 U/ml) y estimuladas con SAC al 0,01%);Cell Culture Supernatant (65 ml of THP-1 cells primed with IFN and (50 ng/ml - 1000 U/ml) and stimulated with 0.01% SAC);
Sustrato Quimioluminiscente SuperSignal ELISA Pico [Pierce, Cat n° 37069].SuperSignal ELISA Pico Chemiluminescent Substrate [Pierce, Cat #37069].
Placas de Revestimiento:Coating Plates:
A 10,5 ml de PBS se añadieron 50 gl de anticuerpo de captura anti-IL23 (p19) (2,5 gg/ml). La mezcla se combinó bien y se añadieron 100 gl de la solución de revestimiento a cada uno de los pocillos de las placas blancas de 96 pocillos de Pierce. Los pocillos se cubrieron y se incubaron durante la noche a 4 °C.To 10.5 ml of PBS was added 50 ml of anti-IL23 (p19) capture antibody (2.5 gg/ml). The mixture was mixed well and 100 ml of the coating solution was added to each well of Pierce 96-well white plates. Wells were covered and incubated overnight at 4°C.
Bloqueo de las placas:Blocking of the plates:
Las placas recubiertas con anticuerpo anti-IL23 (p19) se lavaron 2 veces usando TBST (úsese el lavador de placas), y se bloquearon usando 200 gl de caseína al 0,5% durante 1,5-2 horas a temperatura ambiente con agitación. Adición de Sobrenadante y Detección:Anti-IL23 (p19) antibody-coated plates were washed 2 times using TBST (use plate washer), and blocked using 200 ml 0.5% casein for 1.5-2 hours at room temperature with shaking. . Addition of Supernatant and Detection:
Las placas se lavaron 2 veces usando TBST, y el sobrenadante se transfirió (65 gl/pocillo) a la placa de 96 pocillos prebloqueada/revestida con anticuerpo anti-IL23(p19) anterior, y se incubó a temperatura ambiente durante 1,5 horas con agitación.Plates were washed 2 times using TBST, and the supernatant was transferred (65 ml/well) to the preblocked/anti-IL23(p19) antibody-coated 96-well plate above, and incubated at room temperature for 1.5 hours. with agitation.
Las placas se lavaron 4 veces usando TBST (lavador de placas), y se añadieron 100 gl/pocillo de disolución de anticuerpo de detección preparada a partir de 2 gl de anticuerpo anti-IL-12 (p40/p70) marcado con biotina en 11 ml de disolución de BSA al 1%/PBS (dilución 1-5000). Las placas se incubaron durante 1 hora con agitación a temperatura ambiente.The plates were washed 4 times using TBST (plate washer), and 100 ml/well of detection antibody solution prepared from 2 ml of biotinylated anti-IL-12 (p40/p70) antibody was added in 11 mL of 1% BSA/PBS solution (1-5000 dilution). The plates were incubated for 1 hour with shaking at room temperature.
De nuevo, las placas se lavaron 4 veces con TBST, y se añadieron 100 gl de disolución de estreptavidina marcada con HRP (R&D Systems) (10 gl/10 ml de disolución de BSA al 1%), y las placas se incubaron a temperatura ambiente durante otros 45 minutos con agitación.Again, the plates were washed 4 times with TBST, and 100 ml of HRP-labeled streptavidin solution (R&D Systems) (10 ml/10 ml of 1% BSA solution) was added, and the plates were incubated at room temperature. ambient for another 45 min with shaking.
Después de 45 minutos, las placas se lavaron con TBST 4 veces, y se añadieron 100 gl/pocillo de sustrato quimioluminiscente Super Signal ELISA Pico de Pierce (3,5 ml A 3,5 ml B 3,5 ml de agua MQ). Las placas se agitaron durante 1-2 minutos y luego se leyeron en un lector de placas.After 45 min, the plates were washed with TBST 4 times, and 100 ml/well of Pierce's Super Signal ELISA Pico chemiluminescent substrate (3.5 ml A 3.5 ml B 3.5 ml MQ water) was added. The plates were shaken for 1-2 minutes and then read on a plate reader.
Los resultados de CE50 de los ensayos descritos en los Ejemplos 123 y 125 se muestran en la Tabla 1. Los compuestos 1-72 a 1-77 son compuestos de referencia únicamente, y no forman parte de la invención. The EC 50 results of the tests described in Examples 123 and 125 are shown in Table 1. Compounds 1-72 to 1-77 are reference compounds only, and do not form part of the invention.
V. Realizaciones ejemplares de compuestos de la invención, y sus usosV. Exemplary Embodiments of Compounds of the Invention, and Their Uses
Los siguientes párrafos numerados ilustran realizaciones ejemplares de los compuestos de la invención, y los fines para los que se pueden usar tales compuestos.The following numbered paragraphs illustrate exemplary embodiments of the compounds of the invention, and the purposes for which such compounds may be used.
Párrafo 1. Un compuesto, que tiene una fórmula 1Paragraph 1. A compound, having a formula 1
o una sal del mismo, en la que:or a salt thereof, wherein:
el anillo A es heteroarilo;ring A is heteroaryl;
R1 es H, o alquilo de C1-6 ;R1 is H, or C 1-6 alkyl;
R2 es H, alifático, heteroalifático, o heterociclilo;R2 is H, aliphatic, heteroaliphatic, or heterocyclyl;
cada Z1, Z2, Z3, y Z4, es independientemente N o CR3, en la que al menos uno de Z1, Z2, Z3, y Z4 es N; cada R3 es independientemente H, alifático, o heteroalifático; yeach Z1, Z2, Z3, and Z4, is independently N or CR3, wherein at least one of Z1, Z2, Z3, and Z4 is N; each R3 is independently H, aliphatic, or heteroaliphatic; and
R4 es halógeno, heteroarilo, heterocicloalifático, arilo, -NH-heteroarilo, u -O-heteroarilo.R4 is halogen, heteroaryl, heterocycloaliphatic, aryl, -NH-heteroaryl, or -O-heteroaryl.
Parágrafo 2. [Omitido]Paragraph 2. [Omitted]
Parágrafo 3. El compuesto del párrafo 1, en el que el anillo A es piridinilo o pirazinilo.Paragraph 3. The compound of paragraph 1, wherein ring A is pyridinyl or pyrazinyl.
Párrafo 4. El compuesto de uno cualquiera de los párrafos 1-3, en el que el anillo A es piridin-2-ilo.Paragraph 4. The compound of any one of paragraphs 1-3, wherein ring A is pyridin-2-yl.
Párrafo 5. El compuesto de uno cualquiera de los párrafos 1-4, en el que el anillo A esParagraph 5. The compound of any one of paragraphs 1-4, in which ring A is
cada Q es independientemente CH o N, en el que al menos un Q es N;each Q is independently CH or N, where at least one Q is N;
Rm es Rb;Rm is Rb;
x es 0, 1, o 2; x is 0, 1, or 2;
Ra es independientemente, para cada aparición, H, D, alquilo de Ci-6, cicloalquilo de C3-6, aromático de C5-10, o heterocicloalifático de C3-6;Ra is independently, for each occurrence, H, D, Ci-6 alkyl, C 3-6 cycloalkyl, C 5-10 aromatic, or C 3-6 heterocycloaliphatic;
Rb es independientemente, para cada aparición, -OH, -CF3 , -CN, -ORc, -SO2Rc, -NRdRd, -N(H)SO2 Rc, -C(O)OH, -N(H)C(O)Rc, -C(O)ORc, -C(O)NRdRd, =O, o halógeno;Rb is independently, for each occurrence, -OH, -CF 3 , -CN, -ORc, -SO 2 Rc, -NRdRd, -N(H)SO 2 Rc, -C(O)OH, -N(H) C(O)Rc, -C(O)ORc, -C(O)NRdRd, =O, or halogen;
Rc es independientemente, para cada aparición, alquilo de C1-6, cicloalquilo de C3-6, heteroaliciclilo de C3-6, aralquilo, alquilo de C1-6 sustituido con 1,2 o 3 Re, aromático de Cs-i, aromático de C5-10 sustituido con 1,2 o 3 Re;Rc is independently, for each occurrence, C 1-6 alkyl, C 3-6 cycloalkyl, C 3-6 heteroalicyclyl, aralkyl, C 1-6 alkyl substituted with 1,2 or 3 Re, Cs-aromatic i, C 5-10 aromatic substituted with 1,2 or 3 Re;
Rd es independientemente, para cada aparición, H, alquilo de C1-6 opcionalmente sustituido con 1 ,2 o 3 Re, cicloalquilo de C3-6 opcionalmente sustituido con 1,2 o 3 Re, heteroaliciclilo de C3-6 opcionalmente sustituido con 1,2 o 3 Re, aromático de C5-10 opcionalmente sustituido con 1,2 o 3 Ra o Rb, o dos grupos Rd, junto con el nitrógeno unido a ella, forman un resto heteroaliciclilo de C3-6 opcionalmente sustituido con alquilo de C1-6 y opcionalmente interrumpido con uno o dos -O- o -N(R8), en el que R9 es R70; yRd is independently, for each occurrence, H, C 1-6 alkyl optionally substituted with 1, 2 or 3 Re, C 3-6 cycloalkyl optionally substituted with 1, 2 or 3 Re, C 3-6 heteroalicyclyl optionally substituted with 1,2 or 3 Re, C 5-10 aromatic optionally substituted with 1,2 or 3 Ra or Rb, or two Rd groups, together with the nitrogen attached to it, form an optionally substituted C 3-6 heteroalicyclyl moiety with C 1-6 alkyl and optionally interrupted with one or two -O- or -N(R 8 ), where R 9 is R 7 0; and
Re es independientemente, para cada aparición, halógeno, alquilo de C1-6, cicloalquilo de C3-6, u -ORa. Párrafo 6. El compuesto de uno cualquiera de los párrafos 1 -5, en el que el anillo A esRe is independently, for each occurrence, halogen, C 1-6 alkyl, C 3-6 cycloalkyl, or -ORa. Paragraph 6. The compound of any one of paragraphs 1 -5, in which ring A is
Párrafo 7. [Omitido].Paragraph 7. [Omitted].
Párrafo 8. El compuesto de uno cualquiera de los párrafos 1-6, en el que R2 es H, heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi o heterocicloalifático.Paragraph 8. The compound of any one of paragraphs 1-6, wherein R2 is H, 3- to 10-membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy substituted cyclohexyl and/or hydroxy, unsubstituted C 1-6 alkyl, or C 1-6 alkyl substituted with -OH, amino, alkoxy or heterocycloaliphatic.
Párrafo 9. El compuesto de uno cualquiera de los párrafos 5-8, en el que R2 es Ra, Ra sustituido con Rb, o Ra sustituido con 1 o 2 Rc, o Ra sustituido con Rd.Paragraph 9. The compound of any one of paragraphs 5-8, wherein R2 is Ra, Ra substituted with Rb, or Ra substituted with 1 or 2 Rc, or Ra substituted with Rd.
Párrafo 10. El compuesto de uno cualquiera de los párrafos 1-9, en el que R2 es H, CH3 ,Paragraph 10. The compound of any one of paragraphs 1-9, wherein R2 is H, CH 3 ,
Párrafo 11. El compuesto del párrafo 1, en el que:Paragraph 11. The compound of paragraph 1, in which:
el anillo A es piridinilo o pirazinilo;ring A is pyridinyl or pyrazinyl;
R1 es H; y R1 is H; and
R2 es H, heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi o heterocicloalifático.R2 is H, 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C 1- alkyl. 6 substituted with -OH, amino, alkoxy or heterocycloaliphatic.
Párrafo 12. El compuesto del párrafo 1, en el que:Paragraph 12. The compound of paragraph 1, in which:
cada Z1, Z2, Z3, y Z4, es independientemente N o CR3, en la que al menos uno de Z1, Z2, Z3, y Z4 es N; cada R3 es independientemente H, alifático, o heteroalifático; yeach Z1, Z2, Z3, and Z4, is independently N or CR3, wherein at least one of Z1, Z2, Z3, and Z4 is N; each R3 is independently H, aliphatic, or heteroaliphatic; and
R4 es halógeno, heteroarilo, heterocicloalifático, arilo, -NH-heteroarilo, u -O-heteroarilo.R4 is halogen, heteroaryl, heterocycloaliphatic, aryl, -NH-heteroaryl, or -O-heteroaryl.
Parágrafo 13. El compuesto del párrafo 12, en el que el restoParagraph 13. The compound of paragraph 12, in which the remainder
es piridinilo, pirimidinilo, o pirazinilo.is pyridinyl, pyrimidinyl, or pyrazinyl.
Párrafo 14. El compuesto del párrafo 12 o del párrafo 13, en el que Z1 es N.Paragraph 14. The compound of paragraph 12 or paragraph 13, where Z1 is N.
Párrafo 15. El compuesto de uno cualquiera de los párrafos 12-14, en el que Z1 es N, y Z2, Z3, y Z4 son CR3. Párrafo 16. El compuesto de uno cualquiera de los párrafos 12-14, en el que Z1 y Z2 son N, y Z3 y Z4 son CR3. Párrafo 17. El compuesto del párrafo 12 o del párrafo 13, en el que Z1 y Z3 son N, y Z2 y Z4 son CR3.Paragraph 15. The compound of any one of paragraphs 12-14, wherein Z1 is N, and Z2, Z3, and Z4 are CR3. Paragraph 16. The compound of any one of paragraphs 12-14, wherein Z1 and Z2 are N, and Z3 and Z4 are CR3. Paragraph 17. The compound of paragraph 12 or paragraph 13, where Z1 and Z3 are N, and Z2 and Z4 are CR3.
Párrafo 18. El compuesto del párrafo 12 o del párrafo 13, en el que Z1 y Z4 son N, y Z2 y Z3 son CR3.Paragraph 18. The compound of paragraph 12 or paragraph 13, where Z 1 and Z 4 are N, and Z 2 and Z 3 are CR 3 .
Párrafo 19. El compuesto del párrafo 12 o del párrafo 13, en el que Z3 es N, y Z1, Z2, y Z4 son CR3.Paragraph 19. The compound of paragraph 12 or paragraph 13, where Z 3 is N, and Z1, Z2, and Z4 are CR 3 .
Párrafo 20. El compuesto de uno cualquiera de los párrafos 12-19, en el que R1 es H.Paragraph 20. The compound of any one of paragraphs 12-19, wherein R1 is H.
Párrafo 21. [Omitido].Paragraph 21. [Omitted].
Párrafo 22. El compuesto de uno cualquiera de los párrafos 1 al 20, en el que R4 es Br, heteroarilo de 5 a 10 miembros, heterocicloalifático de 3 a 6 miembros, arilo de 6 a 10 miembros, -NH-(heteroarilo de 5 a 10 miembros), u -O-(heteroarilo de 5 a 10 miembros).Paragraph 22. The compound of any one of paragraphs 1 to 20, wherein R4 is Br, 5- to 10-membered heteroaryl, 3- to 6-membered heterocycloaliphatic, 6- to 10-membered aryl, -NH-(5-membered heteroaryl to 10 membered), or -O-(5 to 10 membered heteroaryl).
Párrafo 23. El compuesto del párrafo 22, en el que R4 es piridinilo, pirimidinilo, pirazolilo, -NH-pirazolilo, pirrolilo, -O-piridinilo, -NH-piridinilo, indolilo, furanilo, -NH-benzopirazolilo, pirrolopiridinilo, fenilo, tetrahidropiridinilo, piperidinilo, o 2-oxo-1,2-dihidropiridinilo.Paragraph 23. The compound of paragraph 22, wherein R4 is pyridinyl, pyrimidinyl, pyrazolyl, -NH-pyrazolyl, pyrrolyl, -O-pyridinyl, -NH-pyridinyl, indolyl, furanyl, -NH-benzopyrazolyl, pyrrolopyridinyl, phenyl, tetrahydropyridinyl, piperidinyl, or 2-oxo-1,2-dihydropyridinyl.
Párrafo 24. El compuesto de uno cualquiera de los párrafos 1o 20, en el que R4 es Br; piridinilo no sustituido; piridinilo sustituido con alquilo de C1-6, haloalquilo, amino, heterocicloalifático, cicloalquilo, -CN, alcoxi, -O-heterocicloalifático, -NH-heterocicloalifático, halógeno, sulfonamida, -O-bencilo, carboxilo, sulfonilo, -NH-cicloalquilo, o amida; pirimidinilo no sustituido; pirazolilo no sustituido; pirazolilo sustituido con alquilo de C1-6; -NH-pirazolilo no sustituido; -NH-pirazolilo sustituido con alquilo de C1-6, o heteroarilo; pirrolilo; -O-piridinilo no sustituido; -O-piridinilo sustituido con amino; -NH-piridinilo sustituido con alquilo de C1-6, haloalquilo, o heterocicloalifático; indolilo no sustituido; indolilo sustituido con alcoxi; furanilo; -NH-benzopirazolilo; pirrolopiridinilo; fenilo no sustituido; fenilo sustituido con halógeno, alquilo de C1-6, alcoxi, -CN, amino, o sulfonamida; tetrahidropiridinilo no sustituido; tetrahidropiridinilo sustituido con terc-butoxicarbonilo; piperidinilo; o 2-oxo-1,2-dihidropiridinilo.Paragraph 24. The compound of any one of paragraphs 1 or 20, wherein R4 is Br; unsubstituted pyridinyl; C 1-6 alkyl-substituted pyridinyl, haloalkyl, amino, heterocycloaliphatic, cycloalkyl, -CN, alkoxy, -O-heterocycloaliphatic, -NH-heterocycloaliphatic, halogen, sulfonamide, -O-benzyl, carboxyl, sulfonyl, -NH-cycloalkyl , or amide; unsubstituted pyrimidinyl; unsubstituted pyrazolyl; C 1 -6 alkyl substituted pyrazolyl; -NH-pyrazolyl unsubstituted; -NH-pyrazolyl substituted with C 1-6 alkyl, or heteroaryl; pyrrolyl; -O-pyridinyl unsubstituted; -O-pyridinyl substituted with amino; -NH-pyridinyl substituted with C 1-6 alkyl, haloalkyl, or heterocycloaliphatic; unsubstituted indolyl; alkoxy substituted indolyl; furanyl; -NH-benzopyrazolyl; pyrrolopyridinyl; unsubstituted phenyl; phenyl substituted with halogen, C 1-6 alkyl, alkoxy, -CN, amino, or sulfonamide; unsubstituted tetrahydropyridinyl; tetrahydropyridinyl substituted with tert-butoxycarbonyl; piperidinyl; or 2-oxo-1,2-dihydropyridinyl.
Párrafo 25. El compuesto de uno cualquiera de los párrafos 1-24, en el que R4 es Br,Paragraph 25. The compound of any one of paragraphs 1-24, wherein R4 is Br,
, en las que y es 0, 1 o 2, y cada Rp es independientemente Ra, Rb, Ra sustituido con Rb, o Ra sustituido con Rc., wherein y is 0, 1, or 2, and each Rp is independently Ra, Rb, Ra substituted with Rb, or Ra substituted with Rc.
Párrafo 26. El compuesto del párrafo 25, en el que cada Rp independientemente es -CH3 , -OCH3 , -NH2 , -CF3 , F, -CN,Paragraph 26. The compound of Paragraph 25, where each Rp independently is -CH 3 , -OCH 3 , -NH 2 , -CF 3 , F, -CN,
Párrafo 27. [Omitido].Paragraph 27. [Omitted].
Párrafo 28. El compuesto de uno cualquiera de los párrafos 1 -27, en el compuesto tiene una fórmula 2,Paragraph 28. The compound of any one of paragraphs 1 -27, in the compound has a formula 2,
en la que cada uno de R5, R6, y R7 independientemente es H o alquilo.wherein each of R5, R6, and R7 independently is H or alkyl.
Párrafo 29. El compuesto del párrafo 28, en el que cada uno de R5, R6, y R7 es H.Paragraph 29. The compound of paragraph 28, wherein each of R5, R6, and R7 is H.
Párrafo 30. El compuesto del párrafo 28 o del párrafo 29, en el que cada uno de R5, R6, y R7 es alquilo de C1-6.Paragraph 30. The compound of paragraph 28 or paragraph 29, wherein each of R5, R6, and R7 is C 1 -6 alkyl.
Párrafo 31. El compuesto de uno cualquiera de los párrafos 28-30, en el que el anillo A es piridin-2-ilo o pirazin-2-ilo. Paragraph 31. The compound of any one of paragraphs 28-30, wherein ring A is pyridin-2-yl or pyrazin-2-yl.
Párrafo 32. El compuesto de uno cualquiera de los párrafos 1 -31, en el compuesto tiene una fórmula 3Paragraph 32. The compound of any one of paragraphs 1 -31, in the compound has a formula 3
y cada uno de R8, R9, R10 y R11 independientemente es H, alifático, halógeno, heterocicloalifático, alcoxi, u -O-heterocicloalifático.and each of R 8 , R 9 , R 10 and R 11 independently is H, aliphatic, halogen, heterocycloaliphatic, alkoxy, or -O-heterocycloaliphatic.
Párrafo 33. El compuesto del párrafo 32, en el que cada uno de R8, R9, R10 y R11 es independientemente H, halógeno, heterocicloalifático de 3 a 6 miembros, alcoxi, u -O-(heterocicloalifático de 3 a 6 miembros).Paragraph 33. The compound of paragraph 32, wherein each of R 8 , R 9 , R 10 and R 11 is independently H, halogen, 3 to 6 membered heterocycloaliphatic, alkoxy, or -O-(3 to 6 membered heterocycloaliphatic) .
Párrafo 34. El compuesto del párrafo 32 o del párrafo 33, en el que R8 es H o halógeno.Paragraph 34. The compound of Paragraph 32 or Paragraph 33, wherein R 8 is H or halogen.
Párrafo 35. El compuesto de uno cualquiera de los párrafos 32-34, en el que R9 y R11 son H.Paragraph 35. The compound of any one of paragraphs 32-34, wherein R9 and R11 are H.
Párrafo 36. El compuesto de uno cualquiera de los párrafos 32-35, en el que R10 es H, F, morfolino, N-metilpiperidin-1-ilo, metoxi, 2-hidroxi-2-metilpropoxi, u -O-oxetanilo.Paragraph 36. The compound of any one of paragraphs 32-35, wherein R10 is H, F, morpholino, N-methylpiperidin-1-yl, methoxy, 2-hydroxy-2-methylpropoxy, or -O-oxetanyl.
Párrafo 37. El compuesto de uno cualquiera de los párrafos 32-36, en el que cada uno de R8, R9, y R11 e Párrafo 38. El compuesto de uno cualquiera de los párrafos 32-37, en el que R9 y R11 son H, y R8 y F.Paragraph 37. The compound of any one of paragraphs 32-36, wherein each of R8 , R9, and R11 e Paragraph 38. The compound of any one of paragraphs 32-37, wherein R9 and R11 are H, and R 8 and F.
Párrafo 39. El compuesto de uno cualquiera de los párrafos 32-36, en el que cada uno de R8, R9, R10 y R11 es H.Paragraph 39. The compound of any one of paragraphs 32-36, wherein R8 , R9, R10, and R11 are each H.
Párrafo 40. El compuesto de uno cualquiera de los párrafos 32-39, en el compuesto tiene una fórmula 9Paragraph 40. The compound of any one of paragraphs 32-39, in the compound has a formula 9
Párrafo 41. El compuesto del párrafo 40, en el que:Paragraph 41. The compound of paragraph 40, in which:
R1 es H;R1 is H;
R2 es H, heteroalifático de 3 a 10 miembros, tetrahidropiranilo, oxetanilo, ciclobutilo, ciclobutilo sustituido con alcoxi y/o hidroxi, ciclohexilo, ciclohexilo sustituido con alcoxi y/o hidroxi, alquilo de C1-6 no sustituido, o alquilo de C1-6 sustituido con -OH, amino, alcoxi, o heterocicloalifático;R2 is H, 3 to 10 membered heteroaliphatic, tetrahydropyranyl, oxetanyl, cyclobutyl, alkoxy and/or hydroxy substituted cyclobutyl, cyclohexyl, alkoxy and/or hydroxy substituted cyclohexyl, unsubstituted C 1-6 alkyl, or C alkyl 1-6 substituted with -OH, amino, alkoxy, or heterocycloaliphatic;
R4 es Br; piridinilo no sustituido; piridinilo sustituido con alquilo de C1-6 , haloalquilo, amino, heterocicloalifático, cicloalquilo, -CN, alcoxi, -O-heterocicloalifático, -NH-heterocicloalifático, halógeno, sulfonamida, -O-bencilo, carboxilo, sulfonilo, -NH-cicloalquilo, o amida; pirimidinilo no sustituido; pirazolilo no sustituido; pirazolilo sustituido con alquilo de C1-6 ; -NH-pirazolilo no sustituido; -NH-pirazolilo sustituido con alquilo de C1-6 , o heteroarilo; pirrolilo; -O-piridinilo no sustituido; -O-piridinilo sustituido con amino; -NH-piridinilo sustituido con alquilo de C1-6 , haloalquilo, o heterocicloalifático; indolilo no sustituido; indolilo sustituido con alcoxi; furanilo;R4 is Br; unsubstituted pyridinyl; C 1-6 alkyl-substituted pyridinyl, haloalkyl, amino, heterocycloaliphatic, cycloalkyl, -CN, alkoxy, -O-heterocycloaliphatic, -NH-heterocycloaliphatic, halogen, sulfonamide, -O-benzyl, carboxyl, sulfonyl, -NH-cycloalkyl , or amide; unsubstituted pyrimidinyl; unsubstituted pyrazolyl; pyrazolyl substituted with C 1-6 alkyl; -NH-pyrazolyl unsubstituted; -NH-pyrazolyl substituted with C 1-6 alkyl, or heteroaryl; pyrrolyl; -O-pyridinyl unsubstituted; -O-pyridinyl substituted with amino; -NH-pyridinyl substituted with C 1-6 alkyl, haloalkyl, or heterocycloaliphatic; unsubstituted indolyl; alkoxy substituted indolyl; furanyl;
-NH-benzopirazolilo; pirrolopiridinilo; fenilo no sustituido; fenilo sustituido con halógeno, alquilo de C1-6 , alcoxi,-NH-benzopyrazolyl; pyrrolopyridinyl; unsubstituted phenyl; phenyl substituted with halogen, C 1-6 alkyl, alkoxy,
-CN, amino, o sulfonamida; tetrahidropiridinilo no sustituido; tetrahidropiridinilo sustituido con tercbutoxicarbonilo; piperidinilo; o 2-oxo-1,2-dihidropiridinilo; -CN, amino, or sulfonamide; unsubstituted tetrahydropyridinyl; tetrahydropyridinyl substituted with tert-butoxycarbonyl; piperidinyl; or 2-oxo-1,2-dihydropyridinyl;
R8 es H o F;R8 is H or F;
R9 y R11 son H; yR9 and R11 are H; and
R10 es H, F, morfolino, N-metilpiperidinilo, metoxi, 2-hidroxi-2-metilpropoxi, u -O-oxetanilo.R10 is H, F, morpholino, N-methylpiperidinyl, methoxy, 2-hydroxy-2-methylpropoxy, or -O-oxetanyl.
Párrafo 42. El compuesto del párrafo 41, en el que cada uno de R8, R9, R10 y R11 es H.Paragraph 42. The compound of paragraph 41, wherein each of R8 , R9, R10, and R11 is H.
Párrafo 43. El compuesto del párrafo 41, en el que R8, R9, y R11 son H, y R10 es morfolino de 3 a 6 miembros o N-metilpiperidinilo, metoxi, 2-hidroxi-2-metilpropoxi, u -O-oxetanilo.Paragraph 43. The compound of paragraph 41, wherein R 8 , R 9 , and R 11 are H, and R 10 is 3- to 6-membered morpholino or N-methylpiperidinyl, methoxy, 2-hydroxy-2-methylpropoxy, or -O- oxetanil.
Párrafo 44. El compuesto del párrafo 41, en el que R8 y R10 son F, y R9 y R11 son H.Paragraph 44. The compound of paragraph 41, wherein R8 and R10 are F, and R9 and R11 are H.
Párrafo 45. Un compuesto seleccionado de los compuestos ejemplares descritos aquí.Paragraph 45. A compound selected from the exemplary compounds described herein.
Párrafo 46. Una composición, que comprende un compuesto de uno cualquiera de los párrafos 1-45, y un excipiente farmacéuticamente aceptable.Paragraph 46. A composition, comprising a compound of any one of paragraphs 1-45, and a pharmaceutically acceptable excipient.
Párrafo 47. La composición del párrafo 46, que comprende además un agente terapéutico adicional.Paragraph 47. The composition of paragraph 46, further comprising an additional therapeutic agent.
Párrafo 48. Un método que comprende administrar a un sujeto que lo necesite una cantidad efectiva de un compuesto de uno cualquiera de los párrafos 1-45, o una composición de uno cualquiera de los párrafos 46 47.Paragraph 48. A method comprising administering to a subject in need thereof an effective amount of a compound of any one of paragraphs 1-45, or a composition of any one of paragraphs 46-47.
Párrafo 49. El método del párrafo 48, para el tratamiento de una enfermedad o afección para la cual está indicado un inhibidor de IRAK.Paragraph 49. The method in paragraph 48, for the treatment of a disease or condition for which an IRAK inhibitor is indicated.
Párrafo 50. El método del párrafo 49, en el que la enfermedad es una enfermedad autoinmune, trastorno inflamatorio, enfermedad cardiovascular, trastorno neurodegenerativo, trastorno alérgico, insuficiencia multiorgánica, enfermedad renal, agregación plaquetaria, cáncer, trasplante, motilidad espermática, deficiencia de eritrocitos, rechazo del injerto, lesión pulmonar, enfermedad respiratoria, afección isquémica, infección bacteriana, infección viral, trastorno regulador inmune, o una combinación de los mismos.Paragraph 50. The method of paragraph 49, wherein the disease is an autoimmune disease, inflammatory disorder, cardiovascular disease, neurodegenerative disorder, allergic disorder, multi-organ failure, renal disease, platelet aggregation, cancer, transplantation, sperm motility, erythrocyte deficiency , graft rejection, lung injury, respiratory disease, ischemic condition, bacterial infection, viral infection, immune regulatory disorder, or a combination thereof.
Párrafo 51. El método del párrafo 49, en el que la enfermedad es esclerosis lateral amiotrófica (ELA), lupus eritematoso sistémico, artritis reumatoide crónica, diabetes mellitus tipo I, enfermedad inflamatoria intestinal, cirrosis biliar, uveítis, esclerosis múltiple, enfermedad de Crohn, colitis ulcerosa, penfigoide ampolloso, sarcoidosis, psoriasis, miositis autoinmune, pancreatitis, sarcoma de Kaposi, síndrome mielodisplásico, granulomatosis de Wegener, ictiosis, oftalmopatía de Graves, o asma.Paragraph 51. The method of paragraph 49, wherein the disease is amyotrophic lateral sclerosis (ALS), systemic lupus erythematosus, chronic rheumatoid arthritis, type I diabetes mellitus, inflammatory bowel disease, biliary cirrhosis, uveitis, multiple sclerosis, Crohn's disease , ulcerative colitis, bullous pemphigoid, sarcoidosis, psoriasis, autoimmune myositis, pancreatitis, Kaposi's sarcoma, myelodysplastic syndrome, Wegener's granulomatosis, ichthyosis, Graves' ophthalmopathy, or asthma.
Párrafo 52. El método del párrafo 50, en el que el trastorno regulador inmune es artritis reumatoide, lupus eritematoso sistémico, tiroiditis de Hashimoto, esclerosis múltiple, esclerosis sistémica, miastenia grave, diabetes tipo I, uveítis, uveítis posterior, encefalomielitis alérgica, glomerulonefritis, enfermedades autoinmunes posinfecciosas que incluyen fiebre reumática y glomerulonefritis posinfecciosa, enfermedades inflamatorias e hiperproliferativas de la piel, psoriasis, dermatitis atópica, dermatitis por contacto, dermatitis eccematosa, dermatitis seborreica, liquen plano, pénfigo, penfigoide ampolloso, epidermólisis ampollosa, urticaria, angioedemas, vasculitis, eritema, eosinofilia cutánea, lupus eritematoso, acné, alopecia areata, queratoconjuntivitis, conjuntivitis vernal, uveítis asociada a la enfermedad de Behcet, queratitis , queratitis herpética, córnea cónica, distrofia epitelial de la córnea, leucoma corneal, pénfigo ocular, úlcera de Mooren, escleritis, oftalmopatía de Graves, síndrome de Vogt-Koyanagi-Harada, sarcoidosis, alergia al polen, enfermedad obstructiva reversible de las vías respiratorias, asma bronquial, asma alérgica, asma intrínseca, asma extrínseca, asma por polvo, asma crónica o inveterada, asma tardía e hiperreactividad de las vías respiratorias, bronquitis, úlceras gástricas, daño vascular causado por enfermedades isquémicas y trombosis, enfermedades isquémicas del intestino, enfermedades inflamatorias del intestino, enterocolitis necrosante, lesiones intestinales asociadas con quemaduras térmicas, celiaquías, proctitis, gastroenteritis eosinofílica, mastocitosis, enfermedad de Crohn, colitis ulcerosa, migraña, rinitis, eccema, nefritis intersticial, síndrome de Goodpasture, síndrome urémico hemolítico, nefropatía diabética, miositis múltiple, síndrome de Guillain-Barré, enfermedad de Meniere, polineuritis, neuritis múltiple, mononeuritis, radiculopatía, hipertiroidismo, enfermedad de Basedow, aplasia pura de glóbulos rojos, anemia aplásica, anemia hipoplásica, púrpura trombocitopénica idiopática, anemia hemolítica autoinmune, agranulocitosis, anemia perniciosa, anemia megaloblástica, aneritroplasia, osteoporosis, sarcoidosis, pulmón fibroide, neumonía intersticial idiopática, dermatomiositis, leucodermia vulgar, ictiosis vulgar, sensibilidad fotoalérgica, linfoma cutáneo de células T, leucemia linfocítica crónica, arteriosclerosis, aterosclerosis, síndrome de aortitis, poliarteritis nodosa, miocardosis, esclerodermia, granuloma de Wegener, síndrome de Sjogren, adiposis, fascitis eosinofílica, lesiones de las encías, periodonto, hueso alveolar, sustancia ósea dental, glomerulonefritis, alopecia de patrón masculino o alopecia senil al prevenir la depilación o facilitar la germinación del cabello y/o promover la generación y el crecimiento del cabello, distrofia muscular, pioderma y síndrome de Sezary, enfermedad de Addison, lesión por isquemia-reperfusión de órganos que se produce tras la conservación, trasplante o enfermedad isquémica, choque por endotoxinas, colitis seudomembranosa, colitis causada por fármacos o radiación, insuficiencia renal aguda isquémica, insuficiencia renal crónica, toxinosis causada por oxígeno pulmonar o fármacos, cáncer de pulmón, enfisema, cataratas, siderosis, retinitis pigmentosa, degeneración macular senil, cicatrización vítrea, quemadura alcalina de la córnea, dermatitis eritema multiforme, dermatitis ampollosa IgA lineal y dermatitis del cemento, gingivitis, periodontitis, septicemia, pancreatitis, enfermedades causadas por la contaminación ambiental, envejecimiento, carcinogénesis, metástasis de carcinoma e hipobaropatía, enfermedad causada por liberación de histaminas o leucotrieno-C4, enfermedad de Behcet, hepatitis autoinmune, cirrosis biliar primaria, colangitis esclerosante, resección hepática parcial, necrosis hepática aguda, necrosis causada por toxina, hepatitis viral, choque o anoxia, hepatitis por virus B, hepatitis no Nno B, cirrosis, cirrosis alcohólica, insuficiencia hepática, insuficiencia hepática fulminante, insuficiencia hepática de inicio tardío, insuficiencia hepática “aguda sobre crónica”, aumento del efecto quimioterapéutico, infección por citomegalovirus, infección por HCMV, SIDA, cáncer, demencia senil, enfermedad de Parkinson, trauma, o infección bacteriana crónica.Paragraph 52. The method of paragraph 50, wherein the immune regulatory disorder is rheumatoid arthritis, systemic lupus erythematosus, Hashimoto's thyroiditis, multiple sclerosis, systemic sclerosis, myasthenia gravis, type I diabetes, uveitis, posterior uveitis, allergic encephalomyelitis, glomerulonephritis , post-infectious autoimmune diseases including rheumatic fever and post-infectious glomerulonephritis, inflammatory and hyperproliferative skin diseases, psoriasis, atopic dermatitis, contact dermatitis, eczematous dermatitis, seborrheic dermatitis, lichen planus, pemphigus, bullous pemphigoid, epidermolysis bullosa, urticaria, angioedema, vasculitis, erythema, cutaneous eosinophilia, lupus erythematosus, acne, alopecia areata, keratoconjunctivitis, vernal conjunctivitis, Behcet's disease-associated uveitis, keratitis, keratitis herpes, cornea conica, epithelial dystrophy of the cornea, corneal leukoma, ocular pemphigus, ulcer of Mooren's, scleritis, Graves' ophthalmopathy, Vogt-Koyanagi-Harada syndrome, sarcoidosis, pollen allergy, reversible obstructive airway disease, bronchial asthma, allergic asthma, intrinsic asthma, extrinsic asthma, dust asthma, chronic or inveterate asthma , late-onset asthma and airway hyperresponsiveness, bronchitis, gastric ulcers, vascular damage caused by ischemic diseases and thrombosis, ischemic bowel diseases, inflammatory bowel diseases, necrotizing enterocolitis, intestinal lesions associated with thermal burns, celiac disease, proctitis, eosinophilic gastroenteritis , mastocytosis, Crohn's disease, ulcerative colitis, migraine, rhinitis, eczema, interstitial nephritis, Goodpasture's syndrome, hemolytic uremic syndrome, diabetic nephropathy, multiple myositis, Guillain-Barré syndrome, Meniere's disease, polyneuritis, multiple neuritis, mononeuritis, radiculopathy, hyperthyroidism, Basedow's disease, pure red cell aplasia, aplastic anemia, hypoplastic anemia, idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia, agranulocytosis, pernicious anemia, megaloblastic anemia, anerythroplasia, osteoporosis, sarcoidosis, fibroid lung, idiopathic interstitial pneumonia, der matomyositis , leukoderma vulgaris, ichthyosis vulgaris, photoallergic sensitivity, cutaneous T-cell lymphoma, chronic lymphocytic leukemia, arteriosclerosis, atherosclerosis, aortitis syndrome, polyarteritis nodosa, myocardosis, scleroderma, Wegener's granuloma, Sjogren's syndrome, adiposis, eosinophilic fasciitis, lesions of gums, periodontium, alveolar bone, dental bone substance, glomerulonephritis, male pattern alopecia or senile alopecia by preventing hair removal or facilitating hair germination and/or promoting hair generation and growth, muscular dystrophy, pyoderma and syndrome of sezary disease Addison's disease, ischemia-reperfusion injury of organs that occurs after preservation, transplantation or ischemic disease, endotoxin shock, pseudomembranous colitis, colitis caused by drugs or radiation, ischemic acute renal failure, chronic renal failure, pulmonary oxygen toxinosis or drugs, lung cancer, emphysema, cataracts, siderosis, retinitis pigmentosa, age-related macular degeneration, vitreous scarring, alkaline burn of the cornea, dermatitis erythema multiforme, linear IgA bullous dermatitis and cementum dermatitis, gingivitis, periodontitis, sepsis, pancreatitis, diseases caused by environmental pollution, aging, carcinogenesis, carcinoma metastasis and hypobaropathy, disease caused by histamine or leukotriene-C4 release, Behcet's disease, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, partial liver resection, acute liver necrosis, toxin necrosis, viral hepatitis, shock or anoxia, hepatitis B virus, non- N non-B hepatitis, cirrhosis, alcoholic cirrhosis, liver failure, fulminant liver failure, late-onset liver failure, “acute on chronic” liver failure, increased of the chemotherapeutic effect, cytomegalovirus infection, HCMV infection, AIDS, cancer, senile dementia, Parkinson's disease, trauma, or chronic bacterial infection.
Párrafo 53. Un método para inhibir una proteína IRAK, que comprende poner en contacto la proteína IRAK con una cantidad efectiva de un compuesto de uno cualquiera de los párrafos 1-45, o una composición de uno cualquiera de los párrafos 46-47.Paragraph 53. A method of inhibiting an IRAK protein, comprising contacting the IRAK protein with an effective amount of a compound of any one of paragraphs 1-45, or a composition of any one of paragraphs 46-47.
Párrafo 54. El método del párrafo 53, en el que el compuesto tiene una CE50 de más de 0 a 5 μM.Paragraph 54. The method of Paragraph 53, wherein the compound has an EC 50 of greater than 0 to 5 µM.
Párrafo 55. El método del párrafo 53, en el que el compuesto tiene una CE50 de más de 0 a 1 μM.Paragraph 55. The method of Paragraph 53, wherein the compound has an EC 50 of greater than 0 to 1 µM.
Párrafo 56. El método de uno cualquiera de los párrafos 53-55, en el que la proteína IRAK está en un sujeto. Paragraph 56. The method of any one of paragraphs 53-55, wherein the IRAK protein is in a subject.
Párrafo 57. El método de uno cualquiera de los párrafos 53-55, en el que la puesta en contacto con la proteína IRAK comprende la puesta en contacto con la proteína IRAK in vitro. Paragraph 57. The method of any one of paragraphs 53-55, wherein contacting with IRAK protein comprises contacting with IRAK protein in vitro.
Claims (18)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662413299P | 2016-10-26 | 2016-10-26 | |
| PCT/US2017/058329 WO2018081294A1 (en) | 2016-10-26 | 2017-10-25 | Pyrazole amide compounds as irak inhibitors |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| ES2946001T3 true ES2946001T3 (en) | 2023-07-11 |
Family
ID=60263148
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| ES17794624T Active ES2946001T3 (en) | 2016-10-26 | 2017-10-25 | Pyrazole amide compounds as IRAK inhibitors |
Country Status (7)
| Country | Link |
|---|---|
| US (4) | US10414753B2 (en) |
| EP (2) | EP4212523A1 (en) |
| JP (2) | JP7170635B2 (en) |
| CN (2) | CN115531387A (en) |
| CA (1) | CA3040526A1 (en) |
| ES (1) | ES2946001T3 (en) |
| WO (1) | WO2018081294A1 (en) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JOP20180011A1 (en) | 2017-02-16 | 2019-01-30 | Gilead Sciences Inc | PYRROLO[1,2-b] PYRIDAZINE DERIVATIVES |
| TWI721483B (en) | 2018-07-13 | 2021-03-11 | 美商基利科學股份有限公司 | Pyrrolo[1,2-b]pyridazine derivatives |
| CN110862375B (en) * | 2018-08-27 | 2022-10-25 | 深圳铂立健医药有限公司 | Pyrazole compound and pharmaceutical composition and application thereof |
| MX2022003830A (en) * | 2019-10-02 | 2022-05-12 | Kainos Medicine Inc | N-(1h-imidazol-2-yl)benzamide compound and pharmaceutical composition comprising the same as active ingredient. |
| EP4125895A4 (en) * | 2020-03-23 | 2024-05-15 | Dana-Farber Cancer Institute, Inc. | Potent and selective irreversible inhibitors of irak1 |
| TW202400250A (en) | 2022-04-12 | 2024-01-01 | 美商健臻公司 | Use of irak4 modulators for gene therapy |
| CN116751195A (en) * | 2023-06-21 | 2023-09-15 | 杭州科兴生物化工有限公司 | Bipyridine compound, pharmaceutically acceptable salt thereof, preparation method and application |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4921475A (en) | 1983-08-18 | 1990-05-01 | Drug Delivery Systems Inc. | Transdermal drug patch with microtubes |
| US5087240A (en) | 1983-08-18 | 1992-02-11 | Drug Delivery Systems Inc. | Transdermal drug patch with conductive fibers |
| US4738851A (en) | 1985-09-27 | 1988-04-19 | University Of Iowa Research Foundation, Inc. | Controlled release ophthalmic gel formulation |
| US5163899A (en) | 1987-03-20 | 1992-11-17 | Drug Delivery Systems Inc. | Transdermal drug delivery system |
| US5312325A (en) | 1987-05-28 | 1994-05-17 | Drug Delivery Systems Inc | Pulsating transdermal drug delivery system |
| GB8804164D0 (en) | 1988-02-23 | 1988-03-23 | Tucker J M | Bandage for administering physiologically active compound |
| US4882150A (en) | 1988-06-03 | 1989-11-21 | Kaufman Herbert E | Drug delivery system |
| US5008110A (en) | 1988-11-10 | 1991-04-16 | The Procter & Gamble Company | Storage-stable transdermal patch |
| US5088977A (en) | 1988-12-21 | 1992-02-18 | Drug Delivery Systems Inc. | Electrical transdermal drug applicator with counteractor and method of drug delivery |
| US5521222A (en) | 1989-09-28 | 1996-05-28 | Alcon Laboratories, Inc. | Topical ophthalmic pharmaceutical vehicles |
| IE66203B1 (en) | 1989-12-04 | 1995-12-13 | Searle & Co | System for transdermal albuterol administration |
| US5077033A (en) | 1990-08-07 | 1991-12-31 | Mediventures Inc. | Ophthalmic drug delivery with thermo-irreversible gels of polxoxyalkylene polymer and ionic polysaccharide |
| DK0495421T3 (en) | 1991-01-15 | 1996-12-09 | Alcon Lab Inc | Use of carrageenans in topical ophthalmic compositions |
| US5352456A (en) | 1991-10-10 | 1994-10-04 | Cygnus Therapeutic Systems | Device for administering drug transdermally which provides an initial pulse of drug |
| JPH07502219A (en) | 1991-12-18 | 1995-03-09 | ミネソタ マイニング アンド マニュファクチャリング カンパニー | Multilayer barrier structure |
| ATE132381T1 (en) | 1992-01-29 | 1996-01-15 | Voelkl Franz Ski | BALL GAME RACKETS, ESPECIALLY TENNIS RACKETS |
| IL114193A (en) | 1994-06-20 | 2000-02-29 | Teva Pharma | Ophthalmic pharmaceutical compositions based on sodium alginate |
| ES2094688B1 (en) | 1994-08-08 | 1997-08-01 | Cusi Lab | MANOEMULSION OF THE TYPE OF OIL IN WATER, USEFUL AS AN OPHTHALMIC VEHICLE AND PROCEDURE FOR ITS PREPARATION. |
| IT1283911B1 (en) | 1996-02-05 | 1998-05-07 | Farmigea Spa | VISCOSIZED OPHTHALMIC SOLUTIONS WITH TAMARIND GUM POLYSACCHARIDES |
| US5800807A (en) | 1997-01-29 | 1998-09-01 | Bausch & Lomb Incorporated | Ophthalmic compositions including glycerin and propylene glycol |
| US6261547B1 (en) | 1998-04-07 | 2001-07-17 | Alcon Manufacturing, Ltd. | Gelling ophthalmic compositions containing xanthan gum |
| US6197934B1 (en) | 1998-05-22 | 2001-03-06 | Collagenesis, Inc. | Compound delivery using rapidly dissolving collagen film |
| BRPI0409949A (en) * | 2003-05-01 | 2006-04-25 | Bristol Myers Squibb Co | aryl-substituted amide pyrazole compounds useful as kinase inhibitors |
| EP2070924A1 (en) * | 2007-12-10 | 2009-06-17 | Bayer Schering Pharma Aktiengesellschaft | New 2 hetarylthiazol-4-carboxylic acid derivatives, their manufacture and use as medicine |
| JP2012254939A (en) * | 2009-10-07 | 2012-12-27 | Astellas Pharma Inc | Oxazole compound |
| EP2556066B1 (en) * | 2010-04-07 | 2016-10-19 | F.Hoffmann-La Roche Ag | Pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| US9586948B2 (en) * | 2012-10-08 | 2017-03-07 | Merck Sharp & Dohme Corp. | Inhibitors of IRAK4 activity |
| AU2014347027A1 (en) * | 2013-11-06 | 2016-06-23 | Bristol-Myers Squibb Company | GSK -3 inhibitors |
| CN105899505B (en) * | 2013-11-08 | 2018-08-28 | 武田药品工业株式会社 | Pyrazoles for treating autoimmune disorder |
| CA2964982C (en) | 2014-11-20 | 2022-07-05 | Merck Patent Gmbh | Heteroaryl compounds as irak inhibitors and uses thereof |
| DK3286181T3 (en) * | 2015-04-22 | 2021-04-06 | Rigel Pharmaceuticals Inc | PYRAZOLE COMPOUNDS AND PROCEDURE FOR PREPARATION AND USE OF THE COMPOUNDS |
| JP2019196309A (en) * | 2016-09-15 | 2019-11-14 | 武田薬品工業株式会社 | Heterocyclic compound |
-
2017
- 2017-10-25 EP EP23158563.9A patent/EP4212523A1/en active Pending
- 2017-10-25 JP JP2019522484A patent/JP7170635B2/en active Active
- 2017-10-25 CN CN202211335278.5A patent/CN115531387A/en active Pending
- 2017-10-25 US US15/793,743 patent/US10414753B2/en active Active
- 2017-10-25 CN CN201780080550.0A patent/CN110234637B/en active Active
- 2017-10-25 ES ES17794624T patent/ES2946001T3/en active Active
- 2017-10-25 WO PCT/US2017/058329 patent/WO2018081294A1/en unknown
- 2017-10-25 CA CA3040526A patent/CA3040526A1/en active Pending
- 2017-10-25 EP EP17794624.1A patent/EP3532465B1/en active Active
-
2019
- 2019-08-02 US US16/529,995 patent/US10947216B2/en active Active
-
2021
- 2021-02-24 US US17/184,199 patent/US11530194B2/en active Active
-
2022
- 2022-11-01 JP JP2022175232A patent/JP7379641B2/en active Active
- 2022-11-09 US US17/983,645 patent/US11939317B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019533687A (en) | 2019-11-21 |
| JP7379641B2 (en) | 2023-11-14 |
| EP4212523A1 (en) | 2023-07-19 |
| CA3040526A1 (en) | 2018-05-03 |
| CN115531387A (en) | 2022-12-30 |
| EP3532465A1 (en) | 2019-09-04 |
| CN110234637B (en) | 2022-11-01 |
| US20190359593A1 (en) | 2019-11-28 |
| US11939317B2 (en) | 2024-03-26 |
| JP7170635B2 (en) | 2022-11-14 |
| US10414753B2 (en) | 2019-09-17 |
| US11530194B2 (en) | 2022-12-20 |
| US20180111917A1 (en) | 2018-04-26 |
| EP3532465B1 (en) | 2023-03-01 |
| WO2018081294A1 (en) | 2018-05-03 |
| US20210214342A1 (en) | 2021-07-15 |
| JP2023011796A (en) | 2023-01-24 |
| CN110234637A (en) | 2019-09-13 |
| US10947216B2 (en) | 2021-03-16 |
| US20230115275A1 (en) | 2023-04-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2020343671B2 (en) | RIP1 inhibitory compounds and methods for making and using the same | |
| ES2946001T3 (en) | Pyrazole amide compounds as IRAK inhibitors | |
| US11059814B2 (en) | Kinase inhibitors and methods for making and using | |
| WO2021203011A1 (en) | Rip1k inhibitors | |
| US11993594B2 (en) | IRAK inhibitors and method for making and using |