EP2297288B1 - Laundry compositions - Google Patents
Laundry compositions Download PDFInfo
- Publication number
- EP2297288B1 EP2297288B1 EP09793904.5A EP09793904A EP2297288B1 EP 2297288 B1 EP2297288 B1 EP 2297288B1 EP 09793904 A EP09793904 A EP 09793904A EP 2297288 B1 EP2297288 B1 EP 2297288B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- alginate
- agents
- granule
- ratio
- citric acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Not-in-force
Links
- 239000000203 mixture Substances 0.000 title claims description 51
- 235000010443 alginic acid Nutrition 0.000 claims description 77
- 229920000615 alginic acid Polymers 0.000 claims description 77
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 72
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 claims description 69
- 229940072056 alginate Drugs 0.000 claims description 69
- 239000008187 granular material Substances 0.000 claims description 51
- 239000003795 chemical substances by application Substances 0.000 claims description 38
- 230000008901 benefit Effects 0.000 claims description 33
- 239000003599 detergent Substances 0.000 claims description 32
- 239000004744 fabric Substances 0.000 claims description 21
- 239000000975 dye Substances 0.000 claims description 18
- 239000004094 surface-active agent Substances 0.000 claims description 17
- 125000002091 cationic group Chemical group 0.000 claims description 14
- 238000000034 method Methods 0.000 claims description 14
- 239000002304 perfume Substances 0.000 claims description 13
- 239000003352 sequestering agent Substances 0.000 claims description 13
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 11
- 102000004190 Enzymes Human genes 0.000 claims description 9
- 108090000790 Enzymes Proteins 0.000 claims description 9
- 150000001768 cations Chemical class 0.000 claims description 8
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 7
- 239000011575 calcium Substances 0.000 claims description 7
- 229910052791 calcium Inorganic materials 0.000 claims description 7
- 229910052751 metal Inorganic materials 0.000 claims description 7
- 239000002184 metal Substances 0.000 claims description 7
- 239000006081 fluorescent whitening agent Substances 0.000 claims description 5
- AEMOLEFTQBMNLQ-VANFPWTGSA-N D-mannopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@@H]1O AEMOLEFTQBMNLQ-VANFPWTGSA-N 0.000 claims description 4
- 239000004599 antimicrobial Substances 0.000 claims description 4
- 102000005701 Calcium-Binding Proteins Human genes 0.000 claims description 3
- 108010045403 Calcium-Binding Proteins Proteins 0.000 claims description 3
- 125000005614 guluronate group Chemical group 0.000 claims description 3
- 239000004753 textile Substances 0.000 claims description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 2
- 229940123973 Oxygen scavenger Drugs 0.000 claims description 2
- 239000006096 absorbing agent Substances 0.000 claims description 2
- 239000003242 anti bacterial agent Substances 0.000 claims description 2
- 239000003429 antifungal agent Substances 0.000 claims description 2
- 229940121375 antifungal agent Drugs 0.000 claims description 2
- 239000003963 antioxidant agent Substances 0.000 claims description 2
- 239000003638 chemical reducing agent Substances 0.000 claims description 2
- 239000000460 chlorine Substances 0.000 claims description 2
- 229910052801 chlorine Inorganic materials 0.000 claims description 2
- 239000013078 crystal Substances 0.000 claims description 2
- 239000002270 dispersing agent Substances 0.000 claims description 2
- 239000003966 growth inhibitor Substances 0.000 claims description 2
- 239000003112 inhibitor Substances 0.000 claims description 2
- 238000004900 laundering Methods 0.000 claims description 2
- 239000000314 lubricant Substances 0.000 claims description 2
- 239000006187 pill Substances 0.000 claims description 2
- 239000002689 soil Substances 0.000 claims description 2
- 238000010186 staining Methods 0.000 claims description 2
- -1 carrageenan polysaccharide Chemical class 0.000 description 20
- 239000007844 bleaching agent Substances 0.000 description 14
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 12
- 239000000463 material Substances 0.000 description 12
- 239000000243 solution Substances 0.000 description 12
- 239000002245 particle Substances 0.000 description 10
- 239000010457 zeolite Substances 0.000 description 10
- 229910021536 Zeolite Inorganic materials 0.000 description 9
- 150000001875 compounds Chemical class 0.000 description 9
- 229910052708 sodium Inorganic materials 0.000 description 9
- 239000011734 sodium Substances 0.000 description 9
- VRVDFJOCCWSFLI-UHFFFAOYSA-K trisodium 3-[[4-[(6-anilino-1-hydroxy-3-sulfonatonaphthalen-2-yl)diazenyl]-5-methoxy-2-methylphenyl]diazenyl]naphthalene-1,5-disulfonate Chemical compound [Na+].[Na+].[Na+].COc1cc(N=Nc2cc(c3cccc(c3c2)S([O-])(=O)=O)S([O-])(=O)=O)c(C)cc1N=Nc1c(O)c2ccc(Nc3ccccc3)cc2cc1S([O-])(=O)=O VRVDFJOCCWSFLI-UHFFFAOYSA-K 0.000 description 9
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 8
- UHXQPQCJDDSMCB-UHFFFAOYSA-L disodium;3-[[9,10-dioxo-4-(2,4,6-trimethyl-3-sulfonatoanilino)anthracen-1-yl]amino]-2,4,6-trimethylbenzenesulfonate Chemical compound [Na+].[Na+].CC1=CC(C)=C(S([O-])(=O)=O)C(C)=C1NC(C=1C(=O)C2=CC=CC=C2C(=O)C=11)=CC=C1NC1=C(C)C=C(C)C(S([O-])(=O)=O)=C1C UHXQPQCJDDSMCB-UHFFFAOYSA-L 0.000 description 8
- 239000002243 precursor Substances 0.000 description 8
- 229940088598 enzyme Drugs 0.000 description 7
- 229920000742 Cotton Polymers 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 239000011149 active material Substances 0.000 description 6
- 229910052783 alkali metal Inorganic materials 0.000 description 6
- 229910000323 aluminium silicate Inorganic materials 0.000 description 6
- 239000001257 hydrogen Substances 0.000 description 6
- 229910052739 hydrogen Inorganic materials 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 125000000217 alkyl group Chemical group 0.000 description 5
- 125000000129 anionic group Chemical group 0.000 description 5
- 239000003752 hydrotrope Substances 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 4
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- 229910019142 PO4 Inorganic materials 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 150000001298 alcohols Chemical class 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 4
- 239000010452 phosphate Substances 0.000 description 4
- 239000001509 sodium citrate Substances 0.000 description 4
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 4
- QUCDWLYKDRVKMI-UHFFFAOYSA-M sodium;3,4-dimethylbenzenesulfonate Chemical compound [Na+].CC1=CC=C(S([O-])(=O)=O)C=C1C QUCDWLYKDRVKMI-UHFFFAOYSA-M 0.000 description 4
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical compound [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 3
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 3
- 239000003945 anionic surfactant Substances 0.000 description 3
- 239000000987 azo dye Substances 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- LLSDKQJKOVVTOJ-UHFFFAOYSA-L calcium chloride dihydrate Chemical compound O.O.[Cl-].[Cl-].[Ca+2] LLSDKQJKOVVTOJ-UHFFFAOYSA-L 0.000 description 3
- 238000004140 cleaning Methods 0.000 description 3
- 125000000664 diazo group Chemical group [N-]=[N+]=[*] 0.000 description 3
- 238000004090 dissolution Methods 0.000 description 3
- 239000007850 fluorescent dye Substances 0.000 description 3
- 238000005342 ion exchange Methods 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 239000011236 particulate material Substances 0.000 description 3
- 125000000864 peroxy group Chemical group O(O*)* 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000011591 potassium Substances 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 150000004760 silicates Chemical class 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 229920002126 Acrylic acid copolymer Polymers 0.000 description 2
- 108010065511 Amylases Proteins 0.000 description 2
- 102000013142 Amylases Human genes 0.000 description 2
- 108010084185 Cellulases Proteins 0.000 description 2
- 102000005575 Cellulases Human genes 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 108090001060 Lipase Proteins 0.000 description 2
- 102000004882 Lipase Human genes 0.000 description 2
- 239000004367 Lipase Substances 0.000 description 2
- 229910000503 Na-aluminosilicate Inorganic materials 0.000 description 2
- 108090000854 Oxidoreductases Proteins 0.000 description 2
- 102000004316 Oxidoreductases Human genes 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- KFSLWBXXFJQRDL-UHFFFAOYSA-N Peracetic acid Chemical compound CC(=O)OO KFSLWBXXFJQRDL-UHFFFAOYSA-N 0.000 description 2
- 108010081873 Persil Proteins 0.000 description 2
- 241000199919 Phaeophyceae Species 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 235000019418 amylase Nutrition 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 235000019864 coconut oil Nutrition 0.000 description 2
- 239000003240 coconut oil Substances 0.000 description 2
- 239000008139 complexing agent Substances 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- VTIIJXUACCWYHX-UHFFFAOYSA-L disodium;carboxylatooxy carbonate Chemical compound [Na+].[Na+].[O-]C(=O)OOC([O-])=O VTIIJXUACCWYHX-UHFFFAOYSA-L 0.000 description 2
- 229960001484 edetic acid Drugs 0.000 description 2
- 238000005538 encapsulation Methods 0.000 description 2
- 229940071087 ethylenediamine disuccinate Drugs 0.000 description 2
- 229940071106 ethylenediaminetetraacetate Drugs 0.000 description 2
- 239000003205 fragrance Substances 0.000 description 2
- 150000002431 hydrogen Chemical class 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- CDOSHBSSFJOMGT-UHFFFAOYSA-N linalool Chemical compound CC(C)=CCCC(C)(O)C=C CDOSHBSSFJOMGT-UHFFFAOYSA-N 0.000 description 2
- UWKAYLJWKGQEPM-LBPRGKRZSA-N linalyl acetate Chemical compound CC(C)=CCC[C@](C)(C=C)OC(C)=O UWKAYLJWKGQEPM-LBPRGKRZSA-N 0.000 description 2
- 235000019421 lipase Nutrition 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 239000002736 nonionic surfactant Substances 0.000 description 2
- 239000000049 pigment Substances 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 230000001376 precipitating effect Effects 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- CZCBTSFUTPZVKJ-UHFFFAOYSA-N rose oxide Chemical compound CC1CCOC(C=C(C)C)C1 CZCBTSFUTPZVKJ-UHFFFAOYSA-N 0.000 description 2
- 235000012217 sodium aluminium silicate Nutrition 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L sodium carbonate Substances [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 229940045872 sodium percarbonate Drugs 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000003760 tallow Substances 0.000 description 2
- 239000001490 (3R)-3,7-dimethylocta-1,6-dien-3-ol Substances 0.000 description 1
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 1
- CDOSHBSSFJOMGT-JTQLQIEISA-N (R)-linalool Natural products CC(C)=CCC[C@@](C)(O)C=C CDOSHBSSFJOMGT-JTQLQIEISA-N 0.000 description 1
- KZYAYVSWIPZDKL-UHFFFAOYSA-N 1,4-diamino-2,3-dichloroanthracene-9,10-dione Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C(N)=C(Cl)C(Cl)=C2N KZYAYVSWIPZDKL-UHFFFAOYSA-N 0.000 description 1
- ZMLPKJYZRQZLDA-UHFFFAOYSA-N 1-(2-phenylethenyl)-4-[4-(2-phenylethenyl)phenyl]benzene Chemical group C=1C=CC=CC=1C=CC(C=C1)=CC=C1C(C=C1)=CC=C1C=CC1=CC=CC=C1 ZMLPKJYZRQZLDA-UHFFFAOYSA-N 0.000 description 1
- ZNQIAQXHADXXQI-UHFFFAOYSA-N 1-anilino-4-hydroxyanthracene-9,10-dione Chemical compound C1=2C(=O)C3=CC=CC=C3C(=O)C=2C(O)=CC=C1NC1=CC=CC=C1 ZNQIAQXHADXXQI-UHFFFAOYSA-N 0.000 description 1
- MPJQXAIKMSKXBI-UHFFFAOYSA-N 2,7,9,14-tetraoxa-1,8-diazabicyclo[6.6.2]hexadecane-3,6,10,13-tetrone Chemical compound C1CN2OC(=O)CCC(=O)ON1OC(=O)CCC(=O)O2 MPJQXAIKMSKXBI-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- CULIYQPRUGMRRT-UHFFFAOYSA-N 2-chloro-n-[2-[(2-cyano-4-nitrophenyl)diazenyl]-5-(diethylamino)phenyl]acetamide Chemical compound ClCC(=O)NC1=CC(N(CC)CC)=CC=C1N=NC1=CC=C([N+]([O-])=O)C=C1C#N CULIYQPRUGMRRT-UHFFFAOYSA-N 0.000 description 1
- JBVOQKNLGSOPNZ-UHFFFAOYSA-N 2-propan-2-ylbenzenesulfonic acid Chemical compound CC(C)C1=CC=CC=C1S(O)(=O)=O JBVOQKNLGSOPNZ-UHFFFAOYSA-N 0.000 description 1
- UWOFGIXNNCPENM-UHFFFAOYSA-N 3,3-difluoropentan-2-one Chemical compound CCC(F)(F)C(C)=O UWOFGIXNNCPENM-UHFFFAOYSA-N 0.000 description 1
- YGUMVDWOQQJBGA-VAWYXSNFSA-N 5-[(4-anilino-6-morpholin-4-yl-1,3,5-triazin-2-yl)amino]-2-[(e)-2-[4-[(4-anilino-6-morpholin-4-yl-1,3,5-triazin-2-yl)amino]-2-sulfophenyl]ethenyl]benzenesulfonic acid Chemical compound C=1C=C(\C=C\C=2C(=CC(NC=3N=C(N=C(NC=4C=CC=CC=4)N=3)N3CCOCC3)=CC=2)S(O)(=O)=O)C(S(=O)(=O)O)=CC=1NC(N=C(N=1)N2CCOCC2)=NC=1NC1=CC=CC=C1 YGUMVDWOQQJBGA-VAWYXSNFSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 239000004382 Amylase Substances 0.000 description 1
- 102100032487 Beta-mannosidase Human genes 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical group [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 108010023736 Chondroitinases and Chondroitin Lyases Proteins 0.000 description 1
- 102000011413 Chondroitinases and Chondroitin Lyases Human genes 0.000 description 1
- 235000019499 Citrus oil Nutrition 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 101710121765 Endo-1,4-beta-xylanase Proteins 0.000 description 1
- 108090000371 Esterases Proteins 0.000 description 1
- 108010003272 Hyaluronate lyase Proteins 0.000 description 1
- 102000001974 Hyaluronidases Human genes 0.000 description 1
- IAJILQKETJEXLJ-SQOUGZDYSA-N L-guluronic acid Chemical compound O=C[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O IAJILQKETJEXLJ-SQOUGZDYSA-N 0.000 description 1
- 108010029541 Laccase Proteins 0.000 description 1
- 241000296380 Laminaria hyperborea Species 0.000 description 1
- 244000178870 Lavandula angustifolia Species 0.000 description 1
- 235000010663 Lavandula angustifolia Nutrition 0.000 description 1
- QPCDCPDFJACHGM-UHFFFAOYSA-N N,N-bis{2-[bis(carboxymethyl)amino]ethyl}glycine Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CC(O)=O QPCDCPDFJACHGM-UHFFFAOYSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical class CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- MQNVHUZWFZKETG-UHFFFAOYSA-N P1(OCCCCCO1)=O.NCCNCCN Chemical compound P1(OCCCCCO1)=O.NCCNCCN MQNVHUZWFZKETG-UHFFFAOYSA-N 0.000 description 1
- WFRXSOIFNFJAFL-UHFFFAOYSA-N P1(OCCCCO1)=O.C(CN)N Chemical compound P1(OCCCCO1)=O.C(CN)N WFRXSOIFNFJAFL-UHFFFAOYSA-N 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- SCKXCAADGDQQCS-UHFFFAOYSA-N Performic acid Chemical compound OOC=O SCKXCAADGDQQCS-UHFFFAOYSA-N 0.000 description 1
- 108700020962 Peroxidase Proteins 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- 108010064785 Phospholipases Proteins 0.000 description 1
- 102000015439 Phospholipases Human genes 0.000 description 1
- 108010059820 Polygalacturonase Proteins 0.000 description 1
- 229920000388 Polyphosphate Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 1
- 108091007187 Reductases Proteins 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 239000004115 Sodium Silicate Substances 0.000 description 1
- BGRWYDHXPHLNKA-UHFFFAOYSA-N Tetraacetylethylenediamine Chemical compound CC(=O)N(C(C)=O)CCN(C(C)=O)C(C)=O BGRWYDHXPHLNKA-UHFFFAOYSA-N 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 102000003425 Tyrosinase Human genes 0.000 description 1
- 108060008724 Tyrosinase Proteins 0.000 description 1
- ZZXDRXVIRVJQBT-UHFFFAOYSA-M Xylenesulfonate Chemical compound CC1=CC=CC(S([O-])(=O)=O)=C1C ZZXDRXVIRVJQBT-UHFFFAOYSA-M 0.000 description 1
- 238000005299 abrasion Methods 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- GTZCVFVGUGFEME-UHFFFAOYSA-N aconitic acid Chemical compound OC(=O)CC(C(O)=O)=CC(O)=O GTZCVFVGUGFEME-UHFFFAOYSA-N 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000004996 alkyl benzenes Chemical class 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 108090000637 alpha-Amylases Proteins 0.000 description 1
- 108010084650 alpha-N-arabinofuranosidase Proteins 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- ANBBXQWFNXMHLD-UHFFFAOYSA-N aluminum;sodium;oxygen(2-) Chemical compound [O-2].[O-2].[Na+].[Al+3] ANBBXQWFNXMHLD-UHFFFAOYSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 229940025131 amylases Drugs 0.000 description 1
- 239000001000 anthraquinone dye Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- JXLHNMVSKXFWAO-UHFFFAOYSA-N azane;7-fluoro-2,1,3-benzoxadiazole-4-sulfonic acid Chemical compound N.OS(=O)(=O)C1=CC=C(F)C2=NON=C12 JXLHNMVSKXFWAO-UHFFFAOYSA-N 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical class [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical class OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 108010055059 beta-Mannosidase Proteins 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 238000004061 bleaching Methods 0.000 description 1
- 239000001045 blue dye Substances 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- MMCOUVMKNAHQOY-UHFFFAOYSA-N carbonoperoxoic acid Chemical compound OOC(O)=O MMCOUVMKNAHQOY-UHFFFAOYSA-N 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 231100000315 carcinogenic Toxicity 0.000 description 1
- 239000000679 carrageenan Substances 0.000 description 1
- 235000010418 carrageenan Nutrition 0.000 description 1
- 229920001525 carrageenan Polymers 0.000 description 1
- 229940113118 carrageenan Drugs 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000010500 citrus oil Substances 0.000 description 1
- 229910052681 coesite Inorganic materials 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000007859 condensation product Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 229910052593 corundum Inorganic materials 0.000 description 1
- 229910052906 cristobalite Inorganic materials 0.000 description 1
- 108010005400 cutinase Proteins 0.000 description 1
- 230000000249 desinfective effect Effects 0.000 description 1
- XSNQECSCDATQEL-UHFFFAOYSA-N dihydromyrcenol Chemical compound C=CC(C)CCCC(C)(C)O XSNQECSCDATQEL-UHFFFAOYSA-N 0.000 description 1
- 229930008394 dihydromyrcenol Natural products 0.000 description 1
- 239000001177 diphosphate Substances 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- NJPXFJXCALXJCX-UHFFFAOYSA-L disodium 7-anilino-3-[[4-[(2,4-dimethyl-6-sulfonatophenyl)diazenyl]-2,5-dimethylphenyl]diazenyl]-4-hydroxynaphthalene-2-sulfonate Chemical compound [Na+].[Na+].Cc1cc(C)c(N=Nc2cc(C)c(cc2C)N=Nc2c(O)c3ccc(Nc4ccccc4)cc3cc2S([O-])(=O)=O)c(c1)S([O-])(=O)=O NJPXFJXCALXJCX-UHFFFAOYSA-L 0.000 description 1
- LARMRMCFZNGNNX-UHFFFAOYSA-L disodium 7-anilino-3-[[4-[(2,4-dimethyl-6-sulfonatophenyl)diazenyl]-2-methoxy-5-methylphenyl]diazenyl]-4-hydroxynaphthalene-2-sulfonate Chemical compound [Na+].[Na+].COc1cc(N=Nc2c(C)cc(C)cc2S([O-])(=O)=O)c(C)cc1N=Nc1c(O)c2ccc(Nc3ccccc3)cc2cc1S([O-])(=O)=O LARMRMCFZNGNNX-UHFFFAOYSA-L 0.000 description 1
- VUJGKADZTYCLIL-YHPRVSEPSA-L disodium;5-[(4-anilino-6-morpholin-4-yl-1,3,5-triazin-2-yl)amino]-2-[(e)-2-[4-[(4-anilino-6-morpholin-4-yl-1,3,5-triazin-2-yl)amino]-2-sulfonatophenyl]ethenyl]benzenesulfonate Chemical compound [Na+].[Na+].C=1C=C(\C=C\C=2C(=CC(NC=3N=C(N=C(NC=4C=CC=CC=4)N=3)N3CCOCC3)=CC=2)S([O-])(=O)=O)C(S(=O)(=O)[O-])=CC=1NC(N=C(N=1)N2CCOCC2)=NC=1NC1=CC=CC=C1 VUJGKADZTYCLIL-YHPRVSEPSA-L 0.000 description 1
- 239000000986 disperse dye Substances 0.000 description 1
- GMSCBRSQMRDRCD-UHFFFAOYSA-N dodecyl 2-methylprop-2-enoate Chemical compound CCCCCCCCCCCCOC(=O)C(C)=C GMSCBRSQMRDRCD-UHFFFAOYSA-N 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 108010093305 exopolygalacturonase Proteins 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 108010002430 hemicellulase Proteins 0.000 description 1
- 229960002773 hyaluronidase Drugs 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 150000004966 inorganic peroxy acids Chemical class 0.000 description 1
- 108010059345 keratinase Proteins 0.000 description 1
- 239000001102 lavandula vera Substances 0.000 description 1
- 235000018219 lavender Nutrition 0.000 description 1
- 108010062085 ligninase Proteins 0.000 description 1
- 229930007744 linalool Natural products 0.000 description 1
- UWKAYLJWKGQEPM-UHFFFAOYSA-N linalool acetate Natural products CC(C)=CCCC(C)(C=C)OC(C)=O UWKAYLJWKGQEPM-UHFFFAOYSA-N 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- NYGZLYXAPMMJTE-UHFFFAOYSA-M metanil yellow Chemical group [Na+].[O-]S(=O)(=O)C1=CC=CC(N=NC=2C=CC(NC=3C=CC=CC=3)=CC=2)=C1 NYGZLYXAPMMJTE-UHFFFAOYSA-M 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- WKGHJBXTMFXUNA-UHFFFAOYSA-N n,n,n',n'-tetrahexadecylethane-1,2-diamine Chemical compound CCCCCCCCCCCCCCCCN(CCCCCCCCCCCCCCCC)CCN(CCCCCCCCCCCCCCCC)CCCCCCCCCCCCCCCC WKGHJBXTMFXUNA-UHFFFAOYSA-N 0.000 description 1
- DMMDCPMHDXAIRV-UHFFFAOYSA-N n-[5-[bis(2-methoxyethyl)amino]-2-[(2-cyano-4-nitrophenyl)diazenyl]phenyl]acetamide Chemical compound CC(=O)NC1=CC(N(CCOC)CCOC)=CC=C1N=NC1=CC=C([N+]([O-])=O)C=C1C#N DMMDCPMHDXAIRV-UHFFFAOYSA-N 0.000 description 1
- ZOCHHNOQQHDWHG-UHFFFAOYSA-N n-hexan-3-ol Natural products CCCC(O)CC ZOCHHNOQQHDWHG-UHFFFAOYSA-N 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- MGFYIUFZLHCRTH-UHFFFAOYSA-N nitrilotriacetic acid Chemical compound OC(=O)CN(CC(O)=O)CC(O)=O MGFYIUFZLHCRTH-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 150000004967 organic peroxy acids Chemical class 0.000 description 1
- 108010087558 pectate lyase Proteins 0.000 description 1
- 229960003330 pentetic acid Drugs 0.000 description 1
- 230000008447 perception Effects 0.000 description 1
- XCRBXWCUXJNEFX-UHFFFAOYSA-N peroxybenzoic acid Chemical compound OOC(=O)C1=CC=CC=C1 XCRBXWCUXJNEFX-UHFFFAOYSA-N 0.000 description 1
- 125000005342 perphosphate group Chemical group 0.000 description 1
- 235000020030 perry Nutrition 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L persulfate group Chemical group S(=O)(=O)([O-])OOS(=O)(=O)[O-] JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- NIXKBAZVOQAHGC-UHFFFAOYSA-N phenylmethanesulfonic acid Chemical class OS(=O)(=O)CC1=CC=CC=C1 NIXKBAZVOQAHGC-UHFFFAOYSA-N 0.000 description 1
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229920000196 poly(lauryl methacrylate) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920005646 polycarboxylate Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000001205 polyphosphate Substances 0.000 description 1
- 235000011176 polyphosphates Nutrition 0.000 description 1
- 150000004804 polysaccharides Chemical class 0.000 description 1
- 229920005996 polystyrene-poly(ethylene-butylene)-polystyrene Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 150000003138 primary alcohols Chemical class 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 150000003219 pyrazolines Chemical class 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 230000001850 reproductive effect Effects 0.000 description 1
- 229930007790 rose oxide Natural products 0.000 description 1
- 238000005185 salting out Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 229940071207 sesquicarbonate Drugs 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 229910001388 sodium aluminate Inorganic materials 0.000 description 1
- 239000000429 sodium aluminium silicate Substances 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 description 1
- 229910052911 sodium silicate Inorganic materials 0.000 description 1
- 235000019832 sodium triphosphate Nutrition 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000992 solvent dye Substances 0.000 description 1
- LJFWQNJLLOFIJK-UHFFFAOYSA-N solvent violet 13 Chemical compound C1=CC(C)=CC=C1NC1=CC=C(O)C2=C1C(=O)C1=CC=CC=C1C2=O LJFWQNJLLOFIJK-UHFFFAOYSA-N 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 229910052682 stishovite Inorganic materials 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 125000001273 sulfonato group Chemical group [O-]S(*)(=O)=O 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 229910021653 sulphate ion Inorganic materials 0.000 description 1
- 108010038851 tannase Proteins 0.000 description 1
- 229910052905 tridymite Inorganic materials 0.000 description 1
- 239000001226 triphosphate Substances 0.000 description 1
- 235000011178 triphosphate Nutrition 0.000 description 1
- UNXRWKVEANCORM-UHFFFAOYSA-N triphosphoric acid Chemical compound OP(O)(=O)OP(O)(=O)OP(O)(O)=O UNXRWKVEANCORM-UHFFFAOYSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 239000008096 xylene Chemical class 0.000 description 1
- 229940071104 xylenesulfonate Drugs 0.000 description 1
- 229910001845 yogo sapphire Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/20—Organic compounds containing oxygen
- C11D3/2075—Carboxylic acids-salts thereof
- C11D3/2086—Hydroxy carboxylic acids-salts thereof
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/0039—Coated compositions or coated components in the compositions, (micro)capsules
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/20—Organic compounds containing oxygen
- C11D3/22—Carbohydrates or derivatives thereof
- C11D3/222—Natural or synthetic polysaccharides, e.g. cellulose, starch, gum, alginic acid or cyclodextrin
Definitions
- This invention relates to an alginate granule. More particularly, the invention is directed to an alginate granule comprising citric acid. The invention further relates to laundry detergent compositions comprising the alginate granules of the invention, to a process to make the alginate granules and to the use of said granules to deliver benefit agents to the fabric.
- Encapsulation or immobilisation of active materials is a well known technique which can offer advantages such as the stabilisation/protection of active materials that are otherwise unstable or reactive.
- Alginates are known as encapsulation materials.
- WO 00/46337 Quest International B.V.
- This document relates to a liquid detergent composition containing greater than 5% by weight of surfactant and an encapsulate containing greater than 10% by weight of active material and a cross-linked anionic gum.
- the anionic gum can be an alginate, and the active material can be a fragrance.
- WO 2007/009621 discloses fabric softening particles which incorporate a fabric softening emulsion into an alginate or carrageenan polysaccharide matrix. These particles do not contain any sequestrant. The alginate particles leave residues cn fabric due to the reliance on sequestrants present in detergent formulations to aid dissolution of the softening particle.
- WO 2004/074422 A discloses a sprayable, acidic, hard surface cleaning and/or disinfecting composition which contains suspended inclusions which appear as visibly discernible, discrete particulate materials, preferably where said discrete particulate materials are based on alginates which are present as two or more classes of particulate materials.
- US 5, 334, 229 discloses alginate gel beads useful for encapsulating plant reproductive units, and a method for production thereof.
- alginate encapsulates/beads A problem that exists with such alginate encapsulates/beads is that they leave visible residues on laundered clothes. Such visible residues are not only problematic for consumers, but are also indicative that the alginate matrix has not released the encapsulated benefit agent or other active material.
- citric acid in the granulation process provides alginate granules that exhibit a reduced level of visible residues in laundry use.
- the present invention provides an alginate granule comprising:
- a second aspect of the invention provides a laundry detergent composition comprising from 0.1 to 25 wt.% of the alginate granule of the first aspect, from 2 to 70 wt.% of a surfactant, and from 1 to 70 wt.% of a builder.
- a third aspect of the invention provides the use of the alginate granule of first aspect, to deliver a fabric benefit agent to a textile during the laundering process.
- a fourth aspect of the invention provides a process for making the alginate granules of the first aspect, wherein the process includes the steps of:-
- the alginate granules preferably have a size range of from 0.05 to 10mm. More preferably the particle size is between 0.1 and 2mm. The granule size can be measured for example using graded sieves.
- the alginate granule comprises:
- Alginate is the general name for alginic acid and its salts. Alginates are linear polysaccharides made up from ⁇ -1,4 linked D-mannuronate (M) residues and its C-5 epimer, ⁇ -1,4 linked L-guluronate (G) residues. The alginates have a block polymeric arrangement of these M and G residues along the linear chain. The arrangement of these blocks can be described as being blocks of repeating M residues, repeating G residues, or alternating M and G residues.
- the ratio of mannuronate to guluronate residues present in the alginate is well known in the art as the M:G ratio.
- the M:G ratio of the alginate can vary due to the source or growth conditions of the alginate.
- One common alginate source is brown seaweed (Phaeophyceae).
- the M:G ratio of the alginate used in the present invention is preferably from 0.1:1 to less than 1:1, for example 0.1:1 to 0.99:1. This means that the alginates used herein preferably contain a greater number of G residues than M residues.
- the M:G ratio is more preferably 0.1:1 to 0.8:1, even more preferably from 0.2:1 to 0.8:1.
- Certain embodiments of the alginate granules of the present invention may comprise alginate having an M:G ratio of from 0.25 to 0.75. Suitable sources for these alginates are those obtained from the fronds and stipes of Laminaria hyperborea.
- the alginate granules comprise preferably alginate with a M:G as defined above. More preferably all of the alginate present in the granule has the aforementioned M:G ratios.
- the molecular weight of the alginate can be between 1,000 to 3,000,000 Daltons.
- the alginate is used in the form of a sodium salt.
- Suitable alginates with the preferred M:G ratio are available under the "Manugel” trade name from International Speciality Products, for example “Manugel GMB”; “Protonal” from FMC Biopolymer; and, “Satialgine”, “Cecalgum” and “Algogel” from Texturant Systems.
- the alginate is present in the granule at a level of from 30 to 80 wt.%.
- the cationic species form the gelled cross-linked matrix with the alginate.
- the cationic species is a divalent or polyvalent metal cation.
- the cationic species forms a gelled network with alginate.
- the cationic species is a calcium salt (e.g. calcium chloride).
- the cationic species is present in the granule at a level of fron 10 to 30 wt.%.
- Citric acid as used herein incorporates the free acid itself as well as its various anionic forms.
- the citric acid is incorporated in the alginate granule as the free acid.
- the citric acid is present in the granule at a level of from 5 to 30 wt.%.
- the fabric benefit agent is selected from the group consisting of: chlorine/oxygen scavengers, antioxidants, non-calcium binding sequestrants, perfumes, antimicrobial agents, antibacterial agents, antifungal agents, lubricants, UV absorbers, shading dyes, fluorescent whitening agents, dispersants, anti-redeposition agents, soil release agents, enzymes (for removing fuzz or pills or preventing staining), dye transfer inhibitors, dye binders, dye fixers, softeners, or crystal growth inhibitors.
- the fabric benefit agent may also be a mixture of two or more of the aforementioned benefit agents.
- the fabric benefit agent is selected from the group consisting of: mild reducing agents, non-calcium binding sequestrants, perfumes, fluorescent whitening agents, shading dyes, antimicrobial agents or mixtures thereof.
- the inclusion level of the fabric benefit agent(s) in the granules is dependant on the amount that is required to achieve the benefit required, the release profile of the agent(s) and the calcium level. Typical ranges of fabric benefit agents in the alginate granule are from 0.001 to 60wt.% of the granule.
- the inclusion level can preferably be between 0.001% to 20wt.% of the granule.
- a suitable process for making the alginate granules of the invention includes the steps of:-
- citric acid is also present in the second solution.
- the solution can use any suitable solvent. Water is preferred.
- the alginate granule is suitably delivered to the fabric via incorporation into laundry detergent composition.
- Suitable laundry detergent compositions comprise from 0.1 to 25 wt.% of the alginate granule and from 2 to 70 wt.% of a surfactant and from 1 to 70 wt.% of a builder.
- the alginate granules are present in the laundry detergent composition at a level of from 0.1 to 25 wt.%, preferably from 0.5 to 10 wt.%.
- the laundry treatment composition may take the form of an isotropic liquid, a surfactant-structured liquid, a granular, spray-dried or dry-blended powder, a tablet, a paste, a molded solid or any other laundry detergent form known to those skilled in the art.
- the composition is preferably a liquid or granular laundry composition, most preferably a granular laundry composition.
- Preferred laundry detergent composition forms which are particularly suitable in combination with the alginate granules of the invention are granular, spray-dried or dry-blended powder compositions.
- the laundry detergent composition comprises between 2 to 70 wt.% of a surfactant, most preferably 10 to 30 wt.%.
- a surfactant most preferably 10 to 30 wt.%.
- the nonionic and anionic surfactants of the surfactant system may be chosen from the surfactants described " Surface Active Agents" Vol. 1, by Schwartz & Perry, Interscience 1949 , Vol. 2 by Schwartz, Perry & Berch, Interscience 1958 , in the current edition of "McCutcheon's Emulsifiers and Detergents” published by Manufacturing Confectioners Company or in " Tenside-Taschenbuch", H. Stache, 2nd Edn., Carl Hauser Verlag, 1981 .
- the surfactants used are saturated.
- Suitable nonionic detergent compounds which may be used include, in particular, the reaction products of compounds having a hydrophobic group and a reactive hydrogen atom, for example, aliphatic alcohols, acids, amides or alkyl phenols with alkylene oxides, especially ethylene oxide either alone or with propylene oxide.
- Specific nonionic detergent compounds are C 6 to C 22 alkyl phenol-ethylene oxide condensates, generally 5 to 25 EO, i.e. 5 to 25 units of ethylene oxide per molecule, and the condensation products of aliphatic C 8 to C 18 primary or secondary linear or branched alcohols with ethylene oxide, generally 5 to 40 EO.
- Suitable anionic detergent compounds which may be used are usually water-soluble alkali metal salts of organic sulphates and sulphonates having alkyl radicals containing from about 8 to about 22 carbon atoms, the term alkyl being used to include the alkyl portion of higher acyl radicals.
- suitable synthetic anionic detergent compounds are sodium and potassium alkyl sulphates, especially those obtained by sulphating higher C 8 to C 18 alcohols, produced for example from tallow or coconut oil, sodium and potassium alkyl C 9 to C 20 benzene sulphonates, particularly sodium linear secondary alkyl C 10 to C 15 benzene sulphonates; and sodium alkyl glyceryl ether sulphates, especially those ethers of the higher alcohols derived from tallow or coconut oil and synthetic alcohols derived from petroleum.
- the preferred anionic detergent compounds are sodium C 11 to C 15 alkyl benzene sulphonates and sodium C 12 to C 18 alkyl sulphates.
- surfactants such as those described in EP-A-328 177 (Unilever), which show resistance to salting-out, the alkyl polyglycoside surfactants described in EP-A-070 074 , and alkyl monoglycosides.
- Preferred surfactant systems are mixtures of anionic with nonionic detergent active materials, in particular the groups and examples of anionic and nonionic surfactants pointed out in EP-A-346 995 (Unilever).
- surfactant system that is a mixture of an alkali metal salt of a C 16 to C 18 primary alcohol sulphate together with a C 12 to C 15 primary alcohol 3 to 7 EO ethoxylate.
- the nonionic detergent is preferably present in amounts greater than 10%, e.g. 25 to 90 wt.% of the surfactant system.
- Anionic surfactants can be present for example in amounts in the range from about 5 wt.% to about 40 wt.% of the surfactant system.
- the laundry detergent composition may comprise from 1 to 70 wt.% of a builder.
- the level of builder is preferably from 1 to 40 wt.%.
- Builder materials may be selected from 1) calcium sequestrant materials, 2) precipitating materials, 3) calcium ion-exchange materials and 4) mixtures thereof.
- the size is in the range 0.1 to 10 microns (as measured by The Mastersizer 2000 particle size analyzer using laser diffraction ex MalvernTM).
- calcium sequestrant builder materials examples include alkali metal polyphosphates, such as sodium tripolyphosphate and organic sequestrants, such as ethylene diamine tetraacetic acid.
- precipitating builder materials examples include sodium orthophosphate and sodium carbonate.
- Examples of calcium ion-exchange builder materials include the various types of water-insoluble crystalline or amorphous aluminosilicates, of which zeolites are the best known representatives, e.g. zeolite A, zeolite B (also known as zeolite P), zeolite C, zeolite X, zeolite Y and also the zeolite P-type as described in EP-A-0,384,070 .
- zeolites are the best known representatives, e.g. zeolite A, zeolite B (also known as zeolite P), zeolite C, zeolite X, zeolite Y and also the zeolite P-type as described in EP-A-0,384,070 .
- the composition may also contain 0-50 wt.% of a builder or complexing agent such as ethylenediaminetetraacetic acid, diethylenetriamine-pentaacetic acid, alkyl- or alkenylsuccinic acid, nitrilotriacetic acid or the other builders mentioned below.
- a builder or complexing agent such as ethylenediaminetetraacetic acid, diethylenetriamine-pentaacetic acid, alkyl- or alkenylsuccinic acid, nitrilotriacetic acid or the other builders mentioned below.
- Many builders are also bleach-stabilising agents by virtue of their ability to complex metal ions.
- Zeolite and carbonate are preferred builders.
- the composition may contain as builder a crystalline aluminosilicate, preferably an alkali metal aluminosilicate, more preferably a sodium aluminosilicate. This is typically present at a level of less than 15 wt.%.
- Aluminosilicates are materials having the general formula: 0.8-1.5 M 2 O. Al 2 O 3 . 0.8-6 SiO 2 where M is a monovalent cation, preferably sodium. These materials contain some bound water and are required to have a calcium ion exchange capacity of at least 50 mg CaO/g.
- the preferred sodium aluminosilicates contain 1.5-3.5 SiO 2 units in the formula above. They can be prepared readily by reaction between sodium silicate and sodium aluminate, as amply described in the literature.
- the ratio of surfactants to aluminosilicate (where present) is preferably greater than 5:2, more preferably greater than 3:1.
- phosphate builders may be used.
- 'phosphate' embraces diphosphate, triphosphate, and phosphonate species.
- Other forms of builder include silicates, such as soluble silicates, metasilicates, layered silicates (e.g. SKS-6 from Hoechst).
- the laundry detergent formulation is a non-phosphate built laundry detergent formulation, i.e., contains less than 1 wt.% of phosphate.
- the laundry detergent composition preferably comprises a blue or violet shading agent in the range from 0.0001 to 0.01 wt.%.
- the shading agents reduce the perception of damage to many coloured garments and increase whiteness of white garments.
- the shading agents are preferably selected from blue and violet dyes of the solvent disperse basic, direct and acid type listed in the colour index ( Society of Dyers and Colourists and American Association of Textile Chemists and Colorists 2002 ).
- a direct violet or direct blue dyes is present.
- the dyes are bis-azo, tris-azo dyes or triphendioxazine dye.
- the carcinogenic benzidene based dyes are not preferred.
- Bis-azo copper containing dyes such as direct violet 66 may be used.
- the most preferred bis-azo dyes have the following structure: or wherein:
- Preferred bis-azo dyes are direct violet 7, direct violet 9, direct violet 11, direct violet 26, direct violet 31, direct violet 35, direct violet 40, direct violet 41, direct violet 51, and direct violet 99.
- Preferred solvent and disperse dyes are selected from, mono-azo or anthraquinone dyes, most preferably, solvent violet 13, disperse violet 27 disperse violet 26, disperse violet 28, disperse violet 63 and disperse violet 77.
- a preferred pigment is pigment violet 23.
- the laundry detergent composition preferably comprises one or more enzymes which provide cleaning performance and/or fabric care benefits.
- suitable enzymes include, but are not limited to, hemicellulases, peroxidases, proteases, cellulases, xylanases, lipases, phospholipases, esterases, cutinases, pectinases, mannanases, pectate lyases, keratinases, reductases, oxidases, phenoloxidases,-lipoxygenases, ligninases, pullulanases, tannases, pentosanases, malanases, arabinosidases, hyaluronidase, chondroitinase, laccase, and amylases, or mixtures thereof.
- a typical combination is an enzyme cocktail that may comprise, for example, a protease and lipase in conjunction with amylase.
- the aforementioned additional enzymes may be present at levels from about 0.00001 wt.% to about 2 wt.%, from about 0.0001 wt.% to about 1 wt.% or even from about 0.001 wt.% to about 0.5 wt.% enzyme protein by weight of the composition.
- Preferred enzymes are cellulases.
- the laundry detergent composition preferably comprises a fluorescent agent (optical brightener).
- fluorescent agents are well known and many such fluorescent agents are available commercially. Usually, these fluorescent agents are supplied and used in the form of their alkali metal salts, for example, the sodium salts.
- the total amount of the fluorescent agent or agents used in the composition is generally from 0.005 to 2 wt.%, more preferably 0.01 to 0.1 wt.%.
- Preferred classes of fluorescer are: Di-styryl biphenyl compounds, e.g. Tinopal (Trade Mark) CBS-X, Diamine stilbene di-sulphonic acid compounds, e.g.
- Preferred fluorescers are: sodium 2-(4-styryl-3-sulfophenyl)-2H-napthol[1,2-d]trazole, disodium 4,4'-bis ⁇ [(4-anilino-6-(N methyl-N-2 hydroxyethyl) amino 1,3,5-triazin-2-yl)]amino ⁇ stilbene-2-2' disulfonate, disodium 4,4'-bis ⁇ [(4-anilino-6-morpholino-1,3,5-triazin-2-yl)]amino ⁇ stilbene-2-2' disulfonate, and disodium 4,4'-bis(2-sulfoslyryl)biphenyl.
- the laundry detergent composition comprises a perfume.
- the perfume is preferably in the range from 0.001 to 3 wt.%, most preferably 0.1 to 1 wt.%.
- CTFA Cosmetic, Toiletry and Fragrance Association
- Many suitable examples of perfumes are provided in the CTFA (Cosmetic, Toiletry and Fragrance Association) 1992 International Buyers Guide, published by CFTA Publications and OPD 1993 Chemicals Buyers Directory 80th Annual Edition, published by Schnell Publishing Co.
- compositions of the present invention it is envisaged that there will be four or more, preferably five or more, more preferably six or more or even seven or more different perfume components.
- top notes are defined by Poucher (Journal of the Society of Cosmetic Chemists 6(2):80 [1955 ]).
- Preferred top-notes are selected from citrus oils, linalool, linalyl acetate, lavender, dihydromyrcenol, rose oxide and cis-3-hexanol.
- Perfume and top note may be used to cue the whiteness benefit of the invention.
- the laundry detergent composition may comprise one or more polymers.
- Examples are carboxymethylcellulose, poly(ethylene glycol), poly(vinyl alcohol), polycarboxylates such as polyacrylates, maleic/acrylic acid copolymers and lauryl methacrylate/acrylic acid copolymers.
- compositions in the form of a liquid it is useful to include a hydrotrope, which prevents liquid crystal formation.
- Suitable hydrotropes include but are not limited to propylene glycol, ethanol, urea, salts of benzene sulphonate, toluene sulphonate, xylene sulphonate or cumene sulphonate.
- Suitable salts include but are not limited to sodium, potassium, ammonium, monoethanolamine, triethanolamine.
- the hydrotrope is selected from the group consisting of propylene glycol, xylene sulfonate, ethanol, and urea to provide optimum performance.
- the amount of the hydrotrope is generally in the range of from 0 to 30%, preferably from 0.5 to 30%, more preferably from 0.5 to 30%, most preferably from 1 to 15%.
- the laundry detergent compositions may also suitably contain a bleach system. If bleach is present, then it is preferred that the compositions of the invention contain peroxy bleach compounds capable of yielding hydrogen peroxide in aqueous solution, for example inorganic or organic peroxyacids, and inorganic persalts such as the alkali metal perborates, percarbonates, perphosphates, persilicates and persulphates. Bleach ingredients are generally post-dosed as powders.
- the peroxy bleach compound for example sodium percarbonate
- the peroxy bleach compound is suitably present in an amount of from 5 to 35 wt.%, preferably from 10 to 25 wt.%.
- the peroxy bleach compound for example sodium percarbonate
- the bleach precursor is suitably present in an amount of from 1 to 8 wt.%, preferably from 2 to 5 wt.%.
- Preferred bleach precursors are peroxycarboxylic acid precursors, more especially peracetic acid precursors and peroxybenzoic acid precursors; and peroxycarbonic acid precursors.
- An especially preferred bleach precursor suitable for use in the present invention is N, N, N', N'-tetracetyl ethylenediamine (TAED).
- a bleach stabiliser may also be present.
- Suitable bleach stabilisers include ethylenediamine tetraacetate (EDTA), ethylenediamine disuccinate (EDDS), and the aminopolyphosphonates such as ethylenediamine tetramethylene phosphonate (EDTMP) and diethylenetriamine pentamethylene phosphonate (DETPMP).
- the granules were then removed from the hardening bath using a 1mm sieve and oven dried at 60°C to constant weight.
- the benefit agent release profile of the three batches of the alginate granules was then measured by placing 1g of granules into 500ml demineralised water adjusted to either pH 4, 7 or 10. The solution was pumped thorough a 10mm quartz flowcell mounted in a Hewlett-Packard 8453 diode array Uv/Vis Spectrophotometer ® . The release of Acid Blue 80 was measured by absorption at 629nm over a period of 90 minutes at room temperature.
- Granule dissolution in a washing machine was assessed using the "black sachet" test, which reproduces the conditions experienced by a granule if it becomes caught in a pocket and is thus suffers less mechanical abrasion than if it was mobile inside the drum.
- 1g of the various alginate granules was placed in between two pieces of black woven cotton and all edges overlocked, thus preventing the alginate granules from escaping.
- the sachet was then attached to a 100x50cm panel of woven cotton sheeting to prevent it becoming lodged in the door seal of the washing machine.
- the panel was then placed in a washing machine along with 800g woven cotton sheeting, 800g of knitted cotton and 800g of 65:35 woven cotton:polyester.
- the particles of batch 1 are considered a fair representation of the disclosure of WO 2007/009621 , in that they contain alginate, benefit agent and calcium.
- the particles of batches 2 and 3 are according to the invention, and show the benefit of adding citric acid as part of the actual particle, as opposed to relying on sequestrant present in the detergent compositions to aid dissolution of the particle.
- the alginate granules that contained citric acid as part of the granule itself exhibited significantly improved performance in that there were reduced or no residues after washing. This the technical advantage in terms of reduced residues for the incorporation of citric acid in a granule according to the invention as opposed to the prior art disclosures.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Molecular Biology (AREA)
- Detergent Compositions (AREA)
Description
- This invention relates to an alginate granule. More particularly, the invention is directed to an alginate granule comprising citric acid. The invention further relates to laundry detergent compositions comprising the alginate granules of the invention, to a process to make the alginate granules and to the use of said granules to deliver benefit agents to the fabric.
- Encapsulation or immobilisation of active materials is a well known technique which can offer advantages such as the stabilisation/protection of active materials that are otherwise unstable or reactive. Alginates are known as encapsulation materials.
- Our co-pending application
WO 2008/083877 relates to gelled alginate beads comprising alginate with a M:G ratio of from 0.1:1 to less than 1:1; one or more cationic species and one or more benefit agents. These are used to slowly release a benefit agent. - Another example of encapsulate use of alginate can be found in
WO 00/46337 -
WO 2007/009621 discloses fabric softening particles which incorporate a fabric softening emulsion into an alginate or carrageenan polysaccharide matrix. These particles do not contain any sequestrant. The alginate particles leave residues cn fabric due to the reliance on sequestrants present in detergent formulations to aid dissolution of the softening particle. -
WO 2004/074422 A discloses a sprayable, acidic, hard surface cleaning and/or disinfecting composition which contains suspended inclusions which appear as visibly discernible, discrete particulate materials, preferably where said discrete particulate materials are based on alginates which are present as two or more classes of particulate materials. -
US 5, 334, 229 discloses alginate gel beads useful for encapsulating plant reproductive units, and a method for production thereof. - A problem that exists with such alginate encapsulates/beads is that they leave visible residues on laundered clothes. Such visible residues are not only problematic for consumers, but are also indicative that the alginate matrix has not released the encapsulated benefit agent or other active material.
- We have found that inclusion of citric acid in the granulation process provides alginate granules that exhibit a reduced level of visible residues in laundry use.
- In one aspect the present invention provides an alginate granule comprising:
- (a) 30-80 wt.% of alginate;
- (b) 10-30 % of one or more cationic species which is a divalent or polyvalent metal cation;
- (c) 5-30 % of citric acid; and
- (d) one or more fabric benefit agents.
- A second aspect of the invention provides a laundry detergent composition comprising from 0.1 to 25 wt.% of the alginate granule of the first aspect, from 2 to 70 wt.% of a surfactant, and from 1 to 70 wt.% of a builder.
- A third aspect of the invention provides the use of the alginate granule of first aspect, to deliver a fabric benefit agent to a textile during the laundering process.
- A fourth aspect of the invention provides a process for making the alginate granules of the first aspect, wherein the process includes the steps of:-
- a) provision of a first solution comprising an admixture of alginate, citric acid, and one or more benefit agents;
- b) forming droplets of the first solution; and,
- c) contacting said droplets with a second solution comprising a cationic species which is a divalent or polyvalent metal cation.
- The amounts of components present in the various compositions quoted herein are given as wt.% of the composition unless otherwise stated.
- Except in the operating and comparative examples, or where otherwise explicitly indicated, all numbers in this description indicating amounts or ratios of material or conditions of reaction, physical properties of materials and/cr use are to be understood as modified by the word "about".
- The alginate granules preferably have a size range of from 0.05 to 10mm. More preferably the particle size is between 0.1 and 2mm. The granule size can be measured for example using graded sieves.
- The alginate granule comprises:
- (a) 30-80 wt.% of alginate;
- (b) 10-30% of one or more cationic species which is a divalent or polyvalent metal cation;
- (c) 5-30% citric acid; and,
- (d) one or more fabric benefit agents.
- "Alginate" is the general name for alginic acid and its salts. Alginates are linear polysaccharides made up from β-1,4 linked D-mannuronate (M) residues and its C-5 epimer, α-1,4 linked L-guluronate (G) residues. The alginates have a block polymeric arrangement of these M and G residues along the linear chain. The arrangement of these blocks can be described as being blocks of repeating M residues, repeating G residues, or alternating M and G residues.
- The ratio of mannuronate to guluronate residues present in the alginate is well known in the art as the M:G ratio. The M:G ratio of the alginate can vary due to the source or growth conditions of the alginate. One common alginate source is brown seaweed (Phaeophyceae).
- The M:G ratio of the alginate used in the present invention is preferably from 0.1:1 to less than 1:1, for example 0.1:1 to 0.99:1. This means that the alginates used herein preferably contain a greater number of G residues than M residues. The M:G ratio is more preferably 0.1:1 to 0.8:1, even more preferably from 0.2:1 to 0.8:1. Certain embodiments of the alginate granules of the present invention may comprise alginate having an M:G ratio of from 0.25 to 0.75. Suitable sources for these alginates are those obtained from the fronds and stipes of Laminaria hyperborea.
- The alginate granules comprise preferably alginate with a M:G as defined above. More preferably all of the alginate present in the granule has the aforementioned M:G ratios.
- Depending on the nature of the benefit agents and the release profile required, the molecular weight of the alginate can be between 1,000 to 3,000,000 Daltons.
- Conveniently, the alginate is used in the form of a sodium salt.
- Suitable alginates with the preferred M:G ratio are available under the "Manugel" trade name from International Speciality Products, for example "Manugel GMB"; "Protonal" from FMC Biopolymer; and, "Satialgine", "Cecalgum" and "Algogel" from Texturant Systems.
- The alginate is present in the granule at a level of from 30 to 80 wt.%.
- The cationic species form the gelled cross-linked matrix with the alginate. The cationic species is a divalent or polyvalent metal cation. The cationic species forms a gelled network with alginate. In a preferred embodiment, the cationic species is a calcium salt (e.g. calcium chloride).
- The cationic species is present in the granule at a level of fron 10 to 30 wt.%.
- Citric acid as used herein incorporates the free acid itself as well as its various anionic forms. Preferably the citric acid is incorporated in the alginate granule as the free acid.
- The citric acid is present in the granule at a level of from 5 to 30 wt.%.
- Preferably the fabric benefit agent is selected from the group consisting of: chlorine/oxygen scavengers, antioxidants, non-calcium binding sequestrants, perfumes, antimicrobial agents, antibacterial agents, antifungal agents, lubricants, UV absorbers, shading dyes, fluorescent whitening agents, dispersants, anti-redeposition agents, soil release agents, enzymes (for removing fuzz or pills or preventing staining), dye transfer inhibitors, dye binders, dye fixers, softeners, or crystal growth inhibitors. The fabric benefit agent may also be a mixture of two or more of the aforementioned benefit agents.
- Most preferably the fabric benefit agent is selected from the group consisting of: mild reducing agents, non-calcium binding sequestrants, perfumes, fluorescent whitening agents, shading dyes, antimicrobial agents or mixtures thereof.
- The inclusion level of the fabric benefit agent(s) in the granules is dependant on the amount that is required to achieve the benefit required, the release profile of the agent(s) and the calcium level. Typical ranges of fabric benefit agents in the alginate granule are from 0.001 to 60wt.% of the granule.
- For certain fabric benefit agents such as perfumes, fluorescent whitening agents cr shading dyes which are effective at low levels, the inclusion level can preferably be between 0.001% to 20wt.% of the granule.
- A suitable process for making the alginate granules of the invention includes the steps of:-
- a) provision of a first solution comprising an admixture of alginate, citric acid, and one or more benefit agents;
- b) forming droplets of the first solution; and,
- c) contacting said droplets with a second solution comprising a cationic species which is a divalent or polyvalent metal cation.
- This is an example of a diffusion setting method and can suitably be carried out at neutral pH.
- In an alternative process, citric acid is also present in the second solution.
- The solution can use any suitable solvent. Water is preferred.
- The alginate granule is suitably delivered to the fabric via incorporation into laundry detergent composition.
- Suitable laundry detergent compositions comprise from 0.1 to 25 wt.% of the alginate granule and from 2 to 70 wt.% of a surfactant and from 1 to 70 wt.% of a builder.
- The alginate granules are present in the laundry detergent composition at a level of from 0.1 to 25 wt.%, preferably from 0.5 to 10 wt.%.
- The laundry treatment composition may take the form of an isotropic liquid, a surfactant-structured liquid, a granular, spray-dried or dry-blended powder, a tablet, a paste, a molded solid or any other laundry detergent form known to those skilled in the art. The composition is preferably a liquid or granular laundry composition, most preferably a granular laundry composition.
- Preferred laundry detergent composition forms which are particularly suitable in combination with the alginate granules of the invention are granular, spray-dried or dry-blended powder compositions.
- The laundry detergent composition comprises between 2 to 70 wt.% of a surfactant, most preferably 10 to 30 wt.%. In general, the nonionic and anionic surfactants of the surfactant system may be chosen from the surfactants described "Surface Active Agents" Vol. 1, by Schwartz & Perry, Interscience 1949, Vol. 2 by Schwartz, Perry & Berch, Interscience 1958, in the current edition of "McCutcheon's Emulsifiers and Detergents" published by Manufacturing Confectioners Company or in "Tenside-Taschenbuch", H. Stache, 2nd Edn., Carl Hauser Verlag, 1981. Preferably the surfactants used are saturated.
- Suitable nonionic detergent compounds which may be used include, in particular, the reaction products of compounds having a hydrophobic group and a reactive hydrogen atom, for example, aliphatic alcohols, acids, amides or alkyl phenols with alkylene oxides, especially ethylene oxide either alone or with propylene oxide. Specific nonionic detergent compounds are C6 to C22 alkyl phenol-ethylene oxide condensates, generally 5 to 25 EO, i.e. 5 to 25 units of ethylene oxide per molecule, and the condensation products of aliphatic C8 to C18 primary or secondary linear or branched alcohols with ethylene oxide, generally 5 to 40 EO.
- Suitable anionic detergent compounds which may be used are usually water-soluble alkali metal salts of organic sulphates and sulphonates having alkyl radicals containing from about 8 to about 22 carbon atoms, the term alkyl being used to include the alkyl portion of higher acyl radicals. Examples of suitable synthetic anionic detergent compounds are sodium and potassium alkyl sulphates, especially those obtained by sulphating higher C8 to C18 alcohols, produced for example from tallow or coconut oil, sodium and potassium alkyl C9 to C20 benzene sulphonates, particularly sodium linear secondary alkyl C10 to C15 benzene sulphonates; and sodium alkyl glyceryl ether sulphates, especially those ethers of the higher alcohols derived from tallow or coconut oil and synthetic alcohols derived from petroleum. The preferred anionic detergent compounds are sodium C11 to C15 alkyl benzene sulphonates and sodium C12 to C18 alkyl sulphates. Also applicable are surfactants such as those described in
EP-A-328 177 EP-A-070 074 - Preferred surfactant systems are mixtures of anionic with nonionic detergent active materials, in particular the groups and examples of anionic and nonionic surfactants pointed out in
EP-A-346 995 - The nonionic detergent is preferably present in amounts greater than 10%, e.g. 25 to 90 wt.% of the surfactant system. Anionic surfactants can be present for example in amounts in the range from about 5 wt.% to about 40 wt.% of the surfactant system.
- The laundry detergent composition may comprise from 1 to 70 wt.% of a builder.
- For laundry compositions in the form of granular, spray-dried or dry-blended powders, the level of builder is preferably from 1 to 40 wt.%.
- Builder materials may be selected from 1) calcium sequestrant materials, 2) precipitating materials, 3) calcium ion-exchange materials and 4) mixtures thereof.
- It is preferred that when an insoluble inorganic builder, e.g., zeolite is used, the size is in the range 0.1 to 10 microns (as measured by The Mastersizer 2000 particle size analyzer using laser diffraction ex Malvern™).
- Examples of calcium sequestrant builder materials include alkali metal polyphosphates, such as sodium tripolyphosphate and organic sequestrants, such as ethylene diamine tetraacetic acid.
- Examples of precipitating builder materials include sodium orthophosphate and sodium carbonate.
- Examples of calcium ion-exchange builder materials include the various types of water-insoluble crystalline or amorphous aluminosilicates, of which zeolites are the best known representatives, e.g. zeolite A, zeolite B (also known as zeolite P), zeolite C, zeolite X, zeolite Y and also the zeolite P-type as described in
EP-A-0,384,070 . - The composition may also contain 0-50 wt.% of a builder or complexing agent such as ethylenediaminetetraacetic acid, diethylenetriamine-pentaacetic acid, alkyl- or alkenylsuccinic acid, nitrilotriacetic acid or the other builders mentioned below. Many builders are also bleach-stabilising agents by virtue of their ability to complex metal ions.
- Zeolite and carbonate (including bicarbonate and sesquicarbonate) are preferred builders.
- The composition may contain as builder a crystalline aluminosilicate, preferably an alkali metal aluminosilicate, more preferably a sodium aluminosilicate. This is typically present at a level of less than 15 wt.%. Aluminosilicates are materials having the general formula:
0.8-1.5 M2O. Al2O3. 0.8-6 SiO2
where M is a monovalent cation, preferably sodium. These materials contain some bound water and are required to have a calcium ion exchange capacity of at least 50 mg CaO/g. The preferred sodium aluminosilicates contain 1.5-3.5 SiO2 units in the formula above. They can be prepared readily by reaction between sodium silicate and sodium aluminate, as amply described in the literature. The ratio of surfactants to aluminosilicate (where present) is preferably greater than 5:2, more preferably greater than 3:1. - Alternatively, or additionally to the aluminosilicate builders, phosphate builders may be used. In this art the term 'phosphate' embraces diphosphate, triphosphate, and phosphonate species. Other forms of builder include silicates, such as soluble silicates, metasilicates, layered silicates (e.g. SKS-6 from Hoechst).
- Preferably the laundry detergent formulation is a non-phosphate built laundry detergent formulation, i.e., contains less than 1 wt.% of phosphate.
- The laundry detergent composition preferably comprises a blue or violet shading agent in the range from 0.0001 to 0.01 wt.%. The shading agents reduce the perception of damage to many coloured garments and increase whiteness of white garments.
- The shading agents are preferably selected from blue and violet dyes of the solvent disperse basic, direct and acid type listed in the colour index (Society of Dyers and Colourists and American Association of Textile Chemists and Colorists 2002).
- Preferably a direct violet or direct blue dyes is present. Preferably the dyes are bis-azo, tris-azo dyes or triphendioxazine dye. The carcinogenic benzidene based dyes are not preferred.
- Bis-azo copper containing dyes such as direct violet 66 may be used.
-
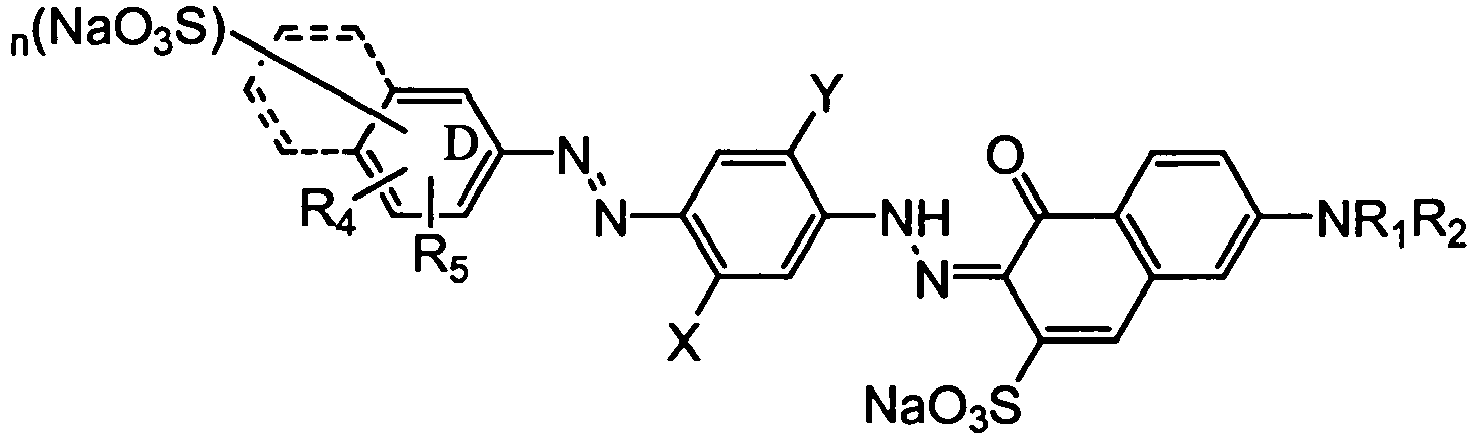
- ring D and E may be independently naphthyl or phenyl as shown;
- R1 is selected from: hydrogen and C1-C4-alkyl, preferably hydrogen;
- R2 is selected from: hydrogen, C1-C4-alkyl, substituted or unsubstituted phenyl and substituted or unsubstituted naphthyl, preferably phenyl;
- R3 and R4 are independently selected from: hydrogen and C1-C4-alkyl, preferably hydrogen or methyl;
- X and Y are independently selected from: hydrogen, C1-C4-alkyl and C1-C4-alkoxy; preferably the dye has X= methyl; and, Y = methoxy and n is 0, 1 or 2, preferably 1 or 2.
- Preferred bis-azo dyes are direct violet 7, direct violet 9, direct violet 11, direct violet 26, direct violet 31, direct violet 35, direct violet 40, direct violet 41, direct violet 51, and direct violet 99.
- Preferred solvent and disperse dyes, are selected from, mono-azo or anthraquinone dyes, most preferably, solvent violet 13, disperse violet 27 disperse violet 26, disperse violet 28, disperse violet 63 and disperse violet 77.
- A preferred pigment is pigment violet 23.
- The laundry detergent composition preferably comprises one or more enzymes which provide cleaning performance and/or fabric care benefits. Examples of suitable enzymes include, but are not limited to, hemicellulases, peroxidases, proteases, cellulases, xylanases, lipases, phospholipases, esterases, cutinases, pectinases, mannanases, pectate lyases, keratinases, reductases, oxidases, phenoloxidases,-lipoxygenases, ligninases, pullulanases, tannases, pentosanases, malanases, arabinosidases, hyaluronidase, chondroitinase, laccase, and amylases, or mixtures thereof. A typical combination is an enzyme cocktail that may comprise, for example, a protease and lipase in conjunction with amylase. When present in a cleaning composition, the aforementioned additional enzymes may be present at levels from about 0.00001 wt.% to about 2 wt.%, from about 0.0001 wt.% to about 1 wt.% or even from about 0.001 wt.% to about 0.5 wt.% enzyme protein by weight of the composition.
- Preferred enzymes are cellulases.
- The laundry detergent composition preferably comprises a fluorescent agent (optical brightener). Fluorescent agents are well known and many such fluorescent agents are available commercially. Usually, these fluorescent agents are supplied and used in the form of their alkali metal salts, for example, the sodium salts. The total amount of the fluorescent agent or agents used in the composition is generally from 0.005 to 2 wt.%, more preferably 0.01 to 0.1 wt.%. Preferred classes of fluorescer are: Di-styryl biphenyl compounds, e.g. Tinopal (Trade Mark) CBS-X, Diamine stilbene di-sulphonic acid compounds, e.g. Tinopal DMS pure Xtra and Blankophor (Trade Mark) HRH, and Pyrazoline compounds, e.g. Blankophor SN. Preferred fluorescers are: sodium 2-(4-styryl-3-sulfophenyl)-2H-napthol[1,2-d]trazole, disodium 4,4'-bis{[(4-anilino-6-(N methyl-N-2 hydroxyethyl) amino 1,3,5-triazin-2-yl)]amino}stilbene-2-2' disulfonate, disodium 4,4'-bis{[(4-anilino-6-morpholino-1,3,5-triazin-2-yl)]amino} stilbene-2-2' disulfonate, and disodium 4,4'-bis(2-sulfoslyryl)biphenyl.
- Preferably the laundry detergent composition comprises a perfume. The perfume is preferably in the range from 0.001 to 3 wt.%, most preferably 0.1 to 1 wt.%. Many suitable examples of perfumes are provided in the CTFA (Cosmetic, Toiletry and Fragrance Association) 1992 International Buyers Guide, published by CFTA Publications and OPD 1993 Chemicals Buyers Directory 80th Annual Edition, published by Schnell Publishing Co.
- It is commonplace for a plurality of perfume components to be present in a formulation. In the compositions of the present invention it is envisaged that there will be four or more, preferably five or more, more preferably six or more or even seven or more different perfume components.
- In perfume mixtures preferably 15 to 25 wt.% are top notes. Top notes are defined by Poucher (Journal of the Society of Cosmetic Chemists 6(2):80 [1955]). Preferred top-notes are selected from citrus oils, linalool, linalyl acetate, lavender, dihydromyrcenol, rose oxide and cis-3-hexanol.
- Perfume and top note may be used to cue the whiteness benefit of the invention.
- The laundry detergent composition may comprise one or more polymers. Examples are carboxymethylcellulose, poly(ethylene glycol), poly(vinyl alcohol), polycarboxylates such as polyacrylates, maleic/acrylic acid copolymers and lauryl methacrylate/acrylic acid copolymers.
- For compositions in the form of a liquid, it is useful to include a hydrotrope, which prevents liquid crystal formation. The addition of the hydrotrope thus aids the clarity/transparency of the composition. Suitable hydrotropes include but are not limited to propylene glycol, ethanol, urea, salts of benzene sulphonate, toluene sulphonate, xylene sulphonate or cumene sulphonate. Suitable salts include but are not limited to sodium, potassium, ammonium, monoethanolamine, triethanolamine. Preferably, the hydrotrope is selected from the group consisting of propylene glycol, xylene sulfonate, ethanol, and urea to provide optimum performance. The amount of the hydrotrope is generally in the range of from 0 to 30%, preferably from 0.5 to 30%, more preferably from 0.5 to 30%, most preferably from 1 to 15%.
- The laundry detergent compositions may also suitably contain a bleach system. If bleach is present, then it is preferred that the compositions of the invention contain peroxy bleach compounds capable of yielding hydrogen peroxide in aqueous solution, for example inorganic or organic peroxyacids, and inorganic persalts such as the alkali metal perborates, percarbonates, perphosphates, persilicates and persulphates. Bleach ingredients are generally post-dosed as powders.
- If present, the peroxy bleach compound, for example sodium percarbonate, is suitably present in an amount of from 5 to 35 wt.%, preferably from 10 to 25 wt.%. The peroxy bleach compound, for example sodium percarbonate, may be used in conjunction with a bleach activator (bleach precursor) to improve bleaching action at low wash temperatures. The bleach precursor is suitably present in an amount of from 1 to 8 wt.%, preferably from 2 to 5 wt.%.
- Preferred bleach precursors are peroxycarboxylic acid precursors, more especially peracetic acid precursors and peroxybenzoic acid precursors; and peroxycarbonic acid precursors. An especially preferred bleach precursor suitable for use in the present invention is N, N, N', N'-tetracetyl ethylenediamine (TAED).
- A bleach stabiliser (heavy metal sequestrant) may also be present. Suitable bleach stabilisers include ethylenediamine tetraacetate (EDTA), ethylenediamine disuccinate (EDDS), and the aminopolyphosphonates such as ethylenediamine tetramethylene phosphonate (EDTMP) and diethylenetriamine pentamethylene phosphonate (DETPMP).
- Three granules were prepared using the diffusion setting process.
-
- Alginate solution - 200ml demineralised water + 4.5g Manugel GMB + 0.1013g Acid Blue 80
- Hardening bath - 500ml demineralised water + 1.75g CaCl2.2H2O + 0.2533g Acid Blue 80
-
- Alginate solution - 200ml demineralised water + 4.5g Manugel GMB + 1g Citric acid + 0.1013g Acid Blue 80
- Hardening bath - 500ml demineralised water + 1.75g CaCl2.2H2O + 2.5g citric acid + 0.2533g Acid Blue 80
-
- Alginate solution - 200ml demineralised water + 4.5g Manugel GMB + 1g Citric acid + 0.1013g Acid Blue 80
- Hardening bath - 500ml demineralised water + 1.75g CaCl2.2H2O + 10.0g citric acid + 0.2533g Acid Blue 80
- The granules were then removed from the hardening bath using a 1mm sieve and oven dried at 60°C to constant weight.
- The benefit agent release profile of the three batches of the alginate granules was then measured by placing 1g of granules into 500ml demineralised water adjusted to either pH 4, 7 or 10. The solution was pumped thorough a 10mm quartz flowcell mounted in a Hewlett-Packard 8453 diode array Uv/Vis Spectrophotometer®. The release of Acid Blue 80 was measured by absorption at 629nm over a period of 90 minutes at room temperature.
Time (mins) Batch 1 pH 7 Batch 2 pH 4 Batch 2 pH 7 Batch 2 pH 10 Batch 3 pH 4 Batch 3 pH 7 Batch 3 pH 10 0 0.000 0.000 0.000 0.000 0.000 0.000 0.000 10 0.134 0.297 0.321 0.469 0.564 0.579 0.829 20 0.206 0.432 0.473 0.718 0.802 0.826 1.141 30 0.248 0.549 0.603 0.937 0.918 0.942 1.311 40 0.276 0.660 0.721 1.252 0.989 1.013 1.399 50 0.302 0.762 0.833 1.456 1.042 1.065 1.444 60 0.312 0.858 0.939 1.629 1.083 1.106 1.468 70 0.325 0.950 1.037 1.772 1.118 1.141 1.481 80 0.336 1.034 1.124 1.815 1.148 1.170 1.486 90 0.345 1.034 1.124 1.815 1.173 1.193 1.491 % released 17.3 51.8 56.2 90.8 58.7 59.7 74.6 - Thus, at pH 10, where the citric acid is converted to sodium citrate, dye release increases significantly compared to pH 4 and pH 7.
- Granule dissolution in a washing machine was assessed using the "black sachet" test, which reproduces the conditions experienced by a granule if it becomes caught in a pocket and is thus suffers less mechanical abrasion than if it was mobile inside the drum.
- 1g of the various alginate granules was placed in between two pieces of black woven cotton and all edges overlocked, thus preventing the alginate granules from escaping. The sachet was then attached to a 100x50cm panel of woven cotton sheeting to prevent it becoming lodged in the door seal of the washing machine. The panel was then placed in a washing machine along with 800g woven cotton sheeting, 800g of knitted cotton and 800g of 65:35 woven cotton:polyester.
- 100g of Persil® Biological washing powder which comprises sodium citrate was placed in the detergent compartment and a 40°C cotton wash cycle carried out (Prenton water, 26°FH). On completion of the wash, the load was removed and tumble dried. The sachet was then opened and the degree of residues assessed on a scale of 1 to 5 (1 = no residues, 5 = high residues).
Batch 1 (no citric acid) Ranking = 5 Batch 2 (low citric acid) Ranking = 2 Batch 3 (high citric acid) Ranking = 1 - As Persil® Biological powder contains sodium citrate, it is clear that inclusion of the sodium citrate (a sequestrant) in the detergent composition is insufficient to stop residues. This confirms the disclosure of
WO 2007/009621 . The particles of batch 1 are considered a fair representation of the disclosure ofWO 2007/009621 , in that they contain alginate, benefit agent and calcium. The particles of batches 2 and 3 are according to the invention, and show the benefit of adding citric acid as part of the actual particle, as opposed to relying on sequestrant present in the detergent compositions to aid dissolution of the particle. - The alginate granules that contained citric acid as part of the granule itself exhibited significantly improved performance in that there were reduced or no residues after washing. This the technical advantage in terms of reduced residues for the incorporation of citric acid in a granule according to the invention as opposed to the prior art disclosures.
Claims (10)
- An alginate granule comprising:(a) 30-80 wt.% of alginate;(b) 10-30% of one or more cationic species which is a divalent or polyvalent metal cation;(c) 5-30% of citric acid; and(d) one or more fabric benefit agents.
- An alginate granule according to claim 1, wherein the alginate has a ratio of mannuronate to guluronate residues (the M:G ratio) of from 0.1:1 to less than 1:1, preferably from 0.1:1 to 0.8:1, more preferably from 0.2:1 to 0.8:1.
- An alginate granule according to claim 1 or claim 2, wherein the cationic species is calcium.
- An alginate granule according to any preceding claim, wherein the fabric benefit is selected from the group consisting of: chlorine/oxygen scavengers, antioxidants, non-calcium binding sequestrants, perfumes, antimicrobial agents, antibacterial agents, antifungal agents, lubricants, UV absorbers, shading dyes, fluorescent whitening agents, dispersants, anti-redeposition agents, soil release agents, enzymes (for removing fuzz or pills or preventing staining), dye transfer inhibitors, dye sequestrants, dye fixers, softeners, crystal growth inhibitors, or mixtures thereof.
- An alginate granule according to claim 4, wherein the fabric benefit is selected from the group consisting of: mild reducing agents, sequestrants, perfumes, fluorescent whitening agents, shading dyes, antimicrobial agents or mixtures thereof.
- An alginate granule according to any preceding claim wherein the fabric benefit agent is water soluble.
- A laundry detergent composition comprising:-(i) from 0.1 to 25 wt.% of the alginate granule of any one of claims 1 to 6,(ii) from 2 to 70 wt.% of a surfactant; and,(iii) from 1 to 70 wt.% of a builder.
- Use of the alginate granule of any one of claims 1 to 6, to deliver a fabric benefit agent to a textile during the laundering process.
- A process for making the granule of any one of claims 1 to 6, comprising the following steps:-a) provision of a first solution comprising an admixture of alginate, citric acid, and one or more benefit agents;b) forming droplets of the first solution; and,c) contacting said droplets with a second solution comprising a cationic species which is a divalent or polyvalent metal cation.
- A process according to claim 9, wherein the alginate has a ratio of mannuronate to guluronate residues (the M:G ratio) of from 0.1:1 to less than 1:1.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP09793904.5A EP2297288B1 (en) | 2008-07-09 | 2009-06-17 | Laundry compositions |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP08160024 | 2008-07-09 | ||
| EP09793904.5A EP2297288B1 (en) | 2008-07-09 | 2009-06-17 | Laundry compositions |
| PCT/EP2009/057537 WO2010003792A1 (en) | 2008-07-09 | 2009-06-17 | Laundry compositions |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2297288A1 EP2297288A1 (en) | 2011-03-23 |
| EP2297288B1 true EP2297288B1 (en) | 2013-05-08 |
Family
ID=39951625
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09793904.5A Not-in-force EP2297288B1 (en) | 2008-07-09 | 2009-06-17 | Laundry compositions |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP2297288B1 (en) |
| CN (1) | CN102083952B (en) |
| BR (1) | BRPI0914211A2 (en) |
| CL (1) | CL2010001323A1 (en) |
| ES (1) | ES2424793T3 (en) |
| WO (1) | WO2010003792A1 (en) |
| ZA (1) | ZA201008049B (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2806062A1 (en) * | 2013-05-13 | 2014-11-26 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| WO2017003025A1 (en) * | 2015-07-01 | 2017-01-05 | 한국생산기술연구원 | Improved method for preparing zeolite-metal chloride hybrid moisture adsorbent and moisture adsorbent prepared by same, and method for preparing moisture adsorbent composition for surface coating comprising same |
| US9624615B2 (en) | 2013-03-15 | 2017-04-18 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US9702074B2 (en) | 2013-03-15 | 2017-07-11 | Whirlpool Corporation | Methods and compositions for treating laundry items |
Families Citing this family (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5944887B2 (en) | 2010-04-26 | 2016-07-05 | ノボザイムス アクティーゼルスカブ | Enzyme granules |
| WO2012175401A2 (en) | 2011-06-20 | 2012-12-27 | Novozymes A/S | Particulate composition |
| WO2012175708A2 (en) | 2011-06-24 | 2012-12-27 | Novozymes A/S | Polypeptides having protease activity and polynucleotides encoding same |
| DK3543333T3 (en) | 2011-06-30 | 2022-02-14 | Novozymes As | METHOD FOR SCREENING ALFA AMYLASES |
| CN107523441A (en) | 2011-07-12 | 2017-12-29 | 诺维信公司 | The enzyme granulate of stable storing |
| US9000138B2 (en) | 2011-08-15 | 2015-04-07 | Novozymes A/S | Expression constructs comprising a Terebella lapidaria nucleic acid encoding a cellulase, host cells, and methods of making the cellulase |
| CN104204200B (en) | 2011-09-22 | 2017-06-09 | 诺维信公司 | Polypeptide and their polynucleotides of coding with proteinase activity |
| JP2015500006A (en) | 2011-11-25 | 2015-01-05 | ノボザイムス アクティーゼルスカブ | Subtilase variant and polynucleotide encoding the same |
| WO2013092635A1 (en) | 2011-12-20 | 2013-06-27 | Novozymes A/S | Subtilase variants and polynucleotides encoding same |
| MX2014008764A (en) | 2012-01-26 | 2014-08-27 | Novozymes As | Use of polypeptides having protease activity in animal feed and detergents. |
| CN104114698A (en) | 2012-02-17 | 2014-10-22 | 诺维信公司 | Subtilisin variants and polynucleotides encoding same |
| WO2013131964A1 (en) | 2012-03-07 | 2013-09-12 | Novozymes A/S | Detergent composition and substitution of optical brighteners in detergent compositions |
| ES2643216T3 (en) | 2012-05-07 | 2017-11-21 | Novozymes A/S | Polypeptides with degradation activity of xanthan and polynucleotides encoding it |
| AU2013279440B2 (en) | 2012-06-20 | 2016-10-06 | Novozymes A/S | Use of polypeptides having protease activity in animal feed and detergents |
| EP2934177B1 (en) | 2012-12-21 | 2017-10-25 | Novozymes A/S | Polypeptides having protease activiy and polynucleotides encoding same |
| EP2941485B1 (en) | 2013-01-03 | 2018-02-21 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| US20160083703A1 (en) | 2013-05-17 | 2016-03-24 | Novozymes A/S | Polypeptides having alpha amylase activity |
| EP3786269A1 (en) | 2013-06-06 | 2021-03-03 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2014207227A1 (en) | 2013-06-27 | 2014-12-31 | Novozymes A/S | Subtilase variants and polynucleotides encoding same |
| WO2014207224A1 (en) | 2013-06-27 | 2014-12-31 | Novozymes A/S | Subtilase variants and polynucleotides encoding same |
| EP3017032A2 (en) | 2013-07-04 | 2016-05-11 | Novozymes A/S | Polypeptides having anti-redeposition effect and polynucleotides encoding same |
| EP3339436B1 (en) | 2013-07-29 | 2021-03-31 | Henkel AG & Co. KGaA | Detergent composition comprising protease variants |
| US9926550B2 (en) | 2013-07-29 | 2018-03-27 | Novozymes A/S | Protease variants and polynucleotides encoding same |
| EP3611260A1 (en) | 2013-07-29 | 2020-02-19 | Novozymes A/S | Protease variants and polynucleotides encoding same |
| US20160177240A1 (en) * | 2013-08-28 | 2016-06-23 | Novozymes A/S | Enzyme Granule with Fluorescent Whitening Agent |
| WO2015049370A1 (en) | 2013-10-03 | 2015-04-09 | Novozymes A/S | Detergent composition and use of detergent composition |
| CN105814200A (en) | 2013-12-20 | 2016-07-27 | 诺维信公司 | Polypeptides having protease activity and polynucleotides encoding same |
| CN106062271A (en) | 2014-03-05 | 2016-10-26 | 诺维信公司 | Compositions and methods for improving properties of cellulosic textile materials with xyloglucan endotransglycosylase |
| US20160348035A1 (en) | 2014-03-05 | 2016-12-01 | Novozymes A/S | Compositions and Methods for Improving Properties of Non-Cellulosic Textile Materials with Xyloglucan Endotransglycosylase |
| CN106103708A (en) | 2014-04-01 | 2016-11-09 | 诺维信公司 | There is the polypeptide of alpha amylase activity |
| CN106414729A (en) | 2014-06-12 | 2017-02-15 | 诺维信公司 | Alpha-amylase variants and polynucleotides encoding same |
| US10550381B2 (en) | 2014-07-04 | 2020-02-04 | Novozymes A/S | Variant proteases and amylases having enhanced storage stability |
| EP3164486B1 (en) | 2014-07-04 | 2020-05-13 | Novozymes A/S | Subtilase variants and polynucleotides encoding same |
| WO2016079305A1 (en) | 2014-11-20 | 2016-05-26 | Novozymes A/S | Alicyclobacillus variants and polynucleotides encoding same |
| US10683491B2 (en) | 2014-12-04 | 2020-06-16 | Novozymes A/S | Subtilase variants and polynucleotides encoding same |
| DK3399031T3 (en) | 2014-12-15 | 2020-01-20 | Henkel Ag & Co Kgaa | DETERGENT COMPOSITION WITH SUBTILASE VARIETIES |
| EP3106508B1 (en) | 2015-06-18 | 2019-11-20 | Henkel AG & Co. KGaA | Detergent composition comprising subtilase variants |
| EP3310912B1 (en) | 2015-06-18 | 2021-01-27 | Novozymes A/S | Subtilase variants and polynucleotides encoding same |
| CN108291212A (en) | 2015-10-14 | 2018-07-17 | 诺维信公司 | Polypeptide variants |
| CN108291215A (en) | 2015-10-14 | 2018-07-17 | 诺维信公司 | Polypeptide with proteinase activity and encode their polynucleotides |
| WO2017207762A1 (en) | 2016-06-03 | 2017-12-07 | Novozymes A/S | Subtilase variants and polynucleotides encoding same |
| EP3485010B1 (en) | 2016-07-13 | 2024-11-06 | The Procter & Gamble Company | Bacillus cibi dnase variants and uses thereof |
| CN117683748A (en) | 2017-10-27 | 2024-03-12 | 宝洁公司 | Detergent compositions comprising polypeptide variants |
| EP3701017A1 (en) | 2017-10-27 | 2020-09-02 | Novozymes A/S | Dnase variants |
| EP3781660A1 (en) | 2018-04-17 | 2021-02-24 | Novozymes A/S | Polypeptides comprising carbohydrate binding activity in detergent compositions and their use in reducing wrinkles in textile or fabric |
| MX2021011287A (en) | 2019-03-21 | 2021-10-13 | Novozymes As | Alpha-amylase variants and polynucleotides encoding same. |
| CN113874499A (en) | 2019-04-10 | 2021-12-31 | 诺维信公司 | Polypeptide variants |
| WO2021037895A1 (en) | 2019-08-27 | 2021-03-04 | Novozymes A/S | Detergent composition |
| WO2021053127A1 (en) | 2019-09-19 | 2021-03-25 | Novozymes A/S | Detergent composition |
| US20220340843A1 (en) | 2019-10-03 | 2022-10-27 | Novozymes A/S | Polypeptides comprising at least two carbohydrate binding domains |
| EP3892708A1 (en) | 2020-04-06 | 2021-10-13 | Henkel AG & Co. KGaA | Cleaning compositions comprising dispersin variants |
| CN116507725A (en) | 2020-10-07 | 2023-07-28 | 诺维信公司 | Alpha-amylase variants |
| EP4291646A2 (en) | 2021-02-12 | 2023-12-20 | Novozymes A/S | Alpha-amylase variants |
| EP4359518A1 (en) | 2021-06-23 | 2024-05-01 | Novozymes A/S | Alpha-amylase polypeptides |
| WO2024131880A2 (en) | 2022-12-23 | 2024-06-27 | Novozymes A/S | Detergent composition comprising catalase and amylase |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03218303A (en) * | 1989-09-26 | 1991-09-25 | Kirin Brewery Co Ltd | Improved alginate gel beads |
| AR043906A1 (en) * | 2003-02-22 | 2005-08-17 | Reckitt Benckiser Inc | CLEANING COMPOSITIONS FOR HARD SURFACES |
| GB0514716D0 (en) * | 2005-07-19 | 2005-08-24 | Unilever Plc | Process to form fabric softening particle,particle obtained and its use |
| ATE474035T1 (en) * | 2007-01-12 | 2010-07-15 | Unilever Nv | LAUNDRY DETERGENT |
-
2009
- 2009-06-17 EP EP09793904.5A patent/EP2297288B1/en not_active Not-in-force
- 2009-06-17 WO PCT/EP2009/057537 patent/WO2010003792A1/en active Application Filing
- 2009-06-17 CN CN200980126208.5A patent/CN102083952B/en not_active Expired - Fee Related
- 2009-06-17 ES ES09793904T patent/ES2424793T3/en active Active
- 2009-06-17 BR BRPI0914211A patent/BRPI0914211A2/en not_active IP Right Cessation
-
2010
- 2010-11-10 ZA ZA2010/08049A patent/ZA201008049B/en unknown
- 2010-11-30 CL CL2010001323A patent/CL2010001323A1/en unknown
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9624615B2 (en) | 2013-03-15 | 2017-04-18 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US9631310B2 (en) | 2013-03-15 | 2017-04-25 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US9644301B2 (en) | 2013-03-15 | 2017-05-09 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US9689101B2 (en) | 2013-03-15 | 2017-06-27 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US9702074B2 (en) | 2013-03-15 | 2017-07-11 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US9758914B2 (en) | 2013-03-15 | 2017-09-12 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US10011935B2 (en) | 2013-03-15 | 2018-07-03 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US10017893B2 (en) | 2013-03-15 | 2018-07-10 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US10072373B2 (en) | 2013-03-15 | 2018-09-11 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| US10266981B2 (en) | 2013-03-15 | 2019-04-23 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| EP2806062A1 (en) * | 2013-05-13 | 2014-11-26 | Whirlpool Corporation | Methods and compositions for treating laundry items |
| WO2017003025A1 (en) * | 2015-07-01 | 2017-01-05 | 한국생산기술연구원 | Improved method for preparing zeolite-metal chloride hybrid moisture adsorbent and moisture adsorbent prepared by same, and method for preparing moisture adsorbent composition for surface coating comprising same |
Also Published As
| Publication number | Publication date |
|---|---|
| ZA201008049B (en) | 2012-02-29 |
| BRPI0914211A2 (en) | 2015-11-03 |
| WO2010003792A1 (en) | 2010-01-14 |
| CL2010001323A1 (en) | 2011-04-08 |
| CN102083952B (en) | 2013-04-10 |
| EP2297288A1 (en) | 2011-03-23 |
| ES2424793T3 (en) | 2013-10-08 |
| CN102083952A (en) | 2011-06-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2297288B1 (en) | Laundry compositions | |
| EP2252680B1 (en) | Laundry treatment composition comprising polymeric lubricants | |
| EP2300589B1 (en) | Shading composition | |
| EP2252678B2 (en) | Laundry treatment compositions | |
| EP3194543B1 (en) | Whitening composition | |
| EP2534206B1 (en) | Dye polymers | |
| EP2294169B1 (en) | Laundry treatment compositions | |
| EP2488622B1 (en) | Dye polymers | |
| EP2534237A1 (en) | Laundry treatment composition comprising bis-azo shading dyes | |
| WO2012098046A1 (en) | Dye polymer for laundry treatment | |
| EP2103677A1 (en) | Laundry treatment compositions | |
| EP2427540B1 (en) | Shading composition | |
| EP2519624B1 (en) | Shading composition | |
| EP2360232A1 (en) | Surfactant ratio in laundry detergents comprising a dye | |
| EP2521764B1 (en) | Detergent formulation containing spray dried granule | |
| EP2252681B2 (en) | Laundry treatment compositions | |
| EP2331670B1 (en) | Cationic isothiazolium dyes | |
| CN108699490B (en) | Whitening composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20101109 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA RS |
|
| DAX | Request for extension of the european patent (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 611109 Country of ref document: AT Kind code of ref document: T Effective date: 20130515 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602009015622 Country of ref document: DE Effective date: 20130704 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2424793 Country of ref document: ES Kind code of ref document: T3 Effective date: 20131008 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 611109 Country of ref document: AT Kind code of ref document: T Effective date: 20130508 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20130508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130808 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130809 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130909 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130908 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130808 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: BE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| 26N | No opposition filed |
Effective date: 20140211 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130630 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130617 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130630 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602009015622 Country of ref document: DE Effective date: 20140211 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20090617 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130617 Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130508 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20160621 Year of fee payment: 8 Ref country code: ES Payment date: 20160614 Year of fee payment: 8 Ref country code: GB Payment date: 20160621 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20160527 Year of fee payment: 8 Ref country code: FR Payment date: 20160627 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20160628 Year of fee payment: 8 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602009015622 Country of ref document: DE |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20170617 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20180228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170617 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170617 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170630 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20181112 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170618 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170617 |