FIELD OF THE INVENTION
-
This invention relates to a silver halide photographic material, and more particularly to a photographic light-sensitive material containing internal latent image type silver halide emulsion(s) having high sensitivity and good graininess and having excellent in reciprocity law characteristics and storage stability.
BACKGROUND OF THE INVENTION
-
For improving both the sensitivity and the image quality of a silver halide photographic material, it is necessary to improve the light absorptivity, quantum yield, and developability of silver halide emulsions.
-
For example, U.S. Patent 3,979,213 (U.S. Patent A) discloses that the inherent desensitization of an internal latent image type silver halide emulsion upon color sensitization is low as compared to a silver halide emulsion of the same grain size wherein, the surface only has been chemically sensitized; therefore, the internal latent image type silver halide emulsion is effectively color-sensitized using a large amount of sensitizing dye(s). However, the internal latent image type silver halide emulsion used in the patent contains the greater part of the radiation sensitive portion (or latent image-forming portion) in the inside of grains, and the ratio of the surface sensitivity to the total sensitivity is less than 10%. When such a silver halide emulsion capable of forming latent images sufficiently inside of grains is developed by a developer for black and white light sensitive materials, color negative photographic materials, or color reversal photographic materials, the development becomes insufficient, showing substantial loss of sensitivity. Furthermore, when a large amount of sensitizing dye is adsorbed on silver halide grains as disclosed in the patent, the development of silver halide grains is further undesirably supressed.
-
Also, in the internal latent image type silver halide emulsions described in Journal of Photographic Science, Vol 13, p.48(1965), ibid., Vol 22, p.174(1974), ibid., Vol. 25, p.19(1977), ibid., Vol 31, p.41(1986), Photographic Science and Engineering, Vol. 19, p.333(1975), U.S. Patents 4,035,185 and 3,850,737, Berichte der Geselshaft fur Physikalishe Chemie, Vol. 67, p.356(1963), the most radiation sensitive portion exists in the silver halide grains at the position deeper than 0.01µm from the surface thereof. Therefore, such silver halide emulsions can not be easily developed by a processing solution practically used and cannot show optimum sensitivity and graininess.
-
Furtheremore, U.S. Patent 3,966,476 discloses a silver halide emulsion which forms latent images in the voids opening to the surface of the grain, and can be developed by a surface developer. However, a silver halide emulsion which shows a sensitivity at least same as that of the surface latent image type emulsion having the same grain size by a surface developer hardly be said as an internal latent image type emulsion, and the excellent color sensitizability of the internal latent image type emulsion described in U.S. Patent A described above cannot be sufficiently realized. Also, in the aforesaid silver halide emulsion, the latent image formed is opened to the surface of the grain and hence the latent image tends to be oxidized during storage of the photographic light-sensitive material containing the emulsion until development processing, thereby substantially reducing the sensitivity.
-
Regarding the production process of silver halide emulsion having a high internal sensitivity, U.S. Patent 3,206,313 describes that the emulsion is prepared by mixing a chemically sensitized large grain silver halide emulsion and a chemically unsensitized fine grain silver halide emulsion and subjecting the mixture to Ostwald ripening. Furthermore, U.S. Patent 3,917,485 describes an emulsion prepared by alternately adding a silver ion and halide ion to chemically sensitized silver halide grains to an excessive extent. By controlling the thickness of the shell of the silver halide grains using the above-described controlling process, the balance of the surface sensitivity and the internal sensitivity can be properly controlled.
-
However, the state of the depth most largely forming latent images in silver halide grains has not yet been clarified, and reagrding processing solutions for practical use, it has never been investigated that what kind of latent image distribution from the surface to the inside of silver halide grains is preferred for obtaining high sensitivity and good image quality.
SUMMARY OF THE INVENTION
-
The object of this invention is, therefore, to provide a photographic light-sensitive material containing internal latent image type silver halide emulsion(s) excellent in sensitivity, graininess, reciprocity law characteristics, and storage stability.
-
As a result of extensive investigations for developing silver halide emulsions satisfying the aforesaid demand, the inventors have discovered that the above-described object can be attained by the present invention as described below.
-
That is, according to this invention, a silver halide photographic material is provided comprising a support having thereon at least one silver halide emulsion layer composed of one or more layers, wherein the silver halide emulsion contained in at least one layer of the silver halide photographic emulsion layer is chemically sensitized so that silver halide grains in the emulsion have at least one maximum value of the distribution of the latent image number in the inside of the silver halide grains and the position (mode) where the maximum value exists is at a depth of less than 0.01 µm from the surface of the silver halide grains, and the emulsion also has a latent image number of at least 1/5 of the largest maximum value but less than the largest maximum value on the surface of the grains.
-
In this case, the latent image distribution is shown by the depth (x µm) of a latent image from the grain surface as an abcissa value and a latent image number (y) as an ordinate value; x is determined by the formula
wherein;
S : Mean diameter (µm) of silver halide grains;
Ag₁ : Amount of remaining silver after processing (described below) a sample coated with an unexposed silver halide emulsion; and
Ag₀ : Amount of coated silver before processing;
and y is the reciprocal of the exposure amount providing a density of fog + 0.2 in the case of applying the following processing to a silver halide emulsion after exposing the emulsion to white light, for 1/100 second.
-
A processing solution composition for obtaining the above-described latent image distribution is as follows.
-
The processing solution is prepared by adding from 0 to 10 g/liter of anhydrous sodium sulfite to a solution having the above-described composition, and silver halide emulsions are processed by such processing solution for 5 minutes at 25°C.
-
In this case, by changing the amount of anhydrous sodium sulfite from 0 to 10 g/liter, the depth of latent images formed in silver halide grains developed during processing from the surface of the grain changes and thus the change of the latent image number in the depth direction can be known.
BRIEF DESCRIPTION OF THE DRAWING
-
- Fig. 1 is a graph showing the latent image distribution, i.e., the relation of the latent image number (y) as ordinate and the depth (x µm) of the latent images as abcissa.
DETAILED DESCRIPTION OF THE INVENTION
-
The invention is explained in further detail below.
-
When the maximum value of the latent image distribution obtained as described above exists at a position in the silver halide grains deeper than 0.01 µm from the surface of the grain, in the case of developing silver halide emulsions by a developer which is practically used for black and white photographic materials, color negative photographic materials, or color reversal photographic materials, the development becomes insufficient and the sensitivity is substantially reduced. The preferable depth is from 0.002 to 0.008 µm, and more preferable depth is from 0.003 to 0.007 µm.
-
According to processes for preparing internal latent image type silver halide emulsions, which have been reported up to this time, the thickness of the shell of silver halide grains is controlled, which results in changing the ratio of the surface sensitivity and the internal sensitivity. However, as a result of the inventors' investigations, it has now been found that for obtaining the optimum sensitivity for processing, it is necessary to independently control the mode of latent image distribution and the ratio of the surface sensitivity to the internal sensitivity by controlling the conditions for forming silver halide grains.
-
For example, even when the mode of latent image distribution is in a position of less than 0.01 µm from the surface of silver halide grain, the effect for the color sensitization of internal latent image type silver halide emulsions as described in U.S. Patent A becomes insufficient if the latent image number at the surface thereof is more than the maximum value. When the latent image distribution at the surface is less than 1/5 of the maximum value, the development using a developer practically used becomes insufficient and the substantial sensitivity is deteriorated. The preferable range of the latent image number is from 0.3 to 0.9 times the maximum value.
-
On the other hand, it has been clarified that a conventional design standard for internal latent image type silver halide grains aiming at only the difference between the sensitivity of applying surface development and the sensitivity of applying internal development is insufficient for attaining the optimum sensitivity.
-
That is, even if the ratio of the surface sensitivity and the internal sensitivity satisfies the conditions of the present invention (e.g., the surface sensitivity is 1/2 of the internal sensitivity), when the maximum value of the latent image distribution exists at a position deeper than 0.01 µm from the surface of the grain, the development by a practical processing becomes insufficient and the potential and the most proper maximum sensitivity of the grains cannot be obtained.
-
As described above, it has been clarified that for obtaining the optimum sensitivity, internal latent image type silver halide grains must be designed by considering both the position of the maximum value of the latent image distribution and the proportion of the maximum value to the latent image number on the surface of the grains.
-
A practical processing solution in this invention is not a developer removing therefrom a silver halide solvent for aiming at the development of surface latent image only and a developer containing a large amount of silver halide solvent for aiming at the development of internal latent images.
-
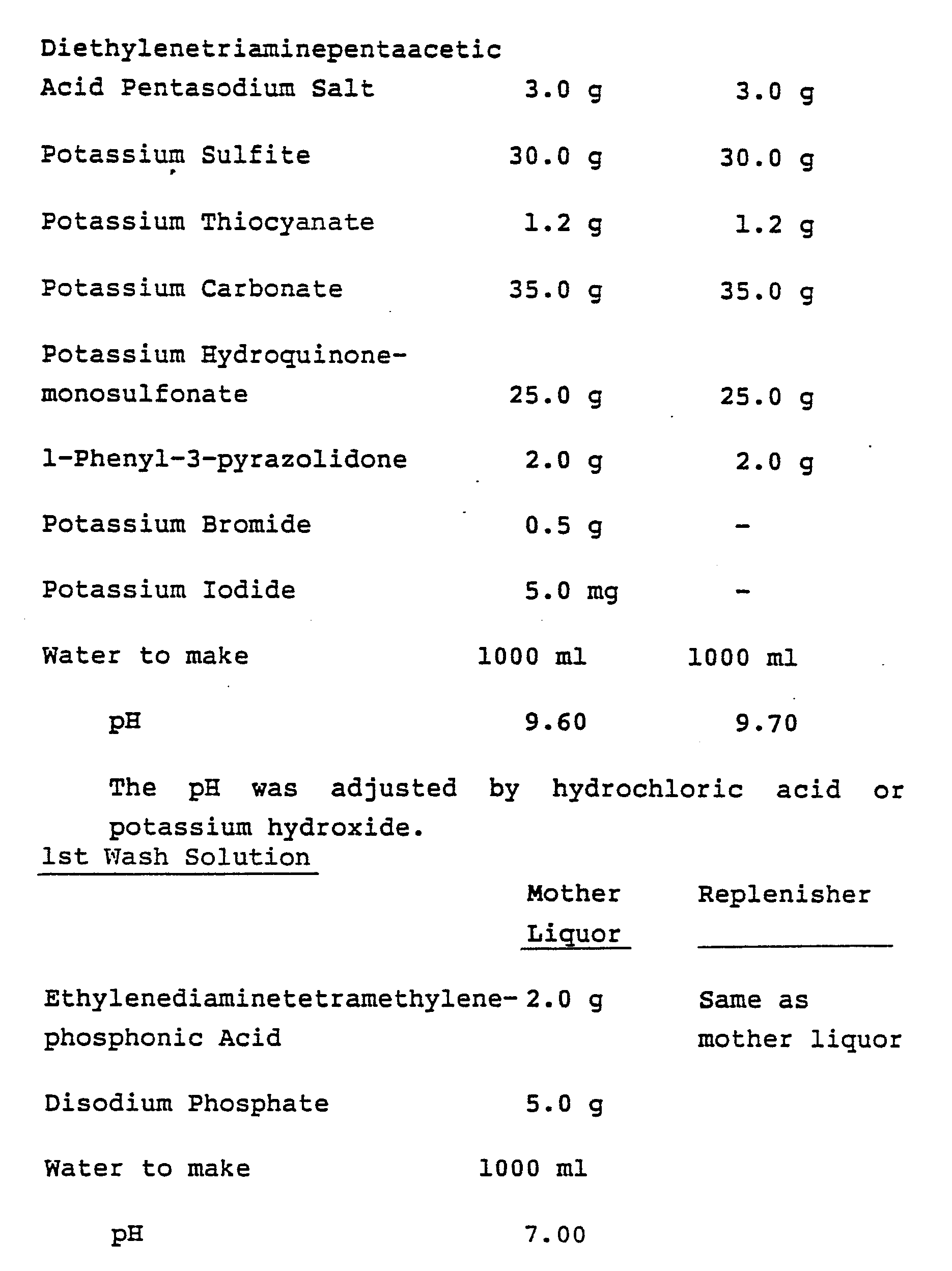
By the former developer, the optimum sensitivity of the internal latent image type silver halide emulsion of this invention cannot be obtained. In the latter case, the dissolution of silver halide during processing proceeds too fast or the grain form is deteriorated by an infectious development. Practically, it is preferred that the developer in this invention contains from about 10 to 100 mg/ℓ of potassium iodide or from about 1 to 100 g/liter of sodium sulfite or potassium sulfite as silver halide solvent. Furthermore, the developer can contain potassium thiocyanate, etc., as silver halide solvent preferably in an amount of from about 10 mg/ℓ to 10 g/ℓ.
-
The silver halide emulsion for use in this invention can be color sensitized by a method well-known in the art.
-
The amount of the color sensitizer(s) shall be the amount for obtaining the maximum minus blue sensitivity but the amount is almost same as the amount for obtaining the maximum minus blue sensitivity in a surface latent image type silver halide emulsion and the addition of a too much amount of dye than the aforesaid amount is undesirable since the development of silver halide grains is suppressed.
-
The silver halide emulsion in this invention can be used without applying color sensitivitizer. In this case, the color sensitizing effect described in U.S. patent A cannot be expected but the effect for improving reciprocity law characteristics and storage stability is obtained.
-
For the silver halide photographic emulsions for use in this invention a silver halide such as silver bromide, silver iodobromide, silver iodochloro-bromide, silver chlorobromide, and silver chloride can be used.
-
A preferred silver halide is silver iodobromide or silver iodochloro-bromide containing from 0 to about 30 mol% silver iodide. A particularly preferred silver halide is silver iodobromide containing from about 0.5 mol% to about 15 mol% silver iodide.
-
The maximum value of the silver iodide distribution in the silver halide grain may be one or plural. Also, the content of silver iodide at the maximum value portion of the grain is preferably twice, more preferably four times the mean silver iodide content of the whole grain, and most preferably silver iodide itself. The position of the maximum value portion may be at any place in the grain if the place is within the chemically sensitized portion in the grain. The change gradient with respect to the direction from the center of the grain to the surface thereof of the silver iodide composition to the maximum value portion is preferably larger and at the extreme case, the grain may have an epitaxial junction.
-
The crystal structure of the silver halide grain other than the portion forming the miximum value of the silver iodide distribution may be uniform, may be composed of a halogen composition of different characteristics, or may have a layer structure.
-
These silver halide grains are, for example, disclosed in British Patent 1,027,146, U.S. Patents 3,505,068, 4,444,877, and Japanese Patent Application No. 248469/83, etc.
-
Also, the silver halide grains of this invention may be jointed with a silver halide having a different composition by epitaxial junction or may be jointed with other compound than silver haldie, such as silver rhodanide, lead oxide, etc.
-
These silver halide grains are, for example, disclosed in U.S. Patents, 4,094,684, 4,142,900, 4,459,353, British Patent 2,038,792, U.S. Patents 4,349,622, 4,395,478, 4,433,501, 4,463,087, 3,656,962, 3,852,067, Japanese Patent Application (OPI) No. 162540/84, etc.
-
The silver halide grains for use in this invention may be so-called regular grains having a regular crystal form such as a cube, an octahedron, a tetradecahedron, etc., irregular grains having an irregular crystal form such as a tabular form, a spherical form, etc., grains having a crystal defect such as twining plane, etc., or a composite form thereof. Regular grains are preferred for controlling the latent image distribution. Also, a mixture of various crystal forms can be used.
-
Tablular grains having an aspect ratio of at least 5 are preferably used in this invention.
-
The silver halide grains may be fine grains having a mean diameter based on prejection area of the grains of less than about 0.1 µm, preferably not less than 0.03 µm or large grains of up to about 10 µm, preferabley from 0.05 to 2.0 µm in the diameter of projected area. Also, the silver halide emulsion for use in this invention may be a monodisperse emulsion having a narrow distribution or a polydisperse emulsion having a wide distribution, but a monodisperse emulsion is preferred for improving the graininess of the emulsion.
-
As a monodisperse emulsion, a silver halide emulsion wherein at least 95% by weight thereof is within ± 40% of the mean grain size is preferable. Silver halide emulsions having a mean grain size of from 0.05 µm to 2 µm, wherein at least 95% by weight or number thereof is in the range of ± 20% of the mean grain size can be used in this invention. The production methods for such emulsions are described e.g., in U.S. Patents, 3,574,628 and 3,655,394 and British Patent 1,413,748. Also, the monodisperse silver halide emulsions as described in Japanese Patent Application (OPI) Nos. 39027/76, 83097/76, 137133/78, 48521/79, 99419/79, 37635/83, 49938/83, corresponding to U.S. Patent 4,497,895 etc., can be preferably used in this invention.
-
The monodisperse hexagonal tabular grains as disclosed in Japanese Patent Application No. 299155/86 are especially preferable.
-
The emulsion of this invention can be used for a same emulsion layer with an ordinary so-called "surface latent image type emulsion". Also, the emulsion of this invention and the above-described ordinary emulsion can be separately used for emulsion layers each having a same or different color sensitivity.
-
The silver halide photographic emulsions for use in this invention can be produced by known methods as described, for example, in Research Disclosure, Vol. 176, RD No. 17643 pages 22-23, "I. Emulsion Preparation and Types", (December, 1978) and ibid, Vol. 187, No. 18716, page 648 (November, 1979).
-
That is, the silver halide photographic emulsions for use in this invention can also be prepared using the methods described in Glafkides, Chimie et Physique Photographique, published by Paul Montel, 1967; G.F. Duffin, Photographic Emulsion Chemistry, published by Focal Press, 1966; V.L. Zelikman et al, Making and Coating Photographic Emulsion, published by Focal Press, 1964; etc. Thus, an acid method, a neutral method, an ammonia method, etc., may be used and also as a system for reacting a soluble silver salt and a soluble halide, a single jet method, a double jet method, or a combination thereof may be used. A so-called reverse mixing method of forming silver halide grains in the presence of excess silver ions can be used. As one system of the double jet method, a so-called controlled double jet method of keeping a constant pAg in a liquid phase wherein silver halide grains can also be used. According to this method, a silver halide emulsion containing silver halide grains having a regular crystal form and substantially uniform grain sizes can be obtained.
-
Also, physical ripening of the silver halide emulsions can be performed in the presence of a known silver halide solvent (e.g., ammonia, potassium rhodanate, and the thioethers described in U.S. Patents 3,271,157, Japanese Patent Application (OPI) Nos. 12360/76, 82408/78, 144319/78, 100711/79, etc.).
-
The above-described silver halide emulsion can be obtained by controlling pAg and pH during the formation of the silver halide grains. Details of the method are described, for example, in Photographic Science and Engineering, Vol. 6, 159-165(1962), Journal of Photographic Science, Vol. 12, 242-251(1964), U.S. Patent 3,655,394, and British Patent 1,413,748.
-
Also, tabular grain silver halide having an aspect ratio of at least 5 can be used in this invention. The tabular grain silver halide can be easily prepared by the methods described in Gutoff, Photographic Science and Engineering, Vol. 14, pp. 248-257(1970), U.S. Patents 4,434,226, 4,414,310, 4,433,048, 4,439,520, British Patent 2,112,157, etc. In the case of using the tabular grain silver halide, there are advantages that the covering power is increased, the color sensitizing effect by sensitizing dye is increased, etc.: such matters are described in detail in U.S. Patent 4,434,226 cited above.
-
Details of the structure and the production process of the monodisperse hexagonal tabular grains in this invention are described in Japanese Patent Application No. 299155/86 and they are briefly as follows. That is, the emulsion is a silver halide emulsion composed of a dispersion medium and silver halide grains. Tabular silver halide grains having a hexagonal form in which the ratio of the length of the longest side to the length of the shortest side is not more than 2 and having two parallel planes as the outer surfaces account for at least 70% of the total projected area of the silver halide grains, furthermore, the silver halide grains have a monodisperse property wherein the variation coefficeint [the value obtained by dividing the deviation (standard deviation) of the grain sizes shown by the diameters calculated as circles having the same area as the projected area by the mean grain size thereof] of the grain size distribution of the hexagonal silver halide grains is not more than 20%, the aspect ratio thereof is at least 2.5, and the grain sizes thereof are at least 0.2 µm.
-
The composition of the hexagonal tabular grains may be silver bromide, silver iodobromide, or silver chloroiodobromide. The content of iodide ions may be from 0 to 30 mol% and the crystal structure thereof may be uniform, may differ in halogen composition between the inside and the outer portion of the grain, or may have a layer structure. Also, it is preferred that the grains contain reduction sensitized silver nuclei.
-
The silver halide grains can be produced by applying the steps of nucleus formation, Ostwald ripening, and grain growth and details thereof are described in Japanese Patent Application No. 299155/86.
-
The properties of silver halide grains can be controlled by performing the precipitation formation of silver halide grains in the existence of various compounds. Such compounds may initially exist in a reaction vessel or may be added to the system together with one or more kinds of salts according to an ordinary manner. As described in U.S. Patents 2,448,060, 2,628,167, 3,737,313, and 3,772,031, and Research Disclosure, Vol. 134, No. 13452 (June, 1975), the characteristics of silver halide can be controlled by performing the precipitation and growth of the silver halide in the existence of the compounds of copper, iridium, lead, bismuth, cadmium, zinc (chalcogen compound such as sulfur, selenium, and tellurium), gold, and noble metals belonging to group VII.
-
Also, as described in Japanese Patent Publication No. 1410/63 and Moiser et al, The Journal of Photographic Science, Vol. 25, pp. 19-27(1977), monodisperse hexagonal tabular grains can be subjected to internal reduction sensitization in the precipitation formation step.
-
The monidisperse hexagonal tabular grains for use in this invention can be formed into a core-shell grain emulsion by covering the silver halide grains with shell by a technique well known in the field of the art. Methods for forming the silver salt sheath are described in U.S. Patents 3,367,776, 3,206,313, 3,317,322, 3,917,485, and 4,164,878.
-
Internal latent image type silver halide emulsions can be prepared by utilizing the methods described in U.S. Patents 3,979,213, 3,966,476, 3,206,313, 3,917,485, Japanese Patent Publication Nos. 29405/68, 13259/70, etc., but in any of these methods, for obtaining the silver halide emulsions having a latent image distribution in accordance with this invention, it is necessary to control the manner of chemical sensitization, the amount of silver halide being precipitated on silver halide grains after chemical sensitization, and the conditions for the precipitation.
-
Practically, an internal latent image type emulsion is prepared by a method of re-precipitating silver halide on a surface-chemically-sensitized silver halide emulsion grains by a controlled double jet method in U.S. Patent 3,979,213. However, when a silver halide of the amount practiced in the patent is deposited on the silver halide grains, the ratio of the surface sensitivity to the whole sensitivity becomes less than 1/10. Therefore, for obtaining the latent image distribution of this invention, the amount of silver halide precipitated after chemical sensitization must be less than the amount practiced in above-described U.S. Patent 3,979,213.
-
Also, in U.S. Patent 3,966,476, a method of precipitating silver halide on silver halide emulsion grains after chemical sensitization by a controlled double jet method is practiced. However, when a silver halide is precipitated on silver halide grains after chemical sensitization by the method as practiced in the aforesaid patent, sensitivity specks cannot be laid in the inside of the silver halide grains. Accordingly, the silver halide emulsion practiced in the aforesaid patent has a sensitivity at least 0.02 logE higher than that of the original surface-chemically-sensitized silver halide emulsion. Therefore, for obtaining the latent image distribution of this invention, it is necessary to increase the amount of silver halide to be precipitated onto silver halide grains after chemical sensitization more than the amount of silver halide practiced in U.S. Patent 3,966,647, or to otherwise control the precipitation conditions (e.g., the solubility of silver halide during precipitation and the rates of adding a soluble silver salt and a soluble halide).
-
For increasing the growth speed of tabular grains at the production of the tabular grains of this invention, a method of increasing the addition rates, addition amounts, and addition concentrations of a silver salt solution (e.g., an aqueous solution of silver nitrate) and a halide solution (e.g., an aqueous solution of potassium bromide) is preferably used.
-
These methods are described, for example, in British Patent 1,335,925, U.S. Patents 3,672,900, 3,650,757, and 4,242,445, Japanese Patent Application (OPI) No. 142329/80.
-
A chemical sensitization for the silver halide emulsions can be performed using active gelatin as described in T.H. James, The Theory of the Photographic Process, 4th edition, Macmillan, 1977, pages 67-76 or can be also performed using sulfur, selenium, tellurium, gold, platinum, palladium, iridium, or a combination of these plural sensitizers at pAg of from 5 to 10, pH of from 5 to 8, and temperature of from 30°C to 80°C as described in Research Disclosure, Vol. 120, No. 12008, April, 1974, ibid., Vol. 34, No. 3452, June, 1975, U.S. Patents 2,642,361, 3,297,446, 3,772,031, 3,857,711, 3,901,714, 4,266,018, and 3,904,415. The chemical sensitization is most suitably performed in the existence of a gold compound and a thiocyanate compound, or in the existence of sulfur-containing compounds described in U.S. Patents 3,857,711, 4,266,018, and 4,054,457 or a sulfur-containing compound such as sodium thiosulfate, a thiourea compound, a rhodanine compound, etc. The chemical sensitization can be also performed in the existence of a chemical sensitization assistant. As the chemical sensitization assistant, compounds which are known to prevent the formation of fog during the chemical sensitization and also increase the sensitivity, such as azaindene, azapyridazine, azapyrimidine, etc., are used. Examples of chemical sensitization assistant modifiers are described in U.S. Patents 2,131,038, 3,411,914, and 3,554,757 and in G.F. Duffin, Photographic Emulsion Chemistry, pages 138-143.
-
In addition to or in palce of the chemical sensitization, a reduction sensitization using, for example, hydrogen as described in U.S. Patents 3,891,446 and 3,984,249 or a reduction sensitization using a reducing agent such as stannous chloride, thiourea dioxide, polyamine, etc., as described in U.S. Patents 2,518,698, 2,743,182, and 2,743,183 or by low pAg (e.g., lower than 5) and/or high pH e.g., higher than 8) treatment can be performed. Also, the color sensitizability can be improved by a chemical sensitization method described in U.S. Patents 3,917,485 and 3,966,476.
-
Also, a sensitization method using an oxidizing agent described in U.S. Patent 4,678,745 can be applied.
-
For removing soluble silver salt from the emulsion before and after physical ripening, a noodle washing method, a flocculation-precipitation method, or a ultrafiltration method can be used.
-
The additives which are used for the chemical ripening and spectral sensitization of the silver halide emulsions for use in this invention are described in above-described Research Disclosure, No. 17643 (December, 1978) and ibid., No. 18716 (November, 1979) and the corresponding portions thereof are summarized in the table described hereinafter.
-
Known photographic additives which can be used in this invention are also described in the above-described two Research Dissclosures and the corresponding portions are shown in the same table.
-
In this invention, spectral sensitizing dyes, antifoggants, and stabilizers can exist in any step for the production of the photographic emulsions or in any stage directly before coating the emulsions after the production thereof. Examples of the former case are a step of forming silver halide grains, a step of physical ripening, a step of chemical ripening, etc. That is, spectral sensitizing dyes, antifoggants, and stabilizers can be used for limiting the position of forming chemical sensitized nculei by utilizing other properties thereof than the essential function thereof, such as a strong adsorptive property to emulsions, etc., or can be used for keeping the junction structure of different halogens by stopping excessive halogen conversion at the formation of junction structure grains of different halogen compositions. These techniques are described in U.S. Patents 3,628,960 and 4,225,666.
-
It is particualrly preferred to add partially or wholly the spectral sensitizing dyes, antifoggants, and stabilizers to the silver halide emulsions before adding chemical sensitizers thereto and then adding thereto chemical sensitizers to perform chemical ripening since in this case, the position of forming chemical sensitized ncueli on the silver halide grains is limited to the portions having no sensitizing dyes, antifoggants, and stabilizers adsorbed thereto, whereby the occurence of dispersion of latent images is prevented and the photographic characteristics are improved. In particular, it is very preferred to add the sensitizing dyes, antifoggants, and stabilizers, which selectively adsorb to the (111) plane of silver halide grains, since the chemical sensitized nuclei are formed at the edge portions only of the hexagonal tabular grains.
-
In general, by the selective adsorption of the aforesaid additives to the surface of crystals forming the main surface of the tabular grains, the chemical sensitized nuclei form on the crystal surfaces which are different from each other.
-
Also, it is effective to perform the chemical sensiti zation in the existence of silver halide solvent. As the silver halide solvent, thiocyanates and the solvents described in Japanese Patent Application No. 299155/86 can be used. The concentration of the silver halide solvent is preferably from 10⁻⁵ mol/liter to 10⁻¹ mol/liter.
-
By using one or both of the above-described techniques or by a third technique independent therefrom, a silver salt capable of forming precipitations on the surface of the silver halide grains, such as silver thiocyanate, silver phosphate, silver carbonate, etc., a soluble silver salt such as silver acetate, silver trifluoroacetate, and silver nitrate, and fine silver halide grains capable of applying Ostwald ripening onto the surface of the tabular grains (e.g., silver bromide, silver iodide, and/or silver chloride) can be introduced into the silver halide emulsion for use in this invention directly before the chemical snesitization or during the chemical sensitization of the emulsion. For example, a Lipmann emulsion can be introduced into the emulsion for use in this invention during the step of the chemical sensitization thereof.
-
Additives which are used for the production of silver halide emulsions are described in
Research Disclosure, No. 17643 and
ibid., No. 18716 and the corresponding portions are summaried in the table described below.
-
In this invention, various kinds of color couplers can be used and practical examples thereof are described in the patents described in Paragraph VII-C to G of Research Disclosure (RD), RD No. 17643 (Dec. 1978).
-
Preferred yellow couplers for use in this invention are described, for example, in U.S. Patents 3,933,501, 4,022,620, 4,326,024, 4,401,752, Japanese Patent Publication No. 10739/83, British Patents 1,425,020 and 1,476,760.
-
As magenta couplers for use in this invention, 5-pyrazoline series compounds and pyrazoloazole series compounds are preferred, and such magenta couplers described in U.S. Patents 4,310,619, 4,351,897, European Patent 73,636, U.S. Patents 3,061,432, 3,725,067, Research Disclosure, No. 24220 (June, 1984), ibid., No. 24230 (June, 1984), Japanese Patent Application (OPI) Nos. 33552/85 and 43659/85, with U.S. Patents 4,500,630 and 4,540,654 being particularly preferred.
-
As cyan couplers, there are phenolic couplers and naphtholic couplers, and the cyan couplers described in U.S. Patent 4,052,212, 4,146,396, 4,228,233, 4,296,200, 2,369,929, 2,801,171, 2,772,162, 2,895,826, 3,772,002, 3,758,308, 4,334,011, 4,327,173, West German Patent Application (OLS) No. 3,329,729, European Patent 121,365A, U.S. Patents 3,446,622, 4,333,999, 4,451,559, 4,427,767, European Patent 161,626A, etc., are preferred.
-
Also, colored couplers for correcting unnecessary absorption of colored dyes can be used in this invention and preferred examples of these colored couplers are described in Research Disclosure, RD No. 17643, Paragraph VII-G, (Jan. 1979), U.S. Patent 4,163,670, Japanese Patent Publication No. 39413/82, U.S. Patents 4,004,929 and 4,138,258, and British Patent 1,146,368.
-
Couplers providing colored dyes having proper diffusibility can be also used in this invention and preferred examples of these couplers are described in U.S. Patent 4,366,237, British Patent 2,125,570, European Patent 96,570, and West German Patent Application (OLS) No. 3,234,533.
-
Furthermore, polymerized dye-forming couplers can be also used in this invention, and typical examples of these couplers are described in U.S. Patents 3,451,820, 4,080,211, 4,367,282, and British Patent 2,102,173.
-
Couplers releasing photographically useful residues with coupling are preferably used in this invention.
-
For example, preferred DIR couplers releasing a development inhibitor are described in the patents cited in Paragraph VII-F of above-described RD No. 17643, Japanese Patent Application (OPI) Nos. 151944/82, 154234/82, 184248/85, and U.S. Patent 4,248,962.
-
Also, preferred examples of couplers imagewise releasing nucleating agent or development accelerator upon development are described in British Patents 2,097,140, 2,131,188, Japanese Patent Application (OPI) Nos. 157638/84 and 170840/84.
-
Other couplers which can be used for the photographic light-sensitive materials of this invention are competing couplers as described in U.S. Patent 4,130,427, etc., polyequivalent couplers as described in U.S. Patents 4,283,472, 4,338,393, 4,310,618, etc., DIR redox compound-releasing couplers described in Japanese Patent Application (OPI) No. 185950/85, etc., and couplers releasing a dye which causes recoloring after release, as described in European Patent 173,302A, etc.
-
The couplers for use in this invention can be introduced into the photographic light-sensitive materials by various known dispersing methods.
-
Examples of high-boiling solvents which are used for an oil drop-in-water dispersion method are described in U.S. Patent 2,322,027, etc.
-
A latex dispersion method can be also used in this invention, and practical examples of the dispersion method, the effect of the method, and latex for impregnation are described in U.S. Patent 4,199,363 and West German Patent Application (OLS) Nos. 2,541,274, 2,541,230, etc.
-
Examples of a proper support for use in this invention are, for example, described in Research Disclosure, No. 17643, page 28 (Dec. 1978) and ibid., page 647, right column to page 648, left column.
-
After development and blixing or fixing, the color photographic light-sensitive materials of this invention are usually subjected to wash processing or stabilization processing.
-
For the wash step, a countercurrent wash step having two or more baths are generally used for saving water. A typical examples of the stabilization processing is a multistage countercurrent stabilization process described in Japanese Patent Application (OPI) No. 8543/82, which is used in place of washing step.
-
The present invention can be applied to various color photographic materials, such as general or cine color negative photographic films, color reversal photographic films for slide or television, color photographic papers, color positive photographic films, and color reversal photographic papers. This invention can be also applied to a black and white photographic material utilizing a mixture of three color couplers described in Research Disclosure, No. 17123 (July, 1978), etc.
-
The following examples serve to illustrate this invention in more detail, without limiting, however, the scope of the invention.
Example 1
-
Fifteen kinds of silver iodibromide emulsions (iodine content 3 mol%) shown in Table 1 below were prepared.
-
The Preparation methods for these emulsions are described below.
(1) Preparation of Monodisperse Emulsion Having (100) Crystal Habit
-
A monodisperse silver halide emulsion having (100) crystal habit was prepared by adding an aqueous solution of silver nitrate and an aqueous solution of potassium bromide and potassium iodide to an aqueous gelatin solution kept at 70°C by double jet method while maintaining pBr at 4.5. Then, the core emulsion thus prepared was split into three portions and shells were formed by the following different conditions to form three emulsions each having a final mean grain size of 0.7 µm and a silver iodide content of 3 mol%.
(Emulsion A):
-
To the aforesaid core emulsion were added sodium thiosulate and potassium chloroaurate to perform chemical sensitization. Thereafter, a shell was deposited on the core emulsion under the same conditions as were used for the formation of the core.
(Emulsion B):
-
After depositing shell on the core emulsion under the same conditions as Emulsion A, the chemical sensitization was applied thereto under the same conditions as above.
(Emulsion C):
-
The chemical sensitization and the shell deposition were applied to the aforesaid core emulsion in the same manner as for Emulsion A, and then the same chemical sensitization as above was further applied thereto.
(Emulsion D):
-
By following the same procedure as in the case of forming Emulsion A, except that a core emulsion having a core smaller than the core used for preparing Emulsions A to C, Emulsion D was prepared.
(Emulsion E):
-
By following the same procedure as in the case of forming Emulsion D, except that the deposition of shell was performed under the condition of increasing pBr to 5.0 and increasing the solubility of silver halide, Emulsion E was prepared.
(Emulsion F):
-
By following the same procedure as in the case of forming Emulsion A, except that the shell deposition was performed under the condition of reducing the pBr to 4.0 and reducing the solubility of silver halide, Emulsion F was prepared.
(Emulsion G and I):
-
By following the same manner as the case of forming Emulsion A, Emulsions G and I each having the final mean grain size of 0.3 µm or 0.9 µm, respectively, were prepared.
(Emulsions H, J, and K):
-
By following the same manner as the case of forming Emulsion B, Emulsions H, J, and K each having the final mean grain size of 0.3 µm, 0.9 µm, or 1.1 µm, respectively was prepared.
(2) Preparation of Monodisperse Emulsion Having (111) Crystal Habit
-
By following the same procedures as the cases of forming Emulsions A, B, C, and D except that pBr at the formation of the emulsions was changed to 3.3, octahedral monodisperse emulsions having different crystal habit from that of Emulsions A to D were prepared, and they were denoted as Emulsions L, M, N, and O, respectively.
-
After adding Sensitizing Dye S-1 shown hereinbelow to each emulsion, the emulsion was coated on a support film at a silver amount of 2 µg/m². Then the latent image distribution was determined for each coated sample. The results obtained are shown in Fig. 1.
-
Each film thus obtained was exposed through a blue filter (BPN-42) for 10 sec., 1/100 sec., or 1/100000 sec., or through a minus blue filter (SC-39) for 1/100 sec., and then processed using the processing solutions shown below.
-
The sensitometric results thus obtained are shown in Table 2 below. In this case, the sensitivity is shown as the relative value of the reciprocal of the exposure amount for providing a density of fog + 0.1.
-
From the results shown in Table 2 below, it can be seen that the photographic materials containing the silver halide emulsions having the latent image distribution of this invention are excellent in sensitivity, storage stability, and reciprocity law characteristics as compared to the comparison samples containing silver halide emulsions having other latent image distribution.
-
For example, Emulsion F wherein the depth of position of the maximum value of the latent image distribution is similar to that of the emulsion of this invention, but the latent image number at the surface is less than that of the emulsion of this invention, lower sensitivity occurs, in contrast to the emulsions of this invention. Also, Emulsion E wherein the maximum value of the latent image distribution and the latent image number at the surface are similar to those of the emulsion in this invention but the position of the maximum value is deeper than that of the emulsions in this invention gives lower sensitivity.
-
The composition of the processing solution used in the example was as follows.
Example 2
-
Multilayer color photographic materials (Samples 101 to 104) were prepared by forming layers having the following compositions on each cellulose triacetate film having a subbing layer.
-
Each layer further contained Gelatin Hardening Agent H-3 and surface active agent in addition to the aforesaid components.
-
The compounds used for making the samples are shown later together with the compounds used in Example 3.
-
Each of Samples 101 to 104 thus obtained was wedge exposed to white light and processed as follows.
-
The compositions of the processing solutions used were as follows:
-
The color reversal sensitivity of each sample was measured and the results were compared based on the relative exposure amount of 0.2 larger in density above the minimum density. The results are shown in the following Table 3.
-
As shown in the above table, the silver halide emulsions having the latent image distribution in this invention show excellent reversal sensitivity.
Example 3
-
Multilayer color photographic materials (Samples 201 and 202) were prepared by forming the layers having the following compositions on a cellulose triacetate film having subbing layer.
-
Each layer further contained Gelatin Hardening Agent H-3 and surface active agent.
-
Each of Samples 201 to 202 thus obtained was subjected to uniform exposure to white light of 4800°K, 7 Lux for 1/100 sec. and processed as follows.
-
The compositions of the processing solutions used for the steps were as follows:
-
The relative sensitivities of the green-sensitive emulsion layers of each sample were measured and the results obtained are shown in Table 4 below.
Sample 201: Comparison sample.
Sample 201: Comparison sample.
Sample 202: Sample of this invention.
-
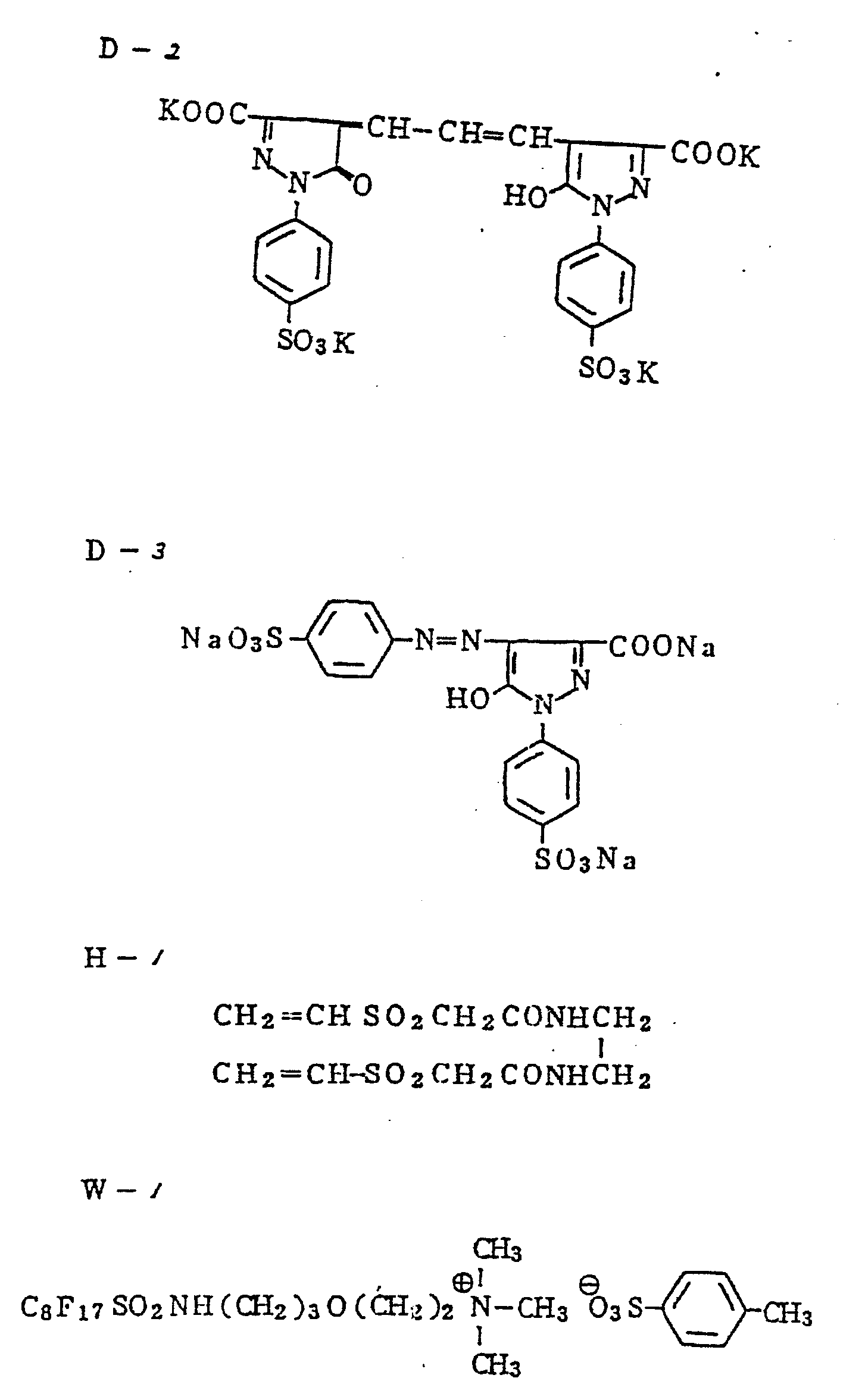
The compounds used in Examples 2 and 3 are shown below.
Example 4
-
A color photographic material was prepared by coating the following layers (Layer 1 through Layer 12) on a paper support having polyethylene layer on both surfaces. In this case, the polyethylene layer at the first layer-carrying side contained titanium white as white pigment and a slight amount of Ultramarine blue as bluish pigment.
Composition of Light-Sensitive Layer
-
Compounds and coating amount in g/m² are shown below The coating amount of silver halide is shown based on the amount of silver.
>
>
-
Furthermore, for each layer, Alkanol XC (made by Du Pont) and sodium alkylbenzenesulfonate were used as an emulsification dispersion aid and a succinic acid ester and Magefac F-120 (made by Dainippon Ink and Chemicals, Inc.) were used as coating aid. For each silver halide emulsion layer or colloid silver-containing layer, Cpd-19, -20, and -21 were used as stabilizers.
-
Then, the compounds used in the example are shown below.
- Solv-1:
Di(2-ethylhexyl) Phthalate. - Solv-2:
Trinonyl Phosphate. - Solv-3:
Di(3-methylhexyl) Phthalate. - Solv-4:
Tricresyl Phosphate. - Solv-5:
Dibutyl Phthalate. - Solv-6:
Trioctyl Phosphate. - Solve-7:
1,2-Bis(vinylsulfonylacetamide) ethane.
-
The sample thus obtained was denoted as Sample 401. Furthermore, Samples 402 to 405 were prepared by replacing the silver halide emulsions in the photosensitive layers as shown in Table 5 below.
- Solv-1:
Di(2-ethylhexyl) Phthalate. - Solv-2:
Trinonyl Phosphate. - Solv-3:
Di(3-methylhexyl) Phthalate. - Solv-4:
Tricresyl Phosphate. - Solv-5:
Dibutyl Phthalate. - Solv-6:
Trioctyl Phosphate. - Solve-7:
1,2-Bis(vinylsulfonylacetamide) ethane.
-
The sample thus obtained was denoted as Sample 401. Furthermore, Samples 402 to 405 were prepared by replacing the silver halide emulsions in the photosensitive layers as shown in Table 5 below.
-
Emulsions ME-11, -14, and -17 shown in Table 5 were prepared by the same manner as Emulsion A in Example 1, in which, however, the spectral sensitizing dyes, halogen compositions, mean grain size, grain size distributions, crystal phases, etc., were same as those of EM-1, EM-4, and EM-7, respectively.
-
Emulsions EM-12, -13, -15, -16, -18, and -19 were prepared by the same manner as Emulsion A except that a tabular emulsion was used as the core emulsion. Furthermore, the spectral sensitizing dyes, halogen compositions, mean grain sizes, grain size distributions, crystal phases, etc., of these emulsions were the same as those of EM-2, -3, -5, -6, -8, and -9, respectively.
-
Each of Samples 401 to 405 was subjected to sensitometric exposure and processes as follows.
-
The compositions of the processing solutions used for the steps were as follows.
-
The test results obtained are shown in Table 6 below.
-
In Table 6 above, the density change by storage and the latent image change by storage are the changes of the values which are 1.00 in color density before storage, and if sensitivity reduction and regression of latent image occur during storage, these values are increased. From the results shown in Table 6, it can be seen that the samples of this invention cause less change of sensitivity and latent images during storage. Furthermore, the red color was evaluated by visual determination of red color in the images and the density measurement. The cyan density shows a cyan component in red having a magenta density of 1.5 and as the value is less, the purity of the red is higher. It can be seen that in the samples of this invention, the reproduction of red color is improved.
Example 5
-
After exposing each of Samples 401 to 405 shown in Example 4 by the same manner as the Example 4, each sample was processed by the processing method described below until the accumulated replenisher amount became thrice the volume of the tank using an automatic processor. The results obtained were same as those in Example 4.
-
In this case, 1st Wash and 3rd Wash were each performed by a countercurrent wash system. That is, wash water is supplied to first wash (2), the overflow solution thereof is introduced to 1st wash (1) or wash water is supplied to 3rd wash (3), the overflow solution thereof is introduced to 3rd wash (2), and the overflow solution from 3rd wash (2) is introduced into 3rd wash (1).
-
The compositions of the processing solutions were as follows.
Example 6
-
Samples 401 to 405 as used in Example 4 were exposed as in Example 4 and processed in the manner as described below using an automatic processor until the accumulated amount of the replenisher became thrice the volume of the tank. The results obtained were same as those in Example 4.
-
In this case, the 1st wash and the 2nd wash each was performed by a countercurrent replenishing system. That is, 1st wash water was supplied to 1st wash (2) and the overflow liquid therefrom was introduced into 1st wash (1). Also, 2nd wash water was supplied to 2nd wash (3), the overflow liquid therefrom was introduced into 2nd wash (2), and the overflow liquid from 2nd wash (2) was introduced into 2nd wash (1).
-
The compositions of the processing solutions used in this example were as follows.
2nd Wash Solution
-
Both the mother liquor and the replenisher were as follows.
-
City water was passed through a mixed bed column packed with an H-type strong acid cation exchange resin (Amberlite IR-120 B, trademark for product made by Rohm and Haas Company) and OH type anion exchange resin (Amberlite IR-400, trademark for product made by Rohm and Haas Company) to reduce the calcium ion concentration and magnesium ion concentration below 3 mg/liter and then 20 mg/liter of sodium dichloroisocyanurate and 1.5 g/liter of sodium sulfate were added to the water. The pH of the solution was in the range, of 6.5 to 7.5.
Example 7
-
After exposing Samples 401 to 405 as used in Example 4, each sample was processed using an automatic processor by the processing steps as shown below until the accumulated amount of each replenisher became thrice the tank volume. The results were the same as those in Example 4.
-
In this case, the 1st wash and the 2nd wash were each performed by a countercurrent replenishing system. That is, 1st wash solution was supplied to 1st Wash (2) and the overflow solution therefrom was introduced into 1st Wash (1). Also, the 2nd wash solution was supplied to 2nd Wash (3), the overflow solution therefrom was introduced into 2nd Wash (2), and the overflow solution from 2nd Wash (2) was introduced into 2nd Wash (1).
-
The compositions of the processing solutions were
2nd Wash Solution
-
Both the mother liquor and the replenisher were as follows.
-
City water was passed through a mixed bed column packed with a H-type strong acid cation exchange resin (Amberlite IR-120 B, trademark for product made by Rohm and Haas Company) and OH-type anion exchange resin (Amberlite IR-400, trademark for product made by Rohm and Haas Company) to reduce the calcium ion concentration and magnesium ion concentration below 3 mg/liter and then 20 mg/liter of sodium dichloroisocyanurate and 1.5 g/liter of sodium sulfate were added to the water. The pH of the solution was in the range of 6.5 to 7.5.
Example 8
-
The influence of the silver iodide distribution in silver halide grains was determined as follows.
Emulsions P, Q, and R
-
The core grain formation step for Emulsion A described above was divided into three stages. In the first stage, silver halide grains having a long edge length of 0.4 µm were formed. In the second stage, a silver iodobromide phase corresponding to the silver amount of 10 mol% of the total silver amount of core and shell is formed and in this case, the content of silver iodide was adjusted to 4.8 mol%, 7.5 mol%, or 15.6 mol%. In these cases, the content of silver iodide in the cores in stage 1 and stage 3 and the content of silver iodide in the shell were same and also the mean silver iodide content in the final grains was 3 mol%. The emulsions thus prepared were defined as Emulsions P, Q, and R.
Emulsion S
-
After forming a monodisperse cubic emulsion of pure silver bromide having a long edge length of 0.4 µm, aqueous solution of potassium iodide corresponding to 3 mol% of the total silver amount of core and shell was added to the emulsion as an aqueous solution to form again pure silver bromide phase, whereby core grains of 0.68 µm were prepared. After chemically sensitizing the emulsion as in the case of Emulsion A described above, a pure silver bromide shell was further formed on the core to provide a cubic emulsion of silver halide grains having a mean silver iodide content of 3 mol% and a long edge length of 0.7 µm. The emulsion was defined as Emulsion S.
Emulsions T, U, and V
-
By following the same procedure as Emulsion S except that the addition time of the aqueous potassium iodide solution was changed after the formation of the silver bromide core and before the chemical sensitization, Emulsion T was prepared. Also, by following the same procedure as Emulsion S except that the aqueous potassium iodide solution was added when the grain sizes during the formation of the shell became 0.69 µm, Emulsion U was prepared. Also, by following the same procedure as Emulsion S except that at the case of adding the aqueous solution of potassium iodide, potassium iodide corresponding to 1.5 mol% of the total silver amount of core and shell was also added, Emulsion V was prepared.
Preparation of Monodisperse Emulsion Having (111) Crystal Habit
-
By following the same procedure for producing each of Emulsions A, S, B, and D described above except that pBr at the formation of grains of each emulsion was changed, monodisperse octahedral emulsions having the same silver iodide content in the grains and latent iamge distribution as those of Emulsions A, S, B, and D were prepared, which were defined as Emulsions L, X, M, and O.
Preparation of Tabular Emulsion having Mean Aspect Ratio of 7
-
Monodisperse tabular grain emulsions (a), (b), (c), and (d) having same grain volumes as those of Emulsions A, S, B, and D, respectively, having same silver iodide content distribution in grains and latent image distribution, and a mean value of ratios of diameters to thickness of grains (mean aspect ratio) of 7 were prepared by the manner described in Japanese Patent Application No. 299155/86.
-
After adding Sensitizing Dye S - 1 to each emulsion, the emulsion was coated on a support at a silver amount of 2 µg/cm².
-
Emulsions S, L, X, (a), and (b) showed almost same latent image distribution as that of Emulsion A, Emulsions M and (c) same as that of Emulsion B, and Emulsions (O) and (d) same as that of Emulsion D.
-
Each emulsion was coated and the exposure and processing were carried out by the same manners as Example 1. The results botained are shown in Table 7 below.
-
As the results, it can be seen that the light-sensitive materials using the emulsions of this invention having the latent image distribution and silver iodide content distribution in this invention are excellent in sensitivity, storage stability and reciprocity law characteristics as compared to the comparison samples using ordianry surface latent image type emulsions.
Example 9
-
Samples 601 and 606 were prepared by forming the layers having the following compositions on each triacetyl cellulose film support having subbing layer, wherein Emulsions A, S, B, (a), (b), and (c) described above were respectively used for Layer 11 as shown in Table 8.
-
Each of the aforesaid layers further contained Gelatin Hardening Agent H - 1 and surface active agent in addition to the above components.
-
The compounds used for preparing the samples were as follows.
-
Using Samples 601 to 606 prepared described above, the tests as shown in Table 8 below were carried out (light exposure amount 20 CMS, processing was same as Example 2). The results obtained are shown in Table 8.
-
As shown in the results, the emulsions of this invention showed excellent photographic properties.
-
While the invention has been described in detail and with reference to specific embodiments thereof, it will be apparent to one skilled in the art that various changes and modifications can be made therein without departing from the spirit and the scope thereof.