EP0066915B1 - Detergent composition containing performance additive and copolymeric compatibilizing agent therefor - Google Patents
Detergent composition containing performance additive and copolymeric compatibilizing agent therefor Download PDFInfo
- Publication number
- EP0066915B1 EP0066915B1 EP82200602A EP82200602A EP0066915B1 EP 0066915 B1 EP0066915 B1 EP 0066915B1 EP 82200602 A EP82200602 A EP 82200602A EP 82200602 A EP82200602 A EP 82200602A EP 0066915 B1 EP0066915 B1 EP 0066915B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- detergent composition
- range
- copolymeric
- alkyl
- detergent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 229920001577 copolymer Polymers 0.000 title claims abstract description 39
- 239000003599 detergent Substances 0.000 title claims abstract description 38
- 239000000654 additive Substances 0.000 title claims abstract description 29
- 239000000203 mixture Substances 0.000 title claims abstract description 25
- 239000003795 chemical substances by application Substances 0.000 title claims abstract description 15
- 230000000996 additive effect Effects 0.000 title claims description 12
- 239000000178 monomer Substances 0.000 claims abstract description 15
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims abstract description 8
- 239000002689 soil Substances 0.000 claims abstract description 8
- 239000011976 maleic acid Substances 0.000 claims abstract description 7
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims abstract description 7
- 150000001412 amines Chemical class 0.000 claims abstract description 6
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims abstract description 6
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 claims abstract description 5
- 239000004094 surface-active agent Substances 0.000 claims abstract description 5
- 239000004615 ingredient Substances 0.000 claims description 30
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 claims description 10
- 125000004432 carbon atom Chemical group C* 0.000 claims description 10
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 claims description 6
- 239000011159 matrix material Substances 0.000 claims description 6
- JZRYQZJSTWVBBD-UHFFFAOYSA-N pentaporphyrin i Chemical compound N1C(C=C2NC(=CC3=NC(=C4)C=C3)C=C2)=CC=C1C=C1C=CC4=N1 JZRYQZJSTWVBBD-UHFFFAOYSA-N 0.000 claims description 6
- 125000000217 alkyl group Chemical group 0.000 claims description 5
- -1 nonionic Chemical group 0.000 claims description 5
- 229920000768 polyamine Polymers 0.000 claims description 5
- 125000003342 alkenyl group Chemical group 0.000 claims description 4
- 150000002763 monocarboxylic acids Chemical class 0.000 claims description 4
- 229910052751 metal Inorganic materials 0.000 claims description 3
- 239000002184 metal Substances 0.000 claims description 3
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 2
- 125000000129 anionic group Chemical group 0.000 claims description 2
- 239000007864 aqueous solution Substances 0.000 claims description 2
- 125000002091 cationic group Chemical group 0.000 claims description 2
- 150000001991 dicarboxylic acids Chemical class 0.000 claims description 2
- 229910052739 hydrogen Inorganic materials 0.000 claims description 2
- 239000001257 hydrogen Substances 0.000 claims description 2
- 125000001424 substituent group Chemical group 0.000 claims description 2
- 239000007844 bleaching agent Substances 0.000 abstract description 5
- 239000012190 activator Substances 0.000 abstract description 2
- 229930195733 hydrocarbon Natural products 0.000 abstract description 2
- 150000002430 hydrocarbons Chemical class 0.000 abstract description 2
- 239000004215 Carbon black (E152) Substances 0.000 abstract 1
- 150000004965 peroxy acids Chemical class 0.000 abstract 1
- 229920001296 polysiloxane Polymers 0.000 abstract 1
- 159000000000 sodium salts Chemical class 0.000 description 8
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 239000003760 tallow Substances 0.000 description 4
- 239000011575 calcium Substances 0.000 description 3
- HNEGQIOMVPPMNR-IHWYPQMZSA-N citraconic acid Chemical class OC(=O)C(/C)=C\C(O)=O HNEGQIOMVPPMNR-IHWYPQMZSA-N 0.000 description 3
- 150000001990 dicarboxylic acid derivatives Chemical class 0.000 description 3
- 235000014113 dietary fatty acids Nutrition 0.000 description 3
- 239000000194 fatty acid Substances 0.000 description 3
- 229930195729 fatty acid Natural products 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- 150000002191 fatty alcohols Chemical class 0.000 description 3
- 239000000835 fiber Substances 0.000 description 3
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 235000013162 Cocos nucifera Nutrition 0.000 description 2
- 244000060011 Cocos nucifera Species 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 2
- 229910021536 Zeolite Inorganic materials 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 239000012876 carrier material Substances 0.000 description 2
- 229940018557 citraconic acid Drugs 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 125000005395 methacrylic acid group Chemical class 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000009919 sequestration Effects 0.000 description 2
- 235000019832 sodium triphosphate Nutrition 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000013042 solid detergent Substances 0.000 description 2
- 238000001694 spray drying Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 239000010457 zeolite Substances 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- WBIQQQGBSDOWNP-UHFFFAOYSA-N 2-dodecylbenzenesulfonic acid Chemical compound CCCCCCCCCCCCC1=CC=CC=C1S(O)(=O)=O WBIQQQGBSDOWNP-UHFFFAOYSA-N 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-M 2-methylbenzenesulfonate Chemical compound CC1=CC=CC=C1S([O-])(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-M 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 229910003252 NaBO2 Inorganic materials 0.000 description 1
- 239000006057 Non-nutritive feed additive Substances 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- 229920000388 Polyphosphate Polymers 0.000 description 1
- 101710194948 Protein phosphatase PhpP Proteins 0.000 description 1
- 239000004115 Sodium Silicate Substances 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- PTFCDOFLOPIGGS-UHFFFAOYSA-N Zinc dication Chemical compound [Zn+2] PTFCDOFLOPIGGS-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000003899 bactericide agent Substances 0.000 description 1
- 229910002056 binary alloy Inorganic materials 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- PVEOYINWKBTPIZ-UHFFFAOYSA-N but-3-enoic acid Chemical compound OC(=O)CC=C PVEOYINWKBTPIZ-UHFFFAOYSA-N 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- YRIUSKIDOIARQF-UHFFFAOYSA-N dodecyl benzenesulfonate Chemical compound CCCCCCCCCCCCOS(=O)(=O)C1=CC=CC=C1 YRIUSKIDOIARQF-UHFFFAOYSA-N 0.000 description 1
- 229940071161 dodecylbenzenesulfonate Drugs 0.000 description 1
- 229940060296 dodecylbenzenesulfonic acid Drugs 0.000 description 1
- 238000007580 dry-mixing Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 229940071106 ethylenediaminetetraacetate Drugs 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- HNEGQIOMVPPMNR-NSCUHMNNSA-N mesaconic acid Chemical compound OC(=O)C(/C)=C/C(O)=O HNEGQIOMVPPMNR-NSCUHMNNSA-N 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- FFQQCJGNKKIRMD-UHFFFAOYSA-N methyl n-(3-hydroxyphenyl)carbamate Chemical compound COC(=O)NC1=CC=CC(O)=C1 FFQQCJGNKKIRMD-UHFFFAOYSA-N 0.000 description 1
- XJRBAMWJDBPFIM-UHFFFAOYSA-N methyl vinyl ether Chemical compound COC=C XJRBAMWJDBPFIM-UHFFFAOYSA-N 0.000 description 1
- HNEGQIOMVPPMNR-UHFFFAOYSA-N methylfumaric acid Natural products OC(=O)C(C)=CC(O)=O HNEGQIOMVPPMNR-UHFFFAOYSA-N 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- MGFYIUFZLHCRTH-UHFFFAOYSA-N nitrilotriacetic acid Chemical compound OC(=O)CN(CC(O)=O)CC(O)=O MGFYIUFZLHCRTH-UHFFFAOYSA-N 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 235000011837 pasties Nutrition 0.000 description 1
- HWGNBUXHKFFFIH-UHFFFAOYSA-I pentasodium;[oxido(phosphonatooxy)phosphoryl] phosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O HWGNBUXHKFFFIH-UHFFFAOYSA-I 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 239000003504 photosensitizing agent Substances 0.000 description 1
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical compound N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 1
- 229920001444 polymaleic acid Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 239000001205 polyphosphate Substances 0.000 description 1
- 235000011176 polyphosphates Nutrition 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 239000003352 sequestering agent Substances 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- NVIFVTYDZMXWGX-UHFFFAOYSA-N sodium metaborate Chemical compound [Na+].[O-]B=O NVIFVTYDZMXWGX-UHFFFAOYSA-N 0.000 description 1
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 description 1
- 229910052911 sodium silicate Inorganic materials 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- MWNQXXOSWHCCOZ-UHFFFAOYSA-L sodium;oxido carbonate Chemical compound [Na+].[O-]OC([O-])=O MWNQXXOSWHCCOZ-UHFFFAOYSA-L 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3746—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/3757—(Co)polymerised carboxylic acids, -anhydrides, -esters in solid and liquid compositions
Definitions
- This invention relates to the detergent utilization of certain copolymeric ingredients in a compatibilizing functionality in conjunction with performance additives.
- the copolymeric ingredient is prepared from an ethylenically unsaturated carboxylic acid and an ethylenically unsaturated dicarboxylic acid.
- Preferred monomers are acrylic and methacrylic acids on one hand and maleic and citraconic acids on the other hand.
- the copolymeric agent was found to be especially suitable for enhancing the compatibility of detergent additives which are known to be sensitive to various conditions inclusive of prolonged storage, temperature, alkalinity, and/or laundry conditions.
- the copolymeric compatibilizing agent is capable of procuring, in contradistinction to their art-established functionality, unexpected transfer properties in relation to specific detergent additives. These transfer properties go against the prevailing opinion according to which comparable copolymeric adjuvants serve as deposition inhibitors, and consequently diminish the physical contact between detergent ingredients and e.g. the fiber.
- the copolymer comprises a large majority of the (meth)acrylic acid monomer FR-A-2388045, The Procter & Gamble Company, published November 17, 1978, pertains to detergent compositions containing surface-active agents, builders and a binary system based on not more than 4% of a polyphosphonate and not more than 4% of a polymeric ingredient which can be represented by the copolymers obtained from the polymerization of (meth)-acrylic acid and maleic anhydride.
- the copolymeric ingredient is claimed to provide effective active oxygen regulation.
- copolymeric ingredient of the invention herein can be used beneficially with a view to secure very desirable performance benefits upon use in combination with particular detergent additives with the proviso that the copolymeric ingredient is preferably used in excess levels in relation to a given performance additive.
- the performance additives are present in an amount from 0.002% to 5% by weight.
- the copolymeric ingredient is prepared from an ethylenically unsaturated carboxylic acid monomer having not more than five carbon atoms and from an ethylenically unsaturated dicarboxylic acid monomer having not more than six carbon atoms, whereby these monomers are copolymerized in a molar proportion of 1:4 to 4:1.
- the weight ratio of the copolymeric ingredient to the performance additives is from 500:1 to 1:5.
- Performance additives for use in combination with the copolymeric ingredients are selected from water-soluble porphine photoactivators such as mixtures of sulfonated metal phthalocyanines; and renewable polyamine or amine oxide soil release agents.
- the invention herein comprises at least three major parameters, namely: a conventional detergent matrix comprising surface-active agents and builders; a detergent performance additive; and a specific copolymeric ingredient.
- major parameters of the invention are described in more detail hereinafter.
- percent indications represent “percent by weight” indications.
- the detergent matrix of the compositions of this invention can also contain other major components according to the particular needs and/or the physical state of the invention.
- the compositions herein can be solid, pasty or liquid.
- Major amounts of pH regulants, inert fillers like sodium sulfate, water and/or organic solvents like hydrocarbons and/or lower alcohols can be applied as is well-known in the art.
- the detergent matrix can also contain major levels of bleaching agents, for example, oxygen bleach agents such as perborate, percarbonate, perpyrophosphate, persilicate or, more in general, all oxygen-bleach agents which are known to be suitable for use in detergent compositions in the established levels.
- the copolymeric ingredient consists of an ethylenically unsaturated monocarboxylic acid monomer having not more than 5, preferably 3 or 4, carbon atoms, and an ethylenically unsaturated dicarboxylic acid monomer having not more than 6, preferably 4 carbon atoms, whereby the molar ratio of the monomers is in the range from 1:4 to 4:1 (i.e., monocarboxylic acid:dicarboxylic acid).
- Suitable examples of the monocarboxylic acid monomer are: acrylic acid, methacrylic acid and vinyl acetic acid. Acrylic and methacrylic acids are preferred.
- Suitable examples of the dicarboxylic acid monomers are: maleic acid; fumaric acid; citraconic acid; and mesaconic acid. Preferred dicarboxylic acids are maleic acid and citraconic acid.
- the copolymeric ingredient can be further defined with the aid of the calcium sequestration value. These values can be measured by nephelometric titration methods (as described in literature
- the sequestration value is expressed in mgCaC0 3 /gram of copolymeric ingredient.
- the calcium sequestration value of the copolymeric ingredient is preferably in the range from 300-900 mgCaC0 3 /g.
- the performance additives herein are present in an amount from 0.002% to 5%. Depending upon the particular functionality of the additive and the planned use of the final detergent composition, the preferred usage ranges for the individual additives vary from additive to additive.
- performance additive as used herein is meant to express that the specific ingredient is added either to cure a deficiency of the general detergent matrix and/or to provide special laundry and cleaning benefits.
- a class of performance additives that can be utilized beneficially in combination with the copolymeric agent is represented by a photoactivator, also frequently termed a photosensitizer.
- the photoactivator is a porphine of a mono-, di-, tri-, or tetraaza porphine solubilized with anionic, nonionic, and/or cationic substituent groups and metal free or metallated with Zn(II), Ca(II), Cd(II), Mg(II), Sc(III), AI(III), or Sn(IV).
- Preferred metal-ions forthe photoactivator are zinc and aluminum.
- the photoactivator is frequently used in low levels, e.g., in the level from about 20 ppm to 2000 ppm.
- the photoactivator is generally used in combination with a carrier material such as polyphosphates, sulfates, a.s.o.
- a carrier material such as polyphosphates, sulfates, a.s.o.
- the level of such photoactivator particles represents from 0.1 % to 1% of the detergent composition.
- the photoactivator is believed to exhibit its activity in the direct environment of the fiber possibly in combination with perborate.
- the copolymeric ingredient favors this transfer of photoactivator to the fiber in presence of the other components in the laundry liquor at the applicable temperatures, e.g., laundry temperatures in the range from up to about 60°C or up to the boil.
- a water-soluble photoactivator used in the specified levels can be represented by the porphine activators of European Patent Application 0003149.
- the weight ratio of copolymer to photoactivator performance additive is frequently in the range from 500:1 to 7:1.
- the copolymeric ingredient was also found to be effective in combination with additive levels of renewable soil release agents as described in EP-A-0042187 and EP-A-0042188.
- the copolymeric ingredient enhances and contributes to a more quantitative deposition of the renewable soil release agent, specifically the polyamines oxidized or not and/or the oxidized monoamines.
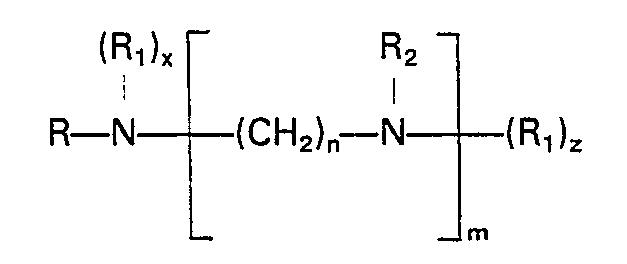
- the renewable soil release agent which frequently is used in levels from 0.1% to 1.5% is represented by polyamines having the formula: wherein R is an alkyl or alkenyl group having 10 to 22 carbon atoms, the R i 's, which are identical or different, are ethylene oxide or propylene oxide, R 2 is hydrogen, C 1 - 4 alkyl or (R i )y, where x, y, and z are numbers such that the sum (x+y+z) is in the range from 2 to 25, n is a number from 1 to 6 and m is a number from 1 to 9; or amine oxides having the formula: wherein R is an alkyl or alkenyl group having 10 to 22 carbon atoms, the R 3 's which are identical or different are selected from C ' - 4 alkyl, ethylene oxide and propylene oxide, k is an integer from 1 to 6, is an integer from 0 to 6, p is 0 or 1, u, v, and w are each 1 for alkyl
- a preferred polyamine for use herein is N-hydrogenated tallow C 16 ⁇ C 18 -N,N',N'-tri-(2-hydroxyethyl)-propylene-1,3-diamine.
- Preferred amine oxide species are N-C 12 - 14 -coconutalkyl-N,N-dimethyl-N-amine oxide; N-tallow C 16-18 -alkyl-N,N',N-tri-(2-hydroxyethyl)-propylene-1,3-diamine-N,N'-dioxide; N-C 12-14- alkyl-N,N',N'-tri-(2-hydroxyethyl)-propylene-1,3-diamine-N,N'-dioxide; N-C 16-18 -tallow-alkyl-N, N-dimethyl-N-amine oxide; N-C 12-14 -coconutalkyl-N,N-di(2-hydroxyethyl)-N-amine
- compositions of this invention can comprise a series of supplementary components to perfect and complement the performance advantages derived from the compositions.
- additional components include brighteners, dyes, perfumes, bactericides, and antioxidants, processing aids, corrosion inhibitors, enzymes and so on.
- processing aids i.e., frequently in the range from 0.1% to 5%.
- the following granular detergent compositions are prepared by conventional spray-drying of a slurry of individual ingredients and subsequent dry-mixing of this base powder with spray-drying sensitive ingredients.
- Solid detergent compositions are prepared having the following formulae.
- a liquid detergent composition is made by mixing the following individual ingredients.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Detergent Compositions (AREA)
Abstract
Description
- This invention relates to the detergent utilization of certain copolymeric ingredients in a compatibilizing functionality in conjunction with performance additives. The copolymeric ingredient is prepared from an ethylenically unsaturated carboxylic acid and an ethylenically unsaturated dicarboxylic acid. Preferred monomers are acrylic and methacrylic acids on one hand and maleic and citraconic acids on the other hand. The copolymeric agent was found to be especially suitable for enhancing the compatibility of detergent additives which are known to be sensitive to various conditions inclusive of prolonged storage, temperature, alkalinity, and/or laundry conditions. In more detail, the copolymeric compatibilizing agent is capable of procuring, in contradistinction to their art-established functionality, unexpected transfer properties in relation to specific detergent additives. These transfer properties go against the prevailing opinion according to which comparable copolymeric adjuvants serve as deposition inhibitors, and consequently diminish the physical contact between detergent ingredients and e.g. the fiber.
- The use of low levels of (co)polymeric additives for detergent application has been known for a long time and has found application in commercial detergent products. European Patent Application 0009171, BASF AG, published April 2, 1980 relates to the incorporation of polymaleic acid as a builder ingredient and/or as an incrustation inhibitor in detergents. European Patent Application 0025551, BASF AG, published March 25, 1981 discloses the utilization of (meth)acryIic · acid-maleic acid copolymeric ingredients as incrustation inhibitors in detergent compositions. The copolymer comprises a large majority of the (meth)acrylic acid monomer FR-A-2388045, The Procter & Gamble Company, published November 17, 1978, pertains to detergent compositions containing surface-active agents, builders and a binary system based on not more than 4% of a polyphosphonate and not more than 4% of a polymeric ingredient which can be represented by the copolymers obtained from the polymerization of (meth)-acrylic acid and maleic anhydride.
- German Patent Application DOS 19 59 272, Procter & Gamble European Technical Center, published July 23, 1970, relates to solid oxygen-bleach detergent compositions containing a copolymeric ingredient based on vinyl methylether and maleic anydride. The copolymeric ingredient is claimed to provide effective active oxygen regulation.
- It has now been discovered that the particular copolymeric ingredient of the invention herein can be used beneficially with a view to secure very desirable performance benefits upon use in combination with particular detergent additives with the proviso that the copolymeric ingredient is preferably used in excess levels in relation to a given performance additive.
- It is an object of this invention to formulate detergent compositions having desirable performance characteristics containing specific detergent additives.
- It is a further object of this invention to formulate laundry products capable of providing superior overall performance in presence of specific additives.
- It has now been discovered that markedly improved detergent compositions can be formulated containing a conventional detergent matrix in combination with detergent performance additives and a copolymeric ingredient. The performance additives are present in an amount from 0.002% to 5% by weight. The copolymeric ingredient is prepared from an ethylenically unsaturated carboxylic acid monomer having not more than five carbon atoms and from an ethylenically unsaturated dicarboxylic acid monomer having not more than six carbon atoms, whereby these monomers are copolymerized in a molar proportion of 1:4 to 4:1. The weight ratio of the copolymeric ingredient to the performance additives is from 500:1 to 1:5. Performance additives for use in combination with the copolymeric ingredients are selected from water-soluble porphine photoactivators such as mixtures of sulfonated metal phthalocyanines; and renewable polyamine or amine oxide soil release agents.
- The invention herein comprises at least three major parameters, namely: a conventional detergent matrix comprising surface-active agents and builders; a detergent performance additive; and a specific copolymeric ingredient. The major parameters of the invention are described in more detail hereinafter.
- Unless indicated to the contrary, the "percent" indications represent "percent by weight" indications.
- Qualitatively and quantitatively suitable surface-active agents for use herein are disclosed in U.S. Patent 4,192,761, column 3, line 49 to column 5, line 42. Qualitatively and quantitatively suitable detergent builder materials can also be taken from U.S. Patent 4,192,761, column 8, line 56 to column 9, line 68.
- It goes without saying that the detergent matrix of the compositions of this invention can also contain other major components according to the particular needs and/or the physical state of the invention. In this respect, the compositions herein can be solid, pasty or liquid. Major amounts of pH regulants, inert fillers like sodium sulfate, water and/or organic solvents like hydrocarbons and/or lower alcohols can be applied as is well-known in the art. The detergent matrix can also contain major levels of bleaching agents, for example, oxygen bleach agents such as perborate, percarbonate, perpyrophosphate, persilicate or, more in general, all oxygen-bleach agents which are known to be suitable for use in detergent compositions in the established levels.
- The copolymeric ingredient consists of an ethylenically unsaturated monocarboxylic acid monomer having not more than 5, preferably 3 or 4, carbon atoms, and an ethylenically unsaturated dicarboxylic acid monomer having not more than 6, preferably 4 carbon atoms, whereby the molar ratio of the monomers is in the range from 1:4 to 4:1 (i.e., monocarboxylic acid:dicarboxylic acid). Suitable examples of the monocarboxylic acid monomer are: acrylic acid, methacrylic acid and vinyl acetic acid. Acrylic and methacrylic acids are preferred. Suitable examples of the dicarboxylic acid monomers are: maleic acid; fumaric acid; citraconic acid; and mesaconic acid. Preferred dicarboxylic acids are maleic acid and citraconic acid.
- The copolymeric ingredient can be further defined with the aid of the calcium sequestration value. These values can be measured by nephelometric titration methods (as described in literature
- -S. Chaberek and A. E. Martell, Organic Sequestering Agents, Wiley, New York, 1959;
- -R. L. Smith, The Sequestration of Metals, Chapman and Hall, London, 1959):
- a calcium nitrate solution is added to a solution containing sequestrant and sodium oxalate until turbidity is produced; the titration is being carried out at constant pH=10 and room temperature.
- The sequestration value is expressed in mgCaC03/gram of copolymeric ingredient.
- The calcium sequestration value of the copolymeric ingredient is preferably in the range from 300-900 mgCaC03/g.
- The performance additives herein are present in an amount from 0.002% to 5%. Depending upon the particular functionality of the additive and the planned use of the final detergent composition, the preferred usage ranges for the individual additives vary from additive to additive. The term performance additive as used herein is meant to express that the specific ingredient is added either to cure a deficiency of the general detergent matrix and/or to provide special laundry and cleaning benefits.
- A class of performance additives that can be utilized beneficially in combination with the copolymeric agent is represented by a photoactivator, also frequently termed a photosensitizer. The photoactivator is a porphine of a mono-, di-, tri-, or tetraaza porphine solubilized with anionic, nonionic, and/or cationic substituent groups and metal free or metallated with Zn(II), Ca(II), Cd(II), Mg(II), Sc(III), AI(III), or Sn(IV). Preferred metal-ions forthe photoactivator are zinc and aluminum. The photoactivator is frequently used in low levels, e.g., in the level from about 20 ppm to 2000 ppm. In solid detergent compositions, the photoactivator is generally used in combination with a carrier material such as polyphosphates, sulfates, a.s.o. Generally, the level of such photoactivator particles (containing a carrier material) represents from 0.1 % to 1% of the detergent composition. The photoactivator is believed to exhibit its activity in the direct environment of the fiber possibly in combination with perborate. The copolymeric ingredient favors this transfer of photoactivator to the fiber in presence of the other components in the laundry liquor at the applicable temperatures, e.g., laundry temperatures in the range from up to about 60°C or up to the boil.
- As an example, a water-soluble photoactivator used in the specified levels can be represented by the porphine activators of European Patent Application 0003149. The weight ratio of copolymer to photoactivator performance additive is frequently in the range from 500:1 to 7:1.
- The copolymeric ingredient was also found to be effective in combination with additive levels of renewable soil release agents as described in EP-A-0042187 and EP-A-0042188. The copolymeric ingredient enhances and contributes to a more quantitative deposition of the renewable soil release agent, specifically the polyamines oxidized or not and/or the oxidized monoamines.
- The renewable soil release agent which frequently is used in levels from 0.1% to 1.5% is represented by polyamines having the formula:
amine oxides having the formula: - A preferred polyamine for use herein is N-hydrogenated tallow C16―C18-N,N',N'-tri-(2-hydroxyethyl)-propylene-1,3-diamine. Preferred amine oxide species are N-C12-14-coconutalkyl-N,N-dimethyl-N-amine oxide; N-tallow C16-18-alkyl-N,N',N-tri-(2-hydroxyethyl)-propylene-1,3-diamine-N,N'-dioxide; N-C12-14- alkyl-N,N',N'-tri-(2-hydroxyethyl)-propylene-1,3-diamine-N,N'-dioxide; N-C16-18-tallow-alkyl-N, N-dimethyl-N-amine oxide; N-C12-14-coconutalkyl-N,N-di(2-hydroxyethyl)-N-amine oxide; or N-C16-18- tallowalkyl-N,N-di-(2-hydroxyethyl)-N-amine oxide. The weight ratio of copolymer to soil release additive is preferably in the range from 10:1 to 1:1.
- In addition to the essential components described herreinbefore, the compositions of this invention can comprise a series of supplementary components to perfect and complement the performance advantages derived from the compositions. These additional components include brighteners, dyes, perfumes, bactericides, and antioxidants, processing aids, corrosion inhibitors, enzymes and so on. These further ingredients are used for their known functionality in the art established levels, i.e., frequently in the range from 0.1% to 5%.
- The following examples illustrate the invention and facilitate its understanding.
- The abbreviations for the individual ingredients have the following meaning:
- LAS: Sodium salt of linear dodecyl benzene sulfonate
- TAS: Sodium salt of tallow alcohol sulfate
- FAE3S: Sodium salt of fatty alcohol (C12-18) (ethoxy)3 sulfate
- AO: C12-14 alkyl dimethylamine oxide
- HLAS: Linear dodecyl benzene sulfonic acid
- TAE11: Tallow alocohol ethoxylated with about 11 moles of ethylene oxide
- FA25E7: Fatty alcohol (C12―C15) ethoxylated with about 7 moles of ethylene oxide
- FA25E4: Fatty alcohol (C12―C15) ethoxylated with about 4 moles of ethylene oxide
- CFA: C12-14 coconut fatty acid
- HFA: Hydrogenerated C16-22 fatty acid
- STPP: Sodium tripolyphosphate
- Zeolite A: Sodium salt of zeolite 4A (average particle size between 2-6 pm)
- NTA: Sodium salt of nitrilotriacetate
- Copolymer: AA40/MA60=copolymer of acrylic acid 40 mol-% and maleic acid 60 mol-% MAA50/MA50=copolymer of methyacrylic acid 50 mol-% and maleic acid 50 mol-%
- CMC: Sodium salt of carboxymethyl cellulose
- MHPC: Sodium salt of methyl hydroxypropyl cellulose
- Silicate 1.6: Sodium silicate Si02/Na20=1.6
- TEA: triethanolamine
- STS: Sodium salt of toluene sulfonate
- EDTA: Sodium salt of ethylene diamine tetra-acetate
- Perborate: NaBO2. H2O2 · 3H20
- P.A.: Photoactivator sulfonated Zn phthalocyanine
- SRS III: oil in water emulsion
- -9% polydimethylsiloxane
- -1% amorphous hydrophobic silica
- -5% coconut fatty acid ethoxylated with 7 moles of ethylene oxide
- -85% water
- The following granular detergent compositions are prepared by conventional spray-drying of a slurry of individual ingredients and subsequent dry-mixing of this base powder with spray-drying sensitive ingredients.
-
-
Claims (4)
an amine oxide having the formula:
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT82200602T ATE30738T1 (en) | 1981-05-30 | 1982-05-17 | DETERGENT COMPOSITION CONTAINING A PERFORMANCE ADDITIVE AND A COPOLYMER TO ENSURE THE SAME TOLERANCE. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB8116607 | 1981-05-30 | ||
| GB8116607 | 1981-05-30 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0066915A2 EP0066915A2 (en) | 1982-12-15 |
| EP0066915A3 EP0066915A3 (en) | 1985-01-16 |
| EP0066915B1 true EP0066915B1 (en) | 1987-11-11 |
Family
ID=10522165
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP82200602A Expired EP0066915B1 (en) | 1981-05-30 | 1982-05-17 | Detergent composition containing performance additive and copolymeric compatibilizing agent therefor |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0066915B1 (en) |
| AT (1) | ATE30738T1 (en) |
| CA (1) | CA1187373A (en) |
| DE (1) | DE3277630D1 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7790664B2 (en) | 2008-10-27 | 2010-09-07 | The Procter & Gamble Company | Methods for making a nil-phosphate liquid automatic dishwashing composition |
| US7867963B2 (en) | 2007-06-12 | 2011-01-11 | Rhodia Inc. | Mono-, di- and polyol phosphate esters in personal care formulations |
| US7919073B2 (en) | 2007-06-12 | 2011-04-05 | Rhodia Operations | Mono-, di- and polyol alkoxylate phosphate esters in oral care formulations and methods for using same |
| US7919449B2 (en) | 2007-06-12 | 2011-04-05 | Rhodia Operations | Detergent composition with hydrophilizing soil-release agent and methods for using same |
| US8293699B2 (en) | 2007-06-12 | 2012-10-23 | Rhodia Operations | Hard surface cleaning composition with hydrophilizing agent and method for cleaning hard surfaces |
| US8993506B2 (en) | 2006-06-12 | 2015-03-31 | Rhodia Operations | Hydrophilized substrate and method for hydrophilizing a hydrophobic surface of a substrate |
Families Citing this family (77)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3323621A1 (en) * | 1982-07-08 | 1984-03-01 | Yamasa Shoyu K.K., Choshi, Chiba | PHARMACEUTICAL PREPARATION WITH A REINFORCING ANTITUMOR EFFECT, A CHEMOTHERAPEUTIC COMPOSITION CONTAINING SUCH A PREPARATION AND USE OF THE PREPARATION TO SUPPORT ANTITUM TREATMENT AND TREATMENT IN MAN |
| ATE20476T1 (en) * | 1983-04-08 | 1986-07-15 | Procter & Gamble | GRANULATED DETERGENT COMPOSITIONS CONTAINING A MIXED POLYMER ADDITIVE SYSTEM. |
| DE3329400A1 (en) * | 1983-08-13 | 1985-02-28 | Henkel KGaA, 4000 Düsseldorf | GRAY PREVENTIVE ADDITIVE FOR PHOSPHATE-FREE AND LOW-PHOSPHASE DETERGENTS |
| GB2177717B (en) * | 1983-09-09 | 1987-09-23 | Godrej Soaps Ltd | Laundry detergent composition containing alpha olefin sulphonate |
| DE3340164C2 (en) * | 1983-11-07 | 1987-03-26 | S.A. Camp, Fábrica de Jabones, Granollers, Barcelona | laundry detergent |
| US4609473A (en) * | 1984-11-26 | 1986-09-02 | Colgate Palmolive Company | Bentonite-sulfate fabric softening particulate agglomerate, processes for manufacture and use thereof, and detergent compositions containing it |
| GB8625475D0 (en) * | 1986-10-24 | 1986-11-26 | Unilever Plc | Detergent composition |
| GB8708312D0 (en) * | 1987-04-07 | 1987-05-13 | Unilever Plc | Detergent powder composition |
| GB8730205D0 (en) * | 1987-12-24 | 1988-02-03 | Unilever Plc | Detergent composition |
| DE4312972A1 (en) * | 1993-04-21 | 1994-10-27 | Basf Ag | Use of alkoxylated polyamines and their N - oxides as auxiliaries for leather dyeing |
| EP0693549A1 (en) | 1994-07-19 | 1996-01-24 | The Procter & Gamble Company | Solid bleach activator compositions |
| FR2723858B1 (en) | 1994-08-30 | 1997-01-10 | Ard Sa | PROCESS FOR THE PREPARATION OF SURFACTANTS FROM WHEAT BY-PRODUCTS AND NOVEL ALKYL XYLOSIDES |
| US5928384A (en) * | 1994-11-10 | 1999-07-27 | The Procter & Gamble Company | Method of cleaning carpets |
| GB2297978A (en) | 1995-02-15 | 1996-08-21 | Procter & Gamble | Detergent compositions containing amylase |
| US5905065A (en) * | 1995-06-27 | 1999-05-18 | The Procter & Gamble Company | Carpet cleaning compositions and method for cleaning carpets |
| EP0778342A1 (en) | 1995-12-06 | 1997-06-11 | The Procter & Gamble Company | Detergent compositions |
| WO1997042282A1 (en) | 1996-05-03 | 1997-11-13 | The Procter & Gamble Company | Detergent compositions comprising polyamine polymers with improved soil dispersancy |
| ATE431844T1 (en) | 2002-02-11 | 2009-06-15 | Rhodia Chimie Sa | DETERGENT WITH BLOCK COPOLYMER |
| FR2887450B1 (en) | 2005-06-23 | 2007-08-24 | Rhodia Chimie Sa | CONCENTRATED INGREDIENT FOR THE TREATMENT AND / OR MODIFICATION OF SURFACES, AND ITS USE IN COSMETIC COMPOSITIONS |
| FR2894585B1 (en) | 2005-12-14 | 2012-04-27 | Rhodia Recherches Et Tech | COPOLYMER COMPRISING ZWITTERIONIC UNITS AND OTHER UNITS, COMPOSITION COMPRISING THE COPOLYMER, AND USE |
| MX2009012327A (en) | 2007-05-17 | 2009-12-01 | Procter & Gamble | Detergent additive extrudates containing alkyl benzene sulphonate. |
| GB0810881D0 (en) | 2008-06-16 | 2008-07-23 | Unilever Plc | Improvements relating to fabric cleaning |
| US20110166370A1 (en) | 2010-01-12 | 2011-07-07 | Charles Winston Saunders | Scattered Branched-Chain Fatty Acids And Biological Production Thereof |
| WO2011120799A1 (en) | 2010-04-01 | 2011-10-06 | Unilever Plc | Structuring detergent liquids with hydrogenated castor oil |
| RU2012147538A (en) | 2010-05-14 | 2014-06-20 | 3Е Сан Продактс Корпорейшн | POLYMER-CONTAINING CLEANING COMPOSITIONS AND METHODS FOR PRODUCING AND USING THEM |
| EP2588655B1 (en) | 2010-07-02 | 2017-11-15 | The Procter and Gamble Company | Method for delivering an active agent |
| RU2607747C1 (en) | 2010-07-02 | 2017-01-10 | Дзе Проктер Энд Гэмбл Компани | Method for producing films from non-woven fabrics |
| BR112013000099A2 (en) | 2010-07-02 | 2016-05-17 | Procter & Gamble | filaments comprising non-woven non-scent active agent fabrics and methods of manufacture thereof |
| CN105332075B (en) | 2010-07-02 | 2017-11-24 | 宝洁公司 | Long filament, non-woven webs comprising activating agent and the method for preparing them |
| CN103003476B (en) | 2010-07-02 | 2016-02-10 | 宝洁公司 | Web material and the method for the manufacture of web material |
| US20120172281A1 (en) | 2010-07-15 | 2012-07-05 | Jeffrey John Scheibel | Detergent compositions comprising microbially produced fatty alcohols and derivatives thereof |
| JP5715251B2 (en) | 2010-07-15 | 2015-05-07 | ザ プロクター アンド ギャンブルカンパニー | Personal care composition comprising a near-terminal branched compound |
| BR112013019685A2 (en) | 2011-02-17 | 2016-10-18 | Procter & Gamble | compositions comprising mixtures of c10 -C13 alkyl phenyl sulfonates |
| WO2012112828A1 (en) | 2011-02-17 | 2012-08-23 | The Procter & Gamble Company | Bio-based linear alkylphenyl sulfonates |
| EP2495300A1 (en) | 2011-03-04 | 2012-09-05 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Structuring detergent liquids with hydrogenated castor oil |
| WO2013002786A1 (en) | 2011-06-29 | 2013-01-03 | Solae | Baked food compositions comprising soy whey proteins that have been isolated from processing streams |
| WO2013043855A2 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | High suds detergent compositions comprising isoprenoid-based surfactants |
| AR088442A1 (en) | 2011-09-20 | 2014-06-11 | Procter & Gamble | DETERGENT COMPOSITIONS THAT INCLUDE PRIMARY SURFACTANT SYSTEMS THAT INCLUDE SURFACTANTS BASED ON HIGHLY RAMIFIED ISOPRENOIDS AND OTHER SURFACTANTS |
| MX2014003280A (en) | 2011-09-20 | 2014-05-13 | Procter & Gamble | Detergent compositions comprising sustainable surfactant systems comprising isoprenoid-derived surfactants. |
| EP2758503A2 (en) | 2011-09-20 | 2014-07-30 | The Procter and Gamble Company | Detergent compositions comprising specific blend ratios of isoprenoid-based surfactants |
| AR088758A1 (en) | 2011-09-20 | 2014-07-02 | Procter & Gamble | EASY DETERGENT COMPOSITIONS RINSE THAT UNDERSTAND ISOPRENOID BASED SURFACTANTS |
| US8541352B2 (en) | 2011-11-11 | 2013-09-24 | The Procter & Gamble Company | Surface treatment compositions including poly(diallyldimethylammonium chloride) and sheilding salts |
| US9139802B2 (en) | 2012-01-04 | 2015-09-22 | The Procter & Gamble Company | Active containing fibrous structures with multiple regions |
| GB2498265B (en) | 2012-01-04 | 2015-04-08 | Procter & Gamble | Fibrous structures comprising particles and methods for making same |
| MX352942B (en) | 2012-01-04 | 2017-12-14 | Procter & Gamble | Active containing fibrous structures with multiple regions having differing densities. |
| MX2015000924A (en) | 2012-07-26 | 2015-04-10 | Procter & Gamble | Low ph liquid cleaning compositions with enzymes. |
| KR102148348B1 (en) | 2012-09-04 | 2020-08-26 | 루브리졸 어드밴스드 머티어리얼스, 인코포레이티드 | Polyurethane/polyacrylic hybrid dispersions for shine applications in home care |
| CN105102600A (en) | 2013-03-28 | 2015-11-25 | 宝洁公司 | Cleaning compositions comprising polyetheramine, soil release polymer and carboxymethylcellulose |
| BR112016013055B1 (en) | 2013-12-09 | 2022-08-02 | The Procter & Gamble Company | BLANKET COMPRISING A FIBROUS STRUCTURE SOLUBLE IN WATER |
| US20150210964A1 (en) | 2014-01-24 | 2015-07-30 | The Procter & Gamble Company | Consumer Product Compositions |
| PL3099775T3 (en) | 2014-01-29 | 2021-03-08 | Cooperatie Koninklijke Cosun U.A. | Aqueous detergent compositions |
| WO2015130653A1 (en) | 2014-02-25 | 2015-09-03 | The Procter & Gamble Company | A process for making renewable surfactant intermediates and surfactants from fats and oils and products thereof |
| WO2015148360A1 (en) | 2014-03-27 | 2015-10-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| US20150275143A1 (en) | 2014-03-27 | 2015-10-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| EP3152288A1 (en) | 2014-06-06 | 2017-04-12 | The Procter & Gamble Company | Detergent composition comprising polyalkyleneimine polymers |
| WO2016048674A1 (en) | 2014-09-25 | 2016-03-31 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| US9388368B2 (en) | 2014-09-26 | 2016-07-12 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| US10421932B2 (en) | 2016-07-21 | 2019-09-24 | The Procter & Gamble Company | Cleaning composition with insoluble quaternized cellulose particles and non-anionic performance polymers |
| WO2018085390A1 (en) | 2016-11-01 | 2018-05-11 | Milliken & Company | Leuco colorants as bluing agents in laundry care compositions |
| CN109890950A (en) | 2016-11-01 | 2019-06-14 | 宝洁公司 | Leuco colorants as bluing agents in laundry care compositions |
| CN109890949B (en) | 2016-11-01 | 2021-10-01 | 宝洁公司 | Leuco colorants as bluing agents in laundry care compositions, packages, kits and methods thereof |
| EP3881900B1 (en) | 2017-01-27 | 2023-01-25 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| US11697906B2 (en) | 2017-01-27 | 2023-07-11 | The Procter & Gamble Company | Active agent-containing articles and product-shipping assemblies for containing the same |
| US11697904B2 (en) | 2017-01-27 | 2023-07-11 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| US11697905B2 (en) | 2017-01-27 | 2023-07-11 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| US20200199801A1 (en) | 2017-06-09 | 2020-06-25 | Conopco, Inc., D/B/A Unilever | Laundry liquid dispensing system |
| WO2019038186A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
| WO2019038187A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
| EP3694980A1 (en) | 2017-10-12 | 2020-08-19 | The Procter and Gamble Company | Leuco colorants in combination with a second whitening agent as bluing agents in laundry care compositions |
| WO2019075146A1 (en) | 2017-10-12 | 2019-04-18 | The Procter & Gamble Company | Leuco colorants as bluing agents in laundry care composition |
| US20190194579A1 (en) | 2017-10-12 | 2019-06-27 | The Procter & Gamble Company | Leuco colorants as bluing agents in laundry care compositions |
| TWI715878B (en) | 2017-10-12 | 2021-01-11 | 美商美力肯及公司 | Leuco colorants and compositions |
| JP7230043B2 (en) | 2017-11-01 | 2023-02-28 | ミリケン・アンド・カンパニー | Leuco compounds, colorant compounds, and compositions containing them |
| EP3894527A1 (en) | 2018-12-14 | 2021-10-20 | The Procter & Gamble Company | Foaming fibrous structures comprising particles and methods for making same |
| US20210148044A1 (en) | 2019-11-15 | 2021-05-20 | The Procter & Gamble Company | Graphic-Containing Soluble Articles and Methods for Making Same |
| WO2022093189A1 (en) | 2020-10-27 | 2022-05-05 | Milliken & Company | Compositions comprising leuco compounds and colorants |
| CA3228918A1 (en) | 2021-08-10 | 2023-02-16 | Nippon Shokubai Co., Ltd. | Polyalkylene-oxide-containing compound |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK130544A (en) * | 1968-11-27 | |||
| DE2816770C2 (en) * | 1977-04-22 | 1984-10-18 | The Procter & Gamble Co., Cincinnati, Ohio | Textile detergent containing builders |
| DE2936984A1 (en) * | 1979-09-13 | 1981-04-02 | Basf Ag, 6700 Ludwigshafen | USE OF (METH) ACRYLIC ACID-MALEIC ACID COPOLYMERISATES AS INCREDIBLE INHIBITORS IN DETERGENTS |
| GR76045B (en) * | 1981-04-08 | 1984-08-03 | Procter & Gamble |
-
1982
- 1982-05-17 EP EP82200602A patent/EP0066915B1/en not_active Expired
- 1982-05-17 DE DE8282200602T patent/DE3277630D1/en not_active Expired
- 1982-05-17 AT AT82200602T patent/ATE30738T1/en not_active IP Right Cessation
- 1982-05-26 CA CA000403734A patent/CA1187373A/en not_active Expired
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8993506B2 (en) | 2006-06-12 | 2015-03-31 | Rhodia Operations | Hydrophilized substrate and method for hydrophilizing a hydrophobic surface of a substrate |
| US7867963B2 (en) | 2007-06-12 | 2011-01-11 | Rhodia Inc. | Mono-, di- and polyol phosphate esters in personal care formulations |
| US7919073B2 (en) | 2007-06-12 | 2011-04-05 | Rhodia Operations | Mono-, di- and polyol alkoxylate phosphate esters in oral care formulations and methods for using same |
| US7919449B2 (en) | 2007-06-12 | 2011-04-05 | Rhodia Operations | Detergent composition with hydrophilizing soil-release agent and methods for using same |
| US8268765B2 (en) | 2007-06-12 | 2012-09-18 | Rhodia Operations | Mono-, di- and polyol phosphate esters in personal care formulations |
| US8293699B2 (en) | 2007-06-12 | 2012-10-23 | Rhodia Operations | Hard surface cleaning composition with hydrophilizing agent and method for cleaning hard surfaces |
| US7790664B2 (en) | 2008-10-27 | 2010-09-07 | The Procter & Gamble Company | Methods for making a nil-phosphate liquid automatic dishwashing composition |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0066915A3 (en) | 1985-01-16 |
| ATE30738T1 (en) | 1987-11-15 |
| EP0066915A2 (en) | 1982-12-15 |
| CA1187373A (en) | 1985-05-21 |
| DE3277630D1 (en) | 1987-12-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0066915B1 (en) | Detergent composition containing performance additive and copolymeric compatibilizing agent therefor | |
| EP0193360B1 (en) | Detergent compositions | |
| EP0378261B1 (en) | Liquid detergent composition containing enzyme and enzyme stabilization system | |
| AU624328B2 (en) | Liquid detergent containing perborate bleach | |
| EP0137669B1 (en) | Detergent compositions | |
| US5494488A (en) | Detergent composition and method of use with surfactant, silicate, and polycarboxylate | |
| EP0663408B1 (en) | Process for preparing water soluble polymers of monoethylenically unsaturated dicarboxylic acids | |
| IE63070B1 (en) | Liquid detergent containing solid peroxygen bleach | |
| EP0124913B1 (en) | Granular detergent compositions containing mixed polymer additive system | |
| US5061396A (en) | Detergent compositions containing polyether polycarboxylates | |
| US5066749A (en) | Hydrophobically-modified polycarboxylates and process for their preparation | |
| EP0378262B1 (en) | Liquid detergent composition containing enzyme and enzyme stabilization system | |
| EP0001853B2 (en) | Detergent compositions having improved bleaching effect | |
| EP0000215A1 (en) | Low-phosphate detergent composition for fabric washing | |
| AU612711B2 (en) | Non-phosphorus detergent bleach compositions | |
| KR920001656B1 (en) | Non-phosphorus detergent bleach compositions | |
| JPH05132696A (en) | Composition and method for preventing sticking of textile | |
| US6310030B1 (en) | Unsaturated carboxylic acid polymer, biodegradable builder, and detergent composition | |
| EP0181180B1 (en) | Detergent compositions | |
| JPH05132697A (en) | Composition and method for preventing sticking of textile |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI NL |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI NL |
|
| 17P | Request for examination filed |
Effective date: 19850704 |
|
| 17Q | First examination report despatched |
Effective date: 19860306 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI NL |
|
| REF | Corresponds to: |
Ref document number: 30738 Country of ref document: AT Date of ref document: 19871115 Kind code of ref document: T |
|
| ITF | It: translation for a ep patent filed | ||
| REF | Corresponds to: |
Ref document number: 3277630 Country of ref document: DE Date of ref document: 19871217 |
|
| ET | Fr: translation filed | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: UNILEVER PLC / UNILEVER N.V. Effective date: 19880726 |
|
| 26 | Opposition filed |
Opponent name: S.A. CAMP Effective date: 19880809 Opponent name: UNILEVER PLC / UNILEVER N.V. Effective date: 19880810 |
|
| R26 | Opposition filed (corrected) |
Opponent name: UNILEVER PLC / UNILEVER N.V. Effective date: 19880810 |
|
| 26 | Opposition filed |
Opponent name: CIBA-GEIGY AG Effective date: 19880811 Opponent name: S.A. CAMP Effective date: 19880809 Opponent name: UNILEVER PLC / UNILEVER N.V. Effective date: 19880810 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: S.A. CAMP Opponent name: UNILEVER PLC / UNILEVER N.V. |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: CIBA-GEIGY AG |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: UNILEVER PLC / UNILEVER N.V. * 880809 S.A. CAMP * Effective date: 19880810 |
|
| NLXE | Nl: other communications concerning ep-patents (part 3 heading xe) |
Free format text: IN PAT.BUL.22/88:CORR.:880810 |
|
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19920429 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19920511 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19920512 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19920525 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19920531 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19920610 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19920616 Year of fee payment: 11 |
|
| RDAG | Patent revoked |
Free format text: ORIGINAL CODE: 0009271 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT REVOKED |
|
| 27W | Patent revoked |
Effective date: 19921007 |
|
| GBPR | Gb: patent revoked under art. 102 of the ep convention designating the uk as contracting state |
Free format text: 921007 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| NLR2 | Nl: decision of opposition | ||
| APAC | Appeal dossier modified |
Free format text: ORIGINAL CODE: EPIDOS NOAPO |
|
| APAC | Appeal dossier modified |
Free format text: ORIGINAL CODE: EPIDOS NOAPO |
|
| APAH | Appeal reference modified |
Free format text: ORIGINAL CODE: EPIDOSCREFNO |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |