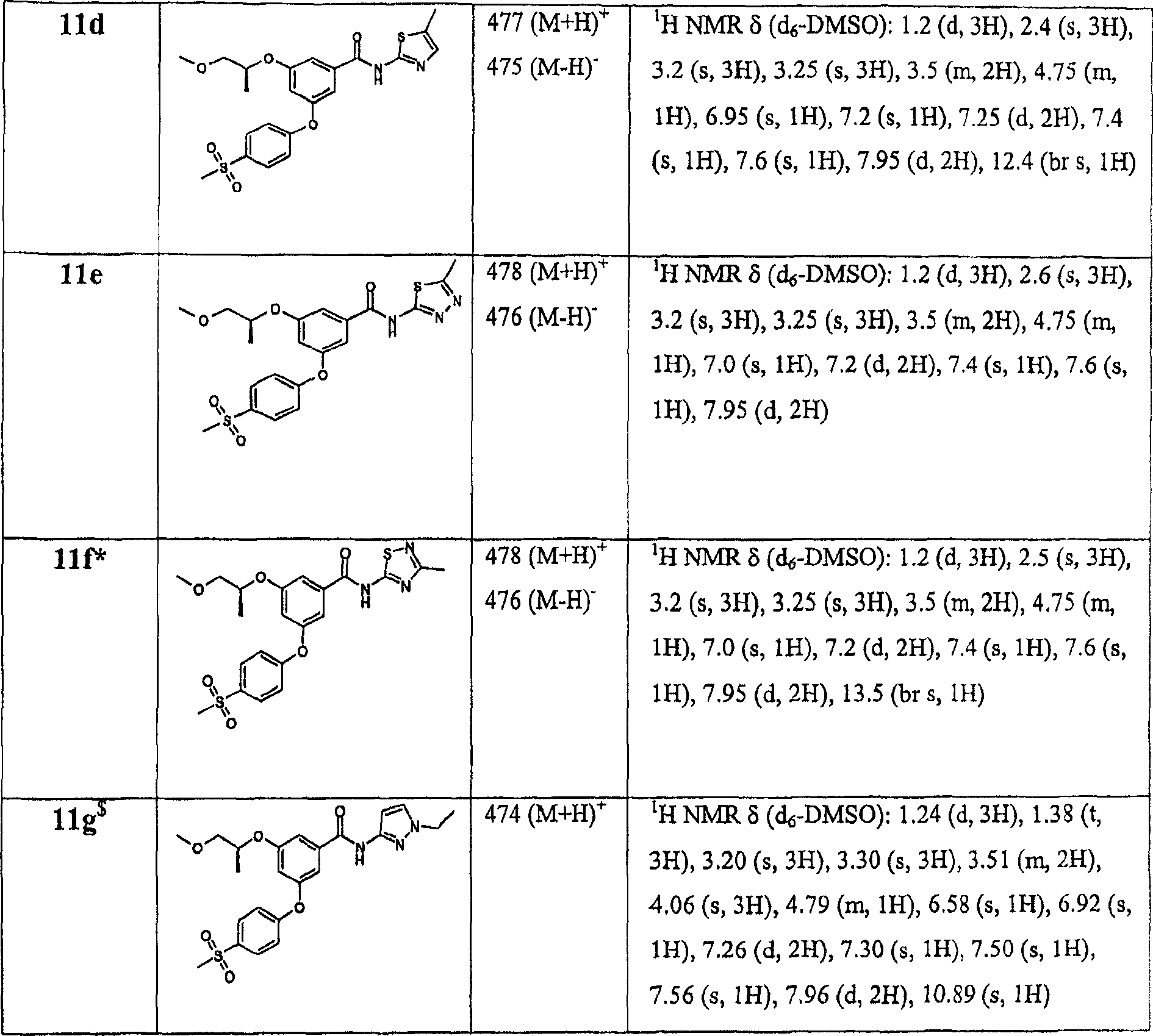
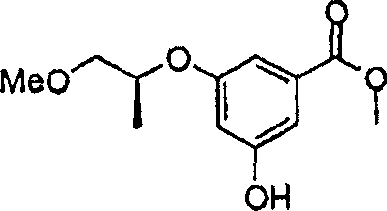
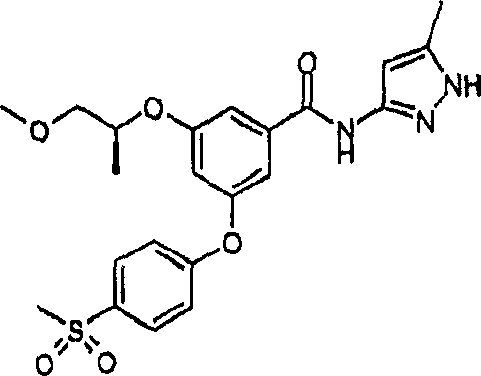
CN1922159A - Benzamide derivatives and their use as glucokinase activating agents - Google Patents
Benzamide derivatives and their use as glucokinase activating agents Download PDFInfo
- Publication number
- CN1922159A CN1922159A CN 200580005262 CN200580005262A CN1922159A CN 1922159 A CN1922159 A CN 1922159A CN 200580005262 CN200580005262 CN 200580005262 CN 200580005262 A CN200580005262 A CN 200580005262A CN 1922159 A CN1922159 A CN 1922159A
- Authority
- CN
- China
- Prior art keywords
- group
- het
- alkyl
- methyl
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Landscapes
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Compounds of Formula (I): wherein: R<1> is methoxymethyl; R<2> is selected from -C(O)NR<4>R<5>, -SO2NR<4>R<5>, -S(O)pR<4> and HET-2; HET-1 is a 5- or 6-membered, optionally substituted C-linked heteroaryl ring; HET-2 is a 4-, 5- or 6-membered, C- or N-linked optionally substituted heterocyclyl ring; R<3> is selected from halo, fluoromethyl, difluoromethyl, trifluoromethyl, methyl, methoxy and cyano; R<4> is selected from for example hydrogen, optionally substituted (1-4C)alkyl and HET-2; R<5> is hydrogen or (1-4C)alkyl; or R<4> and R<5> together with the nitrogen atom to which they are attached may form a heterocyclyl ring system as defined by HET-3; HET-3 is for example an optionally substituted N-linked, 4, 5 or 6 membered, saturated or partially unsaturated heterocyclyl ring; p is (independently at each occurrence) 0, 1 or 2; mis 0 or1; n is 0, 1 or 2; provided that when m is 0, then n is 1 or 2; or a salt, pro-drug or solvate thereof, are described. Their use as GLK activators, pharmaceutical compositions containing them, and processes for their preparation are also described.
Description
The present invention relates to one group of benzoyl-amido heterocyclyl compounds, described compound can be used for treatment or the disease or the illness of glucokinase (GLK or GK) mediation are passed through in prevention, and causes the threshold glucose value of insulin secretion to reduce.In addition, estimate that described compound absorbs and reduces blood-glucose by increasing liver glucose.These compounds can be used for treating diabetes B and obesity.The invention still further relates to the pharmaceutical composition that contains described compound and use the method for described compounds for treating by the disease of GLK mediation.
Membrane plasmapheresis glucose transporter main in pancreas beta cell and liver parenchyma is GLUT2.Under the physiology glucose concn, the speed that GLUT2 transhipment glucose passes film is not the limiting speed that glucose uptake enters total speed of these cells.The speed of glucose uptake is the rate limiting that is become the phosphorylation of G-6-P (G-6-P) by glucose, and this acts on through glucokinase (GLK) catalysis [1].GLK has the Km of height (6-10mM) for glucose, and is not suppressed [1] by the G-6-P of physiological concentration.GLK expresses and is confined to minority tissue and cell type, and modal is pancreas beta cell and liver cell (liver cell) [1].The GLK activity is that the speed limit effect of glucose utilization and the degree of regulating and control the insulin secretion that glucose causes thus and liver starch are synthetic in these cells.These processes are very crucial and both equal functional defecies [2] in diabetes in the keeping of overall glucose homeostasis.
In a kind of hypotype diabetes, in the young 2 type growth perioies outbreak diabetes (MODY-2), these diabetes are [3,4] that the GLK loss by function mutation causes.In MODY-2 patient, hyperglycemia is derived from the damaged property glucose utilization [5] in pancreas and the liver.The threshold value that damaged property glucose utilization causes glucose to stimulate insulin secretion in MODY-2 patient's the pancreas raises.On the contrary, the activated mutant effect of rare GLK reduces this threshold value and causes familial insulism [6,6a, 7].Except the GLK activity of observing reduction in the MODY-2 diabetes, liver glucokinase activity also reduces [8] in diabetes B.Importantly, GLK comprehensively or the overexpression of liver selectivity stop or reverse the deterioration [9-12] of diabetes phenotype in the diet of this disease and the genetic model.In addition, with fructose the quick treatment of diabetes B is used to improve glucose tolerance [13] by stimulating liver glucose.It is believed that this effect is that kytoplasm GLK activity increases [13] that mediate in the liver cell of being brought out by fructose by following mechanism.
Can suppress liver GLK activity by regulating albumen (GLKRP) association with GLK.The GLK/GLKRP mixture is replaced this sugared phosphoric acid stabilization removal by fructose-6-phosphate (F6P) in conjunction with the GLKRP stabilization and by fructose-1-phosphate (F1P).Under the phosphorylation mediation of the food fructose that fructokinase mediates, generate F1P.So the integrity of GLK/GLKRP mixture and liver GLK activity are regulated in nutrition dependence mode, because F6P raises and F1P state after dining is preponderated in postabsorptive state.Form contrast with liver cell, the pancreas beta cell is expressed GLK under the non-existent condition of GLKRP.So beta cell GLK activity is only regulated by the utilizability of its substrate glucose.Small molecules can be directly or by making GLK/GLKRP mixture stabilization removal activate GLK.The compound of last kind estimates to stimulate the glucose utilization in liver and the pancreas and the latter estimates only to work in liver.Yet the compound with a kind of performance estimates to have the treatment benefit of treatment diabetes B, because this genius morbi is the damaged property glucose utilization in above-mentioned two kinds of tissues.
GLK and GLKRP and K
ATPPassage is expressed in hypothalamic neurone, and hypothalamus is to regulate energy balance and control ingestion of food very important brain zone [14-18].Verified these neuron expression appetizing and apocleisis neuropeptides [15,19,20], and inferred it is the interior glucose sensing neurone of hypothalamus, they suppress or excited [17,19,21,22] by the change of environment glucose concn.The ability that these neurone sensation glucose levels change is damaged [23-28] in multiple heredity and the fat model of experiment inductive.Glucalogue, the Intraventricular of the competitive inhibitor of glucokinase (icv) infusion just, the ingestion of food [29,30] that stimulates thin and weak rat.On the contrary, the icv infusion of glucose suppresses feed [31].So the small molecules activator of GLK can reduce ingestion of food and weight increase by the central action to GLK.So the GLK activator can therapeutic be applied to treat the eating disorder except that diabetes, comprises obesity.Hypothalamic effect will be with the effect adduction of these compounds or bring into play the effect that makes glucose homeostasis normalizing synergistically in liver and/or pancreas, for example treats diabetes B.So the GLK/GLKRP system can be described as being potential " diabetes obesity " target (all useful in diabetes and obesity).
GLK also expresses in specific enteroendocrine cell, it is believed that in these cells, GLK control incretin peptide GIP (glucose-dependent-insulinotropic polypeptide) and GLP-1 (glucagon kind polypeptide-1) the glucose-sensitive secretion (32 from intestines K-cell and L-cell respectively, 33,34).Therefore, the small molecules activator of GLK can have other beneficial effect for insulin secretion, beta cell function and survival and body weight, and this is to stimulate GIP and GLP-1 excretory result from enteroendocrine cell.
In WO0058293 and WO 01/44216 (Roche), a series of benzylamino formylation compounds as glucokinase activating agents have been described.The mechanism that this compounds activates GLK be by measure these compounds therein active direct being used for that generates in the relevant test with NADH of GLK assess, and NADH generates the in vitro tests details by optical method measuring-description in vide infra.The compounds of this invention can directly activate GLK or can activate GLK by the interaction that suppresses GLKRP and GLK.
Other GLK activator has been described in WO03/095438 (the phenyl-acetamides compound of replacement; Roche), WO03/055482 (methane amide and sulfone amide derivative; NovoNordisk), WO2004/002481 (aryl carbonyl derivatives; Novo Nordisk) and WO03/080585 (the amino benzoyl-amido heterogeneous ring compound that replaces, Banyu) in.
The applicant's International Application No. WO 03/000267 has been described one group of benzoyl-amido pyridyl carboxylic acid cpd, and described compound activates glucokinase (GLK).
The applicant's International Application No. WO 03/015774 has been described formula (A) compound:
R wherein
3It is the heterocycle except the pyridyl of carboxylic acid-substituted.
International Application No. WO 2004/076420 (Banyu) has been described some compounds, and these compounds generally are the subclass of the compound described among the WO03/015774, wherein R for example
1Be (replacement) alkyl oxide, and R
2It is (replacement) phenoxy group.
We have been surprised to find group's compound, it generally is a group of from the compound that WO 03/015774 describes, selecting, these compounds have good effectiveness usually for the GLK enzyme, more favourable physical properties comprises that for example higher water-soluble, higher perviousness and/or lower plasma proteins are in conjunction with central one or more.Therefore, estimate the such compound of equilibrated, after oral administration, render a service that this is definite by for example activity in oral glucose tolerance test (OGTT) with showing in higher free drug levels and the good body with these character.Therefore, estimate that this group compound will provide good oral availability than low dosage, so be specially adapted to treat or prevent disease or illness by the GLK mediation.
Therefore, according to a first aspect of the invention, provide formula (I) compound or its salt, prodrug or solvate:
Wherein:
R
1It is methoxymethyl;
R
2Be selected from-C (O) NR
4R
5,-SO
2NR
4R
5,-S (O)
pR
4And HET-2; HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1 or 2 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or be independently selected from R by 1 or 2 on theheterocyclic nitrogen atom
6Substituting group replace, condition is that theheterocyclic nitrogen atom is not thus by quaternized; HET-2 is the heterocyclic ring that 4,5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group; R
4Be selected from hydrogen, (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or (1-4C) alkyl;
Perhaps R
4And R
5Can form the heterocyclic radical ring system that defines by HET-3 with the nitrogen-atoms that they connected;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-3 is 4,5 or 6 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 7 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 other heteroatoms (except the N atom that is connected) that is independently selected from O, S and N, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 6-10 unit's two ring fillings or part unsaturated heterocycle basic ring, optional 1 the other nitrogen-atoms (except the N atom that connects) that contains of described heterocyclic ring, wherein-CH
2-group can be chosen wantonly by-C (O)-replacement; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be selected from hydroxyl and R by 1
3Substituting group replace;
R
8Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkylamino, two (1-4C) alkylamino, HET-3 (wherein said ring is unsubstituted), (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate as defined above are provided, condition is the compound that is not included in the WO2004/076420 illustrated, otherwise these illustrational compounds will drop in the scope of the invention.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
R
2Be selected from-C (O)-HET-3 and-SO
2-HET-3;
HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1 or 2 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or be independently selected from R by 1 or 2 on theheterocyclic nitrogen atom
6Substituting group replace, condition is that theheterocyclic nitrogen atom is not thus by quaternized;
HET-2 is the heterocyclic ring that 4,5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group;
R
4Be selected from hydrogen, (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or (1-4C) alkyl; Perhaps
R
4And R
5Can form the heterocyclic radical ring system that defines by HET-3 with the nitrogen-atoms that they connected;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-3 is 4,5 or 6 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 7 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 other heteroatoms (except the N atom that is connected) that is independently selected from O, S and N, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 6-10 unit's two ring fillings or part unsaturated heterocycle basic ring, optional 1 the other nitrogen-atoms (except the N atom that connects) that contains of described heterocyclic ring, wherein-CH
2-group can be chosen wantonly by-C (O)-replacement; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be selected from hydroxyl and R by 1
3Substituting group replace;
R
8Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkylamino, two (1-4C) alkylamino, HET-3 (wherein said ring is unsubstituted), (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate as defined above are provided, wherein:
HET-3 is the saturated or part unsaturated heterocycle basic ring of 4-6 unit that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
R
2Be selected from-C (O) NR
41R
51,-SO
2NR
41R
51With-S (O)
pR
41
HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1 or 2 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or be independently selected from R by 1 or 2 on theheterocyclic nitrogen atom
6Substituting group replace, condition is that theheterocyclic nitrogen atom is not thus by quaternized;
HET-2 is the heterocyclic ring that 4,5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group; R
41Be selected from (1-4C) alkyl [by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
51Be hydrogen or (1-4C) alkyl;
R
4Be selected from (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or (1-4C) alkyl;
Perhaps R
4And R
5Can form the heterocyclic radical ring system that defines by HET-3 with the nitrogen-atoms that they connected;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-3 is 4,5 or 6 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace;
Perhaps
HET-3 is 7 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 other heteroatoms (except the N atom that is connected) that is independently selected from O, S and N, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 6-10 unit's two ring fillings or part unsaturated heterocycle basic ring, optional 1 the other nitrogen-atoms (except the N atom that connects) that contains of described heterocyclic ring, wherein-CH
2-group can be chosen wantonly by-C (O)-replacement; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be selected from hydroxyl and R by 1
3Substituting group replace;
R
8Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkylamino, two (1-4C) alkylamino, HET-3 (wherein said ring is unsubstituted), (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate as defined above are provided, wherein:
R
4Be selected from hydrogen, (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace] and HET-2;
HET-3 is 6-10 unit's two ring fillings or part unsaturated heterocycle basic ring, optional 1 the other nitrogen-atoms (except the N atom that connects) that contains of described heterocyclic ring, wherein-CH
2-group can be chosen wantonly by-C (O)-replacement, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be selected from R by 1
3Substituting group replace.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
R
2Be HET-2;
HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1 or 2 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or is replaced by 1 or 2 substituting group that is independently selected from R6 on theheterocyclic nitrogen atom, and condition is that theheterocyclic nitrogen atom is not thus by quaternized;
HET-2 is the heterocyclic ring that 4,5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group;
R
4Be selected from hydrogen, (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or (1-4C) alkyl;
Perhaps R
4And R
5Can form the heterocyclic radical ring system that defines by HET-3 with the nitrogen-atoms that they connected;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-3 is 4,5 or 6 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace;
Perhaps
HET-3 is 7 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 other heteroatoms (except the N atom that is connected) that is independently selected from O, S and N, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 6-10 unit's two ring fillings or part unsaturated heterocycle basic ring, optional 1 the other nitrogen-atoms (except the N atom that connects) that contains of described heterocyclic ring, wherein-CH
2-group can be chosen wantonly by-C (O)-replacement; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be selected from hydroxyl and R by 1
3Substituting group replace;
R
8Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkylamino, two (1-4C) alkylamino, HET-3 (wherein said ring is unsubstituted), (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
Should be appreciated that and work as R
4Be-C (O) NR
5R
5The time, each R
5Be independently selected from hydrogen and (1-4C) alkyl, so R
4This definition include, but is not limited to-CONH
2,-CONHMe ,-CONMe
2With-CONMeEt.
Should be appreciated that they can be identical or different when formula (I) compound contains an above HET-2 ring.
Should be appreciated that working as formula (I) compound contains an above radicals R
4The time, they can be identical or different.
Should be appreciated that working as formula (I) compound contains an above radicals R
5The time, they can be identical or different.
Should be appreciated that working as formula (I) compound contains an above radicals R
8The time, they can be identical or different.
For all other group and substituting groups on formula (I) compound as defined above, similar convention also is suitable for.
Formula (I) compound can form salt, and it belongs in the scope of the present invention.Preferred pharmacologically acceptable salt, but other salt can be used for for example separating or the purification compound.
In yet another aspect, the present invention relates to formula (I) compound or pharmaceutically acceptable salt thereof as defined above.
In yet another aspect, the present invention relates to formula (I) compound or its prodrug as defined above.The suitable example of the prodrug of formula (1) compound is the interior hydrolyzable ester of the body of formula (I) compound.Therefore, in yet another aspect, the present invention relates to formula (I) compound or the interior hydrolyzable ester of its body as defined above.
In this manual, term " alkyl " comprises straight chain and branched-chain alkyl.Yet,
For example, " C
1-4Alkyl " comprise propyl group, sec.-propyl and the tertiary butyl.Yet, for example only refer in particular to linear form when " propyl group " mentioning individual groups, and for example only refer in particular to the side chain form during tertiary butyl mentioning indivedual branched-chain alkyls.For example, " (1-4C) alkyl " comprises methyl, ethyl, propyl group, sec.-propyl and the tertiary butyl.Similar convention also is applicable to other group.
For fear of query, when mentioning the group HET-1 that contains nitrogen in the 2-position, this is meant the 2-position for group connection amide nitrogen atom thereon.For example, include, but is not limited to down array structure:
The suitable example of the HET-1 of the heteroaryl ring that connects as 5 or 6 yuan of C-as defined above comprise thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly and triazolyl.
Should be appreciated that HET-2 can be saturated or partially or completely undersaturated ring.
The suitable example of HET-2 comprises azetidinyl, furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ azoles base, different _ the azoles base, _ di azoly, morpholino, morpholinyl, piperidyl, piperazinyl, morpholinyl, thio-morpholinyl, pyrryl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, 2-oxo-1,3,4-(4-triazoline base), 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 1,2, the 4-triazolyl, 1,2, the 3-triazolyl, pyranyl and 4-pyriconyl.
Should be appreciated that HET-2 can connect by any suitable available C or N atom, therefore, for example the HET-2 as " imidazolyl " comprises 1-, 2-, 4-and 5-imidazolyl.
The suitable example of the HET-3 of or part unsaturated heterocycle saturated as 4-6 unit is morpholino, piperidyl, piperazinyl, pyrrolidyl and azetidinyl.
To be high piperazinyl, high morpholino, high-sulfur (be oxidized to SO or S (O) with sulphur wherein for morpholino to the suitable example of the HET-3 of or part unsaturated heterocycle saturated as 7 yuan
2The modification of group) and homopiperidinyl.
Suitable example as the HET-3 of 6-10 unit bicyclic heterocycles is two ring fillings or part unsaturated heterocycle basic ring, structure for example as follows (wherein dotted line is meant the point that is connected with the rest part of this molecule):
Particularly, HET-3 is that [2,2,1] ring system for example
(7-azabicyclic [2.2.1] heptan-7-yl).
The suitable example of HET-4 be furyl, pyrryl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ azoles base, different _ azoles base and triazolyl.
Should be appreciated that definition as heterocyclic radical HET-1-HET-4 comprises can be on nitrogen during substituted heteroaryl ring, and such replacement may not cause charged quaternary nitrogen atoms.The definition that should be appreciated that HET-1-HET-4 does not comprise any O-O, O-S or S-S key.The definition that should be appreciated that HET-1-HET-4 does not comprise unsettled structure.
(1-4C) example of alkyl comprises methyl, ethyl, propyl group, sec.-propyl, butyl and the tertiary butyl; (3-6C) example of cycloalkyl comprises cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl; The example of halogen comprises fluorine, chlorine, bromine and iodine; The example of hydroxyl (1-4C) alkyl comprises hydroxymethyl, 1-hydroxyethyl, 2-hydroxyethyl, 2-hydroxypropyl, 3-hydroxypropyl, 1-hydroxyl sec.-propyl and 4-hydroxybutyl; (1-4C) example of alkoxyl group (1-4C) alkyl comprises methoxymethyl, ethoxyl methyl, tert.-butoxy methyl, 2-methoxy ethyl, 2-ethoxyethyl group, methoxy-propyl, 2-methoxy-propyl and methoxyl group butyl; (1-4C) example of alkyl S (O) p (1-4C) alkyl comprises methylsulfinyl methyl, ethyl sulfinyl methyl, ethyl sulfinyl ethyl, methylsulfinyl propyl group, methylsulfinyl butyl, sulfonyloxy methyl ylmethyl, ethylsulfonyl methyl, ethylsulfonyl ethyl, methyl sulphonyl propyl group, methyl sulphonyl butyl, methylthiomethyl, ethylmercapto group methyl, ethylmercapto group ethyl, methylthio group propyl group and methylthio group butyl; The example of amino (1-4C) alkyl comprises amino methyl, amino-ethyl, 2-aminopropyl, 3-aminopropyl, the amino sec.-propyl of 1-and the amino butyl of 4-; (1-4C) example of alkylamino (1-4C) alkyl comprises (N-methyl) amino methyl, (N-ethyl) amino methyl, 1-((N-methyl) amino) ethyl, 2-((N-methyl) amino) ethyl, (N-ethyl) amino-ethyl, (N-methyl) aminopropyl and 4-((N-methyl) amino) butyl; The example of two (1-4C) alkylamino (1-4C) alkyl comprises dimethylaminomethyl, methyl (ethyl) amino methyl, methyl (ethyl) amino-ethyl, (N, the N-diethyl) amino-ethyl, (N, the N-dimethyl) aminopropyl and (N, N-dimethyl) amino butyl; (1-4C) example of alkylamino comprises methylamino, ethylamino, propyl group amino, sec.-propyl amino, butyl amino and tertiary butyl amino; The example of two (1-4C) alkylamino comprises dimethylamino, methyl (ethyl) amino, diethylamino, dipropyl amino, diisopropylaminoethyl and dibutylamino;-C (O) (1-4C) example of alkyl comprises methyl carbonyl, ethyl carbonyl, propyl group carbonyl and tertiary butyl carbonyl.
Be to be understood that, because one or more unsymmetrical carbons, can have optically-active or racemic form in the scope of formula (I) compound as defined above at some, the present invention comprises any so any such optically-active or racemic form that has direct stimulation GLK or suppress the interactional character of GLK/GLKRP in its definition.The synthetic of optically-active form can be undertaken by the well-known vitochemical standard technique in affiliated field, for example by being synthesized by the optically-active raw material or being undertaken by the fractionation of racemic form.It is also understood that some compounds can exist with tautomeric form, and the invention still further relates to any and all tautomeric forms of the The compounds of this invention that can activate GLK.
In one embodiment of the invention, formula (I) compound is provided, in another embodiment, the pharmacologically acceptable salt of formula (I) compound is provided, in another embodiment, the interior hydrolyzable ester of body of formula (I) compound is provided, in another embodiment, provides the pharmacologically acceptable salt of the interior hydrolyzable ester of body of formula (I) compound.
The preferred value of each variable group is as follows.In appropriate circumstances, such value is to use with the embodiment of any value, definition, claims, aspect or contextual definition.Particularly, each value can be used as the most independent restriction of wide definition of formula (I) is used.In addition, each down train value can with one or more other down train values unite to make and be used for the wideest definition of restraint-type (I).
(1) R
1Be methoxymethyl, preferred configuration is preferably (S), and it is
(2) R
2Be-C (O) NR
4R
5
(3) R
2Be-SO
2NR
4R
5
(4) R
2Be-S (O)
pR
4
(5) R
2Be HET-2
(6) m is 1, and R
2In contraposition with respect to ehter bond
(7) m is 1, and n is 0 or 1
(8) m is 1, and n is 0
(9) m is 1, and n is 0, and R
2In contraposition with respect to ehter bond
(10) m is 1, and n is 1, R
2In contraposition with respect to ehter bond, R
3On ortho position with respect to ehter bond
(11) m is 1, and n is 1, R
2In contraposition with respect to ehter bond, R
3Between with respect to ehter bond on the position
(12) n is 0
(13) n is 1
(14) n is 2
(15) n is 2, and two R
3It all is halogen
(16) n is 2, and each R
3Be halogen or methoxyl group independently
(17) m is 1, and n is 2, and R
2In contraposition with respect to ehter bond
(18) m is 1, and n is 2, R
2In contraposition with respect to ehter bond, and each R
3On ortho position with respect to ehter bond
(19) m is 1, and n is 2, two R
3All be halogen, R
2In contraposition with respect to ehter bond, and two R
3On ortho position with respect to ehter bond
(20) R
3Be methyl fluoride or difluoromethyl
(21) R
3Be halogen or trifluoromethyl
(22) R
3It is halogen
(23) R
3Be chlorine or fluorine
(24) R
3It is fluorine
(25) R
3It is methoxyl group
(26) n is 2, and two R
3All be fluorine,
(27) n is 2, two R
3All be fluorine, and on 3-and 5-position (position) with respect to ehter bond
(28) m is 1, and n is 2, R
2In contraposition with respect to ehter bond, two R
3All be fluorine, and on 3-and 5-position with respect to ehter bond
(29) p is 0
(30) p is 1
(31) p is 2
(32) HET-1 is 5 yuan of heteroaryl rings
(33) HET-1 is 6 yuan of heteroaryl rings
(34) HET-1 is independently selected from R by 1 or 2
6Substituting group replace
(35) HET-1 is selected from R by 1
6Substituting group replace
(36) HET-1 is unsubstituted
(37) HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly and triazolyl
(38) HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly
(39) HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl
(40) HET-1 be selected from thiazolyl, pyrazolyl and _ the azoles base
(41) HET-1 be selected from thiadiazolyl group and _ di azoly
(42) HET-1 is selected from 1,3,4-thiadiazolyl group and 1,3,4-_ di azoly
(43) HET-1 is selected from 1,2,4-_ di azoly and 1,2,4-_ di azoly
(44) HET-1 is a pyrazolyl
(45) HET-1 is pyridyl or piperazinyl
(46) HET-1 is selected from thiazolyl, pyrazolyl, thiadiazolyl group and pyridyl;
(47) R
6Be selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4
(48) R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, hydroxymethyl, methoxymethyl, amino methyl, N-methylamino methyl, dimethylaminomethyl
(49) R
6Be selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl and two (1-4C) alkylamino (1-4C) alkyl
(50) R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl
(51) R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, hydroxymethyl and methoxymethyl
(52) R
6Be selected from methyl, ethyl, bromine, chlorine and fluorine
(53) R
6It is methyl
(54) R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl, dimethylaminomethyl, hydroxymethyl and methoxymethyl
(55) R
6Be selected from methyl, ethyl, amino methyl, N-methylamino methyl, dimethylaminomethyl, hydroxymethyl and methoxymethyl
(56) R
6Be selected from methyl, ethyl, sec.-propyl and methoxymethyl
(57) when there being 2 substituent R
6The time, two R
6All be selected from methyl, ethyl, bromine, chlorine and fluorine; Preferably, two R
6It all is methyl
(58) R
6Be selected from (1-4C) alkyl S (O) p (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4
(59) R
6Be HET-4
(60) HET-4 is selected from furyl, pyrryl and thienyl
(61) HET-4 is a furyl
(62) R
4Be hydrogen
(63) R
4Be (1-4C) alkyl [by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace]
(64) R
4Be (1-4C) alkyl [by 1 be selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl and-C (O) NR
5R
5Substituting group replace]
(65) R
4It is (1-4C) alkyl
(66) R
4By-OR
5(1-4C) alkyl that replaces
(67) R
4Replaced (1-4C) alkyl by HET-2
(68) R
4Be (3-6C) cycloalkyl, particularly cyclopropyl
(69) R
4Be selected from R
7(3-6C) cycloalkyl of replacing of group
(70) R
4Be selected from-OR
5(1-4C) (3-6C) cycloalkyl of the group of alkyl replacement
(71) R
4Be HET-2
(72) R
4Be selected from hydrogen, (1-4C) alkyl and quilt-OR
5(1-4C) alkyl that replaces
(73) HET-2 is unsubstituted
(74) HET-2 is independently selected from (1-4C) alkyl, hydroxyl and (1-4C) the substituting group replacement of alkoxyl group by 1 or 2
(75) HET-2 is complete saturated ring system
(76) HET-2 is complete unsaturated ring system
(77) HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl (78) HET-2 are selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, pyrrolidyl, thio-morpholinyl, tetrahydrofuran base and THP trtrahydropyranyl
(79) HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base
(80) HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, piperidyl, piperazinyl, 3-oxo piperazinyl, pyrrolidyl, pyrrolidone-base, 2-_ oxazolidone base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo tetrahydro-thienyl and 2-oxo-imidazole alkyl
(81) HET-2 be selected from morpholino, furyl, imidazolyl, _ the azoles base, different _ the azoles base, _ di azoly, piperidyl, piperazinyl, 3-oxo piperazinyl, pyrrolidyl, 2-Pyrrolidone base, 2-_ oxazolidone base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo tetrahydro-thienyl and 2-oxo-imidazole alkyl
(82) HET-2 be selected from morpholino, furyl, imidazolyl, different _ the azoles base, _ di azoly, piperidyl, piperazinyl, 3-oxo piperazinyl, pyrrolidyl, 2-Pyrrolidone base, THP trtrahydropyranyl, 1,1-dioxo tetrahydro-thienyl and 2-oxo-imidazole alkyl
(83) R
5Be hydrogen
(84) R
5Be (1-4) alkyl, preferably methyl
(85) R
5Be hydrogen or methyl
(86) R
7Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkoxyl group (1-4C) alkyl and hydroxyl (1-4C) alkyl
(87) R
7Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5And hydroxyl (1-4C) alkyl
(88) R
7Be selected from hydroxyl, methoxyl group ,-COMe ,-CONH
2,-CONHMe ,-CONMe
2And hydroxymethyl
(89) R
7Be selected from (1-4C) alkyl, hydroxyl and (1-4C) alkoxyl group
(90) R
7Be selected from methyl, ethyl, methoxyl group and hydroxyl
(91) R
7It is methyl
(92) R
8Be selected from methyl, hydroxyl, methoxyl group ,-COMe ,-CONH
2,-CONHMe ,-CONMe
2, hydroxymethyl, hydroxyethyl ,-NHMe and-NMe
2
(93) R
8Be selected from morpholino, piperidyl, piperazinyl, pyrrolidyl and azetidinyl
(94) R
8Be selected from methyl ,-COMe ,-CONH
2, hydroxyethyl and hydroxyl
(95) R
8It is methyl
(96) HET-3 is full saturated rings
(97) HET-3 is selected from morpholino, piperidyl, piperazinyl, pyrrolidyl and azetidinyl
(98) R
4And R
5Form the ring that defines by HET-3 with the nitrogen-atoms that they connected
(99) HET-3 is selected from pyrrolidyl and azetidinyl
(100) HET-3 is an azetidinyl
(101) HET-3 is 4,5 or 6 yuan of saturated or part unsaturated heterocycles as defined above
(102) HET-3 is 7 yuan of saturated or part unsaturated heterocycles as defined above
(103) HET-3 is 6-10 unit two ring fillings or part unsaturated heterocycle as defined above
(104) HET-3 is 7-azabicyclic [2.2.1] heptan-7-base
(105) HET-3 be selected from morpholino, piperidyl, piperazinyl, pyrrolidyl, azetidinyl and 7-azabicyclic [2.2.1] heptan-the 7-base
(106) HET-3 be selected from piperidyl, pyrrolidyl, azetidinyl and 7-azabicyclic [2.2.1] heptan-the 7-base
The The compounds of this invention of following preferred group is provided according to another aspect of the present invention:
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are provided,
Wherein:
R
1It is methoxymethyl;
R
2Be selected from-C (O) NR
4R
5,-SO
2NR
4R
5,-S (O)
pR
4And HET-2;
HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1,2 or 3 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or be independently selected from R by 1 or 2 on theheterocyclic nitrogen atom
6Substituting group replace, condition is that theheterocyclic nitrogen atom is not thus by quaternized; HET-2 is the heterocyclic ring that 5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group;
R
4Be selected from hydrogen, (1-4C) alkyl [optional quilt-OR
5Replace] and HET-2;
R
5Be hydrogen or (1-4C) alkyl;
Perhaps R
4And R
5Can form first heterocyclic radical ring system with the nitrogen-atoms that they connected by the 4-6 of HET-3 definition;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-OR
5(1-4C) alkyl;
HET-3 is the saturated or part unsaturated heterocycle basic ring of 4-6 unit that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace;
R
8Be selected from-OR
5(1-4C) alkyl;
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are provided,
Wherein:
R
1It is methoxymethyl;
R
2Be selected from-C (O) NR
4R
5,-SO
2NR
4R
5,-S (O)
pR
4And HET-2; HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1,2 or 3 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or be independently selected from R by 1 or 2 on theheterocyclic nitrogen atom
6Substituting group replace, condition is that theheterocyclic nitrogen atom is not thus by quaternized; HET-2 is the heterocyclic ring that 5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group;
R
4Be selected from hydrogen, (1-4C) alkyl [optional quilt-OR
5Replace] and HET-2;
R
5Be hydrogen or (1-4C) alkyl;
Perhaps R
4And R
5Can form the heterocyclic radical ring system that defines by HET-3 with the nitrogen-atoms that they connected;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-OR
5(1-4C) alkyl;
HET-3 is the saturated or part unsaturated heterocycle basic ring of 4-6 unit that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 7 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 other heteroatoms (except the N atom that is connected) that is independently selected from O, S and N, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 6-10 unit's two ring fillings or part unsaturated heterocycle basic ring, optional 1 the other nitrogen-atoms (except the N atom that connects) that contains of described heterocyclic ring, wherein-CH
2-group can be chosen wantonly by-C (O)-replacement; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be selected from R by 1
3Substituting group replace;
R
8Be selected from-OR
5(1-4C) alkyl;
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate as defined above are provided, wherein:
R
1It is methoxymethyl;
R
2Be selected from-C (O) NR
4R
5,-SO
2NR
4R
5,-S (O)
pR
4And HET-2;
HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1 or 2 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or be independently selected from R by 1 or 2 on theheterocyclic nitrogen atom
6Substituting group replace, condition is that theheterocyclic nitrogen atom is not thus by quaternized; HET-2 is the heterocyclic ring that 4,5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group;
R
4Be selected from (1-4C) alkyl [by 1 or 2 be independently selected from HET-2 ,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or (1-4C) alkyl;
Perhaps R
4And R
5Can form first heterocyclic radical ring system with the nitrogen-atoms that they connected by the 4-6 of HET-3 definition;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-3 is the saturated or part unsaturated heterocycle basic ring of 4-6 unit that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace;
R
8Be selected from-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkylamino, two (1-4C) alkylamino, HET-3 (wherein said ring is unsubstituted), (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate as defined above are provided, wherein:
R
1It is methoxymethyl;
R
2Be selected from-C (O) NR
4R
5,-SO
2NR
4R
5,-S (O)
pR
4And HET-2;
HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1 or 2 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or be independently selected from R by 1 or 2 on theheterocyclic nitrogen atom
6Substituting group replace, condition is that theheterocyclic nitrogen atom is not thus by quaternized;
HET-2 is the heterocyclic ring that 4,5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group;
R
4Be selected from (1-4C) alkyl [by 1 or 2 be independently selected from HET-2 ,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or (1-4C) alkyl;
Perhaps R
4And R
5Can form first heterocyclic radical ring system with the nitrogen-atoms that they connected by the 4-6 of HET-3 definition;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-3 is the saturated or part unsaturated heterocycle basic ring of 4-6 unit that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 7 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 other heteroatoms (except the N atom that is connected) that is independently selected from O, S and N, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 6-10 unit's two ring fillings or part unsaturated heterocycle basic ring, optional 1 the other nitrogen-atoms (except the N atom that connects) that contains of described heterocyclic ring, wherein-CH
2-group can be chosen wantonly by-C (O)-replacement; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be selected from R by 1
3Substituting group replace;
R
8Be selected from-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkylamino, two (1-4C) alkylamino, HET-3 (wherein said ring is unsubstituted), (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is 5 or 6 yuan of heteroaryl rings;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
HET-2 is 5 or 6 yuan of heterocyclic rings as defined above, and described heterocyclic ring contains 1 or 2 heteroatoms that is independently selected from O, N and S; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is 5 or 6 yuan of heteroaryl rings;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is 5 or 6 yuan of heterocyclic rings as defined above, and described heterocyclic ring contains 1 or 2 heteroatoms that is independently selected from O, N and S; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be selected from hydrogen, (1-4C) alkyl [optional quilt-OR
5Replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 be selected from morpholino, furyl, imidazolyl, different _ the azoles base, _ di azoly, piperidyl, piperazinyl, 3-oxo piperazinyl, pyrrolidyl, 2-Pyrrolidone base, THP trtrahydropyranyl, 1,1-dioxo tetrahydro-thienyl and 2-oxo-imidazole alkyl; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl and pyridazinyl;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be selected from hydrogen, (1-4C) alkyl [optional quilt-OR
5Replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 be selected from morpholino, furyl, imidazolyl, different _ the azoles base, _ di azoly, piperidyl, piperazinyl, 3-oxo piperazinyl, pyrrolidyl, 2-Pyrrolidone base, THP trtrahydropyranyl, 1,1-dioxo tetrahydro-thienyl and 2-oxo-imidazole alkyl; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be selected from (1-4C) alkyl [optional quilt-OR
5Replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is selected from piperidyl, piperazinyl, 3-oxo piperazinyl, 2-Pyrrolidone base, 2,5-dioxo pyrrolidyl, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 2-oxo-imidazole alkyl and 2,4-dioxo alkyl imidazole base; And
R
7It is (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be selected from (1-4C) alkyl [optional quilt-OR
5Replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is piperidyl or piperazinyl; And
R
7It is (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0;
HET-1 is selected from thiazolyl, thiadiazolyl group and pyrazolyl;
R
2Be-CONR
4R
5
R
4Be optional by methyl substituted piperidyl;
R
5Be hydrogen or methyl;
R
6It is methyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl and pyridazinyl;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be selected from (1-4C) alkyl [optional quilt-OR
5Replace] and HET-2;
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is selected from piperidyl, piperazinyl, 3-oxo piperazinyl, 2-Pyrrolidone base, 2,5-dioxo pyrrolidyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 2-oxo-imidazole alkyl and 2,4-dioxo alkyl imidazole base; And
R
7It is (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl and pyridazinyl;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4Be selected from (1-4C) alkyl [optional quilt-OR
5Replace] and HET-2;
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is piperidyl or piperazinyl; And
R
7It is (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4And R
5Form morpholino, piperidyl, piperazinyl, pyrrolidyl or azetidine basic ring with the nitrogen that they connected, described ring is chosen wantonly on carbon or nitrogen-atoms by R
8Replace;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl; And
R
8Be selected from hydroxyl, (1-4C) alkoxyl group and (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4And R
5Form morpholino, piperidyl, piperazinyl, pyrrolidyl or azetidine basic ring with the nitrogen that they connected, described ring is chosen wantonly on carbon or nitrogen-atoms by R
8Replace;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl; And
R
8Be tetramethyleneimine or piperidines.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4And R
5Form morpholino, piperidyl, piperazinyl, pyrrolidyl or azetidine basic ring with the nitrogen that they connected, described ring is chosen wantonly on carbon or nitrogen-atoms and is replaced by (1-4C) alkyl; And
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl and pyridazinyl;
R
2Be-CONR
4R
5Or-SO
2NR
4R
5
R
3Be halogen or trifluoromethyl;
R
4And R
5Form morpholino, piperidyl, piperazinyl, pyrrolidyl or azetidine basic ring with the nitrogen that they connected, described ring is chosen wantonly on carbon or nitrogen-atoms and is replaced by (1-4C) alkyl; And
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0;
HET-1 is selected from thiazolyl, thiadiazolyl group and pyrazolyl;
R
2Be-CONR
4R
5
R
4And R
5Form piperidyl or piperazinyl ring with the nitrogen that they connected, described ring is chosen wantonly and is replaced by (1-4C) alkyl on carbon or nitrogen-atoms or replaced by the tetramethyleneimine basic ring;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0;
HET-1 is selected from thiazolyl, thiadiazolyl group and pyrazolyl;
R
2Be-CONR
4R
5
R
4And R
5Form the azetidine basic ring with the nitrogen that they connected, described ring is chosen wantonly on carbon atom and is replaced by hydroxyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, hydroxymethyl, amino methyl, N-methylamino methyl and dimethylaminomethyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0;
HET-1 is selected from thiazolyl, thiadiazolyl group and pyrazolyl;
R
2Be-CONR
4R
5
R
4And R
5Form 7 yuan of ring HET-3 with the nitrogen that they connected, described ring is chosen wantonly on carbon or nitrogen-atoms by methyl substituted;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, hydroxymethyl, amino methyl, N-methylamino methyl and dimethylaminomethyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0;
HET-1 is selected from thiazolyl, thiadiazolyl group and pyrazolyl;
R
2Be-CONR
4R
5
R
4And R
5Form the bicyclic heterocycles HET-3 of 6-10 unit with the nitrogen that they connected;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, hydroxymethyl, amino methyl, N-methylamino methyl and dimethylaminomethyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is 5 or 6 yuan of heteroaryl rings;
R
2Be-S (O) pR
4
P is 1 or 2;
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
HET-2 contains 1 or 2 heteroatomic 5 or 6 yuan of heterocyclic ring as defined above that are independently selected from O, N and S; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is 5 or 6 yuan of heteroaryl rings;
R
2Be-S (O) pR
4
P is 1 or 2;
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 contains 1 or 2 heteroatomic 5 or 6 yuan of heterocyclic ring as defined above that are independently selected from O, N and S; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-S (O) pR
4
P is 1 or 2;
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl; And R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-S (O) pR
4
P is 1 or 2;
R
3Be halogen or trifluoromethyl;
R
4Be selected from hydrogen, (1-4C) alkyl [optional quilt-OR
5Replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be-S (O) pR
4
P is 1 or 2;
R
3Be halogen or trifluoromethyl;
R
4Be (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl and-C (O) NR
5R
5Substituting group replace];
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be-S (O) pR
4
P is 1 or 2;
R
3Be halogen or trifluoromethyl;
R
4Be selected from hydrogen, (1-4C) alkyl [optional quilt-OR
5Replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or methyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be-S (O) pR
4
P is 1 or 2;
R
3Be halogen or trifluoromethyl;
R
4It is (1-4C) alkyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from thiazolyl, thiadiazolyl group and pyrazolyl;
R
2Be-S (O) pR
4
P is 1 or 2;
R
4It is (1-4C) alkyl;
R
6It is methyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0;
HET-1 is selected from thiazolyl, thiadiazolyl group and pyrazolyl;
R
2Be-S (O) pR
4
P is 1 or 2;
R
4It is (3-6C) cycloalkyl;
R
6It is methyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be-S (O) pR
4
P is 1 or 2;
R
3Be halogen or trifluoromethyl;
R
4It is (1-4C) alkyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is 5 or 6 yuan of heteroaryl rings;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
5Be hydrogen or (1-4C) alkyl;
HET-2 contains 1 or 2 heteroatomic 5 or 6 yuan of heterocyclic ring as defined above that are independently selected from O, N and S; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
5Be hydrogen or methyl;
HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl; And R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
5Be hydrogen or methyl;
HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
5Be hydrogen or methyl;
HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
5Be hydrogen or methyl;
HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base; And
R
7Be selected from-OR
5(1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl; And
R
7It is (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 be selected from thiazolyl, isothiazolyl, thiadiazolyl group, pyrazolyl, imidazolyl, _ azoles base, different _ azoles base and _ di azoly;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base; And
R
7It is (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 is selected from azetidinyl, morpholino, morpholinyl, piperidyl, piperazinyl, 3-oxo piperazinyl, thio-morpholinyl, pyrrolidyl, pyrrolidone-base, 2,5-dioxo pyrrolidyl, 1,1-dioxo tetrahydro-thienyl, 2-_ oxazolidone base, 2-oxo-tetrahydrofuran base, tetrahydrofuran base, THP trtrahydropyranyl, 1,1-dioxo thiomorpholine generation, 1,3-dioxolane base, 2-oxo-imidazole alkyl, 2,4-dioxo alkyl imidazole base, pyranyl and 4-pyriconyl; And
R
7It is (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 1, and n is 0 or 1;
HET-1 is selected from pyridyl, piperazinyl, pyridazinyl and pyrimidyl;
R
2Be HET-2;
R
3Be halogen or trifluoromethyl;
R
6Be selected from methyl, ethyl, bromine, chlorine, fluorine, amino methyl, N-methylamino methyl and dimethylaminomethyl;
HET-2 be selected from furyl, thienyl, thiazolyl, isothiazolyl, thiadiazolyl group, pyridyl, piperazinyl, pyridazinyl, pyrazolyl, imidazolyl, pyrimidyl, _ the azoles base, different _ the azoles base, _ di azoly, pyrryl, 1,2,4-triazolyl and 1,2,3-triazoles base; And
R
7It is (1-4C) alkyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 0 or 1, and n is 0,1 or 2;
HET-1 is selected from thiazolyl, pyrazolyl, N-methylpyrazole-3-base, N-ethyl pyrazole-3-yl, 5-methylpyrazole-3-base, 4-methylthiazol-2-base, 5-methylthiazol-2-base, 5-methyl isophthalic acid, 3,4-thiadiazoles-2-base, 4-methyl isophthalic acid, 3,5-thiadiazoles-2-base, 4-hydroxymethyl thiazol-2-yl, 4-methoxymethyl thiazol-2-yl and 5-bromopyridine-2-base;
R
3Be selected from chlorine, fluorine and trifluoromethyl;
R
2Be selected from the azetidinyl carbonyl; the methoxy ethyl aminocarboxyl; the imidazolyl methyl aminocarboxyl; N-methyl piperidine-4-base aminocarboxyl; N methyl piperazine-4-base carbonyl; the dimethylamino carbonyl; morpholino carbonyl; the pyrrolidyl carbonyl; 7-azabicyclic [2.2.1] heptan-7-base carbonyl; the dimethylamino alkylsulfonyl; the morpholino alkylsulfonyl; the sec.-propyl amino-sulfonyl; amino-sulfonyl; N methyl piperazine-4-base alkylsulfonyl; the methoxy ethyl amino-sulfonyl; cyano group; ethylsulfonyl; methyl sulphonyl; methylthio group; methylsulfinyl; iprotiazem base and sec.-propyl alkylsulfonyl.
In another aspect of the present invention, formula (I) compound or its salt, prodrug or solvate are as defined above provided, wherein
R
1It is methoxymethyl;
M is 0 or 1, and n is 0,1 or 2;
HET-1 is selected from thiazolyl, pyrazolyl, N-methylpyrazole-3-base, N-ethyl pyrazole-3-yl, 5-methylpyrazole-3-base, 4-methylthiazol-2-base, 5-methylthiazol-2-base, 5-methyl isophthalic acid, 3,4-thiadiazoles-2-base, 4-methyl isophthalic acid, 3,5-thiadiazoles-2-base, 4-hydroxymethyl thiazol-2-yl, 4-methoxymethyl thiazol-2-yl and 5-bromopyridine-2-base;
R
3Be selected from chlorine, fluorine, methoxyl group and trifluoromethyl;
R
2Be selected from the azetidinyl carbonyl; the methoxy ethyl aminocarboxyl; the imidazolyl methyl aminocarboxyl; N-methyl piperidine-4-base aminocarboxyl; N methyl piperazine-4-base carbonyl; the dimethylamino carbonyl; morpholino carbonyl; the pyrrolidyl carbonyl; 7-azabicyclic [2.2.1] heptan-7-base carbonyl; the dimethylamino alkylsulfonyl; the morpholino alkylsulfonyl; the sec.-propyl amino-sulfonyl; amino-sulfonyl; N methyl piperazine-4-base alkylsulfonyl; the methoxy ethyl amino-sulfonyl; cyano group; ethylsulfonyl; methyl sulphonyl; methylthio group; methylsulfinyl; iprotiazem base and sec.-propyl alkylsulfonyl.
Preferred in addition The compounds of this invention is the compound of embodiment, and each these compound provides other independent aspects of the present invention.In aspect other, the invention provides the compound of two or more embodiment then.
In one aspect, concrete The compounds of this invention comprises any or multiple following compounds:
3-(4-{[(2-methoxy ethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-(4-{[(1H-imidazoles-2-ylmethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{4-[(4-methylpiperazine-1-yl) carbonyl] phenoxy group }-N-1,3-thiazol-2-yl benzamide;
3-(3-{[(2-methoxy ethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-(3-{[(1H-imidazoles-2-ylmethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{4-[(4-methylpiperazine-1-yl) carbonyl] phenoxy group }-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(morpholine-4-base carbonyl) phenoxy group] benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(tetramethyleneimine-1-base carbonyl) phenoxy group] benzamide;
3-[4-(7-azabicyclic [2.2.1] heptan-7-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-{2-chloro-4-[(dimethylamino) alkylsulfonyl] phenoxy group }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(2-chloro-4-{[(1-methylethyl) amino] alkylsulfonyl } phenyl) the oxygen base]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-{[2-chloro-4-({ [2-(methoxyl group) ethyl] amino } alkylsulfonyl) phenyl] the oxygen base }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-(2-chloro-4-[(4-methylpiperazine-1-yl) and alkylsulfonyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
The 3-{4-[(dimethylamino) alkylsulfonyl] phenoxy group }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-(4-[(4-methylpiperazine-1-yl) and alkylsulfonyl] phenyl } the oxygen base)-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
The 3-{4-[((1-methylethyl) alkylsulfonyl amino)] phenoxy group }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-(4-{[(2-methoxy ethyl) amino] alkylsulfonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-(4-cyano-benzene oxygen)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-{[4-(aminocarboxyl) phenyl] the oxygen base }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(ethylsulfonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-{[3-(methylthio group) phenyl] the oxygen base } benzamide;
3-(the 4-[(1-methylethyl) and sulfenyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[3-(methyl sulphonyl) phenoxy group] benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[3-(methylsulfinyl) phenoxy group] benzamide;
3-(the 4-[(1-methylethyl) and alkylsulfonyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(methyl sulphonyl) phenoxy group] benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-1,3-thiazol-2-yl benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-(4-methyl isophthalic acid, 3-thiazol-2-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-(5-methyl isophthalic acid, 3-thiazol-2-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-(5-methyl isophthalic acid, 3,4-thiadiazoles-2-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
N-(1-ethyl-1H-pyrazole-3-yl)-3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group] benzamide;
3-(3,5-two fluorophenoxies)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
N-(5-bromopyridine-2-yl)-3-(3,5-two fluorophenoxies)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base] benzamide;
3-(3,5-two fluorophenoxies)-N-[4-(hydroxymethyl)-1,3-thiazoles-2-yl]-5-[(1S)-and 2-methoxyl group-(1-methylethyl) oxygen base] benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(5-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(methyl sulphonyl) phenoxy group] benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-[4-(methoxymethyl)-1,3-thiazoles-2-yl]-5-[4-(methyl sulphonyl) phenoxy group] benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-(4-{[(1-methyl piperidine-4-yl) amino] carbonyl } phenoxy group)-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-(trifluoromethyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide; With
The 3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide; And/or be selected from
The 3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[4-(ethylsulfonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[2-fluoro-4-(methyl sulphonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[4-(ethylsulfonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
The 3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
2-methoxyl group-4-(3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenoxy group)-N-methyl-benzamide;
2-methoxyl group-4-(3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenoxy group)-N, the N-dimethyl benzamide;
3-fluoro-4-{3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-[(1H-pyrazole-3-yl amino) carbonyl] phenoxy group }-N, the N-dimethyl benzamide;
The 3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide; With
3-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(1,2,4-_ diazole-3-yl) phenoxy group] benzamide;
Or its salt, prodrug or solvate.
In yet another aspect, concrete The compounds of this invention comprises any or multiple following compounds:
3-(4-{[(2-methoxy ethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-(4-{[(1H-imidazoles-2-ylmethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-(3-{[(2-methoxy ethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-(3-{[(1H-imidazoles-2-ylmethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-{[2-chloro-4-({ [2-(methoxyl group) ethyl] amino } alkylsulfonyl) phenyl] the oxygen base }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-(4-{[(2-methoxy ethyl) amino] alkylsulfonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-(4-{[(1-methyl piperidine-4-yl) amino] carbonyl } phenoxy group)-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
Or its salt, prodrug or solvate.
In yet another aspect, concrete The compounds of this invention comprises any or multiple following compounds:
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{4-[(4-methylpiperazine-1-yl) carbonyl] phenoxy group }-N-1,3-thiazol-2-yl benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{4-[(4-methylpiperazine-1-yl) carbonyl] phenoxy group }-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(morpholine-4-base carbonyl) phenoxy group] benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(tetramethyleneimine-1-base carbonyl) phenoxy group] benzamide;
3-[4-(7-azabicyclic [2.2.1] heptan-7-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-(2-chloro-4-[(4-methylpiperazine-1-yl) and alkylsulfonyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-(4-[(4-methylpiperazine-1-yl) and alkylsulfonyl] phenyl } the oxygen base)-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-(trifluoromethyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
Or its salt, prodrug or solvate.
In yet another aspect, concrete The compounds of this invention comprises any or multiple following compounds:
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(tetramethyleneimine-1-base carbonyl) phenoxy group] benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-(trifluoromethyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
Or its salt, prodrug or solvate.
In yet another aspect, concrete The compounds of this invention comprises any or multiple following compounds:
2-methoxyl group-4-(3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenoxy group)-N-methyl-benzamide;
2-methoxyl group-4-(3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenoxy group)-N, the N-dimethyl benzamide;
Or its salt, prodrug or solvate.
In yet another aspect, concrete The compounds of this invention comprises:
3-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(1,2,4-_ diazole-3-yl) phenoxy group] benzamide; Or its salt, prodrug or solvate.
In yet another aspect, concrete The compounds of this invention comprises any or multiple following compounds:
3-{2-chloro-4-[(dimethylamino) alkylsulfonyl] phenoxy group }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(2-chloro-4-{[(1-methylethyl) amino] alkylsulfonyl } phenyl) the oxygen base]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
The 3-{4-[(dimethylamino) alkylsulfonyl] phenoxy group }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
The 3-{4-[((1-methylethyl) alkylsulfonyl amino)] phenoxy group }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-(4-cyano-benzene oxygen)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-{[4-(aminocarboxyl) phenyl] the oxygen base }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(ethylsulfonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-{[3-(methylthio group) phenyl] the oxygen base } benzamide;
3-(the 4-[(1-methylethyl) and sulfenyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[3-(methyl sulphonyl) phenoxy group] benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[3-(methylsulfinyl) phenoxy group] benzamide;
3-(the 4-[(1-methylethyl) and alkylsulfonyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(methyl sulphonyl) phenoxy group] benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-1,3-thiazol-2-yl benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-(4-methyl isophthalic acid, 3-thiazol-2-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-(5-methyl isophthalic acid, 3-thiazol-2-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-(5-methyl isophthalic acid, 3,4-thiadiazoles-2-yl) benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
N-(1-ethyl-1H-pyrazole-3-yl)-3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group] benzamide;
3-(3,5-two fluorophenoxies)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
N-(5-bromopyridine-2-yl)-3-(3,5-two fluorophenoxies)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base] benzamide;
3-(3,5-two fluorophenoxies)-N-[4-(hydroxymethyl)-1,3-thiazoles-2-yl]-5-[(1S)-and 2-methoxyl group-(1-methylethyl) oxygen base] benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(5-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(methyl sulphonyl) phenoxy group] benzamide;
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-[4-(methoxymethyl)-1,3-thiazoles-2-yl]-5-[4-(methyl sulphonyl) phenoxy group] benzamide;
The 3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide;
The 3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
The 3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-fluoro-4-{3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-[(1H-pyrazole-3-yl amino) carbonyl] phenoxy group }-N, the N-dimethyl benzamide;
The 3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
3-[4-(ethylsulfonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[2-fluoro-4-(methyl sulphonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide;
3-[4-(ethylsulfonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide;
Or its salt, prodrug or solvate.
The compounds of this invention can be with the form administration of prodrug.Prodrug is bioprecursor or the pharmaceutically acceptable compound (for example ester of The compounds of this invention or acid amides, particularly body in hydrolyzable ester) of degradable to generate The compounds of this invention in body.Multiple multi-form prodrug is known in the art.The example of such prodrug derivant can referring to:
A) Design of Prodrugs, H.Bundgaard writes, (Elsevier, 1985) and Methods in Enzymology, Vol.42, p.309-396, K.Widder waits the people to write (Academic Press, 1985);
B) A Textbook of Drug Design and Development, Krogsgaard-Larsen writes;
c)H.Bundgaard,Chapter?5“Design?and?Application?ofProdrugs”,H.Bundgaard?p.113-191(1991);
d)H.Bundgaard,Advanced?Drug?Delivery?Reviews,8,1-38(1992);
E) H.Bundgaard waits the people, Journal of Pharmaceutical Sciences, 77,285 (1988); With
F) N.Kakeya waits the people, Chem Pharm Bull, 32,692 (1984).
The content of above-mentioned file is incorporated herein by reference.
The example of prodrug is as follows.The interior hydrolyzable ester of body that contains the The compounds of this invention of carboxyl or hydroxyl, for example, hydrolysis is to generate the pharmaceutically acceptable ester of parent acid or alcohol in human or animal body.For carboxyl, suitable pharmaceutically acceptable ester comprises C
1-C
6The alkoxy methyl ester, methoxymethyl ester for example, C
1-6The alkanoyloxymethyl ester, oxy acid methyl neopentyl ester for example, phthalidyl ester, C
3-C
8Cyclo alkoxy carbonyl oxygen base C
1-C
6Alkyl ester, for example 1-cyclohexyl carbonyl oxygen base ethyl ester; 1,3-dioxole-2-ketone group methyl ester, 5-methyl isophthalic acid for example, 3-dioxole-2-ketone group methyl ester; And C
1-6The alkoxy-carbonyl oxy ethyl ester.
The interior hydrolyzable ester of body that contains the The compounds of this invention of hydroxyl comprises inorganic ester for example phosphoric acid ester (comprising phosphamide cyclic ester (phosphoramidic cyclic esters)) and alpha-acyloxy alkyl oxide and allied compound, as the ester result of hydrolysis in vivo, their fractures are to produce parent hydroxy.The example of alpha-acyloxy alkyl oxide comprises acetoxyl group methoxyl group and 2,2-dimethyl propylene acyloxy-methoxyl group.Be used for the hydroxyl organizer in the selection of group of hydrolyzable ester comprise benzoyl and phenyl acetyl, alkoxy carbonyl (to generate alkyl carbonate), dialkyl amido formyl radical and N-(dialkyl amido ethyl)-N-alkyl-carbamoyl (to generate carbamate), dialkyl amido ethanoyl and the carboxyl ethanoyl of alkyloyl, benzoyl, phenyl acetyl and replacement.
The suitable pharmacologically acceptable salt of The compounds of this invention is, for example, has the acid salt of the The compounds of this invention of enough alkalescence, for example, with the acid salt that for example inorganic or organic acid forms, described acid is for example hydrochloric acid, Hydrogen bromide, sulfuric acid, phosphoric acid, trifluoroacetic acid, citric acid or toxilic acid.In addition, suitable pharmacologically acceptable salt with enough tart benzo _ oxazinone derivatives of the present invention is an an alkali metal salt, for example sodium or sylvite, alkaline earth salt, for example calcium or magnesium salts, ammonium salt or can accept the salt that cationic organic bases forms, for example salt that forms with methylamine, dimethylamine, Trimethylamine 99, piperidines, morpholine or three-(2-hydroxyethyl) amine with physiology is provided.
Another feature of the present invention is to comprise the pharmaceutical composition of formula (I) compound or its salt, solvate or prodrug and pharmaceutically acceptable diluent or carrier as defined above.
According to another aspect of the present invention, the invention provides formula as defined above (I) compound as medicine.
The present invention also provides formula (I) compound that is used to prepare the medicine that is used for treating disease, particularly diabetes B by the GLK mediation.
The compounds of this invention suitably is mixed with is used for the pharmaceutical composition that uses in this mode.
According to another aspect of the present invention, the invention provides the method for disease, the especially diabetes of treatment GLK mediation, comprise formula (I) compound or its salt, solvate or prodrug to the administration significant quantity of the such treatment of needs.
Can comprise with the disease specific of The compounds of this invention or combination treatment: under the situation that does not have severe hypoglycemia danger, reduce the blood sugar (and effectively treating 1 type) in the diabetes B; Unusual lipidemia; Fat; Insulin resistance; Metabolism syndrome X; Glucose tolerance lowers.
As mentioned above, therefore the GLK/GLKRP system can be described as being potential " diabetes obesity " target (all useful in diabetes and obesity).Therefore, according to another aspect of the present invention, the invention provides formula (I) compound or its salt, solvate or prodrug are used for the medicine of combination therapy or prevent diabetes and obesity in preparation application.
According to another aspect of the present invention, the invention provides formula (I) compound or its salt, solvate or prodrug preparation be used for the treatment of or the medicine of prevention of obesity in application.
According to another aspect of the present invention, the invention provides the method for the combination therapy that is used for fat and diabetes, comprise formula (I) compound or its salt, solvate or prodrug to the administration significant quantity of the such treatment of needs.
According to another aspect of the present invention, the invention provides the method that is used for the treatment of obesity, comprise formula (I) compound or its salt, solvate or prodrug to the administration significant quantity of the such treatment of needs.
Composition of the present invention can be the form that is fit to an orally use (tablet for example, lozenge, hard or soft capsule, water or oil suspension, emulsion, can disperse powder or granule, syrup or elixir), the local form of using (creme for example, paste, gelifying agent or water or oil solution or suspension), the form of inhalation (for example micro mist powder or liquid aerosol) (for example is used for intravenously by the form (for example micro mist powder) or the form of parenterai administration that is blown into administration, subcutaneous, the aqua sterilisa of intramuscular or intramuscular administration or oil solution or be used for the suppository of rectal administration).The formulation that is suitable for orally using is preferred.
Composition of the present invention can utilize the well-known conventional medicine vehicle in this field to obtain by ordinary method.So the composition that is used to orally use can contain, for example, one or more tinting materials, sweeting agent, correctives and/or sanitas.
The suitable pharmaceutically acceptable vehicle that is used for tablet formulation comprises that for example, inert diluent is lactose, yellow soda ash, calcium phosphate or lime carbonate for example, and granulation agent and disintegrating agent be W-Gum or alginic acid for example; Tackiness agent is starch for example; Lubricant is Magnesium Stearate, stearic acid or talcum powder for example; Sanitas is ethyl p-hydroxybenzoate or propyl ester and oxidation inhibitor xitix for example for example.Tablet formulation can not have dressing or has dressing to change its disintegration and the sorption of activeconstituents in gi tract subsequently, or improves its stability and/or apparent, in any case, uses well-known conventional Drug coating in this field and method.
The composition that orally uses can be the form of hard gelatin capsule, wherein for example lime carbonate, calcium phosphate or kaolin mix activeconstituents with inert solid diluent, or become soft gelatin capsule, for example peanut oil, whiteruss or mixed with olive oil of activeconstituents and water or oil wherein.
Aqeous suspension generally contains activeconstituents and one or more suspension agents, for example Xylo-Mucine, methylcellulose gum, Vltra tears, sodiun alginate, polyvinylpyrrolidone, tragacanth and the gum arabic of micro mist form; Dispersion agent or wetting agent be the condensation product (for example polyoxyethylene stearic acid ester) of Yelkin TTS or oxyalkylene and lipid acid for example, or the condensation product of oxyethane and long chain aliphatic alcohol, 17 ethylene oxide hexadecanols (heptadecaethyleneoxycetanol) for example, or oxyethane and derived from the condensation product of the partial ester of lipid acid and hexitol, polyoxyethylene Sorbitol Powder monooleate for example, or the condensation product of oxyethane and long chain aliphatic alcohol, 17 ethylene oxide hexadecanols (heptadecaethyleneoxycetanol) for example, or oxyethane and derived from the condensation product of the partial ester of lipid acid and hexitol, polyoxyethylene Sorbitol Powder monooleate for example, or oxyethane and derived from the condensation product of the partial ester of lipid acid and hexitan, for example polyethylene sorbitan monooleate.Aqeous suspension can also contain one or more sanitass (for example ethyl p-hydroxybenzoate or propyl ester), antioxidant (for example xitix), tinting material, correctives and/or sweeting agent (for example sucrose, asccharin or aspartame).
Oil suspension can be prepared by activeconstituents being suspended in vegetables oil (for example peanut oil, sweet oil, sesame oil or Oleum Cocois) or the mineral oil (for example whiteruss).Oil suspension also can contain thickening material for example beeswax, paraffinum durum or hexadecanol.Can add sweeting agent for example above-mentioned those and correctives good to eat oral preparations is provided.These compositions can for example xitix be next anticorrosion by adding oxidation inhibitor.
Be fit to make the disperseed powder and the granule of aqeous suspension and generally contain activeconstituents and dispersion agent or wetting agent, suspension agent and one or more sanitass by adding entry.Suitable dispersion agent or wetting agent and suspension agent for example above-mentioned those.Additional vehicle is sweeting agent, correctives and tinting material for example, also can exist.
Pharmaceutical composition of the present invention also can exist with the form of oil-in-water emulsion.Oil phase can be a vegetables oil, for example sweet oil or peanut oil, or mineral oil, for example mixture of whiteruss or any of these.Suitable emulsifying agent can be, for example, natural gum is gum arabic or tragacanth for example, natural phospholipid is soybean lecithin and derived from the condensation product of the ester of lipid acid and hexitan or partial ester (for example sorbitan monooleate) and described partial ester and oxyethane polyoxyethylene sorbitan monooleate for example for example.Emulsion also can contain sweeting agent, correctives and sanitas.
Syrup and elixir can with for example glycerine, propylene glycol, Sorbitol Powder, aspartame or sucrose preparation of sweeting agent, and also can contain negative catalyst, sanitas, correctives and/or tinting material.
Described pharmaceutical composition can also be the form of sterilization injectable water or oil suspension, and it can utilize one or more suitable dispersions or wetting agent and suspension agent to prepare according to currently known methods, and these materials as mentioned above.The sterilization injectable formulation also can be to be present in nontoxic parenteral can accept sterilization Injectable solution or suspension in thinner or the solvent, for example solution in 1,3 butylene glycol.
The composition that is used for inhalation can be the conventional pressurised aerosol that is designed to distribute activeconstituents, and it is the form that contains the aerosol of micro mist solid or drop.Can use conventional aerosol propellant for example volatility fluorinated hydrocarbons or hydrocarbon, and the aerosol device is assembled into the activeconstituents of distribution and computation amount usually.
Other information of relevant preparation can be referring to the 5th volume of Comprehensive Medicinal Chemistry, 25.2 chapters (Corwin Hanschl; Chairman of Editorial Board), Pergamon Press 1990.
Merging with one or more vehicle must be according to being changed by treatment host and concrete route of administration with the amount of the activeconstituents that is prepared as single formulation.For example, be used for the preparation of human oral administration is generally contained, for example, 0.5mg-2g with suitably and the promoting agent of the mixed with excipients of convention amount, it can account for about 5-about 98% of said composition gross weight.Unit dosage generally contains the about 500mg activeconstituents of the 1mg-that has an appointment.About the further information of route of administration and dosage regimen can be referring to the 5th volume of Comprehensive Medicinal Chemistry, 253 chapters (Corwin Hanschl; Chairman of Editorial Board), Pergamon Press1990.
Formula (I) compound for the dosage of treatment or prevention purpose naturally should be according to the character of illness and seriousness, animal or patient's age and sex and route of administration, change according to the well-known principle of medicine.
For treatment or prevention purpose and use formula (I) compound the time generally is with per daily dose administration in the scope of for example 0.5mg-75mg/kg body weight, if desired can the gradation administration.Usually when adopting parenteral route, adopt than low dosage.So, for example,, generally adopt for example interior dosage of 0.5mg-30mg/kg weight range for intravenous administration.Similarly, for inhalation, adopt for example interior dosage of 0.5mg-25mg/kg weight range.Yet preferred oral administration.
The active rising of GLK of the present invention can be used as independent therapy, perhaps for the indication of being treated, can unite use with one or more other materials and/or treatment.When such combination therapy can be treated component by each, the mode of order or separate administration reaches.Treatment can be in single tablet or the tablet that is separating simultaneously.For example in treatment of diabetes, chemotherapy can comprise the treatment of following main type:
1) Regular Insulin and insulin analog;
2) Regular Insulin succagoga comprises sulfonylurea (for example Glyburide, Glipizide), diet glucose conditioning agent (for example repaglinide, nateglinide);
3) improve the promoting agent (for example inhibitors of dipeptidyl IV and GLP-1 agonist) of incretin effect;
4) insulin sensitizer comprises PPAR gamma agonist (for example pioglitazone and rosiglitazone) and has the PPAR α of combination and the promoting agent of gamma activity;
5) regulate liver glucose equilibrated promoting agent (for example N1,N1-Dimethylbiguanide, fructose-1 inhibitor, glycogen phosphorylase inhibitors, glycogen synthase kinase inhibitor);
6) reduce the promoting agent (for example acarbose) that absorbs glucose in the intestines;
7) stop the resorbent promoting agent (SGLT inhibitor) of kidney to glucose;
8) promoting agent (for example aldose reductase inhibitor) of the complication of the long-term hyperglycemia of treatment;
9) antiobesity agent (for example sibutramin and orlistat);
10) antilipidemic disease agent, for example HMG-CoA reductase inhibitor (for example Statins (statins)); PPAR alfa agonists (shellfish special class (fibrates), for example gemfibrozil); Cholic acid chelating agent (Colestyramine); Cholesterol absorption inhibitor (plant Sitosterol (stanol), synthetic inhibitor); Cholic acid absorption inhibitor (IBATi) and nicotinic acid and analogue (nicotinic acid and sustained release preparation);
11) hypotensive agent, for example beta-blocker (for example atenolol USP 23, Proprasylyte); ACE inhibitor (for example lisinopril); Calcium antagonist (for example Nifedipine); Angiotensin receptor antagonist (for example Candesartan), alpha-2 antagonists and diuretic(s) (for example Furosemide, benzthiazide);
12) hemostasis conditioning agent, antithrombotic agent for example, Fibrinolytic activator and anti-platelet agents; The zymoplasm antagonist; The Xa factor inhibitor; The VIIa factor inhibitors); Anti-platelet agents (for example acetylsalicylic acid, clopidogrel); Anti-coagulant (heparin and lower molecular weight analogue, r-hirudin) and warfarin;
13) promoting agent of the effect of antagonism hyperglycemic-glycogenolytic factor; With
14) anti-inflammatory agent, for example nonsteroidal anti-inflammatory (for example acetylsalicylic acid) and steroid antiphlogiston (for example cortisone).
According to another aspect of the present invention, the invention provides all cpds and salt/solvate and the prodrug that makes as end product in the following embodiments.
The compounds of this invention or its salt can prepare by any currently known methods that is used to prepare related compound on this compounds or the structure.Functional group can utilize ordinary method protection and deprotection.For example, for example amino and carboxylic acid protecting group of protecting group (and the mode of formation and last deprotection) can be referring to T.W.Greene and P.G.M.Wuts, " protecting group in the organic synthesis ", the 2nd edition, John Wiley ﹠amp; Sons, New York, 1991.
The method of synthesis type (I) compound provides as additional features of the present invention.Therefore, according to another aspect of the present invention, the invention provides the method for preparation formula (I) compound, described method comprises method a)-d) (wherein unless otherwise defined, otherwise variable define about formula (I) compound as mentioned):
(a) sour or its activated derivatives and formula (IV) compound with formula (III) reacts
Perhaps
(b) with formula V compound and the reaction of formula (VI) compound
X wherein
1Be leavings group, and X
2Be hydroxyl, perhaps X
1Be hydroxyl, and X
2It is leavings group;
Method (b) can also be carried out like this: use wherein P
1Be the intermediate ester of the formula (VII) of protecting group as described below, described and the well-known method of those skilled in the art is carried out the ester hydrolysis and acid amides forms by this paper other places then;
Perhaps
(c) with formula (VIII) compound and the reaction of formula (IX) compound
X wherein
3Be leavings group or organometallic reagent, and X
4Be hydroxyl, perhaps X
3Be hydroxyl, and X
4Be leavings group or organometallic reagent;
Method (c) can also be carried out like this: the intermediate ester of use formula (X), carry out ester hydrolysis and acid amides formation by this paper other places are described with the well-known method of those skilled in the art then; Perhaps
(d) with formula (XI) compound and the reaction of formula (XII) compound
X wherein
5It is leavings group;
And if necessary afterwards:
I) a kind of formula (I) compound is changed into another kind of formula (I) compound;
Ii) remove any protecting group; And/or
Iii) form its salt, prodrug or solvate.
For method b)-d), suitable leavings group X
1-X
5Be any leavings group about these type reaction known in the art, for example halogen, alkoxyl group, trifluoro-methanesulfonyl oxy, mesyloxy or tolysulfonyl oxygen base; Perhaps can change into the group (for example hydroxyl) of leavings group (for example oxygen base triphenyl _ yl) in position.
The commercially available acquisition of formula (III)-(XII) compound, or known in the art perhaps can make by methods known in the art, for example, and as described in the embodiment of the invention and make.About the further information of the method for preparing such compound, referring to our PCT open WO 03/000267, WO 03/015774 and WO 03/000262 and the reference wherein quoted.Usually should be appreciated that any aryl-O or alkyl-O key can choose that the method by nucleophilic substitution or metal catalytic forms in the presence of suitable alkali wantonly.
Those skilled in the art are well-known to be changed into another kind of formula (I) examples for compounds with a kind of formula (I) compound and comprises that functional group changes mutually, for example hydrolysis, hydrogenation, hydrogenolysis, oxidation or reduction and/or by standard reaction for example the coupling of acid amides or metal catalytic or nucleophilic displacement reaction carry out other functionalized.
For above-mentioned reaction, concrete reaction conditions is as follows, wherein works as P
1When being protecting group, P
1Be preferably C
1-4Alkyl is methyl or ethyl for example:
Method a)-amino linked reaction with carboxylic acid is well-known in the art to form acid amides.For example,
(i) use suitable linked reaction, for example, in room temperature, at suitable solvent for example in methylene dichloride (DCM), chloroform or the dimethyl formamide (DMF), in the presence of dimethyl aminopyridine (DMAP), the carbodiimide linked reaction of using EDAC (1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride) to carry out; Or
(ii) wherein by with oxalyl chloride in for example reaction and activated carboxylic is become the reaction of acyl chlorides in the presence of the DCM of suitable solvent.Afterwards can be under 0 ℃-80 ℃ temperature, at suitable solvent for example among chloroform or the DCM, at alkali for example in the presence of triethylamine or the pyridine, with acyl chlorides and the reaction of formula (IV) compound.
Method b)-formula V and (VI) compound can be at suitable solvent for example in DMF or the tetrahydrofuran (THF) (THF), use alkali for example sodium hydride or potassium tert.-butoxide, under 0-200 ℃ of temperature, for example acid chloride (II), palladium on carbon, venus crystals (II) or cupric iodide (I) react for optional use microwave heating or metal catalyst; Perhaps, can with formula V and (VI) compound together at suitable solvent for example among THF or the DCM, use suitable phosphine for example triphenylphosphine and azodiformate for example diethyl azodiformate react.Method b) can also use formula (VII) ester precursor for example aryl nitrile or trifluoromethyl derivative carry out, change into carboxylic acid then as mentioned above and form acid amides;
Method c)-Shi (VIII) and (IX) compound can be at suitable solvent for example among DMF or the THF, use alkali for example sodium hydride or potassium tert.-butoxide, under 0-200 ℃ of temperature, for example acid chloride (II), palladium on carbon, venus crystals (II) or cupric iodide (I) react for optional use microwave heating or metal catalyst; Method c) can also use formula (X) ester precursor for example aryl nitrile or trifluoromethyl derivative carry out, change into carboxylic acid then as mentioned above and form acid amides;
Method d)-Shi (XI) compound and formula (XII) compound can be at polar solvent for example DMF or non-polar solvents for example among the THF, use highly basic for example sodium hydride or potassium tert.-butoxide, under 0-200 ℃ of temperature, for example acid chloride (II), palladium on carbon, venus crystals (II) or cupric iodide (I) react for optional use microwave heating or metal catalyst.
It is believed that some formulas (III), (VI), (VII), (IX) and/or (XI) intermediate be new, and comprise independent aspects of the present invention.
It is believed that wherein R
1Be some formulas (III), (IX) of methoxymethyl and/or (XI) intermediate be new, and comprise independent aspects of the present invention.
During the preparation method, the protecting group that is used for intramolecular functional group may be favourable.Protecting group can by describe in the document or the chemical field any proper method that is suitable for removing the protecting group of being paid close attention to known to the skilled remove, the selection of method should realize this other groups of removing of protecting group and inferior limit ground disturbing molecule.
For the purpose of convenient, provide the specific examples of protecting group below, wherein " rudimentary " represents that this group preferably has 1-4 carbon atom.It is not exhaustive should understanding these examples.Though provide the specific examples of the method for removing protecting group below, these methods equally neither be exhaustive.The use of the protecting group of specifically not mentioning and the method for deprotection obviously belong in the scope of the present invention.
Carboxyl-protecting group can be into the residue of ester aliphatic series or aromatic grease group alcohol or become the residue (described alcohol or silanol preferably contain 1-20 carbon atom) of ester silanol.The example of carboxyl-protecting group comprises straight chain and side chain (C1-12) alkyl (for example sec.-propyl, the tertiary butyl); Lower alkoxy low alkyl group (for example methoxymethyl, ethoxyl methyl, isobutoxy methyl; Lower aliphatic acyloxy low alkyl group (for example acetoxy-methyl, propionyloxy methyl, butyryl acyloxy methyl, oxy acid methyl neopentyl); Elementary alkoxy carbonyl oxygen base low alkyl group (for example 1-methoxycarbonyl oxygen base ethyl, 1-ethoxy carbonyl oxygen base ethyl); Aromatic yl elementary alkyl (for example to methoxy-benzyl, adjacent nitrobenzyl, to nitrobenzyl, diphenyl-methyl and phthalidyl); Three (low alkyl group) silyl (for example trimethyl silyl and t-butyldimethylsilyl); Three (low alkyl group) silyl low alkyl group (for example trimethyl silyl ethyl); (2-6C) alkenyl (for example allyl group and vinyl ethyl).
The method that is particularly suitable for removing carboxyl-protecting group comprise for example acid-, metal-or enzymatic-catalytic hydrolysis.
The example of hydroxyl protecting group comprises low-grade alkenyl (for example allyl group); Low-grade alkane acidyl (for example ethanoyl); Elementary alkoxy carbonyl (for example tert-butoxycarbonyl); Low-grade alkenyl oxygen base carbonyl (for example allyl group oxygen base carbonyl); Aryl-lower alkoxy carbonyl (for example benzoyl oxygen base carbonyl, to methoxy-benzyl oxygen base carbonyl, adjacent nitrobenzyl oxygen base carbonyl, to nitrobenzyl oxygen base carbonyl); Three lower alkyl/aryl groups silyls (for example trimethyl silyl, t-butyldimethylsilyl, t-butyldiphenylsilyl); Aromatic yl elementary alkyl (for example benzyl); With triaryl low alkyl group (for example trityl group).
The example of amino protecting group comprises formyl radical, aralkyl (for example the benzyl of benzyl and replacement, for example to methoxy-benzyl, nitrobenzyl and 2,4-dimethoxy-benzyl, and trityl group); The two pairs of anisyl methyl and furyl methyl; Elementary alkoxy carbonyl (for example tert-butoxycarbonyl); Low-grade alkenyl oxygen base carbonyl (for example allyl group oxygen base carbonyl); The aryl-lower alkoxy carbonyl (for example benzyl oxygen base carbonyl, to methoxy-benzyl oxygen base carbonyl, adjacent nitrobenzyl oxygen base carbonyl, to nitrobenzyl oxygen base carbonyl; Trialkylsilkl (for example trimethyl silyl and t-butyldimethylsilyl); Alkylidene group (for example methylene radical); The benzylidene of benzylidene and replacement.
The method that is fit to remove hydroxyl and amino protecting group comprise for example acid-, alkali, metal-or enzymatic-catalytic hydrolysis, or, use photodissociation, or, use fluorion for silyl for for example adjacent nitrobenzyl oxygen of group base carbonyl.
The example of the protecting group of amide group comprises aralkoxy methyl (for example benzyloxymethyl of benzyl oxygen ylmethyl and replacement); Alkoxy methyl (for example methoxymethyl and trimethylsilylethoxymethyl); Trialkyl/aryl silyl (for example trimethyl silyl, t-butyldimethylsilyl, t-butyldiphenylsilyl); Trialkyl/aryl silyl oxygen ylmethyl (for example t-butyldimethylsilyl oxygen ylmethyl, t-butyldiphenylsilyl oxygen ylmethyl); 4-alkoxyl phenyl (for example 4-p-methoxy-phenyl); 2,4-two (alkoxyl group) phenyl (for example 2,4-Dimethoxyphenyl); 4-alkoxybenzyl (for example 4-methoxy-benzyl); 2,4-two (alkoxyl group) benzyl (for example 2,4-two (methoxyl group) benzyl); And alkane-1-thiazolinyl (for example vinyl of allyl group, but-1-ene base and replacement, for example 2-phenyl vinyl).
The aralkoxy methyl can be incorporated on the amide group with suitable aralkoxy methyl chloride reaction by the latter, and remove by catalytic hydrogenation.Alkoxy methyl, trialkyl/aryl silyl and trialkyl/silyl oxygen ylmethyl can be introduced and remove with acid by acid amides is reacted with suitable muriate; Or in containing the situation of silyl-group, use fluorion.Described alkoxyl phenyl and alkoxybenzyl are by introducing with suitable halogenide arylation or alkylation and by removing with the ceric ammonium nitrate oxidation.At last, alkane-1-thiazolinyl can be by introducing acid amides and suitable aldehyde reaction and remove with acid.
The following example illustrates and does not limit the application's scope.Each compounds represented that exemplifies concrete and independent aspects of the present invention.In following non-limiting examples, unless otherwise indicated:
(i) evaporation is being carried out under the vacuum and aftertreatment is to remove residual solids for example by carrying out after removing by filter siccative by rotary evaporation;
(ii) operation is at room temperature carried out, and just carries out in 18-25 ℃ scope and under the atmosphere of rare gas element such as argon gas or nitrogen;
(iii) yield only is that to illustrate and need not be maximum yield;
(iv) the structure of the end product of formula (I) be by have 300 or nuclear (generally the being proton) mr (NMR) and the mass-spectrometric technique of the field intensity (for proton) of 400MHz determine; The proton resonance chemical displacement value is to measure on the δ scale and the multiplicity at peak shows below: s, and unimodal; D, doublet; T, triplet; M, multiplet; Br, broad peak; Q, quartet; Quin, quintet;
(v) intermediate is generally qualitative fully, and purity is analyzed by thin-layer chromatography (TLC), high performance liquid chromatography (HPLC), infrared (IR) or NMR and assessed;
(vi) Isolute silica gel tube is meant the silica gel tube (40g-400g) of pre-filling, uses biotage pump and level part collector system wash-out; Biotage UK Ltd, Hertford, Herts, UK.
Abbreviation
The DCM methylene dichloride;
The DEAD diethyl azodiformate;
The DIAD diisopropyl azodiformate;
DIPEA N, the N-diisopropyl ethyl amine;
The DMSO methyl-sulphoxide;
The DMA N,N-DIMETHYLACETAMIDE;
The DMF dimethyl formamide;
EDAC 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride;
HATU O-(7-azepine benzo triazol-1-yl)-1,1,3,3-tetramethyl-urea hexafluorophosphate;
The HPLC high performance liquid chromatography;
The HPMC Vltra tears;
LCMS liquid chromatography/mass spectrum;
The NMR nucleus magnetic resonance;
The RT room temperature;
The THF tetrahydrofuran (THF).
All compound titles all are to use the name of ACD NAME computer packages.
Embodiment 1:3-(4-{[(2-methoxy ethyl) amino] carbonyl } phenoxy group)-5-(2-(1S)-methoxyl group-(1-methylethyl) oxygen base)-N-1,3-thiazol-2-yl benzamide
To 4-({ 3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-[(1,3-thiazol-2-yl amino) carbonyl] phenyl the oxygen base) add DIPEA (0.11mL) in phenylformic acid (107mg), HATU (122mg) and the suspension of 2-methoxy ethyl amine (38mg) in DMF (2mL), and with this mixture stirring at room 1 hour.Add entry (30mL), (3 * 15mL) extract with ethyl acetate with this mixture.With the organic extract liquid salt water washing that merges, dry (MgSO
4), and be evaporated to resistates, by the silica gel chromatography purifying, as eluent, obtained required compound (63mg) with ethyl acetate.
1H?NMR?δ(d
6-DMSO):1.2(d,3H),3.3(s,6H),3.4-3.5(m,6H),4.7-4.8(m,1H),6.85(s,1H),7.1(d,2H),7.25(m,2H),7.55(d,2H),7.9(d,2H),8.45(s,1H);m/z?486(M+H)
+
To be similar to above-mentioned method, also made embodiment 1a-1c:
Embodiment 1 required acid is as described below making:
4-(3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-[(1,3-thiazol-2-yl amino) carbonyl] phenyl } the oxygen base) phenylformic acid
With 4-({ 3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-[(1,3-thiazol-2-yl amino) carbonyl] phenyl } the oxygen base) solution of ethyl benzoate (334mg) in THF (10mL) is added in the solution of lithium hydroxide monohydrate (82mg) in water (5mL).Stirring at room 16 hours, and vacuum was removed THF with this mixture.With the water layer acidifying, and filter out solid precipitation with 1M hydrochloric acid (1.83mL), wash with water, and vacuum-drying, required compound (268mg) obtained.
1H?NMR?δ(d
6-DMSO):1.2(d,3H),3.25(s,3H),3.5(m,2H),4.7-4.8(m,1H),6.9(t,1H),7.1(d,2H),7.25(d,1H),7.35(s,1H),7.55(d,2H),7.95(d,2H),12.75(s,1H);m/z?429(M+H)
+
4-(3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-[(1,3-thiazol-2-yl amino) carbonyl] phenyl } the oxygen base) ethyl benzoate
With 3-hydroxyl-5-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-N-1,3-thiazol-2-yl benzamide (1.0g), 4-ethoxy carbonyl phenyl-boron dihydroxide (1.18g), venus crystals (II) are (1.19g), triethylamine (2.25mL) and the new solution of activatory 4_ molecular sieve (4g) in DCM (50mL) stirred 2 days under normal pressure in room temperature.Via diatomite filtration, (2 * 10mL) washings, vacuum is removed DCM, and remaining oily matter is distributed between ethyl acetate (75mL) and 1M hydrochloric acid (30mL) with DCM with this reaction mixture.Isolate ethyl acetate layer, use sodium bicarbonate aqueous solution and salt water washing successively, dry (MgSO
4), and be evaporated to resistates, by the silica gel chromatography purifying, as eluent, obtained required compound (700mg) with the mixture of 30% ethyl acetate in isohexane.
1H?NMR?δ(CDCl
3):1.3(d,3H),1.4(t,3H),3.4(s,3H),3.5-3.6(m,2H),4.35(q,2H),4.5-4.6(m,1H),6.85(s,1H),6.95(d,1H),7.0(d,2H),7.15(s,1H),7.2(d,1H),7.35(d,1H),8.05(d,2H);m/z?457(M+H)
+
The 3-hydroxyl-5-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-N-1,3-thiazol-2-yl benzamide
With 3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-the 5-{[(2-aminomethyl phenyl) methyl] the oxygen base }-N-1,3-thiazol-2-yl benzamide (6.9g) and the solution of thioanisole (10mL) in trifluoroacetic acid (65mL) were in stirring at room 16 hours.Vacuum is removed trifluoroacetic acid, and remaining oily matter is distributed between ethyl acetate (75mL) and sodium bicarbonate aqueous solution (200mL).Isolate water layer, the usefulness ethyl acetate (2 * 75mL) extractions, and with the organic extract liquid salt water washing that merges, dry (MgSO
4), and be evaporated to resistates, by the silica gel chromatography purifying, as eluent, obtained required compound (4.6g) with the mixture of 50% ethyl acetate in isohexane.
1H?NMR?δ(CDCl
3):1.3(d,3H),3.4(s,3H),3.5-3.6(m,2H),4.5-4.6(m,1H),6.65(s,1H),6.95(d,1H),7.05(s,1H),7.1(s,1H),7.25(d,1H);m/z?309(M+H)
+
3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-the 5-{[(2-aminomethyl phenyl) methyl] the oxygen base }-N-1,3-thiazol-2-yl benzamide
To 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[(2-aminomethyl phenyl) methyl] the oxygen base } add oxalyl chloride (2.83mL) in the solution of phenylformic acid (9.55g) in DCM (140mL), add DMF (1) then, and with this mixture stirring at room 16 hours.DCM and excessive oxalul chloride vacuum are removed, remaining oily matter are dissolved among the DCM (25mL), be added in thiazolamine (2.84g) and the solution of triethylamine (7.88mL) in DCM (75mL) at 0-5 ℃, and with this mixture stirring at room 4 hours.DCM and excess of triethylamine vacuum are removed, remaining oily matter is distributed between ethyl acetate (100mL) and 1M hydrochloric acid (100mL).Isolate ethyl acetate layer, use 1M hydrochloric acid, sodium bicarbonate aqueous solution and salt water washing successively, dry (MgSO
4), and be evaporated to resistates, it by the alumina chromatogram purification, as eluent, has been obtained required compound (11.0g) with ethyl acetate.
1H?NMR?δ(CDCl
3):1.3(d,3H),2.35(s,3H),3.4(s,3H),3.5-3.6(m,2H),4.55-4.6(m,1H),5.0(s,2H),6.8(s,1H),6.95(d,1H),7.15(s,1H),7.25(m,5H),7.4(d,1H);m/z?413(M+H)
+
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[(2-aminomethyl phenyl) methyl] the oxygen base } phenylformic acid
With 3-[{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[(2-aminomethyl phenyl) methyl] the oxygen base } solution of methyl benzoate (10.65g) in THF (200mL) and methyl alcohol (50mL) is added in the solution of lithium hydroxide monohydrate (6.0g) in water (100mL).This mixture stirring at room 16 hours, is removed THF and methyl alcohol vacuum.With hydrochloric acid water layer is acidified to pH1, and (3 * 50mL) extract with ethyl acetate.With the organic extract liquid salt water washing that merges, dry (MgSO
4) and evaporation, obtained required compound (9.55g).m/z?329(M-H)
-
3-[{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[(2-aminomethyl phenyl) methyl] the oxygen base } methyl benzoate
With 3-hydroxyl-5-{[(2-aminomethyl phenyl) methyl] the oxygen base } suspension that stirring of triphenylphosphine (39.2g) in anhydrous DCM (900mL) of methyl benzoate (15.3g) and polymkeric substance load cools off in ice bath, drips diisopropyl azodiformate (11.88mL).This reaction mixture was stirred 30 minutes at 0-5 ℃, drip (R)-1-methoxyl group-propan-2-ol.This reaction mixture stirring at room 16 hours, via diatomite filtration, is evaporated to resistates with DCM,, as eluent, has obtained required compound (10.7g) with the mixture of 10% ethyl acetate in isohexane by the silica gel chromatography purifying.
1H?NMR?δ(CDCl
3):1.3(d,3H),2.4(s,3H),3.4(s,3H),3.5-3.6(m,2H),3.9(s,3H),4.55-4.6(m,1H),5.0(s,2H),6.8(s,1H),7.25(m,5H),7.4(d,1H)
3-hydroxyl-5-{[(2-aminomethyl phenyl) methyl] the oxygen base } methyl benzoate
At 0 ℃, to 3, (50g, 0.30mol) add sodium hydride in the solution in DMF (500mL) (10.8g 0.27mol), remains on temperature of reaction below 10 ℃ the 5-methyl dihydroxy benzoate in batches.Allow this reaction be warmed to 15 ℃, and stirred 20 minutes.This mixture is cooled to 0 ℃, with 30 minutes adding 2-methyl-benzyl bromine (36mL, 0.27mol) solution in DMF (50mL).This reaction is warmed to room temperature, and vacuum concentration, with remaining oily matter between ethyl acetate (500mL) and water (250mL) in batches, isolate ethyl acetate layer, water and salt water washing successively, dry (MgSO
4), and be evaporated to resistates, and by the silica gel chromatography purifying, carry out gradient elution with the mixture of 0-100% ethyl acetate in isohexane, obtained required compound (21.9g).
1H?NMR?δ(CDCl
3)2.39(s,3H),3.90(s,3H),5.02(s,2H),5.61(s,1H),6.69(t,1H),7.15-7.42(m,6H)
Embodiment 2:3-(3-{[(2-methoxy ethyl) amino] carbonyl } phenoxy group)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide
To 3-({ 3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-[(1,3-thiazol-2-yl amino) carbonyl] phenyl the oxygen base) add DIPEA (0.11mL) in phenylformic acid (107mg), HATU (122mg) and the suspension of 2-methoxy ethyl amine (38mg) in DMF (2mL), and with this mixture stirring at room 1 hour.Add entry (30mL), (3 * 15mL) extract with ethyl acetate with this mixture.With the organic extract liquid salt water washing that merges, dry (MgSO
4), and be evaporated to resistates, by the silica gel chromatography purifying, as eluent, obtained required compound (85mg) with ethyl acetate.
1H?NMR?δ(d
6-DMSO):1.2(d,3H),3.25(s,3H),3.3(s,3H),3.4-3.5(m,6H),4.7-4.8(m,1H),6.8(s,1H),7.2-7.25(m,3H),7.55(m,4H),7.7(d,1H)8.55(t,1H),12.6(s,1H);m/z486(M+H)
+
In a similar manner, also made embodiment 2a:
Embodiment 2 required acid are as described below making:
3-(3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-[(1,3-thiazol-2-yl amino) carbonyl] phenyl } the oxygen base) phenylformic acid
With 3-({ 3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-[(1,3-thiazol-2-yl amino) carbonyl] phenyl } the oxygen base) solution of ethyl benzoate (319mg) in THF (10mL) is added in the solution of lithium hydroxide monohydrate (78mg) in water (5mL).Stirring at room 16 hours, vacuum was removed THF with this mixture.With the water layer acidifying, filter out solid precipitation with 1M hydrochloric acid (1.75mL), wash with water, and vacuum-drying, required compound (283mg) obtained.
1H?NMR?δ(d
6-DMSO):1.2(d,3H),3.25(s,3H),3.5(m,2H),4.7-4.8(m,1H),6.85(t,1H),7.25(m,2H),7.35(dd,1H),7.55(m,4H),7.75(d,1H);m/z?429(M+H)
+
3-(3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-[(1,3-thiazol-2-yl amino) carbonyl] phenyl } the oxygen base) ethyl benzoate
With 3-hydroxyl-5-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-N-1,3-thiazol-2-yl benzamide (1.0g), 3-ethoxy carbonyl phenyl-boron dihydroxide (1.18g), venus crystals (II) are (1.19g), triethylamine (2.25mL) and the new solution of activatory 4_ molecular sieve (4g) in DCM (50mL) stirred 2 days under normal pressure in room temperature.Via diatomite filtration, (2 * 10mL), vacuum is removed DCM, and remaining oily matter is distributed between ethyl acetate (75mL) and 1M hydrochloric acid (30mL) with the DCM washing with this reaction mixture.Isolate ethyl acetate layer, use sodium bicarbonate aqueous solution and salt water washing successively, dry (MgSO
4), and be evaporated to resistates, by silica gel chromatography purifying (with the mixture wash-out of 30% ethyl acetate in isohexane), obtained required ester (680mg).
1H?NMR?δ(CDCl
3):1.3(d,3H),1.4(t,3H),3.4(s,3H),3.5-3.6(m,2H),4.35(q,2H),4.5-4.6(m,1H),6.8(t,1H),6.95(d,1H),7.1(d,1H),7.2(m,2H),7.3(d,1H),7.4(t,1H),7.7(d,1H),7.85(d,1H),11.6(s,1H);m/z?457(M+H)
+
The 3-hydroxyl-5-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-N-1, the synthetic of 3-thiazol-2-yl benzamide is described in the foregoing description 1.
Embodiment 3:3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{4-[(4-methylpiperazine-1-yl) carbonyl] phenoxy group }-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide
To 4-[(3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl phenyl) the oxygen base] add DIPEA (0.35mL) in phenylformic acid (212mg), HATU (400mg) and the suspension of N methyl piperazine (105mg) in DMF (10mL), and with this mixture stirring at room 24 hours.Add entry (30mL), with this mixture ethyl acetate extraction (3 * 15mL).With the organic extract liquid salt water washing that merges, dry (MgSO
4), and be evaporated to resistates, and by the silica gel chromatography purifying, carry out gradient elution with the mixture of 0-50% methyl alcohol in ethyl acetate, obtained required compound (130mg).
1H?NMRδ(CDCl
3):1.32(d,3H),2.35(s,3H),2.43(m,4H),3.41(s,3H),3.54(m,2H),3.6-3.8(m,4H),3.82(s,3H),4.59(m,1H),6.78(m,2H),7.05(t,3H),7.22(m,1H),7.27(m,1H),7.42(d,2H),8.30(br?s,1H);m/z?508(M+H)
+
To be similar to above-mentioned method, also made embodiment 3a-3d:
Embodiment 3 required acid are as described below making:
4-[(3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base])-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenyl) the oxygen base] phenylformic acid
With 4-[(3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base])-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenyl) the oxygen base] solution of ethyl benzoate (5.45g) in THF (200mL) is added in the solution of lithium hydroxide monohydrate (2.52g) in water (100mL).Stirring at room 48 hours, vacuum was removed THF with this mixture.With the water layer acidifying, and filter out solid precipitation with 1M hydrochloric acid (60mL), wash with water, and vacuum-drying, required acid (5g) obtained.
1H?NMR?δ(d
6-DMSO):1.22(d,3H),3.26(s,3H),3.45(m,2H),3.75(s,3H),4.71(m,1H),6.51(m,1H),6.84(m,1H),7.08(d,2H),7.24(m,1H),7.44(s,1H),7.57(m,1H),7.95(d,2H),10.84(br?s,1H),12.80(br?s,1H);m/z?426(M+H)
+
4-[(3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base])-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenyl) the oxygen base] ethyl benzoate
With 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide (10.0g), 4-ethoxy carbonyl phenyl-boron dihydroxide (9.4g), venus crystals (II) (9g), triethylamine (23mL) and the new solution of activatory 4_ molecular sieve (36g) in DCM (500mL) stirred 2 days under normal pressure in room temperature.Via diatomite filtration, (2 * 50mL), vacuum is removed DCM, and remaining oily matter is distributed between ethyl acetate (500mL) and 1M hydrochloric acid (200mL) with the DCM washing with this reaction mixture.Isolate ethyl acetate layer, use sodium bicarbonate aqueous solution and salt water washing successively, dry (MgSO
4), and be evaporated to resistates, and by the silica gel chromatography purifying, carry out gradient elution with the mixture of 50-100% ethyl acetate in isohexane, obtained required compound (5.47g).
1H?NMR?δ(CDCl
3):1.3(m,3H),1.41(t,3H),3.39(s,3H),3.49(m,1H),3.58(m,1H),3.78(s,3H),4.38(q,2H),4.58(m,1H),6.79(m,2H),7.01-7.1(m,3H),7.26(m,2H),8.01(m,2H),8.61(br?s,1H);m/z?454(M+H)
+
The 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide
To 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[(phenyl methyl) the oxygen base] add the slurries of 10% palladium on carbon (727mg) in THF (1mL) and methyl alcohol (1mL) in the solution of benzamide (7.07g) in THF (50mL) and methyl alcohol (50mL).This mixture is placed under the vacuum, and under nitrogen atmosphere, stirred 70 hours.Via diatomite filtration, (2 * 100mL) wash diatomite, vacuum concentration then with methyl alcohol with this mixture.Resistates is dissolved in the ethyl acetate (10mL), handles with isohexane (40mL), with solid filtering, and with isohexane (50mL) washing, obtained required compound (5.17g), it need not be further purified and directly use.
1H NMR δ (d
6-DMSO): 1.22 (d, 3H), 3.28 (s, 3H are covered by water), 3.38-3.53 (m, 2H), 3.76 (s, 3H), 4.65 (m, 1H), 6.44 (m, 1H), 6.54 (m, 1H), 6.93 (s, 1H), 7.04 (s, 1H), 7.57 (m, 1H), 9.63 (br s, 1H), 10.60 (s, 1H); M/z 306 (M+H)
+, 304 (M-H)
-
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[(phenyl methyl) the oxygen base] benzamide
With 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } solution of phenylformic acid (8.73g) in DCM (150mL) is cooled to 0 ℃.Under agitation add oxalyl chloride (4.81mL) and DMF (0.15mL) lentamente.Allow this mixture be warmed to room temperature and stirred 16 hours, vacuum is removed organic layer then, with resistates with toluene (75mL) azeotropic.This roughage is dissolved among the DCM (75mL), is added to lentamente in 1-methyl isophthalic acid H-pyrazoles-3-amine (3.35g) and the suspension that is stirring of DIPEA (14.4mL) in DCM (75mL).This mixture stirring at room 18 hours, then with organic layer vacuum-evaporation, is dissolved in resistates in the ethyl acetate (150mL).Organic phase is washed with hydrochloric acid (100mL) and salt solution (50mL), and dry (MgSO
4), vacuum-evaporation has then obtained roughage.With it at 200g Biotage Flash 75 SiO
2Pass through chromatography purification (with the mixture wash-out of 30-90% ethyl acetate in isohexane) on the post, and vacuum-evaporation, required compound (7.07g) obtained.
1H NMR δ (d
6-DMSO): 1.23 (d, 3H), 3.28 (s, 3H are covered by water), 3.40-3.52 (m, 2H), 3.77 (s, 3H), 4.70 (m, 1H), 5.03 (s, 2H), 6.56 (m, 1H), 6.71 (m, 1H), 7.18 (s, 1H), 7.24 (s, 1H), 7.32-7.47 (br m, 5H), 7.58 (m, 1H), 10.73 (s, 1H); M/z 396 (M+H)
+.
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } phenylformic acid
With 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base solution of methyl benzoate (77.4mmol) in the mixture of THF (232mL) and methyl alcohol (232mL) handles with 2M sodium hydroxide solution (232mmol), with this reaction mixture stirring at room 4 hours.With gained solution with water (250mL) dilution, vacuum is removed most of organic solvent.(3 * 200mL) washings discard organic washings with ether with gained suspension.Obtained aqueous solution is acidified to pH4 with 2M hydrochloric acid soln and uses ethyl acetate extraction (2 * 200mL).Combining extraction liquid is used the salt water washing, dry (MgSO
4), and evaporation, obtained required compound (99% productive rate).
1H?NMR?δ(d
6-DMSO):1.20(d,3H),3.46(m,2H),4.64(m,1H),5.15(s,2H),6.83(app?t,1H),7.06(s,1H),7.13(s,1H),7.30-7.49(m,5H),12.67(br?s,1H)
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } methyl benzoate
To 3-hydroxyl-5-{[phenyl methyl] the oxygen base add in the solution of methyl benzoate (77.4mmol) in THF the polymkeric substance load triphenylphosphine (51.7g of 3mmol/g charge capacity, 155mmol) and (R)-(-)-1-methoxyl group-2-propyl alcohol (102mmol).This solution that is stirring is covered with argon gas, and in ice bath, cool off.By syringe with the solution that dripped DIAD (116mmol) in 10 minutes.With this solution stirring 20 minutes, and filter, with THF (500mL) debris.Filtrate and washings are merged, and evaporation, having obtained required compound, it need not be further purified and directly use.
1H?NMR?δ(d
6-DMSO):3.26(s,3H),3.44(m,2H),3.82(s,3H),4.63(m,1H),5.14(s,2H),6.85(s,1H),7.05(s,1H),7.11(s,1H),7.30-7.47(m,5H)
1H NMR spectrum also contains and a small amount of hydrazine-1, the signal of 2-dioctyl phthalate two (1-methylethyl) ester unanimity.
3-hydroxyl-5-{[phenyl methyl] the oxygen base } methyl benzoate
To 3, add salt of wormwood (9mol) in the solution that is stirring of 5-methyl dihydroxy benzoate (5.95mol) in DMF (6L), this suspension is stirred under argon gas in room temperature.With 1 hour to wherein adding bromotoluene (8.42mol) lentamente, slight heat release takes place, with this reaction mixture in stirred overnight at room temperature.Use ammonium chloride solution (5L) and water (35L) to end this reaction carefully successively.(1 * 3L and 2 * 5L) extracts with DCM with this aqeous suspension.With extraction liquid water (10L) washing that merges, and dried overnight (MgSO
4).With the evaporation of this solution for vacuum, divide 2 batches to carry out chromatogram purification (post fast, 3 * 2kg silica gel carry out gradient elution to the DCM that contains 50% ethyl acetate with the hexane that contains 10%DCM again to pure DCM) crude product to remove raw material.Thick elutriant with the further chromatogram purification of the batch of 175g (Amicon HPLC, the 5kg purification on normal-phase silica gel is with the isohexane wash-out that contains the 20%v/v ethyl acetate), has been obtained required compound (21% productive rate).
1H?NMRδ(d
6-DMSO):3.8(s,3H),5.1(s,2H),6.65(m,1H),7.0(m,1H),7.05(m,1H),7.3-7.5(m,5H),9.85(br?s,1H)
Embodiment 4: the general method of preparation halo sulphonamide
In the solution of suitable amine (1.8mmol) in DCM (2mL), be added in SULPHURYL CHLORIDE (0.72mmol) among the DCM (2mL), the gained mixture was stirred 18 hours.This mixture is handled with 1M hydrochloric acid (4mL), isolated organic layer.Vacuum-evaporation has obtained fluorine sulphonamide crude product, and it need not be further purified and directly use.
Adding 3-hydroxyl in the solution of fluorine sulphonamide crude product (7.2mmol) in acetonitrile (3mL)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide (0.36mmol) and salt of wormwood (1.8mmol).This mixture ' in 170 ℃ of heating 100 minutes, is being filtered, with the vacuum-evaporation of gained organic phase among the Smith Creator Microwave ' then.Then at the Redisep (12g, the SiO that use Isco Optix chromatographic system
2) on the tube by the chromatography purification resistates, use the mixture of 30-100% ethyl acetate in isohexane to carry out wash-out, and vacuum-evaporation, obtained required compound.
Use above-mentioned general preparation method to synthesize embodiment 4a-4d:
$The necessary sulphonamide of this embodiment is to use the amine of 1: 1 ratio: SULPHURYL CHLORIDE makes, and separates by handling with the 1M aqueous sodium hydroxide solution.
3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide is described in the foregoing description 3.
Embodiment 5: the general method of preparation sulphonamide
The solution of essential Clofenamide (0.12mmol) in THF (5mL) and methyl alcohol (5mL) that derives from the foregoing description 4 is handled with 10% palladium on carbon (6mg) and triethylamine (0.1mL).Flask is placed under the vacuum, under nitrogen atmosphere, stir.The gained mixture is intact until raw material consumption in stirring at room, then via diatomite filtration, use methanol wash.With organic phase vacuum-evaporation, (3 * 5mL) azeotropic, vacuum-drying has then obtained required compound with ether.
Use above-mentioned general method to synthesize embodiment 5a-5d:
Embodiment 6:3-(4-cyano-benzene oxygen)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide
To 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-add the solution (0.164mmol) of 1M hexamethyl-disilazane sodium in THF in N-(the 1-methyl isophthalic acid H-pyrazole-3-yl) solution that is stirring of benzamide (0.164mmol) in DMF (1mL).This is reflected at stirring at room 10 minutes, adds 4-fluorine benzonitrile (0.164mmol) then.This is reflected at stirred overnight at room temperature, is heated to 60 ℃ then, and restir 4 hours.Allow this reaction be cooled to room temperature, handle with 0.2 normal 4-fluorine benzonitrile and hexamethyl-disilazane sodium again, be heated to 70 ℃, under this temperature, stirred 3 hours.Allow this reaction be cooled to room temperature, further handle, be warmed to 70 ℃, under this temperature, stir and spend the night with 0.2 normal hexamethyl-disilazane sodium.Solvent removed in vacuo, and remaining oily matter distributed between ethyl acetate and water.Isolate water layer, extract again with ethyl acetate.With the organic layer salt water washing that merges, dry (MgSO
4), filter and be evaporated to resistates, by the silica gel chromatography purifying, use the mixture of 0-1% methyl alcohol in DCM as eluent, obtained required product (60% productive rate).
1H?NMR?δ(CDCl
3):1.35(d,3H),3.40(s,3H),3.55(m,2H),3.78(s,3H),4.60(m,1H),6.80(m,2H),7.10(m,3H),7.30(m,2H),7.62(d,2H),8.55(br?s,1H);m/z?407(M+H)
+,405(M-H)
-
3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) the synthetic of benzamide be described in the foregoing description 3.
Embodiment 7:3-{[4-(aminocarboxyl) phenyl] the oxygen base }-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide
With 3-(4-cyano-benzene oxygen)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide (0.25mmol), sodiumazide (0.28mmol) and the suspension of zinc bromide (0.25mmol) in water (2mL) is heated to backflow, stirs under this temperature and spend the night.Add Virahol (2mL), should react reheat and reflux 24 hours.This reaction is cooled to room temperature, and vacuum-evaporation distributes resistates to half volume between ethyl acetate and water.Isolate water layer, extract again with ethyl acetate.With the organic layer salt water washing that merges, dry (MgSO
4), filter and be evaporated to resistates, by the silica gel chromatography purifying, as eluent, obtained crude product with the mixture of 0-10% methyl alcohol in DCM.This crude product is dissolved in the ethyl acetate, with 2M sodium hydroxide washing 2 times.With organic layer salt water washing, dry (MgSO
4), filter and evaporation.This resistates is dissolved among the DCM, uses ' Isolute-NH2 ' ion-exchange column purification, use 10% methyl alcohol: the DCM wash-out has obtained required product.
1H?NMR?δ(CDCl
3):1.30(d,3H),3.40(s,3H),3.50(m,2H),3.75(s,3H),4.60(m,1H),6.80(m,2H),7.00(d,2H),7.05(s,1H),7.25(m,2H),7.80(d,2H),8.75(br?s,1H);m/z?423(M-H)
-
3-(4-cyano-benzene oxygen)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-preparation of N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide is described among the embodiment 6.
Embodiment 8:3-[4-(ethylsulfonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide
With 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide (154mg), 4-ethylsulfonyl phenylo boric acid (203mg), venus crystals (II) are (183mg), triethylamine (0.345mL) and the new solution of activatory 4_ molecular sieve (1g) in DCM (10mL) stirred 3 days under normal pressure in room temperature.Via diatomite filtration, with DCM washing (10mL), vacuum is removed DCM, and remaining oily matter is dissolved in the ethyl acetate (50mL) with this reaction mixture.With organic solution 1M hydrochloric acid, saturated sodium bicarbonate aqueous solution, salt water washing, dry then (MgSO
4) and vacuum-evaporation.With resistates on alumina by chromatography purification, with the mixture of 5% methyl alcohol in ethyl acetate as eluent.Further, use the mixture of 50% ethyl acetate in isohexane, obtained required compound (54mg) as eluent by the silica gel chromatography purifying.
1H?NMR?δ(CDCl
3):1.2-1.35(m,6H),3.15(q,2H),3.4(s,3H),3.5-3.6(m,2H),4.5-4.6(m,1H),6.8(s,1H),6.95(d,1H),7.2(d,2H),7.25(d,2H),7.4(s,1H),7.85(d,2H).m/z?477(M+H)
+
Also in a similar manner, by 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide made following compounds:
The 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-1,3-thiazol-2-yl benzamide and 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-be described in respectively in embodiment 1 and 3 synthesizing of N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide.
Embodiment 9a:3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[3-(methyl sulphonyl) phenoxy group] benzamide
Embodiment 9b:3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[3-(methylsulfinyl) phenoxy group] benzamide
To 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-{[3-(methylthio group) phenyl] the oxygen base } benzamide (makes as described in top embodiment 8a, 270mg) add metachloroperbenzoic acid (1.3 equivalent) in the solution in DCM (5mL), this was reflected at stirring at room 1 hour.Add 1.4 normal metachloroperbenzoic acids again, this was reflected at the room temperature restir 30 minutes.This reaction is added in the saturated metabisulfite aqueous solution, and stirred 20 minutes.Isolate organic layer, use the salt water washing, dry (MgSO
4), and evaporation, obtained white foam shape thing.Use this crude mixture of 20g Redisep column purification, use the mixture wash-out of 0-5% methyl alcohol in DCM, obtained required sulfone (117mg).
1H?NMR?δ(d
6-DMSO):1.12(d,3H),3.22(s,3H),3.26(s,3H),3.47(m,2H),3.75(s,3H),4.75(m,1H),6.54(m,1H),6.85(m,1H),7.23(s,1H),7.40(m,1H),7.45(s,1H),7.52(m,1H),7.57(m,1H),7.68(m,2H),10.84(br?s,1H);m/z?460(M+H)
+
Obtained required sulfoxide (105mg) from another grade part.
1H?NMR?δ(d
6-DMSO):1.12(d,3H),2.75(s,3H),3.26(s,3H),3.47(m,2H),3.76(s,3H),4.73(m,1H),6.53(m,1H),6.80(m,1H),7.19(m,2H),7.33(m,1H),7.44(m,2H),7.59(m,2H),10.83(br?s,1H);m/z?444(M+H)
+
Embodiment 10:3-(the 4-[(1-methylethyl) and alkylsulfonyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide
Being similar in the foregoing description 9 method of describing, by 3-({ 4-[(1-methylethyl) sulfenyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide made 3-({ 4-[(1-methylethyl) alkylsulfonyl] phenyl } the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide.
1H?NMR?δ(d
6-DMSO):1.32(m,9H),3.27(m,1H),3.41(s,3H),3.50(dd,1H),3.58(dd,1H),3.80(s,3H),4.61(m,1H),6.82(m,2H),7.09(d,2H),7.17(m,1H),7.28(m,1H),7.33(m,1H),7.84(d,2H),8.86(br?s,1H);m/z?488(M+H)
+
3-({ 4-[(1-methylethyl) sulfenyl] phenyl the oxygen base)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the superincumbent embodiment 8b of synthetic description of N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide in.
Embodiment 11: acid amides synthetic general method-HATU coupling
DIPEA (2.5 equivalent) is added to 3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } in phenylformic acid (1 equivalent), HATU (1.25 equivalent) and the suspension of amine (1.25 equivalent) in DMF (20mL).Initial suspension is dissolved in the darkorange solution.With the gained mixture stirring at room 2 hours.Vacuum is removed DMF, resistates and methylbenzene azeotropic.Add entry, with this mixture ethyl acetate extraction.Combining extraction liquid is used 1M hydrochloric acid, saturated sodium bicarbonate solution and salt water washing successively.With this solution drying (MgSO
4), filter, and vacuum-evaporation, obtained crude product, it by chromatography purification (mixture of 50% ethyl acetate in isohexane), has been obtained required compound (40-70% productive rate).
Embodiment 11a-11g is to use and is similar to that above-mentioned method makes by suitable acid and amino-heterocycles;
*Embodiment 11f can carry out crystallization by the following method: in the system of sealing, allow the isohexane vapor diffusion in the solution of this compound in ethyl acetate, allow this mixture evaporate lentamente 4 days in room temperature then, mp 109-112 ℃.
$The necessary amino-pyrazol of embodiment 11g is as described below making:
Under argon atmospher, with sodium hydride (60% dispersion liquid in mineral oil, 39mg, 0.973mmol) be added to 5-nitro-1H-pyrazoles in dry DMF (2mL) (100mg, 0.885mmol) in.With this solution stirring 5 minutes, add then iodoethane (0.85mL, 1.062mmol), with this be reflected at 80 ℃ warm 3 hours.Add saturated sodium bicarbonate aqueous solution (30mL), this mixture is extracted with ether (40mL).With the organic extract liquid salt water washing that merges, dry (MgSO
4), and be evaporated to resistates, (the isohexane wash-out with containing ethyl acetate 33%v/v), has obtained alkylation pyrazoles (80mg), and it need not be further purified and be directly used in next step by the silica gel chromatography purifying.
1H?NMRδ(CDCl
3):1.58(t,3H),4.26(q,2H),6.91(d,1H),7.48(d,1H).
Under inert atmosphere, (70mg 0.50mmol) adds 10% palladium on carbon (15mg) in the solution in THF (5mL) to the alkylation pyrazoles.Flask is found time, recharge 3 times, room temperature vigorous stirring 3 hours with hydrogen.This reaction mixture is recharged with argon gas, add a part of 10% palladium on carbon (50mg) again, recharge with hydrogen as mentioned above then.Should react and stir 16 hours, via diatomite filtration, and evaporation, obtained this title compound (56mg), be colorless oil, it need not be further purified and directly use.
1H?NMR?δ(CDCl
3):1.42(t,3H),3.58(br.s,2H),3.98(q,2H),5.59(d,1H),7.16(d,1H)
As described belowly made the necessary acid of embodiment s 11a-11g:
3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } phenylformic acid
With 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{[4-(methyl sulphonyl) phenyl] the oxygen base solution of methyl benzoate (60.9mmol) in THF (400mL) handles with 1M sodium hydroxide solution (125mmol), with this reaction mixture stirring at room 13 hours.Vacuum is removed most of organic solvent, and surplus solution water (150mL) is diluted.With the 1M citric acid solution obtained aqueous solution is acidified to pH4, and (2 * 100mL) extract with ethyl acetate.Combining extraction liquid is used the salt water washing, dry (MgSO
4), and evaporation, obtained required compound (83% productive rate).
1H NMR δ (d
6-DMSO): 1.2 (d, 3H), 3.2 (s, 3H), 3.26 (s, 3H), 3.44 (m, 2H), 4.63 (m, 1H), 7.05 (s, 1H), 7.11 (s, 1H), 7.2 (d, 2H), 7.3 (s, 1H), 7.9 (d, 2H); M/z 479 (M-H)
-3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } methyl benzoate
With 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base] methyl benzoate (154mmol), boric acid (1.1 equivalent), venus crystals (II) (1.1 equivalent), triethylamine (5 equivalent) and the new suspension of activatory 4_ molecular sieve (200g) in DCM (500mL) stirred 2 days under normal pressure in room temperature.This reaction mixture is filtered, and vacuum is removed DCM, and remaining oily matter is distributed between ethyl acetate and 1-2M hydrochloric acid.Isolate ethyl acetate layer, with sodium bicarbonate aqueous solution and salt water washing, dry (MgSO
4), and be evaporated to resistates, by silica gel chromatography purifying (using the mixture of 20-60% ethyl acetate in isohexane), obtained required ester (58% productive rate) as eluent.
1H?NMR?δ(d
6-DMSO):1.2(d,3H),3.2(s,3H),3.26(s,3H),3.44(m,2H),3.8(s,3H),4.65(m,1H),7.05(s,1H),7.11(s,1H),7.2(d,2H),7.3(s,1H),7.9(d,2H)
The 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base] methyl benzoate
With 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } methyl benzoate (50.0g; 0.152mmol) be dissolved in THF: in the alcohol mixture (600mL), flask is found time, with nitrogen purging (3 times).Add 10% palladium on carbon (5.0g), flask is found time again, use hydrogen purge at last.With this reaction mixture stirring at room 20 hours until finishing.This reaction mixture is found time, with nitrogen purging (3 times).Filter out catalyzer,, obtained required compound (36.7g) the filtrate vacuum concentration.
1H?NMRδ(d
6-DMSO):1.2(d,3H),3.25(s,3H),3.44(m,2H),3.82(s,3H),4.55(m,1H),6.6(s,1H),6.9(s,1H),6.95(s,1H),9.8(s,1H)
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } the synthetic of methyl benzoate be described among the embodiment 1.
Embodiment 12: acid amides synthetic general method-oxalyl chloride coupling
Under argon atmospher, to 3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{3,5-two fluorophenoxies } drip oxalyl chloride (2 equivalent) and DMF (1) in the solution that is stirring of phenylformic acid (0.285mmol) in anhydrous DCM (2mL).With gained solution at stirring at room 1-2 hour.Solvent removed in vacuo places crude mixture in the pyridine (2mL), and is added in the suitable amine (2.2 equivalent).This reaction mixture in stirring at room or heating if necessary, is monitored by TLC and/or LCMS.Vacuum is removed pyridine, adds entry and ethyl acetate.Organic layer is washed with 1M citric acid and salts solution successively, and dry (MgSO
4), vacuum concentration by silica gel chromatography purifying (with the mixture wash-out of 30-90% ethyl acetate in isohexane), has obtained required product (35-40% productive rate usually) with resistates.
Embodiment 12a ﹠amp; 12b prepares with suitable amine:
$In this embodiment, acyl chlorides is placed in the THF, add pyridine and suitable amine then.
3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{3,5-two fluorophenoxies } benzoic synthetic as described below:
3-{ (1S)-2-methyl-(1-methylethyl) oxygen base }-5-{3,5-two fluorophenoxies } phenylformic acid
This compound is according to top synthetic 3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } benzoic method is by 3-[(3, the 5-difluorophenyl) the oxygen base]-5-[(1S)-and 2-methoxyl group-(1-methylethyl) oxygen base] methyl benzoate makes:
1H NMR δ (d
6-DMSO): 1.21 (d, 3H), 3.26 (s, 3H is covered by solvent peak), 3.46 (m, 2H), 4.67 (m, 1H), 6.81 (d, 2H), 6.96-7.08 (m, 3H), 7.27 (s, 1H), 13.13 (bs, 1H); M/z 337 (M-H)
-
3-[(3, the 5-difluorophenyl) the oxygen base]-5-[(1S)-and 2-methoxyl group-(1-methylethyl) oxygen base] methyl benzoate
At 0 ℃, to 3-[(3,5-difluorophenyl) oxygen base]-add DIAD (18.0mmol) in 5-methyl hydroxybenzoate (15.0mmol), (R)-(-)-1-methoxyl group-2-propyl alcohol (18.75mmol) and the solution of triphenylphosphine (18.0mmol) in anhydrous THF (100mL).This is reflected at stirred overnight at room temperature, and vacuum concentration is the resistates acetoacetic ester: mixture development in 1: 1 of isohexane.By solids removed by filtration,,, obtained this title compound (75% productive rate) by silica gel chromatography purifying (using Biotage Flash 75) with the mixture wash-out of 10-15% ethyl acetate in isohexane the filtrate vacuum concentration.
1H NMR δ (d
6-DMSO): 1.21 (d, 3H), 3.27 (s, 3H is covered by solvent peak), 3.46 (m, 2H), 3.82 (s, 3H), 4.69 (m, 1H), 6.81 (dd, 2H), 7.01-7.07 (m, 2H), 7.10 (s, 1H), 7.28 (s, 1H) 3-[(3,5-difluorophenyl) oxygen base]-the 5-methyl hydroxybenzoate
To 3-[(3,5-difluorophenyl) oxygen base]-the 5-{[(4-aminomethyl phenyl) alkylsulfonyl] the oxygen base } add the methanol solution (13.75g) of 20% potassium hydroxide in the solution of methyl benzoate (16.3mmol) in methyl alcohol (60mL).This mixture 50 ℃ of heating 1 hour, is allowed its cooling then.Add entry (20mL), use 1M hydrochloric acid immediately this mixture acidifying.Vacuum is removed methyl alcohol, uses the ethyl acetate extraction resistates.Isolate organic phase, use the salt water washing, dry (MgSO
4), vacuum concentration has obtained this title compound (92% productive rate).
1H?NMR?δ(d
6-DMSO):3.80(s,3H),6.72(m,1H),6.79(m,2H),6.98-7.05(m,2H),7.19(m,1H),10.18(bs,1H);m/z?279(M-H)
-
3-[(3, the 5-difluorophenyl) the oxygen base]-the 5-{[(4-aminomethyl phenyl) alkylsulfonyl] the oxygen base } methyl benzoate
To 3-hydroxyl-5-{[(4-aminomethyl phenyl) alkylsulfonyl] the oxygen base methyl benzoate (30mmol), venus crystals (II) (36mmol), 3, add triethylamine (150mmol) in 5-difluorophenyl boric acid (42mmol) and the solution of 4_ molecular sieve (30g) in DCM (300mL).Allow this reaction stir 40 hours, filter then, and vacuum concentration.Resistates is dissolved in the ethyl acetate, with 1M citric acid solution, 1M sodium hydrogen carbonate solution and salt water washing, dry then (MgSO
4), and vacuum concentration.Resistates by silica gel chromatography purifying (Biotage Flash75), with the mixture wash-out of 10-25% ethyl acetate in isohexane, has been obtained this title compound (55% productive rate).
1H?NMR?δ(d
6-DMSO):2.39(s,3H),3.83(s,3H),6.74(dd,2H),6.93(m,1H),7.08(m,1H),7.44(m,3H),7.50(s,1H),7.74(d,2H);m/z?452(M+NH
4)
+,433(M-H)
-
3-hydroxyl-5-{[(4-aminomethyl phenyl) alkylsulfonyl] the oxygen base } methyl benzoate
With 3,5-methyl dihydroxy benzoate (0.40g) and 4-toluene sulfonyl chloride (0.45g) in ether (20mL) with saturated sodium bicarbonate aqueous solution (20mL) stirring at room 62 hours.Remove water layer, resistates is used saturated sodium bicarbonate aqueous solution, salt water washing successively, dry (MgSO
4), filter, and vacuum concentration, colorless oil obtained.Crude product is dissolved in the ether,, uses the salt water washing then, dry (MgSO with unsaturated carbonate aqueous solutions of potassium and salt water washing
4), filter and vacuum concentration, obtained colorless oil, it leaving standstill crystallization down, has been obtained this title compound (0.51g).
1H?NMR?δ(d
6-DMSO):2.43(s,3H),3.82(s,3H),6.66(m,1H),6.97(s,1H),7.26(s,1H),7.47(d,2H),7.75(d,2H);m/z?340(M+NH
4)
+
Embodiment 13:3-(3,5-two fluorophenoxies)-N-[4-(hydroxymethyl)-1,3-thiazoles-2-yl]-5-[2-(1S)-methoxyl group-(1-methylethyl) oxygen base] benzamide
To 3-(3,5-two fluorophenoxies)-N-[4-chloromethyl-1,3-thiazoles-2-yl]-5-[(1S)-and 2-methoxyl group-(1-methylethyl) oxygen base] add 0.5M sodium hydroxide solution (1mL) in the solution of benzamide (0.107mmol) in THF (1mL).This was reflected at stirring at room 2 hours, and vacuum is removed organic solvent., distribute between ethyl acetate and water the resistates acidifying with the 1M citric acid.Isolate organic phase, dry (MgSO
4), and vacuum concentration.By silica gel chromatography purifying resistates, with the mixture wash-out of 80% ethyl acetate in isohexane, obtained this title compound, by adding isohexane it is precipitated out from dense diethyl ether solution, obtained solid sample (35% productive rate).
1H NMR δ (d
6-DMSO): 1.24 (d, 3H), 3.28 (s, 3H is covered by solvent peak), 3.48 (m, 2H), 4.49 (s, 2H), 4.75 (m, 1H), 6.83 (d, 2H), 6.93 (s, 1H), 6.98 (s, 1H), 7.04 (m, 1H), 7.32 (s, 1H), 7.54 (s, 1H); M/z 451 (M+H)
+, 449 (M-H)
-
3-(3,5-two fluorophenoxies)-N-[4-chloromethyl-1,3-thiazoles-2-yl]-5-[(1S)-and 2-methoxyl group-(1-methylethyl) oxygen base] benzamide
To 3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{3,5-two fluorophenoxies } drip 3 DMF and oxalyl chloride (6.12mmol in the solution that is stirring of phenylformic acid (3.06mmol) in DCM (20mL); 2.0 equivalent), with the gained mixture stirring at room 5 hours.With this reaction mixture vacuum concentration, with methylbenzene azeotropic, drying under reduced pressure spends the night.Resistates is dissolved among the DCM, adds 4-(chloromethyl)-1,3-thiazoles-2-amine (3.36mmol), triethylamine (3.36mmol) and dimethyl aminopyridine (0.31mmol).With the gained mixture stirring at room 16 hours.This reaction mixture is washed successively dry (MgSO with 2M hydrochloric acid and 1M sodium hydrogen carbonate solution
4) and vacuum concentration.Resistates by chromatography purification (with the mixture wash-out of 15--20% ethyl acetate in isohexane), has been obtained required compound (33% productive rate).
1H NMR δ (d
6-DMSO): 1.24 (d, 3H), 3.28 (s, 3H is covered by solvent peak), 3.49 (m, 2H), 4.76 (m, 3H), 6.84 (dd, 2H), 6.94 (s, 1H), 7.04 (m, 1H), 7.32 (m, 2H), 7.55 (s, 1H), 12.77 (bs, 1H); M/z 469,471 (M+H)
+, 467,469 (M-H)
-
3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{3,5-two fluorophenoxies } among the superincumbent embodiment 12 of benzoic synthetic description.
The preparation of 4-(chloromethyl)-1,3-thiazoles-2-amine is described in the document (J.Indian Chem.Soc.1960,37,241).
Reference example 14:3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group]-N-1H-pyrazole-3-yl benzamide
Trifluoroacetic acid (0.5mL) is added to 3-({ 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate (180mg; 0.330mmol) in the solution in anhydrous DCM (3mL), this solution was stirred 3 hours under argon atmospher.And then add a part of trifluoroacetic acid (0.2mL), should react and stir 30 minutes, vacuum-evaporation then.Resistates is placed ethyl acetate (30mL) and saturated sodium bicarbonate aqueous solution (15mL),, be dissolved in then in DCM and the hexane the resistates evaporation, and evaporation, obtained this title compound (145mg), be colourless foam shape thing.
1H NMR δ (d
6-DMSO): 1.27 (d, 3H), 3.22 (s, 3H), 3.31 (s, 3H), 3.60 (m, 2H are partly covered by HOD), 4.78 (m, 1H), 6.62 (s, 1H), 6.93 (s, 1H), 7.27 (d, 2H), 7.32 (s, 1H), 7.53 (s, 1H), 7.65 (s, 1H), 7.96 (d, 2H), 10.86 (s, 1H); M/z 444 (M-H)
-
3-(3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate
With HATU (375mg; 1.17mmol) be added to 3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } phenylformic acid (300mg; 0.79mmol) in; add then DMF (5mL), DIPEA (0.35mL) and 3-amino-1H-pyrazoles-1-t-butyl formate (155mg, 0.85mmol).This is reflected under the argon gas stirred 4 hours,, resistates is dissolved in saturated sodium bicarbonate aqueous solution (30mL) and the ethyl acetate (50mL) solvent evaporation.Isolate organic layer, with saturated aqueous ammonium chloride (30mL) washing, dry then (MgSO
4), filter and evaporation.By the column chromatography purifying, with 1: 1 ethyl acetate: the hexane wash-out, obtained this title compound (185mg, 43%), be colorless oil.
1H?NMR?δ(CDCl
3):1.37(d,3H),1.63(s,9H),3.09(s,3H),3.40(s,3H),3.58(m,2H),4.61(m,1H),6.85(s,1H),7.08(m,2H),7.15(d,2H),7.30(s,1H),7.92(d,2H),8.01(d,1H),8.58(br.s,1H);m/z?544(M-H)
-
3-amino-1H-pyrazoles-1-t-butyl formate
In 0 ℃, (428mg 5.15mmol) is dissolved among the DMF (5mL), and (206mg 5.15mmol) handles, and stirs then 30 minutes with sodium hydride with 1H-pyrazoles-3-amine.(1.12g 5.15mmol), allows this reaction be warmed to room temperature, and stirred 2 hours to add tert-Butyl dicarbonate with 5 minutes lentamente by syringe then.This reaction is placed in saturated sodium bicarbonate aqueous solution (50mL) and the ethyl acetate (100mL).Isolate organic layer, dry then (MgSO
4), filter and evaporation.By column chromatography purifying (with 1: 1 ethyl acetate: hexane was to purified eluent ethyl acetate), obtained this title compound (117mg), be white solid.
1H?NMR?δ(CDCl
3):1.62(s,9H),4.00(br.s,2H),5.81(d,1H),7.82(d,1H)
3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } among the superincumbent embodiment 11 of benzoic synthetic description.
Embodiment 15:3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(5-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(methyl sulphonyl) phenoxy group] benzamide
Trifluoroacetic acid (1.5mL) is added to 3-({ 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group] benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate (500mg; 0.330mmol) in the solution in anhydrous DCM (6mL), this is reacted under the argon gas stirred 2 hours.Solvent removed in vacuo places ethyl acetate (30mL) and saturated sodium bicarbonate aqueous solution (15mL) with resistates.Isolate organic layer, dry (MgSO
4), to filter, evaporation then with DCM/ hexane revaporization, has obtained this title compound (350mg), is colourless foam shape thing.
1H NMR δ (DMSO-d
6): 1.23 (d, 3H), 2.20 (s, 3H), 3.20 (s, 3H), 3.30 (s 3H) (is covered by HOD), 3.50 (m, 2H) 4.78 (m, 1H), 6.38 (s, 1H), 6.90 (s, 1H), 7.22 (d, 2H), 7.30 (s, 1H), 7.45 (s, 1H), 7.93 (d, 2H), 10.71 (br.s, 1H), 12.08 (br.s, 1H); M/z 458 (M-H)
-
3-(3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group] benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate
With HATU (500mg; 1.31mmol) be added to 3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } phenylformic acid (400mg; 1.05mmol) in; add then DMF (6mL), DIPEA (0.47mL) and 3-amino-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate (380mg, 1.93mmol).This is reflected under the argon gas stirred 72 hours, be dissolved in then in saturated sodium bicarbonate aqueous solution (30mL) and the ethyl acetate (50mL).Isolate organic layer, with saturated aqueous ammonium chloride (30mL) washing, dry then (MgSO
4), filter and evaporation.By the column chromatography purifying, with 1: 1-2: 1 ethyl acetate: the hexane wash-out, obtained this title compound (500mg, 85%), be foam.
1H?NMR?δ(CDCl
3):1.37(d,3H),1.62(s,9H),2.54(s,3H),3.08(s,3H),3.40(s,3H),3.58(m,2H),4.60(m,1H),6.82(m,2H),7.08(m,1H),7.15(d,2H),7.30(s,1H),7.93(d,2H),8.52(brs,1H);m/z?558(M-H)
-
3-amino-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate
(800mg 8.25mmol) is dissolved among the DMF (10mL), and (336mg 8.25mmol) handles, and then stirred 30 minutes with sodium hydride with 5-methyl isophthalic acid H-pyrazoles-3-amine in 0 ℃.(1.80g 8.25mmol), allows this reaction be warmed to room temperature, and restir 1 hour to add warm tert-Butyl dicarbonate with 5 minutes lentamente by syringe then.This reaction is placed in saturated sodium bicarbonate aqueous solution (50mL) and the ethyl acetate (100mL).Dry then (MgSO
4), filter and evaporation.By the column chromatography purifying (with 1: 1 ethyl acetate: hexane to 100% eluent ethyl acetate), obtained this title compound (380mg, 23%), be colorless oil.
1H?NMRδ(CDCl
3):1.62(s,9H),2.43(s,3H),3.87(br.s,2H),5.60(s,1H)
3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } among the superincumbent embodiment 11 of benzoic synthetic description.
Embodiment 16:3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-[4-(methoxymethyl)-1,3-thiazoles-2-yl]-5-[4-(methyl sulphonyl) phenoxy group] benzamide
To N-[4-(chloromethyl)-1,3-thiazoles-2-yl]-3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group] benzamide (280mg; 0.55mmol) add sodium methylate (1.1mmol in the solution that is stirring in methyl alcohol (5mL); 2.0 equivalent; The solution of 25% weight in methyl alcohol), this reaction mixture is heated to 50 ℃, and stirs and spend the night.With this reaction mixture vacuum concentration, (use the 50-70% ethyl acetate: the isohexane wash-out), obtained this title compound (71mg by chromatography purification; 26%).δ (d-DMSO): 1.21 (d, 3H), 2.50 (2s, 6H are covered by water peak part), 3.21 (s, 3H), 3.43-3.54 (m, 2H), 4.40 (s, 2H), 4.78 (m, 1H), 6.98 (s, 1H), 7.12 (s, 1H), 7.25 (d, 2H), 7.37 (s, 1H), 7.58 (s, 1H), 7.95 (d, 2H), 12.69 (br s, 1H); M/z 507 (M+H), 505 (M-H)
1
6
+
-
N-[4-(chloromethyl)-1,3-thiazoles-2-yl]-3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-[4-(methyl sulphonyl) phenoxy group] benzamide
To 3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } drip 1 DMF and oxalyl chloride (2.0mmol in the solution that is stirring of phenylformic acid (1.0mmol) in DCM (10mL); 2.0 equivalent).This reaction mixture was stirred 2 hours under argon gas in room temperature, and any vacuum concentration is with the DCM azeotropic.Resistates is dissolved among the DCM, is added in 4-(chloromethyl)-1,3-thiazoles-2-amine (1.0mmol) and DIPEA (2.5mmol) and dimethyl aminopyridine (0.1mmol) among the DCM.With the gained mixture under argon gas in stirring at room 13 hours, vacuum concentration by chromatography purification (with the mixture wash-out of 50-60% ethyl acetate in isohexane), has obtained this title compound (53% productive rate) then.
1H?NMR?δ(d
6-DMSO):1.3(d,3H),3.2(s,3H),3.25(s,3H)3.45(m,2H),4.75(s,2H),4.8(m,1H),7.0(s,1H),7.25(d,2H),7.3(s,1H),7.4(s,1H),7.6(s,1H),7.95(d,2H),12.80(brs,1H)
3-{ (1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{[4-(methyl sulphonyl) phenyl] the oxygen base } among the superincumbent embodiment 11 of benzoic synthetic description.
Embodiment 17:3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-5-yl) benzamide
To 4-({ 3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-the 5-[(3-methyl isophthalic acid, 2,4-thiadiazoles-2-base is amino) carbonyl] phenyl the oxygen base) add DIPEA (0.68mL) in phenylformic acid (300mg), HATU (336mg) and the suspension of azetidine hydrochloride (190mg) in DMF (5mL), and with this mixture stirring at room 16 hours.Add entry (75mL), and (3 * 25mL) extract with ethyl acetate with this mixture.With organic extract liquid 1M hydrochloric acid (25mL), the saturated sodium hydrogen aqueous solution (25mL), the salt water washing that merges, dry (MgSO
4), and be evaporated to resistates, by the silica gel chromatography purifying, as eluent, obtained required compound (190mg) with ethyl acetate.
1H?NMRδ(d
6-DMSO):1.25(d,3H),2.2-2.3(m,2H),2.5(m,3H),3.3(s,3H),3.5(m,2H),4.O(m,2H),4.3(m,2H),4.8(m,1H),6.95(s,1H),7.1(d,2H),7.35(s,1H),7.55(s,1H),7.65(d,2H),13.35(s,1H);m/z?483(M+H)
+
In a similar manner, embodiment 17a and 17b have also been made.
4-(3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-the 5-[(3-methyl isophthalic acid, 2,4-thiadiazoles-2-base is amino) carbonyl] phenyl } the oxygen base) phenylformic acid
With 4-({ 3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-the 5-[(3-methyl isophthalic acid, 2,4-thiadiazoles-2-base is amino) carbonyl] phenyl } the oxygen base) solution of ethyl benzoate (1.3g) in THF (40mL) is added in the solution of lithium hydroxide monohydrate (310mg) in water (20mL).Stirring at room 16 hours, and vacuum was removed THF with this mixture.With the water layer acidifying, and filter out solid precipitation with 1M hydrochloric acid (6.9mL), wash with water, and vacuum-drying, required compound (1.12g) obtained.
1H?NMRδ(d
6-DMSO):1.2(d,3H),2.45(s,3H),3.25(s,3H),3.5(m,2H),4.7-4.8(m,1H),6.95(s,1H),7.1(d,2H),7.35(s,1H),7.6(s,1H),7.95(d,2H);m/z?444(M+H)
+
4-(3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-the 5-[(3-methyl isophthalic acid, 2,4-thiadiazoles-2-base is amino) carbonyl] phenyl } the oxygen base) ethyl benzoate
With 3-hydroxyl-5-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-2-yl) benzamide (3.23g), 4-ethoxy carbonyl phenyl-boron dihydroxide (3.63g), venus crystals (II) are (3.63g), triethylamine (6.9mL) and the new solution of activatory 4_ molecular sieve (12.5g) in DCM (250mL) stirred 2 days under normal pressure in room temperature.Via diatomite filtration, (2 * 50mL), vacuum is removed DCM, and remaining oily matter is distributed between ethyl acetate (300mL) and 1M hydrochloric acid (200mL) with the DCM washing with this reaction mixture.Isolate ethyl acetate layer, use sodium bicarbonate aqueous solution and salt water washing successively, dry (MgSO
4), and be evaporated to resistates, by the silica gel chromatography purifying, as eluent, obtained required compound (1.35g) with the mixture of 40% ethyl acetate in isohexane.
1H NMR δ (CDCl
3): 1.3 (d, 3H), 1.4 (t, 3H), 2.45 (s, 3H), 3.4 (s, 3H), 3.5-3.6 (m, 2H), 4.35 (q, 2H), 4.5-4.6 (m, 1H), 6.85 (s, 1H), 7.0 (d, 2H), 7.1 (s, 1H), 7.3 (d, 1H), 8.05 (d, 2H), 10.5 (s, 1H); M/z 472 (M+H)
+The 3-hydroxyl-5-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-2-yl) benzamide
With 3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{ phenyl methoxyl group }-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-2-yl) benzamide (9.53g) and the solution of thioanisole (13.9mL) in trifluoroacetic acid (45mL) were in stirring at room 16 hours.Vacuum is removed trifluoroacetic acid, and remaining oily matter is distributed between ethyl acetate (100mL) and sodium bicarbonate aqueous solution (300mL).Isolate water layer, the usefulness ethyl acetate (2 * 100mL) extractions, and with the organic extract liquid salt water washing that merges, dry (MgSO
4), and be evaporated to resistates, by the silica gel chromatography purifying, as eluent, obtained required compound (4.5g) with the mixture of 50% ethyl acetate in isohexane.
1H?NMR?δ(CDCl
3):1.2(d,3H),2.5(s,3H),3.3(s,3H),3.4-3.6(m,2H),4.6-4.7(m,1H),6.6(s,1H),7.05(s,1H),7.1(s,1H),9.85(s,1H),13.2(s,1H);m/z?324(M+H)
+
3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base }-5-{ phenyl methoxyl group }-N-(3-methyl isophthalic acid, 2,4-thiadiazoles-2-yl) benzamide
To 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } add oxalyl chloride (5.24mL) in the solution of phenylformic acid (15.8g) in DCM (260mL), add DMF (1) then, and with this mixture stirring at room 16 hours.DCM and excessive oxalul chloride vacuum are removed, remaining oily matter is dissolved among the DCM (50mL), is added to 5-amino-3-methyl isophthalic acid, 2 at 0-5 ℃, in 4-thiadiazoles (6.05g) and the solution of triethylamine (14.6mL) in DCM (150mL), and with this mixture stirring at room 16 hours.DCM and excess of triethylamine vacuum are removed, and remaining oily matter is distributed between ethyl acetate (250mL) and 1M hydrochloric acid (150mL).Isolate ethyl acetate layer, use 1M hydrochloric acid, sodium bicarbonate aqueous solution and salt water washing successively, dry (MgSO
4), and be evaporated to resistates, and by the alumina chromatogram purification, as eluent, by the silica gel chromatography purifying, use the mixture of 30% ethyl acetate in isohexane then it as eluent with ethyl acetate, obtained required compound (9.6g).
1H?NMRδ(CDCl
3):1.3(d,3H),2.45(s,3H),3.4(s,3H),3.5-3.6(m,2H),4.55-4.6(m,1H),5.05(s,2H),6.8(s,1H),7.1(m,2H),7.25(m,5H),10.7(s,1H);m/z?414(M+H)
+
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } among the superincumbent embodiment 3 of benzoic synthetic description.
Embodiment 18:3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide
To 3-chloro-4-[(3-{[(1S)-2-methoxyl group-(1-methylethyl) oxygen base-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl phenyl) the oxygen base] add DIPEA (0.50mL) in phenylformic acid (344mg), HATU (366mg) and the suspension of azetidine hydrochloride (88mg) in DMF (10mL), and with this mixture stirring at room 24 hours.Add entry (30mL), and (3 * 15mL) extract with ethyl acetate with this mixture.With the organic extract liquid salt water washing that merges, dry (MgSO
4), and be evaporated to resistates, and by the silica gel chromatography purifying, carry out gradient elution with the mixture of 50-100% ethyl acetate in hexane, obtained required compound (197mg).
1H?NMR?δ(CDCl
3):1.3(d,3H),2.4(m,2H),3.4(s,3H),3.5(m,2H),3.8(s,3H),4.2-4.4(m,4H),4.6(m,1H),6.7(d,2H),7.0(m,2H),7.2(m,2H),7.5(d,1H),7.8(d,1H),8.60(br?s,1H);m/z?499(M+H)
+
In a similar manner, also made embodiment 18a-18e:
Be used to prepare embodiment 18 ﹠amp; The required acid of 18a-e is as described below making:
3-chloro-4-[(3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base])-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenyl) the oxygen base] phenylformic acid
To 3-chloro-4-(3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenoxy group) add the solution (11.7mL) of 1M lithium hydroxide monohydrate in water in the solution of methyl benzoate (2.23g) in THF (58mL).Stirring at room 18 hours, vacuum was removed THF with this mixture.With water layer 2M hydrochloric acid (5.85mL) acidifying, and filter out solid precipitation, wash with water, and vacuum-drying, required acid (1.87g) obtained.
1H?NMRδ(CDCl
3):1.4(d,3H),3.4(s,3H),3.6(m,2H),3.8(s,3H),4.7(m,1H),6.95(m,1H),7.05(m,1H),7.1(d,1H),7.3(m,2H),7.6(m,2H),8.1(s,1H),10.75(br?s,1H);m/z460(M+H)
+
Use similar approach to make and be used for the synthetic necessary acid of embodiment 18a-e:
Embodiment 18 ﹠amp; The necessary ester of 18a-e is as described below making:
3-chloro-4-(3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-5-{[(1-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenoxy group) methyl benzoate
To 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide (832mg, 2.72mmol) and 3-chloro-4-fluorophenyl carbamate (504mg, 2.72mmol) add salt of wormwood (364mg in the solution in acetonitrile (20mL), 2.72mmol), with this stirring mixture ' among the Smith CreatorMicrowave ' in 160 ℃ the heating 30 minutes.Allow this mixture be back to room temperature and normal pressure, filter and be evaporated to resistates,, as eluent, obtained required compound (1.11g) with the mixture of 0-50% ethyl acetate in hexane by the silica gel chromatography purifying.m/z?474(M+H)
+
The synthetic required ester of embodiment 18a-e makes with similar approach:
$The precursor that is used for embodiment 18c is to use 1.2 equivalent fluoroesters to make in 4 hours in 150 ℃ of reactions at DMF.The precursor that is used for embodiment 18d-e makes in 150 ℃ of reactions 2 hours at DMF.
The 4-fluoro-O-Anisic Acid methyl esters of preparation that is used for the precursor of embodiment 18d-e is to make according to the method that W098/13332 describes.
3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) the synthetic of benzamide be described among the embodiment 3.
Embodiment 19:3-{4-[(dimethylamino) carbonyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide
To 4-{3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-[(1H-pyrazole-3-yl amino) carbonyl] phenoxy group } phenylformic acid (280mg, 0.55mmol), HATU (260mg, 0.685mmol) and the dimethyl amine (solution of 0.345mL 2.0M in THF, 0.685mmol) add DIPEA (0.238mL in the suspension in DMF (1mL), 1.37mmol), with this reaction mixture stirring at room 16 hours.Then water is added in this reaction mixture, and is extracted into ethyl acetate (in 3 * 25mL).Organic layer is washed with saturated sodium bicarbonate and saturated brine solution, and dry (MgSO
4).With the filtrate vacuum concentration,, obtained white solid (95mg by chromatography purification resistates (mixture of 50-100% ethyl acetate in isohexane); 40%).
1H?NMR?δ(d
6-DMSO):1.2(d,3H),2.95(s,6H),3.3(s,3H),3.5(m,2H),4.75(m,1H),6.6(s,1H),6.8(s,1H),7.05(d,2H),7.2(s,1H),7.4(d,2H),7.45(s,1H),7.6(s,1H),10.8(s,1H).m/z?439(M+H)
+
In a similar manner, also made embodiment 19a-d.
Preparation embodiment 19 and the required acid of 19a are as described below making:
4-{3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-[(1H-pyrazole-3-yl amino) carbonyl] phenoxy group } phenylformic acid
To 3-(3-[4-(ethoxy carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl amino)-(1.75g 3.25mmol) adds 1M sodium hydroxide solution (16mL to 1H-pyrazoles-1-t-butyl formate in the solution in THF (16mL) and water (8mL); 5.0 equivalent), allow this reaction mixture stirring at room 16 hours.Vacuum is removed THF, adds the 1M citric acid until pH 3-4.Filter out light-yellow precipitate, and wash with water, obtained light yellow solid, with its vacuum-drying (1.18g, 71%).
1H NMR δ (d
6-DMSO): 1.2 (d, 3H), 3.25 (s, 3H is covered by the water peak), 3.4-3.5 (m, 2H), 4.75 (m, 1H), 6.55 (s, 1H), 6.85 (s, 1H), 7.1 (d, 1H), 7.25 (s, 1H), 7.45 (s, 1H), 7.6 (d, 1H), 7.95 (d, 1H), 10.85 (s, 1H); M/z 412 (M+H)
+.
Being used to prepare the required acid of embodiment 19b-d makes with similar fashion:
Be used to prepare embodiment 19 and the required ester of 19a is as described below making:
3-(3-[4-(ethoxy carbonyl) phenoxy group]-5-[(1S)-and 2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate
In the presence of new activatory 4_ molecular sieve powder (about 1g), with 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate (391mg, 1mmol), 4-boric acid ethyl benzoate (388mg, 2.0 equivalent), venus crystals (II) (363mg, 2.0 equivalents) and triethylamine (0.7mL; 5.0 equivalent) in anhydrous among the DCM in normal pressure low suspension 7 hours.This reaction mixture via diatomite filtration, is washed (* 3) with DCM.With the filtrate vacuum concentration, place in the ethyl acetate, with 1M hydrochloric acid, saturated sodium bicarbonate, saturated brine washing, and dry (MgSO
4).Filter,, and, obtained brown oil (210mg, 39%) by chromatography purification (0-50% ethyl acetate/isohexane) with the filtrate vacuum concentration.
1H?NMRδ(CDCl
3):1.3(d,3H),1.4(t,3H),1.6(s,9H),3.4(s,3H),3.5(m,2H),4.35(q,2H),4.5(m,1H),6.8(s,1H),7.0(d,2H),7.05(s,2H),7.2(s,1H),8.0(s,1H),8.05(d,2H),9.2(s,br,1H);m/z?440(M+H)
+.
3-(the 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate
(23g, 47.8mmol) solution in THF (140mL) and ethanol (140mL) is found time, with nitrogen purging (* 3) with 3-({ 3-(benzyloxy)-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate.Add 10% palladium on carbon (2.3g; 10%w/w), this reaction mixture is found time, use hydrogen purge at last.Allow this reaction mixture under the hydrogen capsule, stir 16 hours in room temperature.Pd/C via diatomite filtration, the filtrate vacuum concentration, has been obtained white foam shape thing (18g, 97%).
1H NMR δ (d
6-DMSO): 1.2 (d, 3H), 1.55 (s, 9H), 3.25 (s, 3H is covered by the water peak), 3.4-3.5 (m, 2H), 4.7 (m, 1H), 6.5 (s, 1H), 6.95 (d, 1H), 7.0 (s, 1H), 7.1 (s, 1H), 8.2 (d, 1H), 9.65 (s, 1H), 11.2 (s, br, 1H); M/z 392 (M+H)
+
3-(3-(benzyloxy)-5-[(1S)-2-methoxyl group-1-methyl ethoxy] and benzoyl } amino)-1H-pyrazoles-1-t-butyl formate
To 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } phenylformic acid (20.7g, 65.6mmol), HATU (31.2g, 82.0mmol) and 3-amino-1H-pyrazoles-1-t-butyl formate (15.0g, 82.0mmol) add DIPEA (28.5mL in the suspension in DMF (30mL), 164mmol), with this reaction mixture stirring at room 16 hours.Then water (250mL) is added in this reaction mixture, and is extracted into ether (in 3 * 150mL).Organic layer is washed with saturated brine solution, and dry (MgSO
4).With the filtrate vacuum concentration, resistates is crystallization when leaving standstill.With the isohexane washing, obtained yellow crystals (23.4g; 73%).m/z?482(M+H)
+.
3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } benzoic preparation is described among the embodiment 3.
The preparation of 3-amino-1H-pyrazoles-1-t-butyl formate is described among the embodiment 14.
The required ester of preparation embodiment 19b is as described below making:
3-fluoro-4-{3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-[(1H-pyrazole-3-yl amino) carbonyl] phenoxy group } ethyl benzoate
To 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate (587mg; 1.5mmol), cesium carbonate (488mg; 1.5mmol) add 3 in the suspension in DMA (3mL); 4-difluoro-benzoic acid ethyl ester (279mg, 1.5mmol).This mixture was heated 16 hours at 110 ℃.This reaction mixture is filtered and vacuum concentration, then resistates is passed through the silica gel chromatography purifying,, obtained required compound, be yellow oil (271mg, 40%) with the mixture wash-out of 0-70% ethyl acetate in hexane.
1H?NMR(CDCl
3):1.3(d,3H),1.4(t,3H),3.4(s,3H),3.5(m,2H),4.4(q,2H),4.6(m,1H),6.75(s,1H),6.85(s,1H),7.1(s,1H),7.15(s,1H),7.3(s,1H),7.5(d,1H),7.8(d,1H),7.85(d,1H),9.4(s,1H)
The preparation of 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate is described among the embodiment 19.
The required ester of preparation embodiment 19c is as described below making:
3-(3-[4-(ethoxy carbonyl) phenoxy group]-5-[(1S)-and 2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate
New activatory 4_ molecular sieve (1.5g) is added to 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate (1.0g; 2.47mmol), (4-ethoxy carbonyl phenyl) boric acid (718mg; 3.7mmol), venus crystals (II) (672mg; 3.7mmol) and triethylamine (1.7mL is 12.3mmol) in the solution in DCM (40mL).This mixture stirring at room 2 days, is removed DCM via diatomite filtration and vacuum then.Remaining oily matter is distributed between ethyl acetate (35mL) and 1N hydrochloric acid (35mL), isolate ethyl acetate layer, with saturated sodium bicarbonate aqueous solution (35mL), salt solution (35mL) washing, dry (MgSO
4) and be evaporated to resistates, by the silica gel chromatography purifying,, obtained required compound with the mixture wash-out of 40-60% ethyl acetate in hexane, be orange (80mg, 6%).
1H?NMR(CDCl
3):1.3(d,3H),1.4(t,3H),1.6(s,9H),2.55(s,3H),3.4(s,3H),3.5(m,2H),4.4(q,2H),4.6(m,1H),6.8(s,1H),6.9(s,1H),7.05(d,2H),7.2(s,1H),7.35(s,1H),8.05(d,2H),9.4(s,1H)
3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate is the method for describing among the embodiment 19 according to being similar to for preparing 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate, by 3-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-the 5-{[phenyl methyl] the oxygen base } phenylformic acid and 3-amino-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate make.
3-(the 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate
1H NMR δ (d
6-DMSO): 1.2 (d, 3H), 1.55 (s, 9H), (3.2-3.3 s, 3H is covered by the water peak), 3.2-3.3 (s, 3H is covered by the water peak), 3.4-3.5 (m, 2H), 4.65 (m, 1H), 6.45 (s, 1H), 6.75 (s, 1H), 6.95 (s, 1H), 7.1 (s, 1H), 9.65 (s, 1H), 11.05 (brs, 1H); M/z 406 (M+H)
+.
3-(3-(benzyloxy)-5-[(1S)-2-methoxyl group-1-methyl ethoxy] and benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate
1H NMR δ (d
6-DMSO): 1.2 (d, 3H), 1.55 (s, 9H), 3.25 (s, 3H is covered by the water peak), 3.4-3.5 (m, 2H), 4.7 (m, 1H), 5.15 (s, 2H), 6.7 (s, 1H), 6.8 (s, 1H), 7.2 (s, 1H), 7.25 (s, 1H), 7.3-7.5 (m, 5H), 11.15 (brs, 1H); M/z 496 (M+H)
+.
The required ester of preparation embodiment 19d is as described below making:
3-chloro-4-(3-[(1S)-2-methoxyl group-1-methyl ethoxy]-5-{[(5-methyl isophthalic acid H-pyrazole-3-yl) amino] carbonyl } phenoxy group) ethyl benzoate
With 3-chloro-4-ethyl fluoro benzoate (242mg; 1.2mmol) (405mg is 1mmol) and in the suspension of salt of wormwood (1mmol) in butyronitrile (5mL) to be added to 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate.This mixture is placed microwave oven, 190 ℃ of heating 2.5 hours.With this reaction mixture vacuum concentration, resistates is distributed between ethyl acetate and water, use ethyl acetate (3 * 25mL) extractions then.With organic phase drying (MgSO
4) and vacuum concentration.This crude mixture (420mg, 86%) need not be further purified and be directly used in next step.
The preparation of 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate is described among the embodiment 19c.The preparation of 3-chloro-4-ethyl fluoro benzoate be described in document (Journal of Fluorine Chemistry, 1991,53 (2), 301-305) in.
Embodiment 20:3-[4-(ethylsulfonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide
With 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } amino)-1H-pyrazoles-1-t-butyl formate (391mg; 1mmol), cesium carbonate (325mg; 1mmol) with 3; (206mg, 1mmol) suspension in DMA (3mL) was in 120 ℃ of heating 4 hours for 4-difluorophenyl ethyl sulfone.Water (20mL) is added in this reaction mixture, is extracted into ethyl acetate then and (in 3 * 30mL), uses the salt water washing.With organic phase drying (MgSO
4), vacuum concentration by chromatography purification resistates (50-100% ethyl acetate/isohexane), has obtained white solid (120mg, 25%).
1H?NMRδ(d
6-DMSO):1.1(t,3H),1.2(d,3H),3.25(s,3H),3.3(q,2H),3.5(m,2H),4.75(m,1H),6.6(s,1H),6.95(s,1H),7.3(s,1H),7.35(t,1H),7.45(s,1H),7.6(s,1H),7.7(d,1H),7.95(dd,1H),10.8(s,br?1H).m/z?477(M+H)
+
Made the following example with similar approach.
The preparation that is used for 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } the amino)-1H-pyrazoles-1-t-butyl formate of the preparation of embodiment 20 and 20a is described among the embodiment 19.
Be used for the 3-hydroxyl of the preparation of embodiment 20b-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-preparation of N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide is described in embodiment 3.
The preparation that is used for 3-({ 3-hydroxyl-5-[(1S)-2-methoxyl group-1-methyl ethoxy] benzoyl } the amino)-5-methyl isophthalic acid H-pyrazoles-1-t-butyl formate of the preparation of embodiment 20c is described in embodiment 19.
Be used for embodiment 20 and 20b preparation 3,4-difluorophenyl ethyl sulfone is as described below making.
3,4-difluorophenyl ethyl sulfone
To 3, add 75% metachloroperbenzoic acid (2.97g) in the solution of 4-difluorophenyl thioethyl ether (1.50g) in DCM (50mL), and with this mixture stirring at room 16 hours.This mixture is used saturated potassium carbonate (20mL) and salt solution (30mL) washing successively, use dried over mgso then, filter and vacuum concentration.Gained is clarified oily matter by silica gel chromatography purifying (with the mixture wash-out of 0-50% ethyl acetate in isohexane), isolate the product (0.90g) that comparatively fast elutes.Required 3,4-difluorophenyl ethyl sulfone need not further characterize and directly use.
Be used for embodiment 20a preparation 3,4-difluorophenyl methyl sulfone be according to similar approach by 3,4-difluorophenyl methyl sulfide makes.
1-(3, the 4-difluoro benzoyl) azetidine that is used for the preparation of embodiment 20c is as described below making.
1-(3, the 4-difluoro benzoyl) azetidine
(1.05mL 12.0mmol) is added to 3, and (1.58g is 10mmol) in the solution in the DCM (50mL) that contains DMF (1) for the 4-difluoro-benzoic acid with oxalyl chloride.This is reflected at stirring at room 16 hours, is evaporated to dried then.Resistates is dissolved among the DCM (25mL) again, add the azetidine hydrochloride (1.12g, 12.0mmol), add then triethylamine (4.18mL, 30.0mmol).With this mixture at stirring at room 2 hours, vacuum concentration then.Resistates is distributed between ethyl acetate and 1N hydrochloric acid, organic phase is washed with saturated sodium bicarbonate aqueous solution, use the salt water washing then, dry (MgSO
4) and vacuum concentration.With the crystallization from the ethyl acetate/hexane mixture of this title compound, obtained white crystalline solid (1.0g, 51%).
1H?NMR?δ(CDCl
3):2.4(m,2H),4.3(m,4H),7.2(m,1H),7.4(m,1H),7.5(t,1H).
Embodiment 21:3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-1H-pyrazole-3-yl benzamide
To 3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-and 2-methoxyl group-1-methyl ethoxy] phenylformic acid (300mg, 0.75mmol), HATU (356mg, 0.938mmol) and 3-amino-1H-pyrazoles-1-t-butyl formate (172mg, 0.938mmol) add DIPEA (0.326mL in the suspension in DMF (2mL), 1.88mmol), with this reaction mixture stirring at room 16 hours.Then water is added in this reaction mixture, is extracted into ethyl acetate (in 3 * 25mL).Organic layer is washed with saturated sodium bicarbonate and saturated brine solution, and dry (MgSO
4).With the filtrate vacuum concentration, obtained orange.This oily matter is dissolved among the DCM (4mL), adds trifluoroacetic acid (0.445mL, 8.0 equivalents).With this reaction mixture stirring at room 8 hours.Saturated sodium carbonate is added in this reaction mixture, separates each phase.With organic phase drying (MgSO
4) and vacuum concentration, obtained white foam shape thing (26mg, 7%).
1H NMR δ (CDCl
3): 1.3 (d, 3H), 2.4 (m, 2H), 3.4 (s, 3H), 3.55 (m, 2H), 4.2 (m, 2H), 4.35 (m, 2H), 4.6 (m, 1H), 6.75 (s, 1H), 6.8 (app s, 1H), 7.05 (t, 1H), 7.1 (s, 1H), 7.3 (s, 1H), 7.4 (d, 1H), 7.5 (app d, 1H), 7.5 (app s, 1H), 9.6 (s, br, 1H) .m/z 469 (M+H)
+, 467 (M-H)
-3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-and 2-methoxyl group-1-methyl ethoxy] phenylformic acid
To 3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-and 2-methoxyl group-1-methyl ethoxy] (400mg 1mmol) adds 1M sodium hydroxide solution (3mL to methyl benzoate in the solution in THF (6mL) and water (1mL); 5.0 equivalent), allow this reaction mixture stirring at room 3 hours.Vacuum is removed THF, adds the 1M citric acid until pH 3-4.Add ethyl acetate, and separate each phase.With organic phase drying (MgSO
4) and vacuum concentration, obtained clarification oily matter (305mg, 79%).
1H?NMR?δ(CDCl
3):1.3(d,3H),2.4(m,2H),3.4(s,3H),3.5(m,2H),4.2-4.4(m,4H),4.6(m,1H),6.8(s,1H),7.05(t,1H),7.25(s,1H),7.4(s,1H),7.45(d,1H),7.5(d,1H);m/z?403(M+H)
+.
3-[4-(azetidine-1-base carbonyl)-2-fluorophenoxy]-5-[(1S)-and 2-methoxyl group-1-methyl ethoxy] methyl benzoate
To 3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base] methyl benzoate (480mg; 2mmol) add salt of wormwood (552mg in the solution in DMA (2mL); 4mmol) and 1-(3; the 4-difluoro benzoyl) azetidine (394mg, 2mmol) solution in DMA (2mL).This reaction mixture is heated to 110 ℃, and stirred 16 hours.This reaction mixture is filtered, in this reaction mixture, add entry (20mL).Be extracted in the ethyl acetate, with saturated sodium bicarbonate solution and salt water washing.With this solution drying (MgSO
4) and vacuum concentration, obtained oily matter (400mg, 48%).This resistates need not be further purified or characterize and directly use.
3-hydroxyl-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base] preparation of methyl benzoate is described among the embodiment 11.The preparation of 1-(3, the 4-difluoro benzoyl) azetidine is described among the embodiment 20c.
Embodiment 22:3-[4-(azetidine-1-base carbonyl) phenoxy group]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide
With 3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide (100mg, 0.2mmol) and triethylamine (0.139mL, 1mmol) solution in THF (2.5mL) and ethanol (2.5mL) is found time, and with nitrogen purging (* 3).Add 10%w/w palladium on carbon (10mg), this reaction mixture is found time and use hydrogen purge.Allow this reaction mixture under hydrogen, stir 48 hours in room temperature.By just removing solid residue, this mixture is distributed between ethyl acetate and 1M hydrochloric acid via diatomite filtration.With organic phase drying (MgSO
4), and with the filtrate vacuum concentration.Resistates by the silica gel chromatography purifying, is used the mixture wash-out of 0-70% methyl alcohol in ethyl acetate, obtained product (14mg).
1H NMR δ (d
6-DMSO): 1.2 (d, 3H), 2.2 (s, 3H), 2.25 (m, 2H), (3.25 s, 3H is covered by the water peak), 3.5 (m, 2H), 4.00 (m, 2H), 4.3 (m, 2H), 4.75 (m, 1H), 6.35 (s, 1H), 6.8 (s, 1H), 7.05 (d, 2H), 7.2 (s, 1H), 7.4 (s, 1H), 7.65 (d, 2H), 10.7 (s, 1H), 12.1 (s br, 1H) .m/z 465 (M+H)
+, 463 (M-H)
+
3-[4-(azetidine-1-base carbonyl)-2-chlorophenoxy]-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-preparation of N-(5-methyl isophthalic acid H-pyrazole-3-yl) benzamide is described among the embodiment 19d.
Embodiment 23:3-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(1-methyl isophthalic acid H-pyrazole-3-yl)-5-[4-(1,2,4-_ diazole-3-yl) phenoxy group] benzamide
With the 3-{4-[(hydroxyl amino) (imino-) methyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide places in the trimethyl orthoformate (3mL), adds 2 BF3. ether compounds.Gained solution is surveyed in the microwave oven in 55 ℃ of heating 80 minutes at CEM.Volatile matter is removed in decompression, and gained oily matter by the silica gel chromatography purifying, with the mixture wash-out of 0-100% ethyl acetate in isohexane, has been obtained required compound, is white foam shape thing (295mg).
1H?NMRδ(d
6-DMSO)δ1.23(d,3H),3.40-3.58(m,2H),3.75(s,3H),4.71m,1H),6.54(s,1H),6.86(s,1H),7.18-7.28(m,3H),7.44(s,1H),7.57(s,1H),8.06(d,2H),9.65(s,1H),10.82(s,1H);m/z?450(M+H)
+.
The 3-{4-[(hydroxyl amino) (imino-) methyl] phenoxy group }-5-[(1S)-2-methoxyl group-1-methyl ethoxy]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide
With oxyamine (50%w/w solution, 1mL) be added to 3-(4-cyano-benzene oxygen)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide (300mg, 0.74mmol) in the solution in ethanol (3mL), allow this mixture stirring at room 18 hours.Vacuum is removed volatile matter, has obtained required compound, is colourless foam shape thing (325mg).m/z=440(M+H)
+
3-(4-cyano-benzene oxygen)-5-[(1S)-2-methoxyl group-(1-methylethyl) oxygen base]-N-(1-methyl isophthalic acid H-pyrazole-3-yl) benzamide is described among the embodiment 6.
Biological test:
The biological action of formula (I) compound can be tested by following method:
(1) enzymic activity
The enzymic activity of recombinant human pancreas GLK can be measured by cultivating GLK, ATP and glucose.The speed that product (being G-6-P) forms can be by testing and G-6-P desaturase, the coupling of NADP/NADPH system and measure under the 340nm optical density(OD) along with people 1993 such as () Matschinsky measured in the linearity increase of time.Compound can use this test to the activation of GLK, is in or be not in GLKRP and has to get off assessment, and (Diabetes 2004,53,535-541) as described in people such as Brocklehurst.
The preparation of reorganization GLK and GLKRP
People GLK and GLKRP cDNA by human pancreas and liver mRNA, utilize Sambrook respectively, Fritsch ﹠amp by PCR; Maniatis, the technology of the foundation described in 1989 obtains.According to Tanizawa etc. 1991 and Bonthron, GLK and the GLKRP cDNA sequences Design PCR primer described in the D.T. etc. 1994 (latter is at Warner, and J.P.1995 revises).
In Bluescript II carrier, clone
Use pBluescript II people 1998 such as () Short that GLK and GLKRP cDNA are cloned in the intestinal bacteria, pBlueseript II is similar to (1985) used recombinant cloning vector systems such as Yanisch-Perron C, comprise the colEI-base replicon that has the multi-link body dna fragmentation that contains a plurality of unique restriction sites, the side has phage T3 and T7 promoter sequence; Filobactivirus source of duplicating and penbritin resistance marker gene.
Transform
Intestinal bacteria transform and are generally undertaken by electroporation.The 400ml culture of bacterial strain DH5a or BL21 (DE3) grows to OD 600 in L-meat soup be 0.5 and by 2, the centrifugal results under the 000g.Cell is suspended in once more in 1ml 10% glycerine and with sample aliquot and is kept at-70 ℃ with ice-cold deionized water wash 2 times.Connect mixture Millipore V series
TMFilm (0.0025mm) aperture) desalination.The cell of 40ml and 1ml be connected mixture or plasmid DNA was being cultivated 10 minutes in 0.2cm electroporation cuvette on ice, and utilize Gene Pulser subsequently
TMInstrument (BioRad) is at 0.5kVcm
-1, add pulse under the 250mF.On the L-agar that is supplemented with 10mg/ml tsiklomitsin or 100mg/ml penbritin, select transformant.
Express
GLK is expressed in the e. coli bl21 cell by carrier pTB375NBSE, produces recombinant protein, and this recombinant protein contains the 6-His mark with N-terminal methionine next-door neighbour.Perhaps, another kind of appropriate carriers is pET21 (+) DNA, Novagen, registration number 697703.This 6-His mark is used for recombinant protein purifying on being filled with available from the post of nickel-nitrilotriacetic acid(NTA) agarose of Qiagen (cat no 30250).
GLKRP is expressed in the e. coli bl21 cell by carrier pFLAG CTC (IBI Kodak), generates recombinant protein, and this recombinant protein contains the terminal FLAG mark of C-.This albumen is at first by DEAE agarose ion-exchange purification, utilize subsequently FLAG be marked at available from the M2 of Sigma-Aldrich (registration number A1205) anti--carry out final purification on the FLAG immunoaffinity post.
(2) oral glucose tolerance test (OGTT)
Oral glucose tolerance test is that the fat fa/fa rat of Zucker (12-13 age or bigger in week) with Consciousness carries out, and before on-test, feeds high fat diet (45% kilocalorie of fat) at least 2 weeks to rat.Before being used for test, with animal fasting 2 hours.Before orally give glucose solution 120 minutes, with test compounds or carrier dosed administration with the 2g/kg body weight.Glucose level is measured from the tail blood sample with the Accucheck glucose meters, and described blood sample is that before giving glucose and afterwards the different time points of (60 minutes times) is gathered.Produce the time curve of glucose level, calculate 120 minutes area under curve (AUC) (time that gives glucose is the time 0).The AUC of use in the vehicle Control group is 0 inhibition per-cent, determines to suppress per-cent.
Embodiment 11b example II 107
For glucokinase, The compounds of this invention generally has the activity of activation, and EC
50Less than about 500nM.For example, the EC of embodiment 11b
50Be 30nM.
Example II 107 among embodiment 11b and the WO 03/015774 generally has similar EC
50Value.Yet embodiment 11b has good oral administration biaavailability, and shows 29% OGTT activity at 10mg/kg, but the example II among the WO 03/,015,774 107 does not have activity at 10mg/kg.
Reference
1 Printz, R.L., Magnuson, M.A. and Granner, D.K. (1993) AnnualReview of Nutrition 13,463-96
2?DeFronzo,R.A.(1988)Diabetes?37,667-87
3 Froguel, P., Zouali, H., Vionnet, N., Velho, G., Vaxillaire, M., Sun, F., Lesage, S., Stoffel, M., Takeda, J. and Passa, P. (1993) New England Journal of Medicine 328,697-702
4 Bell, G.I., Pilkis, S.J., Weber, I.T. and Polonsky, K.S. (1996) Annual Review of Physiology 58,171-86
5 Velho, G., Petersen, K.F., Perseghin, G., Hwang, J.H., Rothman, D.L., Pueyo, M.E., Cline, G.W., Froguel, P. and Shulman, G.l. (1996) Journal of Clinical Investigation 98,1755-61
6 Christesen, H.B., Jacobsen, B.B., Odili, S., Buettger, C., Cuesta-Munoz, A., Hansen, T., Brusgaard, K., Massa, O., Magnuson, M.A., Shiota, C., Matschinsky, F.M. and Barbetti, F. (2002) Diabetes51,1240-6
6a Gloyn, A.L., Noordam, K., Willemsen, M.A.A.P., Ellard, S., Lam, W.W.K., Campbell, I.W., Midgley, P., Shiota, C., Buettger, C., Magnuson, M.A., Matschinsky, F.M., and Hattersley, A.T.; Diabetes52:2433-2440
7 Glaser, B., Kesavan, P., Heyman, M., Davis, E., Cuesta, A., Buchs, A., Stanley, C.A., Thornton, P.S., Permutt, M.A., Matschinsky, F.M. and Herold, K.C. (1998) New England Journal ofMedicine 338,226-30
8 Caro, J.F., Triester, S., Patel, V.K., Tapscott, E.B., Frazier, N.L. and Dohm, G.L. (1995) Hormone ﹠amp; Metabolic Research 27,19-22
9 Desai, U.J., Slosberg, E.D., Boettcher, B.R., Caplan, S.L., Fanelli, B., Stephan, Z., Gunther, V. J., Kaleko, M. and Connelly, S. (2001) Diabetes 50,2287-95
10 Shiota, M., Postic, C., Fujimoto, Y., Jetton, T.L., Dixon, K., Pan, D., Grimsby, J., Grippo, J.E., Magnuson, M.A. and Cherrington, A.D. (2001) Diabetes 50,622-9
11 Ferre, T., Pujol, A., Riu, E., Bosch, F. and Valera, A. (1996) Proceedings of the National Academy of Sciences of the United Statesof America 93,7225-30
12 Seoane, J., Barbera, A., Telemaque-Potts, S., Newgard, C.B. and Guinovart, J.J. (1999) Journal of Biological Chemistry 274,31833-8
13 Moore, M.C., Davis, S.N., Mann, S.L. and Cherrington, A.D. (2001) Diabetes Care 24,1882-7
14 Alvarez, E., Roncero, I., Chowen, J.A., Vazquez, P. and Blazquez, E. (2002) Journal of Neurochemistry 80,45-53
15 Lynch, R.M., Tompkins, L.S., Brooks, H.L., Dunn-Meynell, A.A. and Levin, B.E. (2000) Diabetes 49,693-700
16 Roncero, I., Alvarez, E., Vazquez, P. and Blazquez, E. (2000) Journal of Neurochemistry 74,1848-57
17 Yang, X.J., Kow, L.M., Funabashi, T. and Mobbs, C.V. (1999) Diabetes 48,1763-1772
18 Schuit, F.C., Huypens, P., Heimberg, H. and Pipeleers, D.G. (2001) Diabetes 50,1-11
19?Levin,B.E.(2001)International?Journal?of?Obesity?25,supp?5,S68-S72
20 Alvarez, E., Roncero, I., Chowen, J.A., Thorens, B. and Blazquez, E. (1996) Journal of Neurochemistry 66,920-7
21 Mobbs, C.V., Kow, L.M. and Yang, X.J. (2001) American Journalof Physiology-Endocrinology ﹠amp; Metabolism 281, E649-54
22 Levin, B.E., Dunn-Meynell, A.A. and Routh, V.H. (1999) AmericanJournal of Physiology 276, R1223-31
23 Spanswick, D., Smith, M.A., Groppi, V.E., Logan, S.D. and Ashford, M.L. (1997) Nature 390,521-5
24 Spanswick, D., Smith, M.A., Mirshamsi, S., Routh, V.H. and Ashford, M.L. (2000) Nature Neuroscience 3,757-8
25 Levin, B.E. and Dunn-Meynell, A.A. (1997) Brain Research 776,146-53
26 Levin, B.E., Govek, E.K. and Dunn-Meynell, A.A. (1998) BrainResearch 808,317-9
27 Levin, B.E., Brown, K.L. and Dunn-Meynell, A.A. (1996) BrainResearch 739,293-300
28 Rowe, I.C., Boden, P.R. and Ashford, M.L. (1996) Journal ofPhysiology 497,365-77
29 Fujimoto, K., Sakata, T., Arase, K., Kurata, K., Okabe, Y. and Shiraishi, T. (1985) Life Sciences 37,2475-82
30 Kurata, K., Fujimoto, K. and Sakata, T. (1989) Metabolism:Clinical﹠amp; Experimental 38,46-51
31 Kurata, K., Fujimoto, K., Sakata, T., Etou, H. and Fukagawa, K. (1986) Physiology ﹠amp; Behavior 37,615-20
32 Jetton T.L., Liang Y., Pettepher C.C., Zimmerman E.C., Cox F.G., Horvath K., Matschinsky F.M. and Magnuson M.A., J.Biol.Chem., Feb 1994; 269:3641-3654.
33 Reimann F. and Gribble F.M., Diabetes 2002 51:2757-2763
34?Cheung?A.T.,Dayanandan?B.,Lewis?J.T.,Korbutt?G.S.,RajotteR.V.,Bryer-Ash?M.,Boylan?M.O.,Wolfe?M.M.,Kieffer?T.J.,Science,Vol?290,Issue?5498,1959-1962,8?December?2000
Claims (17)
1. formula (I) compound or its salt, prodrug or solvate:
Wherein:
R
1It is methoxymethyl;
R
2Be selected from-C (O) NR
4R
5,-SO
2NR
4R
5,-S (O)
pR
4And HET-2;
HET-1 is the heteroaryl ring that 5 or 6 yuan of C-connect, and described heteroaryl ring contains nitrogen-atoms and optionally contains 1 or 2 other ring hetero atom that is independently selected from O, N and S in the 2-position; Described ring is chosen wantonly on can substituted carbon atom or be independently selected from R by 1 or 2 on theheterocyclic nitrogen atom
6Substituting group replace, condition is that theheterocyclic nitrogen atom is not thus by quaternized;
HET-2 is the heterocyclic ring that 4,5 or 6 yuan of C-or N-connect, and described heterocyclic ring contains 1,2,3 or 4 heteroatoms that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-replacement, and wherein the sulphur atom in the heterocycle can be chosen wantonly and is oxidized to S (O) or S (O)
2Group, described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
7Substituting group replace;
R
3Be selected from halogen, methyl fluoride, difluoromethyl, trifluoromethyl, methyl, methoxyl group and cyano group;
R
4Be selected from hydrogen, (1-4C) alkyl [optional by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2;
R
5Be hydrogen or (1-4C) alkyl;
Perhaps R
4And R
5Can form the heterocyclic radical ring system that defines by HET-3 with the nitrogen-atoms that they connected;
R
6Be independently selected from (1-4C) alkyl, halogen, hydroxyl (1-4C) alkyl, (1-4C) alkoxyl group (1-4C) alkyl, (1-4C) alkyl S (O) p (1-4C) alkyl, amino (1-4C) alkyl, (1-4C) alkylamino (1-4C) alkyl, two (1-4C) alkylamino (1-4C) alkyl and HET-4;
R
7Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-3 is 4,5 or 6 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 or 2 other heteroatoms (except the N atom that is connected) that is independently selected from O, N and S, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 7 yuan of saturated or part unsaturated heterocycle basic rings that N-connects, and described ring is chosen wantonly and contained 1 other heteroatoms (except the N atom that is connected) that is independently selected from O, S and N, wherein-and CH
2-group can be chosen wantonly by-C (O)-group and replace, and wherein the sulphur atom in the ring can be chosen wantonly and is oxidized to S (O) or S (O)
2Group; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be independently selected from R by 1 or 2
8Substituting group replace; Perhaps
HET-3 is 6-10 unit's two ring fillings or part unsaturated heterocycle basic ring, optional 1 the other nitrogen-atoms (except the N atom that connects) that contains of described heterocyclic ring, wherein-CH
2-group can be chosen wantonly by-C (O)-replacement; Described ring choose wantonly can substituted carbon atom or nitrogen-atoms on be selected from hydroxyl and R by 1
3Substituting group replace;
R
8Be selected from-OR
5, (1-4C) alkyl ,-C (O) (1-4C) alkyl ,-C (O) NR
4R
5, (1-4C) alkylamino, two (1-4C) alkylamino, HET-3 (wherein said ring is unsubstituted), (1-4C) alkoxyl group (1-4C) alkyl, hydroxyl (1-4C) alkyl and-S (O) pR
5
HET-4 is the unsubstituted heteroaryl ring that 5 or 6 yuan of C-or N-connect, and described heteroaryl ring contains 1,2 or 3 ring hetero atom that is independently selected from O, N and S;
P is (being independently when at every turn occurring) 0,1 or 2;
M is 0 or 1;
N is 0,1 or 2;
Condition is: when m was 0, then n was 1 or 2.
2. the formula of claim 1 (I) compound or its salt, prodrug or solvate, condition be described compound be not included in exemplify among the WO2004/076420 with the compound that is included in the scope of the present invention.
3. the formula of claim 1 or claim 2 (I) compound or its salt, prodrug or solvate, wherein R
1Has (S) configuration.
4. formula (I) compound or its salt, prodrug or the solvate of claim 1, claim 2 or claim 3, wherein HET-1 is 5 yuan of rings.
5. each formula (I) compound or its salt, prodrug or solvate, wherein R of claim 1-4
2Be selected from-C (O) NR
4R
5With-SO
2NR
4R
5, and R
4And R
5Can form the heterocyclic radical ring system that defines by HET-3 with the nitrogen-atoms that they connected.
6. each formula (I) compound or its salt, prodrug or solvate of claim 1-5, wherein HET-3 is a 4-6 unit ring.
7. formula (I) compound or its salt, prodrug or solvate, the wherein R of claim 1, claim 2 or claim 3
2Be selected from-C (O) NR
4R
5With-SO
2NR
4R
5, and R
4Be selected from (1-4C) alkyl [by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2.
8. formula (I) compound or its salt, prodrug or solvate, the wherein R of claim 1, claim 2 or claim 3
2Be-SO
2R
4, and R
4Be selected from (1-4C) alkyl [by 1 or 2 be independently selected from HET-2 ,-OR
5,-SO
2R
5, (3-6C) cycloalkyl (optional is selected from R by one
7Group replace) and-C (O) NR
5R
5Substituting group replace], (3-6C) cycloalkyl (optionally is selected from R by one
7Group replace) and HET-2.
9. formula (I) compound or its salt, prodrug or solvate, the wherein R of claim 1, claim 2 or claim 3
2Be HET-2.
10. pharmaceutical composition, described composition comprise each compound or pharmaceutically acceptable salt thereof, solvate or prodrug and pharmaceutically acceptable diluent or carrier of claim 1-9.
11. as each compound or pharmaceutically acceptable salt thereof, solvate or prodrug of the claim 1-9 of medicine.
12. be used to prepare each the compound of claim 1-9 of the medicine that is used for treating the disease by the GLK mediation.
13. be used to prepare each the compound of claim 1-9 of the medicine that is used for treating diabetes B.
14. the method for the disease of treatment GLK mediation comprises each formula (I) compound or its salt, solvate or the prodrug to the claim 1-9 of the administration significant quantity of the such treatment of needs.
15. the method for claim 14, the disease of wherein said GLK mediation is a diabetes B.
16. each the method for formula (I) compound of preparation claim 1-9, described method comprise (wherein unless otherwise defined, otherwise variable as defined in claim 1):
(a) sour or its activated derivatives and formula (IV) compound with (III) reacts
Perhaps
(b) with formula V compound and the reaction of formula (VI) compound
X wherein
1Be leavings group, and X
2Be hydroxyl, perhaps X
1Be hydroxyl, and X
2It is leavings group;
[perhaps by with P wherein
1Be the intermediate ester reaction of the formula (VII) of protecting group, carry out ester hydrolysis and acid amides then and form]
Perhaps
(c) with formula (VIII) compound and the reaction of formula (IX) compound
X wherein
3Be leavings group or organometallic reagent, and X
4Be hydroxyl, perhaps X
3Be hydroxyl, and X
4Be leavings group or organometallic reagent;
[, carry out ester hydrolysis and acid amides then and form] perhaps by (VIII) reaction with the intermediate ester of formula (X)
Perhaps
(d) with formula (XI) compound and the reaction of formula (XII) compound
X wherein
5It is leavings group;
And if necessary afterwards:
I) a kind of formula (I) compound is changed into another kind of formula (I) compound;
Ii) remove any protecting group; And/or
Iii) form its salt, prodrug or solvate.
17. the compound or its salt that exemplifies herein, prodrug or solvate.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0403593.7 | 2004-02-18 | ||
| GB0403593A GB0403593D0 (en) | 2004-02-18 | 2004-02-18 | Compounds |
| GB0413386.4 | 2004-06-16 | ||
| GB0423039.7 | 2004-10-16 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1922159A true CN1922159A (en) | 2007-02-28 |
Family
ID=32039950
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN 200580005262 Pending CN1922159A (en) | 2004-02-18 | 2005-02-15 | Benzamide derivatives and their use as glucokinase activating agents |
Country Status (3)
| Country | Link |
|---|---|
| CN (1) | CN1922159A (en) |
| GB (1) | GB0403593D0 (en) |
| ZA (1) | ZA200606684B (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102656149A (en) * | 2009-12-11 | 2012-09-05 | 安斯泰来制药株式会社 | Benzamide compound |
| CN102015637B (en) * | 2008-02-06 | 2014-05-21 | 第一三共株式会社 | Phenylpyrrole derivative |
-
2004
- 2004-02-18 GB GB0403593A patent/GB0403593D0/en not_active Ceased
-
2005
- 2005-02-15 CN CN 200580005262 patent/CN1922159A/en active Pending
-
2006
- 2006-08-11 ZA ZA200606684A patent/ZA200606684B/en unknown
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102015637B (en) * | 2008-02-06 | 2014-05-21 | 第一三共株式会社 | Phenylpyrrole derivative |
| CN102656149A (en) * | 2009-12-11 | 2012-09-05 | 安斯泰来制药株式会社 | Benzamide compound |
Also Published As
| Publication number | Publication date |
|---|---|
| ZA200606684B (en) | 2008-12-31 |
| GB0403593D0 (en) | 2004-03-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1264825C (en) | Dihydro-benzo [b][1,4] diazepin-2-one derivatives as mglur 2 antagonists II | |
| CN1520296A (en) | Amino nicotinate derivatives as glucokinase (GLK) modulators | |
| CN1886377A (en) | Benzoyl amino pyridyl carboxylic acid derivatives useful as glucokinase (GLK) activators | |
| CN1188415C (en) | Pyrazolopyrimidinones which inhibit type 5 cyclic guanosine 3', 5'-monophosphate phosphodiesterase (cGMP PDE5) for treatment of sexual dysfunction | |
| CN1568185A (en) | Compounds effecting glucokinase | |
| CN100338061C (en) | Alkyne-aryl phosphodiesterase-4 inhibitors | |
| CN101039915A (en) | Phenoxy benzamide compounds with utility in the treatment of type 2 diabetes and obesity | |
| CN1269813C (en) | Imidazolo-5-yl-2-anilino-pyrimidines as agents for inhibition of cell proliferation | |
| CN1252054C (en) | Qinoline derivatives inhibiting effect of growth factors such as VEGF | |
| CN1219768C (en) | Quinoline derivatives as inhibitors of MEK enzymes | |
| CN1934100A (en) | Substituted quinazoline or pyridopyrimidine derivative | |
| CN1898209A (en) | Pyridine carboxylic acid derivatives as glucokinase modulators | |
| CN1170827C (en) | Benzofurylpyrone derivatives | |
| CN1832928A (en) | 5-membered heterocycle-based p38 kinase inhibitors | |
| CN1863779A (en) | Novel indazole derivative | |
| CN1871240A (en) | Pyrrolopyrimidine thion derivatives | |
| CN1993349A (en) | Quinazoline derivatives as ERBB receptor tyrosine kinases | |
| CN1678586A (en) | Substituted quinoline CCR5 receptor antagonists | |
| CN1930144A (en) | 3-'4-heterocyclyl -1,2,3,-triazol-1-yl!-n-aryl-benzamides as inhibitors of the cytokines production for the treatment of chronic inflammatory diseases | |
| CN1852905A (en) | N-substituted benzimidazolyl c-kit inhibitors | |
| CN1703405A (en) | Aminobenzamide derivatives as glycogen synthase kinase 3 beta inhibitors | |
| CN1494541A (en) | Heterocyclic inhibitors of ERK 2 and uses thereof | |
| CN1906190A (en) | Selective kinase inhibitors | |
| CN1555367A (en) | 3-substituted-4-pyrimidone derivatives | |
| CN1890219A (en) | Benzoyl amino pyridyl carboxylic acid derivatives useful as glucokinase (GLK) activators |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| AD01 | Patent right deemed abandoned |
Effective date of abandoning: 20070228 |
|
| C20 | Patent right or utility model deemed to be abandoned or is abandoned |