环胺衍生物及其作为药物的用途
本申请是发明名称为“环胺衍生物及其作为药物的用途”的中国发明申请(申请日:1998年11月17日,申请号:98811317.1)的分案申请。
发明领域
本发明涉及新颖的环胺衍生物。
本发明也涉及化学因子受体拮抗剂,它们是例如下述的疾病的有效治疗剂和/或预防剂:动脉粥样硬化、类风湿性关节炎、牛皮癣、气喘、溃疡性结肠炎、肾炎(肾病)、多发性硬化、肺纤维症、心肌炎、肝炎、胰腺炎、肉样瘤病、克罗恩氏病、子宫内膜异位、充血性心力衰竭、病毒性脑膜炎、脑梗塞、神经病、川崎病和败血症,这些疾病中血液单核细胞和淋巴细胞等白细胞的组织浸润作用在疾病的发生、发展或维持中起主要作用。
有关领域的说明
化学因子是一类分子量为6-15kD的炎性/免疫调节性多肽因子,是在炎性部位由多种类型细胞产生的,例如巨噬细胞、单核细胞、嗜曙红细胞、嗜中性白细胞、成纤维细胞、血管内皮细胞、平滑肌细胞和肥大细胞。化学因子可以分为两个主要的小类,CXC化学因子(或α化学因子)和CC化学因子(或β化学因子),分类依据是四个保存的半胱氨酸残基的共同位置和编码它们的基因的染色体位置差异。CXC化学因子的前两个半胱氨酸被一个氨基酸分开,CC化学因子的前两种半胱氨酸是相邻的。例如,IL-8(白介素-8的缩写)是一种CXC化学因子,而CC化学因子包括MIP-1α/β(巨噬细胞炎性蛋白-1α/β的缩写)、MCP-1(单核细胞化学引诱蛋白-1的缩写)和RANTES(正常T细胞表达和分泌的活化调节的缩写)。也有不属于这两小类之一的化学因子。它们是:lymphotactin,仅具有两个半胱氨酸,因此定义为C化学因子;和fractalkine,在粘蛋白结构中具有一个化学因子样结构域,其中前两个半胱氨酸被三个氨基酸分开,因此定义为CX3C化学因子。这些化学因子促进趋化性、细胞移行,提高整联蛋白等细胞粘连分子的表达和细胞粘连,被认为是密切参与白细胞向炎性组织等病原部位粘连和浸润的蛋白因子(参考文献例如Vaddi,K.等,《化学因子论文》,Academic Press,1997;《化学引诱剂配体及其受体》,Horuk,R.,Ed.,CRC Press,1996;Ward,G.W.等,《生物化学杂志》,1998,333,457;Luster,A.D.,《新英格兰医学杂志》,1998,338,436;Baggiolini,M.,《自然》,1998,392,565;Rollins,B.J.,《血液》,1997,90,909;Alam,R.,《变态反应临床免疫学杂志》,1997,99,273;Hancock,W.W.,《美国病理学杂志》,1996,148,681;Taub,D.D.,《细胞因子与生长因子评论》,1996,7,335;Strieter,R.M.等,《免疫学杂志》,1996,156,3583;Furie,M.B.等,《美国病理学杂志》,1995,146,1287;Schall,T.J.等,《免疫学中的流行观点》,1994,6,865;Edginton,S.M.,《生物工程》,1993,11,676)。
例如,MIP-1α导致细胞内钙离子浓度水平短暂升高,诱导T淋巴细胞、B淋巴细胞的移行(例如参见Taub,D.D.等,《科学》,1993,260,355;Schall,T.J.等,《实验医学杂志》,1993,177,1821)和嗜曙红细胞的移行(例如参见Rot,A.等,《实验医学杂志》,1992,176,1489),自然杀伤细胞的趋化性(例如参见Maghazachi,A.A.等,《免疫学杂志》,1994,153,4969),整联蛋白的表达(例如参见Vaddi,K.等,《免疫学杂志》,1994,153,4721),和破骨细胞的分化(例如参见Kukita,T.等,《实验室研究》,1997,76,399)。MIP-1α也增加B细胞内IgE和IgG4的产生(例如参见Kimata,H.等,《实验医学杂志》,1996,183,2397)和抑制造血干细胞的增殖(例如参见Mayani,H.等,《实验血液学》,1995,23,422;Keller,J.R.等《血液》,1994,84,2175;Eaves,C.J.等,《美国国家科学院院报》,1993,90,12015;Bodine,D.M.等,《血液》,1991,78,914;Broxmeyer,H.E.等,《血液》,1990,76,1110)。
关于MIP-1α的体内活性和在发病机理中的作用,据报道,它在兔子内是一种热原(例如参见Davatelis,G.等,《科学》1989,243,1066);MIP-1α注入小鼠足垫导致炎性反应,例如嗜中性白细胞和单核细胞的浸润(例如参见Alam,R.等,《免疫学杂志》1994,152,1298);MIP-1α中和抗体对下列动物模型具有抑制作用或治疗作用:肉芽肿(例如参见Lukacs,N.W.等,《实验医学杂志》1993,177,1551),气喘(例如参见Lukacs,N.W.等,《欧洲免疫学杂志》1995,25,245;Lukacs,N.W.等,《免疫学杂志》1997,158,4398),多发性硬化(例如参见Karpus,W.J.等,《免疫学杂志》1995,155,5003;Karpus,W.J.等,《白细胞生物学杂志》1997,62,681),自发性肺纤维症(例如参见Smith,R.E.等,《免疫学杂志》1994,153,4704;Smith,R.E.,《生物学信号》1996,5,223),急性肺损伤(例如参见Shanley,T.P.等,《免疫学杂志》1995,154,4793;Standiford,T.J.等,《免疫学杂志》1995,155,1515),和类风湿性关节炎(例如参见Kasama,T.等,《临床研究杂志》1995,95,2868);柯萨奇病毒诱发的小鼠心肌炎和角膜基质疱疹被破裂的MIP-1α基因抑制(例如参见Cook,D.N.等,《科学1995,269,1583;Tumpey,T.M.等,《病毒学杂志》1998,72,3705);下列患者观察到MIP-1α的显著表达:慢性肺部炎性疾病(例如参见Standiford,T.J.等,《免疫学杂志》1993,151,2852),过敏性肺炎(例如参见Denis,M.,《Am.J.Respir.Crit.Care Med.》1995,151,164),类风湿性关节炎(例如参见Koch,A.E.等,《临床研究杂志》1994,93,921),传染性脑膜炎(例如参见Lahrtz,F.等,《神经免疫学杂志》1998,85,33),和慢性肌肉炎症(例如参见Adams,E.M.等,《Proc.Assoc.Am.Physicians》1997,109,275)。这些研究指出,MIP-1α密切参与了不同白细胞亚型的局部吸引作用和所致炎性反应的发生、发展和维持。
MCP-1(已知也叫MCAF(巨噬细胞趋化性与活化因子的缩写)或JE)是一种由单核细胞/巨噬细胞、平滑肌细胞、成纤维细胞和血管内皮细胞产生的CC化学因子,它引起下列细胞的细胞移行和细胞粘连:单核细胞(例如参见Valente,A.J.等,《生物化学》1988,27,4162;Matsushima,K.等,《实验医学杂志》,1989,169,1485;Yoshimura,T.等,《免疫学杂志》,1989,142,1956;Rollins,B.J.等,《美国国家科学院院报》,1988,85,3738;Rollins,B.J.等,《血液》,1991,78,1112;Jiang,Y.等,《免疫学杂志》,1992,148,2423;Vaddi,K.等,《免疫学杂志》,1994,153,4721),记忆T淋巴细胞(例如参见Carr,M.W.等,《美国国家科学院院报》,1994,91,3652),T淋巴细胞(例如参见Loetscher,P.等,《美国实验生物学会联合会会志》,1994,8,1055),和自然杀伤细胞(例如参见Loetscher,P.等,《免疫学杂志》,1996,156,322;Allavena,P.等,《欧洲免疫学杂志》,1994,24,3233),以及介导嗜碱细胞释放组胺(例如参见Alam,R.等,《临床研究杂志》,1992,89,723;Bischoff,S.C.等,《实验医学杂志》,1992,175,1271;Kuna,P.等,《实验医学杂志》,1992,175,489)。
另外,在某些疾病中报道了MCP-1的高度表达,在这些疾病中,单核细胞/巨噬细胞和/或T细胞的蓄积对疾病的发生或发展来说被认为是重要的,例如动脉粥样硬化(例如参见Hayes,I.M.等,《Arterioscler.Thromb.Vasc.Biol.》,1998,18,397;Takeya,M.等,《人的病理学》,1993,24,534;Yla-Herttuala,S.等,《美国国家科学院院报》,1991,88,5252;Nelken,N.A.,《临床研究杂志》,1991,88,1121),类风湿性关节炎(例如参见Koch,A.E.等,《临床研究杂志》,1992,90,772;Akahoshi,T.等,《类风湿性关节炎》,1993,36,762;Robinson,E.等,《临床实验免疫学》,101,398),肾炎(例如参见Noris,M.等,《实验室研究》,1995,73,804;Wada,T.等,《肾炎研究》,1996,49,761;Gesualdo,L.等,《肾炎研究》,1997,51,155),肾病(例如参见Saitoh,A.等,《临床实验室分析杂志》,1998,12,1;Yokoyama,H.等,《白细胞生物学杂志》,1998,63,493),肺纤维症、肺肉样瘤病(例如参见Sugiyama,Y.等,《内科学》,1997,36,856),气喘(例如参见Karina,M.等,《变应性学临床免疫学研究杂志》,1997,7,254;Stephene,T.H.,《Am.J.Respir.Crit.CareMed.》,1997,156,1377;Sousa,A.R.等,《美国呼吸细胞分子生物学杂志》,1994,10,142),多发性硬化(例如参见McManus,C.等,《神经免疫学杂志》,1998,86,20),牛皮癣(例如参见Gillitzer,R.等,《皮科学研究杂志》,1993,101,127),炎性肠疾病(例如参见Grimm,M.C.等,《白细胞生物学杂志》,1996,59,804;Reinecker,H.C.等,《胃肠病学》,1995,106,40),心肌炎(例如参见Seino,Y.等,《细胞因子》,1995,7,301),子宫内膜异位(例如参见Jolicoeur,C.等,《美国病理学杂志》,1998,152,125),腹膜内粘连(例如参见Zeyneloglu,H.B.等,《人的生殖》,1998,13,1194),充血性心力衰竭(例如参见Aurust,P.等,《循环》,1998,97,1136),慢性肝疾病(例如参见Marra,F.等,《美国病理学杂志》,1998,152,423),病毒性脑膜炎(例如参见Lahrtz,F.等,《欧洲免疫学杂志》,1997,27,2484),川崎病(例如参见Wong,M.等,《风湿病学杂志》,1997,24,1179),和败血症(例如参见Salkowski,C.A.等,《感染性免疫学》,1998,66,3569)。而且据报道,抗MCP-1抗体显示对下列动物模型的抑制作用或治疗作用:类风湿性关节炎(例如参见Schimmer,R.C.等,《免疫学杂志》,1998,160,1466;Schrier,D.J.,《白细胞生物学杂志》,1998,63,359;Ogata,H.等,《病理学杂志》,1997,182,106),多发性硬化(例如参见Karpus,W.J.等,《白细胞生物学杂志》,1997,62,681),肾炎(例如参见Lloyd,C.M.等,《实验医学杂志》,1997,185,1371;Wada,T.等,《美国实验生物学会联合会会志》,1996,10,1418),气喘(例如参见Gonzalo,J.-A.等,《实验医学杂志》,1998,188,157;Lukacs,N.W.等,《免疫学杂志》,1997,158,4398),动脉粥样硬化(例如参见Guzman,L.A.等,《循环》,1993,88(增刊),I-371),延迟型过敏症(例如参见Rand,M.L.等,《美国病理学杂志》,1996,148,855),肺动脉高血压(例如参见Kimura,H.等,《实验室研究》,1998,78,571),和腹膜内粘连(例如参见Zeyneloglu,H.B.等,《美国产科学与妇科学杂志》,1998,179,438)。也有报道MCP-1的肽拮抗剂MCP-1(9-76)抑制小鼠模型关节炎(例如参见Gong,J.-H.,《实验医学杂志》,1997,186,131),以及对MCP-1缺陷小鼠的研究表明,MCP-1是体内单核细胞募集所必需的(例如参见Lu,B.等,《实验医学杂志》,1998,187,601;Gu,L.等,《Moll.Cell》,1998,2,275)。
这些资料指出,MIP-1α和MCP-1等化学因子将单核细胞和淋巴细胞吸引到疾病部位,介导它们的活化作用,因此被认为密切参与了与单核细胞和淋巴细胞密切有关的疾病的发生、发展和维持,例如动脉粥样硬化、类风湿性关节炎、牛皮癣、气喘、溃疡性结肠炎、肾炎(肾病)、多发性硬化、肺纤维症、心肌炎、肝炎、胰腺炎、肉样瘤病、克罗恩氏病、子宫内膜异位、充血性心力衰竭、病毒性脑膜炎、脑梗塞、神经病、川崎病和败血症(例如参见Rovin,B.H.等,《美国肾疾病杂志》,1998,31,1065;Lloyd,C.等,《肾性高血压中的流行观点》,1998,7,281;Conti,P.等,《Allergy and AsthmaProc.》,1998,19,121;Ransohoff,R.M.等,《神经科学趋势》,1998,21,154;MacDermott,R.P.等,《炎性肠疾病》,1998,4,54)。因此,抑制化学因子对靶细胞的作用的药物可能是这些疾病的有效治疗和/或预防药。
编码特异性化学因子受体的基因已经被克隆,现在知道,这些受体是与G蛋白偶联的七种跨膜受体,存在于不同的白细胞种群上。迄今,已经鉴定了至少五种CXC化学因子受体(CXCR1-CXCR5)和八种CC化学因子受体(CCR1-CCR8)。例如,IL-8是CXCR1和CXCR2的配体,MIP-1α是CCR1和CCR5的配体,MCP-1是CCR2A和CCR2B的配体(参考文献例如Holmes,W.E.等,《科学》,1991,253,1278-1280;Murphy,P.M.等,《科学》,253,1280-1283;Neote,K.等,《细胞》,1993,72,415-425;Charo,I.F.等,《美国国家科学院院报》,1994,91,2752-2756;Yamagami,S.等,《生物化学与生物物理学研究通讯》,1994,202,1156-1162;Combadier,C.等,《生物化学杂志》,1995,270,16491-16494;Power,C.A.等,《生物化学杂志》,1995,270,19495-19500;Samson,M.等,《生物化学》,1996,35,3362-3367;Murphy,P.M.,《免疫学年鉴》,1994,12,592-633)。据报道,肺部炎症和肉芽肿形成在CCR1缺陷小鼠中被抑制了(见Gao,J.-L等,《实验医学杂志》,1997,185,1959;Gerard,C.等,《临床研究杂志》,1997,100,2022),巨噬细胞的募集和动脉粥样硬化损害的形成在CCR2缺陷小鼠中减少了(见Boring,L.等,《自然》,1998,394,894;Kuziel,W.A.等,《美国国家科学院院报》,1997,94,12053;Kurihara,T.等,《实验医学杂志》,1997,186,1757;Boring,L.等,《临床研究杂志》,1997,100,2552)。因此,抑制MIP-1α和/或MCP-1等化学因子与这些受体结合的化合物、也就是化学因子受体拮抗剂可用作抑制MIP-1α和/或MCP-1等化学因子对靶细胞作用的药物,但是已知还没有药物具有这样的作用。
由本发明所提供的环胺衍生物是相当新颖的。最近报道,下列化合物对CXCR1、CXCR4、CCR1、CCR2、CCR3和CCR5等化学因子受体具有拮抗活性:二苯基甲烷衍生物(WO 9724325;Hesselgesser,J.等,《生物化学杂志》,1998,273,15687),哌啶衍生物(JP 9-249566),咪唑并苯并二氮杂_衍生物(JP 9-249570),苯并吖辛因衍生物(JP 9-255572),具有环氨基的三环化合物(WO 9804554),吩噻嗪衍生物(Bright,C.等,《生物有机与医药化学快报》,1998,8,771),哌嗪衍生物(WO 9744329),苯并咪唑衍生物(WO9806703),偏端霉素类似物(Howard,O.M.Z.等,《医药化学杂志》,1998,41,2184),双吖啶衍生物(WO 9830218),螺取代的氮杂环(WO 9825604;WO9825605),取代的芳基哌嗪(WO 9825617),氨基喹啉衍生物(WO 9827815),3-芳基哌啶衍生物(WO 9831364),己酰胺衍生物(WO 9838167),和其他小分子(WO 9744329;WO 9802151;WO 9804554)。不过,这些化合物不同于本发明的化合物。
发明概述
因此,本发明的一个目的是提供抑制MIP-1α和/或MCP-1等化学因子与其靶细胞上受体结合的小分子化合物。
本发明的另一个目的是建立抑制MIP-1α和/或MCP-1等化学因子与靶细胞上受体结合和/或抑制其对靶细胞的作用的方法。
本发明还有一个目的是提出用于疾病治疗的方法,该疾病的病因之一是MIP-1α和/或MCP-1等化学因子与靶细胞上受体的结合。
作为深入研究的结果,本发明人发现,具有芳烷基的环胺衍生物、其药学上可接受的C1-C6烷基加成盐或其药学上可接受的酸加成盐对抑制MIP-1α和/或MCP-1等化学因子与靶细胞受体的结合具有极佳的活性,从而完成了本发明。
也就是说,本发明是下式(I)化合物:
其药学上可接受的酸加成盐或其药学上可接受的C1-C6烷基加成盐(发明1),
其中R1是苯基、C3-C8环烷基或具有1-3个杂原子的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,其中该苯基或芳族杂环基可以与苯环或具有1-3个杂原子的芳族杂环基稠合形成稠合环,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,该苯基、C3-C8环烷基、芳族杂环基或稠合环可以被一个或多个下列取代基取代:卤原子、羟基、氰基、硝基、羧基、氨基甲酰基、C1-C6烷基、C3-C8环烷基、C2-C6烯基、C1-C6烷氧基、C1-C6烷硫基、C3-C5亚烷基、C2-C4亚烷氧基、C1-C3亚烷二氧基、苯基、苯氧基、苯硫基、苄基、苄氧基、苯甲酰氨基、C2-C7烷酰基、C2-C7烷氧羰基、C2-C7烷酰氧基、C2-C7烷酰氨基、C2-C7N-烷基氨基甲酰基、C4-C9 N-环烷基氨基甲酰基、C1-C6烷磺酰基、C3-C8(烷氧羰基)甲基、N-苯基氨基甲酰基、哌啶子基羰基、吗啉代羰基、1-吡咯烷基羰基、由式-NH(C=O)O-代表的二价基团、由式-NH(C=S)O-代表的二价基团、氨基、单(C1-C6烷基)氨基、或二(C1-C6烷基)氨基,其中该苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基可选地被一个或多个卤原子、羟基、氨基、三氟甲基、C1-C6烷基或C1-C6烷氧基取代;
R2是氢原子、C1-C6烷基、C2-C7烷氧羰基、羟基或苯基,其中该C1-C6烷基或苯基可以被一个或多个卤原子、羟基、C1-C6烷基或C1-C6烷氧基取代,且当j=0时,R2不是羟基;
j代表0-2的整数;
k代表0-2的整数;
m代表2-4的整数;
n代表0或1;
R3是氢原子或可选被一个或两个苯基取代的C1-C6烷基,该苯基各自可以被一个或多个卤原子、羟基、C1-C6烷基或C1-C6烷氧基取代;
R4和R5是彼此相同或不同的,是氢原子、羟基、苯基或C1-C6烷基,其中该C1-C6烷基可选地被一个或多个下列取代基取代:卤原子,羟基,氰基,硝基,羧基,氨基甲酰基,巯基,胍基,C3-C8环烷基,C1-C6烷氧基,C1-C6烷硫基,可选被一个或多个卤原子、羟基、C1-C6烷基、C1-C6烷氧基或苄氧基取代的苯基,苯氧基,苄氧基,苄氧羰基,C2-C7烷酰基,C2-C7烷氧羰基,C2-C7烷酰氧基,C2-C7烷酰氨基,C2-C7 N-烷基氨基甲酰基,C1-C6烷磺酰基,氨基,单(C1-C6烷基)氨基,二(C1-C6烷基)氨基,或具有1-3个杂原子且可选与苯环稠合的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,或者R4和R5一起形成3至6元环烃;
p代表0或1;
q代表0或1;
G是由下式代表的基团:-CO-、-SO2-、-CO-O-、-NR7-CO-、-CO-NR7-、-NH-CO-NH-、-NH-CS-NH-、-NR7-SO2-、-SO2-NH-、-NH-CO-O-、或-O-CO-NH-,其中R7是氢原子或C1-C6烷基,或者R7与R5一起代表C2-C5亚烷基;
R6是苯基、C3-C8环烷基、C3-C8环烯基、苄基或具有1-3个杂原子的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,其中该苯基、苄基或芳族杂环基可以与苯环或具有1-3个杂原子的芳族杂环基稠合形成稠合环,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,该苯基、C3-C8环烷基、C3-C8环烯基、苄基、芳族杂环基或稠合环可以被一个或多个下列取代基取代:卤原子、羟基、巯基、氰基、硝基、氰硫基、羧基、氨基甲酰基、三氟甲基、C1-C6烷基、C3-C6环烷基、C2-C6烯基、C1-C6烷氧基、C3-C8环烷氧基、C1-C6烷硫基、C1-C3亚烷二氧基、苯基、苯氧基、苯氨基、苄基、苯甲酰基、苯亚磺酰基、苯磺酰基、3-苯脲基、C2-C7烷酰基、C2-C7烷氧羰基、C2-C7烷酰氧基、C2-C7烷酰氨基、C2-C7 N-烷基氨基甲酰基、C1-C6烷磺酰基、苯基氨基甲酰基、N,N-二(C1-C6烷基)氨磺酰基、氨基、单(C1-C6烷基)氨基、二(C1-C6烷基)氨基、苄氨基、C2-C7(烷氧羰基)氨基、C1-C6(烷磺酰)氨基、或双C1-C6(烷磺酰)氨基,其中该苯基、C3-C8环烷基、C3-C8环烯基、苄基、芳族杂环基或稠合环的取代基可选地被一个或多个卤原子、氰基、羟基、氨基、三氟甲基、C1-C6烷基、C1-C6烷氧基、C1-C6烷硫基、单(C1-C6烷基)氨基或二(C1-C6烷基)氨基取代。
本发明也是一种方法,该方法使用含有治疗上有效量的由上式(I)代表的化合物、其药学上可接受的酸加成盐或其药学上可接受的C1-C6烷基加成盐的药物制剂抑制化学因子与靶细胞受体结合和/或抑制其对靶细胞的作用(发明2)。
这里,由上式(I)代表的化合物具有抑制MIP-1α和/或MCP-1等化学因子与靶细胞受体结合的活性和抑制由MIP-1α和/或MCP-1等化学因子引起的细胞生理学活性的活性。
优选实施方式的说明
(1)发明1
上式(I)中,R1是苯基、C3-C8环烷基或具有1-3个杂原子的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,其中该苯基或芳族杂环基可以与苯环或具有1-3个杂原子的芳族杂环基稠合形成稠合环,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,该苯基、C3-C8环烷基、芳族杂环基或稠合环可以被一个或多个下列取代基取代:卤原子、羟基、氰基、硝基、羧基、氨基甲酰基、C1-C6烷基、C3-C8环烷基、C2-C6烯基、C1-C6烷氧基、C1-C6烷硫基、C3-C5亚烷基、C2-C4亚烷氧基、C1-C3亚烷二氧基、苯基、苯氧基、苯硫基、苄基、苄氧基、苯甲酰氨基、C2-C7烷酰基、C2-C7烷氧羰基、C2-C7烷酰氧基、C2-C7烷酰氨基、C2-C7N-烷基氨基甲酰基、C4-C9 N-环烷基氨基甲酰基、C1-C6烷磺酰基、C3-C8(烷氧羰基)甲基、N-苯基氨基甲酰基、哌啶子基羰基、吗啉代羰基、1-吡咯烷基羰基、由式-NH(C=O)O-代表的二价基团、由式-NH(C=S)O-代表的二价基团、氨基、单(C1-C6烷基)氨基、或二(C1-C6烷基)氨基。
R1代表的“C3-C8环烷基”指的是一种环状烷基,例如环丙基、环丁基、环戊基、环己基、环庚基和环辛基,具体包括环丙基、环戊基和己基。
R1代表的“具有1-3个杂原子的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组”具体例如噻吩基、呋喃基、吡咯基、咪唑基、吡唑基、噁唑基、异噁唑基、噻唑基、异噻唑基、吡啶基、嘧啶基、三嗪基、三唑基、噁二唑基(呋咱基)、噻二唑基等,优选包括噻吩基、呋喃基、吡咯基、异噁唑基和吡啶基。
R1代表的“稠合环”指的是,苯基或具有1-3个杂原子的芳族杂环基,杂原子选自由氧原子、硫原子和/或氮原子组成的组,通过在任意可能的位置与苯环或具有1-3个杂原子的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子组成的组,进行稠合作用所得到的环,适当地和具体例如萘基、吲哚基、苯并呋喃基、苯并噻吩基、喹啉基、苯并咪唑基、苯并噁唑基、苯并三唑基、苯并噁二唑基(苯并呋咱基)和苯并噻二唑基。
其中,苯基和异噁唑基可以列为R1代表的优选具体实例。
作为R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基的“卤原子”包括氟原子、氯原子、溴原子和碘原子,适当地包括氟原子、氯原子和溴原子。
作为R1的取代基的“C1-C6环烷基”指的是C1-C6直链或支链烷基,例如甲基、乙基、正丙基、正丁基、正戊基、正己基、正庚基、正辛基、异丙基、异丁基、仲丁基、叔丁基、异戊基、新戊基、叔戊基、异己基、2-甲基戊基、1-乙基丁基等,适当地具体包括甲基、乙基、丙基和异丙基。
作为R1的取代基的“C3-C8环烷基”与上述R1代表的“C3-C8环烷基”所定义的相同,优选的具体实例也可以给出相同的实例。
作为R1的取代基的“C2-C6烯基”指的是C2-C6直链或支链烯基,例如乙烯基、烯丙基、1-丙烯基、2-丁烯基、3-丁烯基、2-甲基-1-丙烯基、4-戊烯基、5-己烯基、4-甲基-3-戊烯基等,适当地具体包括乙烯基和2-甲基-1-丙烯基。
作为R1的取代基的“C1-C6烷氧基”指的是由上述C1-C6烷基和氧基组成的基团,具体例如甲氧基和乙氧基。
作为R1的取代基的“C1-C6烷硫基”指的是由上述C1-C6烷基和硫基组成的基团,具体例如甲硫基和乙硫基。
作为R1的取代基的“C3-C5亚烷基”指的是C3-C5二价亚烷基,例如三亚甲基、四亚甲基、五亚甲基和1-甲基三亚甲基,具体例如三亚甲基和四亚甲基。
作为R1的取代基的“C2-C4亚烷氧基”指的是由上述C2-C4二价亚烷基和氧基组成的基团,例如亚乙氧基(-CH2CH2O-)、三亚甲氧基(-CH2CH2CH2O-)、四亚甲氧基(-CH2CH2CH2CH2O-)和1,1-二甲基亚乙氧基(-CH2C(CH3)2O-),具体例如亚乙氧基和三亚甲氧基。
作为R1的取代基的“C1-C3亚烷二氧基”指的是由C1-C3二价亚烷基和两个氧基组成的基团,例如亚甲二氧基(-OCH2O-)、亚乙二氧基(-OCH2CH2O-)、三亚甲二氧基(-OCH2CH2CH2O-)和亚丙二氧基(-OCH2CH(CH3)O-),具体例如亚甲二氧基和亚乙二氧基。
作为R1的取代基的“C2-C7烷酰基”指的是C2-C7直链或支链烷酰基,例如乙酰基、丙酰基、丁酰基、戊酰基、己酰基、庚酰基、异丁酰基、3-甲基丁酰基、2-甲基丁酰基、新戊酰基、4-甲基戊酰基、3,3-二甲基丁酰基、5-甲基己酰基等,其中优选的和具体的实例包括乙酰基。
作为R1的取代基的“C2-C7烷氧羰基”指的是由上述C1-C6烷氧基和羰基组成的基团,优选地和具体例如甲氧羰基和乙氧羰基。
作为R1的取代基的“C2-C7烷酰氧基”指的是由上述C2-C7烷酰基和氧基组成的基团,具体例如乙酰氧基。
作为R1的取代基的“C2-C7烷酰氨基”指的是由上述C2-C7烷酰基和氨基组成的基团,具体例如乙酰氨基。
作为R1的取代基的“C2-C7 N-烷基氨基甲酰基”指的是由上述C1-C6烷基和氨基甲酰基组成的基团,具体例如N-甲基氨基甲酰基和N-乙基氨基甲酰基。
作为R1的取代基的“C4-C9 N-环烷基氨基甲酰基”指的是由上述C3-C8环烷基和氨基甲酰基组成的基团,具体例如N-环戊基氨基甲酰基和N-环己基氨基甲酰基。
作为R1的取代基的“C1-C6烷磺酰基”指的是由上述C1-C6烷基和磺酰基组成的基团,优选地和具体例如甲磺酰基。
作为R1的取代基的“C3-C8(烷氧羰基)甲基”指的是由上述C2-C7烷氧羰基和甲基组成的基团,优选地和具体例如(甲氧羰基)甲基和(乙氧羰基)甲基。
作为R1的取代基的“单(C1-C6烷基)氨基”指的是被上述C1-C6烷基之一取代的氨基,优选地和具体例如甲氨基和乙氨基。
作为R1的取代基的“二(C1-C6烷基)氨基”指的是被相同或不同的两个上述C1-C6烷基取代的氨基,优选地和具体例如二甲氨基、二乙氨基和N-乙基-N-甲氨基。
其中,卤原子、羟基、C1-C6烷基、C2-C6烯基、C1-C6烷氧基、C1-C6烷硫基、C2-C4亚烷氧基、亚甲二氧基、N-苯基氨基甲酰基、氨基、单(C1-C6烷基)氨基和二(C1-C6烷基)氨基可以列为R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基的优选具体实例。
而且,上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基可选地被一个或多个卤原子、羟基、氨基、三氟甲基、C1-C6烷基或C1-C6烷氧基取代。卤原子、C1-C6烷基和C1-C6烷氧基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
上式(I)中,R2代表氢原子、C1-C6烷基、C2-C7烷氧羰基、羟基或苯基,其中该C1-C6烷基或苯基可以被一个或多个卤原子、羟基、C1-C6烷基或C1-C6烷氧基取代,且当j=0时,R2不是羟基。
R2代表的C1-C6烷基和C2-C7烷氧羰基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
作为R2中C1-C6烷基或苯基的取代基的卤原子、C1-C6烷基和C1-C6烷氧基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
其中,氢原子是R2代表的优选具体实例。
上式(I)中,j代表0-2的整数。特别优选的j是0。
上式(I)中,k代表0-2的整数,m代表2-4的整数。优选使用的是2-取代的吡咯烷、其中k是0且m是3,3-取代的吡咯烷、其中k是1且m是2,3-取代的哌啶、其中k是1且m是3,4-取代的哌啶、其中k是2且m是2,或3-取代的六氢氮杂_、其中k是1且m是4。
上式(I)中的n代表0或1。
尤其,3-酰氨基吡咯烷、其中k是1、m是2且n是0,和4-(酰氨甲基)哌啶、其中k是2、m是2且n是1可以列为特别优选的实例。
上式(I)中的R3代表氢原子或可选被一个或两个苯基取代的C1-C6烷基,该苯基各自可以被一个或多个卤原子、羟基、C1-C6烷基或C1-C6烷氧基取代。
R3代表的C1-C6烷基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,具体例如甲基、乙基和丙基。
R3中C1-C6烷基的取代基是苯基,作为该苯基取代基的卤原子、C1-C6烷基和C1-C6烷氧基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
其中,氢原子是R3代表的优选具体实例。
上式(I)中,R4和R5是彼此相同或不同的,是氢原子、羟基、苯基或C1-C6烷基,其中该C1-C6烷基可选地被一个或多个下列取代基取代:卤原子,羟基,氰基,硝基,羧基,氨基甲酰基,巯基,胍基,C3-C8环烷基,C1-C6烷氧基,C1-C6烷硫基,可选被一个或多个卤原子、羟基、C1-C6烷基、C1-C6烷氧基或苄氧基取代的苯基,苯氧基,苄氧基,苄氧羰基,C2-C7烷酰基,C2-C7烷氧羰基,C2-C7烷酰氧基,C2-C7烷酰氨基,C2-C7 N-烷基氨基甲酰基,C1-C6烷磺酰基,氨基,单(C1-C6烷基)氨基,二(C1-C6烷基)氨基,或具有1-3个杂原子且可选与苯环稠合的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,或者R4和R5一起形成3至6元环烃。
R4和R5代表的C1-C6烷基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
作为R4和R5中C1-C6烷基取代基的卤原子、C1-C6烷氧基、C1-C6烷硫基、C2-C7烷酰基、C2-C7烷氧羰基、C2-C7烷酰氧基、C2-C7烷酰氨基、C2-C7 N-烷基氨基甲酰基、C1-C6烷磺酰基、单(C1-C6烷基)氨基和二(C1-C6烷基)氨基上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
作为R4和R5中C1-C6烷基取代基的C3-C8环烷基和具有1-3个杂原子的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,与上述R1代表的基团所定义的相同,并且相同的实例可以列为优选的具体实例。
R4和R5中C1-C6烷基的取代基是苯基,作为该苯基取代基的卤原子、C1-C6烷基和C1-C6烷氧基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
由R4、R5和相邻碳原子组成的“3至6元环烃”包括环丙烷、环丁烷、环戊烷和环己烷。
其中,氢原子和C1-C6烷基可以列为R4和R5的优选具体实例。
上式(I)中,p代表0或1,q代表0或1。特别优选的是p和q都是0。
上式(I)中,G是由下式代表的基团:-CO-、-SO2-、-CO-O-、-NR7-CO-、-CO-NR7-、-NH-CO-NH-、-NH-CS-NH-、-NR7-SO2-、-SO2-NH-、-NH-CO-O-或-O-CO-NH-,其中R7是氢原子或C1-C6烷基,或者R7与R5一起代表C2-C5亚烷基。
上式中,-CO-指羰基,-SO2-指磺酰基,-CS-指硫代羰基。优选的G基团具体例如由式-NR7-CO-和-NH-CO-NH-代表的基团。
R7代表的C1-C6烷基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
由R5和R7组成的“C2-C5亚烷基”指的是C2-C5直链或支链亚烷基,例如亚甲基、亚乙基、亚丙基、三亚甲基、四亚甲基、1-甲基三亚甲基、五亚甲基等,适当地和具体包括亚乙基、三亚甲基和四亚甲基。
氢原子是R7代表的优选具体实例。
上式(I)中,R6是苯基、C3-C8环烷基、C3-C8环烯基、苄基或具有1-3个杂原子的芳族杂环基,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,其中该苯基、苄基或芳族杂环基可以与苯环或具有1-3个杂原子的芳族杂环基稠合形成稠合环,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,并且该苯基、C3-C8环烷基、C3-C8环烯基、苄基、芳族杂环基或稠合环可以被一个或多个下列取代基取代:卤原子、羟基、巯基、氰基、硝基、氰硫基、羧基、氨基甲酰基、三氟甲基、C1-C6烷基、C3-C6环烷基、C2-C6烯基、C1-C6烷氧基、C3-C8环烷氧基、C1-C6烷硫基、C1-C3亚烷二氧基、苯基、苯氧基、苯氨基、苄基、苯甲酰基、苯亚磺酰基、苯磺酰基、3-苯脲基、C2-C7烷酰基、C2-C7烷氧羰基、C2-C7烷酰氧基、C2-C7烷酰氨基、C2-C7 N-烷基氨基甲酰基、C1-C6烷磺酰基、苯基氨基甲酰基、N,N-二(C1-C6烷基)氨磺酰基、氨基、单(C1-C6烷基)氨基、二(C1-C6烷基)氨基、苄氨基、C2-C7(烷氧羰基)氨基、C1-C6(烷磺酰)氨基、或双C1-C6(烷磺酰)氨基。
R6代表的C3-C8环烷基、具有1-3个杂原子的芳族杂环基和稠合环,杂原子选自由氧原子、硫原子、氮原子或其组合组成的组,与上述R1所定义的相同,并且相同的实例可以列为优选的具体实例。
R6代表的“C3-C8环烯基”指的是环状烯基,例如环丁烯基、环戊烯基、环己烯基、环庚烯基和环辛烯基,具体包括1-环戊烯基和1-环己烯基。
其中,苯基、呋喃基和噻吩基可以列为R6代表的优选具体实例。
作为R6中苯基、C3-C8环烷基、C3-C8环烯基、苄基、芳族杂环基或稠合环的取代基的卤原子、C1-C6烷基、C2-C6烯基、C1-C6烷氧基、C1-C6烷硫基、C1-C3亚烷二氧基、C2-C7烷酰基、C2-C7烷氧羰基、C2-C7烷酰氧基、C2-C7烷酰氨基、C2-C7 N-烷基氨基甲酰基、C1-C6烷磺酰基、单(C1-C6烷基)氨基和二(C1-C6烷基)氨基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
作为R6的取代基的C3-C8环烷基与上述R1代表的C3-C8环烷基所定义的相同,其中优选的具体实例可以给出相同的实例。
作为R6的取代基的“C3-C8环烷氧基”指的是由上述C3-C8环烷基和氧基组成的基团,具体例如环丙氧基、环戊氧基和环己氧基。
作为R6的取代基的“N,N-二(C1-C6烷基)氨磺酰基”指的是被相同或不同的两个上述C1-C6烷基取代的氨磺酰基,优选地和具体例如N,N-二甲基氨磺酰基、N,N-二乙基氨磺酰基和N-乙基-N-甲基氨磺酰基。
作为R6的取代基的“C2-C7(烷氧羰基)氨基”指的是由上述C2-C7烷氧羰基和氨基组成的基团,具体例如(甲氧羰基)氨基和(乙氧羰基)氨基。
作为R6的取代基的“C1-C6(烷磺酰)氨基”指的是由上述C1-C6烷磺酰基和氨基组成的基团,具体例如(甲磺酰)氨基。
作为R6的取代基的“双(C1-C6烷磺酰)氨基”指的是被相同或不同的两个上述C1-C6烷磺酰基取代的氨基,优选地和具体例如双(甲磺酰)氨基。
其中,卤原子、巯基、硝基、氰硫基、三氟甲基、C1-C6烷基、C1-C6烷氧基、苯基、苯磺酰基、C2-C7烷酰氨基或氨基可以列为上述R6中苯基、C3-C8环烷基、C3-C8环烯基、苄基、芳族杂环基或稠合环的取代基的优选具体实例。
而且,上述R6中苯基、C3-C8环烷基、C3-C8环烯基、苄基、芳族杂环基或稠合环的取代基可选地被一个或多个卤原子、氰基、羟基、氨基、三氟甲基、C1-C6烷基、C1-C6烷氧基、C1-C6烷硫基、单(C1-C6烷基)氨基或二(C1-C6烷基)氨基取代。
该卤原子、C1-C6烷基、C1-C6烷氧基、C1-C6烷硫基、单(C1-C6烷基)氨基和二(C1-C6烷基)氨基与上述R1中苯基、C3-C8环烷基、芳族杂环基或稠合环的取代基所定义的相同,并且相同的实例可以列为优选的具体实例。
(2)发明2
由上式(I)代表的化合物、其药学上可接受的酸加成盐或其药学上可接受的C1-C6烷基加成盐可用于制备本发明的化学因子受体拮抗剂,方法是将治疗上的有效量与载体和/或稀释剂配制成药物组合物。因此,由上式(I)表示的环胺衍生物、其药学上可接受的酸加成盐或其药学上可接受的C1-C6烷基加成盐可以口服或胃肠外给药,例如静脉内、皮下、肌内、经皮或直肠内给药。
实现口服给药的剂型可以是片剂、丸剂、颗粒、药粉、溶液、混悬液、胶囊等。
制备片剂的方法例如可以使用赋形剂,如乳糖、淀粉和晶性纤维素;黏合剂,如羧甲基纤维素、甲基纤维素和聚乙烯吡咯烷酮;崩解剂,如藻酸钠、碳酸氢钠和月桂基硫酸钠等。
丸剂、药粉和颗粒剂可以用上述赋形剂按照标准方法制备。溶液或混悬液可以用甘油三辛酸酯和甘油三乙酸酯等甘油酯或乙醇等醇类按照标准方法制备。胶囊可以通过将颗粒、药粉或溶液装填在明胶等内进行制备。
皮下、肌内或静脉内制剂可以用含水或不含水溶液制成注射剂。含水溶液例如可以包括等渗的氯化钠溶液。不含水溶液例如可以包括丙二醇、聚乙二醇、橄榄油、油酸乙酯等,可选地可以加入抗菌剂和稳定剂。注射剂的灭菌方法可以是通过细菌滤器过滤或结合使用消毒剂。
经皮给药可以是软膏或霜剂的剂型,软膏可以用蓖麻油和橄榄油等脂肪油或凡士林按照标准方法制备,霜剂可以用脂肪油或二甘醇和脂肪酸的脱水山梨醇酯等乳化剂进行制备。
直肠内给药可以使用标准的栓剂,用明胶软胶囊等制得。
本发明的环胺衍生物、其药学上可接受的酸加成盐或其药学上可接受的C1-C6烷基加成盐的给药剂量因疾病类型、给药途径、患者年龄与性别、和疾病的严重性而异,不过1-500mg/天对普通成人来说是合适的。
(3)贯穿发明1和发明2的共同物质
上式(I)中环胺化合物的优选具体实例包括具有下表1.1-1.201所示取代基的化合物。
表1.1-1.201中,“手性”指的是环胺上不对称碳原子的构型。“R”表示不对称碳原子具有R构型,“S”表示不对称碳原子具有S构型,“-”指的是外消旋物或含氮环上不具有不对称碳原子的化合物。
表1.1
化合
6
1 2 0 S H
表1.2
化合
12
1 2 0 S H
13
1 2 0 S H
14
1 2 0 S H
18
1 2 0 S H
22
1 2 0 S H
表1.3
化合
26
1 2 0 S H
27
1 2 0 S H
28
1 2 0 S H
29
1 2 0 R H
30
1 2 0 R H
31
1 2 0 R H
表1.4
化合
物号
k m n 手性 R3
39
1 2 0 R H
41
1 2 0 R H
43
1 2 0 R H
44
1 2 0 R H
表1.5
化合
47
1 2 0 R H
48
1 2 0 R H
51
1 2 0 R H
55
1 2 0 R H
表1.6
化合
61
1 2 0 R H
65
1 2 0 R H
表1.7
化合
68
1 2 0 R H
71
1 2 0 R H
74
1 2 0 R H
75
1 2 0 R H
表1.8
化合
80
1 2 0 R H
81
1 2 0 R H
85
1 2 0 - H
86
1 2 0 - H
表1.9
化合
91
1 2 0 S H
96
1 2 0 S H
表1.10
化合
物号
k m n 手性 R3
102
1 2 0 S H
108
1 2 0 S H
表1.11
化合
112
1 2 0 R H
113
1 2 0 R H
116
1 2 0 R H
118
1 2 0 R H
120
1 2 0 R H
121
1 2 0 R H
表1.12
化合
126
1 2 0 R H
表1.13
化合
134
1 2 0 R H
141
1 2 0 R H
143
1 2 0 R H
表1.14
化合
144
1 2 0 R H
145
1 2 0 R H
148
1 2 0 R H
150
1 2 0 R H
151
1 2 0 R H
153
1 2 0 R H
表1.15
化合
158
1 2 0 R H
164
1 2 0 R H
表1.16
化合
166
1 2 0 R H
169
1 2 0 R H
174
1 2 0 R H
175
1 2 0 R H
表1.17
化合
177
1 2 0 R H
183
1 2 0 R H
表1.18
化合
189
1 2 0 R H
192
1 2 0 R H
196
1 2 0 R H
197
1 2 0 R H
表1.19
化合
物号
k m n 手性 R3
201
1 2 0 R H
203
1 2 0 R H
205
1 2 0 R H
表1.20
化合
215
1 2 0 - H
220
1 2 0 - H
表1.21
化合
229
1 2 0 H
231
1 2 0 - H
表1.22
化合
235
1 2 0 - H
236
1 2 0 - H
242
1 2 0 S H
表1.23
化合
247
1 2 0 S H
251
1 2 0 S H
表1.24
化合
254
1 2 0 S H
261
1 2 0 S H
表1.25
化合
265
1 2 0 S H
268
1 2 0 S H
273
1 2 0 S H
表1.26
化合
279
1 2 0 S H
表1.27
化合
288
1 2 0 R H
290
1 2 0 R H
295
1 2 0 R H
297
1 2 0 R H
表1.28
化合
298
1 2 0 R H
299
1 2 0 R H
303
1 2 0 R H
304
1 2 0 R H
308
1 2 0 R H
表1.29
化合
309
1 2 0 R H
311
1 2 0 R H
312
1 2 0 R H
表1.30
化合
320
1 2 0 R H
表1.31
化合
332
0 3 1 - H
337
0 3 1 - H
表132
化合
342
0 3 1 - H
346
0 3 1 - H
350
0 3 1 - H
表1.33
化合
物号
k m n 手性 R3
353
1 2 1 - H
355
1 3 0 - H
356
1 3 0 - H
表1.34
化合
物号
k m n 手性 R3
364
1 3 0 - H
365
1 3 0 - H
368
1 3 0 - H
371
1 3 0 - H
表1.35
化合
378
1 3 0 - H
379
1 3 0 - H
383
1 3 0 - H
表1.36
化合
390
2 2 0 - H
396
2 2 0 - H
表1.37
化合
物号
k m n 手性 R3
398
2 2 0 - H
402
2 2 0 - H
405
2 2 0 - H
表1.38
化合
412
2 2 0 - H
415
2 2 0 - H
416
2 2 0 - H
表1.39
化合
426
2 2 0 - H
429
2 2 0 - H
表1.40
化合
434
1 3 1 - H
435
1 3 1 - H
438
1 3 1 - H
表1.41
化合
物号
k m n 手性 R3
446
1 3 1 - H
450
1 3 1 - H
表1.42
化合
物号
k m n 手性 R3
459
2 2 1 - H
461
2 2 1 - H
表1.43
化合
465
2 2 1 - H
468
2 2 1 - H
473
2 2 1 - H
表1.44
化合
476
2 2 1 - H
484
2 2 1 - H
表1.45
化合
488
2 2 1 - H
490
2 2 1 - H
492
2 2 1 - H
表1.46
化合
497
2 2 1 - H
501
2 2 1 - H
502
2 2 1 - H
504
2 2 1 - H
表1.47
化合
物号
k m n 手性 R3
507
2 2 1 - H
511
2 2 1 - H
514
2 2 1 - H
表1.48
化合
520
2 2 1 - -CH3
522
2 2 1 -
525
2 2 1 - H
527
2 2 1 - H
表1.49
化合
532
2 2 1 - H
536
2 2 1 - H
537
2 2 1 - H
表1.50
化合
540
2 2 1 - H
544
2 2 1 - H
表1.51
化合
物号
k m n 手性 R3
559
2 2 1 - H
561
2 2 1 - H
表1.52
化合
565
2 2 1 - H
566
2 2 1 - H
572
2 2 1 - H
表1.53
化合
物号
k m n 手性 R3
573
2 2 1 - H
577
2 2 1 - H
579
2 2 1 - H
表1.54
化合
587
2 2 1 - H
593
2 2 1 - H
表1.55
化合
596
2 2 1 - H
597
2 2 1 - H
598
2 2 1 - H
599
2 2 1 - H
602
2 2 1 - H
表1.56
化合
物号
k m n 手性 R3
606
2 2 1 - H
607
2 2 1 - H
609
2 2 1 - H
614
2 2 1 - H
615
2 2 1 - H
表1.57
化合
623
2 2 1 - H
625
2 2 1 - H
626
2 2 1 - H
627
2 2 1 - H
表1.58
化合
表1.59
化合
642
2 2 1 - H
646
2 2 1 - H
648
2 2 1 - H
表1.60
化合
物号
k m n 手性 R3
653
2 2 1 - H
654
2 2 1 - H
656
2 2 1 - H
660
2 2 1 - H
表1.61
化合
664
2 2 1 - H
667
2 2 1 - H
669
2 2 1 - H
表1.62
化合
672
2 2 1 - H
表1.63
化合
物号
k m n 手性 R3
683
2 2 1 - H
685
2 2 1 - H
693
2 2 1 - H
表1.64
化合
698
2 2 1 - H
700
2 2 1 - H
702
2 2 1 - H
表1.65
化合
712
2 2 1 - H
715
2 2 1 - H
表1.66
化合
717
2 2 1 - H
722
2 2 1 - H
726
2 2 1 - H
表1.67
化合
物号
k m n 手性 R3
732
2 2 1 - H
735
2 2 1 - H
表1.68
化合
物号
k m n 手性 R3
738
2 2 1 - H
741
2 2 1 - H
743
2 2 1 - H
745
2 2 1 - H
表1.69
化合
751
2 2 1 - H
752
2 2 1 - H
755
2 2 1 - H
表1.70
化合
物号
k m n 手性 R3
762
2 2 1 - H
764
2 2 1 - H
表1.71
化合
773
2 2 1 - H
775
2 2 1 - H
表1.72
化合
789
2 2 1 - H
表1.73
化合
表1.74
化合
物号
k m n 手性 R3
804
2 2 1 - H
811
2 2 1 - H
812
2 2 1 - H
814
2 2 1 - H
表1.75
化合
物号
k m n 手性 R3
818
2 2 1 - H
821
2 2 1 - H
823
2 2 1 - H
表1.76
化合
物号
k m n 手性 R3
828
2 2 1 - H
830
2 2 1 - H
832
2 2 1 - H
835
2 2 1 - H
表1.77
化合
物号
k m n 手性 R3
842
2 2 1 - H
847
2 2 1 - H
表1.78
化合
物号
k m n 手性 R3
854
2 2 1 - H
表1.79
化合
物号
k m n 手性 R3
869
2 2 1 - H
表1.80
化合
物号
k m n 手性 R3
880
2 2 1 - H
表1.81
化合
885
2 2 1 - H
888
2 2 1 - H
表1.82
化合
893
2 2 1 - H
898
2 2 1 - H
899
2 2 1 - H
表1.83
化合
903
2 2 1 - H
表1.84
化合
914
2 2 1 - H
920
2 2 1 - H
924
2 2 1 - H
表1.85
化合
926
2 2 1 - H
934
2 2 1 - H
表1.86
化合
937
2 2 1 - H
938
2 2 1 - H
945
1 4 0 - H
946
1 4 0 - H
表1.87
化合
948
1 4 0 - H
954
1 2 0 R H
表1.88
化合
968
1 2 0 R H
表1.89
化合
975
1 2 0 R H
977
1 2 0 R H
979
1 2 0 R H
表1.90
化合
984
1 2 0 R H
985
1 2 0 R H
988
1 4 0 - H
990
1 4 0 - H
表1.91
化合
物号
k m n 手性 R3
997
2 2 1 - H
表1.92
化合
物号
k m n 手性 R3
1002
2 2 1 - H
1008
2 2 1 - H
1010
2 2 1 - H
1011
2 2 1 - H
表1.93
化合
物号
k m n 手性 R3
1021
2 2 1 - H
表1.94
化合
1026
2 2 1 - H
1032
2 2 1 - H
1034
2 2 1 - H
表1.95
化合
1037
2 2 1 - H
1041
2 2 1 - H
表1.96
化合
1047
2 2 1 - H
1048
2 2 1 - H
1050
2 2 1 - H
1053
2 2 1 - H
表1.97
化合
物号
k m n 手性 R3
1064
2 2 1 - H
1065
2 2 1 - H
1066
2 2 1 - H
表1.98
化合
1068
2 2 1 - H
1071
2 2 1 - H
1072
2 2 1 - H
1074
2 2 1 - H
1077
2 2 1 - H
表1.99
化合
1087
1 2 0 R H
表100
化合
物号
k m n 手性 R3
1092
1 2 0 R H
1096
1 2 0 R H
1098
1 2 0 R H
表1.101
化合
1107
1 2 0 R H
1108
1 2 0 R H
1110
1 2 0 R H
表1.102
化合
1113
2 2 1 - H
1115
2 2 1 - H
1118
1 2 0 R H
表1.103
化合
1124
1 2 0 R H
1129
2 2 1 - H
表1.104
化合
1135
1 2 0 R H
1140
1 2 0 R H
表1.105
化合
1145
1 2 0 R H
1149
1 2 0 R H
表1.106
化合
1157
1 2 0 R H
1158
1 2 0 R H
1163
1 2 0 R H
表1.107
化合
1168
1 2 0 R H
1169
1 2 0 R H
1176
1 2 0 R H
表1.108
化合
1179
1 2 0 R H
1182
1 2 0 R H
1183
1 2 0 R H
表1.109
化合
1189
2 2 1 - H
表1.110
化合
1201
1 2 0 R H
1203
1 2 0 R H
1204
1 2 0 R H
1205
1 2 0 R H
1207
1 2 0 R H
1209
1 2 0 R H
1210
1 2 0 R H
表1.111
化合
1211
1 2 0 R H
1215
2 2 1 - H
1218
1 2 0 R H
1219
1 2 0 R H
1220
1 2 0 R H
表1.112
化合
1223
1 2 0 R H
1224
1 2 0 R H
1225
1 2 0 R H
1226
1 2 0 R H
1231
1 2 0 R H
表1.113
化合
物号
k m n 手性 R3
1235
1 2 0 R H
1238
1 2 0 R H
表1.114
化合
1244
2 2 1 - H
1247
2 2 1 - H
1252
1 2 0 R H
1253
1 2 0 R H
表1.115
化合
物号
k m n 手性 R3
1258
1 2 0 R H
1260
1 2 0 R H
表1.116
化合
物号
k m n 手性 R3
1266
1 2 0 R H
1267
1 2 0 R H
1268
1 2 0 R H
1269
1 2 0 R H
1271
1 2 0 R H
1274
1 2 0 R H
表1.117
化合
物号
k m n 手性 R3
1282
2 2 1 - H
1287
1 2 0 R H
表1.118
化合
1294
1 2 0 R H
表1.119
化合
物号
k m n 手性 R3
1302
1 2 0 R H
表1.120
化合
1315
1 2 0 R H
1320
1 2 0 R H
表1.121
化合
1326
1 2 0 R H
表1.122
化合
物号
k m n 手性 R3
1333
1 2 0 R H
1335
1 2 0 R H
1339
1 2 0 R H
1340
1 2 0 R H
1342
2 2 1 - H
表1.123
化合
1343
2 2 1 - H
1346
2 2 1 - H
表1.124
化合
1360
1 2 0 R H
表1.125
化合
1370
1 2 0 R H
1373
1 2 0 R H
表1.126
化合
1376
1 2 0 R H
1377
1 2 0 R H
1386
2 2 1 - H
表1.127
化合
物号
k m n 手性 R3
1388
1 2 0 R H
1392
1 2 0 R H
表1.128
化合
1398
1 2 0 R H
1399
1 2 0 R H
1401
1 2 0 R H
1404
1 2 0 R H
1406
1 2 0 R H
1407
1 2 0 R H
表1.129
化合
1411
1 2 0 R H
1413
1 2 0 R H
1415
1 2 0 R H
1417
1 2 0 R H
1418
2 2 1 - H
1419
1 2 0 R H
表1.130
化合
1424
1 2 0 R H
1425
1 2 0 R H
1428
2 2 1 - H
1430
2 2 1 - H
表1.131
化合
1433
2 2 1 - H
1435
2 2 1 - H
1439
2 2 1 - H
1440
2 2 1 - H
1441
2 2 1 - H
表1.132
化合
1445
2 2 1 - H
1449
2 2 1 - H
1450
2 2 1 - H
表1.133
化合
1454
2 2 1 - H
1460
2 2 1 - H
1462
2 2 1 - H
表1.134
化合
物号
k m n 手性 R3
1465
2 1 1 - H
1470
2 1 1 - H
1471
2 1 1 - H
1473
1 2 0 R H
表1.135
化合
1480
1 2 0 R H
1482
1 2 0 R H
表1.136
化合
物号
k m n 手性 R3
1490
1 2 0 R H
1492
1 2 0 R H
1493
1 2 0 R H
1496
1 2 0 R H
表1.137
化合
1503
1 2 0 R H
1506
2 1 1 - H
表1.138
化合
1509
2 1 1 - H
1513
2 1 1 - H
1515
2 2 1 - H
1516
2 2 1 - H
1517
2 2 1 - H
1518
2 2 1 - H
表1.139
化合
1519
2 2 1 - H
1521
1 2 0 R H
1523
1 2 0 R H
表1.140
化合
1535
1 2 0 R H
表1.141
化合
表1.142
化合
1555
1 2 0 R H
1557
1 2 0 R H
1559
1 2 0 R H
1560
1 2 0 R H
1561
1 2 0 R H
表1.143
化合
1564
1 2 0 R H
1567
1 2 0 R H
表1.144
化合
1576
2 2 1 - H
1581
2 2 1 - H
表1.145
化合
物号
k m n 手性 R3
1591
1 2 0 R H
1593
1 2 0 R H
表1.146
化合
1596
1 2 0 R H
1598
1 2 0 R H
1600
2 2 1 - H
1605
2 2 1 - H
表1.147
化合
1609
2 2 1 - H
1611
2 2 1 - H
1615
2 2 1 - H
1616
2 2 1 - H
表1.148
化合
1618
1 2 0 R H
1625
1 2 0 R H
表1.149
化合
1635
1 2 0 R H
1636
1 2 0 R H
1638
1 2 0 R H
1639
1 2 0 R H
表1.150
化合
物号
k m n 手性 R3
1640
1 2 0 R H
1645
1 2 0 R H
1648
1 2 0 R H
表1.151
化合
1651
2 2 1 - H
1652
2 2 1 - H
1653
2 2 1 - H
1657
2 2 1 - H
1658
2 2 1 - H
表1.152
化合
1664
2 2 1 - H
1666
2 2 1 - H
1669
2 2 1 - H
1670
2 2 1 - H
表1.153
化合
1677
2 2 1 - H
1683
2 2 1 - H
表1.154
化合
1685
2 2 1 - H
1687
2 2 1 - H
1693
1 2 0 R H
表1.155
化合
1701
1 2 0 R H
1705
1 2 0 R H
表1.156
化合
1707
1 2 0 R H
1708
1 2 0 R H
1709
1 2 0 R H
表1.157
化合
1723
1 2 0 R H
表1.158
化合
1728
1 2 0 R H
1734
1 2 0 R H
1735
1 2 0 R H
表1.159
化合
1739
1 2 0 R H
1741
1 2 0 R H
1745
1 2 0 R H
1749
1 2 0 R H
表1.160
化合
1750
1 2 0 R H
1757
1 2 0 R H
1758
1 2 0 R H
表1.161
化合
1762
1 2 0 R H
1767
1 3 1 - H
1768
1 3 1 - H
表1.162
化合
1774
1 2 0 R H
1777
2 2 1 - H
1778
2 2 1 - H
表1.163
化合
1783
2 2 1 - H
1788
2 2 1 - H
表1.164
化合
1797
2 2 1 - H
1798
2 2 1 - H
1800
2 2 1 - H
表1.165
化合
1805
1 2 0 R H
1807
1 2 0 R H
1812
1 2 0 R H
1813
1 2 0 R H
1814
1 2 0 R H
1815
1 2 0 R H
表1.166
化合
物号
k m n 手性 R3
1817
1 2 0 R H
1822
1 2 0 R H
1823
1 2 0 R H
1824
1 2 0 R H
1826
1 2 0 R H
表1.167
化合
1835
1 2 0 R H
1836
1 2 0 R H
1837
1 2 0 R H
表1.168
化合
1845
1 2 0 R H
表1.169
化合
1850
1 2 0 R H
1851
1 2 0 R H
1858
1 2 0 R H
表1.170
化合
1864
1 2 0 R H
1868
1 2 0 R H
表1.171
化合
1875
1 2 0 R H
表1.172
化合
表1.173
化合
1893
1 2 0 R H
1896
1 2 0 R H
1900
1 2 0 R H
1902
1 2 0 R H
表1.174
化合
物号
k m n 手性 R3
1906
1 2 0 R H
1907
1 2 0 R H
1909
1 2 0 R H
1910
2 2 1 - H
1913
2 2 1 - H
表1.175
化合
物号
k m n 手性 R3
1918
2 2 1 - H
1920
2 2 1 - H
1925
2 2 1 - H
表1.176
化合
1929
2 2 1 - H
1933
2 2 1 - H
表1.177
化合
1943
2 2 1 - H
1944
2 2 1 - H
1945
2 2 1 - H
表1.178
化合
1952
2 2 1 - H
1953
2 2 1 - H
1955
2 2 1 - H
1958
2 2 1 - H
表1.179
化合
物号
k m n 手性 R3
1959
2 2 1 - H
1968
2 2 1 - H
表1.180
化合
物号
k m n 手性 R3
1971
2 2 1 - H
1976
2 2 1 - H
1978
2 2 1 - H
表1.181
化合
1985
2 2 1 - H
1989
2 2 1 - H
1990
2 2 1 - H
表1.182
化合
表1.183
化合
2004
2 2 1 - H
2005
2 2 1 - H
2007
2 2 1 - H
2010
2 2 1 - H
表1.184
化合
物号
k m n 手性 R3
2019
2 2 1 - H
2023
2 2 1 - H
表1.185
化合
2025
2 2 1 - H
2028
2 2 1 - H
2031
2 2 1 - H
2032
2 2 1 - H
2035
2 2 1 - H
表1.186
化合
2045
1 2 0 R H
表1.187
化合
2047
1 2 0 R H
2056
2 2 1 - H
2057
2 2 1 - H
表1.188
化合
2059
2 2 1 - H
2067
2 2 1 - H
表1.189
化合
2071
2 2 1 - H
2076
2 2 1 - H
表1.190
化合
2080
2 2 1 - H
2081
2 2 1 - H
2084
1 2 0 R H
2086
1 2 0 R H
表1.191
化合
2093
2 2 1 - H
2094
2 2 1 - H
表1.192
化合
2105
2 2 1 - H
2112
2 2 1 - H
表1.193
化合
2113
2 2 1 - H
2118
1 2 0 R H
表1.194
化合
2129
1 2 0 R H
表1.195
化合
2141
2 2 1 - H
表1.196
化合
物号
k m n 手性 R3
2146
2 2 1 - H
2147
2 2 1 - H
2150
1 2 0 R H
2152
1 2 0 R H
2156
2 2 1 - H
表1.197
化合
物号
k m n 手性 R3
2157
1 2 0 R H
2164
1 2 0 R H
表1.198
化合
2175
1 2 0 R H
2176
1 2 0 R H
表1.199
化合
物号
k m n 手性 R3
2181
1 2 0 R H
2183
1 2 0 R H
2186
2 2 1 - H
2187
1 2 0 R H
2189
1 2 0 R H
表1.200
化合
2190
2 2 1 - H
表1.201
化合
2201
2 2 1 - H
2208
2 2 1 - H
本发明也可以使用该环胺化合物的酸加成盐,其中的酸例如包括无机酸,如盐酸、氢溴酸、硫酸、磷酸、碳酸等,以及有机酸,如马来酸、柠檬酸、苹果酸、酒石酸、富马酸、甲磺酸、三氟乙酸、甲酸等。
而且,本发明也可以使用该环胺化合物的C1-C6烷基加成盐,例如1-(4-氯苄基)-1-甲基-4-[{N-(3-三氟甲基苯甲酰基)甘氨酰}氨基甲基]哌啶鎓碘化物,其中的烷基例如包括甲基、乙基、正丙基、正丁基、正戊基、正己基、正庚基、正辛基、异丙基、异丁基、仲丁基、叔丁基、异戊基、新戊基、叔戊基、2-甲基戊基、1-乙基丁基等,适当地具体包括甲基和乙基。作为铵阳离子的反离子的优选具体实例,可以列举卤阴离子,例如氟、氯、溴或碘。
本发明可以使用由上式(I)代表的化合物的外消旋物和所有可能的旋光活性形式。
由上述通式(I)代表的化合物可以按照下面给出的任意一般制备方法合成。
制备方法1
该方法要求,将一当量由下式(II)代表的化合物:
(其中R1、R2、R3、j、k、m和n是分别如上式(I)所定义的)用0.1-10当量由下式(III)代表的羧酸或其反应性衍生物进行处理:
(其中R4、R5、R6、G、p和q是分别如上式(I)所定义的),反应在有或没有溶剂的存在下进行。
上式(III)中羧酸的反应性衍生物包括常用于有机化学合成的高度反应性羧酸衍生物,例如酰卤、酸酐、混合酸酐。
使用适量下列试剂可以使该反应进行得更顺利:脱水剂、如分子筛,偶联剂、如二环己基碳二亚胺(DCC)、N-乙基-N’-(3-二甲氨基丙基)碳二亚胺(EDCI或WSC)、羰基二咪唑(CDI)、N-羟基琥珀酰亚胺(HOSu)、N-羟基苯并三唑(HOBt)、苯并三唑-1-基氧基三(吡咯烷基)六氟磷酸鏻(PyBOP_)、2-(1H-苯并三唑-1-基)-1,1,3,3-四甲基六氟磷酸脲(HBTU)、2-(1H-苯并三唑-1-基)-1,1,3,3-四甲基四氟硼酸脲(TBTU)、2-(5-降冰片烯-2,3-二羧基亚氨基)-1,1,3,3-四甲基四氟硼酸脲(TNTU)、O-(N-琥珀酰亚胺基)-1,1,3,3-四甲基四氟硼酸脲(TSTU)、溴三(吡咯烷基)六氟磷酸鏻(PyBroP_)等,或基质,包括无机盐、如碳酸钾、碳酸钠、碳酸氢钠等,胺、如三乙胺、二异丙基乙胺和吡啶等,或聚合物载体基质、如(哌啶子基甲基)聚苯乙烯、(吗啉代甲基)聚苯乙烯、(二乙氨基甲基)聚苯乙烯、聚(4-乙烯基吡啶)等。
制备方法2
该方法要求,将1当量由下式(IV)给出的烷基化剂:
(其中R1、R2和j是分别如上式(I)所定义的;X代表卤原子、烷磺酰氧基或芳磺酰氧基)用0.1-10当量由下式(V)代表的化合物进行处理:
(其中R3、R4、R5、R6、G、k、m、n、p和q是分别如上式(I)所定义的),反应在有或没有溶剂的存在下进行。
如果存在类似于上述制备方法1所用的基质,该反应能进行得更顺利。另外,这些制备方法中的反应也可以用碘化物促进其进行,例如碘化钾、碘化钠等。
上式(IV)中,X代表卤原子、烷磺酰氧基、芳磺酰氧基。该卤原子优选包括氯、溴和碘原子。烷磺酰氧基的适当具体实例包括甲磺酰氧基、三氟甲基磺酰氧基等。芳磺酰氧基的优选具体实例包括甲苯磺酰氧基。
制备方法3
该方法要求,将1当量由下式(VI)代表的醛:
(其中R1和R2是分别如上式(I)所定义的;j代表1或2)或由下式(VII)代表的醛:
R1-CHO (VII)
(其中R1是如上式(I)所定义的;j代表0)用0.1-10当量由式(V)代表的化合物进行处理,反应在还原性条件下,在有或没有溶剂的存在下进行。
该反应一般被称为还原性胺化反应,该还原性条件可以由催化氢化提供,其中使用含有一种金属的催化剂、例如钯、铂、镍、铑等,使用复合氢化物、例如氢化锂铝、硼氢化钠、氰基硼氢化钠、三乙酰氧基硼氢化钠等,硼烷,或由电解还原提供。
制备方法4
该方法要求,将一当量由下式(VIII)代表的化合物:
(其中R1、R2、R3、R4、R5、R7、j、k、m、n、p和q是分别如上式(I)所定义的)用0.1-10当量由下式(IX)代表的羧酸或磺酸或其反应性衍生物进行处理:
HO-A-R6 (IX)
(其中R6是如上式(I)所定义的;“A”代表羰基或磺酰基),反应在有或没有溶剂的存在下进行。
上式(IX)中羧酸或磺酸的反应性衍生物包括常用于有机化学合成的高度反应性羧酸或磺酸衍生物,例如酰卤、酸酐、混合酸酐。
使用适量类似于制备方法1所用的脱水剂、偶联剂或基质可以使该反应进行得更顺利。
制备方法5
该方法要求,将1当量由上式(VIII)代表的化合物用0.1-10当量由下式(X)代表的异氰酸酯或异硫氰酸酯进行处理:
Z=C=N-R6 (X)
(其中R6是如上式(I)所定义的;Z代表氧原子或硫原子),反应在有或没有溶剂的存在下进行。
制备方法6
该方法要求,将1当量由下式(XI)代表的化合物:
(其中R1、R2、R3、R4、R5、j、k、m、n、p和q是分别如上式(I)所定义的;“A”代表羰基或磺酰基)用0.1-10当量由下式(XII)代表的胺进行处理:
R6-NH2 (IX)
(其中R6是如上式(I)所定义的),反应在有或没有溶剂的存在下进行。
使用适量类似于制备方法1所用的脱水剂、偶联剂或基质可以使该反应进行得更顺利。
在一般有机化学合成中,如果用于参加上述各制备方法的反应物含有在各反应条件下反应的取代基或者被认为对反应产生不利影响,该官能团可以用已知的适当保护基团保护起来,然后进行上述制备反应,再用已知操作进行去保护,得到所需化合物。
而且,本发明化合物可以通过进一步转化由上述制备方法1-6制得的化合物的取代基进行制备,转化使用已知的常用于有机化学合成的反应,例如烷基化、酰化、还原等。
上述各制备方法都可以使用反应溶剂,例如卤代烃,如二氯甲烷、氯仿等;芳族烃,如苯、甲苯等;醚,如二乙醚、四氢呋喃等;酯,如乙酸乙酯;极性非质子传递溶剂,如二甲基甲酰胺、二甲基亚砜、乙腈等;醇,如甲醇、乙醇、异丙醇等。
各制备方法中的反应温度应当在-78℃至+150℃范围内,优选为0℃-100℃。反应完成后,可以进行常用的分离和纯化操作,例如浓缩、过滤、萃取、固相萃取、重结晶、色谱等,以分离所需的由上式(I)代表的环胺化合物。利用常用方法可将它们转化为药学上可接受的酸加成盐或C1-C6烷基加成盐。
潜在的工业实用性
含有抑制MIP-1α和/或MCP-1等化学因子对靶细胞作用的本发明环胺化合物、其药学上可接受的酸加成盐或其药学上可接受的C1-C6烷基加成盐的化学因子受体拮抗剂,可用作下列疾病的治疗剂和/或预防剂:动脉粥样硬化、类风湿性关节炎、牛皮癣、气喘、溃疡性结肠炎、肾炎(肾病)、多发性硬化、肺纤维症、心肌炎、肝炎、胰腺炎、肉样瘤病、克罗恩氏病、子宫内膜异位、充血性心力衰竭、病毒性脑膜炎、脑梗塞、神经病、川崎病、败血症等,这些疾病中血液单核细胞、淋巴细胞等的组织浸润作用在疾病的发生、发展和维持中起主要作用。
实施例
现在用下列实施例对本发明进行具体描述。不过,本发明不限于这些实施例中描述的这些化合物。这些实施例中的化合物编号与列在表1.1-1.201中的适当优选化合物实例编号是一致的。
参考例1:二盐酸3-氨基-1-(4-氯苄基)吡咯烷
向3-[(叔丁氧羰基)氨基]吡咯烷(4.81g,25.8mmol)的DMF(50ml)溶液中加入4-氯苄基氯(4.15g,25.8mmol)和iPr2NEt(6.67g,51.6mmol)。反应混合物在70℃下搅拌15小时,在减压下除去溶剂。重结晶(CH3CN,50ml)得到所需物质3-(叔丁氧羰基)氨基-1-(4-氯苄基)吡咯烷,为淡黄色固体(6.43g,80.2%):1H NMR(CDCl3,300Mhz)δ1.37(s,9H),1.5-1.7(br,1H),2.1-2.4(m,2H),2.5-2.7(m,2H),2.83(br,1H),3.57(s,2H),4.1-4.3(br,1H),4.9-5.1(br,1H),7.15-7.35(br,4H);纯度用RPLC/MS测定(98%);ESI/MS m/e 311.0(M++H,C16H24ClN2O2).
将3-(叔丁氧羰基)氨基-1-(4-氯苄基)吡咯烷(6.38g,20.5mmol)的CH3OH(80ml)溶液用1N HCl-Et2O(100ml)处理,在25℃下搅拌15小时。在减压下除去溶剂,得到一固体,用重结晶法(1∶2 CH3OH-CH3CN,150ml)纯化,得到二盐酸3-氨基-1-(4-氯苄基)吡咯烷,为白色粉末(4.939g,84.9%):1H NMR(d6-DMSO,300Mhz)δ3.15(br,1H),3.3-3.75(br-m,4H),3.9(br,1H),4.05(br,1H),4.44(br,1H),4.54(br,1H),7.5-7.7(m,4H),8.45(br,1H),8.60(br,1H);纯度用RPLC/MS测定(99%);ESI/MS m/e 211.0(M++H,C11H16ClN2).
依据上述方法,分别使用相应的试剂,也制备了旋光活性的二盐酸(R)-3-氨基-1-(4-氯苄基)吡咯烷和二盐酸(S)-3-氨基-1-(4-氯苄基)吡咯烷。产物与外消旋物显示相同的1H NMR。
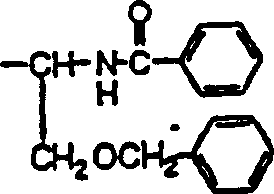
实施例1:3-(N-苯甲酰基甘氨酰)氨基-1-(4-氯苄基)吡咯烷(1号化合物)的制备
向二盐酸3-氨基-1-(4-氯苄基)吡咯烷(14.2mg,0.050mmol)与Et3N(15.2mg)的CHCl3(2.5ml)溶液中加入N-苯甲酰基甘氨酸(9.9mg,0.055mmol)、盐酸3-乙基-1-(3-二甲氨基丙基)碳二亚胺(EDCI)(10.5mg)和1-羟基苯并三唑水合物(HOBt)(7.4mg)。反应混合物在25℃下搅拌16小时,用2N NaOH水溶液(2ml×2)和盐水(1ml)洗涤。通过PTFE滤膜过滤后,在减压下除去溶剂,得到3-(N-苯甲酰基甘氨酰)氨基-1-(4-氯苄基)吡咯烷(1号化合物),为淡黄色油(17.7mg,95%)。纯度用RPLC/MS测定(95%);ESI/MSm/e 372.0(M++H,C20H22ClN3O2)
实施例2-32
依据实施例1的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表2中。
表2
| |
化合物编号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例2 |
2 |
C21 H24 Cl N3 O2 |
386 |
16.4 |
85 |
|
实施例3 |
3 |
C19 H21 Cl N4 O2 |
373 |
18.7 |
100 |
|
实施例4 |
4 |
C21 H21 Cl F3 N3 O2 |
440 |
57.2 |
69 |
|
实施例5 |
82 |
C22 H23 Cl F3 N3 O2 |
454 |
5.6 |
11 |
|
实施例6 |
85 |
C21 H24 Cl N3 O2 |
386 |
22.6 |
59 |
|
实施例7 |
86 |
C21 H23 Cl N4 O4 |
431 |
21.2 |
98 |
|
实施例8 |
214 |
C22 H25 Cl N2 O2 |
385 |
23.9 |
62 |
|
实施例9 |
215 |
C23 H27 Cl N2 O3 |
415 |
17.4 |
84 |
|
实施例10 |
216 |
C20 H23 Cl N2 O2 S |
391 |
21.6 |
quant |
|
实施例11 |
217 |
C23 H27 Cl N2 O4 |
431 |
15.3 |
66 |
|
实施例12 |
218 |
C23 H27 Cl N2 O2 |
399 |
12.8 |
64 |
|
实施例13 |
219 |
C22 H24 Cl F N2 O3 |
419 |
18.1 |
86 |
|
实施例14 |
220 |
C22 H25 Cl N2 O2 |
385 |
16.4 |
85 |
|
实施例15 |
221 |
C21 H23 Cl N2 O2 |
371 |
14.9 |
80 |
|
实施例16 |
222 |
C21 H22 Cl2 N2 O2 |
405 |
13.3 |
65 |
|
实施例17 |
223 |
C25 H31 Cl N2 O3 |
443 |
18.4* |
63 |
|
实施例18 |
224 |
C20 H23 Cl N2 O3 S |
407 |
11.2 |
28 |
|
实施例19 |
225 |
C22 H26 Cl N3 O2 |
400 |
22.7 |
quant |
|
实施例20 |
226 |
C23 H28 Cl N3 O3 |
430 |
21.0 |
98 |
|
实施例21 |
227 |
C22 H25 Cl2 N3 O2 |
434 |
21.9 |
100 |
|
实施例22 |
228 |
C23 H28 Cl N3 O3 |
430 |
20.8 |
97 |
|
实施例23 |
229 |
C25 H32 Cl N3 O2 |
462 |
25.4 |
quant |
|
实施例24 |
230 |
C26 H31 Cl F N3 O2 |
472 |
26.0 |
quant |
|
实施例25 |
231 |
C24 H28 Cl N3 O3 |
442 |
30.3* |
quant |
|
实施例26 |
232 |
C22 H32 Cl N3 O2 |
406 |
3.9 |
19 |
|
实施例27 |
233 |
C23 H28 Cl N3 O2 |
414 |
8.5 |
41 |
|
实施例28 |
234 |
C22 H27 Cl N4 O2 |
415 |
7.3 |
35 |
|
实施例29 |
235 |
C24 H29 Cl2 N3 O2 |
462 |
9.0 |
39 |
|
实施例30 |
236 |
C25 H29 Cl N4 O3 S |
501 |
17.4 |
69 |
|
实施例31 |
237 |
C21 H24 Cl N3 O3 |
402 |
14.2 |
71 |
|
实施例32 |
238 |
C21 H23 Cl2 N3 O3 |
436 |
23.4 |
quant |
*TFA盐的产量
参考例2:(R)-3-[N-(叔丁氧羰基)甘氨酰]氨基-1-(4-氯苄基)吡咯烷的制备
摇动二盐酸(R)-3-氨基-1-(4-氯苄基)吡咯烷(4.54g,16.0mmol)、2NNaOH溶液(80ml)和乙酸乙酯(80ml)的混合物,分离有机层,含水层用乙酸乙酯萃取(80ml×2)。合并了的有机层经无水硫酸钠干燥,过滤,并蒸发,得到游离的(R)-3-氨基-1-(4-氯苄基)吡咯烷(3.35g,99%)。
将(R)-3-氨基-1-(4-氯苄基)吡咯烷(3.35g,16mmol)的CH2Cl2(80ml)溶液用Et3N(2.5ml,17.6mmol)、N-叔丁氧羰基甘氨酸(2.79g,16.0mmol)、EDCI(3.07g,16.0mmol)和HOBt(2.16g,16mmol)处理。反应混合物在25℃下搅拌16小时后,加入2N NaOH溶液(80ml)。分离有机层,含水层用二氯甲烷萃取(100ml×3)。合并了有机层用水(100ml×2)和盐水(100ml)洗涤,经无水硫酸钠干燥,过滤,并浓缩。柱色谱(SiO2,乙酸乙酯)得到所需的(R)-3-[N-(叔丁氧羰基)甘氨酰]氨基-1-(4-氯苄基)吡咯烷(5.40g,92%)。
参考例3:(R)-1-(4-氯苄基)-3-(甘氨酰氨基)吡咯烷的制备
向(R)-3-[N-(叔丁氧羰基)甘氨酰]氨基-1-(4-氯苄基)吡咯烷(5.39g,14.7mmol)的甲醇(60ml)溶液中加入4N HCl的二噁烷(38ml)溶液。溶液在室温下搅拌2小时。反应混合物浓缩,加入2N NaOH溶液(80ml)。混合物用二氯甲烷萃取(80ml×3),合并了的有机萃取液经无水硫酸钠干燥,并浓缩。柱色谱(SiO2,EtOAc/EtOH/Et3N=90/5/5)得到(R)-1-(4-氯苄基)-3-(甘氨酰氨基)吡咯烷(3.374g,86%):1H NMR(CDCl3,270Mhz)δ1.77(dd,J=1.3 and 6.9Hz,1H),2.20-3.39(m,2H),2.53(dd,J=3.3 and 9.6Hz,1H),2.62(dd,J=6.6 and9.6Hz,1H),2.78-2.87(m,1H),3.31(s,2H),3.57(s,2H),4.38-4.53(br,1H),7.18-7.32(m,4H),7.39(br.s,1H).
依据参考例2和3的方法,分别使用相应的试剂,也合成了其他3-酰氨基-1-(4-氯苄基)吡咯烷。
(S)-1-(4-氯苄基)-3-(甘氨酰氨基)吡咯烷:3.45g,79%(2步)。
(R)-3-(β-丙氨酰氨基)-1-(4-氯苄基)吡咯烷:3.79g,85%(2步)。
(S)-3-(β-丙氨酰氨基)-1-(4-氯苄基)吡咯烷:3.72g,86%(2步)。
(R)-3-[(S)-丙氨酰氨基]-1-(4-氯苄基)吡咯烷:368mg,65%(2步)。
(R)-3-[(R)-丙氨酰氨基]-1-(4-氯苄基)吡咯烷:425mg,75%(2步)。
(R)-3-[(2S)-2-氨基-3-噻吩基丙酰]氨基-1-(4-氯苄基)吡咯烷:566mg,78%(2步)。
(R)-3-[(2R)-2-氨基-3-噻吩基丙酰]氨基-1-(4-氯苄基)吡咯烷:585mg,81%(2步)。
(R)-3-(2-氨基-2-甲基丙酰]氨基-1-(4-氯苄基)吡咯烷:404mg,66%(2步)。
(R)-3-[(2S)-2-氨基-4-(甲磺酰基)丁酰]氨基-1-(4-氯苄基)吡咯烷:535mg,72%(2步)。
而且,依据参考例1、2和3的方法,分别使用相应的试剂,也合成了(R)-3-(甘氨酰氨基)-1-(4-甲基苄基)吡咯烷、(R)-1-(4-溴苄基)-3-(甘氨酰氨基)吡咯烷、(R)-1-(2,4-二甲基苄基)-3-(甘氨酰氨基)吡咯烷和(R)-1-(3,5-二甲基异噁唑-4-基甲基)-3-(甘氨酰氨基)吡咯烷。
(R)-3-(甘氨酰氨基)-1-(4-甲基苄基)吡咯烷:4.65g,以3-[(叔丁氧羰基)氨基]吡咯烷为原料的产率为62%。
(R)-1-(4-溴苄基)-3-(甘氨酰氨基)吡咯烷:2.55g,以(R)-3-氨基-1-(4-溴苄基)吡咯烷为原料的产率为68%;1H NMR(CDCl3,270MHz)δ1.37-1.78(m,3H),2.23-2.39(m,2H),2.50-2.67(m,2H),2.80-2.89(m,1H),3.32(s,2H),3.58(s,2H),4.39-4.55(m,1H),7.21(d,J=6.5Hz,2H),7.45(d,J=6.5Hz,2H).
(R)-1-(2,4-二甲基苄基)-3-(甘氨酰氨基)吡咯烷:1.56g,以3-[(叔丁氧羰基)氨基]吡咯烷为原料的产率为58%;1H NMR(CDCl3,270MHz)δ1.55-1.78(m,3H),2.30(s,3H),2.23-2.31(m,2H),2.33(s,3H),2.51-2.63(m,2H),2.78-2.87(m,1H),3.30(s,2H),3.55(s,2H),4.38-4.60(m,1H),6.95(d,J=7.6Hz,1H),6.97(s,1H),7.13(d,J=7.6Hz,1H),7.43(br-s,1H).
(R)-1-(3,5-二甲基异噁唑-4-基甲基)-3-(甘氨酰氨基)吡咯烷:3.14g,以3-[(叔丁氧羰基)氨基]吡咯烷为原料的产率为45%。
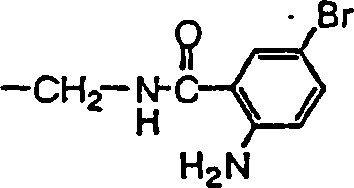
实施例33:(S)-3-[N-{3,5-双(三氟甲基)苯甲酰基}甘氨酰]氨基-1-(4-氯苄基)吡咯烷(5号化合物)的制备
向(S)-1-(4-氯苄基)-3-(甘氨酰氨基)吡咯烷(0.050mmol)与三乙胺(0.070mmol)的氯仿(1.0ml)溶液中加入3,5-双(三氟甲基)苯甲酰氯(0.060mmol)的氯仿(0.4ml)溶液。反应混合物在室温下搅拌2.5小时后,加入(氨甲基)聚苯乙烯树脂(1.04mmol/g,50mg,50mmol),混合物在室温下搅拌12小时。反应混合物过滤,树脂用二氯甲烷(0.5ml)洗涤。合并滤液和洗液,加入二氯甲烷(4ml),溶液用2N NaOH水溶液(0.5ml)洗涤,得到(S)-3-[N-{3,5-双(三氟甲基)苯甲酰基}甘氨酰]氨基-1-(4-氯苄基)吡咯烷(5号化合物)(14.4mg,57%)。纯度用RPLC/MS测定(97%);ESI/MS m/e 508.0(M++H,C22H20ClF6N3O2)
实施例34-239
依据实施例33的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表3中。
表3
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例34 |
5 |
C22H20ClF6N3O2 |
508.0 |
14.4 |
57 |
|
实施例35 |
6 |
C21H21ClF3N3O2 |
440.0 |
17.0 |
77 |
|
实施例36 |
7 |
C20H21BrClN3O2 |
450.0 |
17.7 |
79 |
|
实施例37 |
8 |
C20H21ClFN3O2 |
390.0 |
12.7 |
65 |
|
实施例38 |
9 |
C20H20Cl3N3O2 |
440.0 |
39.0 |
quant |
|
实施例39 |
10 |
C21H24ClN3O3 |
402.5 |
23.5 |
quant |
|
实施例40 |
11 |
C22H26ClN3O4 |
432.5 |
22.4 |
quant |
|
实施例41 |
12 |
C22H26ClN3O4 |
432.5 |
15.9 |
74 |
|
实施例42 |
13 |
C21H21ClF3N3O2 |
440.0 |
13.1 |
60 |
|
实施例43 |
14 |
C21H24ClN3O2 |
386.0 |
16.4 |
85 |
|
实施例44 |
15 |
C20H21Cl2N3O2 |
406.0 |
15.7 |
77 |
|
实施例45 |
16 |
C21H24ClN3O2 |
402.0 |
28.2 |
quant |
|
实施例46 |
17 |
C20H20Cl3N3O2 |
442.0 |
35.6 |
quant |
|
实施例47 |
18 |
C21H21ClN4O2 |
397.5 |
22.8 |
quant |
|
实施例48 |
19 |
C21H22ClN3O4 |
416.0 |
16.3 |
78 |
|
实施例49 |
20 |
C21H20ClF4N3O2 |
458.0 |
24.9 |
quant |
|
实施例50 |
21 |
C21H20ClF4N3O2 |
458.0 |
17.9 |
78 |
|
实施例51 |
22 |
C21H20ClF4N3O2 |
458.0 |
9.4 |
41 |
|
实施例52 |
23 |
C21H20ClF4N3O2 |
458.0 |
15.4 |
67 |
|
实施例53 |
24 |
C21H21ClF3N3O3 |
456.0 |
20.7 |
91 |
|
实施例54 |
25 |
C21H20ClF4N3O2 |
458.0 |
18.5 |
81 |
|
实施例55 |
26 |
C20H21ClN4O4 |
417.0 |
21.9 |
quant |
|
实施例56 |
27 |
C20H21ClN4O4 |
417.0 |
16.8 |
81 |
|
实施例57 |
28 |
C20H21ClN4O4 |
417.0 |
6.8 |
33 |
|
实施例58 |
29 |
C22H20ClF6N3O2 |
508.0 |
20.8 |
82 |
|
实施例59 |
30 |
C21H21ClF3N3O2 |
440.0 |
15.2 |
69 |
|
实施例60 |
31 |
C20H21BrClN3O2 |
450.0 |
15.6 |
69 |
|
实施例61 |
32 |
C20H21ClFN3O2 |
390.0 |
11.8 |
61 |
|
实施例62 |
33 |
C20H20Cl3N3O2 |
440.0 |
15.8 |
72 |
|
实施例63 |
34 |
C21H24ClN3O3 |
402.5 |
33.8 |
quant |
|
实施例64 |
35 |
C22H26ClN3O4 |
432.5 |
56.1 |
quant |
|
实施例65 |
36 |
C22H26ClN3O4 |
432.5 |
37.6 |
quant |
|
实施例66 |
37 |
C21H21ClF3N3O2 |
440.0 |
12.6 |
57 |
|
实施例67 |
38 |
C21H24ClN3O2 |
386.0 |
12.3 |
64 |
|
实施例68 |
39 |
C20H21Cl2N3O2 |
406.0 |
15.9 |
78 |
|
实施例69 |
40 |
C21H24ClN3O2 |
402.0 |
11.6 |
58 |
|
实施例70 |
41 |
C20H20Cl3N3O2 |
442.0 |
17.8 |
81 |
|
实施例71 |
42 |
C21H21ClN4O2 |
397.5 |
22.4 |
quant |
|
实施例72 |
43 |
C21H22ClN3O4 |
416.0 |
30.1 |
quant |
|
实施例73 |
44 |
C21H20ClF4N3O2 |
458.0 |
13.4 |
59 |
|
实施例74 |
45 |
C21H20ClF4N3O2 |
458.0 |
13.2 |
58 |
|
实施例75 |
46 |
C21H20ClF4N3O2 |
458.0 |
14.4 |
63 |
|
实施例76 |
47 |
C21H21ClF3N3O3 |
456.0 |
16.4 |
72 |
|
实施例77 |
48 |
C21H20ClF4N3O2 |
458 |
16.5 |
72 |
|
实施例78 |
49 |
C20H21ClN4O4 |
417.0 |
12.5 |
60 |
|
实施例79 |
50 |
C21H20ClF4N3O2 |
458.0 |
26.3 |
quant |
|
实施例80 |
51 |
C20H21BrClN3O2 |
450.0 |
8.6 |
38 |
|
实施例81 |
52 |
C20H21ClFN3O2 |
390.5 |
4.1 |
21 |
|
实施例82 |
53 |
C20H21Cl2N3O2 |
406.0 |
5.4 |
27 |
|
实施例83 |
54 |
C20H20Cl3N3O2 |
440.0 |
8.8 |
40 |
|
实施例84 |
55 |
C20H20BrCl4N3O2 |
440.0 |
7.7 |
35 |
|
实施例85 |
56 |
C21H24ClN3O2 |
386.0 |
4.8 |
25 |
|
实施例86 |
57 |
C22H26ClN3O4 |
429.5 |
4.9 |
23 |
|
实施例87 |
58 |
C20H21Cl2N3O2 |
406.0 |
4.1 |
20 |
|
实施例88 |
59 |
C20H21BrClN3O2 |
452.0 |
3.5 |
16 |
|
实施例89 |
60 |
C26H26ClN3O2 |
448.5 |
7.3 |
33 |
|
实施例90 |
61 |
C21H21ClF3N3O2 |
440.0 |
7.1 |
32 |
|
实施例91 |
62 |
C21H24ClN3O2 |
386.0 |
10.4 |
54 |
|
实施例92 |
63 |
C22H26ClN3O2 |
400.5 |
6.0 |
30 |
|
实施例93 |
64 |
C21H21ClN4O2 |
397.0 |
7.0 |
35 |
|
实施例94 |
65 |
C24H24ClN3O2 |
422.0 |
7.7 |
36 |
|
实施例95 |
66 |
C24H24ClN3O2 |
422.0 |
6.3 |
30 |
|
实施例96 |
67 |
C20H20ClF2N3O2 |
408.0 |
4.7 |
23 |
|
实施例97 |
68 |
C20H20ClF2N3O2 |
408.0 |
7.8 |
38 |
|
实施例98 |
69 |
C20H20ClF2N3O2 |
408.0 |
7.3 |
36 |
|
实施例99 |
70 |
C20H20ClF2N3O2 |
408.0 |
9.1 |
45 |
|
实施例100 |
71 |
C22H26ClN3O4 |
429.0 |
5.6 |
26 |
|
实施例101 |
72 |
C21H21ClF3N3O2 |
456.0 |
6.2 |
27 |
|
实施例102 |
73 |
C21H21ClF3N3O2 |
456.5 |
16.8 |
74 |
|
实施例103 |
74 |
C22H24ClN3O4 |
430.0 |
16.4 |
76 |
|
实施例104 |
75 |
C21H20ClF4N3O2 |
458.0 |
16.1 |
70 |
|
实施例105 |
76 |
C21H20ClF4N3O2 |
458.0 |
17.0 |
74 |
|
实施例106 |
77 |
C20H15ClF3N3O2 |
426.0 |
16.2 |
76 |
|
实施例107 |
78 |
C20H15ClF3N3O2 |
426.0 |
18.0 |
85 |
|
实施例108 |
79 |
C22H20ClF6N3O2 |
508.0 |
18.8 |
74 |
|
实施例109 |
80 |
C22H20ClF6N3O2 |
508.0 |
16.4 |
65 |
|
实施例110 |
81 |
C22H26ClN3O2 |
400.0 |
13.9 |
70 |
|
实施例111 |
83 |
C20H21ClN4O4 |
417.0 |
16.0 |
77 |
|
实施例112 |
84 |
C20H21ClN4O4 |
417.0 |
21.6 |
quant |
|
实施例113 |
87 |
C23H22ClF6N3O2 |
522.0 |
17.5 |
67 |
|
实施例114 |
88 |
C22H22ClF3N3O2 |
454.0 |
13.9 |
61 |
|
实施例115 |
89 |
C21H23BrClN3O2 |
466.0 |
15.4 |
66 |
|
实施例116 |
90 |
C21H23ClFN3O2 |
404.0 |
10.7 |
53 |
|
实施例117 |
91 |
C21H22Cl3N3O2 |
456.0 |
13.7 |
60 |
|
实施例118 |
92 |
C22H26ClN3O3 |
416.0 |
38.4 |
quant |
|
实施例119 |
93 |
C23H23ClN3O4 |
446.0 |
25.2 |
quant |
|
实施例120 |
94 |
C23H23ClN3O4 |
446.0 |
16.5 |
74 |
|
实施例121 |
95 |
C22H23ClF3N3O2 |
454.0 |
16.3 |
72 |
|
实施例122 |
96 |
C22H26ClN3O2 |
400.5 |
16.7 |
84 |
|
实施例123 |
97 |
C21H23Cl2N3O2 |
420.0 |
11.2 |
53 |
|
实施例124 |
98 |
C22H26ClN3O2 |
416.5 |
11.8 |
57 |
|
实施例125 |
99 |
C22H22Cl3N3O2 |
454.0 |
14.8 |
65 |
|
实施例126 |
100 |
C22H23ClN4O2 |
411.0 |
9.5 |
46 |
|
实施例127 |
101 |
C22H24ClN3O4 |
430.5 |
13.2 |
61 |
|
实施例128 |
102 |
C22H22ClF4N3O2 |
472.0 |
13.1 |
56 |
|
实施例129 |
103 |
C22H22ClF4N3O2 |
472.0 |
36.5 |
quant |
|
实施例130 |
104 |
C22H22ClF4N3O2 |
472.0 |
22.8 |
97 |
|
实施例131 |
105 |
C22H22ClF4N3O2 |
472.0 |
20.1 |
85 |
|
实施例132 |
106 |
C22H23ClF3N3O3 |
470.0 |
27.4 |
quant |
|
实施例133 |
107 |
C22H22ClF4N3O2 |
472.0 |
18.5 |
78 |
|
实施例134 |
108 |
C21H23ClN4O4 |
431.0 |
11.9 |
55 |
|
实施例135 |
109 |
C21H23ClN4O4 |
431.0 |
23.9 |
quant |
|
实施例136 |
110 |
C21H23ClN4O4 |
431.0 |
24.4 |
quant |
|
实施例137 |
111 |
C23H22ClF6N3O2 |
522.0 |
9.5 |
36 |
|
实施例138 |
112 |
C22H23ClF3N3O2 |
454.0 |
3.9 |
17 |
|
实施例139 |
113 |
C21H23BrClN3O2 |
466.0 |
7.5 |
32 |
|
实施例140 |
114 |
C21H23ClFN3O2 |
404.0 |
6.1 |
30 |
|
实施例141 |
115 |
C21H22Cl3N3O2 |
456.0 |
6.6 |
29 |
|
实施例142 |
116 |
C22H26ClN3O3 |
416.0 |
4.8 |
23 |
|
实施例143 |
117 |
C23H28ClN3O4 |
446.0 |
6.4 |
29 |
|
实施例144 |
118 |
C23H28ClN3O4 |
446.0 |
24.6 |
quant |
|
实施例145 |
119 |
C22H23ClF3N3O2 |
454.0 |
5.2 |
23 |
|
实施例146 |
120 |
C22H26ClN3O2 |
400.5 |
4.4 |
22 |
|
实施例147 |
121 |
C21H23Cl2N3O2 |
420.0 |
7.8 |
37 |
|
实施例148 |
122 |
C22H26ClN3O2 |
416.5 |
14.1 |
68 |
|
实施例149 |
123 |
C21H23Cl3N3O2 |
454.0 |
5.4 |
24 |
|
实施例150 |
124 |
C22H23ClN4O2 |
411.0 |
34.0 |
quant |
|
实施例151 |
125 |
C22H24ClN3O4 |
430.5 |
32.0 |
quant |
|
实施例152 |
126 |
C22H22ClF4N3O2 |
472.0 |
4.6 |
19 |
|
实施例153 |
127 |
C22H22ClF4N3O2 |
472.0 |
10.4 |
44 |
|
实施例154 |
128 |
C22H22ClF4N3O2 |
472.0 |
7.3 |
31 |
|
实施例155 |
129 |
C22H22ClF4N3O2 |
472.0 |
13.5 |
57 |
|
实施例156 |
130 |
C22H23ClF3N3O3 |
470.0 |
15.1 |
64 |
|
实施例157 |
131 |
C22H22ClF4N3O2 |
472.0 |
8.6 |
36 |
|
实施例158 |
132 |
C21H23ClN4O4 |
431.0 |
4.4 |
20 |
|
实施例159 |
133 |
C21H23ClN4O4 |
431.0 |
32.0 |
quant |
|
实施例160 |
134 |
C21H23ClN4O4 |
431.0 |
6.9 |
32 |
|
实施例161 |
135 |
C21H23BrClN3O2 |
466.0 |
7.8 |
34 |
|
实施例162 |
136 |
C21H23ClFN3O2 |
404.0 |
13.7 |
68 |
|
实施例163 |
137 |
C21H23Cl2N3O2 |
420.5 |
14.6 |
69 |
|
实施例164 |
138 |
C21H22Cl3N3O2 |
454.0 |
17.7 |
78 |
|
实施例165 |
139 |
C21H22BrCl4N3O2 |
454.0 |
17.2 |
76 |
|
实施例166 |
140 |
C22H26ClN3O2 |
400.0 |
15.0 |
75 |
|
实施例167 |
141 |
C23H28ClN3O4 |
443.5 |
13.9 |
62 |
|
实施例168 |
142 |
C21H23Cl2N3O2 |
420.0 |
13.7 |
65 |
|
实施例169 |
143 |
C21H23BrClN3O2 |
464.0 |
16.1 |
69 |
|
实施例170 |
144 |
C27H28ClN3O2 |
462.0 |
17.6 |
76 |
|
实施例171 |
145 |
C22H23ClF3N3O2 |
454.0 |
16.0 |
71 |
|
实施例172 |
146 |
C22H26ClN3O2 |
400.0 |
14.9 |
75 |
|
实施例173 |
147 |
C23H28ClN3O2 |
414.0 |
16.2 |
78 |
|
实施例174 |
148 |
C22H23ClN4O2 |
411.0 |
14.9 |
73 |
|
实施例175 |
149 |
C25H26ClN3O2 |
436.0 |
17.1 |
78 |
|
实施例176 |
150 |
C25H26ClN3O2 |
436.0 |
13.1 |
60 |
|
实施例177 |
151 |
C21H22ClF2N3O2 |
422.0 |
14.8 |
70 |
|
实施例178 |
152 |
C21H22ClF2N3O2 |
422.0 |
15.3 |
73 |
|
实施例179 |
153 |
C21H22ClF2N3O2 |
422.0 |
15.3 |
73 |
|
实施例180 |
154 |
C21H22ClF2N3O2 |
422.0 |
16.4 |
78 |
|
实施例181 |
155 |
C23H28ClN3O4 |
443.0 |
16.9 |
76 |
|
实施例182 |
156 |
C22H23ClF3N3O2 |
470.5 |
12.6 |
54 |
|
实施例183 |
157 |
C22H23ClF3N3O2 |
470.0 |
20.0 |
85 |
|
实施例184 |
158 |
C23H26ClN3O4 |
444.0 |
17.4 |
78 |
|
实施例185 |
159 |
C22H22ClF4N3O2 |
472.0 |
18.4 |
78 |
|
实施例186 |
160 |
C22H22ClF4N3O2 |
472.0 |
19.6 |
83 |
|
实施例187 |
161 |
C21H21ClF3N3O2 |
440.0 |
17.0 |
77 |
|
实施例188 |
162 |
C21H21ClF3N3O2 |
440.0 |
17.1 |
78 |
|
实施例189 |
163 |
C23H22ClF6N3O2 |
522.0 |
20.8 |
80 |
|
实施例190 |
164 |
C23H22ClF6N3O2 |
522.0 |
2.7 |
10 |
|
实施例191 |
165 |
C23H25ClN3O2 |
414.0 |
16.4 |
79 |
|
实施例192 |
166 |
C22H23ClF3N3O2 |
454.0 |
8.6 |
38 |
|
实施例193 |
167 |
C21H23BrClN3O2 |
464.0 |
11.6 |
50 |
|
实施例194 |
168 |
C21H23Cl2N3O2 |
420.0 |
11.5 |
55 |
|
实施例195 |
169 |
C21H22Cl3N3O2 |
454.0 |
10.0 |
44 |
|
实施例196 |
170 |
C22H22ClF4N3O2 |
472.0 |
10.4 |
44 |
|
实施例197 |
171 |
C21H23Cl2N3O2 |
420.0 |
8.9 |
42 |
|
实施例198 |
172 |
C21H24ClN3O2 |
386.0 |
10.3 |
53 |
|
实施例199 |
173 |
C21H23ClN4O4 |
431.0 |
14.6 |
68 |
|
实施例200 |
174 |
C22H23ClF3N3O2 |
454.0 |
10.4 |
46 |
|
实施例201 |
175 |
C21H23BrClN3O2 |
464.0 |
13.4 |
58 |
|
实施例202 |
176 |
C21H23Cl2N3O2 |
420.0 |
12.7 |
60 |
|
实施例203 |
177 |
C21H22Cl3N3O2 |
454.0 |
13.2 |
58 |
|
实施例204 |
178 |
C22H22ClF4N3O2 |
472.0 |
12.9 |
55 |
|
实施例205 |
179 |
C21H23Cl2N3O2 |
420.0 |
13.3 |
63 |
|
实施例206 |
180 |
C21H24ClN3O2 |
386.0 |
24.2 |
quant |
|
实施例207 |
181 |
C21H23ClN4O4 |
431.0 |
1.0 |
1 |
|
实施例208 |
182 |
C23H25ClF3N3O2 |
468.0 |
15.1 |
65 |
|
实施例209 |
183 |
C22H25BrClN3O2 |
478.0 |
18.0 |
75 |
|
实施例210 |
184 |
C22H25Cl2N3O2 |
434.0 |
16.3 |
75 |
|
实施例211 |
185 |
C22H24Cl3N3O2 |
468.0 |
18.6 |
79 |
|
实施例212 |
186 |
C23H24ClF4N3O2 |
486.0 |
16.5 |
68 |
|
实施例213 |
187 |
C22H25Cl2N3O2 |
434.0 |
14.4 |
66 |
|
实施例214 |
188 |
C22H26ClN3O2 |
400.0 |
14.0 |
70 |
|
实施例215 |
189 |
C22H25ClN4O4 |
445.0 |
16.8 |
76 |
|
实施例216 |
190 |
C26H25ClF3N3O2S |
536.0 |
17.7 |
66 |
|
实施例217 |
191 |
C25H25BrClN3O2S |
546.0 |
20.4 |
75 |
|
实施例218 |
192 |
C25H25Cl2N3O2S |
502.0 |
16.9 |
67 |
|
实施例219 |
193 |
C25H24Cl3N3O2S |
536.0 |
18.3 |
68 |
|
实施例220 |
194 |
C26H24ClF4N3O2S |
554.0 |
19.4 |
70 |
|
实施例221 |
195 |
C25H25Cl2N3O2S |
502.0 |
19.1 |
76 |
|
实施例222 |
196 |
C25H26ClN3O2S |
468.0 |
16.0 |
68 |
|
实施例223 |
197 |
C25H25ClN4O4S |
513.0 |
18.4 |
72 |
|
实施例224 |
198 |
C26H25ClF3N3O2S |
536.0 |
13.9 |
52 |
|
实施例225 |
199 |
C25H25BrClN3O2S |
546.0 |
12.9 |
47 |
|
实施例226 |
200 |
C25H25Cl2N3O2S |
502.0 |
15.6 |
62 |
|
实施例227 |
201 |
C25H24Cl3N3O2S |
536.0 |
17.3 |
64 |
|
实施例228 |
202 |
C26H24ClF4N3O2S |
554.0 |
15.4 |
56 |
|
实施例229 |
203 |
C25H25Cl2N3O2S |
502.0 |
13.5 |
54 |
|
实施例230 |
204 |
C25H25ClN3O2S |
468.0 |
13.7 |
59 |
|
实施例231 |
205 |
C25H25ClN4O4S |
513.0 |
13.9 |
54 |
|
实施例232 |
206 |
C24H27ClF3N3O4S |
546.0 |
10.0 |
37 |
|
实施例233 |
207 |
C23H27BrClN3O4S |
558.0 |
17.1 |
61 |
|
实施例234 |
208 |
C23H27Cl2N3O4S |
512.0 |
17.0 |
66 |
|
实施例235 |
209 |
C23H26Cl3N3O4S |
546.0 |
7.3 |
27 |
|
实施例236 |
210 |
C24H26ClF4N3O4S |
564.0 |
19.2 |
68 |
|
实施例237 |
211 |
C23H27Cl2N3O4S |
512.0 |
7.9 |
31 |
|
实施例238 |
212 |
C23H28ClN3O4S |
478.0 |
13.7 |
57 |
|
实施例239 |
213 |
C23H27ClN4O4S |
523.0 |
5.5 |
21 |
实施例240:(R)-3-[N-{3-氟-5-(三氟甲基)苯甲酰基}甘氨酰]氨基-1-(3,5-二甲基异噁唑-4-基甲基)吡咯烷(1191号化合物)的制备
向(R)-1-(3,5-二甲基异噁唑-4-基甲基)-3-(甘氨酰氨基)吡咯烷(0.050mmol)与(哌啶子基甲基)聚苯乙烯(58mg)在氯仿(0.2ml)与二氯甲烷(0.75ml)中的混合物中加入3-氟-5-(三氟甲基)苯甲酰氯(0.058mmol)的二氯甲烷(1ml)溶液。反应混合物在室温下搅拌2小时后,加入甲醇(1.0ml),混合物在室温下搅拌30分钟。将反应混合物装上VarianTM SCX柱,用CH3OH(16ml)洗涤。用2N NH3的CH3OH(6ml)溶液洗脱产物,并浓缩,得到(R)-3-[N-{3-氟-5-(三氟甲基)苯甲酰基}甘氨酰]氨基-1-(3,5-二甲基异噁唑-4-基甲基)吡咯烷(1191号化合物)(19.5mg,88%)。纯度用RPLC/MS测定(100%);ESI/MS m/e 443.2(M++H,C20H22F4N4O3)
实施例241-265
依据实施例240的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表4中。
表4
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例241 |
1192 |
C20 H22 F4 N4 O3 |
443.2 |
19.2 |
87 |
|
实施例242 |
1193 |
C20 H23 F3 N4 O4 |
441.0 |
17.5 |
79 |
|
实施例243 |
1194 |
C21 H22 F6 N4 O3 |
493.0 |
20.4 |
83 |
|
实施例244 |
1195 |
C19 H23 Br N4 O3 |
435.1 |
16.8 |
77 |
|
实施例245 |
1196 |
C19 H23 N5 O5 |
402.2 |
16.2 |
81 |
|
实施例246 |
1197 |
C20 H22 F4 N4 O3 |
443.2 |
17.6 |
80 |
|
实施例247 |
1198 |
C19 H23 Cl N4 O3 |
391.0 |
16.5 |
84 |
|
实施例248 |
1199 |
C20 H26 N4 O3 |
371.0 |
16.1 |
87 |
|
实施例249 |
1200 |
C19 H22 Cl2 N4 O3 |
425.0 |
18.0 |
85 |
|
实施例250 |
1201 |
C19 H22 F2 N4 O3 |
393.0 |
16.6 |
85 |
|
实施例251 |
1202 |
C20 H22 F4 N4 O3 |
443.2 |
16.8 |
76 |
|
实施例252 |
1203 |
C22 H24 F3 N3 O3 |
436.2 |
17.1 |
79 |
|
实施例253 |
1204 |
C23 H23 F6 N3 O2 |
488.2 |
18.1 |
74 |
|
实施例254 |
1205 |
C21 H24 Br N3 O2 |
430.0 |
17.5 |
81 |
|
实施例255 |
1206 |
C21 H24 N4 O4 |
397.0 |
16.2 |
82 |
|
实施例256 |
1207 |
C22 H23 F4 N3 O2 |
438.2 |
17.5 |
80 |
|
实施例257 |
1208 |
C21 H24 Cl N3 O2 |
386.0 |
15.8 |
82 |
|
实施例258 |
1209 |
C22 H27 N3 O2 |
366.0 |
15.7 |
86 |
|
实施例259 |
1210 |
C21 H23 Cl2 N3 O2 |
420.0 |
17.8 |
85 |
|
实施例260 |
1211 |
C21 H23 F2 N3 O2 |
388.0 |
16.3 |
84 |
|
实施例261 |
1212 |
C22 H23 F4 N3 O2 |
438.2 |
17.4 |
80 |
|
实施例262 |
1213 |
C24 H24 Cl F6 N3 O2 |
536.2 |
24.0 |
90 |
|
实施例263 |
1214 |
C23 H24 Cl F4 N3 O3 |
486.2 |
22.2 |
91 |
|
实施例264 |
1215 |
C22 H24 Cl3 N3 O2 |
467.9 |
20.9 |
89 |
|
实施例265 |
1216 |
C22 H24 Cl F2 N3 O2 |
436.0 |
19.3 |
89 |
实施例266:(R)-1-(4-氯苄基)-3-[{N-(4-(二甲氨基)苯甲酰基)甘氨酰}氨基]吡咯烷(952号化合物)的制备
将(R)-1-(4-氯苄基)-3-(甘氨酰氨基)吡咯烷(13.8mg,0.052mmol)的CHCl3(2ml)溶液用Et3N(0.021ml,0.15mmol)、4-(二甲氨基)苯甲酸(10mg,0.061mmol)、EDCI(10.2mg,0.053mmol)和HOBt(7.5mg,0.055mmol)处理。反应混合物在室温下搅拌16小时。溶液用2N NaOH水溶液(2ml×2)和盐水(2ml)洗涤,用CH2Cl2(3ml)通过PTFE膜过滤进行干燥。浓缩得到所需物质(952号化合物)(24.9mg,定量)。纯度用RPLC/MS测定(91%);ESI/MS m/e415.0(M++H,C22H27ClN4O2)
实施例267-347
依据实施例266的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用固相萃取法(VarianTM SCX柱)或色谱法(HPLC-C18)得到所需物质。ESI/MS数据和产率总结在表5中。
表5
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例267 |
951 |
C22 H24 Cl N3 O4 |
430.0 |
26.3 |
quant |
|
实施例268 |
953 |
C23 H29 Cl N4 O2 |
429.0 |
28.8 |
quant |
|
实施例269 |
954 |
C21 H25 Cl N4 O2 |
401.0 |
27.9 |
quant |
|
实施例270 |
955 |
C22 H27 Cl N4 O2 |
415.0 |
26.8 |
quant |
|
实施例271 |
956 |
C21 H24 Cl N3 O3 |
402.0 |
10.3 |
51 |
|
实施例272 |
957 |
C20 H22 Cl N3 O3 |
388.0 |
1.4 |
7 |
|
实施例273 |
958 |
C21 H24 Cl N3 O3 |
402.5 |
1.2 |
6 |
|
实施例274 |
959 |
C22 H25 Cl N4 O3 |
429.5 |
4.7 |
22 |
|
实施例275 |
960 |
C23 H27 Cl N4 O3 |
443.0 |
10.9 |
49 |
|
实施例276 |
961 |
C21 H25 Cl N4 O2 |
401.0 |
28.4 |
quant |
|
实施例277 |
962 |
C22 H27 Cl N4 O2 |
415.0 |
24.9 |
quant |
|
实施例278 |
963 |
C21 H24 Cl N3 O3 |
402.0 |
4.4 |
22 |
|
实施例279 |
964 |
C22 H24 Cl N3 O4 |
430.0 |
29.5 |
quant |
|
实施例280 |
965 |
C23 H26 Cl N3 O4 |
444.0 |
27.2 |
quant |
|
实施例281 |
966 |
C22 H24 Cl N3 O3 |
414.0 |
27.0 |
quant |
|
实施例282 |
967 |
C23 H26 Cl N3 O3 |
428.0 |
27.0 |
quant |
|
实施例283 |
968 |
C22 H23 Cl N4 O2 |
411.0 |
21.4 |
quant |
|
实施例284 |
969 |
C23 H25 Cl N4 O2 |
425.0 |
27.6 |
quant |
|
实施例285 |
970 |
C22 H27 Cl N4 O2 |
415.0 |
28.6 |
quant |
|
实施例286 |
971 |
C23 H29 Cl N4 O2 |
429.0 |
27.9 |
quant |
|
实施例287 |
972 |
C20 H23 Cl N4 O2 |
387.0 |
26.2 |
quant |
|
实施例288 |
973 |
C21 H25 Cl N4 O2 |
401.0 |
26.8 |
quant |
|
实施例289 |
974 |
C20 H23 Cl N4 O2 |
387.0 |
26.6 |
quant |
|
实施例290 |
975 |
C21 H25 Cl N4 O2 |
401.0 |
28.2 |
quant |
|
实施例291 |
976 |
C22 H23 Cl N4 O2 |
411.0 |
29.2 |
quant |
|
实施例292 |
977 |
C23 H25 Cl N4 O2 |
425.0 |
29.5 |
quant |
|
实施例293 |
978 |
C20 H21 Cl N6 O2 |
413.0 |
2.2 |
11 |
|
实施例294 |
979 |
C21 H23 Cl N6 O2 |
427.0 |
10.2 |
48 |
|
实施例295 |
980 |
C22 H25 Cl N4 O3 |
429.0 |
28.8 |
quant |
|
实施例296 |
981 |
C23 H27 Cl N4 O3 |
443.0 |
11.9 |
54 |
|
实施例297 |
982 |
C22 H27 Cl N4 O2 |
415.0 |
27.4 |
quant |
|
实施例298 |
983 |
C23 H29 Cl N4 O2 |
429.5 |
28.1 |
quant |
|
实施例299 |
984 |
C21 H24 Cl N3 O3 |
402.0 |
27.7 |
quant |
|
实施例300 |
985 |
C22 H26 Cl N3 O3 |
416.0 |
28.6 |
quant |
|
实施例301 |
1149 |
C21 H28 N4 O4 |
401 |
15.5* |
38 |
|
实施例302 |
1150 |
C21 H28 N4 O3 |
385 |
10.9* |
28 |
|
实施例303 |
1151 |
C21 H25 F3 N4 O3 |
439 |
17.3* |
39 |
|
实施例304 |
1152 |
C21 H24 F N5 O3 |
415 |
12.7* |
30 |
|
实施例305 |
1153 |
C21 H24 Cl N5 O3 |
430 |
17.5* |
41 |
|
实施例306 |
1154 |
C22 H27 N5 O3 |
410 |
20.6* |
50 |
|
实施例307 |
1155 |
C19 H23 F3 N4 O4 |
429 |
13.8* |
32 |
|
实施例308 |
1156 |
C21 H30 N4 O4 |
403 |
17.7* |
43 |
|
实施例309 |
1157 |
C18 H24 N4 O3 S2 |
409 |
12.6* |
30 |
|
实施例310 |
1158 |
C19 H23 Cl2 N5 O3 |
440 |
16.9* |
38 |
|
实施例311 |
1159 |
C22 H31 N5 O6 |
462 |
38.6* |
85 |
|
实施例312 |
1160 |
C20 H26 Br N5 O3 |
464 |
20.4 |
45 |
|
实施例313 |
1289 |
C20 H27 N5 O4 |
403 |
5.8* |
14 |
|
实施例314 |
1290 |
C21 H29 N5 O3 |
400 |
6.9* |
17 |
|
实施例315 |
1291 |
C24 H28 N4 O2 |
405 |
22.4 |
68 |
|
实施例316 |
1292 |
C22 H27 Br N4 O2 |
461 |
23.8 |
15 |
|
实施例317 |
1293 |
C22 H23 F4 N3 O2 |
438 |
20.9 |
59 |
|
实施例318 |
1294 |
C22 H23 F4 N3 O2 |
438 |
20.8 |
59 |
|
实施例319 |
1295 |
C23 H31 N3 O3 |
398 |
17.5 |
54 |
|
实施例320 |
1296 |
C20 H25 N3 O2 S2 |
404 |
18.8 |
58 |
|
实施例321 |
1297 |
C21 H24 F3 N3 O3 |
424 |
18.1 |
53 |
|
实施例322 |
1388 |
C21 H32 N6 O3 |
417 |
7.4* |
24 |
|
实施例323 |
1389 |
C19 H22 N6 O4 |
399 |
15.2 |
48 |
|
实施例324 |
1401 |
C23 H25 Cl N4 O2 |
425 |
8.3* |
16 |
|
实施例325 |
1402 |
C24 H32 N4 O5 |
457 |
8.3* |
15 |
|
实施例326 |
1403 |
C20 H24 N4 O2 |
353 |
14.8 |
52 |
|
实施例327 |
1404 |
C20 H24 N4 O2 |
353 |
17.0 |
60 |
|
实施例328 |
1405 |
C21 H26 N4 O2 S |
399 |
17.3 |
54 |
|
实施例329 |
1407 |
C22 H28 N4 O2 S |
413 |
19.1 |
57 |
|
实施例330 |
1410 |
C19 H24 N4 O3 |
357 |
9.7* |
59 |
|
实施例331 |
1769 |
C22 H26 Cl F3 N4 O5 |
519 |
11.6* |
20 |
|
实施例332 |
1770 |
C26 H28 Cl2 N6 O4 |
559 |
13.1* |
21 |
|
实施例333 |
1771 |
C26 H37 N5 O4 |
484 |
12.7* |
23 |
|
实施例334 |
1772 |
C28 H39 N5 O4 |
510 |
5.5* |
9 |
|
实施例335 |
1773 |
C28 H37 N5 O4 |
509 |
6.2* |
11 |
|
实施例336 |
1774 |
C28 H34 N6 O6 |
551 |
13.6* |
22 |
|
实施例337 |
2039 |
C19 H24 N4 O2 |
341 |
5.2* |
14 |
|
实施例338 |
2040 |
C22 H27 N3 O4 |
398 |
2.0* |
5 |
|
实施例339 |
2041 |
C23 H29 N3 O3 |
396 |
6.2* |
15 |
|
实施例340 |
2042 |
C25 H37 N3 O2 |
413 |
2.6* |
6 |
|
实施例341 |
2043 |
C24 H31 N3 O2 |
394 |
6.8* |
17 |
|
实施例342 |
2044 |
C25 H28 N4 O4 |
449 |
8.7* |
16 |
|
实施例343 |
2045 |
C26 H29 Cl N6 O4 |
525 |
11.4* |
19 |
|
实施例344 |
2046 |
C27 H32 N6 O4 |
505 |
7.7* |
13 |
|
实施例345 |
2047 |
C28 H32 N4 O4 |
489 |
10.0* |
18 |
|
实施例346 |
2048 |
C28 H37 N5 O5 |
524 |
3.7* |
6 |
|
实施例347 |
2049 |
C28 H37 N5 O4 |
509 |
5.3* |
9 |
*TFA盐的产量
实施例348:(R)-1-(4-氯苄基)-3-[{N-(2-氨基-5-氯苯甲酰基)甘氨酰}氨基]吡咯烷(1084号化合物)的制备
将(R)-1-(4-氯苄基)-3-(甘氨酰氨基)吡咯烷(0.050mmol)的CHCl3(2ml)溶液用2-氨基-5-氯苯甲酸(0.060mmol)和二异丙基碳二亚胺(0.060mmol)处理。反应混合物在室温下搅拌15小时。将反应混合物装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到(R)-1-(4-氯苄基)-3-[{N-(2-氨基-5-氯苯甲酰基)甘氨酰}氨基]吡咯烷(1084号化合物)(12.7mg,60%)。纯度用RPLC/MS测定(87%);ESI/MS m/e421.0(M++H,C20H22Cl2N4O2)
实施例349-361
依据实施例348的方法,分别使用相应的试剂,合成本发明的化合物。如果残留原料胺,在室温下用异氰酰甲基化的聚苯乙烯(50mg)的CHCl3(1ml)溶液处理,过滤,并浓缩,得到所需物质。ESI/MS数据和产率总结在表6中。
表6
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例349 |
1085 |
C20H22ClN5O4 |
432.0 |
4.1 |
19 |
|
实施例350 |
1086 |
C20H23ClN4O2 |
387.0 |
7.9 |
41 |
|
实施例351 |
1087 |
C22H23ClN4O2 |
411.0 |
15.0 |
73 |
|
实施例352 |
1088 |
C18H20ClN3O3 |
362.0 |
12.9 |
71 |
|
实施例353 |
1089 |
C22H22ClFN4O2 |
429.0 |
16.0 |
75 |
|
实施例354 |
1090 |
C22H26ClN3O3 |
416.0 |
15.8 |
76 |
|
实施例355 |
1091 |
C21H24Cl2N4O2 |
435.0 |
10.9 |
50 |
|
实施例356 |
1092 |
C21H24ClN5O4 |
446.0 |
7.9 |
35 |
|
实施例357 |
1093 |
C21H25ClN4O2 |
401.0 |
9.5 |
47 |
|
实施例358 |
1094 |
C23H25ClN4O2 |
425.0 |
15.8 |
74 |
|
实施例359 |
1095 |
C15H22ClN3O3 |
376.0 |
13.5 |
72 |
|
实施例360 |
1096 |
C23H24ClFN4O2 |
443.0 |
11.8 |
53 |
|
实施例361 |
1097 |
C23H28ClN3O3 |
430.0 |
15.1 |
70 |
实施例362:(R)-1-(4-氯苄基)-3-[{N-(3-溴-4-甲基苯甲酰基)甘氨酰}氨基]吡咯烷(1098号化合物)的制备
将(R)-1-(4-氯苄基)-3-(甘氨酰氨基)吡咯烷(0.050mmol)的CHCl3(1.35ml)与叔丁醇(0.15ml)溶液用3-溴-4-甲基苯甲酸(0.060mmol)、二异丙基碳二亚胺(0.060mmol)和HOBt(0.060mmol)处理。反应混合物在室温下搅拌15小时。将反应混合物装上VarianTM SCX柱,用CH3OH/CHCl3 1∶1(12ml)和CH3OH(12ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到(R)-1-(4-氯苄基)-3-[{N-(3-溴-4-甲基苯甲酰基)甘氨酰}氨基]吡咯烷(1098号化合物)(11.6mg,50%)。纯度用RPLC/MS测定(94%);ESI/MS m/e 466.0(M++H,C21H23BrClN3O2)
实施例363-572
依据实施例362的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC得到所需物质。ESI/MS数据和产率总结在表7中。
作为1415、1416和1417号化合物的副产物,分别得到下列3种化合物。
1419:7.9mg,产率38%;ESI/MS m/e 419.0(C20H23ClN4O2S)
1420:7.1mg,产率36%;ESI/MS m/e 399.2(C21H26N4O2S)
1421:7.4mg,产率37%;ESI/MS m/e 404.2(C19H25N5O3S)
表7
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例363 |
1099 |
C20H20BrClFN3O2 |
470.0 |
3.1 |
13 |
|
实施例364 |
1100 |
C20H20Cl2FN3O2 |
424.0 |
3.1 |
15 |
|
实施例365 |
1101 |
C21H23ClIN3O2 |
512.0 |
12.5 |
49 |
|
实施例366 |
1102 |
C21H23ClN4O4 |
431.2 |
7.7 |
36 |
|
实施例367 |
1103 |
C22H25BrN3O2 |
446.0 |
13.8 |
62 |
|
实施例368 |
1104 |
C21H23BrFN3O2 |
450.0 |
16.5 |
74 |
|
实施例369 |
1105 |
C21H23ClFN3O2 |
404.2 |
14.7 |
73 |
|
实施例370 |
1106 |
C22H36IN3O2 |
492.0 |
18.5 |
75 |
|
实施例371 |
1107 |
C22H26N4O4 |
411.2 |
15.2 |
74 |
|
实施例372 |
1108 |
C20H25BrN4O3 |
449.0 |
12.8 |
57 |
|
实施例373 |
1109 |
C19H22BrFN4O3 |
455.0 |
16.2 |
71 |
|
实施例374 |
1110 |
C19H22ClFN4O3 |
409.2 |
14.4 |
70 |
|
实施例375 |
1111 |
C20H25IN4O3 |
497.0 |
17.9 |
72 |
|
实施例376 |
1112 |
C20H25N5O5 |
416.2 |
14.9 |
72 |
|
实施例377 |
1113 |
C23H27BrClN3O2 |
494.0 |
16.1 |
65 |
|
实施例378 |
1114 |
C22H24BrClFN3O2 |
498.0 |
20.2 |
81 |
|
实施例379 |
1115 |
C22H24Cl2FN3O2 |
452.2 |
18.6 |
82 |
|
实施例380 |
1116 |
C23H27ClIN3O2 |
539.1 |
21.9 |
81 |
|
实施例381 |
1117 |
C23H27ClN4O4 |
459.2 |
18.7 |
81 |
|
实施例382 |
1171 |
C21H23BrClN3O2 |
466.0 |
4.9 |
21 |
|
实施例383 |
1172 |
C22H23ClN4O3 |
427.2 |
16.1 |
75 |
|
实施例384 |
1173 |
C23H25ClN4O3 |
441.2 |
22.8 |
quant |
|
实施例385 |
1174 |
C20H22ClFN4O2 |
405.2 |
21.4 |
quant |
|
实施例386 |
1175 |
C22H26BrN3O2 |
446.0 |
15.8 |
71 |
|
实施例387 |
1176 |
C23H26N4O3 |
407.2 |
17.6 |
87 |
|
实施例388 |
1177 |
C24H28N4O3 |
421.2 |
20.2 |
96 |
|
实施例389 |
1178 |
C21H25FN4O2 |
385.0 |
16.2 |
84 |
|
实施例390 |
1179 |
C21H25N5O4 |
412.2 |
2.3 |
11 |
|
实施例391 |
1180 |
C23H26N4O2 |
391.0 |
21.6 |
quant |
|
实施例392 |
1181 |
C20H25BrN4O3 |
451.0 |
20.1 |
89 |
|
实施例393 |
1182 |
C21H25N5O4 |
412.2 |
13.3 |
65 |
|
实施例394 |
1183 |
C22H27N5O4 |
426.2 |
20.9 |
98 |
|
实施例395 |
1184 |
C15H24FN5O3 |
390.0 |
20.0 |
quant |
|
实施例396 |
1185 |
C15H24N6O5 |
417.2 |
18.2 |
87 |
|
实施例397 |
1186 |
C21H25N5O3 |
396.2 |
17.6 |
89 |
|
实施例398 |
1187 |
C23H27BrClN3O2 |
494.0 |
22.1 |
90 |
|
实施例399 |
1188 |
C24H27ClN4O3 |
455.2 |
17.2 |
76 |
|
实施例400 |
1189 |
C25H25ClN4O3 |
469.2 |
21.1 |
90 |
|
实施例401 |
1190 |
C22H26ClFN4O2 |
433.2 |
20.4 |
94 |
|
实施例402 |
1217 |
C21H20Cl2F3N3O2 |
474.0 |
38.5 |
81 |
|
实施例403 |
1218 |
C21H23ClFN3O2 |
404.2 |
35.6 |
88 |
|
实施例404 |
1219 |
C21H23Cl2N3O2 |
420.0 |
3.7 |
9 |
|
实施例405 |
1220 |
C20H22ClIN4O2 |
513.0 |
53.0 |
quant |
|
实施例406 |
1221 |
C20H21ClF2N4O2 |
423.0 |
38.7 |
92 |
|
实施例407 |
1222 |
C15H23ClN4O2 |
375.2 |
33.6 |
90 |
|
实施例408 |
1223 |
C26H26ClN3O2S |
496.0 |
43.7 |
88 |
|
实施例409 |
1224 |
C20H21ClN4O5 |
433.0 |
40.6 |
94 |
|
实施例410 |
1225 |
C22H23ClF3N3O2 |
454.2 |
18.4 |
41 |
|
实施例411 |
1226 |
C23H26FN3O2 |
384.0 |
17.1 |
45 |
|
实施例412 |
1227 |
C22H26ClN3O2 |
400.2 |
17.5 |
44 |
|
实施例413 |
1228 |
C21H25IN4O2 |
493.0 |
23.3 |
47 |
|
实施例414 |
1229 |
C21H24F2N4O2 |
403.2 |
18.4 |
46 |
|
实施例415 |
1230 |
C20H26N4O2 |
355.2 |
15.7 |
44 |
|
实施例416 |
1231 |
C27H25N3O2S |
476.0 |
20.9 |
88 |
|
实施例417 |
12 32 |
C21H24N4O5 |
413.0 |
19.9 |
96 |
|
实施例418 |
1233 |
C20H22ClF3N4O3 |
459.0 |
19.4 |
85 |
|
实施例419 |
1234 |
C20H25FN4O3 |
389.0 |
17.8 |
92 |
|
实施例420 |
1235 |
C20H25ClN4O3 |
405.2 |
18.7 |
92 |
|
实施例421 |
1236 |
C15H24IN5O3 |
498.0 |
23.9 |
96 |
|
实施例422 |
1237 |
C15H23F2N5O3 |
408.2 |
19.0 |
93 |
|
实施例423 |
1238 |
C18H25N5O3 |
360.0 |
16.3 |
91 |
|
实施例424 |
1239 |
C25H28N4O3S |
481.2 |
21.4 |
89 |
|
实施例425 |
1240 |
C15H23N5O6 |
418.0 |
19.9 |
95 |
|
实施例426 |
1241 |
C23H24Cl2F3N3O2 |
502.0 |
22.5 |
90 |
|
实施例427 |
1242 |
C23H27ClFN3O2 |
432.2 |
21.2 |
98 |
|
实施例428 |
1243 |
C23H27Cl2N3O2 |
448.0 |
21.6 |
96 |
|
实施例429 |
124 4 |
C22H26ClIN4O2 |
541.0 |
26.4 |
98 |
|
实施例430 |
124 5 |
C22H25ClF2N4O2 |
451.0 |
21.3 |
94 |
|
实施例431 |
1246 |
C21H27ClN4O2 |
403.2 |
19.4 |
96 |
|
实施例432 |
1247 |
C28H30ClN3O2S |
524.0 |
24.7 |
94 |
|
实施例433 |
1248 |
C22H25ClN4O5 |
461.0 |
20.7 |
90 |
|
实施例434 |
1249 |
C20 H20 Cl2 N4 O4 |
451.0 |
7.4 |
33 |
|
实施例435 |
1250 |
C21 H23 Cl N4 O4 |
431.2 |
15.5 |
72 |
|
实施例436 |
1251 |
C19 H22 Cl N5 O5 |
436.0 |
22.9 |
quant |
|
实施例437 |
1252 |
C23 H28 Cl N3 O2 |
414.2 |
17.9 |
86 |
|
实施例438 |
1253 |
C24 H31 N3 O2 |
394.2 |
15.8 |
80 |
|
实施例439 |
1254 |
C22 H30 N4 O3 |
399.2 |
17.3 |
87 |
|
实施例440 |
1255 |
C20 H22 Br Cl N4 O2 |
467.0 |
21.3 |
91 |
|
实施例441 |
1256 |
C21 H25 Br N4 O2 |
445.0 |
20.7 |
93 |
|
实施例442 |
1257 |
C19 H24 Br N5 O3 |
450.0 |
21.8 |
97 |
|
实施例443 |
1258 |
C21 H25 Cl N4O2 |
401.2 |
18.1 |
90 |
|
实施例444 |
1259 |
C19 H24 Cl N5 O3 |
406.0 |
20.1 |
99 |
|
实施例445 |
1260 |
C23 H29 N3 O3 |
396.2 |
16.8 |
85 |
|
实施例446 |
1261 |
C23 H30 Cl N3 O3 |
432.2 |
19.8 |
92 |
|
实施例447 |
1262 |
C24 H33 N3 O3 |
412.2 |
17.4 |
85 |
|
实施例448 |
1263 |
C22 H32 N4 O4 |
417.2 |
18.7 |
90 |
|
实施例449 |
1264 |
C25 H26 Cl N3 O3 |
452.2 |
29.1 |
quant |
|
实施例450 |
1265 |
C26 H29 N3 O3 |
432.2 |
18.1 |
84 |
|
实施例451 |
1266 |
C24 H28 N4 O4 |
437.2 |
19.3 |
88 |
|
实施例452 |
1267 |
C23H22ClF3N4O3 |
495.2 |
20.6 |
83 |
|
实施例453 |
1268 |
C21H23Cl2N3O3 |
436.0 |
17.5 |
80 |
|
实施例454 |
1269 |
C20H21BrClN3O3 |
468.0 |
19.2 |
82 |
|
实施例455 |
1270 |
C20H21Cl2N3O3 |
422.2 |
17.3 |
82 |
|
实施例456 |
1271 |
C20H20ClFN4O4 |
435.0 |
17.1 |
79 |
|
实施例457 |
1272 |
C24H25F3N4O3 |
475.2 |
21.7 |
91 |
|
实施例458 |
1273 |
C22H26ClN3O3 |
416.2 |
17.8 |
86 |
|
实施例459 |
1274 |
C21H24BrN3O3 |
448.0 |
19.5 |
87 |
|
实施例460 |
1275 |
C21H24ClN3O3 |
402.2 |
16.7 |
83 |
|
实施例461 |
1276 |
C21H23FN4O4 |
415.2 |
18.1 |
87 |
|
实施例462 |
1277 |
C22H24F3N5O4 |
480.2 |
20.3 |
85 |
|
实施例463 |
1278 |
C20H25ClN4O4 |
421.2 |
18.6 |
88 |
|
实施例464 |
1279 |
C15H23BrN4O4 |
451.0 |
21.3 |
94 |
|
实施例465 |
1280 |
C15H23ClN4O4 |
407.2 |
19.1 |
94 |
|
实施例466 |
1281 |
C15H22FN5O5 |
420.2 |
19.1 |
91 |
|
实施例467 |
1282 |
C25H26ClF3N4O3 |
523.2 |
25.0 |
96 |
|
实施例468 |
1283 |
C23H27Cl2N3O3 |
464.2 |
12.2 |
53 |
|
实施例469 |
1284 |
C22H25BrClN3O3 |
496.0 |
24.1 |
97 |
|
实施例470 |
1285 |
C22H25Cl2N3O3 |
450.2 |
21.8 |
97 |
|
实施例471 |
1321 |
C20H20BrCl2N3O2 |
486.0 |
5.1 |
21 |
|
实施例472 |
1322 |
C21H23Cl2N3O2 |
420.0 |
10.5 |
50 |
|
实施例473 |
1323 |
C20H20Cl2IN3O2 |
532.0 |
7.1 |
27 |
|
实施例474 |
1324 |
C21H24ClN3O3 |
402.2 |
22.2 |
quant |
|
实施例475 |
1325 |
C27H26ClN3O3 |
476.0 |
22.2 |
93 |
|
实施例476 |
1326 |
C20H21ClIN3O3 |
514.0 |
26.9 |
quant |
|
实施例477 |
1327 |
C21H25ClN4O2 |
401.2 |
24.2 |
quant |
|
实施例478 |
1328 |
C21H23BrClN3O2 |
466.0 |
23.1 |
99 |
|
实施例479 |
1329 |
C22H26ClN3O2 |
400.2 |
16.4 |
82 |
|
实施例480 |
1330 |
C21H23ClIN3O2 |
512.2 |
20.8 |
81 |
|
实施例481 |
1331 |
C21H24N3O3 |
382.2 |
19.6 |
quant |
|
实施例482 |
1332 |
C28H25N3O3 |
456.2 |
21.1 |
93 |
|
实施例483 |
1333 |
C21H24IN3O3 |
494.0 |
25.3 |
quant |
|
实施例484 |
1334 |
C22H22N4O2 |
381.2 |
19.0 |
quant |
|
实施例485 |
1335 |
C15H22BrClN4O3 |
471.0 |
25.8 |
quant |
|
实施例486 |
1336 |
C20H25ClN4O3 |
405.2 |
18.5 |
91 |
|
实施例487 |
1337 |
C15H22ClIN4O3 |
517.0 |
23.1 |
89 |
|
实施例488 |
1338 |
C20H26N4O4 |
387.2 |
20.6 |
quant |
|
实施例489 |
1339 |
C26H28N4O4 |
461.2 |
23.7 |
quant |
|
实施例490 |
1340 |
C15H23IN4O4 |
499.0 |
28.2 |
quant |
|
实施例491 |
1341 |
C20H26N4O4 |
386.0 |
20.5 |
quant |
|
实施例492 |
1342 |
C22H24BrCl2N3O2 |
514.0 |
27.2 |
quant |
|
实施例493 |
1343 |
C23H27Cl2N3O2 |
448.0 |
21.4 |
95 |
|
实施例494 |
1344 |
C22H24Cl2IN3O2 |
560.0 |
27.0 |
96 |
|
实施例495 |
1345 |
C23H28ClN3O3 |
430.2 |
23.8 |
quant |
|
实施例496 |
1346 |
C22H25ClIN3O3 |
542.0 |
29.4 |
quant |
|
实施例497 |
1347 |
C15H22ClN3O2S |
392.0 |
16.9 |
43 |
|
实施例498 |
1348 |
C20H25N3O2S |
372.2 |
6.9 |
19 |
|
实施例499 |
1349 |
C18H24N4O3S |
377.2 |
8.1 |
43 |
|
实施例500 |
1350 |
C21H26ClN3O2S |
420.0 |
13.0 |
62 |
|
实施例501 |
1351 |
C22H24BrClN4O3 |
509.2 |
5.0 |
10 |
|
实施例502 |
1352 |
C23H27BrN4O3 |
489.2 |
3.6 |
15 |
|
实施例503 |
1353 |
C21H26BrN5O4 |
494.0 |
2.8 |
11 |
|
实施例504 |
1354 |
C24H28BrClN4O3 |
537.2 |
5.2 |
19 |
|
实施例505 |
1355 |
C21 H22 Cl N5 O2 |
412.0 |
25.5 |
quant |
|
实施例506 |
1356 |
C22 H25 N5 O2 |
392.0 |
16.5 |
84 |
|
实施例507 |
1357 |
C20 H24 N6 O3 |
397.2 |
19.9 |
quant |
|
实施例508 |
1358 |
C23 H26 Cl N5 O2 |
440.2 |
21.8 |
99 |
|
实施例509 |
1368 |
C21H20Cl2F3N3O2 |
474.0 |
18.4 |
78 |
|
实施例510 |
1369 |
C24H24ClF6IN3O4 |
568.0 |
24.1 |
85 |
|
实施例511 |
1370 |
C18H19BrClN3O2S |
458.0 |
19.4 |
85 |
|
实施例512 |
1371 |
C26H26ClN3O4S |
512.2 |
22.1 |
86 |
|
实施例513 |
1372 |
C26H26ClN3O2 |
448.0 |
19.1 |
85 |
|
实施例514 |
1373 |
C22H23ClF3N3O2 |
454.2 |
16.2 |
71 |
|
实施例515 |
1374 |
C25H27F6IN3O4 |
548.2 |
22.1 |
81 |
|
实施例516 |
1375 |
C15H22BrN3O2S |
436.0 |
17.1 |
78 |
|
实施例517 |
1376 |
C27H29N3O4S |
492.0 |
19.4 |
79 |
|
实施例518 |
1377 |
C27H29N3O2 |
428.2 |
18.1 |
85 |
|
实施例519 |
1378 |
C20H22ClF3N4O3 |
459.0 |
17.3 |
75 |
|
实施例520 |
1379 |
C23H26F6IN4O5 |
553.2 |
21.0 |
76 |
|
实施例521 |
1380 |
C17H21BrN4O3S |
443.0 |
16.4 |
74 |
|
实施例522 |
1381 |
C25H28N4O5S |
497.0 |
18.4 |
74 |
|
实施例523 |
1382 |
C25H28N4O3 |
433.2 |
17.3 |
80 |
|
实施例524 |
1383 |
C23H24Cl2F3N3O2 |
502.0 |
20.0 |
80 |
|
实施例525 |
1384 |
C20H23BrClN3O2S |
486.0 |
21.0 |
87 |
|
实施例526 |
1385 |
C28H30ClN3O4S |
540.2 |
23.8 |
88 |
|
实施例527 |
1386 |
C28H30ClN3O2 |
476.0 |
20.0 |
84 |
|
实施例528 |
1411 |
C22H24Cl2N4O3 |
463.0 |
0.4 |
2 |
|
实施例529 |
1412 |
C23H27ClN4O2 |
443.0 |
1.3 |
6 |
|
实施例530 |
1413 |
C21H26ClN5O4 |
448.0 |
1.1 |
5 |
|
实施例531 |
1414 |
C24H28Cl2N4O3 |
491.0 |
0.8 |
3 |
|
实施例532 |
1415 |
C21H23ClN5O2S |
44 4.0 |
6.8 |
31 |
|
实施例533 |
1416 |
C22H25N5O2S |
424.0 |
4.8 |
23 |
|
实施例534 |
1417 |
C20H24N6O3S |
429.2 |
4.5 |
21 |
|
实施例535 |
1418 |
C23H26ClN5O2S |
472.0 |
10.4 |
44 |
|
实施例536 |
1423 |
C27 H26 Cl N3 O3 |
476.0 |
23.9 |
quant |
|
实施例537 |
1424 |
C27 H29 N3 O4 S |
456.2 |
28.0 |
quant |
|
实施例538 |
1425 |
C26 H28 N4 O4 |
461.2 |
22.3 |
97 |
|
实施例539 |
1426 |
C29 H30 Cl N3 O3 |
504.2 |
26.8 |
quant |
|
实施例540 |
1583 |
C21 H22 Cl F3 N4 O2 |
455.0 |
14.6 |
64 |
|
实施例541 |
1584 |
C21 H22 Cl F3 N4 O3 |
471.0 |
17.4 |
74 |
|
实施例542 |
1585 |
C19 H20 Br Cl N4 O2 |
453.0 |
15.6 |
69 |
|
实施例543 |
1586 |
C19 H20 Cl2 N4 O2 |
407.2 |
2.3 |
11 |
|
实施例544 |
1587 |
C26 H26 Cl N3 O3 |
464.0 |
15.4 |
66 |
|
实施例545 |
1588 |
C20 H23 Cl N4 O2 |
387.0 |
14.8 |
77 |
|
实施例546 |
1589 |
C22 H25 F3 N4 O2 |
435.2 |
11.1 |
51 |
|
实施例547 |
1590 |
C20 H25 F3 N4 O3 |
451.2 |
16.3 |
72 |
|
实施例548 |
1591 |
C20 H23 Br N4 O2 |
433.0 |
15.4 |
71 |
|
实施例549 |
1592 |
C20 H23 Cl N4 O2 |
387.0 |
15.6 |
81 |
|
实施例550 |
1593 |
C27 H29 N3 O3 |
444.2 |
14.8 |
67 |
|
实施例551 |
1594 |
C20 H24 F3 N5 O3 |
440.2 |
16.2 |
74 |
|
实施例552 |
1595 |
C20 H24 F3 N5 O4 |
456.2 |
15.4 |
68 |
|
实施例553 |
1596 |
C18 H22 Br N5 O3 |
436.0 |
15.6 |
72 |
|
实施例554 |
1597 |
C18 H22 Cl N5 O3 |
391.8 |
14.4 |
73 |
|
实施例555 |
1598 |
C25 H28 N4 O4 |
449.2 |
15.9 |
71 |
|
实施例556 |
1599 |
C19 H25 N5 O3 |
372.2 |
15.8 |
85 |
|
实施例557 |
1606 |
C21 H21 Cl F3 N3 O2 S |
472.0 |
17.0 |
72 |
|
实施例558 |
1607 |
C21 H21 Cl F3 N3 O2 S |
452.2 |
15.3 |
68 |
|
实施例559 |
1608 |
C20 H23 F3 N4 O3 S |
457.2 |
15.9 |
70 |
|
实施例560 |
1660 |
C21 H22 Br F3 N4 O2 |
501.0 |
19.0 |
76 |
|
实施例561 |
1661 |
C21 H22 Br F3 N4 O3 |
517.0 |
16.2 |
63 |
|
实施例562 |
1662 |
C20 H21 Br F2 N4 O2 |
469.0 |
15.1 |
65 |
|
实施例563 |
1663 |
C20 H22 Br Cl N4 O2 |
467.0 |
14.5 |
62 |
|
实施例564 |
1692 |
C20 H23 Br2 N3 O3 |
514 |
7.3 |
28 |
|
实施例565 |
1693 |
C22 H26 F2 N4 O2 |
417 |
16.2 |
78 |
|
实施例566 |
1694 |
C22 H27 F N4 O2 |
399 |
21.8 |
quant |
|
实施例567 |
1695 |
C22 H27 Br N4 O2 |
459 |
24.5 |
quant |
|
实施例568 |
1696 |
C22 H27 I N4 O2 |
507 |
27.4 |
quant |
|
实施例569 |
1697 |
C22 H27 Cl N4 O2 |
415 |
22.1 |
quant |
|
实施例570 |
1698 |
C23 H27 F3 N4 O3 |
465 |
24.3 |
quant |
|
实施例571 |
1699 |
C23 H27 F3 N4 O2 |
449 |
25.3 |
quant |
|
实施例572 |
1700 |
C22 H25 Br Cl N3 O2 |
480 |
17.8 |
74 |
例如,1583号化合物显示下列NMR谱:
.1H NMR(400MHz,CD3OD)δ1.64-1.72(m,1H),2.20-2.30(m,1H),2.41-2.51(m,2H),2.71-2.78(m,2H),3.59(dd,J=15.4,12.9Hz,2H),3.94(s,2H),4.35-4.41(m,1H),6.82(d,J=8.6Hz,1H),7.29(s,4H),7.40(dd,J=8.6,1.7Hz,1H),7.85(d,J=0.96Hz,1H).
参考例4:(S)-3-[N-{3-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷的制备
将(S)-1-(4-氯苄基)-3-[N-{3-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷(2.93g,6.66mmol)与Pd(OH)2在5%HCO2H/甲醇(70ml)中的混悬液在60℃下搅拌3小时。通过硅藻土滤出Pd催化剂,滤液浓缩。向残余物中加入2NNaOH水溶液(100ml),混合物用乙酸乙酯萃取(100ml×3)。合并了的萃取液用盐水洗涤,经无水硫酸钠干燥,过滤,并浓缩。柱色谱(SiO2,EtOAc/MeOH/Et3N=85/10/5-60/30/5)得到(S)-3-[N-{3-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷(1.70g,81%),为一种油: 1HNMR(CDCl3,270MHz)δ1.76(d,J=7.3Hz,1H),2.07-2.25(m,1H),2.81-2.98(m,2H),3.02-3.11(m,2H),4.12(s,2H),4.41(br,1H),6.90(br,1H),7.45(br,1H),7.58(dd,J=7.3 and 7.3Hz,1H),7.77(d,J=7.3Hz,1H),8.02(d,J=7.3Hz,1H),8.11(s,1H);ESI/MS m/e 316.0(M++H,C14H16F3N3O2).
依据上述方法,使用相应的试剂,也制备了(R)-3-[N-{3-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷:1.49g,68%;该产物显示与(S)-异构体相同的1H NMR和ESI/MS。
依据上述方法,使用相应的试剂,也制备了(R)-3-[N-{2-氨-5-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷:316mg,93%;ESI/MS m/e 331.2(M++H,C14H17F3N4O2)。
依据上述方法,使用相应的试剂,也制备了(R)-3-[N-{2-(叔丁氧羰基氨基)-5-(三氟甲氧基)苯甲酰基}甘氨酰]氨基吡咯烷:定量; 1H NMR(CDCl3,400MHz)δ1.51(s,9H),1.60-1.70(m,2H),2.10-2.25(m,1H),2.80-2.88(m,1H),2.89-2.98(m,1H),3.04-3.18(m,2H),4.05(d,J=4.9Hz,2H),4.43(br,1H),6.15(br,1H),7.03(br,1H),7.32(d,J=9.3Hz,1H),7.38(s,1H),8.42(d,J=9.3Hz,1H).
实施例573:(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4-氯苄基)吡咯烷的制备
将(R)-1-(4-氯苄基)-3-(甘氨酰氨基)吡咯烷(5.0g,18.7mmol)的二氯甲烷(100ml)溶液用Et3N(2.9ml,20.5mmol)、2-(叔丁氧羰基氨基)-5-(三氟甲基)苯甲酸(6.27g,20.5mmol)、EDCI(3.9g,20.5mmol)和HOBt(2.8g,20.5mmol)处理。反应混合物在室温下搅拌过夜。向反应混合物中加入2N NaOH水溶液(80ml),混合物用二氯甲烷萃取。萃取液经无水Na2SO4干燥,过滤,并蒸发。柱色谱(SiO2,己烷/乙酸乙酯=1/1-1/4)得到(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4-氯苄基)吡咯烷(9.41g,91%),为白色无定形固体:ESI/MS m/e 555.2(M++H,C26H30ClF3N4O4)。
参考例5:(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷的制备
将(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4-氯苄基)吡咯烷(6.3g,11.4mmol)、Pd(OH)2(1.68g)、HCO2H(3.7ml)和甲醇(80ml)的混合物在50℃下搅拌过夜。混合物冷却至室温后,通过硅藻土滤出Pd催化剂,滤液浓缩。柱色谱(SiO2,AcOEt,AcOEt/MeOH=5/1-4/1)得到(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(4.42g,90%),为白色固体:
1H NMR(CDCl3,400MHz)δ1.48(s,9H),2.0-2.4(m,2H),3.42-3.71(m,5H),4.00-4.22(m,2H),4.56(br,1H),7.48(d,J=9.0Hz,1H),7.93(s,1H),8.17(br,1H),8.33(d,J=9.0Hz,1H),8.45(br,1H).
实施例574:(S)-1-苄基-3-[N-{3-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷(239号化合物)的制备
向苄基溴(0.050mmol)的CH3CN(0.4ml)溶液中加入(S)-3-[N-{3-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷(0.060mmol)在CH3CN(1.1ml)与哌啶子基甲基聚苯乙烯(2.6-2.8mmol/g,30mg)中的溶液。反应混合物在45℃下搅拌5小时。混合物冷却至室温后,过滤除去树脂,滤液浓缩。将残余物溶于CH3CN(1.0ml),加入异氰酸苯基酯(0.008ml,0.05mmol)。混合物在室温下搅拌1小时,装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3的CH3OH(6ml)溶液洗脱产物,并浓缩,得到(S)-1-苄基-3-[N-{3-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷(239号化合物)(9.0mg,44%):纯度用RPLC/MS测定(99%);ESI/MS m/e 406.0(M++H,C21H22F3N3O2)。
实施例575:(R)-1-(4-丁基苄基)-3-[{N-(3-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(1648号化合物)的制备
向(R)-3-[N-{3-(三氟甲基)苯甲酰基}甘氨酰]氨基吡咯烷(0.050mmol)、4-丁基苯甲醛(0.18mmol)、NaBH3CN(0.23mmol)和甲醇(1.85ml)的混合物中加入乙酸(0.060ml)。反应混合物在60℃下搅拌12小时。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到(R)-1-(4-丁基苄基)-3-[{N-(3-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(1648号化合物)(20.6mg,89%):纯度用RPLC/MS测定(91%);ESI/MS m/e 462.2(M++H,C25H30F3N3O2)。
实施例576-738
依据实施例574或575的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC或色谱法(HPLC-C18)得到所需物质。ESI/MS数据和产率总结在表8中。
表8
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg ) |
产率(%) |
|
实施例576 |
240 |
C21H21F4N3O2 |
424.0 |
10.2 |
48 |
|
实施例577 |
241 |
C21H21ClF3N3O2 |
440.0 |
12.1 |
55 |
|
实施例578 |
242 |
C21H20Cl2F3N3O2 |
474.0 |
13.9 |
59 |
|
实施例579 |
243 |
C21H20Cl2F3N3O2 |
474.0 |
13.8 |
58 |
|
实施例349 |
244 |
C22H24F3N3O2 |
420.0 |
13.1 |
62 |
|
实施例581 |
245 |
C21H21F4N3O2 |
424.0 |
11.9 |
56 |
|
实施例582 |
246 |
C21H21ClF3N3O2 |
440.0 |
8.5 |
39 |
|
实施例583 |
247 |
C21H20Cl2F3N3O2 |
474.0 |
10.5 |
44 |
|
实施例584 |
248 |
C22H24CF3N3O3 |
436.0 |
11.0 |
51 |
|
实施例585 |
249 |
C22H21ClF6N3O2 |
474.0 |
12.8 |
54 |
|
实施例586 |
250 |
C22H24F3N3O2 |
420.0 |
11.0 |
52 |
|
实施例587 |
251 |
C21H21F4N3O2 |
424.0 |
13.5 |
64 |
|
实施例588 |
252 |
C22H24F3N3O3 |
436.0 |
11.8 |
54 |
|
实施例589 |
253 |
C22H34F3N3O2 |
420.0 |
11.1 |
53 |
|
实施例590 |
254 |
C21H20ClF3N4O4 |
485.0 |
2.4 |
10 |
|
实施例591 |
255 |
C21H21F3N4O4 |
451.0 |
12.2 |
54 |
|
实施例592 |
256 |
C21H21F3N4O4 |
451.0 |
11.4 |
51 |
|
实施例593 |
257 |
C22H21F6N3O2 |
474.0 |
11.1 |
47 |
|
实施例594 |
258 |
C24H26F3N3O4 |
478.0 |
15.3 |
64 |
|
实施例595 |
259 |
C22H23ClF3N3O2 |
420.0 |
6.4 |
31 |
|
实施例596 |
260 |
C21H20Cl2F3N3O2 |
474.0 |
12.1 |
51 |
|
实施例597 |
261 |
C22H21ClF6N3O2 |
474.0 |
13.6 |
57 |
|
实施例598 |
262 |
C21H21BrF3N3O2 |
484.0 |
15.2 |
63 |
|
实施例599 |
263 |
C21H21BrF3N3O2 |
484.0 |
14.5 |
60 |
|
实施例600 |
264 |
C27H26F3N3O3 |
498.0 |
9.3 |
37 |
|
实施例601 |
265 |
C21H21BrF3N3O2 |
484.0 |
11.6 |
48 |
|
实施例602 |
266 |
C22H22F3N3O4 |
450.0 |
8.9 |
40 |
|
实施例603 |
267 |
C22H24F3N3O3 |
436.0 |
10.3 |
47 |
|
实施例604 |
268 |
C23H25F3N4O3 |
463.0 |
6.3 |
27 |
|
实施例605 |
269 |
C22H24F3N3O4S |
484.0 |
8.0 |
33 |
|
实施例606 |
270 |
C23H24F3N3O4 |
464.0 |
8.9 |
38 |
|
实施例607 |
271 |
C21H20F5N3O2 |
442.0 |
6.1 |
28 |
|
实施例608 |
272 |
C21H22F3N3O3 |
422.0 |
13.6 |
59 |
|
实施例609 |
273 |
C22H21F3N4O2 |
431.0 |
12.6 |
59 |
|
实施例610 |
274 |
C22H21F3N4O2 |
431.0 |
7.7 |
36 |
|
实施例611 |
275 |
C22H21F3N4O2 |
431.0 |
12.7 |
59 |
|
实施例612 |
276 |
C21H20F5N3O2 |
442.0 |
11.7 |
53 |
|
实施例613 |
277 |
C27H26F3N3O2 |
482.0 |
9.5 |
39 |
|
实施例614 |
278 |
C23H24F3N3O4 |
464.0 |
13.0 |
56 |
|
实施例615 |
279 |
C22H21F6N3O3 |
490.0 |
10.4 |
42 |
|
实施例616 |
280 |
C22H21F6N3O3 |
490.0 |
12.0 |
49 |
|
实施例617 |
281 |
C22H22F3N3O4 |
450.0 |
4.9 |
22 |
|
实施例618 |
282 |
C25H30F3N3O2 |
462.0 |
12.0 |
52 |
|
实施例619 |
283 |
C30H23F3N4O3 |
425.0 |
8.1 |
38 |
|
实施例620 |
284 |
C27H25ClF3N3O2 |
516.0 |
4.8 |
19 |
|
实施例621 |
285 |
C21H22F3N3O2 |
406.0 |
4.8 |
24 |
|
实施例622 |
286 |
C21H21F4N3O2 |
424.0 |
4.5 |
21 |
|
实施例623 |
287 |
C21H21ClF3N3O2 |
440.0 |
5.8 |
26 |
|
实施例624 |
288 |
C23H20Cl2F3N3O2 |
474.0 |
8.1 |
34 |
|
实施例625 |
289 |
C21H20Cl2F3N3O2 |
474.0 |
8.0 |
34 |
|
实施例626 |
290 |
C22H24F3N3O2 |
420.0 |
6.0 |
29 |
|
实施例627 |
291 |
C21H21F4N3O2 |
424.0 |
6.2 |
29 |
|
实施例628 |
292 |
C21H21ClF3N3O2 |
440.0 |
4.5 |
20 |
|
实施例629 |
293 |
C21H24Cl2F3N3O2 |
474.0 |
5.1 |
22 |
|
实施例630 |
294 |
C22H24CF3N3O3 |
436.0 |
4.2 |
19 |
|
实施例631 |
295 |
C22H21ClF6N3O2 |
474.0 |
6.0 |
25 |
|
实施例632 |
296 |
C22H24F3N3O2 |
420.0 |
4.3 |
21 |
|
实施例633 |
297 |
C21H21F4N3O2 |
424.0 |
8.2 |
39 |
|
实施例634 |
298 |
C22H24F3N3O3 |
436.0 |
12.2 |
56 |
|
实施例635 |
299 |
C22H24F3N3O2 |
420.0 |
8.1 |
39 |
|
实施例636 |
300 |
C21H20ClF3N4O4 |
485.0 |
13.7 |
57 |
|
实施例637 |
301 |
C21H21F3N4O4 |
451.0 |
15.1 |
67 |
|
实施例638 |
302 |
C21H21F3N4O4 |
451.0 |
16.6 |
74 |
|
实施例639 |
303 |
C22H21F6N3O2 |
474.0 |
12.6 |
53 |
|
实施例640 |
304 |
C24H26F3N3O4 |
478.0 |
14.5 |
61 |
|
实施例641 |
305 |
C22H23ClF3N3O2 |
420.0 |
8.4 |
37 |
|
实施例642 |
306 |
C21H20Cl2F3N3O2 |
474.0 |
13.5 |
57 |
|
实施例643 |
307 |
C22H21ClF6N3O2 |
474.0 |
3.7 |
16 |
|
实施例644 |
308 |
C21H21BrF3N3O2 |
484.0 |
7.2 |
30 |
|
实施例645 |
309 |
C21H21BrF3N3O2 |
484.0 |
6.7 |
28 |
|
实施例646 |
310 |
C27H26F3N3O3 |
498.0 |
4.2 |
17 |
|
实施例647 |
311 |
C21H21BrF3N3O2 |
484.0 |
6.3 |
26 |
|
实施例648 |
312 |
C22H22F3N3O4 |
450.0 |
2.4 |
11 |
|
实施例649 |
313 |
C22H24F3N3O3 |
436.0 |
1.9 |
9 |
|
实施例650 |
314 |
C23H25F3N4O3 |
463.0 |
5.0 |
22 |
|
实施例651 |
315 |
C22H24F3N3O4S |
484.0 |
2.5 |
10 |
|
实施例652 |
316 |
C23H24F3N3O4 |
464.0 |
3.3 |
14 |
|
实施例653 |
317 |
C21H23F5N3O2 |
442.0 |
4.5 |
20 |
|
实施例654 |
318 |
C21H22F3N3O3 |
422.0 |
7.9 |
34 |
|
实施例655 |
319 |
C22H21F3N4O2 |
431.0 |
6.5 |
30 |
|
实施例656 |
320 |
C22H21F3N4O2 |
431.0 |
14.2 |
66 |
|
实施例657 |
321 |
C22H21F3N4O2 |
431.0 |
14.9 |
69 |
|
实施例658 |
322 |
C21H24F5N3O2 |
442.0 |
13.6 |
62 |
|
实施例659 |
323 |
C27H24F3N3O2 |
482.0 |
3.9 |
16 |
|
实施例660 |
324 |
C23H24F3N3O4 |
464.0 |
15.2 |
66 |
|
实施例661 |
325 |
C23H21F6N3O3 |
490.0 |
16.1 |
66 |
|
实施例662 |
326 |
C22H21F6N3O3 |
490.0 |
13.6 |
56 |
|
实施例663 |
327 |
C22H21F3N3O4 |
450.0 |
5.4 |
24 |
|
实施例664 |
328 |
C25H24F3N3O2 |
462.0 |
10.9 |
47 |
|
实施例665 |
329 |
C20H23F3N4O3 |
425.0 |
12.0 |
57 |
|
实施例666 |
986 |
C27 H25 Cl F3 N3 O2 |
516.0 |
1.5 |
6 |
|
实施例667 |
1118 |
C28 H27 F3 N4 O3 |
525 |
21.5 |
62 |
|
实施例668 |
1119 |
C22 H24 F3 N3 O2 S |
452 |
16.9 |
57 |
|
实施例669 |
1120 |
C23 H26 F3 N3 O4 |
466 |
20.5 |
67 |
|
实施例670 |
1121 |
C22 H23 F3 N4 O4 |
465 |
16.8 |
55 |
|
实施例671 |
1122 |
C28 H36 F3 N3 O2 |
504 |
21.0 |
63 |
|
实施例672 |
1123 |
C25 H23 Br F3 N3 O2 |
534 |
26.6 |
75 |
|
实施例673 |
1124 |
C19 H19 F3 N4 O5 |
441 |
21.3 |
73 |
|
实施例674 |
1133 |
C23 H26 F3 N3 O4 |
467 |
33.6 |
84 |
|
实施例675 |
1134 |
C24 H28 F3 N3 O5 |
496 |
34.8 |
82 |
|
实施例676 |
1135 |
C22 H21 F3 N4 O6 |
495 |
32.6 |
77 |
|
实施例677 |
1136 |
C23 H24 F3 N3 O5 |
480 |
36.6 |
89 |
|
实施例678 |
1137 |
C22 H21 Br F3 N3 O4 |
529 |
30.8 |
69 |
|
实施例679 |
1138 |
C24 H26 F3 N3 O2 |
446 |
32.7 |
86 |
|
实施例680 |
1139 |
C22 H24 F3 N3 O2 |
420 |
18.6 |
51 |
|
实施例681 |
1140 |
C21 H20 F3 N5 O6 |
496 |
20.5 |
49 |
|
实施例682 |
1141 |
C25 H24 F3 N3 O2 |
456 |
22.5 |
58 |
|
实施例683 |
1142 |
C25 H24 F3 N3 O2 |
456 |
21.6 |
55 |
|
实施例684 |
1143 |
C35 H34 F3 N3 O4 |
618 |
27.3 |
53 |
|
实施例685 |
1144 |
C23 H26 F3 N3 O4 |
466 |
25.5 |
64 |
|
实施例686 |
1145 |
C23 H25 F3 N4 O6 |
511 |
38.0 |
88 |
|
实施例687 |
1146 |
C28 H28 F3 N3 O3 |
512 |
38.3 |
89 |
|
实施例688 |
1147 |
C23 H25 F3 N4 O3 |
463 |
27.1 |
62 |
|
实施例689 |
1148 |
C27 H26 F3 N3 O2 |
482 |
22.4 |
57 |
|
实施例690 |
1161 |
C22 H24 F3 N3 O4 |
452 |
13.5 |
58 |
|
实施例691 |
1162 |
C24 H28 F3 N3 O3 |
464 |
16.7 |
70 |
|
实施例692 |
1163 |
C22 H23 F4 N3 O3 |
454 |
15.8 |
68 |
|
实施例693 |
1164 |
C23 H26 F3 N3 O3 |
450 |
15.7 |
68 |
|
实施例694 |
1165 |
C23 H24 F3 N3 O4 |
464 |
16.3 |
68 |
|
实施例695 |
1166 |
C22 H23 Br F3 N3 O3 |
513 |
15.0 |
57 |
|
实施例696 |
1168 |
C17 H17 Cl F3 N5 O2 S |
448 |
6.9* |
23 |
|
实施例697 |
1169 |
C20 H22 F3 N5 O3 S |
470 |
1.7* |
6 |
|
实施例698 |
1170 |
C22 H22 F3 N5 O2 |
446 |
2.3* |
8 |
|
实施例699 |
1286 |
C26 H33 F3 N4 O3 |
507 |
25.3* |
51 |
|
实施例700 |
1287 |
C21 H20 F3 N5 O6 |
496 |
4.0* |
8 |
|
实施例701 |
1288 |
C22 H24 F3 N3 O4 |
452 |
3.6* |
13 |
|
实施例702 |
1298 |
C23 H25 Br F3 N3 O4 |
544 |
28.4 |
quant |
|
实施例703 |
1299 |
C24 H28 F3 N3 O5 |
496 |
1.4 |
6 |
|
实施例704 |
1300 |
C23 H26 F3 N3 O4 |
466 |
7.3 |
33 |
|
实施例705 |
1301 |
C24 H28 F3 N3 O5 |
496 |
12.6 |
53 |
|
实施例706 |
1302 |
C24 H28 F3 N3 O3 |
464 |
24.5 |
quant |
|
实施例707 |
1303 |
C23 H25 Br F3 N3 O4 |
544 |
22.2 |
86 |
|
实施例708 |
1304 |
C29 H30 F3 N3 O4 |
542 |
28.6 |
quant |
|
实施例709 |
1305 |
C26 H26 F3 N3 O3 |
486 |
35.4 |
quant |
|
实施例710 |
1306 |
C24 H28 F3 N3 O4 |
480 |
8.1 |
35 |
|
实施例711 |
1307 |
C23 H26 F3 N3 O5 |
482 |
27.9 |
quant |
|
实施例712 |
1308 |
C23 H24 F3 N3 O3 |
448 |
5.9 |
28 |
|
实施例713 |
1309 |
C23 H25 F3 I N3 O4 |
592 |
24.0 |
85 |
|
实施例714 |
1310 |
C22 H24 F3 N3 O4 |
452 |
3.4 |
16 |
|
实施例715 |
1311 |
C22 H22 F3 N3 O4 |
450 |
3.4 |
16 |
|
实施例716 |
1312 |
C21 H21 F3 I N3 O2 |
532 |
18.1 |
72 |
|
实施例717 |
1313 |
C21 H21 Br F3 N3 O2 |
484 |
17.4 |
76 |
|
实施例718 |
1314 |
C19 H19 F3 N4 O4 5 |
457 |
16.8 |
77 |
|
实施例719 |
1315 |
C20 H22 F3 N3 O3 |
410 |
13.6 |
70 |
|
实施例720 |
1316 |
C22 H20 Cl F6 N3 O2 |
508 |
18.6 |
77 |
|
实施例721 |
1317 |
C21 H20 Cl F3 N4 O4 |
485 |
17.0 |
74 |
|
实施例722 |
1318 |
C21 H20 Cl F4 N3 O2 |
458 |
17.0 |
78 |
|
实施例723 |
1319 |
C21 H20 Cl F4 N3 O2 |
458 |
17.6 |
81 |
|
实施例724 |
1320 |
C21 H20 Br F4 N3 O2 |
502 |
18.5 |
77 |
|
实施例725 |
1390 |
C26 H32 F3 N3 O2 |
476 |
16.1 |
51 |
|
实施例726 |
1391 |
C23 H26 F3 N3 O2 |
434 |
20.0 |
76 |
|
实施例727 |
1392 |
C22 H23 Cl F3 N3 O2 |
454 |
20.0 |
67 |
|
实施例728 |
1393 |
C23 H26 F3 N3 O2 |
434 |
20.1 |
70 |
|
实施例729 |
1394 |
C22 H23 F3 N4 O4 |
465 |
18.4 |
60 |
|
实施例730 |
1395 |
C23 H24 F3 N3 O2 |
432 |
21.4 |
75 |
|
实施例731 |
1396 |
C26 H26 F3 N3 O2 |
470 |
20.4 |
66 |
|
实施例732 |
1397 |
C21 H20 Br2 F3 N3 O2 |
562 |
14.5 |
54 |
|
实施例733 |
1398 |
C22 H22 Cl2 F3 N3 O2 |
488 |
10.8 |
47 |
|
实施例734 |
1399 |
C22 H22 Cl2 F3 N3 O2 |
488 |
9.4 |
40 |
|
实施例735 |
1400 |
C22 H23 Cl F3 N3 O2 |
454 |
19.1 |
88 |
|
实施例736 |
1614 |
C22 H21 F6 N3 S |
506.0 |
24.2 |
96 |
|
实施例737 |
2050 |
C20 H22 F3 N3 O2 S |
426 |
6.0 |
30 |
|
实施例738 |
2051 |
C21 H23 F3 N4 O2 |
421 |
6.5 |
32 |
*TFA盐的产量
实施例739-748
依据实施例738的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC得到所需物质。ESI/MS数据和产率总结在表9中。
表9
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例739 |
1650 |
C24 H28 F3 N3 O2 |
448.0 |
20.4 |
91 |
|
实施例740 |
1706 |
C23 H25 F3 N4 O3 |
463.2 |
3.7 |
11 |
|
实施例741 |
1707 |
C22 H25 F3 N4 O2 S |
467.0 |
10.3 |
29 |
|
实施例742 |
1708 |
C23 H27 F3 N4 O2 |
449.2 |
11.4 |
34 |
|
实施例743 |
1709 |
C24 H29 F3 N4 O2 |
463.2 |
15.2 |
44 |
|
实施例744 |
1775 |
C22 H25 F3 N4 O4 |
467.2 |
9.2 |
26.3 |
|
实施例745 |
1776 |
C22 H25 F3 N4 O4 |
467.2 |
8.9 |
25.4 |
|
实施例746 |
1787 |
C24 H29 F3 N4 O2 |
463.2 |
5.6 |
16.1 |
|
实施例747 |
1802 |
C23 H27 F3 N4 O4 |
481.2 |
11.7 |
32.5 |
|
实施例748 |
1803 |
C22 H25 F3 N4 O3 |
451.2 |
9.6 |
28.4 |
实施例749:(R)-3-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基]-1-(3-羟基-4-甲氧基苄基)吡咯烷(1896号化合物)的制备
向(R)-3-[N-{2-(叔丁氧羰基氨基)-5-(三氟甲氧基)苯甲酰基}甘氨酰]氨基吡咯烷(0.050mmol)、3-羟基-4-甲氧基苯甲醛(0.060mmol)、NaBH3CN(0.15mmol)和甲醇(1.3ml)的混合物中加入乙酸(0.050ml)。反应混合物在60℃下搅拌8小时。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH(10ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。向所得物质中加入4N HCl的1,4-二噁烷溶液,溶液在室温下搅拌过夜。经过浓缩和制备型TLC得到(R)-3-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基]-1-(3-羟基-4-甲氧基苄基)吡咯烷(1896号化合物)(9.1mg,38%):纯度用RPLC/MS测定(93%);ESI/MS m/e 483(M++H,C22H25F3N4O5)。
实施例750-757
依据实施例749的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表10中。
表10
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例750 |
1897 |
C22 H25 F3 N4 O3 S |
483 |
22.7 |
94.1 |
|
实施例751 |
1898 |
C23 H27 F3 N4 O3 |
465 |
12.2 |
52.5 |
|
实施例752 |
1899 |
C24 H29 F3 N4 O3 |
479 |
14.4 |
60.2 |
|
实施例753 |
1900 |
C22 H25 F3 N4 O5 |
483 |
2.6 |
10.8 |
|
实施例754 |
1901 |
C24 H29 F3 N4 O3 |
479 |
14.5 |
60.6 |
|
实施例755 |
1902 |
C23 H25 F3 N4 O4 |
479 |
12.0 |
50.2 |
|
实施例756 |
1915 |
C23 H27 F3 N4 O5 |
467.2 |
2.5 |
6.7 |
|
实施例757 |
1916 |
C22 H25 F3 N4 O4 |
467.2 |
3.1 |
8.9 |
实施例758:(R)-3-[{N-(2-氨基-5-(三氟甲基)苯甲酰基)甘氨酰}氨基]-1-(4-乙烯基苄基)吡咯烷(1701号化合物)的制备
将(R)-3-[{N-(2-氨基-5-(三氟甲基)苯甲酰基)甘氨酰}氨基]吡咯烷(0.050mmol)、4-乙烯基苄基氯(9.9mg,0.065mmol)、哌啶子基甲基聚苯乙烯(60mg)、乙腈(1.0ml)和氯仿(0.30ml)的混合物在50℃下搅拌12小时。反应混合物冷却,装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到(R)-3-[{N-(2-氨基-5-(三氟甲基)苯甲酰基)甘氨酰}氨基]-1-(4-乙烯基苄基)吡咯烷(1701号化合物)(19.6mg,88%):纯度用RPLC/MS测定(92%);ESI/MS m/e 547.2(M++H,C23H25ClF3N4O2)。
实施例759-762
依据实施例758的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC得到所需物质。ESI/MS数据和产率总结在表11中。
表11
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例759 |
1702 |
C22 H25 F3 N4 O3 |
451.2 |
5.3 |
24 |
|
实施例760 |
1703 |
C22 H23 F3 N4 O4 |
465.2 |
5.0 |
22 |
|
实施例761 |
1704 |
C21 H23 F3 N4 O3 |
437.2 |
20.9 |
96 |
|
实施例762 |
1705 |
C21 H21 Cl2 F3 N4 O2 |
489.2 |
9.3 |
38 |
实施例763:(R)-3-[{N-(2-氨基-5-(三氟甲氧基)苯甲酰基)甘氨酰}氨基]-1-(2,4-二氯苄基)吡咯烷(1905号化合物)的制备
将(R)-3-[{N-(2-氨基-5-(三氟甲氧基)苯甲酰基)甘氨酰}氨基]吡咯烷(0.050mmol)、2,4-二氯苄基氯(0.060mmol)、哌啶子基甲基聚苯乙烯(60mg)、乙腈(0.8ml)和氯仿(0.50ml)的混合物在60℃下搅拌12小时。反应混合物冷却,装上VarianTM SCX柱,用50%CHCl3/CH3OH(10ml)和CH3OH(10ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,溶液在室温下搅拌过夜。经过浓缩和制备型TLC,得到(R)-3-[{N-(2-氨基-5-(三氟甲氧基)苯甲酰基)甘氨酰}氨基]-1-(2,4-二氯苄基)吡咯烷(1905号化合物)(17.6mg,70%):纯度用RPLC/MS测定(93%);ESI/MS m/e 505(M++H,C21H21Cl2F3N4O3)。
实施例764-770
依据实施例763的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表12中。
表12
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例764 |
1906 |
C22 H23 F3 N4 O5 |
481 |
9.4 |
39.1 |
|
实施例765 |
1907 |
C21 H23 F3 N4 O4 |
453 |
7.5 |
33.2 |
|
实施例766 |
1908 |
C22 H25 F3 N4 O4 |
467 |
7.7 |
33.0 |
|
实施例767 |
2180 |
C22 H24 Cl F3 N4 O2 |
469 |
1.3 |
26 |
|
实施例768 |
2181 |
C23 H25 F3 N6 O3 |
491 |
4.3 |
52 |
|
实施例769 |
2182 |
C19 H22 F3 N5 O2 S |
442 |
7.0 |
51 |
|
实施例770 |
1909 |
C23 H25 F3 N4 O3 |
463 |
8.7 |
37.6 |
实施例771:(R)-3-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基]-1-(2-氨基-4-氯苄基)吡咯烷(1921号化合物)的制备
将(R)-3-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基]吡咯烷(0.050mmol)、4-氯-2-硝基苄基氯(0.050mmol)、哌啶子基甲基聚苯乙烯(60mg)、乙腈(1.0ml)和氯仿(0.7ml)的混合物在50℃下搅拌过夜。反应混合物冷却,装上VarianTM SCX柱,用50%CHCl3/CH3OH(10ml)和CH3OH(10ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。向所得物质中加入乙醇(3ml)和10%Pd-C(15mg),混合物在室温H2下搅拌1.5小时。经过过滤、浓缩和制备型TLC,得到(R)-3-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基]-1-(2-氨基-4-氯苄基)吡咯烷(1921号化合物)(2.2mg,6%):纯度用RPLC/MS测定(81%);ESI/MS m/e 486.2(M++H,C21H23ClF3N5O3)。
实施例772:(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(2-溴-4-氟苄基)吡咯烷(2120号化合物)的制备
向(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(0.050mmol)、4-溴-2-氟苯甲醛(0.15mmol)、甲醇(1.5ml)和乙酸(0.016ml)的混合物中加入NaBH3CN(0.25mmol)的甲醇(0.50ml)溶液。反应混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。将残余物溶于甲醇(0.25ml),加入4N HCl的二噁烷(0.50ml)溶液。溶液在室温下搅拌5小时,并浓缩。将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。将所得物质溶于乙酸乙酯(0.5ml),装上VarianTM Si柱,用乙酸乙酯/甲醇=5∶1(6ml)洗脱,并浓缩,得到(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(2-溴-4-氟苄基)吡咯烷(2120号化合物)(16.0mg,31%):纯度用RPLC/MS测定(99%);ESI/MS m/e 517.0(M++H,C21H21BrF4N4O2)。
实施例773-793
依据实施例772的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表13中。
表13
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例773 |
2083 |
C22 H24 Br F3 N4 O4 |
545.2 |
2.9 |
11 |
|
实施例774 |
2084 |
C23 H27 F3 N4 O5 |
497.2 |
5.1 |
21 |
|
实施例775 |
2085 |
C22 H25 F3 N4 O4 |
467.2 |
3.1 |
13 |
|
实施例776 |
2086 |
C21 H22 Cl F3 N4 O3 |
471.0 |
4.6 |
20 |
|
实施例777 |
2087 |
C23 H28 F3 N5 O2 |
464.2 |
5.6 |
24 |
|
实施例778 |
2088 |
C25 H32 F3 N5 O2 |
492.2 |
5.9 |
24 |
|
实施例779 |
2089 |
C21 H21 F5 N4 O2 |
457.2 |
4.5 |
20 |
|
实施例780 |
2090 |
C27 H27 F3 N4 O3 |
513.2 |
8.0 |
31 |
|
实施例781 |
2118 |
C21 H23 F3 N4 O4 |
453.1 |
2.7 |
12 |
|
实施例782 |
2119 |
C21 H23 F3 N4 O4 |
453.1 |
4.3 |
19 |
|
实施例783 |
2121 |
C22 H25 F3 N4 O4 |
467.0 |
1.2 |
2 |
|
实施例784 |
2122 |
C21 H21 Cl F4 N4 O2 |
472.9 |
13.1 |
28 |
|
实施例785 |
2123 |
C22 H22 F3 N5 O6 |
510.1 |
13.1 |
51 |
|
实施例786 |
2124 |
C21 H21 Cl F3 N5 O4 |
500.1 |
15.6 |
62 |
|
实施例787 |
2125 |
C22 H24 F3 N5 O5 |
496.0 |
16.0 |
65 |
|
实施例788 |
2126 |
C22 H24 F3 N5 O4 |
480.1 |
15.6 |
65 |
|
实施例789 |
2137 |
C22 H24 Cl F3 N4 O2 |
469.2 |
2.6 |
11 |
|
实施例790 |
2138 |
C26 H29 F3 N6 O2 |
515.3 |
25.1 |
98 |
|
实施例791 |
2139 |
C20 H24 Cl F3 N6 O2 |
473.2 |
25.0 |
98 |
|
实施例792 |
2149 |
C21 H22 F3 N5 O5 |
4 82.3 |
4.9 |
34 |
|
实施例793 |
2157 |
C22 H25 F3 N4 O3 |
451.2 |
15.5 |
70 |
实施例794:(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(2,4-二甲氧基嘧啶-5-基甲基)吡咯烷(2175号化合物)的制备
将(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(17.2mg,0.04mmol)溶于THF(1ml),加入2,4-二甲氧基-5-嘧啶甲醛(6.7mg,0.04mmol),然后加入三乙酰氧基硼氢化钠(12.7mg,0.06mmol)和冰乙酸(2.4mg,0.04mmol)。混合物在室温下搅拌24小时,并蒸发。然后将残余物溶于二氯甲烷(1ml),用1N NaOH溶液(1ml)洗涤。回收有机相,蒸发,然后在室温下用25%三氟乙酸的二氯甲烷(1ml)溶液处理1小时,并蒸发。残余物用LC/MS纯化,得到(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(2,4-二甲氧基嘧啶-5-基甲基)吡咯烷(2175号化合物)(18.6mg,78%):纯度用RPLC/MS测定(98%);ESI/MS m/e 483(M++H,C21H25F3N6O4)。
实施例795-803
依据实施例794的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表14中。
表14
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例795 |
2165 |
C18 H21 F3 N6 O2 |
411 |
2.0 |
27 |
|
实施例796 |
2166 |
C18 H20 F3 N5 O2 S |
428 |
9.9 |
66 |
|
实施例797 |
2167 |
C24 H25 F3 N6 O2 |
487 |
15.1 |
73 |
|
实施例798 |
2169 |
C24 H29 F3 N4 O2 |
463 |
1.2 |
24 |
|
实施例799 |
2170 |
C26 H25 Cl F3 N5 O2 |
520 |
6.0 |
40 |
|
实施例800 |
2171 |
C19 H23 F3 N6 O2 |
425 |
16.8 |
88 |
|
实施例801 |
2174 |
C23 H24 Br F3 N4 O2 S2 |
591 |
5.3 |
53 |
|
实施例802 |
2178 |
C25 H28 F3 N5 O4 |
518 |
5.4 |
62 |
|
实施例803 |
2179 |
C25 H28 F3 N5 O3 |
502 |
6.3 |
60 |
实施例804:(R)-1-(2-氨基-4,5-亚甲二氧基苄基)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(2127号化合物)的制备
将(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4,5-亚甲二氧基-2-硝基苄基)吡咯烷(30.5mg)、10%Pd-活性碳(6mg)和甲醇(3ml)的混合物在室温氢气氛下搅拌10小时。通过硅藻土滤出Pd催化剂,滤液浓缩。固相萃取(Bond ElutTM SI,20%甲醇/AcOEt),得到(R)-1-(2-氨基-4,5-亚甲二氧基苄基)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(2127号化合物)(21.9mg,76%):纯度用RPLC/MS测定(95%);ESI/MS m/e 480.1(M++H,C22H24F3N5O4)。
实施例805和806
依据实施例804的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表15中。
表15
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例805 |
2128 |
C22 H26 F3 N5 O3 |
466.0 |
8.6 |
30 |
|
实施例806 |
2129 |
C22 H26 F3 N5 O2 |
450.1 |
13.1 |
37 |
实施例807:(R)-1-(3-氨基-4-氯苄基)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(2132号化合物)的制备
将(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4-氯-3-硝基苄基)吡咯烷(32.6mg)、10%Pd-活性碳(8mg)、乙酸乙酯(2.7ml)和甲醇(0.3ml)的混合物在室温氢气氛下搅拌15小时。滤出Pd催化剂,滤液浓缩。固相萃取(Bond ElutTM SI,20%甲醇/AcOEt),得到(R)-1-(3-氨基-4-氯苄基)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(2132号化合物)(10.5mg,34%):纯度用RPLC/MS测定(84%);ESI/MS m/e 470.2(M++H,C21H23ClF3N5O2)。
实施例808:(R)-1-(2-氨基-4,5-亚甲二氧基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷的制备
向(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(0.150mmol)、4,5-亚甲二氧基-2-硝基苯甲醛(0.45mmol)、甲醇(4.5ml)和乙酸(0.048ml)的混合物中加入NaBH3CN(0.75mmol)的甲醇(1.50ml)溶液。反应混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩,得到(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4,5-亚甲二氧基-2-硝基苄基)吡咯烷。
将上面制备的(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4,5-亚甲二氧基-2-硝基苄基)吡咯烷、10%Pd-活性碳(22mg)和甲醇(3.0ml)的混合物在室温氢气氛下搅拌过夜。滤出Pd催化剂,滤液浓缩,得到(R)-1-(2-氨基-4,5-亚甲二氧基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(87.1mg,定量):TLC没有检测到任何明显的副产物。
依据实施例808的方法,分别使用相应的试剂,也合成了(R)-1-(3-氨基-4-甲氧基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷和(R)-1-(3-氨基-4-甲基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷。
(R)-1-(3-氨基-4-甲氧基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷:101mg,定量;TLC没有检测到任何明显的副产物。
(R)-1-(3-氨基-4-甲基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷:97.2mg,定量;TLC没有检测到任何明显的副产物。
实施例809:(R)-1-(3-氨基-4-氯苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷的制备
向(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(0.150mmol)、4-氯-3-硝基苯甲醛(0.45mmol)、甲醇(4.5ml)和乙酸(0.048ml)的混合物中加入NaBH3CN(0.75mmol)的甲醇(1.50ml)溶液。反应混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩,得到(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4-氯-3-硝基苄基)吡咯烷。
将上面制备的(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(4-氯-3-硝基苄基)吡咯烷、10%Pd-活性碳(22mg)、乙酸乙酯(2.7ml)和甲醇(0.3ml)的混合物在室温氢气氛下搅拌15小时。滤出Pd催化剂,滤液浓缩,得到(R)-1-(3-氨基-4-氯苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(89.7mg,定量):TLC没有检测到任何明显的副产物。
实施例810:(R)-1-(3-氨基-4-羟基苄基)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(2187号化合物)的制备
将依据实施例808方法制备的(R)-1-(3-氨基-4-羟基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(20mg)的4N HCl二噁烷(2.0ml)溶液在室温下搅拌过夜。溶液浓缩后,将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH洗涤,用2N NH3的CH3OH溶液洗脱。经过浓缩和制备型TLC(SiO2,AcOEt/MeOH=4/1)得到(R)-1-(3-氨基-4-羟基苄基)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(2187号化合物)(9.6mg,59%):纯度用RPLC/MS测定(86%);ESI/MS m/e 452.3(M++H,C21H24F3N5O3)。
实施例811:(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-{4-氯-3-(二甲氨基)苄基}吡咯烷(2133号化合物)的制备
向(R)-1-(3-氨基-4-氯苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(44.9mg)、甲醇(0.95ml)、乙酸(0.05ml)和37%HCHO水溶液(0.15ml)的混合物中加入NaBH3CN(38mg)。反应混合物在50℃下搅拌过夜。混合物冷却至室温,并蒸发。向残余物中加入2N NaOH水溶液和乙酸乙酯,分离有机层,含水层用乙酸乙酯萃取。合并了的有机层干燥并浓缩,将残余物装上VarianTM SCX柱,用CH3OH洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩。将残余物溶于50%浓HCl/二噁烷,溶液在室温下搅拌1小时。用5N NaOH水溶液调反应混合物的pH为10,用乙酸乙酯萃取(2次)。合并了的萃取液经Na2SO4干燥,过滤,并蒸发。经过制备型TLC(SiO2,20% MeOH/AcOEt)得到(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-{4-氯-3-(二甲氨基)苄基}吡咯烷(2133号化合物)(10.9mg,28%):纯度用RPLC/MS测定(95%);ESI/MS m/e 498.3(M++H,C23H27ClF3N5O2)。
实施例812-814
依据实施例811的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表16中。
表16
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例812 |
2134 |
C28H28F3N5O4 |
508.4 |
19.0 |
50 |
|
实施例813 |
2135 |
C24H30F3N5O3 |
494-4 |
21.8 |
50 |
|
实施例814 |
2136 |
C24H30F3N5O2 |
478.4 |
29.2 |
69 |
实施例815:(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(3-甲氨基-4-羟基苄基)吡咯烷(2158号化合物)的制备
向(R)-1-(3-氨基-4-羟基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(27.3mg,0.049mmol)、37% HCHO溶液(4.0mg,0.049mmol)、乙酸(0.10ml)和甲醇(1.3ml)的混合物中加入NaBH3CN(9.2mg)的甲醇(0.2ml)溶液。反应混合物在60℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(8ml)溶液洗脱产物,并浓缩。
将所得物质溶于甲醇(1ml),加入4N HCl的二噁烷(1.0ml)溶液。溶液在室温下搅拌3小时。溶液浓缩后,将残余物溶于甲醇(1ml),装上VarianTM SCX柱,用CH3OH洗涤(5ml×2),用2N NH3的CH3OH(8ml)溶液洗脱。经过浓缩和制备型TLC(SiO2)得到(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(3-甲氨基-4-羟基苄基)吡咯烷(2158号化合物)(4.3mg,19%):纯度用RPLC/MS测定(71%);ESI/MS m/e 480.3(M++H,C22H26F3N5O3)。
实施例816:(R)-1-(3-乙酰氨基-4-甲氧基苄基)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(2152号化合物)的制备
向(R)-1-(3-氨基-4-甲氧基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(50.5mg)的吡啶(1ml)溶液中加入乙酸酐(1ml)。反应混合物在室温下搅拌过夜,加入甲醇。混合物蒸发,加入1N NaOH溶液。混合物用乙酸乙酯萃取,有机层浓缩。经过制备型TLC得到(R)-1-(3-乙酰氨基-4-甲氧基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷。
将所得(R)-1-(3-乙酰氨基-4-甲氧基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷溶于50% 6N盐酸的二噁烷溶液,溶液在室温下搅拌2小时。用5M NaOH溶液调混合物的pH为10,用乙酸乙酯萃取。有机层蒸发,经过制备型TLC(SiO2,AcOEt/MeOH=4∶1)得到(R)-1-(3-乙酰氨基-4-甲氧基苄基)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(2152号化合物)(3.7mg,8%):纯度用RPLC/MS测定(100%);ESI/MS m/e 508.3(M++H,C24H28F3N5O4)。
实施例817-819
依据实施例816的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表17中。
表17
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例817 |
2150 |
C23H25ClF3N5O3 |
512.3 |
3.8 |
9 |
|
实施例818 |
2151 |
C24H26F3N5O5 |
522.2 |
3.1 |
8 |
|
实施例819 |
2153 |
C24H28F3N5O3 |
492.3 |
4.3 |
10 |
实施例820:(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(苯并[d]噁唑-5-基)吡咯烷(2189号化合物)的制备
将依据实施例808方法制备的(R)-1-(3-氨基-4-羟基苄基)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(20mg)的THF(2ml)溶液用原甲酸三乙酯(0.020ml,3.3当量)和吡啶鎓对甲苯磺酸酯(1.2mg,0.4当量)处理。反应混合物在回流下搅拌过夜。冷却至室温后,混合物浓缩。将残余物溶于AcOEt,装上BondElutTM Si柱,用乙酸乙酯/甲醇=4/1洗脱,并浓缩。
将所得物质溶于AcOEt(1.5ml),加入4N HCl的二噁烷(0.5ml)溶液。溶液在室温下搅拌过夜,用5M NaOH水溶液调pH为10,用AcOEt萃取。萃取液浓缩,用PTLC纯化(SiO2,AcOEt/MeOH=4∶1),得到(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(苯并[d]噁唑-5-基)吡咯烷(2189号化合物)(0.5mg,3%):纯度用RPLC/MS测定(97%);ESI/MS m/e 462.3(M++H,C22H22F3N5O3)。
实施例821:(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(苯并[c]噻二唑-5-基)吡咯烷(2183号化合物)的制备
向5-(羟甲基)苯并[c]噻二唑(8.3mg,0.050mmol)、(哌啶子基甲基)聚苯乙烯(86mg)和氯仿(1ml)的混合物中加入甲磺酰氯(0.0042ml),混合物在室温下搅拌1.5小时。加入乙腈(1ml)和(R)-3-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基]吡咯烷(0.060mmol),反应混合物在50℃下搅拌3小时。冷却至室温后,加入异氰酸苯基酯(30mg),混合物在室温下搅拌1小时,装上VarianTM SCX柱,用CH3OH(5ml)和CHCl3(5ml)洗涤。用2N NH3的CH3OH(3ml)溶液洗脱产物,并浓缩。
将所得物质溶于二氯甲烷(1ml),加入1M氯三甲基硅烷和1M苯酚的二氯甲烷(1ml)溶液。溶液在室温下搅拌5小时,装上VarianTM SCX柱,用CH3OH和二氯甲烷洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩。经过制备型TLC(SiO2,AcOEt/MeOH=3∶1)得到(R)-3-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基]-1-(苯并[c]噻二唑-5-基)吡咯烷(2183号化合物)(11.5mg,48%):纯度用RPLC/MS测定(86%);ESI/MS m/e 479.2(m++H,C21H21F3N6O2S)。
参考例6:4-[{N-(1-(9-芴基(fuluorenyl)甲氧羰基)吡咯烷-3-基)氨基甲酰基甲基}氨甲基]-3-甲氧基苯氧基甲基-聚苯乙烯的制备
向盐酸(R)-1-(9-芴基甲氧羰基)-3-甘氨酰氨基吡咯烷(4.38g,10mmol)的DMF(65ml)溶液中加入乙酸(0.3ml)、三乙酰氧基硼氢化钠(1.92g)和4-甲酰基-3-(甲氧基苯氧基甲基)-聚苯乙烯(1mmol/g,200g)。混合物摇动2小时,过滤。树脂用MeOH、DMF、CH2Cl2和甲醇洗涤,干燥,得到所需物质(2.73g)。
实施例822-912:用于3-氨基吡咯烷的固相合成的通用操作
向相应的酸(1.6mmol)、HBTU(1.6mmol)和DMF(6ml)的混合物中加入二异丙基乙胺(3.6mmol),混合物摇动2分钟。加入4-[{N-(1-(9-芴基(fuluorenyl)甲氧羰基)吡咯烷-3-基)氨基甲酰基甲基}氨甲基]-3-甲氧基苯氧基甲基-聚苯乙烯(400mg,0.4mmol),混合物摇动1小时,过滤。树脂用DMF和CH2Cl2漂洗,干燥。
将所得树脂、吡咯烷(原文为哌啶——译者注)(3.2ml)和DMF(12.8ml)的混合物摇动10分钟,过滤。树脂用DMF和CH2Cl2洗涤,干燥。
向干燥树脂(0.05mmol)中加入NaBH(OAc)3(0.25mmol)、AcOH(0.025mmol)和DMF(1ml)的混合物。加入相应的醛(2.5mmol),混合物摇动2小时,然后过滤,用CH3OH、10%二异丙乙胺的DMF溶液、DMF、CH2Cl2和CH3OH洗涤。将树脂、水(0.050ml)和三氟乙酸(0.95ml)的混合物摇动1小时,过滤。树脂用CH2Cl2和CH3OH洗涤。合并滤液和洗液,浓缩。将粗物质装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,浓缩。必要时使用制备型TLC或HPLC,得到所需物质。ESI/MS数据和产率总结在表18中。
表18
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例822 |
1805 |
C21 H21 Br F3 N3 O2 S |
516 |
13.3 |
76 |
|
实施例823 |
1806 |
C22 H24 F3 N3 O3 S |
468 |
12.8 |
81 |
|
实施例824 |
1807 |
C22 H24 F3 N3 O4 S |
484 |
13.7 |
83 |
|
实施例825 |
1808 |
C22 H24 F3 N3 O4 S |
484 |
14.9 |
91 |
|
实施例826 |
1809 |
C21 H22 F3 N3 O3 S |
454 |
12.9 |
84 |
|
实施例827 |
1810 |
C22 H22 F3 N3 O4 S |
482 |
12.9 |
79 |
|
实施例828 |
1811 |
C24 H26 F3 N3 O2 S |
478 |
12.9 |
79 |
|
实施例829 |
1812 |
C22 H24 F3 N3 O2 S2 |
484 |
5.3 |
32 |
|
实施例830 |
1813 |
C23 H26 F3 N3 O2 S |
466 |
12.8 |
81 |
|
实施例831 |
1814 |
C23 H24 F3 N3 O3 S |
480 |
9.7 |
59 |
|
实施例832 |
1815 |
C23 H26 F3 N3 O2 S |
466 |
12.7 |
80 |
|
实施例833 |
1816 |
C24 H28 F3 N3 O2 S |
480 |
14.4 |
88 |
|
实施例834 |
1817 |
C25 H30 F3 N3 O2 S |
494 |
14.1 |
84 |
|
实施例835 |
1818 |
C21 H22 Br F2 N3 O3 |
482 |
13.4 |
82 |
|
实施例836 |
1819 |
C22 H25 F2 N3 O4 |
434 |
11.7 |
79 |
|
实施例837 |
1820 |
C22 H25 F2 N3 O5 |
450 |
11.8 |
77 |
|
实施例838 |
1821 |
C22 H25 F2 N3 O5 |
450 |
13.3 |
87 |
|
实施例839 |
1822 |
C21 H23 F2 N3 O4 |
420 |
11.9 |
83 |
|
实施例840 |
1823 |
C22 H23 F2 N3 O5 |
448 |
11.9 |
78 |
|
实施例841 |
1824 |
C24 H27 F2 N3 O3 |
444 |
9.1 |
60 |
|
实施例842 |
1825 |
C22 H25 F2 N3 O3 S |
450 |
11.3 |
74 |
|
实施例843 |
1826 |
C23 H27 F2 N3 O3 |
432 |
10.8 |
74 |
|
实施例844 |
1827 |
C23 H25 F2 N3 O4 |
446 |
12.7 |
84 |
|
实施例845 |
1828 |
C23 H27 F2 N3 O3 |
432 |
11.7 |
80 |
|
实施例846 |
1829 |
C24 H29 F2 N3 O3 |
446 |
14.3 |
94 |
|
实施例847 |
1830 |
C24 H29 F2 N3 O3 |
446 |
10.0 |
66 |
|
实施例848 |
1831 |
C22 H28 Br N3 O3 |
462 |
4.8 |
31 |
|
实施例849 |
1832 |
C23 H31 N3 O4 |
414 |
10.4 |
74 |
|
实施例850 |
1833 |
C23 H31 N3 O5 |
430 |
12.1 |
83 |
|
实施例851 |
1834 |
C23 H31 N3 O5 |
430 |
12.0 |
82 |
|
实施例852 |
1835 |
C22 H29 N3 O4 |
400 |
7.9 |
58 |
|
实施例853 |
1836 |
C23 H29 N3 O5 |
428 |
11.1 |
76 |
|
实施例854 |
1837 |
C25 H33 N3 O3 |
424 |
13.3 |
92 |
|
实施例855 |
1838 |
C23 H31 N3 O3 S |
430 |
8.7 |
60 |
|
实施例856 |
1839 |
C24 H33 N3 O3 |
412 |
11.3 |
81 |
|
实施例857 |
1840 |
C24 H31 N3 O4 |
426 |
12.9 |
89 |
|
实施例858 |
1841 |
C24 H33 N3 O3 |
413 |
12.8 |
91 |
|
实施例859 |
1842 |
C25 H35 N3 O3 |
426 |
8.7 |
60 |
|
实施例860 |
1843 |
C25 H35 N3 O3 |
426 |
12.2 |
84 |
|
实施例861 |
1844 |
C26 H37 N3 O3 |
440 |
11.3 |
76 |
|
实施例862 |
1845 |
C31 H37 Br N4 O2 |
577 |
6.4 |
30 |
|
实施例863 |
1846 |
C23 H28 F3 N3 O2 S |
480 |
12.8 |
81 |
|
实施例864 |
1847 |
C25 H31 F2 N3 O3 |
460 |
12.2 |
78 |
|
实施例865 |
1848 |
C27 H29 N3 O4 |
460 |
6.1 |
39 |
|
实施例866 |
1849 |
C29 H31 N3 O2 |
454 |
15.1 |
98 |
|
实施例867 |
1850 |
C28 H31 N3 O2 |
442 |
12.7 |
85 |
|
实施例868 |
1851 |
C28 H31 N3 O2 |
442 |
14.3 |
95 |
|
实施例869 |
1852 |
C28 H29 N3 O3 |
456 |
3.4 |
22 |
|
实施例870 |
1853 |
C27 H29 N3 O6 S |
524 |
15.4 |
87 |
|
实施例871 |
1854 |
C29 H31 N3 O4 S |
518 |
15.8 |
90 |
|
实施例872 |
1855 |
C28 H31 N3 O4 S |
506 |
17.0 |
99 |
|
实施例873 |
1856 |
C28 H31 N3 O4 S |
506 |
3.0 |
17 |
|
实施例874 |
1857 |
C28 H29 N3 O5 S |
520 |
10.0 |
57 |
|
实施例875 |
1858 |
C20 H22 Br2 N4 O2 |
511 |
9.3* |
37 |
|
实施例876 |
1859 |
C21 H25 Br N4 O3 |
461 |
6.7* |
29 |
|
实施例877 |
1860 |
C21 H25 Br N4 O4 |
477 |
9.5* |
40 |
|
实施例878 |
1861 |
C21 H25 Br N4 O4 |
477 |
10.0* |
42 |
|
实施例879 |
1862 |
C20 H23 Br N4 O3 |
4 47 |
7.8* |
34 |
|
实施例880 |
1863 |
C21 H23 Br N4 O4 |
475 |
3.4* |
14 |
|
实施例881 |
1864 |
C21 H25 Br N4 O2 S |
477 |
3.9* |
16 |
|
实施例882 |
1865 |
C22 H25 Br N4 O3 |
473 |
6.4* |
27 |
|
实施例883 |
1866 |
C23 H29 Br N4 O2 |
472 |
7.0* |
29 |
|
实施例884 |
1867 |
C23 H29 Br N4 O2 |
473 |
7.6* |
32 |
|
实施例885 |
1868 |
C24 H31 Br N4 O2 |
4 87 |
9.1* |
37 |
|
实施例886 |
1869 |
C20 H22 Br I N4 O2 |
557 |
8.9* |
33 |
|
实施例887 |
1870 |
C21 H25 I N4 O3 |
509 |
9.2* |
37 |
|
实施例888 |
1871 |
C21 H25 I N4 O4 |
525 |
6.3* |
25 |
|
实施例889 |
1872 |
C21 H25 I N4 O4 |
525 |
5.9* |
23 |
|
实施例890 |
1873 |
C20 H23 I N4 O3 |
495 |
7.7* |
31 |
|
实施例891 |
1874 |
C21 H23 I N4 O4 |
523 |
8.2* |
32 |
|
实施例892 |
1875 |
C23 H27 I N4 O2 |
519 |
6.7* |
26 |
|
实施例893 |
1876 |
C21 H25 I N4 O2 |
525 |
4.3* |
17 |
|
实施例894 |
1877 |
C22 H27 I N4 O2 |
507 |
7.9* |
32 |
|
实施例895 |
1878 |
C22 H25 I N4 O3 |
521 |
8.4* |
33 |
|
实施例896 |
1879 |
C23 H29 I N4 O2 |
521 |
8.2* |
32 |
|
实施例897 |
1880 |
C23 H29 I N4 O2 |
521 |
8.1* |
32 |
|
实施例898 |
1881 |
C24 H31 I N4 O2 |
535 |
8.6* |
33 |
|
实施例899 |
1882 |
C20 H22 Br N5 O4 |
476 |
5.3* |
22 |
|
实施例900 |
1883 |
C21 H25 N5 O5 |
428 |
5.7* |
26 |
|
实施例901 |
1884 |
C21 H25 N5 O6 |
444 |
8.2* |
36 |
|
实施例902 |
1885 |
C21 H25 N5 O6 |
444 |
5.0* |
22 |
|
实施例903 |
1886 |
C20 H23 N5 O5 |
414 |
8.7* |
40 |
|
实施例904 |
1887 |
C21 H23 N5 O6 |
442 |
7.8* |
34 |
|
实施例905 |
1888 |
C23 H27 N5 O4 |
438 |
5.6* |
25 |
|
实施例906 |
1889 |
C21 H25 N5 O4 S |
444 |
13.2* |
58 |
|
实施例907 |
1890 |
C22 H27 N5 O4 |
426 |
11.3* |
51 |
|
实施例908 |
1891 |
C22 H25 N5 O5 |
440 |
7.4* |
33 |
|
实施例909 |
1892 |
C22 H27 N5 O4 |
426 |
5.5* |
25 |
|
实施例910 |
1893 |
C23 H29 N5 O4 |
440 |
5.7* |
25 |
|
实施例911 |
1894 |
C23 H29 N5 O4 |
440 |
9.4* |
41 |
|
实施例912 |
1895 |
C24 H31 N5 O4 |
455 |
8.5* |
37 |
*TFA盐的产量
参考例7:2-氨基甲酰基-1-(4-氯苄基)吡咯烷的制备
将盐酸dl-脯氨酰胺(2.5g,21.8mmol)的CH3CN(35ml)溶液用Et3N(7.45ml)和4-氯苄基氯(3.88g,24.1mmol)处理。反应混合物在70℃下搅拌4小时,然后在25℃下搅拌16小时。所得混合物用CH2Cl2(20ml)稀释,用水洗涤(3×30ml)。有机相干燥(MgSO4)并浓缩。经过色谱法(SiO2,1%CH3OH-CH2Cl2)得到2-氨基甲酰基-1-(4-氯苄基)吡咯烷(5.21g,81%)。
参考例8:2-(氨甲基)-1-(4-氯苄基)吡咯烷的制备
将2-氨基甲酰基-1-(4-氯苄基)吡咯烷溶于1M BH3-THF(9.4ml),加热至70℃。16小时和25小时后,另加入0.5当量1M BH3-THF。40小时后,加入1N HCl水溶液(14ml),反应物加热回流3小时,加入3N HCl水溶液(6ml),反应物再加热3小时。反应混合物冷却至25℃,用4N NaOH水溶液碱化,用CH2Cl2萃取(4×15ml)。经过色谱法(SiO2,8∶1∶1 iPrOH-H2O-NH4OH)得到2-(氨甲基)-1-(4-氯苄基)吡咯烷(1.21g,86%)。
依据上述方法,分别使用相应的试剂,也制备了旋光活性的(S)-2-(氨甲基)-1-(4-氯苄基)吡咯烷和(R)-2-(氨甲基)-1-(4-氯苄基)吡咯烷。
(S)-2-(氨甲基)-1-(4-氯苄基)吡咯烷:
1H NMR(CDCl3,400MHz)δ1.40-1.80(m,5H),1.80-1.95(m,1H),2.12-2.21(m,1H),2.48-2.65(m,1H),2.66-2.78(m,2H),2.85-2.95(m,1H),3.26(d,J=13.2Hz,1H),3.93(d,J=13.2Hz,1H),7.20-7.40(m,4H).
(R)-2-(氨甲基)-1-(4-氯苄基)吡咯烷显示与(S)-异构体相同的1H NMR。
实施例913:2-{(N-苯甲酰基亮氨酰)氨基甲基}-1-(4-氯苄基)吡咯烷(344号化合物)的制备
将2-(氨甲基)-1-(4-氯苄基)吡咯烷(22.5mg,0.10mmol)和dl-苯甲酰亮氨酸(0.12mmol)的CHCl3(1ml)溶液用EDCI(23mg)、HOBt(16.2mg)和Et3N(15.2μl)处理,在25℃下搅拌16小时。反应混合物用CH2Cl2(0.5ml)稀释,用2N NaOH水溶液洗涤(2×0.75ml),通过PTFE膜过滤进行干燥,并浓缩,得到2-{(N-苯甲酰基亮氨酰)氨基甲基}-1-(4-氯苄基)吡咯烷(344号化合物)(74mg,定量):纯度用RPLC/MS测定(85%);ESI/MS m/e 442(M++H,C25H32ClN3O2)。
实施例914-935
依据实施例913的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用色谱法(HPLC-C18,CH3CN/H2O/TFA)得到所需物质的TFA盐。ESI/MS数据和产率总结在表19中,339和340号化合物分别显示如下1HNMR谱。
表19
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例914 |
330 |
C21 H24 Cl N3 O2 |
386 |
75* |
quant |
|
实施例915 |
331 |
C22 H26 Cl N3 O2 |
400 |
44* |
70 |
|
实施例916 |
332 |
C24 H30 Cl N3 O5 |
476 |
57 |
quant |
|
实施例917 |
333 |
C20 H23 Cl N4 O2 |
387 |
40 |
quant |
|
实施例918 |
334 |
C22 H26 Cl N3 O2 |
400 |
68 |
quant |
|
实施例919 |
335 |
C21 H23 Cl N4 O4 |
431 |
73 |
quant |
|
实施例920 |
336 |
C22 H23 Cl F3 N3 O2 |
454 |
75 |
quant |
|
实施例921 |
337 |
C22 H26 Cl N3 O2 |
400 |
68 |
quant |
|
实施例922 |
338 |
C22 H26 Cl N3 O2 |
400 |
70 |
quant |
|
实施例923 |
341 |
C22 H26 Cl N3 O2 |
400 |
80* |
quant |
|
实施例924 |
342 |
C22 H26 Cl N3 O2 |
400 |
68 |
quant |
|
实施例925 |
343 |
C24 H30 Cl N3 O2 |
428 |
63 |
quant |
|
实施例926 |
345 |
C23 H27 Cl N2 O2 |
399 |
68* |
quant |
|
实施例927 |
346 |
C23 H26 Cl F N2 O3 |
433 |
51 |
quant |
|
实施例928 |
347 |
C24 H29 Cl N2 O2 |
413 |
47 |
quant |
|
实施例929 |
348 |
C23 H27 Cl N2 O2 |
399 |
26 |
quant |
|
实施例930 |
349 |
C21 H25 Cl N2 O3 S |
421 |
42 |
quant |
|
实施例931 |
350 |
C26 H33 Cl N2 O3 |
457 |
12.4 |
54 |
|
实施例932 |
351 |
C22 H26 Cl N3 O3 |
416 |
34 |
81 |
|
实施例933 |
352 |
C22 H25 Cl2 N3 O3 |
450 |
51 |
quant |
*TFA盐的产量
实施例934:339号化合物:82%; 1H NMR(CDCl3)δ1.52-1.75(m,4H),1.84-1.95(m,1H),2.10-2.20(m,1H),2.67-2.78(m,1H),2.80-2.90(m,1H),3.10-3.20(m,1H),3.25(d,J=13.1Hz,1H),3.50-3.60(m,1H),3.89(d,J=13.1Hz,1H),4.28-4.20(m,2H),7.00-7.05(m,1H),7.12-7.29(m,4H),7.51(t,J=7.8Hz,1H),7.74(d,J=7.8Hz,1H),7.99(d,J=7.8Hz,1H),8.10-8.27(m,2H).
实施例935:340号化合物:68%; 1H NMR(CDCl3)δ1.55-1.73(m,4H),1.86-1.97(m,1H),2.12-2.21(m,1H),2.67-2.76(m,1H),2.86-2.93(m,1H),3.14-3.21(m,1H),3.27(d,J=13.1Hz,1H),3.52-3.59(m,1H),3.89(d,J=13.1Hz,1H),4.09-4.21(m,2H),7.00-7.07(m,1H),7.12-7.30(m,4H),7.50(t,J=7.8Hz,1H),7.73(d,J=7.8Hz,1H),8.01(d,J=7.8Hz,1H),8.10-8.25(m,2H).
参考例9:3-(氨甲基)-1-(4-氯苄基)吡咯烷的制备
向4-羧基-1-(4-氯苄基)吡咯烷-2-酮(5.05g,20mmol)、EDCI(2.85g,22mmol)、HOBt(2.97g,22mmol)和二氯甲烷(100ml)的混合物中加入0.5M氨的二噁烷溶液(60ml,30mmol)。反应混合物在室温下搅拌15小时,用2NHCl(3次)和2N NaOH水溶液(100ml×4)洗涤。有机层经无水硫酸镁干燥,过滤,并浓缩,得到3-氨基甲酰基-1-(4-氯苄基)吡咯烷-2-酮(1.49g),为无色固体。
向3-氨基甲酰基-1-(4-氯苄基)吡咯烷-2-酮(1.45g)的THF(15ml)溶液中加入1.0N BH3的THF(25ml)溶液。反应混合物在65℃下搅拌15小时。冷却至室温后,在减压下除去溶剂。加入水(30ml)和浓HCl(10ml),混合物在100℃下搅拌2小时,在室温下搅拌1小时。加入2NNaOH水溶液(100ml),混合物用AcOEt萃取(50ml×3)。合并了的有机层经K2CO3干燥,过滤,并浓缩。经过柱色谱法(SiO2,15% CH3OH-5% Et3N的CH2Cl2溶液)得到3-(氨甲基)-1-(4-氯苄基)吡咯烷(860mg,19%),为无色的油。
参考例10:1-(4-氯苄基)-3-{(甘氨酰氨基)甲基}吡咯烷的制备
将3-(氨甲基)-1-(4-氯苄基)吡咯烷(860mg,3.8mmol)、Et3N(5.7mmol)、N-叔丁氧羰基甘氨酸(704mg)、EDCI(594mg)、HOBt(673mg)和二氯甲烷(20ml)的混合物在室温下搅拌15小时。加入二氯甲烷(50ml),溶液用2NNaOH溶液洗涤(50ml×2),经无水硫酸钠干燥,过滤,并浓缩,得到3-[{N-(叔丁氧羰基)甘氨酰}氨基甲基]-1-(4-氯苄基)吡咯烷(1.31g,90%)。
向3-[{N-(叔丁氧羰基)甘氨酰}氨基甲基]-1-(4-氯苄基)吡咯烷(804mg,2.11mmol)的甲醇(10ml)溶液中加入4N HCl的二噁烷(5ml)溶液。溶液在室温下搅拌3.5小时。反应混合物浓缩,加入1N NaOH溶液(20ml)。混合物用二氯甲烷萃取(20ml×3),合并了的萃取液经硫酸钠干燥,并浓缩,得到所需的1-(4-氯苄基)-3-{(甘氨酰氨基)甲基}吡咯烷(599mg,100%):纯度用RPLC/MS测定(100%);ESI/MS m/e 282.2(M++H,C14H20ClN3O)。
实施例936:3-[{N-(3-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)吡咯烷(1463号化合物)的制备
向1-(4-氯苄基)-3-{(甘氨酰氨基)甲基}吡咯烷(0.050mmol)和哌啶子基甲基聚苯乙烯(60mg)在氯仿(0.2ml)与二氯甲烷(1ml)中的混合物中加入3-(三氟甲基)苯甲酰氯(0.058mmol)的二氯甲烷(0.2ml)溶液。反应混合物在室温下搅拌2.5小时后,加入甲醇(0.30ml),混合物在室温下搅拌1小时。将反应混合物装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到3-[{N-(3-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)吡咯烷(1463号化合物)(22.4mg,99%):纯度用RPLC/MS测定(97%);ESI/MS m/e 454.2(M++H,C22H23ClF3N3O2)。
实施例937-944
依据实施例936的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表20中。
表20
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例937 |
1464 |
C22 H23 Cl F3 N3 O3 |
470.0 |
21.0 |
89 |
|
实施例938 |
1465 |
C23 H22 Cl F6 N3 O2 |
522.0 |
24.5 |
94 |
|
实施例939 |
1466 |
C21 H23 Br Cl N3 O2 |
466.0 |
20.8 |
90 |
|
实施例940 |
1467 |
C21 H23 Cl2 N3 O2 |
420.0 |
19.6 |
93 |
|
实施例941 |
1468 |
C21 H23 Cl N4 O4 |
431.2 |
19.5 |
91 |
|
实施例942 |
1469 |
C22 H22 Cl F4 N3 O2 |
472.0 |
21.8 |
92 |
|
实施例943 |
1470 |
C21 H22 Cl3 N3 O2 |
456.0 |
22.1 |
97 |
|
实施例944 |
1471 |
C21 H22 Cl F2 N3 O2 |
422.0 |
20.9 |
99 |
实施例945:3-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)吡咯烷(1506号化合物)的制备
将1-(4-氯苄基)-3-{(甘氨酰氨基)甲基}吡咯烷(0.050mmol)在CHCl3(1.35ml)与叔丁醇(0.05ml)中的溶液用2-氨基-4,5-二氟苯甲酸(0.060mmol)、二异丙基碳二亚胺(0.060mmol)和HOBt(0.060mmol)处理。反应混合物在室温下搅拌19小时。将混合物装上VarianTM SCX柱,用CH3OH/CHCl3 1∶1(10ml)和CH3OH(10ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到3-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)吡咯烷(1506号化合物)(22.0mg,定量):纯度用RPLC/MS测定(92%);ESI/MSm/e 437(M++H,C21H23ClF2N4O2)。
实施例946-952
依据实施例945的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表21中。
表21
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例946 |
1506 |
C21 24 Br Cl N4 O2 |
481 |
20.6 |
86 |
|
实施例947 |
1507 |
C21 H24 F Cl N4 O2 |
419 |
21.7 |
quant |
|
实施例948 |
1509 |
C27 H28 Cl N3 O2 |
462 |
26.5 |
quant |
|
实施例949 |
1510 |
C21 H24 Cl I N4 O2 |
527 |
22.0 |
84 |
|
实施例950 |
1511 |
C19 H21 Br Cl N3 O2 S |
472 |
23.7 |
quant |
|
实施例951 |
1512 |
C21 H24 Cl2 N4 O2 |
435 |
22.3 |
quant |
|
实施例952 |
1513 |
C27 H28 Cl N3 O4 S |
526 |
24.6 |
94 |
参考例11:1-(4-氯苄基)-3-哌啶甲酸的制备
向3-哌啶甲酸乙酯(6.29g,40.0mmol)的CH3CN(15ml)的溶液中加入4-氯苄基氯(6.42g,39.9mmol)和iPr2NEt(7.74g,40.0mmol)。反应混合物在70℃下搅拌1.5小时。在减压下除去溶剂。向残余物中加入饱和NaHCO3水溶液(50ml),混合物用乙酸乙酯(100ml)萃取。有机相用饱和NaHCO3水溶液和盐水洗涤,经Na2SO4干燥。在减压下除去溶剂,得到1-(4-氯苄基)-3-哌啶甲酸乙酯,为红黄色油(11.025g,97.8%),不用进一步纯化。纯度用RPLC/MS测定(97%);ESI/MS m/e 382.2(M++H,C15H21ClNO2)。
向1-(4-氯苄基)-3-哌啶甲酸乙酯在THF(60ml)与CH3OH(20ml)中的溶液中加入LiOH(1.66g)的H2O(25ml)溶液。反应混合物在室温下搅拌15小时。在减压下除去溶剂,得到无定形固体,用柱色谱法纯化(SiO2,50%CH3OH-CH2Cl2),得到1-(4-氯苄基)-3-哌啶甲酸(9.75g,98.2%),为淡黄色无定形固体。纯度用RPLC/MS测定(>95%);ESI/MS m/e 254.0(M++H,C13H17ClNO2)。
参考例12:1-(4-氯苄基)-3-{(叔丁氧羰基)氨基}哌啶的制备
将1-(4-氯苄基)-3-哌啶甲酸(7.06g,27.8mmol)的tBuOH(500ml)溶液用Et3N(3.38g)和活化3A分子筛(30g)处理。加入二苯基磷酰叠氮化物(8.58g),反应混合物加热回流18小时。混合物冷却,溶剂回流18小时。混合物冷却,在真空下除去溶剂。将残余物溶于EtOAc(500ml),有机相用饱和NaHCO3水溶液(2×100ml)和盐水(50ml)洗涤,干燥(Na2SO4),并在真空中浓缩。经过色谱法(SiO2,25% EtOAc-己烷)得到1-(4-氯苄基)-3-{(叔丁氧羰基)氨基}哌啶(2.95g,32.6%),为白色晶性固体:H1 NMR(CDCl3,300Mhz)δ1.4-1.75(br,4H),2.2-2.7(br,4H),3.5(br,2H),3.8(br,1H),7.3(br,4H);纯度用RPLC/MS测定(>99%);ESI/MS m/e 269.2(M++H-56,C17H26ClN2O2)。
参考例13:3-氨基-1-(4-氯苄基)哌啶的制备
将1-(4-氯苄基)-3-{(叔丁氧羰基)氨基}哌啶(2.55g,7.85mmol)的CH3OH(25ml)溶液用1N HCl-Et2O(50ml)处理。反应混合物在25℃下搅拌15小时。在减压下除去溶剂,得到二盐酸3-氨基-1-(4-氯苄基)哌啶,为无定形固体(2.49g,定量)。纯度用RPLC/MS测定(>95%);ESI/MS m/e 225.2(M++H,C12H18ClN2)。
实施例953:1-(4-氯苄基)-3-[{N-(3-甲基苯甲酰基)甘氨酰}氨基]哌啶(355号化合物)的制备
向二盐酸1-(4-氯苄基)-3-氨基哌啶(14.9mg,0.050mmol)和Et3N(15.2mg)的CHCl3(2.5ml)溶液中加入N-(3-甲基苯甲酰基)甘氨酸(10.6mg,0.055mmol)、EDCI(10.5mg)和1-羟基苯并三唑水合物(7.4mg)。反应混合物在25℃下搅拌16小时,用2N NaOH水溶液(2ml×2)和盐水(1ml)洗涤。通过PTFE滤膜过滤后,在减压下除去溶剂,得到1-(4-氯苄基)-3-[{N-(3-甲基苯甲酰基)甘氨酰}氨基]哌啶(355号化合物),为淡黄色油(17.4mg,87%):纯度用RPLC/MS测定(97%);ESI/MS m/e 400.0(M++H,C22H26ClN3O2)。
实施例954-982
依据实施例953的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表22中,358号化合物显示如下1H NMR谱。
表22
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例954 |
354 |
C21 H24 Cl N3 O2 |
386 |
16.1 |
83 |
|
实施例955 |
356 |
C20 H23 Cl N4 O2 |
387 |
19.4 |
100 |
|
实施例956 |
357 |
C22 H26 Cl N3 O2 |
400 |
16.8 |
84 |
|
实施例957 |
359 |
C22 H26 Cl N3 O2 |
400 |
8.9 |
17 |
|
实施例958 |
360 |
C22 H25 Cl N4 O4 |
445 |
25.6 |
quant |
|
实施例959 |
361 |
C23 H27 Cl N2 O2 |
399 |
15.5 |
29 |
|
实施例960 |
362 |
C24 H29 Cl N2 O3 |
429 |
12.4 |
58 |
|
实施例961 |
363 |
C21 H25 Cl N2 O2 S |
405 |
22.2 |
quant |
|
实施例962 |
364 |
C24 H29 Cl N2 O4 |
445 |
20.7 |
93 |
|
实施例963 |
365 |
C24 H29 Cl N2 O2 |
413 |
15.6 |
75 |
|
实施例964 |
366 |
C23 H26 Cl F N2 O3 |
433 |
21.6 |
100 |
|
实施例965 |
367 |
C23 H27 Cl N2 O2 |
399 |
11.9 |
60 |
|
实施例966 |
368 |
C22 H25 Cl N2 O2 |
385 |
16.0 |
83 |
|
实施例967 |
369 |
C22 H24 Cl2 N2 O2 |
419 |
13.9 |
60 |
|
实施例968 |
370 |
C26 H33 Cl N2 O3 |
457 |
15.9 |
54 |
|
实施例969 |
371 |
C25 H31 Cl N2 O3 |
443 |
19.6 |
84 |
|
实施例970 |
372 |
C21 H25 Cl N2 O3 S |
421 |
23.0 |
quant |
|
实施例971 |
373 |
C23 H28 Cl N3 O2 |
414 |
19.1 |
92 |
|
实施例972 |
374 |
C24 H30 Cl N3 O3 |
444 |
18.6 |
84 |
|
实施例973 |
375 |
C23 H27 Cl2 N3 O2 |
448 |
18.0 |
80 |
|
实施例974 |
376 |
C24 H30 Cl N3 O3 |
444 |
19.6 |
88 |
|
实施例975 |
377 |
C25 H31 Cl2 N3 O2 |
476 |
20.7 |
87 |
|
实施例976 |
378 |
C27 H33 Cl F N3 O2 |
486 |
23.9 |
98 |
|
实施例977 |
379 |
C25 H30 Cl N3 O3 |
456 |
33.3 |
quant |
|
实施例978 |
380 |
C24 H30 Cl N3 O2 |
428 |
9.8 |
46 |
|
实施例979 |
381 |
C21 H26 Cl N3 O3 S |
436 |
10.3 |
47 |
|
实施例980 |
382 |
C22 H26 Cl N3 O3 |
416 |
24.4 |
quant |
|
实施例981 |
383 |
C22 H25 Cl2 N3 O3 |
450 |
27.5 |
quant |
实施例982:358号化合物:88%;
1H NMR(CDCl3)δ1.53-1.75(m,4H),2.12-2.20(m,1H),2.37-2.50(m,2H),2.53-2.61(m,1H),3.38-3.50(m,2H),2.53-2.61(m,1H),3.38-3.50(m,2H),4.06-4.20(m,3H),7.10-7.13(m,1H),7.18-7.30(m,4H),7.59(t,J=7.8Hz,1H),7.79(d,J=7.8Hz,1H),8.01(d,J=7.8Hz,1H),8.11(s,1H).
参考例14:1-苄基-4-[{N-(叔丁氧羰基)甘氨酰}氨基]哌啶的制备
将4-氨基-1-苄基哌啶(3.80g,20mmol)的CH2Cl2(40ml)溶液用N-(叔丁氧羰基)甘氨酸(3.48g,20mmol)、EDCI(4.02g,21mmol)和HOBt(2.83g,21mmol)处理。反应混合物在室温下搅拌12小时后,加入2N NaOH溶液(20ml)。分离有机层,含水层用二氯甲烷萃取(20ml×2)。合并了的有机层用水(20ml)和盐水(20ml)洗涤,经无水硫酸钠干燥,过滤,并浓缩。经过柱色谱法(SiO2,乙酸乙酯/MeOH/Et3N=85/12/3)得到1-苄基-4-[{N-(叔丁氧羰基)甘氨酰}氨基]哌啶(6.59g,95%)。
参考例15:1-(4-氯苄基)-4-(甘氨酰氨基)哌啶的制备
向1-苄基-4-[{N-(叔丁氧羰基)甘氨酰}氨基]哌啶(6.59g)的甲醇(80ml)溶液中加入4N HCl的二噁烷(19ml)溶液。溶液在室温下搅拌2小时。反应混合物浓缩,加入2N NaOH水溶液(20ml)。混合物用二氯甲烷萃取(40ml×3),合并了萃取液经无水硫酸钠干燥,并浓缩。经过柱色谱法(SiO2,AcOEt/MeOH/Et3N=85/12/3)得到1-(4-氯苄基)-4-(甘氨酰氨基)哌啶(3.91g,83%)
1H NMR(CDCl3,400MHz)d1.47-1.59(m,2H),1.59(br,2H),1.76-1.96(m,2H),2.10-2.19(m,2H),2.75-2.87(m,2H),3.29(s,2H),3.50(s,2H),3.65-3.89(m,1H),7.15-7.23(m,1H),7.23-7.33(m,5H).
依据参考例13和14的方法,分别使用相应的试剂,也合成了其他4-酰氨基-1-苄基哌啶。
4-(β-丙氨酰氨基)-1-苄基哌啶:2.46g,51%(2步)。
1-苄基-4-((S)-亮氨酰氨基)哌啶:1.78g,74%(2步)。
1-苄基-4-((R)-亮氨酰氨基)哌啶:1.48g,61%(2步)。
实施例983:4-(N-苯甲酰基甘氨酰)氨基-1-苄基哌啶(386号化合物)
向1-(4-氯苄基)-4-(甘氨酰氨基)哌啶(0.050mmol)与三乙胺(0.070mmol)的氯仿(1.0ml)溶液中加入苯甲酰氯(0.060mmol)的氯仿(0.4ml)溶液。反应混合物在室温下搅拌2.5小时,加入(氨甲基)聚苯乙烯树脂(1.04mmol/g,50mg,50mmol),混合物在室温下搅拌12小时。反应混合物过滤,树脂用二氯甲烷(0.5ml)洗涤。合并滤液和洗液,加入二氯甲烷(4ml),溶液用2N NaOH水溶液(0.5ml)洗涤,得到4-(N-苯甲酰基甘氨酰)氨基-1-苄基哌啶(386号化合物)(11.3mg,64%):纯度用RPLC/MS测定(94%);ESI/MS m/e 352.0(M++H,C21H25N3O2)。
实施例984-1034
依据实施例983的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表23中。
表23
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例984 |
384 |
C22 H26 Cl N3 O2 |
400 |
60.0 |
quant |
|
实施例985 |
385 |
C21 H23 Cl N4 O4 |
431 |
58.7 |
91 |
|
实施例986 |
387 |
C25 H27 N3 O2 |
402.5 |
15.5 |
77 |
|
实施例987 |
388 |
C21 H24 N4 O4 |
397.0 |
16.2 |
82 |
|
实施例988 |
389 |
C23 H27 N3 O4 |
410.0 |
16.2 |
79 |
|
实施例989 |
390 |
C22 H24 F3 N3 O2 |
420.0 |
17.4 |
83 |
|
实施例990 |
391 |
C22 H23 F4 N3 O2 |
438.0 |
18.4 |
84 |
|
实施例991 |
392 |
C22 H24 F3 N3 O3 |
436.0 |
17.1 |
79 |
|
实施例992 |
393 |
C21 H24 Br N3 O2 |
430.0 |
18.0 |
84 |
|
实施例993 |
394 |
C21 H24 Cl N3 O2 |
386.0 |
16.4 |
85 |
|
实施例994 |
395 |
C21 H24 Br N3 O2 |
430.0 |
17.2 |
80 |
|
实施例995 |
396 |
C21 H23 F2 N3 O2 |
388.0 |
15.1 |
78 |
|
实施例996 |
397 |
C21 H23 Cl2 N3 O2 |
420.0 |
11.7 |
56 |
|
实施例997 |
398 |
C22 H27 N3 O2 |
366.0 |
13.1 |
72 |
|
实施例998 |
399 |
C26 H29 N3 O2 |
416.0 |
15.8 |
76 |
|
实施例999 |
400 |
C22 H26 N4 O4 |
411.0 |
17.4 |
85 |
|
实施例1000 |
401 |
C24 H29 N3 O4 |
424.0 |
16.9 |
80 |
|
实施例1001 |
402 |
C23 H26 F3 N3 O2 |
434.0 |
17.7 |
82 |
|
实施例1002 |
403 |
C23 H25 F4 N3 O2 |
452.0 |
18.6 |
82 |
|
实施例1003 |
404 |
C23 H26 F3 N3 O3 |
450.0 |
17.8 |
79 |
|
实施例1004 |
405 |
C22 H26 Br N3 O2 |
444.0 |
17.9 |
81 |
|
实施例1005 |
406 |
C22 H26 Cl N3 O2 |
400.0 |
15.5 |
78 |
|
实施例1006 |
407 |
C22 H26 Br N3 O2 |
444.0 |
17.8 |
80 |
|
实施例1007 |
408 |
C22 H25 F2 N3 O2 |
402.0 |
15.6 |
78 |
|
实施例1008 |
409 |
C22 H25 Cl2 N3 O2 |
434.0 |
17.6 |
81 |
|
实施例1009 |
410 |
C25 H33 N3 O2 |
408.0 |
16.2 |
79 |
|
实施例1010 |
411 |
C29 H35 N3 O2 |
458.5 |
18.8 |
82 |
|
实施例1011 |
412 |
C25 H32 N4 O4 |
453.0 |
19.4 |
86 |
|
实施例1012 |
413 |
C27 H35 N3 O4 |
466.0 |
19.8 |
85 |
|
实施例1013 |
414 |
C26 H32 F3 N3 O2 |
476.0 |
20.2 |
85 |
|
实施例1014 |
415 |
C26 H31 F4 N3 O2 |
494.0 |
20.5 |
83 |
|
实施例1015 |
416 |
C26 H32 F3 N3 O3 |
492.0 |
19.5 |
79 |
|
实施例1016 |
417 |
C25 H32 Br N3 O2 |
486.0 |
19.1 |
79 |
|
实施例1017 |
418 |
C25 H32 Cl N3 O2 |
442.0 |
17.7 |
80 |
|
实施例1018 |
419 |
C25 H32 Br N3 O2 |
486.0 |
20.3 |
83 |
|
实施例1019 |
420 |
C25 H31 F2 N3 O2 |
444.0 |
18.6 |
84 |
|
实施例1020 |
421 |
C25 H31 Cl2 N3 O2 |
476.0 |
19.4 |
81 |
|
实施例1021 |
422 |
C25 H33 N3 O2 |
408.0 |
14.4 |
71 |
|
实施例1022 |
423 |
C29 H35 N3 O2 |
458.0 |
16.4 |
72 |
|
实施例1023 |
424 |
C25 H32 N4 O4 |
453.0 |
18.1 |
80 |
|
实施例1024 |
425 |
C27 H35 N3 O4 |
466.0 |
16.4 |
70 |
|
实施例1025 |
426 |
C26 H32 F3 N3 O2 |
476.0 |
17.3 |
73 |
|
实施例1026 |
427 |
C26 H31 F4 N3 O2 |
494.0 |
18.8 |
76 |
|
实施例1027 |
428 |
C26 H32 F3 N3 O3 |
492.0 |
18.4 |
75 |
|
实施例1028 |
429 |
C25 H32 Br N3 O2 |
486.0 |
17.9 |
74 |
|
实施例1029 |
430 |
C25 H32 Cl N3 O2 |
442.0 |
15.7 |
71 |
|
实施例1030 |
431 |
C25 H32 Br N3 O2 |
486.0 |
17.7 |
73 |
|
实施例1031 |
432 |
C25 H31 F2 N3 O2 |
444.0 |
16.6 |
75 |
|
实施例1032 |
433 |
C25 H31 Cl2 N3 O2 |
476.0 |
18.7 |
78 |
|
实施例1033 |
1016 |
C22 H23 Cl F3 N3 O2 |
454 |
32.5* |
53 |
|
实施例1034 |
1017 |
C21 H24 Cl N3 O2 |
386 |
55.2* |
quant |
*TFA盐的产量
参考例16:3-氨基甲酰基-1-(4-氯苄基)哌啶的制备
将3-哌啶甲酰胺(6.40g,50mmol)在CH3CN(150ml)与乙醇(20ml)中的溶液用Et3N(7.0ml,50mmol)和4-氯苄基氯(8.05g,50mmol)处理。反应混合物在50℃下搅拌16小时。冷却至室温后,向反应混合物中加入饱和NaHCO3水溶液(50ml)和水(150ml)。混合物用乙酸乙酯萃取(150ml×3),合并了的有机层用盐水洗涤,干燥(Na2SO4),并浓缩,得到淡红色固体。粗固体用乙醚(100ml)洗涤,得到3-氨基甲酰基-1-(4-氯苄基)哌啶(6.98g,54%)。
参考例17:3-(氨甲基)-1-(4-氯苄基)哌啶的制备
将3-氨基甲酰基-1-(4-氯苄基)哌啶(3.80g,15mmol)溶于THF(30ml),向溶液中加入1M BH3-THF(9.4ml)。反应混合物在70℃下搅拌15小时。混合物冷却至0℃后,加入2N HCl水溶液(50ml),混合物在室温下再搅拌3小时,用4N NaOH水溶液碱化,用乙酸乙酯萃取(100ml×3)。合并了萃取液用盐水洗涤,经无水Na2SO4干燥,过滤,并浓缩。经过柱色谱法(SiO2,乙酸乙酯/EtOH/Et3N=80/15/5)得到3-(氨甲基)-1-(4-氯苄基)哌啶(2.05g,55%)
1H NMR(CDCl3,400MHz)δ1.00-1.09(m,1H),1.50-1.87(m,7H),1.97-2.06(m,1H),2.65-2.77(m,2H),3.16-3.26(m,2H),3.32(s,2H),3.40.(d,J=13.3Hz,1H),3.49(d,J=13.3Hz,1H),7.22-7.33(m,5H).
实施例1035:3-{(N-苯甲酰基甘氨酰)氨基}甲基-1-(4-氯苄基)哌啶(434号化合物)的制备
向3-(氨甲基)-1-(4-氯苄基)哌啶(0.050mmol)和三乙胺(0.070mmol)的氯仿(1.0ml)溶液中加入苯甲酰氯(0.060mmol)的氯仿(0.4ml)溶液。反应混合物在室温下搅拌2.5小时后,加入(氨甲基)聚苯乙烯树脂(1.04mmol/g,50mg,50mmol),混合物在室温下搅拌12小时。反应混合物过滤,树脂用二氯甲烷(0.5ml)洗涤。合并滤液和洗液,加入二氯甲烷(4ml),溶液用2N NaOH水溶液(0.5ml)洗涤,得到3-{(N-苯甲酰基甘氨酰)氨基}甲基-1-(4-氯苄基)哌啶(434号化合物)(14.7mg,74%):纯度用RPLC/MS测定(91%);ESI/MS m/e 400(M++H,C22H26ClN3O2)。
实施例1036-1058
依据实施例1035的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表24中。
表24
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1036 |
435 |
C26 H28 Cl N3 O2 |
450 |
16.0 |
71 |
|
实施例1037 |
436 |
C22 H25 Cl N4 O4 |
445 |
18.9 |
85 |
|
实施例1038 |
437 |
C24 H28 Cl N3 O4 |
458 |
18.2 |
79 |
|
实施例1039 |
438 |
C23 H25 Cl F3 N3 O2 |
468 |
19.0 |
81 |
|
实施例1040 |
439 |
C23 H24 Cl F4 N3 O2 |
486 |
20.2 |
83 |
|
实施例1041 |
440 |
C23 H25 Cl F3 N3 O3 |
484 |
18.9 |
78 |
|
实施例1042 |
441 |
C22 H25 Br Cl N3 O2 |
478 |
19.2 |
80 |
|
实施例1043 |
442 |
C22 H25 Cl2 N3 O2 |
434 |
17.3 |
80 |
|
实施例1044 |
443 |
C22 H25 Br Cl N3 O2 |
478 |
18.8 |
79 |
|
实施例1045 |
444 |
C22 H24 Cl F2 N3 O2 |
436 |
16.7 |
77 |
|
实施例1046 |
445 |
C22 H24 Cl3 N3 O2 |
468 |
17.9 |
76 |
|
实施例1047 |
446 |
C23 H28 Cl N3 O2 |
414 |
14.6 |
71 |
|
实施例1048 |
447 |
C27 H30 Cl N3 O2 |
464 |
17.0 |
73 |
|
实施例1049 |
448 |
C23 H27 Cl N4 O4 |
459 |
19.5 |
85 |
|
实施例1050 |
449 |
C25 H30 Cl N3 O4 |
472 |
17.1 |
72 |
|
实施例1051 |
450 |
C24 H27 Cl F3 N3 O2 |
482 |
19.4 |
81 |
|
实施例1052 |
451 |
C24 H26 Cl F4 N3 O2 |
500 |
18.2 |
73 |
|
实施例1053 |
452 |
C24 H27 Cl F3 N3 O3 |
498 |
18.8 |
76 |
|
实施例1054 |
453 |
C23 H27 Br Cl N3 O2 |
492 |
19.4 |
79 |
|
实施例1055 |
454 |
C23 H27 Cl2 N3 O2 |
448 |
16.5 |
74 |
|
实施例1056 |
455 |
C23 H27 Br Cl N3 O2 |
492 |
19.3 |
78 |
|
实施例1057 |
456 |
C23 H26 Cl F2 N3 O2 |
450 |
17.1 |
76 |
|
实施例1058 |
457 |
C23 H26 Cl3 N3 O2 |
482 |
16.9 |
70 |
参考例18:4-(氨甲基)-1-(4-氯苄基)哌啶的制备
将4-(氨甲基)哌啶(7.00g,61.3mmol)的CH3CN(100ml)溶液按顺序用K2CO3(3.02g)和4-氯苄基氯(3.52g,21.8mmol)处理。反应混合物在60℃下加热16小时,冷却至25℃后浓缩。使残余物在CH2Cl2(75ml)与水(50ml)之间分布,用水(2×50ml)和盐水(1×50ml)洗涤。有机相干燥(MgSO4),并浓缩。经过色谱法(SiO2,4%H2O-iPrOH)得到4-(氨甲基)-1-(4-氯苄基)哌啶(3.58g,69%)。
实施例1059:4-{(N-苯甲酰基甘氨酰)氨基}甲基-1-(4-氯苄基)哌啶(458号化合物)的制备
将4-(氨甲基)-1-(4-氯苄基)哌啶(50mg,0.21mmol)的CH2Cl2(1ml)溶液用马尿酸(38mg,0.21mmol)、EDCI(48mg,0.24mmol)、HOBt(31mg,0.23mmol)和Et3N(38μl,0.27mmol)处理。反应混合物在25℃下搅拌16小时,用1ml CH2Cl2稀释,用2N NaOH水溶液洗涤(2×0.75ml),干燥(MgSO4),并浓缩。经过色谱法(SiO2,6至8% CH3OH/CH2Cl2梯度洗脱)得到4-{(N-苯甲酰基甘氨酰)氨基}甲基-1-(4-氯苄基)哌啶(458号化合物),用TFA处理得到TFA盐(105mg,97%):纯度用RPLC/MS测定(85%);ESI/MS m/e 400(M++H,C22H26ClN3O2)。
实施例1060-1086
依据实施例1059的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表25中。
表25
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1060 |
459 |
C23 H28 Cl N3 O2 |
414 |
86* |
78 |
|
实施例1061 |
460 |
C23 H28 Cl N3 O2 |
414 |
55 |
quant |
|
实施例1062 |
461 |
C23 H25 Cl F3 N3 O2 |
468 |
65 |
quant |
|
实施例1063 |
462 |
C23 H28 Cl N3 O2 |
414 |
61 |
quant |
|
实施例1064 |
463 |
C23 H28 Cl N3 O2 |
414 |
54 |
quant |
|
实施例1065 |
464 |
C25 H32 Cl N3 O5 |
490 |
56 |
quant |
|
实施例1066 |
465 |
C21 H25 Cl N4 O2 |
401 |
38 |
96 |
|
实施例1067 |
466 |
C22 H25 Cl N4 O4 |
445 |
15 |
34 |
|
实施例1068 |
557 |
C23 H28 Cl N3 O2 |
414 |
58* |
66 |
|
实施例1069 |
558 |
C23 H28 Cl N3 O2 |
414 |
55 |
quant |
|
实施例1070 |
618 |
C25 H32 Cl N3 O2 |
442 |
58 |
quant |
|
实施例1071 |
686 |
C26 H34 Cl N3 O2 |
456 |
62 |
quant |
|
实施例1072 |
749 |
C34 H37 Cl N4 O2 |
569 |
7.2* |
18 |
|
实施例1073 |
750 |
C24 H30 Cl N3 O3 |
444 |
4.7* |
14 |
|
实施例1074 |
840 |
C24 H29 Cl N2 O2 |
413 |
52* |
58 |
|
实施例1075 |
841 |
C23 H27 Cl N2 O2 |
399 |
52 |
quant |
|
实施例1076 |
842 |
C23 H26 Cl2 N2 O2 |
433 |
55 |
quant |
|
实施例1077 |
843 |
C25 H31 Cl N2 O2 |
427 |
58 |
quant |
|
实施例1078 |
844 |
C24 H29 Cl N2 O2 |
413 |
56 |
quant |
|
实施例1079 |
845 |
C24 H29 Cl N2 O4 S |
477 |
62 |
quant |
|
实施例1080 |
846 |
C29 H31 Cl N2 O3 |
491 |
43 |
88 |
|
实施例1081 |
847 |
C24 H28 Cl F N2 O3 |
447 |
54 |
quant |
|
实施例1082 |
848 |
C25 H31 Cl N2 O2 |
427 |
47 |
quant |
|
实施例1083 |
849 |
C25 H31 Cl N2 O4 |
459 |
55 |
quant |
|
实施例1084 |
850 |
C22 H27 Cl N2 O3 S |
435 |
46 |
quant |
|
实施例1085 |
873 |
C20 H28 Cl N3 O2 |
378 |
44.8 |
quant |
|
实施例1086 |
874 |
C23 H27 Cl2 N3 O3 |
464 |
51 |
quant |
*TFA盐的产量
参考例19:1-(4-氯苄基)-4-{N-(3,3-二苯丙基)氨甲基}哌啶的制备
在NaI(2.6当量)的存在下,在70℃ CH3CN中将4-(氨甲基)-1-(4-氯苄基)哌啶(120mg)用甲磺酸3,3-二苯丙基酯(1.0当量)进行烷基化16小时。经过一般操作和柱色谱法(SiO2)得到1-(4-氯苄基)-4-{N-(3,3-二苯丙基)氨甲基}哌啶(118mg,54%):纯度用RPLC测定(98%)。
参考例20:1-(4-氯苄基)-4-{N-(2,2-二苯乙基)氨甲基}哌啶的制备
在25℃甲醇中,将4-(氨甲基)-1-(4-氯苄基)哌啶(120mg)用2,2-二苯基乙醛(0.66当量)和以聚合物为载体的硼氢化物进行还原性胺化16小时,然后经过一般操作和柱色谱法(SiO2)得到1-(4-氯苄基)-4-{N-(2,2-二苯乙基)氨甲基}哌啶(70mg,49%):纯度用RPLC测定(98%)。
实施例1087:4-{N-(N-苯甲酰基甘氨酰)-N-(2,2-二苯乙基)氨甲基}-1-(4-氯苄基)哌啶(524号化合物)的制备
将1-(4-氯苄基)-4-{N-(2,2-二苯乙基)氨甲基}哌啶(0.084mmol)的CH2Cl2溶液用马尿酸(1.1当量)、HBTU(1.1当量)、HOBt(1.1当量)处理。反应混合物在40℃下搅拌24小时。经过一般操作和制备型TLC(SiO2)得到4-{N-(N-苯甲酰基甘氨酰)-N-(2,2-二苯乙基)氨甲基}-1-(4-氯苄基)哌啶(524号化合物)(8.5mg,17%):纯度用RPLC/MS测定(98%);ESI/MS m/e 580(M++H,C36H38ClN3O2)。
实施例1088-1090
依据实施例1087的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表26中。
表26
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1088 |
521 |
C38 H39 Cl F3 N3 O2 |
662 |
5.5 |
10 |
|
实施例1089 |
522 |
C37 H37 Cl F3 N3 O2 |
648 |
8.6 |
16 |
|
实施例1090 |
523 |
C37 H40 Cl N3 O2 |
594 |
4.8 |
10 |
参考例21:1-(4-氯苄基)-4-{(缬氨酰氨基)甲基}哌啶的制备
将4-(氨甲基)-1-(4-氯苄基)哌啶(1.0g,4.2mmol)的CH2Cl2(21ml)溶液用Et3N(0.76ml,5.44mmol)、dl-N-(叔丁氧羰基)缬氨酸(1.09g,5.03mmol)、EDCI(883mg,4.61mmol)和HOBt(623mg,4.61mmol)处理。反应混合物在25℃下搅拌16小时。所得溶液用CH2Cl2(20ml)稀释,用2N NaOH溶液(2×20ml)和盐水(1×20ml)洗涤,干燥(MgSO4)。经过浓缩和色谱法(SiO2,3%CH3OH/CH2Cl2)得到1-(4-氯苄基)-4-[{(N-Boc-缬氨酰)氨基}甲基]哌啶(1.1g,60%),为淡琥珀色油:ESI/MS m/e 438(M++H)。
将1-(4-氯苄基)-4-[{(N-Boc-缬氨酰)氨基}甲基]哌啶(1.1g,2.51mmol)溶于3M HCl-CH3OH溶液(25ml),在25℃下搅拌1小时。反应混合物浓缩,将所得盐溶于3∶1 tBuOH-H2O(25ml)。加入阴离子(OH-)交换树脂,直到溶液微呈碱性时为止。经过过滤和浓缩得到1-(4-氯苄基)-4-{(缬氨酰氨基)甲基}哌啶(819mg,97%),不需要进一步纯化:RPLC(97%);ESI/MS m/e 338.1(M++H,C18H28ClN3O)。
依据参考例20的方法,分别使用相应的试剂,也合成了其他4-{(酰氨基)甲基}-1-(4-氯苄基)哌啶。
1-(4-氯苄基)-4-{(甘氨酰氨基)甲基}哌啶:0.830g,67%(2步);ESI/MS269(M++H)。
1-(4-氯苄基)-4-{(丝氨酰氨基)甲基}哌啶:0.286g,20%(2步);ESI/MS326(M++H)。
4-{(丙氨酰氨基)甲基}-1-(4-氯苄基)哌啶:1.20g,65%(2步);ESI/MS310(M++H)。
1-(4-氯苄基)-4-{(脯氨酰氨基)甲基}哌啶:1.48g,86%(2步);ESI/MS336(M++H)。
1-(4-氯苄基)-4-{(谷氨酰氨基)甲基}哌啶:0.830g,27%(2步);ESI/MS367(M++H)。
1-(4-氯苄基)-4-{((2-甲基丙氨酰)氨基)甲基}哌啶:2.24g,62%(2步);ESI/MS 324(M++H)。
1-(4-氯苄基)-4-{((O-甲基丝氨酰)氨基)甲基}哌啶:0.686g,38%(2步);ESI/MS 340(M++H)。
1-(4-氯苄基)-4-{((1-氨基环丙基羰基)氨基)甲基}哌啶:2.03g,82%(2步);ESI/MS 322(M++H)。
1-(4-氯苄基)-4-{(亮氨酰氨基)甲基}哌啶:1.30g,58%(2步);ESI/MS352(M++H)。
1-(4-氯苄基)-4-{((O-苄基丝氨酰)氨基)甲基}哌啶:1.34g,56%(2步);ESI/MS 416(M++H)。
参考例22:1-(叔丁氧羰基)-4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶的制备
将4-(氨甲基)-1-(叔丁氧羰基)哌啶(5.72g)的CH2Cl2(150ml)溶液用Et3N(3.51g)、N-(9-芴基甲氧羰基)甘氨酸(7.93g,26.7mmol)、EDCI(3.80g)和HOBt(4.33g)处理。反应混合物在室温下搅拌5小时后,混合物用水(100ml×3)和盐水(100ml×2)洗涤,经无水硫酸钠干燥,过滤,并浓缩。从0℃CH3CN/CH3OH(150ml/1ml)中重结晶,得到1-(叔丁氧羰基)-4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶(5.75g,44%),为淡黄色晶体。
参考例23:4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶的制备
向1-(叔丁氧羰基)-4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶(3.17g,6.42mmol)中加入4N HCl的二噁烷(50ml)溶液。溶液在室温下搅拌5小时。反应混合物浓缩,得到4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶(3.85g),为白色固体。该产物在使用时不经进一步纯化。
参考例24:4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]-1-(4-甲硫基苄基)哌啶的制备
向4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶(1.00g,2.33mmol)的1% AcOH/DMF(15ml)溶液中加入4-甲硫基苯甲醛(1.24g)和NaBH(OAc)3(2.56g)。反应混合物在60℃下搅拌1小时,冷却至室温,并浓缩。加入饱和NaHCO3水溶液(50ml),混合物用AcOEt萃取(50ml×2)。合并后的萃取液经无水硫酸钠干燥,过滤,并浓缩。经过柱色谱法(SiO2,5%-10%CH3OH/CH2Cl2)得到4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]-1-(4-甲硫基苄基)哌啶(602mg),为无色的油。
参考例25:1-(4-乙基苄基)-4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶的制备
向4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶(1.00g,2.33mmol)的2.5% AcOH/CH3OH(15ml)溶液中加入4-乙基苯甲醛(1.09g,8.16mmol)和NaBH3CN(6.59g,10.5mmol)。反应混合物在60℃下搅拌13小时。混合物冷却至室温后,加入1NNaOH水溶液(50ml)和二氯甲烷(50ml)。分离有机层,含水层用二氯甲烷萃取(50ml×3)。合并后的有机层用盐水洗涤,经无水硫酸钠干燥,过滤,并浓缩。经过柱色谱法(SiO2,CH3OH/AcOEt 2∶8)得到1-(4-乙基苄基)-4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶(740mg,62%)。
参考例26:4-{(甘氨酰氨基)甲基}-1-(4-甲硫基苄基)哌啶的制备
将4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]-1-(4-甲硫基苄基)哌啶(590mg)和哌啶(1ml)的DMF(4ml)溶液在室温下搅拌2小时。经过浓缩和柱色谱法(SiO2,Et3N∶CH3OH∶CH2Cl2=1∶1∶9)得到4-{(甘氨酰氨基)甲基}-1-(4-甲硫基苄基)哌啶(365mg),为白色固体:
1H NMR(CDCl3,270MHz)δ1.25(dd,J=12Hz,4.1Hz,2H),1.34(dd,J=12Hz,4.1Hz,2H),1.51(br-s,2H),1.66(d,J=12Hz,2H),1.77(d,J=7.3Hz,1H),1.94(t,J=9.5Hz,2H),2.48(s,3H),2.80(d,J=12Hz,2H),3.18(t,J=6.2Hz,2H),3.35(s,2H),3.45(s,2H),7.18-7.29(m,4H),7.35(br-s,1H).
依据参考例25的方法,使用相应的试剂,也合成了1-(4-乙基苄基)-4-{(甘氨酰氨基)甲基}哌啶:333mg,79%。
参考例27:4-{(甘氨酰氨基)甲基}-1-(4-氟苄基)哌啶的制备
将4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶(1.50g,3.49mmol)、4-氟苄基溴(0.478ml,3.84mmol)和Et3N(1.47ml,10.5mmol)的CH3CN(200ml)溶液在室温下搅拌13小时,浓缩。经过柱色谱法(SiO2,10% CH3OH/CH2Cl2)得到4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]-1-(4-氟苄基)哌啶。
将4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]-1-(4-氟苄基)哌啶和哌啶(5ml)的DMF(5ml)溶液在室温下搅拌17小时。经过浓缩和柱色谱法(SiO2,Et3N∶CH3OH∶CH2Cl2=0.5∶2∶8)得到4-{(甘氨酰氨基)甲基}-1-(4-氟苄基)哌啶(453mg,46%)。
参考例28:4-{(甘氨酰氨基)甲基}-1-{4-(N-苯基氨基甲酰基)苄基}哌啶的制备
向4-[{N-(9-芴基甲氧羰基)甘氨酰}氨基甲基]哌啶(1.27g,2.96mmol)、Et3N(1.25ml,8.88mmol)、KI(50mg,0.30mmol)和CH3CN(200ml)的混合物中滴加4-(N-苯基氨基甲酰基)苄基氯(800mg,3.26mmol)的CH3CN(100ml)溶液。混合物在室温下搅拌19小时,再在60℃下搅拌5小时。经过浓缩和柱色谱法(SiO2,5% CH3OH/CH2Cl2-Et3N∶CH3OH∶CH2Cl2=2∶2∶96)得到4-{(甘氨酰氨基)甲基}-1-{4-(N-苯基氨基甲酰基)苄基}哌啶(340mg,30%)。
实施例1091:1-(4-氯苄基)-4-[{N-(3-氰基苯甲酰基)缬氨酰}氨基甲基]哌啶(619号化合物)的制备
将1-(4-氯苄基)-4-{(缬氨酰氨基)甲基}哌啶(20mg,0.059mmol)的CH2Cl2(0.60ml)溶液用Et3N(0.011ml,0.077mmol)、间氰基苯甲酸(28mg,0.071mmol)、EDCI(13mg,0.065mmol)和HOBt(9mg,0.065mmol)处理。反应混合物在25℃下搅拌16小时。所得溶液用CH2Cl2(0.75ml)稀释,用2NNaOH水溶液洗涤(2×0.75ml),通过PTFE膜过滤进行干燥。经过浓缩得到1-(4-氯苄基)-4-[{N-(3-氰基苯甲酰基)缬氨酰}氨基甲基]哌啶(619号化合物)(24.2mg,88%),不需要进一步纯化:纯度用RPLC/MS测定(85%);ESI/MS m/e 467(M++H,C26H31ClN4O2)。
实施例1092-1543
依据实施例1091的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表27中。
表27
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg ) |
产率(%) |
|
实施例1092 |
467 |
C22 H25 Br Cl N3 O2 |
478 |
11 |
46 |
|
实施例1093 |
468 |
C24 H31 Cl N4 O2 |
443 |
9 |
41 |
|
实施例1094 |
469 |
C23 H28 Cl N3 O3 |
430 |
7* |
27 |
|
实施例1095 |
470 |
C23 H25 Cl N4 O2 |
425 |
21 |
quant |
|
实施例1096 |
471 |
C24 H28 Cl N3 O4 |
458 |
7 |
29 |
|
实施例1097 |
472 |
C29 H31 N3 O3 |
504 |
5* |
21 |
|
实施例1098 |
473 |
C24 H28 Cl N3 O3 |
442 |
16 |
71 |
|
实施例109 |
474 |
C23 H25 Cl F3 N3 O2 |
468 |
14 |
60 |
|
实施例1110 |
475 |
C25 H32 Cl N3 O2 |
442 |
5 |
22 |
|
实施例1101 |
476 |
C22 H25 Cl N4 O4 |
445 |
4 |
17 |
|
实施例1102 |
477 |
C25 H32 Cl N3 O3 |
458 |
10* |
36 |
|
实施例1103 |
478 |
C21 H27 Cl N4 O2 |
403 |
9 |
47 |
|
实施例1104 |
479 |
C20 H24 Cl N3 O3 |
390 |
17 |
87 |
|
实施例1105 |
480 |
C20 H23 Br Cl N3 O3 |
470 |
23 |
quant |
|
实施例1106 |
481 |
C20 H24 Cl N3 O2 S |
406 |
7 |
33 |
|
实施例1107 |
482 |
C21 H26 Cl N3 O2 S |
420 |
9 |
45 |
|
实施例1108 |
483 |
C21 H26 Cl N3 O2 S |
420 |
8 |
40 |
|
实施例1109 |
484 |
C24 H27 Cl N4 O2 |
439 |
9* |
34 |
|
实施例1110 |
485 |
C24 H24 Cl F6 N3 O2 |
536 |
13 |
49 |
|
实施例1111 |
486 |
C23 H25 Cl N4 O2 |
425 |
16 |
74 |
|
实施例1112 |
487 |
C22 H25 Cl2 N3 O2 |
434 |
5 |
24 |
|
实施例1113 |
488 |
C22 H27 Cl N4 O2 |
415 |
7 |
32 |
|
实施例1114 |
489 |
C24 H24 Cl F6 N3 O2 |
536 |
21 |
78 |
|
实施例1115 |
490 |
C24 H30 Cl N3 O3 |
444 |
8 |
35 |
|
实施例1116 |
491 |
C23 H24 Cl F4 N3 O2 |
486 |
19 |
79 |
|
实施例1117 |
492 |
C23 H25 Cl F3 N3 O3 |
484 |
18 |
76 |
|
实施例1118 |
493 |
C23 H24 Cl2 F3 N3 O2 |
502 |
23 |
92 |
|
实施例1119 |
494 |
C23 H24 Cl F4 N3 O2 |
486 |
19 |
79 |
|
实施例1120 |
495 |
C23 H24 Cl F4 N3 O2 |
486 |
20 |
83 |
|
实施例1121 |
496 |
C23 H24 Cl F4 N3 O2 |
486 |
12 |
48 |
|
实施例1122 |
497 |
C25 H32 Cl N3 O3 |
458 |
4 |
16 |
|
实施例1123 |
498 |
C23 H26 Cl F3 N4 O2 |
483 |
13 |
52 |
|
实施例1124 |
499 |
C24 H31 Cl N4 O2 |
443 |
8 |
36 |
|
实施例1125 |
500 |
C23 H28 Cl N3 O3 |
430 |
10 |
48 |
|
实施例1126 |
501 |
C22 H24 Br Cl N4 O4 |
523 |
10 |
39 |
|
实施例1127 |
502 |
C22 H24 Cl F N4 O4 |
463 |
4 |
17 |
|
实施例1128 |
503 |
C22 H24 Cl2 N4 O4 |
479 |
12 |
52 |
|
实施例1129 |
504 |
C24 H30 Cl N3 O4 |
460 |
11 |
43 |
|
实施例1130 |
505 |
C22 H24 Br Cl N4 O4 |
523 |
2 |
8 |
|
实施例1131 |
506 |
C20 H23 Cl N4 O5 |
435 |
2 |
10 |
|
实施例1132 |
507 |
C21 H26 Cl N3 O3 |
404 |
9 |
44 |
|
实施例1133 |
508 |
C24 H26 Cl N3 O2 S |
456 |
1 |
5 |
|
实施例1134 |
509 |
C20 H23 Br Cl N3 O2 S |
484 |
12 |
48 |
|
实施例1135 |
510 |
C22 H28 Cl N3 O3 |
418 |
9 |
44 |
|
实施例1136 |
511 |
C24 H32 Cl N3 O3 |
446 |
9 |
40 |
|
实施例1137 |
512 |
C25 H29 Cl N4 O2 |
453 |
10 |
45 |
|
实施例1138 |
513 |
C24 H28 Cl N3 O3 |
442 |
9 |
41 |
|
实施例1139 |
514 |
C26 H34 Cl N3 O2 |
456 |
11 |
49 |
|
实施例1140 |
515 |
C23 H28 Cl N3 O3 |
430 |
5 |
24 |
|
实施例1141 |
525 |
C23 H28 Cl N3 O4 S |
478 |
20 |
85 |
|
实施例1142 |
526 |
C20 H24 Cl N3 O3 |
390 |
6 |
31 |
|
实施例1143 |
527 |
C20 H24 Cl N3 O2 S |
406 |
8 |
39 |
|
实施例1144 |
528 |
C25 H30 Cl F3 N4 O4 |
543 |
28.2 |
95 |
|
实施例1145 |
529 |
C20 H23 Cl N4 O4 S |
451 |
9 |
39 |
|
实施例1146 |
530 |
C31 H33 Cl N4 O2 |
529 |
5 |
17 |
|
实施例1147 |
531 |
C21 H26 Cl N3 O3 S |
436 |
8 |
37 |
|
实施例1148 |
532 |
C22 H28 Cl N3 O3 |
418 |
8 |
40 |
|
实施例1149 |
533 |
C21 H26 Cl N3 O3 |
404 |
6 |
32 |
|
实施例1150 |
534 |
C21 H25 Cl N4 O5 |
449 |
5 |
20 |
|
实施例1151 |
535 |
C22 H26 Cl N3 O3 S |
448 |
8 |
37 |
|
实施例1152 |
536 |
C23 H31 Cl N4 O2 |
431 |
6 |
28 |
|
实施例1153 |
537 |
C25 H34 Cl N3 O3 |
460 |
8 |
34 |
|
实施例1154 |
538 |
C27 H30 Cl N3 O3 |
480 |
9 |
36 |
|
实施例1155 |
539 |
C22 H25 Cl F3 N3 O3 |
472 |
18 |
75 |
|
实施例1156 |
540 |
C25 H29 Cl N4 O2 |
453 |
8 |
36 |
|
实施例1157 |
541 |
C22 H26 Cl N5 O4 |
460 |
2.4 |
10 |
|
实施例1158 |
542 |
C24 H30 Cl N3 O2 |
428 |
4.6* |
51 |
|
实施例1159 |
543 |
C24 H30 Cl N3 O2 |
428 |
20.6* |
71 |
|
实施例1160 |
544 |
C22 H25 Cl F N3 O2 |
418 |
15.8* |
56 |
|
实施例1161 |
545 |
C22 H24 Cl3 N3 O2 |
468 |
7.3* |
23 |
|
实施例1162 |
546 |
C22 H24 Cl3 N3 O2 |
468 |
17.4* |
55 |
|
实施例1163 |
547 |
C22 H24 Cl3 N3 O2 |
468 |
14.1* |
44 |
|
实施例1164 |
548 |
C22 H24 Cl3 N3 O2 |
468 |
6.8* |
22 |
|
实施例1165 |
549 |
C22 H24 Cl2 N4 O4 |
479 |
5.7* |
18 |
|
实施例1166 |
550 |
C22 H24 Cl2 N4 O4 |
479 |
18.9* |
58 |
|
实施例1167 |
551 |
C24 H30 Cl N3 O2 |
428 |
14.2* |
49 |
|
实施例1168 |
552 |
C24 H27 Cl F3 N3 O2 |
482 |
30.6* |
94 |
|
实施例1169 |
553 |
C25 H26 Cl F6 N3 O2 |
550 |
38.0* |
quant |
|
实施例1170 |
554 |
C24 H26 Cl F N4 O2 |
457 |
0.9* |
3 |
|
实施例1171 |
555 |
C24 H26 Cl2 N4 O2 |
473 |
11.1* |
35 |
|
实施例1172 |
556 |
C25 H29 Cl N4 O2 |
453 |
12.5* |
41 |
|
实施例1173 |
559 |
C25 H26 Cl F6 N3 O2 |
550 |
15 |
72 |
|
实施例1174 |
560 |
C24 H27 Cl N4 O2 |
439 |
12 |
68 |
|
实施例1175 |
561 |
C23 H27 Br Cl N3 O2 |
494 |
14 |
73 |
|
实施例1176 |
562 |
C23 H27 Cl2 N3 O2 |
448 |
13 |
75 |
|
实施例1177 |
563 |
C25 H26 Cl F6 N3 O2 |
550 |
14 |
66 |
|
实施例1178 |
564 |
C25 H32 Cl N3 O3 |
458 |
5 |
28 |
|
实施例1179 |
565 |
C24 H26 Cl F4 N3 O2 |
500 |
12 |
61 |
|
实施例1180 |
566 |
C24 H27 Cl F3 N3 O3 |
498 |
12 |
62 |
|
实施例1181 |
567 |
C24 H26 Cl2 F3 N3 O2 |
516 |
12 |
61 |
|
实施例1182 |
568 |
C24 H26 Cl F4 N3 O2 |
500 |
15 |
77 |
|
实施例1183 |
569 |
C24 H26 Cl F4 N3 O2 |
500 |
11 |
59 |
|
实施例1184 |
570 |
C24 H26 Cl F4 N3 O2 |
500 |
16 |
84 |
|
实施例1185 |
571 |
C26 H34 Cl N3 O3 |
472 |
14 |
77 |
|
实施例1186 |
572 |
C24 H28 Cl F3 N4 O2 |
497 |
11 |
55 |
|
实施例1187 |
573 |
C21 H25 Br Cl N3 O2 S |
500 |
12 |
64 |
|
实施例1188 |
574 |
C21 H25 Br Cl N3 O2 S |
500 |
15 |
75 |
|
实施例1189 |
575 |
C25 H34 Cl N3 O3 |
460 |
16 |
87 |
|
实施例1190 |
576 |
C22 H28 Cl N3 O2 S2 |
466 |
13 |
71 |
|
实施例1191 |
577 |
C22 H28 Cl N3 O3 |
418 |
12 |
72 |
|
实施例1192 |
578 |
C25 H28 Cl N3 O2 S |
470 |
15 |
81 |
|
实施例1193 |
579 |
C25 H29 Cl N4 O2 |
453 |
17 |
94 |
|
实施例1194 |
580 |
C22 H28 Cl N3 O2 S |
434 |
15 |
91 |
|
实施例1195 |
581 |
C21 H26 Cl N3 O2 S |
420 |
13 |
80 |
|
实施例1196 |
582 |
C22 H28 Cl N3 O2 S |
434 |
10 |
59 |
|
实施例1197 |
583 |
C26 H31 Cl N4 O2 |
467 |
6 |
31 |
|
实施例1198 |
584 |
C30 H32 Cl N3 O3 |
518 |
18 |
92 |
|
实施例1199 |
585 |
C24 H27 Cl N4 O2 |
439 |
14 |
85 |
|
实施例1200 |
586 |
C23 H27 Cl2 N3 O2 |
448 |
17 |
97 |
|
实施例1201 |
587 |
C24 H27 Cl F3 N3 O2 |
482 |
17 |
91 |
|
实施例1202 |
588 |
C23 H29 Cl N4 O2 |
429 |
5 |
29 |
|
实施例1203 |
589 |
C27 H36 Cl N3 O2 |
470 |
4 |
24 |
|
实施例1204 |
590 |
C26 H34 Cl N3 O2 |
456 |
6 |
36 |
|
实施例1205 |
591 |
C25 H33 Cl N4 O2 |
457 |
7 |
38 |
|
实施例1206 |
592 |
C24 H30 Cl N3 O3 |
444 |
4 |
20 |
|
实施例1207 |
593 |
C24 H30 Cl N3 O3 |
444 |
2 |
14 |
|
实施例1208 |
594 |
C23 H28 Cl N3 O3 |
430 |
4 |
25 |
|
实施例1209 |
595 |
C25 H30 Cl N3 O4 |
472 |
7 |
38 |
|
实施例1210 |
596 |
C25 H30 Cl N3 O3 |
456 |
7 |
40 |
|
实施例1211 |
597 |
C25 H30 Cl N3 O3 |
456 |
15 |
85 |
|
实施例1212 |
598 |
C21 H26 Cl N3 O3 |
404 |
15 |
94 |
|
实施例1213 |
599 |
C22 H29 Cl N4 O2 |
417 |
5 |
30 |
|
实施例1214 |
600 |
C21 H25 Br Cl N3 O3 |
484 |
6 |
34 |
|
实施例1215 |
601 |
C24 H30 Cl N3 O3 |
444 |
5 |
28 |
|
实施例1216 |
602 |
C25 H33 Cl N4 O2 |
457 |
5 |
28 |
|
实施例1217 |
603 |
C23 H29 Cl N4 O2 |
429 |
4 |
22 |
|
实施例1218 |
604 |
C21 H27 Cl N4 O2 |
403 |
9 |
58 |
|
实施例1219 |
605 |
C21 H26 Cl N3 O3 |
404 |
17 |
87 |
|
实施例1220 |
606 |
C21 H26 Cl N3 O2 S |
420 |
15 |
74 |
|
实施例1221 |
607 |
C22 H28 Cl N3 O3 S |
450 |
31 |
quant |
|
实施例1222 |
608 |
C23 H30 Cl N3 O3 |
432 |
17 |
80 |
|
实施例1223 |
609 |
C22 H28 Cl N3 O3 |
418 |
18 |
89 |
|
实施例1224 |
610 |
C23 H28 Cl N3 O3 S |
462 |
20 |
86 |
|
实施例1225 |
611 |
C26 H36 Cl N3 O3 |
474 |
21 |
90 |
|
实施例1226 |
612 |
C28 H32 Cl N3 O3 |
494 |
20 |
84 |
|
实施例1227 |
613 |
C23 H27 Cl F3 N3 O3 |
486 |
19 |
81 |
|
实施例1228 |
614 |
C24 H33 Cl N4 O2 |
445 |
23 |
quant |
|
实施例1229 |
615 |
C25 H29 Cl N4 O2 |
453 |
4 |
20 |
|
实施例1230 |
616 |
C32 H35 Cl N4 O2 |
543 |
11 |
40 |
|
实施例1231 |
617 |
C25 H27 Cl F3 N3 O2 |
482 |
6.7 |
37 |
|
实施例1232 |
620 |
C25 H31 Br Cl N3 O2 |
520 |
15 |
49 |
|
实施例1233 |
621 |
C25 H31 Cl2 N3 O2 |
476 |
18 |
64 |
|
实施例1234 |
622 |
C27 H37 Cl N4 O2 |
485 |
14 |
50 |
|
实施例1235 |
623 |
C26 H34 Cl N3 O3 |
472 |
19 |
69 |
|
实施例1236 |
624 |
C25 H31 Cl N4 O4 |
487 |
21 |
73 |
|
实施例1237 |
625 |
C25 H33 Cl N4 O2 |
457 |
19 |
69 |
|
实施例1238 |
626 |
C27 H30 Cl F6 N3 O2 |
578 |
8 |
25 |
|
实施例1239 |
627 |
C27 H36 Cl N3 O3 |
486 |
16 |
55 |
|
实施例1240 |
628 |
C27 H34 Cl N3 O4 |
500 |
24 |
80 |
|
实施例1241 |
629 |
C26 H30 Cl F4 N3 O2 |
528 |
18 |
56 |
|
实施例1242 |
630 |
C26 H31 Cl F3 N3 O3 |
526 |
21 |
68 |
|
实施例1243 |
631 |
C26 H30 Cl2 F3 N3 O2 |
544 |
15 |
48 |
|
实施例1244 |
632 |
C26 H30 Cl F4 N3 O2 |
528 |
13 |
41 |
|
实施例1245 |
633 |
C26 H30 Cl F4 N3 O2 |
528 |
20 |
63 |
|
实施例1246 |
634 |
C26 H30 Cl F4 N3 O2 |
528 |
19 |
62 |
|
实施例1247 |
635 |
C28 H38 Cl N3 O3 |
500 |
11 |
36 |
|
实施例1248 |
636 |
C26 H34 Cl N3 O2 |
456 |
21 |
89 |
|
实施例1249 |
637 |
C26 H31 Cl F3 N3 O2 |
510 |
20 |
95 |
|
实施例1250 |
638 |
C26 H31 Cl N4 O2 |
467 |
15 |
54 |
|
实施例1251 |
639 |
C27 H37 Cl N4 O2 |
485 |
19 |
66 |
|
实施例1252 |
640 |
C26 H34 Cl N3 O3 |
472 |
16 |
56 |
|
实施例1253 |
641 |
C27 H34 Cl N3 O4 |
500 |
18 |
59 |
|
实施例1254 |
642 |
C32 H36 Cl N3 O3 |
546 |
24 |
73 |
|
实施例1255 |
643 |
C26 H31 Cl F3 N3 O2 |
510 |
16 |
54 |
|
实施例1256 |
644 |
C29 H40 Cl N3 O2 |
498 |
18 |
61 |
|
实施例1257 |
645 |
C25 H33 Cl N4 O2 |
457 |
22 |
78 |
|
实施例1258 |
646 |
C26 H34 Cl N3 O3 |
472 |
13 |
47 |
|
实施例1259 |
647 |
C27 H34 Cl N3 O3 |
500 |
13 |
46 |
|
实施例1260 |
648 |
C28 H38 Cl N3 O2 |
484 |
17 |
60 |
|
实施例1261 |
649 |
C28 H38 Cl N3 O3 |
500 |
12.5 |
42 |
|
实施例1262 |
650 |
C32 H36 Cl N3 O3 |
546 |
1* |
2 |
|
实施例1263 |
651 |
C28 H35 Cl N4 O2 |
495 |
4* |
12 |
|
实施例1264 |
652 |
C25 H31 Cl N4 O4 |
487 |
5* |
14 |
|
实施例1265 |
653 |
C30 H42 Cl N3 O3 |
528 |
1* |
3 |
|
实施例1266 |
654 |
C27 H34 Cl N3 O3 |
484 |
7* |
21 |
|
实施例1267 |
655 |
C26 H32 Cl F3 N4 O2 |
525 |
6* |
16 |
|
实施例1268 |
656 |
C23 H30 Cl N3 O3 |
432 |
6* |
18 |
|
实施例1269 |
657 |
C23 H30 Cl N3 O2 S |
448 |
4* |
13 |
|
实施例1270 |
658 |
C27 H33 Cl N4 O2 |
48 |
1* |
4 |
|
实施例1271 |
659 |
C23 H29 Cl N4 O4 S |
493 |
4* |
10 |
|
实施例1272 |
660 |
C34 H39 Cl N4 O2 |
571 |
3* |
7 |
|
实施例1273 |
661 |
C24 H32 Cl N3 O3 S |
478 |
3* |
7 |
|
实施例1274 |
662 |
C25 H34 Cl N3 O3 |
460 |
2* |
6 |
|
实施例1275 |
663 |
C24 H32 Cl N3 O3 |
446 |
2* |
5 |
|
实施例1276 |
664 |
C24 H31 Cl N4 O5 |
491 |
2* |
5 |
|
实施例1277 |
665 |
C25 H32 Cl N3 O3 S |
490 |
1* |
3 |
|
实施例1278 |
666 |
C26 H37 Cl N4 O2 |
473 |
3* |
7 |
|
实施例1279 |
667 |
C30 H36 Cl N3 O3 |
522 |
3* |
7 |
|
实施例1280 |
668 |
C25 H31 Cl F3 N3 O3 |
514 |
2* |
6 |
|
实施例1281 |
669 |
C24 H33 Cl N4 O2 |
445 |
15* |
45 |
|
实施例1282 |
670 |
C23 H29 Br Cl N3 O3 |
510 |
3* |
7 |
|
实施例1283 |
671 |
C23 H29 Cl N4 O5 |
477 |
2* |
5 |
|
实施例1284 |
672 |
C23 H31 Cl N4 O2 |
431 |
2* |
7 |
|
实施例1285 |
673 |
C23 H30 Cl N3 O2 S |
448 |
2* |
6 |
|
实施例1286 |
674 |
C24 H32 Cl N3 O2 S |
462 |
3* |
9 |
|
实施例1287 |
675 |
C24 H32 Cl N3 O2 S |
462 |
1* |
4 |
|
实施例1288 |
676 |
C27 H33 Cl N4 O2 |
482 |
2* |
6 |
|
实施例1289 |
677 |
C28 H35 Cl N4 O2 |
495 |
2* |
6 |
|
实施例1290 |
678 |
C24 H32 Cl N3 O3 |
446 |
3* |
9 |
|
实施例1291 |
679 |
C27 H32 Cl N3 O2 S |
498 |
1* |
3 |
|
实施例1292 |
680 |
C23 H29 Br Cl N3 O2 S |
526 |
2* |
6 |
|
实施例1293 |
681 |
C25 H34 Cl N3 O3 |
460 |
2* |
5 |
|
实施例1294 |
682 |
C27 H38 Cl N3 O3 |
488 |
2* |
4 |
|
实施例1295 |
683 |
C24 H32 Cl N3 O2 S2 |
494 |
1* |
4 |
|
实施例1296 |
684 |
C26 H36 Cl N3 O4 S2 |
554 |
2* |
5 |
|
实施例1297 |
685 |
C24 H32 Cl N3 O4 S2 |
526 |
3* |
7 |
|
实施例1298 |
687 |
C25 H30 Cl N3 O2 |
440 |
24 |
quant |
|
实施例1299 |
688 |
C27 H28 Cl F6 N3 O2 |
576 |
28 |
98 |
|
实施例1300 |
689 |
C26 H29 Cl N4 O2 |
465 |
23 |
99 |
|
实施例1301 |
690 |
C25 H29 Br Cl N3 O2 |
518 |
26 |
99 |
|
实施例1302 |
691 |
C27 H35 Cl N4 O2 |
483 |
24 |
97 |
|
实施例1303 |
692 |
C26 H32 Cl N3 O3 |
470 |
24 |
quant |
|
实施例1304 |
693 |
C27 H28 Cl F6 N3 O2 |
576 |
16 |
55 |
|
实施例1305 |
694 |
C27 H34 Cl N3 O3 |
484 |
25 |
quant |
|
实施例1306 |
695 |
C27 H32 Cl N3 O4 |
498 |
12 |
47 |
|
实施例1307 |
696 |
C26 H29 Cl F3 N3 O3 |
524 |
25 |
95 |
|
实施例1308 |
697 |
C26 H29 Cl N4 O2 |
465 |
15 |
64 |
|
实施例1309 |
698 |
C27 H35 Cl N4 O2 |
483 |
24 |
quant |
|
实施例1310 |
699 |
C26 H32 Cl N3 O3 |
470 |
26 |
quant |
|
实施例1311 |
700 |
C27 H32 Cl N3 O4 |
498 |
15 |
62 |
|
实施例1312 |
701 |
C27 H32 Cl N3 O3 |
482 |
11 |
44 |
|
实施例1313 |
702 |
C26 H29 Cl F3 N3 O2 |
508 |
23 |
94 |
|
实施例1314 |
703 |
C28 H36 Cl N3 O2 |
482 |
26 |
quant |
|
实施例1315 |
704 |
C25 H29 Cl N4 O4 |
485 |
11 |
43 |
|
实施例1316 |
705 |
C24 H30 Cl N3 O2 S |
460 |
25 |
quant |
|
实施例1317 |
706 |
C24 H30 Cl N3 O2 S |
460 |
25 |
quant |
|
实施例1318 |
707 |
C26 H29 Cl F3 N3 O2 |
508 |
15 |
55 |
|
实施例1319 |
708 |
C23 H27 Br Cl N3 O2 S |
526 |
25 |
92 |
|
实施例1320 |
709 |
C24 H30 Cl N3 O2 S2 |
492 |
26 |
quant |
|
实施例1321 |
710 |
C23 H27 Br Cl N3 O2 S |
526 |
25 |
94 |
|
实施例1322 |
711 |
C25 H32 Cl N3 O3 |
458 |
26 |
quant |
|
实施例1323 |
712 |
C27 H30 Cl N3 O2 S |
496 |
26 |
quant |
|
实施例1324 |
713 |
C24 H30 Cl N3 O3 |
444 |
26 |
quant |
|
实施例1325 |
714 |
C28 H33 Cl N4 O2 |
493 |
12 |
50 |
|
实施例1326 |
715 |
C23 H28 Cl N3 O2 S |
446 |
24 |
quant |
|
实施例1327 |
716 |
C27 H31 Cl N4 O2 |
479 |
32 |
quant |
|
实施例1328 |
717 |
C23 H27 Cl N4 O5 |
475 |
23 |
95 |
|
实施例1329 |
718 |
C23 H29 Cl N4 O2 |
429 |
24 |
quant |
|
实施例1330 |
719 |
C23 H28 Cl N3 O3 |
430 |
24 |
quant |
|
实施例1331 |
720 |
C23 H27 Br Cl N3 O3 |
510 |
24 |
95 |
|
实施例1332 |
721 |
C24 H31 Cl N4 O2 |
443 |
22 |
98 |
|
实施例1333 |
722 |
C26 H32 Cl N3 O3 |
470 |
9 |
37 |
|
实施例1334 |
723 |
C25 H31 Cl N4 O2 |
455 |
10 |
44 |
|
实施例1335 |
724 |
C29 H38 Cl N3 O2 |
496 |
28 |
quant |
|
实施例1336 |
725 |
C32 H34 Cl N3 O3 |
544 |
26 |
95 |
|
实施例1337 |
726 |
C27 H33 Cl N4 O3 |
497 |
3 |
11 |
|
实施例1338 |
727 |
C25 H29 Cl2 N3 O2 |
474 |
25 |
quant |
|
实施例1339 |
728 |
C25 H31 Cl N4 O2 |
455 |
21 |
92 |
|
实施例1340 |
729 |
C25 H29 Cl N4 O4 |
485 |
26 |
quant |
|
实施例1341 |
730 |
C25 H29 Cl2 N3 O2 |
474 |
21 |
90 |
|
实施例1342 |
731 |
C27 H32 Cl N3 O3 |
482 |
10 |
41 |
|
实施例1343 |
732 |
C26 H28 Cl F4 N3 O2 |
526 |
27 |
quant |
|
实施例1344 |
733 |
C28 H36 Cl N3 O3 |
498 |
22 |
89 |
|
实施例1345 |
734 |
C26 H28 Cl F4 N3 O2 |
526 |
25 |
94 |
|
实施例1346 |
735 |
C26 H28 Cl F4 N3 O2 |
526 |
23 |
87 |
|
实施例1347 |
736 |
C26 H30 Cl F3 N4 O2 |
523 |
24 |
78 |
|
实施例1348 |
737 |
C26 H28 Cl F4 N3 O2 |
526 |
21 |
66 |
|
实施例1349 |
738 |
C25 H32 Cl N3 O3 |
458 |
23 |
84 |
|
实施例1350 |
739 |
C27 H31 Cl N4 O2 |
479 |
19 |
66 |
|
实施例1351 |
740 |
C24 H31 Cl N4 O5 |
489 |
23 |
77 |
|
实施例1352 |
741 |
C23 H27 Cl N4 O4 S |
491 |
26 |
88 |
|
实施例1353 |
742 |
C24 H30 Cl N3 O3 S |
476 |
23 |
82 |
|
实施例1354 |
743 |
C23 H28 Cl N3 O3 |
430 |
21 |
81 |
|
实施例1355 |
744 |
C26 H32 Cl N3 O2 |
454 |
25 |
91 |
|
实施例1356 |
745 |
C27 H36 Cl N3 O3 |
486 |
23 |
80 |
|
实施例1357 |
746 |
C26 H35 Cl N4 O2 |
471 |
27 |
96 |
|
实施例1358 |
747 |
C25 H29 Cl F3 N3 O3 |
512 |
23 |
74 |
|
实施例1359 |
748 |
C23 H28 Cl N3 O2 S |
446 |
22 |
82 |
|
实施例1360 |
751 |
C24 H30 Cl N3 O3 |
444 |
3 |
11 |
|
实施例1361 |
752 |
C25 H26 Cl F6 N3 O3 |
566 |
7 |
20 |
|
实施例1362 |
753 |
C24 H27 Cl N4 O3 |
455 |
6 |
22 |
|
实施例1363 |
754 |
C23 H27 Cl2 N3 O3 |
464 |
8 |
29 |
|
实施例1364 |
755 |
C24 H30 Cl N3 O4 |
460 |
6 |
22 |
|
实施例1365 |
756 |
C23 H27 Cl N4 O5 |
475 |
5 |
18 |
|
实施例1366 |
757 |
C25 H32 Cl N3 O4 |
474 |
5 |
18 |
|
实施例1367 |
758 |
C25 H30 Cl N3 O5 |
488 |
5 |
18 |
|
实施例1368 |
759 |
C24 H27 Cl F3 N3 O4 |
514 |
6 |
20 |
|
实施例1369 |
760 |
C24 H26 Cl F4 N3 O3 |
516 |
6 |
18 |
|
实施例1370 |
761 |
C24 H26 Cl F4 N3 O3 |
516 |
3 |
10 |
|
实施例1371 |
762 |
C24 H27 Cl F3 N3 O3 |
498 |
2 |
95 |
|
实施例1372 |
763 |
C23 H28 Cl N3 O3 |
430 |
4 |
95 |
|
实施例1373 |
764 |
C24 H30 Cl N3 O2 |
428 |
9 |
42 |
|
实施例1374 |
765 |
C25 H32 Cl N3 O2 |
442 |
10 |
47 |
|
实施例1375 |
766 |
C25 H29 Cl F3 N3 O2 |
496 |
10 |
42 |
|
实施例1376 |
767 |
C25 H32 Cl N3 O4 S |
506 |
8 |
32 |
|
实施例1377 |
768 |
C24 H29 Br Cl N3 O2 |
506 |
9 |
35 |
|
实施例1378 |
769 |
C25 H29 Cl F3 N3 O3 |
512 |
6 |
22 |
|
实施例1379 |
770 |
C25 H28 Cl F4 N3 O2 |
514 |
3 |
10 |
|
实施例1380 |
771 |
C25 H28 Cl F4 N3 O2 |
514 |
10 |
37 |
|
实施例1381 |
772 |
C25 H29 Cl F3 N3 O2 |
496 |
8 |
33 |
|
实施例1382 |
773 |
C26 H36 Cl N3 O3 |
474 |
10 |
41 |
|
实施例1383 |
774 |
C23 H30 Cl N3 O2 S2 |
480 |
12 |
50 |
|
实施例1384 |
775 |
C27 H38 Cl N3 O3 |
488 |
14 |
57 |
|
实施例1385 |
776 |
C29 H34 Cl N3 O3 |
508 |
12 |
49 |
|
实施例1386 |
777 |
C24 H29 Cl F3 N3 O3 |
500 |
22 |
87 |
|
实施例1387 |
778 |
C24 H28 Cl2 N4 O4 |
507 |
6 |
22 |
|
实施例1388 |
779 |
C24 H29 Cl2 N3 O2 |
462 |
10 |
46 |
|
实施例1389 |
780 |
C24 H29 Cl N4 O4 |
473 |
15 |
65 |
|
实施例1390 |
781 |
C26 H31 Cl N4 O2 |
467 |
7* |
20 |
|
实施例1391 |
782 |
C25 H32 Cl N3 O3 |
458 |
8* |
23 |
|
实施例1392 |
783 |
C26 H34 Cl N3 O3 |
472 |
7* |
19 |
|
实施例1393 |
784 |
C26 H31 Cl F3 N3 O2 |
510 |
7* |
17 |
|
实施例1394 |
785 |
C26 H34 Cl N3 O4 |
488 |
6* |
17 |
|
实施例1395 |
786 |
C24 H28 Cl N3 O2 |
426 |
22 |
9 |
|
实施例1396 |
787 |
C25 H30 Cl N3 O2 |
440 |
21 |
94 |
|
实施例1397 |
788 |
C25 H27 Cl F3 N3 O2 |
494 |
4* |
14 |
|
实施例1398 |
789 |
C25 H30 Cl N3 O4 S |
504 |
9 |
35 |
|
实施例1399 |
790 |
C24 H27 Cl2 N3 O2 |
460 |
5* |
16 |
|
实施例1400 |
791 |
C24 H27 Cl N4 O4 |
471 |
3* |
10 |
|
实施例1401 |
792 |
C25 H27 Cl F3 N3 O3 |
510 |
5* |
16 |
|
实施例1402 |
793 |
C25 H26 Cl F4 N3 O2 |
511 |
5* |
16 |
|
实施例1403 |
794 |
C25 H26 Cl F4 N3 O2 |
512 |
5* |
16 |
|
实施例1404 |
795 |
C25 H27 Cl F3 N3 O2 |
494 |
6* |
21 |
|
实施例1405 |
796 |
C23 H28 Cl N3 O2 S2 |
478 |
4* |
14 |
|
实施例1406 |
797 |
C27 H36 Cl N3 O3 |
486 |
7* |
29 |
|
实施例1407 |
798 |
C29 H32 Cl N3 O3 |
506 |
3 |
13 |
|
实施例1408 |
799 |
C24 H27 Cl F3 N3 O3 |
498 |
3* |
11 |
|
实施例1409 |
800 |
C24 H26 Cl2 N4 O4 |
505 |
5* |
15 |
|
实施例1410 |
801 |
C26 H29 Cl N4 O2 |
465 |
12 |
41 |
|
实施例1411 |
802 |
C25 H30 Cl N3 O3 |
456 |
5* |
15 |
|
实施例1412 |
803 |
C26 H32 Cl N3 O3 |
470 |
6* |
16 |
|
实施例1413 |
804 |
C26 H29 Cl F3 N3 O2 |
508 |
8* |
20 |
|
实施例1414 |
805 |
C26 H32 Cl N3 O4 |
486 |
6* |
15 |
|
实施例1415 |
806 |
C24 H27 Br Cl N3 O2 |
506 |
5* |
14 |
|
实施例1416 |
807 |
C27 H32 Cl N5 O3 |
510 |
29.7 |
quant |
|
实施例1417 |
808 |
C26 H33 Cl N4 O3 |
485 |
29.9 |
quant |
|
实施例1418 |
809 |
C25 H30 Cl2 N4 O3 |
505 |
30.2 |
quant |
|
实施例1419 |
810 |
C30 H35 Cl N4 O4 |
551 |
31.0 |
quant |
|
实施例1420 |
811 |
C25 H29 Cl2 N5 O5 |
550 |
30.4 |
quant |
|
实施例1421 |
812 |
C24 H31 Cl N4 O3 S2 |
523 |
25.0 |
88 |
|
实施例1422 |
813 |
C26 H30 Cl F3 N4 O3 |
539 |
20.5 |
70 |
|
实施例1423 |
814 |
C26 H30 Cl F3 N4 O4 |
555 |
22.7 |
75 |
|
实施例1424 |
815 |
C26 H29 Cl F4 N4 O3 |
557 |
25.8 |
85 |
|
实施例1425 |
816 |
C26 H30 Cl F3 N4 O3 |
539 |
25.3 |
86 |
|
实施例1426 |
817 |
C26 H29 Cl F4 N4 O3 |
557 |
26.8 |
88 |
|
实施例1427 |
818 |
C25 H30 Br Cl N4 O3 |
551 |
27.1 |
90 |
|
实施例1428 |
819 |
C27 H29 Cl F6 N4 O3 |
607 |
13.9 |
42 |
|
实施例1429 |
820 |
C25 H30 Cl N5 O5 |
516 |
14.1 |
51 |
|
实施例1430 |
821 |
C24 H28 Cl2 N4 O5 |
523 |
40 |
86 |
|
实施例1431 |
822 |
C23 H30 Cl N3 O3 S2 |
496 |
41 |
93 |
|
实施例1432 |
823 |
C26 H31 Cl N4 O3 |
483 |
43 |
quant |
|
实施例1433 |
824 |
C27 H38 Cl N3 O4 |
503 |
37 |
83 |
|
实施例1434 |
825 |
C29 H34 Cl N3 O4 |
524 |
28 |
61 |
|
实施例1435 |
826 |
C24 H29 Cl F3 N3 O4 |
516 |
40 |
87 |
|
实施例1436 |
827 |
C26 H31 Cl N4 O3 |
483 |
31 |
72 |
|
实施例1437 |
828 |
C25 H29 Cl F3 N3 O4 |
528 |
40 |
86 |
|
实施例1438 |
829 |
C25 H28 Cl F4 N3 O3 |
530 |
45 |
97 |
|
实施例1439 |
830 |
C25 H28 Cl F4 N3 O3 |
530 |
35 |
74 |
|
实施例1440 |
831 |
C24 H29 Br Cl N3 O3 |
523 |
45 |
98 |
|
实施例1441 |
832 |
C24 H29 Cl2 N3 O3 |
478 |
38 |
91 |
|
实施例1442 |
833 |
C24 H29 Cl N4 O5 |
488 |
38 |
87 |
|
实施例1443 |
834 |
C25 H29 Cl F3 N3 O3 |
512 |
42 |
93 |
|
实施例1444 |
835 |
C24 H30 Cl N3 O3 |
444 |
43 |
quant |
|
实施例1445 |
836 |
C25 H32 Cl N3 O3 |
458 |
37 |
91 |
|
实施例1446 |
837 |
C25 H29 Cl F3 N3 O3 |
512 |
41 |
91 |
|
实施例1447 |
838 |
C26 H34 Cl N3 O4 |
488 |
34 |
78 |
|
实施例1448 |
839 |
C27 H36 Cl N3 O6 |
534 |
37 |
71 |
|
实施例1449 |
942 |
C27 H30 Cl F6 N3 O2 |
578 |
17 |
48 |
|
实施例1450 |
997 |
C26 H34 Cl N3 O2 |
456 |
7.6* |
23 |
|
实施例1451 |
998 |
C27 H33 Cl F3 N3 O2 |
524 |
6 |
15 |
|
实施例1452 |
999 |
C27 H36 Cl N3 O2 |
470 |
8 |
24 |
|
实施例1453 |
1000 |
C27 H36 Cl N3 O3 |
486 |
9 |
24 |
|
实施例1454 |
1001 |
C28 H38 Cl N3 O3 |
500 |
4 |
10 |
|
实施例1455 |
1002 |
C27 H33 Cl F3 N3 O3 |
540 |
9 |
23 |
|
实施例1456 |
1003 |
C28 H38 Cl N3 O2 |
484 |
7 |
21 |
|
实施例1457 |
1004 |
C28 H38 Cl N3 O4 |
516 |
11 |
30 |
|
实施例1458 |
1005 |
C29 H40 Cl N3 O5 |
547 |
9 |
23 |
|
实施例1459 |
1006 |
C30 H42 Cl N3 O4 |
544 |
8 |
21 |
|
实施例1460 |
1007 |
C32 H46 Cl N3 O5 |
589 |
7 |
17 |
|
实施例1461 |
1008 |
C25 H31 Cl N4 O3 |
471 |
25 |
79 |
|
实施例1462 |
1009 |
C26 H33 Cl N4 O4 |
501 |
35 |
97 |
|
实施例1463 |
1010 |
C27 H35 Cl N4 O4 |
515 |
35 |
9 |
|
实施例1464 |
1011 |
C27 H35 Cl N4 O3 |
499 |
32 |
54 |
|
实施例1465 |
1012 |
C27 H35 Cl N4 O5 |
531 |
27 |
77 |
|
实施例1466 |
1013 |
C28 H37 Cl N4 O6 |
561 |
14 |
37 |
|
实施例1467 |
1014 |
C29 H39 Cl N4 O5 |
559 |
24 |
66 |
|
实施例1468 |
1015 |
C31 H43 Cl N4 O6 |
603 |
25 |
65 |
|
实施例1469 |
1018 |
C26 H34 Cl N3 O4 |
488 |
13.0* |
39 |
|
实施例1470 |
1019 |
C28 H38 Cl N3 O5 |
532 |
13.4* |
37 |
|
实施例1471 |
1020 |
C25 H32 Cl N3 O4 |
474 |
12.7* |
40 |
|
实施例1472 |
1021 |
C26 H28 Cl F6 N3 O4 |
596 |
13.8* |
34 |
|
实施例1473 |
1022 |
C25 H32 Cl N3 O4 |
474 |
14.2* |
37 |
|
实施例1474 |
1023 |
C25 H32 Cl N3 O2 |
442 |
11.5* |
32 |
|
实施例1475 |
1024 |
C26 H34 Cl N3 O5 |
504 |
12.0* |
30 |
|
实施例1476 |
1025 |
C27 H36 Cl N3 O4 |
502 |
14.7* |
37 |
|
实施例1477 |
1026 |
C29 H40 Cl N3 O5 |
546 |
13.5* |
32 |
|
实施例1478 |
1027 |
C26 H34 Cl N3 O4 |
488 |
11.9* |
31 |
|
实施例1479 |
1028 |
C27 H30 Cl F6 N3 O4 |
610 |
14.6* |
31 |
|
实施例1480 |
1029 |
C25 H32 Cl N3 O3 |
458 |
14.0* |
38 |
|
实施例1481 |
1030 |
C24 H27 Cl F3 N3 O3 |
498 |
14.0* |
35 |
|
实施例1482 |
1031 |
C24 H30 Cl N3 O3 |
444 |
10.4* |
29 |
|
实施例1483 |
1032 |
C25 H32 Cl N3 O4 |
474 |
14.9* |
39 |
|
实施例1484 |
1033 |
C25 H32 Cl N3 O2 |
442 |
13.3* |
37 |
|
实施例1485 |
1034 |
C26 H34 Cl N3 O5 |
504 |
13.7* |
34 |
|
实施例1486 |
1035 |
C27 H36 Cl N3 O4 |
502 |
16.7* |
42 |
|
实施例1487 |
1036 |
C29 H40 Cl N3 O5 |
547 |
15.5* |
36 |
|
实施例1488 |
1037 |
C26 H34 Cl N3 O4 |
488 |
14.1* |
36 |
|
实施例1489 |
1038 |
C27 H30 Cl F6 N3 O4 |
610 |
17.5* |
37 |
|
实施例1490 |
1039 |
C25 H32 Cl N3 O3 |
458 |
15.1* |
41 |
|
实施例1491 |
1040 |
C24 H27 Cl F3 N3 O3 |
498 |
15.4* |
39 |
|
实施例1492 |
1041 |
C24 H30 Cl N3 O3 |
444 |
12.7* |
35 |
|
实施例1493 |
1042 |
C22 H26 Br Cl N4 O2 |
495 |
10.4* |
25 |
|
实施例1494 |
1043 |
C22 H26 Cl2 N4 O2 |
449 |
11.1* |
29 |
|
实施例1495 |
1044 |
C23 H29 Cl N4 O2 |
429 |
5.2* |
14 |
|
实施例1496 |
1045 |
C23 H29 Cl N4 O3 |
445 |
12.4* |
33 |
|
实施例1497 |
1046 |
C22 H25 Cl3 N4 O2 |
483 |
10.0* |
25 |
|
实施例1498 |
1047 |
C24 H31 Cl N4 O2 |
443 |
12.1* |
32 |
|
实施例1499 |
1048 |
C25 H33 Cl N4 O5 |
505 |
16.1* |
39 |
|
实施例1500 |
1049 |
C23 H28 Br Cl N4 O2 |
507 |
12.0* |
29 |
|
实施例1501 |
1050 |
C28 H38 Cl N3 O4 |
516 |
39.2* |
quant |
|
实施例1502 |
1051 |
C28 H38 Cl N3 O2 |
484 |
34.0* |
quant |
|
实施例1503 |
1052 |
C29 H40 Cl N3 O5 |
546 |
14.5* |
39 |
|
实施例1504 |
1053 |
C30 H42 Cl N3 O4 |
544 |
11.8* |
32 |
|
实施例1505 |
1054 |
C32 H46 Cl N3 O5 |
588 |
12.2* |
31 |
|
实施例1506 |
1055 |
C29 H40 Cl N3 O4 |
530 |
44.5* |
quant |
|
实施例1507 |
1056 |
C30 H36 Cl F6 N3 O4 |
652 |
46.0* |
quant |
|
实施例1508 |
1057 |
C28 H38 Cl N3 O3 |
500 |
11.2* |
32 |
|
实施例1509 |
1058 |
C27 H36 Cl N3 O3 |
486 |
35.5* |
quant |
|
实施例1510 |
1059 |
C27 H33 Cl F3 N3 O3 |
540 |
41.4* |
quant |
|
实施例1511 |
1060 |
C29 H40 Cl N3 O4 |
530 |
13.6* |
37 |
|
实施例1512 |
1061 |
C30 H36 Cl F6 N3 O4 |
652 |
44.2* |
quant |
|
实施例1513 |
1062 |
C28 H38 Cl N3 O3 |
500 |
39.9* |
quant |
|
实施例1514 |
1063 |
C27 H36 Cl N3 O3 |
486 |
12.0* |
35 |
|
实施例1515 |
1064 |
C27 H33 Cl F3 N3 O3 |
540 |
37.8* |
quant |
|
实施例1516 |
1065 |
C28 H38 Cl N3 O4 |
516 |
12.3* |
34 |
|
实施例1517 |
1066 |
C28 H38 Cl N3 O2 |
484 |
30.7* |
90 |
|
实施例1518 |
1067 |
C29 H40 Cl N3 O5 |
546 |
13.8* |
37 |
|
实施例1519 |
1068 |
C30 H42 Cl N3 O4 |
544 |
13.4* |
35 |
|
实施例1520 |
1069 |
C32 H46 Cl N3 O5 |
589 |
14.1* |
35 |
|
实施例1521 |
1070 |
C29 H34 Cl N3 O3 S2 |
572 |
38.3 |
93 |
|
实施例1522 |
1071 |
C32 H35 Cl N4 O3 |
559 |
39.6 |
98 |
|
实施例1523 |
1072 |
C33 H42 Cl N3 O4 |
580 |
40.9 |
98 |
|
实施例1524 |
1073 |
C35 H38 Cl N3 O4 |
600 |
40.5 |
94 |
|
实施例1525 |
1074 |
C30 H33 Cl F3 N3 O4 |
592 |
38.7 |
91 |
|
实施例1526 |
1075 |
C31 H33 Cl F3 N3 O4 |
604 |
38 |
87 |
|
实施例1527 |
1076 |
C30 H33 Cl N4 O5 |
565 |
38.5 |
94 |
|
实施例1528 |
1077 |
C31 H33 Cl F3 N3 O3 |
588 |
35.8 |
84 |
|
实施例1529 |
1078 |
C30 H34 Cl N3 O3 |
520 |
34.7 |
93 |
|
实施例1530 |
1079 |
C31 H36 Cl N3 O3 |
534 |
38.4 |
quant |
|
实施例1531 |
1080 |
C32 H38 Cl N3 O4 |
564 |
39.3 |
97 |
|
实施例1532 |
1081 |
C33 H40 Cl N3 O6 |
610 |
45.5 |
quant |
|
实施例1533 |
1082 |
C28 H36 Cl N3 O3 |
498 |
4.1* |
10 |
|
实施例1534 |
1083 |
C28 H36 Cl N3 O3 |
498 |
6.4* |
16 |
|
实施例1535 |
1125 |
C30 H32 Cl2 N4 O5 |
599 |
3.4* |
8 |
|
实施例1536 |
1126 |
C30 H32 Br Cl N4 O5 |
644 |
3.4* |
7 |
|
实施例1537 |
1127 |
C32 H35 Cl N4 O3 |
559 |
1.6* |
4 |
|
实施例1538 |
1128 |
C31 H32 Cl F4 N3 O3 |
606 |
4.3* |
10 |
|
实施例1539 |
1129 |
C31 H32 Cl F4 N3 O3 |
606 |
5.9* |
14 |
|
实施例1540 |
1130 |
C30 H33 Br Cl N3 O3 |
599 |
5.7* |
13 |
|
实施例1541 |
1131 |
C30 H33 Cl2 N3 O3 |
554 |
6.4* |
16 |
|
实施例1542 |
1132 |
C31 H33 Cl F3 N3 O3 |
588 |
6.3* |
15 |
|
实施例1543 |
1167 |
C27 H34 Cl N3 O3 |
484 |
1.8* |
4 |
*TFA盐的产量
实施例1544:1-(4-氯苄基)-4-[{N-(3,5-双(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(1213号化合物)的制备
向1-(4-氯苄基)-4-{(甘氨酰氨基)甲基}哌啶(0.050mmol)与哌啶子基甲基聚苯乙烯(58mg)在氯仿(0.2ml)与二氯甲烷(0.75ml)中的混合物中加入3,5-双(三氟甲基)苯甲酰氯(0.058mmol)的二氯甲烷(1ml)溶液。反应混合物在室温下搅拌2小时后,加入甲醇(1.0ml),混合物在室温下搅拌30分钟。将反应混合物装上VarianTM SCX柱,用CH3OH(16ml)洗涤。用2N NH3的CH3OH(6ml)溶液洗脱产物,并浓缩,得到1-(4-氯苄基)-4-[{N-(3,5-双(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(1213号化合物)(24.0mg,90%):纯度用RPLC/MS测定(100%);ESI/MS m/e 536.2(M++H,C24H24ClF6N3O2)。
实施例1545-1547
依据实施例1544的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表28中。
表28
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1545 |
1214 |
C23 H24 Cl F4 N3 O3 |
486.2 |
22.2 |
91 |
|
实施例1546 |
1215 |
C22 H24 Cl3 N3 O2 |
467.9 |
20.9 |
89 |
|
实施例1547 |
1216 |
C22 H24 Cl F2 N3 O2 |
436.0 |
19.3 |
89 |
实施例1548:4-[{N-(3-溴-4-甲基苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)哌啶(1113号化合物)的制备
将1-(4-氯苄基)-4-{(甘氨酰氨基)甲基}哌啶(0.050mmol)在CHCl3(1.35ml)与叔丁醇(0.15ml)中的溶液用3-溴-4-甲基苯甲酸(0.060mmol)、二异丙基碳二亚胺(0.060mmol)和HOBt(0.060mmol)处理。反应混合物在室温下搅拌15小时。将混合物装上VarianTM SCX柱,用CH3OH/CHCl3 1∶1(12ml)和CH3OH(12ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到4-[{N-(3-溴-4-甲基苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)哌啶(1113号化合物)(16.1mg,65%):纯度用RPLC/MS测定(95%);ESI/MS m/e 494.0(M++H,C23H27BrClN3O2)。
实施例1549-1619
依据实施例1548的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC得到所需物质。ESI/MS数据和产率总结在表29中。
所得1422号化合物是1418号化合物的副产物:5.6mg,产率25%;ESI/MS m/e 447.2(C22H27ClN4O2S)。
表29
| | 化合物号 | 分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1549 |
1114 |
C22H24BrClFN3O2 |
498.0 |
20.2 |
81 |
|
实施例1550 |
1115 |
C22H24Cl2FN3O2 |
452.2 |
18.6 |
82 |
|
实施例1551 |
1116 |
C23H27ClIN3O2 |
539.1 |
21.9 |
81 |
|
实施例1552 |
1117 |
C23H27ClN4O4 |
459.2 |
18.7 |
81 |
|
实施例1553 |
1187 |
C23H27BrClN3O2 |
494.0 |
22.1 |
90 |
|
实施例1554 |
1188 |
C24H27ClN4O3 |
455.2 |
17.2 |
76 |
|
实施例1555 |
1189 |
C25H25ClN4O3 |
469.2 |
21.1 |
90 |
|
实施例1556 |
1190 |
C22H26ClFN4O2 |
433.2 |
20.4 |
94 |
|
实施例1557 |
1241 |
C23H24Cl2F3N3O2 |
502.0 |
22.5 |
90 |
|
实施例1558 |
1242 |
C23H27ClFN3O2 |
432.2 |
21.2 |
98 |
|
实施例1559 |
1243 |
C23H27Cl2N3O2 |
448.0 |
21.6 |
96 |
|
实施例1560 |
1244 |
C22H26ClIN4O2 |
541.0 |
26.4 |
98 |
|
实施例1561 |
1245 |
C22H25ClF2N4O2 |
451.0 |
21.3 |
94 |
|
实施例1562 |
1246 |
C21H27ClN4O2 |
403.2 |
19.4 |
96 |
|
实施例1563 |
1247 |
C22H30ClN3O2S |
524.0 |
24.7 |
94 |
|
实施例1564 |
1248 |
C22H25ClN4O5 |
461.0 |
20.7 |
90 |
|
实施例1565 |
1282 |
C25H26ClF3N4O3 |
523.2 |
25.0 |
96 |
|
实施例1566 |
1283 |
C23H27Cl2N3O3 |
464.2 |
12.2 |
53 |
|
实施例1567 |
1284 |
C22H25BrClN3O3 |
496.0 |
24.1 |
97 |
|
实施例1568 |
1285 |
C22H25Cl2N3O3 |
450.2 |
21.8 |
97 |
|
实施例1569 |
1342 |
C22H24BrCl2N3O2 |
514.0 |
27.2 |
quant |
|
实施例1570 |
1343 |
C23H27Cl2N3O2 |
448.0 |
21.4 |
95 |
|
实施例1571 |
1344 |
C22H24Cl2IN3O2 |
560.0 |
27.0 |
96 |
|
实施例1572 |
1345 |
C23H28ClN3O2 |
430.2 |
23.8 |
quant |
|
实施例1573 |
1346 |
C22H25ClIN3O3 |
542.0 |
29.4 |
quant |
|
实施例1574 |
1350 |
C21H26ClN3O2S |
420.0 |
13.0 |
62 |
|
实施例1575 |
1354 |
C24H28BrClN4O3 |
537.2 |
5.2 |
19 |
|
实施例1576 |
1358 |
C23H26ClN5O2 |
440.2 |
21.8 |
99 |
|
实施例1577 |
1383 |
C23H24Cl2F3N3O2 |
502.0 |
20.0 |
80 |
|
实施例1578 |
1384 |
C20H23BrClN3O2S |
486.0 |
21.0 |
87 |
|
实施例1579 |
1385 |
C28H30ClN3O4S |
540.2 |
23.8 |
88 |
|
实施例1580 |
1386 |
C28H30ClN3O2 |
476.0 |
20.0 |
84 |
|
实施例1581 |
1414 |
C24H28Cl2N4O3 |
491.0 |
0.8 |
3 |
|
实施例1582 |
1418 |
C23H26ClN5O2S |
472.0 |
10.4 |
44 |
|
实施例1583 |
1436 |
C29 H30 Cl N3 O3 |
504.2 |
26.8 |
quant |
|
实施例1584 |
1600 |
C23 H26 Cl F3 N4 O2 |
483.2 |
16.5 |
68 |
|
实施例1585 |
1601 |
C23 H26 Cl F3 N4 O3 |
499.0 |
20.0 |
80 |
|
实施例1586 |
1602 |
C21 H24 Br Cl N4 O2 |
481.0 |
18.1 |
75 |
|
实施例1587 |
1603 |
C21 H24 Cl2 N4 O2 |
435.0 |
5.5 |
25 |
|
实施例1588 |
1604 |
C27 H30 Cl N3 O3 |
492.0 |
18.6 |
76 |
|
实施例1589 |
1605 |
C21 H27 Cl N4 O2 |
415.2 |
18.1 |
87 |
|
实施例1590 |
1609 |
C23 H25 N3 O2 S |
500.0 |
18.3 |
73 |
|
实施例1591 |
1659 |
C22 H26 Cl2 N4 O2 |
449.0 |
366.0 |
83 |
|
实施例1592 |
1664 |
C24 H29 F3 N4 O2 S |
495.2 |
13.7 |
55 |
|
实施例1593 |
1665 |
C24 H29 F3 N4 O3 S |
511.2 |
14.9 |
58 |
|
实施例1594 |
1666 |
C23 H28 F2 N4 O2 S |
463.2 |
12.9 |
56 |
|
实施例1595 |
1667 |
C22 H27 Br2 N3 O3 |
542 |
26.1 |
96 |
|
实施例1596 |
1668 |
C24 H30 F2 N4 O2 |
445 |
22.9 |
quant |
|
实施例1597 |
1669 |
C24 H31 F N4 O2 |
427 |
24.0 |
quant |
|
实施例1598 |
1670 |
C24 H31 I N4 O2 |
535 |
28.1 |
quant |
|
实施例1599 |
1671 |
C25 H31 F3 N4 O3 |
493 |
26.8 |
quant |
|
实施例1600 |
1672 |
C25 H31 F3 N4 O2 |
478 |
24.7 |
quant |
|
实施例1601 |
1673 |
C24 H29 Br Cl N3 O2 |
508 |
24.9 |
98 |
|
实施例1602 |
1674 |
C20 H22 Br2 F N3 O3 |
532 |
25.6 |
96 |
|
实施例1603 |
1675 |
C22 H25 F3 N4 O2 |
435 |
21.5 |
99 |
|
实施例1604 |
1676 |
C22 H26 F2 N4 O2 |
417 |
21.4 |
quant |
|
实施例1605 |
1677 |
C22 H26 Br F N4 O2 |
479 |
23.4 |
98 |
|
实施例1606 |
1678 |
C22 H26 F I N4 O2 |
525 |
27.4 |
quant |
|
实施例1607 |
1679 |
C22 H26 Cl F N4 O2 |
433 |
22.4 |
quant |
|
实施例1608 |
1680 |
C23 H26 F4 N4 O3 |
483 |
25.5 |
quant |
|
实施例1609 |
1681 |
C23 H26 F4 N4 O2 |
467 |
23.2 |
99 |
|
实施例1610 |
1682 |
C23 H26 Br Cl F N3 O |
498 |
24.2 |
98 |
|
实施例1611 |
1683 |
C27 H28 Br2 N4 O4 |
633 |
31.8 |
quant |
|
实施例1612 |
1684 |
C29 H31 F2 N5 O3 |
536 |
28.3 |
quant |
|
实施例1613 |
1685 |
C29 H32 F N5 O3 |
518 |
31.1 |
quant |
|
实施例1614 |
1686 |
C29 H32 Br N5 O3 |
578 |
29.6 |
quant |
|
实施例1615 |
1687 |
C29 H32 I N5 O3 |
626 |
32.4 |
quant |
|
实施例1616 |
1688 |
C29 H32 Cl N5 O3 |
534 |
28.2 |
quant |
|
实施例1617 |
1689 |
C30 H32 F3 N5 O4 |
584 |
31.7 |
quant |
|
实施例1618 |
1690 |
C30 H32 F3 N5 O3 |
568 |
30.6 |
quant |
|
实施例1619 |
1691 |
C29 H30 Br Cl N4 O3 |
599 |
31.4 |
quant |
例如,1245和1600号化合物显示下列NMR谱。
1245号化合物:1H NMR(270MHz,CDCl3)δ1.20-1.97(m,7H),2.80-2.86(m,2H),3.19(t,J=6.5Hz,2H),3.43(s,2H),4.02(d,J=5.3Hz,2H),5.52(br s,2H),6.44(d,J=11.9,6.6Hz,1H),7.02(br s,1H),7.21-7.32(m,5H).
1600号化合物:
1H NMR(270MHz,CDCl3)δ1.25-1.97(m,9H),2.82-2.87(m,2H),3.21(t,J=6.5Hz,2H),3.44(s,2H),4.06(d,J=5.1Hz,2H),5.98(br s,1H),6.71(d,J=8.3Hz,1H),6.87(br s,1H),7.26(s,4H),7.43(dd,J=5.9Hz,1H),7.64(s,1H).
实施例1620:1-(4-氯苄基)-4-[{N-(4-异丙基苯磺酰基)甘氨酰}氨基甲基]哌啶(869号化合物)的制备
将1-(4-氯苄基)-4-{(甘氨酰氨基)甲基}哌啶(14.8mg,0.05mmol)的CHCl3(2ml)溶液用(哌啶子基甲基)聚苯乙烯树脂(28mg,2.8mmol/g)、4-异丙基苯磺酰氯(1.5当量)处理,在25℃下搅拌16小时。加入(氨甲基)聚苯乙烯以清除残留的磺酰氯,反应混合物在25℃下搅拌16小时。过滤并浓缩,得到1-(4-氯苄基)-4-[{N-(4-异丙基苯磺酰基)甘氨酰}氨基甲基]哌啶(869号化合物)(22.1mg,92%):纯度用RPLC/MS测定(86%);ESI/MS m/e 478(M++H,C24H32ClN3O3S)。
实施例1621-1627
依据实施例1620的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表30中。
表30
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1621 |
865 |
C22 H28 Cl N3 O3 S |
450 |
16.2 |
72 |
|
实施例1622 |
866 |
C22 H25 Cl F3 N3 O3 S |
504 |
8.8 |
35 |
|
实施例1623 |
867 |
C23 H24 Cl F6 N3 O3 S |
572 |
8.0 |
28 |
|
实施例1624 |
868 |
C23 H30 Cl N3 O3 S |
464 |
9.6 |
41 |
|
实施例1625 |
870 |
C22 H28 Cl N3 O3 S |
450 |
8.8 |
39 |
|
实施例1626 |
871 |
C25 H34 Cl N3 O3 S |
492 |
11.1 |
45 |
|
实施例1627 |
872 |
C21 H26 Cl N3 O3 S |
436 |
9.6 |
44 |
实施例1628:1-(4-氯苄基)-4-[{2-(3-(4-三氟甲基苯基)脲基)乙酰氨基}甲基]哌啶(852号化合物)的制备
将1-(4-氯苄基)-4-{(甘氨酰氨基)甲基}哌啶(14.8mg,0.05mmol)的CHCl3(2ml)溶液用(哌啶子基甲基)聚苯乙烯树脂(28mg,2.8mmol/g)、异氰酸3-(三氟甲基)苯基酯(1.3当量)处理,在25℃下搅拌16小时。加入(氨甲基)聚苯乙烯以清除残留的异氰酸酯,反应混合物在25℃下搅拌16小时。过滤并浓缩,得到1-(4-氯苄基)-4-[{2-(3-(4-三氟甲基苯基)脲基)乙酰氨基}甲基]哌啶(852号化合物)(19mg,78%):纯度用RPLC/MS测定(92%);ESI/MS m/e483(M++H,C23H26ClF3N4O2)。
实施例1629-1641
依据实施例1628的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表31中。
表31
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1629 |
851 |
C23 H26 Cl F3 N4 O2 |
483 |
13.2 |
55 |
|
实施例1630 |
853 |
C22 H27 Cl N4 O2 |
416 |
8.5* |
32 |
|
实施例1631 |
854 |
C23 H29 Cl N4 O2 |
429 |
11.4* |
42 |
|
实施例1632 |
855 |
C23 H29 Cl N4 O2 |
429 |
10.1* |
37 |
|
实施例1633 |
856 |
C24 H29 Cl N4 O3 |
457 |
10.3* |
36 |
|
实施例1634 |
857 |
C23 H29 Cl N4 O3 |
445 |
10.9* |
39 |
|
实施例1635 |
858 |
C23 H29 Cl N4 O3 |
445 |
8.6* |
31 |
|
实施例1636 |
859 |
C22 H26 Cl2 N4 O2 |
449 |
11.0* |
39 |
|
实施例1637 |
860 |
C23 H26 Cl N5 O2 |
440 |
9.2* |
33 |
|
实施例1638 |
861 |
C22 H27 Cl N4 O S |
431 |
13.3 |
62 |
|
实施例1639 |
862 |
C23 H29 Cl N4 O S |
445 |
15.3 |
69 |
|
实施例1640 |
863 |
C23 H29 Cl N4 O2 S |
461 |
14.7 |
64 |
|
实施例1641 |
864 |
C23 H29 Cl N4 O2 S |
461 |
13.1 |
57 |
*TFA盐的产量
实施例1642:1-(4-氯苄基)-4-[{N-(3-乙氧基苯甲酰基)-D-苯丙氨酰}氨基甲基]哌啶(2091号化合物)的制备
将1-(4-氯苄基)-4-(氨甲基)哌啶(100mg)的CHCl3(3ml)溶液用Et3N(0.090ml)、N-(叔丁氧羰基)-D-苯丙氨酸(122mg)、EDCI(89mg)和HOBt(62mg)处理。反应混合物在室温下搅拌17小时。反应混合物用1N NaOH水溶液(2ml×2)和盐水(2ml)洗涤。有机层干燥,并浓缩,得到1-(4-氯苄基)-4-[{N-(叔丁氧羰基)-D-苯丙氨酰}氨基甲基]哌啶。
将所得1-(4-氯苄基)-4-[{N-(叔丁氧羰基)-D-苯丙氨酰}氨基甲基]哌啶溶于甲醇(5ml),加入4N HCl的二噁烷(1.5ml)溶液。溶液在室温下搅拌19小时,浓缩。
将所得物质和3-乙氧基苯甲酸(80mg,0.48mmol)的CHCl3(1ml)溶液用Et3N(0.090ml)、EDCI(90mg)和HOBt(68mg)处理。反应混合物在室温下搅拌11小时。反应混合物用1N NaOH水溶液(1.5ml×2)和盐水(1.5ml)洗涤。有机层干燥并浓缩。经过柱色谱法(SiO2,CH2Cl2/MeOH=95∶5)得到1-(4-氯苄基)-4-[{N-(3-乙氧基苯甲酰基)-D-苯丙氨酰}氨基甲基]哌啶(2091号化合物):纯度用RPLC/MS测定(99%);ESI/MS m/e 534.0(M++H,C31H36ClN3O3)。
实施例1643-1657
依据实施例1642的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表32中。
表32
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1643 |
2092 |
C33 H37 Cl N4 O3 |
572.8 |
152.9 |
64 |
|
实施例1644 |
2093 |
C27 H36 Cl N3 O3 S |
518.0 |
177.4 |
82 |
|
实施例1645 |
2094 |
C29 H34 Cl N3 O3 S |
539.9 |
164.4 |
73 |
|
实施例1646 |
2095 |
C28 H38 Cl N3 O3 |
500.0 |
139.1 |
66 |
|
实施例1647 |
2096 |
C31 H42 Cl N3 O3 |
540.0 |
161.7 |
71 |
|
实施例1648 |
2097 |
C27 H36 Cl N3 O3 |
485.8 |
157.8 |
78 |
|
实施例1649 |
2098 |
C31 H35 Cl2 N3 O3 |
567.9 |
172.2 |
72 |
|
实施例1650 |
2099 |
C30 H34 Cl N3 O3 |
519.8 |
144.7 |
66 |
|
实施例1651 |
2100 |
C32 H38 Cl N3 O4 |
564.0 |
181.5 |
77 |
|
实施例1652 |
2101 |
C38 H42 Cl N3 O4 |
639.9 |
192.3 |
72 |
|
实施例1653 |
2103 |
C33 H40 Cl N3 O4 |
577.8 |
159.9 |
66 |
|
实施例1654 |
2104 |
C28 H36 Cl N3 O5 |
530.1 |
99.7 |
45 |
|
实施例1655 |
2115 |
C27 H36 Cl N3 O3 |
486.2 |
122.9 |
60 |
|
实施例1656 |
2116 |
C28 H38 Cl N3 O3 |
500.1 |
118.3 |
57 |
|
实施例1657 |
2117 |
C28 H34 Cl N5 O3 |
524.1 |
98.3 |
45 |
参考例29:1-(叔丁氧羰基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶的制备
向1-(叔丁氧羰基)-4-(氨甲基)哌啶(4.03g)的无水CH2Cl2(200ml)溶液中加入N-{3-(三氟甲基)苯甲酰基}甘氨酸(4.22g,17.0mmol)、EDCI(4.25g,22.1mmol)、1-羟基苯并三唑水合物(2.99g,22.1mmol)和Et3N(1.72g)。反应混合物在25℃下搅拌20小时。向反应混合物中加入H2O(100ml),混合物用CH2Cl2(2×50ml)萃取。合并后的萃取液用H2O(2×50ml)、盐水(50ml)洗涤,并干燥(MgSO4)。在减压下除去溶剂,得到黄色的油,用柱色谱法纯化(SiO2,70% EtOAc-己烷),得到1-(叔丁氧羰基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶,为白色固体(6.39g,85%):
1H-NMR(CDCl3,300MHz)δ1.4(s,9H),1.0-1.8(m,5H),2.6-2.8(m,2H),3.15-3.3(m,2H),4.0-4.3(m,4H),6.6-6.7(m,1H),7.64(s,1H),7.60(dd,1H,J=7.2,7.2Hz),7.79(d,1H,J=7.2Hz),8.0(d,1H,J=7.2Hz),8.11(s,1H);
纯度用RPLC/MS测定(97%);ESI/MS m/e 444.3(M++H,C21H28F3N3O4)。
参考例30:4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶的制备
将1-(叔丁氧羰基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(2.29g,5.16mmol)的CH3OH(40ml)溶液用1N HCl-Et2O(55ml)处理。反应混合物在25℃下搅拌15小时,在减压下除去溶剂。向反应混合物中加入2N NaOH水溶液(100ml),混合物用EtOAc萃取(3×100ml)。合并了萃取液用盐水洗涤,干燥(K2CO3)。在减压下除去溶剂,得到白色固体,用柱色谱法纯化(SiO2,CH3OH/CH2Cl2/Et3N=7/6/1),得到4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶,为白色固体(1.27g,72%):纯度用RPLC/MS测定(98%);ESI/MS m/e 344.1(M++H,C16H20F3N3O2)。
实施例1658:1-{3-(三氟甲氧基)苄基}-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(927号化合物)的制备
向3-(三氟甲氧基)苄基溴(12.3mg,0.048mmol)的CH3CN(1.0ml)溶液中加入4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(19.9mg,0.058mmol)的CH3CN(1.0ml)溶液和(哌啶子基甲基)聚苯乙烯(55mg,2.7mmol基质/g树脂)。反应混合物在60℃下搅拌2.5小时。向冷却后的反应混合物中加入异氰酸苯基酯(6.9mg,0.048mmol),混合物在25℃下搅拌1小时。将反应混合物装上VarianTM SCX柱,用CH3OH(20ml)洗涤。用2N NH3的CH3OH(6ml)溶液洗脱产物,并浓缩,得到1-{3-(三氟甲氧基)苄基}-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(927号化合物)(22.8mg,91%),为淡黄色油:纯度用RPLC/MS测定(99%);ESI/MS m/e 518.1(M++H,C24H25F6N3O3)。
实施例1659-1710
依据实施例1658的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表33中。
表33
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1659 |
875 |
C23 H26 F3 N3 O2 |
434 |
6.3 |
40 |
|
实施例1660 |
876 |
C23 H25 Br F3 N3 O2 |
512 |
4.3 |
23 |
|
实施例1661 |
877 |
C24 H25 F3 N4 O2 |
459 |
11.3 |
68 |
|
实施例1662 |
878 |
C23 H25 F3 N4 O4 |
479 |
8.3 |
48 |
|
实施例1663 |
884 |
C25 H29 F3 N4 O3 |
491 |
10.8 |
61 |
|
实施例1664 |
885 |
C24 H28 F3 N3 O4 S |
512 |
9.0 |
49 |
|
实施例1665 |
886 |
C23 H25 F4 N3 O2 |
452 |
12.7 |
78 |
|
实施例1666 |
887 |
C24 H25 F6 N3 O2 |
502 |
13.9 |
77 |
|
实施例1667 |
888 |
C23 H26 F3 N3 O3 |
450 |
11.5 |
71 |
|
实施例1668 |
889 |
C29 H30 F3 N3 O2 |
510 |
12.4 |
68 |
|
实施例1669 |
890 |
C27 H28 F3 N3 O2 |
484 |
12.0 |
69 |
|
实施例1670 |
891 |
C23 H24 Cl2 F3 N3 O2 |
502 |
11.4 |
63 |
|
实施例1671 |
892 |
C24 H28 F3 N3 O3 |
464 |
11.7 |
70 |
|
实施例1672 |
893 |
C24 H26 F3 N5 O5 |
522 |
13.9 |
74 |
|
实施例1673 |
894 |
C26 H32 F3 N3 O3 |
492 |
11.3 |
64 |
|
实施例1674 |
895 |
C24 H28 F3 N3 O2 |
448 |
4.8 |
30 |
|
实施例1675 |
896 |
C24 H25 F3 N4 O2 |
459 |
17.5 |
quant |
|
实施例1676 |
897 |
C24 H26 F3 N3 O4 |
478 |
9.2 |
57 |
|
实施例1677 |
898 |
C24 H26 F3 N3 O4 |
478 |
8.9 |
55 |
|
实施例1678 |
899 |
C24 H28 F3 N3 O3 |
464 |
13.7 |
82 |
|
实施例1679 |
900 |
C25 H28 F3 N3 O4 |
492 |
18.6 |
quant |
|
实施例1680 |
901 |
C29 H30 F3 N3 O2 |
510 |
13.7 |
75 |
|
实施例1681 |
902 |
C23 H24 F3 N5 O6 |
524 |
12.6 |
67 |
|
实施例1682 |
903 |
C25 H30 F3 N3 O4 |
494 |
14.0 |
79 |
|
实施例1683 |
906 |
C25 H30 F3 N3 O2 |
462 |
11.2 |
67 |
|
实施例1684 |
907 |
C31 H34 F3 N3 O2 |
538 |
19.6 |
75 |
|
实施例1685 |
908 |
C30 H31 F3 N4 O3 |
553 |
30.4 |
76 |
|
实施例1686 |
909 |
C30 H31 F3 N4 O3 |
553 |
12.6 |
63 |
|
实施例1687 |
910 |
C23 H24 Cl2 F3 N3 O2 |
502 |
11.0 |
61 |
|
实施例1688 |
911 |
C23 H25 Cl F3 N3 O2 |
468 |
20.2 |
89 |
|
实施例1689 |
912 |
C23 H24 Br2 F3 N3 O2 |
590 |
20.2 |
95 |
|
实施例1690 |
913 |
C24 H28 F3 N3 O3 |
464 |
12.6 |
76 |
|
实施例1691 |
914 |
C30 H32 F3 N3 O3 |
540 |
13.9 |
72 |
|
实施例1692 |
915 |
C24 H28 F3 N3 O3 |
464 |
8.3 |
25 |
|
实施例1693 |
916 |
C22 H25 F3 N4 O2 |
435 |
2.5 |
8 |
|
实施例1694 |
917 |
C22 H25 F3 N4 O2 |
435 |
2.7 |
9 |
|
实施例1695 |
918 |
C26 H30 F3 N3 O4 |
506 |
3.9 |
22 |
|
实施例1696 |
919 |
C24 H28 F3 N3 O2 |
448 |
15.9 |
99 |
|
实施例1697 |
920 |
C24 H25 F6 N3 O3 |
518 |
20.3 |
81 |
|
实施例1698 |
921 |
C27 H28 F3 N3 O2 |
484 |
15.5 |
89 |
|
实施例1699 |
922 |
C20 H26 F3 N3 O2 |
398 |
7.3 |
51 |
|
实施例1700 |
923 |
C29 H29 Cl F3 N3 O2 |
544 |
12.5 |
48 |
|
实施例1701 |
928 |
C24 H25 F6 N3 O3 |
518 |
21.4 |
86 |
|
实施例1702 |
929 |
C24 H28 F3 N3 O2 S |
480 |
23.7 |
quant |
|
实施例1703 |
930 |
C24 H28 F3 N3 O2 |
448 |
21.3 |
99 |
|
实施例1704 |
931 |
C24 H25 F3 N4 O2 |
459 |
21.4 |
97 |
|
实施例1705 |
932 |
C23 H24 Cl F3 N4 O4 |
513 |
15.6 |
63 |
|
实施例1706 |
933 |
C24 H28 F3 N3 O2 |
448 |
16.6 |
77 |
|
实施例1707 |
934 |
C22 H25 F3 N4 O2 |
435 |
18.0 |
43 |
|
实施例1708 |
935 |
C23 H25 F3 N4 O4 |
479 |
15.1 |
65 |
|
实施例1709 |
936 |
C23 H25 F3 N4 O4 |
479 |
15.4 |
67 |
|
实施例1710 |
1615 |
C24 H25 F6 N3 O2 S |
534.2 |
26.3 |
99 |
实施例1711:1-{4-(二甲氨基)苄基}-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(937号化合物)的制备
向4-(二甲氨基)苯甲醛(30.4mg,0.204mmol)的5% CH3COOH/CH3OH(1.0ml)溶液中加入4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(20.0mg,0.058mmol)的CH3CN(1.0ml)溶液和NaBH3CN(16.5mg)。反应混合物在60℃下搅拌19小时。蒸发溶剂得到一固体。向该固体加入CH3CN(2.0ml)和异氰酸苯基酯(6.9mg,0.048mmol),混合物在25℃下搅拌1小时。将反应混合物装上VarianTM SCX柱,用CH3OH(20ml)洗涤。用2N NH3-CH3OH(6ml)洗脱产物,浓缩洗脱液,得到1-{4-(二甲氨基)苄基}-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(937号化合物)(13.5mg,49%),为淡黄色油:纯度用RPLC/MS测定(87%);ESI/MS m/e 477.3(M++H,C25H31F3N4O2)。
实施例1712-1729
依据实施例1711的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC(SiO2)得到所需物质。ESI/MS数据和产率总结在表34中。
表34
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1712 |
879 |
C24 H26 F3 N3 O4 |
478 |
13.0 |
62 |
|
实施例1713 |
880 |
C24 H26 F3 N3 O4 |
478 |
16.3 |
78 |
|
实施例1714 |
881 |
C23 H25 Br F3 N3 O2 |
512 |
11.4 |
51 |
|
实施例1715 |
882 |
C29 H30 F3 N3 O3 |
526 |
13.4 |
58 |
|
实施例1716 |
883 |
C23 H25 Cl F3 N3 O2 |
468 |
7.9 |
39 |
|
实施例1717 |
904 |
C23 H26 F3 N3 O3 |
450 |
3.3 |
17 |
|
实施例1718 |
905 |
C21 H23 F3 N4 O4 S |
485 |
27.7 |
98 |
|
实施例1719 |
938 |
C23 H24 Cl F4 N3 O2 |
486 |
8.6 |
30 |
|
实施例1720 |
939 |
C23 H24 Cl F3 N4 O4 |
513 |
11.0 |
37 |
|
实施例1721 |
940 |
C23 H26 F3 N3 O3 |
450 |
5.5 |
21 |
|
实施例1722 |
941 |
C24 H24 Cl F6 N3 O2 |
536 |
11.2 |
36 |
|
实施例1723 |
987 |
C30 H32 F3 N3 O2 |
524 |
17.5 |
76 |
|
实施例1724 |
1449 |
C25 H30 F3 N3 O2 |
462 |
21.6 |
80 |
|
实施例1725 |
1450 |
C26 H32 F3 N3 O2 |
476 |
23.5 |
85 |
|
实施例1726 |
1452 |
C27 H35 F3 N4 O2 |
505 |
5.1 |
17 |
|
实施例1727 |
1453 |
C26 H32 F3 N3 O3 |
492 |
22.0 |
77 |
|
实施例1728 |
1454 |
C25 H30 F3 N3 O3 |
478 |
21.4 |
77 |
|
实施例1729 |
1456 |
C25 H28 F3 N3 O4 |
492 |
23.8 |
83 |
实施例1730:1-(3-羟基-4-甲氧基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(1452号化合物)的制备
向4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(20.0mg,0.058mmol)和3-羟基-4-甲氧基苯甲醛(33mg)的5%CH3COOH/CH3OH(1.0ml)溶液中加入NaBH3CN(16.5mg)的5%CH3COOH/CH3OH(1.0ml)溶液。反应混合物在60℃下搅拌15小时。将反应混合物装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3-CH3OH(5ml)洗脱产物,浓缩洗脱液,得到1-(3-羟基-4-甲氧基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(1452号化合物)(25.8mg,92%):纯度用RPLC/MS测定(91%);ESI/MSm/e 480(M++H,C24H28F3N3O4)。
实施例1731-1733
依据实施例1730的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表35中。
表35
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1731 |
1455 |
C24 H28 F3 N3 O4 |
480 |
24.0 |
86 |
|
实施例1732 |
1647 |
C27 H34 F3 N3 O2 |
490.2 |
23.6 |
96 |
|
实施例1733 |
1649 |
C26 H32 F3 N3 O2 |
476.2 |
23.1 |
97 |
实施例1734:1-(4-苄基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(926号化合物)的制备
向4-(苄基)苯甲醇(8.7mg,0.044mmol)的CHCl3(1.0ml)溶液中加入甲磺酰氯(4.2mg,0.037mmol)的CHCl3(1.0ml)溶液和(哌啶子基甲基)聚苯乙烯(54mg,2.7mmol基质/g树脂)。反应混合物在25℃下搅拌15小时。向反应混合物中加入4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(15.1mg,0.044mmol)的CH3CN(1.0ml)溶液和KI(2mg),混合物在65℃下搅拌5小时。向冷却后的反应混合物中加入异氰酸苯基酯(5.2mg),混合物在25℃下搅拌1小时。将反应混合物装上VarianTM SCX柱,用CH3OH(20ml)洗涤。用2N NH3的CH3OH(6ml)溶液洗脱产物,并浓缩,得到1-(4-苄基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(926号化合物)(5.6mg,29%),为淡黄色油:纯度用RPLC/MS测定(94%);ESI/MS m/e 524.1(M++H,C30H32F3N3O2)。
参考例31:4-[{(N-(苄氧羰基)甘氨酰)氨基}甲基]-1-(叔丁氧羰基)哌啶的制备
将4-(氨甲基)-1-(叔丁氧羰基)哌啶(3.54g,16.5mmol)的CH2Cl2(80ml)溶液用Et3N(2.8ml,20mmol)、N-(苄氧羰基)甘氨酸(3.77g,18mmol)、EDCI(3.45g,18mmol)和HOBt(2.43g,18mmol)处理。反应混合物在室温下搅拌15小时后,加入2N NaOH水溶液(100ml)。分离有机层,含水层用二氯甲烷萃取(100ml×3)。合并了有机层经无水硫酸钠干燥,过滤,并浓缩。经过柱色谱法(SiO2,乙酸乙酯)得到4-[{(N-(苄氧羰基)甘氨酰)氨基}甲基]-1-(叔丁氧羰基)哌啶(6.27g,94%),为无定形固体。
参考例32:4-{(甘氨酰氨基)甲基}-1-(叔丁氧羰基)哌啶的制备
在5%钯碳(620mg)的存在下,将4-[{(N-(苄氧羰基)甘氨酰)氨基}甲基]-1-(叔丁氧羰基)哌啶(6.26g,15.4mmol)的甲醇(100ml)溶液在室温、1atm下氢化7小时。通过硅藻土过滤除去催化剂,合并后的滤液浓缩,得到4-{(甘氨酰氨基)甲基}-1-(叔丁氧羰基)哌啶(3.84g,92%),为一固体。
参考例33:4-[{(N-(2-氨基-5-氯苯甲酰基)甘氨酰)氨基}甲基]-1-(叔丁氧羰基)哌啶的制备
将4-{(甘氨酰氨基)甲基}-1-(叔丁氧羰基)哌啶(1.33g,4.90mmol)的CH2Cl2(25ml)溶液用Et3N(0.75ml,5.4mmol)、2-氨基-5-氯苯甲酸(840mg,4.9mmol)、EDCI(940mg,4.9mmol)和HOBt(660mg,4.9mmol)处理。反应混合物在室温下搅拌3小时后,加入2N NaOH水溶液(20ml)。分离有机层,含水层用二氯甲烷萃取(20ml×3)。合并后的有机层经无水硫酸钠干燥,过滤,并浓缩。经过柱色谱法(SiO2,乙酸乙酯)得到4-[{(N-(2-氨基-5-氯苯甲酰基)甘氨酰)氨基}甲基]-1-(叔丁氧羰基)哌啶(1.63g,78%),为一固体。
参考例34:4-[{(N-(2-氨基-5-氯苯甲酰基)甘氨酰)氨基}甲基]哌啶的制备
向4-[{(N-(2-氨基-5-氯苯甲酰基)甘氨酰)氨基}甲基]-1-(叔丁氧羰基)哌啶(1.63g,3.84mmol)的甲醇(20ml)中加入4N HCl的二噁烷(9.5ml)溶液。溶液在室温下搅拌6小时。反应混合物浓缩,加入2N NaOH水溶液(20ml)。混合物用二氯甲烷萃取(20ml×3),合并后的萃取液经无水硫酸钠干燥,过滤,并浓缩,得到4-[{(N-(2-氨基-5-氯苯甲酰基)甘氨酰)氨基}甲基]哌啶(1.19g,95%)
1H NMR(CDCl3,270MHz)δ1.10-1.76(m,4H),2.55(td,J=2.4 and 12.2Hz,2H),3.00-3.10(m,2H),3.17(t,J=6.2Hz,2H),3.48(s,2H),4.03(d,J=4.9Hz,2H),5.50(br.s,2H),6.11-6.23(m,1H),6.60(d,J=8.8Hz,1H),6.85-7.02(m,1H),7.15(dd,J=2.7 and 8.8Hz,1H),7.38(d,J=2.4Hz,1H);ESI/MS m/e325.2(C15H21ClN4O2).
依据参考例32和33的方法,使用相应的试剂,也合成了4-[{(N-(2-氨基-5-溴苯甲酰基)甘氨酰)氨基}甲基]哌啶:951mg,64%(2步);ESI/MS m/e369.2(C15H21BrN4O2)。
实施例1735:4-[{(N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰)氨基}甲基]-1-(4-氯苄基)哌啶的制备
将二盐酸1-(4-氯苄基)-4-{(甘氨酰氨基)甲基}哌啶(738mg,2mmol)的CH2Cl2(20ml)溶液用Et3N(1.1ml,8mmol)、2-(叔丁氧羰基氨基)-4,5-二氟苯甲酸(607mg,2.2mmol)、EDCI(422mg,2.2mmol)和HOBt(337mg,2.2mmol)处理。反应混合物在室温下搅拌14小时后,加入0.6N NaOH水溶液(50ml),混合物用二氯甲烷萃取(3次)。合并后的有机层经无水硫酸钠干燥,过滤,并浓缩。经过柱色谱法(SiO2,乙酸乙酯然后是乙酸乙酯/甲醇=92/8)得到4-[{(N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰)氨基}甲基]-1-(4-氯苄基)哌啶(1.01g,92%):ESI/MS m/e 551.3(M++H,C27H33ClF2N4O4)。
依据上述方法,使用相应的试剂,也合成了4-[{(N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰)氨基}甲基]-1-(4-氯苄基)哌啶:3.03g,82%;ESI/MS m/e 583.2(M++H,C28H34ClF3N4O4)。
参考例35:4-[{(N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰)氨基}甲基]哌啶的制备
将1-(4-氯苄基)-4-[{(N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰)氨基}甲基]哌啶(447mg,0.93mmol)与Pd(OH)2(60mg,0.23mmol)在5% HCO2H/甲醇(10ml)中的混悬液在50℃下搅拌14小时。通过硅藻土滤出Pd催化剂,浓缩滤液。向残余物中加入1N NaOH水溶液(15ml),混合物用乙酸乙酯萃取(30ml×3)。合并后的萃取液经无水硫酸钠干燥,过滤,并浓缩。经过柱色谱法(SiO2,AcOEt/MeOH/Et3N=70/25/5)得到4-[{(N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰)氨基}甲基]哌啶(284mg,86%):ESI/MS m/e 359.0(M++H,C16H21F3N4O2)。
依据上述方法,分别使用相应的试剂,也制备了4-[{(N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰)氨基}甲基]哌啶、4-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲氧基苯甲酰基)甘氨酰}氨基甲基]哌啶、4-[{(N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰)氨基}甲基]哌啶和4-[{(N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰)氨基}甲基]哌啶。
4-[{(N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰)氨基}甲基]哌啶:564mg,89%;ESI/MS m/e 327.2(M++H,C15H20F2N4O2)。
4-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲氧基苯甲酰基)甘氨酰}氨基甲基]哌啶:定量;
1H NMR(CDCl3,400MHz)δ1.10-1.25(m,2H),1.45-1.73(m,3H),1.51(s,9H),2.53-2.64(m,2H),3.04-3.13(m,2H),3.22(t,J=6.3Hz,2H),4.09(d,J=4.6Hz,2H),5.91(br.s,1H),7.08(br.s.,1H),7.32(d,J=9.0Hz,1H),7.38(s,1H),8.43(d,J=9.0Hz,1H).
4-[{(N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰)氨基}甲基]哌啶:310mg,40%;ESI/MS m/e 427.3(M++H,C20H28F2N4O4)。
4-[{(N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰)氨基}甲基]哌啶:1.35g,57%;ESI/MS m/e 459.3(M++H,C21H29F3N4O4)。
实施例1736:4-[{N-(2-氨基-5-氯苯甲酰基)甘氨酰}氨基甲基]-1-(4-乙氧基苄基)哌啶(1429号化合物)和1-(4-乙氧基苄基)-4-[{N-(2-(4-乙氧基苄基)氨基-5-氯苯甲酰基)甘氨酰}氨基甲基]哌啶(1433号化合物)的制备
向4-[{N-(2-氨基-5-氯苯甲酰基)甘氨酰}氨基甲基]哌啶(0.10mmol)、4-乙氧基苯甲醛(0.10mmol)、乙酸(0.050ml)和甲醇(1.6ml)的混合物中加入氰基硼氢化钠(140mmol)的甲醇(0.4ml)溶液。反应混合物在60℃下搅拌14小时。将反应混合物装上VarianTM SCX柱,用CH3OH(20ml)洗涤。用2N NH3的CH3OH(6ml)溶液洗脱产物,并浓缩。经过制备型TLC(SiO2,AcOEt/CH3OH5∶1)得到4-[{N-(2-氨基-5-氯苯甲酰基)甘氨酰}氨基甲基]-1-(4-乙氧基苄基)哌啶(1429号化合物)和1-(4-乙氧基苄基)-4-[{N-(2-(4-乙氧基苄基)氨基-5-氯苯甲酰基)甘氨酰}氨基甲基]哌啶(1433号化合物)。
1429号化合物:4.5mg,20%;纯度用RPLC/MS测定(95%);ESI/MSm/e 459.2(M++H,C24H31ClN4O3)。
1433号化合物:8.4mg,28%;纯度用RPLC/MS测定(98%);ESI/MSm/e 593.2(M++H,C33H41ClN4O4)。
实施例1737-1779
依据实施例1736的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表36中。
表36
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1737 |
1430 |
C24 H29 Cl N4 O4 |
473.0 |
3.1 |
13 |
|
实施例1738 |
1431 |
C24 H31 Br N4 O3 |
505.2 |
5.8 |
23 |
|
实施例1739 |
1432 |
C24 H29 Br N4 O4 |
517.0 |
4.1 |
16 |
|
实施例1740 |
1434 |
C33 H41 Br N4 O6 |
637.2 |
9.7 |
30 |
|
实施例1741 |
1435 |
C24 H31 Cl N4 O2 |
443.2 |
9.7 |
44 |
|
实施例1742 |
1436 |
C25 H33 Cl N4 O2 |
457.2 |
12.5 |
55 |
|
实施例1743 |
1437 |
C25 H33 Cl N4 O3 |
473.2 |
9.4 |
40 |
|
实施例1744 |
1438 |
C24 H31 Br N4 O2 |
489.2 |
5.9 |
24 |
|
实施例1745 |
1439 |
C25 H33 Br N4 O2 |
503.2 |
15.2 |
61 |
|
实施例1746 |
1440 |
C25 H33 Br N4 O3 |
519.2 |
11.0 |
43 |
|
实施例1747 |
1441 |
C23 H29 Br N4 O2 S |
507.2 |
9.3 |
37 |
|
实施例1748 |
1442 |
C33 H41 Cl N4 O2 |
561.4 |
6.8 |
24 |
|
实施例1749 |
1443 |
C35 H45 Cl N4 O2 |
589.4 |
9.8 |
33 |
|
实施例1750 |
1444 |
C35 H45 Cl N4 O4 |
621.4 |
9.4 |
30 |
|
实施例1751 |
1445 |
C33 H41 Br N4 O2 |
605.2 |
6.5 |
21 |
|
实施例1752 |
1446 |
C35 H45 Br N4 O2 |
635.2 |
10.7 |
34 |
|
实施例1753 |
1447 |
C35 H45 Br N4 O4 |
665.4 |
12.4 |
37 |
|
实施例1754 |
1448 |
C31 H37 Br N4 O2 S2 |
643.2 |
7.6 |
24 |
|
实施例1755 |
1457 |
C24 H32 Cl N5 O2 |
458.2 |
4.5 |
20 |
|
实施例1756 |
1458 |
C23 H29 Cl N4 O4 |
461.2 |
6.0 |
26 |
|
实施例1757 |
1459 |
C24 H32 Br N5 O2 |
504.0 |
6.8 |
27 |
|
实施例1758 |
1460 |
C23 H29 Br N4 O4 |
505.0 |
8.0 |
32 |
|
实施例1759 |
1461 |
C31 H37 Cl N4 O6 |
597.2 |
5.9 |
20 |
|
实施例1760 |
1462 |
C31 H37 Br N4 O6 |
643.2 |
6.0 |
19 |
|
实施例1761 |
1514 |
C26 H36 Cl N5 O2 |
486.2 |
5.5 |
23 |
|
实施例1762 |
1515 |
C23 H29 Cl N4 O4 |
463.0 |
5.8 |
25 |
|
实施例1763 |
1516 |
C26 H36 Br N5 O2 |
530.2 |
4.2 |
16 |
|
实施例1764 |
1517 |
C23 H29 Br N4 O4 |
505.0 |
6.5 |
26 |
|
实施例1765 |
1518 |
C31 H37 Cl N4 O6 |
597.2 |
4.3 |
14 |
|
实施例1766 |
1519 |
C31 H37 Br N4 O6 |
641.2 |
5.3 |
17 |
|
实施例1767 |
1570 |
C23 H29 Cl N4 O2 S |
461.0 |
2.7 |
12 |
|
实施例1768 |
1571 |
C31 H37 Cl N4 O2 S2 |
597.2 |
4.9 |
16 |
|
实施例1769 |
1651 |
C37 H49 Br N4 O2 |
663.2 |
5.5 |
17 |
|
实施例1770 |
1652 |
C26 H35 Br N4 O2 |
515.2 |
6.0 |
23 |
|
实施例1771 |
1653 |
C35 H45 Br N4 O2 |
633.2 |
5.0 |
16 |
|
实施例1772 |
1654 |
C25 H33 Br N4 O2 |
501.0 |
6.2 |
25 |
|
实施例1773 |
1655 |
C37 H49 C1 N4 O2 |
617.4 |
5.6 |
18 |
|
实施例1774 |
1656 |
C26 H35 Cl N4 O2 |
471.2 |
5.9 |
25 |
|
实施例1775 |
1657 |
C35 H45 Cl N4 O2 |
589.2 |
4.6 |
16 |
|
实施例1776 |
1658 |
C25 H33 Cl N4 O2 |
457.2 |
5.3 |
23 |
|
实施例1777 |
1785 |
C26 H33 F3 N4 O2 |
491.2 |
4.7 |
12.8 |
|
实施例1778 |
1786 |
C25 H29 F3 N4 O3 |
491.2 |
3.7 |
10.1 |
|
实施例1779 |
1804 |
C25 H32 F2 N4 O2 |
459.2 |
3.3 |
9.6 |
实施例1780:4-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基甲基]-1-(4-异丙基苄基)哌啶(1903号化合物)的制备
向4-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲氧基)苯甲酰基甘氨酰}氨基甲基]哌啶(0.050mmol)、4-异丙基苯甲醛(0.060mmol)、NaBH3CN(0.15mmol)和甲醇(1.3ml)的混合物中加入乙酸(0.050ml)。反应混合物在60℃下搅拌8小时。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH(10ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。向所得物质中加入4N HCl的1,4-二噁烷(2ml)溶液,溶液在室温下搅拌过夜。经过浓缩和制备型TLC得到4-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基甲基]-1-(4-异丙基苄基)哌啶(1903号化合物)(6.6mg,28%):纯度用RPLC/MS测定(93%);ESI/MS m/e 507(M++H,C26H33F3N4O3)。
实施例1781-1783
依据实施例1780的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表37中。
表37
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg ) |
产率(%) |
|
实施例1781 |
1904 |
C26 H33 F3 N4 O3 |
507 |
9.6 |
37.9 |
|
实施例1782 |
1917 |
C25 H31 F3 N4 O5 |
525.2 |
1.2 |
3.1 |
|
实施例1783 |
1918 |
C24 H29 F3 N4 O4 |
495.2 |
2.8 |
7.5 |
实施例1784:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(5-溴-2-乙氧基苄基)哌啶(2052号化合物)的制备
向4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(0.050mmol)、5-溴-2-乙氧基苯甲醛(0.15mmol)、甲醇(1.2ml)和乙酸(0.030ml)的混合物中加入NaBH3CN(0.25mmol)的甲醇(0.50ml)溶液。反应混合物在50℃下搅拌13小时。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤(5ml×3)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。向所得物质中加入二氯甲烷(1ml)和三氟乙酸(TFA)(0.50ml),溶液在室温下搅拌10分钟。反应混合物浓缩,将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。经过制备型TLC(SiO2,乙酸乙酯/甲醇=10/1)得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(5-溴-2-乙氧基苄基)哌啶(2052号化合物)(10.2mg,38%):纯度用RPLC/MS测定(96%);ESI/MS m/e 539.2(M++H,C24H29F2N4O3)。
实施例1785-1792
依据实施例1784的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表38中。
表38
| | 化合物号 | 分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例1785 |
2053 |
C30 H34 F2 N4 O4 |
553.4 |
12.7 |
46 |
|
实施例1786 |
2054 |
C27 H30 F2 N4 O3 |
497.2 |
13.7 |
55 |
|
实施例1787 |
2055 |
C23 H28 F2 N4 O4 |
463.2 |
10.1 |
44 |
|
实施例1788 |
2056 |
C22 H24 Br F3 N4 O2 |
515.2 |
7.7 |
30 |
|
实施例1789 |
2057 |
C23 H27 Br F2 N4 O3 |
527.0 |
8.6 |
33 |
|
实施例1790 |
2058 |
C24 H30 F2 N4 O4 |
477.2 |
6.4 |
27 |
|
实施例1791 |
2059 |
C28 H30 F2 N4 O3 |
509.4 |
6.7 |
26 |
|
实施例1792 |
2060 |
C25 H32 F2 N4 O5 |
507.2 |
7.2 |
28 |
实施例1793:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3,4-二乙氧基苄基)哌啶(2065号化合物)的制备
向4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(0.050mmol)、3,4-二乙氧基苯甲醛(0.15mmol)、甲醇(1.2ml)和乙酸(0.050ml)的混合物中加入NaBH3CN(0.25mmol)的甲醇(0.50ml)溶液。反应混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。向所得物质中加入二氯甲烷(2ml)和异氰酸苯基酯(0.10ml),溶液在室温下搅拌1小时,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。将残余物溶于甲醇(0.25ml),加入4NHCl的二噁烷(0.125ml)溶液。溶液在室温下搅拌过夜,并浓缩。将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3,4-二乙氧基苄基)哌啶(2065号化合物)(21.2mg,84%):纯度用RPLC/MS测定(97%);ESI/MS m/e 5 05.2(M++H,C26H34F2N4O4)。
实施例1794-1808
依据实施例1793的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表39中。
表39
| | 化合物号 | 分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例1794 |
2061 |
C23 H27 F3 N4 O2 |
449.2 |
12.6 |
56 |
|
实施例1795 |
2062 |
C23 H27 F3 N4 O3 |
465.2 |
19.7 |
85 |
|
实施例1796 |
2063 |
C25 H32 F2 N4 O4 |
491.2 |
19.8 |
81 |
|
实施例1797 |
2064 |
C22 H24 Br F3 N4 O2 |
515.2 |
17.5 |
68 |
|
实施例1798 |
2066 |
C29 H32 F2 N4 O3 |
523.2 |
18.0 |
69 |
|
实施例1799 |
2067 |
C26 H34 F2 N4 O2 |
473.2 |
21.9 |
93 |
|
实施例1800 |
2068 |
C22 H24 Cl F3 N4 O2 |
469.2 |
11.2 |
48 |
|
实施例1801 |
2069 |
C24 H30 F2 N4 O3 |
461.4 |
20.2 |
88 |
|
实施例1802 |
2070 |
C23 H27 Br F2 N4 O3 |
527.2 |
17.7 |
67 |
|
实施例1803 |
2071 |
C24 H30 F2 N4 O4 |
477.2 |
10.9 |
46 |
|
实施例1804 |
2072 |
C25 H32 F2 N4 O3 |
475.2 |
19.3 |
81 |
|
实施例1805 |
2073 |
C29 H32 F2 N4 O3 |
523.2 |
22.8 |
87 |
|
实施例1806 |
2074 |
C29 H32 F2 N4 O4 |
539.2 |
22.5 |
84 |
|
实施例1807 |
2075 |
C23 H27 F3 N4 O3 |
465.2 |
14.9 |
64 |
|
实施例1808 |
2076 |
C22 H24 F4 N4 O2 |
453.2 |
21.9 |
97 |
实施例1809:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(2-羟基-3-甲基苄基)哌啶(2106号化合物)的制备
向4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰)氨基甲基]哌啶(0.050mmol)、2-羟基-3-甲基苯甲醛(0.25mmo1)、甲醇(1.0ml)和乙酸(0.040m1)的混合物中加入NaBH3CN(0.40mmol)的甲醇(0.50ml)溶液。反应混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。将所得物质溶于乙酸乙酯/甲醇=5∶1(1ml),装上VarianTM Si柱,用乙酸乙酯/甲醇=5∶1(5ml)洗脱,并浓缩。将残余物溶于甲醇(2ml),加入4N HCl的二呼烷(0.50ml)溶液。溶液在室温下搅拌过夜,并浓缩。将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。经过制备型TLC得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(2-羟基-3-甲基苄基)哌啶(2106号化合物):纯度用RPLC/MS测定(97%);ESI/MS m/e 447.0(M++H,C23H28F2N4O3)。
实施例1810-1823
依据实施例1809的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表40中。
表40
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例1810 |
2077 |
C22 H25 Cl F2 N4 O3 |
467.2 |
3.7 |
16 |
|
实施例1811 |
2078 |
C24 H30 F2 N4 O4 |
477.2 |
1.9 |
8 |
|
实施例1812 |
2079 |
C30 H34 F2 N4 O4 |
553.4 |
4.8 |
17 |
|
实施例1813 |
2080 |
C22 H25 Cl F2 N4 O3 |
467.2 |
13.5 |
58 |
|
实施例1814 |
2081 |
C22 H25 Cl F2 N4 O3 |
467.2 |
13.8 |
59 |
|
实施例1815 |
2082 |
C23 H28 F2 N4 O4 |
463.2 |
9.6 |
42 |
|
实施例1816 |
2105 |
C23 H28 F2 N4 O4 |
463.2 |
ND |
ND |
|
实施例1817 |
2106 |
C23 H28 F2 N4 O3 |
447.0 |
ND |
ND |
|
实施例1818 |
2107 |
C20 H23 Br F2 N4 O2 S |
503.1 |
ND |
ND |
|
实施例1819 |
2108 |
C25 H28 F2 N4 O2 S |
487.2 |
ND |
ND |
|
实施例1820 |
2109 |
C20 H23 Br F2 N4 O3 |
487.0 |
ND |
ND |
|
实施例1821 |
2110 |
C22 H28 F2 N4 O3 |
435.1 |
ND |
ND |
|
实施例1822 |
2111 |
C22 H24 Cl F3 N4 O2 |
469.0 |
ND |
ND |
|
实施例1823 |
2112 |
C24 H29 Br F2 N4 O4 |
557.0 |
ND |
ND |
ND:没有测定
实施例1824:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-氨基-4-甲基苄基)哌啶(2114号化合物)的制备
向4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(0.050mmol)、4-甲基-3-硝基苯甲醛(0.25mmol)、甲醇(1.2ml)和乙酸(0.050ml)的混合物中加入NaBH3CN(0.50mmol)的甲醇(1.0ml)溶液。反应混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。将所得物质溶于乙酸乙酯/甲醇=2/1(2ml),装上VarianTM Si柱,用乙酸乙酯/甲醇=2/1(6ml)洗脱,并浓缩。将残余物溶于甲醇(1ml),加入4N HCl的二噁烷(0.50ml)溶液。溶液在室温下搅拌过夜,并浓缩。将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物。经过浓缩得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(4-甲基-3-硝基苄基)哌啶。
将上面制备的4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(4-甲基-3-硝基苄基)哌啶、5%钯-活性碳(15mg)和甲醇(2ml)的混合物在室温氢气氛下搅拌4小时。通过硅藻土滤出Pd催化剂,浓缩滤液。经过制备型TLC(SiO2,乙酸乙酯/MeOH=3/1)得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-氨基-4-甲基苄基)哌啶(2114号化合物)(2.9mg,13%):纯度用RPLC/MS测定(100%);ESI/MS m/e 446.1(M++H,C23H29F2N5O2)。
实施例1825:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-氨基-4-甲氧基苄基)哌啶(2113号化合物)的制备
依据实施例1824的方法,使用相应的试剂,合成标题化合物:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-氨基-4-甲氧基苄基)哌啶(2113号化合物):4.6mg,产率20%;ESI/MS m/e 462.2(M++H,C23H29F2N5O3)。
实施例1826:1-(3-氨基-4-羟基苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶的制备
向4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(0.35mmol)、4-羟基-3-硝基苯甲醛(1.22mmol)、甲醇(3.8ml)和乙酸(0.175ml)的混合物中加入NaBH3CN(1.58mmol)的甲醇(3.2ml)溶液。反应混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩。将所得物质溶于乙酸乙酯/甲醇=5/1,装上VarianTM Si柱,用乙酸乙酯/甲醇=5/1(10ml)洗脱,并浓缩,得到4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(4-羟基-3-硝基苄基)哌啶(175mg,87%)。
将上面制备的4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(4-羟基-3-硝基苄基)哌啶、10%钯-活性碳(45mg)和甲醇(5ml)的混合物在室温氢气氛下搅拌2小时。滤出Pd催化剂,浓缩滤液,得到1-(3-氨基-4-羟基苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(100mg,60%)。
实施例1827:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-氨基-4-羟基苄基)哌啶(2141号化合物)的制备
向1-(3-氨基-4-羟基苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(20.0mg,0.035mmol)的甲醇(1ml)溶液中加入4NHCl的二噁烷(0.50ml)溶液,溶液在室温下搅拌过夜。溶液浓缩后,将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH(5ml×2)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物。经过浓缩得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-氨基-4-羟基苄基)哌啶(2141号化合物)(17.6mg,定量):纯度用RPLC/MS测定(85%);ESI/MS m/e 448.3(M++H,C22H27F2N5O3)。
实施例1828-1831
依据实施例1826和1827的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC(SiO2)得到所需物质。ESI/MS数据和最后步骤的产率总结在表41中。
表41
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例1828 |
2140 |
C23 H27 F2 N5 O4 |
476.3 |
6.7 |
28.4 |
|
实施例1829 |
2144 |
C24 H30 F3 N5 O3 |
494.2 |
18.7 |
82.0 |
|
实施例1830 |
2145 |
C23 H28 F3 N5 O3 |
480.3 |
19.8 |
63.7 |
|
实施例1831 |
2146 |
C24 H28 F3 N5 O4 |
508.3 |
13.5 |
81.7 |
实施例1832:1-(3-氨基-4-氯苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶的制备
向4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(0.14mmol)、4-氯-3-硝基苯甲醛(0.50mmol)、甲醇(1.5ml)和乙酸(0.070ml)的混合物中加入NaBH3CN(0.63mmol)的甲醇(1.3ml)溶液。反应混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩。将所得物质溶于乙酸乙酯/甲醇=5/1,装上VarianTM Si柱,用乙酸乙酯/甲醇=5/1(6ml)洗脱,并浓缩,得到4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯-3-硝基苄基)哌啶(44mg,53%):ESI/MS m/e 596.3(M++H)。
将上面制备的4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯-3-硝基苄基)哌啶(121mg,0.20mmol)、10%钯-活性碳(85mg)、乙酸乙酯(10ml)和甲醇(1ml)的混合物在室温氢气氛下搅拌19小时。滤出Pd催化剂,浓缩滤液,得到1-(3-氨基-4-氯苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(78mg,68%)。
实施例1833:1-(3-氨基-4-氯苄基)-4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(2142号化合物)的制备
依据实施例1832的方法,使用相应的试剂,合成标题化合物:1-(3-氨基-4-氯苄基)-4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(2142号化合物):13.7mg,98%;纯度用RPLC/MS测定(83%);ESI/MS m/e466.2(M++H,C22H26ClF2N5O2)。
实施例1834:1-(3-乙酰氨基-4-羟基苄基)-4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(2148号化合物)的制备
向1-(3-氨基-4-羟基苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(27mg,0.049mmol)、(哌啶子基甲基)聚苯乙烯(2.7mmol/g,60mg,0.15mmol)和二氯甲烷(2ml)的混合物中加入乙酸酐(0.12mmol)的二氯甲烷(0.12ml)溶液。反应混合物在室温下搅拌3小时。将混合物装上VarianTM SCX柱,用CH3OH洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩,得到1-(3-乙酰氨基-4-羟基苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(30mg,定量):ESI/MS m/e590.4(M++H,C29H37F2N5O6)。
向上面得到的1-(3-乙酰氨基-4-羟基苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶的甲醇(1ml)溶液中加入4N HCl的二噁烷(0.50ml)溶液,溶液在室温下搅拌过夜。溶液浓缩后,将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH(5ml×2)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物。经过浓缩和制备型TLC(SiO2,AcOEt/MeOH=3∶2)得到1-(3-乙酰氨基-4-羟基苄基)-4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(2148号化合物)(2.3mg,9.2%):纯度用RPLC/MS测定(98%);ESI/MSm/e 490.3(M++H,C24H29F2N5O4)。
实施例1835-1839
依据实施例1826和1834的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表42中。
表42
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例1835 |
2143 |
C25 H29 F2 N5 O5 |
518.3 |
4.8 |
45 |
|
实施例1836 |
2147 |
C25 H31 F2 N5 O4 |
504.3 |
3.0 |
23 |
|
实施例1837 |
2154 |
C26 H32 F3 N5 O4 |
536.4 |
4.1 |
66 |
|
实施例1838 |
2155 |
C25 H30 F3 N5 O4 |
522.3 |
5.5 |
71 |
|
实施例1839 |
2156 |
C26 H30 F3 N5 O5 |
550.3 |
7.0 |
78 |
实施例1840:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-甲氨基-4-羟基苄基)哌啶(2160号化合物)的制备
向1-(3-氨基-4-羟基苄基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(20.4mg,0.037mmol)、37%HCHO溶液(3.0mg,0.037mmol)、乙酸(0.10ml)和甲醇(1.3ml)的混合物中加入NaBH3CN(7.0mg)的甲醇(0.2ml)溶液。反应混合物在60℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(8ml)溶液洗脱产物,并浓缩,得到4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-甲氨基-4-羟基苄基)哌啶。
向上面得到的4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-甲氨基-4-羟基苄基)哌啶的甲醇(1.0ml)溶液中加入4N HCl的二噁烷(1.0ml)溶液,溶液在室温下搅拌3小时。溶液浓缩后,将残余物溶于甲醇(1ml),装上VarianTM SCX柱,用CH3OH(5ml×2)洗涤。用2N NH3的CH3OH(8ml)溶液洗脱产物。经过浓缩和制备型TLC(SiO2)得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3-甲氨基-4-羟基苄基)哌啶(2160号化合物)(3.4mg,20%):纯度用RPLC/MS测定(96%);ESI/MS m/e462.4(M++H,C23H29F2N5O3)。
实施例1841-1844
依据实施例1826和1840的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表43中。
表43
| |
化合物号 |
分子式 |
ESI/MSm/e |
产量(mg) |
产率(%) |
|
实施例1841 |
2159 |
C24 H31 F2 N5 O3 |
476.3 |
7.6 |
48 |
|
实施例1842 |
2161 |
C23 H28 Cl F2 N5 O2 |
480.3 |
7.3 |
45 |
|
实施例1843 |
2162 |
C25 H32 F3 N5 O3 |
508.4 |
6.0 |
24 |
|
实施例1844 |
2163 |
C24 H30 F3 N5 O3 |
494.3 |
4.3 |
15 |
实施例1845:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[c]呋咱-5-基)哌啶(2130号化合物)的制备
将4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(0.050mmol)、5-(溴甲基)苯并[c]呋咱(0.75mmol)、(哌啶子基甲基)聚苯乙烯(2.6-2.8mmol/g,60mg,0.15mmol)、甲醇(0.2ml)、乙腈(1.0ml)和氯仿(0.50ml)的混合物在50℃下搅拌过夜。混合物冷却至室温,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。向所得物质中加入氯仿(1.5ml)和异氰酸苯基酯(0.075ml),溶液在室温下搅拌1小时,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。将残余物溶于甲醇(1ml),加入4N HCl的二噁烷(0.50ml)溶液。溶液在室温下搅拌过夜,并浓缩。将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH(5ml×2)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物。经过浓缩和制备型TLC(SiO2,乙酸乙酯/MeOH=5/1)得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[c]呋咱-5-基)哌啶(2130号化合物)(3.6mg,16%):纯度用RPLC/MS测定(87%);ESI/MS m/e459.3(M++H,C22H24F2N6O3)。
实施例1846:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3,5-二甲基异噁唑-4-基)哌啶(2131号化合物)的制备
依据实施例1845的方法,使用相应的试剂,合成标题化合物:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(3,5-二甲基异噁唑-4-基)哌啶(2131号化合物):3.8mg,产率18%;ESI/MS m/e 436.2(M++H,C21H27F2N5O3)。
实施例1847:4-[{N-(2-氨基-5-氯苯甲酰基)甘氨酰}氨基甲基]-1-{4-(三氟甲硫基)苄基}哌啶(1616号化合物)的制备
将4-[{N-(2-氨基-5-氯苯甲酰基)甘氨酰}氨基甲基]哌啶(16.2mg,0.050mmol)、4-(三氟甲硫基)苄基溴(20.3mg,0.075mmol)、哌啶子基甲基聚苯乙烯(60mg)、乙腈(1.0ml)和氯仿(0.50ml)的混合物在60℃下搅拌15小时。反应混合物冷却,装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到4-[{N-(2-氨基-5-氯苯甲酰基)甘氨酰}氨基甲基]-1-{4-(三氟甲硫基)苄基}哌啶(1616号化合物)(21.9mg,85%):纯度用RPLC/MS测定(96%);ESI/MS m/e 545.2(M++H,C23H26ClF3N4O2S)。
实施例1848-1868
依据实施例1847的方法,使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC得到所需物质。ESI/MS数据和产率总结在表44中。
表44
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1848 |
1617 |
C23 H26 Br F3 N4 O2 S |
559.0 |
21.0 |
75 |
|
实施例1849 |
1777 |
C23 H25 Cl2 F3 N4 O2 |
517.0 |
16.3 |
63.0 |
|
实施例1850 |
1778 |
C24 H29 F3 N4 O2 |
463.2 |
9.5 |
41.1 |
|
实施例1851 |
1779 |
C24 H27 F3 N4 O4 |
493.2 |
12.7 |
51.6 |
|
实施例1852 |
1780 |
C23 H26 Br F3 N4 O2 |
527.0 |
16.4 |
62.2 |
|
实施例1853 |
1781 |
C23 H27 F3 N4 O3 |
465.2 |
10.0 |
28.7 |
|
实施例1854 |
1782 |
C25 H29 F3 N4 O2 |
475.2 |
12.2 |
34.3 |
|
实施例1855 |
1783 |
C24 H26 F3 N5 O2 |
474.2 |
17.2 |
48.4 |
|
实施例1856 |
1784 |
C23 H27 F3 N4 O2 |
449.2 |
11.3 |
33.6 |
|
实施例1857 |
1788 |
C25 H31 F3 N4 O2 |
477.2 |
10.0 |
42.0 |
|
实施例1858 |
1789 |
C24 H29 F3 N4 O3 |
479.2 |
10.0 |
27.9 |
|
实施例1859 |
1792 |
C24 H30 F2 N4 O2 |
445.2 |
5.9 |
26.5 |
|
实施例1860 |
1793 |
C22 H24 Cl2 F2 N4 O2 |
485.2 |
9.2 |
37.9 |
|
实施例1861 |
1794 |
C23 H28 F2 N4 O2 |
431.2 |
5.7 |
26.5 |
|
实施例1862 |
1795 |
C23 H26 F2 N4 O4 |
461.2 |
6.0 |
26.1 |
|
实施例1863 |
1796 |
C22 H25 Br F2 N4 O2 |
497.0 |
10.5 |
42.4 |
|
实施例1864 |
1797 |
C22 H26 F2 N4 O3 |
433.2 |
3.5 |
16.2 |
|
实施例1865 |
1798 |
C23 H28 F2 N4 O3 |
447.2 |
5.6 |
25.1 |
|
实施例1866 |
1799 |
C24 H28 F2 N4 O2 |
443.2 |
5.5 |
24.9 |
|
实施例1867 |
1800 |
C23 H25 F2 N5 O2 |
442.2 |
9.4 |
42.6 |
|
实施例1868 |
1801 |
C22 H26 F2 N4 O2 |
417.2 |
6.5 |
31.2 |
实施例1869:4-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基甲基]-1-(4-溴苄基)哌啶(1910号化合物)的制备
将4-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲氧基苯甲酰基)甘氨酰}氨基甲基]哌啶(0.050mmol)、4-溴苄基溴(0.060mmol)、哌啶子基甲基聚苯乙烯(60mg)、乙腈(0.8ml)和氯仿(0.5ml)的混合物在60℃下搅拌12小时。反应混合物冷却,装上VarianTM SCX柱,用50%CHCl3/CH3OH(10ml)和CH3OH(10ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。向所得物质中加入4N HCl的1,4-二噁烷(2ml)溶液,溶液在室温下搅拌过夜。经过浓缩和制备型TLC得到4-[{N-(2-氨基-5-三氟甲氧基苯甲酰基)甘氨酰}氨基甲基]-1-(4-溴苄基)哌啶(1910号化合物)(6.5mg,24%):纯度用RPLC/MS测定(96%);ESI/MS m/e 545(M++H,C23H26BrF3N4O3)。
实施例1870-1873
依据实施例1869的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表45中。
表45
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1870 |
1911 |
C23 H25 Cl2 F3 N4 O3 |
533 |
10.6 |
39.7 |
|
实施例1871 |
1912 |
C23 H27 F3 N4 O4 |
481 |
12.5 |
52.0 |
|
实施例1872 |
1913 |
C25 H31 F3 N4 O3 |
493 |
7.5 |
30.5 |
|
实施例1873 |
1914 |
C24 H29 F3 N4 O3 |
479 |
11.0 |
46.0 |
实施例1874:4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[d]咪唑-5-基)哌啶(2186号化合物)的制备
将4-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]哌啶(0.060mmol)、1-(叔丁氧羰基)-6-(溴甲基)苯并[d]咪唑(15.6mg,0.050mmol)、(哌啶子基甲基)聚苯乙烯(86mg)和乙腈(2ml)的混合物在50℃下搅拌3小时。冷却至室温后,加入异氰酸苯基酯(30mg),混合物在室温下搅拌1小时,装上VarianTM SCX柱,用CH3OH(5ml)和CHCl3(5ml)洗涤。用2N NH3的CH3OH(3ml)溶液洗脱产物,并浓缩。
将所得物质溶于甲醇(1ml),加入4N HCl的二噁烷(1ml)溶液。溶液在室温下搅拌过夜,装上VarianTM SCX柱,用CH3OH和二氯甲烷洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩。经过制备型TLC(SiO2,AcOEt/MeOH=3∶1)得到4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[d]咪唑-5-基)哌啶(2186号化合物)(1.9mg,7.8%):纯度用RPLC/MS测定(100%);ESI/MS m/e 489.4(M++H,C24H27F3N6O2)。
实施例1875:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[c]噻二唑-5-基)哌啶(2184号化合物)的制备
向5-(羟甲基)苯并[c]噻二唑(8.3mg,0.050mmol)、(哌啶子基甲基)聚苯乙烯(86mg)和氯仿(1ml)的混合物中加入甲磺酰氯(0.0042ml),混合物在室温下搅拌1.5小时。加入乙腈(1ml)和4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(0.060mmol),反应混合物在50℃下搅拌3小时。冷却至室温后,加入异氰酸苯基酯(30mg),混合物在室温下搅拌1小时,装上VarianTM SCX柱,用CH3OH(5ml)和CHCl3(5ml)洗涤。用2N NH3的2CH3OH(3ml)溶液洗脱产物,并浓缩。
将所得物质溶于二氯甲烷(1ml),加入1M氯三甲基硅烷和1M苯酚的二氯甲烷(1ml)溶液。溶液在室温下搅拌5小时,装上VarianTM SCX柱,用CH3OH和二氯甲烷洗涤。用2N NH3的CH3OH溶液洗脱产物,并浓缩。经过制备型TLC(SiO2,AcOEt/MeOH=3∶1)得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[c]噻二唑-5-基)哌啶(2184号化合物)(1.3mg,5.5%):纯度用RPLC/MS测定(100%);ESI/MS m/e 475.2(M++H,C22H24F2N6O2S)。
实施例1876:4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[c]噻二唑-5-基)哌啶(2185号化合物)的制备
依据实施例1875的方法,使用相应的试剂,合成标题化合物:4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[c]噻二唑-5-基)哌啶(2185号化合物):7.2mg,产率28%;ESI/MS m/e 507.4(M++H,C23H25F3N6O2S)。
实施例1877:4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(2-氨基-4-氯苄基)哌啶(1919号化合物)的制备
将4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]哌啶(0.050mmol)、4-氯-2-硝基苄基氯(0.050mmol)、哌啶子基甲基聚苯乙烯(60mg)、乙腈(1.0ml)和氯仿(0.7ml)的混合物在50℃下搅拌过夜。反应混合物冷却,装上VarianTM SCX柱,用50%CHCl3/CH3OH(10ml)和CH3OH(10ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。向所得物质中加入乙醇(3ml)和10%Pd-C(15mg),混合物在室温H2下搅拌1.5小时。经过过滤、浓缩和制备型TLC得到4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(2-氨基-4-氯苄基)哌啶(1919号化合物)(5.1mg,14%):纯度用RPLC/MS测定(90%);1H NMR(400MHz,CDCl3)δ1.09-1.32(m,4H),1.41-1.59(m,1H),1.66(d,J=12.5Hz,2H),1.88(t,J=11.5Hz,2H),2.82(d,J=11.5Hz,2H),3.17(t,J=6.5Hz,2H),3.42(s,2H),4.05(d,J=5.5Hz,2H),4.85(br s,1H),5.92(br s,2H),6.25-6.36(m,1H),6.55-6.66(m,1H),6.70(d,J=8.5Hz,1H),6.85(d,J=8.5Hz,1H),7.26(s,1H),7.42(d,J=8.5Hz,1H),7.68(s,1H);ESI/MS m/e 498.2(M++H,C23H27ClF3N5O3).
实施例1878和1879
依据实施例1877的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表46中。
表46
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1878 |
1920 |
C22 H26 Cl F2 N5 O2 |
466.2 |
3.5 |
10.0 |
|
实施例1879 |
1922 |
C23 H27 Cl F3 N5 O3 |
514.2 |
1.2 |
3.1 |
实施例1880:4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[d]噁唑-5-基)哌啶(2188号化合物)的制备
将依据实施例1826方法制备的1-(3-氨基-4-羟基苄基)-4-[{N-(2-(叔丁氧羰基氨基)-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]哌啶(34.8mg,0.060mmol)的THF(2ml)溶液用原甲酸三乙酯(0.033ml,3.3当量)和对甲苯磺酸吡啶鎓(2mg,0.4当量)处理。反应混合物在回流下搅拌过夜。冷却至室温后,混合物浓缩。将残余物溶于AcOEt,装上BondElutTM Si柱,用乙酸乙酯/甲醇=4/1洗脱,并浓缩。
将所得物质溶于AcOEt(1.5ml),加入4N HCl的二噁烷(0.5ml)溶液。溶液在室温下搅拌过夜,用5M NaOH水溶液调pH为10,用AcOEt萃取。萃取液浓缩,用PTLC纯化(SiO2,AcOEt/MeOH=4∶1),得到4-[{N-(2-氨基-5-三氟甲基苯甲酰基)甘氨酰}氨基甲基]-1-(苯并[d]噁唑-5-基)哌啶(2188号化合物)(1.6mg,5%):纯度用RPLC/MS测定(94%);ESI/MS m/e 490.3(M++H,C24H26F3N5O3)。
实施例1881:4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(2-酮基-2,3-二氢-1,3-苯并噁唑-5-基)哌啶(2190号化合物)的制备
向1-(3-氨基-4-羟基)-4-[{N-(2-(叔丁氧羰基氨基)-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]哌啶(22mg,0.040mmol)、NaHCO3(0.040mmol)、水(0.7ml)和甲醇(1.5ml)的混合物中加入氯甲酸苯基酯(0.046mmol),混合物在室温下搅拌3小时。加入1N NaOH溶液(0.040ml),反应混合物再搅拌1.5小时。混合物用乙酸乙酯萃取,蒸发。将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。
向所得物质中加入1M氯三甲基硅烷和1M苯酚的二氯甲烷(2ml)溶液。溶液在室温下搅拌2小时,蒸发。将残余物溶于甲醇,装上VarianTM SCX柱,用CH3OH洗涤(5ml×2)。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩。经过制备型TLC(SiO2,AcOEt/MeOH=5∶2)得到4-[{N-(2-氨基-4,5-二氟苯甲酰基)甘氨酰}氨基甲基]-1-(2-酮基-2,3-二氢-1,3-苯并噁唑-5-基)哌啶(2190号化合物)(4.1mg,22%):纯度用RPLC/MS测定(100%);ESI/MS m/e474.2(M++H,C23H25F2N5O4)。
实施例1882-1884
依据实施例1881的方法,分别使用相应的试剂(在制备2192和2193号化合物时,用氯硫甲酸苯基酯(chlorothionoformate)代替氯甲酸苯基酯),合成本发明的化合物。ESI/MS数据和产率总结在表47中。
表47
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1882 |
2191 |
C24 H26 F3 N5 O4 |
506.3 |
3.1 |
10 |
|
实施例1883 |
2192 |
C23 H25 F2 N5 O3 S |
490.2 |
6.9 |
35 |
|
实施例1884 |
2193 |
C24 H26 F3 N5 O3 S |
522.2 |
3.6 |
11 |
参考例36:4-[{N-(1-(9-芴基甲氧羰基)哌啶-4-基甲基)氨基甲酰基甲基}氨基甲基]-3-甲氧基苯氧基甲基-聚苯乙烯的制备
向盐酸1-(9-芴基甲氧羰基)-4-(甘氨酰氨基甲基)哌啶(10mmol)的DMF(65ml)溶液中加入乙酸(0.3ml)、三乙酰氧基硼氢化钠(1.92g)和4-甲酰基-3-(甲氧基苯氧基甲基)-聚苯乙烯(1mmol/g,200g)。混合物摇动2小时,过滤。树脂用MeOH、DMF、CH2Cl2和甲醇洗涤,并干燥,得到所需物质。
实施例1885-2000:用于4-氨甲基哌啶的固相合成的通用操作
向相应的酸(1.6mmol)、HBTU(1.6mmol)和DMF(6ml)的混合物中加入二异丙基乙胺(3.6mmol),混合物摇动2分钟。加入4-[{N-(1-(9-芴基甲氧羰基)哌啶-4-基甲基)氨基甲酰基甲基}氨甲基]-3-甲氧基苯氧基甲基-聚苯乙烯(0.4mmol),混合物摇动1小时,过滤。树脂用DMF和CH2Cl2漂洗,干燥。
将所得树脂、哌啶(3.2ml)和DMF(12.8ml)的混合物摇动10分钟,过滤。树脂用DMF和CH2Cl2洗涤,干燥。
向干燥树脂(0.05mmol)中加入NaBH(OAc)3(0.25mmol)、AcOH(0.025mmol)和DMF(1ml)的混合物。加入相应的醛(2.5mmol),混合物摇动2小时,然后过滤,用CH3OH、10%二异丙乙胺的DMF溶液、DMF、CH2Cl2和CH3OH洗涤。将树脂、水(0.050ml)和三氟乙酸(0.95ml)的混合物摇动1小时,过滤。树脂用CH2Cl2和CH3OH洗涤。合并滤液和洗液,浓缩。将粗物质装上VarianTM SCX柱,用CH3OH(15ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,浓缩。必要时使用制备型TLC或HPLC,得到所需物质。ESI/MS数据和产率总结在表48中。
表48
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例1885 |
1923 |
C23 H25 Br F3 N3 O2 S |
544 |
15.7 |
87 |
|
实施例1886 |
1924 |
C24 H28 F3 N3 O3 S |
496 |
14.6 |
89 |
|
实施例1887 |
1925 |
C23 H25 F4 N3 O2 S |
484 |
11.7 |
73 |
|
实施例1888 |
1926 |
C23 H24 F5 N3 O2 S |
502 |
13.9 |
84 |
|
实施例1889 |
1927 |
C23 H26 F3 N3 O3 S |
482 |
10.7 |
67 |
|
实施例1890 |
1928 |
C24 H26 F3 N3 O4 S |
510 |
14.3 |
85 |
|
实施例1891 |
1929 |
C26 H30 F3 N3 O2 S |
506 |
14.7 |
88 |
|
实施例1892 |
1930 |
C24 H28 F3 N3 O2 S2 |
512 |
14.4 |
85 |
|
实施例1893 |
1931 |
C25 H30 F3 N3 O2 S |
494 |
14.3 |
88 |
|
实施例1894 |
1932 |
C25 H28 F3 N3 O3 S |
509 |
7.1* |
35 |
|
实施例1895 |
1933 |
C25 H30 F3 N3 O2 S |
494 |
14.3 |
88 |
|
实施例1896 |
1934 |
C26 H32 F3 N3 O2 S |
509 |
14.4 |
86 |
|
实施例1897 |
1935 |
C23 H25 F3 N4 O4 S |
511 |
14.9 |
88 |
|
实施例1898 |
1936 |
C24 H28 F3 N3 O2 S |
480 |
13.3 |
84 |
|
实施例1899 |
1937 |
C26 H32 F3 N3 O2 S |
509 |
11.1 |
66 |
|
实施例1900 |
1938 |
C23 H27 Br2 N3 O2 |
538 |
5.3* |
25 |
|
实施例1901 |
1939 |
C24 H30 Br N3 O3 |
488 |
5.0* |
25 |
|
实施例1902 |
1940 |
C23 H27 Br F N3 O2 |
476 |
4.9* |
25 |
|
实施例1903 |
1941 |
C23 H26 Br F2 N3 O2 |
494 |
6.1* |
30 |
|
实施例1904 |
1942 |
C23 H28 Br N3 O3 |
474 |
1.7* |
9 |
|
实施例1905 |
1943 |
C24 H28 Br N3 O4 |
502 |
6.6* |
32 |
|
实施例1906 |
1944 |
C26 H32 Br N3 O2 |
498 |
7.0* |
35 |
|
实施例1907 |
1945 |
C24 H30 Br N3 O2 S |
504 |
11.1 |
67 |
|
实施例1908 |
1946 |
C25 H32 Br N3 O2 |
488 |
3.2* |
16 |
|
实施例1909 |
1947 |
C25 H30 Br N3 O3 |
500 |
5.7 |
35 |
|
实施例1910 |
1948 |
C25 H32 Br N3 O2 |
486 |
4.9* |
25 |
|
实施例1911 |
1949 |
C26 H34 Br N3 O2 |
500 |
6.7* |
33 |
|
实施例1912 |
1950 |
C23 H27 Br N4 O4 |
503 |
5.0* |
25 |
|
实施例1913 |
1951 |
C24 H30 Br N3 O2 |
472 |
5.1* |
26 |
|
实施例1914 |
1952 |
C22 H24 Br2 F N3 O2 |
542 |
14.9 |
83 |
|
实施例1915 |
1953 |
C23 H27 Br F N3 O3 |
492 |
13.9 |
86 |
|
实施例1916 |
1954 |
C22 H24 Br F2 N3 O2 |
480 |
12.5 |
79 |
|
实施例1917 |
1955 |
C22 H23 Br F3 N3 O2 |
498 |
13.2 |
80 |
|
实施例1918 |
1956 |
C22 H25 Br F N3 O3 |
478 |
7.0 |
44 |
|
实施例1919 |
1957 |
C23 H25 Br F N3 O4 |
506 |
4.0* |
20 |
|
实施例1920 |
1958 |
C25 H29 Br F N3 O2 |
502 |
14.6 |
88 |
|
实施例1921 |
1959 |
C23 H27 Br F N3 O2 S |
508 |
13.1 |
78 |
|
实施例1922 |
1960 |
C24 H29 Br F N3 O2 |
490 |
13.8 |
85 |
|
实施例1923 |
1961 |
C24 H27 Br F N3 O3 |
504 |
2.7* |
13 |
|
实施例1924 |
1962 |
C24 H29 Br F N3 O2 |
490 |
12.7 |
78 |
|
实施例1925 |
1963 |
C25 H31 Br F N3 O2 |
504 |
13.5 |
81 |
|
实施例1926 |
1964 |
C22 H24 Br F N4 O4 |
507 |
14.8 |
88 |
|
实施例1927 |
1965 |
C23 H27 Br F N3 O2 |
476 |
12.1 |
77 |
|
实施例1928 |
1966 |
C25 H31 Br F N3 O2 |
504 |
13.4 |
80 |
|
实施例1929 |
1967 |
C22 H26 Br F N4 O2 |
477 |
4.7* |
20 |
|
实施例1930 |
1968 |
C23 H29 F N4 O3 |
429 |
6.9* |
32 |
|
实施例1931 |
1969 |
C22 H27 F N4 O3 |
415 |
3.7* |
17 |
|
实施例1932 |
1970 |
C23 H27 F N4 O4 |
443 |
5.4* |
24 |
|
实施例1933 |
1971 |
C25 H31 F N4 O2 |
439 |
4.3* |
20 |
|
实施例1934 |
1972 |
C23 H29 F N4 O2 S |
445 |
6.2* |
28 |
|
实施例1935 |
1973 |
C24 H31 F N4 O2 |
427 |
6.3* |
29 |
|
实施例1936 |
1974 |
C24 H31 F N4 O2 |
427 |
4.9* |
23 |
|
实施例1937 |
1975 |
C22 H26 F N5 O4 |
444 |
5.9* |
27 |
|
实施例1938 |
1976 |
C23 H29 F N4 O2 |
413 |
6.7* |
32 |
|
实施例1939 |
1977 |
C23 H26 F N5 O2 |
424 |
5.1* |
24 |
|
实施例1940 |
1978 |
C25 H33 F N4 O2 |
441 |
6.3* |
29 |
|
实施例1941 |
1979 |
C25 H30 F2 N4 O2 |
457 |
8.0* |
35 |
|
实施例1942 |
1980 |
C24 H28 F2 N4 O3 |
459 |
6.0* |
26 |
|
实施例1943 |
1981 |
C22 H25 F2 N5 O4 |
462 |
9.3* |
41 |
|
实施例1944 |
1982 |
C23 H25 F2 N5 O2 |
442 |
6.0* |
27 |
|
实施例1945 |
1983 |
C25 H32 F2 N4 O2 |
459 |
8.3* |
37 |
|
实施例1946 |
1984 |
C22 H26 Br I N4 O2 |
585 |
9.7* |
36 |
|
实施例1947 |
1985 |
C23 H29 I N4 O3 |
537 |
9.2* |
36 |
|
实施例1948 |
1986 |
C22 H27 I N4 O3 |
523 |
5.8* |
23 |
|
实施例1949 |
1987 |
C23 H27 I N4 O4 |
551 |
8.2* |
32 |
|
实施例1950 |
1988 |
C25 H31 I N4 O2 |
547 |
6.7* |
26 |
|
实施例1951 |
1989 |
C23 H29 I N4 O2 S |
553 |
6.4* |
25 |
|
实施例1952 |
1990 |
C24 H31 I N4 O2 |
535 |
7.2* |
29 |
|
实施例1953 |
1991 |
C24 H29 I N4 O3 |
549 |
5.6* |
22 |
|
实施例1954 |
1992 |
C24 H31 I N4 O2 |
535 |
6.2* |
25 |
|
实施例1955 |
1993 |
C22 H26 I N5 O4 |
552 |
10.2* |
40 |
|
实施例1956 |
1994 |
C23 H29 I N4 O2 |
521 |
7.5* |
30 |
|
实施例1957 |
1995 |
C23 H26 I N5 O2 |
532 |
6.8* |
27 |
|
实施例1958 |
1996 |
C25 H33 I N4 O2 |
549 |
7.1* |
28 |
|
实施例1959 |
1997 |
C25 H33 I N4 O2 |
549 |
3.0* |
12 |
|
实施例1960 |
1998 |
C22 H25 Br Cl N3 O2 |
478 |
7.6* |
39 |
|
实施例1961 |
1999 |
C23 H28 Cl N3 O3 |
430 |
7.0* |
39 |
|
实施例1962 |
2000 |
C22 H25 Cl F N3 O2 |
418 |
14.1 |
102 |
|
实施例1963 |
2001 |
C22 H26 Cl N3 O3 |
416 |
6.3* |
36 |
|
实施例1964 |
2002 |
C23 H26 Cl N3 O4 |
444 |
7.1* |
39 |
|
实施例1965 |
2003 |
C25 H30 Cl N3 O2 |
440 |
15.3 |
105 |
|
实施例1966 |
2004 |
C23 H28 Cl N3 O2 S |
446 |
8.4* |
45 |
|
实施例1967 |
2005 |
C24 H30 Cl N3 O2 |
428 |
7.4* |
41 |
|
实施例1968 |
2006 |
C24 H30 Cl N3 O2 |
428 |
13.8 |
98 |
|
实施例1969 |
2007 |
C22 H25 Cl N4 O4 |
445 |
16.0 |
109 |
|
实施例1970 |
2008 |
C23 H28 Cl N3 O2 |
414 |
14.1 |
103 |
|
实施例1971 |
2009 |
C23 H25 Cl N4 O2 |
425 |
14.8 |
106 |
|
实施例1972 |
2010 |
C25 H32 Cl N3 O2 |
442 |
14.5 |
99 |
|
实施例1973 |
2011 |
C25 H32 Cl N3 O2 |
442 |
14.5 |
99 |
|
实施例1974 |
2012 |
C22 H24 Br2 Cl N3 O2 |
558 |
12.8* |
58 |
|
实施例1975 |
2013 |
C23 H27 Br Cl N3 O3 |
508 |
8.6* |
42 |
|
实施例1976 |
2014 |
C22 H25 Br Cl N3 O3 |
494 |
6.0* |
30 |
|
实施例1977 |
2015 |
C23 H25 Br Cl N3 O4 |
522 |
8.4* |
40 |
|
实施例1978 |
2016 |
C25 H29 Br Cl N3 O2 |
518 |
17.6 |
103 |
|
实施例1979 |
2017 |
C23 H27 Br Cl N3 O2 S |
524 |
17.1 |
99 |
|
实施例1980 |
2018 |
C24 H29 Br Cl N3 O2 |
506 |
14.7 |
88 |
|
实施例1981 |
2019 |
C24 H27 Br Cl N3 O3 |
520 |
8.0* |
38 |
|
实施例1982 |
2020 |
C24 H29 Br Cl N3 O2 |
506 |
14.7 |
88 |
|
实施例1983 |
2021 |
C22 H24 Br Cl N4 O4 |
523 |
12.0* |
57 |
|
实施例1984 |
2022 |
C23 H27 Br Cl N3 O2 |
492 |
8.5* |
42 |
|
实施例1985 |
2023 |
C23 H24 Br Cl N4 O2 |
503 |
6.3* |
31 |
|
实施例1986 |
2024 |
C25 H31 Br Cl N3 O2 |
520 |
9.6* |
46 |
|
实施例1987 |
2025 |
C25 H31 Br Cl N3 O2 |
520 |
15.0 |
87 |
|
实施例1988 |
202 6 |
C22 H23 Br Cl F2 N3 O2 |
514 |
15.8 |
93 |
|
实施例1989 |
2027 |
C22 H26 Br2 N4 O2 |
537 |
10.7* |
42 |
|
实施例1990 |
2028 |
C23 H29 Br N4 O3 |
489 |
8.5* |
36 |
|
实施例1991 |
2029 |
C22 H27 Br N4 O3 |
475 |
7.5* |
32 |
|
实施例1992 |
2030 |
C23 H27 Br N4 O4 |
503 |
6.8* |
28 |
|
实施例1993 |
2031 |
C25 H31 Br N4 O2 |
499 |
6.2* |
26 |
|
实施例1994 |
2032 |
C24 H29 Br N4 O3 |
501 |
8.9* |
37 |
|
实施例1995 |
2033 |
C24 H31 Br N4 O2 |
487 |
9.1* |
39 |
|
实施例1996 |
2034 |
C22 H26 Br N5 O4 |
504 |
6.4* |
26 |
|
实施例1997 |
2035 |
C23 H29 Br N4 O2 |
473 |
6.5* |
28 |
|
实施例1998 |
2036 |
C23 H26 Br N5 O2 |
484 |
6.3* |
27 |
|
实施例1999 |
2037 |
C25 H33 Br N4 O2 |
501 |
5.4* |
22 |
|
实施例2000 |
2038 |
C22 H25 Br F2 N4 O2 |
495 |
5.4* |
23 |
*TFA盐的产量
实施例2001:1-(3-氨基甲酰基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(924号化合物)的制备
向1-(3-羧基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(19.4mg,0.041mmol)的CHCl3(2.5ml)的溶液中加入EDCI(10.7mg)、1-羟基苯并三唑水合物(7.5mg)、Et3N(15.4mg)、0.5M NH3的二噁烷溶液(0.1ml,0.05mmol)和DMF(0.5ml)。反应混合物在25℃下搅拌20小时,用2N NaOH水溶液(2×2ml)和盐水(1ml)洗涤。通过PTFE滤膜过滤后,在减压下除去溶剂,得到1-(3-氨基甲酰基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(924号化合物),为淡黄色固体(17.9mg,92%):纯度用RPLC/MS测定(89%);ESI/MS m/e 447.3(M++H,C24H27F3N4O3)。
实施例2002:1-(4-氨基甲酰基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(925号化合物)的制备
依据实施例2001的方法,使用相应的试剂,合成了925号化合物:14.2mg,72%;纯度用RPLC/MS测定(86%);ESI/MS m/e 447(M++H,C24H27F3N4O3)。
实施例2003:1-(4-氨基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(516号化合物)的制备
在5%钯碳(10mg)的存在下,将1-(4-硝基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(22.4mg,0.047mmol)的EtOH(3ml)溶液在25℃1atm下氢化1小时。过滤除去催化剂,用EtOH(5ml)洗涤。合并后的滤液蒸发,得到1-(4-氨基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(516号化合物),为淡黄色固体(20.1mg,96%)。纯度用RPLC/MS测定(99%);ESI/MS m/e 449.1(M++H,C23H27F3N4O2)。
实施例2004和2005
依据实施例2003的方法,分别使用相应的试剂,合成了517和518号化合物。ESI/MS数据和产率总结在表49中。
表49
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例2004 |
517 |
C23 H27 F3 N4 O2 |
449 |
26.5 |
78 |
|
实施例2005 |
518 |
C23 H27 F3 N4 O2 |
449 |
25.3 |
71 |
实施例2006:1-{4-(苯甲酰氨基)苄基}-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(519号化合物)的制备
向1-(4-氨基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(10.1mg,0.023mmol)的CH2Cl2(2.5ml)溶液中加入EDCI(4.7mg)、1-羟基苯并三唑水合物(3.3mg)、Et3N(2.5mg)和苯甲酸(3.0mg)。反应混合物在25℃下搅拌16小时,用2N NaOH水溶液(2×2ml)和盐水(1ml)洗涤。通过PTFE滤膜过滤后,在减压下除去溶剂,得到1-{4-(苯甲酰氨基)苄基}-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(519号化合物),为无色的油(4.6mg,36%):纯度用RPLC/MS测定(99%);ESI/MS m/e 553.2(M++H,C30H31F3N4O3)。
实施例2007:1-{4-(哌啶子基羰基)苄基}-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(1572号化合物)的制备
向1-(4-羧基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(0.040mmol)的DMF(1.0ml)溶液中加入哌啶(0.048mmol)、二异丙基碳二亚胺(0.45mmol)的DMF(0.15ml)溶液、1-羟基苯并三唑水合物(0.45mmol)的DMF(0.15ml)溶液。反应混合物在室温下搅拌17小时,装上VarianTM SCX柱,用CHCl3/CH3OH 1∶1(5ml)和CH3OH(5ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到1-{4-(哌啶子基羰基)苄基}-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(1572号化合物)(14.3mg,66%):纯度用RPLC/MS测定(99%);ESI/MS m/e 545(M++H,C29H35F3N4O3)。
实施例2008-2015
依据实施例2007的方法,分别使用相应的试剂,合成本发明的化合物。ESI/MS数据和产率总结在表50中。
表50
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例2008 |
1573 |
C31 H33 F3 N4 O4 |
583 |
17.6 |
76 |
|
实施例2009 |
1574 |
C31 H33 F3 N4 O3 |
567 |
18.8 |
83 |
|
实施例2010 |
1575 |
C30 H30 Cl F3 N4 O3 |
587 |
3.2 |
14 |
|
实施例2011 |
1576 |
C28 H33 F3 N4 O4 |
547 |
21.1 |
97 |
|
实施例2012 |
1577 |
C26 H31 F3 N4 O4 |
521 |
5.1 |
24 |
|
实施例2013 |
1578 |
C31 H33 F3 N4 O3 |
567 |
16.9 |
75 |
|
实施例2014 |
1579 |
C31 H33 F3 N4 O3 |
567 |
6.0 |
26 |
|
实施例2015 |
1580 |
C29 H35 F3 N4 O3 |
545 |
15.1 |
69 |
实施例2016:1-[4-(氯甲酰基)苄基]-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶的制备
将1-(4-羧基苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(240mg)和亚硫酰氯(1ml)的混合物在室温下搅拌12小时,在减压下除去过量的亚硫酰氯,得到所需的1-[4-(氯甲酰基)苄基]-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶。酰氯在使用时不经过进一步纯化。
实施例2017:1-[4-{N-(2-甲氧基乙基)氨基甲酰基}苄基]-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(1612号化合物)的制备
将1-[4-(氯甲酰基)苄基]-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(0.042mmol)、2-甲氧基乙胺(3.8mg,0.050mmol)、哌啶子基甲基聚苯乙烯(46mg)和二氯甲烷(1.5ml)的混合物在室温下搅拌17小时。加入水(0.020ml),混合物搅拌30分钟。加入甲醇(1ml),将混合物装上VarianTM SCX柱,用CH3OH(10ml)洗涤。用2N NH3的CH3OH(5ml)溶液洗脱产物,并浓缩,得到1-[4-{N-(2-甲氧基乙基)氨基甲酰基}苄基]-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(1612号化合物)(26.7mg,100%):纯度用RPLC/MS测定(92%);ESI/MS m/e 535.2(M++H,C27H33F3N4O4)。
实施例2018-2020
依据实施例2017的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用制备型TLC得到所需物质。ESI/MS数据和产率总结在表51中。
表51
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例2018 |
1610 |
C31 H30 F6 N4 O3 |
621.2 |
4.4 |
14 |
|
实施例2019 |
1611 |
C30 H29 C12 F3 N4 O3 |
621.2 |
35.7 |
quant |
|
实施例2020 |
1613 |
C32 H35 F3 N4 O3 |
581.2 |
29.9 |
quant |
实施例2021:4-[N-{5-溴-2-(甲氨基)苯甲酰基}甘氨酰]氨基甲基-1-(4-氯苄基)哌啶(1427号化合物)的制备
将4-{N-(2-氨基-5-溴苯甲酰基)甘氨酰}氨基甲基-1-(4-氯苄基)哌啶(1042号化合物)(50mg,0.10mmol)的原甲酸三乙酯(6.5ml)溶液在150℃下搅拌17小时。浓缩得到黄色固体。向该黄色固体的乙醇(3ml)溶液中加入硼氢化钠(7.6mg,0.2mmol),混合物在室温下搅拌14小时。将所得白色沉淀溶于二氯甲烷,溶液用1N NaOH水溶液(2ml)洗涤。分离有机层,经K2CO3干燥,过滤并蒸发。经过柱色谱法(SiO2,20%MeOH/CHCl3)得到4-[N-{5-溴-2-(甲氨基)苯甲酰基}甘氨酰]氨基甲基-1-(4-氯苄基)哌啶(1427号化合物)(40mg,80%):纯度用RPLC/MS测定(100%);ESI/MS m/e 505(M++H,C23H28BrClF6N4O2)。
实施例2022:4-[N-{5-溴-2-(二甲氨基)苯甲酰基}甘氨酰]氨基甲基-1-(4-氯苄基)哌啶(1428号化合物)的制备
向4-{N-(2-氨基-5-溴苯甲酰基)甘氨酰}氨基甲基-1-(4-氯苄基)哌啶(1042号化合物)(67mg,0.14mmol)、37%甲醛水溶液(0.112ml,1.4mmol)、乙腈(2ml)和甲醇(1.5ml)的混合物中连续加入氰基硼氢化钠(26mg,0.42mmol)和乙酸(14μl)。溶液在50℃下搅拌30小时后,加入1N NaOH水溶液和二氯甲烷。分离含水层,有机层经K2CO3干燥,过滤并蒸发。经过柱色谱法(SiO2,20%MeOH/AcOEt)得到4-[N-{5-溴-2-(二甲氨基)苯甲酰基}甘氨酰]氨基甲基-1-(4-氯苄基)哌啶(1428号化合物)(60mg,82%):纯度用RPLC/MS测定(100%);ESI/MS m/e 523(M++H,C24H30BrClF6N4O2)。
实施例2023:4-[{N-(5-溴-2-(甲磺酰氨基)苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)哌啶(1581号化合物)的制备
将4-[{N-(2-氨基-5-溴苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)哌啶(25mg,0.05mmol)、甲磺酰氯(0.0045ml)、三乙胺(0.026ml)和二氯甲烷(2ml)的混合物在室温下搅拌17小时。反应混合物用柱色谱法(SiO2)纯化,装上VarianTM SAX柱,用CH3OH(5ml)洗涤。用0.1N HCl的CH3OH(5ml)溶液洗脱产物,并浓缩,得到4-[{N-(5-溴-2-(甲磺酰氨基)苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)哌啶(1581号化合物)(5.4mg,19%):ESI/MS m/e 573.0(M++H,C23H28BrClN4O4S)。
实施例2024:4-[{N-(5-溴-2-(双(甲磺酰)氨基)苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)哌啶(1582号化合物)的制备
将4-[{N-(2-氨基-5-溴苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)哌啶(57mg,0.10mmol)、甲磺酰氯(0.018ml,0.24mmol)、三乙胺(0.068ml)和二氯甲烷(2ml)的混合物在室温下搅拌8小时。加入1N NaOH水溶液(1ml),混合物用二氯甲烷萃取(2ml×3)。合并后的萃取液经K2CO3干燥,过滤并蒸发。经过柱色谱法(SiO2)得到4-[{N-(5-溴-2-(双(甲磺酰)氨基)苯甲酰基)甘氨酰}氨基甲基]-1-(4-氯苄基)哌啶(1582号化合物)(40mg,62%):ESI/MS m/e 651(M++H,C24H30BrClN4O6S2)。
实施例2025:1-(4-氯苄基)-1-甲基-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶鎓碘化物(461号化合物的甲基碘化铵)的制备
向4-氯苄基氯(11.7mg,0.073mmol)的CH3CN(1.0ml)溶液中加入4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(30mg,0.087mmol)的CH3CN(1.0ml)溶液和(哌啶子基甲基)聚苯乙烯(80mg,2.7mmol基质/g树脂)。反应混合物在60℃下搅拌2小时。向冷却后的反应混合物中加入异氰酸苯基酯(10.4mg,0.087mmol),混合物在25℃下搅拌1小时。将反应混合物装上VarianTM SCX柱,用CH3OH(20ml)洗涤。用2N NH3的CH3OH(6ml)溶液洗脱产物,并浓缩,得到1-(4-氯苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶,为无色的油,使用时不经过纯化。向1-(4-氯苄基)-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶的CH3CN(2.0ml)溶液中加入碘甲烷(28mg,0.20mmol),反应混合物在70℃下搅拌4小时。在减压下除去溶剂,得到1-(4-氯苄基)-1-甲基-4-[{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶鎓碘化物,为淡黄色油(31.7mg,71%):纯度用RPLC/MS测定(99%);ESI/MS m/e 482.1(M+,C24H28ClF3N3O2)。
实施例2026:1-(4-氯苄基)-4-[N-甲基-N-{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(520号化合物)的制备
向1-(4-氯苄基)-4-(氨甲基)哌啶(318mg,1.33mmol)与NaBH3CN(668mg)的10%CH3COOH/CH3OH(3ml)溶液中加入甲醛(108mg,1.33mmol,37wt%H2O溶液)。反应混合物在25℃下搅拌1小时。将反应混合物装上DOWEXTM50W×2柱(10ml),用CH3OH(100ml)洗涤。用2N NH3的CH3OH(100ml)溶液洗脱产物,并浓缩,得到173mg粗的1-(4-氯苄基)-4-{(甲氨基)甲基}哌啶,为无色的油,使用时不经过纯化。
向1-(4-氯苄基)-4-{(甲氨基)甲基}哌啶(111mg,0.44mmol)的CH2Cl2(4ml)溶液中加入EDCI(85mg)、1-羟基苯并三唑水合物(60mg)。反应混合物在25℃下搅拌1小时,然后用2N NaOH水溶液(2ml×2)和盐水(1ml)洗涤。通过PTFE滤膜过滤后,在减压下除去溶剂,得到黄色的油,用制备型TLC纯化(SiO2,5%CH3OH/CH2Cl2),得到1-(4-氯苄基)-4-[N-甲基-N-{N-(3-(三氟甲基)苯甲酰基)甘氨酰}氨基甲基]哌啶(520号化合物),为淡黄色油(14.0mg,3.4%):纯度用RPLC/MS测定(99%);ESI/MS m/e 482.1(M++H,C24H27ClF3N3O2)。
参考例37:3-氨基高哌啶的制备
将DL-α-氨基-ε-己内酰胺(2g,16mmol)的THF(70ml)溶液用1MBH3-THF溶液(80ml)处理,并加热回流3小时。加入2N HCl溶液(50ml),反应物再加热回流一小时,然后冷却至25℃。向反应物中加入4N NaOH溶液进行碱化(pH 10),用EtOAc萃取(3×200ml)。合并后的有机相用饱和NaHCO3水溶液洗涤,干燥(MgSO4),并浓缩,得到所需物质(990mg,54%),使用时不经过任何进一步纯化。
参考例38:3-氨基-1-(4-氯苄基)高哌啶的制备
将3-氨基高哌啶(1.71g,15mmol)的CH3CN(45ml)溶液用对氯苄基氯(463mg,2.9mmol)和K2CO3(828mg(原文为828g——译者注),6mmol)处理,并在70℃下加热9小时。反应混合物冷却至25℃,并浓缩,得到黄色固体。使残余物在H2O(5ml)与EtOAc(50ml)之间分布,用EtOAc萃取(2×50ml)。合并后的有机萃取液用盐水(20ml)洗涤,干燥(Na2SO4),并浓缩。所得黄色的油用色谱法纯化(SiO2,5-20%CH3OH-CH2Cl2梯度洗脱),得到所需产物,为黄色的油(639mg,93%)。
实施例2027:1-(4-氯苄基)-3-{(4-苯甲酰基丁酰)氨基}高哌啶(994号化合物)的制备
将3-氨基-1-(4-氯苄基)高哌啶(24mg,0.10mmol)与4-苯甲酰基丁酸(1.2当量)的CHCl3(1ml)溶液用EDCI(23mg)、HOBt(16.2mg)和Et3N(15.2μl)处理,在25℃下搅拌16小时。反应混合物用CH2Cl2(0.5ml)稀释,用2N NaOH水溶液洗涤(2×0.75ml),通过PTFE膜过滤进行干燥,并浓缩,得到1-(4-氯苄基)-3-{(4-苯甲酰基丁酰)氨基}高哌啶(994号化合物)(43mg,99%):纯度用RPLC/MS测定(98%);ESI/MS m/e 413(M++H,C24H29ClN2O2)。
实施例2028-2042
依据实施例2027的方法,分别使用相应的试剂,合成本发明的化合物。必要时使用色谱法(HPLC-C18)得到所需物质的TFA盐。ESI/MS数据和产率总结在表52中。
表52
| |
化合物号 |
分子式 |
ESI/MS m/e |
产量(mg) |
产率(%) |
|
实施例2028 |
943 |
C23 H25 Cl F3 N3 O2 |
468 |
6 |
28 |
|
实施例2029 |
944 |
C23 H28 Cl N3 O2 |
414 |
5 |
29 |
|
实施例2030 |
945 |
C22 H25 Cl N4 O4 |
445 |
6 |
30 |
|
实施例2031 |
946 |
C23 H27 Cl N4 O4 |
459 |
5 |
24 |
|
实施例2032 |
947 |
C25 H31 Cl N2 O4 |
459 |
4 |
20 |
|
实施例2033 |
948 |
C24 H29 Cl2 N3 O2 |
462 |
6 |
32 |
|
实施例2034 |
949 |
C25 H32 Cl N3 O2 |
442 |
6 |
31 |
|
实施例2035 |
988 |
C23 H25 Cl F3 N3 O2 |
468 |
45 |
92 |
|
实施例2036 |
989 |
C23 H28 Cl N3 O3 |
430 |
44 |
97 |
|
实施例2037 |
990 |
C22 H26 Cl N3 O2 |
400 |
41 |
99 |
|
实施例2038 |
991 |
C23 H27 Cl N2 O2 |
399 |
41 |
97 |
|
实施例2039 |
992 |
C25 H31 Cl N2 O4 |
459 |
47 |
98 |
|
实施例2040 |
993 |
C25 H31 Cl N2 O2 |
427 |
44 |
98 |
|
实施例2041 |
995 |
C25 H31 Cl N2 O3 |
443 |
44 |
95 |
|
实施例2042 |
996 |
C24 H31 Cl N4 O2 |
443 |
5* |
11 |
*TFA盐的产量
实施例2043:供试化合物对MIP-1α与THP-1细胞结合的抑制作用的测量
将人单核细胞白细胞系THP-1悬浮在测定缓冲液(RPMI-1640(Gibco-BRL Co.),含有0.1%BSA和25mM HEPES,pH调为7.4)中,得到浓度为1×107个细胞/ml的细胞混悬液。将供试化合物稀释在测定缓冲液中,用作供试化合物溶液。将碘化人MIP-1α(DuPont NEN Co.)稀释在测定缓冲液中,浓度为250nCi/ml,用作标记配体溶液。在96孔滤板(Millipore Co.)中,向每孔中按顺序等量加入25μl供试化合物溶液、25μl标记配体溶液和50μl细胞混悬液,搅拌(总反应体积为100μl),在18℃下恒温一小时。
反应后,过滤反应溶液,滤器用200μl冷PBS洗涤两次(加入200μl冷PBS,然后过滤)。滤器风干,向每孔中加入25μl液体闪烁剂。用TopCount(Packard Instrument Co.)测量滤器上细胞保留的放射性。
为了计算供试化合物抑制人MIP-1α与THP-1细胞结合的能力,减去由加入100ng未标记的人MIP-1α(Peprotech Co.)代替供试化合物所测定的非特异性结合,将没有加入供试化合物的读数作为100%。
抑制率(%)={1-(A-B)/(C-B)}×100
(A:加入供试化合物的读数;B:加入100ng未标记的人MIP-1α的读数;C:加入[125I]-标记的人MIP-1α的读数)。
在测量本发明的环胺衍生物的抑制作用时,例如,下列化合物在2μM或5μM下分别证实了20%-50%、50%-80%和>80%的抑制活性。这些化合物是:
10μM下抑制率为20%-50%的化合物:29,37,41,45,46,47,50,82,85,107,120,134,214,217,218,220,222,225,226,227,228,229,230,231,233,234,236,237,238, 333,334,335,336,338,340,342,347,348,349,350,352,357,359,361,366,372,374,375,376,380,382,383,385,470,471,472,473,474,483,484,488,489,491,497,499,500,502,506,508,510,514,515,518,524,543,553,554,555,556,563,571,575,576,578,579,580,583,586,587,588,590,591,592,595,596,598,603,610,611,612,614,624,625,626,629,635,638,639,640,641,642,643,644,646,647,648,649,652,653,658,659,660,665,666,669,671,675,677,679,681,682,684,691,695,696,700,702,704,706,711,712,714,717,721,723,724,726,727,728,729,731,737,739,740,741,742,744,746,765,767,772,773,774,775,776,780,781,785,786,787,788,790,791,792,793,795,796,797,798,805,806,807,810,813,820,821,822,824,825,827,829,830,833,834,837,838,844,853,855,873,877,878,880,882,887,888,891,894,901,903,904,905,911,929,932,933,935,938,940,948,993,996,1006,1018,1026,1028,1035,1048,1053,1054,1055,1056,1068,1070,1071,1072,1073,1075,1076,1081,1763,1764.
10μM下抑制率为50%-80%的化合物:1,2,3,4,7,13,22,23,24,25,27,31,32,38,48,83,319,121,123,131,215,216,221,235,337,351,354,358,362,363,365,367,368,369,373,378,381,384,458,459,463,465,466,467,468,478,479,480,482,485,486,487,492,493,494,495,496,498,501,503,504,507,511,512,513,520,523,527,529,530,531,532,533,534,535,536,537,538,539,540,541,542,545,546,547,548,549,550,551,552,558,559,560,561,562,565,567,568,569,570,572,573,574,577,581,582,594,597,599,600,602,604,606,607,608, 609,613,615,616,618,619,620,621,628,630,631,632,633,634,636,637,645,651,654,655,657,661,662,664,673,674,676,678,680,683,685,687,688,689,693,703,705,707,708,709,710,713,716,718,719,720,725,730,732,733,734,735,736,749,750,751,752,753,754,756,758,760,762,763,764,766,768,769,770,771,777,778,779,784,794,799,800,802,804,808,809,811,812,815,816,819,828,831,832,835,836,839,840,845,846,847,848,850,851,854,857,858,859,860,861,862,863,865,866,867,868,872,874,876,886,899,910,942,998,1004,1005,1007,1013,1015,1016,1017,1019,1020,1021,1022,1024,1030,1037,1042,1043,1044,1045,1046,1047,1049,1050,1052,1059,1060,1061,1067,1069,1074,1078,1079,1080,1766.
10μM下抑制率>80%的化合物:461,464,469,481,490,505,509,521,526,528,544,564,566,601,605,617,622,623,627,650,656,663,668,672,686,690,692,694,715,743,747,748,755,757,759,761,782,783,803,814,817,818,826,849,856,864,869,870,871,999,1000,1001,1002,1003,1008,1009,1010,1011,1012,1023,1029,1031,1032,1033,1034,1036,1038,1039,1040,1041,1051,1057,1058,1062,1063,10 64,10 65,1066,1082,1083.
2μM下抑制率为20%-50%的化合物:1042,1043,1244,1245,1416,1435,1436,1438,1441,1480,1570,1583,1584,1589,1590,1594,1595,1601,1660,1672,1687,1724,1779,1780,1787,1795,1796,1798,1799,1802,1893,1894,1898,1900,1915,1919,1920,2092,2096,2098,2100.
2μM下抑制率为50%-80%的化合物:1190,1414,1600,2091,2094,2095.
2μM下抑制率>80%的化合物:2093,2097,2099,2103,2104.
实施例2044:MCP-1与THP-1细胞结合的抑制作用的测量
1、携带人MCP-1基因的重组杆状病毒的构造
基于以前公开的人MCP-1基因序列(例如T.Yoshimura等《FEBSLett.》1989,244,487-493),使用两种侧面连接限制酶部位的合成DNA引物(5’-CACTCTAGACTCCAGCATGA-3’和5’-TAGCTGCAGATTCTTGGGTTG-3’)扩增来源于人内皮细胞cDNA(购自Kurabow Co.)的DNA片段;扩增后的片段用限制酶(PstI和XbaI)切割,与转移载体pVL1393(Invitrogen Co.)结合,将所得载体与传染性杆状病毒共同转染给Sf-9昆虫细胞,上清液进行噬斑测定,得到人MCP-1基因杆状病毒重组体。
2、杆状病毒内表达的[125I]-标记的人MCP-1的合成
利用K.Ishii等的方法(《生物化学与生物物理学研究通讯》1995,206,955-961),用5×107PFU(噬斑形成单位)上述人MCP-1重组杆状病毒感染5×106Sf-9昆虫细胞,在Ex-Cell 401培养基中培养7天。培养物上清液用肝素琼脂糖柱(Pharmacia Co.)亲和法纯化,然后进一步用反相HPLC(Vydac C18柱)纯化,制备纯化后的人MCP-1。纯化后的人MCP-1通过Amersham Co.用Bolton Hunter法进行蛋白质标记,得到[125I]-标记的杆状病毒表达的人MCP-1(比活度为2000Ci/mmol)。
3-1、对[125I]-标记的杆状病毒表达的人MCP-1与THP-1细胞结合的抑制作用的测量(方法1)
将人单核细胞白细胞系THP-1悬浮在测定缓冲液(RPMI-1640(Gibco-BRLCo.),含有0.1%BSA和25mM HEPES,pH调为7.4)中,得到浓度为1×107个细胞/ml的细胞混悬液。将供试化合物稀释在测定缓冲液中,用作供试化合物溶液。将上述[125I]-标记的人MCP-1稀释在测定缓冲液中,浓度为1mCi/ml,用作标记配体溶液。在96孔滤板(Millipore Co.)中,向每孔中按顺序等量加入25μl供试化合物溶液、25μl标记配体溶液和50μl细胞混悬液,搅拌(总反应体积为100μl),在18℃下恒温一小时。
反应后,过滤反应溶液,滤器用200μl冷PBS洗涤两次(加入200μl冷PBS,然后过滤)。滤器风干,向每孔中加入25μl液体闪烁剂。用TopCount(Packard Instrument Co.)测量滤器上细胞保留的放射性。
为了计算供试化合物抑制人MCP-1与THP-1细胞结合的能力,减去由加入100ng未标记的人MCP-1代替供试化合物所测定的非特异性结合,将没有加入供试化合物的读数作为100%。
抑制率(%)={1-(A-B)/(C-B)}×100
(A:加入供试化合物的读数;B:加入100ng未标记的人MCP-1的读数;C:加入[125I]-标记的人MCP-1的读数)。
在测量本发明的环胺衍生物的抑制作用时,例如,下列化合物在1μM、10μM或100μM下分别证实了20%-50%、50%-80%和>80%的抑制活性。这些化合物是
100μM下抑制率为20%-50%的化合物:3,6,11,15,16,19,28,44,88,92,94,104,111,112,124,125,133,219,220,224,228,236,338,343,346,347,348,349,362,363,367,368,371,373,381,618,847,849,850,866,867,869,870,871,872,873.
100μM下抑制率为50%-80%的化合物:1,8,10,12,18,21,26,30,33,35,39,84,89,90,91,96,97,98,99,100,101,103,106,108,109,110,116,122,126,216,218,221,225,226,231,330,332,333,334,337,341,342,350,352,354,356,359,360,361,364,366,374,375,379,382,462,463,464,557,686,840,841,842,843,844,845,846,848,862,863,864,865,868.
100μM下抑制率>80%的化合物:
2,4,5,7,13,14,17,20,22,23,24,25,27,29,31,32,34,36,38,40,41,42,43,45,46,47,48,49,50,83,85,86,95,102,105,107,113,114,115,119,120,121,123,127,128,129,130,131,132,134,214,215,217,227,237,238,331,335,336,339,340,345,351,355,357,358,383,458,459,460,466,558,851,852,861,874.
10μM下抑制率为20%-50%的化合物:12,18,30,34,40,42,43,51,52,53,54,55,56,57,59,60,64,66,75,76,77,78,79,82,8 9,90,97,98,102,103,116,127,128,129,130,132,135,136,140,141,144,156,157,159,160,161,162,163,166,167,168,169,170,171,172,173,174,175,176,178,179,190,191,192,195,197,200,202,203,204,205,208,233,234,235,239,240,241,242,243,245,247,249,250,255,263,264,269,274,278,279,282,306,316,317,323,324,380,404,409,433,446,448,449,451,470,471,473,476,479,486,488,489,497,498,499,501,504,507,508,509,510,512,514,516,519,527,530,532,542,545,560,563,564,565,566,568,569,572,573,574,575,578,583,584,586,587,589,590,599,600,601,603,606,612,613,620,621,622,624,625,627,629,630,632,634,636,637,640,641,642,643,644,645,646,647,648,649,658,678,682,687,692,694,764,775,856,857,860,881,882,883,884,890,892,899,900,903,905,907,908,911,912,916,917,921,922,923,925,927,931,932,935,939,940,968,986,1039,1041,1045,1047,1062,1063,1083.
10μM下抑制率为50%-80%的化合物:7,32,36,61,62,63,65,67,69,70,71,72,73,74,81,91,105,114,121,123,134,137,138,139,146,147,148,149,151,154,165,177,232,244,248,251,252,253,256,259,261,266,267,276,286,292,293,295,301,305,307,310,314,315,320,322,328,434,435,436,437,439,440,443,447,450,452,453,454,455,456,468,469,472,474,475,477,478,480,481,482,483,485,490,493,494,500,505,511,517,520,529,534,540,543,544,548,555,556,561,562,570,576,579,611,617,853,854,855,858,859,875,877,879,880,885,886,887,888,891,894,895,904,906,909,910,913,914,918,928,930,933,937,938,945,970,1040,1044,1046.
10μM下抑制率>80%的化合物:31,45,46,48,58,68,80,83,113,115,142,143,145,150,152,265,268,272,275,283,285,287,288,290,291,294,296,297,302,308,309,313,321,325,326,358,438,441,442,444,445,457,466,467,484,487,491,492,495,496,503,518,537,538,547,554,876,878,919,929,943.
1μM下抑制率为20%-50%的化合物:1118,1121,1136,1143,1146,1158,1159,1167,1170,1359,1361,1362,1363.
1μM下抑制率为50%-80%的化合物:
1133,1134,1137,1141,1156,1161,1162,1163,1164,1166.
1μM下抑制率>80%的化合物:1147.
3-2、对[125I]-标记的杆状病毒表达的人MCP-1与THP-1细胞结合的抑制作用的测量(方法2)
将人单核细胞白细胞系THP-1悬浮在测定缓冲液(50mM HEPES,pH7.4,1.0mM CaCl2,5.0mM MgCl2,0.5%BSA)中,得到浓度为1×107个细胞/ml的细胞混悬液。将供试化合物稀释在测定缓冲液中,用作供试化合物溶液。将上述[125I]-标记的人MCP-1稀释在测定缓冲液中,浓度为1mCi/ml,用作标记配体溶液。在96孔滤板(Millipore Co.)中,向每孔中按顺序等量加入25μl供试化合物溶液、25μl标记配体溶液和50μl细胞混悬液,搅拌(总反应体积为100μl),在18℃下恒温一小时。
反应后,过滤反应溶液,滤器用200μl冷PBS洗涤两次(加入200μl冷PBS,然后过滤)。滤器风干,向每孔中加入25μl液体闪烁剂。用TopCount(Packard Instrument Co.)测量滤器上细胞保留的放射性。
为了计算供试化合物抑制人MCP-1与THP-1细胞结合的能力,减去由加入100ng未标记的人MCP-1代替供试化合物所测定的非特异性结合,将没有加入供试化合物的读数作为100%。
抑制率(%)={1-(A-B)/(C-B)}×100
(A:加入供试化合物的读数;B:加入100ng未标记的人MCP-1的读数;C:加入[125I]-标记的人MCP-1的读数)。
在测量本发明的环胺衍生物的抑制作用时,例如,下列化合物在0.2μM、1μM或10μM下分别证实了20%-50%、50%-80%和>80%的抑制活性。这些化合物是
10μM下抑制率为20%-50%的化合物:1560
10μM下抑制率为50%-80%的化合物:1550.
10μM下抑制率>80%的化合物:541,1042,1043,1559
1μM下抑制率为20%-50%的化合物:
1098,1100,1101,1104,1105,1109,1110,1116,1174,1175,1176,1178,1187,1188,1189,1197,1198,1199,1200,1201,1202,1209,1210,1211,1212,1222,1225,1229,1230,1237,1238,1243,1250,1259,1261,1265,1266,1272,1277,1282,1294,1299,1302,1307,1315,1318,1319,1320,1329,1330,1335,1336,1337,1343,1344,1353,1355,1356,1357,1358,1368,1372,1385,1386,1392,1400,1413,1422,1423,1425,1426,1429,1430,1432,1437,1440,1445,1446,1447,1448,1450,1452,1453,1455,1458,1459,1461,1463,1464,1466,1468,1469,1470,1471,1474,1479,1482,1485,1507,1508,1510,1511,1512,1513,1514,1515,1516,1518,1519,1521,1522,1524,1535,1538,1540,1542,1544,1571,1573,1574,1575,1576,1577,1578,1579,1580,1581,1582,1585,1587,1598,1602,1603,1604,1609,1611,1612,1613,1614,1615,1616,1617,1618,1622,1627,1630,1643,1646,1662,1669,1716,1717,1723,1728,1731,1733,1736,1739,1740,1747,1750,1755,1757,1758,1759,1760,1761,1762,1769,1770,1771,1772,1773,1774,1777,1783,1784,1785,1791,1793,1904,1911,1917,2057,2061,2063,2064,2065,2066,2067,2068,2069,2071,2072,2073,2074,2075,2076,2080,2081,2082,2110,2112,2123,2130,2131,2139.
1μM下抑制率为50%-80%的化合物:
37,298,318,1084,1091,1103,1106,1108,1111,1113,1114,1115,1138,1142,1165,1179,1190,1192,1193,1195,1196,1204,1205,1206,1207,1208,1245,1246,1255,1257,1258,1262,1263,1293,1300,1342,1351,1352,1354,1370,1371,1373,1375,1377,1378,1380,1381,1383,1384,1391,1411,1412,1414,1417,1418,1419,1421,1424,1431,1436,1439,1449,1454,1456,1457,1460,1462,1472,1473,1487,1502,1504,1506,1517,1525,1526,1527,1529,1530,1531,1532,1533,1534,1536,1537,1539,1541,1545,1593,1600,1601,1606,1608,1619,1620,1621,1623,1624,1625,1626,1628,1629,1645,1650,1654,1658,1663,1664,1665,1670,1671,1672,1673,1675,1678,1679,1681,1684,1687,1688,1689,1690,1711,1712,1714,1718,1722,1725,1726,1727,1729,1730,1732,1734,1735,1737,1741,1742,1743,1744,1745,1746,1748,1751,1753,1754,1756,1779,1781,1782,1786,1788,1789,1790,1792,1795,1797,1798,1800,1801,1804,1848,1862,1883,1885,1886,1887,1889,1893,1894,1903,1905,1910,1912,1913,1914,1918,1922,1976,1985,2027,2035,2062,2083,2084,2088,2089,2090,2111,2124,2125,2126,2135.
1μM下抑制率>80%的化合物:299,311,312,329,1042,1043,1085,1119,1191,1203,1220,1228,1236,1244,1256,1288,1295,1308,1310,1376,1382,1393,1395,1415,1416,1420,1435,1438,1441,1480,1481,1570,1583,1584,1589,1590,1594,1595,1607,1634,1660,1661,1666,1668,1695,1696,1697,1698,1699,1701,1702,1703,1704,1705,1706,1707,1708,1709,1713,1724,1749,1752,1775,1776,1778,1780,1787,1794,1796,1799,1802,1803,1841,1869,1870,1871,1872,1876,1877,1892,1896,1897,1898,1899,1900,1901,1902,1906,1907,1908,1909,1915,1916,1919,1920,1921,2085,2086,2087,2113,2114,2118,2119,2120,2121,2122,2127,2128,2129,2132,2133,2136,2137,2138,2159,2161,2162,2187,2189,2193.
0.2μM下抑制率为20%-50%的化合物:
1680,1682,1686,1691,1694,1700,1805,1810,1811,1812,1813,1815,1816,1817,1818,1819,1820,1824,1825,1826,1827,1828,1832,1833,1834,1835,1836,1839,1840,1842,1843,1851,1852,1853,1854,1855,1856,1858,1859,1860,1863,1864,1865,1866,1868,1874,1878,1879,1880,1888,1890,1891,1895,1926,1927,1928,1929,1930,1934,1935,1937,1945,1946,1951,1952,1953,1954,1959,1960,1961,1962,1966,1969,1970,1971,1972,1973,1977,1978,1979,1980,1981,1985,2014,2027,2028,2033,2035,2039,2040,2041,2042,2044,2045,2046.
0.2μM下抑制率为50%-80%的化合物:1677,1678,1679,1681,1687,1688,1689,1690,1695,1697,1808,1809,1841,1848,1861,1862,1869,1870,1871,1872,1873,1876,1877,1883,1884,1885,1886,1887,1889,1893,1894,1976.
0.2μM下抑制率>80%的化合物:1696,1892
实施例2045:对[125I]-标记的人MCP-1与表达MCP-1受体的细胞结合的抑制作用的测量
1、表达MCP-1受体的细胞的衍生
将含有MCP-1受体的cDNA片段(由S.Yamagami等《生物化学与生物物理学研究通讯》1994,202,1156-1162报道)克隆到NotI部位的表达质粒pCEP4(Invitrogen Co.)中,使用脂转染试剂Lipofectamine(Gibco-BRL Co.)将所得质粒转染给人肾上皮细胞系293-EBNA。在选择剂(潮霉素)的存在下培养细胞,得到稳定表达的转染子系。受体的表达通过[125I]-标记的人MCP-1的结合得以确认。
2、对[125I]-标记的杆状病毒表达的人MCP-1与MCP-1受体表达细胞结合的抑制作用的测量
用细胞刮棒刮取组织培养皿上的MCP-1受体表达细胞,将其悬浮在测定缓冲液(D-MEM(Gibco-BRL Co.),含有0.1%BSA和25mM HEPES,pH调为7.4)中,得到浓度为6×106个细胞/ml的细胞混悬液。将供试化合物稀释在测定缓冲液中。其余操作同实施例2044。
在测量本发明某些典型化合物的抑制作用时,抑制活性基本上分别等于实施例2044。
实施例2046:对细胞趋化性的抑制作用的测量
为了测定本发明化合物对细胞趋化性的抑制作用,我们按照Fall等的方法(《免疫学方法杂志》190,33,239-247),使用人单核细胞白细胞系THP-1作为趋化细胞,测量了由单核细胞趋化因子MCP-1引起的细胞趋化性。将2×106个细胞/ml的THP-1细胞(悬浮在RPMI-1640(Flow Laboratories Co.)+10%FCS中)置于96孔微趋化性腔(Neuroprobe,注册商标)的上腔(200μl)中,将在相同溶液中的最终浓度为20ng/ml的人重组MCP-1(Peprotech Co.)置于下腔中,将聚碳酸酯滤器(无PVP,Neuroprobe;注册商标)置于两腔之间。将这些在37℃5%CO2中恒温2小时。
除去滤器,将已经移行至滤器下侧的细胞固定,用Diff Quick(KokusaiShiyaku Co.)染色,然后用平板读数器(Molecular Device Co.)在550nm波长下定量测定细胞移行指数,为3孔的平均值。另外,将供试化合物分别与THP-1和MCP-1置于上腔和下腔,测定对细胞移行的抑制作用(抑制IC50(μM))。抑制作用被定义为{(没有供试化合物的上腔和下腔中由MCP-1诱导的细胞移行)-(没有加入MCP-1的下腔中的细胞移行)=100%},供试化合物给出50%抑制作用的浓度被指定为IC50。
在测量本发明的环胺衍生物的抑制作用时,例如,下列化合物的50抑制作用浓度(IC50)是IC50<0.1μM。
IC50<0.1μM的化合物:4,37,298,299,311,312,318,329,461,886,909,1042,1043,1085,1119,1138,1142,1165,1179,1191,1203,1205,1220,1228,1236,1244,1245,1256,1288,1293,1295,1308,1310,1352,1376,1382,1393,1395,1416,1420,1435,1436,1438,1441,1480,1531,1532,1570,1583,1584,1589,1590,1594,1595,1600,1601,1607,1660,1661,1664,1666,1668,1698,1699,1701,1702,1703,1704,1706,1707,1708,1709,1713,1775,1776,1778,1779,1787,1794,1796,1799,1802,1803,1896,1898,1899,1900,1901,1902,1906,1907,1908,1909,1915,1916,1919,1920,1921,2087,2114,2128,2129,2132,2137,2141,2144,2157,2158,2189.