CN1272324C - 新颖的除草剂 - Google Patents
新颖的除草剂 Download PDFInfo
- Publication number
- CN1272324C CN1272324C CN00813428.6A CN00813428A CN1272324C CN 1272324 C CN1272324 C CN 1272324C CN 00813428 A CN00813428 A CN 00813428A CN 1272324 C CN1272324 C CN 1272324C
- Authority
- CN
- China
- Prior art keywords
- alkyl
- group
- compound
- formula
- hydrogen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D221/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom as the only ring hetero atom, not provided for by groups C07D211/00 - C07D219/00
- C07D221/02—Heterocyclic compounds containing six-membered rings having one nitrogen atom as the only ring hetero atom, not provided for by groups C07D211/00 - C07D219/00 condensed with carbocyclic rings or ring systems
- C07D221/20—Spiro-condensed ring systems
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N35/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having two bonds to hetero atoms with at the most one bond to halogen, e.g. aldehyde radical
- A01N35/06—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having two bonds to hetero atoms with at the most one bond to halogen, e.g. aldehyde radical containing keto or thioketo groups as part of a ring, e.g. cyclohexanone, quinone; Derivatives thereof, e.g. ketals
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/08—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings with oxygen as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/10—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings with sulfur as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/14—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom six-membered rings
- A01N43/16—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom six-membered rings with oxygen as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/14—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom six-membered rings
- A01N43/18—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom six-membered rings with sulfur as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/36—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/36—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings
- A01N43/38—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings condensed with carbocyclic rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/40—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/40—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings
- A01N43/42—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings condensed with carbocyclic rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/80—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,2
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/82—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with three ring hetero atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/86—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms six-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,3
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/90—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having two or more relevant hetero rings, condensed among themselves or with a common carbocyclic ring system
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/02—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having no bond to a nitrogen atom
- A01N47/06—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having no bond to a nitrogen atom containing —O—CO—O— groups; Thio analogues thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N47/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid
- A01N47/08—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom not being member of a ring and having no bond to a carbon or hydrogen atom, e.g. derivatives of carbonic acid the carbon atom having one or more single bonds to nitrogen atoms
- A01N47/10—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof
- A01N47/18—Carbamic acid derivatives, i.e. containing the group —O—CO—N<; Thio analogues thereof containing a —O—CO—N< group, or a thio analogue thereof, directly attached to a heterocyclic or cycloaliphatic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C25/00—Compounds containing at least one halogen atom bound to a six-membered aromatic ring
- C07C25/02—Monocyclic aromatic halogenated hydrocarbons
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C323/00—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups
- C07C323/22—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and doubly-bound oxygen atoms bound to the same carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C49/00—Ketones; Ketenes; Dimeric ketenes; Ketonic chelates
- C07C49/587—Unsaturated compounds containing a keto groups being part of a ring
- C07C49/703—Unsaturated compounds containing a keto groups being part of a ring containing hydroxy groups
- C07C49/747—Unsaturated compounds containing a keto groups being part of a ring containing hydroxy groups containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C49/00—Ketones; Ketenes; Dimeric ketenes; Ketonic chelates
- C07C49/587—Unsaturated compounds containing a keto groups being part of a ring
- C07C49/753—Unsaturated compounds containing a keto groups being part of a ring containing ether groups, groups, groups, or groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C69/00—Esters of carboxylic acids; Esters of carbonic or haloformic acids
- C07C69/013—Esters of alcohols having the esterified hydroxy group bound to a carbon atom of a ring other than a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C69/00—Esters of carboxylic acids; Esters of carbonic or haloformic acids
- C07C69/66—Esters of carboxylic acids having esterified carboxylic groups bound to acyclic carbon atoms and having any of the groups OH, O—metal, —CHO, keto, ether, acyloxy, groups, groups, or in the acid moiety
- C07C69/67—Esters of carboxylic acids having esterified carboxylic groups bound to acyclic carbon atoms and having any of the groups OH, O—metal, —CHO, keto, ether, acyloxy, groups, groups, or in the acid moiety of saturated acids
- C07C69/708—Ethers
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/30—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members
- C07D207/34—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/36—Oxygen or sulfur atoms
- C07D207/38—2-Pyrrolones
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/54—Spiro-condensed
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
- C07D209/96—Spiro-condensed ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/80—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members
- C07D211/84—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen directly attached to ring carbon atoms
- C07D211/86—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/28—Two oxygen or sulfur atoms
- C07D231/30—Two oxygen or sulfur atoms attached in positions 3 and 5
- C07D231/32—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/28—Two oxygen or sulfur atoms
- C07D231/30—Two oxygen or sulfur atoms attached in positions 3 and 5
- C07D231/32—Oxygen atoms
- C07D231/34—Oxygen atoms with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, attached in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/28—Two oxygen or sulfur atoms
- C07D231/30—Two oxygen or sulfur atoms attached in positions 3 and 5
- C07D231/32—Oxygen atoms
- C07D231/36—Oxygen atoms with hydrocarbon radicals, substituted by hetero atoms, attached in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D237/00—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings
- C07D237/02—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings not condensed with other rings
- C07D237/04—Heterocyclic compounds containing 1,2-diazine or hydrogenated 1,2-diazine rings not condensed with other rings having less than three double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D265/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom and one oxygen atom as the only ring hetero atoms
- C07D265/02—1,2-Oxazines; Hydrogenated 1,2-oxazines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D279/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom and one sulfur atom as the only ring hetero atoms
- C07D279/04—1,3-Thiazines; Hydrogenated 1,3-thiazines
- C07D279/06—1,3-Thiazines; Hydrogenated 1,3-thiazines not condensed with other rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D303/00—Compounds containing three-membered rings having one oxygen atom as the only ring hetero atom
- C07D303/02—Compounds containing oxirane rings
- C07D303/12—Compounds containing oxirane rings with hydrocarbon radicals, substituted by singly or doubly bound oxygen atoms
- C07D303/32—Compounds containing oxirane rings with hydrocarbon radicals, substituted by singly or doubly bound oxygen atoms by aldehydo- or ketonic radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/56—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D307/60—Two oxygen atoms, e.g. succinic anhydride
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/94—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom spiro-condensed with carbocyclic rings or ring systems, e.g. griseofulvins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D309/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings
- C07D309/32—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D309/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings
- C07D309/34—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D309/36—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with oxygen atoms directly attached to ring carbon atoms
- C07D309/38—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with oxygen atoms directly attached to ring carbon atoms one oxygen atom in position 2 or 4, e.g. pyrones
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D311/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings
- C07D311/02—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D311/74—Benzo[b]pyrans, hydrogenated in the carbocyclic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D311/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings
- C07D311/02—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D311/94—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings ortho- or peri-condensed with carbocyclic rings or ring systems condensed with rings other than six-membered or with ring systems containing such rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D311/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings
- C07D311/96—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings spiro-condensed with carbocyclic rings or ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/30—Hetero atoms other than halogen
- C07D333/32—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D335/00—Heterocyclic compounds containing six-membered rings having one sulfur atom as the only ring hetero atom
- C07D335/02—Heterocyclic compounds containing six-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/08—Bridged systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/10—Spiro-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/02—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains two hetero rings
- C07D491/10—Spiro-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/12—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains three hetero rings
- C07D491/14—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D493/00—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system
- C07D493/02—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system in which the condensed system contains two hetero rings
- C07D493/10—Spiro-condensed systems
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental Sciences (AREA)
- Wood Science & Technology (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Zoology (AREA)
- Pest Control & Pesticides (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
Abstract
式I化合物,和这些化合物的农用盐、异构体和对映异构体适用于用作除草剂,其中的取代基如权利要求1中所定义。
Description
本发明涉及新的具有除草活性的苯基取代杂环、其制备方法、含有这些化合物的组合物、以及该化合物在控制杂草,特别是在有用的作物中控制杂草,或抑制植物生长方面的应用。
已有报道说3-羟基-4-芳基-5-氧代-吡唑啉衍生物具有除草活性,例如EP-A-0 508 126、WO 96/25395和WO 96/21652所述。
现已发现具有除草活性和抑制植物生长性能的苯基取代杂环。
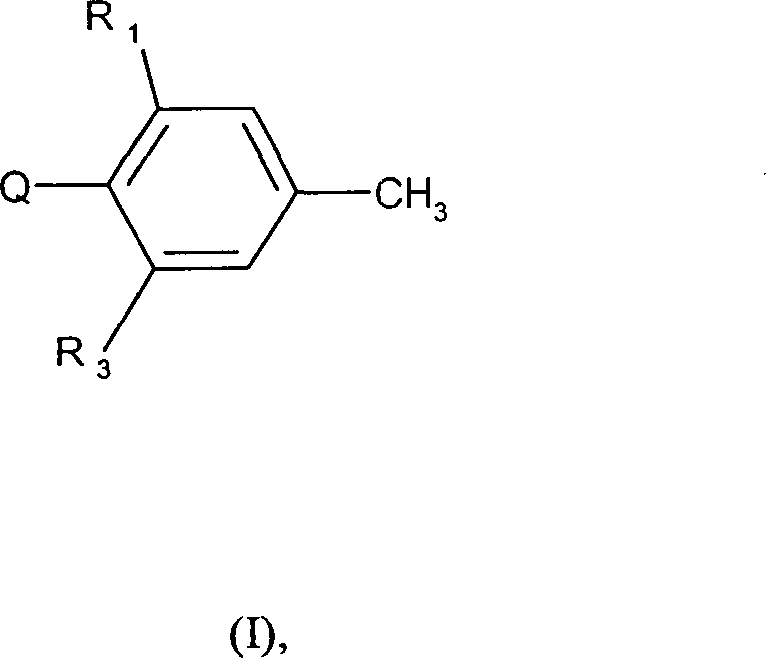
本发明涉及下述化合物,以及这些化合物的农用盐、异构体和对映异构体:
其中
R1和R3各自相互独立地是乙基,卤代乙基,乙炔基,C1-C2烷氧基,C1-C2卤代烷氧基,C1-C2烷基羰基,C1-C2羟基烷基或C1-C2烷氧羰基;
Q是下述基团:
R4和R5各自相互独立地是C1-C10烷基,C2-C10链烯基,C2-C10链炔基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10烷基磺酰基烷基,C2-C10烷基羰基烷基,C2-C10-N-烷氧基亚氨基烷基,C2-C10烷氧羰基烷基,C1-C10氨基烷基,C3-C10二烷基氨基烷基,C2-C10烷基氨基烷基,C1-C10氰基烷基,C4-C10环烷基烷基,C1-C10苯基烷基,C1-C10-杂芳基烷基,C1-C10苯氧基烷基,C1-C10杂芳氧基烷基,C1-C10亚烷基氨基氧基烷基,C1-C10硝基烷基,C1-C10三烷基甲硅烷基烷基,C2-C10烷基氨基羰基烷基,C2-C10二烷基氨基羰基烷基,C2-C10烷基氨基羰氧基烷基,C3-C10二烷基氨基羰氧基烷基,C2-C10烷氧羰基氨基烷基,C1-C10-N-烷氧羰基-N-烷基氨基烷基,C1-C10环烷基,芳基或杂芳基;或
R4和R5与它们所键合的原子一起形成5-至7-元环的基团,该环可含有一个或两个选自氮、氧和硫的杂原子,此外该环还可含有稠合或螺-键合的由2-6个碳原子组成的亚烷基或亚链烯基链,该链又可含有一个或二个选自氧或硫的杂原子,其中的环基可被苯基或苄基取代,而它们又可以被下述基团取代:卤素、C1-C6烷基、C1-C6卤代烷基、C3-C6环烷基、羟基、C1-C6烷氧基、C1-C6烷氧基-C1-C5烷氧基、C1-C6卤代烷氧基或硝基;
R2、R6和R32各自相互独立地是C1-C10烷基,C2-C10链烯基,C2-C10链炔基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10烷基磺酰基烷基,C2-C10烷基羰基烷基,C3-C10-环烷基,芳基或杂芳基;
R7、R31和R33各自相互独立地是氢,C1-C10烷基,C2-C10链烯基,C2-C10链炔基或C2-C10烷氧基烷基;
R8是氢,C1-C10烷基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10烷基磺酰基烷基,C3-C10-环烷基,芳基或杂芳基;或
R6和R7或R2和R31或R32和R33与它们所键合的原子一起形成饱和的3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;或R6和R8与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R9、R10、R11和R12各自相互独立地是C1-C10烷基,C2-C10链烯基,C2-C10-链炔基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10烷基磺酰基烷基,C2-C10烷基羰基烷基,C3-C10环烷基,芳基或杂芳基;或
R9和R11或R9和R10与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R13、R14、R34和R35各自相互独立地是C1-C10烷基,C2-C10链烯基,C2-C10-链炔基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10烷基磺酰基烷基,C2-C10烷基羰基烷基,C3-C10环烷基,芳基或杂芳基;或
R13和R14或R34和R35与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R15是C1-C10烷基,C2-C10链烯基,C2-C10链炔基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10烷基磺酰基烷基,C2-C10烷基羰基烷基,C2-C10烷氧羰基烷基,C1-C10氨基烷基,C3-C10二烷基氨基烷基,C2-C10烷基氨基烷基,C1-C10氰基烷基,C4-C10环烷基烷基,C1-C10苯基烷基,C1-C10杂芳基烷基,C1-C10苯氧基烷基,C1-C10杂芳氧基烷基,C1-C10硝基烷基,C3-C10环烷基,芳基或杂芳基;
R16是C1-C10烷基,C2-C10链烯基,C2-C10链炔基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10-链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10-烷基磺酰基烷基,C3-C10环烷基,芳基或杂芳基;
R17是C1-C10烷基,C2-C10链烯基,C2-C10链炔基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10-链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10烷基磺酰基烷基,C2-C10烷基羰基烷基,C3-C10环烷基,芳基或杂芳基;
R18是氢,C2-C10链烯基,C2-C10链炔基,C1-C10烷基或C1-C10烷氧基烷基;或
R17和R18与它们所键合的原子一起形成3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
Y是氧,硫,C-R19或N-R36;
R19和R36各自相互独立地是C1-C10烷基,C1-C10卤代烷基,苯基或杂芳基;或
R18和R19或R18和R36与它们所键合的原子一起形成饱和的5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
G1、G2、G3、G4、G5、G6、G7、G8、G9和G10各自相互独立地是氢,-C(XI)-R20,-C(X2)-X3-R21,-C(X4)-N(R22)-R23,-SO2-R24,碱金属阳离子,碱土金属阳离子,锍氧离子或铵氧离子,-P(X5)(R25)-R26或-CH2-X6-R27;
XI、X2、X3、X4、X5和X6各自相互独立地是氧或硫;
R20、R21、R22和R23各自相互独立地是氢,C1-C10烷基,C2-C10-链烯基,C2-C10链炔基,C1-C10卤代烷基,C1-C10氰基烷基,C1-C10硝基烷基,C1-C10氨基烷基,C1-C5烷基氨基-C1-C5烷基,C2-C8二烷基氨基-C1-C5烷基,C3-C7环烷基-C1-C5烷基,C2-C10烷氧基烷基,C4-C10链烯氧基烷基,C4-C10链炔氧基烷基,C2-C10烷硫基烷基,C1-C5-烷基亚磺酰基(sulfoxyl)-C1-C5烷基,C1-C6烷基磺酰基-C1-C5烷基,C2-C8亚烷氨基氧基-C1-C5烷基,C1-C5烷基羰基-C1-C5烷基,C1-C5烷氧羰基-C1-C5烷基,C1-C5氨基羰基-C1-C5-烷基,C2-C8二烷基氨基羰基-C1-C5烷基,C1-C5烷基羰基氨基-C1-C5烷基,C1-C5烷基-羰基-(C2-C5烷基)-氨基烷基,C3-C8三烷基甲硅烷基-C1-C5烷基,苯基-C1-C5烷基,杂芳基-C1-C5烷基,苯氧基-C1-C5烷基,杂芳氧基-C1-C5烷基,C2-C5链烯基,C2-C5卤代链烯基,C3-C8环烷基,苯基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的苯基,或杂芳基或杂芳基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的杂芳基或杂芳基氨基,二杂芳基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的二杂芳基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的苯基氨基,二苯基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的二苯基氨基,或C3-C7-环烷基氨基,二-C3-C7环烷基氨基或C3-C7环烷氧基;
R24、R25和R26是氢,C1-C10烷基,C2-C10链烯基,C2-C10链炔基,C1-C10卤代烷基,C1-C10氰基烷基,C1-C10硝基烷基,C1-C10氨基烷基,C1-C5烷基氨基-C1-C5烷基,C2-C8-二烷基氨基-C1-C5烷基,C3-C7环烷基-C1-C5烷基,C2-C10烷氧基烷基,C4-C10链烯氧基烷基,C4-C10链炔氧基烷基,C2-C10烷硫基烷基,C1-C5烷基亚磺酰基-C1-C5烷基,C1-C5烷基磺酰基-C1-C5烷基,C2-C8亚烷基氨基氧基-C1-C5烷基,C1-C5烷基羰基-C1-C5烷基,C1-C5烷氧基-羰基-C1-C5烷基,C1-C5氨基羰基-C1-C5烷基,C2-C8二烷基氨基羰基-C1-C5烷基,C1-C5烷基羰基氨基-C1-C5烷基,C1-C5烷基羰基-(C2-C5烷基)-氨基烷基,C3-C8-三烷基甲硅烷基-C1-C5烷基,苯基-C1-C5烷基,杂芳基-C1-C5烷基,苯氧基-C1-C5烷基,杂芳氧基-C1-C5烷基,C2-C5链烯基,C2-C6卤代链烯基,C3-C8环烷基,苯基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的苯基,或杂芳基或杂芳基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的杂芳基或杂芳基氨基,二杂芳基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的二杂芳基氨基,苯基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的苯基氨基,二苯基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的二苯基氨基,或C3-C7环烷基氨基,二-C3-C7环烷基氨基,C3-C7环烷氧基,C1-C10烷氧基,C1-C10卤代烷氧基,C1-C5烷基氨基,C2-C8二烷基氨基,苄氧基或苯氧基,其中苄氧基和苯基又可被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代;
R27是C1-C10烷基,C2-C10链烯基,C2-C10链炔基,C1-C10卤代烷基,C1-C10氰基烷基,C1-C10硝基烷基,C1-C10氨基烷基,C1-C5烷基氨基-C1-C5烷基,C2-C8二烷基氨基-C1-C5烷基,C3-C7环烷基-C1-C5烷基,C2-C10烷氧基烷基,C4-C10链烯氧基烷基,C4-C10链炔氧基烷基,C2-C10烷硫基烷基,C1-C5烷基亚磺酰基-C1-C5烷基,C1-C5烷基磺酰基-C1-C5烷基,C2-C8亚烷氨基氧基-C1-C5烷基,C1-C5烷基羰基-C1-C5烷基,C1-C5烷氧羰基-C1-C5烷基,C1-C5氨基羰基-C1-C5烷基,C2-C8二烷基氨基羰基-C1-C5烷基,C1-C5烷基羰基-氨基-C1-C5烷基,C1-C5烷基羰基-(C2-C5烷基)-氨基烷基,C3-C6三烷基甲硅烷基-C1-C5烷基,苯基-C1-C5烷基,杂芳基-C1-C5烷基,苯氧基-C1-C5烷基,杂芳氧基-C1-C5烷基,C2-C5链烯基,C2-C6卤代链烯基,C3-C8环烷基,苯基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的苯基,或杂芳基或杂芳基氨基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的杂芳基或杂芳基氨基,二杂芳基氨基,被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的二杂芳基氨基,或苯基氨基,被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的苯基氨基,二苯基氨基,被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的二苯基氨基,C3-C7环烷基氨基,二-C3-C7环烷基氨基,C3-C7环烷氧基或C1-C10烷基-羰基;
Y2是氧,硫,C-R140-R141或N-R142,
R55是C1-C10烷基,C2-C10链烯基,C2-C10链炔基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10-烷基磺酰基烷基,C2-C10烷基羰基烷基,C3-C10环烷基,芳基或杂芳基;
R137是氢,C1-C10烷基,C2-C10链烯基,C2-C10链炔基或C1-C10烷氧基烷基;或
R55和R137与它们所键合的原子一起形成3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R138和R139各自相互独立地是氢,C1-C10烷基,C2-C10链烯基,C2-C10链炔基或C2-C10烷氧基烷基;和
R140和R141各自相互独立地是氢,C1-C10烷基,C2-C10链烯基,C2-C10链炔基或C1-C10烷氧基烷基;或
R55和C-R140与它们所键合的原子一起形成饱和或不饱和的3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R412是氢,C1-C10烷基,C1-C10卤代烷基,C2-C10烷氧基烷基,C3-C10链烯氧基烷基,C3-C10链炔氧基烷基,C2-C10烷硫基烷基,C2-C10烷基亚磺酰基烷基,C2-C10烷基磺酰基烷基,C3-C10环烷基,芳基或杂芳基;或
R55和N-R142与它们所键合的原子一起形成饱和或不饱和的3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子。
出现在所述取代基定义中的烷基基团可以是直链或支链的,例如甲基,乙基,正丙基,异丙基,正丁基,仲丁基,异丁基,叔丁基,和戊基,己基,庚基,辛基,壬基与癸基的异构体。卤代烷基例如是氟甲基,二氟甲基,三氟甲基,氯甲基,二氯甲基,三氯甲基,2,2,2-三氟乙基,2-氟乙基,2-氯乙基,五氟乙基,1,1-二氟-2,2,2-三氯乙基,2,2,3,3-四氟乙基和2,2,2-三氯乙基;优选三氯甲基,二氟氯甲基,二氟甲基,三氟甲基或二氯氟甲基。烷氧基烷基例如是甲氧基甲基,乙氧基甲基,丙氧基乙基,异丙氧基乙基,正丁氧基甲基,异丁氧基-正丁基,仲丁氧基甲基和叔丁氧基异丙基,优选甲氧基甲基和乙氧基甲基。烷氧基,链烯基,链炔基,烷氧基烷基,烷硫基,烷基磺酰基,烷基氨基羰基,二烷基氨基羰基,烷基氨基烷基,苯基烷基,硝基烷基,氨基烷基和N-烷氧羰基-N-烷基氨基烷基基团均是由所述的烷基基团衍生的基团。链烯基和链炔基基团可以是一或多不饱和的。应当理解的是,链烯基例如是乙烯基,烯丙基,甲代烯丙基,1-甲基乙烯基或丁-2-烯-1-基。链炔基例如是乙炔基,炔丙基,丁-2-炔-1-基,2-甲基丁炔-2-基或丁-3-炔-2-基。链炔基例如是乙炔基,炔丙基,丁-2-炔-1-基,2-甲基丁炔-2-基或丁-3-炔-2-基。卤代烷基基团优选链长为1-4个碳原子。卤代烷基例如是氟甲基,二氟甲基,三氟甲基,氯甲基,二氯甲基,三氯甲基,2,2,2-三氟乙基,2-氟乙基,2-氯乙基,五氟乙基,1,1-二氟-2,2,2-三氯乙基,2,2,3,3-四氟乙基或2,2,2-三氯乙基;优选三氯甲基,二氟氯甲基,二氟甲基,三氟甲基或二氯氟甲基。适当的卤代链烯基基团包括被卤素一或多取代的链烯基,卤素是氟、氯、溴或碘,特别是氟或氯,例如2,2-二氟-1-甲基乙烯基,3-氟丙烯基,3-氯丙烯基,3-溴丙烯基,2,3,3-三氟丙烯基,2,3,3-三氯丙烯基和4,4,4-三氟丁-2-烯-1-基。在被卤素一、二或三取代的C2-C6链烯基中,优选链长为3-5个碳原子。烷氧基基团优选链长为1-6碳原子。烷氧基例如是甲氧基,乙氧基,丙氧基,异丙氧基,正丁氧基,异丁氧基,仲丁氧基或叔丁氧基,以及戊氧基和己氧基的异构体,优选甲氧基和乙氧基。烷基羰基优选是乙酰基或丙酰基。烷氧羰基例如是甲氧基羰基,乙氧基羰基,丙氧基羰基,异丙氧基羰基,正丁氧基羰基,异丁氧基羰基,仲丁氧基羰基或叔丁氧基羰基;优选甲氧基羰基或乙氧基羰基。烷硫基基团优选链长是1-4碳原子。烷硫基例如是甲硫基,乙硫基,丙硫基,异丙硫基,正丁硫基,异丁硫基,仲丁硫基或叔丁硫基,优选甲硫基或乙硫基。烷基亚磺酰基例如是甲基亚磺酰基,乙基亚磺酰基,丙基亚磺酰基,异丙基亚磺酰基,正丁基亚磺酰基,异丁基亚磺酰基,仲丁基亚磺酰基或叔丁基亚磺酰基;优选甲基亚磺酰基或乙基亚磺酰基。烷基磺酰基例如是甲基磺酰基,乙基磺酰基,丙基磺酰基,异丙基磺酰基,正丁基磺酰基,异丁基磺酰基,仲丁基磺酰基或叔丁基磺酰基;优选甲基磺酰基或乙基磺酰基。烷基氨基例如是甲基氨基,乙基氨基,正丙基氨基,异丙基氨基或丁胺异构体。二烷基氨基例如是二甲基氨基,甲基乙基氨基,二乙基氨基,正丙基甲基氨基,二丁氨基或二异丙氨基。烷氧基烷基基团优选具有1-6个碳原子。烷氧基烷基例如是甲氧基甲基,甲氧基乙基,乙氧基甲基,乙氧基乙基,正丙氧基甲基,正丙氧基乙基,异丙氧基甲基或异丙氧基乙基。烷硫基烷基例如是甲硫基甲基,甲硫基乙基,乙硫基甲基,乙硫基乙基,正丙硫基甲基,正丙硫基乙基,异丙硫基甲基,异丙硫基乙基,丁硫基甲基,丁硫基乙基或丁硫基丁基。苯基可以是被取代的形式。取代基可以在邻、间和/或对位的位置上。优选取代基的位置在相对于与环连接的位置是邻或对位。
芳基例如是苯基或萘基。这些基团可被取代。在定义中没有特别说明的情况下,苯基也可以作为取代基如苯基烷基的一部分,苯基可被下述基团取代:卤素,硝基,氰基,C1-C4烷基,C1-C4烷氧基,C1-C4烷硫基,C1-C4烷基亚磺酰基,C1-C4烷基磺酰基,羧基,C1-C4烷氧羰基,氨基,C1-C4烷基氨基,C1-C4二烷基氨基或C1-C4烷基羰基氨基。
杂芳基基团通常是优选含有1-3个杂原子,如氮、硫和氧的芳族杂环。适当的杂环和杂芳族化合物的实例包括:吡咯烷酮、哌啶、吡喃、二噁烷、氮杂环丁烷、氧杂环丁烷、吡啶、嘧啶、三嗪、噻唑、噻二唑、咪唑、噁唑、异噁唑以及吡嗪、呋喃、吗啉、哌嗪、吡唑、苯并噁唑、苯并噻唑、喹喔啉和喹啉。这些杂环和杂芳族化合物还可进一步被下述基团取代:卤素,烷基,烷氧基,卤代烷基,卤代烷氧基,硝基,氰基,硫烷基,烷基氨基或苯基。
在本发明的保护范围内,应当理解的是3-至7-元环基团是指环系,除了在取代基Q的环上可能已经存在的杂原子以外,在环系上除了碳原子以外,还可含有一个或多个杂原子如氮、氧和/或硫。它们可以是饱和或不饱和的。例如在基团Q2中可通过取代基R6和R7形成不饱和键。优选这种环系中含有5-7个环原子。3-至7-元环基团包括环烷基,例如环丙基、环丁基、环戊基、环己基、环庚基或环辛基,也可以是被取代的。适当的取代基包括卤素,羟基,硝基,氰基,C1-C4烷基羰基,C1-C4-烷氧羰基,C1-C4烷基,C1-C4卤代烷基,酮基,C2-C4链烯氧基亚氨基,C1-C4烷氧基,C1-C4烷氧基烷氧基,C1-C4烷硫基,或下述三个基团之一:
其中X8是硫或氧,R28是C1-C4烷氧基或两个R28基团和与它们键合的-X8-C-X8桥一起形成5-或6-元环,该环可被甲基、乙基、甲氧基或酮基取代,
R29是C1-C4烷基,C1-C4卤代烷基,C2-C4链烯基或C2-C4卤代链烯基,
R30和R37各自相互独立地是C1-C4烷基,苯基或C2-C4链烯基,或R30和R37与它们所键合的氮原子一起形成5-或6-元环,所述的环可含有选自氮、氧和硫的杂原子。
在取代基定义中,碳原子数目是烷基,链烯基和链炔基基团以及由它们衍生的基团例如卤代烷基或链烯氧基的的碳原子总数。相应的,C2-C3烷氧基烷基包括甲氧基甲基、甲氧基乙基和乙氧基甲基。C3烷氧羰基烷基包括甲氧羰基乙基和乙氧羰基甲基。
取代基G1-G10中的碱金属、碱土金属或铵氧离子例如是钠、钾、镁、钙和铵的氧离子。优选的锍阳离子特别是三烷基锍阳离子,其中的烷基基团优选含有1-4个碳原子。
根据取代基的性质,式I化合物也可以是几何异构体和/或光学异构体和异构体混合物的形式,以及互变异构体和互变异构体混合物的形式。本发明还涉及式I的这些化合物,例如其中Q是Q1和基团G1是氢的式I化合物可以下述互变异构平衡存在:
当G1-G10不是氢以及R4和R5一起形成的环状基团是不对称取代、稠合或螺-键合,例如式I化合物可以下述式Id的异构体形式存在:
本发明同样还包括式I化合物所能形成的盐,优选与胺、碱金属、碱土金属碱或季铵碱形成的盐。适当的成盐物质已有描述,例如WO98/41089中所述。
本发明还包括式I化合物与胺、碱金属、碱土金属碱或季铵碱形成的盐。
在作为成盐物质碱金属和碱土金属氢氧化物之中,值得注意的是锂、钠、钾、镁或钙的氢氧化物,其中特别是钠或钾的氢氧化物。
适合用于形成铵盐的胺的实例包括氨或伯、仲和叔C1-C18烷基胺,C1-C4羟基烷基胺和C2-C4-烷氧基烷基胺,例如甲胺,乙胺,正丙胺,异丙胺,四种丁胺异构体,正戊基胺,异戊基胺,己胺,庚胺,辛胺,壬胺,癸胺,十五烷基胺,十六烷基胺,十七烷基胺,十八烷基胺,甲基-乙基胺,甲基-异丙基胺,甲基-己基胺,甲基-壬基胺,甲基-十五烷基胺,甲基-十八烷基胺,乙基-丁胺,乙基-庚胺,乙基-辛胺,己基-庚胺,己基-辛胺,二甲胺,二乙胺,二正丙基胺,二异丙胺,二正丁基胺,二正戊胺,二异戊基胺,二己基胺,二庚基胺,二辛基胺,乙醇胺,正丙醇胺,异丙醇胺,N,N-二乙醇胺,N-乙基丙醇胺,正丁基乙醇胺,烯丙基胺,正丁烯-2-胺,正戊烯-2-胺,2,3-二甲基丁烯-2-胺,二丁烯-2-胺,正己烯-2-胺,丙二胺,三甲胺,三乙胺,三-正丙基胺,三异丙基胺,三-正丁基胺,三异丁基胺,三-仲丁基胺,三-正戊基胺,甲氧基乙基胺和乙氧基乙基胺;杂环胺,例如吡啶,喹啉,异喹啉,吗啉,哌啶,吡咯烷,二氢吲哚,奎宁环和吖庚因;伯芳基胺,例如苯胺,甲氧基苯胺,乙氧基苯胺,邻-,间-和对-甲苯胺,苯二胺,联苯胺,萘胺,和邻-,间-和对-氯苯胺,特别是三乙胺,异丙胺和二异丙胺。
适于形成相应盐的季铵碱例如是式[N(RaRbRcRd)]OH化合物,其中Ra,Rb,Rc和Rd各自相互独立地是C1-C4烷基。也可以得到具有其它阴离子的其它适当的四烷基铵碱,如阴离子交换反应。
在式I化合物中,优选的是其中的Q是Q1,Q2,Q3,Q4,Q5,Q6,Q7,Q8或Q9的化合物。
优选的式I化合物是其中R4和R5各自相互独立地是C1-C6烷基,C1-C6卤代烷基,C2-C6烷氧基烷基,C4-C8链烯氧基烷基,C4-C6链炔氧基烷基,C2-C6烷硫基烷基,C2-C6烷基亚磺酰基烷基,C2-C6烷基磺酰基烷基,C2-C6烷基羰基烷基,C3-C6-N-烷氧基-亚氨基烷基,C3-C6烷氧羰基烷基,C1-C6氨基-烷基,C2-C6二烷基氨基烷基,C3-C6烷基氨基烷基,C1-C6氰基烷基,C4-C8环烷基烷基,C7-C8苯基烷基,C7-C8杂芳基烷基,C7-C8苯氧基烷基,C7-C8杂芳氧基烷基,C4-C6-亚烷氨基氧基烷基,C1-C6硝基烷基,C4-C8三烷基甲硅烷基烷基,C4-C6烷基氨基羰基,C3-C6二烷基氨基羰基,C4-C8烷基氨基羰氧烷基,C4-C8二烷基氨基羰氧烷基,C4-C8烷氧羰基氨基烷基,C4-C8-N-烷氧羰基-N-烷基氨基烷基,C3-C8环烷基,芳基或杂芳基,或
R4和R5与它们所键合的原子一起形成饱和或不饱和的5-至7-元环基团。
还优选的式I化合物是这些,其中
R2、R6和R32各自相互独立地是C1-C6烷基,C1-C6卤代烷基,C2-C6烷氧基-烷基,C4-C6链烯氧基烷基,C4-C6链炔氧基烷基,C2-C6烷硫基烷基,C2-C6烷基亚磺酰基烷基,C2-C6烷基磺酰基烷基,C3-C6烷基羰基烷基,C3-C8环烷基,芳基或杂芳基;
R7、R31和R33是氢,C1-C6烷基或C1-C6烷氧基烷基;
R8是氢,C1-C6烷基,C1-C6卤代烷基,C2-C6烷氧基烷基,C4-C6链烯氧基烷基,C4-C6-链炔氧基烷基,C1-C6烷硫基烷基,C1-C6烷基亚磺酰基烷基,C1-C6烷基磺酰基烷基,C3-C8-环烷基,芳基或杂芳基;或
R6和R7或R2和R31或R32和R33与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R6和R8与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R9、R10、R11和R12各自相互独立地是C1-C6烷基,C1-C6卤代烷基,C2-C8-烷氧基烷基,C4-C6链烯氧基烷基,C4-C6链炔氧基烷基,C2-C6烷硫基烷基,C2-C6烷基亚磺酰基烷基,C2-C6烷基磺酰基烷基,C3-C6烷基羰基烷基,C3-C8环烷基,芳基或杂芳基;或
R9和R11或R9和R10与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R9和R10与它们所键合的原子一起形成饱和的3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R13、R14、R34和R35各自相互独立地是C1-C6烷基,C1-C6卤代烷基,C2-C6-烷氧基烷基,C4-C6链烯氧基烷基,C4-C6链炔氧基烷基,C2-C6烷硫基烷基,C2-C6烷基亚磺酰基烷基,C2-C6烷基磺酰基烷基,C3-C8烷基羰基烷基,C3-C8环烷基,芳基或杂芳基;或
R13和R14或R34和R35与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R15是C1-C6烷基,C1-C6卤代烷基,C2-C8烷氧基烷基,C4-C6链烯氧基烷基,C4-C8链炔氧基烷基,C2-C6烷硫基烷基,C2-C6烷基亚磺酰基烷基,C2-C6烷基磺酰基烷基,C3-C6烷基羰基烷基,C3-C6烷氧羰基烷基,C2-C6氨基烷基,C4-C6二烷基氨基烷基,C4-C6烷基氨基烷基,C2-C6氰基烷基,C3-C8环烷基烷基,C7-C8苯基烷基,C7-C8杂芳基烷基,C7-C8-苯氧基烷基,C6-C8杂芳氧基烷基,C1-C6硝基烷基,C3-C8环烷基,芳基或杂芳基;
R16是C1-C6烷基,C1-C6卤代烷基,C2-C6烷氧基烷基,C4-C6链烯氧基烷基,C4-C6链炔氧基烷基,C2-C6烷硫基烷基,C2-C6烷基亚磺酰基烷基,C2-C6烷基磺酰基烷基,C3-C8环烷基,芳基或杂芳基;
R17是C1-C6烷基,C1-C6卤代烷基,C2-C6烷氧基烷基,C4-C6链烯氧基烷基,C4-C6链炔氧基烷基,C2-C8烷硫基烷基,C2-C6烷基亚磺酰基烷基,C2-C6烷基磺酰基烷基,C3-C6烷基羰基烷基,C3-C8环烷基,芳基或杂芳基;
R18是氢,C1-C6烷基或C2-C6烷氧基烷基;或
R17和R18与它们所键合的原子一起形成3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R19和R36各自相互独立地是C1-C6烷基,C1-C6卤代烷基,苯基或杂芳基;或
R18和R19或R18和R36与它们所键合的原子一起形成饱和的5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R20、R21、R22、R23和R27各自相互独立地是氢,C1-C8烷基,C1-C8-卤代烷基,C1-C8氰基烷基,C1-C8硝基烷基,C1-C8氨基烷基,C1-C5烷基氨基-C1-C2烷基,C2-C6二烷基氨基-C1-C2烷基,C3-C7环烷基-C1-C2烷基,C2-C8烷氧基烷基,C4-C8链烯氧基-烷基,C4-C8链炔氧基烷基,C2-C8烷硫基烷基,C1-C2烷基亚磺酰基-C1-C2烷基,C1-C2烷基-磺酰基-C1-C2烷基,C2-C8亚烷氨基氧基-C1-C2烷基,C1-C5烷基羰基-C1-C2烷基,C1-C5烷氧羰基-C1-C2烷基,C1-C5氨基-羰基-C1-C2烷基,C2-C8二烷基氨基-羰基-C1-C2烷基,C1-C5烷基羰基氨基-C1-C2烷基,C1-C2烷基羰基-N-C1-C3烷基-C1-C2氨基烷基,C3-C6-三烷基甲硅烷基-C1-C3烷基,苯基-C1-C2烷基,杂芳基-C1-C2烷基,苯氧基-C1-C2烷基,杂芳氧基-C1-C2烷基、C2-C5链烯基,C2-C5卤代链烯基,C3-C8环烷基,苯基或杂芳基;
R24、R25和R26各自相互独立地是氢,C1-C8烷基,C1-C8卤代烷基,C1-C8氰基烷基,C1-C8硝基烷基,C1-C8氨基烷基,C1-C5烷基氨基-C1-C2烷基,C2-C6二烷基氨基-C1-C2烷基,C3-C7环烷基-C1-C2烷基,C2-C8烷氧基烷基,C4-C8链烯氧基烷基,C4-C8-链炔氧基烷基,C2-C8烷硫基烷基,C1-C2烷基亚磺酰基-C1-C2烷基,C1-C2烷基磺酰基-C1-C2烷基,C2-C8亚烷基氨基氧基-C1-C2烷基,C1-C5烷基羰基-C1-C2烷基,C1-C5烷氧基-羰基-C1-C2烷基,C1-C5氨基-羰基-C1-C2烷基,C2-C8二烷基氨基-羰基-C1-C2烷基,C1-C5烷基羰基氨基-C1-C2烷基,C1-C2烷基羰基-N-C1-C3烷基-C1-C2氨基烷基,C3-C8三烷基甲硅烷基-C1-C3烷基,苯基-C1-C2烷基,杂芳基-C1-C2烷基,苯氧基-C1-C2烷基,杂芳氧基-C1-C2烷基,C2-C5链烯基,C2-C5卤代链烯基,C3-C8环烷基,苯基,杂芳基,C1-C6烷氧基,C1-C8卤代烷氧基,C1-C3烷基氨基,C2-C6二烷基氨基,或苄氧基或苯氧基,其中的苄基和苯基又可被下述基团取代:C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基;和
R27是C1-C8烷基,C1-C8卤代烷基,C1-C8氰基烷基,C1-C8硝基烷基,C1-C8氨基烷基,C1-C5-烷基氨基-C1-C2烷基,C2-C6二烷基氨基-C1-C2烷基,C3-C7环烷基-C1-C2烷基,C2-C8-烷氧基烷基,C4-C8链烯氧基烷基,C4-C8链炔氧基烷基,C2-C8烷硫基烷基,C1-C2烷基亚磺酰基-C1-C2烷基,C1-C2烷基磺酰基-C1-C2烷基、C2-C8亚烷基氨基氧基-C1-C2烷基,C1-C5烷基羰基-C1-C2烷基,C1-C5烷氧羰基-C1-C2烷基,C1-C5氨基-羰基-C1-C2-烷基,C2-C8二烷基氨基羰基-C1-C2烷基,C1-C5烷基羰基氨基-C1-C2烷基,C1-C2-烷基羰基-N-C1-C3烷基-C1-C2氨基烷基,C3-C6三烷基甲硅烷基-C1-C3烷基,苯基-C1-C2烷基,杂芳基-C1-C2烷基,苯氧基-C1-C2烷基,杂芳氧基-C1-C2烷基,C2-C5链烯基,C2-C5-卤代链烯基,C3-C8环烷基,苯基,杂芳基,C1-C6烷氧基,C1-C6卤代烷氧基,C1-C8-烷基羰基,C1-C3烷基氨基,C2-C6二烷基氨基,或苄氧基或苯氧基,其中的苄基和苯基又可被下述基团取代:C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基。
特别优选的式I化合物是下述的化合物,其中,
R1和R3各自相互独立地是乙基,卤代乙基,乙炔基,C1-C2烷氧基,C1-C2-卤代烷氧基或C1-C2烷基羰基;
R4和R5各自相互独立地是C1-C6烷基,C1-C8卤代烷基,C2-C6烷氧基烷基,C2-C6烷基羰基烷基,C3-C6烷氧羰基烷基,C1-C8氨基烷基,C2-C6二烷基氨基烷基,C3-C6烷基氨基烷基,C1-C6氰基烷基,C3-C8环烷基,芳基或杂芳基;或
R4和R5与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R2、R6和R32各自相互独立地是C1-C6烷基,C1-C6卤代烷基,C2-C6烷氧基烷基,C3-C8环烷基,芳基或杂芳基;
R7、R31和R33各自相互独立地是氢,C1-C6烷基或C1-C6烷氧基烷基;
R8是氢,C1-C6烷基,C1-C6卤代烷基,C2-C6烷氧基烷基,C1-C6烷硫基烷基,C3-C8环烷基,芳基或杂芳基;或
R6和R7或R2和R31或R32和R33与它们所键合的原子一起形成饱和的3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;或
R6和R8与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R9、R10、R11和R12各自相互独立地是C1-C6烷基,C1-C8卤代烷基,C2-C6-烷氧基烷基,C3-C8环烷基,芳基或杂芳基;或
R9和R11与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R9和R10与它们所键合的原子一起形成饱和的3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R13、R14、R34和R35各自相互独立地是C1-C6烷基,C3-C8环烷基,芳基或杂芳基;或
R13和R14或R34和R35与它们所键合的原子一起形成5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R15是C1-C8烷基,C1-C6卤代烷基,C2-C8烷氧基烷基,C4-C6链烯氧基烷基,C2-C6烷硫基烷基,C2-C6烷基亚磺酰基烷基,C3-C8烷氧羰基烷基,C3-C8环烷基,芳基或杂芳基;
R16是C1-C6烷基,C1-C6卤代烷基,C2-C6烷氧基烷基,C3-C8环烷基,芳基或杂芳基;
R17是C1-C6烷基,C1-C6卤代烷基,C3-C8环烷基,芳基或杂芳基;
R18是氢,C1-C6烷基或C2-C6烷氧基烷基;或
R17和R18与它们所键合的原子一起形成3-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R19和R36各自相互独立地是C1-C6烷基或C1-C6卤代烷基;或
R18和R19或R18和R36与它们所键合的原子一起形成饱和的5-至7-元环基团,该环可含有一个或二个选自氮、氧和硫的杂原子;
R20、R21、R22和R23是各自相互独立地是氢,C1-C8烷基,C1-C8卤代烷基,C3-C7环烷基-C1-C2烷基,C2-C8烷氧基烷基,苯基-C1-C2烷基,杂芳基-C1-C2烷基,苯氧基-C1-C2烷基,杂芳氧基-C1-C2烷基,C2-C5链烯基,C2-C5卤代链烯基,C3-C8环烷基,苯基或杂芳基;
R24、R25和R26是各自相互独立地是氢,C1-C8烷基,C1-C8卤代烷基,C3-C7环烷基-C1-C2烷基,C2-C8烷氧基烷基,苯基-C1-C2烷基,杂芳基-C1-C2烷基,苯氧基-C1-C2烷基,杂芳氧基-C1-C2烷基,C2-C5链烯基,C2-C5卤代链烯基,C3-C8环烷基,苯基,杂芳基,C1-C6烷氧基,C1-C3烷基氨基或C2-C6二烷基氨基;和
R27是C1-C8烷基,C1-C8卤代烷基,C3-C7环烷基-C1-C2烷基,C2-C8烷氧基烷基,苯基-C1-C2-烷基,杂芳基-C1-C2烷基,苯氧基-C1-C2烷基,杂芳氧基-C1-C2烷基,C2-C5链烯基,C2-C5卤代链烯基,C3-C8环烷基,苯基,杂芳基,C1-C6烷氧基,C1-C3烷基氨基,C2-C6-二烷基氨基或C1-C8烷基羰基。
式I化合物可用下述方法制备:
使下述式XXX化合物:
Q-H (XXX)
其中Q是Q1,Q2,Q3,Q4,Q5,Q6,Q7,Q8,Q9或Q10,其取代基除G1,G2,G3,G4,G5,G6,G7,G8,G9和G10以外,具有上述定义,并且G1,G2,G3,G4,G5,G6,G7,G8,G9和G10是氢,
与下述式XXXI化合物进行反应:
其中R1和R3如式I中所定义,Hal是氯、溴或碘,反应在惰性溶剂、碱和钯催化剂存在下,于30至250℃进行。该反应优选在惰性气体气氛中进行。
出人意料的是,该方法对于制备其中的R1和R3是乙基的式I化合物是特别有利的。用于制备式I化合物、其中的R1和R3是乙基并且Hal是氯、溴或碘的式XXXI中间体(式XXXIa)是新的,并且是为了该方法而特别开发的。因此,本发明还涉及这些中间体。
式XXX化合物是已知的,或可按照已知的方法制备,例如可按照J.Chem.Soc.Perkin Trans.1(1987),(4),877-884中所述的方法制备。式XXXI化合物可按照已知的方法制备,例如经由重氮盐制备,例如通过Sandmeyer反应以式XXXII相应的苯胺为原料制备:
其中R1和R3如式I中所定义。这些反应例如描述在《Vogel实用有机化学教材》
Vogel’s Textbook of Practical Organic Chemistry,第5版,B.S.Furniss,A.J.Hannaford,P.W.G.Smith,A.R.Tatchell;LongmanScientific & Technical 1989,第923页。式XXXII化合物是已知的;其中有一些可以买到,或可按照与已知方法类似的方法制备。
适当的反应用碱例如是磷酸的三碱金属盐、碱金属和碱土金属的氢化物、碱金属和碱土金属的氨化物或碱金属醇盐,例如磷酸三钾、氢化钠、二异丙基氨化锂(LDA)、叔丁醇钠或叔丁醇钾。叔丁醇钠、叔丁醇钾和磷酸三钾是特别优选的。
适当的溶剂包括例如芳烃如二甲苯或甲苯,醚如四氢呋喃、二噁烷或乙二醇二甲醚,二甲亚砜,或叔酰胺如二甲基甲酰胺、N-甲基吡咯烷酮或二甲基乙酰胺,以及非环脲如N,N’-二甲基亚丙基脲。
考虑用于式XXX化合物与式XXXI化合物C-C偶合反应的钯催化剂通常是钯(II)或钯(O)的配合物,例如二卤化钯(II)、乙酸钯(II)、硫酸钯(II)、二(三苯膦)二氯化钯(II)、二(三环戊基膦)二氯化钯(II)、二(三环己基膦)二氯化钯(II)、二(二亚苄基丙酮)合钯(O)或四(三苯基膦)合钯(O)。钯催化剂也可由钯(II)或钯(O)化合物现场制备,该制备是通过与所需的配位体复合来完成,例如,使欲配位的钯(II)盐如二氯化钯(II)(PdCl2)或乙酸钯(II)(Pd(OAc)2)与所需的配位体如三苯基膦(PPh3)、三环戊基膦或三环己基膦与所选择的溶剂,以及式XXXI化合物、式XXX化合物和碱相结合。适当的配位体是二齿配位体如1,1’-二(二苯基膦)二茂铁或1,2-二(二苯基膦)乙烷。加热反应介质,C-C偶合反应所需的钯(II)复合物或钯(O)复合物就可在现场制得,然后开始C-C偶合反应。以式XXXI化合物为基础,所用钯催化剂的量为0.001至50mol%,优选0.1-15mol%。
按照所用溶剂,以及如果合适的话,还有压力选择反应温度。优选在大气压下进行。
其中的Q是Q1的式I化合物可按照与WO 96/21652所述类似的方法制备。其中的Q是Q2的式I化合物可按照例如EP-A-0 415 185,EP-A-0 521334,EP-A-0 355 599和EP-A-0 442 077所述方法制备。其中的Q是Q3,Q4,Q6或Q7的式I化合物例如可按照WO 96/35644和WO 97/02243所述的方法制备。其中的Q是Q5的式I化合物例如可按照与WO 97/14667所述类似的方法制备。制备其中Q是Q7的式I化合物的类似方法如WO 97/16436所述。其中Q是Q8的式I化合物可按照与US-A-5 994 274所述类似的方法制备。其中的Q是Q9的式I化合物可按照与JP 11152273 A(优先权:1997.11.19,JP318614)所述类似的方法制备,其中Q是Q10的式I化合物可按照J.Org.Chem.(1979),44(26),4906-4912或J.Org.Chem.(1977),42(7),1163-1169所述的方法或与其类似的方法制备。
制备式I化合物的反应在非质子惰性有机溶剂中进行是有利的。这些溶剂是烃类如苯、甲苯、二甲苯或环己烷,氯代烃如二氯甲烷、三氯甲烷、四氯甲烷或氯苯,醚类如乙醚、乙二醇二甲醚、二乙二醇二甲醚、四氢呋喃或二噁烷,腈类如乙腈或丙腈,和酰胺如N,N-二甲基甲酰胺、二乙基甲酰胺或N-甲基吡咯烷酮。优选反应温度为-20℃至+120℃。反应通常略有放热,并通常在室温下进行。为了缩短反应时间,或为了开始反应,需要时可在短时间内将温度提高到反应混合物的沸点。通过加入几滴碱作为反应催化剂,可缩短反应时间。适当的碱尤其包括叔胺,如三甲胺、三乙胺、奎宁环、1,4-二氮杂双环[2.2.2]辛烷、1,5-二氮杂双环[4.3.0]壬-5-烯和1,5-二氮杂双环[5.4.0]十一碳-7-烯,但是也可以使用无机碱,例如氢化物如氢化钠或氢化钙,氢氧化物如氢氧化钠或氢氧化钾,碳酸盐如碳酸钾或碳酸钠,或碳酸氢盐如碳酸氢钾或钠。
式I化合物可采用浓缩和/或蒸发溶剂的常规手段分离,将固体残余物在其不易溶的溶剂中进行重结晶或研磨以进行纯化,这些溶剂例如乙醚、芳烃或氯代烃。
对本发明式I化合物或含有该化合物的组合物的应用而言,可以任何适当的方式应用于农业,例如芽前应用、芽后应用和拌种,并可使用各种方法和技术,如活性成分的控制释放。在此方法中,将活性成分以溶液的形式应用于无机颗粒载体或聚合的颗粒物(脲/甲醛),并干燥。适当的,也可以使用包衣(包覆的颗粒剂),以使活性成分在特定的时间内定量释放。
式I化合物可以未改性的方式,即以合成中得到的化合物,直接用作除草剂。但优选用常规方法,使用剂型技术中常规的辅剂加工成例如乳油、直可喷雾的或可稀释的溶液、稀乳剂、可湿性粉剂、可溶性粉剂、粉剂、颗粒剂或微胶囊。这些制剂例如如WO 97/34485的第9-13页所述。根据所预期的目的和主要的环境选择组合物的性质、施用的方法如喷雾、弥雾、喷粉、湿润、撒施或浇淋。
剂型加工物(formulations),即组合物、制剂或混合物含有式I的活性成分,或含有至少一种式I化合物的活性成分,并通常含有一种或多种固体或液体的剂型助剂,可用本领域已知的方法在制备,例如使活性成分与剂型助剂一起充分混合和/或研磨,这些辅剂例如是溶剂或固体载体。此外,也可将表面活性化合物(表面活性剂)用于该剂型的制备。溶剂和固体载体的实例例如如WO 97/34485,第6页所述。
根据式I活性成分的性质,适当的表面活性化合物是具有良好的乳化、分散和湿润性能的非离子、阳离子和/或阴离子表面活性剂。适当的阴离子、非离子和阳离子表面活性剂例如如WO 97/34485的第7和8页所述。另外,适合于用于制备本发明除草组合物的表面活性剂是剂型技术中常用的表面活性剂,如在″《Mc Cuteheon洗涤剂和乳化剂年报》
Mc Cutcheon’s Detergents and Emulsifiers Annual″,MCPublishing Corp.,Ridgewood New Jersey,1981;Stache,H.,″Tensid-Taschenbuch″,Carl Hanser Verlag,MunichVienna,1981以及M.和J.Ash,″《表面活性剂大全》
Encyclopedia of Surfactants″,第I-III卷,Chemical Publishing Co.,New York,1980-81中所述。
本发明含有有效量的式I化合物的除草和抑制植物生长的组合物的活性可通过加入喷雾罐混助剂而提高。这些助剂例如可以是非离子表面活性剂,非离子表面活性剂的混合物,阴离子表面活性剂与非离子表面活性剂的混合物,阳离子表面活性剂,有机硅表面活性剂,有或没有表面活性剂的矿物油衍生物,有或没有加入表面活性剂的植物油衍生物,植物油或矿物油的烷基化衍生物,有或没有表面活性剂的鱼油或其它动物油性质的动物油及其烷基化衍生物,优选具有8-28个碳原子的天然存在的高级脂肪酸及其烷基酯衍生物,含有芳环体系和一个或多个羧酸酯的有机酸,及其烷基衍生物,以及乙酸乙烯酯聚合物或乙酸乙烯酯/丙烯酸酯的共聚物的悬浮液,单个助剂相互之间的混合物,以及与有机溶剂的结合可导致活性进一步提高。
适当的非离子表面活性剂例如包括脂族或环脂族醇,饱和或不饱和脂肪酸以及烷基酚的聚二元醇醚的衍生物,优选可含有3-30二元醇醚基团和在(脂族)烃基团中含有8-20个碳原子,以及在烷基酚的烷基基团中含有6-18个碳原子。
进一步的,适当的非离子表面活性剂是水溶性的聚丙二醇、亚乙基二氨基聚丙二醇和烷基聚丙二醇的聚氧化乙烯的加成物,其中优选在烷基链中具有1-10个碳原子,该加成物优选含有20-250个乙二醇醚基团,和10-100丙二醇醚基团。所述的化合物通常是每个丙二醇单元含有1-5个乙二醇单元。
作为非离子表面活性剂进一步的实例,还可以提及的是壬基苯酚聚乙氧基乙醇、蓖麻油聚二元醇醚、聚环氧丙烷/聚环氧乙烷加成物、三丁基苯氧基聚乙氧基乙醇、聚乙二醇和辛基苯氧基聚乙氧基乙醇。
聚氧乙烯脱水山梨糖醇的脂肪酸酯如聚氧乙烯脱水山梨糖醇三油酸酯也是适用的。
优选的非离子表面活性剂特别是烷基硫酸盐、烷基磺酸盐、烷基芳基磺酸盐、烷基化的磷酸,和其乙氧基化的衍生物。其中的烷基基团通常含有8-24个碳原子。
优选的非离子表面活性剂已知有下述商品名称:
聚氧乙烯椰油烷基胺(如AMIET105(Kao Co.)),聚氧乙烯油基胺(如AMIET415(Kao Co.)),壬基苯酚聚乙氧基乙醇,聚氧乙烯硬脂基胺(AMIET320(Kao Co.)),N-聚乙氧基乙胺(GENAMIN(HoechstAG)),N,N,N’,N’-四(聚乙氧基聚丙氧基乙基)亚乙基二胺(如TERRONIL和TETRONIC(BASF Wyandotte Corp.)),BRIJ(AtlasChemicals),ETHYLANCD和ETHYLAND(Diamond Shamrock),GENAPOLC,GENAPOLO,GENAPOLS和GENAPOLX080(Hoechst AG),EMULGEN104P,EMULGEN 109P和EMULGEN 408(Kao Co.);DlSTY 125(Geronazzo),SOPROPHOR CY 18(Rhone Poulenc S.A.);NONISOL(Ciba-Geigy),MRYJ(ICI);TWEEN(ICI);EMULSOGEN(HoechstAG);AMIDOX(Stephan Chemical Co.),ETHOMID(Armak Co.);PLURONlC(BASF Wyandotte Corp.),SOPROPHOR 461 P
PoulencS.A.),SOPROPHOR 496/P(Rhone Poulenc S.A.),ANTAROX FM-63(Rhone Poulenc S.A.),SLYGARD 309(Dow Corning),SILWET 408,SILWET L-7607N(Osi-Specialities)。
阳离子表面活性剂特别是作为N-取代基至少有一个烷基基团具有8-22个碳原子的季铵盐,作为其它的取代基,可以是卤代低级烷基、苄基或羟基-低级烷基基团。盐优选是卤化物、甲基硫酸盐或乙基硫酸盐的形式,例如硬脂基三甲基铵氯化物或苄基二(2-氯乙基)乙基铵溴化物。
所用的油可以是矿物油或天然来源的油。天然的油又可以是动物或植物来源的。动物油中优选是牛油衍生物,但也可以使用鱼油(如沙丁鱼油)和其衍生物。植物油大部分是各种来源的种子的油。所用植物油的实例特别可提及的是椰子油、菜籽油和葵花籽油和其衍生物。
在本发明的组合物中,以喷雾混合物为基础,油添加剂的浓度通常是0.01-2%。例如在制得喷雾混合物之后,以所需的浓度向喷雾罐中加入油添加剂。
本发明组合物中,优选的油添加剂包括植物来源的油,例如菜籽油或葵花籽油,植物来源的油的烷基酯如甲基衍生物,或矿物油。
特别优选的油添加剂包括高级脂肪酸(C8-C22)的烷基酯,特别是C12-C18脂肪酸的甲基衍生物,例如月桂酸、棕榈酸和油酸的甲基酯。这些酯已知是月桂酸甲酯(CAS-111-82-0)、棕榈酸甲酯(CAS-112-39-0)和油酸甲酯(CAS-112-62-9)。
通过将油添加剂与表面活性物质结合使用可改进它们的施用和作用,所述的表面活性物质例如是非离子表面活性剂、阴离子或阳离子表面活性剂。适当的阴离子、非离子或阳离子表面活性剂的例子列于WO 97/34485的第7和8页。
优选的表面活性物质是十二烷基苄基磺酸盐类的阴离子表面活性剂,特别是其钙盐,和脂肪醇乙氧基化物类的非离子表面活性剂。特别优选的是乙氧基化的C12-C22脂肪醇,其乙氧基化度为5-40。可以买到的优选表面活性剂的例子是Genapol类(Clariant AG,Muttenz,Switzerland)。
相对于添加剂总量而言,表面活性物质的浓度通常是1-30重量%。
与表面活性剂一起组成油或矿物油或其衍生物的混合物的油添加剂的实例包括Edenor ME SU,Emery 2231(Henkel subsidiaryCognis GMBH,DE),Turbocharge(Zeneca Agro,Stoney Creek,Ontario,CA),或更特别的是Actipron(BP Oil UK Limited,GB)。
在油添加剂/表面活性剂混合物中加入有机溶剂有可能进一步提高其活性。适当的溶剂例如包括Solvesso(ESSO)和AromaticSolvent(Exxon Corporation)类的溶剂。
这些溶剂的浓度相对于总重量而言,可以是10-80重量%。
在US-A-4834908中所述的这类油添加剂,对本发明组合物来是特别优选的。更优选的油添加剂是已知其商品名称为MERGE的商品,可由BASF公司得到,在US-A-4834 908,第5栏,实施例COC-1中已有基本的描述。本发明中优选的其它油添加剂是SCORE(Novartis CropProtection Canada)。
在剂型和助剂技术中通常所使用的表面活性剂、油,特别是植物油、其衍生物如烷基化的脂肪酸和其混合物,例如优选与阴离子表面活性剂如烷基化的磷酸、烷基硫酸盐和烷基芳基磺酸盐以及还有高级脂肪酸的混合物,在本发明组合物中以及在喷雾剂罐溶液中也可以使用,这些尤其在下述文献中已有描述:″《Mc Cutcheon洗涤剂和乳化剂年报》
Mc Cutcheon’s Detergents and Emulsifiers Annual″MCPublishing Corp.,Ridgewood New Jersey,1998;Stache,H.,″Tensid-Taschenbuch″,Carl Hanser Verlag,MunichNienna,1990;M.和J.Ash,″《表面活性剂大全》
Encyclopedia of Surfactants″,第1-IV卷,Chemical Publishing Co.,New York,1981-89;G.Kapusta,″《除草剂助剂简编》
A Compendium of Herbicide Adjuvants″,SouthernIllinois Univ.,1998;L.Thomson Harvey,″《美国农业喷雾助剂指南》
A Guide to Agricultural Spray Adjuvants Used in the United States″,Thomson Pubns.,1992。
除草制剂通常含有0.1-99重量%,特别是0.1-95重量%的除草剂,1-99.9%,特别是5-99.8重量%的固体或液体制剂助剂,以及0-25重量%,特别是0.1-25重量%的表面活性剂。作为商品优选加工成为浓缩剂,终端用户通常可使用稀释制剂。组合物还可包括其它组份如稳定剂如植物油或环氧化的植物油(环氧化的椰子油、菜籽油或大豆油),消泡剂如硅油、防腐剂、粘度调节剂、粘合剂、增粘剂和肥料或其它活性成分。
在植物或其场所,所用的式I活性成分的施用量通常是0.001-4kg/ha,特别是0.005-2kg/ha。要达到所需效果所需要的浓度可通过试验确定。它取决于作用的类型、作物植物和杂草的生长阶段和施用情况(位置、时间、方法),并且依据这些因素,可在很宽的范围内变化。
式I化合物具有卓越的除草和抑制植物生长的性能,使其适于应用于有用的作物,特别是用于禾谷类、棉花、大豆、甜菜、甘蔗、栽培作物、油菜、玉米和稻,以及非选择性地防治杂草。应当理解的是,作物包括用常规培育方法或遗传工程方法培育的,对除草剂或各类除草剂有耐受性的那些作物,例如包括IMI Maize,Poast ProtectedMaize(耐受稀禾定),Liberty Link Maize,B.t./Liberty Link Maize,IMI/Liberty Link Maize,IMI/Liberty Link/B.t.Maize,RoundupReady Maize and Roundup Ready/B.t.Maize。
欲防治的杂草可以是单子叶或双子叶杂草,例如繁缕属(Stellaria),豆瓣菜属(Nasturtium),剪股颍属(Agrostis),马唐属(Digitaria),燕麦属(Avena),狗尾草属(Setaria),白芥属(Sinapis),黑麦草属(Lolium),茄属(Solanum),稗属(Echinochloa),藨草属(Scirpus),雨久花属(Monochoria),慈菇属(Sagittaria),雀麦属(Bromus),看麦娘属(Alopecurus),石茅(Sorghum halepense),筒轴茅属(Rottboellia),莎草属(Cyperus),苘麻属(Abutilon),黄花稔属(Sida),苍耳属(Xanthium),苋属(Amaranthus),藜属(Chenopodium),番薯属(lpomoea),茼蒿属(Chrysanthemum),猪殃殃属(Galium),堇菜属(Viola)和婆婆纳属(Veronica)。
令人惊讶的是,在US-A-5041157,US-A-5541148,US-A-5006656,EP-A-0 094 349,EP-A-0 551 650,EP-A-0 268 554,EP-A-0 375 061,EP-A-0 174 562,EP-A-492 366,WO 91/7874,WO 94/987,DE-A-19612943,WO 96/29870,WO 98/13361,WO 98/39297,WO 98/27049,EP-A-0 716 073,EP-A-0 613 618,US-A-5 597 776,EP-A-0 430 004,DE-A-4 331 448,WO 99/16744,WO 00/30447和WO 00/00020中给出的特定的安全剂也适用于与本发明的除草组合物进行混合。因此,本发明还涉及用于防治禾本科杂草和有用作物中的杂草,特别是在玉米和谷类作物中的杂草的选择性除草组合物,这些组合物含有式I的除草剂和安全剂(解毒剂),保护有用的植物,而不是杂草免受除草剂的植物毒性作用,本发明还涉及这些组合物在有用作物中防治杂草的应用。
因此,本发明提供了选择性的除草组合物其除了常规的惰性剂型助剂如载体、溶剂和湿润剂之外,作为活性成分,含有下述(a)和(b)的混合物:
a)除草有效量的式I化合物:
其中R1,R3和Q如上文定义,条件是Q不能是Q1;和
b)除草拮抗有效量的下式X化合物:
其中
R37是氢,C1-C8烷基,或C1-C6烷氧基或C3-C6链烯氧基取代的C1-C8烷基;和X7是氢或氯;
或下式XI化合物:
其中E是氮或次甲基;
R38是-CCl3,苯基或卤素取代的苯基;
R39和R40各自相互独立地是氢或卤素;和
R41是C1-C4烷基;
或下式XII化合物:
其中R44和R45各自相互独立地是氢或卤素,R46、R47和R48各自相互独立地是C1-C4烷基;
或下式化合物XIII:
其中A2是下述基团:
R51和R52各自相互独立地是氢、C1-C8烷基、C3-C8环烷基、C3-C.链烯基、C3-C6链炔基,
或R51和R52一起形成C4-C6亚烷基桥,该桥可被氧、硫、SO、SO2、NH或-N(C1-C4烷基)-所间断;
R53是氢或C1-C4烷基;
R49是氢,卤素,氰基,三氟甲基,硝基,C1-C4烷基,C1-C4烷氧基,C1-C4烷硫基,C1-C4烷基亚磺酰基,C1-C4烷基磺酰基,-COORj,-CONRkRm,-CORn,-SO2NRkRm或-OSO2-C1-C4烷基;
Rg是氢,卤素,氰基,硝基,C1-C4烷基,C1-C4卤代烷基,C1-C4烷硫基,C1-C4烷基亚磺酰基,C1-C4烷基磺酰基,-COORj,-CONRkRm,-CORn,-SO2NRkRm或-OSO2-C1-C4烷基,C1-C6烷氧基,或被C1-C4烷氧基或卤素取代的C1-C6烷氧基,C3-C6链烯氧基,或被卤素取代的C3-C6链烯氧基,或C3-C6链炔氧基,或R49和R50一起形成可被C1-C4烷基或卤素取代的C3-C4亚烷基桥,或一起形成可被C1-C4烷基或卤素取代的C3-C4亚链烯基桥,或一起形成可被C1-C4烷基或卤素取代的C4亚二烯基桥;
R50和Rh各自相互独立地是氢,卤素,C1-C4烷基,三氟甲基,C1-C6烷氧基,C1-C6烷硫基或-COORj;
Rc是氢,卤素,硝基,C1-C4烷基或甲氧基;Rd是氢,卤素,硝基,C1-C4烷基,C1-C4烷氧基,C1-C4烷硫基,C1-C4烷基亚磺酰基,C1-C4烷基磺酰基,-COORj,或-CONRkRm;
Re是氢,卤素,C1-C4烷基,-COORj,三氟甲基或甲氧基,或Rd和Re一起形成C3-C4亚烷基桥;
Rp是氢,卤素,C1-C4烷基,-COORj,三氟甲基或甲氧基;Rq是氢,卤素,硝基,C1-C4烷基,C1-C4烷氧基,C1-C4烷硫基,C1-C4烷基亚磺酰基,C1-C4烷基磺酰基,-COORj,或-CONRkRm;或Rp和Rq一起形成C3-C4亚烷基桥;
Rr是氢,卤素,C1-C4烷基,-COORj,三氟甲基或甲氧基;Rs是氢,卤素,硝基,C1-C4烷基,C1-C4烷氧基,C1-C4烷硫基,C1-C4烷基亚磺酰基,C1-C4烷基磺酰基,-COORj,或-CONRkRm;或Rr和Rs一起形成C3-C4亚烷基桥;
Rt是氢,卤素,C1-C4烷基,-COORj,三氟甲基或甲氧基;Ru是氢,卤素,硝基,C1-C4烷基,C1-C4烷氧基,C1-C4烷硫基,C1-C4烷基亚磺酰基,C1-C4烷基磺酰基,-COORj,或-CONRkRm;或Rv和Ru一起形成C3-C4亚烷基桥;
Rf和Rv是氢,卤素或C1-C4烷基;
Rx和Ry各自相互独立地是氢,卤素,C1-C4烷基,C1-C4烷氧基,C1-C4烷硫基,-COOR54,三氟甲基,硝基或氰基;
Rj、Rk和Rm各自相互独立地是氢或C1-C4烷基;或
Rk和Rm一起形成C4-C6亚烷基桥,该桥可被氧、NH或-N(C1-C4烷基)-间断;
Rn是C1-C4烷基,苯基,或被卤素、C1-C4烷基、甲氧基、硝基或三氟甲基取代的苯基;
R54是氢,C1-C10烷基,C1-C4烷氧基-C1-C4烷基,C1-C4烷硫基-C1-C4烷基,二-C1-C4-烷基氨基-C1-C4烷基,卤代-C1-C8烷基,C2-C8链烯基,卤代-C2-C8链烯基,C3-C8链炔基,C3-C7环烷基,卤代-C3-C7环烷基,C1-C8烷基羰基,烯丙基羰基,C3-C7环烷基羰基,苯甲酰基,所述的苯甲酰基是未取代的或是在其苯环上被相同或不同的至多三个选自卤素、C1-C4烷基、卤代-C1-C4烷基、卤代-C1-C4烷氧基或C1-C4烷氧基的取代基所取代;或呋喃甲酰基,噻吩基;或被苯基,卤代苯基,C1-C4烷基-苯基,C1-C4烷氧基苯基,卤代-C1-C4烷基苯基,卤代-C1-C4烷氧基苯基,C1-C6烷氧基-羰基,C1-C4烷氧基-C1-C8烷氧羰基,C3-C8链烯氧基羰基,C3-C8链炔氧基-羰基,C1-C8烷硫基羰基,C3-C8链烯硫基羰基,C3-C8链炔硫基羰基,氨基甲酰基,一-C1-C4烷基氨基羰基,二-C1-C4烷基氨基羰基取代的C1-C4烷基;或苯基氨基-羰基,该基团是未取代的或是在其苯环上被相同或不同的至多三个选自卤素、C1-C4烷基、卤代-C1-C4烷基、卤代-C1-C4烷氧基或C1-C4烷氧基的取代基所取代,或被氰基或硝基取代一次;或二氧戊环-2-基,该基团是未取代的或是被C1-C4烷基取代一或二次,或二氧六环-2-基,该基团是未取代的或是被C1-C4烷基取代一或二次,或被氰基、硝基、羧基或C1-C8烷硫基-C1-C8烷氧基-羰基取代的C1-C4烷基;
或下式XIV化合物:
R60和R61各自相互独立地是C1-C4烷基,或R60和R61一起是-(CH2)5-;
R63,R64,R65,R66,R67,R68,R69,R70,R71,R72,R73,R74,R75,R76,R77和R78各自相互独立地是氢或C1-C4烷基;
或下式XV化合物:
其中R80是氢或氯,和R79是氰基或三氟甲基;
或下式XVI化合物:
其中R81是氢或甲基;
或下式XVII化合物:
其中
R82是氢,C1-C4烷基,或被C1-C4烷基-X2-或C1-C4卤代烷基-X2-取代的C1-C4烷基,或是C1-C4卤代烷基,硝基,氰基,-COOR85,-NR86R87,-SO2NR88R89或-CONR90R91;
R83是氢,卤素,C1-C4烷基,三氟甲基,C1-C4烷氧基或C1-C4卤代烷氧基;
b)两个相邻的环成员U和V、V和W1以及W1和Z4不能同时是氧;
R95和R96各自相互独立地是氢或C1-C8烷基;或
R95和R96一起形成C2-C6亚烷基基团;
A1是R99-Y1-或-NR97R98;
X2是氧或-S(O)s;
Y1是氧或硫;
R99是氢,C1-C8烷基,C1-C8卤代烷基,C1-C4烷氧基-C1-C8烷基,C3-C6链烯氧基-C1-C8烷基,或其中的苯环可被卤素、C1-C4-烷基、三氟甲基,甲氧基或甲基-S(O)s-取代的苯基-C1-C8烷基,或是C3-C6链烯基,C3-C6卤代链烯基,苯基-C3-C6链烯基,C3-C6链炔基,苯基-C3-C6链炔基,氧杂环丁烷基,呋喃基或四氢呋喃基;
R85是氢或C1-C4烷基;
R86是氢,C1-C4烷基或C1-C4烷基羰基;
R87是氢或C1-C4烷基;或
R86和R87一起形成C4-或C5-亚烷基基团;
R88、R89、R90和R91各自相互独立地是氢或C1-C4烷基;或R88与R89一起,或R90与R91一起各自相互独立地是C4-或C5-亚烷基,其中的一个碳原子可被氧或硫代替,或者一个或二个碳原子可被-NR100-代替;
R92、R100和R93各自相互独立地是氢或C1-C8烷基;或
R92和R93一起是C2-C6亚烷基;
R94是氢或C1-C8烷基;
R97是氢,C1-C8烷基,苯基或苯基-C1-C8烷基,其中的苯环可被氟、氯、溴、硝基、氰基、-OCH3、C1-C4烷基或CH3SO2-所取代,或是C1-C4烷氧基-C1-C8烷基,C3-C8链烯基或C3-C6链炔基;
R98是氢,C1-C8烷基,C3-C6链烯基或C3-C8链炔基;或
R97和R98一起是C4-或C5-亚烷基,其中一个碳原子可被氧或硫代替,或者一个或二个碳原子可被-NR101-代替;
R101是氢或C1-C4烷基;
r是0或1;和
s是0、1或2,
或下式化合物XVIII:
其中R103是氢,C1-C6烷基,C3-C6环烷基,C3-C6链烯基或C3-C6链炔基;以及R104、R105和R106各自相互独立地是氢,C1-C6烷基,C3-C8环烷基或C1-C0烷氧基,条件是取代基R104、R105和R106之一不是氢;或下式化合物XIX:
其中Z5是N或CH,当Z5是N时,n是0,1,2或3,当Z5是CH时,n是0,1,2,3或4,
R107是卤素,C1-C4烷基,C1-C4卤代烷基,C1-C4烷氧基,C1-C4卤代烷氧基,硝基,C1-C4烷硫基,C1-C4烷基磺酰基,C1-C4烷氧羰基,苯基或苯氧基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的苯基或苯氧基;
R108是氢或C1-C4烷基,R109是氢,C1-C4烷基,C3-C6环烷基,C2-C6链烯基,C2-C6链炔基,C1-C4卤代烷基,C2-C6卤代链烯基,C2-C6卤代链炔基,C1-C4烷硫基-C1-C4烷基,C1-C4烷基磺酰基-C1-C4烷基,C1-C4烷氧基-C1-C4烷基,C1-C4链烯氧基-C1-C4烷基或C1-C4-链炔氧基-C1-C4烷基;或下式XX化合物:
其中Z6是氧或N-R110,和R110是下式基团:
其中R111和R112各自相互独立地是氰基,氢,C1-C4烷基,C3-C6环烷基,C2-C6链烯基,芳基,苯基或杂芳基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的苯基、芳基或杂芳基,
或下式XXI化合物:
其中Z7是氧,硫,S=O,SO2或CH2,R113和R114各自相互独立地是氢,卤素或C1-C4烷基,W2和W3各自相互独立地是CH2COOR115或COOR0115或-起是基团-(CH2)C(O)-O-C(O)-(CH2)-,和
R115与R0115各自相互独立地是氢,C1-C4烷基,C2-C4链烯基,C2-C6-链炔基,C3-C6环烷基,C1-C4卤代烷基,或金属阳离子或铵阳离子;或下式XXII化合物:
其中R119和R120各自相互独立地是氢,卤素或C1-C4卤代烷基,R121是氢,C1-C4烷基,C3-C4链烯基,C3-C4链炔基,C1-C4卤代烷基,C3-C6环烷基,金属阳离子或铵阳离子,Z8是N,CH,C-F或C-Cl以及W4是下式基团:
其中R122和R123各自相互独立地是氢或C1-C4烷基,和R124与R125各自相互独立地是氢或C1-C4烷基;
或下式XXIII化合物:
其中R126是氢,氰基,卤素,C1-C4烷基,C3-C6环烷基,C1-C4烷氧基,C1-C4-烷氧羰基,C1-C4烷硫基羰基,-NH-R128,-C(O)NH-R0128,芳基或杂芳基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的芳基或杂芳基;
R127是氢,氰基,硝基,卤素,C1-C4烷基,C1-C4卤代烷基,C1-C4烷氧基或C1-C4硫烷基;和
R128和R0128各自相互独立地是C1-C4烷基,C1-C4卤代烷基,C3-C4链烯基,C3-C4链炔基,C3-C4环烷基,芳基或杂芳基,或被C1-C3烷基、C1-C3卤代烷基、C1-C3烷氧基、C1-C3卤代烷氧基、卤素、氰基或硝基取代的芳基或杂芳基,甲酰基,C1-C4烷基羰基或C1-C4烷基磺酰基;或下式XXIV化合物:
其中R129和R130各自相互独立地是氢,C1-C4烷基,C1-C4卤代烷基,C1-C4烷氧基,一-C1-C8-或二-C1-C8-烷基氨基,C3-C6环烷基,C1-C4硫烷基,苯基或杂芳基,R131具有R129的定义,并且另外还可以是OH,NH2,卤素,二-C1-C4氨基烷基,C1-C4烷硫基,C1-C4烷基磺酰基或C1-C4烷氧羰基,R132具有R129的定义,并且另外还可以是氰基,硝基,羧基,C1-C4烷氧羰基,二-C1-C4-氨基烷基,C1-C4烷硫基,C1-C4烷基磺酰基,SO2-OH,i-C1-C4氨基烷基磺酰基或C1-C4-烷氧基磺酰基,R133具有R129的定义,并且另外还可以是OH,NH2,卤素,二-C1-C4-氨基烷基,吡咯烷-1-基,哌啶-1-基,吗啉-1-基,C1-C4烷硫基,C1-C4烷基磺酰基,C1-C4烷氧羰基,苯氧基,萘氧基,苯基氨基,苯甲酰氧基或苯基磺酰氧基;或下式XXV化合物:
其中R134是氢,C4烷基,C1-C4卤代烷基,C2-C4链烯基,C2-C4链炔基或C1-C4烷氧基-C1-C4烷基,R135是氢,卤素,C1-C4烷基,C1-C4卤代烷基或C1-C4烷氧基,和R136是氢,卤素,C1-C4烷基,C1-C4卤代烷基或C1-C4烷氧基,条件是R135和R136不能同时为氢,
或下式XXVI化合物:
其中
R143是氢,碱金属阳离子,碱土金属阳离子,锍阳离子或铵阳离子或乙基;
或下式XXVII化合物:
其中R144和R145各自相互独立地是氢,C1-C6烷基,C2-C6-链烯基,C2-C6链炔基或C3-C6环烷基;
R146是氢,卤素,C1-C4烷基,C1-C6卤代烷基或C1-C6卤代烷氧基;
R147是氢,卤素,C1-C4烷基,C1-C4卤代烷基,C1-C4烷氧基,C1-C4卤代烷氧基,C1-C4-烷硫基,C1-C4烷氧羰基或硝基;
n1是0,1,2或3;和
m是1或2;
或下式XXVIII化合物:
其中
R148是氢,C1-C6烷基,C1-C6烷氧基,C1-C6烷硫基,C3-C8环烷基,苯基,苯基C1-C6烷基或杂芳基;其中所述的基团可被卤素,氰基,硝基,氨基,羟基,羰基,羧基,甲酰基,甲酰胺或磺酰胺取代;
R149是氢,C1-C6烷基或C1-C4卤代烷基;
每个R150各自相互独立地是氢,卤素,C1-C4烷基,C1-C4卤代烷基,C1-C4烷氧基,C1-C4烷硫基,C1-C4烷基磺酰基,氰基,硝基,甲酰基或羧基;
R151是氢,C1-C6烷基或C1-C4卤代烷基;
每个R152各自相互独立地是氢,卤素,C1-C4烷基,C1-C4卤代烷基,C1-C4烷氧基,C1-C4烷硫基,C1-C4烷基磺酰基,氰基,硝基,甲酰基或羧基;
o是0,1,或2,和
p是0,1或2;
或下式XXIX化合物:
其中
R159是氢,甲酰基,C1-6烷基羰基,C1-6链烯基羰基,C1-6链炔基羰基,C1-6烷氧基羰基,C1-6烷硫基羰基,C3-8环烷基羰基,苯基-C1-6烷基羰基,苯基羰基,C1-6烷基磺酰基,C1-6链烯基磺酰基或苯基磺酰基,其中前述的烃基基团可被一个或多个卤素原子、氰基、硝基、氨基、甲氧基、乙氧基或苯基取代;
R153是氢,C1-6烷基,C1-6链烯基,C1-6链炔基,C3-8环烷基,甲酰基,C1-6烷基羰基,C1-6链烯基羰基,C1-6链炔基羰基,C1-6烷氧羰基,C1-6烷硫基羰基,C3-8环烷基羰基,C1-6烷基磺酰基,C1-6链烯基磺酰基或苯基磺酰基,其中前述的烃基基团可被一个或多个卤素原子、氰基、硝基、氨基、甲氧基、乙氧基或苯基取代;
R154是氢,C1-6烷基,C1-6链烯基,C1-6链炔基,C3-8环烷基,甲酰基,C1-6烷基羰基,C1-6链烯基羰基,C1-6链炔基羰基,C1-6烷氧基羰基,C1-6烷硫基羰基,C3-8环烷基羰基,C1-6烷基磺酰基,C1-6链烯基磺酰基或苯基磺酰基,其中前述的烃基基团可被一个或多个卤素原子、氰基、硝基、氨基、甲氧基、乙氧基或苯基取代;
R155、R156、R157和R158各自相互独立地是氢,卤素,氨基,C1-3烷基氨基,C1-6二烷基氨基,羟基,氰基,硝基,甲酰基,羧基,C1-6烷氧基,C1-6卤代烷氧基,C1-6烷基羰基,C1-6烷氧基羧基,C1-6烷基,C1-6卤代烷基,C1-6链烯基或C1-6链炔基;
或R153和R158一起与它们所键连的环原子一起形成5-或6-元的部分饱和或不饱和的环,该环最多可含有两个相同或不同的选自氧、硫和氮的杂原子,该环可被氧代基团取代。
优选本发明的组合物含有拮抗除草剂有效量的式X、XI、XII、XIII、XIV、XV、XVI、XVII、XVIII、XIX、XX、XXI、XXII、XXIII、XXIV或XXV的安全剂。
优选的,按照本发明的选择性除草组合物含有拮抗除草剂有效量的式X化合物:
其中R37是氢,C1-C8烷基,或被C1-C6烷氧基或C3-C6-链烯氧基取代的C1-C8烷基;和X6是氢或氯;
或下式XI化合物:
其中
E是氮或次甲基;R38是-CCl3、苯基或卤素取代的苯基;
R39和R40各自相互独立地是氢或卤素;和
R41是C1-C4烷基;
或下式XII化合物:
其中R44和R45各自相互独立地是氢或卤素,以及R46、R47和R48各自相互独立地是C1-C4烷基。
上述优选的式I化合物也适用于式I化合物与式X-XVIII的安全剂的混合物。本发明优选的组合物含有选自下述各式的安全剂:
式Xa:
式Xb:
和式XIa:
表9、10和11中还列出了其它优选的式X、XI和XII化合物。表9:式X化合物
| 化合物编号 | X6 | R37 |
| 9.01 | Cl | -CH(CH3)-C5H11-n |
| 9.02 | Cl | -CH(CH3)-CH2OCH2CH=CH2 |
| 9.03 | Cl | H |
| 9.04 | Cl | C4H9-n |
优选的式XI化合物列于下表10。
表10:式XI化合物:
| 化合物编号 | R41 | R38 | R39 | R40 | E |
| 10.01 | CH3 | 苯基 | 2-Cl | H | CH |
| 10.02 | CH3 | 苯基 | 2-Cl | 4-Cl | CH |
| 10.03 | CH3 | 苯基 | 2-F | H | CH |
| 10.04 | CH3 | 2-氯苯基 | 2-F | H | CH |
| 10.05 | C2H5 | CCl3 | 2-Cl | 4-Cl | N |
| 10.06 | CH3 | 苯基 | 2-Cl | 4-CF3 | N |
| 10.07 | CH3 | 苯基 | 2-Cl | 4-CF3 | N |
优选的式XII化合物列于下表11。
表11:式XII化合物:
| 化合物编号 | R46 | R47 | R48 | R44 | R45 |
| 11.01 | CH3 | CH3 | CH3 | 2-Cl | 4-Cl |
| 11.02 | CH3 | C2H5 | CH3 | 2-Cl | 4-Cl |
| 11.03 | CH3 | C2H5 | C2H5 | 2-Cl | 4-Cl |
优选的式XIII化合物列于下表12,作为式XIIIa化合物。
表12:式XIIIa化合物:
优选的式XIV化合物列于下表13。
表13:式XIV化合物:
| 化合物编号 | R56 | R57 | R56+R57 |
优选的式XV化合物列于下表14。
表14:式XV化合物:
| 化合物编号 | R80 | R79 |
| 14.01 | H | CN |
| 14.02 | Cl | CF3 |
优选的式XVI化合物列于下表15。
表15:式XVI化合物:
| 化合物编号 | R81 |
| 15.01 | H |
| 15.02 | CH3 |
优选的式XVII化合物列于下表16,作为式XVIIa化合物。
表16:式XVIIa化合物:
优选的式XVII化合物列于下表17,作为式XVIIb化合物。
表17:式XVIIb化合物:
优选的式XVII化合物列于下表18,作为式XVIIc化合物。
表18:式XVIIc化合物:
优选的式XVII化合物列于下表19,作为式XVIId化合物。
表19:式XVIId化合物:
优选的式XVIII化合物列于下表20。
表20:式XVIII化合物:
| 化合物编号 | R103 | R104 | R105 | R106 |
| 20.01 | CH3 | H | 环丙基 | H |
| 20.02 | CH3 | C2H5 | 环丙基 | H |
| 20.03 | CH3 | 环丙基 | C2H5 | H |
| 20.04 | CH3 | CH3 | H | H |
| 20.05 | CH3 | CH3 | 环丙基 | H |
| 20.06 | CH3 | OCH3 | OCH3 | H |
| 20.07 | CH3 | CH3 | OCH3 | H |
| 20.08 | CH3 | OCH3 | CH3 | H |
| 20.09 | CH3 | CH3 | CH3 | H |
| 20.10 | C2H5 | CH3 | CH3 | H |
| 20.11 | C2H5 | OCH3 | OCH3 | H |
| 20.12 | H | OCH3 | OCH3 | H |
| 20.13 | H | CH3 | CH3 | H |
| 20.14 | C2H5 | H | H | CH3 |
| 20.15 | H | H | H | CH3 |
| 20.16 | CH3 | H | H | CH3 |
| 20.17 | CH3 | CH3 | H | CH3 |
在式XXVIII化合物中,优选的是下述定义的化合物:
R148是氢,C1-6烷基,C3-8环烷基或苯基,其中所述基团可被卤素、氰基、硝基、氨基、羟基、羰基、羧基、甲酰基、甲酰胺或磺酰胺所取代;
R149是氢;
每个R150各自相互独立地是氢,卤素,C1-C4烷基,C1-C4卤代烷基,C1-C4烷氧基,C1-C4烷硫基,氰基,硝基或甲酰基;
R151是氢;和
每个R152各自相互独立地是氢,卤素,C1-C4烷基,C1-C4卤代烷基,C1-C4烷氧基,C1-C4烷硫基,氰基,硝基或甲酰基。
特别优选的式XXVIII化合物选自下述一组化合物:
2-甲氧基-N-[4-(2-甲氧基苯甲酰基氨磺酰基)苯基]-乙酰胺,
N-[4-(2-甲氧基苯甲酰基氨磺酰基)苯基]-环丙烷甲酰胺,
N-[4-(2-甲氧基苯甲酰基氨磺酰基)苯基]-环丁烷甲酰胺,
N-[4-(2-氯苯甲酰基氨磺酰基)苯基]-环丙烷甲酰胺,
N-[4-(2-氯苯甲酰基氨磺酰基)苯基]-乙酰胺,
N-[4-(2-三氟甲氧基苯甲酰基氨磺酰基)苯基]-乙酰胺,
N-[4-(2-三氟甲基苯甲酰基氨磺酰基)苯基]-环丙烷甲酰胺,
N-[4-(2-三氟甲氧基苯甲酰基氨磺酰基)苯基]-环丙烷甲酰胺,
N-[4-(2-三氟甲氧基苯甲酰基氨磺酰基)苯基]-环丁烷甲酰胺,和
N-[4-(2-三氟甲基苯甲酰基氨磺酰基)苯基]-乙酰胺。
在式XXIX化合物中,优选的是下述定义的化合物:
R159是氢,甲酰基,C1-6烷基羰基,C1-6链烯基羰基,C1-6链炔基羰基,C1-6烷氧基羰基,C1-6烷硫基羰基,C3-8环烷基羰基,苯基-C1-6烷基羰基或苯基羰基,其中上述烃基可被一或多个下述取代基取代:卤原子、氰基、硝基、氨基、甲氧基、乙氧基或苯基;
R153是氢,C1-6烷基,C1-6链烯基,C1-6链炔基,甲酰基,C1-6烷基羰基或C1-6烷氧基羰基,其中上述烃基可被一或多个下述取代基取代:卤原子、氰基、硝基、氨基、甲氧基、乙氧基或苯基;
R154是氢,C1-6烷基,C1-6链烯基,C1-6炔基,甲酰基,C1-6烷基羰基或C1-6烷氧基羰基,其中上述烃基可被一或多个下述取代基取代:卤原子、氰基、硝基、氨基、甲氧基、乙氧基或苯基;
R155、R156、R157和R158各自相互独立地是氢,卤素,氰基,硝基,甲酰基,羧基,C1-6烷氧基,C1-6卤代烷氧基,C1-6烷氧基羧基,C1-6烷基或C1-6卤代烷基;
或R153和R158,与它们所键连的环原子一起形成5-或6-元部分饱和或不饱和的环,该环最多可含有2个相同或不同的选自氧、硫和氮的杂原子,该环可能被氧代基团取代。
尤其优选这些式XXIX化合物,其中
R159是氢、甲酰基、C1-6烷基羰基、C1-6链烯基羰基、链炔基羰基、C1-6烷氧羰基、C1-6烷硫羰基、C3-8环烷基羰基或苯基羰基;
R153是氢、C1-6烷基、C1-6链烯基、C1-6链炔基、甲酰基、C1-6烷基羰基或C1-6烷氧羰基;
R154是氢、C1-6烷基、C1-6链烯基、C1-6链炔基、甲酰基、C1-6烷基羰基或C1-6烷氧羰基;
R155、R156、R157和R158各自独立地是氢、卤素、氰基、硝基、甲酰基、C1-6烷基、C1-6卤代烷基、C1-6烷氧基或C1-6卤代烷氧基;
或者R153和R158与它们所键连的环原子一起形成5或6元部分饱和或不饱和的环,该环可以含有至多2个相同或不同的选自氧、硫和氮的杂原子,该环可以被氧代基团取代。
特别优选的是选自下述一组的式XXIX化合物:
4-羟基-1-甲基-3-(1H-四唑-5-羰基)-1H-喹啉-2-酮,
1-乙基-4-羟基-3-(1H-四唑-5-羰基)-1H-喹啉-2-酮,
6-羟基-5-(1H-四唑-5-羰基)-1,2-二氢-吡咯并[3.2.1-.ij.]喹啉-4-酮,
3-(1-乙酰基-1H-四唑-5-羰基)-4-羟基-1-甲基-1H-喹啉-2-酮,
6-氯-4-羟基-1-甲基-3-(1H-四唑-5-羰基)-1H-喹啉-2-酮,
6-氟-4-羟基-1-甲基-3-(1H-四唑-5-羰基)-1H-喹啉-2-酮,
4-羟基-1,6-二甲基-3-(1H-四唑-5-羰基)-1H-喹啉-2-酮,
4-羟基-6-甲氧基-1-甲基-3-(1H-四唑-5-羰基)-1H-喹啉-2-酮,
4-羟基-6-甲氧基-1-甲基-3-(1H-四唑-5-羰基)-1H-喹啉-2-酮,
乙酸的1-甲基-2-氧代-3-(1H-四唑-5-羰基)-1,2-二氢-喹啉-4-基酯,
和
2,2-二甲基丙酸的1-甲基-2-氧代-3-(1H-四唑-5-羰基)-1,2-二氢喹啉-4-基酯。
本发明还涉及在有用植物的作物中选择性防治杂草的方法,该方法包括用除草有效量的式I除草剂和拮抗除草剂有效量的式X、XI、XII、XIII、XIV、XV、XVI、XVII、XVIII、XIX、XX、XXI、XXII、XXIII、XXIV、XXV、XXVI、XXVII、XXVIII或XXIX的安全剂或同时,或分别处理有用的植物、其种子或插条,或处理它们的种植区,优选的安全剂是式X、XI、XII、XIII、XIV、XV、XVI、XVII或XVIII化合物。
式X、XI、XII、XIII、XIV、XV、XVI、XVII或XVIII的安全剂可以保护其不受上述除草剂损伤的适当的种植植物尤其是禾谷类、棉花、大豆、甜菜、甘蔗、栽培作物、油菜、玉米或稻,更尤其是玉米或禾谷类。应当理解的是,作物包括用常规培育方法或遗传工程方法培育的而对除草剂或各类除草剂有耐受性的那些作物。
欲防治的杂草可以是单子叶或双子叶杂草,例如单子叶杂草燕麦属,剪股颍属,虉草属,黑麦草属,雀麦属,看麦娘属,狗尾草属,马唐属,臂形草属,稗属,黍属,石茅/高粱,筒轴茅属,莎草属,稗属,草属,雨久花属,慈菇属和繁缕属,以及双子叶杂草白芥属,藜属,猪殃殃属,堇菜属,婆婆纳属,母菊属(Matricaria),罂粟属(Papaver),茄属,苘麻属,黄花稔属,苍耳属,苋属,番薯属和茼蒿属。
种植区是指其上植物已经生长的区域,或其上已经种植了植物的种子的区域,以及,打算种植植物的区域。
根据目的不同,可以使用式X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX的安全剂对这些种植植物的种子进行预处理(对种子和插条进行拌种处理),或在种植前或后掺混入土壤中。但它们也可单独使用,或在植物发芽后与除草剂一起使用。因此,原则上讲,用安全剂处理植物或种子与除草剂的使用时间无关。对植物进行处理时,可将除草剂和安全剂同时使用(例如桶混的形式)。相对于除草剂而言,安全剂的施用量很大程度上取决于施用的方法。在田间进行处理的情况下,施用量会受到是施用安全剂和除草剂结合在一起的桶混物,还是分别使用安全剂和除草剂的影响,除草剂与安全剂的施用比例通常为100∶1-1∶10,优选20∶1-1∶1。在田间进行处理时,通常使用0.001-1.0kg安全剂/公顷,优选0.001-0.25kg安全剂/公顷。
除草剂的施用量通常是0.001-2kg/ha,但优选0.005-0.5kg/ha。
本发明的组合物适合于任何农业上常用的施用方法,例如芽前施用、苗后施用和拌种。
在用于拌种的情况下,通常使用0.001-10克安全剂/千克种子,优选0.05-2克安全剂/千克种子。当在种植前不久施用液体形式的安全剂时,可使种子溶胀,所使用的安全剂溶液的活性成分浓度为1-10000ppm,优选100-1000ppm是有利的。
为了进行施用,式X,Xl,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX的安全剂,或这些安全剂与式I除草剂的结合有利地是与剂型技术中的常规助剂一起加工制成各种剂型,例如乳油、包衣的糊剂、直接可喷雾的或可稀释的溶液剂、稀释乳液剂、可湿性粉剂、可溶性粉剂、粉剂、颗粒剂或微胶囊。
这些剂型例如在WO 97/34485,第9-13页有描述。这些剂型可以常规的方式制备,例如紧密混合/研磨活性成分与液体或固体剂型助剂,如溶剂或固体载体。另外,在制备制剂时还可使用表面活性化合物(表面活性剂)。适合于上述目的的溶剂和固体载体例如在WO 97/34485的第6页有描述。
根据需要进行剂型加工的式I活性成分的性质,作为表面活性化合物应考虑使用具有良好乳化性能、分散和湿润性能的非离子、阳离子和/或阴离子的表面活性剂或其混合物。适用的阴离子、非离子和/或阳离子的表面活性剂的例子例如列于WO 97/34485的第7和8页。还有,适用于制备本发明除草剂组合物的制剂技术中常用的表面活性剂特别描述于″《Mc Cutcheon’s洗涤剂和乳化剂年报》
Mc Cutcheon’s Detergents and Emulsifiers Annual″,MC Publishing Corp.,Ridgewood New Jersey,1981;Stache,H.,″Tensid-Taschenbuch″,Carl Hanser Verlag,MunichNienna,1981以及M.和J.Ash,″《表面活性剂大全》
Encyclopedia of Surfactants″,第I-III卷,ChemicalPublishing Co.,New York,1980-81之中。
除草的制剂中通常含有0.1-99重量%,特别是0.1-95重量%的活性成分混合物,该混合物含有式I化合物和式X,Xl,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX,该制剂中还含有1-99.9%重量的固体或液体的剂型助剂,和0-25重量%,特别是0.1-25重量%的表面活性剂。而商业性的产品通常优选是浓缩剂,终端使用者通常使用稀释的制剂。
该组合物还可含有添加剂,如稳定剂,植物油或环氧化植物油(环氧化椰子油、菜籽油或大豆油),消泡剂如硅油,防腐剂,粘度调节剂,粘合剂,增稠剂和肥料或其它活性成分。可采用多种适当的方法和技术来应用式X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX的安全剂,或含有它们的组合物,以保护种植的植物不受式I除草剂的危害。下面是实例:
i)
拌种
a)用下式活性成分的可湿性粉剂拌种:X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX,在容器中振动,直到制剂在种子表面均匀地分布(干拌种)。每100g种子使用大约1-500g式X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX的活性成分(4g-2kg的可湿性粉剂)。
b)按照a)的方法,用下式活性成分的乳油拌种:X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX(湿拌种)。
c)把种子浸入含有100-1000ppm下式活性成分的液体制剂进行拌种:X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX,时间是1-2小时,如果需要,接着将种子干燥(浸入法拌种)。
拌种或处理发芽的幼苗显然是优选的应用方法,因为用活性成分的处理直接作用于整个的靶标作物。虽然根据所用的方法不同安全剂的用量不同,但通常每100g种子,使用1-1000g安全剂,优选5-250g安全剂,并且还可以加入其它活性成分或微量营养物,也可以采用超过或不足以达到特定浓度限制的量(重复拌种)。
ii)
以桶混物的形式使用
使用安全剂和除草剂混合物的液体制剂(一个与另一个的比例是10∶1-1∶100),除草剂的施用量为每公顷0.005-5.0kg。这种桶混物种植前或种植后使用。
iii)
播种沟施用
以乳油、可湿性粉剂或颗粒剂的形式,将式X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX的活性成分引入开放的,播种种子的垄沟内。播种沟覆盖后,除草剂是以常规的芽前施用的方式施用的。
iv)
活性成分的控制释放
将式X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX的活性成分以溶液的形式应用于无机颗粒载体或聚合的物颗粒(脲/甲醛)并干燥。适当时,也可以涂敷能使活性成分在一定的时间内定量释放的包衣剂(包衣颗粒)。
可通过加入喷雾桶混助剂,可能会提高本发明除草或抑制植物生长的组合物的活性,所述的除草或控制植物生长的组合物含有除草有效量的式I化合物和拮抗除草剂有效量的式X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX化合物。这些助剂例如可以是非离子表面活性剂、非离子表面活性剂的混合物、阴离子表面活性剂与非离子表面活性剂的混合物、阳离子表面活性剂、有机硅表面活性剂、有或没有表面活性剂的矿物油衍生物,有或没有加入表面活性剂的植物油衍生物,有或没有表面活性剂的植物或矿物来源的油的烷基化衍生物,有或没有表面活性剂的动物性质的鱼油或其它动物的油或其烷基化衍生物,优选含有8-28个碳原子的天然的高级脂肪酸,和其烷基酯衍生物,含有芳环系和一个或多个羧酸酯的有机酸,和其烷基衍生物,以及乙酸乙烯酯的聚合物或乙酸乙烯酯/丙烯酸酯的共聚物的悬浮液。单个助剂相互之间的混合物以及与有机溶剂结合可能会使活性进一步提高。
适当的非离子表面活性剂例如包括脂族或环脂族醇、饱和或不饱和脂肪酸和烷基酚的聚二元醇醚的衍生物,优选含有3-30个二元醇醚基团,以及脂族烃基基团中含有8-20个碳原子,烷基酚的烷基基团中具有6-18个碳原子。
其它合适的非离子表面活性剂是水溶性的聚丙二醇、亚乙基氨基聚丙二醇和在烷基链中有1-10个碳原子的烷基聚丙二醇的聚环氧乙烷的加成物,该加成物中优选含有20-250乙二醇醚基团,和10-100丙二醇醚基团。所述的化合物中每个丙二醇单元通常含有1-5个乙二醇单元。
作为非离子表面活性剂其它的实例,还可以提及的是壬基苯酚聚乙氧基乙醇、蓖麻油聚二元醇醚、聚环氧丙烷/聚环氧乙烷加成物、三丁基苯氧基聚乙氧基乙醇、聚乙二醇和辛基苯氧基聚乙氧基乙醇。
聚氧乙烯脱水山梨糖醇的脂肪酸酯如聚氧乙烯脱水山梨糖醇三油酸酯也是适用的。
优选的阴离子表面活性剂特别是烷基硫酸盐、烷基磺酸盐、烷基芳基磺酸盐、烷基化的磷酸,和它们的乙氧基化衍生物。其中的烷基基团通常有8-24个碳原子。
优选的非离子表面活性剂已知有下述商品名称的商品:
聚氧乙烯椰油烷基胺(如AMIET105(Kao Co.)),聚氧乙烯油基胺(如AMIET415(Kao Co.)),壬基苯酚聚乙氧基乙醇,聚氧乙烯硬脂基胺(AMIET320(Kao Co.)),N-聚乙氧基乙胺(GENAMIN(HoechstAG)),N,N,N’,N’-四(聚乙氧基聚丙氧基乙基)亚乙基二胺(如TERRONIL和TETRONIC(BASF Wyandotte Corp.)),BRIJ(AtlasChemicals),ETHYLANCD和ETHYLAND(Diamond Shamrock),GENAPOLC,GENAPOLO,GENAPOLS和GENAPOLX080(Hoechst AG),EMULGEN104P,EMULGEN109P和EMULGEN408(Kao Co.);DISTY125(Geronazzo),SOPROPHORCY 18(Rhone Poulenc S.A.);NONISOL(Ciba-Geigy),MRYJ(ICI);TWEEN(ICI);EMULSOGEN(HoechstAG);AMIDOX(Stephan Chemical Co.),ETHOMID(Armak Co.);PLURONIC(BASF Wyandotte Corp.),SOPROPHOR461P(Rhone PoulencS.A.),SOPROPHOR496/P(Rhone Poulenc S.A.),ANTAROX FM-63(Rhone Poulenc S.A.),SLYGARD 309(Dow Corning),SILWET 408,SILWET L-7607N(Osi-Special ities)。
阳离子表面活性剂特别是作为N-取代基至少有一个烷基基团有8-22个碳原子的季铵盐,作为其它的取代基,可以是卤代低级烷基、苄基或羟基-低级烷基基团。盐优选是卤化物、甲基硫酸盐或乙基硫酸盐的形式,例如硬脂基三甲基铵氯化物或苄基二(2-氯乙基)乙基铵溴化物。
所用的油可以是矿物油或天然来源的油。天然的油还可以是动物或植物来源的油。动物油中优选是牛油衍生物,但也可以使用鱼油(如沙丁鱼油)和其衍生物。植物油大部分是各种来源的种子的油。可以提及的所用植物油的实例包括椰子油、菜籽油和葵花籽油和其衍生物。
在本发明的组合物中,以喷雾混合物为基础,油添加剂的浓度通常是0.01-2%。例如在制得喷雾混合物之后,以所需的浓度向喷雾剂桶中加入油添加剂。
本发明组合物中,优选的油添加剂包括植物来源的油,例如菜籽油或葵花籽油,植物来源的油的烷基酯如甲基衍生物,或矿物油。
特别优选的油添加剂包括高级脂肪酸(C8-C22)的烷基酯,特别是C12-C18脂肪酸的甲基衍生物,例如月桂酸、棕榈酸和油酸的甲基酯。这些酯已知是月桂酸甲酯(CAS-111-82-0)、棕榈酸甲酯(CAS-112-39-0)和油酸甲酯(CAS-112-62-9)。
通过将油添加剂与表面活性物质结合使用可改进它们的施用和作用,所述的表面活性物质例如是非离子表面活性剂、阴离子或阳离子表面活性剂。适当的阴离子、非离子或阳离子表面活性剂列于WO97/34485的第7和8页。
优选的表面活性物质是十二烷基苄基磺酸盐类的阴离子表面活性剂,特别是其钙盐,和脂肪醇乙氧基化物类的非离子表面活性剂。特别优选的是乙氧基化的C12-C22脂肪醇,其乙氧基化度为5-40。可以买到的优选表面活性剂的例子是Genapol类(Clariant AG,Muttenz,瑞士)。
相对于添加剂总量而言,表面活性物质的浓度通常是1-30重量%。
与表面活性剂组成油或矿物油或其衍生物的混合物的油添加剂的实例包括Edenor ME SU,Emery 2231(Henkel subsidiary CognisGMBH,DE),Turbocharge(Zeneca Agro,Stoney Creek,Ontario,CA),或更特别的是Actipron(BP Oil UK Limited,GB)。
在油添加剂/表面活性剂混合物中加入有机溶剂有可能进一步提高其活性。适当的溶剂例如包括Solvesso(ESSO)和Aromatic Solvent(Exxon公司)类的溶剂。
这些溶剂的浓度相对于总重量而言,可以是10-80重量%。
在例如US-A-4834908中所述的这类油添加剂中,对本发明组合物来说是特别优选的。更优选的油添加剂是已知其商品名称为MERGE,可由BASF公司得到,并且在US-A-4834908,第5栏,实施例COC-1中已有基本的描述。本发明中优选的其它油添加剂是SCORE(Novartis CropProtection Canada)。
在制剂和辅剂技术中通常所使用的表面活性剂,油,特别是植物油,其衍生物如烷基化的脂肪酸和其混合物,例如与阴离子表面活性剂如烷基化的磷酸、烷基硫酸盐与烷基芳基磺酸盐和高级脂肪酸的混合物,在本发明组合物中以及在喷雾桶混溶液中也可以使用,这些在下述文献中已有描述:尤其是″《Mc Cutcheon’s洗涤剂和乳化剂年报》
Mc Cutcheon’s Detergents and Emulsifiers Annual″MC PublishingCorp.,Ridgewood New Jersey,1998;Stache,H.,″Tensid-Taschenbuch″,Carl Hanser Verlag,Munich/Vienna,1990;M.和J.Ash,″《表面活性剂大全》
Encyclopedia of Surfactants″,第I-IV卷,Chemical Publishing Co.,New York,1981-89;G.Kapusta,″《除草剂助剂简编》
A Compendium of Herbicide Adjuvants″,SouthernIllinois Univ.,1998;L.Thomson Harvey,″《美国使用的农业喷雾助剂指南》
A Guide to Agricultural Spray Adjuvants Used in the United States″,Thomson Pubns.,1992.
优选的剂型尤其具有下述的组成(%=重量百分数)。
乳油:
活性成分混合物: 1-90%,优选5-20%
表面活性剂: 1-30%,优选10-20%
液体载体: 5-94%,优选70-85%
粉剂:
活性成分混合物: 0.1-10%,优选0.1-5%
固体载体: 99.9-90%,优选99.9-99%
悬浮浓缩液:
活性成分混合物: 5-75%,优选10-50%
水: 94-24%,优选88-30%
表面活性剂: 1-40%,优选2-30%
可湿性粉剂:
活性成分混合物: 0.5-90%,优选1-80%
表面活性剂: 0.5-20%,优选1-15%
固体载体: 5-95%,优选15-90%
颗粒剂:
活性成分混合物: 0.1-30%,优选0.1-15%
固体载体: 99.5-70%,优选97-85%
下述实施例进一步说明了本发明,但不限制本发明。
式I的除草剂与式X、XI、XII、XIII、XIV、XV、XVI、XVII、XVIII、XIX、XX、XXI、XXII、XXIII、XXIV、XXV、XXVI、XXVII、XXVIII或XXIX的安全剂的混合物的剂型实施例(%=重量百分数)
Fl-乳油 a) b) c) d)
活性成分混合物 5% 10% 25% 50%
十二烷基苯磺酸钙 6% 8% 6% 8%
蓖麻油聚乙二醇醚 4% - 4% 4%
(36mol环氧乙烷)
辛基苯酚聚乙二醇醚 - 4% - 2%
(7-8mol环氧乙烷)
环己酮 - - 10% 20%
芳族C9-12烃混合物 85% 78% 55% 16%
由上述浓缩液,通过用水稀释可制备任何所需浓度的乳剂。
F2.乳油 a) b) c) d)
活性成分混合物 5% 10% 25% 50%
1-甲氧基-3-(3-甲氧基- - 20% 20% -
丙氧基)-丙烷
聚乙二醇(分子量400) 20% 10% - -
N-甲基-2-吡咯烷酮 - - 30% 10%
芳族C9-12烃混合物 75% 60% - -
该溶液适用于以微滴的形式施用。
F3.可湿性粉剂 a) b) c) d)
活性成分混合物 5% 25% 50% 80%
木质素磺酸钠 4% - 3% -
月桂基硫酸钠 2% 3% - 4%
二异丁基萘磺酸钠 - 6% 5% 6%
辛基苯酚聚乙二醇醚 - 1% 2% -
(7-8mol环氧乙烷)
高分散硅酸 1% 3% 5% 10%
高岭土 88% 62% 35% -
将活性成分与助剂充分混合,混合物于适当的研磨机中充分研磨,得到可用水稀释的可湿性粉剂,稀释后可得到任何所需浓度的悬浮液。
F4.包衣颗粒剂 a) b) c)
活性成分混合物 0.1% 5% 15%
高分散硅酸 0.9% 2% 2%
无机载体材料 99.0% 93% 83%
(FE 0.1-1mm)
例如CaCO3或SiO2
将活性成分溶解于二氯甲烷,将该溶液喷在载体上,然后真空蒸发溶剂。
F5.包衣颗粒剂 a) b) c)
活性成分混合物 0.1% 5% 15%
聚乙二醇(分子量200) 1.0% 2% 3%
高分散硅酸 0.9% 1% 2%
无机载体材料 98.0% 92% 80%
(FE 0.1-1mm)
例如CaCO3或SiO2
在混合器中,将经过经细研磨的活性成分均匀地施加到用聚乙二醇
湿润的载体材料上,得到无粉尘的包衣颗粒剂。
F6.挤出颗粒剂 a) b) c) d)
活性成分混合物 0.1% 3% 5% 15%
木质素磺酸钠 1.5% 2% 3% 4%
羧甲基纤维素 1.4% 2% 2% 2%
高岭土 97.0% 93% 90% 79%
将活性成分与助剂混合,研磨混合物,用水润湿,挤出,然后在空气流中干燥。
F7.粉剂 a) b) c)
活性成分混合物 0.1% 1% 5%
滑石 39.9% 49% 35%
高岭土 60.0% 50% 60%
将活性成分与载体混合,并在适当的研磨机中研磨该混合物,得到立即可用的粉剂。
F8.悬浮浓缩剂 a) b) c) d)
活性成分混合物 3% 10% 25% 50%
乙二醇 5% 5% 5% 5%
壬基苯酚聚乙二醇醚 - 1% 2% -
(15mol环氧乙烷)
木质素磺酸钠 3% 3% 4% 5%
羧甲基纤维素 1% 1% 1% 1%
37%甲醛水溶液 0.2% 0.2% 0.2% 0.2%
硅油乳液 0.8% 0.8% 0.8% 0.8%
水 87% 79% 62% 38%
将经过精细研磨的活性成分与助剂充分混合,得到悬浮浓缩剂,通过用水稀释可得到任何所需浓度的悬浮液。
经常,更实用的是将式I的活性成分与式X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX的混合配伍成分别加工成制剂,然后,在临使用之前将它们在施用设备中以所需的混合比制成“桶混混合物”的水剂形式。
式X,Xl,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX安全剂保护种植植物不受式I除草剂植物毒性作用危害的能力在下面的实施例中予以说明。
生物试验1:安全作用
在温室条件下,试验植物在塑料盆中生长至4叶期。在此阶段,一方面在试验植物上仅施用除草剂,另一方面施用除草剂与欲试验作为安全剂的物质的混合物。试验物质以水悬浮剂的形式施用,该悬浮剂是由25%的可湿性粉剂制备(实施例F3,b)),施用500升水/公顷。施用2至3周后,除草剂对栽培植物如玉米和禾谷类的毒性以百分数评价,100%是指植物已经死亡,0%是指没有植物毒性。
此试验的结果表明式X,XI,XII,XIII,XIV,XV,XVI,XVII,XVIII,XIX,XX,XXI,XXII,XXIII,XXIV,XXV,XXVI,XXVII,XXVIII或XXIX化合物可显著降低式I除草剂对种植植物的药害。这些结果的一些例子见下表B5。
表B5:本发明的除草剂和安全剂混合物的苗后作用:
| 试验植物 | 化合物编号1.01(60g/ha) | 化合物编号1.01(60g/ha)+化合物编号11.03(15g/ha) |
| 大麦 | 20 | 0 |
| 剪股颖属 | 70 | 70 |
| 看麦娘属 | 70 | 80 |
| 黑麦草属 | 70 | 70 |
由表B5可以看出,化合物编号No.1.01对大麦的植物毒性为20%,这是不能允许的。剪股颖属、看麦娘属和黑麦草属杂草能够很好的被防治。
与此相反,由No.1.01的除草剂和No.11.03的安全剂组成的混合物对种植植物没有植物毒性。与此同时,除草剂对草的作用不仅完全相同,而且令人惊奇的是,在看麦娘属的情况下,甚至还有所增加(与只用除草剂No.1.01达到的70%相比,它可达到80%)。
在把混合物按照实施例F1、F2和F4-F8加工制剂时可得到相同的结果。
将式I化合物与其它一些已知的除草剂混合是有利的。例如其结果是,在很多情况下除草谱更宽,对有用的植物选择性提高。尤其是,式I化合物与至少下述一种除草剂的混合物是重要的:
苯氧基-苯氧基丙酸类的除草剂,例如禾草灵,精吡氟禾草灵,精喹禾灵,噁草酯,炔草酯,氰氟草酯,精噁唑禾草灵,氟吡甲禾灵或氟吡乙禾灵;
羟胺类的除草剂,例如稀禾啶,禾草灭,烯草酮,噻草酮,醌肟草(tepralkoxydim),三甲苯草酮或丁氧环酮;
磺酰脲类的除草剂,例如磺氨黄隆,四唑嘧磺隆,苄嘧磺隆,氯嘧磺隆乙酯,醚磺隆,氯磺隆,氯嘧磺隆,环丙嘧磺隆,胺苯磺隆,乙氧嘧磺隆,fluazasulfuron,氟啶黄隆,唑吡嘧黄隆,iodosulfuron(CAS RN144550-36-7和185119-76-0),甲磺隆,烟嘧磺隆,环丙氧黄隆,氟嘧磺隆,吡嘧黄隆,乙黄黄隆,砜嘧磺隆,噻吩磺隆,醚苯磺隆,苯磺隆,氟胺磺隆,氟磺隆,flucarbazone或tritosulfuron(CAS RN 142469-14-5);
咪唑啉酮类的除草剂,例如咪唑乙烟酸,咪草酸,imazamethapyr,咪唑喹啉酸,咪草啶酸或咪唑烟酸;
嘧啶类的除草剂,例如嘧草硫醚,肟啶草,双草醚;
三嗪类的除草剂,例如莠去津,西玛津,西草净,特丁净,特丁津,;脲类的除草剂,例如异丙隆,绿麦隆,敌草隆,杀草隆,氟草隆,利谷隆,甲基苯噻隆;
膦酸衍生物类的除草剂,例如草甘膦,草铵膦,草硫膦,phosphinothricin;
PPO类的除草剂,例如硝基除草醚,甲羧除草醚,三氟羧草醚,乳氟禾草灵,乙氧氟草醚,ethoxyfen,乙羧氟草醚,氟磺胺草醚,氟硝磺酰胺,唑啶炔草(CAS RN.-68049-83-2),benzfendi zone(CAS RN158755-95-4),butafenacil(US-A-5 183 492中公开,CAS RN158755-95-4),氟酮唑草,cinidon-ethyl(CAS RN 142891-20-1),氟烯草酸,丙炔氟草胺,达草氟,炔丙噁唑草,噁草酮,戊噁唑草,甲磺草胺,fluazolate(CAS RN 174514-07-9)或氟唑草酯;
N-氯乙酰苯胺类的除草剂,例如甲草胺,乙草胺,丁草胺,二甲草胺,二甲吩草胺,S-二甲吩草胺,吡唑草胺,异丙甲草胺,S-异丙甲草胺,丙草胺,毒草胺,异丙草胺,噻醚草胺或pethoamid(CAS RN 106700-29-2);
苯氧基乙酸类的除草剂,例如2,4-滴,氯氟吡氧乙酸,MCPA,MCPP,MCPB,三氯吡氧乙酸或精2甲4氯丙酸;
三嗪酮类的除草剂,例如环嗪酮,苯嗪草酮或嗪草酮;
二硝基苯胺类的除草剂,例如氨磺乐灵,二甲戊灵或氟乐灵;
哒嗪酮类的除草剂,例如杀草敏或哒草伏;
氨基甲酸酯类的除草剂,例如氯苯胺灵,甜菜安,甜菜宁或苯胺灵;
氧乙酰胺类的除草剂,例如苯噻酰草胺或达草氟;
硫代氨基甲酸酯类的除草剂,例如丁草敌,环草敌,燕麦敌,茵草敌,戊草丹,禾草敌,苄草丹,禾草丹或野麦畏;
唑脲(azoloureas)类除草剂,例如fentrazamide(CAS RN158237-07-1)或苯酮唑;
苯甲酸类除草剂,例如麦草畏或氨氯吡啶酸;
N-酰基苯胺除草剂,例如吡氟酰草胺,或敌稗;
腈类除草剂,例如溴苯腈,敌草腈或碘苯腈;
三酮类除草剂,例如磺草酮,mesotrione(由US-A-5006 158已知),异噁氟草或isoxachlortole;
磺酰胺类除草剂,例如flucarbazone(CAS RN 181274-17-9),procarbazone(CAS RN 145026-81-9),chlorasulam,diclosulam(CASRN 145701-21-9),florasulam,阔草伏(flumetsulam)或metosulam;
以及杀草强,呋草黄,灭草松,环庚草醚,异噁草松,chlopyralid,野燕枯,氟硫草定,乙氧呋草黄,flurochloridone,indanofane,异噁草胺,噁嗪草酮(CAS RN 153197-14-9),哒草特,pyridafol(CAS RN40020-01-7),二氯喹啉酸,氯甲喹啉酸,灭草环或氟燕灵。
除非另有说明,上述式I化合物的混合组分是已知的,见《农药手册》The Pesticide Manual,第11版,1997,BCPC。如果需要,上述式I化合物的混合组分也可以是酯或盐的形式,如在《农药手册》,第11版,1997,BCPC中所述。
下面的实施例说明了本发明,但不构成任何限制。
制备实施例
实施例P1:制备下述化合物:
在20g的2-(2,6-二溴-4-甲基-苯基)-丙二酸二甲酯(52.6mmol)的400ml甲苯溶液中(脱气3次,真空/氩气),先加入36.7g(0.116mol)三丁基乙烯基锡烷,然后加入2g四(三苯膦)合钯。然后于90至95℃搅拌反应混合物9小时。用Hyflo过滤,旋转蒸发器浓缩,色谱纯化,得到15.3g(8),是黄色的油状物,该化合物可直接用于下一步反应,而无需进一步纯化。
实施例P2:
将实施例P1得到的15.2g化合物(8)用氢氢化,反应使用钯催化剂(以碳为载体,7g的5%Pd/C),在160ml四氢呋喃中于20至25℃下进行。氢化完成后,通过Hyflo过滤出产物,旋转蒸发器蒸发,得到13.7g化合物(9),为黄色结晶,熔点47-49℃。
实施例P3:
将71.8g(0.71mol)三乙胺加入到40g(0.15mol)(4)于1000ml二甲苯中的悬浮液中,使混合物脱气(4次,真空/氩气)。然后将黄色的悬浮液加热至60℃,搅拌3小时。接着向其中加入42.5g(0.15mol)(5),为了蒸出过量的三乙胺和产生的乙醇,混合物加热至150℃的浴温。3小时后,将反应混合物冷却至40℃,倒入500ml冰/水混合物中。用100ml的1N氢氧化钠水溶液使反应混合物碱化,水相(含有产物)用乙酸乙酯洗涤二次。有机相用1N氢氧化钠溶液洗涤二次之后,合并水相,蒸除残留的二甲苯,合并的水相在冷却下用4NHCl调节pH至2-3。将沉淀的产物倾入抽滤过滤器,过滤的残余物用水和少量己烷洗涤,于60℃用P2O5真空干燥,得到34.6g(6),为浅褐色固体,熔点242-244℃(分解)。
实施例P4:
冷却至0℃,在3g(10.4mmol)的(6)和1.6g(15.8mmol)三乙胺于100ml四氢呋喃溶液中加入催化剂量的4-二甲氨基吡啶,然后向其中滴加1.57g(13.0mmol)新戊酰氯。于0℃拌30分钟后,移去冷却源,继续搅拌60分钟。然后将反应混合物倒入饱和氯化钠水溶液中,分离出有机相,有机相用硫酸镁干燥,过滤和蒸发浓缩。在用色谱纯化和用乙醚重结晶后,得到2.94g(7),熔点135-136℃。
实施例P5:制备2-(2,6-二乙基-4-甲基-苯基)-四氢-吡唑并[1,2-a]
哒嗪-1,3-二酮:
在20℃,将1.39g四氢-吡唑并[1,2-a]哒嗪-1,3-二酮和2.68g叔丁醇钠溶于20ml二甲基甲酰胺,向其中加入3.21g 2,6-二乙基-4-甲基-碘代苯和0.82g Pd(TPP)2Cl2,然后在125℃搅拌2.5小时。冷却至室温后,加入200ml乙酸乙酯和200ml乙醚,将反应混合物倾入抽滤过滤器中。在过滤残余物中加入100ml水和100ml二氯甲烷,用盐酸酸化。分离有机相,干燥和蒸发浓缩。
残余物(1.9g)用硅胶色谱纯化(乙酸乙酯/己烷3∶1)。得到2-(2,6-二乙基-4-甲基-苯基)-四氢-吡唑并[1,2-a]哒嗪-1,3-二酮,为灰褐色结晶,熔点174-175℃。
实施例P6:2-(2,6-二乙基-4-甲基-苯基)-四氢-吡唑并[1,2-a]哒嗪
-1,3-二酮:
在20℃,将1.39g四氢-吡唑并[1,2-a]哒嗪-1,3-二酮和2.68g叔丁醇钠溶于20ml二甲基甲酰胺,向其中加入2.66g 2,6-二乙基-4-甲基-溴代苯和0.82g Pd(TPP)2Cl2,然后在125℃搅拌2.5小时。冷却至室温后,加入200ml乙酸乙酯和200ml乙醚,将反应混合物倾入抽滤过滤器中。在过滤残余物中加入100ml水和100ml二氯甲烷,用盐酸酸化。分离有机相,干燥和蒸发浓缩。
残余物(1.4g)用硅胶色谱纯化(乙酸乙酯/己烷3∶1)。得到2-(2,6-二乙基-4-甲基-苯基)-四氢-吡唑并[1,2-a]哒嗪-1,3-二酮,为灰褐色结晶,熔点174-175℃。
在下表中,熔点以℃表示,Me表示甲基基团。其中给出了取代基G1-G10和R4和R5(各自独立)的结构式,结构式左侧是与杂环Q1-Q10的氧原子的连接点。在R4和R5一起表示取代基含义的情况下,结构式右侧是与杂环Q1的连接点,其余末端价键是甲基。
下表中,″LC/MS:M+″以道尔顿表示分析中通过耦合HPLC(高效液相色谱)和MS(质谱)仪确定的产物的正电荷分子离子。
表1:式Ia化合物:
表2:式Ia化合物:
| 编号 | R1 | R3 | R4 | R5 | G1 | 物理数据 |
| 2.32 | 乙基 | 乙基 | -(CH2)2OH | 烯丙基 | -H | M.p.180-185℃(分解) |
表3:式Ib化合物:
表4:式Ic化合物:
表5:式Id化合物:
表6:式Ie化合物:
表7:式If化合物:
表8:式Ig化合物:
表9:式Ih化合物:
表10:式Ik化合物:
在下表21中,Me是甲基,Et是乙基,Pr是丙基和Bu是丁基。
表21:式Im化合物:
| 化合物编号 | R1 | R3 | R55 | R137 | R138 | R139 | G10 | Y2 | 物理数据 |
| 21.1 | Et | Et | H | H | H | H | H | O | |
| 21.2 | Et | 乙炔基 | H | H | H | H | H | O | |
| 21.3 | Et | Et | Me | Me | Me | Me | H | O | |
| 21.4 | Et | OMe | Me | Me | Me | Me | H | O | |
| 21.5 | Et | Et | Me | H | H | H | H | O | |
| 21.6 | 乙炔基 | Et | Me | H | H | H | H | O | |
| 21.7 | Et | Et | H | H | Me | Me | H | O | |
| 21.8 | OMe | Et | H | H | Me | Me | H | O | |
| 21.9 | Et | Et | Me | H | Me | Me | H | O | |
| 21.10 | Et | 乙炔基 | Me | H | Me | Me | H | O | |
| 21.11 | Et | Et | H | Me | H | Me | H | O | |
| 21.12 | Et | OMe | H | Me | H | Me | H | O | |
| 21.13 | Et | Et | Me | Et | H | H | H | O | |
| 21.14 | 乙炔基 | Et | Me | Et | H | H | H | O | |
| 21.15 | Et | Et | H | Et | H | Et | H | O | |
| 21.16 | OMe | Et | H | Et | H | Et | H | O | |
| 21.17 | Et | Et | H | H | -(CH2)4- | H | O | ||
| 21.18 | Et | 乙炔基 | H | H | -(CH2)4- | H | O | ||
| 21.19 | Et | Et | H | H | H | H | COCMe3 | O | |
| 21.20 | Et | 乙炔基 | H | H | H | H | SO2Me | O | |
| 21.21 | Et | Et | Me | Me | Me | Me | COCMe3 | O | |
| 21.22 | Et | OMe | Me | Me | Me | Me | SO2-n-Pr | O | |
| 21.23 | Et | Et | Me | H | H | H | COCMe3 | O | |
| 21.24 | 乙炔基 | Et | Me | H | H | H | SO2-n-Bu | O | |
| 21.25 | Et | Et | H | H | Me | Me | COCMe3 | O | |
| 21.26 | OMe | Et | H | H | Me | Me | SO2C8H17 | O | |
| 21.27 | Et | Et | Me | H | Me | Me | COCMe3 | O | |
| 21.28 | Et | 乙炔基 | Me | H | Me | Me | SO2Ph | O | |
| 21.29 | Et | Et | H | Me | H | Me | COCMe3 | O | |
| 21.30 | Et | OMe | H | Me | H | Me | SO2Me | O | |
| 21.31 | Et | Et | Me | Et | H | H | COCMe3 | O | |
| 21.32 | 乙炔基 | Et | Me | Et | H | H | COCMe3 | O | |
| 21.33 | Et | Et | H | Et | H | Et | COCMe3 | O | |
| 21.34 | OMe | Et | H | Et | H | Et | COCMe3 | O | |
| 21.35 | Et | Et | H | H | -(CH2)4- | COCMe3 | O | ||
| 21.36 | Et | 乙炔基 | H | H | -(CH2)4- | COCMe3 | O | ||
| 21.37 | Et | Et | H | H | H | H | H | S | |
| 21.38 | Et | 乙炔基 | H | H | H | H | H | S | |
| 21.39 | Et | Et | Me | Me | Me | Me | H | S | |
| 21.40 | Et | OMe | Me | Me | Me | Me | H | S | |
| 21.41 | Et | Et | Me | H | H | H | H | S | |
| 21.42 | 乙炔基 | Et | Me | H | H | H | H | S | |
| 21.43 | Et | Et | H | H | Me | Me | H | S | |
| 21.44 | OMe | Et | H | H | Me | Me | H | S | |
| 21.45 | Et | Et | Me | H | Me | Me | H | S | |
| 21.46 | Et | 乙炔基 | Me | H | Me | Me | H | S | |
| 21.47 | Et | Et | H | Me | H | Me | H | S | |
| 21.48 | Et | OMe | H | Me | H | Me | H | S | |
| 21.49 | Et | Et | Me | Et | H | H | H | S | |
| 21.50 | 乙炔基 | Et | Me | Et | H | H | H | S | |
| 化合物编号 | R1 | R3 | R55 | R137 | R138 | R139 | G10 | Y2 | 物理数据 |
| 21.51 | Et | Et | H | Et | H | Et | H | S | |
| 21.52 | OMe | Et | H | Et | H | Et | H | S | |
| 21.53 | Et | Et | H | H | -(CH2)4- | H | S | ||
| 21.54 | Et | 乙炔基 | H | H | -(CH2)4- | H | S | ||
| 21.55 | Et | Et | H | H | H | H | COCMe3 | S | |
| 21.56 | Et | 乙炔基 | H | H | H | H | SO2Me | S | |
| 21.57 | Et | Et | Me | Me | Me | Me | COCMe3 | S | |
| 21.58 | Et | OMe | Me | Me | Me | Me | SO2-n-Pr | S | |
| 21.59 | Et | Et | Me | H | H | H | COCMe3 | S | |
| 21.60 | 乙炔基 | Et | Me | H | H | H | SO2-n-Bu | S | |
| 21.61 | Et | Et | H | H | Me | Me | COCMe3 | S | |
| 21.62 | OMe | Et | H | H | Me | Me | SO2C8H17 | S | |
| 21.63 | Et | Et | Me | H | Me | Me | COCMe3 | S | |
| 21.64 | Et | 乙炔基 | Me | H | Me | Me | SO2Ph | S | |
| 21.65 | Et | Et | H | Me | H | Me | COCMe3 | S | |
| 21.66 | Et | OMe | H | Me | H | Me | SO2Me | S | |
| 21.67 | Et | Et | Me | Et | H | H | COCMe3 | S | |
| 21.68 | 乙炔基 | Et | Me | Et | H | H | COCMe3 | S | |
| 21.69 | Et | Et | H | Et | H | Et | COCMe3 | S | |
| 21.70 | OMe | Et | H | Et | H | Et | COCMe3 | S | |
| 21.71 | Et | Et | H | H | -(CH2)4- | COCMe3 | S | ||
| 21.72 | Et | 乙炔基 | H | H | -(CH2)4- | COCMe3 | S | ||
| 21.73 | Et | Et | H | H | H | H | H | NCH(CH3)2 | |
| 21.74 | Et | Et | H | H | H | H | H | NCH3 | |
| 21.75 | Et | Et | H | H | H | H | H | NCH2Ph | |
| 21.76 | Et | 乙炔基 | H | H | H | H | H | NCH3 | |
| 21.77 | Et | Et | Me | Me | Me | Me | H | NCH(CH3)2 | |
| 21.78 | Et | OMe | Me | Me | Me | Me | H | NCH3 | |
| 21.79 | Et | Et | Me | H | H | H | H | NCH(CH3)2 | |
| 21.80 | 乙炔基 | Et | Me | H | H | H | H | NCH3 | |
| 21.81 | Et | Et | H | H | Me | Me | H | NCH3 | |
| 21.82 | OMe | Et | H | H | Me | Me | H | NCH(CH3)2 | |
| 21.83 | Et | Et | Me | H | Me | Me | H | NCH2Ph | |
| 21.84 | Et | 乙炔基 | Me | H | Me | Me | H | NCH3 | |
| 21.85 | Et | Et | H | Me | H | Me | H | NCH2Ph | |
| 21.86 | Et | OMe | H | Me | H | Me | H | NCH3 | |
| 21.87 | Et | Et | Me | Et | H | H | H | NCH(CH3)2 | |
| 21.88 | 乙炔基 | Et | Me | Et | H | H | H | NCH3 | |
| 21.89 | Et | Et | H | Et | H | Et | H | NCH2Ph | |
| 21.90 | OMe | Et | H | Et | H | Et | H | NCH(CH3)2 | |
| 21.91 | Et | Et | H | H | -(CH2)4- | H | NCH(CH3)2 | ||
| 21.92 | Et | 乙炔基 | H | H | -(CH2)4- | H | NCH3 | ||
| 21.93 | OMe | Et | Et | Me | H | H | H | NCH3 | |
| 21.94 | Et | Et | H | H | H | H | COCMe3 | NCH(CH3)2 | |
| 21.95 | Et | Et | H | H | H | H | SO2Me | NCH3 | |
| 21.96 | Et | Et | H | H | H | H | COCMe3 | NCH2Ph | |
| 21.97 | Et | 乙炔基 | H | H | H | H | SO2-n-Pr | NCH3 | |
| 21.98 | Et | Et | Me | Me | Me | Me | COCMe3 | NCH(CH3)2 | |
| 21.99 | Et | OMe | Me | Me | Me | Me | SO2-n-Bu | NCH3 | |
| 21.100 | Et | Et | Me | H | H | H | COCMe3 | NCH(CH3)2 | |
| 化合物编号 | R1 | R3 | R55 | R137 | R138 | R139 | G10 | Y2 | 物理数据 |
| 21.101 | 乙炔基 | Et | Me | H | H | H | SO2C8H17 | NCH3 | |
| 21.102 | Et | Et | H | H | Me | Me | COCMe3 | NCH3 | |
| 21.103 | OMe | Et | H | H | Me | Me | SO2Ph | NCH(CH3)2 | |
| 21.104 | Et | Et | Me | H | Me | Me | COCMe3 | NCH2Ph | |
| 21.105 | Et | 乙炔基 | Me | H | Me | Me | SO2Me | NCH3 | |
| 21.106 | Et | Et | H | Me | H | Me | COCMe3 | NCH2Ph | |
| 21.107 | Et | OMe | H | Me | H | Me | COCMe3 | NCH3 | |
| 21.108 | Et | Et | Me | Et | H | H | COCMe3 | NCH(CH3)2 | |
| 21.109 | 乙炔基 | Et | Me | Et | H | H | COCMe3 | NCH3 | |
| 21.110 | Et | Et | H | Et | H | Et | COCMe3 | NCH2Ph | |
| 21.111 | OMe | Et | H | Et | H | Et | COCMe3 | NCH(CH3)2 | |
| 21.112 | Et | Et | H | H | -(CH2)4- | COCMe3 | NCH(CH3)2 | ||
| 21.113 | Et | 乙炔基 | H | H | -(CH2)4- | SO2C8H17 | NCH3 | ||
| 21.114 | OMe | Et | Et | Me | H | H | SO2-n-Bu | NCH3 | |
| 21.115 | Et | Et | H | -(CH2)2- | H | H | CH2 | ||
| 21.116 | Et | 乙炔基 | H | -(CH2)2- | H | H | CH2 | ||
| 21.117 | Et | Et | -(CH2)2- | H | H | H | CH2 | ||
| 21.118 | Et | OMe | -(CH2)2- | H | H | H | CH2 | ||
| 21.119 | Et | Et | H | Me | Me | H | H | CH2 | |
| 21.120 | 乙炔基 | Et | H | Me | Me | H | H | CH2 | |
| 21.121 | Et | Et | Et | H | H | H | H | CH2 | |
| 21.122 | OMe | Et | Et | H | H | H | H | CH2 | |
| 21.123 | Et | Et | H | H | Me | Me | H | CH2 | |
| 21.124 | Et | 乙炔基 | H | H | Me | Me | H | CH2 | |
| 21.125 | Et | Et | H | OMe | H | H | H | CH2 | |
| 21.126 | Et | OMe | H | OMe | H | H | H | CH2 | |
| 21.127 | Et | Et | H | -(CH2)3- | H | H | CH2 | ||
| 21.128 | 乙炔基 | Et | H | -(CH2)3- | H | H | CH2 | ||
| 21.129 | Et | Et | Me | H | Me | Me | H | CH2 | |
| 21.130 | OMe | Et | Me | H | Me | Me | H | CH2 | |
| 21.131 | Et | Et | Me | OMe | H | H | H | CH2 | |
| 21.132 | Et | 乙炔基 | Me | OMe | H | H | H | CH2 | |
| 21.133 | Et | Et | H | SMe | H | H | H | CH2 | |
| 21.134 | Et | OMe | H | SMe | H | H | H | CH2 | |
| 21.135 | Et | Et | Me | Me | Me | Me | H | CH2 | |
| 21.136 | 乙炔基 | Et | Me | Me | Me | Me | H | CH2 | |
| 21.137 | Et | Et | OH | Me | Me | Me | H | CH2 | |
| 21.138 | OMe | Et | OH | Me | Me | Me | H | CH2 | |
| 21.139 | Et | Et | Me | SMe | H | H | H | CH2 | |
| 21.140 | Et | 乙炔基 | Me | SMe | H | H | H | CH2 | |
| 21.141 | Et | Et | Et | Et | H | Me | H | CH2 | |
| 21.142 | Et | 乙炔基 | Et | Et | H | Me | H | CH2 | |
| 21.143 | Et | Et | Me | Me | H | CH2OMe | H | CH2 | |
| 21.144 | Et | OMe | Me | Me | H | CH2OMe | H | CH2 | |
| 21.145 | Et | 乙炔基 | Me | SMe | H | OMe | H | CH2 | |
| 21.146 | Et | Et | Me | SMe | H | OMe | H | CH2 | |
| 21.147 | Et | OMe | Me | SMe | H | OMe | H | CH2 | |
| 21.148 | Et | Et | H | -(CH2)2- | H | COCMe3 | CH2 | ||
| 21.149 | Et | 乙炔基 | H | -(CH2)2- | H | COCMe3 | CH2 | ||
| 21.150 | Et | Et | -(CH2)2- | H | H | SO2-n-Pr | CH2 | ||
| 化合物编号 | R1 | R3 | R55 | R137 | R138 | R139 | G10 | Y2 | 物理数据 |
| 21.151 | Et | OMe | -(CH2)2- | H | H | COCMe3 | CH2 | ||
| 21.152 | Et | Et | H | Me | Me | H | COCMe3 | CH2 | |
| 21.153 | 乙炔基 | Et | H | Me | Me | H | SO2Me | CH2 | |
| 21.154 | Et | Et | Et | H | H | H | COCMe3 | CH2 | |
| 21.155 | OMe | Et | Et | H | H | H | SO2-n-Pr | CH2 | |
| 21.156 | Et | Et | H | H | Me | Me | COCMe3 | CH2 | |
| 21.157 | Et | 乙炔基 | H | H | Me | Me | SO2-n-Bu | CH2 | |
| 21.158 | Et | Et | H | OMe | H | H | COCMe3 | CH2 | |
| 21.159 | Et | OMe | H | OMe | H | H | SO2C8H17 | CH2 | |
| 21.160 | Et | Et | H | -(CH2)3- | H | COCMe3 | CH2 | ||
| 21.161 | 乙炔基 | Et | H | -(CH2)3- | H | COCMe3 | CH2 | ||
| 21.162 | Et | Et | Me | H | Me | Me | SO2-n-Pr | CH2 | |
| 21.163 | OMe | Et | Me | H | Me | Me | COCMe3 | CH2 | |
| 21.164 | Et | Et | Me | OMe | H | H | COCMe3 | CH2 | |
| 21.165 | Et | 乙炔基 | Me | OMe | H | H | SO2Me | CH2 | |
| 21.166 | Et | Et | H | SMe | H | H | COCMe3 | CH2 | |
| 21.167 | Et | OMe | H | SMe | H | H | SO2-n-Pr | CH2 | |
| 21.168 | Et | Et | Me | Me | Me | Me | COCMe3 | CH2 | |
| 21.169 | 乙炔基 | Et | Me | Me | Me | Me | SO2-n-Bu | CH2 | |
| 21.170 | Et | Et | OH | Me | Me | Me | COCMe3 | CH2 | |
| 21.171 | OMe | Et | OH | Me | Me | Me | SO2C8H17 | CH2 | |
| 21.172 | Et | Et | Me | SMe | H | H | COCMe3 | CH2 | |
| 21.173 | Et | 乙炔基 | Me | SMe | H | H | COCMe3 | CH2 | |
| 21.174 | Et | Et | Et | Et | H | Me | COCMe3 | CH2 | |
| 21.175 | Et | 乙炔基 | Et | Et | H | Me | SO2C8H17 | CH2 | |
| 21.176 | Et | Et | Me | Me | H | CH2OMe | SO2-n-Pr | CH2 | |
| 21.177 | Et | OMe | Me | Me | H | CH2OMe | COCMe3 | CH2 | |
| 21.178 | Et | 乙炔基 | Me | SMe | H | OMe | COCMe3 | CH2 | |
| 21.179 | Et | Et | Me | SMe | H | OMe | SO2C8H17 | CH2 | |
| 21.180 | Et | OMe | Me | SMe | H | OMe | COCMe3 | CH2 | |
| 21.181 | Et | Et | H | -(CH2)2- | H | H | CHCH3 | ||
| 21.182 | Et | 乙炔基 | H | -(CH2)2- | H | H | CHCH3 | ||
| 21.183 | Et | Et | -(CH2)2- | H | H | H | CHCH3 | ||
| 21.184 | Et | OMe | -(CH2)2- | H | H | H | CHCH3 | ||
| 21.185 | Et | Et | H | Me | Me | H | H | CHCH3 | |
| 21.186 | 乙炔基 | Et | H | Me | Me | H | H | CHCH3 | |
| 21.187 | Et | Et | Et | H | H | H | H | CHCH3 | |
| 21.188 | OMe | Et | Et | H | H | H | H | CHCH3 | |
| 21.189 | Et | Et | H | H | Me | Me | H | CHCH3 | |
| 21.190 | Et | 乙炔基 | H | H | Me | Me | H | CHCH3 | |
| 21.191 | Et | Et | H | -(CH2)2- | H | COCMe3 | CHCH3 | ||
| 21.192 | Et | 乙炔基 | H | -(CH2)2- | H | COCMe3 | CHCH3 | ||
| 21.193 | Et | Et | -(CH2)2- | H | H | SO2-n-Pr | CHCH3 | ||
| 21.194 | Et | OMe | -(CH2)2- | H | H | COCMe3 | CHCH3 | ||
| 21.195 | Et | Et | H | Me | Me | H | COCMe3 | CHCH3 | |
| 21.196 | 乙炔基 | Et | H | Me | Me | H | SO2Me | CHCH3 | |
| 21.197 | Et | Et | Et | H | H | H | COCMe3 | CHCH3 | |
| 21.198 | OMe | Et | Et | H | H | H | SO2-n-Pr | CHCH3 | |
| 21.199 | Et | Et | H | H | Me | Me | COCMe3 | CHCH3 | |
| 21.200 | Et | 乙炔基 | H | H | Me | Me | SO2-n-Bu | CHCH3 | |
| 化合物编号 | R1 | R3 | R55 | R137 | R138 | R139 | G10 | Y2 | 物理数据 |
| 21.201 | Et | Et | H | -(CH2)2- | H | H | C(CH3)2 | ||
| 21.202 | Et | 乙炔基 | H | -(CH2)2- | H | H | C(CH3)2 | ||
| 21.203 | Et | Et | -(CH2)2- | H | H | H | C(CH3)2 | ||
| 21.204 | Et | OMe | -(CH2)2- | H | H | H | C(CH3)2 | ||
| 21.205 | Et | Et | H | Me | Me | H | H | C(CH3)2 | |
| 21.206 | 乙炔基 | Et | H | Me | Me | H | H | C(CH3)2 | |
| 21.207 | Et | Et | Et | H | H | H | H | C(CH3)2 | |
| 21.208 | OMe | Et | Et | H | H | H | H | C(CH3)2 | |
| 21.209 | Et | Et | H | -(CH2)2- | H | COCMe3 | C(CH3)2 | ||
| 21.210 | Et | 乙炔基 | H | -(CH2)2- | H | COCMe3 | C(CH3)2 | ||
| 21.211 | Et | Et | -(CH2)2- | H | H | SO2-n-Pr | C(CH3)2 | ||
| 21.212 | Et | OMe | -(CH2)2- | H | H | COCMe3 | C(CH3)2 | ||
| 21.213 | Et | Et | H | Me | Me | H | COCMe3 | C(CH3)2 | |
| 21.214 | 乙炔基 | Et | H | Me | Me | H | SO2Me | C(CH3)2 | |
| 21.215 | Et | Et | Et | H | H | H | COCMe3 | C(CH3)2 | |
| 21.216 | OMe | Et | Et | H | H | H | SO2-n-Pr | C(CH3)2 | |
| 21.217 | Et | Et | Me | Me | Me | Me | H | CHCO2Me | |
| 21.218 | Et | Et | H | H | H | H | H | CHCO2Me | |
| 21.219 | Et | Et | Me | Me | Me | Me | COCMe3 | CHCO2Me | |
| 21.220 | Et | Et | H | H | H | H | COCMe3 | CHCO2Me | |
| 21.221 | Et | OMe | -(CH2)2- | H | H | H | CHCO2Me | ||
| 21.222 | Et | OMe | -(CH2)2- | H | H | COCMe3 | CHCO2Me | ||
生物试验实施例
比较试验:
试验下述化合物的除草活性:
按照本发明的化合物编号.1.02
和化合物A
实施例B1:植物发芽前的除草活性(芽前作用)
在塑料盆的标准土壤中种植单子叶和双子叶杂草。种植后立即把实验物质以水悬浮液(由25%可湿性粉剂(实施例F3,b)制备)或乳液(由25%乳油(实施例Fl,c)制备)的形式施用(500升水/ha)。施用量为500g活性物质/公倾。试验植物然后在温室中于最佳条件下生长。评价在施用三周后进行,用9个等级评价(1=全部毁坏,9=无作用)。等级1-4(特别是1-3)表明除草活性好至非常好。试验植物是:看麦娘属(Alo),燕麦属(Ave),黑麦草属(Lol),狗尾草属(Set),黍属(Pan),高粱属(Sor),马唐属(Dig),稗属(Ech)和臂形草属(Bra)。
表B1:芽前作用:
施用量为500g活性成分/公顷时的芽前作用
| 化合物编号 | Alo | Ave | Lol | Set | Pan | Sor | Dig | Ech | Bra |
| 化合物A | 2 | 4 | 1 | 2 | 1 | 4 | 4 | 5 | 3 |
| 1.02 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 1 | 1 |
实施例B2:植物苗后的除草作用(苗后作用):
于温室条件下,在塑料盆的标准土壤中种植单子叶量为和双子叶杂草。在3-6叶期给实验植物施用实验物质。试验物质的施用量为500g活性物质/公顷。施用形式是水悬浮液(由25%可湿性粉剂(ExampleF3,b)制备)或乳液(由25%乳油(Example F1,c)制备)(500升水/公顷)。评价在施用三周后进行,用9个等级评价(1=全部毁坏,9=无作用)。等级1-4(特别是1-3)表明除草活性好至非常好。
试验植物是:看麦娘属(Alo),燕麦属(Ave),黑麦草属(Lol),狗尾草属(Set),黍属(Pan),高粱属(Sor),马唐属(Dig),稗属(Ech)和臂形草属(Bra)。
表B2:苗后作用:
施用量为250g活性成分/公顷时的苗后作用
| 化合物编号 | Alo | Ave | Lol | Set | Pan | Sor | Dig | Ech | Bra |
| 化合物A | 3 | 3 | 2 | 2 | 1 | 3 | 2 | 1 | 2 |
| 1.02 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
将化合物A与本发明化合物No.102进行比较可以看出,出人意料的,虽然化合物No.102与化合物A的不同仅仅在于两个乙基被甲基所代替,但是它对所有的试验杂草的除草活性均有显著提高。
实施例B3:本发明化合物在植物发芽前的除草活性(芽前作用):
在塑料盆的标准土壤中种植单子叶和双子叶杂草。种植后立即把实验物质以水悬浮液(由25%可湿性粉剂(Example F3,b)制备)或乳液(由25%乳油(Example F1,c)制备)的形式施用(500升水/公顷)。施用量为500g活性物质/公顷。然后试验植物在温室中于最佳条件下生长。评价在施用三周后进行,用9个等级评价(1=全部毁坏,9=无作用)。等级1-4(特别是1-3)表明除草活性好至非常好。试验植物是:燕麦属(Ave)、黑麦草属(Lol)、狗尾草属(Set)。
表B3:芽前作用:在浓度为0.7%重量的喷雾混合物中用MERGE作为 油添加剂:
| 试验植物: | |||
| 化合物编号 | Ave | Lol | Set |
| 1.01 | 1 | 1 | 1 |
| 1.02 | 1 | 1 | 1 |
| 1.31 | 1 | 1 | 2 |
| 1.35 | 1 | 1 | 1 |
当式I化合物被加工制成实施例F2和F4-F8的制剂时,得到同样的结果。
实施例B4:本发明化合物的植物苗后除草活性(苗后作用)(如实施例 B2所述):
试验植物:燕麦属(Ave),黑麦草属(Lol),狗尾草属(Set)。结果见下表4。
表B4:苗后作用:在浓度为0.7%重量的喷雾混合物中用MERGF作为
油添加剂。
| 实验植物: | |||
| 化合物编号 | Ave | Lol | Set |
| 1.01 | 1 | 1 | 1 |
| 1.02 | 1 | 1 | 1 |
| 1.04 | 1 | 1 | 1 |
| 1.05 | 1 | 3 | 1 |
| 1.07 | 1 | 1 | 1 |
| 1.08 | 1 | 1 | 1 |
| 1.10 | 1 | 1 | 1 |
| 1.11 | 1 | 1 | 1 |
| 1.14 | 1 | 2 | 2 |
| 1.15 | 1 | 2 | 1 |
| 1.17 | 1 | 1 | 2 |
| 1.19 | 1 | 1 | 1 |
| 1.21 | 1 | 1 | 1 |
| 实验植物: | |||
| 化合物编号 | Ave | Lol | Set |
| 1.23 | 1 | 1 | 1 |
| 1.26 | 1 | 2 | 1 |
| 1.27 | 1 | 1 | 2 |
| 1.30 | 1 | 1 | 1 |
| 1.31 | 1 | 1 | 1 |
| 1.35 | 1 | 1 | 1 |
| 1.37 | 1 | 1 | 1 |
| 1.39 | 1 | 1 | 1 |
| 1.40 | 1 | 1 | 2 |
| 1.43 | 1 | 2 | 2 |
当式I化合物被加工制成实施例F2和F4-F8的制剂时,得到同样的结果。
Claims (8)
1.下式I化合物,或这些化合物的农用盐、异构体和对映异构体:
其中
R1和R3各自相互独立地是乙基、C1-C2烷氧基;
Q是下述基团:
R4和R5与它们所键合的原子一起形成5-至7-元环的基团,该环可含有稠合或螺-键合的由2-6个碳原子组成的亚烷基或亚链烯基链,该链又可含有一个或二个氧原子,其中所述的环基团可以被下述基团取代:卤素、C3-C6环烷基、羟基、C1-C6烷氧基、C1-C6烷氧基-C1-C6烷氧基;
R6是C1-C10烷基;
R7是C1-C10烷基;
R8是氢、C1-C10烷基;或
R6和R7与它们所键合的原子一起形成饱和的3-至7-元环基团,该环基团可含有一个或二个选自氮、氧的杂原子;或R6和R8与它们所键合的原子一起形成5-至7-元环基团;
G1、G2各自相互独立地是氢、-C(X1)-R20;
X1是氧或硫;
R20是C1-C10烷基。
2.制备权利要求1式I化合物的方法,该方法包括使式XXX化合物:
Q-H (XXX)
其中Q是Q1、Q2,除G1、G2以外,它们的取代基具有上述权利要求1中所述定义,G1、G2是氢,
与下式XXXI化合物进行反应:
其中R1和R3如上述权利要求1中所述定义,和Hal是氯、溴或碘,反应在惰性溶剂、碱和钯催化剂存在下于30-250℃下进行。
3.一种除草和抑制植物生长的组合物,该组合物含有除草有效量的式I化合物以及惰性载体。
4.一种控制不需要的植物生长的方法,该方法包括向所述的植物或其场所施用除草有效量的式I的活性成分,或含有这些活性成分的组合物。
5.一种抑制植物生长的方法,该方法包括向所述的植物或其场所施用除草有效量的式I的活性成分,或含有这些活性成分的组合物。
7.权利要求3的组合物,其含有喷雾桶混助剂。
8.权利要求6的组合物,其含有喷雾桶混助剂。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH1642/1999 | 1999-09-07 | ||
| CH164299 | 1999-09-07 | ||
| CH1642/99 | 1999-09-07 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1514829A CN1514829A (zh) | 2004-07-21 |
| CN1272324C true CN1272324C (zh) | 2006-08-30 |
Family
ID=4215237
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN00813428.6A Expired - Fee Related CN1272324C (zh) | 1999-09-07 | 2000-09-05 | 新颖的除草剂 |
Country Status (14)
| Country | Link |
|---|---|
| US (3) | US6894005B1 (zh) |
| EP (2) | EP1210333B1 (zh) |
| CN (1) | CN1272324C (zh) |
| AT (2) | ATE282597T1 (zh) |
| AU (1) | AU767356B2 (zh) |
| CA (1) | CA2382435C (zh) |
| DE (2) | DE50008690D1 (zh) |
| DK (1) | DK1481970T3 (zh) |
| ES (2) | ES2259425T3 (zh) |
| HU (1) | HU229958B1 (zh) |
| PL (1) | PL202547B1 (zh) |
| PT (2) | PT1210333E (zh) |
| RU (1) | RU2269537C2 (zh) |
| WO (1) | WO2001017972A2 (zh) |
Families Citing this family (126)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE60006547T2 (de) * | 1999-09-07 | 2004-09-23 | Syngenta Participations Ag | Herbizide zusammensetzungen |
| DE50008690D1 (de) * | 1999-09-07 | 2004-12-23 | Syngenta Participations Ag | p-Tolyl-Heterocyclen als Herbizide |
| AU762346B2 (en) | 1999-09-07 | 2003-06-26 | Syngenta Participations Ag | Herbicidal composition |
| DE10016544A1 (de) * | 2000-04-03 | 2001-10-11 | Bayer Ag | C2-phenylsubstituierte Ketoenole |
| DE10019145A1 (de) | 2000-04-18 | 2001-10-25 | Bayer Ag | Phenylsubstituierte 4-Hydroxy-tetrahydropyridone |
| DE10139465A1 (de) * | 2001-08-10 | 2003-02-20 | Bayer Cropscience Ag | Selektive Herbizide auf Basis von substituierten, cayclischen Ketoenolen und Safenern |
| US6821971B2 (en) * | 2001-09-20 | 2004-11-23 | The Procter & Gamble Company | Fused pyrazolone compounds which inhibit the release of inflammatory cytokines |
| PL207276B1 (pl) * | 2001-09-27 | 2010-11-30 | Syngenta Participations Ag | Kompozycja chwastobójcza oraz sposób selektywnego zwalczania chwastów i traw w uprawach roślin użytkowych |
| DE10160007A1 (de) | 2001-12-06 | 2003-06-18 | Bayer Cropscience Ag | [1.2]-Oxazin-3,5-dione |
| JP2005516973A (ja) * | 2002-01-22 | 2005-06-09 | シンジェンタ パーティシペーションズ アクチェンゲゼルシャフト | 除草剤として有用なフェニル置換ヘテロ環化合物 |
| DE50304232D1 (de) * | 2002-05-03 | 2006-08-24 | Basf Ag | Fungizide triazolopyrimidine, verfahren zu ihrer herstellung und ihre verwendung zur bek mpfung von schadpilzen sowie sie ent haltende mittel |
| DE10239479A1 (de) | 2002-08-28 | 2004-03-04 | Bayer Cropscience Ag | Substituierte spirocyclische Ketoenole |
| GB0230018D0 (en) | 2002-12-23 | 2003-01-29 | Syngenta Ltd | Fungicides |
| GB0230020D0 (en) | 2002-12-23 | 2003-01-29 | Syngenta Ltd | Fungicides |
| DE10301804A1 (de) * | 2003-01-20 | 2004-07-29 | Bayer Cropscience Ag | 2,4-Dihalogen-6-(C2-C3-alkyl)-phenyl substituierte Tetramsäure-Derivate |
| DE10311300A1 (de) | 2003-03-14 | 2004-09-23 | Bayer Cropscience Ag | 2,4,6-Phenylsubstituierte cyclische Ketoenole |
| DE10326386A1 (de) | 2003-06-12 | 2004-12-30 | Bayer Cropscience Ag | N-Heterocyclyl-phenylsubstituierte cyclische Ketoenole |
| DE10331675A1 (de) | 2003-07-14 | 2005-02-10 | Bayer Cropscience Ag | Hetarylsubstituierte Pyrazolidindion-Derivate |
| DE10337497A1 (de) | 2003-08-14 | 2005-03-10 | Bayer Cropscience Ag | 4-Biphenylsubstituierte-Pyrazolidin-3,5-dion-Derivate |
| DE10354628A1 (de) | 2003-11-22 | 2005-06-16 | Bayer Cropscience Ag | 2-Ethyl-4,6-dimethyl-phenyl-substituierte Tetramsäure-Derivate |
| DE10354629A1 (de) | 2003-11-22 | 2005-06-30 | Bayer Cropscience Ag | 2-Ethyl-4,6-dimethyl-phenyl substituierte spirocyclische Tetramsäure-Derivate |
| DE102004014620A1 (de) | 2004-03-25 | 2005-10-06 | Bayer Cropscience Ag | 2,4,6-phenylsubstituierte cyclische Ketoenole |
| DE102004030753A1 (de) | 2004-06-25 | 2006-01-19 | Bayer Cropscience Ag | 3'-Alkoxy spirocyclische Tetram- und Tretronsäuren |
| DE102004035133A1 (de) | 2004-07-20 | 2006-02-16 | Bayer Cropscience Ag | Selektive Insektizide auf Basis von substituierten, cyclischen Ketoenolen und Safenern |
| DE102004041529A1 (de) * | 2004-08-27 | 2006-03-02 | Bayer Cropscience Gmbh | Herbizid-Kombinationen mit speziellen Ketoenolen |
| DE102004044827A1 (de) | 2004-09-16 | 2006-03-23 | Bayer Cropscience Ag | Jod-phenylsubstituierte cyclische Ketoenole |
| DE102004053192A1 (de) * | 2004-11-04 | 2006-05-11 | Bayer Cropscience Ag | 2-Alkoxy-6-alkyl-phenyl substituierte spirocyclische Tetramsäure-Derivate |
| DE102004053191A1 (de) * | 2004-11-04 | 2006-05-11 | Bayer Cropscience Ag | 2,6-Diethyl-4-methyl-phenyl substituierte Tetramsäure-Derivate |
| DE102005008021A1 (de) * | 2005-02-22 | 2006-08-24 | Bayer Cropscience Ag | Spiroketal-substituierte cyclische Ketoenole |
| DE102005051325A1 (de) | 2005-10-27 | 2007-05-03 | Bayer Cropscience Ag | Alkoxyalkyl spirocyclische Tetram- und Tetronsäuren |
| EP1954699B1 (en) * | 2005-11-22 | 2012-09-19 | Kudos Pharmaceuticals Ltd | PYRIDO-, PYRAZO- AND PYRIMIDOPYRIMIDINE DERIVATIVES AS mTOR INHIBITORS |
| DE102005059469A1 (de) * | 2005-12-13 | 2007-06-14 | Bayer Cropscience Ag | Insektizide Zusammensetzungen mit verbesserter Wirkung |
| DE102005059891A1 (de) * | 2005-12-15 | 2007-06-28 | Bayer Cropscience Ag | 3'-Alkoxy-spirocyclopentyl substituierte Tetram- und Tetronsäuren |
| DE102006000971A1 (de) * | 2006-01-07 | 2007-07-12 | Bayer Cropscience Ag | 2,4,6-Trialkylphenylsubstituierte Cyclopentan-1,3-dione |
| DE102006007882A1 (de) | 2006-02-21 | 2007-08-30 | Bayer Cropscience Ag | Cycloalkyl-phenylsubstituierte cyclische Ketoenole |
| DE102006014653A1 (de) * | 2006-03-28 | 2007-10-04 | Bayer Cropscience Ag | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten durch Angiessen, Tröpfchenapplikation oder Bodeninjektion |
| DE102006018828A1 (de) | 2006-04-22 | 2007-10-25 | Bayer Cropscience Ag | Alkoxyalkyl-substituierte cyclische Ketoenole |
| DE102006022821A1 (de) | 2006-05-12 | 2007-11-15 | Bayer Cropscience Ag | Verwendung von Tetramsäurederivaten zur Bekämpfung von Insekten aus der Ordnung der Käfer (Coleoptera), Thrips (Tysanoptera), Wanzen (Hemiptera), Fliegen (Diptera) und Zikaden (Auchenorrhynchae) |
| DE102006025874A1 (de) | 2006-06-02 | 2007-12-06 | Bayer Cropscience Ag | Alkoxyalkyl-substituierte cyclische Ketoenole |
| DE102006027732A1 (de) | 2006-06-16 | 2008-01-10 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| DE102006027731A1 (de) | 2006-06-16 | 2007-12-20 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| DE102006027730A1 (de) * | 2006-06-16 | 2007-12-20 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| DE102006033154A1 (de) | 2006-07-18 | 2008-01-24 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| RS55881B1 (sr) | 2006-08-23 | 2017-08-31 | Kudos Pharm Ltd | 2-metilmorfolin pirido-,pirazo- i pirimido-pirimidin derivati kao inhibitori mtor-a |
| DE102006050148A1 (de) * | 2006-10-25 | 2008-04-30 | Bayer Cropscience Ag | Trifluormethoxy-phenylsubstituierte Tetramsäure-Derivate |
| GB0621440D0 (en) | 2006-10-27 | 2006-12-06 | Syngenta Crop Protection Ag | Herbicidal compositions |
| DE102006057037A1 (de) | 2006-12-04 | 2008-06-05 | Bayer Cropscience Ag | cis-Alkoxyspirocyclische biphenylsubstituierte Tetramsäure-Derivate |
| DE102006057036A1 (de) | 2006-12-04 | 2008-06-05 | Bayer Cropscience Ag | Biphenylsubstituierte spirocyclische Ketoenole |
| GB0624961D0 (en) * | 2006-12-14 | 2007-01-24 | Syngenta Ltd | Novel herbicides |
| US8680012B2 (en) | 2006-12-14 | 2014-03-25 | Syngenta Crop Protection Llc | 4-phenyl-pyrane-3,5-diones,4-phenyl-thiopyrane-3,6-diones and cyclohexanetriones as novel herbicides |
| DE102007009957A1 (de) * | 2006-12-27 | 2008-07-03 | Bayer Cropscience Ag | Verfahren zur verbesserten Nutzung des Produktionsptentials transgener Pflanzen |
| DE102007001866A1 (de) | 2007-01-12 | 2008-07-17 | Bayer Cropscience Ag | Spirocyclische Tetronsäure-Derivate |
| GB0704652D0 (en) * | 2007-03-09 | 2007-04-18 | Syngenta Participations Ag | Novel herbicides |
| GB0710223D0 (en) | 2007-05-29 | 2007-07-11 | Syngenta Ltd | Novel Herbicides |
| GB0712653D0 (en) | 2007-06-28 | 2007-08-08 | Syngenta Ltd | Novel herbicides |
| EP2014169A1 (de) | 2007-07-09 | 2009-01-14 | Bayer CropScience AG | Wasserlösliche Konzentrate von 3-(2-Alkoxy-4-chlor-6-alkyl-phenyl)-substituierten Tetramaten und ihren korrespondierenden Enolen |
| GB0714981D0 (en) * | 2007-08-01 | 2007-09-12 | Syngenta Ltd | Novel herbicides |
| EP2020413A1 (de) | 2007-08-02 | 2009-02-04 | Bayer CropScience AG | Oxaspirocyclische-spiro-substituierte Tetram- und Tetronsäure-Derivate |
| GB0715454D0 (en) | 2007-08-08 | 2007-09-19 | Syngenta Ltd | Novel herbicides |
| GB0715576D0 (en) | 2007-08-09 | 2007-09-19 | Syngenta Ltd | Novel herbicides |
| GB0717082D0 (en) * | 2007-09-03 | 2007-10-10 | Syngenta Ltd | Novel herbicides |
| EP2039248A1 (de) * | 2007-09-21 | 2009-03-25 | Bayer CropScience AG | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| EP2045240A1 (de) | 2007-09-25 | 2009-04-08 | Bayer CropScience AG | Halogenalkoxyspirocyclische Tetram- und Tetronsäure-Derivate |
| US20100304967A1 (en) * | 2007-11-01 | 2010-12-02 | Syngenta Crop Protection, Inc. | Method of protecting rice crops |
| WO2009074314A1 (en) * | 2007-12-13 | 2009-06-18 | Syngenta Limited | 4-phenylpyrane-3,5-diones, 4-phenylthiopyrane-3,5-diones and 2-phenylcyclohexane-1,3,5-triones as herbicides |
| EP2103615A1 (de) | 2008-03-19 | 2009-09-23 | Bayer CropScience AG | 4'4'-Dioxaspiro-spirocyclisch substituierte Tetramate |
| CN101980601A (zh) * | 2008-03-27 | 2011-02-23 | 拜耳作物科学公司 | 季酮酸衍生物通过地面喷洒、滴灌施用或浸涂施用于抗昆虫和红蜘蛛螨的应用 |
| EP2113172A1 (de) * | 2008-04-28 | 2009-11-04 | Bayer CropScience AG | Verfahren zur verbesserten Nutzung des Produktionspotentials transgener Pflanzen |
| EP2127522A1 (de) | 2008-05-29 | 2009-12-02 | Bayer CropScience AG | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| GB0810728D0 (en) | 2008-06-11 | 2008-07-16 | Syngenta Ltd | Novel herbicides |
| GB0810815D0 (en) * | 2008-06-12 | 2008-07-23 | Syngenta Ltd | Novel herbicides |
| UY31917A (es) | 2008-06-20 | 2010-01-29 | Astrazeneca Ab | Compuestos de pirido-pirimidina inhibidores de mtor, composiciones farmaceuticas que los comprenden, procesos para su preparación y su uso como medicamento. |
| GB0812310D0 (en) | 2008-07-03 | 2008-08-13 | Syngenta Ltd | Novel herbicides |
| US8367873B2 (en) * | 2008-10-10 | 2013-02-05 | Bayer Cropscience Ag | Phenyl-substituted bicyclooctane-1,3-dione derivatives |
| GB0819205D0 (en) | 2008-10-20 | 2008-11-26 | Syngenta Ltd | Novel herbicides |
| TW201031327A (en) | 2008-11-14 | 2010-09-01 | Bayer Cropscience Ag | Active compound combinations having insecticidal and acaricidal properties |
| GB0821167D0 (en) * | 2008-11-19 | 2008-12-24 | Syngenta Ltd | Novel herbicides |
| US8846946B2 (en) | 2008-12-02 | 2014-09-30 | Bayer Cropscience Ag | Germinal alkoxy/alkylspirocyclic substituted tetramate derivatives |
| US8389443B2 (en) | 2008-12-02 | 2013-03-05 | Bayer Cropscience Ag | Geminal alkoxy/alkylspirocyclic substituted tetramate derivatives |
| GB0822834D0 (en) | 2008-12-15 | 2009-01-21 | Syngenta Ltd | Novel herbicides |
| GB0900641D0 (en) | 2009-01-15 | 2009-02-25 | Syngenta Ltd | Novel herbicides |
| GB0901086D0 (en) | 2009-01-22 | 2009-03-11 | Syngenta Ltd | Novel herbicides |
| AR075126A1 (es) * | 2009-01-29 | 2011-03-09 | Bayer Cropscience Ag | Metodo para el mejor uso del potencial de produccion de plantas transgenicas |
| GB0901834D0 (en) | 2009-02-04 | 2009-03-11 | Syngenta Ltd | Novel herbicides |
| GB0901835D0 (en) | 2009-02-04 | 2009-03-11 | Syngenta Ltd | Novel herbicides |
| BRPI1008949B1 (pt) | 2009-03-11 | 2018-07-10 | Bayer Intellectual Property Gmbh | Cetoenóis haloalquilmetilenóxi-fenil-substituídos e seu uso, composição, seu uso e seu método de produção, métodos para combate de pestes animais e/ou crescimento de plantas indesejadas |
| JP5892927B2 (ja) * | 2009-05-19 | 2016-03-23 | バイエル・インテレクチュアル・プロパティ・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツングBayer Intellectual Property GmbH | 除草活性を有するスピロヘテロ環式テトロン酸誘導体 |
| GB0912385D0 (en) | 2009-07-16 | 2009-08-26 | Syngenta Ltd | Novel herbicides |
| DE102009028001A1 (de) | 2009-07-24 | 2011-01-27 | Bayer Cropscience Ag | Wirkstoffkombinationen mit insektiziden und akariziden Eigenschaften |
| US20130137573A1 (en) * | 2009-12-17 | 2013-05-30 | Syngenta Limited | Herbicidal compositions comprising, and methods of use of, herbicidally active pyrandiones |
| JP6151917B2 (ja) | 2010-02-10 | 2017-06-21 | バイエル・インテレクチュアル・プロパティ・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツングBayer Intellectual Property GmbH | スピロヘテロ環置換テトラミン酸誘導体 |
| ES2700996T3 (es) * | 2010-02-10 | 2019-02-20 | Bayer Cropscience Ag | Cetoenoles cíclicos sustituidos con bifenilo |
| DE102010008643A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft, 13353 | Zyklische Ketoenole zur Therapie |
| DE102010008644A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft, 13353 | Zyklische Ketoenole zur Therapie |
| DE102010008642A1 (de) | 2010-02-15 | 2011-08-18 | Bayer Schering Pharma Aktiengesellschaft, 13353 | Zyklische Ketoenole zur Therapie |
| CN102939007B (zh) | 2010-04-20 | 2015-09-02 | 拜耳知识产权有限责任公司 | 基于螺杂环取代的特特拉姆酸衍生物的具有改善活性的杀虫和/或除草组合物 |
| CA2798253A1 (en) | 2010-05-31 | 2011-12-08 | Syngenta Participations Ag | Pesticidal compositions |
| BR112013010810A2 (pt) | 2010-11-02 | 2016-08-09 | Bayer Ip Gmbh | derivados de fenil-substituído bicicloocteno-1, 3-diona. |
| MX343856B (es) | 2011-01-25 | 2016-11-25 | Bayer Ip Gmbh | Procedimiento para la preparación de derivados 1-h-pirrolidin-2,4-diona. |
| DE102011011040A1 (de) | 2011-02-08 | 2012-08-09 | Bayer Pharma Aktiengesellschaft | (5s,8s)-3-(4'-Chlor-3'-fluor-4-methylbiphenyl-3-yl)-4-hydroxy-8-methoxy-1-azaspiro[4.5]dec-3-en-2-on (Verbindung A) zur Therapie |
| DE102011080405A1 (de) | 2011-08-04 | 2013-02-07 | Bayer Pharma AG | Substituierte 3-(Biphenyl-3-yl)-8,8-difluor-4-hydroxy-1-azaspiro[4.5]dec-3-en-2-one zur Therapie |
| EP2675788A1 (de) | 2011-02-17 | 2013-12-25 | Bayer Intellectual Property GmbH | Substituierte 3-(biphenyl-3-yl)-8,8-difluor-4-hydroxy-1-azaspiro[4.5]dec-3-en-2-one zur therapie |
| CA2828639C (en) | 2011-03-01 | 2019-02-12 | Bayer Intellectual Property Gmbh | 2-acyloxypyrrolin-4-ones |
| CN103607895B (zh) | 2011-05-30 | 2017-04-12 | 住友化学株式会社 | 环己酮化合物和包含该化合物的除草剂 |
| AR087008A1 (es) * | 2011-06-22 | 2014-02-05 | Syngenta Participations Ag | Derivados de n-oxi-pirazolo-triazepina-diona |
| DE102011080406A1 (de) | 2011-08-04 | 2013-02-07 | Bayer Pharma AG | Substituierte 3-(Biphenyl-3-yl)-4-hydroxy-8-methoxy-1-azaspiro8[4.5]dec-3-en-2-one |
| CN103857661B (zh) | 2011-08-11 | 2016-11-16 | 拜耳知识产权有限责任公司 | 1,2,4‑三唑基取代的酮烯醇 |
| BR112014012420A2 (pt) * | 2011-12-07 | 2017-06-06 | Akzo Nobel Chemicals Int Bv | formulação herbicida e método para prover proteção herbicida a uma cultura agrícola |
| UA113753C2 (xx) | 2012-01-26 | 2017-03-10 | Фенілзаміщені кетоеноли для боротьби з паразитами риб | |
| RU2015125583A (ru) | 2012-11-28 | 2017-01-11 | Сумитомо Кемикал Компани, Лимитед | Соединения дигидропирона и содержащие их гербициды |
| JP2015205821A (ja) | 2012-11-30 | 2015-11-19 | 住友化学株式会社 | シクロヘキサノン化合物及びそれを含有する除草剤 |
| RS57084B1 (sr) * | 2012-12-21 | 2018-06-29 | Syngenta Ltd | Herbicidno aktivna ciklična dion jedinjenja, ili njihovi derivati, supstituisana sa fenil grupom koja ima supstituent koji sadrži alkinil grupu |
| EA028524B1 (ru) | 2013-04-19 | 2017-11-30 | Зингента Лимитед | Гербицидно активные соединения 2-(замещенный фенил)циклопентан-1,3-диона и их производные |
| GB201310047D0 (en) | 2013-06-05 | 2013-07-17 | Syngenta Ltd | Compounds |
| CN103626704B (zh) * | 2013-11-10 | 2015-09-09 | 上海师范大学 | 1-取代烯基吡唑化合物及制备方法 |
| EP4053097A1 (en) | 2014-12-18 | 2022-09-07 | Corteva Agriscience LLC | Ketone compound as herbicide |
| CN112159321A (zh) * | 2015-06-22 | 2021-01-01 | 拜耳作物科学股份公司 | 新的炔基取代的3-苯基吡咯烷-2,4-二酮及其作为除草剂的用途 |
| JP6837068B2 (ja) | 2016-01-15 | 2021-03-03 | バイエル・クロップサイエンス・アクチェンゲゼルシャフト | 置換された2−アリール−エタノール類の製造方法 |
| RU2611815C1 (ru) * | 2016-03-14 | 2017-03-01 | Общество с ограниченной ответственностью "СоюзХим КО" | Адъювант для увеличения эффективности пестицидов |
| WO2019110613A1 (en) * | 2017-12-05 | 2019-06-13 | Syngenta Participations Ag | Chemical process for the synthesis of herbicidal pyrazolidinedione compounds |
| EP3772938A1 (de) | 2018-04-13 | 2021-02-17 | Bayer CropScience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von tierischen schädlingen durch angiessen oder tröpfchenapplikation |
| WO2019197617A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von tierischen schädlingen durch angiessen, tröpfchenapplikation. pflanzlochbehandlung oder furchenapplikation |
| CN112312768A (zh) | 2018-04-13 | 2021-02-02 | 拜耳作物科学股份公司 | 特特拉姆酸衍生物用于防治特定昆虫的用途 |
| WO2019197652A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Aktiengesellschaft | Feststoff-formulierung insektizider mischungen |
| WO2019197620A1 (de) | 2018-04-13 | 2019-10-17 | Bayer Cropscience Aktiengesellschaft | Verwendung von tetramsäurederivaten zur bekämpfung von speziellen insekten |
| CN108864144B (zh) * | 2018-06-13 | 2021-01-05 | 兰州精细化工高新技术开发公司 | 一种唑啉草酯的合成方法 |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4834908A (en) | 1987-10-05 | 1989-05-30 | Basf Corporation | Antagonism defeating crop oil concentrates |
| DE3939503A1 (de) | 1989-11-30 | 1991-06-06 | Hoechst Ag | Neue pyrazoline zum schutz von kulturpflanzen gegenueber herbiziden |
| DE4109208A1 (de) * | 1991-03-21 | 1992-09-24 | Bayer Ag | 3-hydroxy-4-aryl-5-oxo-pyrazolin-derivate |
| US5358924A (en) | 1991-03-21 | 1994-10-25 | Bayer Aktiengesellschaft | 3-hydroxy-4-aryl-5-oxo-pyrozoline derivatives, compositions and use |
| BR9407006A (pt) * | 1993-07-05 | 1996-08-06 | Bayer Ag | Heterociclos aril cetoenólicos substituidos |
| DE4331448A1 (de) | 1993-09-16 | 1995-03-23 | Hoechst Schering Agrevo Gmbh | Substituierte Isoxazoline, Verfahren zu deren Herstellung, diese enthaltende Mittel und deren Verwendung als Safener |
| JPH10507189A (ja) | 1994-10-17 | 1998-07-14 | ノバルティス アクチエンゲゼルシャフト | 除草剤組成物 |
| CN1175248A (zh) | 1995-01-13 | 1998-03-04 | 诺瓦提斯公司 | 具有农药性能的4-芳基-和4-杂芳基-5-氧代吡唑啉衍生物 |
| AU4715896A (en) * | 1995-02-13 | 1996-09-04 | Bayer Aktiengesellschaft | 2-phenyl-substituted heterocyclic 1,3-ketonols as herbicides and pesticides |
| DE19728568B4 (de) | 1996-07-17 | 2007-06-14 | Bayer Cropscience Ag | Herbizide Mittel auf Basis von (5-Trifluormethyl-1,3,4-thiadiazol-2-yl-oxy)-essigsäure-N-isopropyl-N-(4fluorphenyl)-amid |
| DE69707907T2 (de) | 1996-09-26 | 2002-05-16 | Syngenta Participations Ag, Basel | Herbizid wirkende zusammensetzung |
| DE19742951A1 (de) | 1997-09-29 | 1999-04-15 | Hoechst Schering Agrevo Gmbh | Acylsulfamoylbenzoesäureamide, diese enthaltende nutzpflanzenschützende Mittel und Verfahren zu ihrer Herstellung |
| DK1062217T3 (da) | 1998-03-13 | 2003-10-27 | Syngenta Participations Ag | Herbicidt aktive 3-hydroxy-4-aryl-5-oxopyrazolinderivater |
| DE19853827A1 (de) | 1998-11-21 | 2000-05-25 | Aventis Cropscience Gmbh | Kombinationen aus Herbiziden und Safenern |
| WO2000047585A1 (en) | 1999-02-11 | 2000-08-17 | Novartis Ag | 3-hydroxy-4-aryl-5-pyrazoline derivatives as herbicides |
| IL146337A0 (en) | 1999-06-16 | 2002-07-25 | Syngenta Participations Ag | Substituted arylmalonic acid dinitriles as intermediates for the preparation of herbicides |
| DE50008690D1 (de) | 1999-09-07 | 2004-12-23 | Syngenta Participations Ag | p-Tolyl-Heterocyclen als Herbizide |
| EP1209973B1 (de) | 1999-09-07 | 2004-02-25 | Syngenta Participations AG | Herbizides mittel |
| DE60006547T2 (de) | 1999-09-07 | 2004-09-23 | Syngenta Participations Ag | Herbizide zusammensetzungen |
| AU762346B2 (en) | 1999-09-07 | 2003-06-26 | Syngenta Participations Ag | Herbicidal composition |
| WO2003067984A1 (en) | 2002-02-13 | 2003-08-21 | Syngenta Participations Ag | Herbicidal composition |
-
2000
- 2000-09-05 DE DE50008690T patent/DE50008690D1/de not_active Expired - Lifetime
- 2000-09-05 WO PCT/EP2000/008656 patent/WO2001017972A2/de active IP Right Grant
- 2000-09-05 EP EP00965923A patent/EP1210333B1/de not_active Expired - Lifetime
- 2000-09-05 PT PT00965923T patent/PT1210333E/pt unknown
- 2000-09-05 ES ES04013876T patent/ES2259425T3/es not_active Expired - Lifetime
- 2000-09-05 US US10/070,767 patent/US6894005B1/en not_active Expired - Lifetime
- 2000-09-05 AT AT00965923T patent/ATE282597T1/de active
- 2000-09-05 EP EP04013876A patent/EP1481970B1/de not_active Expired - Lifetime
- 2000-09-05 ES ES00965923T patent/ES2233451T3/es not_active Expired - Lifetime
- 2000-09-05 PT PT04013876T patent/PT1481970E/pt unknown
- 2000-09-05 AT AT04013876T patent/ATE321029T1/de active
- 2000-09-05 HU HU0202573A patent/HU229958B1/hu not_active IP Right Cessation
- 2000-09-05 DE DE50012459T patent/DE50012459D1/de not_active Expired - Lifetime
- 2000-09-05 CN CN00813428.6A patent/CN1272324C/zh not_active Expired - Fee Related
- 2000-09-05 DK DK04013876T patent/DK1481970T3/da active
- 2000-09-05 PL PL354453A patent/PL202547B1/pl unknown
- 2000-09-05 AU AU76503/00A patent/AU767356B2/en not_active Ceased
- 2000-09-05 CA CA002382435A patent/CA2382435C/en not_active Expired - Fee Related
- 2000-09-05 RU RU2002107612/04A patent/RU2269537C2/ru not_active IP Right Cessation
-
2005
- 2005-03-18 US US11/083,415 patent/US7605111B2/en not_active Expired - Fee Related
- 2005-03-18 US US11/083,465 patent/US7459414B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| US20050164883A1 (en) | 2005-07-28 |
| DK1481970T3 (da) | 2006-07-31 |
| ATE282597T1 (de) | 2004-12-15 |
| HU229958B1 (hu) | 2015-03-30 |
| US20050187110A1 (en) | 2005-08-25 |
| AU767356B2 (en) | 2003-11-06 |
| HUP0202573A2 (hu) | 2002-11-28 |
| EP1481970B1 (de) | 2006-03-22 |
| ES2259425T3 (es) | 2006-10-01 |
| US7459414B2 (en) | 2008-12-02 |
| PT1481970E (pt) | 2006-07-31 |
| EP1210333A2 (de) | 2002-06-05 |
| PL354453A1 (en) | 2004-01-12 |
| EP1210333B1 (de) | 2004-11-17 |
| US7605111B2 (en) | 2009-10-20 |
| HU0202573D0 (zh) | 2002-09-28 |
| DE50008690D1 (de) | 2004-12-23 |
| US6894005B1 (en) | 2005-05-17 |
| AU7650300A (en) | 2001-04-10 |
| ATE321029T1 (de) | 2006-04-15 |
| PL202547B1 (pl) | 2009-07-31 |
| HUP0202573A3 (en) | 2002-12-28 |
| ES2233451T3 (es) | 2005-06-16 |
| CA2382435C (en) | 2009-02-03 |
| CA2382435A1 (en) | 2001-03-15 |
| CN1514829A (zh) | 2004-07-21 |
| WO2001017972A2 (de) | 2001-03-15 |
| WO2001017972A3 (de) | 2001-09-27 |
| PT1210333E (pt) | 2005-03-31 |
| DE50012459D1 (de) | 2006-05-11 |
| RU2269537C2 (ru) | 2006-02-10 |
| EP1481970A1 (de) | 2004-12-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1272324C (zh) | 新颖的除草剂 | |
| CN1177532C (zh) | 除草组合物 | |
| CN1185234C (zh) | 具有除草活性的3-羟基-4-芳基-5-氧代吡唑啉衍生物 | |
| CN1615310A (zh) | 可用作除草剂的苯基取代的杂环化合物 | |
| CN1158280C (zh) | 取代的噻吩-3-基磺酰基氨基(硫代)羰基三唑啉(硫)酮类化合物 | |
| CN1027030C (zh) | 含稠合杂环化合物的除草剂及生产方法和用途 | |
| CN1039808A (zh) | 新型除草剂 | |
| CN1064957C (zh) | 用作杀微生物剂的n-磺酰和n-亚磺酰氨基酸酰胺 | |
| CN1064862A (zh) | 具有除草、杀螨和杀虫活性的新化合物 | |
| CN1091738A (zh) | 取代的三唑啉酮 | |
| CN1038643A (zh) | 带杂环的2-烷氧苯氧基硫酰脲类和它们作为除草剂或植物生长调节剂的应用 | |
| CN1058776A (zh) | 农药 | |
| CN1074441A (zh) | 柑桔、油棕、橡胶及其它种植作物的除草剂 | |
| CN1086696C (zh) | 磺酰基氨基(硫代)羰基化合物 | |
| CN1146560C (zh) | 作为除草剂的取代的苯甲酰基吡唑类化合物 | |
| CN1028476C (zh) | 含有尿嘧啶衍生物的除草剂组合物 | |
| CN1048852A (zh) | 新的取代的α-嘧啶氧(硫)基-和α-三嗪氧(硫)基羧酸衍生物,它们的制备方法,以及它们作为除草剂,杀菌剂和植物生长调节剂的应用 | |
| CN1112922A (zh) | 羟氨基-苯磺酰脲类化合物,其制法及作为除草剂和植物生长调节剂的应用 | |
| CN1051287A (zh) | 增效除草剂 | |
| CN1053903C (zh) | 二氢苯并呋喃衍生物及其制备和用途 | |
| CN1195751C (zh) | 噻吩磺酰基化合物 | |
| CN1092770A (zh) | 取代的1-芳基三唑啉酮类化合物 | |
| CN1155311C (zh) | 除草增效组合物及防治杂草的方法 | |
| CN87100152A (zh) | 除草的化合物 | |
| CN1023317C (zh) | 取代的咪唑-5-羧酸衍生物的制备方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20060830 Termination date: 20170905 |