CN1252692A - Fungicide active substance combination - Google Patents
Fungicide active substance combination Download PDFInfo
- Publication number
- CN1252692A CN1252692A CN98804294A CN98804294A CN1252692A CN 1252692 A CN1252692 A CN 1252692A CN 98804294 A CN98804294 A CN 98804294A CN 98804294 A CN98804294 A CN 98804294A CN 1252692 A CN1252692 A CN 1252692A
- Authority
- CN
- China
- Prior art keywords
- general formula
- reactive compound
- weight ratio
- group reactive
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000013543 active substance Substances 0.000 title abstract description 4
- 230000000855 fungicidal effect Effects 0.000 title abstract description 3
- 239000000417 fungicide Substances 0.000 title abstract 2
- 239000000460 chlorine Substances 0.000 claims abstract description 4
- 229910052801 chlorine Inorganic materials 0.000 claims abstract description 4
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims abstract description 3
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 claims abstract description 3
- 229910052794 bromium Inorganic materials 0.000 claims abstract description 3
- 150000001875 compounds Chemical class 0.000 claims description 155
- 239000000203 mixture Substances 0.000 claims description 34
- 230000000844 anti-bacterial effect Effects 0.000 claims description 11
- -1 phosphorus compound Chemical class 0.000 claims description 11
- 238000000034 method Methods 0.000 claims description 6
- 150000003852 triazoles Chemical class 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 5
- 239000011701 zinc Substances 0.000 claims description 5
- 241000894006 Bacteria Species 0.000 claims description 4
- 241000233866 Fungi Species 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 4
- 239000001257 hydrogen Substances 0.000 claims description 4
- 229910052739 hydrogen Inorganic materials 0.000 claims description 4
- 229910052748 manganese Inorganic materials 0.000 claims description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 4
- 230000003641 microbiacidal effect Effects 0.000 claims description 4
- 229940124561 microbicide Drugs 0.000 claims description 4
- 239000002855 microbicide agent Substances 0.000 claims description 4
- JMANVNJQNLATNU-UHFFFAOYSA-N oxalonitrile Chemical compound N#CC#N JMANVNJQNLATNU-UHFFFAOYSA-N 0.000 claims description 4
- 229910052725 zinc Inorganic materials 0.000 claims description 4
- FLBBUIUSCHAKFI-UHFFFAOYSA-N 4-ethoxy-2h-triazole Chemical class CCOC1=CNN=N1 FLBBUIUSCHAKFI-UHFFFAOYSA-N 0.000 claims description 3
- 150000002357 guanidines Chemical class 0.000 claims description 3
- 229940083094 guanine derivative acting on arteriolar smooth muscle Drugs 0.000 claims description 3
- XERJKGMBORTKEO-VZUCSPMQSA-N (1e)-2-(ethylcarbamoylamino)-n-methoxy-2-oxoethanimidoyl cyanide Chemical compound CCNC(=O)NC(=O)C(\C#N)=N\OC XERJKGMBORTKEO-VZUCSPMQSA-N 0.000 claims description 2
- PXMNMQRDXWABCY-UHFFFAOYSA-N 1-(4-chlorophenyl)-4,4-dimethyl-3-(1H-1,2,4-triazol-1-ylmethyl)pentan-3-ol Chemical compound C1=NC=NN1CC(O)(C(C)(C)C)CCC1=CC=C(Cl)C=C1 PXMNMQRDXWABCY-UHFFFAOYSA-N 0.000 claims description 2
- WKBPZYKAUNRMKP-UHFFFAOYSA-N 1-[2-(2,4-dichlorophenyl)pentyl]1,2,4-triazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(CCC)CN1C=NC=N1 WKBPZYKAUNRMKP-UHFFFAOYSA-N 0.000 claims description 2
- IMSODMZESSGVBE-UHFFFAOYSA-N 2-Oxazoline Chemical compound C1CN=CO1 IMSODMZESSGVBE-UHFFFAOYSA-N 0.000 claims description 2
- AIDLAEPHWROGFI-UHFFFAOYSA-N 2-methylbenzene-1,3-dicarboxylic acid Chemical compound CC1=C(C(O)=O)C=CC=C1C(O)=O AIDLAEPHWROGFI-UHFFFAOYSA-N 0.000 claims description 2
- DHJSQJDJTIGMLO-UHFFFAOYSA-N CCC1(C(C1Cl)C)C(=O)N Chemical class CCC1(C(C1Cl)C)C(=O)N DHJSQJDJTIGMLO-UHFFFAOYSA-N 0.000 claims description 2
- 239000005756 Cymoxanil Substances 0.000 claims description 2
- 239000005784 Fluoxastrobin Substances 0.000 claims description 2
- 239000005789 Folpet Substances 0.000 claims description 2
- 239000005790 Fosetyl Substances 0.000 claims description 2
- 239000005906 Imidacloprid Substances 0.000 claims description 2
- 239000005807 Metalaxyl Substances 0.000 claims description 2
- 239000005813 Penconazole Substances 0.000 claims description 2
- 239000005839 Tebuconazole Substances 0.000 claims description 2
- GNVMUORYQLCPJZ-UHFFFAOYSA-M Thiocarbamate Chemical compound NC([S-])=O GNVMUORYQLCPJZ-UHFFFAOYSA-M 0.000 claims description 2
- UELITFHSCLAHKR-UHFFFAOYSA-N acibenzolar-S-methyl Chemical compound CSC(=O)C1=CC=CC2=C1SN=N2 UELITFHSCLAHKR-UHFFFAOYSA-N 0.000 claims description 2
- 150000001412 amines Chemical class 0.000 claims description 2
- OIPMQULDKWSNGX-UHFFFAOYSA-N bis[[ethoxy(oxo)phosphaniumyl]oxy]alumanyloxy-ethoxy-oxophosphanium Chemical compound [Al+3].CCO[P+]([O-])=O.CCO[P+]([O-])=O.CCO[P+]([O-])=O OIPMQULDKWSNGX-UHFFFAOYSA-N 0.000 claims description 2
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 2
- OBISXEJSEGNNKL-UHFFFAOYSA-N dinitrogen-n-sulfide Chemical class [N-]=[N+]=S OBISXEJSEGNNKL-UHFFFAOYSA-N 0.000 claims description 2
- VDLGAVXLJYLFDH-UHFFFAOYSA-N fenhexamid Chemical compound C=1C=C(O)C(Cl)=C(Cl)C=1NC(=O)C1(C)CCCCC1 VDLGAVXLJYLFDH-UHFFFAOYSA-N 0.000 claims description 2
- UFEODZBUAFNAEU-NLRVBDNBSA-N fluoxastrobin Chemical compound C=1C=CC=C(OC=2C(=C(OC=3C(=CC=CC=3)Cl)N=CN=2)F)C=1C(=N/OC)\C1=NOCCO1 UFEODZBUAFNAEU-NLRVBDNBSA-N 0.000 claims description 2
- HKIOYBQGHSTUDB-UHFFFAOYSA-N folpet Chemical compound C1=CC=C2C(=O)N(SC(Cl)(Cl)Cl)C(=O)C2=C1 HKIOYBQGHSTUDB-UHFFFAOYSA-N 0.000 claims description 2
- VUERQRKTYBIULR-UHFFFAOYSA-N fosetyl Chemical compound CCOP(O)=O VUERQRKTYBIULR-UHFFFAOYSA-N 0.000 claims description 2
- YWTYJOPNNQFBPC-UHFFFAOYSA-N imidacloprid Chemical compound [O-][N+](=O)\N=C1/NCCN1CC1=CC=C(Cl)N=C1 YWTYJOPNNQFBPC-UHFFFAOYSA-N 0.000 claims description 2
- 229940056881 imidacloprid Drugs 0.000 claims description 2
- 150000002466 imines Chemical class 0.000 claims description 2
- ZQEIXNIJLIKNTD-UHFFFAOYSA-N methyl N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alaninate Chemical compound COCC(=O)N(C(C)C(=O)OC)C1=C(C)C=CC=C1C ZQEIXNIJLIKNTD-UHFFFAOYSA-N 0.000 claims description 2
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 claims description 2
- 150000002780 morpholines Chemical class 0.000 claims description 2
- 150000002825 nitriles Chemical class 0.000 claims description 2
- 150000002923 oximes Chemical class 0.000 claims description 2
- 239000001301 oxygen Substances 0.000 claims description 2
- 229910052760 oxygen Inorganic materials 0.000 claims description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 2
- 229910052698 phosphorus Inorganic materials 0.000 claims description 2
- 239000011574 phosphorus Substances 0.000 claims description 2
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical class C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 claims description 2
- 229940083082 pyrimidine derivative acting on arteriolar smooth muscle Drugs 0.000 claims description 2
- 150000003230 pyrimidines Chemical class 0.000 claims description 2
- 239000004094 surface-active agent Substances 0.000 claims description 2
- MBBWTVUFIXOUBE-UHFFFAOYSA-L zinc;dicarbamodithioate Chemical compound [Zn+2].NC([S-])=S.NC([S-])=S MBBWTVUFIXOUBE-UHFFFAOYSA-L 0.000 claims description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims 1
- 125000001309 chloro group Chemical group Cl* 0.000 claims 1
- 229910052731 fluorine Inorganic materials 0.000 claims 1
- 239000011737 fluorine Substances 0.000 claims 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 abstract description 3
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 abstract 1
- 229910052736 halogen Inorganic materials 0.000 abstract 1
- 150000002367 halogens Chemical class 0.000 abstract 1
- 230000000857 drug effect Effects 0.000 description 16
- 241000233614 Phytophthora Species 0.000 description 15
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 14
- 240000003768 Solanum lycopersicum Species 0.000 description 14
- 239000003905 agrochemical Substances 0.000 description 12
- 241000196324 Embryophyta Species 0.000 description 9
- 238000009472 formulation Methods 0.000 description 9
- 239000000843 powder Substances 0.000 description 7
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical group CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 239000003995 emulsifying agent Substances 0.000 description 4
- 229910052500 inorganic mineral Inorganic materials 0.000 description 4
- 239000011707 mineral Substances 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000002689 soil Substances 0.000 description 4
- 230000002195 synergetic effect Effects 0.000 description 4
- PNEYBMLMFCGWSK-UHFFFAOYSA-N Alumina Chemical compound [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 241000233639 Pythium Species 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 235000019714 Triticale Nutrition 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 241000228158 x Triticosecale Species 0.000 description 3
- 239000004604 Blowing Agent Substances 0.000 description 2
- 229940126062 Compound A Drugs 0.000 description 2
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 2
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 2
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 2
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 2
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 2
- 239000003899 bactericide agent Substances 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 230000003203 everyday effect Effects 0.000 description 2
- 230000004907 flux Effects 0.000 description 2
- 244000000004 fungal plant pathogen Species 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000002363 herbicidal effect Effects 0.000 description 2
- 239000004009 herbicide Substances 0.000 description 2
- 238000005286 illumination Methods 0.000 description 2
- RONFGUROBZGJKP-UHFFFAOYSA-N iminoctadine Chemical compound NC(N)=NCCCCCCCCNCCCCCCCCN=C(N)N RONFGUROBZGJKP-UHFFFAOYSA-N 0.000 description 2
- 229940043265 methyl isobutyl ketone Drugs 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- QQVIHTHCMHWDBS-UHFFFAOYSA-N perisophthalic acid Natural products OC(=O)C1=CC=CC(C(O)=O)=C1 QQVIHTHCMHWDBS-UHFFFAOYSA-N 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 238000004659 sterilization and disinfection Methods 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 125000005323 thioketone group Chemical group 0.000 description 2
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 1
- WURBVZBTWMNKQT-UHFFFAOYSA-N 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-one Chemical compound C1=NC=NN1C(C(=O)C(C)(C)C)OC1=CC=C(Cl)C=C1 WURBVZBTWMNKQT-UHFFFAOYSA-N 0.000 description 1
- VGPIBGGRCVEHQZ-UHFFFAOYSA-N 1-(biphenyl-4-yloxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-ol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)OC(C=C1)=CC=C1C1=CC=CC=C1 VGPIBGGRCVEHQZ-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- CFWRDBDJAOHXSH-SECBINFHSA-N 2-azaniumylethyl [(2r)-2,3-diacetyloxypropyl] phosphate Chemical compound CC(=O)OC[C@@H](OC(C)=O)COP(O)(=O)OCCN CFWRDBDJAOHXSH-SECBINFHSA-N 0.000 description 1
- 125000001340 2-chloroethyl group Chemical class [H]C([H])(Cl)C([H])([H])* 0.000 description 1
- QUTGXAIWZAMYEM-UHFFFAOYSA-N 2-cyclopentyloxyethanamine Chemical compound NCCOC1CCCC1 QUTGXAIWZAMYEM-UHFFFAOYSA-N 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- RGCKGOZRHPZPFP-UHFFFAOYSA-N Alizarin Natural products C1=CC=C2C(=O)C3=C(O)C(O)=CC=C3C(=O)C2=C1 RGCKGOZRHPZPFP-UHFFFAOYSA-N 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 241000235349 Ascomycota Species 0.000 description 1
- 241000221198 Basidiomycota Species 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 241001465180 Botrytis Species 0.000 description 1
- 229910021532 Calcite Inorganic materials 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 241000760356 Chytridiomycetes Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 241000221785 Erysiphales Species 0.000 description 1
- 241000221787 Erysiphe Species 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 241000192128 Gammaproteobacteria Species 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 239000005802 Mancozeb Substances 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical class CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- 241000233654 Oomycetes Species 0.000 description 1
- 241000233855 Orchidaceae Species 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- 241000233626 Plasmopara Species 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 108010009736 Protein Hydrolysates Proteins 0.000 description 1
- 241001281805 Pseudoperonospora cubensis Species 0.000 description 1
- 241000221300 Puccinia Species 0.000 description 1
- 239000005828 Pyrimethanil Substances 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- 239000004113 Sepiolite Substances 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- 239000005846 Triadimenol Substances 0.000 description 1
- 241000510929 Uncinula Species 0.000 description 1
- 235000009754 Vitis X bourquina Nutrition 0.000 description 1
- 235000012333 Vitis X labruscana Nutrition 0.000 description 1
- 240000006365 Vitis vinifera Species 0.000 description 1
- 235000014787 Vitis vinifera Nutrition 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000642 acaricide Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 238000004026 adhesive bonding Methods 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- HFVAFDPGUJEFBQ-UHFFFAOYSA-M alizarin red S Chemical compound [Na+].O=C1C2=CC=CC=C2C(=O)C2=C1C=C(S([O-])(=O)=O)C(O)=C2O HFVAFDPGUJEFBQ-UHFFFAOYSA-M 0.000 description 1
- 125000002877 alkyl aryl group Chemical group 0.000 description 1
- 150000008051 alkyl sulfates Chemical class 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 239000011717 all-trans-retinol Substances 0.000 description 1
- 235000019169 all-trans-retinol Nutrition 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 239000012874 anionic emulsifier Substances 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 239000000987 azo dye Substances 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 238000009264 composting Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 239000006184 cosolvent Substances 0.000 description 1
- 230000000881 depressing effect Effects 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- WURGXGVFSMYFCG-UHFFFAOYSA-N dichlofluanid Chemical compound CN(C)S(=O)(=O)N(SC(F)(Cl)Cl)C1=CC=CC=C1 WURGXGVFSMYFCG-UHFFFAOYSA-N 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- FPAFDBFIGPHWGO-UHFFFAOYSA-N dioxosilane;oxomagnesium;hydrate Chemical compound O.[Mg]=O.[Mg]=O.[Mg]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O FPAFDBFIGPHWGO-UHFFFAOYSA-N 0.000 description 1
- 239000010459 dolomite Substances 0.000 description 1
- 229910000514 dolomite Inorganic materials 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000003337 fertilizer Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 150000002460 imidazoles Chemical class 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 239000001023 inorganic pigment Substances 0.000 description 1
- 239000002917 insecticide Substances 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- 229950007687 macrogol ester Drugs 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- YKSNLCVSTHTHJA-UHFFFAOYSA-L maneb Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S YKSNLCVSTHTHJA-UHFFFAOYSA-L 0.000 description 1
- 229920000940 maneb Polymers 0.000 description 1
- 239000004579 marble Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 239000011785 micronutrient Substances 0.000 description 1
- 235000013369 micronutrients Nutrition 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- 230000002371 mycocidal effect Effects 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen(.) Chemical compound [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000012875 nonionic emulsifier Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical compound N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 239000005648 plant growth regulator Substances 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920000151 polyglycol Polymers 0.000 description 1
- 239000010695 polyglycol Substances 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 239000003531 protein hydrolysate Substances 0.000 description 1
- ZLIBICFPKPWGIZ-UHFFFAOYSA-N pyrimethanil Chemical compound CC1=CC(C)=NC(NC=2C=CC=CC=2)=N1 ZLIBICFPKPWGIZ-UHFFFAOYSA-N 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 239000010499 rapseed oil Substances 0.000 description 1
- 239000011435 rock Substances 0.000 description 1
- 230000011218 segmentation Effects 0.000 description 1
- 229910052624 sepiolite Inorganic materials 0.000 description 1
- 235000019355 sepiolite Nutrition 0.000 description 1
- 238000007873 sieving Methods 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- YZHUMGUJCQRKBT-UHFFFAOYSA-M sodium chlorate Chemical compound [Na+].[O-]Cl(=O)=O YZHUMGUJCQRKBT-UHFFFAOYSA-M 0.000 description 1
- 235000014347 soups Nutrition 0.000 description 1
- 238000009331 sowing Methods 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 229940042055 systemic antimycotics triazole derivative Drugs 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- HYVWIQDYBVKITD-UHFFFAOYSA-N tolylfluanid Chemical compound CN(C)S(=O)(=O)N(SC(F)(Cl)Cl)C1=CC=C(C)C=C1 HYVWIQDYBVKITD-UHFFFAOYSA-N 0.000 description 1
- BAZVSMNPJJMILC-UHFFFAOYSA-N triadimenol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)OC1=CC=C(Cl)C=C1 BAZVSMNPJJMILC-UHFFFAOYSA-N 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/90—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having two or more relevant hetero rings, condensed among themselves or with a common carbocyclic ring system
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
The new active substance combinations of an halogen benzimidazol of formula (I), in which Z stands for chlorine or bromine, and of the groups of active substances (1 to 25) listed in the description have very good fungicide properties.
Description
The present invention relates to the composition of novel reactive compound, it contains known Halobenzimidazoles and their use as microbicide and other known mycocidal reactive compound, and this composition is suitable for preventing and treating plant pathogenic fungi very much.
The known 1-(3 of people; 5-dimethyl-isoxazoles-4-sulfonyl) 2-bromo-6; 6-two fluoro-[1; 3] oxa--[4-two; 5f]-benzimidazole and 1-(3,5-dimethyl-isoxazole-4-sulfonyl)-2-chloro-6,6-two fluoro-[1; 3]-two oxa--[4,5f]-benzimidazole has sterilization idiocratic (referring to WO 97-06171).The activity of these compounds is good, and is when hanging down consumption, satisfied inadequately in some cases.
And, the also known many triazole derivatives of people, anil, imidodicarbonic diamide and other heterocycle can be used for preventing and treating fungi, and (referring to EP-A 0 040 345, DE-A 2 201 063, DE-A2324010, the agricultural chemicals handbook, 9 editions (1991), p.249 with 827, EP-A382 375 and EP-A 0 515 901).Similarly, when hanging down consumption, the activity of these compounds often is unsatisfied.
At last, also known 1-[(6-chloro-3-pyridine radicals)-methyl]-N-nitro-2-imidazoline imines can be used for preventing and treating for example insect (referring to, agricultural chemicals handbook, 9 editions (1991) p.491) of animal pest., do not find the Fungicidally active of these compounds so far as yet.
Have been found that the halo benzo that contains general formula (I) now
Imidazoles, wherein Z represent chlorine or bromine and
Wherein
X represent chlorine or phenyl and
R
1Represent hydrogen or methyl, and/or
(4) N-[1-of general formula (V) (4-oxygen phenyl)-ethyl]-2,2-two chloro-1-ethyl-3-methyl-cyclopropane carboxamides
And/or
(5) trimethylene-1 of general formula (VI), two (zinc dithiocarbamate) (propinebs) of 2-
N>=1 and/or
Me=Zn or Mn, or the mixture of Zn and Mn and/or
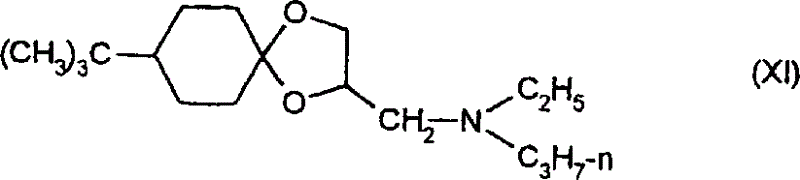
(10) the 8-tertiary butyl-2-of general formula (XI) (N-ethyl-N-n-pro-pyl amido) methyl isophthalic acid, the 4-dioxy spiral shell-luxuriant amine of [5,4]-decane (Luo Evil)
And/or
(12) compound of general formula (XIII) (imines bacterium)
And/or
(14) the cyanogen 9 oxime derivate (cymoxanil) of general formula (XV)
And/or
R
2Represent methylidene or cyclopropyl, and/or
(17) morpholine derivative (dimetomorph) of general formula (XVIII)
And/or
(21) the methyl 1-[(6-chloro-3-pyridine radicals of general formula (XXII))]-N-nitro-2-imidazoline imines (Imidacloprid)
And/or
(22) general formula (XXIII) De oxazoline derovatives (azolactone bacterium)
And/or
(23) heterocyclic carbamate derivatives of general formula (XXIV)
And/or
(24) guanidine derivatives of general formula (XXV)
Wherein
The integer of m representative from 0 to 5 and
(25) triazole derivative (penconazole) of general formula (XXVI)
The active compound combinations of novelty have the good sterilization characteristic.
Surprised is, more much higher than the active summation of each reactive compound according to the bactericidal activity of active compound combinations of the present invention.Therefore, have unexpected, real synergistic effect, and be not only active addition.
General formula (I) comprises 1-(3,5-dimethyl isoxazole-4-sulfonyl)-2-bromo-6,6-two fluoro-[1,3]-two oxa-[4,5f]-benzimidazole, general formula (Ia)
And 1-(3,5-dimethyl isoxazole-4-sulfonyl)-2-chloro-6,6-two fluoro-[1,3]-two oxa--[4,5f]-benzimidazole, general formula (Ib)
General formula (Ia) and Halobenzimidazoles and their use as microbicide (Ib) all are known (referring to WO 97-06171).
General formula (II) comprises the compound 1-(4-chlorophenoxy)-3 of general formula (IIa), 3-dimethyl-1-(1,2, the 4-triazol-1-yl) fourth-2-ketone (triadimefon)
The compound 1-of general formula (IIb) (4-chlorophenoxy)-3,3-dimethyl-1-(1,2, the 4-triazol-1-yl)-Ding-2-alcohol (Triadimenol)
And the compound 1-of general formula (IIc) (4-phenyl phenoxy group)-3,3-dimethyl-1-(1,2, the 4-triazol-1-yl)-Ding-2-alcohol (Bitertanol)
General formula (IV) comprises general formula (IVa) (Euparen)
And general formula (IVb) (tolyfluanid)
Anil.
From obviously visible this compound of the structural formula of the reactive compound of general formula (V) 3 asymmetric substituted carbon atoms are arranged.Therefore this product can be used as various mixture of isomers existence, or exists with the form of single component.
Particularly preferred this compound is general formula (Va)
N-(R)-[1-(4-chlorphenyl)-ethyl]-(1S)-2,2-two chloro-1-ethyls-3t-methyl isophthalic acid r-cyclopropane acid amides
And the N-(R) of general formula (Vb)-[1-(4-chlorphenyl)-ethyl]-(1R)-2,2-two chloro-1-ethyls-3t-methyl isophthalic acid r-cyclopropane acid amides.
General formula (VII) comprises compound
(VIIa) Me=Zn (zineb)
(VIIb) Me=Mn (maneb) and
(VIIc) mixture (mancozeb) (VIIa) and (VIIb).
General formula (XVI) comprises compound
(XVIa) R
2=CH
3(pyrimethanil) and
The ethoxy triazole derivative of general formula (XXI) can general formula (XXIa) " thioketones formula " exist
Or exist with tautomeric " thiol " of general formula (XXIb)
For simplicity, only provide " thioketones formula " in each case
The guanidine derivatives of general formula (XXV) is the mixture of the material of common name guazatine (guazatine).
Being present in the component in the active compound combinations of the present invention, except the Halobenzimidazoles and their use as microbicide of general formula (I), also is known.These reactive compounds have been done introduction especially in following publication:
(1) general formula (II) compound
DE-A?2?201?063
DE-A?2?324?010
(2) general formula (III) compound
EP-A?0?040?345
(3) general formula (IV) compound
The agricultural chemicals handbook, 9 editions [1991] are p.249 with 827
(4) compound of general formula (V) and each derivative thereof
EP-A?0?341?475
(5) compound of general formula (VI)
The agricultural chemicals handbook, 9 editions (1991), p.726.
(6) compound of general formula (VII)
The agricultural chemicals handbook, 9 editions (1991), p.529,531 and 866
(7) compound of general formula (VIII)
EP-A?0?339?418
(8) compound of general formula (IX)
EP-A?0?472?996
(9) compound of general formula (X)
EP-A?0?313?512
(10) compound of general formula (XI)
EP-A?0?281?842
(11) compound of general formula (XII)
EP-A?0?382?375
(12) compound of general formula (XIII)
EP-A?0?515?901
(13) compound of general formula (XIV)
DE-A?196?02?095
(14) compound of general formula (XV)
The agricultural chemicals handbook, 9 editions (1991), p.206
(15) compound of general formula (XVI)
EP-A?0?270?111
EP-A?0?310?550
(16) compound of general formula (XVII)
The agricultural chemicals handbook, 9 editions (1991), p.554
(17) compound of general formula (XVIII)
EP-A?0?219?756
(18) compound of general formula (XIX)
The agricultural chemicals handbook, 9 editions (1991), p.431
(19) compound of general formula (XX)
The agricultural chemicals handbook, 9 editions (1991), p.443
(20) compound of general formula (XXI)
WO?96-16048
(21) compound of general formula (XXII)
The agricultural chemicals handbook, 9 editions (1991), p.491
(22) compound of general formula (XXIII)
EP-A?0?393?911
(23) compound of general formula (XXIV)
EP-A?0?600?629
(24) material of general formula (XXV)
The agricultural chemicals handbook, 9 editions (1991), the p.461 compound of (25) general formula (XXVI)
The agricultural chemicals handbook, 9 editions (1991), p.654
Except the reactive compound of general formula (I), contain a kind of compound (I) group reactive compound to (25) group at least according to active compound combinations of the present invention.In addition, they can contain more bactericidal active constituent.
When the reactive compound in active compound combinations according to the present invention existed with certain weight ratio, synergistic effect was obvious especially., in active compound combinations, the weight ratio of these reactive compounds can change in quite wide scope.Generally, for 0.1~20 part of weight meter of general formula (I) reactive compound use (1) group reactive compound of every part of weight meter, preferred 0.2~10 part of weight meter,
(2) 0.1~20 part of weight meter of group reactive compound, preferred 0.2~10 part of weight meter,
(3) 1~150 part of weight meter of group reactive compound, preferred 1~100 part of weight meter,
(4) 0.1~10 part of weight meter of group reactive compound, preferred 0.2~5 part of weight meter,
(5) 1~150 part of weight meter of group reactive compound, preferred 5~100 parts of weight meters,
(6) 1~150 part of weight meter of group reactive compound, preferred 5~100 parts of weight meters,
(7) 0.1~50 part of weight meter of group reactive compound, preferred 1~20 part of weight meter,
(8) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(9) 0.02~50 part of weight meter of group reactive compound, preferred 0.1~10 part of weight meter,
(10) 0.1~20 part of weight meter of group reactive compound, preferred 0.5~10 part of weight meter,
(11) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(12) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(13) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(14) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(15) 0.2~50 part of weight meter of group reactive compound, preferred 1~20 part of weight meter,
(16) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(17) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(18) 1~150 part of weight meter of group reactive compound, preferred 5~100 parts of weight meters,
(19) 0.1~150 part of weight meter of group reactive compound, preferred 1~100 part of weight meter,
(20) 0.02~50 part of weight meter of group reactive compound, preferred 0.2~10 part of weight meter,
(21) 0.05~20 part of weight meter of group reactive compound, preferred 0.1~10 part of weight meter,
(22) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(23) 0.1~50 part of weight meter of group reactive compound, preferred 0.2~20 part of weight meter,
(24) group reactive compound 0.02~50 part of weight meter, preferred 0.04~10 part of weight meter and/or
(25) 0.2~50 part of weight meter of group reactive compound, preferred 1~20 part of weight meter.
Active compound combinations according to the present invention has excellent bactericidal activity, can be used for preventing and treating plant pathogenic fungi, knee [gluing] Gammaproteobacteria for example, Oomycete, Chytridiomycetes, Zygomycetes, Ascomycetes, Basidiomycetes, deuteromycetes etc.
Active compound combinations according to the present invention is particularly suitable for preventing and treating for example Erysiphe of cereal disease, Puccinia and sickle spore [mould] belong to, the disease of control grape is Uncinula for example, Plasmopara and Botrytis, and the disease-causing organism of in dicotyledonous crops, preventing and treating powdery mildew and Pseudoperonospora cubensis and tikka.
Based under the required concentration of controlling plant diseases, plant has the fact of good tolerability to active compound combinations, can carry out the acrial part of plant and handle breeding rhizome and seed treatment and soil treatment.Composition according to reactive compound of the present invention can be made foliage applying, or makes seed pelleting.
Can be processed into conventional formulation according to active compound combinations of the present invention, solution for example, emulsion, suspending agent, pulvis, foaming agent, paste, granule, aerosol, the coated composition that microcapsule formulations in polymeric material and seed are used, and ULV agent (ultra-low-volume formulation).
These formulations can be produced by known method, for example by mixed active compound or active compound combinations and spreading agent, it is liquid flux, add the liquefied gas of depressing, and/or solid carrier, also can select to use surfactant arbitrarily, i.e. emulsifier and/or dispersant, and/or blowing agent.If used spreading agent is a water, for example also may use organic solvent as cosolvent.Suitable liquid flux consists essentially of: aromatic hydrocarbons is dimethylbenzene for example, toluene or Fluhyzon, chlorinated aromatic hydrocarbons or chlorinated aliphatic hydrocarbon be chlorobenzene for example, chloroethanes or carrene, aliphatic hydrocarbon is cyclohexane or paraffin for example, petroleum cuts for example, alcohol is butanols or ethylene glycol and their ether or ester for example, and ketone is acetone for example, MEK, methyl iso-butyl ketone (MIBK) or cyclohexanone, intensive polar solvent be dimethyl formamide and dimethyl sulfoxide (DMSO) for example, or water.The gas expanded agent or the carrier of liquefaction can be regarded as nominal liquid, and it is a gas under room temperature and atmospheric pressure, for example aerosol propellant such as butane, propane, nitrogen and carbonic acid gas.Suitable solid carrier is: the natural minerals that for example grinds is kaolin for example, clay, and talcum powder, chalk, quartz, Attagel, montmorillonite or diatomite, and the synthetic mineral that grinds is as silica, aluminium oxide and the silicate of segmentation.The suitable solid carrier that granule is used is: the natural rock such as the calcite of for example crushing and sieving, marble, float stone, sepiolite and dolomite, or the particle of synthetic inorganic and organic powder, and organic substance such as sawdust, cocoa shell, the particle of corncob and tobacco rods.Suitable emulsifier and/or blowing agent are: for example non-ionic and anionic emulsifier, and as polyoxyethylene fatty acid ester, polyoxyethylene aliphatic alcohol ether, alkylaryl macrogol ester for example, alkylsulfonate, alkyl sulfate, arylsulphonate, or protein hydrolysate.Suitable dispersant is: for example lignin, sulfite waste liquor and methylcellulose.
Thickener is carboxymethyl cellulose and with powder for example; natural with the synthetic polymer that particle or emulsion form exist, as gum Arabic, polyvinyl alcohol and polyvinylacetate; or natural phosphatide such as lecithin and cephalin, and synthetic phosphatide all can use in formulation.Other additive can be Dormant oils and rape oil.
It is possible as inorganic pigment using pigment, iron oxide for example, titanium oxide and Prussia orchid, and organic dyestuff, alizarin dyes for example, azo dyes and metal phthalocyanine dyestuff, and micronutrient such as iron, magnesium, boron, copper, cobalt, molybdenum and zinc salt.
Various formulations generally contain the reactive compound of 0.1~95% weight meter, and preferred 0.5~90%.
In various formulations, active compound combinations according to the present invention can be used as and other known reactive compound such as bactericide, insecticide, and the mixture of miticide and weed killer herbicide occurs, and also can be used as the mixture appearance with fertilizer or plant growth regulator.
Active compound combinations can be used as their dosage form itself or uses as the type of service for preparing thus, as the solution that can directly use, and missible oil, emulsion, suspending agent, wetting powder, soluble powder and granule.They can be applied by the method for routine, for example by irrigating spraying, dense fog, spreading is dialled and to be spilt, and the powder of handling as dry seeds, as the solution of seed treatment, as the water solube powder of seed treatment, the water solube powder of handling as slurries, or use by dressing.
When using according to active compound combinations of the present invention, amount of application depends on the kind of using, and can change in wide relatively scope.When handling plant part, the amount of application of active compound combinations generally 0.1 and 10000g/ha between, preferably 10 and 1000g/ha between.When seed treatment, the amount of application of active compound combinations is generally between per kilogram seed 0.001 and 50g, between preferred per kilogram seed 0.01 and the 10g.When making soil treatment, the amount of application of active compound combinations generally 0.1 and 10000g/ha between, preferably 1 and 5000g/ha between.
From the obviously visible good bactericidal activity of following example according to active compound combinations of the present invention.And single reactive compound show weak bactericidal activity, the activity of composition surpassed active simply adding and.
When the bactericidal activity of active compound combinations surpasses the summation of activity of the reactive compound of using separately, there is the synergistic effect of bactericide certainly.
Prediction activity for the composition of two given reactive compounds can followingly calculate (referring to Colby, S.R., " synergy of the calculating of herbicidal composition and antagonistic effect ", weeds 15, (1967), 20-22).
If
X is the drug effect when using reactive compound A with amount of application mg/ha,
Y is the drug effect when using reactive compound B with amount of application ng/ha,
E is the drug effect when using reactive compound A and B with amount of application m and ng/ha, so
Drug effect is calculated with %.The 0%th, contrast drug effect accordingly, and 100% mean that infringement is not observed.
If actual bactericidal activity surpasses calculated value, so, the activity of composition adds, and that is to say that synergistic effect exists.In the case, actual observation to drug effect must be numerical value greater than the prediction drug effect of calculating from above-mentioned formula (E).
Following example explanation the present invention.
Example 1
Phytophthora test (tomato)/protection
Solvent: the acetone of 47 parts of weight meters
Emulsifier: the alkylaryl polyglycol ether of 3 parts of weight meters
In order to obtain a kind of appropriate formulations of reactive compound, the reactive compound or the active compound combinations of 1 part of weight meter are mixed mutually with the solvent and the emulsifier of said quantity, and water is diluted to desired concn with dense preparation, or water is diluted to desired concn with the formulation of commercially available reactive compound or active compound combinations.
Active in order to test protection, by said amount of application, with the immature plant of active agent preparations spraying.After the soup drying of spraying, infect former spore fluid suspension inoculation plant with Phytophthora, then plant is placed in the bacterial culture cabinet of about 20 ℃ and 100% relative air humidity and hatches.
Inoculate and estimate after 3 days.0% means the drug effect corresponding to contrast, and 100% mean that infringement is not observed.
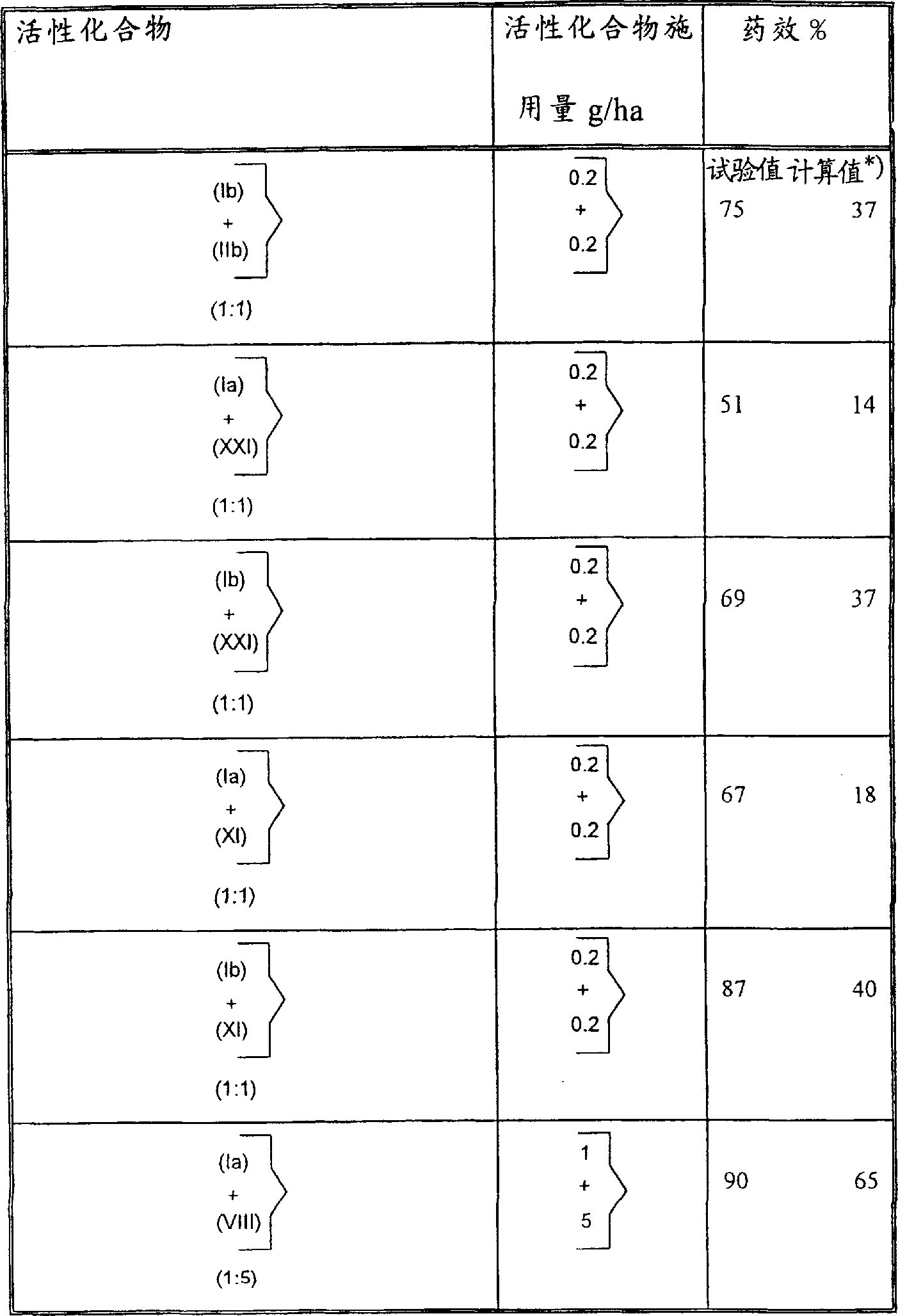
Reactive compound is during amount of application and result of the test are listed in the table below.Table 1 Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
Table 1 (continuing) Phytophthora test (tomato)/protection
The drug effect that the drug effect calculated value of test value=test=employing Coiby formula is calculated
Example 2
Avenge rotten sickle spore test (triticale)/seed treatment
Reactive compound is used as the dry seeds seed coating medicine.Their method for making is that each reactive compound or active compound combinations are scattered in the mixture that obtains fine-powdered in the levigate mineral, and this mixture can guarantee to be evenly distributed in the surface of the seed.
For capsuled seed, seed that will be infected and coating agent for seed shook in airtight glass flask 3 minutes together.
The sowing of 2 * 100 triticales in the standard soil of dark 1cm, and in the greenhouse of about 10 ℃ and relative air humidity about 95%, can be received in every day on the seed trays of illumination in 15 hours and cultivated.
After planting about 3 weeks, the symptom of evaluation plant.0% means the drug effect corresponding to contrast, and 100% drug effect means not infect and observes.
Reactive compound is during application chamber and result of the test are listed in the table below.Table 2 snow rotten sickle spore (triticale)/seed treatment
| Reactive compound | The reactive compound amount of application, the mg/kg seed | Drug effect % |
| Known (Ia) (Ib) (XXII) (IX) (VIIc) (IVb) | ?100 ?500 ?100 ?100 ?500 ?100 ?100 | ?26 ?0 ?0 ?0 ?0 ?0 ?3 |
| According to composition of the present invention, (Ia+VIIc), (1: 1), (Ib+XXII), (1: 1), (Ib+IX), (1: 1), (Ib+VIIc), (1: 1), (Ib+IVb), (1: 1) | ?50+50 ?50+50 ?250+250 ?50+50 ?50+50 | ?66 ?36 ?43 ?32 ?75 |
Example 3
Pythium test (pea)/seed treatment
Reactive compound is used as the dry seeds seed coating medicine.Their method for making is that each reactive compound or active compound combinations are scattered in the mixture that obtains fine-powdered in the mineral that grind, and this mixture can guarantee to be evenly distributed on the surface of the seed.
For capsuled seed, the seed that will infect shakes 3 fens kinds with coating agent for seed in airtight glass flask.
The natural infection of 2 * 50 planting seeds at dark 2cm had in the composting soil of pythium spp, and in about 20 ℃ greenhouse, on 15 hours seed trays of illumination every day, cultivate.
Estimate after 14 days.0% means the drug effect corresponding to contrast, and 100% drug effect means that infringement is not observed.
Reactive compound is during amount of application and result of the test are listed in the table below.Table 3 pythium test (pea)/seed treatment
| Reactive compound | Reactive compound is used application rate in amount mg/kg seed | Drug effect % |
| (Ib) (VIIc) (IVb) for known (Ia) | ?500 ?1000 ?1000 ?1000 ?500 | ?1 ?4 ?8 ?42 ?37 |
| According to (Ib+VIIc) (1: 1) (Ib+IVb) (1: 1) of mixture of the present invention (Ia+IVb) (1: 1) | ?250+250 ?500+500 ?500+500 | ?55 ?38 ?59 |
Claims (5)
1. bactericidal composition is characterized in that they contain a kind of active compound combinations, and it consists of the Halobenzimidazoles and their use as microbicide derivative of general formula (I)
Wherein
Z represent fluorine or bromine and
(1) triazole derivative of general formula (II)
Wherein
(3) anil of general formula (IV)
Wherein
R
1Represent hydrogen or methyl, and/or
(4) N-[1-of general formula (V) (4-oxygen phenyl)-ethyl]-2,2-two chloro-1-ethyl-3-methyl-cyclopropane carboxamides
And/or
(5) trimethylene-1 of general formula (VI), two (zinc dithiocarbamate) (propinebs) of 2-
The thiocarbamate of n>=1 and/or (6) at least a general formula (VII)
Me=Zn or Mn, or the mixture of Zn and Mn and/or
(9) diazosulfide derivative (bendicar) of general formula (X)
And/or
(10) the 8-tertiary butyl-2-of general formula (XI) (N-ethyl-N-n-pro-pyl amido) methyl isophthalic acid, the 4-dioxy spiral shell-luxuriant amine of [5,4]-decane (Luo Evil)
And/or
(11) compound of general formula (XII) (nitrile Fluoxastrobin)
And/or
(15) pyrimidine derivatives of general formula (XVI)
Wherein
R
2Represent methylidene or cyclopropyl, and/or
(21) the methyl 1-[(6-chloro-3-pyridine radicals of general formula (XXII))]-N-nitro-2-imidazoline imines (Imidacloprid)
And/or
(22) general formula (XXIII) De oxazoline derovatives (azolactone bacterium)
And/or
The integer of m representative from 0 to 5 and
2. according to the composition of claim 1, it is characterized in that in active compound combinations, the weight ratio of the reactive compound of general formula (I) and (1) group reactive compound is between 1: 0.1 and 1: 20, and (2) weight ratio of group reactive compound is between 1: 0.1 and 1: 20, and (3) weight ratio of group reactive compound is between 1: 1 and 1: 150, and (4) weight ratio of group reactive compound is between 1: 0.1 and 1: 10, and (5) weight ratio of group reactive compound is between 1: 1 and 1: 150, and (6) weight ratio of group reactive compound is between 1: 1 and 1: 150, and (7) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (8) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (9) weight ratio of group reactive compound is between 1: 0.02 and 1: 50, and (10) weight ratio of group reactive compound is between 1: 0.1 and 1: 20, and (11) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (12) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (13) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (14) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (15) weight ratio of group reactive compound is between 1: 0.2 and 1: 50, and (16) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (17) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (18) weight ratio of group reactive compound is between 1: 1 and 1: 150, and (19) weight ratio of group reactive compound is between 1: 0.1 and 1: 150, and (20) weight ratio of group reactive compound is between 1: 0.02 and 1: 50, and (21) weight ratio of group reactive compound is between 1: 0.05 and 1: 20, and (22) weight ratio of group reactive compound is between 1: 0.1 and 1: 50, and (23) group reactive compound weight ratio be between 1: 0.1 and 1: 50, and (24) group reactive compound weight ratio be between 1: 0.02 and 1: 50 and and (25) group reactive compound weight ratio be between 1: 0.2 and 1: 50.
3. the method for control fungi is characterized in that the active compound combinations according to claim 1 is applied to fungi and/or their habitat.
According to the active compound combinations of claim 1 in the application aspect the control fungi.
5. the method for preparing bactericidal composition is characterized in that the active compound combinations according to claim 1 is mixed mutually with spreading agent and/or surfactant.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19716256.8 | 1997-04-18 | ||
| DE19716256A DE19716256A1 (en) | 1997-04-18 | 1997-04-18 | Fungicidal active ingredient combinations |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1252692A true CN1252692A (en) | 2000-05-10 |
Family
ID=7826915
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN98804294A Pending CN1252692A (en) | 1997-04-18 | 1998-04-06 | Fungicide active substance combination |
Country Status (19)
| Country | Link |
|---|---|
| EP (1) | EP0975221A1 (en) |
| JP (1) | JP2001505924A (en) |
| KR (1) | KR20010006064A (en) |
| CN (1) | CN1252692A (en) |
| AU (1) | AU727180B2 (en) |
| BG (1) | BG103789A (en) |
| BR (1) | BR9809763A (en) |
| CA (1) | CA2286849A1 (en) |
| CO (1) | CO5040019A1 (en) |
| DE (1) | DE19716256A1 (en) |
| HU (1) | HUP0002361A3 (en) |
| ID (1) | ID24677A (en) |
| IL (1) | IL131901A0 (en) |
| NZ (1) | NZ500368A (en) |
| PL (1) | PL336225A1 (en) |
| TR (1) | TR199902450T2 (en) |
| TW (1) | TW385232B (en) |
| WO (1) | WO1998047370A1 (en) |
| ZA (1) | ZA983235B (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103875690A (en) * | 2014-03-14 | 2014-06-25 | 曹荣成 | Propineb and metalaxyl-M compound bactericidal composition |
| CN105475394A (en) * | 2015-12-27 | 2016-04-13 | 胡凡营 | Biological bactericide containing propineb and metalaxyl-M |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| UA70327C2 (en) * | 1998-06-08 | 2004-10-15 | Баєр Акціенгезельшафт | Method of combating phytopathogenic diseases on crop plants and a fungicidal composition |
| DK1085810T3 (en) * | 1998-06-10 | 2006-05-15 | Bayer Cropscience Ag | Means to control plant harmful organisms |
| DE19956098A1 (en) * | 1999-11-22 | 2001-05-23 | Bayer Ag | Synergistic plant fungicide composition containing spiroxamine, quinoxyfen and imidacloprid, thiacloprid and/or thiamethoxam, especially effective against cereal diseases |
| DE10063046A1 (en) * | 2000-12-18 | 2002-06-20 | Basf Ag | Fungicidal mixtures |
| JP2004525880A (en) * | 2001-01-16 | 2004-08-26 | ビーエーエスエフ アクチェンゲゼルシャフト | Bactericidal mixture containing imidazole derivatives |
| JP2004521884A (en) * | 2001-01-16 | 2004-07-22 | ビーエーエスエフ アクチェンゲゼルシャフト | Germicidal mixture |
| WO2002056689A1 (en) * | 2001-01-18 | 2002-07-25 | Basf Aktiengesellschaft | Fungicidal mixtures comprising benzophenone and imidazole derivatives |
| SK286121B6 (en) | 2001-01-22 | 2008-04-07 | Basf Aktiengesellschaft | Fungicide mixtures, method for controlling pathogenic fungi and fungicide compounds |
| FR2832031A1 (en) * | 2001-11-14 | 2003-05-16 | Aventis Cropscience Sa | COMPOSITION FUNGICIDE BASED ON AT LEAST ONE PYRIDYLMETHYLBENZAMIDE DERIVATIVE AND AT LEAST ONE VALINAMIDE-TYPE DERIVATIVE |
| AU2003218790B2 (en) * | 2002-03-21 | 2010-03-25 | Basf Se | Fungicidal mixtures |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6020354A (en) * | 1995-08-10 | 2000-02-01 | Bayer Aktiengesellschaft | Halobenzimidazoles and their use as microbicides |
| DE19609060A1 (en) * | 1995-08-10 | 1997-02-13 | Bayer Ag | Halobenzimidazoles |
-
1997
- 1997-04-18 DE DE19716256A patent/DE19716256A1/en not_active Withdrawn
-
1998
- 1998-04-03 TW TW087105036A patent/TW385232B/en not_active IP Right Cessation
- 1998-04-06 HU HU0002361A patent/HUP0002361A3/en unknown
- 1998-04-06 WO PCT/EP1998/001987 patent/WO1998047370A1/en not_active Application Discontinuation
- 1998-04-06 EP EP98922648A patent/EP0975221A1/en not_active Withdrawn
- 1998-04-06 CA CA002286849A patent/CA2286849A1/en not_active Abandoned
- 1998-04-06 NZ NZ500368A patent/NZ500368A/en unknown
- 1998-04-06 CN CN98804294A patent/CN1252692A/en active Pending
- 1998-04-06 BR BR9809763-6A patent/BR9809763A/en not_active IP Right Cessation
- 1998-04-06 ID IDW991228A patent/ID24677A/en unknown
- 1998-04-06 PL PL98336225A patent/PL336225A1/en unknown
- 1998-04-06 JP JP54492398A patent/JP2001505924A/en not_active Ceased
- 1998-04-06 TR TR1999/02450T patent/TR199902450T2/en unknown
- 1998-04-06 AU AU75221/98A patent/AU727180B2/en not_active Ceased
- 1998-04-06 KR KR1019997009142A patent/KR20010006064A/en not_active Application Discontinuation
- 1998-04-06 IL IL13190198A patent/IL131901A0/en unknown
- 1998-04-16 CO CO98020958A patent/CO5040019A1/en unknown
- 1998-04-17 ZA ZA983235A patent/ZA983235B/en unknown
-
1999
- 1999-10-08 BG BG103789A patent/BG103789A/en active Pending
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103875690A (en) * | 2014-03-14 | 2014-06-25 | 曹荣成 | Propineb and metalaxyl-M compound bactericidal composition |
| CN105475394A (en) * | 2015-12-27 | 2016-04-13 | 胡凡营 | Biological bactericide containing propineb and metalaxyl-M |
Also Published As
| Publication number | Publication date |
|---|---|
| PL336225A1 (en) | 2000-06-19 |
| ID24677A (en) | 2000-07-27 |
| CO5040019A1 (en) | 2001-05-29 |
| HUP0002361A3 (en) | 2002-02-28 |
| NZ500368A (en) | 2000-09-29 |
| ZA983235B (en) | 1998-10-22 |
| BR9809763A (en) | 2000-06-20 |
| DE19716256A1 (en) | 1998-10-22 |
| WO1998047370A1 (en) | 1998-10-29 |
| CA2286849A1 (en) | 1998-10-29 |
| BG103789A (en) | 2000-06-30 |
| IL131901A0 (en) | 2001-03-19 |
| AU727180B2 (en) | 2000-12-07 |
| JP2001505924A (en) | 2001-05-08 |
| KR20010006064A (en) | 2001-01-15 |
| EP0975221A1 (en) | 2000-02-02 |
| TR199902450T2 (en) | 2000-01-21 |
| HUP0002361A2 (en) | 2000-11-28 |
| AU7522198A (en) | 1998-11-13 |
| TW385232B (en) | 2000-03-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1102337C (en) | Fungicidal active compound combinations | |
| CN1112850C (en) | Fungicidal active substance combinations | |
| CN1131670C (en) | Antifungal active compound compsns. | |
| CN1070339C (en) | Fungicidal active compound combinations | |
| CN1159142A (en) | Pesticide | |
| CN1206916C (en) | Fungicide mixtures based on amide compounds and azoles | |
| CN1019260B (en) | Fungicidal agents containing quinazoline derivatives | |
| CN1078043C (en) | Combinations of a fungicide having an azole group with an insecticide having a pyrazole, pyrrole or phenylimidazole group | |
| CN1252692A (en) | Fungicide active substance combination | |
| CN1436044A (en) | Fungicidal active ingredient combinations | |
| CN1335062A (en) | Fungicide composition | |
| CN1244342A (en) | Fungicidal composition for rice crop and plant disease control method for rice crop | |
| CN1214717C (en) | Fungicidal mixture | |
| CN1124487A (en) | Carboxylic acid amide benzothiophene-S-oxides | |
| CN1163143C (en) | Fungicide mixture | |
| CN87107330A (en) | Seed disinfecting compositions | |
| CN1212767C (en) | Fungicide compositions | |
| CN1226929C (en) | Active fungicide composite | |
| CN1127751A (en) | Isothiazole derivatives and their uses | |
| CN1088556C (en) | Fungicidal active compound combinations | |
| CN1192711C (en) | Compound of fungicide and insecticide | |
| CN1711019A (en) | Fungicidal mixtures based on a triazolopyrimidine derivative and azoles | |
| CN1257670C (en) | Sterilization composition and application thereof | |
| CN1028344C (en) | Agricultural chemical compositions | |
| CN1695451A (en) | Fungicidal active compound mixture |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication | ||
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: WD Ref document number: 1026823 Country of ref document: HK |