CN1243385C - Manufacturing method of lithium ion battery positive electrode material - Google Patents
Manufacturing method of lithium ion battery positive electrode material Download PDFInfo
- Publication number
- CN1243385C CN1243385C CNB2004100216587A CN200410021658A CN1243385C CN 1243385 C CN1243385 C CN 1243385C CN B2004100216587 A CNB2004100216587 A CN B2004100216587A CN 200410021658 A CN200410021658 A CN 200410021658A CN 1243385 C CN1243385 C CN 1243385C
- Authority
- CN
- China
- Prior art keywords
- hours
- positive electrode
- ball milling
- lithium
- cooling
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 229910001416 lithium ion Inorganic materials 0.000 title claims abstract description 18
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 title claims abstract description 15
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 15
- 239000007774 positive electrode material Substances 0.000 title abstract 2
- 239000000463 material Substances 0.000 claims abstract description 32
- 238000000034 method Methods 0.000 claims abstract description 28
- 238000004137 mechanical activation Methods 0.000 claims abstract description 18
- 229910003002 lithium salt Inorganic materials 0.000 claims abstract description 7
- 159000000002 lithium salts Chemical class 0.000 claims abstract description 7
- 150000002696 manganese Chemical class 0.000 claims abstract description 7
- 238000002156 mixing Methods 0.000 claims abstract description 5
- 239000002994 raw material Substances 0.000 claims abstract description 5
- 238000012545 processing Methods 0.000 claims abstract description 4
- 238000000498 ball milling Methods 0.000 claims description 24
- 229910015645 LiMn Inorganic materials 0.000 claims description 22
- 238000001816 cooling Methods 0.000 claims description 22
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 16
- 239000011572 manganese Substances 0.000 claims description 15
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 claims description 10
- 230000004913 activation Effects 0.000 claims description 8
- 239000010405 anode material Substances 0.000 claims description 8
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 6
- 238000006243 chemical reaction Methods 0.000 claims description 6
- 239000003960 organic solvent Substances 0.000 claims description 6
- 238000005303 weighing Methods 0.000 claims description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 4
- IIPYXGDZVMZOAP-UHFFFAOYSA-N lithium nitrate Chemical compound [Li+].[O-][N+]([O-])=O IIPYXGDZVMZOAP-UHFFFAOYSA-N 0.000 claims description 4
- NUJOXMJBOLGQSY-UHFFFAOYSA-N manganese dioxide Chemical compound O=[Mn]=O NUJOXMJBOLGQSY-UHFFFAOYSA-N 0.000 claims description 4
- 239000000203 mixture Substances 0.000 claims description 4
- 238000001291 vacuum drying Methods 0.000 claims description 4
- 238000001035 drying Methods 0.000 claims description 3
- 206010013786 Dry skin Diseases 0.000 claims description 2
- 229910013553 LiNO Inorganic materials 0.000 claims description 2
- 101100513612 Microdochium nivale MnCO gene Proteins 0.000 claims description 2
- 150000001875 compounds Chemical class 0.000 claims description 2
- 238000005868 electrolysis reaction Methods 0.000 claims description 2
- 238000000227 grinding Methods 0.000 claims description 2
- XGZVUEUWXADBQD-UHFFFAOYSA-L lithium carbonate Chemical compound [Li+].[Li+].[O-]C([O-])=O XGZVUEUWXADBQD-UHFFFAOYSA-L 0.000 claims description 2
- 229910052808 lithium carbonate Inorganic materials 0.000 claims description 2
- 229940093474 manganese carbonate Drugs 0.000 claims description 2
- 235000006748 manganese carbonate Nutrition 0.000 claims description 2
- 239000011656 manganese carbonate Substances 0.000 claims description 2
- MIVBAHRSNUNMPP-UHFFFAOYSA-N manganese(2+);dinitrate Chemical compound [Mn+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O MIVBAHRSNUNMPP-UHFFFAOYSA-N 0.000 claims description 2
- 229910000016 manganese(II) carbonate Inorganic materials 0.000 claims description 2
- XMWCXZJXESXBBY-UHFFFAOYSA-L manganese(ii) carbonate Chemical compound [Mn+2].[O-]C([O-])=O XMWCXZJXESXBBY-UHFFFAOYSA-L 0.000 claims description 2
- 238000005245 sintering Methods 0.000 claims description 2
- 239000002002 slurry Substances 0.000 claims description 2
- 238000000875 high-speed ball milling Methods 0.000 claims 1
- TYTHZVVGVFAQHF-UHFFFAOYSA-N manganese(3+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Mn+3].[Mn+3] TYTHZVVGVFAQHF-UHFFFAOYSA-N 0.000 claims 1
- GEYXPJBPASPPLI-UHFFFAOYSA-N manganese(III) oxide Inorganic materials O=[Mn]O[Mn]=O GEYXPJBPASPPLI-UHFFFAOYSA-N 0.000 claims 1
- 230000008569 process Effects 0.000 abstract description 10
- 230000008901 benefit Effects 0.000 abstract description 5
- 239000013078 crystal Substances 0.000 abstract description 5
- 229910052596 spinel Inorganic materials 0.000 abstract description 5
- 239000011029 spinel Substances 0.000 abstract description 5
- 230000015572 biosynthetic process Effects 0.000 abstract description 4
- 238000003786 synthesis reaction Methods 0.000 abstract description 4
- 230000002950 deficient Effects 0.000 abstract description 2
- 230000003247 decreasing effect Effects 0.000 abstract 1
- 238000007781 pre-processing Methods 0.000 abstract 1
- 238000001308 synthesis method Methods 0.000 abstract 1
- 238000002360 preparation method Methods 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- 238000010532 solid phase synthesis reaction Methods 0.000 description 8
- 238000005516 engineering process Methods 0.000 description 7
- 238000002441 X-ray diffraction Methods 0.000 description 6
- 238000002389 environmental scanning electron microscopy Methods 0.000 description 6
- 238000003921 particle size analysis Methods 0.000 description 6
- 238000010189 synthetic method Methods 0.000 description 6
- IDSMHEZTLOUMLM-UHFFFAOYSA-N [Li].[O].[Co] Chemical compound [Li].[O].[Co] IDSMHEZTLOUMLM-UHFFFAOYSA-N 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 3
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 3
- 229910052744 lithium Inorganic materials 0.000 description 3
- 229910052748 manganese Inorganic materials 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 229910012851 LiCoO 2 Inorganic materials 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000007791 liquid phase Substances 0.000 description 2
- URIIGZKXFBNRAU-UHFFFAOYSA-N lithium;oxonickel Chemical compound [Li].[Ni]=O URIIGZKXFBNRAU-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 235000001674 Agaricus brunnescens Nutrition 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 229910012820 LiCoO Inorganic materials 0.000 description 1
- 229910013292 LiNiO Inorganic materials 0.000 description 1
- 229910013290 LiNiO 2 Inorganic materials 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- KLARSDUHONHPRF-UHFFFAOYSA-N [Li].[Mn] Chemical compound [Li].[Mn] KLARSDUHONHPRF-UHFFFAOYSA-N 0.000 description 1
- 239000006230 acetylene black Substances 0.000 description 1
- 239000004411 aluminium Substances 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000010406 cathode material Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000001351 cycling effect Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000007599 discharging Methods 0.000 description 1
- 238000000840 electrochemical analysis Methods 0.000 description 1
- 238000003487 electrochemical reaction Methods 0.000 description 1
- 239000007772 electrode material Substances 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000004146 energy storage Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 238000001027 hydrothermal synthesis Methods 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910002102 lithium manganese oxide Inorganic materials 0.000 description 1
- VLXXBCXTUVRROQ-UHFFFAOYSA-N lithium;oxido-oxo-(oxomanganiooxy)manganese Chemical compound [Li+].[O-][Mn](=O)O[Mn]=O VLXXBCXTUVRROQ-UHFFFAOYSA-N 0.000 description 1
- CPABIEPZXNOLSD-UHFFFAOYSA-N lithium;oxomanganese Chemical compound [Li].[Mn]=O CPABIEPZXNOLSD-UHFFFAOYSA-N 0.000 description 1
- LBSANEJBGMCTBH-UHFFFAOYSA-N manganate Chemical compound [O-][Mn]([O-])(=O)=O LBSANEJBGMCTBH-UHFFFAOYSA-N 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000011017 operating method Methods 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 238000004064 recycling Methods 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 238000003980 solgel method Methods 0.000 description 1
- 238000003836 solid-state method Methods 0.000 description 1
- 239000011010 synthetic spinel Substances 0.000 description 1
- 229910000314 transition metal oxide Inorganic materials 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Inorganic Chemistry (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Battery Electrode And Active Subsutance (AREA)
Abstract
The present invention relates to a manufacture method of positive electrode materials of a lithium ion battery. Lithium salt and manganese salt are mixed according to a definite mol metering ratio firstly by the present invention, mechanical activation and material mixing processing are carried out to the mixed materials on a high speed ball mill, preprocessing operation is carried out under low temperature, and finally roasting synthesis is carried out under high temperature to obtain spinel positive pole materials. The high speed ball mill is adopted to carry out the mechanical activation and the material mixing processing, mixing homogeneity of raw materials is ensured, synthesis temperature is reduced, and crystal grains are tiny and uniform. Defective spinel structures synthetized under the low temperature can be repaired and perfected by a two-step synthesis method, pure-phase spinel positive pole materials are easy to obtain, an attenuation rate of high temperature capacity is decreased, and the prices of the made spinel positive pole materials are cheap. The present invention has the advantages of simple process flow, no pollution and easy industrialization scale production.
Description
One, technical field:
The present invention relates to a kind of manufacture method of anode material for lithium-ion batteries, belong to the synthetic field of material.
Two, background technology:
The high sub-battery of lithium has obtained fast development since commercialization in 1991, not only be widely used in portable sets such as mobile phone, video camera, notebook computer, also is listed in the candidate power supply of electric automobile, space flight and aviation, military equipment and energy storage device.Anode material for lithium-ion batteries not only participates in electrochemical reaction as electrode material, but also is " depots " of lithium ion.Therefore, the anode material for lithium-ion batteries progress is directly restricting the development of lithium ion battery.
The research focus of anode material for lithium-ion batteries mainly concentrates on transition metal oxide lithium cobalt oxygen, lithium nickel oxygen, the lithium manganese oxygen (LiCoO of three kinds of rich lithiums at present
2, LiNiO
2And LiMn
2O
4).Wherein because lithium cobalt oxygen preparation is simple, the actual specific capacity height, so advantages such as good cycling stability are the commercialization that takes the lead in.But the resource of cobalt is quite limited, and the world can the amount of adopting only 8,300,000 tons, causes lithium cobalt oxygen number lattice costliness, limits the major obstacle that it further develops so resource scarcity will become; Though lithium nickel oxygen is cheap than lithium cobalt oxygen, obtain electrification LiNiO preferably
2Relatively more difficult, complicated process of preparation should not realize industrialization, as considers the cost of synthesis technique, and its price advantage is also than LiCoO
2Height not what; And spinelle LiMn
2O
4In manganese be seniority among brothers and sisters the 12nd high yield element on the earth, the manganese resource of China more accounts for first of the countries in the world, its aboundresources is cheap, manganese is nontoxic and pollute for a short time in addition, the recycling problem has accumulated rich experience, the LiMn of preparation in primary cell
2O
4The safe advantages such as (quite important aspect electrical source of power) of positive electrode causes many researchers' very big concern, is considered to the anode material for lithium-ion batteries of tool development prospect.Therefore from angle of sustainable development, the restricted degree of lithium ion battery of three kinds of positive electrode preparations increases progressively in the following order: Li-ion (Mn)<Li-ion (Ni)<Li-ion (Co).
Make a general survey of domestic and international spinelle LiMn
2O
4Present Research, present spinelle LiMn as can be known
2O
4The weak point of commercial applications is that capacity is low and the high temperature capacity attenuation is very fast, causes distortion of lattice thereby mainly show as the variation of charge and discharge process phase structure; Lithium ion takes off the embedding difficulty fully, causes circulation volume decay fast etc.And the synthetic method of these weak points and material and preparation technology are closely related, because synthetic method and preparation technology are determining the character such as pattern, granularity, specific area, crystal habit and lattice defect of material.These physicochemical properties directly have influence on the embedding of lithium ion and take off performance, are promptly determining chemical properties such as the charge/discharge capacity of material and cycle life.Therefore, improve the chemical property of material, exploitation is real green, the lithium ion battery manganate cathode material for lithium of high energy, and key will have breakthrough on the synthetic method of material and preparation technology.
Spinelle LiMn
2O
4Synthetic method be broadly divided into solid phase method and liquid phase method two classes.Solid phase method has high temperature solid-state method, melt impregnation, microwave chemical method etc., and liquid phase method has hydrolytic precipitation method, Pechini method, ion-exchange, sol-gel process, hydrothermal synthesis method etc.Though synthetic spinel LiMn
2O
4Synthetic method a lot, but consider the simple degree of technological process, the degree easy to control of preparation condition and be easy to the characteristics that industrialization is produced, great majority or the high temperature solid phase synthesis selected of suitability for industrialized production at present.But traditional high temperature solid phase synthesis exists the reaction diffusion velocity slow, and the product thing is mutually inhomogeneous, the phase structure poor stability, and crystallite dimension is big, and particle size distribution is wide, reaction temperature height, the shortcoming that the reaction time is long.
Mechanochemistry attracts much attention day by day as emerging frontier science, studies show that, the effect by mechanical force not only can make the lattice of material produce various defectives, dislocation, atom vacancy and distortion of lattice etc., helps the diffusion mobility of ion; Can make crystal grain produce new interface again, the surface activity of material is increased, surface free energy reduces, and promotes the carrying out of chemical reaction; Material is fully mixed, and the distribution of control granularity only also can realize some at low temperatures than the reaction that could take place under the exacting terms at high temperature etc.
Three, summary of the invention
1, purpose of the present invention:
The objective of the invention is in order to overcome the deficiency of above-mentioned existing solid phase synthesis law technology, on the basis of traditional high temperature solid phase synthesis, mechanical activation has been proposed---two step solid-phase synthesis, mutually inhomogeneous with the lithium manganese oxide product thing that solves the solid-phase synthesis preparation, the phase structure poor stability, shortcomings such as crystallite dimension is big, and particle size distribution is wide; Be raw material with resource cheap and easy to get simultaneously, under the prerequisite that guarantees material property, reduce production costs; Select technological process simply to be easy to the technology path of suitability for industrialized production.
2, technical scheme of the present invention:
Fig. 1 is a process chart of the present invention.This technological process is earlier lithium salts and manganese salt to be measured than preparing burden by certain mole, the material for preparing carries out mechanical activation and mixing treatment on clipping the ball mushroom machine, carry out preliminary treatment then at low temperatures, carry out mechanical activation again and handle, roasting at high temperature at last synthesizes spinelle LiMn
2O
4Positive electrode.
Concrete technological process operating procedure is as follows:
(1) be reaction raw materials with lithium salts and manganese salt, the mole metering ratio of Li/Mn is 0.95~1.03: between 2, the material for preparing can be undertaken by two kinds of scheme a or b:
In 120~200 ℃ of following vacuumizes 3~12 hours, in case the ball milling caking, natural cooling obtained dry mixture then in vacuum drying chamber for a, the material for preparing;
B, the material for preparing add organic solvent ethanol or acetone, the furnishing pulp-like, and the mass ratio of material and organic solvent is 100: 15;
(2) the above-mentioned compound that obtains is put into ball grinder, with 200~600 rev/mins speed ball milling, carry out mechanical activation and handle, the ball milling time is controlled at 6~30 hours, if slurry after then ball milling is finished, carry out vacuumize to remove organic solvent;
(3) material behind the mechanical activation, put into sintering furnace, earlier 300~500 ℃ of following cryogenic thermostat preliminary treatment 6-12 hour, take out after cooling, carried out the mechanical ball milling activation processing again 30 minutes~2 hours, under 600~850 ℃ temperature, synthesized 12~36 hours at last, with obtaining spinelle LiMn behind the stove natural cooling
2O
4Positive electrode.
Mentioned lithium salts can be a kind of in lithium hydroxide, lithium carbonate, the lithium nitrate among the present invention, and manganese salt can be a kind of in manganese dioxide, manganese carbonate, the manganese nitrate.
The lithium manganese anode material that the present invention is suitable for being prepared as follows: the Li of (1) mole metering ratio and non-mole metering ratio
xMn
2O
4Positive electrode, wherein 0.90≤x≤1.15; (2) the polynary system Li that mixes up
xMaMn
2-aO
4-bN
b, wherein M=Co, Cr, Ni, Al, V etc., N=F, I, S etc., wherein 0.90≤x≤1.15,0≤a≤0.2,0≤b≤0.5.
Present technique is compared with prior art and is had the following advantages:
(1) guaranteed the uniformity that raw material mixes, handled by mechanical activation and reduced synthesis temperature, and can control the size and the distribution of crystal grain, can generate tiny crystal grains by mechanical activation;
(2) can make the deficiency spinel structure that generates under the low temperature obtain reparation and perfect by two step synthetic methods, make the spinelle LiMn of pure phase easily
2O
4Positive electrode reduces its high temperature capacity attenuation rate;
(3) Zhi Bei spinelle LiMn
2O
4Positive electrode is cheap, only is LiCoO
21/10 of positive electrode;
(4) technological process is simple, and is pollution-free, is easy to industrial-scale production.
Four, description of drawings
Fig. 1 is a process chart of the present invention, and Fig. 2,3,4,5 is respectively X-ray diffraction analysis figure, ESEM, laser particle size analysis and the specific discharge capacity curve chart of embodiment 1; Fig. 6,7,8,9 is respectively X-ray diffraction analysis figure, ESEM, laser particle size analysis and the specific discharge capacity curve chart of embodiment 2; Figure 10,11,12,13 is respectively X-ray diffraction analysis figure, ESEM, laser particle size analysis and the specific discharge capacity curve chart of embodiment 3.
Embodiment
Embodiment 1
With LiOH and Mn (NO
3)
2It by Li/Mn 0.95: 2 molar ratio weighing batching, prepare the back 120 ℃ of following vacuumizes 8 hours, treat to put into after its cooling agate jar on planetary ball mill with 450 rev/mins speed ball milling, carrying out mechanical activation handled 15 hours, then earlier 450 ℃ of following constant temperature preliminary treatment 6 hours, cooling back ball milling activation 30 minutes was synthesized 36 hours at 700 ℃ of following constant temperature, then with obtaining spinelle LiMn behind the stove natural cooling
2O
4Positive electrode.To the spinelle LiMn that obtains
2O
4Positive electrode carries out X-ray diffraction analysis, ESEM, laser particle size analysis, and test result is seen Fig. 2,3,4 respectively.
The electrochemical property test of material is assembled into the bipolar electrode simulated battery to carry out.Anode pole piece is pressed LiMn
2O
4: acetylene black: PVDF=85: 8: 7 mixed is even, uses NMP furnishing pulp-like again, be coated on the thick aluminium thin set fluid of 20 μ m being coated with method with cutter on the coating machine, and technology such as drying, rolling, cutting, making diameter is 1cm
2, thickness is the thick positive plates of 130 μ m.It is 99.9% metal lithium sheet that negative pole adopts purity, and barrier film adopts Celgard 2300 PP/PE/PP composite membranes, and electrolyte adopts the 1mol/L LiFP of German Merck company
6-EC+DMC+DEC (1: 1: 1) is assembled into the bipolar electrode simulated battery in vacuum argon gas glove box.Hold up on day BS-9300 secondary cell detection system in Guangzhou at last and carry out electro-chemical test.Charging/discharging voltage 4.3~3.0V, electric current are 0.5mA/cm
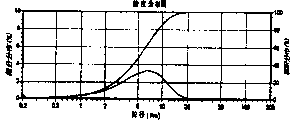
2Test result shows that the 1st time specific discharge capacity is 114.42mAh/g, and its specific discharge capacity curve is seen shown in Figure 5.
Embodiment 2
With LiNO
3And MnCO
3It by Li/Mn 1: 2 molar ratio weighing batching, prepare the back 150 ℃ of following vacuumizes 6 hours, treat to put into after its cooling agate jar on planetary ball mill with 500 rev/mins speed ball milling, carrying out mechanical activation handled 18 hours, then earlier 450 ℃ of following constant temperature preliminary treatment 10 hours, cooling back ball milling activation 1.5 hours was synthesized 30 hours at 800 ℃ of following constant temperature, with obtaining spinelle LiMn behind the stove natural cooling again
2O
4Positive electrode.To obtaining spinelle LiMn
2O
4Positive electrode carries out X-ray diffraction analysis, ESEM, laser particle size analysis, and test result is seen Fig. 6,7,8 respectively.
Electrochemical property test and assembling condition are with embodiment 1.Test result shows that the 1st time specific discharge capacity is 121.59mAh/g, and its specific discharge capacity curve is seen shown in Figure 9.
Embodiment 3
With Li
2CO
3With electrolysis MnO
2It by Li/Mn 1.03: 2 molar ratio weighing batching, mixture for preparing and ethanol are 100: 15 ratio adding ethanol furnishing pulp-like in mass ratio, put into agate jar on planetary ball mill with 600 rev/mins speed ball milling, carrying out mechanical activation handled 25 hours, put into vacuum drying chamber after ball milling is finished and remove ethanol, dry 30 minutes powdering material of material ball milling 180 ℃ of dryings.Then earlier 450 ℃ of following constant temperature preliminary treatment 12 hours, cooling back ball milling activation 2 hours is again 800 ℃ of following constant temperature Synthetic 2s 4 hours, with obtaining spinelle LiMn behind the stove natural cooling
2O
4Positive electrode.To obtaining the LiMn of spinelle
2O
4Positive electrode carries out X-ray diffraction analysis, ESEM, laser particle size analysis, and test result is seen Figure 10,11,12 respectively.
Electrochemical property test and assembling condition are with embodiment 1.Test result shows that the 1st time specific discharge capacity is 131.63mAh/g, and its specific discharge capacity curve is seen shown in Figure 13.
Claims (6)
1, a kind of manufacture method of anode material for lithium-ion batteries is prepared lithium salts and manganese salt in proportion, and carry out mechanical activation and mixing treatment on ball mill, carry out preliminary treatment more at low temperatures, roasting at high temperature at last is synthetic, obtains the spinelle positive electrode, it is characterized in that:
(1) be reaction raw materials with lithium salts and manganese salt, the mole metering ratio of Li/Mn is 0.95~1.03: between 2, the material for preparing can be undertaken by two kinds of scheme a or b:
In 120~200 ℃ of following vacuumizes 3~12 hours, natural cooling obtained dry mixture then in vacuum drying chamber for a, the material for preparing;
B, the material for preparing add organic solvent ethanol or acetone, the furnishing pulp-like, and the mass ratio of material and organic solvent is 100: 15;
(2) the above-mentioned compound that obtains is put into ball grinder, with 200~600 rev/mins speed high speed ball milling, carry out mechanical activation and handle, the ball milling time is controlled at 6~30 hours, if slurry after then ball milling is finished, carry out vacuumize to remove organic solvent;
(3) material behind the mechanical activation is put into sintering furnace, earlier 300~500 ℃ of following cryogenic thermostat preliminary treatment 6-12 hour, take out after cooling, carried out the mechanical ball milling activation processing again 30 minutes~2 hours, under 600~850 ℃ temperature, synthesized 12~36 hours at last, with obtaining spinelle LiMn behind the stove natural cooling
2O
4Positive electrode.
2, the manufacture method of positive electrode according to claim 1 is characterized in that: described lithium salts is a kind of in lithium hydroxide, lithium carbonate, the lithium nitrate.
3, the manufacture method of positive electrode according to claim 1 is characterized in that: described manganese salt is a kind of in manganese dioxide, manganese sesquioxide managnic oxide, manganese carbonate, the manganese nitrate.。
4, the manufacture method of positive electrode according to claim 1 is characterized in that: with LiOH and Mn (NO
3)
2It by Li/Mn 0.95: 2 molar ratio weighing batching, prepare the back 120 ℃ of following vacuumizes 8 hours, treat to put into after its cooling agate jar on planetary ball mill with 450 rev/mins speed ball milling, carrying out mechanical activation handled 15 hours, then earlier 450 ℃ of following constant temperature preliminary treatment 6 hours, cooling back ball milling activation 30 minutes was synthesized 36 hours at 700 ℃ of following constant temperature, then with obtaining spinelle LiMn behind the stove natural cooling
2O
4Positive electrode.
5, positive electrode manufacture method according to claim 1 is characterized in that: with LiNO
3And MnCO
3It by Li/Mn 1: 2 molar ratio weighing batching, prepare the back 150 ℃ of following vacuumizes 6 hours, treat to put into after its cooling agate jar on planetary ball mill with 500 rev/mins speed ball milling, carrying out mechanical activation handled 18 hours, then earlier 450 ℃ of following constant temperature preliminary treatment 10 hours, cooling back ball milling activation 1.5 hours was synthesized 30 hours at 800 ℃ of following constant temperature, with obtaining spinelle LiMn behind the stove natural cooling again
2O
4Positive electrode.
6, positive electrode manufacture method according to claim 1 is characterized in that: with Li
2CO
3With electrolysis MnO
2It by Li/Mn 1.03: 2 molar ratio weighing batching, mixture for preparing and ethanol are 100: 15 ratio adding ethanol furnishing pulp-like in mass ratio, put into agate jar on planetary ball mill with 600 rev/mins speed ball milling, carrying out mechanical activation handled 25 hours, put into vacuum drying chamber after ball milling is finished and remove ethanol 180 ℃ of dryings, dry 30 minutes powdering material of material ball milling, then earlier 450 ℃ of following constant temperature preliminary treatment 12 hours, cooling back ball milling activation 2 hours, again 800 ℃ of following constant temperature Synthetic 2s 4 hours, with obtaining spinelle LiMn behind the stove natural cooling
2O
4Positive electrode.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB2004100216587A CN1243385C (en) | 2004-01-10 | 2004-01-10 | Manufacturing method of lithium ion battery positive electrode material |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB2004100216587A CN1243385C (en) | 2004-01-10 | 2004-01-10 | Manufacturing method of lithium ion battery positive electrode material |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1556552A CN1556552A (en) | 2004-12-22 |
| CN1243385C true CN1243385C (en) | 2006-02-22 |
Family
ID=34351974
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB2004100216587A Expired - Fee Related CN1243385C (en) | 2004-01-10 | 2004-01-10 | Manufacturing method of lithium ion battery positive electrode material |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN1243385C (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102709545A (en) * | 2012-06-11 | 2012-10-03 | 湖南化工研究院 | Lithium manganese oxide cathode material preparation method for lithium ion power battery |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101060173B (en) * | 2006-04-19 | 2011-09-14 | 深圳市比克电池有限公司 | Complex Li-Mn-oxide, manufacture method and battery made of this material |
| CN102148359B (en) * | 2010-02-08 | 2015-12-02 | 清华大学 | The preparation method of lithium manganate anode active material |
| CN102201498B (en) * | 2011-05-18 | 2014-04-16 | 东华大学 | Method for preparing Cu2ZnSnS4 nanocrystalline thin-film solar cell |
| CN103606669B (en) * | 2013-11-28 | 2016-01-20 | 福建师范大学 | Mix the preparation method of the spinel lithium-rich lithium manganate cathode material of trivalent scandium or chromium |
| CN103579613B (en) * | 2013-11-28 | 2017-01-25 | 福建师范大学 | Method for preparing spinel-doped lithium-enriched lithium manganate anode material through doping zirconium |
| CN103594700B (en) * | 2013-11-28 | 2016-03-09 | 福建师范大学 | Mix the preparation method of the rich lithium manganate cathode material for lithium of vanadic spinel |
| CN103606670A (en) * | 2013-12-03 | 2014-02-26 | 苏州科大微龙信息技术有限公司 | Lithium manganate positive electrode material of power lithium ion battery and preparation method thereof |
| CN106784791A (en) * | 2016-12-30 | 2017-05-31 | 湘潭大学 | The preparation method of power type nanometer lithium manganate |
| CN107399764B (en) * | 2017-07-10 | 2019-08-20 | 合肥国轩高科动力能源有限公司 | Submicron lithium manganate for lithium ion battery anode and preparation method thereof |
| CN113443653A (en) * | 2021-06-29 | 2021-09-28 | 哈尔滨工业大学 | Ball milling-heat treatment activation method for lithium dimanganese oxide three-positive electrode material |
| CN114249338B (en) * | 2021-08-20 | 2023-05-02 | 山东泰普锂业科技有限公司 | Preparation method of battery-level high-purity hollow lithium carbonate structure for lithium ion battery |
-
2004
- 2004-01-10 CN CNB2004100216587A patent/CN1243385C/en not_active Expired - Fee Related
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102709545A (en) * | 2012-06-11 | 2012-10-03 | 湖南化工研究院 | Lithium manganese oxide cathode material preparation method for lithium ion power battery |
Also Published As
| Publication number | Publication date |
|---|---|
| CN1556552A (en) | 2004-12-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102683669B (en) | Anode material for lithium-ion batteries and preparation method thereof | |
| CN102263239B (en) | One kind graphene coated adulterated lithium manganate composite positive pole and preparation method thereof | |
| CN101320807B (en) | Positive electrode material of multi-component composite lithium ion cell and its preparation method | |
| CN103762354B (en) | A kind of LiNi0.5Mn1.5O4 material, its preparation method and lithium ion battery | |
| CN102306767B (en) | Method for preparing spinel lithium manganate serving as cathode material of lithium ion power battery | |
| TW200428693A (en) | Positive electrode material, its manufacturing method and lithium secondary battery | |
| Lou et al. | Mg-doped Li1. 2Mn0. 54Ni0. 13Co0. 13O2 nano flakes with improved electrochemical performance for lithium-ion battery application | |
| CN1790782A (en) | Anode material of lithium ion cell and preparation method thereof | |
| CN1243385C (en) | Manufacturing method of lithium ion battery positive electrode material | |
| CN105576231A (en) | High-voltage lithium oil battery positive electrode material with spinel structure and preparation method of high-voltage lithium oil battery positive electrode material | |
| CN103794777A (en) | Preparation method of surface covered nickel lithium manganate positive electrode material | |
| CN104112849A (en) | Light metal element-doped ternary lithium ion battery positive electrode material and synthesis method thereof | |
| CN1803592A (en) | Method for preparing lithium enriched lithium ion phosphate powder | |
| CN106992295B (en) | A kind of preparation method of monodisperse alpha-ferric oxide nanometer sheet | |
| CN110112459B (en) | Preparation method of sulfide solid electrolyte and all-solid-state lithium-sulfur battery | |
| CN110931770A (en) | Cr-doped modified high-voltage spinel cathode material and preparation method thereof | |
| CN102751483A (en) | Layered rich-lithium-manganese-based solid-solution anode material of lithium ion battery and method for preparing anode material | |
| CN101359735A (en) | Method for preparing lithium ionic cell positive pole material by ultrasonic cavitation solid phase synthesis | |
| CN100530779C (en) | Preparing method for spinel potassium manganate as lithium ion battery anode of electric vehicle | |
| CN101719422B (en) | Anode material for lithium ion capacitor or battery and preparation method thereof | |
| CN101286562A (en) | Preparation method of positive electrode material of lithium ion cell | |
| Liu et al. | The influence of surface lithium residue to the performance of LiNi0. 9Co0. 05Mn0. 05O2 cathode materials | |
| CN1834022A (en) | Anode material of lithium ion cell and its prepn method | |
| Zhao et al. | Toward high stability single crystal material by structural regulation with high and low temperature mixing sinter | |
| CN115312754A (en) | Lamellar cobalt-free lithium-rich manganese-based positive electrode material and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| C17 | Cessation of patent right | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20060222 Termination date: 20100210 |